Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/142/02. The contractual start date was in June 2014. The draft report began editorial review in February 2021 and was accepted for publication in July 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Heer et al. This work was produced by Heer et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Heer et al.
Chapter 1 Introduction
Parts of this chapter have been reproduced with permission from Tandogdu et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
Incidence
Bladder cancer is the most frequently occurring tumour of the urinary system, with > 10,500 new cases diagnosed each year in the UK. 2 Overall, bladder cancer is the 11th most common cancer in the UK, accounting for 3% of all new cancer cases. 2 Histologically, > 90% of bladder cancers are of the transitional cell carcinoma type. Bladder cancer is more common in men than in women (5 : 2 ratio), making it the eighth most common cancer in men and the 16th most common cancer in women. 2 Incidence rates for bladder cancer in the UK are highest in people aged 85–89 years, with 8 in 10 cases occurring in people aged ≥ 65 years. Cigarette smoking is causally related to over one-third of bladder cancer diagnoses and is also a risk factor for progression to cancer-related death. 3,4 Time trends in bladder cancer incidence rates over the past 10–20 years are difficult to interpret because of changes in classification. There is a trend towards a reduction in age-standardised incidence rates, currently 11 cases per 100,000 population, which is predicted to continue to decline at a rate of 1% annually. 5 However, the growth in the ageing population will have a substantial impact on the total number of cases, which is projected to rise at an annual rate of > 1%. 5
Pathology
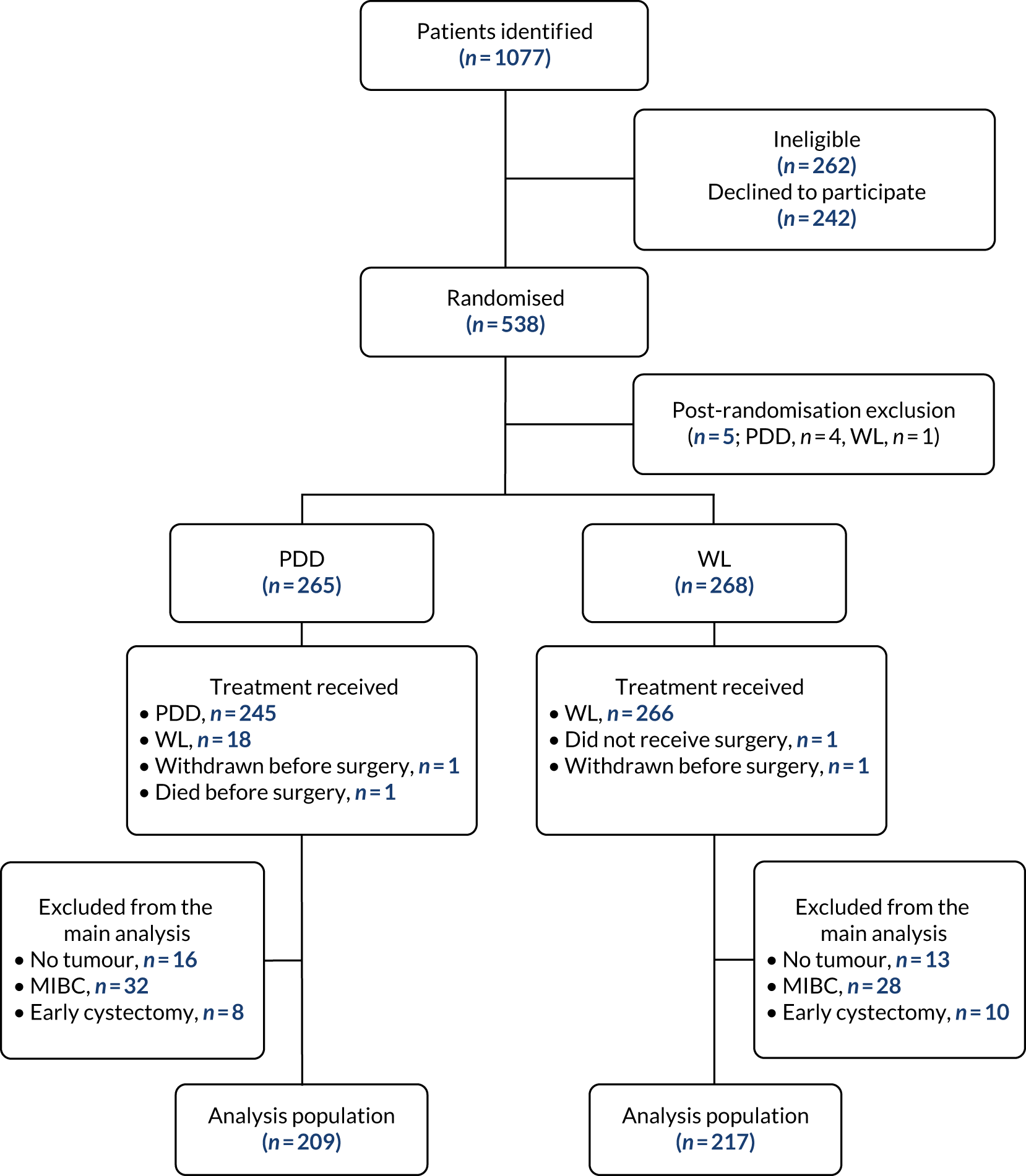
The extent of the spread of cancer is described using the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) tumour node metastasis (TNM) staging system. 6 Tumours confined to the epithelial lining (urothelium) are classified as stage Ta and those invading the lamina propria are classified as stage T1 (Figure 1). Ta and T1 tumours can be easily removed through transurethral resection (TUR) and, therefore, for therapeutic purposes, are grouped together as non-muscle-invasive bladder cancer (NMIBC). NMIBC also includes flat, high-grade tumours that are confined to the epithelium, which are classified as carcinoma in situ (CIS). Grade (microscopic characteristics of the tumour cells) can be used to describe the aggressiveness of cancers, which are characterised as either low grade (relatively benign) or high grade (aggressive). 6
FIGURE 1.
Tumour node metastasis staging of bladder cancer (AJCC TNM staging system). The primary tumour (T) stage shown in this figure is CIS. Node (N) stage and metastasis (M) stage measure cancer spread to local lymph nodes and other parts of the body, respectively.
Presentation and diagnosis of bladder cancer
The most common presentation of bladder cancer is haematuria (presence of blood in the urine), which may be associated with additional symptoms such as dysuria (painful urination), increased frequency/urgency of urination, failed attempts to urinate or urinary tract infections. Haematuria is either visible (frankly blood-stained urine) or non-visible (urine that is clear-looking to the naked eye). Non-visible haematuria is detected by dipstick or microscopic examinations, often included in standard primary care assessments for an NHS Health Check or in the investigation of urinary symptoms. Bladder cancer is detected in approximately 10% of patients with visible haematuria and 3–5% of those with dipstick or microscopic haematuria who are aged > 40 years. 7,8 Therefore, these patients are urgently referred for assessment in rapid-access haematuria clinics in secondary care, where suspected bladder tumours are usually detected by cystoscopy under local anaesthetic or, less frequently, on imaging by ultrasound scanning or computerised tomography (CT). Visual appearances of bladder cancer are confirmed by histological diagnosis using samples taken during cystoscopic transurethral resection of bladder tumour (TURBT), which is conducted as part of the initial management of bladder cancer.
Initial management of non-muscle-invasive bladder cancer
About 80% of people with a new diagnosis of bladder cancer will have NMIBC and will initially be treated by TURBT. The subsequent goal in NMIBC management is the prevention of recurrence and/or progression to higher-stage, life-threatening, muscle-invasive disease. It is thought that failure to identify/appreciate tumours is a factor in 20–40% of the recurrent tumours that are overlooked. 9,10 Tumour seeding following resection and urothelium that may be genetically ‘primed’ for new tumours developing are other factors that are considered relevant and will have an impact on recurrence rates independent of the completeness of resection. Recurrence and stage progression to muscle-invasive or metastatic cancer is more likely to occur in those with high-grade tumours with concomitant CIS. In particular, CIS, which is a flat tumour, can be easily missed using conventional white-light (WL) resection. 11
Risk of recurrences and stage progression
Both clinical and histological parameters can be used to estimate individual risk of recurrence and progression of NMIBC into muscle-invasive bladder cancer (MIBC). Based on this, the European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group has developed an algorithm that calculates probabilities of recurrence and progression, which are integral to the current European Association of Urology (EAU) guideline on the treatment of NMIBC. 12
These probabilities are based on the number of tumours, tumour size, prior recurrence, histological T stage, presence of CIS and tumour grade. The risks of recurrence and progression 3 years after diagnosis are summarised in Table 1. Patients’ cancer management plans are tailored to the risk categories in terms of the intensity of follow-up and the use of adjuvant therapies.
| Recurrence risk group (score) | Probability of recurrence at 3 years (%) | Progression risk group (score) | Probability of progression at 3 years (%) |
|---|---|---|---|
| Low risk (0) | 25 | Low risk (0–1) | 0.8 |
| Intermediate risk (1–9) | 40–56 | Intermediate risk (2–6) | 4 |
| High risk (10–17) | 75 | High risk (7–23) | 11–30 |
Strategies to reduce recurrence and progression
Strategy 1: adjuvant therapy
A meta-analysis of seven randomised trials showed that a single instillation of chemotherapy into the bladder [intravesical mitomycin C (MMC), epirubicin or doxorubicin] leads to a decrease of 39% [standard deviation (SD) ± 8%] in the odds of recurrence (odds ratio 0.61; p < 0.0001). 13 For patients with low-risk disease, no further intravesical treatment is required. 14 For patients with intermediate-risk disease, additional courses of either intravesical chemotherapy or intravesical immunotherapy with bacillus Calmette–Guérin (BCG) for a minimum of 1 year is advised based on clinical evidence and EAU guidelines. 13–17 Use of BCG, which has greater toxicity than chemotherapy, tends to be reserved for those with high-risk disease with higher risk of progression. In some instances, immediate cystectomy is recommended following diagnosis, depending on high-risk factors and patient preference. 12 Intravesical adjuvant therapies are associated with treatment morbidity, affect quality of life (QoL) and have associated costs. 16
Strategy 2: surveillance
Surveillance of NMIBC is carried out using cystoscopy with the aim of detecting recurrence early and allowing treatment before progression. Clinical guidelines tailor follow-up protocols according to the risk groups described above. 12,18 The advised follow-up of low-risk disease is cystoscopy 3 months after initial TURBT; if this is negative, the next cystoscopy is scheduled 9 months later and patients are then discharged if they are clear at that assessment. 18,19 Patients with high-risk tumours have cystoscopy and urine cytology 3 months after TURBT. If this is negative (according to the guidance in place during this study), cystoscopy is repeated every 3 months for 2 years, then every 6 months until 5 years, and annually thereafter. More recently, this guidance was updated by the National Institute for Health and Care Excellence (NICE) to recommend follow-up cystoscopy every 3 months for the first 2 years, then every 6 months for the next 2 years and then once per year thereafter. 18 The recommended intensity of cystoscopic follow-up for patients with intermediate risk was not clearly defined when this trial started. It was recommended that follow-up of patients with tumours considered to fall between low and high risk be adapted according to personal and subjective factors. NICE guidance has since been updated to advise that people with intermediate-risk NMIBC undergo cystoscopic follow-up at 3, 9 and 18 months, and once per year thereafter. Those with intermediate-risk NMIBC can be discharged to primary care after 5 years of disease-free follow-up.
Strategy 3: high-quality resection
A high-quality TURBT aims to completely eradicate Ta–T1 tumours and to accurately stage disease at first presentation. The high between-centre variability in recorded 3-month recurrence rates indicates that TURBT can often be incomplete. 10 Training and technology to improve completeness of resection is thought to be one of the most important modifiable factors in reducing recurrence. 10
Health economic impact of managing non-muscle-invasive bladder cancer
Non-muscle-invasive bladder cancer is one of the costliest cancers to manage because of its high prevalence and high recurrence rate and the need for adjuvant treatments and long-term cystoscopic surveillance. The total cost of treatment and 5-year follow-up of UK patients with NMIBC increased from £73M to £213M from 2001 to 2012 (inflation corrected). This was due to an ageing population and better-defined adjuvant treatment and surveillance regimens requiring additional therapies, with potential mortality and long-term morbidity (e.g. radical surgery). 20,21 Health-related quality of life (HRQoL) is also known to be affected in those receiving morbid treatments for bladder cancer. 22 The cost-effectiveness of NMIBC treatment strategies has not been widely studied.
Rationale for research
Health need
Although many cases of NMIBC are readily treatable with cystoscopic resection, it remains a major health-care burden, for the reasons described above. 11,20 From a patient perspective, there are often considerable anxieties about recurrences, TUR and the impact of adjuvant treatments. 23 TUR is associated with reduced QoL across both mental and physical health domains, although these effects are usually transient. 24 Substantial effects on HRQoL are most likely to result from adjuvant intravesical treatments and radical or palliative treatments for progression. 22 More efficient management strategies to reduce NMIBC recurrence, and hence decrease both the burden to patients and costs to the NHS, are urgently needed. One such approach currently available in the NHS is photodynamic diagnosis (PDD).
Photodynamic diagnosis of non-muscle-invasive bladder cancer
Mechanism
Photodynamic diagnosis can enhance tumour detection during the initial cystoscopic diagnosis and TURBT. 11 PDD exploits photosensitising agents with a high selectivity for accumulation within tumour cells. 25 The photosensitiser can then be excited by a specific electromagnetic wavelength and will re-emit light at a different wavelength for detection. Photosensitising agents that can be administered intravesically include 5-aminolevulinic acid (5-ALA), hexaminolevulinate (HAL) and hypericin. Conversion of 5-ALA to HAL by esterification results in a more rapid cellular uptake and, subsequently, the cancer fluoresces more brightly than with 5-ALA. 26
The HAL product Hexvix® (PhotoCure, Oslo, Norway) is the only agent licensed in the European Union (marketed through Ipsen, Boulogne-Billancourt, France) and the USA (as Cysview™) for PDD in NMIBC.
Diagnostic accuracy
A systematic review funded by the National Institute for Health and Care Research (NIHR) suggested that PDD offered greater diagnostic accuracy in detecting NMIBC than conventional WLC based on a total of 27 studies enrolling 2949 participants. 11,27 The pooled estimates [95% confidence interval (CI)] for patient-level analysis comparing PDD against WL showed increased diagnostic sensitivity from 71% (49–93%) to 92% (80–100%), but decreased specificity from 72% (47–96%) to 57% (36–79%). In particular, PDD was better than WL in detecting intermediate- and high-risk disease, including CIS that otherwise could be easily missed (sensitivity 83%, 95% CI 41% to 100%, vs. 32%, 95% CI 0% to 83%, respectively). The review also suggested that PDD treatment was no better than WL for patients with low-risk disease. 11
Clinical outcomes
Based on data from four studies, the systematic review also showed that the improved diagnostic accuracy with PDD translated into a reduced recurrence rate. 11 Compared with white-light-guided transurethral resection of bladder tumour (WL-TURBT), photodynamic diagnosis-guided transurethral resection of bladder tumour (PDD-TURBT) was associated with fewer tumours at the 3-month follow-up, with a relative risk of 0.37 (95% CI 0.20 to 0.69). The benefit of PDD resection in reducing tumour recurrence in the longer term (12–24 months) was less clear, with effect estimates favouring PDD, but without statistical significance. Variability in the administration of single-dose adjuvant intravesical chemotherapy within 24 hours of resection across the four randomised controlled trials (RCTs) was an important confounding factor in terms of generalising the possible benefit of PDD. A subsequent large RCTshowed that PDD using 5-ALA did not decrease rates of recurrence-free or progression-free survival at 12 months. 28 However, this result conflicted with previous and subsequent studies from the same group, for which the cohort size was 814 and 551 randomised patients, respectively, which did show a decrease in recurrence rates. 29,30 This discrepancy is most likely to be a reflection of the differences in research protocols, including patient selection and the use of HAL rather than 5-ALA. 29,30 There is, therefore, still substantial uncertainty around any potential patient benefit of PDD, particularly when applied to routine care in a pragmatic NHS setting.
Evaluations of the potential health economic impact of photodynamic diagnosis
The NIHR Health Technology Assessment (HTA) review included economic modelling of the cost-effectiveness of PDD and assessment of the performance of urine biomarkers [e.g. nuclear matrix protein 22 (NMP22) and those detected via fluorescence in situ hybridisation (FISH) and immunocytochemistry (immunoCyt)] and cytology for the detection and follow-up of bladder cancer. 11 Although the differences in outcomes and costs between detection methods appeared to be modest, the decision about which strategy to adopt depended on society’s willingness to pay (WTP) for an additional gain. The HTA review was unable to undertake a cost–utility analysis owing to the lack of relevant health utility data. Therefore, although strategies that replaced WL with PDD resulted in a gain in life-years, it was unclear whether this was sufficient to justify the extra costs. 11 To address this, more details on the long-term outcomes of clinical effectiveness, HRQoL data [as quality-adjusted life-years (QALYs)] and a full assessment of all treatment costs were required.
Aims and objectives
We aimed to undertake a pragmatic, patient-randomised controlled trial to compare outcomes of PDD resection with outcomes of standard WL cystoscopic resection for newly diagnosed intermediate- and high-risk NMIBC. Apart from initial treatment, both groups were scheduled to receive usual care, including single-dose adjuvant intravesical MMC, surveillance according to standard risk-adjusted schedules and further adjuvant therapy as indicated by current practice guidelines. 12,18 With the trial results, we aim to deliver a definitive assessment of the benefits, harms and cost-effectiveness of PDD within the NHS to guide decisions around further adoption and implementation.
Primary objectives
Clinical effectiveness
Time to recurrence was compared for the two treatment strategies, with a principal point of interest at 3 years.
Economic evaluation
A within-trial analysis was conducted over a 3-year follow-up period to determine whether or not, as part of the management of people with suspected intermediate- and high-risk cancers confined to the bladder lining, PDD resection was cost-effective for the NHS compared with resection under WL.
Secondary objectives
Clinical effectiveness
-
The relative rates of disease progression at 3 years were measured; a formal comparison was difficult, as progression is rare. Therefore, modelling was undertaken at 3 years using trial and other published data. A projection over the patient lifetime (15–20 years) was also included.
-
Relative harms and safety complications were measured within 30 days of surgery.
Economic evaluation
A model-based economic analysis was planned to estimate the cost-effectiveness of PDD-TURBT over a patient’s lifetime.
Additional objectives
-
Lay the basis for modelling the safest and most cost-effective cystoscopic follow-up surveillance schedules.
-
Evaluate the learning curve for the procedure to account for its effects on outcomes of both PDD and standard WL resections.
-
Establish a well-characterised cohort of patients with intermediate- and high-risk NMIBC, including clinical data and urine, blood and tumour specimens that would be available for separately funded research of genotypic and phenotypic studies.
Chapter 2 Trial design and methods
Parts of this chapter have been reproduced with permission from Tandogdu et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Study design
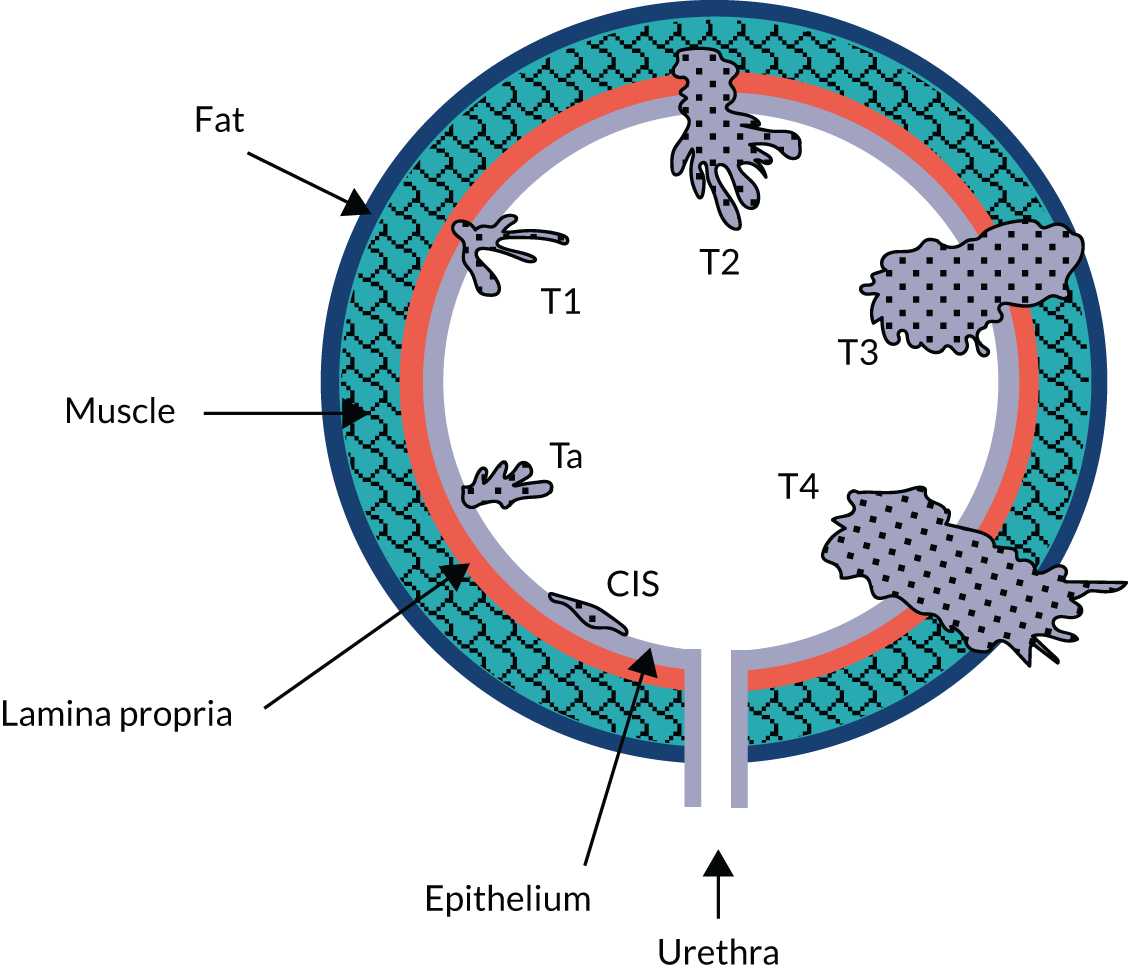
The Photodynamic versus white-light-guided resection of first diagnosis non-muscle-invasive bladder cancer (PHOTO) trial was a multicentre, pragmatic, open-label, parallel-group, non-masked, superiority RCTthat compared the intervention of PDD-TURBT with standard WL resection in patients with newly diagnosed intermediate- or high-risk NMIBC. Apart from initial treatment (initial TURBT with or without second TURBT), both groups received standard care as indicated by current practice guidelines,12,18 including single-dose intravesical MMC within 24 hours of initial resection, risk-adjusted adjuvant therapy and surveillance. The target number of participants was 533, with a trial-specific follow-up of at least 36 months per participant. Further details of the study design have been described previously and are shown in Figure 2.
FIGURE 2.
The PHOTO trial schema. a, Clinical follow-up scheduled from date of second TURBT, if conducted. CTCAE, Common Terminology Criteria for Adverse Events. Adapted with permission from Tandogdu et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Ethics approval and research governance
The Newcastle & North Tyneside 2 ethics committee [Research Ethics Committee (REC) reference number 14/NE/1062] provided a favourable ethics opinion for this research in July 2014. The trial was sponsored by The Newcastle upon Tyne Hospitals NHS Foundation Trust and is registered as ISRCTN84013636.
Participants
Adult patients (aged ≥ 16 years) with a suspected new and first diagnosis of intermediate- or high-risk NMIBC were potentially eligible and were recruited from participating secondary care hospitals, mainly at rapid-access haematuria clinics. On visual diagnosis, it is impossible to confidently predict a final risk category of intermediate or high, as the risk calculators require the results of histological parameters that are not perceptible by macroscopic assessment at flexible cystoscopy, that is, primarily, the microscopic features of low-grade, early-stage papillary tumours (pT) (pTa vs. pT1) and primary or concomitant CIS. Separation of high-risk and intermediate-risk disease can be resolved only retrospectively once the resection and pathological assessment of the tissue have occurred; therefore, separation is included as part of our pre-planned analyses after randomisation. Pragmatically, we can confidently remove low-risk disease on visual parameters alone because the finding of a new solitary lesion of < 3 cm in diameter, irrespective of histological details, would score below the threshold of points (measured using the EORTC calculator; EORTC, Brussels, Belgium; URL: www.eortc.be/tools/bladdercalculator/, accessed 14 January 2022) that indicates ‘higher risk’ disease (intermediate or high risk). Therefore, participants were identified based on the preliminary visual assessment of intermediate- or high-risk NMIBC using cystoscopy or imaging, performed as part of standard evaluation for suspected urinary tract malignancy.
Eligibility criteria
Inclusion criteria
-
Adult men and women aged ≥ 16 years.
-
First suspected diagnosis of bladder cancer.
-
Visual/ultrasound scan (USS)/CT diagnosis of intermediate- to high-risk NMIBC.
-
WL visual appearances of intermediate- or high-risk disease (tumour of ≥ 3 cm in diameter, ≥ 2 tumours or flat velvety erythematous changes giving rise to a clinical suspicion of CIS) or suspicion of papillary bladder tumour of ≥ 3 cm in diameter based on ultrasound or CT scanning (without hydronephrosis).
-
Written informed consent for participation prior to any study-specific procedures.
-
Willing to comply with the following lifestyle guidelines:
-
Female participants must be surgically sterile, be post-menopausal or agree to use effective contraception after joining the study and for 7 days after treatment. (Effective contraception was defined as two forms of contraception, including one barrier method.) Female participants must not breastfeed for 7 days after treatment.
-
Male participants must be surgically sterile or agree to use effective contraception after joining the study and for 7 days after treatment.
-
Exclusion criteria
-
Visual evidence of low-risk NMIBC (solitary tumour of < 3 cm in diameter).
-
Visual evidence of MIBC on preliminary cystoscopy, that is non-papillary or sessile mass (attached directly by its base without a stalk).
-
Imaging evidence of MIBC on CT/USS (including the presence of hydronephrosis).
-
Upper-tract (kidney or ureteric) tumours on imaging.
-
Any other malignancy in the past 2 years [except non-melanomatous skin cancer cured by excision, adequately treated CIS of the cervix, ductal carcinoma in situ (DCIS)/lobular carcinoma in situ (LCIS) of the breast, or prostate cancer in patients who have a life expectancy of > 5 years on trial entry].
-
Evidence of metastases.
-
Porphyria or known hypersensitivity to porphyrins.
-
Known pregnancy (based on history and without formal testing, in keeping with day-to-day NHS practice of PDD use).
-
Any other conditions that in the opinion of the local principal investigator (PI) would contraindicate protocol treatment.
-
Unable to provide informed consent.
-
Unable or unwilling to complete follow-up schedule (including HRQoL questionnaires).
Recruitment procedure
An eligibility checklist was completed by the local PI (or a delegate) to assess fulfilment of the entry criteria for all patients considered for the study. Information from the diagnostic cystoscopy was used to assess eligibility.
Informed consent
All potentially eligible patients were provided with an information sheet to explain why they had been approached and the nature of the study. Eligible patients were asked to provide written informed consent to take part in the study only after they had had sufficient time to consider the trial and the opportunity to have any further questions addressed by the local clinical team.
Interventions
The interventions being compared within the PHOTO trial were:
-
PDD-TURBT (experimental group)
-
standard WL-TURBT (control group).
All participants, unless there were clinical contraindications, received intravesical MMC (40 mg in 40 ml of saline), ideally within 6 hours following TURBT, but otherwise in the inpatient setting before discharge.
Technique of photodynamic diagnosis
Photodynamic diagnosis requires the preliminary instillation of the photosensitiser HAL (85 mg in 50 ml of phosphate-buffered saline) into the participant’s bladder through a urethral catheter. Participants were asked not to pass urine for at least 1 hour after instillation. Following operating theatre preparation in accordance with local standard procedures and under appropriate anaesthesia, participants underwent TURBT of their bladder tumour under blue-light illumination of the bladder (wavelength 380–450 nm). A specialised light source, cystoscope, light cables and cameras were required. When using PDD, normal bladder epithelium appears blue, and red areas are considered suspicious and should be resected.
Technique of standard white-light cystoscopy
The control group did not have any preliminary photosensitiser instillation, and standard tumour localisation and resection took place under WL illumination of the bladder (wavelength 400–800 nm).
Second resection
If, in accordance with the EAU guideline,12 the local PI deemed a second TURBT was required, the same method (PDD or WL) was used as that of the participant’s trial treatment allocation.
Adjuvant therapy
Adjuvant therapy was prescribed according to local clinical judgement in accordance with participant characteristics and the EAU guideline. 12
Treatment allocation (randomisation concealment and blinding)
Eligible consenting patients were centrally randomised using either the secure web-based randomisation system or the 24-hour interactive voice-response randomisation system at the Centre for Healthcare Randomised Trials (CHaRT) in Aberdeen. Minimisation by centre and sex was used to allocate participants 1 : 1 between the control (WL) and experimental (PDD) groups. The minimisation algorithm incorporated a random element to prevent potential deterministic treatment allocation. Participants were randomised by clinical teams at the participating NHS hospitals.
Blinding
Participants reported baseline HRQoL data using self-completed questionnaires before being informed of their randomised allocation. Surgeons and participants could not be blinded to the allocated procedure.
Delivery of the intervention
It was anticipated that allocated treatment would typically occur within the 2 weeks after randomisation to allow reasonable time for planning to meet the NHS 62-day target for cancer treatment. All other aspects of care were left to the discretion of the responsible surgeon.
Outcome measures
The primary clinical outcome measure was the time to recurrence measured as time from the day of randomisation to the day of subsequent biopsy for pathologically proven first recurrence (including progression, cystectomy and death from bladder cancer). The primary objective was to compare the time to recurrence for the two treatment strategies, with a principal point of interest at 3 years.
The primary health economic outcome was to evaluate cost-effectiveness using the incremental cost per recurrence avoided and cost–utility as the incremental cost per QALY gained at 3 years.
Secondary outcome measures
Other clinical outcomes included adverse events (AEs) and complications up to 3 months from initial or second TURBT (as applicable). Direct, surgically related, postoperative events occurring within the 30 days following TURBT were assessed using the Clavien–Dindo classification for surgical complications. 31 Events occurring up to 3 months after TURBT were assessed and recorded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0 framework. 32
The relative changes in HRQoL resulting from the physical and psychological benefit, together with any harms associated with each strategy and any subsequent necessary cancer treatment, were measured using the generic EuroQol-5 Dimensions, three-level version (EQ-5D-3L), questionnaire; the cancer-specific European Organisation for Research and Treatment of Cancer – Quality of Life Questionnaire – Core 30 (EORTC-QLQ-C30); and the disease-specific European Organisation for Research and Treatment of Cancer – Quality of Life Questionnaire – Non-Muscle Invasive Bladder Cancer – 24 items (EORTC-QLQ-NMIBC-24) questionnaire. These were completed by participants on paper at baseline (prior to knowledge of treatment allocation), following surgery and at 3, 6, 12, 18, 24 and 36 months after randomisation.
Disease progression was defined as an increase in stage to MIBC or the development of nodal or metastatic disease. In addition, details of intravesical treatment failure (e.g. BCG) were captured. Overall survival and bladder-cancer-specific survival were compared between the two treatment groups.
Other cost-effectiveness outcomes included the estimation of the incremental cost per recurrence avoided using the economic model over the patients’ lifetime and the estimation of the incremental cost per QALY gained using the economic model over the patients’ lifetime.
Additional exploratory objectives
The following objectives were explored:
-
to model the safest and most cost-effective cystoscopic follow-up surveillance schedule using data from within the trial and, if appropriate, from other relevant sources to describe the risk of recurrence at each interval of surveillance cystoscopy (note that this research is not part of this report; see Mowatt et al. 27)
-
to evaluate the learning curve for the PDD procedure and account for its effects on outcomes of both PDD and standard WL resections
-
to establish a well-characterised cohort of patients with intermediate- and high-risk NMIBC, including clinical data and urine, blood and tumour specimens for separately funded genotypic and phenotypic studies [the Photodynamic versus white-light-guided resection of first diagnosis non-muscle-invasive bladder cancer – Translational (PHOTO-T) study; see Appendix 3].
Data collection and management
The PHOTO trial schedule of assessment and investigations is summarised in Table 2. Eligibility was checked during routine attendance for diagnosis and staging of new bladder cancers, which included obtaining medical history. Eligible patients who consented to join the trial completed HRQoL questionnaires prior to initial TURBT and again prior to discharge from hospital. Intraoperative and postoperative data were reported by the local research teams at the time of the initial and second (as applicable) TURBT. At first recurrence, the date of biopsy and confirmatory histological details were reported by the local team. Details of any cystoscopy checks that were carried out were reported by the local research teams from the routine clinic visits at 3, 6, 9, 12, 18, 24 and 36 months post initial/second TURBT (as applicable), including an assessment of AEs at 3 months. Disease progression was assessed by the local research team using the results of further resection or imaging during follow-up. A short case report form (CRF) was completed annually from routine urology clinic visits following recurrence or progression to collect the disease status (e.g. further recurrence of NMIBC, progression to MIBC or cystectomy) and survival status of participants. The outcome of these assessments for each participant were entered as appropriate by centre staff on electronic CRFs on the central secure database held by CHaRT, where the accruing data were monitored.
| Assessment | Time point | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre randomisation screening | Pre treatment | TURBT | Prior to discharge | Second TURBT (as clinically indicated) | 3 months post treatment | 6 months post treatment | 9 months post treatment | 12 months post treatment | 18 months post treatment | 24 months post treatment | 36 months post treatment | Annually thereafter | At first disease recurrence/progression | |
| Visual diagnosis of intermediate- to high-risk NMIBC | ✗ | In accordance with EAU guideline12 | Treatment in accordance with local practice | |||||||||||
| Medical history | ✗ | In accordance with EAU guideline12 | Treatment in accordance with local practice | |||||||||||
| HRQoL questionnaire | ✗ | ✗ | In accordance with EAU guideline12 | Treatment in accordance with local practice | ||||||||||
| TURBT according to treatment allocation, with post treatment MMC instillation | ✗ | In accordance with EAU guideline12 | Treatment in accordance with local practice | |||||||||||
| Second TURBT, if required, according to treatment allocation | ✗ | In accordance with EAU guideline12 | Treatment in accordance with local practice | |||||||||||
| Assessment of AEs (CTCAE and Clavien–Dindo) | ✗ | In accordance with EAU guideline12 | Treatment in accordance with local practice | |||||||||||
| Cystoscopy | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | In accordance with EAU guideline12 | Treatment in accordance with local practice | |||||
| Histological confirmation of recurrence/progression | In accordance with EAU guideline12 | ✗ | ||||||||||||
Costs and changes in HRQoL were collected via self-completed postal questionnaires sent directly to participants by CHaRT at 3, 6, 12, 18, 24 and 36 months post randomisation. In addition, a participant costs questionnaire was administered by post at 30 months post randomisation.
All recruiting surgeons completed a learning curve questionnaire to elicit their WL- and PDD-resection experience prior to their centre commencing recruitment. The subsequent accruing experience of each surgeon was captured on CRFs.
All CRFs and participant questionnaires are available on the NIHR Journals Library project web page (www.journalslibrary.nihr.ac.uk/programmes/hta/1114202/#/documentation; accessed 16 November 2021).
Tracking and monitoring adverse events
The CHaRT study office was notified of AEs (CTCAEs, primarily grade ≥ 3) by the local research team on the 3-month cystoscopy CRF. Unrelated AEs were not recorded. In the PHOTO trial, ‘relatedness’ was defined as any untoward medical event that had a reasonable causal relationship with PDD-TURBT, standard WL-TURBT or the intravesical MMC. The following events were potentially expected: anaemia, bladder discomfort/pain, bladder perforation, bleeding resulting in clot retention, constipation, diarrhoea, deep-vein thrombosis (DVT), fever, gout, haematuria, headache, increase in white blood cell count, increased level of bilirubin, insomnia, nausea, postoperative dysuria, prolonged catheterisation, skin rash, ureteric obstruction/hydronephrosis, urethral stricture, urinary frequency, urinary retention, urinary tract infection and vomiting.
Any serious adverse events (SAEs) related to the participants’ TURBT treatment that were not further interventions (e.g. being admitted to hospital for an infection) were recorded on the SAE form. All deaths from any cause (related or otherwise) were recorded on the SAE form.
It was a requirement to report to the sponsor any SAEs that were deemed related and unexpected within 24 hours of receiving the signed SAE notification. Such SAEs were also reported to the main REC within 15 days of the chief investigator becoming aware of the event. All related SAEs were summarised and reported to the appropriate authorities as required.
Trial oversight
The day-to-day management of the trial was provided by the Clinical Trials and Statistics Unit at the Institute of Cancer Research (ICR-CTSU) and CHaRT based within the Health Services Research Unit, University of Aberdeen, with ICR-CTSU leading on trial management and CHaRT coordinating data management and statistics. The trial offices provided day-to-day support for the PIs at recruiting centres. The PIs, supported by dedicated research nurses, were responsible for all aspects of local organisation, including recruitment of participants, delivery of the interventions and notification of any problems or unexpected developments during the study.
A core group, chaired by the chief investigator and comprising key members of CHaRT and ICR-CTSU as well as the Health Economics and Biorepository teams at Newcastle University, met monthly. A Trial Management Group (TMG) was established and included the chief investigator, scientific leads (from ICR-CTSU and CHaRT), a health economist, co-investigators and collaborators, a patient representative, CHaRT’s Senior information technology manager, a trial statistician, and senior project managers and trial managers from ICR-CTSU and CHaRT. PIs and other key study personnel were invited to join the TMG, as appropriate, to ensure that there was representation from a range of centres and professional groups. The TMG met regularly and at least annually, and had operational responsibility for the conduct of the trial. The TMG’s terms of reference, roles and responsibilities were defined in a charter issued by ICR-CTSU. The trial was further overseen by the independent Trial Steering Committee (TSC) and an independent Data Monitoring Committee (DMC). The TSC comprised an independent chairperson and four independent members. The DMC comprised an independent chairperson and two independent members.
Patient and public involvement
A co-investigator (a patient with bladder cancer) provided advice based on the service user perspective and contributed to user-led selection of patient-reported outcome measures (PROMs). His experiences of the diagnosis and treatment of bladder cancer were taken into consideration in the design of this trial; in particular, this helped inform the use of QoL questionnaires that included emotional, social and physical domains specific to bladder cancer. As a member of the TMG, he also advised on approaches to recruitment, participant information resources and the dissemination of findings.
One of the independent members of the TSC was also a patient representative. The TSC met throughout the trial and reviewed all study documentation, including patient-facing documents. In addition to being an integral part of the study oversight and contributing to the Plain English summary included in this report, he provided feedback on what he felt were the key impacts of the study and the value of his contributions as the patient and public involvement (PPI) representative on the independent TSC:
As a patient who has lived experience of both a white light and a blue light, PDD-TURBT, I was particularly interested in both the outcome and the conduct of this trial from a patient perspective. Equally, in my role as Chair of the charity Action Bladder Cancer UK, I was keen to support much needed research into bladder cancer, and to use social media and speaking opportunities to raise the profile of the trial and promote participant recruitment. I have supported the TSC since its inception in 2014, being an active part of the committee and helping to make the reporting more patient focused. I was alert to recruitment issues early on (common to all bladder cancer trials) and able to ensure that the TMG, including its own PPI, was doing all it could to improve recruitment rates, paying particular attention to patient-facing materials. Overall, the trial has been very well run.
PPI TSC member
Sample size
The trial aimed to detect an absolute reduction in recurrence of 12% at 3 years: from 40% (under the conservative assumption that all of the patients recruited would be intermediate-risk patients with a probability of recurrence of 0.4 at 3 years) to 28% (similar effect sizes of photodynamic therapy have been reported in both intermediate- and high-risk groups27). This is equivalent to a relative reduction of 30%. Power calculations were based on log-rank analysis of time-to-event data, translating an improvement in the fixed time point recurrence-free rate of 60–72% to a target effect size hazard ratio (HR) of 0.64. The recruitment of 533 participants (214 recurrences) would enable the detection of a HR of 0.64 between experimental and control strategies, and would, using the log-rank test, provide 90% power at a two-sided 5% significance level. This calculation assumed 2.5 years of staggered recruitment (with 6%, 13%, 21%, 29% and 31% of the total number of patients recruited within each 6-month period), a minimum of 3 years’ follow-up and cumulative follow-up attrition rates of 0.56% by the end of year 1, 1% at the end of year 2 and 6.4% at end of year 3, based on unpublished data from the Bladder cyclooxygenase 2 Inhibition Trial (BOXIT).
To achieve the recruitment target, we planned to activate 30 secondary care sites that expected to see approximately 4590 new bladder cancer diagnoses over 2.5 years, from which we would exclude patients with MIBC (20%) and, from the remaining NMIBCs, those with low-risk disease (50%). Furthermore, we predicted that only 30% of these patients would be recruited based on willingness to participate or missed opportunities for recruitment.
Statistical methods
The trial analysis followed a statistical analysis plan. There was no interim analysis of clinical effectiveness data, only a single analysis after the database was locked on 2 June 2020. The main analyses for both safety and clinical effectiveness were based on the intention-to-treat (ITT) principle, that is they were analysed as randomised, regardless of the intervention received. However, after the initial resection, some participants were found to have no tumour, some were found to have MIBC and some had an early cystectomy. These participants were excluded from the ITT population (see Chapter 3 for details). We also used per-protocol analyses restricted to participants who received the treatment to which they were allocated.
Baseline and follow-up data were summarised using appropriate statistics and graphical summaries. All analysis was done using Stata, version 16 (StataCorp LP, College Station, TX, USA), software.
Primary outcome
The primary outcome was time to recurrence of bladder cancer, measured in months from randomisation to recurrence, progression, cystectomy or death due to bladder cancer. The principal time point of interest was 3 years. We used a time-to-event framework to analyse the primary outcome. In the primary analysis, deaths were treated as censored in Cox proportional hazards regression models. The first model adjusted for the minimisation covariate factors of sex and centre (the latter via a random-effects frailty model) and the treatment effect was summarised as a HR with a 95% CI. The second model added the following known prognostic factors: smoking status, risk group, presence or absence of CIS and grade of surgeon (i.e. registrar, non-consultant career grade or consultant). The HRs and 95% CIs for these prognostic factors were given, as was the HR for the treatment effect adjusting for these prognostic factors.
We plotted empirical survival distribution using Kaplan–Meier plots; visually, the proportional hazards assumption was questionable. The proportional hazard assumption was assessed by plotting log [−log (survival)] against log (analysis time). We used accelerated failure time models based on Weibull, exponential, log-logistics, log-normal and generalised gamma distributions, relaxing the proportional hazards assumption. The model with the smallest Akaike information criterion value was considered the best-fitted model and reported.
Secondary outcomes
Secondary outcomes were analysed using the appropriate generalised linear models as follows:
-
Disease progression at 3 years – progression was defined as increased stage to MIBC, development of metastatic disease at other regional sites, development of nodal disease or death due to bladder cancer. The end point was analysed using a time-to-event framework. Analysis was as for the primary outcome.
-
HRQoL – standard measure-specific algorithms were used to derive scores from and handle missing data within each HRQoL outcome. We used a linear mixed model (random effect for centre and participant; fixed effect for nominal time, treatment, sex, smoking status, risk group, presence or absence of CIS and grade of surgeon) to analyse the repeated measures of HRQoL outcomes. Treatment effects at each time were derived from the interaction term for time by treatment. To account for multiple HRQoL outcomes and time points, we report 99% CI around estimates.
-
Bladder-cancer-specific survival – the time to bladder-cancer-specific death was analysed using a competing risks approach (based on the Fine and Gray model). 33 Death from other causes was considered a competing risk in the Cox proportional hazards model, instead of assuming non-informative censoring. The first model was adjusted for sex and centre, and the second was adjusted for sex, centre, smoking status, risk group, CIS and surgeon grade.
Sensitivity analysis
A sensitivity analysis of the primary outcome that treated deaths from non-bladder cancer causes as a competing risk rather than non-informative censoring was performed. We report the subhazard ratio (SHR) for the treatment effect in two models, first adjusted for sex and centre, and then adjusted for sex, centre and prognostic factors as per the primary outcome. We plotted cumulative incidence curves for time to recurrence.
Surgical learning curve
The effect of photodynamic resection experience (learning curve) on clinical effectiveness was examined. Based on the results of a questionnaire completed by participating surgeons prior to centre activation, each centre was classified as PDD experienced or naive.
A subgroup analysis comparing outcomes from experienced or naive centres, including specific PDD- and WL-resection-related outcomes, was used to assess the maximum effect of experience on outcome in an NHS setting. Early recurrence (at 12 weeks) was used as a proxy of incomplete resection. The subsequent accruing experience of each surgeon was captured on a CRF. This allowed each randomised participant to be positioned on an individual surgeon learning curve.
Safety data
The proportion of participants experiencing AEs (CTCAE grade 3 or above) was compared between groups using Poisson regression. The number of AEs by Clavien–Dindo grade was tabulated by group.
Health economics methods
Economic evaluations are conducted as an aid to decision-making. In the PHOTO trial, the economic evaluation aimed to determine whether or not PDD-TURBT was cost-effective compared with WL-TURBT as part of the management of people with suspected intermediate- and high-risk cancers confined to the bladder lining. For the economic evaluation, both a trial-based and model-based cost–utility analysis was planned. The within-trial analysis considered costs and outcomes over a 3-year follow-up. The model-based analysis was planned to have a lifetime time horizon. The model-based analysis was not conducted as it was judged not to add any additional information for decision-makers. See Appendix 2 for information about the proposed modelling component.
The within-trial economic evaluation took an NHS and Personal Social Services (PSS) perspective in line with the NICE reference case. 34 The results were estimated at 3 years following randomisation using the ITT principle. Results are presented in terms of cost, QALYs, incremental cost, incremental QALYs and incremental cost per QALY. The costs and QALYs accruing after the first year and the incremental cost per QALY gained were discounted at a rate of 3.5% per annum.
A time trade-off (TTO) study was commenced to provide estimates of short-term decreases in utility following cystectomy; these data were planned to be used alongside the EQ-5D-3L trial data collected at 6-month intervals. However, the TTO work was paused because of COVID-19 and the nature of the interviews (involving complex instructions, face-to-face contact and lasting approximately 1 hour); it was not feasible to continue this work within COVID-19 restrictions. Eleven patients were recruited, which was substantially short of our target sample size (n = 50) and, hence, meaningful analysis could not be performed. See Appendix 2 for a brief description of the method.
Incremental costs and QALYs were presented as point estimates and 95% CIs using adjusted linear regression. The on-parametric bootstrap approach was also used to produce the cost-effectiveness plane, representing the uncertainty in incremental cost and QALY estimates, and the cost-effectiveness acceptability curve (CEAC), representing the probability that PDD-TURBT was cost-effective compared with WL-TURBT at different cost-effectiveness thresholds for an incremental cost per QALY gained. 35
The within-trial analysis was conducted in Stata version 16.1. The model-based analysis was to be conducted in TreeAge Pro™ 2020 (TreeAge Software, Inc., Williamstown, MA, USA).
Derivation of NHS resource use
The resource use data collected during the PHOTO trial were used to estimate the individual patient costs over the trial. The analysis included the following categories of NHS resource use:
-
Resource use associated with the hospital episode during which the initial intervention was provided. This also included the length of hospital stay.
-
Resource use of hospital associated with readmissions after the initial index admission (follow-up operations and length of stay in hospital) over the 3 years’ follow-up.
-
Outpatient contacts over the 3 years’ follow-up.
-
Use of primary care services, including outpatient/general practitioner (GP)/doctor/nurse consultations and GP/nurse home visits over the 3 years’ follow-up.
The resource use data were collected on an ongoing basis by the clinical investigators, or were self-reported by trial participants at the initial procedure or during the follow-up period (3, 6, 12, 18, 24, 30 and 36 months post randomisation).
Initial procedure
The resources associated with the initial procedure included all of the resources incurred until discharge. The resource use data required to deliver each intervention were collected prospectively for every participant in the study. The operative details were recorded at the time of surgery (e.g. time in theatre, grade of operating surgeon). These data were captured in the operation details CRF. Costs incurred after the TURBT procedure but before discharge were collected using the initial resection CRF and post-treatment participant questionnaire. These forms contained information on the length of hospital stay for the initial TURBT (based on admission and discharge dates), medical procedures and medical events that could occur during the treatment phase.
Subsequent use of services following discharge for the index procedure
After participants were discharged, their resource use was captured using the health service utilisation questionnaire (HSUQ) completed by participants at 3, 6, 12, 18, 24 and 36 months. The use of the HSUQ allowed us to categorise resource use as either secondary or primary care. This included all secondary care (e.g. WLC, flexible cystoscopy, mitomycin, BCG, CT scan, cystectomy, palliative care, inpatient admissions, day admissions, hospital doctor consultation, outpatient consultations, accident and emergency consultations) and primary care contacts with health professionals (e.g. GP consultations, practice and district nurse consultations, other health professional consultations). Visits with these health professionals could occur at the health-care practice, at the participant’s home or over the telephone. We distinguished between the different types of consultation to account for the different costs associated with each setting.
For our analysis, we assumed that any participant who partially completed the questionnaire had left other responses blank because the questions did not apply to them. For participants who had died during the follow-up period, their resource use was automatically imputed as 0. This could cause an underestimation in our costs as these participants may have used some services during the data collection period before they died.
Unit costs of NHS care
National average unit costs were applied to resource use data to generate the total costs to the health service. The sources of unit costs were the British National Formulary (BNF)36 and the NHS Reference Costs 2018–1937 for secondary care resource use data, and the Personal Social Services Research Unit (PSSRU)’s Unit Costs of Health and Social Care for primary care resource use data. 38 See Appendix 2, Table 46, for a list of all unit costs used in the within-trial economic analysis, together with their sources. These costs were reported in 2018–19 Great British pounds (£). For the purpose of inflation, we utilised the Campbell and Cochrane Economics Methods Group Evidence for Policy and Practice Information Centre (CCEMG-EPPI-Centre) Cost Converter, version 1.6 (Campbell and Cochrane Economic Methods Group, London, UK), using International Monetary Fund-reported inflation data. 39
Estimation of NHS costs
For each participant, the total use for each resource was multiplied by the unit cost to calculate the total cost of each resource. For example, the initial length of admission was multiplied by the NHS cost per night for an inpatient stay on a general ward to obtain the cost of hospitalisation for each participant. The cost for each year beyond the first year was discounted at a rate of 3.5% per annum. The total discounted costs from the health services perspective were calculated by summing all intervention treatment and follow-up discounted costs for each participant in the data set.
Participant- and companion-incurred costs and indirect costs
Participant resource utilisation comprised three main elements:
-
costs of accessing and using NHS health services (e.g. petrol, public transport and parking)
-
time costs of accessing and using NHS health services (e.g. time involved away from usual activities or work)
-
indirect costs due to ill health.
Costs of accessing and using NHS health services
The estimation of costs of accessing and using NHS health services required information from participants about the number of visits to, for example, their GP (estimated from health-care utilisation questions in the participant costs questionnaire administered at 30 months) and the unit cost of making a return journey to each type of health-care provider.
Time-off costs of accessing and using NHS health services
The cost of participant time was estimated in a similar manner. The participant was asked, in the participant costs questionnaire, how long they had spent travelling and attending their last visit to each type of health-care provider and if they had been accompanied by a friend or relative (if so, the companion’s time and travel costs were also incorporated into the analysis). These data were recorded in their natural units (e.g. minutes). The unit cost of time lost was obtained from the Department for Transport estimates for the value of work and leisure time. 40 The cost of each visit was then calculated by multiplying the time lost by the time unit cost. The total time cost per patient was then calculated by multiplying the patient’s time cost per visit by the number of health-care contacts obtained from the health-care utilisation questions.
Indirect costs due to ill health
Indirect costs were defined as the production losses when the participant was unable to return to work or was required to take sick leave because of their illness. The cost of days lost was estimated using the UK median gross hourly wage. When a participant’s self-reported costs associated with a specific type of health service visit were missing, the mean cost for that type of visit was imputed. Participants completing the participant costs questionnaire were asked how many days they had been off work in the previous 2 months as a result of health problems. These data were collected using the participant costs questionnaire at the 30-month follow-up point. The data were recorded in their natural units (e.g. days) and multiplied using the unit costs. The total production losses due to time away from work as a result of health problems were estimated and compared between treatment groups.
The data on costs and time-off costs of accessing and using NHS health services and the indirect costs due to ill health were summed to generate a total cost for each participant and their companions. The incremental cost differences between groups from a participant perspective were estimated using the same methods outlined for the NHS perspective (see Health economics methods).
Quality-adjusted life-years
Participants were asked to complete the EQ-5D-3L instrument41 at baseline, discharge and follow-up visits (3, 6, 12, 18, 24 and 36 months). The EQ-5D-3L is a generic instrument used to assess participants’ QoL for the base-case analysis because it is the preferred utility measure of NICE. 34 The EQ-5D-3L measure divides health status into five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Each of these dimensions has three levels, so 243 possible combinations of health states exist. Each combination of levels across the dimensions is associated with an EQ-5D-3L index value. 41 Utility value data derived from the EQ-5D-3L were combined with mortality data from the trial under the standard assumption that all patients who have died in the trial will have a utility value of 0 from the date of death to the end of follow-up. The QALY for each year was then calculated based on these assumptions using the area-under-the-curve approach, and assuming linear extrapolation of utility between time points. QALYs for each year beyond the first year were discounted at a rate of 3.5% per annum. The total discounted QALYs for each participant were calculated by summing the discounted QALYs over the trial follow-up period.
Handling missing data
Missing data are a concern in this study because costs or health outcomes in individuals with missing data may be systematically different from those in individuals with no missing data. A substantial proportion of missing data observed in a trial can pose significant problems for data analysis.
The complete-case analysis is inefficient for the PHOTO study because all of the information from individuals with at least one assessment missing is discarded. In addition, the complete-case analysis cannot be considered an ITT analysis because some randomised patients with follow-up data are excluded. 42 Therefore, the imputed data analysis was used as the base-case analysis and the complete-case analysis was conducted as a scenario analysis in the sensitivity analysis. The imputation was undertaken using Stata’s multiple imputation (MI) procedure.
Multiple imputation was used to impute missing EQ-5D-3L utility values and cost values for individuals with data at baseline or at least one follow-up visit. When missing data are ‘missing at random’ (MAR), valid conclusions can be drawn from the available data using the MI approach. 43 Missing values of total follow-up cost and EQ-5D-3L utility values at each time point were imputed using predictive mean matching by treatment allocation group, accounting for the three closest estimates in terms of baseline EORTC recurrence risk group, age at randomisation and sex. Chained equations were used for the imputations. The imputation procedure predicted 50 plausible alternative imputed data sets, which was found to be a sufficient number to provide stable estimates. An analysis of incremental costs and QALYs was undertaken across the 50 imputed data sets and combined to generate one imputed estimate of incremental costs and QALYs. We drew bootstrap samples from each of the 50 imputed data sets.
Estimation of cost-effectiveness
A seemingly unrelated regression (SUR) approach was used to simultaneously estimate the total discounted costs at 3 years and the total discounted QALYs at 3 years, allowing for the likely correlation of costs and effects. 44 For the QALY outcome variables, baseline EORTC recurrence risk group, age at randomisation, sex and baseline EQ-5D-3L utility value were included as covariates. For the cost outcome variables, baseline EORTC recurrence risk group was included as a covariate.
The results are reported as incremental cost per QALY gained for PDD-TURBT relative to WL-TURBT. The incremental cost per QALY was calculated from the coefficient of treatment effect on costs divided by the coefficient of treatment effect on QALYs from the SUR model.
To address the issue of sampling uncertainty in the data, we used non-parametric bootstrapping methods to estimate 95% CIs for the treatment effects on costs and QALYs, using 2000 repetitions. 45 This imprecision was then presented graphically as a cost-effectiveness plane (see Figure 14). This shows the scatterplot of bootstrapped repetitions for incremental costs and incremental QALY pairs for PDD-TURBT compared with WL-TURBT. 46 The scatterplot is divided into four quadrants, each of which represents one of the following scenarios:
-
PDD-TURBT is less costly and more effective than WL-TURBT.
-
PDD-TURBT is more costly and less effective than WL-TURBT.
-
PDD-TURBT is less costly and less effective than WL-TURBT.
-
PDD-TURBT is more costly and more effective than WL-TURBT.
The proportion of the total bootstrap samples that lie in a quadrant represents the probability associated with that scenario.
The bootstrapped estimates of costs and QALYs were also used to produce CEACs. 47 CEACs were generated using the net monetary benefit (NMB) approach, where:
‘QALY’ and ‘cost’ are the estimated total QALYs and total costs for a treatment strategy, respectively, and λ is the decision-maker’s cost-effectiveness threshold for a QALY gained.
In this analysis, λ was varied over the range £0–60,000. In the tabular presentation of the analysis, λ is presented at the following thresholds: £0, £20,000, £30,000 and £50,000.
The proportion of bootstrap samples in which the net benefit is positive at a given threshold for cost per QALY represents the probability that the treatment is cost-effective. This was repeated for each MI data set. The probability across all MI data sets was averaged for the threshold values stated above.
Sensitivity analysis
The base-case analysis was conducted under the MAR assumption, using MI to impute the missing cost and HRQoL values. It is, however, recognised that participants who failed to complete an EQ-5D-3L questionnaire at a specific follow-up assessment may have been in relatively poorer health than those who did. This means that the chance of observing HRQoL could depend on their actual utility value, that is data are likely to be missing not at random (MNAR). Therefore, it is important to explore the impact of the missing data mechanism [i.e. missing completely at random (MCAR), MAR and MNAR assumptions] on cost-effectiveness outcomes. A sensitivity analysis was conducted to explore the impact on the results of assuming that the data are MNAR, with scenarios for systematic differences between missing and observed values being examined. The sensitivity analysis also explored whether or not this might have differed between randomised groups.
Because we cannot determine the true missing data mechanism based on the observed data, pattern-mixture models were implemented using MI to assess whether or not conclusions are robust to plausible departures from the MAR assumption in the sensitivity analysis. 48,49 These analyses adjusted the imputed values in the base-case analysis by either adding up to 10% to the imputed QALYs and/or total cost, or subtracting up to 10%. This sensitivity parameter was allowed to differ by group, with up to a 5% difference between the two groups (this reflects that the missing data mechanism may not be the same in the two groups, but that it is unlikely to be perfectly MAR in one group and strongly MNAR in the other). The pattern-mixture approach has been favoured in the context of clinical trial sensitivity analysis. 50,51
To explore structural uncertainty in the base-case analysis, the following scenarios around missing data for cost and QALY outcomes were explored:
-
For patients who failed to complete an EQ-5D-3L questionnaire, we assumed their HRQoL could be up to 10% lower than the MAR setting in both groups.
-
For patients who failed to complete an EQ-5D-3L questionnaire, we assumed that their costs could be up to 10% higher than the MAR setting in both groups.
-
For patients who failed to complete an EQ-5D-3L questionnaire, we assumed their HRQoL could be up to 10% lower and their costs could be up to 10% higher than the MAR setting in both groups.
-
For patients who failed to complete an EQ-5D-3L questionnaire, we assumed their HRQoL could be up to 10% lower than the MAR setting in the PDD group.
