Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number NIHR128470. The contractual start date was in January 2020. The draft report began editorial review in June 2022 and was accepted for publication in January 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2024 Todhunter-Brown et al. This work was produced by Todhunter-Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Todhunter-Brown et al.
Chapter 1 Introduction
Background and rationale
The Strategies Used for Constipation in Children – Evidence Synthesis involving Stakeholders (SUCCESS) project was undertaken in response to a National Institute for Health and Care Research (NIHR) commissioned call. The research question (RQ) stated in the NIHR call, and addressed in this project, is ‘What are the most effective interventions, and combinations and sequences of interventions, for childhood chronic functional constipation (CFC), and how can they best be implemented?’.
Definition
The definition of CFC may differ between clinicians, young people and parents but can be defined as a decrease in the frequency of bowel movements, with fewer than three complete stools per week and characterised by the passing of hardened stools associated with straining, pain and episodes of overflow incontinence, which cannot be explained by anatomical, physiological, radiological or histological abnormality. 1–3 The ROME IV criteria4 aim to standardise diagnosis of CFC, defining signs and symptoms which must occur over at least 1 month. While the definition of CFC focuses on the reduced frequency of bowel movements and hard stools, children may present with a range of other signs and symptoms; this can include cycles of stool withholding, abdominal pain and soiling (encopresis). 5
Size and impact of problem
Childhood CFC is a highly prevalent and costly condition. It is estimated to affect between 5% and 30% of school-aged children,2 and to become chronic in around one-third of these cases. 6 In 2017–18, approximately 71,430 people were admitted to hospitals in England with constipation, equivalent to 196 people per day. Children and young people (aged <15 years) accounted for around 20% of these admissions (i.e. 40 admissions/day). 7 However, lack of recognition and embarrassment are likely to contribute to underestimation of prevalence. 5 Despite additional challenges to recognition of symptoms and diagnosis of constipation, prevalence is often reported to be higher in children with intellectual disabilities. 8,9 Prognosis varies, and outcomes are better with prompt identification and treatment. 5
Evidence shows that CFC has negative effects on quality of life (QoL) of children, families and carers, with increasing impact as the child gets older. 10 Although rarely life-threatening,11,12 CFC is an unpleasant and distressing condition, associated with a wide range of complications, including physical discomfort, missed school, poor school performance, social isolation and reduced involvement in group activities. 13,14 In addition, faecal impaction (frequency 40–100%); soiling or encopresis (faecal incontinence) (75–90%); painful defaecation (69%); withholding or straining to stop passage of stools (58%); enuresis or urinary tract infection (30%); fissures or haemorrhoids (5–25%); anal prolapse (3%) may occur. 13–15 More than a third of children with CFC will present clinically with behavioural problems as a result of the constipation;16 attempts at management may focus on behaviour without recognition of the underlying problem, contributing to stress and anxiety amongst children and families. As stated earlier, the healthcare costs of childhood CFC are significant,16 but little is known about the indirect social costs associated with CFC and the economic burden placed on families and wider society. 17
Current clinical guidelines
Currently there are two main clinical guidelines. 1,18 The National Institute for Health and Care Excellence (NICE) guidelines (CG99) on ‘Constipation in children and young people; diagnosis and management’ were published in 2010,1 with a number of subsequent minor updates in 2012,19 20152 and 2017. 20 In addition, a NICE Pathway was updated in 2018 and an exceptional surveillance of constipation in children: diagnosis and management (NICE guideline CG99) was also undertaken. 21 In 2020 a Clinical Knowledge Summary was published. 22 The international guidelines, from the North American and European Societies for Paediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN and ESPGHAN) were published in 2014 (searches to 2011). 18 These guidelines concluded that there was some low-quality evidence which supports the use of laxatives as a first line treatment and other interventions given as adjuncts (i.e. in combination). However, there was insufficient research evidence to support treatment decisions for other interventions, including dietary and lifestyle, psychological and provision of information and advice. Recommendations were based primarily on expert opinion.
Evidence uncertainties
A number of evidence uncertainties are highlighted within existing guidelines and reviews. These include uncertainties relating to several non-pharmacological interventions. While guidelines currently recommend that non-pharmacological interventions (e.g. dietary and lifestyle modifications, support and advice) are given as adjuncts, details of these adjunct interventions or strategies are rarely clearly reported within the synthesised evidence. There is therefore particular uncertainty relating to the effectiveness of delivering interventions in combination which is often the case in the real-world setting.
Why is this review needed now?
There are limitations in the evidence syntheses within guidelines. Consequently, a review is needed to conduct comprehensive, up-to-date searches, with no limitations based on study design or intervention type, providing a clear synthesis and map of all relevant research in this field. This review aims to synthesise current evidence relating to interventions for CFC, enabling clinical practice to become more effective and cost-effective, and to inform future research in this field.
Furthermore, current guidelines and reviews have not systematically considered the impact of adjunct interventions and interventions delivered in combination. There is therefore a need for a review that will enable identification of evidence of the effectiveness of interventions delivered in combination, which is an important information source for all those involved in the care of children with CFC.
Moving beyond evidence of effectiveness
The recent publication of the Medical Research Council (MRC) complex intervention framework,23 has highlighted a clear need to move away from only considering whether a treatment is effective or not towards considering the context in which an intervention is implemented. Establishing evidence of effectiveness of an intervention or a combination of interventions does not guarantee that an intervention or strategy will be successfully implemented, or that guideline recommendations will be adhered to. For example, the majority of physicians surveyed in the USA and the Netherlands were unaware of the ESPGHAN/NASPGHAN guidelines for CFC. 24 If interventions cannot be successfully implemented and sustained in the long term, trials, reviews and guidelines which establish evidence of effectiveness arguably contribute to research waste.
The potential barriers to implementation are diverse, and may include healthcare professionals’ behaviour, individual child factors such as physical or intellectual disability, family dynamics, a carer struggling to get their child to consume relatively large volumes of unappetising fluid (laxative). A better understanding of the barriers and identifying what treatment options might exist to address these barriers is essential for the successful implementation of CFC.
In summary, current guidelines for the treatment of CFC provide a useful foundation but further work to develop recommendations may contribute to improved outcomes. Implementation science is now recognised as an important entity but has not yet applied to this field. Research to address these gaps is urgently needed, in order to improve outcomes for children with CFC and their families.
Research plan
Research questions
The RQ defined in the NIHR call was:
What are the most effective interventions, and combinations and sequences of interventions, for childhood CFC, and how can they best be implemented?
The specific RQs to be answered by this project were:
RQ1: What is the current evidence relating to management strategies for childhood CFC?
RQ2: What are the most effective childhood CFC strategies and combination of strategies in relation to outcomes of importance to stakeholders and/or cost to the patient/NHS?
RQ3: What factors are associated with implementation success or failure of childhood CFC strategies and combination of strategies for different subgroups?
RQ4: What are the evidence gaps for childhood CFC management strategies?
Stakeholder involvement
The active involvement of stakeholders allows cognitive diversity and can enhance quality, relevance and impact of healthcare research. Involvement can also help make to promote uptake of systematic review (SR) findings. 25–28 Stakeholder involvement was central to this project with significant input into development of the protocol and co-production of key elements of this project and this report.
Aims and objectives
In order to answer the above RQs, we worked in partnership with a stakeholder group (SG) to:
-
comprehensively summarise evidence in this field by conducting a scoping review;
-
establish evidence of effectiveness by completing a SR of studies of effectiveness; and
-
determine factors affecting implementation by completing a mixed-method evidence synthesis.
Knowledge translation was facilitated by integrating findings of the above syntheses to summarise evidence and highlight research gaps.
Language and terminology
At the request of our SG, this final report is written in as plain English as possible. Where appropriate we have used consistent terms throughout the report, rather than introducing alternative terms. A glossary of terms is provided. We draw particular attention to the use of the following terms:
-
Child. We use the term child to refer to any person under the age of 18 years. We acknowledge that sometimes terms such as infant or young person may be preferred by some, but – for the sake of consistency – we have used ‘child’ to cover all these different groups. We have used the term child rather than the word ‘patient’, even if the child is (e.g.) a patient in a hospital.
-
Additional needs. We use the term additional needs to describe a child who has additional needs which may include, but are not limited to, learning disabilities, physical disabilities and/or developmental or sensory differences, including autism spectrum disorders, attention deficit hyperactivity disorder and complex health needs.
-
Families/carers. We use the phrase families/carers to describe the people in a child’s life who are responsible for the day-to-day needs and well-being of the child. These people will commonly include parents and other legal guardians, but may include other family members (e.g. siblings or grandparents) or caregivers.
Stakeholder reflection
Members of the SG have provided the following reflections, in their own words, based on their thoughts at the start of this project:
-
As the parent of a severely autistic child with very well hidden but significant CFC, I came to this project with several aims, the main one being to contribute to a more accessible, more consistent provision of treatment for this extremely important and under-reported issue, Questions of recognition and diagnosis were as important as those of treatment – if you can’t diagnose something you can’t treat it! Our son suffered greatly from CFC for some years, and the deterioration in his behaviour, he being unable to communicate his pain, led to significant limitations to his access to school, respite, and made family life extremely difficult. Ideally, my first hope would be to raise the profile of this significant issue especially for those with learning disabilities. Having a better understanding of what treatments are available, and a more consistent access to them, is equally important. This condition is painful, hugely affects quality of life, and is very often quite easily (and probably cheaply) improved, once it is recognised and once its treatment options are better understood. This is what I hoped and expected this project to contribute.
-
I was particularly keen to be involved in this project as my children’s CFC was missed for many years by diagnostic overshadowing. Their faecal incontinence was repeatedly put down to developmental delay rather than investigated. They will have to live with the results of this lack of diagnosis for the rest of their life. The lack of good systems to follow through reports of CFC for all children, including those with developmental delays, who are statistically much more at risk (possibly poor at reporting pain, may have multiple carers and vulnerable to diagnostic overshadowing) is a travesty as early intervention could potentially save so much future pain and cost.
-
There is a lack of recognition of the symptoms of CFC among healthcare professionals, and those affected by it. There is also limited appreciation of the wider costs, to the child and family and to the NHS of under and mismanagement. Successful treatment is based largely on clinical experience rather than research evidence, particularly with respect to which combinations of laxatives work most effectively in which groups of children and young people and which interventions are the most useful treatment adjuncts. This makes this project important.
-
CFC has a significant effect on a large number of children with effects on health, wellbeing and education that may last for decades. The high prevalence means that the burden of the disease is large for the healthcare economy as well as children and their families. Despite this being common and important, there remains a poor understanding of the underlying cause which in turn means treatments are rarely targeted further impairing outcomes. The unglamorous nature of the condition may make it less appealing for health care professionals to look after or for charities to raise money for compounding this effect. The NIHR have provided an excellent opportunity to undertake a structured approach of published data to consider how delivery of care might be best undertaken in the real world.
Summary
The SUCCESS project sought to work in partnership with stakeholders to bring together evidence relating to effectiveness and implementation of strategies to improve important outcomes for children with CFC. Chapter 2 provides an overview of the project.
Chapter 2 Overview of SUCCESS project
Introduction
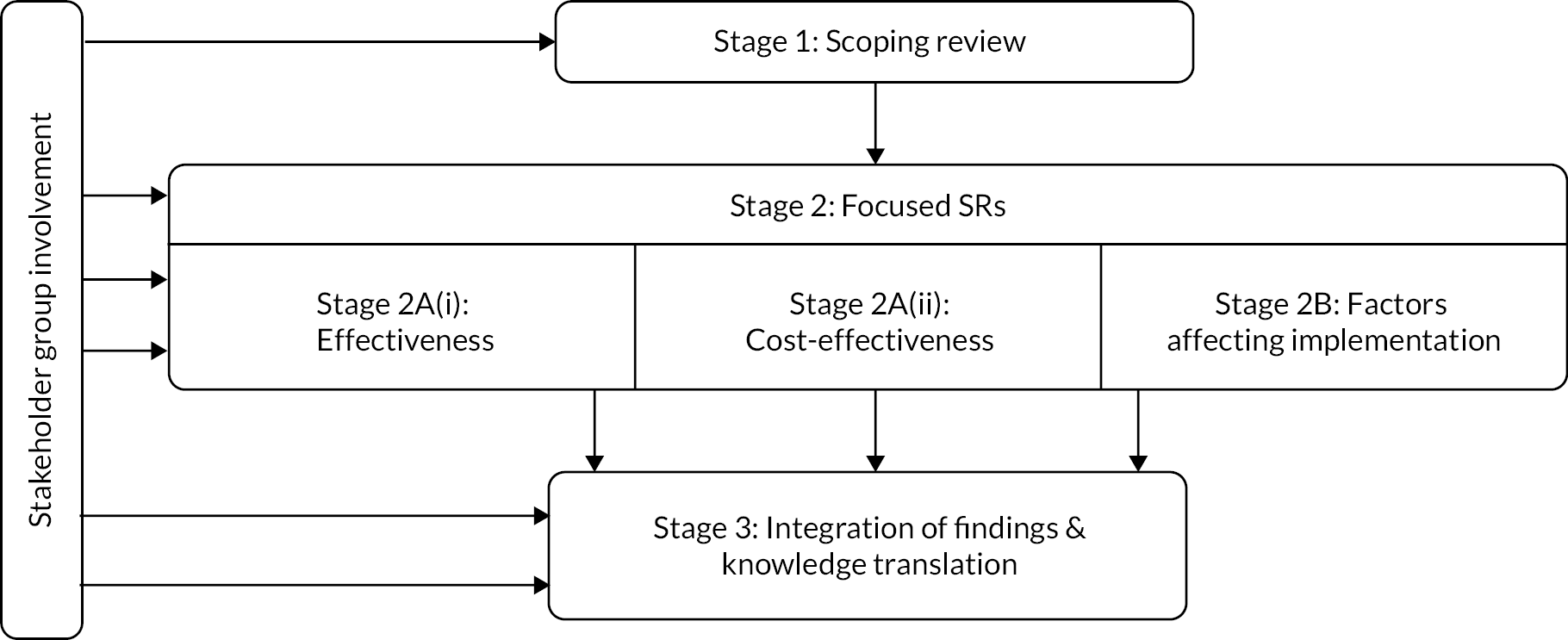
The aim of this chapter is to provide an overview of the SUCCESS project. This is a three-stage project, consisting of (1) a broad scoping review, (2) focused SRs evaluating effectiveness, cost effectiveness and implementation and (3) an integration of findings. This chapter will provide a brief introduction to these three stages and their methods; for an overview see Figure 1. Active stakeholder involvement and co-production was integral to our work, and our approach to involvement is described.
FIGURE 1.
Summary of SUCCESS study design.
Stakeholder group involvement
To ensure that we had meaningful involvement and co-production at every stage of this project, we formed a SG. The SG was involved in the development of the funding application and proposal, and five members were co-applicants on the proposal. INVOLVE guidance29 was followed to ensure that project meetings adhered to key principles of research co-production. In particular, we strove to create an environment which was inclusive, recognised the contributions of all participants, and in which people worked together to achieve a shared understanding. The SG had regular communication, including teleconferences every 4–6 weeks, and additional phone calls and e-mails as required. The opportunity for members to be reimbursed for their time was offered. 29,30 The SG were asked to complete four specific tasks, using methods for involving stakeholders in SRs. 31,32 These tasks included: (1) establishing which interventions, combinations and sequences, are available and being currently used, which are considered most important, and developing an intervention taxonomy, (2) agreeing the most important outcomes for the child, families/carers and health professionals, (3) developing a logic model to describe the effect that interventions, intervention combinations and implementation factors have on important outcomes and (4) reaching consensus over clinical implications and guiding knowledge translation activities.
Details of the SG involvement, and the impact of the involvement on this project, are reported in Chapter 3, a chapter which has been co-written by the SG members. At the end of each chapter in this report, there is also a brief reflection written by the SG members.
SUCCESS Pyramid to describe management strategies for childhood chronic functional constipation
The SG developed a model (‘Pyramid’; see Figure 2) to describe how CFC strategies may be delivered and combined. Details relating to the development of this pyramid are reported in Chapter 3. Informed by our stakeholders, this pyramid is central to all aspects of the SUCCESS project. This pyramid illustrates that CFC strategies may be delivered at a number of different levels:
FIGURE 2.
SUCCESS Pyramid to describe how CFC strategies may be delivered and combined. Note: This model is a simplistic visual of a non-linear complex process. The purpose of this model is to guide review questions and is not intended to advise clinical practice.
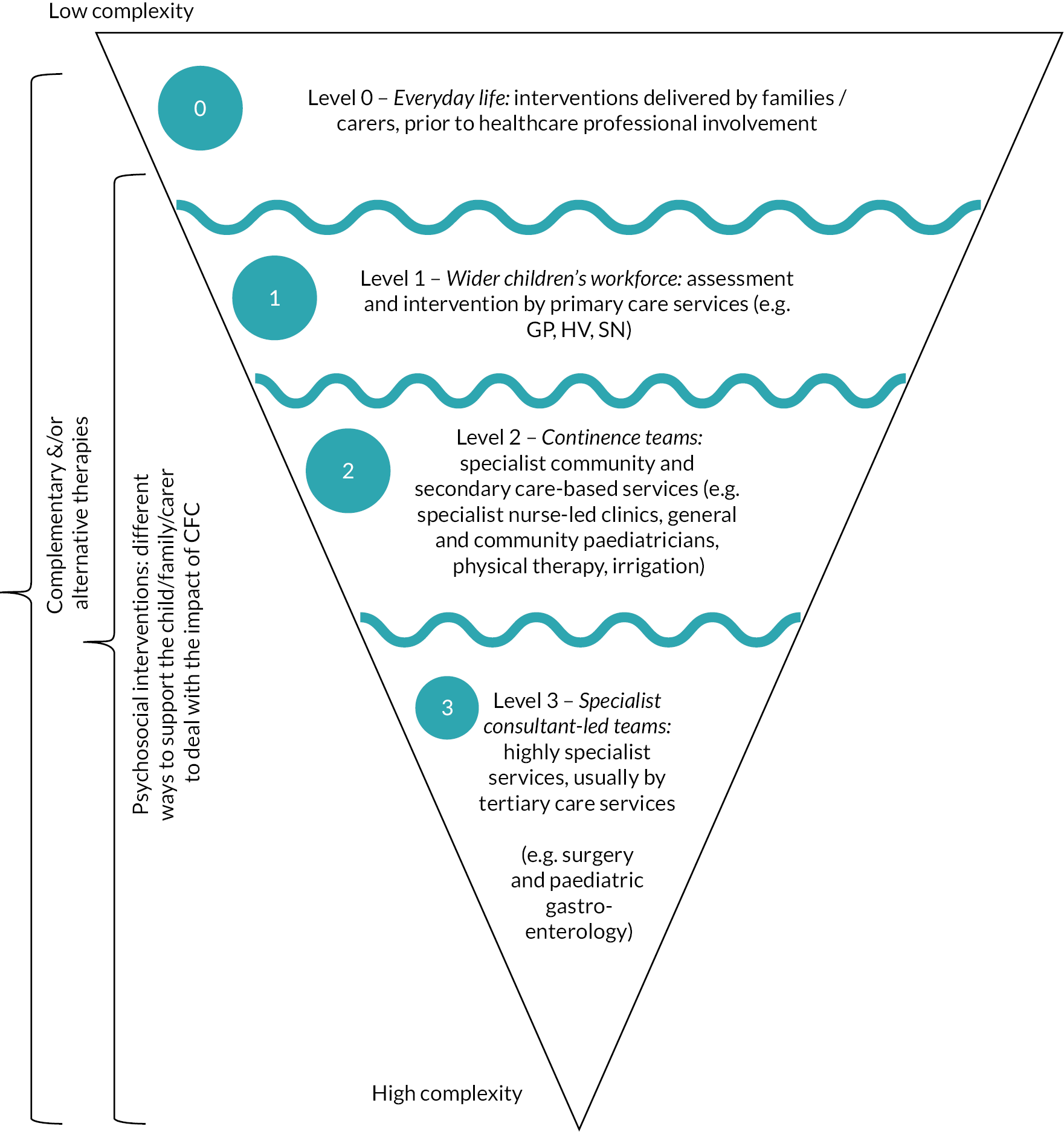
-
Level 0 – Everyday life: interventions delivered by families/carers, prior to healthcare professional involvement. These interventions include lifestyle-related strategies, such as diet, fluid and exercise, and the use of information obtained from sources such as peers, social media and websites.
-
Level 1 – Wider children’s workforce: assessment and intervention by primary care services [e.g. general practitioner (GP), health visitor (HV), school nurse (SN)]. In Level 1, the lifestyle and information-based strategies provided in Level 0 are combined with pharmacological interventions (laxatives). In addition, the wider children’s workforce may provide information/educational strategies to enhance the lifestyle strategies provided by families/carers.
-
Level 2 – Continence teams: specialist community and secondary care-based services (e.g. specialist nurse-led clinics, general and community paediatricians, physical therapy, irrigation). Children referred to Level 2 receive the Level 0 and 1 strategies, combined with specialist interventions such as provision of biofeedback, physical therapy or irrigation.
-
Level 3 – Specialist consultant-led teams: highly specialist services, usually by tertiary care services (e.g. surgery and paediatric gastroenterology). Children referred to Level 3 receive the Level 0, 1 and 2 strategies, combined with highly specialist interventions, such as surgery.
The ‘wiggly’ lines between the levels illustrate that there are often not clear distinctions between services provided at different levels. The ‘journey’ through the levels is not necessarily a linear one, from Level 0–1 to 2–3, with children moving both ‘up’ and ‘down’ the levels according to their symptoms and other factors, such as the availability of services.
In addition, the SG identified that there were a number of types of interventions which may be delivered at any (or all) of the different levels. These may be delivered in combination with the Level 0–3 strategies, and include the following:
-
Service delivery – strategies to organise care provision within or across the different levels.
-
Complementary (and/or alternative) interventions.
-
Psychosocial (including behavioural) interventions.
Throughout this report, evidence has been considered according to these different levels and types of intervention, and the terminology introduced here is used. The terms ‘Level 0’, ‘Level 1’, ‘Level 2’ and ‘Level 3’ are used throughout the report, as defined here.
Interventions within the SUCCESS Pyramid
The SUCCESS Pyramid was designed by stakeholders to cover all/any interventions, or combinations of interventions, which may be delivered for CFC. The Pyramid was designed to reflect that the process was cumulative, with interventions introduced at Level 0 being added to in Level 1, and further added to in Level 2 and then 3. When bringing together evidence about interventions, the interventions were placed within the Level at which it was considered they may first be delivered. For example, stakeholders told us that often the main interventions delivered within Level 2 and 3 were laxatives but acknowledged that the ideal would be to have the ‘right’ laxative prescribed within Level 1. (‘My children’s main interventions at level two and arguably level three (under a paediatric gastroenterologist) were laxatives of one sort or another … but we got to level two and three because we didn’t find the right solution in level one’).
Determining the placement of specific interventions within the Pyramid was done through discussion with stakeholders. Often it was acknowledged that the level at which an intervention could be delivered at could vary (e.g. within different health boards, or according to qualifications and expertise of individual practitioners). There was particular discussion regarding the interventions of dietary fibre, probiotics and laxatives. It was recognised that each of these could be delivered at either Level 0 or Level 1. Through discussion, a number of ‘rules’ were introduced to inform decisions about which Level specific interventions should be placed within. These ‘rules’ included:
-
Probiotics and dietary fibre: Level 0. Evidence relating to probiotics and dietary fibre was synthesised within Level 0, regardless of availability of the intervention. Stakeholders judged that families/carers considered probiotics and dietary fibre a dietary intervention and would potentially ‘buy stuff off the internet if they think it will help’.
-
Cow’s milk-free diet: Level 0. Studies investigating the efficacy of a cow’s milk-free diet for children with constipation are arguably only likely to include children who have been seen by health professionals within Level 1 or above. However, stakeholders highlighted that removing cow’s milk from a child’s diet was something ‘easy’ for families/carers to do. Therefore, evidence relating to a cow’s milk-free diet was synthesised within Level 0, regardless of the person/professional involved in monitoring the delivery of this diet.
-
Laxatives: Level 1. Evidence relating to pharmacological laxatives was synthesised within Level 1, regardless of availability of the intervention. The idea of dividing evidence relating to laxatives into Level 0 [laxatives available ‘over the counter’ (without prescription)] and Level 1 (laxatives only available on prescription) was explored but was considered unfeasible given the nature of the evidence and differences in availability in different countries.
-
Neuromodulation: Levels 2 and 3. Transcutaneous electrical stimulation (TES) and sacral nerve stimulation (SNS) were both considered a form of neuromodulation. However, stakeholders considered that TES should be considered a Level 2 and SNS a Level 3 intervention.
-
Physical exercise and therapy: Levels 0, 1 and 2. Interventions delivered by physiotherapists (also known as physical therapists) were considered Level 2, as these are specialist services. However, advice given to parents relating to more general physical exercises was considered relevant to the wider children’s workforce, and therefore considered Level 1. General physical activity was considered Level 0.
Where there was uncertainty over the placement of a specific intervention, the SG informed the decision-making. There was considerable discussion over the placement of some interventions, and limitations of the Pyramid model were acknowledged. However, despite the challenges and recognised limitations, the use of the Pyramid as a central structure for the evidence synthesised within the SUCCESS project was seen as advantageous, and as a way of ‘instilling some sense into what could just be a jumble of different treatments. This model tells me that I shouldn’t be thinking about Level 2 treatments for my child if I don’t yet know if the options in Level 0 and 1 work for them’.
Stage 1: scoping review
Stage 1 addressed RQ1 (see Research plan): What is the current evidence relating to management strategies for childhood CFC? This involved completion of a broad, comprehensive scoping review. Scoping reviews aim to map a broad field of literature (rather than address a very focused question); thus this approach was appropriate in order to bring together all current evidence relating to management strategies for CFC. Our approach was based on published scoping review guidance and followed a six-stage framework, including thorough searching and use of broad study design inclusion criteria. 33 We included studies of any design (including quantitative and qualitative) which related to a management strategy for children (aged 0–18 years) with CFC, regardless of study setting or outcomes reported. Results were tabulated and summarised narratively. Details of the methods of scoping review are provided in Chapter 4 and results in Chapter 5.
Stage 2: focused systematic reviews
Stage 2 comprised a series of focused SRs. Stage 2A addressed RQ2 (see Research plan): What are the most effective childhood CFC strategies and combination of strategies in relation to outcomes of importance to stakeholders and/or cost to the patient/NHS? For ease of access, we have subdivided these into (1) SRs of evidence of effectiveness of interventions and (2) SR of economic evidence. Stage 2B addressed RQ3 (see Research plan): What factors are associated with implementation success or failure of childhood CFC strategies and combination of strategies for different subgroups?
Stage 2A(i): systematic reviews of evidence of effectiveness of interventions
We conducted a SR of evidence for broad questions which related to each Level of the Pyramid. A stakeholder prioritisation exercise led to agreement as to which questions were of high, medium or low priority for a comprehensive SR of evidence of effectiveness (see Chapter 3). The questions and the agreed priorities were:
-
What is the effectiveness of different models of service delivery? (High priority).
-
What is the effectiveness of ‘everyday life’ interventions delivered by carers, without the involvement of healthcare professionals? (Level 0 interventions) (High priority).
-
What is the effectiveness of interventions delivered/prescribed by the wider children’s workforce (primary care services – GP, HV, SN)? (Level 1 interventions) (Medium priority).
-
What is the effectiveness of interventions delivered by continence teams (specialist secondary care services)? (Level 2 interventions) (Medium priority).
-
What is the effectiveness of interventions delivered by consultant-led teams (tertiary care services)? (Level 3 interventions) (Low priority).
-
What is the effectiveness of complementary interventions? (Medium priority).
-
What is the effectiveness of psychosocial interventions? (Medium priority).
At the request of the SG, these focused SRs included ‘all’/any interventions, or combinations of interventions relevant to each of these questions. The priority placed by stakeholders on the question informed some decisions relating to the comprehensiveness of data extraction and reporting [e.g. intervention details were not extracted into the template for intervention description and replication (TIDieR) template for the Level 3 synthesis, as this was considered low priority].
The outcomes for these focused SRs were identified and prioritised by the SG. The methods for these reviews are presented in Chapter 6 and the results in Chapter 7.
Stage 2A(ii): systematic review of economic evidence
We conducted a SR to summarise the availability and key findings of economic evidence of interventions that aim to improve CFC in children. This addressed the question about the cost of childhood CFC strategies and combinations of strategies to the child, their family/carers and NHS. Based on the synthesised evidence, we produce a brief economic summary. The methods and results of this are presented in Chapter 8.
Stage 2B: systematic review of factors affecting implementation
To explore the factors that are associated with implementation success or failure, we conducted a mixed-method evidence synthesis. This built on the search conducted for the scoping review and its results. The aim was to provide a more in-depth synthesis of evidence relating to barriers and facilitators to implementation of strategies, and the evidence relating to difference subgroups, with a particular focus on equity. The method and results of this synthesis are presented in Chapter 9.
Stage 3: integration of findings
In stage 3, we integrated the findings from stages 1 and 2, working in partnership with our SG to maximise the real-world usefulness of the synthesised evidence. This included development of a ‘logic model’ illustrating the inter-relationships between interventions and implementation; production of interactive evidence maps, bring together key findings and providing an accessible, systematic summary of evidence; comparison of our findings with key guidelines; identification of evidence gaps and generation of recommendations for research.
Details of the methods and results of stage 3 integration of findings are provided in Chapter 10.
Stakeholder reflection
Members of the SG have provided the following reflections, in their own words, based on their thoughts about the overall plan for the project:
-
Any project that is going to clarify the available treatments for CFC – particularly if it is well disseminated to the workforce who are in direct contact with children at risk (this is key) – is long overdue in my mind.
-
My reflections of the overall plan are very positive. I tried to give constructive input during the planning stages of SUCCESS.
-
I thought the project had a very well-considered approach aiming to address stakeholder priority. As a consequence, clinical outcome, process, patient experience and resource use will be considered consistent with the Health Foundation’s ‘Balanced Scorecard’. The use of a scoping review adds academic rigor ensuring that all patient-important aspects are picked up, not merely those that have been written about.
-
This study will provide the opportunity for stakeholders to clarify some of the key issues that may be used to inform future studies, as well as to synthesise the existing evidence, which should benefit those experiencing CFC, as well as those that assess and treat it.
Summary
To determine the most effective interventions and combinations and sequences of interventions for CFC and how they can best be implemented, we planned to carry out a scoping review and series of focused SRs, working in partnership with a SG. We planned to produce an accessible interactive map summarising our findings, a logic model, and a dissemination strategy aimed at supporting optimal implementation of effective interventions and highlighting evidence gaps requiring further research. Chapter 3 details the stakeholder involvement in this project, Chapters 4–10 present details of the methods and results of all project stages, and Chapter 11 provides a discussion and conclusion.
Chapter 3 Stakeholder involvement
Introduction
Involving patients, public and other people (collectively referred to as ‘stakeholders’) in research is widely accepted to be morally right and to enhance the quality, relevance and impact of health research, including SRs. 26,28,34,35 There is no evidence which points to a ‘best’ way of involving stakeholders in a SR, and there are a range of ways in which stakeholders have previously contributed to SRs. 27,28 Patient and public involvement (PPI) was adopted in this study in the form of a SG. The SG engaged with the research team to help inform the study design, methodologies, ongoing PPI evaluation, interpretation and dissemination of findings.
Definitions
For this project, we used the definition of stakeholder proposed by Concannon (2012):36 ‘any individual or group who is responsible for or affected by health- and healthcare-related decisions that can be informed by research evidence’.
Describing stakeholder involvement
Evidence shows that the quality of reporting of stakeholder involvement in SRs has, in the past, tended to be very poor. 28 The Authors and Consumers Together Impacting on eVidencE (ACTIVE) framework has been developed to support clear description of stakeholder involvement in SRs, and it uses five key constructs: (1) who was involved, (2) how were they recruited, (3) when were they involved, (4) what was the level of involvement and (5) what happened?27 These constructs have been used to structure this chapter.
The Guidance for Reporting Involvement of Patients and the Public (GRIPP)2 tool provides guidance for the reporting of stakeholder involvement in health and social care research. 37,38 This involves a checklist to ensure quality, transparency and consistency in reporting of PPI. We have used GRIPP2 to check our reporting, and the completed checklist is available in the project documentation.
Ethical approval and consent
UK guidance39 states that ethical approval is not required for stakeholder involvement activities; however, as we planned to audio record, store and report contributions made, we considered that seeking approval was good practice. Ethical approval was granted by Glasgow Caledonian University’s (GCU’s) School of Health and Life Sciences Nursing Department Research Ethics Committee (HLS/NCH/19/016) (see Project documentation).
Written consent for the recording and reporting of anonymised data was obtained from SG members prior to the first meeting. Verbal consent for the audio was given at the start of meetings. Data were anonymised and written-up, with electronic data stored securely.
Capture of stakeholder impact
After each SG meeting or activity, stakeholders were invited to complete a ‘Record of involvement’ aimed at capturing their views around the activity and the impact on the project. A report of each SG activity was written, describing what happened (based on ACTIVE framework)27 and the results and outcomes of, and reflections on, the SG input [based on Guidance for Reporting Involvement of Patients and the Public (GRIPP) short form]. 37,38 Meetings and activities were audio-recorded, and data used to inform completion of this report. Meeting reports are provided in Report Supplementary Material 1. In addition, on completion of first drafts of all chapters of this report, stakeholders were invited to add their reflections, and these are reported as a section within each chapter.
Training
We provided essential training, including a brief introduction to evidence-based practice and SRs, to all those involved. Stakeholders had opportunities to ask for further information, signposting to relevant online training, clarification and explanation by the research team.
Payment
The PPI members of the SG were offered payment for their time to attend meetings and review documents at NIHR-INVOLVE recommended rates. Eligible expenses, such as travel, were met for all stakeholders.
Description of involvement
Who was involved?
At the stage of developing this project idea and creating our funding application, our SG comprised of:
-
Four care providers (one consultant paediatric surgeon, three continence experts representing the following charities: Bladder and Bowel UK, ERIC Children’s Bladder + Bowel Charity, Association for Continence Advice). One of the continence experts withdrew from the SG in January 2021 due to retirement.
-
Four persons of the public [two who experienced childhood chronic functional constipation (CFC) themselves, and two parents of children experiencing CFC].
We planned that we would involve additional stakeholders throughout the project if this core group identified there was need to include other expertise or voices. During the course of the project, we had collected input from three additional stakeholders with expertise relating to parenthood, clinical commissioning of services within the NHS and general practice.
During the project the core group of stakeholders raised the need to strengthen the ‘voice’ of children and parents. We therefore held conversations with five children and their parents. We were also provided access to a video, which had been recorded within a national paediatric colorectal unit, in which a boy (aged 8 years) talked about living with CFC. Appropriate permissions, ethical approval and consent were granted for these activities (see Project documentation and Report Supplementary Material 3). The content of these conversations was used to inform SG discussions.
How were people recruited?
The public members were recruited through responding to our advert on the NIHR People in Research website. This ensured equality of opportunity as this recruitment process is an open process. The care providers were personally invited, identified because of their known expertise in the field.
During the project, additional stakeholders were identified through personal contacts of one of the other stakeholders.
When were they involved?
Involvement can occur at any one, or all, of 12 ‘stages’ of a SR. 27 The SG had both ‘continuous’ involvement, with informal communication and consultation occurring throughout the project, and ‘one-time’ involvement, with more formal planned meetings attended by the SG members and research team to complete the specific SG activities set at the proposal stage. Activity 1 was conducted at stage 1 (‘Develop question’) of the review process; and Activity 2 at stage 2 (‘Plan methods’). Activity 3 was addressed iteratively, with key meetings held at stage 1–2 (‘Develop question’/‘plan methods’), stage 7 (‘collect data’) and stage 9–11 (‘analyse data’, ‘interpret findings’, ‘write and publish review’). Activity 4 was addressed in a series of meetings held at stages 9–12 (‘analyse data’, ‘interpret findings’, ‘write and publish review’, ‘knowledge translation and impact’). See Stakeholder activities.
Level of involvement
The degree of stakeholder involvement in a review forms a continuum,27 from the active role of ‘leading’ a project (greatest degree of involvement) to the roles of ‘controlling’, ‘influencing’, ‘contributing’ and, finally to the more passive, ‘receiving’ of information or result of the review. Our aim was that the SG and research team members were equal partners in project decision-making, with each individual bringing unique knowledge and experience. For each of the planned tasks, the SG worked in partnership with the research team, with varying degree of control or influence over the review process. We recorded SG members’ involvement and impact during each task and also asked stakeholders to provide feedback after each meeting, describing their perceived level of involvement.
What happened?
Planned stakeholder events and changes due to COVID-19
The original plans had been to hold three face-to-face meetings, each occurring over 1 or 2 whole days, in Glasgow. Each of these events would have incorporated a series of meetings, discussions, presentations and practical exercises interspersed with breaks and opportunities for informal social interaction. The first of these planned events was held (January 2020), but due to COVID-19 restrictions and concerns all remaining meetings were conducted online via Zoom. The move to online meetings led to changes to the planned meeting format, with more frequent, shorter, meetings.
Stakeholder meetings
Appendix 1 provides an overview of the SG meetings held throughout the SUCCESS project.
Meeting participants
The majority of meetings were open to all SG and research team members; however, one meeting was held (13 May 2020) at which only the public members and one researcher attended. This was held soon after the move to online meetings (due to COVID-19 lockdown restrictions) and one of the reasons for this meeting was to explore whether separate meetings for public members would ensure that the public members had sufficient opportunity to contribute. The decision was made that it was preferable to have all SG members invited to all meetings, but it was highlighted that careful chairing was required to ensure that everyone had an equal opportunity to contribute at meetings. One further meeting was held with the public members only (12 April 2021); this was held specifically to explore an idea that public members had about leading a publication relating to PPI in this project.
Meeting conduct
Meetings adhered to key principles of research co-production,29 creating an environment that recognised everyone’s contributions and in which people worked together to achieve a shared understanding. Agendas and meeting material were circulated prior to the meetings. Meetings were chaired by one of the research team. Agendas were planned to ensure substantial time for discussion, and during Zoom meetings the ‘hands-up’ function was used to ensure everyone got the opportunity to speak. The ‘chat’ function was also used to support ‘side’ conversations, and to gain additional comments and questions, during the meetings. Meetings were recorded and a member of the research team listened back to the discussion when writing meeting notes, capturing the outcomes of activities and/or writing sections of the final report.
Stakeholder activities
A description of the stakeholder involvement in, and impact on, each of the four pre-planned activities is provided below.
Activity 1: Establish which interventions, combinations and sequences, are available and being currently used, which are considered most important, and develop an intervention taxonomy.
This activity was addressed through (Activity 1a) a face-to-face meeting was held over 2 days and following that (Activity 1b) an iterative process involving discussions at online meetings, comments on written documents sent by e-mail, and an individual voting/ranking exercise followed by consensus discussion to agree shared priorities. An overview of these Activities can be found in Report Supplementary Material 1. Key outputs from Activity 1a were an intervention taxonomy, listing and categorising all interventions/strategies which were considered to potentially be used to address childhood CFC, and an initial draft of a Pyramid model as a way of classifying intervention combinations and sequences (see Figure 2). The Pyramid model continued to be refined and developed throughout the rest of the project and contributed to the development of the logic model.
The intervention taxonomy listed all types of interventions for CFC known by the SG, grouped under key headings (see Appendix 2). The SG aimed to identify and group all known interventions for CFC within the intervention taxonomy but did not integrate these identified interventions with the Pyramid model, which illustrates how interventions may be delivered within combinations and sequences by different providers.
The key output from Activity 1b was a list of six broad questions – which reflected the key components of the Pyramid model – and agreement that these should be addressed by the SR of effectiveness. Each of the questions was categorised as to whether these were high, medium or low priority, with agreement that the level of priority should be reflected in the ‘depth’ of the evidence syntheses conducted in relation to these questions [see Stage 2A(i): systematic reviews of evidence of effectiveness of interventions].
Activity 2: Agree most important outcomes for the child, parents/carers and health professionals, to inform the SR of effectiveness.
During the scoping review searches, the research team identified a published core outcome set. 40 The SG members reached consensus to use the eight outcomes from the core outcome set project as the outcomes considered for the SR of effectiveness, independently ranking the importance of each of the eight outcomes. The SG considered the combined and individual (anonymised) rankings to reach consensus regarding the prioritisation of outcomes (see Report Supplementary Material 1).
Consensus was reached that the SR of effectiveness should have:
Two primary outcomes (considered of equal importance):
-
painful defaecation;
-
QoL of children and families/carers.
Six secondary outcomes (considered of equal importance):
-
defaecation frequency;
-
stool consistency;
-
side effects of treatment;
-
faecal incontinence, if age appropriate;
-
abdominal pain, if age appropriate;
-
school attendance, if age appropriate.
Activity 3: Develop a logic model which describes the effect that interventions and intervention combinations have on important outcomes, and key factors relating to implementation.
The process of developing the logic model is described fully elsewhere (see Report Supplementary Material 1 and Chapter 10). The SUCCESS Pyramid was central to the content of the final logic model. The stakeholders recognised that the final version of the logic model was complex and required further work, and that the version presented here is enhanced in relation to feedback and new evidence.
Activity 4: Reach consensus over clinical implications and guide knowledge translation activities.
Activities to discuss and agree clinical implications are reported in Report Supplementary Material 1 (Activity 4a). The draft results for each of the different evidence syntheses were presented at a series of meetings. Stakeholders discussed these and proposed clinical implications arising from this evidence and research recommendations. All stakeholders read and commented on the reported implications and research recommendations, ensuring that there was stakeholder input into and oversight of this stage.
Activities to guide knowledge translation are reported in Report Supplementary Material 1 (Activity 4b). An initial draft dissemination plan had been included in the funding proposal. This was re-visited regularly and discussed at several meetings, although the document with the plan was only formally updated twice. Stakeholders led key decisions about dissemination, highlighting the importance of producing a series of outputs, targeted at specific audiences, which can be shared on, or signposted to from, social media platforms. These outputs should comprise ‘layers’ of information, enabling different audiences to access information at different levels of detail: ‘we need pick and mix short presentations’. Stakeholders have contributed to the writing of all sections of this NIHR final report.
Discussion
Stakeholder involvement has controlled and/or influenced key aspects of the SUCCESS project. In particular, the involvement of stakeholders has:
-
Determined the way how evidence of effectiveness was brought together. The grouping of evidence according to the level of the person/organisation responsible for care was the idea of the stakeholders and this has shaped this project, and the outputs from this project.
-
Ensured that evidence (and evidence gaps) were identified relating to interventions delivered by parents/carers/family members. We believe that this is unique and is a key gap in other evidence reviews and guidelines relating to CFC.
-
Informed decisions relating to outcomes of importance to children and family/carers in relation to living with CFC.
-
Identified evidence gaps and influenced the discussion around recommendations for future research.
-
Led to co-production of a ‘logic model’ which reflects the complexity of treatments for CFC. The move away from a traditional ‘linear’ model was initiated by the SG.
-
Informed dissemination strategies.
Overall, stakeholders felt that their involvement was beneficial and they shaped the project in a useful way:
-
‘The stakeholders helped make sure that this project can contribute to the understanding of families and healthcare professionals that CFC is not a straightforward condition to live with or to manage at any level …’
-
‘My PPI experience of SUCCESS has been so valuable. The most important feedback I have given has been around the psychological aspects of constipation which I suffered as a child. My mother was acutely aware of my problems but I believe she normalised constipation and did not seek help …’.
Things that may have improved the impact, or experience, of involvement, included having more face-to-face meetings, with opportunities for open discussion:
-
‘If at all possible I would meet face to face at least once per year, ideally at the beginning and the end of the process. This again was outside the control of everyone’.
-
‘The best meeting we had was when there was much more open discussion …’.
Things that did not work so well and lessons that could be taken to future projects include the following:
-
‘There was no budget for involvement of people with relevant expertise or specialism in the development of dissemination products (e.g. creative arts or digital experts to produce animated films or interactive infographics; experts in information science) … resources to engage and involve suitable experts to maximise the quality and impact of project outputs [would have been good]’.
-
‘… there were no children or young people with lived experience of CFC directly involved. This is perhaps an indication of the embarrassment felt but also a reflection of the difficulty for families to commit to such a project. To try and mitigate this we undertook interviews directly with children and a carer/parent, but possibly we could have done more to hear children’s voices’.
Summary
The involvement of stakeholders has been integral to all aspects of the SUCCESS project. This chapter has provided details of the activities that stakeholders have contributed to, and the impact that this has had on the project. Within subsequent chapters, the role of the stakeholders in shaping the research is not specifically addressed (as it has been fully reported in this chapter) but a reflection from stakeholders on each chapter is included to provide a snapshot of some of the first-hand views and experiences of the members of the SG.
Chapter 4 Scoping review methods
Introduction
In this chapter, we describe the methods that were used to conduct stage 1 of this project. As outlined in Chapter 2, this broad, comprehensive scoping review aimed to identify all of the available evidence (and gaps) relating to management strategies for childhood CFC.
We followed the scoping review framework outlined by Arksey and O’Malley (2005). 33 This is the most commonly used framework for scoping reviews,41 and involves stages of (1) identifying the RQ, (2) identifying relevant studies, (3) selecting study, (4) charting the data, (5) collating, summarising and reporting and (6) consultation. In line with recommended guidance42 and common practice,41 we did not assess methodological quality of included studies, as the aim of the scoping review is to identify whether there is evidence, rather than determine the quality of the evidence base. We co-produced the protocol for the scoping review with our SG using an iterative approach to refine methods, with regular meetings and e-mail communication between the core research team and the stakeholders throughout all stages of the review (see Chapter 3).
The final agreed protocol which specified our selection criteria, methods and analysis was registered on the The International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42019159008) and published online. 43 Our methods and results (see Chapter 5) are reported in accordance with scoping review guidelines [preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (PRISMA-ScR)]. 42
Identifying the research question (scoping review stage 1)
Our broad RQ was pre-defined as What is the current evidence relating to management strategies for childhood CFC? Involvement of our SG (see Chapter 3) informed key aspects of the search strategy, data coding and synthesis.
Identifying relevant studies (scoping review stage 2)
Eligibility criteria
The selection criteria for inclusion were kept deliberately wide. We included any study which investigated, reported or discussed any interventions or management strategies aimed at improving outcomes for childhood CFC (as defined in Chapter 1). The study eligibility criteria are stated in Table 1, with further justification provided below.
| Study | Inclusion | Exclusion |
|---|---|---|
| Design |
|
|
| Participants |
|
Organic cause for constipation including:
|
| Intervention |
|
|
| Comparator | All comparators | None excluded |
| Outcomes | Eight outcomes of interest were prioritised by the SG (see Chapter 3) based on the recently published core outcome set40:
We also noted any other measures that had been reported. |
None excluded |
| Setting | Any geographical location or setting (with plans to categorise based on relevance to UK context during data charting). | None excluded |
One of the main challenges of the project was to ensure how best to apply the definition of CFC when determining whether a study was eligible for inclusion. Although constipation is relatively well defined (e.g. ROME III/IV criteria),4 it is referred to in the literature using a variety of terms (e.g. chronic faecal retention, idiopathic constipation). In addition, whether a child has CFC or whether the constipation is the result of another underlying condition, and therefore not functional, is not always clearly reported.
As one member of the SG stated: ‘This is all a very grey area as children with constipation (regardless of the underlying cause) would technically be treated the same … so the key message we give out is the importance of treating the presenting symptoms not the disease. So, constipation is constipation’.
This view is consistent with NICE guidelines1 which note that children and young people living with some physical and learning disabilities are disproportionately affected by CFC, and that management should be the same as is recommended for all children and young people.
Consequently, a list of potential conditions to include (or exclude) was drafted and discussed by members of the SG and research team (see Report Supplementary Material 2). They also agreed that we should employ a pragmatic, and inclusive, approach to applying the definition (when it was not clearly reported).
We excluded studies which explored issues such as diagnosis or causes of CFC. While scoping reviews generally aim to bring together a broad body of literature, there is a recognised need to have a balance between breadth and depth,41 and this allowed us to focus on studies of interventions for children with CFC, regardless of their study design. This decision aligned with our pre-determined aims and RQs.
Search methods to identify relevant studies
Electronic searches
We developed a comprehensive search strategy based on previous published searches. 19 The search strategy was peer-reviewed by an information specialist (JC) in accordance with peer review of electronic search strategies (PRESS) guidelines44 and the search string for medical literature analysis and retrieval system online (MEDLINE) is shown in Appendix 3. Other searches are available in Report Supplementary Material 2.
Searches were adapted for each of the following major electronic databases:
-
MEDLINE and PREMEDLINE (Medical Literature Analysis and Retrieval System Online);
-
Cochrane library databases including CENTRAL (Cochrane Central Register of Controlled Trials), CDSR (Cochrane Database of Systematic Reviews);
-
EMBASE (Excerpta Medica database);
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature);
-
PsycINFO;
-
AMED (Allied and Complementary Medicine Database);
-
Archived databases: DARE (Database of Abstracts of Reviews of Effects), HTA (Health Technology Assessment) database, NHS EED (NHS Economic Evaluation Database) will be accessed at www.crd.york.ac.uk/CRDWeb/.
Clinical trial registries:
-
World Health Organisation (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/clinical-trials-registry-platform);
-
OpenTrials (https://opentrials.net);
-
NIH US National Library of Medicine ClinicalTrials.gov (https://clinicaltrials.gov).
Electronic bibliographic databases and clinical trial registers were searched from January 2011 (unless otherwise indicated) to 10 March 2020. No language restrictions were applied. The electronic database search date was set from January 2011 following explicit comments from funders during the development of the research proposal. In order to avoid substantive duplication of searching previously conducted for NICE (2012) updated guidelines,19 which had a search date of 3 February 2012, the search date of January 2011 was agreed on.
Searching other resources
We conducted several supplementary searches in order to identify other potentially relevant studies. Dates of when each of the searches were conducted and any date limitations applied to this grey literature is documented in Report Supplementary Material 2. This included searching the following grey literature:
-
Google Scholar (https://scholar.google.com/);
-
Grey Matters (www.cadth.ca/grey-matters-practical-tool-searching-health-related-grey-literature);
-
Open Grey Repository (www.opengrey.eu/);
-
Physiotherapy Evidence Database (PEDro) (https://pedro.org.au/);
-
OTseeker (www.otseeker.com/);
-
PROSPERO International Prospective Register of Systematic Reviews (www.crd.york.ac.uk/prospero/);
-
Online social media platforms identified by members of our SG including Netmums, Scope, Challenging Behaviour Foundation forum.
We also searched relevant journals, conferences, guidelines and websites. This included:
-
International Continence Society (www.ics.org);
-
Digestive Disease Week (https://ddw.org);
-
United European Gastroenterology Week and the European Society for Paediatric Gastroenterology (https://ueg.eu);
-
European Society for Paediatric Gastroenterology, Hepatology and Nutrition (www.espghan.org);
-
North American Society of Pediatric Gastroenterology and Nutrition archives (https://naspghan.org);
-
British Journal of School Nursing (www.magonlinelibrary.com/loi/bjsn);
-
ProQuest Dissertations and Theses.
We also contacted experts, authors of eligible papers, manufacturers, national and international professional organisations and bodies (e.g. British Dietetic Association, School and Public Health Nurses Association) who are involved in the management of CFC to identify other relevant published and unpublished studies. Finally, we looked for studies included within published guidelines, including NICE guidelines1,2,19,20 and European guidelines,45 relevant SRs and reference lists.
Study selection (scoping review stage 3)
One reviewer (PC) ran the search strategy for each of the electronic databases. Two reviewers (LB, CT) conducted the supplementary searches. Using EndNote (v9) data management software, individual libraries were created for each electronic search (where possible); these were then merged into one master Endnote library and the files were de-deduplicated using the method recommended by Bremar (2016). 46 Titles were screened for inclusion with obviously irrelevant titles excluded by one reviewer (PC).
Pairs of reviewers (LB, DM, CT, PC) independently applied the selection criteria (see Table 1) to the remaining abstracts and full papers. Disagreements were resolved through discussion, involving a third reviewer (one of LB, DM, CT, PC, or a content expert from the SG). Exclusion reasons were recorded and reported. 42
Studies that could not be electronically downloaded were screened by one researcher (LB or CT) with relevant data and weblinks entered into Microsoft Excel. Details were checked by a second researcher.
Charting the data (scoping review stage 4)
For all included studies, one reviewer (LB, CT, PC) systematically extracted data from any related papers using a pre-developed data extraction file, focused on categorising the key features of the study. All data extraction was cross-checked by a second reviewer.
We extracted the following items:
-
study demographics: author, year, geographical region using the World Bank data47 categories for geographical region and income group;
-
study aim and design (as stated by authors);
-
participant characteristics (age range, sample size, whether the children had any additional needs);
-
definition of CFC and length of time with CFC (if reported);
-
setting (in which the intervention was delivered);
-
intervention details (as stated by authors) including any combinations or order of delivering combinations of treatments (e.g. laxatives combined with behavioural or dietary interventions);
-
reported outcomes (as stated by authors).
Collating, summarising and reporting (scoping review stage 5)
Following data extraction, the included studies were descriptively coded by two independent reviewers (LB, CT, PC) using predefined codes. A detailed coding manual is available in Report Supplementary Material 2. We also applied a series of tags to all studies that were considered relevant for inclusion in subsequent more focused SRs (see Chapter 6). Coded data were entered into Evidence for Policy and Practice (EPPI)-mapper software and an interactive evidence map generated to visually summarise the evidence. A 2 × 2 matrix was created, combining the interventions based on the taxonomy (columns), the study design (rows) and the volume of evidence (cells). Data were coded so that the number of studies was depicted by the size of a bubble within the cell, summarising the volume of evidence for each intervention and study design. An empty cell indicates a gap in evidence. In addition to the evidence tables and visual maps, data were summarised using figures and the findings discussed narratively.
Consultation (scoping review stage 6)
Data synthesis was informed by the SG and the development of the intervention taxonomy (see Chapter 3). Preliminary findings of the scoping review were presented to the SG during an online meeting (see Chapter 3). The session was audio-recorded to allow the research team the opportunity to take part in the discussion, and to accurately capture the views of the stakeholders to incorporate into the final synthesis.
Stakeholder reflections
Members of the SG have provided the following reflections, in their own words, on the methods and conduct of the scoping review:
-
I commend the researchers for drawing in stakeholder input at every stage of the scoping review. There was no sense of tokenism in this project. The protocol was effectively co-produced and the four PPI co-applicants as well as the professional stakeholders, had significant input.
-
I felt well informed about the work that went on and in terms of the way we co-produced the protocol for the scoping review and the subsequent focused evidence synthesis I felt that I was well involved. I feel this work was definitely well doing and I was very happy with my involvement in this.
-
Co-production allowed the skill sets needed for academic and technical delivery of the review to be combined with lived experience and professional perspectives. In turn, this may make research output more likely to be aligned to patient and service need and less likely to be wasted research. Whilst imperfect, it was more than ‘good enough’.
Summary
This scoping review was conducted to address a broad question and comprehensively identify relevant published and unpublished evidence of interventions delivered for children in the management of CFC, using established rigorous methods. Chapter 5 presents the results of this scoping review.
Chapter 5 Scoping review results
Introduction
In this chapter, we present the findings of the scoping review of interventions for CFC. This scoping review has identified and synthesised the existing evidence, providing an overview of the scope and nature of the evidence, and supporting identification of any evidence gaps.
Results of the search
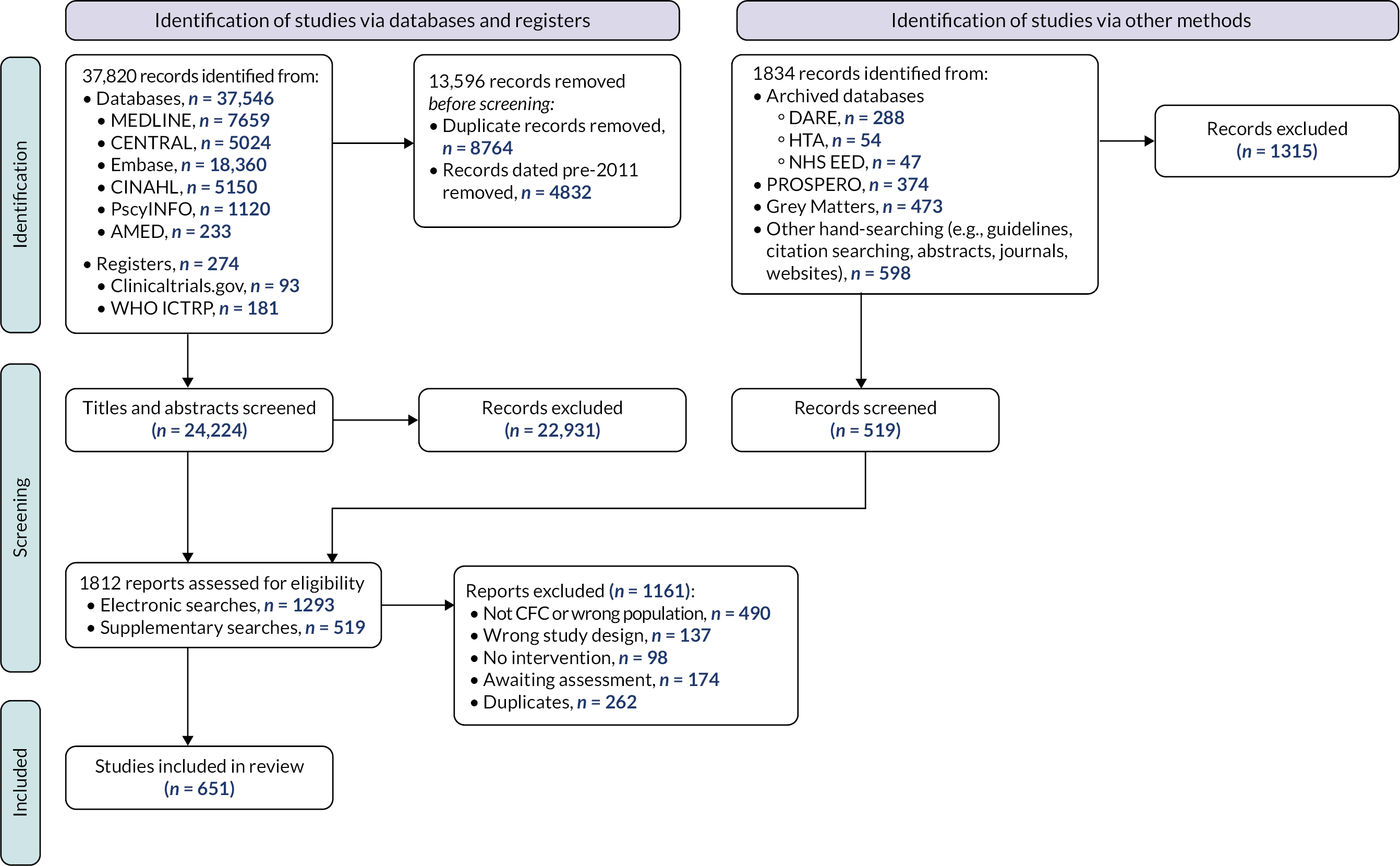
The results of the search are presented in Figure 3.
FIGURE 3.
Results of the search – scoping review.
Included studies
We included the remaining 651 studies in the scoping review. Details of the included studies are provided within a series of evidence tables (see Report Supplementary Material 3) categorised according to the taxonomy developed by the stakeholders in Chapter 3 (see Appendix 2). Section Characteristics of included studies charts the included studies according to study design, place of conduct, participant characteristics, intervention characteristics and study outcomes.
Excluded studies
As illustrated in Figure 3, we excluded 1161 reports following assessment of full texts. Reasons for exclusion are detailed in Figure 3. One hundred and seventy-four studies remain classified as awaiting assessment; 140/174 are published in languages other than English and require translation, we were unable to locate the full text of 16 studies and we have unanswered queries regarding the eligibility of remaining 18 studies (e.g. regarding age of participants or study design).
Characteristics of included studies
Study design and place of conduct
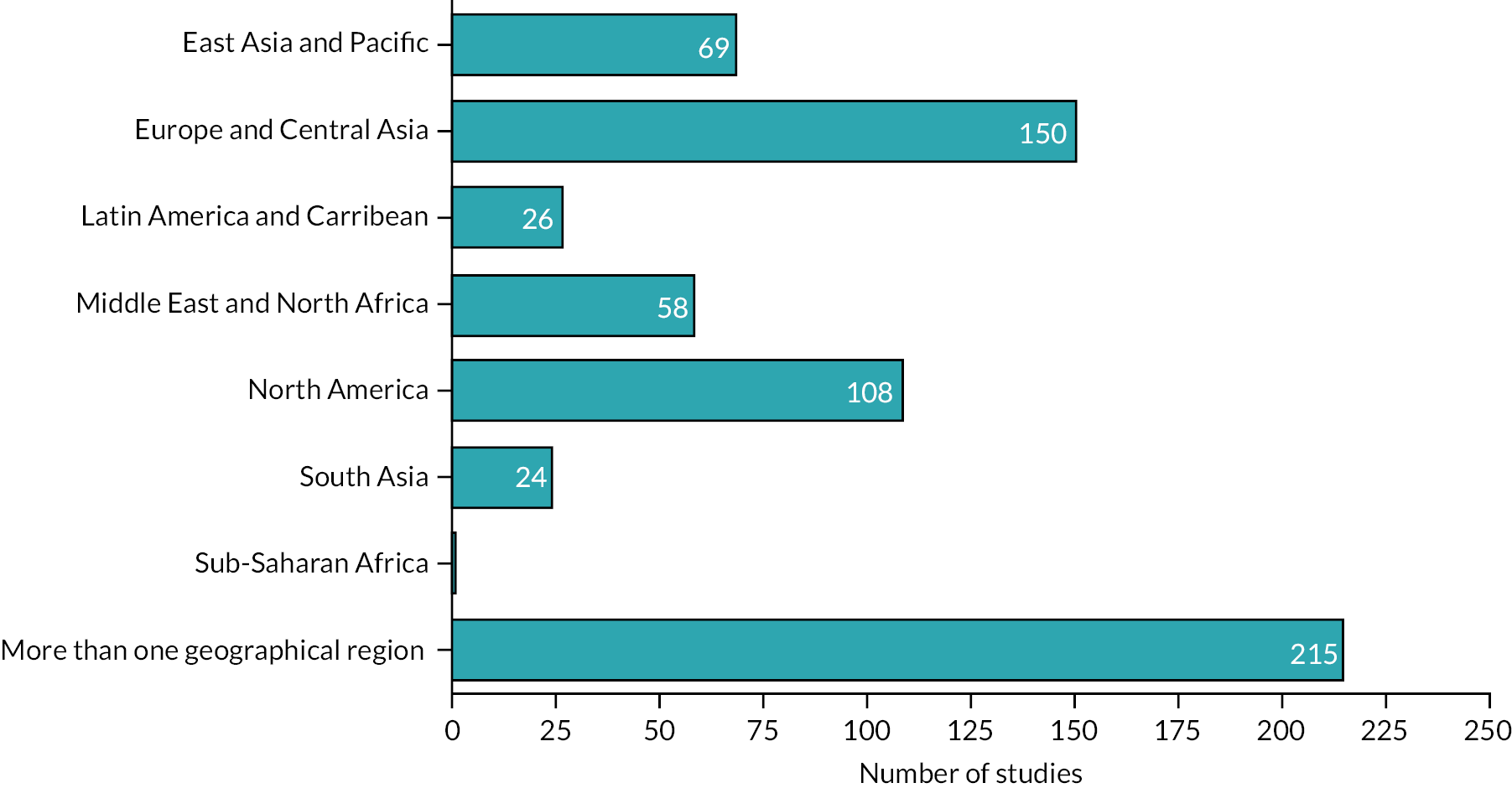
The majority of studies included in the scoping review were categorised as a primary study (n = 236) or used a randomised controlled trial (RCTs) design (n = 190). Almost a third of studies were evidence syntheses: narrative reviews (n = 140) and SRs (n = 71). The studies were conducted in 41 countries, with 215/651 (33%) of studies reporting evidence from more than one country. Most of the studies were conducted in the USA (103/651), UK and Iran (48/651), the Netherlands (29/651) and Australia (26/651). The majority of studies were conducted in high-income countries (46%); no studies from low-income countries47 were identified. Figures 4 and 5 show a more detailed summary of studies by region and income.
Participant characteristics
The number of participants included in the studies varied widely, from single case studies to 14,243 participants in one large multicentre cohort study. 48 The majority of studies focused on children across a wide age range (birth to 18 years). Forty-five studies specifically reported the treatment and management of CFC in children with a variety of additional support needs (ASN) including cerebral palsy (CP), autism, attention deficit disorder/attention hyperactivity disorder and neurodevelopmental disorders. The ROME criteria (II/III or IV) or a variation of these criteria were frequently used to define CFC.
Intervention characteristics
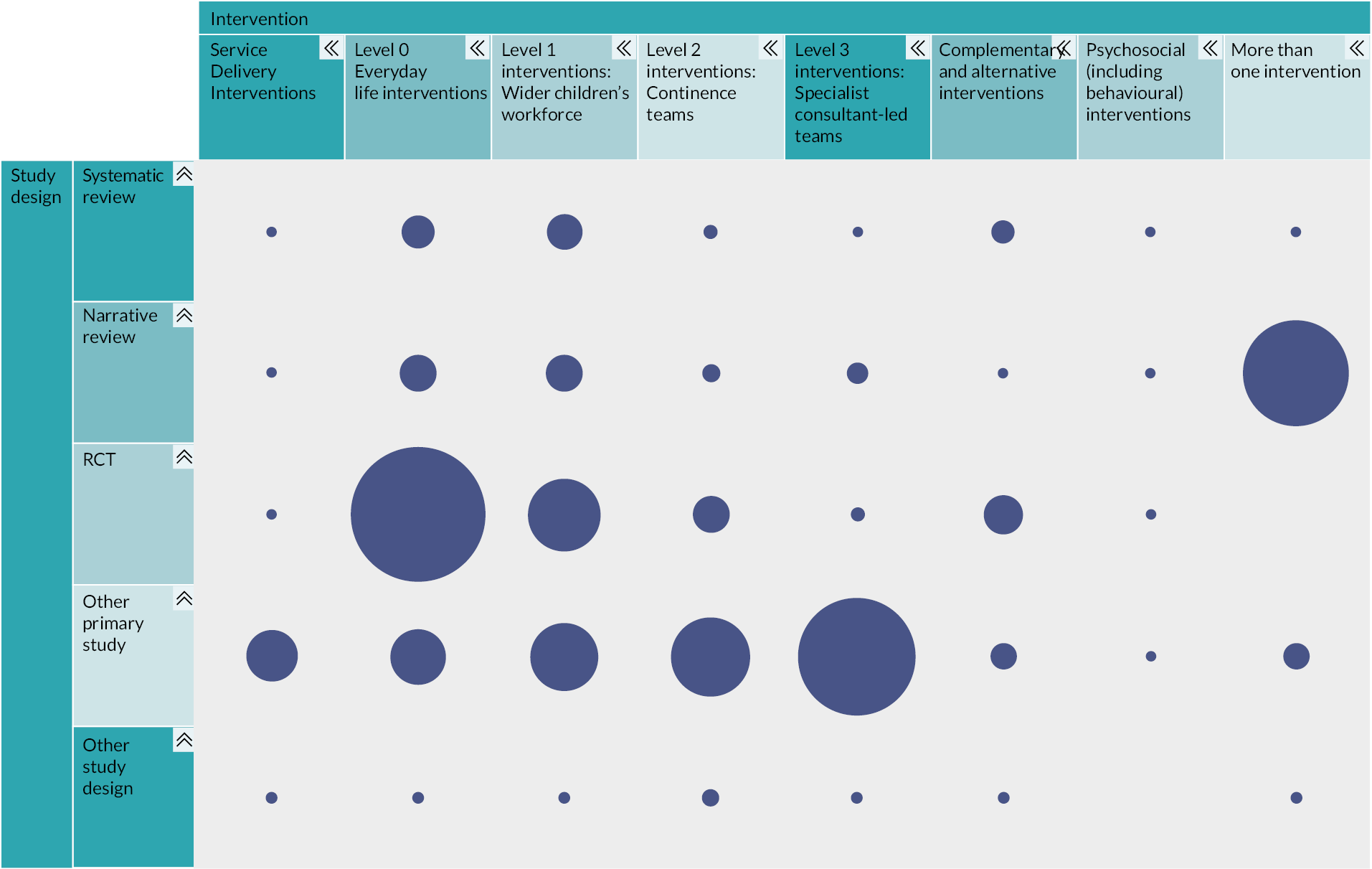
We identified 48 interventions (or combinations of interventions) reported across 651 studies. Details of the interventions are summarised in Report Supplementary Material 3. Figures 6 and 7 illustrate the types of interventions that were explored within the included studies, according to the taxonomy developed by stakeholders (see Chapter 3). An interactive evidence map (www.gcu.ac.uk/success) illustrates the evidence (number of studies and study design) which relates to the different interventions.
FIGURE 6.
Percentage of studies reporting different types of interventions, according to the taxonomy developed by the stakeholders.
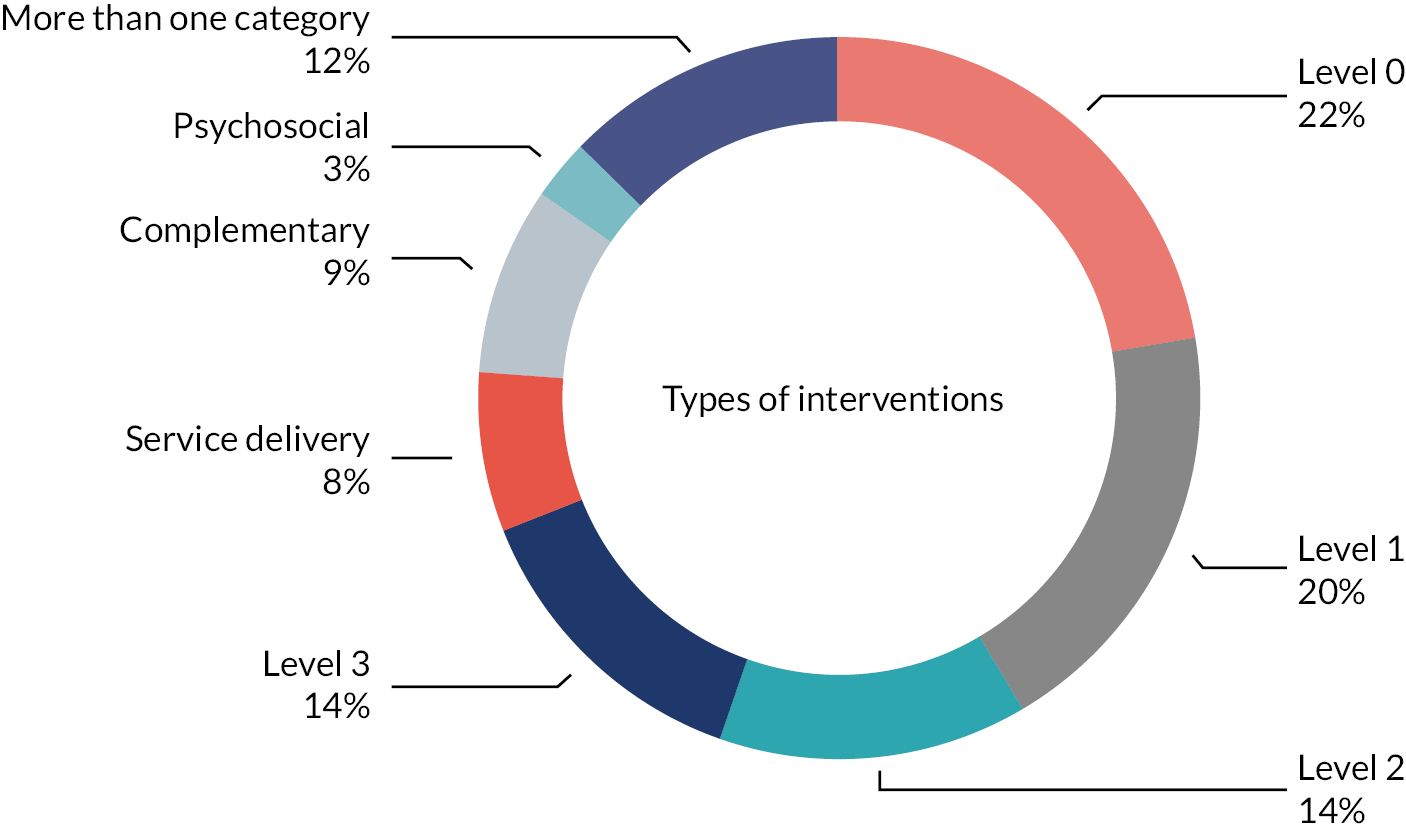
FIGURE 7.
Overview of studies, study design and ‘level’ of intervention (size of bubble represents number of studies; total number = 651).
Children were recruited from a variety of settings including hospitals, clinical outpatients and other community settings. Interventions were delivered at home and within a variety of hospital settings. Interventions were rarely delivered in education settings (e.g. school-based settings) (n = 5) or residential care/looked after population (n = 1) settings.
Reported outcomes
Figure 8 shows the number of outcomes reported across the included studies as mapped to the outcomes that were identified as priorities by our stakeholders (see Chapter 3).
FIGURE 8.
