Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/35/27. The contractual start date was in November 2013. The draft report began editorial review in April 2021 and was accepted for publication in February 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Goldberg et al. This work was produced by Goldberg et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Goldberg et al.
Chapter 1 Introduction
Background
Ankle osteoarthritis is a condition in which the cartilage lining the ankle joint has worn away. The cartilage acts as a shock absorber and allows smooth, gliding motion. Absence of the cartilage and the resultant bone spurs that form (bony projections or osteophytes), and calcification and scarring of the capsule, lead to progressive pain and stiffness.
More than 29,000 patients in the UK present to specialists each year with symptomatic ankle osteoarthritis, a condition that causes major disability and has a similar impact on quality of life (QoL) as end-stage cardiac failure1 and end-stage hip arthritis. 2 The current demand incidence of ankle osteoarthritis has been estimated to be 47.7 per 100,000 per year. 3
The most common aetiological factor in the development of osteoarthritis of the ankle is previous trauma, often following fractures or severe sprains of the ankle. 4 The incidence of both of these is rising; hence, post-traumatic osteoarthritis of the ankle is likely to become an increasing health burden. Indeed, ankle sprains are one of the most common reasons for attendance at emergency departments. Other causes of ankle osteoarthritis include long-standing inflammatory arthropathies (e.g. rheumatoid arthritis, haemochromatosis and haemophiliac arthropathy).
In the early stages of disease, non-operative measures such as a change in activity levels, weight loss, physiotherapy, painkillers and ankle braces should be used. When these conservative management measures have failed for at least 6 months, and providing the surgeon confirms the diagnosis of osteoarthritis (now termed ‘end-stage osteoarthritis’) on the basis of radiological and clinical evidence (i.e. plain radiographs and unrelenting symptoms, respectively), surgery might then be considered.
Although ankle fusion is the most common surgical treatment for end-stage ankle osteoarthritis, surgeons are increasingly performing total ankle replacement (TAR), also known as arthroplasty, in response to patient demand. TAR started in the 1970s, with initial poor results. However, over the last 50 years, several new generations of implants have been developed with far improved results and its use is increasing globally. At least 4000 patients are treated with ankle fusion or TAR each year in the NHS. 5 Every TAR implanted in England, Wales, Northern Ireland, the Isle of Man and Guernsey is captured on the National Joint Registry, which has revision surgery as its only end point. No comprehensive outcome data are captured for ankle fusion patients. All studies comparing TAR with ankle fusion to date are observational and, to the best of our knowledge, there are no prospective randomised trials.
Many studies have shown that ankle fusion provides good short- and medium-term results. However, in the long term, it poses major risk (> 80%) of the development of adjacent joint arthritis owing to the transfer of stresses and motion to other joints. 6,7 Other complications following ankle fusion include pain, dysfunction, non-union and malalignment. 8
On the other hand, TAR can preserve the functional range of ankle motion, relieve pain and might avoid potential osteoarthritis in the adjacent joints. However, it may also result in revision surgery for aseptic loosening, intraoperative fracture, malalignment, impingement and heterotopic ossification. 9–11
To the best of our knowledge, there is no high-quality study comparing the two procedures, and the literature on this subject does not provide conclusive differentiation of the treatments, with varying length of follow-up, sample size and types of technique and implants. 12–19 The studies use a wide range of patient-reported outcome measures, without consistency of reporting or statistical analysis. Many of them have missing data, which makes the interpretation and comparison of results from individual studies next to impossible.
More recently, Daniels et al. 20 looked at 281 TARs and 107 ankle fusions and found comparable outcome scores between the two surgeries at a mean follow-up of 5.5 years. In their study, which was not randomised, patients who received ankle fusion were younger, more likely to be diabetic, less likely to have inflammatory arthritis and more likely to be smokers than those who received TAR. Veljkovic et al. 21 analysed 88 TARs and 150 ankle fusions at a follow-up of 3.6 years and found comparable clinical outcomes between ankle fusion and TAR in patients with non-deformed end-stage ankle arthritis.
In the National Joint Registry, which covers England, Wales, Northern Ireland, the Isle of Man and Guernsey, the most commonly used ankle replacement implants drastically changed between 2014 and 2019. 22 Prior to 2014, the majority of implants used in the UK were mobile bearing. In 2014, the Mobility™ Total Ankle System (DePuy Synthes Companies, Raynham, MA, USA) was withdrawn from the market. By 2019, the majority of implants used in the UK were fixed bearing. In 2019, the most commonly used implant was the Infinity™ Total Ankle System (Stryker, MI, USA) with the STAR™ (DJO, LLC, Vista, CA, USA) and Box® Total Ankle Replacement (MatOrtho Limited, Leatherhead, UK) implants the second and third most popular, respectively. 22 With regard to ankle fusion, there were a heterogeneity of techniques used to perform the ankle fusion, including arthroscopic and open techniques.
Esparragoza et al. 17 conducted a 2-year follow-up study of 30 patients [ankle fusion, n = 16; TAR with Ankle Evolutive System (AES) prosthesis (Transystème JMT Implants SA, Nîmes, France), n = 14], comparing their QoL before and after the procedure. They showed that the third-generation TAR provided greater improvement in QoL (physical conditions, and perception of general health and QoL) at 2 years post surgery. 17 On the other hand, Krause et al. ,18 in their 3 year-follow up study of 161 patients [ankle fusion, n = 27; TAR with Agility™ (DePuy Synthes Companies), HINTEGRA® (DT MedTech, LLC, TN, USA), STAR or Mobility Total Ankle System implants, n = 114], found no significant difference in the mean improvement between the two groups, although the rate of complication was significantly higher after TAR than after ankle fusion. In another short-term follow-up study, Slobogean et al. 16 assessed QoL 1 year after TAR or ankle fusion in 107 patients and demonstrated that preference-based QoL was improved following TAR and ankle fusion, but the improvement was not significantly different between the two procedures. 16
Two systematic reviews comparing outcomes from TAR with ankle fusion, using second-generation prostheses13 or third-generation three-component meniscal-bearing prostheses,12 showed no significant differences in short-, mid- or long-term outcomes between the two treatments. Haddad et al. 13 reported that ankle fusion resulted in a higher risk of lower limb amputation, although they did not include any studies that directly compared TAR with ankle fusion. A systematic review and meta-analysis of 7942 modern TARs by Zaidi et al. 23 reported that TAR has a positive impact on patients’ lives, with benefits lasting 10 years, as judged by improvement in pain and function, and improved gait and increased range of movement. Zaidi et al. 23 reported an overall survivorship at 10 years of 89%, with an annual failure rate of 1.2% [95% confidence interval (CI) 0.7% to 1.6%]. The same authors reported improvements in clinical scores, although the scores used were heterogeneous and without consistency. Radiolucency was identified in up to 23% of TARs after a mean of 4.4 years (95% CI 2.3 to 9.6 years).
Gougouilas et al. 15 also performed a systematic review of the outcome of seven TAR implants that are currently in use [Agility, STAR, Buechel-Pappas™ (Endotec, Inc., Orlando, FL, USA), HINTEGRA, Salto Talaris® Total Ankle Prosthesis (Integra LifeSciences Corporation, Boston, MA, USA), TNK (Kyocera Corporation, Kyoto, Japan) and Mobility implants] and showed that most patients experienced significant improvement, as assessed by the clinical score. In contrast to Zaidi et al. ,23 Gougouilas et al. 15 suggested that the postoperative improvement in the range of ankle motion was relatively small (0–14°). A decision analysis using a Markov model showed that TAR was a better treatment than ankle fusion, as assessed by the quality well-being index score. 19 These systematic reviews have exposed significant bias and a lack of prospective controlled data for either procedure.
A cost-effectiveness evaluation conducted in the USA concluded that TAR has the potential to be a cost-effective alternative to ankle fusion, but reaffirmed the poor quality of the supporting evidence. 24 To the best of our knowledge, there have been no level 1 RCTs to inform this important subject.
Objectives
The total ankle replacement versus ankle arthrodesis (TARVA) trial was a pragmatic, multicentre, parallel-group, non-blinded randomised controlled trial (RCT) that compared the two existing NHS treatment options: TAR and ankle fusion. The trial compared any current TAR implant with any isolated tibiotalar ankle fusion procedure. As a pragmatic trial should reflect the real-world situation, procedures varied in terms of technique owing to the specific requirements of each case and the preference of the operating surgeon. Thus, no surgical technique or type of ankle fusion was specified, although details were captured. Surgeons performing TAR were free to adopt their usual technique within each treatment arm, allowing the results of the trial to be extrapolated across the NHS. All surgeons included in this trial used implants and prostheses commonly used in the NHS only.
The trial assessed the comparative efficacy of the two main surgical treatments for end-stage ankle osteoarthritis: TAR and ankle fusion. It investigated the clinical effectiveness and complication rates of the two procedures in patients aged 50–85 years, measured through self-reported pain-free function using a standardised questionnaire of walking and standing ability at 52 weeks after the surgical intervention. It also aimed to determine whether or not there was a difference in physical function [measured using the Foot and Ankle Ability Measure – Activities of Daily Living (FAAM-ADL)], QoL [measured using the EuroQol 5-Dimensions, five-level version (EQ-5D-5L)] and range of motion (ROM) at 26 and 52 weeks post surgery. Last, we investigated the cost-effectiveness and cost–utility of TAR and ankle fusion. The adoption of a pragmatic trial design with broad entry criteria for the comparison of the two topical therapies means that the results can be generalised to the large number of patients presenting with ankle osteoarthritis who are treated each year.
Chapter 2 Methods
Parts of this chapter are reproduced from the TARVA trial protocol (Goldberg et al. 25). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter are also reproduced from the TARVA trial statistical analysis plan (SAP) (Muller et al. 26). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Design
The TARVA trial was a randomised, multicentre, non-blinded, prospective, parallel-group trial of TAR versus ankle fusion in patients with end-stage ankle osteoarthritis who were aged between 50 and 85 years, comparing clinical outcomes (i.e. pain-free function, QoL, ROM and rate of postoperative complications) and cost-effectiveness.
The trial incorporated an internal feasibility phase to ensure the surgeons’ willingness to randomise and the patients’ willingness to be randomised. The feasibility phase involved four centres and took place over a 6-month period following randomisation of the first patient (24 cumulative months across all centres) to closely monitor eligibility, consent and randomisation rates and ensure that they were adequate. In addition, this provided 5 months’ information on whether or not patients accepted their randomised surgery, and whether or not the surgery took place.
The final protocol has been published previously. 25 All trial analyses were performed in accordance with a predefined SAP. 26
Ethics
London Bloomsbury Research Ethics Committee (REC) reviewed and approved (14/LO/0807) the trial protocol and all material given to prospective participants, including the informed consent forms (ICFs). Subsequent amendments to these documents were submitted for further approval. Before initiation of the trial at each additional clinical site, the same/amended documents were reviewed and approved by local Research and Development.
Patient and public involvement
Patients and the public were involved at all stages of the trial, from the development of the research questions and protocol to the running of the trial. This was important to ensure the salience of the research question and that the methods proposed were acceptable to potential participants, including the frequency of visits and relevance of outcome measures. One patient representative was part of the TARVA Trial Management Group, and one patient and public representative sat on the Trial Steering Committee. We will involve patient organisations and charities such as Versus Arthritis (Chesterfield, UK) and the Arthritis and Musculoskeletal Alliance (London, UK) in the dissemination of the findings to a wider audience, both professionals and patients, through their newsletters, at their annual members’ meetings and on their websites.
Setting
The trial was carried out in 17 UK hospitals, in a mixture of district general hospitals, university teaching hospitals and specialist orthopaedic hospitals (including their adjoining private hospitals) with adequate facilities to carry out the surgical procedures and trial assessments (see Acknowledgements for a list of participating sites).
Participants
The eligibility criteria for participation were patients with end-stage ankle osteoarthritis, aged 50–85 years, who the surgeon believed to be suitable for both TAR and ankle fusion (having considered various patient factors including deformity, stability, bone quality, soft tissue envelope and neurovascular status). The patients had to be able to read and understand the patient information sheet (PIS) and provide written informed consent. Eligible patients were randomised to a surgery type. ‘End-stage’ osteoarthritis is defined as a combination of severe unrelenting symptoms sufficient to make the patient consider surgical intervention, radiological changes consistent with osteoarthritis and failure of at least 6 months of non-operative measures, necessitating a definitive surgical procedure.
Exclusion criteria included patients with previous ipsilateral talonavicular, subtalar or calcaneocuboid fusion or surgery planned within 1 year of index procedure; those with more than four lower-limb joints fused; and those who were unable to undergo magnetic resonance imaging (MRI) or computerised tomography (CT). Those with a history of local bone or joint infection and those who had severe osteoporosis (T-score of < –2.5) with recent fracture (< 12 months previously) were not included in the trial. Patients with any comorbidity that, in the opinion of the investigator, was severe enough to interfere with the patient’s ability to complete the trial assessments or present an unacceptable risk to the patient’s safety were also excluded from the trial.
Interventions
In the UK, two broad types of prostheses are currently used in TAR: a two-component fixed-bearing prosthesis and a three-component mobile-bearing prosthesis. As both are commonly used, no restriction on the type of prosthesis used was stipulated, although data on prosthesis type were captured. The surgical technique followed the standard operative procedure, which involved an anterior approach to the ankle joint, protection of the neurovascular bundle, and talar and tibial preparation according to the prosthesis used and its instrumentation. Intraoperative fluoroscopy was used as required to confirm position, and final implantation used an uncemented technique. Thorough washout was followed by wound closure using the surgeon’s standard technique. Details of the surgeon’s technique were captured on a case report form (CRF). The surgeon’s usual postoperative protocol was followed with respect to method of immobilisation (plaster or walking boot) and weight-bearing status.
Ankle fusion was performed either as an open procedure or arthroscopically, depending on the surgeon’s preference. Tibial and talar joint surfaces were prepared to avoid bleeding from the cancellous bone, any deformity correction was addressed, and the surfaces were opposed and held with screws and/or plates as required to ensure that the foot was plantigrade and appropriately positioned to match the contralateral ankle in axial orientation. If performed arthroscopically, two portals were made, one anteromedially and one anterolaterally, over the ankle joint for access. If arthroscopic access was not favourable, the operation was performed using an open procedure, which involved either a standard anterior approach, two mini anterior incisions or a lateral approach. The surgical technique and implants used were captured on the CRF. The surgeon’s usual postoperative protocol was followed with respect to use of plaster or walking boot and weight-bearing status, and the specific details of these were captured for each patient on the CRF.
Magnetic resonance imaging
Each participant was booked to undergo MRI of the affected ankle, if this had not already been performed as part of routine care, once they had given written informed consent to take part in the trial. If the participant was ineligible for MRI, CT was booked instead. The grade of MRI/CT was determined by an independent radiologist using a methodology published by our group,27 the report of which was sent to the local principal investigator, and a preoperative assessment appointment was scheduled for the participant.
Randomisation
The randomisation process was based on a minimisation algorithm. The algorithm gave an overall chance of 85% of allocating the patient to the treatment arm that was under-represented with respect to three stratifying variables: surgeon, presence of osteoarthritis in subtalar joint and presence of osteoarthritis in talonavicular joint (as determined by preoperative MRI). The research nurse or delegated individual logged on to the Sealed Envelope randomisation service and provided patient information (including information on minimisation variables), and the surgical treatment to be received was supplied immediately. Patients were allocated in a 1 : 1 ratio to the TAR and ankle fusion arms. To protect against allocation bias, the person recruiting the patient to the trial was not aware of the allocation to be assigned prior to contacting the randomisation service. All surgeons were proficient in both surgical procedures, having independently performed ≥ 10 procedures of each type prior to participation.
Blinding
The trial was open (i.e. non-blinded). It was not possible to blind patients, surgeons, radiologists and clinical assessors for the following reasons:
-
Surgeons would have known which procedure they were performing.
-
Radiologists and patients would be able to identify which procedure had taken place from the radiographs.
-
Patients who received ankle fusion would tend to have stiffer ankles and the incisions may also provide clues to the surgery type.
Recruitment and consent
All patients with ankle osteoarthritis who were considering surgery were screened prospectively by principal investigators at 17 UK hospitals. Potentially eligible participants were identified during routine clinic appointments to assess treatment need, or through screening of referral letters/clinic lists. Those identified through screening were sent a study information pack in the post prior to their appointment.
If considered eligible for the TARVA trial, the patient watched a bespoke trial video, and read the PIS and a generic factsheet about ankle arthritis and its treatment options. Participants either consented at that stage or received a follow-up telephone call from the research team to discuss participation. Reasons for non-enrolment in the trial (including lack of equipoise) were recorded. All participants provided written informed consent using an ICF.
Following consent, participants underwent MRI (or, where contraindicated, CT) (if this had not been performed as part of standard care within the previous 6 months), followed by a preoperative assessment 14–30 days prior to surgery. If declared fit for surgery, participants were randomised to one of the two surgical treatments. Participants who were found to be unsuitable for surgery at the preoperative assessment appointment were passed back to their general practitioner (GP) to be re-referred for surgery when they were considered fit.
Baseline visit
Baseline measures were recorded at the point of randomisation, once the participant had been found to be fit for surgery, at their preoperative assessment. Baseline measures included the EQ-5D-5L, MOXFQ, Foot and Ankle Ability Measure (FAAM), Client Service Receipt Inventory (CSRI), ROM and concomitant medication.
Follow-up assessments and treatment
All participants attended routine follow-up, which consisted of visits at 2, 6, 12, 26 and 52 weeks post surgery. These visits were standard care. Patients underwent routine clinical review at 2 weeks, during which the stitches were removed and plaster casts changed. Trial-specific outcome measures, including adverse events (AEs) and postprocedural complications, were recorded at 6, 12, 26 and 52 weeks. Concomitant medications were recorded from preoperative assessment to the 52-week visit. Participants underwent routine physical examination, as per standard care. Participants completed additional questionnaires (the MOXFQ, EQ-5D-5L and FAAM) at 26- and 52-week routine follow-up visits, with the EQ-5D-5L and CSRI additionally completed at 12 weeks. ROM (total floor to tibial shaft plantarflexion and dorsiflexion) was assessed using a goniometer at the preoperative assessment visit and 52 weeks post surgery. 28
To avoid bias, operating surgeons were not involved in measuring ROM. Preoperative and postoperative hindfoot deformity was measured using weight-bearing anteroposterior and lateral radiographs of the ankle and tibia at baseline, and on a postoperative radiograph (between 0 and 26 weeks post surgery) using the methods described by Knupp et al. 29 Plain radiographs were sent to the Royal National Orthopaedic Hospital via the NHS Image Exchange Portal (Sectra Ltd, Stevenage, UK) in one batch (containing preoperative and postoperative radiographs) after the second (postoperative) radiograph was taken. Investigators were blinded to participant treatment allocation when reviewing the preoperative radiographs. Each participant was in the trial from consent until the final 52-week follow-up visit, although long-term follow-up at 2, 5 and 10 years post surgery was part of their informed consent.
Safety
All medical device deficiencies, AEs and serious adverse events (SAEs) occurring during the trial that were observed by the investigator or reported by the participant (whether or not they were attributed to the surgery, surgery-related medications, device or other trial-specific procedures) were recorded in the participants’ medical records. Related AEs over and above what would normally be expected after ankle surgery were recorded on the relevant CRFs. SAEs were reported in line with procedures set out in the protocol. 25
The severity of all AEs (serious and non-serious) was graded using the TARVA trial safety management plan for expected AEs, in conjunction with the most recent version of the Common Terminology Criteria for Adverse Events (at the time the protocol was written, this was version 4.030) for other (unexpected) AEs. The ‘expectedness’ was determined by the list of expected events in the TARVA trial safety management plan.
Outcomes
Primary outcome
The primary outcome measure was the absolute difference between the two treatment arms in self-reported pain-free function, as measured by the Manchester–Oxford Foot Questionnaire (MOXFQ) walking/standing domain score at 52 weeks post surgery (0–100, where lower scores are better). 31 The 52-week score was used if it was taken in the window from 48 to 56 weeks post surgery.
The MOXFQ walking/standing domain score has been found to be a valid and responsive measure to evaluate all types of foot and ankle surgery and it has also been shown to be more responsive for the outcomes of foot and ankle surgery patients than generic QoL measures such as the EQ-5D-5L quality-of-life instrument. 32 The MOXFQ walking/standing domain score was selected by patients as the most important outcome measure. 32
Secondary outcomes
The secondary outcome measures for the trial were the absolute differences between the two treatment arms in:
-
the MOXFQ walking/standing domain score at 26 weeks post surgery
-
self-reported pain and social interaction, measured using the MOXFQ pain and social interaction domain scores at 26 weeks and 52 weeks post surgery
-
physical function, measured using the FAAM-ADL questionnaire at 26 weeks and 52 weeks post surgery (0–100, higher scores better)
-
physical function for patients involved in sport, measured using the FAAM sport subscale score at 26 weeks and 52 weeks post surgery
-
QoL, assessed using the EQ-5D-5L [EQ-5D-5L index value and EQ-5D-5L visual analogue scale (VAS)] at 26 weeks and 52 weeks post surgery
-
total ROM (degrees plantarflexion and dorsiflexion) at 52 weeks post surgery, assessed using a goniometer
-
the proportion of patients experiencing at least one AE
-
the proportion of patients experiencing at least one SAE
-
the proportion of patients with recorded complications (including revision surgery and reoperations other than revision).
Additional outcomes were also collected for a detailed cost and cost-effectiveness analysis of TAR compared with ankle fusion.
The Manchester–Oxford Foot Questionnaire
Responses to each MOXFQ questionnaire item consist of a five-point Likert scale ranging from no limitation (scoring 0) to maximum limitation (scoring 4). Items are grouped into three domains: walking/standing (seven items), pain (five items), and social interaction (four items). Domain scores are computed by summing the patient’s responses to each item within the domain and converting to a 0–100 metric, where higher scores represent greater severity.
If a single item within any domain is unanswered, it will be imputed with the mean of the respondent’s answers to the other items within that domain. If two or more questions on any domain are unanswered, the overall score for that domain will not be calculated and its value will be set to missing. 33 If the entire questionnaire has not been completed, all MOXFQ domain scores for that visit will be set to missing.
The Foot and Ankle Ability Measure – Activities of Daily Living
Each of the 21 items on the FAAM-ADL is scored from 4 (no difficulty) to 0 (difficulty). 34 The overall FAAM-ADL score is then calculated by summing the responses to each completed item, dividing this by the maximum score achievable based on the number of items completed (e.g. 84 if all 21 items are completed), and then multiplying the resulting fraction by 100 to return a 0–100 metric, where higher scores indicate a higher level of physical function. If an answer for one item is missing, its value will be imputed as the mode of the other items; if more than one item is missing, the overall score will be set to missing.
