Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 16/82/01. The contractual start date was in November 2017. The draft manuscript began editorial review in March 2022 and was accepted for publication in November 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Bugge et al. This work was produced by Bugge et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Bugge et al.
Chapter 1 Introduction
Scientific background and current evidence base
Pelvic organ prolapse
Pelvic organ prolapse (hereafter prolapse) is the descent of some, or all, of the female pelvic organs from their usual position in the pelvis into the vagina. 1 Prolapse is common, with one UK survey identifying that 8.4% of community-dwelling women report a vaginal lump or bulge2 and up to 50% of women reported to have prolapse on examination. 3 The prevalence of prolapse increases with age and, with the UK population of older adults increasing, it is anticipated that the prevalence of prolapse will also increase. 4
Two large cohort studies identify that prolapse has a multifactorial aetiology. 5,6 Factors that predict prolapse include pregnancy, vaginal delivery, hereditary factors, ageing, menopause, and factors associated with chronically raised intra-abdominal pressure (caused by, e.g., obesity or heavy lifting). 5,7
Women report symptoms of ‘something coming down’ in their vagina, a dragging sensation in their vagina, a bulge coming down from their vagina, and pelvic and back pain. 1 The movement of the pelvic organs into the vagina can also cause urinary, bowel and sexual problems. 1 Women’s quality of life, body image and ability to function in their day-to-day life are negatively affected by their symptoms in general and the extent to which those symptoms are bothersome. 8–11
Current treatment options for pelvic organ prolapse
Women with prolapse can be treated with surgical or conservative options. Approximately 9.5% of women will undergo surgery for prolapse in their lifetime. 12–14 One study estimated the cost of any prolapse surgery in England, in 2005, as €81,030,907. 15
There are several conservative treatment options: lifestyle advice, pelvic floor muscle training, vaginal lubricants (hormonal and non-hormonal) and vaginal pessary use. Lifestyle interventions include features such as weight management or physical activity, each of which has minimal evidence of effect. 16,17 Pelvic floor muscle training has been shown to be effective across several clinical trials. 18,19 Local oestrogen as a treatment for prolapse may need further evidence to guide practice. 20
Few clinical trials have assessed the effectiveness of vaginal pessaries. 1 However, two-thirds of women will opt to try a vaginal pessary when it is offered. 21 The largest UK-based pessary study reported that 86% of women who successfully retain their pessary at 4 weeks will continue to use a pessary at 5 years. 22 However, other studies have reported lower continuation rates, for example 62.1% among women aged 65–74 years and 37.8% among those aged ≥ 75 at 5 years. 23 Reasons for pessary discontinuation include development of complications, dislike of pessary changes (insertion or removal) and inconvenience of attending appointments. 24–27
Care pathways for women who use a vaginal pessary as treatment for prolapse
Vaginal pessaries are a common, and recommended, treatment option in the UK NHS. 3,28 National Institute for Health and Care Excellence (NICE) guidance recommends that a woman be offered 6-monthly appointments if she is at risk of complications that may make it ‘difficult for them to manage their ongoing pessary care’ (recommendation 1.7.9). 3 The NICE guidance does not offer clear recommendations on self-management; however, joint guidance from the United Kingdom Continence Society and Pelvic, Obstetric and Gynaecological Physiotherapy recommends that women are offered self-management where that option is available. 29
A UK multiprofessional survey found that only 17% of clinicians offered their patients the option of self-managing their pessary, with most pessary care being delivered in clinics. 30 This is a difference in practice from North America, where self-management is offered more routinely. 31 Clinic-based care therefore seems to be the most common way of delivering pessary care in the UK, but alternative models of care delivery do exist.
Evidence for the effectiveness of self-management of vaginal pessaries for prolapse
There are no published trials to date comparing the effectiveness of pessary self-management with that of clinic-based care. 32 One small (n = 88), non-randomised study assessed self-management of vaginal pessaries;33 this study reported gains from self-management, in that women reported higher levels of convenience, ability to access help, support and comfort than those attending clinic. 33 A few observational studies have addressed various features of self-management. For example, Manonai et al. 34 undertook a chart review and found that self-management was a strong predictor of pessary continuation for women in Thailand at 3 years. The observational studies suggest that self-management may be a viable treatment option, but self-management effectiveness has not been tested.
Proposed mechanism of action for why self-management might improve quality of life for women with prolapse
The treatment of prolapse with self-care pessary (TOPSY) trial was developed prior to the publication of the 2021 complex interventions framework35 and therefore draws on the 2008 framework36 and the 2015 process evaluation guidance. 37 We will therefore refer to the mechanism of action of the intervention as opposed to the programme theory.
The mechanism of action of the TOPSY self-management intervention is based within self-efficacy theory38 and self-management theory. 39 Self-efficacy has been argued to be the mechanism through which self-management achieves its goals. 39,40 Self-efficacy focuses on an individual’s beliefs in their abilities to achieve goals. 38 Previous service evaluation has suggested that women who self-manage may feel more in control of managing their prolapse than those who receive clinic-based care. 33 Self-management theory posits that three tasks need to be achieved for individuals to self-manage: medical management of the condition, role management and emotional management. 39 Based within these theoretical constructs it was therefore hypothesised that self-management of a vaginal pessary will lead to improved quality of life for women with prolapse because support at service and professional levels, receipt of information and self-management support will lead to women becoming more confident (self-efficacious) about their pessary management; will improve their understanding of and confidence in their role to self-manage; and will enhance their emotional capacity and confidence to cope with their pessary such that their condition-specific quality of life will be improved more than that of those who receive clinic-based care. The details of the intervention are presented in Chapter 2.
Rationale for the research
It is unclear if self-managing a vaginal pessary, rather than receiving care in a clinic, would improve women’s quality of life. Data from non-pelvic health and pelvic health observational studies suggest that self-management interventions have the potential to improve quality of life. Thus, a robust comparison of a theoretically developed self-management intervention with clinic-based care is imperative to understand if alternative clinical models of pelvic health delivery can support better outcomes for women.
Aims and objectives
This research aims to answer the following research questions (RQs):
-
What is the clinical effectiveness and cost-effectiveness of self-management of vaginal pessaries to treat pelvic organ prolapse, compared with clinic-based pessary care, on condition-specific quality of life? (RQ1).
-
What are the barriers to and facilitators of intervention acceptability, intervention effectiveness, fidelity to delivery, and adherence for women treated with vaginal pessary and the healthcare professionals (HCPs) who treat them, and how does this differ between randomised groups? (RQ2).
The specific objectives are:
-
to undertake a parallel-group, multicentre, individual randomised controlled trial to test for the superiority of pessary self-management compared with clinic-based pessary care in terms of women’s condition-specific quality of life
-
to undertake an internal pilot study to ensure that the trial can recruit, randomise and retain sufficient numbers of participants while delivering the intervention as planned
-
to undertake a process evaluation in parallel with the trial to maximise recruitment; assess eligible but non-randomised women; understand women’s experience and acceptability of the intervention; assess adherence to allocated trial group; describe fidelity to intervention delivery; and identify contextual factors that may interact with intervention effectiveness
-
to undertake an economic evaluation to establish whether pessary self-management is cost-effective compared with clinic-based pessary care.
The structure of the report
Chapter 2 outlines the study design. It is split into three sections outlining the three component parts of the study: the trial, the process evaluation and the economic evaluation. The pilot study was reported previously and therefore is not included in this report, but it is included as part of the Project Documentation. 41 This Project Documentation, which we refer to throughout this report, is listed in Appendix 1 and available to view on the award website41 [https://fundingawards.nihr.ac.uk/award/16/82/01 (accessed June 2022)]. Chapters 3–5 present the findings of the trial, process evaluation and cost-effectiveness analysis, respectively. Chapter 6 brings some key points from across the three components together in a synthesis, and Chapter 7 presents the discussion.
Chapter 2 Study design and methods
Sections of this chapter have been reproduced from the TOPSY protocol papers published by the same authorship group,42,43 published under licence CC-BY-4.0, or by re-use of materials available on the project website and listed in Appendix 1.
The design and methods are explained for each part of the TOPSY study: the randomised controlled trial, the process evaluation and the cost-effectiveness evaluation. Appendix 2 is a study flow diagram that provides an overview of the study. Published protocols are available for the trial and cost-effectiveness evaluation42 and process evaluation. 43 The funder-approved protocol is available on the project website. 41
Internal pilot study
The internal pilot study was undertaken to ensure that the trial could recruit, randomise and retain sufficient numbers of participants while delivering the intervention as planned. Stop/go criteria were applied that focused on recruitment and retention. Both of the pilot study targets, namely to recruit 63 women across six centres over 6 months and for 60% of those in the self-management group to be self-managing at the 2-week follow-up telephone call, were achieved. The study therefore continued as planned.
The internal pilot study findings were reported to the funder in January 2019. That report is available as part of the Project Documentation on the project website. 41 Data from non-randomised women heavily influenced our understanding of recruitment processes and their acceptability, and these are reported in detail in the pilot study report. Participant data gathered in the pilot study were the same as the data gathered in the main trial, process evaluation and cost-effectiveness analysis. As a result, the pilot study used the same methods reported here for all parts of the study, and all pilot study data are included in the analysis presented in this report.
The treatment of prolapse with self-care pessary trial
The trial is reported following guidance from the Consolidated Standards of Reporting Trials (CONSORT)44 and the Template for Intervention Description and Replication (TIDieR). 45
Design
The TOPSY trial was designed to compare vaginal pessary self-management with clinic-based pessary care for pelvic organ prolapse in order to assess improvement in women’s quality of life. 42 It included a multicentre superiority randomised controlled trial comparing two parallel treatment groups: pessary self-management and clinic-based pessary care (the results can be found in Chapter 3).
Recruitment to the trial was completed on 6 February 2020, before the start of the COVID-19 pandemic. Follow-up procedures amended due to the pandemic are detailed in this chapter and included in a COVID-19 annex in the funder-approved protocol.
Participants and setting
The trial recruited women who used a vaginal pessary for the management of pelvic organ prolapse from 21 UK hospital-based centres (see Appendix 3, Table 36).
Inclusion criteria
Women were eligible for inclusion if they:
-
were aged ≥ 18 years
-
were using a pessary of any type/material (except shelf, Gellhorn or cube pessaries)
-
had retained the pessary for at least 2 weeks.
Exclusion criteria
Women were ineligible if they:
-
had limited manual dexterity that would affect their ability to remove and replace their pessary
-
were judged by their healthcare team to have a cognitive deficit such that it was not possible for them to provide informed consent or self-manage
-
were pregnant
-
had insufficient understanding of the English language (the self-management teaching was only available in English).
Recruitment procedure
To identify potential participants, local centre staff reviewed patient notes, clinic lists and caseloads to identify women who were currently using a pessary and were suitable to be approached. In addition, women were identified at appointments when attending for pessary review (existing users) or being fitted with a pessary for the first time (new users). If a woman had previously used a pessary but was having a break in pessary use, she was classed as an existing user, as it was believed that her experience would mean she had existing knowledge of pessary use. Women who learned about the TOPSY study themselves (by the website, posters, word of mouth) could approach their centre or the trial office to enquire about participation.
Women were provided with a recruitment pack (either given in person or sent by post) containing an introductory letter, a participant information leaflet, an expression of interest form and a reply-paid envelope. Once women had made their decision regarding participation, they returned the expression of interest form to the local clinical team. On receiving a positive expression of interest form, a member of the local clinical team discussed the study further with the woman in question and screened her for eligibility.
If the woman was a new pessary user (had used a pessary for ≤ 3 months), eligibility screening included a telephone call to confirm that the pessary had been retained for at least 2 weeks. If not, and the woman remained interested in participating, eligibility was reassessed once the pessary had been retained for 2 weeks.
Consent
If a woman was eligible and willing to take part, she attended a baseline clinic appointment where she provided written, informed consent for randomisation. The trial consent form asked participants if they were willing to be contacted about taking part in interviews for the process evaluation, and if they were willing to have their self-management teaching session or 2-week telephone call digitally recorded. They were also asked if they could be contacted about future research.
Women were informed of their right to withdraw at any time from all or part of the study. Any change to women’s participation was recorded in a study change of status form. Women randomised to self-management could opt to change to clinic-based care, and their reasons for choosing to do this were recorded. A woman randomised to clinic-based care could cross over to self-management only if she received formal TOPSY training and was no longer on a regular clinic-based pathway. If a woman discontinued pessary use, she could remain in the study and continue with the data collection elements of the research.
Participant retention
Active measures to minimise loss to follow-up of participants included:
-
Recording women’s e-mail addresses and mobile phone numbers at the outset, their preferred method of contact (for follow-up) and their preferred method of completing questionnaires. Questionnaires could be completed online (via an e-mail link) or on paper and returned by post.
-
Any participant who did not return their questionnaires within 3 weeks was sent a maximum of three reminders, the first two of which were via the participant’s preferred method. The third reminder was a telephone call in which either the researcher reminded the participant to complete and return the questionnaire or the questionnaire was completed during the call.
Response rates to the participant-completed questionnaires were monitored closely to ensure that they remained above 80%.
Randomisation, concealment and blinding
The trial was supported by the Centre for Healthcare Randomised Trials (CHaRT), a fully registered UK Clinical Research Network clinical trials unit in the Health Services Research Unit, University of Aberdeen. CHaRT developed an internet-based data management system and remote automated computerised randomisation system for the trial.
After informed consent was obtained from a woman, the local clinical staff entered the required information into the data management system to remotely generate the participant’s group allocation. The centralised randomisation of participants after enrolment ensured allocation concealment. The randomisation system assigned women to one of the two trial groups, with an even allocation ratio and naive minimisation by age (< 65/≥ 65 years), pessary user type (new user/existing user) and centre.
Due to the nature of the trial interventions, the trial group to which women were allocated could not be masked from the participants or the centre staff who provided treatment and assessed outcomes after randomisation, and therefore blinding was not possible.
Intervention
Self-management
The self-management of pessary intervention was developed using the Medical Research Council complex intervention framework,36 normalisation process theory46 and self-management theory,39 with the aim of boosting self-efficacy guided by the tasks and skills described by Lorig and Hollman39 as necessary to self-manage a health condition. No previous trials of self-management of pessary had been identified and only one paper outlining a self-management intervention was found. 33 Therefore, informal consensus methodology was used. A draft protocol for pessary self-management support was created by two clinical co-applicants drawing on the sole paper identified during the literature search33 and their own clinical practice. This was subsequently reviewed by clinical and pessary user co-applicants. Feedback was received and reviewed by the co-applicants and changes made accordingly. The protocol was changed to reflect feedback about the language used when discussing the correct positioning of the pessary.
The self-management support documentation was then reviewed by the Royal College of Obstetricians and Gynaecologists Women’s Voices panel. Further amendments were made to ensure that the content offered pragmatic and realistic self-management advice that met women’s information needs. This included the addition of further illustrations and details about pessary insertion and removal.
To support a woman to achieve the three tasks needed for self-management, the intervention was directed at three levels:
-
at a service level to facilitate a supportive culture for a self-management treatment pathway
-
at a professional level to ensure that staff had the necessary self-management teaching and support skills
-
at an individual woman level to ensure that women could achieve the necessary tasks to self-manage.
As many different health professional groups deliver pessary care in the UK, a pragmatic approach was taken to who delivered the intervention based on pessary management practice at the hospital or clinic site to ensure that the intervention was only delivered by a HCP who already delivered pessary care as part of their role. This included doctors, nurses (bands 5–8), and physiotherapists.
A clinical co-applicant delivered intervention delivery training before recruitment opened at all sites. The majority of the training was completed face to face. In a few instances, the training was delivered remotely when either a staff member was absent from the initial site initiation visit or a new site staff member came on board during the recruitment phase and needed to be trained to deliver the intervention at the site. The training presentation covered pessary self-management, each aspect of the intervention, why it was necessary and the information to be included. A reference training manual was also provided, which specified the key components of the self-management intervention, facilitating standardisation of the self-management intervention across all centres. During the site visit, the TOPSY team ensured that the intervention was compatible with how pessary self-management was currently taught (if applicable) and could be feasibly delivered. By ensuring that additional training was not onerous and did not conflict with established working, cognitive participation and collective action were secured among clinicians and key stakeholders in intervention delivery. Following the site visit, HCPs who accepted delegated responsibility for intervention delivery were asked to sign a training record confirming that they had received the training and felt confident in delivering self-management support as part of the trial. All those who delivered the self-management intervention received training and signed the training record.
Women allocated to self-management received a self-management teaching appointment, a self-management information leaflet, a 2-week follow-up telephone call, and a telephone helpline number/e-mail address for their local clinical site. The self-management teaching appointment followed the guidance given in the training manual. 41 During the appointment, women were also given a self-management information leaflet containing written information about pessary self-management. The leaflet included diagrams of various pessary types and pelvic floor anatomy and information about common complications and what to do if these were experienced. The same leaflet was used across all centres.
Participants in the self-management group were asked to remove, clean and reinsert their pessary at least once in the 2 weeks following the self-management teaching appointment. They were telephoned 2 weeks after the appointment and asked if they had been successful in removing, cleaning and reinserting their pessary and wanted to discuss any difficulties experienced. If the participant had not changed the pessary, she was asked to try again during the following week, and a subsequent telephone call was completed. If a participant experienced difficulty that necessitated HCP assessment or had not changed the pessary by the time of the second telephone call, she was offered a second self-management teaching appointment. If, after this second appointment, the participant was unable to self-manage or did not wish to do so, she was given the choice to transfer to clinic-based pessary care. Once it was clear that a participant could remove and reinsert the pessary at least once, she was asked to do this at least once every 6 months.
Participants in the self-management group also received a local telephone number and an e-mail address to contact the intervention HCP at their centre if they experienced any pessary-related problems or had questions. Women in the self-management group using a PVC (polyvinyl chloride) pessary received a new pessary by post or by prescription, or they were given two extra pessaries at the baseline visit. Women using silicone pessaries, which are more durable, had the pessary replaced only if required (e.g. if the pessary became damaged).
Clinic-based care
In the clinic-based care group, pessary management appointments were conducted in accordance with each local centre’s policy (commonly every 6–12 months, but sometimes as often as every 4 months for a new pessary user). Care was delivered during these appointments in accordance with the usual local centre protocols.
Data collection, management and storage
Data were gathered from participants and other sources throughout the trial. An overview of the trial data collected is presented in Table 1. Introductory information and instructions in the questionnaire booklet were drawn from previous trials led by the applicant team. 47 All data collection instruments are available on the project website as part of the Project Documentation. 41
| Data collected | Data type (for outcome measures) | Time point | |||
|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | 18 months | ||
| Consent and randomisation | N/A | X | |||
| Demographics and medical history | N/A | X | |||
| Primary outcome | |||||
| Condition-specific quality of life (PFIQ-7) | Continuous | X | X | X | X |
| Secondary outcomes (validated) | |||||
| Generic quality of life (EQ-5D-5L) | Continuous | X | X | X | X |
| Pelvic floor symptoms (PFDI-20) | Continuous | X | X | X | X |
| Sexual function (PISQ-IR) | Continuous | X | X | X | X |
| General Self-efficacy Scale | Continuous | X | X | ||
| Patient Global Impression of Improvement | Ordinal | X | X | X | X |
| Secondary outcomes (non-validated) | |||||
| Pessary Complications Questionnaire (to assess complications specific to pessary use) | Continuous | X | X | X | X |
| Pessary Use Questionnaire (to assess pessary use, acceptability and benefit)a | Binary/ordinal | X | X | X | X |
| Pessary Confidence Questionnaire (to measure pessary-specific self-efficacy)a | Continuous | X | X | X | X |
| Health Resource Use Questionnaire (uptake of additional prolapse treatment/support) | Binary | X | X | X | |
| Telephone support log (uptake of telephone support related to pessary use) | Continuous | Continuous data collection | |||
| Adherence to randomised protocol | Binary | Continuous monitoring | |||
| Health of vaginal tissues (vaginal examination in clinic)b | Binary | X | X | ||
| COVID-19 surveyc | N/A | Completed at first clinic visit once services resumed | |||
All participants were given an individual trial identification number, which was used on all trial paperwork. After a participant consented, her demographic and medical history data were collected. Table 1 shows the primary and secondary outcome measures collected at each trial time point, and more detailed information is given in Outcome measures.
At 18 months after randomisation, participants in both groups attended a clinic appointment that included an examination of vaginal tissues. All follow-ups were completed by September 2021.
Participants in both trial groups were asked to complete questionnaires at baseline and at 6, 12 and 18 months. Participants who opted to complete the questionnaires on paper posted these back to the TOPSY office where they were checked for completeness and data were entered into the data management system. Women were sent a £10 voucher with their 18-month questionnaire (whether they returned it or not). For those participants who opted to complete questionnaires online, no further data entry was required but a check for completeness was carried out. If any data were missing, checks were undertaken to see if there was any supporting evidence of what the missing data should be. Self-evident correction was made only if there was evidence to allow this. Detailed information on permitted self-evident corrections was documented in the TOPSY data entry guidelines. If a large part of the questionnaire was missing, attempts were made to contact the participant to obtain the information.
Data return rates were continually monitored by the central team for completeness and timeliness of all data returned. The frequency with which those randomised to self-management reverted to clinic-based care was reviewed at every team meeting (usually fortnightly) and reported to the Data Monitoring and Ethics Committee (DMEC).
Outcome measures
Primary outcome measure
The primary outcome of condition-specific quality of life at 18 months post randomisation was measured using the participant-completed Pelvic Floor Impact Questionnaire (PFIQ-7). 48 The PFIQ-7 is a reliable, valid and responsive short-form of the PFIQ that measures condition-specific quality of life in women with pelvic floor disorders including urinary incontinence, prolapse and faecal incontinence. The participant-completed instrument included questions about the effect of bladder, bowel and vaginal symptoms on the woman’s activities, relationships and feelings. There are three subscales (UIQ-7, CRAIQ-7, POPIQ-7), with each subscore ranging from 0 to 100 and the total score ranging from 0 to 300. Data were collected at each time point to allow repeated measures analysis of the PFIQ-7 scores.
Validated secondary outcome measures
The EuroQol-5 Dimensions, five-level version (EQ-5D-5L),49 was used to measure participants’ general health-related quality of life, complementing the primary outcome measure of condition-specific quality of life, and to provide data for the analysis of cost-effectiveness using quality-adjusted life-years (QALYs). The EQ-5D-5L is a two-part instrument. The first section, the EQ-5D descriptive system, contains five items: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The second part, the EQ-5D VAS, is a visual analogue scale. Data were collected at each time point to give a complete profile of QALYs across the trial time points, calculated using an area under the curve method.
The Pelvic Floor Distress Inventory-20 (PFDI-20) measured the severity of pelvic-floor-related symptoms. This was developed and validated in parallel with the PFIQ-7. 48 It comprises 20 questions about the presence of bladder, bowel and prolapse symptoms and how bothersome these are. There are three subscales (Urinary Distress Inventory-6 [UDI-6], ColoRectal Anal Distress Inventory-8 [CRADI-8], Pelvic Organ Prolapse Distress Inventory-6 [POPDI-6]), with each subscore ranging from 0 to 100 and having a total score of 0–300.
The Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire, IUGA-Revised (PISQ-IR),50 was used to assess female sexual function in women with pelvic floor disorders. It contains 10 subscales, of which six are relevant to women who are sexually active and four are relevant to women who are not sexually active. A psychometrically valid summary score can be created for sexually active women only and is calculated as a mean of scores ranging from 1 to 5. 51 The PISQ-IR is a revision based on the PISQ-12, which was the originally planned measure of sexual function (this was the only change to any of the planned outcome measures).
The General Self-efficacy Scale52 was used to assess general self-efficacy (hypothesised to be a moderator of quality of life). This is a 10-item scale with scores ranging from 10 to 40.
Non-validated secondary outcome measures
Pessary Complications Questionnaire
A new pessary questionnaire (listing 15 possible complications of pessary use), developed based on the literature, women’s experiences and the team’s experiences in a previous service evaluation,33 was used to assess women’s pessary-related complications (e.g. discharge, odour, pain, discomfort, bleeding). The same questionnaire was used in both groups.
Pessary Use Questionnaire
A new questionnaire (of nine questions) developed based on the literature and women’s experiences was used to assess the pattern of women’s pessary use, including perceived acceptability and benefit. This included questions on whether or not women were still using a pessary as treatment for prolapse; when they last removed and reinserted their pessary; the reasons for pessary removal; interference of the pessary with everyday life; and if they found the pessary an acceptable treatment. Also included in the questionnaire was a question adapted from the Patient Global Impression of Improvement that was used to assess perceived benefit of the pessary care regimens being evaluated. The Patient Global Impression of Improvement is a single-item tool that rates the change in a condition since having treatment and has been validated for urogenital prolapse. 53,54 An amended version was used that asked women to describe how they felt about their pessary care since taking part in the TOPSY study. The standard range of response options from very much better to very much worse was used. Patterns of pessary use were used to measure the impact of, adherence to and acceptability of the trial interventions.
Pessary Confidence Questionnaire (to measure pessary-specific self-efficacy)
No suitable condition-specific measure existed; thus, questions were developed relating to pessary self-efficacy based on the guidance from Bandura. 38 These six questions were discussed with and reviewed by patient and public involvement (PPI) representatives, statistical experts, the Project Management Group (PMG) and clinical team members before they were used and assessed in the pilot study. We used both the generic validated measure of self-efficacy (the General Self-efficacy Scale) and the responses to the developed pessary-specific self-efficacy questions to measure self-efficacy and to aid understanding of the influence self-efficacy had as a moderating factor on quality of life.
Uptake of additional treatment for prolapse
As an indicator of intervention effectiveness, the uptake of additional treatment for prolapse since the start of the study, or treatment awaited, was recorded in participant questionnaires (e.g. surgery, pelvic floor muscle training, oestrogen use, lifestyle advice). Participants’ access to professional pessary-related support since starting the study was also recorded [e.g. telephone support, a hospital appointment, a general practitioner (GP) appointment]. These data were collected at all trial time points to maximise reliability as they rely on participants recalling events occurring over a period of months.
Uptake of telephone support related to pessary use
Using a telephone support log form, we asked the intervention HCP to record the frequency and details of all participant calls to the telephone support line. In addition, the pessary complication questionnaire included a question to all women about telephone support they had accessed from their local team.
Adherence to randomised protocol
Adherence to the self-management or clinic-based care protocol was monitored throughout the trial. Monitoring was via multiple data sources: questions in the Pessary Use Questionnaire, telephone support contacts and health records. It included monitoring crossover to the other trial group (i.e. self-management group participants crossing over to clinic-based care). Clinic-based care group participants did not have access to the trial self-management teaching and support intervention and therefore did not cross over. However, individual women may choose (without being trained to do so) to remove and replace their pessary at home, and instances of this were recorded in the Pessary Use Questionnaire.
Health of vaginal tissues
At baseline and 18 months, all women in the trial underwent a vaginal examination by a HCP at the clinic to assess the health of the vaginal tissues and identify any problems associated with pessary use. Information was collected on inflammation of vaginal tissues, ulceration, granulation and any other clinical concerns.
Sample size
The aim was to recruit sufficient participants to detect a 20-point difference between groups in the primary outcome measure, namely PFIQ-7 score at 18 months. The potential range of scores on the PFIQ-7 is 0–300, and, in the absence of robust data on minimal clinically important difference, the clinicians in the research team and the wider Trial Steering Committee (TSC) considered 20 points to be an important clinical difference. A sample size of 330 women (165 per group) was required to provide 90% power to detect a difference of 20 points in the PFIQ-7 score at 18 months, assuming a standard deviation (SD) of 50, which was based on previous studies,55,56 two‐sided alpha of 0.05, and 20% loss to follow-up.
COVID-19: changes to follow-up assessment
This section describes the changes to study processes that were implemented from 21 April 2020 due to the COVID-19 pandemic. As recruitment was complete in February 2020, only follow-up processes were amended.
Clinic-based pessary care during the pandemic
All 21 centres postponed pessary clinics for at least 3 months at the start of and intermittently throughout the pandemic, depending on local lockdown procedures. All centres let women know (by either letter or telephone call) what to do if they had any issues with their pessaries. Some centres implemented a new standard procedure of calling women when they would have been due a follow-up appointment to carry this out a remotely over the telephone. If this happened, the clinical staff completed the telephone support log form that captured the same information that would have been collected at clinic. If centres did not call women as part of their standard care pathway, they were not asked to complete this.
Eighteen-month treatment of prolapse with self-care pessary visit: health of vaginal tissues (COVID-19)
The greatest impact of the COVID-19 pandemic was that participants could not attend their 18-month end-of-study TOPSY visit, which included an examination of the vaginal tissues, if pessary clinics were postponed/cancelled. Therefore, from April 2020, if a woman could not receive her 18-month TOPSY end-of-study assessment in person at the appropriate time, the process was changed to the following:
-
part 1 – a telephone call during which all end-of-study questions were completed
-
part 2 – a clinic visit, when clinics resumed, during which the vaginal examination took place.
Questionnaire completion
A letter providing a COVID-19 update was sent to all TOPSY participants. This letter stated that if a woman wanted to complete her questionnaires online, rather than on paper, she could e-mail the TOPSY office to provide her e-mail address.
A batch of questionnaires were issued at the beginning of March 2020, just before the first lockdown, when the TOPSY trial office team commenced working from home. From 11 May 2020, a member of the TOPSY team was granted access to the trial office every 6 weeks to post out batches of questionnaires. For all batches of questionnaires sent as of this date, reminder 1 (which would usually be sent 3 weeks after the initial questionnaire) was not sent. Reminders could be posted out every 6 weeks (which was previously the time of the second reminder), and then the third reminder, if required, was undertaken over the telephone as described previously.
A short-form questionnaire was developed to ensure that the minimum number of primary outcome and other required data could be gathered when data could only be gathered by telephone.
COVID-19 survey: impact of COVID-19 on how women view their pessary management
We developed a COVID-19 survey to assess the impact of the care delivery disruption, such as cancelled clinic visits, on participants’ views of pessary care/self-management. The survey was completed at the woman’s next clinic visit or alternatively posted to participants who had had a clinic-based care or 18-month appointment cancelled or postponed due to COVID-19.
Statistical analysis
Study analyses were conducted in accordance with a prespecified statistical analysis plan (Project Documentation is available on the project website)41 using Stata version 16 (StataCorp LP, College Station, TX, USA). The primary outcome measure (PFIQ-7) and all secondary outcome measures were presented as summaries of descriptive statistics at each time point, and comparisons between the groups were analysed using general linear models. All analyses were adjusted for minimisation covariates (age, pessary user type and centre) and for baseline scores where applicable. The models used to analyse the continuous outcomes were repeated measures mixed models with a compound symmetry covariance matrix and centre fitted as a random effect. Estimates of treatment effect size were expressed as the fixed-effect solutions in the mixed models and odds ratios in the ordinal regression models. For all estimates, 95% confidence intervals (CIs) were calculated and reported.
Planned sensitivity analyses were carried out on the primary outcome measure to investigate the impact of missing data under various assumptions. The first sensitivity analysis was a complete-case analysis that used only cases for which follow-up data were available at the primary end point (18 months). The remaining sensitivity analyses used pattern mixture modelling by increasing and decreasing the imputed PFIQ-7 values in the initial sensitivity analysis by 20 points, equivalent to the minimal clinically important difference. These adjustments were then repeated in one group only and repeated again by applying the adjustments in the other group only. We used 20 points on the PFIQ-7 score as this was the clinically important difference initially assumed and hence a meaningful systematic difference to test in the sensitivity analyses.
A further set of planned sensitivity analyses of the primary outcome measure were conducted to examine crossover, adherence to treatment and the inclusion of previous hysterectomy as a covariate. In addition, a sensitivity analysis of the repeated measures mixed model specification was carried out, applying the constrained longitudinal model57 with the baseline value in the outcome vector, an approach suggested for extension to randomised studies. 58 Finally, a planned sensitivity analysis was carried out to incorporate mode of data collection, which shifted more to electronic submission as a consequence of the COVID-19 pandemic, as it was recognised that the results could be biased if collection method was not addressed in the analysis. 59
Analysis populations
The main analysis was an intention to treat (ITT) analysis, and all participants were analysed as randomised. Two further prespecified per-protocol analyses were conducted. The first analysed all participants, reflecting any change of status resulting in crossovers to the other trial group. The second analysis included only participants defined as ‘on treatment’ at the 18-month follow-up. The definition of ‘on treatment’ is given in the statistical analysis plan and summarised in Intervention adherence.
Missing data
Missing baseline data were not imputed for the reporting of baseline characteristics, but imputation of the primary outcome was carried out prior to the main analysis to improve efficiency. Missing baseline values of the primary outcome were imputed at the overall mean.
Demographic and baseline variables
The baseline characteristics of the participants were tabulated by randomised group. No inferential tests were undertaken when comparing participant baseline characteristics between the trial groups.
Intervention adherence
Intervention adherence was assessed through the definition of ‘on treatment’. The proportions of women adhering to the self-management or clinic-based care protocols for the duration of the 18-month intervention period were reported for each group. Adherence was further analysed as part of the process evaluation (see Chapter 4). Adherence to the intervention was defined in the self-management group as the participant using a pessary for the management of prolapse, the participant having received the TOPSY self-management teaching, and the participant inserting her pessary herself. In the clinic-based care group, adherence was continued use of a pessary and not reporting inserting own pessary.
Analysis of primary outcome measure
The analysis of the primary outcome used a mixed-effects repeated measures model or longitudinal analysis of covariance as described by Twisk. 60 The three follow-up measurements of the outcome variable (PFIQ-7 at 6, 12 and 18 months) were employed as the dependent variable. The analysis adjusted for the baseline value of the dependent variable. The model included ‘time’ (6, 12, 18 months) as a dummy variable because a non-linear development of the outcome over time was anticipated. Interaction effects between treatment (trial group) and time were included in the model. The model also included age group (< 65/≥ 65 years) and pessary user type (new/existing) as fixed effects and participant and recruitment centre as random effects. A random effect of participant was included at the level of the individual to account for the non-independence of observations under repeated measures. In addition, the three PFIQ-7 subscales were each analysed separately using equivalent models.
To assess whether the assumptions behind the mixed-effects model were met, we generated normal quantile plots of residuals and standardised residuals. Where the assumptions of the analysis of covariance appeared to be violated, we explored other modelling strategies such as zero-inflated Poisson models.
