Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/31/53. The contractual start date was in January 2018. The draft report began editorial review in July 2021 and was accepted for publication in March 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Thomson et al. This work was produced by Thomson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Thomson et al.
Chapter 1 Introduction
Scientific background
Anatomy
The cervical (neck) spine consists of seven vertebral bones, termed C1–C7, that are connected to each other by ligaments, the intervertebral discs and the facet joints. Vertebrae have a large vertebral body positioned ventrally (anterior) and in the midline with bilateral lateral masses positioned dorsally (posterior). The pedicles connect the vertebral body to the lateral masses and the laminae connect the lateral masses to the midline and dorsal spinous process. The intervertebral disc is positioned between the vertebral bodies, and the facet joints are positioned between the lateral masses. There are multiple ligaments, the most important of which, in the pathophysiology of brachialgia, is the ligamentum flavum, which is elastic and runs between adjacent laminae.
The spinal cord is positioned within the cervical bones in the spinal canal and gives rise to eight pairs of nerves, one pair for every spinal level. The first section of each nerve is called the nerve root, and it exits from the spinal canal via the nerve root foramen (Figure 1). Each nerve exits above its equivalently named vertebra, with the exception of the C8 nerve, which exits below the C7 vertebra and above the first thoracic vertebra. The nerve exits at a 40–45° angle to the coronal plane and at a 10° angle to the axial plane. 1
FIGURE 1.
An oblique view of a model of the left side of the lower cervical spine to demonstrate the margins of the C5/C6 nerve root foramen. The ligamentum flavum is medial to the FJ and not shown. D, C5/6 interarticular disc; FJ, facet joint; N, C6 nerve root; P, C5 pedicle; VB, C5 vertebral body.

The nerve root foramen is bounded ventrally (anteromedial) by the intervertebral disc, cranially (superior) by the pedicle of the vertebra above, dorsally (posterolateral) by the facet joint and ligamentum flavum and caudally (inferior) by the pedicle of the vertebra below. The nerve root foramen is widest at the cranial end and tapers at the caudal end. The nerve root is normally positioned at the widest section towards the cranial end of the foramen.
Degenerative disease
As people age, degenerative disease affects the intervertebral disc, facet joint and ligamentum flavum; consequently, the nerve root foramen may change shape and the nerve root becomes compressed. Most commonly, the intervertebral joint will develop bony growths called osteophytes that cause ventral compression of the nerve root; alternatively, the facet joint or ligamentum flavum may hypertrophy, causing dorsal compression of the nerve root.
The rate at which degenerative disease develops is related to the amount of movement, especially flexion, which occurs at a joint. In the cervical spine, the C5/C6 and C6/C7 joints flex more than any others;2 consequently, these are the most common locations for cervical degenerative disease. The nerve that exits between C5 and C6 is the C6 nerve, and the nerve that exits between C6 and C7 is the C7 nerve; therefore, these are the most commonly affected nerves.
Symptoms and signs of cervical radiculopathy
Cervical nerve root compression at the nerve root foramen causes a syndrome called cervical radiculopathy. Cervical radiculopathy is characterised by the following:
-
neck pain
-
brachialgia – the symptom of pain coming from the neck and radiating into the upper arm
-
symptoms and signs related to nerve dysfunction, which include sensory symptoms (e.g. pain, pins and needles, and sensory loss), or, less commonly, motor symptoms (weakness, muscle loss and diminished reflexes). These symptoms and signs are in the distribution of the nerve affected (Table 1).
| Bone level | Nerve root | Sensory | Motor |
|---|---|---|---|
| C5/C6 | C6 | Thumb and index finger | Elbow flexion |
| Forearm supination | |||
| C6/C7 | C7 | Middle finger | Elbow extension |
Brachialgia incidence
The reported incidence of cervical brachialgia is 1.79 cases per 1000 per year,3 with > 110,000 cases of brachialgia annually in the UK.
Cervical brachialgia typically affects people aged 40–60 years, with up to 15% of patients unable to work owing to the pain. 4 In a large registry study (1809 patients), patients had significantly worse scores than the general population in all eight Short Form questionnaire-36 items quality-of-life dimensions. 5
Natural history and non-surgical management
In most patients, brachialgia is self-resolving with conservative management including analgesia and physiotherapy. 6 Foraminal injections may also be used to provide analgesia. Surgery is not normally indicated in the first 6 weeks of treatment; however, 26% of patients will undergo surgery if their symptoms are persistent or remain debilitating after at least 6 weeks. 6
Role of surgery in the treatment of cervical radiculopathy
Among patients for whom symptoms persist 6 weeks to 3 months after onset, randomised controlled trials (RCTs) have shown that surgery results in a more rapid recovery than further conservative management, with significantly better pain intensity, muscle weakness and sensory loss at 4 months. 7,8 However, by 12 months after the surgery, the difference between the surgical and the non-surgical groups is smaller,9 and some studies have shown no difference. 7 The loss of benefit is due to both ongoing improvement of the conservative group and late recurrence of symptoms in surgical patients. 7,10 Careful patient selection and informed consent are therefore critically important.
Surgical treatment of cervical radiculopathy is targeted at decompressing the nerve as it passes through the nerve root foramen and, in anterior cervical discectomy (ACD), at fusing the joint so that further degenerative disease cannot occur at this point. Operations may be performed using an anterior approach (ACD or anterior cervical arthroplasty) or a posterior approach [posterior cervical foraminotomy (PCF)].
Anterior cervical discectomy
The most common procedure performed for cervical radiculopathy is ACD. 11–13 The approach is from the front of the neck, passing between the carotid artery laterally and the larynx and pharynx medially. The recurrent laryngeal nerve, vertebral artery and sympathetic plexus are at risk. The intervertebral disc and pathological osteophytes are removed to decompress the nerve root. The disc space may be filled with bone or an implant, or left unfilled. Some surgeons will apply a plate to the front of the vertebra, an addition that changes the name of the procedure to ACD and fusion. It should be noted, however, that bony fusion occurs between the vertebrae whether or not a plate is used.
The procedure is effective, but there is a high incidence of significant, potentially permanent, complications, including dysphagia (swallowing difficulty) (9.5%) and hoarse voice (3.1%),14 which can be devastating. Degenerative disease may occur at adjacent cervical spinal levels, necessitating further surgery (25.6% risk in 10 years). 15 There is an age-dependent effect on health-related quality of life (HRQoL), with younger patients of working age most severely affected. 5
Posterior cervical foraminotomy
Posterior cervical foraminotomy is undertaken from the back of the neck. The approach is either through the muscle (minimal access) or between the muscle and the spinous process of the vertebra. The facet joint is exposed, and the bone and ligament over the nerve is then removed.
Posterior cervical foraminotomy avoids risk to the structures in front of the spine including the carotid artery, sympathetic trunk, recurrent laryngeal nerve, larynx and pharynx, but may result in high levels of postoperative neck pain16,17 and higher reoperation rates. 18–21
Other operations for cervical brachialgia
Anterior cervical arthroplasty is performed in the same way as ACD. At the end of the procedure, an artificial disc is inserted to maintain movement of the joint. The technology has been available for many years but is not often used by surgeons in the UK to treat cervical radiculopathy.
Anterior cervical foraminotomy is performed in the same way as ACD except that only one side of the disc is removed and no implant is inserted. The operation is intended to preserve motion at the operated level, but the vertebral artery may be placed at more risk. The operation is not commonly performed in the UK.
Choice of operation
There are two published scales for assessing the degree of compression on a magnetic resonance imaging (MRI) scan. Park et al. 22 used non-standard oblique sagittal images to measure stenosis in the root canal, whereas Kim et al. 23 used axial slices. Neither method has been assessed with regard to choice of surgical procedure or surgical outcome. Similarly, it is unclear whether or not the position, length or severity of root compression affects whether an anterior or posterior approach should be used. The choice of surgical technique is therefore frequently left to surgeon preference or familiarity.
Scientific rationale
Anterior cervical discectomy and PCF are the most common and second most common operations used to treat cervical brachialgia, respectively. 24 This trial aimed to compare the clinical benefits, cost effectiveness and safety of the two operations.
We performed a systematic review by searching PubMed and EMBASE for all studies published on this topic. Studies were included if they met the following criteria: (1) study design – prospective or retrospective comparative studies; (2) patients with brachialgia due to a lateral disc herniation or foraminal stenosis; (3) clinical outcomes, radiological outcomes, complications, reoperation rates and cost-effectiveness differences were compared between ACD and PCF; and (4) published in English. Studies on tumours, trauma, infection, previous surgeries, revision surgeries, combined anterior and posterior surgeries, and other posterior approaches were excluded. Non-English-language studies were also excluded. The systematic review was last updated on 13 March 2021.
Efficacy
The efficacy of both ACD and PCF in the management of cervical brachialgia is well established.
Matz et al. 25 conducted a systematic review of 13 retrospective and three prospective studies on the surgical outcomes of ACD for cervical brachialgia. They concluded that ACD provides rapid relief (within 3 to 4 months) from arm and neck pain, weakness and/or sensory loss. Improvement in motor function at 12 months was also noted.
Heary et al. 26 conducted a systematic review of 13 studies investigating the surgical outcomes from PCF. Although the studies identified were observational, retrospective and often lacked validated outcome measures, they concluded that PCF is an effective treatment for cervical brachialgia.
Randomised controlled trials
Three RCTs comparing the two techniques directly have been published.
Ruetten et al. 27 conducted a single-centre RCT comparing ACD with minimal access, endoscopic PCF. The authors compared the outcome of 175 participants and found no statistically significant difference between treatment arms. The outcome measures used were the visual analogue scale (VAS) arm pain score, the German version of the North American Spine Society (NASS) Instrument and Hilibrand criteria. The mean VAS scores at 12 months for ACD and PCF were 7 and 8, respectively. The mean NASS Instrument scores were 1.7 and 1.8 for ACD and PCF, respectively. The proportions that reported excellent results based on the Hilibrand criteria were 78% and 80% for ACD and PCF, respectively. This study has important limitations. Standard deviation (SD) is not reported, and the NASS Instrument has been shown to have poor reliability and validity in assessing outcomes of brachialgia patients. 28 In the UK, most PCF procedures are performed using the standard open technique, and the study included patients who have had symptoms for only 5 days, whereas, in the UK, it is standard practice based on clinical evidence6,7 to recommend surgery following at least a 6-week period of conservative management.
Wirth et al. 29 conducted a three-arm RCT comparing PCF, ACD with fusion and ACD without fusion (this was a small underpowered trial recruiting 14 participants to each arm). The proportions of participants reporting complete or partial pain relief (100% vs. 100% vs. 96% for PCF, ACD with fusion and ACD without fusion, respectively), requirement for analgesia (15.9 vs. 13.0 vs. 12.5, respectively), median operative time (139 vs. 98 vs. 120 minutes, respectively) and median length of hospital stay (4.3 vs. 3.9 vs. 4.5 days, respectively) were similar in all three groups. All PCF participants and 96% (13/14) of the ACD participants reported partial or complete relief of radicular pain.
Herkowitz et al. 30 compared outcomes in 28 ACD and 16 PCF patients, with a mean follow-up of 4.2 years, using Odom’s criteria. Good/excellent results were obtained in 95% (26/28) of ACD and 75% (12/16) of PCF patients. They concluded that ACD was the treatment of choice.
Three further RCTs comparing ACD with PCF in the management of cervical radiculopathy are ongoing and yet to report as follows:
-
Foraminotomy ACDF Cost-Effectiveness Trial (FACET) – a Dutch study designed as a non-inferiority trial comparing ACDF with PCF in 308 participants and assessing clinical and cost–benefit outcomes. The trial protocol was published in 2017. 31 The results from this trial have not yet been reported.
-
ForaC trial – a German trial protocol comparing ACDF with PCF in 88 participants and using Neck Disability Index (NDI) as the primary outcome measure was published in trials in 2014. 32 The results from this trial have not yet been reported.
-
A Swedish trial comparing ACD with PCF in the treatment of cervical radiculopathy in 110 participants and using NDI as the primary outcome measure was registered in November 2019. 33 The full protocol was published in 2021. 34 The outcomes have not yet been published; the expected completion date of the trial is January 2026.
Non-randomised studies
There are also several non-randomised studies that have directly compared the two operative techniques.
Korinth et al. 35 compared 124 ACD patients with 168 PCF patients, over a mean follow-up of 6 years (SD 25.9 months), using Odom’s criteria. This non-randomised retrospective study found that 93.6% of ACD and 85.1% of PCF patients had excellent/good outcomes (p < 0.05).
Tumialán et al. 36 compared the costs and efficacy of managing unilateral cervical brachialgia with PCF or ACD and fusion in 38 (19 per arm) American military personnel. The primary outcome measure was the time to return to active duty. PCF patients returned to unrestricted activity faster (a mean of 14.8 weeks faster than ACD patients; p < 0.001).
Selvanathan et al. 13 compared 150 ACD operations with 51 PCF operations in a retrospective study, using the NDI as the primary outcome measure. PCF demonstrated a non-significant mean improvement in NDI score of 21.9 units, compared with ACD (mean improvement of 11.9 units).
Alvin et al. 37 compared clinical outcome using VAS pain scores following ACD (n = 45) or PCF (n = 25) to treat patients with brachialgia. The authors found no difference between the treatment arms in the VAS score, the EuroQol-5 Dimensions (EQ-5D) score or the pain disability questionnaires scores (p = 0.40, p = 0.60 and p = 0.50, respectively).
Scholz et al. 19 retrospectively examined the outcomes of 107 patients who had been treated with ACD or PCF in a single institution. Odom’s criteria, VAS, NDI and subjective satisfaction score were compared. PCF demonstrated better overall outcome (Odom’s criteria), and greater relief of neck and radicular pain. Operative time was less in the PCF group but reoperations were more common.
Mok et al. 12 retrospectively studied 1102 patients in an American national surgical database who had been treated with ACD, PCF or cervical arthroplasty. PCF had the shortest mean operating time [90.72 (SD 43.78) vs. 105.9 (SD 52.12) minutes for ACD] and length of stay [0.86 (SD 1.12) vs. 1.1 (SD 0.90) days for ACD], but the clinical outcomes, including surgical site infection, pneumonia, reintubation, pulmonary embolism, deep-vein thrombosis, re-admissions and reoperations, showed no differences.
Lin et al. 20 retrospectively identified patients treated with ACD (n = 55), PCF (n = 21) or cervical arthroplasty (n = 21). They found all procedures to be equally effective in improving neck disability. The mean 12-month postoperative NDI score was 9.9 (SD 5.1) for ACD and 10.1 (SD 4.4) for PCF. The mean 12-month postoperative VAS arm pain score was 1.5 (SD 0.9) for ACD and 1.6 (SD 1.1) for PCF, and the mean 12-month postoperative VAS neck pain score was 1.4 (SD 1.1) for ACD and 1.2 (SD 1.4) for PCF. The reoperation rate was lowest with ACD (0% vs. 14.3% for ACD and PCF, respectively).
Foster et al. 38 retrospectively reviewed patient-reported outcomes on the Core Outcome Measures Index (COMI) neck questionnaire from 634 ACD and 54 PCF operations from a single UK centre. Both procedures were associated with an improvement in COMI score at 3 and 12 months. The mean pre-operative, 3-month and 12-month COMI scores for ACD were 7.46, 5.15 and 4.53, respectively; the mean pre-operative, 3-month and 12-month COMI scores for PCF were 7.34, 4.94 and 4.3, respectively. The mean pre-operative, 3-month and 12-month VAS arm pain scores for ACD were 7.02, 4.20 and 4.06, respectively; the mean pre-operative, 3-month and 12-month VAS arm pain scores for PCF were 6.86, 3.82 and 4.07, respectively. There were no significant differences between ACD and PCF in intraoperative complications, postoperative complications or length of stay, but operation times were shorter for PCF, with 52% performed in < 1 hour, compared with 23% for ACD.
Dunn et al. 39 retrospectively studied 49 PCF operations and 210 ACD operations from a single American institution using neck disability and VAS scores. The mean follow-up was 42.9 (SD 6.6) and 44.9 (10.3) weeks. The mean pre-operative NDI score for ACD was 35.6 (SD 17.6); at final outcome, this had dropped to 9.7 (SD 4.8). The mean pre-operative NDI score for PCF was 34.2 (SD 13.3); at final outcome, this had dropped to 9.6 (SD 3.9). The mean pre-operative VAS neck pain score for ACD was 6.8 (SD 3.9); at final outcome, this had dropped to 1.4 (SD 0.8). The mean pre-operative VAS neck pain score for PCF was 6.3 (SD 3.4); at final outcome, this had dropped to 1.2 (SD 0.6). The mean pre-operative VAS arm pain score for ACD was 5.9 (SD 3.8); at final outcome, this had dropped to 0.6 (SD 0.3). The mean pre-operative VAS arm pain score for PCF was 5.8 (SD 3.6); at final outcome, this had dropped to 0.4 (SD 0.3).
Lubelski et al. 18 compared reoperation rates within 2 years of ACD and PCF in 627 and 163 patients, respectively. Reoperation rates at the index level were 4.8% for the ACD group and 6.4% for the PCF group within 2 years of the initial surgery (p = 0.7).
Meta-analyses and reviews
In a meta-analysis, Fang et al. 21 compared ACD with PCF. They identified 15 studies: three RCTs27,29,30 and 12 non-randomised studies. 11–13,19,20,35–41
Fang et al. 21 found that postoperative NDI score was reported in three studies;19,20,39 the results were comparable [p = 0.61, weighted mean difference (WMD) 0.28, 95% confidence interval (CI) 0.79 to 1.34; I2 = 16%]. The VAS score for neck pain was reported in two studies,20,39 with no significant difference between the two groups (p = 0.11, WMD 0.15, 95% CI –0.03 to 0.34; I2 = 44%). The VAS score for arm pain was reported satisfactorily in four studies,19,20,37,40 with no significant difference between the two groups (p = 0.11, WMD 0.61, 95% CI –0.14 to 1.35; I2 = 0%).
The reoperation rate was reported in nine studies. 13,19,20,27,29,35,37,39,41 There was a significant difference: the ACD group had lower reoperation rates (p = 0.02, odds ratio 0.54, 95% CI 0.33 to 0.9; I2 = 0%).
The operation time of ACD and open PCF was reported in six studies. 12,19,29,35,36,40 There was a significant difference: the PCF group had shorter operating times (p = 0.001, WMD 12.8 minutes, 95% CI 4.91 to 20.68 minutes; I2 = 65%). Minimal-access PCF, however, had comparable operating times, based on data from a single study. 27
The length of stay was reported in seven studies. 11,12,20,29,35,38,41 There was a significant difference: the PCF group had a shorter length of stay (p = 0.002, WMD 0.28 days, 95% CI 0.23 to 0.34 days; I2 = 24%).
The cost was reported in three studies. 12,36,42 There was a significant difference: PCF is cheaper (p = 0.002, WMD £7063.89, 95% CI £1468.19 to £12,659.60; I2 = 99%).
There were no significant differences in patient satisfaction, complications or intraoperative blood loss.
Several systematic reviews24–26 and a review article on cervical brachialgia in the New England Journal of Medicine6 have all called for a large prospective RCT to compare these two procedures.
Safety
Most authors have reported fewer surgical complications with PCF than with ACD,11,13,35,37,43 although some authors have found comparable complication rates. 12,19,21,38
Korinth et al. 35 found that surgery-related complications (dysphagia, hoarse voice, transient neurological deficit and postoperative haematoma) were observed in 6.5% of ACD patients, compared with 1.8% of PCF patients (cerebrospinal fluid fistula, wound infection and transient neurological deficit) (p < 0.05).
Witiw et al. 11 retrospectively analysed American insurance data; 4851 PCF and 46,147 ACD procedures were included. The reported complications for PCF and ACD were death (0% and 0.01%, respectively), vascular injury (0% and 0.02%, respectively), dysphagia and hoarse voice (0.17% and 1.62% for PCF and ACD, respectively), cerebrospinal fluid (0% and 0.02%, respectively) and deep-vein thrombosis (0.06% and 0.14%, respectively). Wound infections were more common with PCF (2.05% and 0.57%, respectively). The mean length of stay was shorter with PCF [1.47 (SD 1.39) vs. 1.23 (SD 1.06) days].
Degenerative disease occurring at the adjacent spinal level following ACD occurs at a rate of 2.9% per annum; this may be a consequence of the fusion that occurs with this operation. 15 PCF maintains movement,20,39,40 and may therefore reduce the incidence of degenerative disease occurring at adjacent segments.
Health economics
Tumialán et al. 36 found that both ACD and PCF procedures had similar median operating times (151.8 vs. 154.0 minutes), median blood loss (32.6 ml vs. 41.3 ml) and analgesic use, but the mean direct cost of PCF was US$20,094–30,553 lower per case than for ACD.
Alvin et al. 37 performed a retrospective 1-year cost–utility analysis on 45 patients to determine the cost effectiveness of ACD in comparison with PCF for patients with single-level cervical radiculopathy. The authors found PCF to be less costly (US$12,777 vs. US$18,473) and more cost-effective [0.16 vs. 0.14 increase in quality-adjusted life-years (QALYs) over 1 year] than ACD.
Witiw et al. 11 analysed American insurance data for 4851 PCF and 46,147 ACD procedures, and found that the mean PCF cost was US$15,281 whereas the mean ACD cost was US$26,849 (p < 0.001).
Mansfield et al. 41 conducted an American retrospective cohort study and calculated the mean direct cost of PCF and of ACD among 101 patients with cervical radiculopathy. The mean cost of ACD was US$8192; the mean cost of PCF was US$4320.
In our UK institution (Leeds Teaching Hospitals NHS Trust), the average cost of PCF is £4200 and the average cost of ACD is £5380. This is mostly because of the cost of the implant used in an ACD. The average cost of PCF is, therefore, lower, and its widespread implementation could lead to significant savings for the NHS. However, there is no published economic study of these surgical treatments from a UK NHS perspective.
Summary
The posterior cervical FORaminotomy Versus Anterior cervical Discectomy in the treatment of cervical brachialgia (FORVAD) trial aimed to evaluate the clinical effectiveness and cost effectiveness of PCF compared with ACD in the treatment of patients with cervical brachialgia. However, in May 2020, following a review of the internal pilot phase data, the funder made the decision to close down the FORVAD trial, owing to the trial being unlikely to recruit to target on time. Although the results presented in this report are not sufficiently powered to address the original aims, we expect that the controlled data will inform the emerging evidence base and can be included in future meta-analyses.
There is still a need for a high-quality prospective clinical trial comparing the clinical outcomes, complications and costs of ACD with those of PCF in the management of cervical radiculopathy. In addition, the qualitative work performed will help to inform the design of such a trial.
Chapter 2 Trial design and methods
Aims and objectives
The aim of the FORVAD trial was to determine the clinical effectiveness and cost effectiveness of PCF, compared with ACD, in the treatment of patients with cervical brachialgia.
Primary objective
The primary objective was to determine whether or not PCF is superior to ACD in terms of improving clinical outcome as measured by the NDI at 52 weeks post operation.
Secondary objectives
The secondary objectives were to compare PCF and ACD in terms of:
-
NDI scores over 52 weeks post operation
-
neck and upper-limb pain, including the shoulder, arm and hand, over 52 weeks post operation
-
dysphagia (difficulty swallowing) and globus (sensation of a lump in the throat) over 52 weeks post operation, as assessed by the participant
-
hoarse voice over 52 weeks post operation, as assessed by the participant, and at 6 weeks post operation for a randomly selected subset of participants, as assessed by expert review
-
extent and severity of a patient’s spinal cord functional impairment, including upper-limb nerve root function, at day 1 and at 6 weeks post operation
-
incidence of revision surgery over 52 weeks post operation
-
incidence of surgical complications up to 6 weeks post operation
-
cost effectiveness over 52 weeks post operation.
Exploratory objectives
The exploratory objectives were to explore the impact of variations in the optional surgical components of PCF and ACD on the NDI and EuroQol-5 Dimensions, three-level version (EQ-5D-3L) scores. The types of variation in the optional surgical components are as follows:
-
ACD – fusion with a cervical plate in addition to ACD, versus simple ACD without a plate
-
PCF – posterior cervical minimal-access technique versus open-access technique.
Trial design
The FORVAD trial was a UK multicentre, Phase III, parallel-group, superiority, individually RCT of patients with symptomatic unilateral cervical brachialgia for at least 6 weeks, with confirmed nerve root compression on MRI or computerised tomography (CT) myelogram. The FORVAD trial was not blinded to participants, medical staff or clinical trial staff, as blinding was infeasible owing to substantial differences between the two surgical interventions. The trial incorporated an internal pilot phase to assess feasibility of trial delivery (see Internal pilot phase).
It was originally intended that a total of 252 participants would be randomised. Participants were randomised on the day of surgery to receive either PCF or ACD on a 1 : 1 basis. Full descriptions of the trial interventions can be found in Interventions. A registration phase was incorporated into the trial design whereby participants could be registered up to 28 days prior to their planned surgery date. This was to allow sufficient time for the trial baseline assessments to be conducted ahead of the participant’s operation. Participants were followed up in clinic at day 1 and at 6 weeks post operation, and continued to be followed up by post at 12, 26, 39 and 52 weeks post operation.
The FORVAD trial received national ethics approval in the UK from the North West–Greater Manchester Central Research Ethics Committee (reference number 18/NW/0682), and was overseen by an independent Trial Steering Committee (TSC) and a Data Monitoring and Ethics Committee (DMEC). The trial also included patient and public involvement (PPI) throughout its duration, from the initial stages of trial design and development of the protocol through to the analysis and dissemination of results. Full details of PPI in the trial can be found in Patient and public involvement statement.
The FORVAD trial was prospectively registered on the International Standard Randomised Controlled Trial Number (ISRCTN) register (reference number 10133661).
Internal pilot phase
A 12-month internal pilot phase evaluated the feasibility of recruitment within the planned timelines, based on the number of actively recruiting sites and overall average recruitment rate. The pilot also aimed to assess early safety data, data validity, compliance with trial procedures, compliance and completeness of patient-completed quality-of-life questionnaires, eligibility of the clinical teams and subtype of procedure performed (e.g. minimal-access or open-surgery PCF).
At the end of the internal pilot phase, trial progress was reviewed by the trial funder against predefined progression criteria (see Appendix 1) to determine whether or not the trial could proceed in full.
Patient and public involvement statement
Six members of the public, including four postoperative patients, provided feedback on the proposed trial design at the grant application stage. The feedback provided was used to inform the selection of appropriate patient-centred quality-of-life outcomes, resulted in improvements to the proposed patient information sheet and informed appropriate follow-up procedure for data collection.
A PPI representative was a member of the Trial Management Group (TMG) and a second PPI representative was a member of the independent TSC, to provide insight and experience from the patients’ perspective, ensuring that the trial maintained focus on service users and needs. Their key roles involved input into patient information documentation, including drafting and reviewing information leaflets and other patient resources to ensure that patient information was meaningful and clear.
Early trial closure
In May 2020, following a review of the internal pilot phase, the funder made the decision to close down the FORVAD trial, owing to the trial’s slow recruitment rates. The funder’s decision was based on pre-COVID-19 recruitment rates, but it coincided with the first COVID-19 wave in the UK and the TMG considered that accelerated recruitment of elective surgery cases during the COVID-19 pandemic would have been impossible. All participating sites were notified of the decision to close the trial and recruitment to the FORVAD trial formally ceased on 10 June 2020.
The trial team developed a close-down plan, which was ratified by the TSC and DMEC, and approved by the sponsor and funder. The close-down plan consisted of two main elements:
-
follow-up of all randomised participants to continue until the end of the 12-month postoperative follow-up period, as originally planned
-
the introduction of a qualitative substudy to be undertaken during the trial close-down period to identify site-level feedback and the reasons why the FORVAD trial struggled to recruit (see Chapter 5).
Summary of protocol changes
Protocol changes made during the trial are summarised in Table 2.
| Version and date | Summary of changes |
|---|---|
| V1.0, dated 23 August 2018 (never approved for use) |
|
| V2.0, dated 2 November 2018 |
|
| V3.0, dated 15 January 2021 |
|
|
|
|
|
|
Participants
Eligibility waivers were not permitted in this trial.
Inclusion criteria
The trial inclusion criteria were as follows:
-
age ≥ 18 years
-
diagnosis of unilateral cervical brachialgia as confirmed by MRI or CT myelogram taken within the preceding 12 months
-
symptoms of cervical brachialgia present for at least 6 weeks
-
single-level nerve entrapment
-
posterolateral disc and/or foraminal narrowing
-
failed conservative management (including, but not limited to, medication, physiotherapy and modification of daily activities)
-
able and willing to comply with the terms of the protocol, including quality-of-life questionnaires (which were provided in English only; for this reason patients had to be English-speaking)
-
able to provide written informed consent.
Exclusion criteria
The trial exclusion criteria were as follows:
-
cervical disc causing cord compression
-
cervical myelopathy
-
bilateral cervical brachialgia
-
previous cervical spine surgery
-
professionals for whom a hoarse voice would be exceptionally significant (e.g. singers or speakers)
-
skin disease at surgical sites (e.g. eczema)
-
pregnancy
-
cervical deformity
-
not suitable for ACD
-
not suitable for PCF.
Trial setting
The FORVAD trial opened to recruitment at NHS hospital trusts, all of which had to fulfil a set of prespecified criteria and complete a registration form that verified that the research site was willing and able to comply with the trial requirements prior to trial participation.
To be eligible to participate in the trial, research sites had to:
-
be able to perform both ACD and PCF
-
have the capacity to recruit at least 10 participants per year.
Once site eligibility had been confirmed, research sites were required to obtain local management approval, return all essential documentation to the Clinical Trials Research Unit (CTRU) and undertake a site initiation with the CTRU prior to the start of recruitment to the trial.
In addition to site eligibility, participating surgeons were required to have performed a minimum of 10 PCF and 10 ACD operations as the primary surgeon, and to complete a bespoke training package hosted by the e-brain platform (www.ebrain.net).
Interventions
Where components of the described interventions were optional, this is clearly stated.
Pre-operative interventions and preparation
Pre-operative investigations and preparation were as per individual site protocols.
Posterior cervical foraminotomy
Prior to the skin incision
Prior to surgery, clinical assessment and imaging with MRI or cervical myelography were used to identify the location of the affected nerve root and correlate this with the clinical level, and to confirm the absence of cord compression and myelopathy.
Under general anaesthesia, the participant was positioned in a prone position. A MAYFIELD® pin headrest (Integra LifeSciences, Princeton, NJ, USA) was used to secure the head in a flexed position; an alternative headrest that could be used was the Sugita™ head frame (Mizuho Medical Co. Ltd, Tokyo, Japan).
Intraoperative localisation of the spinal level to be operated on was obtained using fluoroscopy prior to an incision being made. This ensured that the incision was correctly placed and not too long. Unless contraindicated, skin preparation was to be done with an alcoholic skin preparation agent; care had to be taken to prevent alcoholic skin preparations from running round into the eyes. Local anaesthetic with adrenaline was used at the incision site.
Incision and exposure
Surgeons could choose to perform a traditional open foraminotomy or use a minimal-access technique, according to their personal preference.
Option A: traditional open foraminotomy
A midline dorsal incision was made overlying the spinal level of interest. The incision was to be kept as short as possible to minimise postoperative neck pain. The incision was deepened until the spinous processes were reached. Subperiosteal dissection was continued unilaterally to expose the spinous processes, lateral masses and laminae above and below the level to be decompressed. A subperiosteal route protected the muscles that can be a source of postoperative pain; excessive use of monopolar diathermy was also to be avoided. Once the laminae and lateral masses had been exposed, fluoroscopy was again employed to confirm the correct level. A cranked retractor system was used to allow surgical access while minimising the size of the wound.
Option B: minimal-access technique
A minimally invasive ‘tube-based’ approach was permitted whereby a 2 cm skin incision was made 2.5 cm lateral to the spinous process with fluoroscopic guidance. Two planar radiographs were to be used for docking the dilators on the lateral mass to avoid the known risk of perforation of the ligamentum flavum with the dilators. The muscle fascia was opened and progressive dilators were directed obliquely under fluoroscopy through the muscle fibres to the facet. After radiographically confirming the two laminae at the level of the pathology, the muscle attachments were coagulated to complete the exposure. The laminae and lateral masses were defined as specified in the standard open approach in the previous section.
Decompression
Bone removal was to begin using small Kerrison punches and/or a high-speed drill to thin the inferior edge of the superior lamina and the superior aspect of the inferior lamina. No more than 50% of the lateral mass was to be removed. Care had to be taken to adequately decompress the nerve root without compromising spinal stability. A thin footplate (usually 1 mm, or maximum 2 mm) upcut punch could be used to dissect the bone off the ligamentum flavum and the nerve underlying it. Instruments with a thick footplate were to be avoided as their insertion may have caused further compression and damaged the nerve. The ligamentum flavum was also removed; the removal of the ligamentum flavum could proceed laterally until the lateral dural sac and the nerve root with its axilla were exposed. Adequate decompression of the neural foramen could be evaluated by very careful palpation using a nerve hook.
Optionally, the nerve root axilla could be explored to expose an osteophyte or soft disc, which could then be removed.
Haemostasis
After decompression, the wound was to be copiously irrigated, followed by meticulous haemostasis. Excessive coagulation of epidural vessels around the nerve root was to be avoided.
Closure
The wound was then closed in layers: first muscles, then fascia, then subcutaneous tissue and, finally, skin. The choice of materials used to close the wound was made according to surgeon preference.
Optionally, a drain could be used, according to surgeon preference.
Anterior cervical discectomy
Prior to skin incision
Prior to surgery, a clinical assessment took place and imaging with MRI or cervical myelography was used to identify the location of the affected nerve root and correlate this with the clinical level, and to confirm the absence of cord compression or myelopathy.
The participant was positioned in a supine position with the neck in extension, and a roll placed behind the scapulae. The shoulders could be depressed using tape for better visualisation of the lower cervical vertebrae on fluoroscopy.
The approach is most commonly performed from the right-hand side, but the left side could be used if the surgeon preferred. The participant was placed in the supine position on a head ring or horseshoe. Depending on the surgeon’s preference, pre-operative traction could be used. If this was not used, intraoperative disc spreading or pin retractors could be used instead.
Pre-operative confirmation of the operative level could be obtained using fluoroscopy to localise the level of the incision. Anatomical landmarks (mandible, hyoid bone, thyroid and cricoid cartilage) could also be used to localise the level of the incision.
Incision
A transverse horizontal incision following a skin crease was made from the medial border of the sternocleidomastoid muscle and approaching the midline.
Exposure
Once the skin incision had been performed, the platysma muscle could be divided horizontally or split vertically. The platysma muscle was elevated at both wound margins and dissection proceeded immediately beneath this muscle.
The approach was on the medial edge of the sternocleidomastoid muscle. This plane was followed to the carotid sheath. Once the carotid artery had been palpated, the trachea/larynx and oesophagus/pharynx were retracted medially. Once this had been performed, the prevertebral fascia became visible and was divided in the midline.
The longus colli muscles overlying the anterolateral edge of the vertebral bodies and discs were then visualised. The affected level thought to be appropriate was selected and verified with fluoroscopy.
Once the correct level had been confirmed, the longus colli muscles were raised bilaterally from the anterior surface of the two vertebral bodies adjacent to the interspace that would be explored. A self-retaining anterior spinal retractor was then inserted underneath the longus colli muscles bilaterally. A window was made into the disc interspace with an 11 blade and extended laterally to the uncovertebral joint.
Resection
An operating microscope was used. The superficial disc material was resected with cervical curettes and rongeurs. For the deeper portion of the discectomy, a high-speed drill was used, especially where there were posterior osteophytes that needed to be removed. Care was to be taken to avoid damaging the bony end plate in the anterior two-thirds of the vertebral bodies. The posterior longitudinal ligament was divided across the entire width of the interspace. The neural foramen was opened to ensure that the nerve root had been decompressed. The medial edge of the nerve root was to be visualised and decompression could be assessed using a blunt hook. The excessive use of bipolar and haemostatic agents that expand was to be avoided.
Insertion of an implant
Once the discectomy and appropriate bony decompression had been completed, the height of the disc space was obtained by measurement with an interbody spacer, and a cage, iliac crest graft or no implant was inserted according to the surgeon’s usual practice. An artificial disc replacement was not permitted. A cage could be packed with some bone matrix or other bone substitute to promote fusion. The choice of cage/spacer or fusion material was at the discretion of the operating surgeon.
Optionally, an anterior cervical plate (made from titanium or resorbable plastic polymer) of adequate length to span the fusion area could be used. The type of plate and screw system used was at the discretion of the surgeon.
Haemostasis and closure
The participant’s carotid pulse was verified and superficial bleeding was controlled with bipolar cauterisation. The platysma and skin were closed. The choice of materials used to close the wound were according to surgeon preference.
Optionally, a surgical drain could be used.
Postoperative care
Postoperative care was as per institutional protocol. Postoperatively, the participant could mobilise immediately as pain allowed. If used, the drain was removed the following day. Unless the participant developed a new neurological deficit, postoperative imaging was not mandatory, but surgeons could elect to perform anterior–posterior and lateral cervical spine radiography if they deemed it necessary. It was anticipated that participants would be discharged the day after physiotherapy review.
Trial procedures
Registration
Following confirmation of written informed consent and eligibility, patients were registered into the trial by authorised members of staff at the trial sites. Registration was required to take place at least 1 day and no more than 28 days prior to the planned surgery date, to allow trial-specific assessments to take place. Registration was performed centrally using the CTRU automated 24-hour telephone randomisation system. Authorisation codes and personal identification numbers (PINs), provided by the CTRU, were required to access the registration system. At the point of registration, participants were allocated a five-digit trial identification number.
Restricted American Spinal Injury Association assessment
All participants were required to undergo a restricted American Spinal Injury Association (ASIA) assessment as part of their trial registration assessments; this assessment was carried out pre operatively, within 28 days of surgery. The patient’s score was based on how much sensation they could feel at multiple points on the body, in addition to tests of motor function, as assessed locally by the examiner. Sensory assessment was restricted to the following regions only: C4, C5, C6, C7, C8, T1, T10, L2, L4 and S1. The sensory assessment was performed twice for each area, once using light-touch sensation and once using pin-prick sensation. Motor function was assessed across all 20 muscles.
Voice-recording sample
A total of 25% of participants were randomly selected at registration to undergo a clinical assessment of hoarse voice using blocked randomisation stratified by centre, with a block size of four. Those participants selected to undergo a hoarse voice assessment had to provide a recording of their voice as part of their registration assessments. Details of the hoarse voice assessment can be found in Appendix 2. Participants were asked whether or not they considered their voice to be ‘normal’ at the time that the recording was performed. If a participant indicated that their voice was not ‘normal,’ the recording was still taken as part of the registration assessments, but an additional recording was taken on the day of surgery.
Day of surgery (day 0)
Randomisation
Participants who had previously been registered, had confirmation of eligibility and had given written informed consent were randomised to the trial by an authorised member of staff at the trial research site on the day of the patient’s surgery. Randomisation was performed centrally using the CTRU automated 24-hour telephone randomisation system. Authorisation codes and PINs, provided by the CTRU, were required to access the randomisation system. Treatment group allocation used a computer-generated minimisation program incorporating a random element of 0.8 and balancing for the following minimisation factors: centre, duration of upper-limb symptoms (6 weeks to just under 6 months, ≥ 6 months to just under 12 months and ≥ 12 months) and smoking status (smoker or non-smoker).
Completion of baseline questionnaire booklet
On the day of surgery, but prior to randomisation, participants were asked to complete a questionnaire booklet containing the NDI, the Eating Assessment Tool-10 items (EAT-10), the Glasgow–Edinburgh Throat Scale (GETS), the Voice Handicap Index-10 items (VHI-10), PainDETECT, Numerical Rating Scale – Neck Pain (NRS-NP), Numerical Rating Scale – Arm Pain (NRS-AP) and the EQ-5D-3L. Further details about the questionnaires used can be found in Outcome measures.
Voice-recording sample
Any participants who were randomly selected at registration to undergo a hoarse voice assessment, and who indicated that they did not consider their voice to be ‘normal’ at the time of the recording taken as part of the registration assessments, were asked to provide a further recording of their voice. The additional recording (if applicable) was required to take place prior to randomisation on the day of surgery. Participants were asked whether or not they considered their voice to be ‘normal’ at the time the recording was performed.
Postoperative follow-up assessments
Participants were reviewed for trial purposes on the ward at day 1 post operation and at 6 weeks in clinic post operation. At both time points, participants underwent the restricted ASIA assessment (described in Restricted American Spinal Injury Association assessment) and completed quality-of-life questionnaire booklets (see Participant-completed questionnaires). In addition, those participants randomly selected at registration to undergo an additional assessment of hoarse voice were asked to provide a recording of their voice at 6 weeks post operation. Participants were not required to indicate whether or not they considered their voice to be ‘normal’ at this time.
Any further clinic visits were according to local standard clinical practice. If participants required any further intervention for brachialgia, as per routine NHS practice, further clinical intervention was permitted. If a participant did receive additional treatment, information on the type of intervention, the details of the treatment received and the reason for the intervention were collected on the trial case report forms.
Participant-completed questionnaires
Participants were asked to complete a number of questionnaires during the course of their involvement in the trial to capture details of their HRQoL and health and social care resource use.
The questionnaires used were as follows:
-
NDI
-
PainDETECT
-
NRS-NP and NRS-AP
-
EAT-10
-
GETS
-
VHI-10
-
EQ-5D-3L
-
health resource use questionnaire.
Participants were asked to complete the questionnaires on the day of surgery (prior to randomisation), at day 1 post operation and at 6, 12, 26, 39 and 52 weeks post operation. The questionnaires were amalgamated into one questionnaire booklet (specific to the follow-up time point) for ease of completion. All of the aforementioned questionnaires were completed at each time point, apart from the health resource use questionnaire, which participants were asked to complete only at 6, 12, 26, 39 and 52 weeks post operation, and the PainDETECT questionnaire, which was completed at each time point except at day 1 post operation.
Further details about each of the questionnaires used in the trial can be found in Secondary outcome measures.
Outcome measures
Primary outcome measure
The primary outcome measure was the NDI44 at 52 weeks post operation. The NDI is a validated45 patient-reported measure consisting of 10 items that assesses different aspects of daily functioning among patients with neck pain. It comprises four items regarding subjective symptoms (pain intensity, headache, concentration and sleeping), four items regarding activities of daily living (lifting, work, driving and recreation) and two items regarding discretionary activities of daily living (personal care and reading). 46 Each item is scored from 0 (best) to 5 (worst) and the total score is expressed either as a raw score (0–50) or rescaled as a percentage score (0–100), with a higher score corresponding to greater disability. In this trial, the primary outcome measure has been expressed as a percentage score. If two or fewer items were missing, the questionnaire was scored using the maximum attainable score as the denominator in the percentage calculation, as per the scoring manual. For example, if 9 out of 10 items were present, the maximum attainable score was 45, instead of 50, but the maximum percentage score remained 100.
Secondary outcome measures
Patient-reported outcomes
-
Neck Disability Index over 52 weeks post operation: the NDI was assessed at day 0 pre randomisation, at day 1, and at 6, 12, 26, 39 and 52 weeks post operation to assess the change in percentage score over time (see Primary outcome measure).
-
The NRS-NP and NRS-AP scores over 52 weeks post operation: NRS-NP and NRS-AP were assessed at day 0 pre randomisation, at day 1, and at 6, 12, 26, 39 and 52 weeks post operation. Both scales are unidimensional 11-step measures of pain intensity, including pain in the cervical and arm areas. It comprises a horizontal line marked in unit integers from 0 to 10 in equidistant intervals, with one end denoting ‘no pain’ (score of 0) and the other ‘worst imaginable pain’ (score of 10). It is self-completed by the respondent who is asked to mark the number on the scale that represents their pain intensity. The score is the number marked by the participant.
-
The EAT-10 swallowing screening tool:47 the EAT-10 was used to assess dysphagia over 52 weeks post operation, and was collected at day 0 pre randomisation, at day 1, and at 6, 12, 26, 39 and 52 weeks post operation. The tool is a validated47 patient-reported outcome measure consisting of 10 items, used to document and monitor the severity of dysphagia. Each item is scored from 0 to 4, with 0 indicating ‘no problem’ for that item and 4 denoting a ‘severe problem’. The overall score is obtained by summing the scores for each individual item and can range from 0 to 40 points, with higher scores corresponding to an increasingly severe swallowing problem.
-
Glasgow–Edinburgh Throat Scale:48 the GETS was used to assess dysphagia symptoms, especially globus, over 52 weeks post operation, and was collected at day 0 pre randomisation, at day 1, and at 6, 12, 26, 39 and 52 weeks post operation. The scale is a validated48 patient-reported outcome measure consisting of 10 items, used to evaluate the presence and severity of common throat complaints, especially symptoms of globus (the sensation of a lump in the throat). Each item is scored from 0 to 7, with 0 indicating ‘no problem’ for that item and 7 denoting a problem that is ‘unbearable’. The overall score is obtained by summing the scores for each individual item and can range from 0 to 70 points, with higher scores corresponding to an increasingly severe sensation of swallowing difficulty.
-
The VHI-10:49 the VHI-10 was used to assess hoarse voice over 52 weeks post operation, and was collected at day 0 pre randomisation, at day 1, and at 6, 12, 26, 39 and 52 weeks post operation. The scale is a validated49 patient-reported outcome measure consisting of 10 items that evaluate the frequency with which an individual experiences each item. Each item is scored from 0 to 4, with 0 indicating ‘never’ for that item and 4 denoting ‘always’. The overall score is obtained by summing the scores for each individual item and can range from 0 to 40 points, with higher scores corresponding to an increasingly severe vocal handicap.
-
PainDETECT:50 a diagnostic questionnaire assessing whether the pain experienced by a patient is neuropathic or nociceptive in nature (questionnaire permission information may be found at www.pfizerpcoa.com). It was collected at day 0 pre randomisation and at 6, 12, 26, 39 and 52 weeks post operation. It consists of a total of 12 items, including three Numerical Rating Scales (NRSs) that range from 0 to 10 to measure pain intensity, seven descriptive scales where the respondent can choose from six possible descriptions that describe the type of pain, and two items aimed at graphically describing the location and course of the respondent’s pain. The first of these requires that the participant marks the picture that best represents the fluctuation of their pain, and the second requires the participant to mark the area of pain on a picture of the human body, and indicate whether or not the pain is radiating. The overall score ranges from 0 to 38, with a score of 0–12 indicating that neuropathic pain is unlikely (< 15% probability), and scores between 19 and 38 indicating that neuropathic pain is likely (> 90% probability). Scores of 13–18 are considered to be ambiguous. A score of –1 is technically possible, but can be achieved only by providing contradictory responses. The overall score is calculated by summing the numerical scores corresponding to each descriptor for the seven descriptive scales, and modifying it based on the responses given for the two graphical items. It is important to note that scores from the NRSs do not contribute to the overall score, but are reported as separate items. In addition, burning (dysaesthesia) pain is a feature of neuropathic pain, but is usually considered to respond poorly to surgery as it reflects nerve root dysfunction, rather than compression. The PainDETECT questionnaire includes a descriptive scale question on the presence and severity of burning pain. If the questionnaire was missing items, the best and worst score scenarios were calculated and participants were given a category score where the two scenarios agreed. In cases where the best- and worst-case scenarios did not agree, participants were assigned an ambiguous score category, and the range of scoring categories that the possible score spans was listed.
Clinical outcomes
-
Restricted ASIA score at the registration assessment, and at day 1 and 6 weeks post operation: the ASIA score is a system of tests, developed by the ASIA, used to define and describe the extent and severity of a patient’s functional impairment as a result of nerve entrapment or other spinal injury. 51 The patient’s sensory score is based on how much sensation the patient can feel at multiple points on the body, as well as tests of motor function, as assessed by the examiner. The sensory assessment is performed twice for each area, once using light-touch sensation and once using pinprick sensation, because these sensory modalities are carried in different parts of the spinal cord. In the FORVAD trial, it was considered excessive to assess all sensory areas, and so sensory assessment was restricted to the following 10 regions: C4, C5, C6, C7, C8, T1, T10, L2, L4 and S1, which were assessed bilaterally. Each test is scored from 0 (sensation is absent) to 2 (sensation is normal), and so the highest possible score for the sensory examination is 40 for each of the two sensations, giving a maximum of 80 overall. Motor function is assessed across 20 different muscles, each scored from 0 (total paralysis) to 5 (active movement, full range of motion, against gravity and provides normal resistance). The maximum possible score for this component is 100. Sensory and motor scores were also calculated for the upper limb on the operated side only, using the following areas: C4, C5, C6, C7, C8 and T1. Therefore, the maximum scores for each of these assessments were 12 (six regions for each of the light-touch and pinprick sensory scores on the operated side only) and 25 (five upper-limb muscles assessed to derive motor score on the operated side only). A lower score is indicative of a greater degree of functional impairment.
-
Grade, roughness, breathiness, asthenia and strain (GRBAS) scale in participants selected for voice-recordings only: the GRBAS scale was used to assess hoarse voice at baseline and at 6 weeks post operation. Participant voice recordings for a subset of participants were collected by sites and sent for central expert review. The expert reviewer was blinded to participant’s treatment allocation. For the recording, participants were asked to perform three vocal exercises, detailed in Appendix 2. The central reviewer was required to score the patient from 0 (‘normal’) to 3 (‘severe’), on the five parameters (i.e. GRBAS). Higher scores indicate a more pronounced vocal problem for the parameter in question. 52
-
Incidence of revision surgery over 52 weeks post operation: the number of participants requiring further revision surgery who were still symptomatic at 6 weeks post operation, as identified from history, examination and persistent nerve compression on repeat MRI.
-
Incidence of intraoperative surgical complications.
-
Incidence of postoperative complications occurring up until 6 weeks post operation, as graded by the Clavien–Dindo classification. 53
Exploratory outcomes
-
Whether participants receiving PCF received minimal- or open-access surgery.
-
Whether participants receiving ACD received surgery using a plate or without using a plate.
Safety monitoring
Information on all complications, whether volunteered by the participant, discovered by the investigator questioning or detected through physical examination or other investigation, were required to be reported, and were monitored throughout the trial. A complication was defined as an untoward medical event in a participant that had a causal relationship to the trial. The trial included the trial-specific interventions as defined in Interventions and any further treatment related to the trial intervention (such as treatment of complications caused by the trial intervention and any trial-specific interventions such as the consent process and completion of questionnaires).
A serious complication was defined as any complication that resulted in death, was life-threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity or a congenital anomaly or birth defect, or was otherwise considered medically significant by the investigator. An unexpected serious complication was defined as any serious complication that was deemed related and unexpected in accordance with the Health Research Authority definitions of relatedness and expectedness.
All serious complications and unexpected serious complications occurring within 6 weeks of the operation were reported to the CTRU within 24 hours of the research staff becoming aware of the event. All complications were followed up until they were resolved or a final outcome had been reached.
Participant withdrawal
Participants could withdraw from the trial at any time without explanation, without affecting their further treatment or care.
Statistical methods
Analysis
Formal statistical analysis of outcome measures was not possible as a result of the limited sample recruited to the trial. For the analysis of the primary outcome measure, it was intended to utilise a multilevel linear regression model incorporating random effects with respect to centre, and adjusting for baseline (day 0) NDI score and minimisation factors (duration of upper-limb symptoms and smoking status). The statistical analysis plan for the trial was updated to account for early trial closure and subsequent limited sample size prior to the final analysis of the trial. All descriptive statistics were computed using SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA).
Analysis populations
Intention-to-treat, per-protocol and safety populations
All patients recruited to the trial were included in the analysis using intention to treat (ITT) principles and summarised according to the randomised allocation. A per-protocol population was also defined: participants who violated the protocol or who did not receive the randomised intervention were to be analysed according to the treatment actually received. Furthermore, all randomised participants who received trial surgery formed the safety population and were to be analysed according to treatment actually received. However, at final analysis, the ITT, per-protocol and safety populations were equivalent, and so results are presented for the ITT population only, to prevent repetition. Unless clearly stated otherwise, outcome measures were analysed in this manner.