-
For patients who failed to complete an EQ-5D-3L questionnaire, we assumed their HRQoL could be up to 10% lower than the MAR setting in the WLC group.
-
For patients who failed to complete an EQ-5D-3L questionnaire, we assumed their costs could be up to 10% higher than the MAR setting in the PDD group.
-
For patients who failed to complete an EQ-5D-3L questionnaire, we assumed their costs could be up to 10% higher than the MAR setting in the WLC group.
-
A complete-case analysis was performed, in which participants with missing data were excluded from analysis.
We also explored the impact of varying the discount rate used for costs and QALYs following NICE best practice recommendations,34 ranging the discount rate from 0% to 6% per annum. Furthermore, a supplementary analysis presents costs from a wider patient/societal perspective.
Chapter 3 Participant baseline characteristics
This chapter describes patient recruitment into the study and baseline characteristics. The subsequent results of the clinical effectiveness and cost-effectiveness analyses are reported in Chapters 4 and 5, respectively. Participants were screened and recruited at 22 NHS hospitals (see Appendix 1, Table 29, for the numbers recruited at each centre).
Study recruitment
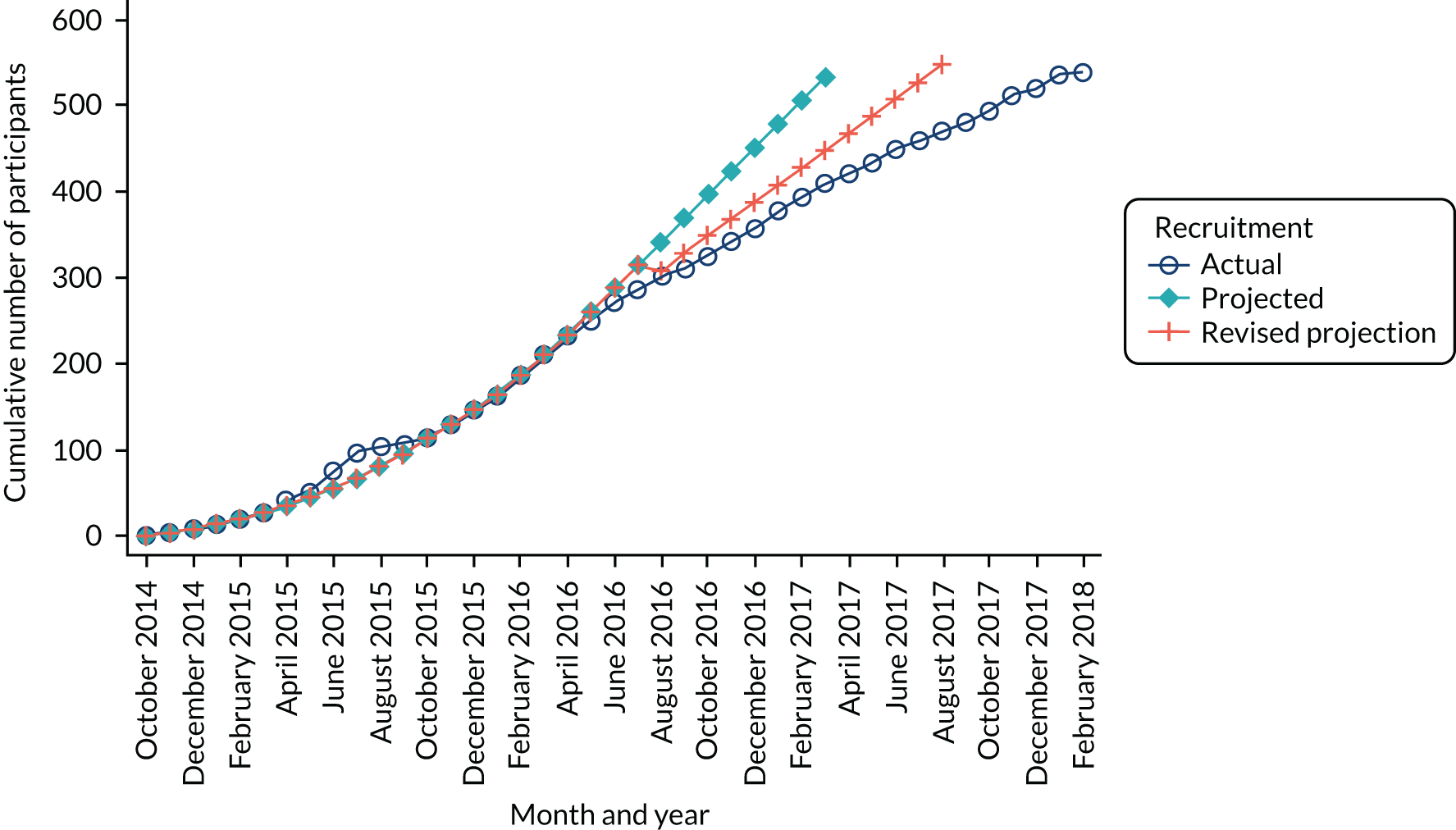
A total of 538 participants were randomised into the study across the 22 participating centres. Participants were recruited between 11 November 2014 and 6 February 2018, and followed up until 28 August 2020. (See Appendix 1, Table 29, for the centre recruitment figures.) Figure 3 shows the trajectory of the number of participants randomised over the recruitment period.
FIGURE 3.
Recruitment graph.
Participant flow
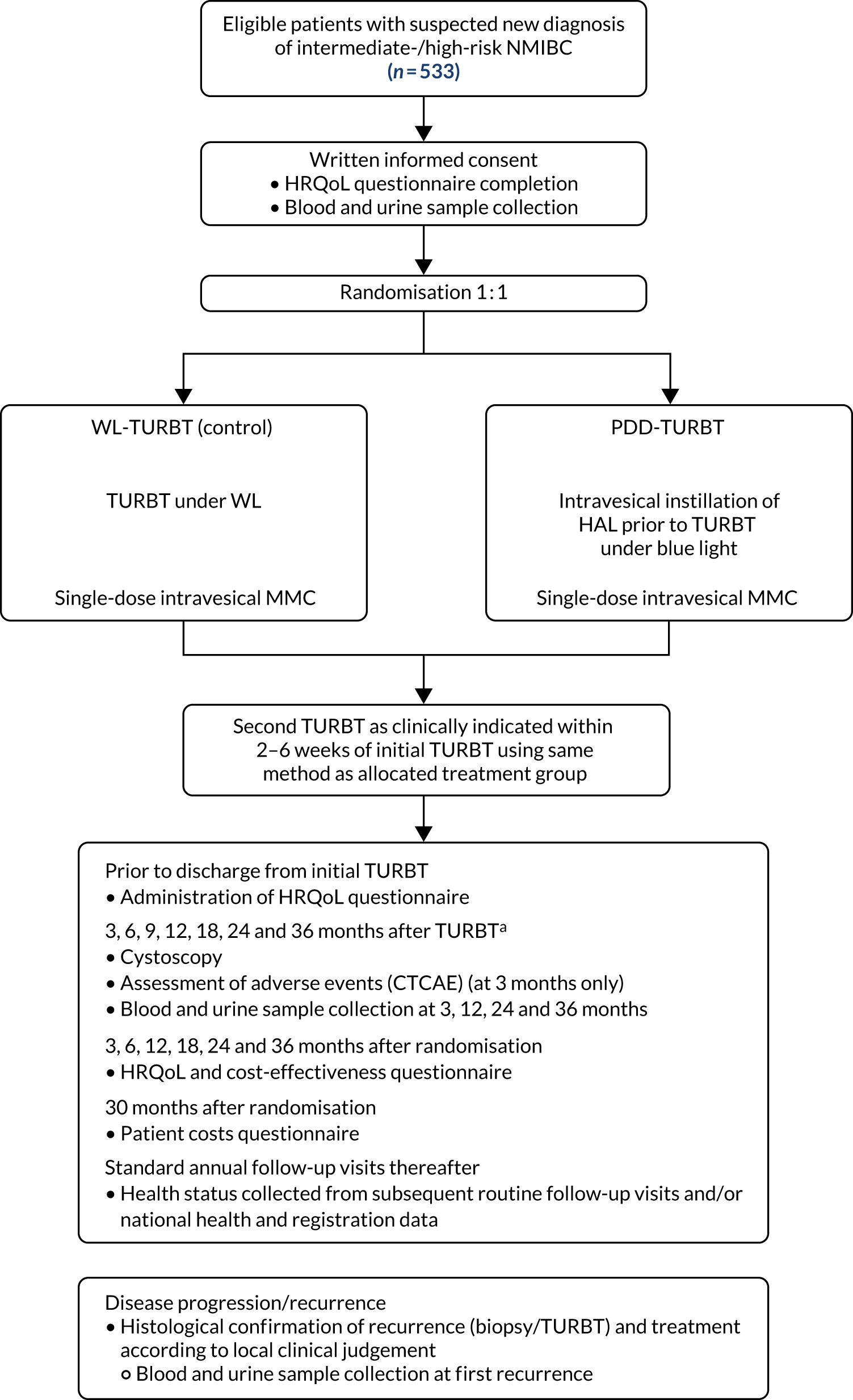
The flow of participants through the trial is summarised in Figure 4, in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement. A total of 1077 patients were reported on ineligible or declined eCRFs and were assessed for eligibility. Of those, 538 were randomised: 269 participants were allocated to PDD and 269 were allocated to WL. There were 226 participants who did not meet the inclusion criteria and 242 who declined to participate in the study. The main reason for ineligibility was visual evidence of low-risk NMIBC (59%) and the most frequent reason for participants declining was because they were not interested in the study (21%). (See Appendix 1, Table 30, for further details of why screened participants were not randomised.)
There were five post-randomisation exclusions, the reasons for which were as follows: participant was consented and randomised and then found to be ineligible; CT report showed left hydronephrosis; CT scans of two patients showed likely MIBC after randomisation; and patients were found to have upper tract disease after randomisation. After the initial TURBT, 29 participants were found to have no histological evidence of tumour, 60 had MIBC and 18 had an early cystectomy. These 107 participants have been excluded from analysis and reporting in the main body of this monograph beyond this point (see Appendix 1 for baseline and follow-up data for these participants). A total of 426 participants (PDD, n = 209; WL, n = 217) were included in the analysis (see Figure 4).
Baseline characteristics
The minimisation variables of centre and sex are shown in Table 3, and participants’ baseline characteristics are shown in Table 4. The groups remain well balanced after the exclusion of participants with MIBC and no tumour. The mean age of the participants was 70 years and the majority were men.
| Minimisation variable | Treatment group, number of participants (%) | |
|---|---|---|
| PDD (N = 209) | WL (N = 217) | |
| Centre | ||
| Newcastle Hospitals NHS Foundation Trust, Newcastle | 22 (10.5) | 21 (9.7) |
| Royal Devon and Exeter NHS Foundation Trust, Exeter | 15 (7.2) | 17 (7.8) |
| Oxford University Hospitals NHS Foundation Trust, Oxford | 12 (5.7) | 9 (4.1) |
| NHS Tayside, Dundee | 5 (2.4) | 4 (1.8) |
| University College London Hospitals NHS Foundation Trust, London | 2 (0.9) | |
| Cwm Taf Morgannwg University Health Board, Bridgend | 1 (0.5) | |
| Ashford and St Peter’s Hospitals NHS Foundation Trust, Ashford | 5 (2.4) | 2 (0.9) |
| NHS Lothian, Edinburgh | 34 (16.3) | 38 (17.5) |
| Hull University Teaching Hospitals NHS Trust, Cottingham | 6 (2.9) | 10 (4.6) |
| Hampshire Hospitals NHS Foundation Trust, Basingstoke | 3 (1.4) | 3 (1.4) |
| South Tees Hospitals NHS Foundation Trust, Middlesbrough | 21 (10.0) | 24 (11.1) |
| Imperial College Healthcare NHS Trust, London | 2 (1.0) | 7 (3.2) |
| Leeds Teaching Hospitals NHS Trust, Leeds | 14 (6.7) | 11 (5.1) |
| Swansea Bay University Health Board, Swansea | 13 (6.2) | 12 (5.5) |
| Dartford and Gravesham NHS Trust, Dartford | 20 (9.6) | 26 (12.0) |
| University Hospital Southampton NHS Foundation Trust, Southampton | 6 (2.9) | 2 (0.9) |
| University Hospitals of North Midlands NHS Trust, Stoke-on-Trent | 10 (4.8) | 10 (4.6) |
| Derby Teaching Hospitals NHS Foundation Trust, Derby | 6 (2.9) | 4 (1.8) |
| Salisbury NHS Foundation Trust, Salisbury | 6 (2.9) | 9 (4.1) |
| NHS Grampian, Aberdeen | 8 (3.8) | 4 (1.8) |
| East and North Hertfordshire NHS Trust, Stevenage | 1 (0.5) | 1 (0.5) |
| Sex | ||
| Male | 167 (79.9) | 172 (79.3) |
| Female | 42 (20.1) | 45 (20.7) |
| Baseline clinical characteristic | Treatment group | |
|---|---|---|
| PDD (N = 209) | WL (N = 217) | |
| Age (years), mean (SD); minimum, maximum | 71 (11); 27, 96 | 70 (10); 31, 89 |
| Smoking status, n (%) | ||
| Current smoker | 33 (15.8) | 30 (13.8) |
| Previous smoker | 117 (56.0) | 123 (56.7) |
| Never | 57 (27.3) | 60 (27.6) |
| Unknown | 1 (0.5) | 3 (1.4) |
| Missing | 1 (0.5) | 1 (0.5) |
| Number of tumours, n (%) | ||
| Single | 66 (31.6) | 81 (37.3) |
| 2–7 | 122 (58.4) | 113 (52.1) |
| ≥ 8 | 17 (8.1) | 21 (9.7) |
| Missing | 4 (1.9) | 2 (0.9) |
| Tumour size at baseline (cm), n (%) | ||
| < 3 | 69 (33.0) | 81 (37.3) |
| ≥ 3 | 133 (63.6) | 129 (59.4) |
| Missing | 7 (3.3) | 7 (3.2) |
| Histological grade at baseline, n (%) | ||
| Grade 1 | 17 (8.1) | 16 (7.4) |
| Grade 2 | 116 (55.5) | 112 (51.6) |
| Grade 3 | 72 (34.4) | 86 (39.6) |
| Missing | 4 (1.9) | 3 (1.4) |
| Histological stage at baseline, n (%) | ||
| pTa | 150 (71.8) | 160 (73.7) |
| pT1 | 64 (30.6) | 66 (30.4) |
| CIS, n (%) | ||
| Present | 27 (12.9) | 24 (11.1) |
| Absent | 180 (86.1) | 190 (87.6) |
| Missing | 2 (1.0) | 3 (1.4) |
| EORTC risk group, n (%) | ||
| Low risk (score 0) | 0 (0.0) | 2 (0.9) |
| Intermediate risk (score 1–9) | 184 (88.0) | 190 (87.6) |
| High risk (score 10–17) | 17 (8.1) | 15 (6.9) |
| Not calculable | 8 (3.8) | 10 (4.6) |
| NICE risk group, n (%) | ||
| Low risk | 10 (4.8) | 8 (3.7) |
| Intermediate risk | 100 (47.8) | 96 (44.2) |
| High risk | 96 (45.9) | 107 (49.3) |
| Not calculable | 3 (1.4) | 6 (2.8) |
Participants were categorised into low-, intermediate- and high-risk groups for recurrence based on EORTC and NICE risk tables. 18 Based on the EORTC risk table > 80% of participants in both treatment groups were in the intermediate-risk group. CIS was absent in > 85% of the participants.
Table 5 summarises the characteristics of the surgeons who performed the initial/second resections. Consultants performed > 65% of the surgeries in both groups. Surgeons with experience of > 40 cases of PDD performed 39% of PDD surgeries and 40% of WL surgeries.
| Surgeon characteristics | Treatment group, number of participants (%) | |
|---|---|---|
| PDD (N = 209) | WL (N = 217) | |
| Grade of surgeon | ||
| Registrar/non-consultant career grade | 46 (22.0) | 65 (30.0) |
| Consultant | 160 (76.6) | 148 (68.2) |
| Missing | 3 (1.4) | 4 (1.8) |
| Surgeon’s PDD experience (number of cases) | ||
| < 10 | 43 (20.6) | 50 (23.0) |
| 10–19 | 46 (22.0) | 46 (21.2) |
| 20–40 | 30 (14.4) | 11 (5.1) |
| > 40 | 81 (38.8) | 87 (40.1) |
| Missing | 9 (4.3) | 23 (10.6) |
Health-related quality of life was assessed using three validated questionnaires: the EQ-5D-3L, EORTC-QLQ-C30 and EORTC-QLQ-NMIBC-24. Data are summarised in Table 6. The mean EQ-5D-3L score was 0.83 in the PDD group and 0.84 in the WL group. Across subscales, median scores ranged between 75.0 and 100.0 in both the PDD and WL groups. For symptom subscales, the scores ranged between 0 and 22.2 (the score for fatigue) in the PDD group and between 0 and 11.1 (the score for fatigue) in the WL group.
| Baseline HRQoL | Treatment group | |
|---|---|---|
| PDD (N = 209) | WL (N = 217) | |
| EQ-5D-3L | ||
| Total score | ||
| Mean (SD); n | 0.83 (0.20); 189 | 0.84 (0.22); 190 |
| Median (25th, 75th percentile) | 0.85 (0.73, 1.00) | 0.87 (0.73, 1.00) |
| Visual analogue scale | ||
| Mean (SD); n | 75.92 (18.37); 183 | 74.58 (18.25); 184 |
| Median (25th, 75th percentile) | 80 (70, 90) | 80 (65.00, 90) |
| EORTC-QLQ-C30 | ||
| Functioning scalesa | ||
| Physical | ||
| Mean (SD); n | 83.50 (20.26); 191 | 85.55 (17.76); 197 |
| Median (25th, 75th percentile) | 93.33 (73.33, 100) | 93.33 (80, 100) |
| Role | ||
| Mean (SD); n | 85.53 (24.95); 190 | 87.65 (21.94); 197 |
| Median (25th, 75th percentile) | 100 (83.33, 100) | 100 (83.33, 100) |
| Cognitive | ||
| Mean (SD); n | 85.44 (18.58); 190 | 87.48 (18.09); 197 |
| Median (25th, 75th percentile) | 83.33 (83.33, 100) | 100 (83.33, 100) |
| Emotional | ||
| Mean (SD); n | 80.01 (21.18); 188 | 81.63 (19.14); 194 |
| Median (25th, 75th percentile) | 83.33 (66.67, 100) | 83.33 (75.00, 100) |
| Social | ||
| Mean (SD); n | 86.61 (22.26); 188 | 88.46 (21.26); 195 |
| Median (25th, 75th percentile) | 100 (83.33, 100) | 100 (83.33, 100) |
| Global QoL | ||
| Mean (SD); n | 73.36 (19.27); 188 | 73.80 (20.29); 195 |
| Median (25th, 75th percentile) | 75.00 (66.67, 83.33) | 75.00 (66.67, 83.33) |
| Symptom scales and/or itemsb | ||
| Fatigue | ||
| Mean (SD); n | 21.84 (22.81); 189 | 19.51 (20.27); 197 |
| Median (25th, 75th percentile) | 22.22 (0, 33.33) | 11.11 (0, 33.33) |
| Nausea and vomiting | ||
| Mean (SD); n | 3.88 (11.89); 189 | 3.13 (9.23); 197 |
| Median (25th, 75th percentile) | 0 (0, 0) | 0 (0, 0) |
| Pain | ||
| Mean (SD); n | 18.76 (25.10); 191 | 17.51 (25.18); 197 |
| Median (25th, 75th percentile) | 16.67 (0, 33.33) | 0 (0, 33.33) |
| Dyspnoea | ||
| Mean (SD); n | 14.29 (22.84); 189 | 13.87 (21.54); 197 |
| Median (25th, 75th percentile) | 0 (0, 33.33) | 0 (0, 33.33) |
| Sleep disturbance | ||
| Mean (SD); n | 22.46 (29.87); 190 | 22.84 (27.20); 197 |
| Median (25th, 75th percentile) | 0 (0, 33.33) | 0 (0, 33.33) |
| Appetite loss | ||
| Mean (SD); n | 12.52 (24.35); 189 | 8.63 (19.89); 197 |
| Median (25th, 75th percentile) | 0 (0, 33.33) | 0 (0, 0) |
| Constipation | ||
| Mean (SD); n | 12.52 (23.36); 189 | 9.14 (20.37); 197 |
| Median (25th, 75th percentile) | 0 (0, 33.33) | 0 (0, 0) |
| Diarrhoea | ||
| Mean (SD); n | 7.17 (18.57); 186 | 5.44 (15.24); 196 |
| Median (25th, 75th percentile) | 0 (0, 0) | 0 (0, 0) |
| Financial difficulties | ||
| Mean (SD); n | 4.46 (15.39); 187 | 4.27 (13.91); 195 |
| Median (25th, 75th percentile) | 0 (0, 0) | 0 (0, 0) |
| EORTC-QLQ-NMIBC-24 | ||
| Functioning scalesa | ||
| Sexual function | ||
| Mean (SD); n | 18.27 (23.37); 166 | 19.32 (24.22); 176 |
| Median (25th, 75th percentile) | 8.33 (0, 33.33) | 0 (0, 33.33) |
| Sexual enjoyment | ||
| Mean (SD); n | 57.78 (36.51); 45 | 54.17 (35.14); 56 |
| Median (25th, 75th percentile) | 66.67 (33.33, 100) | 66.67 (33.33, 66.67) |
| Symptom scales and/or itemsb | ||
| Urinary symptoms | ||
| Mean (SD); n | 26.26 (21.14); 188 | 22.34 (19.46); 195 |
| Median (25th, 75th percentile) | 19.05 (9.52, 38.10) | 19.05 (4.76, 33.33) |
| Malaise | ||
| Mean (SD); n | 4.94 (12.12); 189 | 4.00 (10.39); 196 |
| Median (25th, 75th percentile) | 0 (0, 0) | 0 (0, 0) |
| Future worries | ||
| Mean (SD); n | 33.50 (25.69); 189 | 32.81 (22.18); 196 |
| Median (25th, 75th percentile) | 33.33 (16.67, 41.67) | 33.33 (16.67, 50) |
| Bloating and flatulence | ||
| Mean (SD); n | 18.78 (21.98); 189 | 18.96 (22.56); 196 |
| Median (25th, 75th percentile) | 16.67 (0, 33.33) | 16.67 (0, 33.33) |
| Sexual problems (men) | ||
| Mean (SD); n | 34.28 (38.07); 123 | 29.89 (35.42); 126 |
| Median (25th, 75th percentile) | 16.67 (0, 66.67) | 16.67 (0, 50) |
| Intravesical treatment issues | ||
| Mean (SD); n | 7.57 (16.75); 185 | 5.53 (14.57); 193 |
| Median (25th, 75th percentile) | 0 (0, 0) | 0 (0, 0) |
| Sexual intimacy | ||
| Mean (SD); n | 15.91 (24.37); 44 | 9.84 (21.38); 61 |
| Median (25th, 75th percentile) | 0 (0, 33.33) | 0 (0, 0) |
| Risk of contaminating partner | ||
| Mean (SD); n | 7.94 (21.85); 42 | 8.93 (19.58); 56 |
| Median (25th, 75th percentile) | 0 (0, 0) | 0 (0, 0) |
| Sexual problems (female) | ||
| Mean (SD); n | 33.33 (27.22); 7 | 33.33 (47.14); 8 |
| Median (25th, 75th percentile) | 33.33 (0, 66.67) | 0 (0, 83.33) |
Chapter 4 Clinical results
Treatment received
Overall, 207 participants (99.0%) in the PDD group and 215 (99.1%) in the WL group underwent surgery. The reasons for not receiving surgery are shown in Table 7. Of the participants who underwent surgery, 194 (93.7%) participants in the PDD group received the allocated treatment. In the WL group, all participants who underwent surgery received the allocated treatment. Those who did not receive their allocated treatment in the PDD group received WL treatment. The reasons for PDD not being administered are listed in Table 7. The main reason for not receiving the allocated treatment in the PDD group was communication errors at the site (n = 9). A total of 68 participants in each group underwent a second resection (PDD: 32.5%; WL: 31.3%). In those whose risk group was known, 57 (31.0%) and 58 (30.5%) intermediate-risk group participants in the PDD and WL groups, respectively, underwent a second resection. In the known high-risk group, eight (47.1%) participants in the PDD group and six (40%) participants in the WL group underwent a second resection.
| Surgery/treatment details | Treatment group | |
|---|---|---|
| PDD (N = 209) | WL (N = 217) | |
| Received surgery, n (%) | 207 (99.0) | 215 (99.1) |
| Did not receive surgery, n (%) | 2 (1.0) | 2 (0.9) |
| Reasons for not receiving surgery (n) | ||
| Withdrew before TURBT | 1 | 1 |
| Did not attend any appointment | – | 1 |
| Died before intervention | 1 | – |
| Received surgery (n) | 207 | 215 |
| Received allocated treatment, n (%) | 194 (93.7) | 215 (100.0) |
| Did not receive allocated treatment,a n (%) | 13 (6.3) | – |
| Reasons for not receiving allocated treatment | ||
| Communication errors at site (n) | 9 | – |
| Patient was admitted as an emergency admission (n) | 1 | – |
| Patient could not retain the Hexvix in the bladder (n) | 1 | – |
| Long waiting time for PDD (n) | 1 | – |
| No reason given (n) | 1 | – |
| Second resection, n (%) | 68 (32.5) | 68 (31.3) |
Primary outcome: recurrence of bladder cancer
The median follow-up time was 21 months for the PDD group and 22 months for the WL group. Overall, there were 86 recurrences of bladder cancer in the PDD group and 84 recurrences in the WL group (Table 8). Kaplan–Meier survival curves summarise the raw survival data (Figure 5). Visually, the Cox proportional hazards assumption appeared to be violated, and this was confirmed by plotting [−log (survival)] against log (analysis time). Table 9 shows the results for the analyses of the primary outcome. The ITT analysis of the primary outcome estimated an HR of 0.94 (95% CI 0.69 to 1.28; p = 0.704). The prespecified important difference (HR 0.64) was incompatible with the data. Relaxing the proportional hazards assumption using an accelerated failure time model based on log-normal distribution showed no evidence that the time ratio (TR) for trial participants differed between groups (TR 1.12, 95% CI 0.78 to 1.60; p = 0.550). The 3-year recurrence-free survival rate was 57.8% (95% CI 50.7% to 64.2%) in the PDD group and 61.6% (95% CI 54.7% to 67.8%) in the WL group, with an absolute difference of –3.8% (95% CI –5.59% to 13.37%). The prognostic factors that were included in the analysis are reported in Table 10.
| First event | Treatment group | Total (N = 426) | |
|---|---|---|---|
| PDD (N = 209) | WL (N = 217) | ||
| Any recurrence eventa | 86 | 84 | 170 |
| Recurrences | 82 | 81 | 163 |
| Progression | 3 | 2 | 5 |
| Died from bladder cancer | 1 | 1 | 2 |
| Total events | |||
| Recurrences | 83 | 82 | 165 |
| Progression | 17 | 10 | 27 |
| Cystectomy | 9 | 11 | 20 |
| Died from bladder cancer | 9 | 8 | 17 |
FIGURE 5.
Kaplan–Meier survival estimates for recurrence of bladder cancer. Adapted with permission from Heer et al. 52
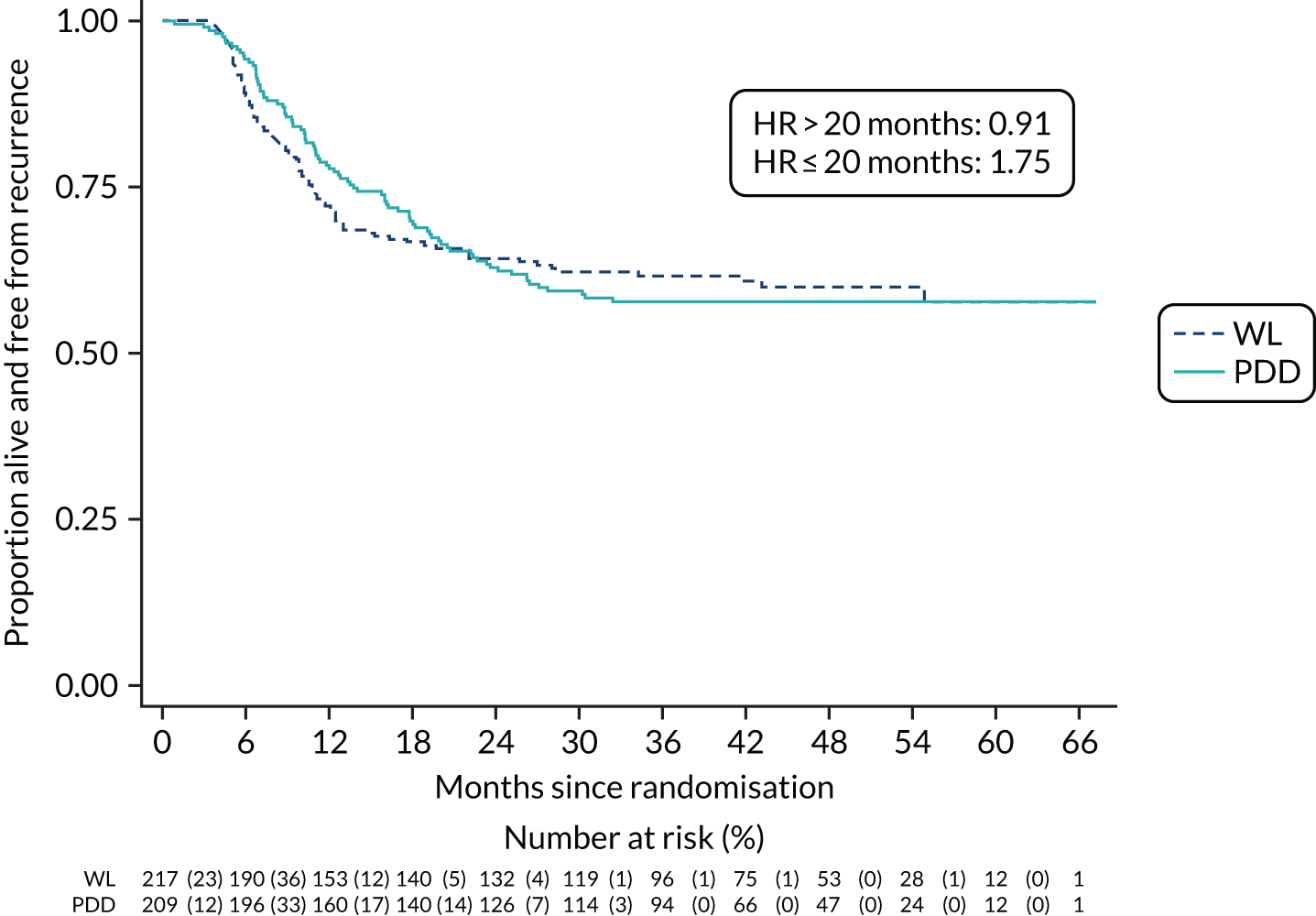
| Analysis | Percentage |
|---|---|
| 3-year recurrence rate | |
| PDD | 42.2 |
| WL | 38.4 |
| Analysis | Effect estimate (95% CI); p-value |
| ITT (HR)b | |
| Unadjusted | 1.01 (0.75 to 1.36); 0.950 |
| Adjusted for minimisation variables | 0.95 (0.70 to 1.28); 0.726 |
| Adjusted for prespecified baseline variablesa | 0.94 (0.69 to 1.28); 0.704 |
| Per protocolb | |
| Unadjusted | 1.01 (0.74 to 1.37); 0.945 |
| Adjusted for minimisation variables | 0.95 (0.70 to 1.30); 0.757 |
| Adjusted for prespecified baseline variablesa | 0.97 (0.71 to 1.32); 0.824 |
| Accelerated failure time (TR)c | |
| Adjusted for minimisation variables | 1.10 (0.76 to 1.59); 0.607 |
| Adjusted for prespecified baseline variablesa | 1.12 (0.78 to 1.60); 0.550 |
| Variablea | HR (95% CI); p-value |
|---|---|
| PDD | 0.94 (0.69 to 1.28); 0.704 |
| Sex: male | 0.98 (0.67 to 1.44); 0.932 |
| Smoking status | |
| Current smoker | Reference category |
| Previous smoker | 1.75 (1.06 to 2.91); 0.030 |
| Never | 1.23 (0.70 to 2.17); 0.465 |
| Unknown | 1.41 (0.30 to 6.65); 0.664 |
| EORTC risk group | |
| Low risk (score 0) | Reference category |
| Intermediate risk (score 1–9) | 0.72 (0.09 to 5.81); 0.755 |
| High risk (score 10–17) | 1.18 (0.14 to 10.17); 0.881 |
| Not calculable | 0.79 (0.08 to 7.93); 0.838 |
| Presence of CIS | 1.06 (0.65 to 1.75); 0.812 |
| Grade of surgeon | |
| Registrar/non-consultant career grade | Reference category |
| Consultant | 0.94 (0.67 to 1.33); 0.736 |
Sensitivity analysis
Death as a competing risk for primary outcome
Of the 57 participants who died, 23 died without having a recurrence of bladder cancer: 10 in the PDD group and 13 in the WL group. Table 11 shows the results for recurrence of bladder cancer when treating death as a competing risk. There was no evidence that the SHR for trial participants differed for either the PDD or the WL group (SHR 1.00, 95% CI 0.74 to 1.35; p = 0.987).
| Analysis | SHR (95% CI); p-value |
|---|---|
| Unadjusted | 1.02 (0.75 to 1.38); 0.901 |
| Adjusted for minimisation variables | 1.02 (0.73 to 1.42); 0.912 |
| Adjusted for prespecified baseline variablesa | 1.00 (0.74 to 1.35); 0.987 |
Secondary outcomes
Progression of bladder cancer
Overall, there were 19 bladder-cancer progressions in the PDD group and 12 progressions in the WL group. There were seven progressions to MIBC, 10 progressions to metastatic disease and two deaths due to bladder cancer in the PDD group. In the WL group, there were three progressions to MIBC, seven progressions to metastatic disease and two deaths due to bladder cancer. The survival curve was plotted as unadjusted Kaplan–Meier estimates (Figure 6). There was no difference in progression of bladder cancer between the PDD and WL group (HR 1.41, 95% CI 0.67 to 2.96; p = 0.369). Table 12 shows the results for the Cox proportional hazard analysis. The assumption of proportional hazards was violated (see Figure 7). The accelerated failure time model based on exponential distribution showed that there was no difference between the PDD and WL groups in terms of time to progression of bladder cancer (TR 0.64, 95% CI 0.30 to 1.36; p = 0.25).
FIGURE 6.
Kaplan–Meier survival estimates for progression of bladder cancer.
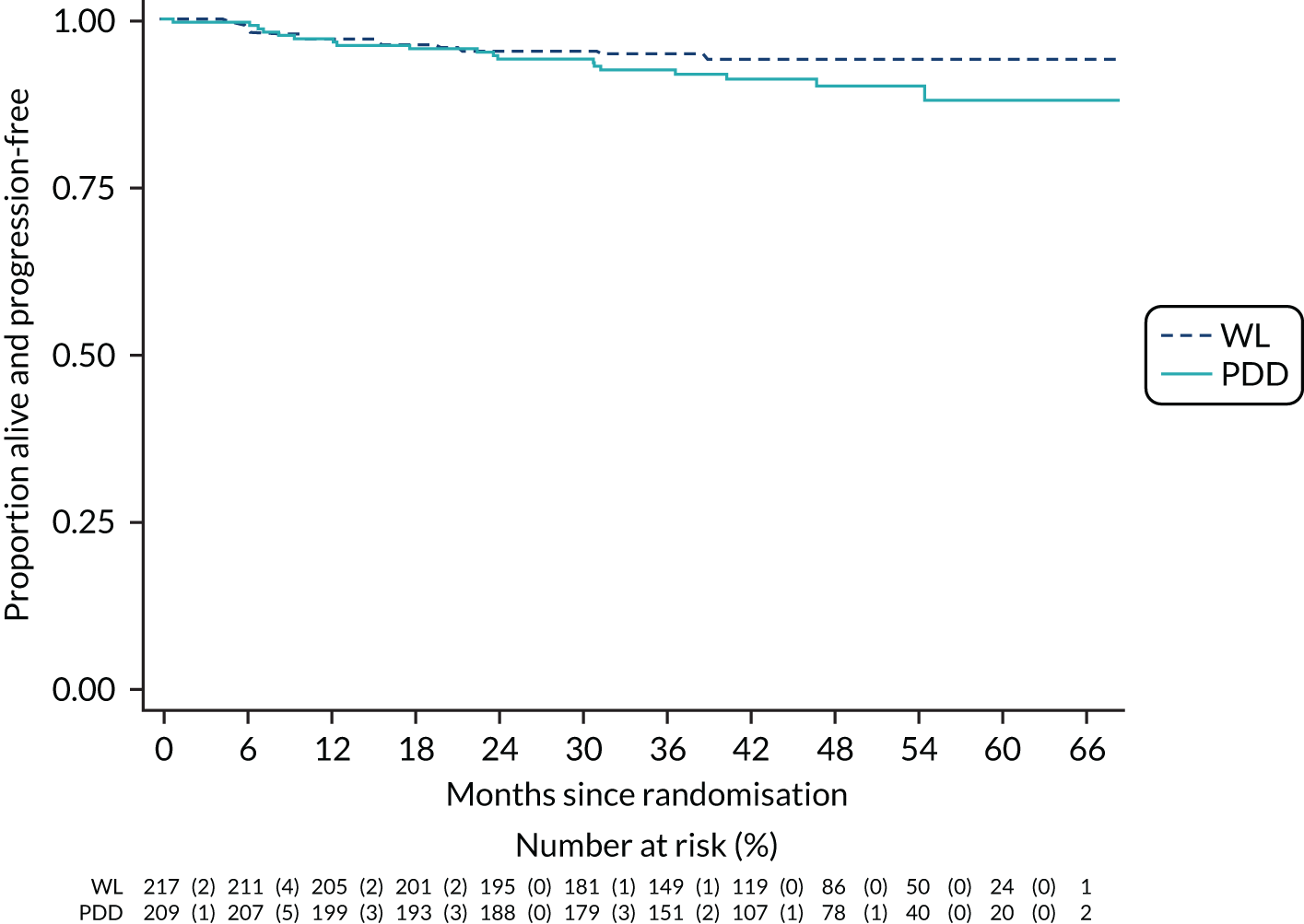
| Analysis | Effect estimate (95% CI); p-value |
|---|---|
| Cox regression model (HR)a | |
| Unadjusted | 1.64 (0.80 to 3.38); 0.180 |
| Adjusted for minimisation variables | 1.63 (0.79 to 3.37); 0.187 |
| Adjusted for prespecified baseline variablesb | 1.41 (0.67 to 2.96); 0.369 |
| Accelerated failure time (TR)c | |
| Adjusted for minimisation variables | 0.61 (0.29 to 1.25); 0.177 |
| Adjusted for prespecified baseline variablesd | 0.69 (0.33 to 1.46); 0.334 |
Bladder-cancer-specific survival
Overall, there were 17 deaths from bladder cancer. Of these, nine deaths were in the PDD group and eight were in the WL group. Table 13 shows the results for the bladder-cancer-specific survival using the Fine and Grey model. There was no evidence that bladder-cancer-specific survival differed between the PDD and WL groups (SHR 0.92, 95% CI 0.40 to 2.14; p = 0.852).
| Analysis | SHR (95% CI); p-value |
|---|---|
| Unadjusted | 1.14 (0.44 to 2.91); 0.790 |
| Adjusted for minimisation variables | 1.13 (0.46 to 2.78); 0.782 |
| Adjusted for prespecified baseline variablesa | 0.92 (0.40 to 2.14); 0.852 |
Overall survival
There was a total of 57 deaths, 27 in the PDD group and 30 in the WL group. Of the 57 participants who died, 17 (29.8%) died from bladder cancer, nine (15.8%) from cardiovascular events, nine (15.8%) from other cancers and 22 (38.6%) from other causes. Figure 7 shows the Kaplan–Meier survival estimates for overall survival. There was no difference in overall survival between the PDD and WL groups (HR 0.83, 95% CI 0.49 to 1.41; p = 0.496) (Table 14). The assumption of proportional hazards was violated. The accelerated failure time model based on log-normal distribution showed no evidence that the TR for trial participants differed for either the PDD or WL group (TR 1.19, 95% CI 0.70 to 2.02; p = 0.512) (see Table 13).
FIGURE 7.
Kaplan–Meier survival estimates for overall survival.
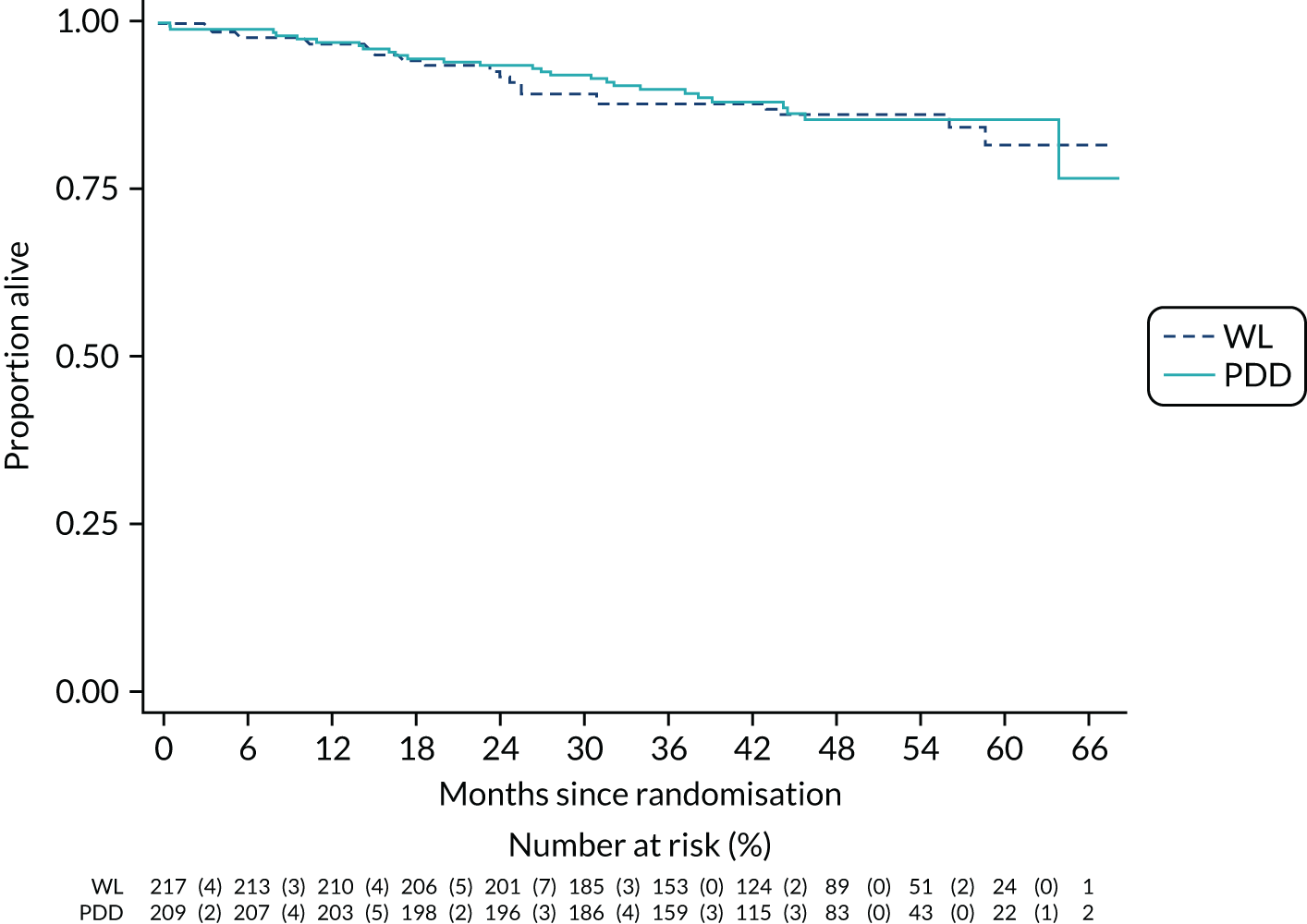
| Analysis | Effect estimate (95% CI); p-value |
|---|---|
| Cox regression model (HR)a | |
| Unadjusted | 0.91 (0.54 to 1.54); 0.734 |
| Adjusted for minimisation variables | 0.91 (0.54 to 1.53); 0.723 |
| Adjusted for prespecified baseline variablesb | 0.83 (0.49 to 1.41); 0.496 |
| Accelerated failure time (TR)c | |
| Adjusted for minimisation variables | 1.09 (0.61 to 1.93); 0.770 |
| Adjusted for prespecified baseline variablesb | 1.19 (0.70 to 2.02); 0.512 |
Health-related quality of life
Tables 15 and 16 report selected time points for each quality-of-life outcome; the full tables are reported with all data at all time points in Appendix 1, Tables 33 and 34.
| Outcome measure | Treatment group, mean (SD); p-value | Estimate (99% CI); p-value | |
|---|---|---|---|
| PDD (N = 209) | WL (N = 217) | ||
| EQ-5D-3L | |||
| Baseline | 0.834 (0.205); 187 | 0.838 (0.223); 188 | |
| Post treatment | 0.706 (0.265); 170 | 0.717 (0.279); 174 | –0.000 (–0.058 to 0.058); 0.995 |
| 36 months | 0.797 (0.251); 95 | 0.825 (0.238); 94 | –0.013 (–0.086 to 0.061); 0.660 |
| EORTC-QLQ-C30 | |||
| Functioning scalesa | |||
| Physical | |||
| Baseline | 83.6 (20.3); 189 | 85.8 (17.7); 195 | |
| Post treatment | 76.0 (24.5); 167 | 78.6 (23.2); 177 | 0.3 (–3.7 to 4.4); 0.829 |
| 36 months | 80.6 (22.6); 100 | 81.8 (21.4); 96 | 0.5 (–4.6 to 5.5); 0.813 |
| Role | |||
| Baseline | 85.7 (24.8); 188 | 87.7 (22.0); 195 | |
| Post treatment | 75.0 (31.3); 171 | 74.5 (32.4); 178 | 2.5 (–4.0 to 9.1); 0.320 |
| 36 months | 78.7 (30.3); 100 | 84.0 (27.4); 96 | –2.7 (–10.9 to 5.6); 0.404 |
| Cognitive | |||
| Baseline | 85.7 (18.3); 188 | 87.5 (18.1); 195 | |
| Post treatment | 82.2 (20.3); 173 | 84.4 (20.3); 181 | –1.6 (–6.1 to 2.8); 0.343 |
| 36 months | 80.2 (19.8); 100 | 83.7 (20.4); 96 | –1.0 (–6.5 to 4.5); 0.630 |
| Emotional | |||
| Baseline | 80.4 (20.8); 186 | 81.5 (19.2); 192 | |
| Post treatment | 80.0 (20.5); 172 | 77.5 (22.9); 180 | 3.3 (–1.2 to 7.9); 0.061 |
| 36 months | 81.2 (21.9); 100 | 83.0 (22.4); 96 | –0.4 (–6.0 to 5.3); 0.872 |
| Social | |||
| Baseline | 87.0 (22.0); 186 | 88.6 (21.2); 193 | |
| Post treatment | 78.4 (25.0); 172 | 77.3 (28.1); 179 | 3.0 (–3.1 to 9.2); 0.198 |
| 36 months | 83.0 (25.3); 100 | 86.6 (22.9); 96 | –2.4 (–10.0 to 5.3); 0.423 |
| Global QoL | |||
| Baseline | 73.7 (19.0); 186 | 73.8 (20.4); 193 | |
| Post treatment | 68.9 (21.3); 172 | 67.9 (21.1); 180 | 1.8 (–2.5 to 6.1); 0.276 |
| 36 months | 73.4 (19.3); 100 | 76.2 (19.2); 96 | –2.3 (–7.6 to 3.0); 0.265 |
| Symptom scales and/or itemsb | |||
| Fatigue | |||
| Baseline | 21.7 (22.9); 187 | 19.4 (20.3); 195 | |
| Post treatment | 28.7 (25.0); 172 | 27.3 (24.9); 180 | –1.8 (–7.0 to 3.3); 0.361 |
| 36 months | 25.3 (22.7); 100 | 24.2 (21.3); 96 | –0.5 (–7.0 to 5.9); 0.827 |
| Nausea and vomiting | |||
| Baseline | 3.9 (12.0); 187 | 3.2 (9.3); 195 | |
| Post treatment | 5.0 (13.0); 172 | 5.2 (12.9); 180 | –0.8 (–4.0 to 2.3); 0.494 |
| 36 months | 6.0 (15.6); 100 | 3.3 (10.2); 96 | 0.1 (–4.0 to 4.1); 0.962 |
| Pain | |||
| Baseline | 18.7 (25.2); 189 | 17.4 (25.2); 195 | |
| Post treatment | 26.4 (29.7); 172 | 23.2 (27.1); 180 | 1.4 (–4.4 to 7.3); 0.523 |
| 36 months | 23.5 (27.0); 100 | 14.1 (23.7); 96 | 6.1 (–1.2 to 13.5); 0.031 |
| Dyspnoea | |||
| Baseline | 14.3 (22.9); 187 | 14.0 (21.6); 195 | |
| Post treatment | 14.3 (24.5); 170 | 12.8 (22.5); 177 | 0.9 (–4.7 to 6.5); 0.667 |
| 36 months | 17.2 (26.7); 99 | 17.7 (24.6); 96 | –2.7 (–9.6 to 4.2); 0.307 |
| Sleep disturbance | |||
| Baseline | 22.0 (29.5); 188 | 23.1 (27.2); 195 | |
| Post treatment | 28.3 (30.7); 171 | 28.9 (29.9); 181 | 1.5 (–5.6 to 8.6); 0.592 |
| 36 months | 29.0 (30.6); 100 | 25.3 (29.9); 95 | 3.7 (–5.3 to 12.7); 0.292 |
| Appetite loss | |||
| Baseline | 12.1 (23.6); 187 | 8.7 (20.0); 195 | |
| Post treatment | 15.7 (24.3); 172 | 12.0 (20.7); 181 | 1.6 (–3.7 to 7.0); 0.425 |
| 36 months | 11.0 (20.7); 100 | 9.4 (19.2); 96 | –1.1 (–7.9 to 5.7); 0.682 |
| Constipation | |||
| Baseline | 12.7 (23.4); 187 | 8.7 (19.4); 195 | |
| Post treatment | 18.1 (27.3); 171 | 18.2 (26.2); 179 | –2.6 (–8.6 to 3.5); 0.275 |
| 36 months | 13.8 (23.3); 99 | 7.4 (17.0); 95 | 3.0 (–4.7 to 10.7); 0.315 |
| Diarrhoea | |||
| Baseline | 7.1 (18.6); 184 | 5.2 (14.7); 194 | |
| Post treatment | 5.6 (15.7); 173 | 5.4 (15.4); 180 | –2.0 (–6.4 to 2.4); 0.240 |
| 36 months | 10.8 (22.8); 99 | 5.2 (12.2); 96 | 2.7 (–3.0 to 8.5); 0.218 |
| Financial difficulties | |||
| Baseline | 4.5 (15.5); 185 | 4.3 (14.0); 193 | |
| Post treatment | 6.8 (19.4); 171 | 5.8 (15.8); 178 | 0.7 (–3.7 to 5.2); 0.663 |
| 36 months | 6.7 (18.3); 100 | 4.9 (16.7); 96 | 0.7 (–4.8 to 6.2); 0.732 |
| Time point | Treatment group, mean (SD); p-value | Estimate (99% CI); p-value | |
|---|---|---|---|
| PDD (N = 209) | WL (N = 217) | ||
| Functioning scalesa | |||
| Sexual function | |||
| Baseline | 18.4 (23.5); 164 | 19.3 (24.3); 174 | |
| 36 months | 23.3 (26.5); 86 | 25.0 (24.7); 80 | 1.1 (–6.2 to 8.4); 0.704 |
| Sexual enjoyment | |||
| Baseline | 57.8 (36.5); 45 | 54.2 (35.1); 56 | |
| Post treatment | 69.3 (30.4); 38 | 56.8 (34.6); 54 | 6.9 (–10.7 to 24.6); 0.312 |
| 36 months | 52.1 (40.3); 39 | 60.8 (30.1); 40 | 10.7 (–10.1 to 31.5); 0.184 |
| Symptom scales and/or itemsb | |||
| Urinary symptoms | |||
| Baseline | 26.0 (21.1); 186 | 22.5 (19.5); 193 | |
| Post treatment | 31.7 (23.2); 169 | 30.3 (22.2); 175 | –0.7 (–5.9 to 4.5); 0.737 |
| 36 months | 23.5 (20.2); 98 | 22.4 (20.0); 95 | –1.0 (–7.4 to 5.5); 0.700 |
| Malaise | |||
| Baseline | 4.8 (12.0); 187 | 4.0 (10.4); 194 | |
| Post treatment | 7.9 (14.6); 171 | 6.9 (15.1); 180 | 0.4 (–3.1 to 3.8); 0.788 |
| 36 months | 5.5 (11.6); 100 | 3.3 (10.2); 95 | 0.5 (–4.0 to 5.0); 0.780 |
| Future worries | |||
| Baseline | 33.0 (25.4); 187 | 33.1 (22.1); 194 | |
| Post treatment | 33.9 (25.6); 172 | 36.4 (25.9); 181 | –3.8 (–9.2 to 1.7); 0.074 |
| 36 months | 24.3 (25.2); 100 | 27.5 (24.4); 95 | –3.9 (–10.7 to 2.8); 0.134 |
| Bloating and flatulence | |||
| Baseline | 18.7 (22.1); 187 | 18.8 (22.6); 194 | |
| Post treatment | 21.8 (22.9); 170 | 21.7 (23.4); 179 | 0.6 (–4.4 to 5.6); 0.758 |
| 36 months | 22.2 (23.2); 100 | 22.5 (21.6); 95 | –1.1 (–7.4 to 5.3); 0.671 |
| Sexual problems (men) | |||
| Baseline | 34.3 (38.2); 122 | 30.0 (35.7); 124 | |
| Post treatment | 36.4 (37.7); 108 | 33.2 (34.4); 110 | 2.5 (–8.4 to 13.5); 0.552 |
| 36 months | 47.6 (39.8); 63 | 36.8 (35.0); 62 | 7.4 (–6.6 to 21.4); 0.174 |
| Intravesical treatment issues | |||
| Baseline | 7.3 (16.3); 183 | 5.6 (14.6); 191 | |
| Post treatment | 9.4 (19.0); 166 | 10.5 (21.1); 175 | –1.9 (–7.0 to 3.2); 0.344 |
| 36 months | 4.7 (15.0); 100 | 6.0 (15.4); 95 | –2.8 (–9.3 to 3.6); 0.257 |
| Sexual intimacy | |||
| Baseline | 15.9 (24.4); 44 | 9.8 (21.4); 61 | |
| Post treatment | 16.7 (22.6); 40 | 18.1 (28.9); 57 | –10.4 (–25.5 to 4.8); 0.078 |
| 36 months | 17.5 (27.2); 40 | 12.2 (25.6); 41 | –2.8 (–21.5 to 16.0); 0.703 |
| Risk of contaminating partner | |||
| Baseline | 7.9 (21.9); 42 | 8.9 (19.6); 56 | |
| Post treatment | 13.5 (24.2); 37 | 14.9 (23.7); 56 | –9.1 (–24.9 to 6.7); 0.138 |
| 36 months | 10.8 (23.1); 40 | 8.9 (18.3); 41 | 1.9 (–17.8 to 21.5); 0.808 |
| Sexual problems (female) | |||
| Baseline | 33.3 (27.2); 7 | 33.3 (47.1); 8 | |
| 36 months | 33.3 (44.1); 9 | 50.0 (57.7); 4 | –1.1 (–47.8 to 45.7); 0.953 |
EQ-5D-3L
The mean baseline EQ-5D-3L score was 0.834 in the PDD group and 0.838 in the WL group. At 36 months, the mean score difference between the groups was –0.013 (99% CI –0.086 to 0.061; p = 0.660) (see Table 15).
EORTC-QLQ-C30
Scores for all of the domains of the EORTC-QLQ-C30 were similar over time between the PDD and WL groups, except for pain at 36 months (see Table 15). The mean pain score was 23.5 in the PDD group and 14.1 in the WL group (mean difference 6.1, 99% CI –1.2 to 13.5; p = 0.031); however, when multiple tests are performed, the probability of seeing a significant difference between treatment groups, and therefore a type I error, increases as the number of tests increases. Therefore, caution is required when interpreting the results.
EORTC-QLQ-NMIBC-24
At 36 months, there were no evidence of a difference between the PDD and WL groups in any domain (see Table 16).