Analysis of secondary outcome measures
Secondary outcomes assessed through continuous measures were analysed in a similar way to the primary outcome measure (see previous section). However, the reporting of subscales includes only descriptive summaries. For binary measures, we estimated odds ratios using mixed-effects binary logistic regression, and for ordinal measures, we estimated odds ratios using mixed-effects ordinal logistic regression with odds determined by the logistic cumulative distribution function. Age group and pessary type were included as fixed effects and participant and centre as random effects in the logistic regression models.
From the Pessary Complications Questionnaire, the unweighted proportion of complication types reported was calculated for each participant, with the summary statistic reported being the mean proportion in each group. Only the 13 categories applicable to both clinic-based care and self-management were used in this calculation. For women not sexually active, only the relevant subset of complication types was included in the calculation (questions 11 and 12 were therefore excluded). Individual items were summarised for the two other non-validated questionnaires (pessary use and pessary confidence), but overall scores were not calculated. Between-group comparisons were made for the confidence to remove pessary and insert pessary as specified in the statistical analysis plan, and an additional analysis of the confidence to manage pessary problems was added as post hoc analysis and is listed in the deviations from the statistical analysis plan.
For the uptake of additional support, regression models were used to estimate the mean difference in the number of telephone support and additional clinic appointments between the groups. Both outcomes included the uptake of additional support over the 18-month follow-up period as a single time point.
Subgroup analysis
Prespecified subgroup analyses of the primary outcome were carried out within the following groups identified at baseline:
-
age (< 65/≥ 65 years)
-
pessary user type (new/existing)
-
previous hysterectomy (yes/no).
Adverse events and data and safety monitoring
All women in the TOPSY trial had a vaginal pessary inserted. As a foreign body placed in the vagina, a pessary is recognised as a potential cause of specific symptoms. Expected events arising from pessary treatment are noted below and were not collected as adverse events but were recorded as secondary outcomes if they occurred:
-
granulation of vaginal tissue
-
involuntary expulsion of pessary
-
vaginal smell
-
vaginal discharge
-
bleeding during pessary change.
The questionnaires completed at the 6-, 12- and 18-month follow-ups included a Pessary Complications Questionnaire in which women indicated any complications they experienced. All participants were asked in the questionnaires if they had been admitted to hospital, had any accidents, used any new medicines or changed medication regimens. In the clinic-based care group, the local clinical TOPSY research team asked about the occurrence of adverse events (AEs)/serious adverse events (SAEs) at every pessary follow-up appointment. Women in the self-management group were asked during the teaching appointment and advised in the information leaflet to call the telephone helpline if they experienced any of the symptoms indicative of an SAE/AE. At the end of data capture, a cross-check of the database and the SAE forms was also carried out to ensure that an SAE was recorded for all women who had self-reported in their follow-up questionnaires that they had been admitted to hospital.
Methods for the process evaluation
Research question for the process evaluation
The process evaluation answers the following research question: what are the barriers to and facilitators of intervention acceptability, intervention effectiveness, fidelity to delivery and adherence among women treated with vaginal pessary and the HCPs who treat them, and how does this differ between trial groups?
The process evaluation objectives are:
-
to undertake an internal pilot study to ensure that the trial could recruit, randomise and retain sufficient numbers of participants while delivering the intervention as planned
-
to undertake a process evaluation in parallel with the trial to maximise recruitment; assess eligible but non-randomised women; understand women’s experience and acceptability of the intervention; assess adherence to allocated trial group; describe fidelity to intervention delivery; and identify contextual factors that may interact with intervention effectiveness.
Study design
The process evaluation is a mixed-methods study that was nested within, and operationalised concurrently with, the trial. 43 The process evaluation samples, methods of data collection, including those that were part of the internal pilot study, and analysis are outlined briefly below. Further details of the design are in the published protocol. 41 Table 2 outlines the links between the purposes laid out in the objectives and the methods used.
| Objectives | Audio-recordings recruitment sessions | Audio-recordings self-management teaching and telephone calls | Checklists | Interviews: randomised women | Interviews: non-randomised women | Interviews: HCPs | Open question in outcome measures |
|---|---|---|---|---|---|---|---|
| Maximise recruitment | |||||||
| Assess non-participating women | |||||||
| Understand women’s experience and acceptability | |||||||
| Assess adherence to group | |||||||
| Describe fidelity | |||||||
| Identify contextual factors linked to effectiveness |
Study methods for recruitment, consent and data collection
The sampling, recruitment and data collection for each method are outlined below. The participant information leaflets and consent forms for each element are part of the Project Documentation on the project website. 41
Audio-recording of recruitment discussions (maximise recruitment)
The target was two or three recruitment sessions in each of the six pilot centres (a total target of 12–18 sessions). If more than one person undertook recruitment at any of the pilot centres, recruitment aimed to sample for diversity of recruiter professional background. Potential participants received a short participant information leaflet from a delegated member of the local TOPSY research team. If a woman was willing to take part, her written consent was obtained prior to audio-recording. If a woman did not want to take part, the recruitment discussion still took place but was not audio-recorded. With the woman’s consent, the recruiter asked to record the discussion using a small, unobtrusive digital recorder.
Audio-recording of self-management teaching appointments and self-management support telephone calls (fidelity)
In the internal pilot study, 5–10 teaching appointments and 5–10 support telephone calls were recorded and analysed. Feedback was given to centres to maximise fidelity to delivery of the self-management protocol. A further 20–25 self-management teaching appointments (total n = 30) and 20–25 telephone calls (total n = 30) were audio-recorded in the main trial, with at least one self-management teaching appointment or one telephone call recorded in each centre. Variation across the sample aimed for diversity in treating HCP (nurse/physiotherapist/doctor) and woman’s age. The main trial participant information leaflet stated that, if the woman was allocated to the self-management group, a self-management teaching appointment or a follow-up call may be recorded with her consent. The main trial consent form asked the woman to indicate ‘yes’ or ‘no’ to these recordings by initialling the relevant box, and her consent was checked verbally prior to the recording. To record the interaction, a small digital recorder was, with the consent of the woman and HCP, placed in the consulting room or attached to the phone.
Checklists (fidelity)
Checklists to assess fidelity were developed to include the key aspects of the intervention content and theory. The checklists were completed by the HCP who delivered the self-management teaching appointment or the 2-week follow-up telephone call for all appointments and all follow-up calls.
Interviews with randomised women (maximise recruitment, women’s experience/acceptability, adherence, contextual factors)
To understand the perspectives of those in receipt of self-management or clinic-based care, a purposive sample of women randomised to the trial were asked to take part in one-to-one, face-to-face, semi-structured interviews. The original aim was to recruit 30 women (10 in the clinic-based care group and 20 in the self-management group). However, early in the trial, the protocol was amended to prevent bias in the trial, so that the same number of women in each group were interviewed. Therefore, the recruitment target was changed to 36 women, 18 in each group. Five women in each group were interviewed as part of the pilot study.
The main trial patient information leaflet advised that some women would be invited to take part in an interview. The trial consent form asked women to tick ‘yes’ or ‘no’ if they were willing to be contacted about the interview. Among those who ticked ‘yes’, women were purposively sampled to achieve variance in centre, age, user status (new/existing) and treating HCP (nurse/physiotherapist/doctor). An interview participant information leaflet was posted to sampled women and a telephone call made a few days later to discuss their possible participation in the interview study. If they consented, an interview study consent form was signed prior to the first interview. Their consent was verbally rechecked prior to the 18-month interview.
Interviews occurred at randomisation and at 18 months post randomisation (the same time point the primary outcome was measured). Interview schedules were developed based on the literature and with guidance from PPI members who were study grant holders or on study committees along with the Royal College of Obstetricians and Gynaecologists’ Women’s Voices group. Interview schedules explored perspectives on recruitment (baseline); symptoms (baseline)/change in symptoms (18 months); experience and acceptability of clinic-based care or self-management (18 months); adherence to the allocated trial group (18 months); and contextual factors perceived to interact with the effectiveness of the intervention (18 months). Where a woman crossed over to receive treatment offered in the group to which she had not been randomised, her reasons for doing so were explored during the 18-month follow-up interview.
All face-to-face interviews were suspended and changed to telephone interviews after March 2020 due to the COVID-19 pandemic and subsequent restrictions placed on interactions and travel. The interviewing of women randomised to the self-management group prior to their self-management teaching appointment was not always possible, as the time between randomisation and appointment was short. This short timeline also meant that it was also not always feasible to interview women face to face, and therefore some pre-COVID-19 interviews were also undertaken over the telephone. Interviews were digitally recorded using a small recorder placed discreetly in the room or attached to the phone.
Interviews with women who declined randomisation (assessment of non-randomised women)
To assess women who were not randomised, those who were eligible for the trial and did not consent to randomisation but did consent to taking part in an interview study were interviewed at baseline and 18 months over the telephone using a semistructured interview schedule. Sampling was by convenience as it relied on women responding to the research team. The aim was to recruit 20 women who declined randomisation (approximately five in the internal pilot). Sampling variance was on woman’s age and centre type (outpatient/community/primary care).
Based on ethics committee requirements, only women invited to take part in the trial in the clinic (as opposed to those who had information posted to them), and declined in clinic, were asked to take part. Women who declined trial participation were asked if they were willing to take a recruitment pack for an interview study with non-randomised women. The recruitment pack contained an introductory letter, a participant information leaflet, an expression of interest form, a consent form and two stamped addressed envelopes. Participants opted into this component of the study by returning the expression of interest form. When a completed form was received, the researcher contacted the woman in question to answer any questions she had, go over the consent process and arrange a baseline telephone interview if the woman was willing to consent. Participants were asked to sign and return the consent form prior to the telephone interview.
Interviews focused on reasons for declining to take part in the trial (baseline); symptoms (baseline)/change in symptoms (18 months); treatment received for prolapse (18 months); and contextual factors that may interact with future service implementation (baseline and 18 months).
Qualitative semistructured interviews with healthcare professionals who recruited to the trial and delivered the interventions (maximise recruitment, fidelity, contextual factors)
Interviews with HCPs aimed to increase understanding of the recruitment, fidelity and contextual factors that affected the intervention. The aim was to interview at least two staff involved in the trial at each centre who recruited to the trial and/or delivered the self-management intervention. Sampling aimed for diversity of professional group for both recruitment and delivery.
Consent started at the site initiation visits, where HCPs who were identified as being part of the local TOPSY research team were advised that they may be approached and invited to take part in an interview as part of the TOPSY trial. Contact details for the local TOPSY research team were collected before the centre was opened to recruitment and as part of the delegation log. Interviews with pilot centre recruiters were undertaken during the pilot study; all other interviews took place towards the end of data collection in each centre. The TOPSY process evaluation researcher contacted HCPs to invite them to participate in the interview, sent them a participant information leaflet and consent form and was available to answer any questions. Willing HCPs were asked to return the consent form. Once written consent was obtained, a suitable date and time for a telephone interview was arranged.
Interviews were semistructured, lasted approximately 30 minutes and were undertaken over the telephone. Interviews with recruiters focused on factors that influenced the identification of potential participants and recruitment, including service structures, contributing to maximising recruitment. Interviews with those involved in delivering clinic-based pessary care and/or self-management focused on experiences of delivering self-management/clinic-based care, including variation in delivery and reasons for the variation, and contextual factors that were perceived to impact on delivery. Interviews were digitally recorded and transcribed.
Secondary outcome measures in questionnaires (experience/acceptability, adherence, contextual factors)
Within the questionnaire booklet at baseline and at 6, 12 and 18 months, women were asked questions about adherence and self-efficacy. In addition, an open question asked women about their experience of their trial group (self-management or clinic-based care). The aim of these questions was to understand the experience and adherence of the wider sample of women involved in the trial.
Data analysis
Recordings of recruitment sessions, teaching sessions and telephone calls and interviews with women were transcribed verbatim and imported into NVivo software (QSR International, Warrington, UK). Each data source was analysed individually in the first instance to reach separate conclusions, and the findings were then synthesised across data sources. Quantitative checklist and coded self-management appointment and follow-up recording data were transferred to Statistical Product and Service Solutions (IBM SPSS Statistics v26, Armonk, NY, USA) for analysis. All analysis was undertaken by the process evaluation subgroup of grant‐holders, which included PPI and clinical representation, with the rest of the team blinded to the analysis.
Analysis process for all interviews and open question in outcome questionnaires
A thematic framework analysis approach61 was applied to interviews and data from the open question. The stages laid out below were applied to each individual data source (randomised interviews, non-randomised interviews, HCP interviews, individual question). The initial level of analysis focused on women’s experience of prolapse and their symptoms at the outset; experience of self-management or clinic-based pessary care; perceptions of prolapse cause; experience of trial processes and participation; perceptions of treatment outcomes; adherence to trial group; and reasons for declining participation in the main trial. At this stage, the aim of the analysis was to identify barriers to and facilitators of adherence to trial group, acceptability of self-management pessary care and acceptability of trial participation (where applicable). The steps are briefly listed below:
-
Based on the research questions, an initial broad thematic framework was developed.
-
Individual transcripts were uploaded into NVivo and read several times so that the content became familiar. Ten per cent of interview transcripts from each data set were coded by a second analyst, and the coding was discussed.
-
Initial framework was applied to all data and iteratively developed as coding progressed.
-
Data extracts for codes were summarised, reviewed and discussed.
-
Preliminary ‘findings’ and case summaries were shared and discussed with the process evaluation management group.
-
Data for each individual source were described and explained.
Interviews with both randomised and non-randomised women were further analysed using a case study approach. Priority was given to complete data sets (cases that had interviews for both time points) during the analysis process. Each case comprised one woman and all of the data gathered about that woman. This is a three-tailed case study, with the tails representing the intervention and control groups of the trial, respectively, plus women who declined participation in the trial but consented to the interview study alone. The analysis approach is briefly summarised below:
-
Case summaries were written for each case. Case summaries were written with a focus on creating an understanding of women’s experience using the key areas of interest driven by the process evaluation analysis plan (see Project Documentation on the project website). 41
-
Additional (to those originally set out) theoretical propositions were developed that were drawn from observations of the data.
-
All of the cases for one group of the interview study (intervention, control, non-randomised) were collected and consistencies/inconsistencies were searched for. The collected data were discussed with the process evaluation group of researchers. The aim of analysis at this stage was to identify the core barriers and facilitators within the trial groups, as well as detailed explanations for them and interactions between them.
-
Study groups were compared. The intervention and control groups of the trial were compared using the theoretical underpinnings of the study. The aim of this part of the analysis was to identify similarities in and differences between the two trial groups. Additionally, cases from the non-randomised interview only group were compared with the trial groups with regard to experiences of treatment and perceptions of treatment outcome.
Self-management teaching sessions and follow-up telephone calls
The self-management teaching sessions and 2-week follow-up telephone calls were transcribed. An a priori analytic grid was developed for the teaching session and 2-week self-management follow-up telephone call. The analytic grid was developed based on the underlying self-management philosophy around which each component part of the intervention was set up, for example to assess if women were offered the practical skills to self-manage. The grid was applied to each transcript from the teaching sessions and 2-week self-management follow-up calls by MD, the primary qualitative researcher. Over 10% transcripts were double coded (by CB) to assess for agreement in coding and discussed with members of the qualitative PMG. Coded data were imported into SPSS and described.
Intervention checklists for self-management teaching session and 2-week telephone call
The HCP-completed checklists for the self-management teaching sessions and 2-week follow-up telephone calls were entered into SPSS. Following the procedures explained in the process evaluation analysis plan, the data were described and analysed.
Methods for the health economic evaluation
A cost–utility analysis was conducted. In this economic evaluation method, costs are attached to resource use for the delivery of the intervention and comparator treatments as well as all healthcare-related resource use for each patient during the follow-up period. Health outcomes are measured in QALYs. The incremental net monetary benefit (INMB) is calculated for the treatment (self-management) versus the comparator group (clinic-based care). The INMB has been proposed62 as a more informative alternative to the incremental cost-effectiveness ratio (ICER), especially in situations where the incremental cost or effectiveness is negative. The INMB is calculated by multiplying incremental effectiveness by the policy-maker cost-effectiveness threshold, which in the UK is £20,000 willingness to pay per QALY gained,63 and then subtracting the incremental cost of the treatment. A positive INMB implies that the treatment is cost-effective, whereas a negative INMB suggests that the alternative or existing approach should be adopted.
For both the within-trial analysis and the decision-analytic model, a prospectively agreed health economics analysis plan was followed (see Project Documentation). 41 As reported in Chapter 3, patients in 21 sites across the UK were randomised to either pessary self-management or clinic-based care. Clinic-based care was not standardised across all sites and each site continued with their regular follow-up appointment schedule, although in practice all sites had a standard 6-month follow-up for outpatient appointments. The details of the women recruited into the trial are reported in Chapter 3. The economic analysis follows the same approach as the main statistical analysis by adopting an ITT methodology. Some women in this trial reverted from self-management to clinic-based care, but this analysis is based on status at randomisation.
Perspective
A health sector payer (NHS) perspective was taken for the cost–utility analysis.
Time horizon and discounting
The primary economic analysis compared the costs and benefits of each group over the first 18 months after randomisation. A secondary analysis over a 5-year time horizon was performed using modelling beyond the trial data collection period. A 5-year horizon was chosen as it was assumed that the conditions and characteristics of patients will be broadly the same across the period while still being relevant to NHS funding cycles.
A discount rate of 3.5% was applied to all costs and outcomes over 1 year as recommended by NICE. 63
Health outcomes
Data about health-related quality of life for use in the cost–utility analysis were collected using the EQ-5D-5L. 49 The EQ-5D-5L is a generic measure of health-related quality of life with five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. For each domain, respondents can select one of five levels ranging from ‘no problems’ to ‘unable or extreme’ based on their health today (the full version of the questionnaire is available as part of the Project Documentation). The raw scores from responses to the EQ-5D-5L domains can be used to generate health state utility values that are used to calculate QALYs. The QALY can be described as 1 year in full health and along with costs forms the basis of this economic evaluation. The utility values were calculated using the procedure recommended by NICE using the crosswalk from the UK EQ-5D-3L tariff. 64
The five questions are followed by EQ-VAS, a visual analogue scale on which respondents are asked to rate their health today between 0 (worst imaginable health) and 100 (best imaginable health). The EQ-5D-5L was completed at baseline and at 6, 12 and 18 months post randomisation alongside the primary outcome and other secondary measures reported in Chapter 2.
Resource use and costs
The intervention for the self-management group was additional training from a specialist nurse at a hospital clinic during the first appointment. This consisted of approximately 30 minutes more than clinic-based care with a specialist nurse, physiotherapist or consultant (see Chapter 2 for full details of the intervention). For the women in the self-management group, the regular follow-up clinic appointments were then scheduled for the 18-month time point only.
Healthcare resource use was collected from the clinic visit [case report form (CRF) 07] and the telephone support (CRF08) CRFs and from a participant-completed Resource Use Questionnaire (RUQ) designed for this study (all are part of the Project Documentation available on the project website). Data on outpatient clinic appointments related to pessary management were captured on the clinic CRF, and telephone support appointments were recorded on a telephone log CRF.
The RUQ consisted of six questions related to the use of secondary care services, primary care services and medications (prolapse-related treatments) and for any personal out-of-pocket expenses resulting from experiencing prolapse or having a pessary. For primary care services participants were asked to record the number of GP appointments in person and home visits, nurse appointments in person and home visits, district nurse home visits, community physiotherapy appointments and community dietitian appointments. For secondary care services participants were asked to record the number of outpatient appointments with a doctor, outpatient appointments with a nurse, attendances at accident and emergency (A&E), and inpatient stays including the number of nights. For both primary and secondary care resource use participants were asked to record this in terms of appointments for prolapse-related reasons and any other health-related reason.
The RUQ was completed by participants at the 6-, 12- and 18-month follow-ups; they were asked to report all resource use over the period since the previous questionnaire. Given the long period between follow-up questionnaires, an aide memoire was given to the participants so that they could note down any appointments or medication during the intervening period that could then be transferred to the main questionnaire.
The unit costs attached to each item of resource use are presented in Table 3. Unit costs were identified using Unit Costs of Health and Social Care for staff and British National Formulary for prescribed medication. 65,66 All costs are in Great British pounds (GBP) in 2019/20 prices. To calculate an A&E unit cost, we used the weighted average of all acute outpatient appointments as described in Unit Costs of Health and Social Care (page 87). 65 For hospital episodes, we used the average cost per non-elective inpatient stay (short stays), which is based on national data and described in Unit Costs of Health and Social Care (page 87). 65 Outpatient doctor appointments were costed based on consultant grade. Outpatient nurse appointments were costed based on 1-hour contact with a band 7 nurse. Outpatient physiotherapist appointments were costed based on a 1-hour appointment with a band 6 physiotherapist. In-person appointments with GPs were costed based on a 9-minute contact time for each appointment. Community nurse appointments were costed as 15-minute appointments. Costing of GP and nurse (band 7) home appointments assumes 1 hour of patient contact, which includes travel to the patient’s home. District nurse at home appointments costing assumes 1 hour with a band 6 nurse. Physiotherapy local clinic visits were costed assuming 1 hour of patient contact with a band 7 community (advanced) physiotherapist. Appointments with a dietitian were costed using a band 7 hospital-based dietitian. Clinic visits assume 45 minutes of patient contact, and telephone support calls assume 10 minutes of patient contact with a band 6 specialist nurse. The costing of the initial training appointment was based on patient-level trial data that were costed using Unit Costs of Health and Social Care depending on the grade of the HCP who provided the training. The statistical analysis accounted for the uncertainty in the unit costs by drawing Monte Carlo samples from normal distributions shown in Table 3.
| Service | Mean | SD | Distribution | Reference |
|---|---|---|---|---|
| A&E | 154 | 30.8 | Normal | Curtis and Burns65 |
| Hospital episode | 602 | 102 | Normal | Curtis and Burns65 |
| Outpatient doctor | 135 | 27 | Normal | Curtis and Burns65 |
| Outpatient nurse | 60 | 12 | Normal | Curtis and Burns65 |
| Outpatient physiotherapist | 50 | 10 | Normal | Curtis and Burns65 |
| GP | 33 | 6.6 | Normal | Curtis and Burns65 |
| Community nurse | 9.5 | 1.9 | Normal | Curtis and Burns65 |
| GP @ home | 223 | 44.6 | Normal | Curtis and Burns65 |
| Nurse @ home | 120 | 24 | Normal | Curtis and Burns65 |
| District nurse @ home | 89 | 17.8 | Normal | Curtis and Burns65 |
| Physiotherapist @ local clinic | 58 | 11.6 | Normal | Curtis and Burns65 |
| Dietitian | 60 | 12 | Normal | Curtis and Burns65 |
| Initial training appointment | 29.90 | 12 | Normal | Curtis and Burns65 |
| Clinic visits | 37 | 7.4 | Normal | Curtis and Burns65 |
| Telephone support | 8.3 | 1.7 | Normal | Curtis and Burns65 |
Data analysis
Within-trial cost–utility analysis
The analysis included all randomised participants based on the definition of ‘on treatment’ for the TOPSY trial, with the results presented based on an ITT sample. Subgroup analysis was not conducted, and no additional adjustments were made to account for how socioeconomic characteristics of participants could impact on the findings.
Unit costs were attached to each item of resource use to calculate the total cost per patient. The mean cost per patient was estimated for each group. The EQ-5D-5L was scored using the process outlined in Health outcomes. The mean number of QALYs associated with each treatment option was calculated. The methods employed account for the uncertainty around the mean estimates of both costs and QALYs and also incorporate the uncertainty in unit costs. Non-parametric bootstrap methods were employed to produce unbiased standard errors given the distribution of cost and effects. 67–69
Using the estimated mean QALYs and costs associated with each treatment option, the incremental cost and incremental QALYs gained from self-management compared with clinic-based care were calculated over the 18-month period. The incremental net benefit was calculated at a willingness-to-pay threshold for a QALY gained of £20,000. The primary economic analysis reports the probability of cost-effectiveness at a willingness-to-pay threshold of £20,000 per QALY gained. It follows that at a higher willingness-to-pay threshold the probability of cost-effectiveness would be higher than what is shown in these results. Two analyses were run: one with fully completed data and a second employing imputation methods, which is described in the following section.
Missing data
Multiple imputation combined with rules-based imputation was employed to maximise the usable data in the economic evaluation. Both costs and outcomes were analysed using methods to account for missing data to reduce bias and ensure that missing data were handled appropriately. Baseline costing data were not available; therefore, we employed different strategies for missing data in costs and outcomes. There were no participants with full EQ-5D-5L data who had missing values in the RUQ. In the RUQ, missing values were considered missing only if participants had not responded to any of the questions (complete missingness). Costing data in this evaluation were generally very well completed, with < 2% non-response to the resource use questions across all participants. Non-response was due to either not reporting the number of events or only responding to resource questions with positive use. For example, a respondent replied that she had seen her GP but did not give the number of times. In these instances, we imputed the value as one visit, taking the most conservative approach of resource use. In cases where respondents only responded to some of the resource use questions, we assumed zero resource use in the unanswered questions. We conducted sensitivity analysis with case deletion to make sure that these decisions did not have a material impact on our results, and our results remained broadly the same. Therefore, the data of participants with non-response to some of the resource questions were included in the baseline analysis. Multiple imputation was used to impute only outcome missing data. 70 The imputation was run 100 times, resulting in 100 different data sets to be used in the cost-effectiveness analysis. The imputation was implemented separately for the intervention and control groups to account for differences in the missing values between the two groups. Multiple imputation was performed using predictive mean matching. 71 The multiple imputation model uses baseline covariates and QALYs at each follow-up point to impute unobserved QALYs, so that, for example, missing QALYs at 12 months are imputed using data on baseline covariates, utility at baseline and 6 months (if available) and QALYs between baseline and 6 months (if available).
Modelling
Decision-analytic modelling was undertaken to extrapolate costs and outcomes beyond the follow-up period of the trial to investigate the potential for cost-effectiveness to deviate from the baseline results under a 5-year horizon. The model simulates progression over time given the baseline analysis. Essentially, the observed data in the first 18 months are extended in time for an extra 5 years with uncertainty allowed. The model was developed using recommended methods. 72
A Markov decision model, referred to as the TOPSY model, with a monthly cycle is used to evaluate the effects of the intervention on costs, any QALY gains and cost-effectiveness over the 5-year horizon. The model is run as both a cohort and a Monte Carlo simulation. 73 The decision model comprises two groups, with one group for each intervention evaluated (intervention vs. clinic-based care). Each group is structured as a Markov model built around health states to which healthcare cost and QALY data collected as part of the trial are linked. The model structure is shown in Figure 1.
FIGURE 1.
State diagram of the TOPSY trial decision-analytic model.

The health states in the TOPSY model simulate the type of patient encountered in the trial. The health states can be related to light or heavy resource use and a small associated change in quality of life. For example, the good health state is associated with low resource use and high quality of life, whereas the poor health state is associated with high resource use and low quality of life. Differences between the quality of life in these states were very small, given the results of the main economic analysis that showed minimal differences between the trial groups. The distribution of total resource use in the two trial groups at each follow-up was examined to inform the level of resource use at each state and the transition probabilities over time. Patients can remain in the same health state throughout the 5-year period or move between states, as shown in the diagram. Patients can change states at the beginning of each month depending on the model parameters. To reflect the fact that patients at the start of a clinical trial should be identical, and to isolate the impact of randomisation, all patients start in the moderate state in both the clinic-based care and self-management groups.
The model parameters were derived from the trial data: (1) transition probabilities between states (depending on observed resource use changes over time), (2) treatment effects of the intervention, (3) quality of life and (4) healthcare costs (see Appendix 4, Table 37). The key transition probability parameters are manually varied to examine the impact on cost-effectiveness shown using an INMB tornado diagram that reports the range of INMBs generated for each parameter’s uncertainty range. We did not manually vary other parameters as transitional probabilities had the most impact and we wanted to show a visual representation of these using the tornado diagram. Probabilistic sensitivity analysis is employed to account for uncertainty across all model parameters, which includes 10,000 Monte Carlo draws of values from cost and patient utility distributions (see Appendix 4). 73
Management of the study
A PMG made up of all co-applicants, additional PPI representatives and research staff employed on the study met regularly, face to face or by teleconference, to review the study’s progress. In addition, approximately weekly meetings were held between the appointed staff and the chief investigators.
An independent TSC and DMEC, approved by the National Institute for Health and Care Research Health Technology Assessment programme, met at least yearly to review study progress. The TSC supervised the trial conduct to ensure that the principles of Good Clinical Practice and relevant regulations were adhered to. The DMEC reviewed accumulating data (e.g. monitoring attrition, adverse events) and ethical issues. No interim analyses were planned or conducted.
A subgroup including CB, one clinical co-applicant (AK), one PPI co-applicant (MG) and the process evaluation research fellow (MD) met monthly to consider process evaluation processes and data analysis. These meetings were closed to protect the integrity of the main trial.
Patient and public involvement
We had active PPI contributions across all areas of the study. The self-management training manual and information leaflet had consistent patient and public representative input from our PPI co-applicant. In addition, both documents were reviewed (and subsequently amended) by a focus group of women from the Royal College of Obstetricians and Gynaecologists PPI group Women’s Voices.
Throughout the study, three PPI women were part of the PMG. There has also been one additional representative on the TSC. One woman withdrew from the TSC half-way through the study and another woman took on the role. The existing PPI PMG members were involved in providing support for the new PPI member who came on board half-way through the study.
Our four PPI representatives meet virtually every few months to maintain contact and share their experiences, and one woman has written an insightful piece on her PPI experiences, which has been submitted for publication. 74
Our PPI co-applicant was involved in the qualitative analysis, and all women were involved in reviewing this final report and our dissemination plans.
Dissemination
A trial publication policy was developed and ratified by the PMG for internal team use. A dissemination and impact policy is being developed.
We maintained collaborator interest in the trial by circulating newsletters, which were also available on our website. The results of the trial were reported first to the PMG, then to the TSC/DMEC and then to study collaborators. A lay summary of the findings was sent in a final newsletter to all participants involved in the trial. To maximise the reach of the findings to the public, we also plan to develop a short video involving our PPI colleagues that will be circulated on social media outlets. We will write posts on social media platforms to highlight the findings to the public. Where possible we will encourage our PPI representatives to be involved in dissemination activities.
Dissemination to clinical and academic colleagues will be through conference presentations and clinical and academic publications. We will present the main trial/cost-effectiveness findings first at the International Continence Society conference, which is attended by clinicians and academics from across the globe. We will then present supporting parts of the trial at other conferences. To maximise the impact of the findings, we will publish our findings in both practice-based and high-quality academic journals (such as The Lancet). Our training manual is freely available and included in Appendix 6 of this report.
Regulatory requirements
The TOPSY trial received ethics approval from the West of Scotland Research Ethics Service, West of Scotland Research Ethics Committee 3 (17/WS/0267), on 17 February 2018 and the NHS Health Research Authority on 9 March 2018. All local NHS approvals were given. All participants gave verbal and written informed consent. The study sponsor was the University of Stirling and the TOPSY trial office was based in the Nursing, Midwifery and Allied Health Professions Research Unit (NMAHP Research Unit) at Glasgow Caledonian University. The TOPSY trial was registered with the International Standard Randomised Controlled Trial Register (International Standard Randomised Controlled Trial Number 62510577) on 6 October 2017. A log of all amendments (substantial and non-substantial) can be found in Appendix 5, Table 38.
Summary
This chapter has described the methods used across the study and for each of the three study components. The results for each component are presented in the following chapters: the trial results (see Chapter 3), the process evaluation findings (see Chapter 4) and the cost-effectiveness evaluation (see Chapter 5).
Chapter 3 Trial results
Trial recruitment
The TOPSY trial recruited 340 participants (10 more than the 330 target) between 16 May 2018 and 7 February 2020, thus completing recruitment before the first COVID-19 lockdown (Figure 2). The 10 additional women were already on various stages of the screening pathway at the time the initial target of 330 was reached, so all 10 were recruited. The original target was to complete recruitment by July 2019 (see Figure 2); however, recruitment was extended by 6 months so that the required number of participants could be randomised.
FIGURE 2.
Projected and actual recruitment.

During the initial 6-month internal pilot, 72 participants were recruited (see the Project Documentation on the project website for a full report of the pilot study). Thereafter, between 12 and 27 participants joined the study during each full month of recruitment (Figure 3). Participants were recruited at 21 centres across the UK (Figure 4). Although centres were given targets for recruitment (based on an initial feasibility assessment at each of the centres’ study set-up), the centres that were recruiting well were permitted to recruit over the agreed target. This meant that the cohort of women could be recruited to target despite some centres struggling to recruit their expected number of women. The greater number of women recruited in two of the centres was discussed at the oversight committees (TSC and DMEC), and both committees agreed that this was acceptable.
FIGURE 3.
Treatment of prolapse with self-care pessary recruitment per month.
FIGURE 4.
TOPSY recruitment by centre.

Participant flow
A total of 2174 women were screened for eligibility, with 770 (35.4%) ineligible and 1404 (64.6%) declining to participate. A total of 340 women were randomised (169 to self-management and 171 to clinic-based care).
Questionnaires were completed by 334 participants (98.2%) at baseline (167/169 in the self-management group, 167/171 in the clinic-based care group). At 6 months, 306 participants (90.0%; 149/169 in the self-management group and 157/171 in the clinic-based care group) provided primary outcome (PFIQ-7) data, dropping slightly to 292 participants (85.9%; 144/169 in the self-management group and 148/171 in the clinic-based care group) at 12 months. At 18 months, the target of 264 questionnaires (80%) with valid primary outcome data set out in the sample size calculation was exceeded (291 returned; 85.6%), with 139 out of 169 (82.2%) in the self-management group and 152 out of 171 (88.9%) in the clinic-based care group. Two participants died during the follow-up period (both deaths were unrelated to the trial). The number of participants at each stage of the trial is summarised in the CONSORT diagram (Figure 5). Data were collected until the trial database was locked on 17 September 2021.
FIGURE 5.
CONSORT flow diagram. Some participants in the self-management group who discontinued the intervention are counted as both discontinuing pessary use and reverting to clinic-based care.