Number of outcome measures reported across included studies as mapped to outcome measures prioritised by the SG.

Narrative synthesis
Our narrative synthesis is structured according to the Pyramid of interventions and intervention taxonomy developed by our stakeholders (see Figure 2). Further details relating to studies and interventions are available in Report Supplementary Material 3.
Service delivery – Strategies to organise care provision within or across the different levels
We identified 49 studies that described different service delivery interventions; 34/49 studies employed a primary study design. The most frequently reported service delivery strategy involved care provision delivered by continence teams (n = 15) (Level 2). These interventions were often provided by a multidisciplinary team with input from dietitians, psychologists, clinical nurse specialists and gastroenterologists. The remaining studies described service delivery interventions aimed at improving constipation care pathways and algorithms (n = 10), evaluating the benefit of nurse-led models of care and/or identifying the role of the nurse in these settings (n = 7); refining service delivery in primary care (n = 8) or highly specialist (‘tertiary’) settings (n = 2). One study described a follow-up regime, and six studies described service delivery interventions that included more than one level of care provision.
Level 0 – Everyday life: interventions delivered by families/carers, prior to healthcare professional involvement
The largest volume of evidence identified by our scoping review were those interventions that were judged as being able to be delivered by family members/carers before the involvement of healthcare professionals (n = 144). These interventions typically fell into one of two categories: lifestyle (n = 133) or education and information provision (n = 11). Lifestyle interventions mainly focused on changes to diet, for example, by using probiotics, increasing fibre intake by changing a child’s diet to consume more fruit, vegetables and water or diet restrictions (e.g. cow’s milk-free diet) or a combination of these approaches. Educational and information interventions generally involved provision of leaflets.
Level 1 – Wider children’s workforce: assessment and intervention by primary care services
Our review identified 127 studies that were categorised as interventions delivered by primary care services. The majority of these studies involved the provision of a pharmacological intervention (126/127); only one study described the use of physical exercise (‘walking in squatting’). Several laxative agents were described [e.g. polyethylene glycol (PEG; with and without electrolytes), lactulose, linaclotide, lubiprostone, prucalopride, senna, sodium picosulphate]. While many of the interventions involved the delivery of one of these laxatives (alone or in combination with another laxative), almost half of the studies involved PEG (with and without electrolytes) (n = 61).
Note: all studies of laxatives were categorised as Level 1 interventions, as it is possible for laxatives to be prescribed by primary care services. However, laxatives (and all other Level 1) interventions may be delivered at both Level 2 and 3.
Level 2 – Continence teams: specialist community and secondary care-based services Level 2 interventions delivered by continence teams
Specialist interventions provided by specialist community and secondary care services were reported in 89 studies. The interventions that were most frequently investigated in the studies were enemas and/or suppositories (n = 29) or TES (home-based and hospital-based) (n = 21). Other common interventions in this category included biofeedback [with and without electromyography (EMG)] (n = 15), physical therapy (or physiotherapy) (n = 11) and irrigation (usually with the Peristeen system) (n = 8).
Level 3 – Specialist consultant-led teams: highly specialist services, usually by tertiary care services
Highly specialist interventions delivered by specialist consultant-led teams were reported in 88 studies. The most frequently investigated interventions in this category were surgical procedures to provide antegrade continence enema (ACE) or Malone antegrade continence enema (MACE) (n = 20), or studies that reported more than one surgical intervention (n = 20). Sacral neuromodulation/stimulation was described in 12 studies and 8 studies reported the use of botulinum toxin in children. The remaining studies described the use of faecal microbiotia transplantation, rectal biopsy, manual evacuation or other specialist interventions.
Complementary (and/or alternative) interventions
Our review identified 56 studies that reported complementary interventions. Most of the interventions described were herbal or traditional medicines (n = 20) or a variety of different massage approaches (n = 12). The remaining studies reported a range of interventions including acupuncture, musculoskeletal manipulation, reflexology and aromatherapy. Two studies reported more than one type of complementary therapy.
Psychosocial (including behavioural) interventions
Psychosocial interventions including behavioural management strategies were reported in 17 studies; 8/17 studies were narrative or SRs. All of the included studies reported more than one psychosocial intervention: describing a complex delivery of different psychotherapy approaches (e.g. cognitive-based therapy or other counselling and talking therapies), incentive-based reward systems and other techniques (e.g. relaxation, breathing, guided mastery).
More than one intervention
Our scoping review also identified 81 studies (12%) which reported more than one intervention. Most of these studies were narrative (n = 55) or SRs (n = 6), which meant that the evidence included within them spanned several interventions. The remaining studies were guidelines or consensus statements (n = 6) or other primary studies (n = 14); of these, 10/14 evaluated healthcare providers, knowledge and adherence to existing constipation guidelines.
Stakeholder reflections
Members of the SG have provided the following reflections, in their own words, on the results of the scoping review:
-
One of the revelations of the scoping review, from a parent point of view, is the gaps in areas studied, and one of the challenges, again from a parent’s point of view, is keeping in mind that gaps in evidence or poor-quality evidence does not directly reflect on the efficacy of a given treatment, unless of course the evidence suggests this.
-
When I was growing up I did not receive any help …. I had thought that by now there would be many more effective treatments …. I remember that when I saw the results of this review and the outcome measures that the studies had used, I was shocked. I am frustrated that there are so few studies that look at absenteeism from school.
-
I understand why all the studies of laxatives have been included in Level 1, as I get that we have made an assumption that if the study was about laxatives they could be prescribed at Level 1. But we need to make this clear and discuss this. Certainly my clinical practice at Level 2 (specialist community nurse-led service) was that the main tool for treatment was laxatives or laxative combinations.
Summary
Our scoping review identified 651 studies which reported evidence relating to interventions for CFC. Around half of these were primary research studies (RCTs or other primary designs). Characteristics of studies (including sample size and age of participants) varied widely. No studies from low-income countries were identified. The most frequently reported management or treatments of childhood CFC were those delivered by families/carers, prior to healthcare professional involvement and mainly focused on lifestyle interventions. Psychosocial interventions were reported in only 2.6% of studies. Defaecation frequency and stool consistency were the most commonly reported outcomes within the studies. School attendance (or absenteeism) was reported in 1% of included studies.
Chapter 6 Systematic review of evidence of effectiveness: methods
Introduction
This SR aims to synthesise the evidence for the effectiveness of strategies and combinations of strategies for childhood CFC in relation to outcomes of importance to stakeholders.
Systematic review questions
A pre-planned co-production process, involving key stakeholders, was conducted and determined the broad questions to be addressed by this SR of effectiveness. These questions reflect the SUCCESS Pyramid (see Figure 2). The broad questions addressed within these SRs, and prioritisation of these, are given in Section Stage 2A(i): Systematic reviews of evidence of effectiveness of interventions.
Interventions addressed by systematic review questions
The stakeholders wished to include ‘all’ interventions, and combinations of interventions, addressed under the broad questions given above. Determining which specific interventions should be addressed under which of the questions was done through discussion with stakeholders. Some general ‘rules’ were introduced to inform where some interventions were placed within the Pyramid (see Interventions within the SUCCESS Pyramid).
Clinical outcomes of importance
A stakeholder co-production process established the most important outcomes for the child, parents and health professionals. The primary clinical problems for this SR, and outcomes of importance to consider within the evidence syntheses, are listed in Section Stakeholder activities.
Search method for the identification of studies
All studies which were identified in the scoping review and ‘tagged’ as studies of effectiveness were included. These studies of effectiveness were tagged according to the broad RQ(s) to which they were related, and by type of study (SR, narrative review, RCT or other primary research study) (see Chapter 4). Studies addressing each broad RQ were then categorised according to the type of intervention that they addressed (using the intervention taxonomy; see Appendix 2).
Selection, data extraction and risk of bias assessment
Selection of studies to include used a step-wise approach, informed by a decision tree (see Figure 9). This approach was designed to avoid research waste by identifying and updating any relevant SRs, and to be comprehensive, by bringing together evidence from non-randomised studies where there were no high-quality SRs or large numbers of RCTs.
a.
Summary of review methods, including decision tree for selection of studies to include in review.

| Data extraction from | SRs | New RCTs + SR (s) | RCTs | Other primary research |
|---|---|---|---|---|
| Quality appraisal tool | ROBIS | ROB-1 for RCTs ROBIS for SR |
ROB-1 | Various (selected according to study design) |
| Data analysis/synthesis | Summarise meta-analyses from SR | New/updated meta-analysesa | New meta-analysesa | Descriptive summary of results |
Systematic reviews
For SRs identified in the tagging process, one reviewer extracted details of the study design, participants, intervention, comparison, outcomes, and checked by a second reviewer. Risk of bias (ROB) was assessed using the risk of bias assessment tool for systematic reviews (ROBIS) tool. 49 ROBIS was applied by two independent reviewers, with differences resolved through discussion, involving a third reviewer if necessary. Systematic reviews judged to be at high or unclear ROB were excluded from any further synthesis. For all SRs judged to be at low ROB, data were extracted on: aim, search strategy, selection criteria, number of included studies, number of participants within included studies, characteristics of participants and interventions within included studies, methodological quality of included studies (as reported by authors), results data (including statistical data for relevant outcomes and subgroups).
Two reviewers independently grouped the included SRs according to the specific RQ which they sought to address, based on the SG developed intervention taxonomy; groupings were discussed and agreed, involving a third reviewer if necessary. Systematic reviews were grouped into a matrix, summarising (1) the question addressed and (2) the search date of the review. Where there were two or more SRs assessing the same question, three reviewers discussed these and reached agreement on which was the most relevant, comprehensive, high quality and up to date, and this review was included and updated, and others excluded. Reasons for all decisions were transparently recorded within an Excel worksheet. When a SR of RCTs was identified and included no additional primary studies addressing that question were sought.
Excluded systematic reviews
Any SRs which did not meet the inclusion criteria were excluded and listed in a table of excluded studies. Papers which had been tagged as SRs during the scoping review, but on appraisal, were found not to have SR methods (i.e. a comprehensive search, systematic selection, data extraction and ROB assessment) were automatically considered to be high ROB (or unclear ROB), and were classed as ‘narrative reviews’ and excluded.
Randomised controlled trials
For RCTs identified in the tagging process, one reviewer extracted details of the study design, participants, intervention, comparison, outcomes and the level(s) of the Pyramid (see Figure 2) to which the intervention related, and this was checked by a second reviewer. Two reviewers independently grouped the studies according to the specific RQ which they sought to address; groupings were discussed, and a final categorisation agreed, involving a third reviewer if necessary. For all RCTs, ROB was assessed using the Cochrane ROB tool for RCTs (ROB1). 50 Assessments were completed by two independent reviewers, and differences resolved through discussion, involving a third reviewer if necessary. Additional data extraction, completed by one reviewer and checked by a second, was completed, including: aim, inclusion criteria, study design, number of participants (retention and drop-outs), demographic variables of included participants, baseline and follow-up results data [summary statistics, e.g. mean and standard deviation (SD)] for relevant outcomes. The TIDieR framework51 was used to summarise details of all trial interventions.
Where there were one or more RCTs addressing the same question and reporting data for one or more of our outcome measures of interest, these were considered for pooling within meta-analysis. For questions where RCTs were combined within meta-analysis, no further primary studies were sought.
Excluded randomised controlled trials
Randomised controlled trials judged found not to meet the inclusion criteria were excluded, with details listed in a table of excluded studies.
Ongoing randomised controlled trials
Where the identified paper was a protocol or study registration, a search was done for any reports of the completed study. If no reports were found, the study was excluded from the review, and details listed in a table of ongoing studies. The search for reports of completed studies was repeated (between 1 May 2022 and 1 June 2022) during the evidence synthesis write-up stage to ensure completed study data were not missing from the final synthesis.
Other primary research studies
For non-randomised primary research studies identified in the tagging process, one reviewer extracted details of the study design, participants, intervention, comparison and outcomes, and this was checked by a second reviewer. Two reviewers independently grouped the studies according to the specific RQ which they sought to address, building on the RQs identified through the assessment of SRs and RCTs. Assessment of the quality of primary research studies was conducted using tools appropriate to the study design. Quality appraisal tools included the critical appraisals skills programme (CASP) tool for cohort studies and qualitative studies,52 risk of bias in non-randomised studies of interventions (ROBINS-I) tool for non-randomised studies,53 Joanna Briggs Institute (JBI) tool for non-comparative studies54 and Ways of Evaluating Important and Relevant Data (WEIRD) tool55 where none of the previous listed tools were judged to be suitable. Assessments were completed by one reviewer and checked by a second. Additional data extraction was completed as for RCTs (see Randomised controlled trials).
Excluded primary research studies
Where a non-randomised primary research study was judged to address the same, or a similar, question as an included SR or as a number of RCTs found, that study was excluded, and not included in the narrative synthesis.
Data analysis
Meta-analyses within systematic reviews
Where we included a SR with a search date more than 12 months previously, we incorporated any new RCTs into the review and meta-analysis results, using the inclusion criteria stated within the original review.
Meta-analysis of randomised controlled trial data
We planned to conduct meta-analyses of pairwise comparisons for primary and secondary outcomes where direct evidence was available. Our plans involved estimation of pooled effect sizes [with 95% confidence intervals (CI)] using data from individual arms of included trials, with estimation of risk ratios for binary outcomes and mean differences (MDs) for continuous outcomes [or standardised mean differences (SMDs) if multiple measures had been used]. We planned to assess heterogeneity by visually inspecting forest plots and assessing I-squared statistics, with random-effects models used to address potential heterogeneity, and to investigate sources of heterogeneity by means of subgroup analysis (e.g. children with or without additional needs).
Network meta-analysis
We planned to explore whether it was possible to combine direct and indirect evidence using network meta-analysis (NMA) in order to estimate treatment effects between all interventions, even where no head-to-head trials had been identified. However, there were insufficient data relating to RCTs with similar clinical and methodological characteristics to enable NMA.
Data synthesis
We produced a narrative synthesis of evidence of effectiveness for each of the broad RQs (see Systematic review questions). Each synthesis comprises a preferred reporting items for systematic reviews and meta-analysis (PRISMA) flowchart summarising the searching and evidence identified for each broad RQ, a table of characteristics of all included reviews/studies, a summary of key characteristics and ROB and a narrative synthesis of the evidence. The narrative synthesis of evidence addresses the specific RQ addressed by the included reviews/studies. Where we have meta-analyses relating to effectiveness (i.e. from existing SRs or from our own analyses), these results are summarised. Our certainty in the findings for each specific question is judged as high, moderate, low, very low or insufficient evidence using a process of considered judgement informed by grading of recommendations, assessment, development and evaluations (GRADE) approach,56,57 and involving consideration of study limitations, inconsistency of results, indirectness of evidence, imprecision and reporting bias across the studies addressing each question. A summary of key findings is provided.
Stakeholder reflections
Members of the SG have provided the following reflections, in their own words, based on their thoughts about the methods for the SRs of effectiveness:
-
I thought this was done in a logical way that was well communicated to us as stakeholders and which used our experience as well as any work like this can. Work like this is much more difficult to involve stakeholders meaningfully but the team have done a good job.
-
It was gratifying that lay stakeholders were so well included including in areas in which we have no direct expertise but have an interest.
-
Considering two PPI members had experience of systematic reviewing and synthesis and two did not, the research team did a great job of keeping us all on the same page. When we asked questions, the team made every endeavour to give comprehensive answers and explanations. These were communicated in various formats – the written word, tables, graphs, charts, pictures. The use of colour coding was particularly helpful. Furthermore, we had opportunity to give detailed written feedback post meetings – an added task for PPI members to complete – but great to promote insight and recollection.
Summary
This chapter describes the methods for the SR of evidence of effectiveness. This SR is structured according to broad questions relating to the SUCCESS Pyramid (see Figure 2). Specific questions addressed by included studies are identified and narrative syntheses of evidence are presented, where appropriate supported by results of meta-analyses from updated SRs.
Chapter 7 Systematic review of evidence of effectiveness: results
Introduction
This chapter provides the results of the SR of evidence of effectiveness. These are presented according to each of the broad questions addressed (see Systematic review questions). Evidence relating the different models of service delivery has been presented first (see What is the effectiveness of different models of service delivery?), as the issue of how a service is delivered was considered a top priority; one stakeholder summed this up by saying ‘It ain’t what you do, it’s the way that you do it’. The evidence relating to Level 0 [see What is the evidence of effectiveness of interventions delivered by families/carers, prior to healthcare professional involvement (everyday life/Level 0 interventions)?], 1 [see What is the effectiveness of assessment and intervention by primary care services (wider children’s workforce/Level 1 interventions)?], 2 [see What is the effectiveness of interventions delivered by secondary specialist care (continence teams/Level 2 interventions)?] and 3 [see Evidence of effectiveness: interventions delivered by consultant-led teams (Level 3/highly specialist tertiary care services)] is then presented, followed by evidence relating to complementary therapies (see What is the effectiveness of complementary therapy interventions?) and psychosocial interventions (see What is the effectiveness of psychosocial interventions?).
What is the effectiveness of different models of service delivery?
Results of the search
Figure 10 illustrates the results of the search. Details of ongoing, excluded and awaiting assessment studies are provided in Report Supplementary Material 4.
FIGURE 10.
Results of the search – service delivery studies.
We included a total of 15 studies within this synthesis: 4 RCTs and 11 other primary studies. We did not include any SRs.
Characteristics of included studies
Characteristics of the included studies are provided in Tables 2 and 14 (see Appendix 4). The ROB is summarised in Report Supplementary Material 4. Details of interventions according to TIDieR51 template are provided in Report Supplementary Material 12.
| Study | Study design | No. of children with CFC recruited | Intervention | Overall ROB | Abstract only? |
|---|---|---|---|---|---|
| Nurse-led models of care ( n = 4) | |||||
| Burnett 200458 | RCT | 102 | Nurse-led vs. physician-led clinic | L-L-H-H-U | |
| Faramarzian 201859 | RCT | 120 | Additional nurse-led input vs. usual care | H-H-H-H-H | |
| Ismail 201160 | Non-comparative | 50 | Nurse-led clinic | Minor concerns | |
| Tappin 201361 | Non-comparative | 75 | Nurse-led clinic vs. physician-led clinic | Very minor concerns | |
| Primary care pathway/algorithm ( n = 3) | |||||
| Bellesheim 201862 | Non-comparative | 82 | Practice pathway comprising framework of flowcharts for children with ASD | Very minor concerns | |
| Mallon 201563 | Retrospective cohort | 61 | Management algorithm aimed at adherence to guidelines | Very minor concerns | |
| Norbedo 201764 | Retrospective cohort | 1020 | Practice within ED | Moderate concerns | |
| Specialist (Level 2) services/models of care ( n = 6) | |||||
| Athanasakos, 202065 | Non-comparative | 112 | Specialist physiology service | Very minor concerns | |
| Costigan 201966 | Retrospective cohort | 15 | Bowel management clinic with TAI | Serious concerns | |
| Gabr 202067 | Non-comparative | 111 | Bowel management programme/management pathway | Minor concerns | |
| Gonring 201968 | Retrospective cohort | 26 | Interdisciplinary, ‘carer-assisted medical-behavioural, group-based intervention’. | Minor concerns | |
| Karagiozoglou-Lampoudi 201269 | RCT | 86 | Model with personalised dietary advice from a registered dietitian | U-U-U-H-U | |
| Poenaru 199770 | Non-comparative | 114 | Multidisciplinary bowel management clinic | Serious concerns | |
| Follow-up regimes ( n = 1) | |||||
| Modin 201671 | RCT | 235 | Website + phone follow-up | L-L-H-H-L | |
| Highly specialist (Level 3) services/models of care ( n = 1) | |||||
| Short 201872 | Retrospective cohort study | 43 | Recovery protocol after colorectal surgery | Moderate concerns | |
A total of 2252 children with constipation (range 15–1020 per study) were included in the 15 studies. 58–72 The age of children ranged between 1 month and 20 years. One study62 included only children additional needs, and two studies explicitly excluded children with additional needs. 58,71 The remaining studies did not explicitly report whether children with additional needs were included or not.
The most frequently reported outcome of interest was faecal incontinence, reported by 10 studies. 58,60,61,63,65–68,70,71 Only four studies assessed painful defaecation59–62 and two assessed QoL. 65,67 No study assessed school attendance. A summary of the outcomes reported and ROB judgements are provided in Report Supplementary Material 4.
Research questions addressed
The included studies were judged to address seven distinct RQs. The following sections provide a summary of the evidence in relation to each question. Studies are summarised in Table 2, with more details provided in Table 14 (see Appendix 4). The outcomes assessed, ROB and judgements of certainty and are summarised in Report Supplementary Material 4.
Evidence of effectiveness: models of service delivery across all levels of pyramid
What is the effect of nurse-led models of care as compared to alternative models of care?
Four studies58,59,60,61 addressed this question (see Table 2). Burnett (2004)58 randomised 102 children for follow-up (after an initial paediatric gastroenterology clinic appointment) by either a nurse-led clinic or a physician-led clinic. Results did not reach statistical significance; however, 65.4% were ‘cured’ in the nurse-led clinic, compared to 50.0% in the physician-led clinic, with a median time to cure of 18.0 months (95% CI 8.5 to 27.5) in the nurse-led clinic and 23.2 months (95% CI 17.3 to 29.2) in the physician-led clinic. Faramarzian (2018)59 allocated 120 children to either additional nurse-led input or usual care. There were no statistically significant differences between groups at 1, 2 or 3 months after the start of the intervention. Ismail (2011)60 explored the impact of a nurse-led clinic for children who had not made satisfactory progress while attending a general paediatric clinic. Of the first 50 children attending the nurse-led clinic, significant self/parent reported improvement was made after 3–4 months. Tappin (2013)61 found no significant differences between 75 children who attended either nurse-led or physician-led clinics for any of the reported outcomes. In summary, there is some very-low-quality evidence that nurse-led clinics are feasible and could result in equivalent (or possibly better) outcomes than traditional physician-led clinics.
Evidence of effectiveness: models of service delivery relating to Level 1 of the Pyramid
What is the effect of a constipation care pathway/algorithm used in primary care/community settings?
Three studies62–65 (see Table 2) explored the effect of a constipation care pathway/algorithm used in primary care/community settings. Mallon (2015)63 found that, compared to before the circulation of a management algorithm, after implementation there were significantly fewer children with faecal impaction and a smaller proportion of children referred on to secondary care (gastrointestinal specialists). Bellesheim (2018)62 explored the effect of a practice pathway, comprising a framework of flowcharts covering assessments, interventions, medication, referrals and follow-up, for children with constipation and autistic spectrum disorder (ASD). The refined pathway led to 85% of 82 children with ASD and constipation achieving at least one personal ‘goal’. Norbedo (2017)64 explored the effects of a constipation care pathway/algorithm used for children presenting in emergency departments (EDs), aiming to determine incidence of functional constipation in children presenting at the ED. Information on treatment and treatment outcomes are limited, although 734/1020 were treated with an enema. In summary, there is very limited evidence that an algorithm, or care pathway, used in primary care settings to guide the management and referral of children with constipation (including children with ASD) may be beneficial. There is insufficient evidence on which to reach conclusions relating to care pathways within ED.
Evidence of effectiveness: models of service delivery relating to Level 2 of the Pyramid
What are the effects of specialist (Level 2) services and models of service delivery?
Six included studies65–70 addressed this question (see Table 2). A RCT69 investigated the effect of a model of care which involved 86 children with, and families, receiving personalised dietary advice from a registered dietitian. Effect on constipation-related outcomes is not reported. Gonring (2019)68 described the delivery and outcomes of an interdisciplinary, ‘carer-assisted medical-behavioural, group-based intervention’. The results demonstrate improvements in stooling patterns (frequency and incontinence) and suggest this programme may be beneficial to children who have failed ‘traditional medical management’. Gabr (2020)67 evaluated the effect of a Bowel Management Program comprising laxatives, diet modification and toilet training delivered as part of a comprehensive management pathway. A statistically significant beneficial change in a QoL score and faecal incontinence score was found after the programme. Costigan (2019)66 described the profile of children attending a bowel management clinic, with a focus on the use of transanal irrigation (TAI) devices. Only 15 of the 192 included children had CFC. However, 10 of the children with CFC who attended the service were able to discontinue washout use and achieve faecal continence. Athanasakos (2020)65 explored the effect of having input from a specialist physiology service, providing scientific investigations, to inform decisions of the multidisciplinary team. The authors reported a significant improvement in subjective condition severity (physical and emotional) scores at 3 months post intervention. Poenaru (1997)70 explored the effect of a multidisciplinary team running a paediatric bowel management clinic. Defaecation frequency, stool consistency and abdominal pain significantly improved but there was no effect on faecal incontinence.
In summary, consistent findings from studies with some limitations provide very-low-quality evidence that specialist services may have a beneficial impact on outcomes of children with chronic constipation, but further research is required.
What is the effect of different follow-up regimes following appointments with specialists?
We identified one RCT71 which randomised 235 children to receive either a phone follow-up, access to a website or standard care. Results at 12 months showed there were 68.4% successfully treated children in the phone follow-up group, 78.5% in the website access group and 72.7% in the control group. The percentage with faecal incontinence in the three groups were 21.5%, 15.6% and 13.0%, respectively. The authors conclude that provision of access to web-based information may be more beneficial for the recovery from constipation.
In summary, low-quality evidence from one RCT suggests that access to web-based information, following an appointment with a specialist, may be more beneficial that a follow-up phone call or no follow-up.
Evidence of effectiveness: models of service delivery relating to Level 3 of the Pyramid
What are the effects of highly specialist (Level 3) services and models of care?
We found one study72 which explored the effect of a recovery protocol for children who underwent colorectal surgery. The majority of children had inflammatory bowel disease, with only seven with a diagnosis of constipation. Compared to pre protocol, the length of stay significantly decreased, with no significant differences for complication and readmission rates. In summary, there is very-low-quality evidence that a recovery protocol may benefit outcomes following colorectal surgery. This evidence does not relate specifically to the population of children with CFC.
Summary of evidence of effectiveness: models of service delivery
Fifteen primary studies,58–72 of which four were RCTs,58,59,69,71 provide evidence of effectiveness of different models of care provision. Generally reporting was poor, limiting ability to draw generalisable conclusions. There is some low-quality evidence that: nurse-led clinics are feasible and could result in equivalent (or possibly better) outcomes than traditional physician-led clinics; an algorithm, or care pathway, used in primary care settings, may be beneficial. Low-quality evidence suggests that access to web-based information, following an appointment with a specialist, may be more beneficial that a follow-up phone call or no follow-up and that specialist (Level 2) services may have a beneficial impact.
Stakeholder reflections on evidence of effectiveness: models of service delivery
Members of the SG have provided the following reflections, in their own words, on the evidence identified in relation to models of service delivery:
-
The service models section is of particular interest to me as a parent, as it is tied in so closely with access. The interventions themselves are important, but having access to them in a convenient and reasonable manner is equally important. And while it isn’t directly my concern, cost effectiveness overall is also very important – models of service delivery are key to this.
-
There is a need for much more robust research to be done in a number of these areas, but I am glad some interventions are evidenced to be effective.
-
I have wondered about white coat syndrome for children regarding nurse-led or physician-led interventions. I like the idea of online resources for post appointment access to information. This may be a way forward for the future. It’d be a less stressful environment to absorb information and of course, you can return to it for recap.
-
It is important to consider how services are delivered along with the who, why, what, where, when. In real world practice variability is often seen between centres for reasons that aren’t immediately apparent and which may well be affected by infrastructure and culture and values. Little research is likely to be done focusing on the size of effect and how to influence but nevertheless this is likely to have as big an impact as many of the interventions studied. It will be of interest to service commissioners and funders as well as families who pass through the service and the staff that work within it.
-
The concept of a bowel management programme to deliver considered intense delivery of care to groups of patients seems sensible and perhaps replicates ‘induction programmes’ for children with newly diagnosed diabetes in Europe. Although traditionally done by surgeons in the US, this approach could perhaps be undertaken by physicians, surgeons or nurse specialist, perhaps with psychology and other healthcare professionals for children with treatment resistant symptoms. Replication in a non fee-for-service environment may produce different results if less health activated families more likely to attend but nevertheless, this may demonstrate benefits to a range of outcomes.
What is the evidence of effectiveness of interventions delivered by families/carers, prior to healthcare professional involvement (everyday life/Level 0 interventions)?
Results of the search
Figure 11 illustrates the results of the search. Details of ongoing, excluded and awaiting assessment studies are provided in Report Supplementary Material 5. Of the 88 studies not meeting the exclusion criteria, 33 were obviously irrelevant; reasons for exclusion of 55 others are listed in the table of excluded studies (see Report Supplementary Material 5). One of the 23 ongoing RCTs (RCT protocols) published results in May 2022, but we have left listed as an ongoing study as results are not available in English; 11 participants were included. 73
FIGURE 11.
Results of the search – Level 0 studies.
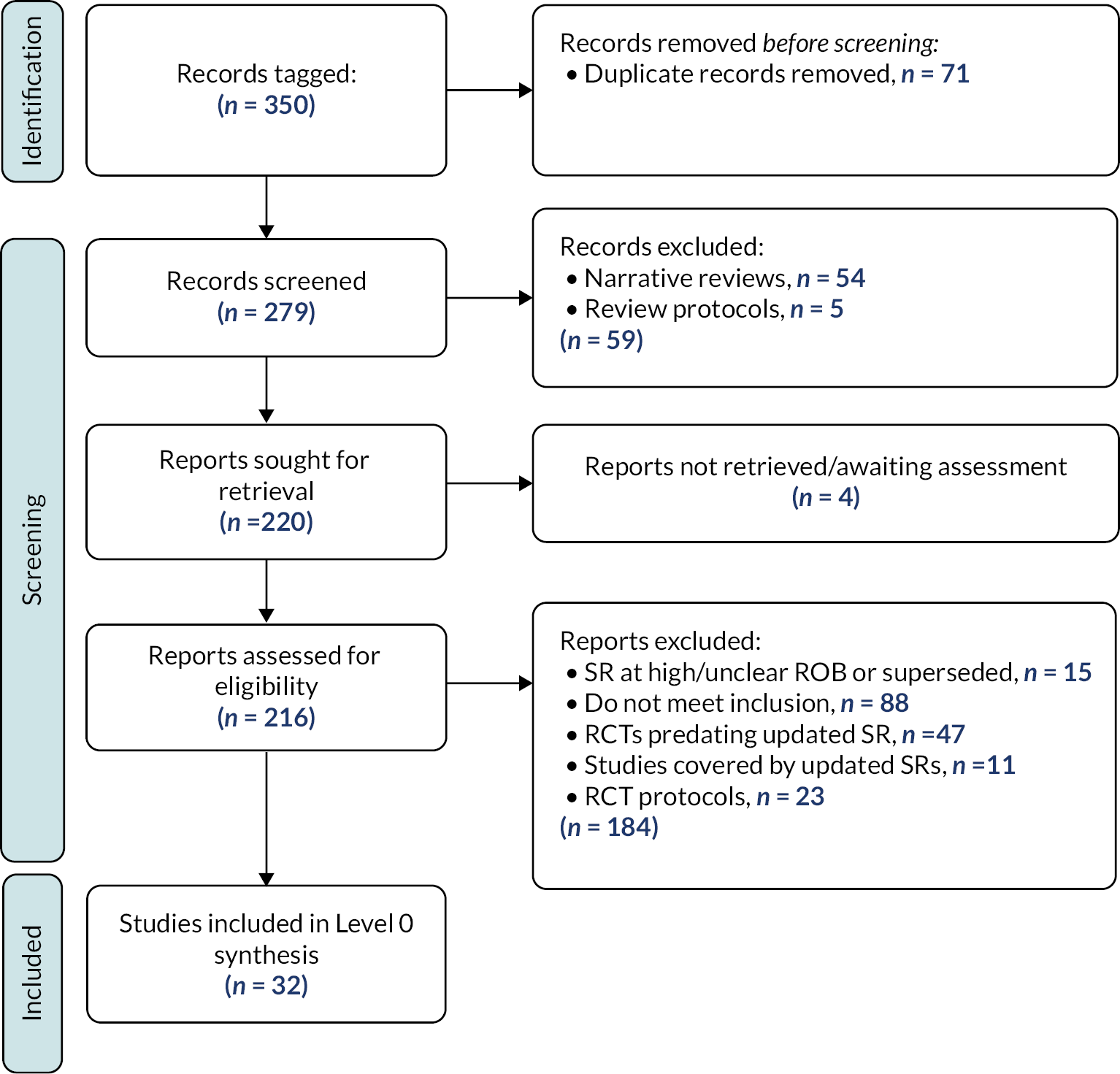
We included a total of 32 studies within this synthesis; 2 SRs which were updated, adding a further 8 RCTs; 15 RCTs and 7 other primary studies.
Characteristics of included studies
Characteristics of the included studies are summarised in Table 3 and Table 15 (see Appendix 4). The ROB is summarised in Report Supplementary Material 5. Details of interventions according to TIDieR51 template are provided in Report Supplementary Material 12.
| Study | Study design | No. of children with CFC recruited | Intervention | Overall ROB | Abstract only? |
|---|---|---|---|---|---|
| Probiotics (1 SR, 3 additional RCTs) | |||||
| Harris 201974 | Systematic review | 1408 (17 RCTs) | Probiotics vs. placebo or treatment as usual | Low | |
| Kubota 202075 | RCT | 60 | Lactobacillus rueteri DSM 17938 and lactose hydrate vs. L. rueteri DSM 17938 and MgO and lactose hydrate vs. placebo | L-L-L-H-U | |
| Sanctuary 201976 | Pilot crossover RCT | 11 (children with ASD) |
Bifidobacterium infantis | L-L-L-H-L | |
| Chao 201777 | RCT | 109 | Magnesium oxide and MIYAIRI-BM vs. magnesium oxide | U-U-U-U-U | |
| Fibre (1 SR, 5 additional RCTs) | |||||
| Piccoli de Mello 201878 | Systematic review | 680 (9 RCTs) | Fibre vs. placebo or laxative | Low | |
| Closa-Monasterolo 201779 | RCT | 17 | Inulin-type fructans derived from chicory vs. placebo | L-L-L-H-L | |
| Mahdavi 201780 | RCT | 79 | Synbiotic + PEG vs. PEG | L-U-H-H-H | |
| Aulia 201681 | RCT | 36 | Glucomannan vs. placebo | L-L-L-H-U | |
| Basturk 201782 | RCT | 146 | Synbiotics and prebiotics mixture vs. placebo | U-L-L-L-U | |
| Cassettari 201983 | RCT | 80 | Green banana biomass vs. different combinations of laxatives | H-H-H-H-L | |
| Different milk formula ( n = 6) | |||||
| Bongers 200784 | Randomised crossover trial | 38 | Infant formula (NF; Nutrilon Omneo) vs. whey-based control formula | U-L-L-H-L | |
| Chao 200785 | RCT | 93 | Magnesium-enriched infant formula. | L-U-H-H-L | |
| Infante 201186 | Cohort study | 30 | Novalac AE (IT) (United Pharmaceuticals SA, France) for 2 weeks | Moderate concerns | |
| Infante Pina 200887 | Cohort study | 3487 | Novalac formulas | Serious concerns | |
| Savino 200388 | Cohort study | 932 | Formula based on a partially hydrolysed bovine protein | Serious concerns | |
| Xinias 201889 | Non-randomised study | 65 | Formula with a partial whey hydrolysate, synbiotics | H-H-H-H-H | |
| Cow’s milk-free diet ( n = 4) | |||||
| Dehghani 201290 | RCT | 140 | 4 weeks cow’s milk-free diet | L-U-H-H-L | |
| Iacono 199891 | Crossover RCT | 65 | Cow’s milk vs. soy milk | L-L-U-H-L | |
| Iacono 199592 | Cohort study | 27 | Cow’s milk-free diet | Minor concerns | |
| Mohammadi Bourkheili 202193 | RCT | 70 | Cow’s milk-free and dairy-free diet | U-H-H-H-L | |
| Sugars (brown sugar, figs syrup, black sugar molasses) ( n = 3) | |||||
| Beleli 201594 | Non-randomised crossover study | 23 | Prebiotic 4ʹ-galactooligosaccharide vs. placebo | H-H-L-L-L | |
| Dehghani 201995 | RCT | 47 | Black sugar molasses syrup vs. PEG | L-U-U-H-L | |
| Tajik 201896 | RCT | 60 | Red (brown) sugar vs. Fijian figs | L-U-H-H-U | |
| Selenium supplements ( n = 1) | |||||
| Tanjung 201697 | RCT | 120 | Selenium vs. placebo | U-U-U-H-L | |
| Other/alternative dietary intake ( n = 3) | |||||
| Hassanein 202198 | RCT | 100 (cerebral palsy) | Oral magnesium sulphate vs. placebo | L-L-L-L-L | |
| Modaresi Saryazdi 201399 | RCT | 58 | PEG and senagol syrup, plus paraffin oil, daily (plus high-fibre diet) vs. PEG and senagol syrup daily (plus high-fibre diet) | U-U-U-U-U | √ |
| Stepurina 2018100 | RCT | 55 | Magnesium-containing mineral water vs. standard care | L-U-U-U-H | √ |
| Fluid intake ( n = 1) | |||||
| Young 1998101 | RCT | 90 | 50% increase in water intake vs. no change | U-U-U-H-U | |
| Educational interventions ( n = 3) | |||||
| Ritterband 2013102 | RCT | 91 | UCanPoopToo internet intervention vs. usual care | U-U-U-H-L | |
| Ritterband 2003103 | RCT (pilot) | 24 | UcanPoopToo internet intervention vs. usual care | U-H-H-H-U | |
| Tayag-Lacsina 2019104 | RCT | 90 | Information leaflet + usual care vs. usual care | U-U-U-H-H | √ |
| Combined dietary and behavioural interventions ( n = 1) | |||||
| Mazzoni 2017105 | Non-randomised study | 52 | Dietary and behavioural rules | H-H-U-U-U | √ |
The SRs, updated to include new trials, contained randomised studies with a total of 1408 (Harris, 2019)74 and 680 (Piccoli de Mello, 2018)78 children, respectively.
The 15 RCTs of interventions not relevant to the SRs included a total of 1141 children, while the 9 (out of 10) other primary studies which reported participant numbers included a total of 4701 children. Studies focused on infant formula included participants from 3 weeks to 6 months old,84–89 one study only included children under 3 years old,92 while other studies generally only included children over 2 years old. One included children with spastic cerebral palsy only. 98
Defaecation frequency and treatment success were meta-analysed by both Harris (2019)74 and Piccoli de Mello (2018). 78 In addition, Harris (2019)74 included adverse events as an outcome and Piccoli de Mello (2018)78 included stool consistency. Within the primary studies, the most frequently reported outcomes of interest were defaecation frequency and stool consistency, reported by 2084,85,87–104 and 1975,84–90,91,93,96,97,99,100–102,104,105 studies, respectively. Only one study assessed QoL89 and school attendance. 102 A summary of the outcomes reported and ROB judgements are provided in Report Supplementary Material 5.
Research questions addressed
The included studies were judged to address 10 distinct RQs relevant to Level 0. The following sections provide a summary of the evidence in relation to each question. The outcomes assessed, ROB and judgements of certainty and are summarised in Report Supplementary Material 5.
Evidence of effectiveness: lifestyle interventions delivered by family/carers (Level 0)
What is the effectiveness of probiotics?
Harris (2019)74 pooled evidence from 14 RCTs80,106–118 which compared probiotics with any control (of which 9 had an ‘active’ and 5 an ‘inactive’ control intervention); we added 2 RCTs to the ‘active’ control comparison75,76 (see Table 3), so there were data from a total of 16 RCTs (1021 participants). Chao (2017)77 did not provide any relevant data. For the outcome of defaecation frequency (in bowel movements/week), the addition of the new trials did not change the direction or significantly change the size of the effect: the weighted mean difference (WMD) between probiotics and active control was 0.14 (−0.14, 0.43) (see Figure 12). Results for the meta-analysis for a measure of ‘treatment success’ remained the same, as neither of the new trials included data relating to this outcome [relative risk (RR) between probiotics and active control: 1.16 (0.93, 1.44)]. The conclusion of Harris (2019)74 that the adverse events observed during treatment with probiotics were few and were balanced between groups, suggesting safety, is not affected by the new trials. In summary, evidence suggests that probiotics may not be more beneficial than control at improving outcomes in children with constipation, but there is no suggestion that probiotics are not safe. We have moderate certainty in this finding.
FIGURE 12.
Forest plot: probiotics vs. control. Defaecation frequency.

What is the effectiveness of additional dietary fibre?
Piccoli de Mello (2018)78 pooled evidence from 9 RCTs119–127 (680 participants) which compared dietary fibre with either a placebo (4 studies) or laxatives (5 studies); we added one pilot placebo-controlled RCT to this comparison79 (see Table 3), so there were data from a total of 10 RCTs (702 participants). The WMDs between fibre and control were 0.18 (−0.10, 0.46) for defaecation frequency (bowel movements per week) (see Figure 13) and 0.04 (−0.33, 0.41) for stool consistency (see Figure 14). The addition of the new trial did not change the direction or significantly change the size of the effect for either outcome. Four further new trials80–83 were relevant to this review but none reported sufficient data for inclusion in the updated meta-analysis. Thus, updating the review of fibre with new evidence from one new RCT did not change conclusions which can be drawn about fibre. In summary, there is very-low-quality evidence that additional dietary fibre is not more beneficial than control or laxatives in improving clinical outcomes.
FIGURE 13.
Forest plot: dietary fibre vs. control. Defaecation frequency.
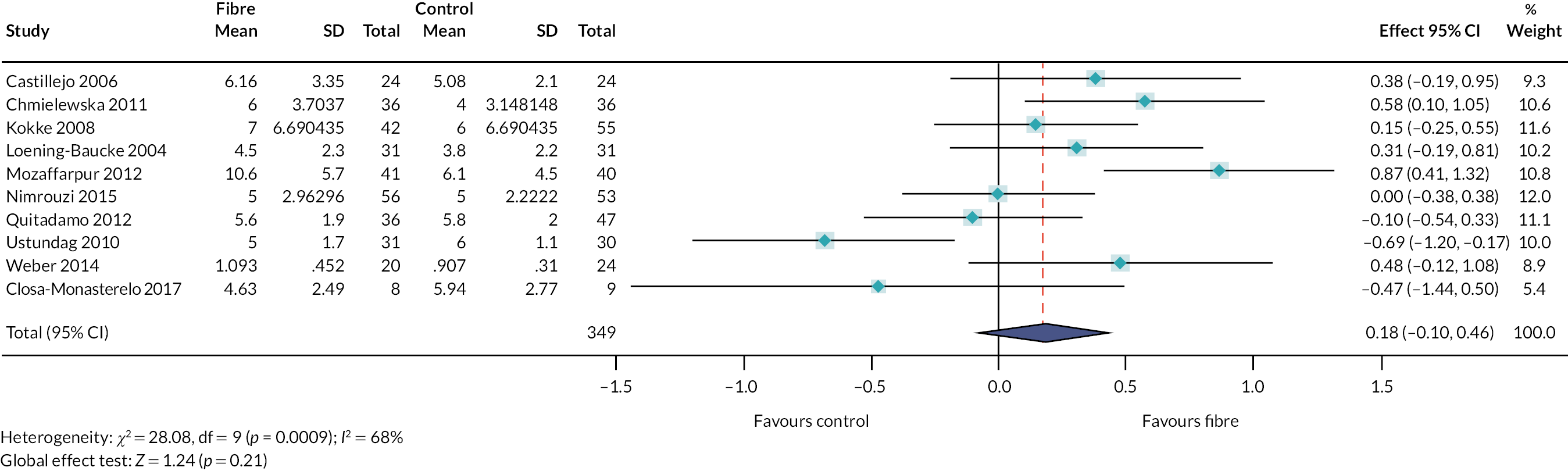
FIGURE 14.
Forest plot: dietary fibre vs. control. Stool consistency.
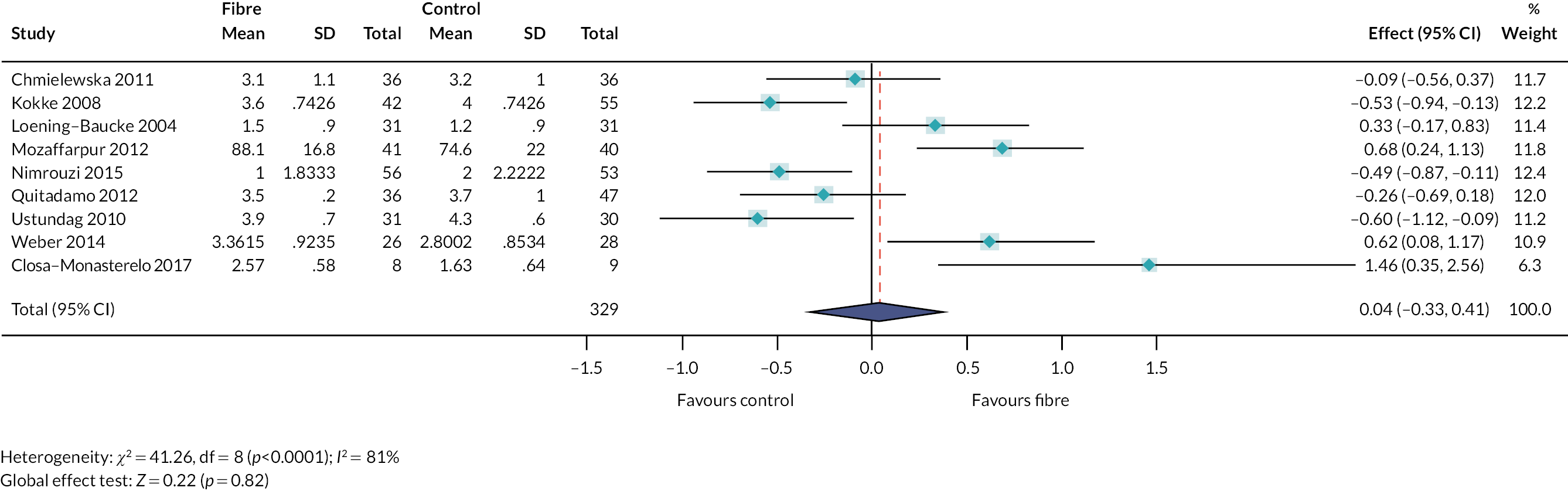
What are the effects of different milk formula in infants?
Six studies84–89 address this question (see Table 3). Two small RCTs, both with methodological concerns, each compared two different formula. 84,85 Four non-randomised studies also explored different milk formulas. 86–89 The heterogeneity between these studies – particularly in terms of the type of milk formula given, but also in terms of whether or not infants had diagnosed constipation, or the length of the reported symptoms – and methodological concerns mean that it is not possible to reach generalised conclusions. In summary, there is insufficient evidence to support any generalised conclusions about the relative effect of different milk formula.
What is the effect of a cow’s milk-free diet?
Four studies90–93 addressed this question (see Table 3). Three were RCTs, all of which recruited children who were referred to gastrointestinal clinics with chronic constipation, having had no benefits from laxatives (total n = 275). In two of these, a cow’s milk free-diet was compared with a diet including cow’s milk, with children in both groups receiving the same dose of PEG. 90,93 The other RCT compared children taking cow’s milk and soy milk, with neither groups receiving laxatives. 91 We also identified one small cohort study. 92 In all studies, there were improvements in symptoms of constipation in the children receiving a cow’s milk-free diet. Two crossover trials (reported in one paper),128 including 52 children, were excluded from our synthesis because the majority of included children had cow’s milk allergy. In summary, there is low certainty that cow’s milk-free diet may be beneficial to outcomes, in children for whom laxatives have been unsuccessful. It is important to note that evidence from studies which included participants who had a diagnosis of cow’s milk allergy were excluded from our review. It will be important to consider this wider body of evidence relating to cow’s milk allergy to inform decisions relating to exclusion of cow’s milk from diet.
What is the effectiveness of sugars (brown sugar, figs syrup, black sugar molasses)?
Three studies94–96 addressed this question (see Table 3). Tajik (2018) compared a daily dose of brown sugar with a daily dose of Fijian figs, in children aged 2–10 years with CFC. 96 Both interventions had a beneficial effect on defaecation frequency and pain, faecal incontinence and abdominal pain. Brown sugar had a greater improvement than Fijian figs on faecal incontinence and abdominal pain. Dehghani (2019) compared black sugar molasses with PEG syrup. 95 Similar outcomes were observed for both groups. Beleli (2015)94 reported that the prebiotic 4ʹ-galactooligosaccharide might be more beneficial than placebo. The heterogeneity between these studies – particularly in terms of the type of sugars and the adjunct treatments given – and methodological concerns mean that it was not possible to reach generalised conclusions. In summary, there is insufficient evidence to support any generalised conclusions about the effectiveness of sugars (including brown sugar, Fijian figs and black sugar molasses).
What are the effects of selenium supplements?
One RCT97 randomised children (from a boarding school) who had constipation to receive a daily selenium supplement or a placebo for 2 weeks. Twenty-three per cent of 530 school students were found to have constipation and were recruited to the study. There were statistically significant differences between groups for frequency of defaecation at days 14 and 21 (but not day 7), and for stool consistency and abdominal pain severity at days 7, 14 and 21. The proportion of children from the boarding school with constipation varies from reported prevalence in other populations, limiting certainty in the findings. In summary, there is very low certainty that selenium supplements may improve outcomes of defaecation frequency, stool consistency and abdominal pain.
What is the effectiveness of other/alternative dietary intake?
Three studies98–100 addressed this question (see Table 3). Hassanein (2021)98 randomised 100 children with cerebral palsy to receive either oral magnesium sulphate or a placebo for 1 month. The oral magnesium sulphate improved outcomes of defaecation frequency and stool consistency compared to placebo. Stepurina (2018) randomised 95 children to receive a ‘magnesium-containing water’ in addition to standard care, or standard care alone, over an 18-day treatment period. 100 Modaresi Saryazdi (2013) randomised 58 children to receive paraffin oil in addition to a combined treatment. 99 Limited information prevents generalised conclusions being drawn from either Stepurina (2018)100 or Modaresi Saryazdi (2013),102 but authors of both studies conclude that the interventions may be safe and beneficial. In summary, there is insufficient evidence relating to support generalised conclusions relating to other/alternative dietary intake.
What is the effect of fluid intake on constipation?
One RCT101 explored the effect of increasing water intake and found no effect. The authors conclude that ‘Advising parents of constipated children to increase liquid intake is not helpful and should not be recommended unless history suggests that the child’s liquid intake is inadequate for a normal child of that age and activity level’. In summary, there is insufficient evidence to support any routine change in fluid intake for children with constipation, unless ‘history suggests that the child’s liquid intake is inadequate for a normal child of that age and activity level’.
Evidence of effectiveness: educational interventions aimed at family/carers (Level 0)
What are the effects of educational interventions (delivered in addition to routine care)?
We identified three studies102–104 (see Table 3). Two RCTs102,103 investigated the effect on an internet-based intervention (www.ucanpooptoo.com/), which gave parents and children access to web-based educational resources and tools designed to support ‘enhanced toilet training’ in children with encopresis. One RCT104 investigated the effect of an information leaflet for parents of children with CFC. These three RCTs concluded that there was evidence of a beneficial effect on the number of bowel movements.
In summary, there is some limited evidence that educational interventions – particularly web-based interventions – used by parents/carers in addition to standard care may have a beneficial effect on clinical outcomes. Due to the small evidence base, limitations in the reporting of these studies and some methodological limitations, we have very low certainty in this finding.
Evidence of effectiveness: combined interventions delivered by family/carers (Level 0)
What is the effect of combined dietary and behavioural interventions?
One study105 addressed this question (see Table 3), providing 25 children with ‘dietary and behavioural rules’ and 27 children with PEG. 105 Parents were involved in deciding which treatment children should receive, and in the intervention delivery and outcome assessment. In summary, there is insufficient evidence to support any generalised conclusions relating to combined dietary and behavioural interventions.
Summary of evidence of effectiveness: interventions delivered carers, prior to health-care professional involvement (Level 0/everyday life interventions)
Two SRs, updated with a further 8 RCTs, and another 22 primary studies, of which 15 were RCTs, provide evidence for the effectiveness of interventions delivered by family/carers (Level 0). There is evidence that the addition of probiotics (moderate quality) and dietary fibre (very low quality) does not lead to any added benefit. There is low-to-very-low certainty that educational interventions, cow’s milk-free diet, selenium supplements may be beneficial to outcomes. There is insufficient evidence to support conclusions about fluid intake, different milk formula, sugars, other dietary supplements and combined dietary and behavioural interventions.
Stakeholder reflections on evidence of effectiveness: interventions delivered carers, prior to healthcare professional involvement (Level 0/everyday life interventions)
Members of the SG have provided the following reflections, in their own words, on the evidence identified in relation to Level 0:
-
I think that we have found that there are significant gaps in the evidence of the effectiveness of many of these interventions and this really cries out for the need for urgent research to be done to produce robust evidence of whether these interventions are effective or not. I say it should be urgent because of the impact on children and their loved ones and on children’s lives going into adulthood as I have experience of not having effective interventions.
-
I was disappointed that we didn’t find stronger evidence regarding some of the interventions. But it’ll be good if we can come up with significant research recommendations. I was always concerned about children and young people suffering from diabetes – some interventions would not be appropriate for them. I am aware of that because as well as developing IBS as an adult, I’ve also been diagnosed with type 2 diabetes.
-
It’s useful to have set out the range of issues that have been considered. Evaluation and synthesis of the findings is useful. Equally useful is to see the gaps which may in turn inform research prioritisation as well as clinical decision making.
-
The absence of evidence around combined dietary and behavioural interventions is really important.
-
It is always useful to reflect that the gaps are as important as the data.
What is the effectiveness of assessment and intervention by primary care services (Wider Children’s workforce/Level 1 interventions)?
Results of the search
Figure 15 illustrates the results of the search. Details of ongoing, excluded and awaiting assessment studies are provided in Report Supplementary Material 6.
FIGURE 15.
Results of the search – Level 1 studies.
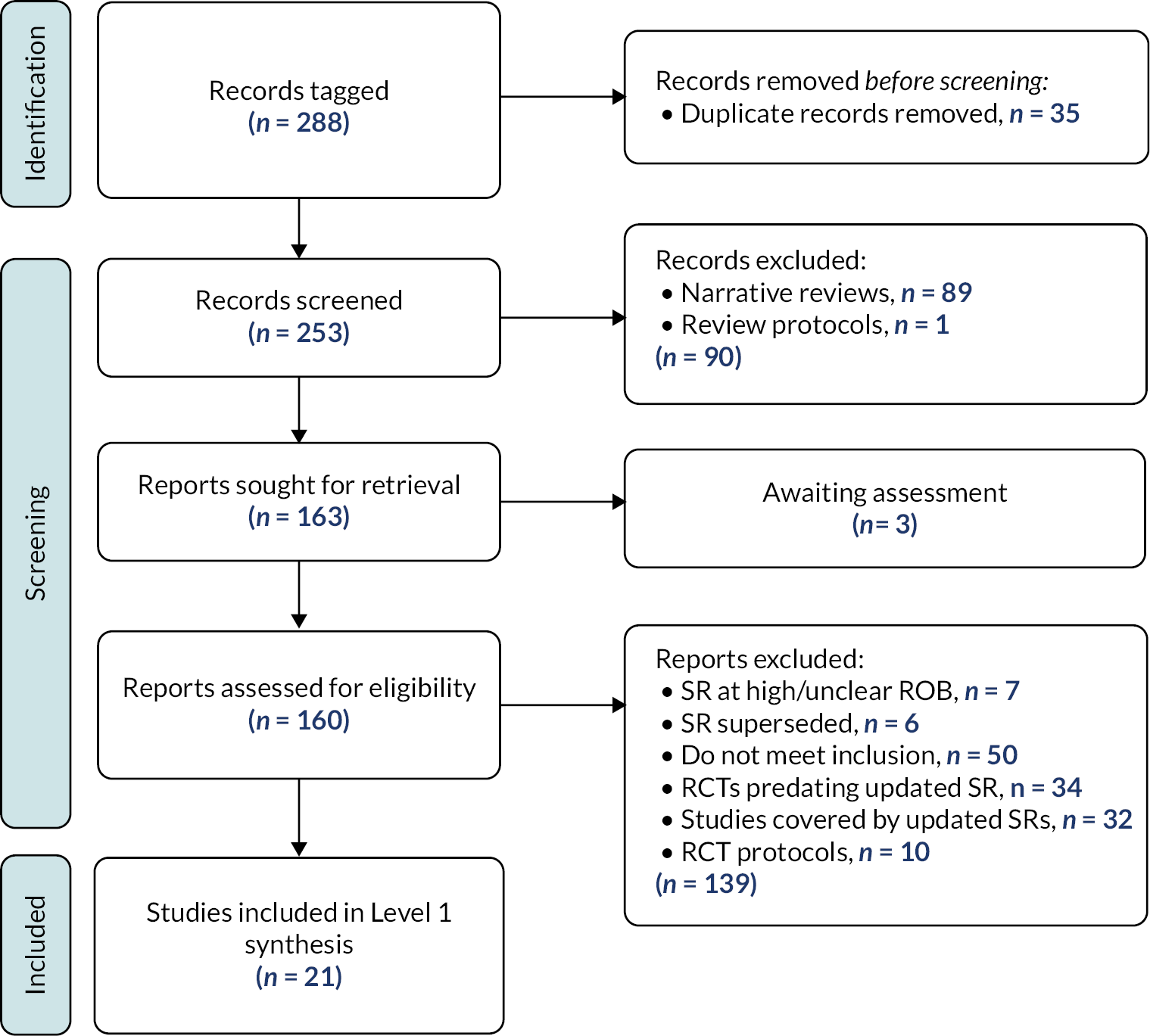
We included a total of 21 studies within this synthesis; 2 SRs; 13 RCTs and 6 other primary studies.
Characteristics of included studies
Characteristics of the included studies are summarised in Tables 4 and 16 (see Appendix 4). The ROB is summarised in Report Supplementary Material 6. Details of interventions according to TIDieR51 template are provided in Report Supplementary Material 12.
| Study | Study design | No. of children with CFC recruited | Intervention | Overall ROB | Abstract only? |
|---|---|---|---|---|---|
| Laxatives (2 SRs, 12 RCTs) | |||||
| Bekkali 2018129 | RCT | 97 | PEG3350 with electrolytes vs. PEG4000 | L-L-L-L-L | |
| Benninga 2022130 NCT02042183 | RCT | 606 | Lubiprostone vs. placebo | L-L-L-L-L | |
| Benninga 2022130 NCT02138136 | RCT | 419 | 12 vs. 24 μg BID, lubiprostone | L-L-L-L-L | |
| Cao 2018131 | RCT | 100 | Lactulose vs. placebo | L-L-L-L-L | |
| Esmaeilidooki 2016132 | RCT | 109 | PEG4000 vs. Cassia fistula emulsion | L-U-H-H-U | |
| Gordon 2016133 | Cochrane review | 2310 (25 RCTs) | Osmotic or stimulant laxative vs. placebo or another intervention | L-L-L-L-L | |
| Hashemi 2015110 | RCT | 120 | PEG vs. probiotic vs. PEG + probiotic | U-U-L-L-U | √ |
| Imanieh 2019134 | RCT | 52 | PEG vs. PEG + Motilium | U-U-U-H-U | |
| Jarzebicka 2019135 | RCT | 102 | PEG3350 vs. lactulose | L-L-H-H-U | |
| Modin 2018136 | RCT | 102 | PEG3350 vs. placebo | L-L-L-L-L | |
| Pranoto 2016137 | RCT | 99 | Oral vs. rectal bisacodyl | L-U-H-U-H | |
| Rachel 2020138 | Systematic review | 468 (5 studies) | PEG3350, with or without electrolytes, vs. PEG4000 | U-L-U-L-L | |
| Shatnawi 2019139 | RCT | 65 | Lactulose vs. PEG4000 | U-U-U-U-H | |
| Torabi 2017140 | RCT | 160 | PEG vs. paraffin | U-U-L-L-U | |
| Physical exercise (focused on pelvic floor muscles) ( n = 1) | |||||
| Farahmand 2015141 | Prospective cohort | 44 | Walking in squatting exercise | Minor concerns | |
| Combined programmes ( n = 6) | |||||
| Axelrod 2016142 | Repeated measures | 2 (with ASD) | Diet, laxatives, toilet training | Serious concerns | |
| Hankinson 2018143 | Prospective cohort | 162 | Diet, laxatives, psychosocial | Moderate concerns | |
| Jordan-Ely 2013144 | Retrospective cohort | 33 | Diet, education, laxatives | Serious concerns | √ |
| Lomas Mevers 2020145 | RCT | 20 (with ASD) | Multidisciplinary intervention | L-L-H-H-U | |
| Soares 2009146 | Cohort | 34 | Diet, laxatives, psychosocial | Moderate concerns | |
| Speridiao 2003147 | Prospective cohort | 25 | Diet, laxatives | Moderate concerns | |
One SR133 originally contained 25 randomised studies121,124–126,133,148–168 with a total of 2310 children, while another138 contained 5 studies (2 randomised) with a total of 468 children.
The 13 RCTs included 1632, and the 6 other primary studies included 300, children with CFC. One study focused specifically on children with cerebral palsy,134 and two on children with other additional needs. 142,145
The SRs explored outcomes including defaecation frequency, faecal incontinence, disimpaction, need for additional therapies and adverse events. 133,138 Within the primary studies, the most frequently reported outcomes of interest were defaecation frequency and faecal incontinence, reported by 14129–132,134–137,140–142,144,146,147 and 13129,130,134,142,145,132,135,136,139–141,144,146,147 studies, respectively. No studies reported QoL or school attendance. A summary of the outcomes reported, and ROB judgements are provided in Report Supplementary Material 6.
Research questions addressed
The included studies were judged to address four distinct RQs (including several comparisons) relevant to Level 1. The following sections provide a summary of the evidence in relation to each question. The outcomes assessed, ROB and judgements of certainty are summarised in Report Supplementary Material 6.
Evidence of effectiveness: pharmacological interventions delivered by wider children’s workforce (Level 1)
What are the effects of laxatives?
Gordon (2016)133 pooled evidence from 25 RCTs121,124–126,148–168; we identified 10 more recent relevant RCTs110,129–132,135–137,140,139 (see Table 4). Four135,136,139,140 investigated comparisons which were explored by Gordon (2016):133
-
PEG versus placebo. Gordon (2016)133 included two studies (101 children). We added one more RCT,136 adding 102 participants. For defaecation frequency, Gordon (2016)133 reported a WMD of 2.61 (CI 1.15 to 4.08) (low certainty). Adding data from Modin (2018)136 changed this to 1.94 (CI 0.44 to 3.44) (see Figure 16). This addition did not change the direction or significantly change the size of the effect.
-
PEG versus lactulose. Gordon (2016)133 included six studies (465 children). We identified two more RCTs,135,139 with a further 148 participants, and were able to add data from 1135 (83 children) to the meta-analysis for defaecation frequency, adding 83 participants. Gordon (2016)133 reported a WMD of 0.7 (CI 0.1 to 1.31) (low certainty). Adding data from Jarzebicka (2019)135 changed this to 1.08 (CI 0.12 to 2.05) (see Figure 17). This addition did not change the direction or significantly change the size of the effect.
-
PEG versus liquid paraffin. Gordon (2016)133 included three studies (299 studies), and pooled data from two, finding no difference in defaecation frequency between groups (low certainty). We identified one further RCT140 but there were no suitable data to combine.
FIGURE 16.
Forest plot: PEG vs. control. Defaecation frequency.
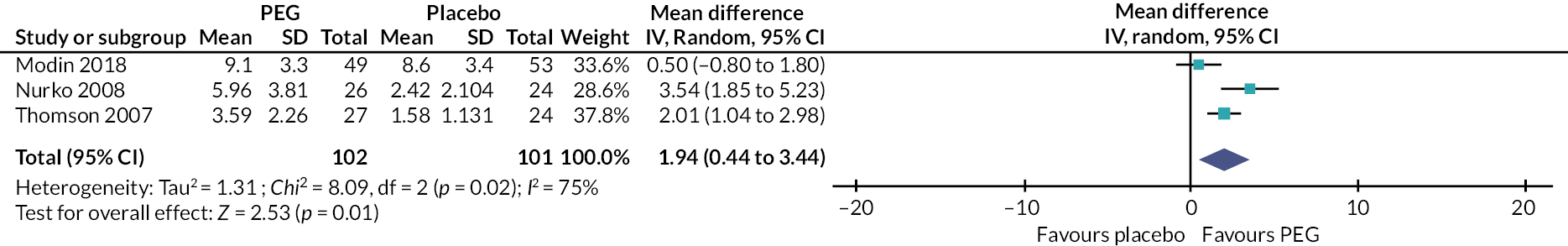
FIGURE 17.
Forest plot. PEG vs. lactulose. Defaecation frequency.
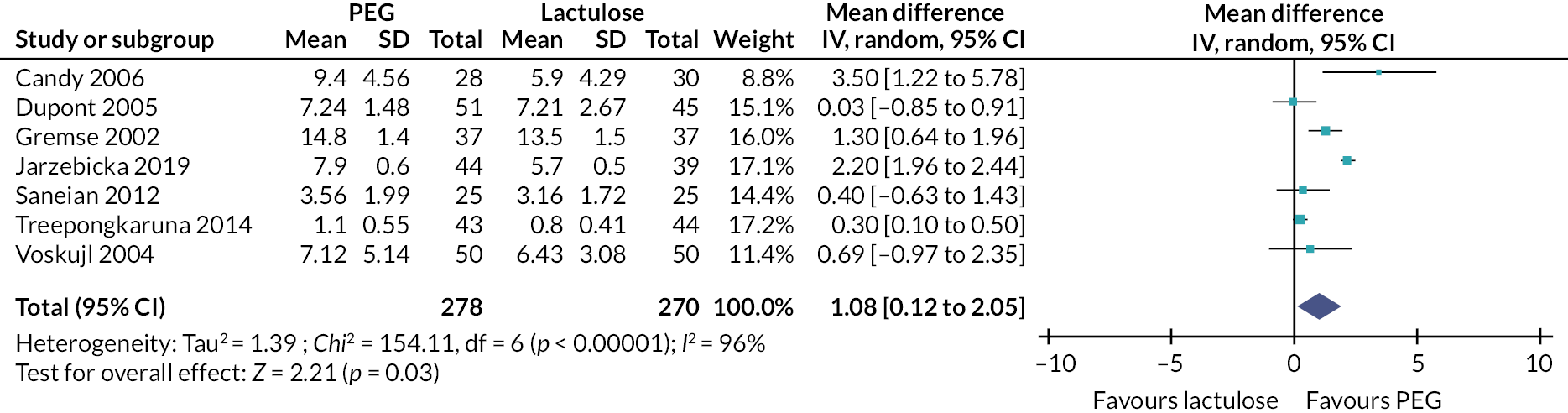
Six of the 10 newly identified studies110,129–132,137 investigated comparisons which were not explored within the trials included in the SR. These were:
-
Polyethylene glycol 3350 (PEG3350) plus electrolytes versus polyethylene glycol 4000 (PEG4000). Bekkali (2018)129 randomised 97 children to receive PEG3350 plus electrolytes or PEG4000 and assessed outcomes after a year. The results demonstrated no difference between PEG3350 plus electrolytes and PEG4000 for a parent/child reported symptom score or adverse events.
-
Lactulose versus placebo. Cao (2018)131 randomised 100 children to receive lactulose or placebo for 6 weeks. Lactulose was more beneficial than placebo for defaecation frequency and stool consistency, and there were no differences in adverse events.
-
Oral bisacodyl versus rectal bisacodyl. Pranoto (2016)137 randomised 99 children to receive an oral or rectal stimulant laxative (5 mg bisacodyl). No differences were found between groups.
-
PEG versus cassia fistula. Esmaelidooki (2016)132 randomised 109 children to receive either PEG4000 or cassia fistula. This trial is included within the synthesis reported in Section Evidence of effectiveness: complementary/alternative medicines.
-
PEG versus probiotics. Hashemi (2015)110 randomised 120 children to receive PEG, probiotics or PEG plus probiotics. No data are reported but the authors conclude that PEG plus probiotics is superior to probiotics alone.
-
Lubiprostone versus placebo, and comparison of doses. Benninga (2022)132 conducted two studies, involving the same children. One study randomised 606 children to lubiprostone or placebo. No differences were found between groups for defaecation frequency (p = 0.22). The second study compared a 36-week treatment of two different doses of lubiprostone.
Gordon (2016)133 identified a number of comparisons which we were not found in other studies. This includes a comparison of PEG with milk of magnesia, including four studies (261 children) and concluding that PEG was superior to milk of magnesia for defaecation frequency (low certainty). Gordon (2016)133 also identified single trials which reported no difference on key outcomes between PEG and enema, PEG and flixweed, PEG and dietary fibre mix, lactulose and lactitol, lactulose and partially hydrolysed guar gum, dietary fibre mix and lactulose, and senna and lactulose; two studies which found liquid paraffin to be more effective than lactulose, and one which found a difference between high- and low-dose PEG. Quality of this evidence was considered low to very low.
We identified a SR138 focused specifically on children aged less than 24 months. Data from two RCTs and three retrospective studies were included,150,129,169–171 and the authors concluded that there is insufficient evidence to determine the optimal dosage of PEG.
In summary, new trials do not change key conclusions from previous reviews, and quality of evidence remains low.
Note: Studies primarily include children without additional needs. There may be high risks of aspiration when using PEG or liquid paraffin in children with additional needs. Guidelines for children with neurological impairment specifically state that laxative prescription for this population should be ‘as in typically developing children, unless there is a risk of aspiration of polyethylene glycol or liquid paraffin’. 172 A review of side effects in children using senna concluded that senna was safe, although a rare side effect of skin blistering may occur where there is skin exposure. 173
Head-to-head comparisons of laxatives
Table 5 illustrates where there are trials with head-to-head comparisons of different laxatives and provides an indication of the relative effectiveness (across multiple outcomes). There have been direct comparisons of PEG with lactulose, milk of magnesia, liquid paraffin and placebo; lactulose with milk of magnesia, senna, liquid paraffin and placebo; and lubiprostone with placebo. PEG appears superior to lactulose and milk of magnesia, milk of magnesia superior to lactulose, and lactulose equivalent to senna. Quality of evidence is low to very low for all findings. Empty cells indicate that no RCT evidence has been identified.
| Placebo | PEG | Lactulose | Milk of magnesia | Bisacodyl | Senna | Lubiprostone | Liquid paraffin | |
|---|---|---|---|---|---|---|---|---|
| Placebo | + | + | 0 | |||||
| PEG | − | 0a | − | − | 0 | |||
| Lactulose | − | + | + | 0 | + | |||
| Milk of magnesia | + | − | ||||||
| Bisacodyl | 0b | |||||||
| Senna | 0 | |||||||
| Lubiprostone | 0 | |||||||
| Liquid paraffin | 0 | − |
What are the effects of laxatives plus domperidone?
We found one RCT which investigated the effect of domperidone in 52 children with cerebral palsy134 (see Table 4). After 2 weeks, slightly more number of children receiving both PEG and domperidone had a satisfactory response than children receiving PEG only. Importantly, in 2014 the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK advised that, due to a small increased risk of serious side effects, domperidone should only be used for specific conditions, and should not be used in children under 16 years of age.
Evidence of effectiveness: lifestyle interventions delivered by wider children’s workforce (Level 1)
What is the effect of physical exercise (focused on pelvic floor muscles)?
A cohort study investigated the effect of an exercise (walking in squatting) designed to exercise the pelvic muscles, twice a day for 8 weeks in 44 children with CFC, for whom other treatments – including laxatives and toilet training – had failed141 (see Table 4). Statistically significant changes were found for measures of overall improvement in symptoms, defaecation frequency and stool consistency. In summary, there is low certainty that physical exercise (focused on pelvic floor muscles) may improve outcomes.
Evidence of effectiveness: combined pharmacological, lifestyle, information and lifestyle interventions delivered by wider children’s workforce (Level 1)
What is the effect of a combined pharmacological, diet and behavioural programme?
Four studies explored combined treatment approaches, including education – including dietary and behavioural – and pharmacological interventions, including children with and without additional needs144,146,147 (see Table 4). These studies provide some very limited data which suggest that a combined pharmacological, dietary and behavioural programme may have some benefits for children with CFC. One small RCT145 randomised 20 children with ASD and encopresis to 10 appointments at a nurse-led behavioural programme compared to a waiting-list control. The study reports a large increase in the number of faecally continent children after treatment for children who received the intervention (treatment group 6/10 continent post-intervention and 5/10 at follow-up; control group 0/10 and 1/10 respectively). Findings from Axelrod (2016)142 are in agreement with this RCT. In summary, there are some very limited data which suggest that a combined pharmacological, dietary and behavioural programme may have some benefits. We have very low certainty in this finding due to the quantity and quality of available studies.
Summary of evidence of effectiveness: interventions delivered by wider children’s workforce (Level 1/primary care services)
Two SRs of laxatives, 13 RCTs and 6 other primary studies are evidence for the effectiveness of interventions delivered by the wider children’s workforce (Level 1). There is low certainty that PEG is more effective than placebo, lactulose and milk of magnesia, but may not be more effective than liquid paraffin, and that PEG3350 plus electrolytes and PEG4000 may be equally effective. There is low to very low certainty that lactulose is more effective than placebo, but that it may be less effective than liquid paraffin. Oral and rectal stimulant laxatives may be equally effective. Lubiprostone may not be more effective than placebo. High-quality head-to-head comparisons of different laxatives, and combinations of laxatives, selected according to biological plausibility are required. There is limited evidence about the effect of physical exercise and combined programmes.