Voice-recording subpopulation
Participants who were randomly selected at registration to provide additional recordings of voice pre and post operation formed the voice-recording subpopulation. Descriptive analysis of the GRBAS outcome was performed using this population.
Posterior cervical foraminotomy and anterior cervical discectomy subpopulations
Patients who actually received PCF as their initial trial surgery formed the PCF subpopulation. Members of this population will have received either open or minimally invasive PCF as their initial trial procedure. Patients who actually received ACD as their initial trial surgery formed the ACD subpopulation. Members of this population will have received ACD either with or without a plate as their initial trial procedure. Descriptive statistics of exploratory outcomes were produced using these populations.
Primary and secondary outcome measures
Primary and all secondary outcome measures are summarised using descriptive statistics by treatment group. For participant-reported outcomes, item-level summaries and overall scores are provided in Chapter 3 and Appendix 5 by time point and treatment group.
Imputation of missing questionnaire responses was not performed, except as described in Patient-reported outcomes for the PainDETECT score categories.
Exploratory outcome measures
Owing to early trial closure, the analysis of exploratory outcomes is limited to a descriptive summary of the predefined PCF variation (i.e. operation performed using open- or minimal-access technique) or the ACD variation (i.e. operation with or without a plate) conducted in the trial.
Sample size
The trial originally aimed to recruit 252 participants (126 per trial arm). This number was required to have 90% power to detect the minimum clinically important difference of an absolute 10% difference in NDI score at 52 weeks post operation, assuming a between-patient SD of 23 units (based on local audit data13), two-sided 5% significance level and 10% loss to follow-up.
Because the trial was multicentre, the possibility of clustering within a centre was considered. Published literature suggests that clustering by surgeon or centre for a range of long-term patient-reported and quality-of-life outcomes is minimal across various types of surgical interventions,54 with minimal impact on sample size. We therefore anticipated that there would be minimal or no clustering for the primary clinical outcome and chose a conservative approach to the sample size calculation to allow for the possibility of zero clustering for the NDI score at 52 weeks.
There was also the possibility of clustering by surgeon. However, owing to the nature of the primary outcome measure and as each surgeon was expected to undertake very few cases, this was again expected to be minimal. Moreover, as the number of surgeons within each centre was expected to be small (two or three surgeons per centre), surgeon clustering is likely to be confounded with centre clustering, and this was addressed by stratifying by centre.
Chapter 3 Trial results
Participant flow
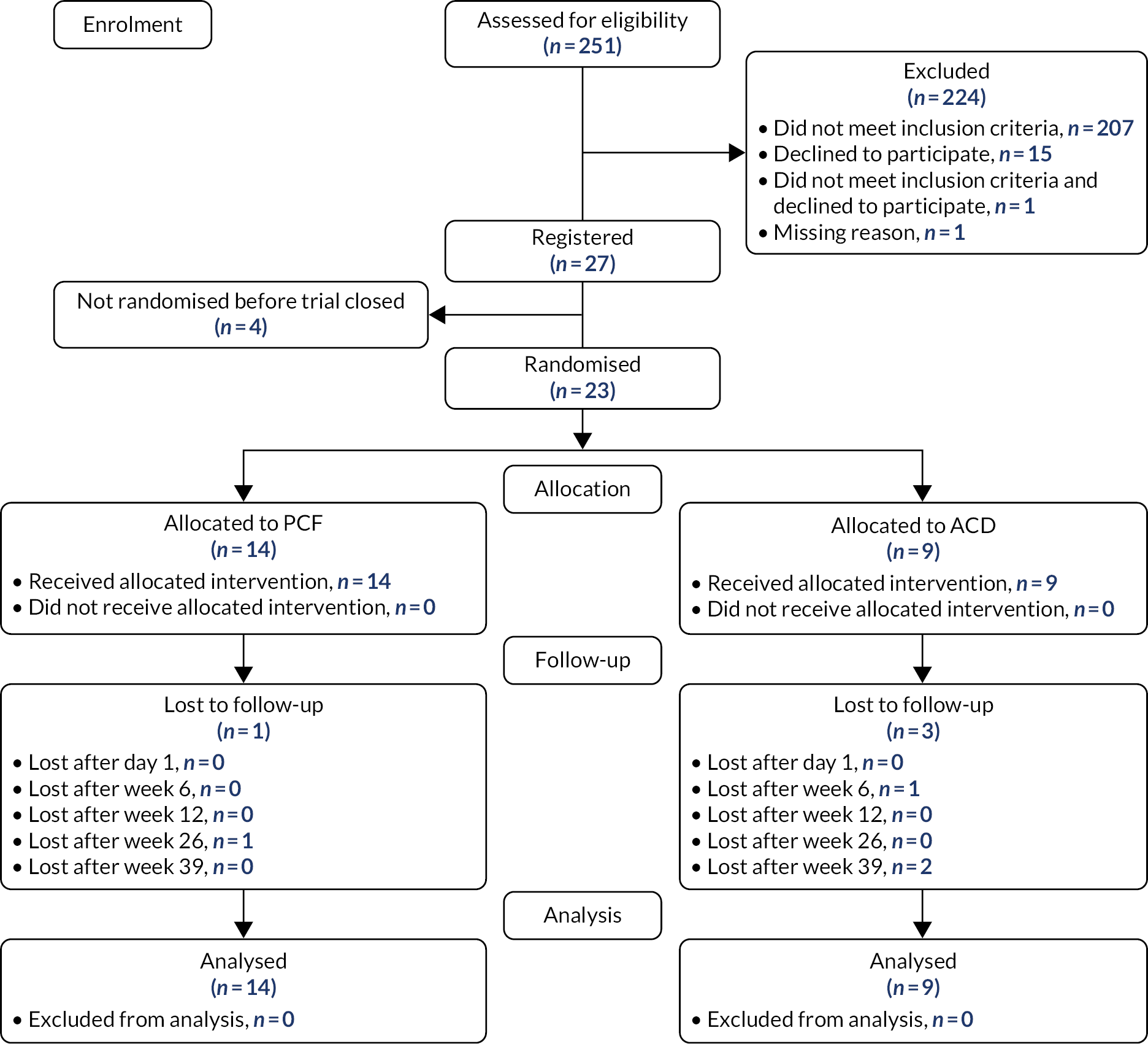
Between January 2019 and March 2020, 251 patients were screened for the trial across 11 centres. A total of 224 patients were excluded prior to registration, during screening, 207 of whom failed to meet the eligibility criteria. The main reasons for failing to meet the eligibility criteria were diagnosis of cervical myelopathy (n = 38), no single-level nerve entrapment (n = 34) and no diagnosis of unilateral cervical brachialgia as confirmed by MRI or CT myelogram taken within the preceding 12 months (n = 30). A full breakdown of non-eligibility reasons is given in Appendix 3, Table 24. A total of 27 patients gave written informed consent and were registered to the trial. Four patients were excluded post registration, of whom two declined to proceed to randomisation, one because they no longer wanted to receive surgery and one because they no longer wanted to be randomised. The other two participants did not receive surgery because of COVID-19; one participant’s surgery was cancelled and the other participant’s surgery was conducted at a non-trial site. Owing to early closure of the trial, only 23 participants were randomised: 14 to receive PCF and nine to receive ACD.
Participant flow is shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 2). Note that participants were considered lost to follow-up from the point at which they last returned a questionnaire pack at the time of trial final analysis, regardless of whether or not the questionnaire pack had been completed. There were no withdrawals of participant consent during the trial.
FIGURE 2.
Consolidated Standards of Reporting Trials flow diagram.
Trial recruitment
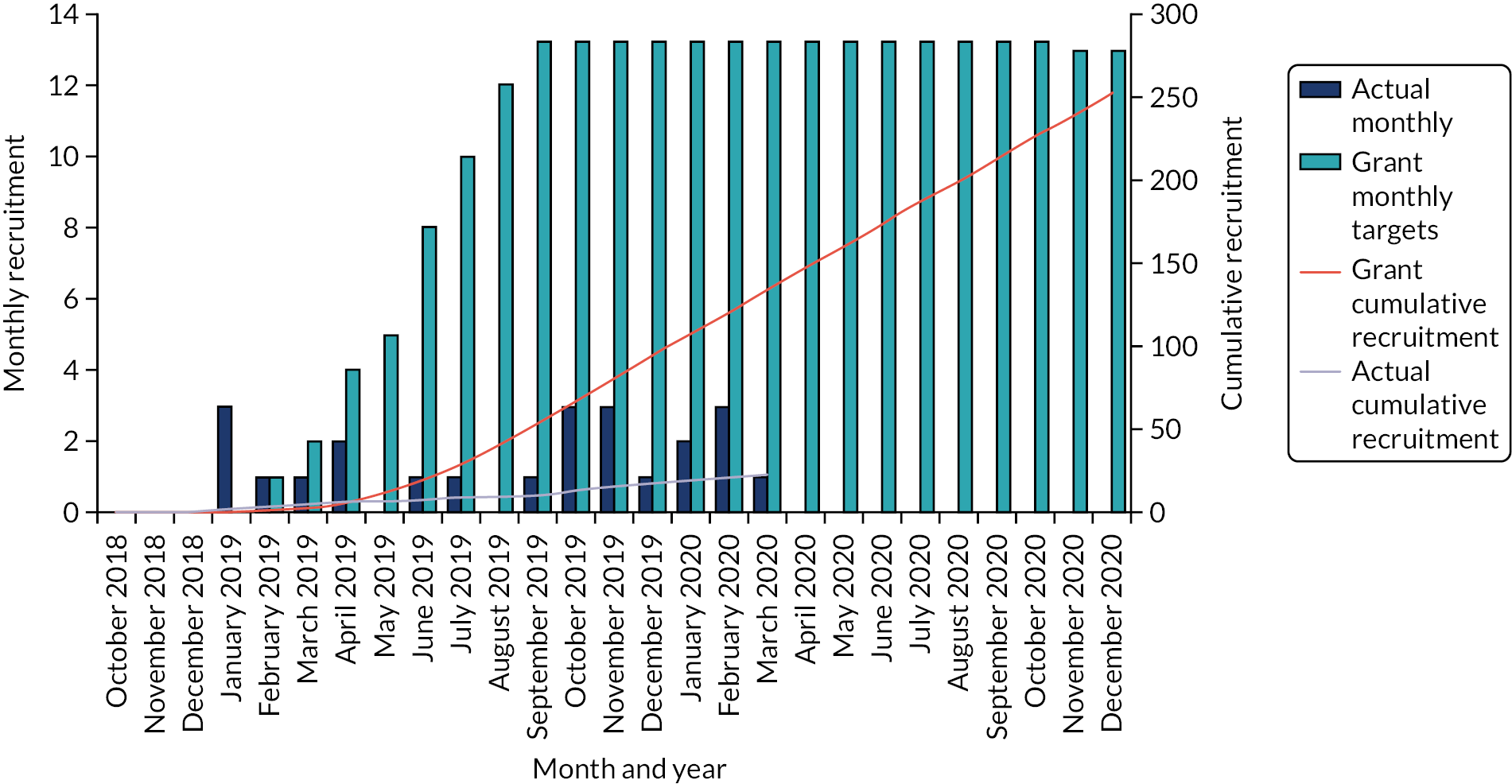
The trial opened to recruitment on 9 January 2019 and closed because of slower than anticipated accrual on 10 June 2020, after registering 27 participants and randomising 23 participants. Figure 3 shows the projected and actual recruitment figures throughout the trial. The first participant was registered on 10 January 2019, and subsequently randomised on 16 January 2019. The final participant was randomised on 5 March 2020. The trial opened in 11 NHS trust hospital sites. In sites that recruited to the trial, the median time from site opening to the first randomisation of a participant was 169 days [interquartile range (IQR) 73–221 days]. An overview of all participating NHS trusts (in the order in which they opened to recruitment) can be found in Table 3. Recruitment by centre can be seen in Table 4.
FIGURE 3.
Projected and actual recruitment in the FORVAD trial.
| Number | Participating centres | Date opened to recruitment | Date of first registered participant |
|---|---|---|---|
| 1 | Leeds Teaching Hospitals NHS Trust | 9 January 2019 | 10 January 2019 |
| 2 | Sheffield Teaching Hospitals NHS Foundation Trust | 30 April 2019 | 9 January 2020 |
| 3 | Lancashire Teaching Hospitals NHS Foundation Trust | 12 June 2019 | 19 November 2019 |
| 4 | The Walton Centre NHS Foundation Trust | 28 June 2019 | 11 November 2019 |
| 5 | University Hospitals Plymouth NHS Trust | 11 September 2019 | N/A |
| 6 | Cambridge University Hospitals NHS Foundation Trust | 12 September 2019 | 13 November 2019 |
| 7 | South Tees Hospitals NHS Foundation Trust | 18 September 2019 | 4 February 2020 |
| 8 | Cardiff and Vale University Health Board | 15 October 2019 | N/A |
| 9 | Whittington Health NHS Trust | 16 October 2019 | N/A |
| 10 | St George’s University Hospitals NHS Foundation Trust | 24 January 2020 | N/A |
| 11 | King’s College Hospital NHS Foundation Trust | 4 February 2020 | N/A |
| Participating NHS trust | Participants (n) | |
|---|---|---|
| Registered | Randomised | |
| Leeds Teaching Hospitals NHS Trust | 18 | 18 |
| The Walton Centre NHS Foundation Trust | 3 | 2 |
| Lancashire Teaching Hospitals NHS Foundation Trust | 3 | 1 |
| Sheffield Teaching Hospitals NHS Foundation Trust | 1 | 1 |
| South Tees Hospitals NHS Foundation Trust | 1 | 1 |
| Cambridge University Hospitals NHS Foundation Trust | 1 | 0 |
| Total | 27 | 23 |
The trial experienced delays at 11 centres owing to a range of set-up obstacles that presented themselves during the site set-up period. These obstacles were site-specific issues, such as a lack of research support or local logistical challenges. When appropriate, the CTRU trial team sought support from the lead Clinical Research Network or local Clinical Research Networks across the UK to help resolve these issues. Further qualitative work to understand the experiences of research centres and participants was performed and is summarised in Chapter 5.
Protocol deviations
Non-COVID-19-related deviations
There are two reported deviations of participants completing the baseline questionnaire on the date of registration instead of the date of randomisation; they were mistakenly not given the envelope to seal the questionnaire booklet in. This occurred once in each treatment group.
COVID-19-related deviations
COVID-19 was explicitly reported as an issue in the conduct of the trial in 10 instances. Six of these relate to postal questionnaires being sent out early, four in the ACD group and two in the PCF group. Two of the issues relate to ASIA assessments that could not be performed because of COVID-19; one of these could not be performed because the participant was assessed over the telephone instead of in clinic. Finally, two registered patients were not randomised as a result of COVID-19, as the pandemic meant that one had their surgery at an external facility and another had their surgery cancelled.
Baseline data
Baseline demographic and clinical data
Summaries of minimisation factors at baseline are reported in Table 5 and data collected at the baseline clinic visit are summarised in Table 6. The mean age was 54.0 years (SD 8.1 years, range 34–70 years). A total of 15 participants (62.5%) were female, and the ethnic background of all trial
| Minimisation factor | Trial group, n (%) | Total (N = 23), n (%) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Site | |||
| Royal Preston Hospital | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sheffield Teaching Hospitals NHS Foundation Trust | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Leeds Teaching Hospitals NHS Trust | 11 (78.6) | 7 (77.8) | 18 (78.3) |
| South Tees Hospitals NHS Foundation Trust | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| The Walton Centre NHS Foundation Trust | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Total | 14 (100.0) | 9 (100.0) | 23 (100.0) |
| Duration of upper-limb symptoms | |||
| 6 weeks to just under 6 months | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ≥ 6 months to just under 12 months | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| ≥ 12 months | 8 (57.1) | 7 (77.8) | 15 (65.2) |
| Total | 14 (100.0) | 9 (100.0) | 23 (100.0) |
| Smoking status in preceding 6 weeks | |||
| Smoker | 5 (35.7) | 3 (33.3) | 8 (34.8) |
| Non-smoker | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| Total | 14 (100.0) | 9 (100.0) | 23 (100.0) |
| Characteristic | Trial group | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Age (years) | |||
| Mean (SD) | 54.5 (6.6) | 53.3 (10.5) | 54.0 (8.1) |
| Median (range) | 53.5 (46.0–67.0) | 52.0 (34.0–70.0) | 52.0 (34.0–70.0) |
| IQR | 50.0–58.0 | 47.0–61.0 | 49.0–61.0 |
| Gender, n (%) | |||
| Male | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Female | 10 (71.4) | 5 (55.6) | 15 (65.2) |
| Total | 14 (100.0) | 9 (100.0) | 23 (100.0) |
| Body mass index (kg/m 2 ) | |||
| Mean (SD) | 30.1 (4.9) | 30.2 (4.3) | 30.1 (4.6) |
| Median (range) | 30.2 (22.5–40.8) | 31.4 (24.3–35.5) | 30.2 (22.5–40.8) |
| IQR | 26.0–33.2 | 26.0–34.3 | 26.0–34.3 |
| Average number of cigarettes smoked per day (smokers only) | |||
| Mean (SD) | 13.6 (6.2) | 18.3 (10.4) | 15.4 (7.7) |
| Median (range) | 12.0 (6.0–20.0) | 15.0 (10.0–30.0) | 13.5 (6.0–30.0) |
| IQR | 10.0–20.0 | 10.0–30.0 | 10.0–20.0 |
| n | 5 | 3 | 8 |
| Type of scan, n (%) | |||
| MRI | 14 (100.0) | 8 (88.9) | 22 (95.7) |
| CT myelography | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Cause of nerve root compression, n (%) | |||
| Soft disk | 6 (42.9) | 5 (55.6) | 11 (47.8) |
| Osteophyte | 5 (35.7) | 3 (33.3) | 8 (34.8) |
| Both | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Dominant hand, n (%) | |||
| Left-handed | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Right-handed | 13 (92.9) | 9 (100.0) | 22 (95.7) |
| Ambidextrous | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Arm that participant has worse pain in, n (%) | |||
| Left arm | 6 (42.9) | 6 (66.7) | 12 (52.2) |
| Right arm | 8 (57.1) | 3 (33.3) | 11 (47.8) |
| Litigation pending for this complaint?, n (%) | |||
| Yes | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| No | 12 (85.7) | 8 (88.9) | 20 (87.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Medications for this presentation?, n (%) | |||
| Yes | 13 (92.9) | 8 (88.9) | 21 (91.3) |
| No | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Other non-operative intervention for cervical brachialgia?, a n (%) | |||
| Yes | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| No | 10 (71.4) | 7 (77.8) | 17 (73.9) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Does the participant have any comorbidities?, n (%) | |||
| Yes | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| No | 11 (78.6) | 9 (100.0) | 20 (87.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Comorbidity list, n (%) | |||
| Chronic obstructive pulmonary disease | 1 (50.0) | 0 (0.0) | 1 (50.0) |
| Peptic ulcer disease | 1 (50.0) | 0 (0.0) | 1 (50.0) |
| Depression in preceding 6 months?, n (%) | |||
| Yes | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| No | 9 (64.3) | 8 (88.9) | 17 (73.9) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
participants as reported at baseline was white. In the 6 months prior to baseline, five participants had received physiotherapy (21.7%), four in the PCF group and one in the ACD group, and one participant in the PCF group had been seen by a chiropractor (4.3%). No participants had been treated with cervical nerve root injections.
Baseline data for outcome measures are reported in Table 7.
| Outcome | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| NDI | |||
| Median score (range) | 44.0 (18.0–76.0) | 35.6 (18.0–78.0) | 40.0 (18.0–78.0) |
| IQR | 36.0–62.0 | 34.0–44.0 | 34.0–58.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Unreturned booklet, n (%) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| NRS-NP | |||
| Median score (range) | 5.5 (2.0–10.0) | 5.0 (3.0–8.0) | 5.0 (2.0–10.0) |
| IQR | 4.0–8.0 | 4.0–7.0 | 4.0–7.0 |
| n | 12 | 9 | 21 |
| Missing (n) | 2 | 0 | 2 |
| NRS-AP | |||
| Median score (range) | 7.0 (1.0–10.0) | 6.0 (3.0–8.0) | 6.5 (1.0–10.0) |
| IQR | 4.0–8.0 | 5.0–7.0 | 4.5–8.0 |
| n | 11 | 9 | 20 |
| Missing (n) | 3 | 0 | 3 |
| PainDETECT score categories, n (%) | |||
| Negative (0–12) | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Unclear (13–18) | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Positive (19–38) | 6 (42.9) | 4 (44.4) | 10 (43.5) |
| Ambiguous (18–19) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| EAT-10 | |||
| Median score (range) | 0.0 (0.0–17.0) | 0.0 (0.0–10.0) | 0.0 (0.0–17.0) |
| IQR | 0.0–0.0 | 0.0–3.0 | 0.0–3.0 |
| n | 13 | 8 | 21 |
| Missing (n) | 1 | 1 | 2 |
| Partially missing questionnaire, n (%) | 0 (0.0) | 1 (100.0) | 1 (50.0) |
| Unreturned booklet, n (%) | 1 (100.0) | 0 (0.0) | 1 (50.0) |
| GETS | |||
| Median score (range) | 0.0 (0.0–24.0) | 0.0 (0.0–20.0) | 0.0 (0.0–24.0) |
| IQR | 0.0–6.0 | 0.0–3.0 | 0.0–6.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Unreturned booklet, n (%) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| VHI-10 | |||
| Median score (range) | 0.0 (0.0–21.0) | 0.0 (0.0–5.0) | 0.0 (0.0–21.0) |
| IQR | 0.0–2.0 | 0.0–2.0 | 0.0–2.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Unreturned booklet, n (%) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| ASIA scores | |||
| Total motor score | |||
| Median (range) | 100.0 (95.0–100.0) | 100.0 (95.0–100.0) | 100.0 (95.0–100.0) |
| IQR | 100.0–100.0 | 99.0–100.0 | 99.0–100.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Total light-touch sensory score | |||
| Median (range) | 39.0 (36.0–40.0) | 37.0 (27.0–40.0) | 38.0 (27.0–40.0) |
| IQR | 38.0–40.0 | 36.0–38.0 | 37.0–40.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Total pinprick sensory score | |||
| Median (range) | 39.0 (36.0–40.0) | 38.0 (27.0–40.0) | 38.5 (27.0–40.0) |
| IQR | 38.0–40.0 | 37.0–39.0 | 38.0–40.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
Comparability of treatment groups at baseline
The baseline characteristics listed in Table 6 appear to be balanced across treatment groups. Notable exceptions to this are the cause of nerve root compression, depression in the previous 6 months, duration of upper-limb symptoms and the arm that participant has worse pain in. A proportionally higher number of participants in the PCF group had depression in the preceding 6 months (28.6%, compared with 11.1% in the ACD group). The duration of upper-limb symptoms is more commonly > 12 months in the ACD group (77.8%, compared with 57.1% in the PCF group), and the arm that participants have worse pain in is more commonly the left arm in the ACD group (66.7%, compared with 42.9% in the PCF group).
For outcome measure data (see Table 7), the median baseline NDI score was 44.0 (IQR 36.0–62.0) in the PCF group and 35.6 (IQR 34.0–44.0) in the ACD group, meaning that, at baseline, the ACD group was observed to have a better (i.e. lower) NDI score; this was influenced by outlying higher scores observed by chance in this small sample.
Outcomes and estimation
Primary outcome: Neck Disability Index score at 52 weeks post operation
Summaries of the primary outcome, percentage NDI score at 52 weeks post operation, are presented in Figure 4, alongside baseline (day 0) summaries of percentage NDI score. Mean scores are denoted in figures by circles for PCF and by triangles for ACD. In general, a lower NDI score is better, that is it corresponds to a lower level of neck pain. The minimum clinically important difference for the NDI is 10%. At 52 weeks post operation, the median NDI score was 25.3 (IQR 20.0–42.0) in the PCF group and 45.0 (IQR 20.0–57.0) in the ACD group. Summary tables of all descriptive statistics computed for this outcome measure can be found in Appendix 4.
FIGURE 4.
Percentage NDI scores at baseline (day 0) and week 52, by treatment group.

Missing data for the primary outcome measure
Item-level responses at baseline and at week 52 post operation are summarised in Appendix 4, Table 26. The most commonly missing item in questionnaires that were at least partially answered is a question about driving, usually because the participant reported that they did not drive. The most common source of missingness is the questionnaire booklet not being returned at all (one booklet was unreturned at baseline, and four were unreturned at week 52). This event is most common in the ACD group at 52 weeks (three unreturned booklets at week 52).
Secondary outcome: repeated Neck Disability Index score assessed over 52 weeks postoperatively
Summaries of percentage NDI scores at baseline, at day 1 and at 6, 12, 26, 39 and 52 weeks post operation are presented in Figure 5. The median NDI score at day 1 post operation is higher, indicating worse functional disability, than at other time points. Thereafter, there does not seem to be a clear trend across time points, or a clear difference between the treatment groups in the NDI scores.
FIGURE 5.
Percentage NDI scores at each time point, by treatment group.

The summary statistics calculated for each time point, including medians and IQRs, can be found in Appendix 4, Table 25.
Missing data for the Neck Disability Index at all time points
Item-level responses at each time point are summarised in Appendix 4, Table 26. Across all time points, only one questionnaire, in the PCF group, could not be scored because of partial missingness at day 1 post operation. The largest source of missingness for this outcome was unreturned questionnaire booklets (n = 29, 18.0%), although a number of questionnaires were wholly missing, that is the questionnaires were returned but were blank. In total, across all time points, 17 (10.6%) questionnaires were wholly missing, seven in the PCF group and 10 in the ACD group. At item level, the most commonly missing response was the driving question, usually because the participant reported that they do not drive.
Secondary outcome: Numerical Rating Scale scores for neck and arm pain
Summaries of NRS scores (0–10) at baseline, at day 1 and at 6, 12, 26, 39 and 52 weeks post operation are presented in Figure 6 (NRS-NP) and Figure 7 (NRS-AP). In general, a lower NRS score is better, that is it corresponds to a lower level of pain. It appears that, following an initial increase in neck pain score at day 1 post operation [median of 8.5 (IQR 6.0–10.0) in the PCF group and 7.0 (IQR 4.0–9.0) in the ACD group], neck pain then decreases over the postoperative period in both treatment groups. At 52 weeks, the median NRS-NP scores were 4.0 (IQR 2.0–5.0) in the PCF group and 5.0 (IQR 3.0–7.0) in the ACD group.
FIGURE 6.
The NRS-NP scores at each time point, by treatment group.
FIGURE 7.
The NRS-AP scores at each time point, by treatment group.
For upper-limb pain, median arm pain reached its lowest level in both groups at 12 weeks post operation; thereafter, median arm pain increased to week 52 post operation in the ACD group and fluctuated over time in the PCF group, although IQRs overlap at each time point. At 52 weeks, the median NRS-AP scores were 5.0 (IQR 2.0–7.0) in the PCF group and 5.0 (IQR 3.0–6.0) in the ACD group.
The median surgical wound length was 32.0 mm (IQR 25–50 mm) in the PCF group and 39.0 mm (IQR 35–41 mm) in the ACD group. Although the median wound length was smaller in the PCF group, it was also much more variable, as demonstrated by the IQRs.
The summary statistics calculated for each time point, including medians and IQRs, can be found in Appendix 5, Table 27.
In addition, the number of participants who took pain medications in the previous 24 hours and the amount by which participants felt that their pain was reduced (on a NRS from 0–10) is presented in Appendix 5, Table 28. At any given time point, it appears as though the majority of participants [15 (65.2%) at 52 weeks] had taken pain medication in the previous 24 hours (across both treatment groups), and that pain was reduced to a greater degree in the PCF arm [median of 6.0 (IQR 2.0–7.0) in the PCF group and 3.5 (IQR 2.5–4.5) in the ACD group] at 52 weeks (see Appendix 5, Figure 15).
Secondary outcome: the PainDETECT questionnaire
Summaries of PainDETECT category scores at baseline and at 6, 12, 26, 39 and 52 weeks post operation are presented in Table 8. Categories represent different types of pain: a score of 0–12 means that pain is nociceptive [neuropathic pain is unlikely (< 15% probability)], a score of 13–18 is an unclear result and a score of 19–38 represents a high probability of neuropathic pain (> 90% probability). Table 8 summarises the score categories after considering best- and worst-case scenarios for missing responses. If best- and worst-case scores agree (are contained within the same scoring zone), participants are reported in the corresponding score category; otherwise, they are reported as ambiguous. It appears that, across time points, PainDETECT scores fluctuate in the PCF group, but reduce in the ACD group.
| Time point and scoring category | Trial arm n (%) | Total (N = 23), n (%) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Day 0 PainDETECT score categories | |||
| Negative (0–12) | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Unclear (13–18) | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Positive (19–38) | 6 (42.9) | 4 (44.4) | 10 (43.5) |
| Ambiguous (between unclear and positive) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Week 6 PainDETECT score categories | |||
| Negative (0–12) | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Unclear (13–18) | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Positive (19–38) | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| Ambiguous (between unclear and positive) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Week 12 PainDETECT score categories | |||
| Negative (0–12) | 3 (21.4) | 4 (44.4) | 7 (30.4) |
| Unclear (13–18) | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Positive (19–38) | 5 (35.7) | 0 (0.0) | 5 (21.7) |
| Ambiguous (between negative and unclear) | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Week 26 PainDETECT score categories | |||
| Negative (0–12) | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Unclear (13–18) | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Positive (19–38) | 5 (35.7) | 1 (11.1) | 6 (26.1) |
| Ambiguous (between negative and unclear) | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Ambiguous (between unclear and positive) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Week 39 PainDETECT score categories | |||
| Negative (0–12) | 1 (7.1) | 4 (44.4) | 5 (21.7) |
| Unclear (13–18) | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Positive (19–38) | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Ambiguous (between negative and unclear) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Week 52 PainDETECT score categories | |||
| Negative (0–12) | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Unclear (13–18) | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Positive (19–38) | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| Ambiguous (between unclear and positive) | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
Summaries of the PainDETECT total scores can be found in Appendix 5, Table 29.
Missing data for the PainDETECT questionnaire
Item-level responses at each time point are summarised in Appendix 5, Table 30. Across all time points, partial completion of the questionnaire is the largest source of missingness for this outcome. At an item level, the most commonly missing responses were the score modifier items (course of pain and pain radiation questions), although beyond this there does not appear to be a discernible pattern. In some cases, it is still possible to ascertain the categorical score for the questionnaire if the achievable total score in the presence of missingness falls into the same category in both best- and worst-case scenarios for the missing items. In cases in which the best- and worst-case scenarios do not agree, these were included as a potential score category of ambiguous, followed by the possible list of categories the score could fall into in either scenario.
Secondary outcome: the Eating Assessment Tool-10 items
Summaries of the EAT-10 total scores at baseline, at day 1 and at 6, 12, 26, 39 and 52 weeks post operation are presented in Figure 8. In general, a lower EAT-10 score is better, that is, it corresponds to less severe swallowing problems. Up until week 6 post operation, the ACD group was observed to have worse (higher) EAT-10 scores, after which there was no clear difference between groups. The shaded section of Figure 8 shows the section of the graph where a participant would not be considered to have a swallowing problem, corresponding to an EAT-10 score of ≤ 3. 47
FIGURE 8.
The EAT-10 total score at each time point, by treatment group.

Summary statistics calculated for each time point, including medians and IQRs, can be found in Appendix 5, Table 31. At 52 weeks, the median EAT-10 scores were 0.0 (IQR 0.0–6.0) in the PCF group and 0.0 (IQR 0.0–1.0) in the ACD group.
Missing data for the Eating Assessment Tool-10 items
Item-level responses at each time point are summarised in Appendix 5, Table 32. Across all time points, completed questionnaires were able to be scored, although partial missingness is the second-largest source of missingness for this outcome, after unreturned questionnaire booklets. At an item level, there does not appear to be a consistent or discernible pattern, and no particular item stands out as being missed more often than others.
Secondary outcome: the Glasgow–Edinburgh Throat Scale
Summaries of the GETS scores at baseline, at day 1 and at 6, 12, 26, 39 and 52 weeks post operation are presented in Figure 9. In general, a lower GETS score is better, that is it corresponds to less severe globus symptoms. At all time points post operation, the median GETS scores are lower in the PCF group, although the IQRs overlap at all time points except day 1. There is an observed spike in median GETS score at day 1 post operation for the ACD group.
FIGURE 9.
Total GETS score at each time point, by treatment group.

Summary statistics calculated for each time point, including medians and IQRs, can be found in Appendix 5, Table 33, as can summaries of additional questionnaire items that did not contribute to the overall score. At 52 weeks, the median GETS scores were 0.0 (IQR 0.0–8.0) in the PCF group and 2.5 (IQR 2.0–5.0) in the ACD group.
Missing data for the Glasgow–Edinburgh Throat Scale
Item-level responses at each time point are summarised in Appendix 5, Table 34. Across all time points, returned questionnaires could be scored, although partial missingness is the second-largest source of missingness for this outcome, after unreturned questionnaire booklets. At an item level, there does not appear to be a consistent or discernible pattern, and no particular item stands out as being missed more often than others.
Secondary outcome: grade, roughness, breathiness, asthenia and strain assessment
Owing to the small sample size and concerns about identifiability in this subgroup, results for this outcome are presented overall, and not by treatment group.
Six participants were selected at registration to provide additional voice recordings. In total, eight voice recordings from five participants were collected during the trial, five at baseline and three at 6 weeks post operation. All collected recordings were considered by the central reviewer to be of adequate quality to review, and all participants performed the sentence production and at least one of the spontaneous speech or alternative reading tasks (see Appendix 2 for details of voice tasks). There was one participant who partially completed the vowel sound task at baseline, and who did not complete the same task at 6 weeks.
Parameters are scored from 0 (‘normal’) to 3 (‘severe’). Higher scores indicate a more pronounced vocal problem for the parameter in question. Across all voice recordings, no participants were judged in any parameter to experience more than a mild degree of issues, and this did not change between baseline and 6 weeks post operation. Summaries of score by parameter are presented in Appendix 5, Table 38.
Secondary outcome: the Voice Handicap Index-10 items
Summaries of the VHI-10 total scores at baseline, at day 1 and at 6, 12, 26, 39 and 52 weeks post operation are presented in Figure 10. In general, a lower VHI-10 score is better, that is it corresponds to less severe vocal problems. At all postoperative time points, the median VHI-10 scores are low or zero across both treatment groups; variability in scores appears to increase over time. At day 1 post operation, the ACD group appears to have a worse (higher) VHI-10 score, although there is no clear difference between groups thereafter.
FIGURE 10.
The VHI-10 total scores at each time point, by treatment group.
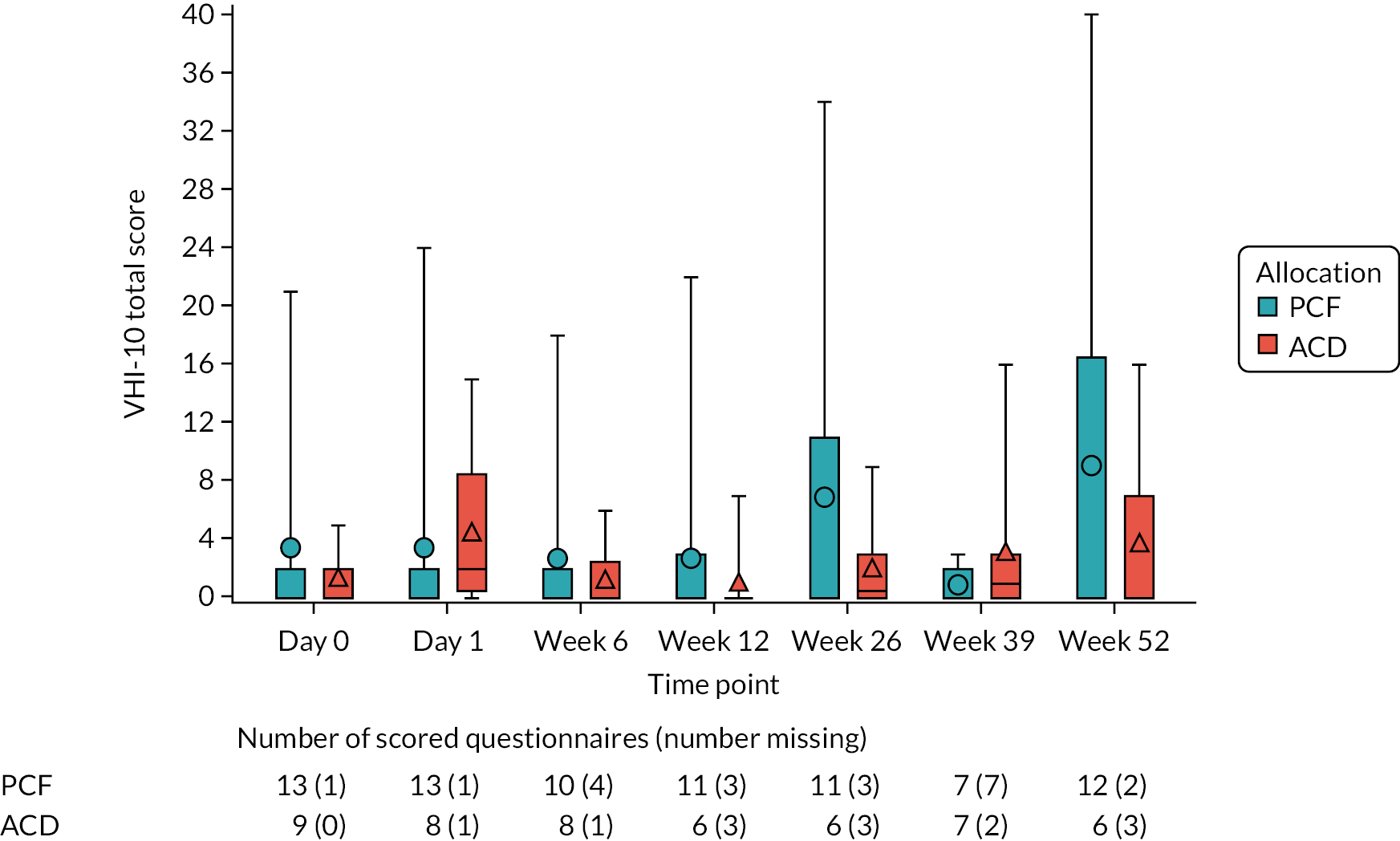
Summary statistics calculated for each time point, including medians and IQRs, can be found in Appendix 5, Table 35. At 52 weeks, the median VHI-10 scores were 0.0 (IQR 0.0–16.5) in the PCF group and 0.0 (IQR 0.0–7.0) in the ACD group.
Missing data for the Voice Handicap Index-10 items
Item-level responses at each time point are summarised in Appendix 5, Table 36. Across all time points, partial missingness is the second-largest source of missingness for this outcome, after unreturned questionnaire booklets. At an item level, there does not appear to be a consistent or discernible pattern, and no particular item stands out as being missed more often than others.
Secondary outcome: Restricted American Spinal Injury Association score
Across all summarised outcomes [the total motor score for the upper limb on the operated side, the total light-touch sensory score for the upper limb on the operated side, the total pinprick sensory score for the upper limb on the operated side, the total motor score (Figure 11), the total light-touch sensory score (Figure 12) and the total pinprick sensory score (Figure 13)], scores remained relatively unchanged and were similar across all time points (i.e. baseline, day 1 and 6 weeks post operation) and both treatment groups. In general, a higher ASIA score for each of these outcomes is better, that is it corresponds to a higher level of motor or sensory function. Additional figures and tables are presented in Appendix 5, Figures 16–18 and Table 37.
FIGURE 11.
Total motor score at each time point, by treatment group.

FIGURE 12.
Total light-touch sensory score at each time point, by treatment group.

FIGURE 13.
Total pinprick sensory score at each time point, by treatment group.
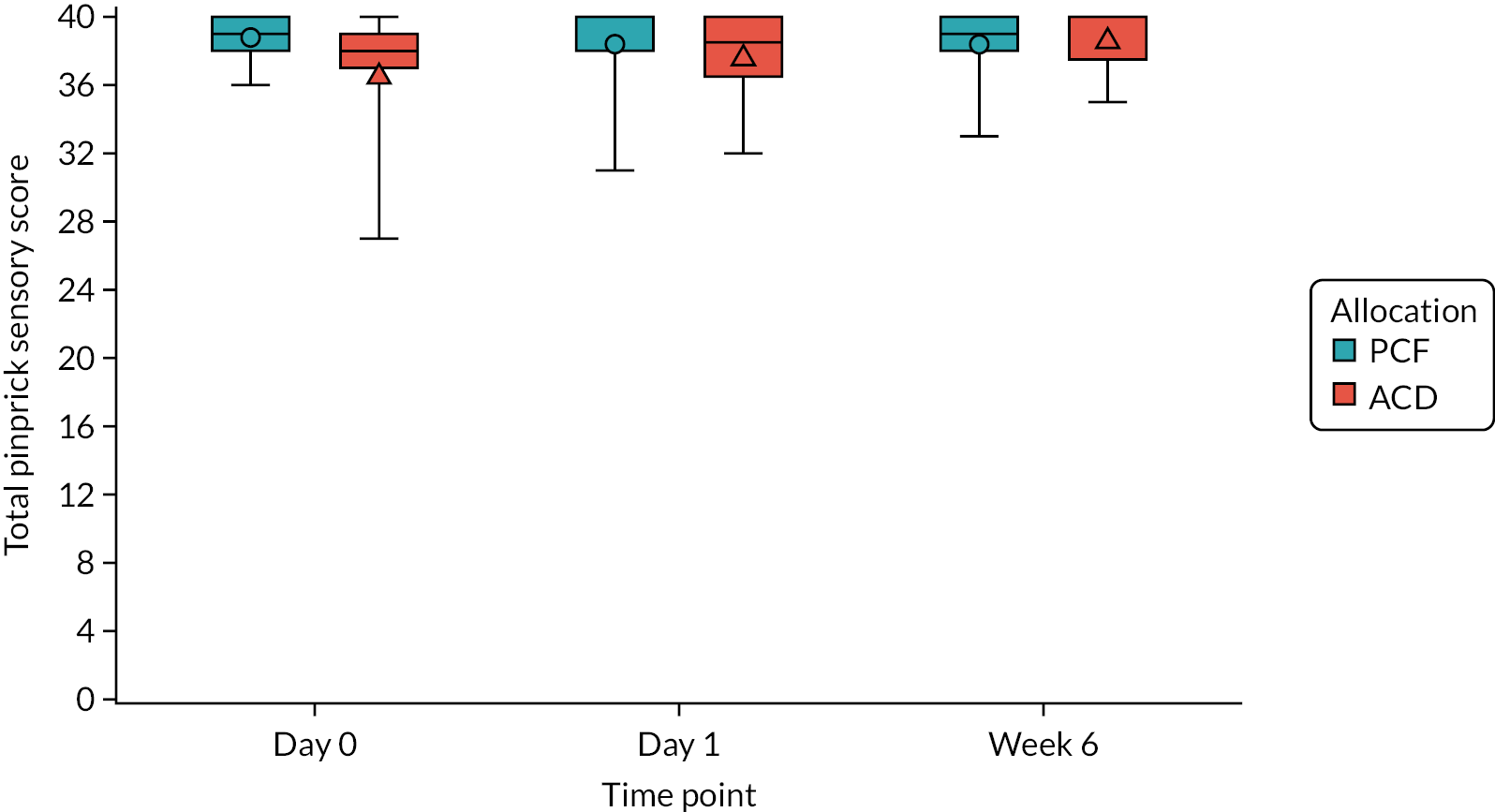
Secondary outcome: incidence of revision surgery within 52 weeks postoperatively
There were no reported reoperations occurring within the trial.
Secondary outcome: impact of variations in the optional surgical components of posterior cervical foraminotomy
All participants in the trial received PCF using an open technique.
Secondary outcome: impact of variations in the optional surgical components of anterior cervical discectomy
The majority of participants receiving ACD in the trial received ACD without a plate (n = 8, 88.9%). The remaining participant received a plate.
Harms
Incidence of intraoperative complications
No intraoperative complications were reported during the trial.
Incidence of postoperative complications within 6 weeks of surgery
Five postoperative complications occurring within 6 weeks of surgery were reported in the trial, all in the ACD group. No serious or unexpected serious complications were reported. Line listings of postoperative complications are presented in Table 9. All listed complications presented in different participants.
| Treatment received | Complication description | Clavien–Dindo classification | Time (in days) between surgery and onset | Serious? | Expected? |
|---|---|---|---|---|---|
| ACD | Dysphagia | I | 4 | No | Yes |
| ACD | Other, wound infection | II | 8 | No | Yes |
| ACD | Other, wound redness stitches overnight | I | 32 | No | No |
| ACD | Other, urinary retention | I | 1 | No | Yes |
| ACD | Dysphagia | IIIa | 22 | No | Yes |
Deaths
No deaths were reported in the trial.
Chapter 4 Health economic evaluation
Original health economic analysis plan
A full economic analysis plan was to be prepared by the trial health economist, and approved as per the statistical analysis plan. When appropriate, missing resource use or health outcome data were to be imputed. Non-parametric bootstrapped 95% CIs would be estimated (10,000 replicates). We also intended to employ simple parametric approaches for analysing cost and QALY data that assume normal distributions. Should the data have indicated otherwise, we would have developed a generalised linear model to deal with problems such as skewness. Total costs were to be combined with QALYs to calculate the incremental cost–utility ratio, which would then be compared with the £20,000- to £30,000-per-QALY threshold of cost effectiveness specified by the National Institute for Health and Care Excellence. We also planned to conduct a range of one-way sensitivity analyses for assessing the robustness of results, and multivariate sensitivity analyses were to be applied where interaction effects were suspected. The joint uncertainty in costs and benefits were to be considered through the application of bootstrapping and the estimation of cost-effectiveness acceptability curves.
Protocol amendment to health economics analysis plan
The original aim of the health economic analysis was to determine the cost effectiveness of PCF, compared with ACD, in the treatment of patients with cervical brachialgia over a 52-week time horizon from the perspectives of the NHS, Personal Social Services and society. Owing to the early termination of the FORVAD trial at the internal pilot phase, the scope of the health economics study has been revised to describe and explore the costs and EQ-5D utility values in each trial arm.
Revision to economic analysis plan
-
Costs and QALYs will no longer be combined into a summary cost-effectiveness ratio measure. Instead, mean quantities of resource use, mean costs and mean EQ-5D-3L utility scores will be reported for each trial arm, including measures of sample variation, but without estimation or statistical testing for differences.
-
The number of missing observations for each item of health-care resource use and of the EQ-5D-3L questionnaire will be presented.
-
Imputation of missing data will no longer be conducted.
-
Regression analysis will no longer be conducted.
-
Uncertainty analysis and both deterministic (one way and multiway) and probabilistic (bootstrapping of mean costs and QALYs) sensitivity analyses will no longer be conducted.
Revised aims
The aims of the health economic study are as follows:
-
Calculate the health-care costs associated with providing PCF and ACD, including surgical staff time, theatre space, devices, tests, consumables (anaesthesia) and hospital bed use.
-
Report the frequency and type of health and social care service use after initial discharge from hospital both in the community [contacts with general practitioner (GP), nurses at general practice or home, physiotherapist, chiropractor/osteopath, walk-in clinic and telephone support] and hospital [ward/outpatient attendance and accident and emergency (A&E) attendance], and estimate their associated costs for each trial arm. Use of prescription of medications is only described. Patients’ out-of-pocket costs and productivity losses from time off work as a result of the initial surgical intervention or its complications are described and their costs estimated.
-
Derive utility values from the EQ-5D-3L for each trial arm.
-
Describe the level of completion of health and social service resource use and HRQoL for each trial arm. This is intended to inform strategies to minimise missing data in a future definitive trial.
These aims reflect the objectives of the internal pilot study in the FORVAD trial, which sought to assess the feasibility of methods of data collection, with the aim to inform the design of a future economic evaluation alongside an adequately powered trial. Because no formal interim analyses were planned, this report is descriptive in scope.
Data collection for the health economics component
Health-related quality-of-life data and health resource use questionnaire data were collected as shown in Table 10.
| Measure | Assessment | |||||
|---|---|---|---|---|---|---|
| Baseline (day 1 for procedure) | Week 6 | Week 12 | Week 26 | Week 39 | Week 52 | |
| Initial operation (resources involved in surgery for both arms) | ✓ | a | ||||
| Health-care resource use questionnaire (records the type and amount of health-care resource use, including clinical contacts, social care and productivity losses) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| EQ-5D-3L (measures HRQoL in adults to estimate utility scores for deriving QALYs) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Methods
Health-care resource use was analysed by the ITT principle and valued from the perspective of the NHS and Personal Social Services. No covariate or subgroup analysis was conducted because of the early termination of the study at the internal pilot phase, when the number of recruited participants was too small to allow estimation and inferential analyses of treatment differences. Data were downloaded into Stata®/SE 16.0 (StataCorp LP, College Station, TX, USA) for analysis.
We calculated the following cost categories from baseline to 52 weeks post surgery:
-
initial surgical intervention and initial hospitalisation stay
-
subsequent health-care services
-
out-of-pocket costs and productivity losses as a result of surgery or its complications.
Intervention and comparator surgery and initial hospitalisation costs
The costs of resources used during the surgical procedures were collected for each trial participant in the trial case report forms;61 these included the type of implant used (cage or plate) for ACD; time in theatre from skin incision to closure for ACD and PCF; the grade of surgeons and anaesthetists involved in the operations; and number of days in the inpatient ward, high-dependency unit and/or intensive care unit.