Adjuvant therapy/intravesical treatment
Tables 17 and 18 show the adjuvant therapy received by participants post operation in those patients whose cancer recurred and in those whose cancer did not recur up to 36 months after the resection. Immediate postoperative intravesical MMC was administered to 132 (63.2%) of participants in the PDD group and 143 (65.9%) in the WL group. There was no difference between the PDD and WL groups in terms of the proportion of participants who received MMC (χ2 = 0.27; p = 0.601). When compared by risk group, MMC was administered in 252 (67.4%) participants in the intermediate-risk group and 13 (40.6%) participants in the high-risk group.
| Immediate post-operative MMC | Risk group, n/N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Low | Intermediate | High | Not calculable | |||||
| PDD (N = 0) | WL (N = 2) | PDD (N = 184) | WL (N = 190) | PDD (N = 17) | WL (N = 15) | PDD (N = 8) | WL (N = 10) | |
| Administered | 122/184 (66.3) | 130/190 (68.4) | 7/17 (41.2) | 6/15 (40.0) | 3/8 (37.5) | 7/10 (70.0) | ||
| Not administered | 2/2 (100.0) | 57/184 (31.0) | 56/190 (29.5) | 10/17 (58.8) | 9/15 (60.0) | 3/8 (37.5) | 1/10 (10.0) | |
| Missing | 5/184 (2.7) | 4/190 (2.1) | 2/8 (25.0) | 2/10 (20.0) | ||||
| Reason for not administering MMC | ||||||||
| Deep resection | 24/57 (42.1) | 28/56 (50.0) | 5/10 (50.0) | 4/9 (44.4) | 1/3 (33.3) | 1/1 (100.0) | ||
| Perforation | 7/57 (12.3) | 3/56 (5.4) | 2/10 (20.0) | 1/9 (11.1) | ||||
| Uncontrollable bleeding | 1/57 (1.8) | 1/56 (1.8) | ||||||
| Irritation | 1/2 (50.0) | 1/56 (1.8) | ||||||
| Physician’s choice | 1/2 (50.0) | 14/57 (24.6) | 15/56 (26.8) | 3/10 (30.0) | 3/9 (33.3) | 1/3 (33.3) | ||
| Other | 7/57 (12.3) | 6/56 (10.7) | 1/9 (11.1) | |||||
| Missing | 4/57 (7.0) | 2/56 (3.6) | 1/3 (33.3) | |||||
| Timing for MMC | ||||||||
| < 6 hours after TURBT | 86/122 (70.5) | 86/130 (66.2) | 5/7 (71.4) | 4/6 (66.7) | 2/3 (66.7) | 7/7 (100.0) | ||
| 6–24 hours after TURBT | 27/122 (22.1) | 33/130 (25.4) | 1/7 (14.3) | 1/6 (16.7) | ||||
| > 24 hours after TURBT | 5/122 (4.1) | 2/130 (1.5) | 1/7 (14.3) | 1/3 (33.3) | ||||
| Missing | 4/122 (3.3) | 9/130 (6.9) | 1/6 (16.7) | |||||
| Adjuvant therapy | Risk group, n/N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Low | Intermediate | High | Not calculable | |||||
| PDD (N = 0) | WL (N = 2) | PDD (N = 184) | WL (N = 190) | PDD (N = 17) | WL (N = 15) | PDD (N = 8) | WL (N = 10) | |
| Adjuvant intravesical treatment for those whose cancer recurred | ||||||||
| BCG induction | 12/74 (16.2) | 11/74 (14.9) | 4/9 (44.4) | 2/7 (28.6) | 1/3 (33.3) | |||
| BCG induction and maintenance | 11/74 (14.9) | 5/74 (6.8) | 2/9 (22.2) | |||||
| MMC weekly (for 6 weeks) | 10/74 (13.5) | 6/74 (8.1) | 2/7 (28.6) | |||||
| None | 1/1 (100.0) | 33/74 (44.6) | 45/74 (60.8) | 2/9 (22.2) | 2/7 (28.6) | 1/2 (50.0) | ||
| Other | 4/74 (5.4) | 3/74 (4.1) | 1/9 (11.1) | 1/7 (14.3) | 1/3 (33.3) | 1/2 (50.0) | ||
| Missing | 4/74 (5.4) | 4/74 (5.4) | 1/3 (33.3) | |||||
| Duration of BCG maintenance (months) | ||||||||
| 12 | 1/23 (4.3) | 1/16 (6.3) | ||||||
| 36 | 1/16 (6.3) | |||||||
| Adjuvant intravesical treatment for those whose cancer did not recur up to 36 months after the operation | ||||||||
| BCG induction | 1/60 (1.7) | 3/67 (4.5) | 1/5 (20.0) | |||||
| BCG induction and maintenance | 16/60 (26.7) | 27/67 (40.3) | 3/4 (75.0) | 2/5 (40.0) | 1/2 (50.0) | 1/2 (50.0) | ||
| MMC weekly (for 6 weeks) | 14/60 (23.3) | 11/67 (16.4) | 2/5 (40.0) | 1/2 (50.0) | ||||
| None | 23/60 (38.3) | 15/67 (22.4) | ||||||
| Other | 5/60 (8.3) | 11/67 (16.4) | 1/4 (25.0) | 1/2 (50.0) | ||||
| Missing | 1/60 (1.7) | |||||||
| Duration of BCG maintenance (months) | ||||||||
| 12 | 1/17 (5.9) | |||||||
| 36 | 2/17 (11.8) | 3/30 (10.0) | ||||||
In those whose cancer recurred, 17 (19.8%) participants in the PDD group and 13 (15.5%) in the WL group received BCG induction, and 13 (15.1%) participants in the PDD group and 5 (6.0%) participants in the WL group received BCG induction and maintenance. There were no differences between the PDD and WL groups in terms of the proportion of participants who received BCG induction (χ2 = 0.54; p = 0.463) and proportion of participants who had BCG induction and maintenance (χ2 = 3.77; p = 0.052). When compared by risk group, 39 (26.4%) participants in the intermediate-risk group and eight (50.0%) participants in the high-risk group received either BCG induction alone or BCG induction and maintenance.
In those whose cancer did not recur up to 36 months after the initial/second TURBT, one (1.5%) participant in the PDD group and four (5.4%) in the WL group received BCG induction, and 20 (30.3%) participants in the PDD group and 30 (40.5%) participants in the WL group received BCG induction and maintenance. There were no differences between the PDD and WL groups in terms of the proportion of participants who received BCG induction (χ2 = 1.53; p = 0.216), and the proportion of participants who received BCG induction and maintenance (χ2 = 1.59; p = 0.207). When compared by risk group, 47 (37.0%) participants in the intermediate-risk group and six (66.6%) participants in the high-risk group had either BCG induction alone or BCG induction and maintenance.
Surgical learning curve
Subgroup analysis for recurrence and second resection
Of the 23 sites that participated, four (17.4%) were classified as PDD naive. Figure 8 shows the prespecified subgroup analyses comparing the outcomes from PDD-experienced and PDD-naive centres. Overall, there was no evidence that the treatment effect was moderated by PDD-naive/PDD-experienced centres. For recurrence, the HR for PDD-experienced centres was 0.91 (99% CI 0.63 to 1.31) and for PDD-naive centres it was 1.19 (99% CI 0.45 to 3.12) (interaction effect, p = 0.504). For second resection, the odds ratio for PDD-experienced centres was 0.73 (99% CI 0.37 to 1.44) and for PDD-naive centres it was 1.42 (99% CI 0.49 to 4.11) (interaction effect, p = 0.175).
FIGURE 8.
Subgroup analyses of (a) recurrence for PDD vs. WL (experienced centres vs. naive centres); and (b) second resection for PDD vs. WL (experienced centres vs. naive centres).
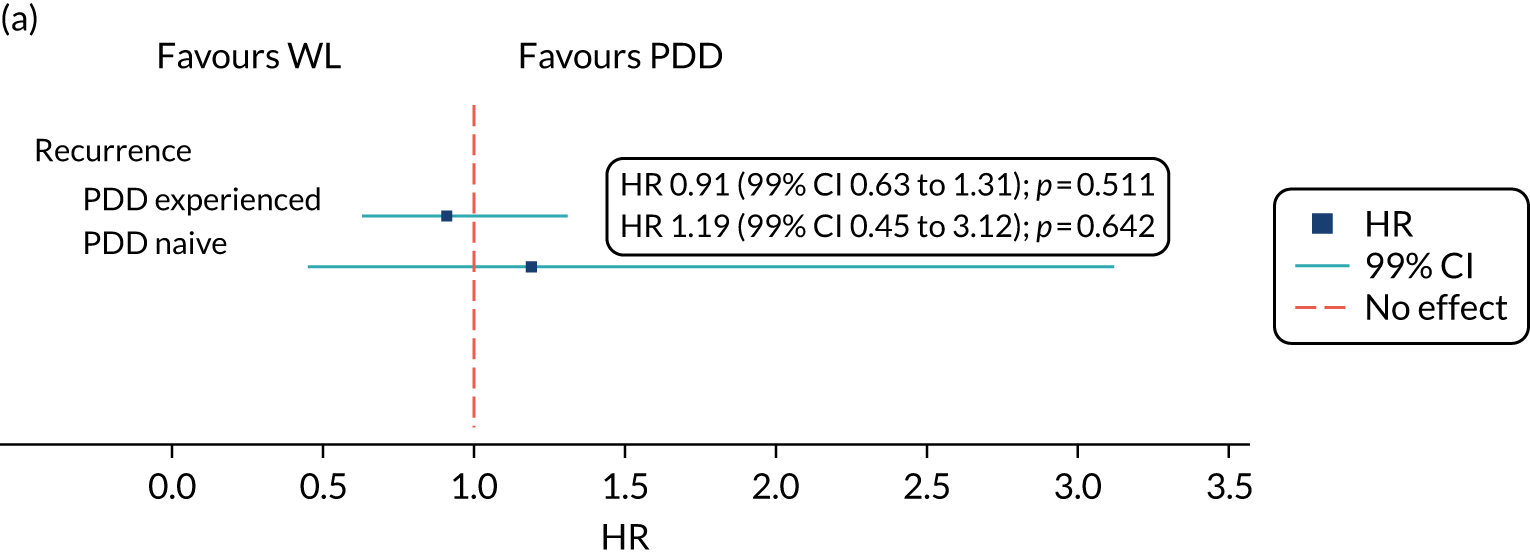
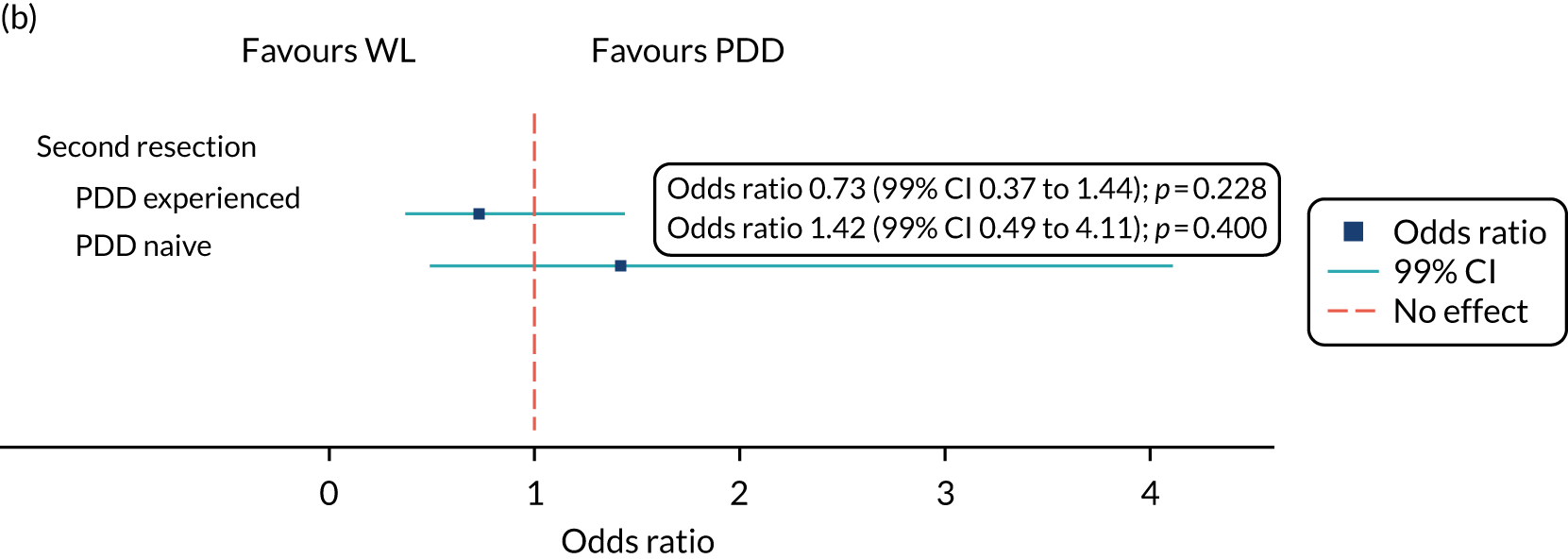
Bystander effect
Table 19 shows the percentage of recurrences by treatment group and surgeon experience (i.e. number of PDDs performed prior to study start). In both groups, > 50% of the participants whose surgery was performed by surgeons with experience of < 10 PDD procedures had recurrence of their bladder cancer.
| Treatment group | Surgeon experience (number of PDD procedures), n (%) | ||||
|---|---|---|---|---|---|
| < 10 | 10–19 | 20–40 | > 40 | Missing | |
| WL | |||||
| Sample size | 50 | 46 | 11 | 87 | 23 |
| No recurrence | 24 (48.0) | 30 (65.2) | 7 (63.6) | 55 (63.2) | 17 (73.9) |
| Recurrence | 26 (52.0) | 16 (34.8) | 4 (36.4) | 32 (36.8) | 6 (26.1) |
| PDD | |||||
| Sample size | 43 | 46 | 30 | 81 | 9 |
| No recurrence | 19 (44.2) | 26 (56.5) | 18 (60.0) | 54 (66.7) | 6 (66.7) |
| Recurrence | 24 (55.8) | 20 (43.5) | 12 (40.0) | 27 (33.3) | 3 (33.3) |
Figure 9 shows the unadjusted Kaplan–Meier survival estimates by surgeon experience. Participants whose surgeries were performed by surgeons with experience of > 40 PDD procedures had a lower risk of recurrence than participants whose surgeries were performed by surgeons with experience of < 10 PDD procedures (HR 0.60, 95% CI 0.40 to 0.92; p = 0.019).
FIGURE 9.
Kaplan–Meier survival estimates for recurrence by surgeon experience.
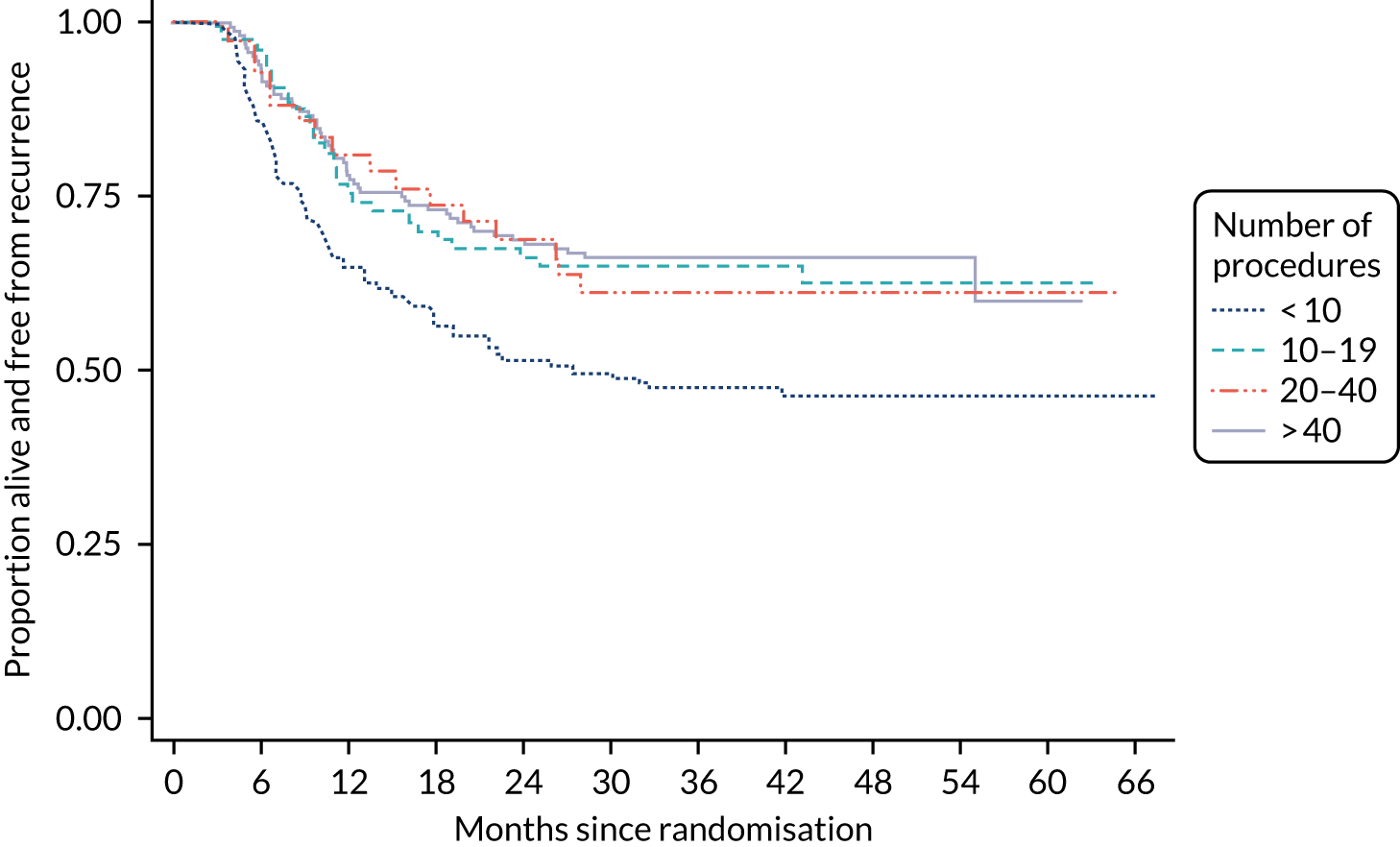
Table 20 shows the results for the Cox proportional hazards analysis. The accelerated failure time model based on log-logistics distribution showed similar findings (TR 1.94, 95% CI 1.08 to 3.46; p = 0.026) (see Table 19).
| Analysis | Percentage |
|---|---|
| 3-year recurrence rate | |
| PDD | 42.2 |
| WL | 38.4 |
| Analysisa | Effect estimate (95% CI); p-value |
| Cox regression model (HR)b | |
| Surgeon PDD experience (number of procedures) | |
| > 10 | Reference category |
| 10–19 | 0.80 (0.52 to 1.23); 0.300 |
| 20–40 | 0.58 (0.31 to 1.09); 0.088 |
| > 40 | 0.60 (0.40 to 0.92); 0.019 |
| Accelerated failure time model (TR)c | |
| Surgeon PDD experience (number of procedures) | |
| > 10 | Reference category |
| 10–19 | 1.35 (0.76 to 2.39); 0.309 |
| 20–40 | 2.22 (0.95 to 5.21); 0.066 |
| > 40 | 1.94 (1.08 to 3.46); 0.026 |
Figure 10 shows the forest plot for the subgroup analysis by surgeon PDD experience. There was no evidence that the treatment effect was moderated by surgeon PDD experience.
FIGURE 10.
Subgroup analysis for recurrence of bladder cancer by surgeon PDD experience.
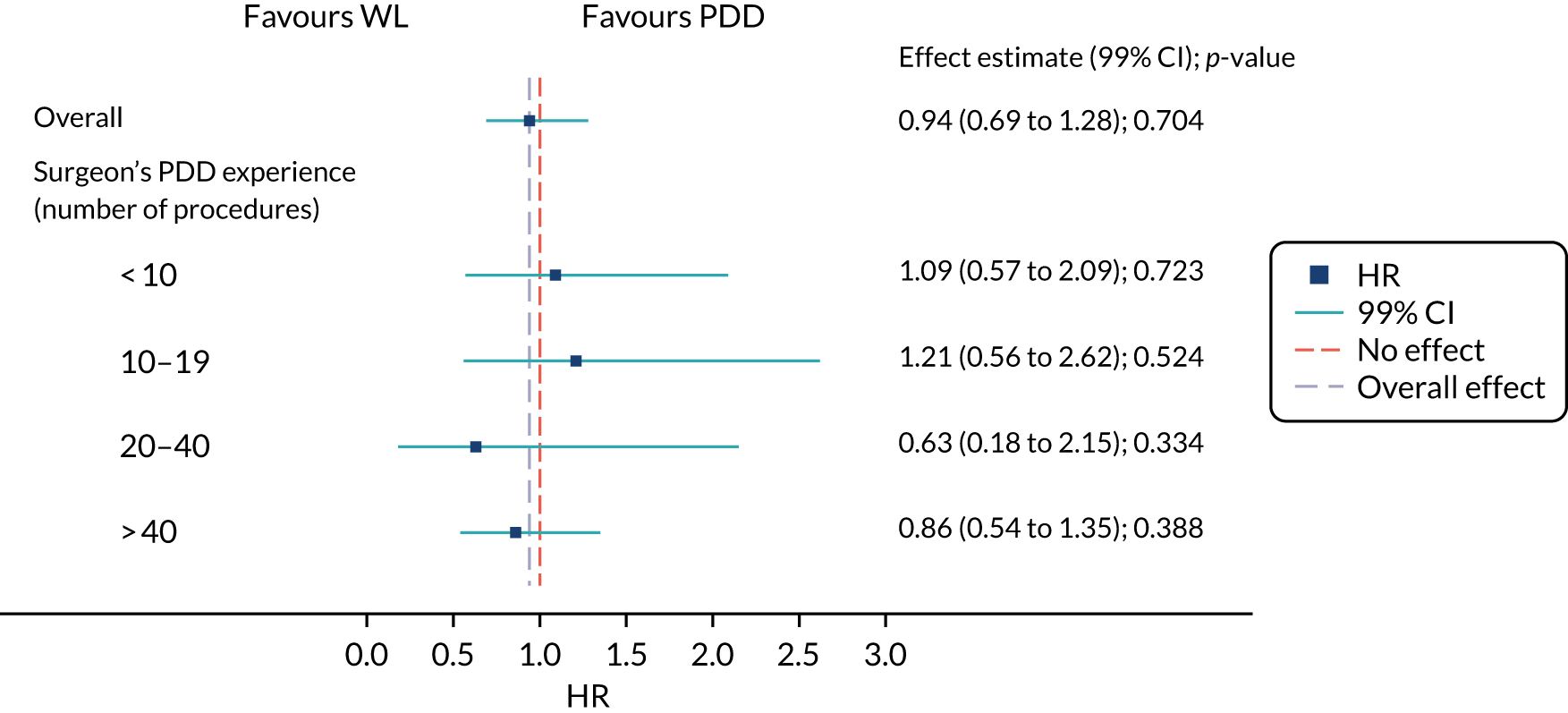
Post hoc subgroup analysis
There was no evidence that the treatment effect was moderated by EAU/EORTC risk group (Figure 11).
FIGURE 11.
Subgroup analysis for recurrence of bladder cancer by risk group at baseline.
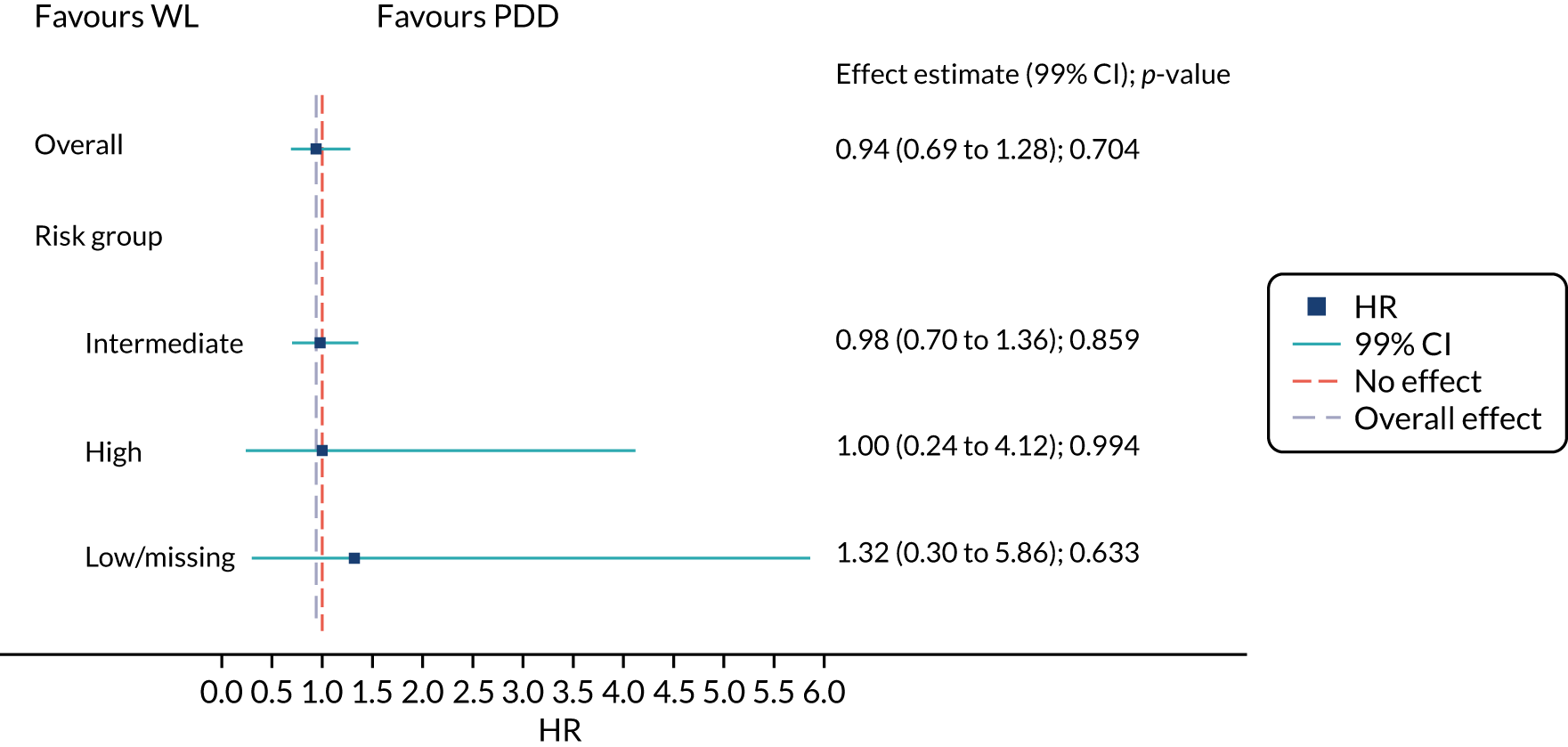
Clavien–Dindo grade, serious adverse events and adverse events (Common Terminology Criteria for Adverse Events grade 3 or above)
The number of AEs by Clavien–Dindo grade, the number of SAEs and the number of CTCAE grade three or above events are reported in the sensitivity analysis.
There were 26 SAEs reported throughout the study (> 1 SAE could be reported per participant). There was no significant difference in the number of SAEs reported between the groups (Table 21). In total, eight participants experienced AEs (CTCAE grade 3 and above) and there was no significant difference in the number of participants who experienced an AE between the groups [rate ratio (RR) 0.62, 95% CI 0.24 to 1.60; p = 0.33]. The expected AEs following TURBT are reported in Appendix 1, Table 37.
| Post-operative event | Treatment group | |
|---|---|---|
| PDD (N = 209) | WL (N = 217) | |
| Clavien–Dindo gradea | ||
| Grade (n) | ||
| I | 28 | 31 |
| II | 16 | 20 |
| IIIa | 2 | 3 |
| IIIb | 2 | 0 |
| IVa | 0 | 0 |
| IVb | 0 | 0 |
| V | 0 | 0 |
| Participants, n (%) | 34 (16.3) | 31 (14.3) |
| SAE | ||
| Participants, n (%) | 12 (5.7) | 12 (5.5) |
| Events (n) | 13 | 13 |
| Event related to TURBT (n) | 13 | 13 |
| Expected events (n) | 13 | 13 |
| Type of SAE | ||
| Prolongation of existing hospitalisation | 6 | 2 |
| Requires rehospitalisation after medical discharge | 6 | 11 |
| Considered medically significant by the investigator | 1 | – |
| AEs (CTCAE grade 3 or above) | ||
| Participants who experienced AEs (CTCAE grade 3 and above), n (%) | 3 (1.4) | 5 (2.3) |
Chapter 5 Cost-effectiveness analysis results
Estimation of NHS costs
Initial procedure
The resource use for each trial group is reported in Table 22. The total costs to the NHS, based on the microcosting approach described in Estimation of NHS costs using resource use data collected within the trial (see Table 22), are presented in Table 23. The sample size varies according to the reported data. The economic evaluation evaluates the cost-effectiveness of using PDD in the treatment of people with suspected intermediate-risk and high-risk bladder cancer. Consequently, the sample size is based on all patients randomised to either WL or PDD, whereas the statistical analysis was based on the patients with NMIBC only. There was no evidence of differences between the groups in terms of staff time and length-of-stay costs. We also conducted a sensitivity analysis that showed that a reduced unit cost (£468 instead of £891) for one night in hospital would not lead to a subsequent difference in cost (£193.62, 95% CI –£142.96 to £530.20). The additional equipment cost for PDD-TURBT is the cost of the photosensitiser (Hexvix), which results in the differences in total intervention costs between groups (£665, 95% CI £28 to £1303).
| Resource use | Treatment group | |||||
|---|---|---|---|---|---|---|
| PDD | WL | |||||
| n | Mean/percentage | SD | n | Mean/percentage | SD | |
| Intervention | ||||||
| First TURBT | ||||||
| Length of operation (minutes) | 244 | 59.60 | 23.30 | 249 | 56.04 | 39.80 |
| Grade of operating surgeon | ||||||
| Registrar | 53 | 21.0% | 71 | 28.5% | ||
| Consultant | 188 | 74.6% | 173 | 69.5% | ||
| Non-consultant | 3 | 1.2% | 5 | 2.0% | ||
| Drugs in theatre: Hexvix | 227 | 93.0% | 1 | 0.4% | ||
| Length of stay (days) | 244 | 3.27 | 4.70 | 249 | 2.86 | 3.32 |
| Post-operative instillation of MMC | 141 | 57.8% | 153 | 61.4% | ||
| Second TURBT | ||||||
| Length of operation (minutes) | 78 | 51.85 | 28.17 | 79 | 47.24 | 27.94 |
| Grade of operating surgeon | ||||||
| Registrar | 7 | 8.6% | 11 | 13.9% | ||
| Consultant | 69 | 85.2% | 67 | 84.8% | ||
| Non-consultant | 2 | 2.5% | 1 | 1.3% | ||
| Follow-up management (1 year) | ||||||
| Secondary care | ||||||
| Inpatient stay (days) | 244 | 2.65 | 5.58 | 249 | 2.77 | 5.15 |
| Cystectomy | 20 | 8.2% | 22 | 8.8% | ||
| Resection surgery | 44 | 18.0% | 61 | 24.5% | ||
| Length of operation (minutes) | 244 | 7.05 | 16.20 | 249 | 10.23 | 19.73 |
| Length of stay (days) | 244 | 0.19 | 0.41 | 249 | 0.25 | 0.45 |
| Cystoscopy | ||||||
| WLC | 244 | 1.30 | 1.00 | 249 | 1.27 | 0.96 |
| PDD | 244 | 0.08 | 0.30 | 249 | 0.02 | 0.18 |
| Narrow-band imaging | 244 | 0 | 0 | 249 | 0 | 0 |
| Other | 244 | 0.08 | 0.40 | 249 | 0.08 | 0.36 |
| Hospital doctor consultation | ||||||
| Telephone | 244 | 0.08 | 0.43 | 249 | 0.08 | 0.44 |
| Out of hours | 244 | 0.03 | 0.20 | 249 | 0.07 | 0.37 |
| Outpatient consultations (face to face) | 244 | 7.29 | 7.28 | 249 | 7.45 | 7.67 |
| A&E consultations (face to face) | 244 | 0.34 | 1.82 | 249 | 0.24 | 0.75 |
| Primary care | ||||||
| Face to face | ||||||
| GP consultations | 244 | 1.02 | 1.81 | 249 | 1.00 | 1.73 |
| GP home visits | 244 | 0.10 | 0.44 | 249 | 0.10 | 0.82 |
| Nurse consultations | 244 | 0.66 | 1.71 | 249 | 0.75 | 1.94 |
| Nurse home visits | 244 | 0.94 | 3.81 | 249 | 0.82 | 3.26 |
| Telephone consultations | ||||||
| GP led | 244 | 0.27 | 0.93 | 249 | 0.35 | 1.18 |
| Nurse led | 244 | 0.66 | 1.51 | 249 | 0.67 | 1.88 |
| Other | 244 | 0.16 | 0.62 | 249 | 0.26 | 1.30 |
| Out-of-hours consultations | ||||||
| GP | 244 | 0.06 | 0.42 | 249 | 0.04 | 0.30 |
| Nurse | 244 | 0.08 | 0.56 | 249 | 0.05 | 0.42 |
| Other | 244 | 0.03 | 0.23 | 249 | 0.02 | 0.15 |
| Follow-up management (2–3 years) | ||||||
| Secondary care | ||||||
| Inpatient stay (days) | 244 | 1.44 | 7.92 | 249 | 0.65 | 3.49 |
| Cystectomy | 6 | 2.5% | 7 | 2.8% | ||
| Resection surgery | 37 | 15.2% | 18 | 7.2% | ||
| Length of operation (minutes) | 244 | 7.19 | 18.41 | 249 | 3.12 | 12.12 |
| Length of stay (days) | 244 | 0.16 | 0.37 | 249 | 0.07 | 0.25 |
| Cystoscopy | ||||||
| WLC | 244 | 1.19 | 1.28 | 249 | 1.21 | 1.31 |
| PDD | 244 | 0.02 | 0.24 | 249 | 0.00 | 0.00 |
| Narrow-band imaging | 244 | 0.00 | 0.00 | 249 | 0.00 | 0.06 |
| Other | 244 | 0.08 | 0.40 | 249 | 0.07 | 0.37 |
| Hospital doctor consultation | ||||||
| Telephone | 244 | 0.05 | 0.34 | 249 | 0.05 | 0.30 |
| Out of hours | 244 | 0.02 | 0.16 | 249 | 0.02 | 0.17 |
| Outpatient consultations (face to face) | 244 | 4.26 | 6.81 | 249 | 4.51 | 10.60 |
| A&E consultations (face to face) | 244 | 0.14 | 1.21 | 249 | 0.06 | 0.44 |
| Primary care | ||||||
| Face to face | ||||||
| GP consultations | 244 | 0.47 | 1.39 | 249 | 0.38 | 1.14 |
| GP home visits | 244 | 0.03 | 0.33 | 249 | 0.11 | 1.47 |
| Nurse consultations | 244 | 0.26 | 1.11 | 249 | 0.42 | 1.45 |
| Nurse home visits | 244 | 0.20 | 0.94 | 249 | 0.15 | 1.07 |
| Telephone consultations | ||||||
| GP led | 244 | 0.13 | 0.71 | 249 | 0.17 | 1.59 |
| Nurse led | 244 | 0.24 | 0.90 | 249 | 0.30 | 1.04 |
| Other | 244 | 0.07 | 0.47 | 249 | 0.02 | 0.20 |
| Out-of-hours consultations | ||||||
| GP | 244 | 0.01 | 0.13 | 249 | 0.00 | 0.06 |
| Nurse | 244 | 0.01 | 0.13 | 249 | 0.02 | 0.25 |
| Other | 244 | 0.01 | 0.19 | 249 | 0.02 | 0.18 |
| Costs (£) | Treatment group | Mean difference (95% CI) | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| PDD | WL | |||||||
| n | Mean | SD | n a | Mean | SD | |||
| Total NHS costs | 244 | 12,927 | 10,994 | 249 | 11,934 | 8235 | 993 (–724 to 2709) | 0.256 |
| Intervention | ||||||||
| First TURBT | ||||||||
| Length of operation | 244 | 92.29 | 45.37 | 249 | 83.84 | 74.93 | 8.45 (–2.54 to 19.44) | 0.132 |
| Drugs in theatre: Hexvix | 244 | 322.82 | 88.53 | 249 | 1.39 | 21.99 | 321.43 (310.07 to 332.79) | < 0.001 |
| Length of stay | 244 | 2914.55 | 4189.33 | 249 | 2545.93 | 2958.97 | 368.63 (–272.17 to 1009.42) | 0.259 |
| Post-operative instillation of MMC | 244 | 520.97 | 446.18 | 249 | 553.95 | 439.68 | –32.99 (–111.38 to 45.4) | 0.409 |
| Subtotal | 244 | 3851 | 4153 | 249 | 3185 | 2964 | 666 (28 to 1303) | 0.041 |
| Second TURBT | ||||||||
| Length of operation | 78 | 88.96 | 53.32 | 79 | 77.49 | 51.42 | 11.48 (–5.04 to 27.99) | 0.172 |
| Subtotal | 78 | 89 | 53 | 79 | 77 | 51 | 11 (–5 to 28) | 0.172 |
| Total intervention costs | 244 | 3879 | 4157 | 249 | 3210 | 2967 | 669 (31 to 1308) | 0.040 |
| Follow-up management (1 year) | ||||||||
| Secondary care | ||||||||
| Inpatient stay | 244 | 2362.61 | 4970.22 | 249 | 2469.04 | 4590.87 | –106.43 (–952.86 to 740.01) | 0.805 |
| Cystectomy | 244 | 853.77 | 2863.14 | 249 | 920.29 | 2962.10 | –66.52 (–582.19 to 449.15) | 0.800 |
| Resection surgery | ||||||||
| Length of operation | 244 | 11.34 | 27.63 | 249 | 15.69 | 32.38 | –4.35 (–9.69 to 0.98) | 0.109 |
| Length of stay | 244 | 167.98 | 367.45 | 249 | 221.86 | 402.31 | –53.88 (–122.1 to 14.34) | 0.121 |
| Cystoscopy | 244 | 1558.66 | 1076.97 | 249 | 1436.79 | 1010.65 | 121.86 (–62.91 to 306.64) | 0.196 |
| Hospital doctor consultation | ||||||||
| Telephone | 244 | 1.24 | 6.45 | 249 | 1.27 | 6.59 | –0.04 (–1.19 to 1.12) | 0.951 |
| Out of hours | 244 | 4.01 | 24.50 | 249 | 8.85 | 45.78 | –4.83 (–11.35 to 1.68) | 0.146 |
| Outpatient consultations (face to face) | 244 | 787.43 | 786.35 | 249 | 805.01 | 828.65 | –17.59 (–160.59 to 125.42) | 0.809 |
| A&E consultations (face to face) | 244 | 57.15 | 306.11 | 249 | 41.16 | 125.35 | 15.99 (–25.26 to 57.24) | 0.447 |
| Subtotal | 244 | 5804 | 6649 | 249 | 5920 | 6323 | –116 (–1264 to 1032) | 0.843 |
| Primary care | ||||||||
| Face to face | ||||||||
| GP consultations | 244 | 33.98 | 60.38 | 249 | 33.17 | 57.56 | 0.82 (–9.62 to 11.25) | 0.878 |
| GP home visits | 244 | 14.29 | 60.98 | 249 | 14.57 | 113.88 | –0.27 (–16.49 to 15.94) | 0.974 |
| Nurse consultations | 244 | 23.75 | 61.48 | 249 | 27.04 | 69.81 | –3.28 (–14.93 to 8.37) | 0.580 |
| Nurse home visits | 244 | 21.92 | 88.60 | 249 | 18.95 | 75.82 | 2.96 (–11.62 to 17.54) | 0.690 |
| Telephone consultations | ||||||||
| GP led | 244 | 4.02 | 14.03 | 249 | 5.34 | 17.86 | –1.31 (–4.16 to 1.53) | 0.365 |
| Nurse led | 244 | 5.08 | 11.66 | 249 | 5.20 | 14.51 | –0.11 (–2.45 to 2.22) | 0.923 |
| Other | 244 | 1.20 | 4.79 | 249 | 2.01 | 10.04 | –0.81 (–2.21 to 0.59) | 0.255 |
| Out-of-hours consultations | ||||||||
| GP | 244 | 4.18 | 30.74 | 249 | 3.22 | 21.97 | 0.96 (–3.76 to 5.68) | 0.689 |
| Nurse | 244 | 5.68 | 41.18 | 249 | 3.51 | 30.51 | 2.16 (–4.24 to 8.57) | 0.507 |
| Other | 244 | 2.09 | 16.73 | 249 | 1.17 | 11.28 | 0.92 (–1.6 to 3.44) | 0.473 |
| Subtotal | 244 | 116 | 195 | 249 | 114 | 228 | 2 (–36 to 40) | 0.916 |
| Follow-up management (2–3 years) | ||||||||
| Secondary care | ||||||||
| Inpatient stay | 244 | 955.03 | 6438.22 | 249 | 552.26 | 2994.50 | 402.77 (–482.95 to 1288.49) | 0.372 |
| Cystectomy | 244 | 244.73 | 1544.99 | 249 | 280.23 | 1651.47 | –35.50 (–318.63 to 247.62) | 0.805 |
| Resection surgery | ||||||||
| Length of operation | 244 | 9.54 | 25.92 | 249 | 4.35 | 18.11 | 5.20 (1.25 to 9.15) | 0.010 |
| Length of stay | 244 | 138.76 | 333.69 | 249 | 60.83 | 225.18 | 77.93 (27.65 to 128.21) | 0.002 |
| Cystoscopy | 244 | 1279.48 | 1287.27 | 249 | 1266.90 | 1322.52 | 12.59 (–218.42 to 243.59) | 0.915 |
| Hospital doctor consultation | ||||||||
| Telephone | 244 | 14.80 | 43.56 | 249 | 12.12 | 36.25 | 2.68 (–4.41 to 9.76) | 0.459 |
| Out of hours | 244 | 3.87 | 44.76 | 249 | 14.36 | 193.83 | –10.50 (–35.51 to 14.51) | 0.410 |
| Outpatient consultations (face to face) | 244 | 8.79 | 37.64 | 249 | 14.51 | 49.96 | –5.72 (–13.56 to 2.12) | 0.152 |
| A&E consultations (face to face) | 244 | 4.44 | 20.60 | 249 | 3.25 | 23.09 | 1.19 (–2.69 to 5.06) | 0.547 |
| Subtotal | 244 | 3089 | 7492 | 249 | 2639 | 4557 | 451 (–644 to 1546) | 0.419 |
| Primary care | ||||||||
| Face to face | ||||||||
| GP consultations | 244 | 14.80 | 43.56 | 249 | 12.12 | 36.25 | 2.68 (–4.41 to 9.76) | 0.459 |
| GP home visits | 244 | 3.87 | 44.76 | 249 | 14.36 | 193.83 | –10.50 (–35.51 to 14.51) | 0.410 |
| Nurse consultations | 244 | 8.79 | 37.64 | 249 | 14.51 | 49.96 | –5.72 (–13.56 to 2.12) | 0.152 |
| Nurse home visits | 244 | 4.44 | 20.60 | 249 | 3.25 | 23.09 | 1.19 (–2.69 to 5.06) | 0.547 |
| Telephone consultations | ||||||||
| GP led | 244 | 1.81 | 10.18 | 249 | 2.46 | 23.27 | –0.64 (–3.83 to 2.55) | 0.692 |
| Nurse led | 244 | 1.73 | 6.52 | 249 | 2.19 | 7.66 | –0.47 (–1.72 to 0.79) | 0.468 |
| Other | 244 | 0.48 | 3.40 | 249 | 0.18 | 1.44 | 0.30 (–0.16 to 0.76) | 0.198 |
| Out-of-hours consultations | ||||||||
| GP | 244 | 0.58 | 9.02 | 249 | 0.28 | 4.46 | 0.29 (–0.96 to 1.55) | 0.645 |
| Nurse | 244 | 0.58 | 9.02 | 249 | 1.13 | 17.86 | –0.55 (–3.07 to 1.96) | 0.665 |
| Other | 244 | 0.87 | 13.53 | 249 | 1.09 | 12.19 | –0.23 (–2.51 to 2.05) | 0.844 |
| Subtotal | 244 | 38 | 101 | 249 | 52 | 257 | –14 (–48.27 to 20.99) | 0.439 |
| Total follow-up costs | 244 | 9048 | 10,071 | 249 | 8724 | 7677 | 323 (–1259 to 1906) | 0.688 |
Subsequent use of services following discharge for the index procedure
Table 23 describes the use of services during follow-up. The additional costs are combined with the costs to the health services over the trial follow-up period for each treatment group; these are also presented in Table 23. Compared with WL-TURBT, PDD-TURBT does not incur additional consumable costs and additional costs are presented only. Each category of cost is presented for full cases within that category. Although PDD-TURBT is more costly than WL-TURBT treatment, there is no evidence of a difference in the total follow-up costs between the two groups.
Total NHS costs
Overall, including intervention and follow-up health-service costs, PDD-TURBT is, on average, £993 (95% CI –£724 to £2709) more costly over the 3 years’ follow-up than WL-TURBT.
Costs directly incurred by participants and indirect costs
We further incorporated both participant costs and indirect costs into the analysis over the 3-year follow-up. Table 24 reports mean costs (from a wider economic perspective) of attending inpatient admissions, outpatient appointments and primary care. Each category of cost is presented for full cases within that category. These are then summed together across all of the available cost categories for participant and companion indirect costs, and presented as the total participant cost at 3 years.
| Costs (£) | Treatment group | Mean difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| PDD | WL | |||||
| Mean | SD | Mean | SD | |||
| Patient and companion time and travel costs | ||||||
| Inpatient appointments | 204.04 | 186.96 | 123.93 | 79.46 | 80.10 (54.77 to 105.44) | < 0.001 |
| Outpatient appointments | 675.75 | 749.95 | 713.20 | 1499.97 | –37.44 (–247.96 to 173.08) | 0.727 |
| Primary care appointments | 39.59 | 78.45 | 50.90 | 129.75 | –11.32 (–30.34 to 7.7) | 0.243 |
| Time off work | 222.19 | 692.87 | 351.88 | 1513.40 | –129.69 (–338.69 to 79.31) | 0.223 |
| Self-purchased health care and medication | 8.31 | 66.54 | 19.25 | 273.02 | –10.94 (–46.27 to 24.39) | 0.543 |
| Total indirect and patient costs | 1150 | 1184 | 1259 | 2737 | –109 (–484 to 265) | 0.567 |
| Total NHS costs | 12,927 | 10,994 | 11,934 | 8235 | 993 (–724 to 2709) | 0.256 |
| Overall NHS, patient and indirect costs | 14,077 | 11,802 | 13,193 | 9630 | 883 (–1024 to 2788) | 0.362 |
As patients with clinical symptoms of recurrence or progression attended a large number of consultations and appointments with the health services, the personal and economic costs were substantial. However, there was no evidence of differences between randomised groups, except for patient costs of accessing and using inpatient appointments. The mean differences in patient costs of accessing and using inpatient, outpatient and primary care appointments were £80.10 (95% CI £54.77 to £105.44; p < 0.001), –£37.44 (95% CI –£247.96 to £173.08; p = 0.727) and –£11.32 (95% CI –£30.34 to £7.70; p = 0.243), respectively.
Furthermore, a small proportion of patients incurred direct private health-care or self-purchased medication costs. However, the majority of patients did not and, as with the analyses above, there was no evidence of a difference between groups. The mean difference was –£10.94 (95% CI –£46.27 to £24.39; p = 0.543).
The mean indirect cost of sick leave taken by participants over 3 years for reasons related to clinical symptoms of recurrence or progression was £351.88 and £222.19 for WL-TURBT and PDD-TURBT, respectively. However, there was no evidence of a difference between the groups. The mean difference was –£129.69 (95%CI –£338.69 to £79.31; p = 0.223).
Combining all of the NHS, patient and indirect costs, we can estimate a wider overall economic cost to society. This is limited, of course, to the costs considered, and the true economic costs may be much higher. Nonetheless, the analysis gives an overall impression of the most immediate wider economic costs associated with the TURBT options considered in the PHOTO study. The total NHS, personal health-care and productivity costs were £13,193 and £14,077 for WL-TURBT and PDD-TURBT, respectively. The difference is consistent with the larger number of resections in the PDD group than in the WLC group, which results in more travel time. However, there was no evidence of a significant difference between the groups. The mean difference was £883 (95% CI –£1024 to £2788; p = 0.362).
EQ-5D-3L scores and quality-adjusted life-years
The proportion of patients with any health problems reported on the EQ-5D-3L measure of generic QoL is shown in Appendix 2, Figures 16–20. These figures present the data as reported by patients across randomised groups at baseline, discharge and follow-up visits (i.e. at 3, 6, 12, 18, 24 and 36 months), and are based on all of the available recorded data. This contrasts with the economic evaluation data in Health economic evaluation of photodynamic diagnosis of bladder tumour in reducing recurrence in primary non-muscle-invasive bladder cancer, which are based on all participants in the base-case analysis, and complete cost and QALY pairs in the sensitivity analysis. This also contrasts with the analysis of EQ-5D-3L at different time points in Chapter 4, which excludes 40 out of 135 participants in the PDD group and 49 out of 143 participants in the WLC group. The clinical analysis excluded patients who were subsequently classified as no tumour, as MIBC or who had an early cystectomy. A substantial proportion of patients appear to have had some pain or discomfort, with a significant increase after the initial surgery. Fewer patients reported problems with self-care than for the other EQ-5D-3L dimensions, with a large proportion of patients reporting no problem. A visual inspection of the graphical data does not indicate any substantial differences between the groups in any of the dimensions of generic QoL at each time point.
Table 25 provides descriptive data of mean utility scores and QALYs, generated by combining utilities with the duration (i.e. length) of life over follow-up. The preliminary utility scores for each treatment group suggest that, on average, the PDD treatment group has similar utility values over short-term (i.e. 6 months) and long-term (i.e. 3 years) follow-up. The results for incremental QALYs gained are presented, comparing PDD-TURBT with WL-TURBT for raw differences between QALY estimates. There was no evidence of a difference in QALYs gained between treatment groups at 3 years (mean difference –0.096, 95% CI –0.342 to 0.151).
| Time point | Treatment group | Mean difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| PDD | WL | |||||
| n (%) | Mean (SD) | n (%) | Mean (SD) | |||
| EQ-5D-3L | ||||||
| Baseline | 242 (99) | 0.823 (0.015) | 235 (94) | 0.820 (0.015) | 0.003 (–0.038 to.045) | 0.886 |
| Discharge | 207 (85) | 0.702 (0.019) | 210 (84) | 0.691 (0.021) | 0.012 (–0.044 to.067) | 0.682 |
| 3 months | 215 (88) | 0.788 (0.017) | 222 (89) | 0.780 (0.016) | 0.008 (–0.037 to.053) | 0.721 |
| 6 months | 203 (83) | 0.802 (0.017) | 208 (84) | 0.792 (0.017) | 0.010 (–0.037 to.057) | 0.684 |
| 12 months | 192 (79) | 0.757 (0.022) | 202 (81) | 0.763 (0.022) | –0.006 (–0.067 to.056) | 0.854 |
| 18 months | 199 (82) | 0.728 (0.023) | 202 (81) | 0.761 (0.022) | –0.033 (–0.096 to.03) | 0.301 |
| 24 months | 188 (77) | 0.684 (0.026) | 194 (78) | 0.717 (0.026) | –0.032 (–0.104 to.04) | 0.379 |
| 36 months | 135 (55) | 0.630 (0.035) | 143 (57) | 0.610 (0.035) | 0.020 (–0.077 to.116) | 0.688 |
| QALYsa gainedb (baseline–3 years) | 86 (35) | 2.112 (0.093) | 85 (34) | 2.207 (0.084) | –0.096 (–0.342 to.151) | 0.444 |
Caution should be taken when interpreting Table 25, as the results are presented for only those participants who completed the EQ-5D-3L at each time point. The number of participants providing utility data in each treatment group decreased by approximately 47% from randomisation to the 1-year visit, with a further 21% decrease between the 1-year and 3-year visits.
Missing data
Missing data were mostly driven by missing EQ-5D-3L data. Utilities, as measured using the EQ-5D-3L, were completed by 89.5% and 52.1% of individuals at baseline and 36 months, respectively. Data completeness for QALYs at 3 years was evenly distributed between the groups, with data missing for 180 of 268 (67%) and 176 of 265 (66%) participants for WL-TURBT and PDD-TURBT, respectively. Furthermore, we investigated the mechanism of missingness of data by exploring the impact of baseline covariates on missing EQ-5D-3L data. Missing EQ-5D-3L data were found to differ significantly among risk categories (p < 0.01) and age groups (p < 0.01).
Complete resource use data were available for 100% of participants at the initial procedure and for 46.3%–95.7% of participants at follow-up visits of those in the ITT population. Resource use data at follow-ups were complete for 98% of participants (all of the health-care data were missing for the remaining 2% at follow-ups). Further analysis shows that the average 3-year cost of PDD-TURBT is less than that of WL-TURBT for participants without complete QALY data. This finding suggests that the complete-case analyses may overestimate the true follow-up costs for the PDD group, and that the cost difference between PDD-TURBT and WL-TURBT may be smaller after MIs.
Cost-effectiveness
Base-case analysis
The primary cost-effectiveness analysis (CEA) of the trial was conducted under the MAR assumption, using MI to impute the missing follow-up cost and HRQoL values. Effectiveness was measured in QALYs, and costs were captured by the total health-care use over the trial period. Table 26 presents the results of the base-case analysis from an NHS and PSS perspective over the 3-year time horizon. On average, PDD-TURBT is more costly and less effective; therefore, an incremental cost-effectiveness ratio (ICER) is not presented. Figure 12 illustrates the scatterplot of incremental costs and incremental QALYs for this analysis. It shows that there is substantial uncertainty in the number of QALYs gained, but also that PDD-TURBT is more costly than WL-TURBT. The CEAC in Figure 13 shows that PDD-TURBT has a 23% and 26% chance of being considered cost-effectiveness at threshold ICERs of £20,000 per QALY gained and £30,000 per QALY gained, respectively. This is a very low probability of being cost-effective. WL-TURBT is far more likely to be cost-effective than PDD-TURBT. PDD-TURBT remains dominated when using a lower unit cost (£468 instead of £891) for 1 night in hospital.
| Analysis | Adjusted, mean (95% CI) | Incremental, mean (95% CI) | ICER (£/QALY) | Probability (%) that intervention is cost-effective for different threshold values for society’s WTP for an additional QALY | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | £0 | £20,000 | £30,000 | £50,000 | ||
| Base case | |||||||||
| Imputed data analysis (3 years), MAR | |||||||||
| WL-TURBT | 12,005 (10,845 to 13,166) | 2.094 (2.010 to 2.178) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 12,881 (11,713 to 14,049) | 2.087 (1.996 to 2.179) | 876 (–766 to 2518) | –0.007 (–0.133 to 0.119) | 21 | 23 | 26 | 30 | |
| Scenario analyses | |||||||||
| Scenario 1: imputed data analysis (3 years), same MNAR parameters in both groups (–10% QoL) | |||||||||
| WL-TURBT | 12,005 (10,845 to 13,166) | 1.956 (1.877 to 2.035) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 12,881 (11,713 to 14,049) | 1.948 (1.861 to 2.034) | 876 (–766 to 2518) | –0.008 (–0.127 to 0.110) | 21 | 21 | 24 | 27 | |
| Scenario 2: imputed data analysis (3 years), same MNAR parameters in both groups (+10% cost) | |||||||||
| WL-TURBT | 12,075 (10,899 to 13,251) | 2.094 (2.010 to 2.178) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 12,948 (11,765 to 14,132) | 2.087 (1.996 to 2.179) | 873 (–791 to 2538) | –0.007 (–0.133 to 0.119) | 21 | 24 | 27 | 30 | |
| Scenario 3: imputed data analysis (3 years), same MNAR parameters in both groups (–10% QoL and +10% cost) | |||||||||
| WL-TURBT | 12,075 (10,899 to 13,251) | 1.956 (1.877 to 2.035) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 12,948 (11,765 to 14,132) | 1.948 (1.861 to 2.034) | 873 (–791 to 2538) | –0.008 (–0.127 to 0.110) | 21 | 21 | 24 | 27 | |
| Scenario 4: imputed data analysis (3 years), different MNAR parameters in both groups (–10% QoL in PDD-TURBT group) | |||||||||
| WL-TURBT | 12,005 (10,845 to 13,166) | 2.094 (2.010 to 2.178) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 12,881 (11,713 to 14,049) | 1.948 (1.861 to 2.035) | 876 (–766 to 2518) | –0.146 (–0.269 to –0.024) | 21 | 0 | 0 | 0 | |
| Scenario 5: imputed data analysis (3 years), different MNAR parameters in both groups (–10% QoL in WL-TURBT group) | |||||||||
| WL-TURBT | 12,005 (10,845 to 13,166) | 1.956 (1.877 to 2.035) | |||||||
| PDD-TURBT | 12,881 (11,713 to 14,049) | 2.087 (1.996 to 2.179) | 876 (–766 to 2518) | 0.131 (0.009 to 0.254) | 6664 | 21 | 85 | 90 | 93 |
| Scenario 6: imputed data analysis (3 years), different MNAR parameters in both groups (+10% cost in PDD-TURBT group) | |||||||||
| WL-TURBT | 12,006 (10,841 to 13,171) | 2.094 (2.010 to 2.178) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 12,949 (11,769 to 14,128) | 2.087 (1.996 to 2.179) | 943 (–711 to 2597) | –0.007 (–0.133 to 0.119) | 19 | 22 | 25 | 29 | |
| Scenario 7: imputed data analysis (3 years), different MNAR parameters in both groups (+10% cost in WL-TURBT group) | |||||||||
| WL-TURBT | 12,074 (10,903 to 13,245) | 2.094 (2.010 to 2.178) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 12,880 (11,709 to 14,052) | 2.087 (1.996 to 2.179) | 806 (–847 to 2459) | –0.007 (–0.133 to 0.119) | 23 | 25 | 28 | 31 | |
| Scenario 8: complete-case analysis (3 years) | |||||||||
| WL-TURBT | 12,265 (10,131 to 14,399) | 2.146 (2.030 to 2.261) | |||||||
| PDD-TURBT | 15,089 (12,577 to 17,602) | 2.168 (2.032 to 2.305) | 3236 (–1081 to 6554) | 0.034 (–0.146 to 0.213) | 95,606 | 2 | 16 | 26 | 38 |
FIGURE 12.
Scatterplot of incremental costs and QALYs for PDD-TURBT compared with WL-TURBT: base case.
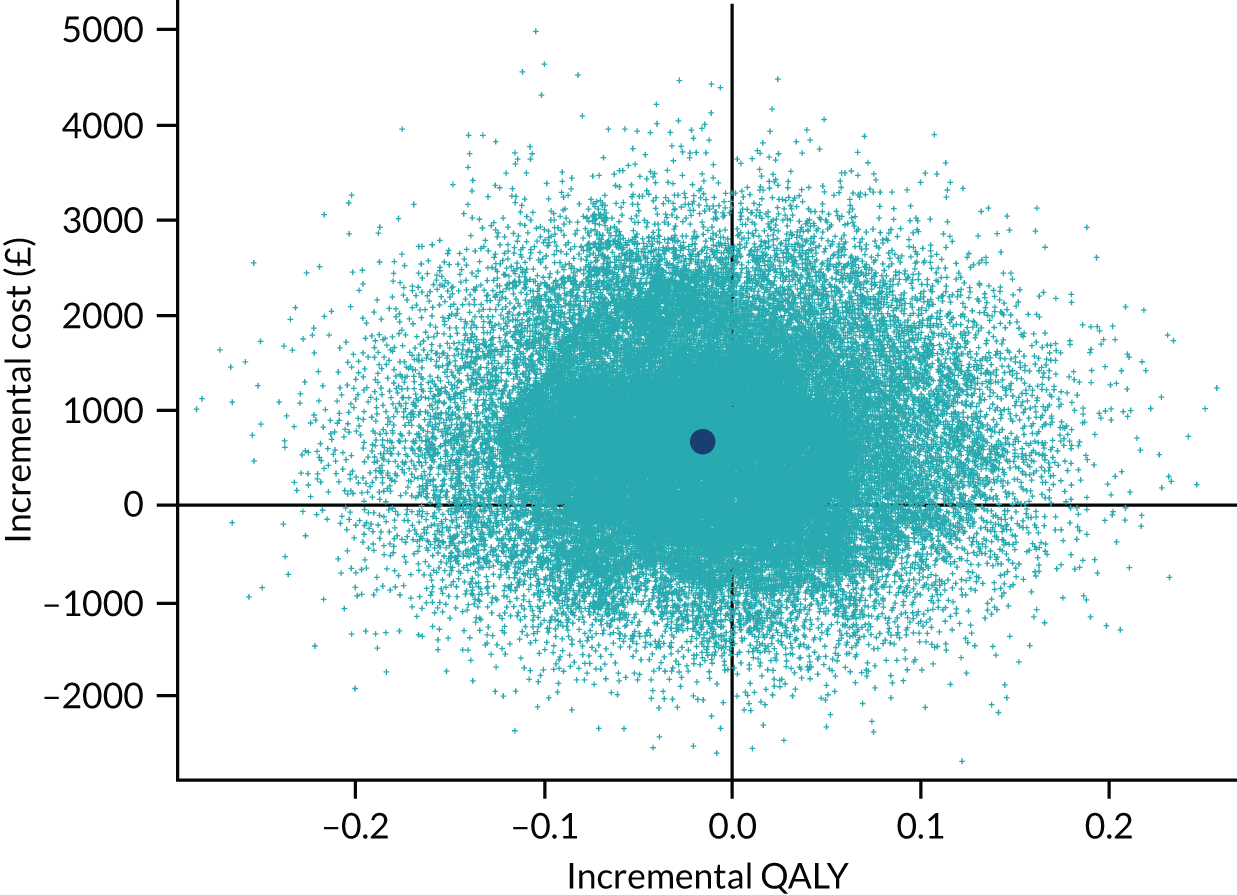
FIGURE 13.