Stakeholder reflections on evidence of effectiveness: interventions delivered by wider children’s workforce (Level 1/primary care services)
Members of the SG have provided the following reflections, in their own words, on the evidence identified in relation to Level 1:
-
The combinations of some of the interventions struck me as being a more holistic approach for treating children with constipation. But to date, evidence does not exist. I’d be delighted to see more research undertaken in this area.
-
I think it is significant that we have found low certainty that physical exercise can help. I, also, find it significant that we have found there is low certainty that a combined pharmacological and diet and behavioural programme could help and it calls into question for me some of the interventions some clinicians recommend and I think further work is called for to identify other interventions.
-
Adult services seem to use pelvic floor muscle training (PFMT) quite a bit. Absence of evidence is striking. Worth flagging this if only to make sure our patients aren’t missing out on something useful because we don’t study it, or that adults aren’t getting something hopeless.
-
In relation to combined pharmacological, diet and behavioural programmes – my understanding is that there isn’t much data so there is low confidence in the data itself, while not making a specific comment on the thing being done but merely the extent to which it is documented (or not as the case may be). I suspect for many autistic people, a combined behavioural and pharmacological and dietary approach would be a really good first step – but studies aren’t done in sufficient numbers to support or not support this. It does raise the question as to why more studies aren’t done …
-
Most striking is the paucity of actionable information for such an important topic … understanding the reasons for this are key. Limited understanding of pathophysiology may impair ability to stratify patients for studies. With better understanding, inclusion criteria and metrics would become clearer and more meaningful, and in turn therapies more personalised. Better outcomes, including clinical outcomes, process, experiential and resource use, would be likely to follow.
What is the effectiveness of interventions delivered by secondary specialist care (continence teams/Level 2 interventions)?
Results of the search
Figure 18 illustrates the results of the search. Details of ongoing, excluded and awaiting assessment studies are provided in Report Supplementary Material 7. We included a total of 31 studies within this synthesis; 1 SR174 which was updated to include one further RCT175; 14 RCTs148,175,192–195,197–205 and 15 other primary studies. 176–189
FIGURE 18.
Results of the search – Level 2 studies.

One RCT190 listed in our table of ongoing studies (Report Supplementary Material 7) was published following completion of our evidence synthesis. We have referred to this within the relevant point in our narrative synthesis, but it has not undergone full data extraction and assessment and is not integrated into the rest of this section.
Characteristics of included studies
Characteristics of the included studies are summarised in Tables 6 and 17 (see Appendix 4). The ROB is summarised in Report Supplementary Material 7. Details of interventions according to TIDieR51 template are provided in Report Supplementary Material 12.
| Study | Study design | No. of children with CFC recruited | Intervention | Overall ROB | Abstract only |
|---|---|---|---|---|---|
| Pharmacological interventions ( n = 6) | |||||
| Bekkali 2009148 | RCT | 90 | Enema vs. PEG | L-U-U-H-L | |
| Bongers 2009191 | RCT | 102 | Enema + PEG vs. PEG | L-U-U-H-L | |
| Garcia 2016192 | RCT | 58 | Oral + enema therapy vs. enema | U-U-U-L-H | √ |
| Ormarsson 2016193 | RCT | 80 | Enema vs. suppository | L-L-L-L-U | |
| Strisciuglio 2021194 EUCTR2015-005111-32-IT |
RCT | 158 | Microenema vs. PEG | L-L-H-H-L | |
| Yoo 2017176 | Retrospective cohort | 28 | Enema | Moderate concerns | |
| TES (1 SR, 1 RCT) | |||||
| Ng 2016174 | Cochrane review | 46 | TES | Low | |
| Sharifi-Rad 2018175 IRCT2016030617876N |
RCT | 90 | TES | L-L-H-L-L | |
| TAI (n = 5) | |||||
| Jorgensen 2017177 | Retrospective cohort | 72 | Alterna TAI | Moderate concerns | |
| Koppen 2017178 | Survey study | 67 | Peristeen TAI | Serious concerns | |
| Nasher 2014179 | Retrospective cohort | 7 | Peristeen TAI | Serious concerns | |
| Patel 2019180 | Cohort | 19 | Peristeen TAI | Serious concerns | √ |
| Sharma 2016181 | Cohort | 11 | Peristeen TAI | Serious concerns | √ |
| Biofeedback ( n = 7) | |||||
| Jarzebicka 2016182 | Cohort | 44 | Anorectal manometer to provide biofeedback | Serious concerns | |
| Loening-Baucke 1990194 | RCT | 43 | External anal sphincter EMG biofeedback | H-U-U-H-U | |
| Nader 2016195 | Retrospective cohort | 25 | External anal sphincter EMG biofeedback | Serious concerns | √ |
| Nolan 1998196 | RCT | 29 | External anal sphincter EMG biofeedback | L-L-U-L-L | |
| Raffaele 2015183 | Cohort | 25 | External anal sphincter EMG biofeedback | Serious concerns | √ |
| Van der Plas 1998197 | RCT | 192 | External anal sphincter EMG biofeedback | U-U-H-H-U | |
| Wald 1987198 | RCT | 50 | Anorectal manometer to provide biofeedback | U-U-H-L-H | |
| Physiotherapy ( n = 6) | |||||
| Silva 2013199 | RCT | 72 | Physiotherapy (including muscular training, abdominal massage and diaphragmatic breathing) | L-L-U-H-L | |
| Van Engelenburg 2017200 | RCT | 53 | Physiotherapy (including core stability and balance training, relaxation and breathing exercises, sensory processing techniques, PFMT and education) | L-L-U-L-L | |
| Van Summeren 2020201 | RCT | 134 | Physiotherapy (including knowledge, toileting behaviour and posture, awareness of sensation of needing to defaecate, relaxation whilst defaecating, pressure and straining during defaecation) | L-L-H-H-L | |
| Awan 2016184 | Prospective cohort | 40 | Physical therapy for cerebral palsy | Minor concerns | |
| Awan 2021202 NCT03379038 |
Randomised crossover trial | 35 | Physical therapy for cerebral palsy | U-U-H-H-U | |
| Eisenberg 2009185 | Non-randomised study | 22 | Standing frame vs. walker for cerebral palsy | Minor concerns | |
| Dietary exclusion ( n = 1) | |||||
| Waingankar 2018186 | Retrospective cohort | 29 | Sugar restriction (health professional supervised) | Serious concerns | |
| Combined interventions ( n = 4) | |||||
| Borowitz 2002203 | RCT | 87 | Intensive medical therapy; intensive medical therapy plus enhanced toilet training; these combined with EMG biofeedback. | L-U-H-H-L | |
| Loening-Bauke (i) 1989187 | Non-comparative study | 97 | Laxatives, diet, toileting programme, rewards. | Moderate concerns | |
| Loening-Baucke 1993188 | Cohort | 174 | Education, disimpaction and toilet training | Serious concerns | |
| Modin 2016189 | Prospective cohort | 132 | Information and disimpaction; focus on behaviour | Serious concerns | √ |
There were a total of 2078 children (range 7–192) with CFC. Three studies184,185,201 only included children with cerebral palsy. The most common pre-specified outcomes reported were faecal incontinence (n = 17) and frequency of defaecation (n = 16). One study assessed school attendance. The ROB judgements and outcomes assessed are summarised in Report Supplementary Material 7.
Research questions addressed
The included studies were judged to address 10 distinct RQs relevant to Level 2. The following sections provide a summary of the evidence in relation to each question. Studies are summarised in Table 6, with more details provided in Table 15. The outcomes assessed, ROB and judgements of certainty and are summarised in Report Supplementary Material 7.
Evidence of effectiveness: pharmacological interventions delivered by continence teams (Level 2)
Six studies148,176,191–193 explored pharmacological interventions delivered at Level 2 (see Table 6).
What is the effect of rectal enemas in children with severe constipation?
Bongers (2009)191 randomised 102 children to receive an addition of 3 rectal enemas a week for 3 months or PEG only. Defaecation frequency improved in both groups at 26- and 52-week follow-up; however, the enema group had significantly higher frequency of defaecation. Abdominal pain, painful defaecation and faecal incontinence improved in both groups, but there were no significant differences between groups. The majority (76%) of the children in the enema group reported to never/seldom feeling worse after the application of the enema, while 11% reported sometimes and 13% reported always. A rectal enema was perceived as very to extremely terrible in 15% of children. Bekkali (2009)148 randomised 90 children to 6 consecutive days of rectal enemas or oral PEG3350 with electrolytes. There were no statistically significant differences in outcomes at follow-up for measures of successful disimpaction, defaecation frequency, stool consistency, faecal incontinence and abdominal pain. Successful disimpaction was achieved in 37 (80%) children receiving enemas and 30 (68%) receiving oral laxatives (p = 0.28). Garcia (2016)204 randomised 58 hospitalised children, to a combined oral and rectal enema 1-day regimen (which included Fleet enema, bisacodyl suppository, and castor oil) or a 3-day Fleet enema regimen. Disimpaction was successful in both groups, but the combined group reported significantly fewer complaints of abdominal pain after treatment compared to the Fleet only group (p = 0.019). Yoo (2017)176 evaluated the efficacy and safety of combined oral (PEG3350 electrolyte solution) and enema solution in children with CFC who were hospitalised after failed attempts at disimpaction as an outpatient (n = 28). Defaecation frequency was found to significantly increase after disimpaction. Abdominal pain was also reduced from 15 children before therapy to 4 children post therapy.
In summary, the addition of regular rectal enemas may increase defaecation frequency in children with severe constipation, but not have any effect on overall treatment success or other outcomes, and may cause discomfort or distress to some. Enemas and high-dose laxatives may be equally effective at reducing rectal faecal impaction. Combined oral and enema therapy may be beneficial for hospitalised children with faecal impaction. We have very low certainty in these findings. There is insufficient evidence to reach any conclusions about the relative effect of different types of enemas or the effectiveness of specific regimens.
Note: A narrative review205 reports that data provided by the Medicines Control Agency (MCA) showed 24 potential serious reactions to phosphate enemas. Further, in 2014 the US Food and Drug Administration (FDA)206 issued a warning that sodium phosphate, delivered orally or rectally in an enema, may cause rare complications and even death. Given that there is currently insufficient research evidence about the relative effect of different types of enemas, these reports support the avoidance of phosphate enemas.
What is the difference in effectiveness of microenemas and oral laxatives for functional constipation in infants?
Strisciuglio (2021)193 randomised 158 infants, aged 6–48 months, with functional constipation, to receive 2 weeks of oral PEG4000 or of Promelaxin (a microenema containing honeys and polysaccharides). For stool frequency, the group receiving Promelaxin had a better outcome [16.5% (CI 1.55% to 31.49%)]. There were no statistically significant differences between groups for other outcomes and no adverse events identified in either group. In summary, there is evidence that Promelaxin microenemas and oral laxatives are equally effective in the treatment of functional constipation in infants (6–48 months). Due to the quantity and quality of the evidence, we have low certainty in this finding.
What is the difference in effectiveness of an enema and a soft suppository for disimpaction?
Ormarsson (2016)192 randomised 54 children receive either low-dose LP101 free fatty acids suppository (n = 23) or Klyx enema (n = 33). In a second phase, a further 21 children received a higher-dose suppository (and were compared to the earlier randomised groups). The higher-dose suppository was equally effective as the Klyx enema for bowel emptying and symptom relief. In summary, there is evidence that enemas and high-dose suppositories may be equally effective at promoting bowel emptying. Due to quantity and quality of evidence, we have low certainty in this finding.
Evidence of effectiveness: other interventions delivered by continence teams (Level 2)
What is the effect of transcutaneous electrical stimulation?
A Cochrane review (Ng, 2016)174 evaluated the effectiveness and safety of transcutaneous electric stimulation. This review included one study (Chase, 2015)207 which randomised 46 children to receive either TES or sham TES. One more recent relevant RCT175 randomised 90 children to receive either interferential electrical stimulation and pelvic floor muscle exercises or sham stimulation and pelvic floor muscle exercises. The number with diagnosed CFC reduced more in TES group (38/45) than the sham group (19/44) after treatment (p < 0.0003). We updated analyses for QoL and improvement in faecal soiling (see Report Supplementary Material 7 for exploration of outcomes available for pooling). For QoL, using the optimal method for estimating SDs from medians,208 the pooled effect size (SMD) is 0.49 (95% CI 0.13 to 0.85) (see Figure 19), demonstrating a statistically significant result in favour of TES. This changes the results presented by Ng (2016) which found no significant difference between TES and sham. For improvement in faecal soiling, the risk ratio is 2.78 (95% CI 1.63 to 4.74) (see Figure 20); this is statistically significant and increases the effect size reported by Ng (2016). In summary, there is very low certainty that TES may reduce the number of soiling episodes and improve self-reported QoL, as compared to sham TES. [Note: evidence relating to SNS is included within Level 3 synthesis – see Evidence of effectiveness: interventions delivered by consultant-led teams (Level 3/highly specialist tertiary care services)].
FIGURE 19.
Forest plot. Transcutaneous electric stimulation vs. placebo. QoL.
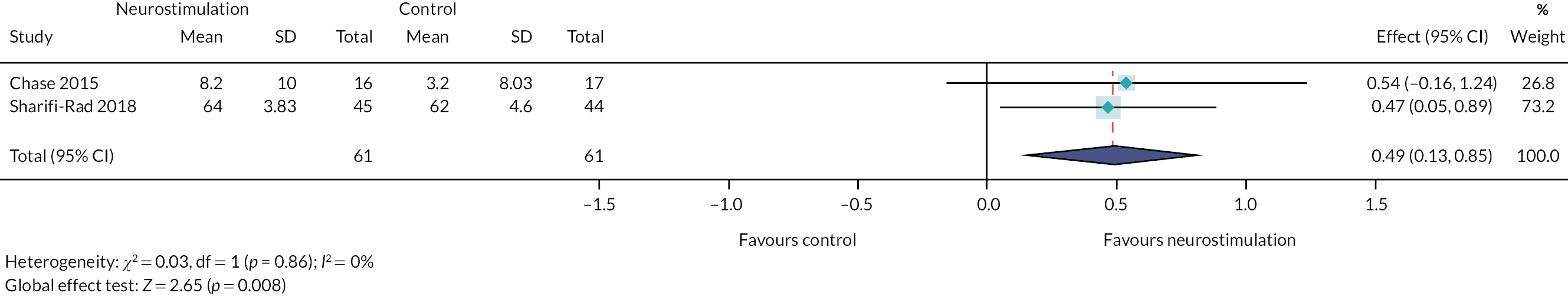
FIGURE 20.
Forest plot. Transcutaneous electric stimulation vs. placebo. Faecal incontinence.
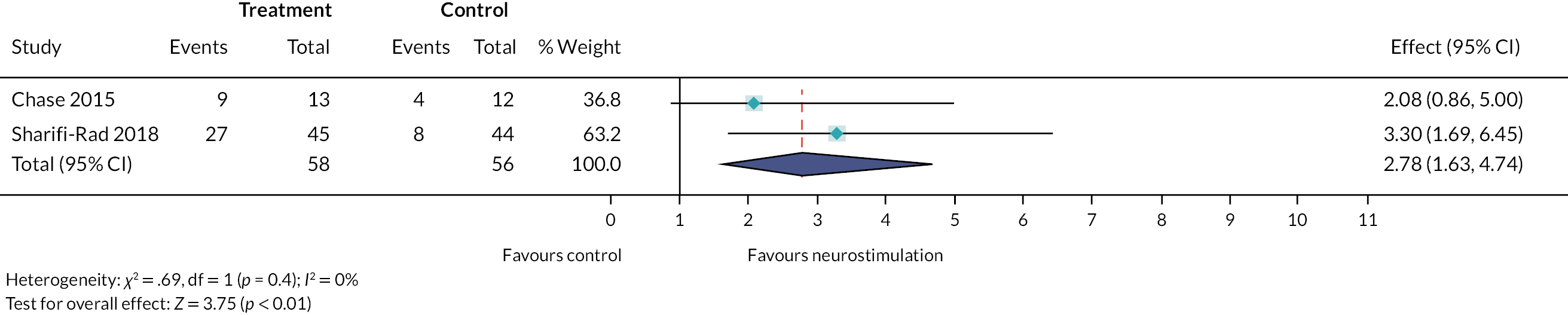
What is the effect of transanal irrigation?
Five studies,177–181 investigating the effect of TAI in a small number of children with CFC (see Table 6), provide very limited evidence about the effectiveness of TAI. These studies provide some very-low-certainty evidence that TAI may be safe, feasible and effective for children with intractable symptoms which have not resolved with long-term conventional laxatives and management.
What is the effect of biofeedback?
Seven studies182,183,194,196–198 addressed this question (see Table 6).
Anorectal manometry
Two studies182,198 investigated the use of anorectal manometer to provide biofeedback. Wald (1987)198 compared biofeedback therapy, using an anorectal manometer, to conventional mineral oil therapy. There were no significant differences between the biofeedback group and mineral oil therapy group for clinical outcomes (including defaecation frequency, incontinence frequency, parental perception of clinical status and overall satisfaction) at 3-, 6- and 12-month follow-up. Mineral oil therapy was suggested to be superior to biofeedback in children with normal defaecation patterns.
External anal sphincter electromyographic biofeedback
Five studies195,183,196,197 investigated the effectiveness of external anal sphincter biofeedback. Nolan (1998)196 compared up to 4, weekly, surface EMG biofeedback to control (no intervention) in 29 children. Number of improved children was similar in both groups at 6 months’ follow-up. Loening-Baucke (1990)194 compared the effectiveness of supplementing conventional treatment with EMG biofeedback training in 31 children. Participants were trained in pushing out a rectal balloon, with verbal, visual and sound reinforcement to produce relaxation of the external anal sphincter. One of 19 children in the conventional group and 12 of 22 in the biofeedback group were judged to have recovered (p < 0.001) at 7 months. Van der Plas (1998)197 compared EMG biofeedback training, combined with conventional treatment, with conventional treatment only in 192 children. Defaecation dynamics improved more in the biofeedback group than the control group (p < 0.001). There were no significant differences in achievement of ‘successful treatment’ between the groups.
Biofeedback plus transcutaneous electrical stimulation
One RCT, published after the completion of our synthesis and not fully integrated, compared, in 40 children aged 5–13 years with faecal incontinence, biofeedback plus TES with TES alone. 190 Both groups received 10 sessions of treatment, delivered twice per week. There were non-significant differences between groups for outcomes of frequency of incontinence and QoL, in favour of the group with biofeedback. There was a significant difference in the number with ‘resolved’ symptoms, with 55% of the TES group reported symptoms had been resolved, compared to 65% of the biofeedback plus TES.
In summary, there may be no additional benefit of supplementing conventional treatment with biofeedback therapy in children with normal defaecation dynamics, but potentially some benefit for the subgroup of children with abnormal defaecation dynamics. Certainty in this finding is very low, due to the quality and quantity of available evidence.
What is the effect of physiotherapy, in combination with conventional treatment?
Three RCTs explore the effectiveness of different physiotherapy regimes199–201 (see Table 6). Silva (2013)199 reported a significant difference in favour of the physiotherapy group, for defaecation frequency at 6-week follow-up (p = 0.01). There was no significant difference between groups for any other outcomes measured. Van Engelenburg (2017)200 concluded that outcomes improved more for the physiotherapy group, for numbers with CFC, use of laxatives, measures of treatment success and QoL. Van Summeren (2020)201 found no statistically significant differences in success rates or QoL between treatment groups, but a difference in favour of physiotherapy for global perceived treatment effect (adjusted RR 1.40; 95% CI 1.00 to 1.73). A subgroup analysis comparing outcomes of children with and without chronic laxative use indicated that children with chronic laxative use may have had some benefit from physiotherapy. The authors compare the results of their study with the findings of Silva (2013) and Van Engelenburg (2017)200 and conclude that there is no evidence of benefit of providing physiotherapy to the ‘whole group of children with CFC consulting in primary care’, but that it was possible that physiotherapy may be beneficial in a subgroup of children with longer duration symptoms. In summary, evidence relating to the effectiveness of physiotherapy is inconsistent. Routine referral to physiotherapy for all children with constipation seen within primary care is not supported. There is some limited evidence that physiotherapy may be beneficial for a subgroup of children, but further research is required to confirm (or refute) this. We have very low certainty in this finding, due to the quantity and quality of studies.
What is the effectiveness of physical therapy for children with cerebral palsy?
Two studies investigate the effect of physical therapy on constipation in children with cerebral palsy184,202 (see Table 6). Awan (2016)184 reported that defaecation frequency and severity of constipation improved significantly after the programme (p < 0.05). Awan (2021)202 reported differences at baseline between groups for defaecation frequency and constipation severity score, and no significant differences between groups after the first intervention period, concluding that there was a significant improvement over the whole intervention period (p < 0.001). Eisenberg (2009)185 compared the use at home of a standing frame and a walker in children with severe cerebral palsy. There was a reduction in that proportion of children with constipation among the walker group, but not in the standing group (p = 0.02). In summary, there is some very-low-quality evidence to suggest that constipation in children with cerebral palsy may be improved with physical therapy. However, evidence is insufficient to support generalised conclusions.
Evidence of effectiveness: lifestyle interventions delivered by continence teams (Level 2)
What is the effect of dietary exclusion of fructose and lactose?
One study,188 explored the effect of expert health professional advice and a diet which excluded an identified positive sugar, for 6–12 months (see Table 6). After exclusion of sugars, consistency of stools and severity of constipation were significantly improved. Seventy-two per cent of parents reported difficulties implementing the diet. In summary, there is insufficient evidence to support any conclusions. One small study provides very-low-certainty evidence that exclusion of fructose and lactose could reduce severity of constipation. However, implementation of this diet was challenging and further research is required to support any conclusions.
Evidence of effectiveness: combined interventions delivered by continence teams (Level 2)
What is effect of a combined therapeutic programme?
Four studies187–189,203 explored a range of combined programmes (see Table 6). Borowitz (2002)203 found no significant differences between groups, but children receiving enhanced toilet training achieved these outcomes whilst using significantly less laxatives than the group receiving intensive medical therapy only. Loening-Baucke (1989)187 reports that 43% of children had recovered at 12 months, and 57% not to have recovered, based on clinical symptoms. Data analysis compares those who did and did not recover. Loening-Baucke (1993)188 interviewed parents of children who had not recovered but does not provide data supporting conclusions about effectiveness of treatment. Modin (2016)189 provides data which demonstrated the impact of constipation on behavioural difficulties, but the study design is not appropriate to support conclusions relating to treatment effectiveness. In summary, there is insufficient evidence to support specific conclusions relating to the effect of a combined treatment programme, but some very-low-certainty evidence that combined programmes may be beneficial for some children.
Summary of evidence of effectiveness: interventions delivered by continence teams (Level 2/specialist secondary care services)
One SR, updated to include two RCTs, a further 13 RCT and 15 other primary studies provided evidence for the effectiveness of interventions delivered by specialist secondary care services (Level 2 of the pyramid). Methodological concerns about these studies limit our certainty in findings.
Stakeholder reflections on evidence of effectiveness: interventions delivered continence teams (Level 2/specialist secondary care services)
Members of the SG have provided the following reflections, in their own words, on the evidence identified in relation to Level 2:
-
I found some of the debate around the interventions in this section very difficult. The thoughts of enemas and even more drastic interventions for children, seemed so distasteful. But I’d personally used suppositories as a teenager. I feel that evidence needs to be more robust for these interventions because of the issue of acceptability for both parents and the children themselves.
-
The amount of uncertainty about many of these interventions is extremely concerning. When one adds the concerns that have been raised about the robustness of some trials it makes me very pessimistic that overall there is much known about effective interventions and makes the need for robust research to be done even more urgent in my view.
-
As before, the key gaps section is very important … but I do find the long lists of interventions for which there is not sufficient data to have confidence in drawing conclusions to be a bit discouraging…
-
Understanding the range of therapies available is itself useful. There seems largely to be an absence of evidence (rather than evidence of absence of effect). By defining what an adequate protocol might look like (considering complex issues such as how to stratify patients with potentially diverse aetiologies), and by undertaking co-ordinated research activity as might be driven by NIHR, some questions may be adequately addressed for the next generation of patients.
Evidence of effectiveness: interventions delivered by consultant-led teams (Level 3/highly specialist tertiary care services)
Results of the search
Figure 21 illustrates the results of the search. Details of ongoing and excluded are provided in Report Supplementary Material 8.
FIGURE 21.
Results of the search – Level 3 studies.
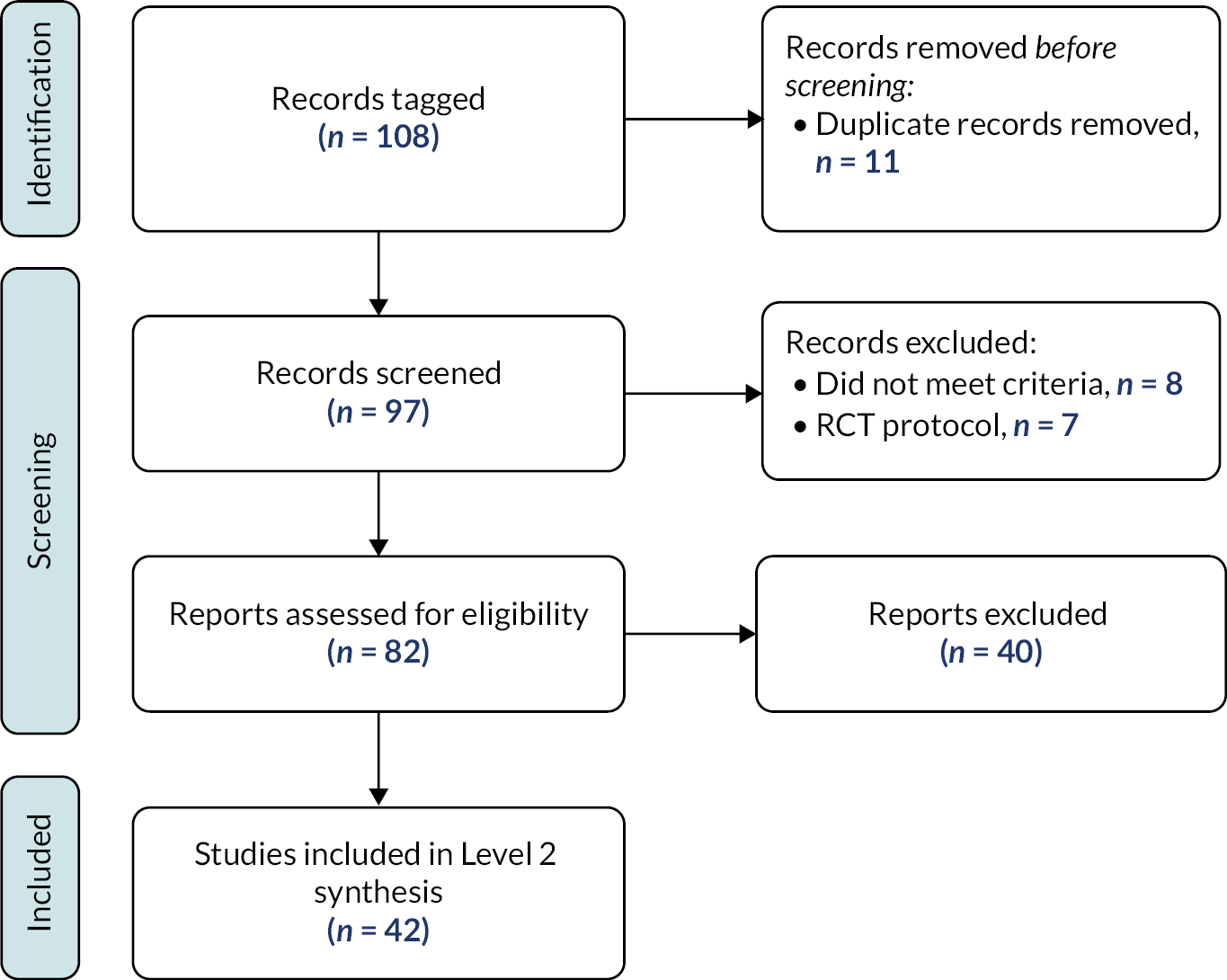
We included no SRs or RCTs. We included a total of 42 other primary studies209–249 within this synthesis.
Characteristics of included studies
Characteristics of the included studies are summarised in Table 7 and Table 18 (see Appendix 4). There were a total of 1886 children (range 10–126) included in the 42 studies which reported participant numbers. The most common pre-specified outcomes reported were side effects (n = 27), faecal incontinence (n = 24) and stool frequency (n = 21). School attendance was only reported in five studies. The outcomes assessed, ROB and judgements of certainty and are summarised in Report Supplementary Material 8.
| Study | Study design | No. of children with CFC recruited | Overall ROB | Abstract only |
|---|---|---|---|---|
| Botulinum toxin ( n = 5) | ||||
| Ahmadi 2013209 | Controlled before-and-after | 40 | Moderate concerns | |
| Basson 2014210 | Retrospective cohort | 29 | Serious concerns | |
| Hallagan 2019211 | Retrospective cohort | 112 | Serious concerns | √ |
| Hameed 2018212 | Controlled before-and-after | 20 | Moderate concerns | |
| Zar-Kessler 2018213 | Cohort | 141 | Very minor concerns | |
| Sacral neuromodulation ( n = 9) | ||||
| Janssen 2018214 | Retrospective cohort | 38 | Minor concerns | |
| Lu 2017215 | Prospective cohort | 17 | Minor concerns | |
| Lu 2016250 | Prospective cohort | 22 | Minor concerns | √ |
| Peeters 2011216 | Retrospective cohort | 13 | Moderate concerns | √ |
| Sulkowski 2015217 | Retrospective cohort | 34 | Minor concerns | |
| Van der Wilt 2016218 | Prospective cohort | 30 | Very minor concerns | |
| Van der Wilt 2017219 | Prospective cohort | 30 | Moderate concerns | |
| Van der Wilt 2014220 | Cohort | 33 | Serious concerns | √ |
| Van Wunnik 2012221 | Retrospective cohort | 13 | Minor concerns | |
| Anorectal myectomy ( n = 4) | ||||
| Mousavi 2014222 | Cohort Study | 44 | Serious concerns | |
| Peyvasteh 2015223 | Cohort study | 48 | Serious concerns | |
| Redkar 2018224 | Cohort Study | 37 | Moderate concerns | |
| Redkar 2012225 | Cohort Study | 28 | Serious concerns | |
| ACE/MACE (n = 17) | ||||
| Basson 2014a226 | Retrospective cohort | 68 | Moderate concerns | |
| Bellomo-Branda 2018227 | Retrospective cohort | 29 | Minor concerns | √ |
| Chong 2016228 | Retrospective cohort | 14 | Very minor concerns | |
| Church 2017229 | Survey | 10 | (no overall assessment) | |
| Dolejs 2017230 | Retrospective cohort | 93 | Very minor concerns | |
| Gomez-Suarez 2016231 | Retrospective cohort | 31 | Minor concerns | |
| Har 2013232 | Survey | 15 | (no overall assessment) | |
| Hoekstra 2011233 | Retrospective cohort | 15 | Serious concerns | |
| Husberg 2011234 | Retrospective cohort | 2 | Moderate concerns | √ |
| King 2005235 | Survey | 42 | (no overall assessment) | |
| Khoo 2017236 | Retrospective cohort | 84 | Very minor concerns | |
| Mousa 2006237 | Cohort | 9 | Serious concerns | |
| Mugie 2012238 | Retrospective cohort | 35 | Serious concerns | |
| Peeraully 2014239 | Retrospective cohort | 45 | Moderate concerns | |
| Randall 2014240 | Prospective cohort | 126 | Serious concerns | |
| Siddiqui 2011241 | Retrospective cohort | 37 | Minor concerns | |
| Youssef 2002b242 | Cohort study | 12 | Minor concerns | |
| MACE compared to caecostomy button (n = 1) | ||||
| Cascio 2004243 | Retrospective cohort | 49 | Very minor concerns | |
| ACE compared to SNS (n = 2) | ||||
| Vriesman 2020244 | Retrospective cohort | 42 | Very minor concerns | |
| Wang 2019245 | Retrospective cohort | 41 (may be same children as Vriesman 2020) | Moderate concerns | √ |
| Colonic resection ( n = 2) | ||||
| Bonilla 2013246 | Cohort study | 12 | Moderate concerns | |
| Tamura 2020247 | Cohort study | 22 | Very minor concerns | |
| Colonic resection combined with Malone appendicostomy ( n = 1) | ||||
| Gasior 2018248 | Cohort study | 31 | Moderate concerns | |
| Surgical intervention (ileostomy, colostomy or (sub)total colectomy) ( n = 1) | ||||
| Kuizenga-Wessel 2017249 | Cohort study | 37 | Minor concerns | |
Research questions addressed
The included studies were judged to address nine distinct RQs relevant to Level 3. The following sections provide a summary of the evidence. An extended narrative synthesis is available in Report Supplementary Material 9 and tables detailing quality assessments in Report Supplementary Material 8.
Evidence of effectiveness: pharmacological interventions delivered by consultant-led teams (Level 3)
What is the effect of botulinum toxin?
Five studies209–213 (see Table 7) provide very-low-quality evidence that botulinum toxin injection for chronic constipation may be an effective method in managing children with CFC. Due to methodological limitations and limitations in the reporting of these studies, we have very low certainty in this finding.
Evidence of effectiveness: surgical interventions delivered by consultant-led teams (Level 3)
Study details are in Table 7.
What is the effect of sacral neuromodulation?
Nine studies214–221 provide some very-low-quality evidence that sacral neuromodulation may be effective in treating the symptoms of CFC. Aspects commonly reported across studies include less abdominal pain, improvement in symptoms, complications.
What is the effect of anorectal myectomy?
Four studies222–225 provide some very-low-quality evidence that suggests anorectal myectomy may be effective at treating CFC, in children who have not responded to medical treatment. However, due to methodological limitations and low number of studies, the evidence is insufficient to support generalisable conclusions. It should be noted that these studies were conducted prior to the availability of botulinum toxin, which may provide a non-surgical alternative.
What is the effect of antegrade continence enema/Malone antegrade continence enema?
Seventeen studies226–242 provide very low certainty that the use of ACE/MACE may be effective for CFC. Although studies were consistent in their findings that ACE/MACE is effective, many of the outcomes of interest were not addressed by the studies. Complications (e.g. granulation, leakage, additional surgery required) arising from ACE/MACE use were common.
What is the effect of MACE compared to caecostomy button?
One study243 provides some very-low-quality evidence that caecostomy button may have less complications than MACE, but this is insufficient to support generalisable conclusions.
What is the effect of ACE compared to sacral nerve stimulation?
Two studies244,245 provide some very limited evidence that SNS may be superior to ACE for faecal incontinence, ACE superior to SNS at improving defaecation frequency in those with reduced bowel movements, and that SNS may have less complications than ACE. This evidence is insufficient to support generalisable conclusions.
What is the effect of colonic resection?
Two studies246,247 provide some very-low-quality evidence which suggests outcomes were mixed for the use of colonic resection to treat the symptoms of CFC. There is insufficient evidence to suggest the use of colonic resection is safe and effective for the treatment of CFC. The population of children to whom this evidence relates is limited to those with CFC for whom other treatments, including ACE, have failed.
What is the effect of colonic resection combined with Malone appendicostomy?
One study248 provides some very-low-quality evidence which suggests colonic resection combined with Malone appendicostomy may be safe and effective to treat the symptoms of CFC. There is only one small study, providing insufficient evidence to support generalisable conclusions.
What is the effect of surgical intervention [ileostomy, colostomy or (sub)total colectomy]?
One study249 assessed outcomes of surgical intervention [ileostomy, colostomy or (sub)total colectomy] in children with CFC. There provides insufficient evidence to support generalisable conclusions about these surgical interventions.
Summary of evidence of effectiveness: interventions delivered by consultant-led teams (Level 3/highly specialist tertiary care services)
Forty-two primary studies (no RCTs) provide some evidence for the effectiveness of interventions delivered by highly specialist tertiary care services (Level 3). Evidence relates to children with CFC for whom medical treatments have failed. Our certainty in findings of the studies is generally limited by the study designs, low numbers of participants and concerns about ROB for a number of these studies. For all these questions, it is essential to consider the heterogeneity between children, and how they may respond to treatments, and aspects relating to service delivery (including team expertise, health professional and family/carer education and support).
Stakeholder reflections on evidence of effectiveness: Level 3
Members of the SG have provided the following reflections, in their own words, on the evidence identified in relation to Level 3:
-
The recurring theme is that data seem scarce. Availability of good quality data addressing key clinical questions would inform treatment and likely improve outcomes such as clinical outcomes, process, resource use and experience.
-
This work illustrates the range of approaches and highlights some of the blocks to evidence based practice. These include understanding of aetiology and (including for subgroups of children with, say neuro-disability or true connective tissue disorder), stratification of patients to ensure meaningful inclusion criteria, well considered protocols that might state treatments that will have been undertaken pre procedure, and well considered metrics to be used before and after intervention and which map to patient and service-important outcomes.
-
The fact that a number of knowledge gaps persist despite a large number of studies and therefore research resource being used, and the highly respected groups involved in some of this work suggests a change of research approach might be needed. We need strong, patient centred, institutions and collaboration between clinicians, families and research bodies.
-
Where there is evidence to support conclusions, there should be dissemination and education to insure these findings are implemented.
What is the effectiveness of complementary therapy interventions?
Results of the search
Figure 22 illustrates the results of the search. Details of ongoing and excluded are provided in Report Supplementary Material 10.
FIGURE 22.
Results of the search – complementary therapy studies.

We included 15 studies; these were 2 SRs,251,252 8 RCTs253–260 and 5 other primary studies261–265 within this synthesis.
Characteristics of included studies
Characteristics of the included studies are summarised in Tables 8 and 19 (see Appendix 4). There were a total of 1755 participants (range 7 months to 19 years) included across 15 studies. Three studies254,255,262 only included children with cerebral palsy and one261 only included children with an identified disability, and one study included a mix of children with and without disabilities and gastric conditions. 263 Seven studies excluded children with additional needs. 251,253,255–260,262,264 The most common pre-specified outcome reported was stool frequency (n = 17 studies). QoL was reported by two studies255,260 and painful defaecation by seven studies. 252,254–257,259,260,264 School attendance was reported in only one study. 261
| Study | Study design | No. of children with CFC recruited | Intervention | Overall ROB | Abstract only |
|---|---|---|---|---|---|
| Non-pharmacological therapies ( n = 8) | |||||
| Bromley 2014261 | Non-comparative | 28 | Abdominal massage | Minor concerns | |
| Canbulat Sahiner 2017253 | RCT | 37 | Reflexology | High | |
| Chase 2011251 | Systematic review | 87 (3 studies) | Non-pharmacological therapies | Low | |
| Duymaz 2020254 | RCT | 50 | Reflexology | High | |
| Elbasan 2018262 | Non-randomised study | 40 | Reflexology | Moderate | |
| Mostamand 2019263 | Prospective cohort | 5 | Abdominal massage. | Serious concerns | √ |
| Orhan 2018255 | RCT | 45 | Connective tissue manipulation and KT | L-L-H-L-L | |
| Shahamat 2016256 | RCT | 118 | Dry cupping therapy vs. laxative | L-U-H-L-U | |
| Complementary/alternative medicines ( n = 7) | |||||
| Aslam 2021252 | Systematic review | 132 (2 RCTs) | Cassia fistula | Low | |
| Babaei 2018264 | Case series | 6 | Traditional Persian medicine (senna, cascara, aloe, rhubarb, Terminalia chebula, Citrullus colocynthis, Ficus carica). | Minor concerns | |
| Cai 2018257 | RCT | 480 | XEBT vs. placebo | Low | |
| Nimrouzi 2015258 | RCT | 120 | PEG vs. Flixweed (Descurainia sophia) | High | |
| Tavassoli 2021259 IRCT20180305038968N1 |
RCT | 140 | PEG vs. Viola flower syrup | Low | |
| Zadpe 2020265 | Non-comparative | 30 | Shunthiyadi syrup (ginger, dried fruit of the Haritaki tree, long pepper plant) | Serious concerns | |
| Qiao 2021260 | RCT | 200 | Chinese herbal medicine (XiaojiDaozhi decoction) vs. placebo |
Low | |
Research questions addressed
The studies we extracted data from were judged to address eight distinct RQs. The following sections provide a summary of the evidence in relation to each question. The outcomes assessed, ROB and judgements of certainty and are summarised in Report Supplementary Material 10.
Evidence of effectiveness: non-pharmacological therapies
Eight studies addressed non-pharmacological complementary therapies (see Table 8).
What is the effect of abdominal massage (in children with or without disabilities)?
We found two small studies of abdominal massage. 261,263 Bromley (2014)261 report on 25 children with an identified disability provided with daily abdominal massage by parents, following training. Constipation was reported to improve by 87.5% of parents, diet by 41%, sleep by 37% and school attendance by 19%. Mostamand (2018)263 reported the results of manometric tracings in children who had received 5 minutes of abdominal massage, and report improvements. In summary, abdominal massage may be beneficial for children with CFC, but there is insufficient evidence to support generalised conclusions.
What is the effect of connective tissue manipulation and kinesio taping in children with cerebral palsy?
One study255 randomised 45 children with cerebral palsy and CFC to receive either connective tissue manipulation (CTM) to the sacral and cervical regions, kinesio taping (KT) to the lumbar sacral area, or control. Outcomes improved in the two treatment groups compared to control. In summary, there is low certainty from one RCT that physiotherapy techniques of CTM and KT may be beneficial components of a programme for children with cerebral palsy who have constipation and are receiving physiotherapy.
What is the effect of chiropractic or osteopathic manipulation?
Chase (2011)251 included three case studies (five children) in a SR of chiropractic manipulation. One of the studies combined spinal manipulation with changes to diet, and another with abdominal massage. In summary, there is insufficient evidence to support conclusions relating to the effectiveness of manipulation interventions.
What is the effect of dry cupping therapy compared to laxatives?
Shahamat (2016)256 compared dry cupping therapy of the abdominal wall with laxative therapy in 120 children with CFC. Findings suggest that cupping therapy was as effective as laxatives. In summary, there is very low certainty that dry cupping therapy of the abdominal wall may be as effective as laxatives.
What is the effect of reflexology?
Chase (2011)251 included one study which demonstrated some benefits of reflexology in 48 children with CFC. We identified three later studies. 253,254,262 Canbulat Sahiner (2017)253 randomised 37 children with CFC to receive reflexology or control group. There were no significant differences between groups. Duymaz (2020)254 and Elbasan (2018)262 explored the effect of the addition of reflexology to neurodevelopmental therapy session in children with cerebral palsy. There were statistically significant differences between groups, in favour of reflexology, for constipation-related outcomes. In summary, studies provide mixed results. There is insufficient evidence to support generalised conclusions relating to the effect of reflexology.
What is the effect of acupuncture?
Chase (2011)251 included one study (n = 10) which investigated the effect acupuncture to points in children with CFC and demonstrated some benefits. This is insufficient evidence to support conclusions about the effect of acupuncture.
Evidence of effectiveness: complementary/alternative medicines
Seven studies addressed complementary medical therapies (see Table 8).
What is the effectiveness of cassia fistula?
Aslam (2021)252 combined results of two studies (n = 132) of cassia fistula. Meta-analyses demonstrated a beneficial effect on outcomes of defaecation frequency (MD 4.22, 95% CIs, CI 2.78 to 5.66),1 severity of pain during defaecation (MD −4.84, 95% CI −8.28 to −1.41) and stool consistency (MD −9.51, 95% CI −13.52 to −5.51), but not for outcomes of faecal incontinence and retentive posturing. In summary, there is some very limited evidence that suggests cassia fistula may have some beneficial effects, but this is insufficient to support any generalised conclusions. We have very low certainty in this finding.
What is the effect of herbal and/or traditional medicines?
Six studies investigated the role of other herbal/traditional medicines. 257–260,264,265 Cai (2018)257 explored the effect and safety of Xiao’er Biantong (XEBT) granules in 487 children with CFC. The study found that XEBT can increase frequency of defaecation and shorten defaecation interval time as well as positive impact on a range of other factors. Qiao (2021)260 randomised 100 children with CFC to receive a Chinese herbal medicine or placebo. There were significant differences between treatment groups for all outcomes, in favour of the herbal medicine, but a small number of side effects in the herbal medicine group. Tavassoli (2021)259 randomised 140 children to receive the medical herb viola flower or PEG4000. After 4 weeks of intervention, there were no differences between the groups for clinical outcomes including of stool consistency, defaecation frequency, faecal retention or soiling. There was a significant difference between group in the number of minor side effects (p = 0.03). Nimrouzi (2015)258 compared the medical herb Flixweed (Descurainia sophia L.) with PEG4000 in 120 children. This RCT is also included in the SR of laxatives which is included in the Level 1 synthesis [see What is the effectiveness of assessment and intervention by primary care services (Wider Children’s workforce/Level 1 interventions)?]. No significant differences were found between the groups after 8 weeks of treatment. Zadpe (2020)265 studied the effect of Shunthyadi syrup in 30 children with CFC, concluding that Shunthyadi was effective at improving outcomes. Babaei (2018)264 studied the effect of behaviour modification and herbal drugs (based on traditional Persian medicine) on the symptoms of constipation in six children.
In summary, collectively, there is very low quality of evidence that herbal/traditional medicine for children with CFC may result in equivalent or improved outcomes. However, studies investigated different interventions, making it difficult to support clinical decisions. Studies provides evidence that XEBT granules and XiaojiDaozhi Decoction may be promising interventions, and that Flixweed may be a suitable alternative to PEG, but these studies had limitations, limiting conclusions.
Summary of evidence of effectiveness: complementary therapies
Studies provide insufficient evidence to support any clinical implications relating to the effectiveness of any type of complementary therapies. Complementary therapies for which there was some – limited – evidence of possible benefit, and which may merit further investigation include some non-pharmacological therapies and XEBT granules.
Stakeholder reflections on evidence of effectiveness: complementary therapies
Members of the SG have provided the following reflections, in their own words, on the evidence identified in relation to complementary therapies:
-
I like this section … the fact that you have included interventions we don’t know about shows you have been open.
-
There could be something in these herbal drugs. But we maybe need to understand how they could possibly be working – what their mechanism is – before we study them more.
-
Complementary therapies are often holistic. It’s important to think about the whole child, and the context that that child is in. Constipation is complex and it makes sense to view everything in a holistic way.
-
There was some evidence about abdominal massage in children with additional needs. This group of children are often not studied, but this is important. If abdominal massage is safe and could help these children then parents should be told about this so that they can try it. You reach a stage where you will try anything, and I would rather it was massage than a drug.
What is the effectiveness of psychosocial interventions?
Results of the search
Figure 23 illustrates the results of the search. Details of ongoing and excluded studies are provided in Report Supplementary Material 11. Two SRs exploring the effectiveness of behavioural interventions for CFC, both judged to be at low ROB, were identified. 266,267 Consensus discussion led to the decision to include Freeman (2014). 267 Justification for this decision is provided in Report Supplementary Material 11.
FIGURE 23.
Results of the search – psychosocial intervention studies.
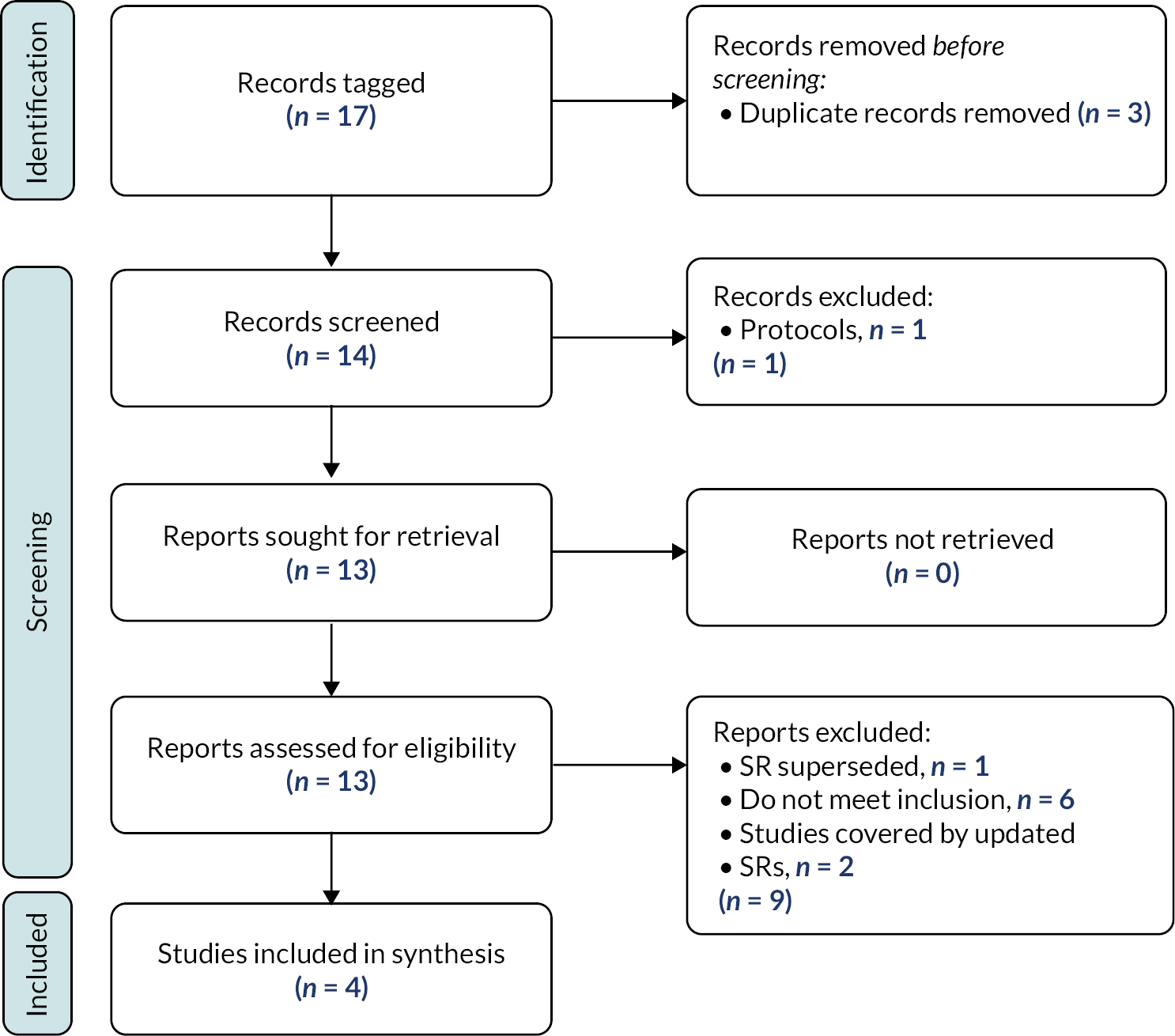
We included four studies in this synthesis; one SR which was updated with one new RCT, and two other primary studies.
Characteristics of included studies
Characteristics of the included studies are summarised in Tables 9 and 20 (see Appendix 4).
| Study | Study design | No. of children with CFC recruited | Intervention | Overall ROB | Abstract only |
|---|---|---|---|---|---|
| Freeman 2014267 | Systematic review | 562 (10 studies) | Behavioural interventions | Low | |
| Santucci 2018268 | Pilot RCT | 21 | Guided mastery vs. control | U-U-H-H-H | √ |
| Silver 1998269 | Retrospective Cohort | 108 | Externalising therapy | Minor concerns | |
| Taitz 1986270 | Survey | 47 | Psychotherapy | Serious concerns |
The SR267 included 10 RCTs, with a total of 562 children. We identified a further three studies with 176 children. In all studies, the behavioural intervention was delivered by a specialist practitioner, such as a behavioural therapist, specialist psychologist or psychiatrist. The outcomes assessed, ROB and judgements of certainty are summarised in Report Supplementary Material 11.
Research questions addressed
The included studies were judged to address three different RQs. The following sections provide a summary of the evidence in relation to each question.
Evidence of effectiveness: behavioural interventions
Four studies addressed behavioural interventions (see Table 9).
What is the effect of behavioural therapy techniques delivered by specialist practitioners?
Freeman (2014)267 included 10 studies in a SR of behavioural interventions for children with faecal incontinence with constipation (see Report Supplementary Material 11 for a summary of the interventions included). Data from four RCTs102,103,203,271 were pooled within meta-analysis, and suggest behavioural interventions are more beneficial than control interventions at achieving ‘author-defined success’. We identified one further RCT266 relevant to this review and were able to combine data on treatment success with the data pooled within Freeman (2014)267 review for this outcome. This did not change the direction of evidence but changed the risk ratio from 1.78 (95% CI 1.25 to 2.55) to 1.68 (95% CI 1.22 to 2.32) (see Figure 24). There were no data suitable for inclusion in other meta-analyses. The pooled data in Freeman (2014)267 indicate that behavioural interventions are more beneficial than control interventions at reducing soiling frequency (MD −2.81, 95% CIs −5.04 to −0.58; low certainty) and defaecation frequency (MD −0.57, 95% CI −2.90 to 1.75; low certainty). In summary, behavioural therapy techniques delivered by specialist practitioners may be beneficial. Our certainty in this finding is very low due to limitations in the reporting of completed studies.
FIGURE 24.
Forest plot. Behavioural interventions vs. control. Treatment success.
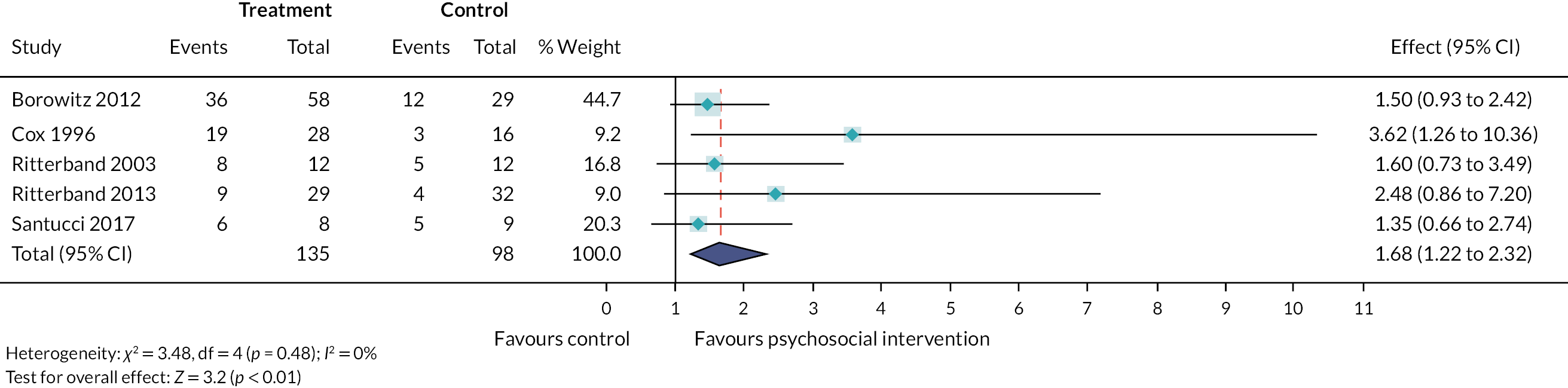
What is the effect of externalising treatment, compared to other behavioural interventions?
Silver (1998)269 conducted a retrospective audit of 108 children treated for soiling problems, including constipation, using externalising treatment or other more traditional, behavioural approaches. Some benefits of externalising therapy were found (42/47 stopped soiling in externalising group compared to 30/43 other; 24/29 found externalising therapy helpful compared to 10/30 other). There is insufficient evidence to support conclusions about the benefits of externalising treatment, compared to other behavioural interventions.
What is the effect of psychotherapy, given in addition to other behavioural therapy?
Taitz (1986)270 compared children with CFC who did and did not receive psychotherapy and found no difference in results between the two groups, but factors such as psychosocial background had an influence on success. There is insufficient evidence to support conclusions about the benefits of providing psychotherapy in addition to other behavioural therapy.
Summary of evidence of effectiveness: psychosocial interventions
A SR, including 10 RCTs, and updated to include 1 further RCT, provides some limited evidence that behavioural therapy, delivered by a specialist practitioner, may improve some outcomes for children. We have low certainty in this finding, with conclusions limited by poor reporting and methodological limitations of RCTs. There was insufficient evidence to support any conclusions relating to the specific nature of the behavioural interventions. There was an absence of evidence about the effectiveness of behavioural interventions delivered by ‘non’ specialists, for example parents and the wider children’s workforce.
Stakeholder reflections on evidence of effectiveness: psychosocial interventions
Members of the SG have provided the following reflections, in their own words, on the evidence identified in relation to psychosocial interventions:
-
It’s so important to know when children need psychological support and to give them – and their families – that support when they need it. Childhood should be carefree and happy – not blighted by pain, discomfort, multiple medical appointments and embarrassment. Having suffered childhood constipation from a young age, resulting in problems throughout adulthood, I favour a holistic approach which integrates psychosocial support.
-
Clinical psychology and similar interventions seem in practice to have a significant effect for some children and some families. The limited data available should be increased through well conducted and well-co-ordinated research to support the ability for children and families that might benefit can access it, and those that won’t don’t waste resource.
-
I feel that there should be more emphasis put on psychosocial interventions including behavioural. I felt very sorry for those who suffer alone and have no-one to turn to.
Summary
This chapter has brought together data from 145 studies which provide evidence about the effectiveness of different models of service delivery, interventions delivered at Level 0, Level 1, Level 2 and Level 3, and complementary and psychosocial interventions. This is a comprehensive overview of all evidence relating to the effectiveness of strategies and combinations of strategies for childhood CFC.
Chapter 8 Brief economic commentary
Introduction
Multiple interventions are currently used to manage constipation; however, little is known about whether these interventions provide good value for money. Identifying treatments that are both clinically effective and cost-effective is key to ensuring a sustainable health service.
In this chapter, we present the methods and results of a SR which aimed to identify all types of economic studies of interventions used to manage CFC in children and young people and to summarise the availability and key findings in terms of costs, resources and cost effectiveness of interventions.
Methods
Search strategy and study identification
Studies tagged as potentially having economic evidence in the scoping review (see Chapter 4) were carried through for more detailed assessment of eligibility.
In addition to the systematic searches outlined in the scoping review, we updated the searches based on guidance from Han (2018). 17 This included additional searches for modelling studies of cost effectiveness, using uncontrolled vocabulary terms, Medical Subject Headings (MeSH) terms ‘costs’ and ‘cost analysis’, building on the existing search strategy (see Appendix 3) and other recent published studies. Backward and forward citation tracking were also used to identify further related/relevant studies.
We included all types of studies detailing costs related to interventions aimed at children with CFC that were published in English, regardless of study design (e.g. cost-effectiveness, cost-consequence, cost-utility and cost-benefit Analysis) (see Table 10). 272
| Type of economic study | Definition |
|---|---|
| Cost-consequence analysis | An economic evaluation where a range of health and potentially other outcome measures are presented alongside the resource use and costs. In cost-consequence analysis, the outcomes are left in a disaggregated format allowing the decision-maker to choose which outcome measure suits the decision-making context. |
| Cost-effectiveness analysis | A cost-effectiveness analysis is a form of economic evaluation in which the outcomes of the intervention and comparator are measured in the same unit. These outcomes are compared against the cost. An intervention is cost-effective if, for a given cost, it produces the largest health gain or it produces a given health gain for the lowest cost and it is more cost-effective than the comparator. |
| Cost-utility analysis | A variant of cost-effectiveness analysis where the health outcome measure of interest is usually expressed as a QALY, a single index that combines length of life with QoL. |
| Cost-benefit analysis | A form of economic evaluation where both costs and benefits of an intervention are measured in corresponding monetary units (normally) to assess whether an intervention is worthwhile. |
| Cost analysis including studies which report cost of illness | These studies aim to identify and measure the total costs attributable to a particular disease. These are not a type of economic evaluation, as they are not used to assess the costs and benefits of alternate courses of action. They may provide useful information, which can be used in the context of an economic evaluation of interventions related to the disease category, although care must be taken as not all costs included in a cost-of-illness study represent resource costs. |
Data extraction
We extracted the following data (where appropriate and available) for each included study:
-
author, year, country of main author;
-
study funder, sponsor, any conflicts of interest;
-
study design;
-
study objectives and perspective (i.e. healthcare sector, society);
-
additional study methods details (i.e. treatment setting, country of study population, study duration, follow-up period, inclusion/exclusion criteria);
-
participants (i.e. target population, number and age of participants, number of participants completing the study);
-
intervention characteristics (e.g. intervention type, intervention provider, number of intervention sessions, frequency and duration of intervention, any equipment used to deliver the intervention);
-
brief details of the comparator group;
-
primary and secondary outcomes reported.
Additional details for economic evaluations were also extracted. These included details regarding the RQ, type of economic design, costs (economic outcome measure, identification of costs, cost categories, cost values, source of cost values) and benefits (i.e. identification of benefits, benefit categories, benefit values), key economic findings, authors’ conclusions about economic findings, whether the intervention was judged as effective and/or cost-effective (based on authors judgements) and incremental cost-effectiveness ratio (ICER) data.
Assessment of reporting quality
The reporting quality of studies categorised as cost consequence, cost effectiveness, cost utility or cost-benefit were evaluated using the consensus health economic criteria (CHEC) checklist. Studies judged as being a cost of illness were not evaluated using CHEC as they are not economic evaluations, so it was not possible to apply the reporting checklist.
Data coding
Included studies were assigned one of the five codes (see Table 10) using well-established definitions for economic evaluations. 272
Data synthesis
A narrative review of relevant studies was undertaken to arrive at overall conclusions regarding the state of knowledge of the cost and resource use of interventions.
Results
Description of included studies
Systematic searches identified 39,380 potential studies; of which 87 full-text papers were identified as potentially relevant for inclusion. Of these, 31 studies (reported across 28 publications) were judged to have met the selection criteria. 17,261,273–296 NICE guidelines27 reported four different economic analyses within the same publication: (1) cost analysis of treatments for disimpaction; (2) cost effectiveness of disimpaction by dose of a specific pharmacological treatment (PEG3350 plus electrolytes); (3) pharmacological treatment for disimpaction: comparing different alternatives; and (4) maintenance phase following disimpaction and initial management. Key study characteristics are summarised in Tables 11 and 21 (see Appendix 5).
| Study | Study design | Intervention | Economic outcomes | Type of economic analysis |
|---|---|---|---|---|
| Alper 2010273 | Narrative review | PEG | NR | Cost-of-illness |
| Bladder and Bowel UK 2017275 | Vignette – single case | Variety | Costs of medication and other interventions, professionals contact time, hospital admission time. | Cost-of-illness |
| Bladder and Bowel UK 2017274 | Vignette – single case | Variety | Costs of medication and other interventions, professionals’ contact time, hospital admission time. | Cost-of-illness |
| BIG 2020277 | Secondary data analysis | Laxative prescribing | Costs based on admissions data for ICD-10 diagnosis code K59.0 (Constipation) | Cost-of-illness |
| Brazzelli 2011270 | Systematic review | Behavioural and/or cognitive interventions | Sought evidence for multiple health economic outcomes | Unable to conduct planned analysis |
| Bromley 2014261 | Service development | Abdominal massage | Bowel movements, medication use and contact with healthcare professionals | Cost-of-illness |
| Choung277 | Nested case-control study | Medical visits | Resource use and associated charges | Cost-of-illness |
| Guest and Clegg 2006279 | Decision model | Disimpaction | Resource use, utility estimates | Cost minimisation analysis |
| Guest 2007278 UK |
Decision model | PEG3350 plus electrolytes |
Data resources | Cost minimisation analysis |
| Han 201817 | Systematic review | Variety | Costs and outcomes of treatments for chronic constipation and cost- effectiveness methods | Other: Systematic review of economic evaluations |
| Liem et al. 2009280 | Secondary data analysis | Laxatives | Service utilisation and expenditures | Cost-of-illness |
| Mahon 2017281 | Systematic review and a cost-of-illness calculation | Medication | Number of admissions, visits to A&E, mean length of stay prescriptions of constipation medicines, appointments with HV, GP | Cost-of-illness |
| Moser 2014 282 |
Retrospective review of data | Psychology | Utilisation of psychology services, programme expenses, collections and offsets | Cost-of-illness |
| NICE 20101 (i) | Economic evaluation | Disimpaction | Resources use, costs and benefits | Cost minimisation analysis |
| NICE 20101 (ii) | Economic evaluation: decision model | Laxatives (PEG3350) | QALYs, cost data | Cost effectiveness/cost-utility analysis |
| NICE 20101 (iii) | Economic evaluation | Pharmacological treatment for disimpaction: comparing different alternative laxatives | Resources use, costs and benefits See Report Supplementary Material 13 |
Cost minimisation analysis |
| NICE 20101 (iv) | Economic evaluation | Maintenance phase following disimpaction and initial management. | Resources use, costs and benefits See Report Supplementary Material 13 |
Cost effectiveness/cost-utility analysis |
| Persels 2016284 | Website | Website | Advice about how to reduce costs | Cost-of-illness |
| Phatak 2014285 | Narrative review | PEG | Cost of medications | Cost-of-illness |
| Ritterband 2013286 USA |
RCT | Internet-based intervention, | Items and events that incurred costs related to encopresis. | Cost-of-illness |
| Rogers 2011287 | Narrative review | NICE recommendations | NR | Cost-of-illness |
| Rogers 2012288 | New care model | Nurse-led clinic | NR | Cost-of-illness |
| Sandweiss 2018289 |
Non-comparative study using quality improvement methodology | ED constipation management | Pathway utilisation, rate of abdominal radiography, ED cost and length of stay, and ED admission rate for constipation | Cost-of-illness |
| Sommers 2015290 | Secondary data analysis | NR | ED visits and associated costs | Cost-of-illness |
| Southwell 2020291 | Review of meta-analyses, SRs, and RCTs | SNS/sacral nerve modulation | Key conclusions about costs | Cost-of-illness |
| Sparks 2018292 | Retrospective cross-sectional study | NR | Number of constipation-related ED visits | Cost-of-illness |
| Stephens 2018293 | Retrospective cohort study |
NR | Number of visits, spending, and prescription services. |
Cost-of-illness |
| Van der Wilt294 | Economic evaluation | Sacral neuromodulation. | Compared costs and QALYs. | Cost effectiveness/cost-utility analysis |
| Van Summeren295 | RCT | Physiotherapy. | Costs, ICER, cost acceptability | Cost effectiveness/cost-utility analysis |
| Wheeler 2019296 | Feasibility study | Manometry | Limited details reported | Cost-of-illness |
| Windell 2020297 | Magazine article | NR | NR | Cost-of-illness |
The flow of studies is shown in Figure 25. Main reasons for exclusion of studies were that they did not report any economic data (n = 23) or were conducted in adults (n = 13). Reasons for exclusion and details of ongoing studies which are summarised in Report Supplementary Material 15.
FIGURE 25.
Results of the search – economic studies.

All of the studies were conducted in high-income countries: the USA (n = 10),276,280,282,284,287,289,290,292,293,298 UK (n = 9),1,261,274,275,277,278,287,288,296 the Netherlands (n = 2)224,294 and Australia (n = 1). 279 Six studies presented limited economic evidence from more than one country;17,273,276,281,285,291 none of the cost data reported within these studies were gathered in low-middle-income countries (LMICs).
The description of participants was highly variable across studies (Report Supplementary Material 13); of these, 2/31 studies were focused on children with additional needs. 261,292 The definition of constipation, where specified, also differed across studies.
Economic evidence and gaps
Cost-of-illness studies
The majority of included studies were categorised as cost-of-illness studies which estimate resource use and calculate costs from a variety of sources (n = 20)261,273–277,282–291 or SRs. 17,270 Most of these studies reported costs from a health sector perspective; 4/20 considered both health and societal perspectives274,275,277,286; however, data about the indirect impact of childhood constipation (e.g. missed days at school, QoL, academic achievement) were sparse (Report Supplementary Material 13).
Reporting of cost/resource descriptions also varied widely across these studies; from those reporting limited information about cost savings or burden of illness in children with constipation (e.g. see references177,282,285,295,296) to those that provided more detailed descriptions of costs associated with treating constipation in children. 276,280,281,290,292,293 Of those studies that provided a richer description of costs, most relied on data sources that are now 5–15 years out of date.
Economic evaluations
Of the remaining eight studies, seven were economic evaluations using decision models1,224,278,279 and one was an economic evaluation conducted alongside a RCT. 294 The reporting of these studies was assessed using the CHEC list. The RQ, economic importance and type of economic evaluation were clearly stated in all of the studies. However, reporting outcomes for the data collection were less reliable, and analysis and interpretation of results for the CHEC criteria were only reported in sufficient detail in one study224 (see Report Supplementary Material 13).
No economic evaluations were identified for interventions delivered by parents/carers; interventions delivered by the wider children workforce; models of delivery or psychosocial/complementary interventions (see Figure 26).
FIGURE 26.
Studies reporting economic evidence mapped across levels of care provision.
Four studies were categorised as partial economic evaluations (i.e. cost minimisation analysis). 1,278,279 These studies all assessed the clinical and economic impact of treating faecal impaction using laxatives or combinations of laxatives. The studies drew on a variety of sources to develop the models including retrospective case notes, interviews with clinicians, clinical expert opinion and a hypothetical case study. These studies all assumed equal effectiveness between the comparators, essentially evaluating the cost differences between the treatment options. All of the studies concluded that PEG plus electrolytes (PEG3350) provided a cost-effective option.
Four studies conducted full economic evaluations; two studies reported in the NICE 2010 guidelines considered the cost effectiveness of disimpaction by dose of PEG3350, and during the maintenance phase following disimpaction1; one evaluated the cost effectiveness of sacral neuromodulation224 and one considered the cost effectiveness of physiotherapy plus conventional treatment. 294 The decision models used in the NICE guidelines reported two cost-effectiveness/utility analysis evaluations,1 which were based on data from a RCT297 and clinical expert opinion. In the disimpaction model, it was concluded that PEG3350 provided cost-savings, but in the maintenance model, the cost of drugs in the treatment alternatives had a greater impact on the total of care than hospitalisation (see Report Supplementary Material 13).
The cost-effectiveness evaluation of sacral neuromodulation employed a Markov model based on a cohort of 30 children with refractory constipation. The authors concluded that the intervention was both effective and cost-effective (see Report Supplementary Material 13). The cost-effectiveness study of physiotherapy plus standard care (education, dietary advice, toilet training and laxative) was based on a concurrent RCT study. 207 Cost-effectiveness data for this study have not been fully reported, but preliminary findings published in an abstract concluded that physiotherapy as first-line treatment for all children with constipation in primary care was not cost-effective compared to standard care.
Stakeholder reflections
Members of the SG have provided the following reflections, in their own words, on the economic evidence:
-
The work done here was definitely work that needed to be done and was done well by the research team. I felt that the research team communicated well with is as a stakeholder group about what they were doing and their results and I felt if needed to we could ask questions and give our reflections which was always were taken on board and considered.
-
The cost of managing CFC is large; there is a likely lifelong effect of impaired education and self-confidence with measurable economic consequence to a young person with chronic soiling. Societal costs follow.
-
In addition to direct costs to families, there are costs for the healthcare environment. Costs in terms of bed occupancy and staff time may also be considerable and one lead community nurse has said that continence was the single biggest challenge her service had. By at least considering the importance of resource use, the current review has highlighted gaps that might be usefully addressed as well as provided some data.
Summary
We identified 31 studies which reported some evidence of cost or resource use; of these, 20 were cost-of-illness studies. Studies were often poorly reported, with limited details and in many cases this literature was 5–15 years out of date. Cost-of-illness studies provide useful context for the burden of illness, but they are not economic evaluations and are frequently criticised for overestimating costs and failing to evaluate a particular intervention or drawing comparisons between alternative treatment options. As such they provide little information regarding the optimal allocation of scarce resources and benefit. What little evidence was presented was primarily focused on cost to the health service.
Chapter 9 Implementation review methods and results
Introduction
In order for complex interventions to be delivered successfully, they must work for the child, and their family, and be implementable and sustainable in clinical practice. The recent MRC guidance framework for developing and evaluating complex interventions clearly highlights the importance of considering the context stating that
complex intervention research requires strong and early engagement with patients, practitioners, and policy makers, shifting the focus from the ‘binary question of effectiveness’ to whether and how the intervention will be acceptable, implementable, cost effective, scalable, and transferable across contexts23
However, we have little understanding of the barriers and facilitators that may impact on the real-world delivery of interventions for childhood constipation. In this chapter, we present our findings of the factors that affect the success or failure of implementation of CFC treatments.
Methods
Search method and study selection
During the data coding for the scoping review, we systematically coded papers reporting data relating to key participant variables, barriers, facilitators, equity factors and adherence. Papers identified in the scoping review which explicitly reported or described factors relating to implementation, including barriers, facilitators, acceptability, fidelity and participant characteristics that stratify health opportunities and outcomes were included in this synthesis.
Data collection and extraction
Two reviewers (PC and JC) independently extracted and coded data identified as a barrier or facilitator to the implementation of interventions (author, year, direct quote, section/page numbers). This was then independently checked by a second review author (CT) and any ambiguities resolved through discussion with each other and other members of the review team.