There were 23 withdrawals from the study: 5 because they discontinued pessary use, 6 because they reverted to clinic-based care, 1 because they had difficulties with the questionnaires, 5 because they opted for surgery, and 6 because they had moved/did not feel they could self-manage/were no longer interested in taking part. In addition to the withdrawals, two participants died.
Quality of participant-completed data
Missing data
Six participants did not complete the baseline questionnaire: two in the self-management group and four in the clinic-based care group. The number of missing responses in the PFIQ-7 questionnaire at each time point is shown in Table 4. A valid total PFIQ-7 score could not be generated for three participants at baseline, three at 6 months, four at 12 months and four at 18 months, because there were more than four missing items on at least one subscale (i.e. more missing items than non-missing items).
| Number of missing items | Baseline (N = 334), n (%) | 6 months (N = 309), n (%) | 12 months (N = 296), n (%) | 18 months (N = 295), n (%) | Total (N = 1234), n (%) |
|---|---|---|---|---|---|
| 0 | 317 (94.9) | 295 (95.5) | 280 (94.6) | 274 (92.9) | 1166 (94.5) |
| 1 | 7 (2.1) | 5 (1.6) | 6 (2.0) | 7 (2.4) | 25 (2.0) |
| 2 | 2 (0.6) | 1 (0.3) | 1 (0.3) | 3 (1.0) | 7 (0.6) |
| 3 | 3 (0.9) | 3 (1.0) | 1 (0.3) | 5 (1.7) | 12 (1.0) |
| 4 | 2 (0.6) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 3 (0.2) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 2 (0.6) | 1 (0.3) | 0 (0.0) | 3 (0.2) |
| 7 | 1 (0.3) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 2 (0.2) |
| 9 | 0 (0.0) | 0 (0.0) | 2 (0.7) | 2 (0.7) | 4 (0.3) |
| 14 | 2 (0.6) | 2 (0.6) | 3 (1.0) | 4 (1.4) | 11 (0.9) |
| 21 | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
Description of the sample
Table 5 summarises the baseline characteristics of the participants. The mean age of participants was 63.7 years [standard deviation (SD) 11.3 years]: 63.2 years (SD 11.6 years) in the self-management group and 64.2 years (SD 11.1 years) in the clinic-based care group.
| Characteristic | Self-management (N = 169) | Clinic-based care (N = 171) | Total (N = 340) | |||
|---|---|---|---|---|---|---|
| n; mean (SD) | Median (IQR) | n; mean (SD) | Median (IQR) | n; mean (SD) | Median (IQR) | |
| Number of births | 164; 2.4 (1.1) | 2 (2–3) | 164; 2.3(1.2) | 2 (2–3) | 328; 2.4 (1.1) | 2 (2–3) |
| Age (years) | 169; 63.2 (11.6) | 66 (57–72) | 171; 64.2 (11.1) | 65 (59–72) | 340; 63.7 (11.3) | 66 (58–72) |
| BMI (kg/m2) | 165; 26.1 (4.3) | 25.1 (23.4–28.4) | 162; 26.6 (4.2) | 25.7 (23.7–28.6) | 327; 26.3 (4.2) | 25.4 (23.6–28.6) |
| Ethnicity, n (column percentage) | ||||||
| Asian/Asian British | 3 (1.8) | 4 (2.3) | 7 (2.1) | |||
| Black/African/Caribbean/Black British | 5 (3.0) | 6 (3.5) | 11 (3.2) | |||
| Mixed/multiple ethnic groups | 1 (0.6) | 0 (0) | 1 (0.3) | |||
| White | 153 (90.5) | 156 (91.2) | 309 (90.9) | |||
| Other ethnic group | 2 (1.2) | 1 (0.6) | 3 (0.9) | |||
| Missing | 5 (3.0) | 4 (2.3) | 9 (2.7) | |||
| Educational qualifications | ||||||
| No formal qualifications | 19 (11.2) | 18 (10.5) | 37 (10.9) | |||
| Secondary/further education | 57 (33.7) | 53 (31.0) | 110 (32.4) | |||
| Higher education | 61 (36.1) | 65 (38.0) | 126 (37.1) | |||
| Missing | 32 (18.9) | 35 (20.5) | 67 (19.7) | |||
| Current employment status | ||||||
| Full-time employment | 32 (18.9) | 28 (16.4) | 60 (17.7) 17.65 |
|||
| Part-time employment | 26 (15.4) | 38 (22.2) | 64 (18.8) | |||
| Student | 1 (0.6) | 3 (1.8) | 4 (1.2) | |||
| Housework | 15 (8.9) | 8 (4.7) | 23 (6.8) | |||
| Seeking work | 0 (0) | 1 (0.6) | 1 (0.3) | |||
| Other | 95 (56.2) | 92 (53.8) | 187 (55.0) | |||
| Missing | 0 (0) | 1 (0.6) | 1 (0.3) | |||
Baseline clinical characteristics and baseline pessary-related issues are reported in Table 6. These variables are taken from the baseline CRF. The PFIQ-7 baseline score taken from the baseline questionnaire ranged from 0 to the maximum of 300, and the mean score pooled across groups was 30.5 (SD 48.3). In general, the groups are well balanced in demographic and clinical characteristics.
| Characteristic | Response | Self-management | Clinic-based care | Total | |||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
| Hormone therapy | Yes | 49 | 29.0 | 60 | 35.1 | 109 | 32.1 |
| No | 120 | 71.0 | 111 | 64.9 | 231 | 67.9 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Systemic hormone replacement therapy | Yes | 3 | 1.8 | 10 | 5.8 | 13 | 3.8 |
| No | 166 | 98.2 | 161 | 94.2 | 327 | 96.2 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Local oestrogen | Yes | 47 | 27.8 | 51 | 29.8 | 98 | 28.8 |
| No | 122 | 72.2 | 120 | 70.2 | 242 | 71.2 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Chronic cough | Yes | 13 | 7.7 | 8 | 4.7 | 21 | 6.2 |
| No | 156 | 92.3 | 163 | 95.3 | 319 | 93.8 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Diabetes | Yes | 7 | 4.1 | 6 | 3.5 | 13 | 3.8 |
| No | 162 | 95.9 | 165 | 96.5 | 327 | 96.2 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Arthritis | Yes | 46 | 27.2 | 42 | 24.6 | 88 | 25.9 |
| No | 123 | 72.8 | 129 | 75.4 | 252 | 74.1 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Constipation | Yes | 35 | 20.7 | 30 | 17.5 | 65 | 19.1 |
| No | 134 | 79.3 | 141 | 82.5 | 275 | 80.9 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Recurrent urinary tract infections | Yes | 14 | 8.3 | 12 | 7.0 | 26 | 7.6 |
| No | 155 | 91.7 | 159 | 93.0 | 314 | 92.4 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Vulvodynia | Yes | 1 | 0.6 | 4 | 2.3 | 5 | 1.5 |
| No | 168 | 99.4 | 167 | 97.7 | 335 | 98.5 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Hysterectomy | Yes | 20 | 11.8 | 18 | 10.5 | 38 | 11.2 |
| No | 149 | 88.2 | 153 | 89.5 | 302 | 88.8 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Pelvic floor surgery | Yes | 20 | 11.8 | 19 | 11.1 | 39 | 11.5 |
| No | 149 | 88.2 | 152 | 88.9 | 301 | 88.5 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Other comorbidities | Yes | 45 | 26.6 | 56 | 32.7 | 101 | 29.7 |
| No | 124 | 73.4 | 115 | 67.3 | 239 | 70.3 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Inflammation of tissues | Yes | 1 | 0.6 | 2 | 1.2 | 3 | 0.9 |
| No | 168 | 99.4 | 169 | 98.8 | 337 | 99.1 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Ulceration | Yes | 1 | 0.6 | 0 | 0.0 | 1 | 0.9 |
| No | 168 | 99.4 | 171 | 100.0 | 339 | 99.1 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Granulation | Yes | 1 | 0.6 | 4 | 2.3 | 5 | 1.5 |
| No | 168 | 99.4 | 167 | 97.7 | 335 | 98.5 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Other clinical concerns | Yes | 14 | 8.3 | 14 | 8.2 | 28 | 8.3 |
| No | 154 | 91.7 | 157 | 91.8 | 311 | 91.7 | |
| Total | 168 | 100.0 | 171 | 100.0 | 339 | 100.0 | |
| Bothersome discharge | Yes | 32 | 18.9 | 27 | 15.8 | 59 | 17.4 |
| No | 137 | 81.1 | 144 | 84.2 | 281 | 82.6 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Bothersome smell | Yes | 15 | 8.9 | 15 | 8.8 | 30 | 8.8 |
| No | 154 | 91.1 | 156 | 91.2 | 310 | 91.2 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Vaginal pain | Yes | 3 | 1.8 | 4 | 2.3 | 7 | 2.1 |
| No | 166 | 98.2 | 167 | 97.7 | 333 | 97.9 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Other pain | Yes | 16 | 9.5 | 16 | 9.4 | 32 | 9.4 |
| No | 153 | 90.5 | 154 | 90.6 | 307 | 90.6 | |
| Total | 169 | 100.0 | 170 | 100.0 | 339 | 100.0 | |
| Urine infection | Yes | 12 | 7.1 | 9 | 5.3 | 21 | 6.2 |
| No | 157 | 92.9 | 162 | 94.7 | 319 | 93.8 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Urinary incontinence | Yes | 55 | 32.5 | 53 | 31.0 | 108 | 31.8 |
| No | 114 | 67.5 | 118 | 69.0 | 232 | 68.2 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Bowel incontinence | Yes | 6 | 3.6 | 15 | 8.8 | 21 | 6.2 |
| No | 163 | 96.4 | 156 | 91.2 | 319 | 93.8 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Difficulty emptying bowel | Yes | 27 | 16.0 | 30 | 17.5 | 57 | 16.8 |
| No | 142 | 84.0 | 141 | 82.5 | 283 | 83.2 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Difficulty having sex | Yes | 4 | 2.4 | 10 | 5.8 | 14 | 4.1 |
| No | 93 | 55.0 | 94 | 55.0 | 187 | 55.0 | |
| N/A | 72 | 42.6 | 67 | 39.2 | 139 | 40.9 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Pain during sex | Yes | 4 | 2.4 | 11 | 6.4 | 15 | 4.4 |
| No | 94 | 55.6 | 92 | 53.8 | 186 | 54.7 | |
| N/A | 71 | 42.0 | 68 | 39.8 | 139 | 40.9 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Pessary fell out | Yes | 11 | 6.5 | 14 | 8.2 | 25 | 7.4 |
| No | 158 | 93.5 | 157 | 91.8 | 315 | 92.6 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
| Non-menstrual bleeding | Yes | 5 | 3.0 | 10 | 5.8 | 15 | 4.4 |
| No | 164 | 97.0 | 161 | 94.2 | 325 | 95.6 | |
| Total | 169 | 100.0 | 171 | 100.0 | 340 | 100.0 | |
Primary outcome measure
The PFIQ-7 score at 18 months was the primary outcome measure in the trial (lower scores indicate poorer quality of life related to pelvic floor dysfunction). In the self-management group, the unadjusted mean (SD) score was 32.3 (50.9) compared with 32.5 (47.8) in the clinic-based care group, with a mean difference (adjusted for baseline score and minimisation covariates) of –0.03 (95% CI −9.32 to 9.25). There was, therefore, no evidence of a difference between the groups in terms of prolapse-related quality of life. Comparable results were found at the 6- and 12-month time points (Table 7).
| Time point | Self-management | Clinic-based care | Unadjusteda interaction termb (95% CI) | Adjustedc interaction termb (95% CI) | Unadjusted mean difference (95% CI) | Adjusted mean difference (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||||
| Baseline | 165 | 32.5 | 49.6 | 166 | 31.7 | 48.0 | ||||
| 6 months | 149 | 22.7 | 36.7 | 157 | 29.4 | 47.7 | −6.71 (−16.31 to 2.89) | 5.90 (−3.20 to 15.00) | –6.71 (–16.31 to 2.89) | –5.90 (–15.00 to 3.20) |
| 12 months | 144 | 30.3 | 52.0 | 148 | 33.1 | 53.3 | −2.87 (−14.92 to 9.18) | −2.46 (–11.92 to 7.01) | 2.78 (–14.90 to 9.35) | –3.45 (–12.71 to 5.82) |
| 18 months | 139 | 32.3 | 50.9 | 152 | 32.5 | 47.8 | 0.10 (−11.15 to 11.35) | −5.87 (–15.34 to 3.60) | –0.17 (–11.55 to 11.22) | –0.03 (–9.32 to 9.25) |
Sensitivity analyses
Analyses of the primary outcome to examine data under differing assumptions relating to non-compliance and missing data (Table 8) all showed very similar results to the primary ITT analysis (see Table 7).
| Type | SAP section | Sensitivity analyses | Coefficient for interaction of trial group and 18-month time point (95% CI) |
|---|---|---|---|
| Covariates | 5.1 | A sensitivity analysis of the primary outcome was conducted with previous hysterectomy included as an additional fixed effect | −5.87 (−15.34 to 3.60) |
| Per protocol (crossover) | 5.7 | Per-protocol analysis carried out using the definitions for crossover in SAP section 5.7 | −7.84 (−17.51 to 1.83) |
| Per protocol (on treatment) | 4.3.5/5.7 | A further per-protocol analysis of the primary outcome measure using the ‘on treatment’ definitions set out in section 4.3.5 of the SAP | 1.44 (−9.62 to 12.50) |
| Data missing completely at random | 6.3 | Observed cases at 18 months only | −5.01 (−14.41 to 4.40) |
| Data missing not at random (pattern mixture models)b | 6.3 | Non-responders assumed to have worse outcomes in both groups | −3.07 (−13.07 to 6.93) |
| Non-responders assumed to have better outcomes in both groups | −0.46 (−10.46 to 9.54) | ||
| Self-management worse | −5.11 (−15.06 to 4.85) | ||
| Self-management better | 1.58 (−8.38 to 11.90) | ||
| Clinic-based care worse | 0.28 (−9.66 to 10.21) | ||
| Clinic-based care better | −3.80 (−13.74 to 6.14) | ||
| Mode of data collection (paper vs. electronic) | 8.2 | Mode of data collection added to the model as a fixed effect Mode of collection with an interaction with treatment allocation |
−5.88 (−15.35 to 3.60) −9.82 (−21.70 to 2.05)a |
| Time frame for exclusion of late returns | 8.3 | Section 8.3 of the SAP sets out rules for handling and excluding data collected outside a 3-month window of the due date, but an additional sensitivity analysis of the primary outcome will be conducted in which these rules will be relaxed to include all data | −5.57 (−14.99 to 3.86) |
| Analysis model | 7.1 | Baseline response is included as part of the outcome vector rather than a covariate | 0.60 (−9.22 to 10.42) |
| Analysis modelb | 5.4 | Alternative model to account for violation of distributional assumptions – zero inflated Poisson | 0.01 (−0.03 to 0.05) |
Subgroup analysis
The prespecified subgroup analysis was conducted by age group, pessary user type (new/existing) and history of hysterectomy at baseline and showed no significant treatment effect by subgroup interactions (Figure 6), that is there was no evidence that any intervention effects are modified by subgroups. However, it is important to note that the study was not powered to investigate subgroups and effects were considered on the primary outcome only. These analyses were therefore exploratory and potentially for hypothesis generation.
FIGURE 6.
Forest plot of interaction effects.

Secondary outcome measures
Pessary complications
Table 9 shows pessary complications by trial group as reported on the Pessary Complications Questionnaire at 18 months. As specified in the SAP, the proportion of complications reported by each participant, of those complications that were applicable to the participant, was calculated (Table 10).
| Complication | Response | Self-management (N = 142) | Clinic-based care (N = 152) | Total (N = 294) | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Vaginal discharge | Yes | 41 | 28.9 | 49 | 32.2 | 90 | 30.6 |
| No | 100 | 70.4 | 102 | 67.1 | 202 | 68.7 | |
| Missing | 1 | 0.7 | 1 | 0.7 | 2 | 0.7 | |
| Vaginal smell | Yes | 26 | 18.3 | 33 | 21.7 | 59 | 20.1 |
| No | 115 | 81.0 | 117 | 77.0 | 232 | 78.9 | |
| Missing | 1 | 0.7 | 2 | 1.3 | 3 | 1.0 | |
| Vaginal pain | Yes | 11 | 7.7 | 17 | 11.2 | 28 | 9.5 |
| No | 130 | 91.5 | 130 | 85.5 | 260 | 88.4 | |
| Missing | 1 | 0.7 | 5 | 3.3 | 6 | 2.0 | |
| Urine infection | Yes | 17 | 12.0 | 16 | 10.5 | 33 | 11.2 |
| No | 124 | 87.3 | 135 | 88.8 | 259 | 88.1 | |
| Missing | 1 | 0.7 | 1 | 0.7 | 2 | 0.7 | |
| Urine incontinence | Yes | 71 | 50.0 | 79 | 52.0 | 150 | 51.0 |
| No | 69 | 48.6 | 73 | 48.0 | 142 | 48.3 | |
| Missing | 2 | 1.4 | 0 | 0.0 | 2 | 0.7 | |
| Difficulty emptying bladder | Yes | 25 | 17.6 | 41 | 27.0 | 66 | 22.4 |
| No | 116 | 81.7 | 106 | 69.7 | 222 | 75.5 | |
| Missing | 1 | 0.7 | 5 | 3.3 | 6 | 2.0 | |
| Bowel incontinence | Yes | 20 | 14.1 | 34 | 22.4 | 54 | 18.4 |
| No | 120 | 84.5 | 118 | 77.6 | 238 | 81.0 | |
| Missing | 2 | 1.4 | 0 | 0.0 | 2 | 0.7 | |
| Difficulty emptying bowels | Yes | 34 | 23.9 | 55 | 36.2 | 89 | 30.3 |
| No | 106 | 74.6 | 96 | 63.2 | 202 | 68.7 | |
| Missing | 2 | 1.4 | 1 | 0.7 | 3 | 1.0 | |
| Unable to remove pessary | Yes | 15 | 10.6 | 11 | 7.2 | 26 | 8.8 |
| No | 117 | 82.4 | 45 | 29.6 | 162 | 55.1 | |
| N/A | 8 | 5.6 | 94 | 61.8 | 102 | 34.7 | |
| Missing | 2 | 1.4 | 2 | 1.3 | 4 | 1.4 | |
| Difficulty removing pessary | Yes | 27 | 19.0 | 12 | 7.9 | 39 | 13.3 |
| No | 100 | 70.4 | 44 | 28.9 | 144 | 49.0 | |
| N/A | 12 | 8.5 | 93 | 61.2 | 105 | 35.7 | |
| Missing | 3 | 2.1 | 3 | 2.0 | 6 | 2.0 | |
| Difficulty having sex | Yes | 5 | 3.5 | 16 | 10.5 | 21 | 7.1 |
| No | 63 | 44.4 | 50 | 32.9 | 113 | 38.4 | |
| N/A | 66 | 46.5 | 82 | 53.9 | 148 | 50.3 | |
| Missing | 8 | 5.6 | 4 | 2.6 | 12 | 4.1 | |
| Pain during sex | Yes | 4 | 2.8 | 9 | 5.9 | 13 | 4.4 |
| No | 66 | 46.5 | 56 | 36.8 | 122 | 41.5 | |
| N/A | 66 | 46.5 | 82 | 53.9 | 148 | 50.3 | |
| Missing | 6 | 4.2 | 5 | 3.3 | 11 | 3.7 | |
| Other | Yes | 9 | 6.3 | 9 | 5.9 | 18 | 6.1 |
| No | 123 | 86.6 | 136 | 89.5 | 259 | 88.1 | |
| Missing | 10 | 7.0 | 7 | 4.6 | 17 | 5.8 | |
| Time point | Self-management | Clinic-based care | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Baseline | 167 | 15.3 | 13.5 | 167 | 17.4 | 15.8 |
| 6 months | 152 | 17.2 | 14.2 | 157 | 18.3 | 16.3 |
| 12 months | 144 | 16.8 | 14.1 | 152 | 21.0 | 17.7 |
| 18 months | 142 | 16.7 | 13.2 | 152 | 22.0 | 17.3 |
In the mixed-effects linear regression on proportion of complications at 18 months adjusted for baseline, there was evidence that participants in the clinic-based care group had a statistically significant higher proportion of pessary-related complications than those in the self-management group (mean difference in proportion 3.83, 95% CI 0.81 to 6.86).
Pelvic Floor Distress Inventory-20 and subscales
Table 11 shows the descriptive statistics for the PFDI-20 and its subscales at 18 months. There are three subscales (UDI‐6, CRADI‐8, POPDI‐6), with each subscore ranging from 0 to 100 and a total score of 0–300. Higher scores indicate greater symptom severity.
| Minimum | Maximum | Mean | SD | Median | P25 | P75 | |
|---|---|---|---|---|---|---|---|
| Self-management | |||||||
| UDI-6 | 0 | 91.67 | 38.01 | 20.53 | 33.33 | 25.00 | 45.83 |
| CRADI-8 | 0 | 87.50 | 29.75 | 16.52 | 28.13 | 21.88 | 37.50 |
| POPDI-6 | 0 | 87.50 | 30.30 | 18.25 | 29.17 | 16.67 | 41.67 |
| PFDI-20 | 6.25 | 240.63 | 98.07 | 47.13 | 96.88 | 68.75 | 125.00 |
| Clinic-based care | |||||||
| UDI-6 | 0 | 100.00 | 38.72 | 21.60 | 37.50 | 25.00 | 50.00 |
| CRADI-8 | 0 | 87.50 | 31.54 | 17.88 | 31.25 | 25.00 | 40.63 |
| POPDI-6 | 0 | 100.00 | 31.77 | 19.93 | 31.25 | 16.67 | 41.67 |
| PFDI-20 | 0 | 269.79 | 102.04 | 52.05 | 101.56 | 70.83 | 130.06 |
A mixed-effects linear regression showed no significant difference between the groups in the severity of prolapse-related symptoms measured by PFDI-20 total score at 18 months adjusted for covariates (adjusted mean difference –0.55, 95% CI –9.17 to 8.08).
Pessary care
Participants were asked whether they had removed their pessary themselves in the last 6 months. At baseline, 42 (25.15%) of participants in the self-management group and 28 (16.77%) in the clinic-based care group had done so. At the 6-, 12- and 18-month follow-up points, the proportions reporting removing their own pessary were 84.87%, 84.03% and 79.58%, respectively, in the self-management group and 19.75%, 23.03% and 25.00%, respectively, in the clinic-based care group.
Participants who reported removing their pessary were asked how often they did so. In the self-management group, the numbers of those who reported removing their pessary at least once a month in the last 6 months were two at baseline, 20 at 6 months, 20 at 12 months and 22 at 18 months. In the clinic-based care group, those who reported removing their pessary at least monthly numbered seven at baseline, nine at 6 months, five at 12 months and 13 at 18 months.
Participants were asked at each time point, ‘Are you planning to continue using a pessary to manage your prolapse symptoms?’ (Table 12). Most participants in both groups intended to continue using a pessary.
| Time point | Self-management | Clinic-based care | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Yes, n (%) | No, n (%) | Not sure, n (%) | Not answered, n (%) | N | Yes, n (%) | No, n (%) | Not sure, n (%) | Not answered, n (%) | |
| Baseline | 167 | 163 (97.6) | 1 (0.6) | 1 (0.6) | 2 (1.2) | 167 | 163 (97.6) | 0 (0.0) | 2 (1.2) | 2 (1.2) |
| 6 months | 152 | 136 (89.5) | 7 (4.6) | 7 (4.6) | 2 (1.3) | 157 | 142 (90.4) | 5 (3.2) | 6 (3.8) | 4 (2.5) |
| 12 months | 144 | 133 (92.4) | 2 (1.4) | 4 (2.8) | 5 (3.5) | 152 | 136 (89.5) | 6 (3.9) | 7 (4.6) | 3 (2.0) |
| 18 months | 142 | 130 (91.5) | 5 (3.5) | 3 (2.1) | 4 (2.8) | 152 | 131 (86.2) | 5 (3.3) | 12 (7.9) | 4 (2.6) |
Participants were asked at each time point to agree or disagree with three statements regarding pessaries. The responses to each statement are presented by group (Tables 13–15). The proportions finding pessary changes comfortable or convenient or pessary care acceptable are similar in the two groups.
| Time point | Self-management | Clinic-based care | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Strongly agree/agree, n (%) | Neither agree nor disagree, n (%) | Disagree/strongly disagree, n (%) | Not answered, n (%) | N | Strongly agree/agree, n (%) | Neither agree nor disagree, n (%) | Disagree/strongly disagree, n (%) | Not answered, n (%) | |
| Baseline | 167 | 83 (49.7) | 42 (25.1) | 26 (15.6) | 16 (9.6) | 167 | 83 (49.7) | 48 (28.7) | 22 (13.2) | 14 (8.4) |
| 6 months | 152 | 73 (48.0) | 38 (25.0) | 34 (22.4) | 7 (4.6) | 157 | 79 (50.3) | 32 (20.4) | 37 (23.6) | 9 (5.7) |
| 12 months | 144 | 69 (47.9) | 33 (22.9) | 36 (25.0) | 6 (4.2) | 152 | 80 (52.6) | 34 (22.4) | 35 (23.0) | 3 (2.0) |
| 18 months | 142 | 64 (45.1) | 40 (28.2) | 31 (21.8) | 7 (4.9) | 152 | 76 (50.0) | 28 (18.4) | 39 (25.7) | 9 (5.9) |
| Time point | Self-management | Clinic-based care | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Strongly agree/agree, n (%) | Neither agree nor disagree, n (%) | Disagree/strongly disagree, n (%) | Not answered, n (%) | N | Strongly agree/agree, n (%) | Neither agree nor disagree, n (%) | Disagree/strongly disagree, n (%) | Not answered, n (%) | |
| Baseline | 167 | 90 (53.9) | 45 (26.9) | 15 (9.0) | 17 (10.2) | 167 | 87 (52.1) | 51 (30.5) | 15 (9.0) | 14 (8.4) |
| 6 months | 152 | 104 (68.4) | 29 (19.1) | 10 (6.6) | 9 (5.9) | 157 | 95 (60.5) | 36 (22.9) | 17 (10.8) | 9 (5.7) |
| 12 months | 144 | 102 | 25 (17.4) | 11 (7.6) | 6 (4.2) | 152 | 98 (64.5) | 34 (22.4) | 16 (10.5) | 4 (2.6) |
| 18 months | 142 | 101 (71.1) | 21 (14.8) | 12 (8.5) | 8 (5.6) | 152 | 92 (60.5) | 34 (22.4) | 17 (11.2) | 9 (5.9) |
| Time point | Self-management | Clinic-based care | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Strongly agree/agree, n (%) | Neither agree nor disagree, n (%) | Disagree/strongly disagree, n (%) | Not answered, n (%) | N | Strongly agree/agree, n (%) | Neither agree nor disagree, n (%) | Disagree/strongly disagree, n (%) | Not answered, n (%) | |
| Baseline | 167 | 128 (76.6) | 18 (10.8) | 5 (3.0) | 16 (9.6) | 167 | 133 (79.6) | 16 (9.6) | 4 (2.4) | 14 (8.4) |
| 6 months | 152 | 129 (84.9) | 12 (7.9) | 3 (2.0) | 8 (5.3) | 157 | 134 (85.4) | 13 (8.3) | 2 (1.3) | 8 (5.1) |
| 12 months | 144 | 125 (86.8) | 7 (4.9) | 5 (3.5) | 7 (4.9) | 152 | 131 (86.2) | 11 (7.2) | 7 (4.6) | 3 (2.0) |
| 18 months | 142 | 122 (85.9) | 12 (8.5) | 1 (0.7) | 7 (4.9) | 152 | 131 (86.2) | 9 (5.9) | 6 (3.9) | 6 (3.9) |
Participants completed the Patient Global Impression of Improvement at each time point to indicate any change in their perception of their pessary care. Responses to the question ‘Compared to before I took part in this study, my pessary care now is …’ are summarised in Table 16.
| Group | Response | Baseline, n (%) | 6 months, n (%) | 12 months, n (%) | 18 months, n (%) |
|---|---|---|---|---|---|
| Self-management | Very much better | 15 (9.0) | 25 (16.4) | 25 (17.4) | 32 (22.5) |
| Much better | 22 (13.2) | 42 (27.6) | 37 (25.7) | 27 (19.0) | |
| A little better | 5 (3.0) | 16 (10.5) | 13 (9.0) | 17 (12.0) | |
| No change | 63 (37.7) | 46 (30.3) | 35 (24.3) | 20 (14.1) | |
| A little worse | 0 (0.0) | 4 (2.6) | 2 (1.4) | 4 (2.8) | |
| Much worse | 0 (0.0) | 0 (0.0) | 1 (0.7) | 1 (0.7) | |
| Very much worse | 0 (0.0) | 0 (0.0) | 1 (0.7) | 0 (0.0) | |
| N/Aa | 5 (3.0) | 1 (0.7) | 21 (14.6) | 25 (17.6) | |
| Not answered | 57 (34.1) | 18 (11.8) | 9 (6.2) | 16 (11.3) | |
| Total | 167 | 152 | 144 | 142 | |
| Clinic-based care | Very much better | 15 (9.0) | 17 (10.8) | 17 (11.2) | 12 (7.9) |
| Much better | 15 (9.0) | 20 (12.7) | 13 (8.6) | 14 (9.2) | |
| A little better | 5 (3.0) | 7 (4.5) | 7 (4.6) | 11 (7.2) | |
| No change | 93 (55.7) | 101 (64.3) | 78 (51.3) | 66 (43.4) | |
| A little worse | 0 (0.0) | 0 (0.0) | 5 (3.3) | 13 (8.6) | |
| Much worse | 0 (0.0) | 0 (0.0) | 2 (1.3) | 1 (0.7) | |
| N/Aa | 2 (1.2) | 0 (0.0) | 23 (15.1) | 26 (17.1) | |
| Not answered | 37 (22.2) | 12 (7.6) | 7 (4.6) | 9 (6.9) | |
| Total | 167 | 157 | 152 | 152 |
A mixed-effects ordinal regression model indicated that participants in the clinic-based care group were significantly more likely to respond in lower categories. The clinic-based care group had increased odds of being in a more dissatisfied category (adjusted odds ratio 3.23, 95% CI 1.47 to 7.13), conditional on all other variables in the model and the random effects.
Pessary confidence
Table 17 shows the responses to ‘How confident are you that you can manage problems related to using a pessary?’, ‘How confident are you that you can (or could if asked) remove your pessary on your own?’ and ‘How confident are you that you can (or could if asked) insert your pessary on your own?’. Responses are on a 0–100 scale, where 0 is no confidence and 100 is highly confident.
| Response | Time point | Group | Mean | SD | n |
|---|---|---|---|---|---|
| Manage pessary problems | Baseline | Self-management | 75.84 | 22.85 | 166 |
| Clinic-based care | 74.85 | 23.53 | 164 | ||
| 6 months | Self-management | 78.94 | 23.43 | 146 | |
| Clinic-based care | 74.74 | 25.09 | 153 | ||
| 12 months | Self-management | 80.73 | 23.17 | 143 | |
| Clinic-based care | 72.81 | 26.63 | 149 | ||
| 18 months | Self-management | 78.95 | 26.03 | 141 | |
| Clinic-based care | 70.86 | 28.10 | 149 | ||
| Remove pessary | Baseline | Self-management | 67.72 | 31.78 | 163 |
| Clinic-based care | 62.68 | 33.30 | 163 | ||
| 6 months | Self-management | 84.24 | 29.85 | 148 | |
| Clinic-based care | 57.07 | 35.55 | 155 | ||
| 12 months | Self-management | 83.86 | 30.87 | 143 | |
| Clinic-based care | 54.91 | 37.98 | 149 | ||
| 18 months | Self-management | 85.28 | 30.24 | 142 | |
| Clinic-based care | 52.63 | 38.63 | 152 | ||
| Insert pessary | Baseline | Self-management | 58.28 | 33.92 | 163 |
| Clinic-based care | 58.61 | 32.52 | 162 | ||
| 6 months | Self-management | 80.99 | 32.04 | 146 | |
| Clinic-based care | 53.22 | 35.65 | 155 | ||
| 12 months | Self-management | 81.25 | 33.14 | 142 | |
| Clinic-based care | 50.01 | 38.37 | 149 | ||
| 18 months | Self-management | 81.28 | 33.31 | 142 | |
| Clinic-based care | 48.36 | 37.74 | 152 |
The adjusted difference between groups at 18 months is statistically significant for all three outcomes. From a mixed-effects analysis there was evidence that those in the selfmanagement group were more confident that they could manage pessary problems (−7.99, 95% CI −14.15 to −1.82), that they could remove their pessary (−32.78, 95% CI –40.45 to –25.10) and that they could insert their pessary (−32.92, 95% CI –40.64 to –25.19).
General self-efficacy
The mean general self-efficacy score (measured on a scale from 10 to 40, where higher scores indicate greater self-efficacy) was similar in the two the groups (Table 18).
| Time point | Minimum | Maximum | Mean | SD | Median | P25 | P75 | n |
|---|---|---|---|---|---|---|---|---|
| Baseline | ||||||||
| Self-management | 15 | 40 | 32.9 | 4.8 | 33 | 30 | 37 | 163 |
| Clinic-based care | 16 | 40 | 33.0 | 4.3 | 33 | 30 | 37 | 163 |
| 18 months | ||||||||
| Self-management | 12 | 40 | 31.3 | 5.4 | 31 | 29 | 35 | 132 |
| Clinic-based care | 18 | 40 | 32.0 | 4.0 | 31 | 29 | 35 | 143 |
In a mixed-effects linear regression, there was no evidence of a difference between the groups in general self-efficacy at 18 months (mean difference 0.77, 95% CI –0.14 to 1.69).
