Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number HTA 13/146/02. The contractual start date was in April 2016. The draft report began editorial review in November 2021 and was accepted for publication in June 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2024 Sohanpal et al. This work was produced by Sohanpal et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Sohanpal et al.
Chapter 1 Introduction
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is a common, incurable, but treatable, condition characterised by the progressive reduction in lung function,1 insidious onset of breathlessness, cough and sputum production, along with increasing fatigue. 2–4 The primary cause of COPD in the UK is tobacco smoking and, globally, indoor biomass fuel and air pollution (including occupational) and, therefore, COPD is largely preventable. In addition, reducing exposure (e.g. quitting smoking) slows progression. 2,3 The progressive course of the disabling symptoms is interspersed by acute exacerbations5 (often associated with respiratory infections), which accelerates the deterioration in health. As the condition becomes more severe, exacerbations lead to increasingly frequent hospitalisations,6 and about one-third of people with COPD will die of the condition. 7
Burden of disease
Burden on healthcare systems
Chronic obstructive pulmonary disease is a major public health problem globally, and is associated with socioeconomic deprivation and with high morbidity and mortality. COPD is currently the third leading cause of death worldwide,8 causing in excess of 3 million deaths annually,4 and this number is predicted to increase over the next decade. 9 In the UK, about 1.2 million people have diagnosed COPD, incurring more than 140,000 hospital admissions, over a million bed-days and about 30,000 deaths each year. 10,11 It is estimated that COPD costs the NHS £1.9B each year, with costs attributed mostly to hospital admissions. 12
Burden on individuals
Symptoms start insidiously, but a diagnosis of COPD is rarely made until the symptoms (especially breathlessness) or exacerbations have become troublesome enough to affect day-to-day activities, typically after the age of 40 years. 5,11 As the condition becomes more severe, breathlessness and the other symptoms of productive cough and fatigue increasingly limit activities and reduce quality of life. The negative impact extends to social isolation, loneliness, embarrassment and loss of independence. 13 Inhaled therapy can ease the symptoms, and pulmonary rehabilitation (PR) aims to reverse the vicious circle of reduced activity causing muscle weakness and further reduction in activity levels. 2,3
Multimorbidity and chronic obstructive pulmonary disease
Multimorbidity is the norm. 14 Smoking-related conditions, such as cardiovascular disease15 and lung cancer,16 are common comorbidities that are responsible for over half the deaths in people with COPD. 7,11,17 COPD is also associated with conditions such as metabolic diseases (including diabetes), obstructive sleep apnoea and osteoporosis (increased by immobility and steroid use), as well as a number of mental health conditions,2,3,9 including an increased suicide risk. 18
Anxiety and depression in chronic obstructive pulmonary disease
The estimated prevalence of anxiety and depression in people with COPD varies widely, depending on the population screened and the definitions used, with rates of up to 80% associated with more severe disease. 19–21 Even in stable COPD, rates of anxiety and depression of 30–40% are cited. 19 Anxiety and depression often overlap and affect quality of life, even in patients with mild to moderate COPD. 22 Anxiety and/or depression reduce the ability to manage the COPD effectively, reduce physical activity capacity and capability, and make patients susceptible to exacerbations, hospital admissions and re-admissions. 21,23 A recent mixed-methods review has highlighted the need to address psychological and emotional morbidity in addition to physical and social domains in supporting people to live with and manage their COPD. 24
Evidence-based management of anxiety and depression in chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease guidelines recommend that comorbid anxiety and/or depression should be treated ‘as usual’. 3 Current recommendations for both anxiety and depression include cognitive–behavioural therapy (CBT) instead of, or as well as, pharmacotherapy. 25,26 In the context of COPD, the potential benefits of physical exercise on mental health morbidity and the importance of promoting PR are specifically highlighted,3 suggesting synergy between these approaches.
Cognitive–behavioural therapy
Developed by Beck27 in the 1960s, CBT is based on the principle that given a triggering event or situation, how one thinks and interprets the event/situation will influence physical, behavioural and emotional response to that situation. These interactions are not ‘one-offs’ but can reinforce and impact on each of the other elements unless the cycle is interrupted. In mood disorders, patients are commonly in a pattern of vicious cycles where each element negatively influences the others. Figure 1 uses an exemplar of a depressive cycle of thoughts, symptoms, behaviours and feelings formulated as a ‘hot cross bun’. 28
FIGURE 1.
‘Hot cross bun’ formulation, illustrating a typical cycle of depressive thoughts, symptoms, behaviours and feelings in the context of COPD.
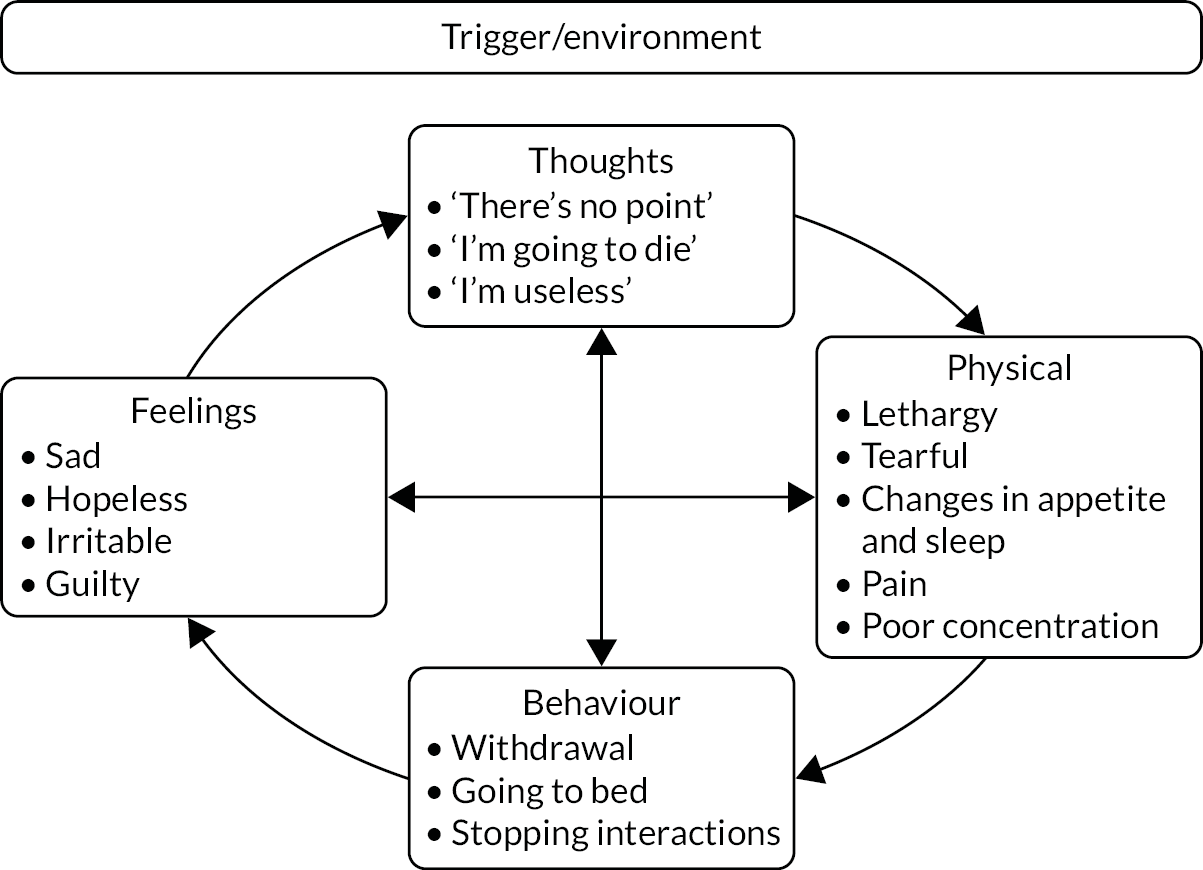
Cognitive–behavioural therapy addresses this unhelpful pattern by working at the level of thoughts, behaviours or symptoms to interrupt and reverse the negative impacts and, hence, improve mood. Low-intensity CBT is focused more around the present than historical events and commonly deals with what are known as negative automatic thoughts (i.e. the constant, sometimes subconscious, internal dialogue that is present for all of us). 29 High-intensity CBT can, however, deal with ingrained thinking patterns, rules for living and schema, which may have evolved at earlier stages of development. Limited in duration, and often delivered by specifically trained healthcare professionals rather than psychologists, low-intensity CBT is usually targeted at people with mild/moderate depression. 29
Cognitive–behavioural therapy improves anxiety and depression
Cognitive–behavioural therapy has been widely evaluated in a broad range of mental health conditions, and it has been adapted and delivered face to face or using a variety of digital health options. (The Cochrane Library lists 168 reviews relating to CBT in anxiety, depression, severe mental illness, substance misuse, self-harming, obsessive compulsive disorders and post-traumatic stress, among many others.) Psychological therapy based on CBT principles for people with anxiety disorders is effective in reducing anxiety, worry and depression symptoms, at least in the short term. 30–34 Similarly, psychological therapy, including CBT, reduced depression scores over the medium/long term35 and, importantly, given the age group affected by COPD, was effective in older patients with depression. 36 On the basis of this evidence, clinical guidelines now recommend CBT (via the internet or face to face) for the treatment of mild to moderate depression25 and generalised anxiety. 26
Benefits of cognitive–behavioural therapy for people with anxiety and/or depression associated with long-term conditions
There is increased recognition of the importance of identifying and treating mental health problems in people with long-term physical conditions. 37,38 Policy initiatives, such as promoting Improving Access to Psychological Therapies (IAPT) services,39 build on systematic review evidence that CBT can reduce symptoms of depression in people with physical conditions (including COPD). 40 Patient selection is important as effects are greater in people with clinically relevant depression at baseline. 40,41 Benefits for symptoms of anxiety have also been demonstrated in people with cardiovascular disease42 and cancer,43 both of which are common comorbidities in people with COPD. A lower-intensity CBT intervention [termed a ‘cognitive–behavioural approach’ (CBA)] delivered by physiotherapists to groups of people with low back pain has also been shown to be effective. 44
A Cochrane review,45 with 13 studies and 1500 participants, and focused on people with COPD, concluded that psychological therapies (using a CBT-based approach) may be effective for treating COPD-related depression. 45 There was a small effect showing the effectiveness of psychological therapies in improving depressive symptoms when compared with no intervention [standardised mean difference (SMD) 0.19, 95% confidence interval (CI) 0.05 to 0.33; six studies, 764 participants] or with education alone (SMD 0.23, 95% CI 0.06 to 0.41; three studies, 507 participants).
Pulmonary rehabilitation
Pulmonary rehabilitation is a comprehensive, multidisciplinary and individually tailored exercise and education intervention, which is designed to reduce the symptom burden associated with the deconditioning induced by COPD. 46,47 National and international COPD guidelines2,3,48 recommend offering PR to patients who are functionally disabled by breathlessness [commonly defined as a Medical Research Council (MRC) Dyspnoea Scale grade 3 and above]. 49 PR comprises an individually prescribed physical exercise training programme with at least twice-weekly supervised sessions, augmented by home-based exercise sessions and strategies to manage breathlessness. 3,46,47 Other components include an educational package to support effective self-management, nutritional advice, and social and psychological support.
Benefits of pulmonary rehabilitation
There is robust evidence from a Cochrane review,50 with 65 studies and 3822 participants, that completion of a course of PR significantly improves health-related outcomes, including increased functional exercise capacity, reduced breathlessness and improved quality of life, and this specifically includes an improvement in the Chronic Respiratory Questionnaire score for ‘emotional functioning’ (mean difference 0.56, 95% CI 0.34 to 0.78), suggesting benefit for people with both COPD and anxiety/depression. These effects are described as ‘moderately large and clinically significant’. 50
In addition, people with COPD report benefits from attending PR beyond health outcomes, such as having a better understanding of COPD and strategies that can help them live better with COPD, including improvements in social functioning. 51,52 Patients who had experienced these benefits wished that they had had been offered PR early in the course of their disease. 51
Barriers to attending pulmonary rehabilitation
There are well-documented delays in healthcare professionals initiating a referral,53–55 and more than one-third of patients referred do not attend the offered assessment. Likewise, assessment completion rates are persistently low,11,55 especially in people from the most deprived quintile, people who are underweight or very severely obese and people with more severe disease. 56 In addition to limited information about PR, and long delays between referral and starting a course, there are multiple practical barriers (e.g. travel, parking arrangements, timing of classes) that reduce people’s capability to attend and/or complete a course of PR. 57 People who had ‘lost hope’ or whose anxiety about their breathlessness ‘made exercise impossible’ were less likely to attend PR,53 suggesting that a focus on addressing these perceptual barriers could benefit people with COPD and anxiety/depression. Providing information and reassurance, addressing practicalities and maintaining contact while waiting to start the course are suggested as strategies to improve attendance at PR. 53
Strategies to increase uptake and completion of pulmonary rehabilitation
A systematic review of interventions (14 studies) to improve uptake and completion of PR had inconsistent outcomes,58 although uptake was improved when the referral was part of a care plan actively supported by the healthcare team59 or when information about the benefits of attending the course was provided to the patient. 60 Qualitative interviews reinforce the potential benefits of ‘repeated discussions’ to facilitate understanding of PR and its perceived benefits and of addressing any emotional and practical limitations associated with attending. 53 A systematic review of qualitative and quantitative evidence (48 studies) in relation to referral, uptake, attendance and/or completion in PR mapped interventions to aspects of the theoretical domains framework. 61 The domains most mapped were ‘environmental, context and resources’, ‘knowledge’ and ‘beliefs about consequences’, again, emphasising the need to support patients’ understanding of the benefits of PR. Potential practical solutions were also addressed (including provision of transport or parking, offering choice of timing of classes and language provision), along with organisational support to increase service capacity.
Synergies of psychological and physical interventions
Both CBT and PR, therefore, have benefits for improving the mental health of people with COPD and anxiety/depression, raising the potential for synergistic and additive effects.
The National Institute for Health and Care Research (NIHR) Health Technology Assessment brief62 was based on a systematic review and meta-analysis of complex interventions that concluded that psychological interventions combined with exercise training (29 studies in the meta-analysis) resulted in clinically significant improvements in symptoms of anxiety and depression in COPD, compared with CBT alone, which was only minimally effective. 63 This effect applied regardless of the severity of the anxiety and depression, although there was a caveat in that some of the studies recruited people with normal mental health scores at baseline. A more recent Cochrane review45 concluded that a psychological therapy combined with a PR programme reduced depressive symptoms more than a PR programme alone (SMD 0.37, 95% CI 0.00 to 0.74; p = 0.05; two studies, 112 participants).
The TANDEM (Tailored intervention for Anxiety and Depression Management in COPD) intervention was, therefore, designed to provide an intervention based on CBT and linked with a referral to PR for people with moderate to very severe COPD and mild to moderate anxiety and/or depression [see Chapter 2, Step 2: theory-informed intervention outline, for an explanation of how we developed the TANDEM intervention using a COPD-focused, lower-intensity CBT approach that addressed negative automatic thoughts (but did not address more ingrained thinking patterns)].
The potential benefit to carers was of interest and, at the request of the funder, we invited carers (who were nominated by patients recruited to the trial) to participate. 62
Aim
Our aim was to evaluate a tailored psychological CBA intervention (i.e. the TANDEM intervention), which precedes, links into and optimises the benefits of attending an existing PR course, with the intention of reducing mild/moderate anxiety and/or depression symptoms in people with COPD and moderate to very severe airflow limitation. 2,3
Objectives
-
To develop and refine the TANDEM intervention to develop a training programme for healthcare professionals who will deliver the programme, and to document the training programme in a manual (see Chapter 2).
-
To undertake a randomised controlled trial of the TANDEM intervention to examine the effectiveness of the TANDEM intervention on clinical outcomes compared with usual care (i.e. guideline-defined care including the offer of PR2,3) (see Chapter 4).
-
To examine the affect of the TANDEM intervention (which is directed at patients) on carers (where appropriate) (see Chapter 4).
-
To determine the cost effectiveness of the TANDEM intervention from an NHS and PSS perspective (see Chapter 5).
-
To conduct a process evaluation to assist interpretation of findings and inform the implementation of the TANDEM intervention if the trial results are positive (see Chapter 6).
Rationale for our approach
Pooler and Beech23 outlined a virtual model in which a cycle develops, starting with anxiety and/or depression, which leads to increased exacerbations and hospitalisations, which leads to reduced ability to cope, leading back to further increases in anxiety/depression. Figure 2 illustrates our proposed logic model, which shows how we aimed to counteract this cycle. An updated version of the logic model following intervention development is described in Chapter 2.
FIGURE 2.
Proposed logic model. We proposed that by addressing cognitions and behaviours the TANDEM intervention would (1) reduce anxiety and depression, (2) lead to increased participation with PR, (3) result in a decrease in exacerbations and improve quality of life and (4) directly feedback to further reduce anxiety and depression, as well as reducing morbidity and healthcare utilisation.

The TANDEM intervention optimised the potential synergy between the psychological one-to-one intervention and PR. The TANDEM therapy preceded PR and targeted individuals’ cognitions and behaviours associated with anxiety and depression both to decrease psychological morbidity and to increase motivation to attend and complete PR, which in itself has a positive effect on anxiety and depression.
Patient and public involvement in the TANDEM intervention
Aligned with our patient-centred approach, patient and public involvement (PPI) was central throughout the TANDEM project, informing the initial proposal, contributing to development of the intervention, advising on the evaluation and contributing to dissemination.
Further details on how PPI has been used in the TANDEM study are provided in Barradell and Sohanpal. 64
How patients and the public were recruited
Patients with COPD and carer advisors were identified via established patient networks. We had worked with some patients/carers before, whereas other patients/carers were introduced to us by study team members as patients who had previously expressed interest in involvement. In addition, we promoted the TANDEM study on social media, specifically Twitter, now renamed X (URL: www.twitter.com; Twitter, Inc., San Francisco, CA, USA). We approached potential advisors by letter, e-mail or telephone, or by attending one of their regular meetings and presenting the purpose of study, inviting them to become an advisor to the TANDEM study and giving options for involvement.
Most of the patients who expressed interest were members of support groups affiliated to the Asthma UK (London, UK) and British Lung Foundation (BLF) (London, UK) partnership (e.g. Breathe Easy patient groups in London, an exercise group in the West Midlands and a dedicated hospital-based PPI committee in Leicestershire). One patient responded to the Twitter invitation and we also recruited a patient representative known to a study team member through previous work.
Flexible options for involvement
We were flexible in our approach of involving patients as advisors. Advice was sought in groups (e.g. a discussion group to inform intervention development) or individually (e.g. to provide comments and feedback on intervention handouts). Communication could use any mode of contact (e.g. in person, telephone, e-mail, post).
Remuneration for involvement was discussed with Steven Towndrow (PPI/Engagement and Communications Officer, NIHR Collaborations for Leadership in Applied Health Research and Care North Thames, hosted by Barts Health NHS Trust). Vouchers to the value of £15 or £30 were provided based on involvement activity (i.e. £15 for reading and providing comments on study documents and £30 for attending a meeting).
The research cycle
The research cycle (Figure 3) highlights the contributions of PPI colleagues to the TANDEM study before, during and after the trial. A PPI co-applicant (who eventually withdrew from involvement in the study) had been with us from inception of the study. At grant application stage, the PPI co-applicant contributed to shaping the research question, methods and intervention, and helped write the lay summary. During the trial, the PPI co-applicant attended and contributed to the study team meetings and also attended the Independent Trial Steering Committee.
FIGURE 3.
The contributions of the PPI colleagues throughout the TANDEM study. Reproduced with permission from Kelly et al. 65 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.

The nature of involvement from other patient or carer advisors varied, as it depended on the requirements at that stage of the trial. Types of involvement included the following:
-
Providing comments and feedback on patient and carer study documentation (i.e. invitation letter, participant information sheet, consent form, study leaflets, interview topic guide, intervention handouts). Advice was sought on clarity, how easy it was to understand what is proposed and suitability of content and images, as well as suggestions for improvement.
-
Providing comments and feedback on terminology, advising on the best words to describe the intervention.
-
Providing comments and feedback on patient and carer recruitment and participant flow into the study.
-
Providing comments and feedback on intervention content and materials, and intervention delivery processes.
-
Role-playing with researchers to improve the process of approaching patients about the study.
-
Completing the patient and carer questionnaire to assess burden and time of completion.
-
Suggesting preferences for:
-
promotional study materials for study participants
-
an electronic database based on looking at screenshots of two types of electronic database.
-
-
Commenting on dissemination plans.
Ethics approvals and research governance
The trial was sponsored by the Joint Research Management Office for Barts Health NHS Trust and Queen Mary University of London (London, UK). The study was approved by the London – Queen Square Research Ethics Committee (reference 17/LO/0095) and by the local research and development departments in each participating trust. Before commencing recruitment, the trial was registered with the International Standard Randomised Controlled Trial Number Register (reference ISRCTN59537391) and NIHR Clinical Research Network Portfolio (reference 32713).
All study participants gave written informed consent. All participants were aware that they could withdraw from the study at any time and that participating in the study, or withdrawing from it, would have no effect on the medical care they were offered.
The trial was monitored by a Trial Steering Committee and a Data Monitoring Committee.
Chapter 2 Developing the TANDEM intervention
A paper has been published elaborating on the process of intervention development. 66
A five-step approach to intervention development
We used the widely cited MRC framework for developing and evaluating complex interventions,67 guidance and a ‘person-based approach’68 to guide the development of the TANDEM intervention.
At the time of TANDEM development, the MRC framework recommended that intervention development should be evidence based, use theory and model processes and outcomes prior to feasibility testing the intervention before formal evaluation. 67 The person-based approach to intervention development was originally designed for use with digital interventions,69 but has subsequently been applied to a more diverse set of interventions. 68 The person-based approach is based on the premise that a full and in-depth understanding of the target population and their involvement throughout the design process will enhance how the theory and evidence base are used. There are two main elements of this approach: (1) a developmental phase in which users provide information and opinions around the behavioural elements of the intervention and (2) a set of guiding principles are identified that ‘highlight the distinctive ways that the intervention will address key context-specific behavioural issues and provide a guiding framework for the intervention’. 68
In the TANDEM programme of work, these two approaches to intervention development were integrated to inform a five-step process that has been described in detail by Steed et al. 66 The steps were (1) building an expert team, (2) theory-informed intervention outline, (3) developing guiding principles informed by qualitative work, (4) developing a detailed intervention design and (5) pre-piloting and refinement (Figure 4).
FIGURE 4.
Schema of the five-step approach to intervention development as applied to the TANDEM intervention. Reproduced with permission from Steed et al. 66 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.

The fit of the TANDEM intervention with the 2021 Medical Research Council guidance for complex interventions
The extended MRC guidance for development and evaluation of complex interventions published in 2021 continues to specify the four key phases: (1) intervention development, (2) feasibility testing, (3) evaluation and (4) implementation. 70 In addition, there are six core elements that should be considered within each phase:
-
How does the intervention interact with its context?
-
What is the underpinning programme theory?
-
How can diverse stakeholder perspectives be included in the research?
-
What are the key uncertainties?
-
How can the intervention be refined?
-
What are the comparative resource and outcome consequences of the intervention?
Although the TANDEM intervention was developed prior to this publication,70 each of these questions was addressed during the course of the developmental work. With regard to how the intervention interacts with its context, consideration was given to where the intervention would be delivered (e.g. within a patient’s home or at a clinic). The programme theory was extensively developed and illustrated in a logic model. Using the person-based approach meant that a full range of stakeholder perspectives were used to inform intervention development from the outset. The pre-pilot identified important uncertainties (e.g. whether or not acceptability and feasibility were influenced by qualification level of the facilitator) and the intervention was refined post pre-pilot. Finally, the TANDEM trial addresses questions of clinical effectiveness and cost effectiveness to understand resource implications.
Step 1: building an expert team
The commissioning brief called for an intervention combining psychological therapy and physical retraining tailored to the severity of the patient’s respiratory and mental health. 62 Important outcomes were defined as measures of depression and anxiety, breathlessness, health-related quality of life and acceptability, as well as impact on carers, smoking status, use of healthcare resources and cost effectiveness. 62 As outlined in Chapter 1, Synergies of psychological and physical interventions, we proposed combining two well-established interventions (i.e. CBT and PR) and considered that efficient intervention design would be best served by building on the work of others in the field, rather than ‘re-inventing’ the wheel. Therefore, we sought, from the beginning, to build a multidisciplinary team with particular expertise in CBT for COPD and PR. In addition to leaders in COPD and primary care research (SJCT, HP and RS), we invited colleagues who had developed and demonstrated the effectiveness of a CBT intervention (i.e. The Lung Manual) for individuals with anxiety and COPD (KH-M)71,72 and colleagues who had developed Self-management Programme of Activity, Coping and Education (SPACE) for COPD® (a self-management manual) (SS)73 to join the team. Other team members included a health psychologist (LS), a clinical psychologist (SW), a qualitative research lead (MJK) and our co-applicant with COPD (see Chapter 1 for a detailed description of the contribution of PPI colleagues). Initially, the team aimed to pool resources from the already developed interventions (i.e. The Lung Manual and SPACE for COPD) to provide a starting point that could act as the bedrock for the intervention. The intervention development team subsequently met at key points along the development journey, forming an expert panel to suggest content, consider theory, review resources, provide feedback and agree revisions to the intervention.
Step 2: theory-informed intervention outline
Cognitive–behavioural therapy: a theoretical approach to influencing mood
The brief specified an intervention be designed that combining psychological therapy with physical retraining that was tailored both to the severity of the patient’s COPD (ranging from moderate to severe COPD) and their mental health (ranging from mild to moderate anxiety and/or depression)’. 62 Arguably, the most commonly used and well-proven psychological therapy for people with mood disorders, either with or without physical health conditions, is CBT25,26,38,74 (see Chapter 1 for an overview of CBT). CBT typically addresses unhelpful cognitive interpretations that negatively influence mood and feelings. By working at the levels of thoughts, behaviours or symptoms CBT aims to interrupt and reverse the negative impacts and, hence, improve mood. A CBA has been effective when delivered by healthcare professionals with expertise in the clinical condition and who are trained to provide low-intensity CBT (i.e. focused on dealing with current negative automatic thoughts, rather than ingrained historical thinking patterns29).
The TANDEM intervention and a cognitive–behavioural approach
The CBT model has, therefore, been shown to have relevance75 and has had some efficacy with patients with COPD,45,52,76,77 where interpretations of physical symptoms, such as breathlessness, and consequent coping (e.g. fear avoidance) are key precipitators of anxiety and depression. CBT was, therefore, selected as the psychological therapy of choice in the TANDEM intervention (see Chapter 1). Although the underlying theory was based on CBT, the skill set of the facilitators as experienced respiratory practitioners trained to deliver a brief course of treatment meant that high-intensity CBT intended to be delivered by a mental health practitioner would not be appropriate. 29 Instead, we considered that facilitators would use a CBA, working at the level of lower-intensity CBT and addressing primarily negative automatic thoughts, but not addressing engrained thinking patterns, such as rules or schema.
Linked pulmonary rehabilitation: additional benefits for anxiety and/or depression
Physical retraining delivered through PR is recommended for people with COPD. 2,3,46,78 PR has a strong evidence base and is known to improve both physical and psychosocial outcomes at all levels of COPD severity. 50 Therefore, it seemed appropriate to link the cognitive–behavioural element of the TANDEM intervention with existing PR services. The core aim of PR is to increase exercise so that Bandura’s self-efficacy model,75 which holds that confidence in ability to change behaviour is an important predictor of whether or not behaviour will be undertaken, is a useful theoretical basis. Bandura states that self-efficacy can be enhanced through mastery (i.e. successful performance of a goal), vicarious learning (i.e. seeing similar others perform the behaviour of interest) and persuasion (i.e. encouragement from a significant other). Bandura’s self-efficacy model was the theoretical basis of SPACE for COPD73 and so that alignment with the SPACE for COPD programme was appropriate. The TANDEM intervention, therefore, provided all intervention participants with SPACE for COPD materials.
The TANDEM intervention and supporting pulmonary rehabilitation
A challenge of PR is poor uptake and retention54,55,79,80 (see Chapter 1 for a summary of the main issues). The TANDEM intervention aimed to address the issues of poor uptake and retention by applying behaviour change theory to the intervention. We selected Leventhal and Leventhal’s81 common sense model of illness self-regulation, as this model is designed to help understand the dynamic processes that an individual goes through when understanding and managing illness. Leventhal and Leventhal81 hypothesised that to understand their condition, individuals develop cognitive illness representations, which are beliefs that revolve around the following six conceptual areas: (1) identity (i.e. the label and symptoms that are attributed to the condition), (2) consequences (i.e. the impact the condition will have on physical and psychosocial areas of the person’s life), (3) causes (i.e. what contributed to causing the condition), (4) timeline (i.e. how long the illness is perceived to last), (5) control (i.e. the extent the individual can exert control over the condition) and (6) coherence (i.e. the extent to which the condition makes sense). People also have emotional representations that, together with the illness representations, influence coping procedures. Coping procedures can include behaviours undertaken to manage a condition (e.g. COPD), such as ‘take medications’ or ‘attend PR’, as well as adopting problem-based coping strategies rather than avoidance strategies. Within the common-sense model, the impact of the coping procedures on both illness and emotional outcomes is then appraised with potential to feedback and influence early aspects of the model. Illness representations have been shown to be important in COPD,82,83 and interventions targeting illness representations have been shown to be successful;84 therefore, this theory was seen as relevant for the TANDEM intervention. A recent extension to the model is the inclusion of treatment representations (i.e. beliefs around effectiveness, worries and concerns of the recommended treatment). 85 Given that PR is a specific treatment that we were targeting, we felt it appropriate to include content around the theoretical construct of treatment representations in addition to illness representations.
The theoretical basis of the TANDEM intervention was, therefore, complex because of integration of several models; however, the models were considered to be complementary and such approaches have previously been used. 86
Healthcare professional training
It was recognised at this stage that it would be necessary to train professionals to deliver the intervention. There is a large body of theory relating to training and adult education, one of the most common being the VARK [visual, auditory, read, kinesthetic (i.e. experience or practice, simulated or real)] model of learning. 87 The VARK model was recommended to us by collaborators from Education for Health (Warwick, UK) and was adopted in the professional training element of the TANDEM intervention. The VARK approach to learning suggests that different learners will have preferences for different ways to assimilate knowledge, and these preferences can be categorised as visual or aural information by reading about things or through simulated learning. By incorporating material in a range of formats (e.g. videos, papers for reading, practical exercises), we aimed to embrace each of the possible learning styles.
Step 3: guiding principles
In line with the person-based approach, exploratory qualitative work was conducted with both patients and healthcare professionals from the outset to understand what would be important to include in the intervention and whether or not certain elements were important for both the effectiveness and implementation of the intervention.
Methods for exploratory qualitative work
Recruitment
A maximum variation sample of respiratory healthcare professionals who had an interest in delivery of psychological interventions to patients were recruited through social media and professional networks. The healthcare professionals were invited to participate in either an individual interview (i.e. face to face or telephone) or a focus group, dependent on participant preference. Similarly, two focus groups for people with COPD and carers were arranged (one for individuals with experience of psychological therapies and one for patients without this experience). Patients and carers were recruited for the focus groups via a respiratory support group (‘Breath Easy’). These formal qualitative approaches were in addition to PPI input on, for example, the one-to-one approach, number of sessions and content of intervention booklets (see Chapter 1, Patient and public involvement in the TANDEM intervention, for further description of the whole integrated PPI contribution).
Topic guides and data collection
Interviews/focus groups were conducted by Liz Steed, Ratna Sohanpal and Karen Heslop-Marshall. Topic guides for both patient and professional groups included (1) perspectives on patients’ difficulties with living with COPD, (2) opinions on the proposed intervention (i.e. we described our ideas as outlined in the grant application to participants) and (3) key strategies that would maximise the chance of implementation if the intervention was shown to be successful. Interviews and focus groups were audio-recorded, transcribed and analysed thematically.
Results of the exploratory qualitative study
Participant characteristics
One focus group with respiratory professionals was conducted, comprising a respiratory consultant, three physiotherapists, an occupational therapist and one exercise practitioner. All participants in the focus group were employees at a tertiary care hospital. In addition, seven individual interviews were conducted with healthcare professionals [one general practitioner (GP), four psychologists and two physiotherapists]. These individuals all had experience of working with respiratory patients within the community, primary care or secondary care.
We were able to conduct only one focus group with patient and carers because of time and governance delays at the site where individuals who had previously received CBT were to be recruited. In total, four individuals with COPD, two individuals with other respiratory conditions and two carers participated.
Guiding principles
Table 1 provides a summary of the guiding principles for both the intervention and its implementation.
| Guiding principle | Illustrating quote |
|---|---|
| Intervention | |
| Depression and anxiety are key topics, but could be introduced via breathlessness |
I think they most often talk about symptoms like breathlessness, rather than saying that they’re anxious or depressed
HCP006 physiotherapist Terminology is important such as ‘dealing with’, ‘living with’ Patient focus group discussion … not much focus is given on … knowing how to deal and what to expect when you experience breathlessness Patient focus group participant |
| The intervention should be tailored/flexible to individuals |
It’s just that patients are all different, and therefore present very differently and the intervention has to be tailored individually to what they’re presenting with
HCP003, psychologist Topic suggestions ‘involve family’, ‘concept of acceptance’, ‘coping strategies’ Patient focus group discussion |
| Sessions could be offered at home or in a clinic, but there may be limitations to the latter. Accessibility is key |
So I think having the capacity to start off at home is certainly a good idea. I think just something about accessible locations
HCP002, psychologist |
| Clear expectations and boundaries should be set at the start of the intervention |
So there needs to be quite clear boundaries about what the intervention offers and doesn’t offer
HCP006, physiotherapist |
| Implementation | |
| Delivery by respiratory professionals rather than psychologists is preferable |
It feels important that other members of the healthcare team are being trained up in these approaches. That can only be a good thing …
HCP003, psychologist |
| Some selection and training of facilitators will be needed |
A lot of people would be attracted to this, but it’s not for everyone to deliver
HCP005, physiotherapist What training would this nurse have? Patient focus group participant |
| Supervision of facilitators delivering the intervention is essential and should be ongoing |
I think that’s important [supervision]
HCP005, physiotherapist |
| The intervention must be deliverable and supported by management |
There’s no point evaluating it, if it’s not something that’s going to be deliverable
HCP FG001 doctor |
In general, both patients and healthcare professionals were very positive about the proposed intervention. Patients and healthcare professionals felt that anxiety and depression were important and relevant for individuals with COPD, and that anxiety and depression were closely linked to breathlessness, which might be an acceptable way to introduce the intervention to potential participants. There was recognition that different people would have different needs and, therefore, being able to offer an intervention tailored to the individual with the possibility of home-based sessions would be ideal. There was consensus that, given the physical complexity of these patients, delivery by respiratory professionals would be preferable to psychologists, but training and supervision would be essential. One of the key concerns was around the symptom of breathlessness and its causation (i.e. was breathlessness anxiety related or an indicator of a developing exacerbation?). For anxiety-related breathlessness, some basic distraction and breathing techniques may be appropriate; however, for an acute exacerbation, assessment and treatment by a healthcare professional would be needed. These management options are diametrically opposed. From a safety perspective, it was considered that training respiratory professionals (familiar with COPD and managing acute exacerbations) in psychological techniques would be preferable to training psychology practitioners in respiratory medicine.
Step 4: developing the detailed intervention design
Patient sessions
The topics for the patient-facing intervention were informed by the underlying theory, discussed with the intervention development team and aligned with the guiding principles. Table 2 provides an overview of the TANDEM intervention. The topics to be addressed in the first session were related to illness representations around COPD and, in particular, the symptom of breathlessness and techniques to manage this. At this initial session (and repeated at the final session of the TANDEM intervention), the facilitator administered short questionnaires that are widely used in clinical practice to assess anxiety and depression status. The Patient Health Questionnaire-9 items (PHQ-9) assesses depression88 and the General Anxiety Disorder-7 (GAD-7) measures anxiety. 89 These assessments were intended to guide the intervention, and were not considered as trial outcome measures.
| Session | Topic covered | Content |
|---|---|---|
| 1 | Introduction, setting expectations Topic 1: what is COPD? Topic 2: taking control of COPD Topic 3: the patient experience of breathlessness |
Eliciting the patient’s understanding of COPD Identifying and working with illness and treatment beliefs and acceptance Teaching basic breathing control |
| 2 | Feedback from home practice Topic 4: introducing mood and COPD |
Conducting a formulation and presentation of a CBA |
| 3–7 (CBA) | Feedback from home practice Topic 5: managing anxiety and COPD Topic 6: managing depression and COPD Topic 7: applying CBA to other problems (optional) |
Up to four sessions to conduct cognitive–behavioural work on anxiety and/or depression dependent on individual need One further session available to discuss other problems if needed |
| Penultimate session | Feedback from home practice Topic 8: living with COPD day to day |
Self-management approaches to COPD Learning to problem solve and set goals |
| Final session | Feedback from home practice Topic 9: preparing for PR |
Expectations of PR, addressing worries and concerns |
| Linking to PR | Reviewing progress and adjusting goals | Fortnightly telephone calls exploring any worries or concerns delivered between the end of the one to one sessions until the end of PR |
The topic of mood and COPD was introduced in session 2, as the premise of CBT is that thoughts, feelings, symptoms and behaviours interact. By the end of session 2, an initial formulation of the primary presenting problem of the individual patient should have been made (see Figure 1 for an illustration of a ‘hot cross bun’ formulation). Dependent on the presenting problems, sessions 3–6 used a CBA that focused on depression and/or anxiety. The penultimate session addressed self-management techniques and the final session addressed treatment representations around PR. An additional, optional, session was designed to apply CBA skills to a non-COPD problem, if needed, and this reflected recognition that many COPD patients have complex lives and may have issues that affect their psychological well-being that are separate from COPD. To acknowledge the importance of other issues, while maintaining the centrality of the intervention on COPD, session 7 (i.e. ‘applying CBA to other problems’) was added.
All topics were coded as core (i.e. applicable and for delivery to all participants), tailored (i.e. addressing depression and/or anxiety as appropriate) or optional (i.e. used only if applicable). Patient-guided self-completion leaflets were written by a member of the team (LS) and revised by members of the intervention development team. The PPI co-applicant then went through the leaflets in detail to ensure they were accessible to the COPD population. The leaflets were modelled on publicly available self-help leaflets designed by Northumberland, Tyne and Wear NHS Foundation Trust (Newcastle upon Tyne, UK), which had been extensively reviewed by lay people. Leaflets were developed for key topics (e.g. mood and COPD, depression and COPD, anxiety and COPD). Any relevant SPACE leaflets (controlling your breathing, diet and COPD etc.) were reformatted to be consistent with the TANDEM intervention style. In addition, we developed a leaflet for ‘caring for someone with COPD’, as this had been highlighted in focus groups as an important topic. Having developed the key topics and related resources, these were then translated into individual facilitated sessions to be delivered by the healthcare professionals trained as TANDEM facilitators, following a similar model to that of guided lower-intensity sessions by the NHS IAPT programme. 90,91
In addition to the face-to-face sessions, telephone contacts were designed for patients who were going on to PR. The telephone calls were designed to be fortnightly, leading up to and throughout the PR. The aim of the telephone calls was to provide continuity of contact between the conclusion of the face-to-face sessions and PR, and to help patients reflect on their goals for PR and review progress. If participants decided not to participate in PR, or if patients were not considered eligible by the PR service, then they did not receive additional telephone calls.
Facilitator training
Following development of the patient-facing intervention, we devised a training programme for respiratory healthcare professionals to deliver the sessions. The training programme drew on training developed in ‘The Lung Manual’. 73 The Lung Manual, in contrast to the TANDEM intervention, did not specify session content or provide an overview structure, rather the manual, supported by 3 days of training, taught ‘a practical and structured guide to using CBT principles with patients who have been identified as experiencing anxiety, panic, low mood or depression as a result of their physical health problems’. 73
Three-day training
For the TANDEM intervention, a 3-day training programme was proposed. The first 2 days of the training programme were delivered consecutively and were designed to cover background theory, introduction to depression and anxiety, and the basic knowledge and skills to use in the TANDEM CBA for patients with COPD. The training was designed to be delivered in a group by two trainers (a respiratory healthcare professional and either a health or clinical psychologist). Learning was interactive, with practical exercises and opportunities to practise skills with a professional actor who had been briefed on the training aims. This type of learning was informed by medical education curricula that suggests that providing students practice with simulated patients is beneficial to clinical competency. 92
A third day was scheduled 4–6 weeks later. During the interim period, all trainees were asked to apply the skills they had learnt within their clinical practice. On the third training day, trainees presented a case study of a patient to illustrate how they had conducted a formulation and used the skills they had learnt. The case studies presentations were discussed, with any difficulties or problems resolved.
Training manual
A comprehensive training manual was developed, comprising three sections:
-
A detailed outline of content and delivery recommendations for each topic.
-
A guide to skills of cognitive–behavioural and self-management assessment and intervention.
-
Background information on COPD and theoretical underpinnings of behaviour change and CBA for trainees who wished to gain additional knowledge.
All resources, such as patient handouts, were appended at the end of the manual. The aim was to make the manual a comprehensive ‘go-to’ resource for delivery of all TANDEM intervention elements.
Study materials (research and clinical documentation)
Facilitators were provided with all materials required to audio-record the intervention sessions, along with video instructions on how to (1) use the encrypted audio-recorder and (2) securely transfer audio-recordings to the study team. Research documents included a CBA-session contact and delivery log. Clinical documents (not for research purposes) were as follows:
-
A CBA-session case notes and supervision document was used to record detailed clinical information (including personal identifiable details of participant) necessary for intervention delivery for each participant, and to facilitate clinical supervision. The clinical notes were stored securely and separately from the TANDEM research data.
-
A summary letter that was sent to patients’ healthcare professionals (with the participant’s permission) following completion of intervention delivery. The letter included a brief summary about the intervention delivery, the PHQ-9 and GAD-7 scores (used by the facilitator to assess the mental health status of the patient at the beginning and end of the CBA sessions) and any other information that was important for healthcare professionals to know about the patient in relation to their ongoing care.
Supervisor training
We recognised that TANDEM intervention facilitators would require clinical supervision to ensure safe and effective delivery of the intervention. Supervision has been shown to be an important factor in reducing ‘therapist drift’, particularly with new therapists. 93,94 All supervisors were required to be qualified as either a cognitive–behavioural therapist or a clinical/health psychologist. The supervisors participated in the 3-day TANDEM training programme and had an additional half-day training in the clinical management of COPD and applying TANDEM CBA to COPD-related issues. The supervisors themselves received support through a lead supervisor who was a member of the research team (SW).
Ensuring fidelity
Throughout step 4 of intervention development, the intervention development team considered actions that would enhance intervention fidelity in line with the National Institutes of Health Behaviour Change Consortium framework and recent recommendations. 95,96 Figure 5 summarises the strategies taken to enhance fidelity (see Chapter 6 for greater detail on fidelity assessment).
FIGURE 5.
Strategies used to enhance fidelity.

