Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR127489. The contractual start date was in January 2020. The draft manuscript began editorial review in April 2022 and was accepted for publication in December 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Rivero-Arias et al. This work was produced by Rivero-Arias et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Rivero-Arias et al.
Chapter 1 Introduction
Background
National population screening programmes are implemented in health systems on the advice of screening committees such as the United Kingdom National Screening Committee (UK NSC), which makes independent, evidence-based recommendations to ministers in the four countries of the UK. Antenatal and newborn screening are covered by 6 of the 11 NHS screening programmes, namely fetal anomaly screening, infectious diseases in pregnancy screening, the newborn and infant physical examination, newborn blood spot screening, newborn hearing screening and sickle cell and thalassaemia screening. The recommendation to adopt a screening programme on a national scale is based on the premise that the benefits associated with screening outweigh the harms to all relevant stakeholders once implemented. 1 Harms of screening associated with false-positive and false-negative results include unnecessary additional resources to conduct further investigations, adverse psychological effects and legal claims, as well as decreased trust and confidence in the healthcare system. 2 In antenatal screening, when a decision to continue a pregnancy is made after a true-positive result, a clear screening benefit is the time it offers expectant parents to prepare for the care of a child with a clinical condition. An informed decision to terminate a pregnancy can also follow after a true-positive result. Both outcomes can sometimes lead to long-lasting psychosocial sequelae for women and their partners, affecting their quality of life and their future pregnancy choices. 3–9 The use of whole genome sequencing for newborn screening presents an opportunity to identify and treat or prevent severe health conditions, thus maximising survival and quality of life of the newborn, but is subject to overdiagnosis and over-treatment that need careful evaluation. 10,11
Screening committees require up-to-date evidence of these benefits and harms as well as data demonstrating that the screening programmes represent value for money. 12 The latter is determined using a health economic assessment confirming that the additional costs of implementing a screening programme are justified by the additional benefits achieved, usually through outcome measures such as the incremental cost per quality-adjusted life-year (QALY) gained, where the QALY combines preference-based health-related quality-of-life weights (health utilities) with data on length of time in the health states of interest. 13 This approach to cost-effectiveness assessment mirrors those recommended more broadly by Health Technology Assessment (HTA) agencies in the UK, such as the National Institute of Health and Care Excellence (NICE) in England and the Scottish Medicines Consortium in Scotland. 14,15 It also mirrors the preferred form of cost-effectiveness assessment adopted by HTA, pricing and reimbursement authorities in several other industrialised countries. 16–18 Although there is established guidance on best practices for economic assessment for screening programmes in general, such as in the area of economic modelling,19 such guidance does not address the challenges of how to incorporate the breadth of potentially relevant benefits and harms into a single assessment, and does not specifically focus on antenatal and newborn screening. Guidance in this area, therefore, remains limited. 20
Furthermore, several methodological factors have constrained capacity to evaluate antenatal and newborn screening programmes using the standard incremental cost per QALY gained metric. These include challenges surrounding the valuation of prenatal life when decisions following antenatal screening and diagnostic testing result in the termination of the fetus or unborn child,21,22 the absence of a multi-attribute utility measure validated for use in infancy and through early childhood23 and the challenges surrounding QALY aggregation across the mother, child and potentially other family members. 24 Attributes of relevance to parents, such as the utility derived from information per se or reassurance following a screen-negative test result, and the disutility associated with anxiety from a false-positive test result or over-diagnosis of disease, are likely to be missed, or at least inadequately covered, by standard approaches to health utility measurement, such as available multi-attribute utility measures [e.g. EuroQol-5 Dimensions (EQ-5D), Short Form 6-Dimension (SF-6D), Health Utilities Index Mark 3]. 25,26 In addition, numerous ethical challenges also compound the technical complexities surrounding economic assessments of antenatal and newborn screening programmes. These emanate from differences in moral perspectives on the status of the fetus or unborn child22 and how society should value disability,27 and differing perspectives on the ownership of genetic information28 and the potential harms of inadequately informed consent processes on parental autonomy. 29 Failure to incorporate all relevant benefits and harms when assessing the cost effectiveness of antenatal and newborn screening programmes may lead screening committees to make decisions based on suboptimal levels of evidence, resulting in a suboptimal allocation of resources.
Previous work has focused on the identification of methodological challenges and the development of good practice guidelines for the purpose of health economic assessments of antenatal and newborn screening programmes. 20,30,31 However, none has specifically focused on the range of benefits and harms incorporated into health economic assessments of antenatal and newborn screening programmes, or the methodological issues surrounding their identification, measurement and valuation.
Objectives
The overall aim of the proposed programme of work was to enhance knowledge about methods for valuing benefits and harms within economic assessments of antenatal and newborn screening and make recommendations about the benefits and harms that should be considered by economic evaluations and the health economic tools that could be employed for this purpose. Our specific objectives were:
-
to systematically identify the benefits and harms adopted by health economic assessments evaluating antenatal and newborn screening programmes, and to assess how they have been measured and valued
-
to identify attributes of relevance to stakeholders (parents/carers, health professionals, other relevant stakeholders) that should be considered for incorporation into future economic assessments using a range of qualitative research methods
-
to make recommendations about approaches for the measurement and valuation of outcomes that should be considered by future economic assessments in these contexts.
A brief explanation of the foundations of health economic assessments for non-health economists.
A note about benefits and harms of screening
The aim of a screening programme is to identify asymptomatic people who may be developing or at a greater risk of developing a condition and offer further investigations and/or treatment. The objective is then to allow individuals to make informed choices and reduce complications in the future. Screening programmes run on a national population scale and every year millions of people in countries with established screening organisations benefit from these programmes. However, no screening test is perfect and when false-negative result (individual with a negative screening result with the target condition) and false-positive result (individual with a positive screening result without the target condition) happen, there is potential to generate harm to the people the programme is intended to help. Consequently, screening programmes are subject to benefits as well as harms. In the UK, the NSC makes recommendations about adding new conditions to the current antenatal and newborn screening programmes based on the premise that the benefits associated with screening outweigh its harms at a population level. Essentially, screening agencies evaluate available evidence about benefits and harms of the screening programme in an attempt to understand whether it does more good than harm to the population.
The benefits of screening are diverse and include better future health for individuals who are identified asymptomatically or at an early onset of the disease, more effective treatment for individuals who are true screen positive, reassurance of women and their families, informed decisions about continuation or end a pregnancy and justifiable allocation of NHS resources to implement the programme. Harms of screening are also diverse and encompass incorrect results and associated anxiety or false reassurance, difficult decisions about continuing or ending a pregnancy, risks associated with treatment or the screening test and over-treatment (people identify with a condition that would never develop into a serious condition over the life course). If harms of screening are not quantified correctly, screening agencies risk that many people could be harmed instead of benefiting from screening.
How these benefits and harms are included in health economic assessments is currently not known and VALENTIA aims to clarify this. In the previous section, we introduce the QALY as an outcome measure widely used in economic evaluation to evaluate the health benefits of screening. We hypothesise in this study that QALYs are a good outcome measure to capture functional and psychological impacts of screening test results for women and their babies in newborn screening, but that present challenges in antenatal screening test results leading ending a pregnancy. Moreover, we also hypothesise that a particular screening result may be seen as a benefit for a group of women and seen as a harm for another group. Whether researchers have attempted to estimate QALYs capturing all these complexities is currently not known.
Organisation of the VALENTIA study
To achieve the objectives set out in Objectives, VALENTIA was organised into four linked work packages (WPs) as described in Figure 1.
FIGURE 1.
Flow diagram of the four linked WPs of the VALENTIA study.
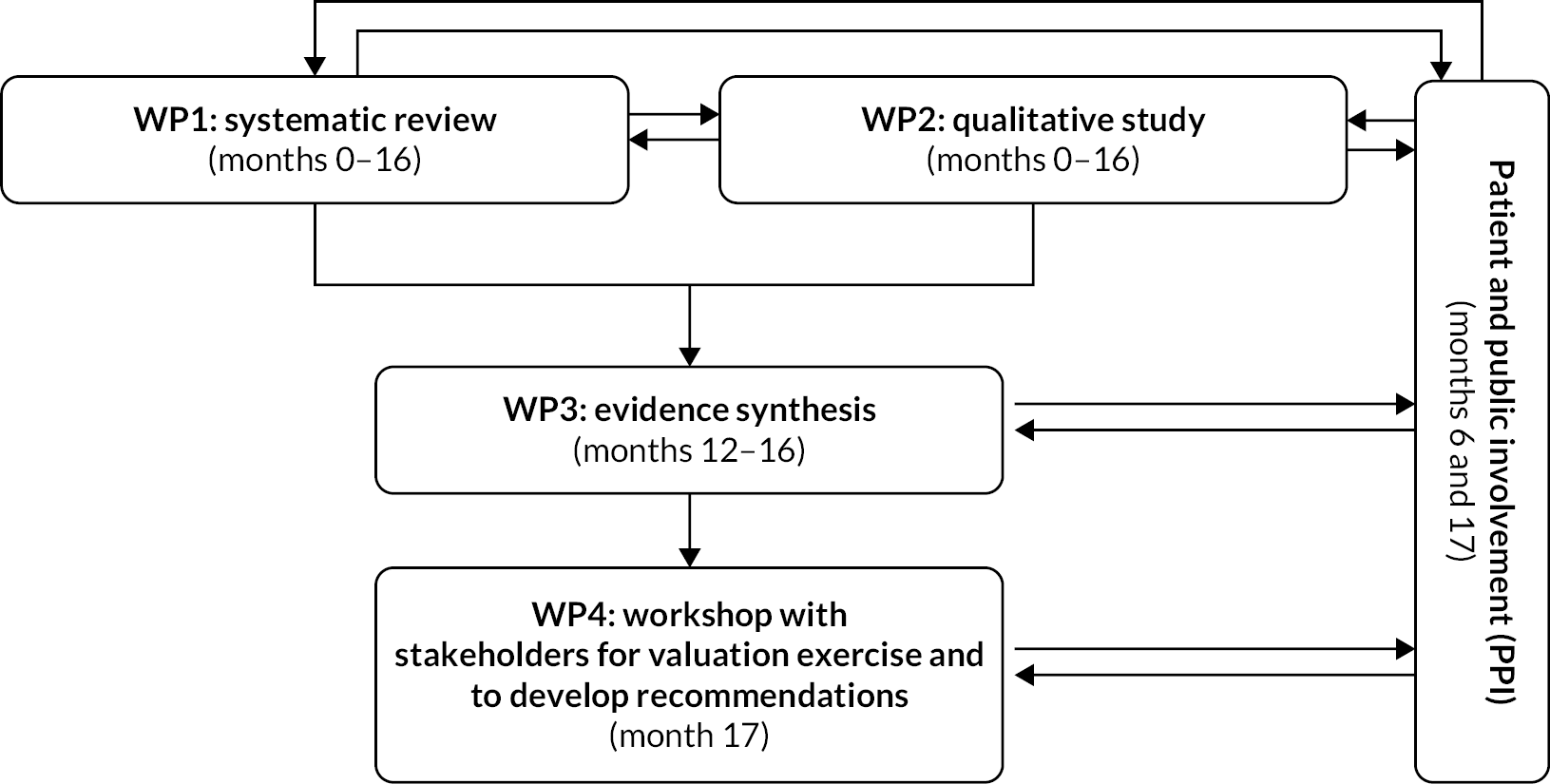
In the first WP1, a systematic review of the published and grey literature identified all health economic assessments evaluating antenatal and newborn screening programmes in Organisation for Economic Co-operation and Development (OECD) countries. Information relevant to the screening programmes, complemented by the reporting items contained within the Consolidated Health Economic Evaluation Reporting Standards (CHEERS), was extracted. This exercise provided a complete picture of the cost-effectiveness evidence assessing antenatal and newborn screening programmes in terms of the clinical areas and the reporting quality of the articles and reports. Chapter 3 describes the methodology of the systematic review with associated results and interpretation. Detailed information about the types of benefits and harms included in each of the studies was also extracted. Using an integrative descriptive analysis, we developed a thematic framework of benefits and harms used in health economic assessments in this area. We applied this thematic framework to the literature identified and discovered that economic evaluations assessing antenatal and newborn screening programmes have generally adopted a narrow range of benefits and harms or ignored important benefits and harms in their evaluations. Our work suggests that there is an immediate need to provide guidance for researchers conducting economic evaluations of antenatal and newborn screening. Our proposed thematic framework of benefits and harms can be used to guide the development of future health economic assessments evaluating antenatal and newborn screening programmes, to prevent exclusion of important potential harms. The development of this framework and its application is presented in Chapter 4.
Work package 2 was a qualitative study conducted using multiple methods to capture stakeholders’ views about the benefits and harms of antenatal and newborn screening that should be incorporated into future economic assessments. The qualitative component of VALENTIA is presented in three separate chapters in this report. Experiences of antenatal screening have been extensively investigated, but newborn screening experiences remained underexplored. Therefore, a meta-ethnography was conducted to better understand what was known about the experiences of newborn bloodspot screening (see Chapter 5). This was followed by secondary analysis of existing individual interviews related to antenatal screening, newborn screening and living with screened-for conditions (see Chapter 6). In this exercise, the goal was to bring together, examine and interpret the findings from disparate qualitative research studies and produce a richer and broader understanding than would be possible by looking at the studies individually. A large body of existing interview data was drawn upon to reflect a range of screening experiences and to better understand how individuals affected by screening discussed their experiences. What emerged was a complex web of individuals, organisations, technologies and discourses that shape the screening landscape. In Chapter 7, a thematic analysis of primary data collected with stakeholders about their experiences with antenatal and newborn screening was carried out. This analysis was conducted with three groups of stakeholders (individuals, charities, professionals working in the field of policy and healthcare professionals) to understand how these groups conceptualised the benefits and harms of screening. This primary data collection further developed the concepts derived from our meta-ethnography and secondary analysis, which developed an understanding of the landscape in which benefits and harms might be conceptualised. By using a range of qualitative methods, the benefits and harms of screening as experienced and perceived by a variety of stakeholders were identified, as well as concepts which did not map neatly onto the harms and benefits identified within existing economic assessments from WP1. The methods and results for each of these pieces of work in individual chapters are presented before summarising critical findings from across components of the qualitative study at the end of Chapter 7.
Work package 3 compared the benefits and harms identified in the quantitative health economics literature (WP1) and the qualitative literature (WP2) using a narrative synthesis exercise. The aim was to understand whether benefits and harms relevant to stakeholders were missing in the health economic assessment conducted in antenatal and newborn screening. In general, we observed good overlap between both types of data, but some gaps were also found. We present this in Chapter 8 and provide a discussion about potential alternatives to incorporate some of the missing benefits and harms in future work.
The final WP4 involved meeting with key stakeholders to discuss potential valuation techniques to value antenatal and newborn screening scenarios following the results from previous WPs and a final meeting to present the results of the study and our recommendations for future work. Although originally planned as face-to-face meetings over 2–3 days with different stakeholders’ groups, this was not possible due to pandemic restrictions at the time of the study. Given this change in format and the challenges of keeping audiences engaged in online settings, we instead conducted two online workshops with participants from our patient and public involvement group where we discussed potential alternative valuation techniques, and a large virtual workshop with the remaining stakeholders to discuss our final results and recommendations. Full details are presented in Chapter 9.
A final set of recommendations for the conduct of economic evaluations assessing antenatal and newborn screening programmes arising from the study is presented in Chapter 10.
An important aspect of the VALENTIA study was our approach to parent and public involvement (PPI). From the design of the study through to delivery, we placed PPI at the centre of our programme of work. Our strategy involved working with our PPI co-investigator to create a group of PPI members at the beginning of the study with different antenatal and newborn screening experiences to support the work carried out in the WPs. We explain this strategy and the areas where we have benefited from input from our PPI members in the next chapter (see Chapter 2).
Chapter 2 Parent and public involvement
Background
The inclusion of the public and patient voice is now widely regarded as an important element of research, ensuring that research is both relevant to the people affected by it and written in a way that is easily understood by the general public. 32 For VALENTIA, it was clear from the conceptualisation of the study that PPI would be fundamental to the identification and interpretation of benefits and harms of screening, since this calls for first-hand experience of antenatal and newborn screening.
There were several key considerations which steered the development of the PPI strategy for the study. Firstly, the many kinds of experiences that a woman and/or her partner may have during their interactions with a screening programme would need to be reviewed to determine how to put together a representative group for the PPI. Secondly, an engagement strategy would be needed since the PPI members would be engaged over a considerable period of time (24 months) and across several of the WPs. Finally, it was crucial that the PPI members were treated sensitively and respectfully, given the potential for traumatic experiences related to screening outcomes. There was particular concern to ensure the emotional well-being of all PPI members, avoiding harm through interactions with other PPI members who may hold different views or have had very different screening experiences.
This chapter describes the development of the PPI strategy, the ‘recruitment’ and characteristics of the group, and reflects on the successes and challenges of the PPI element of the study. Where our PPI members provided input into the specific workstreams, this is expanded in the PPI sections in the relevant chapters. We have reported the methodology and results according to the GRIPP2 guidelines. 33 The GRIPP2 short form table (Table 1) identifies the area(s) in the report where each reporting element can be found.
| Section and topic | Item | Reported on |
|---|---|---|
| 1: Aim | Report the aim of PPI in the study | Chapter 2 |
| 2: Methods | Provide a clear description of the methods used for PPI in the study | Chapter 2 |
| 3: Study results | Outcomes – report the results of PPI in the study, including both positive and negative outcomes | Chapters 2 , 6 , 7 , 9 |
| 4: Discussion and conclusions | Outcomes – comment on the extent to which PPI influenced the study overall. Describe positive and negative effects | Chapters 6 , 7 , 9 |
| 5: Reflections/critical perspective | Comment critically on PPI input in the study, reflecting on the things that went well and those that did not, so others can learn from this experience | Chapter 2 |
Objective
The aim of our PPI was to create a representative group of members of the public with direct experience of the antenatal and newborn screening programmes in the UK. The group was established to help clarify and interpret the study results, the sampling and data collection methods, analysis and synthesis of the results and conclusions of the study. The PPI group was an integral part of the research process, being involved at several stages for input and guidance, and receiving regular study updates.
Methods
In a first step, in collaboration with our PPI co-investigator (JF), the research team mapped out the broad categories of possible antenatal and newborn screening experiences, resulting in 14 different ‘groups’ of experience (Figure 2).
FIGURE 2.
Flow chart of the categories of experience of newborn and antenatal screening.

These groups were shaped and refined according to several factors: whether parents consented or not to the screening, whether the results of the screening tests were positive or negative and whether a condition was diagnosed or not (‘true-’ or ‘false-’ positive or -negative results). Depending on which categories parents’ experiences fall into, their perceptions of the benefits and harms of screening vary. As a result, it was decided that representation across as many of the groups as possible in Figure 2 was needed. We aimed to recruit several members from each category, with the goal of including a minimum number of two members from each experience group, representing 28 potential PPI members. We were also cognisant to include seldom-heard voices within the screening process, including fathers, people with disabilities and those from ethnic minority groups. 34–36 Engaging such a large and diverse group would be a significant undertaking, so early in the study a PPI Coordinator (JSO) joined the study team.
The creation of the PPI group coincided with the start of the global pandemic and affected the PPI element in two ways. Firstly, resources: access to recruitment avenues, and then the available time and energy that potential PPI members had for a study such as this were drastically reduced. Charities with whom we engaged were under resourced and many were not able to support reaching out to their communities in a way we had hoped. Potential PPI members were likely to be parents of young children, some with additional support needs, with dual working from home and child-care responsibilities, which significantly impacted the likelihood of being able to participate. Secondly, restrictions in the UK meant that the planned face-to-face interactions had to take place online, which caused concern over the development of rapport and group cohesion. However, we found that regular communication by e-mail and holding discussions online still provided the depth of response and input we had hoped for.
To begin engaging possible PPI members, we contacted relevant organisations as well as contacts linked to pregnancy, parenting, screened-for conditions and parental support following the diagnosis of a condition, drawing on the networks of our co-investigators. The National Perinatal Epidemiology Unit (NPEU), where several of the research team were based, has long-standing relationships with such organisations. Twenty-eight organisations were identified through these sources and contacted, including several that support parents in socially deprived areas such as Maternity Mates (based in Tower Hamlets). Eleven of the organisations responded to the team’s request to advertise the opportunity to their members. Alongside this, VALENTIA co-investigator JF, who is Director of Antenatal Results and Choices (ARC), a charity that supports parents following the diagnosis of a genetic or physical condition, shared the project with service users, and co-investigator FB identified parents and people with disabilities who had been involved in earlier research relating to screening and who had consented to participate in future research.
These ‘trusted intermediaries’ enabled the team to undertake wide-reaching advertising, which, along with other avenues such as social media posts and snowballing, resulted in 30 PPI members across 9 of the experience groups in Figure 2 volunteering to participate as PPI members. Groups 7, 10, 12, 14 and 15 including those who opted out of NHS screening and either undertook private screening or no screening at all proved more difficult to engage with. Several clinics and privately practising clinicians were contacted for support with reaching those parents who opted for private screening but declined to engage with the study. It was expected that parents opting out of screening would be a harder population to reach,37 given that only a very small number of parents do not have tests. In 2018–9, the NHS reported that 99.1% of eligible parents in England had a fetal anomaly screening test result documented. Of the nine groups that were represented most had between two and four representatives, but it should also be acknowledged that, while parents were ‘assigned’ to a group based on their most recent or prominent experience, many parents had multiple experiences of screening over one or more pregnancies, and therefore members provided a broad and rich depth of perspectives.
To maintain engagement and reflect the value they added to the study, we ensured that all our PPI members were remunerated at a rate consistent with National Institute for Health and Care Research (NIHR) Centre for Engagement and Dissemination guidelines. We maintained regular e-mail communication throughout the project, providing updates on progress and signposting when further input may be needed. We also produced training videos to explain the roles of PPI in research, an overview of the study and an overview of qualitative research. There was concern that not being able to hold face-to-face PPI focus groups would be detrimental to the members’ engagement with the project. Careful thought was given to how to recreate the level of interaction and rapport gained through face-to-face discussions using online communication tools. We chose the platform ‘SLACK’ which allowed PPI members to have own anonymised communication though an individual ‘channel’ where a researcher could ask their input, share resources such as training videos or written material, and to respond to questions and discussion points that were posted by the research team either in their own time or over a specified window. This feature was crucial for the accessibility of the PPI strategy, given the competing demands on members’ time, and our desire not to exclude those with limited availability, for example, due to caring responsibilities. In some ways, it was an advantage over face-to-face discussion groups. Towards the end of the study, we also facilitated two smaller online focus group discussions using Zoom conference facilities, which enabled the researchers and PPI members to interact in real time and enabled more free-flowing discussions (see Chapter 9).
Demographics
It took a significant amount of time and resources, from April to September 2020, to reach sufficient numbers of representatives for the group. Thirty members, representing 10 of the 14 experience groups, ultimately consented to take part; however, 1 member withdrew prior to the first interaction session due to personal reasons, resulting in a final group of 29 members. Their characteristics are shown in Table 2.
| Woman, n (%) | 27 (93.1) |
|---|---|
| Mean age, years | 34.8 |
| Range of age, years | 23–41 |
| Disability, n (%) | |
| No | 27 (93.1) |
| Yes | 1 (3.4) |
| Prefer not to say | 1 (3.4) |
| Religious beliefs, n (%) | |
| Christian | 7 (24.1) |
| Hindu | 1 (3.4) |
| None | 20 (69.0) |
| Other/prefer not to say | 1 (3.4) |
| Nationality, n (%) | |
| British | 22 (75.9) |
| Scottish | 1 (3.4) |
| British and American | 1 (3.4) |
| Other/prefer not to say | 5 (17.2) |
| Ethnicity, n (%) | |
| White | 20 (69.0) |
| Other white background | 4 (13.8) |
| Black | 1 (3.4) |
| Asian/British Asian | 2 (6.9) |
| Mixed/multiple ethnic background | 2 (6.9) |
| Employment status, n (%) | |
| Full-time | 8 (27.6) |
| Part-time | 10 (34.5) |
| Self-employed | 4 (13.8) |
| Furloughed | 1 (3.4) |
| Unemployed/working with your family/caring | 5 (17.2) |
| Other/prefer not to say | 1 (3.4) |
Despite the unprecedented burdens of child care on this group, we managed to build up the PPI group over several months. The PPI group was demographically diverse, although we acknowledge not nationally representative. The group characteristics included a range of age and socioeconomic status, but it was not as diverse as we had hoped for in terms of ethnicity, religion or disability. Efforts were made to engage with people from minority ethnic backgrounds, such as engaging with ‘Maternity Mates’, sharing widely through charities and social media and using wording which encouraged men and lesser heard voices to participate. With the limited resources available and the pressures on this particular population, it was not possible to achieve representativeness in the group. Perhaps if the timing of the study had been different, it might have been easier to obtain a more representative PPI group, and this is acknowledged as a significant challenge and limitation.
Results
The PPI members provided input at several points over the duration of the project, feeding significantly into WP2 and WP4. They reviewed the sampling strategy of the qualitative WP, and identified additional participant groups to include. They also provided feedback on the wording and screening scenarios that would be used as tools to prompt discussions during focus groups and interviews with participants in the qualitative research, and supported the recruitment of participants to the qualitative WP.
We tracked how many PPI members took part in the interactions over the duration of the study to give us a sense of engagement levels with the study. Ninety-seven per cent (28/29) of PPI members responded to the first task and 76% (22/29) to the second task a few weeks later. By the end of the study, around 15 months after the first task, 41% (12/29) of PPI members were still actively involved with the study and responding to communications. Thirty-five per cent (10/29) participated in the last online focus groups. They represented the following categories: one in group 3 (positive antenatal screening and no further tests); two in group 4 (false-positive antenatal screening); one in group 5 (positive antenatal screening and continue pregnancy); three in group 6 (positive antenatal screening and pregnancy termination); two in group 9 (true-negative newborn screening); and one in group 13 (true-positive newborn screening), representing six of the experience groups. Given the global pandemic, the duration of the study and the fact that PPI members had caring responsibilities, the rate of attrition was unsurprising. However, the focus groups did benefit from the breadth of experiences represented by the PPI members.
Discussion and reflections
Despite the limitation of the representativeness of the PPI group, the study benefitted significantly from its input based on their experiences of screening over the perinatal period. Their input included advising on appropriate language for research participants; reviewing and adding to the list of stakeholders; supporting recruitment of participants into the primary research; and ultimately advising on the appropriateness of employing different health economic methods to value antenatal and newborn screening scenarios. Their input shaped the success of the study by not only enhancing the engagement the research team had with the study participants, but also advising on the feasibility of the recommendations to move forward. Specific examples of PPI input that were taken forward and led to an amendment in the methodology or improvement in interpretation are expanded upon in Chapters 6, 7 and 9.
Having a PPI co-ordinator to manage the group proved crucial due to the numbers involved, the extraordinary circumstances of the pandemic and the length of time members needed to be involved. Some aspects of the engagement strategy were labour-intensive, for example, recruitment, creating the online platform; producing information videos to explain the role of PPI in research and another to explain qualitative research; and producing study progress communications. Another aspect was the involvement of JF as a co-investigator, who was able to provide contacts, champion the involvement of the PPI at the regular project meetings and advice on ways that their input could be helpful. PPI engagement was well-maintained for a significant duration, and the quality of the input was high, with the members providing very insightful and thoughtful input throughout, shaping the progress and recommendations of the study.
Our final interaction with the PPI members involved two smaller online group discussions with the health economics researchers (see Chapter 9). The research team gained very useful input on some of the proposed recommendations of methods for valuing benefits and harms of antenatal and newborn screening. The PPI group and the research team did not get to meet other than these final interactions, due to COVID-19 restrictions. Our experience demonstrates that engaging a large PPI group such as this is possible, that it greatly improves the quality of the research. It also demonstrates that group cohesion does not necessarily rely on face-to-face interactions. However, it does require significant resource commitment, thought and effort on behalf of both the research team and PPI group members.
Chapter 3 Work package 1: systematic review of health economic assessments evaluating antenatal and newborn screening
Sections of this chapter have been previously reported in Png et al. (2021). 38
Introduction
In this chapter, we report our systematic review of health economic assessments evaluating antenatal and newborn screening programmes in developed countries. The systematic review had two distinct purposes. The first was to identify all available evidence in the published and grey literature over the last two decades and understand its main characteristics, the clinical areas and conditions covered and the reporting quality of the contributing studies. The second objective was to extract detailed information about the benefits and harms incorporated into these health economic assessments. This chapter covers the first aim and reports a comprehensive overview, whereas the second aim is presented in Chapter 4.
Methods
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist39 when reporting the methods and results of the systematic review. The review protocol has been registered with PROSPERO (CRD42020165236) and published on 13 January 2020. This review is based on data available from secondary sources and published materials with no primary data collection required, so ethics committee approval or written informed consent was not required.
Eligibility criteria
The Population, Intervention, Comparator, Outcome and Study design (PICOS) framework was used to develop the study eligibility criteria (Table 3) and applied to the literature searches. Searches were limited to studies published after 1 January 2000. Studies reporting health economic assessments, such as economic evaluations and studies that use economic frameworks of cost-effectiveness evidence or economic notions of value (e.g. multi-criteria decision analyses, programme budgeting and marginal analyses) of antenatal or newborn screening programmes, were included. Non-English language studies were included, but studies were limited to those conducted in developed countries (defined, for the purposes of this review, as a member of the OECD40).
| Characteristics | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Pregnant women Newborns |
Anyone other than pregnant women or newborns Studies on animals Not conducted in an OECD member countrya |
| Intervention | Antenatal or newborn screening programmeb | Pre-conception screening No screening programme |
| Comparator | No screening or specific form(s) of screening other than experimental intervention(s), as defined by specific conditions | |
| Outcome | Benefits and harms of antenatal or newborn screening that have been identified, measured and valued by economic assessments | |
| Study design | Economic evaluation design:
Economic framework that incorporates cost-effectiveness evidence or economic notion of value (e.g. multi-criteria decision analysis, programme budgeting and marginal analysis) |
Descriptive cost analysis Budget impact analysis Not an economic evaluation |
Other report types:
|
Information sources
Systematic searches of both published and grey literature, including peer-reviewed journal articles controlled by commercial publishers and documents produced by all levels of government, academia, business and industry, were conducted. The following electronic bibliographic databases were searched: MEDLINE (OvidSP) (1946–present), EMBASE (OvidSP) (1974–present), NHS Economic Evaluation Database (via CRDWeb www.crd.york.ac.uk/CRDWeb/) (inception to 31 March 2015), EconLit (Proquest) (1969–present), Science Citation Index, Social Science Citation Index and Conference Proceedings Citation Index – Science (Web of Science Core Collection) (1945–present), CINAHL (EBSCOhost) (1982–present) and PsycINFO (OvidSP) (1806–present). SCOPUS (Elsevier) was used to run forward and backward citation searches once relevant studies were identified. The academic electronic database searches were supplemented by manual reference searching of bibliographies from studies that were included, contacts with experts in the field and author searching based on experts’ opinion. The first full search of published literature was conducted on 24 April 2020 with a top-up search conducted on 2 July 2020 to include the ‘perinatal’ search term while a refresh search was conducted on 22 January 2021.
The list of grey literature searched was derived from a pool of relevant websites that was informed by a recent systematic review of national policy recommendations on newborn screening that identified websites of national and regional screening organisations with documentation about antenatal and/or newborn screening recommendations. 41 This was widened to cover websites reported by the Health Grey Matters checklist and those for national and regional screening organisations, HTA agencies, paediatrics organisations, and obstetrics and gynaecology societies in OECD countries, as well as international decision-making bodies, such as the World Health Organization, the European Council, European Commission and the European Observer. 41,42 A customised web scraping tool that used the Google search engine was built using Python to directly query the stated websites (see Appendix 1) from 18 to 27 January 2021 using English search terms and from 14 to 17 February 2021 using translated search terms for non-English websites, as well as to automate the data extraction processes.
Search strategy
The search strategies applied to the published literature (see Appendix 2, Tables 21–26) were developed using a combination of medical subject headings (MeSH) and free-text keywords related to health economic assessments of antenatal and newborn screening programmes in collaboration with an information specialist (NR) with expertise in conducting systematic literature reviews in the health sciences. A simplified search strategy derived based on the Cochrane guidelines was applied to the grey literature search. 43 Translation of the simplified search terms for non-English websites was performed by professional translators.
Data management
The results of the literature searches were uploaded into the Endnote software package X9 (Clarivate, Philadelphia, PA, USA, 2019), a reference management system specifically designed for managing bibliographies and citations, to remove duplicates. Unique records were subsequently imported into Covidence,44 an online software program that facilitates collaboration among reviewers during the screening and data extraction stages. This software allows importation of references and files to be screened and information can be entered into a pre-created data extraction form after removing duplicates. Screening criteria based on the inclusion and exclusion criteria specified in Table 3 were developed and tested. A calibration exercise was undertaken to pilot and refine the screening criteria before the formal screening process started. For non-English language papers, Google Translate (Google, Mountain View, CA, USA) was used to translate relevant documents.
Selection process
For the published literature, two reviewers (MEP and MY) independently screened the titles and abstracts of all retrieved articles and documented the reasons for study exclusion according to the criteria specified in Table 3. Full texts of potentially relevant articles were reviewed independently by the same reviewers (MEP and MY), and study eligibility based on the inclusion and exclusion criteria was assessed. At each stage of the selection process, any disagreement was resolved by discussion and consensus between the two reviewers. When consensus could not be reached, input from the rest of the review team (ORA and SP) was obtained. For the grey literature, only one reviewer (MEP) did the title/abstract and full-text screening of all the retrieved articles, while another reviewer (SR) screened the titles/abstracts of a random sample of at least 10% (13%) of the retrieved articles due to a change and shortage in work force and limited time.
Data collection process
A data extraction form, which was piloted and refined using 10 randomly selected studies identified in the academic electronic databases, was created using Microsoft Excel following recommendations from the Cochrane Handbook for Systematic Reviews of Interventions. 43 As we had anticipated a large number of articles to data extract, after consulting our Independent Oversight Committee members and Information Specialist (NR), a selection of 10% of the articles/reports was extracted independently by two health economists (MEP and MY), followed by a reconciliation process. During this reconciliation process, MEP and MY had to extract the same key information from a random set of conference abstracts, journal articles and reports before they proceeded to extract the other studies independently and this was observed after assessing around 10% of the papers/reports. The rest of the published literature was subsequently divided between the two reviewers (MEP and MY), while data from the grey literature were extracted by one reviewer (MEP) only. Furthermore, any uncertainties related to data extracted by the two independent reviewers (MEP and MY) was discussed with the two senior investigators (ORA and SP) at weekly meetings. The list of variables extracted from each report included at the final stage of the review process was finalised following the piloting and refinement of the data extraction sheet.
The data extraction form consisted of two parts: (1) a section that contained items from the CHEERS checklist,45 modified where applicable to align with our research focus (i.e. benefits and harms within economic assessments) (see Appendix 3). This included bibliographic details; condition(s) screened; approaches for measuring and valuing health outcome measures; the journal impact factor quartile during the year that the article was published, obtained from Clarivate Analytics and SCImago; whether the authors made any policy recommendation based on their economic evaluation evidence; and whether the authors might have had any potential conflicts of interest in promoting their screening programme or mechanism (defined as a study that was funded by an industry sponsor, unless it was an unrestricted grant, and at least one of the authors being clearly employed by the industry sponsor); and (2) a bespoke form (see Appendix 4) created by the research team to extract benefits and harms adopted by economic assessments evaluating antenatal or newborn screening programmes. This form was created de novo as we could not find any previous examples in the published literature. A detailed description of the process to create the bespoke form is described in Chapter 4. This bespoke form contained consequences as reported by authors by screening test outcome (i.e. true positives, false positives, true negatives and false negatives) and type of data (i.e. probability, cost or outcome), which were captured and categorised as either a benefit or a harm. We also recorded the stage of the disease pathway at which the screening test was administered and the phase(s) of the screening programme using categorisations from recent guidance,1 as well as recorded whether the structure of decision-analytical models had been reported, and the consequences associated with treatment where applicable.
Data items
In order to reduce bias from including data from multiple reports of the same study, multiple articles published by the same authors with similar titles and abstracts were treated as linked companion studies (i.e. multiple reports from a single study) and only the most detailed publication was included in our final outputs. Similarly, if conference abstract(s) and a journal article by a similar group of authors had been published on the same topic, only the journal article was included at the full-text screening stage. Since we were interested in the methodological approaches to the measurement and valuation of benefits and harms and how the results were reported, if the article/report title suggested that an economic evaluation was conducted but neither the methods nor the results were presented in the abstract or full text, the article/report was excluded at the screening stage(s). Articles/reports that did not focus specifically on pregnant women or newborns but reported separate results of screening of pregnant women or newborns within broader populations were still included. In addition, authors were not contacted for missing data on individual data items included in our data extraction sheet, which were instead recorded as ‘not stated’.
Assessment of reporting quality of individual studies
Since only aggregated data and no effect sizes were sought, we did not assess the risk of bias or conduct a formal meta-analysis. Instead, the reporting quality of articles and reports (excluding conference abstracts) was assessed using the CHEERS reporting statement. 45 The items were considered as ‘satisfied’ if reported in full or ‘not satisfied’ if not reported or partially reported. The items were not scored as per the guidance in the CHEERS reporting statement. 45
Deviations from protocol
There were a few of deviations from the protocol. First, we used Endnote and Covidence for different components of the systematic review. Endnote was used to record all the studies identified as part of our searches and employed to identify and remove duplicates. Covidence was used as it can better facilitate collaboration among reviewers during the screening and data extraction stages than Endnote. Second, we did not use any risk of bias tools such as Cochrane ROBEQ tool and Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I) for different study designs because the aim of the systematic review was to understand the benefits and harms of antenatal and newborn screening and not to extract any quantitative information from the papers. Therefore, we excluded any reference to risk of bias assessment from the final published protocol for the systematic review. For the same reason, we did not explore further the external validity of any of the cost-effectiveness results published in the studies. We have used the CHEERS statement to evaluate the reporting quality of the studies since understanding the reporting quality of these studies was a primary aim of our systematic review as it was good indicator of whether a particular study was going to provide all the information in our bespoke form. Last, data extraction was not conducted independently by the two reviewers and a 10% sample was used because the former was not feasible as we ended up including 336 articles and reports and it was decided given our timelines to change the strategy for the data extraction. All deviations were discussed and approved by our Independent Oversight Committee.
Results
Search results
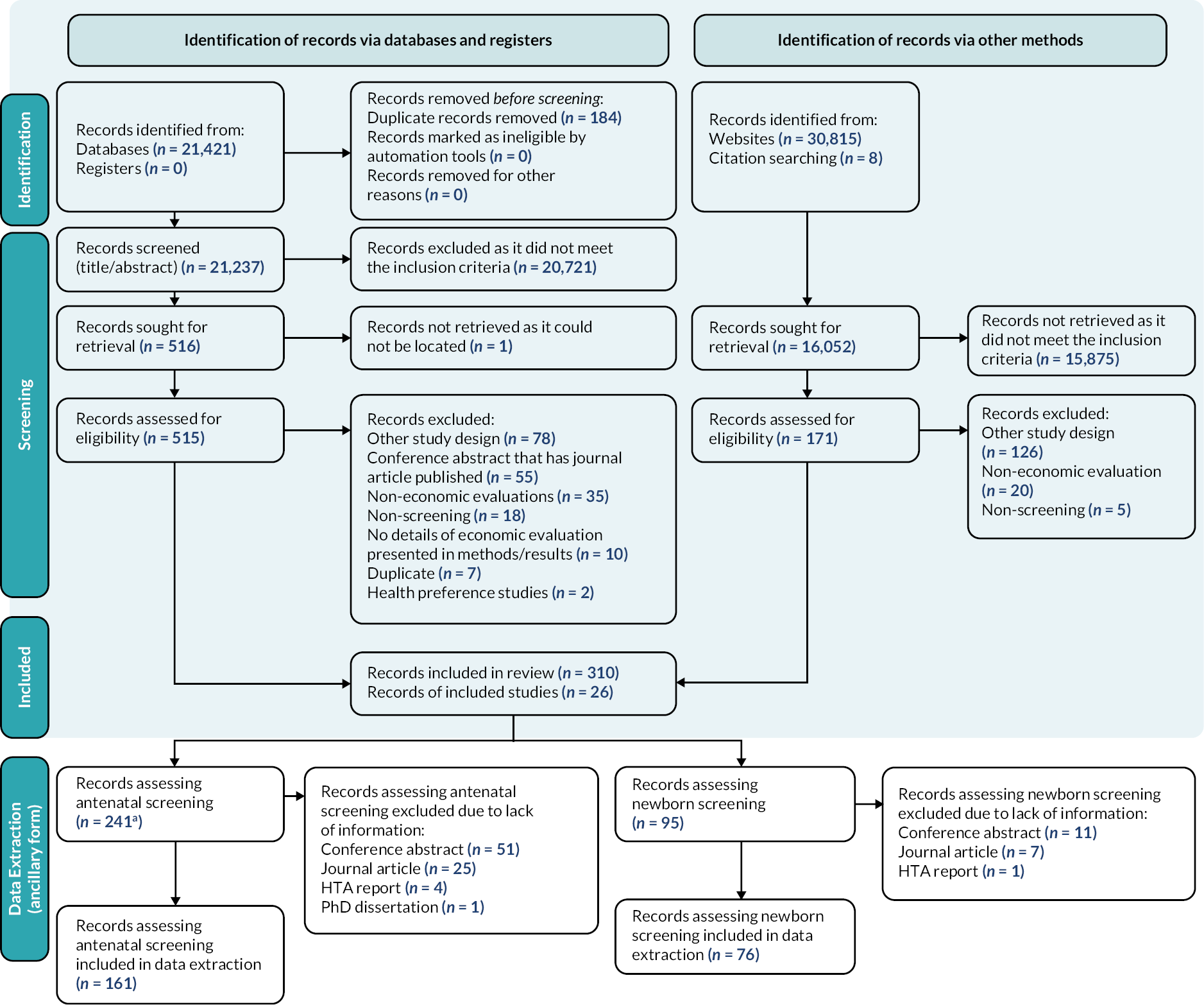
We identified 52,244 articles and reports from the searches of the published and grey literature. Among the 16,052 records that were sought for retrieval based on identification of records via other methods (i.e. grey literature), 7464 records were non-English (46.5%). Thirty-nine studies of the non-English records were assessed for eligibility with five subsequently included in the data extraction phase. A total of 336 records, 310 articles (1.4% of databases) and 26 reports (0.08% of websites), were included in the systematic review. One HTA report included two separate economic evaluations that were separated into two different reports, resulting in 337 outputs. Study selection and reasons for exclusion as well as data extraction of the bespoke form are summarised in the modified PRISMA diagram (Figure 3). The list of studies excluded is summarised in Report Supplementary Material 1.
FIGURE 3.
Modified PRISMA flow diagram. a, One HTA report included two separate economic evaluations that were separated into two different reports, resulting in 242 outputs from the 241 records.
There was no trend in the publication year of the articles and reports (Figure 4). Characteristics of the included articles and reports are presented in Table 4. The majority of the articles and reports included were journal articles (228, 67.7%) and almost half of the studies were conducted in the USA (109, 32.2%) and the UK (43, 12.7%). The majority of the articles and reports also required further information to determine if the authors had potential conflicts of interests (221, 65.6%). Furthermore, the authors did not make any recommendation about the adoption of the screening programme based on the economic evidence generated for the majority of the articles and reports (273, 81.0%). The majority of the articles were published in top quartile medical journals (i.e. quartile one; 129, 38.3%).
FIGURE 4.
Number of articles and reports published from 2000 to January 2021. Note: Dotted lines were used to indicate that only January 2021 was included in this chart.

| Articles and reports assessing antenatal screening (%) (n = 242) |
Articles and reports assessing newborn screening (%) (n = 95) |
Articles and reports assessing either screening type (%) (n = 337) |
|
|---|---|---|---|
| Publication type | |||
| Journal article | 156 (64.5) | 72 (75.8) | 228 (67.7) |
| Conference abstract | 61 (25.2) | 12 (12.6) | 73 (21.7) |
| HTA report | 24 (9.9) | 11 (11.6) | 35 (10.4) |
| PhD dissertation | 1 (0.4) | 0 (0) | 1 (0.3) |
| Country of screening programmea | |||
| USA | 82 (33.7) | 27 (28.4) | 109 (32.2) |
| UK | 32 (13.2) | 11 (11.6) | 43 (12.7) |
| Canada | 17 (7) | 15 (15.8) | 32 (9.5) |
| The Netherlands | 12 (4.9) | 9 (9.5) | 21 (6.2) |
| France | 8 (3.3) | 4 (4.2) | 12 (3.6) |
| Australia | 9 (3.7) | 3 (3.2) | 12 (3.6) |
| Spain | 6 (2.5) | 4 (4.2) | 10 (3.0) |
| Colombia | 3 (1.2) | 3 (3.2) | 6 (1.8) |
| Austria | 3 (1.2) | 1 (1.1) | 4 (1.2) |
| Israel | 4 (1.6) | 0 (0) | 4 (1.2) |
| Italy | 3 (1.2) | 1 (1.1) | 4 (1.2) |
| Germany | 1 (0.4) | 2 (2.1) | 3 (0.9) |
| Belgium | 1 (0.4) | 2 (2.1) | 3 (0.9) |
| Finland | 1 (0.4) | 1 (1.1) | 2 (0.6) |
| Sweden | 1 (0.4) | 3 (3.2) | 4 (1.2) |
| Chile | 1 (0.4) | 0 (0) | 1 (0.3) |
| Czech Republic | 1 (0.4) | 0 (0) | 1 (0.3) |
| Denmark | 1 (0.4) | 0 (0) | 1 (0.3) |
| Ireland | 2 (0.8) | 0 (0) | 2 (0.6) |
| Japan | 0 (0) | 1 (1.1) | 1 (0.3) |
| New Zealand | 1 (0.4) | 1 (1.1) | 2 (0.6) |
| Norway | 2 (0.8) | 0 (0) | 2 (0.6) |
| Switzerland | 1 (0.4) | 0 (0) | 1 (0.3) |
| Not stated | 51 (21) | 7 (7.4) | 58 (17.2) |
| Potential conflicts of interest | |||
| No | 70 (28.9) | 38 (40) | 108 (32.0) |
| Yes | 7 (2.9) | 1 (1.1) | 8 (2.4) |
| More information needed to classify | 165 (68.2) | 56 (58.9) | 221 (65.6) |
| Policy recommendation | |||
| No | 194 (80.2) | 79 (83.2) | 273 (81.0) |
| Yes | 48 (19.8) | 16 (16.8) | 64 (19.0) |
| Journal impact factor quartile (articles only) | |||
| First quartile of medical journals | 36 (10.7) | 93 (27.6) | 129 (38.3) |
| Second quartile of medical journals | 17 (5) | 26 (7.7) | 43 (12.8) |
| Third quartile of medical journals | 11 (3.3) | 27 (8) | 38 (11.3) |
| Fourth quartile of medical journals | 3 (0.9) | 7 (2.1) | 10 (3) |
| Not available | 5 (1.5) | 3 (0.9) | 8 (2.4) |
Target population and setting
The characteristics of screening programmes and populations in the included articles and reports are summarised in Table 5. There were 173 (71.5%) studies on antenatal screening and 63 (66.3%) studies on newborn screening that did not state the setting of the screening (236, 70.0%) or the women’s gestational stage at the time of screening (168, 65.4% of the antenatal screening studies). The majority of the studies were targeted at the general population of pregnant women (197, 57.1%) or infants (91, 26.4%). Many studies were investigations at the symptomless stage with pathologically definable change present (303, 89.9%) or involved all phases of the screening programmes (162, 48.1%).
| Articles and reports assessing antenatal screening (%) (n = 242) |
Articles and reports assessing newborn screening (%) (n = 95) |
Articles and reports assessing either screening type (%) (n = 337) |
|
|---|---|---|---|
| Setting of screeninga | |||
| Home | 0 (0) | 3 (3.2) | 3 (0.9) |
| Primary care | 6 (2.5) | 3 (3.2) | 9 (2.7) |
| Secondary care | 58 (24.0) | 22 (23.2) | 80 (23.7) |
| Primary and secondary care | 5 (2.1) | 4 (4.2) | 9 (2.7) |
| Not stated | 173 (71.5) | 63 (66.3) | 236 (70.0) |
| Populationa | |||
| Healthy pregnancy | 196 (79.0) | 1 (1.0) | 197 (57.1) |
| Pregnant women and their partner/relative | 8 (3.2) | 0 (0) | 8 (2.3) |
| Pregnancy at risk | 37 (14.9) | 1 (1.0) | 38 (11.0) |
| Healthy infant | 7 (2.8) | 84 (86.6) | 91 (26.4) |
| Infant at risk | 0 (0) | 11 (11.3) | 11 (3.2) |
| Gestation stage of pregnant women | |||
| First trimester | 25 (9.7) | 0 (0) | 25 (7.2) |
| First or second trimester | 3 (1.2) | 0 (0) | 3 (0.9) |
| First and second trimesters | 6 (2.3) | 0 (0) | 6 (1.7) |
| First and third trimesters | 1 (0.4) | 0 (0) | 1 (0.3) |
| Second trimester | 25 (9.7) | 0 (0) | 25 (7.2) |
| Second and third trimesters | 10 (3.9) | 0 (0) | 10 (2.9) |
| Third trimester | 19 (7.4) | 0 (0) | 19 (5.5) |
| Not stated | 168 (65.4) | 2 (2.2) | 170 (49.0) |
| Not applicable | 0 (0) | 88 (97.8) | 88 (25.4) |
| Stage of disease pathway | |||
| Person at risk but no pathological changes present | 24 (9.9) | 6 (6.3) | 30 (8.9) |
| Symptomless stage with pathologically definable change present | 217 (89.7) | 86 (90.5) | 303 (89.9) |
| Signs and/or symptoms exist but condition undiagnosed | 1 (0.4) | 2 (2.1) | 3 (0.9) |
| Clinical phase | 0 (0) | 1 (1.1) | 1 (0.3) |
| Phase(s) of screening programme | |||
| Screening and diagnostic | 60 (24.8) | 16 (16.8) | 76 (22.6) |
| Screening and intervention | 59 (24.4) | 10 (10.5) | 69 (20.5) |
| Screening, diagnostic and intervention | 103 (42.6) | 59 (62.1) | 162 (48.1) |
| Not clear | 20 (8.3) | 10 (10.5) | 30 (8.9) |
Medical conditions investigated are summarised in Table 6. Genetic conditions and infectious diseases (153, 63.2%) were the main areas covered by the articles and reports assessing antenatal screening. Metabolic and structural conditions (57, 60.0%) were the main areas covered by health economic assessments evaluating newborn screening programmes.
| Conditions | Articles and reports assessing antenatal screening (%)a (n = 242) |
Articles and reports assessing newborn screening (%)a (n = 95) |
|---|---|---|
| Developmental | 0 (0) | 6 (6.3) |
| Endocrinology | 24 (9.9) | 4 (4.2) |
| Genetic | 77 (31.8) | 12 (12.6) |
| Gestational cardiorenal | 5 (2.1) | 0 (0) |
| Haematology | 18 (7.4) | 12 (12.6) |
| Infection | 76 (31.4) | 3 (3.2) |
| Intrauterine fetal demise/sudden infant death syndrome | 1 (0.4) | 1 (1.1) |
| Maternal mental health | 1 (0.4) | 6 (6.3) |
| Metabolic | 0 (0) | 32 (33.7) |
| Neurodevelopment | 1 (0.4) | 3 (3.2) |
| Neurological | 0 (0) | 1 (1.1) |
| Nutrition | 4 (1.7) | 0 (0) |
| Structural | 36 (14.9) | 25 (26.3) |
| Urology | 1 (0.4) | 0 (0) |
The key methodological characteristics of the health economic assessments from the CHEERS checklist are summarised in Table 7 and in the following subsections.
| Articles and reports assessing antenatal screening (%) (n = 242) |
Articles and reports assessing newborn screening (%) (n = 95) |
|
|---|---|---|
| Study design | ||
| Individual patient-level data analysis | 12 (5.0) | 6 (6.4) |
| Cohort | 10 (4.1) | 5 (5.3) |
| Cross-sectional | 0 (0) | 1 (1.1) |
| Randomised controlled trial | 2 (0.8) | 0 (0) |
| Decision-analytical model | 200 (82.6) | 72 (76.6) |
| Decision tree | 90 (37.2) | 39 (41.5) |
| Decision tree and Markov model | 9 (3.7) | 6 (6.4) |
| Discrete event simulation model | 1 (0.4) | 1 (1.1) |
| Markov model | 10 (4.1) | 15 (16.0) |
| Model type not specified | 83 (34.3) | 8 (8.5) |
| Patient-level simulation model | 7 (2.9) | 3 (3.2) |
| Other | 2 (0.8) | 3 (3.2) |
| Not stated | 28 (11.6) | 13 (13.8) |
| Type of economic evaluationa | ||
| Cost–benefit analysis | 18 (7.4) | 5 (5.3) |
| Cost–consequences analysis | 6 (2.5) | 3 (3.2) |
| Cost-effectiveness analysis | 87 (36.0) | 47 (50.0) |
| Cost-minimisation analysis | 2 (0.8) | 3 (3.2) |
| Cost–utility analysis | 129 (53.3) | 38 (40.4) |
| Perspective of costsa | ||
| Health system or payer | 107 (43.5) | 53 (52.5) |
| Societal | 44 (17.9) | 25 (24.8) |
| Not applicableb | 0 (0) | 1 (1.0) |
| Not stated | 95 (38.6) | 22 (21.8) |
| Time horizon of decision-analytical model | ||
| Up to delivery | 9 (4.5) | 0 (0) |
| From delivery to 1 year from delivery | 26 (13.0) | 7 (9.7) |
| Between 1 year to specific time horizon excluding lifetime | 8 (4.0) | 14 (19.4) |
| Lifetime: infant | 52 (26.0) | 37 (51.4) |
| Lifetime: mother | 16 (8.0) | 0 (0) |
| Lifetime: mother and infant | 12 (6.0) | 0 (0) |
| Not stated | 77 (38.5) | 14 (19.4) |
| Sources to inform health benefits | ||
| Primary data collection | 21 (9.7) | 13 (14.1) |
| Evidence synthesis of secondary data | 167 (77.3) | 62 (67.4) |
| Combination of primary and secondary data | 28 (13.0) | 16 (17.4) |
| Expert opinion only | 0 (0) | 1 (1.1) |
| Main outcome measure used in the economic evaluationa | ||
| Natural units | 187 (59.2) | 73 (65.2) |
| QALYs | 126 (39.9) | 36 (32.1) |
| DALYs | 3 (0.9) | 2 (1.8) |
| Not applicableb | 0 (0) | 1 (0.9) |
| Reporting of preference-based outcomes in cost–utility analysis | ||
| Maternal QALYs/DALYs | 94 (72.9) | 4 (10.5) |
| Infant QALYs/DALYs | 19 (14.7) | 34 (89.5) |
| Maternal and infant QALYs/DALYs | 16 (12.4) | 0 (0) |
Choice and time horizon of model
The most common type of economic evaluation used was ‘cost–utility analysis’, which reports outcomes in terms of QALYS or disability-adjusted life-years (DALYs), for antenatal screening (129, 53.3%), and cost-effectiveness analysis for newborn screening (47, 50.0%). Decision-analytical models were employed in 272 (81.0%) of the articles and reports for the economic evaluations – 200 (82.6%) in antenatal screening and 72 (76.6%) in newborn screening. Among these studies, the majority either employed a lifetime horizon (82, 41.0% for antenatal screening and 37, 51.4% in newborn screening) or did not state the time horizon (75, 37.5% for antenatal screening and 14, 19.4% for newborn screening).