Coding was performed using the Consolidated Framework for Implementation Research (CFIR) guide (https://cfirguide.org) using a best-fit framework synthesis approach combining deductive and inductive thematic approaches to identification of barriers and facilitators. The CFIR was developed as a pragmatic, comprehensive and ‘meta-theoretical’ typology. 298 It has 39 factors, which potentially act as barriers or facilitators to implementation, organised into 5 domains (intervention characteristics, outer setting, inner setting, characteristics of the individuals involved, and process). Using the predefined CFIR constructs, we deductively coded data ensuring barriers and facilitators were linked to the best fit CFIR construct. We employed an inductive approach to theme and subtheme development from data that did not fit easily with one of the predefined codes. Definitions of the each of the CFIR constructs are shown in Table 22 (see Appendix 6).
Data synthesis and assessment of findings
Data were brought together in a narrative synthesis, supported by tables and figures, organised around the CFIR five major domains: intervention characteristics, inner and outer settings, individual characteristics and implementation process characteristics.
Results
Description of studies
One hundred and twenty studies were tagged as potentially relevant from previous searches described. From these studies, multiple barriers and facilitators were described across 106 studies. Table 20 (see Appendix 6) summarises the studies reporting barriers and facilities based on the CFIR framework, with a relevant example (where described). Characteristics of included studies are detailed in Report Supplementary Material 3.
We also extracted data relating to participant factors which could result in inequitable access to interventions using the PROGRESS-plus framework. 299 This included extracting data (where reported) related to: place of residence, race/ethnicity/culture, language, occupation, gender, religion, socioeconomic status, social capital; and data related to personal characteristics potentially associated with discrimination (e.g. age or disability).
Six studies (6%) included in this synthesis had a clear and explicit equity focus143,271,282,300–302; 13 studies (12%) partially addressed equity in their study. 24,102,103,145,144,255,261,303–317 Age and sex were the most commonly reported factors; few studies reported any other social determinant factors. Details reported according to the PROGRESS-Plus framework available in Report Supplementary Material 14.
Qualitative synthesis mapped to the CFIR domains
Intervention characteristics
The evidence base (or lack of) behind ‘successfulness’ of the intervention was an important factor. Sixty-five studies described evidence uncertainties and a limited evidence base as a significant barrier to implementation. 63,64,69,74,84,92,102,103,119,132,133,136,143,145,149,153,154,161,174,178,185,203,211,214,232,243,255,258,270,282,302,303,306–337 Evidence was reported as hampered by variation in diagnostic assessment and inconsistent application of CFC definitions, different outcomes, poor adherence, small sample sizes and general lack of high-quality studies.
Conversely, implementation was described as successful in 41 studies61,65,84,91,102,133,143,145,148–151,153,154,178,185,232,243,246,255,270,282,298,303,305,314–317,319,322,330,333,337–343 when the evidence underpinning an intervention was perceived as ‘credible’ or built on a strong evidence base resulting in a confident clinical message being conveyed. 4–6,8,11,12,15,18–21,23,24 For example, Hankinson (2018) states that
In addition to providing efficacious care, the multidisciplinary model may also help correct the presumption that childhood constipation, and incontinence should be viewed as primarily a medical or behavioral problem. This message may be given to parents of children with constipation and incontinence issues when there are actually many medical issues that may be contributing … the myth that this is solely a physical health or behavioral condition is implicitly debunked. Both of these treatment modalities have empirical support based on previous literature addressing constipation and faecal incontinence (p39).
Intervention adaptability and flexibility was described critical to successful implementation in 38 studies. 61,65,84,92,102,132,143,145,148,149,151,154,178,203,212,215,233,270,282,303,306,314,317,322,324,325,328,331,339,344–350 Nineteen studies24,130,133,149,179,186,246,268,299,303,308,309,312,319,323,326,332,336,342 described interventions that could not be adapted, for example medications with a specific dosage or delivery route, and/or had significant side effects.
Interventions or services that could be tailored to meet the needs of the child and/or family circumstances were more likely to be implemented. Tailoring the intervention to the child and/or family included:
-
multidisciplinary input to provide ‘bespoke’ interventions;65,282,303
-
mode of delivery, for example home-based versus more traditional outpatient clinic based on children and family’s needs;65
-
modifying or planning alternative approaches. 61,65,84,92,102,132,143,145,148,149,151,154,178,203,212,215,233,270,282,303,306,314,315,317,322,325,328,331,339,344–350
Intervention adaptability was often linked to the complexity of the treatments. Interventions which were perceived as simple (i.e. did not involve a lot of steps), not too time-consuming or disruptive to daily life were seen as a facilitator. 63,103,143,149,178,243,294,300,306,314,323,331,337,339,351 These interventions were commonly available, for example foods that could be bought at a supermarket to improve fibre intake in a child’s diet or being able to access the intervention over the counter or at a health food store. Attending appointments was another ‘simple’ intervention as described by Hankinson (2018) ‘the combination of medical and behavioural treatments in a single visit has the potential to address multiple detrimental symptoms associated with constipation in a concise and accessible manner’.
Stakeholders’ perception of what improved as a result of using the intervention compared with an alternative solution was identified as both a barrier and facilitator. This ‘relative advantage’ was reported as a barrier in 33 studies24,74,84,132,154,162,178,247,301,302,306,308,312,314,315,319,321,322,328,330,331,337,343,348–350,352–356 and a facilitator in 47 studies. 24,61,65,102,103,133,148,149,153,154,161,167,145,132,136,143,178,232,243,247,255,294,306–310,313,315,319,322,323,327,328,330,331,334,337,341,348–350,353–355,357,358 Studies where the intervention was perceived to have more severe side effects or adverse events as a consequence of the intervention were more frequently reported as having less of a relative advantage. Studies also weighed the relative advantage based on the target population. For example, additional caution was advised when a child who had additional needs was using the intervention, as described in Romano (2018) who states that ‘reports of severe pneumonia due to aspiration of laxatives and therefore use of macrogol or liquid paraffin must be provided with caution in children with NI [Neurological Impairment] with high risk of aspiration’ (p255). Studies describing fewer side effects or adverse events were frequently reported as having an advantage and were selected for implementation (see Appendix 6, Table 20).
Interventions that were perceived to have good quality, design and packaging of an intervention that fitted the needs of children and their families were reported as a facilitator in 13 studies. 61,65,103,153,162,302,305,313,322,333,341,347 Studies reported better engagement with the intervention when the children and families were able to ‘enjoy themselves’, or ‘go at their own pace’ or used innovative approaches (e.g. video games or internet resources) to receive information. Studies also reported the importance of supporting children and families emotionally, reducing the isolation that many families felt when dealing with constipation. For example, Sullivan (2006) reported that a nurse-led clinic was successful because of the degree of empathy shown by the nurse prescriber and the quality of the information provided.
Outer setting
The needs of children and young people living with CFC were often unmet or overlooked. This presented a major obstacle in 27 studies. 65,69,135,143,161,246,298,300,302,303,307,309,314,315,319,320,328–333,338,342,344,347,351,359 Studies reported the failure of clinicians to recognise or listen to what families were saying as negatively impacting on implementation. Other studies describe a misplaced emphasis on dealing with the physical symptoms over other relevant factors as observed by Athanasakos (2020) who states that the ‘perceived cause and effect relationship between physical and psychological pathologies was deemed to be less important than correctly diagnosing both and effectively treating both in tandems’.
Other studies highlighted the twin impact of stigma and embarrassment on seeking help for constipation, with parents often confused as to who they should talk to and when. Gaps in knowledge and practices of healthcare professionals and knowing who was responsible for management meant that constipation was frequently undetected or identified too late, compounding the initial problem. As Trajanovska (2020) noted that ‘delays in management may result in patients suffering from psychological and social complications arising from their condition. For families experiencing disadvantage, the need for timely and low-cost care in the public setting is important to ensure that all children receive quality care and that socio-economic disparities do not impact child outcomes’. A lack of specific integrated care pathways for children and young people with disabilities, particularly during the transition from paediatric to adult care, was also highlighted.
Multiple studies described the importance of working in partnership with families, children and building close collaboration with other involved agencies (e.g. school/educators) to overcome many of these barriers. Several examples included encouragement of parents, building their confidence in delivering interventions or providing opportunities for children and young people to improve their own self-management (where possible). Many of the examples cited in these studies are closely tied to the constructs of self-efficacy and adaptability discussed earlier. As stated in O’Connor (2012) ‘You have to get the family on board and ask them what is going to work for their child and come up with such a plan’ (p1).
Seventeen studies described a lack of external policy and/or incentives as a barrier to implementation. 24,63,65,148,149,185,314,316,319,330,331,333,337,350,356,359 Studies commented on the lack of guidance or consensus about what constitutes a ‘normal’ bowel motion particularly in younger children (Eicher, 2006). Other studies observed that the complexity of constipation is not fully appreciated as Mallon (2015) states that ‘constipation involves more complex physical and behavioural components and lacks a straightforward objective measure of severity’ (e1304). Studies highlighted the real-world challenges of using the ROME criteria for the diagnosis of CFC in clinical practice. Yang (2013) argues that ‘given the lack of familiarity with NASPGHAN guidelines, finding ways to increase awareness of these guidelines among pediatricians would be beneficial and likely increase consistency in management’.
Inner setting
Culture was identified as a barrier to successful implementation in nine studies,24,69,148,330,347,360–362 and is closely linked to an earlier construct – child’s needs and resources. The taboo nature of constipation and the reluctance of children, parents, healthcare professionals and wider society to openly engage in discussion about the subject negatively impacts on the implementation of interventions. Studies cited examples of families delaying help because of the stigma – hoping that it will resolve spontaneously. The culture of ‘not talking’ about constipation was identified across all levels of healthcare provision; from individual healthcare professional across the wider organisation; and was mirrored by the limited attention that constipation receives at the level of public health. A recent publication from a multidisciplinary group highlighted the costs of constipation, arguing that awareness of constipation urgently needs to be escalated to ‘encourage increased understanding of the condition and start implementing solutions that can begin to alleviate the problem’ (Cost of Constipation, p3). 277
Supporting clinicians caring for children and families who are living with CFC by providing a suitable climate to deliver a seamless ‘integrated’ service was identified as a facilitator in eight studies. 65,69,149,246,282,294,347,362 This frequently involved the vertical and horizontal integration of services involving the collaboration of professionals across disciplines. This was supported by good communication and strong networks described in two studies. 294,362
Another important consideration in the success of an intervention was how compatible the intervention was with the needs of the child. Poor intervention compatibility, as a result of poor tolerance of the intervention was reported in five studies as a barrier. 103,151,243,246,305 Conversely, interventions that were perceived as compatible were described as successful in 11 studies and could result in an improved QoL. 65,102,149,143,243,302,303,325,328,332,359 This frequently involved making sure that the right professional was available at the right time as highlighted by Hankinson (2018) ‘Having a nurse practitioner- and psychologist-led clinic allowed for patients to be seen more frequently (as frequent as monthly) to thoroughly evaluate medical symptoms and adherence to medical and behavioral treatments’ (p10). 143
Understanding the tension for change (i.e. why clinicians and families felt that the changes were needed now) was identified as a facilitator in 19 studies. 61,65,103,148–151,153,143,178,294,303,306,331,348,359,363,364 In most of these studies, it was the failure of treatments to improve CFC which acted as a trigger for clinicians and/or families to consider other treatment options as noted by Eicher (2007) ‘We realized we needed to take drastic measures to get him help’ (p2). 359 Sometimes the driver for change was having the expertise on board to push forward change as O’Connor reported that ‘When United Kingdom specialist paediatric HV and an expert in bowel management in children, Jackie Wade, came to work on the ward, she provided Smith with the impetus and the necessary practice guidelines to make a case for a specialist nursing service’ (p1). 347
The readiness for implementation was described as a barrier and facilitator. Several studies74,305,307,319,330,333,356 highlighted the lack of access to knowledge and information within and across multiple organisations as a barrier to readiness for implementation. Torres (2015) pointed out that ‘knowledge gaps regarding pediatric constipation may be a worldwide problem that should help in educational planning for pediatric residency programs and the pediatrics curricula of medical schools’ (p78). 333 The family role was also highlighted here as ‘the desire for information about their child’s care is usually very high. In some cases, families do not believe that their doctors are providing them with all the information they want or need’ (p646). 305 Improving access to education and knowledge about ‘multimodal’ CFC treatments and raising the awareness of the complex nature of CFC across physical, psychological and sociological factors as well as including families in decision-making was reported as facilitator in six studies. 63,305,328,330,333,342 Yang (2014) argues that we need to ‘… encourage pediatrics and GI societies to establish simple guidelines to be implemented during the course of medical school education’ (p78). 354
Limited available resources including expertise (behavioural and medical), capacity and workload were all cited as impeding implementation in five studies. 145,305,307,333,347 Trajanovska (2020) stated that ‘… most significant obstacle in the provision of timely management for constipation, a relatively low morbidity chronic condition, is wait time. Several factors may contribute to increased wait times, such as high volumes of incoming referrals, referral management and acceptance parameters, triage and scheduling procedures and prioritisation based on severity’ (p301). 307
Seven studies highlighted the importance of adequate resourcing for implementation success. 56,61,145,307,344,346,347 This included ensuring equipment was provided and schools having access to private bathrooms for children to use uninterrupted. Other studies commented on the benefits of redesigning care pathways to maximise available resources which often included streamlining referral and triage processes.
Characteristics of individuals
The ability to engage in the intervention and ensure successful implementation was cited as a barrier in 14 studies. 149,151,154,185,270,300,305,322,323,328,331,361,365,366 Multiple reasons for a lack of compliance or adherence with the intervention were cited across studies and included:
-
child’s refusal to take the medication305,322 because of taste167,361 or adverse events;302
-
lack of time to deliver the intervention305,323 or failure to prioritise treatment ‘I forgot’;302,305
-
financial difficulties;302
-
Family concern about safety and side effects of drugs. 365
Self-efficacy was reported as a key component to the success of implementation in 33 studies,61,69,103,136,143,151,153,161,167,178,185,203,215,244,255,282,394,302,305,306,309,318,319,322,328,333,341,345–347,349,350,360 with several studies reporting innovative strategies to overcome barriers to compliance and improving treatment adherence. These included:
-
provision of individualised easy-to-follow instructions detailing treatment protocols to families;69,136,178,203,255,294,347
-
involving family in the decision-making process;350
-
sending e-mail reminders;305
-
increasing knowledge and improving understanding61,102,333,350,365 [e.g. providing tailored education demonstration of the intervention to the parent and child using of models, visuals (e.g. Bristol stool charts)];136,203
-
normalising the use of any devices needed to improve CFC by encouraging the use in everyday circumstances;185
-
use of bowel diaries;69,153,143,313,318,319,322,328,345,346,360
-
disguising the taste of medications to make them more palatable;341,349
-
supporting the use of more complex interventions178 by means of facilitating home management179,292 and/or free samples to improve compliance;294
-
involving other key stakeholders (e.g. school). 333
Eight studies identified knowledge and beliefs about the intervention as a barrier. 136,307,320,342,343,360,365,367 Studies described a lack of knowledge about current constipation guidelines and what recommendations are for optimal treatment and care plans; a lack of agreed terminology and definitions were also mentioned as a barrier, with one study reporting that how constipation is defined was often a limiting factor in it being operationalised in a clinical setting. As a result, studies reported that care providers relied on other diagnostic measures rather than adopting the clinical features described in the ROME criteria.
A lack of knowledge by some providers also provided a very real barrier in accessing timely care or referral which could exacerbate the condition as Modin (2018)136 stated ‘varying therapeutic approaches, with some children receiving inappropriately short or long treatments, which may cause maladaptation to toilet routines and create unnecessarily complex and costly situation’.
Several studies argued that raising awareness of guideline recommendations and developing clearer treatment protocols would reduce the variability in practice and improve the management of CFC. Studies also called for a clearer and simpler message about treatment. Other studies took a more pragmatic approach, calling for healthcare professionals to start a conversation and actively look for opportunities to engage with, and listen to families, so that the problems with constipation were not dismissed or overlooked. 347
‘As healthcare professionals, I think we have a responsibility to have this conversation with people as often as possible, as a poor functioning gut can have such a negative impact on wellbeing. Everyone should pay attention to their bowel habits to know what their ‘normal’ is and when something is not quite right – and, yes, that means turning around to see what it looks like!’ (p32). 296
Personal (individual) characteristics of healthcare professionals were described as barriers to implementation in seven studies. 167,300,302,320,334,350,365 Some studies argued that healthcare professionals were reluctant to change their habits, particularly their prescribing, or lacked motivation to deal with CFC. Several studies also described the unique challenges of timely diagnosis and clear communication from professionals to families about the importance of regular medication adherence in children. These communication challenges left particularly vulnerable children at a greater risk of having issues with CFC. 350
Process
The involvement of informal champions, such as a parent or carer, or a more formal champion, for example, a nurse or another clinician, was frequently reported as facilitating implementation. 61,63,92,102,136,143,145,148,150,151,154,161,185,204,232,282,294,305,311,317,333,334,346,347,350,359,363,365 Family involvement was reported in these studies as critical for the successful implementation. Parental involvement in their role as a ‘champion’ was extensive and included completing questionnaires, providing detailed histories, attending appointments (remote and face to face), documenting symptoms and recording information about their child’s bowel motions, delivering interventions and increasing input into decision-making about their child’s treatment. Formal ‘champions’ had roles in supporting children and young people, by building their confidence and self-esteem and did this in a variety of ways. For example, the establishment of a code word or signal was reported in one study, so that a child could indicate that they needed to leave a classroom.
We noted that there were little data reported about the process of planning for implementation, or the opportunity for reflecting and evaluating within studies. Some studies touched on the value of forward management planning, but only one described this in detail, highlighting the importance of consultation with all of the involved professionals as successful treatment for constipation is often protracted. 346
Stakeholder reflections
Members of the SG have provided the following reflections, in their own words, on the evidence relating to barriers and facilitators to implementation:
-
The results and conclusions tallies with my own experiences that one of the best facilitators in dealing with constipation at any age is self-efficacy and that one of the greatest barriers is lack of understanding from those around oneself. The conclusions, therefore, do not surprise me but it does sadden me that we are still having to talk about things that are barriers to children and their families managing constipation issues.
-
PPI is difficult to incorporate in the Implementation stage of the research cycle so I commend the research team in drawing us in to these discussions. It has been quite emotional at times, whether we were speaking of our children or recollecting historic experiences from our own childhood. Attitudes and perceptions of parents are always going to be important. I believe my mother ‘normalised’ my constipation. This may well have contributed to psychological and emotional issues, lasting well beyond childhood.
-
Factor like deprivation need to be considered. We lived in a mining community in which there were pockets of deprivation. This may be another factor why I ‘did not do well’.
-
Understanding variables that affect real world implementation allows some chance of improving the outcome of patients and reduced research waste. The use of a framework to do this clearly makes the approach more likely to be rigorous and the items included feel intuitively correct. Traditional researchers may consider ‘how to actually deliver care to optimise outcome’ as service development or quality, but it is just as important as understanding pathophysiology.
-
It’s really good to work with a group that recognise the importance actually improving outcomes in healthcare. Politik (at the interface of policy and politics) and phroenesis (practical wisdom) have existed as concepts outside healthcare for considerable periods of time and I wondered if implementation science is similar.
Summary
Evidence relating to barriers and facilitators highlights that management of CFC is multifactorial and involves a complex interplay of physical and psychosocial factors. Successful delivery and implementation of these multicomponent strategies require strengthening of the current evidence base and successfully imparting this knowledge to everyone who is involved. Parents and families have an important role as informal champions. Studies have highlighted the need to ‘shine a light’ on constipation, encouraging discussion about constipation and raising the profile of constipation so that there is greater awareness of this common problem.
Chapter 10 Integration of findings
Introduction
This review project has worked in partnership with a SG (see Chapter 3) to conduct a series of reviews, employing a pragmatic mixed-method approach. This has included completion of a broad, comprehensive scoping review (see Chapters 4 and 5) and a series of focused SRs focused on effectiveness (see Chapters 6 and 7), economics (see Chapter 8) and implementation (see Chapter 9). The aim of the final stage of our project was to integrate findings from this review project, using innovative technology designed to support knowledge translation, and work with our SG to clearly identify implications from the synthesised evidence. Section Logic model presents the logic model which is central to the structure of the evidence syntheses conducted within the SUCCESS project, and to the integration of findings. Section Interactive evidence maps presents the interactive evidence maps which bring together our key findings, providing an accessible, systematic summary of evidence, and Section Evidence gaps describes how evidence gaps have been systematically identified.
The NIHR commissioned call that supported this project specifically sought an evidence synthesis that would enrich and enhance existing guidance. 1,18–20 In order to clearly identify clinical implications arising from the findings of this project, it was considered important to systematically compare the implications supported by our findings with those stated within previously published guidelines. Section Complementarity of results of evidence synthesis and current guidelines provides a comparison of our findings with key guidelines and Section Integration of findings: evidence of effectiveness provides an integrated narrative summary of the findings.
Logic model
A key activity of the SG during the SUCCESS project was to develop a logic model. The iterative process of developing the model is described in Chapter 3 and Report Supplementary Material 1. The development started with the SUCCESS Pyramid (see Figure 2), and this has been central to the evidence syntheses conducted within the SUCCESS project, and the presentation of findings from the project. The stakeholders agreed that a traditional ‘linear’ or ‘flow diagram’ logic model did not enable the complexity of management of CFC to be captured. This led to development of a circular model, with the child placed at the centre (see Figure 27). Circles were used to illustrate that interventions for a child with CFC are usually delivered in a step-wise, or cumulative, way, within different models of service delivery, across Level 0, 1, 2 or 3, and potentially incorporating complementary therapies and/or psychosocial interventions. A child’s journey through these levels of interventions will be unique and may not be a simple journey of 0–1 to 2–3, but may involve steps ‘down’ as well as ‘up’. Layered on top of the circles were factors relating to implementation, aimed at reflecting that every child is an individual, and that the success of CFC strategies may be affected by individual characteristics, and the characteristics of the inner and outer setting. Details of factors affecting implementation of interventions identified from our evidence synthesis are illustrated; these are fully described in Chapter 9.
FIGURE 27.
Logic model.
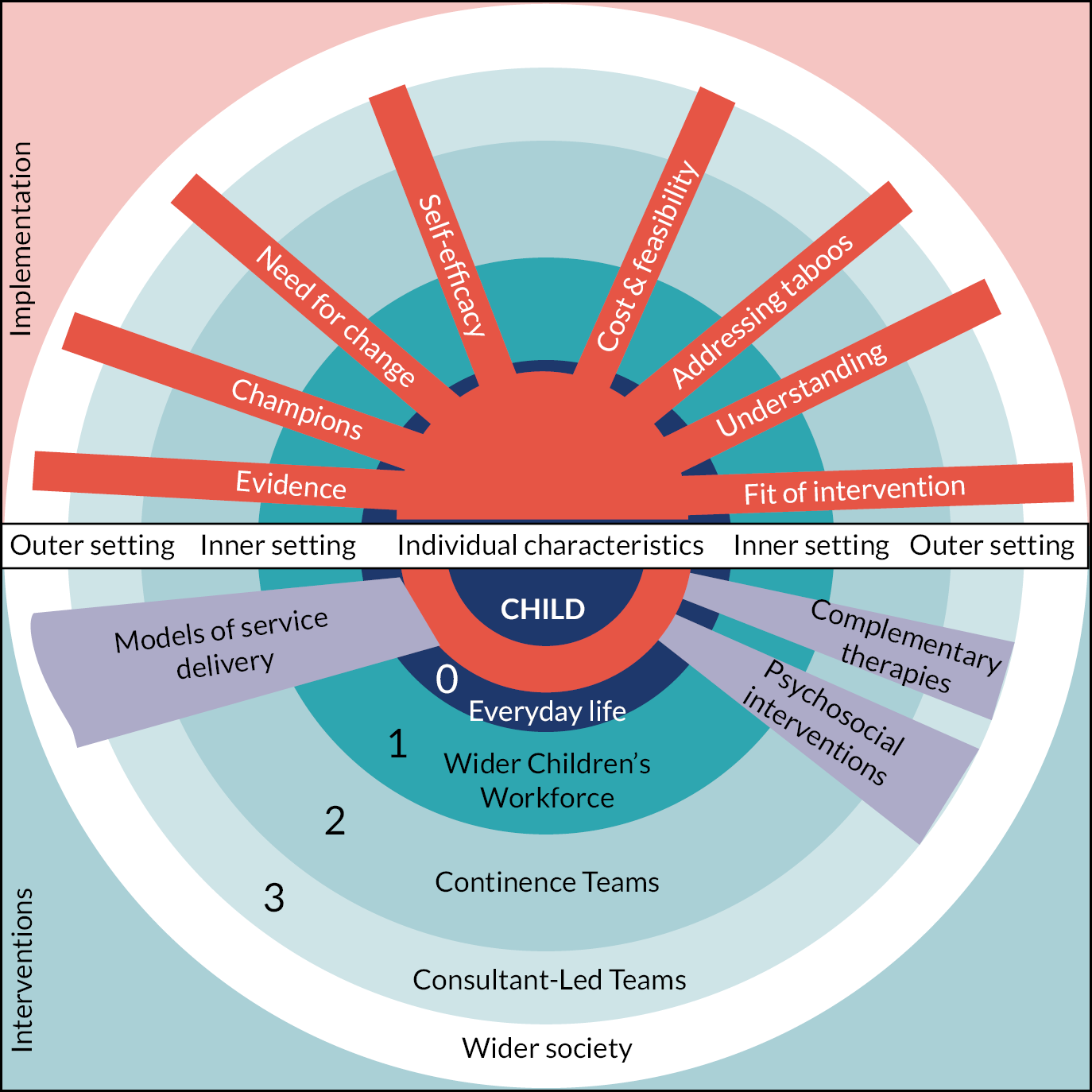
Stakeholders attempted to find ways to incorporate ‘problems’ and ‘outcomes’ into this model, but ultimately came to the conclusion that further work was required to explore this further. The different iterations and key points discussed are presented in Report Supplementary Material 1. In agreeing the final model to be presented in this report, stakeholders concluded, that substantial further work was required to be able to come up with a final model:
there are gaps in terms of knowing what the key outcomes are, we have a really complicated system, there is a massive evidence gap … we don’t know … there are loads of gaps still … loads of questions …
The challenges of capturing the complexity of management of CFC was central to discussions relating to the final model:
I am new to the concept of a logic model but it strikes me that if you put too many things in you are trying to simplify it so that it’s in one graphic representation … it can’t easily be summed up in this way … you don’t want to get carried away with the idea of a logic model encapsulating everything, because it won’t
The final model, and discussions involved in the development of this, and exploration of other iterations, contributed to the final integrations of findings, as described in subsequent sections.
Interactive evidence maps
Planning the evidence maps
To bring together findings from the SUCCESS project and to support knowledge translation, we used interactive digital maps to summarise evidence and highlight gaps. The SG had a key role in informing the content and structure of the maps. Draft maps were presented at a number of SG meetings (see Appendix 1) and feedback gained on optimal format to maximise accessibility of information. The structure of the evidence synthesised within SRs of effectiveness was informed by the SUCCESS Pyramid (which later developed into the logic model), which consequently informed the decision to create separate evidence maps for each of the different evidence syntheses, corresponding to the structure of the logic model. In order to provide a summary of evidence relating to effectiveness we therefore created evidence maps for evidence relating to models of service delivery, Level 0, 1, 2, 3 complementary therapies and psychosocial interventions.
Consideration was given to which types of information would be most useful to support clinical decision making and knowledge translation. Each of the separate evidence syntheses had been structured around a series of questions, and it was considered important to retain these within the development of the maps. A range of information in this evidence was considered including volume, study design, methodological quality, statistical evidence of effect/harm and judgement of certainty. Informed by discussions with stakeholders, the decision was made that key domains to include in the map should relate to the concepts of ‘does the treatment improve outcomes for the child?’ and ‘how confident are we in this finding?’ Further, it was important that the map provided users with the ability to ‘dig’ into the evidence, providing information about the types of research study that had been conducted and access to the references to these studies (‘… you can get to the source data and you feel respected ….’).
Production of interactive digital evidence maps
We used visual analytics software (EPPI-mapper) to produce the interactive digital evidence maps summarising evidence and highlighting gaps. In order to create focused maps summarising the evidence of effectiveness, data were coded so that:
-
Interventions and related questions were key column headings.
-
Quality (or certainty) of evidence were column subheadings.
-
‘Findings’ were row headings; based on the evidence statements supported across the evidence syntheses, the categories of ‘may be beneficial’, ‘may be harmful’, ‘may have no effect (no benefit or harm)’, ‘mixed findings’ and ‘no clear findings’ were used.
-
Types and number of studies were included in the cells, with access to study references. Type of studies was categorised into three broad groups of ‘systematic review’, ‘RCT’ and ‘primary study’. The category of primary study includes all non-randomised trials and studies of other design. Information about the study design was provided in Chapter 7.
Focused evidence synthesis maps: evidence of effectiveness
The interactive evidence maps are available here: www.gcu.ac.uk/success.
Evidence gaps
The SG was central to the identification of evidence gaps, where interventions may be used, or which could potentially be beneficial, but for which there was no evidence identified. Evidence gaps were identified in a number of different ways throughout the project. The intervention taxonomy used in the production of the broad overview of evidence and evidence gaps (scoping review map; Chapter 5), ensured that identified evidence was mapped against all possible interventions (as identified by stakeholders), so that interventions for which there was no evidence could be identified as evidence gaps. The draft findings of each of the focused evidence syntheses were presented to the SG and SG members specifically asked if there were any gaps/missing questions (see Stakeholder activities, Activity 4a). A list of collated evidence gaps was circulated to stakeholders towards the end of the project, who had an opportunity to add further questions, either in writing or during a SG meeting. The final list of evidence gaps is provided in Report Supplementary Material 15.
Stakeholder reflection on these evidence gaps highlighted a number of key issues. Firstly, the mechanisms for generating evidence gaps resulted in evidence gaps which often related to specific, single interventions as this reflected the nature of the research studies. The stakeholders agreed that there was a need for a comprehensive research prioritisation process: ‘this needs to go out for a much wider consultation and involve everybody’. A number of wider RQs were considered relevant across all evidence gaps. These included questions about delivering the ‘right intervention, in the right way, to the right child at the right time’ and questions about how to capture this within well-designed research studies. Broad topics which the stakeholders considered priorities for future research related to (1) recognition of CFC, (2) information provision for families/carers, (3) dietary considerations, (4) laxatives and combinations of laxatives, (5) behavioural interventions, (6) other adjuncts which could be delivered in combination with ‘standard’ care and (7) education of health professionals. Broad questions about optimal service delivery, including when, and how, children should be referred from Level 1 to Level 2 or Level 2 to Level 3, were also raised as important.
Complementarity of results of evidence synthesis and current guidelines
Current guidelines
Our SRs were designed to build on the evidence that contributed to the recommendations within current clinical guidelines. 1,18–20 In order to enhance interpretation of our findings, we systematically explored the complementarity of results of our evidence syntheses and current guidelines. 1,18–20
Methods of comparing results of evidence synthesis with guidelines
We produced a ‘convergence coding matrix’368 to display the key conclusions of the evidence synthesis and any related recommendations from current guidelines1,18–20 within a table. We considered levels of complementarity between each conclusion from the evidence synthesis and any related recommendations from the guidelines, applying categories of:368
-
‘agreement’, where the evidence synthesis conclusion was in agreement with the Guideline recommendation(s);
-
‘partial agreement’, where the evidence synthesis conclusion was partly in agreement with the Guideline recommendation(s);
-
‘dissonance’, where the evidence synthesis conclusion differed from, or conflicted with, the Guideline recommendation(s);
-
‘silence’, where the evidence synthesis conclusion was not addressed within the Guideline recommendations, or vice versa.
Complementarity between evidence syntheses and guideline recommendations
The matrices illustrating the complementarity between the results of the evidence syntheses relating to evidence of effectiveness are available in Report Supplementary Material 16. For the majority of the RQs that our syntheses addressed there was agreement or partial agreement (often classed as ‘partial’ due to some subtle differences in wording) with current guidelines, although many questions in our evidence synthesis were not addressed (‘silence’) within current guidelines. For a small number of questions, we identified some dissonance between statements or recommendations within current guidelines and our evidence synthesis. These included:
-
The NICE (2017) guidelines:20 state that ‘limited recent evidence for dietary interventions suggests that fibre can improve constipation’. It is unclear whether this relates to ‘sufficient’ fibre, as part of a balanced diet, or ‘additional’ fibre. Our evidence synthesis concluded that there is no new research evidence, and no evidence which supports additional fibre intake for children with CFC.
-
Current guidelines do not recommend the use of biofeedback. Our evidence synthesis identified that biofeedback may be beneficial for the subgroup of children who have abnormal defaecation dynamics.
-
NICE (2010) guidelines1 state that rectal medications should only be used for disimpaction if all oral medications have failed. Our evidence synthesis suggests that enemas and suppositories may be suitable alternatives to oral laxatives for some children, and further research is merited.
-
Current guidelines highlight an absence of RCT evidence relating to alternative therapies and recommend against the use of these therapies. Our evidence synthesis has identified a number of RCTs which suggest that some complementary therapies may be promising and warrant further investigation. This includes abdominal massage and CTM for children with cerebral palsy and use of some herbal medicines.
-
Current guidelines18 do not recommend multidisciplinary treatment. Our evidence synthesis provides some low certainty findings that multidisciplinary bowel management programmes may have a beneficial effect on outcomes.
Integration of findings: evidence of effectiveness
Based on the data described in previous sections, an integrated narrative summary of the findings relating to evidence of effectiveness for interventions for CFC is presented below.
Models of service delivery
Interventions which may be beneficial
Our evidence synthesis highlights some interventions which may be beneficial. These include:
-
Nurse-led clinics are feasible and could result in equivalent (or possibly better) outcomes than traditional physician-led clinics. We have very low certainty in this finding. Current guidelines do not make any recommendations relating to nurse-led models of care (but recommend further research to investigate effectiveness).
-
An algorithm, or care pathway, used in primary care settings to guide the management and referral of children with constipation (including children with ASDs) may be beneficial. We have very low certainty in this finding and further research is required. The European Guidelines18 contains algorithms for the evaluation and treatment of children with CFC.
-
Specialist (Level 2) services may have a beneficial effect on outcomes, but we have very low certainty in these findings and further research is required. The specialist services investigated included specialist dietitians, psychologists, physiologists and teams providing specific, generally multidisciplinary, bowel management programmes, including elements such as laxatives, diet and toilet training. NICE guidelines1,2,19,20 refer to combined treatment approaches. There is disagreement with European guidelines18 which do not recommend multidisciplinary treatment.
-
Web-based information, following an appointment with a specialist, may be more beneficial than a phone call or no follow-up. We have low certainty in this finding, which is based on a single RCT. Current guidelines do not specifically recommend use of web-based information.
Evidence gaps
We found studies which investigated the effect of models of primary care services and the use of a constipation care pathway/algorithm within EDs, but limitations in the evidence meant that we were unable to reach generalisable conclusions.
We found no evidence which addressed questions relating to the key components and features of an effective team involved in care of children with CFC, or how a child’s ‘journey’ through the different levels of care could be made as seamless and direct as possible.
Interventions delivered by carers, prior to healthcare professional involvement (everyday life/Level 0 interventions)
Interventions which may be beneficial
Our evidence synthesis highlights some interventions which may be beneficial:
-
A cow’s milk-free diet may be beneficial (but our certainty in these findings is low). This is in partial agreement with NICE (2010) (CG99, 1.5.5)1 which recommends that ‘in children with idiopathic constipation, start a cow’s milk exclusion diet only on the advice of the relevant specialist services’. Further research into which children should be considered for a trial of a cow’s milk-free diet, the best way to implement this, the advice and information required by parents to do this safely, and the role of specialist services (and how parents can access this) is required.
-
Education interventions for parents may be beneficial, particularly web-based interventions focused on education around toilet training (but our certainty in these findings is very low). This is in partial agreement with NICE (2010) (CG99, 1.5.4 and 1.8)1 and NICE (2019)22 which recommends information and advice for parents and children, and in agreement with European Guidelines (2014)18 which recommends (based on expert opinion) guidance on toilet training. Our evidence synthesis highlights that there is a gap in knowledge relating to the optimal content and mode of delivery of information to parents.
-
Selenium supplements may be beneficial (very low certainty). The current guidelines do not make any recommendations relating to use of selenium. Further research is warranted.
Interventions which may not have an effect
Our evidence synthesis identified that the following interventions may not be beneficial:
-
Probiotics (moderate certainty of evidence). These findings are in agreement with current guidelines18 which do not recommend routine use of probiotics.
-
Additional dietary fibre (low certainty of evidence). There is some dissonance with the NICE (2017)20 guidance which states that ‘limited evidence for dietary interventions suggests that fibre can improve constipation’, but agreement with other guidance1,18–20,22 which recommends ‘adequate’ fibre, but not fibre supplements or unprocessed bran. Recommendations for daily fibre intake in children are available369,370; however, research to clearly define ‘adequate’ fibre for children, and to provide parents with this knowledge in a way which is accessible and understandable, is required. Future studies should consider the severity of constipation, and whether additional dietary fibre provided within home settings (i.e. Level 0) may benefit children with mild symptoms.
-
Increased fluid intake. This finding is in agreement with current guidance, which recommends ‘adequate’, but not extra, fluids. 1,18–20,22
Evidence gaps
Our synthesis demonstrated that, although there have been some studies, there remains insufficient evidence to support conclusions relating to different milk formula, sugars, other/alternative dietary intake (such as cassia fistula emulsion, paraffin oil and magnesium-containing mineral water) and combined dietary and behavioural interventions as an alternative to laxatives. No recommendations relating to these interventions are made within current guidelines.
There were no studies identified, and therefore an absence of evidence, relating to a number of topics. These included gaps relating to diet (e.g. breast milk, gluten-free diet, alternatives to cow’s milk), physical activity and exercise, optimal content and format of information and support for children and families, recognition of constipation by children and families, and continence-related equipment and products.
Assessment and intervention by primary care services (Wider Children’s workforce/Level 1 interventions).
Interventions which may be beneficial
Our evidence synthesis highlights some interventions which may be beneficial:
-
Laxatives may be beneficial and are recommended by clinical guidelines. Our update of evidence relating to laxatives does not change the conclusions that can be drawn about laxatives and is in agreement with current guidelines. Our updated analysis re-states the benefit of PEG compared with placebo and with lactulose. However, it should be noted that this finding remains limited by the lack of new head-to-head evidence across multiple treatments, lack of evidence relating to effectiveness and safety of some laxatives, and lack of evidence relating to effectiveness of combinations of laxatives.
-
Physical exercise focused on pelvic floor muscles may improve overall symptoms, defaecation frequency and stool consistency (low certainty of evidence). This is in partial agreement with current guidelines which recommend ‘daily physical activity that is tailored to the child ….’ (NICE, 2010, CG99 1.5.6)1 and normal physical activity levels. 18 Our synthesis identified limited evidence which supports physical exercise, focused on pelvic floor muscles, in additional to normal physical activity. Further research is required to investigate this.
-
A combined pharmacological, diet and behaviour programme may be beneficial for children with and without typical development, including children with ASDs. Current guidelines do not explicitly address combined programmes, or the specific population of children with ASDs. Pragmatic studies to investigate the effect of combined programmes delivered by primary care services are required.
Interventions which may be harmful
Our evidence synthesis identified one trial which suggested that the combination of PEG plus domperidone may be more beneficial than PEG only in children with cerebral palsy. This study does not present data on side effects. There is a mismatch between the aim of the identified study and guidance relating to safety; MHRA advise against use of domperidone in children under 12 years, due to serious side effects. 371
Evidence gaps
There were no studies identified, and therefore an absence of evidence, relating to a number of topics including early identification and diagnosis, behavioural interventions combined with laxatives and information provision.
Interventions delivered by secondary specialist care (continence teams/Level 2 interventions)
Interventions which may be beneficial
Our evidence synthesis highlights some interventions which may be beneficial:
-
Combined oral and enema therapy in hospitalised children may benefit faecal disimpaction, but there is insufficient evidence to reach conclusions about the relative effectiveness of specific regimes. This is in partial agreement with current guidelines which list enemas as an option for disimpaction. 20 Further research is required to explore the optimal regime.
-
Transanal irrigation may be safe, feasible and effective in children who have symptoms which have failed to resolve with conventional treatments. This finding is in partial agreement with the NICE (2018) Exceptional Surveillance21 which concludes that there are ‘promising results’, but further evidence required before the guideline on this topic is updated. Our finding is in agreement with a NICE Medial Technology Guidance372 report which concluded that, while data for children were limited, there was some evidence to suggest TAI may be beneficial for some children. Although we conclude that there is some very low certainty evidence that TAI may be effective, the quality of evidence remains poor and further research required.
-
Biofeedback may be beneficial for children with abnormal defaecation dynamics (very low-certainty evidence). There is dissonance between these finding, and current clinical guidelines which conclude that evidence does not support the use of biofeedback. 1,18 However, how to effectively examine for abnormal defaecation dynamics within clinical practice remains an important uncertainty.
-
Combined treatment programmes, incorporating behavioural therapy with pharmacological, and possibly dietary, interventions may be beneficial (very low certainty), but the evidence is insufficient to support specific conclusions relating to the effective components of a combined programme. Further research is required; however, in exploring combined programmes it is important to consider child-centred care. Further understanding of the pathophysiology of CFC is required in order to develop targeted treatment programmes.
Interventions which may not have an effect
Our evidence synthesis also identified that the following interventions may not be beneficial:
-
Biofeedback for children with normal defaecation dynamics. This is in agreement with current clinical guidance, which does not recommend the use of biofeedback. 1,18 As stated above, how to effectively examine for abnormal defaecation dynamics within clinical practice remains an important uncertainty.
Interventions which may be equally effective
Our evidence synthesis provides some low certainty evidence that promelaxin microenemas may be equally effective to oral laxatives in infants (6–24 months), rectal enemas (dioctyl sulfosuccinate sodium) may be equally effective to oral laxatives, and that a soft suppository (LP101 free fatty acids suppository) may be equally effective to an enema. These findings have some agreement with current clinical guidelines which conclude that oral osmotic laxatives may have a similar effect to rectal enemas but are different from the NICE (2010) guidance1 which states that rectal medications should only be used for disimpaction if all oral medications have failed. Given the barriers to implementation of oral laxatives, further research to establish suitable alternatives to oral laxatives merit further investigation. This could include investigation of efficacy of combined therapies.
Interventions for which evidence is mixed
Our evidence synthesis identified some low-certainty evidence that the addition of regular rectal enemas may increase defaecation frequency in children with severe constipation, but not have any effect on overall treatment success or other outcomes and may cause some discomfort or distress. We found insufficient evidence to reach any conclusions about the relative effect of different types of enemas delivered within an ED. These findings have partial agreement with guidelines which concludes that enemas are an option for disimpaction if all oral medications have failed. 20 The retrospective studies included in our evidence syntheses report use of sodium phosphate enemas; it is important to note that potential serious reactions, including death, have been linked to phosphate enemas. 206 Given that there is currently insufficient research evidence about the relative effect of different types of enemas, these reports support the avoidance of phosphate enemas.
We found mixed findings relating to physiotherapy for children with CFC; current evidence does not support the routine referral to physiotherapy for all children with CFC. Guidelines do not currently make recommendation relating to physiotherapy, and the European guidelines18 state that routine multidisciplinary treatment is not recommended.
Evidence gaps
We found some very-low-quality evidence relating to the effect of TES, the use of physical therapy for children with severe cerebral palsy and dietary exclusion of fructose and lactose, but this was insufficient to support generalisable conclusions. Further research is required to determine whether these interventions have any benefits.
Key evidence gaps arising from where we found mixed, or limited, evidence relate to the relative effect of different interventions. This includes questions such as whether TAI may be an alternative to rectal interventions, and the relative effectiveness of different rectal interventions. There were no studies identified, and therefore an absence of evidence, relating to physiotherapy interventions and the use of tibial nerve stimulation.
Interventions delivered by highly specialist tertiary care services (consultant-led teams/Level 3 interventions)
Interventions which may be beneficial
Our evidence synthesis highlights some interventions which may be beneficial:
-
Botulinum toxin may be effective in the management of CFC (very low certainty). Botulinum toxin is not mentioned in current clinical guidelines,1,18 but the NICE (2018) Surveillance21 does identify the need for further evidence relating to this intervention.
-
ACE/MACE may be safe and effective, but there were high rates of (minor) complications, and our certainty in these findings are very low. This finding is in partial agreement with current guidelines which recommend referring children with unresolved symptoms to specialist centres for assessment of suitability for ACE/MACE.
-
Sacral neuromodulation may be safe and effective, but we have very low certainty in these findings. Current guidelines do not address this intervention.
Further high-quality studies are required to investigate all these interventions.
Evidence gaps
We found some studies which addressed a number of other interventions, but for which the evidence was insufficient to support any generalisable conclusions. These included ACE compared to caecostomy button, ACE compared to SNS, and a range of surgical interventions (including colonic resection, colonic resection combined with appendicostomy, anorectal myectomy, ileostomy, colostomy, colectomy). Current clinical guidelines did not make any recommendations relating to these interventions. There was an absence of evidence relating to long-term implications of surgical procedures, effect of ACE/MACE in children with special needs and manual evacuation.
Complementary therapy interventions
The ESPHGAN guidelines18 state that no RCTs were found and that ‘based on expert opinion, we do not recommend the use of alternative treatments in childhood constipation’. Evidence in this field has changed, and we identified eight RCTs relating to complementary therapy interventions for CFC.
Interventions which may be beneficial
Our evidence synthesis highlights some interventions which may be beneficial:
-
Connective tissue manipulation as a component of a programme for children with cerebral palsy and are receiving physiotherapy (low certainty).
-
Herbal/traditional medicines may improve outcomes in children with CFC, but studies investigate a range of interventions and there is insufficient evidence to reach conclusions about the effect of specific interventions. There is a need for high-quality research studies; these should investigate safety and adverse events, as well as effectiveness.
Interventions which may be equally effective
Evidence from one small RCT suggests that dry cupping therapy of the abdominal wall could be equally effective as laxatives. Further research is required to investigate this finding.
Evidence gaps
We found some studies which addressed a number of other interventions, but for which the evidence was insufficient to support any generalisable conclusions. These included abdominal massage, chiropractic or osteopathic manipulation, reflexology and acupuncture. We found no studies relating to the effect of baby massage on constipation in infants.
Psychosocial interventions
Interventions which may be beneficial
Our evidence synthesis highlights that behavioural therapy techniques delivered by specialist practitioners may be beneficial (very low certainty). This is in partial agreement with the recommendations within current clinical guidelines; however, these are based on expert opinion and the lack of high-quality evidence is acknowledged. The European Guidelines18 recommend behavioural toilet training, but not the routine use of an intensive programme. The NICE (2010)1 guidance recommends referral to specialist psychological support where there is psychological distress.
Evidence gaps
We found studies which compared externalising treatment to other behavioural interventions, and which investigated the addition of psychotherapy to other behavioural therapies, but for both of these the evidence was insufficient to support any generalisable conclusions.
We found no studies which addressed a range of topics, including the best ways to support children and families to deal with the psychological effects of constipation.
Stakeholder reflections
Members of the SG have provided the following reflections, in their own words, on the integration of findings across the project:
-
The process that we have gone through to get to the point that we are at one has been very long and complicated as I am sure anyone who has read this chapter will understand. However, it has been truly collaborative, and I am sure that having such a mixed group as academics and clinicians and mothers of children with constipation and those of us who are adults who had constipation problems as children has made the work we have done and what we have come up with all the richer.
-
Throughout the project, I was keen for the psychological aspect not to be underplayed but to be taken seriously – whether at diagnosis, or ongoing psychological problems persisting throughout childhood or connotations with building personal relationships in teenage years and beyond. I am pleased to say that our discussions have covered all of these issues.
-
The process of reflecting essential concerns and processes in a comprehensive but also coherent logic model has been illuminating for me as a parent of a disabled child with CFC, and with our own experiences of parts of the services and interventions on offer. Finding the balance between comprehensiveness and coherence has been a considerable effort, and I’m gratified that the result fairly finds this balance, and that the contributions of the non-specialist stakeholders have been taken very seriously.
-
NIHR have commissioned this piece of work with the intention of improving the outcomes for a large number of children and their families, many of whom struggle for long periods with embarrassing symptoms. This work usefully demonstrates known knowns, but equally importantly, highlights some of the known unknowns. There is potential to improve a huge amount of morbidity and of course resource use with updated guidance that includes service design. Additionally, with support from the correct research organisations, carefully constructed research combining basic science, clinical research and implementation science may help solve the patient-important problems and make research activity better aligned.
Summary
This chapter has brought together the key findings from the SUCCESS project. Key findings are structured according to the logic model developed by the SG. The identified evidence of effectiveness has been summarised within evidence maps and compared with current guidelines. Evidence gaps have been collated and presented. These findings are discussed in Chapter 11.
Chapter 11 Discussion and Conclusions
Introduction
Every year up to one-third of children experience constipation, causing pain, embarrassment, significant stress and anxiety among children, their parents, carers and families. 373,374 Current national and international guidelines1,18–20 recommend laxatives as ‘first line’ treatment but conclude that there is insufficient research evidence to support treatment decisions for other interventions, including dietary and lifestyle, behavioural and provision of information and advice. The SUCCESS project worked in partnership with stakeholders to bring together the most up-to-date evidence relating to childhood CFC and answer the question ‘What are the most effective interventions, and combinations and sequences of interventions, for childhood CFC, and how can they best be implemented?’.
Summary of findings
Current evidence
Our scoping review identified 651 studies relating to management strategies for childhood CFC; 29% of these were RCTs and 36% studies with other primary research designs. One-third of the studies identified by the scoping review were evidence syntheses [i.e. narrative review (n = 140) or SR (n = 71)]. Relevant studies were included in a series of focused evidence syntheses which addressed effectiveness of interventions (n = 145), cost effectiveness (n = 31) and factors affected implementation (n = 106). Our focused evidence syntheses identified a number of interventions which may be beneficial (see Integration of findings: evidence of effectiveness), and summaries of this evidence can be accessed through a series of interactive evidence maps (see Interactive evidence maps). A small number of differences between the results of our evidence syntheses and current guidelines were identified (see Complementarity between evidence syntheses and guideline recommendations), but our findings were generally in agreement with the majority of guideline recommendations.
We found no evidence which we considered to give us high certainty in the findings; and only had moderate certainty relating to one intervention (probiotics, with evidence demonstrating that probiotics may not have any beneficial – or harmful – effect). While there is evidence which supports the use of laxatives, there is a lack of high-quality trials, and further research is required to explore relative effects of different laxatives and combinations of laxatives. Our certainty about all other findings was low to very low or, in many cases, we judged that the evidence was insufficient to support any generalised conclusions. The current evidence base is seriously limited by small, often poorly reported, studies, which rarely measure outcomes of highest priority to children and families, and many studies fail to describe the complex nature of the treatments that a child may be receiving. This limits the conclusions that can be made from the current evidence. Further, the limitations within the evidence base reduce certainty in recommendations and create a barrier to implementation of best practice.
A summary of evidence of effectiveness of interventions and evidence gaps is provided in Chapter 10. We highlight and discuss the key findings from the SUCCESS project here below.
Management of childhood chronic functional constipation is complex
Management of CFC is multifactorial and involves a complex interplay of physical, and psychosocial factors, including the personal characteristics of the child and the environment in which they live. Our review identified that factors affecting implementation for CFC included:
-
the evidence base (or lack of) behind the ‘successfulness’ of the intervention;
-
whether the intervention was adaptable, flexible and offered an advantage over an alternative solution;
-
understanding the tension for change (i.e. why clinicians and families felt that the changes were needed now);
-
the taboo nature of constipation and the reluctance of children, parents, healthcare professionals and wider society to openly engage in discussion about constipation;
-
a lack of understanding of what children, young people and their families need;
-
self-efficacy, coupled with individual knowledge and beliefs, which were important facilitators;
-
engagement of champions to support children and families.
Recognising this complexity is essential to enhance quality of healthcare provision and research in this field. Future research into any interventions for childhood CFC should take into account relevant evidence relating to the development and evaluation of complex interventions. 23 There will not be a ‘one size fits all’ approach to management and, while treatment algorithms and care pathways have been demonstrated to improve outcomes in some settings, these must be considered a guide to inform decision making and not a singular/set approach. There remains insufficient understanding of the pathophysiology of CFC375 and the biological mechanisms of treatments; it is important that this is considered in future research. Across all future research into interventions for CFC, the question of ‘for whom and when’ is essential.
There is a body of evidence which explores the effectiveness of different models of service delivery (see What is the effectiveness of different models of service delivery?), including ‘who’ delivers the service (e.g. nurses or doctors). Factors other than professional qualification are likely to be important (e.g. experience, enthusiasm and case-load/time).
Behavioural techniques are part of parenting
We identified some evidence that programmes which combine pharmacological, dietary and behavioural interventions may be beneficial. There was evidence for effectiveness of combined programmes when delivered at Levels 1 and 2, but the evidence was insufficient to support specific conclusions relating to the effective components of a combined programme. There was some evidence which supports the use of behavioural therapy techniques delivered by specialist practitioners, but this evidence was generally specific to children for whom Level 1 interventions had failed. In these research studies, the interventions were delivered by specialist practitioners such as psychologists or paediatric specialists, and often delivered to children within Level 3 settings.
There is an absence of evidence around whether, and in what way, behavioural interventions could be an effective component of successful management of CFC by parents/family (Level 0), or members of the wider children’s workforce (Level 1). Information/guidance aimed at supporting successful use of simple behavioural strategies could be a useful component of an educational/information package for parents/carers. 376 Research in this field is considered a high priority.
Laxatives are effective, but are not the only treatment for chronic functional constipation
Our evidence syntheses support the continued use of laxatives as the first-line treatment provided by primary care services, although there is still a lack of direct evidence about the relative effectiveness of different types of laxatives, and combinations of laxatives. However, our work highlights that the first interventions provided to a child with CFC are unlikely to be those provided by a prescribing health professional. The established guidance and practice of laxatives as ‘first line’1,18–20,377 treatment for CFC fail to acknowledge the complex, individualised nature of childhood constipation, and the fact that rarely will a child be given a laxative as the ‘only’ intervention.
Although evidence supports the use of laxatives as an effective treatment, there are many factors which affect successful implementation. Consequently, laxatives may not be an effective treatment for all children. Where laxatives are not an acceptable intervention for a child, specialist continence teams have an important role in identifying alternatives. Currently, there is some evidence of other treatments (e.g. enemas, some complementary therapies) which may provide effective alternatives to laxatives, but this evidence tends to come from populations of children for whom treatment has been unsuccessful and often they have reached ‘crisis’ point, perhaps presenting at an ED or being admitted to hospital. There is a need to conduct research to establish effective alternatives to ‘conventional’ laxatives, including effectiveness of combinations of different laxatives.
Effective primary care support could improve outcomes and resource waste
A key goal for the management of CFC should be to avoid unnecessary use of Level 2 or Level 3 services. There is arguably often the possibility of successful management by identification at Level 0 and assessment and treatment at Level 1. The numbers of children who do not respond to initial interventions at Level 0 and 1 and are subsequently referred to Level 2 or 3 specialist clinics could potentially be significantly reduced if there was increased investment and support for the early recognition and management of CFC at Level 0 and 1. Optimising recognition and management at Levels 0 and 1 will mean that the limited specialist service will be able to see the significantly reduced number of children whose CFC is – for some individualised reason – unresponsive to Level 0 and 1 interventions.
Long-term psychological impact and support needs further attention
Only 17 of 651 (2.6%) studies in our scoping review explored psychosocial interventions, such as cognitive-based therapy, counselling or talking therapies; less than 10% of our identified studies reported measures of QoL and 1% school attendance. We found no studies which addressed the best ways to support children to deal with the psychological effects of constipation, and this was a key evidence gap, considered a high priority for future research.
It is important to consider costs
We did not find any robust data relating to the costs or resource use associated with CFC. Our review highlighted a number of gaps in economic evidence including a lack of economic evaluations for interventions delivered by families/carers, or by the wider children’s workforce or by different models of delivery. Fewer than 30% of the studies identified in our review employed any formal economic evaluation study design. Half of these were cost-minimisation analyses which assume identical effectiveness between the comparators, which is rarely accurate. Furthermore, these studies were based on limited empirical data, relying heavily on expert input rather than clinical evidence for their parameters.
Our review also highlighted the limited economic data in special populations (e.g. children with learning disabilities, complex needs, looked after children or children in lower socioeconomic groups). Such data are urgently required as the prevalence of CFC is much higher in these groups. An ongoing large-scale cluster RCT378 was identified as part of the searches and is anticipated to report in the next year.
Conducting economic evaluations in children brings unique challenges in terms of patterns of health resources, relying on parents/carers as proxies and wider consideration of costs beyond the health system to include school and community costs. However, high-quality economic evaluations from other areas of health care could provide roadmaps for conducting these evaluations.
Research priorities must be addressed with high-quality research
Our review highlights serious limitations in the evidence base relating to management of CFC. Poor research conduct and reporting, studies which fail to address the RQs which are most important to people affected by CFC, and failure to consider previous studies contribute to research waste. We have clearly identified evidence gaps and interventions for which further research is indicated; this work should inform future prioritisation. To avoid further research waste, and promote evidence-based practice, it is essential that future research addresses the questions which are of the highest priority to key stakeholders and has the highest possible standards of conduct and reporting. A robust, comprehensive research prioritisation project, involving a wide range of stakeholders, is recommended.
Comparison of findings with guidelines and other systematic reviews
In Chapter 10 we provided a systematic comparison of our findings with the conclusions of existing guidelines. 1,18–20 This highlighted that the majority of questions addressed by evidence included in our review have not been considered by current guidelines. However, where evidence has been considered there is generally agreement between our findings and current guidelines.
Tian (2016)379 provides an overview and appraisal of guidelines relating to constipation (adults and children). Of 22 international guidelines relating to constipation, three publications related specifically to children. 379 Tian (2016) concluded that the NICE guidelines can be ‘strongly recommended’. 379 This finding supports our conclusion that the current UK1,2,19,20,22,377 guidelines should continue to be used to inform clinical practice. However, whilst the recommendations within the NICE guidelines remain relevant, it is important to note that we identified many questions/interventions that have not been addressed within the current guidelines.
Our conclusion that there is a need for individualised care and improved organisation of service delivery is in line with national aims to deliver patient-centred care. 380 In 2021 NICE published guidance relating to babies, children and young people’s experience of health care. 381 Central to this guideline is the aim of ensuring that every baby, child and young person has ‘the best possible experience of health care’. Care of children with constipation should adhere to the recommendations within this guideline, which clearly highlight the need for ‘a personalised approach to implementation’.
A guideline relating to nutritional complications in children with neurological impairment recommends use of ‘standard treatments as in typically developing children, unless there is a risk of aspiration of polyethylene glycol or liquid paraffin’. 172 The risk of aspiration when using PEG or liquid paraffin is highlighted by studies of prevalence which we did not consider within our review (e.g. ref. 382). We noted this evidence within our narrative summaries to ensure completeness. The guideline also recommends increasing fluid and fibre intake for children with neurological impairment and constipation; although this initially appears contradictory to our findings (that there is no evidence to support fluid or fibre which is additional to routine/‘normal’ recommended intake for age/size of child), this difference is explained by evidence which suggests that fluid and fibre may be inadequate in many children with neurological impairment. 383
A number of SRs have been published since the date of our search. A SR of RCTs of ‘non-pharmacological’ treatments for children with CFC was published in January 2022 (search up to August 2020). 384 This review identified 52 RCTs, exploring the effectiveness of a range of interventions. This review has findings which are broadly in agreement with ours. An earlier SR explored evidence for ‘non-pharmacological auxiliary treatments’ (search up to October 2020); this only included seven RCTs. 385 Our review is substantially more comprehensive, with wider inclusion criteria.
A Cochrane SR that focused on effectiveness of probiotics for treatment of chronic constipation in children was published in March 2022. 386 This review included 14 RCTs. The authors conclude that there is insufficient evidence to determine whether probiotics are effective, and that future research could consider the context in which the intervention is delivered as well as effectiveness. Our conclusions relating to probiotics are in agreement with this Cochrane review.
Liu (2021)387 conducted a SR exploring evidence for safety and effectiveness of ‘Traditional Chinese medicine infant massage’. The searching included key Chinese language databases and grey literature sources which were not searched for our review. The authors identified 23 RCTs, all of which were conducted in China. The authors conclude that infant massage was safe and resulted in statistically significant benefits when compared to either traditional Chinese medicine or to Western medicine. However, there are a number of factors which limit certainty in this finding, and we conclude that further investigation is required. This evidence does indicate that there are a number of Chinese-language studies which were not identified and included in our review.
Consensus, best practice recommendations relating to the use of TAI in children are in agreement with our findings and provide practical recommendations relating to the use of this intervention. 388 A SR focused specifically on children with cerebral palsy concluded that there was insufficient evidence to support any specific treatment modalities in this group of children, supporting our findings in this area. 389 Our findings in relation to the evidence for Level 3 (surgical) interventions agree with previous reviews (e.g. refs390,391).
We are aware of only two reviews that have systematically identified and evaluated the cost effectiveness of treatments for chronic constipation in children. 1,17 The first review was conducted as part of a guideline in 2010 concluded that economic data were ‘sparse’. 1 More recently, Han et al. (2018)17 published a SR of 10 full economic evaluations in adults only: no evidence for children or special populations (e.g. children and young people with ASN) was identified. 17 While our findings are similar to those of these two previous reviews about the lack of high-quality economic evidence, our review did uncover new economic evidence related to sacral neuromodulation and physiotherapy, adding to the current evidence base.
In agreement with our findings relating to barriers and facilitators to implementation, the importance of the context in which the child is living was highlighted by a recent SR which identified 15 primary studies which explored the ‘association between childhood constipation and exposure to stressful events’. 392 The authors conclude that stressful events experienced by children (e.g. parental separation, family illness, being bullied) are associated with CFC.
Strengths and limitations
Overview of key strengths
We involved stakeholders from project inception and throughout the study (see Chapter 3), using current UK guidance and an established framework to plan and describe involvement. 27 This involvement was a key strength, with stakeholders controlling and/or influencing several aspects of the project (see Chapter 3). Stakeholder involvement led to the development of the SUCCESS Pyramid (see Figure 2), and later logic model (see Figure 27), which formed the basis for the structure of our evidence syntheses. A key advantage of this is that this way of bringing evidence together was perceived as clinically relevant and meaningful to knowledge users.
We aimed to work to the highest methodological standards across the project. We adhered to recognised best practice for methodological conduct and reporting. This included use of TIDieR,52 PRISMA,393 PRISMA-A,394 PRISMA-ScR,42 GRIPP2,37 quality appraisal tools50,52–54 and judgement of certainty informed by the GRADE56,57 approach. We established definitions of CFC and developed, in partnership with our stakeholders, a comprehensive taxonomy of potential interventions, these informed review inclusion criteria. We employed robust, rigorous searching, searching key electronic database and range of other sources, including grey literature. With reference to the ROBIS tool,49 we consider our review at low ROB for domains relating to study eligibility criteria, identification and selection of studies and data collection and study appraisal.
The design and conduct of this project adopted a pragmatic approach, aiming to build on (rather than replicate) the existing evidence base. We adopted a step-wise approach, informed by a decision tree, to study selection and inclusion within our reviews of effectiveness (see Chapter 6). The strength of this approach was that it enabled us to benefit from the results of high-quality SRs when these were available (rather than only include primary studies, as is common in many evidence syntheses) and to bring together evidence from non-randomised studies when other studies were not available. This was key to enabling us to complete a comprehensive synthesis of research evidence relating to CFC, whilst avoiding contributing to research waste by duplicating other published evidence syntheses.
Limitations
There are a number of limitations of this project. These principally relate to the following.
Stakeholder involvement
Our SG comprised a small number of people, meaning that input could have been biased and thus not reflective or representative of a national or international viewpoint. We did not take any specific steps to ensure diversity within our SG or to involve people from under-served groups. The limitations relating to our stakeholder involvement have been discussed elsewhere (see Chapter 3).
Focus on treatments for chronic functional constipation
As defined by the NIHR call, this project focused on the effectiveness and implementation of interventions, and combinations and sequences of interventions, for childhood CFC (see Research questions). Consequently, we did not search for or synthesise evidence specifically focused on the recognition and diagnosis of CFC. Recognition and diagnosis of CFC are clearly as essential first step to successful management, and our conclusions are limited as we have not reviewed evidence relating to this key step. Our stakeholders raised concerns about this omission but accepted that this was beyond the scope of the SUCCESS project. Stakeholders highlighted that a child’s parents, carers or family are likely to be the first people who attempt to recognise the cause of a child’s discomfort, altered bowel habit, soiling, straining or changed behaviour, and that the lack of awareness of the problem of constipation, and societal taboos around engaging in open discussion about this, are barriers to identification and early treatment.
Stakeholders highlighted that there was an evidence gap relating to how parents, carers and children can recognise constipation, the relationship between early recognition and outcomes, how health professionals could facilitate the early identification and diagnosis of constipation, and education of professionals across the workforce to support this. Our review did include some limited evidence that suggested that educational interventions, particularly web-based interventions may be beneficial. Further research to identify the optimal content and format of providing information to children and families, including information around what is ‘normal’ and when to seek professional input is needed. This is supported by a recent SR, which concludes that lack of knowledge and understanding is a key issue faced by families/carers of children with CFC, and research to identify effective educational/informational strategies a priority. 392
Our focus was on management of CFC in children, and – while we considered psychosocial and behavioural interventions – we did not consider the psychosocial and behavioural factors which may contribute to poor outcomes. For example, there is evidence that some children may actively avoid using school toilets395 and concern has been raised about the impact of this on children with bowel problems. 396
Methodological approach and rigour
We adopted a step-wise approach to study selection and inclusion. A key limitation of this approach was that it is novel and there is a lack of best practice guidance and few examples to guide implementation. Our protocol lacked sufficient clarity relating to inclusion of different study designs, and as a result we included all relevant RCTs and primary studies for the majority of our evidence syntheses. This was inclusive and resulted in a comprehensive synthesis of evidence, but it took considerable time, and resulted in inclusion of many very-low-quality studies, which have limited generalisability. While our methods benefited from the use of independent reviewers to check study inclusion and data extraction, we failed to collect data which enabled us to calculate and report inter-rater reliability.
Following our step-wise approach, and specifically design to avoid duplication and research waste, where we identified a SR which we judged to be low ROB we included this within our evidence synthesis, rather than the original primary studies. There are a number of established debates relating to the methodological approaches adopted by SRs and meta-analyses. This includes debates relating to the merits of ‘lumping’ versus ‘splitting’ of interventions, comparators and outcomes. 397,398 We did not make any judgement relating to the methodological approach of a review, assuming that we assessed the review as low ROB. This meant that our synthesis could potentially include SRs and meta-analyses which had taken different approaches, and this may have impacted on the specificity of conclusions that could be drawn. For example, we included a review of probiotics [Harris, 2019,74 see Section Evidence of effectiveness: lifestyle interventions delivered by family/carers (Level 0)] which combined (‘lumped’) evidence from trials with a range of active and inactive comparators but, following completion of our review, a Cochrane review of probiotics397 was published which ‘split’ the same body of evidence. Whilst there remains to be consensus within this debate, our ‘neutral’ stance of considering the most up-to-date review, regardless of approach, seems justifiable, but may have impacted on the conclusions that could be drawn from the evidence.
-
Our methodological approach involved the use of one comprehensive search, implemented for our scoping review, with studies then ‘tagged’ for inclusion within for focused reviews of effectiveness and factors relating implementation. This was designed as an efficient approach, avoiding duplication of searching across different evidence synthesis. To avoid duplication of searches from previous evidence syntheses, we included studies identified from the searches for the NICE guidelines and limited our searches to after this time period. There are arguably risks associated with this approach; we assumed that searches for previous projects were suitably comprehensive to address our broad RQ, and we utilised a broad search (focused on terms for constipation and children) which may have potentially lacked the sensitivity to find studies of some select interventions. This approach to searching meant that our electronic searches were from January 2011 onwards, but that we attempted to integrate findings from research published before 2011 by including evidence used to develop national and international guidelines. However, these guidelines often limited their inclusion criteria to SRs or RCTs only. For example, NICE guidelines1,19 only included other study designs if no SRs, meta-analyses or RCTs were identified, and did not search grey literature. Further, systematically identifying and integrating grey literature into our search results was challenging as there is little guidance in this area. Studies published before 2011 represent 15% of the total number of studies included within our review.
We followed a predefined protocol for decisions around statistical analyses and conducted narrative syntheses where results could not be pooled statistically. We extracted and reported details relating to the children included in studies, but we did not systematically group these according to the details of the populations studied. This limits the generalisability of our narrative syntheses. Due to the quality and heterogeneity of studies we were limited in the number of analyses that we could conduct. We did not conduct any subgroup analysis.
Quality of evidence
Findings from this project are limited by the quality of research evidence available. This includes a lack of high-quality studies and poor reporting. Our review could be criticised for synthesising studies which have used a wide variety of study designs. This means we have included all studies that may report some, albeit limited, evidence related to CFC. However, while many of these studies report piecemeal evidence, they do present a realistic picture of the evidence that is currently available in this field. They also highlight the gaps in evidence and underscore the need for enhanced methodological quality.
There are limitations in our approach to judging certainty of evidence. We grouped included studies according to the question that they addressed and judged our certainty in the evidence relating to this question. We used an approach based on the GRADE56,57 approach, in which we applied downgrades where we had concerns relating to our confidence in the findings of the studies addressing each question. The broad nature of our evidence syntheses, including a wide range of different study designs and including studies which addressed heterogeneous outcomes, meant that we were unable to reach judgements using the established GRADE approach, following specific guidance for individual domains of study limitations, inconsistency, indirectness and imprecision. As a consequence, our judgement of certainty in the evidence is arguably a crude categorisation, which falls short of the rigour which would be obtained by using GRADE approach.
Prioritisation of interventions to be addressed by review
In our project proposal we stated that we would work in partnership with stakeholders to identify the top priority interventions for which we should conduct focused evidence syntheses, anticipating prioritisation of a list of interventions, and/or intervention combinations. However, the stakeholders chose to focus on broad RQs rather than specific interventions/intervention combinations.
The stakeholders ranked the broad questions into high, medium and low priority for completion of evidence syntheses. However, the research team found it difficult to conduct ‘light touch’ syntheses for the lower priority questions, as they did not wish to compromise methodological rigour. Consequently, the completed evidence syntheses have been inclusive and comprehensive, providing a broad overview of all research in this field. The limitation of this is that a large volume of evidence has been synthesised, potentially reducing the accessibility of information and impacting on the level of detail that could be provided for individual studies.
Prioritisation of outcomes
A strength of our project was that our stakeholders reached consensus on the outcomes which were of greatest importance to include within our SR of effectiveness. However, our protocol did not clearly specify how we would use these prioritised outcomes to inform selection of studies for inclusion or synthesis of evidence within our review. Further, our synthesis of evidence relating to these outcomes was limited by the fact that studies often presented clinical outcomes as combined scores, for example reporting ‘constipation scores’ or ‘improvement scores’. Consequently, our narrative syntheses report findings relating to a wide range of different outcomes, as reported by the included studies. Our evidence synthesis may have been more concise and informative had we strictly adhered to reporting the prioritised outcomes.
Timelines and search dates
Conducted between January 2020 and May 2022, the project was subject to the working practice impacts of the COVID-19 pandemic and associated restrictions. During 2020–1 we experienced delays to the literature identification and retrieval of full texts due to the temporary closure of the British Library. Clinical trial registers (e.g. WHO ICTRP) and SR registers (e.g. PROSPERO) were frequently unstable as they struggled to cope with the volume of COVID-19-related studies. There were some changes to research staff, and a number of periods of staff absence over the course of the project, creating challenges to continuity. As a result, the project end date was extended. However, in order to facilitate completion within the available resources, it was agreed with our funders (e-mail correspondence 10 May 2021) that an update to the searches (conducted in March 2020) was not essential, meaning searches are more than 12 months out of date. However, our comprehensive search of trials registers meant that we had knowledge of relevant ongoing RCTs. In May 2022, we searched for completed RCTs and updated our syntheses accordingly. This means that we are confident that we are not missing any key evidence which is likely to impact on our conclusions. We may be missing some recent non-randomised studies, but the results of these are unlikely to impact on our conclusions or certainty in the findings. A small number of studies remain as ‘awaiting assessment’ meaning some data may be missing from the synthesis.
Assigning studies to levels based on our Pyramid
Our evidence syntheses were structured around the Pyramid developed by the SG. This introduced a number of challenges, particularly in relation to assigning individual studies to a level on the Pyramid. Studies of some interventions, which could feasibly be delivered within Level 0 or Level 1, were conducted by highly specialist (Level 2 and 3) teams. It was often difficult to judge whether the level at which the intervention was delivered within the study was a factor related to study conduct (e.g. aiding study recruitment by identifying participants through a consultant-led clinic) or was a key factor of the intervention. However, there was strength in the involvement of the wide group of stakeholders to inform consensus decisions.
Recommendations for future research
To address RQ4 (see Research questions) we identified and produced a list of evidence gaps (see Evidence gaps). We explored with our SG whether it was possible to generate and/or prioritise specific recommendations for future research from this collated list of evidence gaps. However, as reported in section Evidence gaps, stakeholders informed our decision not to generate more specific, focused recommendations for future research. The limitations in our SG members (see above) supported this decision. Consequently, our key recommendation for future research is that a ‘robust research prioritisation exercise involving a wide range of stakeholders’ is conducted (see Implications for research), and this project has not generated more specific recommendation for future research.