The CEACs: base case.
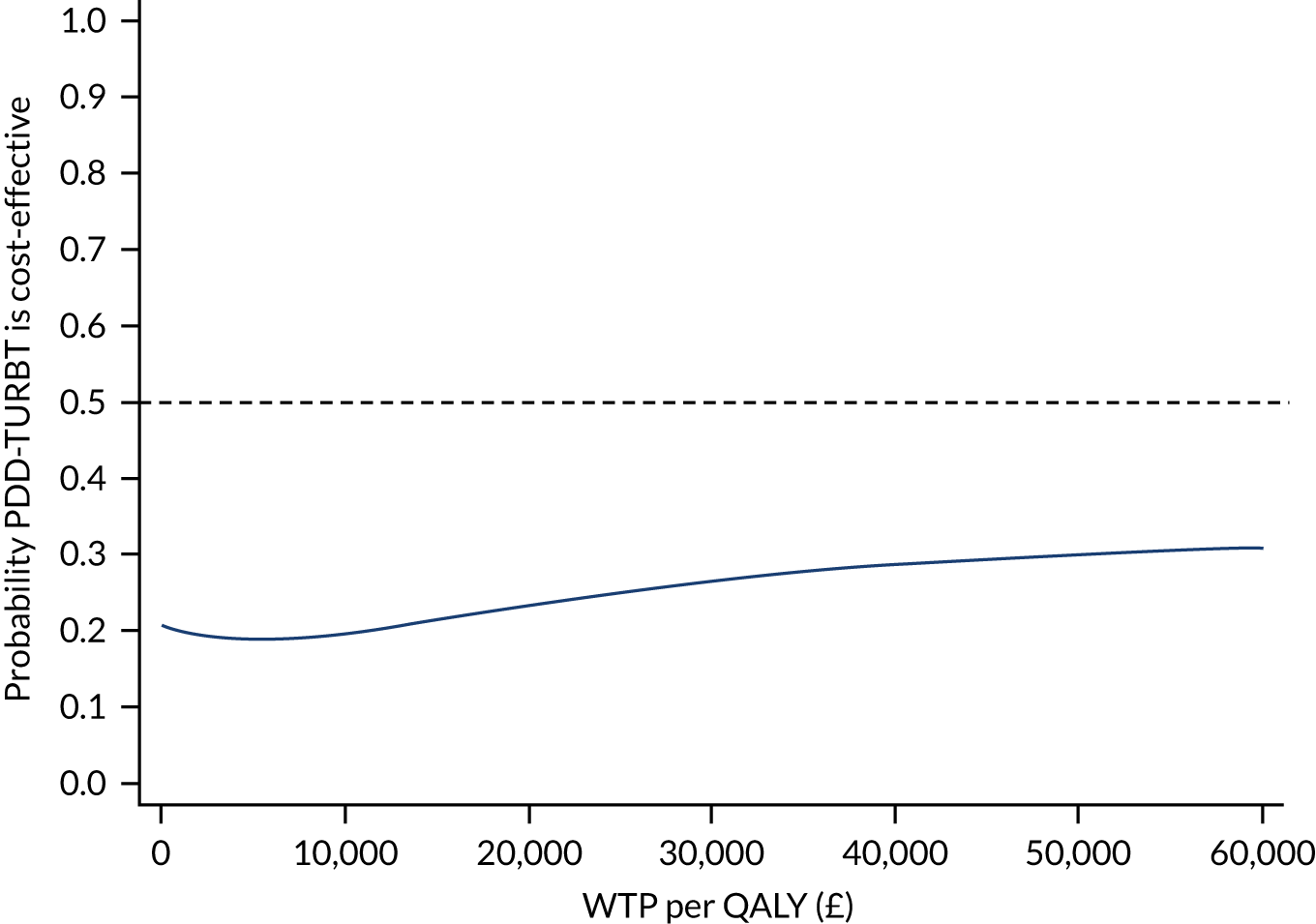
Sensitivity analysis
The results under the seven missing-data scenarios (scenarios 1–7) and the complete-case analysis (scenario 8) are reported in Table 26 and as cost-effectiveness planes in Figure 14. The CEAC (Figure 15) shows that the probability of PDD-TURBT being cost-effective is relatively stable when MAR departures in total costs and HRQoL are assumed to be the same in each group (scenarios 1–3). This is also seen in Table 26, where the alternative departures from MAR had little effect on the incremental costs and QALYs in these scenarios. This will usually be the case when the missing data pattern is broadly similar across treatment groups, as the MNAR bias applies roughly equally to each group and cancels out in the treatment comparison.
FIGURE 14.
Cost-effectiveness planes under different scenarios. (a) Scenario 1; (b) scenario 2; (c) scenario 3; (d) scenario 4; (e) scenario 5; (f) scenario 6; (g) scenario 7; and (h) scenario 8.
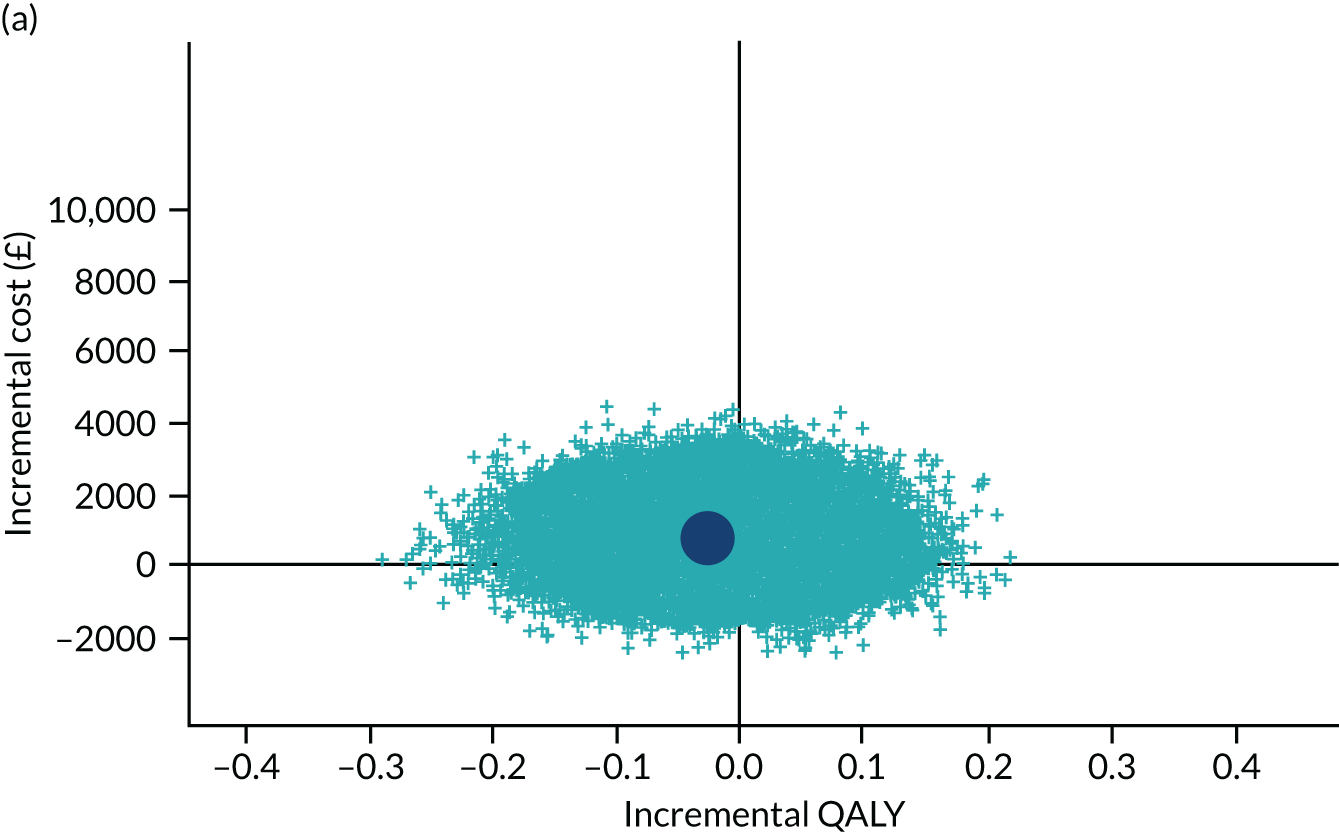
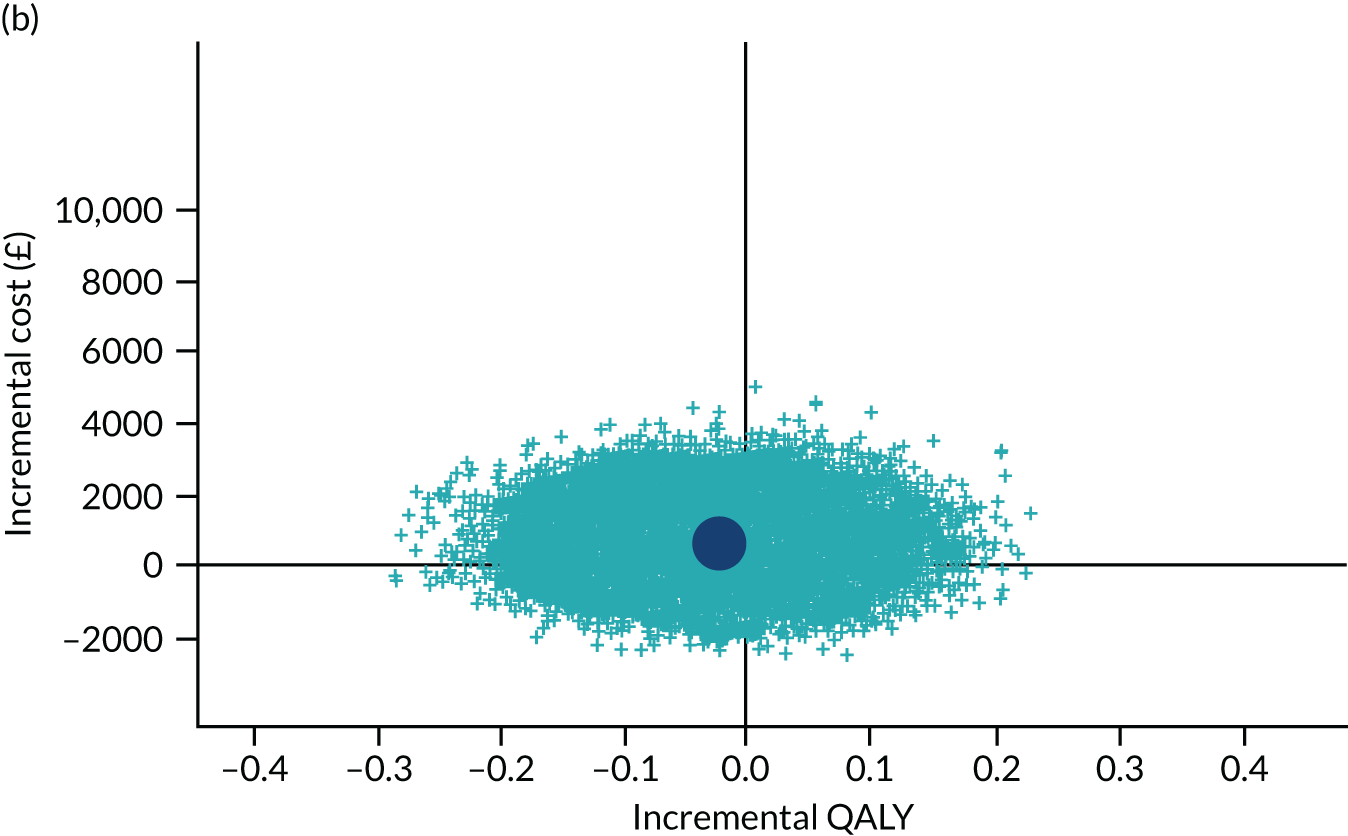
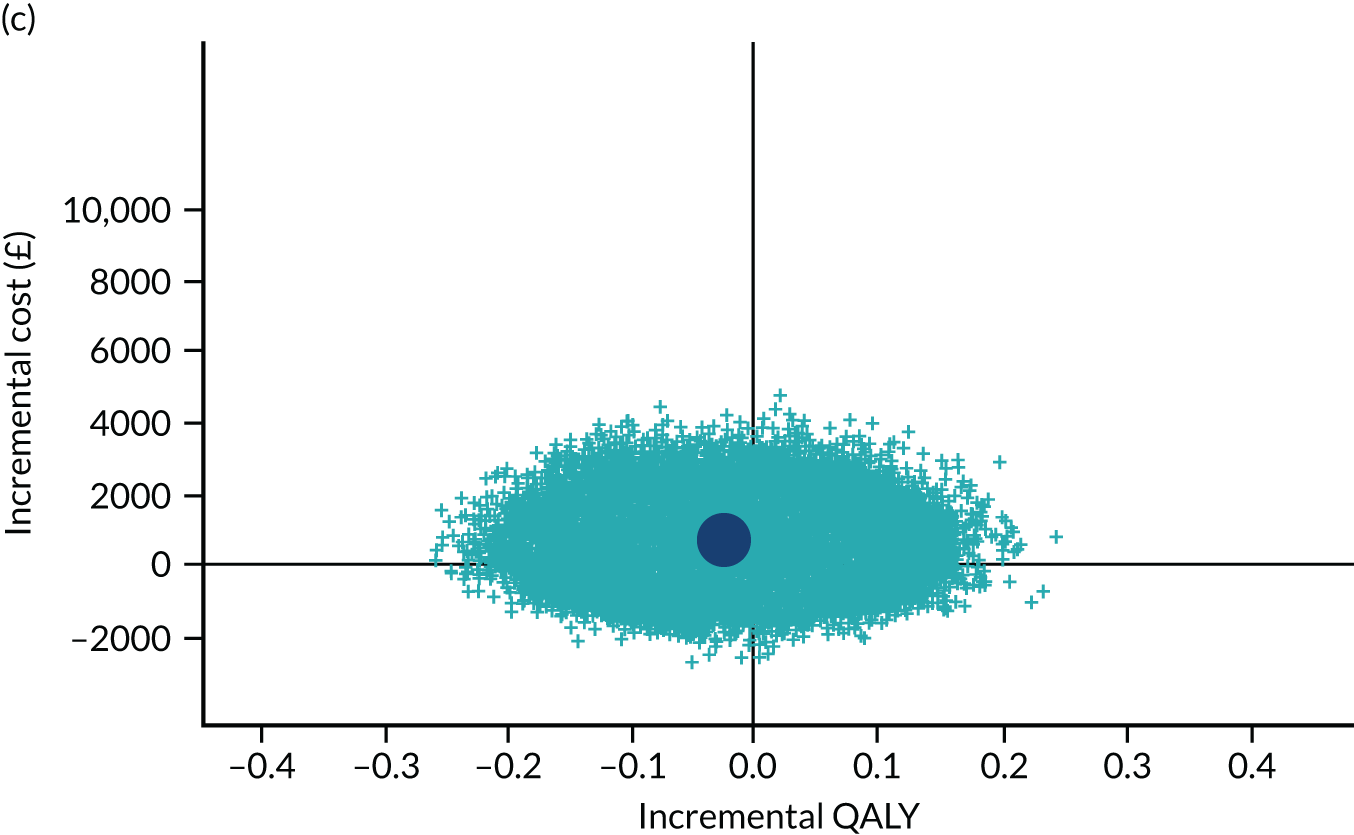
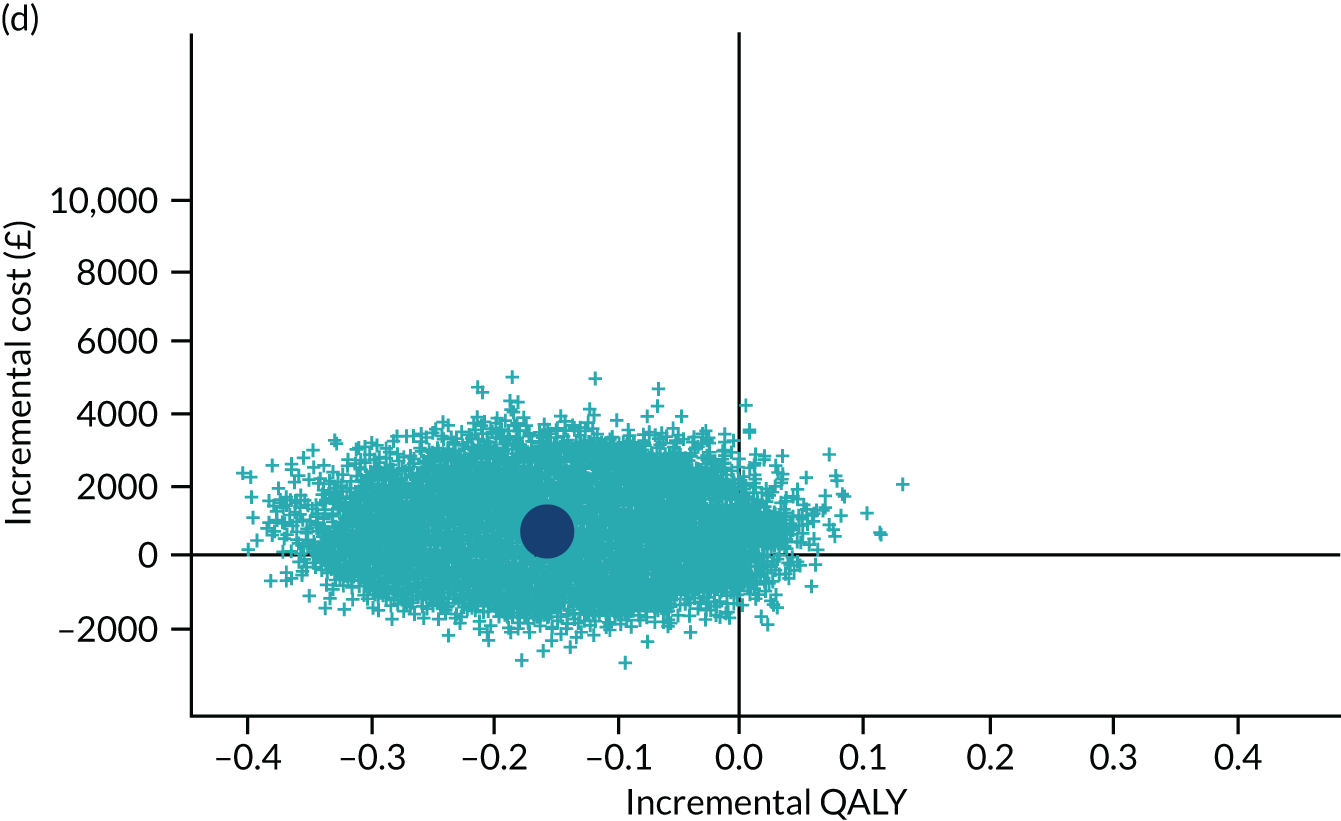
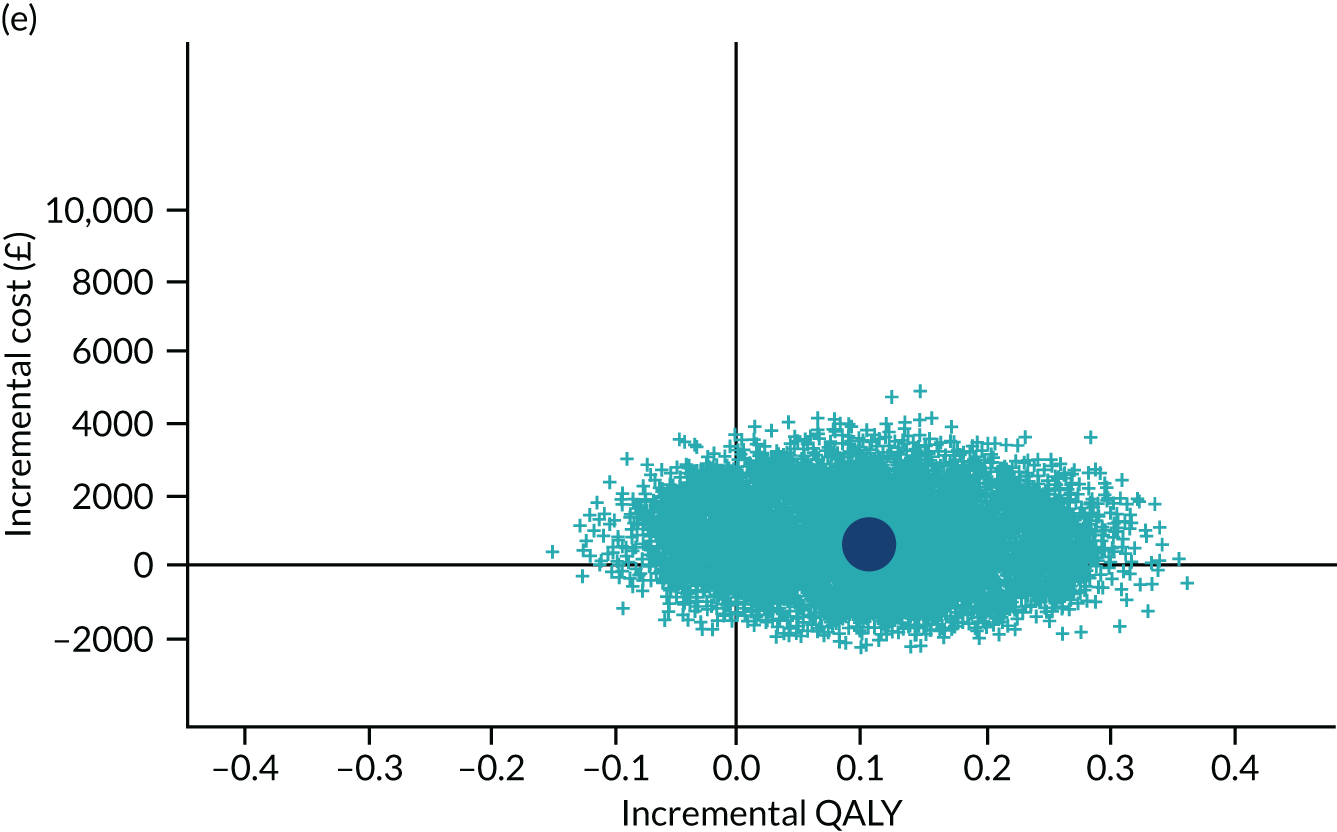
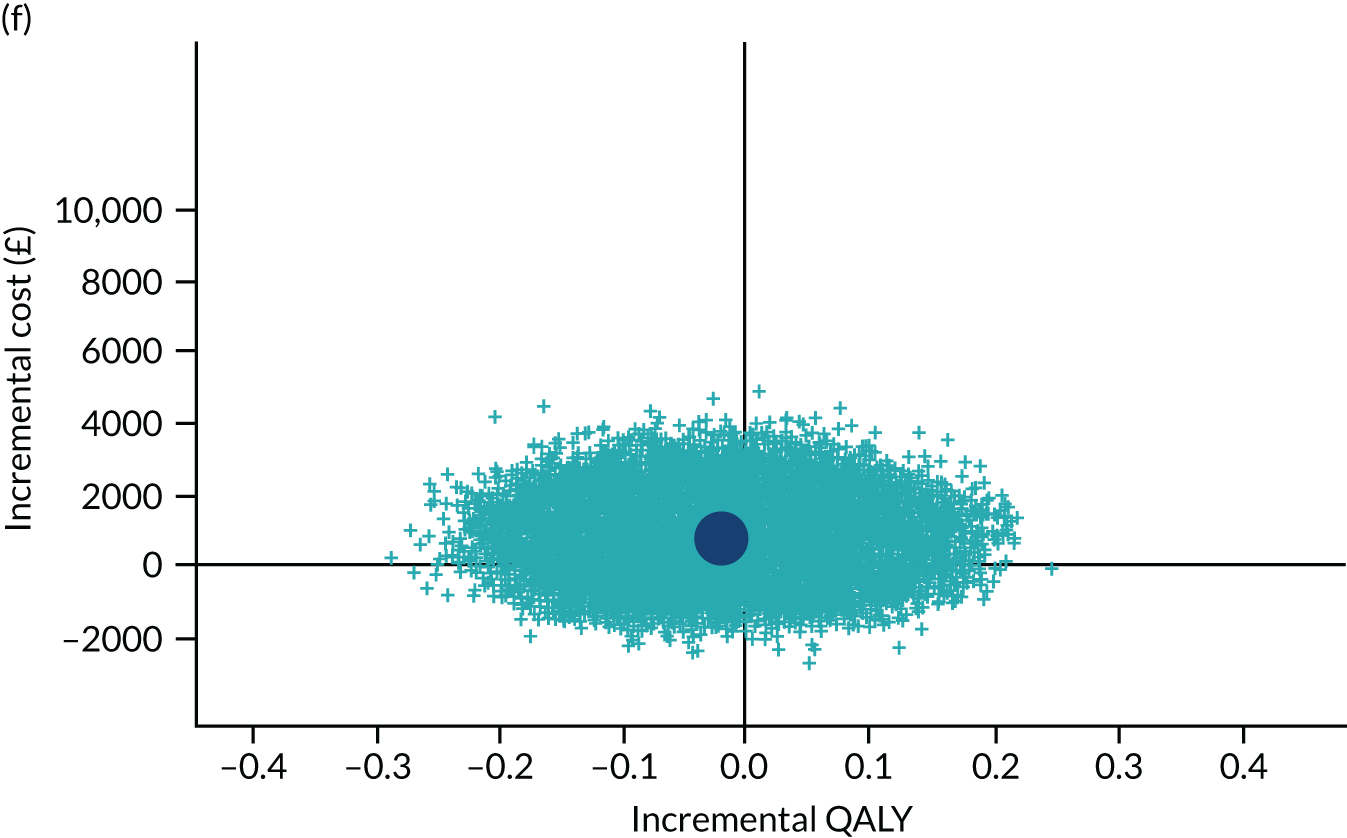
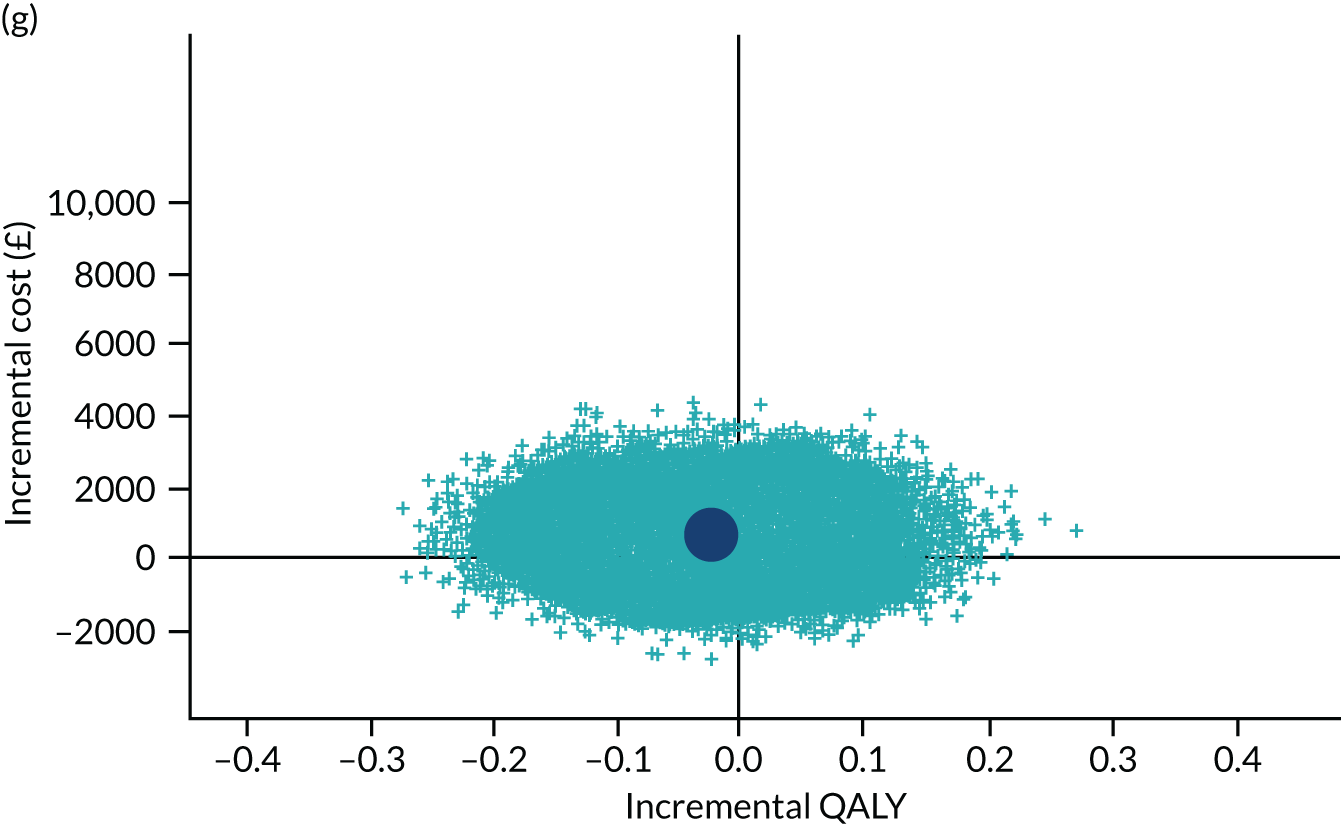
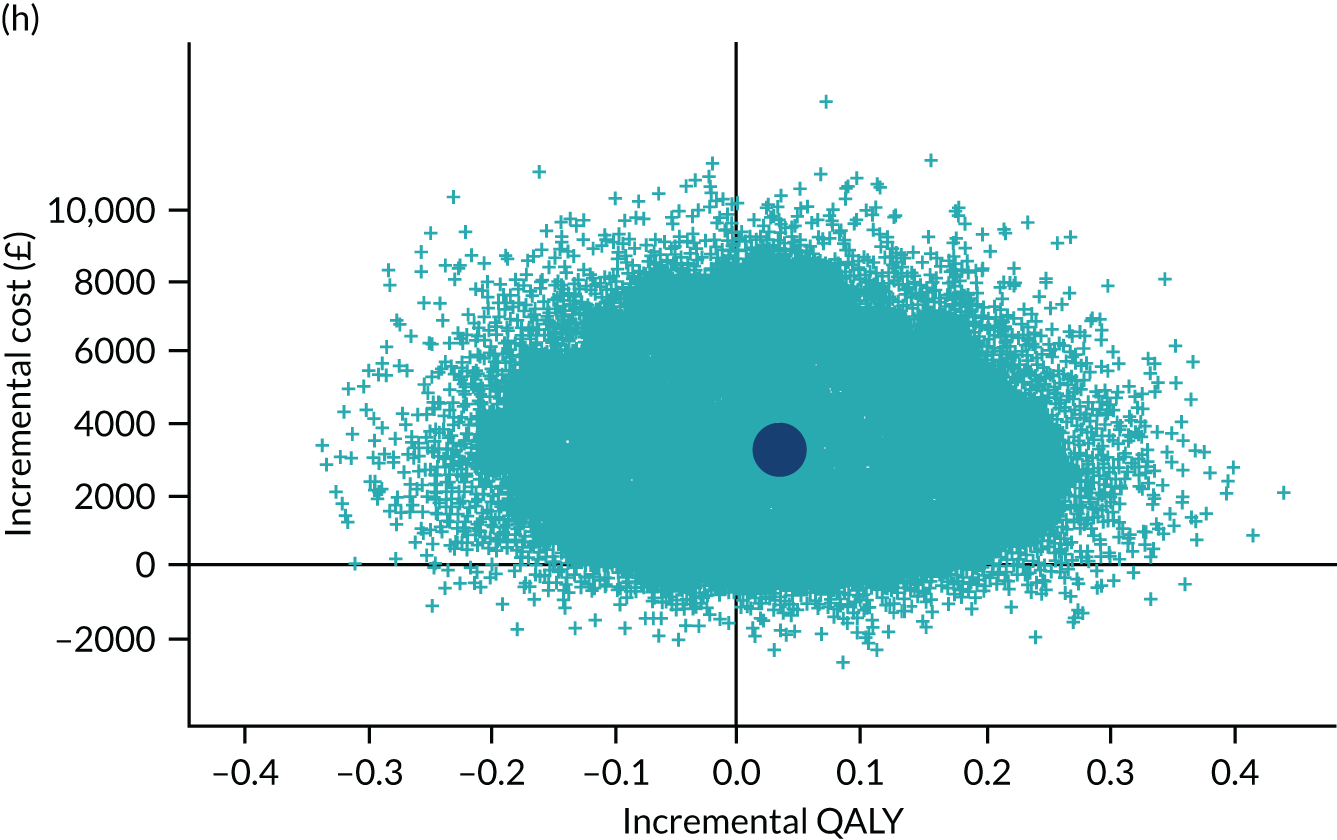
FIGURE 15.
The CEACs under different scenarios.
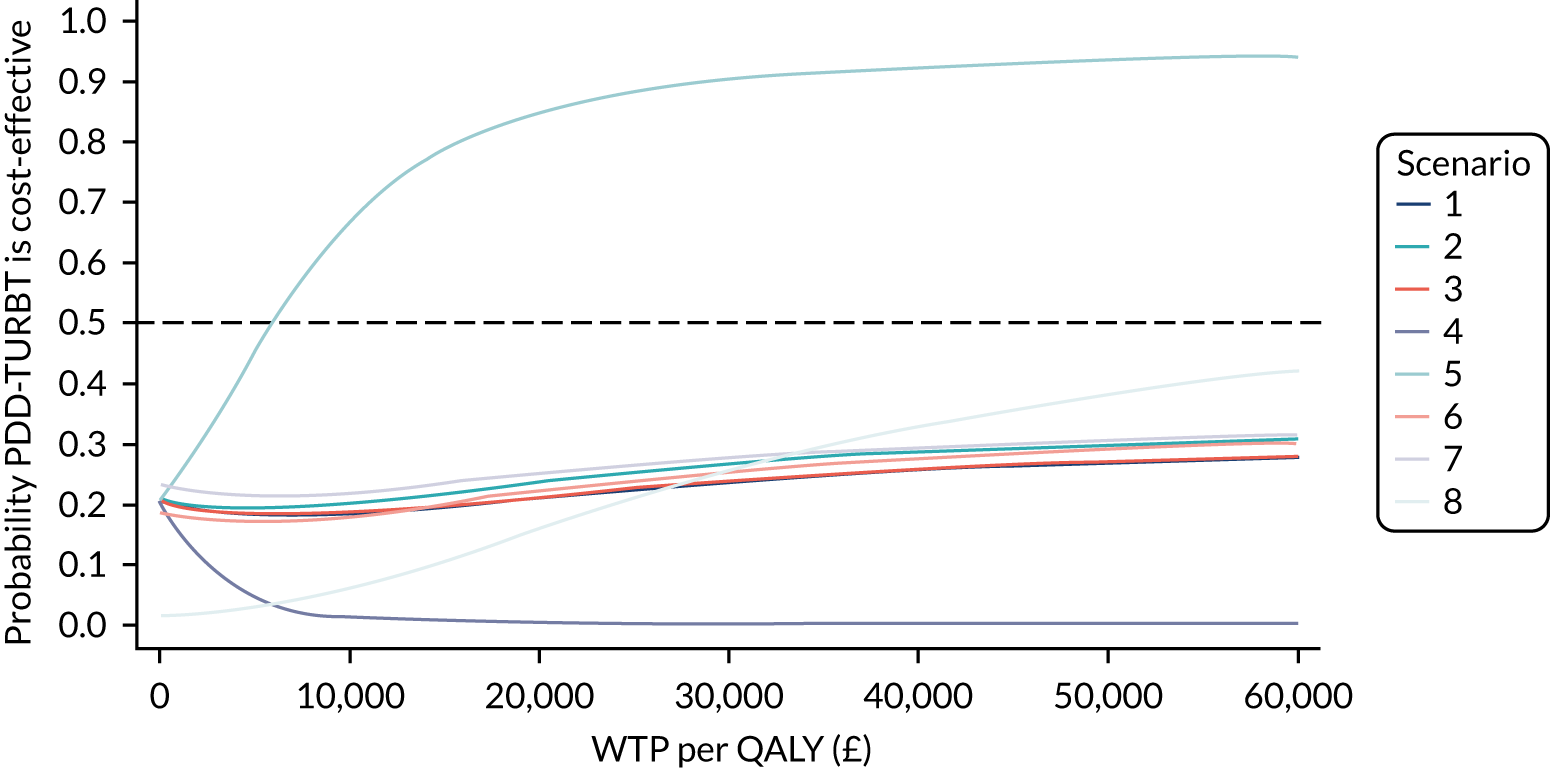
Where the missing data mechanisms differ between groups (see Table 26), this suggests that the departures from MAR for the total cost (scenarios 6 and 7) would have a marginal effect on the overall results only, whereas departures for the QoL (scenarios 4 and 5) can strongly affect the conclusions. For example, PDD-TURBT appeared to be likely to be cost-effective when we assumed stronger MNAR (i.e. lower QoL) in the WL group, with a probability of being cost-effective of around 90% at £30,000 per QALY.
The results of the complete-case analysis (see Table 26) show that QALYs are similar between groups over 3 years, but PDD-TURBT is more costly. The point-estimate incremental cost per QALY gained for PDD-TURBT compared with WL-TURBT is £95,606. However, this estimate should be interpreted in the light of the considerable uncertainty surrounding it. The probability of PDD-TURBT being the preferred treatment option is substantially lower; it never reaches a probability of cost-effectiveness of > 40% at threshold values of up to £50,000 per QALY gained.
Widening the perspective of costs to include those falling on participants, families and wider societal costs changed the incremental cost to £763 (95% CI £1048 to £2574), although there were no differences between treatment groups (see Appendix 2, Table 46).
PDD-TURBT, compared with WL-TURBT, remained unlikely to be considered cost-effective over the range of society’s cost-effectiveness threshold values for a QALY that we considered; the probability that PDD-TURBT would be considered cost-effective was never above 35%.
Our results were also consistent across alternative discount rates applied to costs and QALYs (see Appendix 2, Table 48). The conclusions based on the net-benefit statistics (at a threshold value of £30,000 per QALY gained) remained unchanged for the exploration of alternative discount rates.
Changes in performance due to learning may dynamically influence the results of a technology evaluation through the change in effectiveness and costs. Learning curve analyses in the clinical analysis did not find evidence of a positive/negative learning effect. The conclusions would not change by incorporating the effect of learning in this study. Therefore, we did not conduct a learning curve analysis in the within-trial analysis.
Decision model
An economic model was produced to model the lifetime outcomes utilising the effectiveness data of the trial, but it was considered that the economic model would not provide any additional information for decision-makers. (See Appendix 3 for a description of the model.) Although the model was designed to evaluate the cost-effectiveness of PDD-TURBT, the individual patient simulation model could be edited to evaluate different surveillance strategies, accounting for the cost of a delay in diagnosis through the application of the relative risk of progression for those not receiving treatment compared with those receiving treatment during the undiagnosed period.
The economic model would be driven by the effectiveness of PDD-TURBT in terms of reducing recurrence and progression. PDD-TURBT costs more than WL-TURBT. For it to be cost-effective, PDD-TURBT needs to be more effective than WL-TURBT. The effectiveness evidence showed no statistically significant evidence of a positive effect of PDD-TURBT on recurrence or progression using either proportional hazard or accelerated failure time models. The quality-of-life and resource use outcomes were consistent with the recurrence and progression outcomes. The mean HR or TR estimates were close to 1 for recurrence and > 1 for progression. Owing to the violation of the proportional hazard assumption, the economic model would have used relative risk estimates for recurrence and progression over time periods that were different from those used in the trial, but, overall, there would be no positive effect. An economic model may estimate a greater net benefit for PDD-TURBT than the net-benefit estimate from a within-trial economic analysis if PDD-TURBT is associated with better cost and health outcomes beyond the end of the trial; however, there is no evidence for this from the PHOTO trial. As the objective of the study was to evaluate PDD-TURBT, the cost-effectiveness of different surveillance frequencies of WL-TURBT was not evaluated.
Chapter 6 Discussion and conclusions
Summary of findings
Clinical effectiveness of photodynamic diagnosis of bladder tumour in primary non-muscle-invasive bladder cancer
The PHOTO trial compared PDD-TURBT and WL-TURBT for the treatment of newly diagnosed intermediate-risk and high-risk NMIBC in a pragmatic UK setting. The main clinical outcome was bladder cancer recurrence, assessed as time to recurrence. The findings revealed no significant difference in this primary outcome measure. The CEA showed that the strategy of PDD-TURBT was not more cost-effective than WL-TURBT at 3 years. Similarly, there were no significant differences across the study’s secondary outcomes relating to cancer control, specifically cancer progression (i.e. a recurrence resulting in increased tumour staging to MIBC or metastases) and bladder-cancer-specific death. Other secondary outcomes included PROMs of HRQoL (measured using the EQ-5D-3L, EORTC-QLQ-C30 and EORTC-QLQ-NMIBC-24) and AEs (Clavien–Dindo grade, AEs and SAEs), which also showed no overall differences.
Overall, in the management of primary NMIBC, following pragmatic exclusion of those with low-risk tumours (i.e. solitary, small papillary bladder lesions of < 3 cm in diameter), the use of PDD-TURBT is not recommended.
Health economic evaluation of photodynamic diagnosis of bladder tumour in reducing recurrence in primary non-muscle-invasive bladder cancer
In the earlier evidence synthesis,11 a number of assumptions were made to link the impact of diagnosis to health outcomes. It was acknowledged in that evidence synthesis that, although PDD showed promise based on the modelling, the evidence was not conclusive given the need to splice data from multiple sources and the assumptions made about the mechanism by which costs and QALYs would be generated. It was for this reason that a trial was recommended. However, in our end-to-end evaluation, the promise seen in the modelling was not realised in practice.
Photodynamic diagnosis-guided transurethral resection of bladder tumour was, on average, a more costly procedure, driven by the cost of drugs in theatre. The drug cost excluded an associated equipment cost. It was a challenge to estimate the equipment cost because it varies depending on existing equipment and if a completely new system needs to be purchased. Including the equipment cost makes PDD-TURBT more costly. There was no evidence of a difference in the time to perform the two procedures, nor was there any evidence of a difference in terms of the follow-up care required between the groups. PDD-TURBT was more costly and less effective at 3-year follow-up from an NHS and PSS perspective than WL-TURBT. This finding did not change when different ways of handling missing data were considered, except when assuming that participants in the WL group who failed to complete an EQ-5D-3L questionnaire were likely to have been relatively poorer health.
In conclusion, over a 3-year follow-up, PDD-TURBT was, on average, more costly than WL-TURBT. There was no evidence of a difference in QALYs and it was unlikely that PDD-TURBT would be considered cost-effective compared with WL-TURBT over the range of values for society’s cost-effectiveness threshold for a QALY that we considered. These results remained unchanged over a range of plausible assumptions about missing data.
Acceptability of the intervention
From a patient perspective, complications and HRQoL outcomes in the short term and longer term showed that the use of PDD was not associated with any significant effect over conventional WL resection, suggesting that PDD as part of TURBT is acceptable and well tolerated.
From a service provider perspective, the trial revealed real-world challenges in the current climate of the NHS in establishing a new service in PDD-naive centres. The capital investment involved in setting up a new PDD service includes costs for equipment (e.g. light-generating stacks, bespoke light cables and dedicated scopes). Despite sharing a model business case, interested centres were unable to commit to or unable to secure local funding in the timeframe required for the study. Ultimately, this led to a longer recruitment period and a study extension, as there were only four PDD-naive centres that successfully secured funds. Part of this issue might have reflected uncertainty rooted in the equivocal effectiveness of the PDD approach, which may have deterred some centres from making an investment at the risk of eventually seeing the study show no significant effect. However, some of these cost implications may have extended to reduced acceptability to service providers, unless a compelling cost-effectiveness case was made.
Strengths, limitations and discussion
Trial limitations
Sample size considerations
In planning the study, recruitment of 533 participants (to provide a projected 214 recurrences) was required to detect a HR of 0.64 with a log-rank test (90% power, two-sided, 5% significance). At the time of analysis, the study had accrued 170 events in the analysis population across both groups of the trial.
As it became apparent that the trial was not going to reach the target number of events in the proposed recruitment time, a number of considerations were made. It was appreciable that the event rate had slowed during the study, in keeping with typical patterns of recurrence, with the majority of events occurring early in the first 18 months of follow-up. Accordingly, we estimated that another 30 months would be needed to accrue 214 events. Following review and discussion with the independent DMC, it was agreed that we could accept the number of events falling short of the required 214 for 90% power given that, even in the worst-case scenario, our power to detect the prespecified difference was above 80%.
When considering why we had a shortfall in the number of project events, we explored our original rationale for sample size estimates to see if we had underestimated rates of recurrence. We referenced recurrence rates reported in a recent UK trial with similar inclusion criteria (BOXIT). 53 BOXIT’s contemporary estimate of 40% recurrence at 3 years was replicated in the PHOTO trial. However, what was not anticipated was that a total of 112 participants were excluded from the PHOTO trial’s planned final analyses owing to absence of tumour at TURBT, MIBC disease on histological assessment or preference for an immediate cystectomy because of high-risk NMIBC. In effect, this left 426 participants for the final analysis, with a consequent reduction in statistical power to detect the target effect size. Despite this limitation, the PHOTO trial showed a HR of 0.94 (95% CI 0.69 to 1.28; p = 0.70) when comparing the two treatment groups. Although the precision around the HR estimate is fairly wide, the data were precise enough to rule out the prespecified difference at the start of the trial.
The factors limiting the total number of participants available for the final analyses are discussed below.
False-positive visual diagnosis of intermediate- and high-risk non-muscle-invasive bladder cancer
The nature of routine management of bladder masses, and therefore routine management of this pragmatic study, meant that participants were recruited on a presumptive visual diagnosis of NMIBC and then randomised to treatment. The delivery of TURBT itself is required to provide a tissue biopsy for definitive pathological diagnosis of NMIBC. Therefore, this is a complex intervention that simultaneously provides both treatment and diagnosis on the a priori judgement that there is a mass that appears to be bladder cancer on initial flexible cystoscopy or cross-sectional imaging. Based on previous descriptions of high clinician accuracy in using visual criteria on cystoscopy, we made an informed assumption that the rate of false-negative visual diagnosis would be 5%. 54,55 However, in our real-world experience, the visual assessment of bladder lesions in determining (1) NMIBC compared with MIBC and (2) cancer compared with benign macroscopic changes was not as precise as the 5% false-positive rate previously reported, with a 21% false-positive rate observed in the PHOTO trial [14% MIBC (60/426) and 7% benign (29/426)]. This was still much higher than that reported in a more recent, single-centre cohort,56 in which NMIBC was predicted accurately in 93.4% of cases. However, the PHOTO trial data are more representative of performance across the UK and may reflect day-to-day practice, in which the initial flexible cystoscopy is delivered by trainee urologists or a nurse specialist. This may lead to a reluctance not to biopsy a potential mass because of inexperience, even if it is suspected that it represents inflammation or other benign changes. Understandably, there is a broad culture of maintaining a low threshold for obtaining a formal histological diagnosis with a TURBT/bladder biopsy, as the consequences of missing cancer are disastrous. Similar discordance between visual assessment and histological verification of NMIBC was noted in another contemporary NHS-based trial, CALIBER (a phase II randomized feasibility trial of chemoablation with mitomycin-C vs. surgical management in low-risk non-muscle-invasive bladder cancer). 57
Early radical cystectomy for high-risk non-muscle-invasive bladder cancer
A proportion of participants, 8% (18/221) based on NICE criteria18 or 36% (18/50) based on EAU12/EORTC criteria, elected to undergo treatment for high-risk NMIBC with radical cystectomy (i.e. bladder removal). This reduced the number of participants who were able to experience bladder cancer recurrence within the bladder or, subsequently, as distant metastases, further diluting the originally predicted event rate from our sample size consideration. Both NICE18 and EAU12 guidelines recommend that, for those patients with NMIBC with the highest risk characteristics for progression (stage T1, high grade and concomitant CIS), radical cystectomy should be offered as the primary treatment (this applied to 12% of our cohort). The proportion of patients who undergo upfront cystectomy was previously unknown and was not accounted for in planning this study, affecting the total number in the analysis group. The alternative approach would be intravesical BCG treatment for those participants wishing to spare their bladder. In high-risk participants who experience cancer recurrence and those who did not experience a recurrence at up to 36 months, 50.0% and 66.6%, respectively, underwent BCG induction (± maintenance). These data are in keeping with the BOXIT results,53 which found that the proportion of high-risk participants who received BCG maintenance was 61%. Although it appears not to have affected the number of participants in the PHOTO trial accessing BCG, there was a global BCG shortage during the study and it is difficult to characterise how much this may have affected the rates of early cystectomy we observed. Moreover, radical cystectomy removed a cohort of participants that had the highest risk of recurrence (up to 75%), further diluting the event rate in our final analysis population.
Low rates of progression
A limitation of the study that was recognised from the start, was its ability to characterise progression and, as a consequence, the primary measure focused on recurrence alone. Progression refers to a recurrence associated with an increase in stage (MIBC or metastatic disease), which represents a significant clinical change to life-threatening or palliative cancer and, therefore, is a priority area of research. Low rates of progression were predicted during the design of the study as, in BOXIT, only 10% of participants were seen to develop MIBC at 3 years. 53 As we appreciated that this event rate was likely to be low, we were aware that it was unlikely we would be able to make an appropriately powered comparison without a much larger number of patients and, therefore, progression was made a secondary outcome measure.
According to the EAU guideline that describes management primarily based on EORTC risk characteristics, the expected rate of progression from NMIBC in our study would be 4% for the intermediate-risk patients (i.e. EORTC score 2–6) and 11–30% for the high-risk patients (i.e. EORTC score 7–23). In our study, 4 out of 196 (2.04%) participants progressed in the intermediate-risk group and 22 out of 190 (11.58%) participants progressed in the high-risk group, at a median follow-up of 22 months. Although it remains difficult to make an inference with any certainty with a small number of events, the globally reduced rates of progression compared with the EORTC risk prediction probably relate to differences in modern NMIBC management involving routine second resection and intravesical BCG with maintenance for the high-risk cases. We observed a lower rate of progression than that of BOXIT; however, this difference may simply reflect that in the PHOTO trial a 6% rate was observed with a median follow-up of 22 months, whereas in BOXIT the 10% rate was recorded at 3 years. 53 Another factor that possibly relates to the improved outcomes is the inclusion of patients with NMIBC who received radical cystectomy in our final analyses. This differed from BOXIT, which excluded these patients. Nevertheless, based on both PHOTO and BOXIT, a rate of progression approaching 10% at 3 years is not a negligible event rate, especially when appreciating that cancer-specific survival is 50% at 3–5 years in localised MIBC and approaching 25% at 3–5 years in those with metastatic disease. It remains an area of research interest to describe patient-based preferences in the context of high-risk NMIBC and the options of primary cystectomy compared with intravesical therapy.
Strengths of the trial
Pragmatic study
The PHOTO trial was designed to be an effectiveness study. Efficacy measures performance of an intervention under ideal and controlled circumstances, whereas effectiveness refers to its performance under ‘real-world’ conditions. Therefore, we based the intervention in a pragmatic setting, embedding the trial technology of PDD-TURBT in the routine clinical pathway for the management of presumed new intermediate- and high-risk NMIBC based on flexible cystoscopy. This meant that we used only the available clinical parameters to inform a decision about the likely risk category and, therefore, those most likely to benefit from PDD-TURBT (intermediate- and high-risk cases). Although this would mean the inadvertent inclusion of false-positive diagnoses of higher-risk bladder cancer, it faithfully recreated the real-life situation in which the technology would be used. The inclusion criteria also pragmatically reflected those criteria that would mirror the broad demographic of patients normally presenting and undergoing routine diagnosis with typical investigations in haematuria clinics from a mix of larger teaching hospitals and smaller district general hospitals. Overall, 22 NHS centres were involved, providing a representative experience across the UK. In addition, minimal exclusion criteria were described, again aligned with routine clinical practice, making the patient population in the PHOTO trial very much representative of those seen in secondary care across the UK. The primary outcome measures were captured during routine, scheduled, follow-up bladder cancer surveillance, using routine measures for recurrence (standardised according to contemporary clinical guidelines18). This approach to the study design and conduct ensures the generalisability of our findings to real-life practice across the UK.
The tightly protocolised management seen in efficacy studies can demonstrate clear relationships between an intervention and a clinically important outcome. These data appear compelling, especially in examples where there are clear mechanistic data relating to the associated outcome, strengthening a causal effect. However, in real-life situations, these strict criteria and protocols are difficult to apply and the causal effect can become diluted. Effectiveness (i.e. pragmatic) studies aim to explore the day-to-day benefits that can be realistically expected. In efforts to recreate efficacy data in a pragmatic setting, a reduction of 50% in the effect size is typically seen in the pragmatic data compared with the efficacy data and where there is marginal gain, encroaching on a minimally important clinical difference, the value expected benefit of the intervention could be lost altogether. Given the finite funding resource for the NHS, real-life effect sizes and health economic analyses guide which technologies society is willing and able to afford. Herein lies a key strength of the PHOTO trial.
The use of contemporary, NHS-based estimates of recurrence event rates over those predicted by the EORTC risk tables
Based on the 2006 EORTC risk tables, we were able to calculate the likelihood of recurrence, with or without progression, for participants with intermediate- and high-risk NMIBC. The EORTC risk score is the most widely used and validated tool to predict risk of recurrence, having established the prognostic value of clinical and pathological factors analysed in participants randomised in seven studies between 1979 and 1989. 58 These tables predicted that, for those with disease categorised as intermediate risk (i.e. EORTC scores 1–9) and high risk (i.e. EORTC scores 10–17), recurrence could be expected at 3 years in 40–56% and 75% of participants, respectively. Given that, prospectively, at randomisation, we did not have the pathological details of the tumour (i.e. grade or stage) as these are only available following TURBT, we were unable to use all of the parameters described in the EORTC tables to distinguish between intermediate and high risk of recurrence and progression (i.e. number of tumours, size, grade, stage, presence of CIS and frequency of recurrence). Therefore, we originally planned a weighted prediction to consider a 2 : 1 capture of intermediate-risk to high-risk NMIBCs based on a previous publication. 59 Based on these assumptions, we might have expected a recurrence rate between 63% and 69%. However, we recognised that the EORTC risk score is based on 2596 patients in studies between 1979 and 1989 with associated historical management of the disease. 55 Of relevance to contemporary practice, < 10% of patients in the EORTC cohorts received an immediate instillation of chemotherapy after TURBT, only 7% were treated with BCG (all without maintenance), 21% of the patients did not receive any intravesical treatment and a second resection was not practised. Conscious of modern UK clinical practice, instead we referenced another contemporary clinical trial to predict the risk of recurrence in intermediate- and high-risk NMIBC: BOXIT. 53 In practice, our actual recurrence rate in the WL group was substantially lower than the EORTC risk prediction at 40% (n = 170/426) at 3 years, mirroring the experience from BOXIT. 53
Having used contemporary estimates of recurrence for our sample size calculations, we accurately predicted the true recurrence rate in our trial. This strengthens the validity of our findings, having been conducted in a sample size that would detect a minimally important clinical difference in reducing recurrence in the management of NMIBC. In addition, the final analyses for the PHOTO trial showed that the predicted 2 : 1 distribution ratio of intermediate- to high-risk cases also did not hold true. In the PHOTO trial, 184 (88.0%) and 190 (87.6%) participants had intermediate-risk disease (EORTC scores 1–9), and 17 (8.1%) and 15 (6.9%) participants had high-risk NMIBC (EORTC scores 10–17) in the PDD and WL groups, respectively. By contrast, the more recent NICE criteria, mainly based on expert opinion, categorised participants in the PHOTO trial in an approximately 1 : 1 ratio to the intermediate- and high-risk categories.
There appears to be discordance between historical data that informs guidelines and up-to-date practice, and an apparent difference between the characterisations of risk between the EAU and NICE guidelines. To facilitate risk-group assignment, treatment and follow-up recommendations, simplified EAU risk categories stratification based on the EORTC risk score for progression were introduced in 2013. 60 Compared with EORTC risk stratification, EAU categories reclassified 37.9% of patients into a higher-risk group of recurrence and 11.8% into a higher-risk group of progression, bringing its categorisation closer to that seen with the NICE guidance. More recently, we have seen further updates to the EAU prognostic risk groups, although they are still based on historical data. 61 The overestimation of the risk of recurrence and progression with the EORTC risk tables and EAU risk categories has been previously described, comparing this with data from the scoring model based on 1062 patients with intermediate- and high-risk NMIBC included in four Club Urológico Español de Tratamiento Oncológico (CUETO) trials. 62,63 For recurrence, the calculated risks using the CUETO scoring model were lower than those obtained with the EORTC risk tables. However, a limitation of the CUETO model is that second TURBT and immediate intravesical instillation of chemotherapy were not performed. In addition, the CUETO maintenance schedule (i.e. 12 instillations in 5–6 months) was considerably shorter than the 1–3 years of maintenance currently recommended for BCG by the EAU. 12
All of these predictive tools have limitations in their application to contemporary practice. 58 The data from up-to-date trials, such as PHOTO and BOXIT, could provide a new benchmark to guide current management decisions, updating or even replacing the clinical utility derived from EORTC/EAU/CUETO risk tables in current guidelines. 12,18
Preplanned statistical analyses considering effects of potential confounders
The PHOTO trial was a pragmatic study, with an ITT analysis, providing outcomes relating to the effects of PDD-TURBT in a real-life setting. Randomisation controlled for confounders between the two groups. However, to account for potential imbalances that may still have occurred between the two groups, subsequent analyses included predefined, per-protocol evaluations and adjustment for baseline variables (i.e. sex, centre, smoking status, risk group, presence/absence of CIS and grade of surgeon). There were no significant differences between the groups for these confounders and the final findings of these sequent analyses match those from the ITT analyses, providing a robust conclusion.
Competing risk of death analysis
A sensitivity analysis of the primary outcome included deaths as a competing risk. Bladder cancer occurrence is associated with age and, therefore, multiple competing risks of death. This analysis showed no effect on the results.
Completeness of data
There were no missing data for the primary outcome (i.e. recurrence), proving robust capture of the main measure of interest.
Health economic analysis
The main strength of the trial-based analysis was that a comprehensive costing approach was undertaken, further adding to the generalisability of results across participating centres and the NHS. The incorporation of a wider economic perspective on costs adds value in terms of a broader economic perspective and understanding of the non-health-care costs to patients, their families and the economy. The analysis of QALYs based on EQ-5D-3L patient-level responses followed best-practice methods and is another advantage.
As there were a number of missing data for cost and QALY outcomes, MI of missing cost and EQ-5D-3L data were conducted. Imputation did not alter the cost estimates from the analysis, but did alter the QALY outcomes substantially.
