Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/88/21. The contractual start date was in February 2016. The draft report began editorial review in November 2020 and was accepted for publication in May 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Harding et al. This work was produced by Harding et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Harding et al.
Chapter 1 Introduction (background and objectives)
Parts of this chapter have been reproduced with permission from Forbes et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Scientific background
Urinary tract infections (UTIs) are common in adult women, in both the community and the hospital setting. Inoculation of the periurethral tissues, and subsequently the bladder, by bacteria that originate from the lower gastrointestinal tract or vagina may lead to classic symptoms of infective cystitis such as dysuria, frequency, urgency and suprapubic pain. Around 40% of all adult women will experience a UTI in their lifetime, with peak incidences occurring in the third and ninth decades. Annually, 3% of all adult women will be diagnosed with a UTI and almost half of these women will experience a second episode within 1 year. 2,3 With > 300,000 women per year in the UK requiring treatment for recurrent urinary tract infection (rUTI), this represents a significant health problem. 4 Standard preventative treatment of rUTI usually consists of daily low-dose antibiotic therapy, and a previously published meta-analysis from the Cochrane collaboration5 described a reduction in UTI episodes of > 80%. The relationship between antibiotic prescription and the development of antimicrobial resistance is well described, with documented resistance not only confined to the causative micro-organisms but also observed in commensal flora. 6 The judicious prescribing of antibiotic agents, termed ‘antibiotic stewardship’, is an essential component of national and international strategies to halt the progression of global antimicrobial resistance. 7,8 Current cross-specialty guidelines9 have identified that ‘repeated/prolonged treatment with antibiotics’ is a significant contributing factor to this progression. Consequently, interest has focused on non-antibiotic treatments for the prevention of rUTI, which have the potential to improve public health by minimising the development of antimicrobial resistance in bowel reservoirs.
Summary with implications for trial design
The current body of evidence and the contribution of this study
National and international guidelines on the topic of rUTI prevention currently recommend the use of daily low-dose prophylactic antibiotics as the standard of care. 9–12 Level 1a evidence exists to support their use and this is derived from a Cochrane systematic review. 5 That review included data from 19 studies comprising > 1100 women, and described an 85% reduction in symptomatic urinary infections compared with placebo [risk ratio (RR) 0.15, 95% confidence interval (CI) 0.08 to 0.28]. 5 The authors concluded that continuous antibiotic prophylaxis for a 6- to 12-month period was an effective treatment for rUTI. Despite the documented efficacy of daily prophylactic antibiotics, adverse events (AEs) including vaginal and oral candidiasis and gastrointestinal symptoms were more common in the antibiotic-treated groups. In addition, the benefit of daily prophylactic antibiotics did not appear to be sustained following the cessation of this treatment. Data from two studies in the meta-analysis revealed that infection rates returned to pre-treatment levels in the majority of participants. 5
One of the most promising non-antibiotic treatments for the prevention of rUTI is methenamine hippurate. 13 A meta-analysis from the Cochrane group14 included 13 trials with data from > 2000 patients. A 76% reduction in the incidence of UTIs was described (RR 0.24, 95% CI 0.07 to 0.89) and is comparable to the data described above for daily prophylactic antibiotics. The quality of the included studies in this meta-analysis was mixed, and there was significant underlying heterogeneity in many of the trials. Despite this, the authors concluded that methenamine hippurate may be an effective preventative treatment for those suffering with rUTI, particularly those patients without underlying renal tract abnormalities. A research recommendation was made for large, well-conducted clinical trials involving this promising treatment, particularly in the prevention of recurrent infection.
According to currently available evidence, daily low-dose prophylactic antibiotics appear to be the most effective intervention for rUTI prevention,5 but a number of well-conducted randomised controlled trials (RCTs) have described the emergence of antimicrobial resistance as an unwanted side effect of this treatment compared with placebo. 5,15,16 The pattern of resistance was not only confined to the prescribed antibiotic but often extended to a range of other agents commonly used to treat symptomatic urinary infections, and some trials have described the emergence of antimicrobial resistance after only a few weeks of prophylactic antibiotic treatment. 2,15,16 The detection of multidrug-resistant bacteria has also been shown to increase significantly following prolonged antibiotic treatment, and in one study this increased from 25% to 80% after such treatment. 6
The obvious theoretical advantages of non-antibiotic preventative treatments for rUTIs are underlined by UK, European and US guideline documents, which describe ‘reducing collateral damage’ of antibiotic use by minimising the risk of resistance development. 7,10,17,18 Current UK health policy advises antibiotic avoidance when possible to reduce the rate of antimicrobial resistance nationally and globally. 17 The wider public health issue of health-care-associated infections (HAIs) with organisms such as meticillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile infection (CDI) and, more recently, other types of multiresistant organisms [including those producing extended-spectrum beta-lactamases (ESBLs)] is also believed to be, in part, due to antibiotic overprescribing. This is undoubtedly a global issue and must be tackled by international collaborative effort. A recent report19 concerning uropathogenic Escherichia coli speculated that the overuse of non-prescription antibiotics in Asia was a potential causative factor in the development of a new mechanism of ESBL antibiotic resistance detected in the UK. Limiting the use of broad-spectrum antibiotics is a key measure in addressing this problem and has been the driver for recent UK guideline updates. 9 The development of antimicrobial stewardship programmes that encourage prudent antibiotic prescribing has already been shown to reduce antibiotic use and, consequently, the incidence of HAIs, which until recently had been increasing. 20,21 The avoidance of antibiotic administration, when possible, is believed to be the single most important factor in the observed decline in HAIs in Scotland. 21
Why this research is needed now
A recent meta-analysis22 reviewed the evidence for non-antibiotic treatments as prophylaxis against rUTI but the results were disappointing, mainly owing to a paucity of evidence. One of the conclusions of this report was that ‘Although sometimes statistically significant, pooled findings for the other (non-antibiotic) interventions should be considered tentative until corroborated by more research’. It would appear that one of the barriers to clinicians recommending non-antibiotic alternatives for the treatment of rUTI is the lack of currently available clinical evidence for these. The campaign for antibiotic stewardship and more prudent prescribing of antibiotic agents can be strengthened only by further work exploring the effectiveness of non-antibiotic alternatives. A further conclusion from this meta-analysis22 was that ‘Large head-to-head trials should be performed to optimally inform clinical decision making’.
One of the most comprehensive guidelines published on the subject of UTI is the 2012 Scottish Intercollegiate Guideline Network (SIGN) Guideline 88. 9 The literature review carried out prior to the formulation of this document identified much evidence of variations both in practice and when antibiotic treatment is started for UTI. 9 In addition, one of the constant themes in this report is the need to avoid prescribing antibiotics unnecessarily, which is associated with adverse events such as CDI, MRSA and antibiotic-resistant UTIs. 9 The UK antimicrobial resistance strategy and action plan states ‘the increasing prevalence of antimicrobial resistant micro-organisms is causing international concern’, and identifies that ‘the emergence of resistance represents adaptive selection by micro-organisms which is an inevitable result of therapeutic use of antimicrobial agents’7 (quotations contain public sector information licensed under the Open Government Licence v3.0). This document reflects an urgent need for prudent antibiotic use as one of three key elements of the strategy to control antibiotic resistance. The ALTAR (ALternatives To prophylactic Antibiotics for the treatment of Recurrent urinary tract infection in women) trial aimed to provide high-level contemporary evidence of the relative effectiveness of a non-antibiotic UTI prevention treatment compared with the standard treatment of prolonged low-dose antibiotics in a UK population of women with rUTI in a routine NHS care setting.