Impact of COVID-19
As stated above, COVID-19 impacted on this project. From March 2020, for the remainder of the project, all project work was conducted remotely and there were no face-to-face meetings. Researchers and stakeholders were impacted by a shift to home-based working and changes to caring activities and responsibilities. There were challenges with our stakeholder activities, which had largely been planned as full-day face-to-face meetings. Some stakeholders were unable to attend meetings due to changes to caring responsibilities. Our stakeholders described difficulties in finding their place in the group, taking in the information presented, and using the technology. Shifting to video meetings changed the nature of our meetings and at least temporarily altered communication quality and contribution across meetings. As the project and pandemic progressed, we developed more effective ways of communicating and working together. For example, we amended our plans so that we moved to more frequent, but shorter, meetings.
As a result of our timeline, all studies included within our evidence syntheses were conducted pre-COVID-19. Changes to provision of health care and workforce availability following the pandemic may need to be considered within future research.
Patient and public involvement
Stakeholder involvement was central to this project. Everyone involved had equal status and were involved at all stages of the project. The methods and impact of stakeholder involvement have been fully reported in Chapter 3 (see Report Supplementary Material 1) and the GRIPP2 reporting checklist is available within project documentation. Members of our SG contributed to writing this report.
Equality, diversity and inclusion
Within our review
We included any studies which included children with CFC, placing no restrictions which would exclude any groups. However, as this project comprised a SR, we were limited by the participants included within the studies eligible for our review, and by the descriptions of included participants provided by study authors. Generally, across the studies included in our review, age and gender were commonly reported, but there was very poor reporting of other participant characteristics. This makes us uncertain about whether our results are representative of under-served groups.
Within our stakeholder group
We advertised opportunities for people with experience of childhood constipation to join our SG (see Chapter 3). The individual people recruited had a variety of different backgrounds, geographical locations, circumstances and lived experiences. However, these members were all recruited through online routes, meaning that our SG was not inclusive of people with barriers to participation in this way. Further, none of our stakeholders were representative of specific under-served groups, such as ethnic minority groups. Efforts were made to maintain contact and retain inclusion throughout the project, particularly during COVID restrictions, when some stakeholders experienced additional barriers to attending meetings. For example, some stakeholders had individual catch-up meetings with the research team if they had missed a meeting, and information technology support was provided to support use of online meeting software. We took steps to ensure that information that we shared with our SG was inclusive and accessible, although the involvement of stakeholders in the writing of the final project report did mean that stakeholders had to access and work on lengthy word documents. We offered payment to the PPI contributors at NIHR recommended rates to acknowledge the time spent involved in reviewing documents, preparing for and attending meetings.
Within our research team
Our research team were all academics employed by the Nursing, Midwifery and Allied Health Professions Research Unit (NMAHP RU) based with GCU and University of Stirling. As such, the research team did not include people from groups who are generally under-represented in this field. One of our research team was a parent to a child with complex needs, with lived experience of constipation. There was a range of research expertise across the research team, with more junior members mentored to gain skills in SR methods.
Implications
Implications for clinical practice and policy
Management of childhood CFC should be child-centred, adapted according to the individual characteristics of the child and the context in which they live. Strategies to raise awareness of constipation and support recognition and early management are essential. Families/carers should have access to information about constipation, including what is normal and when to seek help. Health professionals should receive appropriate education to ensure they have the knowledge and understanding to provide evidence-based care.
Our findings do not support changes to current clinical guidance around use of pharmacological interventions, although evidence identifies that alternatives to oral laxatives may sometimes be appropriate. Nurse-led clinics, multidisciplinary programmes, or care pathways for primary care services may be beneficial, having implications for service delivery. Advice around fibre may need clarification as evidence does not support additional fibre intake (but rather, ‘adequate’ fibre as part of a balanced diet). Biofeedback, not currently recommended in guidelines, could be beneficial for some children.
Management strategies which we judge to be low risk and potentially beneficial, and therefore worthy of implementation, include educational interventions for parents (particularly web-based); programmes combining pharmacological, dietary and behavioural interventions, delivered by primary care services (Level 1); physical exercise, focused on pelvic floor muscle, in addition to normal physical activity.
Implications for research
We have collated a list of research gaps (see Evidence gaps). As a next step, a robust research prioritisation exercise involving a wide range of stakeholders is considered essential. Key topics which are considered priorities for future research by the stakeholders involved in this project relate to recognition of CFC, information provision, diet, laxatives and combinations of laxatives, behavioural therapy and psychological support. It is important that research studies address what works for which individual child, and when, including children with and without additional needs. Research to explore the optimal delivery of services across the different levels, including identification of key components and features of effective teams and criteria for referring children from one level to the next, is important.
This project has highlighted that research in this field often does not adhere to recognised standards for conduct and reporting or consider the complexities of interventions for CFC. Future research relating to CFC must adhere to these standards, address questions which are identified priorities, measure outcomes of importance and consider the views of key stakeholders, including children, parents and healthcare professionals.
Stakeholder reflections
Members of the SG have provided the following reflections, in their own words, on the discussion arising from this project:
-
I agree with the recommendations made for future research and I would highlight the need for much more research to be done as soon as it can be on the psychological aspects of childhood constipation and psychological treatments that can be done to help people. I feel that until this is addressed, we are allowing children and their loved ones to suffer unnecessarily and in a world that does not understand. These children become adults who struggle and so not only is their quality of life affected but overall, it is more costly for the health and social care system.
-
The recommendations agree with what I would have expected given our own experience. It is clear to me that CFC is both very common and very often quite effectively treated if recognised and sensitively treated, with recourse to a specialist team (ideally nurse-led) rather than as an adjunct to other services. The practical issues with CFC, the provision of nappies and continence supplies, are important but so is the psychological cost of untreated CFC, and above all the diagnosis and recognition of the extent of the problem, especially among people with additional needs (and in particular, the nonverbal) is life changing.
-
This project was meant to be about how to combine things and clearly, it’s very difficult to get a handle on that. I am wondering about how issues around complexity can be usefully considered when shaping pathways for children, and particularly in allowing combinations of therapy.
-
This project has highlighted areas where there are gaps … the number of children affected justifies further money to look at research prioritisation.
-
At the heart of all this is the child and the family – a lot of these interventions, we know they work, but unless you get the context right, they don’t. And that is the underpinning problem with the current research, I think.
Conclusions
Management of childhood CFC is complex, and there is no simple ‘one size fits all’ approach. The available evidence remains limited, with a preponderance of small studies which are often poorly conducted and reported. Our findings do not indicate that changes are needed to the treatment recommendations within current clinical guidelines but do highlight the need to move away from considering effectiveness of single interventions in isolation. Clinical care and future studies must consider the individual characteristics of each child with constipation, and the context – or environment – within which they live. Key goals of successful management of CFC should be early recognition of symptoms and delivery of interventions by families/carers, achieved by providing children and parents/carers with effective education and support from members of the wider children’s workforce (primary care services). Development, evaluation and implementation of strategies to enhance the delivery of services focused on individualised care, combining lifestyle and behavioural strategies with laxatives, selected with knowledge of biological plausibility, are a priority.
Acknowledgements
We want to acknowledge the invaluable contribution of June Rogers, Gemma Kierczuk, Tracey Stone and Dr Helen Haywood. We would also like to acknowledge the contribution of child and parent experiences captured via video. Gaining insight from people with lived experience is extremely important for any condition but for some conditions we are particularly limited in being able to easily learn from children and their families. The footage was invaluable for increasing our understanding of the impact of childhood constipation.
Contributions of authors
Alex Todhunter-Brown (https://orcid.org/0000-0003-4941-7985) (Senior Research Fellow, Systematic Review Specialist) was involved in the conceptualisation of the application, methodological design and funding acquisition as well as conducting the investigation, formal analysis, project administration, supervision, creation of resources and visualisation, data curation, validation of results, writing original drafts and reviewing and editing the report. She was principal investigator from January 2022.
Lorna Booth (https://orcid.org/0000-0001-5776-3541) (Behaviour Change Specialist, Systematic Reviewer) was involved in the conceptualisation of the application, methodological design and funding acquisition as well as conducting the investigation, validation of results, writing original drafts and reviewing and editing the report.
Pauline Campbell (https://orcid.org/0000-0002-5879-5526) (Research Fellow, Evidence Synthesis) was involved in the conceptualisation of the application, methodological design and funding acquisition as well as conducting the investigation, formal analysis, data curation, creation of resources and visualisation, validation of results, writing original drafts and reviewing and editing the report.
Brenda Cheer (https://orcid.org/0000-0001-5090-6562) (Paediatric Specialist Continence Nurse), a Stakeholder Group member, was involved in the conceptualisation of the study, conducting the investigation, and reviewing and editing the report.
Julie Cowie (https://orcid.org/0000-0002-4653-1283) (Senior Research Fellow, Implementation Specialist) was involved in conducting the investigation, validation of results, and reviewing and editing the report.
Andrew Elders (https://orcid.org/0000-0003-4172-4702) (Statistician Statistics) was involved in the conceptualisation of the application, methodological design and funding acquisition as well as conducting the investigation, formal analysis, data curation, visualisation, validation of results, and reviewing and editing the report.
Suzanne Hagen (https://orcid.org/0000-0002-9741-9160) (Professor of Health Services Research, Trialist) was involved in the conceptualisation of the application, methodological design and funding acquisition as well as conducting the investigation, validation of results, and reviewing and editing the report.
Karen Jankulak (https://orcid.org/0000-0001-5105-228X) (PPI Member), a Stakeholder Group member, was involved in the conceptualisation of the study, conducting the investigation, writing original drafts, reviewing and editing the report.
Helen Mason (https://orcid.org/0000-0002-9303-2794) (Professor, Health Economist) was involved in the conceptualisation of the application, methodological design and funding acquisition as well as conducting the investigation, formal analysis, data curation, validation of results and reviewing and editing the report.
Clare Millington (https://orcid.org/0000-0002-9560-3423) (PPI Member), a Stakeholder Group member, was involved in the conceptualisation of the study, conducting the investigation, writing original drafts, reviewing and editing the report.
Margaret Ogden (https://orcid.org/0000-0002-7644-2310) (PPI Member), a Stakeholder Group member, was involved in the conceptualisation of the study, conducting the investigation, writing original drafts, reviewing and editing the report.
Charlotte Paterson (https://orcid.org/0000-0001-6796-227X) (Research Fellow) was involved in conducting the investigation, data curation, validation of results, and reviewing and editing the report.
Davina Richardson (https://orcid.org/0000-0002-5081-7866) (Children’s Specialist Nurse), a Stakeholder Group member, was involved in the conceptualisation of the study, conducting the investigation, and reviewing and editing the report.
Debs Smith (https://orcid.org/0000-0002-5020-1228) (PPI Member), a Stakeholder Group member, was involved in the conceptualisation of the study, conducting the investigation, writing original drafts, reviewing and editing the report.
Jonathan Sutcliffe (https://orcid.org/0000-0002-2842-2980) (Consultant Paediatric Surgeon), a Stakeholder Group member, was involved in the conceptualisation of the study, conducting the investigation, and reviewing and editing the report.
Katie Thomson (https://orcid.org/0000-0002-2558-2559) (Researcher and Occupational Therapy Lecturer) was involved in the conducting of the investigation, reviewing and editing the report.
Claire Torrens (https://orcid.org/0000-0002-3883-2502) (Research Fellow, Behaviour Change) was involved in the conceptualisation of the application, methodological design and funding acquisition as well as conducting the investigation, validation of results, and reviewing and editing the report.
Doreen McClurg (https://orcid.org/0000-0002-2872-1702) (Professor of Pelvic Floor Physiotherapy) was principal investigator until January 2022. She was involved in the conceptualisation of the application, methodological design and funding acquisition as well as conducting the investigation, formal analysis, project administration, supervision, creation of resources and visualisation, validation of results, writing original drafts and reviewing and editing the report.
Conference Presentations
Booth L, Campbell P, Mason H, Cheer B, Cowie J, Elders A, et al. A systematic review of economic studies used to manage chronic functional constipation in children and young people. International Continence Society, Melbourne, October 2021, Abstract #411. www.ics.org/2021/abstract/411.
Booth L, Cowie J, Campbell P, Mason H, Cheer B, Elders A, et al. A systematic review of the evidence surrounding the use of complementary therapies as a treatment for childhood Chronic Functional Constipation (CFC). International Continence Society, Melbourne, October 2021, Abstract #412. www.ics.org/2021/abstract/412.
McClurg D, Booth L, Campbell P, Mason H, Cheer B, Cowie J, et al. How clinically relevant is our updated evidence on the management of paediatric chronic constipation? Bladder and Bowel UK National Symposium, Manchester, UK, March 2022.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
Ethical approval was granted by GCU’s School of Health and Life Sciences Nursing Department Research Ethics Committee (HLS/NCH/19/016) (see project documentation).
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- National Institute for Health and Care Excellence (NICE) . CG99: Constipation in Children and Young People: Diagnosis and Management 2010. https://www.nice.org.uk/guidance/cg99 (accessed 28 May 2022).
- National Institute for Health and Care Excellence (NICE) . Constipation in Children 2015. https://cks.nice.org.uk/topics/constipation-in-children/ (accessed 28 May 2022).
- Benninga M, Candy DC, Catto-Smith AG, Clayden G, Loening-Baucke V, Di Lorenzo C, et al. The Paris Consensus on Childhood Constipation Terminology (PACCT) Group. J Pediatr Gastroenterol Nutr 2005;40:273-5. https://doi.org/10.1097/01.mpg.0000158071.24327.88.
- ROME Foundation . Rome IV Criteria 2016. https://theromefoundation.org/rome-iv/rome-iv-criteria/ (accessed 28 May 2022).
- Timmerman MEW, Trzpis M, Broens PMA. The problem of defecation disorders in children is underestimated and easily goes unrecognized: a cross-sectional study. Eur J Pediatr 2019;178:33-9. https://doi.org/10.1007/s00431-018-3243-6.
- Pijpers MA, Bongers ME, Benninga MA, Berger MY. Functional constipation in children: a systematic review on prognosis and predictive factors. J Pediatr Gastroenterol Nutr 2010;50:256-68. https://doi.org/10.1097/MPG.0b013e3181afcdc3.
- NHS Digital Statistics . Hospital Admitted Patient Care Activity (2017–18) 2018. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2017-18 (accessed 25 September 2023).
- Staiano A, Del Giudice E. Colonic transit and anorectal manometry in children with severe brain damage. Pediatrics 1994;94:169-73.
- Robertson J, Baines S, Emerson E, Hatton C. Constipation management in people with intellectual disability: a systematic review. J Appl Res Intellect Disabil 2018;31:709-24. https://doi.org/10.1111/jar.12426.
- Collis D, Kennedy-Behr A, Kearney L. The impact of bowel and bladder problems on children’s quality of life and their parents: a scoping review. Child Care Health Dev 2019;45:1-14. https://doi.org/10.1111/cch.12620.
- BBC . Constipation Death ‘Wholly Preventable’ 2018. www.bbc.co.uk/news/uk-england-suffolk-42778884 (accessed 28 May 2022).
- PHE . Making Reasonable Adjustments for People With Learning Disabilities in the Management of Constipation 2016.
- COMPASS . COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care 2012. https://hscbusiness.hscni.net/pdf/52817_HSC_-_Constipation.pdf.
- Auth MK, Vora R, Farrelly P, Baillie C. Childhood constipation. BMJ 2012;345. https://doi.org/10.1136/bmj.e7309.
- Afzal NA, Tighe MP, Thomson MA. Constipation in children. Ital J Pediatr 2011;37. https://doi.org/10.1186/1824-7288-37-28.
- Diaz S, Bittar K, Mendez MD. Constipation. Treasure Island, FL: StatPearls; 2022.
- Han D, Iragorri N, Clement F, Lorenzetti D, Spackman E. Cost effectiveness of treatments for chronic constipation: a systematic review. PharmacoEcon 2018;36:435-49. https://doi.org/10.1007/s40273-018-0609-6.
- Tabbers MM, DiLorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition . Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 2014;58:258-74. https://doi.org/10.1097/MPG.0000000000000266.
- National Institute for Health and Care Excellence (NICE) . Constipation in Children and Young People. Evidence Update June 2012. A Summary of Selected New Evidence Relevant to NICE Clinical Guideline 99 ‘Diagnosis and Management of Idiopathic Childhood Constipation in Primary and Secondary Care’ (2010) 2012.
- National Institute for Health and Care Excellence (NICE) . Constipation in Children and Young People: Diagnosis and Management [Last Updated: 13 July 2017] 2017. www.nice.org.uk/guidance/cg99/chapter/Update-information (accessed 28 May 2022).
- National Institute for Health and Care Excellence (NICE) . 2018 Exceptional Surveillance of Constipation in Children: Diagnosis and Management 2018. www.nice.org.uk/guidance/cg99/resources/2018-exceptional-surveillance-of-constipation-in-children-diagnosis-and-management-nice-guideline-cg99-pdf-6349983078085 (accessed 13 June 2022).
- National Institute for Health and Care Excellence (NICE) . Clinical Knowledge Summaries: Constipation in Children and Young People: Diagnosis and Management 2019. https://cks.nice.org.uk/topics/constipation-in-children/how-up-to-date-is-this-topic/changes/ (accessed 20 June 2022).
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374. https://doi.org/10.1136/bmj.n2061.
- Koppen IJN, Vriesman MH, Tabbers MM, Di Lorenzo C, Benninga MA. Awareness and implementation of the 2014 ESPGHAN/NASPGHAN Guideline for Childhood Functional Constipation. J Pediatr Gastroenterol Nutr 2018;66:732-7. https://doi.org/10.1097/MPG.0000000000001786.
- INVOLVE . Exploring the Impact of Public Involvement on the Quality of Research: Examples 2013. www.invo.org.uk/wp-content/uploads/2013/08/invoNETexamples2013.pdf (accessed 21 March 2022).
- Kreis J, Puhan MA, Schunemann HJ, Dickersin K. Consumer involvement in systematic reviews of comparative effectiveness research. Health Expect 2013;16:323-37. https://doi.org/10.1111/j.1369-7625.2011.00722.x.
- Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Development of the ACTIVE framework to describe stakeholder involvement in systematic reviews. J Health Serv Res Policy 2019;24:245-55. https://doi.org/10.1177/1355819619841647.
- Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Stakeholder involvement in systematic reviews: a scoping review. Syst Rev 2018;7. https://doi.org/10.1186/s13643-018-0852-0.
- INVOLVE . Guidance on Co-Producing a Research Project 2019.
- Hoddinott P, Pollock A, O’Cathain A, Boyer I, Taylor J, MacDonald C, et al. How to incorporate patient and public perspectives into the design and conduct of research. F1000Res 2018;7. https://doi.org/10.12688/f1000research.15162.1.
- Pollock A, Campbell P, Baer G, Choo PL, Morris J, Forster A. User involvement in a Cochrane systematic review: using structured methods to enhance the clinical relevance, usefulness and usability of a systematic review update. Syst Rev 2015;4. https://doi.org/10.1186/s13643-015-0023-5.
- Hickey G, Richards T, Sheehy J. Co-production from proposal to paper. Nature 2018;562:29-31. https://doi.org/10.1038/d41586-018-06861-9.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19-32.
- Camden C, Shikako-Thomas K, Nguyen T, Graham E, Thomas A, Sprung J, et al. Engaging stakeholders in rehabilitation research: a scoping review of strategies used in partnerships and evaluation of impacts. Disabil Rehabil 2015;37:1390-400. https://doi.org/10.3109/09638288.2014.963705.
- INVOLVE . Exploring the Impact of Public Involvement on the Quality of Research: Examples 2013. www.invo.org.uk/wp-content/uploads/2013/08/invoNETexamples2013.pdf (accessed 28 May 2022).
- Concannon TW, Meissner P, Grunbaum JA, McElwee N, Guise JM, Santa J, et al. A new taxonomy for stakeholder engagement in patient-centered outcomes research. J Gen Intern Med 2012;27:985-91. https://doi.org/10.1007/s11606-012-2037-1.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. Res Involv Engagem 2017;3. https://doi.org/10.1186/s40900-017-0062-2.
- Health Research Authority/INVOLVE . Public Involvement in Research and Research Ethics Committee Review 2016. www.invo.org.uk/wp-content/uploads/2016/05/HRA-INVOLVE-updated-statement-2016.pdf (accessed 28 May 2022).
- Kuizenga-Wessel S, Steutel NF, Benninga MA, Devreker T, Scarpato E, Staiano A, et al. Development of a core outcome set for clinical trials in childhood constipation: a study using a Delphi technique. BMJ Paediatr Open 2017;1. https://doi.org/10.1136/bmjpo-2017-000017.
- Pham MT, Rajic A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods 2014;5:371-85. https://doi.org/10.1002/jrsm.1123.
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467-73. https://doi.org/10.7326/M18-0850.
- SUCCESS Team . Strategies Used for Constipation in Children – Evidence Synthesis Involving Stakeholders (SUCCESS): Protocol V3 2022. www.journalslibrary.nihr.ac.uk/programmes/hta/NIHR128470/#/ (accessed 28 May 2022).
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol 2016;75:40-6. https://doi.org/10.1016/j.jclinepi.2016.01.021.
- Tabbers M, DiLorenzo C, Berger M, Faure C, Langendam M, Nurko S, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 2014;58:258-74.
- Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016;104:240-3. https://doi.org/10.3163/1536-5050.104.3.014.
- The World Bank . World Bank Open Data 2022. https://data.worldbank.org (accessed 28 May 2022).
- Librizzi J, Flores S, Morse K, Kelleher K, Carter J, Bode R. Hospital-level variation in practice patterns and patient outcomes for pediatric patients hospitalized with functional constipation. Hosp Pediatr 2017;7:320-7. https://doi.org/10.1542/hpeds.2016-0101.
- Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS Group . ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016;69:225-34. https://doi.org/10.1016/j.jclinepi.2015.06.005.
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- CASP Checklists. Oxford, UK; 2022.
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. https://doi.org/10.1136/bmj.i4919.
- JBI . Critical Appraisal Tools 2022. https://jbi.global/critical-appraisal-tools (accessed 1 June 2022).
- Lewin S, Langlois E, Tuncalp Ö, Portela A. WEIRD (Ways of Evaluating Important and Relevant Data) tool: Questions to Guide Assessment/Critical Appraisal of Programme Descriptions, Implementation Descriptions and Other Mainly Descriptive Types of Evidence. Oslo: Norwegian Institute of Public Health; 2019.
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. GRADE Working Group . What is ‘quality of evidence’ and why is it important to clinicians?. BMJ 2008;336:995-8. https://doi.org/10.1136/bmj.39490.551019.BE.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. https://doi.org/10.1136/bmj.39489.470347.AD.
- Burnett CA, Juszczak E, Sullivan PB. Nurse management of intractable functional constipation: a randomised controlled trial. Arch Dis Child 2004;89:717-22. https://doi.org/10.1136/adc.2002.025825.
- Faramarzian Z, Kargar M, Dehghani M, Zare N. Investigating the effect of nurse-centered strategies on functional chronic constipation of children aged 3–14 years referring to Imam Reza Clinic of Shiraz University of Medical Sciences in 2014. Shiraz E-Med J 2017;19. https://doi.org/10.5812/semj.14874.
- Ismail N, Ratchford I, Proudfoot C, Gibbs J. Impact of a nurse-led clinic for chronic constipation in children. J Child Health Care 2011;15:221-9. https://doi.org/10.1177/1367493511406568.
- Tappin D, Nawaz S, McKay C, MacLaren L, Griffiths P, Mohammed TA. Development of an early nurse led intervention to treat children referred to secondary paediatric care with constipation with or without soiling. BMC Pediatr 2013;13. https://doi.org/10.1186/1471-2431-13-193.
- Bellesheim KR, Cole L, Coury DL, Yin L, Levy SE, Guinnee MA, et al. Family-driven goals to improve care for children with autism spectrum disorder. Pediatrics 2018;142. https://doi.org/10.1542/peds.2017-3225.
- Mallon D, Vernacchio L, Trudell E, Antonelli R, Nurko S, Leichtner AM, et al. Shared care: a quality improvement initiative to optimize primary care management of constipation. Pediatrics 2015;135:e1300-7. https://doi.org/10.1542/peds.2014-1962.
- Norbedo S, Bassanese G, Barbieri F, Barbi E. Acute abdominal pain: recognition and management of constipation in the emergency department. Pediatr Emerg Care 2017;33:e75-8. https://doi.org/10.1097/PEC.0000000000001039.
- Athanasakos E, Dalton S, McDowell S, Shea T, Blakeley K, Rawat D, et al. Scientific solution to a complex problem: physiology and multidisciplinary team improve understanding and outcome in chronic constipation and faecal incontinence. Pediatr Surg Int 2020;36:295-303. https://doi.org/10.1007/s00383-019-04605-y.
- Costigan AM, Orr S, Alshafei AE, Antao BA. How to establish a successful bowel management programme in children: a tertiary paediatric centre experience. Ir J Med Sci 2019;188:211-8. https://doi.org/10.1007/s11845-018-1819-9.
- Gabr AA, Gad MA, Shalaby A. Quality of life in children with pseudoincontinence after implementing a bowel management program in Egypt. J Pediatr Surg 2020;55:261-4. https://doi.org/10.1016/j.jpedsurg.2019.10.048.
- Gonring K, Dolan B, Kapke TL, Begotka A, Sood M, Silverman AH. Group treatment of fecal incontinence: a description of an interdisciplinary intervention. J Pediatr Gastroenterol Nutr 2019;69:e70-4. https://doi.org/10.1097/MPG.0000000000002372.
- Karagiozoglou-Lampoudi T, Daskalou E, Agakidis C, Savvidou A, Apostolou A, Vlahavas G. Personalized diet management can optimize compliance to a high-fiber, high-water diet in children with refractory functional constipation. J Acad Nutr Diet 2012;112:725-9. https://doi.org/10.1016/j.jand.2012.01.021.
- Poenaru D, Roblin N, Bird M, Duce S, Groll A, Pietak D, et al. The Pediatric Bowel Management Clinic: initial results of a multidisciplinary approach to functional constipation in children. J Pediatr Surg 1997;32:843-8. https://doi.org/10.1016/s0022-3468(97)90633-3.
- Modin L, Walsted AM, Rittig CS, Hansen AV, Jakobsen MS. Follow-up in childhood functional constipation: a randomized, controlled clinical trial. J Pediatr Gastroenterol Nutr 2016;62:594-9. https://doi.org/10.1097/MPG.0000000000000974.
- Short HL, Heiss KF, Burch K, Travers C, Edney J, Venable C, et al. Implementation of an enhanced recovery protocol in pediatric colorectal surgery. J Pediatr Surg 2018;53:688-92. https://doi.org/10.1016/j.jpedsurg.2017.05.004.
- JPRN-UMIN000034508 . Examination of the Diet Cure for the Pediatric Constipation – Assessment of the Dietary Fiber Intake and the Cow’s Milk Allergy 2018. https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000039341 (accessed 13 June 2022).
- Harris RG, Neale EP, Ferreira I. When poorly conducted systematic reviews and meta-analyses can mislead: a critical appraisal and update of systematic reviews and meta-analyses examining the effects of probiotics in the treatment of functional constipation in children. Am J Clin Nutr 2019;110:177-95. https://doi.org/10.1093/ajcn/nqz071.
- Kubota M, Ito K, Tomimoto K, Kanazaki M, Tsukiyama K, Kubota A, et al. Lactobacillus reuteri DSM 17938 and magnesium oxide in children with functional chronic constipation: a double-blind and randomized clinical trial. Nutrients 2020;12. https://doi.org/10.3390/nu12010225.
- Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0210064.
- Chao HCH YC, Chen SY, Chen CC. Impact of probiotics on constipation and intestinal microflora in children with functional constipation. World Congress of Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2016;63. https://doi.org/10.1097/01.mpg.0000503536.79797.66.
- de Mello PP, Eifer DA, de Mello ED. Use of fibers in childhood constipation treatment: systematic review with meta-analysis. J Pediatr 2018;94:460-70. https://doi.org/10.1016/j.jped.2017.10.014.
- Closa-Monasterolo R, Ferre N, Castillejo-DeVillasante G, Luque V, Gispert-Llaurado M, Zaragoza-Jordana M, et al. The use of inulin-type fructans improves stool consistency in constipated children. A randomised clinical trial: pilot study. Int J Food Sci Nutr 2017;68:587-94. https://doi.org/10.1080/09637486.2016.1263605.
- Mahdavi M, Esmaeili-Dooki MR, Mehrabani S, Hajiahmadi M, Moghadamnia AA, Moslemi L. The effect of adding synbiotics to polyethylene glycol in childhood functional constipation: a randomized clinical trial study. Int J Pediatr 2017;5:5357-67.
- Aulia I, Supriatmo S, Azlin E, Sinuhaji AB. The role of glucomannan fiber in childhood functional constipation. Paediatr Indone 2016;56:95-100.
- Basturk A, Artan R, Atalay A, Yilmaz A. Investigation of the efficacy of synbiotics in the treatment of functional constipation in children: a randomized double-blind placebo-controlled study. Turk J Gastroenterol 2017;28:388-93. https://doi.org/10.5152/tjg.2017.17097.
- Cassettari VMG, Machado NC, Lourencao P, Carvalho MA, Ortolan EVP. Combinations of laxatives and green banana biomass on the treatment of functional constipation in children and adolescents: a randomized study. J Pediatr (Rio J) 2019;95:27-33. https://doi.org/10.1016/j.jped.2017.10.011.
- Bongers ME, de Lorijn F, Reitsma JB, Groeneweg M, Taminiau JA, Benninga MA. The clinical effect of a new infant formula in term infants with constipation: a double-blind, randomized cross-over trial. Nutr J 2007;6. https://doi.org/10.1186/1475-2891-6-8.
- Chao HC, Vandenplas Y. Therapeutic effect of Novalac-IT in infants with constipation. Nutrition 2007;23:469-73. https://doi.org/10.1016/j.nut.2007.03.006.
- Infante DD, Segarra OO, Redecillas SS, Alvarez MM, Miserachs MM. Modification of stool’s water content in constipated infants: management with an adapted infant formula. Nutr J 2011;10. https://doi.org/10.1186/1475-2891-10-55.
- Infante Pina D, Badia Llach X, Arino-Armengol B, Villegas Iglesias V. Prevalence and dietetic management of mild gastrointestinal disorders in milk-fed infants. World J Gastroenterol 2008;14:248-54. https://doi.org/10.3748/wjg.14.248.
- Savino F, Cresi F, Maccario S, Cavallo F, Dalmasso P, Fanaro S, et al. ‘Minor’ feeding problems during the first months of life: effect of a partially hydrolysed milk formula containing fructo- and galacto-oligosaccharides. Acta Paediatr Suppl 2003;91:86-90. https://doi.org/10.1111/j.1651-2227.2003.tb00653.x.
- Xinias I, Analitis A, Mavroudi A, Roilides I, Lykogeorgou M, Delivoria V, et al. A synbiotic infant formula with high magnesium content improves constipation and quality of life. Pediatr Gastroenterol Hepatol Nutr 2018;21:28-33. https://doi.org/10.5223/pghn.2018.21.1.28.
- Dehghani SM, Ahmadpour B, Haghighat M, Kashef S, Imanieh MH, Soleimani M. The role of cow’s milk allergy in pediatric chronic constipation: a randomized clinical trial. Iran J Pediatr 2012;22:468-74.
- Iacono G, Cavataio F, Montalto G, Florena A, Tumminello M, Soresi M, et al. Intolerance of cow’s milk and chronic constipation in children. N Engl J Med 1998;339:1100-4. https://doi.org/10.1056/NEJM199810153391602.
- Iacono G, Carroccio A, Cavataio F, Montalto G, Cantarero MD, Notarbartolo A. Chronic constipation as a symptom of cow milk allergy. J Pediatr 1995;126:34-9. https://doi.org/10.1016/s0022-3476(95)70496-5.
- Mohammadi Bourkheili A, Mehrabani S, Esmaeili Dooki M, Haji Ahmadi M, Moslemi L. Effect of cow’s-milk-free diet on chronic constipation in children; A randomized clinical trial. Caspian J Intern Med 2021;12:91-6. https://doi.org/10.22088/cjim.12.1.91.
- Beleli CA, Antonio MA, dos Santos R, Pastore GM, Lomazi EA. Effect of 4’galactooligosaccharide on constipation symptoms. J Pediatr (Rio J) 2015;91:567-73. https://doi.org/10.1016/j.jped.2015.01.010.
- Dehghani SM, Bahroloolomifard MS, Yousefi G, Pasdaran A, Hamedi A. A randomized controlled double blinded trial to evaluate efficacy of oral administration of black strap molasses (sugarcane extract) in comparison with polyethylene glycol on pediatric functional constipation. J Ethnopharmacol 2019;238. https://doi.org/10.1016/j.jep.2019.111845.
- Tajik P, Goudarzian AH, Shadnoush M, Bagheri B. Effect of red sugar on functional constipation in children compared to figs syrup; a randomized controlled trial study. Gastroenterol Hepatol Bed Bench 2018;11:313-8.
- Tanjung M, Supriatmo S, Deliana M, Yudiyanto AR, Sinuhaji AB. Selenium and functional constipation in children. Paediatr Indones 2016;56:111-7. https://doi.org/10.14238/pi56.2.2016.111-7.
- Hassanein SMA, Deifallah SM, Bastawy HA. Efficacy of oral magnesium therapy in the treatment of chronic constipation in spastic cerebral palsy children: a randomized controlled trial. World J Pediatr 2021;17:92-8. https://doi.org/10.1007/s12519-020-00401-0.
- Modaresi SV, Moslehiniya M. Comparing two common methods of treatment in children with functional constipation. Iran J Pediatr 2013;23.
- Stepurina L, Zakharova I, Tvorogova T. Use of magnesium-containing mineral waters for treatment of functional constipation among children and teenagers. J Pediatr Gastroenterol Nutr 2018;66:462-3.
- Young RJ, Beerman LE, Vanderhoof JA. Increasing oral fluids in chronic constipation in children. Gastroenterol Nurs 1998;21:156-61. https://doi.org/10.1097/00001610-199807000-00002.
- Ritterband LM, Thorndike FP, Lord HR, Borowitz S, Walker LS, Ingersoll KS, et al. An RCT of an internet intervention for pediatric encopresis with one year follow-up. Clin Pract Pediatr Psychol 2013;1:68-80.
- Ritterband LM, Cox DJ, Walker LS, Kovatchev B, McKnight L, Patel K, et al. An Internet intervention as adjunctive therapy for pediatric encopresis. J Consult Clin Psychol 2003;71:910-7. https://doi.org/10.1037/0022-006X.71.5.910.
- Tayag-Lacsina EC, Castro R, Monreal P, Castro C. The effectiveness of a constipation pamphlet in improving outcomes among pediatric patients with functional constipation: a single-blinded randomized controlled trial. Arch Dis Child 2019;104. https://doi.org/10.1136/archdischild-2019-epa.667.
- Mazzoni F, Navarra D, Maimone M, Bavastrelli M. Childhood functional constipation: evaluation and treatment. Dig Liver Dis 2017;49:e265-6. https://doi.org/10.1016/j.dld.2017.09.063.
- Banaszkiewicz A, Szajewska H. Ineffectiveness of Lactobacillus GG as an adjunct to lactulose for the treatment of constipation in children: a double-blind, placebo-controlled randomized trial. J Pediatr 2005;146:364-9. https://doi.org/10.1016/j.jpeds.2004.10.022.
- Bu LN, Chang MH, Ni YH, Chen HL, Cheng CC. Lactobacillus casei rhamnosus Lcr35 in children with chronic constipation. Pediatr Int 2007;49:485-90. https://doi.org/10.1111/j.1442-200X.2007.02397.x.
- Guerra PVPL, Lima LN, Souza TC, Mazochi V, Penna FJ, Silva AM, et al. Pediatric functional constipation treatment with bifidobacterium-containing yogurt: a crossover, double-blind, controlled trial. World J Gastroenterol 2011;17:3916-21. https://doi.org/10.3748/wjg.v17.i34.3916.
- Hannah M, Juffrie S, Yati S. Effectiveness of synbiotics as laxative agent for constipation in children. Paediatr Indones 2008;48:136-41.
- Hashemi M, Javaheri J, Habibi M, Chaijan PY, Naziri M. Comparing the effect of probiotics and polyethylene glycol in treatment of childhood constipation. Arak Med Univ J 2015;18:78-85.
- Jadresin O, Sila S, Trivic I, Misak Z, Hojsak I, Kolacek S. Lack of benefit of lactobacillus reuteri DSM 17938 as an addition to the treatment of functional constipation. J Pediatr Gastroenterol Nutr 2018;67:763-6. https://doi.org/10.1097/MPG.0000000000002134.
- Jose S, Ismael MK. Effect of probiotics on constipation in children. Int J Contemp Pediatr 2018;5:46-9.
- Khodadad A, Sabbaghian M. Role of synbiotics in the treatment of childhood constipation: a double-blind randomized placebo controlled trial. Iran J Pediatr 2010;20:387-92.
- Russo M, Giugliano FP, Quitadamo P, Mancusi V, Miele E, Staiano A. Efficacy of a mixture of probiotic agents as complementary therapy for chronic functional constipation in childhood. Ital J Pediatr 2017;43. https://doi.org/10.1186/s13052-017-0334-3.
- Sadeghzadeh M, Rabieefar A, Khoshnevisasl P, Mousavinasab N, Eftekhari K. The effect of probiotics on childhood constipation: a randomized controlled double blind clinical trial. Int J Pediatr 2014;2014. https://doi.org/10.1155/2014/937212.
- Tabbers MM, Chmielewska A, Roseboom MG, Crastes N, Perrin C, Reitsma JB, et al. Fermented milk containing Bifidobacterium lactis DN-173 010 in childhood constipation: a randomized, double-blind, controlled trial. Pediatrics 2011;127:e1392-e9. https://doi.org/10.1542/peds.2010-2590.
- Wegner A, Banaszkiewicz A, Kierkus J, Landowski P, Korlatowicz-Bilar A, Wiecek S, et al. The effectiveness of Lactobacillus reuteri DSM 17938 as an adjunct to macrogol in the treatment of functional constipation in children. A randomized, double-blind, placebo-controlled, multicentre trial. Clin Res Hepatol Gastroenterol 2018;42:494-500. https://doi.org/10.1016/j.clinre.2018.03.008.
- Wojtyniak K, Horvath A, Dziechciarz P, Szajewska H. Lactobacillus casei rhamnosus Lcr35 in the management of functional constipation in children: a randomized trial. J Pediatr Gastroenterol Nutr 2017;64. https://doi.org/10.1097/01.mpg.0000516381.25680.b4.
- Castillejo G, Bullo M, Anguera A, Escribano J, Salas-Salvado J. A controlled, randomized, double-blind trial to evaluate the effect of a supplement of cocoa husk that is rich in dietary fiber on colonic transit in constipated pediatric patients. Pediatrics 2006;118:e641-8. https://doi.org/10.1542/peds.2006-0090.
- Chmielewska A, Horvath A, Dziechciarz P, Szajewska H. Glucomannan is not effective for the treatment of functional constipation in children: a double-blind, placebo-controlled, randomized trial. Clin Nutr 2011;30:462-8. https://doi.org/10.1016/j.clnu.2011.01.012.
- Kokke FT, Scholtens PA, Alles MS, Decates TS, Fiselier TJ, Tolboom JJ, et al. A dietary fiber mixture versus lactulose in the treatment of childhood constipation: a double-blind randomized controlled trial. J Pediatr Gastroenterol Nutr 2008;47:592-7. https://doi.org/10.1097/mpg.0b013e318162c43c.
- Loening-Baucke V, Miele E, Staiano A. Fiber (glucomannan) is beneficial in the treatment of childhood constipation. Pediatrics 2004;113:e259-64. https://doi.org/10.1542/peds.113.3.e259.
- Mozaffarpur SA, Naseri M, Esmaeilidooki MR, Kamalinejad M, Bijani A. The effect of cassia fistula emulsion on pediatric functional constipation in comparison with mineral oil: a randomized, clinical trial. Daru 2012;20. https://doi.org/10.1186/2008-2231-20-83.
- Nimrouzi M, Sadeghpour O, Imanieh MH, Shams Ardekani M, Salehi A, Minaei MB, et al. Flixweed vs. polyethylene glycol in the treatment of childhood functional constipation: a randomized clinical trial. Iran J Pediatr 2015;25. https://doi.org/10.5812/ijp.425.
- Quitadamo P, Coccorullo P, Giannetti E, Romano C, Chiaro A, Campanozzi A, et al. A randomized, prospective, comparison study of a mixture of acacia fiber, psyllium fiber, and fructose vs polyethylene glycol 3350 with electrolytes for the treatment of chronic functional constipation in childhood. J Pediatr 2012;161. https://doi.org/10.1016/j.jpeds.2012.04.043.
- Ustundag G, Kuloglu Z, Kirbas N, Kansu A. Can partially hydrolyzed guar gum be an alternative to lactulose in treatment of childhood constipation?. Turk J Gastroenterol 2010;21:360-4. https://doi.org/10.4318/tjg.2010.0121.
- Weber TK, Toporovski MS, Tahan S, Neufeld CB, De Morais MB. Dietary fiber mixture in pediatric patients with controlled chronic constipation. J Pediatr Gastroenterol Nutr 2014;58:297-302. https://doi.org/10.1097/MPG.0000000000000224.
- Crowley ET, Williams LT, Roberts TK, Dunstan RH, Jones PD. Does milk cause constipation? A crossover dietary trial. Nutrients 2013;5:253-66. https://doi.org/10.3390/nu5010253.
- Bekkali NLH, Hoekman DR, Liem O, Bongers MEJ, van Wijk MP, Zegers B, et al. Polyethylene glycol 3350 with electrolytes versus polyethylene glycol 4000 for constipation: a randomized, controlled trial. J Pediatr Gastroenterol Nutr 2018;66:10-5. https://doi.org/10.1097/MPG.0000000000001726.
- Benninga MA, Hussain SZ, Sood MR, Nurko S, Hyman P, Clifford RA, et al. Lubiprostone for pediatric functional constipation: randomized, controlled, double-blind study with long-term extension. Clin Gastroenterol Hepatol 2022;20:602-10.e5. https://doi.org/10.1016/j.cgh.2021.04.005.
- Cao Y, Liu SM. Lactulose for the treatment of Chinese children with chronic constipation: a randomized controlled trial. Medicine (Baltim) 2018;97. https://doi.org/10.1097/MD.0000000000013794.
- Esmaeilidooki MR, Mozaffarpur SA, Mirzapour M, Shirafkan H, Kamalinejad M, Bijani A. Comparison between the Cassia Fistula’s emulsion with polyethylene glycol (PEG4000) in the pediatric functional constipation: a randomized clinical trial. Iran Red Crescent Med J 2016;18. https://doi.org/10.5812/ircmj.33998.
- Gordon M, MacDonald JK, Parker CE, Akobeng AK, Thomas AG. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev 2016;2016. https://doi.org/10.1002/14651858.CD009118.pub3.
- Imanieh MH, Golpayegan MR, Sedighi M, Ahmadi K, Aghaie A, Dehghani SM, et al. Comparison of three therapeutic interventions for chronic constipation in paediatric patients with cerebral palsy: a randomised clinical trial. Prz Gastroenterol 2019;14:292-7. https://doi.org/10.5114/pg.2019.84872.
- Jarzebicka D, Sieczkowska-Golub J, Kierkus J, Czubkowski P, Kowalczuk-Kryston M, Pelc M, et al. PEG 3350 versus lactulose for treatment of functional constipation in children: randomized study. J Pediatr Gastroenterol Nutr 2019;68:318-24. https://doi.org/10.1097/MPG.0000000000002192.
- Modin L, Walsted AM, Dalby K, Jakobsen MS. Polyethylene glycol maintenance Treatment tor childhood functional constipation: a randomized, placebo-controlled trial. J Pediatr Gastroenterol Nutr 2018;67:732-7. https://doi.org/10.1097/MPG.0000000000002070.
- Pranoto WJ, Supriatmo MD, Sinuhaji AB. Oral versus rectal laxatives for functional constipation in children. Paediatr Indones 2016;56:162-6.
- Rachel H, Griffith AF, Teague WJ, Hutson JM, Gibb S, Goldfeld S, et al. Polyethylene glycol dosing for constipation in children younger than 24 months: a systematic review. J Pediatr Gastroenterol Nutr 2020;71:171-5. https://doi.org/10.1097/MPG.0000000000002786.
- Shatnawi MS, Alrwalah MM, Ghanma AM, Alqura’an ML, Zreiqat EN, Alzu’bi MM. Lactulose versus polyethylene glycol for disimpaction therapy in constipated children, a randomized controlled study. Sudan J Paediatr 2019;19:31-6. https://doi.org/10.24911/SJP.106-1546805996.
- Torabi Z, Amiraslani S, Diaz D, Ahmadiafshar A, Eftekhari K. Comparison of paraffin versus polyethylene plycol (PEG) in children with chronic functional constipation. Int Jof Pediatr 2017;5:5843-50.
- Farahmand F, Abedi A, Esmaeili-Dooki MR, Jalilian R, Tabari SM. Pelvic floor muscle exercise for paediatric functional constipation. J Clin Diagn Res 2015;9:SC16-7. https://doi.org/10.7860/JCDR/2015/12726.6036.
- Axelrod MI, Tornehl M, Fontanini-Axelrod A. Co-occurring autism and intellectual disability: a treatment for encopresis using a behavioral intervention plus laxative across settings. Clin Pract Pediatr Psychol 2016;4:1-10. https://doi.org/10.1037/cpp0000131.
- Hankinson JC, Borden L, Allen T, Santo Domingo L, Oliva-Hemker M, Mathews T, et al. Outcomes of combined medical and behavioral treatments for constipation within a specialty outpatient clinic. Clin Pract Pediatr Psychol 2018;6:31-4. https://doi.org/10.1037/cpp0000202.
- Jordan-Ely J, Dobson K, Hutson JM, Southwell BR. MOTIVATE-How to ensure compliance for large volume polyethylene glycol (PEG) for chronic constipation. J Gastroenterol Hepatol 2013;28. https://doi.org/10.1111/jgh.12365-8.
- Lomas Mevers J, Call NA, Gerencser KR, Scheithauer M, Miller SJ, Muething C, et al. A pilot randomized clinical trial of a multidisciplinary intervention for encopresis in children with autism spectrum disorder. J Autism Dev Disord 2020;50:757-65. https://doi.org/10.1007/s10803-019-04305-5.
- Soares AC, Tahan S, Morais MB. Effects of conventional treatment of chronic functional constipation on total and segmental colonic and orocecal transit times. J Pediatr (Rio J) 2009;85:322-8. https://doi.org/10.2223/JPED.1912.
- Speridiao PG, Tahan S, Fagundes-Neto U, Morais MB. Dietary fiber, energy intake and nutritional status during the treatment of children with chronic constipation. Braz J Med Biol Res 2003;36:753-9. https://doi.org/10.1590/s0100-879x2003000600011.
- Bekkali NL, van den Berg MM, Dijkgraaf MG, van Wijk MP, Bongers ME, Liem O, et al. Rectal fecal impaction treatment in childhood constipation: enemas versus high doses oral PEG. Pediatrics 2009;124:e1108-15. https://doi.org/10.1542/peds.2009-0022.
- Candy DC, Edwards D, Geraint M. Treatment of faecal impaction with polyethelene glycol plus electrolytes (PGE + E) followed by a double-blind comparison of PEG + E versus lactulose as maintenance therapy. J Pediatr Gastroenterol Nutr 2006;43:65-70. https://doi.org/10.1097/01.mpg.0000228097.58960.e6.
- Dupont C, Leluyer B, Maamri N, Morali A, Joye JP, Fiorini JM, et al. Double-blind randomized evaluation of clinical and biological tolerance of polyethylene glycol 4000 versus lactulose in constipated children. J Pediatr Gastroenterol Nutr 2005;41:625-33. https://doi.org/10.1097/01.mpg.0000181188.01887.78.
- Dziechciarz P, Horvath A, Szajewska H. Polyethylene glycol 4000 for treatment of functional constipation in children. J Pediatr Gastroenterol Nutr 2015;60:65-8. https://doi.org/10.1097/MPG.0000000000000543.
- Farahmand F. A randomised trial of liquid paraffin versus lactulose in the treatment of chronic functional constipation in children. Acta Medica Iranica 2007;45:183-8.
- Gomes PB, Duarte MA, de Melo MdCB. Comparison of the effectiveness of polyethylene glycol 4000 without electrolytes and magnesium hydroxide in the treatment of chronic functional constipation in children. J Pediatr (Rio J) 2011;87:24-8. https://doi.org/10.2223/JPED.2051.
- Gremse DA, Hixon J, Crutchfield A. Comparison of polyethylene glycol 3350 and lactulose for treatment of chronic constipation in children. Clin Pediatr (Phila) 2002;41:225-9. https://doi.org/10.1177/000992280204100405.
- Karami H, Khademloo M, Niari P. Polyethylene glycol versus paraffin for the treatment of childhood functional constipation. Iran J Pediatr 2009;19:255-61.
- Loening-Baucke V, Pashankar DS. A randomized, prospective, comparison study of polyethylene glycol 3350 without electrolytes and milk of magnesia for children with constipation and fecal incontinence. Pediatrics 2006;118:528-35. https://doi.org/10.1542/peds.2006-0220.
- Nurko S, Youssef NN, Sabri M, Langseder A, McGowan J, Cleveland M, et al. PEG3350 in the treatment of childhood constipation: a multicenter, double-blinded, placebo-controlled trial. J Pediatr 2008;153:254-61. https://doi.org/10.1016/j.jpeds.2008.01.039.
- Perkin JM. Constipation in childhood: a controlled comparison between lactulose and standardized senna. Curr Med Res Opin 1977;4:540-3. https://doi.org/10.1185/03007997709115268.
- Pitzalis G, Deganello F, Mariani P, Chiarini-Testa MB, Virgilii F, Gasparri R, et al. [Lactitol in chronic idiopathic constipation in children]. Pediatr Med Chir 1995;17:223-6.
- Rafati MR, Karami H, Salehifar E, Karimzadeh A. Clinical efficacy and safety of polyethylene glycol 3350 versus liquid paraffin in the treatment of pediatric functional constipation. Daru 2011;19:154-8.
- Ratanamongkol P, Lertmaharit S, Ongpiputvanich S. Polyethylene glycol 4000 without electrolytes versus milk of magnesia for the treatment of functional constipation in infants and young children: a randomized controlled trial. Asian Biomed 2009;3:391-9.
- Saneian H, Mostofizadeh N. Comparing the efficacy of polyethylene glycol (PEG), magnesium hydroxide and lactulosein treatment of functional constipation in children. J Res Med Sci 2012;17:S145-9.
- Thomson MA, Jenkins HR, Bisset WM, Heuschkel R, Kalra DS, Green MR, et al. Polyethylene glycol 3350 plus electrolytes for chronic constipation in children: a double blind, placebo controlled, crossover study. Arch Dis Child 2007;92:996-1000. https://doi.org/10.1136/adc.2006.115493.
- Tolia V, Lin CH, Elitsur Y. A prospective randomized study with mineral oil and oral lavage solution for treatment of faecal impaction in children. Aliment Pharmacol Ther 1993;7:523-9. https://doi.org/10.1111/j.1365-2036.1993.tb00128.x.
- Treepongkaruna S, Simakachorn N, Pienvichit P, Varavithya W, Tongpenyai Y, Garnier P, et al. A randomised, double-blind study of polyethylene glycol 4000 and lactulose in the treatment of constipation in children. BMC Pediatr 2014;14. https://doi.org/10.1186/1471-2431-14-153.
- Urganci N, Akyildiz B, Polat TB. A comparative study: the efficacy of liquid paraffin and lactulose in management of chronic functional constipation. Pediatr Int 2005;47:15-9. https://doi.org/10.1111/j.1442-200x.2004.02001.x.
- Voskuijl W, de Lorijn F, Verwijs W, Hogeman P, Heijmans J, Makel W, et al. PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial. Gut 2004;53:1590-4. https://doi.org/10.1136/gut.2004.043620.
- Wang BX, Wang MG, Jiang MZ, Xu CD, Shao CH, Jia LY, et al. Forlax in the treatment of childhood constipation: a randomized, controlled, multicenter clinical study. Zhongguo Dang Dai Er Ke Za Zhi 2007;9:429-32.
- Loening-Baucke V. Prevalence, symptoms and outcome of constipation in infants and toddlers. J Pediatr 2005;146:359-63. https://doi.org/10.1016/j.jpeds.2004.10.046.
- Loening-Baucke V, Krishna R, Pashankar DS. Polyethylene glycol 3350 without electrolytes for the treatment of functional constipation in infants and toddlers. J Pediatr Gastroenterol Nutr 2004;39:536-9.
- Michail S, Gendy E, Preud’Homme D, Mezoff A. Polyethylene glycol for constipation in children younger than eighteen months old. J Pediatr Gastroenterol Nutr 2004;39:197-9. https://doi.org/10.1097/00005176-200408000-00014.
- Romano C, Van Wynckel M, Hulst J, Broekaert I, Bronsky J, Dall’Oglio L, et al. European Society for Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J Pediatr Gastroenterol Nutr 2017;65:242-64. https://doi.org/10.1097/MPG.0000000000001646.
- Vilanova-Sanchez A, Gasior AC, Toocheck N, Weaver L, Wood RJ, Reck CA, et al. Are Senna based laxatives safe when used as long term treatment for constipation in children?. J Pediatr Surg 2018;53:722-7. https://doi.org/10.1016/j.jpedsurg.2018.01.002.
- Ng RT, Lee WS, Ang HL, Teo KM, Yik YI, Lai NM. Transcutaneous electrical stimulation (TES) for treatment of constipation in children. Cochrane Database Syst Rev 2016;2016. https://doi.org/10.1002/14651858.CD010873.pub4.
- Sharifi-Rad L, Ladi-Seyedian SS, Manouchehri N, Alimadadi H, Allahverdi B, Motamed F, et al. Effects of interferential electrical stimulation plus pelvic floor muscles exercises on functional constipation in children: a randomized clinical trial. Am J Gastroenterol 2018;113:295-302. https://doi.org/10.1038/ajg.2017.459.
- Yoo TB, Sun H. Efficacy and safety of combined oral and enema therapy using polyethylene glycol 3350-electrolyte for disimpaction in pediatric constipation. Pediatr Gastroenterol Hepatol Nutr 2017;20:244-51. https://doi.org/10.5223/pghn.2017.20.4.244.
- Jorgensen CS, Kamperis K, Modin L, Rittig CS, Rittig S. Transanal irrigation is effective in functional fecal incontinence. Eur J Pediatr 2017;176:731-6. https://doi.org/10.1007/s00431-017-2902-3.
- Koppen IJ, Kuizenga-Wessel S, Voogt HW, Voskeuil ME, Benninga MA. Transanal irrigation in the treatment of children with intractable functional constipation. J Pediatr Gastroenterol Nutr 2017;64:225-9. https://doi.org/10.1097/MPG.0000000000001236.
- Nasher OH, Richard E, Peeraully R, Wright A, Singh SJ. Peristeen (c) transanal irrigation system for paediatric faecal incontinence: a single centre experience. Int J Pediatr 2014;2014. https://doi.org/10.1155/2014/954315.
- Patel S, Hopson P, Bornstein JA, Safder S. Impact of transanal irrigation (TAI) device (Peristeen, coloplast) in management of pediatric patients with chronic fecal incontinence and constipation. J Pediatr Gastroenterol Nutr 2019;536. https://doi.org/10.1097/MPG.0000000000002518.
- Sharma V, Gordon K, Stevens S, Mew N, Rodrigues A, Howarth L. A single centre paediatric gastroenterology unit experience of use of peristeen, trans-anal irrigation system for paediatric faecal incontinence. Arch Dis Child 2016;101. https://doi.org/10.1136/archdischild-2016-310863.47.
- Jarzebicka D, Sieczkowska J, Dadalski M, Kierkus J, Ryzko J, Oracz G. Evaluation of the effectiveness of biofeedback therapy for functional constipation in children. Turk J Gastroenterol 2016;27:433-8. https://doi.org/10.5152/tjg.2016.16140.
- Raffaele A, Pasqua N, Fusillo M, Brunero M. The role of biofeedback in the treatment of chronic constipation and incontinence in children. Dig Liver Dis 2015;47:e240-1. https://doi.org/10.1016/j.dld.2015.07.058.
- Awan WA, Masood T. Role of stretching exercises in the management of constipation in spastic cerebral palsy. J Ayub Med Coll Abbottabad 2016;28:798-801.
- Eisenberg S, Zuk L, Carmeli E, Katz-Leurer M. Contribution of stepping while standing to function and secondary conditions among children with cerebral palsy. Pediatr Phys Ther 2009;21:79-85. https://doi.org/10.1097/PEP.0b013e31818f57f2.
- Waingankar K, Lai C, Punwani V, Wong J, Hutson JM, Southwell BR. Dietary exclusion of fructose and lactose after positive breath tests improved rapid-transit constipation in children. JGH Open 2018;2:262-9. https://doi.org/10.1002/jgh3.12079.
- Loening-Baucke V. Factors determining outcome in children with chronic constipation and faecal soiling. Gut 1989;30:999-1006. https://doi.org/10.1136/gut.30.7.999.
- Loening-Baucke V. Constipation in early childhood: patient characteristics, treatment, and longterm follow up. Gut 1993;34:1400-4. https://doi.org/10.1136/gut.34.10.1400.
- Modin LJ,IS, Jakobsen MS. ESPGHAN 49th Annual Meeting of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2016;62:1-890. https://doi.org/10.1097/01.mpg.0000484500.48517.e7.
- Ladi-Seyedian SS, Sharifi-Rad L, Alimadadi H, Nabavizadeh B, Manouchehri N, Allahverdi B, et al. Comparative efficacy of transcutaneous functional electrical stimulation with or without biofeedback therapy on functional non-retentive fecal incontinence in children: a randomized clinical trial. Dig Dis Sci 2022;67:989-96. https://doi.org/10.1007/s10620-021-07012-3.
- Bongers ME, van den Berg MM, Reitsma JB, Voskuijl WP, Benninga MA. A randomized controlled trial of enemas in combination with oral laxative therapy for children with chronic constipation. Clin Gastroenterol Hepatol 2009;7:1069-74. https://doi.org/10.1016/j.cgh.2009.06.018.
- Ormarsson OT, Asgrimsdottir GM, Loftsson T, Stefansson E, Lund SH, Bjornsson ES. Free fatty acid suppositories are as effective as docusate sodium and sorbitol enemas in treating constipation in children. Acta Paediatr Int J Paediatr 2016;105:689-94. https://doi.org/10.1111/apa.13394.
- Strisciuglio C, Coppola V, Russo M, Tolone C, Marseglia GL, Verrotti A, et al. Promelaxin microenemas are non-inferior to oral polyethylene glycol for the treatment of functional constipation in young children: a randomized clinical trial. Front Pediatr 2021;9. https://doi.org/10.3389/fped.2021.753938.
- Loening-Baucke V. Modulation of abnormal defecation dynamics by biofeedback treatment in chronically constipated children with encopresis. J Pediatr 1990;116:214-22. https://doi.org/10.1016/s0022-3476(05)82877-x.
- Nader EA, Zanotti M, Jais JP, Rousselle S, Norsa L, Goulet O, et al. Outcome of biofeedback treatment in children with fecal incontinence and establishment of an “envy score”. World Congress of Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2016;63. https://doi.org/10.1097/01.mpg.0000503536.79797.66.
- Nolan T, Catto-Smith T, Coffey C, Wells J. Randomised controlled trial of biofeedback training in persistent encopresis with anismus. Arch Dis Child 1998;79:131-5. https://doi.org/10.1136/adc.79.2.131.
- van der Plas RN, Benninga MA, Buller HA, Bossuyt PM, Akkermans LM, Redekop WK, et al. Biofeedback training in treatment of childhood constipation: a randomised controlled study. Lancet 1996;348:776-80. http://dx.doi.org/10.1016/s0140-6736(96)03206-0.
- Wald A, Chandra R, Gabel S, Chiponis D. Evaluation of biofeedback in childhood encopresis. J Pediatr Gastroenterol Nutr 1987;6:554-8. https://doi.org/10.1097/00005176-198707000-00011.
- Silva CAG, Motta EFA. The use of abdominal muscle training, breathing exercises and abdominal massage to treat paediatric chronic functional constipation. Colorectal Dis 2013;15:e250-5. https://doi.org/10.1111/codi.12160.
- van Engelenburg-van Lonkhuyzen ML, Bols EM, Benninga MA, Verwijs WA, de Bie RA. Effectiveness of pelvic physiotherapy in children with functional constipation compared with standard medical care. Gastroenterology 2017;152:82-91. https://doi.org/10.1053/j.gastro.2016.09.015.
- van Summeren JJGT, Holtman GA, Kollen BJ, Lisman-van Leeuwen Y, van Ulsen-Rust AHC, Tabbers MM, et al. Physiotherapy for children with functional constipation: a pragmatic randomized controlled trial in primary care. J Pediatr 2020;216. https://doi.org/10.1016/j.jpeds.2019.09.048.
- Ahmed Awan W, Masood T, Kanwal R. Effectiveness of physical therapy for improving constipation in spastic cerebral palsy. Altern Ther Health Med 2021;27:185-9.
- Borowitz SM, Cox DJ, Sutphen JL, Kovatchev B. Treatment of childhood encopresis: a randomized trial comparing three treatment protocols. J Pediatr Gastroenterol Nutr 2002;34:378-84. https://doi.org/10.1097/00005176-200204000-00012.
- Garcia MA, Castro RA, Monreal PD, Choa JM. The effectiveness of COMREST(constipation management, relief and support therapy) regimen for the treatment of fecal impaction among pediatric patients in a tertiary hospital. World Congress of Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2016;63. https://doi.org/10.1097/01.mpg.0000503536.79797.66.
- Davies C. The use of phosphate enemas in the treatment of constipation. Nurs Times 2004;100:32-5.
- US Food and Drug Administration (FDA) . FDA Drug Safety Communication: FDA Warns of Possible Harm Rom Exceeding Recommended Dose of Over-the-Counter Sodium Phosphate Products to Treat Constipation 2014. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-possible-harm-exceeding-recommended-dose-over-counter-sodium (accessed 10 June 2022).
- Chase J, Gibb SM, Clarke MCC, Catto-Smith A, Robertson V, Hutson JM. Transcutaneous electrical current to overcome slow transit constipation in children – an RCT. Neurogastroenterol Motil 2009;21.
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-135.
- Ahmadi J, Azary S, Ashjaei B, Paragomi P, Khalifeh-Soltani A. Intrasphincteric botulinum toxin injection in treatment of chronic idiopathic constipation in children. Iran J Pediatr 2013;23:574-8.
- Basson S, Charlesworth P, Healy C, Phelps S, Cleeve S. Botulinum toxin use in paediatric colorectal surgery. Pediatr Surg Int 2014;30:833-8. https://doi.org/10.1007/s00383-014-3536-4.
- Hallagan APH, Ryan K, Halleran D, Levitt M, Wood R, Bali N, et al. NASPGHAN Annual Meeting Abstracts. J Pediatr Gastroenterol Nutr 2019;69:S1-473. https://doi.org/10.1097/mpg.0000000000002518.
- Hameed AMN LT, Al-Ghafoor BH. A. Evaluation the efficacy and outcome of intersphciteric injection of botulinium toxin in the treatment of children with chronic idiopathic constipation in Iraqi hospital. Int J Pharm Sci Rev Res 2018;52:142-9.
- Zar-Kessler C, Kuo B, Belkind-Gerson J. Botulinum toxin injection for childhood constipation is safe and can be effective regardless of anal sphincter dynamics. J Pediatr Surg 2018;53:693-7. https://doi.org/10.1016/j.jpedsurg.2017.12.007.
- Janssen PTJ, Meyer YM, Van Kuijk SMJ, Benninga MA, Stassen LPS, Bouvy ND, et al. Long-term outcome of intractable constipation treated by sacral neuromodulation: a comparison between children and adults. Colorectal Dis 2018;20:134-43. https://doi.org/10.1111/codi.13837.
- Lu PL, Asti L, Lodwick DL, Nacion KM, Deans KJ, Minneci PC, et al. Sacral nerve stimulation allows for decreased antegrade continence enema use in children with severe constipation. J Pediatr Surg 2017;52:558-62. https://doi.org/10.1016/j.jpedsurg.2016.11.003.
- van Wunnik BP, Peeters B, Govaert B, Nieman FH, Benninga MA, Baeten CG. Sacral neuromodulation therapy; a promising treatment for adolescents with refractory functional constipation. J Pediatr Gastroenterol Nutr 2011;53:S81-2. https://doi.org/10.1097/MPG.0b013e31823cadc6.
- Sulkowski JP, Nacion KM, Deans KJ, Minneci PC, Levitt MA, Mousa HM, et al. Sacral nerve stimulation: a promising therapy for fecal and urinary incontinence and constipation in children. J Pediatr Surg 2015;50:1644-7. https://doi.org/10.1016/j.jpedsurg.2015.03.043.
- van der Wilt AA, van Wunnik BPW, Sturkenboom R, Han-Geurts IJ, Melenhorst J, Benninga MA, et al. Sacral neuromodulation in children and adolescents with chronic constipation refractory to conservative treatment. Int J Colorectal Dis 2016;31:1459-66. https://doi.org/10.1007/s00384-016-2604-8.
- van der Wilt AA, Groenewoud HHM, Benninga MA, Dirksen CD, Baeten C, Bouvy ND, et al. Cost-effectiveness of sacral neuromodulation for chronic refractory constipation in children and adolescents: a Markov model analysis. Colorectal Dis 2017;19:1013-23. https://doi.org/10.1111/codi.13869.
- Van Der Wilt AA, Breukink SO, Han IJ, Melenhorst J, Benninga MA, Baeten CGMI. Sacral neuromoduation in adolescents with constipation refractory to conservative treatment. Colorectal Dis 2014;16. https://doi.org/10.1111/codi.12708.
- Van Wunnik BP, Peeters B, Govaert B, Nieman FH, Benninga MA, Baeten CG. Sacral neuromodulation therapy: a promising treatment for adolescents with refractory functional constipation. Dis Colon Rectum 2012;55:278-85. https://doi.org/10.1097/DCR.0b013e3182405c61.
- Mousavi SA, Karami H, Rajabpoor AA. Intractable chronic constipation in children: outcome after anorectal myectomy. Afr J Paediatr Surg 2014;11:147-9. https://doi.org/10.4103/0189-6725.132810.
- Peyvasteh M, Askarpour S, Talaiezadeh AH, Imani MR, Javaherizadeh H. Results of posterior myectomy for the treatment of children with chronic constipation. Arq Gastroenterol 2015;52:299-302. https://doi.org/10.1590/S0004-28032015000400009.
- Redkar RG, Raj V, Bangar A, Hathiramani V, Chigicherla S, Tewari S. Role of ano rectal myomectomy in children with chronic refractory constipation. Afr J Paediatr Surg 2018;15:31-5. https://doi.org/10.4103/ajps.AJPS_99_17.
- Redkar RG, Mishra PK, Thampi C, Mishra S. Role of rectal myomectomy in refractory chronic constipation. Afr J Paediatr Surg 2012;9:202-5. https://doi.org/10.4103/0189-6725.104720.
- Basson S, Zani A, McDowell S, Athanasakos E, Cleeve S, Phelps S, et al. Antegrade continence enema (ACE): predictors of outcome in 111 patients. Pediatr Surg Int 2014;30:1135-41. https://doi.org/10.1007/s00383-014-3602-y.
- Bellomo-Brandao MAAVPA, Bustorff-Silva JM, Lomazi EA. Long-term outcomes in children with refractory constipation treated by conservative therapy or by antegrade continence enema procedure (ACE). J Pediatr Gastroenterol Nutr 2018;66.
- Chong C, Featherstone N, Sharif S, Cherian A, Cuckow P, Mushtaq I, et al. 5 years after an ACE: what happens then?. Pediatr Surg Int 2016;32:397-401. https://doi.org/10.1007/s00383-016-3857-6.
- Church JT, Simha S, Wild LC, Teitelbaum DH, Ehrlich PF. Antegrade continence enemas improve quality of life in patients with medically-refractory encopresis. J Pediatr Surg 2017;52:778-82. https://doi.org/10.1016/j.jpedsurg.2017.01.042.
- Dolejs SC, Smith JK, Sheplock J, Croffie JM, Rescorla FJ. Contemporary short- and long-term outcomes in patients with unremitting constipation and fecal incontinence treated with an antegrade continence enema. J Pediatr Surg 2017;52:79-83. https://doi.org/10.1016/j.jpedsurg.2016.10.022.
- Gomez-Suarez RA, Gomez-Mendez M, Petty JK, Fortunato JE. Associated factors for antegrade continence enemas for refractory constipation and fecal incontinence. J Pediatr Gastroenterol Nutr 2016;63:e63-8. https://doi.org/10.1097/MPG.0000000000001280.
- Har AF, Rescorla FJ, Croffie JM. Quality of life in pediatric patients with unremitting constipation pre and post Malone Antegrade Continence Enema (MACE) procedure. J Pediatr Surg 2013;48:1733-7. https://doi.org/10.1016/j.jpedsurg.2013.01.045.
- Hoekstra LT, Kuijper CF, Bakx R, Heij HA, Aronson DC, Benninga MA. The Malone antegrade continence enema procedure: the Amsterdam experience. J Pediatr Surg 2011;46:1603-8. https://doi.org/10.1016/j.jpedsurg.2011.04.050.
- Husberg BO-JM, Michanec M, Frenckner B, Svensson J. Lunchtime posters. Colorectal Dis 2011;13:16-27. https://doi.org/10.1111/j.1463-1318.2011.02706.x.
- King SK, Sutcliffe JR, Southwell BR, Chait PG, Hutson JM. The antegrade continence enema successfully treats idiopathic slow-transit constipation. J Pediatr Surg 2005;40:1935-40. https://doi.org/10.1016/j.jpedsurg.2005.08.011.
- Khoo AK, Askouni E, Basson S, Ng J, Cleeve S. How long will I have my ACE? The natural history of the antegrade continence enema stoma in idiopathic constipation. Pediatr Surg Int 2017;33:1159-66. https://doi.org/10.1007/s00383-017-4128-x.
- Mousa HM, van den Berg MM, Caniano DA, Hogan M, Di Lorenzo C, Hayes J. Cecostomy in children with defecation disorders. Dig Dis Sci 2006;51:154-60. https://doi.org/10.1007/s10620-006-3101-7.
- Mugie SM, Machado RS, Mousa HM, Punati JB, Hogan M, Benninga MA, et al. Ten-year experience using antegrade enemas in children. J Pediatr 2012;161:700-4. https://doi.org/10.1016/j.jpeds.2012.04.042.
- Peeraully MR, Lopes J, Wright A, Davies BW, Stewart RJ, Singh SS, et al. Experience of the MACE procedure at a regional pediatric surgical unit: a 15-year retrospective review. Eur J Pediatr Surg 2014;24:113-6. https://doi.org/10.1055/s-0033-1357502.
- Randall J, Coyne P, Jaffray B. Follow up of children undergoing antegrade continent enema: experience of over two hundred cases. J Pediatr Surg 2014;49:1405-8. https://doi.org/10.1016/j.jpedsurg.2014.02.090.
- Siddiqui AA, Fishman SJ, Bauer SB, Nurko S. Long-term follow-up of patients after antegrade continence enema procedure. J Pediatr Gastroenterol Nutr 2011;52:574-80. https://doi.org/10.1097/MPG.0b013e3181ff6042.
- Youssef NN, Barksdale JE, Griffiths JM, Flores AF, Di Lorenzo C. Management of intractable constipation with antegrade enemas in neurologically intact children. J Pediatr Gastroenterol Nutr 2002;34:402-5. https://doi.org/10.1097/00005176-200204000-00016.
- Cascio S, Flett ME, De la Hunt M, Barrett AM, Jaffray B. MACE or caecostomy button for idiopathic constipation in children: a comparison of complications and outcomes. Pediatr Surg Int 2004;20:484-7. https://doi.org/10.1007/s00383-004-1220-9.
- Vriesman MH, Wang L, Park C, Diefenbach KA, Levitt MA, Wood RJ, et al. Comparison of antegrade continence enema treatment and sacral nerve stimulation for children with severe functional constipation and fecal incontinence. Neurogastroenterol Motil 2020;32. https://doi.org/10.1111/nmo.13809.
- Wang L, Vriesman MH, Halleran DR, Diefenbach KA, Levitt MA, Wood RJ, et al. Prospective comparison of sacral nerve stimulation versus antegrade continence enemas for children with severe functional constipation. Gastroenterology 2019;156:S-201. https://doi.org/10.1016/S0016-5085%2819%2937294-4.
- Bonilla SF, Flores A, Jackson CC, Chwals WJ, Orkin BA. Management of pediatric patients with refractory constipation who fail cecostomy. J Pediatr Surg 2013;48:1931-5. https://doi.org/10.1016/j.jpedsurg.2012.12.034.
- Tamura RJ, Jaffray B. Outcomes of colonic resection for chronic idiopathic constipation in childhood. J Pediatr Surg 2020;55:269-72. https://doi.org/10.1016/j.jpedsurg.2019.10.047.