The FAAM sport subscale score provides a complementary, specific assessment of ability to participate in sports based on eight questionnaire items, each also scored from 0–4. A 0–100 metric is then generated using the same approach as for the FAAM-ADL; higher scores indicate a higher level of ability to participate in sports. Missing items will be handled using the same approach as for the FAAM-ADL.
EuroQol-5 Dimensions, five-level version, quality-of-life instrument
The EQ-5D-5L is a generic measure of health-related quality of life (HRQoL) developed by the EuroQol group in 2009. It was introduced to improve on the sensitivity of its predecessor, the EuroQol 5-Dimensions, three-level version (EQ-5D-3L). It is a five-dimension, five-level questionnaire scored 1 (no problem) to 5 (extreme problem). The dimensions are mobility, self-care, usual activities, pain/discomfort and anxiety/depression. It also includes a VAS scored from 0 (worst imaginable health) to 100 (best imaginable health). The EQ-5D-5L was translated into > 130 languages and is available in various modes of administration. 35
The EQ-5D-5L is a descriptive system that defines a unique health state by combining one level from each of the five dimensions. 36 The descriptive system can be converted into a single index value using a value set. 37 The value set was derived from a study that elicited preferences from the general population (n = 3395). 37 The index value can take values from 0 (death) to 1 (perfect health). Currently, a value set is available for the EQ-5D-3L, which was derived directly from the population responses. For the EQ-5D-5L, the National Institute for Health and Care Excellence (NICE) recommends using a ‘crosswalk’ calculator,38 which is a link function that allows researchers to obtain index values using value sets for the EQ-5D-3L. The index values are also used in the calculation of quality-adjusted life-years (QALYs) in the economic evaluation of health interventions.
Another generic measure of HRQoL is the Short Form questionnaire-36 items (SF-36). The SF-36 is a standardised questionnaire comprising 36 items across eight domains. The domains of the SF-36 are physical functioning (10 items), physical role limitations (four items), bodily pain (two items), general health perceptions (five items), energy/vitality (four items), social functioning (two items), emotional role limitations (three items) and mental health (five items). The last item is called ‘self-report health transition’; it is answered by the respondent, but is not included in the scoring system. The SF-36 has a scoring algorithm that generates a score for each of the eight domains and two summary scores (a physical component summary and mental health component summary), but it is not preference based. A study was conducted to create a preference-based measure from the SF-36, which is called the Short Form questionnaire-6 Dimensions (SF-6D). 39 A value set was created by interviewing a representative sample of 611 members of the UK population. There is also a short version of the questionnaire, called the Short Form questionnaire-12 items (SF-12). It is often preferred for routine follow-up. 40
Both measures are widely used in joint replacement registries. 41 The EQ-5D-5L42,43 and SF-3644 were validated to use in patients with osteoarthritis. There are no recommendations as to which one is preferred. 40 There is a mapping function available to convert the SF-12 to EQ-5D-5L index values, which facilitates comparison between the two measures. 45
If any dimension score is missing, the EQ-5D-5L index value will be set to missing. If the entirety of one component of the questionnaire (dimension score or VAS) has not been completed, the associated component score will be set to missing. If the entire questionnaire has not been completed, both the EQ-5D-5L index value and EQ-5D-5L VAS at that visit will be set to missing.
Sample size
The sample size calculation for the primary outcome (change in MOXFQ walking/standing domain score by 52 weeks post surgery) was performed using Stata/IC®, version 12.1 (StataCorp LP, College Station, TX, USA). It was based on achieving 90% power to detect the minimal important difference (MID) in the primary outcome at the 5% level of significance, accounting for expected loss to follow-up.
Dawson et al. 46 previously defined the MID in the MOXFQ when evaluating outcomes following surgery for hallux valgus as the mean change in MOXFQ score of those patients who reported feeling at least ‘slightly better’. They found the MID to be 16, 12 and 24 for the walking/standing, pain and social interaction domains, respectively. 46
A later paper by Dawson et al. 47 discussed the minimal detectable change, which is the smallest change for an individual that is beyond the measurement of error of a given instrument and therefore likely to represent a true change. Although Dawson et al. ’s 2007 paper46 looked at hallux valgus, their later paper47 specifically studied ankle procedures as a subgroup and estimated the MID to be 10.67.
For this trial, we determined that it was important to detect a difference of 12 in the change in the MOXFQ walking/standing domain scores from baseline between the two treatment arms.
The standard deviation (SD) of the MOXFQ walking/standing domain score was estimated to be 27. 46 We took into account an anticipated 10% dropout rate (attrition in orthopaedic trials is about 5–7%, as shown by other similar UK RCTs48). Based on these quantities, the required sample size was estimated to be 118 patients per arm.
However, the trial was multicentre and the outcome was assumed to vary by surgeon, so the sample size was increased to account for clustering by surgeon. The intraclass correlation coefficient (ICC) was estimated from the median of 10 previous surgical studies reporting patient-reported disease-specific measures 12 months post surgery,49 and the initial sample size estimate was inflated by a factor of f = 1 + (m – 1) × ICC. Assuming an average cluster size (m) of 14 (patients per surgeon) and an ICC of 0.03, an inflation factor of f = 1.39 was estimated, leading to a final required sample size of 164 patients per arm or 328 patients in total.
Data collection and management
A member of the research team captured data from patients on paper using the TARVA trial CRFs. The data were entered onto the main trial database (MACRO v4.1; Elsevier, Amsterdam, the Netherlands) by a delegated member of site staff.
The site retained the original paper copies of patient CRFs to allow monitoring and audit by the University College London Comprehensive Clinical Trials Unit (UCL CCTU) trial team. All data queries were resolved prior to trial closure and analysis.
At sites where electronic records were available, the site may have captured some of the data electronically, which were then transcribed onto the paper CRFs to ensure a complete record.
Statistical methods
All trial analyses were performed according to a predefined SAP. 26 All efficacy analyses were conducted following the intention-to-treat (ITT) principle, in which all randomised patients were analysed according to their randomised surgical procedure, irrespective of the type of surgery they received.
In addition, a per-protocol analysis was carried out for the primary outcome, which included only the outcome data that were collected within the protocol-specified time window from patients who underwent surgery according to their randomised surgical procedure, excluding crossover patients.
The baseline characteristics were summarised by randomised treatment arm. The categorical variables were summarised by number and percentage in each category; continuous variables were summarised by mean and SD, or median and interquartile range, as appropriate. No statistical tests of differences in baseline characteristics between arms were undertaken, as in a randomised trial any differences between treatment arms must be due to chance.
Primary outcome analysis
A multilevel repeated-measures linear regression model was used to estimate the difference between the treatment arms in the change in MOXFQ walking/standing domain score from before the operation to 52 weeks post surgery. The model included fixed effects for time, treatment, treatment-by-time interaction, baseline MOXFQ walking/standing domain score and presence of osteoarthritis in each of the two adjacent joints (subtalar and talonavicular). A random patient effect was included to account for clustering by patient. A random surgeon effect was also included to account for clustering by surgeon.
Owing to the heterogeneity of the surgeon cluster sizes, the planned model (which included an additional, random surgeon by-treatment-coefficient) encountered convergence problems. Although randomisation was stratified by surgeon, many of the surgeons treated only a few patients, leading to insufficient data to estimate the random surgeon-by-treatment coefficient. As the primary analysis model failed to converge, the model was refitted after excluding the random surgeon-by-treatment coefficient.
The model used an unstructured covariance structure and was fitted using restricted maximum likelihood. The model makes assumptions about random effects distributions, correlation structure and residuals, which were investigated using appropriate plots.
Secondary outcome analysis: continuous secondary outcomes
Each of the following continuous secondary outcome measures were analysed using a separate multilevel repeated-measures linear regression model:
-
change in MOXFQ pain domain score
-
change in MOXFQ social interaction domain score
-
change in FAAM-ADL
-
change in FAAM sport subscale (for patients involved in sport)
-
change in EQ-5D-5L index value
-
change in EQ-5D-5L VAS
-
change in ROM dorsiflexion
-
change in ROM plantarflexion.
Similar to the primary analysis model, each model included fixed effects for treatment, time, treatment by time, baseline value of the associated score and presence of osteoarthritis in each of the two adjacent joints. A random patient effect and a random surgeon effect were also included in each of the models.
The outcomes ROM dorsiflexion and ROM plantarflexion were measured at baseline and 52 weeks only. Hence, the analyses models included fixed effects for treatment, baseline value of the associated score and presence of osteoarthritis in each of the two adjacent joints, and a random surgeon effect.
Adverse events, serious adverse events and complications
The following absolute differences in proportions were estimated using the treatment coefficient obtained using a binomial regression model with the identity link function:
-
proportion of patients experiencing at least one AE
-
proportion of patients experiencing at least one SAE.
Relative risks were obtained using a binomial regression model with the log link.
The distribution of the AEs and SAEs per patient have also been presented descriptively, but no formal analysis was performed. The descriptive statistics of complications, revisions and reoperations were also presented.
Subgroup analyses
An exploratory subgroup analysis was performed to investigate whether there was any interaction between the effect of treatment and the presence of osteoarthritis in the two adjacent joints on the primary outcome.
The fitted primary analysis model was extended to include the interactions between treatment and presence/absence of osteoarthritis in adjacent joints. As the trial was not powered to detect this, the analysis had limited power and is exploratory.
Further exploratory subgroup analyses were undertaken to investigate whether or not there was any interaction between age and the randomised treatment.
Post hoc analysis
At the time of developing the protocol, only mobile-bearing TAR implants were on the UK market. Between 2014 and 2019, after the study had begun, fixed-bearing implants became the most commonly used implants in the UK. Therefore, a post hoc analysis was carried out as a sensitivity analysis, comparing the most common type of implant in the UK (fixed-bearing TAR) with ankle fusion. The subtypes of TAR patients (those who received fixed-bearing TAR and those who received mobile-bearing TAR) were used as separate groups in the post hoc model and compared with the ankle fusion arm (including both open and arthroscopic ankle fusion patients).
Study oversight
A Trial Steering Committee (TSC) was established, comprising seven independent members, including a patient and public representative, the chief investigator and representatives from among the principal investigators. The trial health economist and senior trial statistician attended meetings as observers. The committee provided advice to the chief investigator, UCL CCTU, the funder and the sponsor on all aspects of the trial.
The UCL CCTU was responsible for the day-to-day management of the trial, with oversight from a Trial Management Group on the design, co-ordination and strategic management of the trial. The Trial Management Group was chaired by the chief investigator.
An Independent Data Monitoring Committee (IDMC) monitored the accumulating data and made recommendations to the TSC on whether or not the trial should continue as planned. The committee consisted of three independent members: a professor of medical statistics, a professor of rehabilitation sciences and a professor of orthopaedic surgery (the chairperson).
All oversight committees had agreed terms of reference.
During the trial, the TSC and IDMC each met six times between August 2014 and July 2019, one of which was a joint meeting of the two committees. The joint meeting led to the abbreviation of the exclusion criteria so that the surgeons’ checklist was shorter. The committees also reviewed the impact of the withdrawal of the Mobility TAR implant, which occurred after the study began but prior to any recruitment. The IDMC and TSC also advised on a recovery plan for slow recruitment, including increasing the number of recruitment sites, extending the recruitment period and a qualitative study to provide insight into recruitment difficulties.
Chapter 3 Trial results
Recruitment
Participants were randomised between 6 March 2015 and 10 January 2019. A total of 1604 patients were screened for eligibility, of whom 303 were randomised: 152 to TAR and 151 to ankle fusion. The numbers of participants recruited and included in the ITT analysis are summarised in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram in Figure 1. Of the 303 patients randomised, 21 withdrew from the trial before receiving surgery, one withdrew before the 26-week follow-up and a further five withdrew/had missing data at week 52. Six of those who received surgery were missing primary outcome measure data at 52 weeks. All patients who received surgery and attended either the 26-week or 52-week visit were included in the ITT analysis. Four patients randomised to arthrodesis did not receive their allocated surgery and crossed over to the TAR arm. All observed outcome data from these patients were analysed according to their randomised surgical procedure.
FIGURE 1.
Trial profile: CONSORT flow diagram.

Of the 282 patients who received surgery, one patient who withdrew before the 26-week follow-up could not contribute data to the primary outcome but was included in the baseline characteristics table. All 281 patients who received surgery and attended at least one follow-up were included in the mixed model for the primary outcome analysis (ITT analysis).
Table 1 lists the 17 sites in order of the date the site opened to recruitment. The first site to open was the Royal National Orthopaedic Hospital in December 2014. This site randomised the largest number of patients (24% of the total randomised).
| Site (site identification number) | Date openeda | Number of patients | |||
|---|---|---|---|---|---|
| Screened | Eligible | Randomised | Per centre per month | ||
| Royal National Orthopaedic Hospital (10) | 23 December 2014 | 287 | 136 | 73 | 1.7 |
| Aintree University Hospital (11) | 10 February 2015 | 109 | 37 | 17 | 0.4 |
| Northern General Hospital (Sheffield) (13) | 13 February 2015 | 101 | 51 | 10 | 0.2 |
| Wrightington Hospital (24) | 21 April 2015 | 147 | 100 | 10 | 0.2 |
| Freeman Hospital (Newcastle) (18) | 19 May 2015 | 116 | 73 | 19 | 0.4 |
| Royal Derby Hospital (28) | 11 June 2015 | 103 | 72 | 30 | 0.7 |
| Royal Surrey County Hospital (27) | 9 July 2015 | 34 | 14 | 12 | 0.3 |
| Cardiff and Vale University Local Health Board (16) | 20 November 2015 | 67 | 27 | 3 | 0.1 |
| Hull and East Yorkshire Hospitals NHS Trust (30) | 1 December 2015 | 51 | 36 | 9 | 0.2 |
| Northumbria Healthcare NHS Foundation Trust (25) | 15 January 2016 | 105 | 73 | 26 | 0.7 |
| Norfolk and Norwich University Hospital NHS Foundation Trust (19) | 15 January 2016 | 99 | 58 | 11 | 0.3 |
| Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust (21) | 29 January 2016 | 128 | 93 | 23 | 0.6 |
| Brighton and Sussex University Hospitals NHS Trust (26) | 4 March 2016 | 37 | 25 | 18 | 0.5 |
| Oxford University Hospitals NHS Foundation Trust (22) | 3 May 2016 | 32 | 17 | 9 | 0.3 |
| Nottingham University Hospitals NHS Trust (20) | 20 May 2016 | 75 | 61 | 16 | 0.5 |
| Royal Cornwall Hospitals NHS Trust (33) | 28 September 2016 | 16 | 14 | 1 | 0.0 |
| North Bristol NHS Trust (14) | 23 January 2017 | 97 | 46 | 16 | 0.7 |
| Total | 1604 | 933 | 303 | ||
Table 2 summarises the losses and exclusions after randomisation, with reasons, for each arm. There have been no losses to follow-up in the trial.
| Reasons for withdrawal | Number of patients | |
|---|---|---|
| TAR arm | Ankle fusion arm | |
| Withdrawal pre surgery | 14 | 7 |
| Declined surgery | 9 | 3 |
| Patient experienced medical complication | 2 | 3 |
| Postponed surgery | 3 | 1 |
| Withdrawal post surgery | 2 | 2 |
| Unable to commit to treatment schedule | 1 | 0 |
| Patient experienced SAE | 1 | 0 |
| Patient died | 0 | 1 |
| Reason not given | 0 | 1 |
| Withdrawal after 52 weeks | 1 | 0 |
| Patient died | 1 | 0 |
A total of 21 randomised patients withdrew from the trial prior to surgery – 14 (9%) in the TAR arm and seven (5%) in the ankle fusion arm. Of the patients who received surgery, four (two in each arm) withdrew from the trial prior to the 52-week follow-up. One patient in the TAR arm died after their 52-week follow-up visit.
Baseline characteristics of participants
The baseline characteristics of participants are presented in Table 3.
| Baseline characteristics | TAR arm (N = 138) | Ankle fusion arm (N = 144) | Total (N = 282) |
|---|---|---|---|
| Age (years), mean (SD) | 68.0 (8.1) | 67.7 (8.0) | 67.9 (8.0) |
| Sex, n (%) | |||
| Female | 34 (25) | 47 (33) | 81 (29) |
| Male | 104 (75) | 97 (67) | 201 (71) |
| Height (m), mean (SD) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) |
| Weight (kg), mean (SD) | 85.8 (13.2) | 88.3 (17.4) | 87.1 (15.5) |
| Body mass index (kg/m2), n (%) | |||
| < 30 | 87 (64) | 70 (49) | 157 (56) |
| ≥ 30 | 50 (37) | 74 (51) | 124 (44) |
| Smoking status | |||
| Current smoker, n (%) | 5 (4) | 5 (4) | 10 (4) |
| Cigarettes/day, mean (SD) | 5.8 (2.4) | 10.4 (7.4) | 8.1 (5.7) |
| Ex-smoker, n (%) | 53 (38) | 57 (40) | 110 (39) |
| Time since cessation (years), mean (SD) | 25.5 (16.0) | 25.9 (15.6) | 25.7 (15.7) |
| Patients’ treatment preference, n (%) | |||
| No preference expressed | 100 (75) | 112 (79) | 212 (77) |
| TAR | 26 (19) | 20 (14) | 46 (17) |
| Ankle fusion | 8 (6) | 9 (6) | 17 (6) |
| Aetiology of osteoarthritis, n (%) | |||
| Post traumatic | 83 (60) | 73 (50) | 156 (55) |
| Primary | 46 (33) | 56 (38) | 102 (36) |
| Rheumatoid arthritis | 6 (4) | 7 (5) | 13 (5) |
| Other inflammatory | 2 (2) | 5 (4) | 7 (3) |
| Other | 1 (1) | 4 (3) | 5 (2) |
| Presence/absence of osteoarthritis, n (%) | |||
| Healthy adjacent joint | 81 (59) | 79 (55) | 160 (57) |
| Osteoarthritis in subtalar or talonavicular | 45 (32) | 52 (36) | 97 (34) |
| Osteoarthritis in both adjacent joints | 12 (9) | 13 (9) | 25 (9) |
| User of assistive device, n (%) | |||
| No | 80 (58) | 79 (55) | 159 (56) |
| Yes | 58 (42) | 65 (45) | 123 (44) |
| Assistive device, n (%) | |||
| Crutches | 12 (9) | 14 (10) | 26 (9) |
| Ankle brace | 16 (12) | 7 (5) | 23 (8) |
| Frame | 2 (2) | 1 (1) | 3 (1) |
| Wheelchair | 3 (3) | 3 (2) | 6 (2) |
| Stick/cane | 33 (24) | 46 (32) | 79 (28) |
| Wheeled walker | 1 (1) | 4 (3) | 5 (2) |
| Knee scooter | 1 (1) | 1 (1) | 2 (1) |
| Other | 8 (6) | 4 (3) | 12 (4) |
| Medical history, n (%) | |||
| Anticoagulants | 24 (17) | 24 (17) | 48 (17) |
| History of cancer | 13 (9) | 20 (14) | 33 (12) |
| Chronic pain | 40 (29) | 46 (32) | 86 (31) |
| Connective tissue disorder | 1 (1) | 4 (3) | 5 (2) |
| Diabetes | 9 (7) | 16 (11) | 25 (9) |
| Gastrointestinal disease | 17 (12) | 22 (15) | 39 (14) |
| Hypertension/hypercholesterolaemia | 61 (44) | 62 (43) | 123 (44) |
| Inflammatory disorder | 8 (6) | 12 (8) | 20 (7) |
| Metabolic disorder | 5 (4) | 3 (2) | 8 (3) |
| Neurological disorder | 2 (2) | 6 (4) | 8 (3) |
| Obesity | 8 (6) | 15 (10) | 23 (8) |
| Peripheral nervous system disorder | 0 (0) | 5 (4) | 5 (2) |
| Peripheral vascular disease | 2 (2) | 3 (2) | 5 (2) |
| Renal pathology | 7 (5) | 3 (2) | 10 (4) |
| Respiratory pathology | 12 (9) | 20 (14) | 32 (11) |
| Thromboembolic disease | 7 (5) | 7 (5) | 14 (5) |
| Other condition affecting mobility | 39 (28) | 43 (30) | 82 (29) |
| Degree of deformity, n (%) | |||
| 16–30° varus | 13 (10) | 7 (5) | 20 (7) |
| 5–15° varus | 36 (26) | 43 (30) | 79 (28) |
| Physiological neutral | 47 (34) | 51 (35) | 98 (35) |
| 5–15° valgus | 20 (15) | 18 (13) | 38 (14) |
| 16–30° valgus | 10 (7) | 6 (4) | 16 (6) |
| Not available | 11 (8) | 19 (13) | 30 (11) |
| Fixed flexion deformity of knee, n (%) | 2 (1.4) | 3 (2.1) | 5 (1.8) |
| Fixed equinus, n (%) | 7 (5.1) | 5 (3.5) | 12 (4.3) |
| ROM dorsiflexion (degrees), mean (SD) | 14.3 (9.5) | 14.2 (9.3) | 14.2 (9.4) |
| ROM plantarflexion (degrees), mean (SD) | 25.4 (8.3) | 26.3 (10.5) | 25.9 (9.5) |
| Outcome measures at baseline, mean (SD) | |||
| MOXFQ walking/standing | 81.6 (16.6) | 81.5 (16.8) | 81.5 (16.7) |
| MOXFQ pain | 66.7 (16.8) | 67.6 (17.5) | 67.2 (17.1) |
| MOXFQ social interaction | 54.4 (26.1) | 56.3 (21.7) | 55.4 (24.0) |
| MOXFQ summary indexa | 70.1 (15.4) | 70.9 (14.8) | 70.5 (15.1) |
| FAAM-ADL | 47.0 (16.7) | 44.1 (16.6) | 45.5 (16.7) |
| FAAM sport subscale | 28.3 (19.7) | 25.6 (21.3) | 27.3 (20.2) |
| EQ-5D-5L index value | 0.5 (0.2) | 0.5 (0.2) | 0.5 (0.2) |
| EQ-5D-5L VAS | 72.7 (20.2) | 67.5 (21.4) | 70.0 (21.0) |
The mean (SD) age of the participants was similar in each treatment arm: 68.0 (8.1) years in the TAR arm and 67.7 (8.0) in the ankle fusion arm. In total, 81 (29%) participants were female. The rate of obesity (body mass index of ≥ 30 kg/m2) was 37% in the TAR arm and 51% in the ankle fusion arm.