Design/methods
The ALTAR trial tests the null hypothesis that the non-antibiotic treatment methenamine hippurate (Hiprex®; Mylan NV, Canonsburg, PA, USA) is inferior to the standard treatment of an extended course of prophylactic antibiotic for the prevention of rUTI in women, and is less cost-effective to the NHS. The alternative hypothesis is that methenamine hippurate is as good at preventing rUTI and is as cost-effective as antibiotic prophylaxis. We have chosen to investigate the possible non-inferiority of methenamine hippurate in order to clarify an alternative treatment choice to antibiotics in treating rUTIs, which, if used more routinely, may prevent increases in antibiotic-resistant strains of infection.
Estimates of prevalence, effectiveness and harms from Cochrane reviews5,14 have informed the power calculation conservatively based on what we, guided by a patient panel, considered to be a minimum threshold difference that would drive patient and clinician acceptability together with change of practice prompted by the inclusion of trial results in future meta-analyses and guidance for management of rUTI in the NHS and internationally.
Aims and objectives
Primary objective
The primary objective is to:
-
Determine the relative clinical effectiveness and cost-effectiveness for the NHS of two types of licensed preventative treatments for women with recurrent uncomplicated UTI over a 12-month treatment period. Uncomplicated UTI refers to UTIs occurring in patients with no structural or functional urinary tract abnormalities that could contribute to their infective episodes.
Secondary objectives
The secondary objectives are to determine:
-
the relative impact on the incidence of symptomatic antibiotic-treated UTI self-reported by patients during the 6-month follow-up period after completion of 12 months of allocated treatment
-
the total number of days spent taking urinary-specific antibiotics (prophylactic or treatment) during the 12-month treatment period and 6 months of follow-up
-
if there is any longitudinal ecological change in terms of phenotype and genotype of bacteria and their resistance patterns in isolates from individual participants’ (1) urine and (2) faecal reservoir during the 12-month treatment period and in the 6 months following completion of treatment
-
the number of microbiologically proven UTIs during the 12-month treatment and 6-month follow-up periods
-
the incidence of asymptomatic bacteriuria during the study period
-
the incidence rate of hospitalisation due to UTIs during the study period
-
overall patients satisfaction with antibiotic compared with antiseptic treatment
-
patients’ and clinicians’ views regarding trial processes and participation using an embedded qualitative study
-
the incremental cost per quality-adjusted life-year (QALY) gained at the 18-month time point based on responses to the EuroQol-5 Dimensions, five-level version (EQ-5D-5L)
-
the incremental costs to the NHS and Personal Social Services measured at the end of the 18-month study period
-
the relative health economic efficiency over the patient’s lifetime using a modelling exercise.
Chapter 2 Methods
Parts of this chapter have been reproduced with permission from Forbes et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
This chapter covers general trial methods, statistical analysis and governance; details of methods and findings of the qualitative and health economic analyses are provided in Chapters 3 and 5, respectively.
Summary of study design
The ALTAR trial is a multicentre, pragmatic, open-label randomised non-inferiority trial evaluating the clinical effectiveness and cost-effectiveness of the urinary antiseptic methenamine hippurate, a non-antibiotic treatment for the prevention of rUTI, and comparing it with the current standard treatment of low-dose antibiotic prophylaxis. Outside the trial medication, both arms received usual care and any breakthrough UTIs were treated by discrete courses of antibiotic treatment, as required.
Sites
From June 2016 to June 2017, we established eight research sites comprising NHS organisations across England and Scotland (Table 1). All sites were large, secondary care urology or urogynaecology centres known to have a consistent clinical assessment pathway for women with rUTI. We initially opened six sites: Newcastle upon Tyne Hospitals NHS Foundation Trust, Cambridge University Hospitals NHS Foundation Trust, Mid Yorkshire Hospitals NHS Trust (Wakefield), Leeds Teaching Hospitals NHS Trust, NHS Greater Glasgow and Clyde and Central Manchester University Hospitals NHS Foundation Trust (now known as Manchester University NHS Foundation Trust). In addition, a further two sites, Royal Liverpool and Broadgreen University Hospitals NHS Trust (now known as Liverpool University Hospitals NHS Foundation Trust) and Pennine Acute Hospitals NHS Trust (Oldham), were opened to support recruitment to the trial.
| Site | Number of participants randomised |
|---|---|
| Newcastle upon Tyne Hospitals NHS Foundation Trust | 84 |
| Cambridge University Hospitals NHS Foundation Trust | 43 |
| Manchester University NHS Foundation Trust (formerly known as Central Manchester University Hospitals NHS Foundation Trust) | 20 |
| NHS Greater Glasgow and Clyde | 24 |
| Leeds Teaching Hospitals NHS Trust | 22 |
| Mid Yorkshire Hospitals NHS Trust | 31 |
| Liverpool University Hospitals NHS Foundation Trust (formerly known as Royal Liverpool and Broadgreen University Hospitals NHS Trust) | 8 |
| Pennine Acute Hospitals NHS Trust | 8 |
| Total | 240 |
Trial management
The central trial office was established at the Newcastle Clinical Trials Unit (NCTU) at Newcastle University, Newcastle upon Tyne. NCTU was responsible for obtaining approvals, trial registration, trial management, provision of the randomisation service, database construction and database management. The trial statistics, health economic evaluation and qualitative research teams were also based at Newcastle University.
Participants
The target population for the ALTAR trial was women (aged ≥ 18 years) with rUTI, for whom, after discussion with their responsible clinician, prophylactic antibiotics were considered as a therapeutic option. The definition of rUTI used was at least three episodes of symptomatic antibiotic-treated urinary infection in the previous 12 months, two episodes of UTI in the last 6 months or a single occurrence of severe UTI requiring hospital admission in the preceding year.
Patients were approached and introduced to the trial by clinical staff at sites during routine clinic visits. If interested, patient eligibility was assessed in accordance with the following eligibility criteria.
Inclusion criteria
-
Women aged ≥ 18 years.
-
Women with rUTI who, in consultation with a clinician, had decided that prophylaxis was an appropriate option (to include women who had suffered at least three episodes of symptomatic UTI within the preceding 12 months or two episodes in the last 6 months or a single severe infection requiring hospitalisation).
-
Women able to take a once-daily oral dose of at least one of nitrofurantoin or trimethoprim or cefalexin.
-
Women able to take methenamine hippurate.
-
Women who agreed to take part in the trial but were already taking methenamine hippurate or antibiotic prophylaxis. These participants were consented for participation and had their preventative therapy stopped for a 3-month washout period. The women were reassessed, and if they were still eligible to take part, then they underwent the baseline assessment and randomisation.
-
Women able to give informed consent for participation in the trial.
-
Women able and willing to adhere to an 18-month trial protocol.
Exclusion criteria
-
Women unable to take methenamine hippurate, for example because they had a known allergy to methenamine hippurate, severe hepatic impairment (Child–Pugh class C, score of ≥ 10 see Appendix 1, Table 26), gout, estimated glomerular filtration rate (eGFR) of < 10 ml/minute/1.73 m2 and Proteus spp. as consistent proven causative organism for rUTIs.
-
Women who were unable to take any of the trial antibiotics.
-
Women with correctable urinary tract abnormalities that were considered to be contributory to the occurrence of rUTI.
-
Presence of symptomatic UTI; this was treated and symptoms resolved prior to randomisation.
-
Pregnancy or intended pregnancy in the next 12 months.
-
Women who were breastfeeding.
-
Women already taking methenamine hippurate or antibiotic prophylaxis and who declined a 3-month washout period.