- Gasior A, Reck C, Vilanova-Sanchez A, Diefenbach KA, Yacob D, Lu P, et al. Surgical management of functional constipation: an intermediate report of a new approach using a laparoscopic sigmoid resection combined with malone appendicostomy. J Pediatr Surg 2018;53:1160-2. https://doi.org/10.1016/j.jpedsurg.2018.02.074.
- Kuizenga-Wessel S, Koppen IJN, Zwager LW, Di Lorenzo C, de Jong JR, Benninga MA. Surgical management of children with intractable functional constipation; experience of a single tertiary children’s hospital. Neurogastroenterol Motil 2017;29. https://doi.org/10.1111/nmo.13005.
- Lu PL, Koppen IJ, Orsagh-Yentis DK, Leonhart K, Ambeba EJ, Deans KJ, et al. Sacral nerve stimulation for treatment of constipation in children: long-term outcomes, patient benefit and parent satisfaction. World Congress of Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2016;63. https://doi.org/10.1097/01.mpg.0000503536.79797.66.
- Chase J, Shields N. A systematic review of the efficacy of non-pharmacological, non-surgical and non-behavioural treatments of functional chronic constipation in children. Aust N Z Continence J 2011;17:40-5.
- Aslam MS. Application of genus Cassia in the treatment of constipation: a systematic review. F1000Res 2021;8. https://doi.org/10.12688/f1000research.17893.2.
- Canbulat Sahiner N, Demirgoz Bal M. A randomized controlled trial examining the effects of reflexology on children with functional constipation. Gastroenterol Nurs 2017;40:393-400. https://doi.org/10.1097/SGA.0000000000000196.
- Duymaz T. The effectiveness of reflexology in children with cerebral palsy with constipation. Ann Clin Anal Med 2020;11:120-4. https://doi.org/10.4328/acam.6173.
- Orhan C, Kaya Kara O, Kaya S, Akbayrak T, Kerem Gunel M, Baltaci G. The effects of connective tissue manipulation and Kinesio Taping on chronic constipation in children with cerebral palsy: a randomized controlled trial. Disabil Rehabil 2018;40:10-2. https://doi.org/10.1080/09638288.2016.1236412.
- Shahamat M, Daneshfard B, Najib KS, Dehghani SM, Tafazoli V, Kasalaei A. Dry cupping in children with functional constipation: a randomized open label clinical trial. Afr J Tradit Complement Altern Med 2016;13:22-8. https://doi.org/10.21010/ajtcam.v13i4.4.
- Cai QH, Ma R, Hu SY, Li XM, Xiang XX, Ding Y, et al. A randomized controlled trial of Chinese Patent Medicine Xiao’er Biantong Granules in the treatment of functional constipation in children. Evid Based Complement Alternat Med 2018;2018. https://doi.org/10.1155/2018/4941505.
- Nimrouzi M, Sadeghpour O, Imanieh MH, Ardekani MS, Salehi A, Minaei MB, et al. Flixweed vs. polyethylene glycol in the treatment of childhood functional constipation: a randomized clinical trial. Iran J Pediatr 2015;25. https://doi.org/10.5812/ijp.425.
- Tavassoli S, Eftekhari K, Karimi M, Ghobadi A, Shati M, Naddaf A, et al. Effectiveness of viola flower syrup compared with polyethylene glycol in children with functional constipation: a randomized, active-controlled clinical trial. Evid Based Complement Alternat Med 2021;2021. https://doi.org/10.1155/2021/9915289.
- Qiao L, Wang LJ, Wang Y, Chen Y, Zhang HL, Zhang SC. A randomized, double-blind, and placebo-controlled trial of Chinese herbal medicine in the treatment of childhood constipation. Clin Transl Gastroenterol 2021;12. https://doi.org/10.14309/ctg.0000000000000345.
- Bromley D. Abdominal massage in the management of chronic constipation for children with disability. Community Pract 2014;87:25-9.
- Elbasan B, Bezgin S. The effects of reflexology on constipation and motor functions in children with cerebral palsy. Pediatr Neonatol 2018;59:42-7. https://doi.org/10.1016/j.pedneo.2017.01.005.
- Mostamand S, Busing L, Sicolo AR, Punati J, Danialifar TF. Initial experience with abdominal massage during pediatric colonic manometry. Gastroenterology 2019;156:S-777. https://doi.org/10.1016/S0016-5085%2819%2938894-8.
- Babaei AH, Nabavizadeh SS, Nimrouzi M. Traditional Persian medicine s measures for treatment of childhood constipation. Pak J Med Health Sci 2018;12:426-8.
- Zadpe A, Rathi R, Rathi B, Umate R. Study on the efficacy of Shunthyadi syrup in children with Vibandha (constipation). Wutan Huatan Jisuan Jishu 2020;XVI:555-69.
- Brazzelli M, Griffiths PV, Cody JD, Tappin D. Behavioural and cognitive interventions with or without other treatments for the management of faecal incontinence in children. Cochrane Database Syst Rev 2011;12.
- Freeman KA, Riley A, Duke DC, Fu R. Systematic review and meta-analysis of behavioral interventions for fecal incontinence with constipation. J Pediatr Psychol 2014;39:887-902. https://doi.org/10.1093/jpepsy/jsu039.
- Santucci N, Hyman P, Reuther E, Schindler M, Rein L, Van Tilburg M. A pilot randomized study to assess the effect of guided mastery, a specific behavioral intervention on outcomes in children with functional constipation. J Pediatr Gastroenterol Nutr 2018;67:S316-8. https://doi.org/10.1097/MPG.0000000000002164.
- Silver E, Williams A, Worthington F, Phillips N. Family therapy and soiling: an audit of externalizing and other approaches. J Fam Ther 1998;20:413-22.
- Taitz LS, Wales JK, Urwin OM, Molnar D. Factors associated with outcome in management of defecation disorders. Arch Dis Child 1986;61:472-7. https://doi.org/10.1136/adc.61.5.472.
- Cox DJ, Sutphen J, Ling W, Quillian W, Borowitz S. Additive benefits of laxative, toilet training, and biofeedback therapies in the treatment of pediatric encopresis. J Pediatr Psychol 1996;21:659-70. https://doi.org/10.1093/jpepsy/21.5.659.
- Cochrane Methods Economics . Glossary of Terms for Health Economics and Systematic Review 2022. https://methods.cochrane.org/economics/methods-training/glossary (accessed 22 March 2022).
- Alper A, Pashankar DS. Polyethylene glycol: a game-changer laxative for children. J Pediatr Gastroenterol Nutr 2013;57:134-40. https://doi.org/10.1097/MPG.0b013e318296404a.
- Bladder & Bowel UK . The Right Care Approach for Treating Idiopathic Constipation (Michael) 2017. www.bbuk.org.uk/wp-content/uploads/2017/12/The-Right-Care-Approach-for-Treating-Idiopathic-Constipation-Michael.pdf (accessed 28 May 2022).
- Bladder & Bowel UK . The Right Care Approach for Treating Intractable Constipation (James) 2017. www.bbuk.org.uk/wp-content/uploads/2017/12/The-Right-Care-Approach-for-Treating-Intractable-Constipation-James.pdf (accessed 28 May 2022).
- Choung RS, Shah ND, Chitkara D, Branda ME, Van Tilburg MA, Whitehead WE, et al. Direct medical costs of constipation from childhood to early adulthood: a population-based birth cohort study. J Pediatr Gastroenterol Nutr 2011;52:47-54. https://doi.org/10.1097/MPG.0b013e3181e67058.
- BIG 2020.
- Guest JF, Candy DC, Clegg JP, Edwards D, Helter MT, Dale AK, et al. Clinical and economic impact of using macrogol 3350 plus electrolytes in an outpatient setting compared to enemas and suppositories and manual evacuation to treat paediatric faecal impaction based on actual clinical practice in England and Wales. Curr Med Res Opin 2007;23:2213-25. https://doi.org/10.1185/030079907X210462.
- Guest JF, Clegg JP. Modelling the costs and consequences of treating paediatric faecal impaction in Australia. Curr Med Res Opin 2006;22:107-19. https://doi.org/10.1185/030079905X65583.
- Liem O, Harman J, Benninga M, Kelleher K, Mousa H, Di Lorenzo C. Health utilization and cost impact of childhood constipation in the United States. J Pediatr 2009;154:258-62. https://doi.org/10.1016/j.jpeds.2008.07.060.
- Mahon J, Lifschitz C, Ludwig T, Thapar N, Glanville J, Miqdady M, et al. The costs of functional gastrointestinal disorders and related signs and symptoms in infants: a systematic literature review and cost calculation for England. BMJ 2017;7. https://doi.org/10.1136/bmjopen-2016-015594.
- Moser NL, Plante WA, LeLeiko NS, Lobato DJ. Integrating behavioral health services into pediatric gastroenterology: a model of an integrated health care program. Clin Pract Pediatr Psychol 2014;2:1-12. https://doi.org/10.1037/cpp0000046.
- National Institute for Health and Care Excellence (NICE) . Constipation in Children and Young People: Diagnosis and Management [CG99] 2010. www.nice.org.uk/guidance/cg99 (accessed 25 September 2023).
- Goldman J. Learning about Constipation 2016. https://www.medicalhomeportal.org/living-with-child/diagnoses-and-conditions—faqs/constipation (accessed 25 September 2023).
- Phatak UP, Pashankar DS. Role of polyethylene glycol in childhood constipation. Clin Pediatr (Phila) 2014;53:927-32. https://doi.org/10.1177/0009922813505699.
- Ritterband LM, Thorndike FP, Lord HR, Borowitz S, Walker LS, Ingersoll KS, et al. An RCT of an Internet intervention for pediatric encopresis with one year follow-up. Clin Pract Pediatr Psychol 2013;1:68-80. https://doi.org/10.1037/cpp0000007.
- Rogers J. Functional constipation in childhood. Nurse Prescribing 2011;9:326-31.
- Rogers J. Working with families to boost children’s continence. Nurs Times 2012;108:16-8.
- Sandweiss DR, Allen L, Deneau M, Harnsberger J, Pasmann A, Smout R, et al. Implementing a standardized constipation-management pathway to reduce resource utilization. Acad Pediatr 2018;18:957-64. https://doi.org/10.1016/j.acap.2018.07.011.
- Sommers T, Corban C, Sengupta N, Jones M, Cheng V, Bollom A, et al. Emergency department burden of constipation in the United States from 2006 to 2011. Am J Gastroenterol 2015;110:572-9. https://doi.org/10.1038/ajg.2015.64.
- Southwell BR. Electro-neuromodulation for colonic disorders-review of meta-analyses, systematic reviews, and RCTs. Neuromodulation 2020;23:1061-81. https://doi.org/10.1111/ner.13099.
- Sparks B, Cooper J, Hayes C, Williams K. Constipation in children with autism spectrum disorder associated with increased emergency department visits and inpatient admissions. J Pediatr 2018;202:194-8. https://doi.org/10.1016/j.jpeds.2018.05.004.
- Stephens JR, Steiner MJ, Dejong N, Rodean J, Hall M, Richardson T, et al. Constipation-related health care utilization in children before and after hospitalization for constipation. Clin Pediatr (Phila) 2018;57:40-5.
- Van Summeren J, Holtman GA, Lisman-Van Leeuwen Y, Van Ulsen-Rust AHC, Vermeulen KM, Tabbers MM, et al. Cost-effectiveness of physiotherapy in childhood functional constipation: a pragmatic randomized controlled trial in primary care. J Pediatr Gastroenterol Nutr 2019;68.
- Wheeler J, Blumenthal K, Martinez M, Zobell S, Rollins M. Bedside anorectal manometry to guide management in pediatric patients with defecation disorders. J Pediatr Gastroenterol Nutr 2019;69. https://journals.lww.com/jpgn/toc/2019/11002.
- Windell J. Constipation: struggling in silence. Community Pract 2020;93:30-2.
- Youssef NN, Peters JM, Henderson W, Shultz-Peters S, Lockhart DK, Di Lorenzo C. Dose response of PEG 3350 for the treatment of childhood fecal impaction. J Pediatr 2002;141:410-4. https://doi.org/10.1067/mpd.2002.126603.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-50.
- Cochrane Methods Equity . PROGRESS-Plus 2021. https://methods.cochrane.org/equity/projects/evidence-equity/progress-plus (accessed 25-09-2023).
- Rogers J, Patricolo M. Addressing continence in children with disabilities. Nurs Times 2014;110:22-4.
- Romano C, van Wynckel M, Hulst J, Broekaert I, Bronsky J, Dall’Oglio L, et al. ESPGHAN-guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J Pediatr Gastroenterol Nutr 2017;65:242-64. https://doi.org/10.1097/MPG.0000000000001646.
- Steiner SA, Torres MRF, Penna FJ, Gazzinelli BF, Corradi CGA, Costa AS, et al. Chronic functional constipation in children: adherence and factors associated with drug treatment. J Pediatr Gastroenterol Nutr 2014;58:598-602. https://doi.org/10.1097/MPG.0000000000000255.
- Aquino A, Perini M, Cosmai S, Zanon S, Pisa V, Castagna C, et al. Osteopathic manipulative treatment limits chronic constipation in a child with Pitt-Hopkins syndrome. Case Rep Pediatr 2017;2017. https://doi.org/10.1155/2017/5437830.
- Inoue R, Sakaue Y, Kawada Y, Tamaki R, Yasukawa Z, Ozeki M, et al. Dietary supplementation with partially hydrolyzed guar gum helps improve constipation and gut dysbiosis symptoms and behavioral irritability in children with autism spectrum disorder. J Clin Biochem Nutr 2019;64:217-23. https://doi.org/10.3164/jcbn.18-105.
- Ritterband LM, Borowitz S, Cox DJ, Kovatchev B, Walker LS, Lucas V, et al. Using the internet to provide information prescriptions. Pediatrics 2005;116:e643-7. https://doi.org/10.1542/peds.2005-0404.
- Russell KW, Barnhart DC, Zobell S, Scaife ER, Rollins MD. Effectiveness of an organized bowel management program in the management of severe chronic constipation in children. J Pediatr Surg 2015;50:444-7. https://doi.org/10.1016/j.jpedsurg.2014.08.006.
- Trajanovska M, Liew A, Gibb S, Goldfeld S, King SK. Retrospective audit of referral and triage pathways of paediatric patients with constipation and soiling. J Paediatr Child Health 2020;56:298-303. https://doi.org/10.1111/jpc.14601.
- Aboumarzouk OM, Agarwal T, Antakia R, Shariff U, Nelson RL. Cisapride for intestinal constipation. Cochrane Database Syst Rev 2011;1. https://doi.org/10.1002/14651858.CD007780.pub2.
- Ahmed M, Pai B, Reynolds T. Use of polyethylene glycol in children less than 3 years of age. J Coll Physicians Surg Pak 2012;22:267-8.
- Boles EE, Gaines CL, Tillman EM. Comparison of polyethylene glycol-electrolyte solution vs polyethylene glycol-3350 for the treatment of fecal impaction in pediatric patients. J Pediatr Pharmacol Ther 2015;20:210-6. https://doi.org/10.5863/1551-6776-20.3.210.
- Berg I, Forsythe I, Holt P, Watts J. A controlled trial of ‘Senokot’ in faecal soiling treated by behavioural methods. J Child Psychol Psychiatry 1983;24:543-9. https://doi.org/10.1111/j.1469-7610.1983.tb00131.x.
- Dziechciarz P, Wojtyniak K, Horvath A, Szajewska H. Enema versus polyethylene glycol for the management of rectal faecal impaction in children with constipation – a systematic review of randomised controlled trials. Prz Gastroenterol 2015;10:234-8. https://doi.org/10.5114/pg.2015.52184.
- Erickson BA, Austin JC, Cooper CS, Boyt MA. Polyethylene glycol 3350 for constipation in children with dysfunctional elimination. J Urol 2003;170:1518-20. https://doi.org/10.1097/01.ju.0000083730.70185.75.
- Fujii Y, Morimoto T. Utility of lubiprostone for Japanese children with functional constipation. Iran J Pediatr 2019;29. https://doi.org/10.5812/ijp.81563.
- Hahn TW, Lee J. What is the safest treatment for constipation in children?. Evid Based Pract 2015;18.
- Heemskerk SCM, Rotteveel AH, Benninga MA, Baeten CIM, Masclee AAM, Melenhorst J, et al. Sacral neuromodulation versus personalized conservative treatment in patients with idiopathic slow-transit constipation: study protocol of the No. 2-trial, a multicenter open-label randomized controlled trial and cost-effectiveness analysis. Int J Colorectal Dis 2018;33:493-501. https://doi.org/10.1007/s00384-018-2978-x.
- Iacono G, Bonventre S, Scalici C, Maresi E, Di Prima L, Soresi M, et al. Food intolerance and chronic constipation: manometry and histology study. Eur J Gastroenterol Hepatol 2006;18:143-50. https://doi.org/10.1097/00042737-200602000-00006.
- Jang HJ, Shim JO, Seo JH, Chung JY. ESPGHAN 52nd annual meeting abstracts. J Pediatr Gastroenterol Nutr 2019;68:1-1243. https://doi.org/10.1097/mpg.0000000000002403.
- Kuizenga-Wessel S, Benninga MA, Tabbers MM. Reporting outcome measures of functional constipation in children from 0 to 4 years of age. J Pediatr Gastroenterol Nutr 2015;60:446-56. https://doi.org/10.1097/MPG.0000000000000631.
- Kuizenga-Wessel S, Heckert SL, Tros W, van Etten-Jamaludin FS, Benninga MA, Tabbers MM. Reporting on outcome measures of functional constipation in children – a systematic review. J Pediatr Gastroenterol Nutr 2016;62:840-6. https://doi.org/10.1097/MPG.0000000000001110.
- Li C, Shanahan S, Livingston MH, Walton JM. Malone appendicostomy versus cecostomy tube insertion for children with intractable constipation: a systematic review and meta-analysis. J Pediatr Surg 2018;53:885-91. https://doi.org/10.1016/j.jpedsurg.2018.02.010.
- Loening-Baucke V. Polyethylene glycol without electrolytes for children with constipation and encopresis. J Pediatr Gastroenterol Nutr 2002;34:372-7. https://doi.org/10.1097/00005176-200204000-00011.
- Ok JH, Kurzrock EA. Objective measurement of quality of life changes after ACE Malone using the FICQOL survey. J Pediatr Urol 2011;7:389-93. https://doi.org/10.1016/j.jpurol.2011.02.012.
- Parnell Prevost C, Gleberzon B, Carleo B, Anderson K, Cark M, Pohlman KA. Manual therapy for the pediatric population: a systematic review. BMC Complement Altern Med 2019;19. https://doi.org/10.1186/s12906-019-2447-2.
- Rodriguez L, Nurko S, Flores A. Factors associated with successful decrease and discontinuation of antegrade continence enemas (ACE) in children with defecation disorders: a study evaluating the effect of ACE on colon motility. Neurogastroenterol Motil 2013;25:140-e81. https://doi.org/10.1111/nmo.12018.
- Sadjadei N, Hosseinmardy S, Hakimzadeh M, Ziaei Kajbaf T, Javaherizadeh H. Anti-TTG among children with chronic functional constipation unresponsive to 6 weeks of treatment of constipation. Arq Gastroenterol 2017;54:197-200. https://doi.org/10.1590/s0004-2803.201700000-22.
- Saneian H, Tavakkol K, Adhamian P, Gholamrezaei A. Comparison of Lactobacillus Sporogenes plus mineral oil and mineral oil alone in the treatment of childhood functional constipation. J Res Medi Sci 2013;18:85-8.
- Santucci NRR, Lauren E, van Tilburg MA, Karpinski A, Rosenberg A, Amado-Feeley A, et al. Self-Efficacy in children with functional constipation is associated with treatment success. J Pediatr 2020;216:19-24. https://doi.org/10.1016/j.jpeds.2019.08.062.
- Sood M, Lichtlen P, Perez C. Unmet needs in pediatric functional constipation. J Pediatr Gastroenterol Nutr 2017;65:S259-60. https://doi.org/10.1097/MPG.0000000000001805.
- Sood M, Lichtlen P, Perez MC. Unmet needs in pediatric functional constipation. Clin Pediatr (Phila) 2018;57:1489-95. https://doi.org/10.1177/0009922818774343.
- Stewart ML, Schroeder NM. Dietary treatments for childhood constipation: efficacy of dietary fiber and whole grains. Nutr Rev 2013;71:98-109. https://doi.org/10.1111/nure.12010.
- Sullivan PB, Burnett CA, Juszczak E. Parent satisfaction in a nurse led clinic compared with a paediatric gastroenterology clinic for the management of intractable, functional constipation. Arch Dis Child 2006;91:499-501. https://doi.org/10.1136/adc.2005.087486.
- Torres MR, de Melo MdCB, Purcino FAC, Maia JC, Aliani NA, Rocha HC. Knowledge and practices of pediatricians regarding functional constipation in the State of Minas Gerais, Brazil. J Pediatr Gastroenterol Nutr 2015;61:74-9. https://doi.org/10.1097/MPG.0000000000000768.
- Tran K, Staller K, Macklin E, Goldstein A, Belkind-Gerson J, Kuo B. Need for rectal biopsy for childhood constipation predicts severity of illness and need for laxatives. J Pediatr Gastroenterol Nutr 2016;62:834-9. https://doi.org/10.1097/MPG.0000000000001033.
- van Ginkel R, Reitsma JB, Büller HA, van Wijk MP, Taminiau JA, Benninga MA. Childhood constipation: longitudinal follow-up beyond puberty. Gastroenterology 2003;125:357-63. https://doi.org/10.1016/s0016-5085(03)00888-6.
- van Schaick E, Benninga MA, Levine A, Magnusson M, Troy S. Development of a population pharmacokinetic model of prucalopride in children with functional constipation. Pharmacol Res Perspect 2016;4. https://doi.org/10.1002/prp2.236.
- Gardiner P, Kemper K. For GI complaints, which herbs and supplements spell relief?. Contemp Pediatr 2005;22:50-5.
- Habib S, Agarwal H. Milk of molasses enema-induced anaphylaxis in a pediatric patient. Crit Care Med 2019;47.
- Hardikar W, Cranswick N, Heine RG. Macrogol 3350 plus electrolytes for chronic constipation in children: a single-centre, open-label study. J Paediatr Child Health 2007;43:527-31. https://doi.org/10.1111/j.1440-1754.2007.01116.x.
- Herguner S, Herguner A. Atomoxetine for encopresis in 2 children with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 2012;32:302-3. https://doi.org/10.1097/JCP.0b013e3182499aba.
- Pashankar DS, Loening-Baucke V, Bishop WP. Safety of polyethylene glycol 3350 for the treatment of chronic constipation in children. Arch Pediatr Adolesc Med 2003;157:661-4. https://doi.org/10.1001/archpedi.157.7.661.
- Yang CH, Punati J. Practice patterns of pediatricians and trainees for the management of functional constipation compared with 2006 NASPGHAN guidelines. J Pediatr Gastroenterol Nutr 2015;60:308-11. https://doi.org/10.1097/MPG.0000000000000591.
- Yik YI, Leong LCY, Hutson JM, Southwell BR. The impact of transcutaneous electrical stimulation therapy on appendicostomy operation rates for children with chronic constipation – a single-institution experience. J Pediatr Surg 2012;47:1421-6. https://doi.org/10.1016/j.jpedsurg.2012.01.017.
- Firestone Baum C, John A, Srinivasan K, Harrison P, Kolomensky A, Monagas J, et al. Colon manometry proves that perception of the urge to defecate is present in children with functional constipation who deny sensation. J Pediatr Gastroenterol Nutr 2013;56:19-22. https://doi.org/10.1097/MPG.0b013e31826f2740.
- Jordan-Ely J, Dughetti LD, Dobson K, Stathopoulos L, Lamanna A, Leal M, et al. Paediatrics. J Gastroenterol Hepatol 2016;31:177-9. https://doi.org/10.1111/jgh.13527.
- Mosca NW, Schatz ML. Encopresis: not just an accident. NASN Sch Nurse 2013;28:218-21. https://doi.org/10.1177/1942602X13500994.
- O’Connor T. Supporting children to be ‘boss of their bowels’. Nurs N Z (Wellington, NZ: 1995) 2012;18.
- Radwan AB, El-Debeiky MS, Abdel-Hay S. Contrast enema as a guide for senna-based laxatives in managing overflow retentive stool incontinence in pediatrics. Pediatr Surg Int 2015;31:765-71. https://doi.org/10.1007/s00383-015-3741-9.
- Savino F, Viola S, Erasmo M, Di Nardo G, Oliva S, Cucchiara S. Efficacy and tolerability of peg-only laxative on faecal impaction and chronic constipation in children. A controlled double blind randomized study vs a standard peg-electrolyte laxative. BMC Pediatr 2012;12. https://doi.org/10.1186/1471-2431-12-178.
- Thomson MA, Tighe MP, Afzal NA. Constipation in children. Ital J Pediatr 2011;37. https://doi.org/10.1186/1824-7288-37-28.
- Turner-Bowker DM, Lindner E, Mareya S, Losch-Beridon T, Elash CA, Paty J, et al. Development and content validity of a pediatric functional constipation daily diary. Value Health 2015;18.
- Navarro F, Liu Y, Rhoads JM. Can probiotics benefit children with autism spectrum disorders?. World J Gastroenterol 2016;22:10093-102. https://doi.org/10.3748/wjg.v22.i46.10093.
- Tabbers MM, Boluyt N, Berger MY, Benninga MA. Constipation in children. BMJ Clin Evid 2010;2010.
- Tatsuki M, Miyazawa R, Tomomasa T, Ishige T, Nakazawa T, Arakawa H. Serum magnesium concentration in children with functional constipation treated with magnesium oxide. World J Gastroenterol 2011;17:779-83. https://doi.org/10.3748/wjg.v17.i6.779.
- Tort S, Pettersen K. How does polyethylene glycol (PEG) compare with placebo and other interventions for the management of childhood constipation?. Cochrane Clinical Answers 2016. http://dx.doi.org/10.1002/cca.1492.
- Yang CH, Punati J. Naspghan guidelines for functional constipation compared to the current practices of pediatricians. Gastroenterology 2014;146. https://doi.org/10.1016/S0016-5085%2814%2960612-0.
- Medaer E, Hoffman I, Van Laere K, Deroose CM. Colon transit scintigraphy: high impact on clinical decision making in severely constipated children. J Nucl Med 2019;60.
- Nath D. Complementary and alternative medicine in the school-age child with autism. J Pediatr Health Care 2017;31:393-7. https://doi.org/10.1016/j.pedhc.2016.12.001.
- Eicher P, Vitello L, Roche W, Martorana P, Kalderon V, Kalderon A. Children and constipation: poop is not a four letter word: a parent and professional perspective. Except Parent 2007;37:72-5.
- Jang HJ, Chung JY, Seo JH, Moon JS, Choe BH, Shim JO. Nationwide survey for application of ROME IV criteria and clinical practice for functional constipation in children. J Korean Med Sci 2019;34. https://doi.org/10.3346/jkms.2019.34.e183.
- Traslavina GAA, Ciampo LAD, Ferraz IS. Acute urinary retention in a pre-school girl with constipation. Rev Paul Pediatr 2015;33:488-92. https://doi.org/10.1016/j.rppede.2015.08.003.
- Wolfe I, Lemer C, Heys M, Ingrassia A, Newham J, Forman J, et al. Implementing and evaluating the CYPHP Evelina London new care model to improve health, healthcare quality, and patterns of service use among children and young people. Int J Integr Care (IJIC) 2019;19:1-2. https://doi.org/10.5334/ijic.s3253.
- Chaney R, Albright C, Howe D, Lu P, Yacob D, Manini ML, et al. NASPHAN Abstracts. J Pediatr Gastroenterol Nutr 2017;65:S1-359. https://doi.org/10.1097/mpg.0000000000001805.
- Dehghani SM, Askarian M, Kaffashan HA. Oral domperidone has no additional effect on chronic functional constipation in children: a randomized clinical trial. Indian J Gastroenterol 2014;33:125-30. https://doi.org/10.1007/s12664-013-0375-5.
- Sobhani Shahmirzadi M. Challenges and new treatment in childhood constipation. Int J Pediatr 2014;2.
- Steiner SA, Penna FJ, Gazzinelli BF, Corradi CGA, Costa AS, Ribeiro IG, et al. Poor adherence in the treatment of functional constipation in children: a population-based study. J Pediatr Gastroenterol Nutr 2011;53.
- MacGeorge CA, Williams DC, Vajta N, Morella K, Thacker PG, Russell S, et al. Understanding the constipation conundrum: predictors of obtaining an abdominal radiograph during the emergency department evaluation of pediatric constipation. Pediatr Emerg Care 2019;35:680-3. http://dx.doi.org/10.1097/PEC.0000000000001206.
- O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4587.
- Scientific Advisory Committee on Nutrition . Carbohydrate and Health 2015. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf (accessed 10 June 2022).
- Knowledge for Policy . Dietary Recommendations for Dietary Fibre Intake 2022. https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/dietary-fibre-recommendations-2_en#:~:text=‘The%20average%20population%20intake%20of,years%20about%2030g%2F%20day (accessed 10 June 2022).
- MHRA . Domperidone: Risks of Cardiac Side Effects 2014. www.gov.uk/drug-safety-update/domperidone-risks-of-cardiac-side-effects (accessed 25 September 2023).
- Dale M, Morgan H, Carter K, White J, Carolan-Rees G. Peristeen transanal irrigation system to manage bowel dysfunction: a NICE medical technology guidance. Appl Health Econ Health Policy 2019;17:25-34. http://dx.doi.org/10.1007/s40258-018-0447-x.
- NHS Inform . Constipation 2022. www.nhsinform.scot/illnesses-and-conditions/stomach-liver-and-gastrointestinal-tract/constipation (accessed 10 June 2022).
- ERIC . Bowel Problems 2022. www.eric.org.uk/pages/category/bowel-problems (accessed 10 June 2022).
- Avelar Rodriguez D, Popov J, Ratcliffe EM, Toro Monjaraz EM. Functional constipation and the gut microbiome in children: preclinical and clinical evidence. Front Pediatr 2020;8. https://doi.org/10.3389/fped.2020.595531.
- Thompson AP, Wine E, MacDonald SE, Campbell A, Scott SD. Parents’ experiences and information needs while caring for a child with functional constipation: a systematic review. Clin Pediatr (Phila) 2021;60:154-69. https://doi.org/10.1177/0009922820964457.
- National Institute for Health and Care Excellence (NICE) . Constipation in Children and Young People: Quality Standard [QS62] 2014. www.nice.org.uk/guidance/qs62/chapter/Quality-statement-2-First-line-treatment-with-laxatives (accessed 10 June 2022).
- Newham JJ, Forman J, Heys M, Cousens S, Lemer C, Elsherbiny M, et al. Children and Young People’s Health Partnership (CYPHP) Evelina London model of care: protocol for an opportunistic cluster randomised controlled trial (cRCT) to assess child health outcomes, healthcare quality and health service use. BMJ 2019;9. https://doi.org/10.1136/bmjopen-2018-027301.
- Tian H, Ding C, Gong J, Ge X, McFarland LV, Gu L, et al. An appraisal of clinical practice guidelines for constipation: a right attitude towards to guidelines. BMC Gastroenterol 2016;16. https://doi.org/10.1186/s12876-016-0466-8.
- NHS . The NHS Long Term Plan 2019. www.longtermplan.nhs.uk (accessed 14 June 2022).
- National Institute for Health and Care Excellence (NICE) . Babies, Children and Young People’s Experience of Healthcare 2021.
- Veugelers R, Benninga MA, Calis EA, Willemsen SP, Evenhuis H, Tibboel D, et al. Prevalence and clinical presentation of constipation in children with severe generalized cerebral palsy. Dev Med Child Neurol 2010;52:e216-21. https://doi.org/10.1111/j.1469-8749.2010.03701.x.
- Fischer M, Adkins W, Hall L, Scaman P, Hsi S, Marlett J. The effects of dietary fibre in a liquid diet on bowel function of mentally retarded individuals. J Ment Defic Res 1985;29:373-81. https://doi.org/10.1111/j.1365-2788.1985.tb00363.x.
- Wegh CAM, Baaleman DF, Tabbers MM, Smidt H, Benninga MA. Nonpharmacologic treatment for children with functional constipation: a systematic review and meta-analysis. J Pediatr 2022;240:136-149.e5. https://doi.org/10.1016/j.jpeds.2021.09.010.
- Tang J, Li H, Tang W. Efficacy of non-pharmacologic auxiliary treatments in improving defecation function in children with chronic idiopathic constipation: a systematic review and network meta-analysis. Front Pediatr 2021;9. https://doi.org/10.3389/fped.2021.667225.
- Wallace C, Sinopoulou V, Gordon M, Akobeng AK, Llanos-Chea A, Hungria G, et al. Probiotics for treatment of chronic constipation in children. Cochrane Database Syst Rev 2022;3. https://doi.org/10.1002/14651858.CD014257.pub2.
- Liu Z, Gang L, Yunwei M, Lin L. Clinical edfficacy of infantile massage in the treatment of infant functional constipation: a meta-analysis. Front Public Health 2021;9. https://doi.org/10.3389/fpubh.2021.663581.
- Mosiello G, Marshall D, Rolle U, Cretolle C, Santacruz BG, Frischer J, et al. Consensus review of best practice of transanal irrigation in children. J Pediatr Gastroenterol Nutr 2017;64:343-52. https://doi.org/10.1097/MPG.0000000000001483.
- Vande Velde S, Van Renterghem K, Van Winckel M, De Bruyne R, Van Biervliet S. Constipation and fecal incontinence in children with cerebral palsy. Overview of literature and flowchart for a stepwise approach. Acta Gastroenterol Belg 2018;81:415-8.
- Siminas S, Losty PD. Current surgical management of pediatric idiopathic constipation: a systematic review of published studies. Ann Surg 2015;262:925-33. https://doi.org/10.1097/SLA.0000000000001191.
- Wood RJ, Yacob D, Levitt MA. Surgical options for the management of severe functional constipation in children. Curr Opin Pediatr 2016;28:370-9. https://doi.org/10.1097/MOP.0000000000000345.
- Liyanarachchi H, Rajindrajith S, Kuruppu C, Chathurangana P, Ranawaka R, Devanarayana NM, et al. Association between childhood constipation and exposure to stressful life events: a systematic review. Neurogastroenterol Motil 2022;34. https://doi.org/10.1111/nmo.14231.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65-94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136.
- PRISMA-A . PRISMA 2020 for Abstracts 2022. https://prisma-statement.org//Extensions/Abstracts (accessed 14 June 2022).
- ESSITY . Bottom of the Class: Are Hygiene Standards Failing Primary School Children? 2022. www.essity.com/Images/Essity-Bottom-of-the-Class_tcm339-84689.pdf (accessed 8 November 2022).
- Bladder & Bowel UK . Managing Bladder and Bowel Issues in Nurseries, Schools and Colleges: Guidance for School Leaders, Proprietors, Governors, Staff and Practitioners 2022. https://eric.org.uk/wp-content/uploads/2022/10/Managing-Bowel-and-Bladder-issues-in-nurseries-colleges-and-schools-2022.pdf (accessed 8 November 2022).
- Gotzsche PC. Why we need a broad perspective on meta-analysis. BMJ 2000;321:585-6.
- Weir MC, Grimshaw JM, Mayhew A, Fergusson D. Decisions about lumping vs. splitting of the scope of systematic reviews of complex interventions are not well justified: a case study in systematic reviews of health care professional reminders. J Clin Epidemiol 2012;65:756-63. https://doi.org/10.1016/j.jclinepi.2011.12.012.
- Cox DJ, Sutphen J, Borowitz S, Kovatchev B, Ling W. Contribution of behavior therapy and biofeedback to laxative therapy in the treatment of pediatric encopresis. Ann Behav Med 1998;20:70-6. https://doi.org/10.1007/BF02884451.
- van Wunnik BP, Visschers RG, van Asselt AD, Baeten CG. Cost-effectiveness analysis of sacral neuromodulation for faecal incontinence in The Netherlands. Colorectal Dis 2012;14:e807-14. https://doi.org/10.1111/codi.12002.
Appendix 1 Overview of the stakeholder meetings held throughout the SUCCESS project
The following table provides an overview of the SG meetings held throughout the SUCCESS project:
| Date | Format – key focus | Length | Key agenda items | Key outcomes |
|---|---|---|---|---|
| 28 and 29 January 2020 | Face-to-face – Activity 1 | 2 days (second day finished early to allow travel) |
Project information Role of PPI Group Creation of taxonomy Research training Combination and sequences of treatment |
1. Draft taxonomy created 2. Draft pyramid of intervention sequence 3. Discussion around prevention and diagnosis |
| 31 March 2020 | Online – Activity 2 | 3 hours | Progress on abstract screening Confirmed outcomes for project How to prioritise treatments for effectiveness review |
1. Outcomes agreed following voting exercise 2. Pyramid should guide prioritisation |
| 13 May 2020 | Online – PPI stakeholders | 2 hours | Group membership Terminology Laxatives Online experience Pyramid model Research questions |
1. Terminology agreed 2. Pyramid model amended 3. Research questions amended |
| 6 July 2020 | Online – update | 3 hours | Project update Scoping review update Effectiveness review update Dissemination ideas Statistical analyses training |
Ranking of RQs to prioritise |
| 9 September 2020 | Online – task | 2 hours | Project update Scoping review Interventions discussion Dissemination ideas |
Categorisation agreed for scoping review papers |
| 1 December 2020 | Online – update | 1.5 hours | Project update Care provision effective review results Implications for clinicians and carers |
Missed opportunities for early intervention highlighted Evidence gaps identified |
| 9 February 2021 | Online – Activity 3 | 2 hours | Summary of intervention review Logic model |
Guidelines available but not being implemented Child protection issues important research gap Implementation review draft to be circulated for feedback |
| 08 March 2021 | Online – Activity 4 | 1 hour | Authorship order Effectiveness review Level 0 and 1 |
Gaps identified for Level 0 |
| 12 April 2021 | Online – PPI stakeholders | 1 hour | Project update PPI paper Meeting format |
Decision not to progress with a PPI-led paper at this stage |
| 28 April 2021 | Online – update | 1 hour | Application for extension Dissemination plans |
Dissemination plan amended |
| 29 June 2021 | Online – Activity 4 | 2 hours | ICS Abstracts Level 0 and 1 results Cost-effectiveness results |
Implications of Level 0 and Level 1 results highlighted Gaps identified |
| 13 September 2021 | Online – Activity 4 | 2 hours | Project update Economic evaluation Interactive maps Report writing |
Need to include videos alongside maps Report writing plan |
| 16 November 2021 | Online – Activity 4 | 1.5 hours | Feedback on Level 3 review | Implications of review discussed |
| 30 November 2021 | Online – Activity 4 | 1.5 hours | Feedback on Level 2 review | Implications of review discussed Research gaps identified |
| 25 January 2022 | Online – Activity 3 and 4 | 1.5 hours | NIHR report update Stakeholder involvement reporting GRIPP2 format Report writing Logic Model |
Report writing plans including stakeholder chapter GRIPP2 LF as reporting guideline |
| 15 March 22 | Online – Activity 3 and 4 | 1.5 hours | NIHR report writing Logic model |
Report writing plans Updated version of logic model |
| 20 April 22 | Online – Activity 3 and 4 | 1.5 hours | NIHR report writing Stakeholder involvement reporting Logic model Interpretation of findings 5. Dissemination plan |
Report writing plans updated Version of logic model agreed Dissemination plan updated |
| 24 May 2022 | Online – update | 1.5 hours | NIHR report writing Logic model Research recommendations |
Clarification of terminology for final report Presentation of logic model within final report agreed Grouping of research recommendation for final report. Key research priorities. |
Appendix 2 Intervention taxonomy
Figure 28 identifies the main headings under which interventions were grouped.
FIGURE 28.
Intervention taxonomy.
Table 13 identifies subgroups of interventions under each of the main headings and, where appropriate, specific named interventions under the subheadings.
| Main heading | Subheading | Specifics |
|---|---|---|
| Surgical | Anorectal myectomy | |
| Anal and pelvic floor interventions | ||
| Colon resection with anastomosis and rectal operations | ||
| Operations that provide ACE | ACEs | |
| MACE | ||
| Permanent or long-term stoma |
Cecostomy/caecostomy button | |
| Manual evacuation | ||
| Rectal biopsy | ||
| Irrigation | Colonic irrigation | |
| ACEs | ||
| MACE | ||
| Pharmacological | Laxatives | PEG
|
| Senakot (Senna concentration) | ||
| Oral bisacodyl | ||
| Glucomannan | ||
| Lactulose | ||
| Macrogol (e.g. Movicol) | ||
| Cassia fistula’s emulsion | ||
| Sodium picosulphate | ||
| Ducosate sodium | ||
| Milk of magnesia | ||
| Mineral oil | ||
| Botox injection in the anal sphincter | ||
| Enema | Paraffin | |
| Saline | ||
| Sodium-dioctyl sulfosuccinate and Sorbitol | ||
| Milk and molasses | ||
| Soap enema | ||
| Suppository | Glycerol suppositories | |
| Domperidone | ||
| Lubiprostone | ||
| Osmotic bulk forming stimulants | ||
| Lubricating agents | ||
| Lifestyle | Exercise | Standing |
| Yoga | ||
| Strength training | ||
| Physical therapies | ||
| Diet | Tailored diet management | |
| Dietary fibre and whole grains | ||
| Probiotics – Lactobacillus GG – Bifidobacteria – other micro-organisms |
||
| Prebiotics | ||
| Soy milk | ||
| Diet restriction/diet replacement (e.g. removing cow’s milk from diet) | ||
| Milk formulas | ||
| Goat’s yoghurt | ||
| Supplements | ||
| Fluids | ||
| Toileting programmes | ||
| Information | Wider children’s workforce | |
| Peer support | ||
| Parental training and advice | ||
| Educational leaflets | ||
| Lifestyle advice | ||
| Psychosocial | Psychotherapy | |
| Counselling and talking therapies | ||
| Incentives (e.g. reward system or financial) | ||
| Interventions aimed at social issues | Social stories | |
| Care provision | Consistency of care | |
| Continuity of care | ||
| Model of care | Nurse-led clinics | |
| Consultant-led clinics | ||
| Bowel management clinics | ||
| Other | Complementary and/or alternative therapies | Reflexology |
| Connective tissue massage | ||
| Acupuncture | ||
| Mind-body therapy | ||
| Homeopathy | ||
| Musculoskeletal manipulations (e.g. osteopathy, chiropractic manipulation) | ||
| Neuromodulation | TES | |
| Sacral modulation | ||
| Tibial nerve stimulation | ||
| Feedback | EMG biofeedback | |
| Biofeedback at home | ||
| Biofeedback – video games controlled by external sphincter activity | ||
| Manometry | ||
| External anal sphincter EMG biofeedback | ||
| Equipment | Continence containment products | |
| Toilet posture equipment | ||
| Other | ||
| Other | Kinesio taping |
Appendix 3 Search strategy
MEDLINE(R) ALL (Ovid) from January 2011 to 10 March 2020
-
exp Child/ 1881370
-
exp Child, Preschool/ 904222
-
exp Infant/ 1123826
-
exp Infant, Newborn/ 599339
-
exp Infant, Low Birth Weight/or exp Infant, Very Low Birth Weight/or exp Infant, Premature 78406
-
exp Infant, Postmature/ 380
-
exp Adolescent/ 1994792
-
exp Pediatrics/ 57079
-
(child$ or infant$ or infancy or newborn$ or neonat$ or baby or babies or preschool or pre school or pre-school or pubescen$ or teen$ or adolescen$ or puber$ or prepubert$ or juvenil$ or p?ediatric$ or youth$ or schoolchild$ or school age$ or schoolage$ or elementary school or high school$ or highschool$ or kindergar$ or boy or boys or girl$ or minors or underag$ or under ag$ or kid or kids or toddler$ or preteen$ or pre-teen$ or young).ti,ab. 2707405
-
or/1-9 4499018
-
exp Constipation/ 13797
-
exp Encopresis/ 663
-
exp Intestinal Obstruction/ or exp Fecal Incontinence/or exp Fecal Impaction/ 56251
-
exp Megacolon/ 7353
-
(constipat$ or obstipat$ or coprostasis or soiling or encopresis or fecal incontin$ or faecal incontin$ or feces incontin$ or fecal impact$ or faecal impact$ or fecally impact$ or faecally impact$ or feces impact$ or faeces impact$ or fnrfi or functional defecation disorder$ or functional defaecation disorder$ or functional gastrointestinal disorder$).ti,ab. 32722
-
((difficult$ or retent$ or delay$ or irregular$ or infrequent$ or pain$ or strain$) adj3 (defecat$ or stool$ or faeces or feces or bowel movement$ or evacuat$ or anorectal$)).ti,ab. 3970
-
(fecalith$ or faecalith$ or coprolith$ or stercolith$).ti,ab. 315
-
(megacolon$ or megarectum$).ti,ab. 2811
-
or/11-18 95145
-
10 and 19 28849
-
exp animals/not humans.sh. 4675679
-
20 not 21 28649
-
limit 22 to yr= ‘2011 -Current’ 7569
The search strategy adapted a previously published search (see NICE 2012 guidelines19).
Appendix 4 Characteristics of included studies: effectiveness reviews
| Study | Aim | Inclusion criteria | Study design | No. of participants | Participant demographics | Intervention | Findings: painful defaecation | Findings: QoL |
|---|---|---|---|---|---|---|---|---|
| RCTs | ||||||||
| Burnett 200458 | To evaluate the effectiveness of a NLC compared with a consultant-led PGC in the management of chronic constipation | Children between the ages of 1 and 15 years presenting to the paediatric gastroenterology with constipation were potentially eligible. | RCT | 102 | Nurse-led group: median age 55.5 months (range 13–164). Physician-led group: median age 58 months (range 24–175). |
Nurse-led clinic: an evidence-based treatment algorithm for the management of CFC based on published guidelines, was constructed. | NR | NR |
| 55 boys, 47 girls. Children with organic or neurological disease were not included. | This standardised treatment algorithm provided the basis for management decisions in all consultations in both the nurse-led clinic and the paediatric gastroenterology clinic. The initial phases in this algorithm involved child and parent education about diet | |||||||
| (fibre and fluid), exercise, toilet training and the actions of the laxatives prescribed. Laxative therapy comprised a combination of stool softeners (e.g. lactulose, docusate sodium) and stimulants. Stimulants of different potencies | ||||||||
| (senna, bisacodyl, sodium picosulphate) were prescribed according to the clinical response as indicated by the bowel diaries. If there was an inadequate clinical response to this initial phase, the patient moved on to an advanced treatment regime which might include, enemas, intestinal lavage, manual removal of faeces under general anaesthesia, or psychological referral as was appropriate in each case. | ||||||||
| Duration: median number of visits was 6. | ||||||||
| Faramarzian 201859 | To investigate the impact of nurse-based approaches on FC in children | 3- to 14-year-old children via Imam Reza Clinic of Shiraz diagnosed with chronic FC based on the data gathering form (including ROME III criteria items) and confirmed by a paediatric gastroenterologist | RCT | 120 recruited. 95 after intervention | Mean age in experimental and control groups were 6.25 ± 2.71 and 6.21 ± 2.68, respectively. 56 boys, 39 girls. Mean duration of constipation in experimental and control groups was respectively 35.80 ± 28.55 and 44.33 ± 34.6 months |
Nurse-led clinic: a comprehensive nurse-centred programme in addition to usual treatment that was prescribed by the doctor. Each child participated in three training sessions with his/her parents at the Clinic. In each of these sessions, certain subjects were discussed and three training pamphlets containing the required training notes (points on introducing constipation and points related to nutrition and behaviour) were given to the parents. | Self-reported painful defaecation incorporated into ‘improvement’ score, and reported as percentage of children improved: after 4 weeks: 70.2% in the experimental group and 70.8% in the control subjects (p value = 0.947). After 8 weeks, 83% and 68.8%, respectively (p value = 0.106). | NR |
| In addition, the researcher nurse was available 1 day a week; she offered the child’s parents necessary recommendations and advice if required. Duration: The follow-up period continued for 3 months. During this period, the nurse contacted the children and their parents by telephone, assessing their status and offering advice. | After 12 weeks: 83 and 72.9%, respectively (p value = 0.237). | |||||||
| Karagiozoglou-Lampoudi 201269 | To evaluate adherence of paediatric patients with refractory functional constipation to a high-water, high-fibre diet following the physician’s dietary instructions based on official treatment guidelines or personalised dietetic management according to patient’s needs, based on the same official treatment guidelines | Patients referred to the paediatric gastroenterology clinic with FC diagnosed according to the NASPGHAN. Patients were referred because the constipation was refractory to treatment by paediatrician for several months and/or presented with complications. | RCT | 86 | Mean age: 4.4 years. Range 1–11 years. 44 boys, 42 girls. |
In both groups: parents were given written instructions about their children’s diet with examples explained by a paediatric gastroenterologist. Children also received lactulose. In intervention group: addition of a personalised diet management by a registered dietitian – each child and his or her parents had a further appointment the same day with a registered dietitian who prescribed a personalised diet (7-day diet plan) based on the Mediterranean-type eating plan, and calculated to cover the personal energy, nutrient, water and fibre requirements of paediatric patients. In addition, the dietitian provided high-fibre recipes. Duration: one clinic visit. | NR | NR |
| Modin 201671 | To evaluate whether follow-up by phone or self-management through web-based information improved treatment outcomes. | Children, ages 2–16 years, referred from GPs to the paediatric outpatient clinic between June 2012 and September 2013. To be included, children had to fulfil two ROME III criteria for FC for 2 months: defaecation frequency <3 times per week, 1 episode of faecal incontinence per week, retentive posturing, painful bowel movements, large faecal mass in the rectum and large-diameter stools. | RCT | 235 recruited. 206 after intervention | Age, median (range) for the three groups, respectively: 6.3 (2.1–12.8), 6.3 (2.3–15.8), 5.8(2–14.3). 123 boys, 112 girls. Constipation Duration, median (range) in months, for the 3 groups, respectively 12 (2–72), 12 (2–36), 12 (2–48) |
All groups: treated according to the first three steps in the treatment algorithm comprising information, disimpaction and maintenance treatment. Information included facts about CFC pathophysiology, instructions for PEG3350 disimpaction (1.5 g/kg/day), PEG maintenance treatment, and the use of the Bristol stool chart. Phone group: two scheduled phone contacts after 1 and 4 weeks. During phone call – provided information on constipation and advice regarding adjustment of PEG treatment. |
NR | NR |
| Web group: provided a password to a website containing information about CFC (pathophysiology, treatment principles, Bristol stool scale, PEG administration, toilet training and common treatment pitfalls and solutions). | ||||||||
| Other primary study designs | ||||||||
| Athanasakos, 202065 | To assess the impact of an innovative CAPS focusing on improving outcomes in children with CFC and faecal incontinence | All patients referred to CAPS. Children with CFC and FI. | Non-comparative study | 112 patients: 89 (79%) had CFC/FI; 9 (8%), Hirschsprung’s disease; 12 (11%), anorectal malformations and 2 (2%), trauma. | Median 9 years (17 months to 16 years). 66 males (59%); 46 females |
Children’s anorectal physiology service performs investigations: colonic transit studies, awake high-resolution anorectal manometry, endoanal ultrasound, defaecating proctogram and bowel, psychological and QoL assessments. A multidisciplinary team | NR | Paediatric QoL inventory: ‘PedsQol of life was found poor in 57 (55%)’ (no other relevant data presented) |
| (clinical physiologist, clinical nurse specialists, clinical psychologist, play team specialist, paediatric gastroenterologist and paediatric surgeon) meets weekly to review referrals, discuss results of physical investigations, as well as psychological and sociological assessments. The team will then formulate a bespoke treatment that aims to manage physical, psychological and sociological aspects simultaneously and effectively. Management strategies included toileting/medical modification, surgery, irrigation, biofeedback, intersphincteric botulinum toxin injection, psychological | ||||||||
| (narrative therapy, hypnotherapy, behavioural modification, cognitive behavioural therapy, desensitisation, psychotropic medication), therapeutic play (coping skills, emotional support, support challenging behaviour, education, reassurance and confidence) and neuromodulation. | ||||||||
| Duration/frequency of visits/treatments not reported. | ||||||||
| Bellesheim 201862 | To present lessons learned by the Learning Collaborative and any process improvements made to the practice pathways. Secondary aim is to report rates of improvement associated with the insomnia and constipation practice pathways in a sample of children with ASD. | Children with ASD with constipation by the presence of <3 stools per week and/or painful stools. | Non-comparative study | 82 children. Dropout = 4. | Not reported | Constipation care pathway – involved routine screenings, behavioural and educational-based interventions when possible, medication or referral to specialists when needed, and follow-up and re-evaluation. ‘Baseline packets’ were sent to families, which included a Constipation Action Plan. Families answered questions regarding symptoms, daily routine, and medications. Baseline packets were reviewed by each site team to determine treatment strategies and a communication plan |
‘82 families set intervention goals and were treated per the guidelines that were presented in the constipation practice pathway.’ Elimination of pain was one of two most common goals. Data are presented in relation to the meeting of goals, but not relating to specific changes in painful defaecation. |
NR |
| (method and frequency) with the Learning Collaborative. A variety of communication methods were used, including Research Electronic Data Capture, Google Drive, FluidSurveys, text messaging, phone calls and electronic medical records. After screening, Learning Collaborative teams helped families identify up to four intervention goals. Goals were initially chosen from a predefined list of constipation goals | ||||||||
| (e.g. more frequent stooling, decreased pain with stooling, presence of soft stools and decreased encopresis). On a weekly basis, families communicated with the site Learning Collaborative team to review progress towards goals, discuss what worked and what was less useful, and plan next steps. | ||||||||
| Costigan 201966 | To describe the profile of children attending a bowel management clinic in a large tertiary paediatric hospital, present their treatment protocols and outcomes, and evaluate the contributory factors for a successful bowel management programme. | All children attending a bowel management clinic in a paediatric centre over a 5-year period (2010–15) | Retrospective cohort | 192, of whom 15 had idiopathic constipation | 55% male, 45% female. Mean age at start of trans-anal irrigation was 7 years (range 2–17 years) | Clinic: individualised bowel management plan commenced after a complete history, clinical examination, review of medical and operative records, and a baseline plain film of the abdomen. Follow-up by clinical nurse specialist. All children complete the first stage of the BMP before TAI is considered. If faecal loading is identified on the baseline abdominal X-ray, it is recommended to treat this with oral medications or rectal enemas. | NR | NR |
| After successful disimpaction, maintenance medication is prescribed. This first stage involves monitoring the patient’s fluid and fibre intake, and timed toileting. The child and parent at home document this for at least a 1-week period. The child and parent are educated on the anatomy and physiology of the bowel. After a period of 6 months, the child’s progress is reviewed, and if there is little or no improvement, then TAI is instituted, using a washout suitability tool, to decide which irrigation equipment is most suitable. | ||||||||
| Gabr 202067 | To evaluate the impact of implementing a Bowel Management Program on the QoL in children with pseudo incontinence. | Children more than 3 years of age with pseudo incontinence who were referred to the centre and enrolled to the Bowel Management Program | Non-comparative study | 115 children with pseudo incontinence. 111 were secondary to functional constipation | 86 males, 29 females. Mean age 7.51 ± 2.48 years (range 2.5–13 years). | BMP: set up within a dedicated paediatric colorectal surgery outpatient clinic, composed a comprehensive management pathway of three elements: medications (laxatives), diet modification and toilet training. Duration: usually provided for a ‘6-month cycle’. |
NR | QoL questionnaire total scoring ranged from 0 to 16. QoL score before BMP: 2.45 ± 1.57, and after BMP: 14.36 ± 1.37, p ˂ 0.05. |
| Gonring 201968 | To describe an interdisciplinary group-based treatment for faecal incontinence in school-aged children | Children referred for behaviour therapy after failing standard medical management provided through a paediatric gastroenterology clinic. Children included met diagnostic criteria for faecal incontinence with functional constipation; agreed to participate; English speaking; no developmental delays; no psychiatric diagnosis (including ASDs). | Retrospective cohort | 26 | 20 males, 6 females. Age range 5–8 years. | ‘Poop Group’: caregiver-assisted, protocolised, group-based intervention combining behaviour therapy and medical management for families of children with faecal incontinence. Group sessions focus on the gastrointestinal system, medication, toilet sitting posture, hydration, fibre and behaviour contracts. Parents and children met in separate groups. Weekly assignments and sticker charts reinforced the care regimen were given to families. Educational materials were provided as handouts. | NR | NR |
| Duration: Families met weekly for 90-minute sessions for 6 weeks. Children had weekly examinations. Upon completion of group treatment, patients returned to their medical provider for individual care. Families also continued to receive individual treatment from one of the group psychologists for ongoing behavioural treatment 1-month post group. |
||||||||
| Ismail 201160 | To assess the impact of involving experienced children’s outpatient nurses in the management of children with FC who were not making satisfactory progress, despite regularly attending a general paediatric clinic. | Children referred to the nurse-led clinic had been constipated for a minimum of 6 months and had attended at least three appointments at a general paediatric clinic. All the children had been assessed as suffering from FC and not making satisfactory progress with constipation at the time of referral. | Non-comparative study | 50 participants | 1.5–10 years (median, 4.0 years). Gender NR. Constipated for between 0.4 and 8 years (median, 1.5 years). |
Nurse-led clinic: At the first clinic visit, and again at subsequent visits if necessary, the nurses spent time educating parents (and older children) about constipation, including basic bowel physiology, pathogenesis of CFC, and management, including the mode of action of commonly used laxatives, the rationale for their use and the importance of complying with treatment. | At first clinic visit: 35 children (70%) reported painful defaecation. At third clinic visit: 9 children (18%); p < 0.001. Parents graded their child’s pain on defaecation on a scale of 1 (no pain) to 10 (unbearable pain). At first clinic visit: median pain score 8 (range 1–10). At third clinic visit; median pain score of 1 (range 1–4). |
NR |
| The emphasis during these first three clinic visits was to encourage compliance with the currently prescribed doses of laxatives The benefit of a good diet and fluid intake, and establishment of a good toileting regime and behavioural issues related to toileting both in the home and at school were discussed. A range of leaflets complimented the educational discussions. In formats suitable for children or for their parents. | ||||||||
| Duration/frequency: At least 3 clinic visits: second clinic was held 4–6 weeks after the first, and the third 6–8 weeks later. After the third clinic visit, further appointments were arranged at between 6-week and 3-month intervals, depending on progress. | ||||||||
| Mallon 201563 | To investigate whether Shared Care (a collaborative quality improvement initiative) reduces referrals and improves adherence to established clinical guidelines | Patients 1–18 years old, new visit to BCH GI during the 6-month periods before and after implementation of Shared Care, primary or secondary diagnosis of constipation, faecal impaction, encopresis, or IBS, constipation type | Retrospective cohort study | 61 | median age = 6.7 years. 46 males (75%) | Intervention: decision-making algorithm plus information about algorithm, pagers, telephones, computers for e-mails, materials for didactic education sessions to explain algorithm. Information about algorithms was sent electronically to all providers and in print to all practices, and was embedded as a linked document for point-of-care access. Advice lines with a Rapid Response pager line to a lead nurse a secure e-mail portal. Detailed Algorithm used to guide care. Including laxative use and dietary advice. | NR | NR |
| Norbedo 201764 | To investigate the incidence and the clinically relevant features of functional constipation in patients evaluated for acute abdominal pain in a tertiary care paediatric ED | Children presenting at ED with abdominal pain, with diagnosed functional constipation | Retrospective cohort | 4394 children with abdominal pain, of which 1020 were diagnosed with functional constipation | 52.7% male; mean age 8 ± 4.4 years | Diagnostic assessment methods on arrival in ED: 2% children received X-ray, 3.7% received abdominal ultrasound, 4.5% received blood test, 3% received surgical consult. 72% were treated with a n enema. All patients with CFC were discharged with dietary ad-vice and toilet training and were prescribed PEG; 43% of CFC patients were discharged with advice to continue the enema therapy at home for the first day. Duration/frequency: single ED visit. |
NR | NR |
| Poenaru 199770 | To present the experience of the first 16 months of a multidisciplinary clinic for the treatment of functional constipation | Patients referred to the bowel management clinic (multidisciplinary team) who had all previously treated unsuccessfully for constipation. | Non comparative study | 114 | Average age was 5.4 ± 3.8 years (range: 4 months to 19 years). Equal gender distribution. Mean length of symptoms related to constipation was 20.3 months with 38% of children having been constipated for over 1 year. |
Bowel Management Clinic: children assessed by the physician and the clinic nurse, with further referral to the other clinic staff as needed. The only compulsory treatment modality is patient education. Follow-up visits are used to monitor progress and continue the education process. Patients who show no progress are reassessed by the physician and may become candidates for diagnostic testing. Laxatives constitute the mainstay of medical therapy. The choice is based on patient compliance and the nature of symptoms. Most patients are treated with senna. docusate sodium, or mineral oil Multiple laxatives are avoided. | NR | NR |
| Follow-up is arranged by each healthcare professional as needed. Individualised dosage is maintained for a minimum of 3–6 months, during which time dietary and psychosocial issues are dealt with. The patient is then slowly weaned off the medications. Discharge from the clinic occurs when the patient is asymptomatic and off medications. | ||||||||
| Short 201872 | To compare outcomes before and after implementation of an ERP in children undergoing colorectal surgery. | Patients aged 5–20 years who underwent an elective major colon and rectal operation by 2 board-certified paediatric surgeons | Retrospective cohort study | 43 patients in the pre-ERP period (2012–4) and 36 patients in the post-ERP period (2015–16). Only three (10%) had diagnosis of colonic dysmotility, constipation | Mean age 14.5 (13, 15) males – 20 (56%) |
Enhanced recovery protocol: standardised instructional handouts were distributed to all patients at the time of their preoperative clinic visit. Protocol covered: preset discharge criteria, Bowel preparation medication, pre-operative management on day of operation, intraoperative management, postoperative care. |
NR | NR |
| Tappin 201361 | To describe the development of a nurse-led early intervention within Glasgow for children referred by their GP with constipation. The project was a feasibility study that included an audit, design of a nurse-led intervention and a service evaluation | Eligible patients were GP referrals, aged 0–13 years, from postcode areas in the City of Glasgow. To be included the main complaint in the referral letter had to be constipation. | Non-comparative study | 75 recruited. 45 with outcome data (16 + weeks) |
Median age 3.5years (1.9, 7.3). 35 males, 40 females | Nurse-led intervention: First appointments were one hour, follow-up appointments 30 minutes. History and examination reviewed by paediatrician. Education for child and parents. Duration/frequency: initial 16 weeks treatment period. After 16 weeks, agreement was reached on further support or a 6-month break. |
After 16 weeks treatment: 10/45 (22%) participants had pain on defaecation. This was 26/58 (45%) for those not receiving intervention. | NR |
| Study | Aim | Country | Study design | Key inclusion criteria | Number of participants | Participant demographics | Intervention 1 (active intervention) |
Intervention 2 (control) | Question addressed | Findings: painful defaecation | Findings: QoL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Systematic reviews ( n = 2) | |||||||||||
| Harris 201974 | To review evidence on the efficacy of probiotics in the treatment of functional constipation in children. | (Any – no language restrictions) | Systematic review | RCTs including children (aged <18 years of age) with functional constipation according to ROME II or III criteria or variations of these. Excludes studies of patients with an organic cause for constipation or with a history of colorectal surgery. | 1408 children, included within 17 RCTs | Not reported as combined result | Probiotics | Placebo (7 RCTs) or treatment as usual (10 RCTs) | What is the effectiveness of probiotics? | NR (review authors investigated ‘treatment success’, using RCT author definition of this; painful defaecation is reported to be part of ‘treatment success’ for 3/11 included RCTs.) | NR |
| Piccoli de Mello 201878 | To gather evidence on the use of fibre in the treatment of functional constipation in paediatric patients | (Studies written in Portuguese, English, Spanish, French and German) | Systematic review | RCTs including participants 1 and 18 years, without breast milk consumption and with a diagnosis of functional constipation receiving. Excludes studies with incomplete data or other interventions | 680 children included within 9 RCTs | Age range 1–16 years. 45% male. | Fibre | Placebo or laxative | What is the effectiveness of additional dietary fibre? | NR | NR |
| RCTs which were incorporated into an included/updated SR ( n = 9) | |||||||||||
| Aulia 201681 | To determine the role of glucomannan for the treatment of functional constipation in children. | Indonesia | RCT | Children aged 7–12 years with functional constipation based on ROME III not attributable to organic/pathological abnormalities or intake of medication | 36 | Mean age 9.8 (SD 1.52) | Glucomannan (dietary fibre) | Maltodextrin as placebo | What is the effectiveness of additional dietary fibre? | NR | NR |
| Basturk 201782 | Efficacy of synbiotic [Lactobacillus casei, L. rhamnosus, L. plantarum and Bifidobacterium lactis and prebiotics (fibre, polydextrose, fructo-oligosaccharides and galacto-oligosaccharides)] treatment in children with functional constipation | Turkey | RCT | Paediatric patients aged between 4 and 18 years. Patients diagnosed with functional constipation according to the ROME III diagnostic criteria |
146 | Mean age 9.2 (SD 3.5), 95% female | Synbiotics and prebiotics (fibre) (a mixture including 4´109 colony-forming units of L. casei, L. rhamnosus, L. plantarum, B. lactis and prebiotics at a dose of 1996.57 mg (fibre, polydextrose, fructo-oligosaccharides, and galacto-oligosaccharides) sachet once a day). | Placebo | What is the effectiveness of additional dietary fibre? | Probiotic group: number with painful defaecation before treatment: 38 (52.7%) and after treatment 16 (22.2%) (p < 0.001). Same data for placebo group: 34 (45.95) and 27 (36.4%) (p = 0.207). | NR |
| Cassettari 201983 | Effects of green banana biomass with different combinations of laxatives in the management of children/adolescents with functional constipation | Brazil | Multiarm RCT | Toilet-trained children and adolescents, aged 5–15 years; functional constipation according to ROME IV criteria | 80 | Mean age 9.2 (SD 3.5), 46% female | Green banana biomass (fibre) | Different combinations of laxatives | What is the effectiveness of additional dietary fibre? | Fibre group: number with painful defaecation before treatment: 14/15, and after treatment 4/15 (p < 0.05). Same data for PEG laxative group: 16/16 and 4/16 (p < 0.05) |
NR |
| Chao 201777 | To compare the differences of faecal microflora between constipated and non-constipated healthy children, and evaluate the efficacy of probiotics in reducing symptoms of constipation and the influence of intestinal microflora in children with functional constipation | China | RCT | Children aged 6 months to 10 years with constipation according to ROME III criteria. Exclusion Criteria: gastro-oesophageal reflux disease; inflammatory bowel disease; cardiopulmonary diseases; liver disease; renal disease; genetic diseases; endocrinal diseases; received abdominal surgeries. | 153 109 with constipation; 44 healthy. 82/109 completed. |
Age. treatment group: mean 2.75 years (SD 1.25). Control: mean 3.92 years (SD 1.83). 47 female, 36 males |
Magnesium oxide and MIYAIRI-BM Magnesium oxide 125 mg twice per day for children with weight <15 kg, 250 mg twice per day for weight <15–30 kg, and 500 mg twice per day for weight >30 kg for 12 weeks. MIYAIRI-BM 1 package (1 g) divided as 0.5 g twice per day for children with weight <15 kg, 2 packages divided as 1 g twice per day for weight 15–30 kg, and 3 packages divided as 1.5 g twice per day for weight >30 kg for 12 weeks. |
Active comparator: Magnesium oxide | What is the effectiveness of probiotics? | NR (outcomes presented as a severity score, which included ‘difficulties with defaecation’). | NR |
| Closa-Monasterolo 201779 | To investigate possible benefits of supplementing the daily diet with inulin-type fructans in 2–5-year-old constipated children | Spain | Pilot RCT | 2–5-year-old constipated children | 17 | Mean age 9.2 (SD 3.5), 95% female | Inulin-type fructans derived from chicory (70 : 30 combination of OraftiVR P95 oligofructose and OraftiVR GR inulin) | Placebo | What is the effectiveness of additional dietary fibre? | Pain during defaecation: evaluated using Wong-Baker FACES Pain Rating Scale. ‘Pain during defaecation’ clearly decreased (p = 0.014) in the two groups and this effect of independent of the treatment (interaction p value = 0.736). | NR |
| Kubota 202075 | Efficacy of the probiotic Lactobacillus (L.)reuteriDSM17938 and magnesium oxide (MgO) for relieving CFC in children | Japan | Multiarm RCT | Patients had to be more than 6 months old or under 6 years of age, with a diagnosis of functional constipation according to the ROME IV criteria | 60 | Mean age 9.2 (SD 3.5), 95% female | Group A (n = 20) received L. rueteri DSM 17938 and lactose hydrate as a placebo of MgO Group B (n = 19) received L. rueteri DSM 17938 and MgO and lactose hydrate | Group C (n = 21) received a placebo of L. rueteri DSM 17938 and MgO and lactose hydrate. | What is the effectiveness of probiotics? | NR | NR |
| Mahdavi 201780 | To determine effects of synbiotics on treatment of functional constipation in children aged 2–10 years old. | Iran | RCT | Aged between 2- and 10-year-old and diagnosed with functional constipation according to the ROME III criteria | 79 | Mean age 9.2 (SD 3.5), 95% female | Synbiotic in combination with PEG | PEG | What is the effectiveness of additional dietary fibre? | Severity of pain during defaecation (visual analogue scale). Median score for intervention and control group, at: baseline: 88.15 and 85.12 (p = 0.50). 4-week: 4.73 and 6.58 (p = 0.58). 8-week: 3,51 and 3.33 (p = 0.93). 12-week 13.48 and 15.45 (p = 0.81). |
NR |
| Sanctuary 201976 | Tolerability of aprobiotic (Bifidobacterium infantis) combined with a bovine colostrum product as a source of prebiotic oligosaccharides and to evaluate GI, microbiome and immune factors in children with ASDs and gastrointestinal comorbidities. | USA | Pilot crossover RCT | Children with a previous diagnosis of ASD, ages 2–11 with a history of frequent gastrointestinal symptoms including chronic constipation, diarrhoea, and/or IBS | 11 | Mean age 9.2 (SD 3.5), 95% female | Bovine colostrum product plus + Bifidobacterium infantis | Bovine colostrum product (BCP) | What is the effectiveness of probiotics? | Frequency of pain during defaecation, Treatment group (combined group): mean −0.75 (95%CI −1.341 to −0.159). BCP only group: mean −0.938 (95%CI −1.843 to −0.032) | NR |
| RCTs included within narrative synthesis ( n = 15) | |||||||||||
| Mohammadi Bourkheili 201293 | To determine the effect of cow’s milk-free diet on chronic constipation in children who are not responding to laxatives | Iran | RCT | Children aged 4–14 years with chronic constipation (Rome III criteria), taking high-fibre foods; no response to PEG after a 3-month treatment. Having no red flag symptoms. | 70 | Cow’s milk-free group: mean age 5 (SD 2) years. Cow’s milk group: mean age 6 (SD 2) years. |
Cow’s milk-free and dairy-free diet plus 30 mg/kg/day of calcium syrup for 4 weeks. PEG (1 g/kg/day) | No restrictions in consuming cow’s milk and dairy products. PEG (1 g/kg/day) |
What is the effect of a cow’s milk-free diet? | History of painful defaecation or hard bowel movements: Cow’s milk-free group: ‘10(55.6)’ Cow’s milk group: ‘2(18.2)’. NB: unclear what data are presented in table. p-value for difference between groups = 0.004. |
NR |
| Bongers 200786 | To evaluate the effect of a new infant formula on the stool characteristics of constipated infants | Netherlands | Randomised crossover trial | Healthy, term infants with constipation, between 3 and 20 weeks of age, who received at least 2 bottles of milk-based formula per day. Constipation was defined as presence of at least one of the following symptoms: | 38 | Median age 1.7 months (range 0.7–5.0 months). 19 male; 19 female. Median age at onset of symptoms = 2 weeks (range 0–20 weeks) |
A new infant formula (NF; Nutrilon Omneo, Nutricia Nederland BV, Zoetermeer, the Netherlands) was developed which contains modified vegetable oil with a high proportion (41%) of palmitic acid at the sn-2 position, | A whey-based control formula was partly mixed with a formula based on hydrolysed whey protein (mixture of 75% Nutrilon 1 and 25% Aptamil HA l). | What are the effects of different milk formula in infants? | Number with painful defaecation after treatment: New formula group: 35% (7/20). Standard formula group: 33% (5/15). RR and 95% CI: 1.0(0.4 to 2.7), p = 0.92. |
NR |
| (1) frequency of defaecation <3/week; (2) painful defaecation (crying); (3) abdominal or rectal palpable mass. Children with Hirschsprung’s disease, spinal or anal anomalies, previous colonic surgery, metabolic, cerebral and renal abnormalities were excluded. Children who were treated with laxatives at enrolment were also excluded. | a mixture of prebiotic oligosaccharides, partially hydrolysed whey protein and a reduced lactose content. The oligosaccharides mixture consists of 90% short-chain GOS and 10% lcFOS, 0.8 g/100 ml, and resembles human milk oligosaccharides with respect to its molecular weight distribution and high galactose content | ||||||||||
| Chao 200785 | To evaluate the therapeutic and nutritional effects of a magnesium-enriched infant formula and a 20% strengthened infant formula | Taiwan | RCT | Healthy infants 2–6 months of age with constipation. Participation in the trial was proposed before a more complete diagnostic workup for cow’s milk protein allergy, Hirschsprung’s disease, and others. | 93 | Mean age of 3.8 ± 1.7 months. 47 boys and 46 girls. Constipation for at least 2 weeks |
Magnesium-enriched infant formula. | 20% strengthened infant formula (20% extra formula) | What are the effects of different milk formula in infants? | NR | NR |
| Dehghani 201290 | To investigate the role of cow’s milk allergy as a cause of chronic constipation and effect of CMFD on its treatment in children | Iran | RCT | <14 years old Referred to GI clinic with chronic constipation (ROME III criteria). Previous unsuccessful laxatives for at least 3 months. |
140 | Age 1–13 years (mean 4.6, SD 2.7 years) Typically developing |
4 weeks cow’s milk-free diet + PEG solution 0.5 g/kg/day | PEG solution 0.5 g/kg/day | What is the effect of a cow’s milk-free diet? | History of painful or hard bowel movements (number of participants). Before cow’s milk-free diet: 69/70 participants. After 4 weeks on cow’s milk-free diet: 9/70 participants. p = 0.000 |
NR |
| Dehghani 201995 | To evaluate efficacy and safety of oral intake of BSM syrup on the treatment of children functional constipation in comparison with PEG syrup | Iran | RCT | Age 4–12 years; CFC. Exclusion: ‘Taking any type of laxative 4 weeks before the study; diabetes, autoimmune diseases, hypothyroidism, cystic fibrosis, history of intestinal surgery’. | 47 | 29 girls; 37 boys. Mean age in years – BSM group: 7.24 ± 2.63, PEG group: 6.21 ± 2.33. Mean duration of constipation (days) – BSM group: 37.00 ± 32.79. PEG group = 27.91 ± 26.25 | BSM syrup (40%w/v) with a dose of 1 ml/kg body weight/day for 1 month. Plus behaviour modifications training and nutritional advice. | PEG syrup (40% w/v) with a dose of 1 ml/kg body weight/day for 1 month. Plus behaviour modifications training and nutritional advice. | What is the effectiveness of sugars (brown sugar, figs syrup, black sugar molasses)? | Painful or hard stool: number (per cent). After 2 weeks treatment: BSM group: 4/41 (9.8%). PEG group: 4/45 (8.9%). p = 0.89 |
NR |
| Hassanein 202195 | To study the therapeutic and adverse effects of oral magnesium sulphate therapy on spasticity and constipation in infants and children with cerebral palsy | Egypt | RCT | Children aged 2–12 years, spastic cerebral palsy, and meeting constipation criteria in neurologically disabled children. Exclusion: Children with organic aetiology of constipation, congenital gastrointestinal malformation, inflammatory bowel disease, inborn errors of metabolism, impaired renal function and non-compliant | 100 randomised (97 completed) | Age – treatment group: mean 6.28 (SD 2.67) years. Placebo: mean 6.49 (SD 2.55) years. 43 females: 44 males |
Oral magnesium sulphate solution 4% (4 mg elemental magnesium per millilitre solution). A daily morning dose of 1 ml/kg/day given for 1 month. | Placebo – an equivalent saline solution in similar container was given to the placebo group | What is the effectiveness of other/alternative dietary intake? | NR | NR |
| Iacono 199891 | To ascertain whether there is a relation between chronic constipation and cow milk protein allergy | Italy | Cross-over RCT | <6 years old. Referred to GI clinic with chronic constipation. Previous laxative treatment unsuccessful. | 65 | Age 11–72 months; mean 34.6 months. Typically developing | Cow’s milk for 2 weeks (laxatives stopped) | Soy milk for 2 weeks (laxatives stopped) | What is the effect of a cow’s milk-free diet? | ‘44 children with a response to the cow’s-milk–free diet underwent a double-blind, placebo- controlled challenge with cow’s milk in the hospital… None of those who received the placebo (soy milk) | NR |
| had a clinical reaction. No patient who received cow’s milk had an acute reaction, but in all patients constipation associated with hard stools and discomfort on defaecation reappeared after 5–10 days of the diet.’ | |||||||||||
| Modaresi Saryazdi 201399 | Not stated (abstract only) | Iran | RCT (abstract only) | Children ‘habitual constipating’. No other details. | 58 | No details reported | PEG and senagol syrup, plus paraffin oil, daily (plus high-fibre diet) | PEG and senagol syrup daily (plus high-fibre diet) | What is the effectiveness of other/alternative dietary intake? | Outcomes included ‘painless without fear defaecation’. Data not presented in abstract. | NR |