Cost perspective
The costing perspective adopted was not stated in 117 (33.7%) articles and reports. Among those that stated a costing perspective, the majority adopted a health system or payer perspective (107, 43.5% for antenatal screening and 53, 52.5% for newborn screening).
Main outcome measures used in the economic evaluations
The source to inform the main outcome measures in the economic evaluations came predominantly from evidence synthesis of secondary data for both antenatal (167, 77.3%) and newborn (62, 67.4%) screening. Natural units such as number of cases averted and number of cases detected were the more commonly reported outcome measure in both antenatal (187, 59.2%) and newborn (73, 65.2%) screening studies. QALYs were used as the main outcome measure in 129 (39.9%) of antenatal screening and 36 (32.1%) of newborn screening studies. The DALY metric (an outcome measure that combines years of life lost due to premature mortality and years lived with a disability) was used in five studies across both types of screening programmes. Maternal preference-based outcomes (QALYs/DALYs) were reported in 94 (72.9%) of the antenatal screening evaluations, whereas infant preference-based outcomes were reported in 34 (89.5%) of the newborn screening evaluations.
Preference-elicitation methods for valuation of outcomes
Thirty out of 162 studies generated QALYs based on preferences for relevant health states using direct valuation exercises or preference-based instruments based on individual patient-level data. Thirteen out of the 65 studies (20%) reported that they had used a standard gamble and/or time trade-off method to obtain preferences directly from individuals within their studies; of which, 10/47 (21.3%) were antenatal screening programme assessments and 3/18 (16.7%) newborn screening programme evaluations. The use of preference-based instrument to describe health-related quality of life outcomes was limited with only 9/47 studies (19.1%) that investigated antenatal screening programmes and 7/18 (38.9%) that investigated newborn screening programmes stating clearly the instrument used and included the EQ-5D, Health Utilities Index 2 (HUI2), HUI3, 16-Dimension (16D) or the Quality of Well-Being Scale (QWB). There were two studies (one each for antenatal and newborn screening programmes) that used mapping of a non-preference-based survey [i.e. Edinburgh Postnatal Depression Scale and Adrenoleukodystrophy-Disability rating scale (ALD-DRS)] onto a generic preference-based measure.
Assessment of reporting quality
Reporting quality assessed using the CHEERS checklist was heterogeneous among the 264 full-length articles and reports (as summarised in Appendix 5). The top five items not satisfied among the studies for antenatal screening programmes were ‘Abstract’ (160, 88.4%), ‘Time horizon’ (153, 84.5%), ‘Choice of model’ (153, 84.5%), ‘Discount rate’ (130, 71.8%) and ‘Study funding, limitation, generalisability, and current knowledge’ (123, 68.0%). Similar results were found among studies assessing newborn screening programmes. The top five items not satisfied among these studies were ‘Abstract’ (69, 83.1%), ‘Time horizon’ (67, 80.7%), ‘Study funding, limitation, generalisability, and current knowledge’ (59, 71.1%), ‘Choice of model’ (55, 66.3%), ‘Discount rate’ (53, 63.9%) and ‘Setting and location’ (53, 63.9%). The majority of these items were partially satisfied as authors failed to justify the rationale of their methodology as required by the CHEERS checklist.
Discussion
This is the first systematic review to synthesise the evidence surrounding the benefits and harms adopted by health economic assessments evaluating antenatal and newborn screening programmes in OECD countries. Almost half of the articles were published in first-quartile journals, indicating interest in the topic by high-impact journals. Most of the economic evidence of antenatal screening programmes focused on screening for genetic conditions or infectious diseases, while that surrounding newborn screening programmes primarily focused on screening for metabolic or structural conditions.
We found clear evidence that decision-analytic models represent the main vehicle for the conduct of these studies, unsurprisingly given the nature of the evidence synthesis needed. Almost half of the articles and reports used standard health economic measures of QALYs or DALYs to measure the health benefits of the screening programmes. Only 30 of the studies using QALYs attempted to estimate preferences for relevant health states using valuation exercises or employing a preference-based instruments or mapping exercise on participant-level data sets. Therefore, the main source of information to inform utility values used in QALY estimations was the published literature.
A key strength of this review includes the focus on a comprehensive set of antenatal and newborn screening programmes across OECD countries. We did not restrict our search to English-only records to avoid language bias and did not restrict to the published literature only to avoid publication bias. However, this study has its limitations. We did not perform dual extraction of data, as currently recommended,43 due to the large amount of information to extract from the final included articles and reports and the timelines to complete the project. For practical purposes and quality assurance, dual data extraction was performed for 10% of the papers after consulting our Independent Oversight Committee and information specialist (NR) using a reconciliation process that ended in a high-level agreement between reviewers. Furthermore, reporting quality was assessed using the original CHEERS checklist and not the CHEERS 2022 checklist that was published after the completion of the systematic review. 46 Arguably, application of the CHEERS 2022 checklist, which includes requirements to report on the use of health economic analysis plans, the contributions of patients and members of the public to study design and reporting, and trade-offs between efficiency and equity concerns, would have led to different assessments of reporting quality.
We found that many of these studies did not adhere to recent reporting guidance for health economic evaluations. Time horizon, choice of model and discount rates were poorly reported in general. Related to time horizon, we observed that authors employed longer time horizons to estimate health benefits than their associated costs counterparts. It was common to observe studies that used a lifetime horizon for the estimation of QALYs but a shorter time frame (e.g. up to delivery or when a case was detected) for the costs included in the model. Current lack of long-term data to inform accurate costs of living with a condition over time partly explains this result,11 but it highlights a serious limitation of these studies. It also indicates that these studies did not adhere to recognised methods guidelines for the conduct of economic evaluations for the purposes of assessing the value for money of screening programmes. 14 This suggests that policy-makers using cost-effectiveness information from these studies to inform local decision-making should read these reports with caution.
Chapter 4 Work package 1: developing a thematic framework of benefits and harms to use in health economics assessments evaluating antenatal and newborn screening programmes
Introduction
The systematic review presented in the previous chapter identified all the health economic assessments evaluating antenatal and newborn screening programmes over the last two decades and described their characteristics and reporting quality. This chapter presents the methodology used to understand the benefits and harms adopted in these studies.
Methods
Development of bespoke form
A rapid review was conducted to identify previous checklists in the area of identification of benefits and harms associated with screening programmes. We evaluated checklists for the conduct of economic evaluations of screening programmes [i.e. Consensus Health Economic Criteria for trial-based studies and International Society of Pharmacoeconomics and Outcomes Research (ISPOR) checklist for model-based studies], and report of harms in clinical studies [i.e. PRISMA-Harms, PRIO-Harms, McHarm and Consolidated Standards of Reporting Trials (CONSORT)-Harm checklists]. This review suggested that no previous checklist was fit-for-purpose to identify benefits and harms associated with screening and adopted by economic evaluations. We therefore developed a de novo bespoke form for this purpose. Our starting point was that benefits and harms can manifest across the screening pathway and that ideally studies should report the following: the stage of the screening pathway; information on different phases of the screening programme such as the screening test, confirmatory diagnosis (if needed) and treatment; and description of consequences included, depending on the outcomes of the screening test (i.e. true positives, false positives, true negatives, false negatives as well as inconsequential conditions that will remain without symptoms during lifetime among the ‘true positives’ and ‘false negatives’) as set out in recent guidance. 1 Therefore, a bespoke form based on the aforementioned was created (see Appendix 3).
Development of the thematic framework
For all the studies included in the systematic review, we attempted to complete the bespoke form. The information captured in the bespoke form was used to create a framework of benefits and harms adopted by health economic assessments using a process of grouping themes derived from the data collected in the bespoke form. 47 An integrative descriptive analysis48 of the collated themes within each category was then conducted, resulting in a taxonomy of benefits and harms consisting of a primary theme and up to four levels of subtheme(s). In the first step, the description of consequences was categorised into specific themes by ST-P. This pool of themes was the starting point of an iterative process where members of the study team (ST-P, MEP, OR-A and SP) merged, separated and refined the wording of themes and subthemes. The iterative process was maintained until consensus was reached among the study team (ST-P, MEP, OR-A and SP). Articles and reports were categorised into themes and subtheme(s) according to the condition and screening type. Bar charts were generated to illustrate the framework across and by medical condition(s).
Results
We identified 86 unique descriptions of consequences across all articles and reports up to January 2021. Our thematic analysis resulted in seven core themes around benefits and harms with each core theme including up to four levels of subtheme(s). An update of the search strategies up to November 2021 identified an additional 18 articles but no new themes on benefits and harms emerged (see Report Supplementary Material 2). An abridged version of the thematic framework with a description of each theme and key examples is presented in Table 8 with the full version up to subtheme level 4 presented in Table 27 (see Appendix 6). All the themes listed are applicable to both antenatal and newborn screening except for theme 6 – pregnancy loss, which is only relevant to antenatal screening programmes.
| Theme no. | Theme | Description | Key selected examples |
|---|---|---|---|
| 1 | Diagnosis of screened for condition | Related to the process of identifying a condition through screening. For example, cases diagnosed or missed, confirmatory tests (necessary and unnecessary), reduction in infants born with condition through effective treatment, or pregnancy termination | Infants born with condition Confirmatory test and additional tests to reach diagnosis of screened for condition Cases missed at screening Cases diagnosed at screening Screened for condition-related complications Additional screening of partners Additional testing to reach diagnosis in the absence of screening (links to diagnostic odyssey) |
| 2 | Life-years and health status adjustments | Impact of identifying a condition on the health of women, infants and other family members and included, for example, standard health measures such as QALYs, DALYs, life-years or impact of anxiety on parents after a false-positive result | Infant life-years post birth (including QALYs) Maternal life-years (including QALYs) Parental QALYs Psychological (anxiety/disutility from false-positive results, genetic variants of unclear penetrance, or knowledge of disease) |
| 3 | Treatment | Caused by harms of adverse reactions, unnecessary interventions and antibiotic resistance, or benefits of adverse complications averted due to timely interventions | Comparison of earlier treatment after screen detection and later after symptomatic detection Additional health care post diagnosis Hospital stay Missed due to false negative Prevention of screened for condition (infectious) Psychological (counselling about screening/confirmatory test/genetic diagnosis) Screened for condition-related treatment/management Treatment-related harm (disutility/anxiety/adverse reaction/antibiotic resistance) Unnecessary due to false positive |
| 4 | Long-term cost associated with screened for condition | Impact on long-term healthcare and non-healthcare costs related to identifying a condition through screening | Direct healthcare cost Direct non-healthcare cost (education/social care/caregiving) Productivity gains Societal cost |
| 5 | Overdiagnosis | Impact on costs and consequences of detecting a condition that would never develop into symptomatic disease | QALY decrement Unnecessary test/treatment |
| 6 | Pregnancy loss | Caused by treatment or an invasive diagnostic procedure, or an informed decision of termination after a true-positive result | Spontaneous Termination (of unaffected fetus due to false-positive test result/prevent downstream adverse maternal outcomes/psychological consequences) Treatment/test related |
| 7 | Spillover effects | Health and well-being effects to parents and other relevant stakeholders as a direct consequence of the child’s diagnosis | Health impacts to parents and siblings from child’s diagnosis with genetic condition, through knowledge of their own genetic status |
The benefits and harms incorporated within health economic assessments are presented in Figures 5 and 6 by screening type using the thematic framework. Limited information about benefits and harms was provided in 81 (33.5%) out of the 242 antenatal screening evaluations and 19 (20.0%) out of 95 newborn screening evaluations (e.g. conference abstracts). These included 51 (63.0%) antenatal screening evaluations and 11 (57.9%) newborn screening evaluations described in conference abstracts. Across all conditions targeted by antenatal screening, represented in Figure 5 (n = 242), 115 (47.5%) incorporated benefits and harms related to the diagnosis of screened for condition (theme 1). Ninety (37.2%) of the evaluations included benefits and harms related to life-years and health status adjustments (theme 2). Eighty-eight (36.4%) of the antenatal screening evaluations included benefits and harms associated with treatment (theme 3). In general, for antenatal screening, benefits and harms associated with the long-term costs of screened for conditions (theme 4) were only adopted in 68 (28.1%) of the evaluations. Only 21 out of the 242 (8.7%) antenatal screening evaluations adopted benefits and harms from all of themes 1 to 4. The condition in the antenatal screening programmes which reported the widest range of themes was infectious diseases (see Report Supplementary Material 2). Among the 76 antenatal infectious diseases programmes, 22 (28.9%) did not have any themes and the remaining studies reported at least one theme from themes 1 to 6. However, none of them reported any spillover effects (theme 7).
FIGURE 5.
Benefits and harms adopted by health economic assessments evaluating antenatal screening programmes using thematic framework. a, Limited information about benefits and harms could be extracted from 81 (33.5%) out of the 242 antenatal screening evaluations to inform our bespoke form.

FIGURE 6.
Benefits and harms adopted by health economic assessments evaluating newborn screening programmes using thematic framework. a, Limited information about benefits and harms could be extracted from 19 (20.0%) out of 95 newborn screening evaluations to inform our bespoke form.
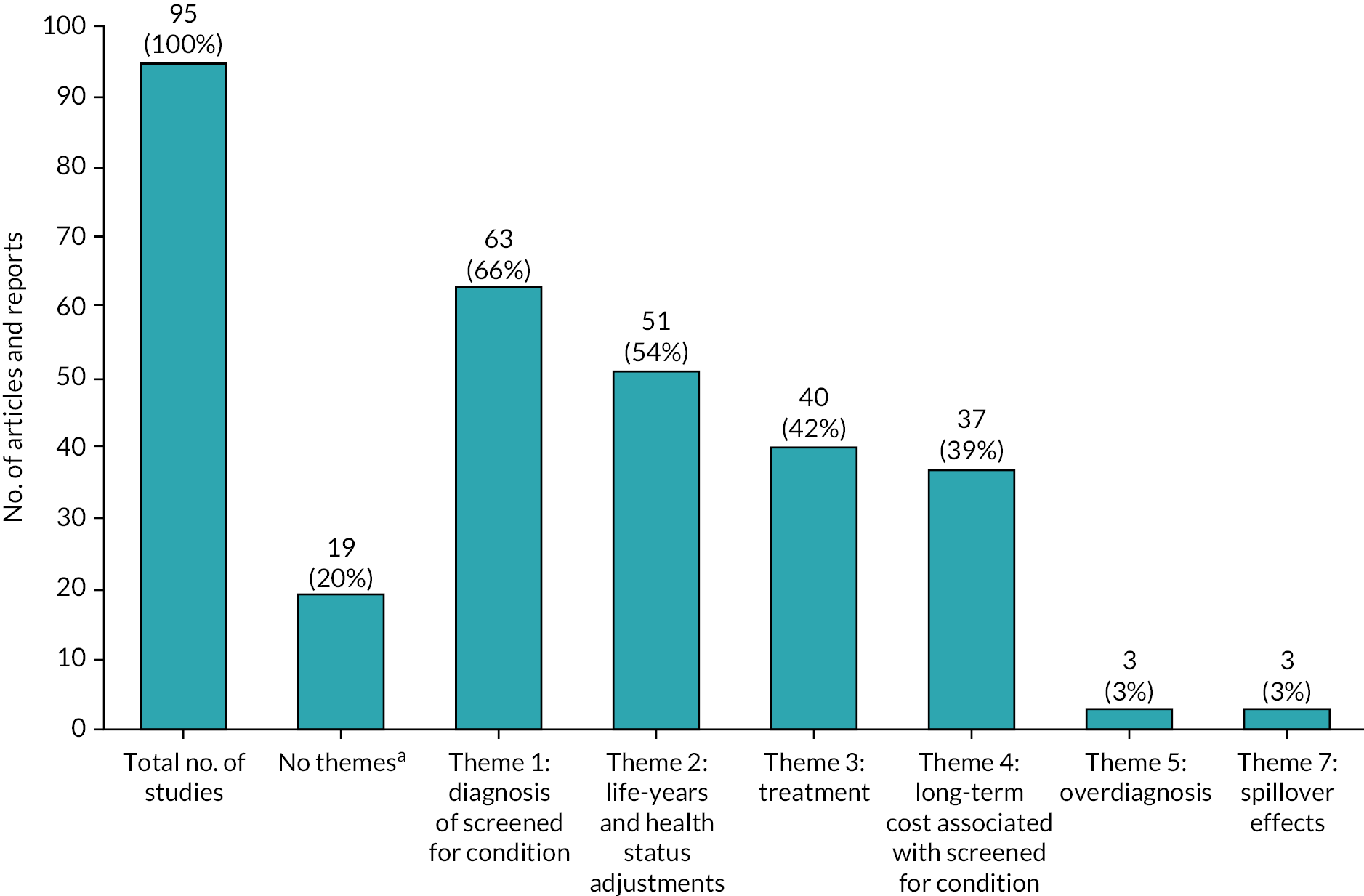
In newborn screening in Figure 6 (n = 95), 63 (66.3%) evaluations incorporated benefits and harms related to the diagnosis of screened for condition (theme 1). Fifty-one (53.7%) evaluations included life-years and health status adjustments as benefits or harms (theme 2). Forty (42.1%) of the antenatal screening evaluations included benefits and harms associated with treatment (theme 3). Benefits and harms associated with the long-term costs of screened for conditions (theme 4) were only adopted in 37 (38.9%) of the evaluations. Only 17 out of the 95 (17.9%) newborn screening evaluations adopted benefits and harms from all of themes 1 to 4. Benefits and harms related to overdiagnosis (5, 1.5%) and spillover effects (1, 0.3%) were largely absent from the studies. The condition category in the newborn screening programmes for which the widest range of themes was reported was metabolic conditions (see Report Supplementary Material 2). Among the 32 newborn, metabolic screening programmes, 9 (28.1%) did not have any themes and the remaining studies reported at least one theme from themes 1 to 7 (but note that theme 6 which is on pregnancy loss was not applicable to newborn screening programmes).
Table 2 in Report Supplementary Material 2 summarises the benefits and harms adopted in the articles and reports for specific conditions. Health economic assessments evaluating antenatal screening programmes for infectious diseases adopted the broadest spectrum of benefits and harms compared to the other conditions.
Discussion
Our thematic analysis summarised a wide range of benefits and harms adopted by the studies identified in our systematic review and summarised them into seven core themes. There is no consistency on the selection of benefits and harms across and within conditions, suggesting that additional guidance is needed in this field. In general, articles and reports assessing antenatal and newborn screening programmes have considered benefits and harms that reflect the processes of identifying a condition in their health economic assessments. This includes, for example, cases correctly identified or missed or the number of unnecessary tests due to false positives. This result is not surprising because benefits and harms associated with the diagnosis of screened for conditions provide the first line of clinical evidence about these programmes and are of key interest to screening organisations. Around half of the articles and reports evaluating newborn screening programmes across all conditions did not consider benefits and harms associated with life-years and health status adjustments. Our review also found that benefits and harms identified as important by screening agencies and international health organisations, including overdiagnosis and spillover effects on family members, have rarely been adopted by these economic evaluations. 1,49 In the case of spillover effects, the only relevant subtheme identified was benefits to parents that inform future reproductive decisions from discovering carrier status as a consequence of the child’s diagnosis. 50
Our analysis of the application of the thematic framework to each study identified in the systematic review made an implicit assumption that all themes were relevant across the target condition of screening. Arguably, that may not the case. For instance, incorporating long-term costs and spillover effect for a condition, where health status is effectively restored to healthy levels after treatment for a true-positive screen result, may not be needed. However, we argue that for most of the conditions assessed by screening agencies including the UK NSC that is not the case. In general, we expect themes 1–4 to cover the main effects of screening associated with correct/incorrect results, impact on health on women and their baby, consequences of treatment and implications for the healthcare system in terms on costs. All economic evaluation ought to incorporate benefits and harms associated with these themes at a minimum. Themes 5 and 7 might not be relevant to all target conditions but authors should explain their rationale to exclude these benefits and harms from their health economic assessments. Theme 6 and pregnancy loss, as already eluded in the methods, only target conditions of antenatal screening and is a key outcome of interest of the fetal anomaly scan in the UK. 51
Authors did not generally refer to ‘benefits’ and ‘harms’ when describing the utilities and disutilities included in their evaluations. In addition, what constitutes a benefit or harm depends on the perspective of the particular stakeholder involved in the decision-making. For instance, a reduction in the number of infants born with a condition through pregnancy termination may be seen by some as a societal benefit in economic terms due to healthcare savings and reduced societal comorbidity. However, this may well be considered a devastating harm for families who value living with an infant with a condition. Therefore, we had to extract and interpret detailed information about the consequences included in the studies and reports for the thematic analysis without value judgements and recognising that the same consequence as described above could be categorised as a benefit or harm.
To our knowledge, this is the first time a detailed account of benefits and harms adopted by health economic assessments has been conducted in the literature. Previous work has focused on the identification of methodological challenges and the development of good practice guidelines in the development of health economic assessments of antenatal and newborn screening programmes. 20,30,31 Our work suggests that there is an immediate need to provide guidance for researchers conducting these types of studies in the future. This is addressed in later chapters in the report. Our proposed framework of benefits and harms can be used as a starting point to guide the development of health economic assessments evaluating antenatal and newborn screening for specific conditions.
Chapter 5 Work package 2: systematic search and meta-ethnography of parents’ experiences of newborn screening
Sections of this chapter have been previously reported in White et al. (2021). 52
Introduction
This meta-ethnography49 used a systematic review process to identify qualitative studies that focus on parents’ experiences of newborn screening published in English-language academic journals from 2000 to 2019 (n = 36). The included studies represented a range of moments, outcomes, screening programmes and conditions that illuminated discrete elements of the newborn screening journey. We drew on these varied studies to construct a diagram of possible newborn screening pathways that parents may experience and identified a ‘critical window’ of time in parents’ accounts that occurred between the signalling of a positive newborn screen and the outcome of confirmatory testing or diagnosis. During this critical window, families navigate complex emotional reactions, information, and decisions. From an in-depth analysis of this data, we developed the concept of ‘absorptive capacity’ as a lens through which to understand parents’ experiences of, and reactions to, new and emerging information during this critical window. We also identified how the ‘concertinaing of time’ – the various ways that parents experience the expansion and compression of time throughout and beyond the screening pathway – directly impacts their absorptive capacities. This study underscored the need to move away from viewing newborn screening as a discrete series of clinical events, but rather a process that can have far-reaching implications across time, space and family groups. Further, it informed us of potential benefits and harms for associated with newborn screening that needed to be considered for the subsequent pieces of qualitative work.
Methods
While the larger project is solely focused on the UK, we reviewed the international literature to interpret existing findings and develop a fuller conceptual understanding of the way newborn screening is experienced. We approached the review sensitised to the possibility that different sociocultural, geographical and health system contexts will impact the screening experience but sought to identify cross-cutting themes that transcended these differences. This review did not require ethical approval since it drew on existing publications.
Deviation from protocol
As we developed our approach to reviewing the literature on experiences of newborn screening, we decided that using a meta-ethnography, rather than a scoping review, would allow give us more sophisticated purchase on the literature we sought to synthesise. The goal of this approach is to bring together, and interpret, insights from qualitative research and producing an in-depth understanding of their collective findings. This approach supported identification of the key themes of absorptive capacity, reported in the article in Social Science and Medicine. 52
Approach to meta-synthesis
We opted not to conduct a meta-ethnography of experiences of antenatal screening due to the existence of several reviews, meta-syntheses and meta-ethnographies in this area,53–56 including those published recently. 57,58 This literature highlights the sense of responsibility felt by parents to participate in antenatal screening,55,56 the shock and devastation experienced when an unanticipated result was returned54,55 as well as the significance of technology, and relationships with healthcare professionals as mediators of the antenatal screening experience. 57
Unlike antenatal screening, parents’ experiences of newborn screening have been less extensively reviewed. This is in spite of the newborn genomes programme gaining momentum59 and increasing calls to expand the newborn bloodspot test to include more conditions (e.g. SMA Newborn Screening Alliance, 2021). As such, the scope and delivery of newborn screening is currently high on both the research and policy agenda in the UK. In light of this context, our meta-ethnography was designed to analytically interrogate parents’ experiences of newborn screening to contribute to debates around expanded screening, and to interpret the findings as a contrast to the existing antenatal screening literature, where decisions, timeframes and outcomes of screening are entirely different.
The goal of a meta-synthesis is to bring together, examine and interpret findings from disparate qualitative research studies and produce a more in-depth understanding than is possible from looking at the studies individually. 50,60 It offers the opportunity to identify patterns, processes and contexts, as well as omissions from a body of work. 61 While there are multiple ways of synthesising qualitative research,62 we followed the stages of meta-ethnography as described by Toye et al. (2014). 63
Stages of meta-ethnography
We wanted to bring together qualitative studies that explored parents’ experiences of newborn screening. We opted to conduct a systematic literature search to provide evidence that we sought to capture as much of the evidence within the scope of our research question as possible. 63 Recognising that it can be challenging to locate qualitative research studies, we began with a broad, systematic search strategy. 64 We searched for any instance of the terms ‘newborn screen*’, ‘neonatal screen*’ or ‘newborn bloodspot’ in the title or abstract of academic journals published in English from January 2000 until December 2019 across five databases accessed through the University of Oxford Libraries (Table 9).
| Search terms in title or abstract | Databases |
|---|---|
| Newborn screena | CINAHL Complete |
| Neonatal screena | JSTOR |
| Newborn bloodspot | PsycINFO |
| Sociological Abstracts | |
| Web of Science |
After running the search, AW removed duplicate records and reviewed titles and abstracts for eligibility. If it was unclear whether or not a record should be included based on the title and abstract, it remained in the pool until the next step. Next, AW read the remaining studies in their entirety to assess eligibility. LH provided a secondary ruling for articles that AW was uncertain about. As the final step, AW hand-searched the reference lists of included studies to identify additional research not identified through the search strategy. AW maintained a database of all decisions about inclusion and exclusion (Figure 7).
FIGURE 7.
Flow diagram of included and excluded studies.

Studies were eligible for inclusion if they focused on parental experiences of newborn bloodspot screening programmes and used qualitative methods. Non-research publications, such as commentaries or letters to editors, were excluded. Similarly, mixed-methods research was excluded, as the qualitative analysis was often secondary to reporting statistical findings. As we were interested in how parents experienced varying aspects of the newborn screening process, studies that included other stakeholders (e.g. genetic counsellors or midwives) alongside parents were excluded, as it was challenging to separate findings between stakeholder types. We also excluded studies that were beyond the scope of the review, including those focused on storing newborn blood spot cards, antenatal genetic counselling, being diagnosed with a screened-for condition in later life or living with a screened-for condition. We did not exclude any papers based on quality. We were aware that the authors of included papers were writing with varying aims for different audiences, and they ranged from healthcare providers to ethnographers. The papers included in this synthesis reflect the wide-ranging disciplinary backgrounds and purposes of the authors contributing to this field.
Once we had a finalised list of included studies, we divided the work of reviewing among the research team. AW read all of the studies and maintained an Excel database tracking study characteristics. We split the number of studies (n = 36) evenly among the remaining four members of the research team (AM, FB, LH, LL) so that each was responsible for focusing on nine studies. We read the included studies in their entirety, with particular attention focused on the findings and discussion sections of studies. We used a combination of computer-aided and paper-based reading and coding processes. AW uploaded PDFs for the included studies to NVivo 12 Pro (QSR International, Warrington, UK). She progressed from a line-by-line coding approach to organising codes into descriptive themes and then refining codes into conceptual categories. The remaining researchers used a paper-based approach to coding, where they read the studies and hand-coded higher-level concepts as they emerged. They made a note of these concepts and shared them with the research team during analysis meetings.
After we analysed the studies individually, we set about synthesising the literature and identifying meta-themes. This was an iterative process that took place over several months. We arranged evidence synthesis meetings to discuss our analysis and identify cross-cutting themes. We discussed overarching concepts and considered how they might apply to various other studies in the synthesis. Even though individuals were responsible for different subsets of studies, we generally found that we had developed overlapping concepts, albeit under slightly different names. As we reached consensus on the core experience dimensions drawn from the compiled literature, we synthesised these findings into higher-order interpretations across screening contexts and conditions from which substantive conclusions about the experiences and implications of newborn screening from a parental perspective could be drawn.
Results
Mapping newborn screening pathways from included studies
Our systematic review yielded a total of 36 studies (see Appendix 7). Studies ranged from descriptive accounts of the newborn screening experience to theory generation about the meanings attached to those experiences. Given the complexity of newborn screening, many studies were focused on discrete points in the pathway, although collectively they covered the newborn bloodspot ‘journey’. Through a cross-study analysis, a broader, richer picture takes shape compared to what can be provided in the individual papers.
Newborn screening has become an embedded part of the neonatal experience for parents. Drawing on the included studies, we mapped the various pathways that parents and babies might take when experiencing newborn screening (Figure 8). The consent process varies across, and even within, countries. In the USA, for example, screening is compulsory across nearly, but not, all of the 50 states. In other countries, parents are nominally required to consent to newborn screening. For example, in the UK under the NHS guidelines, healthcare providers (HCPs) should offer parents screening, and parents may verbally agree. 65 In practice, however, the extent to which parents are aware of their ability to refuse newborn screening is unclear, as a mother whose child screened negative in England reported, ‘It’s a very, very quick process and you’re not given any option to think about it’. 66
FIGURE 8.
Map of newborn screening pathways.

Regardless of the differences in the consent process, our review suggests that newborn screening is poorly understood, and its potential ramifications are not readily considered by parents. Parents frequently reported not recalling the consent process, or much about the purpose of the screen. 66–69 Parents described putting their trust in the healthcare system and medical authority, with newborn screening being largely seen as something that ‘just happens’ after having a baby, rather than an active choice. For these screen-negative families, newborn screening is ushered in by trusted medical authorities and is typically an experience that passes with little concern or complication.
While the majority of families exit their newborn screening journey swiftly, there is a subset of families who will receive the news that their baby screened positive, and these families are typically offered further testing or investigations. This is a moment of no return for many families, which has implications not only for the infant and parents, but also for the family writ large. Before receiving the news, parents may not have realised or appreciated how much impact the ‘heel prick’ test could have on their lives. Most parents – unless they are known carriers or are living with a condition – tend to have limited knowledge of the various screened-for conditions. 70 This is exacerbated by the fact that for the vast majority of conditions identified through the newborn screening ‘heel prick’, such as inherited metabolic diseases like phenylketonuria (PKU), the infant is typically asymptomatic at the point a positive result is received. However, sometimes non-specific symptoms may have already been observed by the parent(s). 71 Regardless of context or condition, such news ushered families into a compressed, critical window of time characterised by waiting periods, strong affective responses and a need for more focused communication. 71–75
Positive screening results are followed up by the offer of confirmatory diagnostic testing, with the nature of this testing varying by condition. From the result of the diagnostic test, the condition is either confirmed (the screening result was a ‘true positive’) or ruled out (the screening result was a ‘false positive’). However, for a subset of families, the results of diagnostic testing are somewhat more ambiguous, indicating either a carrier status or a gene variant for which the link to phenotype is neither clear nor certain. Even in cases where a precise diagnosis is made, the broad spectrum of severities associated with the conditions screened for, combined with lack of experience with symptoms at the point of diagnosis (or potential lack of symptoms), can dramatically heighten uncertainty for parents, despite being presented with the seeming certainty of a confirmed diagnosis.
Assessing parents’ absorptive capacity
Across studies, participants frequently used descriptions and metaphors of ‘absorption’ and ‘digestion’ to describe their processing of screening and testing information. For example, ‘Your brain is a sponge’ (mother of child with cystic fibrosis76) and ‘There was just too much at that time to absorb’ (mother of child with cystic fibrosis77). These metaphors and descriptions were common and prompted us to apply the concept of ‘absorptive capacity’ to screening contexts. ‘Absorptive capacity’ is a term used widely in management studies to refer to a company’s ability to ‘recognise the value of new, external information, assimilate it and apply it’. 78 The term is not commonly used at the level of the individual but has value as a lens through which to examine and explain parents’ ability to process new diagnostic information.
For the subset of families that have a positive or inconclusive newborn ‘heel prick’ screen, the initial affective responses tended to be ones of shock and anxiety. 79–81 Parents were not necessarily aware of what they consented to (or did not give consent for), so hearing that their child tested positive ushered in a period of shock, confusion and fear. Before receiving the news, parents largely conceptualised their child as healthy and perfect, particularly if they had also gone through the process of antenatal screening without any positive screening results. Parents had to reconcile to the fact that their child might not be symptomatic and instead ‘looked’ healthy, yet still had a positive newborn screening test. In such cases, parents’ distress and shock limited their ability to absorb information in the moments following the news that their child screened positive and at later points in the diagnostic process.
Absorptive capacity is also dependent on an individual’s prior related knowledge, including familiarity with medical concepts and language. 78 Based on the studies in this review, we argue that even if parents remember consenting to, or being notified about, newborn screening, they do not necessarily have the tools to understand what it means. Parents’ distress and subsequent inability to absorb information were augmented by their own unfamiliarity with screened-for conditions and genetics. 70,72,82 Tluczek (2006)76 points out that even the language surrounding screening and testing can be fraught with confusion, including the counterintuitive meaning of the terms ‘positive’ and ‘negative’ when describing test results, compared to an everyday conversation where these indicate ‘good’ and ‘bad’, respectively. However, even this interpretation overlooks the complexity of ways these terms can be understood and experienced by families. There has been a more recent push from families living with a range of screened-for conditions to employ neutral language in screening contexts that do not pre-empt the parents’ reception of the news as either ‘good’ or ‘bad’ which has now filtered into professional guidelines. Nevertheless, the use of inaccessible medical language to describe screening and testing results and processes was a widespread concern across the data set, which in turn impacted on parents’ absorptive capacities.
Beyond parents’ (un)familiarity, the format of information presentation also influenced parents’ absorptive capacity. To effectively absorb information ‘it is insufficient merely to expose an individual briefly to the relevant prior knowledge’ (p. 131). 78 Consent, or lack thereof, for newborn screening is taken at a time when new parents are simultaneously exhausted, distracted and busy caring for their new child. New mothers may also be experiencing postnatal physical and mental health concerns of their own. Any information given about the screen at that point does not seem to be experienced as a choice or an active conversation, but rather a minimal (if any) conveyance of information. 66–69 For the subset of families who are notified of a positive or inconclusive screen, the limited prior communication about the potential implications of newborn screening sets the stage for what is often perceived to be a period of problematic communication. 72,75,80,83–85
By looking across the studies, we considered how parents’ information needs varied by condition detected and context. We identified a continuum of informational needs, with parents preferring different levels of information at different points in the screening process, based on their absorptive capacities at that time. Some parents wanted to dive in and ‘consume’ as much information as possible, while others wanted ‘bite-sized’ chunks. Others wanted to hold back from obtaining information until there was diagnostic clarity. Unfortunately, parents’ needs were not always met. In some cases, parents reported being given a volume of information that they were ill-prepared to receive and unable to absorb. In other cases, parents reported wanting more information than was given by their HCPs. As a result, they sought other sources – primarily the internet or support organisations – in an attempt to increase their knowledge. 86–88
Informational needs became more complicated in instances where HCPs themselves, who were looked to as the experts, did not have the expertise to provide answers to parents’ questions. 81,86,89,90 HCPs may be counselling families with a rare condition for the first time, and this could undermine parents’ confidence in the information provided. Although we acknowledge that there may be a discrepancy between what HCPs say and what parents hear, it seems there is room for improving communication and information provision during this critical window when absorptive capacity is in its highest state of flux. 91
Newborn screening, uncertainty and the concertinaing of time
There is a crucial temporal component to parents’ experiences of newborn screening. Throughout the critical window, time expands and contracts, generating ripple effects into the past, present, and future. During the period between a positive screen and (potentially) receiving definitive diagnostic results, families are often living through a state of ambiguity or disorientation. How people approached living in the liminal space during the critical window varied considerably.
Families who viewed ambiguity as a negative state desired definitive answers as a means of gaining control. Such families may want to find out as much as possible about a potential condition and turn to information seeking online and from those with lived experience of the condition. 82,87,92 Once these families received a diagnosis, they were more able to make sense of the condition and how to manage it in the future. 70 Even among those with an ambiguous diagnosis, there may be a drive towards labelling as an attempt to gain control and make the situation more concrete, as explored in a study of families whose children screened positive for cystic fibrosis, yet received an inconclusive diagnosis. 90 For these families, knowledge about the condition allowed them to feel prepared to manage the health and social needs of their infant moving forward. However, such families must still live with the uncertainty of how/whether the condition will manifest in their child in the future and hold out hope that they will not need to use the information in the future.
While some families sought to learn, as much as possible, other families viewed the inherent ambiguity of this liminal period in a more positive light. In such cases, families rejected in-depth information about the condition in order to retain the hope that they will never need it. 92,93 These families adopted a ‘wait and see’ approach to the period between screening positive and receiving a diagnosis, on the basis that ‘too much information can be hurtful in a sense’. 92 If they are notified that the initial ‘heel prick’ result was a false positive, such families will have avoided the anxiety and stress that additional information can bring. However, if the child is diagnosed with a condition, these families may opt for ‘ “easy-to digest” information and “just the facts, because you can’t handle anything else” ’,83 reflecting again the concept of absorptive capacity. These families continued to look towards the future as a coping mechanism, hopeful that the condition will not manifest or will be of limited severity, or perhaps that treatments will improve over time.
While families were found to take a range of differing approaches as they moved through the critical window, they all shared a common experience that was found to have long-term ramifications for everyone involved. Indeed, for screen-positive families, the experience had ripple effects that stretched both backwards and forward in time. If an inheritable condition was detected, families found themselves looking backwards in their family tree, in an expansion of time, trying to work out where the trait might have ‘come from’. 70,82,94 As families looked backwards, some grappled with shame, blame, and guilt as they considered what might have been for their child, particularly when a child inherits a condition. 77,82,86,90,94,95
The impact of newborn screening was also found to flow forwards in time from the moment of notification of positive results, as parents found themselves reconsidering their expectations of the life they had imagined for their child and for themselves as parents. In quick succession, parents had to initiate a chain of emotional processing, discussion and medical appointments, involving not just the child but sometimes also the parents and wider family members. 73,74,94 For the parents of children who were asymptomatic at the time of the results, one particularly significant part of the screening journey was a loss of what should have been a happy time with their child – of ‘blissful ignorance’. This impact was particularly pronounced when compared to the experiences of families who received a later diagnosis. 73 For some, the pre-symptomatic diagnosis ushered in by newborn screening interrupted, and limited, the joyful time families were experiencing at the birth of their child. For these families, the positive or ambiguous screening result effectively extended the length and reach of the condition earlier into the infant’s life than otherwise would have been the case.
Newborn screening also has ripple effects that extended into the future, and across generations. As families moved away from receiving a diagnosis, time expands into uncertainty and the unknown. Parents of children living with conditions or ambiguous health statuses (e.g. cystic fibrosis screen positive, inconclusive diagnosis) have to consider how, when and to whom they disclose their child’s condition. 90 Parents also have to consider their own future reproductive intentions, including whether or not they want further children and, if so, what role antenatal or newborn screening may play in any subsequent pregnancies. 96 Parents of children who are found to be carriers of a condition through newborn screening also need to consider if, when, and how they will tell their child about their result. 97 These planning conversations were found to take place even as children were still newborns, even though disclosure might not take place for many years to come. Looking further into the future, the child will also have to consider how they manage their condition or carrier status, how/whether to disclose it to others, as well as their own reproductive intentions. 86 The implications of newborn screening therefore play out in the months, years and even decades following diagnosis, suggesting that the aspects of screening are enduring over the life course.
Discussion
By looking across the range of moments, outcomes and conditions across international contexts, our synthesis characterised the critical features of the broader newborn screening experience from the familial perspective. Our findings demonstrate that newborn bloodspot screening is a familial experience. While currently most families will receive negative newborn ‘heel prick’ screening results, it is also important to consider the experiences of those who receive positive, inconclusive or ambiguous screening outcomes, particularly as we move into an era of genomic sequencing, with the potential to generate an exponential rise in the number of ‘positive’ and unexpected newborn screening results. Given the ‘urgency narrative’ often used to promote newborn screening programmes, it can be difficult to critique the expansion of newborn screening panels. 98 However, here we have provided evidence that the experience of screening is highly variable across families. We have identified and focused on a ‘critical window’ of time between being alerted to a positive or inconclusive newborn screening result and further testing wherein families must process a range of emotions, determine their informational needs, and shift through rapidly alternating periods of waiting and activity. We have developed the concept of absorptive capacity – the ability to recognise, assimilate and apply new information – to capture the abilities of parents, and crucially also the limits of those abilities, to comprehend their child’s screening results or condition. We have synthesised detailed qualitative evidence to explain the various ways that parents experience the expansion and compression of time throughout and beyond the screening pathway, demonstrating the far-reaching implications of screening across time, as well as to wider family and kin. We used these findings to begin to understand possible benefits and harms of the screening experience, which we explored further in subsequent pieces of qualitative work (see Chapters 6 and 7).
Chapter 6 Work package 2: secondary analysis of existing interviews exploring experiences of antenatal and newborn screening
Introduction
We conducted a secondary analysis of existing qualitative interviews exploring experiences of, and attitudes towards, antenatal and newborn screening, as well as the experience of living with screened-for conditions (n = 256). Here, we present findings derived from a situational analysis mapping exercise. 99 Findings demonstrate the wide-ranging elements inherent in discussions about antenatal and newborn screening. We used this information to inform our own primary data collection (see Chapter 7).
Methods
Data
We conducted secondary analysis of qualitative interviews derived from previously conducted studies exploring antenatal and newborn screening experiences and attitudes (n = 256; Table 10). These in-depth narrative interviews were conducted over the last 13 years by FB at the University of Warwick, and LH, LL et al. at the Medical Sociology and Health Experience Research Group, University of Oxford. While some interviews were conducted over 10 years ago, the majority were conducted within the past 5 years. Interviews included perspectives of people with a wide range of experiences in relation to screening, testing, pregnancy termination and continuation, as well as parents and affected adults living with genetic and chromosomal conditions.
| Type of participant | Number of transcripts | Source of data | Year(s) of collection |
|---|---|---|---|
| Parents who have undergone antenatal or newborn screening | 45 (37 women, 8 couples) | Oxford | 2005 |
| Parents whose child received a diagnosis of cystic fibrosis following newborn screening | 6 | Warwick | 2018 |
| Parents whose fetus received a diagnosis of thalassaemia following antenatal screening | 5 | Warwick | 2017–8 |
| Parents who have undergone pregnancy termination for fetal anomaly (screening or family history) | 48 (40 Oxford, 12 Warwick) | Oxford, Warwick | 2006–8 |
| Parents who refused prenatal testing for condition in family | 12 | Warwick | 2012–8 |
| Parents who continued with a pregnancy following prenatal diagnosis | 9 | Warwick | 2012–8 |
| Adults living with conditions that are currently screened for | 20 (10 cystic fibrosis, 10 thalassaemia) | Warwick | 2017–8 |
| Parents of children with conditions that are currently screened for | 20 (7 cystic fibrosis, 7 thalassaemia, 6 Down syndrome) | Warwick | 2017–8 |
| Parents with experience of neonatal surgery | 13 | Oxford | 2017 |
| Participants with experience of late miscarriage | 3 | Oxford | 2018 |
| Families (parents and affected adults) living with genetic diseases that are not yet screened for | 75 (36 spinal muscular atrophy, 22 haemophilia, 17 fragile X syndrome) | Warwick | 2012–8 |
Analysis
We used a situational mapping exercise to analyse this large data set. Situational maps ‘lay out the major human, non-human, discursive, and other elements in the research situation of concern and provoke analyses of relations among them’ (p. 554). 99 Situational maps are a reflexive, subjective form of data interpretation which aim to capture the complexities of a situation and their relations. 99,100 They are useful for rendering large data sets manageable and identifying complexity within the data. Our aim was to understand the people, objects, places and discourses that influenced antenatal and newborn screening experiences and attitudes. AW read the interview transcripts and used concept coding to assign meanings to chunks of the data. 91 These concepts represented higher-order themes that went beyond an individual narrative, and they typically included nouns (e.g. time) or processes (e.g. conceptualising normality). Concept coding was deemed appropriate since we were interested in meta-themes across the vast data sets and progressing towards an understanding of key concepts surrounding antenatal and newborn screening. 101
We used the concept codes to develop a situational map through an iterative, increasingly ordered representation of the elements related to antenatal and newborn screening. 99 Initially, this was a messy list of elements rooted in the empirical evidence from the conceptual codes. Over several weeks, we iteratively discussed the elements and considered the content of the data and boundaries of this project. 100 We refined the elements and organised them based on Clarke’s (2003)99 categories. Our situational map has both human elements and non-human elements. Human elements include the key individuals, groups and institutions involved in screening. Non-human elements include the technologies, materials and information that ‘structurally condition the interactions within the situation through their specific properties and requirements’ (p. 561). 99 We also considered the ‘ideas, concepts, ideologies, discourses, symbols, sites of debate’ that are involved in antenatal and newborn screening (p. 563). 99 Thus, we developed categories for economic elements (e.g. funding), sociocultural elements (e.g. gender or ethnicity), temporal elements (e.g. waiting periods), spatial elements (e.g. geographical differences) and related discourses (e.g. conceptualising quality of life).
As the situational map took shape, we conducted several rounds of revision to condense and clarify the categories. We asked PPI members to review the list of human elements (see Human elements involved in antenatal and newborn screening) and we also presented the entire situational map to our independent oversight committee. We used the input of these groups to make final minor adjustments to our meta-themes (e.g. naming conventions) but they deemed that no other adjustments were necessary.
Stakeholder mapping
We identified stakeholders for inclusion in our primary data collection as part of the situational analysis exercise (i.e. the individual and collective human elements). We compiled the list of stakeholders and presented it to our PPI members. We asked PPI members to review the list and brainstorm if there were any missing stakeholders based on their knowledge and/or experiences. The PPI members named different types of HCPs, but we have condensed these into broad categories in our situational analysis map (e.g. doctor, nurse). They also named different types of charities. Again, we condensed these into a broad category (i.e. ‘charity organisations’); however, we did seek out the identified charities for our later primary data collection (see Chapter 7). We also sought approval for our stakeholder list from our independent oversight committee. Nothing further was added and the stakeholder list was approved.
Using comments submitted to the National Screening Committee
Additionally, we reviewed comments sent to the UK NSC for previous reviews of antenatal and newborn screening that went to consultation within the last 5 years. We wanted to verify that the stakeholder groups identified in our secondary analysis were reflective of the stakeholders that typically submit comments. We observed good correlation between our stakeholder list and those submitting responses to UK NSC policy reviews, and as such were satisfied with the robustness of our approach.
Results
Our situational map has 11 categories comprised of nearly 100 elements, which we describe in Table 11.
| Individual human elements | Collective human elements | Implicated actors | Discursive constructions of human elements |
|---|---|---|---|
| Non-human elements | Discursive constructions of non-human elements | Temporal elements | Spatial elements |
|
|
|
|
|
|
|
|
| Sociocultural elements | Economic elements | Related discourses and debates | |
|
|
|
Human elements involved in antenatal and newborn screening
The secondary analysis revealed that there are many varied human elements involved in antenatal and newborn screening. During antenatal screening, the mother’s well-being is at the centre of these discussions. During newborn screening, the baby’s well-being is at the centre of these discussions. Other family actors include the father, other children, and extended family members such as grandparents, aunts, or uncles. Friends can also be closely involved in screening conversations, as sources of information or support. In addition to the family members, our analysis shows the myriad types of HCPs involved in screening. This list includes, but is not limited to, general practitioners (GPs), specialist consultants, nurses, midwives, obstetricians, gynaecologists, genetic counsellors, physiotherapists, pharmacists and psychologists.
While HCPs provide tangible medical services to the mother, fetus/baby and other family members, our data also revealed the importance of other actors on the periphery. Data highlighted the importance of employers for understanding pregnancy and maternal health needs, particularly when things did not go as expected. Social workers were essential for connecting families with disparate services to manage living with conditions. Religious leaders, and church organisations, were named as sources of comfort and community, again especially important when screening resulted in the unexpected. Charity organisations and online communities that specialised in providing information about screening or screened-for conditions were named, repeatedly, as vital elements for (potential) parents on their screening journey. These organisations not only provide support and information, but also provide a collective sense of community for people at a time that may be emotionally challenging. Charities and online communities also link people going through screening to people who are living (or caring for someone) with a condition. People living with a condition, or those caring for someone with a condition, are a vital source of experiential knowledge that is valued as separate from what one may hear from other sources – such as the media or HCPs.
In addition to the named individual and collective human elements, our secondary analysis also implicated three important actors. The first is academic or scientific researchers – including ourselves. Researchers discuss what is (un)known about screening and screened-for conditions. Findings from research may be used by others to make political, healthcare or social care decisions. Researchers can develop treatment breakthroughs and give voice to those affected by screening. The second implicated actor is the UK NSC. The UK NSC advises the NHS and Members of Parliament (MPs) about screening programmes. An independent panel of experts, they weigh evidence to make recommendations on expanding screening programmes and support the implementation of existing programmes. While not always named in the data, the UK NSC makes decisions that shape the screening landscape. Similarly, MPs may go unnamed but are the decision-makers who determine screening and healthcare policies.
In addition to the individual and collective human elements, we noticed a number of recurring discourses constructed about humans and screening. Within the data, parents made assumptions made about their (partner’s) pregnancy or newborn child. For those who had not previously had a screen-positive experience, participants generally assumed that nothing would come of the antenatal or newborn screening (i.e. ‘everything will be fine’). Conversely, for those who had previously experienced unexpected news from antenatal or newborn screening, there were assumptions that perhaps their pregnancy or child would continue to have problems. For such people, this feeling of ‘something could be wrong’ continued through subsequent pregnancies.
Alongside the assumptions about one’s pregnancy or newborn were regularly expressed points about what it was like to live with a condition or as a carrier. Constructions of what it was like to live with a condition varied based on perceived severity and nature of the condition as well as whether the participant had direct lived experience with the condition itself. Constructions of what it was like to be a carrier also varied based on whether the participant had direct experience with the condition but tended to centre on reproductive decision-making. These discourses intersected with (generally negative) stereotypes about what it was like to live with a disability, such as inability to join the workforce, having to attend specialist education, or leading a life that was somehow ‘less than’. Similarly, there were stereotypes about who might be diagnosed with particular conditions which tended to focus on ethnicity (e.g. linking being of African or Caribbean background with sickle cell disease) or maternal age (e.g. linking ‘older’ motherhood with Down syndrome).
Within the data of people who had screen-positive experiences and/or lived experience of a condition, much was said about how little public awareness there is of screened-for conditions. This lack of awareness was perceived to make positive screening results more difficult to absorb, while also perpetuating stereotypes about life with the conditions. These same participants also talked regularly about what made HCPs (un)supportive. Supportive HCPs were constructed as listening to concerns, providing sufficient information, and being available to answer questions. Unsupportive HCPs, on the other hand, were those who tended to do the opposite. This included saying things that participants perceived as negative about a given condition, or focusing solely on clinical complications, rather than abilities and potential. Such unsupportive actions by HCPs ran counter to the recurring parental narratives that their child was special or a gift, and that their experiences had been far more positive than anticipated at the point of diagnosis. Finally, narratives tended to construct antenatal and newborn screening as having ‘spillover effects’. There were regular references to how screening had significant personal, relational and resource implications on individuals above and beyond the medical purview of screening.
Non-human elements related to antenatal and newborn screening
The secondary analysis revealed a range of recurring non-human elements, such as technologies and materials, related to antenatal and newborn screening. These included the screening tools themselves [i.e. antenatal screens, non-invasive prenatal testing (NIPT), and the heel prick test] as well as diagnostic tests [i.e. amniocentesis and chorionic villus sampling (CVS)]. For antenatal screening, there were differing interpretations of the purpose of ultrasound scans. For those who had not had an unexpected screening result or pregnancy complications, scans were constructed as an opportunity to ‘see’ their unborn baby and viewed in a positive manner. For those who had pregnancy complications, scans were something that were medically necessary and worrying. If a diagnostic test was offered, pregnant women and their partners considered the invasiveness and potential risks in their decision-making. The policies and procedures around termination were of vital importance as were ideas about whether or not termination would be appropriate. The decision about whether or not to terminate was sometimes complicated by the wide spectrums of presentation associated with the conditions, such as Down syndrome. Similarly, concerns about, or experiences of, miscarriage were also central to testing decisions, particularly for conditions like Edwards syndrome or Patau syndrome where there were higher chances of not carrying to term. Women who terminated pregnancies for medical reasons or those who experienced a miscarriage (or stillbirth) described them as bereavements involving mourning and adjustment.
Individuals who received positive screening result frequently recalled the exact moment of ‘getting the phone call’ which first alerted them to the finding, and some held onto the hope that the screen was a false positive. ‘Getting the phone call’ was often followed up by considering one’s family history and looking online or for other sources of information. Medical histories and notes might be accessed. Participants stressed how communication could drastically alter their experience of receiving this result, depending on what was said, how and by whom. Good communication was essential since the science behind screening, genetics and probability was constructed as mystifying. While people placed their trust in the NHS, this trust could be violated if communication was poor or if they felt misled. The NHS and private non-NHS services were sites of screening and follow-up, while nurseries and schools influenced how children living with screened-for conditions might learn and grow.
Temporal elements related to antenatal and newborn screening
Temporality was an important category related to antenatal and newborn screening. Within screening stories, the timing and journey to pregnancy were important factors. Some pregnancies were unintended; in these circumstances, antenatal screening was considered alongside decisions about proceeding with pregnancy overall. Other people spent months or years trying to conceive. These individuals had more time to think about pregnancy, screening and parenthood. Besides the timing of the pregnancy itself, it is important to consider overall the reproductive life course. Individuals bring their experiences with fertility, pregnancy, contraception, disability, health and relationships to their screening narratives. The moment of having blood drawn, or an ultrasound scan, and experiencing the results does not exist in a vacuum. Rather, the entirety of a person’s history over time is implicated.
The timing of screening itself was also important. People discussed antenatal screening moments as pregnancy milestones for themselves and their unborn child. Newborn screening, on the other hand, was seen as a blurred moment in time where the tired parent is adjusting to caring for a baby, potentially for the first time. As a result, it became less of an event, particularly as it was often folded into a consultation with other well-baby checks (e.g. weight gain or feeding). For both antenatal and newborn screening, the period of time between receiving a positive screen result and getting additional (often diagnostic) information was a slowing down of time where hours felt like days and days like years.
For families who receive positive screening results, their feelings about the condition may vary over time. How people feel about the condition varies based on the condition, timing of diagnosis, and decision-making trajectory. For people living with a screened-for condition, or their carers, the cumulative effects of a diagnosis – the emotional, social, financial and physical toll – were important discussion points. These cumulative effects intersect with knowledge of historical eugenic practices in the past, which some contend continue into the present. People living with screened-for conditions, and their carers, also have to think about when it might be appropriate to disclose their diagnosis to others; a decision, which varies, again based on the condition and the nature of the relationship. This can be complicated by the future uncertainty over what an individual’s future with the condition might look like.