Sexual activity
Participants were asked at baseline and 18 months whether they were sexually active or not (Table 19).
| Time point | Response | Self-management, n (%) | Clinic-based care, n (%) |
|---|---|---|---|
| Baseline | Not sexually active | 85 (50.9) | 82 (49.1) |
| Sexually active | 78 (46.7) | 79 (47.3) | |
| Missing | 4 (2.4) | 6 (3.6) | |
| Total | 167 | 167 | |
| 18 months | Not sexually active | 72 (50.7) | 77 (50.7) |
| Sexually active | 57 (40.1) | 61 (40.1) | |
| Missing | 13 (9.2) | 14 (9.2) | |
| Total | 142 | 152 |
There was very little difference between the groups in the proportion of participants who were sexually active at each time point: just over half said at both time points that they were not sexually active. The PISQ-IR50 was used to assess participants’ sexual symptoms. There are five subscales for women who are sexually active with or without a partner. Higher scores indicate better sexual function. Responses were very similar in the two groups at both time points (Table 20).
| Time point | Min | Max | Mean | SD | Median | P25 | P75 | n |
|---|---|---|---|---|---|---|---|---|
| Baseline | ||||||||
| Self-management | ||||||||
| SA Partner Related | 1.33 | 4.00 | 3.37 | 0.57 | 3.67 | 3.33 | 4.00 | 73 |
| SA Condition Specific | 2.33 | 5.00 | 4.68 | 0.53 | 5.00 | 4.33 | 5.00 | 62 |
| SA Global Quality | 1.00 | 4.75 | 3.61 | 1.08 | 3.88 | 3.00 | 4.75 | 74 |
| SA Condition Impact | 1.00 | 4.00 | 3.27 | 0.83 | 3.50 | 2.75 | 4.00 | 75 |
| SA Desire | 1.33 | 4.67 | 2.90 | 0.67 | 3.00 | 2.33 | 3.33 | 75 |
| Clinic-based care | ||||||||
| SA Partner Related | 1.67 | 4.00 | 3.46 | 0.52 | 3.33 | 3.33 | 4.00 | 73 |
| SA Condition Specific | 3.33 | 5.00 | 4.76 | 0.42 | 5.00 | 4.67 | 5.00 | 65 |
| SA Global Quality | 1.50 | 5.00 | 3.58 | 1.02 | 3.75 | 2.75 | 4.75 | 76 |
| SA Condition Impact | 1.00 | 4.00 | 3.21 | 0.72 | 3.25 | 2.75 | 4.00 | 76 |
| SA Desire | 1.33 | 4.33 | 2.75 | 0.59 | 2.67 | 2.33 | 3.00 | 76 |
| 18 months | ||||||||
| Self-management | ||||||||
| SA Partner Related | 2.00 | 4.00 | 3.41 | 0.52 | 3.33 | 3.00 | 4.00 | 49 |
| SA Condition Specific | 3.67 | 5.00 | 4.69 | 0.43 | 5.00 | 4.67 | 5.00 | 53 |
| SA Global Quality | 1.00 | 4.75 | 3.51 | 1.10 | 3.50 | 2.75 | 4.50 | 57 |
| SA Condition Impact | 1.00 | 4.00 | 3.37 | 0.73 | 3.75 | 2.75 | 4.00 | 57 |
| SA Desire | 1.33 | 5.00 | 2.91 | 0.69 | 2.67 | 2.33 | 3.33 | 57 |
| Clinic-based care | ||||||||
| SA Partner Related | 1.67 | 4.00 | 3.39 | 0.61 | 3.33 | 3.00 | 4.00 | 50 |
| SA Condition Specific | 1.00 | 5.00 | 4.54 | 0.77 | 5.00 | 4.33 | 5.00 | 59 |
| SA Global Quality | 1.25 | 4.75 | 3.39 | 0.94 | 3.50 | 2.75 | 4.00 | 62 |
| SA Condition Impact | 1.5 | 4.00 | 3.25 | 0.79 | 3.25 | 2.65 | 4.00 | 64 |
| SA Desire | 1.33 | 4.00 | 2.66 | 0.62 | 2.67 | 2.33 | 3.00 | 62 |
A linear mixed-effects model of the total PISQ-IR score for participants who were sexually active showed no difference between the groups at 18 months (mean difference –0.34, 95% CI –9.87 to 7.19).
Adverse events
No suspected unexpected serious adverse reactions were reported by participants in the trial. There were 32 SAEs in total (17 reported in the self-management group, 14 reported in the clinic-based care group). There was also one SAE reported by a woman in the non-randomised interview group relating to her pessary and her prolapse symptoms. This was reported and included despite her not being in the randomised cohort.
Fourteen of the reported SAEs were for surgery: seven for prolapse surgery and seven for non-urogynaecology surgery. Two deaths were reported: one due to COVID-19 and one due to ischaemic small-bowel infarction. Two women were hospitalised due to COVID-19. The other 14 reported SAEs, including the SAE for the non-randomised woman, were all hospitalisations for a variety of other reasons such as cancer, ectopic pregnancy and chronic obstructive pulmonary disease.
Health of vaginal tissues
In the self-management group 136 women attended their final 18-month clinic visit, and in the clinic-based care group 154 women attended. We have data on health of vaginal tissues for 135 and 153 participants from each group, respectively (Table 21).
| Self-management (N = 135), n (%) | Clinic-based care (N = 153), n (%) | Total (N = 288), n (%) | |
|---|---|---|---|
| Inflammation of tissues | 9 (6.7) | 17 (11.1) | 26 (9.0) |
| Ulceration | 3 (2.2) | 9 (5.9) | 12 (4.2) |
| Granulation | 10 (7.4) | 8 (5.2) | 18 (6.3) |
| Other clinical concerns | 15 (11.1) | 26 (17.0) | 41 (14.2) |
Additional telephone support
In the self-management group 28 participants received at least one additional telephone support call. In the clinic-based care group 26 received at least one additional call. The difference in the number of additional telephone calls between the groups was not statistically significant (effect size 0.01, 95% CI −0.02 to 0.03). The mean number of additional clinic visits per participant was 0.25 in the self-management group and 0.19 in the clinic-based care group (effect size −0.07, 95% CI −0.20 to 0.07).
Adherence to intervention
Crossover
Twenty-eight participants reverted from self-management to clinic-based care before the 6-month follow-up, and six reverted from self-management to clinic-based care between the 6- and 12- month follow-ups. An analysis was conducted in which these participants were recoded as clinic-based care and the groups were compared by treatment received, employing an identical linear mixed model to that used in the main analysis of the primary outcome, and this is shown in row 2 of Table 5. This showed an interaction effect between the groups and the 18-month time point of –7.84 (95% CI –17.51 to 1.83), which was not statistically significant.
A complier-average causal effect estimate was obtained using instrumental variable methods, where randomisation was the exogenous instrument and treatment received under the crossover definition was the endogenous variable. The estimate of the complier-average causal effect was −0.66 (95% CI –12.59 to 11.28), which was not statistically significant.
On treatment
Very few clinic-based care participants met the original 'on treatment' definition (n = 2) because there were large numbers of missing data for the question of whether they had inserted their pessary themselves in the last 6 months. Using a modified definition of ‘on treatment’, which was specified in the SAP, an additional analysis was carried out whereby those in the clinic-based care group who had missing responses to the question about pessary insertion were treated as if they had responded ‘no’. Using this definition, there were 141 (83.4%) self-management participants on treatment and 103 (60.2%) clinic-based care participants on treatment for the 18 months. The low proportion of clinic-based care participants on treatment is primarily due to a sizeable number who had responded ‘yes’ to inserting their pessary for at least one time point. A total of 44 participants in the clinic-based care group (26.0%) reported inserting their pessary themselves at the 6-, 12- or 18-month time point, and a further 16 had discontinued pessary use.
Post hoc analysis: disruption to treatment due to COVID-19 pandemic
Primary outcome
Some participants in the clinic-based care group did not receive their clinic appointments while clinics were cancelled during the COVID-19 pandemic. The primary analysis was re-run excluding participants in the clinic-based care group who missed appointments for this reason. There were 11 participants who missed 6-month appointments and 15 who missed 12-month appointments. All data for these participants were removed from an analysis of the primary outcome at each time point, the results of which are summarised in Table 22.
| Time point | Trial group | n | Minimum | Maximum | Mean | SD | Median | P25 | P75 |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Self-management | 165 | 0 | 271.4 | 32.5 | 49.6 | 9.5 | 0 | 42.9 |
| Clinic-based care | 142 | 0 | 228.6 | 30.6 | 47.2 | 14.3 | 0 | 38.1 | |
| 6 months | Self-management | 149 | 0 | 238.1 | 22.7 | 36.6 | 9.5 | 0 | 23.8 |
| Clinic-based care | 134 | 0 | 266.7 | 30.0 | 48.1 | 9.5 | 0 | 33.3 | |
| 12 months | Self-management | 144 | 0 | 290.5 | 30.3 | 52.0 | 9.5 | 0 | 33.3 |
| Clinic-based care | 126 | 0 | 285.7 | 33.0 | 54.0 | 9.5 | 0 | 42.9 | |
| 18 months | Self-management | 139 | 0 | 295.2 | 32.3 | 50.9 | 9.5 | 0 | 38.1 |
| Clinic-based care | 128 | 0 | 223.8 | 34.5 | 50.3 | 14.3 | 0 | 42.9 |
The primary analysis by mixed-effects regression showed no difference between the groups at 18 months after these data were removed (interaction effect between trial group and 18-month time point −4.38, 95% CI −14.22 to 5.47).
Pessary complications
The analysis of pessary complications was repeated with participants in clinic-based care who had missed clinic appointments due to COVID-19 removed from the analysis. The proportion of complications experienced by participants in each group at each time point is summarised in Table 23.
| Time point | Trial group | n | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Baseline | Self-management | 167 | 15.3 | 13.5 | 0 | 63.6 |
| Clinic-based care | 143 | 17.6 | 16.0 | 0 | 63.6 | |
| 6 months | Self-management | 152 | 17.2 | 14.2 | 0 | 54.6 |
| Clinic-based care | 135 | 19.0 | 17.0 | 0 | 69.2 | |
| 12 months | Self-management | 146 | 16.8 | 14.0 | 0 | 54.6 |
| Clinic-based care | 129 | 21.4 | 18.1 | 0 | 81.8 | |
| 18 months | Self-management | 143 | 16.8 | 13.2 | 0 | 63.6 |
| Clinic-based care | 130 | 22.9 | 18.1 | 0 | 76.9 |
The analysis of the proportion of complications experienced at 18 months by group showed that the participants in the clinic-based care group reported a significantly higher proportion of complications than the self-management group (4.76, 95% CI 1.56 to 7.96).
COVID-19 questionnaire
Participants in either group who had a clinic appointment postponed or cancelled due to COVID-19 restrictions were sent a survey about their experiences. There were 80 responses to the survey: 26 from the self-management group and 54 from the clinic-based care group.
Thirty-seven participants gave the dates of their cancelled appointments, and these were from 1 March 2020 to 15 December 2020 inclusive.
Participants were asked ‘When your pessary appointment was cancelled were you given clear instructions on what to do if there was a problem with your pessary?’, and 65.4% (17/26) of the self-management participants and 55.6% (30/54) of the clinic-based care participants responded ‘yes’ to this question.
Responses to the question ‘How worried were you about NOT having your pessary changed due to your face-to-face clinic appointment being delayed?’ are shown in Table 24.
| Level | Self-management | Clinic-based care | ||
|---|---|---|---|---|
| Frequency | Per cent | Frequency | Per cent | |
| Very high | 0 | 0 | 1 | 1.85 |
| High | 3 | 11.54 | 6 | 11.11 |
| Moderate | 5 | 19.23 | 19 | 35.19 |
| Low | 4 | 15.38 | 12 | 22.22 |
| Very low or none | 13 | 50.00 | 14 | 25.93 |
| Missing | 1 | 3.85 | 2 | 3.70 |
| Total | 26 | 100.00 | 54 | 100.00 |
A higher proportion of participants in the clinic-based care group reported being moderately worried about not having their pessary changed.
Summary of findings
-
There is no evidence of a significant difference between the groups in the primary outcome measure, the PFIQ-7, at 18 months.
-
A sensitivity analysis of the primary outcome showed no significant difference between the groups under a range of different assumptions and analysis methods.
-
A subgroup analysis of the primary outcome showed no significant effect of treatment group by subgroup interactions (subgroups were age < 65 vs. ≥ 65 years, new vs. existing pessary user and hysterectomy at baseline vs. no hysterectomy at baseline).
-
A significantly higher proportion of pessary complications was reported at 18 months in the clinic-based care group.
-
Women in the self-management group were significantly more confident in their ability to manage pessary-related problems.
-
An analysis adjusting for clinic-based care appointments cancelled due to the COVID-19 pandemic did not alter the findings.
Chapter 4 Process evaluation
Introduction
The process evaluation aimed to answer the following research question: What are the barriers to and facilitators of intervention acceptability, intervention effectiveness, fidelity to delivery, and adherence among women treated with vaginal pessary and the HCPs who treat them, and how does this differ between randomised groups?
In this chapter, the sample obtained for each method of data collection will be described; findings will then be presented under each key area identified in the research questions, specifically, acceptability, effectiveness (from a qualitative perspective), fidelity and adherence, with consideration given to group difference in each section. The analysis was undertaken as documented in the process evaluation analysis plan (see Project Documentation). 41 A mediational analysis of self-efficacy was planned; however, this was not undertaken as there was no significant direct effect of self-management on either the primary outcome or the potential mediating factor.
The pilot study process evaluation specifically focused on recruitment processes. The recruitment materials and processes were found to be acceptable to potential participants and recruiting HCPs, and findings suggested that the processes would support successful recruitment to the trial. For a more detailed account of the findings, please see the pilot study report in the Project Documentation on the project website. 41
Data collected
Table 25 provides an overview of the data gathered for the process evaluation. As planned, 36 interviews were undertaken with women randomised to the trial at baseline. The interview sample showed a similar distribution in age, parity and deprivation index at baseline to that of the main trial sample. There was a slightly larger number of new users in the clinic-based group at baseline (n = 12) than there was in the self-management group (n = 7) for the interviews. Twenty-three women agreed to a follow-up interview: 12 from the self-management group and 11 from the clinic-based care group. The analysis of randomised interviews presented in this chapter is based on the analysis of the 23 complete cases. Of the 12 women in the complete self-management cases, 5 were < 65 years old and 7 were > 65 years old, 5 were new pessary users and 7 were existing users, 1 was in Scottish Index of Multiple Deprivation75 or Index of Multiple Deprivation76 category 1–5 and 11 were in categories 6–10, and parity ranged from one to three births. Of the 11 women in the complete clinic-based care cases, 4 were < 65 years and 7 were > 65 years, 7 were new pessary users and 4 were existing users, 3 were in SIMD or IMD categories 1–5 and 8 were in categories 6–10, and parity ranged from one to six births.
| Interviews | ||
| With randomised women | Self-management 18 interviews at baseline
12 interviews at 18 months |
Clinic-based care 18 at baseline
11 interviews at 18 months |
| With non-randomised women | 20 women interviewed at baseline (of whom 18 were on a clinic-based care pathway and 2 were on a non-TOPSY self-management pathway)
18 follow-up interviews conducted (17 clinic-based care women and 1 self-management woman) |
|
| With HCPs | 36 interviews with HCPs:
|
|
| Audio-recordings | ||
| Of recruitment discussions | 13 recordings | |
| Of self-management support sessions | 22 recordings (self-management group only) | |
| Of 2-week follow-up self-management support telephone calls | 34 recordings (self-management group only) | |
| HCP-completed checklists (self-management group only) | ||
| For self-management support sessions | 156 checklists completed | |
| For 2-week follow-up self-management support telephone calls | 145 checklists completed | |
Non-randomised interview participants were women who were eligible for the trial but had declined to participate. As planned, 20 baseline interviews were conducted with this group. At baseline, women were predominantly receiving clinic-based care (n = 18), were over the age of 65 years and were existing pessary users. Women had a similar distribution to the randomised sample with regard to parity, and a slightly higher proportion resided in areas of higher deprivation than in the randomised sample. A complete data set for analysis was available for 18 women, of whom 5 were < 65 years of age and 11 were > 65 years, with 2 women’s ages missing; 2 women were new pessary users and 16 were existing pessary users; 8 resided in SMID or IMD categories 1–5 and 10 resided in categories 6–10; and parity ranged from one to four births (four missing).
Interviews were undertaken with at least one HCP involved with the TOPSY study from each participating centre; the HCPs were involved in TOPSY recruitment (n = 19) or intervention delivery (n = 17). HCPs recruiting to the TOPSY trial and those delivering the intervention were highly experienced, with a range of 6 to > 20 years’ clinical experience, and represented various professional backgrounds such as medical consultants, specialist nurses and physiotherapists.
As planned, one or more recruitment discussions were audio-recorded at each of the six pilot centres (total n = 13). The target to audio-record 30 self-management support sessions was not met, with 22 sessions recorded. This was because either women did not consent to have their session recorded or centres delivered the self-management teaching appointment directly following randomisation to minimise women’s need to return to clinic, which did not allow the researcher time to contact the centre to request that the session be recorded. Thirty-four follow-up calls were recorded, four more than originally planned. At least one self-management session or follow-up call was recorded for each centre.
The HCPs who delivered the self-management intervention, either the support session or the telephone call, were asked to complete a checklist for each session or call. A total of 156 checklists were completed for the self-management support sessions and 145 were completed for the 2-week follow-up support telephone calls.
Process evaluation findings
The findings presented in this chapter were documented prior to the results of the trial and cost-effectiveness analysis being revealed. The relevant data from each of the data sources are drawn on to offer a synthesis of the main points for each of the four main areas identified in the research questions.
Intervention acceptability
Intervention acceptability was explored for self-management and clinic-based care. Self-management was an acceptable intervention, as was clinic-based care. However, the underlying reasons for acceptability differed.
Self-management
One hundred and sixty-nine women were randomised to the self-management group, of whom 156 received the self-management support session (92.3%); 145 (85.8%) received the 2-week follow-up call and 133 (78.7%) were still self-managing their pessary after they had received the complete TOPSY intervention package (teaching appointment, leaflet and contact telephone number, and 2-week follow-up call).
Self-management for vaginal pessaries was consistently reported as an acceptable treatment pathway for women and HCPs. HCPs delivering the intervention commented on the benefits of self-management for women and services. For example, they felt that self-management would allow women to gain confidence in their abilities and use the pessary in a way that suited their own personal preference and needs:
I know, but it would be good for it to be rolled out nationwide, giving the confidence to the patients to look after themselves, and be in control of it.
Self-management deliverer [SMD] 53
HCPs, women randomised to the trial and those who were eligible for the trial but declined to participate all reported benefits of self-management to service delivery with regard to the availability of clinic spaces and the pressures on the service more generally. Women participating in the trial and those participating in only the interview component also spoke about the cost–benefit to the NHS if more women were to self-manage:
Yeah, and it works for them and for us really ‘cause, obviously, it will keep our clinic numbers down a little bit, and they’ve, you know, they can get on with it as and when it suits them, so to me the whole idea of it is quite interesting.
SMD 56
It seemed to me that if the pessary clinic, the lassie [girl] that runs the pessary clinic, if she had no … she literally had no appointments from the June right round to the following April and it seemed to me that if I self-managed then I wouldn't be taking up an appointment. And if more people did they wouldn't be taking up an appointment.
Dahlia, self-management, randomised
There were components of the intervention that made pessary self-management acceptable to women. The key feature of the intervention emphasised by women who received self-management was having a dedicated HCP instruct them with empathy and calm professionalism, which enhanced the acceptability of self-management for them as they felt reassured and cared for during the teaching session. Given this emphasis, the relationship between women and HCPs was seen as crucial. Women in the self-management group often commented on how the reassurance from the HCP delivering the intervention gave them the confidence to start and continue to self-manage. The women randomised to the self-management group also spoke about having confidence in their care team in case they needed to see them or had any concerns while self-managing. For many women in the self-management group, this confidence in the care team provided them with an extra level of autonomy to self-manage their vaginal pessary as they felt that they continued to be ‘cared for’ and not left behind in case a need arose:
And knowing that if there were any problems I could ring was always useful. I think that’s quite reassuring when you know you can do that.
Liana, self-management, randomised
It was important to some self-management women to have this continued HCP support by having a contact telephone number available in case of any problems and to continue to be ‘part of the system’ rather than being discharged and potentially having to go through a referral process again if they needed further HCP input in the future:
I mean, the service has always been good. My frustration has been that it’s an effort for me to go to the clinic because it’s a, sort of, at least an hour there and an hour back for an appointment that sometimes takes 5 minutes, but I don’t want to not be part of the process because I don’t want to, kind of, come out of the system.
Jasmine, self-management, randomised
This emotional labour performed by the HCP should be noted as a vital component of the self-management intervention delivery in the TOPSY trial.
Pragmatic benefits, such as reduced travel and parking costs and not having to attend an appointment every 6 months, also contributed to acceptability for women randomised to self-management. Aside from practical benefits, women could see other benefits for themselves. These included feeling in control, being able to readjust the pessary properly if it moved out of position, being able to remove the pessary when needed, avoiding pain and discomfort during pessary removal, feeling less embarrassed and feeling ‘cleaner’. These advantages align with the proposed mechanism of action of the intervention, specifically women having the self-efficacy to manage their own pelvic health.
Clinic-based care
Women receiving clinic-based care found this to be acceptable, and women from the non-randomised interview sample overwhelmingly preferred it to self-management. Women in the non-randomised sample provided additional data on clinic-based care, demonstrating that clinic-based care in the trial was the same as that outside the trial. Women randomised to the clinic-based group and those women who received clinic-based care in the non-randomised sample also placed considerable value on their relationship with the HCP who provided pessary care. While responsiveness of the HCP was a factor common to both groups, those in the clinic-based care group also wanted to be looked after by the HCP, with the HCP holding the responsibility:
They are great and they directed me in the right way, the right direction so they looked after me and I’m grateful for that.
Clementine, clinic-based care, randomised
I really like them because I can just call them and say, it’s pinching, can I come? And she just lets me come in without even an official appointment and she fixes it, she takes it out and cleans it and puts it back in, all of them, both. So I am very happy with my … What do you call it? Clinical management?
>Iris, clinic-based care, randomised
Women receiving clinic-based care favoured receiving care from the same HCP for every pessary change but understood that this was not always possible, and care delivery generally did not reduce in quality with different HCPs. There was only one instance of a non-randomised clinic-based care woman saying that the quality of care was lower when she did not see her usual HCP.
[…] I had a new nurse on my last appointment and when she replaced the pessary, she said oh, this is a very, very strange colour, you know, like do you have any idea why and I said well, it's because I'm still menstruating. And she had never seen, you know, the fact that they discolour if, you know, if the patients, you know, and I felt a little bit embarrassed by the fact that she said, oh I've never seen this before […].
Sage, clinic-based care, non-randomised
This strengthens the argument that the HCP–woman interaction is important in all pessary care delivery, and it provides guidance for HCPs about communication with women who may already feel vulnerable.
Multiple other factors supported acceptability of clinic-based care for women randomised to this group. One of these was a history of prolonged pessary fitting or of recurrent urinary tract infections, which increased women’s need to be reassured by HCPs and receive routine visual examinations of the vaginal tissues by a trained HCP. Women’s lack of knowledge about their anatomy was linked to a lack of confidence in their ability to correctly self-manage their pessary and their worries that the pessary could possibly get lost or inserted in the wrong way. This was further linked to a lower level of self-efficacy and a higher level of comfort with a more paternalistic model of care where women could hand over their medical problem and care to a HCP. The latter could be associated with women struggling with accepting their prolapse and showing avoidance behaviour by handing over pessary care to a HCP:
I’m in denial about it slightly, so that’s why I don’t want to take the pessary out or put it back in again because I feel like I don’t like interacting with that part of my body because I feel as though it’s not, it’s let me down maybe.
Sage, clinic-based care, non-randomised
In summary, self-management was reported to be an acceptable intervention for all who participated in the study. Support from HCPs was valued by women in both randomised groups and by non-randomised women. Self-management women valued HCP support in giving them the confidence to self-manage, and clinic-based care women valued HCP support in looking after them.
Fidelity to delivery
Self-management
Data to assess fidelity to the self-management intervention derived from interviews with women randomised to self-management, interviews with intervention deliverers and HCP-completed checklists at the support sessions and 2-week follow-up calls. The TOPSY intervention was compared with the usual practice of centres that delivered self-management prior to TOPSY, and it was clear that self-management delivery in the trial looks like it does outside the trial, with the addition in the trial of a 2-week follow-up call.
Intervention deliverers described the TOPSY intervention teaching as very structured. Some HCPs welcomed this detailed structure, whereas others found it somewhat restricting:
It’s very comprehensive and I thought it was a good, clear guide that made it very simple and easy to understand, clear instructions, tips and tricks in there that I could embellish on through the consultation, and there was very little that the ladies didn’t understand, but if there was, it was very easy to clarify for them in the consultation, I think the guide was comprehensive, it was good.
55SMD
It was more rigid, I would say, than conversational, because you’re having to do, like, the checklist. Whereas when we do it, it’s more conversational.
58SMD
Analysis of the checklists suggests that there was fidelity to intervention delivery, with 87–100% of HCP-completed checklists indicating that HCPs felt that they had delivered all elements of the intervention across the support session and 2-week follow-up call. The checklist data were corroborated by data from self-management teaching appointments and follow-up telephone call recordings. However, the recordings highlighted small deviations from the teaching manual. Analysis of the recordings showed that 68% of HCPs delivered ≥ 70% of the required elements of the intervention. The remaining 32% of HCP recordings indicated that HCPs delivered < 70% of the intervention elements, often solely focusing on the practicalities of self-managing a pessary. Specifically, on the recordings HCPs could not always be heard in teaching sessions discussing what to do in the case of pregnancy, introducing themselves or explaining their role in the study. It is possible that the introductions and role explanations had occurred previously or before the recorder had been switched on, and pregnancy might not have been discussed because the HCP knew that the woman in question was post-menopausal.
The original study protocol (V1.29.11.17) documented that the HCP delivering the intervention would also undertake the 2-week self-management follow-up telephone call. Not all participating sites could adhere to this requirement due to the limited capacity of consultants and specialist nurses. Centres that followed an alternative approach, with a different HCP undertaking the 2-week follow-up call, were identified during the site initiation visit and provided with detailed instructions to ensure patient safety; for example, the intervention deliverer would call the woman if any questions, concerns or complications were highlighted during the follow-up call.
Although there were some deviations from the protocol, these were not detrimental to the proposed mechanism of action of the intervention, and nor did they impact on patient safety.
Contextual factors that influenced self-management delivery
Facilitators of intervention fidelity included attitudes to self-management, initial positivity about self-management or the ability to overcome initial negativity, provision of a local support number, clarity and content of the TOPSY protocol, and the ability to practise insertion and removal. The most apparent facilitator of successful intervention delivery was staff and patient attitudes towards pessary self-management. HCPs delivering the intervention voiced their support for pessary self-management:
I think it’s been well overdue doing a study like this to see because I think overall for patients throughout, you know speaking to other areas where self-care hasn’t been a big thing, and I think for women all over it will have a positive outcome hopefully, that more patients will be offered the self-care option. And then it takes the pressure off the health service and things a little bit if more patients are doing self-care.
54SMD
In addition, HCPs described how women responded to being instructed in pessary self-management:
She was really keen for it and managed no problem and has had no problem since.
59SMD
I think, a personal acceptance in preparation for it is more important than actually how you do it, because I don’t think how you do it, is very difficult. As just, whether you’re mentally, as a patient, prepared to go down that route. And, as I said, I was pleasantly surprised, so. Yeah … no, the teaching, sort of, as far as I’m aware the teaching was easy and straightforward.
53SMD
When talking about their experiences teaching the TOPSY intervention, HCPs frequently commented on their perceptions of women’s responses to being instructed in pessary self-management. Intervention deliverers reported that women’s initial reactions ranged from fear and embarrassment to great enthusiasm beyond what the woman herself had expected. The ability to overcome the less positive initial reactions was a facilitator of fidelity:
There’s a lot of fear initially because it’s an area that people don’t talk about a lot, it’s quite embarrassing for the patients and a lot of them think, oh I don’t think I could do that, that’s not something I could do. But when they actually see the pessary and how simple it is, they think oh my goodness, why did I not do that sooner, they’re really, really positive about it.
61SMD
A lot of the patients don’t want to try and do it within the clinic, they feel rather embarrassed, and they do it at home.
54SMD
I was expecting some objection, or when I do it, I’m not sure I’m the right person for this, or can I change my mind. I haven’t had any of that, so … no. I think I … I was pleasantly surprised by the acceptance is what I’m trying to say.
51SMD
Regardless of how women initially responded to the prospect of being taught how to self-manage their pessary, reassurance that they could get in touch with a HCP if they were to experience any problems was a crucial facilitator of acceptance and subsequent fidelity:
Yes, it just gives them reassurance there’s somebody at the end of the phone that they can talk to, yeah.
58SMD
I suppose they are all thinking the same, what if I can’t get it out. Just the usual things, I can’t get it out, I can’t do it. What if there’s problems? But again, it’s just to reinforce that we’re just a phone call away and they can, you can come up and see us.
62SMD
Three barriers to successful delivery of the self-management intervention were the physical space in clinics, the manual dexterity of the women and difficulty in removing the pessary. Some HCPs delivering the intervention thought that the room used for the self-management teaching appointment could have an impact on successful removal/insertion of the pessary during the practical element of the appointment:
We’re a bit short on rooms sometimes, depending on what times the ladies are coming. So maybe the room setup could influence them, I don’t know. Some rooms have more room, you know, a better bed. I don’t know, maybe that? […] some rooms don’t even have a sink, so that’s a bit of a struggle, but we manage.
57SMD
Women’s physical limitations, such as not being able to pull the pessary out, also impacted successful delivery:
We had the clothes and everything off. She was [inaudible 17:10] with the chairs out, we changed the furniture, we got ourselves locked in and we were getting on. She just couldn’t do it … her hands … Unfortunately, with her … it’s full-body osteoarthritis. Her hands were just so bad she could not … just any strength to pull, to do moves … even with pressure, you know … coughing and, sort of, helping … the wee soul. She persevered for so long. It’s just … But it just wasn’t to be, unfortunately.
56SMD
All women randomised to the self-management group who participated in the interview component confirmed that they had been provided with a local support telephone number in case of problems. This was a uniformly appreciated aspect of the intervention and part of the intervention delivery.
The majority of women who participated in interviews stated that they found insertion of the pessary easier than removal, the main reason being that once the pessary was in situ it could not be manipulated for removal, but it could be manipulated for insertion.
Those who received the TOPSY intervention commented that the intervention elements provided enough information, practical guidance and follow-up for them to confidently self-manage their pessary over the duration of the trial. The clarity and content of delivery acted as a facilitator, with all women who attended the teaching appointment commenting that they found the material delivered to be acceptable and comprehensive:
She [the nurse] showed me what to do, and I did it while I was there. It worked. And I had no problems with the changing of them.
Daisy, self-management, randomised
It [the teaching appointment] was very straightforward, [name of HCP] just basically showed me how I should do it, what I should do, sent me off behind a curtain and let me try. I did struggle to start with but perseverance and tried and I did it. Then we tried it again and after that I was quite fine with it.
Zahara, self-management, randomised
All women in the self-management group who were interviewed said that during their teaching appointment they had received a demonstration of how to remove and insert the pessary and had received replacement pessaries or instructions on how to receive replacement pessaries. Women particularly valued the opportunity to practise removing, inserting and positioning the pessary in clinic with a HCP present to encourage them and provide confidence. Women equally valued being able to handle a pessary prior to practising removal, insertion and positioning. Being able to ask questions during the intervention delivery appointment allowed women to have any concerns addressed and points of uncertainty clarified before they started on this new care pathway.
Opinions about the frequency of clinic appointments for women self-managing their pessary varied among the women interviewed. Some women liked the idea of having a visual examination once a year, while others felt that a clinic appointment every 2 years would be appropriate and cost-effective for both them and the NHS. A few women commented that they felt confident enough to not have routine clinic appointments at all as long as they continued to have access to services when they needed them. Women’s views about the frequency of routine clinic appointments while self-managing is reflected in practice, with centres that offered self-management prior to trial participation either recalling women annually for a physical exam or discharging them without any arrangements for a follow-up appointment.
In summary, there was fidelity to the self-management intervention and it was possible to identify barriers and facilitators supporting that fidelity. The clinic-based care intervention was akin to what is delivered in the NHS and was consistent. Therefore, the trial was a true test of the intervention.
Clinic-based care
A qualitative comparison between interviews with women in the clinic-based control trial group and those with non-randomised women who received clinic-based care from the same centre demonstrated that the care received was the same. Clinic-based care was delivered to women based on local centre protocols, with some, albeit minimal, variation between centres. The main clinic-based care variation between centres was whether pessary clinics were led by medical consultants or nurses. There was also variation in the frequency of clinics offered at centres, ranging from one pessary clinic once a month to several pessary clinics weekly. The most frequent interval for women returning to clinic for pessary changes was 6 months across all participating centres, with some exceptions based on pessary type, where the interval between pessary changes was reduced to every 3 or 4 months.
The treatment of prolapse with self-care pessary did not stipulate requirements for clinic-based care delivery. What is evident from the data is that there was minimal variance in clinic-based care delivery within or between centres. It is therefore reasonable to conclude that the care given in the trial control (clinic-based care) group was akin to clinic-based care usually given within the NHS and that care was relatively uniform.
Adherence to trial group
Avoiding surgery was a considerable motivator for women to choose a pessary as a treatment option. This also affected their adherence to pessary use more broadly and was not particular to women in one specific trial group, but rather cut across women in both trial groups. Analysis of the interview data did not show that avoidance of surgery was a facilitator of trial group adherence depending on randomisation, apart from for one woman.
The majority of women who participated in the interview component were on treatment and adhered to their trial group allocation over the duration of the trial. However, there was variation within and between the trial groups, with some women fully adherent, some partially adherent and some non-adherent across both groups. For clinic-based women full adherence was defined as attending all clinic appointments (as far as was possible within COVID-19 restrictions) and not manipulating their pessary themselves between these clinic visits. Five women from the interview sample were deemed fully adherent to their trial group over the trial duration. In comparison, nine self-management women from the interview sample were fully adherent to their trial group. Full adherence for self-management group women was determined as independently changing the pessary over the 18 months of the trial and contacting the care team if necessary.
In addition, a number of women were partially adherent to their trial group allocation. Five women from the clinic-based group fell into this category. These women continued to attend all clinic appointments, but they also removed, cleaned and reinserted their pessary themselves between these appointments. Only one woman randomised to the self-management group was partially adherent. In this instance, the woman independently self-managed her pessary for 12 months, at which point she developed bothersome complications and changed to a surgical pathway, discontinuing her pessary use.