Consent procedures
The trial was conducted according to the principles of Good Clinical Practice (GCP) and the 2013 Declaration of Helsinki. 23 Participants provided informed consent for randomisation, trial participation, telephone interviews and the storage of blood, urine and swabs for future research, and about whether or not they agreed to be contacted about similar research studies. The obtaining of informed consent was undertaken by appropriately trained and delegated staff at each trial site. The consent process involved providing participants with balanced, written information about the need for and overall benefit of the trial, followed by a discussion with a local trial co-ordinator. In relation to the qualitative interviews, the recruiting staff explained why it was important to understand why people do and do not participate, and how an interview study can help to improve the way that trials are conducted. Participants who were willing to be approached were provided with a separate information sheet with details about the study interview. Following receipt of the trial information, participants were given at least 24 hours to decide whether or not they wanted to participate. Written informed consent was obtained prior to randomisation by participants signing and dating the trial consent form. Women who agreed to take part in the trial but who were already taking methenamine hippurate or antibiotic prophylaxis were consented for participation and advised to stop their preventative therapy for a 3-month washout period, but they were not randomised or asked to complete the other baseline measures until the 3-month washout period was complete. Their continued consent and eligibility were reassessed post washout and, if eligible, they were then accepted into the trial.
Randomisation
Participant allocation
Randomisation was administered centrally by the NCTU secure web-based system. Permuted random blocks of variable length (two, four, six or eight) were used to allocate participants on a 1 : 1 basis to the antibiotic prophylaxis and methenamine hippurate arms. An individual not otherwise involved with the trial produced the final randomisation schedule. Stratification by two variables, namely prior frequency of UTI (fewer than four episodes per year, or four or more episodes per year) and menopausal status of participants (pre menopausal or menopausal/post menopausal), was performed prior to randomisation to ensure balanced allocation with regard to these factors. Following randomisation, an appointment was arranged, facilitated by trial staff, with the prescribing clinician to commence allocated treatment and ensure continued supply for the 12-month treatment period, usually through hospital prescription or through the participant’s general practitioner (GP). The antibiotic selected for use as prophylaxis was chosen by both the participant and the clinician while taking into account the individual participant’s characteristics, their previous urine culture results, local guidance and standardised trial information, with preferred agents being, first, nitrofurantoin, second, trimethoprim and, third, cefalexin.
Blinding
This was a pragmatic, open-label trial; therefore, participants, clinicians, local research staff and NCTU staff were not masked to treatment allocation. Central laboratory staff assessing microbiological outcomes were unaware of treatment assignment. Where possible, the clinical research staff conducting the within-trial assessments were blinded to treatment allocation. The trial statistician who performed the final analysis was responsible for preparing unblinded reports to the Data Monitoring Committee (DMC) and had access to unblinded primary outcome data prior to the final database lock. The senior statistician was responsible for approving the statistical analysis plan (SAP) and remained blind to treatment allocation until after the final database lock. Primary outcome assignment was assessed by the trial statistician following the methods defined in the SAP [see the National Institute for Health and Care Research (NIHR) Journals Library project web page – URL: www.journalslibrary.nihr.ac.uk/programmes/hta/138821/#/], with a 10% sample of cases independently assessed by a clinician who was not otherwise involved in the trial.
Progress on trial
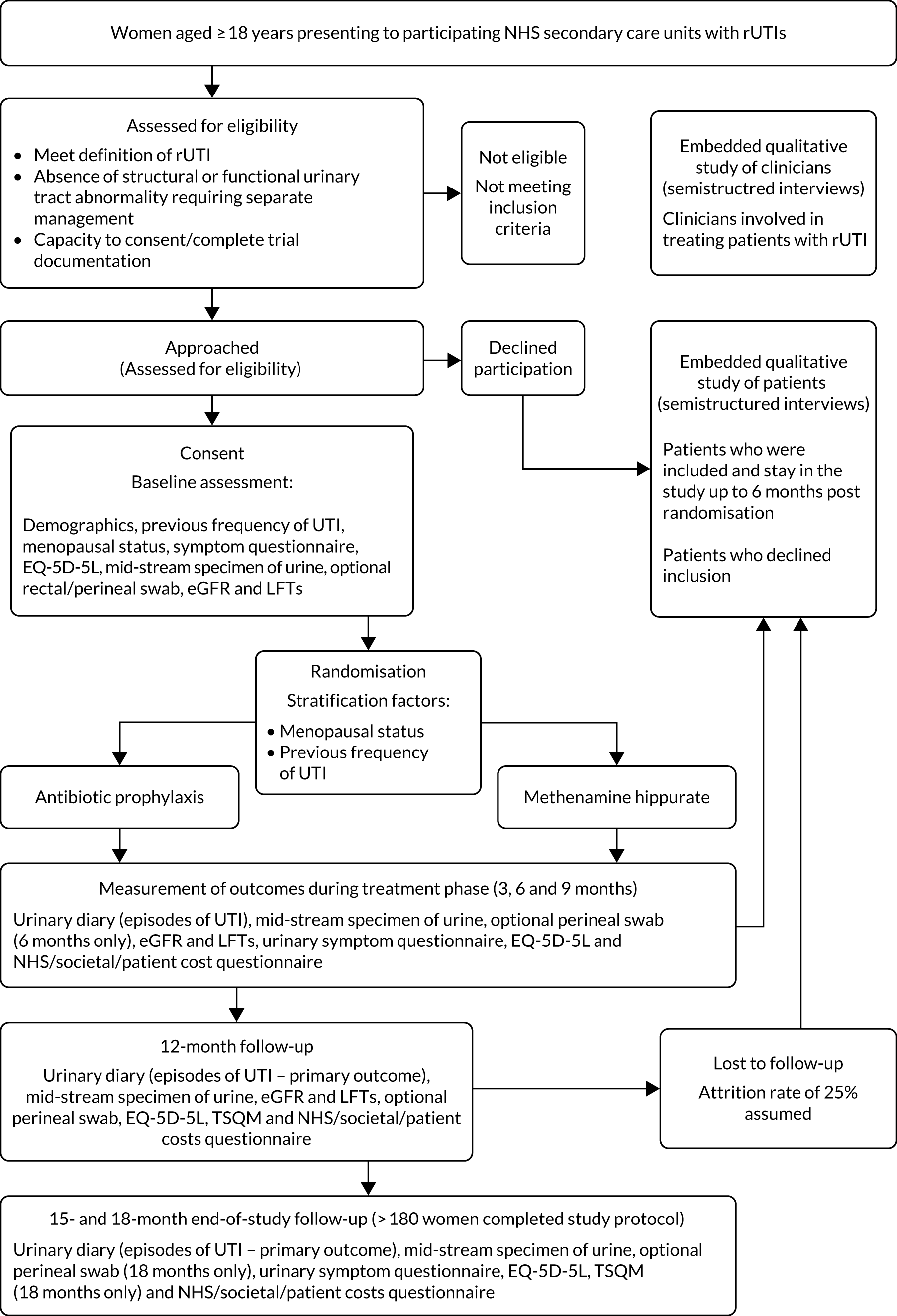
The trial duration for each participant was 18 months. The patient flow through the trial is shown in Figure 1; for the schedule of events for trial participants, see Table 3.
FIGURE 1.
Participant flow. LFT, liver function test; TSQM, Treatment Satisfaction Questionnaire for Medication.
Participant expenses
Participants were reimbursed reasonable travel expenses incurred as a result of taking part in the ALTAR trial. NHS prescription charges for trial medication were reimbursed or managed via hospital prescription or the participant’s GP. Participants were given a £30 thank-you gift voucher when they reached the 3-month time point.
Withdrawal of participants
Participants remained on the trial unless they withdrew their consent, or their participation was deemed inappropriate by trial site staff. Reasons for withdrawal were recorded if agreement was provided by the participant. If a participant chose to withdraw from the trial, their permission was sought to continue to collect primary outcome relevant data through routine health-care records. Participants providing primary outcome data for at least 6 months post randomisation were included in the primary outcome analyses.