Spatial elements related to antenatal and newborn screening
When it came to spatial elements, the data included many references to the sites of antenatal and newborn screening tests. People considered how rooms looked, noting that ‘nicer’ rooms were the ones where ‘bad’ news might be received. People also discussed how prior experiences in a particular room, unit or hospital, such as being told of a miscarriage, could haunt them in subsequent visits to the same place. These feelings held for sites of specialist intervention, such as neonatal intensive care units, the availability of which depended on location. Our data also touched on the need to travel to and from healthcare settings; the ease of travel depended on individual’s geographic location, employment circumstances, financial resources, health (physical and mental), and caring responsibilities. The need to travel intersected with recognised local and regional variations in care. Differences in medical practices, facilities and expertise were consistently referenced as important to the outcomes of antenatal and newborn screening. Further, international differences in approaches to screening and health care were referenced. The data mentioned the more liberal approaches to screening as performed in other countries, primarily other European nations or the USA, but also the benefits of free (at point of access) healthcare that is available in the UK but not in other countries.
Sociocultural elements related to antenatal and newborn screening
The secondary analysis revealed a range of recurring sociocultural elements related to antenatal and newborn screening. Much of the data reflected traditional gendered caring practices. Pregnant women and mothers of newborns were constructed as having heightened responsibility for reproduction – as needing to reduce working hours, manage the household and do the emotional work of being a (potential) parent. Male partners, on the other hand, were constructed as being less involved with pregnancy, including understanding the intricacies of screening, although they might provide ‘strong but silent’ emotional support. Regardless of gender, parents were constructed as accepting of making sacrifices for their pregnancy or child, wanting to provide the ‘best’. However, there were varying interpretations of what the ‘best’ was; for some it was having all possible screening or diagnostic tests, for others it was declining what was offered.
In addition to gender and parenting, religious practices were also important to people on their screening journeys. While religious beliefs can affect how an individual approaches medical care, in the data it most often came up related to beliefs around termination or coping with loss. Similarly, the data indicated the importance of ethnicity as part of the antenatal or newborn screening experience. Ethnicity was implicated in discussions of an individual’s chance of having a condition or being a carrier. The data held numerous references of people saying they were (not) likely to have a condition because they were (not) of a certain ethnic background. Ethnicity intersects with the practice of arranged marriages. While not common among those of white-European descent, arranged marriage continues to be practised among certain ethnic groups, including (but not limited to) people of Indian, Japanese and Pakistani descent. Arranged marriages take place in communities that have higher rates of carriers for certain conditions, such as sickle cell and thalassaemia. Participants discussed the importance of knowing one’s carrier status prior to such arranged marriages in order to prevent passing conditions on to their unborn children.
Economic elements related to antenatal and newborn screening
Our situational mapping exercise revealed personal, organisational and structural economic elements related to antenatal and newborn screening. Personal financial and employment resources dictated whether or not individuals could afford or access private healthcare coverage to bypass or supplement what was available on the NHS. Additionally, greater financial resources could make travel, time off work, or caring for a child with a condition more feasible. From an organisational standpoint, much was made of the role of charities in providing essential information and support for people receiving unexpected screening news, yet the ability of charities to provide services is directly related to the financial and other resources they may have.
Our data regularly implicated the NHS infrastructure and practices, which are dependent on funding and other limited resources – including the number of HCPs. Related to screening, the structure of the NHS influences the amount of time people have with HCPs to ask questions, the availability of specialists to provide care and the types of treatments available. In some cases, people may consider private healthcare options. Typically, this meant paying for additional scans beyond the standard dating and anomaly scans, or paying for NIPT. While the NHS is dependent on government funding decisions, so too are medical and scientific researchers who often depend on grant dispensing organisations (e.g. NIHR) to drive forward screening and treatment innovations. Finally, while private pharmaceutical companies may not compete for government funding, they are also part of this landscape. These companies sell technologies and treatments related to screened-for conditions then reinvest the profits to continue development and production activities.
Related discourses and debates surrounding antenatal and newborn screening
There were several larger discourses and debates related to screening. These include conversations about the rapidly expanding genetic and genomic technologies and the role of screening. The UK NSC currently has a more conservative set of criteria than other countries; they take into account the condition, the test, the intervention and available scientific evidence. As other countries expand their screening programmes, it is easy to imagine that pressure will correspondingly continue to mount to do so in the UK, especially as new treatments become available and methods of delivering screening (e.g. whole genome sequencing) expand. The debate about the role of screening intersects with conversations about what it means to live a ‘quality life’ and how people conceptualise (dis)ability. Within the data, these perceptions seem to be heavily based on individuals’ (lack of) first-hand experiences with conditions. However, these conversations continue to be a source of debate within society.
Antenatal screening also comes with additional caveats that centre on termination. Currently, England, Scotland and Wales allow termination up to 24 weeks of pregnancy, although this limit does not apply if there is a risk to maternal life or if there is evidence of ‘serious’ fetal anomalies. Termination has long been a contested social arena. Debates position beliefs in women’s rights to have bodily autonomy against beliefs about the rights of the (disabled) fetus/baby. These debates continue to shape the personal and social landscape of antenatal screening.
Strengths and limitations of the data set
Our analysis is strengthened by the large number of qualitative interview transcripts included, collected over an extended time frame. These transcripts covered a variety of (non)screened-for conditions and the views of people living with conditions and their family members. While the data set does not represent all conditions, those included are highly diverse, and the data therefore do provide rich insights into the practical and emotional experiences related to a range of screening outcomes from people of different genders, ethnicities and ages.
Discussion
The findings from the secondary analysis demonstrate that conversations about antenatal and newborn screening involve a complicated weaving of individuals, organisations, materials and discourses. By developing a situational map, we were able to identify elements that may (not) be involved in an individual’s situation and consider implicated environments that shape the landscape of screening. This was an iterative, reflexive, subjective exercise. Its strength is that we could reduce a large, rich data set into a manageable representation that is not depleted by its simplicity. Instead, we have identified elements that may work in harmony or at cross-purposes, vary over time or space, and are subject to charged societal debates.
The primary output of the secondary analysis was to inform our primary data collection activities (see Chapter 7). By compiling the human elements, we generated a list of stakeholders that are central to screening conversations, such as various types of HCPs and charity organisations. Further, the data highlighted that the NSC is an important silent actor that we need to consider moving forward. These pieces of information were confirmed by our PPI and independent oversight committee members. Given the large number of temporal, spatial and discursive elements in our situational map, it also became apparent that our data collection activities with people who had gone through screening needed to solicit comprehensive reproductive histories, not just discuss screening itself. In this way, we could gain a broad understanding of how people consider the benefits and harms of antenatal screening within a larger framework of their lives.
Chapter 7 Work package 2: understanding stakeholders’ experiences of screening: a thematic analysis using primary data collection
Introduction
Building on our meta-ethnography (see Chapter 5) and secondary analysis (see Chapter 6), we conducted a thematic analysis of primary data of stakeholders’ screening experiences. We set out to speak with three prominent stakeholder groups that were informed by our analysis in the secondary analysis:
-
people who have made decisions about undertaking antenatal or newborn screening
-
charity and professional stakeholders involved in supporting those undergoing antenatal or newborn screening
-
HCPs involved in delivering antenatal or newborn screening.
We were mindful that this project would cover potentially contentious terrain with groups and individuals that hold strong and sometimes opposing views. We therefore used a variety of data collection methods to allow us to explore how views on screening are socially and culturally shaped without exposing participants to confrontation. We sampled purposively, to include a range of perspectives, both lay and expert, to give voice to these different views. Here we present the methods and findings for each of these subgroups in turn before summarising overarching themes from across the subgroups.
Methods
Ethical considerations
This study was approved by the University of Oxford’s Central University Research Ethics Committee (approval R70422) on 22 July 2020 and was carried out in compliance with the research and information governance policies of the University of Oxford. Potential participants were directed to a study website that provided information about the research and AW’s contact information. Interested people got in contact and AW sent a participant information sheet that detailed the aims and methods of the study (see Appendix 8). Participants had the opportunity to discuss participation with AW before agreeing to take part and gave consent to taking part in an interview or focus group. AW conducted the focus groups, with LH and AM also in attendance to support. AW collected all the interviews. We carefully planned the focus groups around shared experiences in order to minimise the risk of emotional harm, and participants were offered the option of a one-to-one interview if they preferred. All participants were informed that they were under no obligation to take part and could withdraw at any stage of the data collection process until the point of analysis, without questions asked. Each participant was offered a £20 voucher to thank them for their time. All research data have been treated in strict adherence to the General Data Protection Regulation. We have given participants pseudonyms and use those names here. While we have attempted to remove all personally identifiable information from the data, participants were made aware that in some cases – particularly if they are high-profile stakeholders or speaking about a rare condition – full anonymity could not be guaranteed.
Group 1: people who have made decisions about antenatal or newborn screening
This group was made up of people who had decided to use, or not use, antenatal or newborn screening within the past 4 years. Potential participants needed to be 18 years or older and live in the UK. We recruited participants through social media posts, our PPI network, professional contacts and paid Facebook advertising. We asked people who were interested in participating to complete a questionnaire to ascertain their experiences with screening and their demographic characteristics. We used this information to purposively sample a variety of people to participate in focus groups about screening experiences. We sought to recruit participants with a range of screening trajectories, including:
-
people who declined (aspects of) screening
-
people who received false-positive results from screening
-
people who terminated a pregnancy for medical reasons following antenatal diagnosis
-
people who continued a pregnancy following antenatal diagnosis
-
people who experienced miscarriage or stillbirth
-
people who received a positive newborn screening result.
We completed six focus groups from February to March 2021 (n = 30). We carefully planned the focus groups around shared experiences in order to minimise the risk of emotional harm. For example, we convened a focus group entirely of parents whose children are living with Down syndrome and another entirely with people who were pregnant for the first time. In addition, we reminded participants that they could decline to answer any question and that they were at liberty to leave the focus group at any point without giving a reason. Focus groups took place on a bespoke online platform over two, 1-hour-long sessions that took place several days apart. Each focus group had between three and six participants. For each chat, two or three researchers acted as moderators (AW, LH, AM) and drove the conversation. Our PPI partners helped shaped the focus group questions (see Appendix 9). The text from the chat functioned as the data from the focus groups.
After the first focus group session, we asked participants to respond to three fictional screening scenarios on a forum. Scenarios covered: (1) finding out about carrier status, (2) finding out about a positive newborn screening result and (3) finding out there is a high chance of a pregnancy resulting in a trisomy condition. We asked participants to read the scenarios and reflect on what they might be feeling or want to know if this happened to them. Participants could respond at any point between their first focus group chat session and the second chat session. The text from their responses was integrated into our analysis. Our PPI partners helped shaped the fictional scenarios.
We also offered participants who had sensitive or complex experiences the chance to take part in a telephone interview at a time of their choosing instead of participating in a focus group (n = 19). Our PPI partners helped shape the interview questions (see Appendix 9). AW reminded participants that they could decline to answer questions and end the interview at any point. AW attended to signs of emotional distress and offered to pause or halt interviews when participants became upset. Interviews lasted an average of 66 minutes (range 35–93 minutes). All interviews were audio-recorded and professionally transcribed verbatim.
Group 2: charity and professional stakeholders involved in antenatal or newborn screening
This group was made up of stakeholders involved in supporting those going through screening, or supporting families affected by conditions that can be identified through screening (n = 17). It also included people involved in shaping screening policy and practice within the UK. We purposively recruited stakeholder groups who were named in our situational mapping exercise or named by our PPI partners as important. We recruited some stakeholders through personal networking and others through cold e-mailing. AW e-mailed individuals or organisations, provided information about the study, and requested to conduct a 1-hour interview. Interviews took place between February and April 2021 over the telephone or using Microsoft Teams depending on participants’ preferences. AW obtained consent to audio-record conversations (see Appendix 9). Interviews lasted an average of 60 minutes (range 42–76 minutes). All interviews were professionally transcribed verbatim.
Group 3: healthcare professionals involved in antenatal or newborn screening
We had intended to interview up to 20 HCPs involved in screening, such as midwives, sonographers, obstetricians and genetic counsellors. However, the ongoing pandemic during our recruitment period (spring 2021) meant that HCPs were overworked and had little time or energy to take part. We exhausted multiple recruitment avenues, including personal networks, multiple rounds of social media posts, and advertising with professional colleges and organisations. We managed to generate interest from a small number of midwives over several months but had no other leads as of June 2021.
After discussing within the research team and our independent oversight committee members, we decided that the best course was to conduct a focus group with the midwives who volunteered to take part at the end of June 2021 (n = 4). The purpose of the focus group was to sense-check the themes we had identified in the data collected from groups 1 and 2. AW and LH worked together to compile a discussion guide and met with the midwives for approximately 90 minutes using Microsoft Teams (see Appendix 9). Participants had the option of turning their cameras on or off; all opted to keep them on. With participants’ permission, we recorded both the video and audio of the meeting. The audio was used to create a transcript. The video was used to confirm the identity of speakers during transcription before being deleted.
Analytical approach
All focus groups and interviews were recorded for transcription and analysis, and anonymised. We used thematic analysis to identify key themes in the data, including the benefits and harms of antenatal and newborn screening. 102,103 AW completed the initial data coding using NVivo12 software. The qualitative team (FB, LH, LL, AM, AW) met several times to discuss the coding progress and develop themes. While the codebook primarily focused on drawing out the benefits and harms of screening, it also captured other additional themes, such as emotional impacts, temporality, consent and information needs. These results are presented below (see Methods) but also informed WP3 (see Chapter 8).
Results
Group 1: results
We collected data from 49 individuals in total; 30 participated in focus groups and 19 participated in interviews (Table 12). Participants were between 24 and 48 years old, with a mean age of 35 years old. While most of our participants were women (reflecting trends observed in other research of screening), we also spoke with nine men about their experiences as current or expectant fathers. The majority of participants lived in England, were married, and had completed at least a bachelor’s degree. We made concerted efforts to recruit people from ethnically diverse groups through multiple rounds of targeted recruitment; however, the majority of our participants identified as white. These participants represented a diversity of nationalities – including people who identified as American, English, Irish, Scottish, South African and Welsh – but the study may have been strengthened by those from additional ethnicities. We did our best to build a purposive sample that reflected a range of varying screening pathways from a variety of points of view (Table 13). Our data cover a variety of (non)screened-for conditions and the views of people living with conditions and their family members. While the data set does not represent all conditions, those included are highly diverse, and the data therefore do provide rich insights into the practical and emotional experiences related to a range of screening experiences and outcomes. See Appendix 10 for additional demographic details.
| Pseudonym | Sex | Age | Country | Time since last birth | Household yearly income (£) | Relationship status | Ethnicity | Highest level of education | Data type |
|---|---|---|---|---|---|---|---|---|---|
| Ada | Woman | 24 | Wales | Between 13 and 18 months | 30,000–39,999 | Married | White | Sixth form/vocational | Interview |
| Alexander | Man | 41 | Wales | Between 2 and 4 years | 70,000–79,999 | Married | White | Advanced degree | Interview |
| Aria | Woman | 29 | England | < 3 months | 50,000–59,999 | Married | White | Advanced degree | Focus group |
| Beth | Woman | 34 | England | Currently pregnant | 70,000–79,999 | Married | White | Advanced degree | Interview |
| Daisy | Woman | 31 | England | Currently pregnant | 40,000–49,999 | Married | White | Bachelor’s degree | Focus group |
| David | Man | 41 | Wales | Between 2 and 4 years | 70,000–79,999 | Married | White | Advanced degree | Focus group |
| Donna | Woman | 36 | England | < 3 months | 60,000–69,999 | Married | White | Bachelor’s degree | Interview |
| Elizabeth | Woman | 41 | Scotland | Between 2 and 4 years | > 100,000 | Married | White | Bachelor’s degree | Focus group |
| Ella | Woman | 38 | England | Currently pregnant | 70,000–79,999 | Married | White | Bachelor’s degree | Focus group |
| Elsie | Woman | 32 | England | Currently pregnant | Not reported | Married | White | Advanced degree | Focus group |
| Emily | Woman | 31 | England | Between 19 and 24 months | 80,000–89,999 | Married | White | Bachelor’s degree | Interview |
| Erin | Woman | 32 | England | < 3 months | 50,000–59,999 | Partnered | White | Sixth form/vocational | Interview |
| Eva | Woman | 38 | England | Currently pregnant | 70,000–79,999 | Married | White | Advanced degree | Focus group |
| Evie | Woman | 48 | England | Between 2 and 4 years | 30,000–39,999 | Married | White | Bachelor’s degree | Focus group |
| Florence | Woman | 42 | England | Between 2 and 4 years | Not reported | Married | White | Sixth form/vocational | Focus group |
| Hailey | Woman | 39 | Wales | < 3 months | 30,000–39,999 | Partnered | White | Bachelor’s degree | Interview |
| Harper | Woman | 32 | England | Currently pregnant | 80,000–89,999 | Partnered | White | Advanced degree | Focus group |
| Heather | Woman | 40 | England | Between 13 and 18 months | > 100,000 | Married | White | Advanced degree | Interview |
| Imogen | Woman | 32 | England | Between 4 and 6 months | 30,000–39,999 | Partnered | White | Advanced degree | Focus group |
| Isabella | Woman | 34 | England | < 3 months | 70,000–79,999 | Married | White | Advanced degree | Focus group |
| Isla | Woman | 34 | England | Between 7 and 12 months | > 100,000 | Married | White | Advanced degree | Interview |
| Ivy | Woman | 35 | England | Between 19 and 24 months | 80,000–89,999 | Married | White | Bachelor’s degree | Focus group |
| James | Man | 32 | England | Currently pregnant | 70,000–79,999 | Married | White | Advanced degree | Focus group |
| Jessica | Woman | 27 | England | Currently pregnant | 30,000–39,999 | Married | White | Bachelor’s degree | Interview |
| Kelly | Woman | 37 | Scotland | Between 4 and 6 months | 80,000–89,999 | Partnered | White | Advanced degree | Interview |
| Layla | Woman | 34 | Wales | Between 4 and 6 months | 30,000–39,999 | Married | White | Advanced degree | Focus group |
| Lily | Woman | 44 | England | Between 7 and 12 months | 20,000–29,999 | Single | White | Sixth form/vocational | Focus group |
| Luna | Woman | 32 | Wales | < 3 months | 70,000–79,999 | Partnered | White | Advanced degree | Focus group |
| Maisie | Woman | 31 | England | < 3 months | 80,000–89,999 | Married | White | Advanced degree | Focus group |
| Matilda | Woman | 31 | England | Currently pregnant | 90,000–99,999 | Partnered | White | Bachelor’s degree | Focus group |
| Maya | Woman | 27 | England | Currently pregnant | 40,000–49,999 | Married | White | Bachelor’s degree | Focus group |
| Mia | Woman | 42 | England | Between 2 and 4 years | Not reported | Married | Asian | Bachelor’s degree | Interview |
| Michael | Man | 37 | England | < 3 months | 30,000–39,999 | Married | White | Bachelor’s degree | Focus group |
| Owen | Man | 31 | England | < 3 months | 70,000–79,999 | Married | White | Advanced degree | Interview |
| Phoebe | Woman | 32 | England | Currently pregnant | > 100,000 | Married | White | Advanced degree | Focus group |
| Poppy | Woman | 27 | England | Between 7 and 12 months | 60,000–69,999 | Married | White | Bachelor’s degree | Interview |
| Quinn | Woman | 39 | England | Between 19 and 24 months | 40,000–49,999 | Partnered | White | Advanced degree | Interview |
| Robert | Man | 31 | England | Currently pregnant | 80,000–89,999 | Partnered | White | Advanced degree | Focus group |
| Rosie | Woman | 36 | England | Between 4 and 6 months | < 20,000 | Married | White | Bachelor’s degree | Interview |
| Ruby | Woman | 30 | England | Currently pregnant | 60,000–69,999 | Married | White | Bachelor’s degree | Focus group |
| Scarlett | Woman | 37 | England | Currently pregnant | 70,000–79,999 | Married | White | Advanced degree | Focus group |
| Sienna | Woman | 27 | England | Currently pregnant | 30,000–39,999 | Married | White | Sixth form/vocational | Focus group |
| Sophia | Woman | 31 | England | Between 13 and 18 months | 60,000–69,999 | Married | White | Bachelor’s degree | Focus group |
| Sophie | Woman | 43 | England | Between 4 and 6 months | > 100,000 | Married | Arab | Advanced degree | Focus group |
| Thomas | Man | 29 | England | Between 4 and 6 months | 40,000–49,999 | Married | White | Advanced degree | Interview |
| Tim | Man | 34 | England | Currently pregnant | 40,000–49,999 | Married | White | Sixth form/vocational | Interview |
| Vivian | Woman | 34 | Scotland | Between 19 and 24 months | 50,000–59,999 | Married | White | Bachelor’s degree | Interview |
| William | Man | 42 | England | Between 2 and 4 years ago | 50,000–59,999 | Partnered | White | Bachelor’s degree | Focus group |
| Willow | Woman | 40 | England | Between 19 and 24 months | 30,000–39,999 | Married | White | Sixth form/vocational | Focus group |
| Description | Number |
|---|---|
| Women who are currently pregnant | 12 |
| Women whose children do not have a known condition | 13 |
| Women whose children are living with a condition | 11 |
| Women who experienced perinatal loss | 4 |
| Men whose partners are currently pregnant | 4 |
| Men whose children do not have a known condition | 4 |
| Men whose children are living with a condition | 1 |
Individuals discuss screening benefits
Participants named a number of screening benefits, namely (see Report Supplementary Material 3 for supporting quote details):
-
Screening may give people information so they can ask questions, consider termination, prepare, and adjust expectations.
-
Screening may give reassurance.
-
Screening allows people to connect with their pregnancy.
-
Screening may prevent harm (includes treatment).
-
Screening tests work and are done for a reason.
-
Screening is free at point of service.
-
Screening enables people to learn about conditions.
Across focus groups and interviews, the ability to get information was the most consistently referenced benefit of antenatal and newborn screening, reflecting a wide cultural belief that knowledge is power. Participants valued the information so that they could ask questions and consider pathway options. As Quinn (mother to child with rare genetic condition) said, ‘What am I going to do from here? Am I going to seek support from somewhere, seek further information?’ Participants could potentially use the information to consider if termination was the right choice for them. Elsie (currently pregnant) shared, ‘I would have wanted more information and would have to consider termination if I was to have a child with potential health conditions’. Participants discussed using the information to prepare themselves and others to continue a pregnancy with the knowledge that it might (or will) result in the birth of a child with a condition. Florence (mother of child with Down syndrome) touched on this saying, ‘I wanted the diagnosis to be able to prepare and learn as much as I could, and prepare my son’s & also my children’s grandparents’. Finally, the information from screening might also allow people to adjust their pregnancy and/or parenting expectations. Ella (mother, no positive screening results) discussed being ‘glad that I had all the available scans and antenatal screenings offered’ as it allowed her to ‘manage my expectations and plan for the future’.
The second most referenced benefit of antenatal and newborn screening was the reassurance it gave participants. Participants like Elizabeth (mother, no positive screening results) mentioned that ‘the screening journey helped me to feel that the pregnancy was more real’. Imogen (mother, no positive screening results) echoed this sentiment, calling screening tests ‘milestones’ that made her feel reassured that her ‘babies were doing okay’. Some participants mentioned being worried through their pregnancies, but that the screens – especially the dating and anomaly scans – helped provide a sense of relief that things were going as expected. Thomas (father, no positive screening results) spoke about the security that screening brought him:
The various different checks you have it definitely makes you feel like there is a safety net there, that like a lot of the investigation, the, feeling constantly worried with newborns that there’s something desperately wrong with them, and that they’re poorly or something, and I think knowing that there are like this series of tests that have been taken that like basically guarantee like, whether that, I think both pre-birth and post-birth, that you know that there is this regular screening, that things that might go wrong have been screened for and picked up give you that additional sense of sort of security. And sort of calms a lot of worries, I think. At least that did for me.
In addition to the sense of reassurance, another regularly mentioned benefit of antenatal scans was that they allowed participants to connect with their pregnancy. Participants mentioned being able to learn the sex of the fetus/baby as a ‘nice little surprise’ (Poppy, high chance antenatal screen, child born without condition). William (father, no positive screening results) was one of the few participants who acknowledged that the ultrasound scans may have resulted in unexpected news:
To be honest I think both of us actually looked forward to the scans. Even though they were times we could find out really serious news about the baby, we just saw them as an amazing chance to see an image of our child. Probably a bit naïve of us!
While William called his perspective on scans ‘naïve’, most participants saw these scans as positive opportunities. Participants appreciated being able to ‘connect’ with their pregnancy even when they didn’t ‘feel the baby moving yet’ (Harper, currently pregnant). And, seeing the baby and ‘hearing his hear was everything’ for Lily (mother of child with Down syndrome) when she was ‘frightened’ about her pregnancy.
In addition to giving information and letting participants connect with their pregnancies, participants also mentioned that screening could prevent harm and possibly save lives. Lily discussed how a prenatal diagnosis of Down syndrome meant ‘when my baby was born the specialists were there ready if he needed immediate care’. Emily, whose children were diagnosed with PKU from the heel prick test, discussed how screening would ‘maybe not have saved their lives but has you know dramatically enhanced their lives’ by giving her early information about her children, paving the way to treatment. As Owen (father of child with unknown condition identified postnatally) said, you can’t ‘sort it out’ if ‘you don’t look’.
The rhetoric that screening prevents harm aligns closely with another benefit mentioned, namely that screening tests work and are done for a reason. Several participants voiced their support for screening tests because ‘you trust in the science and the doctors so they can give you and your child the best care’ (Maisie, mother, no positive screening results). As Ada (high-chance pregnancy, termination for medical reasons) said, ‘They’re not just a tick box exercise’. The benefit is that the screening tests are effective. Moreover, another benefit is that, within the NHS, screening is free at point of service. This means that pregnant women don’t need to say ‘well I can’t afford all these tests’ (Kelly, mother, no positive screening results) as, on paper, they should be available for everyone.
The last benefit participants regularly mentioned was that screening allowed them to learn about conditions. As Aria (mother, no positive screening results) said, ‘I had never heard of Edwards syndrome so without screening I would never have had to consider my baby could have it, but that is far outweighed by the positives – knowledge is power in my opinion!’ The presence of screening programmes mean that people going through screening can learn about what might happen, giving them knowledge they otherwise might not get. Also, as Emily (mother to children with PKU) mentioned, ‘learning about different conditions that you know that people have to sort of deal with I think gives you a bit more empathy’, which was regarded as a good thing.
Individuals discuss screening harms
Participants named a number of screening harms (see Report Supplementary Material 3 for supporting quote details):
-
Standard screening practices do not suit everyone.
-
Screening for conditions may imply that high-chance pregnancies should be terminated.
-
People may be unprepared for unexpected results.
-
Screening may cause emotional distress by introducing anxieties that were not there previously.
-
False-positive results may cause residual emotional distress.
-
It may be hard for people to understand what they are consenting to.
-
People may not understand that screening tests are not diagnostic tests.
-
Communication about results may be unsatisfactory.
-
Communication and support for high-chance results may not be appropriate.
Interestingly, participants felt that additional harm is that the harms of screening are not often acknowledged. Poppy (high-chance antenatal screen, child born without condition) said it was ‘almost like there’s nothing bad’s gonna come out of’ screening. While Owen (father of child with unknown condition identified postnatally) said, ‘As with any screening, there is a potential harm of doing it. I’m not convinced it’s acknowledged consistently at least’. Below we give voice to the various harms that participants described.
Participants discussed how standard screening pathways do not suit everyone. As Matilda (currently pregnant) put it, ‘I suppose screening in the NHS has to be what is best for most women or most babies but it doesn’t always mean that it is right for each of us’. Indeed, some participants pointed out that when they stepped outside of common pathways through screening, they felt that ‘it has given people, or might potentially give people, reasons or excuses to find something wrong with my pregnancy or my conduct’ (Daisy, currently pregnant). Similarly, there were some participants who felt that screening programme pathways implied that high-chance pregnancies should be terminated. Evie, a mother to a child with Down syndrome, shared:
I also agree that there should be openness about why screening happens. It is done with the language of choice but that immediately infers termination. There should be greater clarity about how it is all optional, which most people seem unaware of … To repeatedly offer termination as an option is not offering choice; it is putting pressure on parents who are already perhaps worried or upset about the diagnosis … by providing [this] literature when a diagnosis is given, it is presented as if that is the route it is assumed people are taking.
Participants, particularly parents of children living with Down syndrome, called into question why antenatal screening programmes exist at all. For them, it seemed as if programmes were in place to discourage births of babies with specific conditions like theirs.
Participants also mentioned that it could be difficult to understand what they were consenting to. Maya (currently pregnant) explained that the ‘language they use to label the tests is confusing’ and ‘there’s so many tests you read about that you’ve never heard of before’. For people like Sienna (currently pregnant), this meant ‘I never know what they’re testing me for’. Difficulty comprehending the purpose of screening created another harm: people are unprepared for unexpected results. As Imogen (mother, no positive screening results) suggested, ‘I would find myself just going along with everything and not prepared at all for bad news’.
Being unaware of tests, or being unprepared for what they might show, contributed to one of the most significant harms, that of emotional distress. Participants repeatedly described how screening caused anxiety and upset that can endure throughout the pregnancy and in subsequent pregnancies. Ella (mother, prior miscarriages) summarised this feeling:
Yes and I think once you have had bad news at a scan, or known someone have bad news, it is hard to ever see them in the same way. I cried my way through all scans until I saw a heartbeat, and then the reassurance they gave was only fleeting. I saw them very much as part of a journey, rather than an end point. I remember even when my babies were in my arms as newborns I was worrying about the cystic fibrosis screening on the heel prick for instance.
The emotional toll of screening may be fleeting for some but linger for others. Residual emotional distress after experiencing a false-positive screening result is another harm. Poppy (high-chance antenatal screen, child born without condition) explained that she always had the false-negative result ‘at the back of (her) mind’. While she doesn’t have these thoughts anymore, she worried through the first month ‘could there actually still be something wrong with (baby) that they’ve just not discovered yet?’ We discuss the emotional responses to screening further in Group 1: results.
Another harm of screening that some participants discussed was that people do not understand that screening tests are not definitive, diagnostic tests. Participants touched on how screening tests can give reassurance, but they are not always accurate. This meant that people could expect one outcome, but actually experience a different one. Or, perhaps, people may have made a decision on incorrect information. Sophie (mother of child with Down syndrome) discussed this:
Some of the harms – you could end up making a decision based on information that is constantly changing – e.g. our baby was not expected to live to term, and at any point I could have chosen to terminate based on this information, but in the end the baby was born with no health concerns whatsoever. So what was missing was for the medics to inform me that these scans/tests are not always 100% accurate and that babies are constantly growing and changing, so what they see 1 week might not be there at the next scan.
The remaining harms centred on communication. Participants discussed how communication about screening results is often lacking. In many cases, we heard about the ‘no news is good news’ approach to screening where ‘communication is lacking’ and ‘they seem to treat it on a need to know basis’ (Scarlett, currently pregnant). As Harper (currently pregnant) said, ‘It would be much better if they just let you know everything is okay’. While the lack of communication for negative screening results was deemed harmful, so too was the lack of communication and support for high-chance results. Ruby (currently pregnant, high-chance antenatal results) explained that she was ‘blindsided by getting results I didn’t even know I was tested for, for a problem I didn’t know existed’. Further, there was a feeling that HCPs may not react as desired after receiving screening results. As Isla (mother, no positive screening results) shared, ‘They wash their hands of it a little bit, that’s it. You do the screening and then there’s no follow-up or support’. While there are certainly HCPs who do provide support, the fact that some people receive results without expectation or pastoral care was deemed a significant harm of screening programmes.
Individuals questioning informed choice
With few exceptions, our participants opted to take up all offered screening tests. While accepting screening tests were right for some, our data suggest that women who declined, and therefore differed from the norm, met resistance from professionals. Isabella had declined blood tests for carrier screening and to check for anaemia during the third trimester. Drawing on her additional experience as a midwife, she felt that they were unnecessary for her. Isabella (mother) had also considered declining the newborn blood spot test but ended up accepting. She shared:
There is an expectation that you should have these things and I did not want to have to explain myself, so I just consented (to the heel prick) … I had declined other things and found it quite exhausting to keep explaining why I hadn’t done the norm. Sometimes people were generally interested but other times just considered me a rebel.
Isabella’s experience helps us understand the subtle pressures and expectations that exist when women are asked to decide whether or not to accept screening tests. Since the tests are offered, people perceive them as important or necessary, and thus it becomes the norm to accept them. As more people accept, the tests become further normalised and more difficult for people, like Isabella, to decline, particularly as it might call into question how ‘good’ of a parent they are. Thus, some people may wish to decline but ultimately conform, which begs the question of whether screening itself is actually a choice.
Similarly, our data suggest that participants faced difficulties when it came to receiving information about screening tests, diagnostic tests and screened-for conditions. For example, some participants felt that the information they were given was insufficient for their needs. Lily, whose combined test results indicated a higher chance of Down syndrome, shared: ‘They talked about “making an informed decision” but actually you aren’t really given helpful information’. Lily, and others whose pregnancies had higher chances of a condition, felt that information was overly medicalised and did not address what it might be like for a person to live with a condition. As a result, Lily sought out additional information from charities, support groups and the internet in order to feel that she was appropriately informed.
At the same time that some participants felt information was insufficient, others felt that there was almost too much information to understand and absorb. The participants reported receiving a cascade of new information, not just about the screening tests but about pregnancy and parenthood itself. It could be overwhelming to try to understand it all, let alone make an informed decision. As Isabella (mother) shared, ‘I do not think it is fair to give some women so much info they are just lost and then step back and say “you now need to choose” ’. In cases where participants felt overwhelmed by the sheer volume of information, they looked for simplified explanations from trusted sources – particularly peers who had recently been pregnant – before endeavouring to make decisions.
While the prior examples focus on antenatal screening, we also asked participants to reflect on what they knew about newborn screening. Below is an excerpt from a focus group discussion with women who gave birth within the last 6 months:
EVIE: I had no idea it was a choice. It’s like antenatal blood tests. They are presented as something you do. I don’t remember ever being told things were optional (apart from CVS of course).
LILY: I said yes to all tests really as I always feel like it’s a good thing they’re available.
IVY: It felt like something we had to get done. Same as Evie – I had no idea it was a choice.
RESEARCHER: Do you all remember being asked for your consent for the heel prick?
WILLOW: In a fairly casual way yes.
FLORENCE: I’m sure we were, but I don’t think it’s made crystal clear that it’s optional.
IVY: No I don’t remember giving consent, but it was all a bit of a blur to be honest.
EVIE: I was asked for consent but we were in Special Care Baby Unit (SCBU) so there was a lot going on. I just agreed to everything!
SOPHIE: I don’t remember the heel prick being optional.
LILY: It didn’t concern me, so perhaps I didn’t understand what it was. Maybe I still don’t?
Evie shared she did not know it was a choice, and that newborn screening is something you do. Ivy too said she had no idea it was a choice. We then asked if participants remembered giving consent for the heel prick. Willow said consent was a casual thing, while Florence agreed with earlier statements that it’s not crystal clear that it was optional. Ivy did not remember giving consent, and Sophie again reiterated not knowing that it was option. Taken together, this excerpt highlights issues around consent around existing newborn screening programmes that must be considered moving forward.
These examples shed light on the complex informational needs of pregnant women and parents as they go through the screening process. It causes us to question how knowledge is conveyed and understood, and ultimately, how informed people are when presented with decision points. Increasingly, parents and pregnant women are asked to absorb information about genetics, screening tests, diagnostic tests and then make decisions. This information comes from multiple sources and is presented with varying details and quality. Our data suggest that while women are at the centre of such screening decisions, their so-called ‘individual informed choice’ is instead one made within potentially partnered relationships, existing family structures, and broader communities. The implications of which may potentially affect the futures of the woman, her pregnancy, her partner, her other children, her extended family and others in her social network.
Individuals discussing the importance of time
While screening tests are presented as discrete events on the timeline, our data suggest that the tests actually have potentially long-reaching ramifications that spill out backwards and forwards in time. We interviewed a mother called Mia. Mia and her partner had tried to become pregnant for several years; she was concerned about the possibility of having a child with a genetic condition because of her age. Mia opted to have the combined test and her results indicated there was a low chance of a genetic condition. Mia had not thought about a diagnostic test because of her screening test results. Her daughter was diagnosed with Down syndrome postnatally. Mia shared,
Now, I also know that these tests aren’t always accurate as (women) believe they are. They are just screening tests. Unless you have the amniocentesis nothing is accurate … it’s so difficult isn’t it to say what, say what the right thing is, but I, I do wish we’d had the (diagnostic) test but then I, maybe I would have made a difficult decision.
While Mia spoke at length about how much she loved her daughter, she couldn’t help but wonder what might have been different had she had a diagnostic test. This retrospective conflict continues to be a source of emotional distress that Mia carries with her, generated by and through the very existence of the screening process.
Participants who decided to have antenatal testing and received results that indicated everything was as expected often described their relief. And yet, there was an acknowledgement that the reassurance might be fleeting. Ella, who had no positive screening results, described this phenomenon as, ‘I think with all the scans and prenatal testing now it almost gives false reassurance of a clean bill of health/development when of course there are so many issues and conditions that can’t be screened for or only emerge later’. Even as antenatal and newborn screening tests seek to rule out possible conditions, they are not sureties. And, as Ella said, they do not guarantee a future without potential unexpected challenges. Indeed, while screening tests might minimise potential concerns in the present, they cannot fully predict how futures might unfold.
Questions still remain about the long-term futures of people living with conditions. Jessica’s pre-teen daughter has a rare genetic condition which is not part of the newborn bloodspot test in the UK. She imagined what her daughter’s life might be like in the years to come, saying,
We don’t know how things like pregnancy will affect her. So, I guess it’s little things like that where research isn’t done. So that’s a worry … It’s sort of as you hit, as she gets older and starts to become an adult what will happen with the normal things?
Jessica and other parents of people living with conditions often project forwards to imagine the long-term futures of their children. Among other things, this includes imaging the care their child will need, picturing how their child would perform so-called ‘normal’ activities, and even their own child’s reproductive future imagining how the condition might be passed to future generations. Some conditions have the potential to have far-reaching implications that continue to exist long after they have been discovered, whether through screening or symptomatic diagnosis. One of the inherent difficulties of characterising the impact of screening programmes is that it can be difficult to separate concerns and anxieties related to the moment(s) of screening, the waiting periods, and possibly the condition(s) diagnosed.
Individuals facing complex emotional responses
In addition to the aforementioned themes, our data also touched on the complex emotional responses that people may have through their screening journey. For example, we asked participants who did not have lived experience with a condition to imagine their reactions to a positive newborn screening test for that condition. Many stated that, along with anxiety, they expected there would be a bit of relief to have the knowledge. Sienna, who was pregnant, shared, ‘I think I would feel very worried, scared and out of my depth but also relieved to know early on’. While information from the newborn screening was valued, the information was connected to feelings of stress, frustration, and panic. This was exacerbated by the knowledge that ‘the heel prick tests for some serious conditions’ (Imogen, mother, no positive screening results). In order to combat these feelings, participants stated they would want to start learning what they could about a condition:
I feel like at the phone call I would be heartbroken and angry and worried and devastated and just in a spiral of worry and sadness … at the diagnosis, I’d feel quite overwhelmed with anxiety, protectiveness and a feeling that I need to rapidly get up to speed with how to look after it. (Elizabeth, mother, no positive screening results)
While these participants had not gone through the experience of learning that their child tested positive, our data illustrate that there are potentially complex emotional needs that need to be recognised.
These findings of complex and sometimes contradictory emotional reactions were also observed amongst parents with children living with screened-for conditions. Elsie, who has two daughters with PKU, shared that while she had anxiety and fears about antenatal screening, she had never given much thought to the implications of newborn screening, knowing that it was for rare conditions. She went on to describe what it felt like to get the news that her first daughter tested positive, she said,
I think just that you have this, and I remember thinking at the time that you have this little tiny little miracle, you know an eight day old, nine day old little baby who you’ve waited nine months to see, and you’ve been through it, and I think obviously at the time as well, you’re in sort of baby blues of no sleep and exhaustion, and then to be told that your little baby is not well, I mean she is still perfect, but you know at the time it felt like suddenly they had just taken that away. And I remember thinking that they’d taken the joy away of having this newborn. ‘Cos we were in our own little dream and bubble of that newborn phase, and then yeah, I think it felt like I’d been hit by a bus a little bit.
Our data reveal that parents are often unprepared to receive unexpected news about newborn screening results, particularly after not receiving any positive antenatal screening results, and there are very real emotional harms that need to be attended to that may be heightened by the physical and emotional aftermath of birth.
Group 2: results
We interviewed 17 stakeholders about the benefits and harms of antenatal and newborn screening (Table 14). We had conversations with people who represented single-condition charities and multiple-condition charities, both screened for and not screened for. We also spoke with people involved in charities with a wider focus around pregnancy or parenthood as well as a number of people involved in policy development (see Table 14). We cannot provide further details about our participants because they may become identifiable.
| Description | Number |
|---|---|
| Single-condition charity | 6 |
| Multiple-condition charity | 3 |
| Wider scope charity | 3 |
| Policy stakeholder | 5 |
Charity and professional stakeholder views on the benefits of screening
Stakeholders named a variety of screening benefits (see Report Supplementary Material 3 for supporting quote details):
-
Screening may provide information for people to make informed choices.
-
Screening may allow people to make informed decisions about their future reproductive intentions.
-
Screening may prevent harm (includes treatment).
-
Screening may provide reassurance.
-
Screening is free at point of service.
As with the people we interviewed who had experiences with screening, the stakeholders suggested that the most important benefit of screening is the information it gives pregnant women or new parents about their pregnancy or baby. Participants mentioned that antenatal screening may allow people the opportunity to seek a diagnostic test, terminate a pregnancy, continue a pregnancy and prepare for a child with a condition. A stakeholder [9] said,
I think what is beneficial is that it allows people to make what’s known as informed choice, right. Screening is not there to tell you don’t have a baby with [condition]. It’s there to give you all the information so that as parents-to-be you are able to make your own choices about whether if you’re at risk of having a child with [condition], how you will deal with it, how you might need to care for it. So that’s, to me, that’s the benefit.
While information from antenatal screening may lead someone down one of several trajectories, the information from newborn screening was viewed as more straightforward. Namely, newborn screening gives parents information about the health of their newborn. If the child has a condition, then treatment or management can begin. The ability to prevent harm through treatment was one of the other benefits stakeholders regularly mentioned. A stakeholder [4] summarised this point:
It is sensible to maintain health and wellbeing as long as possible and your best chance of doing that is by identifying the condition as early as possible and screening is the best way to do that by a long way.
Stakeholders also mentioned how information from screening may allow people to make informed decisions about their future reproductive intentions. Participants shared that ‘it’s helpful for families to know that they have the genetic potential to have a child’ with a condition because sometimes the ‘decision to whether to have a second child’ might be ‘incredibly difficult’ [4]. This information was considered ‘a tremendous benefit’ [10] for families as they considered what might be best for parents, living children, and potentially extended family members.
Stakeholders drew attention to the fact that, in the UK, screening is provided on the NHS as free at point of service. This means that people with ‘rare or very serious health conditions’ can be treated by ‘one of the world’s leading experts’ free of charge [2]. Further, the NHS system was seen as one that can provide uniform care where the clinical community has produced ‘consensus documents, guidelines, patient information leaflets’ about screening and screened-for conditions [4].
Finally, stakeholders also suggested that screening may provide a sense of reassurance or connect people to their pregnancy. Stakeholders, like the individuals interviewed, suggested that screening was beneficial because it let people know their pregnancy or newborn was as expected. One stakeholder [6] shared,
For some women it is you know they, they’re utterly grateful and relieved to know that you know they can have screening and you know once they’ve got you know positive results then they can relax. Then they’re going to be okay.
Stakeholders expressed that finding out screening results were as expected could be a source of reassurance.
Charity and professional stakeholder views on the harms of screening
Interestingly, some stakeholders expressed the idea that a harm of screening can be its absence. These participants mentioned conditions that are currently screened for in other countries but not offered as part of the standardised NHS programmes in the UK. As one stakeholder said, these conditions ‘could have, should have been prevented if only [children were] born in France or Germany or America or Singapore’ [8]. Another stakeholder went on to say, ‘I think the harm from our community’s viewpoint is just where we’re not screening’ [10]. This subset of stakeholders suggested that by not screening for certain conditions, pregnant women or newborns might come to harm. Thus, this is a harm associated with how screening is implemented, and not screening itself.
Regarding screening programmes themselves, stakeholders named a variety of screening harms (see Report Supplementary Material 3 for supporting quote details):
-
Screening may provide unexpected news.
-
Screening may lead to devastating emotional impacts.
-
It is difficult to achieve fully informed consent.
-
Screening may imply pressure to have a termination.
-
Communication about screened-for conditions may not be handled well.
-
Screening may identify carriers.
-
It may be difficult for HCPs to retain specialist knowledge about conditions.
Stakeholders regularly mentioned the emotional toll that screening might have on individuals and families. They mentioned that it could be considered harmful to receive unexpected news from screening. Generally, stakeholders felt that people were ‘nowhere near the position of being able to hear’ unexpected news [1]. Another stakeholder shared that ‘raising the possibility that that child is affected by a lifelong, you know, potentially a lethal condition through a screening test is potentially really, really difficult conversation to have’ [4]. The unexpected news and fallout from screening may lead to devastating emotional impacts. Sometimes, ‘women will blame themselves’ and ‘fall into a pit of guilt and shame’ [6]. The effect, as one stakeholder [2] shared, can have long-term implications:
I think it’s very difficult to explain, explain to people just how devastated parents are when they are faced with this sort of information and it leads to the loss of their baby, it completely destroys people’s lives, not only in the short term but also in the very long term … it just devastates people and that requires, when you have to make a choice or when you are, you feel you’re forced into making a choice about whether your baby lives or dies, that is a choice you have to live with for the rest of your life and all of the questions and things that surround that and that is, yeah, that is one of the hardest things people I think would ever be faced with.
The emotional harms are exacerbated because of two other potential harms: (1) people may not understand what screening tests are for, and (2) it is difficult to achieve fully informed consent. Stakeholders routinely questioned what people knew about screening. As one participant said, ‘I’m just a bit concerned that do people really know what they’re screening for? Do they have an idea in their heads of why this is a good thing and what they might do as a result?’ [12]. Similarly, stakeholders discussed that it was difficult for HCPs to ensure informed consent because of the ‘amount of information and tasks they have to perform in those initial [pregnancy] booking appointments’ [5]. While HCPs might intend to ‘have a proper discussion about screening’, the fact is that ‘various studies have shown that the average length of time that they spend talking about screening is a few minutes. And that probably isn’t sufficient’ [5]. We discuss the challenges with information provision further in Charity and professional stakeholder views on the harms of screening.
Another potential harm of screening stakeholders discussed was that offering screening may imply pressure to have a termination. While stakeholders generally affirmed the right to have a termination, some questioned whether people were making the decision to terminate ‘without adequate information and support’ [3]. They worried that screening inferred a judgement on ‘which lives matter’ [12] and suggested that in the future ‘there’ll be less and less people’ with specific conditions [13]. This harm intersects with the harm that communication about screened-for conditions may not be well-handled. Stakeholders mentioned how HCPs might ‘talk about anomalies, abnormality, disorder, problem, you know, and all of that has negative connotations and, you know, is prejudicial and it influences a person’s perspective’ [3]. Poor communication may be exacerbated by the fact that it can be difficult for HCPs to retain specialist knowledge about conditions. While this was considered a harm, it was understandable since HCPs would not be ‘seeing cases on a sort of regular basis’ [1].
Charity and professional stakeholder discussed information provision challenges
Stakeholders consistently brought up the challenges of providing information about screening programmes and associated conditions (see Report Supplementary Material 3 for supporting quote details). This discussion was not necessity a benefit or a harm, but rather the articulation of a difficult reality. As one participant stated, the challenge is to ‘make sure the information is absolutely correct and up to data, but you also want to make it accessible’ [2]. Later in the interview, this participant also reflected on the fact that, while the NHS does provide information, it does not always do so in a way that is accessible. They conclude, the ‘NHS tries to provide information on absolutely everything and it can’t be good at absolutely everything’. Participants felt that charities played a large role in filling the information and support gap for people who received unexpected news from screening tests.
The difficulty though becomes how to pitch the information so it is accessible, accurate and appropriate. Stakeholders discussed that initial information probably ‘has to be quite general and quite superficial’ but the information provided when someone gets a higher chance result ‘still needs quite a lot of work’ [1]. This is complicated by the diversity of families’ needs; some ‘just wanting to know the very basics’ while ‘some families would want to know everything’ [4]. Participants suggested that it is ‘really helpful to be guided by the family’s questions’ and to ‘follow up questions very quickly’ [4]. Further, people ‘will contact us at various stages’ and their information needs may change over the course of their screening journey [5]. HCPs and support organisations recognised a need to be agile in responding to these needs but that ‘we have to be pragmatic and realistic’ [7]. Indeed, while stakeholders affirmed the right of people to make informed decisions, they drew attention to the point that ‘if you wanted a women to be fully informed, you’d have to sit her down and go through a list of all the potential things’ which would ‘make it a white-knuckle ride for every single woman’ [7]. It is a difficult line to walk, especially when ‘it’s a small percentage that will actually be confronted’ with a positive screening result [7].
Group 3: results
We conducted an online focus group with four midwives to sense check the themes derived from people who have experienced screening and other stakeholders. The four women work in different regions in the UK: London, Birmingham, Yorkshire and Belfast. Each had experiences as a midwife involved in screening and two had additional training in sonography. Below we discuss how these participants considered the themes pulled from focus groups and interviews with the other two groups (see Report Supplementary Material 3 for supporting quote details).
Midwife focus group participants corroborated that antenatal and newborn screening can be emotionally challenging. Participants reflected that it was their job to sometimes tell ‘devastating news’. In these cases, participants recognised it was good practice to ensure that patients ‘know your contact details’ so they could absorb information and get back in touch as needed. Participants also discussed how these emotionally laden moments with patients stick with them, sometimes for years. As one participant shared, ‘that’s probably the hardest part of the job’.
Participants also verified that people may feel pressured to accept screening because it was offered. One participant discussed having patients who have declined antenatal screening but have ‘gone on to consultant appointments’ where ‘they are pressured quite a lot’ to have had things done. Another participant countered that perhaps patients perceive pressure, but it is ‘literally just trying to explain to them what could actually happen’. While participants were quick to affirm the right of anyone to make decisions about screening, they themselves wished that people would take it up, if only to catch any potential conditions or anomalies. In the event that someone does decline screening, one participant shared that it is her job to say ‘you have the right to make this choice. And it is my responsibility to make sure that you understand the implications’. This way a patient’s wishes are respected, but the midwife leaves feeling like ‘I’ve done the best I can for them’.
Participants spent much of the conversation reflecting on the challenges of providing appropriate information while still maintaining informed choice. Participants mentioned the difficulty of providing comprehensive screening information when pregnant women and their partners are being ‘bombarded’ with ‘the vast amount of information we’re giving them at [the booking] appointment’. Participants realise they are ‘up against Google’ and wish that patients would ask HCPs to put information ‘in context’ rather than fixating on things that are statistically unlikely. Participants explained that it was difficult to know just how much information to give, saying, ‘It’s like where do you start and where do you stop?’. They did not want to scare patients but hold that ‘a woman should know what she’s consenting to’. Ultimately, participants questioned whether sharing all possible outcomes was ‘necessary and does it add anything to it? I don’t know’. There were no easy answers and participants acknowledged there was not one solution; much depended on how information was delivered and whom it was delivered to.
Summarised benefits and harms across all primary data groups
We have summarised the primary benefits and harms as discussed across our three primary data groups (Table 15). The ‘X’ marks indicate where one of the groups discussed a benefit or harm as primary theme in the data. The empty cells do not necessarily indicate that there was no evidence in our data of a topic, but rather that it was not a prominent theme within our data. While different stakeholders named different benefits and harms, there was a substantial amount of overlap between groups. Consistently named benefits included screening’s ability to get information, prevent harm, and provide reassurance. Consistently named harms included possible pressure to have termination, lack of preparation for unexpected results, emotional distress and not understanding what screening tests are for.
| Benefits | Individuals | Stakeholders | Midwives |
|---|---|---|---|
| Screening may provide information for people to make informed choices about their current pregnancy or newborn child | X | X | X |
| Screening may allow people to make informed decisions about their future reproductive intentions | X | ||
| Screening may prevent harm | X | X | X |
| Screening may connect people to their pregnancy | X | X | |
| Screening may provide reassurance | X | X | X |
| Screening is free at point of service | X | X | |
| Screening tests work and are done for a reason | X | X | |
| Screening enables people to learn about conditions | X | ||
| Harms | |||
| Screening harms may not be acknowledged | X | ||
| Normative screening practices do not suit everyone | X | X | |
| Screening may imply pressure to have a termination | X | X | X |
| It is difficult to achieve fully informed consent | X | X | X |
| People may be unprepared for unexpected results | X | X | X |
| Screening may cause emotional distress | X | X | X |
| Communication and support may be unsatisfactory | X | X | |
| People may not understand what screening tests are for | X | X | X |
| Screening may not be implemented presently in the UK | X | ||
| Screening may identify carriers | X | ||
| It may be difficult for HCPs to retain specialist knowledge about conditions | X |
Strengths and limitations
This primary qualitative study captured perspectives from a wide range of parents and stakeholders. For the parents we acknowledge that the sample was predominantly white, with two participants from an Asian and Arabic backgrounds. All participants spoke English as that was the only language that the two researchers undertaking data collection spoke and we were not able include funds for translation. However, our focus was on achieving diversity across a number of different domains, including socioeconomic status as well as education level and screening experience. In this, we have achieved a good range. We did not approach private companies/HCPs directly to support with recruitment due to pandemic restrictions, although we did circulate recruitment notices through social and professional organisations, which could have included private practices. However, people in our sample had made some use of private companies, primarily for additional scans for reassurance or to find out the sex. Only a handful reported they had sought private NIPT. Private companies were not central to where our data led.
Reflexivity
We considered our reflexivity consistently throughout this highly sensitive project and discussed this regularly among the team of experienced post-doctoral qualitative researchers, all of whom are female (AW, LH, FB, AM). The researcher principally collecting the data, AW, is a US citizen and was well positioned as an outsider to the NHS to explore experiences of screening openly. She is a woman of reproductive age and therefore shared on the surface several characteristics with our participants, which could have aided building rapport. She is also experienced at interviewing men about their reproductive health and has found that they often find this topic easier to discuss with a woman. The experience of conducting focus groups and interviews on these sensitive topics was emotionally intense, particularly during COVID-19 lockdowns. The qualitative team undertook regular debriefs to both aid the analysis and provide mutual support to each other. During these debriefs we discussed our individual experiences, characteristics, decisions and attitudes. We were consistently attentive to what we brought into the research and strove to maintain an atmosphere where varying voices and views were respected and heard.
Qualitative study discussion
Every year the NHS offers over a million antenatal and newborn screening tests every year to assess their chances of having or developing a health condition. Pregnant women and parents of babies identified by an antenatal or newborn screening programme receive information and are potentially offered further tests and intervention. While the UK has traditionally adopted a more conservative approach to antenatal and newborn screening compared to other countries, the rapid development of genetic and genomic technologies, and their ability to detect large numbers of conditions simultaneously, has prompted new interest in screening policies. Debates around screening have tended to favour quantitative economic and clinical meta-analyses, with the potential of qualitative research to capture the complexity and nuance of personal experience largely overlooked. 99 Yet, such perspectives are vital in understanding how antenatal and newborn screening programmes are experienced and perceived by those offered such tests.