Only three women from the interview sample for whom we had a complete data set were non-adherent to their assigned trial group. Two self-management women changed to clinic-based care following their self-management teaching appointment as they were unable to remove their pessary themselves. One woman randomised to clinic-based care did not attend any clinic appointments and independently self-managed her pessary throughout her trial participation. She did complete her questionnaires at all time points throughout the trial, which provided safety data, and her care was handed back to the local care team.
Factors that affect adherence to trial group
Contextual factors were identified that supported trial group adherence; some were specific to one trial group and others were applicable to both groups (Figure 7). Those applicable to both groups were good general health, a supportive network, an absence of complications and treatment pathway preference.
FIGURE 7.
Contextual factors supporting women’s adherence to trial group.

Good general health
Good general health acted as a facilitator of trial group adherence for both clinic-based care and self-management and, conversely, poor health was a barrier. Good general health allowed women to self-manage without being restricted by other health concerns. Being in good general health was also a facilitator of adherence in the clinic-based group, as it meant that women were able to attend clinic appointments for pessary changes. In both trial groups, good general health also aided women to stay physically active.
A supportive network
Having a supportive social network was a facilitator of trial group adherence. For women in the clinic-based trial group, a supportive social network often took the form of a spouse accompanying or driving them to clinic appointments. Some women were accompanied by other family members, most notably their daughters, to clinic appointments. This was even more prevalent following the pandemic as many women tried to avoid using public transport. However, support was not limited to transport and clinic appointments. Women in both trial groups valued being able to talk to family members and spouses about their prolapse and felt emotionally supported in their journey. This emotional support allowed women to feel comfortable with the pessary and care pathway they were randomised to for the trial, and beyond.
Absence of side effects or complications of using a pessary
Not experiencing complications was a strong facilitator of women in the self-management group continuing to self-manage for the trial duration. Women randomised to self-management who experienced some complications consulted with their local care team as per the self-management instructions. Complications led to pessary discontinuation for one woman in the self-management group and one woman in the clinic-based group from the interview sample. This shows the impact the absence of side effects had on pessary continuation in both trial groups. Women in the clinic-based group did not differ from women in the self-management group in whether they sought medical help for complications when these occurred. One group difference in relation to complications, highlighted by the interview data, was that women in the self-management group would try to address mild side effects, such as discharge, on their own by removing the pessary and reinserting it once symptoms calmed down, before they contacted their care team:
I didn’t have a discharge as such that was bothering me, but I sort of got … because you get a little bit of discharge anyway, because at the end of the day you’ve got a foreign body in there, haven’t you, so obviously you do get tiny little bits. But at one point it seemed to increase so I took it out and cleaned it and popped it back in again, and then that seemed to help that little bit of increased discharge at the time.
Hazel, self-management, randomised
Preference for a care pathway
A preference for self-management was a facilitator of adherence among women randomised to the self-management group, as they received the treatment they desired, and the same was true for clinic-based care women. Preferring self-management but being randomised to the clinic-based group acted as a barrier to trial group adherence. The divergence from clinic-based trial group adherence because of a preference for self-management ranged from mild to high. Women who diverged mildly from adherence continued to attend clinic appointments but also removed and reinserted their pessary on their own between these appointments. By contrast, high non-adherence encompassed forgoing all clinic-appointments and fully self-managing the pessary. Women randomised to self-management who preferred clinic-based care sometimes reverted to clinic-based care following the teaching appointment.
All non-randomised women had a strong preference for their current care pathway and, with the exception of one woman, none deviated from the care pathway they were on over the 18-month period:
I felt so much, kind of relieved that this one was a lot better, and I was coping with it, I sort of didn’t want to upset the apple cart in any way.
Fleur, clinic-based care, non-randomised
Wouldn’t want to do it myself, I wouldn’t have the confidence to put me own pessary in and out.
Holly, clinic-based care, non-randomised
In addition to these contextual factors facilitating trial group adherence irrespective of trial group assignment, there were group-specific factors that supported adherence. Self-efficacy for self-management positively contributed to women’s adherence to self-management. Self-efficacy differed between the groups, with women in the clinic-based group needing more reassurance, as detailed below. In the self-management group, self-efficacy featured in the actions women took to solve problems while self-managing their pessary. High to moderate self-efficacy for self-management acted as a facilitator of self-management. High self-efficacy was demonstrated in women who adapted the teaching to suit themselves, such as finding a comfortable position in which to remove/reinsert the pessary:
I find the position that I’m doing it in now is more comfortable than I was doing it initially, so I think the more I’ve done it, the more confident I’ve got with it.
Margarite, self-management, randomised
In addition, high self-efficacy was displayed in women who took the initiative to address mild side effects, such as discharge or the pessary causing discomfort, before they contacted their local care team. Women who stated that they felt comfortable not being seen by a HCP unless they felt that there were problems that needed to be addressed (such as a change in pessary size) or developing complications also exhibited high levels of self-efficacy.
Women in the clinic-based group, having not received formal instructions in self-management, resorted to different approaches to working out how best to remove and reinsert their pessary in instances where they wanted to do so. These women’s accounts suggested that they lacked self-efficacy to change their own pessary. One woman reasoned that if the pessary needs to be squeezed going in, then the same should be true when removing it:
The very, very first time you go they [the nurses] show you that it bends. And she [the nurse] says, oh, you need to bend it to put it in and then it just springs open. So I just logically thought, well, if you have to bend it to put it in you’ve obviously got to try and bend it to bring it totally out.
Willow, clinic-based care, randomised
Another woman appeared to have received pessary self-management instructions before participating in the trial and continued to self-manage throughout the entire trial without attending clinic appointments.
Two contextual factors specifically supported trial group adherence to the clinic-based group: having a ‘day out’ and needing reassurance.
-
A ‘day out’
For some women, having to attend a clinic-appointment provided the opportunity to leave their house with a clear destination and purpose. Sometimes this was the only ‘outing’ women had and they welcomed the opportunity to see someone who was not part of their immediate social network. In these cases, women preferred clinic-based care and adhered to clinic-based trial group assignment. The social interaction provided by a clinic appointment was often intertwined with a woman’s lack of self-efficacy with regard to pessary management and a more sedentary and sedate lifestyle.
-
Needing reassurance
The need for the reassurance from a HCP provided during, and as a result of, routine clinic appointments was a facilitator of adherence to clinic-based care. Women found that the contact with an authority figure in the form of a trained HCP, whether a consultant or a nurse, and the visual inspection of their vaginal tissues was comforting and alleviated their anxieties about the pessary potentially harming their vagina. This need for reassurance demonstrated a lack of self-efficacy and a comfort with a more paternalistic model of healthcare delivery in the clinic-based care group:
So I was quite happy to go to the hospital because they just had a look to make sure everything was all right and I found that really quite reassuring.
Viola, clinic-based care, randomised
In summary, trial group adherence varied between and within the groups. Several contextual factors acted as facilitators of or barriers to trial group adherence.
Intervention effectiveness and quality of life
There was variation in intervention effectiveness, with multiple contextual factors affecting this, as demonstrated by the quality-of-life outcomes across both trial groups. There was variation in each group, with women exhibiting good, moderate and poor quality of life. Good quality of life for women in the self-management group was demonstrated by symptom control, elimination of painful pessary changes in clinic and being able to move house without the additional burden of having to worry about immediately having to arrange pessary care in the new location. Symptom control and an improvement in symptoms or sex life, as well as the alleviation of concerns about their pessary due to the routine input from a trained HCP, all contributed to good quality of life for women in the clinic-based group. Where some prolapse symptoms persisted despite the pessary, women in both trial groups displayed moderate quality of life. However, for women in the self-management group, being able to self-manage the pessary contributed to an overall increase in well-being.
Quality of life did not improve for all women, and sometimes it worsened. This was evident in both trial groups. Where women experienced decreased quality of life, this commonly resulted in pessary discontinuation irrespective of trial group assignment.
In fact, I think it’s probably better now the pessary is out.
Viola, clinic-based care, randomised
There was no obvious group difference, in terms of qualitative comparison, for the primary outcome of quality of life.
Contextual factors that influenced intervention effectiveness
There were contextual factors that influenced effectiveness as conceptualised by quality of life, with some factors common to both groups, some unique to self-management and some unique to clinic-based care (Figure 8).
FIGURE 8.
Contextual factors affecting quality of life.
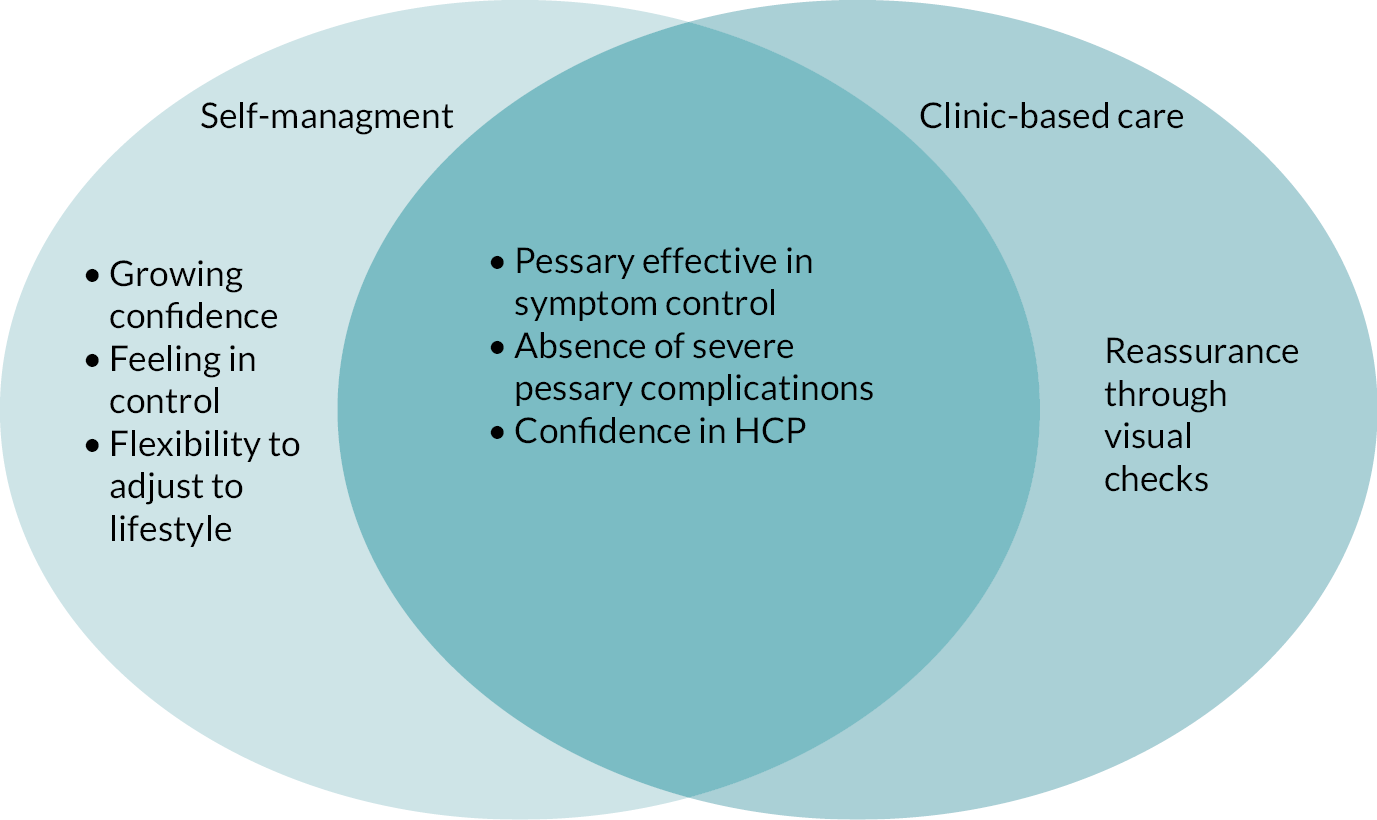
Factors that influenced quality of life and were common to the groups were the effect of the pessary, the absence of severe complications and confidence in the HCP.
-
The effect of the pessary
The pessary itself was effective for symptom control and therefore improved women’s quality of life regardless of their trial group assignment. The impact of the pessary itself meant that it was sometimes difficult to distinguish between the effect of the pessary and the effect of the trial intervention on quality of life.
-
Absence of severe complications
Complications such as non-menstrual bleeding, bothersome smell or discharge, recurrent urinary tract infections or an embedded pessary had a negative impact on a woman’s quality of life, both physically and emotionally. In the case of non-menstrual bleeding this was a distressing experience for a woman, especially as women are routinely informed that non-menstrual bleeding can be a sign of other, more serious, health issues. The possible worries and discomfort caused by vaginal bleeding impacted on a woman’s emotional well-being and quality of life. Similarly, urinary tract infection symptoms were unpleasant and could limit physical activity and social interaction as women may experience discomfort while urinating and need to urinate more frequently. Discharge or a bothersome smell equally could limit a woman’s physical activity and social interactions if she felt unclean, smelly and uncomfortable, again negatively impacting on her quality of life. In the very rare scenario of an embedded pessary, severe pain and other physical symptoms considerably affected a woman’s quality of life and caused emotional distress on top of the physical pain.
-
Confidence in the healthcare professional
All women participating in the interviews expressed confidence in their HCP, asserting that this gave them peace of mind regardless of the trial group they had been randomised to. Women in the self-management group said that they were confident that they would receive help if they needed it by calling the HCP on the telephone number provided. This allowed them to confidently self-manage their pessary without unnecessary worries, consequently enhancing their quality of life. Women in the clinic-based group stated that they felt confident that they received good-quality care from their HCP and that seeing the HCP routinely alleviated their concerns about possible internal abrasions, which they would not be able to see themselves, again improving their quality of life by enhancing their mental well-being.
Self-management and quality of life
For some women in the interview sample there was distinct evidence that pessary self-management had contributed to an improvement in their quality of life beyond the pessary itself. The factors that enabled that improvement were an increase in physical activity and social engagement, self-efficacy to manage the pessary, feeling cleaner, improved sexual functioning, and less travel time and costs. Several of these factors acted in ways that mediated improvements in quality of life; for example, women’s quality of life improved because they were able to improve their social participation.
Increased physical activity and social engagement
Some women commented that self-managing their pessary allowed them to become more physically active and engage in social activities in which they would not have felt comfortable partaking had they not self-managed their pessary. This greater ability to participate improved women’s quality of life:
I mean, in the 18 months of the study I’ve also taken up running again, so I feel more comfortable when I’m using the pessary, certainly, when I’m running, so I have to … if for some reason I’m running when I’ve got my period then I … you know, it’s in out, in out, depending on what I’m doing, but I have that flexibility to take it out and put it back in when I need to at my own choice.
Jasmine, self-management, randomised
Enhanced confidence and empowerment to be in control of the pessary and prolapse
Over the duration of the trial, the women in the self-management group who were interviewed stated that their confidence in self-managing their pessary had grown as time had gone on. This increased confidence in their abilities generated a feeling of empowerment and being in control of their condition, which in turn improved their quality of life:
It’s just been feeling more empowered myself to take care of myself and to not have to go to the hospital as regularly, so every 6 months. Now, I can go annually. For me, that’s just so much easier in terms of my lifestyle.
Jasmine, self-management, randomised
Yes, I’m extremely confident. I had my appointment at the hospital on Wednesday so I got checked out. Everything was fine … He asked me if I wanted to go back and be checked again or when would I want to be checked again, and I said, to be honest, if I don’t need to come back, I know the signs to watch out for if there is a problem, and I would obviously go back to my GP if that was the case and get re-referred so he’s discharged from the clinic now and he’s going to write to my GP so I can get new pessaries on prescription and therefore I’ll be completely self-managing now.
Hazel, self-management, randomised
Improved feeling of cleanliness
Other women expressed an enhanced feeling of cleanliness as self-managing allowed them to remove and clean their pessary more frequently than at the 6-monthly clinic appointments. These additional opportunities to clean the pessary led to women feeling more hygienic:
And of course, when you are … when you take it out, you can have a good bath and everything before you put another … put it back in, you know, which is nice … Yes, well, it is nice just to feel that, you know, everything is clean.
Hyacinth, self-management, randomised
Sexual functioning
A few women commented on improved sexual functioning due to self-managing their pessary. In a few instances women described how being able to remove the pessary for sexual intercourse had had a positive effect on their sex life, enabling them to engage in sex more frequently than had been the case before they received the self-management instructions:
Taking it out for sex is, like, quite good because obviously it sits there, so being able to do that was quite good as well.
Hazel, self-management, randomised
However, other women who received the self-management intervention stated that they did not remove the pessary for sexual intercourse as they would feel uncomfortable during sex without the pessary in situ to hold organs in place:
My husband says he never feels it but I don’t know as such.
Gladys, self-management, randomised
Less travel time and costs
Reduced travel time and costs due to self-managing the pessary also positively impacted on quality of life, especially for women who had to travel long distances to be seen in clinic. In some instances, removing the need to travel every 6 months for a short clinic appointment fitted better with women’s busy and demanding lives:
As far as I’m concerned it’s sorted, I’m extremely grateful I don’t have to keep going back to the hospital every 6 months, which seemed to come around far too quickly.
Hyacinth, self-management, randomised
Clinic-based care and quality of life
One factor that influenced quality of life was unique to the clinic-based care group. Women in the clinic-based group valued the reassurance provided by a trained HCP carrying out routine visual checks during their pessary change appointments. This reassurance considerably contributed to women's quality of life as it limited anxieties that they would miss changes in their vaginal tissues that could lead to complications.
Self-management activities undertaken by women
The proposed mechanism of action was that greater self-efficacy would support women to make the health choices that suit them and their life, and this was demonstrated for the women in the self-management group. Women valued self-management in ways anticipated, with women’s self-management of their pessary varying over the duration of the trial. Among women participating in the interview component who self-managed, this ranged from the bare minimum of removing the pessary once every 6 months to removing it every few weeks:
I only do it once every 6 months … I don’t see the need to, though. I really don’t see the need to remove it, clean it and put it back.
Cassia, self-management, randomised
I would say I take it out every maybe 3 or 4 weeks and give it a clean.
Lily, self-management, randomised
While there is a temporal element to these patterns of pessary care, for other women pessary removal was more situational, as in the following example:
Other than when, there was just a couple of times when I felt that I needed to take it out, ‘cause it had kind of dropped, and when I tried to just push it back, it would just drop again so I’d take it out and start from scratch then.
Daisy, self-management, randomised
Women’s pessary self-management also varied with regard to how long they removed the pessary for. Some women chose to immediately reinsert the pessary after they had cleaned it, whereas others selected to leave the pessary out for days or even weeks:
I just take it out, I clean out and then I put it back in again.
Margarite, self-management, randomised
So I took it out at the end of April and I didn’t put it back in because I didn’t feel I needed it. I wasn’t aware of the cystocele or the other one that’s at the back of whatever, not aware of them at all, I was getting on fine … I reinserted it at the end of May.
Dahlia, self-management, randomised
In summary, quality of life varied, with no obvious group difference emerging from the qualitative analysis. However, the qualitative analysis did suggest that there were contextual factors for women in the self-management group that had the potential to improve their quality of life over and above the effect of the pessary itself.
Impact of COVID-19 pandemic on care receipt
The COVID-19 pandemic affected several patients in the TOPSY trial. For women randomised to clinic-based care, and non-randomised women on a clinic-based care pathway, the COVID-19 pandemic sometimes resulted in the cancellation of at least one clinic appointment. Depending on centre protocols, some women received a telephone call from a member of their care team to ensure patient safety and check that no complications had arisen that required the woman to be seen urgently in clinic. Receiving a telephone call from a member of their healthcare team was appreciated by, and often reassured, women.
However, not all participating centres took this approach, leaving some women without clinical contact until their centres had reopened. Some women felt concerned about not having been seen in clinic once they had reached 12 months since they last had their pessary changed. Despite reassurances from clinical staff, some women questioned the safety of keeping the pessary in situ for longer than 6 months considering that they would usually have a clinic appointment at these time points. In very few instances, women who received clinic-based care as part of the TOPSY trial resorted to removing, cleaning and inserting their pessary themselves without having been formally instructed in pessary self-management.
One woman randomised to clinic-based care removed and reinserted the pessary during the pandemic as her appointment had been cancelled due to the restrictions, and she had felt that she needed to clean the pessary because it was approaching 6 months since this had last been done. Before randomisation she had been asked by her HCP to try to remove and reinsert her pessary. One non-randomised woman inserted the pessary on her own after it accidentally came out when she was removing a tampon. This woman called her local HCP and received coaching over the telephone so she could reinsert the pessary on her own. Both these women did not feel comfortable self-managing their pessary despite being able to do so, and they continued to receive clinic-based care. Their experiences of self-managing the pessary differ from those of women who received formal instructions in clinic by a qualified HCP and who demonstrated self-efficacy in self-management. This highlights the moderating effect of self-efficacy on a woman’s proficiency in self-managing her vaginal pessary.
Women in the self-management group did not experience this level of care disruption. Several women commented that being able to self-manage the pessary during national lockdown had been an additional benefit. Receiving replacement pessaries had not been a problem during the pandemic for the majority of women randomised to the self-management group. This was because women either received the pessaries in the post or already had been given the required pessaries during the teaching appointment.
Conclusion
The multiple data sets show that pessary self-management was an acceptable care pathway for women and HCPs. The data suggest that the TOPSY intervention can be implemented in existing service structures. There was variation in clinic-based care delivery, but the differences within and between centres were minimal. The intervention was delivered as per study protocols and therefore the trial is a true test of the intervention.
There was variation in trial group adherence and intervention effectiveness. Variation was present in both trial groups, and the development of pessary complications considerably affected both adherence and effectiveness. Self-management had the potential to improve some women’s quality of life beyond pessary effectiveness. How women chose to self-manage their pessary was based on their own preferences and needs, which is what would be expected in a self-management intervention. Self-efficacy appeared to influence trial group adherence, acceptability and intervention effectiveness differently in each of the trial groups.
Chapter 5 Economic evaluation
Introduction
This chapter describes the economic evaluation analysis conducted alongside the main statistical analysis. The economic analysis includes a decision-analytic model that extends the time horizon over which cost-effectiveness was considered. The primary objective of the economic evaluation was to calculate the cost-effectiveness of self-management to treat pelvic organ prolapse compared with standard clinic-based NHS treatment in a within-trial-economic evaluation. A secondary objective was to estimate the long-term cost-effectiveness by using decision-analytic modelling to examine the costs and outcomes of pessary self-management compared with clinic-based pessary care beyond the trial period and over a period of 5 years. The methods used are presented in Chapter 2, and the health economics analysis plan is provided as part of the Project Documentation. 41
Results
Within-trial cost–utility analysis
EuroQol-5 Dimensions, five-level
A total of 333 participants at baseline and 293 at 18 months completed the EQ-5D-5L. The final sample excluded participants who dropped out at baseline and had full missing data in either the EQ-5D-5L or the resource questions. The final sample comprised 264 patients, with utility scores calculated for both groups at each follow-up (Table 26).
| Assessment | Self-management | Clinic-based care | Self-management | Clinic-based care | p-valuea |
|---|---|---|---|---|---|
| Mean (SD); nb | Mean (SD); nb | Median | Median | ||
| Index score | |||||
| Baseline | 0.851 (0.170); 125 | 0.840 (0.185); 139 | 1.000 | 1.000 | 0.732 |
| 6 months | 0.841 (0.187); 125 | 0.829 (0.190); 139 | 0.814 | 0.814 | 0.593 |
| 12 months | 0.833 (0.193); 125 | 0.811 (0.192); 139 | 0.814 | 0.814 | 0.301 |
| 18 months | 0.823 (0.190); 125 | 0.819 (0.188); 139 | 0.814 | 0.814 | 0.856 |
| EQ-VAS | |||||
| Baseline | 83.28 (12.65); 125 | 82.40 (15.50); 139 | 85 | 85 | 0.912 |
| 6 months | 80.83 (14.61); 125 | 80.39 (15.84); 139 | 85 | 85 | 0.903 |
| 12 months | 79.59 (15.08); 125 | 79.50 (17.95); 139 | 80 | 80 | 0.524 |
| 18 months | 78.56 (17.35); 125 | 79.15 (16.80); 139 | 80 | 81 | 0.608 |
No significant difference was found between participants’ scores over time or between the treatment and control groups at any time point. This was tested with a non-parametric Mann–Whitney test, and it was not possible to reject the null hypothesis that the two groups are equal at any time point for both EQ-5D-5L index scores (utility) and VAS.
Intervention cost
The cost of the intervention, that is the initial self-management training given to women, consisted of an additional 30-minute appointment with a specialist nurse (band 5, 6 or 7), physiotherapist (band 7 or 8) or consultant-level doctor. The majority of appointments involved a specialist nurse, with only 14% of appointments undertaken by a consultant. This is reflected in the mean cost of the appointments, which was estimated to be £29.90. To calculate the intervention cost, we examined 156 initial appointments in the self-management group that were available in the data. The values ranged from £20 to £59.50 per appointment, and this formed the basis for the distribution of values defined as the intervention cost shown in Table 3.
Resource use data
Resource use assessment was completed for 310 participants at 6 months, 298 participants at 12 months and 297 participants at 18 months. Healthcare resource use is reported by the participants by trial group and separately for their prolapse and other health reasons (reported in Table 27 over the 18-month period). It is clear from the raw data that clinic-based care patients had more contacts with healthcare services over the 18-month period. This was the case across most categories in Table 27 for both prolapse or other reasons. One exception is district nurse home visits, although this was driven by a single patient in the self-management group (who had reverted to clinic-based care), with 30 home visits due to prolapse and 16 visits for other reasons, 89% of total visits in this group. Another exception is hospital bed-days due to prolapse; however, careful examination of this variable showed that there might have been a measurement error, with only six of these nights correctly reported. To minimise measurement error, the analysis is based on hospital episodes rather than reported bed-days. This decision makes our estimated probability of cost-effectiveness of self-management conservative given that the clinic-based care group reported more total bed-days.
| Self-management group | Clinic-based care group | Total self-management | Total clinic-based care | |||
|---|---|---|---|---|---|---|
| Prolapse-related appointment | Other health reason | Prolapse-related appointment | Other health reason | |||
| N a | 158 | 152 | ||||
| Clinic appointment for pessary fitting and check-up | N/A | N/A | N/A | N/A | 91 | 333 |
| Telephone support calls | N/A | N/A | N/A | N/A | 30 | 29 |
| GP surgery appointment | 37 | 319 | 71 | 424 | 356 | 495 |
| Nurse surgery appointment | 17 | 189 | 16 | 213 | 206 | 229 |
| GP home visit | 0 | 5 | 1 | 6 | 5 | 7 |
| Nurse home visit | 0 | 4 | 1 | 12 | 4 | 13 |
| District nurse home visit | 31 | 21 | 1 | 6 | 52 | 27 |
| Physiotherapy | 53 | 33 | 34 | 89 | 86 | 123 |
| Clinic dietitian | 0 | 0 | 0 | 11 | 0 | 11 |
| Outpatient doctor | 70 | 139 | 112 | 207 | 209 | 319 |
| Outpatient nurse | 110 | 87 | 180 | 94 | 197 | 274 |
| A&E visits | 5 | 53 | 12 | 60 | 58 | 72 |
| Hospital bed-daysb | 24c | 47 | 5 | 99 | 71 | 104 |
| Hospital episodes | 15 | 15 | ||||
The unit costs detailed in Table 3 were applied to the healthcare resource use to estimate the mean cost per patient by trial group (Table 28). The initial training appointment was applied only to the self-management group. The costs of medications prescribed for prolapse-related conditions were calculated based on information reported in the RUQ. The difference in resource use between the trial groups is driven mostly by outpatient and GP surgery appointments. The heaviest resource use was reported by two self-management patients who had reverted to clinic-based care early in the trial. For the calculation of prescribed medication costs, we excluded medications for long-term conditions such as diabetes. We focused on medications related to prolapse, infection, bowels, bladder and gynaecological conditions. These kinds of medications were reported by 110 patients (46% self-management), with average spending of £49 in the self-management group compared with £67 in clinic-based care out of the patients who reported medications or appliances. Only one patient reported the use of appliances, which were urinary catheters; this patient was in the clinic-based care group. Twenty-five per cent of drug use in the clinic-based care group was related to antibiotics, compared with 20% in the self-management group. The most commonly reported drug in both groups was prescribed oestrogen (systemic and vaginal), at 68% in self-management and 53% in clinic-based care. The proportions of vaginal oestrogen prescriptions were similar in the groups (at 6 months: self-management group, n = 9; clinic-based care, n = 17; at 12 months: self-management group, n = 15 and clinic-based care, n = 22; at 18 months: self-management group, n = 14 and clinic-based care, n = 17). Twelve per cent of clinic-based care patients reported drug use for constipation as opposed to 5% in self-management. A Cramér’s V statistic of 0.17 shows a small association between the reported types of drug use and the two trial groups, which implies significant differences between the groups in terms of the types of drugs they consumed during this trial.
| Self-management | Clinic-based care | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Meana (£, 2019 prices | SD | Minimum | Maximum | Obs | Meana (£, 2019 prices) | Obs | Minimum | Maximum | |
| Initial appointmentb | 125 | 31.77 | 9.98 | 20 | 56.88 | 139 | 0 | 125 | – | – |
| Clinic visitsc | 16.81 | 39.54 | 0 | 324.59 | 77.45 | 42.37 | 0 | 338.41 | ||
| Telephone supportc | 1.45 | 3.51 | 0 | 17.09 | 1.76 | 4.07 | 0 | 18.85 | ||
| NHS costs | 528.27 | 588.34 | 0 | 3743.29 | 649.63 | 654.02 | 0 | 3542.48 | ||
| Medications | 15.52 | 45.57 | 0 | 348.00 | 24.90 | 79.88 | 0 | 667.88 | ||
Estimation of incremental net benefit
The primary analysis was based on ITT group allocation and excluded participants with missing data; this resulted in a sample of 264 patients (47% in the self-management group), with 58 excluded because they had missing data on the EQ-5D-5L. No participants were excluded because of complete missingness in the resource use data. Some of the questions had non-response, which we assumed meant one visit if non-response was to the number of visits after a positive response or zero resource use if there was no response at all. This assumption was applied to nine self-management and 15 clinic-based participants whose non-responses we had imputed. In total, < 2% of the resource use questions across all participants were imputed in this way. When we excluded these patients (not shown), our results remained very similar to what is shown below.
The incremental cost and incremental effectiveness (QALYs) of self-management compared with clinic-based care are presented in Table 29 along with the ICER and INMB.
| Trial group | Total cost (£) | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER | INMB (£) (SE) |
|---|---|---|---|---|---|---|
| Self-management | 578.30 | 1.241 | –150.53 | 0.021 | Dominated | 564.32 (581.50) |
| Clinic-based care | 728.84 | 1.221 |
As can be seen in Table 29, clinic-based care is dominated by self-management, and therefore the negative ICER is not shown as it is not possible to interpret it. This means that self-management was less costly than clinic-based care and was not less effective in terms of the number of QALYs gained from treatment. The INMB was estimated at £564.32 at a £20,000 willingness-to-pay threshold per QALY gained value. The INMB was calculated by translating both effectiveness and cost into a monetary valuation that depends on policy-makers’ willingness to pay per QALY gained; therefore, the INMB should be thought as value added rather than in simple cost terms. This is the baseline value added per patient by self-management at a £20,000 willingness to pay per QALY gained value. When the INMB is positive, the intervention is cost-effective when compared to the alternative. In other words, this result means that the cost to derive the benefit from self-management is lower than the maximum amount that the decision-maker would be willing to pay for this benefit.
This analysis is based on the costing of individual hospital episodes rather than the costing of individual nights in hospital as a more conservative estimate in terms of self-management cost-effectiveness (see Table 27 for a breakdown of hospital bed-days). A large number of nights were reported for non-prolapse-related reasons and therefore it was decided that to include the cost per hospital night would dominate the overall costing and not reflect the impact of pessary and prolapse required treatment options.
Probability of cost-effectiveness
The probability of cost-effectiveness can be described as the probability that an individual (random) patient will have a positive individual INMB. Essentially, this will tell us how likely it is that the baseline result (INMB = £564.32) applies to the average patient in the population, or simply how likely it is for self-management to be a cost-effective intervention when compared with clinic-based care based on the available evidence.
To calculate this probability, non-parametric bootstrapping methods were used to estimate the distribution of incremental costs and effects associated with self-management compared with clinic-based care. The results of the 10,000-bootstrap resample are shown in Table 30.
| Self-management compared with clinic-based care | Observed coefficient | Bootstrap SE |
|---|---|---|
| Incremental cost (£) | −150.53 | 77.22 |
| Incremental benefit (QALYs) | 0.021 | 0.031 |
| Incremental net benefit (£) | 564.31 | 648.37 |
| Probability of cost-effectiveness at £20,000 WTP | 80.81% | |
According to these results, the probability of cost-effectiveness of self-management is 80.81% at a willingness-to-pay threshold of £20,000 per QALY gained. The visual representation of these results is shown in Figure 9. We randomly selected 5% of the bootstrapped values to make the figure legible, and so the figure shows 500 points (dots) rather than the actual 10,000.
FIGURE 9.
Incremental cost-effectiveness scatterplot of self-management compared with clinic-based care for 10,000 sampled individuals (5% of values shown).