Patient and public involvement
The identification and prioritisation of the research topic were directly patient driven. A patient interest group was set up locally at the lead site to help refine the methodology of the trial and, in particular, select an appropriate patient-defined non-inferiority margin. Patient input had previously been central to the development of both the specialist UTI clinic and a standardised protocol in Newcastle for treating recurrent cystitis. Patients highlighted variation in treatment and inequality in the use and availability of non-antibiotic alternatives as drivers for carrying out this research. A significant number of patients expressed a desire not to use antibiotics and asked specifically for alternatives. The alternative treatment (methenamine hippurate) was selected on the basis of this feedback and the existence of level 1 evidence to support its use. This patient group specifically helped to define the strict non-inferiority margin of one single UTI in a year chosen for this trial, which was used in the sample size calculation. This was a stark indication of the morbidity associated with rUTIs from the patients’ perspective. The patient information sheets (PISs) and documentation pro formas were finalised with feedback from patients attending the UTI clinic at the lead site (Newcastle). We also obtained feedback from patient representatives from the Cystitis and Overactive Bladder (COB) Foundation. The COB Foundation previously advised the team on issues relating to the reporting of research, and assisted with the dissemination of research in the national press and in its own publication A Wee Ray of Hope. A member of this patient interest group was invited to join the Trial Steering Committee (TSC). Following the conclusion of the trial, our links with patient groups, such as the COB Foundation and Bladder Health UK, will be utilised to ensure widespread dissemination of results.
Details of trial medication
Planned interventions
Both prophylactic antibiotics and methenamine hippurate are licensed and approved for routine NHS use; this was detailed clearly in the trial’s PIS.
Antibiotic prophylaxis
For those women randomised to receive antibiotics, a once-daily prophylactic low dose was prescribed for 12 months. The agent used was selected by the responsible clinician, with input from the participant, based on patient characteristics such as previous use, allergy, renal function, liver function, prior urine cultures and local guidance. Available evidence suggested the use of 50 mg or 100 mg of nitrofurantoin, 100 mg of trimethoprim or 250 mg of cefalexin, in that order of preference. Renal function was determined by eGFR at baseline, and if this was < 45 ml/minute/1.73 m2 then nitrofurantoin was not used. Participants randomised to receive antibiotic prophylaxis had blood samples taken at 3, 6, 9, 12, 15 and 18 months to monitor kidney and liver function [eGFR and liver function test (LFT): creatinine, bilirubin, alkaline phosphatase and alanine transaminase (ALT) levels]. If there were any abnormalities in these tests during the period of treatment, appropriate action was taken by the responsible clinician. If clinically indicated, the blood tests were taken more frequently. Participants were asked to take once-daily antibiotic prophylaxis as a single dose at bedtime. If there were specific and intolerable adverse effects (e.g. nausea with nitrofurantoin or candidiasis with cefalexin), switching to an alternative antibiotic agent was advised in consultation with the relevant clinician and the reasons for the change were recorded. The aim was to maintain participants on antibiotic prophylaxis using any one of the three agents for as long as possible during the 12-month treatment period within tolerance and safety constraints. Participants intolerant of prophylactic antibiotic despite trying alternative agents had the opportunity to discontinue the medication and were offered an alternative treatment, which may have been a switch to methenamine hippurate. This information was recorded and the participant continued in the trial. If a participant in the antibiotic prophylaxis arm developed symptoms and signs suggestive of breakthrough UTI, they were advised to seek treatment in their usual way mostly by contacting their GP and starting a discrete treatment course of antibiotics. In this scenario, participants were instructed to stop the prophylactic antibiotic while they were taking a treatment course and restart it the day following the last dose of the antibiotic treatment course. Clinicians and participants were advised to use a different agent for treatment from the one taken for prophylaxis. Details of all antibiotic treatment courses were recorded, including the agent used and the number of days participants took the prescribed antibiotic.
Methenamine hippurate
For those women randomised to receive methenamine hippurate, a twice-daily dose of 1 g to be taken 12 hours apart was prescribed for 12 months [as recommended in the British National Formulary (BNF)]. 24 Participants randomised to receive methenamine hippurate had blood samples taken at 3, 6, 9, 12, 15 and 18 months to monitor kidney and liver function (eGFR and LFT: creatinine, bilirubin, alkaline phosphatase and ALT). If there were any abnormalities in these tests during the period of treatment, appropriate action was taken by the responsible clinician. If clinically indicated, blood tests were taken more frequently. If there were specific and intolerable side effects, such as nausea, gastrointestinal disturbance, itching or skin rashes, participants were given the opportunity to discontinue treatment and were offered the alternative treatment of prophylactic antibiotics. This information was recorded and the participant continued in the trial. If a participant in the methenamine hippurate arm developed symptoms and signs suggestive of breakthrough UTI then they were advised to seek treatment in their usual way, predominantly by contacting their GP and starting a discrete treatment course of antibiotics. They were instructed to continue taking methenamine hippurate during this antibiotic treatment course. Details of all antibiotic treatment courses were recorded, including the agent used and the number of days participants took the prescribed antibiotic.
Standard care for both arms
The ALTAR trial was pragmatic in design and, apart from random allocation of treatment option and participant completion of questionnaires, participant care followed standard pathways in participating secondary care NHS sites. During the trial, participants had access as desired to the use of other measures to reduce the risk of UTI, such as adequate fluid intake, avoidance of constipation and, for postmenopausal women, vaginally administered oestrogen supplements. Participants were also informed of the possible benefit of other alternative options, including cranberry extract. Participants in both trial arms received on-demand discrete courses of antibiotics for symptomatic UTI, as decided by the responsible clinician. The use of all adjunctive treatments was recorded on the relevant case report forms (CRFs).
Delivery of interventions
Local NHS clinicians at the site of randomisation were responsible for initiating and maintaining the delivery of trial medication.
Funding of trial interventions
The interventions were funded by standard NHS contracting mechanisms, having been sanctioned by local commissioning groups through local trial approval mechanisms. The NHS excess treatment costs were approved by the sponsor and, for primary care, the local Clinical Commissioning Group. Any prescription charges incurred by participants for trial drugs were reimbursed from research costs. The trial interventions were of low cost.
Outcome measurement
Outcomes were collected for each participant over the 12-month treatment period following randomisation and during a follow-up period of 6 months after completion of the planned course of preventative treatment (resulting in a total observation period of 18 months for each participant).
Primary clinical outcome measures
The primary clinical outcome for the trial was incidence of symptomatic antibiotic-treated UTI self-reported by participants over the 12-month treatment period.
An episode of UTI was defined as the presence of at least one patient-reported or clinician-recorded symptom from a predefined list encompassing the recommendations of the British Infection Association, together with taking a discrete treatment course of antibiotic for UTI prescribed by a clinician or as part of patient-initiated self-start treatment. 25 Symptom diary format conformed to the recommendations of the British Infection Association. 25 The symptoms recorded were fever, shivers, cloudy urine, smelly urine, visible blood in urine, urinary leakage, lower abdominal pain, feeling generally unwell, frequent passing of urine and pain when passing urine. The end of a single episode of UTI was defined as 14 days after the end of the final treatment course of antibiotics. If a further course of antibiotics was prescribed or symptoms restarted before the end of the 14 days this was counted as the same UTI episode.