Information on unit costs of resources involving surgical procedures was obtained from published sources, including Hutton. 55 The hourly cost of theatre (Information Services Division Scotland 2012), surgeon56 and anaesthetist salaries,57 and the mean cost per day spent in an inpatient ward and high-dependency and intensive care units58 were also obtained. The cost of theatre is from Scotland, which was the only identified source of data on costs in time units available for the UK. The cost of medications was obtained from the British National Formulary59 for typical regimens prescribed to patients according to the opinion of clinical experts in the research team. Costs were reflated to 2020 prices using published price indices56,60 as needed. Table 11 presents further details.
| Item | Cost (£) | Unit | Details |
|---|---|---|---|
| ACD devices | |||
| Cervical cage price | 533.54 | Item | Median price and range across trusts by brand (2017/2018); purchase price index and benchmark tool cited in Hutton;55 £220 (minimum)–£1255 (maximum) |
| Level 1: plates, screws and pins | 377.15 | Item | Same as above; £154 (minimum)–£1494 (maximum) |
| Caspar distractor pins | 145.20 | Instance | Market price G.S. Online Store www.ninelife.uk/products/gs-caspar-cervical-distractor-lift-with-screws-pins-neurosurgi-instrument-op016-best-quality?gclid=Cj0KCQ jw9ZGYBhCEARIsAEUXITXd4o4yKx8JxHY0lsgOKVQ5hILTlyTxsOR2epHtkXwuhjLHmRHN5R8aAmTaEALw_wcB |
| Equipment | |||
| Microscope | 26.17 | Per surgery | Assumed £150,000 acquisition price for a productive life of 10 years, discounted at 3% per year and used for 500 surgical operations per year on average; used for both procedures |
| Drill tips | 16.15 | Per tip | Bone drill bit £134.64 + value-added tax, 10 pieces per pack [www.orthopedicdrills.com/ (accessed 17 May 2022)] |
| Theatre costs | |||
| Theatre | 1220.00 | Hour | Net cost per theatre hour used, April 2018–March 2020, ISD Scotland61 |
| Drugs and tests | |||
| Plain radiography | 45.00 | Occasion | Plain Film, IMAGOTH (image: other), NHS Reference costs 2019/2060 |
| General anaesthesia | |||
| Propofol | 16.79 | 262.5 mg (induction + 2-hour maintenance) injection | 70-kg adult; Propofol-®Lipuro 0.5% emulsion for injection 20-ml ampoules (B. Braun, Melsungen, Germany), 5 mg per 1-ml ampoules, five ampoules £17.77 (hospital only). Induction: 0.75 mg/kg; maintenance: 3 mg/kg/hour (BNF62). Same for both procedures |
| Remifentanil | 13.98 | 1.89 mg (1 mg induction + 2-hour maintenance infusion) | 70-kg adult; remifentanyl hydrochloride (Wockhardt Ltd, Mumbai, India), 1 mg of powder for concentrate for solution for injection vials, £25.60, five vials (hospital only). Induction: infusion of 30 μg/kg/hour; maintenance: 12 μg/kg/hour (BNF62). Same for both procedures |
| Perioperative antibiotic prophylaxis | |||
| Aminoglycoside | 2.29 | 105-mg injection (three vials) | 70-kg adult; gentamicin (Cidomycin®, Sanofi S.A., Paris, France), 80-mg/2-ml solution for injection vials, five vials, £6.88 (hospital only). Slow i.v. injection, 1.5 mg/kg; i.v. injection administered over at least 3 minutes within 30 minutes of operation; one dose (BNF62)63 |
| Cephalosporin | 5.05 | 1.5-g injection vial | Cefuroxime, 1.5 g of powder for injection vials (Flynn Pharma Ltd, Stevenage, UK), one vial (hospital only) administered up to 30 minutes before the procedure (BNF62) |
| Penicillin | 6.00 | 2-g injection vial | Flucloxacillin, 2 g of powder for solution for injection vials (Bowmed Ibisqus Ltd, Chirk, UK), one vial (hospital only), administered up to 30 minutes before the procedure |
| Glycopeptide | 7.57 | 400-mg injection vial | Teicoplanin, 400 mg of powder and solvent for solution for injection vials (Kent Pharmaceuticals Ltd, Ashford, UK), NHS indicative price £7.57, administered up to 30 minutes before the procedure |
| Clinical staff | |||
| Surgeon | 114.00 | Hour | Cost per working hour (surgeon). Curtis and Burns,56 table 14, p. 158. Includes overheads and accountsa |
| Assistant to surgeon | 50.00 | Hour | Registrar. Curtis and Burns,56 table 14, p. 158a |
| Nurse assistant in surgery | 50.00 | Hour | Hospital-based nurse, band 6, Curtis and Burns,56 table 13, p. 155;a two nurses were costed in every operation |
| Anaesthetist | 114.00 | Hour | Surgical consultant, Curtis and Burns,56 table 14, p. 158a |
| Operating department professional | 50.00 | Hour | Hospital-based operating department practitioner, band 6, Curtis and Burns,56 table 13, p. 155a |
Subsequent health-care resource use
Resource use
Quantities of health-care services were collected for each trial participant at weeks 6, 12, 26, 39 and 52 of follow-up using self-completed questionnaires. The questionnaire included the following items:
-
hospital services, including A&E, and ward or outpatient hospital attendance
-
primary care and/or community-based services, including GP, nurse at general practice, district nurse at home, physiotherapist, chiropractor/osteopath, walk-in centres and telephone support (e.g. NHS 111)
-
treatments received, including cervical nerve root injections, and prescribed medications, including antibiotics, painkillers, dressings and other. Owing to the lack of information on regimens prescribed, the costs of medications are not calculated; only frequencies of reported use are available and reported herein.
The questionnaire also includes whether or not the participant had to take time off work because of surgery or its complications and the number of working days that occurred since the last follow-up point or, for the 6-week questionnaire, since baseline.
Unit costs
Primary care consultations and community services were costed using the Unit Costs of Health and Social Care 2020. 56 The unit costs of Agenda for Change hospital services were obtained from the national reference costs60 and Agenda for Change. 64 Table 12 presents further details.
| Item | Cost (£) | Unit | Details |
|---|---|---|---|
| Hospital services | |||
| Standard ward | 385.16 + 503.96 per day | Day | NHS reference costs 2017/1865 |
| Intensive therapy unit and recovery room | 834.00 | Day | Adult critical care, 0 organs supported. CCU02, NCC 2019/2060 |
| Outpatient appointment | 126.85 | Visit | NCC 2018/1966 (weighted average of all consultantled and non-consultant-led outpatient attendances) |
| Day-case treatment | 751.90 | Encounter | NCC 2018/1966 (weighted average of all day-case episodes) |
| A&E attendance | 166.05 | Visit | NCC 2018/1966 (weighted average of all A&E episodes) |
| Primary care and/or community-based services a | |||
| GP | 39.00 | Consultation | Includes costs of qualifications and direct care (nurse) staff, consultation lasting 9.22 minutes; Curtis and Burns56 |
| Practice nurse at surgery/health centre | 12.43 | Visit | Curtis67 and Curtis and Burns68 (£37 per hour, 15.5 minutes per contact, 1 : 0.30 direct-to-indirect time ratio) |
| Practice nurse via telephone | 7.80 | Contact | Curtis and Burns56 |
| Practice nurse at home | 39.68 | Visit | NCC 2018/1966 (N02AF – district nurse, face to face) |
| Chiropractor/osteopath | 42.50 | Contact | Mid-point of two costs (£40.06 and £44.95); Newell et al. (2016),70 General Osteopathic Council 202171 |
| Physiotherapist | 62.90 | Contact | NCC 2018/1966 (A08A1) |
| Walk-in centre | 21.00 | Visit | Curtis and Burns68 (assume 15 minutes of band-6 nurse time) |
| Productivity losses | |||
| Work days lost to surgery or its complications | 141.66 (full time) 55.08 (part time) | Per day | Annual Survey of Hours and Earnings 2019, Office for National Statistics;69 reflated to 2020 values (instead of COVID-distorted values for 2020); gross hourly wage times 8 (full-time) or 5 (part-time) hours |
Health-related quality of life
The EQ-5D measures the patient HRQoL for obtaining health states and deriving preference-based assessments or utility scores for health states. Utility scores are typically used to estimate QALYs. The EQ-5D-3L questionnaire was given to families in paper format and utilities were derived using the UK tariff values. 72
Analysis
We estimated the costs of intervention, health-care resource use and productivity losses using the unit costs presented in Tables 11 and 12. Out-of-pocket costs were presented as reported by participants in the trial.
Average health-care resource use was estimated for each treatment arm. The frequency of contact for each health service was tabulated. The mean and SD and median and IQR for the frequencies and costs of health-care resource use elements, productivity costs and EQ-5D-3L dimensions and scores in each trial arm were calculated at baseline, at day 1 and at 6, 12, 26, 39 and 52 weeks post surgery. For the EQ-5D-3L, the differences in the mean score from baseline at each follow-up point are also presented for each trial arm.
Health-care resource use data were combined with unit costs to calculate health-care service use costs. Recorded use of medications is described, but, in the absence of information on regimens administered, these resources were not costed. The analysis is based on complete-case data. When aggregating the costs of intervention and health care, no adjustment was made for patients who were missing data. Uncertainty around the means of EQ-5D scores and health-care and productivity costs are described using 95% CIs for each treatment arm.
We present per-patient cost estimates from an NHS and Personal Social Services perspective, and, separately, from a societal perspective. The societal perspective includes, in addition to the costs included in the NHS and Personal Social Services (i.e. health-care costs of providing the initial surgical intervention and downstream health-care and Personal Social Services costs post initial hospital discharge), the costs borne and reported by the participant (i.e. out-of-pocket costs) and the productivity costs of time taken off work as a result of their surgery and its complications.
Because all costs and utilities occur within a 12-month period, no discounting is applied to costs. Results are presented in Great British pounds at 2019–20 prices.
Completeness of health economics data
Questionnaire response rates were calculated for each item of health-care resource use and EQ-5D classification system. The number of returned questionnaires was recorded and the response rate percentage was calculated relative to a maximum response rate of 14 participants for the PCF arm and nine participants for the ACD arm, at baseline and each follow-up point.
We present costs for all those participants with available data for each cost item, whereas, when we aggregate into total costs for each category of resource (i.e. intervention, downstream health-care costs, out-of-pocket costs and productivity costs), only participants with complete data for all items in the respective category are included (complete-case analysis). As high attrition after the 6-week follow-up resulted in few observations with complete cost data across the full 52-week period of follow-up, we have presented total costs from the NHS and Personal Social Services perspective and, separately, total costs from the societal perspective up to the 6-week follow-up point, in a complete-case analysis.
Results
Surgical intervention costs
The mean duration of the operation was 61 (SD 24) and 90 (SD 24) minutes in the PCF and ACD arms, respectively (Table 13). The mean duration of anaesthesia administration was 98 and 124 minutes for the PCF and ACD arms, respectively. Principal surgeons operating on participants in the trial were assisted by a surgical registrar and nursing staff. Principal anaesthetists were assisted by middle-grade anaesthetists and an operating department professional. No blood transfusions were recorded, and the operation involved two sets of radiographs in the PCF arm and one set in the ACD arm for the median participant.
| Operation time | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean time (SD) | Median time (IQR) | n | Mean time (SD) | Median time (IQR) | |
| Start to stop of anaesthesia | 13 | 98 (24) | 90 (79–109) | 9 | 124 (20) | 127 (116–142) |
| Knife to skin to last stitch | 13 | 61 (22) | 52 (49–60) | 8 | 90 (23) | 100 (75–109) |
Perioperative antibiotic prophylaxis was administered to 93% (13/14) of participants in the PCF arm and 88% (8/9) in the ACD arm. Combination therapy with a penicillin and an aminoglycoside was the most common antibiotic regimen used: 69% (9/13) in the PCF arm and 75% (6/8) in the ACD arm. Two participants in the PCF arm and one participant in the ACD arm were given an aminoglycoside with a glycopeptide; cephalosporin was given alone (one participant in the PCF arm), in combination with penicillin (one participant in the PCF arm) or with glycopeptides (one participant in the ACD arm). Further details on the devices used and tests performed are presented in Table 14.
| Resource use | Participants, n (%) | |
|---|---|---|
| PCF arm (N = 14) | ACD arm (N = 9) | |
| Perioperative antibiotics | ||
| Used | 13 (92.9) | 8 (88.9) |
| Not used | 1 (7.1) | 1 (11.1) |
| Drill | ||
| Used | 8 (57.1) | 7 (77.8) |
| Not used | 6 (42.9) | 2 (22.2) |
| Number of drill tips | ||
| Zero | 6 (46.1) | 2 (25.0) |
| One | 6 (46.1) | 6 (75.0) |
| Two | 1 (7.7) | 0 (0.0) |
| Missing data | 1 | 1 |
| ACD implant | ||
| Cage alone | N/A | 5 (55.6) |
| Cage and bone substitute | 4 (44.4) | |
| ACD plate and screws | ||
| Plate applied | N/A | 1 (11.1) |
| Plate not applied | 8 (88.9) | |
| Postoperative drain | ||
| Drain used | 14 (100.0) | 8 (88.9) |
| Drain not used | 0 (0.0) | 1 (11.1) |
| Skin closure | ||
| Staples | 2 (14.3) | 1 (11.1) |
| Dissolvable suture | 10 (71.4) | 8 (88.9) |
| Non-dissolvable suture | 2 (14.3) | 0 (0.0) |
| Blood loss (ml) | ||
| 0–100 | 6 (46.1) | 7 (87.5) |
| 101–250 | 6 (46.1) | 1 (12.5) |
| 251–500 | 1 (7.7) | 0 (0.0) |
| Missing data | 1 | 1 |
| Sets of intraoperative radiographs | ||
| One | 2 (15.4) | 8 (88.9) |
| Two | 10 (76.9) | 1 (11.1) |
| 10 | 1 (7.7) | 0 (0.0) |
| Missing data | 1 | 0 |
Among those who were recorded to have received analgesics on day 1 postoperatively, one participant in the ACD arm had missing information on the type of analgesic administered. All participants with available information received paracetamol (12 participants in the PCF arm and nine in the ACD arm). Two-thirds of PCF participants and 87.5% of ACD participants received additional medications: weak opioids (three and one participants in the PCF and ACD arms, respectively), weak opioids and neuromodulating agents (NMAs) (two participants in each arm), both weak and strong opioids with (one participant in each arm) and without NMAs (one participant in each arm), weak and strong opioids with non-steroidal anti-inflammatory drugs (NSAIDs) (one participant in the PCF arm) and strong opioids with (one ACD participant) and without (one ACD participant) NMAs.
At 6 weeks’ follow-up, eight (66.6%) and seven (77%) participants in the PCF and ACD arms, respectively, had a recorded medication, with one instance in each arm being the use of an antibiotic (Table 15). The other participants used medication for pain, which most often included drug combinations involving paracetamol and weak opioids, either without an additional analgesic (two ACD participants) or with the addition of strong opioids, NMAs and NSAIDs (one PCF participant); strong opioids (one PCF participant); NMAs and NSAIDs (two PCF participants); or NMAs (two ACD participants). The remaining participants used a single drug: paracetamol (one PCF participant), strong opioids (two participants, one in each arm), NSAIDs (one PCF participant) or NMAs (one ACD participant).
| Resource use at follow-up | Participants, n (%) | |
|---|---|---|
| PCF arm (N = 14) | ACD arm (N = 9) | |
| Day 1 after surgery | ||
| Use of analgesics since surgery | 13 (100.0) | 8 (88.9) |
| Missing data | 1 | 0 |
| MRI/CT of spine post-surgery (%) | 0 (0.0) | 0 (0.0) |
| Missing data | 0 | 0 |
| Sets of postoperative radiographs | ||
| One | 9 (69.2) | 5 (55.6) |
| Two | 3 (23.1) | 4 (44.4) |
| Three | 1 (7.7) | 0 (0.0) |
| Missing data | 1 | 0 |
| Week 6 follow-up assessment | ||
| Clinic appointment occurred | 12 (92.3) | 8 (88.9) |
| Did not occur | 1 (7.7) | 1 (11.1) |
| Missing data | 1 | 0 |
| GP or hospital attendance | ||
| Yes | 5 (41.7) | 7 (77.8) |
| No | 7 (58.3) | 2 (22.2) |
| Missing data | 2 | 0 |
| Postoperative medication | ||
| Yes | 8 (66.7) | 7 (77.8) |
| No | 4 (33.3) | 2 (22.2) |
| Missing data | 2 | 0 |
| MRI/CT of spine | ||
| Yes | 2 (16.7) | 3 (33.3) |
| No | 10 (83.3) | 6 (66.7) |
| Missing data | 2 | 0 |
The mean length of hospital stay was 1.2 (SD 0.3) and 1.3 (SD 1) days in the PCF and ACD groups, respectively; the IQR was fixed at 1 day in both groups. The total mean cost of the initial surgical therapy was £4294 (SD £631, 95% CI £2344 to £3147) per participant in the PCF arm and £4295 (SD £1028, 95% CI £3436 to £5154) per participant in the ACD arm (Table 16). In contrast, the median total costs were £2622 (IQR £2402–2824) and £4423 (IQR £3849–4821) in the PCF and ACD arms, respectively, which were dominated by the costs of theatre. It is noted that one participant in each arm was missing information on the duration of the operation, and therefore was missing data on the cost of surgical staff input and theatre use; two participants in the PCF arm were missing data on date of discharge from hospital and on costs of hospital stay.
| Cost | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean cost (SD) | Median cost (IQR) | Participants (n) | Mean cost (SD) | Median cost (IQR) | |
| Surgeonsa | 13 | 200 (101) | 154 (143–214) | 8 | 261 (64) | 287 (205–297) |
| Anaesthetistsb | 13 | 240 (117) | 178 (157–285) | 9 | 377 (173) | 278 (249–496) |
| Implants | 14 | 0 (0) | 0 (0) | 9 | 502 (294) | 722 (189–723) |
| Medicationsc | 14 | 65 (3) | 65 (65–65) | 9 | 65 (3) | 65 (65–65) |
| Theatre | 13 | 1265 (456) | 1091 (1013–1247) | 8 | 1870 (486) | 2065 (1559–2260) |
| Other resources in operationd | 13 | 85 (18) | 78 (78–97) | 9 | 91 (9) | 97 (81–97) |
| Inpatient stay | 12 | 995 (200) | 909 (909–909) | 9 | 1081 (515) | 909 (909–909) |
| Total costs of surgical intervention | 12 | 2745 (631) | 2622 (2402–2824) | 8 | 4294 (1028) | 4423 (3849–4821) |
Health-care and social care resource use, and out-of-pocket and productivity costs
At week 6, the mean costs in the ACD arm were driven by a single participant with a recorded attendance at A&E on seven separate occasions; this participant was missing data on some of the other costs elements (ward or outpatient attendances and telephone support), and was therefore excluded in the aggregate total health-care cost variable. As a result, the total health-care costs were more than halved relative to what they would have been had the participant been included with zero values imputed for the missing cost data. However, most use of health-care services related to GP visits and contacts with the nurse at the general practice (see Appendix 6, Table 41). Apart from these two service categories, the majority of participants incurred no resource utilisation, resulting in zero costs for the median participant. The most prescribed medications were analgesics, reported by nine and four participants in the PCF and ACD arms, respectively, and antibiotics were prescribed for one and two participants in the PCF and ACD arms, respectively (see Appendix 6, Table 40). In the ACD arm, one participant recorded receipt of an analgesic and antihistamine drugs, and another recorded receipt of an antibiotic and codeine. Of the six participants who reported having paid for medications, only three provided the amount they spent on them: one participant in the PCF group spent £12; in the ACD group, one participant reported spending £5.50 and another spent £0.70. Most of these expenses were for painkillers (data not presented in Table 17). Table 17 presents further details.
| Resource use and cost | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean (SD), unless stated as % or median (IQR) | Missing (n) | Participants (n) | Mean (SD), unless stated as % or median (IQR) | Missing (n) | |
| Hospital services | ||||||
| Cost (£) of A&E attendances | 10 | 0 (0) | 4 | 8 | 169.72 (416) | 1 |
| Cost (£) of ward/outpatient attendances | 10 | 77.79 (125) | 4 | 7 | 55.56 (69) | 2 |
| Primary care and/or community-based services | ||||||
| Cost (£) of GP at general practice | 9 | 43.3 (45) | 5 | 8 | 102.00 (182) | 1 |
| Cost (£) of nurse at general practice | 9 | 19.84 (13) | 5 | 8 | 3.19 (6) | 1 |
| Cost (£) of nurse at home | 9 | 0.00 (0) | 5 | 8 | 81.12 (229) | 1 |
| Cost (£) of telephone support | 10 | 0.78 (2) | 4 | 7 | 2.92 (6) | 2 |
| Cost (£) of physiotherapist | 10 | 12.80 (27) | 4 | 8 | 0.00 (0) | 1 |
| Cost (£) of chiropractor/osteopath | 10 | 0.00 (0) | 4 | 8 | 0.00 (0) | 1 |
| Cost (£) of walk-in clinic attendances | 10 | 2.15 (7) | 4 | 4 | 0.00 (0) | 5 |
| Total NHS health-care costs (£) | 8 | 183.77 (206) | 6 | 7 | 125.80 (143) | 2 |
| Out-of-pocket costs | ||||||
| Paid for any other medication (%) | 11 | 27.3 | 3 | 8 | 37.5 | 1 |
| Out-of-pocket costs, if positive (£) | 1 | 12.00 | 2 | 2 | 3.10 (3) | 1 |
| Total out-of-pocket costs (£) | 9 | 1.33 (4) | 5 | 7 | 0.89 (2) | 2 |
| Productivity losses | ||||||
| Taken time off work as a result of surgery since hospital discharge (%) | 11 | 54.5 | 3 | 8 | 62.5 | 1 |
| Not taken time off work as a result of surgery since hospital discharge (%) | 11 | 18.2 | 3 | 8 | 12.5 | 1 |
| Time off work question not applicable (%) | 11 | 27.3 | 3 | 8 | 25.0 | 1 |
| Number of working days off, if time off work | 5 | 36.4 (18) | 1 | 4 | 32.00 (7) | 1 |
| Median (IQR) | 5 | 40.00 (30–42) | 1 | 4 | 32.5 (26.5–37.5) | 1 |
| Total productivity losses (£) | 10 | 1942.75 (2320) | 1 | 7 | 1520.51 (1596) | 1 |
| Median (IQR) (£) | 10 | 708.29 (0–4250) | 1 | 7 | 1591.20 (0–3007) | 1 |
When the number of days off work as a result of surgery or its complications were evaluated at the gross hourly wage in the UK according to whether the participant worked part time or full time and their age, the mean productivity costs during the first 6 weeks after surgery amounted to £1942.75 (SD £2320, 95% CI £283 to £3602) per participant in the PCF arm and £1520.51 (SD £1596, 95% CI £44 to £2997) per participant in the ACD arm (see Table 17). Since the mean figure for PCF was affected by outliers, the median productivity costs are also presented in Table 17 and are £708.29 (IQR £0–4250) in the PCF arm and £1591.20 (IQR £0–3007) in the ACD arm.
Most use of health-care services during the period was between weeks 6 and 12 post surgery and was related to GP visits (see Appendix 6, Table 42). Apart from these service categories, the majority of participants incurred no resource utilisation, resulting in zero costs for the median participant. Owing to incomplete data, the aggregate total health-care cost variable was available for only nine out of 14 participants in the PCF arm and three out of nine participants in the ACD arm, and had mean values of £115 (SD £96, 95% CI £41 to £189) and £125 (SD £71, 95% CI –£52 to £303) for the PCF and ACD arms, respectively (Table 18).
| Resource use and cost | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean (SD), unless stated as % or median (IQR) | Missing (n) | Participants (n) | Mean (SD), unless stated as % or median (IQR) | Missing (n) | |
| Hospital services | ||||||
| Cost (£) of A&E attendances | 10 | 16.97 (53) | 4 | 4 | 0.00 (0) | 5 |
| Cost (£) of ward/outpatient attendances | 11 | 47.14 (65) | 3 | 4 | 32.41 (65) | 5 |
| Primary care and/or community-based services | ||||||
| Cost (£) of GP at general practice | 11 | 31.90 (38) | 3 | 4 | 68.25 (19) | 5 |
| Cost (£) of nurse at general practice | 10 | 6.38 (7) | 4 | 4 | 3.19 (6) | 5 |
| Cost (£) of nurse at home | 10 | 0.00 (0) | 4 | 4 | 0.00 (0) | 5 |
| Cost (£) of telephone support | 10 | 3.12 (5) | 4 | 4 | 0.00 (0) | 5 |
| Cost (£) of physiotherapist | 9 | 14.29 (28) | 5 | 4 | 0.00 (0) | 5 |
| Cost (£) of chiropractor/osteopath | 10 | 0.00 (0) | 4 | 4 | 0.00 (0) | 5 |
| Cost (£) of walk-in clinic attendances | 10 | 2.15 (7) | 4 | 4 | 0.00 (0) | 5 |
| Total health-care costs (£) | 9 | 115.30 (96) | 5 | 3 | 125.47 (71) | 6 |
| Out-of-pocket costs | ||||||
| Paid for any other medication (%) | 12 | 33.3 | 2 | 6 | 16.7 | 3 |
| Out-of-pocket costs, if positive (£) | 4 | 9.65 (5) | 0 | 1 | 1.4 (0) | 0 |
| Out-of-pocket costs (£) | 12 | 3.22 (5) | 2 | 6 | 0.23 (0) | 3 |
| Productivity losses | ||||||
| Taken time off work as a result of surgery in previous 12 weeks (%) | 12 | 33.3 | 2 | 6 | 33.3 | 3 |
| Not taken time off work as a result of surgery in previous 12 weeks (%) | 12 | 41.7 | 2 | 8 | 50.0 | 1 |
| Time off work question not applicable (%) | 12 | 25.0 | 2 | 8 | 16.7 | 1 |
| Number of working days off, if time off work | 2 | 35.0 (35) | 2 | 2 | 67.5 (53) | 0 |
| Median (IQR) | 2 | 35.0 (10–60) | 2 | 2 | 67.5 (30–105) | 0 |
| Productivity losses (£) | 10 | 991.60 (2675) | 4 | 6 | 2227.27 (4719) | 3 |
| Median (IQR) (£) | 10 | 0.00 (0–0) | 4 | 6 | 0.00 (0–1591) | 3 |
At week 12, the most prescribed medications were analgesics, reported by nine and two participants in the PCF and ACD arms, respectively, and antibiotics were prescribed to three and two participants in the PCF and ACD arms, respectively. One participant in each trial arm also reported being prescribed dressings. In the PCF arm, one participant recorded receipt of a mucolytic and in the ACD arm one participant reported use of ‘tranexamic acid for periods’. The five participants who reported having paid for medications provided the amount they spent for them: four participants in the PCF group spent a mean of £9.65 and one participant in the ACD group reported spending £1.40. All of these expenses were for analgesics, except for one participant in the PCF arm who reported use of antidepressants.
The number of days off work as a result of surgery or its complications occurring between weeks 6 and 12 was evaluated at the median gross hourly wage in the UK corresponding to the age of the individual participant and the type of work, part or full time, reported at baseline. The resulting mean productivity costs amounted to £991.60 (SD £2675, 95% CI –£992 to £2905) and £2227.27 (SD £4719, 95% CI –£2725 to £7180) per participant in the PCF and ACD groups, respectively (see Table 18). However, most participants had zero productivity costs, as only two participants in each arm out of the 10 and six in the PCF and ACD arms, respectively, with available data had incurred any costs.
Between weeks 13 and 26, the mean cost of health-care service use was £96 in the PCF arm and £284 in the ACD arm. These resulted primarily from four and five ward or outpatient attendances in the PCF and the ACD arm, respectively, two visits to A&E in the ACD arm and at least one GP visit for the majority of the participants in both arms (see Appendix 6, Table 43). The rate of missing data on health-care services used was 21% (3/14) in the PCF arm and 44% (4/9) in the ACD arm.
Taking time off work as a result of surgery or its consequences between postoperative weeks 13 and 26 was reported by two participants in the PCF arm and one participant in the ACD arm (Table 19). This information was not available for 21% (3/14) and 22% (2/9) of participants enrolled in the PCF and ACD arms, respectively. Data on the number of days of work lost for those who took time off work were missing for one participant in each arm. The one participant with available data was in the PCF arm, and reported 2 days of work lost as a consequence of the surgery or its complications over the previous 3 months.
| Resource use and cost | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean (SD), unless stated as % or median (IQR) | Missing (n) | Participants (n) | Mean (SD), unless stated as % or median (IQR) | Missing (n) | |
| Hospital services | ||||||
| Cost (£) of A&E attendances | 11 | 0.00 (0) | 3 | 5 | 67.89 (93) | 4 |
| Cost (£) of ward/outpatient attendances | 11 | 47.14 (65) | 3 | 5 | 129.65 (159) | 4 |
| Primary care and/or community-based services | ||||||
| Cost (£) of GP at general practice | 11 | 39.00 (46) | 3 | 6 | 52.00 (40) | 3 |
| Cost (£) of nurse at general practice | 11 | 2.32 (5) | 3 | 5 | 0.00 (0) | 4 |
| Cost (£) of nurse at home | 11 | 0.00 (0) | 3 | 5 | 0.00 (0) | 4 |
| Cost (£) of telephone support | 11 | 2.13 (7) | 3 | 5 | 1.56 (3) | 4 |
| Cost (£) of physiotherapist | 11 | 0.00 (0) | 3 | 5 | 38.57 (35) | 4 |
| Cost (£) of chiropractor/osteopath | 11 | 3.95 (15) | 3 | 5 | 0.00 (0) | 4 |
| Cost (£) of walk-in clinic attendances | 11 | 1.95 (6) | 3 | 5 | 0.00 (0) | 4 |
| Total health-care costs (£) | 11 | 96.49 (94) | 3 | 5 | 284.47 (251) | 4 |
| Out-of-pocket costs | ||||||
| Paid for any other medication (%) | 9 | 22.2 | 5 | 6 | 33.3 | 3 |
| Out-of-pocket costs, if positive (£) | 2 | 6.45 (5) | 0 | 2 | 2.32 (1) | 0 |
| Out-of-pocket costs (£) | 9 | 1.43 (3) | 5 | 6 | 0.77 (1) | 3 |
| Productivity losses | ||||||
| Taken time off work as a result of surgery in previous 13 weeks (%) | 11 | 18.2 | 3 | 6 | 16.7 | 3 |
| Not taken time off work as a result of surgery in previous 13 weeks (%) | 11 | 36.4 | 3 | 6 | 66.7 | 3 |
| Time off work question not applicable (%) | 11 | 45.4 | 3 | 6 | 16.7 | 3 |
| Number of working days off, if time off work | 1 | 2 (–) | 1 | 0 | – | 1 |
| Median (IQR) | 1 | 2 (2–2) | 1 | 0 | – | 1 |
| Productivity losses (£) | 10 | 25.80 (81) | 4 | 6 | 0.00 (0) | 3 |
| Median (IQR) | 10 | 0.00 (0–0) | 4 | 6 | 0.00 (0.00–0.00) | 3 |
Between weeks 27 and 39, the mean costs of health-care service use were £163 in the PCF arm and £144 in the ACD arm. These resulted mainly from four ward or outpatient attendances in each arm, two visits to A&E and eight sessions with a physiotherapist in the ACD arm, and one or two GP visits for the majority of the participants in both arms (see Appendix 6, Table 44). The rate of missing data on health-care services used was 50% (7/14) in the PCF arm and 22% (2/9) in the ACD arm.
Taking time off work because of the surgery or its consequences between postoperative weeks 27 and 39 was reported by two participants in the PCF arm and one participant in the ACD arm (Table 20). This information was not available for 42% (6/14) and 22% (2/9) of participants enrolled in the PCF and ACD arms, respectively. Data on the number of days of work lost for those who took time off work were missing for one participant in the PCF arm. In each arm, one participant with available data reported 1 day of work lost as a consequence of the surgery or its complications over the previous 3 months.
| Resource use and cost | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean (SD), unless stated as % or median (IQR) | Missing (n) | Participants (n) | Mean (SD), unless stated as % or median (IQR) | Missing (n) | |
| Hospital services | ||||||
| Cost (£) of A&E attendances | 7 | 48.49 (83) | 7 | 5 | 0.00 (0) | 4 |
| Cost (£) of ward/outpatient attendances | 7 | 74.09 (102) | 7 | 5 | 103.72 (169) | 4 |
| Primary care and/or community-based services | ||||||
| Cost (£) of GP visits at general practice | 7 | 55.71 (50) | 7 | 6 | 45.50 (29) | 3 |
| Cost (£) of nurse at general practice | 7 | 7.29 (10) | 7 | 5 | 5.10 (7) | 4 |
| Cost (£) of nurse at home | 7 | 0.00 (0) | 7 | 5 | 0.00 (0) | 4 |
| Cost (£) of telephone support | 6 | 1.30 (3) | 8 | 5 | 6.24 (10) | 4 |
| Cost (£) of physiotherapist | 6 | 0.00 (0) | 8 | 5 | 102.86 (133) | 4 |
| Cost (£) of chiropractor/osteopath | 6 | 0.00 (0) | 8 | 4 | 0.00 (0) | 4 |
| Cost (£) of walk-in clinic attendances | 7 | 3.07 (0) | 7 | 5 | 0.00 (0) | 4 |
| Total health-care costs (£) | 6 | 163.05 (191) | 8 | 4 | 144.27 (89) | 5 |
| Out-of-pocket costs | ||||||
| Paid for any other medication (%) | 8 | 25.0 | 6 | 7 | 14.3 | 2 |
| Out-of-pocket costs, if positive (£) | 2 | 21.65 (5) | 0 | 1 | 15.00 (–) | 0 |
| Out-of-pocket costs (£) | 8 | 5.41 (10) | 6 | 7 | 2.14 (6) | 2 |
| Productivity losses | ||||||
| Taken time off work as a result of surgery in previous 13 weeks (%) | 8 | 25.0 | 6 | 7 | 14.3 | 2 |
| Not taken time off work as a result of surgery in previous 13 weeks (%) | 8 | 50.0 | 6 | 7 | 57.1 | 2 |
| Time off work question not applicable (%) | 8 | 25.0 | 6 | 7 | 28.6 | 2 |
| Number of working days off, if time off work | 1 | 1 (0) | 1 | 1 | 1 (0) | 0 |
| Median (IQR) | 1 | 1 (1–1) | 1 | 1 | 1 (1–1) | 0 |
| Productivity losses (£) | 8 | 16.13 (46) | 6 | 7 | 16.02 (42) | 2 |
| Median (IQR) (£) | 8 | 0.00 (0–0) | 6 | 7 | 0.00 (0–0) | 2 |
Between weeks 40 and 52, the mean cost of health-care service use was £92 in the PCF arm and £1290 in the ACD arm. Similarly to previous follow-up time points, the majority of participants had between one and two GP visits, and there were three visits to A&E in the PCF arm and three outpatient or ward attendances in the ACD arm. However, the mean costs in the ACD arm were influenced by a single participant who reported 95 contacts with a physiotherapist (see Appendix 6, Table 45). The rate of missing data on health-care services used was 29% (4/14) in the PCF arm and 44% (4/9) in the ACD arm.
No participant in either arm reported taking time off work because of surgery or its consequences between postoperative weeks 40 and 52 (Table 21). This information was not available for 21% (3/14) and 33% (3/9) of participants enrolled in the PCF and ACD arms, respectively.
| Resource use and cost | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean (SD), unless stated as % or median (IQR) | Missing (n) | Participants (n) | Mean (SD), unless stated as % or median (IQR) | Missing (n) | |
| Hospital services | ||||||
| Cost (£) of A&E attendances | 10 | 50.92 (82) | 4 | 5 | 0.00 (0) | 4 |
| Cost (£) of ward/outpatient attendances | 9 | 0.00 (0) | 5 | 5 | 77.79 (174) | 4 |
| Primary care and/or community-based services | ||||||
| Cost (£) of GP at general practice | 10 | 54.60 (46) | 4 | 6 | 58.50 (41) | 3 |
| Cost (£) of nurse at general practice | 10 | 1.28 (4) | 4 | 5 | 0.00 (0) | 4 |
| Cost (£) of nurse at home | 10 | 0.00 (0) | 4 | 5 | 0.00 (0) | 4 |
| Cost (£) of telephone support | 9 | 1.73 (3) | 5 | 5 | 2.60 (6) | 4 |
| Cost (£) of physiotherapist | 10 | 12.86 (41) | 4 | 6 | 996.49 (2347) | 3 |
| Cost (£) of chiropractor/osteopath | 10 | 0.00 (0) | 4 | 6 | 0.00 (0) | 3 |
| Cost (£) of walk-in clinic attendances | 10 | 2.15 (7) | 4 | 5 | 0.00 (0) | 4 |
| Total health-care costs (£) | 8 | 92.27 (120) | 6 | 5 | 1289.61 (2541) | 4 |
| Out-of-pocket costs | ||||||
| Paid for any other medication (%) | 13 | 23.1 | 1 | 6 | 16.7 | 3 |
| Out-of-pocket costs, if positive (£) | 3 | 3.89 (5) | 0 | 1 | 0.80 (–) | 0 |
| Out-of-pocket costs (£) | 13 | 0.90 (3) | 1 | 6 | 0.13 (0) | 3 |
| Productivity losses | ||||||
| Taken time off work as a result of surgery in previous 13 weeks (%) | 11 | 0 | 3 | 6 | 0 | 3 |
| Not taken time off work as a result of surgery in previous 13 weeks (%) | 11 | 54.5 | 3 | 6 | 33.3 | 3 |
| Time off work question not applicable (%) | 11 | 45.5 | 3 | 6 | 66.7 | 3 |
| Number of working days off, if time off work | 0 | – | 0 | 0 | – | 0 |
| Median (IQR) | 0 | – | 0 | 0 | – | 0 |
| Productivity losses (£) | 12 | 0.00 (0) | 2 | 6 | 0.00 (0) | 3 |
| Median (IQR) (£) | 12 | 0.00 (0–0) | 2 | 6 | 0.00 (0–0) | 3 |
In Table 22 we summarise the results of the costs analysis. There is high sensitivity of the results to attrition in the data and outliers because of the low numbers, most evident in the lack of complete data from any participant in the ACD arm to be able to calculate the total cumulative costs of health care at 52 weeks after the initial surgery. Nevertheless, the median costs up to week 12 point to productivity costs that decline to zero after week 6, and represent 26% and 37% of the costs of the initial operation in the PCF and ACD arms, respectively. The median health-care costs are of much smaller orders of magnitude and the median out-of-pocket costs are zero in both arms. Furthermore, although positive median costs were observed in both arms during weeks 1–6, the median total productivity costs for each group were zero because the three cases in each arm who were lost to subsequent follow-up were among those with positive costs in weeks 1–6.
| Costs (£) | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean (SD) | Median (IQR) | Participants (n) | Mean (SD) | Median (IQR) | |
| Total costs of surgical intervention | 12 | 2745.00 (631) | 2622.00 (2402–2824) | 8 | 4295.00 (1028) | 4423.00 (3849–4821) |
| Health-care costs post intervention | ||||||
| Weeks 1–6 | 8 | 183.77 (207) | 84.00 (26–350) | 7 | 125.80 (143) | 47.00 (13–208) |
| Weeks 7–12 | 9 | 115.30 (96) | 99.00 (39–207) | 3 | 125.47 (71) | 91.00 (78–208) |
| Weeks 13–52 | 5 | 376.05 (403) | 298.41 (125–360) | 3 | 2395.33 (3533) | 453.23 (259–6473) |
| Total | 3 | 793.00 (584) | 900.00 (164–1317) | 0 | – | – |
| Out-of-pocket costs | ||||||
| Weeks 1–6 | 9 | 1.33 (4) | 0.00 (0–0) | 7 | 0.89 (2) | 0.00 (0–1) |
| Weeks 7–12 | 12 | 3.22 (5) | 0.00 (0–6) | 6 | 0.23 (0) | 0.00 (0–0) |
| Weeks 13–52 | 6 | 8.75 (14) | 0.00 (0–25) | 5 | 4.09 (8) | 0.00 (0–3) |
| Total | 3 | 30.00 (23) | 25.00 (10–55) | 5 | 4.51 (9) | 0.00 (0–3) |
| Productivity costs | ||||||
| Weeks 1–6 | 10 | 1942.75 (2320) | 708.29 (0–4250) | 7 | 1520.51 (1596) | 1591.20 (0–3007) |
| Weeks 7–12 | 10 | 991.60 (2675) | 0.00 (0–0) | 6 | 2227.27 (4719) | 0.00 (0–1591) |
| Weeks 13–52 | 8 | 16.13 (46) | 0.00 (0–0) | 7 | 16.02 (42) | 0.00 (0–0) |
| Totala | 7 | 1971.00 (2613) | 0.00 (0–5418) | 4 | 928.20 (1856) | 0.00 (0–1856) |
Estimating the total costs over the 52-week follow-up duration of the trial was not feasible because of high levels of attrition after week 6. Therefore, costs were aggregated up to week 6 only. The mean costs from the NHS and Personal Social Services perspective were £2716 (95% CI £2345 to £3087; missing 6 out of 14) in the PCF arm and £4133 (95% CI £3099 to £5167; missing 3 out of 9) in the ACD arm. The median costs were £2634 (IQR £2444–2741) and £4214 (IQR £3602–4994) in the PCF and ACD arms, respectively. Combining out-of-pocket costs, productivity losses, and NHS and Personal Social Services costs produced mean per-participant total costs to society of £4608 (95% CI £2514 to £6703; missing 7 out of 14) in the PCF arm and £5015 (95% CI £2286 to £7743; missing 4 out of 9) in the ACD arm; the median costs were £4097 (IQR £2448–6591) and £4143 (IQR £4126–4284) in the PCF and ACD arms, respectively.
Health-related quality of life
On day 0 pre surgery, the baseline EQ-5D-3L scores were 0.291 (SD 0.34, 95% CI 0.07 to 0.51) in the PCF arm and 0.595 (SD 0.25, 95% CI 0.40 to 0.78) in the ACD arm. The corresponding median scores were 0.210 (IQR –0.01 to 0.60) in the PCF arm and 0.689 (IQR 0.66 to 0.69) in the ACD arm. Median changes from baseline at day 1 and at weeks 6, 12, 26, 39 and 52 were, respectively, 0.06, 0.10, 0.00, 0.00, 0.29 and 0.06 in the PCF arm and 0.00, 0.02, 0.00, 0.00, –0.03 and –0.09 in the ACD arm. The rate of missing EQ-5D-3L score data varies from a lowest rate of 14% (2/14) at baseline to a highest rate of 64% (9/14) at 39 weeks in the PCF arm and from 0% (0/9) at baseline to 33.3% (3/9) at weeks 12 and 39 in the ACD arm; no imputation was applied in the analysis. Figure 14 illustrates the variation in EQ-5D-3L scores at each follow-up point for each treatment, and a summary table of results is presented in Appendix 6, Table 39.
FIGURE 14.
The EQ-5D-3L scores at each time point, by treatment group.
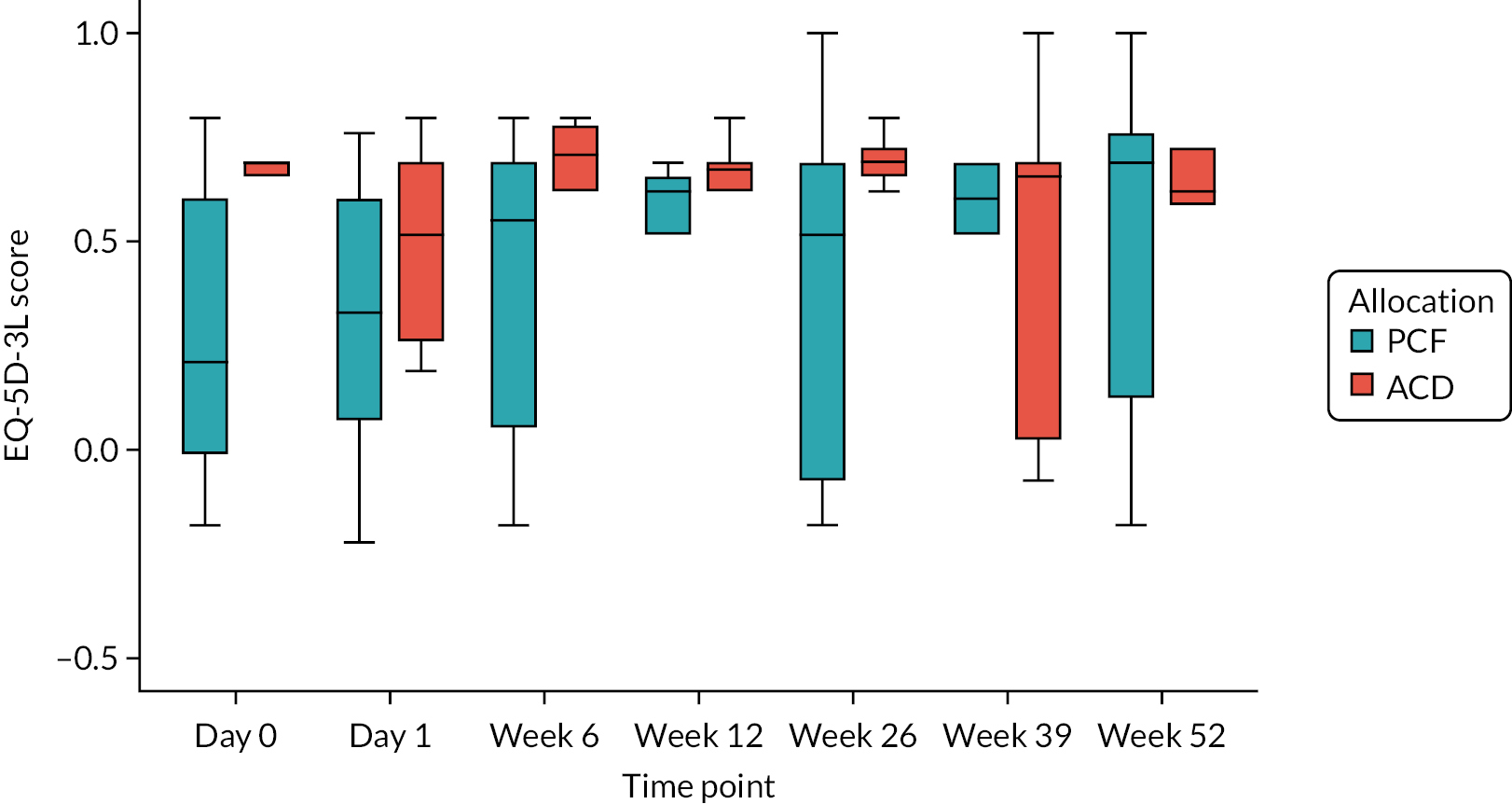
Appendix 6, Table 46 presents the number of participants reporting the level of problems in each of the five EQ-5D dimensions. The distribution of frequencies of reported problems across the two arms is illustrated in Appendix 6, Figure 19, for baseline, day 1 and week 6.
Summary and discussion
This chapter presents the methods and results of the costs and utility outcomes of the surgical interventions investigated in the FORVAD trial, with data collected on 23 trial participants recruited up to the early termination of the trial. It is estimated that PCF costs £2622 (IQR £2402–2824) and ACD costs £4423 (IQR £3849–4821) for the median participant in each trial arm. Subject to the inherent limitations of the small numbers (12 PCF and nine ACD participants), these costs are driven by the cost of implants for ACD and by the shorter time in theatre with PCF.
Similarly, subject to the small numbers available, the results presented herein on productivity losses at 6 weeks for the median participant of £708 (IQR £0–4250) with PCF and £1591 (IQR £0–3007) with ACD suggest possibly fewer days of paid work lost following PCF. In this trial, 54% of participants in the PCF group reported having taken time off work in the first 6 weeks after surgery as a result of the operation, and 62% reported doing so in the ACD group. The figures at week 12 were 33% in both arms; beyond that, there were more instances of missing data than at the previous follow-up points.
Out-of-pocket costs were reported by a minority of participants and were mostly a result of purchasing analgesics. Instances of such costs were few after 12 weeks.
The distribution of EQ-5D-3L scores was observed to be imbalanced across the trial arms at baseline, with median scores of 0.21 (IQR –0.01 to 0.60) in the PCF arm and 0.69 (IQR 0.66 to 0.69) in the ACD arm. This difference is larger than what is considered a clinically important difference, which may be conservatively assumed to be 0.10. 73 Taken at face value, these figures would suggest that the difference in costs may have been larger if the trial had continued with sufficiently larger numbers to achieve a balanced distribution in baseline confounders.
Unlike the report of Tumialán et al.,36 who found that both ACD and PCF procedures had similar median operating times (151.8 vs. 154 minutes, respectively) for unilateral cervical radiculopathy, this trial found that PCF took half an hour less than ACD from skin incision to closure. There was no need for blood transfusion for any of the 23 participants enrolled in the trial. On the other hand, the previous study36 results of large productivity gains from earlier return to normal activities (active duty of military personnel in their case) with PCF are consistent with the results of this small trial. Consistent with the study by Alvin et al.,37 the EQ-5D-3L score gains postoperatively were larger with PCF than with ACD, although, given the much lower baseline score observed in the PCF arm, our results are likely to be affected by ceiling effects. 74 There is evidence of floor effects in the pain and discomfort dimension responses of the PCF trial arm at baseline and at day 1 postoperatively, when the majority of respondents reported a severe level of pain/discomfort, and to some extent at week 6. Future research using the EQ-5D-5L questionnaire may be warranted to address such effects.
The rates of missing data were high, as manifested by the finding that no participant in the ACD arm had the complete data required to estimate the cumulative costs of health-care service use at 52 weeks. This is not surprising given the large number of items involved in the resource use questionnaire; in the light of our results up to 12 weeks, a shortlist of resource items, such as GP visits, A&E, ward and outpatient hospital attendances, and physiotherapy sessions, may be sufficient to capture most of the difference in costs and reduce response fatigue by trial participants. On the other hand, data were missing in an intermittent pattern and at a small rate up to week 6, and the rate of missing data was moderate for weeks 12, 26 and 52. Similar patterns were found for responses to the EQ-5D questionnaire and productivity costs questions.
Besides the small sample of the trial, our analysis was limited by the lack of information on the dosages of medications consumed during the trial. However, this is unlikely to affect the results of an economic analysis, which will be driven by the costs of the operation, and the indirect costs of lost paid work, as described in our results presented herein.
In conclusion, this is the first published report, to our knowledge, of the costs of providing PCF and ACD and their downstream cost and HRQoL consequences from a UK perspective. It highlights the potential for PCF to incur fewer costs and to result in gains similar to or larger than ACD in terms of preference-based HRQoL outcomes. A larger trial will be required to confirm these findings.
Chapter 5 Qualitative study
Introduction
Qualitative research conducted alongside RCTs can help to explain trial findings, illuminate the mechanisms by which interventions are or are not effective and elucidate the intended and unintended consequences of intervention implementation. 75 In addition, qualitative research can help us understand processes such as recruitment, participant retention, trial set-up, and barriers to and facilitators of taking part in RCTs. 76 However, qualitative research has not been used extensively in trials involving surgical interventions. 77 Although recent studies have found that qualitative research can illuminate surgical trials, surgical interventions are inherently complex in nature, requiring an understanding of context in order for trials to successfully fit into established clinical pathways, while maintaining high levels of research integrity and ethics standards. 78,79
Qualitative research has been used to offer insight into patient and health-care professionals’ perspectives of surgical RCTs. 80–83 As a result, there is more understanding of the barriers to and facilitators of successful surgical trial recruitment. However, a number of trials have focused on trial set-up and implementation (such as recruitment),84,85 motivation to take part in trials83,86 and sense-making when understanding expectations of taking part in a RCT,82,87 rather than exploring the barriers to and facilitators of successful trial implementation across multiple sites. 88
Poor recruitment to surgical RCTs is a well-known issue within clinical research,89–92 with many sites failing to reach their expected recruitment targets. 93 Recruitment is often marred by the limited number of patients eligible for recruitment, especially in trials of a complex surgical nature. 94,95 Conversely, the number of potential patients identified as eligible for a trial can vary from site to site, based on surgeon preference alone. 96 In addition, previous studies have shown that delays in resourcing designated trial staff hindered trial set-up, affecting the time available to recruit participants. 93 Key factors influencing patient participation in trials include trust in the trial, positive communication between patient and health professionals,80,97 being able to weigh-up the benefits of taking part and the risk of surgical intervention98 and being able to help further research in surgical areas that have been previously overlooked. 81 The importance of the initial contact between health professionals and patients is an important factor when participating in surgical trials, as is a clear understanding of what patients are to expect if participating in a surgical RCT. 99
In addition, the clinician role in research recruitment is important, particularly when there are issues regarding clinical equipoise and coping with the demands of both a clinical and a research role. 84,97 In addition to issues surrounding equipoise, especially with regard to pragmatic trials, RCTs in surgery may prove challenging because of unfamiliarity with study design, a lack of understanding about the value of randomisation in minimising bias, and aspects of surgical culture and training that are not favourable when conducting a RCT. 100 In recent years there has been an increase in the role of surgeons recruiting patients for RCTs, yet surgeons may find it challenging to convey equipoise during the recruitment phase. 101 If a surgeon has a strong preference for one particular treatment, this may be communicated as a preferred treatment, undermining equipoise and creating bias within a trial. 96,101,102
Understanding the roles and responsibilities of the clinical staff involved in clinical trials enables better understanding of how their roles affect trial success. For example, studies have shown the role of the research nurse to be a crucial aspect of successful trial recruitment. 87,103 Use of research nurses has also been found to be more cost-effective, justifying economic evaluations of clinical staff in randomised trials concerning surgical intervention. 85 This review also stated that research nurses should be considered for enhanced roles when recruiting potential participants;85 nevertheless, this was dependent on resources such as extra training being made available to assist with the increased demands of an enhanced role. Other studies have also reported the benefits of increased training and support during the early stages of recruitment to maximise potential recruitment numbers. 92,104,105 Where surgeons play an active part in the screening and recruitment process, their knowledge and expertise may help to encourage patients to consider taking part in trials. 91,106
The way research sites conduct trials also affects the potential outcome of trials. For example, sites identified logistical issues that made trial recruitment difficult. 96 In addition, sites that recruited from specialist centres were more likely to be able to recruit than sites that did not have specialist facilities. 102 Trial organisation and implementation is often affected by site heterogeneity and facilities. 88,107 In a report by Hackshaw et al.,108 it was suggested that set-up measures should be implemented to streamline trials that had already been approved at national level. Interventions, notably the QuinteT Recruitment Intervention,109 have been developed to optimise trial recruitment. However, there is still a lack of consensus regarding how best to facilitate surgical trials to allow for efficient set-up and incorporation of any clinical pathways that might be in place. Moreover, there is a need to understand how this affects trials pertaining to neurosurgery specifically.