Learning curve
Effect of the photodynamic diagnosis experience
Those new to PDD can face technical issues related to using hardware and interpretation issues related to equivocal or false-positive fluorescence (e.g. from inflammation). Previous studies had suggested that surgeons new to PDD resection required approximately 20 cases to build experience to maximise diagnostic performance with this technology. 64,65 Accordingly, the PHOTO trial, as part of a prespecified analysis, looked to characterise and account for the potential effect of this in outcomes.
In the PHOTO trial, the effect of surgeons with previous PDD experience was compared with that of surgeons with experience of < 20 cases, who were defined as PDD naive. The outcomes for this comparison were (1) recurrence and (2) positive second resection for residual disease as a measure of complete first resection. Of 22 participating centres, four (17.4%) were classified as PDD naive. Overall, there was no evidence that the treatment effect was moderated by PDD-naive/PDD-experienced centres for either of the two outcome measures.
Evidence supporting PDD naivety affecting the ability to detect tumours when using blue-light cystoscopy is lacking; the main issue initially appears to be the detection and resection of false-positive lesions, not of missing a tumour. The phenomenon of reduced specificity was evident in a systematic review involving a total of 27 studies, enrolling 2949 participants and reporting PDD test performance. 27 In the pooled estimates for biopsy-level analysis, based on pathological confirmation, PDD had a significantly lower specificity than WLC (60%, 95% CI 49% to 71%, vs. 81%, 95% CI 73% to 90%). When surgeons start to use PDD, their surgical learning curve does not appear to affect the rate of recurrence. However, as there is no observed difference in the rate of second-resection positivity, the lack of change in recurrence rate does not seem to be related to the surgeons missing tumours because of technical naivety.
Although there appears to be convincing systematic review data (n = 27 studies; n = 2949 participants)27 using biopsy-level analysis to show that PDD has a higher sensitivity than WLC (93%, 95% CI 90% to 96%, vs. 65%, 95% CI 55% to 74%), this difference was not borne out in the PHOTO trial by reducing the rate of recurrence. An alternative explanation for this finding of no evidence of an effect may be that there is still an effect from PDD-TURBT, but that there is an associated improvement in the quality of WL resection that closes the difference in the respective event rates in each group, as explored below.
Exploration of a potential bystander effect
One possible explanation of centres reporting an initial added value of implementing PDD-TURBT, but reporting no lasting effect over time, is that practising PDD may make surgeons appreciate the shortcomings in their own ability, as well as the risk of missed tumours. This has two potential effects: by training the surgeon to (1) have a greater level of concern regarding more subtle lesions and (2) look more thoroughly, they appreciate the potential to miss lesions through experience with PDD. Ultimately, it has been speculated that this leads to a bystander effect where the quality of standard resection improves and, perhaps, accounts for observations in trials showing no difference in PDD compared with WL in highly PDD-experienced centres. 66 In the PHOTO trial, we actively planned to explore this phenomenon.
To test this hypothesis, we considered the effect on overall rates of recurrence based on surgeon’s experience of PDD. Participants whose surgeries were performed by surgeons with experience of > 40 PDD procedures had a lower risk of recurrence than participants whose surgeries were performed by surgeons with experience of < 10 PDD procedures (HR 0.60, 95% CI 0.40 to 0.92; p = 0.019). In both groups, > 50% of participants whose surgeries were performed by surgeons with experience of < 10 PDD procedures experienced a recurrence of bladder cancer. These data point to a role of PDD in improving WL-based detection and resection of NMIBC. Previously, there was a case for experience improving recurrence outcomes with TURBT, with consultants providing better outcomes than trainees. 67 The ability to improve rates of recurrence, related to PDD experience, implies that there is room to improve TURBT performance. Of note is the fact that, in sites where quality performance indicators for TURBT were implemented, no differences were observed between grades of surgeons. 68 The data from the PHOTO trial, which involved centres across the UK, provided a generalisable snapshot of TURBT outcomes and showed that improvements in TURBT are possible. These data highlight a role for better training, which could involve mandating exposure to > 40 cases of PDD-TURBT as a quality-assurance measure.
Implications for health care
Recommendation for the disinvestment in photodynamic diagnosis-guided transurethral resection of bladder tumour
A number of previous randomised trials and systematic reviews showed increased sensitivity in the detection of NMIBC with PDD that translated into a meaningful reduction in bladder cancer recurrence. 10,11,69–73 The included trials had differing protocols, such as use of immediate postoperative intravesical chemotherapy, second resections or approaches to adjuvant intravesical treatments (i.e. chemotherapy and BCG), making it difficult to extrapolate these findings to current practice. 11 Furthermore, differences in inclusion criteria, trial design and outcome measurement limit accurate meta-analytical comparison. 11 Nevertheless, these previous data had led to the uptake of PDD technology across the UK, showing added value in reducing recurrence in ‘real-life’ practice when comparing cohorts treated with PDD and those treated with high-quality WL-TURBT. 73,74 Similarly, there was uptake across Europe and the USA, where strong expert recommendations were made on both the reduction of recurrence and health economic evaluations. 75–78 However, to further substantiate the recommendations from efficacy trials and the subsequent non-randomised reporting of ‘real-life’ practice, a pragmatic randomised trial was required, taking into account current standard treatment approaches, including immediate postoperative intravesical chemotherapy, second resections or adjuvant intravesical treatments.
The PHOTO study addresses the outstanding questions outlined above, providing clear and strong evidence that, in primary intermediate- and high-risk NMIBC managed in the day-to-day UK setting, there is no reduction in recurrence at 3 years with PDD resection compared with standard-of-care WL resection. The main aim of this trial was to provide a precise, unbiased measure of benefit for PDD-TURBT in reducing recurrence and it was rigorously designed accordingly. The highest quality evidence regarding clinical effectiveness is essential, as systematic reviews informing clinical guidance recommendations are affected by the risk of bias and low methodological quality of previous trials. The PHOTO trial findings do not support the use of PDD resection for primary intermediate- and high-risk NMIBC.
Up-to-date information for clinicians, patients and service providers on current treatments for non-muscle-invasive bladder cancer, their outcomes and costs
Although guideline recommendations are designed to use evidence-based medicine to instruct best clinical practice, their implementation can be patchy. Some of the reasons for this are that there can be limitations in the quality of the information available to inform guidelines or that the recommendations are not acceptable/relatable to current clinical practice. 79,80 For bladder cancer guidelines, such as those of NICE,18 EAU12 and the American Urological Association (AUA),59 we have an excellent resource, comprising authoritative and comprehensive systematic reviews, meaning that advice is generally consistent across these major guidelines and is well accepted. 81,82 Despite the outstanding standard of these guidelines, questions remain about the expected outcomes in treating NMIBC and they are essential to counselling patients regarding options and outcomes. This is mainly because there is a strong reliance on historic EORTC data to inform NMIBC management, despite recent efforts to update the prognostic risk groups. 61 Here, we can now provide new up-to-date data based on contemporary management and outcomes of NMIBC in accordance with current NICE18 and EAU guidance. 12 These new data are further explored below.
Intravesical treatments
In the PHOTO trial, participants were managed as per routine clinical practice in accordance with guidelines (EAU12/NICE18) and, therefore, the data provide an accurate characterisation of what can be expected, not only in terms of clinical outcomes, but also compliance with treatment and follow-up. In addition, there are areas of guideline advice where the recommendations are not prescriptive, such as the choice and duration of intravesical treatment in intermediate-risk disease; however, in the new iteration of the guidelines, this has been tightened, albeit still with a choice to be made between chemotherapy and BCG. 60,83 Specifically, new data of interest include the choices made between intravesical chemotherapy and BCG, and the specific regimes of adjuvant intravesical chemotherapy prescription (i.e. six doses vs. maintenance) and BCG (i.e. induction with six doses, followed by 1 year or 3 years of maintenance treatment) and how these compare with guideline recommendations, where these are clearly described. From our work with the PHOTO trial, we have seen that there is good uptake of the guideline-recommended practice of an adjuvant single dose of intravesical chemotherapy (i.e. MMC in our cohort). When compared by risk group, MMC was administered in 67.4% of participants in the intermediate-risk group and 40.6% of participants in the high-risk group (Table 17). There were well documented features of deep resection, or other clinical contraindications or justifications, that limited higher rates of administration. There is EAU guidance for the management of tumours with visual features of high-risk or suspected MIBC, in which immediate single dose intravesical chemotherapy can be omitted as further treatments may be required, including BCG and courses of chemotherapy, making one dose of chemotherapy redundant. 83 The rates of BCG treatment were consistent with other contemporary series, such as BOXIT. 53 There may have been contraindications or a preference-based judgement that limited even higher uptake, as the treatment has a high risk of morbidity, with significant lower urinary tract symptoms. Some researchers have reported a major discrepancy between the care provided by urologists in daily practice and that described in the EAU guidelines in Europe-wide surveys. 84,85 However, given the higher rates in the use of MMC, second resections, and initiated BCG that we report here, it appears that UK clinicians are following guidelines better than most and that there are patient-related factors, such as side effects of treatment, that may be more significant than clinical considerations. Historically, compliance with BCG has been poor: in the Southwest Oncology Group (SWOG) trial’s maintenance protocol, after induction, only 10% of patients completed all 21 treatments86 and, in the EORTC 30911 study, up to 29% of patients completed all 36 months of treatment. 87 Similar patterns of compliance with BCG with increasing treatment duration were seen in the PHOTO trial, mirroring the phenomenon also reported in BOXIT. 53 Increasing patient compliance with BCG has been a priority area of research interest, with studies exploring the effects of (1) reduced dosage and (2) reduced duration to minimise side effects and improve tolerability. 88,89 However, these studies show no change in side effects and a diminished efficacy. The PHOTO study provides realistic expectations of what can be delivered; despite guidelines’ recommendations, these factors are predominantly related to patient tolerability rather than a lack of clinician compliance with the high-quality evidence that supports guideline advice.
Recurrence, progression and survival in patients with non-muscle-invasive bladder cancer
In addition, as already discussed, the PHOTO trial showed lower rates of recurrence than those predicted with EAU/EORTC risk assessment tools, providing a modern-day snapshot of outcomes in intermediate- and high-risk NMIBC. These outcomes point towards an improvement in the management of NMIBC over the past couple of decades, mostly likely through multiple marginal gains across the diagnostic and treatment pathway. This appears to translate to an improved rate of cancer-specific mortality of 3% (13/426), compared with systematic reviews of intermediate- and high-risk NMIBC from seven trials involving 1880 patients showing a rate of 7% at a median follow-up of 4.8 years. 76 This finding is also consistent with the recent BOXIT, showing a mortality rate of 4% at 3 years. 53 However, high-risk NMIBC remains a concern, where a systematic review of 19 trials, with a total of 3088 patients, showed 21% of patients progressing to MIBC and 14% of patients dying of bladder cancer, with median follow-up ranging from 48 to 123 months. 90 This translated into a long-term cancer-specific survival rate of 35% for patients with high-risk NMIBC and tumour progression.
Safety and morbidity in the management of non-muscle-invasive bladder cancer
Overall, TURBT was well tolerated, with only 1–2% of individuals experiencing a major complication (i.e. Clavien–Dindo grade III). There were no significant differences in the WL-TURBT and PDD-TURBT outcomes; nevertheless, AEs were reported in 22% of patients (Clavien–Dindo grades I and II). The data, with the frequency of specific events, provide an excellent reference for informed consent for patients undergoing TURBT and information leaflets for patients awaiting surgery. The most common issues were haematuria and bladder discomfort/pain. (see Appendix 1, Table 37). In addition, looking at the HRQoL outcomes, there were substantial effects across the domains measured immediately post operation, but these recovered, with a generalised trend of all domains returning to baseline thereafter. Interestingly, regarding longer term effects, the PROMs looking specifically at bladder-related effects in the EORTC-QLQ-NMIBC-24 HRQoL questionnaires showed persistent issues extending to at least 3 years. These were most likely related to intravesical treatments.
Future research implications
Other clinical utility for photodynamic diagnosis in non-muscle-invasive bladder cancer
Despite our findings in the specific context of the trial question, the role for PDD-TURBT in other contexts remains an open question. We have shown that PDD-TURBT is safe and well tolerated, with rates of complication and AEs that are no different from conventional WL-TURBT. The current EAU guideline12 recommends PDD in certain clinical situations, graded as strong recommendations, which remain areas for future research to generate more substantial evidence to support their role. The EAU guideline describes taking biopsies from visually abnormal regions and normal-looking epithelium when urine cytology is positive, non-papillary tumours are seen or there is a previous history of high-grade cancers. The guideline states that, if available, PDD-guided biopsies should be taken. In cases where mapping biopsies are indicated, such as those with positive cytology and normal-looking cystoscopy with no upper-tract urothelial cancer, the EAU recommends that PDD-guided biopsies can be used instead of mapping biopsies. Similarly, in the follow-up of patients with normal cytoscopy but positive cytology, a PDD-guided biopsy, where available, can be used instead of a mapping biopsy.
The next generation of cystoscopic bladder imaging with or without novel photosensitisers
The accurate detection of bladder tumours remains a major clinical need and an active research focus. Future research regarding light technologies (e.g. narrow-band imaging, multispectral imaging, optical coherence tomography and artificial intelligence-enhanced visualisation) and the next generation of photosensitiser technology remain of interest to improve the detection and staging of NMIBC. 91–95
Rapid biomarker assessment
The PHOTO trial’s health economic model contains up-to-date UK costs associated with the NMIBC management pathway. This model establishes diagnostic and cost thresholds that could be presented to make a case for NICE guideline implementation and could inform rapid assessment of new biomarkers to replace cystoscopy for diagnosis or more accurate and/or effective approaches to resection (e.g. adjuvant treatments such as immuno-oncology, or novel imaging or resection approaches such as en bloc laser resection).
There are a number of established, commercially available urinary biomarkers [e.g. BTA STAT® (Polymedco Inc., Cortlandt, NY, USA), BTA TRAK® (Polymedco Inc.), Alere NMP22 BladderCheck® (Abbott Molecular, Abbott Park, IL, USA), ImmunoCyt/UCyt+™ test (Diagnocure Inc., Quebec City, Canada) and UroVysion Bladder Cancer Kit™ (Abbott Molecular)] and a plethora of emerging next-generation ‘omic’-based tests that include urinary deoxyribonucleic acid (DNA) and proteomic reads. 96 Rapid urinary biomarker assessment for the presence of bladder cancer followed by cystoscopy and resection for positive biomarker cases could be a cheaper, but possibly less accurate, method for diagnosing NMIBC. Therefore, there is uncertainty in the cost-effectiveness of this diagnostic approach. False-negative results from rapid biomarker tests could result in a delay in diagnosis until the cancer had progressed, as the cancer would not be identified until either an opportunistic retest or the patient became symptomatic. The cost-effectiveness of a rapid biomarker test in this role would depend on the proportion of cystoscopies that could be avoided by using the biomarker test. The PHOTO trial’s health economics model could, potentially, be adapted to evaluate the cost-effectiveness of a rapid biomarker test in this role and the budget impact of a cheaper diagnostic approach. Evidence of the diagnostic accuracy of a rapid biomarker test in this role compared with cystoscopy would be required. Evidence on the stage of bladder cancer for symptomatic and asymptomatic evidence would also be required. If the cancer stage remains non-invasive when the patient becomes symptomatic, then evidence on the time to recurrence for symptomatic compared with asymptomatic NMIBC patients would also be useful to characterise. For any rapid biomarker test to be cost-effective, the risk of a missed diagnosis and the delay to diagnosis would need to be low. Some of these data for specific tests are emerging in the literature and would be of interest to explore in the PHOTO trial’s health economic model in future work.
Modelling alternative surveillance strategies
The cost-effectiveness of different cystoscopic surveillance schedules, possibly in combination with the rapid biomarker tests discussed in the preceding section, could also be evaluated as alternatives to the current surveillance schedule approach by adapting the PHOTO trial’s health economics model. The current schedule for cystoscopy follow-up is based on expert recommendation, relating to the observation that most recurrences occur early, which is apparent in the sharp drop-off shape of the Kaplan–Meier curves for recurrence-free survival presented in this report. There are now emerging data looking at conditional probabilities for recurrence, where the longer an individual goes without recurrence, the more likely they are to avoid recurrence. 97 These data, along with our contemporary recurrence-free survival and those of other up-to-date studies, such as BOXIT, could lay the foundation of a ‘lighter’, dynamic, risk-adaptive follow-up protocol that could produce savings in the massive finance burden currently incurred with cystoscopy surveillance.
In future research, the rapid biomarker test could be evaluated to replace cystoscopy either at every time point in the surveillance schedule or at some time points (e.g. every other time point). Once again, the rapid biomarker test surveillance strategy could be cheaper than the cystoscopy surveillance strategy, but the delayed diagnosis in some cases would result in worse health outcomes for some patients. For the rapid biomarker test to be cost-effective, the risk of a missed diagnosis would need to be low and the delay to diagnosis would need to be small. This analysis would require similar evidence to that described above, although it is possible that the diagnostic accuracy of the rapid biomarker test may differ during surveillance from the accuracy at initial diagnosis.
Updated non-muscle-invasive bladder cancer recurrence, progression and survival prediction tools
As discussed above, the tools currently available for predicting clinical outcomes are reliant on historical data and appear to overestimate recurrence, progression and survival, as shown in our PHOTO trial data. Previous assessments of EORTC prognostic groups performance have been tested in large, external, Spanish and US data sets. 63,98 Data on 1062 patients with NMIBC treated with BCG in the CUETO clinical trials were analysed, showing that the EORTC groups successfully stratified recurrence and progression risks, but overestimated risks of recurrence and progression after BCG therapy. 63 Interestingly, the CUETO scoring system exhibited similarly poor discrimination for recurrence and progression. 99 These overestimations remained in BCG-treated patients, especially for the EORTC tables. External validation of the EORTC risk calculator, in comparison to the National Comprehensive Cancer Network (NCCN) risk groups, was also undertaken in a contemporary US population of 1491 NMIBC patients; it showed that there is overestimated progression among the highest-risk group, which is consistent with prior publications. 98 Additional studies of the EORTC tables’ performance have included a comparison with the simplified, treatment-directed, EAU risk group stratification (which are also based on the EORTC tables) in a multi-institutional database of 5122 patients. The EAU categories reclassified 37.9% of patients into a higher risk group for recurrence and 11.8% of patients into a higher risk group for progression, but, pragmatically, assigned most patients to the same treatment recommendations.
The main issues identified with these established tools were that none used data that reflected current standards of treatment, and recommendations were made to update scoring models with previously unavailable data. 58 In response, updated EAU prognostic factor risk groups were defined from 3401 retrospectively collected individual patient’s data for primary NMIBC patients from the institutions of members of the guidelines panel. 61 Multivariable Cox proportional-hazards regression models were fitted to the primary end point: the time to progression to muscle-invasive disease or distant metastases. A new, very high-risk group was identified and lower risks were assigned for progression to the remaining low, intermediate and high groups. Herein lies an opportunity to combine data from contemporary, prospective, randomised trials (e.g. PHOTO, BOXIT53 and more recent EORTC trials addressing BCG treatment in higher risk individuals100) to generate a more robust prediction tool. Models including competing risk of death would also provide utility when making clinical judgements regarding treatments with a high morbidity rate, such as early cystectomy.
Translational projects accessing the tissue biobank (PHOTO-T project)
Previously, experts have reported accurate visual diagnosis of cancerous compared with benign tissue, and reliable prediction of the stage and grade of cancer; however, in our ‘real-world’ pragmatic study, visual diagnosis was not shown to be reliable using cystoscopy alone. New, more accurate approaches involving biomarkers could be developed for this assessment and be based on urine containing cancer cells that have shed from the tumour or cellular contents [i.e. DNA, ribonucleic acid (RNA) or protein]. There is already a growing body of evidence providing diagnostic tools and the ability to distinguish NMIBC from MIBC. 96 In addition, these tools could have a role in earlier diagnosis, staging and expedited management of bladder cancer. To accelerate the development of such biomarkers, well-annotated tissue biobanks with detailed clinic-pathological data are required; this formed the rationale for archiving urine, blood, and bladder tissue from consenting PHOTO trial participants as part of the PHOTO-T project.
There are a number of separately funded translational projects currently accessing the PHOTO-T archive, either (1) developing new biomarkers {e.g. using tumour-specific mitochondrial deoxyribonucleic acid (mtDNA) mutations (Biomedical Research Centre funded) and exploring urinary volatile agents for diagnosis [Cancer Research UK (CRUK) funded]} or (2) providing external validation of diagnostic markers [e.g. Medical Research Council (MRC)- and CRUK-funded projects looking at urinary DNA methylation and RNA markers]. In the future, other projects may look to access this resource for new discovery or validation work.
Enhanced training in cystoscopy
In the PHOTO trial, we saw an interesting phenomenon in which the use of PDD in previously PDD-naive centres saw a reduction of cancer recurrence in their WL-treated participants. This bystander effect of PDD improving WL detection of tumour and its resection requires further exploration. If this effect is confirmed, PDD may provide a tool for enhancing the clinical training of junior urologists, producing a better standard of WL resection for the next generation of urologists and patents.
Long-term outcome data collection
The PHOTO study has detailed clinical outcomes and costs based on 3 years’ follow-up, and extended those analyses to include a modelled lifetime projection for the health economic assessments. Ideally, we would like to collect longer-term outcomes to more accurately inform our lifetime model. Initially, we had proposed linkage of the trial data with the national British Association of Urological Surgeons (BAUS) Oncology Data and Audit registry101 (Nuvola) over the next 10 years to further inform the HTA model; however, this registry is now being dissolved. There are ongoing discussions within BAUS to establish mechanisms for future procedure and outcomes data. Possible ‘snapshot’ audits, in which centres look at longer-term outcomes for patients and that could include a focus on those enrolled in the PHOTO trial, have been proposed by the BAUS Audit Steering Group. It is most likely that separate grant applications will look to update the HTA model with longer-term data in time, aiming to link to Public Health England (PHE)-maintained National Cancer Registration and Analysis Service (NCRAS). These longer-term data would further support the PHOTO trial’s health economic model in making more accurate 5–10 year predictions for changes to improve the NMIBC pathway.
Conclusions
The PHOTO trial found no evidence of an improvement in clinical effectiveness associated with PDD. The CEA demonstrated that PDD was not more cost-effective than WL at 3 years. Overall, the use of PDD-TURBT is not supported in the management of primary intermediate-high risk NMIBC.
Acknowledgements
The authors wish to thank the patients who participated in the PHOTO trial.
We particularly acknowledge the late Rob Pickard for his contribution to the inception and design of the trial.
We also wish to thank:
-
Helen Green (ICR-CTSU) for her secretarial support
-
the CHaRT programming team, led by Gladys McPherson (until 2016) and Mark Forrest (2016–present)
-
Lorna Henderson (CHaRT), who was involved in the statistical analysis of the trial until 2018
-
Jing Shen (Newcastle), who was involved in the health economic analysis until 2019
-
Laura Wilson for supporting the PHOTO-T study (2019 to present)
-
other staff in the CHaRT/Health Services Research Unit for their assistance with the trial (Lorna Aucott, Maria Ntessalen and Rebecca Bruce)
-
members of the TMG for their ongoing advice and support of the trial
-
the independent members of the TSC and DMC
-
the staff at the recruitment centres who facilitated the recruitment, treatment and follow-up of trial participants.
Finally, we would like to thank NIHR and the HTA programme for funding the PHOTO trial.
Trial Management Group
Rakesh Heer (chairperson); grant holders Joanne Cresswell, Emma Hall, John Kelly, Henry Lazarowicz, Param Mariappan, John McGrath, Hugh Mostafid, Ghulam Nabi, James N’Dow, John Norrie, Craig Ramsay, Zafer Tandogdu, Ernest Taylor and Luke Vale; and study team Angela Allan, Matthew Breckons, Karen Campbell, Louise Campbell, Anne Duncan, Andy Feber, Rebecca Lewis, Graeme Maclennan, Alison McDonald, Steven Penegar, Stephen Rice, Laura Wilson and Ge Yu.
Transient service on the TMG by Anthony Koupparis and Rhidian Hurle is also acknowledged.
Independent members of the Trial Steering Committee
Peter Whelan (chairperson), Allen Knight, Per-Uno Malmstrom, Dan Sjoberg and Andrew Vickers.
Independent members of the Data Monitoring Committee
Angela Casbard (chairperson), Robert Mills, Paul Silcocks, Edward Wilson and Diane Witham.
Recruitment sites
We would like to thank the staff and participants at the following centres:
-
NHS Grampian (Aberdeen, UK) – Sarfraz Ahmad (2015–19) and Justine Royle (PI)
-
Royal Free London NHS Foundation Trust (London, UK) – Paras Singh (PI)
-
Hampshire Hospitals NHS Foundation Trust (Basingstoke, UK) – Hugh Mostafid (PI)
-
Hull University Teaching Hospitals NHS Trust (Hull, UK) – Mathew Simms (PI)
-
Imperial College Healthcare NHS Trust (London, UK) – Norma Gibbbons (PI)
-
Oxford University Hospitals NHS Foundation Trust (Oxford, UK) – Jeremy Crewe (PI)
-
Dartford and Gravesham NHS Trust (Dartford, UK) – Sanjeev Madaan (PI)
-
Newcastle Hospitals NHS Foundation Trust (Newcastle upon Tyne, UK) – Andrew Thorpe (PI)
-
South Tees Hospitals NHS Foundation Trust (Middlesbrough, UK) – Joanne Cresswell (PI)
-
East and North Hertfordshire NHS Trust (Stevenage, UK) – Nikhil Vasdev (PI)
-
Swansea Bay University Health Board (Swansea, UK) – Jonathan Featherstone (2015–16) and Pradeep Bose (PI)
-
NHS Tayside (Dundee, UK) – Ghulam Nabi (PI)
-
Cwm Taf Morgannwg University Health Board (Bridgend, UK) – Rhidian Hurle (PI)
-
Derby Teaching Hospitals NHS Foundation Trust (Derby, UK) – Amjad Peracha (PI)
-
Royal Devon and Exeter NHS Foundation Trust (Wonford, UK) – John McGrath (PI)
-
University Hospitals of North Midlands NHS Trust (Stoke-on-Trent, UK) – Lyndon Gommersall (PI)
-
Salisbury NHS Foundation Trust (Salisbury, UK) – Melissa Davies (PI)
-
University Hospital Southampton NHS Foundation Trust (Southampton, UK) – Bhaskar Somani (PI)
-
Leeds Teaching Hospitals NHS Trust (Leeds, UK) – Sunjay Jain (PI)
-
Ashford and St Peter’s Hospitals NHS Foundation Trust (Ashford, UK) – Sachin Agrawal (PI)
-
University College London Hospitals NHS Foundation Trust (London, UK) – Mark Feneley (PI)
-
NHS Lothian (Edinburgh, UK) – Param Mariappan (PI).
Contributions of authors
Rakesh Heer (https://orcid.org/0000-0003-1952-7462) (Professor and Chief Investigator) contributed to the conception and design of the trial, the conduct of the trial, the interpretation of the results and the writing/editing of the report.
Rebecca Lewis (https://orcid.org/0000-0001-9222-5263) (Clinical Trials Programme Manager, Triallist) was responsible for oversight of the day-to-day management of the trial and contributed to the design of the trial, the interpretation of the results and the writing/editing of the report.
Anne Duncan (https://orcid.org/0000-0001-7644-006X) (Trial Manager, Triallist) was responsible for the day-to-day management of the trial and contributed to the interpretation of the data and the writing/editing of the report.
Steven Penegar (https://orcid.org/0000-0002-3768-0236) (Trial Manager, Triallist) was responsible for the day-to-day management of the trial and contributed to the interpretation of the data and the writing/editing of the report.
Thenmalar Vadiveloo (https://orcid.org/0000-0001-5531-6289) (Statistician, Triallist) conducted the statistical analyses and contributed to the interpretation of the data and the writing/editing of the report.
Emma Clark (https://orcid.org/0000-0003-0065-1463) (Translational Researcher) contributed to the design of the PHOTO-T study, oversaw the day-to-day management and follow-up of samples for the PHOTO-T study, and contributed to the writing/editing of the report.
Ge Yu (https://orcid.org/0000-0002-0891-2501) (Senior Research Associate, Health Economist) conducted the economic analyses and contributed to the interpretation of the data and the writing/editing of the report.
Paramananthan Mariappan (https://orcid.org/0000-0001-8221-4640) (Consultant Urologist) contributed to the design and conduct of the trial, the recruitment and follow-up of participants, the interpretation of the results and the writing/editing of the report.
Joanne Cresswell (https://orcid.org/0000-0003-1699-0339) (Consultant Urologist) contributed to the design and conduct of the trial, the recruitment and follow-up of participants, the interpretation of the results and the review of the report.
John McGrath (https://orcid.org/0000-0001-9416-9912) (Consultant Urologist) contributed to the design and conduct of the trial, the recruitment and follow-up of participants, the interpretation of the results and the review of the report.
James N’Dow (https://orcid.org/0000-0001-5340-0081) (Professor, Consultant Urologist) contributed to the design of the trial, the interpretation of the results and the writing/editing of the report.
Ghulam Nabi (https://orcid.org/0000-0001-9406-5195) (Professor, Consultant Urologist) contributed to the design and conduct of the trial, the recruitment and follow-up of participants, the interpretation of the results and the writing/editing of the report.
Hugh Mostafid (https://orcid.org/0000-0002-5095-2929) (Consultant Urologist) contributed to the design and conduct of the trial, the recruitment and follow-up of participants, the interpretation of the results and the writing/editing of the report.
John Kelly (https://orcid.org/0000-0002-7036-1923) (Professor, Consultant Urologist) contributed to the design and conduct of the trial, the interpretation of the results and the writing/editing of the report.
Craig Ramsay (https://orcid.org/0000-0003-4043-7349) (Professor, Statistician, Triallist) contributed to the conception and design of the trial, the conduct of the trial, the statistical analysis, the interpretation of the results and the writing/editing of the report.
Henry Lazarowicz (https://orcid.org/0000-0003-0792-2983) (Consultant Urologist) contributed to the design and conduct of the trial, the interpretation of the results and the writing/editing of the report.
Angela Allan (https://orcid.org/0000-0001-7410-5778) (Research Nurse) contributed to the conduct of the trial, the recruitment and follow-up of participants, the interpretation of the results and the review of the report.
Matthew Breckons (https://orcid.org/0000-0003-3057-6767) (Research Associate, Health Economist) contributed to the conduct of the trial, the economic analyses, the interpretation of the data and the writing/editing of the report.
Karen Campbell (https://orcid.org/0000-0003-1215-8991) (Assistant Trial Manager) was responsible for the day-to-day management of the trial and contributed to the interpretation of the data and the writing/editing of the report.
Louise Campbell (https://orcid.org/0000-0002-6440-6486) (Trial Data Coordinator) was responsible for the day-to-day management of the trial and contributed to the interpretation of the data and the review and approval of the report.
Andy Feber (https://orcid.org/0000-0001-5282-0498) (Senior Lecturer, Translational Researcher) oversaw the day-to-day management and follow up of samples for the PHOTO-T study, and contributed to the conduct of the trial and the review of the final report.
Alison McDonald (https://orcid.org/0000-0002-0256-2889) (Trial Manager, Triallist) contributed to the design and conduct of the trial and provided commentary on the draft chapters of the report.
John Norrie (https://orcid.org/0000-0001-9823-9252) (Professor, Statistician, Triallist) contributed to the conception, design and conduct of the trial, the interpretation of the results and the review of the report.
Giovany Orozco-Leal (https://orcid.org/0000-0002-7473-7318) (Research Assistant, Health Economist) contributed to the design of the health economic analysis and the interpretation of the results.
Stephen Rice (https://orcid.org/0000-0002-6767-0813) (Senior Research Associate, Health Economist) supervised the economic analysis and contributed to the interpretation of results and the writing of the report.
Zafer Tandogdu (https://orcid.org/0000-0002-5309-3656) (Specialist Urologist) contributed to the conception, design and conduct of the trial, the interpretation of the results and the review of the report.
Ernest Taylor (Patient Representative) contributed to the conduct of the trial, the interpretation of the results and the review of the report.
Laura Wilson (https://orcid.org/0000-0002-7919-2230) (Research Technician, Translational Researcher) oversaw the day-to-day management and follow-up of samples for the PHOTO-T study and contributed to the writing/editing of the report.
Luke Vale (https://orcid.org/0000-0001-8574-8429) (Professor, Health Economist) contributed to the conception, design and conduct of the trial, the interpretation of the results and the review of the report.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (Professor, Statistician and Triallist) contributed to the conduct of the trial, the statistical analysis, the interpretation of the results and the writing/editing of the report.
Emma Hall (https://orcid.org/0000-0001-5999-5020) (Professor, Statistician and Triallist) contributed to the conception, design and conduct of the trial, the interpretation of the results and the writing/editing of the report.
Publications
Tandogdu Z, Lewis R, Duncan A, Penegar S, McDonald A, Vale L, et al. Photodynamic versus white light-guided treatment of non-muscle invasive bladder cancer: a study protocol for a randomised trial of clinical and cost-effectiveness. BMJ Open 2019;9:e022268.
Heer R, Lewis R, Vadiveloo T, Yu G, Mariappan P, Cresswell J, et al. Randomized trial of PHOTOdynamic surgery in non-muscle-invasive bladder cancer. NEJM Evid 2022;1:10.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Tandogdu Z, Lewis R, Duncan A, Penegar S, McDonald A, Vale L, et al. Photodynamic versus white light-guided treatment of non-muscle invasive bladder cancer: a study protocol for a randomised trial of clinical and cost-effectiveness. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-022268.
- Cancer Research UK . Bladder Cancer Statistics 2020. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bladder-cancer (accessed June 2021).
- Aveyard P, Adab P, Cheng KK, Wallace DM, Hey K, Murphy MF. Does smoking status influence the prognosis of bladder cancer? A systematic review. BJU Int 2002;90:228-39. https://doi.org/10.1046/j.1464-410X.2002.02880.x.
- Puente D, Hartge P, Greiser E, Cantor KP, King WD, González CA, et al. A pooled analysis of bladder cancer case-control studies evaluating smoking in men and women. Cancer Causes Control 2006;17:71-9. https://doi.org/10.1007/s10552-005-0389-0.
- Mistry M, Parkin DM, Ahmad AS, Sasieni P. Cancer incidence in the United Kingdom: projections to the year 2030. Br J Cancer 2011;105:1795-803. https://doi.org/10.1038/bjc.2011.430.
- Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 1998;22:1435-48. https://doi.org/10.1097/00000478-199812000-00001.
- Edwards TJ, Dickinson AJ, Natale S, Gosling J, McGrath JS. A prospective analysis of the diagnostic yield resulting from the attendance of 4020 patients at a protocol-driven haematuria clinic. BJU Int 2006;97:301-5. https://doi.org/10.1111/j.1464-410X.2006.05976.x.
- Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000;163:524-7. https://doi.org/10.1016/S0022-5347(05)67916-5.
- Bryan RT, Collins SI, Daykin MC, Zeegers MP, Cheng KK, Wallace DM, et al. Mechanisms of recurrence of Ta/T1 bladder cancer. Ann R Coll Surg Engl 2010;92:519-24. https://doi.org/10.1308/003588410X12664192076935.
- Brausi M, Collette L, Kurth K, van der Meijden AP, Oosterlinck W, Witjes JA, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol 2002;41:523-31. https://doi.org/10.1016/S0302-2838(02)00068-4.
- Mowatt G, N’Dow J, Vale L, Nabi G, Boachie C, Cook JA, et al. Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: systematic review and meta-analysis. Int J Technol Assess Health Care 2011;27:3-10. https://doi.org/10.1017/S0266462310001364.
- European Association of Urology . Non-Muscle-Invasive Bladder Cancer 2021. https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/ (accessed June 2021).
- Sylvester RJ, Oosterlinck W, van der Meijden APM. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol 2004;171:2186-90. https://doi.org/10.1097/01.ju.0000125486.92260.b2.
- Sylvester RJ, Oosterlinck W, Witjes JA. The schedule and duration of intravesical chemotherapy in patients with non-muscle-invasive bladder cancer: a systematic review of the published results of randomized clinical trials. Eur Urol 2008;53:709-19. https://doi.org/10.1016/j.eururo.2008.01.015.
- Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette–Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol 2002;168:1964-70. https://doi.org/10.1097/01.ju.0000034450.80198.1c.
- Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette–Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol 2003;169:90-5. https://doi.org/10.1097/01.ju.0000039680.90768.b3.
- Shelley MD, Court JB, Kynaston H, Wilt TJ, Fish RG, Mason M. Intravesical bacillus Calmette–Guerin in Ta and T1 bladder cancer. Cochrane Database Syst Rev 2000;4. https://doi.org/10.1002/14651858.CD001986.
- National Institute for Health and Care Excellence (NICE) . Bladder Cancer: Diagnosis and Management 2015. www.nice.org.uk/guidance/ng2 (accessed 11 June 2021).
- Mariappan P, Smith G. A surveillance schedule for G1Ta bladder cancer allowing efficient use of check cystoscopy and safe discharge at 5 years based on a 25-year prospective database. J Urol 2005;173:1108-11. https://doi.org/10.1097/01.ju.0000149163.08521.69.
- Sangar VK, Ragavan N, Matanhelia SS, Watson MW, Blades RA. The economic consequences of prostate and bladder cancer in the UK. BJU Int 2005;95:59-63. https://doi.org/10.1111/j.1464-410X.2005.05249.x.
- Leal J, Luengo-Fernandez R, Sullivan R, Witjes JA. Economic burden of bladder cancer across the European Union. Eur Urol 2016;69:438-47. https://doi.org/10.1016/j.eururo.2015.10.024.
- Porter MP, Penson DF. Health related quality of life after radical cystectomy and urinary diversion for bladder cancer: a systematic review and critical analysis of the literature. J Urol 2005;173:1318-22. https://doi.org/10.1097/01.ju.0000149080.82697.65.
- Tan WS, Teo CH, Chan D, Ang KM, Heinrich M, Feber A, et al. Exploring patients’ experience and perception of being diagnosed with bladder cancer: a mixed-methods approach. BJU Int 2020;125:669-78. https://doi.org/10.1111/bju.15008.
- Yoshimura K, Utsunomiya N, Ichioka K, Matsui Y, Terai A, Arai Y. Impact of superficial bladder cancer and transurethral resection on general health-related quality of life: an SF-36 survey. Urology 2005;65:290-4. https://doi.org/10.1016/j.urology.2004.09.050.
- Zaak D, Karl A, Knüchel R, Stepp H, Hartmann A, Reich O, et al. Diagnosis of urothelial carcinoma of the bladder using fluorescence endoscopy. BJU Int 2005;96:217-22. https://doi.org/10.1111/j.1464-410X.2005.05604.x.
- Marti A, Jichlinski P, Lange N, Ballini JP, Guillou L, Leisinger HJ, et al. Comparison of aminolevulinic acid and hexylester aminolevulinate induced protoporphyrin IX distribution in human bladder cancer. J Urol 2003;170:428-32. https://doi.org/10.1097/01.ju.0000075054.38441.2d.
- Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths TR, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14040.
- Stenzl A, Penkoff H, Dajc-Sommerer E, Zumbraegel A, Hoeltl L, Scholz M, et al. Detection and clinical outcome of urinary bladder cancer with 5-aminolevulinic acid-induced fluorescence cystoscopy: a multicenter randomized, double-blind, placebo-controlled trial. Cancer 2011;117:938-47. https://doi.org/10.1002/cncr.25523.
- Stenzl A, Burger M, Fradet Y, Mynderse LA, Soloway MS, Witjes JA, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol 2010;184:1907-13. https://doi.org/10.1016/j.juro.2010.06.148.
- Grossman HB, Stenzl A, Fradet Y, Mynderse LA, Kriegmair M, Witjes JA, et al. Long-term decrease in bladder cancer recurrence with hexaminolevulinate enabled fluorescence cystoscopy. J Urol 2012;188:58-62. https://doi.org/10.1016/j.juro.2012.03.007.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
- National Cancer Institute . CTCAEs v4: Common Terminology Criteria for Adverse Events (CTCAE) 2010. http://ctep.cancer.gov/ (accessed 12 February 2021).
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. https://doi.org/10.1080/01621459.1999.10474144.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. 2013. www.nice.org.uk/process/pmg9 (accessed June 2021).
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. https://doi.org/10.1002/1097-0258(20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Joint Formulary Committee . British National Formulary 2018.
- NHS Improvement . NHS Reference Costs 2018–19 2019.
- Curtis L, Burns A. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2018.
- International Monetary Fund . World Economic Outlook Database (October 2018) n.d. www.imf.org/external/pubs/ft/weo/2019/01/weodata/index.aspx (accessed 23 April 2019).
- Department of Transport . Transport Analysis Guidance (TAG) Data Book v1.13. 1 2020. www.gov.uk/government/publications/tag-data-book (accessed June 2021).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. https://doi.org/10.1136/bmj.d40.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Chichester: John Wiley & Sons; 2004.
- Greene WH. Econometric Analysis: Global Edition. London: Pearson Education UK; 2014.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40. https://doi.org/10.1002/(SICI)1099-1050(199707)6:4<327::AID-HEC282>3.0.CO;2-W.
- Briggs AH, Gray AM. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess 1999;3. https://doi.org/10.3310/hta3020.
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401. https://doi.org/10.1146/annurev.publhealth.23.100901.140534.
- National Research Council (US) Panel on Handling Missing Data in Clinical Trials . The Prevention and Treatment of Missing Data in Clinical Trials 2010.
- Little RJ. Pattern-mixture models for multivariate incomplete data. J Am Stat Assoc 1993;88:125-34. https://doi.org/10.1080/01621459.1993.10594302.
- Ratitch B, O’Kelly M, Tosiello R. Missing data in clinical trials: from clinical assumptions to statistical analysis using pattern mixture models. Pharm Stat 2013;12:337-47. https://doi.org/10.1002/pst.1549.
- Permutt T. Sensitivity analysis for missing data in regulatory submissions. Stat Med 2016;35:2876-9. https://doi.org/10.1002/sim.6753.
- Heer R, Lewis R, Vadiveloo T, Yu G, Mariappan P, Cresswell J, et al. Randomized trial of PHOTOdynamic surgery in non-muscle-invasive bladder cancer. NEJM Evid 2022;1. https://doi.org/10.1056/EVIDoa2200092.
- Kelly JD, Tan WS, Porta N, Mostafid H, Huddart R, Protheroe A, et al. BOXIT – a randomised phase III placebo-controlled trial evaluating the addition of celecoxib to standard treatment of transitional cell carcinoma of the bladder (CRUK/07/004). Eur Urol 2019;75:593-601. https://doi.org/10.1016/j.eururo.2018.09.020.
- Oosterlinck W, Kurth KH, Schröder F, Bultinck J, Hammond B, Sylvester R. A prospective European Organization for Research and Treatment of Cancer Genitourinary Group randomized trial comparing transurethral resection followed by a single intravesical instillation of epirubicin or water in single stage Ta, T1 papillary carcinoma of the bladder. J Urol 1993;149:749-52. https://doi.org/10.1016/S0022-5347(17)36198-0.
- Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466-5. https://doi.org/10.1016/j.eururo.2005.12.031.
- Mariappan P, Lavin V, Phua CQ, Khan SAA, Donat R, Smith G. Predicting grade and stage at cystoscopy in newly presenting bladder cancers-a prospective double-blind clinical study. Urology 2017;109:134-9. https://doi.org/10.1016/j.urology.2017.08.007.
- Mostafid AH, Porta N, Cresswell J, Griffiths TRL, Kelly JD, Penegar SR, et al. CALIBER: a phase II randomized feasibility trial of chemoablation with mitomycin-C vs surgical management in low-risk non-muscle-invasive bladder cancer. BJU Int 2020;125:817-26. https://doi.org/10.1111/bju.15038.
- Soukup V, Čapoun O, Cohen D, Hernández V, Burger M, Compérat E, et al. Risk stratification tools and prognostic models in non-muscle-invasive bladder cancer: a critical assessment from the European Association of Urology Non-muscle-invasive Bladder Cancer Guidelines Panel. Eur Urol Focus 2020;6:479-89. https://doi.org/10.1016/j.euf.2018.11.005.
- Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO Guideline. J Urol 2016;196:1021-9. https://doi.org/10.1016/j.juro.2016.06.049.
- Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013;64:639-53. https://doi.org/10.1016/j.eururo.2013.06.003.
- Sylvester RJ, Rodríguez O, Hernández V, Turturica D, Bauerová L, Bruins HM, et al. European Association of Urology (EAU) prognostic factor risk groups for non-muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: an update from the EAU NMIBC Guidelines Panel. Eur Urol 2021;79:480-8. https://doi.org/10.1016/j.eururo.2020.12.033.
- Lammers RJ, Palou J, Witjes WP, Janzing-Pastors MH, Caris CT, Witjes JA. Comparison of expected treatment outcomes, obtained using risk models and international guidelines, with observed treatment outcomes in a Dutch cohort of patients with non-muscle-invasive bladder cancer treated with intravesical chemotherapy. BJU Int 2014;114:193-201. https://doi.org/10.1111/bju.12495.
- Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Ojea A, et al. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette–Guérin: external validation of the EORTC risk tables. Eur Urol 2011;60:423-30. https://doi.org/10.1016/j.eururo.2011.05.033.
- Gravas S, Efstathiou K, Zachos I, Melekos MD, Tzortzis V. Is there a learning curve for photodynamic diagnosis of bladder cancer with hexaminolevulinate hydrochloride?. Can J Urol 2012;19:6269-73.
- Kruck S, Bedke J, Hennenlotter J, Amend B, Merseburger A, Stenzl A, et al. Virtual bladder tumor transurethral resection: an objective evaluation tool to overcome learning curves with and without photodynamic diagnostics. Urol Int 2011;87:138-42. https://doi.org/10.1159/000328218.
- O’Brien T, Ray E, Chatterton K, Khan MS, Chandra A, Thomas K. Prospective randomized trial of hexylaminolevulinate photodynamic-assisted transurethral resection of bladder tumour (TURBT) plus single-shot intravesical mitomycin C vs conventional white-light TURBT plus mitomycin C in newly presenting non-muscle-invasive bladder cancer. BJU Int 2013;112:1096-104. https://doi.org/10.1111/bju.12355.
- Mariappan P, Finney SM, Head E, Somani BK, Zachou A, Smith G, et al. Good quality white-light transurethral resection of bladder tumours (GQ-WLTURBT) with experienced surgeons performing complete resections and obtaining detrusor muscle reduces early recurrence in new non-muscle-invasive bladder cancer: validation across time and place and recommendation for benchmarking. BJU Int 2012;109:1666-73. https://doi.org/10.1111/j.1464-410X.2011.10571.x.
- Mariappan P, Johnston A, Padovani L, Clark E, Trail M, Hamid S, et al. Enhanced quality and effectiveness of transurethral resection of bladder tumour in non-muscle-invasive bladder cancer: a multicentre real-world experience from Scotland’s Quality Performance Indicators Programme. Eur Urol 2020;78:520-30. https://doi.org/10.1016/j.eururo.2020.06.051.
- Kausch I, Sommerauer M, Montorsi F, Stenzl A, Jacqmin D, Jichlinski P, et al. Photodynamic diagnosis in non-muscle-invasive bladder cancer: a systematic review and cumulative analysis of prospective studies. Eur Urol 2010;57:595-606. https://doi.org/10.1016/j.eururo.2009.11.041.
- Rink M, Babjuk M, Catto JW, Jichlinski P, Shariat SF, Stenzl A, et al. Hexyl aminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: a critical review of the current literature. Eur Urol 2013;64:624-38. https://doi.org/10.1016/j.eururo.2013.07.007.
- Burger M, Grossman HB, Droller M, Schmidbauer J, Hermann G, Drăgoescu O, et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur Urol 2013;64:846-54. https://doi.org/10.1016/j.eururo.2013.03.059.
- Konecki T, Kutwin P, Łowicki R, Juszczak AB, Jabłonowski Z. Hexaminolevulinate in the management of nonmuscle invasive bladder cancer: a meta-analysis. Photobiomodul Photomed Laser Surg 2019;37:551-8. https://doi.org/10.1089/photob.2019.4634.
- Gallagher KM, Gray K, Anderson CH, Lee H, Stewart S, Donat R, et al. ‘Real-life experience’: recurrence rate at 3 years with Hexvix® photodynamic diagnosis-assisted TURBT compared with good quality white light TURBT in new NMIBC-a prospective controlled study. World J Urol 2017;35:1871-7. https://doi.org/10.1007/s00345-017-2077-6.
- Mariappan P, Rai B, El-Mokadem I, Anderson CH, Lee H, Stewart S, et al. Real-life experience: early recurrence with Hexvix® photodynamic diagnosis-assisted transurethral resection of bladder tumour vs good-quality white light TURBT in new non-muscle-invasive bladder cancer. Urology 2015;86:327-31. https://doi.org/10.1016/j.urology.2015.04.015.
- Witjes JA, Babjuk M, Gontero P, Jacqmin D, Karl A, Kruck S, et al. Clinical and cost effectiveness of hexaminolevulinate-guided blue-light cystoscopy: evidence review and updated expert recommendations. Eur Urol 2014;66:863-71. https://doi.org/10.1016/j.eururo.2014.06.037.
- Malmström PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette–Guérin for non-muscle-invasive bladder cancer. Eur Urol 2009;56:247-56. https://doi.org/10.1016/j.eururo.2009.04.038.
- Palou J, Hernández C, Solsona E, Abascal R, Burgués JP, Rioja C, et al. Effectiveness of hexaminolevulinate fluorescence cystoscopy for the diagnosis of non-muscle-invasive bladder cancer in daily clinical practice: a Spanish multicentre observational study. BJU Int 2015;116:37-43. https://doi.org/10.1111/bju.13020.
- Daneshmand S, Schuckman AK, Bochner BH, Cookson MS, Downs TM, Gomella LG, et al. Hexaminolevulinate blue-light cystoscopy in non-muscle-invasive bladder cancer: review of the clinical evidence and consensus statement on appropriate use in the USA. Nat Rev Urol 2014;11:589-96. https://doi.org/10.1038/nrurol.2014.245.
- Cabana MD, Rand CS, Powe NR, Wu AW, . Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458-65. https://doi.org/10.1001/jama.282.15.1458.
- Sylvester RJ, Canfield SE, Lam TB, Marconi L, MacLennan S, Yuan Y, et al. Conflict of evidence: resolving discrepancies when findings from randomized controlled trials and meta-analyses disagree. Eur Urol 2017;71:811-19. https://doi.org/10.1016/j.eururo.2016.11.023.
- Power NE, Izawa J. Comparison of guidelines on non-muscle invasive bladder cancer (EAU, CUA, AUA, NCCN, NICE). Bladder Cancer 2016;2:27-36. https://doi.org/10.3233/BLC-150034.
- Leiblich A, Bryant RJ, R McCormick R, Crew J. The management of non-muscle-invasive bladder cancer: a comparison of European and UK guidelines. J Clin Urol 2018;11:144-8. https://doi.org/10.1177/2051415818757339.
- Babjuk M, Burger M, Compérat EM, Gontero P, Mostafid AH, Palou J, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) – 2019 Update. Eur Urol 2019;76:639-57. https://doi.org/10.1016/j.eururo.2019.08.016.
- Hendricksen K, Aziz A, Bes P, Chun FK, Dobruch J, Kluth LA, et al. Discrepancy between European Association of Urology guidelines and daily practice in the management of non-muscle-invasive bladder cancer: results of a European survey. Eur Urol Focus 2019;5:681-8. https://doi.org/10.1016/j.euf.2017.09.002.
- Witjes JA, Palou J, Soloway M, Lamm D, Kamat AM, Brausi M, et al. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette–Guérin (BCG): results of an international individual patient data survey (IPDS). BJU Int 2013;112:742-50. https://doi.org/10.1111/bju.12012.
- Sylvester RJ, Brausi MA, Kirkels WJ, Hoeltl W, Calais Da Silva F, Powell PH, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette–Guérin, and bacillus Calmette–Guérin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol 2010;57:766-73. https://doi.org/10.1016/j.eururo.2009.12.024.
- Tapiero S, Helfand A, Kedar D, Yossepowitch O, Nadu A, Baniel J, et al. Patient compliance with maintenance intravesical therapy for nonmuscle invasive bladder cancer. Urology 2018;118:107-13. https://doi.org/10.1016/j.urology.2018.04.039.
- Brausi M, Oddens J, Sylvester R, Bono A, van de Beek C, van Andel G, et al. Side effects of bacillus Calmette–Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol 2014;65:69-76. https://doi.org/10.1016/j.eururo.2013.07.021.
- Oddens J, Brausi M, Sylvester R, Bono A, van de Beek C, van Andel G, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette–Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 2013;63:462-72. https://doi.org/10.1016/j.eururo.2012.10.039.
- van den Bosch S, Alfred Witjes J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol 2011;60:493-500. https://doi.org/10.1016/j.eururo.2011.05.045.
- Chen C, Huang H, Zhao Y, Liu H, Luo Y, Sylvester RJ, et al. Diagnostic accuracy of photodynamic diagnosis with 5-aminolevulinic acid, hexaminolevulinate and narrow band imaging for non-muscle invasive bladder cancer. J Cancer 2020;11:1082-93. https://doi.org/10.7150/jca.34527.
- Chen C, Huang H, Zhao Y, Liu H, Sylvester R, Lin T, et al. Diagnostic performance of image technique based transurethral resection for non-muscle invasive bladder cancer: systematic review and diagnostic meta-analysis. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-028173.
- Bolenz C, Rother J, Meessen S, Grychtol B, Majlesara A, Gharabaghi N, et al. The development of real-time multispectral imaging for the diagnostics of bladder cancer. Urologe A 2019;58:1435-42. https://doi.org/10.1007/s00120-019-01037-3.
- Brunckhorst O, Ong QJ, Elson D, Mayer E. Novel real-time optical imaging modalities for the detection of neoplastic lesions in urology: a systematic review. Surg Endosc 2019;33:1349-67. https://doi.org/10.1007/s00464-018-6578-1.
- Ikeda A, Nosato H, Kochi Y, Negoro H, Kojima T, Sakanashi H, et al. Cystoscopic imaging for bladder cancer detection based on stepwise organic transfer learning with a pretrained convolutional neural network. J Endourol 2021;35:1030-5. https://doi.org/10.1089/end.2020.0919.
- Tan WS, Tan WP, Tan MY, Khetrapal P, Dong L, deWinter P, et al. Novel urinary biomarkers for the detection of bladder cancer: a systematic review. Cancer Treat Rev 2018;69:39-52. https://doi.org/10.1016/j.ctrv.2018.05.012.
- Leitner CV, Ederer IA, de Martino M, Hofbauer SL, Lucca I, Mbeutcha A, et al. Dynamic prognostication using conditional recurrence and progression estimates for patients with nonmuscle invasive bladder cancer. J Urol 2016;196:46-51. https://doi.org/10.1016/j.juro.2016.01.102.
- Leo MC, McMullen CK, O’Keeffe-Rosetti M, Weinmann S, Garg T, Nielsen ME. External validation of the EORTC and NCCN bladder cancer recurrence and progression risk calculators in a U.S. community-based health system. Urol Oncol 2020;38:39.e21-7. https://doi.org/10.1016/j.urolonc.2019.10.003.
- Xylinas E, Kent M, Kluth L, Pycha A, Comploj E, Svatek RS, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer 2013;109:1460-6. https://doi.org/10.1038/bjc.2013.372.
- Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus Calmette–Guérin. Eur Urol 2016;69:60-9. https://doi.org/10.1016/j.eururo.2015.06.045.
- British Association of Urological Surgeons . BAUS Data &Amp; Audit Programme n.d. www.baus.org.uk/professionals/baus_business/data_audit.aspx (accessed 14 January 2022).
- Dindyal S, Nitkunan T, Bunce CJ. The economic benefit of photodynamic diagnosis in non-muscle invasive bladder cancer. Photodiagnosis Photodyn Ther 2008;5:153-8. https://doi.org/10.1016/j.pdpdt.2008.05.001.
- National Institute for Health and Care Excellence (NICE) . Narrow Band Imaging for Barrett’s Oesophagus 2019. www.nice.org.uk/advice/mib179 (accessed 11 June 2021).
- Curtis L. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2009.
- National Audit Office . Out-of-Hours GP Services in England 2014. www.nao.org.uk/serarch/out-of-hours/ (accessed 12 January 2022).
- HM Revenue and Customs . Travel – Mileage and Fuel Rates and Allowances 2019. www.gov.uk/government/publications/rates-and-allowances-travel-mileage-and-fuel-allowances/travel-mileage-and-fuel-rates-and-allowances (accessed 11 June 2021).
- Department of Health and Social Care . NHS Reference Costs 2009–2010 2011. www.gov.uk/government/publications/nhs-reference-costs-2009-2010 (accessed 11 June 2021).
- Office for National Statistics (ONS) . Annual Survey of Hours and Earnings Time Series of Selected Estimates 2020. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/ashe1997to2015selectedestimates (accessed 11 June 2021).
- NHS Pay Review Body . NHS Pay Review Body Twenty-Sixth Report 2012 2012. www.gov.uk/government/publications/nhs-pay-review-body-twenty-sixth-report-2012 (accessed 11 June 2021).
- Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Med Decis Making 2012;32:690-70. https://doi.org/10.1177/0272989X12455463.
- Office for National Statistics (ONS) . National Life Tables, UK: 2016 to 2018 2019. www.ons.gov.uk/releases/nationallifetablesuk2016to2018 (accessed June 2021).
- EuroQol Research Foundation . EQ-5D-3L User Guide, 2018 2018.
- Kaltenthaler E, Tappenden P, Paisley S, Squires H. NICE DSU Technical Support Document 13: Identifying and Reviewing Evidence to Inform the Conceptualisation and Population of Cost-effectiveness Models. Sheffield: Decision Support Unit, ScHARR, University of Sheffield; 2011.
- Gray AM, Clarke PM, Wolstenholme J, Wordsworth S. Applied Methods of Cost-Effectiveness Analysis in Healthcare. Oxford: Oxford University Press; 2011.
- Committee for Medicinal Products for Human Use . Guideline on Adjustment for Baseline Covariates in Clinical Trials 2015.
- Shen J, Hill S, Mott D, Breckons M, Vale L, Pickard R. Conducting a time trade-off study alongside a clinical trial: a case study and recommendations. Pharmacoecon Open 2019;3:5-20. https://doi.org/10.1007/s41669-018-0084-1.
- Dolan P, Gudex C, Kind P, Williams A. Valuing health states: a comparison of methods. J Health Econ 1996;15:209-31. https://doi.org/10.1016/0167-6296(95)00038-0.
- Gandhi M, Rand K, Luo N. Valuation of health states considered to be worse than death – an analysis of composite time trade-off data from 5 EQ-5D-5L valuation studies. Value Health 2019;22:370-6. https://doi.org/10.1016/j.jval.2018.10.002.
- Torrance GW. Measurement of health state utilities for economic appraisal: a review. J Health Econ 1986;5. https://doi.org/10.1016/0167-6296(86)90020-2.
- Janssen BM, Oppe M, Versteegh MM, Stolk EA. Introducing the composite time trade-off: a test of feasibility and face validity. Eur J Health Econ 2013;14:5-13. https://doi.org/10.1007/s10198-013-0503-2.
- Gudex C. Time Trade-off User Manual: Props and Self-completion Methods. York: Centre for Health Economics, University of York; 1994.