Important elements to ensure fidelity were provision of a manual for facilitators (with a comprehensive description of the content to cover in each session), reinforcement of the skills taught in the training and background information on the theoretical basis of the intervention. The aim of the manual was to (1) increase facilitator confidence and (2) ensure consistency of delivery between facilitators. Similarly, standardised materials were developed for patients. Patient materials included both information and self-completion exercises to ensure that topics were covered consistently while also allowing tailoring to the individual. All materials were discussed with PPI colleagues to ensure appropriateness for people with COPD.
Step 5: pre-pilot
Having developed the draft patient intervention and facilitator training, a pre-pilot study was conducted. The pre-pilot study allowed delivery of the intervention as a whole and considered how different parts of the intervention worked together. The pre-pilot study was explicitly part of the intervention development (in contrast to feasibility studies, which are typically conducted after the main intervention development phase). Two key questions were asked in the pre-pilot study:
-
Is the TANDEM intervention acceptable and appropriate for patients when delivered by a therapist previously trained and qualified in CBT (when it can be assumed the intervention is delivered with fidelity)?
-
Is the TANDEM intervention acceptable and appropriate when delivered by a facilitator who receives the TANDEM training (i.e. does the TANDEM training provide facilitators with sufficient skills to deliver the intervention appropriately and, therefore, has it potential for implementation)?
To answer these questions, the TANDEM training was delivered to three purposively sampled respiratory healthcare facilitators (one facilitator was a qualified CBT therapist, one facilitator had received a previous 3-day training in CBT and had used the techniques in practice, and one facilitator was a complete novice to CBT). To replicate the group element of the training programme, key members of the research team, including the trial manager, the principal investigators and the qualitative lead, also participated. At the end of each day of training the trainees had a group discussion about strength and weaknesses of the training and what they perceived should change. Each facilitator was then given the task of delivering the TANDEM intervention to two of their patients who fitted TANDEM intervention criteria (i.e. mild/moderate anxiety and or depression and moderate to very severe COPD). Following intervention delivery, the three facilitators and six patients were invited for qualitative interview.
Feedback from the training sessions
The quotations below have been reproduced from Steed et al. 97 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Group members provided constructive feedback at the end of each training day, which highlighted the following themes:
-
A greater need for an overview of the TANDEM intervention at the outset of day 1.
-
A sense that role-play with a professional actor within the first 2 days of training was unhelpful, as participants did not feel confident enough to gain from this role-play at such an early stage and, instead, it was perceived as threatening.
-
A desire for more case study practice to develop formulations of patients’ presenting difficulties.
Two of the three respiratory healthcare professionals went on to deliver the TANDEM intervention. One healthcare professional (the CBT qualified professional) delivered sessions to two patients and one healthcare professional (the briefly CBT-trained professional) delivered sessions to one patient. The complete novice to CBT did not manage to deliver any TANDEM sessions to patients because of governance delays and a change in his work responsibilities, and this facilitator was also not available for interview. Interviews with the facilitators who delivered the TANDEM intervention reported that patients were engaged:
Yeah, I mean the two patients who I had were very, very enthusiastic about all elements of the intervention.
PP-HCP01
This engagement was corroborated by the patients who reported the intervention to be beneficial and acceptable:
And then [facilitator] and I just seemed to get on very well, he’s a likeable chap, very laid back. And so it went from there. And then we started doing the things that you asked in TANDEM. Planning ... They’re just small things, but marvellous.
PP-P01, male participant
The facilitators found that the manual and session materials were too complex at certain points:
I mean section nine, it’s got ‘identifying maintenance factors’, and it talks about ‘safety behaviour’, ‘avoidance and escape’, ‘catastrophic interpretation’, ‘scanning or hypervigilance’, ‘self-fulfilling prophecies’, ‘fear of fear’, ‘reductions’, ‘affectionism’, ‘short-term rewards’. If you’re trying to talk to a patient and remember what it says in the manual you might get yourself a little bit flustered.
PP-HCP02
Recommendations were made to simplify things, with a tool box of techniques for anxiety and depression and crib cards given as examples of additional useful resources:
I feel that people who come away from the training need to have something like a virtual toolbox of techniques that they can refer to ... they expected quite a lot of you ... I made myself a crib sheet type of thing.
PP-HCP01
Adherence to the sessions was good, but one element of the whole intervention that fell below expected levels was use of supervision. Neither facilitator contacted or responded to calls from the supervisor while delivering sessions and this was of concern, as supervision was perceived as a core safety element of the intervention. On further exploration, the facilitators divulged that they did receive supervision but got this from their normal CBT supervisor who worked at their clinical site. As the site supervisor also knew the patients recruited to receive the pre-pilot intervention, a judgement had been made that it was preferable to use the local supervisor rather than the TANDEM supervisor. Although understandable, this highlighted that more focus was needed on the importance of how and when supervision should be received.
Refinements to facilitator training after the pre-pilot study
Table 3 shows the finalised training programme with the amendments made after the pre-pilot study. An additional session on the importance and mechanism of supervision was added. Role-play with an actor was removed from the core training days and, instead, each facilitator conducted an individual video-recorded role-play with a professional actor on day 3. In this role play, the facilitator was asked to conduct an initial interview and formulation with the simulated patient and feed this back to them. This recorded role play served two purposes. First, the role play allowed an assessment of therapeutic competency of the facilitator, which is important for ensuring fidelity in treatment delivery. Second, role play served as a further training opportunity. All facilitators were visited by one of the trainers (LS) who showed them their video-recording and discussed strengths and areas for development. Several of the facilitators commented that although they found this a difficult process it was important for their learning and, in many cases, increased their confidence in the skills they had learnt.
| Day 1 | Day 2 | Day 3 |
|---|---|---|
| Introductions aTANDEM overview The patient’s experience of COPD (group exercise) What are depression and anxiety? (group exercise) Depression and anxiety in COPD Introduction to CBA (group exercise) Core therapeutic skills (avideo demonstration) Making an assessment: recognising thoughts, feelings behaviours, symptoms (practical) Sharing ideas with patients (practical) Feedback on worries and concerns after day 1 |
Practical CBA techniques Psychoeducation Breathing control Distraction Monitoring Problem-solving Goal-setting Graded practice/simple behavioural experiments Challenging thoughts (avideo demonstration) aToolbox for anxiety aToolbox for depression Preparation for case studies |
Case study feedback aIndividual practice with actor (videoed) Delivering TANDEM session by session, including: Changing behaviour Preparing for PR (using a photobook) aImportance of supervision Risk assessment Research requirements aProvision of crib cards |
A further innovation designed to support facilitators and to enhance fidelity of delivery was design of TANDEM-specific clinical case notes. TANDEM-specific clinical case notes served the same purpose as routine clinical practice notes, but were designed to guide the facilitators in key things they should consider in formulation and intervention. For example, in early sessions, facilitators were prompted to record a ‘hot cross bun’ formulation. In later sessions, there were prompts for not only recording what intervention was being undertaken, but also why. Therefore, facilitators were encouraged to be reflective in their practice.
Arrangements for facilitator recruitment
To enhance fidelity of facilitator training and delivery we also put in place a recruitment process for the facilitator role. The recruitment process involved a formal job advertisement and job specification. Facilitators were invited to apply for the role by sending the study team their curriculum vitae. Facilitators who met eligibility criteria were invited to a structured telephone interview focused around their engagement with a biopsychosocial model of care, flexibility to deliver TANDEM sessions and respiratory experience.
Iterative refinements to the TANDEM patient-facing intervention
As a result of the pilot, it became apparent that some patients could have exacerbations during the 6- to 8-week delivery period, necessitating a break in the delivery of the intervention. The decision was, therefore, made to allow one additional review session if there had been a gap in delivery, as this would also allow an adjustment to the formulation, if necessary, and intervention techniques, if appropriate.
A practical problem that occurred was if the participant was invited to start PR before completion of the full TANDEM CBA intervention. As delay in attending PR would not represent best clinical care, the decision was taken that topics could be re-ordered to address the beliefs and concerns around PR at an earlier stage if needed. However, it was stipulated that topics 1, 5 and 6 should have been delivered before initiation of PR to allow for a sufficient dose of the TANDEM intervention to be received first.
Final logic model
Following the finalised intervention design, a logic model was specified outlining movement from initial problem to change in outcomes via hypothesised mechanisms (Figure 6).
FIGURE 6.
The TANDEM logic model. ADL, activities of daily living, HCU, healthcare use.

Chapter 3 Methods: clinical effectiveness study
The trial protocol and statistical analysis plan are published. 98,99
Study design
The TANDEM study was a multicentre pragmatic two-arm individual patient randomised control trial with an internal pilot phase. Following collection of baseline data, participants were randomised with full allocation concealment in a ratio of 1.25 : 1 to the TANDEM intervention (see Chapter 2 for a detailed description of the development) or to usual care. All healthcare providers, including PR teams, outcome assessors and statisticians, were blind to the allocation arm of participants. An economic evaluation (see Chapter 5) and a process evaluation (see Chapter 6) were conducted in parallel.
Research sites
The TANDEM trial was run from what is now the Wolfson Institute of Population Health and Barts, The London School of Medicine and Dentistry, Queen Mary University of London (London, UK) where one of the co-chief investigators, the trial manager, a research assistant and the trial administrator were based. Trial support was provided by the Pragmatic Clinical Trials Unit (PCTU) at Queen Mary University of London.
Research assistants were based alongside co-applicant Investigators in the School of Population Health and Environmental Sciences, King’s College London (London, UK), Centre for Exercise and Disease Science, University Hospitals of Leicester NHS Trust (Leicester, UK) and Warwick Clinical Trials Unit, Warwick Medical School (Coventry, UK).
As the TANDEM intervention was designed to precede the opportunity to attend routine PR, recruitment sites had to involve a participating PR service(s). Initial recruitment sites were local (London, Leicester and Warwick) to the co-applicant investigators, but a number of sites approached the study team and asked to be involved either through their local Clinical Research Networks or because their local respiratory clinicians were particularly interested in participating in the study.
Recruitment of TANDEM facilitators and cognitive–behavioural therapy supervisors
Facilitators were experienced respiratory healthcare professionals with portfolio careers in respiratory health. Potential TANDEM facilitators were identified via news articles and advertising campaigns across respiratory healthcare networks and associated social media. In addition, TANDEM researchers ran a stall about the study at two successive Primary Care Respiratory Society annual conferences.
To avoid any risk of contamination and to preserve blinding of healthcare providers, TANDEM facilitators were recruited from staff not involved in either the provision of routine COPD care or the delivery of PR for COPD at participating sites while the study was running. Typically, TANDEM facilitators were recruited from neighbouring trusts that were not participating in the study, often from part-time staff who were willing to work an extra day per week for the duration of the study (see Chapter 2 and Steed et al. 66 for a description of screening, training and assessment of TANDEM facilitators).
The co-ordinating supervisor was already involved in the study as a co-applicant (SW). The CBT supervisors were either already known to the study team and invited to join as a supervisor or recruited from an advertisement in the British Association for Behavioural and Cognitive Psychotherapies’ (Bury, UK) official membership magazine CBT Today (delivered free to over 10,000 members across the UK and Ireland) [URL: https://babcp.com/Membership/Join-Us (accessed 26 October 2022)]. Each facilitator was assigned a supervisor based on matching availability of a day that was mutually convenient for clinical supervision.
Trial training for facilitators and practical considerations
The facilitators were good clinical practice-trained to deliver the research aspects of intervention delivery, and a NHS letter of access was arranged if facilitators were seeing patients outside their usual trust. The facilitators were informed about the concept of blinding and the arrangements in place to prevent the researcher who would be collecting outcome data knowing the allocation of the patient.
The trial manager (RS) delivered training in practical aspects of intervention delivery, provided TANDEM intervention materials and organised brief telephone catch-ups following first patient assignment, and then approximately fortnightly, to resolve any queries or concerns related intervention delivery.
Participant pathway
The TANDEM intervention (see Chapter 2) is a stand-alone intervention designed to precede the opportunity to attend routine PR. There is often a delay between referral to PR and starting a course, and the TANDEM intervention was designed to take place in this hiatus. 46 In 2017, when the study was conceived, the median waiting time form referral was around 11 weeks. 55
A course of PR is always preceded by an assessment of the patient and not all referred patients are deemed eligible to attend a course when assessed by their local PR team. 46,78 Therefore, ‘referral to PR’ is actually ‘referral to assessment for PR’. Assessment of who is fit for PR may vary between sites, depending on the range of courses the sites offer (e.g. some sites may offer the option of seated PR or attendance once a week instead of twice weekly sessions). We included participants who met our eligibility criteria and were eligible for referral for assessment for a course of PR. Some TANDEM intervention patients would, therefore, not be offered subsequent PR. However, as the TANDEM intervention is a stand-alone, talking-based intervention, and our primary outcome was mood, patients still had the opportunity to benefit from the intervention.
At the time of this study, UK national guidelines defined eligibility for PR as:46
-
patients with chronic respiratory disease who are functionally limited because of dyspnoea, including patients with a Modified Medical Research Council (mMRC) Dyspnoea score of ≥ 2 and a mMRC score of 1 if they are functionally limited.
The main exclusion criteria were:
-
unstable cardiac disease, locomotor or neurological difficulties precluding exercise (e.g. severe arthritis or peripheral vascular disease)
-
patients in a terminal phase of their illness
-
significant cognitive or psychiatric impairment.
Study participants
People with chronic obstructive pulmonary disease
In the internal pilot we included adults with a confirmed diagnosis of COPD and with moderate to severe airflow limitation,2,3 but the recruiting team felt that there were otherwise eligible patients with very severe airflow limitation who were missing out on the opportunity to participate in the study. In the main trial, after discussion with the Trial Management Group and Trial Steering Committee, we extended eligibility to include patients with very severe airflow limitation.
In addition to meeting the eligibility criteria for referral to their local PR service, eligible participants had to have a Hospital Anxiety and Depression Scale (HADS) score at the baseline screening suggestive of mild to moderate anxiety or depression, or both [i.e. Hospital Anxiety and Depression Scale – anxiety (HADS-A) or Hospital Anxiety and Depression Scale – depression (HADS-D) scores in the range of ≥ 8 to ≤ 15100]. Participants with HADS suggesting severe anxiety or depression were advised (and supported) to seek advice from their GP. Patients who were receiving a psychological intervention, or who had received such an intervention within the preceding 6 months, were excluded; however, patients taking prescribed medication for anxiety or depression remained eligible.
A full list of inclusion and exclusion criteria are presented in Table 4.
| Inclusion criterion | Exclusion criterion |
|---|---|
|
|
Carers of people with chronic obstructive pulmonary disease
Recruited participants were asked if they could identify a ‘particular family caregiver or friend who helps them’ whom they would be happy for us to invite to join the study to examine the effect of the patient-directed TANDEM intervention on carers. In this report, we use ‘carers’ as a shorthand to describe this group. Inclusion criteria for carers, identified by a patient participant, were willingness to participate in the study and being sufficiently fluent in English to be able to complete the questionnaires.
Intervention
Chapter 2 provides a detailed description of the TANDEM intervention and its development, and see also Steed et al. 66 In brief, the intervention consisted of a tailored manualised intervention based on CBAs and self-management support delivered one to one by respiratory healthcare professionals (i.e. physiotherapists, occupational therapists, nurses, physiologists or psychologists working in respiratory services) experienced in working with patients with COPD, and trained to deliver the TANDEM intervention as facilitators. A course of therapy lasted six to eight sessions (plus an optional catch-up session if there had been a break in delivery), depending on the needs of the individual participants, and was delivered in patients’ own homes or at a local clinical setting, according to the patient’s choice. Sessions were 40–60 minutes in duration. Facilitators received regular structured telephone supervision from a CBT therapist supervisor throughout intervention delivery who themselves were overseen by a senior clinical psychologist who acted as a co-ordinating supervisor (co-applicant SW).
On recruitment, all intervention and control arm participants were given a BLF DVD on living with COPD and BLF booklets on COPD and PR. At the time of recruitment, these resources were freely available via the BLF website [now the charity Asthma UK + Lung UK URL: https://shop.auk-blf.org.uk/collections/new-shop-hcp (accessed 26 October 2022)]. If local services preferred, then we substituted the BLF PR booklet with local PR resources.
Participants were directed to their usual healthcare providers if they reported that their clinical condition had deteriorated or if they had developed new health problems. In addition, the TANDEM facilitators could (with participants’ permission) discuss with the co-lead applicants (SJCT or HP, both of whom are GPs) any concerns about the participant’s health or social circumstances that may be affecting the participant’s health.
After completing the course of face-to-face sessions, with the participant’s permission, the TANDEM facilitator sent their healthcare providers a brief, structured written case summary documenting progress and highlighting any need for further support. Between completing the face-to-face intervention and up to 2 weeks after completing PR, facilitators also offered brief (15 minutes or less) weekly or less frequent (dependent on participant preference) CBA telephone support.
Usual care
In addition to the BLF materials, participants randomised to the control arm received usual care that followed local arrangements for the provision of guideline-recommended care, including PR. 2 Control participants were eligible for referral to IAPT services or any other psychological or mental health services at the discretion of their usual healthcare providers.
Recruitment of participants with chronic obstructive pulmonary disease
Potential participants with COPD were recruited from secondary, community and primary care, and from referrals to PR services in participating sites. Advertisements in the form of study leaflets were placed in respiratory clinics and other relevant clinical settings. Potentially eligible people with COPD were asked by their clinicians or clinical research staff if they were interested in being contacted by the study team to learn more about the study and to discuss participation. In addition, primary care teams in participating sites identified potentially eligible participants by searching their primary care COPD registers. The primary care teams wrote to the potentially eligible participants, informing them about the study and inviting them to contact the study team if they might be interested in participating.
After learning more about the trial from the study team, potential participants who remained interested were offered a visit at home or at a convenient NHS location (based on the preference of participants) to assess their eligibility for the study. At this visit, with the potential participant’s verbal consent, a TANDEM researcher asked the individual to complete the HADS and checked their lung function using portable spirometry equipment [Microloop with SPCS software (later discontinued) or CareFusion Microlab MK& Desktop, both Becton Dickinson, San Diego, CA, USA] to confirm eligibility. The researchers were trained in operating the spirometers, and conducted the tests and interpreted results in accordance with the spirometry standard operating procedure developed for the study [based on advice from the Association of Respiratory Technology and Physiology (Lichfield, UK),101 Berkshire Healthcare NHS Foundation Trust (Bracknell, UK)102 and Education for Health]. Any unclear results or other uncertainties regarding eligibility were referred to a clinician with expertise in respiratory management and interpreting spirometry (HP or SJCT). Eligible participants then provided written informed consent to join the study and, with supervision, self-completed the baseline assessment questionnaire at the same visit or at a booked second visit with the researcher, if preferred.
Carers
If the study participants identified a ‘carer’ whom they were happy for us to approach we contacted the carer by letter or in person (if they were present when the TANDEM researcher visited the participant) and invited them to consider participating in the study.
Randomisation, concealment and blinding
After completion of baseline data collection, patient participants were randomised to intervention or control arms in a 1.25 : 1 ratio. Randomisation was stratified by NHS trust, and within each trust minimisation was used to balance allocations according to baseline HADS-A and HADS-D scores100 [categories for each were 0–7, 8–10, 11–15 (see Outcomes for details of scoring)], breathlessness, which was assessed with the mMRC scale (0–2 vs. 3–4),103 and self-reported smoking status (smoker vs. non-smoker). A random element was included in the minimisation, such that the allocation that reduced the imbalance was selected with probability 0.8. Randomisation was conducted using a central online randomisation service maintained by the PCTU at the Queen Mary University of London to ensure allocation concealment.
To maintain blinding, the TANDEM researcher who informed patients by telephone of their allocation was based at another study site (therefore, preserving blinding of the local research team who would collect the outcome data). The trial manager (RS) was also unblinded and so was also able to inform patients of their allocation. When collecting outcome data, research staff used a standardised explanation at the beginning of data collection, asking participants not to reveal if they had received the TANDEM intervention. All instances of accidental unblinding during outcome data collection were recorded. If the participant was randomised to the intervention arm, then the TANDEM researcher or the trial manager informed a local TANDEM facilitator with capacity to take on a new participant so that delivery of the intervention could be started.
With participants’ permission, participants’ GPs and respiratory consultants (if recruited from secondary care) were informed by letter that the patient had joined the study, but they were not informed of allocation. The letters also included a brief description about the study and the study inclusion and exclusion criteria, as well as copies of the participant’s spirometry results and HADS-A and HADS-D scores (with a note on interpretation of the HADS). If participants had not yet received a referral to PR (all participants being eligible for a referral), the clinicians were asked to make the referral. GPs were also sent a copy of the signed consent form, which included patients’ consent to allow the study team to access their primary care records.
Data collection and management
Three types of trial data were collected for all patient participants:
-
Patient-reported data, which included validated questionnaire outcomes and reported resource use, were collected at baseline and at 6 and 12 months post randomisation.
-
Healthcare resource use data from primary care records.
-
Data from PR services on the uptake and completion of PR.
Participants self-completed questionnaires at baseline and at 6 and 12 months post randomisation, supervised by researchers at the study sites, with provision for postal data collection or by telephone if requested by participants. If the participant had difficulty reading or otherwise physically completing the questionnaires, then the researcher offered to read the questions and, if necessary, act as an amanuensis. We had permission to attempt telephone follow-up of our primary clinical and health economic outcomes if participants failed to complete questionnaires.
With the exception of the visual analogue scale of the EuroQoL-5 Dimensions, five-level version (EQ-5D-5L) (which has to be recorded on paper), we intended that participants would enter questionnaire data directly using OpenClinica software (OpenCLinica, LLC, Waltham, MA, USA) via a study tablet computer (with 3G/4G connection), unless the participant preferred to use a paper version of the questionnaire. In practice, delays in delivery of the 3G/4G-enabled tablets or patient preference meant that most participants completed paper data collection forms. Carer baseline and outcome data were collected either face to face or by postal questionnaires.
Data from primary care were collected at 12–14 months from the date of randomisation. In accordance with our data management plan, we offered a range of modes of data collection:
-
The researcher visited the practice and collected the data on a paper form.
-
The researcher visited the practice and transferred the data electronically into the secure study server via the secure file transfer protocol (SFTP).
-
The researcher visited the practice and downloaded the data onto an encrypted drive and uploaded it via SFTP.
-
A member of the practice team sent the data securely via nhs.net to nhs.net accounts, or via the SFTP.
Data on attendance and completion at PR were collected directly from the PR services for each study participant at 1 year after randomisation.
All study data were uploaded onto a dedicated folder on the secure virtualised environment at the Barts Cancer Centre, Queen Mary University of London. The Barts Cancer Centre is where all data analysis by the PCTU is conducted. The Barts Cancer Centre environment requires dual-factor authentication to access the portal, and the folders where the data are stored are accessible to only appropriate members of the PCTU and the TANDEM study team.
Outcomes
Primary outcomes
The co-primary outcomes are participant depression and anxiety at 6 months after randomisation, measured using the HADS-A and HADS-D scores. 100 The 14-item HADS questionnaire has two subscales: (1) seven questions related to anxiety (i.e. HADS-A) and (2) seven questions related to depression (i.e. HADS-D). Each item is scored from 0 to 3 and, therefore, participants score between 0 and 21 on each subscale, with higher scores indicating worse symptoms. HADS has validated cut-off points for probable mild anxiety or depression (i.e. a score of ≥ 8 on each subscale) and for severe anxiety or depression (i.e. a score of > 15 on each subscale). 104 Using a score of ≥ 8 as a cut-off point for anxiety/depression, the HADS has high sensitivity (80%) and specificity (90%),103 although, probably in common with most single instruments, it does not provide good separation between symptoms of anxiety and depression. 105
The HADS is commonly used in studies of COPD, specifically in the context of CBT interventions for breathlessness in COPD106,107 and PR for anxiety and depression. 108,109
Secondary outcomes
The HADS-A and HADS-D scores were collected at 12 months after randomisation. In addition, the following measures were obtained at both 6 and 12 months.
Beck Depression Inventory II and Beck Anxiety Inventory
The Beck Depression Inventory II (BDI II)110 and Beck Anxiety Inventory (BAI)111 are both widely used in psychiatric research. BDI II scores range from 0 to 63, with higher scores indicating worse symptoms. BDI II scores in the range of 14–19 indicate mild depression and scores in the range of 20–28 suggest moderate depression. 112 BAI scores range from 0 to 63, with higher scores indicating worse symptoms. BAI scores of ≤ 21 suggest low anxiety and scores in the range of 21–35 suggest moderate anxiety. 113
Health-related quality of life
Health-related quality of life was collected with the validated, respiratory-specific St George’s Respiratory Questionnaire (SGRQ). 114 The SGRQ measures health impairment in people with COPD, incorporating three subscales (i.e. symptoms, activity and impacts). 115 A total score is computed (out of 100), which represents the percentage of overall impairment (where 100 represents the worst overall possible health status).
Social engagement and support
To assess social engagement and support we used the five-question Social Integration and Support subscale of the Health Education Impact Questionnaire (heiQ). 116 Social activity was measured using an adapted version of the UK Time Use Survey,117 which listed activities people might do in their spare time (including social visits) and asked how many times, and for how long, the interviewee had engaged in the activity in the previous week and whether this was alone or with another person. 118
Brief Illness Perception Questionnaire
To understanding how the intervention might be working we used the Brief Illness Perception Questionnaire (B-IPQ). The B-IPQ is a nine-item questionnaire that examines the cognitive and emotional representations an individual holds of their illness. 119
Smoking status and tobacco consumption
Past and current smoking status and tobacco consumption and e-cigarette use was assessed using the Office for National Statistics survey report on smoking-related behaviour and attitudes,120 and the National Asthma and COPD audit programme. 121
Health status
The EQ-5D-5L was used to assess health status for the health economic evaluation. 122
Other data collected at baseline and at 6 and 12 months
A modified Client Services Receipt Inventory123 was used to collect data from patients on their primary care and hospital use and any equipment supplied or bought to help with their respiratory condition (e.g. stair lift, oxygen concentrator, walking frame) in the previous 6 months. In addition, we collected data on current medication use and any current help with personal care (e.g. washing, dressing, housework, shopping).
Baseline assessment
In the baseline questionnaire we also collected:
-
demographic data (i.e. gender, marital status, employment status)
-
educational status (i.e. formal education, age on completion of full-time education)
-
home oxygen status
-
age when COPD was first diagnosed
-
presence of other comorbidities
-
previous attendance at/completion of a PR course
-
mMRC Dyspnoea score. 49
Pulmonary rehabilitation data
For all study participants, data on attendance at assessment for PR, attendance at and adherence to a PR course and completion of the PR course (as defined by the local service) were collected between 6 and 12 months after randomisation.
Healthcare resource use
Data on COPD-related healthcare resource use, both scheduled reviews and unscheduled consultations in primary and secondary care, and medication use for the 12 months from baseline to follow-up, were collected from primary care electronic health records, where possible.
Carer data
Consenting carers identified by study participants completed questionnaires at baseline and at the 6- and 12-month follow-ups. At baseline, the questionnaires also asked for the carer’s age, gender and their relationship to the study participant.
The questionnaire consisted of only two instruments: (1) the Warwick–Edinburgh Mental Well-being Scale (WEMWBS)124 and (2) the Zarit Caregiver Burden Scale (ZBI), which assesses perceived burden of caring125 and was the most commonly used quantitative instrument in a review of caregiver burden in COPD. 126
Delivery of the TANDEM intervention
The TANDEM facilitator completed a proforma-based log each time they delivered a session of the CBA intervention to a participant. The proforma-based log detailed:
-
the date and session number [i.e. 1–8, occasionally more if a session was repeated due to (say) intervening participant illness]
-
the location of the session (i.e. patient’s home/GP clinic/hospital or community clinic)
-
any reasons for failure to deliver the session as planned (e.g. participant was unwell)
-
the names of the topics delivered in that session (based on the TANDEM facilitators’ training and manual)
-
any patient educational materials and handouts delivered in that session
-
the presence of a carer in the session.
At the final session, the facilitator reported the date of discharge from the one-to-one sessions and details of the patient’s consent (or otherwise) to share a proforma summary letter of the TANDEM intervention they had received with their GP and/or respiratory healthcare team.
Recordings of the TANDEM intervention sessions
With the consent of participant, TANDEM sessions were recorded onto encrypted digital recording devices for fidelity assessment (see Chapter 6).
Sample size
We calculated that, in a trial with no clustering, 153 participants in each trial arm would be sufficient to detect a difference of 1.7 points on the HADS-A and a difference of 1.5 points on the HADS-D, with 90% power at the 2.5% significance level, assuming standard deviations (SDs) of 4.2 for anxiety and 3.6 for depression. 127 These effect sizes are equivalent to a SMD of around 0.4 and are in line with the minimum clinically important difference for HADS in COPD. 128 We assumed an intracluster correlation between therapists of 0.01 and 24 participants per therapist, giving a design effect (sample size inflation factor) of 1.23, meaning that the number of participants in the intervention arm would increase to 189, with 153 participants in the control arm, making a total of 342 participants. To allow for a 20% drop-out rate, we set out to recruit 430 participants in total, allocated in a ratio 1.25 : 1 (intervention arm, n = 240; control arm, n = 190). We kept the ratio at 1.25 : 1 because an allocation ratio of 1 : 1 would have required an even larger sample size. 129
Statistical analysis
General analysis principles
The primary analysis was by intention to treat. The intention-to-treat approach does not require that every participant should have an outcome recorded, although every effort should always be made to collect complete data. 130 The analysis included all non-missing outcomes, an approach that is valid under an assumption that outcomes are missing at random (i.e. missingness depends systematically on only variables that are incorporated in the analysis). To investigate the sensitivity of our conclusions to this assumption, we followed the simple strategy, suggested by White et al. ,131 of modelling the impact of differences between missing and non-missing outcomes in the two trial arms on the estimated treatment effect, for the two primary outcomes. For example, if the HADS-A outcomes that were missing at 6 months were (had we been able to observe them) 5 scale points lower, on average, than the non-missing outcomes, then what would be the impact on our estimated treatment effect? We also present tables of baseline characteristics of participants according to whether or not they were included in the analysis of each of the primary outcomes, that is whether they had at least one assessment at 6 or 12 months or were missing both assessments (and in the latter case we distinguish participants who died before 6 months).
A secondary analysis of each of the primary outcomes estimated the complier-average causal effect (CACE), that is the effect of the intervention in participants who would have complied with the intervention, estimated in a way that respects the randomisation. 132 A participant was considered to have complied with the intervention (for the purpose of this CACE analysis) if they completed two or more CBA sessions.
Participants identified post randomisation as having been ineligible at the time they were randomised were excluded from all analyses and these participants were counted separately from the randomised groups in the CONSORT (Consolidated Standards of Reporting Trials) flow chart.
Treatment effects are presented with 95% CIs and p-values. We have avoided dichotomising results as being statistically ‘significant’ or otherwise, although the statistical analysis plan did prespecify that in the event a decision rule was required to determine statistical significance for the two co-primary outcomes, then a Hochberg procedure would be employed (i.e. if either outcome had a p-value of < 0.025 then that outcome would be considered statistically significant, and if both outcomes had p-values of < 0.05 then both would be considered significant). 133
Analyses of primary and secondary outcomes
All outcomes other than smoking were analysed in a similar way (the analysis of smoking is described below). Each of the measures assessed at 6 and/or 12 months was analysed using a mixed linear regression model with adjustment for fixed effects of baseline HADS-A, HADS-D, breathlessness (0–2 vs. 3–4 on the mMRC scale), smoking (smoker vs. non-smoker), NHS trust and (for outcomes other than HADS-A and HADS-D) the measurement of that outcome at baseline. All non-missing observations of the outcome at 6 or 12 months were included in the analysis, which included a random effect of participant. The treatment effects at 6 and 12 months (i.e. the mean difference between intervention and control at each time point) were each extracted from the joint model of 6- and 12-month outcomes. Analyses also allowed for clustering by facilitator in the intervention arm by adjusting for a random effect of facilitator. Where a participant’s facilitator changed during the course of the trial, for example if a facilitator withdrew and a new facilitator was assigned, then the participant was clustered with the facilitator who delivered the majority of their sessions. If a participant had equal numbers of sessions under multiple facilitators, then they were clustered with their first facilitator. Where a participant randomised to the intervention arm did not have a facilitator, then that participant was treated as a cluster by themselves. Individual participants in the control arm were also treated as clusters of size one. The mixed model allowed for heteroscedasticity (i.e. a different variance) to distinguish between clusters that were defined by a facilitator in the intervention arm on the one hand, and clusters of just one individual in the control or intervention arm on the other hand. 134 A Satterthwaite correction was applied to correct for the relatively small number of clusters, as recommended by Candlish et al. 134
We intended the analysis of smoking (smoker vs. non-smoker) at 6 and 12 months to work in a similar way, using a mixed logistic regression model, although we anticipated that if there were few changes over time in this binary outcome then there could be problems with model convergence. The statistical analysis plan prespecified a strategy of successive steps for simplifying analyses in this eventuality by (1) removing adjustment for the minimisation factors, (2) removing adjustment for the baseline measurement of outcome, (3) splitting the two time points into two separate models and (4) removing the random effect of facilitator, as required for convergence. The analysis presented in this report used separate models for the 6- and 12-month smoking outcome, and did not adjust for any covariates, but did include a random effect of facilitator.
The CACE analysis for each of the two primary outcomes was conducted using a latent class modelling approach, with the help of the gllamm command in Stata® (StataCorp LP, College Station, TX, USA) [URL: www.gllamm.org/books/cace.html (accessed 26 October 2022)]. The latent class modelling approach used the same mixed modelling approach as described above, except that we analysed the 6- and 12-month outcomes separately for each of HADS-A and HADS-D, as a multilevel mixed model proved too difficult to parameterise into the specification of the CACE analysis in gllamm.
Sensitivity analyses
We conducted the following sensitivity analyses for the two primary outcomes:
-
A complete-case analysis, that is an analysis that included only participants with outcome data at 6 and 12 months.
-
An analysis in which participants with partially incomplete HADS-A or HADS-D data (i.e. with some items of the scale missing) at 6 and 12 months had their missing outcomes imputed (see below).
-
An analysis excluding participants with baseline HADS-A or HADS-D score of < 8.
-
An analysis with time to rehabilitation as an additional covariate.
-
An analysis excluding participants from the internal pilot stage of the trial.
To impute HADS-A or HADS-D outcomes when some items of the scale were incomplete at 6 or 12 months, we followed the ‘half-rule’ suggested by Bell et al.,135 in which scores are imputed using the relevant subscale mean, as long as half the items in the subscale are non-missing.
Anomaly in allocation ratio
During the trial, an anomaly in the allocation ratio produced by the online randomisation system became apparent. Specifically, over the first 69 randomisations the observed allocation ratio was around 1.25 : 1, as expected, but over the next 70 randomisations the observed allocation ratio was around 5 : 1. The deviation from the expected allocation ratio during this second period of randomisations triggered a corrective action and prevention plan overseen by the sponsor and, with the approval of the Data Monitoring and Ethics Committee, that included migrating the randomisation system to a new platform. For the remainder of randomisations, an allocation ratio of 1 : 1 was specified to return the overall allocation ratio to a figure closer to 1.25 : 1, and the observed allocation ratio over this third randomisation period was close to 1 : 1. In addition, as an additional sensitivity analysis for the two primary outcomes, we included randomisation period (i.e. period 1, period 2 or period 3) as an additional covariate. We also present tables of baseline characteristics of participants in different randomisation periods (and in different trial arms) to confirm the balance of characteristics and to allow an exploration of whether or not these characteristics might have differed in different periods; however, as the trial recruited fairly rapidly, we did not expect this to be the case.
COVID-19 and impact of lockdown
In late March 2020, the UK Government imposed ‘lockdown’ restrictions to reduce the spread of COVID-19. We performed additional sensitivity analyses for the two primary outcomes to explore the impact of the COVID-19 pandemic on our findings. For this purpose, the data were split in two different ways: (1) according to whether data were collected before or after 19 March 2020 and (2) according to whether the mode of intervention delivery was fully face to face or fully/partially via remote sessions (or, alternatively, no CBA at all, in which case we could not define the mode of delivery). For each way of splitting the data, we conducted a sensitivity analysis that included the split as an additional covariate and we also investigated the split as a potential treatment effect modifier. In addition, we present tables of baseline characteristics of participants divided according to the same data splits to allow an exploration of whether or not characteristics of trial participants changed.
Adverse events
All adverse events (AEs) and serious adverse events (SAEs) were recorded and reported in accordance with the Data Monitoring Ethics Committee’s and sponsors’ requirements, and following the standard operating procedures of the Joint Research Management Office for Barts Health NHS Trust and Queen Mary University of London.
Any changes to the project protocol
Changes to the statistical analysis plan
Version 1.0 of the statistical analysis plan was signed off in February 2020, and formed the basis of a publication in the journal Trials in October 2020. 99 Subsequently, in May 2021, prior to the database being locked and unblinded for analysis, version 2.0 of the statistical analysis plan was agreed by the senior statistician and chief investigator, and approved by the independent statistician on the Trial Steering Committee. Substantive changes from version 1.0 to version 2.0 were the inclusion of CACE analyses, sensitivity analyses for the COVID-19 pandemic and randomisation periods, the use of the ‘half-rule’ for imputing partial HADS scores and the detail of the sequential strategy for managing problems with model convergence, all of which are described above.
Changes to the protocol
Any changes made to the protocol underwent ethics and governance approvals. Table 5 lists the changes made to the protocol over the course of the trial
| Document date and protocol version number | Details of amendment |
|---|---|
| 4 April 2017: version 2.0 | The change proposed was at the randomisation level only. The decision was made that randomisation would be performed by stratification by NHS trust, and using minimisation within each stratum with a random element, balanced for important patient characteristics to ensure treatment groups are well matched at baseline |
| 13 June 2017: version 3.0 | Update of recruitment strategy (i.e. producing a new letter for approved study leaflet and clinical teams being able to approach patients who have previously declined PR) |
| 17 August 2017: version 4.0 | Addition of ‘very severe COPD’ and ‘FEV1 predicted < 80%’, FEV1 predicted < 30–80%’ where appropriate |
| 22 April 2018: version 6.0 (approved in subcommittee meeting) following submission of version 5.0 for approval | Refinements and revisions to the study protocol and study documentation to be used in the main trial based on learning from the pilot and the addition of new study sites. The revisions mostly include changes in the form of clarifications that would be used by the research team to operationalise the protocol. One change in the study documentation related to informing and requesting consent from participants about contacting them 5 years following completion of the study to assess their long-term quality of life/survival |
| 29 May 2019: version 8.0 (approved in subcommittee meeting) following submission of version 7.0 for approval | Collection of healthcare use data from NHS Digital as part of the approved study protocol (version 8.0; 29 May 2019). To operationalise this, we sought written permission from study participants. In addition, a brief statement has been added in the screening results letter to ensure safety of study team members doing fieldwork (i.e. study visits conducted in patient homes). The application to use NHS Digital was eventually withdrawn because of extensive delays by NHS Digital in reviewing the application |
| 6 March 2020: version 9.0 | Extending the study timeline, following approval from funder, by 12 months, with study due for completion by 30 June 2021 (original study timeline is 30 June 2020). Reason for the extension is to complete patient recruitment and then completion of the 6- and 12-month follow-up assessments and analysis |
Chapter 4 Results: clinical effectiveness trial
Participating sites
Twelve NHS trusts (including four trusts that had been involved in the pilot) and 51 primary care practices from five NHS Clinical Commissioning Groups were recruited into the study (Figure 7). Some NHS trust sites did not want recruitment to be extended into primary care because their PR services were at capacity and could not take any more referrals. Overall, we had 30 recruitment sites, including a wide range of urban, suburban and rural areas. Table 6 provides a list of participating sites and the date they joined the study (presented as their site approval dates). All participating sites remained involved until the end of the study.
FIGURE 7.
Map of study sites (NHS trusts and NHS Clinical Commissioning Groups).