| Ritterband 2003103 | To examine the utility and effectiveness of an internet intervention, in addition to treatment-as-usual, in children with encropresis. | USA | RCT (pilot) | Ages of 6 and 12 years, soiling at least once a week, and have no medical diagnosis, other than constipation, that could explain their faecal incontinence | 24 | Mean age of 8.46 (SD 1.81) years. 16/24 participants had been taking laxatives for an average of 19.18 months | Use of UCanPoopToo internet intervention + usual care | Usual care only | What are the effects of educational interventions (delivered in addition to routine care)? | NR | NR |
| Ritterband 2013102 | To investigate the effect of an internet intervention, in addition to treatment-as-usual, in children with encropresis. | USA | RCT | 6–12 years of age, symptoms of encopresis for >3 months, >1 accident in the previous 2 weeks, had a home computer and Internet access | 91 (47 allocated to intervention group, 43 allocated to control group) | Mean age 8.47 years in intervention group. 9.66 years on control group. 81% male |
Use of (updated version of) UCanPoopToo internet intervention + usual care | Usual care only | What are the effects of educational interventions (delivered in addition to routine care)? | Data were collected (from diaries) on symptoms including defaecation pain. These are not reported in the paper. | NR |
| Stepurina 2018100 | To substantiate use of magnesium-containing mineral water for optimisation of prevention and treatment of functional constipations among children and teenagers (abstract only) | Russia | RCT | Children who had the ‘functional constipation’ diagnosis (ROME IV criteria) | 55 | Not reported | ‘Magnesium-containing mineral water – internal administration of magnesium-containing water “Donat Мg” with individual calculation of single doses (3 µl/kg) plus basic treatment (see control intervention)’ | ‘basic treatment only (the sparing/training regime, nutritional therapy, exercise therapy, and revitalizing massage)’ | What is the effectiveness of other/alternative dietary intake? | NR | NR |
| Tajik 201896 | To investigate the effect of red (brown) sugar on functional constipation in children compared to figs syrup | Iran | RCT | Aged 2–10 years. Functional constipation. | 60 | 40 boys, 20 girls. Aged 2–10 years. (No other participant details provided.) | Red (brown) sugar – 2cc per kg body weigh/day, dissolved in water. Daily for 1 month. | Fijian figs, containing figs and senna extract (dose based on body weight – 2 g/kg/day) | What is the effectiveness of sugars (brown sugar, figs syrup, black sugar molasses)? | After treatment, no participants in either group reported painful defaecation. | NR |
| Tanjung 201697 | To determine the effect of selenium on functional constipation in children | Indonesia | RCT | Children aged 12–17 years, diagnosis of functional constipation according to ROME III criteria, no organic abnormalities. | 120 (114 completed) | Mean age 13.5 years 37 males, 67 females |
40 micrograms selenium per day for those aged 11–14 years, and 50 micrograms per day for those aged 15–17 years, given once per day after breakfast for 2 weeks | Placebo – the control group was similarly given one capsule daily after breakfast for 2 weeks | What are the effects of selenium supplements? | NR | NR |
| Tayag-Lacsina 2019104 | To investigate the effect of an information leaflet in children with functional constipation | Philippines | RCT (abstract only) | 2–18 years old, fulfilling ROME IV criteria for functional constipation, or with Blethyn grade 2 or 3 on abdominal radiograph | 90 | Not reported | Information leaflet + usual care | Usual care only | What are the effects of educational interventions (delivered in addition to routine care)? | NR | NR |
| Young 1998101 | To identify whether an effect on stooling characteristics would be noted with a concerted effort to increase liquid intake. | USA | RCT | Children between 2 and 12 years of age were screened for inclusion in the study. Only pre-pubertal children. | 90 recruited (108 needed for power analysis) | Mean age 7.5 years (range, 2.5–12.5 years). 59 girls (52.54%) and 31 boys (47.46%). |
INTERVENTION 1: Instructed to increase water intake by 50% on the basis of total measured oral liquid intake during the first week. | Control (no change) | What is the effect of fluid intake? | NR | NR |
| Exclusion: children receiving specialised diets, hypercalcaemia, Hirschsprung’s disease, hypothyroidism and cardiac or renal disorders. Malnourished children already receiving stool softeners or laxative preparations, and children who were physically or intellectually challenged or who had an underlying central nervous system disease. | The mean age at which constipation began was 2 years 3 months (range, 1 month to 12 years) based on maternal recall. | INTERVENTION 2: Supplemental liquid in the form of Kool-Aid, juice, soda pop, or other liquids known to contain more than 600 mOsm/l. This number was chosen because it was considered to be a level above which a significant osmotic load in the small bowel would result in significant plasma to lumen flux. | |||||||||
| Other primary studies ( n = 10) | |||||||||||
| Beleli 201594 | To determine the effects of galactooligosaccharide in paediatric patients with chronic constipation | Brazil | Non-randomised crossover study | 4–16 years old, diagnosis of constipation | 23 | Mean age 8.8 years (SD 4.1 years) | Prebiotic 4ʹ-Galactooligosaccharide (GOS). Daily 6 ml volume, containing 1.7 g GOS | Daily 6 ml maltodextrin solution (placebo) | What is the effectiveness of sugars (brown sugar, figs syrup, black sugar molasses)? | Defaecation discomfort combined with bowel frequency and faecal consistency within a clinical score. Before crossover period: treatment group mean clinical score 3.09 (SD 1.64); and placebo group 5.78 (SD 1.39), p = 0.0004. |
NR |
| Iacono 199592 | To ascertain whether there is a relation between chronic constipation and cow milk protein allergy | Italy | Cohort study | <3 years old with chronic constipation, referred to GI clinic | 27 | Mean age 20.6 months (SD 13.5 months). Typically developing |
Cow’s milk-free diet (use of laxatives not reported) | - | What is the effect of a cow’s milk-free diet? | Score based on diaries: ‘3 = hard faeces, difficulty and pain in passing stools; 2 = soft faeces, no pain; and 1 = mushy or liquid stool’. | NR |
| Six patients did not get any improvement on cow’s milk-free diet (score remained at 3). The other 21 participants did a second trial of cow’s milk-free diet, with before and after scores: 2.75 (SD 0.11) and 1.85 (SD 0.10), p < 0.001. | |||||||||||
| Infante 201186 | To evaluate the impact of a formula with high levels of lactose and magnesium, in compliance with the official regulations, on stool water content, as well as a parental assessment of constipation | Spain | Cohort study | Formula-fed infants aged 4–10 weeks, with constipation without any organic cause. None had a relevant medical history or pathological neonatal conditions. Preterm and low-birthweight infants were excluded. | 30 | Aged 4–10 weeks | Novalac AE (IT) (United Pharmaceuticals SA, France) for 2 weeks. | What are the effects of different milk formula in infants? | ‘Questionnaire about crying during defaecation’. Before formula: 90% described defaecation as painful. After 2 weeks of formula this was 10% (p < 0.0001). |
NR | |
| Infante Pina 200887 | To assess the prevalence of mild gastrointestinal disorders in milk-fed infants in paediatric practice, and to evaluate the effectiveness and satisfaction with dietetic treatment. | Spain | Cohort study | (1) Infants up to 4 months of age fed with artificial milk formulas; (2) the presence of MGDs; (3) the possibility of feeding the infants with some product of the Novalac line of formulas; and (4) continuation of these formulas on an exclusive basis for at least 30 days (with no incorporation of other foods to the diet). | 3487 | Age – between under 1- and 17-week 52.2% boys. Colic manifested at earlier ages (6.2 weeks on average) followed by constipation (7.6 weeks), regurgitation (8.6 weeks) and diarrhoea (10.4 weeks). |
Formulas belonging to the Novalac line of products (United Pharmaceuticals, France; Chiesi España S. A,Spain) [Novalac Anti-Colic (low lactose, adapted formula)] | - | What are the effects of different milk formula in infants? | Presence of pain or discomfort reported by 90% of parents at baseline and 10.4% after 30 days. | NR |
| Novalac Anti-Regurgitation (thickened with starch and enriched amylopectins), Novalac Anti-Diarrhoea (lactose-free and with adapted concentration of electrolytes), or Novalac Anti-Constipation (adapted concentration of magnesium and lactose)] | |||||||||||
| Mazzoni 2017105 (abstract only) | To determine the role of non-pharmacological therapy (dietary rules and behavioural rules) in treatment of childhood chronic functional constipation. | Italy | Non-randomised study | 3–15-year-olds, functional constipation | 52 | Not reported | Dietary and behavioural rules | PEG | What are the effects of combined dietary and behavioural interventions? | NR | NR |
| Savino 200388 | To describe the effects of a formula containing fructo- and galacto-oligosaccharides, partially hydrolysed proteins, low levels of lactose and palmitic acid in the position and higher density, in infants with minor gastrointestinal disorders. | Italy | Cohort study | Formula-fed healthy term infants, up to 3 months of age, who were seen by a paediatrician because of colic and/or constipation and/or regurgitation | 932 | Age – mean 35 (SD 0.77) months. Constipation was defined when stool frequency was below 1 stool/day. |
Milk formula – based on a partially hydrolysed bovine protein that can accelerate the gastrointestinal transit time (11). The lipid fraction contains palmitic acid in the position of the triglyceride molecule, higher (41%) than in normally used vegetable lipids, which should significantly reduce the build-up of calcium soaps in the gut (12, 13). In addition, a bifidogenic oligosaccharide mixture was added to stimulate the growth of faecal bifidobacteria and lactobacilli (13–15) | - | What are the effects of different milk formula in infants? | NR | NR |
| Xinias 201889 | To evaluate the efficacy of synbiotic formula with partial whey hydrolysate and high magnesium content in infants presenting with functional constipation. | Greece | Non-randomised study | Starter formula-fed term born infants between 3 and 13 weeks old, suffering from constipation since at least 1 week without any clinical evidence for an organic cause | 65 | Age – intervention and control: 7.5 ± 3.9 vs. 6.2 ± 3.6 weeks | infant formula with a partial whey hydrolysate, synbiotics (Bifido-bacterium lactis and GOS supplemented with magnesium | Reassurance and anticipatory guidance was the only intervention in the control group, which remained on the standard infant starter formula the infant had been fed with. | What are the effects of different milk formula in infants? | NR | Parents completed a QoL questionnaire at baseline and after 1 month. After 1 month, daily QoL for the intervention group was: excellent (45%), very well (40%), moderate (15%), poor (0%). And for the control group: excellent (8%), very well (56%), moderate (36%), poor (0%). p = 0.002. |
| Study | Aim | Country | Study design | Key inclusion criteria | Number of participants | Participant demographics | Intervention 1 (active intervention) | Intervention 2 (control) | Question addressed | Findings: painful defaecation | Findings: QoL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Systematic reviews ( n = 2) | |||||||||||
| Gordon 2016133 | To evaluate the efficacy and safety of osmotic and stimulant laxatives used to treat functional childhood constipation | International | Cochrane SR | RCTs comparing osmotic or stimulant laxatives to placebo/other intervention, participants aged 0–18 years old. | 25 RCTs 2310 participants | (Varied across trials) | Any osmotic or stimulant laxative | Placebo or another intervention | What are the effects of laxatives? | NR | NR |
| Rachel 202038 | To identify the optimum dose of PEG) to manage chronic constipation in children aged younger than 24 months. | - | Systematic review | PEG therapy for children with functional constipation (excluding dysfunction of organic cause), children younger than 24 months, English language, and PEG dosage, safety, or efficacy. Additional criteria for full-text screening included a placebo or comparison group. | Five studies (2 RCTs, 3 retrospective studies), including 468 participants | Data considered related to participants aged 24 months or under. | PEG3350, with or without electrolytes, or PEG4000 | PEG3350, with or without electrolytes, or PEG4000 | What are the effects of laxatives? | Study results reported narratively, including painful defaecation where reported by study authors. | NR |
| RCTs which were incorporated into an included/updated SR ( n = 11) | |||||||||||
| Bekkali 2018129 | To compare PEG3350 + electrolytes with PEG4000 | Netherlands | RCT | Children 6 months to 16 years, with <3 bowel movements/week. Excluded: children with organic causes, additional needs. |
97 (82 completed) | PEG3350 + electrolyte group. Age: mean 5.5 (SD 3.9) years. PEG4000 group. Age: mean: 5.0 (SD 3.3) years. 40 males/57 females |
PEG3350 with electrolytes | PEG4000 | What are the effects of laxatives? | NR (data reported as ‘total sum score’ which incorporated assessment of painful defaecation.) | NR |
| Benninga 2022130 (study 1; NCT02042183) | To evaluate the efficacy and safety of lubiprostone | USA, Canada, Europe | RCT | Children 6–17 years of age who had a confirmed diagnosis of CFC according to the Rome III criteria | 606 (404 treatment, 202 placebo) (444 completed) | Treatment. Age: mean 11.2 (SD 3.25) years. Placebo. Age: mean 11.1 (SD 3.20) years. 283 male, 323 female | 12 μg BID, lubiprostone 24 μg BID (depending on their weight) (for 12 weeks, 2 weeks follow-up) | Placebo (for 12 weeks, 2 weeks follow-up) |
What are the effects of laxatives? | NR (data reported as overall response score, which incorporated ratings of straining and pain). | NR |
| Benninga 2022130 (study 2; NCT02138136) | To evaluate the long-term safety, efficacy and pharmacokinetics of oral lubiprostone | USA, Canada, Europe | RCT | Children 6–17 years of age who had a confirmed diagnosis of CFC according to the ROME III criteria | 419 (280 completed) | Age. Mean 11.4 (SD 3.15) years. 190 male, 229 female. |
12 μg BID, lubiprostone (for 36 weeks, 4 weeks follow-up) |
24 μg BID lubiprostone (for 36 weeks, 4 weeks follow-up) |
What are the effects of laxatives? | NR (data reported as overall response score, which incorporated ratings of straining and pain). | NR |
| Cao 2018131 | To investigate the efficacy and safety of lactulose | China | RCT | Aged 2–6 years, with constipation for at least 3 months (ROME II criteria). Excluded: children with organic causes, severe neurological disorders |
100 | Treatment group. Age: mean 3.9 (SD 0.6) years. Placebo group. Age: mean 4.0 (SD 0.7) years. 54 males, 46 females. |
Lactulose | Placebo | What are the effects of laxatives? | NR | NR |
| Esmaeilidooki 2016132 | To compare cassia fistula emulsion with PEG4000 | Iran | RCT | Aged between 2 and 15 years with a diagnosis of FC according to the ROME III criteria. Exclusion: organic causes, other diseases |
109 | M/F: 63/46; mean age ± SD: 59.7 ± 28.8 months | Cassia fistula emulsion | PEG4000 | What are the effects of laxatives? | Severity of pain, using visual analogue scale. After 4 weeks of treatment: treatment group mean 4.74 (SD 8.66). PEG group mean 6.54 (SD 11.98). p = 0.407. |
NR |
| Hashemi 2015110 (abstract only; full text in Iranian) |
To evaluate and compare effect of PEG treatment and probiotics | Iran | RCT | Children aged 2–16 years with CFC enrolled the study based on ROME III criteria | 120 | Not stated | Group 1: PEG + placebo Group 2: Probiotic + placebo | Group 3: PEG + probiotic | What are the effects of laxatives? | NR | NR |
| Jarzebicka 2019135 | To compare the clinical efficacy of PEG3350 and lactulose | Poland | RCT | Age 6 months to 6 years. Constipation diagnosed (Rome III criteria). Excluded: children with organic cause, comorbidities. |
102 | Mean age 3.62 (SD 1.42) years. 57 male, 45 female |
PEG3350 | Lactulose | What are the effects of laxatives? | Number of participants reporting defaecations with pain after 12 weeks: PEG group: 2/44 (5%). Lactulose group: 2/39 (5%). RR 0.88 (95% CI 0.16 to 4.86), p = 0.99. |
NR |
| Modin 2018136 | To investigate the long-term effect of PEG maintenance treatment | Denmark | RCT | Aged 2–6 years, CFC (ROME III criteria). Excluded: children with organic causes. |
102 | PEG group. Age: median 6.2 (range 2.5–12.3) years. Placebo group. Age: median 6.1 (range 2.0–15.1) years. 57 male, 45 female |
PEG3350. Maintenance dose was 0.8 g/kg/day, adjusted to maintain daily soft stools | Placebo | What are the effects of laxatives? | NR (successful treatment defined as absence of any ROME III criteria). | NR |
| Pranoto 2016137 | Effectiveness of oral and rectal laxatives in terms of recovery and recurrence in children with functional constipation | Indonesia | RCT | Children aged 8–17 years who met the ROME III criteria for functional constipation | 99 (91 completed) | 13 male, 78 girls | 5 mg of oral bisacodyl once daily for three consecutive days. | A single dose of 5 mg of rectal bisacodyl | What are the effects of laxatives? | NR | NR |
| Shatnawi 2019139 | To evaluate the safety and efficacy of lactulose in faecal impaction management in children with constipation | Jordan | RCT | Aged 1–14 years and had diagnosis of functional constipation according to ROME III criteria. Exclusion: organic causes. |
65 | Lactulose group. Age: mean 5.2 (SD 2.6) years. PEG group. Age: mean 5.4 (SD 3.1) years. 36 male, 29 female. |
Lactulose (10 g/15 ml) 4–6 ml/kg/day (max. 120 ml/day) | Macrogol (PEG4000) 1–1.5 g/kg (max. 30 g/day) | What are the effects of laxatives? | NR | NR |
| Torabi 2017140 | To compare the effect of oral paraffin and PEG | Iran | RCT | Children aged 2–12 years old; CFC (ROME III criteria) for at least 6 months. Excluded: children with organic causes |
160 | Mean (SD) age: 5.28 ± 1.4 and 5.24 ± 1.9, respectively. 69 male, 91 female. |
PEG | Paraffin | What are the effects of laxatives? | NR (response to treatment categorised, including reports of painful defaecation) | NR |
| RCTs ( n = 2) | |||||||||||
| Imanieh 2019134 | To compare three therapeutic methods in the treatment of chronic constipation in CP children. | Iran | RCT | Children with diagnosed cerebral palsy and chronic constipation | 52 | Mean ± SD age of 5.2 ± 2.91 years 29 (58%) male and 21 (42%) female children | PEG (0.5 g/kg/dose) three times daily | Intervention 2: PEG and Motilium three times daily Intervention 3: Motilium (0.2 mg/kg/dose) three times daily | What are the effects of laxatives plus motilium? | NR | NR |
| Lomas Mevers 2020145 | To evaluate the preliminary efficacy of a multidisciplinary intervention of encopresis in children with ASD. | USA | RCT | Children with ASD and encopresis. Excluded: children who had other neurological disorders that effect anal functioning, or prolonged or recurrent gastrointestinal infectious disease | 20 | Mean age 7.6 years ± SD 3.46 years, range 5–16 years. 15 male, 5 female. |
Multidisciplinary intervention for encropresis – 10 sessions of 1–4 hours. Includes scheduled sitting on toilet, praise, incentives, glycerine suppository, caregiver training. | Waiting list control. | What is the effect of a combined pharmacological and behavioural programme for children with ASDs? | NR | NR |
| Other primary studies ( n = 9) | |||||||||||
| Axelrod 2016142 | To investigate treatment protocols for individuals with ASD and intellectual disability, and constipation | USA | Repeated measures design | Two adolescent boys with history of encopresis with constipation and incontinence overflow. | 2 | 13 and 14 years old | Intervention at home and school. Daily milk of magnesia, 30-minute pants checks; toilet training (regular scheduled sits, rewards etc.) plus laxatives (PEG3350 every morning) | N/A | What is the effect of a combined pharmacological and behavioural programme for children with ASDs? | NR | NR |
| Farahmand 2015141 | This study was designed to investigate the effectiveness of pelvic floor muscle exercise on treatment of FC | Iran | Cohort study | Children with chronic constipation aged 4–18 years referred to Children’s Medical Center with a diagnosis of FC and had previously tried and failed adequate treatment for constipation, including toilet training and laxative therapy | 44 (40 completed intervention) | Mean age 5.6 ± 1.03 years. 19 maleS, 25 femaleS |
We instructed the patients to perform sessions of pelvic muscle exercise at home twice a day for 8 weeks. The exercise consisted of walking in a semi-sitting (squatting) position for 5 minutes under supervision of parents. | N/A | What is the effect of physical exercise (focused on pelvic floor muscles)? | Number reporting painful defaecation: Baseline 35/44 (87.5%). After treatment 1/44 (2.5%). p < 0.05. | NR |
| Hankinson 2018143 | To evaluate the outcomes associated with providing a combination of medical and behavioural treatments in a specialty outpatient clinic consisting of a nurse practitioner and behavioural psychologist. | USA | Cohort study | Diagnosis of functional constipation, referred to multidisciplinary chronic constipation clinic. Exclusion criteria: organic cause of constipation (e.g. Hirschsprung’s disease, cystic fibrosis) | 162 recruited; 57 attended at least 2 appointments | 1–15 years, mean 6.18 (SD 3.24) years | Clinical evaluation, medical intervention (protocols of PEG, bisacodyl, clear diet, stimulant laxative); behavioural psychology (psychoeducation – e.g. behavioural methods, toilet sit schedule, rewards, pain management) | N/A | What is the effect of a combined pharmacological and behavioural programme for children with constipation, with or without typical development? | ‘59.6% reported pain during bowel movements’ Mean pain during bowel movement (paediatric constipation score) before: 1.67, after; 1.49 (non-significant). |
NR |
| Jordan-Ely 2013144 (abstract only) |
To review outcomes of oral bowel disimpaction with PEG administered in a nurse‐led clinic using the MOTIVATE method | Australia | Retrospective cohort study | Not reported | 33 | Age 2–17 years. 17 males, 16 females. |
Patients and carers were given information on DELD method during two × 30-minute sessions. Taught how to take PEG + E (Movicol) combined with sodium picosulphate (Dulcolax SP). | N/A | What is the effect of a combined pharmacological and behavioural programme for children with constipation, with or without typical development? | NR | NR |
| Soares 2009146 | To evaluate the effects of conventional treatment of CFC on total and segmental colonic transit times and on orocecal transit time. | Brazil | Cohort study | Children with CFC | 34 | Median age (25th and 75th percentiles) of 93.7 (74.3–107.4) months. Range 3–13 years. 19 male; 15 female. |
Therapy regime included dietary prescription (high fibre), mineral oil and behavioural training. | N/A | What is the effect of a combined pharmacological and behavioural programme for children with constipation, with or without typical development? | Pain during defaecation: Baseline 32/33 (97.0%). After 6 weeks 2/33 (6.0%). p = 0.001. | NR |
| Speridiao 2003147 | To determine dietary fibre and energy intake and nutritional status during the treatment of chronic constipation in children. | Brazil | Cohort study | 2–12 years old, chronic constipation | 25, of whom 16 completed study | Patients completing study: median age 56 months (25th and 75th percentiles 44, 100). 4 males; 12 females |
Rectal disimpaction, ingestion of mineral oil and diet therapy | - | What is the effect of a combined pharmacological and behavioural programme for children with constipation, with or without typical development? | Pain on evacuation. Baseline 14/16 participants. After 45 days 4/16 participants. After 90 days 0/16 participants. p < 0.05 ‘for all’. |
NR |
| Study | Focus | Inclusion criteria | Study design | No. of participants | Participant demographics | Intervention | Findings: painful defaecation | Findings: QoL |
|---|---|---|---|---|---|---|---|---|
| Systematic review ( n = 1) | ||||||||
| Ng 2016 174 |
To explore the effectiveness and safety of transcutaneous electric stimulation to improve bowel function and constipation-related symptoms in children with constipation. | Studies: RCTs. Participants: children aged 0–18 years with diagnosed functional constipation. Interventions: TES. Comparison: no treatment, placebo treatment, any other intervention for constipation. | Systematic review | 1 study (46 participants) | Aged 8–18 years, with slow transit constipation for more than 2 years. Children with Hirschsprung’s disease, coeliac disease, hypothyroidism or allergies that may impact on bowel function were excluded. | Authors planned to include any ‘TES treatment, administered either in clinical setting or at home, and applied either transabdominally, sacrally or via other means are compared to no treatment, a sham TES treatment, other forms of nerve stimulation or any other pharmaceutical or non-pharmaceutical measures used to treat constipation were considered for inclusion. | ‘Improvement in symptoms associated with constipation’ is stated as an outcome, but no data identified. | Number of participants with improved QoL: RR 4.00 (95% CI 0.56 to 28.40). Self-perceived QoL. MD 5.00 (95% CI −1.21 to 11.21). Parent-perceived QoL MD 0.20 (95% CI −7.57 to 7.17) |
| We accepted all types of devices used for the purpose of TES and all dosing regimens (i.e. using TES in different intensities such as number of times applied per day).’ | ||||||||
| One study was identified – Chase 2015 which was TES delivered by trained physiotherapist, 2 channels of AC with 4 electrodes on belly and back. Simulation for 20 minutes/session delivered 3× week for 4 weeks. Children were followed up for 2 months. | ||||||||
| RCTs in review update ( n = 1) | ||||||||
| Sharifi-Rad 2018175 IRCT2016030617876N |
To compare interferential electrical stimulation and pelvic floor muscle exercises with sham stimulation and pelvic floor muscle exercises. | Children aged 5–13 years | RCT | 90 | Mean age – treatment group: 6.5 (SD 2.3) years, range 5–12 years. Control group: 6.1 (SD 1.9) years, range 5–13 years. Gender: M : F Ratio: 43 : 46 |
Interferential electrical stimulation plus PFM exercises. PFM exercises were described by a paediatric physiotherapist. Regular exercises were demonstrated: 15 minutes/day, 7 days/week. Exercises were continued as ‘homework’ under the supervision of parents. Children in the intervention group were also given 10 sessions of inferential electrical stimulation – 20 minutes per session, 2× week for 5 weeks. |
Pain score (0–10 visual analogue score). At end of treatment: PFM group. Median 0 (IQR 0). Control group, median 0 (IQR 6). P = 0.032 |
QoL score. At end of treatment: PFM group. Median 64 (IQR 5). Control group, median 62 (IQR 6). P = 0.069 |
| Children in the control group received 10 ‘sham’ inferential electrical stimulation sessions. | After 6 months: PFM group. Median 0 (IQR 4). Control group, Median 2 (IQR 4). P = 0.037 | After 6 months: PFM group. Median 65 (IQR 5). Control group, Median 63 (IQR 6). P = 0.074 | ||||||
| Children in both groups received conventional care for constipation including education, discussion around diet and correct toileting position. | ||||||||
| RCTs ( n = 14) | ||||||||
| Awan 2021202 NCT03379038 |
To determine the effectiveness of physical therapy in relieving constipation in children with spastic cerebral palsy | Children with spastic cerebral palsy on oral feeding with constipation between ages 2 and 12 years, spasticity higher than 1+ grade on the MAS | Randomised crossover trial | 35 | Physical therapy group. Age: mean 4.58 (SD 1.88). Control group. Age mean 4.98 (SD 2.43). Gender: M : F Ratio: 25 : 10 |
Two groups: progressive physical therapy vs. maintenance physical therapy. Progressive physical therapy involved 5–10 active assisted sit-ups; use of chair and standing frame, reflex and inhibiting postures (sitting and lying down position). Each session lasted approx. 40 minutes performed daily for 6 weeks duration (i.e. 42 sessions). |
NR | NR |
| Children in both groups received similar exercises but the goals were different for children in the progressive physical therapy group (i.e. to improve current activity level) compared to children in the control group who were asked to ‘maintain’ their current activity levels. | ||||||||
| Bekkali 2009148 | To evaluate the efficacy and tolerability of enemas vs. high doses of oral PEG in disimpaction of children with functional constipation and rectal faecal impaction | Children with CFC and evidence of rectal faecal impaction on rectal examination | RCT | 90 | Mean age: enema group 7.9 (SD 2.9) years; PEG group 7.2 (SD 2.6) years. Gender: M : F Ratio: 60 : 30 |
One group received rectal enemas (dioctylsulfosuccinate sodium) once daily for 6 consecutive days, children <6 years 60 ml and children ≥ 6 years 120 ml. The other group received oral PEG3350 with electrolytes for 6 consecutive days. Maintenance treatment was started after 6 days disimpaction treatment and consisted of oral PEG3350 with electrolytes for at least 2 weeks (follow-up). | NR | NR |
| Bongers 2009191 | To assess the effectiveness of routine use of rectal enemas | Children between 8 and 18 years with functional constipation for at least 2 years and unresponsive to conventional treatment | RCT | 102 | Control group Median age 11 years (range 9.5–12.5). Enema group Median age 10.5 years (range 9.2–11.8). Control group Gender: | All children underwent rectal disimpaction by rectal enema for 3 days. Conventional treatment consisted of education, behavioural strategies and oral laxatives (PEG). | Percentages of children with painful defaecation at baseline, week 12, 26, 39 and 52, respectively, for: | Children in the intervention group filled out questions from a QoL questionnaire about feelings about application of rectal enemas. |
| M : F Ratio: 33 : 17. Enema group Gender: M : F Ratio: 32 : 18 |
Oral laxative therapy consisted of PEG, with a starting dose of 0.5 g/kg. If treatment was considered insufficient, the dose was optimised to a maximum of 1.5 g/kg. In the CG, a rectal enema or bisacodyl suppository of 5 mg was only prescribed in case of reoccurrence of faecal impaction. | Intervention group: 60.4%, 13.8%, 11.4%, 17.0%, 8.8%. Control group: 52.0%, 16.2%, 18.0%, 19.3%, 20.9%. p = 0.35 (overall test for differences between groups at all time points). |
Focus is on feelings about rectal enemas (15% reported this to be very to extremely terrible, 11% as quite terrible, and 74% no problem). | |||||
| In the intervention arm, children received, in addition to conventional treatment, 3 rectal enemas weekly during the first 3 months which was reduced by 1 enema per week every 3 months | ||||||||
| Borowitz 2002203 | To compare effectiveness of three additive treatment protocols in children experiencing chronic encopresis | Children, 6–15 years of age, who experienced at least weekly faecal soiling for 6 months | RCT | 87 | Mean age: 8.6 (SD 2.0) years. Gender M : F Ratio: 72 : 15. Mean duration of symptoms 58.2 (SD 38.5) months. |
Three-arm RCT: children were randomised to receive (1) intensive medical therapy; (2) intensive medical therapy plus enhanced toilet training; or |
NR | NR |
| (3) Intensive medical therapy plus enhanced toilet training combined with EMG biofeedback. | ||||||||
| Intensive medical therapy included colonic disimpaction using enemas plus laxative therapy. | ||||||||
| Enhanced toilet training: involved clinical psychologists instructing children and parents about psychophysiology of constipation and strategies to establish toileting. Routines 8–12 minutes toilet time were established after meals. | ||||||||
| Biofeedback: surface EMG biofeedback with children asked to tighten and relax the external anal sphincter. 15–20 minutes training typically involved while viewing a ‘game’ on screen. | ||||||||
| Children in each group were observed 10–14 days post assessment and reviewed weekly as required. Follow-up for 12 months. | ||||||||
| Garcia 2016204 (abstract only) | To validate a 1-day novel combination of oral and rectal laxatives for faecal impaction. | Paediatric patients aged 2–18 years old admitted with faecal impaction | RCT | 58 | Age: NR Gender: NR |
Oral + enema therapy vs. enema | NR | NR |
| COMREST (Constipation management relief and support therapy) regimen. One-day novel combination of oral and rectal laxatives for the treatment of faecal impaction that consists of Fleet enema, bisacodyl suppository, and castor oil. | ||||||||
| Details about this intervention are limited – abstract. | ||||||||
| Loening-Baucke 1990194 | To determine whether outcome in chronically constipated and encopretic children with abnormal defaecation dynamics could be improved with biofeedback training | Children aged 5–16 years with abnormal defaecation dynamics, and at least 2 soiling episodes per week and evidence of a huge amount of faecal material in the rectal ampulla at rectal examination. Faecal soiling for >1 year. | RCT | 43 | Age: Biofeedback group 9.2 (SD 2.6) years; conventional group 8.6 (SD 2.2) years. Gender M : F Ratio: 33 : 10. Participants had constipation for >1 year |
All patients received conventional laxative treatment for encopresis and constipation similar to that used for the previous 10 years in our encopresis clinic plus A biofeedback training session included approximately 30–35 defaecation trials and lasted approximately 45 minutes. | NR | NR |
| Abnormal defaecation dynamics was defined as an abnormal contraction of the external anal sphincter and pelvic floor during defaecation attempts. Excluded: children with Hirschsprung’s disease, hypothyroidism, mental deficiency, chronic debilitating diseases, or neurological abnormalities and children who had had previous surgery of the colon | 2–6 training sessions (2 days apart) were given. The number of training sessions given depended on how soon the child learnt to relax the EAS. Biofeedback training sessions were stopped after 10 relaxations of the EAS without visual feedback could be accomplished in each of two successive biofeedback training sessions. | |||||||
| Nolan 1998 | To assess the efficacy of surface EMG biofeedback training compared to conventional medical treatment | Children aged 4 years and over, who had received 3 months or more of conventional multimodal therapy, had continuing soiling with or without laxative treatment | RCT | 29 | Mean age: Biofeedback group 9.2 years, control group: 8.4 years. Gender: M : F Ratio: 24 : 5 |
External anal sphincter EMG biofeedback. No bowel preparation. Training procedure was conducted with the patient in the left lateral position. External anal sphincter activity was displayed using the EMG procedure. |
NR | NR |
| (more than once a month) or had achieved remission from soiling but could not sustain continence without continued laxative treatment, and had anismus on EMG during anorectal manometry. | Both recording devices could be seen by the patient, but the child was asked to concentrate on the EMG recorder that indicated external sphincter contraction or relaxation both visually and aurally. | |||||||
| Up to four sessions at weekly intervals were conducted for each patient, each session consisting of ~30–35 defaecation attempts. The aim was to achieve 10 relaxations of the external anal sphincter without visual feedback in two successive sessions. | ||||||||
| Ormarsson 2016192 | To determine the efficacy of LP101 suppositories (Lipid Pharmaceuticals, Reykjavik, Iceland) as a treatment for constipation in children referred to a paediatric ED and compare them to Klyx docusate sodium and sorbitol enemas (Ferring Pharmaceuticals, Prague, Czech Republic). | All children from the age of 1–17 years referred to the paediatric ED with abdominal pain, and diagnosed with constipation | RCT | 80; 77 completed | Age range 1–16 years. Gender M : F Ratio: 37 : 40 |
Compared the efficacy of two different types of enemas. The intervention group received the LP101 suppositories with free fatty acids; or the control group received Klyx enemas docusate sodium and sorbitol enemas. | NR | NR |
| Suppositories (2 sizes; 1 g and 2 g) were administered based on children’s weight and calculated from the clinically recommended dose. Interventions were delivered by study nurses. Intervention duration is unclear. |
||||||||
| Silva 2013199 | To assess the efficacy of physiotherapy, including muscular training, abdominal massage and diaphragmatic breathing | Patients aged 4–18 years old with functional constipation according to the ROME III criteria | RCT | 72 | Mean age not provided. In intervention group 25 were between 4 and 10 years, 11 were between 11 and 18 years. In control group, 20 were between 4 and 10 years, 16 were between 11 and 18 years. Gender: M : F Ratio: 30 : 42 |
Physiotherapy (including muscular training, abdominal massage and diaphragmatic breathing). In the physiotherapy group, exercises including isometric training of the abdominal muscles, | Number of participants with painful defaecation after treatment. Physiotherapy group: 9/36 (25%). Medication-only group 10/36 (27.7%). P = 1.00 |
NR |
| diaphragmatic breathing exercises and abdominal massage were used with conventional treatment including disimpaction, when necessary, a high fibre diet, laxatives and toilet training. | ||||||||
| Physiotherapy was conducted by a single generalist physiotherapist who was specially trained to perform the exercises over the 3 months prior to the study. Twelve individual 40-minute sessions were held 2× week and adherence was confirmed only if patients attended all 12 sessions. A 1-minute rest period was observed between each series of exercises | ||||||||
| Strisciuglio 2021193 EUCTR2015-005111-32-IT |
To assess whether Promelaxin microenemas would be non-inferior to PEG4000 in young children with functional constipation | Children aged 6–48 months, with functional constipation according to ROME III criteria. Exclusion criteria were: suspicion or diagnosis of organic diseases causing constipation such as inflammatory bowel disease, motility disorders, neurological disorders, inherited and metabolic disorders, surgical disorders, anal fissures and Hirschsprung’s disease | RCT | 153 | PEG group – mean age 795.49 days (SD 400.17). Promelaxin group – mean age 729.18 days (SD 342.87). Gender: M : F Ratio: 71 : 82 |
Microenema vs. PEG | NR | Percentage of parents with improved QoL. Immediately after treatment. PEG group – fathers 44%, mothers 44% Promelaxin group – fathers 49%, ynami 52%. ‘QoL was similar between the treatment arms at all time points’ |
| Van der Plas 1996197 | To evaluate the effect of biofeedback training and conventional treatment on defaecation dynamics and outcome in chronically constipated children. | Children of at least 5 years old with a diagnosis of constipation. They had to fulfil at least two of these four criteria: stool frequency less than three per week, two or more soiling and/or encopresis episodes per week, periodic passage of very large amounts of stool at least once every 7–30 days, or a palpable abdominal or rectal mass. Had to have been using laxatives for at least a month. Patients with pathological causes of constipation or learning difficulties were excluded. |
RCT | 192 | Conventional treatment (n = 94) – median age 8 years (range 5–16), 68% male. Biofeedback group (n = 98) – median age 8 years (range 5–16), 63% male. |
The biofeedback group (CT + BF) had five outpatient visits, including conventional treatment, in combination with five biofeedback training sessions. Conventional laxative treatment (CT) had five outpatient visits lasting approximately 30 minutes during which laxative treatment and information from a diary containing defaecation frequency and encopresis and/or soiling episodes were discussed. | NR | NR |
| A high-fibre diet was advised but additional fibre supplements were not prescribed, and patients were instructed to try to defaecate on the toilet for 5 minutes immediately after each meal. During the first 3 days of conventional treatment, patients were instructed to use daily enemas. Motivation was enhanced by praise and small gifts. | ||||||||
| Van Engelenburg 2017200 | To evaluate the effect of pelvic physiotherapy, in combination with standard medical care, compared to standard medical care only | (1) Age 5–17 years, (2) functional constipation according to the ROME III criteria, (3) attending regular schools, (4) parent(s) sign(s) an informed consent, (5) children aged over 11 years sign an informed consent themselves and (6) the child and parent(s) are motivated to participate in the study. |
RCT | 53 | Age: 5–15 years. Physio group 8.8 (SD 2.3); standard care group 8.3 (SD 2.1) years. Gender M : F Ratio: 24 : 29 47/53 participants had constipation for >6 months. |
Standard Medical Care plus PPT (Or Standard Medical Care Alone) Physiotherapy (including core stability and balance training, relaxation and breathing exercises, sensory processing techniques, PFMT, and education). |
NR | Numeric rating scales quantifying influence on daily life as perceived by parents and estimated for child. For parents – adjusted MD between groups = 1.8 (95% CI 0.7 to 3.5), p = 0.047. For children; adjusted MD 2.0 (95% CI 0.2 to 3.8), p = 0.028. |
| Van Summeren 2020201 | To evaluate the effectiveness and cost effectiveness of adding physiotherapy to conventional treatment for children with functional constipation in primary care. | Children were eligible for inclusion if aged 4–17 years and diagnosed with FC by a GP or general paediatrician. Specifically, children were required to have experienced FC symptoms or to have used laxatives in the 4 weeks before enrolment. | RCT | 134 (115 completed at least one follow-up assessment) | Physiotherapy group (n = 67) – mean age 7.3 years (SD 3.4). 57% female. Conventional group (n = 67) – mean age 7.8 years (SD 3.5). 66% female. |
Physiotherapy (including knowledge, toileting behaviour and posture, awareness of sensation of needing to defaecate, relaxation whilst defaecating, pressure and straining during defaecation). Physiotherapy that was carried out by specialist physiotherapists |
NR | Emotional and social functioning subdomains of defaecation disorder list. At 4 months: physiotherapy group – median 82 (IQR 75–88). Conventional group – median 84 (IQR 74–88). |
| The exclusion criteria were psychopathology affecting protocol adherence, severe disease (physician determined), and physiotherapy or urotherapy for constipation in the past 3 years | (i.e. with a master’s degree in paediatric or pelvic physiotherapy and certified after additional postgraduate training in the treatment of bladder and bowel dysfunction in children) | At 8 months: physiotherapy group – median 85 (IQR 79–92). Conventional group – median 85 (IQR 77–90). RR 0.1 (95% CI −4.0 to 4.3), p = 0.675 |
||||||
| Wald 1987198 | To assess the efficacy of biofeedback compared to mineral oil therapy | Children with encopresis for a minimum of 6 months | RCT | 50 | Mean age 7.4 years (range 6–15 years). Gender: M : F Ratio: 40 : 10 |
Biofeedback therapy: children were allowed to view the manometric recordings and given a simple explanation of the recording with specific attention to the responses of the external anal sphincter during contraction and simulated defaecation. Children with normal expulsion patterns were simply asked to reproduce the pattern repeatedly, first with and then without visual feedback. | NR | NR |
| All sessions lasted between 25 and 30 minutes. Following biofeedback, children were instructed to use the technique whenever they attempted to defaecation after breakfast and dinner for at least 5–10 minutes. Reinforcement biofeedback sessions were conducted at 2, 4 and 8 weeks. Progress was discussed at those sessions, which lasted approximately 30 minutes. | ||||||||
| Other primary study designs ( n = 16) | ||||||||
| Awan 2016184 | To determine the role of stretching exercises in improving constipation symptoms in children with spastic cerebral palsy | Children with spastic cerebral palsy and ‘complaints of constipation’ | Prospective cohort | 40 | Mean age: 7.55 ± 1.33. Gender: M : F Ratio: 19 : 11 20 (66.7%) quadriplegic 9 (30%) were diplegic and 1 (3.3%) hemiplegic. |
Physical therapy for cerebral palsy | NR | NR |
| Eisenberg 2009185 | To compare outcomes (including constipation) between children in a passive standing programme and those using a walking device (Dan Hart Walker) | Children with spastic quadriplegic cerebral palsy and categorised by the GMFCS as level 4 or 5; unable to stand and walk with a traditional walker/rollator because of insufficient upper extremity control. | Non-randomised study | 22 | Mean age: Walker group: 6.1 (SD 2.1) years. Standing group: 6.7 (SD 1.6) years. Gender: M : F Ratio: 12 : 10 |
Standing frame vs. walker for cerebral palsy. Before the study began, all subjects were receiving physical therapy based on neurodevelopmental treatment of movement while working on motor functions (including lower-extremity weight-bearing activities). A standing programme in a SF 4 times a week for 30 minutes was part of the NDT treatment. Parents were encouraged to use a SF at home, for example during the time the child watches television. |
NR | NR |
| All the children in the study underwent the NDT treatment programme. The control subjects continued with the programme which included the standing sessions. | ||||||||
| Children in the study group, however, did not use the SF but practiced a standing and stepping programme in the HW-gait trainer that provides support to allow the child to walk. The programme began with 30-minute sessions, 4 times a week but the children as well as parents were encouraged to use the HW device | ||||||||
| Jarzebicka 2016182 | To evaluate the effectiveness of biofeedback therapy n children with constipation and pelvic floor dyssynergia (PFD). | Children diagnosed with CFC and PFD | Cohort | 44 | Mean age: 12 years (range 7–18 years). Gender: M : F Ratio: 33 : 11 |
Anorectal manometer to provide biofeedback | NR | NR |
| Jorgensen 2017177 | To evaluate the feasibility and efficacy of TAI | Children with functional faecal incontinence treated with TAI | Retrospective cohort | 72 | Mean age: 9.2 ± 2.2 years Gender: M : F Ratio: 47 : 25 |
Transanal irrigation: Alterna TAI All children accepted treatment and 35% (n = 25) were titrated to daily sessions. |
NR | NR |
| Koppen 2017178 | To explore the treatment efficacy and parental satisfaction in children with FC who are treated with Peristeen. | Children with CFC | Survey study | 67 responses | Time of survey = Mean age: 11.2 years (range 4–19 years). Gender: M : F Ratio: 37 : 30 |
Peristeen TAI. Information about Peristeen and how to use the irrigation system were provided. This included patient-tailored instructions on how to insert and inflate the balloon (e.g. maximum amount of air inflations based on age). |
NR | NR |
| During the first time of irrigation, the balloon was inflated until there was no more water leakage from the anus, and this indicates that the balloon seals the anal canal. The patients (and their parents) were then supported during outpatient clinic visits until they were able to use Peristeen at home, from then on follow-up by the paediatric gastroenterology nurse consisted mainly of telephone contacts. | ||||||||
| See Nasher 2014 entry for a more complete description about the Peristeen device. | ||||||||
| Loening-Baucke 1993188 | To describe characteristics of children receiving a treatment programme | Subgroup of a cohort of 285 children, excluding children with pathological reasons for constipation, and those less than 6 years old. | Cohort study, questionnaire | 174 | Mean age at onset of symptoms = 11 (SD 13) months. 87 males, 87 females | Education, disimpaction, and toilet training | NR | NR |
| Loening-Bauke (i) 1989187 | To evaluate factors thought to contribute to treatment failure of CFC | Children with chronic constipation and overflow incontinence. | Non-comparative study | 97 | Mean age: 9 years Gender: M : F Ratio: 69 : 28 |
Laxatives, diet, toileting programme, rewards. Phosphate enemas, milk of magnesia, a high-fibre diet and instructions in bowel training techniques. Parents and children were instructed and encouraged to increase intake of high fibre or bran-containing cereals and breads, fruits and vegetables. | NR | NR |
| Modin 2016189 (abstract only) | To evaluate behavioural difficulties in children with CFC, based on treatment outcomes | Children aged 5–16 years, referred to paediatric department with FC. Exclusion – organic causes of constipation |
Prospective cohort study | 132 recruited (data for 116 analysed) | Age: range 5–14 years. Gender: 46.6% female. Duration of symptoms: median 12 months. |
Conventional treatment, including information and disimpaction (1.5 g/kg/day) and maintenance treatment (1 g/kg/day) with PEG3350 | NR | NR |
| Nader 2016197 (abstract only) | To explore outcomes of children who have been treated with biofeedback for encopresis | Children aged over 6 years old who had faecal incontinence or encropresis secondary to functional constipation and who had been managed using enemas, PEG and weekly biofeedback sessions. | Retrospective cohort | 25 | Median age 10 years (range 7–17). 10 male, 15 female. | External anal sphincter EMG biofeedback. Initial management included enemas, PEG treatment and weekly biofeedback sessions after ano-rectal manometry to rule out Hirschsprung’s disease. |
NR | NR |
| Nasher 2014179 | To evaluate the efficacy of the Peristeen TAI system when treating faecal incontinence in children due to chronic idiopathic constipation | Children with CIC and FI, seeing no improvements on conventional medical therapy. Children had to be able to co-operate with the procedure. | Cohort (retrospective) | 13 (7 with CFC) | Mean age of 10 participants: 11.1 years (range 10–18 years). Gender: M : F Ratio: 7 : 3 |
Peristeen as described in the guidelines published by the manufacturing company. Families were then supported at home by the local paediatric community nurse who had been educated in the TAI system. The device was easily accessible to patients, as it is provided by the UK NHS on a free-of-charge prescription. If patients were unhappy with the procedure, they did not have to continue with it. | NR | ‘…90% having improvement in their “social problems” or QoL score and 60% achieving a normal score with no social problems following Peristeen treatment’. (No other QoL data provided) |
| The Peristeen system consists of a control unit with a pump, a water bag, and a rectal catheter. Tap water is warmed (36–38 °C) and introduced into the colon via the rectal catheter. Once the rectal catheter has been inserted, an inflatable balloon ensures that it remains in situ until the balloon is deflated. The water, along with the stools in the lower portion of the bowel, is then emptied into the toilet. | ||||||||
| Patel 2019180 (abstract only) | To evaluate the efficacy of the Peristeen TAI system in children who failed to respond to conservative measures for stool incontinence and constipation. | Children with faecal incontinence and constipation including neurogenic bowel related to spina bifida, chronic constipation, or congenital anorectal malformations. | Cohort | 97 (19 with CFC) | Mean age NR Gender NR |
Peristeen TAI as per manufacturers guidelines. No details about who provided the intervention. See Nasher 2014 entry for a more complete description about the Peristeen device. |
NR | NR |
| Raffaele 2015183 (abstract only) | To evaluate the role of biofeedback in the clinical improvement of these children with chronic constipation and incontinence | Not stated | Cohort | 25 | Aged 4–18 years | External anal sphincter EMG biofeedback Limited details about the intervention in this abstract. |
NR | NR |
| Sharma 2016181 | To evaluate the efficacy and safety of TAI system (Peristeen) treatment in children with CFC | Children with CFC who had not responded to long-term laxative management | Cohort | 11 | Mean age = 12.2 years Gender: M : F Ratio: 8 : 3 |
Peristeen TAI as per manufacturers guidelines. Provided at home usually 2× week by the caregiver. Intervention was stopped or the child was weaned off therapy if the Peristeen was successful. See Nasher 2014 entry for a more complete description about the Peristeen device. |
NR | Refers to ‘social activity data’, ‘anxiety and self-confidence’. No data presented. |
| Waingankar 2018186 | To explore the effect of exclusion of FODMAPs from the diet | Patients who attended a surgical paediatric bowel clinic with ‘severe chronic constipation that has not resolved with years of treatment by GPs, paediatricians, or gastroenterologists’. | Cohort study, questionnaire | 29 | Age range 5–15 years 70% male |
Sugar restriction (health professional supervised) Level of sugar tolerance/intolerance was assessed using breath tests. Children with levels >20 ppm of hydrogen or methane above baseline on two consecutive breath measurements 15–30 minutes apart were identified, enrolled to start a diet excluding the positive sugar. Parents monitored the child’s diet. | Visual analogue scale for pain on defaecation. Before: mean 5.8 (SEM 0.6). After: mean 2.6 (SEM 0.5). p < 0.0001. |
NR |
| Multiple providers were involved in this intervention with parents asked to contact their local providers. The intervention lasted 6–12 months, after which time sugars were reintroduced to the diet and closely monitored. | ||||||||
| Yoo 2017176 | To evaluate the efficacy and safety of combined oral and enema therapy using PEG3350E. | Children diagnosed with CFC admitted for inpatient treatment | Retrospective cohort | 28 | Mean age: 8.9 years (SD 0.8, range 2–17). Gender: M : F Ratio 20 : 8 Mean duration of constipation = 41.6 (SD 5.0) months |
Combined oral and enema therapy using PEG3350 with electrolyte solution. Disimpaction using the combined therapy involved oral PEG3350E administered was 50–70 ml/kg/d (PEG, 3–4.1 g/kg/d) divided into intervals of 1–2 times a day with each dose taken within 3 hours each time. An enema was administered 1–2 times a day with a single dose of 15–25 ml/kg (PEG, 0.975–1.625 g/kg/day). |
NR | NR |
| Treatment was administered once or twice a day over 1–3 days No details about the provider. All interventions were delivered in a hospital setting. |
||||||||
| Study | Focus | Inclusion criteria | Study design | No. of participants | Participant demographics | Findings: painful defaecation | Findings: QoL |
|---|---|---|---|---|---|---|---|
| Ahmadi 2013209 | To determine the utility of intra sphincteric injection of botox in the treatment of children with refractory constipation | Children who suffered from chronic constipation for more than 3 months, and who had not responded to medical treatment. | Controlled before-and-after study | Intervention group = 40 | Intervention group, median age 5 years (range 2–12 years). Gender: M : F Ratio: 22 : 18 |
Percentage of patients with symptom of painful defaecation – Before treatment = 87.5% After treatment = 15% p = 0.0001 |
NR |
| Basson 2014210 | To evaluate outcomes of ISBTI in children with intractable constipation. | Patients ≤ 16 years of age undergoing injection of Clostridium botulinum toxin type A injection to the anal sphincter | Cohort study | Total 43. CFC group 29 | CFC group median age at first injection was 5 years 9 months (range 1 year 11 months to 13 years 5 months). Gender: M : F Ratio: 20 : 9 |
NR | NR |
| Basson 2014a226 | To evaluate outcomes of ACEs and identify predictors of outcome | Patients ≤ 16 years of age undergoing an ACE procedure for intractable constipation or faecal soiling | Cohort study | Total 111. CFC group 68 | CFC group median age at ACE was 9 years 8 months (range 3 years 2 months to 16 years). Gender: M : F Ratio: 41 : 27 |
NR | NR |
| Bellomo-Branda 2018227 | To determine the effect of ACE procedure on children with refractory constipation and ORSI | Children with refractory constipation (defn: ORSI persistence 12 months follow-up under conservative therapy) | Cohort study | 29 | Age: median age 94 months (minimum 27, maximum 142). Gender: NR | NR | NR |
| Bonilla 2013246 | To describe long-term outcomes of patients with caecostomy failure | Subgroup of paediatric patients who failed to improve after caecostomy | Cohort study | 12 | Age: mean age 14.7 years (range 11–23 years). Gender: M : F Ratio: 7 : 5 |
NR | NR |
| Cascio 2004243 | To compare the results, complications, and outcomes of patients who had either MACE or CB performed. | Paediatric patients with intractable constipation and faecal soiling that had failed conventional treatment who had MACE or CB performed | Cohort study | 49 | Age: MACE Group: mean age at surgery 9.9 years (range 3–18 years). CB group: mean age at surgery 9.8 years (range 3–15 years). Gender: MACE group: M : F Ratio: 15 : 22. CB group: M : F Ratio 9 : 3 |
NR | NR |
| Chong 2016228 | To determine long-term outcome of ACE | Patients who had ACE formation conducted. | Cohort study | 133 in total. 14 patients with CFC (10%) | Age: CFC Group: median age at surgery 8 years (range 5–12 years). Gender: CFC group: M : F Ratio: 11 : 3. |
NR | NR |
| Church 2017229 | To investigate the success of ACE | Children with encopresis who had received ACE through appendicostomy or caecostomy tube | Survey study | 10 | Age: mean age 8.9 years, at time of surgery. Gender: M : F Ratio: 6 : 4 |
NR | Paediatric QoL score (mean and SD): Before ACE 52.45 (22.75). After ACE 77.17 (19.76). p = 0.005 Disease-specific QoL score (mean and SD): Before ACT 8.38 (3.38). After ACE 13.5 (1.31). p = 0.004 |
| Dolejs 2017230 | To determine short- and long-term outcome of ACE | Patients who had ACE for unremitting constipation and faecal incontinence | Cohort study | 93 | Age: median age 10 years (range 7–11 years). Gender: M : F Ratio: 56 : 37 |
NR | NR |
| Gasior 2018248 | To determine outcome of laparoscopic sigmoid resection combined with Malone appendicostomy |
Patients with FC that failed medical management and underwent laparoscopic colon resection with antegrade flush | Cohort study | 31 | Age: median age 12 years (range 4–18 years). Gender: M : F Ratio: 17 : 14 |
NR | NR |
| Gomez-Suarez 2016231 | To determine predictors of poor outcome after ACE procedure | Patients who had ACE performed | Cohort study | 40 (31 with CFC) | Age: mean age 9.5 ± 4.4 years. Gender: M : F Ratio: 20 : 20 |
NR | NR |
| Hallagan 2019211 | To determine outcome of botulinum toxin injection | Children with various anorectal and colonic disorders who received anal sphincter botox injection | Cohort study | 303 (36.8% with CFC) | Age: median age 5.24 years (range 3 weeks to 19 years) Gender: M : F Ratio: 187 : 116 |
NR | NR |
| Hameed 2018212 | To determine the utility of intrasphincteric injection of botox in the treatment of children with refractory constipation | Children who suffered from chronic constipation for more than 3 months, and who had not responded to medical treatment. | Controlled before-and-after study | Total = 50. Intervention group = 20 | Intervention group, median age 4 years (range 2–8 years). Gender: M : F Ratio: 12 : 8 |
Percentage of patients reporting painful defaecation: Before treatment = 88% After treatment = 15% (control group = 90% and 86%, respectively). p = 0.0001 |
NR |
| Har 2013232 | Changes in QoL scores after MACE procedure | Children with unremitting FC and a normal evaluation, including both anorectal manometry and colonic manometry, who decided to undergo a MACE procedure | Survey Study | 15 | Age: mean age 9.8 years (range 7.0–11.1 years). Gender: M : F Ratio: 10 : 5 |
NR | ‘Mean QoL score pre-MACE was 64.1. At 6 months post-MACE the mean overall QoL score was 90.2, and it was 92.0 at 12 months. All 15 patients at the 6-month follow-up had significant improvement in their QoL (p = 1.9 × 10−7) and all subcategories of QoL were significantly improved as well’. |
| Hoekstra 2011233 | To evaluate outcome of MACE | Patients with intractable constipation and/or faecal incontinence who received a MACE stoma | Cohort study | 23 (15 with CFC) | Age: median age at surgery 7.3 years (range 2–17). Gender: M : F Ratio: 14 : 9 |
NR | QoL questionnaire: ‘evaluated the indication for the MACE procedure, complications, reoperation(s), use of MACE, social functioning, patient satisfaction, choice for surgery in retrospect, and the patients’ recommendation to other patients’. |
| Results: ‘86% of the patients were satisfied with the results of the Malone stoma, with a median score of 8 (range, 6–10). No restrictions in daily life after the surgical procedure were seen in 60% of patients. A minority of the patients experienced some restrictions at school and with sports.’ | |||||||
| Husberg 2011234 | To assess long-term outcomes of MACE | Adolescents who had received ACE for gastrointestinal functional disturbances | Cohort study | 27 (2 with CFC) | Age: mean age 11 years (at surgery) (range 5–21 years). Gender: NR |
NR | NR |
| Janssen 2018214 | To determine how the long-term results of SNM for FC between children and adults compare | Between 10 and 18 years, at least 3 months of FC symptoms, failure of conservative treatment. ROME III met for constipation. | Retrospective cohort study | 38 children | Age: mean 15.8 years (10.4–17.9). Gender: male 6.7% | NR | Assessed using SF-36. 10 of 22 children still receiving SNM returned SF-36. ‘children scored worse on BP (45.5, SD 23.2), GH (50.4, SD 22.4) and V (42, SD 20.6) compared with the Dutch population [BP 74.9, SD 23.4 (p = 0.003); GH 70.7, SD 20.7 (p = 0.019); V 68.6, SD 19.3 (p = 0.003)]’. |
| King 2005235 | To determine whether ACE procedures are successful for idiopathic paediatric STC. | Patients with an appendicostomy for idiopathic chronic constipation | Survey study | 42 | Age: mean age 13.1 years (range 6.9–25). Gender: M : F Ratio: 31 : 11 |
NR | Quantitative assessments of QoL were performed using a modified Templeton score (maximum score, 3).’ Templeton score: Pre-ACE – mean 1.4 (median 1.5, range 0.5–3.0). Post ACE – mean 2.2 (median 2.5, range 0.5–3.0). p < 0.0001. 39/42 families ‘felt there was a significant improvement in their child’s QoL’ |
| Khoo 2017236 | To evaluate the effectiveness of ACE | Paediatric patients undergoing ACE formation for idiopathic constipation | Cohort study | 84 | Age: median age at ACE 9.2 years (range 3–16.6 years). Gender: M : F Ratio: 52 : 32 |
NR | NR |
| Kuizenga-Wessel 2017249 | To assess surgical management of CFC. | Children with CFC who were unresponsive to conservative management and had undergone surgery for their FC. | Cohort study | 37 | Age: median age 12 years (range 1.6–17.6) at time of surgery. Gender: 12 : 25 |
NR | NR |
| Lu 2017215 | To assess how well does SNM work for children with constipation severe enough to be treated with ACE | Up to 21 years old who initiated SNS >2 years ago for treatment of constipation | Prospective cohort study | 25 (17 with FC) | Age: mean 14.0 years. Gender: male 52% | NR | Gastrointestinal Symptom Scale (higher score = improvement) Baseline: 66.7 (44.4–75.0) 12 months 62.5 (52.8–84.8) 24 months 61.1 (47.2–63.9) |
| Lu 2016250 | To determine the long-term outcomes of SNM | Up to 21 years of age treated with ACE for constipation refractory to conventional treatment who underwent SNS implantation | Prospective observational cohort study | 22 | Age: mean age 12 years (range 6–19). Gender: male 52% | NR | Gastrointestinal Symptom Scale Baseline 59.7 (42.4 – 72.2) Follow-up 80.6 (55.6 – 88.9) |
| Mousa 2006237 | To compare the clinical outcome of caecostomy in children with defection disorders secondary to functional constipation, imperforate anus and spinal abnormalities. | Children who had ACE performed via caecostomy due to defaecation disorders. | Retrospective cohort study | Total = 31 Only 9 had CFC. |
CFC group mean age at caecostomy 12 years (range 3–16). Gender: M : F Ratio: NR |
NR | QoL was assessed by scoring limitations of activity (none, mild, moderate and severe), global health score and global emotional score (poor, fair, good, very good and excellent). Data presented within graph. p < 0.01 between pre and post scores. |
| Mousavi 2014222 | To evaluate effectiveness of anorectal myectomy | Children with refractory chronic constipation who did not respond to diet, laxative, or enema who received anorectal myectomy | Cohort study | 44 | Age: median age 4.6 years (range 1–12 years). Gender: NR |
NR | NR |
| Mugie 2012238 | To evaluate effectiveness of ACE | Children with chronic constipation, faecal incontinence or both who received ACE. | Cohort study | 99 (35 with CFC, remaining had organic causes). | Age: median 8 years (range 2–22 years). Gender: M : F Ratio: 57 : 42 |
NR | NR |
| Peeraully 2014239 | To assess the effectiveness of MACE for children with constipation or faecal incontinence | Under 16 that had undergone MACE | Retrospective cohort study | 45 | Age: mean age (8–23 years range). Gender: NR | NR | NR |
| Peeters 2011216 | To assess the short-term results of conducting sacral neuromodulation therapy in adolescents with CFC | FC according to the ROME III criteria not responding to intensive oral and rectal laxative treatment | Retrospective cohort study | 13 | Age: 10–18 years. Gender: 100% female | NR | NR |
| Peyvasteh 2015223 | To evaluate effectiveness of anorectal myectomy | Children with refractory chronic constipation who did not respond to diet, laxative, or enema who received anorectal myectomy | Cohort study | 48 | Age: mean age 4.4 years (range 1.5–11 years). Gender: M : F Ratio: 21 : 27 |
NR | NR |
| Randall 2014240 | To assess long-term outcomes of ACE | Children with persistent constipation or soiling | Cohort study | 203 (126 with CFC 60%) | Age: median 9 years 7 months (range 3–17 years). Gender: M : F Ratio: NR |
NR | NR |
| Redkar 2018224 | To evaluate the diagnostic and therapeutic effectiveness of anorectal myectomy | Children with chronic refractory constipation | Cohort study | 107 (37 with normal histology) | Age: mean age 4 years 1 month (range 7 months to 9 years). Gender: M : F Ratio: 71 : 36 |
NR | NR |
| Redkar 2012225 | To evaluate the diagnostic and therapeutic effectiveness of anorectal myectomy | Children presenting with chronic constipation and showing no response to rigorous medical management | Cohort study | 28 | Age: (range 11 months to 9 years). Gender: M : F Ratio: 17 : 11 |
NR | NR |
| Siddiqui 2011241 | To evaluate long-term outcome of ACE | Children who had undergone ACE procedure | Cohort study | 117 (37 with CFC) | Age: median age 11.1 years. Gender: M : F Ratio: 46 : 59 |
NR | NR |
| Sulkowski 2015217 | To assess the short-term results of SNS on patient-reported symptoms and medical management in children with BBD | Chronic constipation, urinary retention associated with neurogenic bladder, and both faecal and urinary incontinence. | Retrospective cohort study | 34 | Age: median 12.1 years (IQR): (9.4, 14.3). Gender: 44.9% female | NR | Paediatric QoL GI symptom scale (n = 22). Before treatment. Median 15 (IQR 2.3, 20). After treatment. Median 8 (IQR 3, 14). p = 0.016. |
| Tamura 2020247 | To evaluate outcome of colonic resection and compare three different methods of resection | Children with idiopathic constipation who had undergone colonic resection. | Cohort study | 22 | Age: median age 13.7 years. Gender: M : F Ratio: 9 : 13 |
NR | NR |
| Van der Wilt 2017219 | To assess the cost effectiveness of sacral modulation compared to other treatments | Aged 10–18 years suffering from CRC. All patients had FC and met ROME-3 criteria for CFC | Prospective cohort study | 30 | Age: mean 16 years. Gender: female 100% | NR | NR |
| Van der Wilt 2014220 | To assess the effectiveness of sacral neuromodulation | Patients with constipation refractory to conservative treatment according to the ROME III were included (and adolescent) | Cohort study | 33 | Age: 10–20 years. Gender: 1 boy, 32 girls | NR | NR |
| Van der Wilt 2016218 | To determine whether short-term effects of sacral neuromodulation in children and adolescents with constipation are sustained over prolonged period of time | Aged 10–20 years, with refractory constipation, fulfilling the ROME III criteria | Prospective cohort study | 30 | Age: mean 16 (range 10–20). Gender: 100% female | Painful defaecation – 5-point Likert scale. Data not presented. (‘symptoms such as pain at defaecation, straining, and incomplete evacuation showed a comparable decrease …’) |
EQ-5D Visual analogue scale Individual patient data scores provided. ‘The EQ5D VAS score was 69.90 (SD 17.96) at a median follow-up of 12 months, which is lower than the norm score for healthy Dutch females aged 15 – 19 (mean 76.73, SD 12.58)’. |
| Van Wunnik 2012221 | To assess the short-term results of conducting sacral neuromodulation therapy in adolescents with CFC | ROME III criteria for FC, not responding to intensive conservative treatment (oral laxatives, enemas or colonic lavage, and behavioural approaches). | Retrospective cohort study | 13 | Age: median age of 15.2 years (range 10–18). Gender: 100% female | NR | NR |
| Vriesman 2020244 | To compare effectiveness of ACE treatment compared to SNS in | Children with intractable FC and FI. | Cohort study | 42 (ACE = 23, SNS = 19) | Age: median age ACE 10 years (range 6–17 years), SNS 10 years (range 7–16 years). Gender: M : F Ratio: ACE 11 : 12, SNS 5 : 14 |
NR | NR |
| Wang 2019245 | To compare outcomes of SNS and ACE for children with severe FC | Children with severe FC. | Cohort study | 41 (ACE = 10, SNS = 31) | Age: median age ACE 10 years (range 5–16 years), SNS 9 years (range 6–16 years). Gender: M : F Ratio: ACE 4 : 6, SNS 11 : 20 |
NR | NR |
| Youssef 2002b242 | To assess the benefit of ACE | Patients with CFC who had undergone caecostomy placement for administration of ACE and who had no evidence of neurological handicap. | Cohort study | 12 | Age: mean age 8.7 years. Gender: M : F Ratio: 9 : 3 |
NR | ‘Caretakers rated their children’s overall health and emotional state on a 1–5 scale’ Before treatment: 1.7 (0.9) After treatment: 3.6 (0.9) NB – unclear if data are mean (SD) or another measure. p < 0.005. |
| Zar-Kessler 2018213 | To assess botulinum toxin | Children with chronic constipation unresponsive to medication management. |
Cohort study | 141 | Age: median age 7.3 years (range 1–18 years). Gender: M : F Ratio 85 : 56 |
After treatment 62/141 (44%) had decreased pain with defaecation. | NR |
| Study | Focus | Inclusion criteria | Study design | No. of participants | Setting and delivered by | Participant demographics | Intervention | Findings: painful defaecation | Findings: QoL |
|---|---|---|---|---|---|---|---|---|---|
| Aslam 2021252 Country: multiple, NR |
To review evidence on the laxative effect of genus Cassia in children with constipation | RCTs including children aged 2–15 years with FC (ROME III criteria) | Systematic review | 132 children, included within 2 RCTs | Setting: NR (presumed secondary care) Delivered by: NR |
Age 2–15 years; 115/132 male | Intervention: Cassia fistula Frequency/duration: NR |
Average of severity of pain of defaecation by visual analogue scale. | NR |
| Analysis of data from 2 trials (n = 180). After treatment: MD −4.84 (95% CI −8.28 to −1.41) (favours treatment). | |||||||||
| Babaei 2018264 Country: Pakistan |
The efficacy of behaviour modification and some herbal drugs based on TPM in the treatment of childhood constipation. | Aged 2–12 years with FC (based on ROME III) with symptoms ≥ 3 months | Non-comparative study (case series) | 6 | Setting: Traditional Medicine Clinic Delivered by: N/R | Age: mean age of patients was 5 (± 2.7) Gender: 4 females |
Intervention/frequency/duration: Behaviour modification and herbal drugs based on TPM. According to TPM – massage with oil, Senna Cascara, aloe, Rhubarb, Terminalia chebula, Citrullus colocynthis and Ficus carica. | NR (reports improvement in symptoms, which included pain during defaecation, but no separate data presented) | NR |
| Not report ROME III for IBS. No mental disorders, or diseases leading to gastrointestinal problems. Currently, not receiving drugs affecting gastrointestinal motility or undergone gastrointestinal-related surgeries. Free of medication and had not previously responded to conventional drugs. |
Reason for constipation: FC. ASN: Not clear if some had ASN implication is they would be included. | Advice around slow chewing of foods, drinking adequate water but not during or just after meals, avoid junk food and toilet training. In the case of obesity, dyspepsia, abdominal skin coldness in palpation (cold diathesis), thirst, hyperactivity and abdominal skin warmness in palpation (hot diathesis), D. Sophia or Oxymel was added to their therapeutic regimen. Duration: 1 month. Frequency: not reported | |||||||
| Bromley 2014261 Country: UK |
Role of abdominal massage in the management of chronic constipation for children with disability? | Aged 3 months to 19 years. Presentation of an identified disability. Diagnosis of chronic constipation with symptoms ≥ 8weeks | Non-comparative study (service development) | 28 | Setting: Community Delivered by: Specialist HV | Age: 3–19 years Gender; NR Reason for constipation: ASN: Children with known disabilities and/or learning needs. |
Intervention/frequency/duration: Training session on abdominal massage (for parents). Abdominal massage performed for 20 minutes a day (as preferred). Professional support available over phone/face-to-face. Duration: 6 weeks | NR | Percentage of parents reporting an improvement in their child’s ‘QoL’, relating to: Dietary intake (64%) |
| Sleep patterns (66%) Pain (70%) Fluid intake (48%) Use of continence products (87.5%) |
|||||||||
| Chase 2011220 Country: multiple, NR |
Review examining the efficacy of non- pharma/surgical/behavioural treatment of FC in children. | Children (0–18 years) with diagnosis of FC, with symptoms ≥ 8 weeks. | Systematic review | 87 | Setting: NR (presumed secondary care) Delivered by: NR (presumed trained HCP) |
Age: range 7 months–16 years Gender: 79% male Reason for constipation: N/R |
Intervention: 3 studies: spinal manipulation Frequency/duration: varied from 1 to 15 months 3 studies: reflexology to feet, acupuncture, and electrical stimulation |
NR | NR |
| Cai 2018257 Country: China |
Effect and safety of XEBT granules for treating chronic constipation in children. | Children (1–14 years) with diagnosis of FC (based on ROME IV) and classified with food retention syndrome in traditional Chinese Medicine. | RCT | 480 | Setting: 7 medical centres Delivered by; N/R |
Age: XEBT: mean, SD = 5.54, 3.07, median 4.83. Placebo: mean, SD = 5.69, 3.02, median 5 | Intervention: XEBT vs. placebo Frequency/duration: children 1–3 years 2.5 g 3 times a day, 4–6 years 5 g 2 times a day, >7 years 5 g 3 times a day. All delivered over 14 days |
‘Difficult defaecation’: number of participants in each group whose symptoms (disappeared) after treatment. | NR |
| Gender: XEBT: male 46.52%(167), placebo 50.42%(60) Reason for Constipation: all organic cases excluded |
XEBT group 238/356 (66.8%) Placebo group 11/119 (9.2%) p = 0.0001 |
||||||||
| Canbulat Sahiner 2017253 Country: Turkey |
Effect of reflexology in treating children with FC | Aged 3–6 years, diagnosis of FC. Absence of metabolic or chronic illness. | RCT | 37 | Setting: hospital Delivered by: diagnosis by physician then intervention delivered by reflexology specialist |
Age: mean 5.24 ± 1.01 years Gender: intervention (M = 11 65%), control (M = 8 40%) Reason for constipation: FC only. Children with metabolic or chronic illness excluded |
Intervention/frequency/duration: foot massage (10 minutes, 5 days a week), toilet/diet/motivation training (30 minutes once a week), consultancy service via home visits and phone calls, all delivered over 4 vs. control: toilet/diet/motivation training over 4 weeks |
NR | NR |
| Duymaz 2020254 Country: Turkey |
Reflexology on constipation severity, defaecation frequency, pain and QoL in children with cerebral palsy | Aged 4–12 years, with cerebral palsy and chronic constipation (based on ROME III). No surgical intervention in the last 6 months. No laxatives or enemas for 4 weeks. Excluded: children with congenital malformations, GI diseases; impaired joint deformation to prevent reflexology. |
RCT | 50 | Setting: special education centre. Delivered by: ‘therapist’ |
Age: reflexology group – mean 7.33 (SD 3.44) years; control group – mean 8.16 (SD 1.94) years. Gender: 60% female. 19 severe diplegia, 31 quadriplegic. |
Intervention/frequency/duration: patients in both groups received neurodevelopmental therapy, 2 sessions/week for 12 weeks. Sessions were 45 minutes. Patients in the reflexology group, in addition to the neurodevelopmental therapy, received reflexology – 24 sessions (2 sessions/week for 12 weeks). Sessions were 20 minutes. Reflexology involved pressure to reflex points of the soles of the feet using six different techniques. |
Pain scored using visual analogue scale. After treatment. Reflexology group: mean 1.5 (SD 1.04). Control group: mean 7.16 (SD 0.77). p = 0.004 |
NR |
| Elbasan 2018262 Country: Turkey |
Effects of reflexology on constipation and motor functions on children with cerebral palsy? | Aged 3–15 years. Diagnosed with CP. Children with GMFCS levels of 3/4/5 without open wounds in reflexology application area of the foot. | Non-RCT | 40 | Setting: individuals referred by experienced specialist in paediatric Neurology to Physio rehabilitation clinic Delivered by: physiotherapists |