The FORVAD trial experienced challenges in recruiting enough participants. To better understand the nature of these challenges, a rapid qualitative study was conducted during trial close-down to learn more about the set-up, recruitment and delivery of the FORVAD trial, from the perspectives of both patients and health-care professionals, with a view to informing future studies in cervical brachialgia, and neurosurgery in general.
Aims and objectives
The aim of this qualitative study was to understand the set-up, recruitment and delivery of the FORVAD trial, from the perspectives of both patients and health-care professionals, with a view to informing future studies in cervical brachialgia and neurosurgery in general. At present, there is a paucity of surgical trials in neurosurgery specifically. 110,111 In addition, to the best of our knowledge, no studies have considered patient and health-care professionals’ experiences of surgical trials in cervical brachialgia. Therefore, this study aimed to expand the knowledge base in this particular area.
The objectives were as follows:
-
to learn more about the FORVAD trial and the barriers and facilitators when conducting a trial of this nature
-
to understand more about the FORVAD research question from the perspective of each site that took part
-
to gain insight into the set-up and recruitment of the FORVAD trial, from the perspective of health-care professionals that took part in the trial
-
to understand the experience of patients who were recruited to the trial and what taking part in the FORVAD trial was like for them.
Methods and design
A rapid qualitative study was conducted during the close-down phase, involving semistructured interviews with health-care professionals and patients who participated in the FORVAD trial. Interviews were conducted remotely using Microsoft Teams (Microsoft Corporation, Redmond, WA, USA). The interviews explored patients’ experiences of the FORVAD trial and their reasons for taking part, and explored staff experiences of recruiting to the FORVAD trial and neurosurgery trials in general. Interviews were audio-recorded and transcribed verbatim.
Recruitment of sites
Three groups of NHS sites were approached for participation; we aimed to include some sites from each group:
-
those that took part in the FORVAD trial and recruited patients to the trial
-
those that took part in the FORVAD trial but did not recruit any patients to the trial
-
those that were approached about the FORVAD trial but did not open to recruitment.
For the qualitative study, all sites that were open and all sites that had returned a completed feasibility form and were in the process of set-up were approached to take part (11 open sites and six in set-up). In total, 17 NHS sites were approached to take part in the FORVAD qualitative study. Of these, two sites were not able to confirm continued capacity and capability for the study amendment and so declined to take part in the study. Seven sites did not respond to recruitment e-mails or follow-up recruitment e-mails. Eight sites responded to the recruitment e-mails and took part in the qualitative interview phase of the study.
Recruitment of health-care professionals
Participants were identified from authorised personnel (delegation) logs for sites that were willing to take part in the qualitative substudy. All staff identified as working on the trial were invited to participate in an interview. Initial e-mail invitations were sent out to each member of staff along with a copy of the FORVAD qualitative study participant information sheet. A follow-up e-mail was sent after 3–5 days if there had not been a response from the initial e-mail invitation.
All staff who responded to the e-mail invitation were offered a Microsoft Teams interview at a time and date that was convenient to them. A copy of the verbal consent checklist was sent by e-mail prior to the interview. At the start of the Microsoft Teams call, participants were given an opportunity to ask any questions about the study. Consent was recorded verbally at the start of the interview.
Recruitment of patients
Owing to the limited time available to conduct the qualitative study during trial close-down, recruitment for the patient interviews focused on the site with the most participants (18 out of a total of 23 randomised patients), as all other sites had recruited only a very small number of patients. All trial participants from this site were sent an invitation letter and participant information sheet for the qualitative study, along with an expression of interest (EOI) form to return if interested in taking part in the study. Reminder letters were sent out approximately 2 weeks after the initial invitations to anyone who had not returned the EOI form by this point.
Once an EOI form was received, an e-mail was sent to the participant to arrange a date and time for a Microsoft Teams interview. If a response to this e-mail was not received, a reminder was sent 1 week later; if no response to the reminder was received, an attempt was made to contact the participant by telephone. A copy of the verbal consent checklist was sent by e-mail prior to the interview. At the start of the Microsoft Teams call, participants were given an opportunity to ask any questions about the study. Consent was recorded verbally at the start of the interview.
Owing to the limited time available for the qualitative study, the patient interviews were conducted by junior doctors working at the trial site, as they did not require a research passport to conduct the interviews. These junior doctors received training in qualitative interviewing from the FORVAD qualitative research team, as well as ongoing support throughout the fieldwork period. However, it proved challenging to organise training and patient interviews around busy junior doctor schedules, which resulted in a small number of patient interviews being conducted.
Interview procedure
Topic guides were developed based on existing research, our sensitising theoretical framework [normalisation process theory (NPT)] and conversations with the FORVAD TMG members. Two of the three members of the qualitative team (NR and RH) were experienced triallists (although without prior involvement in the FORVAD trial); this helped to direct questions and analysis towards lines of enquiry that were most likely to be fruitful. The third member of the team (RT) was new to the trial context and this helped to ensure that data collection and analysis retained an inductive element. Topic guides covered the following areas: views on the FORVAD trial, setting up the FORVAD trial, surgical approach, experiences of delivering treatments and measuring eligibility and outcomes. The topic guides were used flexibly to allow questions to be tailored to the experience and role of the participant, and were adapted in response to the developing analysis (e.g. specific questions about randomisation on the day of surgery were added to both staff and patient interviews). To maintain rigour and cohesion throughout the interviews, interview principles112 were used as a guide, ensuring good research practice throughout the data collection and analysis process. The interviews were audio-recorded and audio copies of each interview were sent to a transcription service approved for use for the FORVAD qualitative study. All staff interviews were conducted by one of two experienced qualitative researchers (RT or RH); patient interviews were conducted by one of two junior doctors.
Data analysis
The study was undertaken using rapid qualitative analysis, an approach that has been used to enable rapid evaluation of interventions (e.g. clinical and health service models) to inform policy and practice. 113 The use of rapid qualitative analysis allowed the researchers to undertake data collection and analysis together within a short study time frame while maintaining rigour and the facility for findings to be developed iteratively throughout the data collection period.
Data analysis took place concurrently with data collection and was informed by NPT. 114 Meetings of the qualitative team (RT, RH and NR) took place once or twice a week throughout the data collection period to discuss the developing analysis and to make decisions about further data collection. Interviewers shared their reflections of recent interviews so that similarities and differences across the interviews could be identified and categories for the analysis developed. After the first few interviews had been conducted, an initial rapid assessment procedure (RAP) sheet113,115 was developed. Interviewers subsequently used the RAP sheet to summarise each interview as relevant to the developing analysis, to aid understanding of emerging data and to share findings with other members of the research team. As per the process outlined by Vindrola-Padros et al.,113 the RAP sheet formed a working document whereby the research team could explore ongoing findings emerging from these data and aid further refinement of the questions asked during the interview phase of the study. Further familiarisation with the data began when the data had been transcribed verbatim and the process of reading and rereading the transcripts occurred, and annotations were made to highlight items or quotations that were potentially interesting or significant.
The next stage of analysis required collating data from each participant at each site into a table, to help identify and summarise the main issues being discussed at each site, and to compare data across sites to identify whether or not there were any commonalities between sites that could potentially inform the original research question. Each data set was analysed and exploratory comments were divided into three sections: theme name, which focused on a potential main theme; subthemes, which explored subordinate issues emerging from the data; and description of the theme, and descriptive examples from the transcripts that supported the emerging themes. Emergent themes from the data were cross-checked with the transcript to ensure that what was being said was consistent with the preliminary themes and that there was no information missed from the initial analysis of the transcripts. The verification process included detailed discussions among the research team, during which the relevance of each theme was established and the themes/subthemes were further refined and organised into the finalised themes used in the results part of the study. Several analysis sessions more explicitly considered how the emerging findings mapped to (1) existing research on trial conduct and (2) NPT. To help with this process, a table was developed that mapped questions from the FORVAD study to each of the NPT concepts and components.
Results
Participants
Eighteen semistructured interviews were conducted with FORVAD site staff: four with a site principal investigator (PI), five with other surgeons and nine with research nurses/study co-ordinators (Table 23). Six patients responded to the initial e-mail contact; two participated in an interview. Interviews lasted between 12 and 55 minutes. In total, 12 male and 6 female health-care professionals were interviewed. Both of the patients were female, aged between 50 and 59 years and of white British ethnicity and had received PCF at randomisation. All interviews took place between March and May 2021.
| Site | Number interviewed per site | Job roles per site interviewed | Site status | Recruited patients | Randomised patients |
|---|---|---|---|---|---|
| Site A | 5 | Surgeons, research nurses, PI | Open | Yes | Yes |
| Site B | 3 | Surgeon, research nurses | Open | Yes | Yes |
| Site C | 2 | PI, surgeon | Open | No | No |
| Site D | 2 | PI, research nurse | Open | Yes | Yes |
| Site E | 1 | Research nurse | Open | Yes | No |
| Site F | 2 | Surgeon, research nurse | Open | Yes | Yes |
| Site G | 2 | PI, research nurse | Set-up only | No | No |
| Site H | 1 | Set-up co-ordinator | Set-up only | No | No |
In the findings that follow, all participants interviewed have been given pseudonyms and all research sites that took part in the study have been assigned a letter (e.g. site A) to maintain anonymity. The gender implied by the pseudonym does not necessarily match that of the person who took part in the study. Quotation descriptors provide job roles only to help protect the identity of the participant.
Findings
Three main themes were identified in the data analysis: equipoise in the FORVAD trial and in neurosurgical trials, organisation and implementation, and integration of clinical and recruitment pathways.
Equipoise in the FORVAD trial and in neurosurgical trials
The theme of equipoise in the FORVAD trial and in neurosurgical trials includes three subthemes: (1) theory versus applied practice, (2) preferences for surgical approach and (3) considering the patient.
Theory versus applied practice
Although the primary research question for the FORVAD trial made sense to participants and they supported the idea that there was clinical or collective equipoise regarding the two FORVAD interventions, many surgeons had treatment preferences and lacked individual equipoise.
Participants frequently discussed the extent to which considerations regarding equipoise translated into the practical delivery of trial recruitment. Understanding why each site took part in the FORVAD trial and each site’s primary motivation for taking part in the trial revealed whether or not there was collective equipoise across sites, and, if there was little or no equipoise, how the FORVAD trial could help change opinions about what type of procedure might be more effective. Some comments indicated varying opinions regarding equipoise between sites; furthermore, participants remained sceptical about whether or not the research question could be suitably addressed at their site if there was not sufficient equipoise. In addition, participants reflected on whether or not the FORVAD trial was workable in practice if there were differing views regarding equipoise. Nevertheless, the FORVAD trial was overwhelmingly viewed as a worthwhile trial that would provide further information and help inform the evidence base for trials in neurosurgery:
What is equipoise? And you start to argue, is it equipoise for a surgeon, is it equipoise for the department, is it equipoise for the entire community?
Surgeon
I think it was a good study. But I think I was worried from the beginning that because of the difficulty in equipoise everywhere … that it was going to be a struggle to actually enrol patients effectively.
Research nurse
I think the idea for the whole trial itself was a pretty good one, which is why, I thought it was quite a good idea to join in and actually try and answer this question.
Surgeon
We’ve not had anything in this area, not while I’ve been a research nurse, we’ve not had anything like that. That’s why it was quite exciting for us.
Research nurse
One patient interviewed reflected on both procedures, trusting that the information given to them about the trial was enough to make a decision about taking part. This patient had no preference for the type of surgery performed:
Once I had read about both of them and I don’t think either one of them were any better than the other, so I was quite happy to go ahead with it.
Patient B
Preference for surgical approach
Participants reflected on how skills, training and experience are dominant over community equipoise in the surgical environment, suggesting that theoretical notions of equipoise will be overlooked in favour of individual surgical opinion. The tendency to opt for a procedure that had been taught and passed down from surgeon to surgeon affected preferences in individuals and at sites. The participants’ experiences, as a result of performing ACD or PCF (or both) at their sites, were a contributing factor to this preference:
You need to find surgeons who have equipoise, and I certainly had it and a lot of the people who were excited about this trial had it. But if you were to go to a centre where the tradition is to do everything one particular way, there won’t be any equipoise. So, I don’t know if [other trial site] actually agreed to participate but that’s an example of a centre where they do everything through the back of the neck and they won’t have any equipoise, so they wouldn’t want to participate.
Surgeon
Why we are struggling to recruit, the reason is er … with regards to the training we had and what, all we do, there was no ambiguity in choosing foraminotomy or an ACD.
Surgeon
To be honest with having talked already to the consultants, it’s a personal choice, it’s whoever decides, if the consultant is more confident to do the anterior rather than the posterior, it’s their choice, it’s just how comfortable they feel with regards to the approach from a surgical point of view.
Research nurse
Even though surgeons were able to deliver both surgeries across most sites, with regard to the surgical procedure and complications, there were differing views on which procedure was best:
The complication, the potential complications, are much more serious going through the front. That’s not to say we have frequent serious complications, but when it does go wrong, it goes catastrophically wrong, which it doesn’t tend to be the case going through the back of the neck.
Research co-ordinator
And the complications within ACD can be quite, can be sometimes quite significant. So, if you can do a foraminotomy which has its own complications, but it is reasonably easy, well, it’s a better approach, I would say, to have the symptoms sorted, that’s the reason to see whether it helps.
Surgeon
You know, it was interesting that, in theory, they thought they know that there’s no evidence that one’s better than the other. But actually, when it comes down to cutting someone’s neck open, they think that the anterior approach is better. I don’t know how you get over that.
Research nurse
The patients interviewed displayed no preference for either surgical approach. Information given by the surgeon during the surgical consultation, coupled with the surgeon’s knowledge of each procedure, was enough to encourage the patients to take part in the FORVAD trial:
So, he said basically it’s because they were trying to get an idea of how people recovered and how well that surgery went through different procedures, so I were quite happy to do that because, to be fair, he talked me through both operations and both of them sounded, you know, well as good as each other sort of thing.
Patient B
Given this context in variation between sites, it was clear that justification for the FORVAD trial was clearly understood and findings relating to equipoise clearly demonstrated the value of a trial to further inform current practice.
Considering the patient
In addition to a general preference for one or the other procedure, surgeons might also consider that a specific procedure (which might not necessarily be the one that they had a more general preference for) was best for an individual patient, for various reasons. Conceptualisations of patient choice were discussed, suggesting that issues around patient criteria and postoperative outcomes, such as pain, were important for both patient and surgeon when deciding what type of procedure would be best. In addition to variation between sites, there was variation within sites in terms of approaching patients for inclusion in the trial. This related to the numbers of possible patients to recruit and whether or not it was feasible to recruit such numbers in the applied clinical setting. This perception highlighted the reality of recruiting, and offered knowledge about how some health-care providers felt about the practicalities of surgical trial recruitment:
I always have a problem with equipoise and the neurosurgeons. And I literally say to them, ‘Are you telling me that there is equipoise here and that we will be able to recruit to these studies?’, and they say, ‘Yes, Jackie, there are patients who could go on either arm and we will recruit to it’. And I don’t know what more I can do to investigate that with them, because they are the neurosurgeons.
Research nurse
So identifying the patients was fine. Not all patients were particularly keen on the idea. I think one of the difficulties that was identified, and we couldn’t really find a solution to, was it’s a stressful thing for somebody to have an operation, you know, on their neck especially. They have been warned that they might end up paralysed as a consequence of their surgery and everybody is going to be very nervous and apprehensive about that. And then if you throw into the mix of that, the fact that they are not going to know which operation they are going to have on the day, and the risk profile is different between the two, I think that put off a lot of patients.
Surgeon
Patients also discussed their motivations for taking part in the FORVAD trial, discussing how their daily lives were affected by their condition and how taking part in the trial might affect their future quality of life. Considerations were also made about how patient participation in a trial of this nature could benefit others:
I just wanted to get the pain under control and get it done and I were quite happy to do the questions and go through the survey and if you could get anything from it, great.
Patient B
I said, ‘Well yeah, you know, you want to get your surgery done and you need to help others’. Without these trials, you know, things aren’t going to improve are they? You know, they’re not going to find out things.
Patient A
… If one operation works, they found one operation works better than the other, then obviously they’ll probably opt for that most times if they can. So I were quite happy to, if it were, you know, going to help others later on then that would be fine, you know.
Patient B
Organisation and implementation
The FORVAD trial required considerable collective organisation in both its initial set-up and its subsequent implementation.
Set-up and recruitment
Comments relating to the organisation at each individual site were variable and it was apparent that each site experienced its own challenges, especially regarding set-up and recruitment. Experience of previous trial set-up was considered helpful for some sites. The input of the research nurse at each site was integral to the smooth running of the trial. Implementation of the FORVAD trial meant that the research nurse would sometimes need to go above and beyond their job role to ensure that the trial set-up was delivered on time. This meant adapting to the needs of the trial, sometimes coming in to work on a day off or taking on extra responsibilities, such as site initiation visits. In addition, the surgeons would take ownership of screening the first patients for the trial and liaising with the research and development departments to get set-up done in time. The role of the research nurse was clearly defined and a number of participants reflected on how research nurse input contributed to the overall set-up and recruitment during the trial:
You see, I would be lost without a research nurse, that’s the first thing to say. I think, by and large, on the sort of erm … let’s say the paperwork and the minutiae of things and the actual X, Y and Z and the process and all of that, that is where they keep you right. And I think that is very important.
Surgeon
So at times you’ll find that, like when it came to follow-ups post, and most of the times they would have surgery on a Friday, which means Saturday I was not going to be working, but then because of the following day post op[eration follow-up] … I had to come to work so that I could come and just do that so that we don’t miss the patients as well and so that we don’t miss the paperwork as well.
Research nurse
Prolonged set-up and a lack of resources affected recruitment, leading to a loss of momentum and feelings of frustration for some participants:
We had a problem, how to recruit somebody to collect the data, like from research perspective, like research nurse I think so, that was, for some time it was pending but if this is a pending, you know, like, and all the paperwork is quite laborious, whatever you want to do, the research and development, it takes time.
Surgeon
… but to start recruiting in [month] that was a long time, like 8, 9 months, and the information had actually gone from the minds of the surgeons, and gone from the mind of the trainees, so it was, wow, very, very challenging.
Research nurse
One research nurse explained how these logistical challenges affected the recruitment approach on site:
They just, just never seem to be able to keep it in the top of their heads. And we then also would get the lists and go through them, and then say, ‘Oh, you know, what about Mrs X? You know, you saw her in clinic last week, don’t you think she’d be suitable?’, ‘Oh, yeah, yeah, she’d be suitable, yeah’.
Research nurse
This account is important as it demonstrates how delays in set-up could potentially affect recruitment to the trial over time, as momentum is potentially lost when set-up is delayed. Nevertheless, the consensus across sites, with regard to recruitment and set-up, was overwhelmingly positive, with sites making a concerted effort to screen and recruit patients to the trial:
They were quite sort of diligent at trying to identify suitable patients and recruit suitable patients, but I know from talking to her that the numbers were quite low.
Research nurse
… I think, as a team, we were complementing each other pretty well, it worked very well for us that way.
Surgeon
Integration of the clinical and recruitment pathways
This section will focus on the theme of the integration of the clinical and recruitment pathways, which comprises three subthemes: (1) support needs, (2) timing of randomisation and (3) identification and screening of patients.
Support needs
The requirements of the FORVAD trial challenged some of the established patterns of work across sites, especially with regard to making the FORVAD trial fit with established clinical pathways. The participants discussed how the trial could suitably fit in to the site clinical pathway and examined the feasibility of being able to meet the trial protocol regarding supporting participants during follow-up and being confident in understanding trial paperwork (e.g. ASIA assessments) and outcome measures (e.g. hoarse throat assessments):
I think the nurse support is also important because, from the specialist nurse and the research nurse about helping follow-up so that the patients engage with the research and that they’ll carry on and then, so that when you contact them in the future with questionnaires and things that they’re still willing to engage and carry on with the follow-ups.
Research nurse
… Needed a follow-up appointment 6 weeks, but we don’t do our follow-up appointments until 3 months, so we were looking actually, are we going to have to set up a separate specific follow-up clinic and we have a research room … how we could kind of use that room around the other studies who were using that to see research patients?
Research trial co-ordinator
In addition, sites discussed their needs for additional support, and how they accessed further support and learning through networks outside the trial [e.g. research nurse WhatsApp groups (Meta Platforms, Menlo Park, CA, USA) and social media pages]:
… Some trials I work on we’ve got a WhatsApp group, so you could offer support or if you worked on a specific trial, some trusts that work 7 days a week, there was always someone there to ask a question … One trial I’m thinking of, they did set up the WhatsApp group themselves at co-ordinating centre.
Research nurse
Participants described the FORVAD trial as addressing an important research question. However, some neurosurgeons explained the challenges of integrating the FORVAD trial into clinical practice and finding an acceptable balance between research interests and their daily work tasks:
The other thing is, of course …. We don’t have academic sessions in my job plan … your role as a neurosurgeon takes precedence over any research needs, so it’s finding the balance.
Surgeon
So it was like, you know, we could bring the patient in, but then how would it work with the surgeon having time? Would it be, would he have protected time? If you’ve got only one patient to come back a week, how would that then work with his sort of other, his planned surgeries?
Research co-ordinator
Timing of randomisation
Randomisation on the day of surgery raised both ethics and practical concerns at some sites, and often did not ‘fit’ with the general approach to preparation for surgery. For some, randomising on the day of surgery could prove difficult, especially during the day-to-day running of the site and with other research studies that were also open. Identifying which procedure patients had been randomised to was important to clinical staff, because patients would have to be prepared differently in theatre. Integrating the trial pathway into the clinical pathway in this instance provided challenges at some sites.
Some comments also indicated that there were mixed views about randomisation on the day of surgery. This was especially prevalent at sites where there was limited time to communicate surgery preference, especially when setting up theatres for surgery:
Having to do it short notice on the day, of course, because you would have had to, you know … oh I can just imagine the bureaucracy when you know … ‘OK, what operation this patient having?’ Yeah, you then end up putting ‘Well … they might be having one or two’ and you know, then going to the nursing staff. It’s tough to access an ACD opposed to a PCF. There are different instruments.
Surgeon
Participants described the process of randomising patients on the day and the potential risks and benefits to this process. One concern expressed was how waiting lists could delay randomisation at some sites. For example, if there were long waiting lists for either routine procedure, then this increased the chances of the operation being allocated outside the site, to a private hospital. This could lead to potential participants leaving the trial if their operation was delegated to a private hospital, as this was outside the trial protocol. In addition, any emergency admissions took precedence over routine surgery, increasing the likelihood of operations being cancelled if operating theatres were required for other surgery. Furthermore, the process of randomising patients highlighted differing views across sites. Some participants explained that a trial that randomised on the day might not allow enough time for a patient to weigh up the potential risks and benefits about participating in a trial of this nature. Conversely, some participants felt that if the patient was appropriately informed about the trial before the day of surgery, then randomisation on the day of surgery would not pose an issue:
It might have been better to randomise them in advance. Because, you know, it’s the issue of consent, you know, what risks to warn a patient about and there’s no denying the risks are different between the two operations.
Surgeon
It will be easier if you have randomisation on the day because you don’t have time to think the patient, it’s not that you shouldn’t think, but you have already thought about that, and then you randomise on the day and get on with the procedure, whatever comes.
Research nurse
The patient actually should understand what they’re signing up for, what the procedure is, what the potential complications are, all of that. And I accept that you’ve told them all of that and you’ve also told them that they will be randomised on the day to one or the other, and they’ve agreed to it. But I think, overall, I would have been happier and most of my colleagues might have been happier if the randomisation had occurred pretty much during the time of recruitment.
Surgeon
Patients’ reflections on the process of randomisation during the trial suggested that there were some thoughts concerning patient autonomy, yet this did not prove to be a barrier to taking part. Participating patients’ views about randomisation were generally positive and there were no major issues with randomisation on the day:
I was quite happy to do it to be fair. It were a little bit … not having that control over what operation you have … not that either of them are great because you don’t want to go through an operation at any time, do you? But, you know, just not having that choice because obviously it’s randomly picked, isn’t it, as to which you have done.
Patient B
One patient took a rather light-hearted view of randomisation while discussing its outcome with their partner:
On the day of surgery, me and my husband were having a bit of a bet saying, I said I bet it’s going through the back. He goes, no, he’s going to go in through the front.
Patient A
Screening patients
Sites took different approaches to the process of identifying and approaching potential patients, with some choosing to direct potentially eligible patients to particular research clinics and others taking a more ad hoc approach:
So we did something called a trial clinic; so instead of seeing suitable patients, you know, randomly, what we did was we pulled all the suitable patients to a specific trial clinic, and the patients were informed of the trial and were consented for both procedures and on the day of surgery, depending on the randomisation, we then removed the consent if they’re not randomised to it.
Surgeon
So I mean we looked in the past about a research clinic but we didn’t, we couldn’t see how we could logistically go about doing it.
Research nurse
Yeah, more ad hoc. We didn’t set up a specific time. We might have made ourselves available if there was like a clinic, if we knew that it was that particular clinic, but that’s where the spinal nurse comes in that they can assess them straight away and not ring us, you know, unnecessarily. We do make ourselves available. No, we didn’t have a dedicated clinic.
Research nurse
However, ad hoc measures sometimes caused problems, especially when identifying suitable patients to take part in the trial. The pressures between the objectives and priorities of the clinical staff reinforced some of the issues affecting the FORVAD trial:
… after we’d opened, those were the meetings we would go to, to say, ‘Come on, guys’, you know, ‘you told us there were loads of patients, you told us we were going to do alright. And now, you know, I’m literally squeezing these names out of you of these people who’ve been to clinic, who would seem to be, you know, suitable for the study’.
Research nurse
Like I say, without the spinal nurse we would have been lost, so I don’t know how, if we didn’t have them I would have literally had to sit in the clinics with the doctors and wait to find those patients. We can pre screen them, but we have to be careful not to … they’ve not consented to us to look into that information so we have to look at vague information first of all. It’s not easy to say ‘oh that one will be eligible’ ‘til we’ve actually spoken.
Research nurse
In addition, there were different perspectives across sites about who should make the initial approach to patients, with some sites preferring to let the research nurse obtain consent, some sites having a strong PI presence at the screening stage and some sites finding the support of the clinical trials assistant/site co-ordinator a positive factor in ensuring that the overall recruitment process proceeded as planned. These differing accounts are helpful in understanding the role of the clinical staff during a surgical trial and provide insight into their daily responsibilities:
Yeah, I would say a research nurse can … can very well approach us, because a research nurse has most of the information or all of the information … at some point, once all the initial information has been filtered to different departments, it would be better to the surgeon to give a talk or something in the, as a group, to encourage … so that it piques interest, in whatever form it can be.
Surgeon
The PI consultants mostly identify those patients, whereas the larger more common conditions the research nurses tend to identify quite quickly and approach. And I think with the rarer conditions there’s more reliance on the PI and they’ve probably got a relationship with that patient group.
Research nurse
In addition, participants commented on how additional visits for consent could have been simplified to make the recruitment process easier for both staff and patients:
So, no, what would happen is that we would see people in the outpatients’ clinic and then we would contact them and invite them in to be consented. So what you’re having there is two appointments, isn’t it? I mean, in my ideal world, the surgeon would have seen the patient, thought they were relevant, thought they were fit for the study, give them the information sheet there, say, ‘This is a study we’re doing, here we are’, and then we could call them back for a nice calm consent visit in our lovely clinical research unit. But that’s not, that’s not how it worked.
Research nurse
So a lot of surgeons will offer an operation, send the patient off with an information leaflet, bring them back to a consent clinic in advance of their surgery and then on the day of surgery, then confirm the consent with them. So that’s three steps. I think that’s a little bit overkill, so personally I do it as a two-step process.
Surgeon
Discussion
The thematic analysis outlined in this chapter suggests that there was no one specific issue that made recruitment to the FORVAD trial problematic. Rather, as has been found in other studies,84,97 there were a range of interconnected factors that combined to impede recruitment. Notably, these factors combined to a greater or lesser extent at different sites; no one site described experiencing every issue to the same degree, but all experienced some of them to some extent. None of the individual issues identified appears to have been insurmountable, as sites that recruited successfully described experiencing many of the same issues as those that failed to recruit. Likewise, the thematic analysis suggests that some facilitators, although identified as being important, may be necessary but not sufficient for successful recruitment. For example, some surgeons interviewed at sites that failed to recruit reported being supportive of and engaged with the research question and were willing and capable of delivering both interventions. Conversely, surgeons at sites that did recruit described struggling to convince all of their colleagues of the benefits of both interventions. Thus, although ‘equipoise in theory and practice’ is identified in our research findings as a key theme, it is not immediately clear the extent to which this can be directly linked to recruitment performance.
In this context, drawing conclusions about how, why and to what extent the factors identified in the thematic analysis affected recruitment is challenging. NPT is helpful in this regard, as it provides a structured framework with a focus on understanding implementation and integration that can be used as a theoretical ‘lens’ for exploring the results of the thematic analysis. This discussion section considers the results in the context of the four NPT core constructs (coherence, cognitive participation, collective action and reflective monitoring) and identifies key aspects of the FORVAD trial that challenged the ability of sites to embed the trial processes in their day-to-day practice.
Coherence (meaning and sense-making by participants)
Overall, there appears to have been widespread support for the aims of the FORVAD trial, and surgeons felt that the research question was important and that the results would have influenced clinical practice. The differences between the two procedures were well understood and surgeons discussed the relative risks and benefits of each, identifying certain characteristics or circumstances that would make one better than the other for specific patients. However, participating surgeons also felt that there were some patients for whom either procedure could be appropriate, and that there is a lack of evidence as to which procedure is better in these circumstances. The trial eligibility criteria were generally felt to be appropriate and identified patients for whom it was unclear which procedure would be best (i.e. when there is collective clinical equipoise).
There does not appear to have been any other significant issues with the FORVAD trial in terms of communal or individual specification. Site staff did not report any concerns around information provision, training or understanding of the trial aims or benefits, and generally both surgeons and research support staff appeared to be clear of their roles and how they fit in with the trial overall. However, one issue that did appear to present a significant challenge in terms of coherence was the timing of randomisation, which conflicted with a general move towards earlier surgical consent because of ethics and legal concerns.
Randomising on the day of surgery also challenged surgeons’ clinical relationships with patients by removing the opportunity to discuss the specific procedure the patient would have at their pre-operative outpatient clinics. Although none of the surgeons interviewed specifically identified this as a key factor in their recruitment problems, it clearly limited their ability to make sense of the trial in the context of their legal and clinical obligations. The potential impact on surgeons’ willingness to recruit and enthusiasm for the trial should therefore not be discounted, particularly for those surgeons who may have been less engaged to begin with (and who are likely to be unrepresented or under-represented in the interview data).
Cognitive participation (commitment and engagement by participants)
Commitment and engagement emerged from the thematic analysis as a crucial factor in sites’ ability to set up and recruit successfully to the FORVAD trial. This is not to say, however, that commitment and engagement was necessarily lacking at sites that struggled with recruitment, as all interviewees described being enthusiastic about the trial and keen to take part. Rather, it appears that the considerable logistical challenges of delivering a surgical trial could (in some cases) be overcome by an elevated and sustained level of commitment and engagement. Conversely, these challenges appear to have had the effect of dampening enthusiasm at some sites, and it may be that being moderately committed and engaged with the trial initially was not sufficient to maintain the level of motivation required to push through the numerous barriers to operationalising the trial in practice. The importance of having an engaged, experienced and motivated site team that was prepared to ‘go the extra mile’ to deliver the trial clearly cannot be underestimated. However, the experience of one interviewee struggling to access research nurse support despite working in a large, research-intensive hospital highlights the fact that this is not something that can always be relied on for surgical trials, and this issue has been reflected in previous literature. 116 Similarly, the role of a specialty research nurse, working specifically in clinical trials, is considered a fundamental asset of successful trial management and delivery. 117 When designing future trials, therefore, careful consideration should be given to making the trial as easy to set up and deliver as possible [taking into account the specific implementation challenges discussed under Collective action (i.e. the work participants do to make the trial function)], so that site staff can focus their limited time and energy on recruitment. It may also be beneficial to consider whether or not a model of fewer sites with more dedicated support provided to deliver the trial might be more successful in some circumstances (e.g. where there are sufficient patient numbers and the limiting factor is likely to be research capacity at sites). The benefits of this approach, however, would have to be weighed up against the risk of some of these sites still failing to recruit, leaving fewer sites overall to compensate for any shortfall in recruitment numbers.
Another important factor to consider in terms of cognitive participation is legitimisation, and in particular whether or not there is sufficient equipoise among clinical staff at sites. Some hospitals and/or surgeons may not have taken part in the FORVAD trial because of strong preferences for one of the trial interventions over the other. However, it is unlikely that this was the key factor limiting recruitment, as sites that recruited successfully did so with only a small of number of surgeons delivering the trial interventions, and the total number of sites that opened or were in set-up should have been sufficient if those sites had recruited at the same rate as the more successful sites. Furthermore, in line with their general support for the aims of the trial and agreement that there is collective clinical equipoise, the surgeons interviewed largely appeared to have sufficient individual equipoise to deliver the trial successfully. Likewise, in contrast to research on other surgical trials that found equipoise to be the key factor limiting recruitment,118 the research nurses and other research support staff interviewed for the FORVAD qualitative study did not report any significant issues with surgeon preference that affected their ability to communicate the trial to patients, yet they felt that, at times, they had to prompt the surgeon to look for potential patients during consultations as this was overlooked. When surgeons did talk to patients about the FORVAD trial, it appears that they were able to communicate equipoise successfully enough for patients to consent to taking part. The key limiting factor appears not to be the ability of the team to communicate equipoise in a trial recruitment conversation, but rather appears to be the timely identification of eligible patients so that the recruitment conversation could take place. There was some suggestion, however, that surgeons who preferred one procedure over the other might have been less open to considering specific patients for the trial, and been less able to convey equipoise to their patients during consent discussions.
Overall, this suggests that, although equipoise was certainly a factor in the FORVAD trial recruitment difficulties, there is no strong evidence that this was more of a problem than would be expected for a surgical trial of this type, and it is unlikely to have been the only (or even most significant) factor that limited recruitment. Nevertheless, adopting an expertise-based design for future trials in the area could potentially help to address some of the issues caused by a lack of individual surgeon equipoise. 119 It should be noted, however, that this design would not have addressed the issue of entire sites being unwilling to participate because they conduct only one of the interventions, as the standard expertise-based design requires both procedures to be delivered at all sites. An expertise-based design would also not necessarily improve any issues caused by surgeons struggling to convey equipoise to patients during consent discussions, and could, in fact, exacerbate these issues as surgeons are less likely to be able to talk confidently about the risks and benefits of both procedures if they are experienced in delivering only one. It would, however, allow surgeons who are in equipoise overall but do not feel personally able to deliver both of the interventions to participate, increasing the potential pool of surgeons at each site, and it could potentially make surgeons more willing to put patients forward for the trial. These benefits, however, would need to be weighed up against the additional logistical challenges introduced by an expertise-based design,120 particularly in the context of the interactional workability challenges that sites experienced with the FORVAD trial (discussed in more detail in the following section).
Collective action (i.e. the work participants do to make the trial function)
The thematic analysis and, in particular, the issues explored under the Integration of the clinical and recruitment pathways theme, suggests that interactional workability (how the trial was operationalised and its compatibility with existing work practices) is likely to have been one of the most important factors affecting recruitment to the FORVAD trial. The process for screening and approaching patients, the timing of randomisation, fitting follow-up visits into standard care pathways, the length of waiting lists, and the availability of theatre facilities and/or use of private facilities were all important aspects of operationalising the trial. Importantly, sites that recruited successfully were either not affected by these issues (e.g. waiting lists were relatively short because the site had access to theatres dedicated to minor procedures, or follow-up visits happened to fall within their usual clinical pathway) or described putting processes in place that overcame them (identifying potentially eligible patients from referral letters and triaging these patients to dedicated clinics run by participating trial surgeons). Conversely, sites that recruited less well described having issues with some or all of these activities. For example, one site experienced a significant delay in opening because of concerns about randomisation on the day of surgery, and another interviewee described arranging follow-up visits that were outside the usual care pathway taking up time that could have been spent screening. Even in cases in which interviewees felt that their recruitment problems were predominantly due to not seeing enough eligible patients, it was apparent that the site may in fact have simply failed to identify those patients because of the lack of a structured process for screening referrals. Although none of these individual issues around interactional workability would necessarily have made recruitment impossible, the cumulative effect of a number of issues combined is likely to have contributed to the low levels of recruitment seen at most sites.
In terms of skill set workability and contextual and relational integration, the thematic analysis highlighted the importance of site clinical teams and research support working together effectively, and, in particular, the crucial role played by research nurses. In addition to the importance of having surgeons with individual equipoise and an experienced and motivated team that is willing to ‘go the extra mile’ (as discussed previously), learning from each other and establishing support networks appeared to be particularly valuable for many interviewees. However, as this was reported by sites that failed to recruit as well as those that did, it is unlikely to have played a significant or direct role in recruitment, although it may have been an underlying contributory factor in maintaining the enthusiasm and motivation that has been identified as important for successful recruitment.
Reflexive monitoring (i.e. participants reflect on or appraise the trial)
The potential for reflexive monitoring of the FORVAD trial was limited to some extent by the circumstances in which the trial closed. In particular, three interlinked factors are likely to have affected participants’ ability to reflect on and appraise the trial: (1) the short length of time many sites were open limited their opportunity to reflect on the trial processes; (2) the impact of COVID-19 in the last few months of the trial meant that clinical services were severely disrupted, making it difficult to reflect on the trial itself; and (3) the early closure of recruitment means that the research question has not been answered, so participants are not able to reflect meaningfully on the outcome of the trial and the potential for changing clinical practice. Notwithstanding this, however, the thematic analysis does provide some indication of reflexive monitoring, and suggests some tentative conclusions that may be of value for future research in this area.
The surgeons interviewed were generally still supportive of the aims of the trial and felt that this is still an important clinical question to be answered, which suggests that communal appraisal of the value of learning more about ACD and PCF would not be a barrier to future research in this area. In terms of individual appraisal, and in particular the impact of the trial on individuals and their work environment, there were some concerns about the amount of time the trial required and how difficult it was for surgeons to fit this into an already full clinical schedule. This aligns with the previous discussion about the importance of having an engaged team and research support infrastructure to enable surgeons to participate effectively in trials. It does not appear, however, that the FORVAD trial was uniquely challenging in this regard and although there were some aspects of the protocol that created additional work, this does not appear to have been a significant factor that limited recruitment. It is likely, therefore, that this concern is largely applicable to trials in surgery in general, rather than being a specific issue for the FORVAD trial. It does suggest, however, that it is important to take into account the context in which site teams are working when designing surgical trials, and to ensure that site staff can focus their limited resources on screening and recruitment as much as possible. In particular, assessments and data collection should be kept to the minimum required to answer the research question and visits should be aligned as far as possible to standard care pathways to minimise the additional work required at sites.
In line with this, the thematic analysis suggests that sites had only limited ability for reconfiguration and adapting the trial procedures on the basis of their experience. Although there were some positive examples of this, such as one site setting up a follow-up visit tracker to prompt clinic visits to be arranged in the correct window after a visit had been missed, the limited flexibility allowed for in a trial protocol generally made it hard for sites to adapt trial processes to their local clinical pathways. This further increased the reliance on team members being willing to go above and beyond their usual roles, for instance research nurses coming in on the weekend to ensure that post-surgery follow-up assessments were completed on time. Again, these issues will not necessarily have directly affected recruitment rates, but will potentially have limited the time and energy available for screening and recruitment. The nature of RCTs mean that some inflexibility is inevitable, as there needs to be some consistency across sites. However, consideration should be given when designing future trials to identifying aspects of the protocol for which flexibility can be allowed without significantly affecting scientific validity, so that sites can adapt the trial to their working practices as much as possible. In addition, as one interviewee suggested, engagement with as many clinical sites as possible during the protocol design would help to ensure that the trial pathway fits in with as many variations of local clinical pathways as possible. Again, there will be a limit to how far it is practical to accommodate this recommendation. Consultation with sites takes time, and will therefore need to be balanced against the need to get the trial set up and open. Our results do suggest, however, that allocating at least some time and resources to consulting with sites earlier in the set-up process would be beneficial.
In terms of individual appraisal from the patient perspective, there are some indications that patients particularly valued being able to have their surgery as quickly as possible. The actual or perceived impact of trial participation on waiting times (whether positive or negative) is therefore likely to be an important factor in determining how appealing future trials in this area will be to patients. As discussed previously, this study was not designed to provide a comprehensive understanding of patient perspectives on the FORVAD trial, so the results cannot provide any conclusive findings on this issue. However, there are indications that this could be an important area for future research, particularly in the context of the ongoing disruption to elective services as a result of COVID-19.
Qualitative study strengths and limitations
A strength of the qualitative study was that we recruited participants from both the key professional groups involved in the FORVAD trial at sites: research nurses and surgeons. At most sites we were able to speak to at least one individual from each professional role, facilitating a comprehensive understanding of the implementation of the FORVAD trial at that site. We were successful in recruiting participants from the three groups of sites that we identified: those that had opened to recruitment and had successfully recruited patients to the FORVAD trial, those that had opened to recruitment but had not recruited and those that were still in set-up at the point that the FORVAD trial closed. A limitation was that we conducted the research at the end of the FORVAD trial, so some participants may have remembered less about challenges encountered earlier in the trial. In particular, this limited the extent to which we were able to ‘unpick’ the different factors contributing to recruitment challenges: ethnographic work at sites during recruitment might have enabled us to better understand the complex interaction between, for example, surgical preferences and trial organisation. However, sites were at varying stages in set-up and implementation and we were able to use the accumulated knowledge about the trial of other TMG members to help us to direct our recruitment and data collection effectively. We were less successful at recruiting patients, and were able to speak only to patients who had agreed to participate in the FORVAD study; any future trial in this area would benefit from including strong PPI and/or a qualitative feasibility component with patients to identify and address potential patient barriers to recruitment.
Conclusion
The results of the qualitative study suggest that, overall, there was support and enthusiasm for the FORVAD trial and for RCTs in this clinical area in general. Despite this, clinical equipoise appears to have been an issue both at an overall site level and at individual surgeon level, although the extent to which this affected recruitment is not clear, and all participating sites had some surgeons who were willing and able to deliver both procedures. However, the lack of more widespread equipoise in the clinical community limited the number of sites that, and surgeons who, could take part, and may have made it more difficult for participating surgeons to engage their colleagues in the trial.
In addition to this, the difficulties in recruiting appear to have been caused by a combination of interlinked factors related to the interactional workability of the trial. Randomisation on the day of surgery appears to have been an issue in various ways, slowing down set-up at some sites, limiting the number of surgeons and sites willing to take part and potentially discouraging some patients from participating. The process for screening and approaching potential participants also appears to have been a crucial factor, and a structured approach to identifying eligible patients and ‘funnelling’ them to dedicated trial clinics allowed for successful recruitment even in sites with limited numbers of eligible participants and participating surgeons. Delivering the trial successfully also required individuals and teams to do more than just their day-to-day role. Support, engagement and motivation are crucial for this, and elements of the protocol that were hard to incorporate into usual clinical pathways are likely to have made this harder to maintain.
Importantly, there does not appear to have been one specific factor that made recruitment to the FORVAD trial difficult at some sites and more successful at others. Rather, there were a range of factors that facilitated or impeded recruitment to a greater or lesser extent at each site. Some of these issues can potentially be addressed in the design of future trials, for instance using expertise-based designs to overcome surgeon preference, encouraging sites to adopt a triage system to ‘funnel’ potentially eligible patients to trial clinics and avoiding randomisation on the day of surgery unless there is a compelling scientific justification. In addition to these specific suggestions, however, when designing future trials, careful consideration should be given to ensuring that the protocol is as flexible as possible, thus allowing sites to address specific interactional workability issues at a local level.
A rapid qualitative study conducted by experienced triallists and informed by a theoretical framework (NPT) was able to quickly identify key issues in the implementation of the FORVAD trial. Rapid qualitative approaches could usefully be employed in other clinical trials, particularly those with a feasibility or internal pilot component.
Chapter 6 Discussion
The FORVAD trial closed early as a result of failure to adequately recruit participants. As a consequence, the trial is underpowered and the results are discussed in descriptive form.
Comparing the efficacy of anterior cervical discectomy and posterior cervical foraminotomy
Neck Disability Index
The primary outcome measure of the FORVAD trial was NDI score at 52 weeks after surgery. In the PCF group, the median NDI score was 44.0 (IQR 36.0–62.0) pre-operatively and 25.3 (IQR 20.0–42.0) at 52 weeks. In the ACD group, the median NDI score was 35.6 (IQR 34.0–44.0) pre operatively and 45.0 (IQR 20.0–57.0) at 52 weeks, meaning that the PCF group had a worse pre-operative score and improved by 52 weeks, whereas the ACD group had a better pre-operative score and did not improve by 52 weeks. However, there is overlap in the IQRs, and the low sample size may mean that there are influential observations that influence the median. The minimum clinically important difference in NDI score is 10%, but the number of participants was too small to conclude that there is a significant difference in outcome between the two groups.
In the PCF group, the median NDI score deteriorated from 44.0 (IQR 36.0–62.0) at baseline to 61.3 (IQR 41.7–69.4) at day 1, and then sequentially improved to 45.0 (IQR 30.0–48.9) at 6 weeks, 40.0 (IQR 20.0–54.0) at 12 weeks, 42.2 (IQR 22.0–68.0) at 26 weeks, 24.0 (IQR 20.0–42.2) at 39 weeks, and 25.3 (IQR 20.0–42.0) at 52 weeks. This is in contrast to the ACD group, of which the median NDI score was 35.6 (IQR 34.0–44.0) at baseline, deteriorating to 46.0 (IQR 36.0–64.4) at day 1 then improving to the best (lowest) median score of 14.0 (IQR 8.0–53.3) at 6 weeks. Thereafter, the NDI score deteriorates again to 29.0 (IQR 13.0–49.0) at 12 weeks, 28.0 (IQR 16.0–47.0) at 26 weeks, 38.0 (IQR 32.0–44.0) at 39 weeks and 45.0 (IQR 20.0–57.0) at 52 weeks.
The data suggest that ACD may provide better early functional outcomes, whereas PCF may provide better longer-term functional outcomes. The finding that the early functional improvements following ACD are not maintained is supported by the findings of DePalma et al. 9 and Persson et al.,7 who showed that, by 12 months after the surgery, the difference between surgical and non-surgical patients diminishes. The loss of benefit was due to both ongoing improvement of the conservative group and late recurrence of symptoms in surgical patients. 7,10 Change in functional outcome over time following PCF is not well documented.
Neck and arm pain
Neither PCF nor ACD are usually considered to be effective in the treatment of neck pain. In the FORVAD trial, median neck pain scores deteriorated from a baseline of 5.5 (IQR 4.0–8.0) in the PCF arm and 5.0 (IQR 4.0–7.0) in the ACD arm to 8.5 (IQR 6.0–10.0) in the PCF arm and 7.0 (IQR 4.0–9.0) in the ACD arm on the first postoperative day. This is considered to be because of the surgical wound and provides some support for the view that PCF is a more painful procedure. 16,17 We consider that postoperative neck pain can be minimised by using small wounds, microsurgery and good operative technique. In this trial there were no cases for which a PCF minimal-access technique was used. This approach minimises muscle trauma,27 and may therefore also be effective in reducing postoperative neck pain.
Neck pain scores fell to their lowest level at week 12 [median scores of 3.0 (IQR 2.0–7.0) and 2.5 (IQR 1.0–5.0) in the PCF and ACD arms, respectively] and then increased back towards baseline levels by 52 weeks [median scores of 4.0 (IQR 2.0–5.0) and 5.0 (IQR 3.0–7.0) in the PCF and ACD arms, respectively]. The PainDETECT questionnaire was used to assess whether pain was neuropathic or nociceptive.
In the spine, nociceptive pain is usually considered to be due to muscle, joint or ligamental pathologies and responds poorly to surgical decompression, whereas neuropathic pain results from nerve compression, neurological dysfunction or injury. Neuropathic pain can be divided121 into dysaesthetic (burning) pain that does not normally respond to surgical intervention, and radiating pain that is associated with paraesthesias (pins and needles) and improves with surgical decompression. More recently, a third type of pain, termed nociplastic pain, has been described for the pain predominantly associated with central sensitisation. 122 Spinal pain may be ‘mixed pain’, for which all three components are present. 121
In the FORVAD trial data, the percentage of participants with nociceptive pain in the ACD group increased from 11.1% pre operation to 33.3% after 52 weeks, whereas in the PCF group the increase was from 14.3% pre operation to 21.4% at 52 weeks. The failure to control nociceptive neck pain in the longer term and the change in the character of the pain from neurogenic to nociceptive further suggests that the early benefits of surgery may not be maintained in the longer term. We consider that the PainDETECT questionnaire was inadequate to distinguish between dysaesthetic and radiating pain and was unable to identify any nociplastic component, which limits its utility as a tool for patient selection. Other authors have published similar conclusions relating to the lumbar spine. 123
The median arm pain score improved in both groups, from a baseline of 7.0 (IQR 4.0–8.0) in the PCF group and 6.0 (IQR 5.0–7.0) in the ACD group to 3.0 (IQR 2.0–8.0) in the PCF group and 4.0 (IQR 0.5–5.0) in the ACD group on the first postoperative day, with the lowest median scores recorded at week 12 [median scores of 3.0 (IQR 2.0–8.0) and 2.5 (IQR 0.0–5.0) in the PCF and ACD arms, respectively]. However, as with neck pain, longer follow-up demonstrated slow deterioration in median arm pain scores to 5.0 in both arms of the trial by 52 weeks (IQRs of 2.0–7.0 and 3.0–6.0 in the PCF and ACD arms, respectively).
Other markers of outcome
A total of 54% of participants reported taking time off work following PCF, whereas 62% of participants reported taking time off work following ACD, although the numbers in each arm are small.
The median changes in the HRQoL score (EQ-5D-3L) from baseline at day 1 and at weeks 6, 12, 26, 39 and 52 were, respectively, 0.06, 0.10, 0.00, 0.00, 0.29 and 0.06 in the PCF arm and 0.00, 0.02, 0.00, 0.00, –0.03 and –0.09 in the ACD arm. The baseline EQ-5D score was 0.291 (SD 0.34, 95% CI 0.07 to 0.51) in the PCF arm and 0.595 (SD 0.25, 95% CI 0.40 to 0.78) in the ACD arm; ceiling effects will have had some influence.
The mean length of hospital stay was 1.2 days (SD 0.3 days) in the PCF arm and 1.3 days (SD 1 day) in the ACD arm. The median length of stay was 1 day for both groups. Minimal-access PCF is now commonly performed as a day-case procedure,124 but no minimal-access PCF cases were included in the FORVAD trial. If day-case minimal-access PCF was to be widely adopted in the UK, a further reduction in the length of stay, and therefore in the cost of PCF, would be expected.
Arm strength and sensation was assessed using the ASIA score at day 1 and at 6 weeks. Very few participants had clinically detectable weakness or sensory loss pre operation in either group, and this did not change in the postoperative period.
Efficacy summary
The data in this study suggest that, 52 weeks after ACD, there is limited residual benefit in neck pain, arm pain, pain character or functional outcome. Similarly, 52 weeks after PCF, there is little residual benefit in neck pain or arm pain, but functional outcome improves.