We conducted three different qualitative studies: a meta-ethnography, a secondary analysis and primary data collection with three different stakeholder groups. While we summarise the consistently named benefits and harms in Results, we also identified overarching themes. Each aspect of our qualitative work highlighted concerns over informed consent and the challenges of providing accurate, accessible information while still making clear that screening programmes are a choice for individuals to make. This is complicated by the fact that people come to screening with varying absorptive capacities and information needs.
Our data also highlight the importance of temporal considerations. Screening is not only a clinical moment in time but may also involve critical windows of waiting and receiving results, with backwards, and forwards implications. It is important to consider that people come to screening with a variety of life experiences and may return to screening multiple times throughout their life course. Individuals bring their experiences with fertility, pregnancy, contraception and relationships to their screening narratives. The moment of having blood drawn, or an ultrasound scan done, and experiencing the results does not exist in a vacuum. Rather, the entirety of a person’s history over time is implicated.
The lure of expanding genomic technologies to potentially initiate early treatment and ultimately save lives is an appealing narrative of future medical success. However, our data highlight the already complex reality of participation by parents and parents-to-be which would likely be exacerbated by an expansion of screening programmes which may detect a far greater number of conditions, and therefore increase the number of people who receive positive or uncertain screening results. We find that the emotional fallout of receiving unexpected news is amplified when participants are not prepared for the possibility, particularly when they do not recall receiving an explanation about or consenting to the procedures. In the event of unexpected news, charities function as a vital source of information and support for individuals as they try to understand and move forward in the way that seems best for them.
Chapter 8 Work package 3: evidence synthesis
Introduction
We have conducted a systematic review of the benefits and harms considered by antenatal and newborn screening programmes (WP1) and a qualitative study to capture how stakeholders understand and experience screening programmes (WP2). This chapter brings together evidence from both WPs to understand how economic assessment methods and qualitative experiences of the public overlap and diverge in relation to identifying and characterising benefits and harms, ultimately highlighting areas of methodological strengths and limitations.
Methods
The thematically coded data from people who experienced screening (focus groups and interviews) and stakeholders (interviews) in WP2 were mapped onto the completed thematic framework (see Appendix 6, Table 27) derived in WP1. Our qualitative researcher (AW) reviewed the thematic framework in WP1 and, where possible, summarised the collected qualitative evidence providing illustrative quotes related to each subcategory from all the data available in WP2. LH and FB assisted in reviewing the evidence and refining the mapping exercise. In some cases, the qualitative data collected in WP2 did not cover theme subcategories, and we noted the absence. There were also themes from the qualitative data that could not be easily mapped onto the thematic framework. We have made a note of these and included them in the results below.
Results
The results of this mapping exercise presented as broad themes and first level of subthemes from WP1 and WP2 are summarised in Table 16. There were six themes related to antenatal and newborn screening programmes that were unique to WP2. Specific to antenatal screening, WP2 participants consistently discussed ‘the importance of information for antenatal screening’. That is, people going through screening valued the information they received. The information allowed them to consider pathways, decisions and futures for themselves and their families. For both antenatal and newborn screening, WP2 participants consistently recognised the ‘challenge of information provision to make sure choice is “informed” ’. People come to screening with varying absorptive capacities – varying abilities to recognise, assimilate and apply new information – so it is difficult to determine how much information is needed to ensure choices are informed. Not being able to make an informed decision is potentially a harm of screening. The decision to accept or decline screening is further complicated by ‘representations of conditions and disability’. Screening for conditions implies that they are concerning, and that a fetus/baby with a condition would potentially be problematic. Similarly, WP2 also found that ‘having screening available indicates that it is endorsed/normative’. By offering screening tests, the NHS is endorsing that the tests are worth having. Thus, these tests become the normative standard and people may feel an implied pressure to accept, which is potentially harmful.
| Type | Theme | Subtheme level 1 | WP1 | WP2 |
|---|---|---|---|---|
| Antenatal | Challenge of information provision to make sure choice is ‘informed’ | No | Yes | |
| Antenatal | Diagnosis of screened for condition | Additional screening of partners | Yes | Yes |
| Antenatal | Diagnosis of screened for condition | Additional testing to reach diagnosis in the absence of screening (links to diagnostic odyssey) | Yes | No |
| Antenatal | Diagnosis of screened for condition | Born with condition | Yes | Yes |
| Antenatal | Diagnosis of screened for condition | Cases diagnosed at screening | Yes | Yes |
| Antenatal | Diagnosis of screened for condition | Cases diagnosed at screening rather than later symptomatically | Yes | Yes |
| Antenatal | Diagnosis of screened for condition | Cases missed at screening | Yes | Yes |
| Antenatal | Diagnosis of screened for condition | Confirmatory test and additional tests to reach diagnosis of screened for condition | Yes | Yes |
| Antenatal | Diagnosis of screened for condition | Confirmatory test and additional tests to reach diagnosis of screened for condition [invasive] | Yes | Yes |
| Antenatal | Having screening available indicates that it is endorsed/normative | No | Yes | |
| Antenatal | Importance of information for antenatal screening | No | Yes | |
| Antenatal | Importance of time | No | Yes | |
| Antenatal | Life-years and health status adjustments | Infant life-years post birth | Yes | Yes |
| Antenatal | Life-years and health status adjustments | Maternal life-years | Yes | Yes |
| Antenatal | Life-years and health status adjustments | Psychological | Yes | Yes |
| Antenatal | Long-term cost associated with screened for condition | Cost savings from averted births of fetuses with anomalies | Yes | Yes |
| Antenatal | Long-term cost associated with screened for condition | Direct healthcare and non-healthcare cost | Yes | Yes |
| Antenatal | Long-term cost associated with screened for condition | Direct healthcare cost | Yes | Yes |
| Antenatal | Long-term cost associated with screened for condition | Direct non-healthcare cost | Yes | Yes |
| Antenatal | Long-term cost associated with screened for condition | Productivity gains | Yes | Yes |
| Antenatal | Long-term cost associated with screened for condition | Societal cost | Yes | Yes |
| Antenatal | Overdiagnosis | QALY decrement | Yes | No |
| Antenatal | Overdiagnosis | Unnecessary treatment | Yes | No |
| Antenatal | Pregnancy loss | Spontaneous | Yes | Yes |
| Antenatal | Pregnancy loss | Termination | Yes | Yes |
| Antenatal | Pregnancy loss | Treatment/test related | Yes | Yes |
| Antenatal | Representations of conditions and disability | No | Yes | |
| Antenatal | Taking a life course approach to screening journey | No | Yes | |
| Antenatal | Treatment | Additional health care post diagnosis | Yes | Yes |
| Antenatal | Treatment | Comparison of earlier treatment after screen detection | Yes | Yes |
| Antenatal | Treatment | Comparison of earlier treatment after screen detection and later after symptomatic detection | Yes | Yes |
| Antenatal | Treatment | Hospital stay | Yes | Yes |
| Antenatal | Treatment | Missed due to false negative | Yes | Yes |
| Antenatal | Treatment | Prevention of screened for condition (infectious) | Yes | No |
| Antenatal | Treatment | Psychological | Yes | Yes |
| Antenatal | Treatment | Screened for condition-related treatment/management | Yes | Yes |
| Antenatal | Treatment | Treatment-related harm | Yes | No |
| Antenatal | Treatment | Unnecessary due to false positive | Yes | No |
| Newborn | Benefits to parents from child’s diagnosis with genetic condition, through knowledge of their own genetic status | Yes | Yes | |
| Newborn | Challenge of information provision to make sure choice is ‘informed’ | No | Yes | |
| Newborn | Diagnosis of screened for condition | Additional testing to reach diagnosis in the absence of screening (links to diagnostic odyssey) | Yes | Yes |
| Newborn | Diagnosis of screened for condition | Born with condition | Yes | Yes |
| Newborn | Diagnosis of screened for condition | Cases diagnosed at screening | Yes | Yes |
| Newborn | Diagnosis of screened for condition | Cases diagnosed at screening rather than later symptomatically | Yes | Yes |
| Newborn | Diagnosis of screened for condition | Cases diagnosed at screening that would have become symptomatic | Yes | Yes |
| Newborn | Diagnosis of screened for condition | Cases missed at screening | Yes | Yes |
| Newborn | Diagnosis of screened for condition | Confirmatory test and additional tests to reach diagnosis of screened for condition | Yes | Yes |
| Newborn | Diagnosis of screened for condition | Screened for condition-related complications | Yes | Yes |
| Newborn | Having screening available indicates that it is endorsed/normative | No | Yes | |
| Newborn | Importance of time | No | Yes | |
| Newborn | Life-years and health status adjustments | Infant life-years post birth | Yes | Yes |
| Newborn | Life-years and health status adjustments | Maternal life-years | Yes | Yes |
| Newborn | Life-years and health status adjustments | Parental QALYs | Yes | Yes |
| Newborn | Life-years and health status adjustments | Psychological | Yes | Yes |
| Newborn | Life-years and health status adjustments | Screened for condition associated mortality/treatment associated mortality/other causes mortality | Yes | Yes |
| Newborn | Long-term cost associated with screened for condition | Direct healthcare and non-healthcare cost | Yes | Yes |
| Newborn | Long-term cost associated with screened for condition | Direct healthcare cost | Yes | Yes |
| Newborn | Long-term cost associated with screened for condition | Direct non-healthcare cost | Yes | Yes |
| Newborn | Long-term cost associated with screened for condition | Productivity gains | Yes | No |
| Newborn | Long-term cost associated with screened for condition | Societal cost | Yes | Yes |
| Newborn | Overdiagnosis | QALY decrement | Yes | No |
| Newborn | Overdiagnosis | Unnecessary treatment | Yes | No |
| Newborn | Representations of conditions and disability | No | Yes | |
| Newborn | Taking a life course approach to screening journey | No | Yes | |
| Newborn | Treatment | Additional health care post diagnosis | Yes | Yes |
| Newborn | Treatment | Comparison of earlier treatment after screen detection | Yes | Yes |
| Newborn | Treatment | Comparison of earlier treatment after screen detection and later after symptomatic detection | Yes | Yes |
| Newborn | Treatment | Hospital stay | Yes | Yes |
| Newborn | Treatment | Screened for condition-related treatment/management | Yes | Yes |
| Newborn | Treatment | Treatment-related harm | Yes | Yes |
Across both antenatal and newborn screening, WP2 also identified broader factors that influence screening experiences, which are not accounted for in current economic assessment methods. It is vital to consider ‘the importance of time’ in relation to screening. People’s experiences will vary based on when screening happens in a person’s life (e.g. being 25 vs. being 40 years old), the duration between having a screening test and waiting for results (e.g. 24 hours or 1 week) and the duration between unexpected results and follow-up options (e.g. same day, next day, a week). Other important temporal considerations may include the number of weeks gestation (antenatal) or the time child might have before a condition manifests (newborn). Similarly, WP2 highlighted the importance of ‘taking a life course approach to the screening journey’. People bring an accumulation of experiences into screening and screening experiences (and potential outcomes) are carried forward.
Discussion
This chapter provides an evidence synthesis drawn from quantitative and qualitative evidence of benefits and harms associated with antenatal and newborn screening. We found a high degree of overlap between the quantitative and qualitative evidence. Notably, however, we identified specific subthemes that had been considered by previous economic assessments but not identified by the qualitative research. Similarly, we identified specific subthemes considered important to stakeholders in our primary data collection, but that had been excluded by previous economic assessments in this field. This overarching evidence synthesis provides a framework that can be used as a springboard for identifying the benefits and harms for inclusion within future economic assessments. The selection of benefits and harms for inclusion in future economic assessments should ultimately be informed by the condition being targeted by screening, and the methodological requirements for economic assessment set by the national screening agency. Where there is a requirement for cost–utility analysis or cost–benefit analysis to inform adoption decisions, the tractability of benefits and harms to valuation using preference-based techniques should additionally inform the selection process.
Chapter 9 Work package 4: stakeholder workshops
A crucial final element of the VALENTIA research programme involved an assessment of whether and how economic valuation methods can be used to elicit preferences for scenarios of benefits and harms associated with antenatal and newborn screening. The intention here was to provide an early assessment of the feasibility of applying alternative economic valuation methods in future economic assessments of antenatal and newborn screening. This was achieved through two workshops held with patient and public members, which aimed to explore the use of alternative economic (preference-based) techniques to value plausible scenarios of benefits and harms. A separate workshop was held with a broad set of stakeholders to review the findings of the VALENTIA research programme and contribute to a set of recommendations about approaches for the measurement and valuation of outcomes that should be considered by future economic assessments of antenatal and newborn screening, and to highlight areas for future methodological enquiry.
Patient and public members’ workshops
Invitations to participate in online workshops were sent to all 29 patient and public members that remained actively engaged with the VALENTIA research programme through to the final quarter of 2021. The invitations specified that the VALENTIA research team was seeking patient and public member perspectives on scenarios involving antenatal and newborn screening. The invitations specified that the workshops would each involve a maximum of six patient and public members and would be held on separate evenings. They were also informed that each online workshop would last for a maximum of 2 hours. A total of 10 out of the 29 patient and public members (34.5%) agreed to participate in the workshops with 6 attending the first workshop and 4 attending the second workshop. The patient and public members were reimbursed for their time following guidance from the NIHR Centre for Engagement and Dissemination. At each online workshop, members of the VALENTIA research team initially provided a brief overview of the VALENTIA study methods and the results of the systematic review, qualitative research and evidence synthesis. The patient and public members were then presented with alternative scenarios of antenatal and newborn screening that were delineated in terms of benefits and harms identified by the thematic framework outlined in Chapter 8. The views of the 29 patient and public members were then sought on the feasibility of applying alternative economic (preference-based) techniques to value those scenarios. Discussion at each online workshop was recorded and subsequently transcribed. Additionally, several patient and public members sent separate reflections to the VALENTIA research team via e-mail. The insights presented below are thus a combination of direct quotes from the online workshops and separate feedback from the patient and public members.
Developing scenarios for valuation
To assess the feasibility of applying alternative economic (preference-based) techniques to scenarios of antenatal and newborn screening, we developed two scenarios, one describing a scenario of antenatal screening and the other describing a scenario of newborn screening with each scenario reflecting benefits and harms identified by the thematic framework outlined in Chapter 8. It would clearly have been possible to have generated far more scenarios that reflect the permutations of benefits and harms described by the thematic framework. However, increasing the number of scenarios would have added significantly to participant burden. The final scenarios were presented as hypothetical, but nevertheless representative of the types of decisions people might have to make about antenatal or newborn screening.
The preamble for the antenatal screening scenario asked participants to imagine a hypothetical women named Sophie and her partner Jamie who had been trying to have a baby for 3 years. The preamble for this antenatal screening scenario was presented as follows:
Sophie is currently pregnant after having two miscarriages. She had the combined test that showed there was a higher than average chance that Sophie’s pregnancy would result in having a baby with an inherited genetic condition. Sophie had an amniocentesis test to see if the blood test was right. Results from the amniocentesis test confirmed that Sophie’s pregnancy was affected by this condition. Sophie’s doctor explained that pregnancies affected by this condition may end in miscarriage or still birth. The doctor also said that children born with the condition will require specialist care. The doctor says Sophie can work with a team to continue her pregnancy or she can terminate the pregnancy for medical reasons. The doctor tells Sophie about some support organisations and tells her to think about what she wants to do.
The patient and public members were then presented with seven attributes that characterised this hypothetical antenatal screening scenario: history of prior miscarriages, accuracy of screening test, time to wait for screening test results, the risk of miscarriage associated with the diagnostic test, the psychological/emotional impact of a termination decision on the woman and her partner (Sophie and Jamie), the perceived severity of the diagnosed condition and the parents’ ability to make a decision about screening. The potential attributes and levels of those attributes that might influence the antenatal screening decision are presented in Table 17.
| Attributes | Levels |
|---|---|
| History of prior miscarriages | No prior miscarriages 1 prior miscarriage 2 or more prior miscarriages |
| Accuracy of screening test | 90% accurate 95% accurate 99% accurate |
| Time to wait for screening test results | < 24 hours wait Between 1 and 3 days wait > 3 days wait |
| Diagnostic test risk of miscarriage | No risk of miscarriage < 1% risk of miscarriage > 1% risk of miscarriage |
| Psychological/emotional impact of termination decision on woman and her partner | No psychological impact Minor psychological impact Major psychological impact |
| Perceived severity of diagnosed condition | Condition considered minor Condition considered moderate Condition considered severe |
| Parents’ ability to make a decision | It would be very easy to make a decision It would be easy to make a decision It would be hard to make a decision It would be very hard to make a decision |
The preamble for the newborn screening scenario asked participants to imagine a hypothetical newborn baby named Ella. The preamble for the newborn screening scenario framed around a parent’s consent to the heel prick test for newborn Ella was presented as follows:
Thea has a newborn baby named Ella. Five days after Ella is born a midwife comes to the house. The midwife does a physical examination and asks Thea if she wants Ella to have newborn bloodspot screening. Sometimes this is called the ‘heel prick’ test. The midwife explains that newborn blood spot screening involves taking a blood sample to find out if Ella might have one of a range of health conditions. Often, it is too early to tell if Ella might have a health condition any other way. The midwife explains that the blood spot screening is optional. Thea needs to decide what she wants to do.
The patient and public members were then presented with six attributes that characterised this hypothetical newborn screening scenario: age of child when symptoms manifest, timing of start of treatment, the likelihood of success of treatment, perceived severity of diagnosed condition, likelihood of a false-positive result, and likelihood of overdiagnosis. The potential attributes and levels of those attributes that might influence the newborn screening decision are presented in Table 18.
| Characteristic | Levels |
|---|---|
| Age of child when symptoms manifest | Symptoms develop in infancy Symptoms develop in childhood Symptoms develop in adulthood Symptoms do not manifest |
| Start of treatment | Treatment begins within a few weeks of birth Treatment begins within the first year of life Treatment begins during childhood |
| Success of treatment | Treatment will cure the condition Treatment will prevent the condition from getting worse Treatment will slow the worsening of the condition |
| Perceived severity of diagnosed condition | Condition considered minor Condition considered moderate Condition considered severe |
| False-positive result | There are many false-positive results There are few false-positive results |
| Overdiagnosis | There are many infants who are overdiagnosed There are few infants who are overdiagnosed |
Selection of valuation methods
The VALENTIA research team reviewed alternative economic (preference-based) techniques that can be used to value the hypothetical antenatal and newborn screening scenarios described in Developing scenarios for valuation. A pool of 10 potential valuation techniques previously used by economists to value healthcare processes was initially identified: allocation of points, analytical hierarchical process, best–worst scaling, contingent valuation, discrete choice experiments, measure of value, person trade-off, rating scale, standard gamble and time trade-off. 104,105 The VALENTIA research team applied a number of criteria to prioritise the valuation techniques that could be applied in the patient and public member workshops. These included broader application of the valuation technique in health economics or healthcare decision-making contexts; foundation of the valuation technique in economic theory; applicability of the technique to the valuation of antenatal and newborn screening scenarios delineated in terms of benefits and harms described by our thematic framework; and practicality of application given the mode and time constraints of the online workshops. Allocation of points, analytical hierarchical process and measure of value were excluded on the basis of limited previous application in health economics or healthcare decision-making contexts. The rating scale approach and its variants were excluded because of disagreement among health economists of their theoretical basis as preference-based techniques. 106 The standard gamble and time trade-off approaches were excluded because of the challenges surrounding the labelling of upper and lower anchors in the context of the hypothetical antenatal and newborn screening scenarios where there is no meaningful risk of death or severe disability. Concerns around the practicality of the number of valuation techniques that could be applied within the online workshops then led to the selection of best–worst scaling and discrete choice experiments, which had previously been used to value scenarios of antenatal and newborn screening. 107 For both the hypothetical antenatal and newborn screening scenarios, the patient and public members were presented with a best–worst scaling task and a discrete choice experiment task. They were then asked to provide their insights on: (1) whether they were able to imagine the scenario that was being valued and what factors influenced their ability to imagine the scenario; (2) whether they understood each task; (3) their main considerations when attempting to complete the tasks; (4) whether they drew on personal experiences or experiences of their families/friends when completing the tasks; and (5) which valuation method they preferred and why. The best–worst scaling task that related to the hypothetical newborn screening scenario is presented in Table 19, while the discrete choice experiment task that related to the hypothetical antenatal screening scenario is presented in Table 20.
| Which characteristic you consider best | Alternative | Which characteristic you consider worst |
|---|---|---|
| □ | Symptoms develop in infancy | □ |
| □ | Treatment begins within a few weeks of birth | □ |
| □ | Treatment will prevent the condition from getting worse | □ |
| □ | Condition considered minor | □ |
| □ | There are few false-positive results | □ |
| □ | There are many infants who are overdiagnosed | □ |
| Alternative 1 | Alternative 2 |
|---|---|
| Two or more prior miscarriages | No prior miscarriages |
| Screening test 90% accurate | Screening test 99% accurate |
| Waiting time for screening test results < 24 hours | Waiting time for screening test results > 3 days |
| No risk of miscarriage following diagnostic test | > 1% risk of miscarriage following diagnostic test |
| Major psychological impact if pregnancy terminated | Minor psychological impact if pregnancy terminated |
| Diagnosed condition considered moderate in severity | Diagnosed condition considered moderate in severity |
| Very easy for parents to make a decision about screening | Hard for parents to make a decision about screening |
| □ | □ |
Insights from patient and public member workshops
When assessing the hypothetical antenatal and newborn screening scenarios that were being valued, participants could generally imagine the scenarios, although for some participants this highlighted the complexity of the screening decisions often made in the antenatal and newborn screening contexts:
Yeah. I think it was, in my opinion, easy to put yourself in either scenario and say, yes, that could apply with lots of, obviously, differing factors. But yes.
Participant 5
I find it easy enough to imagine myself in each of the scenarios, but I don’t think … I don’t know if this is what you’re after, but for me, it wouldn’t have been clear cut what the decision would’ve been for either [00:31:30] scenario. I don’t think that was particularly easy to imagine the outcome, I guess, and how each would progress.
Participant 2
The level of understanding of each valuation task varied between patient and public members with some participants reporting that their level of understanding increased through the course of workshop and clarification provided by members of the VALENTIA research team and other patient and public members:
I think the more we’ve talked about it, the more I’ve understood it. I don’t think I understood it to begin with, but with everybody having a discussion around the pair comparison I’m talking about, mostly. I have to say, I didn’t understand it completely to begin with, but now I think I do.
Participant 5
Participants considered a number of factors when attempting to complete the valuation tasks or, at least, expressed a desire for more information on factors that would influence their preference structures. These factors included the background and reproductive history of the hypothetical parents described in the examples, the number of attributes reflected in the tasks, the labelling of adjectives such as ‘minor’ or ‘major’, which carried subjective connotations and timing at which a preference elicitation study would be conducted:
I think there’s maybe additional information about the parents themselves that could be explored a little more, and their history, not just their history with pregnancy, but their history in a wider context.
Participant 2
I just was going to just agree with that point really. I found it a bit overwhelming some of the information in the two alternatives. And I agree, a lot of these things are quite hard. Well, take a lot of thought, and maybe just some of the variables, kind of reducing the difference in the number of variables would’ve been helpful.
Participant 6
And one of the things that is constantly asked is this idea of major or minor, and whether your participants understand what that means. Because a major psychological impact will mean something very, very different to different people. And they need to understand the context of what is meant by that and whether that’s going to needs to be provided to them before they understand what does it mean? What does a major psychological impact mean?
Participant 6
Just quickly also, I guess one of the questions is you might be asking these, giving these surveys at a time that’s quite a stress. And I don’t know when you’re going to give these to parents, for example, it might be quite a stressful time.
Participant 6
Several patient and public members revealed that they drew on their personal experiences or experiences of their families/friends when completing the tasks, perhaps reflecting the composition of the sample that was selected from:
But actually, I was pretty clueless in terms of how it did affect me. So this is where my personal experiences come in to definitely influence certain parts of my answer.
Participant 4
I felt like my own experiences and priorities probably came into that quite a lot. But, in terms of kind of empathizing with the person in that situation, I could totally put myself in that context.
Participant 9
Finally, in terms of the two valuation methods that were assessed, there appeared to be a general preference for the best–worst scaling method with several participants describing it as easier to understand and process, although the one participant disputed the ‘best’ and ‘worst’ labels in the hypothetical scenarios provided:
The paired comparison, I felt, could be quite confusing. Because, the attributes that were there, some of them weren’t really comparable. For me, it felt quite challenging to think ‘I’ve got to choose between this one or this one.’ Because, I think people value different things in different ways and what might be higher value to someone else is lower value to another.
Participant 10
And I really like the best-case, worst-case scenario. It’s [inaudible] for me for just ease of understanding, and I could do it whilst sitting somewhere.
Participant 6
I have to say, I think the best worst scenarios are … I think the wording is potentially, I don’t know, because there’s no best scenario, I think, in some of them. So it may be the wording of how it’s come across, but it’s more easy to understand from my point of view. If that makes sense.
Participant 5
In summary, the patient and public participants in the online workshops highlighted a number of factors that influence their preferences for antenatal and newborn screening scenarios, but generally understood the two preference-based techniques presented. Notably, however, they were not presented with some methods developed by economists, such as contingent valuation, which could plausibly be applied to value scenarios delineated in terms of benefits and harms from our thematic framework (see Chapter 4).
Broader stakeholder workshop
A half-day online workshop with a broad set of stakeholders was held in January 2022 to disseminate the findings of the VALENTIA research programme, contribute to a set of recommendations about approaches for the measurement and valuation of outcomes that should be considered by future economic assessments of antenatal and newborn screening, and to highlight areas for future methodological enquiry. Discussion at the workshop was recorded and subsequently transcribed. Members of the VALENTIA research team and the VALENTIA Independent Oversight Committee nominated stakeholders from a breadth of backgrounds with a potential interest in the research and its methodological and policy implications. Snowballing techniques through academic and professional networks were used to widen the pool of individuals that were approached. The initial plan was to recruit a total of 30 individuals to participate in the workshop. A total of 31 stakeholders encompassing healthcare professionals, representatives from relevant academic disciplines, representatives from charities, outreach services and support groups, and representatives from policy-making bodies subsequently participated in the workshop. The workshop followed an overview of the methods and the results of the systematic review, qualitative research and evidence synthesis, and seeking feedback on the thematic framework of benefits and harms outlined in Chapter 4. The recommendations presented in the next chapter resulted from a combination of lessons drawn by the VALENTIA research team and feedback provided by the stakeholder group.
Chapter 10 Study recommendations and areas for future research
The VALENTIA research programme aimed to enhance knowledge about methods for valuing the benefits and harms of antenatal and newborn screening within economic assessments and make recommendations about health economic measurement tools that should be applied in this area in the future. The systematic review presented in Chapter 3 identified health economic assessments that evaluated antenatal and newborn screening programmes in OECD countries over the past two decades. It described the benefits and harms adopted by those health economic assessments. Chapter 4 presented a thematic framework of benefits and harms adopted by those studies and summarised the benefits and harms into seven core themes. Chapter 5–7 summarise evidence from a body of qualitative research around attributes of relevance to stakeholders that should be considered for incorporation into future economic assessments of antenatal and newborn screening. Specifically, Chapter 5 describes a systematic review of qualitative studies that focus on parents’ experiences of newborn screening. Chapter 6 reports a secondary analysis of existing qualitative interviews exploring experiences of, and attitudes towards, antenatal and newborn screening, as well as the experience of living with screened-for conditions. Chapter 7 outlines a thematic analysis of primary data of stakeholders’ screening experiences. Chapter 8 synthesises the evidence extracted from the quantitative and qualitative WPs with the view to describing overlap and divergence between the two strands of evidence. Finally, Chapter 9 provides an early assessment of the feasibility of applying alternative economic valuation methods in future economic assessments of antenatal and newborn screening. This feasibility work was conducted with patients and members of the public but was supplemented by input from a broad set of stakeholders. Each WP was informed by a PPI advisory group (see Chapter 2).
Each chapter of the report outlines the strengths and limitations of the methodological approaches applied, which require consideration when interpreting the results of each constituent element. Notably, we highlight the potential limitations arising from the composition of the PPI group (see Chapter 2); restriction of double data extraction to a 10% random sample of the articles and reports identified by the systematic review of economic assessments (see Chapter 3); lack of representation of all conditions in the secondary analysis of existing interviews exploring experiences of antenatal and newborn screening (see Chapter 6); restriction of primary data collection amongst healthcare professionals aimed at understanding stakeholders’ experiences to midwives with the exclusion of input from sonographers, obstetricians and genetic counsellors (see Chapter 7); and restriction of valuation methods within the benefit valuation exercises to discrete choice experiments and best worst scaling (see Chapter 9).
This chapter presents recommendations for future research, drawing upon evidence from across the VALENTIA programme.
Overarching study recommendations
The VALENTIA study has identified a range of benefits and harms related to antenatal and newborn screening using mixed-methods research that triangulated evidence from a systematic review of economic assessments identified from the published and grey literature and a qualitative study that described the impacts of antenatal and newborn screening of importance to a range of stakeholders.
It is incumbent on researchers designing economic assessments of antenatal and newborn screening to consider the benefits and harms that we have identified that are likely to be of relevance to the research questions they are addressing. The thematic framework presented in Chapter 4 and the synthesis of evidence presented in Chapter 8, and their accompanying appendices, provide a framework that can be used as a springboard for identifying the benefits and harms to consider within partial economic assessments, such as discrete choice experiments and best–worst scaling studies designed to elicit preferences for forms of antenatal and newborn screening. Notably, the evidence base we have generated can also be used to inform the benefits and harms that should be included within economic evaluations of antenatal and newborn screening targeted at specific conditions. This identification process should, in turn, inform the appropriate evaluative framework(s) that could be applied. There will be several circumstances where the breadth and nature of benefits and harms identified are likely to preclude the estimation of net health benefits within the QALY paradigm. For example, attributes of relevance to parents, such as the utility derived from information per se or reassurance following a screen-negative test result, and the disutility associated with a false-positive test result or overdiagnosis of disease, are likely to be missed, or at least inadequately covered, by standard approaches to health utility measurement, such as available multi-attribute utility measures (e.g. EQ-5D, SF-6D, HUI Mark 3). 25,26 In these circumstances, we recommend that alternative evaluative frameworks be considered, for example, cost–benefit analysis that provides a framework for valuing disparate benefits and harms in monetary terms, or cost–consequences analysis that provides a framework for disaggregating disparate consequences that might not necessarily be tractable to economic valuation. Methods guidance from screening organisations, such as the UK NSC, should specify the limitations of cost–utility analysis as an evaluative framework from antenatal newborn screening, at least using the approaches to health utility measurement that are currently available, and the circumstances in which alternative evaluate frameworks should be considered.
In the following sections, we outline areas of further research around identification, measurement and valuation of benefits of harms of antenatal and newborn screening that would enhance knowledge in this area.
Future research
Identification of benefits and harms
Our evidence synthesis around benefits and harms related to antenatal and newborn screening was informed by a systematic review of economic assessments identified from the published and grey literature and qualitative research that took the form of a meta-ethnography, a secondary analysis and primary data collection with three different stakeholder groups. As part of our primary data collection, we had intended to interview healthcare professionals from a breadth of healthcare backgrounds involved in antenatal and newborn screening, such as midwives, sonographers, obstetricians, neonatologists, neonatal nurses and genetic counsellors. However, despite multiple approaches through different routes, the ongoing pandemic during our recruitment period (Spring 2021) meant that healthcare professionals were overworked and had little time or energy to take part. This meant that our primary data collection from healthcare professionals was limited to a focus group conducted with four midwives. Clearly, this represents a limitation of our research and primary data collection from healthcare professionals operating at different stages of the screening pathways is likely to offer new perspectives and highlight potential gaps in our synthesis of benefits and harms. Their inclusion would most likely have offered further insight into our synthesis of relevant benefits and harms. Beyond the composition of the stakeholders from which we collected primary data, the topic guides for our focus groups and one-on-one interviews were necessarily constrained by the limited time available for each research contact. Further research around how people’s feelings about positive screening results and/or diagnosis change over time after receiving results, and around how people’s relationship to screening varies over the reproductive life course, may offer new perspectives on our study outputs.
Measurement of benefits and harms
Economic evaluations of antenatal and newborn screening are constrained by a number of methodological limitations that our research has highlighted. There is a particular need for further research, or methods guidance from screening organisations, in the following areas:
-
Where benefits and harms associated within antenatal screening are synthesised within composite outcome measures such as life-years or QALYs gained or DALYs averted, methods guidance is needed around approaches to aggregating consequences across the mother, fetus or unborn child and potentially other family members. This will clearly be influenced by moral perspectives on the status of the fetus or unborn child with different moral perspectives on when human life commences likely to have a significant impact upon cost effectiveness or cost–benefit estimates. 21,22 Beyond consideration of when to commence ‘counting’ the life of the fetus or unborn child in the calculus, this still leaves the issues of what weight to place on prenatal time lost or gained compared with postnatal time, and what weights to place on measures of consequence to the fetus or unborn child, the mother and potentially to other family members.
-
Where benefits and harms associated within newborn screening are synthesised within QALY metrics, there is a need for development of patient-reported outcome measures (PROMs) with preference-based value sets validated for use in infancy and across other stages of childhood. A recent system review108 of generic multidimensional childhood PROMs revealed 17 measures (incorporating two versions each for the HUI2 and HUI3 and two variants of the EQ-5D-Y) designed to be accompanied by preference-based value sets. Notably, only 2 of the 17 measures, the Infant health-related Quality of Life Instrument (IQI)109 and the Toddler and Infant health-related Quality of Life Instrument (TANDI),110 have been validated for use during infancy and their preference-based value sets are still under development. No preference-based measure is validated for use across all developmental stages from infancy to adolescence, which constrains the potential for QALY estimation in economic evaluations that extrapolate cost effectiveness over a childhood time horizon or beyond.
Valuation of benefits and harms
Economists have developed several possible methods for valuing antenatal and newborn screening scenarios. Our feasibility work focused on testing the application of discrete choice experiments and best–worst scaling in these contexts. Other valuation methods, such as contingent valuation, could conceivably be applied in these contexts,111,112 However, the application of such methods will need to be assessed drawing upon the broad sets of benefits and harms that our research has identified. Valuation techniques such as standard gamble and time trade-off approaches are widely used in other areas of health economics to value health states without consideration for non-health outcomes and process attributes. We identified methodological challenges surrounding the labelling of upper and lower anchors within standard gamble and time trade-off tasks aimed at valuing antenatal and newborn screening scenarios that do not contain a meaningful risk of death or severe disability. Research is required to test the application of these approaches in two-stage processes where the first stage is used to value scenarios of benefits and harms that our research has identified, and the second stage is used to score anchors on the full health-immediate death scale. Finally, consideration should be given to the development of methods for weighting disparate consequences of antenatal and newborn screening, drawn from our taxonomy and accompanying qualitative research, within a cost–consequences analysis framework.
Additional information
Acknowledgements
We would like to express our gratitude to the members of the Independent Oversight Committee – Professor Luke Vale (Chair) (University of Newcastle), Professor Tom Shakespeare (London School of Hygiene and Tropical Medicine), Professor Christopher Megone (University of Leeds), Dr Chris Day (Bradford Teaching Hospitals NHS), Dr Andrew Booth (University of Sheffield), Dr Gareth Thomas (Cardiff University), Mr Nick Meade (Genetic Alliance UK) and Associate Professor Shalini Ojha – for their guidance and advice throughout the study. Their top-rate critical thinking and wealth of knowledge in the field of antenatal and newborn screening have been tremendously helpful to the team and our approach to this study has been significantly improved by their guidance.
The qualitative research team would like to thank the women and men who took part in the interviews and focus groups for sharing their journeys. We also thank the midwives, charity stakeholders and policy-makers who supported the study by sharing their views and experiences with us. We thank Professor Louise Locock for her immensely helpful guidance to the qualitative team.
We would also like to thank our PPI members for their time and input throughout the study. We are also grateful to participants attending our final stakeholder’s workshop.
Contributions of authors
Oliver Rivero-Arias (https://orcid.org/0000-0003-2233-6544) (Associate Professor of Health Economics) was the co-principal investigator. He was involved in the conceptualisation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, visualisation, writing – original draft, and writing – review and editing of this report.
May Ee Png (https://orcid.org/0000-0001-5876-9363) (Senior Researcher, Health Economics) was involved in the data curation, formal analysis, investigation, methodology, project administration, resources, visualisation, writing – original draft, and writing – review and editing of this report.
Ashley White (https://orcid.org/0000-0003-4519-2280) (Qualitative Researcher) was involved in the data curation, formal analysis, investigation, methodology, project administration, resources, visualisation, writing – original draft, and writing – review and editing of this report.
Miaoqing Yang (https://orcid.org/0000-0001-7975-374X) (Researcher in Health Economics) was involved in the formal analysis, investigation, methodology and writing – review and editing of this report.
Sian Taylor-Phillips (https://orcid.org/0000-0001-9843-4736) (Professor of Population Health) was involved in the conceptualisation, formal analysis, funding acquisition, methodology and writing – review and editing of this report.
Lisa Hinton (https://orcid.org/0000-0002-6082-3151) (Senior Research Associate, Qualitative Methodology) was involved in the conceptualisation, funding acquisition, methodology and writing – review and editing of this report.
Felicity Boardman (https://orcid.org/0000-0002-3268-6276) (Professor of Social Science in Medicine) was involved in the conceptualisation, funding acquisition, methodology and writing – review and editing of this report.
Abigail McNiven (https://orcid.org/0000-0001-5041-2095) (Senior Qualitative Researcher) was involved in the methodology and writing – review and editing of this report.
Jane Fisher (https://orcid.org/0000-0003-1318-0074) (Director, Antenatal Results and Choices) was involved in the conceptualisation, funding acquisition, methodology and writing – review and editing of this report.
Baskaran Thilaganathan (https://orcid.org/0000-0002-5531-4301) (Professor of Fetal Medicine) was involved in the conceptualisation, funding acquisition, methodology and writing – review and editing of this report.
Sam Oddie (https://orcid.org/0000-0001-8701-4912) (Consultant Neonatologist) was involved in the conceptualisation, funding acquisition and writing – review and editing of this report.
Anne-Marie Slowther (https://orcid.org/0000-0002-3338-8457) (Professor of Clinical Ethics) was involved in the conceptualisation, funding acquisition and writing – review and editing of this report.
Svetlana Ratushnyak (https://orcid.org/0000-0001-7967-5112) (Researcher in Health Economics) was involved in the methodology and writing – review and editing of this report.
Nia Roberts (https://orcid.org/0000-0002-1142-6440) (Senior Outreach Librarian) was involved in the resources and writing – review and editing of this report.
Jenny Shilton Osborne (https://orcid.org/0000-0001-9057-3395) (Project Manager, PPI Coordinator) was involved in the methodology, project administration, resources, writing – original draft, and writing – review and editing of this report.
Stavros Petrou (https://orcid.org/0000-0003-3121-6050) (Professor of Health Economics) is the co-principal investigator. He was involved in the conceptualisation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, visualisation, writing – original draft, and writing – review and editing of this report.
All authors contributed to the report and approved the final version.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/PYTK6591.
Primary conflicts of interest: Oliver Rivero-Arias, Stavros Petrou, Abigail McNiven, Anne-Marie Slowther, Felicity Boardman, Sian Taylor-Phillips and Lisa Hinton report receipt of funding from NIHR and other entities over the last 3 years, outside the submitted work. Anne-Marie Slowther and Jane Fisher sit on the UK National Screening Committee (UK NSC). Oliver Rivero-Arias, Jane Fisher, Basky Thilaganathan and Felicity Boardman are members of the Fetal, Maternal and Child Health reference group of the UK NSC. Jane Fisher is a member of the NHS Fetal Anomaly Screening Programme Advisory Group. Sian Taylor-Phillips is a member of the UK NSC Adult Reference Group, supported by a National Institute for Health and Care Research (NIHR) Career Development Fellowship. Sam Oddie serves on the UK NSC expert group on implementing saturation screening. Stavros Petrou receives support as a NIHR Senior Investigator and from the NIHR Applied Research Collaboration Oxford and Thames Valley. Lisa Hinton is based at the Healthcare Improvement Studies Institute (THIS Institute), University of Cambridge. THIS Institute is supported by the Health Foundation, an independent charity committed to bringing about better health and healthcare for people in the UK. Abigail McNiven is supported by grants from the NIHR Policy Research Programme and Nuffield Foundation. Basky Thilaganathan is the clinical lead for the SAFE test (non-invasive prenatal testing) laboratory at St Georges Hospital (www.theSAFEtest.co.uk) but has no pecuniary interest in this service, and he receives funding from Tommy’s Charity for the National Centre for Maternity Improvement based at the Royal College of Obstetricians and Gynaecologist and Royal College of Midwives. Oliver Rivero-Arias is a shareholder and director of Maths in Health, a health economics consultancy company. Anne-Marie Slowther is a member of the Board of Trustees of the Institute of Medical Ethics and of the ULK Clinical Ethics Network. The remaining authors declare that they have no competing interests.
Inclusive language
We use the term ‘women’ throughout this publication to refer to those who are planning to become pregnant, are pregnant and give birth. We acknowledge that not all people who are pregnant and give birth identify as women, and it is important that evidence-based care for maternity, perinatal and postnatal health is inclusive. These should be taken to include people who do not identify as women but are pregnant or have given birth.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to data may be granted following review of data-sharing applications.
Ethics statement
Ethics approval was received for the studies included in the secondary analysis in Chapter 6:Warwick University studies: Ethical Application Reference: BSREC 105/18-19. Biomedical and Scientific Research Ethics Committee, University of Warwick. 2019.Oxford University studies: The studies were approved by the Berkshire Research Ethics Committee (12/SC/0495 12/09/2012).Ethics approval was also received for the primary data collection reported in Chapter 7:Ethics Approval Reference: R70422/RE001. Research Ethics Approval – CUREC 2. Medical Sciences Interdivisional Research Ethics Committee, University of Oxford. 2020.
Information governance statement
The Universities of Oxford and Warwick are committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, the Universities of Oxford and Warwick are the Data Controllers, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer for the University of Oxford here [https://www.phc.ox.ac.uk/intranet/information-governance], and the University of Warwick here [infocompliance@warwick.ac.uk]. Some data included in analysis were collected before the Data Protection Act (2018) and General Data Protection Regulation (EU GDPR) 2016/679 but complied with data protection rules, and ethics requirements, at the time of collection. Data were collected under the legal basis 9(2)j necessary for archiving purposes in public interest, scientific or historical research purposes.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by the interviewees in this publication are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the HTA programme or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Raffle A, Muir Gray JA. Screening: Evidence and Practice. Oxford: Oxford University Press; 2019.
- Petticrew M, Sowden A, Lister-Sharp D, Wright K. False-negative results in screening programmes: systematic review of impact and implications. Health Technol Assess 2000;4:1-20.
- Kuppermann M, Nease JR, Gates E, Learman L, Blumberg B, Gildengorin V, et al. How do women of diverse backgrounds value prenatal testing outcomes?. Prenat Diagn 2004;24:424-9.
- Kuppermann M, Norton M, Thao K, O’Leary A, Nseyo O, Cortez A, et al. Preferences regarding contemporary prenatal genetic tests among women desiring testing: implications for optimal testing strategies. Prenat Diagn 2016;36:469-75.
- Kaimal AJ, Norton ME, Kuppermann M. Prenatal testing in the genomic age: clinical outcomes, quality of life, and costs. Obstet Gynecol 2015;126:737-46.
- Woolf-King S, Anger A, Arnold E, Weiss S, Teitel D. Mental health among parents of children with critical congenital heart defects: a systematic review. J Am Heart Assoc 2017;6.
- Fuller A, Horváth-Puhó E, Ray J, Ehrenstein V, Sørensen H, Cohen E. Mortality among parents of children with major congenital anomalies. Pediatrics 2021;147.
- Davies V, Gledhill J, McFadyen A, Whitlow B, Economides D. Psychological outcome in women undergoing termination of pregnancy for ultrasound-detected fetal anomaly in the first and second trimesters: a pilot study. Ultrasound Obstet Gynecol 2005;25:389-92.
- Korenromp M, Christiaens G, van den Bout J, Mulder E, Hunfeld J, Bilardo C, et al. Long-term psychological consequences of pregnancy termination for fetal abnormality: a cross-sectional study. Prenat Diagn 2005;25:253-60.
- Friedman J, Cornel M, Goldenberg A, Lister K, Sénécal K, Vears D. Genomic newborn screening: public health policy considerations and recommendations. BMC Med Genomics 2017;10:1-3.
- Phillips K, Deverka P, Marshall D, Wordsworth S, Regier D, Christensen K, et al. Methodological issues in assessing the economic value of next-generation sequencing tests: many challenges and not enough solutions. Value Health 2018;21:1033-42.
- Public Health England . UK NSC: Evidence Review Process n.d. www.gov.uk/government/publications/uk-nsc-evidence-review-process/uk-nsc-evidence-review-process (accessed 11 May 2020).
- Waugh NR, Shyangdan D, Taylor-Phillips S, Suri G, Hall B. Screening for type 2 diabetes: a short report for the National Screening Committee. Health Technol Assess 2013;35:1-90.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 3 October 2019).
- Scottish Medicines Consortium . Guidance to Manufacturers for Completion of New Product Assessment Form n.d. www.scottishmedicines.org.uk/making-a-submission/ (accessed 23 July 2021).
- Haute Autorité de Santé . Choices in Methods for Economic Evaluation n.d. http://has-sante.fr/upload/docs/application/pdf/2012-10/choices_in_methods_for_economic_evaluation.pdf (accessed 11 May 2020).
- Australian Government Department of Health . Guidelines for Preparing Submissions to the Pharmaceutical Benefits Advisory Committee (PBAC) n.d. https://pbac.pbs.gov.au (accessed 11 May 2020).
- Canadian Agency for Drugs and Technologies in Health (CADTH) . Guidelines for the Economic Evaluation of Health Technologies: Canada n.d. www.cadth.ca/dv/guidelines-economic-evaluation-health-technologies-canada-4th-edition (accessed 11 May 2020).
- Weinstein M, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice of decision analytic modeling in health care evaluation: report of the ISPOR Task Force on Good Research Practices-Modeling Studies. Value Health 2003;6:9-17.
- Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al. A review and critique of modelling in prioritising and designing screening programmes. Health Technol Assess 2007;11:iii-137.
- Simon J, Petrou S, Gray A. The valuation of prenatal life in economic evaluations of perinatal interventions. Health Econ 2009;18:487-94.
- Petrou S. Methodological limitations of economic evaluations of antenatal screening. Health Econ 2001;10:775-8.
- Kwon J, Kim SW, Ungar WJ, Tsiplova K, Madan J, Petrou S. A systematic review and meta-analysis of childhood health utilities. Med Decis Making 2018;38:277-305.
- Al-Janabi H, van Exel J, Brouwer W, Coast J. A framework for including family health spillovers in economic evaluation. Med Decis Making 2016;36:176-86.
- Wright SJ, Ulph F, Dharni N, Payne K. Eliciting preferences for information provision in newborn bloodspot screening programs. Value Health 2017;20:651-61.
- Beulen L, Grutters JPC, Faas BHW, Feenstra I, Groenewoud H, van Vugt JMG, et al. Women’s and healthcare professionals’ preferences for prenatal testing: a discrete choice experiment. Prenat Diagn 2015;35:549-57.
- Nuffield Council on Bioethics . Non-Invasive Prenatal Testing: Ethical Issues n.d. www.nuffieldbioethics.org/assets/pdfs/NIPT-ethical-issues-full-report.pdf (accessed 20 July 2020).
- Hasegawa LE, Fergus KA, Ojeda N, Au SM. Parental attitudes toward ethical and social issues surrounding the expansion of newborn screening using new technologies. Public Health Genomics 2011;14:298-306.
- Silcock C, Liao LM, Hill M, Chitty LS. Will the introduction of non-invasive prenatal testing for Down’s syndrome undermine informed choice?. Health Expect 2015;18:1658-71.
- Cacciatore P, Visser LA, Buyukkaramikli N, van der Ploeg CPB, van den Akker-van Marle ME. The methodological quality and challenges in conducting economic evaluations of newborn screening: a scoping review. Int J Neonatal Screen 2020;6.
- Langer A, Holle R, John J. Specific guidelines for assessing and improving the methodological quality of economic evaluations of newborn screening. BMC Health Serv Res 2012;12.
- McMillan I. The Listening Series: Including Everyone in Public Engagement With Research. Guidance for Researchers n.d. https://tv.ndph.ox.ac.uk/wp-content/uploads/2022/01/Listening-Series-Guide-for-Researchers.pdf (accessed 22 March 2022).
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358.
- Hinton L, Miller T. Mapping men’s anticipations and experiences in the reproductive realm: (in)fertility journeys. Reprod Biomed Online 2013;27:244-52.
- Locock L, Kai J. Parents’ experiences of universal screening for haemoglobin disorders: implications for practice in a new genetics era. Br J Gen Pract 2008;58:161-8.
- Boardman FK. The expressivist objection to prenatal testing: the experiences of families living with genetic disease. Soc Sci Med 2014;107:18-25.
- Public Health England . Antenatal Screening Standards: Data Report 1 April 2018 to 31 March 2019 n.d. www.gov.uk/government/statistics/antenatal-screening-standards-data-report-2018-to-2019/antenatal-screening-standards-data-report-1-april-2018-to-31-march-2019--2 (accessed 28 March 2022).
- Png ME, Yang M, Roberts N, Taylor-Phillips S, Rivero-Arias O, Petrou S. Methods for evaluating the benefits and harms of antenatal and newborn screening programmes adopted by health economic assessments: protocol for a systematic review. BMJ Open 2021;11.
- Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372.
- Organisation for Economic Co-operation and Development (OECD) . List of OECD Member Countries – Ratification of the Convention on the OECD n.d. www.oecd.org/about/document/list-oecd-member-countries.htm (accessed 12 November 2020).
- Taylor-Phillips S, Stinton C, di Ruffano LF, Seedat F, Clarke A, Deeks JJ. Association between use of systematic reviews and national policy recommendations on screening newborn babies for rare diseases: systematic review and meta-analysis. BMJ 2018;361.
- Canadian Agency for Drugs and Technologies in Health (CADTH) (2011) . Grey Matters: A Practical Search Tool for Evidence-based Medicine n.d. https://cadth.ca/resources/finding-evidence/grey-matters (accessed 20 December 2022).
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 2023. www.training.cochrane.org/handbook (accessed 22nd August 2023).
- Veritas Health Innovation . Covidence Systematic Review Software 2024. www.covidence.org (accessed March 2024).
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. CHEERS Task Force . Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346.
- Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ 2022;376.
- Morse J. Confusing categories and themes. Qual Health Res 2008;18:727-8.
- Sandelowski M. What’s in a name? Qualitative description revisited. Res Nurs Health 2010;33:77-84.
- White A, Boardman F, McNiven A, Locock L, Hinton L. Absorbing it all: a meta-ethnography of parents’ unfolding experiences of newborn screening. Soc Sci Med 2021;287.
- Bearman M, Dawson P. Qualitative synthesis and systematic review in health professions education. Med Educ 2013;47:252-60.
- Alt S, McHugh A, Permalloo N, Pandya P. Fetal anomaly screening programme. Obstet Gynaecol Reprod Med 2020;30:395-7.
- White AL, Boardman F, McNiven A, Locock L, Hinton L. Absorbing it all: a meta-ethnography of parents’ unfolding experiences of newborn screening. Soc Sci Med 2021;287.
- Yu J. A systematic review of issues around antenatal screening and prenatal diagnostic testing for genetic disorders: women of Asian origin in western countries. Health Soc Care Community 2012;20:329-46.
- Lafarge C, Mitchell K, Fox P. Termination of pregnancy for fetal abnormality: a meta-ethnography of women’s experiences. Reprod Health Matters 2014;22:191-20. https://doi.org/101016/S0968-8080(14)44799-2.
- Dheensa S, Metcalfe A, Williams RA. Men’s experiences of antenatal screening: a metasynthesis of the qualitative research. Int J Nurs Stud 2013;50:121-33.
- Reid B, Sinclair M, Barr O, Dobbs F, Crealey G. A meta-synthesis of pregnant women’s decision-making processes with regard to antenatal screening for Down syndrome. Soc Sci Med 2009;69:1561-73.
- Moncrieff G, Finlayson K, Cordey S, McCrimmon R, Harris C, Barreix M, et al. First and second trimester ultrasound in pregnancy: a systematic review and metasynthesis of the views and experiences of pregnant women, partners, and health workers. PLOS ONE 2021;16.
- Johnson J, Dunning A, Sattar R, Arezina J, Karkowsky EC, Thomas S, et al. Delivering unexpected news via obstetric ultrasound: a systematic review and meta-ethnographic synthesis of expectant parent and staff experiences. Sonography 2020;7:61-77.
- Genomics England . Genomics England n.d. www.genomicsengland.co.uk/ (accessed 12 September 2022).
- Finfgeld D. Metasynthesis: the state of the art – so far. Qual Health Res 2003;13:893-904.
- Erwin E, Brotherson M, Summers J. Understanding qualitative metasynthesis: issues and opportunities in early childhood intervention research. J Early Interv 2011;33:186-200.
- Barnett-Page E, Thomas J. Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol 2009;9.
- Toye F, Seers K, Allcock N, Briggs M, Carr E, Barker K. Meta-ethnography 25 years on: challenges and insights for synthesising a large number of qualitative studies. BMC Med Res Methodol 2014;14:1-4.
- Ludvigsen M, Hall E, Meyer G, Fegran L, Aagaard H, Uhrenfeldt L. Using Sandelowski and Barroso’s meta-synthesis method in advancing qualitative evidence. Qual Health Res 2016;26:320-9.
- Public Health England . Newborn Blood Spot Screening: Programme Handbook n.d. www.gov.uk/government/publications/hea lth-professional-handbook-newborn-blood-spot-screening (accessed 7 September 2021).
- Nicholls S. Proceduralisation, choice and parental reflections on decisions to accept newborn bloodspot screening. J Med Ethics 2012;38:299-303.
- Nicholls S. Knowledge or understanding? Informed choice in the context of newborn bloodspot screening. Public Health Ethics 2010;3:128-36.
- Nicholls S, Southern K. Parental decision-making and acceptance of newborn bloodspot screening: an exploratory study. PLOS ONE 2013;8.
- Parsons E, King J, Israel J, Bradley D. Mothers’ accounts of screening newborn babies in Wales (UK). Midwifery 2007;23:59-65.
- Chudleigh J, Buckingham S, Dignan J, O’Driscoll S, Johnson K, Rees D, et al. Parents’ experiences of receiving the initial positive newborn screening (NBS) result for cystic fibrosis and sickle cell disease. J Genet Couns 2016;25:1215-26.
- Buchbinder M, Timmermans S. Newborn screening and maternal diagnosis: rethinking family benefit. Soc Sci Med 2011;73:1014-8.
- Buchbinder M, Timmermans S. Newborn screening for metabolic disorders: parental perceptions of the initial communication of results. Clin Pediatr (Phila) 2012;51:739-44.
- Grob R. Parenting in the genomic age: the ‘cursed blessing’ of newborn screening. New Genet Soc 2006;25:159-70.