The primary outcome was determined by the collection of data from multiple sources. At the time of the UTI, participants were asked to record symptoms and antibiotic treatment courses taken on a UTI record form, which was posted to the central trial office for data entry. Participants were also asked to notify local research staff of the occurrence of a UTI using a telephone number with answerphone. Symptoms and antibiotic treatment courses were then recorded on a phone-reported UTI CRF by site staff. Participants were also asked about the occurrence of symptomatic UTI episodes at the time of each 3-monthly participant review with local research staff. Symptoms and antibiotic treatment courses were recorded on the CRF. In addition, antibiotic treatment courses for UTIs were recorded on the 3-monthly participant-completed questionnaires. The occurrence of symptomatic UTI could also be obtained from health-care records, if required.
To ensure consistent attribution and to avoid multiple counting of any UTI episodes reported across different data sources, a hierarchy of evidence was utilised. First, data from health-care records were used as these data should have been the most accurate. Second were UTI record forms and phone-reported UTI CRFs, as these were completed at the time of the UTI. Finally, data from the 3-monthly participant review and participant-completed questionnaires were used. These were deemed the lowest source of evidence because of their retrospective completion, which may have been subject to recall bias.
Episodes of symptomatic UTI were identified by the trial statistician using statistical programming. A clinician not otherwise involved in the trial independently reviewed completed CRFs for a 10% sample of cases, blind to treatment allocation, and was asked to identify symptomatic UTI episodes following the hierarchy of evidence described above. In all cases the number of UTI episodes were found to match that determined by statistical programming when following the predefined criteria. In the event that discrepancies in the number of symptomatic UTI episodes were identified, a second review of the coding algorithm would have been undertaken and a further sample checked; however, this was not required. In the protocol, it was originally anticipated that a randomly selected 10% sample of positive primary outcome episodes would be reported during the first 6 months of the trial and presented as vignettes to the clinical members of the TSC, without details of allocated group, for them to determine whether or not the primary outcome was fulfilled. However, the DMC subsequently advised, as agreed by the TSC, that this role should be fulfilled by an independent clinician not otherwise involved in the trial and that a 10% sample of all cases should be reviewed, not just those observed in the first 6 months of the trial.
The incidence of symptomatic, antibiotic-treated UTI during the 12-month preventative treatment period is primarily defined simply in each group as:
(1)Total number of symptomatic antibiotic-treated UTI episodes÷Total observational period(in years).
The ‘observational period’ was calculated for each participant as the time from randomisation to the date of the 12-month participant review. If the 12-month review took place more than 1 year from randomisation, the observational period was capped at 1 year. If the participant did not attend their 12-month visit, the observational period was calculated as the time from randomisation to their last attended monthly visit or the date of the last UTI record form or telephone-reported UTI CRF prior to 12 months, whichever was later. For participants who had withdrawn from the trial but allowed continued use of their health-care data, sites were requested to check health-care records for symptomatic UTI episodes once the participant would have reached the 12-month time point. Where this was done, the participant observation time was 1 year.
Subsequent analysis of the primary outcome was based on the incident density rate over the 12-month treatment period. This removed time taking therapeutic antibiotics for UTI from the observational period and was calculated in each group as:(2)Total number of symptomatic antibiotic-treated UTI episodes÷Total observational period (in years)–Total time taking therapeutic antibiotics for UTI (in years).
For each participant, the time spent taking therapeutic antibiotics for UTI was calculated from antibiotic treatment courses identified following data processing, using the hierarchy of evidence, for the primary outcome measure. Antibiotic treatment courses for UTIs for which no symptoms were reported were included. Where the end date of a treatment course was missing, a period of 5 days was used as a surrogate.
Secondary outcomes
The occurrence of symptomatic antibiotic-treated UTI in the 6-month follow-up period after stopping the allocated preventative therapy
Episodes of symptomatic UTI occurring between 12 and 18 months post randomisation were defined as for the primary outcome. The observational period was defined as the time (in years) from 1 year post randomisation to the 18-month participant review date. If the 18-month review took place more than 18 months from randomisation, the observational period was capped at 6 months (0.5 years). If the participant did not attend their 18-month visit, the observational period was calculated as the time from 1 year post randomisation to the 15-month visit date or the date of the last UTI record form or phone-reported UTI CRF completed between 12 and 18 months post randomisation, whichever was later. For participants who had withdrawn from the trial but allowed continued use of their health-care data, sites were requested to check health-care records for symptomatic UTI episodes once the participant would have reached the 18-month time point. Where this was done, the participant’s observation time was 6 months (0.5 years).
Antibiotic use
The use of both prophylactic and therapeutic antibiotics was recorded.
Antibiotic prophylaxis
The duration of prophylactic antibiotic use was defined as the number of days for which patients were prescribed antibiotics at a low dose intended for prophylaxis against UTIs. This was measured from the date of first prescription for antibiotic prophylaxis (or the date of randomisation if the first prescription date was unavailable and the participant was randomised to the antibiotic prophylaxis arm) to the patient’s 12-month review, or to the date of stopping treatment, trial withdrawal or switching to the alternative treatment group. For those lost to follow-up during the 12-month treatment period, the last reported treatment compliance assessment date was used. Although for one arm of the trial this was the allocated treatment, measuring this outcome was intended to capture the prophylactic antibiotic use of patients who were initially allocated to the methenamine hippurate arm and needed to change treatment for any reason.
Therapeutic antibiotics
The use of therapeutic antibiotics was defined as the number of days for which patients were prescribed therapeutic (as opposed to prophylactic) doses of antibiotics for breakthrough UTIs during the treatment period of 12 months (following allocation to either the prophylactic antibiotic arm or the methenamine hippurate arm) and, separately, for the 6-month follow-up period (including previous prescription for self-start therapy). Antibiotic treatment courses were those identified following data processing from the primary outcome measure, utilising the hierarchy of evidence to avoid multiple counting of the same treatment course. Antibiotic treatment courses for UTI where there were no associated symptoms reported were included. Where an end date of a treatment course was missing, a period of 5 days was used as a surrogate. The rate of therapeutic antibiotic use was calculated as the total number of days for which therapeutic antibiotics were prescribed divided by the total observational period (in days).
Antibiotics taken for reasons other than UTI were also recorded, given their potential activity against uropathogens. The number of days for which participants were prescribed therapeutic antibiotics for reasons other than UTI was calculated. The rate of therapeutic antibiotic use for reasons other than UTI was calculated as the total number of days for which therapeutic antibiotics were prescribed for reasons other than UTI divided by the total observational period (in days).
Number of microbiologically proven symptomatic antibiotic-treated urinary tract infections
Participants were requested to submit urine samples to the central laboratory in Newcastle when they suspected a UTI, based on symptoms. Symptomatic UTI episodes, identified as per the primary outcome, were considered to be microbiologically proven if a positive urine culture from a urine sample sent to the central laboratory, or, if no sample was received, a positive culture reported by a local laboratory, was available from between 14 days prior to starting antibiotic treatment up to the end of antibiotic treatment. A positive culture was classified according to the current standard Public Health England definitions: the laboratory report of a single isolate at ≥ 104 colony-forming unit (CFU)/ml or two isolates at ≥ 105 CFU/ml. 26
Antimicrobial resistance and multidrug resistance
Ecological change in terms of type of bacteria and their resistance patterns in isolates from (1) mid-stream urine (MSU) samples and (2) the faecal reservoir (via optional perineal swabs) was explored during the 12-month treatment period and in the 6 months following completion of treatment.