The proportion of patients with respiratory pathology, diabetes or obesity was lower in the ankle fusion arm than in the TAR arm at baseline. However, there was more deformity in the TAR arm than in the ankle fusion arm (see Table 3). Overall, the two randomised arms were considered generally similar with regard to medical history factors and smoking habits. In terms of American Society of Anesthesiologists (ASA) grade (Table 4), there were slightly more ASA grade 3 patients (severe systemic disease) in the ankle fusion arm than in the TAR arm (17.4% vs. 14.5%, respectively).
| Surgery characteristic | TAR arm (N = 138) | Ankle fusion arm (N = 144) | Total (N = 282) |
|---|---|---|---|
| Surgery type,a n (%) | |||
| Mobile-bearing TAR | 65 (47.1) | 1 (25.0)a | 66 (46.5) |
| Fixed-bearing TAR | 73 (52.9) | 3 (75.0)a | 76 (53.5) |
| Arthroscopic ankle fusion | – | 85 (60.7) | – |
| Open ankle fusion | – | 55 (39.3) | – |
| Tourniquet duration (minutes),b mean (SD) | 117 (23.8) | 92 (28.0) | 105 (28.8) |
| Operation duration (minutes), mean (SD) | 121 (31.6) | 103 (36.2) | 112 (35.2) |
| Drain used, n (%) | |||
| No | 125 (92.6) | 139 (98.6) | 264 (95.6) |
| Yes | 10 (7.4) | 2 (1.4) | 12 (4.4) |
| Post surgery weight-bearing recommendation, n (%) | |||
| Full weight-bearing | 9 (6.6) | 1 (0.7) | 10 (3.6) |
| Partial weight-bearing | 17 (12.4) | 6 (4.2) | 23 (8.2) |
| Non-weight-bearing until 2 weeks | 81 (59.1) | 40 (28.0) | 121 (43.2) |
| Non-weight-bearing until 6 weeks | 13 (9.5) | 69 (48.3) | 82 (29.3) |
| Other | 17 (12.4) | 27 (18.9) | 44 (15.7) |
| Immobilisation type, n (%) | |||
| Backslab | 104 (75.9) | 114 (79.2) | 218 (77.6) |
| Walker boot | 11 (8.1) | 13 (9.0) | 24 (8.6) |
| Other | 21 (15.4) | 19 (13.2) | 40 (14.3) |
| ASA grade, n (%) | |||
| Healthy patient | 18 (13.0) | 25 (17.4) | 43 (15.3) |
| Mild systemic disease | 100 (72.5) | 94 (65.3) | 194 (68.8) |
| Severe systemic disease | 20 (14.5) | 25 (17.4) | 45 (16.0) |
| Prior fracture around index joint, n (%) | |||
| No | 87 (63.0) | 111 (77.1) | 198 (70.2) |
| Yes | 45 (32.6) | 28 (19.4) | 73 (25.9) |
| Not available | 6 (4.4) | 5 (3.5) | 11 (3.9) |
| Previous surgery on index joint, n (%) | |||
| None | 92 (66.7) | 92 (63.9) | 184 (65.3) |
| Internal fixation | 22 (16.0) | 18 (12.5) | 40 (14.2) |
| Other | 14 (10.4) | 16 (11.1) | 30 (10.6) |
| Thromboprophylaxis given, n (%) | |||
| None | 2 (1.5) | 3 (2.1) | 5 (1.8) |
| Chemical | 31 (22.5) | 34 (23.6) | 65 (23.1) |
| Mechanical | 2 (1.5) | 0 (0.0) | 2 (0.7) |
| Both | 103 (74.6) | 107 (74.3) | 210 (74.5) |
Participants appeared to be equally distributed between treatment arms with regard to the minimisation factors, that is the presence of osteoarthritis in the two adjacent joints (subtalar and talonavicular). A total of 122 patients (34%) had osteoarthritis in the adjacent joints. For 25 (9%) of these patients, the osteoarthritis was in both adjacent joints.
Prior to their surgery, 44% of patients reported that they used assistive devices. The majority of those using an assistive device used a stick or cane. Patients also reported using other forms of assistive devices such as crutches (9%) and ankle braces (8%).
The majority of patients (77%) did not express a treatment preference, 17% of patients stated a preference for TAR and 6% expressed a preference for ankle fusion.
The baseline mean (SD) MOXFQ walking/standing score was 82 (16.6) in TAR and 82 (16.8) in ankle fusion patients. The baseline values for the outcome measures were similar in the two treatment arms.
Surgery details
The duration of the procedure was slightly longer for TAR (121 minutes) than ankle fusion (103 minutes). Patients were immobilised for longer in the ankle fusion arm than in the TAR arm: 26 (19%) patients in the TAR arm compared with seven (5%) in the ankle fusion arm were allowed to weight bear within 2 weeks of the surgery.
The arms were broadly similar in terms of previous surgery, although the TAR arm had slightly more patients who had previously had internal fixation on the index joint than the ankle fusion arm (16% vs. 12.5%, respectively).
For those patients who underwent TAR, 76 (53.5%) had a fixed-bearing TAR and 66 (46.5%) had a mobile-bearing TAR (Table 5). In the ankle fusion arm, 60% of the procedures were performed arthroscopically. For those patients who underwent an open ankle fusion, seven (14%) received a lateral approach (Table 6).
| Type of implant | n (N = 142) | Percentage |
|---|---|---|
| Infinity Total Ankle System | 76 | 53.5 |
| STAR | 24 | 16.9 |
| BOX Total Ankle Replacement | 23 | 16.2 |
| Zenith (Corin Group, Circencester, UK) | 18 | 12.7 |
| Salto Talaris Total Ankle Replacement | 1 | 0.7 |
| Procedure type | n (N = 140) | Percentage |
|---|---|---|
| Arthroscopic | 85 | 60.7 |
| Open anterior/anteromedial | 48 | 34.3 |
| Open lateral | 7 | 5.0 |
The proportion of patients who had an associated procedure was higher in the TAR arm than in the ankle fusion arm (35% vs. 18%, respectively). The most common procedure was Achilles tendon lengthening, which was undertaken in 17 (12.3%) patients in the TAR arm (Table 7). Six patients (4.3%) in the TAR arm required a lateral ligament repair. No patients in the ankle fusion arm underwent ligament repair.
| Procedure | TAR arm (N = 138), n (%) | Ankle fusion arm (N = 144), n (%) | Total, n (%) |
|---|---|---|---|
| None | 90 (65.2) | 118 (81.9) | 208 (73.8) |
| Calcaneal osteotomy | 1 (0.7) | 0 (0.0) | 1 (0.4) |
| Achilles tendon lengthening | 17 (12.3) | 2 (1.4) | 19 (6.7) |
| Fibula osteotomy | 0 (0.0) | 6 (4.2) | 6 (2.1) |
| Lateral ligament repair | 6 (4.3) | 0 (0.0) | 6 (2.1) |
| Bone grafting | 2 (1.4) | 5 (3.5) | 7 (2.5) |
| Removal of metalwork | 4 (2.9) | 2 (1.4) | 6 (2.1) |
| Other | 18 (13.0) | 11 (7.6) | 29 (10.3) |
The distribution of procedures by surgeon is shown in Table 8. Recruitment ended at the Royal National Orthopaedic Hospital in June 2018, 6 months prior to closure of recruitment.
| Surgeon number | Site | Type of surgery (n) | Total (n) | |
|---|---|---|---|---|
| TAR | Ankle fusion | |||
| 1 | Aintree | 2 | 3 | 5 |
| 2 | Aintree | 4 | 6 | 10 |
| 3 | Brighton | 10 | 8 | 18 |
| 4 | Bristol | 4 | 3 | 7 |
| 5 | Bristol | 1 | 1 | 2 |
| 6 | Bristol | 3 | 4 | 7 |
| 7 | Cardiff | 1 | 2 | 3 |
| 8 | Cornwalla | 0 | 2 | 2 |
| 9 | Derby | 14 | 15 | 29 |
| 10 | Guildford | 5 | 4 | 9 |
| 11 | Hull | 4 | 4 | 8 |
| 12 | Newcastle | 5 | 6 | 11 |
| 13 | Newcastle | 3 | 4 | 7 |
| 14 | Northumbria | 6 | 3 | 9 |
| 15 | Northumbria | 4 | 1 | 5 |
| 16 | Northumbria | 6 | 4 | 10 |
| 17 | Norwich | 2 | 6 | 8 |
| 18 | Norwich | 1 | 0 | 1 |
| 19 | Nottingham | 5 | 6 | 11 |
| 20 | Nottingham | 2 | 3 | 5 |
| 21 | Oswestry | 10 | 9 | 19 |
| 22 | Oswestry | 3 | 1 | 4 |
| 23 | Oxford | 4 | 2 | 6 |
| 24 | Oxford | 1 | 1 | 2 |
| 25 | Oxford | 1 | 0 | 1 |
| 26 | Sheffield | 4 | 4 | 8 |
| 27 | Stanmore | 5 | 3 | 8 |
| 28 | Stanmore | 24 | 24 | 48 |
| 29 | Stanmore | 1 | 1 | 2 |
| 30 | Stanmore | 4 | 4 | 8 |
| 31 | Wigan | 0 | 2 | 2 |
| 32 | Wigan | 0 | 1 | 1 |
| 33 | Wigan | 3 | 2 | 5 |
| 34 | Wigan | 0 | 1 | 1 |
| Total | 142 | 140 | 282 | |
Numbers analysed
Owing to the nature of the model used in the analysis of primary and secondary continuous outcomes (i.e. a mixed model), all patients with a baseline visit and at least one follow-up visit were included in the analysis. Therefore, the final primary outcome analysis was based on 281 patients: 137 in TAR and 144 in ankle fusion (Table 9).
| Analysis | TAR arm (N = 138)a | Ankle fusion arm (N = 144) |
|---|---|---|
| Primary outcome (ITT) | 137 | 144 |
| Sensitivity of primary outcome (per protocol) | 135 | 134 |
| Secondary outcome | ||
| MOXFQ pain | 137 | 144 |
| MOXFQ social interaction | 137 | 144 |
| EQ-5D-5L index value | 137 | 144 |
| EQ-5D-5L VAS | 137 | 144 |
| FAAM-ADL | 137 | 143 |
| FAAM sport subscale | 43 | 24 |
| ROM dorsiflexion | 132 | 131 |
| ROM plantarflexion | 132 | 131 |
Primary outcome
Findings for the primary outcome, MOXFQ (walking/standing domain), are presented in Table 10.
| Outcome | TAR arm | Ankle fusion arm | Adjusted difference in change from baseline (95% CI)a | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Value at 52 weeks, mean (SD) | Change from baseline, mean (SD) | n | Value at 52 weeks, mean (SD) | Change from baseline, mean (SD) | |||
| Primary outcome (ITT) | ||||||||
| MOXFQ walking/standing score | 136 | 31.4 (30.4) | –49.9 (30.7) | 140 | 36.8 (30.6) | –44.4 (31.9) | –5.56 (–12.49 to 1.37) | 0.12 |
| Sensitivity analysis of primary outcome (per protocol) | ||||||||
| MOXFQ walking/standing score | 135 | 31.4 (30.5) | –49.9 (30.8) | 134 | 36.4 (30.8) | –45.0 (32.4) | –4.84 (–11.96 to 2.28) | 0.18 |
The mean (SD) MOXFQ walking/standing domain score at 52 weeks was 31 (30.4) in the TAR arm and 37 (30.6) in the ankle fusion arm. The mean (SD) change in scores between pre-surgery baseline and 52 weeks was –50 (30.7) in the TAR arm and –44 (31.9) in the ankle fusion arm. The adjusted difference in change score of –5.56 (95% CI –12.49 to 1.37) suggests that, on average, the improvement in the MOXFQ score from baseline to 52 weeks post surgery was 5.56 points greater in TAR patients than in ankle fusion patients (p = 0.12). The 95% CI for this difference includes both a difference of zero and the MID of 12. The MOXFQ scores improved following surgery in both arms (TAR: mean change –50, 95% CI –55 to –45; ankle fusion: mean change –44, 95% CI –50 to –39), but there was not a statistically significantly greater improvement in the TAR arm than in the ankle fusion arm. The proportion of patients with a reduction in MOXFQ score of at least 12 points from baseline at 52 weeks was very similar in the two arms: 82% of TAR patients compared with 80% of ankle fusion patients.
Four patients crossed over from ankle fusion to TAR after randomisation. Three patients had their 52-week visit outside of the protocol window and an additional five patients had missing 52-week scores. We carried out a per-protocol analysis as a sensitivity analysis for the primary outcome by excluding these 12 patients. The per-protocol analysis did not change the ITT conclusions.
Secondary outcomes
Findings for the secondary outcomes are presented in Table 11.
| Secondary outcomes | TAR arm | Ankle fusion arm | Adjusted difference in change from baseline (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Value at follow-up, mean (SD) | Change from baseline, mean (SD) | n | Value at follow-up, mean (SD) | Change from baseline, mean (SD) | |||
| 52 weeks | ||||||||
| MOXFQ pain | 136 | 26.7 (24.7) | –40.2 (28.0) | 140 | 30.6 (25.7) | –36.7 (24.6) | –4.20 (–9.80 to 1.39) | 0.14 |
| MOXFQ social interaction | 136 | 17.0 (20.1) | –37.0 (30.0) | 140 | 22.4 (24.4) | –33.7 (28.0) | –5.06 (–10.37 to 0.26 | 0.06 |
| MOXFQ summary indexa | 136 | 26.4 (24.5) | –43.7 (26.1) | 140 | 31.2 (25.5) | –39.3 (25.6) | –4.95 (–10.61 to 0.72) | 0.09 |
| FAAM-ADL | 135 | 81.2 (20.5) | 33.8 (22.7) | 141 | 73.8 (20.7) | 29.7 (20.7) | 6.16 (1.54 to 10.78) | 0.01 |
| FAAM sport subscale | 37 | 71.3 (28.8) | 41.9 (31.8) | 22 | 75.6 (23.2) | 52.7 (26.8) | –4.98 (–18.60 to 8.64) | 0.47 |
| EQ-5D-5L index value | 136 | 0.7 (0.2) | 0.3 (0.3) | 140 | 0.7 (0.2) | 0.2 (0.2) | 0.02 (–0.02 to 0.07) | 0.32 |
| EQ-5D-5L VAS | 136 | 81.9 (15.2) | 9.1 (19.9) | 141 | 77.0 (17.3) | 9.4 (22.3) | 3.41 (–0.30 to 7.11) | 0.07 |
| ROM dorsiflexion | 132 | 15.3 (7.2) | 1.1 (10.1) | 131 | 9.1 (5.8) | –4.9 (7.9) | 6.09 (4.61 to 7.57) | < 0.001 |
| ROM plantarflexion | 132 | 27.3 (7.9) | 1.9 (9.8) | 131 | 14.4 (7.2) | –11.7 (11.1) | 13.01 (11.24 to 14.77) | < 0.001 |
| 26 weeks | ||||||||
| MOXFQ walking/standing | 134 | 35.8 (29.9) | –45.8 (31.0) | 141 | 44.6 (29.6) | –36.9 (31.2) | –8.21 (–15.14 to –1.27) | 0.02 |
| MOXFQ pain | 134 | 32.9 (24.3) | –33.8 (25.9) | 140 | 36.2 (24.8) | –31.4 (23.8) | –2.45 (–8.06 to 3.16) | 0.39 |
| MOXFQ social interaction | 134 | 22.3 (24.7) | –32.1 (29.5) | 140 | 26.5 (24.4) | –29.6 (26.9) | –3.38 (–8.71 to 1.95) | 0.21 |
| MOXFQ summary indexa | 134 | 31.5 (25.0) | –38.6 (25.6) | 140 | 37.5 (24.9) | –33.2 (24.9) | –5.13 (–10.80 to 0.55) | 0.08 |
| FAAM-ADL | 132 | 77.1 (20.0) | 30.0 (21.4) | 140 | 70.9 (22.1) | 26.8 (21.9) | 4.56 (–0.08 to 9.20) | 0.05 |
| FAAM sport subscale | 39 | 56.6 (28.1) | 27.7 (26.2) | 19 | 62.9 (28.7) | 37.3 (35.7) | –7.17 (–21.11 to 6.76) | 0.31 |
| EQ-5D-5L index value | 134 | 0.7 (0.2) | 0.2 (0.2) | 141 | 0.7 (0.2) | 0.2 (0.2) | 0.04 (–0.004 to 0.09) | 0.08 |
| EQ-5D-5L VAS | 134 | 81.3 (14.8) | 8.7 (21.5) | 142 | 76.0 (19.2) | 8.1 (22.2) | 4.14 (0.43 to 7.85) | 0.03 |
On average, patients in both arms reported an improvement in the MOXFQ pain and social interaction domains at 26 weeks, and on all MOXFQ domains at 52 weeks, but improvement was not greater in the TAR arm than in the ankle fusion arm. The difference between the TAR and ankle fusion arms in the change in MOXFQ walking/standing domain score at 26 weeks was statistically significant (p = 0.02).
The difference between the TAR and ankle fusion arms in the change in FAAM-ADL scores at 52 weeks was statistically significant (p = 0.01). There were substantial improvements from baseline in both arms, with a difference of 6.16 (95% CI 1.54 to 10.78) between the two arms.
The change in EQ-5D-5L index values between the two treatment arms was not significantly different at 26 weeks (p = 0.08) or 52 weeks (p = 0.32). The change in EQ-5D-5L VAS was statistically significant at 26 weeks (p = 0.03), but not at 52 weeks (p = 0.07).
Changes from baseline in ROM dorsiflexion and ROM plantarflexion were greater in the ankle fusion arm than in the TAR arm. Although ROM (dorsiflexion and plantarflexion) improved from baseline to 52 weeks in the TAR arm, it decreased in ankle fusion patients and the difference between arms was statistically significant (p < 0.001).
Subgroup analyses
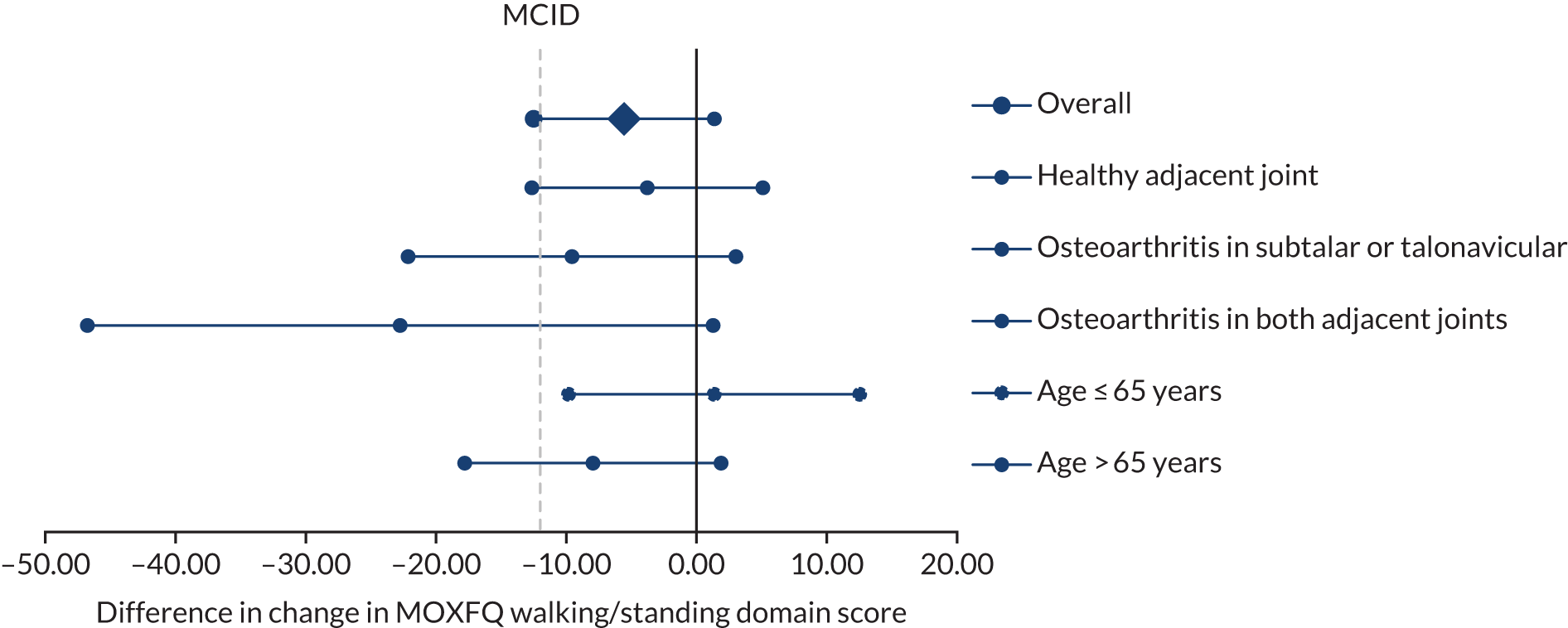
A total of 45 patients (33%) in the TAR arm and 50 (36%) in the ankle fusion arm had osteoarthritis in one adjacent joint at baseline; 11 patients (8%) in the TAR arm and 12 patients (9%) in the ankle fusion arm had osteoarthritis in both the subtalar and talonavicular joints. Adjusted MOXFQ scores were lower in the TAR arm than in the ankle fusion arm at 52 weeks in the subgroup analyses (Table 12). However, we did not find a significant interaction caused by this factor. There was also no evidence to suggest that the effect of treatment was moderated by age. The subgroup analyses are presented in Figure 2.
| Subgroup analyses | TAR arm (n) | Ankle fusion arm (n) | Difference | 95% CI | p-value |
|---|---|---|---|---|---|
| Change in walking/standing score | |||||
| Overall effect | 136 | 140 | –5.56 | –12.49 to 1.37 | 0.12 |
| Age (years) | |||||
| ≤ 65 | 45 | 46 | 1.36 | –9.82 to 12.53 | 0.13 |
| > 65 | 91 | 94 | –7.95 | –17.79 to 1.88 | |
| Adjacent osteoarthritis | |||||
| Healthy adjacent joint | 80 | 78 | –3.78 | –12.64 to 5.09 | |
| Osteoarthritis in subtalar or talonavicular | 45 | 50 | –9.56 | –22.14 to 3.03 | 0.92 |
| Osteoarthritis in both adjacent joints | 11 | 12 | –22.75 | –46.77 to 1.27 | 0.11 |
FIGURE 2.
Forest plot showing subgroup analysis by treatment arm.