- Timmermans S, Buchbinder M. Patients-in-waiting: living between sickness and health in the genomics era. J Health Soc Behav 2010;51:408-23.
- Ulph F, Cullinan T, Qureshi N, Kai J. Parents’ responses to receiving sickle cell or cystic fibrosis carrier results for their child following newborn screening. Eur J Human Genet 2015;23:459-65.
- Tluczek A. Psychosocial issues associated with genetic testing in cystic fibrosis newborn screening. Nurs Health Sci 2006;8.
- Grob R. Is my sick child healthy? Is my healthy child sick?: changing parental experiences of cystic fibrosis in the age of expanded newborn screening. Soc Sci Med 2008;67:1056-64.
- Cohen W, Quarterly DL. Absorptive capacity: a new perspective on learning and innovation. Adm Sci Q 1990;35:128-52.
- Moran J, Quirk K, Duff A, Fibrosis KB. Newborn screening for CF in a regional paediatric centre: the psychosocial effects of false-positive IRT results on parents. J Cyst Fibrosis 2007;6:250-4.
- Salm N, Yetter E, Tluczek A. Informing parents about positive newborn screen results: parents’ recommendations. J Child Health Care 2012;16:367-81.
- Schwan K, Youngblom J, Weisiger K, Kianmahd J, Waggoner R, Fanos J. Family perspectives on newborn screening for X-linked adrenoleukodystrophy in California. Int J Neonatal Screen 2019;5.
- Tluczek A, McKechnie AC, Lynam PA. When the cystic fibrosis label does not fit: a modified uncertainty theory. Qual Health Res 2010;20:209-23.
- Boyse K, Gardner M, Marvicsin DJ, Sandberg DE. It was an overwhelming thing’: parents’ needs after infant diagnosis with congenital adrenal hyperplasia. J Pediatr Nurs 2014;29:436-41.
- Locock L, Kai J. Parents’ experiences of universal screening for haemoglobin disorders: implications for practice in a new genetics era. Br J Gen Pract 2008;58:161-8.
- Tluczek A, Orland K, Nick SW, Brown RL. Newborn screening: an appeal for improved parent education. J Perinat Neonatal Nurs 2009;23.
- Carpenter K, Wittkowski A, Hare DJ, Medford E, Rust S, Jones SA, et al. Parenting a Child with Phenylketonuria (PKU): an Interpretative Phenomenological Analysis (IPA) of the experience of parents. J Genet Couns 2018;27:1074-86.
- Patterson RP, Roedl SJ, Farrell MH. Internet searching after parents receive abnormal newborn screening results. J Commun Healthc 2015;8:303-15.
- Raz A, Amano Y, Timmermans S. Parents like me: biosociality and lay expertise in self-help groups of parents of screen-positive newborns. New Genet Soc 2018;37:97-116.
- Pruniski B, Lisi E, Ali N. Newborn screening for Pompe disease: impact on families. J Inherit Metab Dis 2018;41:1189-203.
- Johnson F, Southern K, Ulph F. Psychological impact on parents of an inconclusive diagnosis following newborn bloodspot screening for cystic fibrosis: a qualitative study. Int J Neonatal Screen 2019;5.
- Hart J, Cox C, Kuhn I, Fritz Z. Communicating diagnostic uncertainty in the acute and emergency medical setting: a systematic review and ethical analysis of the empirical literature. Acute Med 2021;20:204-18.
- DeLuca J, Kearney M, Norton S, Arnold GL. Parents’ experiences of expanded newborn screening evaluations. Pediatrics 2011;128:53-61.
- Tluczek A, Koscik RL, Modaff P, Pfeil D, Rock MJ, Farrell PM, et al. Newborn screening for cystic fibrosis: parents’ preferences regarding counseling at the time of infants’ sweat test. J Genet Couns 2006;15:277-91.
- Tluczek A, Orland KM, Cavanagh L. Psychosocial consequences of false-positive newborn screens for cystic fibrosis. Qual Health Res 2011;21:174-86.
- Buchbinder M, Timmermans S. Medical technologies and the dream of the perfect newborn. Med Anthropol 2011;30:56-80.
- Raz AE, Amano Y, Timmermans S. Coming to terms with the imperfectly normal child: attitudes of Israeli parents of screen-positive infants regarding subsequent prenatal diagnosis. J Community Genet 2019;10:41-50.
- Ulph F, Cullinan T, Qureshi N. Informing children of their newborn screening carrier result for sickle cell or cystic fibrosis: qualitative study of parents’ intentions, views and support needs. J Genet Couns 2014;23:409-20.
- Grob R. Qualitative research on expanded prenatal and newborn screening: robust but marginalized. Hastings Cent Rep 2019;49:S72-81.
- Clarke AE. Situational analyses: grounded theory mapping after the postmodern turn. Symb Interact 2003;26:553-76.
- Grzanka PR. The shape of knowledge: situational analysis in counseling psychology research. J Couns Psychol 2021;68:316-30.
- Saldana J. The Coding Manual for Qualitative Researchers. London: SAGE Publications; 2016.
- Pope C, Ziebland S, Mays N. Qualitative research in health care: analysing qualitative data. BMJ 2000;320:114-6.
- Ziebland S, McPherson A. Making sense of qualitative data analysis: an introduction with illustrations from DIPEx (personal experiences of health and illness). Med Educ 2006;40:405-14.
- Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al. Eliciting public preferences for healthcare: a systematic review of techniques. Health Technol Assess 2001;5.
- Ryan M, Kinghorn P, Entwistle VA, Francis JJ. Valuing patients’ experiences of healthcare processes: towards broader applications of existing methods. Soc Sci Med 2014;106:194-203.
- Parkin D, Devlin N. Is there a case for using visual analogue scale valuations in cost–utility analysis?. Health Econ 2006;15:653-64.
- Vass CM, Georgsson S, Ulph F, Payne K. Preferences for aspects of antenatal and newborn screening: a systematic review. BMC Pregnancy Childbirth 2019;19:1-11.
- Kwon J, Freijser L, Huynh E, Howell M, Chen G, Khan K, et al. Systematic review of conceptual, age, measurement and valuation considerations for generic multidimensional childhood patient-reported outcome measures. Pharmacoeconomics 2022;40:379-431.
- Jabrayilov R, van Asselt ADI, Vermeulen KM, Volger S, Detzel P, Dainelli L, et al. A descriptive system for the Infant health-related Quality of life Instrument (IQI): measuring health with a mobile app. PLOS ONE 2018;13.
- Verstraete J, Ramma L, Jelsma J. Item generation for a proxy health related quality of life measure in very young children. Health Qual Life Outcomes 2020;18:1-15.
- Donaldson C, Shackley P, Abdalla M, Miedzybrodzka Z. Willingness to pay for antenatal carrier screening for cystic fibrosis. Health Econ 1995;4:439-52.
- Lin PJ, Yeh WS, Neumann PJ. Willingness to pay for a newborn screening test for spinal muscular atrophy. Pediatr Neurol 2017;66:69-75.
- Rouse DJ, Stringer JS. An appraisal of screening for maternal type-specific herpes simplex virus antibodies to prevent neonatal herpes. Am J Obstet Gynecol 2000;183:400-6.
- Vintzileos AM, Ananth CV, Smulian JC, Beazoglou T, Knuppel RA. Routine second-trimester ultrasonography in the United States: a cost–benefit analysis. Am J Obstet Gynecol 2000;182:655-60.
- Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM. First and second trimester antenatal screening for Down syndrome: the results of the serum, urine and ultrasound screening study (SURUSS). Health Technol Assess 2003;7:1-77.
- Walker B, Jackson B, LaGrave D, Ashwood E, Schmidt R. A cost-effectiveness analysis of cell free DNA as a replacement for serum screening for Down syndrome. Prenat Diagn 2015;35:440-6.
- Berrigan P, Andrew G, Reynolds JN, Zwicker JD. The cost-effectiveness of screening tools used in the diagnosis of fetal alcohol spectrum disorder: a modelled analysis. BMC Public Health 2019;19.
- Abbey R, Dunsmoor-Su R. Cost–benefit analysis of indirect antiglobulin screening in Rh(D)-negative women at 28 weeks of gestation. Obstet Gynecol 2014;123:938-45.
- Albright CM, Emerson JB, Werner EF, Hughes BL. Third-trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol 2015;126:479-85.
- Zaric GS, Bayoumi AM, Brandeau ML, Owens DK. The cost effectiveness of voluntary prenatal and routine newborn HIV screening in the United States. JAIDS J Acquir Immune Defic Syndr 2000;25:403-16.
- Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, et al. Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness. Health Technol Assess 2009;13:1-154, iii.
- Doyle NM, Levison JE, Gardner MO. Rapid HIV versus enzyme-linked immunosorbent assay screening in a low-risk Mexican American population presenting in labor: a cost-effectiveness analysis. Am J Obstet Gynecol 2005;193:1280-5.
- Duplantie J, Gonzales OM, Bois A, Nshimyumukiza L, Gekas J, Bujold E, et al. Cost-effectiveness of the management of Rh-negative pregnant women. J Obstet Gynaecol Canada 2013;35:730-40.
- Gomez M. A comparison of three screening strategies for prevention of perinatal HIV infection in Colombia: a decision analysis model. Pan Am J Public Health 2008;24:256-64.
- Immergluck LC, Cull WL, Schwartz A, Elstein AS. Cost-effectiveness of universal compared with voluntary screening for human immunodeficiency virus among pregnant women in Chicago. Pediatrics 2000;105.
- Mol BWJ, van der Veen F, Bossuyt PMM. Symptom-free women at increased risk of ectopic pregnancy: should we screen?. Acta Obstet Gynecol Scand 2002;81:661-72.
- Ortved D, Hawkins TL, Johnson JA, Hyett J, Metcalfe A. Cost-effectiveness of first-trimester screening with early preventative use of aspirin in women at high risk of early-onset pre-eclampsia. Ultrasound Obstet Gynecol 2019;53:239-44.
- Pinot D, e M, oira A, Edmunds WJ, Breuer J. The cost-effectiveness of antenatal varicella screening with post-partum vaccination of susceptibles. Vaccine 2006;24:1298-307.
- Poncet B, Touzet S, Rocher L, Berland M, Orgiazzi J, Colin C. Cost-effectiveness analysis of gestational diabetes mellitus screening in France. Eur J Obstet Gynecol Reprod Biol 2002;103:122-9.
- Rivera-Alsina ME, Rivera CC, Rollene N, Kirby RS, Ayres A, McNamara M. Voluntary screening program for HIV in pregnancy. Cost effectiveness. J Reprod Med 2001;46:243-48.
- Stan CM, Boulvain M, Bovier PA, Auckenthaler R, Berner M, Irion O. Choosing a strategy to prevent neonatal early-onset group B streptococcal sepsis: economic evaluation. BJOG 2001;108:840-7.
- Wastlund D, Moraitis AA, Dacey A, Sovio U, Wilson ECF, Smith GCS. Screening for breech presentation using universal late-pregnancy ultrasonography: a prospective cohort study and cost effectiveness analysis. PLOS Med 2019;16.
- Williams M, Zantow E, Turrentine M. Cost effectiveness of latest recommendations for group B streptococci screening in the United States. Obstet Gynecol 2020;10.
- Sansom S. Human immunodeficiency virus retesting during pregnancy: costs and effectiveness in preventing perinatal transmission. Obstet Gynecol 2003;102:782-90.
- Mrus JM, Tsevat J. Cost-effectiveness of interventions to reduce vertical HIV transmission from pregnant women who have not received prenatal care. Med Decis Making 2004;24:30-9.
- Park F, Deeming S, Bennett N, Hyett J. Cost effectiveness analysis of a model of first trimester prediction and prevention for preterm preeclampsia against usual care. Ultrasound Obstet Gynecol 2020;58:688-97.
- Rodriguez PJ, Roberts DA, Meisner J, Sharma M, Owiredu MN, Gomez B, et al. Cost-effectiveness of dual maternal HIV and syphilis testing strategies in high and low HIV prevalence countries: a modelling study. Lancet Glob Health 2021;9:e61-71.
- Arentz-Hansen H, Brurberg K, Kvamme M, Stoinska-Schneider A, Hofmann B, Ormstad S, et al. Rhesus Typing Av Foster Basert På blodprøve Fra Rhesus Negative Gravide n.d. www.fhi.no/globalassets/dokumenterfiler/rapporter/2014/rapport_2014_25_rhesustyping_foster.pdf (accessed 4 March 2024).
- Huntington S, Weston G, Adams E. Repeat Screening for Syphilis in Pregnancy As an Alternative Screening Strategy in the UK: A Cost-Effectiveness Analysis n.d. https://legacyscreening.phe.org.uk/policydb_download.php?doc=1306%0A (accessed 20 February 2021).
- Castilla-Rodriguez I, Cela E, Vallejo-Torres L, Valcarcel-Nazco C, Dulin E, Espada M, et al. Cost-effectiveness analysis of newborn screening for sickle-cell disease in Spain. Expert Opin Orphan Drugs 2016;4:567-75.
- van den Akker-van Marle ME, Dankert HM, Verkerk PH, Dankert-Roelse JE. Cost-effectiveness of 4 neonatal screening strategies for cystic fibrosis. Pediatrics 2006;118:896-905.
- Benn P, Curnow KJ, Chapman S, Michalopoulos SN, Hornberger J, Rabinowitz M. An economic analysis of cell-free DNA non-invasive prenatal testing in the US general pregnancy population. PLOS ONE 2015;10.
- Beulen L, Grutters JP, Faas BH, Feenstra I, van Vugt JM, Bekker MN. The consequences of implementing non-invasive prenatal testing in Dutch national health care: a cost-effectiveness analysis. Eur J Obstet Gynecol Reprod Biol 2014;182:53-61.
- Biggio JR Jr, Morris TC, Owen J, Stringer JS. An outcomes analysis of five prenatal screening strategies for trisomy 21 in women younger than 35 years. Am J Obstet Gynecol 2004;190:721-9.
- Bramley D, Graves N, Walker D. The cost effectiveness of universal antenatal screening for HIV in New Zealand. AIDS 2003;17:741-8.
- Christiansen M, Olesen Larsen S. An increase in cost-effectiveness of first trimester maternal screening programmes for fetal chromosome anomalies is obtained by contingent testing. Prenat Diagn 2002;22:482-6.
- Chung EH, Lim SL, Havrilesky LJ, Steiner AZ, Dotters-Katz S. The cost-effectiveness of prenatal congenital heart defect screening methods in IVF pregnancies. Ultrasound Obstet Gynecol 2020;18.
- Dhaifalah I, Majek O. Cost effectiveness, the economic considerations of prenatal screening strategies for trisomy 21 in the Czech Republic. Ceska Gynekol 2012;77:39-51.
- Dormandy E, Bryan S, Gulliford M, Roberts T, Ades A, Calnan M, et al. Antenatal screening for haemoglobinopathies in primary care: a cohort study and cluster randomised trial to inform a simulation model. The Screening for Haemoglobinopathies in First Trimester (SHIFT) trial. Health Technol Assess 2010;14:1-60.
- Gekas J, Durand A, Bujold E, Vallee M, Forest JC, Rousseau F, et al. Cost-effectiveness and accuracy of prenatal Down syndrome screening strategies: should the combined test continue to be widely used?. Am J Obstet Gynecol 2011;204:175.e1-8.
- Gekas J, Gagne G, Bujold E, Douillard D, Forest JC, Reinharz D, et al. Comparison of different strategies in prenatal screening for Down’s syndrome: cost effectiveness analysis of computer simulation. BMJ 2009;338.
- Gilbert RE, Augood C, Gupta R, Ades AE, Logan S, Sculpher M, et al. Screening for Down’s syndrome: effects, safety, and cost effectiveness of first and second trimester strategies. BMJ 2001;323:423-5.
- Graves N, Walker DG, McDonald AM, Kaldor JM, Ziegler JB. Would universal antenatal screening for HIV infection be cost-effective in a setting of very low prevalence? Modelling the data for Australia. J Infect Dis 2004;190:166-74.
- Hacker F, Griffin E, Shaffer B, Caughey A. Role of sequential genetic sonogram and cellfree fetal DNA after EIF detection: a cost-effectiveness analysis. Am J Obstet Gynecol 2017;216:S95-6.
- Hacker F, Griffin E, Shaffer B, Caughey A. Role of genetic sonogram and NIPT after EIF detection: a cost-effectiveness analysis. Am J Obstet Gynecol 2015;212:S171-2.
- Hacker FM, Griffin EE, Shaffer BL, Caughey AB. Role of genetic ultrasonogram after choroid plexus cyst detection a cost-effectiveness analysis. Obstet Gynecol 2015;125.
- Harris AH. The cost effectiveness of prenatal ultrasound screening for trisomy 21. Int J Technol Assess Health Care 2004;20:464-8.
- Health Quality Ontario . Noninvasive prenatal testing for trisomies 21, 18, and 13, sex chromosome aneuploidies, and microdeletions: a Health Technology Assessment. Ont Health Technol Assess Ser 2019;19:1-166.
- Norman R, van Gool K, Hall J, Delatycki M, Massie J. Cost-effectiveness of carrier screening for cystic fibrosis in Australia. J Cyst Fibrosis 2012;11:281-7.
- Odibo AO, Stamilio DM, Nelson DB, Sehdev HM, Macones GA. A cost-effectiveness analysis of prenatal screening strategies for Down syndrome. Obstet Gynecol 2005;106:562-8.
- Resch S, Altice FL, Paltiel AD. Cost-effectiveness of HIV screening for incarcerated pregnant women. J Acquir Immune Defic Syndr 2005;38:163-73.
- Ritchie K, Bradbury I, Slattery J, Wright D, Iqbal K, Penney G. Economic modelling of antenatal screening and ultrasound scanning programmes for identification of fetal abnormalities. BJOG 2005;112:866-74.
- Udeh B, Udeh C, Graves N. Perinatal HIV transmission and the cost-effectiveness of screening at 14 weeks gestation, at the onset of labour and the rapid testing of infants. BMC Infect Dis 2008;8.
- Vanara F, Bergeretti F, Gaglioti P, Todros T. Economic evaluation of ultrasound screening options for structural fetal malformations. Ultrasound Obstet Gynecol 2004;24:633-9.
- Vintzileos AM, Ananth CV, Smulian JC, Day-Salvatore DL, Beazoglou T, Knuppel RA. Cost–benefit analysis of prenatal diagnosis for Down syndrome using the British or the American approach. Obstet Gynecol 2000;95:577-83.
- Xie X, Wang M, Goh ES, Ungar WJ, Little J, Carroll JC, et al. Noninvasive prenatal testing for trisomies 21, 18, and 13, sex chromosome aneuploidies, and microdeletions in average-risk pregnancies: a cost-effectiveness analysis. J Obstet Gynaecol Canada 2020;31.
- Nielsen R, Gyrd-Hansen D. Prenatal screening for cystic fibrosis: an economic analysis. Health Econ 2002;11:285-99.
- Kessels S, Morona J, Mittal R, Vogan A, Newton S, Schubert C, et al. Testing for Hereditary Mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Gene n.d. www1.health.gov.au/internet/msac/publishing.nsf/Content/D54C11627FEA9785CA25801000123BDC/$File/1216-MSAC-CA-CFTR.pdf%0A (accessed 20 February 2021).
- Ayres AC, Whitty JA, Ellwood DA. A cost-effectiveness analysis comparing different strategies to implement noninvasive prenatal testing into a Down syndrome screening program. Aust N Z J Obstet Gynaecol 2014;54:412-7.
- Bayon JC, Orruno E, Portillo MI, Asua J. The consequences of implementing non-invasive prenatal testing with cell-free foetal DNA for the detection of Down syndrome in the Spanish National Health Service: a cost-effectiveness analysis. Cost Eff Resour Alloc 2019;17.
- Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al. Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views. Health Technol Assess 2000;4:i-vi.
- Caughey AB, Kuppermann M, Norton ME, Washington AE. Nuchal translucency and first trimester biochemical markers for Down syndrome screening: a cost-effectiveness analysis. Am J Obstet Gynecol 2002;187:1239-45.
- DeVore GR, Romero R. Genetic sonography: a cost-effective method for evaluating women 35 years and older who decline genetic amniocentesis. J Ultrasound Med 2002;21:5-13.
- Di Cianni G, Volpe L, Casadidio I, Bottone P, Marselli L, Lencioni C, et al. Universal screening and intensive metabolic management of gestational diabetes: cost-effectiveness in Italy. Acta Diabetol 2002;39:69-73.
- Fairbrother G, Burigo J, Sharon T, Song K. Prenatal screening for fetal aneuploidies with cell-free DNA in the general pregnancy population: a cost-effectiveness analysis. J Matern-Fetal Neonatal Med 2016;29:1160-4.
- Gekas J, van den Berg DG, Durand A, Vallee M, Wildschut HI, Bujold E, et al. Rapid testing versus karyotyping in Down’s syndrome screening: cost-effectiveness and detection of clinically significant chromosome abnormalities. Eur J Hum Genet 2011;19:3-9.
- Juvet LK, Ormstad SS, Stoinska-Schneider A, Solberg B, Arentz-Hansen H, Kvamme MK, et al. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2016.
- Nshimyumukiza L, Beaumont JA, Duplantie J, Langlois S, Little J, Audibert F, et al. Cell-free DNA-based non-invasive prenatal screening for common aneuploidies in a Canadian province: a cost-effectiveness analysis. J Obstet Gynaecol Canada 2018;40:48-60.
- Ohno M, Caughey A. The role of noninvasive prenatal testing as a diagnostic versus a screening tool – a cost-effectiveness analysis. Prenat Diagn 2013;33:630-5.
- O’Leary P, Maxwell S, Murch A, Hendrie D. Prenatal screening for Down syndrome in Australia: costs and benefits of current and novel screening strategies. Aust N Z J Obstet Gynaecol 2013;53:425-33.
- Ong JJ, Chen M, Hocking J, Fairley CK, Carter R, Bulfone L, et al. Chlamydia screening for pregnant women aged 16–25 years attending an antenatal service: a cost-effectiveness study. BJOG 2016;123:1194-202.
- Pinto NM, Nelson R, Puchalski M, Metz TD, Smith KJ. Cost-effectiveness of prenatal screening strategies for congenital heart disease. Ultrasound Obstet Gynecol 2014;44:50-7.
- Song K, Musci TJ, Caughey AB. Clinical utility and cost of non-invasive prenatal testing with cfDNA analysis in high-risk women based on a US population. J Matern-Fetal Neonatal Med 2013;26:1180-5.
- Walker B, Nelson R, Jackson B, Grenache D, Ashwood E, Schmidt R. A cost-effectiveness analysis of first trimester non-invasive prenatal screening for fetal trisomies in the United States. PLOS ONE 2015;10.
- Taylor-Phillips S, Freeman K, Geppert J, Madan J, Uthman O, Agbebiyi A, et al. Systematic Review and Cost-Consequence Assessment of Cell-Free DNA Testing for T21, T18 and T13 in the UK: Final Report n.d. https://legacyscreening.phe.org.uk/policydb_download.php?doc=1265%0A (accessed 20 February 2021).
- Hoogendoorn M, Hamberg-van Reenen H, van Genugten M, de Wit G, Schielen P. Vergelijking Van Kosten En Effecten Van Prenatale Screeningsmethoden Voor Down Syndroom En Neuraalbuisdefecten n.d. https://www.rivm.nl/bibliotheek/rapporten/230041001.pdf%0A (accessed 20 February 2021).
- Institute of Health Economics . First and Second Trimester Prenatal Screening Update n.d. http://www.inahta.org/upload/2014/14043_IHE_First and second trimester prenatal screening update.pdf (accessed 17 February 2021).
- Hulstaert F, Neyt M, Gyselaers W. The Non-Invasive Prenatal Test (NIPT) for Trisomy 21: Health Economic Aspects n.d. https://kce.fgov.be/sites/default/files/atoms/files/KCE_222_Non_invasive_prenatal_ test_Report.pdf (accessed 20 February 2021).
- National Institute for Health and Care Excellence (NICE) . Diabetes in Pregnancy: Management of Diabetes and Its Complications from Preconception to the Postnatal Period. NG3, Feb 2015 (Appendix N.3) n.d. www.nice.org.uk/guidance/ng3/evidence/appendices-ag-and-in-pdf-3784286%0A (accessed 20 February 2021).
- Boshuizen HC, van der Lem GJ, Kauffman-de Boer MA, van Zanten GA, Oudesluys-Murphy AM, Verkerk PH. Costs of different strategies for neonatal hearing screening: a modelling approach. Arch Dis Child Fetal Neonatal Ed 2001;85:F177-81.
- Burke MJ, Shenton RC, Taylor MJ. The economics of screening infants at risk of hearing impairment: an international analysis. Int J Pediatr Otorhinolaryngol 2012;76:212-8.
- Chicaiza-Becerra L, Garcia-Molina M, Oviedo-Ariza S, Gomez-Marin JE, Gomez-Sanchez PI. Cost effectiveness of various diagnostic strategies for detecting congenital toxoplasmosis in newborns. Infectio 2013;17:53-60.
- Hessel F, Grill E, Schnell-Inderst P, Siebert U, Kunze S, Nickisch A, et al. Economic evaluation of newborn hearing screening: modelling costs and outcomes. Ger Med Sci 2003;1.
- Institute of Health Economics . Newborn Blood Spot Screening for Galactosemia, Tyrosinemia Type I, Homocystinuria, Sickle Cell Anemia, Sickle Cell Beta-Thalassemia, Sickle Cell Hemoglobin C Disease, and Severe Combined Immunodeficiency 2016;3.
- Kemper AR, Downs SM. A cost-effectiveness analysis of newborn hearing screening strategies. Arch Pediatr Adolesc Med 2000;154:484-8.
- Londono Trujillo D, Sandoval Reyes NF, Taborda Restrepo A, Chamorro Velasquez CL, Dominguez Torres MT, Romero Ducuara SV, et al. Cost-effectiveness analysis of newborn pulse oximetry screening to detect critical congenital heart disease in Colombia. Cost Eff Resour Alloc 2019;17.
- Nshimyumukiza L, Bois A, Daigneault P, Lands L, Laberge AM, Fournier D, et al. Cost effectiveness of newborn screening for cystic fibrosis: a simulation study. J Cyst Fibrosis 2014;13:267-74.
- Raimond V, Sambuc C, Pibouleau L. Ethics evaluation revealing decision-maker motives: a case of neonatal screening. Int J Technol Assess Health Care 2018;34:189-95.
- Ramwadhdoebe S, Van Merode GG, Boere-Boonekamp MM, Sakkers RJ, Buskens E. Implementation by simulation; strategies for ultrasound screening for hip dysplasia in the Netherlands. BMC Health Serv Res 2010;10.
- Schmidt M, Werbrouck A, Verhaeghe N, De Wachter E, Simoens S, Annemans L, et al. A model-based economic evaluation of four newborn screening strategies for cystic fibrosis in Flanders, Belgium. Acta Clin Belg 2019;75:1-9.
- Seror V, Cao C, Roussey M, Giorgi R. PAP assays in newborn screening for cystic fibrosis: a population-based cost-effectiveness study. J Med Screen 2016;23:62-9.
- Zupancic JAF, Ying GS, Alba C, ampomanes A de, Tomlinson LA, Binenbaum G. G-ROP Study Group . Evaluation of the economic impact of modified screening criteria for retinopathy of prematurity from the Postnatal Growth and ROP (G-ROP) study. J Perinatol 2020;40:1100-8.
- Tran K, Banerjee S, Li H, Noorani H, Mensinkai S, Dooley K. Newborn Screening for Medium Chain Acyl-CoA Dehydrogenase Deficiency Using Tandem Mass Spectrometry: Clinical and Cost-Effectiveness n.d. https://pubmed.ncbi.nlm.nih.gov/17222812/ (accessed 4 March 2024).
- Institute of Health Economics . Screening Newborns for Hearing n.d. www.ihe.ca/download/screening_newborns_for_hearing.pdf%0A (accessed 20 February 2021).
- Hillman SC, Barton PM, Roberts TE, Maher ER, McMullan DM, Kilby MD. BAC chromosomal microarray for prenatal detection of chromosome anomalies in fetal ultrasound anomalies: an economic evaluation. Fetal Diagn Ther 2014;36:49-58.
- Kott B, Dubinsky TJ. Cost-effectiveness model for first-trimester versus second-trimester ultrasound screening for Down syndrome. J Am Coll Radiol 2004;1:415-21.
- Le Bras A, Salomon LJ, Bussieres L, Malan V, Elie C, Mahallati H, et al. Cost-effectiveness of five prenatal screening strategies for trisomies and other unbalanced chromosomal abnormalities: model-based analysis. Ultrasound in Obstet Gynecol 2019;54:596-603.
- Feuchtbaum L, Cunningham G. Economic evaluation of tandem mass spectrometry screening in California. Pediatrics 2006;117:S280-6.
- Griebsch I, Knowles RL, Brown J, Bull C, Wren C, Dezateux CA. Comparing the clinical and economic effects of clinical examination, pulse oximetry, and echocardiography in newborn screening for congenital heart defects: a probabilistic cost-effectiveness model and value of information analysis. Int J Technol Assess Health Care 2007;23:192-204.
- Keren R, Helfand M, Homer C, McPhillips H, Lieu TA. Projected cost-effectiveness of statewide universal newborn hearing screening. Pediatrics 2002;110:855-64.
- Kezirian EJ, White KR, Yueh B, Sullivan SD. Cost and cost-effectiveness of universal screening for hearing loss in newborns. Otolaryngol Head Neck Surg 2001;124:359-67.
- Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C. Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis. Health Technol Assess 2005;9:1-152, iii.
- Lanting CI, van Tijn DA, Loeber JG, Vulsma T, de Vijlder JJ, Verkerk PH. Clinical effectiveness and cost-effectiveness of the use of the thyroxine/thyroxine-binding globulin ratio to detect congenital hypothyroidism of thyroidal and central origin in a neonatal screening program. Pediatrics 2005;116:168-73.
- Narayen IC, Te Pas AB, Blom NA, van den Akker-van Marle ME. Cost-effectiveness analysis of pulse oximetry screening for critical congenital heart defects following homebirth and early discharge. Eur J Pediatr 2019;178:97-103.
- Roberts T, Pickering K, Barton P, Ewer A. Pulse Oximetry As a Screening Test for Critical Congenital Heart Defects and Other Significant Diagnoses in Newborn Infants: A Cost Effectiveness Analysis n.d. https://legacyscreening.phe.org.uk/documents/pulse-oximetry/PO Health Economics Report.pdf%0A (accessed 20 February 2021).
- Hubbard HB. Expanded newborn screening for genetic and metabolic disorders: modeling costs and outcomes. Nurs Econ 2007;25:345-52.
- Hopkins MK, Dugoff L, Durnwald C, Havrilesky LJ, Dotters-Katz S. Cell-free DNA for Down syndrome screening in obese women: is it a cost-effective strategy?. Prenat Diagn 2020;40:173-8.
- Sinkey RG, Odibo AO. Vasa previa screening strategies: decision and cost-effectiveness analysis. Ultrasound Obstet Gynecol 2018;52:522-29.
- Mukerji A, Shafey A, Jain A, Cohen E, Shah PS, Sander B, et al. Pulse oximetry screening for critical congenital heart defects in Ontario, Canada: a cost-effectiveness analysis. Can J Public Health 2020;06.
- De Laet C, Hanquet G, Hendrickx E. Multi Criteria Decision Analysis to Select Priority Diseases for Newborn Blood Screening n.d. https://kce.fgov.be/sites/default/files/atoms/files/KCE_267_Newborn_blood_screening.pdf%0A (accessed 20 February 2021).
- Cipriano LE, Rupar CA, Zaric GS. The cost-effectiveness of expanding newborn screening for up to 21 inherited metabolic disorders using tandem mass spectrometry: results from a decision-analytic model. Value Health 2007;10:83-97.
- Bergevin A, Zick CD, McVicar SB, Park AH. Cost–benefit analysis of targeted hearing directed early testing for congenital cytomegalovirus infection. Int J Pediatr Otorhinolaryngol 2015;79:2090-3.
- Bonds DE, Freedberg KA. Cost-effectiveness of prenatal screening for postpartum thyroiditis. J Womens Health Gend Based Med 2001;10:649-58.
- Dosiou C, Barnes J, Schwartz A, Negro R, Crapo L, Stagnaro-Green A. Cost-effectiveness of universal and risk-based screening for autoimmune thyroid disease in pregnant women. J Clin Endocrinol Metab 2012;97:1536-46.
- Dosiou C, Sanders GD, Araki SS, Crapo LM. Screening pregnant women for autoimmune thyroid disease: a cost-effectiveness analysis. Eur J Endocrinol 2008;158:841-51.
- Tasillo A, Eftekhari Y, azdi G, Nolen S, Schillie S, Vellozzi C, et al. Short-term effects and long-term cost-effectiveness of universal hepatitis C testing in prenatal care. Obstet Gynecol 2019;133:289-300.
- Bessey A, Chilcott JB, Leaviss J, Sutton A. Economic impact of screening for X-linked adrenoleukodystrophy within a newborn blood spot screening programme. Orphanet J Rare Dis 2018;13.
- Peterson C, Grosse SD, Oster ME, Olney RS, Cassell CH. Cost-effectiveness of routine screening for critical congenital heart disease in US newborns. Pediatrics 2013;132:e595-603.
- Tiwana SK, Rascati KL, Park H. Cost-effectiveness of expanded newborn screening in Texas. Value Health 2012;15:613-21.
- Cahill AG, Odibo AO, Stamilio DM, Macones GA. Screening and treating for primary cytomegalovirus infection in pregnancy: where do we stand? A decision-analytic and economic analysis. Am J Obstet Gynecol 2009;201:466.e1-7.
- Ohno M, Cheng YW, Shaffer B, Caughey AB. A new test to diagnose Down syndrome using maternal serum: at what specificity, sensitivity, and cost is it cost-effective?. Am J Obstet Gynecol 2011;204:S236-7.
- Chowers M, Shavit O. Economic evaluation of universal prenatal HIV screening compared with current ‘at risk’ policy in a very low prevalence country. Sex Transm Infect 2017;93:112-7.
- Cipriano LE, Barth WH, Zaric GS. The cost-effectiveness of targeted or universal screening for vasa praevia at 18–20 weeks of gestation in Ontario. BJOG 2010;117:1108-8.
- Darlington M, Carbonne B, Mailloux A, Brossard Y, Levy-Mozziconacci A, Cortey A, et al. Effectiveness and costs of non-invasive foetal RHD genotyping in rhesus-D negative mothers: a French multicentric two-arm study of 850 women. BMC Pregnancy Childbirth 2018;18.
- Farrar D, Simmonds M, Griffin S, Duarte A, Lawlor DA, Sculpher M, et al. The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta-analyses and an economic evaluation. Health Technol Assess 2016;20:1-348.
- Gantt S, Dionne F, Kozak FK, Goshen O, Goldfarb DM, Park AH, et al. Cost-effectiveness of universal and targeted newborn screening for congenital cytomegalovirus infection. JAMA Pediatr 2016;170:1173-80.
- Hauspurg A, Albright C, Rouse D, Werner E. Third trimester fetal growth ultrasound: a cost–benefit analysis. Am J Obstet Gynecol 2015;212.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol 2018;132:699-707.
- Imaz-Iglesia I, Miguel LGS, Ayala-Morillas LE, Garcia-Perez L, Gonzalez-Enriquez J, Blasco-Hernandez T, et al. Economic evaluation of chagas disease screening in Spain. Acta Trop 2015;148:77-88.
- Kowada A. Cost effectiveness of interferon-gamma release assay for TB screening of HIV positive pregnant women in low TB incidence countries. J Infect 2014;68:32-4.
- Little SE, Janakiraman V, Kaimal A, Musci T, Ecker J, Caughey AB. The cost-effectiveness of prenatal screening for spinal muscular atrophy. Am J Obstet Gynecol 2010;202:253.e1-7.
- Marseille E, Lohse N, Jiwani A, Hod M, Seshiah V, Yajnik CS, et al. The cost-effectiveness of gestational diabetes screening including prevention of type 2 diabetes: application of a new model in India and Israel. J Matern-Fetal Neonatal Med 2013;26:802-10.
- Mistry H, Gardiner HM. The cost-effectiveness of prenatal detection for congenital heart disease using telemedicine screening. J Telemed Telecare 2013;19:190-6.
- Plunkett BA, Grobman WA. Routine hepatitis C virus screening in pregnancy: a cost-effectiveness analysis. Am J Obstet Gynecol 2005;192:1153-61.
- Round JA, Jacklin P, Fraser RB, Hughes RG, Mugglestone MA, Holt RI. Screening for gestational diabetes mellitus: cost–utility of different screening strategies based on a woman’s individual risk of disease. Diabetologia 2011;54:256-63.
- Rozenbaum MH, Verweel G, Folkerts DK, Dronkers F, van den Hoek JA, Hartwig NG, et al. Cost-effectiveness estimates for antenatal HIV testing in the Netherlands. Int J STD AIDS 2008;19:668-75.
- Selvapatt N, Ward T, Bailey H, Bennett H, Thorne C, See LM, et al. Is antenatal screening for hepatitis C virus cost-effective? A decade’s experience at a London centre. J Hepatol 2015;63:797-804.
- Shmueli A, Meiri H, Gonen R. Economic assessment of screening for pre-eclampsia. Prenat Diagn 2012;32:29-38.
- Turner ML, Bessos H, Fagge T, Harkness M, Rentoul F, Seymour J, et al. Prospective epidemiologic study of the outcome and cost-effectiveness of antenatal screening to detect neonatal alloimmune thrombocytopenia due to anti-HPA-1a. Transfusion (Paris) 2005;45:1945-56.
- Wetzel S, Miller ES, Cirino N, Dukhovny D, Ameel B, Caughey AB. Routine antenatal screening for depression: what are the outcomes and is it cost-effective?. Am J Obstet Gynecol 2016;214:S383-4.
- Wu O, Robertson L, Twaddle S, Lowe GD, Clark P, Greaves M, et al. Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study. Health Technol Assess 2006;10:1-110.
- Zhang W, Mohammadi T, Sou J, Anis AH. Cost-effectiveness of prenatal screening and diagnostic strategies for Down syndrome: a microsimulation modeling analysis. PLOS ONE 2019;14.
- Eijsink JFH, Al Khayat M, Boersma C, Horst PGJ ter, Wilschut JC, Postma MJ. Cost-effectiveness of hepatitis C virus screening, and subsequent monitoring or treatment among pregnant women in the Netherlands. Eur J Health Econ 2021;22:75-88.
- National Collaborating Centre for Women’s and Children’s Health . Antenatal Care Routine Care for the Healthy Pregnant Woman (Appendix G) n.d. https://stratog.rcog.org.uk/sites/default/files/Induction of labour and prolonged pregnancy/nice_antenatalcare_62_2008_pg15.pdf%0A (accessed 20 February 2021).
- Carroll AE, Downs SM. Comprehensive cost–utility analysis of newborn screening strategies. Pediatrics 2006;117:S287-95.
- Castillo-Riquelme MC, Lord J, Moseley MJ, Fielder AR, Haines L. Cost-effectiveness of digital photographic screening for retinopathy of prematurity in the United Kingdom. Int J Technol Assess Health Care 2004;20:201-13.
- Chan K, Davis J, Pai SY, Bonilla FA, Puck JM, Apkon M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID). Mol Genet Metab 2011;104:383-9.
- Ding Y, Thompson JD, Kobrynski L, Ojodu J, Zarbalian G, Grosse SD. Cost-effectiveness/cost–benefit analysis of newborn screening for severe combined immune deficiency in Washington state. J Pediatr 2016;172:127-35.
- Fox DA, Ronsley R, Khowaja AR, Haim A, Vallance H, Sinclair G, et al. Clinical impact and cost efficacy of newborn screening for congenital adrenal hyperplasia. J Pediatr 2020;7.
- Hamers FF, Rumeau-Pichon C. Cost-effectiveness analysis of universal newborn screening for medium chain acyl-CoA dehydrogenase deficiency in France. BMC Pediatr 2012;12.
- Herrero C, Moreno-Ternero JD. Hospital costs and social costs: a case study of newborn hearing screening. Invest Econ 2005;29:203-16.
- Insinga RP, Laessig RH, Hoffman GL. Newborn screening with tandem mass spectrometry: examining its cost-effectiveness in the Wisconsin Newborn Screening Panel. J Pediatr 2002;141:524-31.
- Masucci L, Schreiber RA, Kaczorowski J, Collet JP, Bryan S. Universal screening of newborns for biliary atresia: cost-effectiveness of alternative strategies. J Med Screen 2019;26:113-9.
- McGhee SA, Stiehm ER, McCabe ER. Potential costs and benefits of newborn screening for severe combined immunodeficiency. J Pediatr 2005;147:603-8.
- Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S. Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review. Health Technol Assess 2004;8:1-121.
- Panepinto JA, Magid D, Rewers MJ, Lane PA. Universal versus targeted screening of infants for sickle cell disease: a cost-effectiveness analysis. J Pediatr 2000;136:201-8.
- Pfeil J, Listl S, Hoffmann GF, Kolker S, Lindner M, Burgard P. Newborn screening by tandem mass spectrometry for glutaric aciduria type 1: a cost-effectiveness analysis. Orphanet J Rare Dis 2013;8.
- Prosser LA, Kong CY, Rusinak D, Waisbren SL. Projected costs, risks, and benefits of expanded newborn screening for MCADD. Pediatrics 2010;125:e286-94.
- Schoen EJ, Baker JC, Colby CJ, To TT. Cost–benefit analysis of universal tandem mass spectrometry for newborn screening. Pediatrics 2002;110:781-6.
- Schreiber RA, Masucci L, Kaczorowski J, Collet JP, Lutley P, Espinosa V, et al. Home-based screening for biliary atresia using infant stool colour cards: a large-scale prospective cohort study and cost-effectiveness analysis. J Med Screen 2014;21:126-32.
- Simpson N, Anderson R, Sassi F, Pitman A, Lewis P, Tu K, et al. The cost-effectiveness of neonatal screening for cystic fibrosis: an analysis of alternative scenarios using a decision model. Cost Eff Resour Alloc 2005;3.
- Vallejo-Torres L, Castilla I, Couce ML, Perez-Cerda C, Martin-Hernandez E, Pineda M, et al. Cost-effectiveness analysis of a national newborn screening programme for biotinidase deficiency. Pediatrics 2015;136:e424-32.
- van der Hilst CS, Derks TG, Reijngoud DJ, Smit GP, TenVergert EM. Cost-effectiveness of neonatal screening for medium chain acyl-CoA dehydrogenase deficiency: the homogeneous population of the Netherlands. J Pediatr 2007;151:115-20.
- van der Ploeg CPB, Blom M, Bredius RGM, van der Burg M, Schielen P, Verkerk PH, et al. Cost-effectiveness of newborn screening for severe combined immunodeficiency. Eur J Pediatr 2019;178:721-9.
- van der Ploeg CP, van den Akker-van Marle ME, Vernooij-van Langen AM, Elvers LH, Gille JJ, Verkerk PH, et al. Cost-effectiveness of newborn screening for cystic fibrosis determined with real-life data. J Cyst Fibrosis 2015;14:194-02.
- Venditti LN, Venditti CP, Berry GT, Kaplan PB, Kaye EM, Glick H, et al. Newborn screening by tandem mass spectrometry for medium-chain Acyl-CoA dehydrogenase deficiency: a cost-effectiveness analysis. Pediatrics 2003;112:1005-5.
- Yoo B, Grosse S. The cost effectiveness of screening newborns for congenital adrenal hyperplasia. Public Health Genomics 2009;12:67-72.
- Zupancic JA, Triedman JK, Alexander M, Walsh EP, Richardson DK, Berul CI. Cost-effectiveness and implications of newborn screening for prolongation of QT interval for the prevention of sudden infant death syndrome. J Pediatr 2000;136:481-9.
- Bessey A, Chilcott J, Pandor A, Paisley S. The cost-effectiveness of expanding the UK newborn bloodspot screening programme to include five additional inborn errors of metabolism. Int J Neonatal Screen 2020;6.
- Richardson JS, Kemper AR, Grosse SD, Lam WKK, Rose AM, Ahmad A, et al. Health and economic outcomes of newborn screening for infantile-onset Pompe disease. Genet Med 2020;23:758-66.
- Mogul D, Zhou M, Intihar P, Schwarz K, Frick K. Cost-effective analysis of screening for biliary atresia with the stool color card. J Pediatr Gastroenterol Nutr 2015;60:91-8.
- Bessey A, Chilcott J, Leaviss J, de la C, ruz C, Wong R. A cost-effectiveness analysis of newborn screening for severe combined immunodeficiency in the UK. Int J Neonatal Screen 2019;5.
- Health Partners Consulting Group . Cost-Effectiveness of Newborn Screening for Severe Combined Immune Deficiency n.d. www.nsu.govt.nz/system/files/resources/cost-effectiveness-newborn-screening-severe-combined-immune-deficiency.pdf (accessed 20 February 2021).
- Turcotte C, Blancquaert I, Brabant J, St-Louis M. Évaluation De La Pertinence Du dépistage néonatal Sanguin Par spectrométrie De Masse En Tandem De l’homocystinurie Classique (HCY) n.d. www.inesss.qc.ca/fileadmin/doc/INESSS/Rapports/Depistage/INESSS_DepistageNeonatal_HCY.pdf%0A (accessed 20 February 2021).
- Ball RH, Caughey AB, Malone FD, Nyberg DA, Comstock CH, Saade GR, et al. First and Second Trimester Evaluation of Risk (FASTER) research consortium. First- and second-trimester evaluation of risk for Down syndrome. Obstet Gynecol 2007;110:10-7.
- Han C, Werner E, Pettker C, Norwitz E, Funai E, Thung S. Universal antenatal screening for spinal muscular atrophy (SMA): a cost–utility analysis. Am J Obstet Gynecol 2009;201.
- Hollingsworth B, Harris A. Economic evaluation of prenatal population screening for fragile X syndrome. Community Genet 2005;8:68-72.
- Musci TJ, Caughey AB. Cost-effectiveness analysis of prenatal population-based fragile X carrier screening. Am J Obstet Gynecol 2005;192:1905-12; discussion 1912.
- Wald NJ, Rudnicka AR, Bestwick JP. Sequential and contingent prenatal screening for Down syndrome. Prenat Diagn 2006;26:769-77.
- Pfeil J, Listl S, Hoffmann GF, Kolker S, Lindner M, Burgard P. Newborn screening by tandem mass spectrometry for glutaric aciduria type 1: a cost-effectiveness analysis. Mol Genet Metab 2014;111.
- Boyd KA, Briggs AH, Fenwick E, Norrie J, Stock S. Power and sample size for cost-effectiveness analysis: fFN neonatal screening. Contemp Clin Trials 2011;32:893-901.
- Cahill AG, Odibo AO, Caughey AB, Stamilio DM, Hassan SS, Macones GA, et al. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: a decision and economic analysis. Am J Obstet Gynecol 2010;202:548.e1-8.
- Berger LM. Estimating the benefits and costs of a universal substance abuse screening and treatment referral policy for pregnant women. J Soc Serv Res 2002;29:57-84.
- Killie MK. Cost-effectiveness of antenatal screening for neonatal alloimmune thrombocytopenia. BJOG 2007;114.
- Bak GS, Shaffer BL, Madriago E, Allen A, Kelly B, Caughey AB, et al. Impact of maternal obesity on fetal cardiac screening: which follow-up strategy is cost-effective?. Ultrasound Obstet Gynecol 2020;56:705-16.
- Baker D, Brown Z, Hollier LM, Wendel GD, Hulme L, Griffiths DA, et al. Cost-effectiveness of herpes simplex virus type 2 serologic testing and antiviral therapy in pregnancy. Am J Obstet Gynecol 2004;191:2074-84.
- Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades A, et al. Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses. Health Technol Assess 2007;11:1-226.
- Danyliv A, Gillespie P, O’Neill C, Tierney M, O’Dea A, McGuire BE, et al. The cost-effectiveness of screening for gestational diabetes mellitus in primary and secondary care in the Republic of Ireland. Diabetologia 2016;59:436-44.
- Miller ES, Grobman WA. Cost-effectiveness of transabdominal ultrasound for cervical length screening for preterm birth prevention. Am J Obstet Gynecol 2013;209:546.e1-6.
- Mission JF, Ohno MS, Cheng YW, Caughey AB. Gestational diabetes screening with the new IADPSG guidelines: a cost-effectiveness analysis. Am J Obstet Gynecol 2012;207:326.e1-9.
- Mone F, O’Mahony JF, Tyrrell E, Mulcahy C, McParland P, Breathnach F, et al. Preeclampsia prevention using routine versus screening test-indicated aspirin in low-risk women. Hypertension 2018;72:1391-6.
- Nicholson WK, Fleisher LA, Fox HE, Powe NR. Screening for gestational diabetes mellitus: a decision and cost-effectiveness analysis of four screening strategies. Diabetes Care 2005;28:1482-4.
- Straub HL, Antoniewicz LW, Riggs JW, Plunkett BA, Hollier LM. Cost-effectiveness analysis of rubella screening strategies using electronic medical records. Am J Perinatol 2013;30:759-64.
- Thung SF, Grobman WA. The cost-effectiveness of routine antenatal screening for maternal herpes simplex virus-1 and -2 antibodies. Am J Obstet Gynecol 2005;192:483-8.
- Tuite AR, McCabe CJ, Ku J, Fisman DN. Projected cost-savings with herpes simplex virus screening in pregnancy: towards a new screening paradigm. Sex Transm Infect 2011;87:141-8.
- Wastlund D, Moraitis AA, Thornton JG, Sanders J, White IR, Brocklehurst P, et al. The cost-effectiveness of universal late-pregnancy screening for macrosomia in nulliparous women: a decision analysis. BJOG 2019;126:1243-50.
- Werner EF, Pettker CM, Zuckerwise L, Reel M, Funai EF, Henderson J, et al. Screening for gestational diabetes mellitus: are the criteria proposed by the international association of the Diabetes and Pregnancy Study Groups cost-effective?. Diabetes Care 2012;35:529-35.
- Ontario H. Noninvasive fetal RhD blood group genotyping: a Health Technology Assessment. Ont Health Technol Assess Ser 2020;20:1-160.
- Albright CM, MacGregor C, Sutton D, Theva M, Hughes BL, Werner EF. Group B streptococci screening before repeat cesarean delivery: a cost-effectiveness analysis. Obstet Gynecol 2017;129:111-9.
- Dunbar JA, Hsu V, Christensen M, Black B, Williams P, Beauchamp G. Cost–utility analysis of screening and laser treatment of retinopathy of prematurity. J Am Assoc Pediatr Ophthalmol Strabismus 2009;13:186-90.
- Hopkins RB, Paradis J, Roshankar T, Bowen J, Tarride JE, Blackhouse G, et al. Universal or targeted screening for fetal alcohol exposure: a cost-effectiveness analysis. J Stud Alcohol Drugs 2008;69:510-9.
- Malec LM, Sidonio RF, Smith KJ, Cooper JD. Three cost–utility analyses of screening for intracranial hemorrhage in neonates with hemophilia. J Pediatr Hematol Oncol 2014;36:474-9.
- Socialstyrelsen . Screening för X-Bunden Adrenoleukodystrofi (X-ALD) n.d. www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/nationella-screeningprogram/2020-12-7078-halsoekonomisk-analys.pdf%0A (accessed 20 February 2021).
- Postma MJ, van den Hoek JA, Beck EJ, Heeg B, Jager JC, Coutinho RA. Pharmaco-economic evaluation of mandatory HIV-screening in pregnancy; a cost-efficacy analysis in Amsterdam. Ned Tijdschr Geneeskd 2000;144:749-54.
- Urbanus AT, van Keep M, Matser AA, Rozenbaum MH, Weegink CJ, van den Hoek A, et al. Is adding HCV screening to the antenatal national screening program in Amsterdam, the Netherlands, cost-effective?. PLOS ONE 2013;8.
- Modell V, Knaus M, Modell F. An analysis and decision tool to measure cost benefit of newborn screening for severe combined immunodeficiency (SCID) and related T-cell lymphopenia. Immunol Res 2014;60:145-52.
- Quaglini S, Rognoni C, Spazzolini C, Priori SG, Mannarino S, Schwartz PJ. Cost-effectiveness of neonatal ECG screening for the long QT syndrome. Eur Heart J 2006;27:1824-32.
- Shermock KM, Gildea TR, Singer M, Stoller JK. Cost-effectiveness of population screening for alpha-1 antitrypsin deficiency: a decision analysis. COPD J Chron Obstruct Pulmon Dis 2005;2:411-8.
- Yeh J, Stout NK, Chaudhry A, Gooch M, McMahon P, Christensen KD, et al. Population-based cancer predisposition testing as a component of newborn screening: a cost effectiveness analysis. J Clin Oncol 2019;37.
- Kanga I, Williams D, Hatchette T, MacKinnon S, Jung H, Black C, et al. Screening for Chlamydia trachomatis and Neisseria gonorrhoeae during pregnancy: Health Technology Assessment. Can Agency Drugs Technol Health 2018.
- Bak GS, Shaffer BL, Madriago E, Allen A, Kelly B, Caughey AB, et al. Detection of fetal cardiac anomalies: is increasing the number of cardiac views cost-effective?. Ultrasound Obstet Gynecol 2020;16.
- Donnay Candil S, Balsa Barro JA, Alvarez Hernandez J, Crespo Palomo C, Perez-Alcantara F, Polanco Sanchez C. Cost-effectiveness analysis of universal screening for thyroid disease in pregnant women in Spain. Endocrinol Nutr 2015;62:322-30.
- Dorius A, Worstell T, Griffin E, Little S, Sparks T, Caughey A. Routine antenatal testing for pregnancies at 40 weeks gestation: a cost-effectiveness analysis. Am J Obstet Gynecol 2014;210:S214-5.
- Ginsberg GM, Eidelman AI, Shinwell E, Anis E, Peyser R, Lotan Y. Should Israel screen all mothers-to-be to prevent early-onset of neonatal group B streptococcal disease? A cost–utility analysis. Isr J Health Policy Res 2013;2.
- Lee BY, Wiringa AE, Mitgang EA, McGlone SM, Afriyie AN, Song Y, et al. Routine pre-cesarean Staphylococcus aureus screening and decolonization: a cost-effectiveness analysis. Am J Manag Care 2011;17:693-700.
- Odibo AO, Coassolo KM, Stamilio DM, Ural SH, Macones GA. Should all pregnant diabetic women undergo a fetal echocardiography? A cost-effectiveness analysis comparing four screening strategies. Prenat Diagn 2006;26:39-44.
- Rours GI, Smith-Norowitz TA, Ditkowsky J, Hammerschlag MR, Verkooyen RP, de Groot R, et al. Cost-effectiveness analysis of Chlamydia trachomatis screening in Dutch pregnant women. Pathog Glob Health 2016;110:292-30.
- Scott RK, Crochet S, Huang CC. Universal rapid human immunodeficiency virus screening at delivery: a cost-effectiveness analysis. Infect Dis Obstet Gynecol 2018;2018.
- Sicuri E, Munoz J, Pinazo MJ, Posada E, Sanchez J, Alonso PL, et al. Economic evaluation of Chagas disease screening of pregnant Latin American women and of their infants in a non endemic area. Acta Trop 2011;118:110-7.
- Turrentine MA, Ramirez MM, Mastrobattista JM. Cost-effectiveness of universal prophylaxis in pregnancy with prior group B streptococci colonization. Infect Dis Obstet Gynecol 2009;2009.