| Study site | Site approval date/date first practice approved |
|---|---|
| Participating NHS trust | |
| Homerton University Hospital NHS Foundation Trust | 25 April 2017 and 23 May 2018 |
| Imperial College Healthcare NHS Trust | 27 July 2017 and 7 June 2018 |
| Guy’s and St Thomas’ NHS Foundation Trust | 20 August 2018 |
| King’s College Hospital NHS Foundation Trust | 26 February 2019 |
| Berkshire Healthcare NHS Foundation Trust | 14 January 2019 |
| Southern Health NHS Foundation Trust | 26 October 2018 |
| Sandwell and West Birmingham Hospitals NHS Trust | 3 December 2018 |
| South Warwickshire NHS Foundation Trust | 16 July 2018 |
| University Hospitals Coventry and Warwickshire NHS Trust | 25 October 2018 |
| Leicestershire Partnership NHS Trust | 11 April 2017 and 21 June 2018 |
| University Hospitals of Leicester NHS Trust | 18 May 2017 and 4 June 2018 |
| North Bristol NHS Trust | 2 April 2019 |
| Participating NHS CCGs | |
| NHS City and Hackney CCG (seven general practices) | 6 July 2017 |
| NHS South London CCGs (12 general practices) | 14 January 2019 |
| NHS West London CCGs (nine general practices) | 10 January 2019 |
| NHS Coventry and Rugby CCG (11 general practices) | 7 December 2018 |
| NHS Leicestershire CCGs (12 general practices) | 10 August 2017 |
Participant recruitment and flow through the study
The recruitment, baseline characteristics and outcomes for TANDEM intervention participants and their carers are reported in this chapter. Details of the recruitment and the characteristics of the TANDEM intervention facilitators, the number of TANDEM intervention courses delivered by the facilitators and the fidelity of TANDEM intervention delivery are reported in Chapter 6.
Initial recruitment strategies
Figure 8 illustrates the initial approach to potential participants.
FIGURE 8.
Initial approach and eligibility checking.
A total of 4491 potentially eligible participants were approached by clinical teams in primary, community or secondary care. Most participants were approached face to face by their clinician as they attended outpatient or primary care appointments or were recruited from the wards following an admission for an exacerbation of COPD, but about one-third (1487/4491) of participants were approached by mailings based on COPD registers in primary care. A total of 2191 (49%) participants agreed to be contacted by the research team to find out more about the study and 49% (n = 1062) of these participants were potentially eligible and proceeded to formal screening for the study (see Figure 8). Of the participants screened, 441 (42%) met the spirometric and HADS eligibility criteria, but 15 of the participants were not randomised because they decided not to participate (n = 8), because another individual in the same household had already been recruited to the study (and, therefore, there was a risk of contamination) (n = 2) or because the participant was later found to have been eligible for randomisation but had been deemed ineligible in error (n = 5). A total of 425 participants were randomised. Two participants were randomised (both to the intervention arm) in error, as they did not meet the eligibility criteria; however, these errors were picked up between the statistician and trial manager during the ongoing cleaning process for Data Monitoring and Ethics Committee reports and, therefore, were found in an unbiased way. As described in Chapter 3, the two participants were excluded from all analyses and are counted separately from the analysed randomised groups in the CONSORT flow chart (see Figure 9).
FIGURE 9.
The CONSORT flow diagram.

Flow through the study
The flow of participants through the trial is illustrated in Figure 9. Blinding of data collection to allocation was maintained in 409 (96.5%) cases. Unblinding in 15 cases occurred because a participant contacted the researcher to reschedule an appointment with their facilitator.
Loss to follow-up, study withdrawals and deaths
Follow-up data were collected for both arms at 6 and 12 months post randomisation. At 6 months, HADS-A and HADS-D (primary outcome data) were available for 205 (85%) and 204 (84%) of participants randomised to the intervention, respectively, and 164 (91%) of control participants. At 12 months, HADS-A and HADS-D (secondary outcome data) were available for 191 (79%) and 190 (79%) participants randomised to the intervention, respectively, and for 150 (83%) and 152 (84%) control participants, respectively. More participants withdrew from the intervention arm of the study (n = 16, 6.6%) than from the control arm (n = 5, 2.8%), and there were more deaths in the intervention arm than in the control arm [13 (5.4%) and 3 (1.7%), respectively].
Baseline characteristics of participants and carers
Baseline characteristics of the 423 recruited participants are shown in Table 7. The median age of participants was 69 [interquartile range (IQR) 62–75] years, 213 (51%) were male and 176 (42%) lived alone. Only 40 (10%) participants described themselves as working or in paid employment, and the majority (329/416, 79%) had completed full-time education between the ages of 13 and 16 years. The median age at diagnosis of COPD was 60 (IQR 53–67) years and few were on home oxygen (31/419, 7.4%). Participants reported significant breathlessness, with 340 (77%) participants being at mMRC level 2 or worse (i.e. walking slower than others of the same age on the level or worse) and 78 (18%) participants reporting being too breathless to leave the house. Fewer than half (175/422, 41%) of the participants had previously attended PR, with 70% (121/173) of the participants who answered the question reporting that they had been able to complete the course. Comorbidities were common, particularly arthritis (161/423, 38%) and hypertension (162/423, 38%), and 30% (n = 128) of participants were still smoking. Overall, the intervention and control participants had similar baseline characteristics.
| Summary measurea | Trial arm | Overall | Data available: N (intervention, n; usual care, n) | |
|---|---|---|---|---|
| Intervention (N = 242) | Usual care (N = 181) | |||
| Age (years), median (IQR) | 68 (61–76) | 69 (63–74) | 69 (62–75) | 411 (237; 174) |
| Gender, n (%) | 422 (242; 180) | |||
| Male | 130 (53.7) | 83 (46.1) | 213 (50.5) | |
| Female | 112 (46.3) | 97 (53.9) | 209 (49.5) | |
| Living circumstances, n (%) | 419 (239; 180) | |||
| Lives alone | 108 (45.2) | 68 (37.8) | 176 (42.0) | |
| Lives with spouse or partner | 83 (34.7) | 72 (40.0) | 155 (37.0) | |
| Lives with adult family member | 33 (13.8) | 22 (12.2) | 55 (13.1) | |
| In paid employment/working, n (%) | 26 (10.8) | 14 (7.8) | 40 (9.5) | 420 (241; 179) |
| If working, hours per week in paid employment/working, median (IQR) | 27 (15–37) | 36 (35–38) | 35 (16–37) | 39 (26; 13) |
| Formal education and age completed full-time education, n (%) | 416 (238; 178) | |||
| Had formal education | 238 (98.3) | 178 (98.9) | 416 (98.6) | 422 (242; 180) |
| ≤ 12 years | 4 (1.7) | 5 (2.8) | 9 (2.2) | |
| 13–16 years | 190 (79.8) | 139 (78.1) | 329 (79.1) | |
| 17–18 years | 27 (11.3) | 13 (7.3) | 40 (9.6) | |
| > 18 years | 17 (7.1) | 21 (11.8) | 38 (9.1) | |
| COPD | 401 (234; 167) | |||
| Age (years) first diagnosed with COPD, median (IQR) | 60 (53–68) | 60 (53–67) | 60 (53–67) | |
| Recent (previous 6 months) hospitalisation for exacerbation COPD, n (%) | 58 (24.0) | 50 (27.6) | 108 (25.5) | |
| On home oxygen, n (%) | 18 (7.5) | 13 (7.3) | 31 (7.4) | 419 (240; 179) |
| Attended PR previously, n (%) | 99 (40.9) | 76 (42.2) | 175 (41.5) | 422 (242; 180) |
| Other long-term health problems (may have more than one), n (%) | 423 (242; 181) | |||
| Heart disease | 36 (14.9) | 25 (13.8) | 61 (14.4) | |
| Diabetes | 39 (16.1) | 25 (13.8) | 64 (15.1) | |
| Arthritis | 91 (37.6) | 70 (38.7) | 161 (38.1) | |
| High blood pressure | 90 (37.2) | 72 (39.8) | 162 (38.3) | |
| Asthma | 60 (24.8) | 49 (27.1) | 109 (25.8) | |
| Epilepsy | 7 (2.9) | 2 (1.1) | 9 (2.1) | |
| Other | 120 (49.6) | 91 (50.3) | 211 (49.9) | |
| None | 26 (10.7) | 16 (8.8) | 42 (9.9) | |
| Smoking status, n (%) | 423 (242; 181) | |||
| Current smoker | 74 (30.6) | 54 (29.8) | 128 (30.3) | |
| Ex-smoker | 162 (66.9) | 124 (68.5) | 286 (67.6) | |
| Never smoked | 6 (2.5) | 3 (1.7) | 9 (2.1) | |
| Current smoker: pack years, median (IQR) | 14.5 (7.2–30.0) | 25.0 (5.0–46.2) | 17.1 (6–34.7) | 107 (62; 45) |
| Current vaper (including e-cigarettes), n (%) | 7 (14.0) | 10 (21.3) | 17 (17.5) | 97 (50; 47) |
| mMRC Breathlessness Scale, n (%) | 443 (242; 181) | |||
| Not troubled by breathlessness except on strenuous exercise | 3 (1.2) | 0 (0.0) | 3 (0.7) | |
| Short of breath when hurrying on the level or walking up a slight hill | 42 (17.4) | 38 (21.0) | 80 (18.9) | |
| Walks slower than other people of the same age on the level | 79 (32.6) | 57 (31.5) | 136 (32.2) | |
| Stops for breath after walking about 100 yards or after a few minutes on the level | 75 (31.0) | 51 (28.2) | 126 (29.8) | |
| Too breathless to leave the house or breathless when dressing or undressing | 43 (17.8) | 35 (19.3) | 78 (18.4) | |
We recruited 43 carers to the study. The carers were randomised with the participants (intervention arm, n = 24; control arm, n = 19). The characteristics of carers are summarised in Table 8. Characteristics are similar between intervention and control arm participants.
| Summary measurea | Trial arm | Overall | Data available: N (intervention, n; usual care, n) | |
|---|---|---|---|---|
| Intervention (N = 23) | Usual care (N = 19) | |||
| Age (years), median (IQR) | 66.5 (54.5–74) | 68 (64–74) | 68 (62, 74) | 42 (24; 18) |
| Gender, n (%) | 43 (24; 19) | |||
| Male | 6 (25.0) | 6 (31.6) | 12 (28.0) | |
| Female | 18 (75.0) | 13 (68.4) | 31 (72.1) | |
| Relationship to participant, n (%) | 42 (23; 19) | |||
| Son | 1 (4.3) | 0 (0.0) | 1 (2.3) | |
| Daughter | 2 (8.7) | 1 (5.3) | 3 (7.1) | |
| Other family member | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Spouse/partner | 20 (87.0) | 18 (94.7) | 38 (90.5) | |
Baseline patient questionnaire scores are shown in Table 9 (see Chapter 3 for a description of the instruments used).
| Summary measure | Trial arm | Overall | Data available: N (intervention, n; usual care, n) | |
|---|---|---|---|---|
| Intervention (N = 242) | Usual care (N = 181) | |||
| HADS, mean (SD) | 422 (242; 180) | |||
| HADS-A total score | 9.7 (3.1) | 9.9 (3.3) | 9.8 (3.2) | 423 (242; 181) |
| HADS-D total score | 9.2 (3.1) | 9.1 (3.1) | 9.1 (3.1) | 423 (242; 181) |
| BDI II and BAI, mean (SD) | ||||
| BDI II total score | 20.2 (8.8) | 20.7 (10.2) | 20.4 (9.4) | 402 (234; 168) |
| BAI total score | 16.6 (10.3) | 16.6 (10.2) | 16.6 (10.2) | 389 (223; 166) |
| SGRQ, mean (SD) | ||||
| Overall score | 59.6 (15.1) | 58.6 (15.4) | 59.2 (15.2) | 418 (240; 178) |
| Symptoms score | 63.8 (20.7) | 62.4 (23.2) | 63.2 (21.8) | 422 (242; 180) |
| Activity score | 78.6 (18.2) | 77.6 (15.9) | 78.2 (17.2) | 419 (240; 179) |
| Impact score | 47.4 (17.0) | 46.7 (18.1) | 47.1 (17.4) | 419 (240; 179) |
| B-IPQ, mean (SD) | ||||
| Consequences score | 6.4 (2.1) | 6.6 (2.2) | 6.5 (2.2) | 418 (240; 178) |
| Timeline score | 9.5 (1.3) | 9.4 (1.5) | 9.5 (1.4) | 417 (240; 179) |
| Personal control score | 4.7 (2.7) | 4.7 (2.8) | 4.7 (2.7) | 416 (239; 179) |
| Treatment control score | 6.5 (2.4) | 6.8 (2.5) | 6.6 (2.5) | 418 (240; 178) |
| Identity score | 6.8 (1.9) | 6.8 (2.1) | 6.8 (2.0) | 420 (241; 179) |
| Concern score | 7.4 (2.6) | 7.5 (2.5) | 7.4 (2.6) | 419 (240; 179) |
| Coherence score | 7.2 (2.7) | 7.3 (2.5) | 7.2 (2.7) | 419 (240; 179) |
| Emotional response score | 6.4 (2.7) | 6.5 (2.8) | 6.4 (2.7) | 420 (241; 179) |
| heiQ, mean (SD) | ||||
| Social engagement score | 2.5 (0.5) | 2.6 (0.6) | 2.6 (0.6) | 417 (237; 180) |
| Time Use Survey, median (IQR) | ||||
| Time (minutes) spent doing activities over last 4 days | 270 (135–540) | 300 (143–570) | 270 (135–540) | 369 (209; 160) |
The mean HADS-A and HADS-D scores, overall, were 9.8 (SD 3.2) and 9.1 (SD 3.1), respectively, which is in the mild symptoms range (8–10). 105 The mean BDI II score at baseline for TANDEM study participants was 20.4 (SD 9.4), suggesting symptoms of moderate depression. 112 The mean BAI score at baseline for TANDEM participants was 16.6 (SD 10.2), suggesting symptoms of mild anxiety. 113 At baseline, TANDEM participants had a high total SGRQ score (mean 59.2, SD 15.2), suggesting significant overall health impairment. 115 (For comparison, a normal score in a healthy individual is around 6.)
The scores for all the instruments were similar between the two groups.
Baseline carer questionnaire scores for the ZBI and WEMWBS are presented in Table 10. Both groups’ ZBI scores are in the ‘mild to moderate’ burden score range (i.e. 20–40). 125 Comparison of the ZBI scores between the two arms indicated that caregivers in the intervention arm tended to report, on average, higher burden than those in the control arm. The WEMWBS score ranges from 14 to 70, with higher scores representing better mental well-being and with the average population score being 50. 136 Carers from the control arm of the study tended to have higher WEMWBS scores than carers from the intervention arm.
| Summary measurea | Trial arm | Overall | Data available: N (intervention, n; usual care, n) | |
|---|---|---|---|---|
| Intervention (N = 25), mean (SD) | Usual care (N = 19), mean (SD) | |||
| ZBI | 27.3 (12.5) | 20.6 (11.2) | 24.3 (12.3) | 38 (21; 17) |
| WEMWBS | 46.5 (10.5) | 55.5 (11.3) | 50.9 (11.7) | 39 (20; 19) |
Participants’ primary and secondary outcomes
Data completeness
Appendix 1, Table 24, provides a summary of completeness of questionnaire data at 6 and 12 months.
Primary outcome analysis
Results are presented in Table 11 as a mean difference between the intervention and control arms at 6 months. As higher HADS-A and HADS-D scores indicate increasingly severe anxiety or depression, a negative difference indicates a beneficial effect of the intervention.
| Outcome | Intervention (n = 242), mean (SD) | Usual care (n = 181), mean (SD) | Mean difference | 95% CI | p-value | Data available: N (intervention, n; usual care, n) |
|---|---|---|---|---|---|---|
| Participants with COPD | ||||||
| HADS-A and HADS-D | ||||||
| HADS-A at 6 months | 8.09 (3.85) | 8.94 (4.19) | –0.60 | –1.40 to 0.21 | 0.145 | 369 (205; 164) |
| HADS-A at 12 months | 8.14 (3.94) | 8.77 (4.47) | –0.42 | –1.25 to 0.40 | 0.314 | 341 (191; 150) |
| HADS-D at 6 months | 7.49 (3.83) | 8.20 (3.71) | –0.66 | –1.39 to 0.07 | 0.074 | 368 (204; 164) |
| HADS-D at 12 months | 8.17 (4.06) | 8.72 (4.07) | –0.46 | –1.21 to 0.28 | 0.220 | 342 (190; 152) |
| BDI II and BAI | ||||||
| BDI II at 6 months | 17.27 (10.63) | 17.65 (10.68) | –0.12 | –2.16 to 1.91 | 0.904 | 336 (191; 145) |
| BDI II at 12 months | 16.85 (10.26) | 17.46 (10.14) | –0.63 | –2.75 to 1.50 | 0.559 | 288 (158; 130) |
| BAI at 6 months | 13.96 (10.32) | 14.55 (10.76) | –0.38 | –2.27 to 1.51 | 0.692 | 327 (180; 147) |
| BAI at 12 months | 12.80 (9.10) | 13.47 (10.12) | –0.95 | –2.92 to 1.01 | 0.339 | 288 (155; 133) |
| SGRQ | ||||||
| SGRQ symptoms at 6 months | 58.65 (22.71) | 58.66 (24.87) | –0.40 | –4.85 to 4.06 | 0.860 | 352 (194; 158) |
| SGRQ symptoms at 12 months | 55.94 (25.90) | 55.61 (26.10) | 0.97 | –3.70 to 5.64 | 0.682 | 210 (170; 140) |
| SGRQ activity at 6 months | 76.00 (18.40) | 75.37 (20.00) | 0.02 | –3.36 to 3.39 | 0.992 | 350 (193; 157) |
| SGRQ activity at 12 months | 76.18 (20.62) | 77.70 (18.14) | –1.36 | –4.89 to 2.17 | 0.446 | 307 (167; 140) |
| SGRQ impacts at 6 months | 42.84 (19.77) | 44.28 (19.22) | –1.24 | –4.57 to 2.09 | 0.460 | 347 (193; 154) |
| SGRQ impacts at 12 months | 42.73 (19.80) | 43.60 (19.97) | –0.15 | –3.61 to 3.31 | 0.932 | 306 (165; 141) |
| SGRQ total score at 6 months | 55.50 (17.23) | 56.04 (17.34) | –0.68 | –3.64 to 2.29 | 0.652 | 347 (193; 154) |
| SGRQ total score at 12 months | 55.00 (18.40) | 55.74 (17.08) | –0.51 | –3.61 to 2.59 | 0.745 | 300 (164; 136) |
| B-IPQ | ||||||
| B-IPQ consequences at 6 months | 6.31 (2.16) | 6.66 (2.57) | –0.27 | –0.73 to 0.19 | 0.248 | 349 (194; 155) |
| B-IPQ consequences at 12 months | 6.34 (2.37) | 6.64 (2.31) | –0.29 | –0.76 to 0.19 | 0.233 | 312 (172; 140) |
| B-IPQ timeline at 6 months | 9.61 (1.29) | 9.54 (1.36) | 0.01 | –0.28 to 0.30 | 0.948 | 351 (195; 156) |
| B-IPQ timeline at 12 months | 9.62 (1.31) | 9.64 (1.11) | –0.03 | –0.33 to 0.26 | 0.816 | 312 (172; 140) |
| B-IPQ personal control at 6 months | 5.04 (2.56) | 5.08 (2.83) | –0.04 | –0.65 to 0.57 | 0.892 | 346 (193; 153) |
| B-IPQ personal control at 12 months | 5.29 (2.50) | 5.19 (2.48) | 0.14 | –0.49 to 0.77 | 0.661 | 312 (171; 141) |
| B-IPQ treatment control at 6 months | 6.68 (2.51) | 6.48 (2.85) | 0.29 | –0.26 to 0.83 | 0.299 | 349 (194; 155) |
| B-IPQ treatment control at 12 months | 6.67 (2.32) | 6.60 (2.57) | 0.33 | –0.24 to 0.90 | 0.258 | 312 (171; 141) |
| B-IPQ identity at 6 months | 6.76 (2.03) | 6.81 (2.14) | –0.08 | –0.53 to 0.37 | 0.729 | 348 (194; 154) |
| B-IPQ identity at 12 months | 6.41 (2.29) | 6.66 (2.02) | –0.23 | –0.70 to 0.23 | 0.320 | 310 (170; 140) |
| B-IPQ concern at 6 months | 6.72 (2.87) | 6.81 (2.95) | –0.05 | –0.56 to 0.46 | 0.856 | 348 (192; 156) |
| B-IPQ concern at 12 months | 6.63 (2.88) | 7.18 (2.58) | –0.53 | –1.06 to 0.01 | 0.054 | 312 (171; 141) |
| B-IPQ coherence at 6 months | 8.10 (2.20) | 7.61 (2.43) | 0.58 | 0.12 to 1.03 | 0.014 | 349 (194; 155) |
| B-IPQ coherence at 12 months | 8.20 (2.09) | 8.18 (2.20) | 0.12 | –0.36 to 0.60 | 0.620 | 313 (172; 141) |
| B-IPQ emotional response at 6 months | 5.88 (2.89) | 6.03 (2.91) | –0.22 | –0.78 to 0.34 | 0.438 | 348 (194; 156) |
| B-IPQ emotional response at 12 months | 6.12 (2.76) | 6.13 (2.92) | –0.06 | –0.64 to 0.52 | 0.833 | 313 (172; 141) |
| heiQ and Time Use Survey | ||||||
| heiQ at 6 months | 2.66 (0.56) | 2.61 (0.63) | 0.07 | –0.05 to 0.18 | 0.272 | 352 (189; 153) |
| heiQ at 12 months | 2.60 (0.61) | 2.54 (0.62) | 0.08 | –0.04 to 0.20 | 0.198 | 301 (164; 137) |
| Time Use Survey at 6 months | 499.9 (708.2) | 410.8 (562.2) | 108.5 | –52.2 to 269.1 | 0.184 | 253 (149; 104) |
| Time Use Survey at 12 months | 430.2 (601.4) | 384.7 (684.3) | 39.2 | –149.3 to 227.7 | 0.682 | 176 (97; 79) |
| Carer | ( n = 25) | ( n = 19) | ||||
| ZBI and WEMWBS | ||||||
| ZBI at 6 months | 20.92 (13.00) | 25.29 (15.13) | –6.68 | –21.83 to 8.46 | 0.353 | 27 (13; 14) |
| ZBI at 12 months | 23.21 (16.53) | 24.00 (16.07) | –5.60 | –20.61 to 9.41 | 0.421 | 27 (14; 13) |
| WEMWBS at 6 months | 48.36 (9.43) | 55.00 (8.81) | 1.72 | –5.60 to 9.03 | 0.626 | 26 (11; 15) |
| WEMWBS at 12 months | 45.29 (7.99) | 52.08 (9.84) | 2.45 | –4.62 to 9.51 | 0.470 | 27 (14; 13) |
The mean difference between the two arms was less than the minimal clinically important difference cited in the sample size calculation, namely a difference of 1.7 on the HADS-A and a difference of 1.5 on the HADS-D. 137,138 The limits of the 95% CI ruled out clinically important effects on HADS-A or HADS-D at 6 months.
Secondary outcome analysis
As in the primary outcome analysis, CIs for HADS-A and HADS-D at 12 months, and other questionnaire scores at 6 and 12 months, ruled out clinically important effects of the intervention.
Carer outcomes
Carer outcomes are also shown in Table 11. There were no detectable differences in outcomes between carers of participants in the two arms of the study, but the CIs were wide.
Smoking status at 6 and 12 months
Treatment effect on smoking is shown in Table 12. Overall, the prevalence of smoking fell across the 12 months of the study, but there was no discernible difference between participants in the two study arms, and around one-quarter of participants were still smoking at 12 months. The estimated odds ratios for the intervention effect at 6 and 12 months are close to 1, but CIs were wide, consistent with a reduction in the odds of smoking by almost 50%, but also consistent with an increase in the odds of smoking by 50%.
| Follow-up | Trial arm | OR (95% CI) | p-value | Data available: N (intervention, n; usual care, n) | |
|---|---|---|---|---|---|
| Intervention (N = 242), n (%) | Usual care (N = 181), n (%) | ||||
| 6 months | 1.11 (0.69 to 1.78) | 0.660 | 360 (201; 159) | ||
| Current smoker | 56 (27.9) | 41 (25.8) | |||
| Non-smoker | 145 (72.1) | 118 (74.2) | |||
| 12 months | 0.90 (0.54 to 1.50) | 0.684 | 321 (175; 146) | ||
| Current smoker | 42 (24.0) | 38 (26.0) | |||
| Non-smoker | 133 (76.0) | 108 (74.0) | |||
Receipt of the intervention and attendance at pulmonary rehabilitation
Among the intervention participants, 217 (90%) received at least some of the intervention and 196 (81%) received at least two sessions, which was our predetermined minimum ‘dose’ of the intervention (see Figure 9). Full details on the number of intervention sessions received by participants are given in Chapter 6, Results of fidelity assessment.
In the intervention arm, 122 (50%) participants were referred to PR and 121 participants attended at least one PR session, of whom 73 (30%) completed the course (defined as attending 75% of the scheduled sessions). In the control arm, a similar proportion (n = 88, 49%) of participants were referred to PR and 77 (43%) participants attended at least one session, of whom 54 (30%) completed the course. The mean time to commencing PR was 114 (SD 68.3) days in the intervention arm and 106 (SD 88.6) days in the control arm.
Sensitivity analyses
Appendix 1, Tables 25 and 26, shows the estimated treatment effects from the sensitivity analyses.
Appendix 1, Tables 27–30, shows the baseline characteristics of participants divided into subgroups of participants defined by sensitivity analyses.
Hospital Anxiety and Depression Scale – anxiety
The estimated mean differences and 95% CIs from each sensitivity analysis for HADS-A at 6 months are presented graphically in Figure 10. None of the sensitivity analyses altered the qualitative interpretation of the primary analysis, that is the CI ruled out clinically important effects, with the possible exception of the analysis that excluded participants with a score of < 8 on HADS-A scale, and the analysis of the intervention effect specific to participants who attended sessions that were partially or fully remote, although the CI in the latter case was wide.
FIGURE 10.
Estimated mean differences and 95% CIs for each sensitivity analysis for HADS-A at 6 months.
Hospital Anxiety and Depression Score – depression
The estimated mean difference and 95% CI for each sensitivity analysis for HADS-D at 6 months are presented graphically in Figure 11. None of the analyses have CIs that suggest a clinically important effect of the intervention.
FIGURE 11.
Estimated mean differences and 95% CIs for each sensitivity analysis for HADS-D at 6 months.
Complier-average causal effect analysis
Estimates of the CACE of the intervention had much wider CIs than the primary analysis and in one instance the analysis did not converge to a solution [mean difference in HADS-A at 6 months –1.09 (95% CI –43.16 to 40.99; p = 0.960); HADS-A at 12 months –0.20 (95% CI –15.42 to 15.01; p = 0.979); HADS-D at 6 months did not converge; HADS-D at 12 months –2.29 (95% CI –10.25 to 5.66; p = 0.572)]. The wider CIs may be due, in part, to the necessity of analysing 6- and 12-month outcomes separately rather than in a longitudinal analysis, and also to the inherent challenge of estimating an effect among compliers when compliance is a latent (unobserved) variable in the control arm. The analysis did not converge to a solution in one instance.
Missing data
Some primary outcome data were missing at 6 and 12 months (see Appendix 1, Tables 31 and 32, for the results of analyses looking at the sensitivity of our conclusion to the assumption that these data were ‘missing not at random’). The tables show that a bias in the HADS-A or HADS-D outcomes that was not observed (i.e. the difference in mean outcome between participants with missing data compared with those with complete data) would need to be very large across both trial arms (of the order of 10 scale points), or would need to be strongly heterogeneous between trial arms (e.g. 3.5 points lower in the intervention arm and 1.5 points higher in the control arm, or 5 points lower in the intervention arm and no different in the control arm) before the CI for the treatment effect included values of 2 scale points or more.
Adverse events
A breakdown of SAEs is shown in Table 13. Thirty participants had a total of 40 SAEs in the intervention arm and seven participants had just one AE each in the control arm. There were no SAEs that were linked to the intervention in either arm of the study. Admissions due to acute exacerbations of COPD and deaths are common in people with COPD and were identified a priori as ‘expected’ events.
| SAEa | Trial arm | |
|---|---|---|
| Intervention (N = 242), n (%)b | Usual care (N = 181), n (%)b | |
| Number of people with an event | 30 | 7 |
| Total number of events | 40 | 7 |
| Severity | ||
| Resulted in death | 13 (32.5) | 3 (42.9) |
| Life-threatening | 3 (7.5) | 0 (0.0) |
| Resulted in hospitalisation or prolongation of hospitalisation | 24 (60.0) | 4 (57.1) |
| Persistent or significant disability or incapacity | 0 (0.0) | 0 (0.0) |
| Other important medical event | 0 (0.0) | 0 (0.0) |
| Related to one of the study procedures | ||
| Related | 0 (0.0) | 0 (0.0) |
| Unrelated | 40 (100.0) | 7 (100.0) |
| Expectedness | ||
| Expected | 20 (50.0) | 1 (14.3) |
| Unexpected | 20 (50.0) | 6 (85.7) |
| Due to the progression of an underlying illnessc | ||
| Yes | 30 (76.9) | 5 (71.4) |
| No | 9 (23.1) | 2 (28.6) |
| Action taken with study intervention | ||
| Continued | 10 (25.0) | 4 (57.1) |
| Reduced | 0 (0.0) | 0 (0.0) |
| Temporary stop | 15 (37.5) | 0 (0.0) |
| Permanent stop | 15 (37.5) | 3 (42.9) |
| Related to the trial conduct | ||
| Yes | 0 (0.0) | 0 (0.0) |
| No | 40 (100.0) | 7 (100.0) |
| Principal investigator withdrew the patient from the study | ||
| Yes | 7 (17.5) | 2 (28.6) |
| No | 33 (82.5) | 5 (71.4) |
| Outcome | ||
| Resolved | 19 (47.5) | 4 (57.1) |
| Resolved with sequelae | 3 (7.5) | 0 (0.0) |
| Improved | 1 (2.5) | 0 (0.0) |
| Persisting | 1 (2.5) | 0 (0.0) |
| Worsened | 0 (0.0) | 0 (0.0) |
| Fatal | 13 (32.5) | 3 (42.9) |
| Unknown | 3 (7.5) | 0 (0.0) |
Serious adverse events were collected opportunistically when the study team became aware of them. The differential arises in part because participants in the intervention arm had a lot more contact with the study (via the TANDEM facilitators delivering the intervention), whereas participants in the control arm were contacted at only 6 and 12 months following randomisation (see Appendix 1, Table 33, for a breakdown of AEs, which includes the SAEs because there is an AE recorded for every SAE).
Chapter 5 Health economics: methods and results
Aim
The aim of the health economic evaluation was to assess the incremental cost effectiveness of the addition of the TANDEM intervention to usual care, applicable to the trial population, compared with usual care alone, over a 12-month follow-up period post randomisation (i.e. a within-trial analysis), from the perspective of the NHS/PSS.
Methods
All analyses for the economic evaluation were undertaken using Stata.
The analyses were carried out on trial data measured at the participant level and according to an intention-to-treat principle. The evaluation adopted a cost–utility framework,139 with the incremental resource impact of the TANDEM intervention over usual care quantified from an NHS/PSS perspective, and patient outcomes quantified as incremental quality-adjusted life-years (QALYs) gained. Intervention cost effectiveness was evaluated with reference to the incremental net health benefit (INHB)140 of the TANDEM intervention combined with usual care compared with usual care alone (expressed in QALY units) and estimated using policy cost-effectiveness thresholds (CETs) of £20,000–30,000 per QALY gained, as per the National Institute for Health and Care Excellence (NICE) recommendation. 141 Decision uncertainty is presented using a cost-effectiveness acceptability curve (CEAC),142 describing the probability that the TANDEM intervention is preferable to usual care on cost-effectiveness grounds across a plausible range of CETs. All costs are reported at 2020/21 prices.
Service utilisation measurement
Healthcare utilisation by trial participants was measured using a combination of data from patients’ general practice records and a modified version of the Client Service Receipt Inventory,143 which was administered as part of the trial questionnaires (see Chapter 3 for details of questionnaire administration). Data obtained from primary care covered an 18-month period (i.e. 6 months pre randomisation to 12 months post randomisation) and included all hospital appointments, admissions, contacts with community-based health services (e.g. GP and community teams, smoking cessation programmes) and prescribing data. The self-report questionnaire covered the same range of healthcare contacts as the GP data, with the addition of questions about contacts made with other social care professionals and services. The GP data acted as the primary source of information on health service contacts, with the self-reported data used to supplement the service use data with details of service utilisation not covered by the GP records. This was needed when the GP data were not available for participants at a certain time point or for certain types of social care services.
For participants in the intervention arm, both the number and length of therapeutic contacts with TANDEM facilitators were obtained from clinical report forms completed by the facilitators for each individual participant. Data on the frequency of participant attendance at PR programmes were extracted at 12 months post randomisation from PR service case records. Implementation of the TANDEM programme also requires investment in the training of facilitators and time allocated to the clinical psychologists’ supervisory activity. To cost training activities, the TANDEM project team provided the following data: (1) the number and length of TANDEM training sessions held, (2) the number of TANDEM facilitators attending each training session, (4) the clinical grades of those attending and running the training sessions and (4) costs of accommodation and travel. Similarly, to cost supervision, information was provided on the time used to train the supervisors and the clinical time allocated by supervisors and facilitators to supervisory activity linked to the TANDEM programme.
To calculate the training cost per participant, we estimated the potential annual treatment caseload for the trained TANDEM facilitators (i.e. 42 cases in total) based on extrapolation of the number of participants in the intervention arm of the trial, assuming a completed episode of the intervention would take 8 weeks (i.e. the maximum anticipated length of a completed course) and 42.6 working weeks over a year (see Appendix 1, Table 34, for details of the assumptions used to estimate training and supervision costs).
Valuation of resource use
Health and social care utilisation were costed using applicable unit costs derived from national NHS reference costs144 and the unit costs of health and social care. 145 The appropriate Agenda for Change pay bands were used, with further adjustment for staff overheads and salary ‘on-costs’, as detailed in the Unit Costs of Health and Social Care,145 to estimate the full economic cost of professional time allocated by TANDEM facilitators to delivery of the intervention.
Cost estimation
For each service item, the cost per participant is estimated as the total ‘units’ of service use or health/social care professional time per patient multiplied by its respective unit cost. For each trial participant, we then calculated a total cost as the sum of all service utilisation costs per trial participant (including TANDEM intervention and training costs for those in the intervention arm of the trial).
Participant outcomes
Quality-adjusted life-years over the 12-month follow-up were estimated based on self-reported multiattribute health states at baseline and at 6 and 12 months follow-up using the EQ-5D-5L instrument. 146 Following recent guidelines issued by NICE on use of the EQ-5D-5L,147 health state utility scores applicable to the EQ-5D-5L were ‘cross-walked’ back to their equivalent three-level version values using a recommended algorithm. 148 QALYs for each participant were quantified with respect to the entire 12 months follow-up, using the ‘area under the curve’ method by use of time-weighted averages of the EuroQoL-5 Dimensions indices. 142
Analytical approach
The economic evaluation is an INHB analysis140 carried out within a cost–utility framework. The INHB of the TANDEM intervention is presented in QALY units by translating the monetary equivalent incremental cost per participant associated with the addition of the TANDEM intervention to usual care into its QALY-based opportunity cost-equivalent value:
where λ is a predefined CET. For our base-case analysis, we adopted a CET of £20,000 per QALY gained (i.e. the lower value used by NICE when identifying which health programmes are cost-effective). 141 If the per participant INHB of the TANDEM intervention is > 0, then this would indicate that the programme is ‘cost-effective’, at least with respect to the 12-month time horizon covered.
Uncertainty
Following convention, we analyse and present combined uncertainty around cost and effects and the appropriate CET to adopt using a CEAC. The CEAC describes the probability that the TANDEM intervention will be cost-effective (i.e. a INHB > 0) across varying assumed CET values. The range includes £30,000 per QALY gained (i.e. the upper threshold limit used by NICE) and £13,000 per QALY gained (i.e. close to the threshold recently argued to be a more realistic representation of the opportunity cost of incremental resource use in the NHS). 149 In addition, we visually present uncertainty by plotting the simulated distribution of incremental cost and QALY outcomes pairings for the TANDEM intervention (also used to identify the CEAC) on to the cost-effectiveness plane.
Statistical modelling
The incremental effect of the TANDEM intervention on the total cost of treatment and wider service contacts and participant QALYs over 12-months were estimated using multivariate statistical modelling. This enabled adjustment for baseline differences in covariates when comparing costs and QALYs between the trial arms, and accommodation of clustering (at the TANDEM facilitator level) within the trial data. In line with the main statistical analysis, the economic evaluation adjusted for the following covariates across both cost and QALY multivariate models: smoking status, breathlessness score and mental health status (using HADS-A and HADS-D scores). In addition, baseline health state utility scores (from the EuroQoL-5 Dimensions, three-level version, cross-walked values) and baseline total cost of service contacts were used to adjust estimated mean differences in QALY and total cost, respectively. Multilevel modelling was used to handle clustering effects in the trial data. QALY differences were estimated using a mixed-effects random intercepts model. Total cost differences were evaluated using a mixed-effects generalised linear modelling specification (gamma family, log-link function). As with the main statistical analysis of clinical outcome, TANDEM facilitators were identified as clusters in the intervention arm, with individual participants acting as their own cluster in the control arm.
To generate the sampling distribution of the joint incremental cost and QALY effect of the TANDEM intervention, we applied non-parametric bootstrapping to generate 1000 sample replications drawn from the trial data to which we applied the two multilevel models described above. 150 This enabled identification of the CEAC for the TANDEM intervention and the associated distribution of paired incremental cost and QALY outcomes on the cost-effectiveness plane.
Missing data
The primary sources of missing data were study withdrawal or loss to follow-up (details are provided in Figure 9). In a small number of cases where follow-up was successfully completed, incomplete data on service contacts meant that total costs could not be estimated. In this instance, a simple mean imputation applied to the missing cost item (by trial arm) was used to enable the total cost of intervention and service contacts to be calculated. To facilitate adherence to an intention-to-treat analysis (modelling cost and QALY outcomes inclusive of all participants randomised), we followed guidelines provided by Faria et al. 151 for handling missing data due to withdrawal and loss to follow-up. Multiple imputation was used to replace missing items of follow-up data. In adopting the multiple imputation approach, data are assumed missing at random. Multiple imputation was performed using the chained equations method, generating five imputed data sets based on predictive mean matching to obtain imputed values. Variables entered to the imputations model included covariates within the main analysis models for costs and QALYs. The revised data, inclusive of multiple imputations, formed the basis for the ‘base-case’ evaluation of the cost effectiveness of the TANDEM intervention.
Results
Table 14 contains the unit costs applied to service contacts to estimate total participant costs of the 12-month follow-up (for brevity we report main items of service contact). Appendix 1, Table 35, shows the full inventory of service contacts and unit costs.
| Service | Cost (£) | Source |
|---|---|---|
| Community-based services | ||
| GP visit | 40.10 | PSSRU 2020 section 10.3b145 |
| GP home visit | 78.39 | PSSRU 2015 sections 10.8a and 10.8b152 |
| Practice nurse visit | 19.18 | PSSRU 2015 section 10.1152 |
| Practice nurse home visit | 30.26 | PSSRU 2015 section 10.1152 |
| Counselling support/talking therapy | 46.54 | PSSRU 2015 section 10.1152 |
| Stop smoking service (cost per head) | 143.61 | PSSRU 2020 section 8.3145 (average of men and women) |
| Hospital-based care | ||
| Inpatient admission (bed-days) | 923.83 | NHS reference costs 2015/16153 |
| A&E not admitted (attendance) | 141.04 | NHS reference costs 2018/19.154 Average of all A&E services (not admitted) |
| A&E admitted (attendance) | 188.37 | NHS reference costs 2018/19.154 Average of all A&E services (admitted) |
| Hospital outpatient clinic: physical (appointments) | 168.70 | NHS reference costs 2018/19.154 Average of physical outpatient services (excluding paediatric services) |
| Hospital outpatient clinic: mental (appointments) | 243.97 | NHS reference costs 2018/19.154 Average of mental outpatient services (excluding paediatric services) |
| Day hospital (attendance) | 785.50 | NHS reference costs 2018/19154 (index sheet) |
Tables 15 and 16 provide descriptive data on categories of service utilisation at baseline (referring to self-reported contacts over the 6-month period prior to baseline interview) and over the 12-month follow-up period.
| Resource | Unit | Trial arm | Data available: N (intervention, n; usual care, n) | |
|---|---|---|---|---|
| Intervention (N = 242), mean (SD) | Usual care (N = 181), mean (SD) | |||
| Hospital-based health and social care | ||||
| Inpatient stay | Bed-days | 2.08 (7.59) | 1.87 (4.82) | 422 (242; 180) |
| A&E not admitted | Attendances | 0.17 (0.43) | 0.15 (0.41) | 423 (242; 181) |
| A&E admitted | Attendances | 0.27 (0.79) | 0.34 (0.82) | 423 (242; 181) |
| Hospital outpatient clinic | Appointments | 1.26 (1.13) | 1.17 (1.13) | 423 (242; 181) |
| Community-based health and social care | ||||
| GP (surgery) | Attendance | 3.52 (3.77) | 3.30 (4.71) | 422 (242; 180) |
| Nurse (surgery) | Attendance | 2.17 (4.22) | 1.50 (2.280) | 421 (241; 180) |
| Counselling/therapy | Session | 0.12 (0.95) | 0.27 (3.60) | 419 (241; 178) |
| Stop smoking service | Session | 0.21 (0.87) | 0.21 (1.09) | 417 (238; 179) |
| Other community healthcare services | N/A | 0.33 (1.74) | 0.19 (0.82) | 412 (241; 171) |
| Resource | Unit | Trial arm | Data available: N (intervention, n; usual care, n) | |
|---|---|---|---|---|
| Intervention (N = 242), mean (SD) | Usual care (N = 181), mean (SD) | |||
| Hospital-based health and social care | ||||
| Inpatient stay | Bed-days | 2.20 (6.20) | 2.71 (10.81) | 338 (192; 146) |
| A&E not admitted | Attendances | 0.32 (0.62) | 0.33 (0.69) | 338 (192; 146) |
| A&E admitted | Attendances | 0.31 (0.73) | 0.39 (1.07) | 338 (192; 146) |
| Hospital outpatient clinic | Appointments | 2.53 (2.14) | 2.14 (2.04) | 338 (192; 146) |
| Community-based health and social care | ||||
| GP (surgery) | Attendance | 5.08 (5.24) | 5.12 (5.98) | 338 (192; 146) |
| Nurse (surgery) | Attendance | 4.24 (7.94) | 3.26 (3.97) | 338 (192; 146) |
| Counselling/therapy | Session | 0.12 (0.60) | 0.38 (1.37) | 338 (192; 146) |
| Stop smoking service | Session | 0.29 (1.40) | 0.11 (0.53) | 338 (192; 146) |
| Other community healthcare services | N/A | 2.12 (6.62) | 1.28 (2.99) | 338 (192; 146) |
Table 17 provides descriptive information on the cost of the TANDEM intervention per participant randomised to the programme. Costs include those relating to the training and supervision of facilitators (i.e. £71.41 per participant) and the delivery of TANDEM intervention sessions. The mean intervention cost was £277.21 per participant, corresponding to a mean of 4.77 therapeutic sessions.
| Variable | Mean (SD) | Median (IQR) | n (data available) |
|---|---|---|---|
| Number of TANDEM sessions (session) | 4.77 (2.59) | 6 (3–6) | 242a |
| Cost (£) of sessions | 205.80 (110.97) | 256.55 (128.48–266.14) | 242 |
| Training time (hour) | 29.53 (35) | 21.75 (21.5–24.5) | 49b |
| Training cost (£) without supervision | 1580.95 (2351.04) | 1008.63 (859.39–1137.18) | 49 |
| Training cost (£) with cost of supervision | 1878.06 (2982.73) | 1017.1 (859.39–1171.86) | 49 |
| Training cost (£) per case | 71.41 | ||
| Total cost (£) of delivering the TANDEM intervention per programme participant | 277.21 (110.97) | 327.96 (199.89–337.55) | 242 |
Table 18 presents descriptive data on the costs of wider service contacts for the both intervention and control participants at baseline and over 12 months of follow-up. There was considerable variation in costs within the trial sample for both groups (as indicated by the large SDs). Compared with all other categories of service contact, hospital admissions were, on average, the most costly single item of service contact at both baseline and during follow-up.
| Resource category | Trial arm | Data available: N (intervention, n; usual care, n) | |
|---|---|---|---|
| Intervention (N = 242), mean (SD) | Usual care (N = 181), mean (SD) | ||
| Community-based services | |||
| Baseline | 298.2 (343.6) | 279.5 (366.8) | 423 (242; 181) |
| 12 months | 502.8 (501.8) | 443.4 (396.8) | 338 (192; 146) |
| A&E | |||
| Baseline | 103.8 (225.4) | 122.3 (248.0) | 423 (242; 181) |
| 12 months | 144.4 (238.9) | 181.9 (350.4) | 338 (192; 146) |
| Hospital care | |||
| Baseline | 2000.3 (7050.3) | 1804.9 (4485.4) | 423 (242; 181) |
| 12 months | 2195.5 (5837.2) | 2682.8 (10,051.5) | 338 (192; 146) |
| Outpatient attendances | |||
| Baseline | 346.3 (407.3) | 373.0 (587.5) | 423 (242; 181) |
| 12 months | 687.0 (716.7) | 591.4 (665.4) | 338 (192; 146) |
| PR | |||
| 12 months | 762.18 (341.12) | 738.29 (373.18) | 309 (179; 130) |
| Intervention delivery and staff training | |||
| 12 months | 227.21 (110.97) | N/A | 242 (242; N/A) |
Table 19 describes cost comparisons between intervention and control participants based on observed data (inclusive of imputed cost data). After adjustments made for differences in baseline covariates (inclusive of baseline costs), the mean cost for TANDEM participants was £770.24 higher than that of control participants, although this difference is estimated with a wide margin of uncertainty (95% CI –£27.91 to £1568.39).
| Time point | Mean health and social care cost (£) | Intervention – control | Data available: N (intervention, n; usual care, n) | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | |||||||
| Unadjusted mean difference | Adjusted mean differencea | 95% CI | ||||||
| Baselineb | 2748.60 | 7363.90 | 2579.80 | 4733.10 | N/A | N/A | N/A | 423 (242; 181) |
| 12 monthsb | 4400.40 | 6222.60 | 4454.20 | 10,397.30 | N/A | N/A | N/A | 238 (192; 146) |
| 12 monthsc | 4401.85 | 407.36 | 5100.42 | 1164.22 | –698.56 | 770.24 | –27.91 to 1568.39 | 423 (242; 181) |
Table 20 reports mean health state ‘utility’ values applicable to self-reported health states identified at baseline and at each follow-up point (i.e. 6 and 12 months), using the EQ-5D-5L instrument. The utility scores are based on wider UK population preferences for the varying states of health described by the EQ-5D-5L, with scores located on a scale anchored at 1 (full health) and 0 (death). Negative values are also possible for states of health regarded as being less preferable to death. Comparisons of mean utility scores measured at baseline and follow-up suggest that health-related quality of life, on average, declined for both intervention and control participants over the period of study. Table 20 also presents QALYs over follow-up (estimated directly from the utility scores), using the observed data (inclusive of imputed values where QALY data were missing). Based on the imputed sample with adjustment for baseline covariates (including health utility values at baseline), TANDEM participants were estimated to accumulate marginally fewer QALYs over 12 months than control participants [a difference of –0.010, (95% CI –0.042 to 0.021) QALYs, which is equivalent to 3.65 fewer days spent in full health over 12 months].
| EuroQoL-5 Dimensions | Intervention, mean (SD) | Usual care, mean (SD) | Intervention – control | Data available: N (intervention, n; usual care, n) | ||
|---|---|---|---|---|---|---|
| Unadjusted mean difference | Adjusted mean differencea | 95% CI | ||||
| Utility scores | ||||||
| Baselineb | 0.552 (0.230) | 0.550 (0.268) | N/A | N/A | N/A | 423 (242; 181) |
| 12 monthsb | 0.546 (0.264) | 0.534 (0.265) | N/A | N/A | N/A | 368 (208; 160) |
| 12 monthsc | 0.515 (0.281) | 0.523 (0.284) | N/A | N/A | N/A | 347 (197; 150) |
| QALYs | ||||||
| Baseline to 6 monthsb | 0.274 (0.106) | 0.269 (0.116) | N/A | N/A | N/A | 368 (208; 160) |
| 6–12 monthsb | 0.268 (0.121) | 0.272 (0.119) | N/A | N/A | N/A | 327 (187; 140) |
| Baseline to 12 monthsb | 0.546 (0.216) | 0.549 (0.223) | N/A | N/A | N/A | 327 (187; 140) |
| Baseline to 12 monthsc | 0.537 (0.0148) | 0.549 (0.016) | –0.011 | –0.010 | –0.042 to 0.021 | 423 (242; 181) |
Table 21 combines information on the cost and QALY differences between the intervention and control arms to make inferences regarding the cost effectiveness of the TANDEM intervention. Across a range of plausible CETs (including those currently adopted by NICE), the expected INHB of the TANDEM intervention is negative, ranging from –0.0697 to –0.0361 QALYs, depending on the CET applied, and this suggests that the TANDEM intervention would not be a cost-effective addition to the standard package of care normally provided to the trial population. Accounting for sampling uncertainty within the trial data, a high degree of confidence can be placed in this finding. The probability that the TANDEM intervention does offer a cost-effective addition to usual practice is estimated to be no more than 5.5% across each value of the CET (see Table 21).
| CET | INHB | 95% CI | Probability cost-effective |
|---|---|---|---|
| £13,000 | –0.0697 | –0.0726 to –0.0680 | 0.030 |
| £20,000 | –0.0489 | –0.0512 to –0.0477 | 0.037 |
| £30,000 | –0.0361 | –0.0380 to –0.0352 | 0.055 |
Figure 12 (a scatterplot of potential incremental cost and QALY pairings on the cost-effectiveness plane) and Figure 13 (the CEAC for the TANDEM intervention) present a visual summary of sampling uncertainty. A scatterplot of potential incremental cost and QALY pairings on the cost-effectiveness plane using only available case data (rather than imputed data) and a CEAC using only available case data are presented in Appendix 2, Figures 23 and 24, with the estimate of INHB for available case data shown in Appendix 1, Table 36.
FIGURE 12.
Cost-effectiveness plane (base-case results). A scatterplot showing the bootstrapped mean differences in healthcare costs and effects (QALYs) between the intervention and control arms. Estimates based on multiple imputation for missing data.