Participants were requested to submit urine samples to the central laboratory when they suspected a UTI based on symptoms, and routinely at baseline and at 3, 6, 9, 12, 15 and 18 months. Antimicrobial sensitivity testing against a panel of antibiotics was carried out on all significant urinary isolates (i.e. a single isolate at ≥ 104 CFU/ml or up to two isolates growing at concentrations ≥ 105 CFU/ml). We also assessed antimicrobial resistance in E. coli within the faecal reservoir by obtaining isolates from optional perineal swabs sent to the central laboratory at baseline and at 6, 12 and 18 months. For the purposes of our study, resistance was defined as resistance to at least one antimicrobial agent. Multidrug resistance (MDR) in E. coli was defined as resistance to at least one antimicrobial agent in at least three antimicrobial categories, following the principles described by Magiorakos et al. 27 For this trial, the antimicrobial agents and categories have been tailored to be specific to uropathogens and are set out in Table 2. For each antibiotic tested, development of ‘(known) resistance’ since baseline was first defined as patients or samples showing resistance post baseline as a proportion of those known to be sensitive at baseline. Owing to the expected low number of positive urine cultures at baseline, we also defined ‘resistance since baseline’ as patients or samples showing resistance post baseline comprising both bacterial isolates confirmed as sensitive at baseline and also those urine samples with no isolates identified in the baseline sample. Development of MDR since baseline was defined similarly.
| Antimicrobial category | Antimicrobial agent |
|---|---|
| Aminoglycosides | Gentamicin |
| Antipseudomonal penicillin | Piperacillin/tazobactam |
| Carbapenems | Ertapenem; meropenem |
| Non-extended-spectrum cephalosporins | Cefuroxime; cefalexin |
| Fluoroquinolones | Ciprofloxacin |
| Folate pathway inhibitors | Trimethoprim–sulphamethoxazole (co-trimoxazole); trimethoprim |
| Monobactams | Aztreonam |
| Penicillins | Amoxicillin; mecillinam |
| Penicillins + β-lactamase inhibitors | Amoxicillin–clavulanic acid (co-amoxiclav) |
| β-Lactamase-resistant penicillin | Temocillin |
| Phosphonic acids | Fosfomycin |
| Nitrofurans | Nitrofurantoin |
Occurrence of asymptomatic bacteriuria
The occurrence of asymptomatic bacteriuria was defined as a positive urine culture in the absence of symptoms. This was detected from the routine urine samples taken during 3-monthly hospital visits throughout the 18-month trial period where participants confirmed no UTI symptoms. Samples taken within 5 days of the onset of UTI symptoms were not included. A positive culture was classified as the presence of a single isolate at ≥ 104 CFU/ml or two isolates at ≥ 105 CFU/ml.
Hospitalisation due to urinary tract infection
Hospitalisation due to UTI was defined as an unplanned visit to hospital for treatment of a UTI. These data were collected from health-care record review and checked from participant report.
Participant satisfaction with treatment
This was measured using the Treatment Satisfaction Questionnaire for Medication (TSQM) administered at both the end of the 12-month treatment period and then again at 18 months, at the end of follow-up. 28 Four separate subscale scores (effectiveness, side effects, convenience and global satisfaction) were calculated as per the scoring algorithm. Possible scores range from 0 to 100, with higher scores indicating increased satisfaction. The effectiveness, convenience and global satisfaction subscales comprised three items and the side effects subscale four. A score was not calculated if more than one item was missing from each subscale.
Economic analysis
The objective of the economics analysis in the ALTAR trial was to determine the relative efficiency of methenamine hippurate compared with antibiotic prophylaxis in the management of rUTI. The economic analysis comprised both a within-trial (18-month time horizon) analysis and a model-based analysis (lifetime time horizon). Costs were estimated using trial-specific estimates and unit costs from routine sources. Effectiveness was measured using the primary outcome (incidence of UTIs) and QALYs, which were estimated based on responses to the EQ-5D-5L. The differences in costs were equated to the differences in effectiveness (incidence of UTIs and QALYs) to estimate incremental cost-effectiveness. Stochastic and probabilistic sensitivity analyses determined the probability of methenamine hippurate being considered cost-effective. The inclusion of a within-trial and model-based economic analysis as part of the ALTAR trial was essential to help address uncertainties surrounding the effectiveness and efficiency of each management strategy in the short term (18 months) and over the longer term. For more details of the economic analysis, see Chapter 5.
Data collection
Summary
Outcome data were collected using CRFs, participant-completed questionnaires and UTI records, and information retrieved from participants’ medical notes. Data from the CRFs were entered into the trial-specific database set up using the MACRO clinical data management system (Elsevier BV, Amsterdam, the Netherlands) by delegated research staff at each site. Participant-completed questionnaires and UTI records were returned by post to the central NCTU trial office and entered into the MACRO database by NCTU staff. The results of urine and perineal swab analysis were processed and uploaded into the MACRO database by the database manager from reports produced by the central laboratory. The NCTU trial team worked closely with sites to ensure data completeness and accuracy.
Trial events
Screening
Potentially eligible participants were identified through direct contact or by searches of electronic records held in each participating NHS trust. Participants were provided with trial information via the ALTAR PIS (see the NIHR Journals Library project web page – URL: www.journalslibrary.nihr.ac.uk/programmes/hta/138821/#/) and consent was taken prior to randomisation (see the NIHR Journals Library project web page – URL: www.journalslibrary.nihr.ac.uk/programmes/hta/138821/#/). All patients approached were recorded on site-specific screening logs and, where provided, reasons for non-participation were documented.
Participants experiencing symptomatic UTI were treated with standard antibiotic therapy and not randomised until they were symptom free.
Randomisation
Randomisation was performed as close as possible to the date of consent (normally this was immediately after). For consented participants who completed a 3-month washout period, eligibility was rechecked prior to randomisation.
Follow-up
The schedule of events for the ALTAR trial is shown in Table 3.
| Procedures | Screening | Baseline | Treatment phase | Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 months | 6 months | 9 months | 12 months | At time of UTI | Monthly checks | 15 months | 18 months | |||
| Informed consent | ✓ | |||||||||
| Demographics | ✓ | ✓a | ||||||||
| Medical history | ✓ | |||||||||
| Physical examination | ✓ | |||||||||
| eGFR and LFTs (a sample for DNA analysis will be taken at one of these time points) | ✓ | ✓a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| MSU (local lab) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| MSU (central lab) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Perineal swab | ✓ | ✓ | ✓ | ✓ | ||||||
| Concomitant medications | ✓ | ✓a | ||||||||
| Eligibility assessment | ✓ | |||||||||
| Randomisation | ✓ | |||||||||
| Dispensing of trial drugs | ✓ | ✓ | ✓ | ✓ | ||||||
| Compliance | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| UTI record | ✓ | |||||||||
| UTI questionnaire | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| EQ-5D-5L | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Health resource use questionnaire | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| TSQM | ✓ | ✓ | ||||||||
| AE assessments | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| CRF completion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Data handling and record-keeping
Case report form data were entered by site staff into the trial-specific clinical data management system, MACRO. Participant-completed questionnaires and UTI records, identifiable only by participant identifier, were returned by post to the central NCTU trial office, where they were entered by NCTU staff and stored securely. As per participant consent processes, identifiable data were stored in a separate, password-protected database within NCTU, with access limited to members of the trial team responsible for the preparation and sending of follow-up questionnaires and logging their return. Two reminders, each with an additional copy of the questionnaires, were sent to participants to prompt their return. Central laboratory results of urine and perineal swab analysis were processed and uploaded into the MACRO database by the database manager. Participants were allocated an individual trial number at randomisation and all trial data and laboratory samples were identified by this unique trial number. Essential data will be retained for a period of at least 10 years following close of the trial, in line with the sponsor’s policy and the latest European Directive on GCP (2005/28/EC). 29 Data were handled, digitalised and stored in accordance with the Data Protection Act 199830 and the General Data Protection Regulation31 (GDPR) from 25 May 2018.