Adverse events
A total of 20.8% of randomised patients experienced at least one SAE and 53.5% of patients experienced at least one AE during the course of their trial pathway (Table 13). The risks of patients experiencing a SAE or an AE were not significantly different between the two arms (p = 0.19 and p = 0.84, respectively).
| Event | TAR arm (N = 152) | Ankle fusion arm (N = 151) | Total (N = 303) | Difference in proportion (95% CI) | p-value |
|---|---|---|---|---|---|
| Number (%) of patients experiencing a SAE | 27 (17.8) | 36 (23.8) | 63 (20.8) | 0.74 (0.48 to 1.16) | 0.19 |
| Total SAEs (n) | 31 | 43 | 75 | ||
| Number (%) of patients experiencing an AE | 82 (54.3) | 80 (52.6) | 162 (53.5) | 1.02 (0.83 to 1.26) | 0.84 |
| Total AEs (n) | 162 | 168 | 330 |
All the AEs and SAEs reported during the trial have been summarised as postoperative complications in Table 14. One patient in the ankle fusion arm died during the follow-up period and one patient in the TAR arm died after the 52-week visit (not presented in Table 14). Both events were unrelated to surgery.
| Complication | TAR arm, n (N = 152) | Ankle fusion arm, n (N = 151) | Total, n (N = 303) |
|---|---|---|---|
| Total surgeries (by procedure, not randomisation) | 142 | 140 | 282 |
| Complications (1–11, larger numbers thought to lead to worse outcome) | |||
| 1: Intraoperative bone fracture | 3 | 0 | 3 |
| 2: Wound-healing problemsb | 20 | 8 | 28 |
| A: Not requiring antibiotics | 3 | 3 | 6 |
| B: Requiring antibiotics | 17 | 4 | 21 |
| C: Requiring debridement | 0 | 1 | 1 |
| 3: Pain undiagnosedc | 17 | 23 | 40 |
| 4: Nerve injuryc | 8 | 1 | 9 |
| 5: Postoperative bone fracture | 3 | 0 | 3 |
| 6: Technical error | 0 | 0 | 0 |
| 7: Reoperation other than revision | 5 | 4 | 9 |
| 8: Bone union issues | 0 | 4 | 4 |
| A: Aseptic loosening for TAR | 0 | – | 0 |
| B: Non-union for ankle fusion | – | 17 | 17 |
| 9: Subsidence | 0 | 0 | 0 |
| 10: Deep infection | 0 | 0 | 0 |
| 11: Implant failured | 1 | 0 | 1 |
| Not related to implantc | |||
| Medical complication unrelated to implant (including cardiopulmonary) | 73 | 92 | 165 |
| Worsening of pre-existing musculoskeletal issue | 35 | 35 | 70 |
| Death | 0 | 1 | 1 |
| Thromboembolic events | |||
| 1: Deep-vein thrombosise | 2 | 5 | 7 |
| 2: Pulmonary embolisme | 2 | 4 | 6 |
| Otherc | |||
| Trauma | 1 | 3 | 4 |
| Stiffness | 3 | 1 | 4 |
| Plaster/immobilisation/mobility issues | 11 | 8 | 19 |
| Tendon complications after surgery | 2 | 2 | 4 |
| Swelling | 8 | 7 | 15 |
There were 20 wound-healing problems in 19 (13.4%) patients in the TAR arm and eight wound-healing problems in eight (5.7%) patients in the ankle fusion arm. One patient in the ankle fusion arm and none in the TAR arm required debridement for the infection, although the majority of TAR patients were administered prophylactic antibiotics to treat the superficial wound infections.
There were eight nerve injury events reported in six patients (4.2%) in the TAR arm and one nerve injury event reported in one (< 1%) patient in the ankle fusion arm. Two events were reported twice.
There were 17 non-unions (12.1%) in the ankle fusion arm, which were diagnosed through the presence of a lucent line on plain radiographs at the 52-week follow-up. Of the 17 patients, eight were expected to be revised (47%), two (12%) were symptomatic but not planning to be revised due to serious comorbidities and seven (41%) were completely asymptomatic. Hence, 10 (7.1%) of 140 patients who received ankle fusion went on to symptomatic non-union.
There were 13 thromboembolic events in 11 patients: four (2.9%) patients in the TAR arm and seven (4.9%) in the ankle fusion arm. Two (1.4%) patients in the TAR arm experienced deep-vein thrombosis events and there were five events in four (2.8%) patients in the ankle fusion arm. Two (1.4%) patients experienced pulmonary embolism events in the TAR arm and there were four events in three (2.1%) patients in the ankle fusion arm. None of these events was fatal.
At 52 weeks’ follow-up, nine patients (3.2%) required further unplanned reoperation other than revisions: five in the TAR arm and four in the ankle fusion arm. In the TAR arm, one revision took place within the 52-week window. This was due to a traumatic fall, leading to a fracture and conversion to a tibiotalocalcaneal fusion. We are aware of several other revisions that will take place outside the 52-week window and these data will be reported in the 2-year results. Table 15 lists reoperations and revisions.
| Reoperation/revision | TAR arm (N = 152) | Ankle fusion arm (N = 151) |
|---|---|---|
| Total surgery (n) (by procedure, not randomisation) | 142 | 140 |
| Cases with no reoperations or revision, n (%) | 136 (95.8) | 136 (97.1) |
| Cases with reoperation, n (%) | 5 (3.5) | 4 (2.9) |
| Cases with revision, n (%) | 1 (0.7) | 0 |
| Reoperations/revisions by type (n) | ||
| Type 2: hardware removal | 0 | 2 |
| Type 3: unplanned procedures related to the TAR | 2 | 2 |
| Type 4: debridement of gutters or heterotopic ossification | 3 | 0 |
| Type 5: exchange of polyethylene bearing | 0 | 0 |
| Type 6: debridement of osteolytic cysts | 0 | 0 |
| Type 7: deep infection requiring debridement, no metal component removal | 0 | 0 |
| Type 9: revision of metal components for aseptic loosening, fracture or malposition | 1 | 0 |
| Type 10: revision of metal components secondary to infection | 0 | 0 |
| Type 11: amputation above the level of the ankle | 0 | 0 |
Post hoc analysis
The baseline characteristics of each of the subtypes of TAR (fixed and mobile bearing) and ankle fusion (open and arthroscopic) are reported in Appendix 3. Of those who received TAR, 53.5% received fixed-bearing TAR and 46.5% received mobile-bearing TAR. Of the ankle fusion patients, 61% received arthroscopic ankle fusion and 39% received open ankle fusion. Overall, all four subtypes of patients appeared similar with respect to baseline factors and baseline outcome measures. We carried out a post hoc comparison of each TAR subtype (those who received fixed-bearing TAR and those who received mobile-bearing TAR) with the ankle fusion arm (including both open and arthroscopic ankle fusion patients).
The mean (SD) MOXFQ walking/standing domain score at 52 weeks was 25.9 (28.3) in the fixed-bearing TAR group, compared with 36.8 (30.6) in the ankle fusion arm (Table 16). The adjusted difference of –11.1 (95% CI –19.3 to –2.9) suggests that, on average, the MOXFQ score at 52 weeks post surgery was 11.1 points lower in those who received fixed-bearing TAR than in those who underwent ankle fusion. This difference is statistically significant (p = 0.008). Comparing mobile-bearing TAR patients with ankle fusion patients, the adjusted difference is 2.1 (95% CI –6.6 to 10.8). This difference is not statistically significant (p = 0.64). There was no difference in the change in MOXFQ score at 52 weeks (p = 0.83) between open and arthroscopic patients in the ankle fusion arm (see Appendix 4).
| Ankle fusion arm (n = 136) | TAR arm | ||
|---|---|---|---|
| Fixed bearing (n = 75) | Mobile bearing (n = 64) | ||
| Operation duration (minutes), mean (SD) | 103 (36.2) | 121 (30.6) | 122 (32.7) |
| MOXFQ at 52 weeks, mean (SD) | 36.8 (30.6) | 25.9 (28.3) | 38.5 (31.6) |
| Change from baseline at 52 weeks, mean (SD) | –44.4 (31.9) | –55.9 (27.7) | –42.0 (32.1) |
| Adjusted difference in change from baseline (95% CI) | – | –11.1 (–19.3 to –2.9) | 2.1 (–6.6 to 10.8) |
| p-value | – | 0.008 | 0.64 |
The subgroup analyses by subtype of TAR patients (fixed and mobile bearing) compared with ankle fusion patients are shown in Figure 3.
FIGURE 3.
Forest plots showing subgroup analysis by treatment arm and TAR subtype. (a) Fixed-bearing TAR vs. ankle fusion; and (b) mobile-bearing TAR vs. ankle fusion.

Chapter 4 Economic evaluation
Overview
The main objective of the economic evaluation was to assess the cost-effectiveness of TAR compared with ankle fusion for patients with end-stage osteoarthritis. We compared the costs and outcomes in the TAR and ankle fusion arms over a 52-week time horizon. The primary analysis was conducted in accordance with the ITT principle from the NHS and personal social services (PSS) perspective. Sensitivity analysis included per-protocol analysis and analysis from a societal perspective. The per-protocol analysis included only patients who received the surgery to which they were randomised. The societal perspective included out-of-pocket costs incurred by participants and productivity loss. The analytical approach took the form of cost–utility analysis. The main result of the analysis was the mean incremental cost per QALY gained. Costs and outcomes were not discounted because of the short time horizon (i.e. 52 weeks) of the within-trial economic evaluation.
We estimated a long-term cost-effectiveness of TAR compared with ankle fusion using decision-analytic modelling. The effect of TAR on participants’ QoL is expected to last longer than the time horizon of the trial and is affected by the rate of future revisions. We constructed a simple Markov model that simulated patients’ pathways after TAR and ankle fusion. The costs were taken from the trial data. The transition probabilities, cost of revision and EQ-5D-5L index values were obtained from published sources detailed in the subsequent section. The rate of revision was based on the clinicians’ opinion.
Methods
Cost of surgery
The cost of surgery in both the TAR and ankle fusion arm is based on the surgery duration, grade of operating surgeon, cost of operating theatre, prices of devices, duration of hospital stay, duration of immobilisation and cost of materials for immobilisation (plaster or boot). The information was obtained from the trial CRF. The cost of each component was then calculated using the unit costs for each component (Table 17).
| Resource item | Unit cost (£) | Unit of analysis | Source of unit cost |
|---|---|---|---|
| Operating theatre | 11.39 | Per minute | Patient-level costing and information systems data 2014/1550 |
| Operating surgeon | |||
| Consultant | 109.00 | Per hour | Unit Costs of Health and Social Care 201951 |
| Specialty doctor | 108.00 | Per hour | Unit Costs of Health and Social Care 201951 |
| Specialty registrar, stage of training 3–8 | 47.00 | Per hour | Unit Costs of Health and Social Care 201951 |
| Hospital stay | 1380.84 | Per day | Patient-level costing and information systems data 2014/1550 (HRG group HN32A, HN32B, HN32C) |
| Walking boot | 100.00 | Per procedure | Estimated by clinician |
| Plaster | 105.16 | Per procedure | NHS Reference Costs 2017/1852 (HRG VB09, service non-admitted) |
| Average cost of TAR implant | 4055.98 | Per implant | Manufacturer quotations |
| Average cost of devices for ankle fusion | 2441.89 | Per patient | Hospital quotation, including disposables (K-wires, drills, arthroscopic shavers) |
All costs are reported in Great British pounds and valued in 2018/19 prices. Where needed, costs were adjusted for inflation using the NHS cost inflation index. 51 Unit costs of operating theatre and operating surgeon’s time were multiplied by the surgery duration to calculate the overall cost. The average duration of TAR operations was 121 (range 60–244) minutes; the average duration of ankle fusion operations was 103 (range 45–240) minutes. The cost of hospital stay is the unit cost per day multiplied by the duration of stay. The average hospital stay was 2.5 (range 0–12) days in the TAR arm and 2.1 (range 0–17) days in the ankle fusion arm. The unit cost was obtained from patient-level costing and information systems data50 and were specifically for foot procedures.
If a walking boot was prescribed, use was assumed for the duration of immobilisation. Participants were wearing a boot for an average of 8.9 (range 0–46) weeks in the TAR arm and for an average of 13.8 (range 0–52) weeks in the ankle fusion arm. If plaster was prescribed, the unit cost was multiplied by the number of times it was applied. The plaster was assumed to be changed every 6 weeks. Participants were wearing plaster for an average of 3 (range 0–52) weeks in the TAR arm and for an average of 4.6 (range 0–26) weeks in the ankle fusion arm.
Cost of health-care resource use
The data on health service resource use were collected using the CSRI. The CSRI was adapted to the trial’s needs and was collected at baseline and at 12, 26 and 52 weeks post surgery. The components include inpatient care, outpatient care, community care and PSS. Community care includes GP surgery and home visits, GP phone calls, GP nurse practice visits and phone calls, district nurse visits and community physiotherapist visits. PSS includes social worker visits and phone calls, home help and using Meals on Wheels. Components were costed for each patient using unit costs from NHS Reference Costs 2017/1852 and Unit Costs of Health and Social Care. 51,53,54 Unit costs are presented in Appendix 2, Table 31.
The CSRI questionnaire also collects data on costs borne by the patient, including transportation costs incurred in the receipt of care, equipment, mobility aids, home adaptations, patients’ time off work and family and friends’ time off work or usual activities because of care. The cost of lost productivity for TAR compared with ankle fusion was calculated using the human capital approach. The number of hours by which patients had to reduce their employment was multiplied by a unit cost. Unit costs were the average gross hourly earnings for men/women and full-time/part-time employees. 55 We also estimated the cost of family and friends’ time using average gross hourly earnings multiplied by the number of hours.
Equipment costs were included in both the NHS and PSS perspective and the societal perspective, as we have information on whether these costs were paid by the PSS or out of pocket. Transportation unit costs include fuel costs for private car journeys only, as we have precise information on how much patients paid out of pocket for parking and taxi, bus and train journeys. We include the cost of replacing an employee for information only, as this unit cost comes from a private study conducted by Oxford Economics and income protection provider Unum, and has not been confirmed by any peer-reviewed publications. 56 All unit costs associated with out-of-pocket costs are presented in Appendix 2, Table 32.
We provided descriptive statistics for resource use variables by treatment arm and follow-up. Between-group differences were estimated using two-sample t-tests. Statistical significance was assessed at the 5% significance level.
Information on concomitant medications was collected in the trial CRF, including duration, dosage and frequency of prescriptions. Unit costs of medications were obtained from the British National Formulary. 57 When medication dosage was missing, we assumed that the participant received the same dosage as other participants who received the same medication.
Total costs from the NHS and PSS perspective include cost of surgery, health-care resource use, concomitant medications, and mobility aids and home adaptations paid for by the PSS. All costs were reported in 2018/19 Great British pounds. The overall mean cost per patient per arm was calculated. We adjusted for baseline values and minimisation factors. The factors are surgeon and presence of osteoarthritis in two adjacent joints (subtalar and talonavicular) as determined by preoperative MRI/CT. We used bias-corrected bootstrapping to calculate 95% CIs. Total costs from the societal perspective include all costs from the section above, and transportation costs, costs of equipment, mobility aids and adaptations paid out-of-pocket and cost of lost productivity. We calculated the total costs from the societal perspective in the same way.
Outcomes
The primary outcome was QALYs, which were calculated as the area under the curve using the EQ-5D-5L index values at baseline and at 12, 26 and 52 weeks post surgery. The EQ-5D-5L is a five-item, five-level questionnaire, scored 1 (no problem) to 5 (extreme problems). The EQ-5D-5L Crosswalk Index Value Calculator was used to estimate the index values. 38 It maps the EQ-5D-5L to the EQ-5D-3L value set and is recommended by NICE. 38 We estimated mean index values at each time point for TAR compared with ankle fusion, mean unadjusted QALYs from baseline to the end of the trial period and mean QALYs adjusted for baseline index values and minimisation factors using regression analysis. 58 We accounted for uncertainty by applying the bootstrapping technique and reporting 95% CIs. The EQ-5D-5L index values are shown in Table 11.
Cost–utility analysis methods
The cost and QALY data were combined to calculate an incremental cost-effectiveness ratio (ICER). Uncertainty in the point estimate of cost per QALY was quantified using bootstrapping methods to calculate CIs around the ICER. 59 Bootstrap ICERs were presented on the cost-effectiveness plane to determine in which quadrant TAR is located compared with ankle fusion and if a decision rule is required.
The bootstrapping results were used to construct the cost-effectiveness acceptability curve (CEAC):58 the probability that TAR is cost-effective compared with ankle fusion at 52 weeks for a range of cost-effectiveness thresholds. The analysis was complete case as < 15% of participants were missing an ICER.
Sensitivity analysis involved a pre-protocol analysis, which included patients who received the surgery to which they were randomised only. We also adopted a societal perspective that included out-of-pocket costs incurred by participants, loss of earnings and productivity loss.
Long-term economic modelling
We used a modelling approach to extrapolate to a lifetime horizon. Our literature search identified two relevant cost-effectiveness studies comparing TAR with ankle fusion. 24,60 Based on these studies, we constructed a simple Markov model that simulated patients’ pathways after TAR or ankle fusion. The structure of the model is shown in Figure 4.
FIGURE 4.
Model structure.
The Markov model, based on 1-year cycles, was used to simulate the impact of TAR and ankle fusion on patients’ health for the lifetime horizon. There are 17 cycles in the model, as average life expectancy for a cohort aged 50–85 years is 17 years. 61 After surgery (TAR or ankle fusion) in year 1, patients stay in good health, move to revision or die. A patient can be in a revision state for 1 year only and then they can move to the good health or death state. We specify the ‘good health after revision’ state as we assume that revision reduces the QoL of a patient. Transition probabilities are reported in Tables 18 and 19.
| Transition probability (%) | Good health | Revision | Good health after revision | Death |
|---|---|---|---|---|
| Good health | 95.8 | 1.2 | 0.000 | 3.00 |
| Revision | 0.00 | 0.00 | 97.00 | 3.00 |
| Good health after revision | 0.00 | 0.00 | 97.00 | 3.00 |
| Death | 0 | 0 | 0 | 100 |
| Transition probability (%) | Good health | Revision | Good health after revision | Death |
|---|---|---|---|---|
| Good health | 92.0 | 5.0 | 0.00 | 3.00 |
| Revision | 0.00 | 0 | 97 | 3.00 |
| Good health after revision | 0.00 | 0 | 97 | 3.00 |
| Death | 0 | 0 | 0 | 100 |
Revision rates are based on clinicians’ opinion. They are comparable to the previous cost-effectiveness model of TAR compared with ankle fusion. 60,62 The revision rate for ankle fusion was assumed to be 5% in the first 3 years (see Table 19) and 0% thereafter. The death rate is based on Public Health England’s Life Expectancy Calculator 2021. 63 Each health state in the model was assigned a cost and a QALY outcome. These are reported in Table 20.
| State reward | Good health | Revision | Good health after revision | Death |
|---|---|---|---|---|
| Cost (£) | ||||
| TAR | 275 | 7218 | 275 | 0 |
| Ankle fusion | 316 | 7218 | 316 | 0 |
| EQ-5D-5L index value | ||||
| TAR | 0.74 | 0.59 | 0.59 | 0 |
| Ankle fusion | 0.71 | 0.66 | 0.66 | 0 |
We assume that the resource use estimated at baseline is a good estimate of what patients would use in ‘good health’ and ‘good health after revision’ states. The cost of revision is based on the cost of ankle fusion. We assumed that patients continue to have the same EQ-5D-5L index values in the subsequent years after the surgery while they are in good health. Decrements in index values after revision are based on estimates in SooHoo and Kominski62 as this is the only available source of these data. 62 The QoL decreases after revision surgery and patients have the same QoL for the rest of their life. We discount costs and QALYs at the rate of 3.5% recommended by NICE. 64
A cost-per-QALY ratio was calculated using the data from the trial for year 1 and data from the Markov model for years 2–17. Probabilistic sensitivity analysis (PSA) was conducted to account for parameter uncertainty. We assigned probability distributions to parameters in the model and then used Monte Carlo simulations to obtain ICERs. We plotted the incremental costs and QALYs on a cost-effectiveness plane. We also estimated the probability of the intervention being cost-effective at a range of cost-effectiveness thresholds. The probabilities were plotted against the thresholds on a CEAC.
Results
This section presents the results of the health economic analysis of TAR compared with ankle fusion. We compare the cost of surgery, cost of health-care resource use, out-of-pocket costs, total costs, QALYs and cost–utility results of the base-case and sensitivity analyses.
Cost of surgery
Table 21 summarises the components of the cost of surgery: devices, operating theatre, orthopaedic surgeon’s time, hospital stay and immobilisation. The total cost of surgery is a sum of the components, and 95% CIs are obtained using bootstrapping.
| Cost component | Cost (£), mean (SD) | Mean difference | p-value | Bootstrap 95% CI | |
|---|---|---|---|---|---|
| TAR arm (n = 138) | Ankle fusion arm (n = 144) | ||||
| Devices | 4055.98 (387.47) | 2441.89 (466.51) | 1614.09 | 0.000 | 1511.60 to 1716.58 |
| Operating theatre | 1457.33 (363.80) | 1227.98 (430.63) | 229.34 | 0.000 | 142.36 to 328.22 |
| Orthopaedic surgeon’s time | 221.78 (55.36) | 184.38 (63.58) | 37.39 | 0.000 | 23.81 to 51.20 |
| Hospital stay | 3562.17 (3133.79) | 3164.43 (4879.62) | 397.74 | 0.418 | –595.96 to 1227.41 |
| Immobilisation | 193.80 (50.99) | 209.58 (65.38) | –15.78 | 0.025 | –29.94 to –1.59 |
| Total cost of surgery | 9491.06 (3166.44) | 7218.45 (5129.87) | 2272.61 | 0.000 | 1282.55 to 3262.67 |
On average, TAR surgery took longer than ankle fusion surgery (121 minutes vs. 103 minutes, respectively). Therefore, the costs of using the operating theatre and surgeon’s time are higher in the TAR arm than in the ankle fusion arm. TAR devices are also more expensive than ankle fusion devices (£4055.98 vs. £2441.89, respectively). The difference in the cost of devices, using the operating theatre and orthopaedic surgeon’s time was statistically significant. The cost of hospital stay was higher in the TAR arm than in the ankle fusion arm (£3562.17 vs. £3164.43, respectively), as TAR patients stayed in hospital longer after the surgery. However, this difference was not statistically significant (p = 0.418). The duration of immobilisation was shorter in the TAR arm, so the cost was lower in the TAR arm than the ankle fusion arm (£193.80 vs. £209.58, respectively) and was statistically significant (p = 0.025). The difference in surgery costs was £2272.61 (95% CI £1282.55 to £3262.67) and was statistically significant.