FIGURE 13.
A CEAC (base-case results) showing the probability that the TANDEM intervention is cost-effective compared with usual care. CEAC based on data that includes multiple imputation for missing data.

Sensitivity analysis
Two post hoc sensitivity analyses were performed. The first analysis repeated the base-case cost-effectiveness analysis on data ‘trimmed’ of one outlying control participant with an extreme cost value (arising because of a high total inpatient admission cost) and an imputed QALY value. The second analysis considered the effect of using a more conservative estimate of the costs of training TANDEM facilitators, based on a larger treatment caseload (5591 cases/year instead of 1288 cases/year) suggested by lead trial clinicians as a realistic facilitator caseload if the intervention were implemented in a routine clinical service. Trimming the outlier from the sample increased the incremental cost of the TANDEM intervention (from £770 to £1057) and led to a zero QALY effect of the TANDEM intervention over usual care only (compared with the small negative effect on QALYs when the outlier was included). Neither trimming the outlier or using a revised estimate of training costs changed core conclusions regarding intervention cost effectiveness or probabilities relating to decision uncertainty (see Appendix 1, Tables 37 and 38, for these analyses).
Summary of the health economic evaluation
The within-trial economic evaluation of the TANDEM intervention suggests that it is highly unlikely to be a cost-effective means of improving mental health outcomes in patients with chronic respiratory disease. After jointly considering incremental effects on costs and QALYs and allowing for sampling uncertainty in the trial data, there was a high degree of certainty that the TANDEM intervention would not offer sufficient value for money based on cost-effectiveness criteria routinely applied to assess whether or not new healthcare technologies should be funded by the NHS. Had we also factored into this analysis the anticipated costs involved with implementing a programme like TANDEM at scale within routine care settings (i.e. beyond the costs of training facilitators), then these conclusions would have been only strengthened. The higher average cost for TANDEM participants was largely explained by higher service utilisation costs after adjusting for baseline covariates [i.e. the mean intervention cost (£277.21 per participant) amounted to over one-third (35%) of the mean total cost differential between intervention and control participants (£770)]. There was no evidence that the TANDEM intervention would generate health-related quality-of-life improvements sufficient to justify these additional costs to the NHS.
Chapter 6 Process evaluation including fidelity assessment: methods and results
The protocol for the process evaluation has been published in Kelly et al. 65
Role of the process evaluation in the TANDEM intervention
Process evaluations are viewed as necessary elements of the evaluation of complex interventions, providing information for interpreting trial results beyond effectiveness. 59,155 Process evaluations include evaluation of fidelity, quality of implementation, mechanisms of change and context. The TANDEM process evaluation includes assessment of fidelity and quality of intervention delivery; experiences and perspectives of health professional facilitators and supervisors, patient participants and carers, and other stakeholders; and relevant quantitative data, such as completion rates. Analysis of the processes involved in delivery helps to explain how an intervention was delivered and, in the context of a negative trial, whether a lack of effectiveness was due to an ineffective intervention or failure to implement the intervention as planned. Investigation of context in qualitative interviews allows for a broader consideration of factors influencing intervention mechanisms.
The process evaluation contributed to the TANDEM intervention’s hybrid design,156 which involves testing a clinical intervention while gathering information on delivery during the effectiveness trial and/or on its potential for real-world implementation. The process evaluation built on initial developmental work and formative evaluation reported by Steed et al. 66 (see Chapter 2).
Underpinning theory
We created a multidimensional conceptual framework that presents how theory underpins and informs our process evaluation (Figure 14).
FIGURE 14.
Conceptual framework for the TANDEM process evaluation. Reproduced with permission from Kelly et al. 65 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.

Our process evaluation was underpinned by realist evaluation principles of context, mechanism and outcome157 to describe and interpret how the intervention interacts with the context in which it has been implemented. Potential contextual influences on effectiveness and implementation of the TANDEM intervention include facilitators’ professional practice, facilitator training and supervision, the patient–facilitator relationship, multimorbidity and the patients’ (and carers’, where relevant) complex lives, policy-level priorities and organisational stakeholders’ perspectives on the delivery context.
Normalisation process theory was used as a theoretical framework to guide data collection and analysis of how the intervention might become embedded or ‘normalised’ (or not) into routine practice. 158,159 There has been an emphasis on engaging from the early stages of study development with those who would receive the intervention (i.e. patients and carers), and those who would deliver it (i.e. healthcare professionals).
Aim and objectives of the process evaluation
The overall aim of our process evaluation was to describe and understand the processes by which the trial and intervention were conducted (specifically including fidelity and acceptability to recipients and professionals) and to consider the effect of these on the outcomes of the study.
Objectives
The objectives address acceptability, fidelity and implementation from a broad range of perspectives. The objectives are as follows:
-
To assess the acceptability of the intervention to patients and carers, including consideration of content (e.g. sessions, home practice), therapeutic alliance and practicalities (e.g. location, timing).
-
To assess the acceptability of the intervention to facilitators delivering the intervention and the supervisors with respect to:
-
patient-facing TANDEM sessions (including content, structure, logistics, telephone support and integration of components)
-
facilitator training (including content, logistics, supervision, perceived confidence to deliver the TANDEM sessions)
-
management of workload
-
supervisors’ training and workload.
-
-
To monitor the fidelity with which the intervention was delivered.
-
Was the facilitator training delivered as intended with respect to professional competence?
-
Were the CBA sessions delivered as intended with respect to adherence and competency?
-
-
To assess the feasibility of implementing the intervention with respect to recruitment/training of facilitators and uptake/completion by participants. Specifically, we measured:
-
the recruitment, training and retention of facilitators and organisation of clinical supervision
-
rates of completed delivery of at least two TANDEM therapy sessions (i.e. the predetermined estimate of minimal clinically important ‘dose’ of intervention) per patient
-
the numbers of patients seen by facilitators and the numbers of sessions delivered to patients, as well as reasons for intervention non-attendance/no delivery of sessions
-
intervention dropout or disruption to delivery and reason for dropout or disruption.
-
-
To explore the experiences and perspectives of patients, carers, facilitators and supervisors regarding the intervention and post-trial implementation.
-
What are patients, carers, facilitators and supervisors’ experiences of the intervention and what are their views about its potential impact on health and quality of care?
-
What are the barriers to and facilitators of implementation and how do these vary according to context and/or other factors?
-
Were there any unexpected consequences?
-
-
To explore the views of organisational stakeholders regarding post-trial implementation of the intervention.
-
What are the barriers to and facilitators of implementation and how do these vary according to context and/or other factors?
-
What resources and partnerships are necessary for implementation?
-
To understand whether or not adaptations to the intervention are necessary depending on the clinical context in which it takes place (e.g. if the intervention is delivered through primary care, secondary care or solely via PR services).
-
Methods for the process evaluation
We used a mixed-methods design, incorporating qualitative and quantitative methods. Qualitative and quantitative data were collected from the intervention arm of the trial and quantitative data from the control arm. The structure of the process evaluation is illustrated in Figure 15.
FIGURE 15.
Schema for structure of the TANDEM process evaluation.