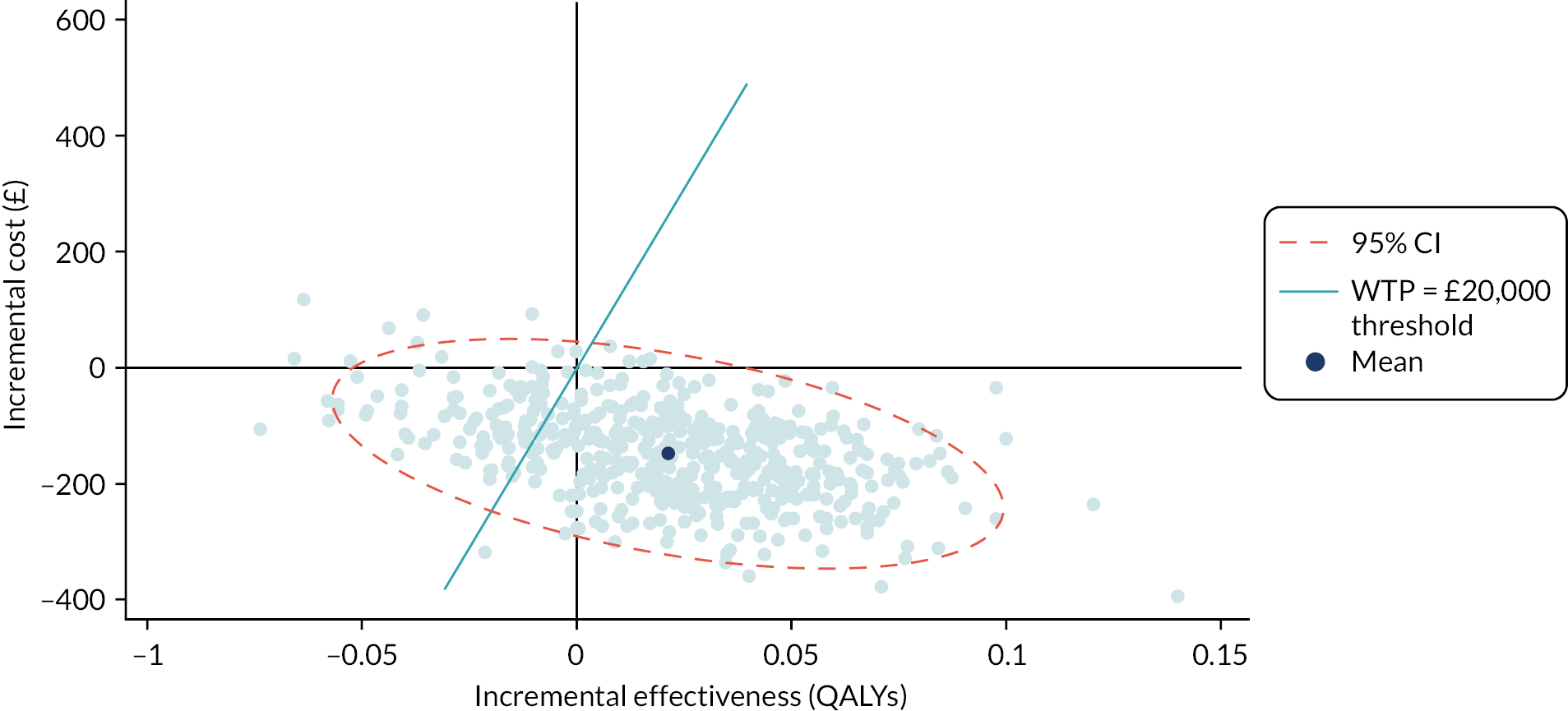
Secondary analysis with multiple imputation
To maximise the data, the analysis was repeated using the multiple imputation methods described in Chapter 2 (data analysis) to estimate EQ-5D-5L values for participants who had completed a baseline EQ-5D-5L but had missing data at a follow-up time point. This increased the sample size to 320 participants who had baseline EQ-5D-5L data [158 (49%) self-management]. Table 31 presents the mean costs and QALYs for the self-management and clinic-based care groups with multiple imputation.
| Trial group | Mean | SE | |
|---|---|---|---|
| Cost | Self-management | 527.85 | 46.11 |
| Clinic-based care | 713.84 | 54.58 | |
| QALYs | Self-management | 1.217 | 0.021 |
| Clinic-based care | 1.208 | 0.022 | |
| n = 320 (100 imputations) | |||
Multiple imputation regression estimates of the impact of self-management on costs and effectiveness, taking into account baseline utility, are shown in Table 32. Essentially, this is the estimated marginal effect of the intervention. The methods used to undertake the estimation are described elsewhere. 77,78
| Coefficient | SE | ||
|---|---|---|---|
| Cost | Self-management | –185.99 | 71.38 |
| Constant | 713.84 | 50.16 | |
| Effectiveness | Self-management | 0.002 | 0.019 |
| Baseline utility | 1.036 | 0.047 | |
| Constant | 0.348 | 0.042 | |
| INMB (SD) | 226.06 (400.14) | ||
| Probability of cost-effectiveness at £20,000 WTP | 71.40% | ||
To validate the results presented in Table 32, which is based on a parametric regression methodology, the probability of cost-effectiveness using a non-parametric method was calculated. A total of 1000 bootstrap samples was drawn from each of the 100 imputed data sets, creating 100,000 samples, and the difference in net benefit was estimated between the trial groups in each bootstrap sample (at £20,000 per QALY gained). The proportion of bootstrap samples in which the net benefit is positive represents the probability that the treatment is cost-effective for each multiply imputed data set. This probability is then averaged across all multiply imputed data sets and is shown in Table 33. 70
| Mean | SD | |
|---|---|---|
| Average incremental costs | −185.52 | 68.57 |
| Average incremental QALYs | 0.002 | 0.017 |
| Probability of cost-effectiveness at £20,000 | 71% | |
The intervention appears cost-effective when compared with clinic-based care given the estimated negative incremental cost, which implies that self-management is a cost-saving intervention. This result is similar to the previous result where missing values were not imputed. However, the imputation, together with the regression (or bootstrap) approach that took into account starting utility in both groups, reduced the probability of cost-effectiveness by approximately 9%.
Decision-analytic modelling results
A model was constructed as described in Chapter 2 to extend the main analysis for a further 5-year period. The results of the baseline analysis are shown in Table 34.
| Trial group | Total cost (£) | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER | INMB (£) |
|---|---|---|---|---|---|---|
| Self-management | 2044 | 4.92 | –494 | 0.19 | Dominated | 4221 |
| Clinic-based care | 2538 | 4.73 |
Self-management remains a cost-effective intervention when compared with clinic-based care 5 years after the initial trial period. The modelling results are consistent with the main analysis.
The cost-effectiveness acceptability curve (Figure 10) is based on 10,000 Monte Carlo probabilistic sensitivity analysis samples. This graph summarises the impact of uncertainty on the results by showing the probability of cost-effectiveness across a range of willingness to pay per QALY gain values for the two strategies. At a willingness-to-pay threshold of £20,000, the probability of self-management being a cost-effectiveness intervention is 69.74%, reflecting the probability of self-management remaining cost-effective for 5 years after the end of the trial.
FIGURE 10.
Cost-effectiveness acceptability curve at 5 years post trial.
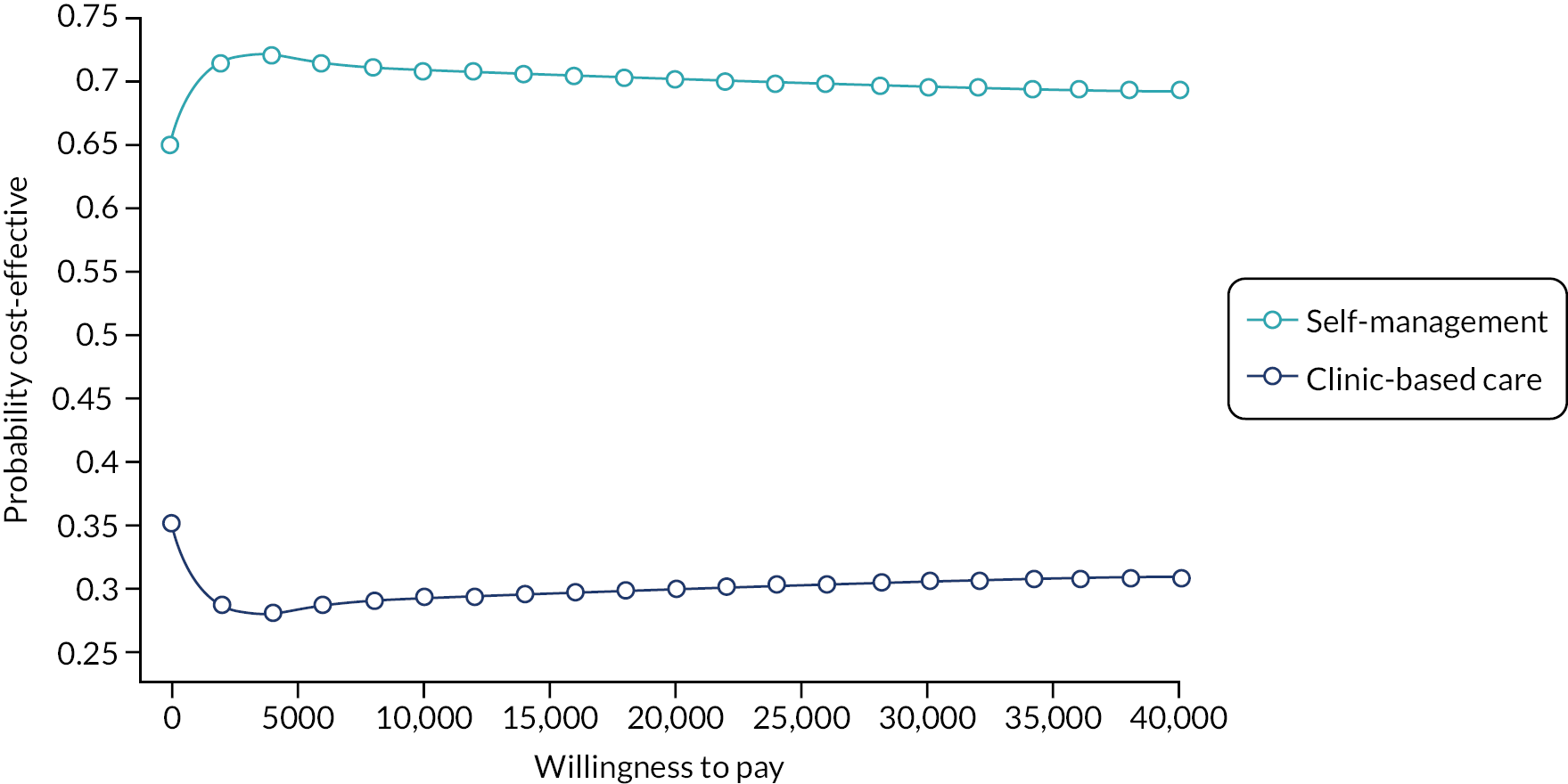
The corresponding cost-effectiveness scatterplot showing the visual representation of the probabilistic sensitivity analysis can be seen in Figure 11.
FIGURE 11.
Incremental cost-effectiveness scatterplot of self-management compared with clinic-based care at 5 years post trial.
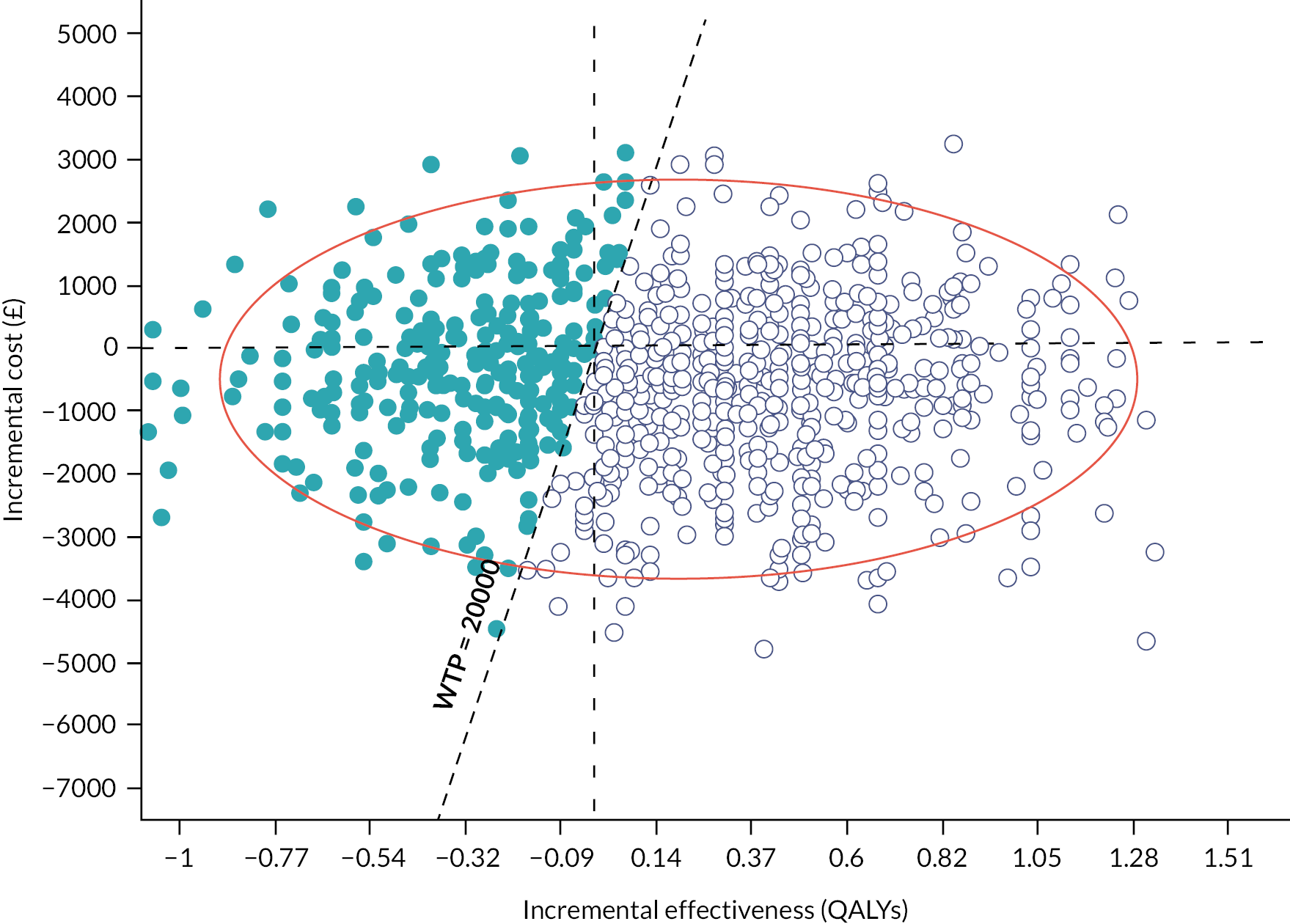
A deterministic sensitivity analysis is presented that examines how uncertainty changes outputs from the base-case values using the best estimate for each parameter considered (Figure 12). This focuses on transition probability parameters, which were derived from observed patient transitions in level of resource use between 12 and 18 months. The sensitivity analysis is presented in the form of a tornado diagram reporting the range of INMBs generated for each parameter’s uncertainty range. The top bar represents the probability of a self-management patient transitioning from moderate to poor over time, which has a base case of 5% per month and an uncertainty range of 1–9%. The blue portion of the bar represents the low part of the uncertainty range (from 1% to 5%), while the red portion of the bar represents the high part of the uncertainty range (from 5% to 9%). Therefore, decreasing the parameter increases the INMB calculation, which makes sense because decreasing this parameter implies that self-management patients are less likely to transition to the poor state over time. The second bar represents the probability of a self-management patient transitioning from poor to moderate over time, which has a base case of 3% per month and an uncertainty range of 0–10%. Similarly, the blue portion of the bar represents the low part of the uncertainty range while the red portion of the bar represents the high part of the uncertainty range. In this case, increasing the parameter increases the INMB calculation, which again makes sense because increasing this parameter implies that self-management patients are more likely to improve from poor to moderate.
FIGURE 12.
INMB tornado diagram. CBC, clinic-based care; SM, self-management.
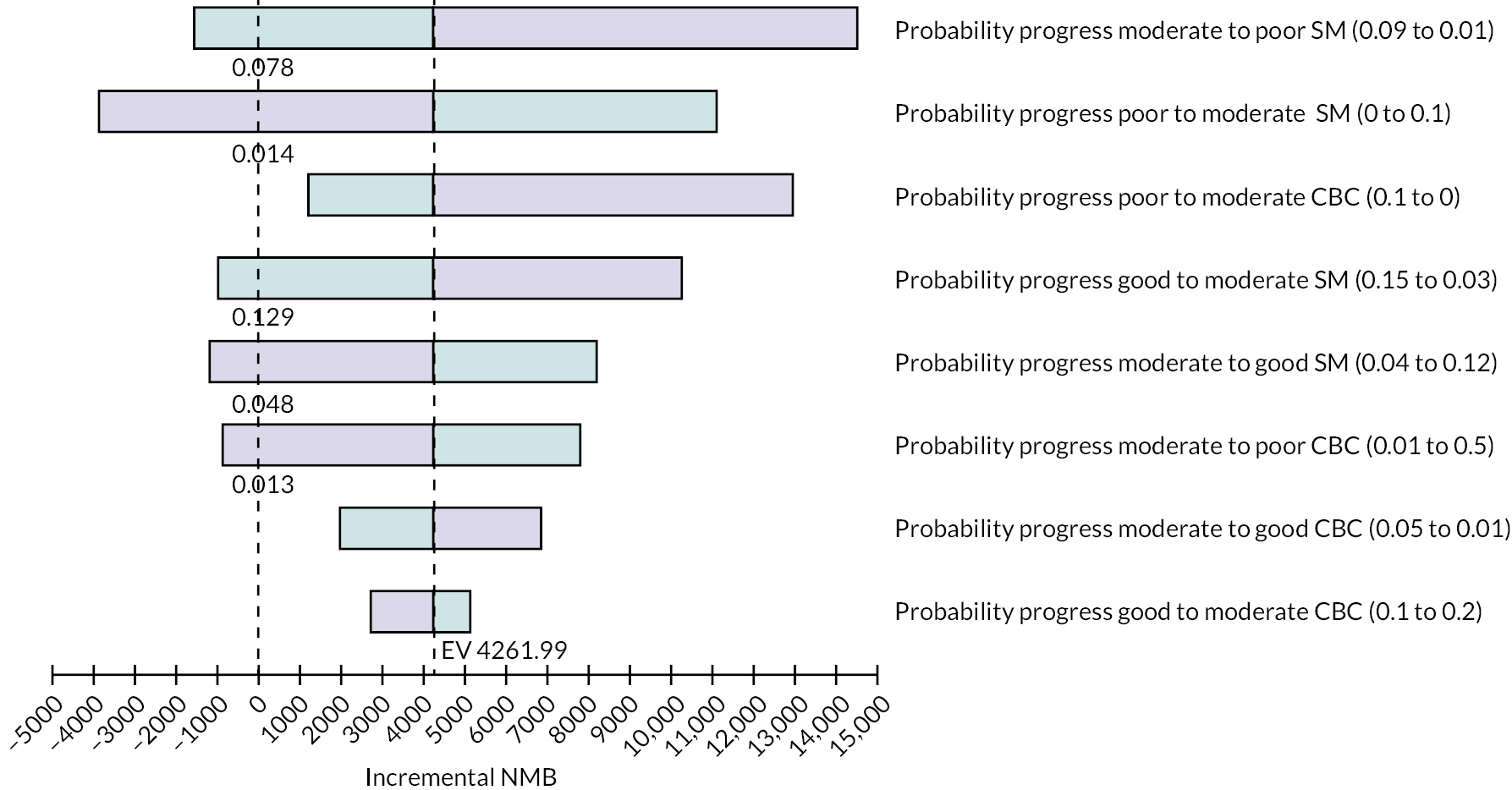
This sensitivity analysis suggests that varying these parameters has a moderate impact on the INMB, which remains positive for most values in the uncertainty range. The probabilities of self-management patients changing between moderate and poor states appear to have the biggest impact. This means that cost-effectiveness depends on the intervention’s impact on patients’ health status, which is then associated with higher or lower resource use. This impact mostly relates to the ability of self-management to keep patients from transitioning into a poor state rather than keeping them in a good or moderate state.
Summary
The results suggest that self-management is cost-effective at a willingness-to-pay threshold of £20,000 per QALY gained. Decision-analytic modelling supports this result and suggests that the intervention remains a cost-effective option for the health service for longer durations than the actual trial. A discussion of these points is presented in Chapter 7.
Chapter 6 Synthesis
This chapter outlines the synthesis of the study findings.
Aim of the synthesis
The TOPSY study had three main components: a trial, a process evaluation and an economic evaluation (see Appendix 2). The aim of synthesising these three components was to reach overall conclusions by identifying domains where there was agreement or disagreement between the findings. The following domains were assessed through synthesis:
-
Does self-management improve quality of life for women more than clinic-based care?
-
Did self-management improve self-efficacy for women more than clinic-based care?
-
Was there adherence to the intervention in each group?
-
What were the effects of complications on adherence to the intervention and quality of life?
Methods
To assess the levels of agreement or disagreement, a triangulation protocol was used, which is an approach that supports the integration of results in multimethod research. 79 In the TOPSY trial, the term triangulation was used to describe a process that brings data from different methods together to improve understanding in a more holistic way, and this was undertaken at the interpretation stage of the study. O’Cathain et al. 79 describe a convergent coding matrix as a way of bringing the findings together. In that matrix, the findings for each individual method are described in a table; where an individual method does not have any findings that relate to a particular domain, the term ‘silence’ is used. The table has a final column offering an interpretation of whether there is agreement, partial agreement or disagreement between the findings.
Results
The findings of the triangulation are presented in Table 35. There was full agreement across data sources that self-management did not improve quality of life more than clinic-based care at 18 months. This held true for prolapse-specific quality of life as well as for generic quality of life. However, there were women in the randomised interview sample, and who received self-management, who reported that their quality of life had improved because they were self-managing. These findings suggested that self-management had the potential to improve quality of life of individual women over and above the improvements seen from the pessary alone.
| Domain | Trial | Economic evaluation | Process evaluation | Agreement |
|---|---|---|---|---|
| Does SM improve quality of life over CBC at 18 months? | No. There is no group difference (as measured using the PFIQ-7) in prolapse-specific quality of life at 18 months | No. There is no group difference (as measured using the EQ-5D-5L) in generic health-related quality of life at 18 months | Wide variance seen in both groups in terms of quality-of-life outcomes at 18 months. However, SM had the potential to improve prolapse-specific and generic quality of life over and above effect of pessary alone | Agreement. Quality of life outcomes were not different between women who received SM and women who received CBC in any of the data sets. The qualitative data from women did suggest there was potential for quality-of-life improvement, but this was not demonstrated by the measurement tools used |
| Did SM improve self-efficacy more than CBC at 18 months? | There was no difference in general self-efficacy (as measured on the General Self-efficacy Scale) between the groups. Women who self-managed were (at 18 months) more confident:
|
Silence | A wide variance in self-efficacy was seen across women in both groups. However, self-efficacy appeared to be underpinned by different factors in the groups. Self-efficacy in ability to manage the pessary was a factor in explaining the potential for improved quality of life for women who self-managed. Women in the CBC group, although talking in ways that suggests they had self-efficacy towards prolapse management generally, rarely talked about having self-efficacy in SM | Agreement. General self-efficacy did not differ between the groups with each group exhibiting different factors that underpinned that self-efficacy. There was a difference between the groups in women’s self-efficacy to manage their pessary themselves |
| Was there adherence to the intervention in each group? | Receipt: 92.3% of those randomised to SM received the teaching session. 78.7% of women randomised to SM were still self-managing their pessary after receiving the complete TOPSY intervention package On treatment: 83.4% of those in SM group self-managing by the on-treatment definition and 60.2% CBC participants on treatment at 18 months (mainly due to removing their own pessary at least once) Crossover: 34 women crossed over from SM to CBC by 18 months; there were no crossovers from CBC to SM (based on strict criteria of receiving the TOPSY intervention) |
The resource use data indicate that women who remained in their allocated trial group followed the clinical pathway as expected. The SM group attended fewer outpatient appointments than the CBC group | The fidelity analysis demonstrated that the women randomised to SM did get the SM intervention (i.e. intervention was delivered as intended) Qualitative data from randomised women suggested that women in SM and women in CBC got different interventions Adherence of women: There was variance to adherence within and between trial groups with some women fully adherent, some partially adherent and some non-adherent across both groups. There were women in the CBC group who reported removing and replacing the pessary themselves |
There are three separate points of agreement Agreement: women in the SM and CBC groups received different interventions Agreement: there was variance in adherence in each of the groups Agreement: a large proportion of women in the CBC group did, at times, self-manage their pessary |
| Complications | Women in the CBC group had a higher proportion of complications than those in the SM group | Silence | Women in the SM group addressed mild side effects, such as discharge or slippage, on their own. Women in CBC were less inclined to self-manage problems they experienced. Complications affected pessary continuation in both groups | Agreement. Women experience fewer complications if self-managing and this may be explained by SM women taking actions to address problems by themselves |
The proposed mechanism of action was that being supported to self-manage would improve women’s self-efficacy more than clinic-based care. Women who self-managed would therefore have improved self-efficacy to manage their own health and well-being (in this case manage their pessary) and this would in turn improve their quality of life. Data were available to consider this mechanism of action from two components: the trial and the process evaluation. There was agreement that there was no group difference in women’s reports of general self-efficacy. Women in the self-management group were more confident in removing and reinserting their pessary and more confident in managing problems related to their pessary than women in the clinic-based care group. These differences in self-efficacy for pessary self-management were echoed in the process evaluation data, where women talked about an increase in confidence in their ability to manage their pessary in a way that suited their life. Although a few women in the clinic-based care interview group did report undertaking self-management, their self-efficacy as they described it qualitatively was different from that of women who had received the TOPSY self-management intervention in that the former group could self-manage but felt less confident to do so.
Adherence was considered in terms of fidelity to intervention delivery (whether women received self-management or clinic-based care as intended) and women’s adherence to their allocated trial group. In terms of fidelity, women in both groups received the intervention to which they had been allocated. Among the self-management group, > 90% received the first teaching session and > 70% received all components of the intervention. Within the self-management intervention components, very high proportions of the required elements were delivered (as measured through self-report checklists and objective observation). None of the women in the clinic-based care group received the TOPSY intervention, and all received clinic-based care as would be usually delivered in their centre. There was consistency across centres in the care delivered in a clinic-based care model. The economic evaluation identified that self-management cost less to deliver than clinic-based care because women in the self-management group made less use of health care over 18 months. The resource use data support the findings that women in each group received a different care pathway. It is therefore possible to conclude that the women in the self-management and clinic-based care groups received different interventions.
There was crossover from self-management to clinic-based care, with 34 women crossing over in this way. Some women in the self-management group also requested clinic appointments for other reasons. Based on the strict definition of receipt of the TOPSY self-management intervention, there were no crossovers from clinic-based care to self-management. However, many clinic-based care women were not ‘on treatment’ because they had chosen to remove their pessary during at least one time point over the 18 months’ follow-up. Interview data also suggested that women in the clinic-based care group did, now and again, remove and reinsert their pessary. Therefore, although they received different interventions, women themselves sometimes chose to undertake elements of the other intervention.
An important safety-related finding was that women in the self-management group reported a lower proportion of complications than women in the clinic-based care group. This finding was supported by data from the process evaluation where women talked about their experience of complications and, for the self-management group women only, their ability to manage those complications. Where complications were experienced and not well managed, women in the interview study linked this directly to discontinuation of the pessary. A higher proportion of clinic-based care participants reported being moderately worried about not having their pessary changed due to pessary clinic being cancelled or postponed during the COVID-19 pandemic.
More detailed discussion of these points is considered in Chapter 7.
Chapter 7 Discussion
Statement of principal findings
We did not find evidence that self-management was better or worse than clinic-based care in the way it affected women’s quality of life. The self-management intervention was delivered as planned and what women received was, as intended, different to the delivery of clinic-based care. The trial was therefore a true test of the self-management intervention. Self-management was reported, by women and HCPs, to be an acceptable intervention. Self-management was cost-effective at WTP threshold of £20,000 per QALY gained. Cost-effectiveness results were driven by women’s resource use and health-seeking behaviour, which was lower in the self-management group. This held true when extrapolated beyond the 18-month follow-up, with economic modelling suggesting that self-management would remain cost-effective at 5 years.
There were no differences between the groups in general self-efficacy, the proposed mechanism of action. However, women in the self-management group had greater self-efficacy in relation to managing problems associated with their pessary, as well as more confidence in their ability to remove and replace their own pessary, than women in the clinic-based care group. This quantitative finding was supported by qualitative data from randomised women who were interviewed. Women in the self-management group valued self-management because it allowed them to manage their pessary in ways that suited their lifestyle.
Women who self-managed reported a lower proportion of complications associated with pessary use than women who received clinic-based care. Self-managing women found removing their pessary more difficult than inserting it. Qualitatively, complications were reported to be associated with pessary discontinuation and were a barrier to effective self-management. Women valued having a telephone number to call if they experienced complications.
A range of contextual factors acted as barriers to or facilitators of trial group adherence and intervention effectiveness. Some contextual factors were common to the groups, such as absence of complications and good general health. Others were group-specific; for example, quality of life for women in the self-management group was influenced by growing self-efficacy over time, feeling in control and having flexibility in managing their lifestyle, whereas in the clinic-based care group women’s quality of life was influenced by reassurance from having a HCP visually inspect their vaginal tissues for any problems.
Robustness of primary outcome analysis
There was no published minimal clinically important difference for the PFIQ-7 suitable for a clinical trial in this population at the outset of the study to guide sample size calculation, but, following recommendations from our PPI representatives and clinical collaborators (and their colleagues), we agreed that a 20-point difference in PFIQ-7 score was meaningful. We then set an objective in the internal pilot to survey a wider group of clinicians for their views regarding the minimal clinically important difference, which resulted in a broader consensus that a difference of 20 points was meaningful. The TOPSY trial was statistically powered to detect a clinically important group difference of 20 points if one existed.
Secondary analyses of the primary outcome data under differing assumptions relating to non-compliance and missing data all showed very similar results to the primary ITT analysis. An analysis removing data for women whose clinic-based care was cancelled due to the COVID-19 pandemic also led to the same finding. Based on the above, we can conclude that the primary outcome analysis and its findings were robust.
Further analysis of the primary outcome
In further prespecified primary outcome analyses, we considered whether or not specific subgroups benefited from self-management compared with clinic-based care. Studies have shown that younger age and hysterectomy status are influencing factors in pessary management, with pre-menopausal women and women after hysterectomy more likely to discontinue or fail pessary management than older women and women with a uterus. 80,81 As hysterectomy can anatomically alter vaginal length, it was felt to be clinically relevant to assess if this anatomical change influenced women’s ability to self-manage. Kearney and Brown33 found that women who had been attending clinic-based care for pessary management prior to being offered self-management were less likely to self-manage than women who were offered self-management at the outset of pessary use. Therefore, we chose age, hysterectomy status and pessary user status as subgroups to investigate.
Subgroup analysis of the primary outcome showed no significant treatment group by subgroup interactions (subgroups were age < 65 vs. ≥ 65 years, new vs. existing pessary user and hysterectomy at baseline vs. no hysterectomy at baseline).
Strengths and limitations
Strengths
The key strengths of this study are its uniqueness in the field, the robust implementation and the holistic perspectives gained from using a mixed-methods design. To the best of our knowledge, no other trials worldwide have compared self-management with clinic-based care. There are studies suggesting that self-management supports pessary continuation33,34 and an ongoing trial comparing self-management for two different pessary types,32 but nothing that compares the process of offering self-management as an alternative service structure. TOPSY therefore offers a unique perspective for clinical practice.
The TOPSY trial was large enough to have detected a meaningful difference in quality of life had one existed. The trial, process evaluation and cost-effectiveness analysis were undertaken using robust methods that were implemented with scientific rigor, and the response rates at all time points were > 87%. The core findings of the study were consistent across methods and when sensitivity analyses were applied. The sample was drawn from geographically spread and diverse locations across the UK. The appointed staff team were the same throughout the study, offering consistency in implementation. The mixed-methods design ensured a holistic perspective on self-management in a way that supported understanding of the trial findings. Overall, the study design and its implementation were strengths that support confidence in the validity of the findings.
Limitations
Limitations focus on the skew in the baseline data for the PFIQ-7, the influence of the global pandemic, the potential effects of crossover and the lack of ethnic diversity in the recruited sample.
A total of 32.9% of the recruited sample scored zero at baseline on the primary outcome measure (PFIQ-7), which suggests that these women had the best possible prolapse-specific quality of life, the implication being that are that there was no room to improve that outcome by implementing self-management. It raises the question of whether the population from whom the sample was recruited was the ‘right’ population and/or whether the chosen outcome measure was the best measure. In terms of population, TOPSY was a pragmatic trial and therefore efforts were made to ensure that the inclusion criteria were as wide as possible and exclusion criteria were minimised. In practice, clinical collaborators confirmed that the recruited sample were representative of those to whom they would offer self-management. They would, for example, still offer an individual self-management to a woman whose prolapse-specific quality of life scored zero. Thus, although the skew to zero may have made it less likely that a difference would be found, the population was the correct population. Previous self-management studies have used disease-specific quality of life to measure outcome. There are many outcome measures for pelvic floor dysfunction symptoms,82 with the PFIQ being one commonly used to measure prolapse-specific quality of life in pessary trials. 1 It is possible that the measure used was not sensitive enough to identify changes in prolapse-specific quality of life over and above the effects caused of the pessary itself.
The global COVID-19 pandemic had the potential to limit the study. However, TOPSY had completed recruitment before the first UK lockdown. Although some clinic appointments were cancelled, and some were held later than planned, sensitivity analysis revealed that this did not affect the findings. Qualitative data suggested that some women in the clinic-based care group removed and replaced their pessary themselves because their appointment was not available. However, women also did this sometimes pre-pandemic. Therefore, although the pandemic had the potential to alter the intervention, it did not seem to do so.
Thirty-four women (20.1%) crossed over from self-management to clinic-based care, and nearly 40% of those randomised to clinic-based care inserted their pessary themselves at least once over the 18 months’ follow-up. Both these actions could potentially dilute the prolapse-specific quality of life effect of self-management in an ITT analysis. TOPSY was a pragmatic trial, and therefore crossovers between treatments occurred as they would in routine pessary management. As a result, this is an important part of the assessment of effectiveness.
The recruited sample in the TOPSY trial had minimal ethnic diversity (see Table 5). This will in part have been because the self-management information was only available in English. In hindsight, funds could have been requested to enable materials to be translated into the other common languages spoken in the UK. The implications of the underrepresentation of women from different ethnic groups is that the findings may not be generalisable beyond women from white ethnic backgrounds. Future studies of pessary self-management should undertake translation of materials to allow the inclusion of women who represent the wide variety of ethnic groups in UK society.