Cost of health-care resource use
The response rate for the CSRI questionnaire was high and we have complete data collection on costs for 93.5% (n = 129) of TAR patients and 93.8% (n = 135) of ankle fusion patients. Components of health-care resource use by treatment arm and follow-up period are summarised in Table 22.
| Cost category by period | Cost (£), mean (SD) | Mean difference | p-value | |
|---|---|---|---|---|
| TAR arm (n = 129) | Ankle fusion arm (n = 135) | |||
| Baseline | ||||
| Inpatient care | 39 (285) | 112 (527) | –73 | 0.16 |
| Outpatient care | 98 (158) | 110 (179) | –12 | 0.57 |
| Community care | 121 (429) | 77 (108) | 44 | 0.24 |
| PSS | 0 (0) | 3 (22) | –3 | 0.14 |
| 12 weeks | ||||
| Inpatient care | 498 (2,201) | 115 (326) | 383 | 0.04 |
| Outpatient care | 199 (326) | 134 (191) | 65 | 0.04 |
| Community care | 183 (650) | 86 (319) | 96 | 0.12 |
| PSS | 6 (50) | 7 (66) | –1 | 0.87 |
| 26 weeks | ||||
| Inpatient care | 180 (1493) | 61 (508) | 118 | 0.39 |
| Outpatient care | 70 (180) | 86 (165) | –15 | 0.45 |
| Community care | 177 (593) | 135 (734) | –8 | 0.92 |
| PSS | 0 (0) | 0 (0) | 0 | N/A |
| 52 weeks | ||||
| Inpatient care | 125 (516) | 78 (386) | 46 | 0.41 |
| Outpatient care | 106 (421) | 80 (168) | 26 | 0.50 |
| Community care | 138 (544) | 191 (847) | –52 | 0.55 |
| PSS | 2 (18) | 2 (17) | –0.1 | 0.97 |
The differences in resource use are due to chance and are not statistically significant. Nevertheless, we account for the difference in baseline values in the analysis of the total costs. Post surgery, TAR patients used more resources than ankle fusion patients, except for community care use at 52 weeks, which was higher in the ankle fusion arm. The only statistically significant differences were in inpatient care and outpatient care costs at 12 weeks: the costs were higher in the TAR arm than in the ankle fusion arm.
Societal costs
Societal costs include equipment, mobility aids and home adaptations that were paid out of pocket, loss of earnings due to time off work, family and friends’ time and transportation costs. These are summarised in Table 23. The costs are shown for participants with complete cost data collection.
| Cost category | Cost (£), mean (SD) | Mean difference | p-value | |
|---|---|---|---|---|
| TAR arm (N = 129) | Ankle fusion arm (N = 135) | |||
| Baseline | ||||
| Equipment, mobility aids and home adaptations | 30.29 (330.17) | 24.55 (191.40) | 5.74 | 0.86 |
| Loss of earnings | 233.33 (776.65) | 228.32 (842.86) | 5.01 | 0.96 |
| Family and friends’ time | 535.00 (1625.31) | 606.33 (1482.53) | –68.32 | 0.72 |
| Transportation costs | 4.29 (17.89) | 7.10 (34.36) | –2.80 | 0.41 |
| 52 weeks post surgery | ||||
| Equipment, mobility aids and home adaptations | 7.35 (48.54) | 7.58 (34.81) | –0.23 | 0.96 |
| Leave (TAR, n = 40; ankle fusion, n = 45) | 2807.07 (1343.37) | 2946.77 (1283.84) | –139.70 | 0.63 |
| Loss of earnings (TAR, n = 40; ankle fusion, n = 45) | 683.69 (2087.20) | 1034.92 (2974.46) | –351.22 | 0.27 |
| Family and friends’ time | 1716.22 (3340.69) | 3707.916 (6403.59) | –1990.95 | 0.00 |
| Transportation costs | 8.69 (24.01) | 11.04 (45.69) | –2.34 | 0.60 |
The differences at baseline are due to chance and are not statistically significant. We assumed that all patients who were employed part or full time at baseline had to take 6 weeks of leave because of the surgery. This assumption was based on the data collected on the duration of immobilisation. This resulted in lost earnings of £2807.07 in the TAR arm and £2946.77 in the ankle fusion arm. A total of 85 participants were employed, 40 in the TAR arm and 45 in the ankle fusion arm. The average loss of earnings over 52 weeks was £683.69 in the TAR arm and £1034.92 in the ankle fusion arm. This difference was not statistically significant (p = 0.27). Two patients in the TAR arm and three patients in the ankle fusion arm had to retire because of their ankle problem. The out-of-pocket spending on equipment, aids and adaptations, and the transportation costs were similar in both arms. Participants in the ankle fusion arm were using more help from their family or friends than those in the TAR arm, which resulted in a higher cost of family’s and friends’ time. On average, this cost £1716.22 in the TAR arm and £3707.92 in the ankle fusion arm. This difference is statistically significant (p = 0.00). Patients in the TAR arm used, on average, 9 hours of their friends’ or family’s time; patients in the ankle fusion arm used, on average, 20 hours.
Total costs
Table 24 summarises the cost components discussed above and presents the total costs by treatment arm from the NHS and PSS perspective.
| Cost component | Cost (£), mean (SD) | Mean difference | p-value | Bootstrap 95% CI | |
|---|---|---|---|---|---|
| TAR arm (n = 129) | Ankle fusion arm (n = 135) | ||||
| Surgery | 9488.61 (3107.47) | 7258.51 (5281.82) | 2230.10 | 0.00 | 1024.22 to 3102.77 |
| Health-care resource use over 52 weeks | 1689.82 (3620.35) | 1047.59 (1591.92) | 642.24 | 0.06 | –18.21 to 1302.68 |
| Concomitant medications | 676.60 (839.68) | 893.09 (1370.38) | –216.49 | 0.12 | –512.23 to 38.97 |
| Mobility aids and home adaptations provided by PSS | 1.54 (6.84) | 18.94 (207.07) | –17.39 | 0.34 | –68.33 to 1.26 |
| Total cost unadjusted | 11,856.59 (5549.61) | 9218.13 (5992.82) | 2638.45 | 0.00 | 1191.20 to 3942.11 |
| Total cost adjusted | 11,824.76 | 9248.55 | 2576.21 | 0.00 | 1181.39 to 3988.13 |
The total costs are reported for participants with complete cost data collection; therefore, these values differ from those in Table 21 as it reported costs for all patients. The total cost of TAR from the NHS and PSS perspective was £2638.45 higher than the total cost of ankle fusion. This is statistically significant (p = 0.00). When we adjusted for baseline values and minimisation factors, the difference was reduced slightly to £2576.21 and was statistically significant. The main driver for the cost difference was the cost of surgery, which was £2230.10 higher in the TAR arm than in the ankle fusion arm. Other differences in cost components were not statistically significant.
We conducted a subgroup analysis of total costs based on the type of TAR implant used (fixed bearing vs. mobile bearing) as there were differences noted in the statistical analysis. The results are presented in Table 25.
| Measure | Ankle fusion arm (n = 131) | TAR arm | |
|---|---|---|---|
| Fixed bearing (n = 72) | Mobile bearing (n = 61) | ||
| Total cost (unadjusted) | 9222.44 (6071.35) | 10,868.10 (3458.01) | 12,841.07 (7088.72) |
| Total cost (adjusted) | 9241.65 | 10,878.31 | 12,787.77 |
| Mean difference | – | 1636.66 | 3578.71 |
| Bootstrap 95% CI | – | 243.09 to 2824.29 | 1744.83 to 5889.34 |
| p-value | – | 0.014 | 0.000 |
The total costs in the TAR arm were higher than those in the ankle fusion arm; however, the total cost in the mobile-bearing TAR group was higher than that of both the ankle fusion arm and the fixed-bearing TAR group.
Quality-adjusted life-years
Quality-adjusted life-years were the outcome in the cost–utility analysis. We present unadjusted and adjusted difference in QALYs by treatment arm in Table 26.
| Analysis | QALYs at 52 weeks | Mean difference | p-value | Bootstrap 95% CI | |
|---|---|---|---|---|---|
| TAR arm (n = 135) | Ankle fusion arm (n = 141) | ||||
| Unadjusted | 0.68 (SD 0.15) | 0.65 (SD 0.17) | 0.03 | 0.09 | –0.004 to 0.07 |
| Adjusted for baseline values | 0.68 | 0.66 | 0.02 | 0.14 | –0.008 to 0.05 |
The QALY is an outcome measure that combines quantity and QoL; 1 year in perfect health is equal to 1 QALY. Patients in the TAR and ankle fusion arms had, on average, 0.68 and 0.65 QALYs, respectively. The difference between the arms was not statistically significant. Adding minimisation factors to the model did not change the result.
We conducted a subgroup analysis by TAR implant type. The results are presented in Table 27.
| Measure | QALYs at 52 weeks | ||
|---|---|---|---|
| Ankle fusion arm (n = 137) | TAR arm | ||
| Fixed bearing (n = 76) | Mobile bearing (n = 64) | ||
| Unadjusted | 0.65 (0.17) | 0.69 (0.14) | 0.67 (0.15) |
| Adjusted | 0.66 | 0.69 | 0.66 |
| Mean difference | – | 0.04 | 0.006 |
| Bootstrap 95% CI | – | –0.004 to 0.07 | –0.03 to 0.04 |
| p-value | – | 0.053 | 0.754 |
The mobile-bearing TAR group did not differ from the ankle fusion arm in terms of QALYs (p = 0.754). By contrast, there was some evidence that fixed-bearing TAR generated more QALYs than ankle fusion (0.69 vs. 0.66, respectively); however, it would not be considered statistically significant at the conventional significance level of 5% (p = 0.053).
Cost–utility analysis
Primary within-trial analysis
Although orthopaedic surgery lasts patients for many years, if we analyse the data over the 52 weeks of the study, the mean incremental cost per QALY gained was £127,931.50 from the NHS and PSS perspective. Using the bootstrapping technique, we generated an empirical distribution of ICERs and presented them on the cost-effectiveness plane (Figure 5).
FIGURE 5.
Cost-effectiveness plane: ITT, NHS and PSS perspective.
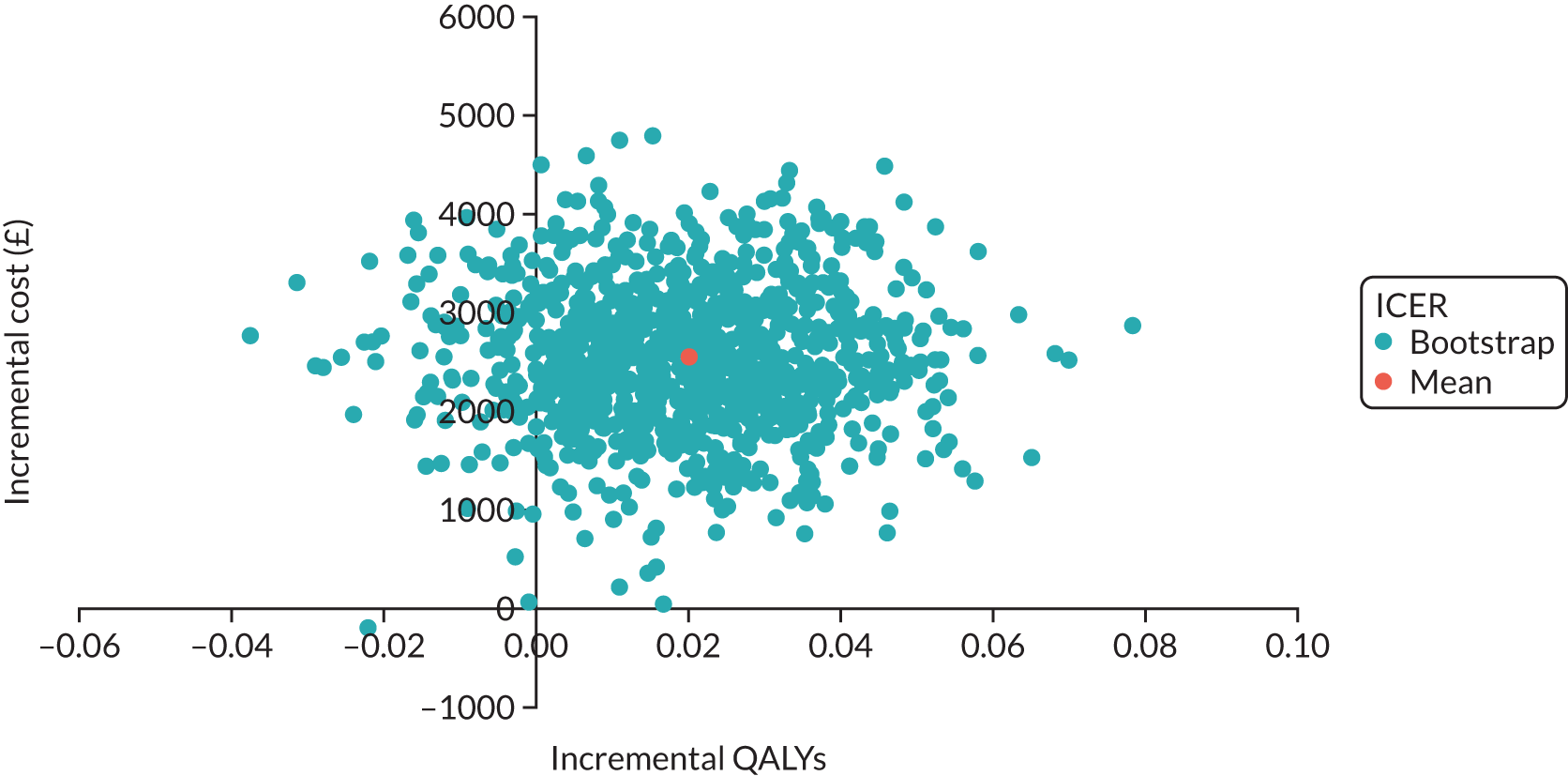
Virtually all ICERs are above the x-axis; therefore, TAR is more expensive than ankle fusion. However, with respect to QALYs, most ICERs suggest that TAR generates more QALYs than ankle fusion. However, some ICERs are on the negative side of the x-axis, implying that TAR generates fewer QALYs than ankle fusion. Hence, there is a high degree of uncertainty in the data. In total, 95% of iterations fall between –£253,647.40 and £182,814.20, implying that TAR may be more expensive or cost-saving.
We also used the bootstrapping results to estimate the probability of TAR being cost-effective compared with ankle fusion at various cost-effectiveness thresholds. The probability is low: 1.3% at the threshold of £30,000 per QALY gained. The probability increases and reaches 37.6% at the threshold of £100,000 per QALY gained (Figure 6).
FIGURE 6.
Cost-effectiveness acceptability curve: ITT, NHS and PSS perspective.
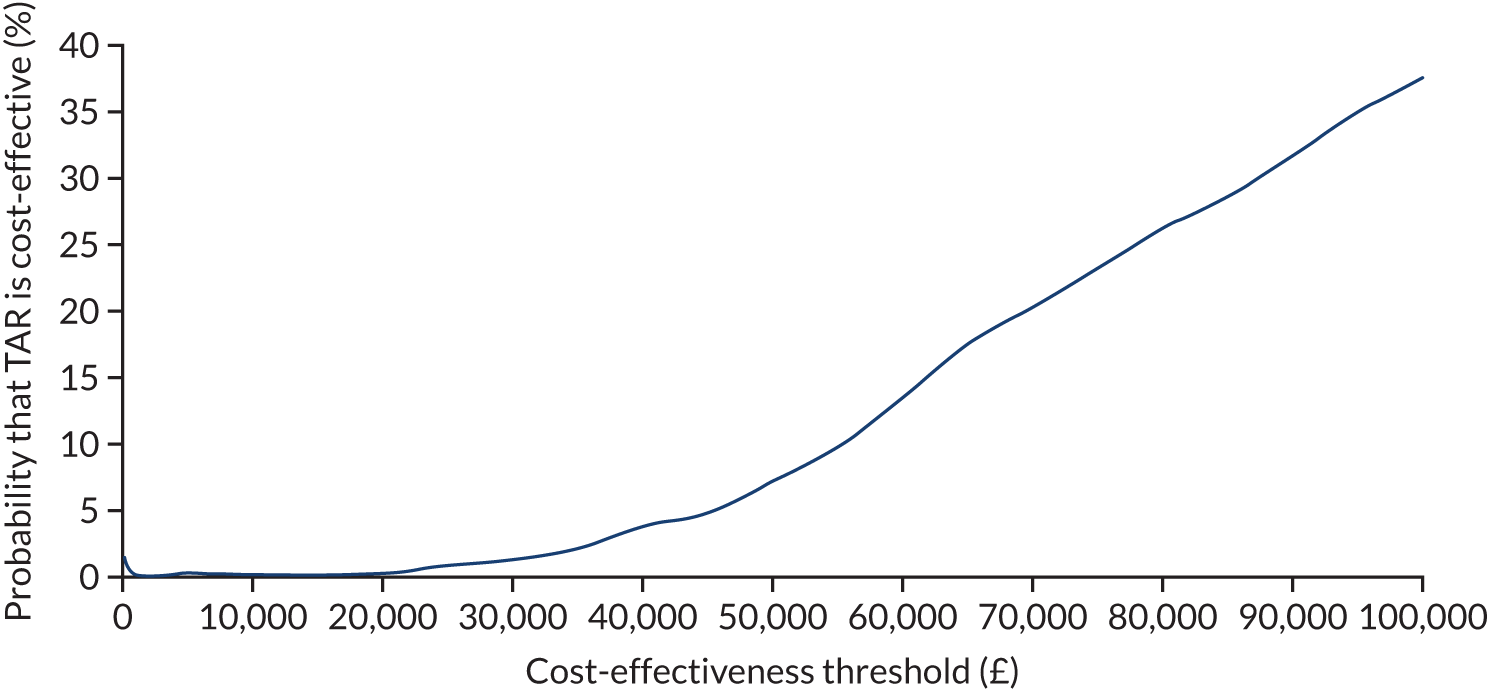
It is important to note that the benefits of the surgery begin after the 52-week window. Therefore, the results need to be interpreted with caution and we conducted long-term economic modelling to account for this.
Sensitivity analysis
The total societal costs were £15,142.95 (SD £7820.42) for TAR and £14,961.09 (SD £9978.75) for ankle fusion. The unadjusted mean difference was £181.86. This difference was not statistically significant. When we adjusted for baseline values and minimisation factors, the mean difference was £198.55. The difference in costs between the two arms was lower than that in the NHS and PSS perspective and it lost significance.
Over 52 weeks, the mean incremental cost per QALY gained was £9927.50 from the societal perspective. This ICER is considerably lower than the base-case result and would be recommended under NICE’s threshold of £20,000–30,000 per QALY gained. 65 However, costs from the societal perspective introduced considerable uncertainty as they were difficult to estimate. Using the bootstrapping technique, 95% of iterations fall between –£41,024.23 and £184,899.90. The cost-effectiveness plane shows that the ICERs can be in any quadrant of the plane, which implies a high degree of uncertainty (Figure 7).
FIGURE 7.
Cost-effectiveness plane: ITT, societal perspective.
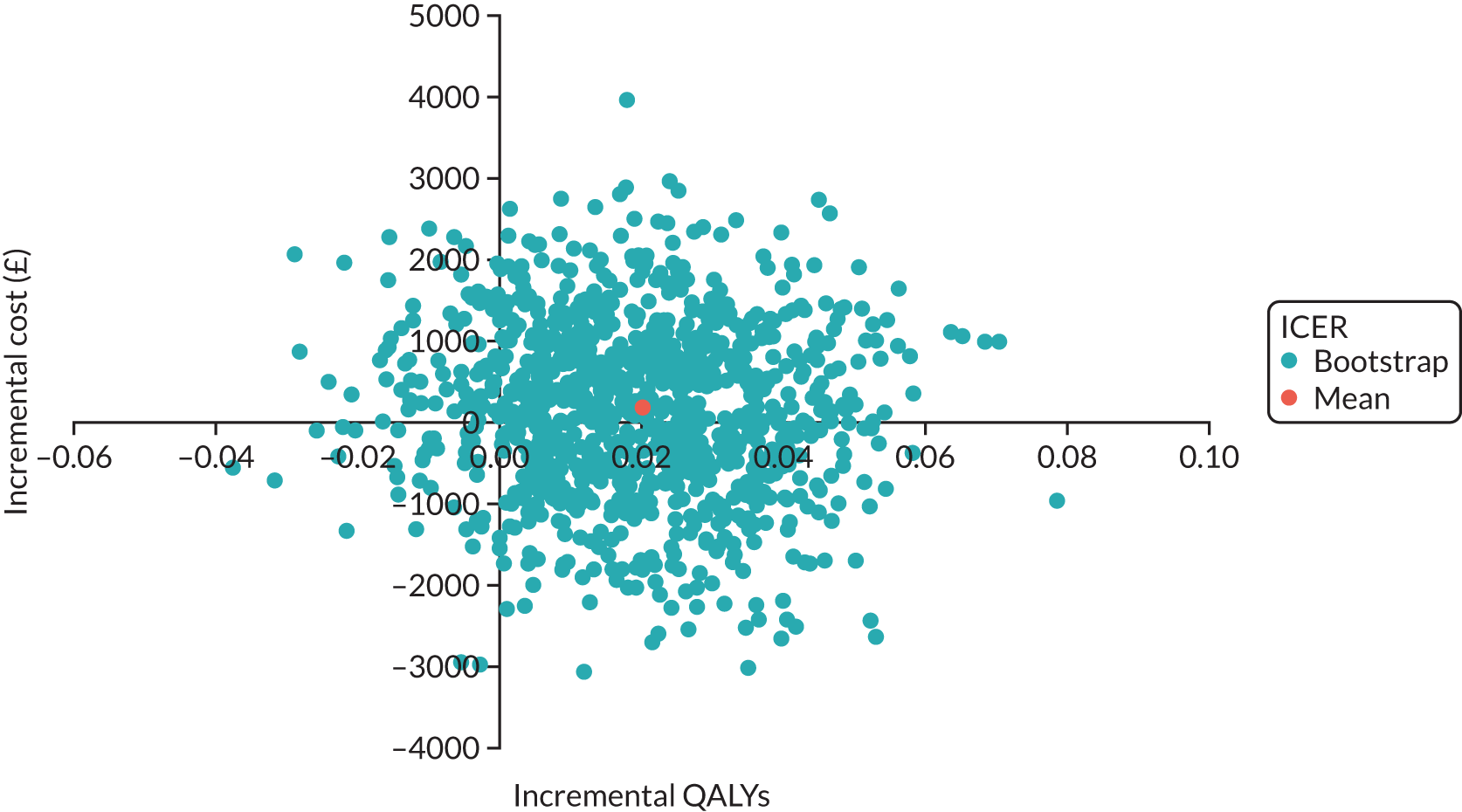
The CEAC shows that the probability of TAR being cost-effective compared with ankle fusion at £30,000 per QALY gained is 61.6% and increases to 80.6% when the threshold increases to £100,000. The CEAC is shown in Figure 8.
FIGURE 8.
Cost-effectiveness acceptability curve: ITT, societal perspective.