The process evaluation team (MJK, LS, RS, VW, Sian Newton, AB, CDD, K-MM, VR, Anna Moore, HP and SJCT) met monthly to discuss all aspects of the evaluation, including design, data collection and analysis. Four subgroups were set up within the larger process evaluation team to undertake different elements of the research (i.e. facilitators and supervisors, patients and carers, organisational stakeholders, fidelity). Organising the work in this way addressed both practical and quality issues, with small teams working closely together on analysis, yet having access to the main process evaluation team for feedback and regular critical discussion throughout.
Qualitative methods
We conducted qualitative interviews with patients and carers, healthcare professionals and supervisors, and organisational stakeholders (i.e. clinical commissioners, GPs, PR specialists, nurses and psychologists). Sampling frames were drawn up based on a purposive sampling approach to gain a full range of views. Sampling was reviewed during data collection and data analysis to pick up unexpected issues and themes (including the impact of the COVID-19 pandemic). Table 22 provides details of the interviews conducted and an outline of the topic guide. Interviews were by telephone or in person, depending on the preference of the research participant and the COVID-19 pandemic restrictions. Open questions were used to explore issues from the perspective of participants, and to allow for unexpected issues to emerge.
| Interviewee | Sample | Main issues addressed by topic guide |
|---|---|---|
| Patients | Five participants who completed face-to-face TANDEM sessions (after the 6-month follow-up assessment) Five participants who dropped out of the TANDEM programme (with four or fewer CBA sessions and after the 6-month follow-up assessment) Five participants who completed the TANDEM CBA sessions and PR programme (after the 6- and 12-month follow-up assessments) Five participants who completed the TANDEM programme but dropped out or did not attend PR (after the 12-month follow-up assessment) |
Current experience of COPD/breathlessness Experience of being in the TANDEM study Relationship and working with the TANDEM facilitator Experience of attending PR Suggested improvements to the TANDEM intervention experience Perspectives on receiving the TANDEM intervention as part of routine care |
| Carers of intervention participants | Five carers (after the 6-month follow-up assessment) | Relationship with patient/role Understanding of the TANDEM intervention Perspectives on CBA sessions Experience of care role in the study Any observed improvements in patient’s condition/quality of life |
| Facilitators | Up to 14 facilitators All facilitators to be invited, but aim for range of professional groups and number of patients seen |
Training sessions CBA sessions with patients Supervision Professional identity Perspectives on post-trial implementation |
| Clinical supervisors | Up to four clinical supervisors All invited |
Training Clinical supervision sessions Logistics of organising supervision sessions Providing clinical supervision for healthcare professionals in professions who do not usually receive it |
| Organisational stakeholders | Up to 20 interviews Range of organisational context and roles |
Organisation and role Issues faced in delivering and improving COPD services Perspectives on the value of PR for people with COPD Understanding of the TANDEM intervention Views on the TANDEM intervention approach to care Perceived differences with current care approaches for COPD Perspectives on post-trial implementation of TANDEM Facilitators of and barriers to implementation Facilitators of and barriers to commissioning |
Data were analysed thematically using an inductive approach and constant comparison. Analysis was a reflexive, iterative process involving review and multidisciplinary discussion. NVivo 12 software (QSR International, Warrington, UK) was used to assist the organisation and analysis of the data. A thematic narrative was constructed for each qualitative substudy.
Methods for assessing fidelity
Fidelity can commonly be understood as the assessment of whether or not an intervention has been delivered and received as intended. In 2004, the National Institutes of Health Behaviour Change Consortium identified the following five key elements of fidelity: (1) treatment design, (2) training providers, (3) delivery of treatment, (4) receipt of treatment and (5) enactment of treatment skills. 95,160 For each element, strategies are recommended for both the enhancement and assessment of fidelity. 161 We used these definitions and recommendations to form the basis of our work on fidelity. Strategies for enhancing fidelity are reported in Chapter 2, Ensuring fidelity.
Measuring fidelity
Fidelity assessment was appropriate for all fidelity elements other than treatment design, which we considered purely in relation to enhancement. Methods used included post-training competency assessment of facilitators, assessment of treatment delivery, treatment receipt and enactment.
Training providers: competency assessment
On completion of the 3-day training programme, all facilitators underwent a role-play assessment with a professional actor (note that the same actor presented the same prespecified case to all facilitators). Trainees were given the task of conducting a 20-minute assessment and formulation with presentation back to the ‘patient’. All assessments were video-recorded and independently coded by two psychologists using the Cognitive First Aid Rating Scale (CFARS). 103 The CFARS is a validated measure for assessment of therapeutic competency based on the revised Cognitive Therapy Rating Scale. The CFARS includes 10 items, each with a 0–6 scale (with a maximum score of 60). Therapeutic competency is deemed to be achieved if a minimum total score of 30 is acquired with no individual item falling below 2. In the post-training assessment, we omitted item 9, relating to delivery of appropriate intervention, as we did not require facilitators to show application of change techniques within the role-play assessment and, therefore, set a minimum score of 27 to indicate therapeutic competency.
Treatment delivery
A bespoke fidelity treatment delivery framework was created for this trial, including the CFARS to assess therapeutic competency (minimum level rated at 30 because of the inclusion of all 10 items) and an intervention-specific adherence measure. The adherence measure included assessment of whether or not core components were delivered across each session, according to a 1–3 scale (1 = not at all, 2 = partial, 3 = complete), and, additionally, whether or not topic-specific sessions were delivered (0 = no, 1 = yes).
Up to two entire TANDEM therapy courses (i.e. all of the therapy sessions provided for one participant) were assessed for each facilitator. A random selection of courses were selected by random number sequence from the second to the fifth participants for each facilitator (the first participant was used when a facilitator saw only one participant). For facilitators who delivered sessions to more than nine participants, a random selection from the 10th to the 15th participants were selected. This assessment was used to capture therapy sessions delivered when TANDEM facilitators were less experienced and when they were (relatively) more experienced. For each participant, all sessions within the course were coded. In the event of missing/unclear evidence from the audio-recording (e.g. when it was unclear which resource was being discussed), then this could be clarified from the facilitator-completed trial case report forms that noted, for example, provision of leaflets.
Coding was conducted by a psychologist (VW) who was trained in the psychological intervention and cognitive–behavioural methods more generally. Seven (19.4%) cases were duplicate coded by a member of the study team (LS) for quality assurance; however, to ensure consistency, the scores of the primary coder were used for analysis.
Treatment receipt and enactment
Qualitative interviews were coded for treatment receipt. A quantitative measure of social engagement (i.e. the heiQ)116 was used as a proxy indicator of treatment enactment. Enactment will be further explored in the qualitative interviews with patients.
Results of the process evaluation
We present the findings in three main sections: (1) qualitative research with patients, carers, facilitators, supervisors and organisational stakeholders, (2) the process of recruitment and training and (3) the fidelity analysis.
Patient and carer experiences and perspectives
Participant characteristics
Interviews were conducted with 19 patients (of 29 patients invited for interview) between September 2019 and December 2020 (nine interviews were conducted by telephone during the COVID-19 pandemic). Sampling reflected engagement with TANDEM therapy sessions and PR, including participants who had completed the TANDEM sessions (n = 5), participants who completed the TANDEM sessions and PR (n = 5), participants who dropped out of the TANDEM sessions (with 0–4 therapy sessions completed) (n = 5) and participants who completed TANDEM sessions but had not attended/completed PR by 12 months from baseline (n = 4). Of 10 carer participants invited, four agreed to be interviewed and all interviews were completed during the COVID-19 lockdown period.
Acceptability of the TANDEM intervention to patients and carers (objective 1)
Patient and carer experience of the intervention was mostly positive. Participants described developing supportive and caring relationships with facilitators. Rapport-building at the start enabled a therapeutic alliance with the facilitator. Patients and carers commented on the knowledge and skills of the facilitators and liked the facilitators’ friendly, empathetic and supportive nature, which led patients and carers to talk more freely about their thoughts and feelings in relation to their COPD, other conditions or social situations:
Yeah, and maybe asked questions that I would’ve not fancied talking about possibly. So she did probe. But it wasn’t putting me on the spot. Then it made me think, oh yeah, I don’t mind talking about that.
Female, 56–65 years, anxious and depressed, very severe COPD, PAT18
Content of sessions and practicalities
Information and self-management skills provided in the TANDEM sessions increased awareness and better understanding of COPD and anxiety/depression. The practical tools were helpful for reflection and application in managing health conditions. Resources, such as leaflets and handouts, were sometimes considered too much, but could be referred to if needed.
The ‘hot cross bun’ formulation diagram was typically introduced in session 2, although, on some occasions, it could not be introduced until as late as the fourth session. In addition, the diagram took some time to complete, as patients needed to be guided to identify and explore their thoughts, feelings and behaviours in a new way. This was also true of homework tasks. Exploring emotions and behaviours was not always straightforward, therefore, the efforts of facilitators to build a therapeutic alliance as the basis for delving into new areas with patients was important to help patients to engage in this process. The format of the sessions was considered acceptable by the patients:
I remember the doughnut [the TANDEM hot cross bun], about how to try and go about things to make sure that I was less out of breath and options like stopping ... she also ... I had, which I’ve still got, two videos talking through different experiences and what would I do if say I was stuck in here and I wanted to go down to the bottom of the garden, and I couldn’t make it, ways to get over that.
Female, 66–75 years, anxious and depressed, moderate COPD, PAT14
Many of the interviews were conducted during the COVID-19 pandemic. Some participants were coping reasonably well, whereas others felt that the pandemic was having a negative effect on their health. The TANDEM model (and content) was new to participants and was not felt to be comparable to other NHS services currently available to them. Specifically, the option of having sessions in their homes, and discussions on mood and COPD, including practical advice and management strategies, were highly valued by participants, and participants felt that the TANDEM intervention could make a useful addition to their care.
The carers’ role comprised provision of personal care, practical and emotional support in line with the needs of the participant, and this affected carers in various ways. Opinions were divided about whether or not the CBA sessions were beneficial for the participant:
No, not at all, no. No changes really [after the TANDEM sessions for patient participant].
Male, spouse, 66–75 years, CAR2
Experiences of the TANDEM intervention
Patients developed a good rapport and a positive relationship with their facilitator over the course of the CBA sessions. Several participants seemed surprised about how much the facilitator was able to get them to open up and talk about issues that they had not considered or did not want to talk about previously. The same view was also expressed by a carer. The TANDEM intervention made some patients realise the connection between physical and mental health for the first time and gave patients confidence to move forward and to see the value of life again:
Other things she [the facilitator] reinforced was ‘don’t over think things, but analyse your reaction or your feelings, and try and figure out why you react a certain way’. And then if you consider that it’s not the way you want to act, you can find an alternative strategy to accept what’s happening.
Male, 76–85 years, depressed, moderate COPD, PAT16
But he seemed to open up, they seemed to have a nice chat in there. She was here about an hour I think, a good hour. Perhaps a bit longer some days. I don’t know, I can’t remember now. But it was just the regular activity and he used to sit in the room on his own and I used to leave him to it, because I knew he’d open up more.
Female, 66–75 years, spouse, CAR4
A few participants said that they were not comfortable reading handouts and/or writing things in between sessions for home practice and, therefore, the facilitator had modified their approach, which was helpful.
Two interviewees completed no sessions. For two interviewees, the sessions stopped at the right time because they were not affected by their mood, and were already familiar with the information provided on COPD. For one patient, the participant wanted to prioritise attending PR, as they had been unable to complete it previously because of a bad back and the programme sessions were going to overlap with the TANDEM sessions:
I sort of knew most of what she was saying really. I’d sort of been doing it myself. That’s the main thing. Went on the breathing thing [PR programme] for 6 weeks, and that was very good ... Yeah, it was sort of a joint decision [to stop the TANDEM sessions]. If I had any problems I could give her a ring. But then I more or less carried on with the breathing thing [PR programme].
Interviewer: And how soon did you join that exercise class after your one-to-one sessions?
More or less straightaway ... I think the thing for me was probably the actual breathing session ... Exercise and information.
Male, age unknown, anxious, severe COPD, PAT11
Therefore, five interviewees had completed fewer than four CBA sessions.
The effect of the TANDEM intervention on patients
The perceived effects of the CBA sessions for patients were wide-ranging. Participants described improvements in mental health, breathlessness and symptom management, knowledge, confidence and social life/activities, and in being able to accept their condition. Increased awareness about their own mental health was highlighted, as some participants realised or acknowledged for the first time that they had depression or anxiety. The TANDEM sessions were described as helpful to shift negative thoughts to positive ones and to improve feelings of anxiety relating to their COPD:
I didn’t realise I was depressed at the time because I was going to the bed most days in the afternoon, and that was more because I was ... I retired last year, so I think it’s because I’d recently retired. I was just bored. So I was going to bed in the afternoons just to kill time. And then I realised through talking to [facilitator’s name] that that was more me being depressed ... I’m going to say it changed my outlook on life. I didn’t realise how depressed I was and how down I was.
Male, age group unknown, anxious, moderate COPD, PAT6
And that’s [TANDEM sessions] helped a lot. So yeah, I don’t really get that anxious anymore ... I mean I’m not saying I don’t get anxious at all. I do occasionally. But not to the extent whereas before I’d be up all night.
Female, age group unknown, anxious and depressed, moderate COPD, PAT12
Participants also described learning skills/techniques and behaviours that some applied to improve the daily management of their COPD, for example integrating relaxation and breathing techniques to manage COPD-related anxiety. The sustainability of perceived improvements varied. The learning became embedded for some participants, whereas for other participants the positive effect was not sustained because of personal and external factors.
A few of the participants interviewed did not perceive any benefit. A couple of participants stated that nothing had changed or helped their condition and one participant did not believe that they could be helped and discontinued the TANDEM sessions:
I wouldn’t say it’s [the sessions] helped me, because I don’t think I can be helped ... to get better. Because I know I ain’t going to. But I live with it and put up with it. I’ve had cancer three times, so that ain’t beat me ... I didn’t expect to learn ... What can you learn about something you’ve got which ain’t going to have a cure?
Male, 66–71 years, depressed, moderate COPD, PAT17
Eleven interviewees completed the PR course and described health benefits. The four participants who did not attend/complete PR explained that this was because of competing comorbidities that needed to prioritised or because of health service-related barriers. Participants remembered discussions with the facilitator about PR focusing on how it may benefit them and what they could expect to gain by attending the programme. In a few cases, the facilitator helped participants to gain a referral to PR by liaising with participants’ clinical teams:
She [the facilitator] told me what it was all about and it was exercising to try and improve my breathing. And I did keep up the exercises for probably, I don’t know, 6 weeks maybe after the sessions finished. I don’t feel any worse for not doing them now. But at the time, again, it was something different. It was an hour or 2 hours a week out the house. We had information sessions and we had exercise sessions. And a little bit of competitiveness between the fitter versions of human beings there. So yeah, it was pretty good and I bought myself some dumbbells so I can exercise whenever I want to. I use the stairs. And step exercises on the bottom stair. If I go and use the loo, if I remember I’ll go up to the top of the stairs, turn around and come back down again and then go back up to use the loo. So I get double stairs exercise.
Male, 76–85 years, depressed, moderate COPD, PAT16
I think through [facilitator’s name] she also put me then in touch with the COPD exercise programme which I attended. So that was good.
Female, 66–75 years, anxious and depressed, severe COPD, PAT10
One carer felt that the patient had not applied any of the TANDEM learning recently because of the onset of the pandemic. However, the patient had attended PR after completing the sessions, which their partner found very helpful.
Interviewer: Before the lockdown, after the one-to-one sessions, was there a referral for [name] to go to the pulmonary rehab classes? The exercise classes?
Female, 66–75 years, spouse, CAR3: Oh yes, he did. He went to ... now let me get this ... You did, didn’t you? You went to the rehab clinic at [name] Hospital. Yes, he did, and that was very good, that was ...
The views and experiences of the telephone support sessions were explored among participants who completed PR; however, by the time participants were interviewed, the majority of participants were not able to remember this aspect of the support provided.
Complexity of patients’ lives
The fluctuation of COPD symptoms and multimorbidity had a cumulative affect on the physical, psychological and social health of the individual. Participants were aware that their COPD was not always visible to others, except when they suffered from noticeable symptoms, which led to feelings of being judged or embarrassment. Participants described social responsibilities that they prioritised over their own health, for example events that required them to look after loved ones and deal with traumatic events, which meant thinking less about themselves and their needs:
The thing that gets me down is I’ve got Parkinson’s as well, which is making walking difficult at the moment. It’s got much worse recently. And I get out of breath ... Although I’m walking slowly it takes a lot of effort.
Male, 66–75 years, depressed, moderate COPD, PAT5
I’ve got an awful lot ... still have, an awful lot of unfortunate stuff going on with my daughter ... But it’s not been a good time ... I know that’s not anything to do with COPD, but in effect it probably is because it’s all the stress and everything.
Female, 66–75 years, anxious and depressed, moderate COPD, PAT14
Carers reflected this complexity and described the care they provided as diverse, including a mix of personal care and practical and emotional support. The type of care they provided was dependent on what their partner might need for management of their condition(s) or and any changes in personality attributed to the partner living with a long-term condition, and this, in turn, could affect their relationship:
Well, yes. I think it’s more frustrating now for the both of us. Really it is frustrating because I suppose at the end of the day you don’t want ... I don’t want him to have dementia. So I’m angry. And I’m sure [name] is feeling depressed with the fact that he’s been diagnosed with the early stages of dementia.
Female, 66–75 years, spouse, CAR3
Effects of COVID-19
People living with COPD described a need to tap into their resilience and adapt to the lockdown restrictions, but overall they felt that their motivation was low. Ongoing or planned physical and social activities had stopped because of the pandemic and although some participants were drawing on techniques learnt in the TANDEM intervention to cope with their mood, other participants highlighted the negative impact on their health:
... I’ve brought into play some of my COPD ... the TANDEM thing. That way you look at your depression, why you’re depressed, let’s get out of it, let’s do something. So I actually wrote down things to do today and a timetable of what to do and when to do it. So I’m feeling a little bit better today ... I’m going to do things to make me feel better.
Male, unknown age group, anxious, moderate COPD, PAT6
A common concern of the carers (all interviewed during the COVID-19 pandemic) was the health of their partner in lockdown. Most daily and social activities had stopped, for example exercise stopped because of loss of interest or the inability to replicate the exercises learnt in their small space at home.
Patient perspective on implementing the TANDEM intervention (objective 5)
The TANDEM intervention was considered to be very different from other services currently available for people living with COPD. Most participants had never received any psychological therapy previously for their anxiety or depression in relation to their COPD. The linking of mood with COPD and practical advice delivered by respiratory health professionals was viewed as very beneficial. When reflecting on whether or not the TANDEM intervention should be delivered as part of usual care, many participants felt that it could be offered by the respiratory healthcare professionals they see regularly.
The TANDEM sessions were available at home or at a general practice or hospital (with the offer of transport). Having flexibility in location was highly valued among patients and carers for multiple reasons:
... at the time it was the right environment for her to come to my house, because I couldn’t get out. I mean if they’d said to me ‘oh, you’ve got to go to [place name] every week’, I wouldn’t have been able to have done it. Because I said I don’t drive, I can’t afford the taxi fare. I can’t use public transport.
Female, age group unknown, anxious and depressed, moderate COPD, PAT12
Participants highlighted that symptoms of COPD fluctuate and this influenced their perception of the stage of the illness when the TANDEM intervention might be of most benefit. Several participants suggested offering the TANDEM intervention to patients at various points of the illness trajectory, such as at diagnosis, when affected by severity of physical or psychological symptoms or alongside the PR pathway. However, participants felt that acceptance of the TANDEM intervention would be highest when it was most meaningful to the patient and would be useful to those who wanted to talk about their mood and feelings. TANDEM sessions should not be compromised if integrated into current NHS services. For example, participants felt that maintaining the option for regular home visits was important. However, a couple of participants commented on the cost and the time impact on NHS services.
Experiences and perspectives of facilitators
Facilitator characteristics
We interviewed 14 facilitators (i.e. six nurses, four physiotherapists and four occupational therapists). All of the facilitators interviewed were female and had been in their clinical professional roles for between 11 and 37 years. Nine facilitators had delivered the TANDEM intervention to four or more patients and five facilitators had delivered the TANDEM intervention to fewer than four patients.
Acceptability of training and delivering the TANDEM intervention (objective 2)
Experience of delivering sessions
Facilitators explained that it was important to build rapport and trust with patients in early sessions, developing a therapeutic alliance, which encouraged open communication in subsequent sessions. Although this was challenging, the TANDEM structure provided the necessary time and space for this to happen:
So he wasn’t actually doing many activities to get breathless because he was avoiding getting breathless ... So while all we’d really done was talk about it and allow him to open up about being scared about being breathless, by him talking about it changed his whole perception of it, and he realised that he could do more. Without even setting small goals he kind of just moved on in leaps and bounds, and was getting on public transport and everything.
Nurse 1
Patients’ engagement with the TANDEM intervention was variable, and facilitators mentioned factors, such as illness perception, age and illness trajectory, that could have contributed to this. Talking with the facilitator helped patients to make connections between their feelings and their COPD, although some patients did not feel that they were anxious or depressed or did not find their breathlessness an issue and may, therefore, have engaged less as a result:
It’s quite interesting to see that they don’t feel that the COPD is their main issue. They think that it’s the other stuff. But then actually when you’re talking to them then they realise that actually the COPD is a problem and that it’s been a bit of an eye opener for them.
Occupational therapist 1
Most facilitators highlighted how the complex lives of patients with COPD affected their day-to-day lives, including management of COPD. Social factors and multimorbidity were prominent in people’s lives. Facilitators worked with patients to identify and address concerns relevant to the patient in a case management approach within the context of the TANDEM intervention. A holistic patient-centred approach to psychological care was viewed as important in tackling the complex medical and social needs of patients with COPD and for improving quality of life:
So one of the challenges I’ve had is that a lot of my patients, breathlessness is a problem. But it’s not necessarily their main problem, because they’ve got lots of other things going on in their lives that have had huge impacts that you can’t really put totally to one side.
Physiotherapist 1
Contact with carers
Some carers (usually partners) sat in on TANDEM sessions. A few facilitators commented on their experience of carers being present during the TANDEM sessions. In some cases, involving the carer positively contributed towards the patient–carer relationship, whereby the facilitator was able to help the carer understand the patient’s experience of living with a chronic condition like COPD:
On the occasion where it was formally done I think it was useful because there was a definitive discrepancy between the thoughts and expectations of the patient compared to the spouse ... think that the participant was more confident than the spouse about their abilities. But there was a mid-point really between the two, because I think the participant was perhaps a little unrealistic. But the spouse was a little bit negative. So I think there was a common bit in between that we were able to reach.
Physiotherapist 4
In some cases, the presence of the carer reassured the patient and the carer could facilitate the session if the patient had problems with speech, hearing or memory:
But the second patient, the more complex one, his wife was always present. And he wanted her present because he has got a bit of a hearing problem as well. And he says that ‘sometimes she hears and remembers things that I don’t. So I always like her with me’. It wasn’t a problem. She didn’t interfere and keep talking or anything like that. It wasn’t a problem. In fact, I think it helped.
Nurse 3
However, the presence of carers could sometimes obstruct the communication between facilitator and patient, although facilitators were usually confident in dealing with this.
One facilitator observed that it was similar to taking on a social support role, particularly when dealing with problems within the patient–carer relationship, and this may highlight a potential need for some training in managing patient–carer dyads:
I find being in someone’s house, telling the husband and the wife how they ought to relate to each other after 45 years or 50 years of marriage is a very unusual ... it’s a very unusual dynamic. Sometimes it almost felt like couple’s therapy. Which I certainly didn’t feel equipped for.
Physiotherapist 3
Experience of facilitator training
The overall experience of the 3-day training for facilitators was positive. Although facilitators found the training challenging, with a lot of material covered, the training prepared the facilitators for the complexities of this specialised role and, in doing so, developed their skills in providing psychological care for patients using a collaborative decision-making approach:
It was, yeah. And I think it was great that they made it so difficult. Because I have had participants who have been as challenging as that. And so it did help to feel that I was on the right track with that role-play and that videotaping. And I could use what I’d learnt from that process.
Nurse 2
Facilitators valued the tools they were provided with during the training, describing the importance of the tools and the time for detailed exploration of anxiety and depression with patients. Facilitators suggested some improvements to the training, such as observing or speaking to experienced TANDEM facilitators, learning about different patient scenarios and some of the challenges to expect (as well as how to address these challenges), and local support and feedback when first delivering the intervention.
Experience of supervision
Facilitators found themselves tackling difficult conversations with patients, which could be overwhelming at times given the complexity of the lives of many of the patients they saw. Supervision was generally considered to be essential in reinforcing their new skills, developing confidence in delivering psychological care and in identifying how to proceed with more complex cases. Supervision was also considered important to debrief when providing psychological care:
... there was one, that tricky lady with the other mental health problems. I was quite worried about her in terms of actually, was my input beneficial, or were there higher levels of stuff going on that were more complicated? So, speaking to [supervisor] about that and helping me work out the best plan of what to do to safety net my patient, that was helpful.
Nurse 5
Impact on professional role
Facilitators felt that it was important to address psychological, as well as physical, aspects of the illness experience of people living with COPD, and were keen to develop skills to address psychological health. This new skill set involved an intentional behaviour change to that of the facilitator’s usual clinical practice. Some facilitators found this change a challenge to start with, with some facilitators experiencing an initial lack of confidence in confronting patients’ mental health issues. However, facilitators also felt that their new TANDEM skill set had enabled them to work collaboratively in a patient-centred way with other patients, improving their clinical practice outside the TANDEM setting:
... from a nursing perspective I can help them physically, I can help you with your emotional psychological needs to a certain limit, but prior to TANDEM I didn’t know those skills and I was passionate and still am passionate about COPD. So the patients for me always felt that I’m really interested and I had that feedback when I left, it was brilliant ... So I imagine those who are interested but not looking outside the box to try and do it that way, they wouldn’t even think to try to find the skills that TANDEM could offer them and ultimately just help the patients.
Nurse 6
Conversely, some facilitators described that they found it challenging to step out of their normal clinical roles while acting as a TANDEM facilitator, and it could sometimes be difficult not to simply provide patients with answers or medical advice linked to their professional role. This was considered to be a potential problem if the TANDEM intervention was to be delivered as part of the facilitator’s usual clinical practice role.
With the time spent with each patient over a number of sessions and in telephone support sessions afterwards, facilitators were able to recognise how patients benefited from the TANDEM intervention and this, in turn, created a learning feedback loop for the facilitator.
Facilitator perspective on implementing the TANDEM intervention (objective 5)
Facilitators believed in the value of the TANDEM intervention and observed the benefits of a tailored psychological intervention for this population. However, about half of the facilitators interviewed mentioned that they could not see the TANDEM intervention being implemented in their current clinical role because of a lack of funding, time and resources:
As a whole programme ... to run it as it is as a programme I think it would be too much bearing in mind I’m one OT [occupational therapist] on 3 days a week and I’m already going out to see people, with you know with lots of different needs and the mood and management part is part of what the condition is, so it would be a staff ... it might be a resource thing as well. But I think it could work ... it would definitely work and it’s also, it’s very much the role to do that it’s just how that works practically.
Occupational therapist 4
Many of the facilitators interviewed believed that convincing commissioners would be the main barrier to implementation within clinical practice. Reasons provided for this barrier included competition for limited funds, psychoeducational interventions can be seen as ‘touchy-feely’, the TANDEM intervention would need to be cost-effective with long-term benefits to patients and the TANDEM intervention would need to fit into the long-term plan for things to change on the ground:
So it’s a lovely thing that we’re doing, but do I think in reality it will carry on? I’m not sure. I’m really ... because of funding, if I’m honest, perfectly honest. I think funding will be a big issue. And CCGs [Clinical Commissioning Groups], will buy into it? I don’t know. They’re all about the pounds, shillings and pence, aren’t they? So I don’t know about that. Sorry, I sound really negative here ...
Nurse 4
Facilitators had opposing views on how acceptable the TANDEM intervention would be to other healthcare professionals. Some facilitators felt that the TANDEM intervention would be accepted by others, whereas other facilitators thought that some professionals may see it as a challenge to their current way of working, particularly if it a was a novel holistic approach to care that required a commitment of time and additional funding.
Many facilitators felt strongly that the training, supervision and the patient-centred TANDEM intervention would need to be replicated at a local level for implementation to maintain the intervention:
But I think the difficulty with integrating it would just be, (a) having the time to do so, and as I was saying, the training is very comprehensive and you need that. But you need everyone to be able to have that. And the clinical time. Because initially just ... I would be just looking over the materials before seeing people, just to try and refresh it each time. Because like I say, anything new just takes a while to embed, doesn’t it? And I think also having that clinical supervision, so making sure that you’re ... Where you’re using it appropriately, you’re using it with the right people in the right way.
Occupational therapist 3
Some facilitators stated that COPD management would benefit from both mental health and physical health support and, therefore, the TANDEM intervention should be delivered as intended. For this reason, facilitators felt that IAPT professionals should not deliver the TANDEM intervention, as the focus may shift purely to mental health:
My concern is if you said like ‘cognitive–behavioural approach’, my worries about that is IAPT to jump on it. And it would also be that it would then be like ‘oh, we can only do talking therapies. We’re only going to talk, and we’re not going to ...’ Whereas actually it was about the home practice. Sometimes it’s about that, the equipment or a graded approach. And it’s that functional. I don’t know, I just ... I know cognitive–behavioural therapy, it is an umbrella term. But I do think that TANDEM’s got that ... because it’s more of an approach, you’ve got flexibility within it. That you want to ... I don’t know. It’s important not to lose that I guess I’m saying.
Occupational therapist 2
A couple of facilitators strongly felt that respiratory professionals or professionals working with respiratory/COPD patients would be more suited to deliver the TANDEM intervention. Several facilitators saw the potential of the TANDEM intervention to reach specific groups of COPD patients in various settings (e.g. frequent hospital attendees).
Perspectives of organisational stakeholders
Participant characteristics
Interviews were conducted with 20 people with a direct or indirect interest in COPD management, including GPs, psychologists, respiratory doctors, nurses, physiotherapists, psychiatrists and IAPT practitioners (Table 23).
| Professional role | n |
|---|---|
| GP | 2 |
| GP with CCG role | 2 |
| Respiratory consultant | 2 |
| Respiratory consultant with CCG role | 1 |
| Psychiatrist | 2 |
| Respiratory nurse | 3 |
| Respiratory physiotherapist | 3 |
| Psychologist | 2 |
| Psychologist with IAPT lead role | 1 |
| CBT therapist | 1 |
| CBT therapist with IAPT lead role | 1 |
Stakeholder perspective on implementing the TANDEM intervention (objective 6)
Perceived enablers of implementation
There was appreciation that for many people with COPD there were several barriers to engaging with healthcare services, including, for example, difficulty travelling to appointments. Attending separate appointments with different healthcare professionals was highlighted as particularly challenging and, therefore, the number of services to which people are exposed should be reduced. Stakeholders felt that a strength of TANDEM intervention was the provision of integrated physical and psychological care within one healthcare service:
Physical and mental health, they come together. They come as a package. So, I think we should treat it as a package.
Participant 4 respiratory physiotherapist
Stakeholders recognised that reaching people at home improved the opportunity for engagement and enabled services to understand wider social determinants, which ultimately could improve uptake of PR:
When people go out to the house you often get all the added benefit of that social interaction... this person’s really struggling at home, we need to get the social work involved and stuff.
Participant 6 respiratory consultant
Furthermore, home visits enable services to understand contextual issues, such as social well-being of the patients, that could affect uptake of PR and be communicated to other primary care services:
And also, you can always feed back to us if they’ve got any issues at home. Because we can’t visit everyone.
Participant 1 GP
Several stakeholders were confident that respiratory professionals could be trained to deliver the TANDEM intervention and it was considered that the existing relationship with patients related to their physical care was a good basis for engagement with a psychological intervention:
But there’s a lot of trust straight away with the respiratory practitioner and I think already, because it’s coming from physical health initially, they’re happy then to engage with the mental health. Rather than starting the other way around really.
Participant 14 PR clinical lead/physiotherapist
Perceived obstacles to implementation
Stakeholders appreciated that the TANDEM intervention was an additional service to existing provision and that the TANDEM intervention would need to be resourced properly; however, concerns were expressed about the availability of resources and competing commissioning demands. There was agreement that the TANDEM intervention must demonstrate cost effectiveness and that this would be demonstrated by reduced healthcare utilisation:
And they will have to fight against their highly specialised respiratory colleagues ... who are more interested in bright, shiny, sexy respiratory [services].
Participant 11 GP and CCG commissioner
Does it work? Is it cost effective?
Participant 3 respiratory consultant and CCG commissioner
The key metrics are going to be, does it reduce hospital admissions ... and does it reduce GP visits.
Participant 2 respiratory consultant and respiratory lead
Organisational change barriers were also raised and it was highlighted that successful implementation would require ongoing support and supervision to ensure adherence to the approach:
But this stuff takes practice, doesn’t it? And you have to keep repeating the things that you learnt in order to make them your default setting. And I think that when people go back into their working environments, there’s so much pressure to behave like they used to...
Participant 3 respiratory consultant and CCG commissioner
Recruitment of TANDEM facilitators and cognitive–behavioural therapy-trained supervisors
Potential TANDEM facilitators were identified successfully via news articles and advertising campaigns across respiratory healthcare networks and associated social media. In addition, TANDEM researchers ran a stall about the study at two successive Primary Care Respiratory Society annual conferences.
To avoid any risk of contamination and to preserve blinding of healthcare providers, TANDEM facilitators were recruited from staff not involved in either the provision of routine COPD care or the delivery of PR for COPD at participating sites while the study was running. Typically, TANDEM facilitators were recruited from neighbouring trusts that were not participating in the study, often from part-time staff who were willing to work an extra day per week for the duration of the study. Other facilitators were experienced respiratory healthcare professionals with portfolio careers in respiratory health based outside the study areas (see Chapter 2 and Steed et al. 66 for a detailed description of screening, training and assessment of TANDEM facilitators).
The senior clinical psychologist was already involved in the study as a co-applicant (SW). The CBT-trained supervisors were either already known to the study team and invited to join as a supervisor or recruited from an advertisement in the British Association for Behavioural and Cognitive Psychotherapies’ official membership magazine CBT Today (delivered free to over 10,000 members across the UK and Ireland) [URL: https://babcp.com/Membership/Join-Us (accessed 28 October 2022)].
Facilitator recruitment (objective 4)
Of 91 healthcare professionals who expressed an interest in the TANDEM facilitator role, 52 (57%) applied formally and had a telephone interview with one of the study leads (SJCT/HP) and/or the TANDEM trainer (LS) (Figure 16). Of the 42 healthcare professionals who attended the TANDEM training course training, 39 were deemed ready for the facilitator role on completion of the training and assessment of competence, including 2 healthcare professionals who left following training in the pilot study (1 for maternity leave and 1 because of family commitments), but both were later retrained and contributed to the main trial. Of the 39 trained facilitators, 8 left the study before being assigned any participants and the reasons for leaving were moving to a new job (n = 4), unhappy with the research governance administrative burden (n = 2) and because of other NHS work commitments (n = 2).
FIGURE 16.
Recruitment of facilitators. CV, curriculum vitae; HCP, healthcare professional.

Characteristics of facilitators
The professional status of the 31 facilitators who delivered the intervention were specialist nurse (n = 9), physiotherapist (n = 13), occupational therapist (n = 6) and physiologist (n = 1). All 31 facilitators had a respiratory background.
Facilitator training (objective 3)
Mean competency score on the CFARS,162 on post-training assessment, was 33 (range 21–41). Only three of the 34 potential facilitators did not meet the threshold competency score of 27 after initial training; however, all three facilitators underwent additional training and two were subsequently deemed competent, but one did not become a TANDEM facilitator. Two further healthcare professionals had been trained for the pilot; however, for personal reasons, were initially unable to deliver the TANDEM intervention in the main trial. The two healthcare professionals later approached the study team to re-join as facilitators and both underwent training for a second time. The competencies that scored most highly were ‘collaborative relationship’ (3.9) and ‘interpersonal skills’ (3.9), with ‘guided discovery’ (3.3) and ‘closure’ (3.4) scoring lower.
Results of fidelity assessment
Receipt of the TANDEM intervention
Twenty-two (9%) intervention arm participants did not receive any CBA sessions, a further 24 (10%) received just one session and 136 (56%) received six or more sessions (see Appendix 1, Table 23, for details). The number of TANDEM intervention sessions received by the 242 participants in the intervention arm of the study is shown in Figure 17.
FIGURE 17.
Number of intervention sessions received by TANDEM intervention participants (n = 242).
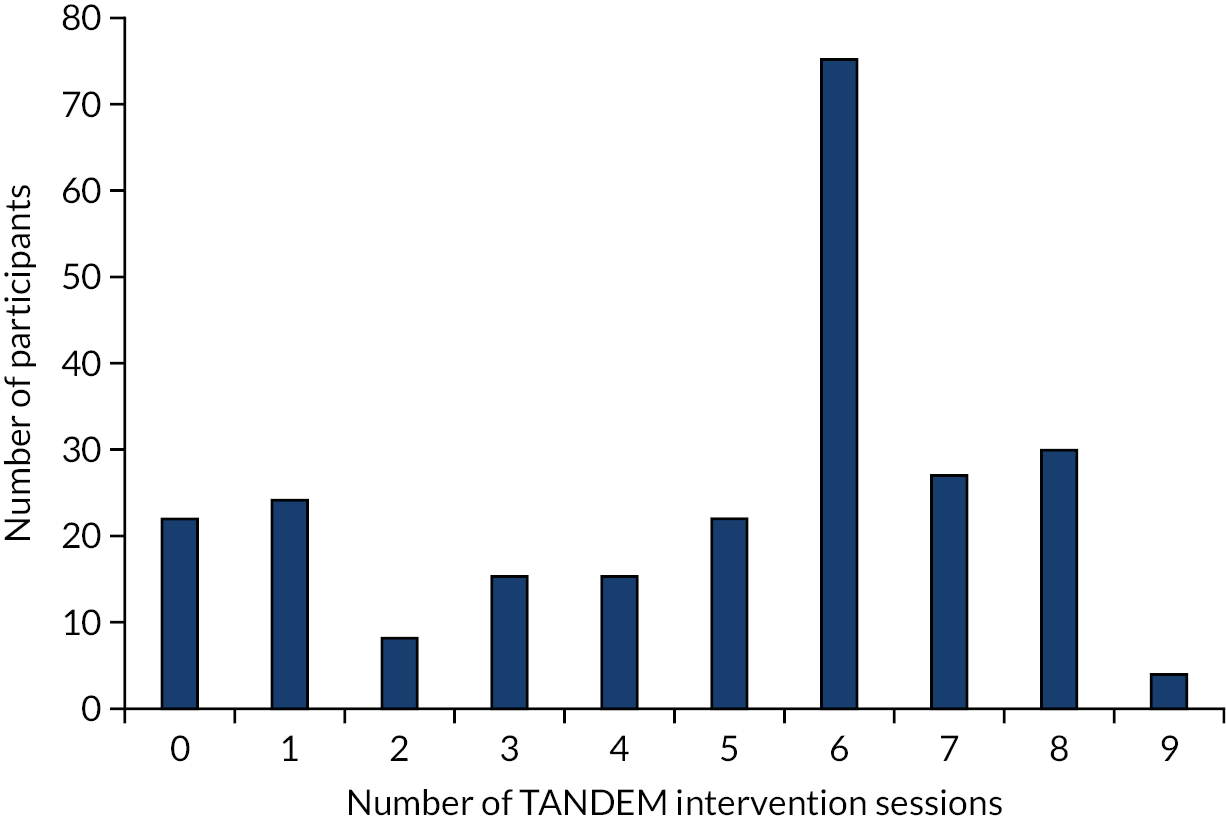
From the caseloads of the 28 facilitators who delivered the intervention in the main trial (i.e. after the conclusion of the internal pilot in which the intervention was delivered by three additional facilitators), 37 cases were randomly selected for inclusion. Twenty-eight cases were selected from the first five participants and nine cases were selected from the participants 10–15. Consent for audio-recording was not available from one participant and so data are reported for 36 cases delivered by 27 facilitators. The median number of audio sessions per case was 5 (IQR 4–6), providing a total of 180 audio-recorded TANDEM treatment sessions.
Cognitive First Aid Rating Scale
The mean therapeutic competence (i.e. CFARS) total score across facilitators when delivering the intervention in the main trial (Figure 18) was 35.3 (SD 7.2). Twenty eight (78%) of 36 cases achieved fidelity, with a mean score of 38.2 (SD 5.4), which is comparable to other trials (a mean score ≥ 30 reflects performance on all 10 items). 162,163
FIGURE 18.
Mean CFARS score for the 36 cases (27 facilitators).
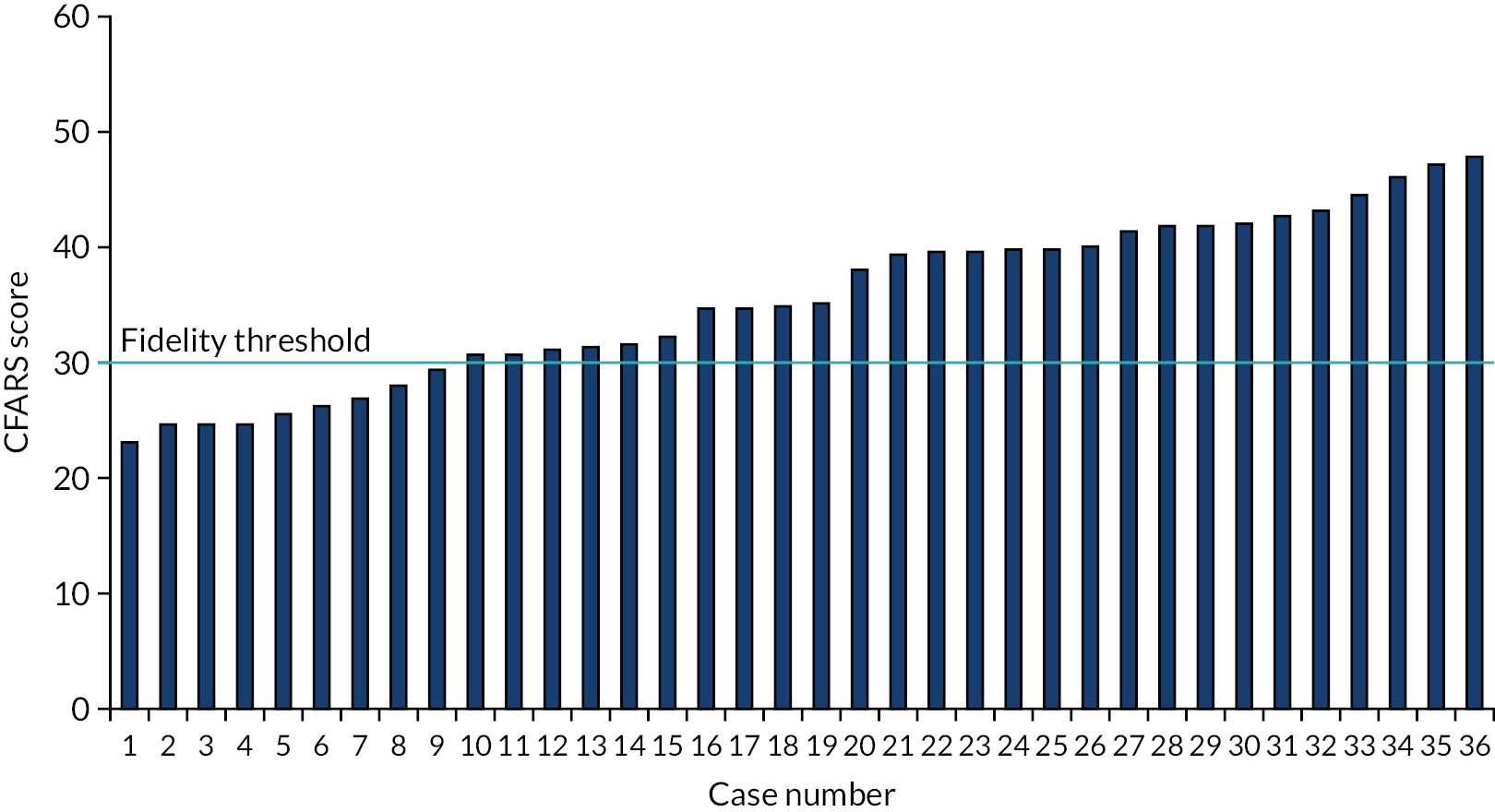
Mean scores for individual items on the CFARS are reported in Figure 19. Higher competency was observed for focus and structure of the session, collaborative relationship and interpersonal effectiveness. In contrast, facilitators demonstrated lower competency for guided discovery and application of appropriate change techniques.
FIGURE 19.
Mean scores for individual items of the CFARS for the 36 cases (27 facilitators).

Adherence to TANDEM intervention core components
All core intervention components across sessions reached > 80% adherence, with the exception of agenda-setting (Figure 20), and this is considered high fidelity. 161
FIGURE 20.
Proportion of cases reaching fidelity for the TANDEM intervention core components.

Anxiety content was delivered to 25 (69%) of the 36 participants and depression content was delivered to 21 (58%) participants. Fourteen (39%) participants received both anxiety and depression content. Four cases received no anxiety or depression content, as treatment was discontinued because it was no longer required.
Handouts
Handouts were provided by facilitators for patients to use between sessions. All 36 patients received the ‘control your breathing’ handout and 34 patients received the ‘mood and COPD’ handout. The remaining handouts, including handouts for anxiety and depression, were tailored to content (see Figure 21 for frequency of use).
FIGURE 21.
Proportion of participants provided with the tailored session handouts during the course.