Posterior cervical foraminotomy does appear to be associated with worse postoperative pain on day 1, but this does not affect length of stay, as PCF participants had a shorter mean length of stay. These findings need to be interpreted cautiously in view of the small numbers in the trial, and care should be taken to consider the spread of the data. Nevertheless, the data on NDI score and neck and arm pain are consistent with the findings of other authors27 that PCF is likely to be at least equivalent to ACD in improving outcome after 1 year.
Complications
Both operations are safe. There were no intraoperative or neurological complications among any of the participants and no deaths. Complications were reported in 5 out of 9 participants following ACD and in 0 out of 14 participants following PCF. The reported complications following ACD were wound related complications, urinary retention and dysphagia.
Dysphagia (difficulty swallowing)
Dysphagia was reported as a complication in two out of nine participants following ACD. Furthermore, the EAT-10 dysphagia assessment tool shows clinically significant swallowing problems in most participants on the first postoperative day. This improved by 6 weeks and resolved by 12 weeks.
Globus is the sensation of a lump in the throat, and was assessed using the GETS. Postoperatively, there was a deterioration in the ACD group that became milder over time, but remained higher than in the PCF group throughout the whole trial period, although IQRs overlapped at multiple postoperative time points.
The finding that postoperative dysphagia and globus are common after ACD is consistent with the literature. 125 Patient-reported instruments are effective at identifying globus. The incidence of dysphagia after anterior cervical spine surgery in the literature has been reported as between 1% and 79%. Dysphagia is known to improve over time and is associated with multilevel surgery, female gender, increased operative time and older age (usually > 60 years). 125
Hoarse voice
The VHI-10 and GRBAS scores were used to assess postoperative hoarse voice. The data are similar to the dysphagia data in that the VHI-10 score appears elevated in the ACD group on the first postoperative day, but this difference resolves at later time points.
The incidence of hoarseness in this trial reflects the known incidence in the literature following ACD, which is reported as 0.06–11%, but most will resolve over time. It fails to resolve in 0–3.5% of cases. 126 Hoarseness is caused by damage to the recurrent laryngeal nerve; however, many unilateral recurrent laryngeal nerve injuries are asymptomatic, so the incidence of postoperative hoarseness is an underestimate of the incidence of recurrent laryngeal nerve palsies. 127
Reoperations
There were no reoperations in the trial. Treatment failure may sometimes necessitate that surgery be performed at the same level, but from the other aspect of the neck. However, this is uncommon within 1 year of the index surgical procedure.
In the literature, the risk of adjacent segment disease following ACD is around 3% per annum. 128 The risk of symptomatic adjacent segment disease following PCF is 1.43% per year in the literature. However, with only 1 year of follow-up, it is unlikely that adjacent segment disease will have had time to present. 129
Therefore, the absence of reoperations is likely to be due to relatively short follow-up of the participants; future studies should consider using longer follow-up periods of 2, or even 5, years to capture these data.
In addition, it is possible that the small sample size, in conjunction with low rates of reoperation in the first year, contributed to the lack of observed reoperations.
Health economics
The FORVAD trial data support the conclusion that providing initial surgical intervention is cheaper with PCF (median £2622) than with ACD (median £4423). The difference is largely due to the cost of implants for ACDs and the shorter operating time with PCF. The health economics data also show that the PCF arm had shorter median lengths of stay and less time off work. A full cost-effectiveness analysis has not been undertaken as the numbers are too small and the economic analysis is impaired by high numbers of missing data during trial follow-up.
The finding that PCF costs 59% of the cost of ACD is in keeping with published literature that has shown that PCF costs are between 53% and 69% of the cost of ACD. 11,36,37,41 All the previous literature is from the USA; we believe that this is the first time that the potential cost saving has been demonstrated in the UK.
Failure to recruit
Trial investigators screened 251 patients, but 224 (89%) were excluded before registration. Of these, 208 (83%) failed to meet the inclusion criteria. The most common reasons for failing to meet the eligibility criteria were ‘diagnosis of cervical myelopathy’ (n = 38), ‘no single-level nerve entrapment’ (n = 34) and ‘no diagnosis of unilateral cervical brachialgia as confirmed by MRI or CT myelogram taken within the preceding 12 months’ (n = 30).
The funding for the trial was withdrawn because of failure to recruit to target. Slow recruitment was multifactorial, including a delay in opening the trial, long lag time between recruiting some sites and opening them, long waiting lists for elective surgery and slow recruitment in some sites.
Notification that the funding was to be withdrawn was received in May 2020, which coincided with the first wave of the COVID-19 pandemic in the UK. As a consequence of COVID-19, elective spinal surgery had stopped, and we therefore considered that we would not be able to catch up with recruitment. Consequently, the research team chose not to appeal against the funder’s decision. Instead, a qualitative study was undertaken to explore the reasons for slow recruitment and to inform the design of future trials on this topic.
The qualitative study results demonstrated that the FORVAD trial investigators were enthusiastic about this trial and that the trial was well designed and run. There were three aspects of the trial design that were commonly cited as contributing to slow recruitment: lack of individual equipoise, randomisation on the day of surgery and regional differences in pathways for elective patients.
The presence of ‘clinical equipoise’ across specialists is an ethics requirement for RCTs and is normally established by the trial team and the ethics committee,130 and the presence of clinical equipoise for the choice of surgical approach in the treatment of cervical brachialgia is well established. 6,21,25,26 However, individual surgeons will not always have ‘individual equipoise’: they may have preferences for one or other of these two operations, making them unwilling to randomise patients. The risk around equipoise was identified before the trial opened and investigators tried to counter the risk by presenting on equipoise at national meetings and including equipoise training in the mandatory trial e-learning package. Nevertheless, the qualitative study identified individual equipoise as an issue that contributed to slow recruitment to this trial.
Randomisation on the day of surgery was used as part of the FORVAD trial protocol to minimise the number of patients crossing over or withdrawing from the trial. It is unfortunate that, since the trial was designed, there has been a legal case in which a high court judgment advised that obtaining surgical consent on the day was ‘… neither the place nor the occasion for a surgeon for the first time to explain to a patient undergoing elective surgery the risks and benefits’. 132 Consequently, trial randomisation for elective surgery on the day of the operation was also viewed by some as inappropriate and legally risky. Consent for surgery within the trial was obtained for both operations prior to the day of surgery, but randomisation occurred on the day of surgery and the qualitative substudy found that trial randomisation on the day of surgery was a factor in slow recruitment.
Finally, the pathways that patients follow as they approach an elective operation on the cervical spine are different in different UK centres. The most successful recruiting centre triaged all spinal referrals and ran a pre-operative consenting and research clinic. This model was not adopted by any other centre and was the key organisational arrangement that allowed rapid recruitment in the leading centre.
Lessons for future trials
In our opinion, a prospective RCT comparing the functional outcomes, surgical complications and cost effectiveness of PCF and ACD should remain a high priority, as it is still unclear which procedure is superior, and an effectively powered trial may demonstrate that PCF has improved outcomes and reduced cost.
The FORVAD trial adopted the correct outcome measures and the trial design was appropriately powered. The NDI provided a functional score that was more clinically relevant than pain scores or Odom criteria because the PainDETECT questionnaire is not designed to identify patients with dysaesthetic or nociplastic pain, it is not adequate for assessing the nature of spinal pain pre or postoperatively.
Slow recruitment was problematic; those designing future RCTs should consider whether or not to abandon randomisation on the day of surgery, and should also consider mandating the use of a preoperative consent and research clinic. Resolving the problem of individual surgical equipoise remains a challenge that may require the publication of more unrandomised or meta-analysis studies before surgeons are willing to randomise a greater proportion of their patients. The alternative of using an expertise-based design may resolve the equipoise concerns, but would require special considerations specific to this type of trial design to be robust.
Reoperation within 1 year of the index procedure is uncommon. Furthermore, the initial benefits of surgery appear to diminish over time. Therefore, studies in the future should consider following up participants for 2, or even 5, years to effectively capture the reoperation rate and to understand what the longer-term benefits of the two procedures are.
Assessment of the imaging has not been part of the FORVAD trial, but the morphology and cause of cervical foraminal stenosis may well affect the outcomes of ACD and PCF procedures. Analysis of pre-operative MRI scans according to the Park et al. 22 or Kim et al. 23 methods should be considered as an option in future studies.
Key recommendations for future research
-
Better imaging protocols, such as oblique MRI scans of the cervical nerve root foramen, should be studied to assess whether or not they could inform which surgical approach is better for different types of nerve root compression.
-
Diffusion tensor imaging (i.e. a type of diffusion-weighted MRI) of the nerve root should be studied to assess whether or not it can help identify when compression is functionally important.
-
In this trial, complication rates seem higher and benefits less sustained than in some of the published literature. A large prospective cohort study of all operations for cervical radiculopathy in multiple centres in the UK might better inform surgeons as to the real-life outcomes.
Trial limitations
The trial recruited only a small number of participants before it was closed; therefore, all data are presented in descriptive form and no firm conclusions can be drawn. Owing to the COVID-19 pandemic, there are moderately high numbers of missing data, which especially affects the health economics section of the trial.
The participants were recruited from five UK neurosurgery centres. However, 18 out of 23 (78%) were recruited in a single centre, and all operations in this centre were performed by only two surgeons.
This is a UK trial; elective spinal patients in the NHS frequently spend many months on waiting lists before surgery is performed. Consequently, care should be taken in generalising the findings to other countries where surgical treatment of cervical brachialgia may occur sooner after the onset of symptoms.
Chapter 7 Conclusions
The FORVAD trial was closed by the funder early after only 23 participants had been recruited; consequently, it is underpowered and definitive conclusions cannot be drawn.
The primary outcome measure was NDI score at 52 weeks; the median scores were 25.3 (IQR 20.0–42.0) in the PCF group and 45.0 (IQR 20.0–57.0) in the ACD group. The minimum clinically important difference in NDI score is 10%, with a low score being better than a high score. The data therefore suggest that, when compared with ACD, PCF may be associated with better functional outcome 52 weeks after the operation.
Anterior cervical discectomy was associated with more surgical complications overall, including postoperative swallowing difficulty and hoarse voice complications. Swallowing and hoarse voice complications were seen to resolve, but globus, as measured on the GETS, was more common throughout the follow-up period, although the IQRs overlap at all time points except day 1. Day-1 neck pain was more severe in the PCF group than in the ACD group.
Providing initial surgical intervention is cheaper with PCF (median £2622) than with ACD (£4423), largely because of the cost of implants for ACDs and the shorter operating time with PCF. In relation to cost implications for society, in the PCF group, the length of hospital stay was shorter than in the ACD group, and the participants required less time off work than those in the ACD group. Nevertheless, the low numbers behind the results do not allow us to draw conclusions generalisable to NHS practice.
The qualitative study explored the reasons for slow recruitment. It has shown that the trial was considered important and relevant by the UK neurosurgery community, but that trial recruitment was impaired by the lack of individual equipoise and concern about randomisation on the day of surgery.
A large prospective multicentre trial comparing ACD with PCF in the treatment of cervical brachialgia is still required.
Acknowledgements
We are indebted to all the patients who participated in this trial. We would also like to thank the TSC (Professor David Mendelow, Mr Robin Johnston, Professor Graeme MacLennan, Dr Catherine Pinnell and Mr Philip Van Hille), the DMEC (Professor Jeremy Fairbank, Mr Conor Mallucci and Mrs Natalie Rowland), the TMG (Miss Gemma Ainsworth, Ms Sarah Brown, Mr Howard Collier, Ms Julie Croft, Mr Martin Gledhill, Dr Ruchi Higham, Professor Peter Hutchinson, Miss Rachel Kelly, Mr James Meacock, Dr Ruben Mujica-Mota, Mr Debasish Patel, Dr Nikki Rousseau, Mr Senthil Selvanathan, Professor Deborah Stocken, Ms Rebecca Talbot, Mr Simon Thomson, Dr Armando Vargas-Palacios and Mr Martin Wilby) and Dr Olympia Papachristofi for their important contributions. We would additionally like to express our gratitude to Victoria Halstead for having undertaken the central review of the hoarse voice assessments.
Patient and public involvement
The FORVAD trial had PPI throughout its duration, from the grant application stage through to trial completion. Mr Martin Gledhill served on the TMG and Dr Catherine Pinnell was a member of the TSC.
Both PPI representatives attended trial oversight committee meetings and were actively involved in trial discussions, providing valued opinions and ideas from a patient and public perspective. PPI input also fed into the protocol design, the drafting/reviewing of participant information resources, the interpretation of the results and the write-up of the final report.
Principal investigators
Thank you to all of the PIs who participated in the trial: Mr Yahia Al-Tamimi (Sheffield Teaching Hospitals NHS Foundation Trust), Mr Neil Buxton (The Walton Centre NHS Foundation Trust), Mr Nicholas Haden (University Hospitals Plymouth NHS Trust), Mr Nitin Mukerji (South Tees Hospitals NHS Foundation Trust), Mr Ravindra Nannapaneni (Cardiff and Vale University Health Board), Professor Marios Papadopoulos (St George’s University Hospitals NHS Foundation Trust), Mr Anantharaju Prasad (Lancashire Teaching Hospitals NHS Foundation Trust), Mr Ivan Timofeev (Cambridge University Hospitals NHS Foundation Trust), Mr Christos Tolias (King’s College Hospital NHS Foundation Trust), Mr Senthil Selvanathan (Leeds Teaching Hospitals NHS Trust) and Mr Nitin Shetty (Whittington Health NHS Trust).
We would also like to thank all other members of the local research teams at each and every one of the FORVAD trial sites for their valued contributions to the set-up and delivery of the trial.
Recruiting surgeons
The following surgeons randomised participants to the trial: Mr Anantharaju Prasad (Lancashire Teaching Hospitals NHS Foundation Trust), Mr Simon Thomson and Mr Senthil Selvanathan (Leeds Teaching Hospitals NHS Trust), Mr Yahia Al-Tamimi (Sheffield Teaching Hospitals NHS Foundation Trust), Mr Nitin Mukerji (South Tees Hospitals NHS Foundation Trust) and Mr Nick Carleton-Bland (The Walton Centre NHS Foundation Trust).
Contributions of authors
Simon Thomson (https://orcid.org/0000-0003-4827-1961) (Consultant Neurosurgeon) was the chief investigator; was responsible for the design, conduct, co-ordination and management of the trial; and contributed to data interpretation and preparation of the final report. He also made a significant contribution to recruitment as a participating surgeon at Leeds Teaching Hospitals NHS Trust.
Gemma Ainsworth (https://orcid.org/0000-0002-9952-8440) (Senior Medical Statistician) was the trial statistician and made a substantial contribution to the analysis, interpretation and reporting of the trial, including preparation of the final report.
Senthil Selvanathan (https://orcid.org/0000-0002-3172-295X) (Consultant Spinal Neurosurgeon) contributed to the design, conduct and management of the trial, in addition to data interpretation and the review of the final report. He was also the PI at the Leeds Teaching Hospitals NHS Trust and made a significant contribution to recruitment.
Rachel Kelly (https://orcid.org/0000-0002-2912-2859) (Senior Trial Co-ordinator) played a central role in trial co-ordination and contributed to the preparation of the final report.
Howard Collier (https://orcid.org/0000-0002-0107-0604) (Senior Data Manager) was responsible for data management and contributed to the preparation of the final report.
Ruben Mujica-Mota (https://orcid.org/0000-0002-7430-2744) (Associate Professor in Health Economics) was responsible for the health economics analysis and contributed to the preparation of the final report.
Rebecca Talbot (https://orcid.org/0000-0002-3020-7585) (PhD candidate) contributed to the conduct, analysis and write-up of the qualitative substudy and was involved in the preparation of the final report.
Sarah Brown (https://orcid.org/0000-0002-1840-3786) (Principal Statistician) supervised the statistical aspects of the trial and contributed to the design, analysis, interpretation and reporting of the trial, including preparation of the final report.
Julie Croft (https://orcid.org/0000-0001-7586-3394) (Head of Trial Management) was the project delivery lead for the trial and contributed to the preparation of the final report.
Nikki Rousseau (https://orcid.org/0000-0001-8826-3515) (University Academic Fellow in Healthcare Technology Evaluation) was the qualitative substudy lead. She oversaw and contributed to the conduct, analysis and write-up of the qualitative substudy, and was involved in the preparation of the final report.
Ruchi Higham (https://orcid.org/0000-0002-0529-1041) (Qualitative Researcher) contributed to the conduct, analysis and write-up of the qualitative substudy, and was involved in the preparation of the final report.
Yahia Al-Tamimi (https://orcid.org/0000-0002-9014-2728) (Consultant Neurosurgeon) was the PI at the Sheffield Teaching Hospitals NHS Foundation Trust and contributed to participant recruitment and the interpretation and reporting of the results.
Neil Buxton (https://orcid.org/0000-0003-0589-9863) (Consultant Neurosurgeon) was the PI at the Walton Centre NHS Foundation Trust and contributed to the interpretation and reporting of the results.
Nick Carleton-Bland (https://orcid.org/0000-0002-9253-0599) (Consultant Neurosurgeon) was a participating surgeon at the Walton Centre NHS Foundation Trust and contributed to participant recruitment, and the interpretation and reporting of the results.
Martin Gledhill (https://orcid.org/0000-0002-8530-9597) (PPI representative) was involved in the trial design, advised on the PPI aspects of the trial and contributed to the preparation of the final report.
Victoria Halstead (https://orcid.org/0000-0003-2514-7351) (Speech and Language Therapist) was responsible for the hoarse voice analysis and contributed to data interpretation and the reporting of the trial.
Peter Hutchinson (https://orcid.org/0000-0002-2796-1835) (Consultant Neurosurgeon) contributed to the design, conduct and management of the trial, and the interpretation and reporting of the results.
James Meacock (https://orcid.org/0000-0002-2775-1838) contributed to the conduct and management of the trial, and the interpretation and reporting of the results.
Nitin Mukerji (https://orcid.org/0000-0002-7427-9230) (Consultant Neurosurgeon) was the PI at South Tees Hospitals NHS Foundation Trust and contributed to participant recruitment, and the interpretation and reporting of the results.
Debasish Pal (https://orcid.org/0000-0003-4120-7692) (Consultant Spinal Neurosurgeon) contributed to the design, conduct and management of the trial, and the interpretation and reporting of the results.
Armando Vargas-Palacios (https://orcid.org/0000-0002-6503-0134) (Health Economist) was involved in the health economic aspects of the trial and contributed to data interpretation and the reporting of the results.
Anantharaju Prasad (https://orcid.org/0000-0002-7245-0110) (Consultant Neurosurgeon) was the PI at Lancashire Teaching Hospitals NHS Foundation Trust and contributed to participant recruitment, and the interpretation and reporting of the results.
Martin Wilby (https://orcid.org/0000-0001-6647-9040) (Consultant Spinal Neurosurgeon) contributed to the design, conduct and management of the trial, and the interpretation and reporting of the results.
Deborah Stocken (https://orcid.org/0000-0001-8031-1738) (Professor of Clinical Trials Research) was the scientific lead and provided senior oversight in the methodology and delivery of the trial, and contributed to data interpretation and the preparation of the final report.
All authors contributed to data interpretation and the writing and/or review of the manuscript.
Data-sharing statement
All data-sharing requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Schroeder GD, Suleiman LI, Chioffe MA, Mangan JJ, McKenzie JC, Kepler CK, et al. The effect of oblique magnetic resonance imaging on surgical decision making for patients undergoing an anterior cervical discectomy and fusion for cervical radiculopathy. Int J Spine Surg 2019;13:302-7. https://doi.org/10.14444/6041.
- Gavin TM, Carandang G, Havey R, Flanagan P, Ghanayem A, Patwardhan AG. Biomechanical analysis of cervical orthoses in flexion and extension: a comparison of cervical collars and cervical thoracic orthoses. J Rehabil Res Dev 2003;40:527-37. https://doi.org/10.1682/jrrd.2003.11.0527.
- Schoenfeld AJ, George AA, Bader JO, Caram PM. Incidence and epidemiology of cervical radiculopathy in the United States military: 2000 to 2009. J Spinal Disord Tech 2012;25:17-22. https://doi.org/10.1097/BSD.0b013e31820d77ea.
- Sampath P, Bendebba M, Davis JD, Ducker T. Outcome in patients with cervical radiculopathy. Prospective, multicenter study with independent clinical review. Spine 1999;24:591-7. https://doi.org/10.1097/00007632-199903150-00021.
- Daffner SD, Hilibrand AS, Hanscom BS, Brislin BT, Vaccaro AR, Albert TJ. Impact of neck and arm pain on overall health status. Spine 2003;28:2030-5. https://doi.org/10.1097/01.BRS.0000083325.27357.39.
- Carette S, Fehlings MG. Clinical practice. Cervical radiculopathy. N Engl J Med 2005;353:392-9. https://doi.org/10.1056/NEJMcp043887.
- Persson LC, Moritz U, Brandt L, Carlsson CA. Cervical radiculopathy: pain, muscle weakness and sensory loss in patients with cervical radiculopathy treated with surgery. Physiotherapy or Cervical Collar. A Prospective, Controlled Study. Eur Spine J 1997;6:256-66. https://doi.org/10.1007/BF01322448.
- Fouyas IP, Statham PF, Sandercock PA. Cochrane review on the role of surgery in cervical spondylotic radiculomyelopathy. Spine 2002;27:736-47. https://doi.org/10.1097/00007632-200204010-00011.
- DePalma AF, Subin DK. Study of the cervical syndrome. Clin Orthop Relat Res 1965;38:135-42. https://doi.org/10.1097/00003086-196500380-00020.
- Lunsford LD, Bissonette DJ, Jannetta PJ, Sheptak PE, Zorub DS. Anterior surgery for cervical disc disease. Part 1: treatment of lateral cervical disc herniation in 253 cases. J Neurosurg 1980;53:1-11. https://doi.org/10.3171/jns.1980.53.1.0001.
- Witiw CD, Smieliauskas F, O’Toole JE, Fehlings MG, Fessler RG. Comparison of anterior cervical discectomy and fusion to posterior cervical foraminotomy for cervical radiculopathy: utilization, costs, and adverse events 2003 to 2014. Neurosurgery 2019;84:413-20. https://doi.org/10.1093/neuros/nyy051.
- Mok JK, Sheha ED, Samuel AM, McAnany SJ, Vaishnav AS, Albert TJ, et al. Evaluation of current trends in treatment of single-level cervical radiculopathy. Clin Spine Surg 2019;32:E241-5. https://doi.org/10.1097/BSD.0000000000000796.
- Selvanathan SK, Beagrie C, Thomson S, Corns R, Deniz K, Derham C, et al. Anterior cervical discectomy and fusion versus posterior cervical foraminotomy in the treatment of brachialgia: the Leeds spinal unit experience (2008–2013). Acta Neurochir 2015;157:1595-600. https://doi.org/10.1007/s00701-015-2491-8.
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, Smisson HF, Johnston KW, Grigorian AA, et al. Anterior cervical discectomy and fusion associated complications. Spine 2007;32:2310-7. https://doi.org/10.1097/BRS.0b013e318154c57e.
- Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am 1999;81:519-28. https://doi.org/10.2106/00004623-199904000-00009.
- Wang MC, Kreuter W, Wolfla CE, Maiman DJ, Deyo RA. Trends and variations in cervical spine surgery in the United States: Medicare beneficiaries, 1992 to 2005. Spine 2009;34:955-61. https://doi.org/10.1097/BRS.0b013e31819e2fd5.
- Albert TJ, Vacarro A. Postlaminectomy kyphosis. Spine 1998;23:2738-45. https://doi.org/10.1097/00007632-199812150-00014.
- Lubelski D, Healy AT, Silverstein MP, Abdullah KG, Thompson NR, Riew KD, et al. Reoperation rates after anterior cervical discectomy and fusion versus posterior cervical foraminotomy: a propensity-matched analysis. Spine J 2015;15:1277-83. https://doi.org/10.1016/j.spinee.2015.02.026.
- Scholz T, Geiger MF, Mainz V, Blume C, Albanna W, Clusmann H, et al. Anterior cervical decompression and fusion or posterior foraminotomy for cervical radiculopathy: results of a single-center series. J Neurol Surg A Cent Eur Neurosurg 2018;79:211-7. https://doi.org/10.1055/s-0037-1607225.
- Lin GX, Rui G, Sharma S, Kotheeranurak V, Suen TK, Kim JS. Does the neck pain, function, or range of motion differ after anterior cervical fusion, cervical disc replacement, and posterior cervical foraminotomy?. World Neurosurg 2019;129:e485-3. https://doi.org/10.1016/j.wneu.2019.05.188.
- Fang W, Huang L, Feng F, Yang B, He L, Du G, et al. Anterior cervical discectomy and fusion versus posterior cervical foraminotomy for the treatment of single-level unilateral cervical radiculopathy: a meta-analysis. J Orthop Surg Res 2020;15. https://doi.org/10.1186/s13018-020-01723-5.
- Park HJ, Kim SS, Lee SY, Park NH, Chung EC, Rho MH, et al. A practical MRI grading system for cervical foraminal stenosis based on oblique sagittal images. Br J Radiol 2013;86. https://doi.org/10.1259/bjr.20120515.
- Kim S, Lee JW, Chai JW, Yoo HJ, Kang Y, Seo J, et al. A new MRI grading system for cervical foraminal stenosis based on axial T2-weighted images. Korean J Radiol 2015;16:1294-302. https://doi.org/10.3348/kjr.2015.16.6.1294.
- Liu WJ, Hu L, Chou PH, Wang JW, Kan WS. Comparison of anterior cervical discectomy and fusion versus posterior cervical foraminotomy in the treatment of cervical radiculopathy: a systematic review. Orthop Surg 2016;8:425-31. https://doi.org/10.1111/os.12285.
- Matz PG, Ryken TC, Groff MW, Vresilovic EJ, Anderson PA, Heary RF, et al. Techniques for anterior cervical decompression for radiculopathy. J Neurosurg Spine 2009;11:183-97. https://doi.org/10.3171/2009.2.SPINE08721.
- Heary RF, Ryken TC, Matz PG, Anderson PA, Groff MW, Holly LT, et al. Cervical laminoforaminotomy for the treatment of cervical degenerative radiculopathy. J Neurosurg Spine 2009;11:198-202. https://doi.org/10.3171/2009.2.SPINE08722.
- Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine 2008;33:940-8. https://doi.org/10.1097/BRS.0b013e31816c8b67.
- Fankhauser CD, Mutter U, Aghayev E, Mannion AF. Validity and responsiveness of the Core Outcome Measures Index (COMI) for the neck. Eur Spine J 2012;21:101-14. https://doi.org/10.1007/s00586-011-1921-4.
- Wirth FP, Dowd GC, Sanders HF, Wirth C. Cervical discectomy. A prospective analysis of three operative techniques. Surg Neurol 2000;53:340-6. https://doi.org/10.1016/S0090-3019(00)00201-9.
- Herkowitz HN, Kurz LT, Overholt DP. Surgical management of cervical soft disc herniation. A comparison between the anterior and posterior approach. Spine 1990;15:1026-30. https://doi.org/10.1097/00007632-199015100-00009.
- Broekema AE, Kuijlen JM, Lesman-Leegte GA, Bartels RH, van Asselt AD, Vroomen PC, et al. Study protocol for a randomised controlled multicentre study: the Foraminotomy ACDF Cost-Effectiveness Trial (FACET) in patients with cervical radiculopathy. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-012829.
- Tschugg A, Neururer S, Scheufler KM, Ulmer H, Thomé C, Hegewald AA. Comparison of posterior foraminotomy and anterior foraminotomy with fusion for treating spondylotic foraminal stenosis of the cervical spine: study protocol for a randomized controlled trial (ForaC). Trials 2014;15. https://doi.org/10.1186/1745-6215-15-437.
- Holy M. Örebro Multicenter Study on Operative Treatment of Cervical Radiculopathy (OMSAP) n.d. https://clinicaltrials.gov/ct2/show/NCT04177849 (accessed 13 March 2021).
- Holy M, MacDowall A, Sigmundsson FG, Olerud C. Operative treatment of cervical radiculopathy: anterior cervical decompression and fusion compared with posterior foraminotomy: study protocol for a randomized controlled trial. Trials 2021;22. https://doi.org/10.1186/s13063-021-05492-2.
- Korinth MC, Krüger A, Oertel MF, Gilsbach JM. Posterior foraminotomy or anterior discectomy with polymethyl methacrylate interbody stabilization for cervical soft disc disease: results in 292 patients with monoradiculopathy. Spine 2006;31:1207-14. https://doi.org/10.1097/01.brs.0000217604.02663.59.
- Tumialán LM, Ponton RP, Gluf WM. Management of unilateral cervical radiculopathy in the military: the cost effectiveness of posterior cervical foraminotomy compared with anterior cervical discectomy and fusion. Neurosurg Focus 2010;28. https://doi.org/10.3171/2010.1.FOCUS09305.
- Alvin MD, Lubelski D, Abdullah KG, Whitmore RG, Benzel EC, Mroz TE. Cost–utility analysis of anterior cervical discectomy and fusion with plating (ACDFP) versus posterior cervical foraminotomy (PCF) for patients with single-level cervical radiculopathy at 1-year follow-up. Clin Spine Surg 2016;29:E67-72. https://doi.org/10.1097/BSD.0000000000000099.
- Foster MT, Carleton-Bland NP, Lee MK, Jackson R, Clark SR, Wilby MJ. Comparison of clinical outcomes in anterior cervical discectomy versus foraminotomy for brachialgia. Br J Neurosurg 2019;33:3-7. https://doi.org/10.1080/02688697.2018.1527013.
- Dunn C, Moore J, Sahai N, Issa K, Faloon M, Sinha K, et al. Minimally invasive posterior cervical foraminotomy with tubes to prevent undesired fusion: a long-term follow-up study. J Neurosurg Spine 2018;29:358-64. https://doi.org/10.3171/2018.2.SPINE171003.
- Cho TG, Kim YB, Park SW. Long term effect on adjacent segment motion after posterior cervical foraminotomy. Korean J Spine 2014;11:1-6. https://doi.org/10.14245/kjs.2014.11.1.1.
- Mansfield HE, Canar WJ, Gerard CS, O’Toole JE. Single-level anterior cervical discectomy and fusion versus minimally invasive posterior cervical foraminotomy for patients with cervical radiculopathy: a cost analysis. Neurosurg Focus 2014;37. https://doi.org/10.3171/2014.8.FOCUS14373.
- Church EW, Halpern CH, Faught RW, Balmuri U, Attiah MA, Hayden S, et al. Cervical laminoforaminotomy for radiculopathy: symptomatic and functional outcomes in a large cohort with long-term follow-up. Surg Neurol Int 2014;30:536-43.
- Jagannathan J, Sherman JH, Szabo T, Shaffrey CI, Jane JA. The posterior cervical foraminotomy in the treatment of cervical disc/osteophyte disease: a single-surgeon experience with a minimum of 5 years’ clinical and radiographic follow-up. J Neurosurg Spine 2009;10:347-56. https://doi.org/10.3171/2008.12.SPINE08576.
- Rodine RJ, Vernon H. Cervical radiculopathy: a systematic review on treatment by spinal manipulation and measurement with the Neck Disability Index. J Can Chiropr Assoc 2012;56:18-2.
- Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther 1991;14:409-15. https://doi.org/10.1037/t35122-000.
- Wainner RS, Fritz JM, Irrgang JJ, Boninger ML, Delitto A, Allison S. Reliability and diagnostic accuracy of the clinical examination and patient self-report measures for cervical radiculopathy. Spine 2003;28:52-6. https://doi.org/10.1097/00007632-200301010-00014.
- Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol 2008;117:919-24. https://doi.org/10.1177/000348940811701210.
- Deary IJ, Wilson JA, Harris MB, MacDougall G. Globus pharyngis: development of a symptom assessment scale. J Psychosom Res 1995;39:203-13. https://doi.org/10.1016/0022-3999(94)00104-D.
- Rosen CA, Lee AS, Osborne J, Zullo T, Murry T. Development and validation of the Voice Handicap Index-10. Laryngoscope 2004;114:1549-56. https://doi.org/10.1097/00005537-200409000-00009.
- Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911-20. https://doi.org/10.1185/030079906X132488.
- Singh AP. ASIA Score and Spinal Injury Classification n.d. http://boneandspine.com/what-is-asia-score-and-how-it-helps-in-classification-of-spinal-injury/ (accessed 13 March 2021).
- Hirano M. Clinical Examination of Voice: Disorders of Human Communication. New York, NY: Springer-Verlag; 1981.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
- Cook JA, Bruckner T, MacLennan GS, Seiler CM. Clustering in surgical trials – database of intracluster correlations. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-2.
- Hutton M. Spinal Services: GIRFT Programme National Specialty Report n.d. https://gettingitrightfirsttime.co.uk/wp-content/uploads/2019/01/Spinal-Services-Report-Mar19-L1.pdf (accessed 17 May 2022).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: Personal Social Services Research Unit, University of Kent; 2020.
- Gomes M, Soares MO, Dumville JC, Lewis SC, Torgerson DJ, Bodenham AR, et al. Cost-effectiveness analysis of general anaesthesia versus local anaesthesia for carotid surgery (GALA Trial). Br J Surg 2010;97:1218-25. https://doi.org/10.1002/bjs.7110.
- Department of Health and Social Care . 2010–11 Reference Costs Publication n.d. www.gov.uk/government/publications/2010-11-reference-costs-publication (accessed 13 March 2021).
- Joint Formulary Committee . British National Formulary 2020.
- NHS England . 2019/20/National/Cost/Collection/Data/Publication n.d. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/ (accessed 13 March 2021).
- Information Services Division Scotland . Theatres: Costs – Detailed Tables. R140X: Theatre Services n.d. www.isdscotland.org/Health-Topics/Finance/Costs/Detailed-Tables/Theatres.asp (accessed 17 May 2022).
- Joint Formulary Committee . British National Formulary 2016.
- Anderson PA, Savage JW, Vaccaro AR, Radcliff K, Arnold PM, Lawrence BD, et al. Prevention of surgical site infection in spine surgery. Neurosurgery 2017;80:S114-23. https://doi.org/10.1093/neuros/nyw066.
- NHS . Agenda for Change – Pay Rates n.d. www.healthcareers.nhs.uk/working-health/working-nhs/nhs-pay-and-benefits/agenda-change-pay-rates (accessed 18 May 2022).
- Department of Health and Social Care . Archived Reference Costs n.d. https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed 18 May 2022).
- NHS England . 2018/19/National/Cost/Collection/Data/Publication n.d. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/ (accessed 18 May 2022).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- Office for National Statistics . Employee Earnings in the UK: 2019 n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2019 (accessed 18 May 2022).
- Newell D, Diment E, Bolton JE. An electronic patient-reported outcome measures system in UK chiropractic practices: a feasibility study of routine collection of outcomes and costs. J Manipulative Physiol Ther 2016;39:31-4.
- General Osteopathic Council . Frequently Asked Question: How Much Does Treatment Cost? 2021. www.osteopathy.org.uk/faqs/how-much-does-treatment-cost/ (accessed 13 March 2021).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res 2014;14:221-33. https://doi.org/10.1586/14737167.2014.894462.
- Thompson AJ, Turner AJ. A Comparison of the EQ-5D-3L and EQ-5D-5L. PharmacoEconomics 2020;38:575-91. https://doi.org/10.1007/s40273-020-00893-8.
- Oakley A, Strange V, Bonell C, Allen E. Stephenson J, RIPPLE Study Team. Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-6. https://doi.org/10.1136/bmj.332.7538.413.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002889.
- Clement C, Edwards SL, Rapport F, Russell IT, Hutchings HA. Exploring qualitative methods reported in registered trials and their yields (EQUITY): systematic review. Trials 2018;19. https://doi.org/10.1186/s13063-018-2983-y.
- Clement C, Rapport F, Seagrove A, Alrubaiy L, Williams J. Healthcare professionals’ views of the use and administration of two salvage therapy drugs for acute ulcerative colitis: a nested qualitative study within the CONSTRUCT trial. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014512.
- Blencowe NS, Brown JM, Cook JA, Metcalfe C, Morton DG, Nicholl J, et al. Interventions in randomised controlled trials in surgery: issues to consider during trial design. Trials 2015;16. https://doi.org/10.1186/s13063-015-0918-4.
- Phelps EE, Tutton E, Griffin X, Baird J. TrAFFix study co-applicants . Facilitating trial recruitment: a qualitative study of patient and staff experiences of an orthopaedic trauma trial. Trials 2019;20. https://doi.org/10.1186/s13063-019-3597-8.
- Phelps EE, Tutton E, Griffin X, Baird J. A mixed-methods systematic review of patients’ experience of being invited to participate in surgical randomised controlled trials. Soc Sci Med 2020;253. https://doi.org/10.1016/j.socscimed.2020.112961.
- Ziebland S, Featherstone K, Snowdon C, Barker K, Frost H, Fairbank J. Does it matter if clinicians recruiting for a trial don’t understand what the trial is really about? Qualitative study of surgeons’ experiences of participation in a pragmatic multi-centre RCT. Trials 2007;8. https://doi.org/10.1186/1745-6215-8-4.
- Featherstone K, Donovan JL. ‘Why don’t they just tell me straight, why allocate it?’ The struggle to make sense of participating in a randomised controlled trial. Soc Sci Med 2002;55:709-19. https://doi.org/10.1016/s0277-9536(01)00197-6.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-5.
- Donovan JL, Peters TJ, Noble S, Powell P, Gillatt D, Oliver SE, et al. Who can best recruit to randomized trials? Randomized trial comparing surgeons and nurses recruiting patients to a trial of treatments for localized prostate cancer (the ProtecT study). J Clin Epidemiol 2003;56:605-9. https://doi.org/10.1016/S0895-4356(03)00083-0.
- McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-31.
- Horwood J, Johnson E, Gooberman-Hill R. Understanding involvement in surgical orthopaedic randomized controlled trials: a qualitative study of patient and health professional views and experiences. Int J Orthop Trauma Nurs 2016;20:3-12. https://doi.org/10.1016/j.ijotn.2015.05.002.
- Lawton J, Jenkins N, Darbyshire J, Farmer A, Holman R, Hallowell N. Understanding the outcomes of multi-centre clinical trials: a qualitative study of health professional experiences and views. Soc Sci Med 2012;74:574-81. https://doi.org/10.1016/j.socscimed.2011.11.012.
- Cook JA. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials 2009;10. https://doi.org/10.1186/1745-6215-10-9.
- Mittal R, Harris IA, Adie S, Naylor JM. Factors affecting patient participation in orthopaedic trials comparing surgery to non-surgical interventions. Contemp Clin Trials Commun 2016;3:153-7. https://doi.org/10.1016/j.conctc.2016.05.007.
- Newington L, Metcalfe A. Researchers’ and clinicians’ perceptions of recruiting participants to clinical research: a thematic meta-synthesis. J Clin Med Res 2014;6:162-72. https://doi.org/10.14740/jocmr1619w.
- Newington L, Metcalfe A. Factors influencing recruitment to research: qualitative study of the experiences and perceptions of research teams. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-10.
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7. https://doi.org/10.1186/1745-6215-7-9.
- Wade J, Donovan JL, Lane JA, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018-28. https://doi.org/10.1016/j.socscimed.2009.02.023.
- Kaur G, Hutchison I, Mehanna H, Williamson P, Shaw R, Tudur Smith C. Barriers to recruitment for surgical trials in head and neck oncology: a survey of trial investigators. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002625.
- Hamilton DW, de Salis I, Donovan JL, Birchall M. The recruitment of patients to trials in head and neck cancer: a qualitative study of the EaStER trial of treatments for early laryngeal cancer. Eur Arch Otorhinolaryngol 2013;270:2333-7. https://doi.org/10.1007/s00405-013-2349-8.
- Paleri V, Patterson J, Rousseau N, Moloney E, Craig D, Tzelis D, et al. Gastrostomy versus nasogastric tube feeding for chemoradiation patients with head and neck cancer: the TUBE pilot RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22160.
- Mills N, Donovan JL, Wade J, Hamdy FC, Neal DE, Lane JA. Exploring treatment preferences facilitated recruitment to randomized controlled trials. J Clin Epidemiol 2011;64:1127-36. https://doi.org/10.1016/j.jclinepi.2010.12.017.
- Garcea G, Lloyd T, Steward WP, Dennison AR, Berry DP. Differences in attitudes between patients with primary colorectal cancer and patients with secondary colorectal cancer: is it reflected in their willingness to participate in drug trials?. Eur J Cancer Care 2005;14:166-70. https://doi.org/10.1111/j.1365-2354.2005.00535.x.
- Davies G, Mills N, Holcombe C, Potter S. iBRA Steering Group . Perceived barriers to randomised controlled trials in breast reconstruction: obstacle to trial initiation or opportunity to resolve? A qualitative study. Trials 2020;21. https://doi.org/10.1186/s13063-020-4227-1.
- Rooshenas L, Elliott D, Wade J, Jepson M, Paramasivan S, Strong S, et al. Conveying equipoise during recruitment for clinical trials: qualitative synthesis of clinicians’ practices across six randomised controlled trials. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002147.
- Whybrow P, Pickard R, Hrisos S, Rapley T. Equipoise across the patient population: optimising recruitment to a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-016-1711-8.
- McDermott C, Vennik J, Philpott C, le Conte S, Thomas M, Eyles C, et al. Maximising recruitment to a randomised controlled trial for chronic rhinosinusitis using qualitative research methods: the MACRO conversation study. Trials 2021;22. https://doi.org/10.1186/s13063-020-04993-w.
- Hernon O, Dalton R, Dowling M. Clinical research nurses’ expectations and realities of their role: a qualitative evidence synthesis. J Clin Nurs 2020;29:667-83. https://doi.org/10.1111/jocn.15128.
- Maxton F, Darbyshire P, Thompson DR. Research nurses rising to the challenges of COVID-19. J Clin Nurs 2021;30:e13-e15. https://doi.org/10.1111/jocn.15504.
- Potter S, Mills N, Cawthorn SJ, Donovan J, Blazeby JM. Time to be BRAVE: is educating surgeons the key to unlocking the potential of randomised clinical trials in surgery? A qualitative study. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-80.
- Legrand C, Ducrocq V, Janssen P, Sylvester R, Duchateau L. A Bayesian approach to jointly estimate centre and treatment by centre heterogeneity in a proportional hazards model. Stat Med 2005;24:3789-804. https://doi.org/10.1002/sim.2475.
- Hackshaw A, Farrant H, Bulley S, Seckl MJ, Ledermann JA. Setting up non-commercial clinical trials takes too long in the UK: findings from a prospective study. J R Soc Med 2008;101:299-304. https://doi.org/10.1258/jrsm.2008.070373.
- Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials 2016;17. https://doi.org/10.1186/s13063-016-1391-4.
- Larson AN, Floccari LV, Garg S, Erickson MA, Sponseller PD, Brito JP, et al. Willingness to enroll in a surgical randomized controlled trial: patient and parent preferences regarding implant density for adolescent idiopathic scoliosis fusion. Spine Deform 2020;8:957-63. https://doi.org/10.1007/s43390-020-00143-z.
- Mansouri A, Cooper B, Shin SM, Kondziolka D. Randomized controlled trials and neurosurgery: the ideal fit or should alternative methodologies be considered?. J Neurosurg 2016;124:558-68. https://doi.org/10.3171/2014.12.JNS142465.
- Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. London: SAGE Publications Ltd; 2013.
- Vindrola-Padros C, Andrews L, Dowrick A, Djellouli N, Fillmore H, Bautista Gonzalez E, et al. Perceptions and experiences of healthcare workers during the COVID-19 pandemic in the UK. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-040503.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- Beebe J. Basic concepts and techniques of rapid appraisal. Hum Organ 2008;54:42-51. https://doi.org/10.17730/humo.54.1.k84tv883mr2756l3.
- Hastings CE, Fisher CA, McCabe MA, Allison J, Brassil D, Offenhartz M, et al. Clinical research nursing: a critical resource in the national research enterprise. Nurs Outlook 2012;60:149-56.e1. https://doi.org/10.1016/j.outlook.2011.10.003.
- Ness EA, Royce C. Clinical trials and the role of the oncology clinical trials nurse. Nurs Clin North Am 2017;52:133-48. https://doi.org/10.1016/j.cnur.2016.10.005.
- Griffin XL, Costa ML, Achten J, Dritsaki M, Baird J, Parsons N. Trial of Acute Femoral Fracture Fixation (TrAFFix): study protocol for a randomised controlled feasibility trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-2265-0.
- Cook JA, Elders A, Boachie C, Bassinga T, Fraser C, Altman DG, et al. A systematic review of the use of an expertise-based randomised controlled trial design. Trials 2015;16. https://doi.org/10.1186/s13063-015-0739-5.
- Cook JA, Campbell MK, Gillies K, Skea Z. Surgeons’ and methodologists’ perceptions of utilising an expertise-based randomised controlled trial design: a qualitative study. Trials 2018;19. https://doi.org/10.1186/s13063-018-2832-z.
- Freynhagen R, Rey R, Argoff C. When to consider ‘mixed pain’? The right questions can make a difference!. Curr Med Res Opin 2020;36:2037-46. https://doi.org/10.1080/03007995.2020.1832058.
- Nijs J, Lahousse A, Kapreli E, Bilika P, Saraçoğlu İ, Malfliet A, et al. Nociplastic pain criteria or recognition of central sensitization? Pain phenotyping in the past, present and future. J Clin Med 2021;10. https://doi.org/10.3390/jcm10153203.
- Morsø L, Kent PM, Albert HB. Are self-reported pain characteristics, classified using the PainDETECT questionnaire, predictive of outcome in people with low back pain and associated leg pain?. Clin J Pain 2011;27:535-41. https://doi.org/10.1097/AJP.0b013e318208c941.
- Singrakhia MD, Malewar NR, Deshmukh S, Deshmukh S. Clinical and radiological outcomes of day-care posterior foraminotomy and decompression of the cervical spine. Asian J Neurosurg 2018;13:1118-22. https://doi.org/10.4103/ajns.AJNS_14_17.
- Anderson KK, Arnold PM. Oropharyngeal dysphagia after anterior cervical spine surgery: a review. Global Spine J 2013;3:273-86. https://doi.org/10.1055/s-0033-1354253.
- Kahraman S, Sirin S, Erdogan E, Atabey C, Daneyemez M, Gonul E. Is dysphonia permanent or temporary after anterior cervical approach?. Eur Spine J 2007;16:2092-5. https://doi.org/10.1007/s00586-007-0489-5.
- Winslow CP, Meyers AD. Otolaryngologic complications of the anterior approach to the cervical spine. Am J Otolaryngol 1999;20:16-27. https://doi.org/10.1016/S0196-0709(99)90046-7.
- Saavedra-Pozo FM, Deusdara RA, Benzel EC. Adjacent segment disease perspective and review of the literature. Ochsner J 2014;14:78-83.
- Kong L, Cao J, Wang L, Shen Y. Prevalence of adjacent segment disease following cervical spine surgery: a PRISMA-compliant systematic review and meta-analysis. Medicine 2016;95. https://doi.org/10.1097/MD.0000000000004171.
- Freedman B. Equipoise and the ethics of clinical research. N Engl J Med 1987;317:141-5. https://doi.org/10.1056/NEJM198707163170304.
- Avery KN, Williamson PR, Gamble C, O’Connell Francischetto E, Metcalfe C, Davidson P, et al. Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013537.
- de Bono QCJ. Thefaut V Johnston – A Game Changer for Consent in Elective Surgery 2017. www.ukhealthcarelawblog.co.uk/thefaut-v-johnston-a-game-changer-for-consent-in-elective-surgery/ (accessed 5 June 2022).
- Fairbanks G. Voice and Articulation Drillbook. New York, NY: Harper & Row; 1960.
Appendix 1 Internal pilot phase document
Internal pilot phase
The trial will include an internal pilot phase to evaluate the feasibility of recruitment within the planned timelines, based on the number of actively recruiting sites and overall average recruitment rate per site per month.
The internal pilot phase will also assess early safety data, data validity, compliance with trial procedures, eligibility of the clinical teams and type of procedure performed (e.g. minimal access or open surgery).
Recruitment progression criteria
The internal pilot phase is in line with the recommendations set out by Avery et al. 131 We have specified decision criteria that will be used to indicate whether or not recruitment and randomisation targets have been met during the internal pilot phase, which will then inform the future progression of the trial. The criteria are set out in the form of stop/amend/continue, similar to a red/amber/green traffic light decision system. Recruitment targets are defined in terms of the expected number of patients with cervical brachialgia referred for surgery per year, and the expected proportion of eligible and consenting patients. Randomisation targets are defined in terms of the overall mean recruitment rate per centre per month and include targets on the number of centres to be opened and actively recruiting within the first 12 months of the recruitment phase.
Recruitment targets
The survey conducted across 18 centres reported a mean of 45 patients with cervical brachialgia requiring surgery per year. Based on local audit data,13 we expect that 70% of patients referred for surgery will meet the eligibility criteria for the trial. Of those patients who are eligible, we expect a minimum consent rate for randomisation of 27%. These conservative recruitment targets are aligned with the minimum randomisation target to be achieved.
Randomisation targets
Stop criteria (red)
If the overall mean recruitment rate is < 0.7 patients per centre per month and there are fewer than eight actively recruiting centres, then recruitment is very unlikely to reach the target sample size of 252 patients, even with a 12-month recruitment extension. Therefore, in this scenario, we would consider that it is futile to continue recruitment and will recommend stopping the trial.
Continue (green)
If the overall mean recruitment rate is at least 1.1 patients per centre per month across 12 actively recruiting centres, then we expect to reach the recruitment target of 252 patients with no remedial action required. The team will be confident that there are no concerning issues that may threaten recruitment to the trial within the planned recruitment timelines.
Amend (amber)
If the overall mean recruitment rate is between 0.7 and 1.1 patients per centre per month or fewer than 12 actively recruiting centres are opened, then a recovery plan detailing remedial actions, including increasing the number of centres and other recruitment initiatives, will be submitted; if approved, the trial will proceed with caution.
Assumptions
Based on our experience of surgical trials, we assume the following:
-
duration of 12 months (44% of the recruitment period)
-
staggered opening of centres
-
a delay of 1 month between a centre opening and the first patient identified and approached
-
a delay of 3 months between patient approach and randomisation (first patient randomised in month 5).
Moreover, the first eight centres opened to recruitment will be a mix of co-applicant and non-co-applicant centres, to represent a range of experience in conducting surgical trials, and will therefore provide a realistic estimate of the expected recruitment rate following the internal pilot phase in the substantive phase of the trial.
Appendix 2 Hoarse voice assessment script
For participants randomly selected at registration to provide voice recordings, the attending trial team will collect a voice recording. The trial team will ask the participant to complete the following tasks.
1. Sustained vowel sounds
Ask the participant to make the following vowel sounds for 3–5 seconds:
-
/a/ – pronounced ‘aaah’
-
/i/ – pronounced ‘eee’.
2. Sentence production
Ask the participant to read the following sentences, which can be found on the flash cards located in the investigator site file.
-
The blue spot is on the key again.
-
How hard did he hit him?
-
We were away a year ago.
-
We eat eggs every Easter.
-
My mama makes lemon muffins.
-
Peter will keep at the peak.
3. Spontaneous speech
This section requires the participant to give 20–30 seconds of free-flowing speech. To facilitate this, the below question should be asked:
-
Tell me about your journey here today.
Optional speech collection
If the researcher is unable to gain 20–30 seconds of speech from the participant in the spontaneous speech section, a scripted passage may be read by the participant as an alternative. The passage used will be ‘The Rainbow Passage’. The script is as follows:
When the sunlight strikes raindrops in the air, they act like a prism and form a rainbow. The rainbow is a division of white light into many beautiful colours. These take the shape of a long round arch, with its path high above, and its two ends apparently beyond the horizon. There is according to legend, a boiling pot of gold at one end. People look, but no one ever finds it. When a man looks for something beyond his reach, his friends say he is looking for the pot of gold at the end of the rainbow.