- Walker A, Caughey A. Positivity thresholds of HbA1c assay as a screening test for diabetes mellitus in the first trimester in high-risk populations. J Matern-Fetal Neonatal Med 2020:1-5.
- Hacker FM, Hersh AR, Shaffer BL, Caughey AB. Isolated echogenic intracardiac foci and the role of cell-free fetal DNA: a cost-effectiveness analysis. Prenat Diagn 2020;40:1517-24.
- Albright CM, Werner EF, Hughes BL. Cytomegalovirus screening in pregnancy: a cost-effectiveness and threshold analysis. Am J Perinatol 2019;36:678-87.
- Neovius M, Tiblad E, Westgren M, Kublickas M, Neovius K, Wikman A. Cost-effectiveness of first trimester non-invasive fetal RHD screening for targeted antenatal anti-D prophylaxis in RhD-negative pregnant women: a model-based analysis. BJOG 2016;123:1337-46.
- Ditkowsky J, Shah KH, Hammerschlag MR, Kohlhoff S, Smith-Norowitz TA. Cost–benefit analysis of Chlamydia trachomatis screening in pregnant women in a high burden setting in the United States. BMC Infect Dis 2017;17.
- Doyle NM, Gardner MO. Prenatal cystic fibrosis screening in Mexican Americans: an economic analysis. Am J Obstet Gynecol 2003;189:769-74.
- El Helali N, Giovangrandi Y, Guyot K, Chevet K, Gutmann L, Durand-Zaleski I. Cost and effectiveness of intrapartum group B streptococcus polymerase chain reaction screening for term deliveries. Obstet Gynecol 2012;119:822-9.
- Kekki M, Kurki T, Kotomaki T, Sintonen H, Paavonen J. Cost-effectiveness of screening and treatment for bacterial vaginosis in early pregnancy among women at low risk for preterm birth. Acta Obstet Gynecol Scand 2004;83:27-36.
- Postma MJ, Bakker A, Welte R, Van Bergen JEAM, Van den Hoek JAR, De Jong-Van den Berg LTW, et al. Screening for asymptomatic infection with Chlamydia trachomatis in pregnancy; favourable cost-effectiveness if prevalence is 3% or more. Ned Tijdschr Geneeskd 2000;144:2350-4.
- Binquet C, Lejeune C, Seror V, Peyron F, Bertaux AC, Scemama O, et al. The cost-effectiveness of neonatal versus prenatal screening for congenital toxoplasmosis. PLOS ONE 2019;14.
- Haberland CA, Benitz WE, Sanders GD, Pietzsch JB, Yamada S, Nguyen L, et al. Perinatal screening for group B streptococci: cost–benefit analysis of rapid polymerase chain reaction. Pediatrics 2002;110:471-80.
- Picchiassi E, Coata G, Babucci G, Giardina I, Summa V, Tarquini F, et al. Intrapartum test for detection of group B streptococcus colonization during labor. J Matern-Fetal Neonatal Med 2018;31:3293-300.
- Geelhoed EA, Lewis B, Hounsome D, O’Leary P. Economic evaluation of neonatal screening for phenylketonuria and congenital hypothyroidism. J Paediatr Child Health 2005;41:575-9.
- Magnusson G, Persson U. Screening for congenital cataracts: a cost-consequence analysis of eye examination at maternity wards in comparison to well-baby clinics. Acta Paediatr 2005;94:1089-95.
- Stillwaggon E, Carrier CS, Sautter M, McLeod R. Maternal serologic screening to prevent congenital toxoplasmosis: a decision-analytic economic model. PLoS Negl Trop Dis 2011;5.
- Brown J, Dezateux C, Karnon J, Parnaby A, Arthur R. Efficiency of alternative policy options for screening for developmental dysplasia of the hip in the United Kingdom. Arch Dis Child 2003;88:760-6.
- Brancazio L, Paglia M, Kuller J, Wells S. Prenatal Down syndrome screening: a cost analysis of different strategies. Am J Obstet Gynecol 2003;189.
- Dillard JP, Shen L, Robinson JD, Farrell PM. Parental information seeking following a positive newborn screening for cystic fibrosis. J Health Commun 2010;15:880-94.
- Kerruish NJ. Parents’ experiences of newborn screening for genetic susceptibility to type 1 diabetes. J Med Ethics 2011;37:348-53.
- Kerruish N. Parents’ experiences 12 years after newborn screening for genetic susceptibility to type 1 diabetes and their attitudes to whole-genome sequencing in newborns. Genet Med 2016;18:249-58.
- Priddis L, Dougall A, Balding E, Barrett A. Cystic fibrosis diagnosis: impact on mothers of affected Australian children. Neonatal Paediatr Child Health Nurs 2009;12:20-5.
- Priddis L, Dunwoodie J, Balding E, Barrett A, Douglas T. Paternal experiences of their children’s diagnosis of Cystic Fibrosis following newborn screening diagnosis. Neonatal Paediatr Child Health Nurs 2010;13:3-9.
- Schmidt JL, Castellanos-Brown K, Childress S, Bonhomme N, Oktay JS, Terry SF, et al. The impact of false-positive newborn screening results on families: a qualitative study. Genet Med 2012;14:76-80.
Appendix 1 List of websites searched
| Type | Country | Organisation | Website link |
|---|---|---|---|
| Screening | Australia | Australian COAG Health Council Health Technology Reference Group | www.coaghealthcouncil.gov.au |
| Screening | Australia | Australian Government Department of Health, Medical Services Advisory Committee | www.msac.gov.au |
| Screening | Australia | Australian Government Department of Health, Standing Committee on Screening | www.health.gov.au |
| Screening | Belgium | Belgian Health Care KCE | https://kce.fgov.be/en |
| Screening | Belgium | Superior Health Council (Hoge Gezondheidsraad/Conseil Supérieur de la Santé) | www.health.belgium.be/en |
| Screening | Canada | Alberta Health Services | www.albertahealthservices.ca |
| Screening | Canada | Canadian Task Force on Preventive Health Care | https://canadiantaskforce.ca |
| Screening | Canada | HQCA | www.hqca.ca |
| Screening | Canada | Health Quality Ontario | www.hqontario.ca |
| Screening | Canada | INESSS (formerly AETMIS) | www.inesss.qc.ca/en/index.html |
| Screening | Canada | Public Health Agency of Canada | www.canada.ca/en.html |
| Screening | Canada | THETA Collaborative | https://theta.utoronto.ca |
| Screening | Denmark | National Board of Health (Sundhedsstyrelsen) | www.sst.dk/en/English/Corona-eng |
| Screening | Finland | NSC, Ministry of Health and Social Affairs (Social- och hälsovårdsministeriet) | http://stm.fi/en/frontpage |
| Screening | France | Haute Autorité de Santé | www.has-sante.fr/jcms/pprd_2986129/en/home |
| Screening | Germany | German Institute of Medical Documentation and Information (DIMDI) | www.dimdi.de/dynamic/en/homepage |
| Screening | Germany | The Federal Joint Committee (Gemeinsamer Bundesausschuss) | www.g-ba.de/english/ |
| Screening | Ireland | HIQA | www.hiqa.ie |
| Screening | Italy | Osservatorio nazionale screening (National Centre for Screening Monitoring) | www.osservatorionazionalescreening.it |
| Screening | The Netherlands | The Health Council (Gezondheidsraad) | www.gezondheidsraad.nl |
| Screening | The Netherlands | Zorginstituut Nederland (National Health Care Institute Netherlands) | www.zorginstituutnederland.nl |
| Screening | New Zealand | National Screening Advisory Committee, National Screening Unit | www.nsu.govt.nz |
| Screening | Norway | NIPH | www.fhi.no/en |
| Screening | Spain | Instituto de Salud Carlos III (ISCIII) | https://eng.isciii.es/eng.isciii.es/Paginas/Inicio.html |
| Screening | Spain | Ministry of Health, Social Services and Equality (Ministerio de Sanidad, Servicios Sociales E Igualdad) | www.mscbs.gob.es/en/home.htm |
| Screening | Sweden | The National Board of Health and Welfare (Socialstyrelsen) | www.socialstyrelsen.se/en/ |
| Screening | UK | Healthcare Improvement Scotland | www.healthcareimprovementscotland.org |
| Screening | UK | UK NSC | https://legacyscreening.phe.org.uk |
| Screening | USA | Advisory Committee on Heritable Disorders in Newborns and Children: Bloodspot | www.hrsa.gov/advisory-committees/heritable-disorders |
| Screening | USA | ACMG | www.acmg.net |
| Screening | USA | U.S. Preventive Services Task Force | www.uspreventiveservicestaskforce.org |
| Screening | USA | Washington State HCA | www.hca.wa.gov |
| Referred to/from initial organisation or experts | Australia | Australian Office of Population Health Genomics | https://ww2.health.wa.gov.au/Home |
| Referred to/from initial organisation or experts | Australia | Human Genetics Society of Australasia | www.hgsa.org.au/ |
| Referred to/from initial organisation or experts | Canada | Canadian Organization for Rare Disorders | www.raredisorders.ca |
| Referred to/from initial organisation or experts | Denmark | Danish Statens Serum Institut | https://en.ssi.dk |
| Referred to/from initial organisation or experts | Finland | Finnish Office for HTA | www.inahta.org |
| Referred to/from initial organisation or experts | Italy | Società Italiana Studio Malattie Metaboliche Ereditarie (Italian Society for the Study of Hereditary Metabolic Diseases and Neonatal Screening) | www.simmesn.it |
| Referred to/from initial organisation or experts | Sweden | Karolinska Universitetssjukhuset (Karolinska University Hospital) | www.karolinska.se/en/karolinska-university-hospital |
| Public organisation | The Netherlands | Rijksinstituut voor Volksgezondheid en Milieu (Dutch National Institute of Public Health and the Environment) | www.rivm.nl/en |
| Public organisation | Spain | Gobierno de Canarias | www3.gobiernodecanarias.org/sanidad/scs/contenidoGenerico.jsp?idDocument=6e55e15a-02e0-11e5-9e16-d107cd1682ec&idCarpeta=993a9b1d-7aed-11e4-a62a-758e414b4260 |
| Paediatrics organisation | Australia | Paediatrics and Child Health Division, The Royal Australasian College of Physicians (2016–8) | www.racp.edu.au/about/racps-structure/paediatrics-child-health-division |
| Paediatrics organisation | Austria | Austrian Society of Pediatrics and Adolescent Medicine | www.paediatrie.at/ |
| Paediatrics organisation | Belgium | Belgian Society of Pediatrics | https://bvk-sbp.be |
| Paediatrics organisation | Canada | CPS | www.cps.ca |
| Paediatrics organisation | Chile | Sociedad Chilena De Pediatria (Pediatric Society of Chile) | www.sochipe.cl/v3 |
| Paediatrics organisation | Czech Republic | Czech National Pediatric Society | www.pediatrics.cz/en |
| Paediatrics organisation | Denmark | Dansk Paediatrisk Selskab (Danish Paediatric Society) | www.paediatri.dk |
| Paediatrics organisation | France | SFP | www.sfpediatrie.com |
| Paediatrics organisation | Germany | German Society of Pediatrics and Adolescent Medicine (DGKJ) | www.dgkj.de |
| Paediatrics organisation | Greece | Hellenic Pediatric Society (Greek Pediatric Society) | https://e-child.gr |
| Paediatrics organisation | Hungary | Hungarian Pediatric Association | www.gyermekorvostarsasag.hu |
| Paediatrics organisation | Ireland | IPA | www.irishpaediatricassociation.ie |
| Paediatrics organisation | Israel | Israel Paediatric Association | www.pediatrics.org.il/english |
| Paediatrics organisation | Italy | Societa Italiana di Pediatria (Italian Society of Pediatrics) | www.sip.it |
| Paediatrics organisation | Japan | The JPS | www.jpeds.or.jp/modules/en/index.php?content_id=1 |
| Paediatrics organisation | Latvia | Latvian Pediatric Association | www.lpa.lv |
| Paediatrics organisation | Lithuania | Lithuanian Paediatric Association | www.pediatrija.org |
| Paediatrics organisation | Mexico | AMP A.C. | www.amp.org.mx |
| Paediatrics organisation | Mexico | CONAPEME | www.conapeme.org/v2 |
| Paediatrics organisation | The Netherlands | NVK (Paediatric Association of the Netherlands) | www.nvk.nl |
| Paediatrics organisation | New Zealand | Paediatric Society of New Zealand | www.paediatrics.org.nz |
| Paediatrics organisation | Norway | Norwegian Pediatric Association | www.legeforeningen.no/foreningsledd/fagmed/norsk-barnelegeforening |
| Paediatrics organisation | Poland | Polish Pediatric Society | https://ptp.edu.pl |
| Paediatrics organisation | Portugal | Sociedade Portuguesa de Pediatria (Portuguese Society of Pediatrics) | www.spp.pt |
| Paediatrics organisation | Slovenia | Slovenian Paediatric Society | https://zzp.si |
| Paediatrics organisation | South Korea | The Korean Pediatric Society | www.pediatrics.or.kr/english/ |
| Paediatrics organisation | Sweden | Swedish Pediatric Society | www.barnlakarforeningen.se |
| Paediatrics organisation | Turkey | Turk Pediatri Kurumu (Turkish Pediatric Association) | http://turkpediatri.org.tr |
| Paediatrics organisation | Turkey | Turkish National Pediatric Society | www.millipediatri.org.tr |
| Paediatrics organisation | UK | RCPCH | www.rcpch.ac.uk |
| Paediatrics organisation | USA | APA | www.academicpeds.org |
| Paediatrics organisation | USA | AAP | www.aap.org |
| Paediatrics organisation | USA | APS | www.aps1888.org |
| Obstetrics and gynaecology organisation | Australia | Royal Australian New Zealand College Obstetricians Gynaecologists | https://ranzcog.edu.au/ |
| Obstetrics and gynaecology organisation | Austria | Oesterreichische Gesellschaft fur Gynakologie und Geburtshilfe Austrian Society Gynaecology Obstetrics | www.oeggg.at/ |
| Obstetrics and gynaecology organisation | Belgium | GGOLFB | www.ggolfb.be |
| Obstetrics and gynaecology organisation | Belgium | Royal Belgian Society for Obstetrics and Gynaecology (VVOG) | www.vvog.be |
| Obstetrics and gynaecology organisation | Canada | Society Obstetricians Gynaecologists Canada/Societé des Obstétriciens et Gynécolgues du Canada | www.sogc.org |
| Obstetrics and gynaecology organisation | Chile | Sociedad Chilena de Obstetricia y Ginecología | https://sochog.cl |
| Obstetrics and gynaecology organisation | Czech Republic | Czech Gynecological Obstetrical Society | www.cgps.cz/en |
| Obstetrics and gynaecology organisation | Denmark | Dansk Selskab for Obstetric og Gynaekologi | www.dsog.dk |
| Obstetrics and gynaecology organisation | Estonia | Society Estonian Gynaecologists | www.ens.ee/en |
| Obstetrics and gynaecology organisation | Finland | Finnish Gynecological Association | https://gynekologiyhdistys.fi/in-english |
| Obstetrics and gynaecology organisation | France | Collège National des Gynécologues et Obstétriciens Français | www.cngof.fr |
| Obstetrics and gynaecology organisation | Germany | Deutsche Gesellschaft für Gynäkologie und Geburtshilfe | www.dggg.de |
| Obstetrics and gynaecology organisation | Greece | Hellenic Obstetrical Gynaecological Society | http://hsog.gr |
| Obstetrics and gynaecology organisation | Hungary | Hungarian Society Obstetrics Gynaecology | http://mnt.olo.hu |
| Obstetrics and gynaecology organisation | Iceland | Icelandic Society Obstetrics Gynecology | www.figo.org |
| Obstetrics and gynaecology organisation | Ireland | Institute Obstetricians Gynaecologists the Royal College Physicians Ireland | www.rcpi.ie |
| Obstetrics and gynaecology organisation | Israel | Israel Society Obstetrics Gynecology | https://gynecology.mednet.co.il |
| Obstetrics and gynaecology organisation | Italy | Società Italiana di Ginecologia e Ostetricia | www.sigo.it |
| Obstetrics and gynaecology organisation | Japan | Japan Society Obstetrics Gynecology | www.jsog.or.jp/modules/en/index.php?content_id=1 |
| Obstetrics and gynaecology organisation | Lithuania | Lithuanian Association Obstetricians Gynecologists | www.lagd.lt |
| Obstetrics and gynaecology organisation | Luxembourg | Société Luxembourgeoise de Gynécologie et d’Obstétrique | www.slgo.lu |
| Obstetrics and gynaecology organisation | Mexico | FEMECOG | https://femecog.org.mx |
| Obstetrics and gynaecology organisation | The Netherlands | Dutch Society Obstetrics Gynaecology | www.nvog.nl |
| Obstetrics and gynaecology organisation | Norway | Norsk gynekologisk Forening Norwegian Society for Gynecology Obstetrics | www.legeforeningen.no/foreningsledd/fagmed/norsk-gynekologisk-forening |
| Obstetrics and gynaecology organisation | Poland | Polish Society Gynecologists Obstetricians. | www.ptgin.pl |
| Obstetrics and gynaecology organisation | Portugal | FSPOG | www.fspog.com/en |
| Obstetrics and gynaecology organisation | South Korea | Korean Society Obstetrics Gynecology | www.ksog.org/eng |
| Obstetrics and gynaecology organisation | Spain | Sociedad Espanõla de Ginecología y Obstetricia | https://sego.es |
| Obstetrics and gynaecology organisation | Sweden | Svensk Förening För Obstetrik and Gynekologi (Swedish Society of Obstetrics and Gynecology) | www.sfog.se/start |
| Obstetrics and gynaecology organisation | Switzerland | Schweizerische Gesellschaft für Gynäkologie Geburtshilf/Société Suisse de Gynécologie and Obstétrique | www.sggg.ch |
| Obstetrics and gynaecology organisation | Turkey | Turkish Society Obstetrics Gynecology | www.tjod.org |
| Obstetrics and gynaecology organisation | UK | Royal College Obstetricians Gynaecologists UK | www.rcog.org.uk |
| Obstetrics and gynaecology organisation | USA | American College Obstetricians Gynecologists | www.acog.org |
| Medical science organisation | Germany | Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (German Association of the Scientific Medical Societies) | www.awmf.org |
| HTA organisation | Austria | ITA of the Austrian Academy of Sciences | www.oeaw.ac.at/en |
| HTA organisation | Canada | Alberta Health Evidence Reviews | www.alberta.ca/health-evidence-reviews.aspx |
| HTA organisation | Canada | CADTH | www.cadth.ca |
| HTA organisation | Canada | IHE | www.ihe.ca |
| HTA organisation | Finland | Finnish Office for HTA (Finohta), National Institute for Health and Welfare | https://thl.fi/en/web/thlfi-en |
| HTA organisation | France | CEDIT | http://cedit.aphp.fr |
| HTA organisation | Ireland | NCPE Ireland | www.ncpe.ie |
| HTA organisation | Spain | Galician Agency for HTA (Avalia-T) | http://avalia-t.sergas.es |
| HTA organisation | Spain | Health and Quality Assessment Agency of Catalonia (AQuAS) | https://aquas.gencat.cat/ca/sobre_aquas |
| HTA organisation | Sweden | Sahlgrenska Universitetssjukhuset – HTA-centrum | www.sahlgrenska.se/en |
| HTA organisation | Sweden | Swedish Agency for HTA and Assessment of Social Services (SBU) | www.sbu.se/en |
| HTA organisation | UK | NICE | www.nice.org.uk |
| HTA organisation | UK | NIHR Journals Library – HTA programme | www.journalslibrary.nihr.ac.uk |
Appendix 2 Literature search strategy
| # | Query | Result |
|---|---|---|
| 1 | Ultrasonography, prenatal/ | 32,214 |
| 2 | exp Prenatal diagnosis/ | 74,833 |
| 3 | (ultrasound* or ultra-sound or ultrasonogra* or ultra-sonogra* or sonogra* or echocardiogra* or nuchal translucen* or amniocentesis or chorionic villus sampl* or cvs or (((noninvasive prenatal or non-invasive prenatal) adj2 (test* or screen*)) or nipt)).ti,ab. | 539,593 |
| 4 | ((fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal or pregnant or pregnancy or trimester?) adj3 (screen* or test* or diagnos* or scan* or structural assessment* or structural survey*)).ti,ab. | 74,649 |
| 5 | screen*.ti. | 183,100 |
| 6 | exp Abortion, Induced/ | 40,453 |
| 7 | ((induced or therap*) adj3 abortion?).ab,kw. or abortion?.ti. | 31,275 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 831,058 |
| 9 | exp Congenital Abnormalities/ | 599,655 |
| 10 | primary dysautonomias/ or dysautonomia, familial/ or Tay-Sachs Disease/ | 2826 |
| 11 | Muscular Atrophy, Spinal/ | 3883 |
| 12 | (dysautonomia? or tay sachs).ti,ab,kw. | 3951 |
| 13 | (congenital* adj2 (defect? or malformation? or abnormalit* or anomal* or aneuploid*)).ti,ab. | 64,716 |
| 14 | ((fetal or foetal or fetus or foetus) adj2 (defect? or malformation? or abnormalit* or anomal* or aneuploid*)).ti,ab. | 10,709 |
| 15 | ((structural or neural tube?) adj2 (defect? or malformation? or abnormalit* or anomal*)).ti,ab. | 25,396 |
| 16 | ((non-chromosom* or nonchromosom* or chromosom*) adj2 (defect? or malformation? or abnormalit* or anomal*)).ti,ab. | 24,057 |
| 17 | (((down* or patau* or edward*) adj2 syndrome*) or trisomy 13 or trisomy 18 or trisomy 21).ti,ab. | 28,599 |
| 18 | spinal muscular atrophy.ti,ab,kw. | 5274 |
| 19 | 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 | 678,982 |
| 20 | 8 and 19 | 74,766 |
| 21 | exp Congenital Abnormalities/di, dg | 169,523 |
| 22 | Prenatal Care/ or Perinatal Care/ | 32,940 |
| 23 | (fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal or pregnant or pregnancy or trimester?).ti,ab. | 781,391 |
| 24 | 22 or 23 | 788,430 |
| 25 | 21 and 24 | 23,353 |
| 26 | ((fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal) adj (screen* or test* or diagnos*)).ti. | 16,689 |
| 27 | 20 or 25 or 26 | 86,726 |
| 28 | Diabetes, Gestational/ or exp Hypertension, Pregnancy-Induced/ | 47,602 |
| 29 | (eclampsia or preeclampsia or pregnancy induced hypertension).ti,ab,kw. | 38,775 |
| 30 | ((gestational or pregnan* or maternal) adj2 diabet*).ti,ab,kw. | 22,881 |
| 31 | exp Obstetric Labor, Premature/ or Vasa Previa/ or Placenta Previa/ or Fetal Death/ | 53,095 |
| 32 | ((preterm or premature) adj2 labo?r).ti,ab,kw. | 11,027 |
| 33 | (f?etal death? or stillbirth? or still birth?).ti,ab,kw. | 21,594 |
| 34 | ((placenta or vasa) adj pr?evia).ti,ab,kw. | 3849 |
| 35 | anemia, hemolytic, congenital/ or exp anemia, sickle cell/ or exp thalassemia/ | 45,108 |
| 36 | (sickle cell or thalass?emia?).ti,ab,kw. | 43,368 |
| 37 | exp Syphilis/ | 27,968 |
| 38 | syphilis.ti,ab,kw. | 26,895 |
| 39 | exp Hepatitis B/ | 59,076 |
| 40 | Hepatitis B virus/ | 27,584 |
| 41 | (hepatitis b or hbv).ti,ab,kw. or hepatitis.ti. | 181,492 |
| 42 | exp HIV/ | 100,335 |
| 43 | exp HIV Infections/ | 287,519 |
| 44 | (hiv or human immunodeficiency virus).ti,ab,kw. | 339,214 |
| 45 | exp Chlamydia Infections/ or exp Chlamydia/ | 25,264 |
| 46 | chlamydia.ti,ab,kw. | 25,588 |
| 47 | exp Cytomegalovirus Infections/ | 26,032 |
| 48 | cytomegalovirus.ti,ab,kw. | 43,114 |
| 49 | exp Streptococcal Infections/ | 79,648 |
| 50 | (group b strep or strep b or (streptococc* adj infection?)).ti,ab,kw. | 5123 |
| 51 | exp Parvoviridae Infections/ | 6086 |
| 52 | parovirus.ti,ab,kw. | 21 |
| 53 | Rubella/ or Rubella virus/ or Rubella Syndrome, Congenital/ | 9813 |
| 54 | rubella.ti,ab,kw. | 12,936 |
| 55 | Toxoplasmosis/ or Toxoplasmosis, Congenital/ | 13,136 |
| 56 | toxoplasmosis.ti,ab,kw. | 15,606 |
| 57 | exp Anemia/ | 162,962 |
| 58 | exp Blood Group Antigens/ | 45,846 |
| 59 | exp Thrombophilia/ | 25,631 |
| 60 | thrombophilia?.ti,ab,kw. | 6407 |
| 61 | an?emia?.ti,ab,kw. | 148,777 |
| 62 | (blood group? or rhd status or rhesus positive or rhesus negative or rhesus status).ti,ab,kw. | 23,199 |
| 63 | exp Urinary Tract Infections/ | 47,147 |
| 64 | (‘urinary tract infection*’ or ‘urine infection*’ or uti or cystitis or bacteriuria).ti,ab,kw. | 58,627 |
| 65 | Vaginosis, Bacterial/ | 3115 |
| 66 | vaginosis.ti,ab,kw. | 4193 |
| 67 | domestic violence/ or spouse abuse/ | 13,326 |
| 68 | ((spous* or intimate partner or domestic) adj2 (violence or abuse)).ti,ab,kw. | 14,475 |
| 69 | or/28-68 | 1,320,845 |
| 70 | exp pregnancy/ or pregnant women/ | 907,431 |
| 71 | exp fetus/ | 158,466 |
| 72 | (pregnan$ or f?etal or f?etus or FVS).ti,ab. | 707,872 |
| 73 | preconception care/ or prenatal care/ or perinatal care/ | 34,729 |
| 74 | (pregnan* or preconception* or pre-conception* or antenat* or ante-nat* or antepart* or ante-part* or prenat* or pre-nat* or prepart* or pre-part* or perinatal or maternal or mother*).ti,ab. | 875,611 |
| 75 | 70 or 71 or 72 or 73 or 74 | 1,403,268 |
| 76 | Mass Screening/ | 105,761 |
| 77 | screen*.ti,ab. | 786,058 |
| 78 | exp Population Surveillance/ | 70,787 |
| 79 | Self Report/ | 34,734 |
| 80 | (selfreport* or self-report* or ((oral or tak*) adj3 history)).ti,ab. | 175,659 |
| 81 | exp Hematologic Tests/ or Diagnostic Tests, Routine/ or Serologic Tests/ | 285,709 |
| 82 | ((h?ematolog* or blood or serum or serologic*) adj3 (test* or assay*)).ti,ab. | 122,020 |
| 83 | ((sero* adj5 (test* or screen* or diagnos*)) or (serotest* or seroscreen* or serodiagnos*)).ti,ab. | 54,196 |
| 84 | exp immunoassays/ | 490,512 |
| 85 | Polymerase Chain Reaction/ | 244,333 |
| 86 | (immuno-assay* or immunoassay* or elisa or eia or Fluorescent antibody to membrane antibod* or fama or trfia).ti,ab. | 239,119 |
| 87 | (enzyme linked immunosorbent assay* or elisa or enzyme immunoassay* or eia or recombinant immunoblot assay* or riba).ti,ab. | 246,491 |
| 88 | (polymerase chain reaction or pcr).ti,ab. | 658,262 |
| 89 | (routine adj5 (test* or screen* or diagnos*)).ti,ab. | 49,621 |
| 90 | (test* or diagnos* or assay*).ti. | 1,117,861 |
| 91 | 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 | 3,540,051 |
| 92 | 69 and 75 and 91 | 53,922 |
| 93 | Prenatal diagnosis/ or maternal serum screening tests/ | 37,872 |
| 94 | ((pregnan* or preconception* or pre-conception* or antenat* or ante-nat* or antepart* or ante-part* or prenat* or pre-nat* or prepart* or pre-part* or perinatal or maternal or mother*) adj5 (screen* or diagnos* or test*)).ti,ab. | 90,463 |
| 95 | 93 or 94 | 108,115 |
| 96 | 69 and 95 | 26,099 |
| 97 | Diabetes, Gestational/di or exp Hypertension, Pregnancy-Induced/di or exp Obstetric Labor, Premature/di or Vasa Previa/di or Placenta Previa/di or Fetal Death/di or anemia, hemolytic, congenital/di or exp anemia, sickle cell/di or exp thalassemia/di | 17,294 |
| 98 | exp Syphilis/di or exp Hepatitis B/di or exp HIV Infections/di or exp Chlamydia Infections/di or exp Cytomegalovirus Infections/di or exp Streptococcal Infections/di or exp Parvoviridae Infections/di or Rubella/di or Rubella Syndrome, Congenital/di or Toxoplasmosis/di or Toxoplasmosis, Congenital/di | 75,310 |
| 99 | exp Anemia/di or exp Blood Group Antigens/di or exp Thrombophilia/di | 25,632 |
| 100 | exp Urinary Tract Infections/di or Vaginosis, Bacterial/di | 9057 |
| 101 | 97 or 98 or 99 or 100 | 120,483 |
| 102 | 75 and 101 | 26,681 |
| 103 | 92 or 96 or 102 | 74,483 |
| 104 | Neonatal Screening/ | 10,420 |
| 105 | (heelprick* or heel prick*).ti,ab,kw. | 373 |
| 106 | ((neonat* or newborn) adj2 screen*).ti,ab,kw. | 11,756 |
| 107 | exp Infant, Newborn/ | 616,683 |
| 108 | (newborn? or neonat* or infant?).ti,ab,kw. | 670,401 |
| 109 | 107 or 108 | 982,857 |
| 110 | Physical Examination/ | 41,234 |
| 111 | (physical adj3 exam*).ti,ab,kw. | 74,051 |
| 112 | Mass screening/ | 105,761 |
| 113 | screen*.ti,ab,kw. | 789,062 |
| 114 | Genetic testing/ | 39,073 |
| 115 | early diagnosis/ | 27,345 |
| 116 | diagnostic tests, routine/ | 12,791 |
| 117 | (routine adj5 (test* or diagnos*)).ti,ab,kw. | 35,319 |
| 118 | Serologic Tests/ | 20,745 |
| 119 | serologic.ti,ab,kw. | 27,433 |
| 120 | ((sero* adj5 diagnos*) or (serotest* or seroscreen* or serodiagnos*)).ti,ab,kw. | 23,181 |
| 121 | Dried Blood Spot Testing/ | 1569 |
| 122 | (blood spot* or bloodspot*).ti,ab,kw. | 6142 |
| 123 | exp Hearing Tests/ | 46,951 |
| 124 | ((hearing or auditor* or acoustic* or otoacoustic*) adj3 (test* or diagnos*)).ti,ab,kw. | 12,865 |
| 125 | (automated auditory brainstem response? or aabr or otoacoustic emission? or aoae).ti,ab,kw. | 5609 |
| 126 | 110 or 111 or 112 or 113 or 114 or 115 or 116 or 117 or 118 or 119 or 120 or 121 or 122 or 123 or 124 or 125 | 1,111,501 |
| 127 | anemia, hemolytic, congenital/ or anemia, sickle cell/ or exp thalassemia/ | 43,774 |
| 128 | (sickle cell or thalass?emia?).ti,ab,kw. | 43,368 |
| 129 | eye diseases/ or eye diseases, hereditary/ or cataract/ or vision disorders/ or exp blindness/ | 113,166 |
| 130 | (cataract? or blind* or ((eye? or vision?) adj2 (disease? or disorder?))).ti,ab,kw. | 375,117 |
| 131 | exp Heart Defects, Congenital/ | 153,550 |
| 132 | ((heart or cardi* or septal or atrial or ventric*) adj2 (defect? or anomal* or malformation?)).ti,ab,kw. | 49,837 |
| 133 | ((coarctat* adj2 aorta) or (valv* adj2 stenosis) or ‘transdisposition of the great arter*’ or patent ductus arteriosus or ebstein* anomal* or ‘tetralogy of fallot’ or hypoplastic left heart syndrome or tricuspid atresia or truncus arteriosus or anomalous pulmonary venous connection).ti,ab,kw. | 34,089 |
| 134 | Hip Dislocation/ | 6429 |
| 135 | (hip? adj2 (dysplasia? or dislocat*)).ti,ab,kw. | 7649 |
| 136 | exp testicular diseases/ | 39,067 |
| 137 | (((undescend* or retract*) adj2 testic*) or cryptorchid*).ti,ab,kw. | 6394 |
| 138 | exp Hearing Loss/ | 70,334 |
| 139 | ((hearing adj2 (loss or disorder?)) or deaf*).ti,ab,kw. | 80,587 |
| 140 | Cystic Fibrosis/ | 35,889 |
| 141 | cystic fibrosis.ti,ab,kw. | 45,649 |
| 142 | Congenital Hypothyroidism/ | 4531 |
| 143 | congenital hypothyroid*.ti,ab,kw. | 3518 |
| 144 | Biliary Atresia/ | 3167 |
| 145 | biliary atresia.ti,ab,kw. | 4585 |
| 146 | exp Genetic Diseases, Inborn/ or exp ‘Sex Chromosome Disorders of Sex Development’/ | 644,292 |
| 147 | Muscular Dystrophy, Duchenne/ or exp Muscular Atrophy, Spinal/ | 10,773 |
| 148 | (phenylketonuria? or medium chain acyl coa dehydrogenase deficien* or medium chain acylcoa dehydrogenase deficien* or mcadd or maple syrup urine disease? or msud or isovaleric acid?emia? or iso-valeric acid?emia? or glutaric aciduria? or homocystinuria? or amino acid metabolism disorder? or biotinidase deficiency or congenital adrenal hyperplasia or duchenne muscular dystrophy or oxidation disorder? or thrombocytop?enia? or galactos?emia? or kernicterus or dehydrogenase deficiency or lchadd or mucopolysaccharidosis or severe combined immunodeficienc* or spinal muscular atrophy or tyrosin?emia? or adrenoleukodystrophy or ccald or canavan or klinefelter syndrome or 22q11 deletion syndrome or digeorge syndrome).ti,ab. | 102,379 |
| 149 | 127 or 128 or 129 or 130 or 131 or 132 or 133 or 134 or 135 or 136 or 137 or 138 or 139 or 140 or 141 or 142 or 143 or 144 or 145 or 146 or 147 or 148 | 1,466,960 |
| 150 | 109 and 126 and 149 | 21,205 |
| 151 | 104 or 105 or 106 or 150 | 27,708 |
| 152 | 27 or 103 or 151 | 178,276 |
| 153 | Economics/ | 27,280 |
| 154 | exp ‘costs and cost analysis’/ | 241,793 |
| 155 | Economics, Dental/ | 1915 |
| 156 | exp economics, hospital/ | 24,905 |
| 157 | Economics, Medical/ | 9116 |
| 158 | Economics, Nursing/ | 4002 |
| 159 | Economics, Pharmaceutical/ | 2969 |
| 160 | (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. | 840,136 |
| 161 | (expenditure$ not energy).ti,ab. | 31,192 |
| 162 | value for money.ti,ab. | 1799 |
| 163 | budget$.ti,ab. | 30,588 |
| 164 | ‘Value of Life’/ | 5730 |
| 165 | quality-adjusted life years/ | 12,795 |
| 166 | Decision Theory/ | 943 |
| 167 | (financ* or fiscal or funding or fee* or charge* or budget*).ti,ab. | 1,023,207 |
| 168 | (value adj2 (money or monetary)).ti,ab. | 2520 |
| 169 | (‘Value of life’ or ‘quality adjusted life year*’ or qaly* or qald* or qale* or ‘disability adjusted life year*’ or daly).ti,ab. | 19,592 |
| 170 | (short form* or shortform*).ti,ab. | 35,456 |
| 171 | (sf6* or sf-6* or sf8 or sf-8 or sf10 or sf-10 or sf12 or sf-12 or sf16 or sf-16 or sf20 or sf-20 or sf36 or sf-36).ti,ab. | 30,575 |
| 172 | (euroqol or euro qol or ‘euro quality of life’ or euroqual or euro qual or eq5d or eq-5d).ti,ab. | 12,134 |
| 173 | (AQoL* or ‘Assessment of Quality of Life’).ti,ab. | 1986 |
| 174 | (‘16D Health Related Quality of Life’ or 16D HRQoL or ‘17D Health Related Quality of Life’ or 17D HRQoL).ti,ab. | 4 |
| 175 | (‘Child Health Utility 9 Dimension’ or CHU9D or ‘CHU-9D’).ti,ab. | 66 |
| 176 | 15 dimensional instrument.ti,ab. | 7 |
| 177 | (‘quality of wellbeing*’ or ‘quality of well being*’ or qwb).ti,ab. | 486 |
| 178 | (hye or health* year equivalent*).ti,ab. | 64 |
| 179 | (health utilit* or disutilit*).ti,ab. | 2675 |
| 180 | (hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab. | 1600 |
| 181 | (health* adj2 priorities).ti,ab. | 2924 |
| 182 | (Adolescent Health Utility Measure or AHUM).ti,ab. | 3 |
| 183 | (preference* adj3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or instrument or instruments)).ti,ab. | 11,459 |
| 184 | ‘preference based measure of HRQoL’.ti,ab. | 2 |
| 185 | (willingness adj2 pay).ti,ab. | 6075 |
| 186 | standard gamble.ti,ab. | 856 |
| 187 | (time trade off or time tradeoff or tto).ti,ab. | 1929 |
| 188 | (vas or visual analog*).ti,ab. | 83,193 |
| 189 | discrete choice*.ti,ab. | 2241 |
| 190 | (utility elicitation or direct elicitation).ti,ab. | 102 |
| 191 | scoring algorithm.ti,ab. | 712 |
| 192 | best worst scaling.ti,ab. | 237 |
| 193 | (multi attribute utility or multiattribute utility).ti,ab. | 306 |
| 194 | (markov or monte carlo method).ti,ab. | 25,785 |
| 195 | exp Resource Allocation/ | 17,956 |
| 196 | Health Priorities/ | 10,971 |
| 197 | ((multicriteria or multi-criteria) adj2 (decision or analys* or decision aid* or decision making)).ti,ab. | 1429 |
| 198 | (benefit risk asessment or risk benefit assessment).ti,ab. | 734 |
| 199 | weighted product.ti,ab. | 22 |
| 200 | ((analytic* hierarchy or analytic* network) adj process*).ti,ab. | 1181 |
| 201 | (‘measuring attractiveness by a categorical based evaluation technique’ or ‘goal programming’ or ‘elimination and choice expressing reality’ or ELECTRE or ‘preference ranking organization method of enrichment evaluation’ or PROMETHEE or ‘technique for order preference by similarity to ideal solution’ or TOPSIS or ‘measuring attractiveness by a categorical based evaluation technique’ or MACBETH).ti,ab. | 643 |
| 202 | ‘Accountability for reasonableness’.ti,ab. | 131 |
| 203 | (decision* adj (tree* or model* or analysis)).ti,ab. | 16,329 |
| 204 | (resource* adj2 (use* or utilisation or allocat*)).ti,ab. | 37,159 |
| 205 | (ration or rationing).ti,ab. | 11,346 |
| 206 | exp Comparative Effectiveness Research/ | 3673 |
| 207 | Comparative Effectiveness Research.ti,ab. | 1790 |
| 208 | or/153-207 | 2,099,102 |
| 209 | 152 and 208 | 12,338 |
| 210 | ((energy or oxygen) adj cost).ti,ab. | 4210 |
| 211 | (metabolic adj cost).ti,ab. | 1476 |
| 212 | ((energy or oxygen) adj expenditure).ti,ab. | 25,875 |
| 213 | 210 or 211 or 212 | 30,566 |
| 214 | 209 not 213 | 12,335 |
| 215 | (comment or letter or editorial or historical article).pt. | 2,265,941 |
| 216 | 214 not 215 | 12,003 |
| 217 | exp animals/ not humans/ | 4,779,072 |
| 218 | 216 not 217 | 11,821 |
| 219 | limit 218 to yr=‘2000 -Current’ | 8933 |
| 220 | (2020* or 2021*).ed,ez,yr. | 2,333,538 |
| 221 | 219 and 220 | 1125 |
Database EMBASE (OvidSP) 1974 to present
| # | Query | Result |
|---|---|---|
| 1 | exp fetus echography/ | 26,549 |
| 2 | exp prenatal diagnosis/ | 110,715 |
| 3 | (ultrasound* or ultra-sound or ultrasonogra* or ultra-sonogra* or sonogra* or echocardiogra* or nuchal translucen* or amniocentesis or chorionic villus sampl* or cvs or (((noninvasive prenatal or non-invasive prenatal) adj2 (test* or screen*)) or nipt)).ti,ab. | 834,781 |
| 4 | ((fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal or pregnant or pregnancy or trimester?) adj3 (screen* or test* or diagnos* or scan* or structural assessment* or structural survey*)).ti,ab. | 99,268 |
| 5 | screen*.ti. | 239,563 |
| 6 | exp induced abortion/ | 29,297 |
| 7 | ((induced or therap*) adj3 abortion?).ab,kw. or abortion?.ti. | 29,267 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 1,206,080 |
| 9 | exp *congenital malformation/ or *congenital disorder/ | 460,966 |
| 10 | *dysautonomias/ or *Tay Sachs Disease/ | 989 |
| 11 | *spinal muscular atrophy/ | 4401 |
| 12 | (dysautonomia? or tay sachs).ti,ab,kw. | 5531 |
| 13 | (congenital* adj2 (defect? or malformation? or abnormalit* or anomal* or aneuploid*)).ti,ab. | 83,139 |
| 14 | ((fetal or foetal or fetus or foetus) adj2 (defect? or malformation? or abnormalit* or anomal* or aneuploid*)).ti,ab. | 15,887 |
| 15 | ((structural or neural tube?) adj2 (defect? or malformation? or abnormalit* or anomal*)).ti,ab. | 33,831 |
| 16 | ((non-chromosom* or nonchromosom* or chromosom*) adj2 (defect? or malformation? or abnormalit* or anomal*)).ti,ab. | 32,975 |
| 17 | (((down* or patau* or edward*) adj2 syndrome*) or trisomy 13 or trisomy 18 or trisomy 21).ti,ab. | 36,445 |
| 18 | spinal muscular atrophy.ti,ab,kw. | 7501 |
| 19 | or/9-18 | 605,880 |
| 20 | 8 and 19 | 88,834 |
| 21 | exp *congenital disorder/di | 170,139 |
| 22 | Prenatal Care/ or Perinatal Care/ | 54,527 |
| 23 | (fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal or pregnant or pregnancy or trimester?).ti,ab. | 964,183 |
| 24 | 22 or 23 | 974,555 |
| 25 | 21 and 24 | 21,664 |
| 26 | ((fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal) adj (screen* or test* or diagnos*)).ti. | 20,735 |
| 27 | 20 or 25 or 26 | 108,969 |
| 28 | *maternal hypertension/ or exp *pregnancy diabetes mellitus/ or exp *‘eclampsia and preeclampsia’/ | 54,333 |
| 29 | (eclampsia or preeclampsia or pregnancy induced hypertension).ti,ab,kw. | 57,146 |
| 30 | ((gestational or pregnan* or maternal) adj2 diabet*).ti,ab,kw. | 34,209 |
| 31 | *premature labor/ or *fetus death/ or *placenta previa/ or *vasa previa/ | 24,921 |
| 32 | ((preterm or premature) adj2 labo?r).ti,ab,kw. | 15,776 |
| 33 | (f?etal death? or stillbirth? or still birth?).ti,ab,kw. | 27,946 |
| 34 | ((placenta or vasa) adj pr?evia).ti,ab,kw. | 5095 |
| 35 | *hereditary hemolytic anemia/ or exp *sickle cell anemia/ or exp *thalassemia/ | 46,844 |
| 36 | (sickle cell or thalass?emia?).ti,ab,kw. | 59,341 |
| 37 | exp *Syphilis/ | 12,446 |
| 38 | syphilis.ti,ab,kw. | 24,347 |
| 39 | exp *Hepatitis B/ | 59,048 |
| 40 | *Hepatitis B virus/ | 24,060 |
| 41 | (hepatitis b or hbv).ti,ab,kw. or hepatitis.ti. | 242,504 |
| 42 | exp *Human immunodeficiency virus/ | 99,310 |
| 43 | exp *Human immunodeficiency virus infection/ | 261,668 |
| 44 | (hiv or human immunodeficiency virus).ti,ab,kw. | 434,224 |
| 45 | *chlamydia trachomatis/ or *chlamydia/ | 10,817 |
| 46 | chlamydia.ti,ab,kw. | 32,612 |
| 47 | *Cytomegalovirus Infection/ | 15,364 |
| 48 | cytomegalovirus.ti,ab,kw. | 53,800 |
| 49 | *streptococcus infection/ or exp *group b streptococcal infection/ | 13,442 |
| 50 | (group b strep or strep b or (streptococc* adj infection?)).ti,ab,kw. | 5909 |
| 51 | exp *Parvovirus Infection/ | 2617 |
| 52 | parvovirus.ti,ab,kw. | 10,983 |
| 53 | *Rubella virus/ or *rubella/ | 6551 |
| 54 | rubella.ti,ab,kw. | 13,206 |
| 55 | exp *Toxoplasmosis/ | 13,980 |
| 56 | toxoplasmosis.ti,ab,kw. | 15,765 |
| 57 | *Anemia/ | 35,165 |
| 58 | exp *Blood Group Antigen/ | 6299 |
| 59 | exp *Thrombophilia/ | 4525 |
| 60 | thrombophilia?.ti,ab,kw. | 12,487 |
| 61 | an?emia?.ti,ab,kw. | 212,496 |
| 62 | (blood group? or rhd status or rhesus positive or rhesus negative or rhesus status).ti,ab,kw. | 24,849 |
| 63 | exp *Urinary Tract Infection/ | 40,185 |
| 64 | (‘urinary tract infection*’ or ‘urine infection*’ or uti or cystitis or bacteriuria).ti,ab,kw. | 86,304 |
| 65 | *Vaginosis/ | 5104 |
| 66 | vaginosis.ti,ab,kw. | 5890 |
| 67 | *domestic violence/ or *battered woman/ or *exp partner violence/ | 6245 |
| 68 | ((spous* or intimate partner or domestic) adj2 (violence or abuse)).ti,ab,kw. | 17,475 |
| 69 | or/28-68 | 1,407,917 |
| 70 | exp *pregnancy/ or pregnant woman/ | 248,097 |
| 71 | exp *fetus/ | 22,840 |
| 72 | (pregnan$ or f?etal or f?etus or FVS).ti,ab. | 873,457 |
| 73 | prepregnancy care/ or prenatal care/ or perinatal care/ | 56,204 |
| 74 | (pregnan* or preconception* or pre-conception* or antenat* or ante-nat* or antepart* or ante-part* or prenat* or pre-nat* or prepart* or pre-part* or perinatal or maternal or mother*).ti,ab. | 1,082,572 |
| 75 | 70 or 71 or 72 or 73 or 74 | 1,273,058 |
| 76 | Mass Screening/ or screening/ or screening test/ | 302,122 |
| 77 | screen*.ti,ab. | 1,099,879 |
| 78 | Self Report/ | 126,013 |
| 79 | (selfreport* or self-report* or ((oral or tak*) adj3 history)).ti,ab. | 236,603 |
| 80 | exp blood examination/ or diagnostic test/ or serology/ | 412,389 |
| 81 | ((h?ematolog* or blood or serum or serologic*) adj3 (test* or assay*)).ti,ab. | 179,376 |
| 82 | ((sero* adj5 (test* or screen* or diagnos*)) or (serotest* or seroscreen* or serodiagnos*)).ti,ab. | 69,144 |
| 83 | exp *immunoassay/ | 62,192 |
| 84 | exp *Polymerase Chain Reaction/ | 57,101 |
| 85 | (immuno-assay* or immunoassay* or elisa or eia or Fluorescent antibody to membrane antibod* or fama or trfia).ti,ab. | 364,591 |
| 86 | (enzyme linked immunosorbent assay* or elisa or enzyme immunoassay* or eia or recombinant immunoblot assay* or riba).ti,ab. | 362,170 |
| 87 | (polymerase chain reaction or pcr).ti,ab. | 897,453 |
| 88 | (routine adj5 (test* or screen* or diagnos*)).ti,ab. | 74,953 |
| 89 | (test* or diagnos* or assay*).ti. | 1,261,580 |
| 90 | 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 | 4,029,117 |
| 91 | 69 and 75 and 90 | 59,357 |
| 92 | Prenatal diagnosis/ or prenatal screening/ | 65,586 |
| 93 | ((pregnan* or preconception* or pre-conception* or antenat* or ante-nat* or antepart* or ante-part* or prenat* or pre-nat* or prepart* or pre-part* or maternal or mother*) adj5 (screen* or diagnos* or test*)).ti,ab. | 120,372 |
| 94 | 92 or 93 | 147,539 |
| 95 | 69 and 94 | 32,614 |
| 96 | maternal hypertension/di or exp pregnancy diabetes mellitus/di or exp ‘eclampsia and preeclampsia’/di or premature labor/di or fetus death/di or placenta previa/di or vasa previa/di | 11,080 |
| 97 | exp Syphilis/di or exp Hepatitis B/di or exp Human immunodeficiency virus infection/di or chlamydia trachomatis/di or chlamydia/di or Cytomegalovirus Infection/di or streptococcus infection/di or exp group b streptococcal infection/di or exp Parvovirus Infection/di or rubella/di or exp Toxoplasmosis/di | 61,600 |
| 98 | Anemia/di or exp Blood Group Antigen/di or exp Thrombophilia/di | 8922 |
| 99 | exp Urinary Tract Infection/di or Vaginosis/di | 11,271 |
| 100 | 96 or 97 or 98 or 99 | 92,192 |
| 101 | 75 and 100 | 17,938 |
| 102 | 91 or 95 or 101 | 79,289 |
| 103 | Newborn Screening/ | 19,656 |
| 104 | (heelprick* or heel prick*).ti,ab,kw. | 517 |
| 105 | ((neonat* or newborn) adj2 screen*).ti,ab,kw. | 18,452 |
| 106 | Newborn/ | 539,425 |
| 107 | (newborn? or neonat* or infant?).ti,ab,kw. | 791,794 |
| 108 | 106 or 107 | 1,009,940 |
| 109 | exp Physical Examination/ | 256,612 |
| 110 | (physical adj3 exam*).ti,ab,kw. | 132,154 |
| 111 | Mass Screening/ or screening/ or screening test/ | 302,122 |
| 112 | screen*.ti,ab,kw. | 1,114,541 |
| 113 | Genetic screening/ | 90,457 |
| 114 | early diagnosis/ | 110,269 |
| 115 | diagnostic test/ | 79,976 |
| 116 | (routine adj5 (test* or diagnos*)).ti,ab,kw. | 53,921 |
| 117 | Serology/ | 75,357 |
| 118 | serologic.ti,ab,kw. | 33,995 |
| 119 | ((sero* adj5 diagnos*) or (serotest* or seroscreen* or serodiagnos*)).ti,ab,kw. | 26,940 |
| 120 | Dried Blood Spot Testing/ | 4177 |
| 121 | (blood spot* or bloodspot*).ti,ab,kw. | 9565 |
| 122 | exp Hearing Tests/ | 46,681 |
| 123 | ((hearing or auditor* or acoustic* or otoacoustic*) adj3 (test* or diagnos*)).ti,ab,kw. | 16,131 |
| 124 | (automated auditory brainstem response? or aabr or otoacoustic emission? or aoae).ti,ab,kw. | 6533 |
| 125 | 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116 or 117 or 118 or 119 or 120 or 121 or 122 or 123 or 124 | 1,851,177 |
| 126 | *hereditary hemolytic anemia/ or exp *sickle cell anemia/ or exp *thalassemia/ | 46,844 |
| 127 | (sickle cell or thalass?emia?).ti,ab,kw. | 59,341 |
| 128 | *eye diseases/ or *eye diseases, hereditary/ or *cataract/ or *vision disorders/ or exp *blindness/ | 55,094 |
| 129 | (cataract? or blind* or ((eye? or vision?) adj2 (disease? or disorder?))).ti,ab,kw. | 518,908 |
| 130 | *congenital heart disease/ or exp *congenital heart malformation/ | 93,263 |
| 131 | ((heart or cardi* or septal or atrial or ventric*) adj2 (defect? or anomal* or malformation?)).ti,ab,kw. | 67,027 |
| 132 | ((coarctat* adj2 aorta) or (valv* adj2 stenosis) or ‘transdisposition of the great arter*’ or patent ductus arteriosus or ebstein* anomal* or ‘tetralogy of fallot’ or hypoplastic left heart syndrome or tricuspid atresia or truncus arteriosus or anomalous pulmonary venous connection).ti,ab,kw. | 46,272 |
| 133 | *hip dysplasia/ | 3762 |
| 134 | (hip? adj2 (dysplasia? or dislocat*)).ti,ab,kw. | 8501 |
| 135 | *cryptorchism/ | 6210 |
| 136 | (((undescend* or retract*) adj2 testic*) or cryptorchid*).ti,ab,kw. | 8073 |
| 137 | hearing impairment/ or exp congenital deafness/ | 62,953 |
| 138 | ((hearing adj2 (loss or disorder?)) or deaf*).ti,ab,kw. | 94,074 |
| 139 | *Cystic Fibrosis/ | 48,582 |
| 140 | cystic fibrosis.ti,ab,kw. | 69,317 |
| 141 | *Congenital Hypothyroidism/ | 3789 |
| 142 | congenital hypothyroid*.ti,ab,kw. | 4927 |
| 143 | *bile duct atresia/ | 3927 |
| 144 | biliary atresia.ti,ab,kw. | 6796 |
| 145 | *congenital disorder/ or *DiGeorge syndrome/ or *canavan disease/ or *leukodystrophy/ or *‘disorder of sex development’/ or *congenital adrenal hyperplasia/ or *klinefelter syndrome/ or *enzyme deficiency/ or *biotinidase deficiency/ or *medium chain acyl coenzyme a dehydrogenase deficiency/ or *multiple acyl coa dehydrogenase deficiency/ or exp *‘inborn error of metabolism’/ or *congenital disorder/ or *maple syrup urine disease/ or *aminoaciduria/ or *phenylketonuria/ | 206,199 |
| 146 | *duchenne muscular dystrophy/ or *muscular dystrophy/ | 19,137 |
| 147 | (phenylketonuria? or medium chain acyl coa dehydrogenase deficien* or medium chain acylcoa dehydrogenase deficien* or mcadd or maple syrup urine disease? or msud or isovaleric acid?emia? or iso-valeric acid?emia? or glutaric aciduria? or homocystinuria? or amino acid metabolism disorder? or biotinidase deficiency or congenital adrenal hyperplasia or duchenne muscular dystrophy or oxidation disorder? or thrombocytop?enia? or galactos?emia? or kernicterus or dehydrogenase deficiency or lchadd or mucopolysaccharidosis or severe combined immunodeficienc* or spinal muscular atrophy or tyrosin?emia? or adrenoleukodystrophy or ccald or canavan or klinefelter syndrome or 22q11 deletion syndrome or digeorge syndrome).ti,ab. | 152,173 |
| 148 | or/126-147 | 1,249,017 |
| 149 | 108 and 125 and 148 | 27,457 |
| 150 | 103 or 104 or 105 or 149 | 38,434 |
| 151 | 27 or 102 or 150 | 214,131 |
| 152 | Health Economics/ | 33,292 |
| 153 | exp Economic Evaluation/ | 314,254 |
| 154 | exp Health Care Cost/ | 298,571 |
| 155 | pharmacoeconomics/ | 7480 |
| 156 | (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. | 1,098,743 |
| 157 | (expenditure$ not energy).ti,ab. | 42,295 |
| 158 | value for money.ti,ab. | 2489 |
| 159 | budget$.ti,ab. | 40,241 |
| 160 | quality adjusted life year/ | 28,123 |
| 161 | Decision Theory/ | 1753 |
| 162 | (financ* or fiscal or funding or fee* or charge* or budget*).ti,ab. | 1,235,512 |
| 163 | (value adj2 (money or monetary)).ti,ab. | 3409 |
| 164 | (‘Value of life’ or ‘quality adjusted life year*’ or qaly* or qald* or qale* or ‘disability adjusted life year*’ or daly).ti,ab. | 31,384 |
| 165 | (short form* or shortform*).ti,ab. | 48,359 |
| 166 | (sf6* or sf-6* or sf8 or sf-8 or sf10 or sf-10 or sf12 or sf-12 or sf16 or sf-16 or sf20 or sf-20 or sf36 or sf-36).ti,ab. | 50,819 |
| 167 | (euroqol or euro qol or ‘euro quality of life’ or euroqual or euro qual or eq5d or eq-5d).ti,ab. | 22,332 |
| 168 | (AQoL* or ‘Assessment of Quality of Life’).ti,ab. | 3225 |
| 169 | (‘16D Health Related Quality of Life’ or 16D HRQoL or ‘17D Health Related Quality of Life’ or 17D HRQoL).ti,ab. | 4 |
| 170 | (‘Child Health Utility 9 Dimension’ or CHU9D or ‘CHU-9D’).ti,ab. | 90 |
| 171 | 15 dimensional instrument.ti,ab. | 7 |
| 172 | (‘quality of wellbeing*’ or ‘quality of well being*’ or qwb).ti,ab. | 600 |
| 173 | (hye or health* year equivalent*).ti,ab. | 130 |
| 174 | (health utilit* or disutilit*).ti,ab. | 4541 |