Changes to protocol
Changes made to the protocol during the trial are listed in Table 4.
| Change to protocol | Protocol version | Date |
|---|---|---|
|
1.1 | 7 April 2016 |
|
1.2 | 24 May 2017 |
|
1.3 | 13 Dec 2017 |
|
2.0 | 7 June 2019 |
|
3.0 | 19 Dec 2019 |
Sample size calculation
The ALTAR trial had a planned recruitment target of 240 patients, 120 in each of the treatment arms.
A non-inferiority design was chosen, as we believed that an oral urinary antiseptic (methenamine hippurate) would be acceptable to the patient group provided that its effectiveness for UTI prevention was no worse than antibiotic prophylaxis and that the burden of adverse effects was similar or better. There is also the key added potential theoretical benefit of reduced rates of resistant organisms and subsequent collateral harm to the individual and community.
Semistructured interviews with a patient panel of 12 women identified that any reduction in UTI episodes, even one per year, would be deemed worthwhile. Therefore, the minimum clinically important difference between the treatment arms of one UTI per 12 months was set as our strict, non-inferiority margin.
Two existing meta-analyses of studies examining prophylactic antibiotics5 and methenamine hippurate14 both quoted a mean relative risk of UTI compared with placebo of 0.15 and 0.24, respectively. Using these values and data from a local audit (Charlotte Cuff, University of Newcastle, Biomedical Sciences Thesis 2014; n = 200) suggesting that the average number of UTI episodes per year in this patient group is 6.5, we estimated that the average number of UTI episodes per year during preventative treatment would be 0.975 in those randomised to antibiotic prophylaxis and 1.56 in those randomised to methenamine hippurate. This equates to an estimated difference in UTI episodes per year between prophylactic antibiotics and methenamine hippurate of around 0.6 episodes (in favour of antibiotics).
The standard deviation (SD) of UTI episodes per year is taken from the placebo groups in the studies included in the Cochrane meta-analyses and was conservatively estimated at 0.9 episodes per year. 5,14
Using a two-sample t-test and assuming an actual difference of 0.6 UTI episodes per year (in favour of treatment with antibiotics) and a SD of 0.9 UTI episodes per year, then two groups of 87 patients would be required to be 90% sure that the lower limit of a one-sided 95% CI (or equivalently a 90% two-sided CI) was above the non-inferiority limit of one. The attrition rate was conservatively estimated at 25%; therefore, the total sample size required was 232, rounded up to 240.
Statistical analysis
A SAP that includes full details of all statistical analyses was finalised and agreed prior to the final database lock and analysis (see the NIHR Journals Library project web page – URL: www.journalslibrary.nihr.ac.uk/programmes/hta/138821/#/). The statistician responsible for approving the SAP remained blind to treatment allocation until after the final database lock. The main analysis of the primary outcome measure was performed in the modified intention-to-treat (mITT) population and contained all randomised participants with an observational period of at least 6 months. Sensitivity analyses were performed in a strict intention-to-treat (ITT) population and a per-protocol population that included all participants achieving at least 90% compliance with any trial preventative treatment. A post hoc sensitivity analysis in a stricter per-protocol population, including all participants achieving at least 90% compliance with their initial randomised trial treatment, was also performed.
Analyses of symptomatic antibiotic-treated UTIs in the 6-month follow-up period (12–18 months) was conducted in all participants in active follow-up at 15 or 18 months plus those with data available from health-care record review.
For all analyses, with the exception of AE data, participants were analysed according to their randomised treatment assignment.
Primary outcome
The simple incidence rate of symptomatic antibiotic-treated UTI episodes over the 12-month treatment period was calculated in each randomised treatment group and reported with a 95% CI calculated using a resampling (bootstrap) procedure with 5000 replicates. The difference between groups (methenamine hippurate vs. antibiotic) was estimated along with a 90% CI calculated using the same resampling (bootstrap) procedure with 5000 replicates. Provided that the upper 90% confidence limit was lower than the inferiority limit of one, non-inferiority of treatment with antiseptic (methenamine hippurate) compared with antibiotic would be inferred. Analyses were repeated using the incident density rate.
The relative difference between treatment groups in the incidence of symptomatic antibiotic-treated UTI episodes over the 12-month treatment period was estimated using a negative binomial regression model with differences between centre included as a random effect and prior annual UTI frequency (< four vs. ≥ four episodes) and menopausal status (premenopausal vs. menopausal/postmenopausal) included as fixed effects. Poisson and zero-inflated negative binomial models were also considered, with model fit compared using Akaike information criterion (AIC) and Bayesian information criterion (BIC) and by graphical examination of the observed and predicted number of events using each model. An estimate of the incidence rate ratio (IRR) was obtained and presented with a 95% CI, calculated using a robust/sandwich estimator of variance. An IRR > 1 would indicate an increased risk of UTI in the methenamine hippurate arm compared with the antibiotic prophylaxis arm.
A binary indicator of at least one episode of symptomatic antibiotic-treated UTI was analysed using a logistic regression model, adjusted for centre, prior UTI frequency and menopausal status, as above. The odds ratio (OR) was reported with 95% CI. Note that in all cases mixed-effects logistic regression models were not preferred to the unadjusted model, as assessed using AIC and BIC.
Secondary outcomes
Symptomatic antibiotic-treated urinary tract infection in the 6-month follow-up period
This outcome was analysed using the same approach as for the primary outcome measure but with 95% CI reported for the difference in the incidence rate between groups. For analyses in the 6-month follow-up period a Poisson model was used rather than a negative binomial model, as this provided a better model fit.
Microbiologically confirmed symptomatic antibiotic-treated urinary tract infection
This outcome was analysed using the same approach as for the primary outcome measure but with 95% CI reported for the difference in the incidence rate between groups.
Antibiotic use
The number and proportion of participants receiving prophylactic antibiotic, therapeutic antibiotics for UTI and therapeutic antibiotics for other reasons was reported. Of those receiving treatment, the total duration (in days) was summarised descriptively. The rate of antibiotic use (number of days of treatment over the observational period) was calculated for all participants and summarised descriptively.
Antimicrobial resistance
The number of perineal swabs available at each time point was reported, along with the number of samples with E. coli isolated. Of the samples with E. coli isolated, the numbers of resistant antibiotic agents and antimicrobial categories (as per the MDR definition) were summarised descriptively. The number and proportion of participants demonstrating any antibiotic resistance in E. coli at baseline, 6 or 12 months and 18 months were tabulated with comparisons made between groups using a chi-squared test. The number and proportion of participants demonstrating MDR were presented similarly but with comparisons between groups made using a Fisher’s exact test. The proportion of participants demonstrating antibiotic resistance in E. coli to eight antibiotics used against UTIs (i.e. amoxicillin, cefalexin, cefuroxime, ciprofloxacin, co-amoxiclav, co-trimoxazole, nitrofurantoin and trimethoprim) was graphically summarised over time along with 95% CIs. For each antibiotic, the number and proportion of participants with E. coli isolates demonstrating antibiotic resistance since baseline (of those known to be sensitive at baseline or with no E. coli isolated at baseline) and known antibiotic resistance since baseline (of those known to be sensitive at baseline) were presented. Similarly, development of MDR since baseline and development of known MDR since baseline were also reported.
The availability of routine urine samples at baseline and 3-monthly follow-up visits was reported along with the number of samples with E. coli isolated. Of those samples in which E. coli was grown, the isolate’s antimicrobial resistance was summarised descriptively, noting the number of antibiotic agents and antimicrobial categories to which it was resistant (as per the MDR definition, i.e. resistance to at least one antimicrobial agent in at least three antimicrobial categories, following the principles described by Magiorakos et al. 27).