The Rainbow Passage, a public domain text, can be found on page 127 of the 2nd edition of Grant Fairbanks’ Voice and Articulation Drillbook (New York, NY: Harper & Row). 133
Appendix 3 Reasons for non-eligibility
| Site name | Reason (n) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cervical myelopathy | No single nerve entrapment | No diagnosis of unilateral cervical brachialgia as confirmed by MRI or CT myelography within the previous 12 months | Previous cervical spine surgery | No symotoms of cervical brachialgia in the previous 6 weeks | Cervical disc causing cord compression | Reason missing | Bilateral cervical brachialgia | Not failed conservative management | Not suitable for PCF | Other (specify) | Unable to provide written informed consent | Cervical deformity | Declined to participate before eligibility assessment performed | No posterolateral disc or foraminal narrowing | Unable to comply with the terms of the protocol | |
| Whittington | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Preston | 12 | 10 | 1 | 8 | 0 | 8 | 1 | 9 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 0 |
| Leeds | 3 | 11 | 19 | 4 | 21 | 3 | 11 | 1 | 5 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Cardiff | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cambridge | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Walton Centre | 23 | 12 | 10 | 13 | 1 | 6 | 2 | 3 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 38 | 34 | 30 | 25 | 22 | 18 | 16 | 14 | 8 | 4 | 3 | 3 | 1 | 1 | 1 | 1 |
Appendix 4 Additional results tables for the Neck Disability Index outcome
Contact information and permission to use the NDI were obtained from Mapi Research Trust, Lyon, France (URL: https://eprovide.mapi-trust.org).
| NDI score | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Day 0 | |||
| Mean (SD) | 45.8 (17.7) | 41.7 (17.3) | 44.1 (17.3) |
| Median (range) | 44.0 (18.0–76.0) | 35.6 (18.0–78.0) | 40.0 (18.0–78.0) |
| IQR | 36.0–62.0 | 34.0–44.0 | 34.0–58.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Unreturned booklet, n (%) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| Day 1 | |||
| Mean (SD) | 55.1 (19.3) | 49.7 (19.5) | 52.8 (19.1) |
| Median (range) | 61.3 (22.0–78.0) | 46.0 (26.0–76.0) | 56.0 (22.0–78.0) |
| IQR | 41.7–69.4 | 36.0–64.4 | 36.0–68.9 |
| n | 12 | 9 | 21 |
| Missing (n) | 2 | 0 | 2 |
| Partially missing questionnaire, n (%) | 1 (50.0) | 0 (0.0) | 1 (50.0) |
| Unreturned booklet, n (%) | 1 (50.0) | 0 (0.0) | 1 (50.0) |
| Week 6 | |||
| Mean (SD) | 42.4 (12.1) | 30.7 (29.0) | 38.5 (19.2) |
| Median (range) | 45.0 (22.0–62.0) | 14.0 (8.0–70.0) | 44.0 (8.0–70.0) |
| IQR | 30.0–48.9 | 8.0–53.3 | 22.0–51.1 |
| n | 10 | 5 | 15 |
| Missing (n) | 4 | 4 | 8 |
| Unreturned questionnaire, n (%) | 0 (0.0) | 1 (25.0) | 1 (12.5) |
| Wholly missing questionnaire, n (%) | 1 (25.0) | 2 (50.0) | 3 (37.5) |
| Unreturned booklet, n (%) | 3 (75.0) | 1 (25.0) | 4 (50.0) |
| Week 12 | |||
| Mean (SD) | 39.3 (19.2) | 31.0 (24.5) | 37.1 (20.2) |
| Median (range) | 40.0 (14.0–72.0) | 29.0 (4.0–62.0) | 36.0 (4.0–72.0) |
| IQR | 20.0–54.0 | 13.0–49.0 | 20.0–54.0 |
| n | 11 | 4 | 15 |
| Missing (n) | 3 | 5 | 8 |
| Unreturned questionnaire, n (%) | 1 (33.3) | 0 (0.0) | 1 (12.5) |
| Wholly missing questionnaire, n (%) | 0 (0.0) | 2 (40.0) | 2 (25.0) |
| Unreturned booklet, n (%) | 2 (66.7) | 3 (60.0) | 5 (62.5) |
| Week 26 | |||
| Mean (SD) | 42.4 (26.0) | 31.5 (25.6) | 39.0 (25.4) |
| Median (range) | 42.2 (6.0–82.0) | 28.0 (4.0–66.0) | 30.0 (4.0–82.0) |
| IQR | 22.0–68.0 | 16.0–47.0 | 22.0–66.0 |
| n | 9 | 4 | 13 |
| Missing (n) | 5 | 5 | 10 |
| Wholly missing questionnaire, n (%) | 2 (40.0) | 2 (40.0) | 4 (40.0) |
| Unreturned booklet, n (%) | 3 (60.0) | 3 (60.0) | 6 (60.0) |
| Week 39 | |||
| Mean (SD) | 28.0 (13.2) | 37.2 (25.8) | 31.9 (19.0) |
| Median (range) | 24.0 (8.0–44.0) | 38.0 (0.0–72.0) | 34.0 (0.0–72.0) |
| IQR | 20.0–42.2 | 32.0–44.0 | 21.0–43.1 |
| n | 7 | 5 | 12 |
| Missing (n) | 7 | 4 | 11 |
| Wholly missing questionnaire, n (%) | 1 (14.3) | 2 (50.0) | 3 (27.3) |
| Unreturned booklet, n (%) | 6 (85.7) | 2 (50.0) | 8 (72.7) |
| Week 52 | |||
| Mean (SD) | 36.1 (24.9) | 38.5 (27.5) | 36.8 (24.6) |
| Median (range) | 25.3 (12.0–90.0) | 45.0 (0.0–64.0) | 33.3 (0.0–90.0) |
| IQR | 20.0–42.0 | 20.0–57.0 | 20.0–50.0 |
| n | 10 | 4 | 14 |
| Missing (n) | 4 | 5 | 9 |
| Wholly missing questionnaire, n (%) | 3 (75.0) | 2 (40.0) | 5 (55.6) |
| Unreturned booklet, n (%) | 1 (25.0) | 3 (60.0) | 4 (44.4) |
| Time point and NDI items | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Baseline: day 0 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (9.0–10.0) | 10.0 (9.0–10.0) | 10.0 (9.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| Missing (n) | 1 | 0 | 1 |
| n | 13 | 9 | 22 |
| Pain intensity, n (%) | |||
| I have no pain at the moment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| The pain is very mild at the moment | 2 (14.3) | 4 (44.4) | 6 (26.1) |
| The pain is moderate at the moment | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| The pain is fairly severe at the moment | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| The pain is very severe at the moment | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| The pain is the worst imaginable at the moment | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Personal care, n (%) | |||
| I can look after myself normally without causing extra pain | 2 (14.3) | 4 (44.4) | 6 (26.1) |
| I can look after myself normally, but it causes extra pain | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| It is painful to look after myself and I am slow and careful | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I need some help but can manage most of my personal care | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I need help every day in most aspects of self-care | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I do not get dressed; I wash with difficulty and stay in bed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Lifting, n (%) | |||
| I can lift heavy weights without extra pain | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I can lift heavy weights, but it gives extra pain | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Pain prevents me lifting heavy weights off the floor, but I can manage if they are conveniently placed, for example on a table | 0 (0.0) | 5 (55.6) | 5 (21.7) |
| Pain prevents me from lifting heavy weights, but I can manage light to medium weights if they are conveniently positioned | 5 (35.7) | 1 (11.1) | 6 (26.1) |
| I can lift only very light weights | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I cannot lift or carry anything | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Reading, n (%) | |||
| I can read as much as I want to with no pain in my neck | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I can read as much as I want to with slight pain in my neck | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I can read as much as I want to with moderate pain in my neck | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| I cannot read as much as I want to because of moderate pain in my neck | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| I can hardly read at all because of severe pain in my neck | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I cannot read at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Headaches, n (%) | |||
| I have no headaches at all | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| I have slight headaches, which come infrequently | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| I have moderate headaches, which come infrequently | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| I have moderate headaches, which come frequently | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I have severe headaches, which come frequently | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| I have headaches almost all the time | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Concentration, n (%) | |||
| I can concentrate fully when I want to with no difficulty | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| I can concentrate fully when I want to with slight difficulty | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| I have a fair degree of difficulty in concentrating when I want to | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| I have a lot of difficulty in concentrating when I want to | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I have a great deal of difficulty in concentrating when I want to | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I cannot concentrate at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Work, n (%) | |||
| I can do as much work as I want to | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I can do only my usual work, but no more | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I can do most of my usual work, but no more | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| I cannot do my usual work | 5 (35.7) | 4 (44.4) | 9 (39.1) |
| I can hardly do any work at all | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I cannot do any work at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Driving, n (%) | |||
| I can drive my car without any neck pain | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I can drive my car as long as I want with slight pain in my neck | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| I can drive my car as long as I want with moderate pain in my neck | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I cannot drive my car as long as I want because of moderate pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can hardly drive at all because of severe pain in my neck | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I cannot drive my car at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Missing | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Sleeping, n (%) | |||
| I have no trouble sleeping | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| My sleep is slightly disturbed (< 1 hour sleepless) | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| My sleep is mildly disturbed (1–2 hours sleepless) | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| My sleep is moderately disturbed (2–3 hours sleepless) | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| My sleep is greatly disturbed (3–5 hours sleepless) | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| My sleep is completely disturbed (5–7 hours sleepless) | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Recreation, n (%) | |||
| I am able to engage in all my recreation activities with no neck pain at all | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I am able to engage in all my recreation activities, with some pain in my neck | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I am able to engage in most, but not all, of my usual recreation activities because of pain in my neck | 4 (28.6) | 5 (55.6) | 9 (39.1) |
| I am able to engage in a few of my usual recreation activities because of pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can hardly do any recreation activities because of pain in my neck | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I cannot do any recreation activities at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Day 1 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (7.0–10.0) | 10.0 (9.0–10.0) | 10.0 (7.0–10.0) |
| IQR | 9.0–10.0 | 10.0–10.0 | 9.0–10.0 |
| Missing (n) | 1 | 0 | 1 |
| n | 13 | 9 | 22 |
| Pain intensity, n (%) | |||
| I have no pain at the moment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| The pain is very mild at the moment | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| The pain is moderate at the moment | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| The pain is fairly severe at the moment | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| The pain is very severe at the moment | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| The pain is the worst imaginable at the moment | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Personal care, n (%) | |||
| I can look after myself normally without causing extra pain | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I can look after myself normally but it causes extra pain | 3 (21.4) | 4 (44.4) | 7 (30.4) |
| It is painful to look after myself and I am slow and careful | 5 (35.7) | 1 (11.1) | 6 (26.1) |
| I need some help but can manage most of my personal care | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| I need help every day in most aspects of self-care | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I do not get dressed, I wash with difficulty and stay in bed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Lifting, n (%) | |||
| I can lift heavy weights without extra pain | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I can lift heavy weights, but it gives extra pain | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Pain prevents me lifting heavy weights off the floor, but I can manage if they are conveniently placed, for example on a table | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Pain prevents me from lifting heavy weights, but I can manage light to medium weights if they are conveniently positioned | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| I can only lift very light weights | 6 (42.9) | 4 (44.4) | 10 (43.5) |
| I cannot lift or carry anything | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Reading, n (%) | |||
| I can read as much as I want to with no pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can read as much as I want to with slight pain in my neck | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I can read as much as I want to with moderate pain in my neck | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| I cannot read as much as I want to because of moderate pain in my neck | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I can hardly read at all because of severe pain in my neck | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| I cannot read at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Headaches, n (%) | |||
| I have no headaches at all | 8 (57.1) | 3 (33.3) | 11 (47.8) |
| I have slight headaches, which come infrequently | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| I have moderate headaches, which come infrequently | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| I have moderate headaches, which come frequently | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I have severe headaches, which come frequently | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I have headaches almost all the time | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Concentration, n (%) | |||
| I can concentrate fully when I want to with no difficulty | 5 (35.7) | 1 (11.1) | 6 (26.1) |
| I can concentrate fully when I want to with slight difficulty | 2 (14.3) | 5 (55.6) | 7 (30.4) |
| I have a fair degree of difficulty in concentrating when I want to | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I have a lot of difficulty in concentrating when I want to | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I have a great deal of difficulty in concentrating when I want to | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I cannot concentrate at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Work, n (%) | |||
| I can do as much work as I want to | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I can only do my usual work, but no more | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| I can do most of my usual work, but no more | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I cannot do my usual work | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| I can hardly do any work at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I cannot do any work at all | 5 (35.7) | 3 (33.3) | 8 (34.8) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Driving, n (%) | |||
| I can drive my car without any neck pain | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I can drive my car as long as I want with slight pain in my neck | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I can drive my car as long as I want with moderate pain in my neck | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| I cannot drive my car as long as I want because of moderate pain in my neck | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I can hardly drive at all because of severe pain in my neck | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I cannot drive my car at all | 8 (57.1) | 3 (33.3) | 11 (47.8) |
| Missing | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Sleeping, n (%) | |||
| I have no trouble sleeping | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| My sleep is slightly disturbed (< 1 hour sleepless) | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| My sleep is mildly disturbed (1–2 hours sleepless) | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| My sleep is moderately disturbed (2–3 hours sleepless) | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| My sleep is greatly disturbed (3–5 hours sleepless) | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| My sleep is completely disturbed (5–7 hours sleepless) | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Recreation, n (%) | |||
| I am able to engage in all my recreation activities with no neck pain at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I am able to engage in all my recreation activities, with some pain in my neck | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I am able to engage in most, but not all of my usual recreation activities because of pain in my neck | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| I am able to engage in a few of my usual recreation activities because of pain in my neck | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I can hardly do any recreation activities because of pain in my neck | 3 (21.4) | 4 (44.4) | 7 (30.4) |
| I cannot do any recreation activities at all | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Week 6 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) |
| IQR | 9.0–10.0 | 0.0–10.0 | 9.0–10.0 |
| Missing (n) | 3 | 2 | 5 |
| n | 11 | 7 | 18 |
| Pain intensity, n (%) | |||
| I have no pain at the moment | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| The pain is very mild at the moment | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| The pain is moderate at the moment | 5 (35.7) | 0 (0.0) | 5 (21.7) |
| The pain is fairly severe at the moment | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| The pain is very severe at the moment | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| The pain is the worst imaginable at the moment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Personal care, n (%) | |||
| I can look after myself normally without causing extra pain | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| I can look after myself normally, but it causes extra pain | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| It is painful to look after myself and I am slow and careful | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I need some help but can manage most of my personal care | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I need help every day in most aspects of self-care | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I do not get dressed, I wash with difficulty and stay in bed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Lifting, n (%) | |||
| I can lift heavy weights without extra pain | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I can lift heavy weights, but it gives extra pain | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| Pain prevents me lifting heavy weights off the floor, but I can manage if they are conveniently placed, for example on a table | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| Pain prevents me from lifting heavy weights, but I can manage light to medium weights if they are conveniently positioned | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I can only lift very light weights | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| I cannot lift or carry anything | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Reading, n (%) | |||
| I can read as much as I want to with no pain in my neck | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| I can read as much as I want to with slight pain in my neck | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I can read as much as I want with moderate pain in my neck | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I cannot read as much as I want because of moderate pain in my neck | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I can hardly read at all because of severe pain in my neck | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I cannot read at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Headaches, n (%) | |||
| I have no headaches at all | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| I have slight headaches, which come infrequently | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I have moderate headaches, which come infrequently | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I have moderate headaches, which come frequently | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I have severe headaches, which come frequently | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I have headaches almost all the time | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Concentration, n (%) | |||
| I can concentrate fully when I want to with no difficulty | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| I can concentrate fully when I want to with slight difficulty | 5 (35.7) | 0 (0.0) | 5 (21.7) |
| I have a fair degree of difficulty in concentrating when I want to | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I have a lot of difficulty in concentrating when I want to | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I have a great deal of difficulty in concentrating when I want to | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I cannot concentrate at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Work, n (%) | |||
| I can do as much work as I want to | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I can only do my usual work, but no more | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| I can do most of my usual work, but no more | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I cannot do my usual work | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I can hardly do any work at all | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I cannot do any work at all | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Missing | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Driving, n (%) | |||
| I can drive my car without any neck pain | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| I can drive my car as long as I want with slight pain in my neck | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I can drive my car as long as I want with moderate pain in my neck | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I cannot drive my car as long as I want because of moderate pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can hardly drive at all because of severe pain in my neck | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I cannot drive my car at all | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 8 (57.1) | 5 (55.6) | 13 (56.5) |
| Sleeping, n (%) | |||
| I have no trouble sleeping | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| My sleep is slightly disturbed (< 1 hour sleepless) | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| My sleep is mildly disturbed (1–2 hours sleepless) | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| My sleep is moderately disturbed (2–3 hours sleepless) | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| My sleep is greatly disturbed (3–5 hours sleepless) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| My sleep is completely disturbed (5–7 hours sleepless) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Recreation, n (%) | |||
| I am able to engage in all my recreation activities with no neck pain at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I am able to engage in all my recreation activities, with some pain in my neck | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I am able to engage in most, but not all of my usual recreation activities because of pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I am able to engage in a few of my usual recreation activities because of pain in my neck | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I can hardly do any recreation activities because of pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I cannot do any recreation activities at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Week 12 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (9.0–10.0) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) |
| IQR | 10.0–10.0 | 0.0–10.0 | 10.0–10.0 |
| Missing (n) | 3 | 3 | 6 |
| n | 11 | 6 | 17 |
| Pain intensity, n (%) | |||
| I have no pain at the moment | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| The pain is very mild at the moment | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| The pain is moderate at the moment | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| The pain is fairly severe at the moment | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| The pain is very severe at the moment | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| The pain is the worst imaginable at the moment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 5 (55.6) | 8 (34.8) |
| Personal care, n (%) | |||
| I can look after myself normally without causing extra pain | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I can look after myself normally, but it causes extra pain | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| It is painful to look after myself and I am slow and careful | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I need some help but can manage most of my personal care | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I need help every day in most aspects of self-care | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I do not get dressed, I wash with difficulty and stay in bed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 5 (55.6) | 8 (34.8) |
| Lifting, n (%) | |||
| I can lift heavy weights without extra pain | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I can lift heavy weights, but it gives extra pain | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Pain prevents me lifting heavy weights off the floor, but I can manage if they are conveniently placed, for example on a table | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Pain prevents me from lifting heavy weights but I can manage light to medium weights if they are conveniently positioned | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I can only lift very light weights | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I cannot lift or carry anything | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 5 (55.6) | 8 (34.8) |
| Reading, n (%) | |||
| I can read as much as I want to with no pain in my neck | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I can read as much as I want to with slight pain in my neck | 5 (35.7) | 1 (11.1) | 6 (26.1) |
| I can read as much as I want to with moderate pain in my neck | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I cannot read as much as I want to because of moderate pain in my neck | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I can hardly read at all because of severe pain in my neck | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I cannot read at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 5 (55.6) | 8 (34.8) |
| Headaches, n (%) | |||
| I have no headaches at all | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I have slight headaches, which come infrequently | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| I have moderate headaches, which come infrequently | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I have moderate headaches, which come frequently | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I have severe headaches, which come frequently | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I have headaches almost all the time | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 5 (55.6) | 8 (34.8) |
| Concentration, n (%) | |||
| I can concentrate fully when I want to with no difficulty | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I can concentrate fully when I want to with slight difficulty | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I have a fair degree of difficulty in concentrating when I want to | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I have a lot of difficulty in concentrating when I want to | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I have a great deal of difficulty in concentrating when I want to | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I cannot concentrate at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 5 (55.6) | 8 (34.8) |
| Work, n (%) | |||
| I can do as much work as I want to | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can only do my usual work, but no more | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can do most of my usual work, but no more | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I cannot do my usual work | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I can hardly do any work at all | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I cannot do any work at all | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 3 (21.4) | 5 (55.6) | 8 (34.8) |
| Driving, n (%) | |||
| I can drive my car without any neck pain | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I can drive my car as long as I want with slight pain in my neck | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I can drive my car as long as I want with moderate pain in my neck | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I cannot drive my car as long as I want because of moderate pain in my neck | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I can hardly drive at all because of severe pain in my neck | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I cannot drive my car at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 5 (35.7) | 5 (55.6) | 10 (43.5) |
| Sleeping, n (%) | |||
| I have no trouble sleeping | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| My sleep is slightly disturbed (< 1 hour sleepless) | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| My sleep is mildly disturbed (1–2 hours sleepless) | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| My sleep is moderately disturbed (2–3 hours sleepless) | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| My sleep is greatly disturbed (3–5 hours sleepless) | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| My sleep is completely disturbed (5–7 hours sleepless) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 5 (55.6) | 8 (34.8) |
| Recreation, n (%) | |||
| I am able to engage in all my recreation activities with no neck pain at all | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I am able to engage in all my recreation activities, with some pain in my neck | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| I am able to engage in most, but not all of my usual recreation activities because of pain in my neck | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I am able to engage in a few of my usual recreation activities because of pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can hardly do any recreation activities because of pain in my neck | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| I cannot do any recreation activities at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 5 (55.6) | 8 (34.8) |
| Week 26 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) |
| IQR | 9.0–10.0 | 0.0–10.0 | 9.0–10.0 |
| Missing (n) | 3 | 3 | 6 |
| n | 11 | 6 | 17 |
| Pain intensity, n (%) | |||
| I have no pain at the moment | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| The pain is very mild at the moment | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| The pain is moderate at the moment | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| The pain is fairly severe at the moment | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| The pain is very severe at the moment | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| The pain is the worst imaginable at the moment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 5 (35.7) | 5 (55.6) | 10 (43.5) |
| Personal care, n (%) | |||
| I can look after myself normally without causing extra pain | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I can look after myself normally, but it causes extra pain | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| It is painful to look after myself and I am slow and careful | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I need some help but can manage most of my personal care | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I need help every day in most aspects of self-care | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I do not get dressed, I wash with difficulty and stay in bed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 5 (35.7) | 5 (55.6) | 10 (43.5) |
| Lifting, n (%) | |||
| I can lift heavy weights without extra pain | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I can lift heavy weights, but it gives extra pain | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Pain prevents me lifting heavy weights off the floor, but I can manage if they are conveniently placed, for example on a table | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Pain prevents me from lifting heavy weights, but I can manage light to medium weights if they are conveniently positioned | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I can only lift very light weights | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I cannot lift or carry anything | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 5 (35.7) | 5 (55.6) | 10 (43.5) |
| Reading, n (%) | |||
| I can read as much as I want to with no pain in my neck | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I can read as much as I want to with slight pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can read as much as I want with moderate pain in my neck | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I cannot read as much as I want because of moderate pain in my neck | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I can hardly read at all because of severe pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I cannot read at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 5 (35.7) | 5 (55.6) | 10 (43.5) |
| Headaches, n (%) | |||
| I have no headaches at all | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I have slight headaches, which come infrequently | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| I have moderate headaches, which come infrequently | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I have moderate headaches, which come frequently | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I have severe headaches, which come frequently | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I have headaches almost all the time | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 5 (35.7) | 5 (55.6) | 10 (43.5) |
| Concentration, n (%) | |||
| I can concentrate fully when I want to with no difficulty | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I can concentrate fully when I want to with slight difficulty | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| I have a fair degree of difficulty in concentrating when I want to | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I have a lot of difficulty in concentrating when I want to | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I have a great deal of difficulty in concentrating when I want to | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I cannot concentrate at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 5 (35.7) | 5 (55.6) | 10 (43.5) |
| Work, n (%) | |||
| I can do as much work as I want to | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can only do my usual work, but no more | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I can do most of my usual work, but no more | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| I cannot do my usual work | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I can hardly do any work at all | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I cannot do any work at all | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 5 (35.7) | 5 (55.6) | 10 (43.5) |
| Driving, n (%) | |||
| I can drive my car without any neck pain | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I can drive my car as long as I want with slight pain in my neck | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I can drive my car as long as I want with moderate pain in my neck | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| I cannot drive my car as long as I want because of moderate pain in my neck | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I can hardly drive at all because of severe pain in my neck | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I cannot drive my car at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 8 (57.1) | 5 (55.6) | 13 (56.5) |
| Sleeping, n (%) | |||
| I have no trouble sleeping | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| My sleep is slightly disturbed (< 1 hour sleepless) | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| My sleep is mildly disturbed (1–2 hours sleepless) | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| My sleep is moderately disturbed (2–3 hours sleepless) | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| My sleep is greatly disturbed (3–5 hours sleepless) | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| My sleep is completely disturbed (5–7 hours sleepless) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 5 (35.7) | 5 (55.6) | 10 (43.5) |
| Recreation, n (%) | |||
| I am able to engage in all my recreation activities with no neck pain at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I am able to engage in all my recreation activities, with some pain in my neck | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I am able to engage in most, but not all of my usual recreation activities because of pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I am able to engage in a few of my usual recreation activities because of pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can hardly do any recreation activities because of pain in my neck | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I cannot do any recreation activities at all | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 5 (35.7) | 5 (55.6) | 10 (43.5) |
| Week 39 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) |
| IQR | 9.5–10.0 | 0.0–10.0 | 9.0–10.0 |
| Missing (n) | 6 | 2 | 8 |
| n | 8 | 7 | 15 |
| Pain intensity, n (%) | |||
| I have no pain at the moment | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| The pain is very mild at the moment | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| The pain is moderate at the moment | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| The pain is fairly severe at the moment | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| The pain is very severe at the moment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| The pain is the worst imaginable at the moment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| Personal care, n (%) | |||
| I can look after myself normally without causing extra pain | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| I can look after myself normally, but it causes extra pain | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| It is painful to look after myself and I am slow and careful | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I need some help but can manage most of my personal care | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I need help every day in most aspects of self-care | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I do not get dressed, I wash with difficulty and stay in bed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| Lifting, n (%) | |||
| I can lift heavy weights without extra pain | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I can lift heavy weights but it gives extra pain | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Pain prevents me lifting heavy weights off the floor, but I can manage if they are conveniently placed, for example on a table | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Pain prevents me from lifting heavy weights, but I can manage light to medium weights if they are conveniently positioned | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I can only lift very light weights | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I cannot lift or carry anything | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| Reading, n (%) | |||
| I can read as much as I want to with no pain in my neck | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I can read as much as I want to with slight pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can read as much as I want to with moderate pain in my neck | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I cannot read as much as I want to because of moderate pain in my neck | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I can hardly read at all because of severe pain in my neck | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I cannot read at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| Headaches, n (%) | |||
| I have no headaches at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I have slight headaches, which come infrequently | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I have moderate headaches, which come infrequently | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I have moderate headaches, which come frequently | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| I have severe headaches, which come frequently | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I have headaches almost all the time | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| Concentration, n (%) | |||
| I can concentrate fully when I want to with no difficulty | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can concentrate fully when I want to with slight difficulty | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| I have a fair degree of difficulty in concentrating when I want to | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I have a lot of difficulty in concentrating when I want to | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I have a great deal of difficulty in concentrating when I want to | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I cannot concentrate at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| Work, n (%) | |||
| I can do as much work as I want to | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can only do my usual work, but no more | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I can do most of my usual work, but no more | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I cannot do my usual work | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can hardly do any work at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I cannot do any work at all | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| Driving, n (%) | |||
| I can drive my car without any neck pain | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I can drive my car as long as I want with slight pain in my neck | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I can drive my car as long as I want with moderate pain in my neck | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I cannot drive my car as long as I want because of moderate pain in my neck | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| I can hardly drive at all because of severe pain in my neck | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I cannot drive my car at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 8 (57.1) | 4 (44.4) | 12 (52.2) |
| Sleeping, n (%) | |||
| I have no trouble sleeping | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| My sleep is slightly disturbed (< 1 hour sleepless) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| My sleep is mildly disturbed (1–2 hours sleepless) | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| My sleep is moderately disturbed (2–3 hours sleepless) | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| My sleep is greatly disturbed (3–5 hours sleepless) | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| My sleep is completely disturbed (5–7 hours sleepless) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| Recreation, n (%) | |||
| I am able to engage in all my recreation activities with no neck pain at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I am able to engage in all my recreation activities, with some pain in my neck | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I am able to engage in most, but not all of my usual recreation activities because of pain in my neck | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| I am able to engage in a few of my usual recreation activities because of pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can hardly do any recreation activities because of pain in my neck | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I cannot do any recreation activities at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| Week 52 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) |
| IQR | 9.0–10.0 | 0.0–10.0 | 0.0–10.0 |
| Missing (n) | 1 | 3 | 4 |
| n | 13 | 6 | 19 |
| Pain intensity, n (%) | |||
| I have no pain at the moment | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| The pain is very mild at the moment | 5 (35.7) | 0 (0.0) | 5 (21.7) |
| The pain is moderate at the moment | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| The pain is fairly severe at the moment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| The pain is very severe at the moment | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| The pain is the worst imaginable at the moment | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 5 (55.6) | 9 (39.1) |
| Personal care, n (%) | |||
| I can look after myself normally without causing extra pain | 6 (42.9) | 1 (11.1) | 7 (30.4) |
| I can look after myself normally, but it causes extra pain | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| It is painful to look after myself and I am slow and careful | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I need some help but can manage most of my personal care | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I need help every day in most aspects of self-care | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I do not get dressed, I wash with difficulty and stay in bed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 5 (55.6) | 9 (39.1) |
| Lifting, n (%) | |||
| I can lift heavy weights without extra pain | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can lift heavy weights but it gives extra pain | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Pain prevents me lifting heavy weights off the floor, but I can manage if they are conveniently placed, for example on a table | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Pain prevents me from lifting heavy weights, but I can manage light to medium weights if they are conveniently positioned | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I can only lift very light weights | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| I cannot lift or carry anything | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 5 (55.6) | 9 (39.1) |
| Reading, n (%) | |||
| I can read as much as I want to with no pain in my neck | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I can read as much as I want to with slight pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can read as much as I want to with moderate pain in my neck | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| I cannot read as much as I want to because of moderate pain in my neck | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| I can hardly read at all because of severe pain in my neck | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I cannot read at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 5 (55.6) | 9 (39.1) |
| Headaches, n (%) | |||
| I have no headaches at all | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I have slight headaches, which come infrequently | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| I have moderate headaches, which come infrequently | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I have moderate headaches, which come frequently | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| I have severe headaches, which come frequently | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| I have headaches almost all the time | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 5 (55.6) | 9 (39.1) |
| Concentration, n (%) | |||
| I can concentrate fully when I want to with no difficulty | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| I can concentrate fully when I want to with slight difficulty | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I have a fair degree of difficulty in concentrating when I want to | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I have a lot of difficulty in concentrating when I want to | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I have a great deal of difficulty in concentrating when I want to | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I cannot concentrate at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 5 (55.6) | 9 (39.1) |
| Work, n (%) | |||
| I can do as much work as I want to | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can only do my usual work, but no more | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| I can do most of my usual work, but no more | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I cannot do my usual work | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I can hardly do any work at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I cannot do any work at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Missing | 4 (28.6) | 5 (55.6) | 9 (39.1) |
| Driving, n (%) | |||
| I can drive my car without any neck pain | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I can drive my car as long as I want with slight pain in my neck | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| I can drive my car as long as I want with moderate pain in my neck | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I cannot drive my car as long as I want because of moderate pain in my neck | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I can hardly drive at all because of severe pain in my neck | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I cannot drive my car at all | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 6 (42.9) | 5 (55.6) | 11 (47.8) |
| Sleeping, n (%) | |||
| I have no trouble sleeping | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| My sleep is slightly disturbed (< 1 hour sleepless) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| My sleep is mildly disturbed (1–2 hours sleepless) | 5 (35.7) | 2 (22.2) | 7 (30.4) |
| My sleep is moderately disturbed (2–3 hours sleepless) | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| My sleep is greatly disturbed (3–5 hours sleepless) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| My sleep is completely disturbed (5–7 hours sleepless) | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Missing | 4 (28.6) | 5 (55.6) | 9 (39.1) |
| Recreation, n (%) | |||
| I am able to engage in all my recreation activities with no neck pain at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I am able to engage in all my recreation activities, with some pain in my neck | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| I am able to engage in most, but not all of my usual recreation activities because of pain in my neck | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I am able to engage in a few of my usual recreation activities because of pain in my neck | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| I can hardly do any recreation activities because of pain in my neck | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| I cannot do any recreation activities at all | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 5 (55.6) | 9 (39.1) |
Appendix 5 Additional results tables for secondary outcomes
| NRS and time point | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| NRS-NP | |||
| Day 0 | |||
| Mean (SD) | 5.9 (2.5) | 5.4 (1.7) | 5.7 (2.2) |
| Median (range) | 5.5 (2.0–10.0) | 5.0 (3.0–8.0) | 5.0 (2.0–10.0) |
| IQR | 4.0–8.0 | 4.0–7.0 | 4.0–7.0 |
| n | 12 | 9 | 21 |
| Missing (n) | 2 | 0 | 2 |
| Day 1 | |||
| Mean (SD) | 7.8 (2.5) | 6.5 (3.0) | 7.3 (2.7) |
| Median (range) | 8.5 (3.0–10.0) | 7.0 (2.0–10.0) | 8.0 (2.0–10.0) |
| IQR | 6.0–10.0 | 4.0–9.0 | 5.5–10.0 |
| n | 12 | 8 | 20 |
| Missing (n) | 2 | 1 | 3 |
| Week 6 | |||
| Mean (SD) | 4.8 (2.5) | 4.1 (2.2) | 4.5 (2.3) |
| Median (range) | 4.5 (0.0–9.0) | 4.0 (0.0–7.0) | 4.0 (0.0–9.0) |
| IQR | 4.0–7.0 | 3.0–6.0 | 3.0–6.0 |
| n | 10 | 8 | 18 |
| Missing (n) | 4 | 1 | 5 |
| Week 12 | |||
| Mean (SD) | 4.2 (3.2) | 3.0 (2.1) | 3.8 (2.8) |
| Median (range) | 3.0 (0.0–9.0) | 2.5 (1.0–6.0) | 3.0 (0.0–9.0) |
| IQR | 2.0–7.0 | 1.0–5.0 | 2.0–5.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| Week 26 | |||
| Mean (SD) | 4.7 (2.7) | 3.0 (2.0) | 4.1 (2.5) |
| Median (range) | 4.5 (1.0–10.0) | 3.0 (0.0–6.0) | 4.0 (0.0–10.0) |
| IQR | 2.0–6.0 | 2.0–4.0 | 2.0–6.0 |
| n | 10 | 6 | 16 |
| Missing (n) | 4 | 3 | 7 |
| Week 39 | |||
| Mean (SD) | 2.9 (2.2) | 4.2 (2.9) | 3.4 (2.5) |
| Median (range) | 3.0 (0.0–6.0) | 4.5 (0.0–7.0) | 3.0 (0.0–7.0) |
| IQR | 1.0–4.5 | 2.0–7.0 | 2.0–6.0 |
| n | 8 | 6 | 14 |
| Missing (n) | 6 | 3 | 9 |
| Week 52 | |||
| Mean (SD) | 4.2 (2.6) | 4.5 (2.7) | 4.3 (2.6) |
| Median (range) | 4.0 (1.0–10.0) | 5.0 (0.0–7.0) | 4.0 (0.0–10.0) |
| IQR | 2.0–5.0 | 3.0–7.0 | 2.0–7.0 |
| n | 13 | 6 | 19 |
| Missing (n) | 1 | 3 | 4 |
| NRS-AP | |||
| Day 0 | |||
| Mean (SD) | 6.4 (2.6) | 5.9 (1.7) | 6.2 (2.2) |
| Median (range) | 7.0 (1.0–10.0) | 6.0 (3.0–8.0) | 6.5 (1.0–10.0) |
| IQR | 4.0–8.0 | 5.0–7.0 | 4.5–8.0 |
| n | 11 | 9 | 20 |
| Missing (n) | 3 | 0 | 3 |
| Day 1 | |||
| Mean (SD) | 4.6 (3.0) | 3.4 (2.9) | 4.1 (2.9) |
| Median (range) | 3.0 (1.0–9.0) | 4.0 (0.0–8.0) | 3.0 (0.0–9.0) |
| IQR | 2.0–8.0 | 0.5–5.0 | 2.0–6.0 |
| n | 13 | 8 | 21 |
| Missing (n) | 1 | 1 | 2 |
| Week 6 | |||
| Mean (SD) | 4.0 (2.9) | 4.0 (2.0) | 4.0 (2.4) |
| Median (range) | 5.0 (0.0–8.0) | 4.0 (1.0–7.0) | 4.0 (0.0–8.0) |
| IQR | 1.0–6.0 | 2.5–5.5 | 2.0–6.0 |
| n | 9 | 8 | 17 |
| Missing (n) | 5 | 1 | 6 |
| Week 12 | |||
| Mean (SD) | 4.1 (3.3) | 2.5 (2.4) | 3.5 (3.0) |
| Median (range) | 3.0 (0.0–9.0) | 2.5 (0.0–5.0) | 3.0 (0.0–9.0) |
| IQR | 2.0–8.0 | 0.0–5.0 | 1.0–5.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| Week 26 | |||
| Mean (SD) | 5.2 (3.1) | 3.8 (1.9) | 4.7 (2.7) |
| Median (range) | 5.0 (1.0–10.0) | 4.5 (1.0–6.0) | 4.5 (1.0–10.0) |
| IQR | 2.0–7.0 | 2.0–5.0 | 2.0–6.5 |
| n | 10 | 6 | 16 |
| Missing (n) | 4 | 3 | 7 |
| Week 39 | |||
| Mean (SD) | 4.6 (1.1) | 4.3 (2.8) | 4.5 (1.9) |
| Median (range) | 4.5 (3.0–6.0) | 4.5 (0.0–7.0) | 4.5 (0.0–7.0) |
| IQR | 4.0–5.5 | 3.0–7.0 | 3.0–6.0 |
| n | 8 | 6 | 14 |
| Missing (n) | 6 | 3 | 9 |
| Week 52 | |||
| Mean (SD) | 4.5 (3.0) | 4.5 (2.8) | 4.5 (2.8) |
| Median (range) | 5.0 (0.0–10.0) | 5.0 (0.0–8.0) | 5.0 (0.0–10.0) |
| IQR | 2.0–7.0 | 3.0–6.0 | 2.0–7.0 |
| n | 13 | 6 | 19 |
| Missing (n) | 1 | 3 | 4 |
FIGURE 15.
The NRS pain reduction at each time point, by treatment group.