Interpretation of the results
Chapters 3–5 contain the findings of each individual component of the study and Chapter 6 contains the synthesis of the findings. In this discussion chapter, the focus is on interpreting these findings collectively to reach conclusions that are useful for women, clinical practice, policy and future research.
Self-management and quality of life
There was no evidence that self-management improved or worsened prolapse-specific quality of life (measured using the PFIQ-7) in comparison with clinic-based care at 18 months. This finding held true at the 6- and 12-month time points; when the primary outcome was examined under different assumptions; when COVID-19 alterations to clinic-based care pathways were considered in the analysis; and for different subgroups. Generic health-related quality of life (measured using the EQ-5D-5L) also demonstrated no difference between the groups at any time point. A range of quality-of-life outcomes were articulated by randomised women in both groups throughout the interviews. However, women who self-managed appeared to have the potential to improve their quality of life to a greater extent than women in the clinic-based care group because they managed the pessary in ways that suited their lifestyle.
One possible explanation for this finding of no difference is that there was a skew to zero (best quality-of-life score on the PFIQ-7) in the primary outcome data at baseline. This makes it difficult to detect improvement, which could be due to the primary outcome measure not capturing the full range of the intended quality-of-life constructs when used in this population. 83 However, in a post hoc sensitivity analysis, all participants with a baseline PFIQ-7 score of zero were removed and the analysis of the primary outcome was rerun. The outcome remained the same, with no group difference in PFIQ-7 score. Another possible explanation could be that the intervention effect is diluted by women crossing over from self-management to clinic-based care. However, although not technically crossing over as they were not given formal self-management teaching, women in the clinic-based care group did at times remove their pessary themselves. Guidance was followed for a treatment policy strategy that ignored these intercurrent events in the primary analysis by following the ITT principle. 84
Two small observational studies have been identified that focus on pessary self-management. One offers a non-randomised comparison of self-management of vaginal pessary with clinic-based care33 but did not measure quality of life. The authors did report higher levels of pessary changes being comfortable and higher convenience for the self-management group. The second study is a retrospective chart review of 289 women, which identified self-management as a strong predictor of continuation but did not measure quality of life. 34 A 2020 Cochrane review of pessary effectiveness identified uncertainty about there being a difference in prolapse-specific quality of life when pessary was compared with pelvic floor muscle training. 1 However, prolapse-specific quality of life was improved when pessary was added to pelvic floor muscle training compared with pelvic floor muscle training alone (moderate certainty of evidence). Some previous reviews of self-management interventions in other long-term condition contexts have found improvements in quality of life, albeit with low-quality evidence,85,86 and others have not,87 again with low-quality evidence. Therefore, variance in the effect on disease-specific quality of life has been reported for both pessary use generally and self-management programmes more widely, and the TOPSY trial adds further data to these debates about self-management’s links to quality of life.
Some self-management interventions may act to improve quality of life by improving symptoms, for example decreasing exacerbation days for chronic obstructive pulmonary disease,85 whereas in this trial the effective treatment component for symptoms (the pessary) was delivered prior to recruitment. The tool selected to measure prolapse-specific quality of life (PFIQ-7) is a measure of how much ‘symptoms affect the quality of life of women with pelvic floor disorders’. 88 The TOPSY trial aimed to evaluate a self-management versus clinic-based care model that may not have impacted on the symptoms enough to be detected by the primary outcome measure, which focused on quality of life through symptoms. However, generic quality of life also did not differ in ways that might have been expected from a self-management programme that targeted self-efficacy. It may be that more sensitive tools for measuring quality of life factors that are important to women need to be developed for pelvic floor dysfunction studies.
Overall, we can conclude that self-management of a vaginal pessary did not improve disease-specific or generic quality of life more than clinic-based care.
Clinical implications of self-management
A key finding was that women in the clinic-based care group experienced proportionally more complications than women in the self-management group. This finding held true when analysis excluded women who had missed appointments due to COVID-19; was not caused by differences in pessary material (as pessary materials were similar between the groups); was not linked to different proportions of additional appointments between the groups; and was not linked to differences in prescriptions for local vaginal oestrogen (which were similar between the groups). There are few other studies that report on the different rates of complications between women who self-manage and those who do not. NICE guidance identifies a lack of data on pessary complications. 28 One small observational study of 100 women with prolapse identified self-management as a means of reducing adverse events, with 16% of self-managing women experiencing adverse events compared with 62% of non-self-managing women;89 and another small observational study (n = 74 participants) notes low complication rates of 6.8% in women who self-manage but did not offer a comparison group. 90 Complication rates of vaginal pessaries have been reported to be > 50% in some studies91,92 and are reported to be markedly higher than those experienced by women who are treated using pelvic floor muscle training. 1 Women in the TOPSY study, regardless of group allocation, and evidence from other studies link complications to pessary discontinuation. 34,91
Fewer complications in the self-management group may be explained by these women having greater confidence in their abilities to manage their pessary (remove and insert it) and manage any problems associated to it. Women having the confidence and ability to manage the pessary and pessary problems is coherent with the self-management theory on which the self-management intervention was based. 39 One small randomised study identified that vaginal pessary complications were lower when pessary changes occur every 3 months, as opposed to every 6 months, but the sample was small (n = 60) and the difference was not statistically significant. 93 Complications have also been linked to the duration the pessary is in situ. 94 Self-efficacy may support women in changing their pessary more frequently and using it for less time (e.g. only when they felt they needed to), which may be one possible mechanism through which complication rates are lowered. Further research is needed to understand the mechanisms through which self-management reduces complication rates.
Women in the TOPSY study reported that removing their pessary was more problematic than inserting it because once it was inside the vagina the pessary was more difficult to manipulate. Discontinuation of pessary use in women who self-manage has been linked to difficulties inserting or removing the pessary. 28 Most self-management training for pessaries (including our own) places greater emphasis on insertion than removal. The HCPs delivering the intervention instructed women to try different positions that would allow for a more comfortable insertion or removal of the pessary; however, despite these instructions, the findings from women in TOPSY suggest that further emphasis is needed on removal during self-management training. The role of pessary removal devices in increasing patient confidence and reducing discomfort when removing the pessary is an area for future research.
Clinically, self-management of vaginal pessaries seems to have a valid place in reducing the complications women experience. Providing further information in self-management training about pessary removal may be helpful. Each of these actions may support a reduction in pessary discontinuation; however, this assertion needs tested in future studies.
The cost-effectiveness of self-management
The results suggest that self-management is cost-effective at a WTP threshold of £20,000 per QALY gained. Given the economic analyses presented, a recommendation can be made that self-management should become an established NHS intervention as it is very likely to be cost-effective. Decision-analytic modelling supports this result and suggests that the intervention remains a cost-effective option for the health service for longer durations than the actual trial. The nature of this intervention meant that there was a rather small impact on quality of life, and the difference between the groups was not statistically significant. One reason for this may be that the EQ-5D-5L was not sensitive enough to capture differences between the groups. Therefore, our results were mostly driven by differences in resource use, which were clearly in favour of self-management in all of the examined cost categories. This picture remained consistent across all methods and data examined either fully completed or with imputed values. There is a degree of uncertainty in our results, and this is captured by the probability of cost-effectiveness and the incremental cost-effectiveness scatterplots (see Figures 9 and 11). The probability of cost-effectiveness results show that self-management is likely to be a cost-effective alternative to clinic-based care. The scatterplots can also be interpreted as the distribution of individual patients who received the intervention compared with clinic-based care. These show that most individual (random) patients will have a positive individual incremental net benefit if they receive this intervention. It can also be seen as the proportion of all patients in the population who have positive individual incremental net benefits when practising self-management rather than receiving clinic-based care. Results across all methodologies presented suggest that self-management was successful in reducing contacts with the health service without compromising patient quality of life. This was the case for both clinic visits and overall contact with primary and secondary care services. The observed lower resource use in the self-management group could have been because self-management patients either felt more confident to deal with their medical issues on their own or had less need for medical care than patients in clinic-based care. Our results suggest that some self-managing women gained in quality of life and at the same time had less need for health services than those in clinic-based care. The data and methods employed were not designed to give us more details of the characteristics of these patients who gained the most from this intervention. Further research is needed to establish the mechanism by which self-management reduces demand for health services and the type of patient who will gain the most from self-management. In the meantime, however, we can be relatively confident that pessary self-management is a cost-effective option for the majority of patients being treated for pelvic organ prolapse.
Self-efficacy as a mechanism of action
The proposed mechanism of action of the self-management intervention was that self-management support (implemented as a teaching session, a follow-up telephone call, a leaflet and as-required telephone support) would improve women’s self-efficacy more than clinic-based care. However, general self-efficacy did not differ between the groups at 18 months. Self-efficacy in elements of pessary self-management specifically did differ between the groups, with women in the self-management group having more confidence in their ability to remove and insert their pessary as well as manage pessary problems and manage their own lifestyle to include pessary care. There is little available global literature to support or dispute these findings in the context of pelvic floor dysfunction. One study of 60 participants compared women’s understanding of pessary care when they were given an educational brochure alongside verbal instruction to support understanding compared with verbal instruction alone. 95 Those who received the education brochure were found to be more confident in self-management 1 week and 3 months after teaching was delivered. Murray et al. ’s95 study included only women who self-managed, and therefore no comparison with clinic-based care was available. It may be that condition-specific elements of self-efficacy are improved through self-management, programmes but overall self-efficacy may be less likely to be influenced.
Self-management theory purports that self-efficacy is a mediator for quality of life. 39 It has already been discussed that the links between self-management and quality of life are uncertain. The same is true for the links between self-management and self-efficacy. For example, a Cochrane review of self-management programmes for people with stroke reported that self-management did improve self-efficacy (low-quality evidence),86 but digital interventions for chronic obstructive pulmonary disease management did not improve self-efficacy (very uncertain evidence). 96 Based on the TOPSY findings and findings from these other studies in other clinical contexts, the links between self-efficacy and quality of life seem uncertain.
Effects of the COVID-19 pandemic on the treatment of prolapse with self-care pessary study
Both the TOPSY trial and process evaluation had completed recruitment by the time the first national lockdown occurred. Delivery of the self-management intervention was also completed, apart from one woman whose teaching was delayed (at her request) due to COVID-19. Therefore, effects on the study were limited to the delivery of clinic-based care, the final 18-month clinic appointment for both groups and participant follow-up.
The data presented in Chapters 3 and 5 show that some clinic-based care appointments were cancelled, with most of these appointments replaced by telephone calls. Data from the process evaluation (see Chapter 4) show that some women in the clinic-based care group did remove their pessary themselves while awaiting an appointment. However, the effect of this on the trial outcome overall was likely to be minimal as, even without missed appointments, some women removed their pessary themselves. The primary outcome findings when those women who had missed appointments were removed was the same as those of the main ITT analysis. The economic analysis took into account that some appointments were cancelled or changed from in-person to over the telephone due to COVID-19. These changes were small and, given that COVID-19 affected both groups in a similar manner, should not have an impact on the cost-effectiveness results.
Women in the clinic-based care group also reported being moderately worried about their pessary not being changed because of COVID-19 restrictions, whereas self-management women reported less worry. Self-management was, therefore, a useful care pathway for women during the unanticipated pandemic.
Therefore, although there were some unanticipated changes to care pathways due to the pandemic, it is believed that these did not unduly influence the findings beyond the influence of individual and service variance that would be normally found with a pragmatic trial design.
Conclusion
NICE guidance (20193 and 202128) recommends that vaginal pessaries be considered as a treatment for women who have symptomatic pelvic organ prolapse. At 18 months, 91.5% and 86.2% of self-management and clinic-based care women, respectively, intended to continue pessary use in the TOPSY study. Previous observational, longitudinal studies have found that 86.1% of women continue pessary use in the UK at 5 years. 22 Pessary is confirmed as a treatment option that is used in the NHS and that women intend to use in the long-term.
Given that pessary treatment is potentially long term, pathways that support women are needed. The 2019 NICE guidance does not mention self-management specifically. The 2021 guidance mentions self-management in relation to offering support if a woman is self-managing. UK Clinical Guideline for Best Practice in the Use of Vaginal Pessaries for Pelvic Organ Prolapse29 recommends that women who are assessed, willing and suitable be offered self-management. The TOPSY study has robustly identified that self-management neither improves nor worsens quality of life more than clinic-based care. Self-management did reduce the proportion of complications experienced, was cost-effective and was acceptable to women and to HCPs. Therefore, it is recommended that self-management is offered as a care pathway for women who have the cognitive and physical capacity to self-manage and who choose a pessary as treatment for pelvic organ prolapse.
Implications for health care
-
Healthcare professionals and policy-makers can be confident that in offering self-management as an option to women who use a vaginal pessary to manage pelvic organ prolapse they are offering an acceptable intervention that will not make women’s quality of life better or worse than clinic-based care. Self-management will, however, reduce the pessary-related complications women experience and will cost the NHS less to deliver than standard clinic-based care models. Self-management of vaginal pessaries should be offered as part of NHS services from the outset of pessary care and as part of routine, ongoing care.
-
In offering self-management to women, HCPs should explain the lower complication rates experienced by women who self-manage and the possible mechanisms that may lead to that reduction (such as women’s confidence in removing the pessary when they experience discomfort).
-
HCPs who deliver self-management training may wish to add further information about options for pessary removal into that training, as women found pessary removal more difficult than pessary insertion.
Future research implications
-
Future research is needed to identify constructs that are important to women in measuring their prolapse-specific quality of life. This may necessitate the generation of a new measure that has greater sensitivity to quality-of-life constructs that are beyond the symptomatic changes linked to the pessary itself.
-
Future trials of self-management should test the effectiveness of self-management with a wide range of ethnic groups and with women of different abilities to allow the assessment of effectiveness in these populations. This may include testing of devices that support pessary removal or insertion.
-
Future research is needed to focus on self-management follow-up. For example, can follow-up be women-initiated or does it need to be planned at specific intervals?
-
Future research on pessary self-management is needed in terms of the possible links between pessary continuation and complications, including which specific complications are more likely to lead to discontinuation.
Additional information
Equality, diversity and inclusion statement
Twenty-one centres participated in the TOPSY study. The centres covered a diverse geographical area. Sociodemographic data were collected from the study participants, including on ethnicity, education and employment status. While a broad range of women covering different education and employment status were recruited, the ethnicity data suggested some bias, with over 90% of the sample self-identifying as ‘white’. Funds for language translations of all the study materials were not available, which could have contributed to the lack of diversity in ethnicity. To the best of our knowledge, no UK-based data are available that identify the ethnic diversity of the women who use a pessary for prolapse.
Role of funder
The TOPSY trial was funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment programme (16/82/01). The funders of the trial had no role in study design, data collection, data analysis, data interpretation, or report writing.
TOPSY study website
Acknowledgements
We are extremely grateful to all the women who gave up their time to take part in the study and shared their experiences. Thanks to each member of staff in the 21 centres (including principal investigators, urogynaecology nurse specialists, research nurses and administrative staff) who delivered on TOPSY even during a global pandemic. Special thanks to Lucy Dwyer, who was key in the intervention development and assisted the trial manager with the 21 site initiation visits.
We are greatly appreciative of the wonderful patient and public representatives in our Project Management Group (Margaret, Angela and Jane), who provided their lived experiences, their expertise and a smile throughout. Special thanks to the support and advice given by all members of the TSC and DMEC as both oversight committees were fantastic in the support offered throughout the study. Special thanks also go to the team at CHaRT: Mark Forrest and his team in the development of the study database and assisting the study team with their continued requests throughout and Suzanne Breeman for trial support. We also want to thank our original trial statistician, Anastasia Karachalia Sandri, for her contribution at the beginning of the trial and to the internal pilot.
Contributions of authors
Carol Bugge (https://orcid.org/0000-0002-4071-0803) (Professor of Nursing) led the design of the study, had overall management responsibility for all aspects of the study, managed the process evaluation, drafted several chapters, edited all chapters and approved the final version. CB has overall accountability for the study and this report.
Suzanne Hagen (https://orcid.org/0000-0002-9741-9160) (Professor of Health Services Research) contributed to the study design, led all aspects of the trial, drafted text, edited all chapters and approved the final version.
Andrew Elders (https://orcid.org/0000-0003-4172-4702) (Senior Statistician) contributed to the study design, led the trial analysis, drafted text, edited all chapters and approved the final version.
Helen Mason (https://orcid.org/0000-0002-9303-2794) (Lead Health Economist) contributed to the study design, led the health economic aspects of the study, drafted text, edited all chapters and approved the final version.
Kirsteen Goodman (https://orcid.org/0000-0002-6590-2748) (Trial Manager) contributed to the study design, led the day-to-day management of the trial, led site visits, drafted text, edited all chapters and approved the final version.
Melanie Dembinsky (https://orcid.org/0000-0002-1448-5358) (Process Evaluation Researcher and Analyst) contributed to the study design, led the day-to-day management of the process evaluation and undertook the process evaluation analysis (with CB), drafted text, edited all chapters and approved the final version.
Lynn Melone (https://orcid.org/0000-0003-0127-2684) (Data Coordinator) contributed to the study design, managed the collection of trial data, drafted text, edited all chapters and approved the final version.
Catherine Best (https://orcid.org/0000-0002-3652-2498) (Statistician) contributed to the study design, conducted the trial analysis, drafted Chapter 3 (with AE and SH), edited all chapters and approved the final version.
Sarkis Manoukian (https://orcid.org/0000-0002-5057-8236) (Health Economist) contributed to the study design, conducted the health economic analysis, drafted Chapter 4 (with HM), edited all chapters and approved the final version.
Lucy Dwyer (https://orcid.org/0000-0002-0284-873X) (Clinical Lead for Self-management) contributed to the study design, co-led the development of the self-management intervention (with RK), edited all chapters and approved the final version.
Aethele Khunda (https://orcid.org/0000-0003-4574-9675) (Clinical Co-applicant and Principal Investigator) contributed to the study design, provided expert clinical guidance, was a member of the qualitative project management group contributing to the process evaluation data analysis, edited all chapters and approved the final version.
Margaret Graham (PPI) contributed to the design and delivery of the study as an ‘expert by experience’, was a member of the qualitative project management group contributing to the process evaluation data analysis and reviewed the report for clarity and content.
Wael Agur (https://orcid.org/0000-0003-4836-6581) (Clinical Co-applicant and Principal Investigator) contributed to the study design, provided expert clinical guidance, edited all chapters and approved the final version.
Suzanne Breeman (https://orcid.org/0000-0001-9950-7079) (Co-applicant and CHaRT Senior Trial Manager) contributed to the study design, provided trial governance oversight on behalf of the Clinical Trials Unit, edited all chapters and approved the final version.
Jane Culverhouse (PPI) contributed to the design and delivery of the study as an ‘expert by experience’ and reviewed the report for clarity and content.
Angela Forrest (PPI) contributed to the design and delivery of the study as an ‘expert by experience’ and reviewed the report for clarity and content.
Mark Forrest (https://orcid.org/0000-0002-2395-8823) (Co-applicant and CHaRT Senior Programmer) contributed to the study design, led the development of the trial databases and their management to CTU standards, edited all chapters and approved the final version.
Karen Guerrero (https://orcid.org/0000-0001-9591-059X) (Clinical Co-applicant and Principal Investigator) contributed to the study design, provided expert clinical guidance, edited all chapters and approved the final version.
Christine Hemming (https://orcid.org/0000-0003-0075-9036) (Clinical Co-applicant and Principal Investigator) contributed to the study design, provided expert clinical guidance, edited all chapters and approved the final version.
Doreen McClurg (https://orcid.org/0000-0002-2872-1702) (Co-applicant) contributed to the study design, provided expert clinical guidance, edited all chapters and approved the final version.
John Norrie (https://orcid.org/0000-0001-9823-9252) (Co-applicant) contributed to the study design, provided expert clinical trial guidance, edited all chapters and approved the final version.
Ranee Thakar (https://orcid.org/0000-0002-5279-141X) (Clinical Co-applicant and Principal Investigator) contributed to the study design, provided expert clinical guidance, edited all chapters and approved the final version.
Rohna Kearney (https://orcid.org/0000-0002-1489-4397) (Clinical Co-applicant and Principal Investigator) contributed to the study design, led all clinical aspects of the trial, co-developed the self-management intervention (with LD), drafted text, edited all chapters and approved the final version.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/NWTB5403.
Primary conflicts of interest: Carol Bugge reports grants from the Chief Scientist Office (CSO), the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme, NHS Lothian and NHS Grampian during the conduct of the study. Suzanne Hagen reports grants from the CSO, NIHR HTA programme and Academy of Medical Sciences during the conduct of the study. Lucy Dwyer reports that she was a member of the National Institute for Health and Care Excellence (NICE) guideline committee for non-surgical management and prevention of pelvic floor dysfunction and a member of the UK Clinical Guideline for best practice in the use of vaginal pessaries for pelvic organ prolapse guidelines committee during the conduct of the study. Aethele Khunda reports that he received supported travel and accommodation funds from Olympus (Southend-on-Sea, UK) to attend a laparoscopic urogynaecology workshop (7–8 March 2019) during the conduct of the study. Wael Agur reports grants from NIHR, consulting fees and payment for testimony from Oaklaw Consultancy Ltd for medico-legal consultancy and financial/non-financial interest associated with Medical Innovation Systems during the conduct of the study. Karen Guerrero reports that she is chair of the UK Continence Society, treasurer of the British Society of Urogynaecology and chair of the Royal College of Obstetricians and Gynaecologists (RCOG) subspecialty training committee. Christine Hemming reports that she is chair of the pilot Pelvic Floor Registry Implementation Group and accountable officer for NHS Grampian for pelvic floor mesh complications. Doreen McClurg reports that she is topic advisor on the NICE guideline ‘Pelvic floor dysfunction prevention and non-surgical management’ (NG10123). John Norrie reports being a member of the following committees: NIHR Clinical Trials Unit Standing Advisory Committee (2018–23); NIHR HTA and Efficacy and Mechanism Evaluation (EME) Editorial Board (2015–19); EME Funding Committee (2019–22); HTA General Committee (2016–19); HTA Post-Funding Committee (2016–19); HTA Funding Committee Policy Group (2016–19); and COVID-19 Reviewing (2020). Ranee Thakar reports a voluntary role in the International Urogynaelogical Association and being President of the RCOG (2022–present). Rohna Kearney reports consulting fees from the British Standards Institution, payment for testimony for medico-legal work in urogynaecology, being a clinical member of the RCOG Women’s Network, a member of NHS England Clinical Research Facility for Women’s Services and a charity trustee for Birth-Aid (Manchester, UK). Sarkis Manoukian is a co-investigator on the NIHR grant ‘Common Health Assets: a mixed methods realist evaluation and economic appraisal of how community led organisations impact on the health and wellbeing of people living in deprived areas in the UK’.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
The TOPSY trial received ethics approval from the West of Scotland Research Ethics Service, West of Scotland REC 3 (17/WS/0267) on 17 February 2018 and the NHS Health Research Authority on 9 March 2018. A log of all amendments can be found in Appendix 5, Table 38.
Information governance statement
The University of Stirling was the study Sponsor and is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (UK GDPR) 2016/679. Under the Data Protection legislation, the University of Stirling is the Data Controller, and you can find out more about how they handle personal data, including how to exercise your individual rights and the contact details for the Data Protection Officer here (https://www.stir.ac.uk/about/professional-services/student-academic-and-corporate-services/policy-and-planning/legal-compliance/data-protectiongdpr/) or by emailing data.protection@stir.ac.uk.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by the interviewees in this publication are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the HTA programme or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Publications
Bugge C, Kearney R, Dembinsky M, Khunda A, Graham M, Agur W, et al. The TOPSY pessary self-management intervention for pelvic organ prolapse: a study protocol for the process evaluation. Trials 2020;21:836.
Hagen S, Kearney R, Goodman K, Melone L, Elders A, Manoukian S, et al. Clinical and cost-effectiveness of vaginal pessary self-management compared to clinic-based care for pelvic organ prolapse: protocol for the TOPSY randomised controlled trial. Trials 2020;21:837.
Dwyer L, Bugge C, Hagen S, Goodman K, Agur W, Dembinsky M, et al. ; TOPSY Team. Theoretical and practical development of the TOPSY self-management intervention for women who use a vaginal pessary for pelvic organ prolapse. Trials 2022;23:742.
Graham, M, Goodman K. Commentary on my personal experience of patient and public involvement in the TOPSY trial. Trials 2023;24:228.Hagen S, Kearney R, Goodman K, Best C, Elders A, Melone L et al. Clinical effectiveness of vaginal pessary self-management vs. clinic-based care for pelvic organ prolapse (TOPSY): a randomised controlled superiority trial. eClinicalMedicine 2023;66:102326.
Manoukian S, Mason H, Hagen S, Kearney R, Goodman K, Best C et al. Cost-effectiveness of 2 Models of Pessary Care for Pelvic Organ Prolapse: Findings From the TOPSY Randomized Controlled Trial. Value Health 2024; in press.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Bugge C, Adams EJ, Gopinath D, Stewart F, Dembinsky M, Sobiesuo P, et al. Pessaries (mechanical devices) for managing pelvic organ prolapse in women. Cochrane Database Syst Rev 2020; 11;CD004010; based on Bugge C, Adams L, Gopinath D,Reid F. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database Syst Rev 2013;2013.
- Cooper J, Annappa M, Dracocardos D, Cooper W, Muller S, Mallen C. Prevalence of genital prolapse symptoms in primary care: a cross-sectional survey. Int Urogynecol J 2015;26:505-10.
- National Institute for Health and Care Excellence . Urinary Incontinence and Pelvic Organ Prolapse in Women: Management 2019. www.nice.org.uk/guidance/ng123 (accessed January 2022).
- Wu JM, Kawasaki A, Hundley AF, Dieter AA, Myers ER, Sung VW. Predicting the number of women who will undergo incontinence and prolapse surgery, 2010 to 2050. Am J Obstet Gynaecol 2011;205:230.e1-5.
- Gyhagen M, Åkervall S, Milsom I. Clustering of pelvic floor disorders 20 years after one vaginal or one cesarean birth. Int Urogynecol J 2015;26:1115-21. https://doi.org/10.1007/s00192-015-2663-3.
- MacArthur C, Wilson D, Herbison P, Lancashire RJ, Hagen S, Toozs-Hobson P, et al. Prolong Study Group . Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG 2016;123:1022-9. https://doi.org/10.1111/1471-0528.13395.
- Jelovsek JE, Chagin K, Gyhagen M, Hagen S, Wilson D, Kattan MW, et al. Predicting risk of pelvic floor disorders 12 and 20 years after delivery. Am J Obstet Gynecol 2018;218:222.e1-222.e19. https://doi.org/10.1016/j.ajog.2017.10.014.
- Manchana T, Bunyavejchevin S. Impact on quality of life after ring pessary use for pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2012;23:873-7.
- Mendes LC, Bezerra LRPS, Bilhar APM, Neto JAV, Vasconcelos CTM, Saboia DM, et al. Symptomatic and anatomic improvement of pelvic organ prolapse in vaginal pessary users. Int Urogynecol J 2021;32:1023-9. https://doi.org/10.1007/s00192-020-04540-w.
- Lowder JL, Ghetti C, Nikolajski C, Oliphant SS, Zyczynski HM. Body image perceptions in women with pelvic organ prolapse: a qualitative study. Am J Obstet Gynecol 2011;204:441.e1-5. https://doi.org/10.1016/j.ajog.2010.12.024.
- Pakbaz M, Rolfsman E, Mogren I, Löfgren M. Vaginal prolapse – perceptions and healthcare-seeking behavior among women prior to gynecological surgery. Acta Obstet Gynecol Scand 2011;90:1115-20. https://doi.org/10.1111/j.1600-0412.2011.01225.x.
- Abdel-Fattah M, Familusi A, Fielding S, Ford J, Bhattacharya S. Primary and repeat surgical treatment for female pelvic organ prolapse and incontinence in parous women in the UK: a register linkage study. BMJ Open 2011;1.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 1997;89:501-6.
- Løwenstein E, Møller LA, Laigaard J, Gimbel H. Reoperation for pelvic organ prolapse: a Danish cohort study with 15–20 years’ follow-up. Int Urogynecol J 2018;29:119-24. https://doi.org/10.1007/s00192-017-3395-3.
- Subramanian D, Szwarcensztein K, Mauskopf JA, Slack MC. Rate, type, and cost of pelvic organ prolapse surgery in Germany, France, and England. Eur J Obstet Gynecol Reprod Biol 2009;144:177-81. https://doi.org/10.1016/j.ejogrb.2009.03.004.
- National Institute for Health and Care Excellence . Pelvic Floor Dysfunction: Prevention and Nonsurgical Management: Weight Loss Interventions 2021. www.nice.org.uk/guidance/ng210/evidence/j-weight-loss-interventions-pdf-392138593237 (accessed January 2022).
- National Institute for Health and Care Excellence . Pelvic Floor Dysfunction: Prevention and Nonsurgical Management: Physical Activity for the Management of Symptoms 2021. www.nice.org.uk/guidance/ng210/evidence/l-physical-activity-for-the-management-of-symptoms-pdf-392138593239 (accessed January 2022).
- National Institute for Health and Care Excellence . Pelvic Floor Dysfunction: Prevention and Nonsurgical Management: Pelvic Floor Muscle Training for the Management of Symptoms 2021. www.nice.org.uk/guidance/ng210/evidence/m-pelvic-floor-muscle-training-for-the-management-of-symptoms-pdf-392138593240 (accessed January 2022).
- Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database Syst Rev 2011;2011. https://doi.org/10.1002/14651858.CD003882.pub4.
- Weber MA, Kleijn MH, Langendam M, Limpens J, Heineman MJ, Roovers JP. Local oestrogen for pelvic floor disorders: a systematic review. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0136265.
- Kapoor DS, Thakar R, Sultan AH, Oliver R. Conservative versus surgical management of prolapse: what dictates patient choice?. Int Urogynaecol J 2009;20:1157-61.
- Lone F, Thakar R, Sultan AH, Karamalis G. A 5-year prospective study of vaginal pessary use for pelvic organ prolapse. Int J Gynaecol Obstet 2011;114:56-9.
- Ramsay S, Tu LM, Tannenbaum C. Natural history of pessary use in women aged 65–74 versus 75 years and older with pelvic organ prolapse: a 12-year study. Int Urogynaecol J 2016;27:1201-7.
- Gorti M, Hundelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol 2009;29:129-31.
- Chan MC, Momoe H, Maryna Y, Schulz JA. What are the clinical factors that are predictive of persistent pessary use at 12 months?. J Obstet Gynaecol Can 2019;41:1276-81.
- Li B, Zhang J, Yu C, Zhang L, Chen L, Chen Q. A prospective study of pessary use for severe pelvic organ prolapse: 3-year follow-up outcomes. Arch Gynecol Obstet 2020;301:1213-8.
- Mao M, Xu T, Kang J, Zhang Y, Ai F, Zhou Y, et al. Factors associated with long-term pessary use in women with symptomatic pelvic organ prolapse. Climacteric 2019;22:478-82.
- National Institute for Health and Care Excellence . Pelvic Floor Dysfunction: Prevention and Non-Surgical Management 2021 n.d. www.nice.org.uk/guidance/ng210 (accessed February 2022).
- Pelvic Obstetric and Gynaecological Physiotherapy. UK Clinical Guideline for Best Practice in the Use of Vaginal Pessaries for Pelvic Organ Prolapse 2021. https://thepogp.co.uk/_userfiles/pages/files/resources/uk_pessary_guideline_final_april21.pdf (accessed March 2022).
- Bugge C, Hagen S, Thakar R. Vaginal pessaries for pelvic organ prolapse and urinary incontinence: a multiprofessional survey of practice. Int Urogynaecol J 2013;24:1017-24.
- Hanson LM, Schultz J, Flood CG, Cooley B, Tam F. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynaecol J 2006;17:155-9.
- Bugge C, Dembinsky M, Kearney R, Hagen S. Is self-management of vaginal support pessary for pelvic organ prolapse safe and does it improve women’s quality of life. BMJ 2021;372.
- Kearney R, Brown C. Self-management of vaginal pessaries for pelvic organ prolapse. BMJ Qual Improv Rep 2014;U206180.
- Manonai J, Sarit-apirak S, Udomsubpayakul U. Vaginal ring pessary use for pelvic organ prolapse: continuation rates and predictors of continued use. Menopause 2018;26:665-9.
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council Guidance. BMJ 2021;374.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council guidance. Developing and evaluating complex interventions: the new Medical Research Council Guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191-215.
- Lorig KR, Holman HR. Self management education: history, definition, outcomes and mechanisms. Ann Behav Med 2003;26:1-7.
- Zwerink M, Brusse-Keizer M, Van Der Valk PDLPM, Zielhuis GA, Monninkhof EM, Van Der Palen J, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014;3. https://doi.org/10.1002/14651858.CD002990.pub3.
- National Institute for Health and Care Research n.d. www.fundingawards.nihr.ac.uk/award/16/82/01 (accessed June 2022).
- Hagen S, Kearney R, Goodman K, Melone L, Elders A, Manoukian S, et al. Clinical and cost-effectiveness of vaginal pessary self-management compared to clinic-based care for pelvic organ prolapse: protocol for the topsy randomised controlled trial. Trials 2020;21.
- Bugge C, Kearney R, Dembinsky M, Khunda A, Graham M, Agur W, et al. The TOPSY pessary self-management intervention for pelvic organ prolapse: a study protocol for the process evaluation. Trials 2020;21.
- Schulz KF, Altman DG, Moher D. for the CONSORT Group . CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials 2010. www.consort-statement.org/ (accessed March 2022).
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8.
- Hagen S, Bugge C, Dean SG, Elders A, Hay-Smith J, Kilonzo M, et al. Basic versus biofeedback-mediated intensive pelvic floor muscle training for women with urinary incontinence: the OPAL RCT. Health Technol Assess 2020;24.
- Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol 2005;193:103-13. https://doi.org/10.1016/j.ajog.2004.12.025.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Rogers RG, Rockwood TH, Constantine ML, Thakar R, Kammerer-Doak DN, Pauls RN, et al. A new measure of sexual function in women with pelvic floor disorders (PFD): the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire, IUGA-Revised (PISQ-IR). Int Urogynecol J 2013;24:1091-103. https://doi.org/10.1007/s00192-012-2020-8.