| 175 | (hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab. | 2424 |
| 176 | (health* adj2 priorities).ti,ab. | 3389 |
| 177 | (Adolescent Health Utility Measure or AHUM).ti,ab. | 3 |
| 178 | (preference* adj3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or instrument or instruments)).ti,ab. | 15,017 |
| 179 | ‘preference based measure of HRQoL’.ti,ab. | 3 |
| 180 | (willingness adj2 pay).ti,ab. | 9455 |
| 181 | standard gamble.ti,ab. | 1109 |
| 182 | (time trade off or time tradeoff or tto).ti,ab. | 2856 |
| 183 | (vas or visual analog*).ti,ab. | 126,460 |
| 184 | discrete choice*.ti,ab. | 3208 |
| 185 | (utility elicitation or direct elicitation).ti,ab. | 165 |
| 186 | scoring algorithm.ti,ab. | 1241 |
| 187 | best worst scaling.ti,ab. | 348 |
| 188 | (multi attribute utility or multiattribute utility).ti,ab. | 401 |
| 189 | (markov or monte carlo method).ti,ab. | 32,415 |
| 190 | Resource Allocation/ | 21,579 |
| 191 | Health Priorities/ | 92,152 |
| 192 | ((multicriteria or multi-criteria) adj2 (decision or analys* or decision aid* or decision making)).ti,ab. | 1881 |
| 193 | (benefit risk asessment or risk benefit assessment).ti,ab. | 1009 |
| 194 | weighted product.ti,ab. | 21 |
| 195 | ((analytic* hierarchy or analytic* network) adj process*).ti,ab. | 1628 |
| 196 | (‘measuring attractiveness by a categorical based evaluation technique’ or ‘goal programming’ or ‘elimination and choice expressing reality’ or ELECTRE or ‘preference ranking organisation method of enrichment evaluation’ or PROMETHEE or ‘technique for order preference by similarity to ideal solution’ or TOPSIS or ‘measuring attractiveness by a categorical based evaluation technique’ or MACBETH).ti,ab. | 808 |
| 197 | ‘Accountability for reasonableness’.ti,ab. | 146 |
| 198 | (decision* adj (tree* or model* or analysis)).ti,ab. | 23,197 |
| 199 | (resource* adj2 (use* or utilisation or allocat*)).ti,ab. | 50,101 |
| 200 | (ration or rationing).ti,ab. | 13,511 |
| 201 | Comparative Effectiveness/ | 96,471 |
| 202 | Comparative Effectiveness Research.ti,ab. | 2494 |
| 203 | or/152-202 | 2,846,296 |
| 204 | 151 and 203 | 18,388 |
| 205 | ((energy or oxygen) adj cost).ti,ab. | 4479 |
| 206 | (metabolic adj cost).ti,ab. | 1583 |
| 207 | ((energy or oxygen) adj expenditure).ti,ab. | 32,825 |
| 208 | 205 or 206 or 207 | 37,761 |
| 209 | 204 not 208 | 18,383 |
| 210 | (editorial or letter or note).pt. | 2,677,181 |
| 211 | 209 not 210 | 17,760 |
| 212 | (exp animals/ or nonhuman/) not human/ | 6,670,870 |
| 213 | 211 not 212 | 17,491 |
| 214 | limit 213 to yr=‘2000 -Current’ | 15,132 |
| 215 | (2020* or 2021*).dc,dd,yr. | 2,435,504 |
| 216 | 214 and 215 | 1572 |
| Query | Result |
|---|---|
| (preconcept* or pre-concept* or fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal or maternal or pregnant or pregnancy or prepregnancy or trimester* or neonat* or newborn*) AND ((screen* or test* or diagnos* or scan* or structural assessment* or structural survey*) OR (Heelprick or heel prick or dried blood spot test* or dried blood spot diagnos*)) – limited to 2000–21 | 777 |
| # | Query | Result |
|---|---|---|
| S1 | ((preconcept* or pre-concept* or fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or pregnant or pregnancy or prepregnancy or trimester* or neonat* or newborn*) NEAR/5 (screen* or test* or diagnos* or scan* or ‘structural assessment*’ or ‘structural survey*’)) | 106 |
| S2 | Heelprick OR ‘heel prick’ OR ‘dried blood spot test*’ OR ‘dried blood spot diagnos*’ | 0 |
| S3 | S1 AND S2 | 106 |
| S4 | S1 and S2 – limited to 2000 onwards | 94 |
| # | Query | Result |
|---|---|---|
| 1 | TS=(((preconcept* or pre-concept* or fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal or pregnant or pregnancy or prepregnancy or trimester* or neonat* or newborn*) NEAR/5 (screen* or test* or diagnos* or scan* or ‘structural assessment*’ or ‘structural survey*’))) OR TS=(Heelprick OR ‘heel prick’ OR ‘dried blood spot test*’ OR ‘dried blood spot diagnos*’) | 118,575 |
| 2 | TS=(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic*) OR TS=(financ* or fiscal or funding or fee* or charge* or budget*) OR TS=(‘Value of life’ or ‘quality adjusted life year*’ or qaly* or qald* or qale* or ‘disability adjusted life year*’ or daly) OR TS=(‘health utilit*’ or disutilit*) | 5,191,685 |
| 3 | #2 AND #1 | 8016 |
| 4 | (#2 AND #1) AND LANGUAGE: (English) | 6999 |
| 5 | (#2 AND #1) AND LANGUAGE: (English) Refined by: [excluding] DOCUMENT TYPES: (EDITORIAL MATERIAL OR REVIEW OR LETTER OR NEWS ITEM OR BOOK CHAPTER) AND PUBLICATION YEARS: (2021 OR 2009 OR 2020 OR 2008 OR 2019 OR 2007 OR 2018 OR 2006 OR 2017 OR 2005 OR 2016 OR 2004 OR 2015 OR 2003 OR 2014 OR 2002 OR 2013 OR 2001 OR 2012 OR 2000 OR 2011 OR 2010) | 6019 |
| # | Query |
|---|---|
| S1 | (MH ‘Prenatal Diagnosis+’) |
| S2 | TI ((ultrasound* or ultra-sound or ultrasonogra* or ultra-sonogra* or sonogra* or echocardiogra* or nuchal translucen* or amniocentesis or chorionic villus sampl* or cvs or (((noninvasive prenatal or non-invasive prenatal) N2 (test* or screen*)) or nipt))) OR AB ((ultrasound* or ultra-sound or ultrasonogra* or ultra-sonogra* or sonogra* or echocardiogra* or nuchal translucen* or amniocentesis or chorionic villus sampl* or cvs or (((noninvasive prenatal or non-invasive prenatal) N2 (test* or screen*)) or nipt))) |
| S3 | TI (((fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal or pregnant or pregnancy or trimester?) N3 (screen* or test* or diagnos* or scan* or structural assessment* or structural survey*))) OR AB (((fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal or pregnant or pregnancy or trimester?) N3 (screen* or test* or diagnos* or scan* or structural assessment* or structural survey*))) |
| S4 | TI screen* |
| S5 | (MH ‘Abortion, Induced’) |
| S6 | AB (((induced or therap*) N3 abortion?)) OR TI abortion* |
| S7 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 |
| S8 | (MH ‘Congenital, Hereditary, and Neonatal Diseases and Abnormalities+’) |
| S9 | TI ((dysautonomia? or tay sachs)) OR AB ((dysautonomia? or tay sachs)) |
| S10 | TI ((congenital* N2 (defect? or malformation? or abnormalit* or anomal* or aneuploid*)).ti,ab.) OR AB ((congenital* N2 (defect? or malformation? or abnormalit* or anomal* or aneuploid*)).ti,ab.) OR TI (((fetal or foetal or fetus or foetus) N2 (defect? or malformation? or abnormalit* or anomal* or aneuploid*))) OR AB (((fetal or foetal or fetus or foetus) N2 (defect? or malformation? or abnormalit* or anomal* or aneuploid*))) OR TI (((structural or neural tube?) N2 (defect? or malformation? or abnormalit* or anomal*))) OR AB (((structural or neural tube?) N2 (defect? or malformation? or abnormalit* or anomal*))) OR TI (((non-chromosom* or nonchromosom* or chromosom*) N2 (defect? or malformation? or abnormalit* or anomal*))) OR AB (((non-chromosom* or nonchromosom* or chromosom*) N2 (defect? or malformation? or abnormalit* or anomal*))) OR TI ((((down* or patau* or edward*) N2 syndrome*) or trisomy 13 or trisomy 18 or trisomy 21)) OR AB ((((down* or patau* or edward*) N2 syndrome*) or trisomy 13 or trisomy 18 or trisomy 21)) OR TI spinal muscular atrophy OR AB spinal muscular atrophy |
| S11 | S8 OR S9 OR S10 |
| S12 | S7 AND S11 |
| S13 | (MH ‘Congenital, Hereditary, and Neonatal Diseases and Abnormalities+/DI/US’) |
| S14 | (MH ‘Prenatal Care’) or (MH ‘Perinatal Care’) |
| S15 | TI ((fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal or pregnant or pregnancy or trimester?)) OR AB ((fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal or pregnant or pregnancy or trimester?)) |
| S16 | S14 OR S15 |
| S17 | S13 AND S16 |
| S18 | TI ((fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal) N1(screen* or test* or diagnos*)) |
| S19 | S12 OR S17 OR S18 |
| S20 | (MH ‘Diabetes Mellitus, Gestational’) OR (MH ‘Pregnancy-Induced Hypertension+’) |
| S21 | TI ((eclampsia or preeclampsia or pregnancy induced hypertension)) OR AB ((eclampsia or preeclampsia or pregnancy induced hypertension)) OR TI (((gestational or pregnan* or maternal) N2 diabet*)) OR AB (((gestational or pregnan* or maternal) N2 diabet*)) |
| S22 | (MH ‘Labor, Premature’) OR (MH ‘Placenta Praevia’) OR (MH ‘Childbirth, Premature’) OR (MH ‘Perinatal Death’) |
| S23 | TI (((preterm or premature) N2 labo?r)) OR AB (((preterm or premature) N2 labo?r)) OR TI ((f?etal death? or stillbirth? or still birth?)) OR AB ((f?etal death? or stillbirth? or still birth?)) OR TI (((placenta or vasa) adj pr?evia)) OR AB (((placenta or vasa) adj pr?evia)) |
| S24 | (MH ‘Hemoglobinopathies+’) |
| S25 | TI ((sickle cell or thalass?emia?)) OR AB ((sickle cell or thalass?emia?)) |
| S26 | (MH ‘Syphilis+’) OR (MH ‘Chlamydia Infections+’) OR (MH ‘Human Immunodeficiency Virus+’) OR (MH ‘HIV Infections+’) OR (MH ‘Cytomegalovirus Infections+’) OR (MH ‘Hepatitis B+’) |
| S27 | TI syphilis OR AB syphilis OR AB (‘hepatitis b’ or hbv) OR TI hepatitis OR TI (hiv OR ‘human immunodeficiency virus’) OR AB (hiv OR ‘human immunodeficiency virus’) OR TI chlamydia OR AB chlamydia OR TI cytomegalovirus OR AB cytomegalovirus |
| S28 | (MH ‘Streptococcal Infections+’) OR (MH ‘Rubella’) OR (MH ‘Toxoplasmosis+’) |
| S29 | TI ((group b strep or strep b or (streptococc* N1 infection?))) OR AB ((group b strep or strep b or (streptococc* N1 infection?))) OR TI parovirus OR AB parovirus OR TI rubella OR AB rubella OR TI toxoplasmosis OR AB toxoplasmosis |
| S30 | (MH ‘Anemia’) |
| S31 | TI thrombophilia? OR AB thrombophilia? OR TI an?emia? OR AB an?emia? OR TI ((blood group? or rhd status or rhesus positive or rhesus negative or rhesus status)) OR AB ((blood group? or rhd status or rhesus positive or rhesus negative or rhesus status)) |
| S32 | (MH ‘Urinary Tract Infections+’) OR (MH ‘Vaginosis, Bacterial’) |
| S33 | TI ((‘urinary tract infection*’ or ‘urine infection*’ or uti or cystitis or bacteriuria)) OR AB ((‘urinary tract infection*’ or ‘urine infection*’ or uti or cystitis or bacteriuria)) OR TI vaginosis OR AB vaginosis |
| S34 | (MH ‘Domestic Violence’) |
| S35 | TI (((spous* or intimate partner or domestic) N2 (violence or abuse))) OR AB (((spous* or intimate partner or domestic) N2 (violence or abuse))) |
| S36 | S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 |
| S37 | (MH ‘Pregnancy+’) OR (MH ‘Expectant Mothers’) OR (MH ‘Fetus+’) OR (MH ‘Prepregnancy Care’) OR (MH ‘Prenatal Care’) or (MH ‘Perinatal Care’) |
| S38 | TI ((pregnan$ or f?etal or f?etus or FVS)) OR AB ((pregnan$ or f?etal or f?etus or FVS)) OR TI ((pregnan* or preconception* or pre-conception* or antenat* or ante-nat* or antepart* or ante-part* or prenat* or pre-nat* or prepart* or pre-part* or perinatal or maternal or mother*)) OR AB ((pregnan* or preconception* or pre-conception* or antenat* or ante-nat* or antepart* or ante-part* or prenat* or pre-nat* or prepart* or pre-part* or perinatal or maternal or mother*)) |
| S39 | S37 OR S38 |
| S40 | (MH ‘Health Screening’) OR (MH ‘Population Surveillance’) |
| S41 | TI screen* OR AB screen* OR TI ((selfreport* or self-report* or ((oral or tak*) N3 history))) OR AB ((selfreport* or self-report* or ((oral or tak*) N3 history))) |
| S42 | (MH ‘Hematologic Tests+’) OR (MH ‘Diagnostic Tests, Routine’) OR (MH ‘Serologic Tests+’) OR (MH ‘Polymerase Chain Reaction+’) |
| S43 | TI (((h?ematolog* or blood or serum or serologic*) 3 (test* or assay*))) OR AB (((h?ematolog* or blood or serum or serologic*) 3 (test* or assay*))) OR TI (((sero* N5 (test* or screen* or diagnos*)) or (serotest* or seroscreen* or serodiagnos*))) OR AB (((sero* N5 (test* or screen* or diagnos*)) or (serotest* or seroscreen* or serodiagnos*))) OR TI ((immuno-assay* or immunoassay* or elisa or eia or Fluorescent antibody to membrane antibod* or fama or trfia)) OR AB ((immuno-assay* or immunoassay* or elisa or eia or Fluorescent antibody to membrane antibod* or fama or trfia)) OR TI ((enzyme linked immunosorbent assay* or elisa or enzyme immunoassay* or eia or recombinant immunoblot assay* or riba)) OR AB ((enzyme linked immunosorbent assay* or elisa or enzyme immunoassay* or eia or recombinant immunoblot assay* or riba)) OR TI ((polymerase chain reaction or pcr)) OR AB ((polymerase chain reaction or pcr)) OR AB ((routine N5 (test* or screen* or diagnos*))) OR TI ((test* or diagnos* or assay*)) |
| S44 | S40 OR S41 OR S42 OR S43 |
| S45 | S36 AND S39 AND S44 |
| S46 | (MH ‘Prenatal Diagnosis’) |
| S47 | TI (((pregnan* or preconception* or pre-conception* or antenat* or ante-nat* or antepart* or ante-part* or prenat* or pre-nat* or prepart* or pre-part* or perinatal or maternal or mother*) N5 (screen* or diagnos* or test*))) OR AB (((pregnan* or preconception* or pre-conception* or antenat* or ante-nat* or antepart* or ante-part* or prenat* or pre-nat* or prepart* or pre-part* or perinatal or maternal or mother*) N5 (screen* or diagnos* or test*))) |
| S48 | S46 OR S47 |
| S49 | S36 AND S48 |
| S50 | S45 OR S49 |
| S51 | (MH ‘Neonatal Assessment+’) |
| S52 | TI ((heelprick* or heel prick*)) OR AB ((heelprick* or heel prick*)) OR TI (((neonat* or newborn) N2 screen*)) OR AB (((neonat* or newborn) N2 screen*)) |
| S53 | (MH ‘Infant, Newborn+’) |
| S54 | TI ((newborn? or neonat* or infant?)) OR AB ((newborn? or neonat* or infant?)) |
| S55 | S53 OR S54 |
| S56 | (MH ‘Physical Examination’) OR (MH ‘Health Screening’) OR (MH ‘Serologic Tests’) OR (MH ‘Diagnostic Tests, Routine’) OR (MH ‘Genetic Screening’) |
| S57 | TI (physical N3 exam*) OR AB (physical N3 exam*) OR TI screen* OR AB screen* OR TI ((routine N5 (test* or diagnos*))) OR AB ((routine N5 (test* or diagnos*))) OR TI (((sero* N5 diagnos*) or (serotest* or seroscreen* or serodiagnos*))) OR AB (((sero* N5 diagnos*) or (serotest* or seroscreen* or serodiagnos*))) OR TI ((blood spot* or bloodspot*)) OR AB ((blood spot* or bloodspot*)) |
| S58 | (MH ‘Hearing Tests+’) |
| S59 | TI (((hearing or auditor* or acoustic* or otoacoustic*) N3 (test* or diagnos*))) OR AB (((hearing or auditor* or acoustic* or otoacoustic*) N3 (test* or diagnos*))) OR TI ((automated auditory brainstem response? or aabr or otoacoustic emission? or aoae)) OR AB ((automated auditory brainstem response? or aabr or otoacoustic emission? or aoae)) |
| S60 | S56 OR S57 OR S58 OR S59 |
| S61 | (MH ‘Hemoglobinopathies+’) |
| S62 | TI ((sickle cell or thalass?emia?)) OR AB ((sickle cell or thalass?emia?)) |
| S63 | (MH ‘Eye Diseases, Hereditary’) OR (MH ‘Cataract’) |
| S64 | TI ((cataract? or blind* or ((eye? or vision?) N2 (disease? or disorder?)))) OR AB ((cataract? or blind* or ((eye? or vision?) N2 (disease? or disorder?)))) |
| S65 | (MH ‘Heart Defects, Congenital+’) |
| S66 | TI (((heart or cardi* or septal or atrial or ventric*) N2 (defect? or anomal* or malformation?))) OR AB (((heart or cardi* or septal or atrial or ventric*) N2 (defect? or anomal* or malformation?))) OR TI (((coarctat* N2 aorta) or (valv* N2 stenosis) or ‘transdisposition of the great arter*’ or patent ductus arteriosus or ebstein* anomal* or ‘tetralogy of fallot’ or hypoplastic left heart syndrome or tricuspid atresia or truncus arteriosus or anomalous pulmonary venous connection)) OR AB (((coarctat* N2 aorta) or (valv* N2 stenosis) or ‘transdisposition of the great arter*’ or patent ductus arteriosus or ebstein* anomal* or ‘tetralogy of fallot’ or hypoplastic left heart syndrome or tricuspid atresia or truncus arteriosus or anomalous pulmonary venous connection)) |
| S67 | (MH ‘Hip Dislocation, Congenital’) |
| S68 | TI ((hip? N2 (dysplasia? or dislocat*))) OR AB ((hip? N2 (dysplasia? or dislocat*))) |
| S69 | (MH ‘Cryptorchidism’) |
| S70 | TI ((((undescend* or retract*) N2 testic*) or cryptorchid*)) OR AB ((((undescend* or retract*) N2 testic*) or cryptorchid*)) |
| S71 | (MH ‘Hearing Disorders+’) |
| S72 | TI (((hearing N2 (loss or disorder?)) or deaf*)) OR AB (((hearing N2 (loss or disorder?)) or deaf*)) |
| S73 | (MH ‘Cystic Fibrosis’) OR (MH ‘Congenital Hypothyroidism’) OR (MH ‘Metabolism, Inborn Errors+’) OR (MH ‘Biliary Atresia’) OR (MH ‘Muscular Dystrophy, Duchenne+’) OR (MH ‘Sex Chromosome Disorders of Sex Development+’) |
| S74 | TI cystic fibrosis OR AB cystic fibrosis OR TI congenital hypothyroid* OR AB congenital hypothyroid* OR TI biliary atresia OR AB biliary atresia OR TI ((phenylketonuria? or medium chain acyl coa dehydrogenase deficien* or medium chain acylcoa dehydrogenase deficien* or mcadd or maple syrup urine disease? or msud or isovaleric acid?emia? or iso-valeric acid?emia? or glutaric aciduria? or homocystinuria? or amino acid metabolism disorder? or biotinidase deficiency or congenital adrenal hyperplasia or duchenne muscular dystrophy or oxidation disorder? or thrombocytop?enia? or galactos?emia? or kernicterus or dehydrogenase deficiency or lchadd or mucopolysaccharidosis or severe combined immunodeficienc* or spinal muscular atrophy or tyrosin?emia? or adrenoleukodystrophy or ccald or canavan or klinefelter syndrome or 22q11 deletion syndrome or digeorge syndrome)) OR AB ((phenylketonuria? or medium chain acyl coa dehydrogenase deficien* or medium chain acylcoa dehydrogenase deficien* or mcadd or maple syrup urine disease? or msud or isovaleric acid?emia? or iso-valeric acid?emia? or glutaric aciduria? or homocystinuria? or amino acid metabolism disorder? or biotinidase deficiency or congenital adrenal hyperplasia or duchenne muscular dystrophy or oxidation disorder? or thrombocytop?enia? or galactos?emia? or kernicterus or dehydrogenase deficiency or lchadd or mucopolysaccharidosis or severe combined immunodeficienc* or spinal muscular atrophy or tyrosin?emia? or adrenoleukodystrophy or ccald or canavan or klinefelter syndrome or 22q11 deletion syndrome or digeorge syndrome)) |
| S75 | S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 |
| S76 | S55 AND S60 AND S75 |
| S77 | S51 OR S52 OR S76 |
| S78 | S19 OR S50 OR S77 |
| S79 | (MH ‘Economic Value of Life’) OR (MH ‘Resource Allocation+’) |
| S80 | (MH ‘Quality-Adjusted Life Years’) |
| S81 | TI ((economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$)) OR AB ((economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$)) OR TI ((expenditure$ not energy)) OR AB ((expenditure$ not energy)) OR TI ‘value for money’ OR AB ‘value for money’ OR TI ((financ* or fiscal or funding or fee* or charge* or budget*)) OR AB ((financ* or fiscal or funding or fee* or charge* or budget*)) OR TI ((value N2 (money or monetary))) OR AB ((value N2 (money or monetary))) OR TI ((‘Value of life’ or ‘quality adjusted life year*’ or qaly* or qald* or qale* or ‘disability adjusted life year*’ or daly)) OR AB ((‘Value of life’ or ‘quality adjusted life year*’ or qaly* or qald* or qale* or ‘disability adjusted life year*’ or daly)) |
| S82 | TI ((short form* or shortform*)) OR AB ((short form* or shortform*)) OR TI ((sf6* or sf-6* or sf8 or sf-8 or sf10 or sf-10 or sf12 or sf-12 or sf16 or sf-16 or sf20 or sf-20 or sf36 or sf-36)) OR AB ((sf6* or sf-6* or sf8 or sf-8 or sf10 or sf-10 or sf12 or sf-12 or sf16 or sf-16 or sf20 or sf-20 or sf36 or sf-36)) OR TI ((euroqol or euro qol or ‘euro quality of life’ or euroqual or euro qual or eq5d or eq-5d)) OR AB ((euroqol or euro qol or ‘euro quality of life’ or euroqual or euro qual or eq5d or eq-5d)) OR TI ((AQoL* or ‘Assessment of Quality of Life’)) OR AB ((AQoL* or ‘Assessment of Quality of Life’)) OR TI ((‘16D Health Related Quality of Life’ or 16D HRQoL or ‘17D Health Related Quality of Life’ or 17D HRQoL)) OR AB ((‘16D Health Related Quality of Life’ or 16D HRQoL or ‘17D Health Related Quality of Life’ or 17D HRQoL)) OR TI ((‘Child Health Utility 9 Dimension’ or CHU9D or ‘CHU-9D’ or ‘15 dimensional instrument’)) OR AB w,((‘Child Health Utility 9 Dimension’ or CHU9D or ‘CHU-9D’ or ‘15 dimensional instrument’)) |
| S83 | ((‘quality of wellbeing*’ or ‘quality of well being*’ or qwb)) OR ((‘quality of wellbeing*’ or ‘quality of well being*’ or qwb)) OR ((hye or health* year equivalent*)) OR ((hye or health* year equivalent*)) OR ((health utilit* or disutilit*)) OR VI ((health utilit* or disutilit*)) OR ((hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3)) OR ((hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3)) OR (health* N2 priorities).ti,ab. OR (health* N2 priorities).ti,ab. OR ((Adolescent Health Utility Measure or AHUM)) OR VI ((Adolescent Health Utility Measure or AHUM)) |
| S84 | TI ((preference* N3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or instrument or instruments))) OR AB ((preference* N3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or instrument or instruments))) OR TI (‘preference based measure of HRQoL’ or (willingness N2 pay) or standard gamble or ‘time trade off’ or ‘time tradeoff’ or tto or vas ir visual analog or discrete choice or utility elicitation or direct elicitation or scoring algorithm or best worst scaling or ‘multi attribute utility’ or ‘multiattribute utility’ or markov or monte carlo) OR AB (‘preference based measure of HRQoL’ or (willingness N2 pay) or standard gamble or ‘time trade off’ or ‘time tradeoff’ or tto or vas ir visual analog or discrete choice or utility elicitation or direct elicitation or scoring algorithm or best worst scaling or ‘multi attribute utility’ or ‘multiattribute utility’ or markov or monte carlo) OR TI (((multicriteria or multi-criteria) N2 (decision or analys* or decision aid* or decision making))) OR AB (((multicriteria or multi-criteria) N2 (decision or analys* or decision aid* or decision making))) OR TI ((benefit risk asessment or risk benefit assessment or weighted product)) OR AB ((benefit risk asessment or risk benefit assessment or weighted product)) OR TI (((analytic* hierarchy or analytic* network) N1 process*)) OR AB (((analytic* hierarchy or analytic* network) N1 process*)) OR TI ((‘measuring attractiveness by a categorical based evaluation technique’ or ‘goal programming’ or ‘elimination and choice expressing reality’ or ELECTRE or ‘preference ranking organisation method of enrichment evaluation’ or PROMETHEE or ‘technique for order preference by similarity to ideal solution’ or TOPSIS or ‘measuring attractiveness by a categorical based evaluation technique’ or MACBETH)) OR AB ((‘measuring attractiveness by a categorical based evaluation technique’ or ‘goal programming’ or ‘elimination and choice expressing reality’ or ELECTRE or ‘preference ranking organization method of enrichment evaluation’ or PROMETHEE or ‘technique for order preference by similarity to ideal solution’ or TOPSIS or ‘measuring attractiveness by a categorical based evaluation technique’ or MACBETH)) |
| S85 | TI ‘Accountability for reasonableness’ OR AB ‘Accountability for reasonableness’ OR TI ((decision* N1 (tree* or model* or analysis))) OR AB ((decision* N1 (tree* or model* or analysis))) OR TI ((resource* N2 (use* or utilisation or allocat*))) OR AB ((resource* N2 (use* or utilisation or allocat*))) OR TI ((ration or rationing)) OR AB ((ration or rationing)) OR TI Comparative Effectiveness Research OR AB Comparative Effectiveness Research |
| S86 | S79 OR S80 OR S81 OR S82 OR S83 OR S84 OR S85 |
| S87 | S78 AND S86 |
| S88 | TI (((energy or oxygen) N1 cost)) OR AB (((energy or oxygen) N1 cost)) OR TI (metabolic N1 cost) OR AB (metabolic N1 cost) OR TI (((energy or oxygen) N1 expenditure)) OR AB (((energy or oxygen) N1 expenditure)) |
| S89 | S87 NOT S88 |
| S90 | S87 NOT S88 Limiters – Publication Type: Anecdote, Book Review, Commentary, Editorial, Historical Material, Interview, Letter, Response, Teaching Materials |
| S91 | S89 NOT S90 Limiters – Published Date: 20000101–20211231 |
| # | Query | Result |
|---|---|---|
| 1 | exp Prenatal diagnosis/ | 710 |
| 2 | (ultrasound* or ultra-sound or ultrasonogra* or ultra-sonogra* or sonogra* or echocardiogra* or nuchal translucen* or amniocentesis or chorionic villus sampl* or cvs or (((noninvasive prenatal or non-invasive prenatal) adj2 (test* or screen*)) or nipt)).ti,ab. | 6786 |
| 3 | ((fetal or foetal or fetus or foetus or prenat* or pre-nat* or prepart* or pre-part* or antenatal or ante-natal or perinatal or pregnant or pregnancy or trimester?) adj3 (screen* or test* or diagnos* or scan* or structural assessment* or structural survey*)).ti,ab. | 4089 |
| 4 | screen*.ti. | 20,929 |
| 5 | Induced Abortion/ | 2692 |
| 6 | ((induced or therap*) adj3 abortion?).ab. or abortion?.ti. | 2551 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 34,112 |
| 8 | exp Neonatal Disorders/ or exp Congenital Disorders/ | 15,798 |
| 9 | [(dysautonomia? or tay sachs).ti,ab,kw.] | 0 |
| 10 | (congenital* adj2 (defect? or malformation? or abnormalit* or anomal* or aneuploid*)).ti,ab. | 1728 |
| 11 | ((fetal or foetal or fetus or foetus) adj2 (defect? or malformation? or abnormalit* or anomal* or aneuploid*)).ti,ab. | 409 |
| 12 | ((structural or neural tube?) adj2 (defect? or malformation? or abnormalit* or anomal*)).ti,ab. | 2597 |
| 13 | ((non-chromosom* or nonchromosom* or chromosom*) adj2 (defect? or malformation? or abnormalit* or anomal*)).ti,ab. | 698 |
| 14 | (((down* or patau* or edward*) adj2 syndrome*) or trisomy 13 or trisomy 18 or trisomy 21).ti,ab. | 7671 |
| 15 | spinal muscular atrophy.ti,ab. | 522 |
| 16 | 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 22,001 |
| 17 | 7 and 16 | 1195 |
| 18 | exp obstetrical complications/ | 1587 |
| 19 | (eclampsia or preeclampsia or pregnancy induced hypertension).ti,ab. | 641 |
| 20 | ((gestational or pregnan* or maternal) adj2 diabet*).ti,ab. | 765 |
| 21 | Premature Birth/ | 5735 |
| 22 | ((preterm or premature) adj2 labo?r).ti,ab. | 271 |
| 23 | (f?etal death? or stillbirth? or still birth?).ti,ab. | 905 |
| 24 | ((placenta or vasa) adj pr?evia).ti,ab. | 26 |
| 25 | exp obstetrical complications/ | 1587 |
| 26 | (sickle cell or thalass?emia?).ti,ab. | 1666 |
| 27 | exp Sexually Transmitted Diseases/ | 46,974 |
| 28 | syphilis.ti,ab. | 1674 |
| 29 | (hepatitis b or hbv).ti,ab. or hepatitis.ti. | 2795 |
| 30 | (hiv or human immunodeficiency virus).ti,ab. | 53,140 |
| 31 | chlamydia.ti,ab. | 922 |
| 32 | cytomegalovirus.ti,ab. | 515 |
| 33 | (group b strep or strep b or (streptococc* adj infection?)).ti,ab. | 254 |
| 34 | parovirus.ti,ab. | 1 |
| 35 | viral disorders/ or rubella/ | 2700 |
| 36 | rubella.ti,ab. | 424 |
| 37 | toxoplasmosis.ti,ab. | 244 |
| 38 | thrombophilia?.ti,ab. | 72 |
| 39 | an?emia?.ti,ab. | 2033 |
| 40 | (blood group? or rhd status or rhesus positive or rhesus negative or rhesus status).ti,ab. | 209 |
| 41 | (‘urinary tract infection*’ or ‘urine infection*’ or uti or cystitis or bacteriuria).ti,ab. | 869 |
| 42 | vaginosis.ti,ab. | 80 |
| 43 | domestic violence/ or intimate partner violence/ | 21,067 |
| 44 | ((spous* or intimate partner or domestic) adj2 (violence or abuse)).ti,ab. | 18,740 |
| 45 | or/18-44 | 103,937 |
| 46 | exp pregnancy/ | 43,025 |
| 47 | exp fetus/ | 2134 |
| 48 | (pregnan$ or f?etal or f?etus or FVS).ti,ab. | 55,568 |
| 49 | prenatal care/ | 1914 |
| 50 | (pregnan* or preconception* or pre-conception* or antenat* or ante-nat* or antepart* or ante-part* or prenat* or pre-nat* or prepart* or pre-part* or perinatal or maternal or mother*).ti,ab. | 192,754 |
| 51 | 46 or 47 or 48 or 49 or 50 | 207,329 |
| 52 | screening/ or exp health screening/ or exp screening tests/ | 29,936 |
| 53 | screen*.ti,ab. | 100,420 |
| 54 | Self Report/ | 18,358 |
| 55 | (selfreport* or self-report* or ((oral or tak*) adj3 history)).ti,ab. | 130,100 |
| 56 | ((h?ematolog* or blood or serum or serologic*) adj3 (test* or assay*)).ti,ab. | 4809 |
| 57 | ((sero* adj5 (test* or screen* or diagnos*)) or (serotest* or seroscreen* or serodiagnos*)).ti,ab. | 1493 |
| 58 | (immuno-assay* or immunoassay* or elisa or eia or Fluorescent antibody to membrane antibod* or fama or trfia).ti,ab. | 4582 |
| 59 | (enzyme linked immunosorbent assay* or elisa or enzyme immunoassay* or eia or recombinant immunoblot assay* or riba).ti,ab. | 4746 |
| 60 | (polymerase chain reaction or pcr).ti,ab. | 10,291 |
| 61 | (routine adj5 (test* or screen* or diagnos*)).ti,ab. | 3748 |
| 62 | (test* or diagnos* or assay*).ti. | 148,890 |
| 63 | or/52-62 | 385,529 |
| 64 | 45 and 51 and 63 | 2487 |
| 65 | Prenatal diagnosis/ | 710 |
| 66 | ((pregnan* or preconception* or pre-conception* or antenat* or ante-nat* or antepart* or ante-part* or prenat* or pre-nat* or prepart* or pre-part* or perinatal or maternal or mother*) adj5 (screen* or diagnos* or test*)).ti,ab. | 9424 |
| 67 | 65 or 66 | 9593 |
| 68 | 45 and 67 | 1259 |
| 69 | 64 or 68 | 2966 |
| 70 | (heelprick* or heel prick*).ti,ab. | 33 |
| 71 | ((neonat* or newborn) adj2 screen*).ti,ab. | 781 |
| 72 | newborn/ | 0 |
| 73 | (newborn? or neonat* or infant?).ti,ab. | 90,167 |
| 74 | 72 or 73 | 90,167 |
| 75 | (physical adj3 exam*).ti,ab. | 7333 |
| 76 | screening/ or exp health screening/ or exp screening tests/ | 29,936 |
| 77 | screen*.ti,ab. | 100,420 |
| 78 | Genetic testing/ | 1820 |
| 79 | (routine adj5 (test* or diagnos*)).ti,ab. | 1888 |
| 80 | serologic.ti,ab. | 340 |
| 81 | ((sero* adj5 diagnos*) or (serotest* or seroscreen* or serodiagnos*)).ti,ab. | 330 |
| 82 | (blood spot* or bloodspot*).ti,ab. | 279 |
| 83 | ((hearing or auditor* or acoustic* or otoacoustic*) adj3 (test* or diagnos*)).ti,ab. | 5708 |
| 84 | (automated auditory brainstem response? or aabr or otoacoustic emission? or aoae).ti,ab. | 785 |
| 85 | or/75-84 | 122,164 |
| 86 | sickle cell disease/ | 1097 |
| 87 | (sickle cell or thalass?emia?).ti,ab. | 1666 |
| 88 | (cataract? or blind* or ((eye? or vision?) adj2 (disease? or disorder?))).ti,ab. | 56,607 |
| 89 | ((heart or cardi* or septal or atrial or ventric*) adj2 (defect? or anomal* or malformation?)).ti,ab. | 665 |
| 90 | ((coarctat* adj2 aorta) or (valv* adj2 stenosis) or ‘transdisposition of the great arter*’ or patent ductus arteriosus or ebstein* anomal* or ‘tetralogy of fallot’ or hypoplastic left heart syndrome or tricuspid atresia or truncus arteriosus or anomalous pulmonary venous connection).ti,ab. | 172 |
| 91 | (hip? adj2 (dysplasia? or dislocat*)).ti,ab. | 57 |
| 92 | (((undescend* or retract*) adj2 testic*) or cryptorchid*).ti,ab. | 75 |
| 93 | ((hearing adj2 (loss or disorder?)) or deaf*).ti,ab. | 23,914 |
| 94 | cystic fibrosis.ti,ab. | 1216 |
| 95 | congenital hypothyroid*.ti,ab. | 138 |
| 96 | biliary atresia.ti,ab. | 19 |
| 97 | (phenylketonuria? or medium chain acyl coa dehydrogenase deficien* or medium chain acylcoa dehydrogenase deficien* or mcadd or maple syrup urine disease? or msud or isovaleric acid?emia? or iso-valeric acid?emia? or glutaric aciduria? or homocystinuria? or amino acid metabolism disorder? or biotinidase deficiency or congenital adrenal hyperplasia or duchenne muscular dystrophy or oxidation disorder? or thrombocytop?enia? or galactos?emia? or kernicterus or dehydrogenase deficiency or lchadd or mucopolysaccharidosis or severe combined immunodeficienc* or spinal muscular atrophy or tyrosin?emia? or adrenoleukodystrophy or ccald or canavan or klinefelter syndrome or 22q11 deletion syndrome or digeorge syndrome).ti,ab. | 3299 |
| 98 | or/86-97 | 86,003 |
| 99 | 74 and 85 and 98 | 663 |
| 100 | 70 or 71 or 99 | 1061 |
| 101 | 17 or 69 or 100 | 5071 |
| 102 | economics/ or health care economics/ or pharmacoeconomics/ | 24,376 |
| 103 | exp ‘costs and cost analysis’/ | 43,479 |
| 104 | resource allocation/ | 3391 |
| 105 | (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. | 220,424 |
| 106 | (expenditure$ not energy).ti,ab. | 8321 |
| 107 | value for money.ti,ab. | 526 |
| 108 | budget$.ti,ab. | 8938 |
| 109 | (financ* or fiscal or funding or fee* or charge* or budget*).ti,ab. | 346,980 |
| 110 | (value adj2 (money or monetary)).ti,ab. | 986 |
| 111 | (‘Value of life’ or ‘quality adjusted life year*’ or qaly* or qald* or qale* or ‘disability adjusted life year*’ or daly).ti,ab. | 2617 |
| 112 | (short form* or shortform*).ti,ab. | 14,029 |
| 113 | (sf6* or sf-6* or sf8 or sf-8 or sf10 or sf-10 or sf12 or sf-12 or sf16 or sf-16 or sf20 or sf-20 or sf36 or sf-36).ti,ab. | 6173 |
| 114 | (euroqol or euro qol or ‘euro quality of life’ or euroqual or euro qual or eq5d or eq-5d).ti,ab. | 2355 |
| 115 | (AQoL* or ‘Assessment of Quality of Life’).ti,ab. | 586 |
| 116 | (‘16D Health Related Quality of Life’ or 16D HRQoL or ‘17D Health Related Quality of Life’ or 17D HRQoL).ti,ab. | 1 |
| 117 | (‘Child Health Utility 9 Dimension’ or CHU9D or ‘CHU-9D’).ti,ab. | 31 |
| 118 | 15 dimensional instrument.ti,ab. | 1 |
| 119 | (‘quality of wellbeing*’ or ‘quality of well being*’ or qwb).ti,ab. | 296 |
| 120 | (hye or health* year equivalent*).ti,ab. | 30 |
| 121 | (health utilit* or disutilit*).ti,ab. | 765 |
| 122 | (hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab. | 598 |
| 123 | (health* adj2 priorities).ti,ab. | 644 |
| 124 | (Adolescent Health Utility Measure or AHUM).ti,ab. | 1 |
| 125 | (preference* adj3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or instrument or instruments)).ti,ab. | 7890 |
| 126 | ‘preference based measure of HRQoL’.ti,ab. | 0 |
| 127 | (willingness adj2 pay).ti,ab. | 2028 |
| 128 | standard gamble.ti,ab. | 219 |
| 129 | (time trade off or time tradeoff or tto).ti,ab. | 409 |
| 130 | (vas or visual analog*).ti,ab. | 8086 |
| 131 | discrete choice*.ti,ab. | 1079 |
| 132 | (utility elicitation or direct elicitation).ti,ab. | 45 |
| 133 | scoring algorithm.ti,ab. | 228 |
| 134 | best worst scaling.ti,ab. | 125 |
| 135 | (multi attribute utility or multiattribute utility).ti,ab. | 262 |
| 136 | (markov or monte carlo method).ti,ab. | 3931 |
| 137 | ((multicriteria or multi-criteria) adj2 (decision or analys* or decision aid* or decision making)).ti,ab. | 466 |
| 138 | (benefit risk asessment or risk benefit assessment).ti,ab. | 105 |
| 139 | weighted product.ti,ab. | 8 |
| 140 | ((analytic* hierarchy or analytic* network) adj process*).ti,ab. | 514 |
| 141 | (‘measuring attractiveness by a categorical based evaluation technique’ or ‘goal programming’ or ‘elimination and choice expressing reality’ or ELECTRE or ‘preference ranking organization method of enrichment evaluation’ or PROMETHEE or ‘technique for order preference by similarity to ideal solution’ or TOPSIS or ‘measuring attractiveness by a categorical based evaluation technique’ or MACBETH).ti,ab. | 369 |
| 142 | ‘Accountability for reasonableness’.ti,ab. | 29 |
| 143 | (decision* adj (tree* or model* or analysis)).ti,ab. | 3717 |
| 144 | (resource* adj2 (use* or utilisation or allocat*)).ti,ab. | 12,294 |
| 145 | (ration or rationing).ti,ab. | 1031 |
| 146 | Comparative Effectiveness Research.ti,ab. | 248 |
| 147 | or/102-104 | 67,563 |
| 148 | 101 and 147 | 82 |
| 149 | ((energy or oxygen) adj cost).ti,ab. | 278 |
| 150 | (metabolic adj cost).ti,ab. | 99 |
| 151 | ((energy or oxygen) adj expenditure).ti,ab. | 2628 |
| 152 | 149 or 150 or 151 | 2925 |
| 153 | 148 not 152 | 82 |
| 154 | (comment reply or editorial or letter or ‘review book’ or ‘review media’ or ‘review software other’).dt. | 317,639 |
| 155 | 153 not 154 | 78 |
| 156 | limit 155 to yr=‘2000-Current’ | 70 |
Appendix 3 Fields in the data extraction form – Consolidated Health EconomicEvaluation Reporting Standards checklist
| Item no. | Section | Data field | Data field description |
|---|---|---|---|
| 1 | General | Completed by | State the name initials of person who has filled out the data extraction sheet. |
| 2 | General | Literature type | Published or grey literature. |
| 3 | General | First author | State first author’s last name. |
| 4 | General | Year | State year of publication. |
| 5 | General | Publication type | Describe type of publication (e.g. journal article, HTA report, conference abstract, book chapters, thesis). |
| 6 | Title | Title | Identify the study as an economic evaluation or use more specific terms such as ‘cost-effectiveness analysis’, and describe the interventions compared. |
| 7 | Abstract | Abstract | Indicate if article provided a structured summary of objectives, perspective, setting (geographical or organisational), methods (including study design and inputs), results (including base-case and uncertainty analyses) and conclusions. |
| 8 | Introduction | Research question/objective | Specify the main research question(s) or objective(s). |
| 9 | Methods | Country/jurisdiction | State country/jurisdiction (or region if available) that the study was conducted in. |
| 10 | Methods | Setting of screening | Describe setting of screening (e.g. inpatient, outpatient, home, community). |
| 11 | Methods | Population | Indicate if population is healthy pregnancy, pregnancy at risk, pregnant women and their partner/relative, healthy infant and/or infant at risk (state gestational week if available). |
| 12 | Methods | Condition(s) screened | Specify condition(s) screened using specified test(s). |
| 13 | Methods | Study type | State if study is a type A, B, or C evaluation:
|
| 14 | Methods | Size of study population | If type A evaluation, specify the sample size. If type B or C evaluation, specify the number of people in the starting cohort. |
| 15 | Methods | Design | If type A evaluation, state design of underpinning study (e.g. randomised controlled trial, retrospective cohort, and prospective cohort). If type C evaluation, state design of underpinning model. If type B evaluation, (1) state the type of underpinning model (e.g. decision-analytic model, transition state probability model, time-dependent multistage transition probability model, Markov model); and (2) indicate whether they have stated the reasons. |
| 16 | Methods | Type of economic evaluation | State type of economic evaluation (e.g. cost–utility analysis, cost-effectiveness analysis, cost–benefit analysis, cost-minimisation analysis, cost-consequences analysis). |
| 17 | Methods | Perspective | State perspective of study (e.g. health care, payer, societal) in base-case analysis. |
| 18 | Methods | Comparators |
|
| 19 | Methods | Time horizon |
|
| 20 | Methods | Discount rate for costs |
|
| 21 | Methods | Discount rate for outcome |
|
| 22 | Methods | Choice of outcomes | Indicate whether the article expressed outcomes in natural units and/or adjusted in utility weights. |
| 23 | Methods | Measurement: Approaches for measuring outcomes (benefits and harms) | Describe approaches for measuring outcomes (benefits and harms). |
| 24 | Methods | Measurement and valuation of preference-based outcomes | If applicable, describe the population and methods used to elicit preferences for outcomes. If applicable, describe the preference-based technique used to value benefits and harms. |
| 25 | Methods | Reporting of preference-based outcomes in cost-utility analysis | If applicable, state if preference-based outcome was reported for mother (maternal) and/or infant. |
| 26 | Methods | Estimating resources and costs | If type A/C evaluation, indicate whether the article described sources and approaches used to estimate resource use and valuing each resource item in terms of its unit cost for all interventions. If type B evaluation, indicate whether the article described sources and approaches used to estimate resource use and valuing each resource item in terms of its unit cost for model health states. |
| 27 | Methods | Currency, reference year | State currency and reference year of currency. |
| 28 | Methods | Conversion | Describe methods for converting costs into a common currency base and the exchange rate or purchasing power parity if applicable. |
| 29 | Methods | Model assumptions | Describe all structural or other assumptions underpinning the decision-analytical model. |
| 30 | Methods | Analytical methods | Describe all analytical methods supporting the evaluation. If type A/C evaluation, indicate whether the article reported the methods and results of regression models that disentangle differences in costs, outcomes, or cost effectiveness that can be explained by variations between subgroups of patients. If type B evaluation, indicate whether the article described and reported how they estimated parameters (e.g. how they transformed transition probabilities between events or health states into functions of age or disease severity); the handling of uncertainty and separation of heterogeneity from uncertainty; and time-dependent input parameters (e.g. in relation to uptake of prenatal diagnosis and termination of pregnancy) where applicable. |
| 31 | Results | Study parameters | Indicate if article reported the (1) values, ranges, references and, if used, probability distributions for all parameters; (2) reasons or sources for distributions used to represent uncertainty where appropriate; and (3) a table to show the input values (strongly recommended). |
| 32 | Results | Incremental costs and outcomes |
|
| 33 | Results | Characterising uncertainty | If type A/C evaluation, indicate whether the article described the effects of sampling uncertainty for the estimated incremental cost and incremental effectiveness parameters, together with the impact of methodological assumptions (such as discount rate, study perspective). If type B evaluation, indicate whether the article described the effects on the results of uncertainty for all input parameters, and uncertainty related to the structure of the model and assumptions. |
| 34 | Results | Characterising heterogeneity | If applicable, indicate whether the article reported differences in costs, outcomes, or cost effectiveness that can be explained by variations between subgroups of patients with different baseline characteristics or other observed variability in effects that are not reducible by more information. |
| 35 | Discussion | Study findings, limitations, generalisability and current knowledge | Indicate whether the article summarised key study findings and describe how they support the conclusions reached; as well as discussed limitations and the generalisability of the findings and how the findings fit with current knowledge. |
| 36 | Discussion | Recommendation | Indicate if the authors made any policy recommendation based on their cost-effective evidence. |
| 37 | General | Source of funding | Describe how the study was funded and the role of the funder in the identification, design, conduct and reporting of the analysis. Describe other non-monetary sources of support. |
| 38 | General | Affiliations | Indicate if affiliation is industry, non-industry, or both. |
| 39 | General | Conflicts of interest | Indicate any potential for conflict of interest of study contributors in accordance with journal policy. In the absence of a journal policy, we recommend authors comply with International Committee of Medical Journal Editors recommendations. |
| 40 | General | Potential conflicts of interest | Based on ‘Source of funding’, ‘Conflicts of interest’ and ‘Affiliations’, is there potential vested interest in promoting screening/particular screening mechanism? For a ‘yes’, two conditions need to be met: Condition 1: a study is funded by an industry sponsor unless it is an unrestricted grant AND Condition 2: at least one of the authors is clearly employed by the industry sponsor. |
| 41 | General | Industry-sponsored | Based on ‘Source of funding’, is the study sponsored by industry? |
| 42 | Methods/Results | PPI in economic evaluation | Was there any PPI? If yes, describe the role of PPI in the study. |
| 43 | General | JIF quartile | JIF quartile during year that the article was published (sources: Clarivate and SCImago) |
Appendix 4 Fields in the data extraction form – bespoke form
| Item no. | Section | Data field | Data field description |
|---|---|---|---|
| 0 | – | Completed by | Completed by |
| 0.1 | – | First author | First author |
| 0.2 | – | Year | Year |
| 0.3 | – | Publication type | Publication type |
| 1 | – | Screening population | What was the eligible screening population? |
| 2 | – | Stage of disease pathway | What stage of the disease pathway, as defined by Raffle and Gray,1 was the screening test administered?
|
| 3 | – | Phase(s) of screening programme | What phases of a screening programme were included in the health economic assessment/model?
|
| 4 | – | Structure of model reported | Was the structure of the model/decision tree reported? |
| 5 | – | True positive | Did the authors include consequences for true positives in the screening/diagnostic phase? If yes, answer 5.1, 5.2 and 5.3. |
| 5.1 | – | True positive – benefit | Were benefits included in the assessment? If yes, answer 5.1.1. |
| 5.1.1 | – | True positive – benefit, specify | What was/were the benefit(s) and how was it included? |
| 5.2 | – | True positive – harm | Were harms evaluated in the assessment (e.g. adverse events of the test)? If yes, answer 5.2.1. |
| 5.2.1 | – | True positive – harm, specify | What was/were the harm(s) and how was it included? |
| 5.3 | – | True positive – inconsequential disease | Were outcomes for true positives in the case of inconsequential disease included? Inconsequential disease refers to those who ‘feel exactly the same as the true positives, are managed in the same way, and are indistinguishable from them because all have to be offered treatment. It is not possible to distinguish those with inconsequential conditions from those whose condition would progress’, as defined by Raffle and Gray.
1
If yes, answer 5.3.1. |
| 5.3.1 | – | True positive – inconsequential disease, specify | What were the benefits and/or harms and how were they included? |
| 6 | – | True negative | Did the authors include consequences for true negatives in the screening/diagnostic phase that did not mirror the benefits/harms for the true positives? If yes, answer 6.1 and 6.2. |
| 6.1 | – | True negative – benefit | Were benefits included in the assessment? If yes, answer 6.1.1. |
| 6.1.1 | – | True negative – benefit, specify | What was/were the benefit(s) and how was it included? |
| 6.2 | – | True negative – harm | Were harms evaluated in the assessment (e.g. adverse events of the test)? If yes, answer 6.2.1. |
| 6.2.1 | – | True negative – harm, specify | What was/were the harm(s) and how was it included? |
| 7 | – | False positive | Did the authors include consequences for false positives in the screening/diagnostic phase that did not mirror the benefits/harms for the true positives? If yes, answer 7.1. |
| 7.1 | – | False positive – harm, specify | What harms were included (e.g. additional tests, increase anxiety) and how was it included? |
| 8 | – | False negative | Did the authors include consequences for false negatives in the screening/diagnostic phase that did not mirror the benefits/harms for the true positives? If yes, answer 8.1 and 8.2. |
| 8.1 | – | False negative – harm, specify | What harms were included and how was it included? |
| 8.2 | – | False negative – inconsequential disease | Were outcomes for false negative in the case of inconsequential disease included? Inconsequential disease refers to those who ‘are better off not being picked up on screening because they avoid the psychological and physical consequences from being part of the overdiagnosis problem, involving pointless investigation and treatment for a condition that never would have caused any illness’ as defined by Raffle and Gray.