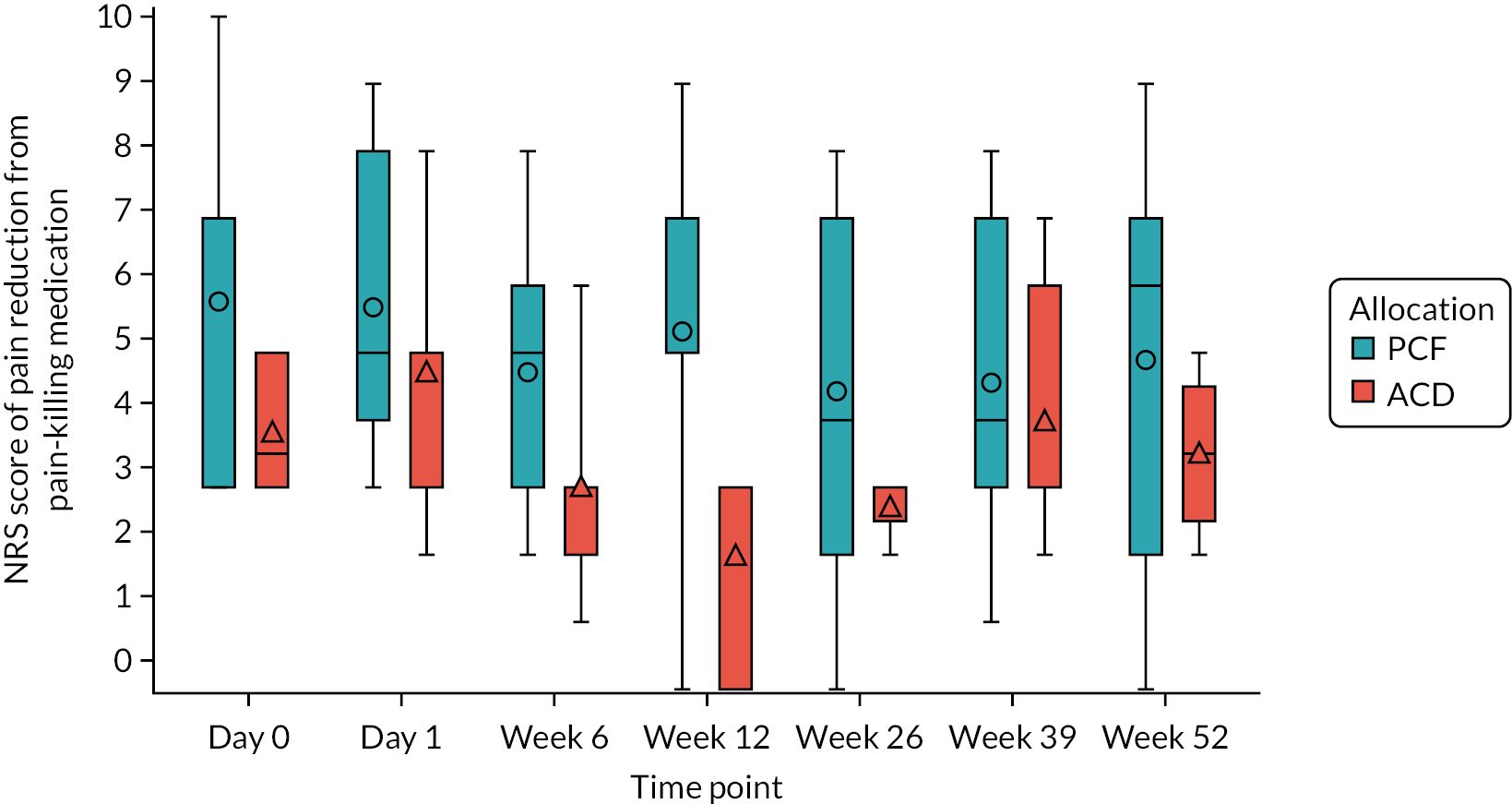
| Time point and responses | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Day 0 | |||
| Taken any pain-killing medications in the previous 24 hours?, n (%) | |||
| Yes | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| No | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Reduction in pain | |||
| Mean (SD) | 5.8 (2.6) | 3.8 (1.0) | 5.0 (2.3) |
| Median (range) | 7.0 (3.0–10.0) | 3.5 (3.0–5.0) | 4.0 (3.0–10.0) |
| IQR | 3.0–7.0 | 3.0–5.0 | 3.0–7.0 |
| n | 9 | 6 | 15 |
| Missing (n) | 1 | 0 | 1 |
| Day 1 | |||
| Taken any pain-killing medications in the previous 24 hours?, n (%) | |||
| Yes | 13 (92.9) | 9 (100.0) | 22 (95.7) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Reduction in pain | |||
| Mean (SD) | 5.7 (2.1) | 4.7 (1.9) | 5.4 (2.0) |
| Median (range) | 5.0 (3.0–9.0) | 5.0 (2.0–8.0) | 5.0 (2.0–9.0) |
| IQR | 4.0–8.0 | 3.0–5.0 | 4.0–7.5 |
| n | 13 | 7 | 20 |
| Missing (n) | 0 | 2 | 2 |
| Week 6 | |||
| Taken any pain-killing medications in the previous 24 hours?, n (%) | |||
| Yes | 11 (78.6) | 6 (66.7) | 17 (73.9) |
| No | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Reduction in pain | |||
| Mean (SD) | 4.7 (1.8) | 3.0 (1.7) | 4.1 (1.9) |
| Median (range) | 5.0 (2.0–8.0) | 3.0 (1.0–6.0) | 4.0 (1.0–8.0) |
| IQR | 3.0–6.0 | 2.0–3.0 | 3.0–5.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 0 | 0 | 0 |
| Week 12 Taken any pain-killing medications in the previous 24 hours?, n (%) |
|||
| Yes | 10 (71.4) | 3 (33.3) | 13 (56.5) |
| No | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Reduction in pain | |||
| Mean (SD) | 5.3 (2.7) | 2.0 (1.7) | 4.5 (2.8) |
| Median (range) | 5.0 (0.0–9.0) | 3.0 (0.0–3.0) | 5.0 (0.0–9.0) |
| IQR | 5.0–7.0 | 0.0–3.0 | 3.0–6.5 |
| n | 9 | 3 | 12 |
| Missing (n) | 1 | 0 | 1 |
| Week 26 | |||
| Taken any pain-killing medications in the previous 24 hours?, n (%) | |||
| Yes | 9 (64.3) | 4 (44.4) | 13 (56.5) |
| No | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Reduction in pain | |||
| Mean (SD) | 4.4 (2.7) | 2.8 (0.5) | 3.9 (2.4) |
| Median (range) | 4.0 (0.0–8.0) | 3.0 (2.0–3.0) | 3.0 (0.0–8.0) |
| IQR | 2.0–7.0 | 2.5–3.0 | 2.0–6.0 |
| n | 9 | 4 | 13 |
| Missing (n) | 0 | 0 | 0 |
| Week 39 | |||
| Taken any pain-killing medications in the previous 24 hours?, n (%) | |||
| Yes | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| No | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Reduction in pain | |||
| Mean (SD) | 4.6 (2.5) | 4.0 (2.0) | 4.3 (2.2) |
| Median (range) | 4.0 (1.0–8.0) | 3.0 (2.0–7.0) | 3.0 (1.0–8.0) |
| IQR | 3.0–7.0 | 3.0–6.0 | 3.0–6.0 |
| n | 7 | 6 | 13 |
| Missing (n) | 0 | 0 | 0 |
| Week 52 | |||
| Taken any pain-killing medications in the previous 24 hours?, n (%) | |||
| Yes | 11 (78.6) | 4 (44.4) | 15 (65.2) |
| No | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Reduction in pain | |||
| Mean (SD) | 4.9 (2.7) | 3.5 (1.3) | 4.5 (2.4) |
| Median (range) | 6.0 (0.0–9.0) | 3.5 (2.0–5.0) | 5.0 (0.0–9.0) |
| IQR | 2.0–7.0 | 2.5–4.5 | 2.0–6.0 |
| n | 11 | 4 | 15 |
| Missing (n) | 0 | 0 | 0 |
| Time point and PainDETECT scores | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Day 0 | |||
| PainDETECT total score | |||
| Mean (SD) | 18.6 (8.1) | 18.5 (6.8) | 18.6 (7.5) |
| Median (range) | 20.0 (6.0–33.0) | 17.5 (11.0–30.0) | 18.0 (6.0–33.0) |
| IQR | 13.0–23.0 | 13.0–22.0 | 13.0–23.0 |
| n | 11 | 6 | 17 |
| Missing (n)a | 3 | 3 | 6 |
| Wholly missing questionnaire, n (%) | 1 (50.0) | 0 (0.0) | 1 (20.0) |
| Partially missing questionnaire, n (%) | 1 (50.0) | 3 (100.0) | 4 (80.0) |
| 1–6 of the total score questions missing | 0 (0.0) | 1 (33.3) | 1 (25.0) |
| Pain radiate missing | 1 (100.0) | 2 (66.7) | 3 (75.0) |
| Potential score categories, n (%) | |||
| Negative (0–12) | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Unclear (13–18) | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Positive (19–38) | 6 (42.9) | 4 (44.4) | 10 (43.5) |
| Ambiguous (between unclear and positive) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Week 6 | |||
| PainDETECT total score | |||
| Mean (SD) | 18.1 (9.6) | 14.6 (7.1) | 16.5 (8.4) |
| Median (range) | 17.0 (6.0–30.0) | 14.0 (4.0–24.0) | 14.0 (4.0–30.0) |
| IQR | 10.0–27.5 | 10.0–23.0 | 10.0–24.0 |
| n | 8 | 7 | 15 |
| Missing (n)a | 6 | 2 | 8 |
| Partially missing questionnaire, n (%) | 3 (100.0) | 1 (100.0) | 4 (100.0) |
| 1–6 of the total score questions missing | 1 (33.3) | 0 (0.0) | 1 (25.0) |
| Pain radiate missing | 2 (66.7) | 0 (0.0) | 2 (50.0) |
| Course of pain missing | 0 (0.0) | 1 (100.0) | 1 (25.0) |
| Potential score categories, n (%) | |||
| Negative (0–12) | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Unclear (13–18) | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Positive (19–38) | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| Ambiguous (between unclear and positive) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Week 12 | |||
| PainDETECT total score | |||
| Mean (SD) | 19.3 (9.9) | 10.8 (3.0) | 16.2 (9.0) |
| Median (range) | 21.0 (6.0–34.0) | 10.0 (8.0–15.0) | 15.0 (6.0–34.0) |
| IQR | 9.0–28.0 | 9.0–12.5 | 9.0–21.0 |
| n | 7 | 4 | 11 |
| Missing (n)a | 7 | 5 | 12 |
| Wholly missing questionnaire, n (%) | 1 (20.0) | 0 (0.0) | 1 (14.3) |
| Partially missing questionnaire, n (%) | 4 (80.0) | 2 (100.0) | 6 (85.7) |
| 1–6 of the total score questions missing | 1 (25.0) | 0 (0.0) | 1 (16.7) |
| Pain radiate missing | 1 (25.0) | 1 (50.0) | 2 (33.3) |
| Course of pain missing | 1 (25.0) | 1 (50.0) | 2 (33.3) |
| Both score modifications missing | 1 (25.0) | 0 (0.0) | 1 (16.7) |
| Potential score categories, n (%) | |||
| Negative (0–12) | 3 (21.4) | 4 (44.4) | 7 (30.4) |
| Unclear (13–18) | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Positive (19–38) | 5 (35.7) | 0 (0.0) | 5 (21.7) |
| Ambiguous (between negative and unclear) | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Week 26 | |||
| PainDETECT total score | |||
| Mean (SD) | 19.1 (11.3) | 13.7 (6.4) | 17.5 (10.1) |
| Median (range) | 21.0 (3.0–34.0) | 11.0 (9.0–21.0) | 17.0 (3.0–34.0) |
| IQR | 9.0–30.0 | 9.0–21.0 | 9.0–24.0 |
| n | 7 | 3 | 10 |
| Missing (n)a | 7 | 6 | 13 |
| Partially missing questionnaire, n (%) | 4 (100.0) | 3 (100.0) | 7 (100.0) |
| 1–6 of the total score questions missing | 1 (25.0) | 1 (33.3) | 2 (28.6) |
| Pain radiate missing | 3 (75.0) | 1 (33.3) | 4 (57.1) |
| Course of pain missing | 0 (0.0) | 1 (33.3) | 1 (14.3) |
| Potential score categories, n (%) | |||
| Negative (0–12) | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Unclear (13–18) | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Positive (19–38) | 5 (35.7) | 1 (11.1) | 6 (26.1) |
| Ambiguous (between negative and unclear) | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Ambiguous (between unclear and positive) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Week 39 | |||
| PainDETECT total score | |||
| Mean (SD) | 14.8 (4.6) | 11.0 (5.4) | 12.9 (5.0) |
| Median (range) | 14.0 (10.0–21.0) | 10.5 (5.0–18.0) | 12.5 (5.0–21.0) |
| IQR | 12.0–17.5 | 7.5–14.5 | 10.0–16.0 |
| n | 4 | 4 | 8 |
| Missing (n)a | 10 | 5 | 15 |
| Wholly missing questionnaire, n (%) | 1 (25.0) | 1 (33.3) | 2 (28.6) |
| Partially missing questionnaire, n (%) | 3 (75.0) | 2 (66.7) | 5 (71.4) |
| Pain radiate missing | 2 (66.7) | 1 (50.0) | 3 (60.0) |
| Course of pain missing | 1 (33.3) | 0 (0.0) | 1 (20.0) |
| Both score modifications missing | 0 (0.0) | 1 (50.0) | 1 (20.0) |
| Potential score categories, n (%) | |||
| Negative (0–12) | 1 (7.1) | 4 (44.4) | 5 (21.7) |
| Unclear (13–18) | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Positive (19–38) | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Ambiguous (between negative and unclear) | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Week 52 | |||
| PainDETECT total score | |||
| Mean (SD) | 19.7 (9.6) | 12.0b | 18.8 (9.3) |
| Median (range) | 18.0 (8.0–38.0) | 12.0 (12.0–12.0) | 16.5 (8.0–38.0) |
| IQR | 14.0–25.0 | 12.0–12.0 | 13.0–22.5 |
| n | 7 | 1 | 8 |
| Missing (n)a | 7 | 8 | 15 |
| Partially missing questionnaire, n (%) | 6 (100.0) | 5 (100.0) | 11 (100.0) |
| Pain radiate missing | 4 (66.7) | 3 (60.0) | 7 (63.6) |
| Course of pain missing | 1 (16.7) | 1 (20.0) | 2 (18.2) |
| Both score modifications missing | 1 (16.7) | 1 (20.0) | 2 (18.2) |
| Potential score categories, n (%) | |||
| Negative (0–12) | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Unclear (13–18) | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Positive (19–38) | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| Ambiguous (between unclear and positive) | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Time point and PainDETECT items | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Day 0 | |||
| Number of questions answered | |||
| Median (range) | 9.0 (0.0–9.0) | 9.0 (7.0–9.0) | 9.0 (0.0–9.0) |
| IQR | 9.0–9.0 | 8.0–9.0 | 9.0–9.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Suffer from a burning sensation in the marked area, n (%) | |||
| Not at all | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| Hardly noticed | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Slightly | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Moderately | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Strongly | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Very strongly | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Have tingling/prickling sensation in area of pain, n (%) | |||
| Not at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hardly noticed | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Slightly | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Moderately | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Strongly | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Very strongly | 5 (35.7) | 1 (11.1) | 6 (26.1) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Light touching in this area is painful, n (%) | |||
| Not at all | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Hardly noticed | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Slightly | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Moderately | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| Strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Have sudden pain attacks in the area of your pain, n (%) | |||
| Not at all | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Hardly noticed | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Slightly | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| Moderately | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Strongly | 5 (35.7) | 1 (11.1) | 6 (26.1) |
| Very strongly | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Cold or heat in this area is occasionally painful, n (%) | |||
| Not at all | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Hardly noticed | 5 (35.7) | 2 (22.2) | 7 (30.4) |
| Slightly | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Moderately | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Strongly | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Very strongly | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Suffer from sensation of numbness in area marked, n (%) | |||
| Not at all | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hardly noticed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Slightly | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Moderately | 3 (21.4) | 5 (55.6) | 8 (34.8) |
| Strongly | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Very strongly | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Slight pressure in this area triggers pain, n (%) | |||
| Not at all | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Hardly noticed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Slightly | 1 (7.1) | 4 (44.4) | 5 (21.7) |
| Moderately | 5 (35.7) | 4 (44.4) | 9 (39.1) |
| Strongly | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Description of pain course, n (%) | |||
| Persistent pain with slight fluctuations | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Persistent pain with pain attacks | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| Pain attacks without pain between them | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Pain attacks with pain between them | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Pain radiating to other regions of your body, n (%) | |||
| Yes | 5 (35.7) | 4 (44.4) | 9 (39.1) |
| No | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Week 6 | |||
| Number of questions answered | |||
| Median (range) | 9.0 (6.0–9.0) | 9.0 (8.0–9.0) | 9.0 (6.0–9.0) |
| IQR | 8.0–9.0 | 9.0–9.0 | 9.0–9.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| Suffer from a burning sensation in the marked area, n (%) | |||
| Not at all | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Hardly noticed | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Slightly | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Moderately | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Strongly | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Very strongly | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Have tingling/prickling sensation in area of pain, n (%) | |||
| Not at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Hardly noticed | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Slightly | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Moderately | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Strongly | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Very strongly | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Light touching in this area is painful, n (%) | |||
| Not at all | 3 (21.4) | 4 (44.4) | 7 (30.4) |
| Hardly noticed | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Slightly | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Moderately | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| Strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Have sudden pain attacks in the area of your pain, n (%) | |||
| Not at all | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Hardly noticed | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Slightly | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Moderately | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Strongly | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Very strongly | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Missing | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Cold or heat in this area is occasionally painful, n (%) | |||
| Not at all | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Hardly noticed | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Slightly | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Moderately | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Very strongly | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Suffer from sensation of numbness in area marked, n (%) | |||
| Not at all | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| Hardly noticed | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Slightly | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Moderately | 6 (42.9) | 0 (0.0) | 6 (26.1) |
| Strongly | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Very strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Slight pressure in this area triggers pain, n (%) | |||
| Not at all | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Hardly noticed | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| Slightly | 1 (7.1) | 4 (44.4) | 5 (21.7) |
| Moderately | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Description of pain course, n (%) | |||
| Persistent pain with slight fluctuations | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Persistent pain with pain attacks | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Pain attacks without pain between them | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Pain attacks with pain between them | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Pain radiating to other regions of your body, n (%) | |||
| Yes | 5 (35.7) | 6 (66.7) | 11 (47.8) |
| No | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Missing | 6 (42.9) | 1 (11.1) | 7 (30.4) |
| Week 12 | |||
| Number of questions answered | |||
| Median (range) | 9.0 (0.0–9.0) | 9.0 (8.0–9.0) | 9.0 (0.0–9.0) |
| IQR | 7.5–9.0 | 8.0–9.0 | 8.0–9.0 |
| n | 12 | 6 | 18 |
| Missing (n) | 2 | 3 | 5 |
| Suffer from a burning sensation in the marked area, n (%) | |||
| Not at all | 3 (21.4) | 4 (44.4) | 7 (30.4) |
| Hardly noticed | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Slightly | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Moderately | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Strongly | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Very strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Have tingling/prickling sensation in area of pain, n (%) | |||
| Not at all | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Hardly noticed | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Slightly | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| Moderately | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Strongly | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| Very strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Light touching in this area is painful, n (%) | |||
| Not at all | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Hardly noticed | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Slightly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderately | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Have sudden pain attacks in the area of your pain, n (%) | |||
| Not at all | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Hardly noticed | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Slightly | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Moderately | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| Strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Cold or heat in this area is occasionally painful, n (%) | |||
| Not at all | 5 (35.7) | 3 (33.3) | 8 (34.8) |
| Hardly noticed | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Slightly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderately | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Very strongly | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Suffer from sensation of numbness in area marked, n (%) | |||
| Not at all | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Hardly noticed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Slightly | 1 (7.1) | 4 (44.4) | 5 (21.7) |
| Moderately | 5 (35.7) | 0 (0.0) | 5 (21.7) |
| Strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Very strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Slight pressure in this area triggers pain, n (%) | |||
| Not at all | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Hardly noticed | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Slightly | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Moderately | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Very strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Description of pain course, n (%) | |||
| Persistent pain with slight fluctuations | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| Persistent pain with pain attacks | 5 (35.7) | 0 (0.0) | 5 (21.7) |
| Pain attacks without pain between them | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Pain attacks with pain between them | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 5 (35.7) | 4 (44.4) | 9 (39.1) |
| Pain radiating to other regions of your body, n (%) | |||
| Yes | 7 (50.0) | 5 (55.6) | 12 (52.2) |
| No | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 5 (35.7) | 4 (44.4) | 9 (39.1) |
| Week 26 | |||
| Number of questions answered | |||
| Median (range) | 9.0 (8.0–9.0) | 8.5 (8.0–9.0) | 9.0 (8.0–9.0) |
| IQR | 8.0–9.0 | 8.0–9.0 | 8.0–9.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| Suffer from a burning sensation in the marked area, n (%) | |||
| Not at all | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Hardly noticed | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Slightly | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Moderately | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Very strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Have tingling/prickling sensation in area of pain, n (%) | |||
| Not at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Hardly noticed | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Slightly | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Moderately | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Very strongly | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Light touching in this area is painful, n (%) | |||
| Not at all | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Hardly noticed | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Slightly | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Moderately | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Have sudden pain attacks in the area of your pain, n (%) | |||
| Not at all | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Hardly noticed | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Slightly | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Moderately | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Strongly | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Very strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 4 (44.4) | 7 (30.4) |
| Cold or heat in this area is occasionally painful, n (%) | |||
| Not at all | 5 (35.7) | 3 (33.3) | 8 (34.8) |
| Hardly noticed | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Slightly | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Moderately | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Very strongly | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Suffer from sensation of numbness in area marked, n (%) | |||
| Not at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Hardly noticed | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Slightly | 1 (7.1) | 4 (44.4) | 5 (21.7) |
| Moderately | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| Strongly | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Very strongly | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Slight pressure in this area triggers pain, n (%) | |||
| Not at all | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Hardly noticed | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Slightly | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Moderately | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Strongly | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| Very strongly | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Description of pain course, n (%) | |||
| Persistent pain with slight fluctuations | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Persistent pain with pain attacks | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Pain attacks without pain between them | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Pain attacks with pain between them | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Missing | 3 (21.4) | 4 (44.4) | 7 (30.4) |
| Pain radiating to other regions of your body, n (%) | |||
| Yes | 3 (21.4) | 4 (44.4) | 7 (30.4) |
| No | 5 (35.7) | 1 (11.1) | 6 (26.1) |
| Missing | 6 (42.9) | 4 (44.4) | 10 (43.5) |
| Week 39 | |||
| Number of questions answered | |||
| Median (range) | 8.5 (0.0–9.0) | 9.0 (0.0–9.0) | 9.0 (0.0–9.0) |
| IQR | 8.0–9.0 | 7.0–9.0 | 8.0–9.0 |
| n | 8 | 7 | 15 |
| Missing (n) | 6 | 2 | 8 |
| Suffer from a burning sensation in the marked area, n (%) | |||
| Not at all | 1 (7.1) | 4 (44.4) | 5 (21.7) |
| Hardly noticed | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Slightly | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Moderately | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Strongly | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Have tingling/prickling sensation in area of pain, n (%) | |||
| Not at all | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Hardly noticed | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Slightly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderately | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Strongly | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Light touching in this area is painful, n (%) | |||
| Not at all | 2 (14.3) | 4 (44.4) | 6 (26.1) |
| Hardly noticed | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Slightly | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Moderately | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Have sudden pain attacks in the area of your pain, n (%) | |||
| Not at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Hardly noticed | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Slightly | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Moderately | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Strongly | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Very strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Cold or heat in this area is occasionally painful, n (%) | |||
| Not at all | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Hardly noticed | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Slightly | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Moderately | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Suffer from sensation of numbness in area marked, n (%) | |||
| Not at all | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Hardly noticed | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Slightly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Moderately | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Strongly | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Slight pressure in this area triggers pain, n (%) | |||
| Not at all | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Hardly noticed | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Slightly | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Moderately | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Very strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Description of pain course, n (%) | |||
| Persistent pain with slight fluctuations | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Persistent pain with pain attacks | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Pain attacks without pain between them | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Pain attacks with pain between them | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 8 (57.1) | 4 (44.4) | 12 (52.2) |
| Pain radiating to other regions of your body, n (%) | |||
| Yes | 5 (35.7) | 4 (44.4) | 9 (39.1) |
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| Week 52 | |||
| Number of questions answered | |||
| Median (range) | 9.0 (7.0–9.0) | 8.0 (7.0–9.0) | 8.0 (7.0–9.0) |
| IQR | 8.0–9.0 | 8.0–8.0 | 8.0–9.0 |
| n | 13 | 6 | 19 |
| Missing (n) | 1 | 3 | 4 |
| Suffer from a burning sensation in the marked area, n (%) | |||
| Not at all | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Hardly noticed | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Slightly | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Moderately | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Strongly | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| Very strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Have tingling/prickling sensation in area of pain, n (%) | |||
| Not at all | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Hardly noticed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Slightly | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Moderately | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Strongly | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Very strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Light touching in this area is painful, n (%) | |||
| Not at all | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Hardly noticed | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Slightly | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Moderately | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Strongly | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Very strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Have sudden pain attacks in the area of your pain, n (%) | |||
| Not at all | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Hardly noticed | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Slightly | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Moderately | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Strongly | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Very strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Cold or heat in this area is occasionally painful, n (%) | |||
| Not at all | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Hardly noticed | 5 (35.7) | 2 (22.2) | 7 (30.4) |
| Slightly | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Moderately | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Strongly | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Very strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Suffer from sensation of numbness in area marked, n (%) | |||
| Not at all | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Hardly noticed | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Slightly | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| Moderately | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Strongly | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| Very strongly | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Slight pressure in this area triggers pain, n (%) | |||
| Not at all | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Hardly noticed | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Slightly | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| Moderately | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Very strongly | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Description of pain course, n (%) | |||
| Persistent pain with slight fluctuations | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Persistent pain with pain attacks | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| Pain attacks without pain between them | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Pain attacks with pain between them | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 5 (55.6) | 8 (34.8) |
| Pain radiating to other regions of your body, n (%) | |||
| Yes | 7 (50.0) | 2 (22.2) | 9 (39.1) |
| No | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 6 (42.9) | 7 (77.8) | 13 (56.5) |
| Time point and EAT-10 total score | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Day 0 | |||
| Mean (SD) | 3.6 (6.9) | 2.0 (3.5) | 3.0 (5.8) |
| Median (range) | 0.0 (0.0–17.0) | 0.0 (0.0–10.0) | 0.0 (0.0–17.0) |
| IQR | 0.0–0.0 | 0.0–3.0 | 0.0–3.0 |
| n | 13 | 8 | 21 |
| Missing (n) | 1 | 1 | 2 |
| Partially missing questionnaire, n (%) | 0 (0.0) | 1 (100.0) | 1 (50.0) |
| Unreturned booklet, n (%) | 1 (100.0) | 0 (0.0) | 1 (50.0) |
| Day 1 | |||
| Mean (SD) | 2.3 (4.1) | 11.1 (8.0) | 5.8 (7.3) |
| Median (range) | 0.0 (0.0–10.0) | 13.5 (0.0–23.0) | 1.0 (0.0–23.0) |
| IQR | 0.0–4.0 | 3.5–16.0 | 0.0–11.5 |
| n | 12 | 8 | 20 |
| Missing (n) | 2 | 1 | 3 |
| Partially missing questionnaire, n (%) | 1 (50.0) | 1 (100.0) | 2 (66.7) |
| Unreturned booklet, n (%) | 1 (50.0) | 0 (0.0) | 1 (33.3) |
| Week 6 | |||
| Mean (SD) | 3.4 (7.3) | 3.7 (4.8) | 3.5 (6.3) |
| Median (range) | 0.0 (0.0–20.0) | 1.0 (0.0–12.0) | 0.0 (0.0–20.0) |
| IQR | 0.0–1.0 | 0.0–8.0 | 0.0–5.0 |
| n | 11 | 7 | 18 |
| Missing (n) | 3 | 2 | 5 |
| Partially missing questionnaire, n (%) | 0 (0.0) | 1 (50.0) | 1 (20.0) |
| Unreturned booklet, n (%) | 3 (100.0) | 1 (50.0) | 4 (80.0) |
| Week 12 | |||
| Mean (SD) | 5.1 (10.3) | 0.3 (0.8) | 3.4 (8.5) |
| Median (range) | 0.0 (0.0–29.0) | 0.0 (0.0–2.0) | 0.0 (0.0–29.0) |
| IQR | 0.0–5.0 | 0.0–0.0 | 0.0–0.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| Wholly missing questionnaire, n (%) | 1 (33.3) | 0 (0.0) | 1 (16.7) |
| Unreturned booklet, n (%) | 2 (66.7) | 3 (100.0) | 5 (83.3) |
| Week 26 | |||
| Mean (SD) | 8.0 (12.8) | 0.7 (0.8) | 5.3 (10.6) |
| Median (range) | 0.0 (0.0–38.0) | 0.5 (0.0–2.0) | 0.0 (0.0–38.0) |
| IQR | 0.0–13.0 | 0.0–1.0 | 0.0–5.0 |
| n | 10 | 6 | 16 |
| Missing (n) | 4 | 3 | 7 |
| Partially missing questionnaire, n (%) | 1 (25.0) | 0 (0.0) | 1 (14.3) |
| Unreturned booklet, n (%) | 3 (75.0) | 3 (100.0) | 6 (85.7) |
| Week 39 | |||
| Mean (SD) | 2.0 (4.0) | 2.8 (3.2) | 2.4 (3.5) |
| Median (range) | 0.0 (0.0–10.0) | 2.5 (0.0–7.0) | 0.0 (0.0–10.0) |
| IQR | 0.0–2.0 | 0.0–5.0 | 0.0–5.0 |
| n | 6 | 6 | 12 |
| Missing (n) | 8 | 3 | 11 |
| Wholly missing questionnaire, n (%) | 1 (12.5) | 1 (33.3) | 2 (18.2) |
| Partially missing questionnaire, n (%) | 1 (12.5) | 0 (0.0) | 1 (9.1) |
| Unreturned booklet, n (%) | 6 (75.0) | 2 (66.7) | 8 (72.7) |
| Week 52 | |||
| Mean (SD) | 5.4 (12.2) | 0.7 (1.2) | 3.7 (9.9) |
| Median (range) | 0.0 (0.0–40.0) | 0.0 (0.0–3.0) | 0.0 (0.0–40.0) |
| IQR | 0.0–6.0 | 0.0–1.0 | 0.0–1.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| Partially missing questionnaire, n (%) | 2 (66.7) | 0 (0.0) | 2 (33.3) |
| Unreturned booklet, n (%) | 1 (33.3) | 3 (100.0) | 4 (66.7) |
| Time point and EAT-10 items | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Day 0 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (10.0–10.0) | 10.0 (1.0–10.0) | 10.0 (1.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| My swallowing problem has caused me to lose weight, n (%) | |||
| 0 | 11 (78.6) | 9 (100.0) | 20 (87.0) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| My swallowing problem interferes with my ability to go out for meals, n (%) | |||
| 0 | 11 (78.6) | 8 (88.9) | 19 (82.6) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Swallowing liquids takes extra effort, n (%) | |||
| 0 | 10 (71.4) | 8 (88.9) | 18 (78.3) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Swallowing solids takes extra effort, n (%) | |||
| 0 | 10 (71.4) | 7 (77.8) | 17 (73.9) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Swallowing pills takes extra effort, n (%) | |||
| 0 | 11 (78.6) | 7 (77.8) | 18 (78.3) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Swallowing is painful, n (%) | |||
| 0 | 10 (71.4) | 8 (88.9) | 18 (78.3) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Pleasure of eating is being affected by swallowing, n (%) | |||
| 0 | 10 (71.4) | 7 (77.8) | 17 (73.9) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| When I swallow food sticks in my throat, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| I cough when I eat, n (%) | |||
| 0 | 12 (85.7) | 6 (66.7) | 18 (78.3) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Swallowing is stressful, n (%) | |||
| 0 | 11 (78.6) | 8 (88.9) | 19 (82.6) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Day 1 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (7.0–10.0) | 10.0 (9.0–10.0) | 10.0 (7.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| My swallowing problem has caused me to lose weight, n (%) | |||
| 0 | 12 (85.7) | 8 (88.9) | 20 (87.0) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| My swallowing problem interferes with my ability to go out for meals, n (%) | |||
| 0 | 10 (71.4) | 7 (77.8) | 17 (73.9) |
| 1 | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Swallowing liquids takes extra effort, n (%) | |||
| 0 | 10 (71.4) | 3 (33.3) | 13 (56.5) |
| 1 | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Swallowing solids takes extra effort, n (%) | |||
| 0 | 9 (64.3) | 2 (22.2) | 11 (47.8) |
| 1 | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| 2 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 3 | 0 (0.0) | 4 (44.4) | 4 (17.4) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Swallowing pills takes extra effort, n (%) | |||
| 0 | 10 (71.4) | 2 (22.2) | 12 (52.2) |
| 1 | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| 2 | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| 3 | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Swallowing is painful, n (%) | |||
| 0 | 9 (64.3) | 1 (11.1) | 10 (43.5) |
| 1 | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 1 (7.1) | 4 (44.4) | 5 (21.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Pleasure of eating is being affected by swallowing, n (%) | |||
| 0 | 9 (64.3) | 1 (11.1) | 10 (43.5) |
| 1 | 2 (14.3) | 4 (44.4) | 6 (26.1) |
| 2 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 3 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 4 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| When I swallow food sticks in my throat, n (%) | |||
| 0 | 11 (78.6) | 4 (44.4) | 15 (65.2) |
| 1 | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I cough when I eat, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Swallowing is stressful, n (%) | |||
| 0 | 10 (71.4) | 4 (44.4) | 14 (60.9) |
| 1 | 3 (21.4) | 4 (44.4) | 7 (30.4) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Week 6 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (10.0–10.0) | 10.0 (9.0–10.0) | 10.0 (9.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| My swallowing problem has caused me to lose weight, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| My swallowing problem interferes with my ability to go out for meals, n (%) | |||
| 0 | 10 (71.4) | 7 (77.8) | 17 (73.9) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Swallowing liquids takes extra effort, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Swallowing solids takes extra effort, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Swallowing pills takes extra effort, n (%) | |||
| 0 | 8 (57.1) | 6 (66.7) | 14 (60.9) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Swallowing is painful, n (%) | |||
| 0 | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Pleasure of eating is being affected by swallowing, n (%) | |||
| 0 | 9 (64.3) | 7 (77.8) | 16 (69.6) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| When I swallow food sticks in my throat, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I cough when I eat, n (%) | |||
| 0 | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Swallowing is stressful, n (%) | |||
| 0 | 9 (64.3) | 7 (77.8) | 16 (69.6) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Week 12 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (0.0–10.0) | 10.0 (10.0–10.0) | 10.0 (0.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 12 | 6 | 18 |
| Missing (n) | 2 | 3 | 5 |
| My swallowing problem has caused me to lose weight, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| My swallowing problem interferes with my ability to go out for meals, n (%) | |||
| 0 | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Swallowing liquids takes extra effort, n (%) | |||
| 0 | 8 (57.1) | 6 (66.7) | 14 (60.9) |
| 1 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Swallowing solids takes extra effort, n (%) | |||
| 0 | 8 (57.1) | 6 (66.7) | 14 (60.9) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Swallowing pills takes extra effort, n (%) | |||
| 0 | 8 (57.1) | 6 (66.7) | 14 (60.9) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Swallowing is painful, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Pleasure of eating is being affected by swallowing, n (%) | |||
| 0 | 8 (57.1) | 6 (66.7) | 14 (60.9) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| When I swallow food sticks in my throat, n (%) | |||
| 0 | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| I cough when I eat, n (%) | |||
| 0 | 8 (57.1) | 5 (55.6) | 13 (56.5) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Swallowing is stressful, n (%) | |||
| 0 | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Week 26 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (1.0–10.0) | 10.0 (10.0–10.0) | 10.0 (1.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| My swallowing problem has caused me to lose weight, n (%) | |||
| 0 | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| 1 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| My swallowing problem interferes with my ability to go out for meals, n (%) | |||
| 0 | 6 (42.9) | 6 (66.7) | 12 (52.2) |
| 1 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Swallowing liquids takes extra effort, n (%) | |||
| 0 | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Swallowing solids takes extra effort, n (%) | |||
| 0 | 6 (42.9) | 6 (66.7) | 12 (52.2) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Swallowing pills takes extra effort, n (%) | |||
| 0 | 6 (42.9) | 5 (55.6) | 11 (47.8) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Swallowing is painful, n (%) | |||
| 0 | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Pleasure of eating is being affected by swallowing, n (%) | |||
| 0 | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| When I swallow food sticks in my throat, n (%) | |||
| 0 | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| I cough when I eat, n (%) | |||
| 0 | 6 (42.9) | 4 (44.4) | 10 (43.5) |
| 1 | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Swallowing is stressful, n (%) | |||
| 0 | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Week 39 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) |
| IQR | 9.5–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 8 | 7 | 15 |
| Missing (n) | 6 | 2 | 8 |
| My swallowing problem has caused me to lose weight, n (%) | |||
| 0 | 7 (50.0) | 5 (55.6) | 12 (52.2) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| My swallowing problem interferes with my ability to go out for meals, n (%) | |||
| 0 | 5 (35.7) | 6 (66.7) | 11 (47.8) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Swallowing liquids takes extra effort, n (%) | |||
| 0 | 6 (42.9) | 6 (66.7) | 12 (52.2) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Swallowing solids takes extra effort, n (%) | |||
| 0 | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| 1 | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 8 (57.1) | 3 (33.3) | 11 (47.8) |
| Swallowing pills takes extra effort, n (%) | |||
| 0 | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| 1 | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Swallowing is painful, n (%) | |||
| 0 | 6 (42.9) | 5 (55.6) | 11 (47.8) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Pleasure of eating is being affected by swallowing, n (%) | |||
| 0 | 6 (42.9) | 6 (66.7) | 12 (52.2) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| When I swallow food sticks in my throat, n (%) | |||
| 0 | 5 (35.7) | 3 (33.3) | 8 (34.8) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 3 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| I cough when I eat, n (%) | |||
| 0 | 5 (35.7) | 3 (33.3) | 8 (34.8) |
| 1 | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Swallowing is stressful, n (%) | |||
| 0 | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Week 52 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (8.0–10.0) | 10.0 (10.0–10.0) | 10.0 (8.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 13 | 6 | 19 |
| Missing (n) | 1 | 3 | 4 |
| My swallowing problem has caused me to lose weight, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| My swallowing problem interferes with my ability to go out for meals, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Swallowing liquids takes extra effort, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Swallowing solids takes extra effort, n (%) | |||
| 0 | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Swallowing pills takes extra effort, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Swallowing is painful, n (%) | |||
| 0 | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Pleasure of eating is being affected by swallowing, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| When I swallow food sticks in my throat, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| I cough when I eat, n (%) | |||
| 0 | 9 (64.3) | 4 (44.4) | 13 (56.5) |
| 1 | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Swallowing is stressful, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| GETS and time points | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| GETS total score | |||
| Day 0 | |||
| Mean (SD) | 5.7 (9.2) | 3.7 (6.6) | 4.9 (8.1) |
| Median (range) | 0.0 (0.0–24.0) | 0.0 (0.0–20.0) | 0.0 (0.0–24.0) |
| IQR | 0.0–6.0 | 0.0–3.0 | 0.0–6.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Unreturned booklet, n (%) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| Day 1 | |||
| Mean (SD) | 5.3 (5.3) | 17.9 (9.1) | 10.5 (9.3) |
| Median (range) | 3.0 (0.0–15.0) | 15.0 (7.0–33.0) | 10.0 (0.0–33.0) |
| IQR | 1.0–10.0 | 10.0–25.0 | 3.0–15.0 |
| n | 10 | 7 | 17 |
| Missing (n) | 4 | 2 | 6 |
| Unreturned questionnaire, n (%) | 0 (0.0) | 1 (50.0) | 1 (16.7) |
| Wholly missing questionnaire, n (%) | 1 (25.0) | 0 (0.0) | 1 (16.7) |
| Partially missing questionnaire, n (%) | 2 (50.0) | 1 (50.0) | 3 (50.0) |
| Unreturned booklet, n (%) | 1 (25.0) | 0 (0.0) | 1 (16.7) |
| Week 6 | |||
| Mean (SD) | 6.5 (14.9) | 7.6 (7.2) | 6.9 (12.2) |
| Median (range) | 0.0 (0.0–46.0) | 7.0 (0.0–16.0) | 0.0 (0.0–46.0) |
| IQR | 0.0–1.0 | 0.0–16.0 | 0.0–12.0 |
| n | 11 | 7 | 18 |
| Missing (n) | 3 | 2 | 5 |
| Partially missing questionnaire, n (%) | 0 (0.0) | 1 (50.0) | 1 (20.0) |
| Unreturned booklet, n (%) | 3 (100.0) | 1 (50.0) | 4 (80.0) |
| Week 12 | |||
| Mean (SD) | 7.4 (22.0) | 5.5 (5.2) | 6.7 (17.4) |
| Median (range) | 0.0 (0.0–70.0) | 6.0 (0.0–12.0) | 0.0 (0.0–70.0) |
| IQR | 0.0–0.0 | 0.0–9.0 | 0.0–6.5 |
| n | 10 | 6 | 16 |
| Missing (n) | 4 | 3 | 7 |
| Partially missing questionnaire, n (%) | 2 (50.0) | 0 (0.0) | 2 (28.6) |
| Unreturned booklet, n (%) | 2 (50.0) | 3 (100.0) | 5 (71.4) |
| Week 26 | |||
| Mean (SD) | 11.5 (22.0) | 8.3 (10.5) | 10.3 (18.1) |
| Median (range) | 0.5 (0.0–70.0) | 6.0 (0.0–28.0) | 2.5 (0.0–70.0) |
| IQR | 0.0–19.0 | 0.0–10.0 | 0.0–14.5 |
| n | 10 | 6 | 16 |
| Missing (n) | 4 | 3 | 7 |
| Partially missing questionnaire, n (%) | 1 (25.0) | 0 (0.0) | 1 (14.3) |
| Unreturned booklet, n (%) | 3 (75.0) | 3 (100.0) | 6 (85.7) |
| Week 39 | |||
| Mean (SD) | 4.8 (7.7) | 7.4 (9.6) | 6.0 (8.4) |
| Median (range) | 0.0 (0.0–20.0) | 2.0 (0.0–25.0) | 0.0 (0.0–25.0) |
| IQR | 0.0–9.0 | 0.0–13.0 | 0.0–13.0 |
| n | 8 | 7 | 15 |
| Missing (n) | 6 | 2 | 8 |
| Unreturned booklet, n (%) | 6 (100.0) | 2 (100.0) | 8 (100.0) |
| Week 52 | |||
| Mean (SD) | 9.0 (19.6) | 4.7 (5.8) | 7.6 (16.4) |
| Median (range) | 0.0 (0.0–70.0) | 2.5 (0.0–16.0) | 2.0 (0.0–70.0) |
| IQR | 0.0–8.0 | 2.0–5.0 | 0.0–8.0 |
| n | 13 | 6 | 19 |
| Missing (n) | 1 | 3 | 4 |
| Unreturned booklet, n (%) | 1 (100.0) | 3 (100.0) | 4 (100.0) |
| Time spent thinking about your throat | |||
| Day 0 | |||
| Mean (SD) | 0.6 (1.2) | 0.2 (0.4) | 0.5 (1.0) |
| Median (range) | 0.0 (0.0–3.0) | 0.0 (0.0–1.0) | 0.0 (0.0–3.0) |
| IQR | 0.0–0.0 | 0.0–0.0 | 0.0–0.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Day 1 | |||
| Mean (SD) | 0.7 (0.9) | 2.1 (2.2) | 1.2 (1.6) |
| Median (range) | 0.0 (0.0–2.0) | 2.0 (0.0–6.0) | 1.0 (0.0–6.0) |
| IQR | 0.0–1.0 | 0.0–3.5 | 0.0–2.0 |
| n | 13 | 8 | 21 |
| Missing (n) | 1 | 1 | 2 |
| Week 6 | |||
| Mean (SD) | 1.0 (2.2) | 0.6 (1.4) | 0.8 (1.9) |
| Median (range) | 0.0 (0.0–6.0) | 0.0 (0.0–4.0) | 0.0 (0.0–6.0) |
| IQR | 0.0–0.0 | 0.0–0.5 | 0.0–0.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| Week 12 | |||
| Mean (SD) | 1.1 (2.2) | 0.5 (0.5) | 0.9 (1.8) |
| Median (range) | 0.0 (0.0–7.0) | 0.5 (0.0–1.0) | 0.0 (0.0–7.0) |
| IQR | 0.0–1.0 | 0.0–1.0 | 0.0–1.0 |
| n | 12 | 6 | 18 |
| Missing (n) | 2 | 3 | 5 |
| Week 26 | |||
| Mean (SD) | 1.4 (2.2) | 0.7 (0.8) | 1.1 (1.9) |
| Median (range) | 0.0 (0.0–7.0) | 0.5 (0.0–2.0) | 0.0 (0.0–7.0) |
| IQR | 0.0–2.0 | 0.0–1.0 | 0.0–1.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| Week 39 | |||
| Mean (SD) | 0.4 (0.8) | 0.7 (1.1) | 0.6 (0.9) |
| Median (range) | 0.0 (0.0–2.0) | 0.0 (0.0–3.0) | 0.0 (0.0–3.0) |
| IQR | 0.0–1.0 | 0.0–1.0 | 0.0–1.0 |
| n | 7 | 7 | 14 |
| Missing (n) | 7 | 2 | 9 |
| Week 52 | |||
| Mean (SD) | 1.0 (2.1) | 0.7 (0.8) | 0.9 (1.8) |
| Median (range) | 0.0 (0.0–7.0) | 0.5 (0.0–2.0) | 0.0 (0.0–7.0) |
| IQR | 0.0–1.0 | 0.0–1.0 | 0.0–1.0 |
| n | 13 | 6 | 19 |
| Missing (n) | 1 | 3 | 4 |
| Annoyance of your throat sensation | |||
| Day 0 | |||
| Mean (SD) | 1.2 (2.0) | 0.6 (1.3) | 0.9 (1.7) |
| Median (range) | 0.0 (0.0–5.0) | 0.0 (0.0–4.0) | 0.0 (0.0–5.0) |
| IQR | 0.0–2.0 | 0.0–0.0 | 0.0–1.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Day 1 | |||
| Mean (SD) | 0.8 (1.1) | 3.0 (1.5) | 1.7 (1.6) |
| Median (range) | 0.0 (0.0–3.0) | 3.5 (1.0–5.0) | 1.0 (0.0–5.0) |
| IQR | 0.0–2.0 | 1.5–4.0 | 0.0–3.0 |
| n | 13 | 8 | 21 |
| Missing (n) | 1 | 1 | 2 |
| Week 6 | |||
| Mean (SD) | 1.1 (2.5) | 1.0 (1.8) | 1.1 (2.1) |
| Median (range) | 0.0 (0.0–7.0) | 0.0 (0.0–5.0) | 0.0 (0.0–7.0) |
| IQR | 0.0–0.0 | 0.0–1.5 | 0.0–1.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| Week 12 | |||
| Mean (SD) | 1.2 (2.3) | 0.5 (0.5) | 0.9 (1.9) |
| Median (range) | 0.0 (0.0–7.0) | 0.5 (0.0–1.0) | 0.0 (0.0–7.0) |
| IQR | 0.0–1.0 | 0.0–1.0 | 0.0–1.0 |
| n | 12 | 6 | 18 |
| Missing (n) | 2 | 3 | 5 |
| Week 26 | |||
| Mean (SD) | 1.5 (2.4) | 1.0 (1.5) | 1.4 (2.1) |
| Median (range) | 0.0 (0.0–7.0) | 0.5 (0.0–4.0) | 0.0 (0.0–7.0) |
| IQR | 0.0–2.0 | 0.0–1.0 | 0.0–2.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| Week 39 | |||
| Mean (SD) | 0.5 (0.8) | 0.6 (1.1) | 0.5 (0.9) |
| Median (range) | 0.0 (0.0–2.0) | 0.0 (0.0–3.0) | 0.0 (0.0–3.0) |
| IQR | 0.0–1.0 | 0.0–1.0 | 0.0–1.0 |
| n | 8 | 7 | 15 |
| Missing (n) | 6 | 2 | 8 |
| Week 52 | |||
| Mean (SD) | 1.2 (2.2) | 0.7 (1.0) | 1.1 (1.9) |
| Median (range) | 0.0 (0.0–7.0) | 0.0 (0.0–2.0) | 0.0 (0.0–7.0) |
| IQR | 0.0–1.0 | 0.0–2.0 | 0.0–2.0 |
| n | 13 | 6 | 19 |
| Missing (n) | 1 | 3 | 4 |
| Time point and GETS items | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Day 0 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (10.0–10.0) | 10.0 (10.0–10.0) | 10.0 (10.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Feeling of something stuck in the throat, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Pain in the throat, n (%) | |||
| 0 | 10 (71.4) | 7 (77.8) | 17 (73.9) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Discomfort/irritation in the throat, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Difficulty in swallowing food, n (%) | |||
| 0 | 10 (71.4) | 8 (88.9) | 18 (78.3) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Throat closing off, n (%) | |||
| 0 | 11 (78.6) | 7 (77.8) | 18 (78.3) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Swelling in the throat, n (%) | |||
| 0 | 13 (92.9) | 9 (100.0) | 22 (95.7) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Catarrh down throat, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Cannot empty throat when swallowing, n (%) | |||
| 0 | 11 (78.6) | 7 (77.8) | 18 (78.3) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Want to swallow all the time, n (%) | |||
| 0 | 10 (71.4) | 9 (100.0) | 19 (82.6) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Food sticking when swallowing, n (%) | |||
| 0 | 10 (71.4) | 8 (88.9) | 18 (78.3) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Day 1 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) | 10.0 (0.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Feeling of something stuck in the throat, n (%) | |||
| 0 | 7 (50.0) | 1 (11.1) | 8 (34.8) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| 3 | 0 (0.0) | 4 (44.4) | 4 (17.4) |
| 4 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 5 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Pain in the throat, n (%) | |||
| 0 | 7 (50.0) | 0 (0.0) | 7 (30.4) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| 3 | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| 4 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 5 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Discomfort/irritation in the throat, n (%) | |||
| 0 | 4 (28.6) | 0 (0.0) | 4 (17.4) |
| 1 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 2 | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| 3 | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| 4 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 5 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Difficulty in swallowing food, n (%) | |||
| 0 | 5 (35.7) | 0 (0.0) | 5 (21.7) |
| 1 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 2 | 4 (28.6) | 2 (22.2) | 6 (26.1) |
| 3 | 0 (0.0) | 4 (44.4) | 4 (17.4) |
| 4 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Throat closing off, n (%) | |||
| 0 | 10 (71.4) | 4 (44.4) | 14 (60.9) |
| 1 | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Swelling in the throat, n (%) | |||
| 0 | 10 (71.4) | 3 (33.3) | 13 (56.5) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 4 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Catarrh down throat, n (%) | |||
| 0 | 5 (35.7) | 5 (55.6) | 10 (43.5) |
| 1 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 4 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| Cannot empty throat when swallowing, n (%) | |||
| 0 | 10 (71.4) | 4 (44.4) | 14 (60.9) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Want to swallow all the time, n (%) | |||
| 0 | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 4 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Food sticking when swallowing, n (%) | |||
| 0 | 7 (50.0) | 2 (22.2) | 9 (39.1) |
| 1 | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Week 6 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (10.0–10.0) | 10.0 (9.0–10.0) | 10.0 (9.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| Feeling of something stuck in the throat, n (%) | |||
| 0 | 9 (64.3) | 4 (44.4) | 13 (56.5) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Pain in the throat, n (%) | |||
| 0 | 9 (64.3) | 4 (44.4) | 13 (56.5) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Discomfort/irritation in the throat, n (%) | |||
| 0 | 9 (64.3) | 3 (33.3) | 12 (52.2) |
| 1 | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Difficulty in swallowing food, n (%) | |||
| 0 | 9 (64.3) | 3 (33.3) | 12 (52.2) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Throat closing off, n (%) | |||
| 0 | 9 (64.3) | 7 (77.8) | 16 (69.6) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Swelling in the throat, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Catarrh down throat, n (%) | |||
| 0 | 10 (71.4) | 5 (55.6) | 15 (65.2) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 4 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Cannot empty throat when swallowing, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Want to swallow all the time, n (%) | |||
| 0 | 8 (57.1) | 7 (77.8) | 15 (65.2) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Food sticking when swallowing, n (%) | |||
| 0 | 9 (64.3) | 4 (44.4) | 13 (56.5) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Week 12 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (8.0–10.0) | 10.0 (10.0–10.0) | 10.0 (8.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 12 | 6 | 18 |
| Missing (n) | 2 | 3 | 5 |
| Feeling of something stuck in the throat, n (%) | |||
| 0 | 10 (71.4) | 3 (33.3) | 13 (56.5) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Pain in the throat, n (%) | |||
| 0 | 10 (71.4) | 4 (44.4) | 14 (60.9) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Discomfort/irritation in the throat, n (%) | |||
| 0 | 9 (64.3) | 3 (33.3) | 12 (52.2) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Difficulty in swallowing food, n (%) | |||
| 0 | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Throat closing off, n (%) | |||
| 0 | 10 (71.4) | 4 (44.4) | 14 (60.9) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Swelling in the throat, n (%) | |||
| 0 | 11 (78.6) | 5 (55.6) | 16 (69.6) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Catarrh down throat, n (%) | |||
| 0 | 9 (64.3) | 2 (22.2) | 11 (47.8) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Cannot empty throat when swallowing, n (%) | |||
| 0 | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Want to swallow all the time, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Food sticking when swallowing, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Week 26 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (9.0–10.0) | 10.0 (10.0–10.0) | 10.0 (9.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| Feeling of something stuck in the throat, n (%) | |||
| 0 | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Pain in the throat, n (%) | |||
| 0 | 7 (50.0) | 5 (55.6) | 12 (52.2) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 4 (28.6) | 3 (33.3) | 7 (30.4) |
| Discomfort/irritation in the throat, n (%) | |||
| 0 | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Difficulty in swallowing food, n (%) | |||
| 0 | 6 (42.9) | 5 (55.6) | 11 (47.8) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Throat closing off, n (%) | |||
| 0 | 9 (64.3) | 4 (44.4) | 13 (56.5) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Swelling in the throat, n (%) | |||
| 0 | 10 (71.4) | 5 (55.6) | 15 (65.2) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Catarrh down throat, n (%) | |||
| 0 | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| 3 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 4 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Cannot empty throat when swallowing, n (%) | |||
| 0 | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| 1 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Want to swallow all the time, n (%) | |||
| 0 | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 5 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Food sticking when swallowing, n (%) | |||
| 0 | 7 (50.0) | 5 (55.6) | 12 (52.2) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Week 39 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (10.0–10.0) | 10.0 (10.0–10.0) | 10.0 (10.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 8 | 7 | 15 |
| Missing (n) | 6 | 2 | 8 |
| Feeling of something stuck in the throat, n (%) | |||
| 0 | 5 (35.7) | 4 (44.4) | 9 (39.1) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Pain in the throat, n (%) | |||
| 0 | 8 (57.1) | 5 (55.6) | 13 (56.5) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Discomfort/irritation in the throat, n (%) | |||
| 0 | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Difficulty in swallowing food, n (%) | |||
| 0 | 5 (35.7) | 4 (44.4) | 9 (39.1) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Throat closing off, n (%) | |||
| 0 | 6 (42.9) | 6 (66.7) | 12 (52.2) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Swelling in the throat, n (%) | |||
| 0 | 8 (57.1) | 6 (66.7) | 14 (60.9) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Catarrh down throat, n (%) | |||
| 0 | 6 (42.9) | 4 (44.4) | 10 (43.5) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Cannot empty throat when swallowing, n (%) | |||
| 0 | 6 (42.9) | 4 (44.4) | 10 (43.5) |
| 1 | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Want to swallow all the time, n (%) | |||
| 0 | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Food sticking when swallowing, n (%) | |||
| 0 | 6 (42.9) | 4 (44.4) | 10 (43.5) |
| 1 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 2 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 3 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Week 52 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (10.0–10.0) | 10.0 (10.0–10.0) | 10.0 (10.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 13 | 6 | 19 |
| Missing (n) | 1 | 3 | 4 |
| Feeling of something stuck in the throat, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 2 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Pain in the throat, n (%) | |||
| 0 | 10 (71.4) | 5 (55.6) | 15 (65.2) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Discomfort/irritation in the throat, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Difficulty in swallowing food, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Throat closing off, n (%) | |||
| 0 | 9 (64.3) | 4 (44.4) | 13 (56.5) |
| 1 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 2 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Swelling in the throat, n (%) | |||
| 0 | 11 (78.6) | 5 (55.6) | 16 (69.6) |
| 1 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Catarrh down throat, n (%) | |||
| 0 | 11 (78.6) | 1 (11.1) | 12 (52.2) |
| 1 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 2 | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| 3 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Cannot empty throat when swallowing, n (%) | |||
| 0 | 10 (71.4) | 5 (55.6) | 15 (65.2) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Want to swallow all the time, n (%) | |||
| 0 | 10 (71.4) | 5 (55.6) | 15 (65.2) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Food sticking when swallowing, n (%) | |||
| 0 | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Time point and VHI-10 total score | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Day 0 | |||
| Mean (SD) | 3.5 (7.1) | 1.4 (1.9) | 2.7 (5.6) |
| Median (range) | 0.0 (0.0–21.0) | 0.0 (0.0–5.0) | 0.0 (0.0–21.0) |
| IQR | 0.0–2.0 | 0.0–2.0 | 0.0–2.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Unreturned booklet, n (%) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| Day 1 | |||
| Mean (SD) | 3.5 (7.2) | 4.6 (5.4) | 4.0 (6.4) |
| Median (range) | 0.0 (0.0–24.0) | 2.0 (0.0–15.0) | 1.0 (0.0–24.0) |
| IQR | 0.0–2.0 | 0.5–8.5 | 0.0–5.0 |
| n | 13 | 8 | 21 |
| Missing (n) | 1 | 1 | 2 |
| Partially missing questionnaire, n (%) | 0 (0.0) | 1 (100.0) | 1 (50.0) |
| Unreturned booklet, n (%) | 1 (100.0) | 0 (0.0) | 1 (50.0) |
| Week 6 | |||
| Mean (SD) | 2.8 (5.9) | 1.4 (2.2) | 2.2 (4.6) |
| Median (range) | 0.0 (0.0–18.0) | 0.0 (0.0–6.0) | 0.0 (0.0–18.0) |
| IQR | 0.0–2.0 | 0.0–2.5 | 0.0–2.0 |
| n | 10 | 8 | 18 |
| Missing (n) | 4 | 1 | 5 |
| Partially missing questionnaire, n (%) | 1 (25.0) | 0 (0.0) | 1 (20.0) |
| Unreturned booklet, n (%) | 3 (75.0) | 1 (100.0) | 4 (80.0) |
| Week 12 | |||
| Mean (SD) | 2.8 (6.6) | 1.2 (2.9) | 2.2 (5.6) |
| Median (range) | 0.0 (0.0–22.0) | 0.0 (0.0–7.0) | 0.0 (0.0–22.0) |
| IQR | 0.0–3.0 | 0.0–0.0 | 0.0–0.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| Partially missing questionnaire, n (%) | 1 (33.3) | 0 (0.0) | 1 (16.7) |
| Unreturned booklet, n (%) | 2 (66.7) | 3 (100.0) | 5 (83.3) |
| Week 26 | |||
| Mean (SD) | 7.0 (11.0) | 2.2 (3.5) | 5.3 (9.2) |
| Median (range) | 0.0 (0.0–34.0) | 0.5 (0.0–9.0) | 0.0 (0.0–34.0) |
| IQR | 0.0–11.0 | 0.0–3.0 | 0.0–7.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| Unreturned booklet, n (%) | 3 (100.0) | 3 (100.0) | 6 (100.0) |
| Week 39 | |||
| Mean (SD) | 1.0 (1.3) | 3.3 (5.8) | 2.1 (4.2) |
| Median (range) | 0.0 (0.0–3.0) | 1.0 (0.0–16.0) | 0.5 (0.0–16.0) |
| IQR | 0.0–2.0 | 0.0–3.0 | 0.0–3.0 |
| n | 7 | 7 | 14 |
| Missing (n) | 7 | 2 | 9 |
| Partially missing questionnaire, n (%) | 1 (14.3) | 0 (0.0) | 1 (11.1) |
| Unreturned booklet, n (%) | 6 (85.7) | 2 (100.0) | 8 (88.9) |
| Week 52 | |||
| Mean (SD) | 9.2 (13.6) | 3.8 (6.6) | 7.4 (11.8) |
| Median (range) | 0.0 (0.0–40.0) | 0.0 (0.0–16.0) | 0.0 (0.0–40.0) |
| IQR | 0.0–16.5 | 0.0–7.0 | 0.0–13.0 |
| n | 12 | 6 | 18 |
| Missing (n) | 2 | 3 | 5 |
| Partially missing questionnaire, n (%) | 1 (50.0) | 0 (0.0) | 1 (20.0) |
| Unreturned booklet, n (%) | 1 (50.0) | 3 (100.0) | 4 (80.0) |
| Time point and VHI-10 items | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Day 0 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (10.0–10.0) | 10.0 (10.0–10.0) | 10.0 (10.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| My voice makes it difficult for people to hear me, n (%) | |||
| Never | 10 (71.4) | 8 (88.9) | 18 (78.3) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I run out of air when I talk, n (%) | |||
| Never | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| People have difficulty understanding me in a noisy room, n (%) | |||
| Never | 10 (71.4) | 8 (88.9) | 18 (78.3) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| The sound of my voice varies throughout the day, n (%) | |||
| Never | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| Almost never | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Sometimes | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| My family has difficulty hearing me when I call them throughout the house, n (%) | |||
| Never | 11 (78.6) | 9 (100.0) | 20 (87.0) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I use the phone less often than I would like, n (%) | |||
| Never | 11 (78.6) | 9 (100.0) | 20 (87.0) |
| Almost never | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I am tense when talking with others because of my voice, n (%) | |||
| Never | 11 (78.6) | 9 (100.0) | 20 (87.0) |
| Almost never | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I tend to avoid groups of people because of my voice, n (%) | |||
| Never | 12 (85.7) | 9 (100.0) | 21 (91.3) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| People seem irritated with my voice, n (%) | |||
| Never | 13 (92.9) | 8 (88.9) | 21 (91.3) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| People ask, ‘What’s wrong with your voice?’, n (%) | |||
| Never | 12 (85.7) | 9 (100.0) | 21 (91.3) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Day 1 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (10.0–10.0) | 10.0 (9.0–10.0) | 10.0 (9.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| My voice makes it difficult for people to hear me, n (%) | |||
| Never | 11 (78.6) | 7 (77.8) | 18 (78.3) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I run out of air when I talk, n (%) | |||
| Never | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| Almost never | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Sometimes | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| People have difficulty understanding me in a noisy room, n (%) | |||
| Never | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| Almost never | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Sometimes | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| The sound of my voice varies throughout the day, n (%) | |||
| Never | 10 (71.4) | 2 (22.2) | 12 (52.2) |
| Almost never | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| Sometimes | 0 (0.0) | 4 (44.4) | 4 (17.4) |
| Almost always | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| My family has difficulty hearing me when I call them throughout the house, n (%) | |||
| Never | 10 (71.4) | 6 (66.7) | 16 (69.6) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Almost always | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I use the phone less often than I would like, n (%) | |||
| Never | 11 (78.6) | 7 (77.8) | 18 (78.3) |
| Almost never | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I am tense when talking with others because of my voice, n (%) | |||
| Never | 12 (85.7) | 6 (66.7) | 18 (78.3) |
| Almost never | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| I tend to avoid groups of people because of my voice, n (%) | |||
| Never | 12 (85.7) | 6 (66.7) | 18 (78.3) |
| Almost never | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| People seem irritated with my voice, n (%) | |||
| Never | 13 (92.9) | 7 (77.8) | 20 (87.0) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| People ask, ‘What’s wrong with your voice?’, n (%) | |||
| Never | 12 (85.7) | 5 (55.6) | 17 (73.9) |
| Almost never | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Week 6 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (9.0–10.0) | 10.0 (10.0–10.0) | 10.0 (9.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| My voice makes it difficult for people to hear me, n (%) | |||
| Never | 10 (71.4) | 7 (77.8) | 17 (73.9) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I run out of air when I talk, n (%) | |||
| Never | 8 (57.1) | 6 (66.7) | 14 (60.9) |
| Almost never | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| People have difficulty understanding me in a noisy room, n (%) | |||
| Never | 8 (57.1) | 7 (77.8) | 15 (65.2) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| The sound of my voice varies throughout the day, n (%) | |||
| Never | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| My family has difficulty hearing me when I call them throughout the house, n (%) | |||
| Never | 9 (64.3) | 6 (66.7) | 15 (65.2) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I use the phone less often than I would like, n (%) | |||
| Never | 11 (78.6) | 8 (88.9) | 19 (82.6) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I am tense when talking with others because of my voice, n (%) | |||
| Never | 10 (71.4) | 8 (88.9) | 18 (78.3) |
| Almost never | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| I tend to avoid groups of people because of my voice, n (%) | |||
| Never | 9 (64.3) | 8 (88.9) | 17 (73.9) |
| Almost never | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (28.6) | 1 (11.1) | 5 (21.7) |
| People seem irritated with my voice, n (%) | |||
| Never | 10 (71.4) | 7 (77.8) | 17 (73.9) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| People ask, ‘What’s wrong with your voice?’, n (%) | |||
| Never | 10 (71.4) | 8 (88.9) | 18 (78.3) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Week 12 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (9.0–10.0) | 10.0 (10.0–10.0) | 10.0 (9.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 12 | 6 | 18 |
| Missing (n) | 2 | 3 | 5 |
| My voice makes it difficult for people to hear me, n (%) | |||
| Never | 11 (78.6) | 5 (55.6) | 16 (69.6) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| I run out of air when I talk, n (%) | |||
| Never | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| People have difficulty understanding me in a noisy room, n (%) | |||
| Never | 10 (71.4) | 5 (55.6) | 15 (65.2) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| The sound of my voice varies throughout the day, n (%) | |||
| Never | 8 (57.1) | 5 (55.6) | 13 (56.5) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| My family has difficulty hearing me when I call them throughout the house, n (%) | |||
| Never | 10 (71.4) | 5 (55.6) | 15 (65.2) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| I use the phone less often than I would like, n (%) | |||
| Never | 11 (78.6) | 6 (66.7) | 17 (73.9) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| I am tense when talking with others because of my voice, n (%) | |||
| Never | 11 (78.6) | 6 (66.7) | 17 (73.9) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| I tend to avoid groups of people because of my voice, n (%) | |||
| Never | 11 (78.6) | 6 (66.7) | 17 (73.9) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| People seem irritated with my voice | |||
| Never | 11 (78.6) | 5 (55.6) | 16 (69.6) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| People ask, ‘What’s wrong with your voice?’, n (%) | |||
| Never | 11 (78.6) | 5 (55.6) | 16 (69.6) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| Week 26 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (10.0–10.0) | 10.0 (10.0–10.0) | 10.0 (10.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 11 | 6 | 17 |
| Missing (n) | 3 | 3 | 6 |
| My voice makes it difficult for people to hear me, n (%) | |||
| Never | 6 (42.9) | 5 (55.6) | 11 (47.8) |
| Almost never | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| I run out of air when I talk, n (%) | |||
| Never | 6 (42.9) | 4 (44.4) | 10 (43.5) |
| Almost never | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Sometimes | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Almost always | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| People have difficulty understanding me in a noisy room, n (%) | |||
| Never | 6 (42.9) | 5 (55.6) | 11 (47.8) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| The sound of my voice varies throughout the day, n (%) | |||
| Never | 7 (50.0) | 3 (33.3) | 10 (43.5) |
| Almost never | 0 (0.0) | 3 (33.3) | 3 (13.0) |
| Sometimes | 3 (21.4) | 0 (0.0) | 3 (13.0) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| My family has difficulty hearing me when I call them throughout the house, n (%) | |||
| Never | 7 (50.0) | 4 (44.4) | 11 (47.8) |
| Almost never | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Sometimes | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| I use the phone less often than I would like, n (%) | |||
| Never | 8 (57.1) | 6 (66.7) | 14 (60.9) |
| Almost never | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| I am tense when talking with others because of my voice, n (%) | |||
| Never | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| I tend to avoid groups of people because of my voice, n (%) | |||
| Never | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| People seem irritated with my voice, n (%) | |||
| Never | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| People ask, ‘What’s wrong with your voice?’, n (%) | |||
| Never | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Week 39 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (9.0–10.0) | 10.0 (10.0–10.0) | 10.0 (9.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 8 | 7 | 15 |
| Missing (n) | 6 | 2 | 8 |
| My voice makes it difficult for people to hear me, n (%) | |||
| Never | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| I run out of air when I talk, n (%) | |||
| Never | 4 (28.6) | 4 (44.4) | 8 (34.8) |
| Almost never | 1 (7.1) | 2 (22.2) | 3 (13.0) |
| Sometimes | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| People have difficulty understanding me in a noisy room, n (%) | |||
| Never | 6 (42.9) | 5 (55.6) | 11 (47.8) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (50.0) | 2 (22.2) | 9 (39.1) |
| The sound of my voice varies throughout the day, n (%) | |||
| Never | 6 (42.9) | 5 (55.6) | 11 (47.8) |
| Almost never | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Sometimes | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| My family has difficulty hearing me when I call them throughout the house, n (%) | |||
| Never | 7 (50.0) | 5 (55.6) | 12 (52.2) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 0 (0.0) | 2 (22.2) | 2 (8.7) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| I use the phone less often than I would like, n (%) | |||
| Never | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| I am tense when talking with others because of my voice, n (%) | |||
| Never | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| I tend to avoid groups of people because of my voice, n (%) | |||
| Never | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| People seem irritated with my voice, n (%) | |||
| Never | 8 (57.1) | 6 (66.7) | 14 (60.9) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| People ask, ‘What’s wrong with your voice?’, n (%) | |||
| Never | 7 (50.0) | 6 (66.7) | 13 (56.5) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (42.9) | 2 (22.2) | 8 (34.8) |
| Week 52 | |||
| Number of questions answered | |||
| Median (range) | 10.0 (9.0–10.0) | 10.0 (10.0–10.0) | 10.0 (9.0–10.0) |
| IQR | 10.0–10.0 | 10.0–10.0 | 10.0–10.0 |
| n | 13 | 6 | 19 |
| Missing (n) | 1 | 3 | 4 |
| My voice makes it difficult for people to hear me, n (%) | |||
| Never | 9 (64.3) | 4 (44.4) | 13 (56.5) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| I run out of air when I talk, n (%) | |||
| Never | 8 (57.1) | 4 (44.4) | 12 (52.2) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Almost always | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| People have difficulty understanding me in a noisy room, n (%) | |||
| Never | 8 (57.1) | 4 (44.4) | 12 (52.2) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 3 (21.4) | 2 (22.2) | 5 (21.7) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| The sound of my voice varies throughout the day, n (%) | |||
| Never | 8 (57.1) | 5 (55.6) | 13 (56.5) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| My family has difficulty hearing me when I call them throughout the house, n (%) | |||
| Never | 8 (57.1) | 4 (44.4) | 12 (52.2) |
| Almost never | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Sometimes | 2 (14.3) | 2 (22.2) | 4 (17.4) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| I use the phone less often than I would like, n (%) | |||
| Never | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| Almost never | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Sometimes | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| I am tense when talking with others because of my voice, n (%) | |||
| Never | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| I tend to avoid groups of people because of my voice, n (%) | |||
| Never | 9 (64.3) | 5 (55.6) | 14 (60.9) |
| Almost never | 0 (0.0) | 1 (11.1) | 1 (4.3) |
| Sometimes | 2 (14.3) | 0 (0.0) | 2 (8.7) |
| Almost always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
| People seem irritated with my voice, n (%) | |||
| Never | 10 (71.4) | 5 (55.6) | 15 (65.2) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 1 (7.1) | 1 (11.1) | 2 (8.7) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 2 (14.3) | 3 (33.3) | 5 (21.7) |
| People ask, ‘What’s wrong with your voice?’, n (%) | |||
| Never | 10 (71.4) | 5 (55.6) | 15 (65.2) |
| Almost never | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sometimes | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| Almost always | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Always | 1 (7.1) | 0 (0.0) | 1 (4.3) |
| Missing | 1 (7.1) | 3 (33.3) | 4 (17.4) |
FIGURE 16.