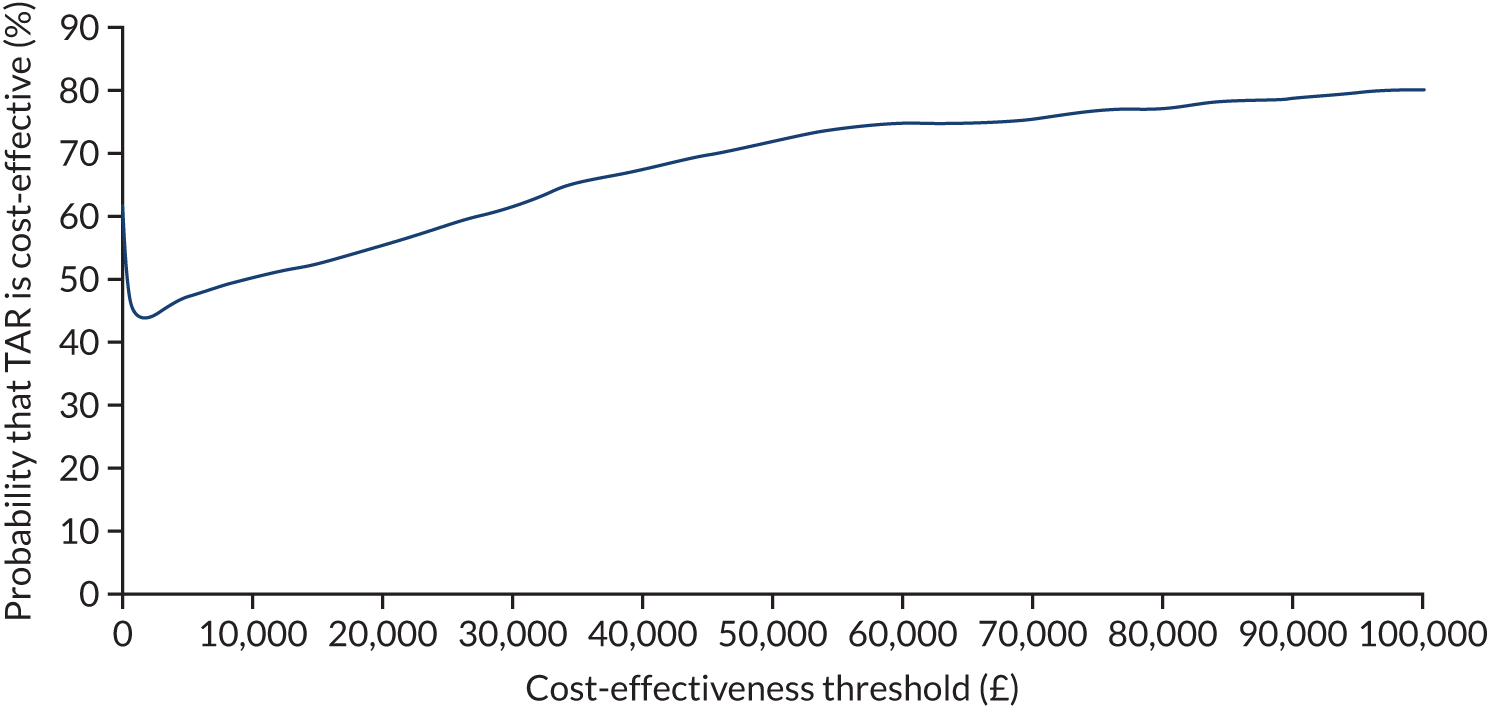
The probability is equal to about 60% when the cost-effectiveness threshold is zero because 60% of ICERs on the cost-effectiveness plane suggest that TAR is cost-saving compared with ankle fusion.
When we included the cost of replacing an employee, total societal costs were £15,616.05 (SD £8854.13) for TAR and £15,622.46 (SD £11,071.48) for ankle fusion. The unadjusted difference between arms was small (£6.42) and not statistically significant (p-value 0.996). Adjusting for the baseline values and minimisation factors did not change the result. We applied this cost to patients who retired after the surgery and reported that they retired because of their ankle problem. There were two such patients in the TAR arm and three in the ankle fusion arm.
The per-protocol analysis resulted in very minor differences in total costs and QALYs. The ICER was £127,154.60 per QALY gained, which is a lot higher than the threshold used by NICE. 65 In total, 95% of bootstrap values fall between –£165,764.40 and £654,921.60. The cost-effectiveness plane shows that TAR is more expensive than ankle fusion, as all ICERs are on the positive side of the y-axis (Figure 9).
FIGURE 9.
Cost-effectiveness plane: per-protocol, NHS and PSS perspective.
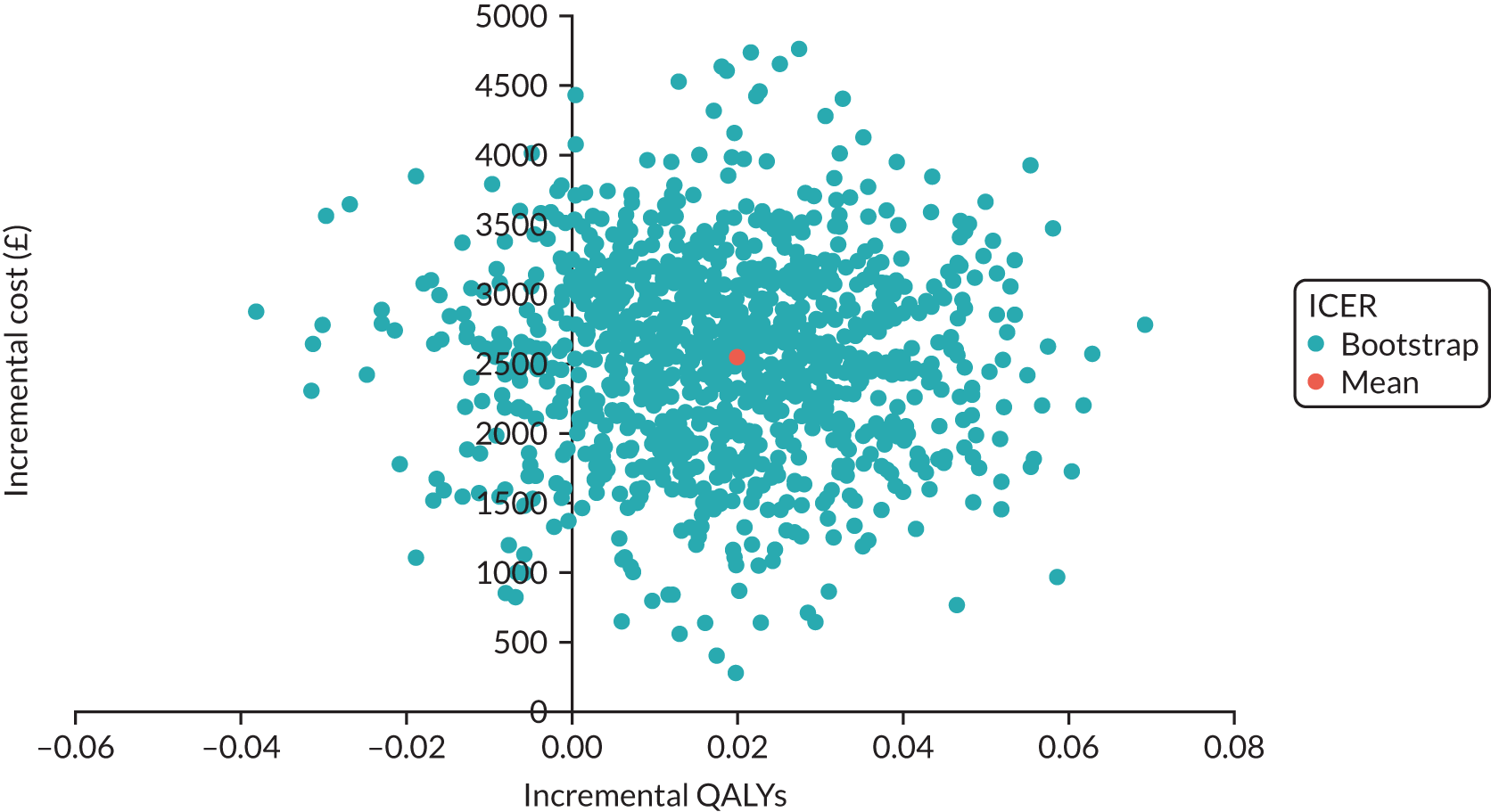
Therefore, the within-trial probability of TAR being cost-effective at 52 weeks was 1% at the cost-effectiveness threshold of £30,000 per QALY gained, increasing to 35.7% if the threshold increases to £100,000 per QALY gained. The CEAC is shown in Figure 10. The results suggest that it is important to account for societal costs when comparing TAR and ankle fusion, as these have a large impact on the ICER. However, societal costs also introduce a high degree of uncertainty. When considering implants and definitive surgery, 52-week data need to be interpreted with caution, as the benefits begin after the 52-week window; hence, the more important analysis relates to longer-term modelling.
FIGURE 10.
Cost-effectiveness acceptability curve: per-protocol, NHS and PSS perspective.
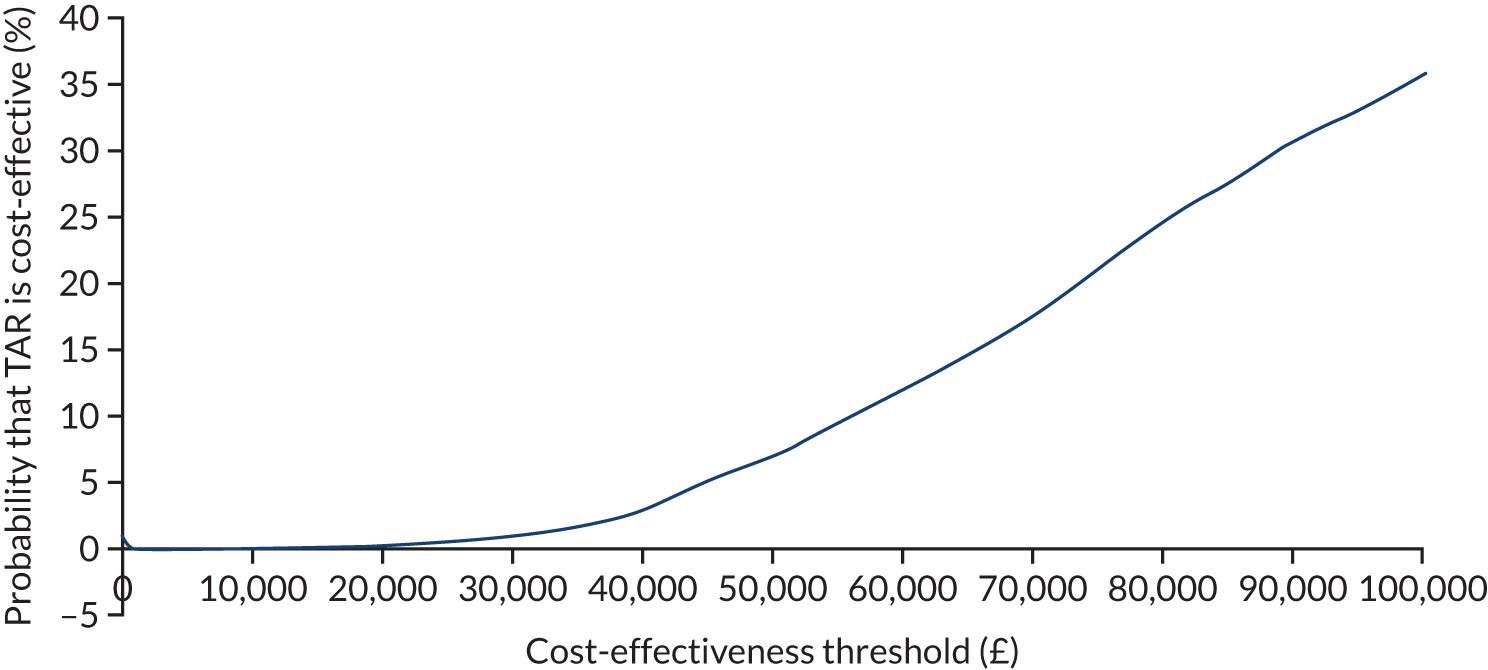
Long-term economic modelling
The model-based analysis suggested that TAR is more expensive than ankle fusion, but generates more QALYs when extrapolated to a lifetime horizon. The ICER was estimated to be £4201.81. Cost and QALY differences are presented in Table 28.
| Measure | TAR arm (n = 129) | Ankle fusion arm (n = 134) | Difference |
|---|---|---|---|
| Total cost (£) | 2,138,343 | 1,878,140 | 260,202 |
| Total QALYs | 1110 | 1048 | 61 |
The result of Monte Carlo simulation (n = 5000) is presented in the cost-effectiveness plane in Figure 11.
FIGURE 11.
Cost-effectiveness plane: lifetime horizon.
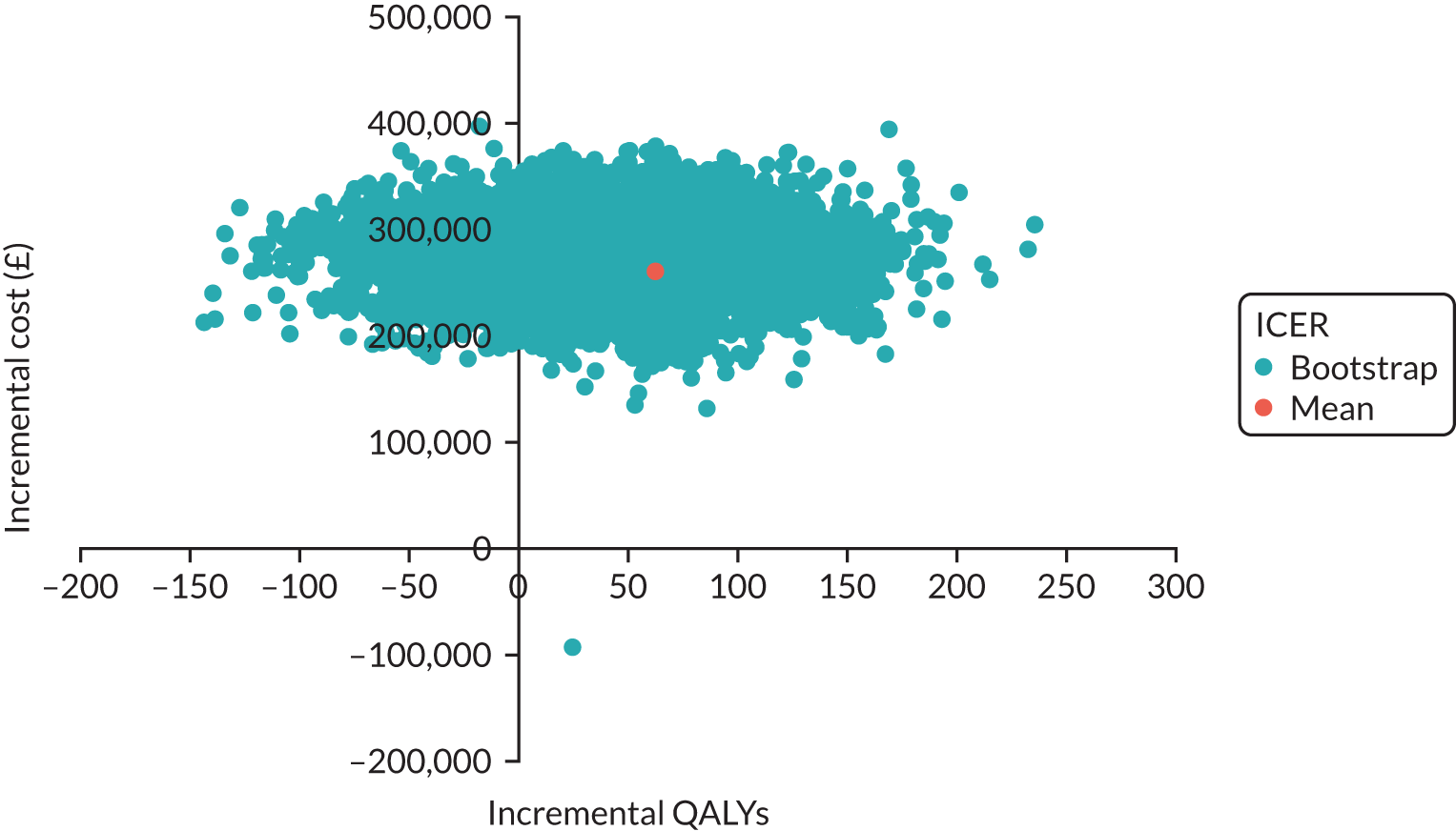
The mean ICER is in the north-east quadrant. This means that TAR is more expensive and also generates more QALYs than ankle fusion. When we varied cost and QoL parameters in the model, we observed that most points still lay in the north quadrant. Therefore, TAR is likely to be more expensive than ankle fusion over the lifetime horizon. However, there was uncertainty regarding the number of QALYs attained as some ICERs were in the north-west quadrant, implying that TAR may generate fewer QALYs and be more expensive than ankle fusion. Hence, there is considerable uncertainty around the lifetime ICER, and longer-term data are required to obtain a more robust result.
Although there is uncertainty, over the lifetime horizon there was a 69% probability that TAR is cost-effective compared with ankle fusion at the cost-effectiveness threshold of £20,000 per QALY gained (Figure 12).
FIGURE 12.
Cost-effectiveness acceptability curve: lifetime horizon.

As seen in the statistical and economic analysis, the fixed-bearing TAR group performed better than the mobile-bearing TAR group and the ankle fusion arm. If we assume that all patients receive fixed-bearing TAR and assign their QoL to all TAR patients, the difference in QALYs between the two arms increases (Table 29).
| Measure | TAR arm: fixed-bearing group (n = 129) | Ankle fusion arm (n = 134) | Difference |
|---|---|---|---|
| Total cost (£) | 2,138,343 | 1,878,140 | 260,202 |
| Total QALYs | 1150 | 1048 | 102 |
Total ankle replacement is still more expensive and generates more QALYs than ankle fusion. The ICER was £2535.32. When we conducted a PSA for this result, the probability of TAR being cost-effective compared with ankle fusion was 72.2% at the cost-effectiveness threshold of £20,000 per QALY gained.
Chapter 5 Discussion
To the best of our knowledge, this is the first RCT to compare TAR with ankle fusion for patients with end-stage ankle osteoarthritis. This was a pragmatic, multicentre, parallel-group, non-blinded RCT that aimed to ensure that the outputs were generalisable and focused on the needs of patients and the public. Our aim was to determine the clinical effectiveness and cost-effectiveness of two recognised treatments for end-stage ankle arthritis in patients aged between 50 and 85 years.
There was a significant improvement in the primary outcome measure at 52 weeks after surgery in both the TAR and ankle fusion arms, with the TAR arm improving, on average, by 49.9 (30.7) points and the ankle fusion arm improving by 44.4 (31.9) points. Although the proportion of patients who met the MID of 12 points was higher in the TAR arm than in the ankle fusion arm (82% vs. 80%, respectively), the adjusted difference in MOXFQ walking/standing domain scores of –5.56 (95% CI –12.49 to 1.37) was not statistically significantly different (p = 0.12). Therefore, we have shown that there was no greater improvement in the TAR arm than in the ankle fusion arm at 52 weeks, whether analysed by ITT or per protocol. At 26 weeks, the adjusted difference in MOXFQ walking/standing domain score was –8.21 (–15.4 to –1.27), which was statistically significant (p = 0.02), but this difference had reduced by 52 weeks.
The difference between TAR and ankle fusion in the change in FAAM-ADL scores at 52 weeks was statistically significant (p = 0.01), with both arms showing substantial improvements from baseline and a difference of 6.16 (95% CI 1.54 to 10.78) between the arms. The changes in EQ-5D-5L index values between the two treatment arms were not significantly different at 26 weeks (p = 0.08) or 52 weeks (p = 0.32). The EQ-5D-5L VAS was statistically significant at 26 weeks (p = 0.03), but not at 52 weeks (p = 0.07).
The arms were similar at baseline in terms of age, sex, comorbidity and clinical scores. Owing to chance, some differences were noted, such as a slightly larger number of obese patients in the ankle fusion arm than in the TAR arm, and more patients who had deformity or previous internal fixation for trauma in the TAR arm than in the ankle fusion arm. We do not, however, believe these differences to be material. Participants appeared to be equally distributed between treatment arms with regard to the minimisation factors, that is the presence of osteoarthritis in the subtalar or talonavicular adjacent joints. The planned subgroup analysis did not identify a significant interaction between the treatment effect and the presence of arthritis in one or both adjacent joints at the 52-week time point, nor did we find a significant interaction with age.
Most studies that have compared TAR and ankle fusion to date have been observational. Daniels et al. 20 looked at 281 TARs and 107 ankle fusions and found comparable outcome scores between the two surgeries at a mean follow-up of 5.5 years. In their study, which was not randomised, patients treated with ankle fusion were younger, more likely to be diabetic, less likely to have inflammatory arthritis and more likely to be smokers than those treated with TAR. 20 Veljkovic et al. 21 analysed 88 TARs and 150 ankle fusions at a follow-up of 3.6 years and found that ankle fusion had comparable clinical outcomes to TAR for patients with non-deformed end-stage ankle arthritis.
Attempts have been made to run RCTs in this area. Norvell et al. 66 reported a prospective study in which 386 TARs were compared with 93 ankle fusions. Although it was designed at the outset as an RCT, patients were unwilling to agree to randomisation, which forced a change from a RCT to a cohort design. This led to an imbalance in the study arms, with differences in baseline characteristics. At 2 years, the study showed both treatments to be effective, with a difference in FAAM-ADL score between TAR and ankle fusion of 9 points. 66 This compares favourably with our study, which showed a difference in FAAM-ADL score of 6.16 (1.54 to 10.78) between TAR and ankle fusion at 52 weeks.
In this trial, 54% of TAR and 53% of ankle fusion patients experienced at least one AE during the trial, although the vast majority were medical complications unrelated to the type of surgery. It is difficult to compare this finding with the literature, which reports only implant-specific complications.
We did not find a difference between the TAR and ankle fusion arms in terms of the risk of patients experiencing an AE overall, but we did find differences in the types of AEs. A total of 19 (13.4%) patients in the TAR arm and eight (5.7%) in the ankle fusion arm had wound-healing problems, although only one patient in the ankle fusion arm required a reoperation as a result. Six patients (4.2%) in the TAR arm and one (< 1%) in the ankle fusion arm had nerve injuries.
There were fewer patients with thromboembolic events in the TAR arm than in the ankle fusion arm [four (2.9%) vs. seven (4.9%), respectively], which might be explained by prolonged immobilisation. Two patients in the ankle fusion arm had multiple events (two events each). There were no fatal pulmonary embolism events. There are sparse comparative data on thromboembolism following ankle surgery. Although the incidence of thromboembolism has been reported as low,67 Hospital Episode Statistics-based studies are confounded because deep-vein thrombosis invariably does not lead to admission,68 meaning that data are not captured in national databases. In our study, 98% of patients received chemical or mechanical prophylaxis, so our data provide pragmatic figures of thromboembolic risk.