Pulmonary rehabilitation-specific intervention content
Twenty-three of 36 (64%) participants confirmed their intention to attend PR. Discussing what to expect from PR was delivered in 20 (87%) of these cases and eliciting attitudes to PR (i.e. thoughts, feelings and expectations about PR) was delivered in 22 (96%) cases. The photobook (i.e. a bespoke book of photographs to enable facilitators to familiarise participants with their local PR services and staff) was used in only five cases and problem-solving was delivered in 35% of cases (Figure 22).
FIGURE 22.
Proportion of patients provided with the specific support to prepare for PR.
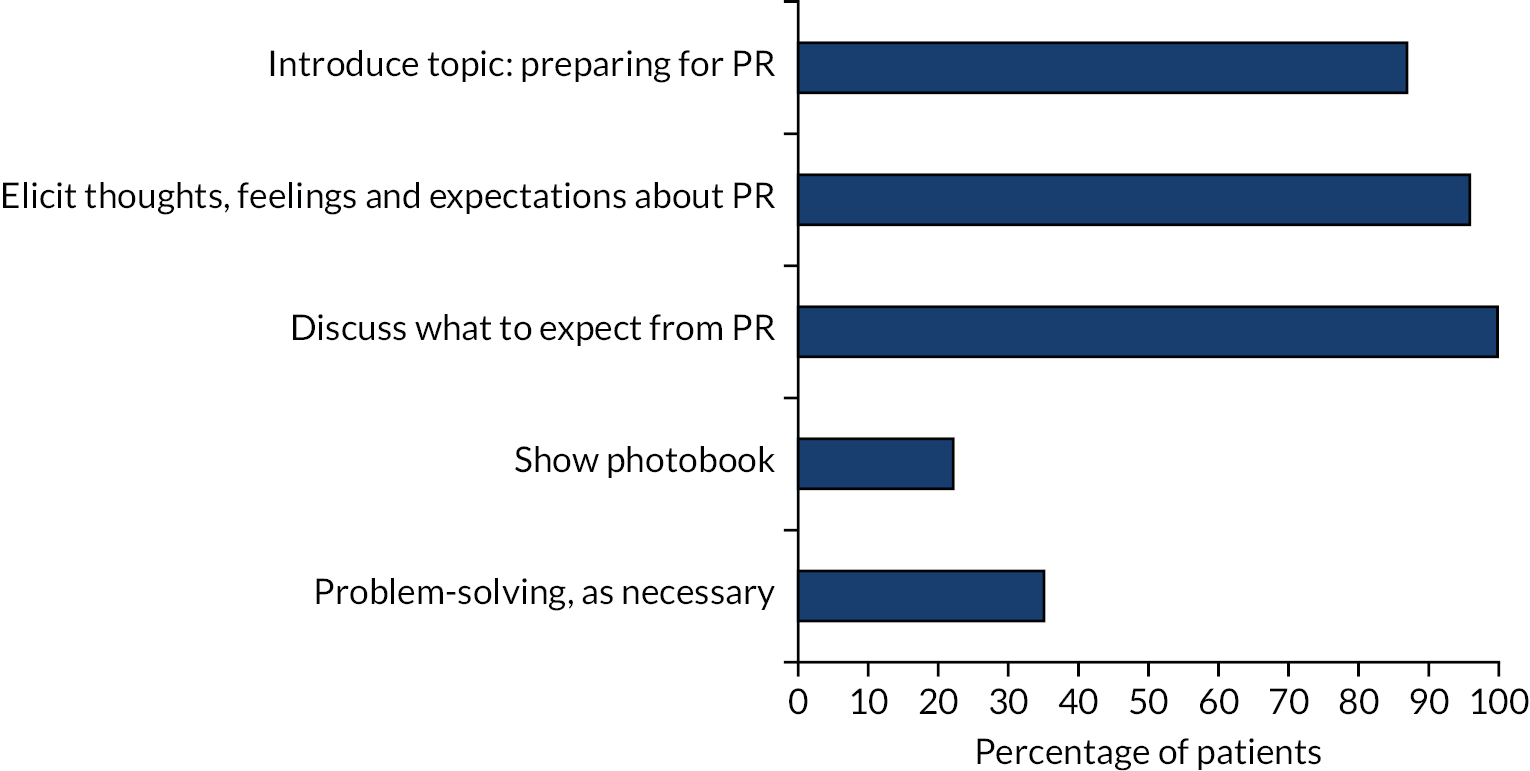
Summary of themes from the process evaluation related to the objectives
Objective 1: themes related to acceptability of the intervention to patients and carers.
Participating in the TANDEM therapy sessions
-
The format and length of weekly sessions were acceptable, and receiving the intervention at home (or having a choice of location) was often valued, although some participants felt that it did not help them.
-
Participants appreciated the knowledge and skills of the facilitators, and liked the facilitators’ friendly, empathetic and supportive nature, which enabled participants to feel safe discussing their thoughts and feelings in relation to their COPD, other conditions or social situations.
-
Initial TANDEM sessions and homework tasks involved patients taking time to consider their thoughts, feelings and behaviours in a new way. A few participants were not comfortable with the reading and writing involved, but the facilitators were able to adapt to this. Homework ‘tasks’ were often not completed.
-
Practical tools were helpful for reflection and were used in management of COPD (and other conditions). The linking of mood and COPD with practical strategies was seen as very beneficial and increased awareness and understanding of COPD and anxiety/depression.
Unexpected themes relating to the complexity of patient lives
-
Lack of understanding about COPD and its effects by family and society leads to feelings of being judged and embarrassment.
-
COPD and multimorbidity have a cumulative effect on physical, psychological and social health, although patients have adapted and become resilient to living and coping with their condition(s).
-
Patients often prioritised competing family and social responsibilities over their own health and needs.
-
Carers provided a mix of personal, practical and emotional support based on the changing needs of the patient.
Impact of living with COPD during the COVID-19 pandemic
-
Ongoing or planned physical and social activities stopped because of the COVID-19 pandemic, with challenges for the caring role. Some patients drew on techniques learnt in the TANDEM intervention to cope with their mood, but other patients described a negative impact of the pandemic on their health.
Objective 2: themes relating to the acceptability of the intervention to facilitators and supervisors
Practicalities of facilitating the TANDEM therapy sessions
-
The TANDEM intervention was viewed as an acceptable and effective way of delivering psychological care to patients with COPD, which was generally seen as bringing value to patients.
-
Training and clinical supervision were integral to embedding new skills and building confidence to deliver the TANDEM intervention, and, more specifically, enabled development of valuable skills in psychological care using collaborative decision-making.
-
Most facilitators were not confident to provide psychological care initially, but felt that their competence developed over a series of sessions, which enabled them to experience a feedback loop where they could see impact on the patient. Clinical supervision was necessary for delivering psychological care and embedding the new skills.
-
Most facilitators were able to manage the workload effectively, although some facilitators found it difficult to balance time on the TANDEM intervention with their clinical roles.
-
The TANDEM intervention provided the time and space to deliver valuable holistic care using skills that could potentially enhance interactions in the Facilitators’ routine care, but there was discussion about NHS resource constraints.
Unexpected themes arising
-
The social complexity of patients’ lives was highlighted. Through the TANDEM process, facilitators were able to identify the concerns relevant to patients in the context of their lives, which raised social, as well as psychological, issues.
-
Some facilitators highlighted the challenge of managing TANDEM therapy sessions that involved carers, and, although this was not necessarily negative, specific training in this would be helpful.
Objective 3: fidelity of training for and delivery of the intervention
-
Only 3 of the 42 potential facilitators who attended the 3-day training did not meet the threshold competency (assessed on a role play with a professional actor); however, two of these potential facilitators went onto to achieve the required standard after additional training.
-
Higher competency for delivery of the intervention was observed for ‘focus and structure of the session’, ‘collaborative relationship’ and ‘interpersonal effectiveness’. In contrast, facilitators demonstrated lower competency for ‘guided discovery’ and ‘application of appropriate change techniques’.
-
Core intervention components across the TANDEM sessions were delivered with high fidelity, with all components reaching > 80%, except for agenda-setting.
Objective 4: feasibility of implementing the intervention with respect to the process of recruitment and training of facilitators, and uptake and completion by participants
-
There was attrition at every stage of the facilitator recruitment and training process. Of 52 respiratory healthcare professionals who applied formally, 31 completed training and facilitated at least 1 TANDEM therapy course.
-
Of 242 participants allocated to the intervention, 219 (90%) attended at least 1 TANDEM therapy session and 196 (81%) completed 2 or more sessions (i.e. our predefined minimal clinically effective dose).
Objective 5: summary of the patients’, carers’, facilitators’ and supervisors’ experience of the intervention
Patient experiences and perspectives regarding the intervention
-
Participants were positive about facilitators’ knowledge of COPD and mental health, and valued the facilitators’ personalised communication and therapeutic skills.
-
Participants felt that they had established a good rapport and positive relationship with facilitators, and were able to open up and talk freely about their mood, which raised awareness for some participants that they had anxiety/depression.
-
Most participants reported improvements in mental health, breathlessness and social activities, although some participants did not perceive any benefit. Perceived improvements became embedded for some patients and helped with managing their COPD, whereas for other patients the positive impact was not sustained because of personal and external factors.
-
The care received in the TANDEM intervention was different from current services offered to people with COPD.
Facilitator experiences and perspectives regarding the intervention
-
Holistic care, integrating mental and physical health was viewed as important in caring for people living with COPD.
-
Facilitators felt that it was important to address psychological, as well as physical, aspects of illness, and engaging with patients to address anxiety and depression using collaborative, personalised care delivery had the potential to tackle complex medical and social care needs.
Objective 6: summary of the views of all stakeholders regarding implementation of the intervention
Patient and carer perspectives on implementation
-
Uptake of the TANDEM intervention may be influenced by perceived need owing to a patient’s condition or level of anxiety/depression and perceived individual benefits.
-
Flexibility in the location for intervention delivery was highly valued.
-
Patients could potentially be offered the TANDEM intervention at different times over the course of the illness trajectory.
Facilitator perspectives on implementation
-
Resource and organisational constraints are likely to impede undertaking the TANDEM intervention as part of usual clinical roles.
-
Wider changes are needed within the current COPD political/healthcare system context for successful TANDEM intervention implementation.
-
Most stakeholders agreed that the TANDEM intervention would be best delivered by respiratory health professionals, but training and supervision would need to be replicated at local level.
Organisational stakeholder views on implementation
-
A strength of the TANDEM intervention was the provision of integrated physical and psychological care within one healthcare service, but it was an additional service and would need to be resourced properly. Clinical effectiveness and cost effectiveness would need to be demonstrated.
-
Reaching people at home improves the opportunity for engagement and ultimately could improve uptake of PR.
Chapter 7 Discussion
Summary of study and findings
Following careful intervention development based on previous work and adhering to MRC guidance for complex interventions,67,70,86 we developed the TANDEM intervention for people with both (1) symptoms of mild to moderate anxiety and/or depression and (2) COPD with moderate to very severe airways obstruction (COPD Gold criteria). 2,3 The intervention is manualised and tailored to participants, and is designed to be delivered by trained healthcare professionals with experience of working with people with COPD while receiving structured supervision throughout from a trained, experienced clinical psychologist or CBT therapist. The TANDEM intervention consists of six to eight structured face-to-face sessions and follow-up telephone calls based on a CBA and includes practical self-management advice and materials, as required, from the SPACE manual73 and a range of COPD patient and carer advice materials (e.g. DVD, leaflets) from the BLF. The TANDEM intervention was designed to precede and ‘bolt on’ to an offer of routine PR. As a result of the COVID-19 pandemic, a small number of participants received all or some of the TANDEM intervention remotely, as in-person PR was not consistently available for participants between March 2020 and the end of the study in June 2021. The services that offered remote PR took time to get started after the cessation of face-to-face PR, reporting to us that face-to-face PR commenced between May 2020 and September 2020.
Trial clinical outcomes (objective 1)
The TANDEM intervention was evaluated in a well-powered individual-patient randomised controlled trial (n = 423; 1.25 : 1, intervention: control), which included an internal pilot. At 6 months post randomisation, no benefit was seen from the intervention on the co-primary outcomes of anxiety and depression, as determined by the HADS subscales. 100 The 95% CIs effectively ruled out any possibility of a clinically important effect (and similar results were seen at 12 months). In addition, no benefit from the intervention was seen on any of the following secondary outcomes at 6 or 12 months: anxiety and depression as measured by the BAI and the BDI II,110,113 quality of life as measured by the SGRQ (i.e. total score or any of its subscales of activity, emotion and impact),114 social engagement as measured by the social integration and support subscale of the heiQ116 and social activity as measured by the Time Use Survey. 117 There was no evidence of any consistent benefit on the subscales of the B-IPQ119 across the follow-up period, nor was there any improvement in smoking cessation or attendance/completion at PR associated with the intervention. No difference in healthcare resource use was observed between intervention and control arm participants at 12 months’ follow-up.
A number of pre-planned sensitivity analyses, which included examining the effect of the COVID-19 pandemic, failed to show any evidence of benefit in specific subgroups or in specific contexts.
Impact on carers (objective 2)
There were no detectable differences in outcomes between carers of participants in the two arms of the study, although numbers were small and the CIs were wide.
Cost effectiveness (objective 3)
A health economic evaluation of the TANDEM intervention conducted in parallel to the clinical effectiveness trial found that the intervention is highly unlikely to be cost-effective and there was a high degree of certainty that the intervention would not offer sufficient value for money to be funded by the NHS.
Process evaluation (objective 4)
Fidelity
The TANDEM intervention was delivered with therapeutic competency and key tasks were delivered with fidelity and, therefore, the lack of effect is unlikely to be explained by a failure to deliver the intervention as planned.
Uptake and attrition
Eighty-one per cent of intervention participants received at least two CBA sessions (i.e. our predefined minimal clinically effective ‘dose’ of the intervention) and 56% received six or more sessions (a full course of the intervention was usually six to eight sessions tailored to the needs of the participant).
Attrition from the study was relatively low and overall 93% of participants were followed up at 6 months (i.e. the time frame for the primary outcome) and just 13% of participants were lost to follow-up at 12 months, which includes deaths, withdrawals and patients loss to follow-up. At both time points, attrition was slightly higher in the intervention arm than in the control arm (6-month follow-up: 90% vs. 97%; 12-month follow-up: 80% vs. 88%, respectively).
Perceptions of the TANDEM intervention
Respiratory health professionals recruited to train as TANDEM facilitators recognised the need for holistic care for patients with COPD and were keen to develop their knowledge and skills in addressing psychological health needs. Facilitators considered their training to be a challenging but effective way of developing skills in psychological care.
The TANDEM intervention was generally well received by study participants. Although some participants did not feel that the TANDEM intervention could offer them anything, many other participants felt that the TANDEM intervention clearly enabled engagement with issues arising in the contexts of their (often complex) lives and many instances of positive effects on quality of life were described. Developing a therapeutic alliance was considered necessary by patients, carers and facilitators, but it took time to build rapport, and the complexity of the therapeutic task was highlighted in the patient, carer and facilitator interviews. The relationship between patient and facilitator built through the TANDEM process enabled therapeutic collaborative work to be carried out, linking physical and mental health with social aspects of people’s lives. Patients and facilitators considered the home environment to be most appropriate for delivery of the TANDEM therapy sessions.
Potential for implementation
Although most patients and health professionals felt that TANDEM improved health care and should be available for people living with COPD, some of the patients and most of the health professionals considered that resource constraints would affect post-trial implementation. Health professionals felt that it would not be possible to deliver the TANDEM intervention as part of their usual clinical respiratory health role.
Strengths and limitations
Strengths
In this well-powered trial, we recruited individuals with COPD and moderate to very severe airways to receive a theoretically informed, manualised intervention tailored to their symptoms of mild to moderate anxiety and/or depression. Allocation was fully concealed and participants were randomised only after collection of baseline data. Although it was not possible to blind the participants, their carers or the facilitators from the arm of the study to which individuals had been allocated, outcome assessment was blinded by using researchers from an adjacent TANDEM team to undertake randomisation and communicate allocation. Accidental unblinding, which was meticulously recorded, was rare (15/423 participants, 3.5%). Trial analysis was blind to allocation. All healthcare professionals (i.e. primary care teams, secondary care teams and PR teams) were unaware of the arm of the study to which participants had been allocated. The trial protocol and statistical analysis plan were published in advance of any data analysis.
The TANDEM intervention was conceived and designed with fidelity of intervention delivery in mind, and our multiperspective process evaluation included a detailed assessment of facilitator training and the fidelity with which the intervention was delivered, enabling us to be confident that the lack of effect was not due to limited or poor implementation. In addition, our year-long pragmatic trial, with a health economic evaluation, considered implementation from the outset. We trained respiratory specialists (mostly nurses and physiotherapists) rather than employing a dedicated cognitive–behavioural therapist. Although this meant that we delivered a low-intensity CBA intervention, rather than high-intensity CBT,29 the strategy reflected a likely strategy if the intervention were to be implemented in the NHS and had the added advantage of using professionals who were already well aware of COPD, its management and the consequences of the condition for patients and their supporters.
Patient and public involvement colleagues were central to the project, shaping the initial conception, steering the invention design and advising on conduct of the trial. In addition, PPI colleagues will support and advise us on the dissemination of our results. Along with the theoretically based patient-centred approach to intervention development, PPI ensured that the TANDEM intervention was generally acceptable and valued by most (although not all) participants who agreed to be interviewed.
Limitations
There were, however, some limitations. We had very few exclusion criteria [principally people whose anxiety or depression required specialist psychological input or who would be unlikely to be able to gain benefit from the TANDEM intervention (e.g. because of cognitive impairment)]. Despite this, only half of the nearly 4500 people contacted by their clinical team as potentially eligible responded, and we screened over 1062 patients to recruit only 426 participants. This limits generalisability, as the population in our trial were self-selected as being potentially interested in a ‘talking therapy’. Although, participants participating in interviews may be more cognitively able, some were not able to recall details of the intervention, perhaps because of the 6 months that had elapsed from completion of the therapy sessions and the interview or, in some cases, because of new health-related or other significant events.
Although we did not exclude potential facilitators able to deliver the TANDEM therapy in other languages, the trial required participants to be able to communicate in English (for completion of the outcome questionnaires). Our recruitment sites covered very different geographical areas, but we did not collect participants ethnicity and so we are unable to comment on the mix within our study. Written English was not a requirement. We were aware that many people with COPD come from deprived backgrounds and that some participants might be less literate than others. Therefore, we provided information for all participants in the form of a DVD and our researchers were able sensitively to support completion of questionnaires.
At the request of our funders we included a substudy looking at carers, but, as anticipated from our previous work,164 we recruited very few carers and, understandably, carers withdrew if the index participant withdrew or died. We conclude that if carers of people with COPD are to be invited to participate in a research study, then researchers should recruit them directly rather than via the patient for whom they are caring. 165 The small number (n = 43) of carers recruited in this study limited our ability to identify any indirect impact of the intervention on carers.
The pandemic disrupted both the delivery of the intervention, which had been designed as face to face, and collection of the outcomes. We were able to adapt to virtual delivery of the CBA sessions and some participants agreed to receive the intervention by telephone, although others did not. We were not able to explore perceptions of remote delivery, as we had completed the patient interviews in line with our pre-planned sampling frame and timeline for completing the process evaluation. We already had arrangements for postal or telephone collection of follow-up questionnaire data to maximise data collection in the event that participants requested this rather than a face-to-face visit, but the lack of face-to-face supervision of questionnaire completion may have led to some questions being missed. Collection of data from primary care records was challenging, as researchers were unable to visit practices during lockdown and we were reliant on practice staff who were preoccupied reorganising their clinical care and often working remotely, and this greatly reduced the health service usage data we were able to obtain after March 2020.
Limitations of the health economic evaluation
The economic evaluation has limitations. The within-trial analysis by implication does not consider outcomes extrapolated beyond the period of the trial. In principle, this can be important when evaluating interventions that focus on people with long-term conditions. Notwithstanding the uncertainty this can generate, it would seem implausible based on the short-term (12-month) differences in economic and clinical outcomes observed in this study that a consideration of longer-term effects would have altered the conclusions reached.
Given analytical time constraints, the analysis for this report prioritised the costing of health and social care contacts, as this included by far the most expensive items of resource use (e.g., inpatient admissions) that could in principle have affected the cost effectiveness of the TANDEM intervention. The cost of antidepressants and other medication that the TANDEM intervention could have affected were excluded. Although this limits the comprehensiveness of our analysis of costs for trial participants, we do not anticipate that this omission would have altered the main conclusion reached, as antidepressants are comparatively inexpensive compared with other areas of health service utilisation and the TANDEM intervention was also found to have no effect on mental health outcomes as a vehicle for reducing the need for antidepressant prescribing.
Limitations of the process evaluation
Although we purposively sampled a broad range of patients, carers, facilitators, supervisors and other stakeholders, we may not have heard all opinions of the TANDEM intervention. We may, for example, have recruited people with a positive bias towards the TANDEM intervention and those who did not agree to be interviewed may have reported different experiences and views. In addition, patients who agreed to be interviewed may have been more cognitively able.
Although we were open to all experiences related to the TANDEM intervention, one focus of the interviews was to explore implementation, which may have led to an assumption that the intervention would be effective and encouraged positive comments. Some of the facilitators, however, did express the concern that the TANDEM intervention was an expensive intervention that would be difficult to implement within limited NHS resources. Specifically, most facilitators considered that it would not be possible to integrate the TANDEM intervention within their usual clinical role.
The detailed fidelity assessment was a major strength of the study and encompassed both facilitator training and delivery of the intervention, although we did not assess the homework.
We were aware of reflexivity, and although the process evaluation was managed separately to the main trial there was overlap with some personnel. The multidisciplinary discussions aided a balanced interpretation.
Strengths and weaknesses in relation to other studies, discussing particularly any differences in results
Recruitment, attrition and outcome measures
To the best of our knowledge, this is by far the largest randomised controlled trial of any psychological intervention for people with COPD published to date, and one of the very few randomised controlled trials to include only patients with symptoms of anxiety and depression. 45 Recruitment to the study was very close to that predicted in our original application (i.e. we estimated that we might need to approach 4720 potential participants with COPD). Most participants were recruited via mail outs from their GPs, with 2191 (49%) participants agreeing to be approached by the research team to learn more about the study, of whom nearly half 1062 (49%) were found to be potentially eligible. A total of 441 (42%) of the participants screened were found to be eligible, most of whom joined the study.
We had low attrition, unlike several of the studies in the recent Cochrane review of psychological interventions for people with COPD, which reported high attrition rates. 166–171
The TANDEM intervention did not succeed in improving mood in participants with COPD with symptoms of mild to moderate anxiety and/or depression, as assessed by the HADS instrument. The HADS instrument has been found to be relatively poor at discriminating between anxiety and depression in COPD, although overall it appears to be a good measure of emotional distress and a useful screening tool in medical populations. 172,173 The HADS is one of the most common screening tools for depression in COPD studies and appears to perform as well as the BDI II and the other commonly used screening tools. 104,174 In addition, we measured BAI and BDI II scores at baseline and follow-up, and the baseline results for these instruments suggest that the HADS instrument successfully identified people with symptoms of mild to moderate anxiety and depression in our study.
Impact on mood
The TANDEM intervention failed to both improve mood (i.e. the primary outcome) and increase uptake and completion of PR (i.e. a very important secondary outcome). We had anticipated that if the TANDEM intervention had succeeded in both these outcomes them there would be synergy in the effect on mood, as PR also has as a positive effect on mood, improving both anxiety and depression. 50,175
We are aware of only seven other published randomised controlled trials that have examined similar interventions in participants with COPD and anxiety and/or depressive symptoms at baseline. Five randomised controlled trials were delivered to individuals71,167,176–178,179 and randomised controlled trials two investigated forms of group CBT. 166,180
Lamers et al. 167 tested a ‘minimal psychological intervention’ based on the Chronic Disease Self-Management Programme,181 Goldberg’s reattribution model (which is also part of CBT)182 and other work by the group. Set in the Netherlands, 187 participants with COPD were randomised to intervention or usual care. The intervention was delivered by a trained nurse in the patient’s home over 3 months in up to 10 visits. Participants had been formally screened for depression before joining the study. Although the severity of their COPD was not recorded, baseline BDI II scores suggested that participants had, on average, mild depression. After 9 months, but not after 3 months, the BDI II was significantly lower (i.e. improved) in the intervention arm of the study, as were symptoms of anxiety and SGRQ total scores. However, the relevance of the of the study is hard to determine, as no primary outcome was stated and attrition was very high (40% in the intervention arm).
Doyle et al. 176 conducted a small randomised controlled trial (n = 110), comparing telephone-administered CBT and befriending (as an ‘active social control’) for people diagnosed with COPD and mild levels of anxiety and/or depression in Australia. Both CBT and befriending were delivered over the telephone across eight weekly sessions following an initial rapport-building session. Unlike the TANDEM intervention, CBT sessions were delivered by registered, or provisionally registered, psychologists experienced in delivering telephone CBT. As in the TANDEM evaluation, there were co-primary outcomes measuring anxiety (using the BAI) or depression (using the PHQ-9). The study was underpowered according to the authors’ power calculation and no significant between-group differences for anxiety or depression were seen.
In Hynninen et al.’s180 small, underpowered trial (n = 52), intervention arm participants attended 7-weekly 2-hour group CBT sessions in a hospital outpatient clinic in Norway. At 6 months’ follow-up, the decrease in anxiety (BAI) and depression (BDI II) was greater in the intervention arm than in the control arm (note that patients in the control arm received 2-weekly 5- to 10-minute telephone calls from the study team).
A randomised controlled trial (n = 238) by Kunik et al. 166 compared mostly male (> 95%) participants with COPD and anxiety or depressive symptoms (as determined by the BAI or BDI II, respectively) randomised to either eight 1-hour group sessions or eight 45-minute educational lectures, each with 15 minutes for discussion about COPD. Both the CBT and lectures were delivered by psychology interns and post-doctoral fellows with experience of delivering CBT. Attrition was very high (> 50% at 4 months) and there was no significant difference in anxiety, depression or quality of life (i.e. the primary outcome) at all follow-up periods up to 1 year.
Bove et al. 177,178 led a small randomised controlled trial (n = 66), comparing a 1-hour home visit psychoeducational session ‘based on... CBT’ and a telephone ‘booster session’ 2 week’ later, involving Socratic questions and discussion of models linking thoughts, emotions, sensations and behaviours, compared with usual care. Study participants had severe COPD and symptoms of anxiety (HADS-A scores ≥ 8). Not all baseline HADS-A scores were collected prior to randomisation. At 1 and 3 months post intervention, there were significantly better HADS-A scores in the intervention arm than in the control arm, but the risk of bias was high, as outcomes were collected in person by the principal investigator who also delivered the intervention.
Heslop-Marshal et al. 71 randomised 279 participants with COPD and symptoms of anxiety (HADS-A scores ≥ 8) to receive either an active control (a self-help leaflet addressing anxiety management) or a brief CBT intervention of 2- to 6-fortnightly sessions delivered in either outpatients or the patient’s home by a trained nurse. The primary outcome was change in HADS-A at 3 months’ follow-up. Attrition at 3 months was low and there was a statistically significant reduction in HADS-A score of a size suggesting clinical significance in the CBT arm compared with the leaflet arm at 3 months. At 6 and 12 months’ follow-up, the difference in favour of the CBT participants was much smaller and not clinically significant.
In a randomised controlled trial, Pumar et al. 179 attempted to evaluate CBT (first two sessions face to face followed by four sessions via telephone) delivered by psychologists to people with chronic lung disease undergoing PR who screened positive for anxiety and or depression. Approximately 75% of participants had COPD. Slower than anticipated recruitment led to the study falling short of its intended sample size, with 65 participants rather than 100 participants randomised in total, and only 24 participants were randomised to the intervention. Attrition was high, especially in the intervention arm and only nine intervention arm participants provided data at 12 months’ follow-up. No significant differences in anxiety or depression were seen between participants in the study arms at 3 and 12 months’ follow-up, but the small sample size makes the findings hard to interpret.
Overall, the Cochrane review of all psychological interventions for people with COPD and depressive symptoms concluded that there was some evidence for the effectiveness of psychological therapies with a CBT‐based approach in depression associated with COPD. However, the effect sizes were small and the evidence was of low quality and at risk of bias. 45 The review called for larger, more robust studies that consider AEs, health service use and cost-effectiveness outcomes. 45
Implications for patient and public involvement methodology
The contribution made by our PPI advisors helped us to deliver a high-quality trial. It was rewarding to work with patients and carers, and having patients and carers as part of the study team helped to ensure that we conducted the trial in line with patients’ perspectives and needs. The PPI members were enthusiastic and motivated to help benefit other people with COPD. We presented abstracts about our PPI work in local meetings and at an international conference, which generated interest and were well received.
Owing to the nature of COPD and its burden on patients, we involved several different PPI advisors to spread the load and contribute to different aspects of the trial as it progressed (see Chapter 1, Figure 3, for an overview of PPI throughout the lifecycle of the project). We discussed expectations and roles and offered PPI advisors choices on the types of involvement and methods of contributing, and we also asked PPI advisors how long they wanted to be involved. Being flexible in arranging meetings with PPI advisors and in the mode of communication was important. Organisation of the type of PPI involvement required, and when it might be required, was improved by planning PPI activities into the project timelines.
A key lesson from this trial is that it is useful to ask PPI advisors how often or at what stages of the trial they would like to get a progress update. In addition to sharing the progress of trial, regular contact with advisors (arranged according to their preference) allowed us to show advisors where and how their contribution had been used to foster better engagement and partnership for the current trial, but also for future collaborations. 183 In addition, we found Bagley et al.’s184 toolkit useful in improving planning and in supporting and evaluating the effects of PPI.
Meaning of the study: possible mechanisms
In our study, the TANDEM intervention did not improve anxiety and/or depression at 6 months for people with moderate to very severe COPD and symptoms of mild to moderate depression or anxiety, or both. This result cannot be explained by a poorly designed or executed trial. We took steps to ensure that the risk of bias was low and the trial was well powered, and we had little attrition. The outcome measures were carefully chosen, and the primary outcome measure is commonly used in trials involving people with COPD and is sensitive to change. 106–109 Participants in both arms of the study were similar at baseline.
Developing the intervention
A possible explanation for our results could lie in the content or focus of the intervention (i.e. if the intervention was not suited to the needs of people with COPD). Our intervention, however, was built on evidence-based and guideline-recommended CBT,25,26,30,45,76 adapted to a low-intensity approach,29 in line with effective CBA intervention in other clinical contexts,44 and linked with potentially synergistic evidence-based PR. 50 Given our extensive efforts at PPI and theoretically based development, this seems unlikely. Moreover, the content of the TANDEM intervention and the aims of our evaluation are strongly endorsed and in line with the research and healthcare priorities of patients with COPD identified in a recent Canadian survey. 185
Ensuring and measuring fidelity
Other potential explanations for our results could lie in the fidelity with which the intervention was implemented or its uptake within the trial. We undertook a comprehensive assessment of fidelity and are confident that fidelity was high and maintained throughout treatment delivery, indicating that recruitment and training of facilitators was effective and that the intervention was implemented as planned. However, our intervention was delivered by trained healthcare professionals, not trained psychologists and, although our measures of fidelity suggested that overall the facilitators delivered the intervention well, their lack of a background or previous experience in psychological interventions might have reduced effectiveness, even though they received regular supervision from experienced clinical psychologists. Perhaps the psychological complexities of some participants were beyond the scope of the facilitators? This is possible, although the Cochrane review of psychological therapies for COPD, which found some weak evidence that such interventions may improve depression in people with COPD, included both interventions delivered by psychologists and by trained nurses. 45 There is also evidence from other areas that healthcare professionals can deliver cognitive–behavioural interventions. For example, a systematic review44,186 of interventions for low back pain suggested that, with suitable training, physiotherapists could deliver effective cognitive–behavioural interventions.
An adequate dose of the intervention
Even a well-designed and well-delivered intervention may have a reduced affect on participants if they do not receive an adequate dose. Our predetermined minimal effective ‘dose’ of two sessions was based on the finding that, in some cases, improvement has been reported following two sessions of a psychological treatment,187 and 81% of participants achieved this, although, in retrospect, this may have been too low a threshold. Only 64% of participants received at least four sessions and 56% of participants received six or more sessions. In addition, it was noted that, although both facilitators and participants interviewed were positive about the intervention, there was some lack of alignment of facilitator and patient (and carer) priorities at the commencement of the TANDEM sessions. Agenda-setting was one area that was not successfully delivered, as observed in the fidelity analysis, although a ‘collaborative relationship’ was established overall. The health professionals (i.e. facilitators and stakeholders) commented on the unmet mental healthcare needs of patients with COPD, framing this as a care deficit. Most patients (and carers), on the other hand, did not identify themselves as being in need of psychological care. Patients were also frequently not used to talking about psychological issues and did not have experience of being offered psychological therapy, which contributed to the considerable work that needed to go into rapport-building in the early TANDEM sessions and making links between breathlessness and anxiety and/or depression. Producing a formulation often happened much later than anticipated in the course of sessions, which effectively reduced the sessions available to deliver the CBA intervention.
Therefore, it is possible that our intervention was not long enough or that a sufficient number of intervention participants did not receive a sufficient dose. None of the other published studies of interventions, which are similar to the TANDEM intervention, in participants with depression have reported how many session participants actually received the intervention and so it is difficult to compare our results with these studies. 70,166,176,180 In Heslop-Marshall et al.’s71 study of a nurse-delivered CBT intervention for patients with COPD and anxiety, the mean number of sessions delivered was four, which the author noted met the IAPT service specification for a brief intervention. 188 Heslop-Marshall et al. found the nurse-delivered CBT intervention to be effective in reducing anxiety, compared with a leaflet control, at 3 months. The process evaluation suggested that participants were not always keen on doing the home practice (the name TANDEM facilitators used to describe homework) associated with the CBA approach, which may have reduced the effectiveness of the intervention, as a meta-analysis189 has found that CBT is more effective with homework than without.
No impact on uptake of pulmonary rehabilitation
The TANDEM intervention failed to increase uptake of, or retention in, PR. Although disappointing, this is perhaps not surprising, as two recent systematic reviews58,190 of interventions to increase the uptake of PR revealed the paucity of evidence of effective interventions and called for further research in the area.
Complexity of lives and the challenge of multimorbidity
An important issue that may help to explain the trial outcome was the complexity of the lives of the patients, which emerged as a frequent theme in the qualitative interviews with patients and facilitators. This complexity included multimorbidity and social factors, such as caring responsibilities and previous trauma. As in previous studies,191,192 patients had lived with COPD for many years and had developed a level of resilience and coping strategies. Many patients did not necessarily consider their COPD to be their primary problem (or not at that point in time), nor believed that their anxiety or low mood was related to their breathlessness or COPD. In this complex context, the TANDEM intervention, which was delivered by respiratory healthcare professionals and designed primarily to address anxiety and/or depression related to COPD, may have had too narrow a focus. Certainly, many of the facilitators observed the breadth of the problems (relating to other conditions, historical issues or social context) presented by the participants.
We may have overestimated the power of the TANDEM intervention, that is a low-intensity CBA intervention delivered at a single, relatively late, point in a long-standing illness trajectory, when people’s problems were deeply embedded and when, over many years, they had established their own coping mechanisms to enable them to live with their slowly progressive respiratory disability. Significantly reducing levels of long-standing anxiety or depression in patients, like patients with complex multimorbidity, may have required a different or much more sustained intervention. It may be that there is a need for awareness-building about the psychological effects of COPD and the availability of support, such as CBA, to be woven into good long-term condition care from the time of diagnosis. 193 Although increasingly important in health care,194,195 complex multimorbidity involving physical and mental health conditions is particularly difficult to address successfully. 196,197
Adverse events
More AEs and SAEs were reported in the intervention arm of the study than in the control arm, but this is likely to be an artefact of the way that trial events are reported, as intervention participants had much more contact with the study through home visits and telephone calls with the TANDEM facilitators (who reported any AEs in the intervention group to the study team). In contrast, the healthcare resource use data (derived from primary care records) reported in Chapter 5 (see Table 16) showed similar or slightly lower mean inpatient stay bed-days, accident and emergency attendances and emergency admissions in the intervention arm participants, compared with the control arm participants. No AEs or SAEs were assessed as being related to the intervention.
Specifically, there were more deaths in the intervention arm, but none was assessed as being related to the intervention and the majority (9/13, 69%) arose in the second 6 months of follow-up (i.e. some time after the intervention was delivered). In addition, as we were able to obtain about only half of the medical records from general practices because of the pandemic, it is possible that some of the patients lost to follow-up had actually died. Owing to the contact the facilitators had with study participants, we would have been more likely to identify a death in the intervention arm than in the control arm.
Recommendations for future research
The study revealed a very high level of unmet need among TANDEM study participants. Further research is needed to identify effective ways of improving mood and outcomes in people with moderate to very severe COPD and anxiety or depression.
There is good evidence for CBAs in other long-term conditions, and this study has confirmed that healthcare professionals can be successfully trained to deliver lower-intensity interventions with fidelity and, therefore, it is worth exploring if an intervention like the TANDEM intervention might be effective among people far earlier in their COPD disease trajectory. It would be useful to consider illness perceptions over time, for example at diagnosis, and to examine how this might relate to the optimum time to intervene with psychoeducational self-management interventions.
Before they joined the study, TANDEM facilitators all recognised the need to address the physical, emotional and psychological aspects of COPD, and after being trained the facilitators spoke of using the skills they had learnt outside the study with other patients with COPD or other long-term respiratory conditions. We suggest evaluating the development of cognitive–behavioural skills as part of undergraduate and postgraduate training for a variety of different healthcare professionals, with the aim of integrating this approach into routine healthcare delivery for long-term conditions.
Whether or not randomised controlled trials are really the best way to determine the clinical effectiveness and value of complex interventions in multimorbidity is unclear, and we suggest that there is a need to identify novel integrated approaches to care and different study designs and research methods for patients with complex health conditions and complicated and, sometimes, difficult lives. 198
Our process evaluation produced rich and detailed data and we hope to explore these data further to better understand our results and how our findings can contribute to the understanding of effective healthcare responses to multimorbidity.
Process evaluation
A number of areas will be explored in more depth:
-
Analysis of the facilitator interviews to explicate more fully facilitators’ experiences of the TANDEM intervention, including developing and applying skills in psychological care for people living with COPD.
-
Further analysis of the process evaluation data to investigate how contextual factors manifested and how the factors may influence mechanisms and outcomes in the TANDEM intervention.
-
Comparative analysis of patient and facilitator interviews to explore the development of a therapeutic alliance in the context of complex multimorbidity, drawing on patient-centred care and behaviour change literature.
-
Exploration of implementation of a psychological intervention for people with COPD in the qualitative data, using normalisation process theory.
Acknowledgements
Oversight committees
We are very grateful to the chairpersons and members of our Trial Steering Committee [Professor Christopher Butler (chairperson), Professor Deborah Fitzsimmons, Dr Robert Stone, Dr Shona Fielding, Dr Louise Restrick] and Data Monitoring Committee [Professor Toby Prevost (chairperson), Dr Sally Hopewell, Dr William Man] for their advice and support throughout the study.
Colleagues who contributed throughout the TANDEM trial
We thank the many people from all the trial sites who contributed to recruitment, delivery of the intervention, data collection and administrative support throughout the trial.
Teams from participant study sites: Katherine Barr, Laura Graham, Clare Maloney, Leyla Osman, Lisa Pritchard, Maria Kouloupoulou, Gemma Kerslake, Cath Darby, Tricia Monroe, Danielle McCracken, Jane Dare, Cheryl Ritchie, Nicky Maritz, Gordon McGregor, Louisa Stonehewer, Jayanth Bhat, Angela O’Sullivan, Charandeep Dehele, Dymphna Medlock, Fiona Shally, Arvind Rajasekaran, Irene Echavez Naguicnic, James Dodd, Daniel Higbee, Sara-Jayne Willets, Caroline Kilby, Elizabeth Habershon, Nicole Bausch, Eva Vernon, Loraine Chowdhury, Jenny Lee, Scott Regan, Emma Padfield, Xiaoli Jia, Aska Matsunga, Priya Varma, Marie Thompson, Lee Berney, Sue Elwell and Sylvia Turner.
TANDEM facilitators and supervisors: Katherine Longhurst, Adam Lound, Sarah Cerezo, Ekta Dhillon, Claire Collins, Gill Gilworth, Nicole Brander, Fiona Conduit, Helen Creasy, Erica Evans, Karen Villabona, Dee Caulfield, Anna McCall, Anne Rodman, Claire Ellis, Yvonne Livie, Helen Beadle, Jack Hayward, Zoe Stone, Cassandra Lee, Liz Harte and Lynette Snowden.
Study administrators: Camille Paulsen and Colin Houlihan. Ms Sian Newton and Dr Anna Moore who contributed to the process evaluation.
Contributions of authors
Ratna Sohanpal (https://orcid.org/0000-0002-1732-0305) (Health Services Researcher) was the trial manager, managed all aspects of the TANDEM study and supported preparation of the report.
Hilary Pinnock (https://orcid.org/0000-0002-5976-8386) (Professor of Primary Care Respiratory Medicine) was the co-principal investigator, and supported Stephanie JC Taylor to develop the original funding proposal, conduct the trial and write the report.
Liz Steed (https://orcid.org/0000-0003-1926-3196) (Senior Lecturer in Health Psychology) was a co-investigator, led the development of the intervention, training of facilitators and delivery of TANDEM therapy, assessment of fidelity and writing of relevant sections of the report.
Karen Heslop-Marshall (https://orcid.org/0000-0002-5990-6961) (Respiratory Nurse Consultant) was a co-investigator, contributed her expertise in nurse-led CBT to the development of the intervention, facilitator training and supervision, and to relevant sections of the report.
Moira J Kelly (https://orcid.org/0000-0001-7911-1149) (Senior Lecturer in Medical Sociology) is a co-investigator. She led the conduct and analysis of the process evaluation and led the writing of relevant sections of the report.
Claire Chan (https://orcid.org/0000-0002-0821-4068) (Statistician) provided statistical support and contributed to relevant sections of the report.
Vari Wileman (https://orcid.org/0000-0002-9673-8047) (Health Psychologist) carried out the fidelity analysis and contributed to relevant sections of the report.
Amy Barradell (https://orcid.org/0000-0002-3688-8879) (Research Associate) carried out recruitment and data collection, and contributed to relevant sections of the report.
Clarisse Dibao-Dina (https://orcid.org/0000-0002-1750-2846) (Professeur des Universités de Médecine Générale) undertook stakeholder interviews and commented on relevant sections of the report.
Paulino Font Gilabert (https://orcid.org/0000-0002-6280-5483) (Health Economist, Research Associate) contributed to the health economic analysis and commented on relevant sections of the report.
Andy Healey (https://orcid.org/0000-0003-2013-3161) (Senior Health Economist) led the conduct and analysis of the health economic study, and led the writing of relevant sections of the report.
Richard Hooper (https://orcid.org/0000-0002-1063-0917) (Professor of Medical Statistics) led final trial analysis and the writing of relevant sections of the report.
Kristie-Marie Mammoliti (https://orcid.org/0000-0003-3614-7453) (Research Assistant) contributed to trial recruitment and data collection, and commented on relevant sections of the report.
Stefan Priebe (https://orcid.org/0000-0001-9864-3394) (Professor for Social and Community Psychiatry) was a co-investigator, provided expertise on mental health aspects of the intervention and trial, and commented on the report.
Mike Roberts (https://orcid.org/0000-0001-8212-1460) (Professor of Respiratory Medicine, Chief Executive Officer Safer Care Victoria) was a co-investigator, provided clinical expertise and commented on the report.
Vickie Rowland (https://orcid.org/0000-0003-2838-1485) (Researcher) contributed to trial recruitment and data collection, and commented on relevant sections of the report.
Sarah Waseem (Clinical Psychologist) was a co-investigator, contributed expertise in clinical psychology to the development of the intervention and facilitator training and supervision, and commented on relevant sections of the report.
Sally Singh (https://orcid.org/0000-0002-9834-0366) (Professor of Pulmonary and Cardiac Rehabilitation) was a co-investigator, provided clinical expertise in PR and commented to relevant sections of the report.
Melanie Smuk (https://orcid.org/0000-0002-1594-1458) (Assistant Professor Program Director of Health Data Science) provided statistical support and contributed to relevant sections of the report.
Martin Underwood (https://orcid.org/0000-0002-0309-1708) (Professor Warwick Clinical Trials Unit) was a co-investigator, contributed trial expertise to the development and conduct of the trial, and commented on the report.
Patrick White (https://orcid.org/0000-0002-2047-8787) (Professor in Primary Care Respiratory Medicine) was a co-investigator, provided primary care and trial expertise to the development of the intervention and conduct of the trial, and commented on the report.
Nahel Yaziji (https://orcid.org/0000-0002-2273-7957) (Health Economist, Research Associate) contributed to the health economic analysis and commented on relevant sections of the report.
Stephanie JC Taylor (https://orcid.org/0000-0001-7454-6354) (Professor in Public Health and Primary Care) was co-principal investigator, and led the development of the original funding proposal, overall conduct of the trial and writing of the report.
Publications
Barradell A, Sohanpal R. Patient and public involvement and its vital role in the TANDEM COPD study: a randomised controlled trial. EMJ Respir 2017;5:56–8.
Chan C, Smuk M, Sohanpal R, Pinnock H, Taylor SJC. Tailored, psychological intervention for anxiety or depression in people with chronic obstructive pulmonary disease (COPD), TANDEM (Tailored intervention for ANxiety and DEpression Management in COPD): statistical analysis plan for a randomised controlled trial. Trials 2020;21:1–2.
Sohanpal R, Pinnock H, Steed L, Heslop-Marshall K, Chan C, Kelly M, et al. Tailored, psychological intervention for anxiety or depression in people with chronic obstructive pulmonary disease (COPD), TANDEM (Tailored intervention for ANxiety and DEpression Management in COPD): protocol for a randomised controlled trial. Trials 2020;21:18.
Kelly M, Steed L, Sohanpal R, Pinnock H, Barradell A, Dibao-Dina C, et al. The TANDEM trial: protocol for the process evaluation of a randomised trial of a complex intervention for anxiety or depression in people living with chronic obstructive pulmonary disease. Trials 2021;22:495.
Steed L, Heslop-Marshall K, Sohanpal R, Saqi-Waseem S, Kelly MJ, Pinnock H, et al. Developing a complex intervention while considering implementation: the Tailored intervention for ANxiety and DEpression Management (TANDEM) for patients with chronic obstructive pulmonary disease (COPD). Trials 2021;22:252.
Steed L, Wileman V, Sohanpal R, Kelly MJ, Taylor SJC. Enhancing and assessing fidelity in the TANDEM (Tailored intervention for ANxiety and DEpression Management in COPD) trial: development of methods and recommendations for research design. BMC Med Res Methodol 2022;22:163.
Wileman V, Rowland V, Kelly M, Steed, Sohanpal R, Pinnock H, Taylor SJC. Implementing psychological interventions delivered by respiratory professionals for people with COPD. A stakeholder interview study. npj Primary Care Respiratory Medicine 2023;33:35. https://doi.org/10.1038/s41533-023-00353-8
Taylor SJC, Sohanpal R, Steed, Marshall K, Chan C, Yaziji N et al. Tailored psychological intervention for anxiety or depression in COPD (TANDEM): a randomised controlled trial. Eur Resp J 2023;62(5):2300432. https://doi.org/10.1183/13993003.00432-2023
Published abstract review
Barradell A, Sohanpal R. Patient and public involvement and its vital role in the TANDEM COPD study: a randomised controlled trial. EMJ Respir 2017;5:56–8.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
Data generated from clinical trials can be used to address many important research questions beyond those planned in the original trial, and this has the potential to provide real benefit to patients and the scientific community. The PCTU has developed a PCTU data-sharing policy to facilitate controlled access to data from PCTU studies. For all data-sharing requests, please complete a PCTU Data Sharing Request Form v1.0 in the first instance and send to the Data Sharing Committee. For any queries please contact the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med 2015;373:111-22. https://doi.org/10.1056/NEJMoa1411532.
- National Institute of Health and Care Excellence . Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management n.d. www.nice.org.uk/guidance/ng115 (accessed 24 October 2021).
- Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for Prevention, Diagnosis and Management of COPD 2020 Report n.d. https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.1wms.pdf (accessed 24 October 2021).
- World Health Organization . Chronic Obstructive Pulmonary Disease (COPD) n.d. www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed 22 October 2021).
- Hoogendoorn M, Feenstra TL, Hoogenveen RT, Al M, Mölken MR. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis 2010;5:435-44. https://doi.org/10.2147/COPD.S13826.
- Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012;67:957-63. https://doi.org/10.1136/thoraxjnl-2011-201518.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89.
- Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Abrams EM, Adedoyin RA, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2020;8:585-96.
- Quaderi SA, Hurst JR. The unmet global burden of COPD. Glob Health Epidemiol Genom 2018;3.
- British Lung Foundation . Chronic Obstructive Pulmonary Disease (COPD) Statistics n.d. https://statistics.blf.org.uk/copd?_ga=2.219875801.1367299004.1527163268-1758129798.1527163268 (accessed 24 October 2021).
- Public Health England . The 2nd Atlas of Variation in Risk Factors and Healthcare for Respiratory Disease in England n.d. https://fingertips.phe.org.uk/profile/atlas-of-variation (accessed 24 October 2021).
- The Lancet . UK COPD treatment: failing to progress. Lancet 2018;391.
- Early F, Lettis M, Winders SJ, Fuld J. What matters to people with COPD: outputs from Working Together for Change. NPJ Prim Care Respir Med 2019;29. https://doi.org/10.1038/s41533-019-0124-z.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. https://doi.org/10.1016/S0140-6736(12)60240-2.
- Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med 2015;3:631-9. https://doi.org/10.1016/S2213-2600(15)00241-6.
- de Torres JP, Marín JM, Casanova C, Cote C, Carrizo S, Cordoba-Lanus E, et al. Lung cancer in patients with chronic obstructive pulmonary disease – incidence and predicting factors. Am J Respir Crit Care Med 2011;184:913-9. https://doi.org/10.1164/rccm.201103-0430OC.
- Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J 2006;28:1245-57.
- Sampaio MS, Vieira WA, Bernardino ÍM, Herval ÁM, Flores-Mir C, Paranhos LR. Chronic obstructive pulmonary disease as a risk factor for suicide: a systematic review and meta-analysis. Respir Med 2019;151:11-8.
- Tselebis A, Pachi A, Ilias I, Kosmas E, Bratis D, Moussas G, et al. Strategies to improve anxiety and depression in patients with COPD: a mental health perspective. Neuropsychiatr Dis Treat 2016;12:297-328. https://doi.org/10.2147/NDT.S79354.
- Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL. Anxiety and depression – important psychological comorbidities of COPD. J Thorac Dis 2014;6:1615-31. https://doi.org/10.3978/j.issn.2072-1439.2014.09.28.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev 2014;23:345-9. https://doi.org/10.1183/09059180.00007813.
- Koskela J, Kilpeläinen M, Kupiainen H, Mazur W, Sintonen H, Boezen M, et al. Co-morbidities are the key nominators of the health related quality of life in mild and moderate COPD. BMC Pulm Med 2014;14. https://doi.org/10.1186/1471-2466-14-102.
- Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis 2014;9:315-30. https://doi.org/10.2147/COPD.S53255.
- Gardener AC, Ewing G, Kuhn I, Farquhar M. Support needs of patients with COPD: a systematic literature search and narrative review. Int J Chron Obstruct Pulmon Dis 2018;13:1021-35. https://doi.org/10.2147/COPD.S155622.
- National Institute for Health and Care Excellence . Depression in Adults: Recognition and Management n.d. www.nice.org.uk/guidance/cg90 (accessed 25 October 2021).
- National Institute for Health and Clinical Excellence . Generalised Anxiety Disorder and Panic Disorder in Adults: Management n.d. www.nice.org.uk/guidance/cg113 (accessed 30 October 2021).
- Beck AT. The Diagnosis and Management of Depression. Philadelphia, PA: University of Pennsylvania Press; 1967.
- Greenberger D, Padesky C. Mind over Mood. A Cognitive Therapy Treatment Manual for Clients. New York, NY: Guilford Press; 1995.
- Shafran R, Myles-Hooton P, Bennett S, Öst LG. The concept and definition of ‘low intensity’ cognitive behaviour therapy. Behaviour Res Therapy 2021;138.
- Olthuis JV, Watt MC, Bailey K, Hayden JA, Stewart SH. Therapist-supported internet cognitive behavioural therapy for anxiety disorders in adults. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD011565.pub2.