Total motor score for the upper limb on the operated side at each time point, by treatment group (ASIA).

FIGURE 17.
Total light-touch sensory score for the upper limb on the operated side at each time point, by treatment group (ASIA).

FIGURE 18.
Total pinprick sensory score for the upper limb on the operated side at each time point, by treatment group (ASIA).
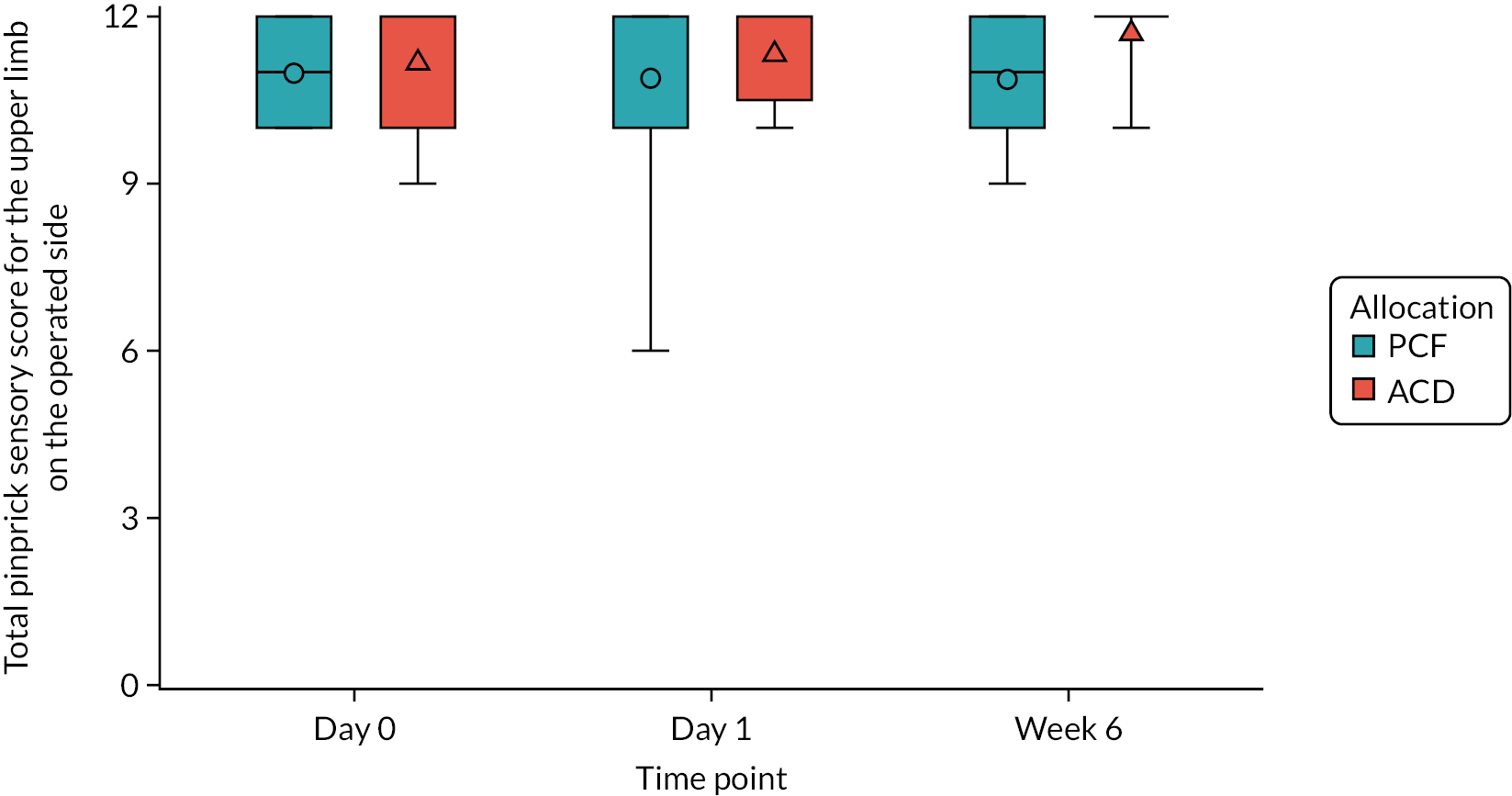
| ASIA assessment | Trial arm | Total (N = 23) | |
|---|---|---|---|
| PCF (N = 14) | ACD (N = 9) | ||
| Side of approach, n (%) | |||
| Left | 6 (42.9) | 0 (0.0) | 6 (26.1) |
| Right | 8 (57.1) | 9 (100.0) | 17 (73.9) |
| Total motor score for the upper limb on the operated side | |||
| Day 0 | |||
| Left operation side | |||
| Mean (SD) | 23.8 (2.2) | – | 23.8 (2.2) |
| Median (range) | 25.0 (20.0–25.0) | – | 25.0 (20.0–25.0) |
| IQR | 24.0–25.0 | – | 24.0–25.0 |
| n | 5 | 0 | 5 |
| Missing (n) | 1 | 0 | 1 |
| Right operation side | |||
| Mean (SD) | 24.8 (0.7) | 25.0 (0.0) | 24.9 (0.5) |
| Median (range) | 25.0 (23.0–25.0) | 25.0 (25.0–25.0) | 25.0 (23.0–25.0) |
| IQR | 25.0–25.0 | 25.0–25.0 | 25.0–25.0 |
| n | 8 | 9 | 17 |
| Missing (n) | 0 | 0 | 0 |
| Total | |||
| Mean (SD) | 24.4 (1.4) | 25.0 (0.0) | 24.6 (1.1) |
| Median (range) | 25.0 (20.0–25.0) | 25.0 (25.0–25.0) | 25.0 (20.0–25.0) |
| IQR | 25.0–25.0 | 25.0–25.0 | 25.0–25.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Day 1 | |||
| Left operation side | |||
| Mean (SD) | 24.3 (1.2) | – | 24.3 (1.2) |
| Median (range) | 25.0 (23.0–25.0) | – | 25.0 (23.0–25.0) |
| IQR | 23.0–25.0 | – | 23.0–25.0 |
| n | 3 | 0 | 3 |
| Missing (n) | 3 | 0 | 3 |
| Right operation side | |||
| Mean (SD) | 24.9 (0.4) | 25.0 (0.0) | 24.9 (0.3) |
| Median (range) | 25.0 (24.0–25.0) | 25.0 (25.0–25.0) | 25.0 (24.0–25.0) |
| IQR | 25.0–25.0 | 25.0–25.0 | 25.0–25.0 |
| n | 8 | 8 | 16 |
| Missing (n) | 0 | 1 | 1 |
| Total | |||
| Mean (SD) | 24.7 (0.6) | 25.0 (0.0) | 24.8 (0.5) |
| Median (range) | 25.0 (23.0–25.0) | 25.0 (25.0–25.0) | 25.0 (23.0–25.0) |
| IQR | 25.0–25.0 | 25.0–25.0 | 25.0–25.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| Week 6 | |||
| Left operation side | |||
| Mean (SD) | 25.0 (0.0) | – | 25.0 (0.0) |
| Median (range) | 25.0 (25.0–25.0) | – | 25.0 (25.0–25.0) |
| IQR | 25.0–25.0 | – | 25.0–25.0 |
| n | 3 | 0 | 3 |
| Missing (n) | 3 | 0 | 3 |
| Right operation side | |||
| Mean (SD) | 24.8 (0.4) | 25.0 (0.0) | 24.9 (0.3) |
| Median (range) | 25.0 (24.0–25.0) | 25.0 (25.0–25.0) | 25.0 (24.0–25.0) |
| IQR | 25.0–25.0 | 25.0–25.0 | 25.0–25.0 |
| n | 6 | 8 | 14 |
| Missing (n) | 2 | 1 | 3 |
| Total | |||
| Mean (SD) | 24.9 (0.3) | 25.0 (0.0) | 24.9 (0.2) |
| Median (range) | 25.0 (24.0–25.0) | 25.0 (25.0–25.0) | 25.0 (24.0–25.0) |
| IQR | 25.0–25.0 | 25.0–25.0 | 25.0–25.0 |
| n | 9 | 8 | 17 |
| Missing (n) | 5 | 1 | 6 |
| Total light-touch sensory score for the upper limb on the operated side | |||
| Day 0 | |||
| Left operation side | |||
| Mean (SD) | 11.2 (1.1) | – | 11.2 (1.1) |
| Median (range) | 12.0 (10.0–12.0) | – | 12.0 (10.0–12.0) |
| IQR | 10.0–12.0 | – | 10.0–12.0 |
| n | 5 | 0 | 5 |
| Missing (n) | 1 | 0 | 1 |
| Right operation side | |||
| Mean (SD) | 10.8 (0.7) | 11.0 (1.6) | 10.9 (1.2) |
| Median (range) | 11.0 (10.0–12.0) | 12.0 (8.0–12.0) | 11.0 (8.0–12.0) |
| IQR | 10.0–11.0 | 10.0–12.0 | 10.0–12.0 |
| n | 8 | 9 | 17 |
| Missing (n) | 0 | 0 | 0 |
| Total | |||
| Mean (SD) | 10.9 (0.9) | 11.0 (1.6) | 11.0 (1.2) |
| Median (range) | 11.0 (10.0–12.0) | 12.0 (8.0–12.0) | 11.0 (8.0–12.0) |
| IQR | 10.0–12.0 | 10.0–12.0 | 10.0–12.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Day 1 | |||
| Left operation side | |||
| Mean (SD) | 11.3 (0.6) | – | 11.3 (0.6) |
| Median (range) | 11.0 (11.0–12.0) | – | 11.0 (11.0–12.0) |
| IQR | 11.0–12.0 | – | 11.0–12.0 |
| n | 3 | 0 | 3 |
| Missing (n) | 3 | 0 | 3 |
| Right operation side | |||
| Mean (SD) | 11.8 (0.7) | 11.5 (0.9) | 11.6 (0.8) |
| Median (range) | 12.0 (10.0–12.0) | 12.0 (10.0–12.0) | 12.0 (10.0–12.0) |
| IQR | 12.0–12.0 | 11.0–12.0 | 12.0–12.0 |
| n | 8 | 8 | 16 |
| Missing (n) | 0 | 1 | 1 |
| Total | |||
| Mean (SD) | 11.6 (0.7) | 11.5 (0.9) | 11.6 (0.8) |
| Median (range) | 12.0 (10.0–12.0) | 12.0 (10.0–12.0) | 12.0 (10.0–12.0) |
| IQR | 11.0–12.0 | 11.0–12.0 | 11.0–12.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| Week 6 | |||
| Left operation side | |||
| Mean (SD) | 11.3 (0.6) | – | 11.3 (0.6) |
| Median (range) | 11.0 (11.0–12.0) | – | 11.0 (11.0–12.0) |
| IQR | 11.0–12.0 | – | 11.0–12.0 |
| n | 3 | 0 | 3 |
| Missing (n) | 3 | 0 | 3 |
| Right operation side | |||
| Mean (SD) | 10.7 (1.2) | 11.8 (0.7) | 11.3 (1.1) |
| Median (range) | 10.5 (9.0–12.0) | 12.0 (10.0–12.0) | 12.0 (9.0–12.0) |
| IQR | 10.0–12.0 | 12.0–12.0 | 10.0–12.0 |
| n | 6 | 8 | 14 |
| Missing (n) | 2 | 1 | 3 |
| Total | |||
| Mean (SD) | 10.9 (1.1) | 11.8 (0.7) | 11.3 (1.0) |
| Median (range) | 11.0 (9.0–12.0) | 12.0 (10.0–12.0) | 12.0 (9.0–12.0) |
| IQR | 10.0–12.0 | 12.0–12.0 | 11.0–12.0 |
| n | 9 | 8 | 17 |
| Missing (n) | 5 | 1 | 6 |
| Total pinprick sensory score for the upper limb on the operated side | |||
| Day 0 | |||
| Left operation side | |||
| Mean (SD) | 11.2 (1.1) | – | 11.2 (1.1) |
| Median (range) | 12.0 (10.0–12.0) | – | 12.0 (10.0–12.0) |
| IQR | 10.0–12.0 | – | 10.0–12.0 |
| n | 5 | 0 | 5 |
| Missing (n) | 1 | 0 | 1 |
| Right operation side | |||
| Mean (SD) | 10.9 (0.8) | 11.2 (1.2) | 11.1 (1.0) |
| Median (range) | 11.0 (10.0–12.0) | 12.0 (9.0–12.0) | 11.0 (9.0–12.0) |
| IQR | 10.0–11.5 | 10.0–12.0 | 10.0–12.0 |
| n | 8 | 9 | 17 |
| Missing (n) | 0 | 0 | 0 |
| Total | |||
| Mean (SD) | 11.0 (0.9) | 11.2 (1.2) | 11.1 (1.0) |
| Median (range) | 11.0 (10.0–12.0) | 12.0 (9.0–12.0) | 11.5 (9.0–12.0) |
| IQR | 10.0–12.0 | 10.0–12.0 | 10.0–12.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Day 1 | |||
| Left operation side | |||
| Mean (SD) | 11.3 (0.6) | – | 11.3 (0.6) |
| Median (range) | 11.0 (11.0–12.0) | – | 11.0 (11.0–12.0) |
| IQR | 11.0–12.0 | – | 11.0–12.0 |
| n | 3 | 0 | 3 |
| Missing (n) | 3 | 0 | 3 |
| Right operation side | |||
| Mean (SD) | 10.8 (2.1) | 11.4 (0.9) | 11.1 (1.6) |
| Median (range) | 12.0 (6.0–12.0) | 12.0 (10.0–12.0) | 12.0 (6.0–12.0) |
| IQR | 10.0–12.0 | 10.5–12.0 | 10.0–12.0 |
| n | 8 | 8 | 16 |
| Missing (n) | 0 | 1 | 1 |
| Total | |||
| Mean (SD) | 10.9 (1.8) | 11.4 (0.9) | 11.1 (1.5) |
| Median (range) | 12.0 (6.0–12.0) | 12.0 (10.0–12.0) | 12.0 (6.0–12.0) |
| IQR | 10.0–12.0 | 10.5–12.0 | 10.0–12.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| Week 6 | |||
| Left operation side | |||
| Mean (SD) | 11.3 (0.6) | – | 11.3 (0.6) |
| Median (range) | 11.0 (11.0–12.0) | – | 11.0 (11.0–12.0) |
| IQR | 11.0–12.0 | – | 11.0–12.0 |
| n | 3 | 0 | 3 |
| Missing (n) | 3 | 0 | 3 |
| Right operation side | |||
| Mean (SD) | 10.7 (1.2) | 11.8 (0.7) | 11.3 (1.1) |
| Median (range) | 10.5 (9.0–12.0) | 12.0 (10.0–12.0) | 12.0 (9.0–12.0) |
| IQR | 10.0–12.0 | 12.0–12.0 | 10.0–12.0 |
| n | 6 | 8 | 14 |
| Missing (n) | 2 | 1 | 3 |
| Total | |||
| Mean (SD) | 10.9 (1.1) | 11.8 (0.7) | 11.3 (1.0) |
| Median (range) | 11.0 (9.0–12.0) | 12.0 (10.0–12.0) | 12.0 (9.0–12.0) |
| IQR | 10.0–12.0 | 12.0–12.0 | 11.0–12.0 |
| n | 9 | 8 | 17 |
| Missing (n) | 5 | 1 | 6 |
| Total motor score | |||
| Day 0 | |||
| Mean (SD) | 99.4 (1.4) | 99.2 (1.6) | 99.3 (1.5) |
| Median (range) | 100.0 (95.0–100.0) | 100.0 (95.0–100.0) | 100.0 (95.0–100.0) |
| IQR | 100.0–100.0 | 99.0–100.0 | 99.0–100.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Day 1 | |||
| Mean (SD) | 99.7 (0.6) | 100.0 (0.0) | 99.8 (0.5) |
| Median (range) | 100.0 (98.0–100.0) | 100.0 (100.0–100.0) | 100.0 (98.0–100.0) |
| IQR | 100.0–100.0 | 100.0–100.0 | 100.0–100.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| Week 6 | |||
| Mean (SD) | 99.9 (0.3) | 100.0 (0.0) | 99.9 (0.2) |
| Median (range) | 100.0 (99.0–100.0) | 100.0 (100.0–100.0) | 100.0 (99.0–100.0) |
| IQR | 100.0–100.0 | 100.0–100.0 | 100.0–100.0 |
| n | 9 | 8 | 17 |
| Missing (n) | 5 | 1 | 6 |
| Total light-touch sensory score | |||
| Day 0 | |||
| Mean (SD) | 38.8 (1.2) | 36.3 (4.0) | 37.8 (2.9) |
| Median (range) | 39.0 (36.0–40.0) | 37.0 (27.0–40.0) | 38.0 (27.0–40.0) |
| IQR | 38.0–40.0 | 36.0–38.0 | 37.0–40.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Day 1 | |||
| Mean (SD) | 39.6 (0.7) | 38.1 (2.5) | 39.0 (1.8) |
| Median (range) | 40.0 (38.0–40.0) | 39.0 (33.0–40.0) | 40.0 (33.0–40.0) |
| IQR | 39.0–40.0 | 37.0–40.0 | 38.0–40.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| Week 6 | |||
| Mean (SD) | 38.4 (2.2) | 38.8 (2.1) | 38.6 (2.1) |
| Median (range) | 39.0 (33.0–40.0) | 40.0 (35.0–40.0) | 39.0 (33.0–40.0) |
| IQR | 38.0–40.0 | 37.5–40.0 | 38.0–40.0 |
| n | 9 | 8 | 17 |
| Missing (n) | 5 | 1 | 6 |
| Total pinprick sensory score | |||
| Day 0 | |||
| Mean (SD) | 38.8 (1.2) | 36.7 (4.1) | 38.0 (2.9) |
| Median (range) | 39.0 (36.0–40.0) | 38.0 (27.0–40.0) | 38.5 (27.0–40.0) |
| IQR | 38.0–40.0 | 37.0–39.0 | 38.0–40.0 |
| n | 13 | 9 | 22 |
| Missing (n) | 1 | 0 | 1 |
| Day 1 | |||
| Mean (SD) | 38.5 (2.8) | 37.8 (2.8) | 38.2 (2.7) |
| Median (range) | 40.0 (31.0–40.0) | 38.5 (32.0–40.0) | 39.0 (31.0–40.0) |
| IQR | 38.0–40.0 | 36.5–40.0 | 37.0–40.0 |
| n | 11 | 8 | 19 |
| Missing (n) | 3 | 1 | 4 |
| Week 6 | |||
| Mean (SD) | 38.4 (2.2) | 38.8 (2.1) | 38.6 (2.1) |
| Median (range) | 39.0 (33.0–40.0) | 40.0 (35.0–40.0) | 39.0 (33.0–40.0) |
| IQR | 38.0–40.0 | 37.5–40.0 | 38.0–40.0 |
| n | 9 | 8 | 17 |
| Missing (n) | 5 | 1 | 6 |
| GRBAS scale | Total (N = 6), n (%) |
|---|---|
| Baseline | |
| Grade | |
| Normal | 2 (33.3) |
| Mild degree | 3 (50.0) |
| Moderate degree | 0 (0.0) |
| Severe | 0 (0.0) |
| Missing | 1 (16.7) |
| Roughness | |
| Normal | 3 (50.0) |
| Mild degree | 2 (33.3) |
| Moderate degree | 0 (0.0) |
| Severe | 0 (0.0) |
| Missing | 1 (16.7) |
| Breathiness | |
| Normal | 4 (66.7) |
| Mild degree | 1 (16.7) |
| Moderate degree | 0 (0.0) |
| Severe | 0 (0.0) |
| Missing | 1 (16.7) |
| Asthenia | |
| Normal | 5 (83.3) |
| Mild degree | 0 (0.0) |
| Moderate degree | 0 (0.0) |
| Severe | 0 (0.0) |
| Missing | 1 (16.7) |
| Strain | |
| Normal | 2 (33.3) |
| Mild degree | 3 (50.0) |
| Moderate degree | 0 (0.0) |
| Severe | 0 (0.0) |
| Missing | 1 (16.7) |
| Week 6 | |
| Grade | |
| Normal | 2 (33.3) |
| Mild degree | 1 (16.7) |
| Moderate degree | 0 (0.0) |
| Severe | 0 (0.0) |
| Missing | 3 (50.0) |
| Roughness | |
| Normal | 3 (50.0) |
| Mild degree | 0 (0.0) |
| Moderate degree | 0 (0.0) |
| Severe | 0 (0.0) |
| Missing | 3 (50.0) |
| Breathiness | |
| Normal | 3 (50.0) |
| Mild degree | 0 (0.0) |
| Moderate degree | 0 (0.0) |
| Severe | 0 (0.0) |
| Missing | 3 (50.0) |
| Asthenia | |
| Normal | 3 (50.0) |
| Mild degree | 0 (0.0) |
| Moderate degree | 0 (0.0) |
| Severe | 0 (0.0) |
| Missing | 3 (50.0) |
| Strain | |
| Normal | 2 (33.3) |
| Mild degree | 1 (16.7) |
| Moderate degree | 0 (0.0) |
| Severe | 0 (0.0) |
| Missing | 3 (50.0) |
Appendix 6 Additional tables and figures for the health economic evaluation
The most salient feature of the resource use data in the 3-monthly follow-up points, other than the recorded visit to the GP for the majority of participants at least once every quarter, is the recorded use of 90 sessions with the physiotherapist by one participant in the ACD arm during weeks 40–52 (Table 45).
| Arm | Time point | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Day 1 post operation | Week 6 | Week 12 | Week 26 | Week 39 | Week 52 | |
| PCF | |||||||
| VAS | |||||||
| Participants who responded (n) | 10 | 8 | 6 | 7 | 9 | 5 | 8 |
| Missing (n) | 4 | 6 | 8 | 7 | 5 | 9 | 6 |
| Mean (SD) score | 51.5 (20) | 50.1 (19) | 54 (19) | 68 (11) | 55 (21) | 65.8 (14) | 62 (17) |
| Mean (SD) changea | – | –0.5 (35) | 4.0 (34) | 18.0 (25) | 7.5 (34) | 23.8 (26) | 16.6 (23) |
| Median (IQR) score | 57 (40 to 70) | 48 (39 to 62) | 62 (34 to 70) | 69 (60 to 70) | 55 (35 to 70) | 70 (67 to 70) | 56 (48 to 79) |
| Median (IQR) changea | – | –1 (–28 to 30) | 1 (–6 to 10) | 15 (0 to 40) | –3 (–17 to 30) | 10 (5 to 40) | 10 (0 to 42) |
| EQ-5D | |||||||
| Participants who responded (n) | 12 | 12 | 10 | 9 | 11 | 6 | 11 |
| Missing (n) | 2 | 2 | 4 | 5 | 3 | 8 | 3 |
| Mean (SD) score | 0.291 (0.34) | 0.322 (0.31) | 0.410 (0.35) | 0.477 (0.40) | 0.392 (0.40) | 0.654 (0.18) | 0.525 (0.37) |
| Mean (SD) changea | – | 0.001 (0.51) | 0.081 (0.36) | 0.152 (0.31) | 0.102 (0.34) | 0.291 (0.32) | 0.193 (0.44) |
| Median (IQR) score | 0.210 (–0.01 to 0.60) | 0.329 (0.07 to 0.60) | 0.550 (0.05 to 0.69) | 0.620 (0.52 to 0.66) | 0.516 (–0.07 to 0.69) | 0.602 (0.52 to 0.69) | 0.689 (0.12 to 0.76) |
| Median (IQR) changea | – | 0.065 (–0.53 to 0.34) | 0.104 (0 to 0.13) | 0 (–0.05 to 0.35) | 0 (–0.11 to 0.28) | 0.204 (0 to 0.59) | 0.127 (0 to 0.42) |
| ACD | |||||||
| VAS | |||||||
| Participants who responded (n) | 7 | 7 | 5 | 4 | 5 | 5 | 6 |
| Missing (n) | 2 | 2 | 4 | 5 | 4 | 4 | 3 |
| Mean (SD) score | 59.7 (25) | 62.1 (17) | 70.6 (18) | 83.2 (11) | 59.0 (28) | 53.6 (12) | 71.7 (18) |
| Mean (SD) changea | – | 2.4 (34) | 5 (27) | 16 (37) | –2.8 (37) | –4 (34) | 12 (17) |
| Median (IQR) score | 59 (40 to 84) | 60 (50 to 70) | 70 (60 to 80) | 84 (74 to 92) | 70 (30 to 70) | 50 (48 to 60) | 60 (60 to 60) |
| Median (IQR) changea | – | 0 (–24 to 20) | 0 (–4 to 1) | 4 (–7 to 39) | 0 (–10 to 0) | 0 (–32 to 24) | 8 (5 to 20) |
| EQ-5D | |||||||
| Participants who responded (n) | 9 | 9 | 8 | 6 | 5 | 7 | 5 |
| Missing | 0 | 0 | 1 | 3 | 4 | 2 | 4 |
| Mean (SD) score | 0.595 (0.25) | 0.495 (0.24) | 0.636 (0.23) | 0.579 (0.28) | 0.697 (0.07) | 0.500 (0.39) | 0.624 (0.29) |
| Mean (SD) changea | – | –0.100 (0.25) | 0.012 (0.09) | –0.023 (0.04) | –0.021 (0.03) | –0.115 (0.31) | –0.036 (0.14) |
| Median (IQR) score | 0.689 (0.66 to 0.69) | 0.516 (0.26 to 0.69) | 0.708 (0.62 to 0.78) | 0.672 (0.62 to 0.69) | 0.691 (0.66 to 0.72) | 0.656 (0.02 to 0.69) | 0.620 (0.59 to 0.72) |
| Median (IQR) changea | – | 0 (–0.22 to 0.07) | 0.018 (–0.03 to 0.06) | 0 (–0.07 to 0) | 0 (–0.03 to 0) | 0 (–0.17 to 0) | –0.069 (–0.10 to –0.03) |
| Time point and medication | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| n | % | Cumulative % | n | % | Cumulative % | |
| Day 1 | 27 | 21 | ||||
| Paracetamol | 12 | 44.4 | 44.4 | 8 | 38.1 | 38.1 |
| Weak opioids | 8 | 29.6 | 74.1 | 5 | 23.8 | 61.9 |
| Strong opioids | 3 | 11.1 | 85.2 | 4 | 19.0 | 80.9 |
| NMAs | 3 | 11.1 | 96.3 | 4 | 19.0 | 100.0 |
| NSAIDs | 1 | 3.7 | 100.0 | 0 | 0.0 | 100.0 |
| Week 6 | 20 | 13 | ||||
| Paracetamol | 5 | 25.0 | 25.0 | 3 | 23.1 | 23.1 |
| Weak opioids | 4 | 20.0 | 45.0 | 4 | 30.8 | 53.8 |
| Strong opioids | 2 | 10.0 | 55.0 | 1 | 7.7 | 61.5 |
| NMAs | 3 | 15.0 | 70.0 | 3 | 23.1 | 84.6 |
| NSAIDs | 4 | 20.0 | 90.0 | 0 | 0.0 | 84.6 |
| Othera | 2 | 10.0 | 100.0 | 2 | 15.4 | 100.0 |
| Resource use questionnaires | ||||||
| Week 6 (previous 6 weeks) | 14 | 9 | ||||
| Painkillers | 1 | 7.1 | 7.1 | 2 | 22.2 | 22.2 |
| Antibiotics | 9 | 64.3 | 71.4 | 4 | 44.4 | 66.7 |
| Dressings | 3 | 21.4 | 92.9 | 0 | 0.0 | 66.7 |
| Otherb | 1 | 7.1 | 100.0 | 3 | 33.3 | 100.0 |
| Week 12 (previous 6 weeks) | 14 | 5 | ||||
| Painkillers | 1 | 7.1 | 7.1 | 2 | 40.0 | 40.0 |
| Antibiotics | 9 | 64.3 | 71.4 | 2 | 40.0 | 80.0 |
| Dressings | 2 | 14.3 | 85.7 | 0 | 0.0 | 80.0 |
| Otherc | 2 | 14.3 | 100.0 | 1 | 20.0 | 100.0 |
| Week 26 (previous 3 months) | 10 | 9 | ||||
| Painkillers | 1 | 10.0 | 10.0 | 0 | 0.0 | 0.0 |
| Antibiotics | 7 | 70.0 | 80.0 | 3 | 33.3 | 33.3 |
| Dressings | 2 | 20.0 | 100.0 | 1 | 11.1 | 44.4 |
| Otherd | 0 | 0.0 | 100.0 | 5 | 55.6 | 100.0 |
| Week 39 (previous 3 months) | 16 | 6 | ||||
| Painkillers | 1 | 6.2 | 6.2 | 1 | 16.7 | 16.7 |
| Antibiotics | 6 | 43.7 | 43.7 | 5 | 83.3 | 100.0 |
| Dressings | 1 | 50.0 | 50.0 | 0 | 0.0 | 100.0 |
| Othere | 8 | 100.0 | 100.0 | 0 | 0.0 | 100.0 |
| Week 52 (previous 3 months) | 14 | 8 | ||||
| Painkillers | 1 | 7.1 | 7.1 | 1 | 12.5 | 12.5 |
| Antibiotics | 9 | 64.3 | 71.4 | 3 | 37.5 | 50.0 |
| Dressings | 0 | 0.0 | 71.4 | 0 | 0.0 | 50.0 |
| Otherf | 4 | 28.6 | 100.0 | 4 | 50.0 | 100.0 |
| Resource | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean resource use (SD) unless stated as % | Missing participants (n) | Participants (n) | Mean resource use (SD) unless stated as % | Missing participants (n) | |
| Hospital services | ||||||
| A&E attendances (n) | 10 | 0.00 (0.0) | 4 | 8 | 1.00 (2.4) | 1 |
| Ward/outpatient attendances (n) | 10 | 0.60 (1.0) | 4 | 7 | 0.43 (0.5) | 2 |
| Cervical root injection (%) | 11 | 0.0 | 3 | 7 | 0.0 | 2 |
| Given prescription by surgical team (%) | 11 | 9.1 | 3 | 8 | 25.0 | 1 |
| Primary care and/or community-based services | ||||||
| GP visits at general practice (n) | 9 | 1.11 (1.2) | 5 | 8 | 2.62 (4.7) | 1 |
| Nurse visits at general practice (n) | 9 | 1.56 (2.4) | 5 | 8 | 0.25 (0.5) | 1 |
| Given prescription by someone else (%) | 11 | 90.9 | 3 | 8 | 75.0 | 1 |
| Saw nurse at home (n) | 10 | 0.00 (0.0) | 4 | 8 | 2.00 (5.7) | 1 |
| Telephone support calls (n) | 10 | 0.10 (0.3) | 4 | 8 | 0.37 (0.7) | 1 |
| Saw physiotherapist (n) | 10 | 0.20 (0.4) | 4 | 8 | 0.00 (0.0) | 1 |
| Saw chiropractor/osteopath (n) | 10 | 0.00 (0.0) | 4 | 8 | 0.00 (0.0) | 1 |
| Walk-in clinic attendances (n) | 10 | 0.00 (0.0) | 4 | 8 | 0.00 (0.0) | 1 |
| Resource | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean resource use (SD) unless stated as % | Missing participants (n) | Participants (n) | Mean resource use (SD) unless stated as % | Missing participants (n) | |
| Hospital services | ||||||
| A&E attendances (n) | 10 | 0.10 (0.3) | 4 | 4 | 1.00 (2.4) | 5 |
| Ward/outpatient attendances (n) | 11 | 0.36 (0.5) | 3 | 4 | 0.25 (0.5) | 5 |
| Cervical root injection (%) | 12 | 0.0 | 2 | 5 | 0.0 | 4 |
| Given prescription by surgical team (%) | 12 | 0.0 | 2 | 6 | 0.0 | 3 |
| Primary care and/or community-based services | ||||||
| GP visits at general practice (n) | 11 | 0.82 (1.0) | 3 | 4 | 1.75 (0.5) | 5 |
| Nurse visits at general practice (n) | 10 | 0.50 (0.5) | 4 | 4 | 0.25 (0.5) | 5 |
| Given prescription by someone else (%) | 12 | 83.3 | 2 | 6 | 66.7 | 3 |
| Saw nurse at home (n) | 10 | 0.00 (0.0) | 4 | 4 | 0.00 (0.0) | 5 |
| Telephone support calls (n) | 10 | 0.40 (0.7) | 4 | 4 | 0.00 (0.0) | 5 |
| Saw physiotherapist (n) | 9 | 0.22 (0.4) | 5 | 4 | 0.00 (0.0) | 5 |
| Saw chiropractor/osteopath (n) | 10 | 0.00 (0.0) | 4 | 4 | 0.00 (0.0) | 5 |
| Walk-in clinic attendances (n) | 10 | 0.10 (0.3) | 4 | 4 | 0.00 (0.0) | 5 |
| Resource | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean resource use (SD) unless stated as % | Missing participants (n) | Participants (n) | Mean resource use (SD) unless stated as % | Missing participants (n) | |
| Hospital services | ||||||
| A&E attendances (n) | 11 | 0.00 (0.0) | 3 | 5 | 0.40 (0.5) | 4 |
| Ward/outpatient attendances (n) | 11 | 0.36 (0.5) | 3 | 5 | 1.00 (1.2) | 4 |
| Cervical root injection (%) | 10 | 0.00 (0.0) | 4 | 6 | 0.00 (0.0) | 3 |
| Given prescription by surgical team (%) | 10 | 0.00 (0.0) | 4 | 6 | 0.00 (0.0) | 3 |
| Primary care and/or community-based services | ||||||
| GP visits at general practice (n) | 11 | 1.00 (1.2) | 3 | 6 | 1.33 (1.0) | 3 |
| Nurse visits at general practice (n) | 11 | 0.18 (0.4) | 3 | 5 | 0.00 (0.0) | 4 |
| Given prescription by someone else (%) | 10 | 70.0 | 4 | 6 | 66.7 | 3 |
| Saw nurse at home (n) | 11 | 0.00 (0.0) | 3 | 5 | 0.00 (0.0) | 4 |
| Telephone support calls (n) | 11 | 0.27 (0.9) | 3 | 5 | 0.20 (0.4) | 4 |
| Saw physiotherapist (n) | 11 | 0.00 (0.0) | 3 | 5 | 0.60 (0.5) | 4 |
| Saw chiropractor/osteopath (n) | 11 | 0.09 (0.0) | 3 | 5 | 0.00 (0.0) | 4 |
| Walk-in clinic attendances (n) | 11 | 0.09 (0.3) | 3 | 5 | 0.00 (0.0) | 4 |
| Resource | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean resource use (SD) unless stated as % | Missing participants (n) | Participants (n) | Mean resource use (SD) unless stated as % | Missing participants (n) | |
| Hospital services | ||||||
| A&E attendances (n) | 7 | 0.29 (0.5) | 7 | 7 | 0.00 (0.0) | 2 |
| Ward/outpatient attendances (n) | 7 | 0.57 (0.8) | 7 | 5 | 0.80 (1.0) | 4 |
| Cervical root injection (%) | 8 | 0.0 (0.0) | 6 | 7 | 0.0 (0.0) | 2 |
| Given prescription by surgical team (%) | 8 | 0.0 (0.0) | 6 | 7 | 0.0 (0.0) | 2 |
| Primary care and/or community-based services | ||||||
| GP visits at general practice (n) | 7 | 1.43 (1.0) | 7 | 7 | 1.43 (1.0) | 2 |
| Nurse visits at general practice (n) | 7 | 0.57 (0.8) | 7 | 5 | 0.40 (0.5) | 4 |
| Given prescription by someone else (%) | 8 | 87.5 | 6 | 7 | 85.7 | 2 |
| Saw nurse at home (n) | 7 | 0.00 (0.0) | 7 | 5 | 0.00 (0.0) | 4 |
| Telephone support calls (n) | 6 | 0.17 (0.4) | 8 | 5 | 0.80 (1.3) | 4 |
| Saw physiotherapist (n) | 6 | 0.00 (0.0) | 8 | 5 | 1.60 (0.0) | 4 |
| Saw chiropractor/osteopath (n) | 6 | 0.00 (0.0) | 8 | 4 | 0.00 (0.0) | 5 |
| Walk-in clinic attendances (n) | 7 | 0.14 (0.4) | 7 | 5 | 0.00 (0.0) | 4 |
| Resource | PCF arm (N = 14) | ACD arm (N = 9) | ||||
|---|---|---|---|---|---|---|
| Participants (n) | Mean resource use (SD) unless stated as % | Missing participants (n) | Participants (n) | Mean resource use (SD) unless stated as % | Missing participants (n) | |
| Hospital services | ||||||
| A&E attendances (n) | 10 | 0.30 (0.5) | 4 | 5 | 0.00 (0.0) | 4 |
| Ward/outpatient attendances (n) | 9 | 0.00 (0.0) | 5 | 5 | 0.60 (1.0) | 4 |
| Cervical root injection (%) | 13 | 0.00 (0.0) | 1 | 6 | 0.00 (0.0) | 3 |
| Given prescription by surgical team (%) | 13 | 0.00 (0.0) | 1 | 6 | 0.00 (0.0) | 3 |
| Primary care and/or community-based services | ||||||
| GP visits at general practice (n) | 10 | 1.40 (1.0) | 4 | 6 | 1.50 (1.0) | 3 |
| Nurse visits at general practice (n) | 10 | 0.10 (0.3) | 4 | 5 | 0.00 (0.0) | 4 |
| Given prescription by someone else (%) | 13 | 76.9 | 1 | 6 | 66.7 | 3 |
| Saw nurse at home (n) | 10 | 0.00 (0.0) | 4 | 5 | 0.00 (0.0) | 4 |
| Telephone support calls (n) | 9 | 0.22 (0.4) | 5 | 6 | 0.30 (1.0) | 3 |
| Saw physiotherapist (n) | 10 | 0.20 (1.0) | 4 | 6 | 15.50 (36.0) | 3 |
| Saw chiropractor/osteopath (n) | 6 | 0.00 (0.0) | 8 | 6 | 0.00 (0.0) | 3 |
| Walk-in clinic attendances (n) | 10 | 0.10 (0.3) | 4 | 6 | 0.00 (0.0) | 3 |
| Time point, trial arm and responses | EQ-5D-3L dimensions | ||||
|---|---|---|---|---|---|
| Mobility | Self-care | Usual activities | Pain/discomfort | Anxiety/depression | |
| Baseline: day 0 | |||||
| PCF arm | |||||
| n | 13 | 13 | 12 | 13 | 13 |
| No problems | 5 | 6 | 3 | 0 | 4 |
| Some/moderate | 8 | 7 | 6 | 5 | 8 |
| Unable/extreme | 0 | 0 | 3 | 8 | 1 |
| ACD arm | |||||
| n | 9 | 9 | 9 | 9 | 9 |
| No problems | 7 | 7 | 1 | 0 | 4 |
| Some/moderate | 2 | 2 | 6 | 9 | 4 |
| Unable/extreme | 0 | 0 | 2 | 0 | 1 |
| Day 1 | |||||
| PCF arm | |||||
| n | 13 | 12 | 13 | 13 | 13 |
| No problems | 6 | 4 | 1 | 0 | 5 |
| Some/moderate | 7 | 7 | 8 | 8 | 7 |
| Unable/extreme | 0 | 1 | 4 | 5 | 1 |
| ACD arm | |||||
| n | 9 | 9 | 9 | 9 | 9 |
| No problems | 3 | 5 | 1 | 0 | 5 |
| Some/moderate | 6 | 4 | 4 | 9 | 4 |
| Unable/extreme | 0 | 0 | 4 | 0 | 0 |
| Week 6 | |||||
| PCF arm | |||||
| n | 11 | 11 | 11 | 10 | 11 |
| No problems | 6 | 6 | 1 | 0 | 5 |
| Some/moderate | 5 | 5 | 9 | 6 | 5 |
| Unable/extreme | 0 | 0 | 1 | 4 | 1 |
| ACD arm | |||||
| n | 8 | 8 | 8 | 8 | 8 |
| No problems | 4 | 7 | 3 | 0 | 4 |
| Some/moderate | 4 | 1 | 5 | 8 | 3 |
| Unable/extreme | 0 | 0 | 0 | 0 | 1 |
| Week 12 | |||||
| PCF arm | |||||
| n | 12 | 11 | 11 | 11 | 11 |
| No problems | 5 | 4 | 1 | 1 | 4 |
| Some/moderate | 7 | 7 | 10 | 8 | 5 |
| Unable/extreme | 0 | 0 | 0 | 2 | 2 |
| ACD arm | |||||
| n | 6 | 6 | 6 | 6 | 6 |
| No problems | 3 | 4 | 1 | 0 | 3 |
| Some/moderate | 3 | 2 | 4 | 6 | 2 |
| Unable/extreme | 0 | 0 | 1 | 0 | 1 |
| Week 26 | |||||
| PCF arm | |||||
| n | 11 | 11 | 11 | 11 | 11 |
| No problems | 6 | 5 | 1 | 1 | 3 |
| Some/moderate | 5 | 6 | 8 | 7 | 5 |
| Unable/extreme | 0 | 0 | 2 | 3 | 3 |
| ACD arm | |||||
| n | 6 | 6 | 6 | 5 | 6 |
| No problems | 3 | 4 | 2 | 0 | 3 |
| Some/moderate | 3 | 2 | 3 | 5 | 2 |
| Unable/extreme | 0 | 0 | 1 | 0 | 1 |
| Week 39 | |||||
| PCF arm | |||||
| n | 6 | 7 | 7 | 7 | 7 |
| No problems | 3 | 4 | 1 | 1 | 1 |
| Some/moderate | 3 | 3 | 5 | 5 | 5 |
| Unable/extreme | 0 | 0 | 1 | 1 | 1 |
| ACD arm | |||||
| n | 7 | 7 | 7 | 7 | 7 |
| No problems | 3 | 3 | 1 | 1 | 3 |
| Some/moderate | 4 | 4 | 4 | 5 | 3 |
| Unable/extreme | 0 | 0 | 2 | 1 | 1 |
| Week 52 | |||||
| PCF arm | |||||
| n | 12 | 12 | 11 | 12 | 12 |
| No problems | 7 | 8 | 3 | 2 | 3 |
| Some/moderate | 5 | 4 | 8 | 7 | 8 |
| Unable/extreme | 0 | 0 | 0 | 3 | 1 |
| ACD arm | |||||
| n | 6 | 5 | 6 | 6 | 6 |
| No problems | 2 | 4 | 2 | 1 | 2 |
| Some/moderate | 4 | 1 | 3 | 5 | 2 |
| Unable/extreme | 0 | 0 | 1 | 0 | 2 |
FIGURE 19.
Proportion of responses to EQ-5D-3L stating problems at baseline, at day 1 and at week 6. (a) PCF arm, baseline; (b) ACD arm, baseline; (c) PCF arm, day 1; (d) ACD arm, day 1; (e) PCF arm, week 6; and (f) ACD arm, week 6. (continued)


List of abbreviations
- A&E
- accident and emergency
- ACD
- anterior cervical discectomy
- ASIA
- American Spinal Injury Association
- CI
- confidence interval
- COMI
- Core Outcome Measures Index
- CT
- computerised tomography
- CTRU
- Clinical Trials Research Unit
- DMEC
- Data Monitoring and Ethics Committee
- EAT-10
- Eating Assessment Tool-10 items
- EOI
- expression of interest
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FORVAD
- posterior cervical FORaminotomy Versus Anterior cervical Discectomy in the treatment of cervical brachialgia
- GETS
- Glasgow–Edinburgh Throat Scale
- GP
- general practitioner
- GRBAS
- grade, roughness, breathiness, asthenia and strain
- HRQoL
- health-related quality of life
- IQR
- interquartile range
- ITT
- intention to treat
- MRI
- magnetic resonance imaging
- NASS
- North American Spine Society
- NDI
- Neck Disability Index
- NMA
- neuromodulating agent
- NPT
- normalisation process theory
- NRS
- Numerical Rating Scale
- NRS-AP
- Numerical Rating Scale – Arm Pain
- NRS-NP
- Numerical Rating Scale – Neck Pain
- NSAID
- non-steroidal anti-inflammatory drug
- PCF
- posterior cervical foraminotomy
- PI
- principal investigator
- PIN
- personal identification number
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RAP
- rapid assessment procedure
- RCT
- randomised controlled trial
- SD
- standard deviation
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- VHI-10
- Voice Handicap Index-10 items
- WMD
- weighted mean difference