Age: 5.85 ± 2.35 years (Group 1) 5.1 ± 1.89 for (Group 2) Gender: 16 females, 24 males Reason for constipation: organic due to CP ASN: children only included if diagnosed with cerebral palsy |
Intervention/frequency/duration: Control (Group 1): neurodevelopmental therapy vs. Intervention (Group 2): neurodevelopmental therapy and reflexology Two sessions per week for 8 weeks. Neurodevelopmental therapy (45–60 minutes), reflexology (20 minutes) |
NR | NR |
| Mostamand 2019263 Country: not stated (abstract only) |
To evaluate the effects of abdominal massage on colonic motility in patients receiving colonic manometry testing for various indications. | Paediatric patients undergoing colonic manometry | Non-comparative study (cohort) | 8 | Setting: hospital Delivered by: N/R |
Age: 2–16 years, mean 8.63 years. Gender: 63% female. Reason for constipation: varied, including children with disabilities and gastric conditions. |
Intervention/frequency/duration: a standardised 5-minute abdominal massage. Duration: one session |
NR | NR |
| Nimrouzi 2015258 Country: Iran |
To clinically compare Flixweed (Descurainia sophia L.) with PEG4000 (without electrolyte) in paediatric constipation and to assess its efficacy and side effects. | Aged 2–12 years with constipation (Rome III criteria) for at least 3 months; free of other medications. Excluded: children with organic causes for constipation. |
RCT | 120 | Setting: specialised medical clinic. Provider: paediatric gastroenterologist |
Age: 5.01 ± 2.38 years. Gender: 48 male, 72 female |
Intervention/frequency/duration: PEG group: PEG (40% solution without electrolytes), 0.4 g/kg, once daily for 8 weeks. Flixweed group: Descurainia sophia L, 2 g for 2–4 years old and 3 g for 4–12 years olds, once daily for 8 weeks. |
NR | NR |
| Orhan 2018255 | Effects of CTM and KT | Children with cerebral palsy aged 4 years or over, free for laxative medications and enemas for at least 4 weeks before the study, and having a diagnosis of chronic constipation according to paediatric ROME III criteria. | RCT | 45 | Setting: university department Delivered by: experienced physiotherapist |
15 participants were randomised to the CTM group [6 females, 7 males; 8 years 6 months (SD 3 years 4 months) range 4–11 years], 15 participants to the KT group [7 females, 7 males; 8 years 7 months | Intervention/frequency/duration: Connective tissue manipulation – all connective tissue areas in the back (sacral, lumbar, lower thoracic, scapular, inter-scapular and cervical) were manipulated from the sacral to the cervical regions. Three days per week for a total of 12 sessions over the |
Post-treatment. Visual analogue scale for pain - median (IQR). CTM group: 3.2 (1.0–4.6). KT group: 3.2 (1.4–3.8). Control group: 6.4 (5.5–7.1). Difference in change from baseline – p < 0.001 Number with pain: |
Paediatric QoL Inventory. median (IQR). CTM group: 42.3 (32.7–58.5). KT group: 43 (31.9–68.4). Control group: 36.9 (20.7–54.2) |
| (SD 3 years 5 months) range 5–11 years] and 15 participants to the control group [6 females, 7 males; 8 years 3 months (SD 3 years 6 months) range 4–11] |
4 weeks of the study period. Each treatment session lasted 15–20 minutes. Kinesio taping – KT (Kinesio Tex, Gold; Kinesio UK, Newcastle upon Tyne, UK) was performed three times per week over the study period of 4 weeks. The tape was kept in position for 2 days and parents were instructed to remove the tape prior to next treatment session. Thus, children remained without taping for just 1 day each week. Taping was applied on lumbosacral area, which is the reflex zone of the bowel and the lower abdominal area | CTM group 2/13 (15.4%). KT group 2/14 (14.3%). Control group 10/13 (76.9%) | Difference in change from baseline – p < 0.001 | ||||||
| Qiao 2021260 Country: China |
To explore the clinical efficacy and safety of Chinese herbal medicine Xiaojidaozhi Decoction in the treatment of childhood constipation | Aged 4–14 years. Meet ROME IV criteria for childhood constipation. Able to tolerate the odour of the Chinese herbal medicine. Exclusion – congenital or acquired intestinal diseases, anorectal diseases, neurological diseases. |
RCT | 200 (153 completed – 82 in Chinese herbal medicine group; 71 in placebo group) | Setting: constipation clinic Delivered by: not stated, but inferred that parent/guardian gives the dose of intervention |
Chinese herbal medicine group: mean age 6.24 (SD 2.20). Gender: 49% female. Duration of symptoms: 6.51 (SD 2.80) months. Placebo group: mean age 6.61 (SD 2.79). Gender: 52% female. Duration of symptoms: 6.78 (SD 3.10) months. |
Intervention/frequency/duration: Medication given twice/day, with fasting, before a meal, for 8 weeks. Chinese herbal medicine group: received a ‘mixture of 12 herbs, including Raphanus sativus L., Areca catechu L., Fructus aurantll immaturus, Citrus aurantium L., Crataegus pinnatifida, Magnolia officinalis Rehd, Cannabis sativa L., Atractylodes macrocephala Koidz., Semen armeniacae amarum, Paeonia lactiflora Pall, Radix et rhizoma rhei, and Honey mel’. (Dosage stated in paper). |
Number with painful or hard bowel movement. At end of treatment: Chinese herbal medicine group: 32/100 (32%). Placebo group 47/100 (47%). p < 0.05 |
NR |
| Placebo group: ‘received a placebo designed to match the CHM group based on appearance, weight, colour, taste, and odour, including 5% drug ingredients and 95% dextrin’ Both groups: ‘basic treatment’ – defaecation training, adjusting diet, fibre intake (20 g/day) and conventional medication. |
|||||||||
| Shahamat 2016256 Country: Iran |
Efficacy of dry cupping therapy of the abdominal wall in children with FC | Aged 4–18 years. Diagnosis of FC (based on ROME III) with symptoms ≥ 3 months. Children having ROME III criteria for inflammatory bowel disease or organic causes excluded. | RCT | 120 | Setting: paediatric gastroenterology clinic. Examined by podiatrist first. Intervention delivered by: Expert in nutritional and behavioural recommendation for children’s constipation and registered operator of cupping therapy. |
Age: cupping group: 6.3 ± 2.1, PEG: 6.4 ± 2.3 0.96 Gender: cupping: (M/F) 25/23, PEG (M/F) 31/29 Reason for constipation: organic causes of constipation excluded |
Intervention/frequency/duration: 3 months Both groups: received routine nutritional and behavioural recommendations for children’s constipation. Intervention group: session 1: training on cupping therapy, session 2: practice session on cupping therapy, 12 sessions parent conduct cupping by parents every other day (total 28 days). PEG administered daily for 4 weeks. |
Number with painful or hard bowel movement. At 12 weeks. Cupping group 5/58 (8.6%). PEG group 10/60 (16.7%). p = 0.190 |
NR |
| Tavassoli 2021259 Country: Iran IRCT20180305038968N1 |
To evaluate the efficacy of VFS compared with PEG) in children with FC. | Aged 4–10 years, FC (ROME III criteria). Excluded: children with organic cause of CFC, other conditions. |
RCT | 140 | Setting: Paediatric gastroenterology clinic. | Age. PEG group: 6.29 ± 2.13 years. Viola flower group: 7.01 ± 2.23 years. Gender: 69 males, 71 females. |
Intervention/frequency/duration: PEG group: PEG solution 40% (1 g/kg/day) Viola flower group: viola flower syrup (5 cc 3 times per day) |
Number of painful defaecations/week are reporting graphically. | NR |
| Zadpe 2020265 Country: India |
Effectiveness of Shunthyadi Syrup in children with Vibandha (constipation) | Aged 5–15 years. Common signs of constipation selected from OPD, IPD of the Department of Kaumarbhritya and Health Camps. | Non-comparative study | 30 | Setting: OPD, IPD, Paediatric clinic Delivered by: N/R |
Age: 14 (46.66%) were 9–11 years. 13 (43.33%) were 5–8 years. 3 (10%) were 12–15 years. Gender: 60% male Reason for constipation: N/R |
Intervention/frequency/duration: Administration of Shunthiyadi Syrup twice a day before food for 7 days. Consists of Ginger, dried fruit of the Haritaki tree, long pepper plant | NR | NR |
| Study | Focus | Inclusion criteria | Study design | No. of participants | Setting and delivery by | Participant demographics | Intervention | Findings: painful defaecation | Findings: QoL |
|---|---|---|---|---|---|---|---|---|---|
| Freeman 2014267 Country: 5 USA, 1 Netherlands, 2 Australia, 1 UK, 1 Mexico |
To examine the evidence on the effect of behavioural treatment of faecal incontinence with constipation | Children between the ages of 4–18 years with a history of faecal incontinence with constipation were included | Systematic review | 562 | Setting: 8 conducted in ambulatory practices, 2 conducted via the internet Delivered by: N/R |
Age: Mean age was 7.9 years Gender: 74.3% male Reason for constipation: FC was idiopathic only |
Intervention/frequency/duration: Multiple intervention components, rarely constrained to one category of intervention |
NR | NR |
| Santucci 2018268 Country: USA |
To examine the effect of guided mastery on self-efficacy and treatment outcomes for children with FC. | ROME 4 criteria for FC | Pilot RCT | 21 | Setting: paediatric gastroenterology clinic Delivered by: psychologist |
Age: 7–16 years Mean age: 10.62 ± 3.09 years, Gender: 38% males 33% were white Reason for constipation: not detailed. |
Duration: 3 weeks. Intervention: intervention group received guided mastery (through a stepwise protocol) Control group: information on dietary changes Frequency: N/R |
NR | ‘QoL on the Peds QoL improved in both groups. There is a clear difference in number of treatment responders and increase in QoL in the intervention vs. control group. This is not statistically significant but that is likely due to lack of a larger sample’. |
| Silver 1998269 Country: UK |
Retrospective audit of the therapy outcome of 108 children with soiling and their families | Referrals included ‘faecal soiling’, ‘encopresis’, ‘psychological soiling’, ‘failed toileting’, ‘constipation with overflow’ and ‘deliberate soiling’. |
Retrospective cohort study | 108 | Setting: Child and Family Consultation Service Delivered by: Therapist |
Age: EXT group: 22 (3–5), 32 (6+), avg (6.98). Other group: 23 (3–5), 31 (6+), avg (6.68) Gender: EXT group (12 F, 42 M), other treatments (16 F, 38 M) Reason for constipation: Excluded if organic cause |
Intervention/frequency/duration Intervention: externalising therapy and this was established at 1st interview with the therapist, narrative developed, no reward system used, family seen as a whole at least once. Other treatments: mixed treatments, a behavioural approach in a family systems context. Duration: externalisation: average 7.8 months, other treatments 6.6 months. Frequency: externalisation: average 8.2 appointments, other treatments 10 appointments. |
NR | NR |
| Taitz 1986270 Country: UK |
To test the effectiveness of behaviour therapy or psychotherapy, or both, that would not seriously tax the resources of busy district paediatric or child psychiatry services. | None had previously been treated by psychotherapy or behaviour modification techniques. They were typical of cases referred to a paediatric outpatient service. All the children were assessed clinically by one individual and then by a child psychiatrist. | Survey | 47 | Setting: paediatric clinic Delivered by: child psychiatrist |
Age: not reported Gender: 26 M, 21 F Reason for constipation: N/R |
Intervention: behaviour therapy and psychotherapy. Control: behaviour therapy only. Frequency/duration: child psychiatrist seen at roughly monthly intervals for between 2 and 12 months. |
NR | NR |
Appendix 5 Characteristics of included studies – economic synthesis
| Author, year (country) |
Study objectives (perspective) |
Methods | Participants | Intervention characteristics |
Comparator | Economic outcomes | Funding or CoI | Type of economic analysis | Key findings |
|---|---|---|---|---|---|---|---|---|---|
| Alper, 2010272 (International evidence) |
Describes biochemistry, efficacy, safety and pharmacoeconomics of PEG studies (Health) |
Narrative review | ‘Children’. No other details. PEG studies vs. other laxatives (n = 1089, 12 studies), PEG for faecal disimpaction (n = 485, 5 studies), PEG studies for bowel prep for colonoscopy, n = 728, 8 studies) |
Intervention: PEG Provider: NR Number of sessions: Daily Frequency and duration: Varied. Range: comparison 1: 2 weeks – 12 months; comparison 2: 3–7 days; comparison 3: varied based on dose but ranged from 3 hours to 4 days Equipment: NR |
Limited details. Table 1 reports comparator as placebo, lactulose, liquid paraffin, fibre and fructose, MoM | NR | Funding: NR COI: none |
Cost-of-illness | Highlighted slightly higher costs of PEG but– refer to the intervention as a ‘game-changer’ because of the intervention characteristics which mean it has a higher acceptability among children (i.e. can be mixed/no taste) |
| Bladder and Bowel UK, 2017275 (UK) |
What is the right care approach: comparing optimal and suboptimal patient journey’s (James) (Health and Society) |
Vignette – single case | Single case scenario for male starting at age 2 years and 3 months. Based on an amalgam of cases who were supported by a third sector charity | Intervention type: Variety of laxatives, toilet training, educational support, behavioural training, rectal biopsy Provider: In the suboptimal journey, child is seen by a variety of professionals as need escalates incl GP, A&E, gastroenterologist. In the optimal journey, seen by HV and continence team |
Optimal journey vs. suboptimal journey | Costs of medication and other interventions, professionals contact time, hospital admission time. Sources: Drug Tariff Sept 2017, PSSRU 2016 |
Funding: NR COI: NR |
Cost-of-illness | Concluded that the difference was £6769.40 between optimal and suboptimal treatment for this case scenario. Authors also point out value to NHS plus improved outcomes for family |
| Number of sessions: NA Frequency and duration: Varied as the different treatments progress Equipment: Telephone |
|||||||||
| Bladder and Bowel UK, 2017274 (UK) |
What is the right care approach: comparing optimal and suboptimal patient journey’s (Michael) (Health and Society) |
Vignette – single case | Single case scenario for male starting at age 3 years. Based on an amalgam of cases who were supported by a third sector charity | Intervention type: Toilet training, educational support, behavioural training, medication, variety of hospital procedures incl. ACE Provider: In the suboptimal journey, child is seen by a variety of professionals as need escalates including HV, GP, SN, ED, hospital-based paediatrician, CAHMS team, dietitian, gastroenterologist. In the optimal journey, seen by HV, continence team, GP |
Optimal journey vs. suboptimal journey | Costs of medication and other interventions, professionals contact time, hospital admission time. Sources: Drug Tariff Sept 2017, PSSRU 2016 |
Funding: NR COI: NR |
Cost-of-illness | Concluded that the difference was £16,701.08 between optimal and suboptimal treatment for this case scenario. Authors also point out value to NHS plus improved outcomes for family |
| Number of sessions: NA Frequency and duration: Varied as the different treatments progress Equipment: Telephone, catheters, Peristeen system |
|||||||||
| BIG, 2020277 (UK) |
To provide useful and up-to-date resources to healthcare professionals in order to benefit patients with bowel conditions. This is alongside our work to increase awareness of constipation and other bowel-related conditions in patients. (Health and Society) |
Secondary data analysis | Adults and children | Intervention: Mainly refer to laxative prescribing. Limited child-specific data reported. Providers: Mainly based on data from GP but also refer to hospital admissions Number of sessions: NA Frequency and duration: NA Equipment: NA |
NA | Costs are based on admissions data for ICD-10 diagnosis code K59.0 (Constipation) (April 2016 to January 2020), Big GP survey and Laxative prescribing data. Detailed on page 16 | Funding: NR COI: NR |
Cost-of-illness | Estimate 1 in 3 children will have constipation (nhsinform.scot/illnesses-and-conditions/stomach-liver-and-gastrointestinal-tract/constipation); ‘Children (age less than 15) accounted for around 18% of admissions (14,000) but less than 10% of the bed days (13,000) in 2018–19’ |
| Brazzelli, 2011270 (International evidence) |
To assess the effects of behavioural and/or cognitive interventions for the management of faecal incontinence in children | Systematic review | Children with and without constipation [‘constipation is used to indicate difficulty or delay in the passage of stools [not a description of the consistency of stool)’] | Intervention: Planned to include any RCTs or quasi-RCT that delivered behavioural and/or cognitive interventions Providers: NA Number of sessions: NA Frequency and duration: NA Equipment: NA |
No treatment, conventional treatment, another behavioural and/or cognitive intervention | Sought evidence for multiple health economic outcomes including resource implications, intervention costs (on health services and patients/families and carers) and cost effectiveness of interventions including cost per episode or soiling avoided, cost per unit of health gain/preference (e.g. cost per QALY) | Funding: CSO, Scottish Government COI: NR |
Other: Planned to conduct CE but only identified one study reporting cost-of-illness | Identified one multiarm RCT (n = 14 included studies) that also reported the average costs of therapy399 (see Table 1). They found that children receiving behaviour modifications plus laxative, required significantly fewer medical assessments than those receiving laxative therapy alone (MD: −0.96, 95% CI −1.87 to 0.05) as a result the estimated costs for this intervention was lower ($213 vs. $246). |
| Bromley, 2014261 (UK) |
Described the costs associated with a complementary therapy (abdominal massage) service development | Service development | 25 disabled children with constipation | Intervention: Abdominal massage Providers: Performed by parents supported by specialist HV Number of sessions: Daily Frequency and duration: 20 minutes a day (distributed across the day as preferred) for 6 weeks Equipment: NA |
NA | Data about bowel movements, medication use and contact with healthcare professionals was collected | Funding: Queens Nursing Institute’s fund Government COI: None |
Cost-of-illness | Parents reported that constipation was improved in the majority of children (87.5%) along with improved school attendance. Annual cost saving of £1322.03 projected for 10 children who reduced their laxative medication at the end of week 6. |
| Choung, 2011276 (USA) |
To estimate the incremental direct medical costs and types of healthcare use associated with constipation from childhood to early adulthood | Nested case-control study | 250/5718 children in a population-based birth cohort born during 1976–82, USA who presented with constipation. |
Intervention: Medical visits and costs that were delivered to improve constipation. Specific details not reported Providers: NA Number of sessions: NA Frequency and duration: NA Equipment: NA |
Age- and sex-matched controls | Resource use and associated charges (e.g. outpatient visits, inpatient stays or ED visits) using a micro costing approach for each service or procedure provided. Source for costs: Olmsted County Healthcare Expenditure and Utilisation Database, USA | Funding: Takeda Pharmaceuticals; Rochester Epidemiology Project COI: None |
Cost-of-illness | Individuals with a diagnosis of constipation consistently had higher health-care costs from childhood to early adulthood |
| Guest and Clegg, 2006279 (Australia) |
To compare the costs and consequences of treating paediatric faecal impaction in Australia (Health and Society) |
Decision model | Data sources drew on a SR and interviews with clinicians (n = 14) for information about treatment patterns and associated resource utilisation. Based on children aged 4–11 years. |
Intervention: Disimpacting and managing children over 12 weeks post-disimpaction using oral macrogel 3350 Providers: Clinicians (inpatient and outpatient settings) Number of sessions: NA Frequency and duration: 12 weeks Equipment: NA |
Compared with enemas/suppositories, manual evacuation and NG administration of macrogel (NGA-PEG) lavage solution in treating faecal impaction in children | Resource use based on clinician interviews and utility estimates obtained from published studies. See Suppl. File 3 for more details | Funding: Norgine Ltd (UK manufacturers of Movicol) – macrogel 3350 plus electrolytes COI: none |
Cost minimisation analysis | Treating children diagnosed with faecal impaction using oral macrogel 3350 in an outpatient setting is cost-effective compared with other treatments |
| Guest, 2007278 (UK) |
To estimate the clinical and economic impact of treating paediatric faecal impaction in England and Wales | Decision model | Children were aged between 2 and 11 years diagnosed with intractable constipation and disimpacted between 1 January 2001 and 31 January 2006 | Intervention: Using macrogel 3350 plus electrolytes in an outpatient setting to treat paediatric faecal impaction. Providers: NA Number of sessions: NA Frequency and duration: NA Equipment: NA |
Compared with enemas/suppositories, manual evacuation | Data resources drew on a chart review of 112 case notes of children treated with macrogel 3350 (in an outpatient setting), 101 case notes of children who received enemas and suppositories and 11 who underwent manual evacuation. See Suppl. File 3 for more details | Funding: Norgine Ltd (UK manufacturers of Movicol) – macrogel 3350 plus electrolytes COI: none |
Cost minimisation analysis | Macrogel 3350 plus electrolytes is clinically effective and cost-effective for treating faecal impaction compared with enemas and suppositories and manual evacuation |
| Han, 201817 (International evidence) |
To comprehensively evaluate the cost effectiveness of treatments for chronic constipation | Systematic review of full economic evaluations of constipation management | Adults and children | Intervention: Authors included full economic evaluations that evaluated a treatment for constipation. Identified 10 studies with various Interventions: Lifestyle advice, dietary treatments and abdominal massage were each compared with current care with laxatives, while PEG and senna–fibre combination was compared with lactulose. | See details in intervention characteristics | Costs and outcomes of treatments for chronic constipation and cost- effectiveness methods | Funding: NR COI: None |
Other: Systematic review of economic evaluations | Identified 10 relevant studies – all of these were conducted in adults. Multiple definitions of constipation were variable as were the number of outcomes reported. The authors found no economic evaluations in children, that included all comparators of laxatives, or for patients who did not respond to laxatives. |
| Newer treatments in patients who had not responded to laxatives: prucalopride was compared with continuing laxatives, and linaclotide was compared with lubiprostone. | |||||||||
| Liem et al., 2009280 (USA) |
To estimate the total costs and healthcare use for children with constipation in the USA. | Secondary data analysis | Children aged <18 years N = 21,778 |
Intervention type: Medications (laxatives), management of constipation Provider: NA Number of sessions: NA Frequency and duration: 2 years of data collected using MEPS survey data Equipment: NA |
NA | Service utilisation and expenditures. Parents reported using a survey. Specific outcomes included: (school/work missed (school, parents’ work); service utilisation) | Funding: NR COI: none |
Cost-of-illness | Constipation in children has a substantial economic impact. The authors noted that the findings reported probably underestimated the actual burden of childhood constipation because it did not ‘account for decreased QoL, undiagnosed constipation, dietary changes, non-traditional therapeutic interventions, clothing/diaper expenses, and OTC laxatives’. |
| (outpatient visits, inpatient visits, emergency room visits, laxative prescriptions); expenditures (total, outpatient, inpatient, emergency room, laxative prescriptions, all other prescriptions, out of pocket); number of other medical conditions | It also excluded children who were in care who are more likely to have higher rates of constipation. | ||||||||
| Mahon, 2017281 (International evidence) |
To estimate the cost of functional gastrointestinal disorders and related signs and symptoms in infants to the third-party payer and to parents. | Systematic review and a cost-of-illness calculation | Infants (<12 months age) | Intervention type: Medications, management of constipation Provider: HV, staff in A&E and hospital Number of sessions: NA Frequency and duration: NA Equipment: NA |
NA | Number of admissions, visits to A&E, mean length of stay (based on ICD-10 codes), prescriptions of constipation medicines (based on analysis 2014/15), appointments with HV, GP | Funding: funded by Nutricia Research COI: TL -employee of Nutricia Research. ILW is an employee of Danone SA. HW, JM, JG and ME are employees of YHEC. HS reports no conflicts of interest for this piece of work.CL, HS, MM, NT and QSH have served as consultants, advisory board members and/or speakers for companies manufacturing infant formulas, foods and probiotics or prebiotics. MS has served as a consultant for a medical food company. |
Cost-of-illness | ‘Evidence from the USA identified in the literature review suggested that 9.4% of all ED visits in the USA due to constipation were in those aged <12 months. If a similar pattern is seen in England and for all FGIDs, then this means that the estimated attendances we have calculated are likely to be a significant underestimate’ |
| Moser, 2014 282 (USA) |
To examine psychology service feasibility, acceptance by medical providers and utilisation patterns (Health) |
Retrospective review of existing billing data of visits to GI-Psychology service | 111/291 children were seen for encopresis, constipation and painful stools Mean age (SD): 10.58 (4.25) years at first visit; Range: 2.77–18.65 years Sex (F): 30.63% |
Intervention type: Psychology appointment Provider: Psychologist Number of sessions: mean (SD): 5.6 (4.61) Frequency and duration: Mean (SD) no. months in treatment = 6.21 (6.44); sessions varied from 20–30 minutes to 45–50 minutes Equipment: NR |
NA | Utilisation of psychology services, programme expenses, collections and offsets | NR | Cost-of-illness | Yes. Expenditure on psychological services was covered up to 97.5% across the 7 years |
| NICE, 2010 (A) (UK) |
Aim of the health economic analysis for this guideline was to develop a model to compare all the pharmacological interventions and combinations of interventions that could be offered to a child with idiopathic constipation. | Economic evaluation | Hypothetical case of a 5-year-old child treated in primary care. | Intervention type: Treatments for disimpaction covered oral pharmacological treatments, in various preparations and dosages, as well as other methods of treatment such as suppositories, enemas and manual evacuation. | Four different pharmacological treatment groups were compared: (a) PEG3350 plus electrolytes, (b) picosulphate, (c) Senna and (d) Enemas | Resources use was calculated for each pathway, including pharmacological treatment costs and hospitalisation costs (related to manual evaluation and enemas only). See Suppl. File 3 for more details |
Funded: NICE guidelines COI: Unclear |
Cost minimisation analysis | Cost of disimpaction by success rate; ‘model showed that treatments with a high chance |
| This was a cost analysis for disimpaction assuming high, medium and low levels of effectiveness | Treatments for the maintenance phase once disimpaction has been achieved included lower dose pharmacological treatments as first line treatment, with higher doses, combinations of treatments and other more invasive procedures where pharmacological treatments fail Provider: Number of sessions: Frequency and duration: Equipment: |
(80%) of success cost less than treatment with a low chance of success (20%), regardless of the price of drugs used or the dose provided. Also, the cost of failure (changing doses, combining drugs and manual evacuation as a last resort) was a far greater determinant of overall cost than the cost of initial treatment. | |||||||
| NICE, 2010 (B) (UK) |
Aim as described in NICE 2010 (A). This involved the analysis of a macrogol [PEG plus electrolytes (Movicol Paediatric Plain – Norgine)] alone to assess the cost effectiveness of different doses of treatment (Health) |
Economic evaluation: decision model | Clinical outcomes and treatment doses came from a RCT297 which aimed to investigate the effectiveness and safety of four different doses of PEG3350 plus electrolytes in the treatment of childhood faecal disimpaction. | Intervention type: macrogol [PEG plus electrolytes (Movicol Paediatric Plain – Norgine)] alone Provider: Number of sessions: Frequency and duration: Equipment: |
None. Different doses of PEG 3350 plus electrolytes were compared | QALYs, cost data based on a 25-kg child. See Suppl. File 3 for more details | Funded: NICE guidelines COI: Unclear |
Cost effectiveness/cost-utility analysis | Analysis by dose of PEG3350 plus electrolytes showed that highly effective strategies will lead to cost savings due to the high downstream costs of invasive treatment requiring hospitalisation that are saved. Effectiveness is determined both by the type of drug used and by the dose given. The data we have been able to identify on doses of treatment suggest that higher doses of PEG3350 plus electrolytes that lead to effectiveness levels of 95% compared with 55% for lower doses would be cost saving to the NHS. |
| NICE, 2010 (C) (UK) |
Aim as described in NICE 2010 (A). This analysis involved the development of a decision analytic model of strategies for disimpaction and initial maintenance in the first 3 months of treatment | Economic evaluation | Clinical outcomes and resource used values obtained from GDG consensus, a model was constructed considering the decision to treat in primary care setting constipated children aged 2–11 years with no flag to a serious underlying disorder after history and physical examination. |
Intervention type:
Provider: Number of sessions: Frequency and duration: Equipment: |
Compared different alternatives | As above – maintenance model. See Suppl. File 3 for more details | Funded: NICE guidelines COI: Unclear |
Cost minimisation analysis | Disimpaction model based on a consensus of treatment pathways developed by the GDG showed that oral pharmacological alternatives were more than 10 times cheaper than enemas which were assumed to be less effective and require hospitalisation. |
| At a 20% failure rate, oral pharmacological treatment provided a mean benefit of 0.23 QALYs per child. The threshold analysis showed that the effectiveness of PEG3350 plus electrolytes would have to be 2.6% higher than the next best alternative in order for it to be the preferred option on cost-effectiveness grounds. | |||||||||
| NICE, 2010 (D) (UK) |
Aim as described in NICE 2010 (A). This evaluation focused on a decision analytic model of strategies for ongoing maintenance after disimpaction (including treatment for reimpaction) in the following 3 months after disimpaction and initial maintenance, 1 year later and 2 years later |
Economic evaluation | X |
Intervention type:
Provider: Number of sessions: Frequency and duration: Equipment: |
As above – maintenance model See Suppl. File 3 for more details |
Funded: NICE guidelines COI: Unclear |
Cost effectiveness/cost-utility analysis | Maintenance model showed that, unlike the disimpaction model, the cost of drugs in the pharmacological treatment alternatives had a greater impact on the total of care than hospitalisation, which widened the gap between the cheapest and most expensive options. | |
| Persels, 2016284 (USA) |
Website providing information about the management and prevention of constipation (Health) |
Website providing general information | Children. | Intervention type: Website detail information about causes of constipation and different management approaches across different age groups Provider: Internet based information provided by/updated by Medical Home Portal Family Consultant Number of sessions: As needed Frequency and duration: As needed Equipment: Home computer, internet access |
NA | Advice about how to reduce costs | Funding: NR CoI: NR |
Cost-of-illness | Provide tips and weblinks for parents/caregivers about how to reduce costs for laxatives and help for diaper costs |
| Phatak, 2014285 (International evidence) |
Reviews ‘mechanism of action, efficacy, safety, and acceptance of PEG as reported in recent paediatric studies. Also compare PEG with previously used laxatives in regard to efficacy, safety, patient acceptance, and cost in children with constipation’ (Health) |
Narrative review | Children. Age range varies between studies reviewed. | Intervention type: PEG Provider: NA Number of sessions: NA Frequency and duration: NA Equipment: NA |
Comparing PEG with other laxatives using the costs based on a 3-year-old weighing 15 kg. | Cost of medications | Funding: none. CoI: none |
Cost-of-illness | Authors report that ‘daily use of a standard dose of mineral oil, MoM, and lactulose will cost approx. 25, 28, and 45 cents daily, respectively, in the US … PEG is slightly more expensive, costing about 60 cents per day’. …’Despite slightly higher costs, the use of PEG is increasing in children in the USA and many countries because of greater efficacy and patient acceptance compared with other laxatives’ |
| Ritterband, 2013286 (USA) |
To evaluate the effectiveness of an updated web-based educational resource with a larger sample (Health and Society) |
RCT (12-month follow-up data) | Children with encopresis and constipation. Age range: 6–12 years N : 91 |
Intervention type: Internet-based intervention, which gave parents and children access to web-based educational resources (‘UCanPoopToo’ version 2) and tools designed to support ‘enhanced toilet training’. Version 2 was enhanced with additional content, online assessment and online diaries, and a game room that included web-based arcade-style games for the children to play as rewards for completing components of the programme. Provider: Internet-based |
Routine care | Pre and Post to quantify items and events that incurred costs related to encopresis. Items relevant to the present analysis include number of diapers used, number of school days missed, trips to school by parents, and clean-out procedures. The per unit cost estimates assigned to the quantified items/events were derived from health economic analysis practices recommended by Finkelstein and colleagues | Funding: CoI: Three of the authors are equity holders of BeHealth Solutions who hold the license for the software program |
Cost-of-illness | Authors reported that children receiving the intervention used fewer incontinence products, missed less school and fewer trips to school by the family. |
| Number of sessions: Number of modules = 22; Participants were instructed to complete the three main Cores of UCanPoopToo. One week following the completion of the intervention Cores, participants completed a ‘Follow-Up’ questionnaire within UCanPoopToo that, based on their answers, assigned additional Modules. This same step of completing a ‘Follow-Up’ questionnaire and assigning subsequent tailored Modules occurred for the next 2 weeks (for a total of three Follow-Ups). | |||||||||
| Frequency and duration: Weekly follow-up and asked to continue to follow guidance of their clinician Equipment: Home computer, internet access |
|||||||||
| Rogers, 2011287 (International evidence) |
To provide an overview of the management of functional or idiopathic constipation in childhood while reflecting on the NICE guidelines and discussing the keys to successful treatment | Narrative review | Children. No other details | Intervention type: Laxatives based on NICE recommendations but also refer to dietary/fluid advice and lifestyle/behavioural advice Provider: Focus is on NICE recommendations, but author also highlight the important role of nurses Number of sessions: NA Frequency and duration: NA Equipment: NA |
NA | NR. Briefly refer to findings reported in Guest 2007278 | Funding: none. CoI: none |
Cost-of-illness | Evidence-based treatment strategies may lead to service improvements and potential cost savings |
| Rogers, 2012288 (UK) |
Discuss new care model for managing idiopathic paediatric continence problems. (Health) |
Article about new care model | Children. No other details reported. | Intervention type: Nurse-led clinic Provider: Nurse Number of sessions: Initial assessment in clinic, then one face-to-face follow-up (nurse/parents/child) in clinic, then rest via telephone support Frequency and duration: NR Equipment: Telephone |
NA | NR | Funding: NR CoI: NR |
Cost-of-illness | Shorter treatment times, more children being offered toilet training programmes; DNA rate reduced to less than 1%; elimination of acute care referral for idiopathic constipation could potentially save more than £250,000 |
| Sandweiss, 2018289 (USA) |
Implemented a standardised approach to caring for patients presenting to a paediatric ED with symptoms consistent with constipation | Non-comparative study using quality improvement methodology (Plan-Do-Study-Act) cycles | Children seen in paediatric ED | Intervention type: Multidisciplinary group developed an ED constipation management pathway, encouraging less reliance on abdominal radiography for diagnosis and promoting home management over inpatient bowel cleanout. Pathway included a home management ‘gift basket’ containing over-the-counter medications and educational materials to promote successful bowel cleanout | NA | Pathway utilisation, rate of abdominal radiography, ED cost and length of stay, and ED admission rate for constipation | Funding: NR CoI: NR |
Cost-of-illness | Significant decrease in abdominal radiography usage (73.3–24.6%, p < 0.001), average per-patient cost ($637.42–538.85), length of stay (223–196 minutes, p < 0.001), and ED admission rate (15.3−5.4%, p < 0.001), with no concerning missed diagnoses or increases in ED revisit rate |
| Provider: Multidisciplinary team Number of sessions: NA Frequency and duration: NA Equipment: Gift basket with medication and educational materials |
|||||||||
| Sommers, 2015290 (USA) |
To analyse trends related to chronic constipation in the USA with respect to ED visits, patient and hospital characteristics, and associated costs. (Health) |
Secondary data analysis | All age groups with diagnosis of constipation who visited ED between 2006 and 2011 | Intervention type: NR Study focus was on number of constipation-related ED visits. Provider: NA Number of sessions: NA Frequency and duration: NA Equipment: NA |
NA | ED visits and associated costs | Funding: NR CoI: none |
Cost-of-illness | Burden of constipation for ED visits was highest in infants (<12 months); but the 1–17-year-old group reported a 50.7% increase in constipated-related usage from 2006 to 2011 |
| Southwell, 2020291 (International evidence) |
Brings together SRs and meta-analyses of electrical stimulation used to treat colonic disorders (faecal incontinence, constipation, slow transit constipation, IBS, and spina bifida- neurogenic bowel). (Health) |
Review of meta-analyses, SRs, and RCTs | Adult and children | Intervention type: SNS/sacral nerve modulation Provider: Hospital based providers – usually involve surgeon but depends on type of stimulation Number of sessions: NA Frequency and duration: NA Equipment: NA |
NA | Authors have cited 6 studies in Table 2 and highlighted the key conclusions about costs | Funding: none CoI: Lead author was employed by GI Therapies as a Consultant- clinical and scientific specialist. They also hold patents for TES and constipation. |
Cost-of-illness | Authors note the relatively high cost of SNS and cite multiple studies (see Table 2) regarding costs, lifespan, follow-up care. Most of these studies have been conducted in adults although one study is cited that is based on costs of SNS for children400 |
| Sparks, 2018292 (USA) |
To evaluate whether constipation in children with is associated with increased ED visits and inpatient admissions compared with constipation in children without ASD (Health) |
Retrospective cross-sectional study Treatment setting: ED Study duration and follow-up period: |
3–18 years olds, primary diagnosis of constipation, secondary diagnosis code of ASD N: not reported |
Intervention type: NR Study focus was on number of constipation-related ED visits. Provider: NA Number of sessions: NA Frequency and duration: NA Equipment: NA |
Children with ASD, children with a chronic condition but without ASD, and children with no chronic conditions. | Number of constipation-related ED visits based on the Nationwide ED Sample | Funding: NR CoI: none |
Cost-of-illness | More hospital charges were incurred by children with ASD and constipation compared with children without chronic conditions and constipation, suggesting that improved outpatient management of constipation in this population could result in significant cost savings. |
| Stephens, 2018293 (USA) |
Describe the demographic and clinical characteristics of children insured by Medicaid who were hospitalised for constipation. Also describe constipation-related health-care utilisation for these children in the 12 months before and after hospitalisation (Health) | Retrospective cohort study |
≤17 years of age with first in-patient visit N : 780 Approx. 54% of children hospitalised for constipation had other complex conditions |
Intervention type: NR Study focus was on number of constipation-related healthcare visits. Based on first inpatient visit for constipation in the years 2010–1. Follow-up: 24 months (12 months before hospitalisation; 12 months after) Provider: NA Number of sessions: NA Frequency and duration: NA Equipment: NA |
Compared data 12 months pre and post hospitalisation | Number of visits, spending, and prescription services. Based on ICD-9 codes |
Funding: Agency for Healthcare Research and Quality CoI: none |
Cost-of-illness | Hospitalisations for constipation were costly, with median spending for 1 hospitalisation being almost 50 times the median spending for 12 months of outpatient constipation visits. Authors also highlighted a gap in outpatient management and point to the need for improved recognition of and treatment for constipation |
| Van der Wilt223 (The Netherlands) |
To examine cost effectiveness of Sacral neuromodulation (Health) |
Economic evaluation using a Markov model based on a cohort study | Children and adolescents aged 10–18 years (mean age: 16 years) with refractory constipation based on ROME III criteria N: 30 |
Intervention type: Sacral neuromodulation. If feasible, then a permanent implantable stimulator was implanted under local anaesthesia Provider: Surgeon Number of sessions: two with follow-up at month 1, 3, 6, 12 and 24 months after implantation |
Compared with conservative treatment in children and adolescents with constipation refractory to conservative management | Compared costs and QALYs. See Suppl. File 3 for more details |
Funding: supported by a non-restrictive grant from Medtronic. CoI: none |
Cost effectiveness/cost-utility analysis | Benefits of the intervention seemed ‘to be sustained over prolonged period of time’. Preliminary evidence suggests that SNM can improve symptoms and QoL at a reasonable cost. |
| Frequency and duration: Based on adult settings which ‘could be adjusted if there were any unpleasant sensations or suboptimal treatment effects’ Equipment: sacral neuromodulation machine (Medtronic Interstim model 3889); X-ray. |
|||||||||
| Van Summeren, 2019294 (The Netherlands) |
To determine the effectiveness and cost effectiveness of physiotherapy plus conventional treatment (Health) |
RCT | Children aged 4–17 years in primary care diagnosed with functional constipation by their primary care physician | Intervention type: Conventional treatment plus physiotherapy. A structured physiotherapy programme was developed and tailored for each child. ‘Six defaecation-related goals: (1) improving knowledge about defaecation and the role of the child and/or parent in symptom persistence; | Compared with conventional treatment alone which involved education, dietary advice, toilet training, and laxative prescribing according to Dutch guidelines | Limited details reported – abstract only. Costs, ICER, cost acceptability See Suppl. File 3 for more details |
Funding: U CoI: U |
Cost effectiveness/cost-utility analysis | No objective benefit from adding physiotherapy to conventional treatment for the whole group of children with functional constipation consulting in primary care, although parents were more satisfied with physiotherapy. Authors concluded that physiotherapy as first line treatment for all children with CFC in primary care was not cost-effective compared to standard care |
| (2) improving toilet behaviour and posture; (3) increasing awareness of the sensation of needing to defaecate; (4) learning to relax while defaecating; (5) learning to generate adequate intra-abdominal pressure during defaecation; and (6) teaching effective straining during defaecation’. | |||||||||
| Provider: Specialist physiotherapists (i.e. with a master’s degree in paediatric or pelvic physiotherapy and certified after additional postgraduate training in the treatment of bladder and bowel dysfunction in children) | |||||||||
| Number of sessions: Maximum of nine sessions Frequency and duration: Up to nine 30-minute sessions. Physiotherapy was stopped earlier if treatment was successful or no improvement was expected. Equipment: NR |
|||||||||
| Wheeler, 2019295 (USA) |
To describe application of bedside ARM (Health and Society) |
Feasibility study | Children aged 6–18-year-olds N = 50 Mean age (SD): 10.1 years |
Intervention type: Bedside AR manometry. Children were treated with high dose stimulant laxatives or large volume enemas. Those with dyssynergia were referred to physical therapy for pelvic floor rehabilitation Provider: NR Number of sessions: NR Frequency and duration: NR Equipment: ARM device |
NA | Limited details reported – abstract only | Funding: NR COI: NR |
Cost-of-illness | ‘AR manometry is a feasible testing modality to guide management of pediatric patients with defaecation disorders … potentially providing time and cost savings for patients’ |
| Windell, 2020296 (UK) |
NR (Health) |
Magazine article | NR | Intervention type: NR | NR | NR | Funding: NR COI: NR |
Cost-of-illness | Cites the BIG 2019 report … condition was ‘under-reported and often poorly managed, leading to a significant cost to the NHS and having a negative impact on patients’ overall health and QoL’.’ |
Appendix 6 Barriers and facilitators to the implementation of interventions
| Domain | CFIR construct/description | Barriers Number of references Illustrative quote |
Facilitators Number of references Illustrative quote |
|---|---|---|---|
|
INTERVENTION
CHARACTERISTICS |
Intervention source: Perception of key stakeholders about whether the intervention is externally or internally developed. | 1 study320 ‘Differences in definitions and treatment out- comes could be due to the fact that some studies may be initiated by health care practitioners whereas other studies are industry-driven, and consequently have different interests. It is, therefore, important to involve all stakeholders in the development of COS in an early stage’ (p845). |
None reported |
| Evidence strength and quality: Stakeholders’ perceptions of the quality and validity of evidence supporting the belief that the intervention will have desired outcomes | 65 studies63,64,69,74,84,92,102,103,119,132,133,136,143,145,149,153,154,161,174,178,187,203,211,214,233,243,258,255,270,282,302,303,306–337 ‘As noted in the introduction, trials comparing inpatient with outpatient constipation treatments are lacking’ (p44).339. |
41 studies61,65,84,91,102,133,143,145,148–151,153,154,178,185,232,243,246,255,270,282,298,303,305,314,317,319,322,330,333,337–343 ‘These results are in accordance with other studies in which success with high doses of oral PEG was reached in 92-97% of cases’ (p130).149 |
|
| Relative advantage: Stakeholders’ perception of the advantage of implementing the intervention vs. an alternative solution. | 33 studies24,74,84,154,162,132,178,247,301,302,306,308,310,312,314,315,319,321,322,328,330,331,337,343,348,352–356 ‘Excessive fiber intake during childhood has the potential to negatively impact energy and nutrient intake by increasing fecal energy losses, reducing energy intake due to increased satiety, and decreasing bioavailability of minerals’ (Stewart 2013, p101). |
47 studies24,61,65,102,103,132,133,136,143,145,149,150,153,154,161,167,178,232,243,247,255,294,306–310,313,315,319,322,323,327,328,330,331,334,337,341,348–350,353–355,357,358 ‘This is a strong point for this new medicine, but needs more clinical trials to endorse’ (Esmaeilidooki 2016, p5). |
|
| Adaptability: The degree to which an intervention can be adapted, tailored, refined, or reinvented to meet local needs. | 19 studies24,148,151,167,178,185,247,270,301,305,310,311,314,321,325,328,334,338,344 ‘transanal irrigation is likely to fail because it is time consuming, which requires commitment and effort from both patients and parents’ (Koppen 2017, p4). |
38 studies61,65,84,92,102,132,143,145,148,149,151,154,178,204,211,214,232,270,282,303,306,314,315,317,322,324,328,331,344–350 ‘At the follow-up visit, behavioral psychologists obtained a status update from patients and parents and problem solved around the respective interventions (e.g. compliance with sit schedule; modifying rewards to increase compliance)’ (Hankinson 2018, p3). |
|
| Trialability: The ability to test the intervention on a small scale in the organisation, and to be able to reverse course (undo implementation) if warranted. | 4 studies151,185,246,322 ‘The familiarization process using the HW and the training session as in any active treatment is based on communication with the child and the child’s motivation. In addition, those differences might reflect wider real differences between groups in other parameters that can affect the results of the study’ (Eisenberg 2009, p5).185 |
7 studies84,149,151,145,187,232,243 ‘Caregivers rated the intervention as acceptable as evidenced by the high acceptability scores captured by the modified TARF-R. The high rate of attendance, low attrition, and caregiver acceptability ratings indicate that caregivers did not find the intervention, including the use of suppositories, overly invasive. Therapists delivered MIE (multidisciplinary intervention for encopresis) with 99.4% fidelity. Taken together, these data support the viability of conducting a larger scale clinical trial evaluating the effectiveness of MIE treatment’ (Lomas-Mevers, p763).145 |
|
| Complexity: Perceived difficulty of implementation, reflected by duration, scope, radicalness, disruptiveness, centrality, and intricacy and number of steps required to implement. | 10 studies24,63,148,150,151,154,243,247,306,364 ‘Our bowel management program is very involved and requires the support of the entire family. Thirty-six percent of our patients were either non-adherent or lost to follow-up’. |
16 studies63,103,149,143,178,243,294,300,306,314,323,331,337,341,351 ’Common fiber-rich food sources consumed by children include vegetables, fruits, breads/cereals, and potatoes’ (Stewart 2013, p101). |
|
| Design quality and packaging: Perceived excellence in how the intervention is bundled, presented, and assembled. | 1 study161 ‘Parents of patients who received MOM recorded that their children did not like the taste of MOM, even if it was mixed with juice’.161 |
13 studies61,65,103,153,162,302,305,313,322,333,341,347 ‘Biofeedback consisted of interactive computerized video games controlled by external sphincter activity’ (Erikson 2009, p1). |
|
| Cost: Costs of the intervention and costs associated with implementing the intervention including investment, supply and opportunity costs. | 6 studies103,143,270,298,302,365 ‘There was a failure of 62% and 70%, respectively, in adherence to treatment in the first and second moments of the study. The main reasons reported for this were lack of administration of medication because of financial difficulty (23.2% of cases) and drug adverse effects (40.2%)’ (Steiner 2014, p600). |
11 studies102,103,143,149,282,294,307,333,343,362 ‘The hospitalizations for constipation were costly, with median spending for 1 hospitalization being almost 50 times the median spending for 12 months of outpatient constipation visits. Given the large relative expense of inpatient vs. outpatient constipation treatment in children and the unknown clinical superiority of one setting vs. the other, the practice of hospitalization for childhood constipation deserves further exploration’ (Stephens 2018, p43). |
|
| OUTER SETTING | Patient Needs and Resources: The extent to which patient needs, as well as barriers and facilitators to meet those needs, are accurately known and prioritised by the organisation. | 27 studies65,69,135,161,143,246,298,300,302,303,307,310,314,315,319,320,328,333,338,342,344,347,351,359 ‘Working at Hawke’s Bay Regional Hospital’s paediatric ward, Smith was “vexed” for the children coming in for bowel clear outs. “”I don’t think the health professionals in contact with these families appreciated the stress the situation created for the kids and their families’ (O’Connor 2012, p1). |
14 studies65,69,294,303,304,310,314,319,328,344,346,362,364 ‘an increasing awareness of the importance of parental factors in the treatment of childhood FC. This awareness may affect treatment outcome and require different treatment approaches to reach satis- factory treatment goals’ (Modin 2018, p736). |
| Cosmopolitanism: The degree to which an organisation is networked with other external organisations. | None reported | 1 study346 ‘Every DHB needs to have a nurse-led bowel management service. There’s plenty of evidence of their success. Many public health nurses are running with it now. I might do myself out of a job – that would be real success’. |
|
| Peer pressure: Mimetic or competitive pressure to implement an intervention; typically, because most or other key peer or competing organisations have already implemented or are in a bid for a competitive edge. | None reported | None reported | |
| External Policy and Incentives: A broad construct that includes external strategies to spread interventions, including policy and regulations (governmental or other central entity), external mandates, recommendations and guidelines, pay-for-performance, collaboratives and public or benchmark reporting. | 17 studies24,63,65,148,149,185,314,316,319,330,331,337,350,356,359 ‘The Guideline of Pediatric Chronic Functional Constipation Treatment, which was published during November 2013 in Japan (4), recommends lubiprostone for pediatric FC. However, this laxative has never been used in pediatric medicine in Japan because there is no clinical usage experience in children. No pediatric study on lubiprostone has been reported in Japan. Moreover, few studies on lubiprostone administered for FC in children have been published worldwide’ (Fujii 2019, p1). |
5 studies308,319,333,353,357 ‘..is the most-prescribed drug by GPs, according to the present study, perhaps because of its low cost and ready availability in our public health facilities’ (Torres 2015, p77). |
|
| INNER SETTING | Structural characteristics: The social architecture, age, maturity and size of an organisation. | None reported | None reported |
| Network and communications: The nature and quality of webs of social networks and the nature and quality of formal and informal communications within an organisation. | None reported | 2 studies294,362 ‘Real-time feedback in the form of e-mail and staff meeting updates facilitated ongoing dialogue and allowed for rapid dissemination and adoption of changes in the pathway, which in turn fostered a high level of team engagement and rapid uptake of the pathway’ (Sandweiss 2018, p963). |
|
| Culture: Norms, values, and basic assumptions of a given organisation. | 9 studies24,69,148,330,347,360,362 ‘Implementation has taken two years longer than anticipated due to technical and cultural challenges of health system strengthening’ (Wolfe 2019, p1). |
None reported | |
| Implementation climate: The absorptive capacity for change, shared receptivity of involved individuals to an intervention, and the extent to which use of that intervention will be rewarded, supported, and expected within their organisation. | None reported | 8 studies65,69,149,246,282,294,347,362 ‘Although there were initial pharmacy department barriers to implementing this medication-dispensing process change, the QI team felt this management option was central to addressing the goals of the project and were able to address the pharmacy department’s concerns without any adverse events’ (Sandweiss 2018, p963). |
|
| Tension for change: The degree to which stakeholders perceive the current situation as intolerable or needing change. | None reported | 19 studies61,65,103,148–151,153,143,178,294,303,306,331,333,348,359,363,364 ‘The majority of patients that came to the clinic had previously been treated by pediatricians and pediatric gastroenterologists for constipation; yet, as reflected by patients seeking an alternative treatment, these prior medical interventions did not typically achieve lasting improvement in constipation and fecal incontinence’ (Hankinson, 2018, p7). |
|
| Compatibility: The degree of tangible fit between meaning and values attached to the intervention by involved individuals, how those align with individuals’ own norms, values and perceived risks and needs, and how the intervention fits with existing workflows and systems. | 5 studies103,151,243,246,305 ‘complex and heterogeneous group and some patients will have continued issues’ (Bonilla 2013, p4). |
11 studies65,102,149,143,243,302,303,325,328,332,359 ‘Although there were participants who dropped from the study, the participants who dropped tended to be those who never used the program rather than participants trying it and then deciding to no longer use it. This suggests that those who try UCanPoopToo, tend to continue to use it more substantively. In addition, those who use it provide positive reports and experiences in using it, including finding it useful, easy, understandable, and convenient’ (Ritterband 2013, p8). |
|
| Relative priority: Individuals’ shared perception of the importance of the implementation within the organisation. | None reported | None reported | |
| Organisational Incentives and Rewards: Extrinsic incentives such as goal-sharing awards, performance reviews, promotions, and raises in salary, and less tangible incentives such as increased stature or respect. | None reported | None reported | |
| Goals and feedback: The degree to which goals are clearly communicated, acted upon, and fed back to staff, and alignment of that feedback with goals. | None reported | None reported | |
| Learning climate: A climate in which: (1) leaders express their own fallibility and need for team members’ assistance and input; (2) team members feel that they are essential, valued, and knowledgeable partners in the change process; (3) individuals feel psychologically safe to try new methods; and (4) there is sufficient time and space for reflective thinking and evaluation. | None reported | 2 studies294,329 ‘Developing a system to allow ED nurses to dispense over-the-counter constipation medications was a major change in workflow but one that had great benefits to patients and physicians alike’ (Sandweiss 2018, p963). |
|
| Readiness for implementation: Tangible and immediate indicators of organisational commitment to its decision to implement an intervention. | None reported | 1 study307 ‘Community providers, such as GPs, are in an ideal position to be able to intervene early and monitor treatment. According to an audit of GP availability, 78% of appointments for non-urgent paediatric conditions could be offered on the same day’ (p301).305 |
|
| Leadership engagement: Commitment, involvement, and accountability of leaders and managers with the implementation. | None reported | 1 study346 ‘The service has proved very popular – ‘the paediatricians have welcomed me with open arms because these children used to take up so much of their time’ (p1).346 |
|
| Available resources: The level of resources dedicated for implementation and on-going operations, including money, training, education, physical space, and time. | 5 studies145,305,307,333,347 ‘In the middle of last year I emailed the paediatricians saying I couldn’t take any new referrals in the foreseeable future because I wanted to give the children already enrolled the best possible service’ (O’Connor 2012, p1). |
7 studies61,56,145,307,344,346,347 ‘The multidisciplinary team meets weekly and is crucial to simultaneous, timely and effective treatment of children with complex CC/FI needs’ (Athanasakos 2020, p297). |
|
| Access to knowledge and information: Ease of access to digestible information and knowledge about the intervention and how to incorporate it into work tasks. | 7 studies74,305,307,319,330,333,356 ‘Our results show that more education regarding the role of medication in the management of functional constipation is necessary; specifically, the use of medication along with behavioral interventions reducing time to remission, the necessity of disimpaction prior to maintenance therapy, and misconceptions regarding the side effects of osmotics and stimulants’ (Yang 2014, S-173). |
6 studies63,305,328,330,333,342 ‘Improved education and awareness of multimodal treatment strategies can facilitate better diagnosis and management of PFC’ (Sood 2018, p1494). |
|
| CHARACTERISTICS OF INDIVIDUALS | Knowledge and beliefs about the intervention: Individuals’ attitudes towards and value placed on the intervention as well as familiarity with facts, truths, and principles related to the intervention. | 8 studies136,307,320,342,343,360,365,367 ‘awareness of the NASPGHAN guidelines remains poor, with 84.3% of our respondents reporting that they are unfamiliar or slightly familiar with the NASPGHAN guidelines. Previous studies have found that only 6.5% to 17% of physicians surveyed are even aware of the existence of the guidelines’ Yang 2015 (p309). |
8 studies24,103,302,307,346,350,367 ‘Community practitioners (CPs) have a key role to play in encouraging people to talk about their bowel movements and to understand what is normal for them. “Tell them what a healthy stool should look like,” says Jodie. “Pointing them towards the Bristol stools chart is a good way to do this. And reassure them that ‘regular’ means different things to different people. For some, once every two days is normal and for others it’s twice a day. This way, the CP will quickly be able to identify if there are any problems and then dig deeper to identify potential solutions, such as increasing fluid intake, wholegrains, vegetables and so on.” Benjamin also feels CPs can help break down the barrier. ‘It is about raising awareness of constipation, ensuring that people don’t feel embarrassed to talk about it, and that they feel able to see a health-care professional when needed’ (Windell 2020, p32). |
| Self-efficacy: Individual belief in their own capabilities to execute courses of action to achieve implementation goals. | 14 studies149,151,154,185,270,300,305,322,323,328,331,361,365,366 ‘The low morbidity of the initial picture of constipation, lack of knowledge regarding the children’s normal pattern of evacuation by their parents, lack of an individualized nutritional plan and the prescription of unpalatable laxatives may explain poor treatment adherence’ (Traslaviña 2015, p491). |
33 studies61,69,102,136,143,151,153,161,167,178,185,203,214,243,255,282,294,302,305,306,309,318,319,322,328,333,341,345–347,349,350,360 ‘Pediatricians should seek the involvement of the patients and their families in the treatment, including them in some decisions related to the treatment, which would contribute to better adherence. Qualitative studies about constipation showed that the patient’s perception of his or her disease process is an important factor in the treatment’ (Steiner 2014, p602). |
|
| Individual stage of change: Characterisation of the phase an individual is in, as he or she progresses towards skilled, enthusiastic, and sustained use of the intervention. | 1 study303 ‘limited collaboration of the patient that restricted the possibility of relying on a proper technique procedure’ (p4).303 |
None reported | |
| Individual identification with organisation: A broad construct related to how individuals perceive the organisation, and their relationship and degree of commitment with that organisation. | 1 study350 ‘non-responders came from families with increased degree of psychosocial problems where reduced compliance of medications was suspected’ (p8).350 |
None reported | |
| Other personal attributes: A broad construct to include other personal traits such as tolerance of ambiguity, intellectual ability, motivation, values, competence, capacity and learning style. | 7 studies167,300,302,320,334,350,365 ‘Another reason can be related to barriers of individual physicians. They may not follow recommendations because of difficulties of changing habits or old routines or having a lack of motivation’ (Kuizenga-Wessel 2016, p845). |
None reported | |
| PROCESS | Planning: The degree to which a scheme or method of behaviour and tasks for implementing an intervention are developed in advance, and the quality of those schemes or methods. | None reported | None reported |
| Engaging: Attracting and involving appropriate individuals in the implementation and use of the intervention through a combined strategy of social marketing, education, role modelling, training and other similar activities. | None reported | None reported | |
| Engaging; Opinion leaders: Individuals in an organisation who have formal or informal influence on the attitudes and beliefs of their colleagues with respect to implementing the intervention. | None reported | None reported | |
| Engaging: Formally Appointed Internal Implementation Leaders: Individuals from within the organisation who have been formally appointed with responsibility for implementing an intervention as co-ordinator, project manager, team leader or other similar role. | None reported | None reported | |
| Engaging: Champions: ‘Individuals who dedicate themselves to supporting, marketing, and ‘driving through’ an (implementation)’ (101) (p. 182), overcoming indifference or resistance that the intervention may provoke in an organisation. | 3 studies322,328,365 ‘An important factor for failed treatment may be unhelpful parent behaviour’ (Santucci 2020, p22). |
28 studies61,63,92,102,136,143,145,148,150,151,154,161,185,203,232,282,294,305,311,317,333,334,346,347,350,359,363,365 ‘Given that children with depressed mothers were less likely to complete post assessment diaries, clinicians may want to exert additional effort with these families to confirm that these families engage with the program. This point is further stressed by the fact that most users who initially engaged with the program went on to use it in a substantive way. If clinicians can help their patients to the point of initial engagement, it appears that the program may be able to capture and maintain interest’ (Ritterband 2013, p10). |
|
| Engaging; External change agents: Individuals who are affiliated with an outside entity who formally influence or facilitate intervention decisions in a desirable direction. | None reported | None reported | |
| Executing: Carrying out or accomplishing the implementation according to plan. | None reported | None reported | |
| Reflecting and evaluating: Quantitative and qualitative feedback about the progress and quality of implementation accompanied with regular personal and team debriefing about progress and experience. | None reported | None reported |
Glossary
- Additional needs
- We use the term additional needs to include learning disability, physical disability or any other additional support needs.
- Adjunct
- Something added to a more important thing.
- Anal fissures
- A tear just inside the anus.
- Anal stenosis/atresia
- A narrowing of the anus.
- Anatomical
- In relation to the anatomy of the body.
- Anorectal manometry
- A test that looks at the muscles and nerves in the rectum and anus.
- Anticholinergics
- Drugs that block the action of acetylcholine. Acetylcholine is a neurotransmitter, or a chemical messenger. It transfers signals between certain cells to affect how your body functions.
- Botox
- A drug made from botulinum toxin.
- Child
- We use the term child for any person under the age of 18 years.
- Clinical guideline
- A systematically developed statement for practitioners and participants about appropriate health care for specific clinical circumstances.
- Complementary interventions
- A wide range of treatments for medical conditions that people use instead of or in addition to ordinary medicine.
- Disimpaction
- Disimpaction is the treatment to give relief from bad constipation, by using medicines or by a method to remove the stool/faeces.
- Effectiveness
- How well a treatment works.
- Electrolytes
- A substance, usually a liquid, that electricity can go through, or that separates into its parts when electricity goes through it.
- Empirical
- Based on what is experienced or seen (through research).
- Endocrine
- Relating to any of the organs of the body that make hormones (= chemicals that make the body grow and develop) and put them into the blood, or to the hormones that they make.
- Evidence synthesis
- The development of techniques to combine multiple sources of quantitative and qualitative data to derive best evidence for use in health care.
- Faecal impaction
- A large mass of solid waste that gets stuck.
- Faecal microbiota transplantation
- A stool transplant from a healthy donor.
- Gastroenterology
- The branch of medicine concerned with diseases of the digestive system.
- Grey literature
- Grey literature is material that is less formal than an article in a peer-review journal or a chapter in a book – so it is not easily tracked down.
- Haemorrhoids
- The veins of the anus become swollen, painful and sometimes bleed.
- Hepatology
- The area of medicine concerned with the liver, gallbladder, biliary tree and pancreas.
- Hirschsprung’s disease
- It is a rare condition that causes faeces to become stuck in the bowels.
- Histological
- Relating to the study of the structure of cells and tissue.
- Intervention
- Treatment used by health professionals.
- Irrigation
- Washing of a body cavity with a gentle stream of water.
- Knowledge translation
- Involves using high-quality knowledge in the process of decision-making.
- Laxatives
- A substance that makes it easier for the waste from the bowels to come out.
- Manual evacuation
- Manual evacuation of faeces.
- Markov model
- An analytical framework that is frequently used in decision analysis.
- Megarectum
- A large rectum (last section of the large bowel).
- Meta-analysis
- Combining data from multiple independent studies. May be undertaken in evidence syntheses.
- Metabolic
- Relating to metabolism (the chemical processes within the body required for life).
- Muscular dystrophy
- A disease in which a person’s muscles gradually become weaker.
- Narrative review
- A thorough and critical evaluation of previous research on a topic. The review summarises a particular area of research.
- Non-pharmacological
- Not relating to treatment that uses drugs.
- Opiate
- A drug that contains opium.
- Overview (study design)
- Use of explicit and systematic methods to search for and identify multiple systematic reviews on related research questions in the same topic area for the purpose of extracting and analysing their results across important outcomes.
- Paediatric
- Medical care of children.
- Pharmacological
- Treatment that uses drugs.
- Physiological
- Relating to the way in which the body works.
- Polyethylene glycol
- A drug to treat constipation.
- Primary study
- Experimental studies generating new data.
- Probiotics
- A food or pill that contains good bacteria.
- Prognosis
- A judgement of the likely/expected development of disease or chance of getting better.
- Psychological
- Relating to the human mind or feelings.
- Radiological
- Relating to radiology (a medical specialty using radiation for diagnosis or treatment).
- Rectal biopsy
- A small piece of tissue is taken from the lining of the rectum.
- Rectal prolapse
- Where the rectum (last section of the large bowel) has moved down out of its usual position.
- ROME criteria
- Criteria used to diagnose gastroenterology disorders.
- Sacral nerve stimulation/neuromodulation
- Use of low-voltage electricity (via an electrode implanted in the lower back) to make nerves work better.
- Secondary data analysis
- Secondary data analysis refers to the analysis of existing data collected by others.
- Scoping review
- Exploratory projects that systematically map the literature available on a topic, identifying key concepts, theories, sources of evidence and gaps in the research.
- Systematic review
- A review of a clearly formulated question that uses systematic and explicit methods to identify, select and critically appraise relevant research, and to collect and analyse data from the studies that are included in the review. Statistical methods (meta-analyses) may or may not be used to analyse and summarise the results of the included studies.
- Taxonomy
- A system for naming and organising things that share similar qualities.
- Tertiary
- Tertiary care refers to highly specialised treatment.
- Transcutaneous electrical stimulation
- A method of pain relief involving the use of a mild electrical current.
- Visual analytic software
- Software to analyse data and give a visual representation (picture).
List of abbreviations
- ACE
- antegrade continence enema
- AMED
- allied and complementary medicine database
- ASD
- autistic spectrum disorder
- ASN
- additional support needs
- BID
- bis in die; twice a day
- BIG
- bowel interest group
- CASP
- critical appraisals skills programme
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CFC
- chronic functional constipation
- CFIR
- consolidated framework for implementation research
- CHEC
- consensus health economic criteria
- CI
- confidence interval
- CINAHL
- cumulative index to nursing and allied health literature
- CP
- cerebral palsy
- CTM
- connective tissue manipulation
- DARE
- database of abstracts of reviews of effects
- ED
- emergency departments
- EMBASE
- excerpta medica database
- EMG
- electromyography (measures muscle response to stimulation)
- EPPI
- Evidence for Policy and Practice
- EQ
- EuroQol
- ERIC
- The Children’s Bowel and Bladder Charity
- ESPGHAN
- European Society for Paediatric Gastroenterology Hepatology and Nutrition
- GCU
- Glasgow Caledonian University
- GP
- general practitioner
- GRADE
- grading of recommendations, assessment, development and evaluations
- GRIPP
- Guidance for Reporting Involvement of Patients and the Public
- HTA
- Health Technology Assessment
- HV
- health visitor
- IBS
- irritable bowel syndrome
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- ICTRP
- International Clinical Trials Registry Platform
- JBI
- Joanna Briggs Institute
- KT
- kinesio taping
- MACE
- Malone antegrade continence enema
- MCA
- Medicines Control Agency
- MD
- mean difference
- MeSH
- Medical Subject Headings
- MEDLINE
- medical literature analysis and retrieval system online
- MHRA
- Medicines and Healthcare Products Regulatory Agency
- MRC
- Medical Research Council
- NASPGHAN
- North American Society for Pediatric Gastroenterology, Hepatology and Nutrition
- NDT
- neurodevelopmental training
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NMA
- network meta-analysis
- NMAHP RU
- Nursing, Midwifery and Allied Health Professions Research Unit
- PEG3350
- polyethylene glycol (drug to treat constipation)
- PFMT
- pelvic floor muscle training
- PI
- principal investigator
- PPI
- patient and public involvement
- PRESS
- peer review of electronic search strategies
- PRISMA-E
- preferred reporting items for systematic reviews and meta-analysis equity-focused extension
- PRISMA-ScR
- preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews
- PROSPERO
- The International Prospective Register of Systematic Reviews
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- ROB
- risk of bias
- ROBINS-I
- risk of bias in non-randomised studies of interventions
- ROBIS
- risk of bias assessment tool for systematic reviews
- RQ
- research questions
- RR
- relative risk
- SD
- standard deviation
- SG
- stakeholder group
- SMD
- standardised mean difference
- SN
- school nurse
- SNM
- sacral neuromodulation
- SNS
- sacral nerve stimulation
- SR
- systematic review
- SUCCESS
- Strategies Used for Constipation in Children – Evidence Synthesis Involving Stakeholders
- TAI
- transanal irrigation
- TES
- transcutaneous electrical stimulation
- TIDieR
- template for intervention description and replication
- WEIRD
- Ways of Evaluating Important and Relevant Data
- WHO
- World Health Organisation
- WMD
- weighted mean difference
- XEBT
- Xiao’er Biantong
Notes
-
Evidence of effectiveness: extended narrative synthesis, Level 3
-
Complementarity between evidence syntheses and guideline recommendations
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/PLTR9622).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.