It is important to state, however, that, to the best of our knowledge, there are very few papers that report the complications of ankle fusion and none that have compared the complications of both treatments in a randomised cohort. Glazebrook et al. 69 classified complications following TAR in terms of risk to implant survival, referring to high-grade complications such as deep infection, medium-grade complications such as subsidence and low-grade complications such as intraoperative fractures and wound-healing issues. The higher the grade, the more likely the complication would result in implant failure. Gadd et al. 70 later suggested a simpler classification of high- and low-grade complications. We have adapted these classifications to enable the comparison with ankle fusion, which, to the best of our knowledge, has not been undertaken in a randomised trial before now.
There were five further unplanned reoperations other than revisions in the TAR arm and four in the ankle fusion arm. Although only one revision procedure took place within the 52-week window, we are aware of at least four patients who will require revision (TAR, n = 1; ankle fusion, n = 3).
Robust outcome studies on ankle fusion are sparse, but the risk of an ankle fusion going on to non-union has previously been estimated to be between 7.8%71 and 10%. 13 In this study, there were 17 non-unions (12%), which were diagnosed by the presence of a lucent line on plain radiographs at the 52-week follow-up. Seven of these patients had no symptoms whatsoever; hence, 10 (7%) of the 140 patients who received ankle fusion went on to symptomatic non-union. Although none of the non-unions were revised in the first 52 weeks following surgery, it is likely that the 10 symptomatic patients may go on to have further investigation and revision surgery.
Haddad et al. ’s13 meta-analysis of the literature showed that TAR and ankle fusion have similar intermediate-term outcomes for clinical scores, patient satisfaction, complications and revision rate, although they did not include any studies that directly compared TAR with ankle fusion. A more recent systematic review and meta-analysis comparing TAR with ankle fusion showed no statistically significant difference between the groups, but commented on significant methodological flaws and the heterogeneity of outcome measures. 72
In clinical practice in the UK, the most common implant type currently used is a fixed-bearing prosthesis, with a > 70% market share. 22 The shift from three-component mobile-bearing prostheses to two-component fixed-bearing prostheses has taken place over the last 5 years during the trial. As a result, it was important for us to perform a post hoc analysis of mobile-bearing and fixed-bearing TAR compared with ankle fusion.
In this trial, 54% of patients in the TAR arm received a fixed-bearing prosthesis and 46% received a mobile-bearing prosthesis. We found an adjusted difference in MOXFQ walking/standing score of 2.1 points (95% CI –6.6 to 10.8 points; p = 0.64) between mobile-bearing TAR and ankle fusion, which suggests that, on average, patients who received mobile-bearing TAR had MOXFQ walking/standing scores 2.1 points higher than those in the ankle fusion arm at 52 weeks post surgery.
In the assessment of fixed-bearing TAR, we found an adjusted difference in MOXFQ walking/standing score of –11.1 (95% CI –19.3 to –2.9; p = 0.008) between fixed-bearing TAR and ankle fusion, which suggests that, on average, patients who received fixed-bearing TAR had MOXFQ walking/standing scores 11.1 points lower than those in the ankle fusion arm at 52 weeks post surgery. This difference was statistically significant (p = 0.008) and we believe this to be clinically meaningful, especially as the FAAM-ADL score also showed a statistically significant improvement between baseline and 52 weeks.
It appears that, when fixed-bearing TAR is compared with ankle fusion, TAR outperforms ankle fusion based on our primary outcome measure, a finding that was not apparent when assessing mobile-bearing TAR as a separate group. In the ankle fusion arm, 60% of patients underwent an arthroscopic approach, but the results for the ankle fusion arm appeared to be similar whether or not an open or an arthroscopic technique was used.
Recruitment
Our aim was to recruit one patient per centre per month. Overall, the recruitment rate achieved was 0.46 patients per centre per month. The lead site achieved a recruitment rate of 1.7 patients per month and the other 16 sites achieved an average recruitment of 0.38 patients per month. There were several challenges to recruitment, which is not unusual for surgical trials. A qualitative study was conducted that identified four common obstacles: (1) patient preference for an intervention, (2) a complex recruitment pathway, (3) logistical issues and (4) lack of equipoise and role conflicts. Clinicians in the study felt that they could predict that specific patients may achieve better outcomes with either TAR or ankle fusion.
A total of 22 (7.3%) randomised patients were excluded from our ITT analysis, which was well within the anticipated 10% drop-out rate from our power calculation. Our trial attrition is similar to that of other reported orthopaedic trials, which had attrition rates of between 5.3% and 18.2%. 73–75 The original sample size calculation for the TARVA trial made a number of assumptions. Based on the data available now, loss to follow-up at 52 weeks was slightly lower than predicted: 9% rather than 10%. The number of recruiting surgeons was 34 rather than 17, so the average number of patients per surgeon was nine rather than 14. Therefore, the power achieved with our 276 patients who had data available at 52 weeks for the ITT analysis was > 88%, very close to our desired power of 90%. The slightly lower power achieved is unlikely to have influenced our conclusions.
Economic evaluation
Over the first 52 weeks following primary surgery, TAR was more expensive than ankle fusion, which was expected owing to the higher prices of the implants and longer duration of the surgery. However, after accounting for other costs associated with the surgery, including mobility aids and home adaptation, productivity loss and transportation cost, the difference between the two arms was no longer statistically significant and the ICER reduced considerably. This suggests that TAR is likely to have a wider impact on patients’ lives that is not accounted for in the effectiveness and QoL measures.
To the best of our knowledge, there is sparse published health economic data regarding the cost-effectiveness of ankle surgery. Slobogean et al. 16 estimated index values in patients after TAR and ankle fusion using a prospective non-randomised cohort of TAR and ankle fusion patients. Their baseline values were higher than those of the TARVA trial for both TAR (0.67, 95% CI 0.64 to 0.69) and ankle fusion (0.66, 95% CI 0.63 to 0.68). At 52 weeks, their index values (TAR 0.73, 95% 0.71 to 0.76; ankle fusion 0.73, 95% 0.70 to 0.76) were comparable with those of the TARVA trial (TAR 0.74, 95% CI 0.70 to 0.77; ankle fusion 0.71, 95% CI 0.67 to 0.74). In their cohort, the authors were unable to account for medical comorbidities. 16
Extrapolating the results further than 1 year after the surgery is common in the orthopaedic literature. 76–78 Two models have been used to explore the cost-effectiveness of TAR compared with ankle fusion. 60,62 SooHoo and Kominski62 implemented a simple decision-tree model, which suggested that TAR had the potential to be cost-effective compared with ankle fusion if the implant survived more than 7 years, but these data were obtained when TAR surgery was in its infancy. Courville et al. 60 built a Markov model and, at the lifetime horizon, showed the cost-effectiveness of TAR compared with ankle fusion in a similar hypothetical cohort of patients aged 60 years with end-stage ankle osteoarthritis. The researchers highlighted the lack of data on the QoL of these patients and the requirement for more detailed estimates of both direct and indirect medical costs.
Our study provided the index values using the EQ-5D-5L at baseline, and at 26 and 52 weeks, which allowed us to estimate QALYs and detailed cost estimates from both health-care and PSS, and societal perspectives. Therefore, the results of the model-based analysis are more robust than those of existing studies and we estimate a 69% probability of TAR being cost-effective compared with ankle fusion at the NICE cost-effectiveness threshold of £20,000 per QALY gained. 65 This increases to 72.2% probability when comparing fixed-bearing TAR implants with ankle fusion.
Patient and public involvement
This study had a significant impact on the patients and members of the public who were involved at all stages of the trial. Almost all patients asked to be kept informed, and high-quality newsletters were developed and sent out at regular intervals, summarising recruitment updates and featuring interviews with the research and oversight team and educational insights. More than 1350 people followed the TARVA trial’s Twitter account (@TARVA_Trial, twitter.com; Twitter, Inc., San Francisco, CA, USA). One patient recorded a video log (vlog) for their own blog channel and many patients remain in communication with the trial team. Following publication, the authors intend to present the results of the study widely and work closely with relevant charities to relay the findings to their members.
Limitations
The limitations relate to the pragmatic nature of this study. There is always a conflict between pragmatic studies and perceived robustness. It could be argued that the arms were too heterogenous because surgeons were allowed to use any implant for TAR and any technique for ankle fusion. However, a design in which surgeons used only one implant and one ankle fusion technique would be logistically difficult, especially across sites, and far less generalisable.
A further limitation relates to the use of a patient-reported outcome as the primary outcome measure, which may be insensitive to a clinically meaningful outcome even if one were present. There are many methods used to assess patient-reported outcome measures. In anchor-based methodology, the outcome of interest is ‘anchored’ to someone’s clinical judgement, typically that of a patient or clinician, to define the important difference. In a distribution-based methodology, two approaches are used. The first looks at measurement error and tries to find a consistent difference that patients would consider meaningful and that is also greater than the imprecision of the measurement. The second distribution approach uses a ‘rule of thumb’; for example, a 10% change may be considered important.
The magnitude of the target difference on a standardised scale (standardised effect size) is commonly used to infer the value of detecting this difference in comparison with other possible standardised effects. 79,80 Cohen’s d has been used as de facto justification for this, using a standardised effect size of 0.2, 0.5 and 0.8 for small, medium and large effects, respectively. 81
For our primary outcome measure, the MOXFQ walking/standing domain, there had been no previous RCTs and, hence, the literature used pertained to studies published by the author of the tool. Dawson et al. evaluated the utility of the measure in several cohorts of patients with forefoot, midfoot and hindfoot disorders. 31,32,46,82–84 These studies looked at the change in score from baseline to post surgery. Two main approaches were used to estimate the smallest change on the measure that was likely to be meaningful or important. The first approach was distribution based, that is based on the statistical characteristics of the sample under study. Examples include the effect size, the standard error of measurement and the minimal detectable change. This approach aimed to identify the smallest change for an individual that is beyond the measurement of error of a given instrument and therefore likely to represent a true change. Although Dawson et al. ’s 2007 paper46 looked at hallux valgus, later papers31,32,82–84 specifically studied surgical ankle procedures as a subgroup, estimating the MID to be 10.67 for the MOXFQ walking/standing domain.
Based on this information, we determined that it was important to detect a difference of 12 in the change in MOXFQ walking/standing domain score from baseline between the two treatment arms; it was on this premise that our power calculation was performed. Cohen’s d for a small, medium and large effect size would be between 6 and 24 points based on the SD in our series, which is not too different from the MID determined by Dawson et al. 31,32,46,82–84 More than 80% of patients in our study achieved the MID when comparing their pre-surgery scores with their postsurgery scores. In fact, they exceeded their MID severalfold, with a mean (SD) improvement for TAR patients of 49.9 (30.7) and 44.3 (31.9) for the ankle fusion patients. However, the difference in the changes between the TAR and ankle fusion arms was 5.56 points, with TAR having, on average, an improvement of 5.56 points more than ankle fusion (because a negative score is better). Our CI for the difference in the improvement was –1.37 to 12.49, which included both the 10.67- and 12-point differences defined by Dawson et al. 31,32,46,82–84 Hence, we cannot rule out this being meaningful. It is important to be aware that the MID of 12 was an estimate only.
Another method for determining clinical importance involves opinion-seeking. A value, a range of plausible values or a prior distribution for the target difference is sought by asking one or more ‘experts’ to state their opinion on what would be an important and/or realistic value for a difference. It is possible that once patients’ scores have improved by > 40 points from baseline to 52 weeks an additional 5.56 points may still be clinically relevant. However, on the basis of our estimated MID of 12, overall, the current study showed no significant difference between the groups in our primary outcome measure at 52 weeks post surgery.
Total ankle replacement is more expensive than ankle fusion at 52 weeks. Resource use for these costs were collected from patients during the trial and hence bias due to missing data and loss to follow-up is limited. We considered the societal perspective and the analysis showed that the difference in costs between TAR and ankle fusion may be lower and not statistically significant. Estimating costs from the societal perspective requires more assumptions, such as the length of time off work for those patients who were employed. Because it was considered that most patients would not be at work, patients were not asked this question directly; therefore, the value was estimated based on the average duration of immobilisation after surgery. The cost of lost productivity was calculated using national average gross hourly earnings,55 accounting for sex. The cost of informal care, which is based on time spent taking care of the patient by family and friends, is difficult to estimate and different approaches are used in the literature. 85 We used national average gross hourly earnings as unit costs.
As joint replacements last for several years, not just 52 weeks, we extrapolated our results to the patients’ lifetime horizon. In this situation, TAR was still more expensive than ankle fusion, but the ICER was low, at £4401.81. The model structure was based on existing literature. However, it was simplified and did not account for, for example, possible below-knee amputation or developing ipsilateral arthritis. Important assumptions were made regarding the revision rate in the ankle fusion arm as data on this in the literature are scarce. Decrements in EQ-5D-5L index values after revision are based on estimates in SooHoo and Kominski,62 as these estimates are the only available source of these data. 62 The model has parameter uncertainty, which we accounted for by conducting a PSA. We made the parameters probabilistic by randomly selecting them from appropriate distributions. The results of the PSA show that there is uncertainty in the QALY estimates, but there is a 69% probability of TAR being cost-effective at the NICE threshold of £20,000 per QALY gained. 65 The model is a simplification of reality and the results may change as new evidence becomes available. However, based on the sensitivity analysis, current results are fairly robust.
Generalisability
We designed the trial with the aim of ensuring that the decision-making streams reflected the usual standard of care as closely as possible. Our 17 centres were widely dispersed across the NHS, including district general hospitals, university teaching hospitals and specialist orthopaedic hospitals. Recruitment was performed by experienced surgeons who chose to use the specific technique that they also used in regular NHS practice. Other than MRI, which is invariably part of standard of care, there were no requirements for extra tests or hospital visits. The use of fixed-bearing TAR implants is now dominant in the NHS, with the National Joint Registry (NJR), which covers England, Wales, Northern Ireland, the Isle of Man and Guernsey, showing that these implants were used in over 70% of cases in 2019. 22 The results should therefore be generalisable to standard NHS care. Although one centre recruited 24% of the total patients, several other centres also recruited well, which enhances the generalisability of the results.
Interpretation
Both TAR and ankle fusion improve patients’ QoL at 1 year, but we have not shown one group to be superior in terms of clinical scores at 52 weeks using either ITT or per-protocol analysis. The TARVA trial is inconclusive in terms of the superiority of TAR, as the 95% CI for the adjusted treatment effect includes both a difference of 0 and the MID of 12, but it can rule out superiority of ankle fusion. Both operations appear to be safe, but there were more wound-healing problems and nerve injuries in the TAR arm than in the ankle fusion arm. Seven per cent of patients in the ankle fusion arm went on to symptomatic non-union and are likely to require revision surgery in the future.
When we excluded mobile-bearing TAR and assessed the most common type of implant in the UK (fixed-bearing TAR, representing a 70% market share), we showed a statistically significant improvement of TAR over ankle fusion, suggesting that fixed-bearing TAR may outperform ankle fusion. However, it is important to point out that this is a post hoc analysis and may be inadequately powered. The reason for using post hoc analysis is that, at the time the study began, fixed-bearing TAR was not used in the UK. In 2014, the study onset was delayed owing to the withdrawal of the most commonly used implant in the UK (the Mobility mobile-bearing implant). Therefore, no Mobility implants were used in this study. Between 2014 and 2019, fixed-bearing implants became the dominant implant used and hence this post hoc analysis was considered essential by the investigators.
Using long-term economic modelling, we estimate that there is a 69% probability of TAR being cost-effective compared with ankle fusion at the NICE cost-effectiveness threshold of £20,000 per QALY gained over a patient’s lifetime. 65 This increases to a 72% probability when analysing fixed-bearing implants against ankle fusion.
Recommendations for research
To the best of our knowledge, this is the first level 1 RCT in this area and we would recommend longer-term follow-up of this important cohort of patients with end-stage ankle arthritis. There is a strong case for continuing follow-up, in particular to study the radiological and clinical progress of these patients, and the need for revision surgery.
Although there is a focus on selecting outcome measures that matter to patients, it is clear that studies such as these have to select MIDs based on observations between baseline and a postsurgery time point. Researchers have assumed that the MIDs within groups are the same as the MIDs between groups when both groups have already improved significantly from their baseline scores. We would recommend that studies explore the sensitivity of clinically important differences to patients in this situation.
Acknowledgements
We would like to acknowledge and thank the following for their contribution to the study.
| Site | Principal investigator | Co-investigators and other site staff |
|---|---|---|
| Royal National Orthopaedic Hospital NHS Trust | Andrew J Goldberga | Deirdre Brooking, Nicholas Cullen*, Dishan Singh*, Karen Alligan, Paul O’Donnell, Amanda Swann, Shiraz Sabah, Neil Segaren, Shelain Patel, Tom Quick, Michael Khoo, Lydia Milnes, Barry Rose, Karan Malhotra, Ali Najefi, Susanne Spas, Wajid Aslam, Sarah Bolton, Alana Pentlow, Matthew Welck*, Sally Wright, Asef Al-Ani, Luckshmana Jeyasellan, Edmund Ieong, Jagwinder Dhaliwal, Razi Zaidi, Puja Bhatt, Pearl Tawana, Shane Ranawaka and Iva Hauptmannova |
| Aintree University Hospitals NHS Foundation Trust | Andy Molloy | Clifford Butcher*, Phil Ellison, Lyndon Mason, Pearly George and Sharon Griffiths |
| Sheffield Teaching Hospitals NHS Foundation Trust | Mark Davies | Chris Blundell* (main operating surgeon), James Tomlinson, Matthew Barnes, Joanne Badloe, Elizabeth Hurditch, Laura Cockayne, Carol Peel, Angela Green, Julie Walker, Diane Swift, Julie Sorrell, Howard Davies*, Carolyn Chadwick*, Richard Stevens and Rachel Sellars |
| North Bristol NHS Trust | Steve Hepple | Elizabeth Barnett, Steven Barnfield, Ruth Halliday, Ian Winson*, William Harries*, Stephen Lines, Lizzy Shaw, Josephine Morley, Katherine Coates and James Bassett |
| Wrightington, Wigan and Leigh NHS Foundation Trust | Mike Karski | Timothy Clough*, Tariq Karim, Tracey Taylor, Valerie Parkinson, Louise Winter, Claire Hill, Robert Smith*, James Davenport*, Sharon Glynn, Mark Gaskell, Christopher Moore, Maria Moffatt, Caroline Tierney, Ann Birch, Anne Evans, Shannon Briggs and Michelle Lee |
| Newcastle upon Tyne Hospitals NHS Foundation Trust | Malik Siddique | Paulo Torres*, Nicola Ashworth, Katie Merrie, Jayasree Ramaskandhan, Andrew Cutts, Alice Mellan, Heather Hunter, Michelle Bardgett, Sherron Furtado, Heidi McColm, Karen Smith, Victoria Cunningham, Jennifer Baron, Claire Humphrey, Christine Dobb, Nicholas Aitken and Steven Galloway |
| University Hospitals of Derby and Burton NHS Foundation Trust | Steve Milner | Charlotte Downes, Lynsey Havill, Claire Stevens, Tracy Brear, Kayleigh Hunt, Ryan Humphries, Aariana Sohal, Charlene Otieno and Mona Mohamed |
| Royal Surrey NHS Foundation Trust | Paul Halliwell | Kate Jardine, Iwona Kolodziejczyk, Erica Gethen-Smith, Alexander Dinneen and Jadranka Jovanovic and Anthony Sakellariou |
| Cardiff and Vale University Health Board | Rhys Thomas | Helen Hodgson, Cheryl Cleary, Claire Nott, Paul Hodgson*, Jessica Whiteman and Matthew Williams |
| Hull and East Yorkshire Hospitals NHS Trust | Viren Mishra | Charde Naylor, Sarah Wilson, Emma Clarkson, Hemant Sharma, Javed Salim, Lisa Wilson and Kim Dearnley |
| Northumbria Healthcare NHS Foundation Trust | Dave Townshend | Deborah Bunn, Christine Dobb, Norma Murray, Sue Bell, Chris Herriott, Asaad Asaad, Rajesh Kakwani*, Anthony Richardson, Rachel Browell, Nicola McLarty, Gail Waddell, An Murty*, Rumina Begum, Sarah Eastwood, Lindsey Cunningham, Jonathan Coorsh, Caroline Varrall, Elizabeth Corbishley, Benjamin Drake, Nicole Abdul, Laura Clifton, Blair Tweedie and Colin Shaw |
| Norfolk and Norwich University Hospital NHS Foundation Trust | David Loveday | Tracey Potter, Angela Bullough, Elizabeth Saunders, Sue Butters, Kelly Waterfield, George Smith*, Denise Archer, Celia Whitehouse and Helen Piffero |
| Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust | Andrew Bing | Sarah Turner, Megan Hyne, Jayne Edwards, Lisa Burgess-Collins, Barbara Linklater-Jones, Tessa Rowlands, Victoria Darke, Nilesh Makwana*, Christopher Marquis*, Simon Hill*, Tim Knight, Andrea Bailey, Susie Morris, Jean Denton, Theresa Garratt, Rajesh Gilla, Claire Nicholas, Alaine Done, Ciara Egan, John Blackwell, Charlotte Perkins, Thomas Hunter and Catriona Heaver |
| Brighton and Sussex University Hospitals NHS Trust | Stephen Bendall | Julie Newman, Jane Gaylard, Sharon Casey, Dee Mullan, Ben Rogers and Joel Vernois |
| Oxford University Hospitals NHS Foundation Trust | Mark Rogers | Karen Doig, Tamsin Hughes, Constantinos Loizou, Paul Cooke*, Martin Raglan, Claudio Pereira, Arul Ramasamy, Rick Brown* and Edmund Ieong |
| Nottingham University Hospitals NHS Trust | Sunil Dhar | Katie Lee, Martin Raglan* and Hatem Salem |
| Royal Cornwall Hospitals NHS Trust | Michael Butler | Gabbie Young, Jessica Summers, Richard Walter, Robert Walker, Fiona Hammonds, Nicki Devooght-Johnson, Benita Adams, Benjamin Kent and Toby Nisbett |
We would also like to thank:
-
The UCL CCTU –
-
Caroline J Doré (Senior Statistician)
-
Elin Rees (Data Manager)
-
Simon S Skene (Senior Statistician)
-
Michelle Tetlow (Clinical Project Manager)
-
Jeff Round (Health Economist)
-
Claire Thomson (Trial Manager)
-
Philip Bakobaki (Programmer)
-
James Blackstone (Data Manager and Trial Manager)
-
Torsten Chandler (Health Economist)
-
Ekaterina Bordea (Health Economist)
-
Elizabeth L Deane (Clinical Project Manager)
-
Roseanna Hamilton (Data Manager)
-
Miriam Pollard (Data Manager)
-
Sophie Connor (Clinical Project Manager)
-
Kashfia Chowdhury (Statistician)
-
Suzie Cro (Medical Statistician)
-
Rumana Jalil (Trial Manager)
-
Alexa King (Data Manager)
-
Susan Tebbs (Deputy Director)
-
Patrick Muller (Medical Statistician)
-
Dominic Hague (Clinical Project Manager).