- Hunot V, Churchill R, Silva de Lima M, Teixeira V. Psychological therapies for generalised anxiety disorder. Cochrane Database Syst Rev 2007;1. https://doi.org/10.1002/14651858.CD001848.pub4.
- Norton PJ, Price EC. A meta-analytic review of adult cognitive-behavioral treatment outcome across the anxiety disorders. J Nerv Ment Dis 2007;195:521-31. https://doi.org/10.1097/01.nmd.0000253843.70149.9a.
- Stewart RE, Chambless DL. Cognitive–behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol 2009;77:595-606. https://doi.org/10.1037/a0016032.
- Hofmann SG, Smits JA. Cognitive–behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry 2008;69:621-32.
- Ijaz S, Davies P, Williams CJ, Kessler D, Lewis G, Wiles N. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst Rev 2018;5. https://doi.org/10.1002/14651858.CD010558.pub2.
- Wilson KC, Mottram PG, Vassilas CA. Psychotherapeutic treatments for older depressed people. Cochrane Database Syst Rev 2008;1. https://doi.org/10.1002/14651858.CD004853.pub2.
- National Collaborating Centre for Mental Health . The Improving Access to Psychological Therapies (IAPT) Pathway for People With Long-Term Physical Health Conditions and Medically Unexplained Symptoms. Full Implementation Guidance 2018.
- National Institute for Health and Care Excellence . Depression in Adults With a Chronic Physical Health Problem: Recognition and Management n.d. www.nice.org.uk/guidance/cg91 (accessed 19 October 2021).
- Clark DM. Implementing NICE guidelines for the psychological treatment of depression and anxiety disorders: the IAPT experience. Int Rev Psychiatry 2011;23:318-27. https://doi.org/10.3109/09540261.2011.606803.
- Beltman MW, Voshaar RC, Speckens AE. Cognitive–behavioural therapy for depression in people with a somatic disease: meta-analysis of randomised controlled trials. Br J Psychiatry 2010;197:11-9. https://doi.org/10.1192/bjp.bp.109.064675.
- van Straten A, Geraedts A, Verdonck-de Leeuw I, Andersson G, Cuijpers P. Psychological treatment of depressive symptoms in patients with medical disorders: a meta-analysis. J Psychosom Res 2010;69:23-32. https://doi.org/10.1016/j.jpsychores.2010.01.019.
- Reavell J, Hopkinson M, Clarkesmith D, Lane DA. Effectiveness of cognitive behavioral therapy for depression and anxiety in patients with cardiovascular disease: a systematic review and meta-analysis. Psychosom Med 2018;80:742-53. https://doi.org/10.1097/PSY.0000000000000626.
- Sanjida S, McPhail SM, Shaw J, Couper J, Kissane D, Price MA, et al. Are psychological interventions effective on anxiety in cancer patients? A systematic review and meta-analyses. Psychooncology 2018;27:2063-76. https://doi.org/10.1002/pon.4794.
- Lamb SE, Lall R, Hansen Z, Castelnuovo E, Withers EJ, Nichols V, et al. A multicentred randomised controlled trial of a primary care-based cognitive behavioural programme for low back pain. The Back Skills Training (BeST) trial. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14410.
- Pollok J, van Agteren JE, Esterman AJ, Carson-Chahhoud KV. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2019;3. https://doi.org/10.1002/14651858.CD012347.pub2.
- Bolton CE, Bevan-Smith EF, Blakey JD, Crowe P, Elkin SL, Garrod R, et al. BTS guideline on pulmonary rehabilitation in adults. Thorax 2013;68:ii1-ii30.
- Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, et al. ATS/ERS statement: enhancing implementation, use and delivery of PR. Am J Respir Crit Care Med 2105;192:1373-86.
- Primary Care Respiratory Society . Chronic Obstructive Pulmonary Disease (COPD) Pathway n.d. www.england.nhs.uk/rightcare/products/pathways/chronic-obstructive-pulmonary-disease-copd-pathway (accessed 24 October 2021).
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959;2:257-66.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;2. https://doi.org/10.1002/14651858.CD003793.pub3.
- Guo SE, Bruce A. Improving understanding of and adherence to pulmonary rehabilitation in patients with COPD: a qualitative inquiry of patient and health professional perspectives. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0110835.
- Williams MT, Johnston KN, Paquet C. Cognitive behavioral therapy for people with chronic obstructive pulmonary disease: rapid review. Int J Chron Obstruct Pulmon Dis 2020;15:903-19. https://doi.org/10.2147/COPD.S178049.
- Early F, Wilson PM, Deaton C, Wellwood I, Haque HW, Fox SE, et al. Pulmonary rehabilitation referral and uptake from primary care for people living with COPD: a mixed-methods study. ERJ Open Res 2020;6:00219-2019.
- Steiner M, Lowe D, Searle L, Skipper E, Welham S, Roberts CM. Pulmonary Rehabilitation: Time to Breath Better n.d. www.rcplondon.ac.uk/projects/outputs/pulmonary-rehabilitation-time-breathe-better (accessed October 2021).
- Steiner M, McMillan V, Lowe D, Holzhauer-Barrie J, Mortier K, Riordan J, et al. Pulmonary Rehabilitation: An Exercise in Improvement – Combined Clinical and Organisational Audit 2017 n.d. www.rcplondon.ac.uk/projects/outputs/pulmonary-rehabilitation-exercise-improvement-combined-clinical-and-organisational (accessed October 2021).
- Stone PW, Hickman K, Steiner MC, Roberts CM, Quint JK, Singh SJ. Predictors of pulmonary rehabilitation completion in the UK. ERJ Open Res 2021;7:00509-2020.
- Liu Y, Dickerson T, Early F, Fuld J, Jiang C, Clarkson PJ. Understanding the influences of COPD patient’s capability on the uptake of pulmonary rehabilitation in the UK through an inclusive design approach. Int J Chron Obstruct Pulmon Dis 2021;16:1717-40. https://doi.org/10.2147/COPD.S305145.
- Early F, Wellwood I, Kuhn I, Deaton C, Fuld J. Interventions to increase referral and uptake to pulmonary rehabilitation in people with COPD: a systematic review. Int J Chron Obstruct Pulmon Dis 2018;13:3571-86. https://doi.org/10.2147/COPD.S172239.
- Zwar NA, Hermiz O, Comino E, Middleton S, Vagholkar S, Xuan W, et al. Care of patients with a diagnosis of chronic obstructive pulmonary disease: a cluster randomised controlled trial. Med J Aust 2012;197:394-8.
- Harris M, Smith BJ, Veale AJ, Esterman A, Frith PA, Selim P. Providing reviews of evidence to COPD patients: controlled prospective 12-month trial. Chron Respir Dis 2009;6:165-73. https://doi.org/10.1177/1479972309106577.
- Cox NS, Oliveira CC, Lahham A, Holland AE. Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: a systematic review using the theoretical domains framework. J Physiother 2017;63:84-93.
- National Institute for Health and Care Research . Research Award: A Tailored, Cognitive Behavioural Approach Intervention for Mild to Moderate Anxiety and Or Depression in People With Chronic Obstructive Pulmonary Disease (COPD): A Randomised Controlled Trial (TANDEM Tailored Intervention for ANxiety and DEpression Management in COPD) n.d. https://fundingawards.nihr.ac.uk/award/13/146/02 (accessed 19 October 2021).
- Coventry PA, Bower P, Keyworth C, Kenning C, Knopp J, Garrett C, et al. The effect of complex interventions on depression and anxiety in chronic obstructive pulmonary disease: systematic review and meta-analysis. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0060532.
- Barradell A, Sohanpal R. Patient and public involvement and its vital role in the TANDEM COPD study: a randomised controlled trial. EMJ Respir 2017;5:56-8.
- Kelly M, Steed L, Sohanpal R, Pinnock H, Barradell A, Dibao-Dina C, et al. The TANDEM trial: protocol for the process evaluation of a randomised trial of a complex intervention for anxiety and/or depression in people living with chronic obstructive pulmonary disease (COPD). Trials 2021;22. https://doi.org/10.1186/s13063-021-05460-w.
- Steed L, Heslop-Marshall K, Sohanpal R, Saqi-Waseem S, Kelly M, Pinnock H, et al. Developing a complex intervention whilst considering implementation: the TANDEM (Tailored intervention for ANxiety and DEpression Management) intervention for patients with chronic obstructive pulmonary disease (COPD). Trials 2021;22. https://doi.org/10.1186/s13063-021-05203-x.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Yardley L, Ainsworth B, Arden-Close E, Muller I. The person-based approach to enhancing the acceptability and feasibility of interventions. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/s40814-015-0033-z.
- Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res 2015;17. https://doi.org/10.2196/jmir.4055.
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374. https://doi.org/10.1136/bmj.n2061.
- Heslop-Marshall K, Baker C, Carrick-Sen D, Newton J, Echevarria C, Stenton C, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res 2018;4:00094-2018.
- Heslop K, Newton J, Baker C, Burns G, Carrick-Sen D, De Soyza A. Effectiveness of cognitive behavioural therapy (CBT) interventions for anxiety in patients with chronic obstructive pulmonary disease (COPD) undertaken by respiratory nurses: the COPD CBT CARE study: (ISRCTN55206395). BMC Pulm Med 2013;13. https://doi.org/10.1186/1471-2466-13-62.
- Apps LD, Mitchell KE, Harrison SL, Sewell L, Williams JE, Young HM, et al. The development and pilot testing of the self-management programme of activity, coping and education for chronic obstructive pulmonary disease (SPACE for COPD). Int J Chron Obstruct Pulmon Dis 2013;8:317-27. https://doi.org/10.2147/COPD.S40414.
- National Institute for Health and Care Excellence . Common Mental Health Problems: Identification and Pathways to Care n.d. www.nice.org.uk/guidance/cg123 (accessed 19 October 2021).
- Bandura A. Self-efficacy: The Exercise of Control. New York, NY: Freeman; 1997.
- Smith SM, Sonego S, Ketcheson L, Larson JL. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respir Res 2014;1. https://doi.org/10.1136/bmjresp-2014-000042.
- Ma RC, Yin YY, Wang YQ, Liu X, Xie J. Effectiveness of cognitive behavioural therapy for chronic obstructive pulmonary disease patients: a systematic review and meta-analysis. Complement Ther Clin Pract 2020;38.
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13-64. https://doi.org/10.1164/rccm.201309-1634ST.
- National Asthma and Chronic Obstructive Pulmonary Disease Audit Programme (NACAP) . Pulmonary Rehabilitation Clinical and Organisational Audits 2019 n.d. www.nacap.org.uk/nacap/welcome.nsf/reportsPR.html (accessed 19 October 2021).
- Sohanpal R, Steed L, Mars T, Taylor SJ. Understanding patient participation behaviour in studies of COPD support programmes such as pulmonary rehabilitation and self-management: a qualitative synthesis with application of theory. NPJ Prim Care Respir Med 2015;25. https://doi.org/10.1038/npjpcrm.2015.54.
- Leventhal L, Leventhal E. Handbook of Health Psychology. New York, NY: Erlbaum; 1997.
- Vaske I, Kenn K, Keil DC, Rief W, Stenzel NM. Illness perceptions and coping with disease in chronic obstructive pulmonary disease: effects on health-related quality of life. J Health Psychol 2017;22:1570-81. https://doi.org/10.1177/1359105316631197.
- Tiemensma J, Gaab E, Voorhaar M, Asijee G, Kaptein AA. Illness perceptions and coping determine quality of life in COPD patients. Int J Chron Obstruct Pulmon Dis 2016;11:2001-7. https://doi.org/10.2147/COPD.S109227.
- Howard C, Dupont S. ‘The COPD breathlessness manual’: a randomised controlled trial to test a cognitive–behavioural manual versus information booklets on health service use, mood and health status, in patients with chronic obstructive pulmonary disease. NPJ Prim Care Respir Med 2014;24. https://doi.org/10.1038/npjpcrm.2014.76.
- Hagger MS, Orbell S. The common sense model of illness self-regulation: a conceptual review and proposed extended model [published online ahead of print February 01 2021]. Health Psychol Rev 2021. https://doi.org/10.1080/17437199.2021.1878050.
- O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-029954.
- Fleming N. Helping students understand how they learn. Teach Prof 1992;7.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7.
- University of Exeter . LI-IAPT Handbooks and Resources n.d. https://cedar.exeter.ac.uk/iapt/lihandbook (accessed 19 October 2021).
- Rothman S, Pilling S. The Competencies Required to Deliver Effective Cognitive and Behavioural Therapy for People with Depression and with Anxiety Disorders. London: University College London; 2007.
- Williams B, Song JJY. Are simulated patients effective in facilitating development of clinical competence for healthcare students? A scoping review. Adv Simul 2016;1. https://doi.org/10.1186/s41077-016-0006-1.
- Waller G, Turner H. Therapist drift redux: why well-meaning clinicians fail to deliver evidence-based therapy, and how to get back on track. Behav Res Ther 2016;77:129-37. https://doi.org/10.1016/j.brat.2015.12.005.
- Alfonsson S, Parling T, Spännargård Å, Andersson G, Lundgren T. The effects of clinical supervision on supervisees and patients in cognitive behavioral therapy: a systematic review. Cogn Behav Ther 2018;47:206-28. https://doi.org/10.1080/16506073.2017.1369559.
- Bellg AJ, Borelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Treatment Fidelity Workgroup of the NIH Behavior Change Consortium. Enhancing treatment fidelity in health behaviour change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51.
- Toomey E, Hardeman W, Hankonen N, Byrne M, McSharry J, Matvienko-Sikar K, et al. Focusing on fidelity: narrative review and recommendations for improving intervention fidelity within trials of health behaviour change interventions. Health Psychol Behav Med 2020;8:132-51. https://doi.org/10.1080/21642850.2020.1738935.
- Steed L, Heslop-Marshall K, Sohanpal R, Saqi-Waseem S, Kelly M, Pinnock H, et al. A Five-Step Process for Designing Implementable Complex Interventions: A Worked Example Using the Tailored Intervention for ANxiety and DEpression Management (TANDEM) for Patients With Chronic Obstructive Pulmonary Disease (COPD) n.d. www.researchsquare.com/article/rs-29318/v1 (accessed 25 October 2022).
- Sohanpal R, Pinnock H, Steed L, Heslop Marshall K, Chan C, Kelly M, et al. Tailored, psychological intervention for anxiety or depression in people with chronic obstructive pulmonary disease (COPD), TANDEM (Tailored intervention for ANxiety and DEpression Management in COPD): protocol for a randomised controlled trial. Trials 2020;21. https://doi.org/10.1186/s13063-019-3800-y.
- Chan CL, Smuk M, Sohanpal R, Pinnock H, Taylor SJC. TANDEM Investigators . Tailored, psychological intervention for anxiety and/or depression in people with chronic obstructive pulmonary disease (COPD), TANDEM (Tailored intervention for ANxiety and DEpression Management in COPD): statistical analysis plan for a randomised controlled trial. Trials 2020;21. https://doi.org/10.1186/s13063-020-04786-1.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Association of Respiratory Technology and Physiology . Guidance on Quality Assured Diagnostic Spirometry n.d. www.artp.org.uk/Guidelines/05ea8b6f-f5d7-4fd2-828f-128e06d2111b (accessed 7 November 2021).
- Berkshire Healthcare NHS Foundation Trust . Protocol and Procedure for Undertaking Spirometry in a Community Setting n.d. www.berkshirewestccg.nhs.uk/media/1597/spirometry.pdf (accessed 7 November 2021).
- Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med 2007;167:60-7.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77.
- Cameron IM, Crawford JR, Lawton K, Reid IC. Psychometric comparison of PHQ-9 and HADS for measuring depression severity in primary care. Br J Gen Pract 2008;58:32-6. https://doi.org/10.3399/bjgp08X263794.
- Howard C, Dupont S, Haselden B, Lynch J, Wills P. The effectiveness of a group cognitive-behavioural breathlessness intervention on health status, mood and hospital admissions in elderly patients with chronic obstructive pulmonary disease. Psychol Health Med 2010;15:371-85. https://doi.org/10.1080/13548506.2010.482142.
- Livermore N, Sharpe L, McKenzie D. Prevention of panic attacks and panic disorder in COPD. Eur Respir J 2010;35:557-63. https://doi.org/10.1183/09031936.00060309.
- Griffiths TL, Burr ML, Campbell IA, Lewis-Jenkins V, Mullins J, Shiels K, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000;355:362-8.
- Dowson C, Laing R, Barraclough R, Town I, Mulder R, Norris K, et al. The use of the Hospital Anxiety and Depression Scale (HADS) in patients with chronic obstructive pulmonary disease: a pilot study. N Z Med J 2001;114:447-9.
- Arnau RC, Meagher MW, Norris MP, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol 2001;20:112-9. https://doi.org/10.1037//0278-6133.20.2.112.
- Kim HF, Kunik ME, Molinari VA, Hillman SL, Lalani S, Orengo CA, et al. Functional impairment in COPD patients: the impact of anxiety and depression. Psychosomatics 2000;41:465-71.
- PsychVisit . Beck Depression Inventory – 2nd Edition (BDI-II) n.d. https://www.pearsonclinical.co.uk/store/ukassessments/en/Store/Professional-Assessments/Personality-%26-Biopsychosocial/Beck-Depression-Inventory-II/p/P100009013.html (accessed 24 September 2023).
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988;56:893-7. https://doi.org/10.1037//0022-006x.56.6.893.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321-7. https://doi.org/10.1164/ajrccm/145.6.1321.
- Jones P. St George’s Respiratory Questionnaire for COPD Patients (SGRQ-C) n.d. www.sgul.ac.uk/about/our-institutes/infection-and-immunity/research-themes/research-centres/health-status/sgrq (accessed 7 November 2021).
- Osborne RH, Elsworth GR, Whitfield K. The Health Education Impact Questionnaire (heiQ): an outcomes and evaluation measure for patient education and self-management interventions for people with chronic conditions. Patient Educ Couns 2007;66:192-201.
- Sullivan O, Gershuny J. Speed-up society? Evidence from the UK 2000 and 2015 time use diary surveys. Sociology 2018;52:20-38.
- Priebe S, Savill M, Wykes T, Bentall RP, Reininghaus U, Lauber C, et al. Effectiveness of group body psychotherapy for negative symptoms of schizophrenia: multicentre randomised controlled trial. Br J Psychiatry 2016;209:54-61. https://doi.org/10.1192/bjp.bp.115.171397.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-7.
- Office of National Statistics . Opinions Survey Report No. 40 Smoking Related Behaviour and Attitudes, 2008 09 n.d. https://webarchive.nationalarchives.gov.uk/ukgwa/20160109212138/www.ons.gov.uk/ons/rel/lifestyles/smoking-related-behaviour-and-attitudes/2008-09/index.html (accessed 30 October 2021).
- Royal College of Physicians . National Asthma and COPD Audit Programme n.d. www.rcplondon.ac.uk/projects/national-asthma-and-copd-audit-programme-nacap (accessed 25 October 2021).
- EQ-5D . About EQ-5D-5L n.d. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about (accessed 15 October 2021).
- Personal Social Services Research Unit . Client Service Receipt Inventory n.d. www.pssru.ac.uk/csri/client-service-receipt-inventory (accessed 19 October 2021).
- Maheswaran H, Weich S, Powell J, Stewart-Brown S. Evaluating the responsiveness of the Warwick Edinburgh Mental Well-Being Scale (WEMWBS): group and individual level analysis. Health Qual Life Outcomes 2012;10. https://doi.org/10.1186/1477-7525-10-156.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649-55. https://doi.org/10.1093/geront/20.6.649.
- Grant M, Cavanagh A, Yorke J. The impact of caring for those with chronic obstructive pulmonary disease (COPD) on carers’ psychological well-being: a narrative review. Int J Nurs Stud 2012;49:1459-71. https://doi.org/10.1016/j.ijnurstu.2012.02.010.
- Gudmundsson G, Gislason T, Janson C, Lindberg E, Hallin R, Ulrik CS, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J 2005;26:414-9.
- Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes 2008;6. https://doi.org/10.1186/1477-7525-6-46.
- Moerbeek M, Wong WK. Sample size formulae for trials comparing group and individual treatments in a multilevel model. Stat Med 2008;27:2850-64. https://doi.org/10.1002/sim.3115.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. https://doi.org/10.1136/bmj.d40.
- White IR, Carpenter J, Evans S, Schroter S. Eliciting and using expert opinions about dropout bias in randomized controlled trials. Clin Trials 2007;4:125-39.
- Hewitt CE, Torgerson DJ, Miles JN. Is there another way to take account of noncompliance in randomized controlled trials?. Can Med J 2006;175.
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988;75:800-2.
- Candlish J, Teare MD, Dimairo M, Flight L, Mandefield L, Walters SJ. Appropriate statistical methods for analysing partially nested randomised controlled trials with continuous outcomes: a simulation study. BMC Med Res Methodol 2018;18. https://doi.org/10.1186/s12874-018-0559-x.
- Bell ML, Fairclough DL, Fiero MH, Butow PN. Handling missing items in the Hospital Anxiety and Depression Scale (HADS): a simulation study. BMC Research Notes 2016;9:479-88.
- Warwick Medical School . Warwick–Edinburgh Mental Well-Being Scales – WEMWBS User Guide Version 1 n.d. https://warwick.ac.uk/fac/sci/med/research/platform/wemwbs/ (accessed 7 November 2021).
- Lemay KR, Tulloch HE, Pipe AL, Reed JL. Establishing the minimal clinically important difference for the Hospital Anxiety and Depression Scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev 2019;39:E6-11. https://doi.org/10.1097/HCR.0000000000000379.
- Smid DE, Franssen FM, Houben-Wilke S, Vanfleteren LE, Janssen DJ, Wouters EF, et al. Responsiveness and MCID estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: a prospective analysis. J Am Med Dir Assoc 2017;18:53-8.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:68-80. https://doi.org/10.1177/0272989X98018002S09.
- National Institute for Health and Care Excellence . NICE Health Technology Evaluations: The Manual 2022. www.nice.org.uk/process/pmg36/resources/nice-health-technology-evaluations-the-manual-pdf-72286779244741 (accessed 25 February 2022).
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2015.
- Beecham J, Knapp M. Measuring Mental Health. London: Gaskell; 2000.
- NHS Digital . Reference Costs 2019 20 n.d. https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/reference-costs (accessed 6 November 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2020 (accessed 6 November 2021).
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. https://doi.org/10.1007/s11136-012-0322-4.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019) n.d. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 6 November 2021).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res 2006;6.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2015/ (accessed 27 October 2022).
- GOV.UK . NHS Reference Costs 2015 to 2016 n.d. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 27 October 2022).
- NHS England . 2018/19/National/Cost/Collection/Data/Publication n.d. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/ (accessed 27 October 2022).
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness–implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217-26. https://doi.org/10.1097/MLR.0b013e3182408812.
- Pawson R, Tilley N. Realistic Evaluation. London: SAGE Publications Ltd; 1997.
- May CR, Cummings A, Girling M, Bracher M, Mair FS, May CM, et al. Using Normalization Process Theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0758-1.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- Borrelli B, Sepinwall D, Ernst D, Bellg AJ, Czajkowski S, Breger R, et al. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. J Consult Clin Psychol 2005;73:852-60.
- Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent 2011;71:52-63.
- Mannix KA, Blackburn IM, Garland A, Gracie J, Moorey S, Reid B, et al. Effectiveness of brief training in cognitive behaviour therapy techniques for palliative care practitioners. Palliat Med 2006;20:579-84.
- Moorey S, Cort E, Kapari M, Monroe B, Hansford P, Mannix K, et al. A cluster randomized controlled trial of cognitive behaviour therapy for common mental disorders in patients with advanced cancer. Psychol Med 2009;39:713-23. https://doi.org/10.1017/S0033291708004169.
- Taylor SJC, Sohanpal R, Bremner SA, Devine A, McDaid D, Fernández J-L, et al. Self management support for moderate to severe COPD: a pilot randomised controlled trial. Br J Gen Pract 2012;63:e687-95. https://doi.org/10.3399/bjgp12X656829.
- Farquhar M. Improving support of informal carers of respiratory patients. Respirology 2021;1.
- Kunik ME, Veazey C, Cully JA, Souchek J, Graham DP, Hopko D, et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: a randomized controlled trial. Psychol Med 2008;38:385-96.
- Lamers F, Jonkers CC, Bosma H, Chavannes NH, Knottnerus JA, van Eijk JT. Improving quality of life in depressed COPD patients: effectiveness of a minimal psychological intervention. COPD 2010;7:315-22. https://doi.org/10.3109/15412555.2010.510156.
- Lee H, Yoon JY, Lim Y, Jung H, Kim S, Yoo Y, et al. The effect of nurse-led problem-solving therapy on coping, self-efficacy and depressive symptoms for patients with chronic obstructive pulmonary disease: a randomised controlled trial. Age Ageing 2015;44:397-403. https://doi.org/10.1093/ageing/afu201.
- Perkins-Porras L, Riaz M, Okekunle A, Zhelezna S, Chakravorty I, Ussher M. Feasibility study to assess the effect of a brief mindfulness intervention for patients with chronic obstructive pulmonary disease: a randomised controlled trial. Chronic Respir Dis 2018;15:400-10.
- Rosser R, Denford J, Heslop A, Kinston W, Macklin D, Minty K, et al. Breathlessness and psychiatric morbidity in chronic bronchitis and emphysema: a study of psychotherapeutic management. Psychol Med 1983;13:93-110. https://doi.org/10.1017/s0033291700050108.
- Schüz N, Walters JA, Cameron-Tucker H, Scott J, Wood-Baker R, Walters EH. Patient anxiety and depression moderate the effects of increased self-management knowledge on physical activity: a secondary analysis of a randomised controlled trial on health-mentoring in COPD. COPD 2015;12:502-9. https://doi.org/10.3109/15412555.2014.995289.
- Norton S, Cosco T, Doyle F, Done J, Sacker A. The Hospital Anxiety and Depression Scale: a meta confirmatory factor analysis. J Psychosom Res 2013;74:74-81. https://doi.org/10.1016/j.jpsychores.2012.10.010.
- Brennan C, Worrall-Davies A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. J Psychosom Res 2010;69:371-8. https://doi.org/10.1016/j.jpsychores.2010.04.006.
- Bock K, Bendstrup E, Hilberg O, Løkke A. Screening tools for evaluation of depression in chronic obstructive pulmonary disease (COPD). A systematic review. Eur Clin Respir J 2017;4. https://doi.org/10.1080/20018525.2017.1332931.
- Gordon CS, Waller JW, Cook RM, Cavalera SL, Lim WT, Osadnik CR. Effect of pulmonary rehabilitation on symptoms of anxiety and depression in COPD: a systematic review and meta-analysis. Chest 2019;156:80-91.
- Doyle C, Bhar S, Fearn M, Ames D, Osborne D, You E, et al. The impact of telephone-delivered cognitive behaviour therapy and befriending on mood disorders in people with chronic obstructive pulmonary disease: a randomized controlled trial. Br J Health Psychol 2017;22:542-56. https://doi.org/10.1111/bjhp.12245.
- Bove DG, Overgaard D, Lomborg K, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention versus usual care for managing anxiety and dyspnoea in patients with severe chronic obstructive pulmonary disease: a randomised controlled trial protocol. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008031.
- Bove DG, Lomborg K, Jensen AK, Overgaard D, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention in patients with advanced COPD: a randomised controlled trial. Respir Med 2016;121:109-16.
- Pumar MI, Roll M, Fung P, Rolls TA, Walsh JR, Bowman RV, et al. Cognitive behavioural therapy (CBT) for patients with chronic lung disease and psychological comorbidities undergoing pulmonary rehabilitation. J Thorac Dis 2019;11:S2238-53.
- Hynninen MJ, Bjerke N, Pallesen S, Bakke PS, Nordhus IH. A randomized controlled trial of cognitive behavioral therapy for anxiety and depression in COPD. Respir Med 2010;104:986-94. https://doi.org/10.1016/j.rmed.2010.02.020.
- Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003;26:1-7. https://doi.org/10.1207/S15324796ABM2601_01.
- Goldberg D, Gask L, O’Dowd T. The treatment of somatization: teaching techniques of reattribution. J Psychosom Res 1989;33:689-95. https://doi.org/10.1016/0022-3999(89)90084-6.
- Selman LE, Clement C, Douglas M, Douglas K, Taylor J, Metcalfe C, et al. Patient and public involvement in randomised clinical trials: a mixed-methods study of a clinical trials unit to identify good practice, barriers and facilitators. Trials 2021;22. https://doi.org/10.1186/s13063-021-05701-y.
- Bagley HJ, Short H, Harman NL, Hickey HR, Gamble CL, Woolfall K, et al. A patient and public involvement (PPI) toolkit for meaningful and flexible involvement in clinical trials – a work in progress. Res Involv Engagem 2016;2. https://doi.org/10.1186/s40900-016-0029-8.
- Michalovic E, Sweet SN, Jensen D. Research and healthcare priorities of individuals living with COPD. COPD 2021;18:133-46. https://doi.org/10.1080/15412555.2021.1901271.
- Hall A, Richmond H, Copsey B, Hansen Z, Williamson E, Jones G, et al. Physiotherapist-delivered cognitive–behavioural interventions are effective for low back pain, but can they be replicated in clinical practice? A systematic review. Disabil Rehabil 2018;40:1-9. https://doi.org/10.1080/09638288.2016.1236155.
- Shalom JG, Aderka IM. A meta-analysis of sudden gains in psychotherapy: outcome and moderators. Clin Psychol Rev 2020;76.
- Care Services Improvement Partnership . IAPT Outline Service Specification n.d. www.uea.ac.uk/documents/246046/11991919/iapt-pathfinder-outline-service-specification.pdf/9fc11891-ecc5-48e4-a974-9bbc8ab0c690 (accessed 10 November 2021).
- Kazantzis N, Whittington C, Dattilio F. Meta-analysis of homework effects in cognitive and behavioral therapy: a replication and extension. Clin Psychol Sci Pract 2010;17:144-56.
- Jones AW, Taylor A, Gowler H, O’Kelly N, Ghosh S, Bridle C. Systematic review of interventions to improve patient uptake and completion of pulmonary rehabilitation in COPD. ERJ Open Res 2017;3:00089-2016.
- Pinnock H, Kendall M, Murray SA, Worth A, Levack P, Porter M, et al. Living and dying with severe chronic obstructive pulmonary disease: multi-perspective longitudinal qualitative study. BMJ 2011;342. https://doi.org/10.1136/bmj.d142.
- Habraken JM, Pols J, Bindels PJ, Willems DL. The silence of patients with end-stage COPD: a qualitative study. Br J Gen Pract 2008;58:844-9. https://doi.org/10.3399/bjgp08X376186.
- Kendall M, Buckingham S, Ferguson S, MacNee W, Sheikh A, White P, et al. Exploring the concept of need in people with very severe chronic obstructive pulmonary disease: a qualitative study. BMJ Support Palliat Care 2018;8:468-74. https://doi.org/10.1136/bmjspcare-2015-000904.
- Langan J, Mercer SW, Smith DJ. Multimorbidity and mental health: can psychiatry rise to the challenge?. Br J Psychiatry 2013;202:391-3. https://doi.org/10.1192/bjp.bp.112.123943.
- National Institute of Health and Care Excellence . Multimorbidity: Clinical Assessment and Management n.d. www.nice.org.uk/guidance/ng56 (accessed 1 October 2021).
- Salisbury C, Man MS, Bower P, Guthrie B, Chaplin K, Gaunt DM, et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet 2018;392:41-50.
- Mercer SW, Fitzpatrick B, Guthrie B, Fenwick E, Grieve E, Lawson K, et al. The CARE Plus study – a whole-system intervention to improve quality of life of primary care patients with multimorbidity in areas of high socioeconomic deprivation: exploratory cluster randomised controlled trial and cost-utility analysis. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0634-2.
- Sturmberg JP, Getz LO, Stange KC, Upshur RE, Mercer SW. Beyond multimorbidity: what can we learn from complexity science?. J Eval Clin Pract 2021;27:1187-93.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2018/ (accessed 27 October 2022).
- Manthorpe J, Samsi K, Joly L, Crane M, Gage H, Bowling A, et al. Service provision for older homeless people with memory problems: a mixed-methods study. Health Serv Deliv Res 2019;7.
- York Health Economic Consortium . A Return on Investment Tool for the Assessment of Falls Prevention Programmes for Older People Living in the Community 2018.
- Curtis L. Unit Costs of Health and Social Care 2014 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2014/ (accessed 27 October 2022).
- Grant P. How much does a diabetes out-patient appointment actually cost? An argument for PLICS. J Health Organ Manag 2015;29:154-69.
- Saf BQ. Support for people starting a new medication for a long-term condition through community pharmacies: a pragmatic randomised controlled trial of the New Medicine Service. BMJ 2020;286.
- Curtis L. Unit Costs of Health and Social Care 2013 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2013/ (accessed 27 October 2022).
Appendix 1 Tables
| Data | Number (%) with one or more items complete (N = 423) | Number (%) with all items complete (N = 423) | Mean (range) number of items completeda |
|---|---|---|---|
| At 6 months | |||
| HADS-A (7 items) | 373 (88.2) | 369 (87.2) | 7.0 (3–7) |
| HADS-D (7 items) | 373 (88.2) | 368 (87.0) | 7.0 (4–7) |
| BDI II (21 items) | 354 (83.7) | 336 (79.4) | 20.8 (1–21) |
| BAI (21 items) | 350 (82.7) | 327 (77.3) | 20.8 (2–21) |
| SGRQ (50 items) | 354 (83.7) | 292 (69.0) | 49.1 (2–50) |
| B-IPQ (8 items) | 423 (100.0) | 423 (100.0) | 8.0 (8–8) |
| heiQ (5 items) | 344 (81.3) | 342 (80.9) | 5.0 (4–5) |
| Time Use Survey (12 items)b | 351 (83.0) | 337 (79.7) | 11.9 (4–12) |
| WEMWBS (14 items) | 29 (65.9) | 26 (59.1) | 13.9 (13–14) |
| ZBI (22 items) | 29 (65.9) | 27 (61.4) | 21.9 (21–22) |
| At 12 months | |||
| HADS-A (7 items) | 345 (81.6) | 341 (80.6) | 7.0 (3–7) |
| HADS-D (7 items) | 345 (81.6) | 241 (80.9) | 7.0 (2–7) |
| BDI II (21 items) | 314 (74.2) | 288 (68.1) | 20.8 (8–21) |
| BAI (21 items) | 314 (74.2) | 288 (68.1) | 20.7 (2–21) |
| SGRQ (50 items) | 316 (74.7) | 226 (53.4) | 48.8 (15–50) |
| B-IPQ (8 items) | 423 (100.0) | 423 (100.0) | 8.0 (8–8) |
| heiQ (5 items) | 302 (71.5) | 301 (71.2) | 5.0 (4–5) |
| Time Use Survey (12 items)b | 306 (72.3) | 293 (69.3) | 11.8 (1–12) |
| WEMWBS (14 items) | 28 (63.6) | 27 (61.4) | 14.0 (13–14) |
| ZBI (22 items) | 28 (63.6) | 27 (61.4) | 21.9 (18–22) |
| Sensitivity analysis | Treatment effect | 95% CI | p-valuea |
|---|---|---|---|
| Main analysis | –0.60 | –1.40 to 0.21 | 0.145 |
| Complete-case analysis | –0.69 | –1.48 to 0.11 | 0.090 |
| Missing items imputed for participants with a partially incomplete HADS-A | –0.61 | –1.41 to 0.19 | 0.137 |
| Excluding participants with a score of < 8 on the HADS-A subscale | –0.95 | –1.88 to –0.01 | 0.048 |
| Time to PR as an additional covariate | 0.60 | –1.40 to 0.20 | 0.143 |
| Excluding internal pilot participants | –0.50 | –1.35 to 0.35 | 0.246 |
| Randomisation period as an additional covariate | –0.60 | –1.41 to 0.21 | 0.144 |
| Pre-pandemic vs. during pandemic as an additional covariate | –0.63 | –1.45 to 0.19 | 0.130 |
| Pre-pandemic vs. during pandemic as a treatment effect modifier | |||
| Pre-pandemic | –0.72 | –1.59 to 0.16 | 0.565 |
| During pandemic | –0.33 | –1.62 to 0.96 | |
| Fully face-to-face delivery vs. remote delivery vs. no CBA as additional covariate | 0.52 | –1.07 to 2.12 | 0.519 |
| Fully face-to-face vs. delivery remote delivery vs. no CBA as a treatment effect modifier | |||
| Fully face-to-face delivery | –0.11 | –0.91 to 0.70 | 0.606 |
| Partially or fully remote delivery | –0.85 | –2.40 to 0.70 | |
| No CBA | 0.61 | –1.14 to 2.36 | |
| Sensitivity analysis | Treatment effect | 95% CI | p-valuea |
|---|---|---|---|
| Main analysis | –0.66 | –1.39 to 0.07 | 0.074 |
| Complete-case analysis | –0.77 | –1.51 to –0.03 | 0.041 |
| Missing items imputed for participants with a partially incomplete HADS-D | –0.70 | –1.42 to 0.02 | 0.058 |
| Excluding participants with a score of < 8 on the HADS-D subscale | –0.66 | –1.52 to 0.20 | 0.129 |
| Time to PR as an additional covariate | –0.64 | –1.35 to 0.08 | 0.079 |
| Excluding internal pilot participants | –0.63 | –1.44 to 0.17 | 0.120 |
| Randomisation period as an additional covariate | –0.65 | –1.37 to 0.07 | 0.078 |
| Pre-pandemic vs. during pandemic as an additional covariate | –0.64 | –1.35 to 0.08 | 0.082 |
| Pre-pandemic vs. during pandemic as a treatment effect modifier | |||
| Pre-pandemic | –0.66 | –1.43 to 0.11 | 0.867 |
| During pandemic | –0.56 | –1.71 to 0.58 | |
| Fully face-to-face delivery vs. remote delivery vs. no CBA as additional covariate | 0.37 | –1.15 to 1.88 | 0.634 |
| Fully face-to-face delivery vs. remote delivery vs. no CBA as a treatment effect modifier | |||
| Fully face-to-face delivery | –0.29 | –0.96 to 0.39 | 0.634 |
| Partially or fully remote delivery | –0.05 | –1.47 to 1.37 | |
| No CBA | 0.68 | –0.98 to 2.33 | |
| Participant demographic | Participants included in HADS-A primary analysis | Participants missing HADS-A at 6 and 12 months | Participants missing HADS-A 6 months because of death | |||
|---|---|---|---|---|---|---|
| Intervention (n = 215) | Usual care (n = 173) | Intervention (n = 27) | Usual care (n = 8) | Intervention (n = 4) | Usual care (n = 0) | |
| Age (years), median (IQR) | 68 (61–76) | 69 (62–74) | 69 (60–80) | 69 (67–87) | 76 (69.5–80.5) | – |
| Gender, n (%) | ||||||
| Male | 112 (52.1) | 77 (44.8) | 18 (66.7) | 6 (75.0) | 4 (100.0) | – |
| Female | 103 (47.9) | 95 (55.2) | 9 (33.3) | 2 (25.0) | 0 (0.0) | – |
| Smoking status, n (%) | ||||||
| Current smoker | 64 (29.8) | 52 (30.1) | 10 (37.0) | 2 (25.0) | 2 (50.0) | – |
| Ex-smoker | 145 (67.4) | 118 (68.2) | 17 (63.0) | 6 (75.0) | 2 (50.0) | – |
| Never smoked | 6 (2.8) | 3 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Degree of breathlessness (mMRC Breathlessness Scale), n (%) | ||||||
| Not troubled by breathlessness except on strenuous exercise | 2 (0.9) | 0 (0.0) | 1 (3.7) | 0 (0.0) | 0 (0.0) | – |
| Short of breath when hurrying on the level or walking up a slight hill | 38 (17.7) | 35 (20.2) | 4 (14.8) | 3 (37.5) | 0 (0.0) | – |
| Walks slower than other people of the same age on the level | 75 (34.9) | 56 (32.4) | 4 (14.8) | 1 (12.5) | 0 (0.0) | – |
| Stops for breath after walking about 100 yards or after a few minutes on the level | 69 (32.1) | 49 (28.3) | 6 (22.2) | 2 (25.0) | 2 (50.0) | – |
| Too breathless to leave the house or breathless when dressing or undressing | 31 (14.4) | 33 (19.1) | 12 (44.4) | 2 (25.0) | 2 (50.0) | – |
| HADS, mean (SD) | ||||||
| HADS-A total score | 9.7 (3.1) | 9.9 (3.3) | 10.3 (3.0) | 8.6 (3.5) | 11.0 (2.7) | – |
| HADS-D total score | 9.1 (3.0) | 9.1 (3.1) | 9.2 (3.5) | 8.4 (3.3) | 9.5 (2.6) | – |
| BDI II, mean (SD) | ||||||
| BDI II total score | 20.0 (8.8) | 20.9 (10.2) | 21.8 (8.8) | 15.3 (9.5) | 27.5 (12.1) | – |
| BAI total score | 16.4 (10.5) | 17.0 (10.1) | 17.8 (8.8) | 8.6 (7.0) | 16.0 (9.4) | – |
| SGRQ, mean (SD) | ||||||
| Total score | 59.2 (15.3) | 58.9 (15.1) | 62.6 (13.9) | 52.2 (20.4) | 66.4 (10.2) | – |
| Symptoms | 63.7 (21.0) | 62.7 (23.4) | 64.7 (18.4) | 56.9 (17.6) | 63.1 (26.3) | – |
| Activity | 78.2 (18.3) | 77.9 (15.5) | 81.2 (17.2) | 70.8 (24.7) | 77.5 (16.3) | – |
| Impact | 46.9 (16.9) | 47.0 (17.8) | 51.3 (17.4) | 39.5 (24.4) | 61.1 (20.6) | – |
| B-IPQ, mean (SD) | ||||||
| Consequences | 6.3 (2.2) | 6.6 (2.3) | 7.0 (1.9) | 6.3 (1.7) | 8.0 (0.8) | – |
| Timeline | 9.5 (1.3) | 9.4 (1.6) | 9.6 (1.0) | 9.9 (0.4) | 9.5 (0.6) | – |
| Personal control | 4.6 (2.7) | 4.7 (2.8) | 5.8 (2.5) | 4.3 (3.4) | 4.3 (3.1) | – |
| Treatment control | 6.4 (2.4) | 6.9 (2.4) | 6.7 (2.4) | 3.9 (3.1) | 6.7 (2.1) | – |
| Identity | 6.8 (1.9) | 6.8 (2.1) | 7.0 (2.3) | 6.5 (2.5) | 6.3 (2.6) | – |
| Concern | 7.4 (2.6) | 7.5 (2.6) | 7.7 (2.6) | 7.8 (2.2) | 8.8 (0.5) | – |
| Coherence | 7.2 (2.8) | 7.3 (2.7) | 6.9 (2.5) | 7.6 (3.4) | 5.5 (1.9) | – |
| Emotional response | 6.4 (2.7) | 6.5 (2.8) | 6.4 (2.9) | 6.3 (2.6) | 7.5 (1.9) | – |
| heiQ, mean (SD) | ||||||
| heiQ social engagement | 2.6 (0.5) | 2.6 (0.6) | 2.4 (0.7) | 2.7 (0.6) | 2.1 (0.1) | – |
| Time Use Survey, median (IQR) | ||||||
| Time (minutes) spent doing activities over last 4 days | 270 (135–540) | 300 (150–600) | 245 (155–480) | 277.5 (125–465) | 270 (180–660) | – |
| Participant demographic | Participants included in HADS-D primary analysis | Participants missing HADS-D at 6 and 12 months | Participants missing HADS-D at 6 months because of death | |||
|---|---|---|---|---|---|---|
| Intervention (n = 216) | Usual care (n = 172) | Intervention (n = 26) | Usual care (n = 9) | Intervention (n = 4) | Usual care (n = 0) | |
| Age (years), median (IQR) | 68 (61–76) | 69 (62–74) | 68.5 (60–80) | 73.5 (67.5–83.5) | 76 (69.5–80.5) | – |
| Gender, n (%) | ||||||
| Male | 113 (52.3) | 76 (44.4) | 17 (65.4) | 7 (77.8) | 4 (100.0) | – |
| Female | 103 (47.7) | 95 (55.6) | 9 (34.6) | 2 (22.2) | 0 (0.0) | – |
| Smoking status, n (%) | ||||||
| Current smoker | 64 (29.6) | 52 (30.2) | 10 (38.5) | 2 (22.2) | 2 (50.0) | – |
| Ex-smoker | 146 (67.6) | 117 (68.0) | 16 (61.5) | 7 (77.8) | 2 (50.0) | – |
| Never smoked | 6 (2.8) | 3 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Degree of breathlessness (mMRC Breathlessness Scale), n (%) | ||||||
| Not troubled by breathlessness except on strenuous exercise | 2 (0.9) | 0 (0.0) | 1 (3.8) | 0 (0.0) | 0 (0.0) | – |
| Short of breath when hurrying on the level or walking up a slight hill | 38 (17.6) | 34 (19.8) | 4 (15.4) | 4 (44.4) | 0 (0.0) | – |
| Walks slower than other people of the same age on the level | 75 (34.7) | 56 (32.6) | 4 (15.4) | 1 (11.1) | 0 (0.0) | – |
| Stops for breath after walking about 100 yards or after a few minutes on the level | 69 (31.9) | 49 (28.5) | 6 (23.1) | 2 (22.2) | 2 (50.0) | – |
| Too breathless to leave the house or breathless when dressing or undressing | 32 (14.8) | 33 (19.2) | 11 (42.3) | 2 (22.2) | 2 (50.0) | – |
| HADS, mean (SD) | ||||||
| HADS-A total score | 9.7 (3.1) | 9.9 (3.3) | 10.3 (3.0) | 9.3 (3.9) | 11.0 (2.7) | – |
| HADS-D total score | 9.1 (3.0) | 9.1 (3.1) | 9.0 (3.5) | 8.6 (3.2) | 9.5 (2.6) | – |
| BDI II, mean (SD) | ||||||
| BDI II total score | 20.0 (8.8) | 21.0 (10.2) | 22.0 (8.9) | 14.9 (8.7) | 27.5 (12.1) | – |
| BAI total score | 16.4 (10.5) | 17.1 (10.1) | 17.9 (9.0) | 8.1 (6.7) | 16.0 (9.4) | – |
| SGRQ, mean (SD) | ||||||
| Total score | 59.2 (15.2) | 58.9 (15.2) | 62.7 (14.1) | 53.6 (19.3) | 66.4 (10.2) | – |
| Symptoms | 63.7 (21.0) | 62.8 (23.4) | 65.4 (18.4) | 55.1 (17.3) | 63.1 (26.3) | – |
| Activity | 78.3 (18.3) | 77.8 (15.5) | 80.7 (17.4) | 73.5 (24.1) | 77.5 (16.3) | – |
| Impact | 46.9 (16.8) | 46.9 (17.9) | 51.5 (17.8) | 41.3 (23.2) | 61.1 (20.6) | – |
| B-IPQ, mean (SD) | ||||||
| Consequences | 6.3 (2.2) | 6.6 (2.3) | 7.0 (1.9) | 6.6 (1.8) | 8.0 (0.8) | – |
| Timeline | 9.5 (1.3) | 9.4 (1.6) | 9.6 (1.0) | 9.4 (1.3) | 9.5 (0.6) | – |
| Personal control | 4.6 (2.7) | 4.7 (2.7) | 6.0 (2.4) | 4.8 (3.6) | 4.3 (3.1) | – |
| Treatment control | 6.4 (2.4) | 6.9 (2.4) | 6.8 (2.4) | 4.4 (3.4) | 6.7 (2.1) | – |
| Identity | 6.8 (1.9) | 6.8 (2.1) | 7.1 (2.3) | 6.9 (2.6) | 6.3 (2.6) | – |
| Concern | 7.4 (2.6) | 7.5 (2.6) | 7.8 (2.6) | 8.0 (2.2) | 8.8 (0.5) | – |
| Coherence | 7.2 (2.8) | 7.3 (2.7) | 6.9 (2.5) | 7.9 (3.3) | 5.5 (1.9) | – |
| Emotional response | 6.4 (2.7) | 6.5 (2.8) | 6.5 (2.9) | 6.7 (2.7) | 7.5 (1.9) | – |
| heiQ, mean (SD) | ||||||
| heiQ social engagement | 2.6 (0.5) | 2.6 (0.6) | 2.5 (0.7) | 2.5 (0.7) | 2.1 (0.1) | – |
| Time Use Survey, median (IQR) | ||||||
| Time (minutes) spent doing activities over last 4 days | 270 (135–540) | 300 (150–600) | 250 (130–480) | 330 (130–510) | 270 (180–660) | – |
| Participant demographics | Period 1 | Period 2 | Period 3 | |||
|---|---|---|---|---|---|---|
| Intervention (n = 37) | Usual care (n = 31) | Intervention (n = 55) | Intervention (n = 13) | Usual care (n = 150) | Intervention (n = 137) | |
| Age (years), median (IQR) | 68 (58–78) | 69 (62–73) | 66 (58–75) | 72 (67–77) | 69 (63–76) | 69 (62–74) |
| Gender, n (%) | ||||||
| Male | 18 (48.6) | 15 (48.4) | 23 (41.8) | 6 (46.2) | 89 (59.3) | 62 (45.6) |
| Female | 19 (51.4) | 16 (51.6) | 32 (58.2) | 7 (53.8) | 6 (40.7) | 74 (54.4) |
| Smoking status, n (%) | ||||||
| Current smoker | 14 (37.8) | 13 (41.9) | 19 (34.5) | 2 (15.4) | 41 (27.3) | 39 (28.5) |
| Ex-smoker | 22 (59.5) | 17 (54.8) | 35 (63.6) | 11 (84.6) | 105 (70.0) | 96 (70.1) |
| Never smoked | 1 (2.7) | 1 (3.2) | 1 (1.8) | 0 (0.0) | 4 (2.7) | 2 (1.5) |
| Degree of breathlessness (mMRC Breathlessness Scale), n (%) | ||||||
| Not troubled by breathlessness except on strenuous exercise | 0 (0.0) | 0 (0.0) | 1 (1.8) | 0 (0.0) | 2 (1.3) | 0 (0.0) |
| Short of breath when hurrying on the level or walking up a slight hill | 10 (27.0) | 4 (12.9) | 10 (18.2) | 3 (23.1) | 22 (14.7) | 31 (22.6) |
| Walks slower than other people of the same age on the level | 14 (37.8) | 12 (38.7) | 15 (27.3) | 3 (23.1) | 50 (33.3) | 42 (30.7) |
| Stops for breath after walking about 100 yards or after a few minutes on the level | 12 (32.4) | 11 (35.5) | 20 (36.4) | 5 (38.5) | 43 (28.7) | 35 (25.5) |
| Too breathless to leave the house or breathless when dressing or undressing | 1 (2.7) | 4 (12.9) | 9 (16.4) | 2 (15.4) | 33 (22.0) | 29 (21.2) |
| HADS, mean (SD) | ||||||
| HADS-A total score | 9.9 (2.7) | 9.9 (3.4) | 9.2 (3.5) | 11.1 (3.3) | 9.9 (3.0) | 9.8 (3.3) |
| HADS-D total score | 9.4 (3.8) | 9.2 (3.4) | 9.6 (2.6) | 10.1 (2.4) | 8.9 (3.0) | 8.9 (3.0) |
| BDI II, mean (SD) | ||||||
| BDI II total score | 22.7 (11.7) | 21.4 (12.0) | 20.2 (8.1) | 25.4 (9.3) | 19.6 (8.1) | 20.0 (9.8) |
| BAI total score | 17.5 (8.9) | 17.5 (10.7) | 16.1 (10.8) | 16.5 (11.4) | 16.5 (10.5) | 16.4 (10.0) |
| SGRQ, mean (SD) | ||||||
| Total score | 58.2 (16.7) | 59.7 (16.3) | 61.1 (13.7) | 60.6 (14.4) | 59.3 (15.3) | 58.2 (15.3) |
| Symptoms | 61.9 (21.7) | 60.5 (27.8) | 63.8 (21.3) | 60.6 (17.4) | 64.3 (20.3) | 63.0 (22.6) |
| Activity | 73.0 (18.8) | 77.8 (14.1) | 81.6 (13.4) | 81.1 (13.0) | 78.8 (19.3) | 77.2 (16.6) |
| Impact | 48.8 (19.3) | 49.0 (20.0) | 48.5 (15.8) | 48.8 (18.0) | 46.7 (16.9) | 45.9 (17.7) |
| B-IPQ, mean (SD) | ||||||
| Consequences | 6.3 (2.1) | 6.7 (2.3) | 6.6 (2.1) | 6.8 (1.5) | 6.3 (2.2) | 6.6 (2.3) |
| Timeline | 9.3 (1.6) | 9.7 (1.1) | 9.5 (1.2) | 8.5 (2.9) | 9.6 (1.2) | 9.4 (1.4) |
| Personal control | 4.6 (2.7) | 4.7 (2.9) | 4.8 (2.6) | 5.2 (2.9) | 4.7 (2.7) | 4.7 (2.8) |
| Treatment control | 6.2 (2.3) | 6.7 (2.2) | 6.3 (2.5) | 7.3 (1.8) | 6.6 (2.4) | 6.8 (2.6) |
| Identity | 6.6 (2.2) | 6.9 (2.2) | 7.1 (1.9) | 7.2 (1.5) | 6.7 (1.9) | 6.7 (2.2) |
| Concern | 7.0 (3.1) | 7.3 (2.5) | 7.6 (2.3) | 8.2 (2.1) | 7.4 (2.6) | 7.5 (2.6) |
| Coherence | 7.2 (2.7) | 7.3 (2.7) | 7.5 (2.6) | 6.8 (3.0) | 7.5 (2.6) | 6.8 (3.0) |
| Emotional response | 6.2 (3.1) | 6.0 (3.3) | 6.6 (2.6) | 6.3 (2.7) | 6.6 (2.6) | 6.3 (2.7) |
| heiQ, mean (SD) | ||||||
| heiQ social engagement | 2.4 (0.6) | 2.6 (0.6) | 2.5 (0.5) | 2.4 (0.6) | 2.6 (0.5) | 2.6 (0.6) |
| Time Use Survey, median (IQR) | ||||||
| Time (minutes) spent doing activities over last 4 days | 240 (135–540) | 300 (120–480) | 266 (120–480) | 360 (60–945) | 300 (155–540) | 285 (150–525) |
| Participant demographic | 6-month data collected before 19 March 2020 | 6-month data collected after 19 March 2020 | Fully face-to-face delivery (n = 195) | Partly remote delivery (n = 22) | ||
|---|---|---|---|---|---|---|
| Intervention (n = 162) | Usual care (n = 119) | Intervention (n = 44) | Usual care (n = 44) | |||
| Age (years), median (IQR) | 68 (61–76) | 70 (63–74) | 69 (62–76) | 65 (59–74) | 69 (62–77) | 68 (62.5–74) |
| Gender, n (%) | ||||||
| Male | 78 (48.1) | 55 (46.6) | 30 (68.2) | 17 (38.6) | 104 (53.3) | 15 (68.2) |
| Female | 84 (51.9) | 63 (53.4) | 14 (31.8) | 27 (61.4) | 91 (46.7) | 7 (31.8) |
| Smoking status, n (%) | ||||||
| Current smoker | 46 (28.4) | 38 (31.9) | 13 (29.5) | 9 (20.5) | 53 (27.2) | 7 (31.8) |
| Ex-smoker | 111 6 (8.5) | 80 (67.2) | 30 (68.2) | 35 (79.5) | 137 (70.3) | 14 (63.6) |
| Never smoked | 5 (3.1) | 1 (0.8) | 1 (2.3) | 0 (0.0) | 5 (2.6) | 1 (4.5) |
| Degree of breathlessness (mMRC Breathlessness Scale), n (%) | ||||||
| Not troubled by breathlessness except on strenuous exercise | 2 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.0) | 1 (4.5) |
| Short of breath when hurrying on the level or walking up a slight hill | 31 (19.1) | 24 (20.2) | 6 (13.6) | 6 (13.6) | 37 (19.0) | 3 (13.6) |
| Walks slower than other people of the same age on the level | 54 (33.3) | 34 (28.6) | 15 (34.1) | 19 (43.2) | 64 (32.8) | 6 (27.3) |
| Stops for breath after walking about 100 yards or after a few minutes on the level | 54 (33.3) | 38 (31.9) | 14 (31.8) | 11 (25.0) | 61 (31.3) | 6 (27.3) |
| Too breathless to leave the house or breathless when dressing or undressing | 21 (13.0) | 23 (19.3) | 9 (20.5) | 8 (18.2) | 31 (15.9) | 6 (27.3) |
| HADS, mean (SD) | ||||||
| HADS-A total score | 9.5 (3.2) | 9.8 (3.1) | 10.3 (2.6) | 10.5 (3.5) | 9.6 (3.2) | 9.6 (2.3) |
| HADS-D total score | 9.1 (3.1) | 9.0 (3.0) | 9.3 (3.0) | 9.1 (3.3) | 9.1 (3.0) | 8.7 (3.9) |
| BDI II, mean (SD) | ||||||
| BDI II total score | 19.9 (8.8) | 21.0 (10.4) | 20.3 (8.2) | 19.6 (10.1) | 19.6 (8.4) | 20.6 (10.7) |
| BAI total score | 16.5 (10.7) | 16.1 (10.0) | 16.9 (9.7) | 19.2 (9.9) | 16.5 (10.2) | 16.1 (11.2) |
| SGRQ, mean (SD) | ||||||
| Total score | 58.2 (15.0) | 58.1 (15.4) | 63.5 (15.1) | 60.8 (15.3) | 59.4 (14.9) | 56.8 (19.3) |
| Symptoms | 61.3 (20.8) | 61.5 (24.4) | 71.0 (19.7) | 65.5 (22.0) | 63.8 (21.0) | 63.8 (19.9) |
| Activity | 77.4 (18.1) | 76.8 (15.6) | 83.5 (16.3) | 81.1 (13.4) | 78.1 (18.3) | 76.9 (23.3) |
| Impact | 46.3 (16.8) | 46.4 (17.9) | 49.7 (16.7) | 47.7 (19.2) | 47.4 (16.5) | 43.2 (21.4) |
| B-IPQ, mean (SD) | ||||||
| Consequences | 6.3 (2.2) | 6.6 (2.3) | 6.4 (2.0) | 6.6 (2.3) | 6.4 (2.1) | 6.3 (2.6) |
| Timeline | 9.5 (1.4) | 9.4 (1.5) | 9.7 (0.8) | 9.3 (1.7) | 9.5 (1.4) | 9.9 (0.5) |
| Personal control | 4.8 (2.7) | 4.7 (2.8) | 3.9 (2.7) | 4.7 (2.6) | 4.7 (2.7) | 5.0 (2.5) |
| Treatment control | 6.5 (2.5) | 6.9 (2.2) | 6.3 (2.2) | 6.7 (2.8) | 6.4 (2.4) | 6.8 (2.6) |
| Identity | 6.7 (2.0) | 6.8 (2.2) | 7.1 (1.7) | 6.6 (1.8) | 6.8 (1.9) | 6.6 (1.9) |
| Concern | 7.2 (2.7) | 7.4 (2.6) | 7.8 (2.2) | 7.7 (2.3) | 7.4 (2.5) | 7.4 (2.9) |
| Coherence | 7.3 (2.6) | 7.3 (2.6) | 6.6 (3.2) | 7.0 (3.0) | 7.3 (2.7) | 7.5 (2.7) |
| Emotional response | 6.3 (2.9) | 6.3 (2.9) | 6.9 (1.7) | 6.8 (2.7) | 6.3 (2.7) | 6.1 (2.1) |
| heiQ, mean (SD) | ||||||
| heiQ social engagement | 2.6 (0.5) | 2.6 (0.6) | 2.6 (0.6) | 2.6 (0.6) | 2.6 (0.5) | 2.5 (0.7) |
| Time Use Survey, median (IQR) | ||||||
| Time (minutes) spent doing activities over last 4 days | 335 (160–570) | 330 (150–630) | 240 (120–420) | 300 (180–480) | 270 (135–540) | 190 (120–420) |
| Trial arm | Treatment effect | 95% CI | ||
|---|---|---|---|---|
| Usual care | Intervention | |||
| Assumed difference in mean responses for participants with missing data compared with participants with complete data | –10 | –15 | –2.04 | –2.83 to –1.25 |
| –10 | –1.28 | –2.06 to –0.49 | ||
| –5 | –0.51 | –1.30 to 0.28 | ||
| –5 | –10 | –1.75 | –2.53 to –0.96 | |
| –5 | –0.98 | –1.77 to –0.19 | ||
| 0 | –0.22 | –1.00 to 0.57 | ||
| –1.5 | –6.5 | –1.54 | –2.33 to –0.75 | |
| –1.5 | –0.78 | –1.56 to 0.01 | ||
| 3.5 | –0.01 | –0.80 to 0.78 | ||
| 0 | –5 | –1.45 | –2.24 to –0.66 | |
| 0 | –0.69 | –1.47 to 0.10 | ||
| 5 | 0.08 | –0.71 to 0.86 | ||
| 1.5 | –3.5 | –1.36 | –2.15 to –0.58 | |
| 1.5 | –0.60 | –1.39 to 0.19 | ||
| 6.5 | 0.17 | –0.62 to 0.95 | ||
| 5 | 0 | –1.16 | –1.94 to –0.37 | |
| 5 | –0.39 | –1.18 to 0.40 | ||
| 10 | 0.37 | –0.41 to 1.16 | ||
| 10 | 5 | –0.86 | –1.65 to –0.07 | |
| 10 | –0.10 | –0.88 to 0.69 | ||
| 15 | 0.67 | –0.12 to 1.45 | ||
| Trial arm | Treatment effect | 95% CI | ||
|---|---|---|---|---|
| Usual care | Intervention | |||
| Assumed difference in mean responses for participants with missing data compared with participants with complete data | –10 | –15 | –2.19 | –2.92 to –1.46 |
| –10 | –1.40 | –2.13 to –0.67 | ||
| –5 | –0.62 | –1.35 to 0.11 | ||
| –5 | –10 | –1.87 | –2.60 to –1.14 | |
| –5 | –1.09 | –1.82 to –0.36 | ||
| 0 | –0.30 | –1.03 to 0.43 | ||
| –1.5 | –6.5 | –1.65 | –2.38 to –0.92 | |
| –1.5 | –0.87 | –1.60 to –0.14 | ||
| 3.5 | –0.08 | –0.81 to 0.65 | ||
| 0 | –5 | –1.56 | –2.29 to –0.83 | |
| 0 | –0.77 | –1.50 to –0.04 | ||
| 5 | 0.01 | –0.72 to 0.74 | ||
| 1.5 | –3.5 | –1.46 | –2.19 to –0.73 | |
| 1.5 | –0.68 | –1.41 to 0.05 | ||
| 6.5 | 0.11 | –0.62 to 0.84 | ||
| 5 | 0 | –1.16 | –1.94 to –0.37 | |
| 5 | –0.39 | –1.18 to 0.40 | ||
| 10 | 0.37 | –0.41 to 1.16 | ||
| 10 | 5 | –0.86 | –1.65 to –0.07 | |
| 10 | –0.10 | –0.88 to 0.69 | ||
| 15 | 0.67 | –0.12 to 1.45 | ||
| SAE | Trial arm | |
|---|---|---|
| Intervention (N = 242), n (%)b | Usual care (N = 181), n (%)b | |
| Number of people with an event | 44 | 7 |
| Total number of events | 68 | 7 |
| Event made known to researcher during patient assessmentc | ||
| Yes | 5 (25.0) | 3 (100.0) |
| No | 15 (75.0) | 0 (0.0) |
| Event made known to facilitator during intervention deliveryc | ||
| Yes | 10 (50.0) | N/A (N/A) |
| No | 10 (50.0) | N/A (N/A) |
| Severity | ||
| Mild | 16 (23.5) | 0 (0.0) |
| Moderate | 27 (39.7) | 0 (0.0) |
| Severe | 11 (16.2) | 4 (57.1) |
| Life-threatening | 3 (4.4) | 1 (14.3) |
| Death | 11 (16.2) | 2 (28.6) |
| Causality | ||
| Unlikely | 67 (98.5) | 7 (100.0) |
| Possibly | 0 (0.0) | 0 (0.0) |
| Probably | 1 (1.5) | 0 (0.0) |
| Definitely | 0 (0.0) | 0 (0.0) |
| Outcome | ||
| Unresolved | 2 (2.9) | 0 (0.0) |
| Resolving | 24 (35.3) | 0 (0.0) |
| Resolved | 24 (35.3) | 4 (57.1) |
| Fatal | 14 (20.6) | 3 (42.9) |
| Unknown | 4 (5.9) | 0 (0.0) |
| Expectedness | ||
| Expected | 34 (50.0) | 1 (14.3) |
| Unexpected | 34 (50.0) | 6 (85.7) |
| Costs and unit estimation | Cost (£ 2020/21) | Notes |
|---|---|---|
| Hourly rate | 23.44 | Based on the median salaries for Agenda for Change bands |
| Salary oncosts | 8.21 | Based on the NHS contribution rate for the period 1 April 2019 to 31 March 2023 of 20.6% of pensionable pay for both the 1995–2008 scheme and the 2015 scheme, and 13.8% employer national insurance rates for the 2021–2 tax year |
| Overheads | 14.77 | Management and other non-care staff are 24.5% of direct care salary costs and include administration and estates staff, section 9.145 Non-staff costs are 38.2% of direct care salary costs, and include costs to the provider for office, travel/transport, publishing, training courses and conferences, supplies and services (clinical and general), and utilities (e.g. water, gas and electricity) |
| Training hours | 21.75 | Based on the median hours of receiving and delivering the training |
| Total training cost | 92,025.18 | This is the cost of training 42 facilitators. The cost includes the opportunity cost of attending a training session, the hourly cost of delivering a training session and the cost of clinical supervision (£14,558.61) |
| Caseload | 1288.65 | This is the expected number of cases per working year. The facilitators in the study dealt with 242 cases during 8 weeks of the intervention period. The number of working weeks per year is 42.6 (i.e. 37.5 hours per week minus annual, 8 statutory leave days, days sickness leave and study/training days) |
| Cost per case | 71.41 |
| Service | Cost (£) | Source |
|---|---|---|
| Community-based services | ||
| GP visit | 40.10 | PSSRU 2020, section 10.3b145 |
| GP home visit | 78.39 | PSSRU 2015, section 10.8a and 10.8b152 |
| GP (telephone) | 15.86 | PSSRU 2020, section 10.5145 |
| GP (out-of-hours home visit) | 116.86 | PSSRU 2018, Supplementary Table 1199 |
| Practice nurse visit | 19.18 | PSSRU 2015, section 10.1152 |
| Practice nurse home visit | 30.26 | PSSRU 2015, section 10.1152 |
| Practice nurse (telephone) | 7.97 | PSSRU 2020, section 10.5145 |
| Out-of-hours service: telephone call (and phone back) | 7.76 | Manthorpe et al.200 |
| Counselling support/talking therapy | 46.54 | PSSRU 2015, section 12.1152 |
| Stop smoking service (cost per head) | 143.61 | PSSRU 2020, section 8.3.145 Average of men and women |
| Dietitian | 94.03 | PSSRU 2020, section 7.1145 |
| Diabetic nurse | 20.78 | PSSRU 2015, section 10.7 (advanced nurse)152 |
| Diabetic phone | 9.09 | PSSRU 2015, section 10.4152 |
| ACERS Respiratory Rapid Response team | 76.02 | PSSRU 2015, section 7.5152 |
| Other healthcare provider for immunisation | 38.78 | National schedule of NHS costs 2018/19.154 Code N03N |
| Community matron | 27.70 | PSSRU 2015, section 10.7152 |
| ACERS home visit | 89.43 | PSSRU 2015, section 7.5152 |
| Integrated independence team | 46.61 | Average of social workers adult services (PSSRU 2020, section 11.1145), community occupational therapist (PSSRU 2020, section 11.4145), support and outreach worker (PSSRU 2020, section 11.7145), peer intern (PSSRU 2020, section 11.8145) and physiotherapy (PSSRU 2020, section 7.1145) |
| Healthcare assistant | 28.92 | PSSRU 2018, Supplementary Table 1199 |
| Healthcare assistant: home visit | 42.13 | PSSRU 2018, Supplementary Table 1199 |
| ACE telephone consultation (community cardiology and respiratory service) | 10.08 | PSSRU 2015, section 10.7 (advanced nurse, 6 minutes telephone call)152 |
| Walk-in clinic | 84.62 | National schedule of NHS costs 2018/19.154 Currency code VB11Z (Emergency Medicine, No Investigation with No Significant Treatment) |
| My Care My Way integrated care service | 29.64 | Average GP and nurse |
| My Care My Way integrated care service: home visit | 54.33 | Average GP and nurse (home visit) |
| Telephone consultation with pharmacist | 8.40 | Manthorpe et al.200 |
| Meeting pharmacist | 8.40 | Manthorpe et al.200 |
| Palliative care | 149.23 | PSSRU 2020, section 7.1145 (average of outpatient medical specialist and non-medical specialist) |
| Falls service | 471.68 | York Health Economic Consortium201 |
| Occupational therapist | 50.08 | PSSRU 2020, section 11.4145 |
| Occupational therapist: home visit | 63.29 | PSSRU 2020, section 11.4145 |
| Physiotherapy | 65.41 | PSSRU 2020, section 7.1145 |
| Called NHS 111 | 7.76 | PSSRU 2015, section 7.1152 |
| Podiatry, district nurse, community therapy services | 41.89 | Podiatry £54 (national schedule of NHS costs 2018/19: service code 653154); district nurse £17.31 (PSSRU 2015, section 10.1152); community occupational therapist (local authority) £49 (PSSRU 2020, section 11.4152) |
| Dietitian nurse | 20.78 | PSSRU 2015, section 10.4.152 Average of a 15-minute consultation in surgery (PSSRU 2015, section 10.7152) |
| Orthopaedics | 125.61 | National schedule of NHS costs 2018/19, service 110 in total outpatient attendance154 |
| Alcohol and drug addiction | 136.28 | Manthorpe et al.200 |
| Out-of-hours urgent visit to general practice | 94.18 | Manthorpe et al.200 |
| Intermediate care visits | 131.85 | Manthorpe et al.200 |
| Healthcare support worker | 28.92 | PSSRU 2018, Supplementary Table 1199 |
| Podiatry | 56.41 | National schedule of NHS costs 2018/19: service code 653154 |
| Mental health clinic | 154.28 | PSSRU 2014, section 9.5202 |
| ACE pathway self-referral | 117.01 | National schedule of NHS costs 2018/19, CMDT154 |
| Diabetes and cardiac centre | 89.75 | Grant203 |
| Anticoagulation clinic | 27.25 | Saf204 |
| Musculoskeletal clinic | 153.57 | NHS schedule of NHS costs 2018/19: service code 410 outpatient154 |
| Nutrition: home visit | 30.26 | PSSRU 2015, Section 10.1 |
| Ambulance call | 7.15 | PSSRU 2020, section 7.1145 |
| Phlebotomist | 20.78 | PSSRU 2015, section 10.4 (nurse specialist).152 Average of a 15-minute consultation in surgery [PSSRU 2015, section 10.7 (advance nurse)152] |
| Rapid response | 40.69 | PSSRU 2013, section 7.5205 |
| Drug counsellor | 123.67 | PSSRU 2020, Drug services – community contacts. Section 3.1 misuse of drugs or alcohol145 |
| Optician | 63.90 | Manthorpe et al.200 |
| Speech therapist | National schedule of NHS costs 2015/16: service code 652153 | |
| Hospital-based care | ||
| Inpatient admission (bed-days) | 923.83 | National schedule of NHS costs 2015/16153 |
| A&E not admitted (attendance) | 141.04 | NHS schedule of NHS costs 2018/19.154 Average of all A&E services (not admitted). |
| A&E admitted (attendance) | 188.37 | NHS schedule of NHS costs 2018/19.154 Average of all A&E services (admitted). |
| Hospital outpatient clinic: physical (appointments) | 168.70 | NHS schedule of NHS costs 2018/19.154 Average of physical outpatient services (excluding paediatric services) |
| Hospital outpatient clinic: mental (appointments) | 243.97 | NHS National Schedule 2018/20. Average of mental outpatient services (excluding paediatric services) |
| Day hospital (attendance) | 785.50 | NHS schedule of NHS costs 2018/19154 (index sheet) |
| CET | INHB (mean) | 95% CI | Probability cost-effective |
|---|---|---|---|
| £13,000 | –0.0976 | –0.1002 to –0.0951 | 0.005 |
| £20,000 | –0.0660 | –0.0680 to –0.0641 | 0.014 |
| £30,000 | –0.0465 | –0.0481 to –0.0448 | 0.041 |
| CET | INHB (mean) | 95% CI | Probability cost-effective |
|---|---|---|---|
| £13,000 | –0.0809 | –0.0816 to –0.0780 | 0.000 |
| £20,000 | –0.0524 | –0.0529 to –0.0499 | 0.013 |
| £30,000 | –0.0348 | –0.0352 to –0.0325 | 0.058 |
| CET | INHB (mean) | 95% CI | Probability cost-effective |
|---|---|---|---|
| £13,000 | –0.0665 | –0.0689 to –0.0642 | 0.038 |
| £20,000 | –0.0470 | –0.0488 to –0.0453 | 0.053 |
| £30,000 | –0.0350 | –0.0364 to –0.0335 | 0.058 |
| Number of CBA sessions received | Intervention participants (N = 242) | |
|---|---|---|
| n (%) | Cumulative % | |
| 0 | 22 (9) | 9 |
| 1 | 24 (10) | 19 |
| 2 | 8 (3) | 22 |
| 3 | 15 (6) | 28 |
| 4 | 15 (6) | 34 |
| 5 | 22 (9) | 43 |
| 6 | 75 (31) | 74 |
| 7 | 27 (11) | 85 |
| 8 | 30 (12) | 97 |
| 9 | 4 (2) | 99 |
Appendix 2 Figures
FIGURE 23.
A cost-effectiveness plane (available case results).