1
If yes, answer 8.2.1. |
| 8.2.1 | – | False negative – inconsequential disease, specify | What were the benefits and/or harms and how were they included? |
| 9 | – | Treatment | Did the authors include consequences associated with the treatment? If yes, answer 9.1. |
| 9.1 | – | Treatment – specify | What benefits/harms were included and how was it included? |
| 10 | – | Notes of interest | Notes mentioned by the authors that might be of interest but not covered by the questions in the ancillary form. |
Appendix 5 Summary of reporting quality of articles and reports (excluding conference abstracts) assessed using Consolidated Health EconomicEvaluation Reporting Standards checklist
| CHEERS item no. | CHEERS item | Articles and reports assessing antenatal screening (%) | Articles and reports assessing newborn screening (%) | ||||
|---|---|---|---|---|---|---|---|
| Satisfied | Not satisfied | Not applicable | Satisfied | Not satisfied | Not applicable | ||
| Title and abstract | |||||||
| 1 | Title | 155 (85.6) | 26 (14.4) | 0 (0) | 70 (86.4) | 11 (13.6) | 0 (0) |
| 2 | Abstract | 21 (11.6) | 160 (88.4) | 0 (0) | 13 (15.7) | 69 (83.1) | 1 (1.2) |
| Introduction | |||||||
| 3 | Background and objectives | 181 (100) | 0 (0) | 0 (0) | 83 (100) | 0 (0) | 0 (0) |
| Methods | |||||||
| 4 | Target population and subgroups | 181 (100) | 0 (0) | 0 (0) | 83 (100) | 0 (0) | 0 (0) |
| 5 | Setting and location | 65 (35.9) | 116 (64.1) | 0 (0) | 30 (36.1) | 53 (63.9) | 0 (0) |
| 6 | Study perspective | 134 (74) | 47 (26) | 0 (0) | 67 (80.7) | 15 (18.1) | 1 (1.2) |
| 7 | Comparators | 159 (87.8) | 22 (12.2) | 0 (0) | 75 (90.4) | 8 (9.6) | 0 (0) |
| 8 | Time horizon | 28 (15.5) | 153 (84.5) | 0 (0) | 15 (18.1) | 67 (80.7) | 1 (1.2) |
| 9 | Discount rate | 51 (28.2) | 130 (71.8) | 0 (0) | 29 (34.9) | 53 (63.9) | 1 (1.2) |
| 10 | Choice of health outcomes | 181 (100) | 0 (0) | 0 (0) | 82 (98.8) | 0 (0) | 1 (1.2) |
| 11 | Measurement of effectiveness | 178 (98.3) | 3 (1.7) | 0 (0) | 82 (98.8) | 1 (1.2) | 0 (0) |
| 12 | Measurement and valuation of preference-based outcomes | 48 (26.5) | 34 (18.8) | 99 (54.7) | 19 (22.9) | 13 (15.7) | 51 (61.4) |
| 13 | Estimate resources and cost | 168 (92.8) | 13 (7.2) | 0 (0) | 79 (95.2) | 3 (3.6) | 1 (1.2) |
| 14 | Currency, price date, and conversion | 139 (76.8) | 42 (23.2) | 0 (0) | 64 (77.1) | 18 (21.7) | 1 (1.2) |
| 15 | Choice of model | 28 (15.5) | 153 (84.5) | 0 (0) | 28 (33.7) | 55 (66.3) | 0 (0) |
| 16 | Assumptions | 131 (72.4) | 20 (11) | 30 (16.6) | 60 (72.3) | 6 (7.2) | 17 (20.5) |
| 17 | Analytic method | 116 (64.1) | 65 (35.9) | 0 (0) | 63 (75.9) | 20 (24.1) | 0 (0) |
| Results | |||||||
| 18 | Study parameters | 155 (85.6) | 26 (14.4) | 0 (0) | 73 (88) | 9 (10.8) | 1 (1.2) |
| 19 | Incremental costs and outcomes | 168 (92.8) | 13 (7.2) | 0 (0) | 75 (90.4) | 7 (8.4) | 1 (1.2) |
| 20 | Characterising uncertainty | 161 (89) | 20 (11) | 0 (0) | 69 (83.1) | 13 (15.7) | 1 (1.2) |
| 21 | Characterising heterogeneity | 24 (13.3) | 3 (1.7) | 154 (85.1) | 4 (4.8) | 0 (0) | 79 (95.2) |
| Discussion | |||||||
| 22 | Study funding, limitation, generalisability and current knowledge | 58 (32.0) | 123 (68.0) | 0 (0) | 23 (27.7) | 59 (71.1) | 1 (1.2) |
| Other | |||||||
| 23 | Source of funding | 100 (55.2) | 81 (44.8) | 0 (0) | 55 (66.3) | 28 (33.7) | 0 (0) |
| 24 | Conflict of interest | 107 (59.1) | 74 (40.9) | 0 (0) | 51 (61.4) | 32 (38.6) | 0 (0) |
Appendix 6 Results of thematic framework analysis
| Theme no. | Theme | Subtheme level 1 | Subtheme level 2 | Subtheme level 3 | Subtheme level 4 | Antenatal screening | Newborn screening |
|---|---|---|---|---|---|---|---|
| 1 | Diagnosis of screened for condition | Additional screening of partners | 113 | – | |||
| 1 | Diagnosis of screened for condition | Additional testing to reach diagnosis in the absence of screening (links to diagnostic odyssey) | 114–116 | 117 | |||
| 1 | Diagnosis of screened for condition | Born with condition | Reduction in children born with condition through effective treatment of the screened for condition | 118–139 | 140,141 | ||
| 1 | Diagnosis of screened for condition | Born with condition | Reduction in children born with condition through termination of pregnancy | 142–168 | – | ||
| 1 | Diagnosis of screened for condition | Cases diagnosed at screening | 5,116,142–144,146,151,157,158,168–189 | 117,190–204 | |||
| 1 | Diagnosis of screened for condition | Cases diagnosed at screening rather than later symptomatically | 150,164,166,205–207 | 208–215 | |||
| 1 | Diagnosis of screened for condition | Cases diagnosed at screening that would have become symptomatic | – | 216 | |||
| 1 | Diagnosis of screened for condition | Cases diagnosed at screening | Maternal | 145 | – | ||
| 1 | Diagnosis of screened for condition | Cases missed at screening | 126,162,171,176,177,187,188,217,218 | 219,220 | |||
| 1 | Diagnosis of screened for condition | Cases missed at screening | Legal cost of reimbursing false negatives | – | 221 | ||
| 1 | Diagnosis of screened for condition | Confirmatory test and additional tests to reach diagnosis of screened for condition | 142–144,164,169,222–226 | 117,192,194,227–229 | |||
| 1 | Diagnosis of screened for condition | Confirmatory test and additional tests to reach diagnosis of screened for condition (invasive) | 115,162 | – | |||
| 1 | Diagnosis of screened for condition | Confirmatory test and additional tests to reach diagnosis of screened for condition | Early vs. late | – | 194 | ||
| 1 | Diagnosis of screened for condition | Confirmatory test and additional tests to reach diagnosis of screened for condition | Unnecessary due to false positive | 5,113,116,118,120–124,128–130,133–135,137–139,145,148,150,151,153,158,162,163,166,170,171,174,176,181,182,184,205–207,230–254 | 141,190,191,193,195–204,208,210–214,216,219,221,229,255–284 | ||
| 1 | Diagnosis of screened for condition | Confirmatory test and additional tests to reach diagnosis of screened for condition | Unnecessary due to false positive (invasive) | 115,152,157,159,160,165,172,173,175,177,178,180,183,185,187,188,217,285–289 | – | ||
| 1 | Diagnosis of screened for condition | Confirmatory test and additional tests to reach diagnosis of screened for condition | Unnecessary due to false positive | Time spent attending | – | 190 | |
| 1 | Diagnosis of screened for condition | Screened for condition-related complications | Morbidity | – | 197 | ||
| 2 | Life-years and health status adjustments | Infant life-years post birth | DALYs | 137,242 | 229,290 | ||
| 2 | Life-years and health status adjustments | Infant life-years post birth | Morbidity | 133,233,291,292 | 203 | ||
| 2 | Life-years and health status adjustments | Infant life-years post birth | Mortality | 121,132,133,139,147,189,238,254,291,293,294 | 196,203,280 | ||
| 2 | Life-years and health status adjustments | Infant life-years post birth | QALYs | 121,133,135,139,147,225,230–232,239,243,248,295–309 | 140,202,203,219,229,256,257,260–262,264,267,271,272,276,282,310–313 | ||
| 2 | Life-years and health status adjustments | Infant life-years post birth | Replacement of affected aborted fetus with subsequent unaffected pregnancy and life | 167 | – | ||
| 2 | Life-years and health status adjustments | Infant life-years post birth | Years | 120,123,125,134,145,163,233,246,247,249,293,296,314,315 | 117,140,141,194,203,208,221,228,257,258,260,263,265,266,270,273–278,281,283,284,313,316,317 | ||
| 2 | Life-years and health status adjustments | Infant life-years post birth | Years | Monetary value | – | 318 | |
| 2 | Life-years and health status adjustments | Maternal life-years | Mortality | 128,133 | 319 | ||
| 2 | Life-years and health status adjustments | Maternal life-years | QALYs | 5,135,147,154–156,172,179,181,189,218,223–226,230,233,235,239–241,244,245,247,250,252–254,285,286,288,292,294,295,300,302,306,320–332 | 227,229,269,276,279,280,318 | ||
| 2 | Life-years and health status adjustments | Maternal life-years | QALYs | Decrement due to caring for child with screened for condition | 333 | – | |
| 2 | Life-years and health status adjustments | Maternal life-years | Years | 120,145,163,233,246,247,249,314,315 | 140,141,203,228,258,260,263,265,270,273–276,281,283,284,313 | ||
| 2 | Life-years and health status adjustments | Parental QALYs | – | 208,221,255,268 | |||
| 2 | Life-years and health status adjustments | Psychological | Anxiety from genetic variants of unclear penetrance | – | 227 | ||
| 2 | Life-years and health status adjustments | Psychological | Disutility due to knowledge of disease | 244 | – | ||
| 2 | Life-years and health status adjustments | Psychological | Disutility due to knowledge of disease in those with positive screening results (stress and anxiety) | 181 | – | ||
| 2 | Life-years and health status adjustments | Psychological | Early diagnosis-induced anxiety | – | 227 | ||
| 2 | Life-years and health status adjustments | Psychological | False-positive anxiety | 183 | 198,257 | ||
| 2 | Life-years and health status adjustments | Psychological | False-positive anxiety | Parental QALYs | – | 221 | |
| 2 | Life-years and health status adjustments | Psychological | Genetic variants of unclear penetrance | Unclear harms including behavioural changes | QALYs | – | 227 |
| 2 | Life-years and health status adjustments | Screened for condition associated mortality/treatment associated mortality/other causes mortality | – | 317 | |||
| 3 | Treatment | Additional health care post diagnosis | 119,291 | 215,227,256 | |||
| 3 | Treatment | Comparison of earlier treatment after screen detection and later after symptomatic detection | Increase in treatment required for false negative | 320,333 | 199,228 | ||
| 3 | Treatment | Comparison of earlier treatment after screen detection and later after symptomatic detection | Increase in treatment required for false negative | Prior to diagnosis | – | 271 | |
| 3 | Treatment | Comparison of earlier treatment after screen detection and later after symptomatic detection | Reduction in treatment required | 114,334 | – | ||
| 3 | Treatment | Comparison of earlier treatment after screen detection and later after symptomatic detection | Reduction in treatment required | Costs | 128,129,181,235,248,296,308,322,325,335–337 | 194,203,221,228,258,259,265,269,276,277,281,283 | |
| 3 | Treatment | Comparison of earlier treatment after screen detection | Adverse complications of screened for condition averted | 113,127–129,170,174,226,236,238,242,247,251,306,307,315,320,322,327,329,331,337–339 | 140,212,216,274,280,340 | ||
| 3 | Treatment | Hospital stay | 237,301,341 | 264,278 | |||
| 3 | Treatment | Missed due to false negative | 130 | – | |||
| 3 | Treatment | Prevention of screened for condition (infectious) | Increase in future earning potential | 341 | – | ||
| 3 | Treatment | Prevention of screened for condition (infectious) | Unnecessary prophylaxis in false positives | 127,130,131,133,135,138,232,248,304,309,320,334,337,341 | – | ||
| 3 | Treatment | Prevention of screened for condition (infectious) | Unnecessary prophylaxis in false positives | Allergic reaction | 330 | – | |
| 3 | Treatment | Psychological | Counselling about genetic diagnosis | 142,157 | – | ||
| 3 | Treatment | Psychological | Counselling about screening or confirmatory test | False positive | 135,165,166,183,304 | – | |
| 3 | Treatment | Psychological | Counselling about screening or confirmatory test | 113,157 | – | ||
| 3 | Treatment | Screened for condition-related treatment/management | 113,137,142,169,181,218,224–226,234,306,342 | 194,228,229,284,317 | |||
| 3 | Treatment | Screened for condition-related treatment/management | Unnecessary in false positives with no confirmatory test | 119–123,126,130,136,139,145,163,181,230,235,238–240,244,247,250,291,292,296,299–301,303,307,308,314,315,323–325,328,331,333,339 | 210,215,221,258,261,274,278,282,311,312,318,343 | ||
| 3 | Treatment | Treatment-related harm | Adverse reaction to treatment | 131,133,239,245,249,251,309,322,330,333 | 263,266,270,274,278,281,344 | ||
| 3 | Treatment | Treatment-related harm | Antibiotic resistance | 119 | – | ||
| 3 | Treatment | Treatment-related harm | Disutility of treatment | 247 | – | ||
| 3 | Treatment | Treatment-related harm | Treatment-related anxiety | – | 276 | ||
| 3 | Treatment | Unnecessary due to false positive | 134,233,305,306 | – | |||
| 4 | Long-term cost associated with screened for condition | Cost savings from averted births of fetuses with anomalies | 114 | – | |||
| 4 | Long-term cost associated with screened for condition | Direct healthcare and non-healthcare cost | Health care and productivity gains | 167 | – | ||
| 4 | Long-term cost associated with screened for condition | Direct healthcare and non-healthcare cost | Health care and social services | – | 279 | ||
| 4 | Long-term cost associated with screened for condition | Direct healthcare and non-healthcare cost | Health care, education and social services | – | 227,262 | ||
| 4 | Long-term cost associated with screened for condition | Direct healthcare and non-healthcare cost | Social services | 139 | – | ||
| 4 | Long-term cost associated with screened for condition | Direct healthcare and non-healthcare cost | Treatment and caregiving | 163 | – | ||
| 4 | Long-term cost associated with screened for condition | Direct healthcare cost | 120,122,123,125,135,153,159,161,167,217,222,233,238,243,246,253,293,294,296,305,308,309,314,324,328,337,341,342 | 141,208,209,212,221,256,260,261,264,265,274,275,345,346 | |||
| 4 | Long-term cost associated with screened for condition | Direct non-healthcare cost | 145 | – | |||
| 4 | Long-term cost associated with screened for condition | Direct non-healthcare cost | Caregiving | 144,232,287,347 | 343 | ||
| 4 | Long-term cost associated with screened for condition | Direct non-healthcare cost | Child protective services investigation and foster care placements if mothers successfully completed substance abuse treatment | 293 | – | ||
| 4 | Long-term cost associated with screened for condition | Direct non-healthcare cost | Education and social services | – | 194 | ||
| 4 | Long-term cost associated with screened for condition | Direct non-healthcare cost | Social care | – | 265 | ||
| 4 | Long-term cost associated with screened for condition | Productivity gains | 129,324 | 311 | |||
| 4 | Long-term cost associated with screened for condition | Societal cost | 116,172,184,241,285,288 | 210,255,311 | |||
| 5 | Overdiagnosis | QALY decrement | 239 | 276 | |||
| 5 | Overdiagnosis | Unnecessary test/treatment | 236,239 | 221,262,276 | |||
| 6 | Pregnancy loss | Spontaneous | 116,119,143,144,147,162,166,186,237,252,333 | – | |||
| 6 | Pregnancy loss | Termination | 115,147,178,189,332 | – | |||
| 6 | Pregnancy loss | Termination | Date/trimester | 157,172,285 | – | ||
| 6 | Pregnancy loss | Termination | Of unaffected fetus due to false-positive test result | 5,146–148,150,151,160,165,175,176,179,183,186 | 141,340 | ||
| 6 | Pregnancy loss | Termination | Of unaffected fetus due to false-positive test result | Psychological consequences | 178 | – | |
| 6 | Pregnancy loss | Termination | Prevent downstream adverse maternal outcomes | 289 | – | ||
| 6 | Pregnancy loss | Termination | Prevent later miscarriage | 119,157 | – | ||
| 6 | Pregnancy loss | Termination | Psychological consequences | 178,289,333 | – | ||
| 6 | Pregnancy loss | Treatment/test-related | 5,115,116,143,144,148–152,154,155,157,158,160,162,164–166,168,169,171–173,175,176,178–180,183–188,206,207,217,231,241,252,288,289,308,332,333 | – | |||
| 6 | Pregnancy loss | Treatment/test related | Unaffected | 115,142 | – | ||
| 7 | Spillover effects | Benefits to parents from child’s diagnosis with genetic condition, through knowledge of their own genetic status | – | 318 |
Appendix 7 Characteristics of studies included in meta-ethnography (n = 36)
| # | Author (date) | Date | Country | Condition(s) addressed | Research aim | Participants | Data |
|---|---|---|---|---|---|---|---|
| 1 | Boyse et al. | 2014 | USA | CAH | To characterise the experiences and expressed needs of parents following diagnosis of their newborn with CAH | Parents of children diagnosed with CAH (n = 6) | Individual interviews |
| 2 | Buchbinder and Timmermans | 2011 | USA | MCADD | To examine how parents and clinical staff work out the social significance of uncertain newborn screening results | Representative case study of one family with positive newborn screen for MCADD | Ethnographic observation, individual interviews |
| 3 | Buchbinder and Timmermans | 2011 | USA | Metabolic conditions | To explore the potential for newborn screening to diagnose mothers with genetic disorders, requiring a reconceptualisation of traditional views of family ‘benefit’ | Parents of newborn screening patients (n = 7 families) | Ethnographic observation, individual interviews |
| 4 | Buchbinder and Timmermans | 2012 | USA | Metabolic conditions | To explore parents’ perceptions of the initial communication of newborn screening results | Parents of newborn screening patients (n = 75 families) | Ethnographic observation, individual interviews |
| 5 | Carpenter et al. | 2018 | UK | PKU | To explore the experiences of parents of children with PKU under the age of 2 | Parents of children with PKU under the age of 2 (n = 7) | Individual interviews |
| 6 | Chudleigh et al. | 2016 | UK | Cystic fibrosis or sickle cell disease | To explore parents’ experiences of receiving the initial positive newborn screening result for their child with cystic fibrosis or sickle cell disease | Parents whose children had been diagnosed with cystic fibrosis or sickle cell disease and were < 1 year old at time of interview (n = 22) | Individual interviews |
| 7 | DeLuca et al. | 2011 | USA | Metabolic conditions | To describe parents’ experiences with testing for rare metabolic conditions | Parents of children undergoing testing for metabolic conditions (n = 44); 9 children with positive diagnoses, 8 negative, 13 equivocal confirmatory results | Longitudinal interviews during and after metabolic testing process |
| 8 | Dillard and Carson 348 | 2005 | USA | Cystic fibrosis | To identify how family members communicatively manage the uncertainty created by a positive newborn screening result | Families of children who had a positive newborn screening test result for cystic fibrosis (n = 17) | Video recordings of medical interactions with families |
| 9 | Grob | 2006 | USA | Cystic fibrosis | To examine parents’ experiences of newborn screening | Parents of children who received genetic diagnoses via newborn screening for cystic fibrosis (n = 25) | Individual interviews |
| 10 | Grob | 2008 | USA | Cystic fibrosis | To examine parents’ experiences of newborn screening | Parents of children who were diagnosed with cystic fibrosis via newborn screen (n = 16); prenatally (n = 4); or after the development of symptoms (n = 15) | Individual interviews |
| 11 | Johnson et al. | 2019 | UK | Cystic fibrosis | To explore the psychological impact of receiving a ‘CFSPID’ result on parents | Parents of children who received CFSPID (n = 8) | Individual interviews |
| 12 | Kerruish 349 | 2011 | New Zealand | Type 1 diabetes | To explore the psychosocial impact of screening newborns for genetic susceptibilities using type 1 diabetes as an example of a common disorder with multiple significant genetic contributors to its aetiology | Parents of children who had received increased risk results in a study that involved newborn screening for genetic susceptibility to type 1 diabetes (n = 11) | Individual interviews |
| 13 | Kerruish 350 | 2016 | New Zealand | Type 1 diabetes | To explore the later effects of screening for genetic susceptibility to a single, complex disorder: type 1 diabetes | Parents of children who had been tested for genetic susceptibility to type 1 diabetes 12 years previously (n = 15) | Individual interviews |
| 14 | Locock and Kai | 2008 | UK | Sickle cell, thalassaemia, other haemoglobin variants | To explore parents’ experiences and attitudes towards antenatal and newborn screening for haemoglobin disorders | Parents who had experienced gene-carrier identification through antenatal and newborn screening for sickle cell, thalassaemia, and other haemoglobin variants within the previous 2 years (n = 39) | Individual interviews |
| 15 | Moran et al. | 2007 | UK | Cystic fibrosis | To investigate the emotional impact of false-positive diagnoses | Parents who received false-positive IRT cystic fibrosis test result (n = 21) | Individual interviews |
| 16 | Nicholls | 2010 | UK | None | To highlight differences between parental knowledge of newborn screening and their understanding of what actually took place | Parents whose children had newborn screening tests (n = 18) | Individual interviews |
| 17 | Nicholls | 2012 | UK | None | To explore parents’ experiences with the newborn screening consent process | Parents who had children born in the prior 2 years (n = 18) | Individual interviews |
| 18 | Nicholls and Southern | 2013 | UK | None | To understand the factors that influence parental decisions in accepting newborn screening and roles they play in the process | Parents who had children born in the prior 2 years (n = 18) | Individual interviews |
| 19 | Parsons et al. | 2007 | UK | None | To explore mothers’ experiences with newborn screening | Mothers who were offered newborn screening and had negative results (n = 18) | Individual interviews |
| 20 | Patterson et al. | 2015 | USA | Cystic fibrosis or sickle cell disease | To explore the role of the internet after parents receive abnormal newborn screening results | Parents who received abnormal newborn screening results and mentioned the internet in their interview (n = 146) | Secondary analysis of existing individual interviews |
| 21 | Priddis et al. 351 | 2009 | Australia | Cystic fibrosis | To explore the experiences of mother’s whose children were diagnosed with cystic fibrosis through newborn screening | Mothers whose children were diagnosed with cystic fibrosis (n = 19) | Individual interviews |
| 22 | Priddis et al. 352 | 2010 | Australia | Cystic fibrosis | To explore the impact of cystic fibrosis diagnosis on fathers | Fathers whose children were diagnosed with cystic fibrosis (n = 15) | Individual interviews |
| 23 | Pruniski et al. | 2018 | USA | PD | To examine the effects of receiving a positive newborn screening result for PD on families | Mothers of children who were diagnosed with PD through newborn screening (n = 9) | Individual interviews |
| 24 | Raz et al. | 2019 | Israel | PKU, CAH, hypothyroidism, MSUD, homocystinuria, or G6PD | To examine the interface between newborn screening and prenatal diagnosis from the point of view of parents of screen-positive children | Parents whose child was screen positive (n = 34) | Individual interviews |
| 25 | Raz et al. | 2018 | Israel | PKU, CAH, hypothyroidism, MSUD, homocystinuria, or G6PD | To examine the patterns of communication and interaction for peer support among parents of screen-positive children | Parents whose child was screen positive (n = 34) | Individual interviews |
| 26 | Salm et al. | 2012 | USA | Cystic fibrosis or hypothyroidism | To examine parents’ reactions to newborn screening results and their recommendations for improving communication | Parents of screen-positive children (n = 106 interviews, 203 parents) | Individual or couple interviews |
| 27 | Schmidt et al. 353 | 2012 | USA | None | To describe the experiences of families who receive a false-positive newborn screening result in an attempt to discover ways to help improve the newborn screening communication process for families | Parents whose children (ages 6–16 months) underwent follow-up testing after newborn screening and whose follow-up test results indicated that the newborn screening result was a false positive (n = 27) | Individual interviews and focus groups |
| 28 | Schwan et al. | 2019 | USA | X-linked ALD | To examine the impact of a positive newborn screening result for ALD on families | Mothers of children who were identified via newborn screening for ALD (n = 10) | Individual interviews |
| 29 | Timmermans and Buchbinder | 2010 | USA | Metabolic conditions | To examine how parents and clinical staff work out the social significance of uncertain newborn screening results | Families of children who visited metabolic genetic disorder clinic, 24 families had ‘deeply ambiguous’ diagnosis (n = 55) | Ethnographic observation, individual interviews |
| 30 | Tluczek et al. | 2006 | USA | Cystic fibrosis | To understand parents’ perceptions of genetic counselling while awaiting their child’s sweat test results | Parents of children who had at least one CFTR mutation at time of sweat test (n = 31 couples and 2 single mothers); 25 false positives, 8 true positives | Individual or couple interviews |
| 31 | Tluczek et al. | 2009 | USA | Cystic fibrosis or hypothyroidism | To understand how parents learnt about newborn screening and their suggestions for improving the process | Parents of 100 newborns recruited from four groups: cystic fibrosis diagnosis, cystic fibrosis carriers, hypothyroidism diagnosis or normal screens (n = 194) | Content analysis of prior individual interviews |
| 32 | Tluczek et al. | 2010 | USA | Cystic fibrosis | To examine the psychosocial consequences of newborn screening when cases are clinically ambiguous | Parents of five infants who received abnormal newborn screening results with gene mutations (n = 10) | Individual interviews |
| 33 | Tluczek et al. | 2011 | USA | Cystic fibrosis | To understand parents’ perspectives about the psychosocial consequences of false-positive newborn screening results for cystic fibrosis | Parents of children who had false-positive screening results for cystic fibrosis (n = 87) | Individual or couple interviews |
| 34 | Ulph et al. | 2011 | UK | Haemoglobin disorders | To explore the origins and content of service users’ prior knowledge of universal antenatal and newborn screening for haemoglobin disorders | People who used antenatal and newborn screening for haemoglobin disorders (n = 37) | Individual interviews |
| 35 | Ulph et al. | 2014 | UK | Cystic fibrosis or sickle cell disease | To examine parents’ intentions to inform their child of newborn screening carrier results | Family members of children who received a carrier result following newborn screening (n = 67) | Individual interviews |
| 36 | Ulph et al. | 2015 | UK | Cystic fibrosis or sickle cell disease | To explore parents’ responses to receiving sickle cell or cystic fibrosis carrier results for their child following newborn screening | Family members of children who received a carrier result following newborn screening (n = 67) | Individual interviews |
Appendix 8 Participant recruitment materials
FIGURE 9.
Participant information sheet.




FIGURE 10.
Participant questionnaire.



Appendix 9 Focus group guide
(A) Group 1 People’s Experiences Focus Group Discussion Guide
INITIAL CHAT CONVERSATION
Hello and thank you everyone for joining us today. We will get started with some questions in just a moment but wanted to establish a few ground rules before we get into it.
-
Please be respectful of each other and the different opinions you may have . We understand that people may have different views on certain topics we will discuss today, and we want to hear about those perspectives. However, we will not condone attacks on someone’s ethnicity, religion, sexual orientation, political views, etc. Please try to remain civil.
-
Please share your thoughts but also allow space for others to share, too . We want to hear about everyone’s experiences. If you have been taking over the conversation for a while, sit back for a moment to allow someone else a chance to join in.
-
Anything you share is at your discretion . Everyone has been assigned a fake name (except for the researchers). No other participants will know who you are. Please try not to use other people’s real names (e.g. the name of your doctor). Also, be mindful of how much you share about your personal location, family, and/or experiences. For example, instead of saying, ‘I am a 30-year-old married woman living in Reading with a 10-month-old daughter with cystic fibrosis and we see Dr. Smith’, you could say, ‘I am in my 30s, live in the southeast, and my daughter has cystic fibrosis. We see a specialist consultant’. However, it is up to you how much to disclose to the other participants.
That’s it – only three rules to follow.
So thank you everyone for joining us tonight. (We have XX people in the room. We understand that you all have XX experiences.)
We appreciate your time and look forward to what you have to share. We are going to ask some questions about what it has been like to be pregnant during these strange times. We are particularly interested to hear about the scans you might have had, the tests you might have been offered, and what you hope might happen in the future.
-
What kind of antenatal scans or screens did you/your partner have?
-
Probe: Where did participant and/or their partner receive antenatal care and why? (e.g. through the NHS, private care, combination).
-
Probe: Ask about specific tests by name
-
Blood for sickle cell and/or thalassaemia
-
Combined test or quadruple tests for T21/T18/T13
-
Dating, 20 week, other scans
-
Amnio or CVS
-
NIPT.
-
-
-
One of the things we want to learn more about is why people do or do not have screening tests. Can you tell me more about why you decided to (have/not have) (tests mentioned above)?
-
Probe: Whether participant was aware of screening and purposes.
-
Probe: Was it a choice, or something that ‘just happened’?
-
Probe: What people or beliefs influenced their decisions?
-
Probe: Explore why people might have had some and not others.
-
-
For those of you who had specific antenatal tests, can you tell me more about how you found out the results?
-
Probe: How and when results were delivered?
-
Probe: Who – if anyone – did they talk with after getting their results?
-
Probe: Solicit narrative about chain of decision-making processes (if applicable).
-
-
(Ask only if we have group where participants miscarried) I was wondering if you might be able to say a bit about your experience with miscarriage(s)? It is up to you how much you share. If you do not want answer, you can skip this question.
-
Probe: How do they feel about the experience?
-
Probe: Who – if anyone – did they talk with before/after?
-
-
(Ask only if we have group where participants terminated pregnancy) I was wondering if you might be able to tell me a bit about your experience with terminating a pregnancy(ies). Again, you do not need to answer if you do not want to.
-
Probe: How they/their partner made that decision?
-
Probe: Who – if anyone – did they talk with before/after?
-
-
(Ask only if we have group where participants gave birth but baby did not survive perinatal period) I was wondering if you might share a bit more about what that was like losing (baby/name)? It is okay if you do not want to talk about this.
-
Probe: Had screening pre-identified a condition? If so, was it better or worse to know in advance?
-
Probe: Who – if anyone – did they talk with before/after?
-
Probe: What – if any – funeral/memorial events unfolded?
-
-
(Ask only if we have group where participants gave birth and baby survived) Once your baby was born, do you remember having a heel prick test?
-
Probe: Circumstances around heel prick.
-
Probe: Circumstances around consent versus did it ‘just happen’.
-
Probe: Whether participant was aware of screening and purposes.
-
-
(Ask only if we have group where participants gave birth and baby survived) Do you remember if you were notified about the results of the heel prick test?
-
Probe: How and when results were delivered?
-
Probe: Who – if anyone – did they talk with after getting their results?
-
Probe: Solicit narrative about chain of decision-making processes (if applicable).
-
-
(Ask only if we have group where participants’ children have had positive newborn screen) Can you tell me more about what happened after you got the positive screen results?
-
Probe: Emotional responses to screening results.
-
Probe: What was the condition and how/if it was diagnosed?
-
Probe: Whether they gathered information on the condition and how?
-
Probe: Had child become symptomatic and impact result had at that point?
-
Probe: What their/their child’s life is like living with the condition?
-
Probe: How the condition has been received by others and how that might change in the future?
-
Those are all of our prepared questions for today. Before we wrap up, is there anything that someone wants to ask about before we end our conversation? (Respond accordingly).
As a reminder, we will be posting three discussion questions for you to read and respond to in just a moment. You have until our next live chat to answer those. They are under the ‘Forum’ tab on the website. In the final discussion we will be asking you to think about the benefits and harms of screening.
Our next live chat will take place on (date and time).
Please let us know if you have any questions in the meantime by getting in touch at ashley.white@phc.ox.ac.uk.
That is it from us. Thank you for your time today everyone!
SECOND CHAT CONVERSATION
Hello and thank you everyone for joining us today.
Just like last time, we want to remind you that the same three ground rules apply. Please respect others, share the conversation, and remember that anything you share is at your discretion.
With that out of the way, we are going to ask you to think back again on your own experiences with antenatal and newborn screening.
Last time we chatted about your specific experiences with antenatal and newborn screening. We really appreciated your ideas about how to better communicate screening result! You have also had a chance to read and reflect on the vignettes. We appreciate you sharing your experiences and thoughts with us.
We have a few questions to wrap up everything today but I wanted to see if anyone had any reflections on the forum questions? Thank you again for taking time to answer those! (Transition to questions below).
-
Looking back at your overall journey, what were some of the benefits – or good things – about having had antenatal screening tests? Only antenatal.
-
Again, looking back at your overall journey, what were some of the harms – or bad or difficult things – about having had antenatal screening tests? Only antenatal.
-
For everyone – did antenatal screening feel like an end point? Or just a stop on the journey?
-
-
Looking back at your overall journey, what were some of the benefits – or good things – about having had newborn screening? Only newborn.
-
Again, looking back at your overall journey, what were some of the harms – or bad or difficult things – about having had newborn screening? Only newborn.
-
Thinking about your own experiences with the screening process overall, what could have been better?
-
Probe: What could have been better for partner and/or child?
-
-
Thinking about your own experiences with the screening process overall, what information or support do you wish you had beforehand?
-
Probe: What could have been communicated/how it could have been communicated?
-
Probe: Are there any people or sources of support you wished you had to consult?
-
Those are all of our prepared questions for today. Before we wrap up, is there anything else that someone wants to talk about? (Respond accordingly).
As a reminder, our finance officer will be sending along electronic vouchers to thank you for your time. You should get those in the next few days. Please let me know if you have any questions by getting in touch at ashley.white@phc.ox.ac.uk.
That is it from us, thank you!
(B) Group 1 People’s Experiences Focus Group Scenarios
The following questions are made up scenarios about how people might experience antenatal or newborn screening. Please imagine yourself living through these scenarios. What might you do if this happened to you? What might you want to know? Who might you want to speak to? There is no right or wrong answer.
SCENARIO 1
You and your partner Sam have been trying to have a baby for several years. You are 38 and Sam is 41. The first time you got pregnant you were 35, but you miscarried before 10 weeks. You got pregnant a second time a year later, and again had a miscarriage before 10 weeks. You and Sam worry that you are running out of time to conceive.
You are now pregnant for a third time and just had an early scan at 8 weeks. You hope that you do not experience another miscarriage as you really want to have a child.
You are being offered a blood test on the NHS to see if you are a ‘carrier’ for thalassaemia or sickle cell disease – two inherited blood conditions. People can be a ‘carrier’ of these conditions, which is also known as having the ‘trait’. If you are a carrier, you generally will not have any health problems, but there is a chance that your child might inherit the condition.
People living with thalassaemia produce either no or too little haemoglobin, which is used by red blood cells to carry oxygen around the body. This can make them very anaemic (tired, short of breath and pale). There are several types of thalassaemia, each requiring varying degrees of treatment. Although the main health problems associated with thalassaemia can often be managed with treatment, it’s still a serious health condition that can have a significant impact on a person’s life. In the past, severe thalassaemia was often fatal by early adulthood. But with current treatments, people are likely to live into their 50s, 60s and beyond.
Sickle cell disease is the name for a group of inherited health conditions that affect the red blood cells. The most serious type is called sickle cell anaemia. People with sickle cell disease produce unusually shaped red blood cells that can cause problems because they do not live as long as healthy blood cells and can block blood vessels. Sickle cell disease varies between individuals from mild to serious. Most people with it lead happy and normal lives but the illness can be serious enough to have a significant effect on a person’s life. Overall, the life expectancy for someone with sickle cell disease tends to be shorter than normal, but this can vary depending on the exact type of sickle cell disease they have, how it’s treated and what problems they experience.
What might you do? What might you want to know? Who might you want to speak to?
SCENARIO 2
You recently gave birth to a little boy named Tyler. During pregnancy, you decided to have all the antenatal screens offered on the NHS. All your tests and scans came back with reassuring results and you had no concerns about your baby’s health. Your labour and birth went smoothly and you were able to take your son home the next day.
A few days later, a midwife came to your house to check how Tyler was doing. You watched as the midwife did a heel prick test to check Tyler’s blood. The midwife said everything seemed fine and then left shortly afterwards.
One week after the midwife came to your house, you received a phone call at 4 pm and were told that you need to come see the GP the next day. The person on the phone did not tell you much information but indicated that something happened with the heel prick test.
The next morning, you took Tyler to the GP. The GP told you that Tyler tested positive for a rare but potentially serious inherited condition called PKU. This condition means that Tyler will have a hard time digesting foods high in protein, such as meat, eggs and dairy products. Tyler will require a special diet and regular blood tests for the rest of his life. If the condition remains untreated, Tyler might develop an intellectual disability or seizures.
How might you feel at this moment? What might you want to know? Who might you want to speak to?
SCENARIO 3
You and your partner Mo have a 4-year-old daughter. You are pregnant again, and are 12 weeks along.
You were offered the ‘combined test’ as part of your antenatal care on the NHS. This is a screen that combines a blood test and scan to check the chances of a having a baby with Down syndrome (also known as Trisomy 21 or T21), Edwards syndrome (Trisomy 18/T18) or Patau syndrome (Trisomy 13/T13). You and Mo talked about it and decided that you wanted to do the screening test.
The screening test showed that there was a higher-than-average chance of having a baby with Down syndrome. Your midwife explains that there is a 1 in 90 chance of your baby having the condition. Your midwife explains that Down syndrome is a lifelong genetic condition that causes delays in learning and development. It may also cause medical conditions such as heart problems. However, it is a variable condition and some people are more seriously affected than others.
You and Mo would have to wait 1 week for further tests on the NHS, so you decided to have a further private screening test the next day. You had a NIPT at your local private hospital. NIPT is a blood test that can be used to assess the chance of having a baby with Down syndrome, Edwards syndrome or Patau syndrome (among others). It is a newer test than the ‘combined test’ you already had. NIPT is also more accurate than the ‘combined test’.
The NIPT result tells you there is a > 9 in 10 chance your baby has Down syndrome. Put another way, the NIPT result tells you that there is a > 90% chance your baby has Down syndrome.
What might you do next? What might you want to know? Who might you want to speak to?
(C) Group 1 People’s Experiences Interview Discussion Guide
(Interviewer script)
Thank you for taking time to speak with me about your experiences with antenatal and newborn screening programmes. We are going to start with a few questions about your background and then shift into talking about your experiences with screening programmes specifically. You have previously signed a consent form, but I want to be sure that you are happy to continue with the interview?
(Wait for affirmative)
Great, thank you. As a reminder, we will remove any identifying details about you or your family members. We will only refer to you using a fake name to protect your anonymity. If I ask a question and you do not want to answer it, we can skip it, which is no problem. We can also end the interview at any point. It is entirely up to you. You can tell me as much or as little as you want. After the interview, I will send you a typed transcript of the conversation and you can change anything that you want to edit. Is that all okay?
(Wait for affirmative)
Is it okay for me to record our conversation so that I have an accurate record of what we talked about?
(If no, then proceed to question #2)
(If yes, then proceed with last statement)
I am going to start the recording in just a moment. The first thing I am going to ask is for you to confirm that you consent to having the conversation recorded. Then we will roll into the questions. Do you have any questions for me before we get going?
(If no, then proceed to question #1)
(If yes, then answer before starting question #1)
-
Okay, this is (interviewer) speaking with participant (number). Can you confirm that I have your permission to record this conversation?
Thank you for confirming. Now, I am going to ask you some questions about what it was like when (you/your partner) were pregnant. In particular, we are interested to hear about the scans you might have had, the tests you might have been offered, and what happened in your experience.
-
So, to start with, can you tell me a bit about your pregnancy(ies)? (Probe for each pregnancy).
-
Probe: How participant found out about their/their partner’s pregnancy(ies)?
-
Probe: Overall picture of how pregnancy went: including time to conception, age, fertility treatments.
-
Probe: Where did participant and/or their partner receive antenatal care and why? (e.g. through the NHS, private care, combination).
-
Probe: What antenatal screens were they offered?
-
Blood for sickle cell and/or thalassaemia
-
Combined test or quadruple tests for T21/T18/T13
-
Dating, 20 week, other scans
-
Amnio or CVS
-
NIPT.
-
-
-
One of the things we want to learn more about is why people do or do not have screening tests. Can you tell me more about why you decided to (have/not have) (tests mentioned above)?
-
Probe: Whether participant was aware of screening and purposes.
-
Probe: Was it a choice, or something that ‘just happened’?
-
Probe: What people or beliefs influenced their decisions?
-
-
(Skip if no tests at all) Can you tell me more about how you found out the results of (tests mentioned above)?
-
Probe: How and when results were delivered?
-
Probe: How the test results impacted the rest of pregnancy, if at all?
-
Probe: Who – if anyone – did they talk with after getting their results?
-
Probe: Solicit narrative about chain of decision-making processes (if applicable).
-
-
(Ask only if participant miscarried) I was wondering if you might be able to tell me a bit about your experience with miscarriage(s)? It is up to you how much you share, and we can skip this question if you want.
-
Probe: How do they feel about the experience?
-
Probe: Who – if anyone – did they talk with before/after?
-
(SKIP TO 12).
-
-
(Ask only if participant terminated pregnancy) I was wondering if you might be able to tell me a bit about your experience with terminating a pregnancy(ies). It is up to you how much you share and we can skip this question if you want.
-
Probe: How they/their partner made that decision?
-
Probe: Who – if anyone – did they talk with before/after?
-
(SKIP TO 12).
-
-
(Ask only if carried to term) Can you tell me about what your/your partner’s labour and birth experience was like?
-
Probe: Who was involved in delivery (partner, midwife, specialists, etc.)?
-
Probe: What was it like for your partner (if applicable)?
-
-
(Ask only if participant gave birth but baby did not survive perinatal period) I was wondering if you might tell me a bit more about what that was like losing (baby/name)? It is okay if you do not want to talk about this, we can skip it.
-
Probe: Had screening pre-identified a condition? If so, was it better or worse to know in advance?
-
Probe: Who – if anyone – did they talk with before/after?
-
Probe: What – if any – funeral/memorial events unfolded?
-
(SKIP TO 12).
-
-
(Ask only if participant gave birth and baby survived) Once your baby was born, do you remember him/her having a heel prick test?
-
Probe: Circumstances around heel prick.
-
Probe: Circumstances around consent versus did it ‘just happen’.
-
Probe: Whether participant was aware of screening and purposes.
-
-
(Ask only if participant’s child had heel prick) Do you remember if you were notified about the results of the heel prick test?
-
Probe: How and when results were delivered?
-
Probe: Who – if anyone – did they talk with after getting their results?
-
Probe: Solicit narrative about chain of decision-making processes (if applicable).
-
-
(Ask only if participant’s child had positive newborn screen) Can you tell me more about what happened after you got the positive screen results?
-
Probe: Emotional responses to screening results.
-
Probe: Family communication around getting screening result/diagnosis – did they share it with others? When?
-
Probe: What was the condition and how/if it was diagnosed?
-
Probe: What did they know about the condition at the time it was first brought up?
-
Probe: Whether they gathered information on the condition and how.
-
Probe: Had child become symptomatic and impact result had at that point?
-
Probe: What their/their child’s life is like living with the condition?
-
Probe: How the condition has been received by others and how that might change in the future?
-
(Ask all)
-
Thank you for telling me about your experiences. I have just a few more questions for you. Looking back at your overall experience, what were some of the benefits – or good things – about having had (specific antenatal/newborn) screening tests?
-
Probe: Do they believe this would be the case for other people?
-
Probe: What were the benefits of their own specific approach?
-
-
Again, looking back at your overall experience, what were some of the harms – or bad or difficult things– about having had (specific antenatal/newborn) screening tests?
-
Probe: Do they believe this would be the case for other people?
-
-
Thinking about your own experiences with the (specific antenatal/newborn) screen process, what could have been better?
-
Probe: What could have been better for partner and/or child?
-
-
Thinking about your own experiences with the (antenatal/newborn) screen process, what information or support do you wish you had beforehand, if any?
-
Probe: What could have been communicated/how it could have been communicated?
-
Probe: Specific services or people they could have consulted.
-
Those are all my prepared questions. Before we wrap up, is there anything that I have not asked about that you think is important to share?
(Respond accordingly)
Is there anything that you would like to ask me before we end our conversation?
(Respond accordingly)
A professional transcriber is going to type up our conversation. Once they are finished, I will send you a copy and you can take a look and make any changes, if you wish. Please feel free to get in touch with any other questions or concerns. Again, thank you very much for your time today!
(End recording)
(D) Group 2 Stakeholders Interview Discussion Guide
(Interviewer script)
Thank you for taking time to speak with me about your experiences with antenatal and newborn screening programmes. We are going to start with a few questions about your background and then shift into talking about your experiences with screening programmes specifically. You have previously signed a consent form, but I want to be sure that you are happy to continue with the interview?
(Wait for affirmative)
Great, thank you. As a reminder, we will remove any identifying details about you or your family members. We will only refer to you using a fake name, to protect your anonymity. If I ask a question and you do not want to answer it, we can skip it, which is no problem. We can also end the interview at any point. It is entirely up to you. You can tell me as much or as little as you want. After the interview, I will send you a typed transcript of the conversation and you can change anything that you want to edit. Is that all okay?
(Wait for affirmative)
Is it okay for me to record our conversation so that I have an accurate record of what we talked about? (and facilitate transcription)
(If no, then proceed to question #2)
(If yes, then proceed with last statement)
I am going to start the recording in just a moment. The first thing I am going to ask is for you to confirm that you consent to having the conversation recorded. Then we will roll into the questions. Do you have any questions for me before we get going?
(If no, then proceed to question #1)
(If yes, then answer before starting question #1)
-
Okay, this is (interviewer) speaking with participant (number). Can you confirm that I have your permission to record this conversation?
-
Great, thank you. Could you please tell me about how you are involved in antenatal and newborn screening?
-
Probe to understand their role, time devoted, who they typically interact with, their goals, route to involvement.
-
Thank you for that. I have some questions now specifically about antenatal screening. We will talk about newborn screening shortly but the focus for now is antenatal screening.
-
Based on your experiences, what are some potential benefits of antenatal screening?
-
Probe to determine how benefits might apply to specific people/groups/conditions/pregnancy stage.
-
-
Based on your experiences, what are some potential harms of antenatal screening?
-
Probe to determine how harms might apply to specific people/groups/conditions/pregnancy stage.
-
-
Thinking about the antenatal screening system in (England), what about the process works well?
-
Probe to see about any variation based on specific people/groups/conditions.
-
-
What, if anything, could be improved about the current approach to antenatal screening?
-
Probe to see about any variation based on specific people/groups/conditions.
-
Thank you for that. I have some questions now specifically about newborn screening. So, as much as you can, think just about newborn screening for these next questions.
-
Based on your experiences, what are some potential benefits of newborn screening?
-
Probe to determine how benefits/positives might apply to specific people/groups/conditions.
-
-
Based on your experiences, what are some potential harms of newborn screening?
-
Probe to determine how harms/negatives might apply to specific people/groups/conditions.
-
-
Thinking about the newborn screening system in (England), what about the process works well?
-
Probe to see about any variation based on specific people/groups/conditions.
-
-
What, if anything, could be improved about the current approach to newborn screening?
-
Probe to see about any variation based on specific people/groups/conditions.
-
Okay, I have just a few more questions for you. We have been thinking about antenatal and newborn screening separately but now I want you to think about screening and testing programmes overall.
-
If you were given the opportunity, and the funding, what aspects of the current screening programmes might you change?
-
Probe around scope for training or other resources to support their work/role.
-
Those are all my prepared questions. Before we wrap up, is there anything that I have not asked about that you would like to share?
(Respond accordingly)
Is there anything that you would like to ask me before we end our conversation?
(Respond accordingly)
A professional transcriber is going to type up our conversation. Once they are finished, I will send you a copy and you can take a look and make any changes, if you wish. Please feel free to get in touch with any other questions or concerns. Again, thank you very much for your time today!
(End recording)
(E) Group 3 Midwives Focus Group Discussion Guide
(INTERVIEWER SCRIPT)
Thank you for joining us this evening. Before we get started, I wanted to remind everyone that we would like to record this session so we can revisit it and create an accurate transcript of the conversation. I have an audio recorder on my desk to do this, and will send this file to our typist. I would also like to do a video recording of my screen, just to be sure that I can accurately capture who is speaking at any given time. The video will only be used to check the accuracy of the written text transcript, and then will be destroyed. In any written reports or presentations, we will only refer to you using a fake name with experience as a midwife. If you prefer, you can turn your camera off during the conversation. Are there any questions before we start? Is it ok if I begin recording?
(BEGIN RECORDINGS)
As you know, this is a project assessing the benefits and harms of antenatal and newborn screening programmes here in the UK. This study has a few parts. One part involved speaking with nearly 50 people who were either pregnant or recently gave birth; or, in some cases their partners. Another part of the study involved conducting interviews with approximately 20 charity leaders or policy-making stakeholders. We have been working to make sense of the things these research participants have shared with us, and some of what they’ve shared will come up in our conversation this evening.
Now, we are turning to healthcare professionals like you to get an idea of whether we are missing anything. Everyone on the call this evening is a midwife. We are going to start with a few questions about your professional background and then shift into talking about screening programmes more specifically.
(INTRODUCTIONS)
-
Just to start, let’s go around the call and have everyone introduce themselves. Could you please share a bit about your training and current position?
-
Probe: What type of patients do you typically work with in your role?
-
Probe: How are you involved in aspects of antenatal screening?
-
Probe: How are you involved in newborn screening?
-
Probe: What is the typical screening journey for a patient that you see?
-
(SENSE CHECKING)
As I mentioned earlier, we have also heard from a number of people who have gone through screening themselves and we are starting to make sense of their experiences. I have some questions about what they have shared, and want to see if it makes sense to you, as midwives.
-
One of the important things people have shared with us how important prior reproductive histories can be when it comes to making decisions about screening. For example, if someone had a very negative experience with a sonographer, they might feel quite anxious about going back for scans in the future.
-
Probe: How do you account for prior pregnancies in your practice?
-
Probe: Do you think other types of healthcare professionals approach prior reproductive experiences the same way?
-
-
Another important thing we keep hearing is how women might think of screening as both a positive and negative thing. For example, we had one participant who had multiple miscarriages, and because of her history she got weekly scans after 12 weeks. She described how she was terrified of going for her scans, because she was afraid the fetus would not have a heartbeat. But, once she was in the room and the sonographer found a heartbeat, she felt joy because, in that moment, everything was okay. She would have that feeling for about an hour afterwards but then doubt would creep back in, and then following week when she went in she was right back to being terrified. Does this sort of experience seem familiar to you?
-
Probe: Are there other examples of where screening can be both beneficial and harmful?
-
-
We have also focused on how people get information about antenatal and newborn screening. Could you describe what you think the average woman knows prior to pregnancy?
-
Probe: What might they know once pregnant about antenatal screening? Newborn screening?
-
Probe: How do you handle providing information about screening tests? How much information is appropriate?
-
Probe: Have you had training on this? What were you told?
-
Probe: What have you amended during years of practice?
-
Probe: Is it possible to ensure that pregnant people are fully informed about the screening tests?
-
Probe: How do you handle a patient who does not want some of the standard screening tests? Ex – combined test? Heel prick?
-
Probe: What would you say? What would you do?
-
Probe: How might you involve partners in these conversations?
-
-
The implications of screening, or not screening, have also been on our minds. We’ve been thinking about how key decisions can have potentially long-term effects on individuals and their families. Could you describe what you think some of those long-term effects might be? Examples?
-
Probe: Do women or their partners ever raise these with you?
-
(REFLECTIONS ON BENEFITS AND HARMS)
-
Thank you for everything you’ve shared with us so far. As we start to wrap up, I want you to think about antenatal and newborn screening overall.
-
What, if anything, are some of the good things about the current approaches to antenatal screening?
-
What, if anything, could be improved about the current approach to antenatal screening?
-
What, if anything, are some of the good things about the current approaches to newborn screening?
-
What, if anything, could be improved about the current approach to newborn screening?
-
Those are all our prepared questions. Before we wrap up, is there anything that we have not touched on that you would like to share?
(Respond accordingly)
Thank you all again for your time this evening. We really appreciate it. I’ll be asking our finance team to send out vouchers to thank you for your time, so please keep an eye on your inbox for those in the next few days. Please feel free to get in touch with any other questions or concerns. Again, thank you very much for your time today!
(End recording)
Appendix 10 Group 1 participants’ demographic characteristics (n = 49)
| No. or range | % or mean (SE) | |
|---|---|---|
| Age | 24–48 | 34.7 (0.75) |
| Gender | ||
| Female | 40 | 81.6 |
| Male | 9 | 18.4 |
| Country | ||
| England | 40 | 81.6 |
| Scotland | 3 | 6.1 |
| Wales | 6 | 12.2 |
| Time since last pregnancy | ||
| I/my partner is currently pregnant | 16 | 32.7 |
| < 4 months | 9 | 18.4 |
| Between 4 and 6 months | 6 | 12.2 |
| Between 7 and 12 months | 3 | 6.1 |
| Between 13 and 18 months | 3 | 6.1 |
| Between 19 and 24 months | 5 | 10.2 |
| Between 2 and 4 years ago | 7 | 14.3 |
| Household income (£) | ||
| 10,000–19,999 per year | 1 | 2.0 |
| 20,000–29,999 per year | 1 | 2.0 |
| 30,000–39,999 per year | 9 | 18.4 |
| 40,000–49,999 per year | 5 | 10.2 |
| 50,000–59,999 per year | 4 | 8.2 |
| 60,000–69,999 per year | 4 | 8.2 |
| 70,000–79,999 per year | 10 | 20.4 |
| 80,000–89,999 per year | 6 | 12.2 |
| 90,000–99,999 per year | 1 | 2.0 |
| > 100,000 per year | 5 | 10.2 |
| Prefer not to say | 3 | 6.1 |
| Relationship | ||
| Married | 38 | 77.6 |
| Cohabitating | 10 | 20.4 |
| Single | 1 | 2.0 |
| Ethnicity | ||
| Arab | 1 | 2.0 |
| Asian/Asian British | 1 | 2.0 |
| White | 47 | 95.9 |
| Education | ||
| Sixth form/vocational qualification | 7 | 14.3 |
| Bachelor’s degree | 19 | 38.8 |
| Advanced university degree | 23 | 46.9 |
List of abbreviations
- 16D
- 16-Dimension
- ALD-DRS
- Adrenoleukodystrophy-Disability rating scale
- ARC
- Antenatal Results and Choices
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CVS
- chorionic villus sampling
- DALY
- disability-adjusted life-year
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-Y
- EuroQol-5 Dimensions-Youth
- HCP
- healthcare providers
- HIV
- human immunodeficiency virus
- HTA
- Health Technology Assessment
- HUI
- Health Utilities Index
- IQI
- Infant health-related Quality of Life Instrument
- MP
- member of parliament
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NIPT
- non-invasive prenatal testing
- NPEU
- National Perinatal Epidemiology Unit
- NSC
- National Screening Committee
- OECD
- Organisation for Economic Co-operation and Development
- PICOS
- Population, Intervention, Comparator, Outcome and Study design
- PKU
- phenylketonuria
- PPI
- parent and public involvement
- PROM
- patient-reported outcome measure
- QALY
- quality-adjusted life-year
- QWB
- Quality of Well-Being Scale
- SCBU
- special care baby unit
- SF-6D
- Short Form 6-dimension
- TANDI
- Toddler and Infant health-related Quality of Life Instrument
- WP
- work package
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/PYTK6591).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