-
-
The IDMC –
-
Justin Cobb
-
Mike Hurley
-
Linda Sharples.
-
-
The TSC –
-
Amar Rangan
-
Julia Bradshaw
-
Chris Blundell
-
Stephen Brealey
-
Marion Campbell
-
Paul Cooke
-
Marion Cumbers
-
Mark Davies
-
Caroline J Doré
-
Andrew J Goldberg
-
Damian Griffin
-
Iva Hauptmannova
-
Alison McGregor
-
Steve Morris
-
Nachiappan Chockalingham
-
Hamish Simpson
-
Claire Thomson
-
Nick Welch.
-
-
All participating patients and the data they have provided for successful completion of the trial.
The study was sponsored by University College London.
Contributions of authors
Andrew J Goldberg (https://orcid.org/0000-0002-8650-4503) (Consultant Orthopaedic Surgeon and Visiting Professor) contributed to the study conception and design, the analysis and interpretation of results and draft manuscript preparation.
Kashfia Chowdhury (https://orcid.org/0000-0002-8185-5152) (Medical Statistician) contributed to the analysis and interpretation of results and draft manuscript preparation.
Ekaterina Bordea (https://orcid.org/0000-0002-3772-7049) (Health Economist) contributed to the analysis and interpretation of results and draft manuscript preparation.
James Blackstone (https://orcid.org/0000-0003-4335-5269) (Clinical Project Manager, UCL CTU) contributed to the data collection and draft manuscript preparation.
Deirdre Brooking (R&D Manager, Royal National Orthopaedic Hospital) contributed to the data collection.
Elizabeth L Deane (https://orcid.org/0000-0002-1503-7768) (Clinical Project Manager) contributed to the data collection.
Iva Hauptmannova (Director for R&D, Royal National Orthopaedic Hospital) contributed to the analysis and interpretation of results and draft manuscript preparation.
Paul Cooke (Consultant Orthopaedic Surgeon, Nuffield Orthopaedic Centre) contributed to the study conception and design.
Marion Cumbers (Patient Representative) contributed to the analysis and interpretation of results and draft manuscript preparation.
Simon S Skene (https://orcid.org/0000-0002-7828-3122) (Professor of Medical Statistics) contributed to the study conception and design.
Caroline J Doré (https://orcid.org/0000-0001-9796-4970) (Professor of Clinical Trials and Statistics) contributed to the study conception and design, the analysis and interpretation of results and draft manuscript preparation.
All authors reviewed the results and approved the final version of the manuscript.
Publications
Goldberg AJ, Zaidi R, Thomson C, Doré CJ, Skene SS, Cro S, et al. Total ankle replacement versus ankle fusion (TARVA): protocol for a multicentre randomised controlled trial. BMJ Open 2016;6:e012716.
Thornton J, Sabah S, Segaren N, Cullen N, Singh D, Goldberg A. Validated method for measuring functional range of motion in patients with ankle arthritis. Foot Ankle Int 2016;37:868–73.
Muller P, Skene SS, Chowdhury K, Cro S, Goldberg AJ, Doré CJ, on behalf of the TARVA Study Group. A randomised, multi-centre trial of total ankle replacement versus ankle arthrodesis in the treatment of patients with end stage ankle osteoarthritis (TARVA): statistical analysis plan. Trials 2020;21:197.
Zaidi R, Hargunani R, Calleja M, Foley J, Goldberg A. MRI classification of subtalar joint osteoarthritis using a novel scoring system. Open J Radiol 2020;10:69–78.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
Appendix 1 Changes to the protocol
| Version and date | Reason for amendment | Amendment number | Substantial or non-substantial | REC approval date |
|---|---|---|---|---|
| V1.0, 9 April 2014 | Original application | N/A | N/A | 10 June 2014 |
| V2.0, 23 June 2014 | Responses to REC additional conditions | N/A | N/A | 24 June 2014 |
| V3.0, 19 August 2014 | To account for review by TSC/IDMC; change to imaging procedures | 1 | Substantial | 26 September 2014 |
| V4.0, 20 November 2014 | Response to review by investigators; clarification of AE reporting procedures and inclusion of reference to Safety Management Plan | 2 | Substantial | 18 December 2014 |
| V5.0, 23 June 2015 |
|
3 | Substantial | 29 July 2015 |
| V6.0, 17 August 2016 |
|
6 | Substantial | 14 November 2016 |
Appendix 2 Health economics
| Resource item | Unit cost (£) | Unit of analysis | Source of unit cost |
|---|---|---|---|
| GP | |||
| Surgery visit | 33 | Per consultation (average length of contact 9.22 minutes) | Unit Costs of Health and Social Care 201951 |
| Home visit | 184 | Per hour (average visit 23.4 minutes) | Unit Costs of Health and Social Care 201951 |
| Telephone call | 15.32 | Per telephone call (average length of contact 6.56 minutes) | Unit Costs of Health and Social Care 201951 |
| Nurse practice visit | 37 | Per hour (average visit 15.5 minutes) | Unit Costs of Health and Social Care 201951 |
| Nurse telephone call | 6 | Per telephone call (average call 4 minutes) | Unit Costs of Health and Social Care 201951 |
| District nurse visit | 41.73 | Per consultation | Unit Costs of Health and Social Care 201854 |
| Occupational therapist | |||
| Surgery visit | 43 | Per hour (average visit 30 minutes) | Unit Costs of Health and Social Care 201951 |
| Home visit | 44 | Per hour (average visit 60 minutes) | Unit Costs of Health and Social Care 201951 |
| Community physiotherapist | |||
| Home visit | 55 | Per hour (average visit 60 minutes) | Unit Costs of Health and Social Care 201951 |
| Surgery visit | 55 | Per hour (average visit 30 minutes) | Unit Costs of Health and Social Care 201951 |
| Clinical nurse telephone call | 102.50 | Per hour (average call time assumed to be 4 minutes) | Unit Costs of Health and Social Care 201951 |
| Inpatient stay | 631 | Per day | NHS Reference Costs 2017/1852 (based on NHS trust) |
| Outpatient visit | 135 | Per attendance | Unit Costs of Health and Social Care 201951 |
| Social worker | |||
| Visit | 45 | Per hour (average length of contact is not possible to estimate) | Unit Costs of Health and Social Care 201951 |
| Telephone call | 45 | Per hour (average length of contact is not possible to estimate) | Unit Costs of Health and Social Care 201951 |
| Home help | 28 | Per hour, weekday | Unit Costs of Health and Social Care 201951 |
| Meals on Wheels | 6 | Per meal (£44 per week) | Unit Costs of Health and Social Care 201353 |
| Resource item | Unit cost (£) | Unit of analysis | Source of unit cost |
|---|---|---|---|
| Equipment | |||
| Back support cushion | 2.70 | Per item | Mobility Smart Ltd (Preston, UK), URL: www.mobilitysmart.co.uk/back-support-cushion.html |
| Bath board | 3.20 | Per item | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/bathroom-aids/benches-seats-and-stools/bath-and-shower-boards.html?product_list_order = price_desc |
| Bath cushion | 1.30 | Per item | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/bathroom-aids/bath-pillows-and-cushions.html |
| Bathtub mat | 0.83 | Per item | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/non-slip-bathtub-mat.html |
| Commode | 11.98 | Per item | Unit Costs of Health and Social Care 201353 |
| Cushion | 3.80 | Per item | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/cushions-and-supports/lower-limb-support.html |
| Food trolley | 4.80 | Per item | Unit Costs of Health and Social Care 201353 |
| Foot stool | 6.20 | Per item | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/daily-living-aids/steps-and-stools/foot-stools.html |
| Leg guards | 4.10 | Per item | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/foldalite-pro-replacement-leg-guards-pair.html |
| Legs wedge | 2.80 | Per item | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/putnams-8-wedge-cushion-beige-14x14x3.html |
| Perching stool | 3.14 | Per item | Unit Costs of Health and Social Care 201353 |
| Porta Potti® | 16.10 | Per item | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/porta-potti-165-toilet-frame.html |
| Shoe raise | 4.60 | Per item | Physique Management Company Limited (Havant, UK), URL: www.physique.co.uk/Orthotics-Footcare/Orthotics-Insoles/Vasyli-Blue-Custom-34-Orthotics-Medium-Density?gclid = Cj0KCQiA48j9BRC-ARIsAMQu3WS2vTe4pRfEnXz0IWgXfipF7KBpNcULyq4-jG4I-Pjl8dr6khUKv5kaAn2kEALw_wcB#fo_c = 2689&fo_k = 1ab3590be76f6a4a5d67d57732e6378d&fo_s = gplauk&fo_oid = 9737 |
| Shower chair | 7.67 | Per item | Unit Costs of Health and Social Care 201353 |
| Toilet frame | 4.18 | Per item | Unit Costs of Health and Social Care 201353 |
| Toilet seat | 4.18 | Per item | Unit Costs of Health and Social Care 201353 |
| Urine bottle | 1.10 | Per item | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/toileting-aids/bed-pans-urinals/male-female-urinals.html |
| Washing tray | 2.60 | Per item | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/hair-washing-tray-with-strap.html |
| Home adaptations | |||
| Hand rail(s) | |||
| Bathroom | 2.50 | Per item | Unit Costs of Health and Social Care 201951 |
| Inside | 4 | Per item | Unit Costs of Health and Social Care 201951 |
| Outside | 5.70 | Per item | Unit Costs of Health and Social Care 201951 |
| Moving bed | 5.70 | Per item | Unit Costs of Health and Social Care 201951 |
| New bedroom | 3750 | Per item | Unit Costs of Health and Social Care 201951 |
| New shower | 15 | Per item | Unit Costs of Health and Social Care 201951 |
| New toilet | 1383 | Per item | Unit Costs of Health and Social Care 201951 |
| New wet room | 2191 | Per item | Unit Costs of Health and Social Care 201086 |
| New driveway | 327.50 | Per item | Unit Costs of Health and Social Care 201951 |
| Painting floors | 80.60 | Per item | Painter.co.uk, URL: www.painter.co.uk/prices/ |
| Ramp | 44 | Per item | Unit Costs of Health and Social Care 201951 |
| Shower replacing bath | 357 | Per item | Unit Costs of Health and Social Care 201086 |
| Stair lift | 263 | Per item | Unit Costs of Health and Social Care 201086 |
| Stair rail | 4 | Per item | Unit Costs of Health and Social Care 201086 |
| Mobility aids | |||
| Crutches | 4.40 | Per pair | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/walking-aids/crutches.html?p=3 |
| Mobility scooter | 172.10 | Per item | Mobility World Ltd (Letchworth, UK), URL: www.mobilityworld.co.uk/pages/mobility-scooters-1 |
| Knee scooter | 22.10 | Per item | YourCare (Croydon, UK), URL: www.yourcare.org.uk/category_schemes/97-product-category/categories/3891-rollators/products/663-knee-walker |
| Walking frame | 11.60 | Per item | Mobility Smart Ltd, URL: www.mobilitysmart.co.uk/walking-aids/zimmer-frames.html |
| Wheelchair | 206 | Per item | Unit Costs of Health and Social Care 201951 |
| Transportation costs | |||
| Fuel cost | 0.115 | Per mile | Guidance: Advisory Fuel Rates87 |
| Productivity loss | |||
| Gross earnings: full time, men | 17.41 | Per hour | EARN08: Distribution of Gross Hourly Earnings of Employees55 |
| Gross earnings: full time, women | 14.75 | Per hour | EARN08: Distribution of Gross Hourly Earnings of Employees55 |
| Gross earnings: part time, men | 11.65 | Per hour | EARN08: Distribution of Gross Hourly Earnings of Employees55 |
| Gross earnings: part time, women | 12.09 | Per hour | EARN08: Distribution of Gross Hourly Earnings of Employees55 |
| Gross earnings: all employees, all people | 15.26 | Per hour | EARN08: Distribution of Gross Hourly Earnings of Employees55 |
| Replacing an employee | 30,614 | Per person | Oxford Economics and income protection provider Unum (Surrey, UK)56 |
Appendix 3 Baseline characteristics by subtype of total ankle replacement and ankle fusion
| Baseline characteristic | Treatment arm | |||
|---|---|---|---|---|
| TAR | Ankle fusion | |||
| Fixed (N = 76) | Mobile (N = 66) | Arthroscopic (N = 85) | Open (N = 55) | |
| Age (years), mean (SD) | 66.4 (7.6) | 70.0 (8.2) | 67.8 (8.6) | 67.4 (7.2) |
| Sex, n (%) | ||||
| Female | 16 (21.1) | 18 (27.3) | 26 (30.6) | 21 (38.2) |
| Male | 60 (78.9) | 48 (72.7) | 59 (69.4) | 34 (61.8) |
| Height (metres), mean (SD) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) |
| Weight (kilograms), mean (SD) | 85.5 (13.3) | 86.1 (13.6) | 89.4 (15.9) | 86.8 (19.4) |
| Smoking status | ||||
| Current smoker, n (%) | 3 (3.9) | 2 (3.0) | 2 (2.4) | 3 (5.5) |
| Cigarettes per day, mean (SD) | 4.7 (0.6) | 7.5 (3.5) | 10.5 (13.4) | 10.3 (4.5) |
| Ex-smoker, n (%) | 24 (31.6) | 29 (43.9) | 40 (47.1) | 17 (30.9) |
| Time since cessation (years), mean (SD) | 26.0 (16.1) | 25.1 (16.2) | 24.6 (15.9) | 29.4 (14.4) |
| Patients’ treatment preference, n (%) | ||||
| No preference expressed | 52 (68.4) | 51 (77.3) | 65 (76.5) | 44 (80.0) |
| TAR | 18 (23.7) | 9 (13.6) | 9 (10.6) | 10 (18.2) |
| Ankle fusion | 4 (5.3) | 4 (6.1) | 9 (10.6) | 0 (0.0) |
| Aetiology of osteoarthritis, n (%) | ||||
| Post traumatic | 51 (67.1) | 34 (51.5) | 39 (45.9) | 32 (58.2) |
| Primary | 17 (22.4) | 30 (45.5) | 37 (43.5) | 18 (32.7) |
| Rheumatoid arthritis | 5 (6.6) | 2 (3.0) | 3 (3.5) | 3 (5.5) |
| Other inflammatory | 2 (2.6) | 0 (0.0) | 5 (5.9) | 0 (0.0) |
| Other | 1 (1.3) | 0 (0.0) | 1 (1.2) | 3 (5.5) |
| Subtalar joint osteoarthritis, n (%) | ||||
| Absent | 51 (67.1) | 41 (62.1) | 48 (56.5) | 36 (65.5) |
| Present | 25 (32.9) | 25 (37.9) | 37 (43.5) | 19 (34.5) |
| Talonavicular joint osteoarthritis, n (%) | ||||
| Absent | 65 (85.5) | 58 (87.9) | 74 (87.1) | 44 (80.0) |
| Present | 11 (14.5) | 8 (12.1) | 11 (12.9) | 11 (20.0) |
| Presence/absence of osteoarthritis, n (%) | ||||
| Healthy adjacent joint | 46 (60.5) | 39 (59.1) | 45 (52.9) | 30 (54.5) |
| Osteoarthritis in subtalar or talonavicular | 24 (31.6) | 21 (31.8) | 32 (37.6) | 20 (36.4) |
| Osteoarthritis in both adjacent joints | 6 (7.9) | 6 (9.1) | 8 (9.4) | 5 (9.1) |
| User of assistive device, n (%) | ||||
| No | 43 (56.6) | 39 (59.1) | 44 (51.8) | 33 (60.0) |
| Yes | 33 (43.4) | 27 (40.9) | 41 (48.2) | 22 (40.0) |
| Assistive device, n (%) | ||||
| Crutches | 10 (13.2) | 2 (3.0) | 10 (11.8) | 4 (7.3) |
| Ankle brace | 11 (14.5) | 5 (7.6) | 5 (5.9) | 2 (3.6) |
| Frame | 1 (1.3) | 1 (1.5) | 0 (0.0) | 1 (1.8) |
| Wheelchair | 1 (1.3) | 2 (3.0) | 3 (3.5) | 0 (0.0) |
| Stick/cane | 15 (19.7) | 20 (30.3) | 30 (35.3) | 14 (25.5) |
| Wheeled walker | 0 (0.0) | 1 (1.5) | 3 (3.5) | 1 (1.8) |
| Knee scooter | 1 (1.3) | 0 (0.0) | 0 (0.0) | 1 (1.8) |
| Other | 5 (6.6) | 3 (4.5) | 3 (3.5) | 1 (1.8) |
| Medical history, n (%) | ||||
| Anticoagulants | 11 (14.5) | 14 (21.2) | 18 (21.2) | 5 (9.1) |
| History of cancer | 10 (13.2) | 4 (6.1) | 13 (15.3) | 6 (10.9) |
| Chronic pain | 16 (21.1) | 24 (36.4) | 28 (32.9) | 18 (32.7) |
| Connective tissue disorder | 0 (0.0) | 1 (1.5) | 3 (3.5) | 1 (1.8) |
| Diabetes | 6 (7.9) | 4 (6.1) | 7 (8.2) | 8 (14.5) |
| Gastrointestinal disease | 8 (10.5) | 9 (13.6) | 13 (15.3) | 9 (16.4) |
| Hypertension/hypercholesterolaemia | 36 (47.4) | 27 (40.9) | 38 (44.7) | 22 (40.0) |
| Inflammatory disorder | 3 (3.9) | 5 (7.6) | 8 (9.4) | 4 (7.3) |
| Metabolic disorder | 3 (3.9) | 2 (3.0) | 3 (3.5) | 0 (0.0) |
| Neurological disorder | 0 (0.0) | 2 (3.0) | 4 (4.7) | 2 (3.6) |
| Obesity | 2 (2.6) | 6 (9.1) | 11 (12.9) | 4 (7.3) |
| Peripheral nervous system disorder | 0 (0.0) | 0 (0.0) | 2 (2.4) | 3 (5.5) |
| Peripheral vascular disease | 0 (0.0) | 2 (3.0) | 0 (0.0) | 3 (5.5) |
| Renal pathology | 2 (2.6) | 5 (7.6) | 3 (3.5) | 0 (0.0) |
| Respiratory pathology | 4 (5.3) | 8 (12.1) | 15 (17.6) | 5 (9.1) |
| Thromboembolic disease | 2 (2.6) | 5 (7.6) | 6 (7.1) | 1 (1.8) |
| Other condition affecting mobility | 20 (26.3) | 20 (30.3) | 21 (24.7) | 21 (38.2) |
| Degree of deformity, n (%) | ||||
| 16–30° varus | 9 (11.8) | 4 (6.1) | 3 (3.5) | 4 (7.3) |
| 5–15° varus | 18 (23.7) | 19 (28.8) | 26 (30.6) | 16 (29.1) |
| Physiological neutral | 28 (36.8) | 21 (31.8) | 35 (41.2) | 14 (25.5) |
| 5–15° valgus | 10 (13.2) | 11 (16.7) | 11 (12.9) | 6 (10.9) |
| 16–30° valgus | 5 (6.6) | 5 (7.6) | 3 (3.5) | 3 (5.5) |
| Not available | 5 (6.6) | 6 (9.1) | 7 (8.2) | 12 (21.8) |
| Fixed flexion deformity of knee, n (%) | 2 (2.6) | 1 (1.5) | 2 (2.4) | 0 (0.0) |
| Fixed equinus, n (%) | 4 (5.3) | 3 (4.5) | 4 (4.7) | 1 (1.8) |
| ROM dorsiflexion (degrees) | 14.2 (10.5) | 14.6 (7.9) | 14.8 (10.2) | 13.0 (7.9) |
| ROM plantarflexion (degrees) | 25.2 (7.8) | 25.9 (8.7) | 27.1 (11.4) | 24.7 (9.1) |
| Outcome measures at baseline, mean (SD) | ||||
| MOXFQ walking/standing | 81.8 (14.4) | 81.0 (19.0) | 82.6 (15.4) | 80.4 (18.8) |
| MOXFQ pain | 67.1 (16.8) | 66.1 (17.2) | 68.4 (16.2) | 66.6 (18.9) |
| MOXFQ social interaction | 53.8 (26.0) | 55.2 (25.8) | 55.5 (20.9) | 57.5 (23.6) |
| FAAM-ADL | 47.7 (15.0) | 46.3 (18.4) | 43.0 (15.9) | 45.5 (17.9) |
| FAAM sport subscale | 26.2 (16.4) | 30.1 (25.7) | 26.2 (24.6) | 28.3 (13.9) |
| EQ-5D-5L index value | 0.5 (0.2) | 0.5 (0.2) | 0.5 (0.2) | 0.5 (0.2) |
| EQ-5D-5L VAS | 71.6 (20.4) | 74.1 (19.6) | 65.3 (22.1) | 70.2 (20.7) |
Appendix 4 Manchester–Oxford Foot Questionnaire walking/standing score at 52 weeks post surgery, by ankle fusion subtype
| Outcome | Ankle fusion subtype | Difference in change from baseline (95% CI) | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Arthroscopic | Open | |||||||
| n | Value at follow-up, mean (SD) | Change from baseline, mean (SD) | n | Value at follow-up, mean (SD) | Change from baseline, mean (SD) | |||
| MOXFQ walking/standing | 83 | 36.3 (30.4) | –46.0 (33.2) | 53 | 37.3 (31.5) | –42.5 (30.8) | –1.15 (–11.44 to 9.15) | 0.83 |
Appendix 5 Manchester–Oxford Foot Questionnaire
Anybody wanting to use the MOXFQ must contact the copyright owners Oxford University Innovation at healthoutcomes@innovation.ox.ac.uk or via the online licence request portal at https://process.innovation.ox.ac.uk/.
List of abbreviations
- AE
- adverse event
- ASA
- American Society of Anesthesiologists
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CSRI
- Client Service Receipt Inventory
- CT
- computerised tomography
- EQ-5D-3L
- EuroQol 5-Dimensions, three-level version
- EQ-5D-5L
- EuroQol 5-Dimensions, five-level version
- FAAM
- Foot and Ankle Ability Measure
- FAAM-ADL
- Foot and Ankle Ability Measure – Activities of Daily Living
- GP
- general practitioner
- HRQoL
- health-related quality of life
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ICF
- informed consent form
- IDMC
- Independent Data Monitoring Committee
- ITT
- intention to treat
- MID
- minimal important difference
- MOXFQ
- Manchester–Oxford Foot Questionnaire
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- PIS
- patient information sheet
- PSA
- probabilistic sensitivity analysis
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- ROM
- range of motion
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- TAR
- total ankle replacement
- TARVA
- total ankle replacement versus ankle arthrodesis
- TSC
- Trial Steering Committee
- UCL CCTU
- University College London Comprehensive Clinical Trials Unit
- VAS
- visual analogue scale