FIGURE 24.
A CEAC (available case results).

List of abbreviations
- AE
- adverse event
- BAI
- Beck Anxiety Inventory
- BDI II
- Beck Depression Inventory II
- B-IPQ
- Brief Illness Perception Questionnaire
- BLF
- British Lung Foundation
- CACE
- complier-average causal effect
- CBA
- cognitive–behavioural approach
- CBT
- cognitive–behavioural therapy
- CEAC
- cost-effectiveness acceptability curve
- CET
- cost-effectiveness threshold
- CFARS
- Cognitive First Aid Rating Scale
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- DVD
- digital video disc
- EQ-5D-5L
- EuroQoL-5 Dimensions, five-level version
- GAD-7
- General Anxiety Disorder-7
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HADS-A
- Hospital Anxiety and Depression Scale – anxiety
- HADS-D
- Hospital Anxiety and Depression Scale – depression
- heiQ
- Health Education Impact Questionnaire
- IAPT
- Improving Access to Psychological Therapies
- INHB
- incremental net health benefit
- IQR
- interquartile range
- mMRC
- Modified Medical Research Council
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- PCTU
- Pragmatic Clinical Trials Unit
- PHQ-9
- Patient Health Questionnaire-9 items
- PPI
- patient and public involvement
- PR
- pulmonary rehabilitation
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- SAE
- serious adverse event
- SD
- standard deviation
- SFTP
- secure file transfer protocol
- SGRQ
- St George’s Respiratory Questionnaire
- SMD
- standardised mean difference
- SPACE
- Self-management Programme of Activity, Coping and Education
- TANDEM
- Tailored intervention for Anxiety and Depression Management in COPD
- VARK
- visual, auditory, read, kinesthetic
- WEMWBS
- Warwick–Edinburgh Mental Well-being Scale
- ZBI
- Zarit Caregiver Burden Scale