- Constantine ML, Pauls RN, Rogers RR, Rockwood TH. Validation of a single summary score for the Prolapse/Incontinence Sexual Questionnaire-IUGA revised (PISQ-IR). Int Urogynecol J 2017;28:1901-7. https://doi.org/10.1007/s00192-017-3373-9.
- Schwarzer R, Jerusalem M. Measures in Health Psychology: A User’s Portfolio – Causal and Control Beliefs. Slough: NFER-Nelson; 1995.
- Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynaecol 2003;189:98-101.
- Srikrishna S, Robinson D, Cardozo L. Validation of the Patient Global Impression of Improvement (PGI-I) for urogenital prolapse. Int Urogynaecol J 2010;21.
- Panman CMCR, Weigersma M, Kollen BJ, Berger MY, Leeuwen YL, Vermeulen KM, et al. Effectiveness and cost-effectiveness of pessary treatment compared with pelvic floor muscle training in older women with pelvic organ prolapse: 2-year follow-up of a randomized controlled trial in primary care. Menopause 2016;23.
- Weigersma M, Panman CM, Kollen BJ, Berger MY, Lisman-Van Leeuwen Y, Dekker JH. Effect of pelvic floor muscle training compared with watchful waiting in older women with symptomatic mild pelvic organ prolapse: randomised controlled trial in primary care. BMJ 2014;349.
- Liang KY, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhyā: Ind J Stat, Ser B 2000;1:134-48.
- Dinh P, Yang P. Handling baselines in repeated measures analyses with missing data at random. J Biopharm Stat 2011;21:326-41.
- Hood K, Robling M, Ingledew D, Gillespie D, Greene G, Ivins R, et al. Mode of data elicitation, acquisition and response to surveys: a systematic review. J Health Technol Assess 2012;16:1-162.
- Twisk J, Bosman L, Hoekstra T, Rijnhart J, Welten M, Heymans M. Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trials Commun 2018;10:80-5.
- Spencer L, Ritchie J, O’Connor W, Morrell G, Ormoston R. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications; 2014.
- Craig BA, Black MA. Incremental cost-effectiveness ratio and incremental net-health benefit: two sides of the same coin. Exp Rev Pharmacoecono Outc Res 2001;1:37-46. https://doi.org/10.1586/14737167.1.1.37.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/process/pmg9/chapter/foreword (accessed February 2022).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: Personal Social Services Research Unit, University of Kent; 2020.
- . British National Formulary 81. London: BMJ Publishing and the Royal Pharmaceutical Society; 2021.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II: an ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices – overview: a report of the ispor-smdm modeling good research practices task force-1. Value Health 2012;15:796-803.
- Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD. ISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health 2012;15:835-42.
- Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices – modeling studies. Value Health 2003;6:9-17.
- Graham M, Goodman K. Commentary on my personal experience of patient and public involvement in the TOPSY trial. Trials 2023;24. https://doi.org/10.1186/s13063-023-07254-8.
- Communities Analysis Division, Scottish Government . Scottish Index of Multiple Deprivation 2020. www.gov.scot/collections/scottish-index-of-multiple-deprivation-2020/ (accessed November 2021).
- Ministry of Housing, Communities and Local Government . National Statistics English Indices of Deprivation 2019. www.gov.uk/government/statistics/english-indices-of-deprivation-2019 (accessed November 2021).
- Zellner A, Huang DS. Further properties of efficient estimators for seemingly unrelated regression equations. Int Econ Rev 1962;3:300-13. https://doi.org/10.2307/2525396.
- Cameron AC, Trivedi PK. Microeconometrics Using Stata. College Station, TX: Stata Press; 2010.
- O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ 2010;341. https://doi.org/10.1136/bmj.c4587.
- Turel Fatakia F, Pixton S, Caudwell Hall J, Dietz HP. Predictors of successful ring pessary use in women with pelvic organ prolapse. Aust N Z J Obstet Gynaecol 2020;60:579-84. https://doi.org/10.1111/ajo.13152.
- Mutone MF, Terry C, Hale DS, Benson JT. Factors which influence the short-term success of pessary management of pelvic organ prolapse. Am J Obstet Gynecol 2005;193:89-94. https://doi.org/10.1016/j.ajog.2004.12.012.
- Gray TG, Vickers H, Krishnaswamy P, Jha S. A systematic review of English language patient-reported outcome measures for use in urogynaecology and female pelvic medicine. Int Urogynecol J 2018;32:2033-92. https://doi.org/10.1007/s00192-021-04810-1.
- Boulton AJ, Williford A. Analyzing skewed continuous outcomes with many zeros: a tutorial for social work and youth prevention science researchers. J Soc Soc Work Res 2018;9:721-40.
- E9 (R1) Statistical Principles for Clinical Trials: Addendum: Estimands and Sensitivity Analysis in Clinical Trials. Washington, DC: US Food and Drug Administraion; 2021.
- Schrijver J, Lenferink A, Brusse-Keizer M, Zwerink M, van der Valk PDLPM, van der Palen J, et al. Self‐management interventions for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2022;1. https://doi.org/10.1002/14651858.CD002990.pub4.
- Fryer CE, Luker JA, McDonnell MN, Hillier SL. Self management programmes for quality of life in people with stroke. Cochrane Database Syst Rev 2016;2016. https://doi.org/10.1002/14651858.CD010442.pub2.
- Kroon FPB, van der Burg LRA, Buchbinder R, Osborne RH, Johnston RV, Pitt V. Self‐management education programmes for osteoarthritis. Cochrane Database Syst Rev 2014;2014. https://doi.org/10.1002/14651858.CD008963.pub2.
- Barber MD, Chen Z, Lukacz E, Markland A, Wai C, Brubaker L, et al. Further validation of the short form versions of the Pelvic Floor Distress Inventory (PFDI) and Pelvic Floor Impact Questionnaire (PFIQ). Neurourol Urodyn 2011;30:541-6. https://do.org/10.1002/nau.20934.
- Manchana T. Ring pessary for all pelvic organ prolapse. Arch Gynecol Obstet 2011;284:391-5.
- Lammers K, Tewest NI, Allen W, Parkin K, Moore KH. Does monthly self-management of vaginal ring pessaries reduce complication rates?. Int Urogynecol J 2019;30:S271-2.
- Sarma S, Ying T, Moore KH. Long-term vaginal ring pessary use: discontinuation rates and adverse events. BJOG 2009;116:1715-21. https://doi.org/10.1111/j.1471-0528.2009.02380.x.
- Chien CW, Lo TS, Tseng LH, Lin YH, Hsieh WC, Lee SJ. Long-term outcomes of self-management Gellhorn pessary for symptomatic pelvic organ prolapse. Female Pelvic Med Reconstr Surg 2020;26:e47-53. https://doi.org/10.1097/SPV.0000000000000770.
- Tam MS, Lee VYT, Yu ELM, Wan RSF, Tang JSM, He JMY, et al. The effect of time interval of vaginal ring pessary replacement for pelvic organ prolapse on complications and patient satisfaction: a randomised controlled trial. Maturitas 2019;128:29-35. https://doi.org/10.1016/j.maturitas.2019.07.002.
- Abdulaziz M, Stothers L, Lazare D, Macnab A. An integrative review and severity classification of complications related to pessary use in the treatment of female pelvic organ prolapse. Can Urol Assoc J 2015;9:E400-6. https://doi.org/10.5489/cuaj.2783.
- Murray C, Thomas E, Pollock W. Vaginal pessaries: can an educational brochure help patients to better understand their care?. J Clin Nurs 2017;26:140-7. https://doi.org/10.1111/jocn.13408.
- Janjua S, Banchoff E, Threapleton CJD, Prigmore S, Fletcher J, Disler RT. Digital interventions for the management of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2021;2021. https://doi.org/10.1002/14651858.CD013246.pub2.
Appendix 1 Project documentation list
All of the following documents are classed as project documentation. As stipulated in the NIHR guidance, these documents are not submitted with the report for peer review but are all uploaded to the TOPSY project website page and can be viewed at https://fundingawards.nihr.ac.uk/award/16/82/0141
-
Patient information leaflets (01–06)
-
Consent forms (01–05)
-
Interview schedules (01–06)
-
Statistical analysis plan for main trial
-
Statistical analysis plan deviations
-
Process evaluation analysis plan
-
Health economics analysis plan
-
TOPSY questionnaire booklets (baseline, 6, 12 and 18 months)
-
All study case report forms (combined into one document)
-
Protocol version 7 dated 11 November 2020
-
Pilot study report
Appendix 2 Treatment of prolapse with self-care pessary study flow chart

Appendix 3 List of Treatment of prolapse with self-care pessary study centres
| Full trust name | Centre name | Centre number | Name of hospital |
|---|---|---|---|
| NHS Ayrshire and Arran | Ayrshire and Arran | 11 and 12 | Ayr Hospital and University Hospital Crosshouse |
| NHS Grampian | Aberdeen | 13 | Aberdeen Royal Infirmary |
| Croydon Health Services NHS Trust | Croydon | 14 | Croydon University Hospital |
| Manchester University NHS Foundation Trust | Manchester | 15 | St Mary’s Hospital |
| South Tees Hospitals NHS Foundation Trust | Middlesbrough | 16 | James Cook University Hospital |
| NHS Greater Glasgow and Clyde | Glasgow | 17 | Queen Elizabeth Hospital and Glasgow Royal Infirmary |
| King’s College Hospital NHS Foundation Trust | London | 18 | King’s College Hospital |
| Hampshire Hospitals NHS Foundation Trust | Basingstoke | 19 | Basingstoke and North Hampshire Hospitals |
| Cambridge University Hospitals NHS Foundation Trust | Addenbrookes | 20 | Addenbrookes Hospital |
| Sheffield Teaching Hospitals NHS Foundation Trust | Sheffield | 21 | Sheffield Teaching Hospitals – The Jessop Wing |
| Birmingham Women’s and Children’s NHS Foundation Trust | Birmingham | 22 | Birmingham Women’s Hospital |
| Liverpool Women’s NHS Foundation Trust | Liverpool | 23 | Liverpool Women’s Hospital |
| NHS Lothian | Lothian | 24 | St John’s Hospital and Edinburgh Royal Infirmary |
| NHS Fife | Fife | 26 | Queen Margaret Hospital |
| County Durham and Darlington NHS Foundation Trust | County Durham | 27 | University Hospital of North Durham |
| University Hospitals Plymouth NHS Trust | Plymouth | 28 | Derriford Hospital |
| Yeovil District Hospital NHS Foundation Trust | Yeovil | 30 | Yeovil District Hospital |
| Taunton and Somerset NHS Foundation Trust | Taunton and Somerset | 31 | Musgrove Park Hospital |
| The Newcastle upon Tyne Hospitals NHS Foundation Trust | Newcastle | 32 | Royal Victoria Hospital |
| NHS Lanarkshire | Lanarkshire | 33 | University Hospital Wishaw and Hairmyers Hospital |
| Norfolk and Norwich University Hospitals NHS Foundation Trust | Norwich | 34 | Norfolk and Norwich University Hospital |
Appendix 4 Treatment of prolapse with self-care pessary decision-analytic modelling
| Parameter type | Parameter | Description | Value | Plausible range | Distribution | Notes |
|---|---|---|---|---|---|---|
| States | Good | Good state | N/A | N/A | N/A | In trial data this state was associated with low resource use |
| Moderate | Moderate state | N/A | N/A | N/A | In trial data this state was associated with moderate resource use | |
| Poor | Poor state | N/A | N/A | N/A | In trial data this state was associated with high resource use | |
| Monthly transition probabilities | pGoodtoModerateSM | Probability of progression from good to moderate state for self-management patients | 0.07 | 0.03–0.15 | Beta | Based on trial data that showed that self-management patients with low starting resource use were less likely to have increased resource use in later follow-ups than clinic-based patients |
| pGoodtoModerateUC | Probability of progression from good to moderate state in clinic-based care | 0.15 | 0.10–0.20 | Beta | ||
| pModeratetoGoodSM | Probability of progression from moderate to good in self-management | 0.08 | 0.04–0.12 | Beta | Based on trial data that showed that self-management patients with moderate starting resource use were more likely to have lower resource use in later follow-ups than clinic-based patients | |
| pModeratetoGoodUC | Probability of progression from moderate to good in clinic-based care | 0.03 | 0.01–0.05 | Beta | ||
| pModeratetoPoorSM | Probability of progression from moderate to poor in self-management | 0.05 | 0.01–0.09 | Beta | Based on trial data that showed that self-management patients with moderate starting resource use were less likely to have higher resource use in later follow-ups than clinic-based patients | |
| pModeratetoPoorUC | Probability of progression from moderate to poor in clinic-based care | 0.03 | 0.01–0.05 | Beta | ||
| pPoortoModerateSM | Probability of progression from poor to moderate in self-management | 0.03 | 0–0.10 | Beta | Based on trial data that showed that self-management patients with high starting resource use were less likely to have lower resource use in later follow-ups than clinic-based patients | |
| pPoortoModerateUC | Probability of progression from poor to moderate in clinic-based care | 0.05 | 0–0.10 | Beta | ||
| Cost parameters | cGoodSM | Yearly cost at good state for SM patients | £53.04 | SD: £5.07 | Gamma | From observed cost distribution in trial data. Divided by CyclesPerYear parameter to calculate monthly values |
| cGoodUC | Yearly cost at good state for clinic-based care patients | £112.66 | SD: £29.84 | Gamma | ||
| cModerateSM | Yearly cost at moderate state for SM patients | £239.59 | SD: £88.41 | Gamma | ||
| cModerateUC | Yearly cost at moderate state for clinic-based care patients | £271.21 | SD: £89.25 | Gamma | ||
| cPoorSM | Yearly cost at poor state for SM patients | £607.59 | SD: £321.08 | Gamma | ||
| cPoorUC | Yearly cost at poor state for clinic-based care patients | £757.75 | SD: £521.01 | Gamma | ||
| Utilities | uGoodSM | Utility at good state for self-management patients | 0.97 | 0.75–1.00 | Beta | From observed trial EQ-5D-5L data. Divided by CyclesPerYear parameter to calculate monthly values |
| uGoodUC | Utility at good state for clinic-based care patients | 0.96 | 0.75–1.00 | Beta | ||
| uModerateSM | Utility at moderate state for self-management patients | 0.75 | 0.70–0.90 | Beta | ||
| uModerateUC | Utility at moderate state for clinic-based care patients | 0.76 | 0.70–0.90 | Beta | ||
| uPoorSM | Utility at poor state for self-management patients | 0.58 | 0.25–0.75 | Beta | ||
| uPoorUC | Utility at poor state for clinic-based care patients | 0.58 | 0.25–0.75 | Beta | ||
| Modelling variables | disc_rate | Discount rate | 0.035 | N/A | N/A | 3.5% discount rate that was applied to costs and utilities as recommended by NICE |
| CyclesPerYear | Number of cycles per year | 12 | N/A | N/A | Used to calculate monthly costs and utilities | |
| Time_Horizon | Cycles of model | 78 | N/A | N/A | Model was run for 78 months, which includes 18-month trial period plus 5 years |
Appendix 5 Log of all study amendments
| Study title | Treatment of prolapse with self-care pessary: the TOPSY trial |
|---|---|
| Chief investigator | Dr Carol Bugge |
| Sponsor internal reference | 234662 (17/WS/0267) |
| Original protocol and version number | Original ethical approval on 23 February 2018; Protocol V2, 8 February 2018. All centres were given version 2 of the protocol or subsequent versions depending on date of centre set-up |
| Amendment number | Date submitted | Where submitted | Classification | Purpose of amendment | Name/version/date of amended documents | Date approved | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REC | HRA | NRSPCC | Each Local centre | Substantial | Non-substantial | New | Old | REC | HRA | Each local centre | |||
| AM01 | 13 June 2018 | Y | N/A | Y | N/A | N | Y | Addition of nine new NHS sites | N/A | N/A | 14 June 2018 | N/A | N/A |
| AM02 and AM04 * | 28 June 2018 | Y | Y | Y | Y | N | Y | Minor clarifications (protocol version 3, expression of interest form, e-mail text to be sent at all questionnaire time points, updated interview schedule for non-randomised, GDPR information included to all for information only) HRA classified the amendment twice Amendment Category A |
See appendix 1 for list of all documents | 25 July 2018 | 11 July 2018 | Yes | |
| HRA made an error in its classification and referenced the same amendment as AM 02 and AM04. Protocol version 3, dated 27 June, approved and sent to all centres open to recruitment at the time and PI signed PI declaration | |||||||||||||
| AM05– see ** | 27 September 2018 | Y | N/A | Y | N/A | N/A | Y | Addition of new sites | N/A | N/A | 27 September 2018 | N/A | N/A |
| ** AM05 – we submitted this with ref AM03 but, as HRA had already classified previous as AM04 in error, HRA classified this as AM05. NO AM03 at all for TOPSY | |||||||||||||
| AM06 | 12 December 2018 | Y | Y | Y | No (as not approved) | Y | Y | Increased flexibility in the recruitment process – not approved | Not approved | Not approved | |||
| AM07 | 17 January 2019 | Y | N/A | Y | N/A | N/A | Y | Addition of new sites | N/A | N/A | 17 January 2019 | N/A | N/A |
| AM08 (Rec REF AM06-01) | 23 January 2019 | Y | Y | Y | Y | Y | No | Modified amendment of AM06 | See appendix 2 for list of all documents | 19 January 2019 – amended letter 1 February 2019 | 7 February 2019 then re-issued with correct details on 25 February 2019 | All rolled out and all approved | |
| AM09 (non-substantial) | 8 August 2019 | Y | Y | Y | Y | N | Y | Extension to recruitment; question added to interviews with HCPs; 12- and 18-month questionnaire booklet – clarification in question; protocol clarifications in timings | See appendix 3 for list of all documents | 13 August 2019 | 20 August 2019 | Still waiting for some centres to approve as 12 September 2019. Implementation date 19 September 2019 | |
| AM10 | August 2020 | Y | Y | Y | Y | Y | N | Due to the COVID-19 pandemic routine pessary clinics were suspended for at least 3 months at participating centres. We would like to ask participants to complete a short questionnaire during their next clinic visit to assess the impact of this disruption to normal care delivery on their views on pessary care/self-management | See appendix 4 for list of documents | Reissued 11 September 2020 (provide on 3 September but wrong date on letter) | 10 September 2020 | Implemented to all sites on 10 September 2020 | |
Appendix 6 Treatment of prolapse with self-care pessary training manual
Training manual for delivering pessary self-management intervention for the TOPSY study
Version 2.0, 30 January 2018
‘Self-management support can be viewed in two ways: as a portfolio of techniques and tools that help patients choose healthy behaviours; and a fundamental transformation of the patient-caregiver relationship into a collaborative partnership’ (Bodenheimer T, MacGregor K, Shafiri C. Helping Patients Manage Their Chronic Conditions. California Healthcare Association. 2005).
This manual is written for the TOPSY study. It provides instruction on teaching the intervention of pessary self-management to women. All staff involved in the TOPSY study who will teach women the self-management intervention should read and apply the techniques described in this manual. The manual covers three main aspects that are critical to teaching this intervention:
-
information on pelvic organ prolapse and pessary use
-
theory underpinning self-management
-
guide to teaching the TOPSY pessary self-management intervention.
Pelvic organ prolapse and pessary use
Pelvic organ prolapse affects about 40% of women over 40 years of age and the number of women affected is expected to rise. The distressing symptoms include a sensation of ‘something coming down’ in the vagina, urinary, bowel and sexual problems and pelvic and back pain. These symptoms impact negatively on a woman’s quality of life. Women presenting with prolapse are most commonly offered the option of conservative management (such as a vaginal pessary) or surgery. About 9.5% of women will undergo surgery for prolapse in their lifetime. Over 29,000 prolapse repairs were performed in England in 2012–3 costing over £60 million. However, surgery is not always effective or durable, with 30% of women requiring at least one further procedure. With the high reoperation rates and the controversy surrounding mesh surgery, it is a good time to consider the evidence supporting conservative options in more detail.
Currently women who have prolapse of all types and stages can receive pessary treatment. Two-thirds of women will opt to try a pessary when offered, but it is not clear if younger women may be offered/use pessaries more often, if alternatives were available with less reliance on follow-up appointments and easier integration with lifestyle. Although previous research indicates that the ring pessary is most commonly used in practice, a wide range of pessaries are available and are used. The most common service model for women who use a pessary is to return to clinic for that pessary to be removed and changed. The most common process seems to be inviting women back to clinic every 6 months for pessary changes but time between changes does vary (3–12 months). The largest UK-based study reported that 86% of women who successfully retain a pessary at 4 weeks will continue to use a pessary at 5 years. However, other studies have reported much lower continuation rate. Reasons for discontinuation of pessary use include developing complications such as bleeding or infection, dislike of the pessary changing procedure and inconvenience of attending appointments.
A UK multiprofessional survey in 2013 found that only 17% of clinicians offered their patients the option of self-managing their pessary. This is a significant difference in practice compared with North America, where the majority of clinicians teach women pessary self-care.
There is only one small (n = 88) non-randomised study that assesses self-management of vaginal pessaries, which reported gains from self-management, in that women reported higher levels of convenience, ability to access help, support and comfort than those attending clinic. Women who were self-managing had one clinic appointment scheduled at 2 years, compared with clinic-based care, where women attended every 4–6 months for pessary changes. While these may be promising findings, there is an urgent need to robustly investigate whether pessary self-management is more clinically effective and cost-effective than clinic-based pessary care.
Previous pessary trials, where women are randomised prior to pessary fitting, have an attrition rate of approximately 40%. As we are aiming to assess the effectiveness of self-management and not of the pessary itself, it will be important to minimise the early attrition associated with pessary treatment (e.g. discontinuation due to discomfort or failure to retain the pessary) and on which self- management would have no effect.
To maximise the likelihood of improving public health and increasing NHS efficiencies, the TOPSY study will pragmatically recruit women of any age, who use any pessary type/material (except Shelf, Gellhorn and cube, which are difficult to self-manage) and have retained the pessary for at least 2 weeks. All of this will be undertaken with a view to improving women’s quality of life.
Introduction to self-management theory
What is self-management and how does it fit with patient-centred care?
Self-management support is an important component of person-centred care. The four principles of person-centred care are:
-
affording people dignity, compassion and respect
-
offering coordinated care, support or treatment
-
offering personalised care, support or treatment
-
supporting people to recognise and develop their own strengths and abilities to enable them to live an independent and fulfilling life (see De longh 2015 under Further reading).
The Health Foundation
It is clear that self-management is a critical part of person-centred care. Self-management focuses on things that people do for themselves to manage their health and illness. We now want to find out if self-management of a vaginal pessary can improve a woman’s quality of life. The current evidence suggests that self-management helps to improve outcomes by supporting people to feel more confident (self-efficacious).
To become more self-efficacious and hence successfully self-manage a pessary women will need self-management support, that is the actions taken by health professionals to support people to self-manage. This manual focuses on those supports that the health professionals delivering the self-management intervention will offer.
How do we support women to self-manage their pessary?
As part of a self-management programme, women have three tasks to achieve and six skills to learn in order to enable the self-efficacy that is needed to self-manage. The three tasks are:
-
Medical management of the condition. For pessary self-management this is women’s medical management of prolapse using a pessary.
-
Role management. For pessary self-management this is maintaining, changing or creating new behaviours such as altering exercise activities, or removing the pessary (if required) for sexual intercourse.
-
Emotional management. For pessary self-management, this is learning to manage the emotions of having prolapse and using a pessary and is a part of the work required to manage the prolapse (e.g. coping with fear of the prolapse getting worse with age or worries about being able to successfully remove and replace the pessary).
For TOPSY the logic of how pessary self-management will improve quality of life is as follows: via information received, teaching and support, women will become more confident (self-efficacious) about the medical management of their prolapse using a vaginal pessary, they will understand their role in relation to self-managing their prolapse and have confidence in their ability to do so, and they will have the emotional capacity and confidence to cope with the prolapse and pessary such that their quality of life will be improved more than those who are followed up as per clinic-based pessary care.
The six skills are:
-
problem-solving, for example women knowing how to troubleshoot pessary problems such as discomfort or discharge
-
decision-making, for example women knowing when to remove and insert the pessary and knowing to call the advice line if they experience non-menstrual bleeding
-
resource utilisation, for example making sure women know what resources are available for example by providing more than one source of information such as a telephone number, information leaflet and online video
-
the formation of a patient–provider partnership role, for example the health professional role changing to that of a teacher and health partner rather than just a health care provider so that women can feel a partner in their care
-
action planning, for example a woman deciding to remove the pessary to have sex
-
self-tailoring, for example the woman using the pessary in a way that works for her.
Section 3 in the guide outlines how we are asking those delivering the self-management intervention to support women in gaining the skills to be competent in the tasks.
Why do this study now?
Self-management has been researched in other conditions. In patients with chronic obstructive pulmonary disease, self-management interventions have been proven to improve their quality of life, reduce hospital admissions and improve the symptom of shortness of breath. Further research is ongoing in patients after acute brain injury, cardiac event and long-term management of diabetes (www.health.org.uk). This evidence supports the idea that self-management can improve quality of life.
A quality improvement project assessing the impact of the introduction of a self-management pathway for women using pessaries for vaginal prolapse in the UK reported that women in the self-management pathway reported higher levels of convenience, ability to access help, support and comfort than women attending clinics for pessary changes. This supports the idea that the gains seen through self-management in other conditions might be possible for women who use a pessary for prolapse, but we need robust evidence before this can be implemented in practice.
The TOPSY study aims to evaluate self-management in a randomised control trial to provide high-quality evidence on the effect of self-management on the quality of life of women using pessaries.
Guide to teaching the TOPSY pessary self-management intervention for women with prolapse
There are three parts to the TOPSY self-management intervention: a teaching appointment between a health professional and the woman; a follow-up phone call by the health professional to the woman and a telephone helpline. A TOPSY intervention training checklist should be completed (by whoever is delivering the training) to make sure all aspects of teaching have been covered. At each teaching appointment, give the woman the self-management patient information leaflet and the IUGA leaflet on prolapse at the start of the session and refer to these in the points listed below.
Appointment to teach self-management (teaching appointment)
The following should be discussed with the woman prior to teaching self-management technique:
Anatomy of the vagina, pelvis, their prolapse and how a pessary supports this
-
Use a diagram showing a pessary in situ. (Use IUGA leaflet to and give this to the woman to take home: www.iuga.org/?page=patientleaflets.)
Discussion about prolapse
-
Discuss that prolapse is a very common condition and up to 40% of women will experience prolapse symptoms. Also discuss that many women choose to use a pessary to manage their symptoms as an alternative to surgery. Again refer to IUGA leaflet.
Address common concerns
-
Inserting pessary incorrectly – explain that the pessary cannot go anywhere other than outside of the vagina, and therefore women do not need to be fearful of putting it somewhere it shouldn’t be.
-
Hygiene – advise the woman to wash and dry her hands before and after inserting or removing a pessary. The pessary can be cleaned with warm water and a mild, non-perfumed soap. A pessary can be worn through a menstrual period. It is also safe for women to use a tampon/moon-cup with a pessary in place.
-
Sexual intercourse – advise that the woman can have sex with a support pessary in place or she can remove it if she prefers.
-
The pessary can be removed and inserted as often as she wishes but it must be removed, cleaned and reinserted at least once every 6 months.
-
Some women choose to wear a pessary continuously whereas other women insert it when they feel it is needed, for example exercising, doing housework, going out for the day, certain times of the menstrual cycle.
Demonstration
-
Show how a ring pessary is compressed into a figure of 8 (vinyl pessary) or an oval (silicone pessary).
-
Provide the woman with a pessary to handle throughout self-management teaching.
-
Explain that the compressed pessary should be inserted into the vagina using the dominant hand, and once inserted the index finger on the other hand should push the pessary into the vagina towards the direction of the coccyx.
-
Refer to the pictures in the self-management participant information leaflet. Circle the picture of the pessary the woman is using and cross out the pictures in the participant information leaflet of the non-relevant pessaries.
Lubrication
Advise the woman to ensure that they use adequate lubrication when inserting the pessary. Any vaginal lubricant can be used, or if the woman has previously been prescribed an oestrogen cream, this can be used instead. When inserting the pessary the lubrication should coat the leading edge of the pessary. When removing the pessary the woman may lubricate their finger prior to insertion into the vagina if she finds this more comfortable.
Positioning
Discuss different positioning which can be used for pessary insertion/removal; these include:
-
standing with legs apart
-
standing with one leg raised (e.g. on the side of a bath or chair)
-
lying on the back
-
lying on the side
-
squatting
-
in a warm shower
-
insertion
Advise the woman to part the labia with her non-dominant hand and insert the pessary with her dominant hand as explained above. Once inside, the pessary can be pushed up further if necessary and that the aim is to position it behind the pubic bone. When it is in the correct position the woman should not feel any discomfort.
Removal
Advise the woman to insert a finger of the dominant hand into the vagina until she feels the edge of the pessary. Advise her to hook the forefinger over the edge of the pessary and pull it down slowly but firmly until it is removed from the vagina.
Cleaning the pessary
Advise to clean the pessary with warm water and a non-perfumed soap after removal and dry with a clean cloth or paper towel. Explain that using perfumed or strong cleaning products on the pessary may affect the natural bacteria within the vagina or damage the pessary. Advise that it is important to rinse thoroughly any soap used.
Pessary storage
Advise that the pessary can be stored in any clean and dry container with a lid but must be kept at room temperature and should not be boiled or sterilised.
Discolouration/damage to the pessary
Advise that it is common for pessaries to become discoloured by vaginal secretions, particularly if the woman is still menstruating. Discolouration of the pessary is not a problem; however, if the pessary is visibly damaged with cracks or no longer holds its shape it should be replaced.
Discharge/odour
Advise that it is common to experience mild discharge or odour with a pessary in situ. Explain that the discharge and odour will most likely be the vagina’s reaction to the pessary. Inform the woman that removing and leaving the pessary out for a few days may help the discharge to clear up. If on reinsertion the discharge continues to be bothersome or if it is blood stained, she should contact the telephone helpline number.
Replacement of pessary
Ensure the woman knows how to obtain a new pessary when required.
Pregnancy
Inform the woman that if she becomes pregnant during the study, her involvement in the study would stop. If she becomes pregnant, she should phone the centre telephone number. Pessary care would then continue according to local centre practice for pregnant women.
What to do in case of problems
Give the woman the centre-specific advice helpline telephone number to call if they have any concerns or problems with their pessary. They must also call this number if they become pregnant during the study.
Make sure they know the times this helpline is open (working hours etc.) and emphasise that it is not 24 hours and what they should do in an emergency. Discuss common pessary-related problems such as the pessary continually falling out, difficulty voiding or opening bowels, discomfort, bleeding, bothersome discharge and odour. All this is in the self-management patient information leaflet.
Resources for the women
Emphasise that the women can take the following home and use as information guides:
-
Intervention participant information leaflet
-
IUGA leaflet on prolapse.
Discuss a suitable day/time to call in a few weeks to complete the follow-up call (in particular, ask if the woman is going to be contactable during the day: working commitments and also ask about holidays, etc).
Removal and insertion practice
Having had the above discussion observe the woman insert and remove her pessary giving verbal instruction if required.
If the woman is unable to do this, discuss the perceived difficulties and ways to address these. Ask her to practise over the following week at home.
Once a woman is successful in removing and replacing her pessary ask her to remove and reinsert at least once over the following 2 weeks at home.
Follow-up phone call
The purpose of the follow-up phone call is to find out how the woman is getting on with self-managing her pessary and to check that she has removed it and replaced it at least once.
-
Contact the woman by telephone approximately 2 weeks after the appointment to teach self-management.
-
Check that the woman has managed to remove, clean and replace their pessary at least once since her appointment and record this in the telephone call CRF.
-
Check that the woman did not have any issues removing or replacing her pessary.
-
Check that the woman does not have any complications, for example problems opening her bowels or passing urine, bleeding, pain or discomfort.
-
Check whether the woman has any questions at this point.
-
Ensure the woman has the appropriate clinic telephone number to call in case of any problems.
-
Remind the woman to remove, clean and reinsert the pessary at least every 6 months.
Helpline
The purpose of the centre specific advice helpline is to offer a forum for self-managing women to report any concerns they have. All women will have the self-management information leaflet that tells them the symptoms that they ought to report.
If the woman contacts the helpline with any problems, document the problem they are reporting and either provide reassurance over the phone if clinically appropriate or arrange an appointment for her to attend and be assessed within a suitable timeframe depending on the issue as per standard clinical care.
Other resources
-
TOPSY information leaflets
-
Video (www.cuh.nhs.uk/our-services/gynaecology/urogynaecology/pessary-self-management-video/)
-
the trial team.
Further reading
-
Bodenheimer T, MacGregor K, Shafiri C. Helping Patients Manage Their Chronic Conditions. Sacramento, CA: California Healthcare Association; 2005.
-
De Iongh A, Fagan P, Fenner J, Kidd L. A Practical Guide to Self-management Support. The Health Foundation; 2015. URL: www.health.org.uk/publications/a-practical-guide-to-self-management-support (accessed 30 January 2018).
-
Lorig KR, Holman HR. Self management education: history, definition, outcomes and mechanisms. Ann Behav Med 2003:26:1–7.
-
Kearney R, Brown C. Self-management of vaginal pessaries for pelvic organ prolapse. BMJ Qual Improvement Reports 2014;U206180.w2533.
-
Lone F, Thakar R, Sultan AH, Karamalis G. A 5-year prospective study of vaginal pessary use for pelvic organ prolapse. Int J Gynaecol Obstet 2011;114:56–9.
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level
- GP
- general practitioner
- HCP
- healthcare professional
- ICER
- incremental cost-effectiveness ratio
- INMB
- incremental net monetary benefit
- ITT
- intention to treat
- PFDI-20
- Pelvic Floor Distress Inventory-20
- PFIQ-7
- Pelvic Floor Impact Questionnaire-7
- PISQ-IR
- Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire, IUGA-Revised
- PMG
- Project Management Group
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RUQ
- Resource Use Questionnaire
- SAE
- serious adverse event
- SD
- standard deviation
- TOPSY
- treatment of prolapse with self-care pessary
- TSC
- Trial Steering Committee