- Miremami J, Kyprianou N. The promise of novel molecular markers in bladder cancer. Int J Mol Sci 2014;15:23897-908. https://doi.org/10.3390/ijms151223897.
- The Cancer Genome Atlas Research Network . Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315-22. https://doi.org/10.1038/nature12965.
- Great Britain . Human Tissue Act 2004 2004.
- NHS Health Research Authority . Hyperthermia for Intermediate Risk Bladder Cancer (HIVEC-II) n.d. www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/hyperthermia-for-intermediate-risk-bladder-cancerhivec-ii/ (accessed 14 January 2022).
- Tan WS, Feber A, Dong L, Sarpong R, Rezaee S, Rodney S, et al. DETECT I & DETECT II: a study protocol for a prospective multicentre observational study to validate the UroMark assay for the detection of bladder cancer from urinary cells. BMC Cancer 2017;17. https://doi.org/10.1186/s12885-017-3758-7.
Appendix 1 Additional information for the clinical results
Risk of recurrence and progression scoring systems
Table 27 shows the EORTC scoring systems for risk of recurrence and progression.
| Factor | EORTC score | |
|---|---|---|
| Recurrence | Progression | |
| Number of tumours | ||
| 1 | 0 | 0 |
| 2–7 | 3 | 3 |
| ≥ 8 | 6 | 3 |
| Tumour size | ||
| < 3 cm | 0 | 0 |
| ≥ 3 cm | 3 | 3 |
| Prior recurrence rate | ||
| Primary | 0 | 0 |
| ≤ 1 recurrence/year | 2 | 2 |
| > 1 recurrence/year | 4 | 2 |
| T category | ||
| Ta | 0 | 0 |
| T1 | 1 | 4 |
| CIS | ||
| No | 0 | 0 |
| Yes | 1 | 6 |
| Grade | ||
| 1 | 0 | 0 |
| 2 | 1 | 0 |
| 3 | 2 | 5 |
| Total score | 0–17 | 0–23 |
Table 28 shows the NICE risk categories for recurrence and progression.
| Risk category | Description |
|---|---|
| Low risk | Urothelial cancer with any of:
|
| Intermediate risk | Urothelial cancer that is not low risk or high risk, including:
|
| High risk | Urothelial cancer with any of:
|
Study recruitment
The number of patients recruited at each site and the number of months over which the site recruited are shown in Table 29. The details of reasons for non-inclusion and ineligibility of screened patients are shown in Table 30.
| Centre name | Patients randomised (n) | Percentage of total recruitment (N = 538) | Months recruiting (n) |
|---|---|---|---|
| Newcastle Hospitals NHS Foundation Trust, Newcastle | 52 | 9.67 | 40 |
| Royal Devon and Exeter NHS Foundation Trust, Exeter | 49 | 9.11 | 39 |
| Oxford University Hospitals NHS Foundation Trust, Oxford | 30 | 5.58 | 37 |
| NHS Tayside, Dundee | 10 | 1.86 | 37 |
| University College London Hospitals NHS Foundation Trust, London | 3 | 0.56 | 37 |
| Cwm Taf Morgannwg University Health Board, Bridgend | 1 | 0.19 | 37 |
| Ashford and St Peter’s Hospitals NHS Foundation Trust, Ashford | 8 | 1.49 | 36 |
| NHS Lothian, Edinburgh | 84 | 15.61 | 37 |
| Hull University Teaching Hospitals NHS Trust, Cottingham | 18 | 3.35 | 37 |
| Hampshire Hospitals NHS Foundation Trust, Basingstoke | 6 | 1.12 | 37 |
| South Tees Hospitals NHS Foundation Trust, Middlesbrough | 53 | 9.85 | 36 |
| Imperial College Healthcare NHS Trust, London | 12 | 2.23 | 35 |
| Leeds Teaching Hospitals NHS Trust, Leeds | 35 | 6.51 | 28 |
| Swansea Bay University Health Board, Swansea | 26 | 4.83 | 25 |
| Dartford and Gravesham NHS Trust, Dartford | 57 | 10.59 | 33 |
| University Hospital Southampton NHS Foundation Trust, Southampton | 10 | 1.86 | 29 |
| University Hospitals of North Midlands NHS Trust, Stoke-on-Trent | 24 | 4.46 | 25 |
| Derby Teaching Hospitals NHS Foundation Trust, Derby | 11 | 2.04 | 19 |
| Salisbury NHS Foundation Trust, Salisbury | 21 | 3.9 | 23 |
| NHS Grampian, Aberdeen | 20 | 3.72 | 19 |
| Royal Free London NHS Foundation Trust, London | 1 | 0.19 | 14 |
| East and North Hertfordshire NHS Trust, Stevenage | 7 | 1.3 | 9 |
| Total | 538 | 100 | 669 |
| Reason | n (%) |
|---|---|
| For non-inclusion | |
| Patient not approached | 55 (10.11) |
| Ineligible | 226 (41.54) |
| Unable to give informed consent | 18 (3.31) |
| Unable to complete study questionnaires | 3 (0.55) |
| Patient not interested in the study | 113 (20.77) |
| Patient did not want to be randomised | 36 (6.62) |
| Patient opted for PDD | 3 (0.55) |
| Other | 90 (16.54) |
| Total | 544 |
| For ineligibilitya | |
| Visual evidence of low risk NMIBC (solitary tumour of < 3 cm in diameter) | 134 (59.29) |
| Visual evidence of MIBC on preliminary cystoscopy | 26 (11.50) |
| Imaging evidence of MIBC: CT/USS | 31 (13.72) |
| Upper-tract tumours on imaging | 4 (1.77) |
| Any other malignancy in the past 2 years | 30 (13.27) |
| Evidence of metastases | 4 (1.77) |
Baseline minimisation variables
Centre and sex were included as minimisation variables. Table 31 shows the baseline minimisation variables for all of the participants who were randomised.
| Minimisation variable | Treatment group, n (%) | |
|---|---|---|
| PDD (N = 265) | WL (N = 268) | |
| Centre | ||
| Newcastle Hospitals NHS Foundation Trust, Newcastle | 25 (9.4) | 26 (9.7) |
| Royal Devon and Exeter NHS Foundation Trust, Exeter | 24 (9.1) | 24 (9.0) |
| Oxford University Hospitals NHS Foundation Trust, Oxford | 15 (5.7) | 15 (5.6) |
| NHS Tayside, Dundee | 5 (1.9) | 5 (1.9) |
| University College London Hospitals NHS Foundation Trust, London | 1 (0.4) | 2 (0.7) |
| Cwm Taf Morgannwg University Health Board, Bridgend | – | 1 (0.4) |
| Ashford and St Peter’s Hospitals NHS Foundation Trust, Ashford | 5 (1.9) | 3 (1.1) |
| NHS Lothian, Edinburgh | 41 (15.5) | 42 (15.7) |
| Hull University Teaching Hospitals NHS Trust, Cottingham | 8 (3.0) | 10 (3.7) |
| Hampshire Hospitals NHS Foundation Trust, Basingstoke | 3 (1.1) | 3 (1.1) |
| South Tees Hospitals NHS Foundation Trust, Middlesbrough | 27 (10.2) | 26 (9.7) |
| Imperial College Healthcare NHS Trust, London | 5 (1.9) | 7 (2.6) |
| Leeds Teaching Hospitals NHS Trust, Leeds | 18 (6.8) | 16 (6.0) |
| Swansea Bay University Health Board, Swansea | 13 (4.9) | 13 (4.9) |
| Dartford and Gravesham NHS Trust, Dartford | 27 (10.2) | 29 (10.8) |
| University Hospital Southampton NHS Foundation Trust, Southampton | 6 (2.3) | 4 (1.5) |
| University Hospitals of North Midlands NHS Trust, Stoke-on-Trent | 12 (4.5) | 12 (4.5) |
| Derby Teaching Hospitals NHS Foundation Trust, Derby | 6 (2.3) | 5 (1.9) |
| Salisbury NHS Foundation Trust, Salisbury | 10 (3.8) | 11 (4.1) |
| NHS Grampian, Aberdeen | 10 (3.8) | 10 (3.7) |
| Royal Free London NHS Foundation Trust, London | – | 1 (0.4) |
| East and North Hertfordshire NHS Trust, Stevenage | 4 (1.5) | 3 (1.1) |
| Sex | ||
| Male | 213 (80.4) | 214 (79.9) |
| Female | 52 (19.6) | 54 (20.1) |
Baseline EQ-5D-3L
Table 32 shows the baseline EQ-5D-3L domains for participants who were included in the analysis.
| EQ-5D-3L domain | Treatment group, n (%) | |
|---|---|---|
| PDD (N = 209) | WL (N = 217) | |
| Morbidity | ||
| No problem | 142 (67.9) | 152 (70.0) |
| Some problem | 50 (23.9) | 43 (19.8) |
| I am confined to bed | – | 1 (0.5) |
| Missing | 17 (8.1) | 21 (9.7) |
| Self-care | ||
| No problem | 180 (86.1) | 183 (84.3) |
| Some problem | 9 (4.3) | 11 (5.1) |
| Missing | 20 (9.6) | 23 (10.6) |
| Usual activities | ||
| No problem | 153 (73.2) | 158 (72.8) |
| Some problem | 36 (17.2) | 35 (16.1) |
| I am unable to perform my usual activities | 2 (1.0) | 1 (0.5) |
| Missing | 18 (8.6) | 23 (10.6) |
| Pain/discomfort | ||
| No pain | 111 (53.1) | 132 (60.8) |
| Moderate pain | 75 (35.9) | 56 (25.8) |
| I have extreme pain or discomfort | 6 (2.9) | 7 (3.2) |
| Missing | 17 (8.1) | 22 (10.1) |
| Anxiety/depression | ||
| Not anxious | 141 (67.5) | 149 (68.7) |
| Moderate | 47 (22.5) | 41 (18.9) |
| I am extremely anxious or depressed | 3 (1.4) | 5 (2.3) |
| Missing | 18 (8.6) | 22 (10.1) |
| HRQoL outcome | Treatment group, mean (SD); n | Estimate (99% CI); p-value | |
|---|---|---|---|
| PDD (N = 209) | WL (N = 217) | ||
| EQ-5D-3L | |||
| Baseline | 0.834 (0.205); 187 | 0.838 (0.223); 188 | |
| Post treatment | 0.706 (0.265); 170 | 0.717 (0.279); 174 | –0.000 (–0.058 to 0.058); 0.995 |
| 3 months | 0.793 (0.242); 178 | 0.806 (0.226); 190 | –0.005 (–0.063 to 0.054); 0.842 |
| 6 months | 0.806 (0.237); 176 | 0.817 (0.223); 179 | –0.001 (–0.060 to 0.057); 0.950 |
| 12 months | 0.796 (0.263); 161 | 0.819 (0.243); 170 | –0.018 (–0.078 to 0.042); 0.449 |
| 18 months | 0.802 (0.242); 161 | 0.831 (0.219); 166 | –0.019 (–0.080 to 0.041); 0.412 |
| 24 months | 0.762 (0.284); 148 | 0.827 (0.241); 151 | –0.064 (–0.126 to –0.001); 0.009 |
| 36 months | 0.797 (0.251); 95 | 0.825 (0.238); 94 | –0.013 (–0.086 to 0.061); 0.660 |
| EORTC-QLQ-C30 | |||
| Functioning scalesa | |||
| Physical | |||
| Baseline | 83.6 (20.3); 189 | 85.8 (17.7); 195 | |
| Post treatment | 76.0 (24.5); 167 | 78.6 (23.2); 177 | 0.3 (–3.7 to 4.4); 0.829 |
| 3 months | 79.5 (22.5); 183 | 82.4 (20.9); 196 | –1.3 (–5.4 to 2.7); 0.390 |
| 6 months | 79.6 (22.9); 183 | 81.9 (20.8); 187 | –1.1 (–5.1 to 3.0); 0.495 |
| 12 months | 78.7 (24.1); 166 | 82.4 (21.1); 174 | –2.1 (–6.2 to 2.1); 0.203 |
| 18 months | 79.6 (22.2); 164 | 83.0 (20.5); 166 | –2.1 (–6.3 to 2.2); 0.209 |
| 24 months | 79.1 (22.9); 154 | 80.9 (22.1); 156 | –0.9 (–5.1 to 3.4); 0.609 |
| 36 months | 80.6 (22.6); 100 | 81.8 (21.4); 96 | 0.5 (–4.6 to 5.5); 0.813 |
| Role | |||
| Baseline | 85.7 (24.8); 188 | 87.7 (22.0); 195 | |
| Post treatment | 75.0 (31.3); 171 | 74.5 (32.4); 178 | 2.5 (–4.0 to 9.1); 0.320 |
| 3 months | 75.2 (30.1); 183 | 81.4 (27.1); 196 | –4.4 (–10.9 to 2.1); 0.084 |
| 6 months | 79.0 (29.0); 183 | 83.2 (25.1); 186 | –2.5 (–9.0 to 4.1); 0.337 |
| 12 months | 79.8 (30.9); 166 | 83.4 (25.1); 174 | –1.9 (–8.6 to 4.9); 0.473 |
| 18 months | 80.1 (28.1); 164 | 84.0 (25.9); 166 | –2.2 (–9.0 to 4.7); 0.415 |
| 24 months | 76.5 (29.4); 155 | 82.8 (27.0); 156 | –5.0 (–11.9 to 2.0); 0.066 |
| 36 months | 78.7 (30.3); 100 | 84.0 (27.4); 96 | –2.7 (–10.9 to 5.6); 0.404 |
| Cognitive | |||
| Baseline | 85.7 (18.3); 188 | 87.5 (18.1); 195 | |
| Post treatment | 82.2 (20.3); 173 | 84.4 (20.3); 181 | –1.6 (–6.1 to 2.8); 0.343 |
| 3 months | 82.1 (20.4); 185 | 84.8 (19.0); 198 | –1.7 (–6.1 to 2.8); 0.335 |
| 6 months | 81.4 (22.1); 184 | 84.5 (17.3); 189 | –1.6 (–6.1 to 2.9); 0.354 |
| 12 months | 83.4 (19.4); 166 | 82.5 (19.7); 174 | 2.2 (–2.4 to 6.8); 0.214 |
| 18 months | 82.2 (20.8); 164 | 83.1 (20.0); 167 | 0.0 (–4.6 to 4.6); 0.993 |
| 24 months | 80.8 (20.1); 154 | 80.4 (22.8); 157 | 2.0 (–2.7 to 6.7); 0.278 |
| 36 months | 80.2 (19.8); 100 | 83.7 (20.4); 96 | –1.0 (–6.5 to 4.5); 0.630 |
| Emotional | |||
| Baseline | 80.4 (20.8); 186 | 81.5 (19.2); 192 | |
| Post treatment | 80.0 (20.5); 172 | 77.5 (22.9); 180 | 3.3 (–1.2 to 7.9); 0.061 |
| 3 months | 81.8 (20.7); 185 | 80.5 (20.8); 196 | 2.3 (–2.3 to 6.8); 0.195 |
| 6 months | 80.3 (22.2); 183 | 82.0 (19.1); 188 | –0.5 (–5.0 to 4.1); 0.790 |
| 12 months | 81.7 (22.7); 164 | 81.3 (21.4); 174 | 1.2 (–3.5 to 5.9); 0.499 |
| 18 months | 84.0 (22.4); 164 | 82.1 (21.7); 166 | 2.5 (–2.2 to 7.2); 0.173 |
| 24 months | 80.1 (24.2); 151 | 83.0 (20.6); 155 | –1.8 (–6.7 to 3.1); 0.341 |
| 36 months | 81.2 (21.9); 100 | 83.0 (22.4); 96 | –0.4 (–6.0 to 5.3); 0.872 |
| Social | |||
| Baseline | 87.0 (22.0); 186 | 88.6 (21.2); 193 | |
| Post treatment | 78.4 (25.0); 172 | 77.3 (28.1); 179 | 3.0 (–3.1 to 9.2); 0.198 |
| 3 months | 79.9 (25.7); 185 | 83.3 (23.9); 197 | –1.0 (–7.0 to 5.0); 0.673 |
| 6 months | 81.0 (27.4); 182 | 83.6 (24.8); 189 | –0.6 (–6.6 to 5.5); 0.813 |
| 12 months | 82.2 (27.5); 164 | 85.4 (22.8); 174 | –1.7 (–7.9 to 4.6); 0.492 |
| 18 months | 82.2 (25.4); 164 | 84.6 (24.9); 166 | –1.4 (–7.7 to 4.9); 0.577 |
| 24 months | 81.8 (26.4); 151 | 84.9 (25.6); 155 | –2.1 (–8.6 to 4.4); 0.412 |
| 36 months | 83.0 (25.3); 100 | 86.6 (22.9); 96 | –2.4 (–10.0 to 5.3); 0.423 |
| Global QoL | |||
| Baseline | 73.7 (19.0); 186 | 73.8 (20.4); 193 | |
| Post treatment | 68.9 (21.3); 172 | 67.9 (21.1); 180 | 1.8 (–2.5 to 6.1); 0.276 |
| 3 months | 71.8 (18.7); 185 | 71.2 (19.4); 196 | 0.7 (–3.6 to 4.9); 0.685 |
| 6 months | 74.0 (20.2); 183 | 72.9 (18.6); 189 | 0.8 (–3.5 to 5.1); 0.634 |
| 12 months | 72.5 (19.3); 164 | 74.0 (20.0); 174 | –1.0 (–5.4 to 3.4); 0.546 |
| 18 months | 73.7 (19.2); 165 | 73.7 (20.3); 166 | –0.2 (–4.6 to 4.2); 0.900 |
| 24 months | 70.9 (20.3); 152 | 72.5 (20.3); 156 | –0.7 (–5.2 to 3.9); 0.704 |
| 36 months | 73.4 (19.3); 100 | 76.2 (19.2); 96 | –2.3 (–7.6 to 3.0); 0.265 |
| Symptom scales and/or itemsb | |||
| Fatigue | |||
| Baseline | 21.7 (22.9); 187 | 19.4 (20.3); 195 | |
| Post treatment | 28.7 (25.0); 172 | 27.3 (24.9); 180 | –1.8 (–7.0 to 3.3); 0.361 |
| 3 months | 27.4 (24.5); 184 | 26.6 (23.8); 197 | –1.0 (–6.1 to 4.1); 0.616 |
| 6 months | 27.9 (25.0); 182 | 26.8 (23.8); 187 | –0.7 (–5.9 to 4.5); 0.733 |
| 12 months | 27.4 (25.8); 166 | 25.2 (23.4); 174 | –0.4 (–5.8 to 4.9); 0.831 |
| 18 months | 25.5 (23.6); 164 | 25.0 (24.2); 166 | –0.9 (–6.3 to 4.4); 0.659 |
| 24 months | 27.5 (24.3); 153 | 25.9 (24.8); 156 | –0.2 (–5.7 to 5.3); 0.928 |
| 36 months | 25.3 (22.7); 100 | 24.2 (21.3); 96 | –0.5 (–7.0 to 5.9); 0.827 |
| Nausea and vomiting | |||
| Baseline | 3.9 (12.0); 187 | 3.2 (9.3); 195 | |
| Post treatment | 5.0 (13.0); 172 | 5.2 (12.9); 180 | –0.8 (–4.0 to 2.3); 0.494 |
| 3 months | 4.5 (12.0); 184 | 4.8 (13.1); 198 | –0.8 (–3.9 to 2.3); 0.494 |
| 6 months | 5.4 (12.3); 182 | 3.9 (11.7); 187 | 0.1 (–3.1 to 3.2); 0.952 |
| 12 months | 4.9 (13.8); 165 | 3.4 (10.5); 174 | –0.3 (–3.5 to 2.9); 0.805 |
| 18 months | 4.9 (12.2); 164 | 4.8 (13.7); 166 | –1.6 (–4.9 to 1.7); 0.209 |
| 24 months | 5.7 (13.1); 154 | 5.9 (16.9); 156 | –1.7 (–5.0 to 1.7); 0.204 |
| 36 months | 6.0 (15.6); 100 | 3.3 (10.2); 96 | 0.1 (–4.0 to 4.1); 0.962 |
| Pain | |||
| Baseline | 18.7 (25.2); 189 | 17.4 (25.2); 195 | |
| Post treatment | 26.4 (29.7); 172 | 23.2 (27.1); 180 | 1.4 (–4.4 to 7.3); 0.523 |
| 3 months | 21.9 (27.6); 184 | 18.9 (25.5); 198 | 1.3 (–4.5 to 7.0); 0.569 |
| 6 months | 19.8 (25.2); 183 | 16.7 (23.4); 187 | 1.8 (–4.0 to 7.6); 0.428 |
| 12 months | 21.6 (28.0); 166 | 15.8 (25.0); 174 | 3.9 (–2.1 to 9.9); 0.090 |
| 18 months | 21.2 (26.1); 164 | 16.4 (24.1); 166 | 3.1 (–2.9 to 9.2); 0.186 |
| 24 months | 22.3 (27.2); 154 | 16.6 (24.9); 156 | 4.1 (–2.1 to 10.3); 0.088 |
| 36 months | 23.5 (27.0); 100 | 14.1 (23.7); 96 | 6.1 (–1.2 to 13.5); 0.031 |
| Dyspnoea | |||
| Baseline | 14.3 (22.9); 187 | 14.0 (21.6); 195 | |
| Post treatment | 14.3 (24.5); 170 | 12.8 (22.5); 177 | 0.9 (–4.7 to 6.5); 0.667 |
| 3 months | 17.8 (26.5); 184 | 17.7 (25.5); 198 | –1.0 (–6.6 to 4.5); 0.635 |
| 6 months | 18.7 (27.9); 182 | 18.4 (26.1); 187 | –0.8 (–6.4 to 4.8); 0.704 |
| 12 months | 17.7 (26.5); 164 | 17.4 (26.0); 174 | –0.9 (–6.7 to 4.8); 0.681 |
| 18 months | 17.1 (26.1); 162 | 17.8 (25.4); 165 | –3.1 (–8.9 to 2.7); 0.169 |
| 24 months | 18.6 (26.7); 154 | 18.2 (26.1); 156 | –1.6 (–7.5 to 4.3); 0.479 |
| 36 months | 17.2 (26.7); 99 | 17.7 (24.6); 96 | –2.7 (–9.6 to 4.2); 0.307 |
| Sleep disturbance | |||
| Baseline | 22.0 (29.5); 188 | 23.1 (27.2); 195 | |
| Post treatment | 28.3 (30.7); 171 | 28.9 (29.9); 181 | 1.5 (–5.6 to 8.6); 0.592 |
| 3 months | 29.3 (31.2); 183 | 26.0 (30.0); 195 | 3.9 (–3.1 to 11.0); 0.153 |
| 6 months | 29.3 (32.0); 183 | 25.3 (27.5); 187 | 4.6 (–2.5 to 11.7); 0.098 |
| 12 months | 27.6 (31.1); 163 | 23.3 (27.9); 173 | 4.2 (–3.1 to 11.6); 0.140 |
| 18 months | 26.2 (28.1); 164 | 22.6 (28.5); 165 | 2.9 (–4.5 to 10.3); 0.309 |
| 24 months | 29.2 (30.7); 155 | 26.9 (29.8); 156 | 2.7 (–4.9 to 10.3); 0.358 |
| 36 months | 29.0 (30.6); 100 | 25.3 (29.9); 95 | 3.7 (–5.3 to 12.7); 0.292 |
| Appetite loss | |||
| Baseline | 12.1 (23.6); 187 | 8.7 (20.0); 195 | |
| Post treatment | 15.7 (24.3); 172 | 12.0 (20.7); 181 | 1.6 (–3.7 to 7.0); 0.425 |
| 3 months | 11.7 (22.9); 183 | 9.8 (21.9); 198 | 0.7 (–4.6 to 5.9); 0.750 |
| 6 months | 12.3 (21.9); 182 | 8.4 (19.1); 187 | 2.5 (–2.8 to 7.8); 0.228 |
| 12 months | 11.4 (22.5); 166 | 9.8 (21.8); 174 | –0.3 (–5.8 to 5.2); 0.892 |
| 18 months | 10.5 (20.2); 162 | 10.4 (22.9); 166 | –0.9 (–6.5 to 4.6); 0.665 |
| 24 months | 14.5 (23.2); 154 | 10.6 (22.1); 154 | 1.6 (–4.1 to 7.3); 0.478 |
| 36 months | 11.0 (20.7); 100 | 9.4 (19.2); 96 | –1.1 (–7.9 to 5.7); 0.682 |
| Constipation | |||
| Baseline | 12.7 (23.4); 187 | 8.7 (19.4); 195 | |
| Post treatment | 18.1 (27.3); 171 | 18.2 (26.2); 179 | –2.6 (–8.6 to 3.5); 0.275 |
| 3 months | 16.0 (25.0); 181 | 15.5 (23.7); 198 | –2.1 (–8.1 to 3.9); 0.362 |
| 6 months | 16.4 (24.5); 181 | 12.4 (20.1); 186 | 1.3 (–4.7 to 7.4); 0.573 |
| 12 months | 16.4 (25.7); 165 | 13.9 (24.4); 173 | –0.0 (–6.3 to 6.2); 0.984 |
| 18 months | 15.7 (23.5); 163 | 16.6 (24.6); 165 | –2.8 (–9.1 to 3.5); 0.253 |
| 24 months | 13.7 (23.5); 151 | 14.7 (22.8); 156 | –2.5 (–8.9 to 3.9); 0.316 |
| 36 months | 13.8 (23.3); 99 | 7.4 (17.0); 95 | 3.0 (–4.7 to 10.7); 0.315 |
| Diarrhoea | |||
| Baseline | 7.1 (18.6); 184 | 5.2 (14.7); 194 | |
| Post treatment | 5.6 (15.7); 173 | 5.4 (15.4); 180 | –2.0 (–6.4 to 2.4); 0.240 |
| 3 months | 6.3 (16.0); 181 | 6.4 (17.3); 197 | –1.1 (–5.4 to 3.3); 0.526 |
| 6 months | 6.8 (17.4); 182 | 6.6 (15.8); 186 | –0.6 (–5.0 to 3.8); 0.721 |
| 12 months | 9.1 (18.2); 165 | 7.1 (17.8); 174 | 1.3 (–3.2 to 5.9); 0.444 |
| 18 months | 7.7 (18.0); 160 | 6.6 (16.1); 166 | 0.7 (–3.9 to 5.3); 0.707 |
| 24 months | 7.8 (20.2); 150 | 6.9 (17.3); 154 | 0.8 (–3.9 to 5.5); 0.660 |
| 36 months | 10.8 (22.8); 99 | 5.2 (12.2); 96 | 2.7 (–3.0 to 8.5); 0.218 |
| Financial difficulties | |||
| Baseline | 4.5 (15.5); 185 | 4.3 (14.0); 193 | |
| Post treatment | 6.8 (19.4); 171 | 5.8 (15.8); 178 | 0.7 (–3.7 to 5.2); 0.663 |
| 3 months | 7.4 (20.9); 184 | 7.0 (20.9); 196 | 0.4 (–4.0 to 4.7); 0.833 |
| 6 months | 8.3 (21.7); 180 | 6.6 (18.5); 188 | 2.2 (–2.2 to 6.6); 0.201 |
| 12 months | 6.5 (17.7); 163 | 6.2 (17.7); 172 | –1.4 (–5.9 to 3.1); 0.426 |
| 18 months | 6.3 (20.1); 164 | 6.2 (18.6); 166 | –0.4 (–5.0 to 4.1); 0.808 |
| 24 months | 5.8 (19.6); 150 | 6.2 (18.9); 156 | –1.6 (–6.2 to 3.1); 0.390 |
| 36 months | 6.7 (18.3); 100 | 4.9 (16.7); 96 | 0.7 (–4.8 to 6.2); 0.732 |
| EORTC-QLQ-NMIBC-24 | Treatment group, mean (SD); n | Estimate (99% CI); p-value | |
|---|---|---|---|
| PDD (N = 209) | WL (N = 217) | ||
| Functioning scalesa | |||
| Sexual function | |||
| Baseline | 18.4 (23.5); 164 | 19.3 (24.3); 174 | |
| Post treatment | 18.2 (23.3); 143 | 19.3 (25.8); 153 | –0.6 (–6.4 to 5.2); 0.774 |
| 3 months | 18.5 (22.6); 158 | 20.9 (24.0); 176 | –3.8 (–9.6 to 1.9); 0.087 |
| 6 months | 20.5 (24.4); 154 | 21.4 (25.3); 167 | –1.3 (–7.2 to 4.5); 0.557 |
| 12 months | 22.6 (24.1); 137 | 24.2 (25.2); 157 | –2.3 (–8.3 to 3.7); 0.328 |
| 18 months | 23.6 (24.9); 134 | 23.0 (25.5); 147 | –0.8 (–6.9 to 5.3); 0.729 |
| 24 months | 20.8 (24.7); 124 | 22.8 (24.7); 136 | –3.0 (–9.2 to 3.3); 0.220 |
| 36 months | 23.3 (26.5); 86 | 25.0 (24.7); 80 | 1.1 (–6.2 to 8.4); 0.704 |
| Sexual enjoyment | |||
| Baseline | 57.8 (36.5); 45 | 54.2 (35.1); 56 | |
| Post treatment | 69.3 (30.4); 38 | 56.8 (34.6); 54 | 6.9 (–10.7 to 24.6); 0.312 |
| 3 months | 54.8 (39.7); 45 | 61.3 (34.2); 62 | 7.9 (–9.8 to 25.5); 0.252 |
| 6 months | 53.0 (37.5); 56 | 63.3 (33.2); 59 | –5.1 (–22.2 to 12.0); 0.442 |
| 12 months | 55.0 (37.5); 57 | 65.2 (30.5); 69 | –3.1 (–19.9 to 13.7); 0.631 |
| 18 months | 59.2 (35.3); 58 | 68.5 (32.0); 56 | –0.3 (–16.9 to 16.4); 0.964 |
| 24 months | 54.1 (36.4); 45 | 65.2 (30.3); 47 | –3.8 (–23.1 to 15.5); 0.614 |
| 36 months | 52.1 (40.3); 39 | 60.8 (30.1); 40 | 10.7 (–10.1 to 31.5); 0.184 |
| Symptom scales and/or itemsb | |||
| Urinary symptoms | |||
| Baseline | 26.0 (21.1); 186 | 22.5 (19.5); 193 | |
| Post treatment | 31.7 (23.2); 169 | 30.3 (22.2); 175 | –0.7 (–5.9 to 4.5); 0.737 |
| 3 months | 29.6 (23.4); 185 | 29.7 (22.9); 196 | –2.3 (–7.4 to 2.8); 0.249 |
| 6 months | 24.8 (23.1); 182 | 23.4 (20.7); 189 | –0.6 (–5.8 to 4.6); 0.761 |
| 12 months | 26.0 (22.8); 162 | 22.5 (20.3); 174 | 1.7 (–3.6 to 7.1); 0.406 |
| 18 months | 24.9 (20.7); 162 | 21.3 (18.5); 164 | 2.4 (–3.0 to 7.7); 0.260 |
| 24 months | 25.2 (22.2); 148 | 23.4 (21.2); 154 | 0.6 (–4.9 to 6.1); 0.774 |
| 36 months | 23.5 (20.2); 98 | 22.4 (20.0); 95 | –1.0 (–7.4 to 5.5); 0.700 |
| Malaise | |||
| Baseline | 4.8 (12.0); 187 | 4.0 (10.4); 194 | |
| Post treatment | 7.9 (14.6); 171 | 6.9 (15.1); 180 | 0.4 (–3.1 to 3.8); 0.788 |
| 3 months | 6.3 (12.4); 185 | 6.9 (14.6); 197 | –0.7 (–4.0 to 2.7); 0.611 |
| 6 months | 6.7 (13.4); 183 | 5.6 (13.8); 189 | 0.2 (–3.2 to 3.6); 0.869 |
| 12 months | 5.7 (13.7); 163 | 4.4 (12.4); 173 | 0.8 (–2.7 to 4.4); 0.542 |
| 18 months | 5.9 (14.3); 164 | 4.6 (10.7); 164 | 0.4 (–3.2 to 4.0); 0.764 |
| 24 months | 6.1 (12.9); 150 | 4.0 (10.7); 156 | 1.2 (–2.5 to 4.9); 0.399 |
| 36 months | 5.5 (11.6); 100 | 3.3 (10.2); 95 | 0.5 (–4.0 to 5.0); 0.780 |
| Future worries | |||
| Baseline | 33.0 (25.4); 187 | 33.1 (22.1); 194 | |
| Post treatment | 33.9 (25.6); 172 | 36.4 (25.9); 181 | –3.8 (–9.2 to 1.7); 0.074 |
| 3 months | 35.1 (27.1); 185 | 36.7 (25.6); 197 | –2.1 (–7.4 to 3.3); 0.325 |
| 6 months | 30.6 (26.7); 183 | 33.3 (25.7); 189 | –3.4 (–8.8 to 2.0); 0.107 |
| 12 months | 29.5 (26.8); 163 | 30.1 (23.7); 174 | –1.1 (–6.7 to 4.5); 0.603 |
| 18 months | 26.3 (26.0); 164 | 30.8 (24.9); 166 | –3.9 (–9.5 to 1.8); 0.078 |
| 24 months | 26.7 (25.3); 151 | 28.8 (25.0); 156 | –2.2 (–8.0 to 3.5); 0.320 |
| 36 months | 24.3 (25.2); 100 | 27.5 (24.4); 95 | –3.9 (–10.7 to 2.8); 0.134 |
| Bloating and flatulence | |||
| Baseline | 18.7 (22.1); 187 | 18.8 (22.6); 194 | |
| Post treatment | 21.8 (22.9); 170 | 21.7 (23.4); 179 | 0.6 (–4.4 to 5.6); 0.758 |
| 3 months | 21.5 (22.0); 185 | 21.5 (23.2); 197 | –0.8 (–5.8 to 4.1); 0.660 |
| 6 months | 21.2 (21.6); 183 | 20.2 (23.0); 189 | 0.5 (–4.5 to 5.5); 0.810 |
| 12 months | 21.0 (22.1); 164 | 19.3 (21.1); 174 | –0.4 (–5.6 to 4.8); 0.842 |
| 18 months | 20.4 (20.2); 164 | 19.8 (24.0); 166 | –0.1 (–5.3 to 5.1); 0.973 |
| 24 months | 21.7 (22.9); 151 | 21.3 (22.9); 156 | –0.3 (–5.7 to 5.1); 0.886 |
| 36 months | 22.2 (23.2); 100 | 22.5 (21.6); 95 | –1.1 (–7.4 to 5.3); 0.671 |
| Sexual problems (men) | |||
| Baseline | 34.3 (38.2); 122 | 30.0 (35.7); 124 | |
| Post treatment | 36.4 (37.7); 108 | 33.2 (34.4); 110 | 2.5 (–8.4 to 13.5); 0.552 |
| 3 months | 39.3 (38.7); 106 | 29.2 (34.4); 128 | 10.0 (–1.0 to 20.9); 0.019 |
| 6 months | 39.7 (35.4); 108 | 37.3 (39.1); 121 | 0.4 (–10.8 to 11.6); 0.925 |
| 12 months | 41.7 (37.7); 94 | 38.5 (36.2); 120 | 1.0 (–10.5 to 12.4); 0.829 |
| 18 months | 41.2 (37.6); 98 | 39.7 (38.2); 110 | 0.2 (–11.3 to 11.8); 0.963 |
| 24 months | 45.1 (38.5); 92 | 39.3 (35.4); 100 | 1.4 (–10.5 to 13.3); 0.759 |
| 36 months | 47.6 (39.8); 63 | 36.8 (35.0); 62 | 7.4 (–6.6 to 21.4); 0.174 |
| Intravesical treatment issues | |||
| Baseline | 7.3 (16.3); 183 | 5.6 (14.6); 191 | |
| Post treatment | 9.4 (19.0); 166 | 10.5 (21.1); 175 | –1.9 (–7.0 to 3.2); 0.344 |
| 3 months | 11.6 (20.6); 184 | 9.5 (21.2); 193 | –0.2 (–5.2 to 4.8); 0.927 |
| 6 months | 8.9 (18.9); 179 | 8.8 (17.7); 189 | –0.6 (–5.7 to 4.4); 0.754 |
| 12 months | 10.8 (23.2); 161 | 8.4 (17.7); 174 | 0.0 (–5.2 to 5.2); 0.991 |
| 18 months | 7.1 (16.8); 164 | 6.1 (16.7); 163 | –0.0 (–5.3 to 5.2); 0.989 |
| 24 months | 5.8 (16.8); 149 | 7.5 (18.0); 155 | –2.0 (–7.4 to 3.4); 0.343 |
| 36 months | 4.7 (15.0); 100 | 6.0 (15.4); 95 | –2.8 (–9.3 to 3.6); 0.257 |
| Sexual intimacy | |||
| Baseline | 15.9 (24.4); 44 | 9.8 (21.4); 61 | |
| Post treatment | 16.7 (22.6); 40 | 18.1 (28.9); 57 | –10.4 (–25.5 to 4.8); 0.078 |
| 3 months | 20.4 (29.5); 49 | 18.2 (30.2); 64 | –5.8 (–20.7 to 9.1); 0.315 |
| 6 months | 14.0 (26.7); 57 | 13.8 (25.8); 63 | –4.7 (–19.5 to 10.1); 0.415 |
| 12 months | 17.9 (29.1); 56 | 8.1 (18.3); 70 | –6.3 (–21.2 to 8.5); 0.272 |
| 18 months | 18.1 (29.9); 59 | 16.1 (28.4); 56 | –4.1 (–18.6 to 10.5); 0.472 |
| 24 months | 16.7 (27.5); 48 | 14.9 (28.5); 47 | –3.9 (–21.0 to 13.1); 0.555 |
| 36 months | 17.5 (27.2); 40 | 12.2 (25.6); 41 | –2.8 (–21.5 to 16.0); 0.703 |
| Risk of contaminating partner | |||
| Baseline | 7.9 (21.9); 42 | 8.9 (19.6); 56 | |
| Post treatment | 13.5 (24.2); 37 | 14.9 (23.7); 56 | –9.1 (–24.9 to 6.7); 0.138 |
| 3 months | 17.7 (28.9); 49 | 18.5 (31.2); 65 | 0.5 (–15.0 to 15.9); 0.939 |
| 6 months | 11.1 (23.0); 57 | 11.3 (21.7); 62 | 1.2 (–14.0 to 16.3); 0.845 |
| 12 months | 10.1 (21.9); 56 | 8.0 (19.1); 71 | 1.6 (–13.6 to 16.8); 0.784 |
| 18 months | 10.3 (24.3); 58 | 12.5 (23.4); 56 | 0.5 (–14.3 to 15.4); 0.926 |
| 24 months | 8.8 (21.3); 49 | 13.0 (26.7); 46 | –6.0 (–23.3 to 11.3); 0.370 |
| 36 months | 10.8 (23.1); 40 | 8.9 (18.3); 41 | 1.9 (–17.8 to 21.5); 0.808 |
| Sexual problems (female) | |||
| Baseline | 33.3 (27.2); 7 | 33.3 (47.1); 8 | |
| Post treatment | 16.7 (19.2); 4 | 30.0 (39.9); 10 | –14.4 (–45.7 to 17.0); 0.238 |
| 3 months | 42.9 (46.0); 7 | 19.0 (37.8); 7 | –14.4 (–45.7 to 17.0); 0.238 |
| 6 months | 21.2 (34.2); 11 | 25.0 (38.8); 8 | –27.2 (–58.9 to 4.5); 0.027 |
| 12 months | 24.2 (39.7); 11 | 8.3 (23.6); 8 | –14.4 (–45.7 to 17.0); 0.238 |
| 18 months | 4.2 (11.8); 8 | 66.7 (38.5); 4 | –32.2 (–69.6 to 5.2); 0.026 |
| 24 months | 23.3 (35.3); 10 | 33.3 (47.1); 4 | –14.4 (–45.7 to 17.0); 0.238 |
| 36 months | 33.3 (44.1); 9 | 50.0 (57.7); 4 | –1.1 (–47.8 to 45.7); 0.953 |
Non-bladder-cancer causes of death
There was a total of 57 deaths, of which 40 were non-bladder-cancer deaths. The cause of death for these participants is shown in Table 35.
| Number | Cause of death/details of event |
|---|---|
| 1 | Perforated sigmoid colon, metastatic squamous cell carcinoma with lung metastases, ischaemic heart disease and aortic stenosis |
| 2 | Pneumonia, atrial fibrillation and bronchiectasia |
| 3 | Pneumonia and acute kidney injury on a background of COPD and CKD |
| 4 | Left-sided aspiration pneumonia, progressive ureteric transition cell carcinoma |
| 5 | Bilateral ureteric cancer with metastasis |
| 6 | Acute kidney injury and pulmonary oedema |
| 7 | Prostate cancer |
| 8 | Acute kidney injury, UTI, congestive cardiac failure, chronic kidney disease and AF |
| 9 | Heart failure and metastatic bladder cancer |
| 10 | Myocardial infarction |
| 11 | Massive pulmonary embolus with deep-vein thrombus and generalised atherosclerosis |
| 12 | Heptopulmonary syndrome and end-stage liver failure secondary to alcoholic liver disease |
| 13 | Aspiratory pneumonia and urinary tract infection |
| 14 | Myocardial infarction and ischaemic heart disease |
| 15 | Bony metastasis – primary likely to be rectal cancer or some unknown site |
| 16 | Respiratory tract infection, old age/Alzheimer’s disease, early-onset dementia |
| 17 | Myocardial infarction |
| 18 | Respiratory failure secondary to influenza A (H1N1) pneumonitis, acute kidney injury, atrial fibrillation, hypertension |
| 19 | Pneumonia and acute tubular necrosis of kidneys |
| 20 | Worsening heart failure pneumonia, frailty of old age and carcinomatosis |
| 21 | Respiratory failure |
| 22 | Sepsis |
| 23 | Cardiac arrest |
| 24 | Urosepsis, peripheral vascular disease and chronic kidney disease |
| 25 | Subdural brain haemorrhage |
| 26 | Metastatic lung cancer |
| 27 | Lung adenocarcinoma (believed primary), also metastases to right adrenal gland |
| 28 | Multiple organ failure, bilateral pneumonia, congestive cardiac failure |
| 29 | COPD |
| 30 | Metastatic disease from a primary of unknown origin – probably lung |
| 31 | Pancreatic cancer |
| 32 | Left total anterior circulation stroke |
| 33 | Pneumonia and frailty |
| 34 | Prostate cancer, contributed by a second malignancy TCC of the bladder |
| 35 | Ischaemic cerebral injury following cardiac arrest |
| 36 | Bronchopneumonia |
| 37 | Neutropenic sepsis. Acute myeloid leukaemia |
| 38 | Sepsis |
| 39 | Pneumonia |
| 40 | Empyema and renal failure |
Serious adverse events for participants who were included in the analysis
A total of 26 events were reported in 24 participants. Table 36 shows the details of the SAEs reported during the study.
| SAE number | Details of SAE | Seriousness criterion | Expected | Caused by taking part in the PHOTO trial |
|---|---|---|---|---|
| 1 | Lower urinary tract symptoms due to decreased flow | Hospitalisation | Yes | Yes |
| 2 | Episode of macroscopic haematuria following TURBT surgery, also found to have a urine infection from a urine dipstick | Hospitalisation | Yes | Yes |
| 3 | Warfarin recommenced in community, resulted in elevated INR to 7. Haematuria developed and settled when INR normalised to recommended threshold of three | Hospitalisation | Yes | Yes |
| 4 | Dysuria. Admitted with presumed UTI | Hospitalisation | – | Yes |
| 5 | Haematuria which worsened a few days before admission and unable to pass urine for a day with associated suprapubic pain | Hospitalisation | Yes | Yes |
| 6 | Haematuria, urine retention with associated pain | Hospitalisation | Yes | Yes |
| 7 | Haematuria post-surgery and prolonged hospital stay. Developed septic obstruction of right kidney | Prolongation of hospitalisation | Yes | Yes |
| 8 | Patient admitted to hospital with haematuria 8 days after having TURBT | Hospitalisation | Yes | Yes |
| 9 | Admitted due to urosepsis and acute kidney injury | Hospitalisation | Yes | Yes |
| 10 | Failed trial without catheter | Prolongation of hospitalisation | Yes | Yes |
| 11 | Failed trial without catheter post op then spiked a temperature, blood culture showed bacterial infection | Prolongation of hospitalisation | Yes | Yes |
| 12 | Patient still hospitalised 6 days after TURBT under PDD | Prolongation of hospitalisation | Yes | Yes |
| 13 | Patient readmitted to hospital following TURBT with haematuria, retention and low Hb | Hospitalisation | Yes | Yes |
| 14 | TURBT procedure abandoned. Surgeon unable to advance resectoscope into bladder due to tight urethra. False passage created. Urinary catheter eventually passed in order to drain bladder | Medically significant | Yes | Yes |
| 15 | Patient had TURBT but failed TWOC. Patient unable to pass urine so was re-catheterised | Prolongation of hospitalisation | Yes | Yes |
| 16 | Patient was admitted to hospital for meatal and urethral dilatation for submeatal stricture 1 cm from meatus | Hospitalisation | Yes | Yes |
| 17 | Patient admitted with a few hour history of haematuria and increased frequency | Hospitalisation | Yes | Yes |
| 18 | 1 week after surgery (6 days post discharge), patient was re-hospitalised with haematuria and clot retention | Hospitalisation | Yes | Yes |
| 19 | Patient was unable to pass urine, urinary retention | Prolongation of hospitalisation | Yes | Yes |
| 20 | Patient admitted to a&e department with dysuria | Hospitalisation | Yes | Yes |
| 21 | Urinary retention, for prolonged period following TURBT procedure | Prolongation of hospitalisation | Yes | Yes |
| 22 | Acute retention of urine following discharge home | Hospitalisation | Yes | Yes |
| 23 | Treated for two UTIs while awaiting BCG treatment required simple analgesia | Hospitalisation | Yes | Yes |
| 24 | Admitted to A&E after 3-day history of frank haematuria (dark red with clots) 2 weeks after TURBT. Coincided with restart of warfarin anticoagulation after pause for TURBT. Condition improved. Successful TWOC before discharge home | Hospitalisation | Yes | Yes |
| 25 | Presented to A&E for urinary retention, haematuria and discovered a blood clot three weeks post TURBT surgery | Hospitalisation | Yes | Yes |
| 26 | Endoscopic removal of blood clot from bladder | Prolongation of hospitalisation | Yes | Yes |
Table 37 shows the expected AEs collected following TURBT. The most common AEs were haematuria and bladder discomfort/pain.
| AE | Treatment group (n) | |
|---|---|---|
| PDD (N = 209) | WL (N = 217) | |
| Bladder discomfort/pain | 11 | 9 |
| Postoperative dysuria | 4 | 2 |
| Urinary retention | 8 | 10 |
| Urinary tract infection | 9 | 8 |
| Nausea | 1 | – |
| Urinary frequency | 8 | 2 |
| Increase in white blood cell count | 1 | 1 |
| Anaemia | – | 1 |
| DVT | 1 | – |
| Urethral stricture | – | 1 |
| Haematuria | 9 | 13 |
| Bleeding resulting in clot retention | 1 | 2 |
| Bladder perforation | – | 1 |
| Diarrhoea | 2 | – |
| Constipation | 4 | 2 |
| Fever | 1 | 1 |
| Prolonged catheterisation | 4 | 6 |
Participants who had no tumour, muscle-invasive bladder cancer or early cystectomy
Baseline characteristics
A total of 29 participants were found to have no tumour, 60 had MIBC and 18 had an early cystectomy after the initial TURBT. The minimisation variables, centre and sex of these participants are shown in Table 38.
| Minimisation variables | Treatment group, n (%) | |
|---|---|---|
| PDD (N = 56) | WL (N = 51) | |
| Centre | ||
| Newcastle Hospitals NHS Foundation Trust, Newcastle | 3 (5.4) | 5 (9.8) |
| Royal Devon and Exeter NHS Foundation Trust, Exeter | 9 (16.1) | 7 (13.7) |
| Oxford University Hospitals NHS Foundation Trust, Oxford | 3 (5.4) | 6 (11.8) |
| NHS Tayside, Dundee | – | 1 (2.0) |
| University College London Hospitals NHS Foundation Trust, London | 1 (1.8) | – |
| Ashford and St Peter’s Hospitals NHS Foundation Trust, Ashford | – | 1 (2.0) |
| NHS Lothian, Edinburgh | 7 (12.5) | 4 (7.8) |
| Hull University Teaching Hospitals NHS Trust, Cottingham | 2 (3.6) | – |
| South Tees Hospitals NHS Foundation Trust, Middlesbrough | 6 (10.7) | 2 (3.9) |
| Imperial College Healthcare NHS Trust, London | 3 (5.4) | – |
| Leeds Teaching Hospitals NHS Trust, Leeds | 4 (7.1) | 5 (9.8) |
| Swansea Bay University Health Board, Swansea | – | 1 (2.0) |
| Dartford and Gravesham NHS Trust, Dartford | 7 (12.5) | 3 (5.9) |
| University Hospital Southampton NHS Foundation Trust, Southampton | – | 2 (3.9) |
| University Hospitals of North Midlands NHS Trust, Stoke-on-Trent | 2 (3.6) | 2 (3.9) |
| Derby Teaching Hospitals NHS Foundation Trust, Derby | – | 1 (2.0) |
| Salisbury NHS Foundation Trust, Salisbury | 4 (7.1) | 2 (3.9) |
| NHS Grampian, Aberdeen | 2 (3.6) | 6 (11.8) |
| Royal Free London NHS Foundation Trust, London | – | 1 (2.0) |
| East and North Hertfordshire NHS Trust, Stevenage | 3 (5.4) | 2 (3.9) |
| Sex | ||
| Male | 46 (82.1) | 42 (82.4) |
| Female | 10 (17.9) | 9 (17.6) |
The baseline characteristics of participants who had MIBC, early cystectomy or no tumour are shown in Table 39.
| Baseline clinical characteristics | Treatment group | |
|---|---|---|
| PDD (N = 56) | WL (N = 51) | |
| Age (years), mean (SD) | 68.7 (12.4) | 67.2 (10.4) |
| Smoking status, n (%) | ||
| Current smoker | 10 (17.9) | 8 (15.7) |
| Previous smoker | 23 (41.1) | 29 (56.9) |
| Never | 23 (41.1) | 13 (25.5) |
| Unknown | – | 1 (2.0) |
| CIS, n (%) | ||
| Present | 6 (10.7) | 9 (17.6) |
| Absent | 34 (60.7) | 29 (56.9) |
| Missing | 16 (28.6) | 13 (25.5) |
| Grade of surgeon, n (%) | ||
| Registrar/non-consultant career grade | 14 (25.0) | 13 (25.5) |
| Consultant | 42 (75.0) | 37 (72.5) |
| Missing | – | 1 (2.0) |
The baseline HRQoL of participants who had MIBC, early cystectomy or no tumour are shown in Table 40.
| Baseline HRQoL | Treatment group | |
|---|---|---|
| PDD (N = 56) | WL (N = 51) | |
| EQ-5D-3L, mean (SD); n | ||
| Total score | 0.79 (0.29); 53 | 0.74 (0.27); 45 |
| Visual analogue scale | 73.86 (20.23); 49 | 70.34 (20.89); 47 |
| EORTC-QLQ-C30, median (25th percentile, 75th percentile) | ||
| Functioning scalesa | ||
| Physical | 91.7 (73.3, 100.0) | 86.7 (73.3, 100.0) |
| Role | 100.0 (66.7, 100.0) | 100.0 (66.7, 100.0) |
| Cognitive | 83.3 (83.3, 100.0) | 83.3 (83.3, 100.0) |
| Emotional | 91.7 (75.0, 91.7) | 83.3 (66.7, 91.7) |
| Social | 100.0 (83.3, 100.0) | 100.0 (66.7, 100.0) |
| Global QoL | 79.2 (58.3, 83.3) | 75.0 (58.3, 83.3) |
| Symptom scales and/or itemsb | ||
| Fatigue | 22.2 (0.0, 33.3) | 11.1 (0.0, 33.3) |
| Nausea and vomiting | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Pain | 16.7 (0.0, 33.3) | 16.7 (0.0, 50.0) |
| Dyspnoea | 0.0 (0.0, 33.3) | 0.0 (0.0, 33.3) |
| Sleep disturbance | 0.0 (0.0, 33.3) | 33.3 (0.0, 66.7) |
| Appetite loss | 0.0 (0.0, 33.3) | 0.0 (0.0, 0.0) |
| Constipation | 0.0 (0.0, 16.7) | 0.0 (0.0, 33.3) |
| Diarrhoea | 0.0 (0.0, 0.0) | 0.0 (0.0, 33.3) |
| Financial difficulties | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| EORTC-QLQ-NMIBC-24, median (25th percentile, 75th percentile) | ||
| Functioning scalesa | ||
| Sexual function | 0.0 (0.0, 50.0) | 16.7 (0.0, 33.3) |
| Sexual enjoyment | 66.7 (33.3, 66.7) | 66.7 (0.0, 100.0) |
| Symptom scales and/or itemsb | ||
| Urinary symptoms | 28.6 (19.0, 47.6) | 28.6 (14.3, 61.9) |
| Malaise | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Future worries | 33.3 (16.7, 41.7) | 33.3 (25.0, 58.3) |
| Bloating and flatulence | 16.7 (0.0, 33.3) | 16.7 (16.7, 50.0) |
| Sexual problems (men) | 33.3 (0.0, 50.0) | 33.3 (0.0, 66.7) |
| Intravesical treatment issues | 0.0 (0.0, 33.3) | 0.0 (0.0, 33.3) |
| Sexual intimacy | 0.0 (0.0, 33.3) | 0.0 (0.0, 16.7) |
| Risk of contaminating partner | 0.0 (0.0, 33.3) | 0.0 (0.0, 33.3) |
| Sexual problems (female) | 66.7 (0.0, 100.0) | 0.0 (0.0, 0.0) |
Outcomes
Of the 78 participants who had MIBC at baseline or an early cystectomy, 41 participants experienced recurrence of bladder cancer, 22 experienced progression, 15 died because of bladder cancer and 21 underwent cystectomy (Table 41). The HRQoL is reported in Tables 42 and 43. At 36 months, participants in the WL group experienced more financial difficulties than those in the PDD group (mean difference –14.5, 95% CI –32.9 to 3.9; p-value 0.04).
| Event | MIBC | Early cystectomy | Total | |||
|---|---|---|---|---|---|---|
| PDD (N = 32) | WL (N = 28) | PDD (N = 8) | WL (N = 10) | PDD (N = 40) | WL (N = 38) | |
| Recurrence | 18 | 21 | – | 2 | 18 | 23 |
| Progression | 10 | 11 | – | 1 | 10 | 12 |
| Death due to bladder cancer | 6 | 8 | – | 1 | 6 | 9 |
| Cystectomy | 11 | 10 | – | – | 11 | 10 |
| HRQoL | Treatment group, mean (SD); n | Estimate (99% CI); p-value | |
|---|---|---|---|
| PDD (N = 40) | WL (N = 38) | ||
| EQ-5D-3L | |||
| Baseline | 0.814 (0.240); 34 | 0.769 (0.278); 32 | |
| Post treatment | 0.692 (0.300); 30 | 0.592 (0.364); 30 | 0.111 (–0.048 to 0.269); 0.072 |
| 3 months | 0.774 (0.213); 32 | 0.622 (0.264); 28 | 0.146 (–0.015 to 0.306); 0.020 |
| 6 months | 0.785 (0.223); 23 | 0.722 (0.268); 21 | 0.081 (–0.102 to 0.265); 0.254 |
| 12 months | 0.767 (0.257); 21 | 0.697 (0.365); 16 | 0.122 (–0.075 to 0.319); 0.112 |
| 18 months | 0.779 (0.293); 18 | 0.755 (0.263); 15 | 0.052 (–0.151 to 0.256); 0.508 |
| 24 months | 0.833 (0.213); 17 | 0.763 (0.305); 14 | 0.133 (–0.080 to 0.346); 0.108 |
| 36 months | 0.830 (0.330); 10 | 0.811 (0.263); 9 | 0.076 (–0.191 to 0.343); 0.465 |
| EORTC-QLQ-C30 | |||
| Functioning scalesa | |||
| Physical | |||
| Baseline | 82.2 (19.5); 35 | 81.1 (23.0); 35 | |
| Post treatment | 77.6 (23.0); 28 | 72.6 (27.8); 33 | 3.9 (–7.5 to 15.3); 0.378 |
| 3 months | 73.7 (21.6); 31 | 62.9 (24.1); 29 | 12.4 (0.8 to 24.0); 0.006 |
| 6 months | 73.3 (18.2); 23 | 62.8 (27.9); 19 | 14.8 (0.8 to 28.8); 0.006 |
| 12 months | 71.1 (25.9); 21 | 71.7 (26.8); 17 | 7.0 (–7.5 to 21.5); 0.216 |
| 18 months | 72.6 (28.6); 18 | 79.3 (25.5); 15 | –0.7 (–16.3 to 14.8); 0.905 |
| 24 months | 77.3 (24.5); 17 | 75.2 (28.0); 14 | 9.8 (–6.3 to 25.9); 0.118 |
| 36 months | 78.3 (24.3); 12 | 76.3 (28.5); 9 | 3.7 (–16.0 to 23.3); 0.631 |
| Role | |||
| Baseline | 84.3 (25.5); 35 | 79.5 (29.7); 35 | |
| Post treatment | 66.1 (37.1); 29 | 66.2 (32.4); 33 | –2.2 (–20.7 to 16.3); 0.761 |
| 3 months | 65.6 (35.2); 31 | 52.3 (36.7); 29 | 13.5 (–5.3 to 32.4); 0.065 |
| 6 months | 63.8 (30.0); 23 | 53.3 (35.3); 20 | 9.7 (–12.5 to 31.8); 0.262 |
| 12 months | 69.8 (36.0); 21 | 68.6 (38.6); 17 | 3.2 (–20.0 to 26.4); 0.724 |
| 18 months | 73.1 (35.8); 18 | 73.3 (37.2); 15 | 4.7 (–20.1 to 29.5); 0.623 |
| 24 months | 73.5 (34.4); 17 | 72.6 (38.5); 14 | 8.7 (–16.8 to 34.3); 0.379 |
| 36 months | 80.6 (28.3); 12 | 77.8 (33.3); 9 | 4.3 (–26.6 to 35.2); 0.718 |
| Cognitive | |||
| Baseline | 86.7 (17.1); 35 | 83.8 (17.8); 35 | |
| Post treatment | 86.2 (14.1); 29 | 79.7 (21.5); 32 | 4.9 (–6.6 to 16.3); 0.273 |
| 3 months | 84.4 (16.4); 32 | 72.4 (26.8); 29 | 9.8 (–1.8 to 21.3); 0.030 |
| 6 months | 78.3 (22.2); 23 | 77.0 (22.0); 21 | 1.9 (–11.3 to 15.0); 0.715 |
| 12 months | 79.4 (16.6); 21 | 77.5 (25.6); 17 | 1.2 (–12.6 to 15.0); 0.820 |
| 18 months | 82.4 (20.2); 18 | 70.0 (36.3); 15 | 12.1 (–2.4 to 26.7); 0.031 |
| 24 months | 83.3 (17.7); 17 | 77.4 (25.8); 14 | 6.0 (–8.9 to 20.9); 0.303 |
| 36 months | 79.2 (17.6); 12 | 83.3 (16.7); 9 | 0.7 (–16.8 to 18.2); 0.922 |
| Emotional | |||
| Baseline | 80.5 (20.4); 35 | 76.2 (22.8); 35 | |
| Post treatment | 74.8 (25.8); 29 | 72.4 (27.4); 32 | –0.1 (–12.2 to 11.9); 0.975 |
| 3 months | 79.0 (20.7); 32 | 61.9 (26.7); 28 | 16.8 (4.5 to 29.1); 0.000 |
| 6 months | 83.0 (16.6); 23 | 65.6 (24.8); 21 | 15.1 (1.4 to 28.9); 0.005 |
| 12 months | 83.2 (17.1); 21 | 67.2 (27.7); 17 | 13.6 (–0.7 to 28.0); 0.015 |
| 18 months | 83.8 (22.4); 18 | 69.4 (27.9); 15 | 13.3 (–1.8 to 28.4); 0.024 |
| 24 months | 83.8 (19.4); 17 | 71.4 (25.5); 14 | 16.1 (0.6 to 31.6); 0.007 |
| 36 months | 84.7 (22.4); 12 | 82.4 (21.0); 9 | 7.6 (–10.5 to 25.7); 0.279 |
| Social | |||
| Baseline | 85.2 (24.5); 35 | 80.0 (27.1); 35 | |
| Post treatment | 75.9 (26.9); 29 | 71.4 (27.2); 32 | 2.6 (–13.5 to 18.6); 0.681 |
| 3 months | 66.1 (30.7); 32 | 56.5 (33.7); 28 | 9.3 (–7.0 to 25.7); 0.142 |
| 6 months | 65.9 (29.1); 23 | 61.9 (33.8); 21 | 2.3 (–16.5 to 21.2); 0.749 |
| 12 months | 73.0 (26.6); 21 | 61.8 (33.2); 17 | 11.4 (–8.6 to 31.4); 0.141 |
| 18 months | 78.7 (33.7); 18 | 68.9 (37.7); 15 | 8.3 (–13.0 to 29.6); 0.316 |
| 24 months | 73.5 (30.1); 17 | 64.3 (34.5); 14 | 12.3 (–9.7 to 34.3); 0.149 |
| 36 months | 81.9 (25.1); 12 | 85.2 (32.7); 9 | –1.8 (–28.2 to 24.6); 0.859 |
| Global QoL | |||
| Baseline | 73.6 (19.2); 35 | 71.4 (17.2); 35 | |
| Post treatment | 62.4 (25.8); 29 | 63.3 (17.4); 32 | –1.2 (–13.1 to 10.7); 0.795 |
| 3 months | 68.2 (17.1); 32 | 52.7 (23.8); 28 | 15.0 (2.8 to 27.1); 0.002 |
| 6 months | 65.6 (18.0); 23 | 65.1 (22.3); 21 | –0.5 (–14.5 to 13.5); 0.929 |
| 12 months | 72.2 (21.0); 21 | 65.2 (20.2); 17 | 7.5 (–7.3 to 22.4); 0.192 |
| 18 months | 75.5 (22.0); 18 | 64.4 (24.3); 15 | 12.2 (–3.6 to 28.0); 0.047 |
| 24 months | 79.4 (16.7); 17 | 67.9 (25.3); 14 | 14.8 (–1.5 to 31.1); 0.019 |
| 36 months | 75.7 (20.6); 12 | 71.3 (24.0); 9 | 7.3 (–12.3 to 26.8); 0.337 |
| Symptom scales and/or itemsb | |||
| Fatigue | |||
| Baseline | 24.6 (22.9); 35 | 23.3 (24.5); 35 | |
| Post treatment | 30.7 (23.9); 29 | 31.3 (22.3); 33 | –4.6 (–17.7 to 8.5); 0.369 |
| 3 months | 40.5 (24.8); 31 | 52.7 (29.7); 29 | –14.8 (–28.2 to –1.4); 0.004 |
| 6 months | 40.6 (19.4); 23 | 51.1 (27.8); 20 | –14.5 (–30.1 to 1.1); 0.017 |
| 12 months | 38.9 (28.8); 21 | 39.9 (29.7); 17 | –7.3 (–23.5 to 9.0); 0.251 |
| 18 months | 31.8 (21.4); 18 | 40.7 (27.1); 15 | –16.7 (–34.0 to 0.6); 0.013 |
| 24 months | 26.8 (26.1); 17 | 37.3 (31.9); 14 | –20.3 (–38.1 to –2.4); 0.003 |
| 36 months | 30.6 (22.8); 12 | 38.3 (27.3); 9 | –14.0 (–35.2 to 7.1); 0.088 |
| Nausea and vomiting | |||
| Baseline | 3.8 (10.8); 35 | 3.4 (9.0); 34 | |
| Post treatment | 4.0 (8.5); 29 | 10.6 (16.6); 33 | –10.2 (–20.6 to 0.2); 0.012 |
| 3 months | 5.4 (10.0); 31 | 18.4 (26.9); 29 | –16.3 (–26.9 to –5.7); 0.000 |
| 6 months | 8.0 (15.0); 23 | 13.3 (26.8); 20 | –10.7 (–22.9 to 1.6); 0.025 |
| 12 months | 5.6 (15.2); 21 | 11.8 (26.9); 17 | –10.0 (–23.0 to 3.0); 0.048 |
| 18 months | 0.9 (3.9); 18 | 5.6 (10.3); 15 | –10.5 (–24.3 to 3.4); 0.052 |
| 24 months | 4.9 (11.4); 17 | 2.4 (6.1); 14 | –4.4 (–18.7 to 10.0); 0.433 |
| 36 months | 4.2 (10.4); 12 | 5.6 (11.8); 9 | –9.5 (–26.9 to 7.9); 0.159 |
| Pain | |||
| Baseline | 18.1 (26.0); 35 | 26.2 (29.8); 35 | |
| Post treatment | 27.6 (30.6); 29 | 32.8 (31.0); 33 | –0.6 (–17.3 to 16.1); 0.925 |
| 3 months | 28.5 (27.3); 31 | 36.2 (29.6); 29 | –1.9 (–18.9 to 15.1); 0.773 |
| 6 months | 19.6 (29.6); 23 | 41.7 (34.8); 20 | –18.9 (–38.3 to 0.5); 0.012 |
| 12 months | 15.9 (26.1); 21 | 31.4 (36.3); 17 | –14.7 (–34.9 to 5.5); 0.061 |
| 18 months | 15.7 (23.2); 18 | 24.4 (32.7); 15 | –9.1 (–30.5 to 12.3); 0.273 |
| 24 months | 12.7 (23.2); 17 | 22.6 (36.8); 14 | –16.5 (–38.5 to 5.5); 0.053 |
| 36 months | 20.8 (32.7); 12 | 18.5 (30.6); 9 | –11.1 (–37.2 to 15.0); 0.274 |
| Dyspnoea | |||
| Baseline | 20.0 (25.8); 35 | 17.1 (28.4); 35 | |
| Post treatment | 21.4 (27.5); 28 | 20.2 (30.0); 33 | –0.3 (–15.1 to 14.4); 0.952 |
| 3 months | 25.8 (28.2); 31 | 27.6 (28.3); 29 | –6.8 (–21.9 to 8.2); 0.243 |
| 6 months | 33.3 (28.4); 23 | 25.0 (28.4); 20 | 2.8 (–14.8 to 20.4); 0.677 |
| 12 months | 27.0 (29.1); 21 | 19.6 (29.0); 17 | –0.8 (–19.2 to 17.6); 0.910 |
| 18 months | 27.5 (24.3); 17 | 17.8 (24.8); 15 | –0.1 (–19.9 to 19.6); 0.988 |
| 24 months | 23.5 (30.7); 17 | 21.4 (31.0); 14 | –3.7 (–23.8 to 16.4); 0.638 |
| 36 months | 25.0 (25.1); 12 | 22.2 (23.6); 9 | 2.5 (–21.5 to 26.5); 0.788 |
| Sleep disturbance | |||
| Baseline | 26.7 (33.1); 35 | 34.3 (37.5); 35 | |
| Post treatment | 31.0 (36.7); 29 | 42.4 (31.5); 33 | –8.2 (–25.0 to 8.6); 0.207 |
| 3 months | 28.0 (37.6); 31 | 44.8 (35.9); 29 | –13.0 (–30.1 to 4.1); 0.051 |
| 6 months | 30.4 (26.4); 23 | 28.3 (29.2); 20 | 5.7 (–14.4 to 25.7); 0.465 |
| 12 months | 20.6 (24.7); 21 | 31.4 (32.2); 17 | –14.7 (–35.6 to 6.3); 0.071 |
| 18 months | 20.4 (25.9); 18 | 28.9 (27.8); 15 | –14.2 (–36.5 to 8.1); 0.102 |
| 24 months | 21.6 (26.2); 17 | 21.4 (24.8); 14 | –6.9 (–29.9 to 16.1); 0.438 |
| 36 months | 33.3 (34.8); 12 | 25.9 (32.4); 9 | –2.2 (–29.9 to 25.5); 0.838 |
| Appetite loss | |||
| Baseline | 9.5 (19.1); 35 | 13.7 (28.6); 34 | |
| Post treatment | 17.2 (27.6); 29 | 23.2 (25.7); 33 | –3.3 (–20.2 to 13.6); 0.613 |
| 3 months | 17.2 (20.9); 31 | 37.9 (33.0); 29 | –21.8 (–39.0 to –4.6); 0.001 |
| 6 months | 21.7 (37.1); 23 | 31.7 (36.6); 20 | –11.1 (–30.9 to 8.8); 0.151 |
| 12 months | 22.2 (38.5); 21 | 19.6 (31.3); 17 | 1.0 (–20.0 to 22.0); 0.902 |
| 18 months | 7.4 (18.3); 18 | 17.8 (33.0); 15 | –10.6 (–33.0 to 11.7); 0.221 |
| 24 months | 3.9 (11.1); 17 | 16.7 (28.5); 14 | –16.4 (–39.5 to 6.7); 0.067 |
| 36 months | 13.9 (26.4); 12 | 18.5 (33.8); 9 | –8.5 (–36.4 to 19.4); 0.434 |
| Constipation | |||
| Baseline | 11.4 (22.8); 35 | 21.9 (32.3); 35 | |
| Post treatment | 25.3 (32.9); 29 | 30.3 (33.7); 33 | –1.4 (–20.1 to 17.3); 0.844 |
| 3 months | 25.8 (30.7); 31 | 44.0 (36.3); 28 | –14.3 (–33.5 to 4.9); 0.055 |
| 6 months | 23.2 (25.5); 23 | 33.3 (35.1); 19 | –7.8 (–30.3 to 14.6); 0.370 |
| 12 months | 22.2 (28.5); 21 | 27.5 (31.7); 17 | –6.6 (–29.8 to 16.6); 0.462 |
| 18 months | 14.8 (23.5); 18 | 26.7 (33.8); 15 | –14.1 (–38.9 to 10.6); 0.141 |
| 24 months | 21.6 (23.4); 17 | 21.4 (24.8); 14 | –4.6 (–30.2 to 20.9); 0.641 |
| 36 months | 27.8 (23.9); 12 | 8.3 (15.4); 8 | 8.4 (–23.4 to 40.2); 0.498 |
| Diarrhoea | |||
| Baseline | 6.9 (21.4); 34 | 14.3 (27.2); 35 | |
| Post treatment | 9.5 (20.0); 28 | 12.1 (20.1); 33 | –1.0 (–15.0 to 13.0); 0.855 |
| 3 months | 20.4 (30.6); 31 | 18.4 (26.1); 29 | –3.3 (–17.6 to 10.9); 0.549 |
| 6 months | 15.9 (31.6); 23 | 20.0 (33.2); 20 | 1.5 (–14.9 to 17.9); 0.810 |
| 12 months | 11.1 (21.9); 21 | 7.8 (14.6); 17 | 1.3 (–15.7 to 18.3); 0.842 |
| 18 months | 9.3 (19.2); 18 | 6.7 (13.8); 15 | 1.6 (–16.4 to 19.7); 0.816 |
| 24 months | 5.9 (13.1); 17 | 7.1 (14.2); 14 | –3.7 (–22.2 to 14.9); 0.612 |
| 36 months | 2.8 (9.6); 12 | 7.4 (22.2); 9 | –6.1 (–28.2 to 16.1); 0.480 |
| Financial difficulties | |||
| Baseline | 4.8 (18.3); 35 | 10.5 (23.9); 35 | |
| Post treatment | 1.1 (6.2); 29 | 11.5 (27.6); 32 | –8.4 (–20.1 to 3.4); 0.066 |
| 3 months | 6.3 (15.7); 32 | 19.0 (27.9); 28 | –11.6 (–23.5 to 0.3); 0.012 |
| 6 months | 2.9 (9.6); 23 | 14.3 (27.0); 21 | –11.8 (–25.4 to 1.7); 0.025 |
| 12 months | 3.2 (10.0); 21 | 13.7 (16.9); 17 | –11.6 (–25.9 to 2.6); 0.036 |
| 18 months | 1.9 (7.9); 18 | 17.8 (33.0); 15 | –14.9 (–30.0 to 0.2); 0.011 |
| 24 months | 2.0 (8.1); 17 | 14.3 (21.5); 14 | –10.4 (–25.9 to 5.2); 0.086 |
| 36 months | 2.8 (9.6); 12 | 18.5 (24.2); 9 | –14.5 (–32.9 to 3.9); 0.043 |
| HRQoL | Treatment group, mean (SD); n | Estimate (99% CI); p-value | |
|---|---|---|---|
| PDD (N = 40) | WL (N = 38) | ||
| Functioning scalesa | |||
| Sexual function | |||
| Baseline | 27.5 (33.8); 34 | 18.1 (22.6); 34 | |
| Post treatment | 22.2 (31.7); 27 | 13.9 (18.6); 30 | 3.4 (–9.0 to 15.7); 0.485 |
| 3 months | 11.1 (20.2); 30 | 9.6 (19.5); 26 | –2.3 (–14.9 to 10.3); 0.632 |
| 6 months | 6.9 (15.7); 17 | 15.7 (23.9); 17 | –9.2 (–24.9 to 6.6); 0.133 |
| 12 months | 18.8 (29.7); 16 | 16.7 (16.0); 14 | 0.7 (–16.0 to 17.4); 0.917 |
| 18 months | 21.8 (34.3); 13 | 15.3 (20.7); 12 | 4.6 (–13.6 to 22.7); 0.515 |
| 24 months | 18.1 (32.9); 12 | 18.1 (19.4); 12 | 2.7 (–15.9 to 21.2); 0.709 |
| 36 months | 25.8 (36.0); 11 | 19.0 (20.2); 7 | 1.6 (–20.0 to 23.2); 0.848 |
| Sexual enjoyment | |||
| Baseline | 61.1 (39.8); 12 | 55.6 (43.4); 12 | |
| Post treatment | 74.1 (32.4); 9 | 36.4 (34.8); 11 | 38.8 (10.8 to 66.8); 0.000 |
| 3 months | 73.3 (14.9); 5 | 38.1 (40.5); 7 | 14.8 (–21.1 to 50.7); 0.288 |
| 6 months | 66.7 (0.0); 2 | 53.3 (38.0); 5 | –1.5 (–42.8 to 39.9); 0.927 |
| 12 months | 53.3 (38.0); 5 | 50.0 (19.2); 4 | 28.2 (–9.2 to 65.7); 0.052 |
| 18 months | 53.3 (38.0); 5 | 75.0 (31.9); 4 | –1.4 (–40.5 to 37.7); 0.928 |
| 24 months | 50.0 (43.0); 4 | 41.7 (16.7); 4 | 33.7 (–7.9 to 75.4); 0.037 |
| 36 months | 50.0 (43.0); 4 | 44.4 (38.5); 3 | 6.2 (–46.4 to 58.7); 0.763 |
| Symptom scales and/or itemsb | |||
| Urinary symptoms | |||
| Baseline | 35.8 (21.8); 35 | 37.8 (28.7); 34 | |
| Post treatment | 38.5 (24.1); 29 | 46.0 (28.1); 32 | –4.7 (–17.1 to 7.7); 0.331 |
| 3 months | 42.3 (26.5); 27 | 46.0 (24.5); 26 | –2.9 (–16.3 to 10.4); 0.571 |
| 6 months | 27.1 (25.6); 20 | 32.2 (29.4); 18 | –0.1 (–15.8 to 15.7); 0.993 |
| 12 months | 21.4 (22.3); 17 | 27.9 (30.2); 12 | –2.5 (–20.3 to 15.4); 0.722 |
| 18 months | 14.3 (20.2); 13 | 20.7 (29.1); 14 | –4.8 (–22.9 to 13.3); 0.494 |
| 24 months | 13.5 (19.0); 12 | 22.3 (28.5); 12 | –8.3 (–27.6 to 10.9); 0.264 |
| 36 months | 14.3 (21.0); 9 | 30.6 (36.4); 7 | –11.0 (–34.8 to 12.8); 0.235 |
| Malaise | |||
| Baseline | 7.6 (20.7); 35 | 8.3 (19.4); 34 | |
| Post treatment | 6.9 (10.5); 29 | 11.5 (17.2); 32 | –5.4 (–14.7 to 3.9); 0.135 |
| 3 months | 5.6 (13.4); 30 | 19.6 (23.2); 28 | –15.7 (–25.3 to –6.1); 0.000 |
| 6 months | 9.5 (12.4); 21 | 15.8 (18.8); 19 | –6.4 (–17.9 to 5.2); 0.157 |
| 12 months | 1.9 (5.4); 18 | 10.7 (16.8); 14 | –8.2 (–21.0 to 4.5); 0.097 |
| 18 months | 5.9 (10.1); 17 | 9.5 (10.8); 14 | –3.4 (–16.4 to 9.5); 0.497 |
| 24 months | 3.3 (6.9); 15 | 9.7 (15.0); 12 | –7.3 (–21.2 to 6.7); 0.180 |
| 36 months | 10.0 (17.9); 10 | 9.5 (18.9); 7 | –1.6 (–19.5 to 16.3); 0.815 |
| Future worries | |||
| Baseline | 37.4 (23.2); 35 | 40.7 (31.0); 34 | |
| Post treatment | 42.8 (28.8); 29 | 48.1 (32.8); 31 | –1.0 (–14.9 to 13.0); 0.857 |
| 3 months | 39.8 (26.1); 31 | 52.1 (29.0); 28 | –11.2 (–25.4 to 3.0); 0.042 |
| 6 months | 31.7 (21.9); 22 | 45.3 (26.1); 20 | –9.6 (–26.2 to 6.9); 0.135 |
| 12 months | 34.9 (26.0); 19 | 46.0 (32.9); 16 | –2.7 (–20.4 to 15.1); 0.700 |
| 18 months | 30.6 (22.2); 17 | 46.4 (35.6); 14 | –9.2 (–27.9 to 9.5); 0.203 |
| 24 months | 26.0 (22.6); 17 | 37.5 (26.5); 12 | –5.4 (–24.9 to 14.1); 0.478 |
| 36 months | 30.0 (19.7); 10 | 36.5 (30.2); 8 | –2.7 (–26.9 to 21.4); 0.771 |
| Bloating and flatulence | |||
| Baseline | 17.1 (19.6); 35 | 33.8 (28.6); 34 | |
| Post treatment | 29.3 (24.3); 29 | 37.1 (25.0); 31 | 1.9 (–13.1 to 17.0); 0.740 |
| 3 months | 25.3 (21.9); 31 | 46.4 (28.8); 28 | –17.8 (–33.1 to –2.5); 0.003 |
| 6 months | 23.5 (27.5); 22 | 40.8 (30.8); 20 | –6.7 (–24.4 to 11.0); 0.331 |
| 12 months | 19.2 (23.1); 20 | 27.1 (20.1); 16 | –1.2 (–19.9 to 17.4); 0.865 |
| 18 months | 10.8 (21.2); 17 | 33.3 (24.4); 15 | –10.9 (–30.3 to 8.6); 0.151 |
| 24 months | 17.6 (24.6); 17 | 23.6 (30.5); 12 | –3.7 (–24.2 to 16.9); 0.646 |
| 36 months | 19.7 (29.6); 11 | 20.8 (21.4); 8 | 5.0 (–19.8 to 29.9); 0.603 |
| Sexual problems (men) | |||
| Baseline | 39.5 (37.0); 27 | 39.7 (38.0); 26 | |
| Post treatment | 37.9 (36.1); 22 | 39.4 (36.9); 22 | –8.5 (–34.8 to 17.8); 0.405 |
| 3 months | 55.3 (37.2); 22 | 52.0 (39.0); 17 | –1.7 (–31.2 to 27.8); 0.880 |
| 6 months | 60.3 (40.0); 13 | 71.7 (35.2); 10 | –21.3 (–58.2 to 15.6); 0.137 |
| 12 months | 56.9 (43.5); 12 | 69.7 (34.8); 11 | –16.7 (–52.4 to 19.0); 0.228 |
| 18 months | 60.4 (47.1); 8 | 91.7 (15.4); 8 | –33.1 (–76.0 to 9.7); 0.046 |
| 24 months | 64.6 (49.1); 8 | 72.9 (23.5); 8 | –18.5 (–61.3 to 24.3); 0.266 |
| 36 months | 72.9 (41.7); 8 | 66.7 (31.2); 5 | –10.7 (–58.4 to 37.1); 0.565 |
| Intravesical treatment issues | |||
| Baseline | 7.5 (14.2); 31 | 13.7 (24.8); 34 | |
| Post treatment | 10.7 (24.1); 28 | 15.1 (20.8); 31 | –1.4 (–15.9 to 13.0); 0.798 |
| 3 months | 12.9 (20.5); 31 | 22.2 (26.1); 27 | –10.7 (–25.7 to 4.4); 0.067 |
| 6 months | 6.7 (13.7); 20 | 11.1 (19.8); 18 | –4.3 (–22.4 to 13.9); 0.545 |
| 12 months | 16.7 (26.2); 18 | 26.7 (28.7); 15 | –3.9 (–22.8 to 15.0); 0.596 |
| 18 months | 10.4 (29.1); 16 | 23.8 (33.1); 14 | –2.7 (–22.4 to 17.1); 0.728 |
| 24 months | 0.0 (0.0); 15 | 19.4 (30.0); 12 | –11.6 (–32.4 to 9.3); 0.153 |
| 36 months | 0.0 (0.0); 10 | 28.6 (48.8); 7 | –17.7 (–44.1 to 8.7); 0.084 |
| Sexual intimacy | |||
| Baseline | 11.1 (29.6); 12 | 14.3 (21.5); 14 | |
| Post treatment | 16.7 (35.6); 8 | 16.7 (22.5); 12 | 17.5 (–11.7 to 46.8); 0.123 |
| 3 months | 13.3 (18.3); 5 | 38.1 (35.6); 7 | 7.2 (–30.5 to 44.9); 0.625 |
| 6 months | 16.7 (23.6); 2 | 50.0 (35.0); 6 | –21.3 (–63.7 to 21.1); 0.195 |
| 12 months | 6.7 (14.9); 5 | 75.0 (50.0); 4 | –44.0 (–86.3 to –1.7); 0.007 |
| 18 months | 13.3 (29.8); 5 | 33.3 (27.2); 4 | –12.2 (–53.5 to 29.2); 0.448 |
| 24 months | 25.0 (50.0); 4 | 33.3 (27.2); 4 | –21.4 (–65.9 to 23.1); 0.215 |
| 36 months | 11.1 (19.2); 3 | 0.0 (0.0); 3 | 5.3 (–54.5 to 65.0); 0.821 |
| Risk of contaminating partner | |||
| Baseline | 19.4 (38.8); 12 | 22.2 (35.8); 12 | |
| Post treatment | 25.9 (36.4); 9 | 24.2 (36.8); 11 | 12.0 (–13.1 to 37.0); 0.218 |
| 3 months | 13.3 (29.8); 5 | 4.8 (12.6); 7 | 25.4 (–8.3 to 59.1); 0.052 |
| 6 months | 0.0 (0.0); 2 | 46.7 (38.0); 5 | –28.0 (–62.8 to 6.8); 0.038 |
| 12 months | 16.7 (19.2); 4 | 16.7 (33.3); 4 | 51.0 (17.0 to 84.9); 0.000 |
| 18 months | 0.0 (0.0); 5 | 8.3 (16.7); 4 | 7.0 (–31.4 to 45.4); 0.638 |
| 24 months | 0.0 (0.0); 4 | 0.0 (0.0); 4 | 35.9 (–2.5 to 74.4); 0.016 |
| 36 months | 0.0 (0.0); 4 | 0.0 (0.0); 3 | 29.7 (–14.2 to 73.5); 0.081 |
| Sexual problems (female) | |||
| Baseline | 66.7 (–); 1 | 0.0 (0.0); 3 | |
| Post treatment | 66.7 (–); 1 | 11.1 (19.2); 3 | |
| 3 months | – (–); 0 | 33.3 (–); 1 | |
| 6 months | – (–); 0 | 66.7 (0.0); 2 | |
| 12 months | 33.3 (–); 1 | – (–); 0 | |
| 18 months | 66.7 (–); 1 | 100.0 (.); 1 | |
| 24 months | 100.0 (–); 1 | 100.0 (.); 1 | |
| 36 months | – (–); 0 | 0.0 (.); 1 | |
Serious adverse events
The number of SAEs is reported in Table 44. Overall, there were five participants who experienced SAEs (more than one SAEs could be reported per participant) after the initial or second resection. The details of the SAEs reported during the study are shown in Table 45.
| SAE | Treatment group | |
|---|---|---|
| PDD (N = 56) | WL (N = 51) | |
| Participants, n (%) | 3 (5.4) | 2 (3.9) |
| Events, n | 4 | 2 |
| Event related to the TURBT surgery | 4 | 2 |
| Expected events | 4 | 2 |
| Type of event, n | ||
| Prolongation of existing hospitalisation | 2 | 1 |
| Required rehospitalisation after medical discharge | 2 | 1 |
| SAE number | Details of SAE | Seriousness criterion | Expected | Caused by taking part in PHOTO |
|---|---|---|---|---|
| 1 | Patient admitted via A&E with haematuria 7 days post TURBT, without pain or fever | Hospitalisation | Yes | Yes |
| 2 | Developed retention post TURBT | Prolongation of hospitalisation | Yes | Yes |
| 3 | Admitted with UTI and left kidney hydronephrosis | Hospitalisation | Yes | Yes |
| 4 | Clot retention following discharge home | Hospitalisation | Yes | Yes |
| 5 | Increased blood in urine seen. Bladder washout showed large blood clots | Prolongation of hospitalisation | Yes | Yes |
| 6 | The patient developed a postoperative UTI and urinary retention | Prolongation of hospitalisation | Yes | Yes |
Appendix 2 Within-trial economic evaluation
EQ-5D-3L results at each time point
FIGURE 16.
The EQ-5D-3L mobility domain by treatment group and follow-up visit. (a) PDD; and (b) WLC. Analysis based on all of the available EQ-5D-3L data points.
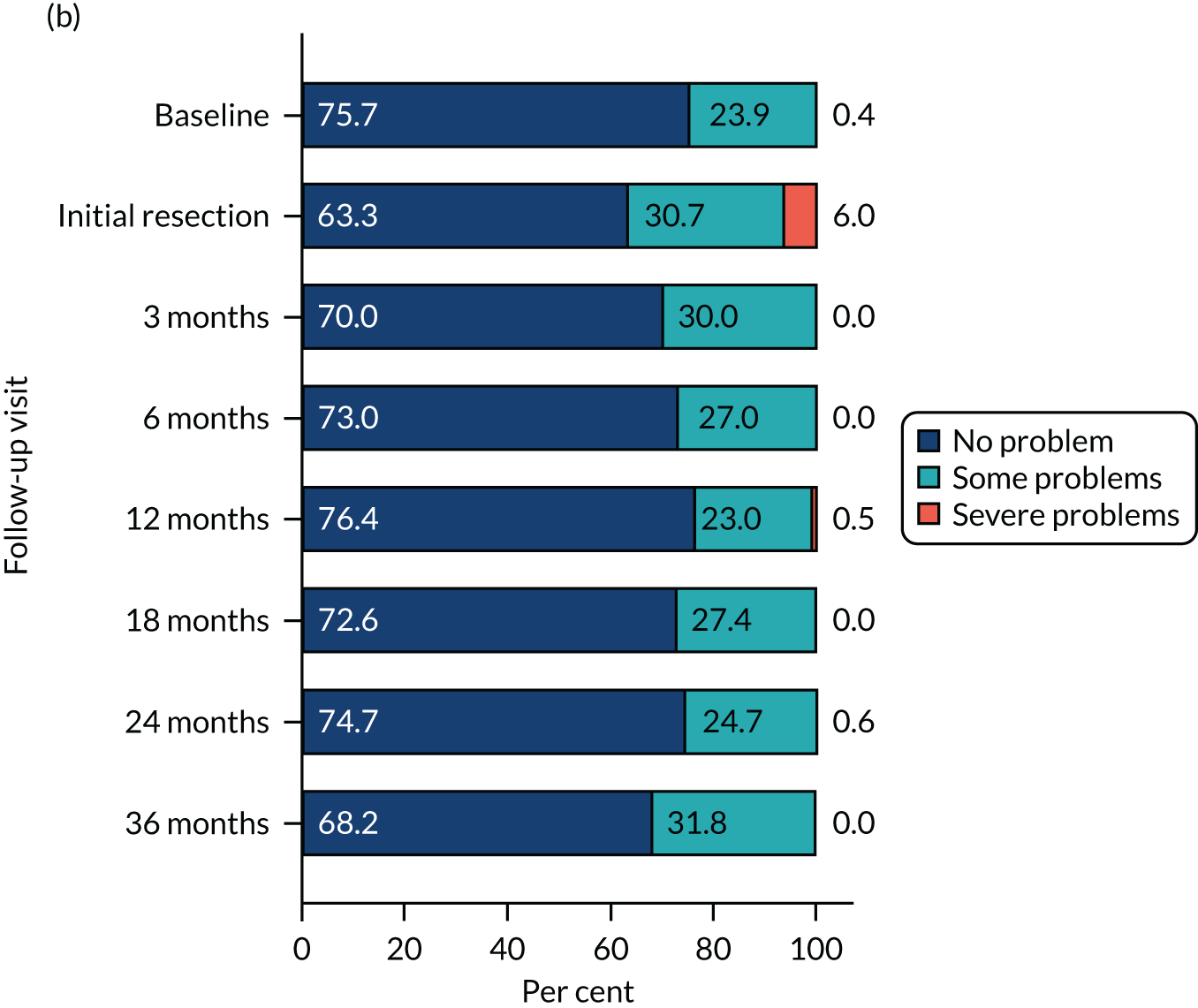
FIGURE 17.
The EQ-5D-3L self-care domain by treatment group and follow-up visit. (a) PDD; and (b) WLC. Analysis based on all of the available EQ-5D-3L data points.
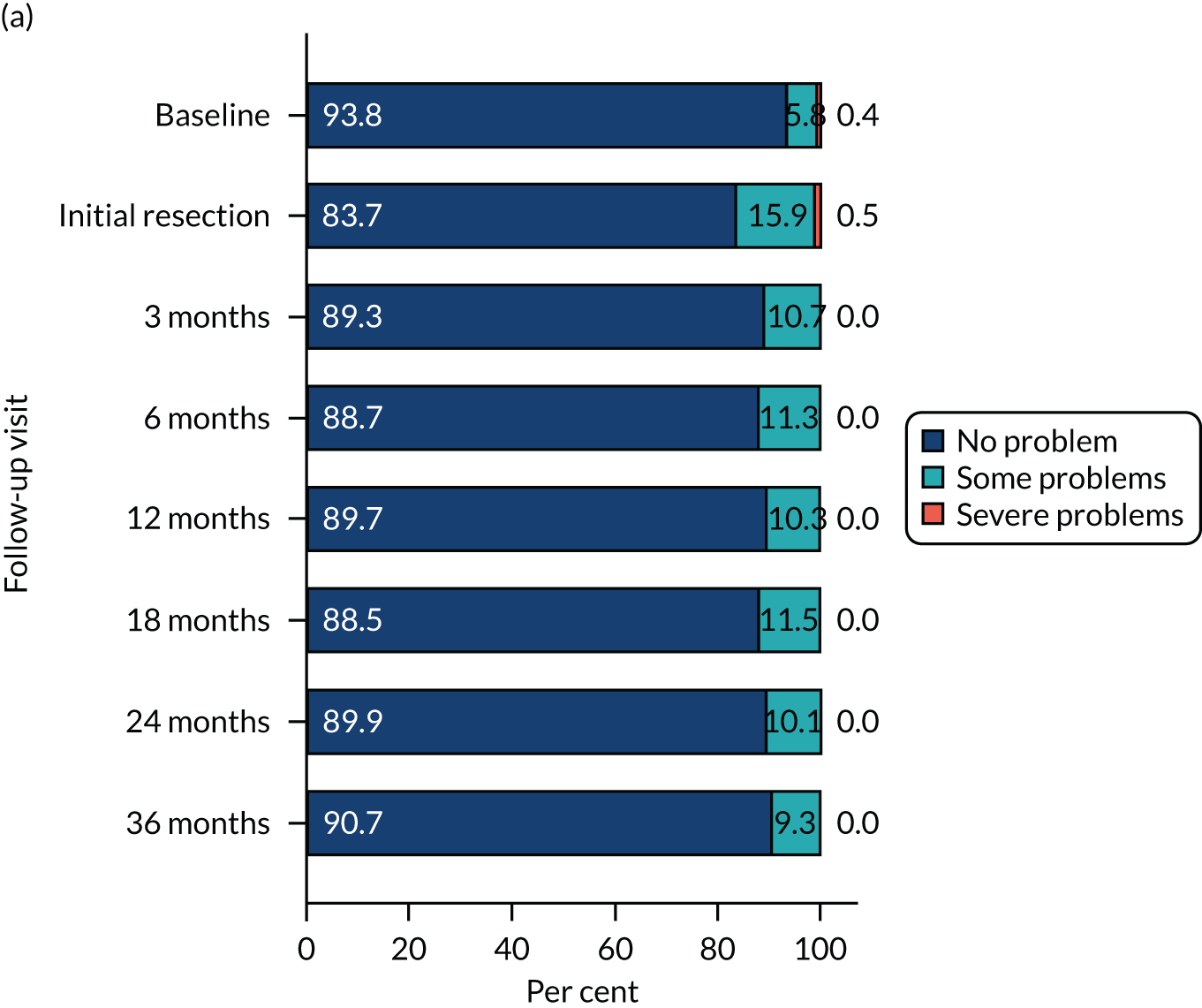
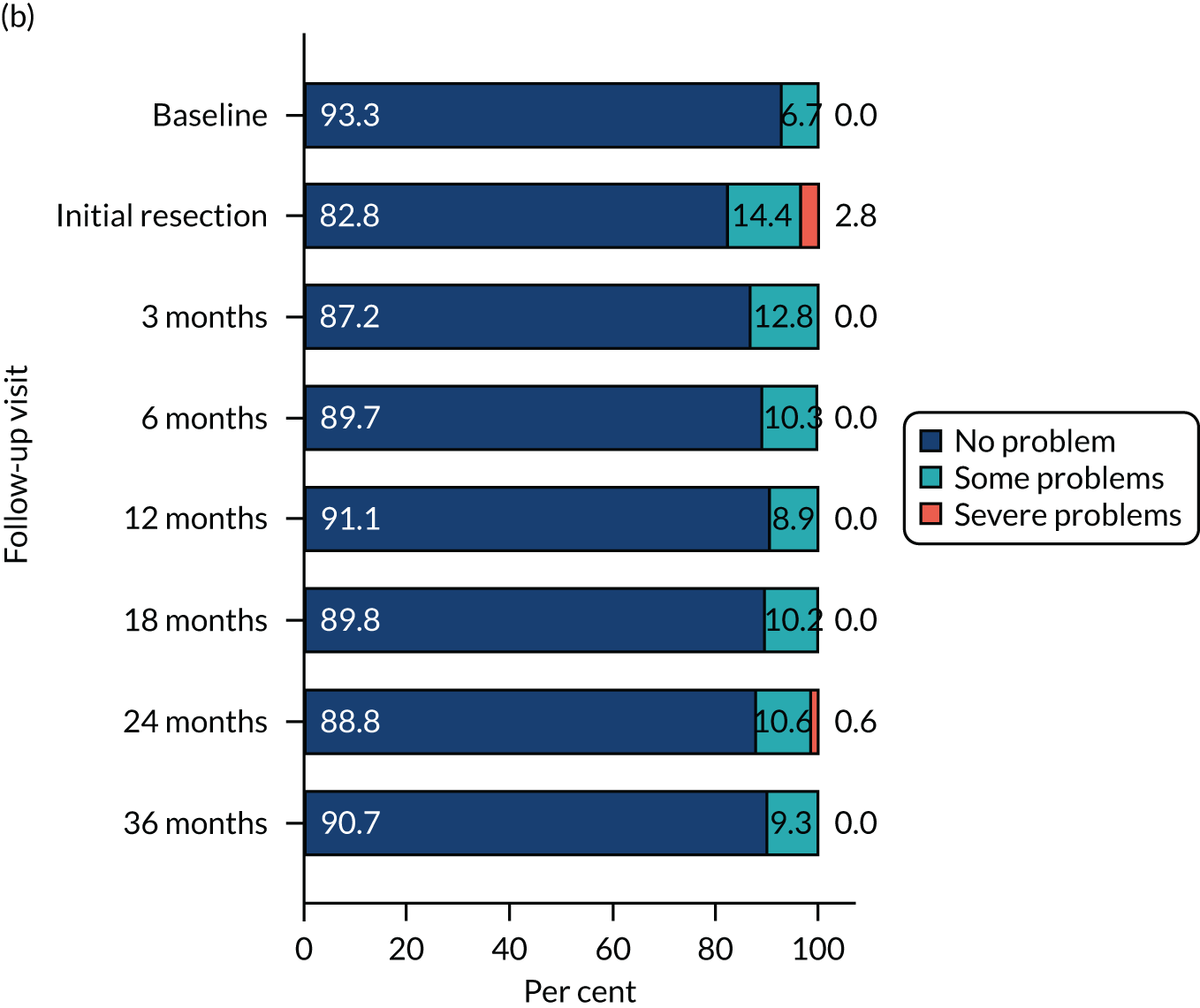
FIGURE 18.
The EQ-5D-3L usual activity domain by treatment group and follow-up visit. (a) PDD; and (b) WLC. Analysis based on all of the available EQ-5D-3L data points.
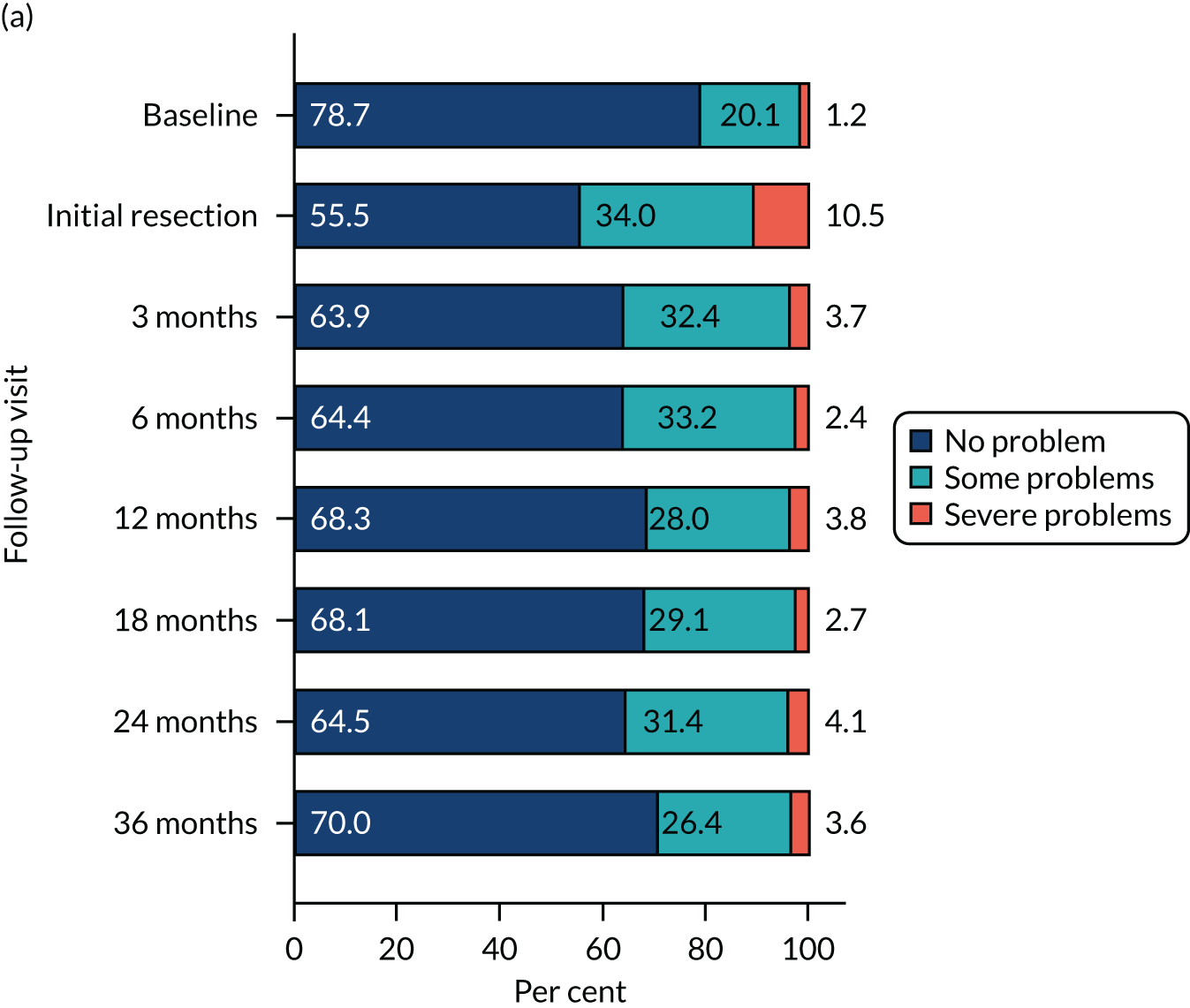
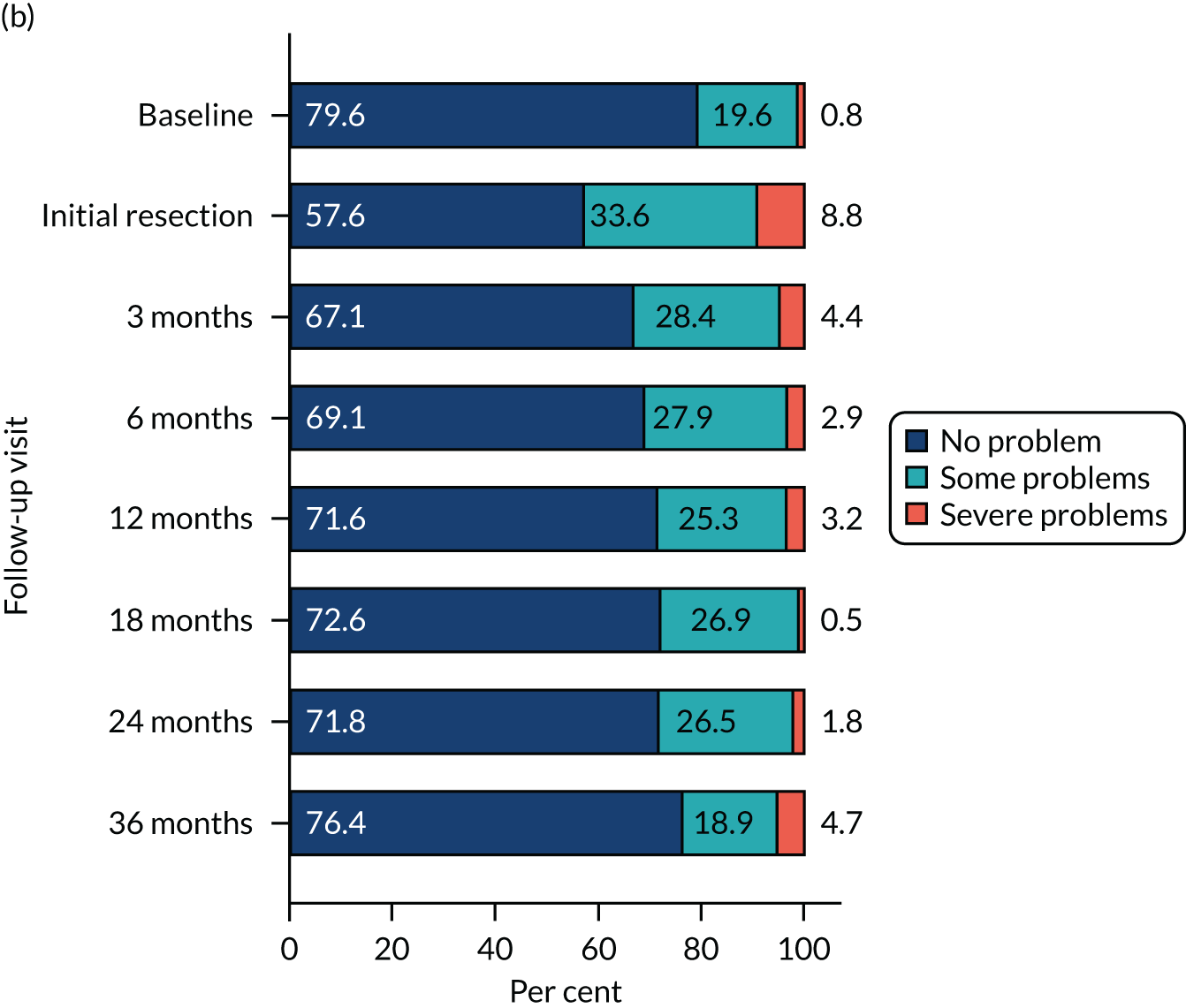
FIGURE 19.
The EQ-5D-3L pain and discomfort domain by treatment group and follow-up visit. (a) PDD; and (b) WLC. Analysis based on all of the available EQ-5D-3L data points.
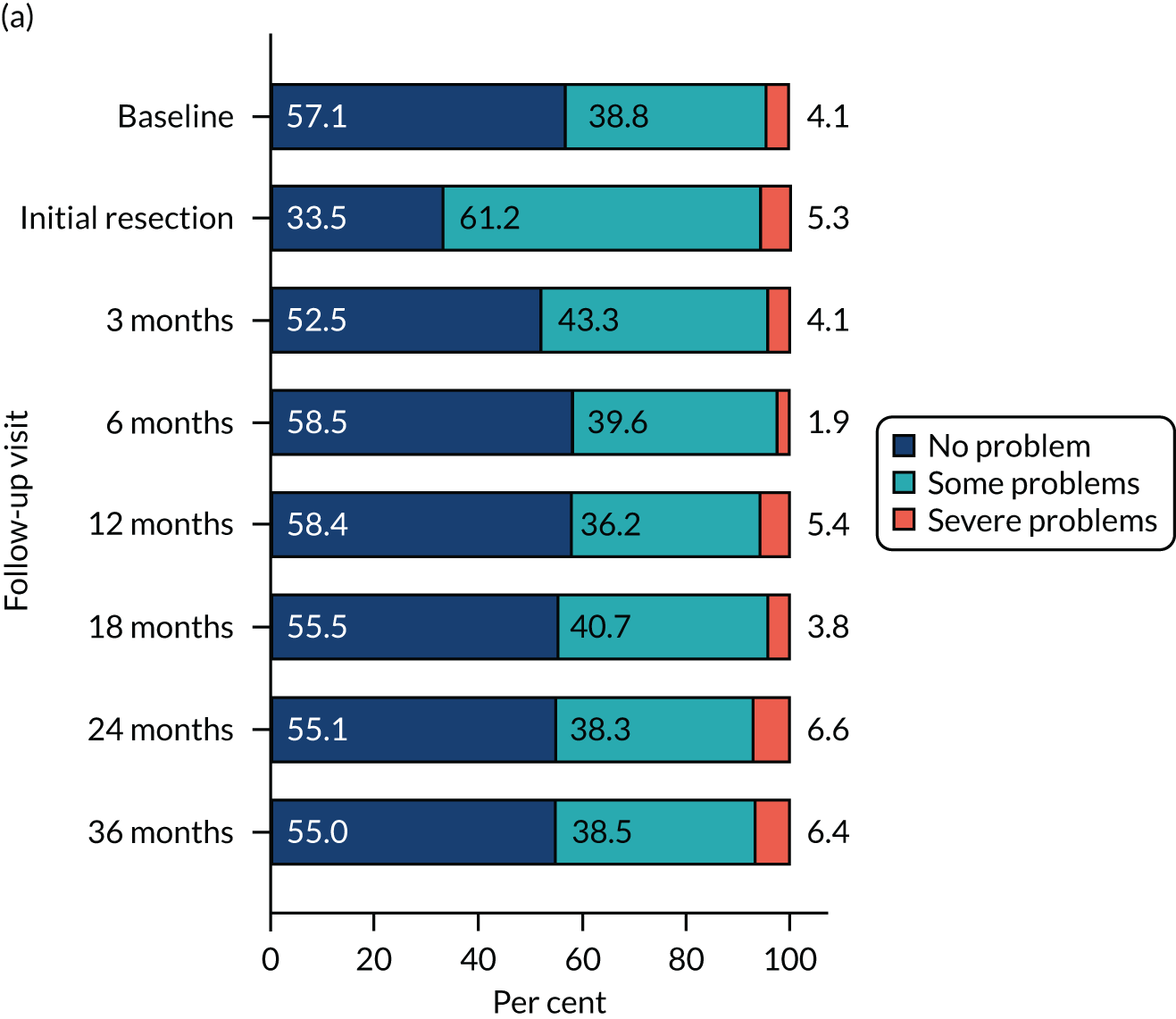
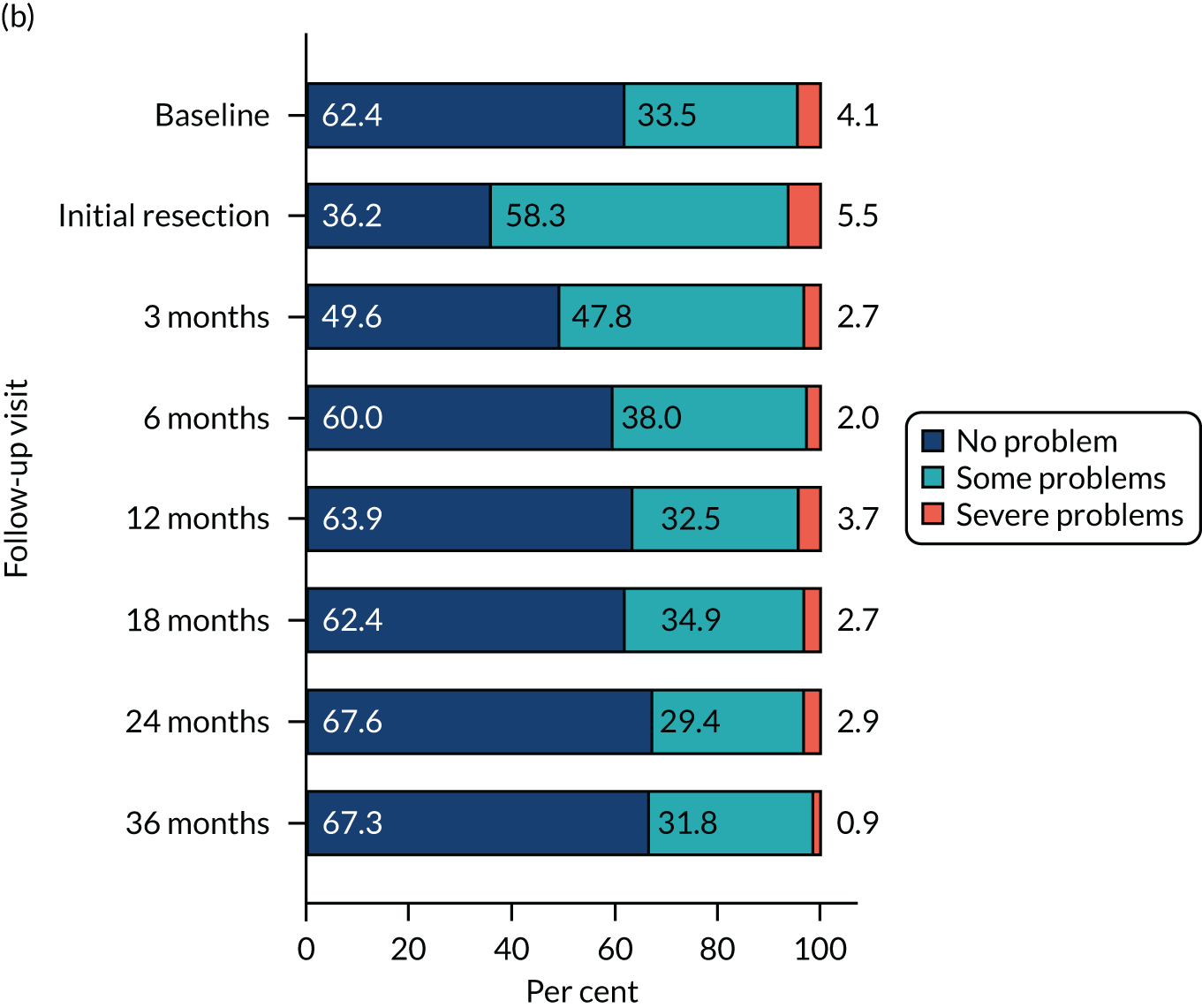
FIGURE 20.
The EQ-5D-3L anxiety and depression domain by treatment group and follow-up visit. (a) PDD; and (b) WLC. Analysis based on all of the available EQ-5D-3L data points.
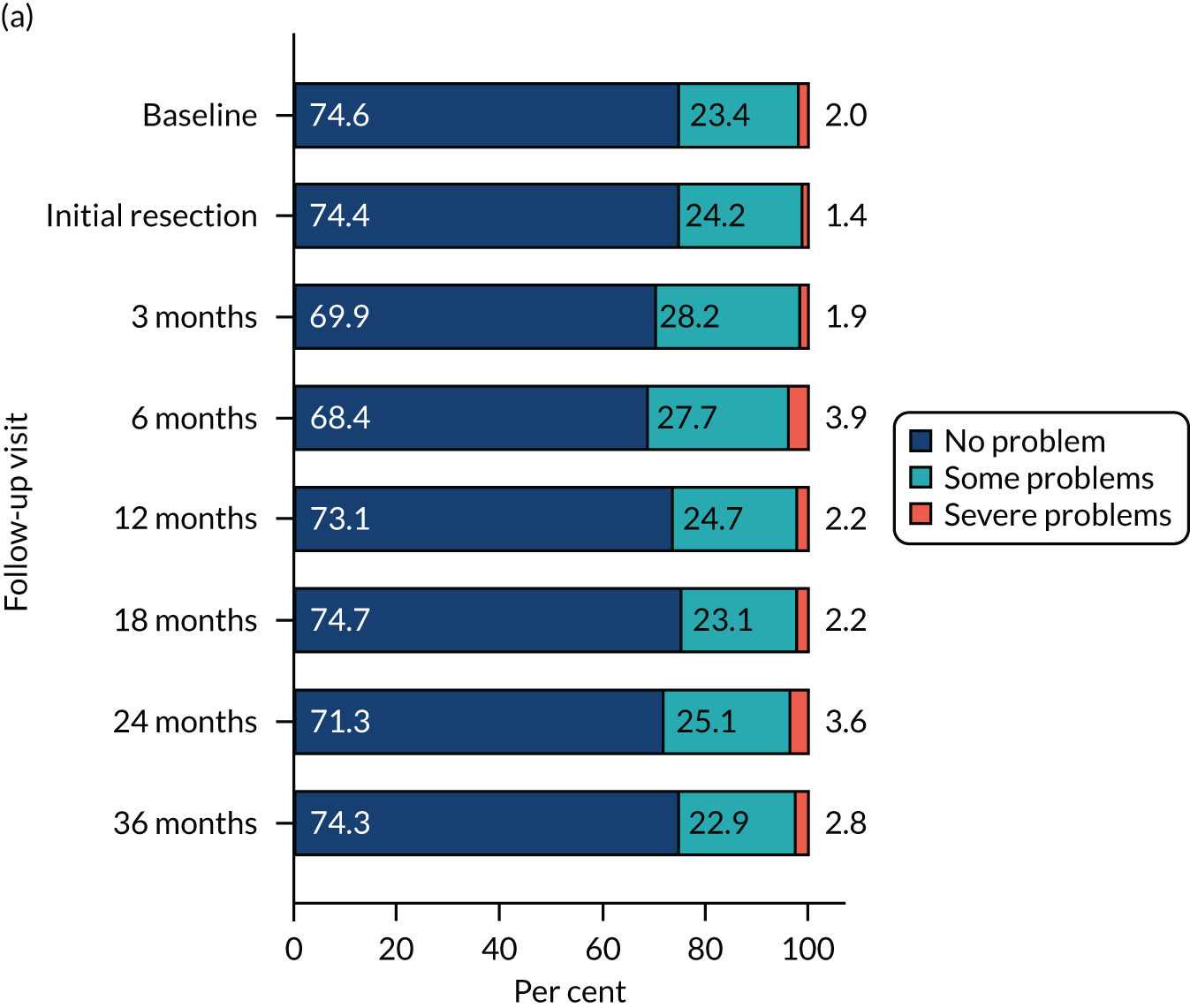
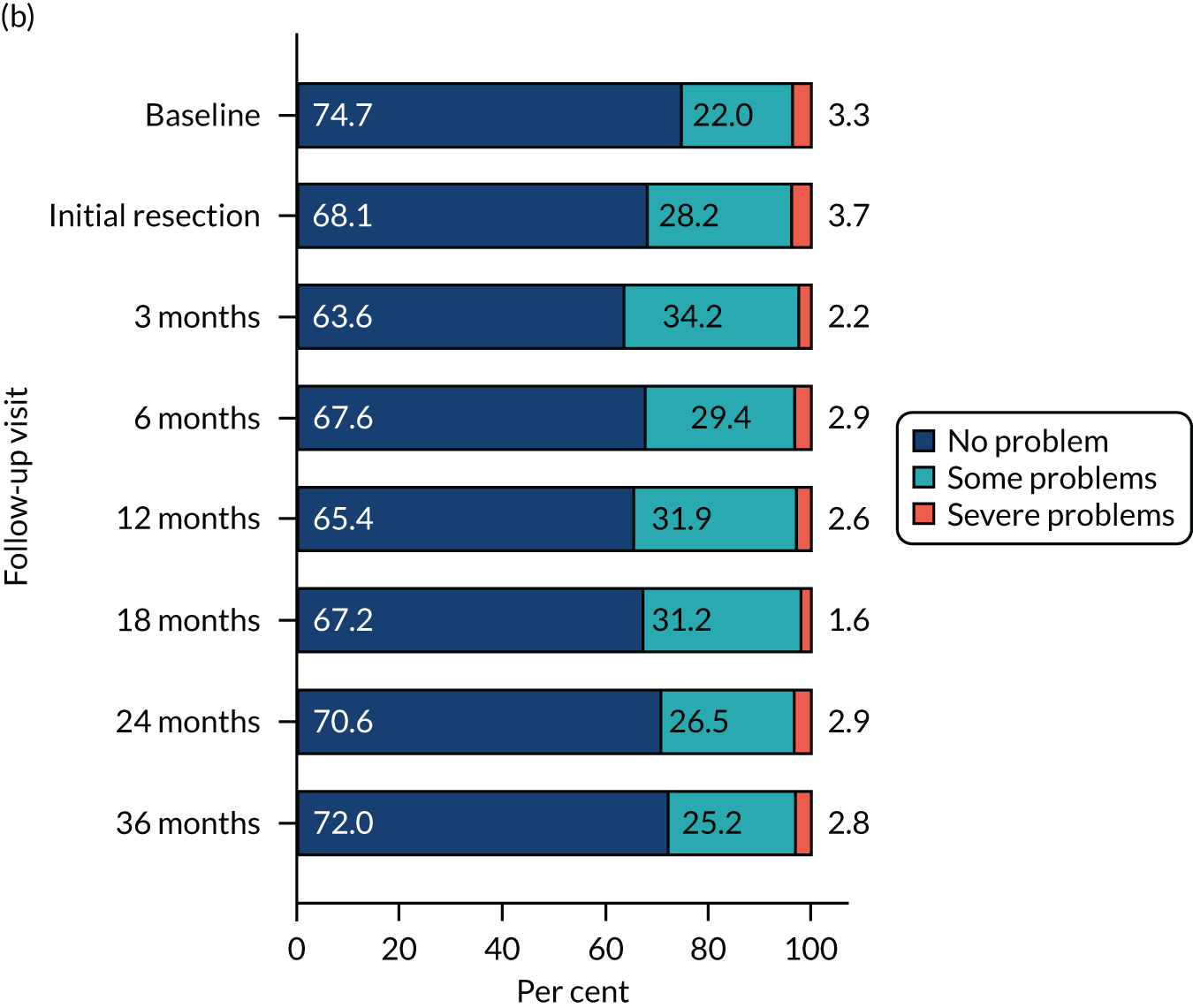
| Resource use item | Cost per unit (£, 2018/19) | Source |
|---|---|---|
| Initial TURBT | ||
| Hexvix | 347.00 | Dindyal et al.,102 inflated to 2018/19 prices using the CCEMG-EPPI-Centre Inflation Calculator |
| Operating surgeon | ||
| Consultant | 108.00 | PSSRU 2018,38 14. Hospital-based doctors: consultant surgical |
| Registrar | 43.00 | PSSRU 2018,38 14. Hospital-based doctors: registrar |
| Non-consultant | 105.00 | PSSRU 2018,38 14. Hospital-based doctors: associate specialist |
| MMC dose (cost per 40-mg vial) | 135.00 | BNF 76th edition,36 NHS indicative price: Mitomycin 40mg powder and solvent for intravesical solutions vials (medac UK) |
| MMC deliver in theatre | ||
| Mito-In system (Laboratorios Inibsa SA, Barcelona, Spain) | 4.33 | £4.00 in 2012, NICE guideline,18 inflated to 2018/19 prices using the CCEMG-EPPI-Centre Inflation Calculator |
| Surgical consultant time (estimate of 2 minutes) | 5.06 | £4.67 in 2012, NICE guideline,18 inflated to 2018/19 prices using the CCEMG-EPPI-Centre Inflation Calculator |
| Secondary care | ||
| WLC | 1072.76 | £937, HTA Report 2010,11,27 inflated to 2018/19 prices using the CCEMG-EPPI-Centre Inflation Calculator |
| PDD cystoscopy | 1569.65 | £1371 HTA Report 2010,11,27 inflated to 2018/19 prices using the CCEMG-EPPI-Centre Inflation Calculator |
| Narrow-band imaging | 1120.00 | NICE, Narrow Band Imaging for Barrett’s Oesophagus103 |
| Flexible cystoscopy (day case) | 467.58 | NHS Reference Costs 2018–19,37 HRG (day case) code LB72 A: Diagnostic Flexible Cystoscopy, 19 years and over |
| Flexible cystoscopy (outpatient) | 186.79 | NHS Reference Costs 2018–19,37 HRG (outpatient) code LB72 A: Diagnostic Flexible Cystoscopy, 19 years and over |
| Induction BCG drug cost (6 doses) | 429.66 | BNF 75,36 NHS indicative price (hospital only): OncoTICE 12.5mg powder for reconstitution for instillation vials (Merck Sharp and Dohme Ltd) |
| Induction BCG delivery cost | 1464.88 | £1324.42 in 2012, NICE guideline,18 inflated to 2018/19 prices using the CCEMG-EPPI-Centre Inflation Calculator |
| Maintenance BCG drug cost (3 doses, one every 6 months) | 214.83 | |
| Maintenance BCG delivery cost | 732.44 | £662.21 at 2012, NICE guideline 2015,18 inflated to 2018/19 prices using CCEMG-EPPI-Centre Inflation Calculator |
| CT scan | 83.23 | NHS Reference Costs 2018–19,37 HRG (diagnostic imaging) code RD20 A: Computerised Tomography Scan of One Area, without Contrast, 19 years and over |
| Magnetic resonance imaging scan | 136.00 | NHS Reference Costs 2018–19,37 HRG (diagnostic imaging) code RD01 A: Magnetic Resonance Imaging Scan of One Area, without Contrast, 19 years and over |
| Neoadjuvant chemotherapy | 1207.30 | £1091.54 in 2012, NICE guideline,18 inflated to 2018/19 prices using the CCEMG-EPPI-Centre Inflation Calculator |
| Radical cystectomy | 10,416.00 | NHS Reference Costs 2018–19,37 HRG (elective inpatient) code LB39D: Cystectomy with Urinary Diversion and Reconstruction, with CC Score 0–2 |
| Blood tests (kidney and PSA tests) | 22.12 | £20 at 2012, NICE guideline,18 inflated to 2018/19 prices using the CCEMG-EPPI-Centre Inflation Calculator |
| Urethroscopy | 961.73 | NHS Reference Costs 2018–19,37 HRG (day case) code LB55 A: Minor or Intermediate, Urethra Procedures, 19 years and over |
| Urology consultant | 110.82 | NHS Reference Costs 2018–19,37 code 101 (consultant led) |
| Radical radiotherapy | 1156.00 | HTA report27 inflated to 2018/19 prices using the CCEMG-EPPI-Centre Inflation Calculator |
| A&E visit | 168.00 | NHS Reference Costs 2018–19,37 HRG (total unit cost) service code 180 |
| Day case | 752.00 | NHS Reference Costs 2018–19,37 Day case (unit cost) |
| Inpatient attendance | 891.00 | NHS Reference Costs 2018–19,37 minor bladder procedures, age 19 years and over (HRG code LB15E), elective inpatients (an alternative unit cost of excess bed-day for elective care, £468, was used in a sensitivity analysis) |
| Outpatient attendance | 108.00 | NHS Reference Costs 2018–19,37 urology outpatient attendance (service code, 101), TOA |
| Primary care | ||
| GP | ||
| At practice | 33.30 | PSSRU, Unit Costs of Health and Social Care, 2018,38 II Community-based health care staff: 10.3 General Practitioner |
| At home | 139.49 | PSSRU, Unit Costs of Health and Social Care, 2009,104 (£120), home visit lasting 23.4 minutes, inflated using the CCEMG-EPPI-Centre Inflation Calculator |
| Telephone | 15.10 | PSSRU, Unit Costs of Health and Social Care, 2018,38 II Community-based health care staff: 10.5 Telephone triage – GP-led and Nurse-led |
| Out of hours | 72.91 | £68.30, Out-of-hours GP services in England, Department of Health and Social Care and NHS England (2014),105 inflated to 2018/19 prices using CCEMG-EPPI-Centre Inflation Calculator |
| Nurse | ||
| At hospital | 28.00 | PSSRU, Unit Costs of Health and Social Care, 2018,38 VI Hospital-based health care staff: 13. Hospital-based nurses ‘Band 2’ |
| At practice | 36.00 | PSSRU, Unit Costs of Health and Social Care, 2018,38 II Community-based health care staff: 10.2 Nurse (GP-practice) |
| At home | 23.25 | PSSRU, Unit Costs of Health and Social Care, 2009104 (£20), inflated using the CCEMG-EPPI-Centre Inflation Calculator |
| Telephone | 7.70 | PSSRU, Unit Costs of Health and Social Care, 2018,38 II Community-based health care staff: 10.5 Telephone triage – GP-led and Nurse-led |
| Out of hours | 72.91 | Assumed to be the same as GP out of hours |
| Hospital doctor | 43.00 | PSSRU, Unit Costs of Health and Social Care, 2018,38 VI Hospital-based health care staff: 14. Hospital-based doctors, ‘Registrar’ |
| Hospital doctor: telephone | 15.10 | Assumed to be the same as GP-led phone triage |
| Participant and companion travel | ||
| Cost per mile travelled by car | 0.45 | HMRC, Travel – Mileage and Fuel Rates and Allowances106 |
| Car parking charges | Various | Participant-reported data |
| Cost of public transport fares (e.g. bus, train, taxi) | Various | Participant-reported data |
| Cost of non-emergency patient transport service (via ambulance) | 47.67 | NHS Reference Costs 2009–2010107 (not included in reference costs since 2011), inflated to 2018/19 prices using the CCEMG-EPPI-Centre Inflation Calculator |
| Participant and companion time | ||
| Paid work | 12.71 | ONS Annual Survey of Hours and Earnings Time Series of Selected Estimates, 2020108 (all employees: median hourly earnings, excluding overtime) |
| Full-time employment | 14.31 | ONS Annual Survey of Hours and Earnings Time Series of Selected Estimates, 2020108 (full-time employees: mean hourly earnings, excluding overtime) |
| Part-time employment | 9.34 | ONS Annual Survey of Hours and Earnings Time Series of Selected Estimates, 2020108 (part-time employees: median hourly earnings, excluding overtime) |
| Housework | 11.24 | NHS Pay Review Body Twenty-sixth Report 2012,109 inflated to 2018/19 prices using the CCEMG-EPPI-Centre Inflation Calculator |
| Child care | 12.71 | ONS Annual Survey of Hours and Earnings Time Series of Selected Estimates, 2020108 (as paid work) |
| Caring for someone | 12.71 | ONS Annual Survey of Hours and Earnings Time Series of Selected Estimates, 2020108 (as paid work) |
| Voluntary work | 12.71 | ONS Annual Survey of Hours and Earnings Time Series of Selected Estimates, 2020108 (as paid work) |
| Student | 5.20 | Transport Analysis Guidance (TAG) Data Book v1.13.1.40 (value of non-working time: other, 2010 values, inflated using the CCEMG-EPPI-Centre Inflation Calculator) |
| Leisure activities | 5.20 | Transport Analysis Guidance (TAG) Data Book v1.13.1.40 (value of non-working time: other, 2010 values, inflated using the CCEMG-EPPI-Centre Inflation Calculator) |
| Retired | 5.20 | Transport Analysis Guidance (TAG) Data Book v1.13.1.40 (value of non-working time: other, 2010 values, inflated using the CCEMG-EPPI-Centre Inflation Calculator) |
| Unemployed | 5.20 | Transport Analysis Guidance (TAG) Data Book v1.13.1.40 (value of non-working time: other, 2010 values, inflated using the CCEMG-EPPI-Centre Inflation Calculator) |
| Analysis | Adjusted, mean (95% CI) | Incremental, mean (95% CI) | ICER (£/QALY) | Probability (%) that intervention is cost-effective for different threshold values for society’s WTP for an additional QALY | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | £0 | £20,000 | £30,000 | £50,000 | ||
| Base case | |||||||||
| Imputed data analysis (3 years), MAR | |||||||||
| WL-TURBT | 13,249 (11,954 to 14,545) | 2.098 (2.015 to 2.182) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 14,012 (12,719 to 15,306) | 2.095 (2.005 to 2.186) | 763 (–1048 to 2574) | –0.003 (–0.123 to 0.116) | 28 | 27 | 30 | 32 | |
| Scenario analyses | |||||||||
| Scenario 1: imputed-data analysis (3 years), same MNAR parameters in both groups (–10% QoL) | |||||||||
| WL-TURBT | 13,249 (11,954 to 14,545) | 1.959 (1.881 to 2.038) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 14,012 (12,719 to 15,306) | 1.955 (1.869 to 2.040) | 763 (–1048 to 2574) | –0.005 (–0.117 to 0.108) | 28 | 25 | 27 | 30 | |
| Scenario 2: imputed-data analysis (3 years), same MNAR parameters in both groups (+ 10% cost) | |||||||||
| WL-TURBT | 13,327 (12,012 to 14,641) | 2.098 (2.015 to 2.182) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 14,087 (12,776 to 15,398) | 2.095 (2.005 to 2.186) | 760 (–1075 to 2595) | –0.003 (–0.123 to 0.116) | 28 | 28 | 30 | 33 | |
| Scenario 3: Imputed data analysis (3 years), same MNAR parameters in both groups (–10% QoL and + 10% cost) | |||||||||
| WL-TURBT | 13,327 (12,012 to 14,641) | 1.959 (1.881 to 2.038) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 14,087 (12,776 to 15,398) | 1.955 (1.869 to 2.040) | 760 (–1075 to 2595) | –0.005 (–0.117 to 0.108) | 29 | 26 | 27 | 30 | |
| Scenario 4: imputed-data analysis (3 years), different MNAR parameters in both groups (–10% QoL in PDD-TURBT group) | |||||||||
| WL-TURBT | 13,249 (11,954 to 14,545) | 2.098 (2.015 to 2.182) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 14,012 (12,719 to 15,306) | 1.955 (1.870 to 2.040) | 763 (–1048 to 2574) | –0.143 (–0.259 to –0.027) | 28 | 1 | 0 | 0 | |
| Scenario 5: imputed-data analysis (3 years), different MNAR parameters in both groups (–10% QoL in WL-TURBT group) | |||||||||
| WL-TURBT | 13,249 (11,954 to 14,545) | 1.959 (1.881 to 2.038) | |||||||
| PDD-TURBT | 14,012 (12,719 to 15,306) | 2.095 (2.004 to 2.185) | 763 (–1048 to 2574) | 0.136 (0.019 to 0.252) | 5624 | 28 | 88 | 93 | 95 |
| Scenario 6: imputed-data analysis (3 years), different MNAR parameters in both groups (+ 10% cost in PDD-TURBT group) | |||||||||
| WL-TURBT | 13,250 (11,950 to 14,551) | 2.098 (2.015 to 2.182) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 14,087 (12,781 to 15,393) | 2.095 (2.005 to 2.186) | 837 (–985 to 2659) | –0.003 (–0.123 to 0.116) | 25 | 26 | 29 | 32 | |
| Scenario 7: imputed-data analysis (3 years), different MNAR parameters in both groups (+ 10% cost in WL-TURBT group) | |||||||||
| WL-TURBT | 13,326 (12,016 to 14,636) | 2.098 (2.015 to 2.182) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 14,011 (12,713 to 15,310) | 2.095 (2.005 to 2.186) | 686 (–1139 to 2510) | –0.003 (–0.123 to 0.116) | 31 | 30 | 31 | 34 | |
| Scenario 8: complete–case analysis (3 years) | |||||||||
| WL-TURBT | 14,147 (11,554 to 16,740) | 2.146 (2.030 to 2.261) | |||||||
| PDD-TURBT | 16,583 (13,657 to 19,508) | 2.168 (2.032 to 2.305) | 2715 (–1101 to 6530) | 0.035 (–0.145 to 0.214) | 78,682 | 2 | 15 | 25 | 38 |
| Analysis | Adjusted, mean (95% CI) | Incremental, mean (95% CI) | ICER (£/QALY) | Probability (%) that intervention is cost-effective for different threshold values for society’s WTP for an additional QALY | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | £0 | £20,000 | £30,000 | £50,000 | ||
| Base case | |||||||||
| Imputed data analysis (3 years), MAR, 3.5% discount rate | |||||||||
| WL-TURBT | 12,005 (10,845 to 13,166) | 2.094 (2.010 to 2.178) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 12,881 (11,713 to 14,049) | 2.087 (1.996 to 2.179) | 876 (–766 to 2518) | –0.007 (–0.133 to 0.119) | 21 | 23 | 26 | 30 | |
| Scenario analyses | |||||||||
| Imputed-data analysis (3 years), MAR, 0% discount rate | |||||||||
| WL-TURBT | 12,165 (10,975 to 13,356) | 2.169 (2.083 to 2.255) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 13,055 (11,843 to 14,266) | 2.168 (2.072 to 2.264) | 889 (–787 to 2566) | –0.001 (–0.130 to 0.127) | 21 | 26 | 29 | 33 | |
| Imputed-data analysis (3 years), MAR, 6% discount rate | |||||||||
| WL-TURBT | 11,879 (10,739 to 13,019) | 2.047 (1.969 to 2.126) | WL-TURBT dominates PDD-TURBT | ||||||
| PDD-TURBT | 12,745 (11,603 to 13,887) | 2.044 (1.958 to 2.131) | 866 (–733 to 2465) | –0.003 (–0.119 to 0.113) | 20 | 24 | 27 | 31 | |
Appendix 3 Health economic model-based analysis plan
This section provides details of the proposed model-based analysis, including the economic model structure, main drivers of the model and data that will be used to populate the model.
Clinical pathway
Lifelong surveillance and treatment of recurrences is required owing to the high recurrence rates. The pathway begins with an initial TURBT (either WL or PDD) for newly diagnosed NMIBC. According to the NICE guideline,18 the standard UK follow-up treatments offered to intermediate-risk NMIBC patients are a course of at least six doses of MMC, and cystoscopy checks at 3, 9, and 18 months, and then once per year thereafter. Patients are discharged to primary care after 5 years of disease-free follow-up.
For high-risk NMIBC patients, another TURBT is offered as soon as possible, and no later than 6 weeks after their first resection. In addition, they may be offered further CT scans or magnetic resonance imaging (MRI) scans. The TURBT operation and the scans are used to double-check how far a patient’s cancer has grown before administering possible treatments, which are as follows: an induction course of six intravesical BCG instillations, followed by a maintenance regimen of a further 21 instillations over a 3-year period, or an operation to remove the bladder (i.e. a radical cystectomy):
-
BCG – a urologist carries out the treatment and a clinical nurse specialist provides a consultation. After the treatment, a cystoscopy check is performed every 3 months for the first 2 years, then every 6 months for the next 2 years, then once per year after that. If the cancer does not respond to intravesical BCG, the residual or recurrent cancer may be NMIBC or MIBC. Patients are then offered radical cystectomy or some form of bladder-sparing treatment.
-
Radical cystectomy – a surgeon carries out the treatment, a clinical nurse specialist provides a consultation, and a nurse (also called a stoma care nurse) takes care of the patient. A patient may be offered neoadjuvant chemotherapy using a cisplatin combination regimen if the cystectomy shows that the cancer has progressed to MIBC. After the treatment, CT scans of the abdomen, pelvis and chest are performed 6 months after the operation and once per year after that; blood tests are performed at least once per year; and tests to check the urethra are performed once per year for 5 years.
For MIBC patients, a CT or MRI scan may be offered, and then one of the following treatments: a radical cystectomy, or radiotherapy in combination with drugs called radiosensitisers [e.g. MMC in combination with fluorouracil (5-FU), or carbogen in combination with nicotinamide]. A urologist carries out the operation; an oncologist and a clinical nurse specialist provide a consultation. After radiotherapy, a cystoscopy check is performed every 3 months for the first 2 years, then every 6 months for the next 2 years and then once per year thereafter.
For metastatic bladder cancer patients, a course of chemotherapy with a combination of drugs is offered. An oncologist should check the patient’s health regularly while they are undergoing chemotherapy. Patients should be offered treatments to help relieve the side effects of chemotherapy. Palliative care is offered if the cancer cannot be cured.
Methods
A microsimulation model will be used to estimate the relative cost-effectiveness of the two treatment groups over 3 years and over the patient’s lifetime horizon. We will project the population’s recurrence and progression incidences, mortality, health-care costs, QALYs and ICERs in the years from baseline up to 2042 (i.e. 25 years after the PHOTO baseline in 2017). A 3-month cycle length will be adopted as it takes up to 3 months for high-risk cancer patients to find out their surgical results and decide to undergo further treatment if required. As the simulated patients age or incur either recurrence or progression, their subsequent risks of cancer-specific death, recurrence and progression are revised. The advantage of a microsimulation model of this nature enables transition probabilities and cost and utility values to be determined by the characteristics of the simulant (i.e. age, sex, treatment group), as well as the clinical history of the simulant (e.g. cumulative recurrence case).
FIGURE 21.
Clinical pathways for bladder cancer. (a) NMIBC; (b) MIBC; and (c) metastases.
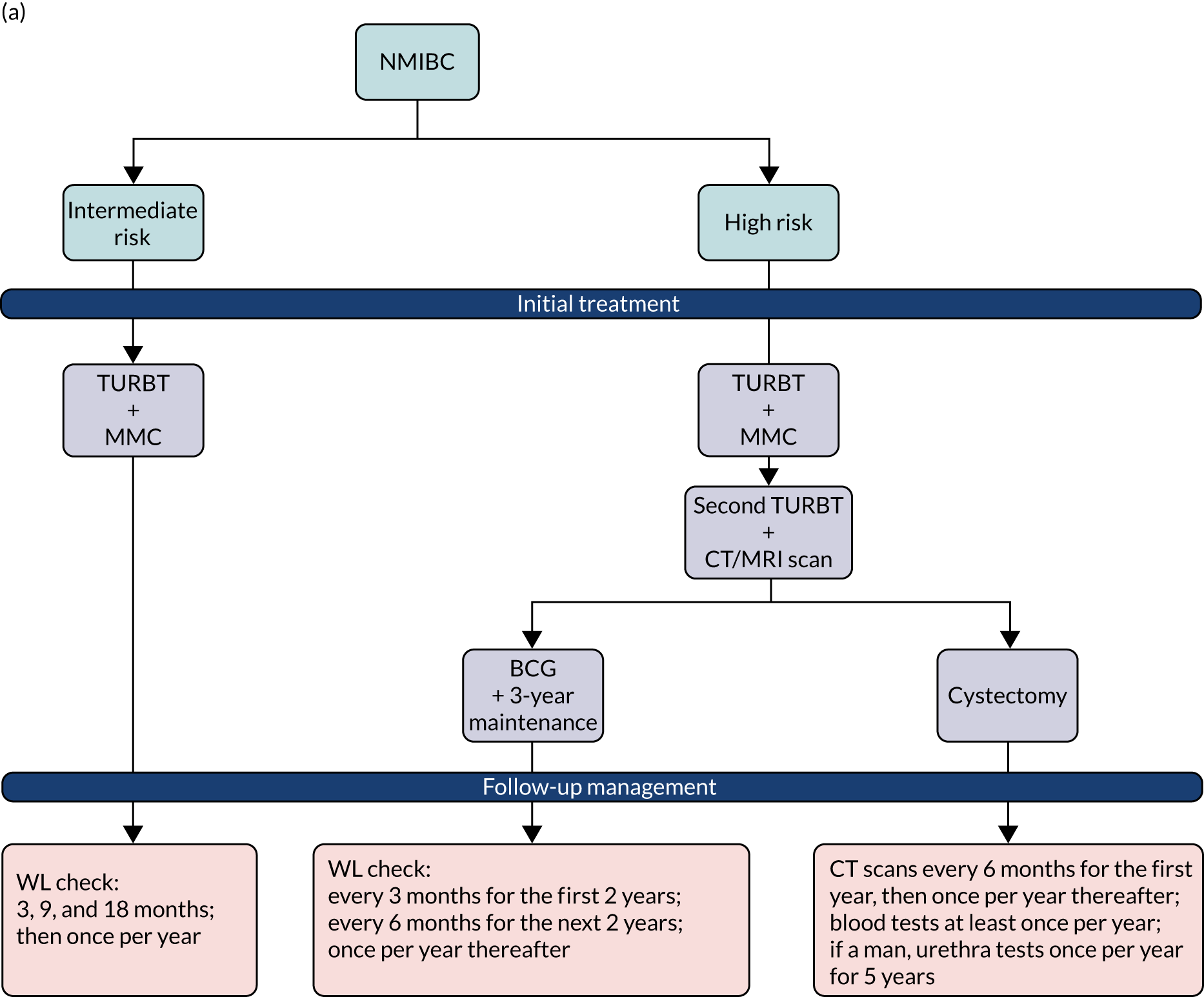
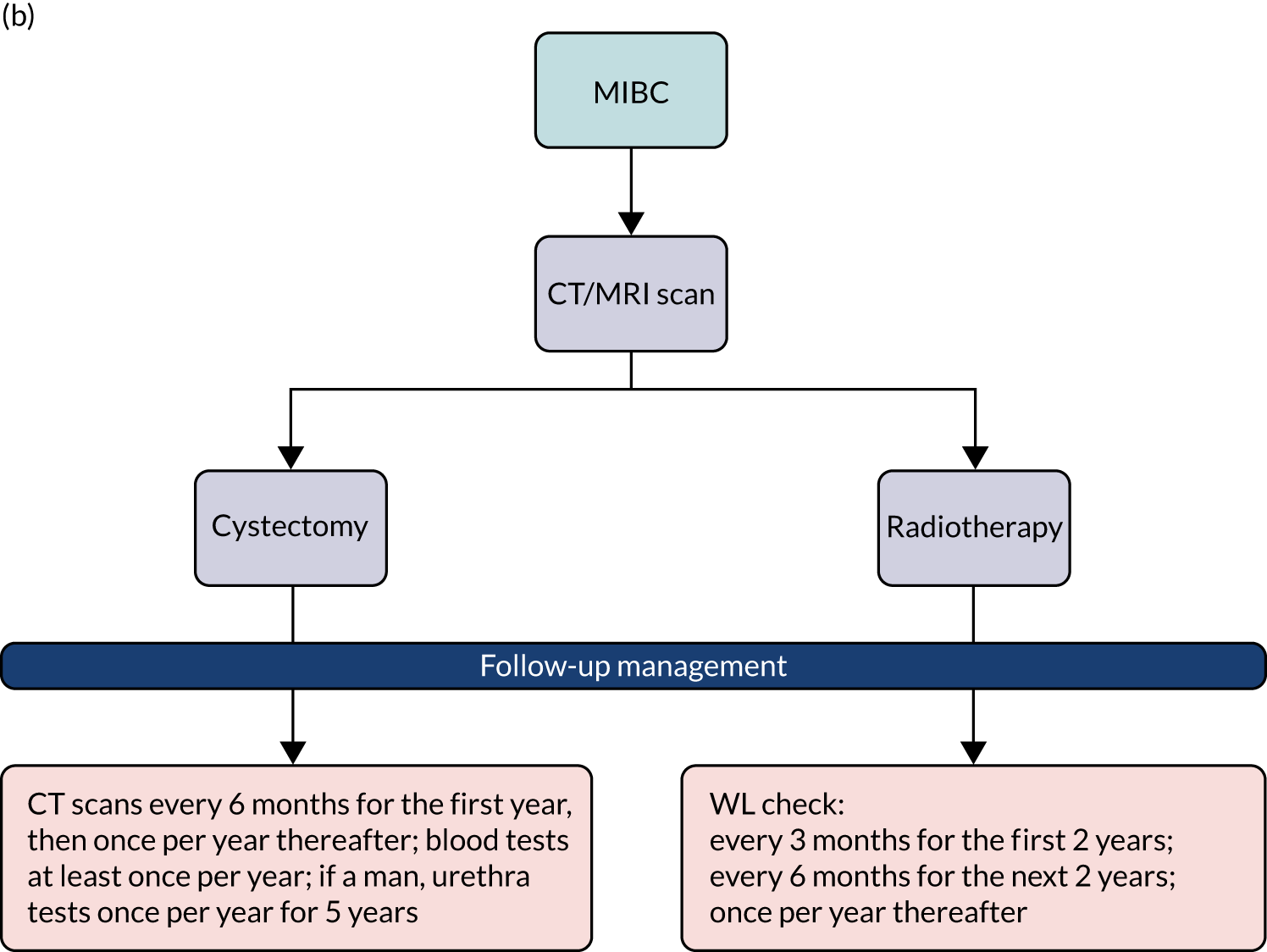
The model
The PHOTO model consists of two linked modules: bladder cancer development and surveillance, and follow-up treatment (Figure 22). An individual patient enters the bladder cancer development and surveillance module at the beginning of the simulation. During each model cycle, a patient could remain or move to other states. After presenting with clinical symptoms of recurrence or progression, they will receive a treatment. The model will estimate the cases of NMIBC recurrences and progression to MIBC for the first 3 years after the initial treatment and over lifetime. Relative risk rates will be applied to differentiate the risk of recurrence and progression depending on the patient’s follow-up management. The relative risk for PDD-TURBT compared with WL-TURBT would be derived from trial data. The model has a 3-month cycle length and a lifetime horizon, and is programmed with decision analysis software (TreeAge Pro 2020).
FIGURE 22.
State-transition diagram. Ovals indicate health states. Arrows indicate a state in which patients begin each cycle and point to a state that a patient enters during a Markov cycle. After receiving TURBT, patients entered the ‘No ReCur1’ state. The rectangle indicates follow-up treatments. The states ‘No ReCur1’, ‘No ReCur2’, and ‘No ReCur3’ represent year 1, 2, and 3 without recurrence following successful treatment. As the cycle length is 3 months, a patient may return to ‘No ReCur1’ three times before moving to ‘No ReCur2’. Following a successful treatment, a patient may move from no recurrence to NMIBC recurrence or progress to MIBC or metastases. A patient may die at any time regardless of their health state.
As an example of NMIBC, even after treatment for recurrence, further recurrence may occur within a cycle (i.e. 3 months), in which case a patient may stay in NMIBC or move to MIBC or metastases. If recurrence does not recur within the first 3 months, then the patient moves to the ‘No ReCur1’ state. Similar transitions are possible following treatment for MIBC. Once a patient has metastases, the patient either remains in metastases or dies.
Assumptions
Key assumptions with regard to NMIBC onset and progression will include the following:
-
The base-case scenario in the model assumes that all patients undergoing WL-TURBT or PDD-TURBT have NMIBC.
-
There is a risk of death associated with the surgical procedures due to using general anaesthesia and the invasive nature of the procedure. The surgical mortality is assumed to be the same between WL-TURBT and PDD-TURBT.
-
It is assumed that PDD-TURBT is not an option after the first TURBT.
-
The risk of NMIBC recurrence and MIBC progression is classified in patients who are diagnosed with NMIBC and assumed to be the same throughout the model. For example, if a patient with a tumour was classified as being at high risk and then has a tumour recurrence during follow-up, the recurrent tumour will be assumed to be a high-risk tumour, and the patient will receive the same treatment as high-risk patients with a tumour who are undergoing their initial treatment.
-
Patient management differs depending on which category they are placed in.
-
Patients are not followed in the model beyond progression to metastases as this is considered to be outside the scope of the decision problem. A one-off cost is applied to these patients.
-
We assume that the only difference between WL-TURBT and PDD-TURBT groups is the transition probabilities in the first recurrence/progression.
-
It is not known when transition occurs within the cycle; we assume that, on average, it will occur about halfway through the cycle.
-
There is no significant difference in QoL and health-care resource use associated with the different categories of resection.
-
Treatment effectiveness and costs are assumed to be homogenous. This assumption may be relaxed where necessary.
Data input
The following sections detail the data sources and base-case values for transition probabilities, resource use, costs and utility that drive the model results.
Starting cohort
The model follows a cohort of 10,000 patients – the estimated number of patients with NMIBC, which was estimated by taking the estimated number of new bladder cancer cases in 2017 and assuming that 75% of these have NMIBC. The base-case analysis does not consider tumour-free patients. This assumption may be explored in the sensitivity analysis. In the start year of the simulation (i.e. 2017), simulated patients will be generated at random with respect to age, sex and risk group, based on the respective distribution of the baseline characteristics of PHOTO trial participants, and identical simulated individuals will be passed through the model for both groups.
States
On entry to the model, all NMIBC participants will initially undergo one of the two TURBT strategies. After their first TURBT, it is assumed that all patients have received successful TURBT and recurrence has not occurred if they did not die as a result of the operation. Therefore, patients enter the model in a ‘disease-free’ state following an initial TURBT with six doses of MMC. At each model cycle, they may experience NMIBC recurrence, progression to MIBC, metastases, bladder-cancer-related death or other-cause death. If the recurrence or progression is detected, patients will undergo a further treatment and return to a disease-free state. We defined the event of interest as NMIBC recurrence, MIBC progression or metastases. The absorbing state is death from either bladder cancer or other causes, which can be reached at any time.
Time horizon
The model will have a lifetime horizon in the base-case analysis. We will create three termination conditions (i.e. life expectancy of 25 years, aged 100 years, or > 99.9% of the cohort are dead) to stop the simulation analysis. The duration of the simulation model will be 25 years, or quasi-lifetime given that the mean starting age of participants is 71 years. Shorter time horizons (5, 10, and 20 years) will also be considering in a sensitivity analysis.
Cycle length
The period from the initial treatment to death will be divided into 3-month time intervals, known as the model cycle. The proportion of participants in each state in the model is calculated at the start of each cycle. It takes up to 6 months for intermediate-risk patients and 3 months for high-risk patients to find out about surgical results and decide to undergo further treatment if required. A shorter cycle length should be considered, even if an event for intermediate-risk participants does not warrant it. 110
Model symmetry
The PHOTO model is symmetric, ensuring that the disease process is presented consistently across the two TURBT strategies.
Recurrence, progression, metastases and death
The analyses will use data from the PHOTO trial, combined with the best available UK-relevant evidence, to estimate the event rates for patients in each of the risk groups for the first 3 years after WL-TURBT. These rates will be estimated using regression analysis of events (i.e. recurrence, progression, metastases and death) during data collection intervals. This will facilitate a non-linear risk of event over time. A probit model will be used for the dichotomous variable (i.e. event or no event) at each time point.
The probability of an event will be assessed with respect to the participant’s treatment group, age, sex and count of previous recurrence and progression (as they occur within the model). Treatment group, sex and age transition probabilities will then be predicted from the resulting regression equation, along with the average marginal effect of the cumulative recurrence and progression. Transition probabilities for beyond the trial duration will be extrapolated for the model based on the ≥ 25-month regression equations or other existing data source (e.g. data sets which we may access and a structured systematic review of long-term outcomes of bladder cancer treatments55,88), and expert opinions. The range and distribution of values will be used in the sensitivity analysis. In addition, input parameters may be calibrated through a critical review of the published medical literature and expert opinion. Co-authors with clinical expertise in bladder cancer (RH) provided guidance regarding model assumptions, model structure and input parameter estimates. Colleagues with expertise in decision analysis (LV and SR) provided guidance regarding model construction, calibration and interpretation of results.
Background mortality
Background mortality will be assumed to be independent of treatment history and will be derived from the published UK life tables for the years 2016–2018 (Office for National Statistics). 111
Resource use
Assumptions will be made about resource use at the initial TURBT procedure and during follow-up, consistent with the NICE guideline. 18
At initial TURBT:
-
Patients diagnosed with intermediate-risk NMIBC are assumed to incur the cost of WL-TURBT or PDD-TURBT, and six doses of MMC.
-
Patients diagnosed with high-risk NMIBC are assumed to incur the cost of WL-TURBT or PDD-TURBT, and six doses of MMC. Then they undergo an early re-resection and a CT or MRI scan, followed by a BCG induction course and 3-year maintenance, or cystectomy with or without adjuvant chemotherapy.
At follow-up:
-
The PDD technology, Hexvix, is considered for use in only those patients with newly diagnosed cancer in the model. It is not considered for use with patients undergoing a subsequent TURBT (i.e. for patients undergoing an early re-resection) or for recurrent tumours that may occur during follow-up. Thus, in the model, patients with a recurrence or who undergo an early re-resection undergo WL-TURBT.
-
Patients who have progressed to metastases incur a one-off cost of flexible cystoscopy, WL-TURBT, CT scan and treatment (i.e. chemotherapy or palliative care), weighted depending on whether patients can be cured or not.
-
Patients are followed up in the model and incur the costs of disease management at each follow-up appointment depending on their risk classification and treatment history.
Costs
The estimation of the total cost will be mainly based on the initial treatment and follow-up management (e.g. frequency of follow-up visits, surveillance, intravesical treatment), the recurrence rate, the type of recurrence, the probability of re-recurrence, the probability of receiving treatment, the compliance during follow-up and the survival time. To estimate the cost of the health service provided per person per cycle, we will disaggregate the total costs incurred in each year within the trial follow-up period to each 3-month cycle. Then, we will assume that the mean costs beyond the final year of follow-up are the same as those incurred in the final year. For each treatment in the model, separate cost models will be developed that include the costs of medication, CT scanning, complications, and treatment by nurses, pharmacists and medical practitioners. Treatment costs will be incorporated as transition costs for those modelled to experience this event.
Utility
To estimate QALYs, HRQoL will be assigned to each health state in the model. The HRQoL weights are based on the health state, along with the average marginal effect of age and sex. The mean QALYs for each TURBT strategy will be calculated by multiplying the amount of time that patients spend in each health state by the associated HRQoL. HRQoL will also be adjusted to account for the effect of ageing on patient’s HRQoL using the value set provided by the EuroQol Research Foundation. 112
Analysis
The model will be developed using TreeAge or Stata to estimate costs, QALYs, recurrence and progression rates, and survival. The QALYs for each TURBT strategy (WL or PDD) will be calculated by multiplying the amount of time that patients spend in each health state by the associated HRQoL. The cost will be calculated by summing the costs incurred in each cycle and the initial TURBT costs. Costs and QALYs will be discounted by an annual rate of 3.5%, as recommended by NICE. 34 Time dependency in the calculation of probabilities of recurrence and death will be captured in the model by using trackers and tables.
Half-cycle correction
Survival and QALYs will be half-cycle corrected. For costs, standard half-cycle corrections will not be modelled, but will be modelled indirectly by using the PHOTO trial data to estimate treatment costs, considering compliance and mortality.
Distribution
The shape and type of distribution will depend on the trial data, literature and recommendations for good practice in modelling. 113 Beta distributions will be employed for utility data, and gamma distributions will be employed for cost data. 114 HRs will be sampled using a log-normal distribution.
Modelled incremental cost-effectiveness ratio
The ICER will be estimated as the incremental costs divided by the incremental QALYs. In this analysis, QALYs are estimated as the time (i.e. number of cycles) multiplied by the simulated patients’ utility score (at each cycle). The ICER will be determined from the difference in mean costs and QALYs between simulated patients receiving WL-TURBT and those receiving PDD-TURBT. The simulation will be undertaken for 10,000 simulated patients. The total expected bladder cancer costs, life-years, QALYs and ICER will be reported for an average NMIBC patient aged 70 years for different time horizons according to the TURBT strategy.
Sensitivity analysis
We will analyse the PHOTO model as a microsimulation to examine first-order uncertainty, which characterises the random variability in individual outcomes conditional on underlying parameter values. We will examine the effect of second-order uncertainty, which characterises the imprecision of knowledge regarding parameter values. We will perform one-way and multiway sensitivity analysis to explore parameter-, methodological- and model-structure uncertainty.
For the model-based analysis, a probabilistic sensitivity analysis will be conducted on our modelled results using the Monte Carlo simulation, in which the simulation model (of 7650 patients) is run 1000 times. This will allow us to vary all of our parameters simultaneously to determine what effect this has on the probability of each treatment being cost-effective. Distributions for each model parameter, along with the information to define those distributions, will be presented. The results of this analysis will be presented in a similar fashion to those from the within-trial bootstrapped analysis. A 95% CI for the ICER will be estimated based on the 1000 model runs.
A deterministic sensitivity analysis will be carried out to test for the effect of our assumptions and variability, such as including out-of-pocket costs, an exploration of alternative unit costs applied to the different resources used and the number of visits a participant has with a health professional. Several analyses will be performed (e.g. one way and/or multiway), depending on the results obtained from the deterministic analysis. We will conduct the deterministic sensitivity analysis as part of our model-based analysis because, in addition to the assumptions that will be made in our base-case analysis, we will make assumptions with regard to the costs estimated over the 3-year period. By conducting this analysis as part of the model analysis, we can capture all of these assumptions and make amendments in one analysis.
To explore the possibility that the baseline imbalance is due to a possible risk factor, sensitivity analyses will be performed that include the baseline outcome as a covariate to assess the robustness of the primary analysis. 115 Additional sensitivity analyses related to QoL will apply the duration QoL weights for patients with bladder cancer varying from 1 to 10 years. A sensitivity analysis of short-term QoL effects related to treatment may also be performed.
Model validation
Model validation will comprise face validity (i.e. setting parameters to extreme values to assess predictable effects on outputs), internal validity (i.e. running the model for 3 years and comparing the simulated results with those of the trial) and external validity (i.e. review by external experts).
Appendix 4 Health economics: time trade off study
Parts of this appendix have been reproduced with permission from Shen et al. 116 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
The collection of EQ-5D-3L data in the PHOTO trial was carried out after patients’ initial resection and then subsequently at 6-month intervals. However, the impact of further treatment for tumour recurrence may be relatively short-lived and, therefore, potentially not captured using fixed interval questionnaires. A TTO study was developed to estimate the short-term loss of utility.
Time trade-off is a preference elicitation method used to derive utility values for different health states, where values usually range between 0 (death) and 1 (perfect health), although they may also include negative values where health states are considered worse than death (WTD). 117,118
Although conventional TTO methods are typically used to value health states lasting a number of years, a chained version, in which an ‘anchor state’ is used as a bridge between the temporary states and the death state, may be more appropriate for health states of short duration. 119
Methods
Ethics approval
Health Research Authority (HRA) approval was provided, and ethics approval was granted by the Berkshire B REC (Reference 17/SC/0519).
Outcomes
‘Health states’ were developed to describe a spectrum of health consequences following bladder resection from ‘best case’ to ‘worst case’. This process involved discussion with a cancer nurse specialist and a consultant urologist (i.e. the study’s chief investigator) to capture the main symptoms and side-effects that were likely to be experienced. These were presented as vignettes describing the health states. Piloting took place with four members of the public and, after refinement, the process and materials were subsequently piloted with 15 patients and further refined. The main timeframe of the health states was 2 weeks as this was considered to be the likely duration of these symptoms; however, within this were timeframes for specific components of the health states (e.g. duration of catheter or length of hospital stay). Nine profiles were used to reflect different combinations of symptoms and the impact of symptoms. An example health state is shown below:
Following surgery, the patient is required to wear a catheter for the duration of an overnight hospital stay and for up to 3 days at home, during which time they do not feel like leaving the house. They see blood and experience a mild burning sensation while urinating. They also have a frequent and urgent need to pass urine during both the day and night, and have moderate pain in the abdomen. They experience mild anxiety awaiting test results on whether treatment is complete or if further treatment for the cancer is required.
Piloting highlighted difficulties in striking a balance when designing the anchor state to be worse than the health states being valued, but better than death. This led to a revision of the methods to allow participants to value the anchor state as WTD through use of the composite TTO method, which uses a lead-time trade-off in the case of participants considering an anchor state to be WTD;120 the lead-time trade-off adds a period of full health to both scenarios. The valuation of the anchor state used a time period of 10 years. A brief sociodemographic questionnaire was also completed by participants.
Participants
Following the piloting work, participants were approached between March 2019 and February 2020 by research nurses in rapid-access haematuria clinics, where they were provided with an information pack and asked to return an expression of interest form if they were willing to speak to a researcher about the study. Following recruitment difficulties, an ethics amendment allowed the researcher to directly contact those given an information pack if they agreed to this at the initial discussion with the research nurse. Follow-up contact was made by phone, which gave potential participants the opportunity to decline to proceed, or ask questions and arrange an interview if they wished. Our intention was to recruit 50 patients to conduct meaningful analyses.
The inclusion and exclusion criteria for the TTO study were:
-
Inclusion criteria –
-
adult men and women aged ≥ 18 years
-
first suspected diagnosis (visual/USS/CT diagnosis) of NMIBC (treated or untreated) on or after 1 October 2014
-
-
Exclusion criteria –
-
unable to communicate complex constructs in English
-
unable to provide informed consent.
-
Interviews
Interviews took place face to face either in patients’ own homes or on university premises. Interviews lasted approximately 1 hour. Written consent was taken immediately prior to interviews.
The interview process followed the ‘props’ method121 and the process described by Shen et al. :116 using a ‘decision board’ and health states displayed on coloured A6 cards.
Three practice profiles were selected from EQ-5D-3L profiles and used in a practice exercise ahead of valuing the health states. The chained TTO comprised two stages. In the first stage, participants were asked to compare temporary health states with the anchor state. The time spent in the temporary state was fixed at 2 weeks, whereas the time period of the anchor state was varied, followed by a return to full health. Participants were asked to imagine both of the scenarios and to find a time point (x1) between 0 and 2 weeks for the anchor state where they felt that the scenarios were equivalent. In the second stage, the anchor state was valued in a conventional TTO, where participants were asked to compare the anchor state and a ‘perfect-health’ state. The time period in the perfect-health state was varied between 0 and 10 years, whereas the anchor state was fixed at 10 years, followed by death. Participants were asked to imagine themselves in both of the scenarios and to find a time point (x2) between 0 and 10 years for the perfect-health state where they felt that the scenarios were equivalent. As described in Outcomes, piloting indicated that some participants valued the anchor state as WTD and a lead-time trade-off was used in this instance. The utilities of the temporary health states being valued were planned to be calculated based on x1 and x2.
Appendix 5 The PHOTO-T study
Background
In most cases, bladder cancer presents as a non-muscle-invasive growth that can be excised endoscopically. However, recurrence rates can approach 70% in patients with high-risk disease and endoscopic surveillance is very costly to provide. Frequent invasive assessment and the ongoing mental health burden of the threat of recurrence in patients living with disease calls for urgent effective and non-invasive approaches to monitoring treatment outcome and predicting disease progression. An NIHR HTA evidence synthesis (HTA 07/02/01),27 conducted prior to the NIHR commissioned call that led to the PHOTO trial, called for additional diagnostic studies as a priority area of clinical need.
The aim of the PHOTO-T study was to establish a well-characterised biorepository of longitudinal, serially collected tissue samples, with associated clinical data collected as part of the PHOTO RCT. This valuable resource of blood, urine and formalin-fixed, paraffin-embedded (FFPE) tumour tissue could then be used in complementary translational research. 122,123 Projects that aim to validate rapid, non-invasive prognostic, diagnostic and predictive biomarkers are prioritised for sample access. The sample collection has the scope to provide promising clinical value in currently unmet areas of need, such as surveillance and diagnosis, risk stratification and understanding molecular mechanisms of disease. If reliable and validated predictive, prognostic or diagnostic biomarkers were identified, this could help to reduce or replace the more invasive surveillance regimens that are currently in place.
This tissue archive will provide a cohort of valuable samples for retrospective biomarker discovery, providing greater insight into the natural history of bladder cancer and creating the opportunity to examine molecular markers associated with treatment responsiveness to a number of adjuvant approaches (i.e. intravesical mitomycin and BCG treatments) and progression to advanced disease. This may help to personalise or stratify treatments for clinical and patient benefit.
Methods
The PHOTO-T study was conducted at a subset of PHOTO trial centres. Informed consent to provide samples for the PHOTO-T collection was obtained from participants at the same time as consent for the PHOTO trial. This consent included advance authorisation for future research on the stored samples, with the understanding that patients would not be identifiable from these samples and that prior approval of an ethics committee would be obtained for any future work. PHOTO-T participation was recorded at the time of central randomisation to the PHOTO trial.
The PHOTO-T study participants were asked to provide up to 20 samples at five time points over a 3-year trial period from randomisation (Table 49). Blood samples were collected for circulating DNA analysis (i.e. using Cell-Free DNA BCT®, Streck Inc., La Vista, NE, USA) and circulating RNA analysis (i.e. using PAXgene® Blood RNA Tube, BD Biosciences, Franklin Lakes, NJ, USA).
| Sample | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre TURBT | TURBT | Prior to discharge | Second TURBT (as clinically indicated) | 3 months post treatment | 12 months post treatment | 24 months post treatment | 36 months post treatment | At disease recurrence/progression | |
| Routinely obtained FFPE tumour tissue | ✗ | ✗ | |||||||
| Urine (2 × 100-ml samples) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Blood (1 × 10-ml Cell-Free DNA BCT and 1 × 2.5-ml PAXgene Blood RNA Tube) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
Sample collection
Research staff at participating sites took the serial blood samples during participants’ clinic visits. Urine samples were provided by participants using specialist home collection kits. FFPE blocks were requested retrospectively from the histopathology departments of participating sites following completion of treatment.
One block containing a representative tumour sample, a block containing normal tissue and the corresponding H & E slide were requested for each case. Following receipt of the blocks and slides, the PHOTO-T study lead pathologist confirmed and marked the area of tumour for sectioning.
Samples were also collected at the time of suspected recurrence. If the recurrence was histologically proven, it would be considered the last sample collection for the patient. However, if a suspected recurrence was not histologically proven, the patient continued sample collection as planned. If a patient had MIBC, then no further samples were required. If a patient commenced BCG treatment, scheduled home-collected urine samples were requested at least 1 week subsequent to the end of treatment.
If participants withdrew consent for the storage of their samples within the PHOTO-T study, samples were either withdrawn and destroyed or returned to the site as appropriate.
Sample processing and storage
All samples were sent to receiving labs at University College London (UCL) (London, UK) and the Northern Institute for Cancer Research (NICR) at Newcastle University (Newcastle upon Tyne, UK).
One urine sample per time point was sent to UCL for immediate processing to release DNA and capture methylation-based measures associated with bladder cancer (see Project 4: Kelly/Feber, University College London). All other samples were received by NICR and processed for long-term storage at –80 °C. All sample transfer, tracking, processing and storage was conducted in accordance with the relevant institute’s standard laboratory operating procedures. Storage of all samples was in accordance with good laboratory practice and adhered to the Human Tissue Act guidelines. 124
Governance and tissue-access requests
While the trial remains open, samples are held at a trial-associated bladder-cancer biorepository within NICR under the custodianship of Professor Rakesh Heer. The PHOTO trial’s TMG and independent TSC are responsible for reviewing and approving tissue access requests from researchers wishing to use samples in the repository. See Approved tissue access requests for a summary of approved projects to the date of this report’s submission.
After declaration of the end of the trial, all samples collected and received by NICR as a trial-associated bladder cancer biorepository will be transferred to the Newcastle Biomedicine Biobank Research Tissue Bank (NBBRTB REC: 12/NE/0395), a fully Human Tissue Act-licensed facility (section 16, Human Tissue Act 2004,124 licence 12534). When the PHOTO trial’s TMG has been disbanded following trial closure, an access approval committee, including independent representatives from university research institutes, will assess requests for the release and use of the biorepository samples.
Results
Recruitment
The first PHOTO trial site opened the PHOTO-T study on 3 February 2016. Twelve of 22 PHOTO trial sites took part in the PHOTO-T study, and seven recruited PHOTO-T study participants. In total, 67 PHOTO-T study participants were recruited.
Samples collected
The number of participants who provided a full set of samples (i.e. blood and urine) per time point was as follows:
-
baseline: 62 (93%)
-
3 months: 45 (67%)
-
12 months: 33 (49%)
-
24 months: 17 (25%)
-
36 months: 11 (16%).
The number of participants who provided partial sample collection (i.e. only a urine or a blood was collected per time point) was as follows:
-
baseline: 3 (4.5%)
-
3 months: 5 (7.5%)
-
12 months: 1 (1.5%)
-
24 months: 1 (1.5%)
-
36 months: 2 (3%).
The number of participants by disease progression status were as follows:
-
recurrence: 22 (34%)
-
MIBC: 8 (11.9%)
-
no tumour on initial TURBT: 4 (6%)
-
metastatic disease: 1 (1.5%).
In total, 516 samples were collected (174 blood, 181 urine and 161 FFPE).
Eight participants provided a complete longitudinal/serial set of samples.
Approved tissue-access requests
The following projects have been approved access to samples from the PHOTO-T study biorepository.
Project 1: Turnbull, Newcastle University – urinary mitochondrial deoxyribonucleic acid mutations: naturally occurring tumour ‘barcodes’ to trace bladder cancer recurrence
Objectives
The objectives are as follows:
-
to describe and characterise the mtDNA mutation signature in bladders from > 100 patients with age-related bladder dysfunction
-
to correlate mtDNA mutations/burden with clinical and bladder physiological measures from formal urodynamic assessments
-
to conduct mtDNA lineage tracing by benchmarking mtDNA mutations that are unique to bladder tumours
-
to interrogate field change characteristics.
Funding
This project is funded by a NIHR BRC Doctor of Philosophy studentship (Newcastle Award).
Samples shared
The samples to be shared from each PHOTO-T study participant at each collection time point are as follows:
-
50 ml of urine supernatant plus cell pellet
-
1 ml of red blood cell fraction.
Project 2: Bryan, University of Birmingham – AmpseqUr: Amplicon deep sequencing of Urinary deoxyribonucleic acid for the detection of bladder cancer
Objectives
The objectives are as follows:
-
to improve the sensitivity of our existing multiplex-polymerase chain reaction (PCR) targeted next-generation sequencing (NGS) assay (‘AmpseqUr’) with little or no deterioration in specificity
-
to validate the improved urine assay for the diagnosis of primary and recurrent disease in prospectively collected clinical trial samples.
Funding
This is a CRUK Biomarker Project.
Samples to be shared
A urine supernatant sample (20 ml) from each PHOTO-T study participant for each episode of NMIBC surveillance is to be shared.
Project 3: Probert, Liverpool University – VOID: Volatile OrganIc compounDs for diagnosing bladder cancer
Objectives
The objectives are as follows:
-
to validate the volatile organic compound (VOC)-based model for the diagnosis of bladder cancer
-
to determine how the VOC profile changes after the tumour has been removed/when it recurs.
Funding
This is a CRUK Biomarker Project.
Samples shared
Urine samples are to be shared (12 ml in total, at baseline and at each review for all PHOTO-T study participants):
-
fifty-nine baseline aliquots shared 14 February 2018
-
forty-seven 3-month aliquots shared 11 October 2018
-
thirty-six 12-month aliquots shared 25 April 2019.
Project 4: Kelly/Feber, University College London – Photo-T urinary study; UroMark: a urinary biomarker assay for the detection of bladder cancer
Objectives
The objectives are as follows:
-
to develop the UroMark assay as a high-throughput NGS assay
-
to use samples from the PHOTO trial, together with those collected in CALIBER,57 HIVEC (Hyperthermia for Intermediate risk bladder cancer)125 and DETECTII (Detecting Bladder Cancer Using the UroMark Test),126 to assess assay sensitivity in patients undergoing surveillance cystoscopy for recurrent bladder cancer.
Samples shared
Urine samples at baseline, and at 3, 12, 24 and 36 months post treatment or at the point of first recurrence from all PHOTO-T study participants are to be shared.
Conclusions
The PHOTO-T study biorepository provides a valuable longitudinal archive that will, hopefully, provide researchers with critical insights into the molecular natural history of bladder cancer and, with that, biomarkers for diagnosis and prognostication of recurrence and progression.
Glossary
- T1
- A tumour that has spread to the connective tissue (called the lamina propria) that separates the lining of the bladder from the muscles beneath, but does not involve the bladder wall muscle.
- T2
- A tumour that has spread to the muscle of the bladder wall.
- T3
- A tumour that has grown into the perivesical tissue (the fatty tissue that surrounds the bladder).
- T4
- A tumour that has spread outside the bladder to any of the following: the abdominal wall, the pelvic wall, the prostate or seminal vesicle (the tubes that carry semen), or the uterus or vagina (as applicable).
- Ta
- Cancer that is a non-invasive papillary carcinoma. It has grown towards the hollow centre of the bladder, but has not grown into the connective tissue or muscle of the bladder wall.
- Tis
- A flat, non-invasive carcinoma, also known as flat carcinoma in situ. The cancer is growing in the inner lining layer of the bladder only; it has not grown inward towards the hollow part of the bladder, nor has it invaded the connective tissue or muscle of the bladder wall.
List of abbreviations
- 5-ALA
- 5-aminolevulinic acid
- AE
- adverse event
- AJCC
- American Joint Committee on Cancer
- BAUS
- British Association of Urological Surgeons
- BCG
- bacillus Calmette–Guérin
- BNF
- British National Formulary
- BOXIT
- Bladder cyclooxygenase 2 Inhibition Trial
- BRC
- Biomedical Research Centre
- CALIBER
- A phase II randomized feasibility trial of chemoablation with mitomycin-C vs surgical management in low-risk non-muscle-invasive bladder cancer
- CCEMG-EPPI
- Campbell and Cochrane Economics Methods Group Evidence for Policy and Practice Information Centre
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CIS
- carcinoma in situ
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CRUK
- Cancer Research UK
- CT
- computerised tomography
- CTCAE
- Common Terminology Criteria for Adverse Events
- CUETO
- Club Urológico Español de Tratamiento Oncológico
- DCIS
- ductal carcinoma in situ
- DMC
- Data Monitoring Committee
- DNA
- deoxyribonucleic acid
- DVT
- deep-vein thrombosis
- EAU
- European Association of Urology
- EORTC
- European Organisation for Research and Treatment of Cancer
- EORTC-QLQ-C30
- European Organisation for Research and Treatment of Cancer – Quality of Life Questionnaire – Core 30
- EORTC-QLQ-NMIBC-24
- European Organisation for Research and Treatment of Cancer – Quality of Life Questionnaire – Non-Muscle Invasive Bladder Cancer – 24 items
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FFPE
- formalin-fixed, paraffin-embedded
- GP
- general practitioner
- HAL
- hexaminolevulinate
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HSUQ
- health service utilisation questionnaire
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICR-CTSU
- Clinical Trials and Statistics Unit at the Institute of Cancer Research
- ITT
- intention to treat
- LCIS
- lobular carcinoma in situ
- MAR
- missing at random
- MI
- multiple imputation
- MIBC
- muscle-invasive bladder cancer
- MMC
- mitomycin C
- MNAR
- missing not at random
- MRI
- magnetic resonance imaging
- mtDNA
- mitochondrial deoxyribonucleic acid
- NGS
- next-generation sequencing
- NICE
- National Institute for Health and Care Excellence
- NICR
- Northern Institute for Cancer Research
- NIHR
- National Institute for Health and Care Research
- NMB
- net monetary benefit
- NMIBC
- non-muscle-invasive bladder cancer
- NMP22
- nuclear matrix protein 22
- PDD
- photodynamic diagnosis
- PDD-TURBT
- photodynamic diagnosis-guided transurethral resection of bladder tumour
- PHOTO
- Photodynamic versus white-light-guided resection of first diagnosis non-muscle-invasive bladder cancer
- PHOTO-T
- Photodynamic versus white-light-guided resection of first diagnosis non-muscle-invasive bladder cancer – Translational
- PI
- principal investigator
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- pT
- papillary tumour
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RNA
- ribonucleic acid
- RR
- rate ratio
- SAE
- serious adverse event
- SD
- standard deviation
- SHR
- subhazard ratio
- SUR
- seemingly unrelated regression
- TMG
- Trial Management Group
- TNM
- tumour node metastasis
- TR
- time ratio
- TSC
- Trial Steering Committee
- TTO
- time trade-off
- TUR
- transurethral resection
- TURBT
- transurethral resection of bladder tumour
- UCL
- University College London
- USS
- ultrasound scan
- VOC
- volatile organic compound
- WL
- white light
- WLC
- white-light cystoscopy
- WL-TURBT
- white-light-guided transurethral resection of bladder tumour
- WTD
- worse than death
- WTP
- willingness to pay