The number and proportion of participants demonstrating any antibiotic resistance in E. coli isolated from any urine sample submitted during periods of symptomatic UTI within the 12-month treatment period and 6-month follow-up period were tabulated. Comparisons between groups were performed using a Fisher’s exact test. The number and proportion of participants with E. coli isolates demonstrating MDR was presented similarly. In addition, the number and proportion of E. coli isolates (rather than participants) demonstrating any antimicrobial resistance and MDR were reported.
For each antibiotic, the number and proportion of participants with urinary E. coli isolates demonstrating antibiotic resistance isolated from any symptomatic urine sample during the 12-month treatment period and 6-month follow-up period were presented. Data were also presented as the number and proportion of resistant E. coli isolates (rather than participants). The cumulative proportion of resistant E. coli isolates was plotted, with 95% CIs, over the 18-month trial period for each antibiotic.
For each antibiotic, utilising all submitted urine samples, the number and proportion of participants demonstrating likely antibiotic resistance since baseline (out of those known to be sensitive at baseline or with no E. coli isolated at baseline) and known antibiotic resistance since baseline (out of those known to be sensitive at baseline) were presented. In the same way, development of MDR since baseline and development of known MDR since baseline were also reported.
All analyses of antimicrobial resistance data from urine samples (except MDR) were repeated based on any bacteria isolated, rather than only E. coli.
Asymptomatic bacteriuria
The number and proportion of positive urine cultures detected from routine samples taken in the absence of symptoms was presented by treatment group. Data were summarised over the whole 18-month trial period and also grouped by time periods (baseline, 3–12 months and 15–18 months). Post hoc comparisons between randomised treatment groups were performed using chi-squared tests.
Hospitalisation due to urinary tract infection
Hospitalisation due to UTI was summarised descriptively.
Participant satisfaction with treatment
Four separate subscale scores (effectiveness, side effects, convenience and global satisfaction) from the TSQM were calculated as per the scoring algorithm at 12 and 18 months, and summarised descriptively. Comparisons between treatment groups were made using a two-sample t-test and an analysis of covariance model adjusted for baseline stratification factors: prior UTI frequency and menopausal status.
Adverse events
Adverse events were reported throughout the 18-month trial period. All events were graded as mild, moderate or severe, and relationship to trial medication was reported as unrelated, unlikely, possible, probable or definite. All events were coded systematically at the end of the trial using the Medical Dictionary for Regulatory Activities (MedDRA), version 22.0.
To account for any participants who were switched to the alternative treatment group, AEs are primarily reported by treatment received (antibiotic prophylaxis or methenamine hippurate) at the time of AE onset. Data were also summarised by randomised treatment group.
The numbers of AEs and adverse reactions (ARs) (possibly, probably or definitely related to treatment) reported per participant were summarised descriptively. The worst grade of AE and AR reported per participant was also presented.
The number of participants reporting each type of AE was tabulated. Only those occurring in at least 3% of participants in either treatment group are shown, owing to a large number of different AEs being reported in a small number of participants.
Serious adverse events (SAEs) and serious ARs were listed.
Trial progress and monitoring
The monitoring of trial conduct and data collected was performed by a combination of off-site, on-site and central monitoring to ensure that the trial was conducted in accordance with the trial protocol and GCP trial monitoring. This included the review of consent procedures, source data verification, SAEs, trial conduct and essential documentation, and was undertaken by members of the Trial Management Group (TMG).
Microbiological methods
Urine samples
Participants were requested to submit MSU samples to the central laboratory when they suspected a UTI, based on symptoms, and routinely at baseline and at 3, 6, 9, 12, 15 and 18 months. The specimens were sent to the central reference laboratory (Freeman Hospital, Newcastle upon Tyne) in a standard urine specimen container pre-loaded with boric acid at a concentration of 18 g/l (International Scientific Supplies Ltd, Bradford, UK), within secure packaging (Safebox™; Royal Mail Ltd, London, UK) and accompanied by a sample shipment checklist, completed by the participant, identifying the time point and type of sample. On arrival at the laboratory, the specimens underwent semiquantitative urine culture and antimicrobial sensitivity testing was carried out in triplicate against a panel of agents on significant isolates. Isolates were deemed significant if semiquantitative bacterial culture yielded ≥ 104 CFU/ml of a single organism or ≥ 105 CFU/ml of up to two different organisms.
To measure microbiologically proven UTI and altered bacterial phenotype and genotype (secondary outcome measures), MSU specimens were collected. Participants collected their own samples, in accordance with standard instructions for MSU collection (which were included in their trial information packs) at baseline and at 3, 6, 9, 12, 15 and 18 months post randomisation, and during the early part of any episodes of UTI prior to antibiotic treatment. The specimens were sent to the central reference laboratory (Freeman Hospital, Newcastle upon Tyne, UK) in a standard urine specimen container pre-loaded with boric acid at a concentration of 18 g/l (International Scientific Supplies Ltd), within secure packaging (Safebox) and accompanied by a sample shipment checklist, completed by the participant, identifying the time point and type of sample. On arrival at the laboratory, a semiquantitative urine culture was conducted, with samples deemed significant if they achieved specified criteria: ≥ 104 CFU/ml of a single isolate or ≥ 105 CFU/ml of up to two isolates.
Perineal swabs
To measure altered gut commensal bacterial phenotype and genotype (secondary outcome measures), optional perineal swabs were collected from participants. Perineal swabs were chosen as a surrogate for rectal swabs as they were felt to be less invasive and, therefore, potentially more acceptable to participants, while still enabling sampling of the individual’s enteric flora. Participants collected their own samples, according to standard instructions for perineal swab collection (included in their trial information packs), at baseline and at 6, 12 and 18 months after randomisation. Using the labels and Safeboxes provided, the samples were sent to the central reference laboratory using surface mail. On arrival at the laboratory, the samples were cultured to establish the presence of E. coli and isolates were tested for antibiotic sensitivity in triplicate.
Any isolated E. coli were temporarily stored in the laboratory for later transfer to the UTI laboratory (Cookson Building, Faculty of Medical Sciences, Newcastle University). Where consent had been obtained from participants for storage and further analysis of samples, deoxyribonucleic acid (DNA) was extracted and stored in the laboratory for analysis of genotypes related to the UTI host response. Samples will be destroyed once the trial and necessary analyses are complete.
Definition of end of trial
The definition of the end of the trial was the last recruited participant’s last follow-up visit at 18 months post randomisation, which took place on 27 January 2020. With approval from the TSC, a 9-month extension to the trial recruitment period was granted by the trial funder in October 2017 (variation to contract, January 2018) to accommodate additional time to meet the trial recruitment target.
Compliance and withdrawal
Assessment of trial adherence
Outcome data were collected by both participant-reported completion of trial questionnaires and attendance at regular trial clinic visits. Trial clinic visits were used to ensure participant compliance with the completion of trial documentation, the provision of samples and adherence to medication allocation.
Participant attrition rates were monitored regularly throughout the trial and appropriately reported to the trial oversight committees (TSC and DMC) when required.
Some participants or their clinicians sought to change their allocated group at some point during trial participation because of either lack of efficacy or adverse effects of either treatment. The trial literature emphasised the need to adhere to the allocated strategy during the 12-month trial period, if possible, and any deviations were recorded. Multiple switching between prophylactic antibiotic agents was allowed. If participants stopped their allocated treatment within the 12-month treatment period or if they recommenced prophylaxis during the subsequent 6-month observation period, this was recorded and the participant continued on the trial unless they withdrew consent.
Data monitoring, quality control and assurance
Data Monitoring Committee
An independent DMC was established, which included two consultant urological surgeons not otherwise involved in the trial and an independent statistician. The DMC reviewed emerging efficacy and safety data by randomised treatment group. Prior to final database lock, only the trial statistician and independent DMC members (i.e. those who were not trial team members) had access to outcome data by randomised treatment group. The DMC operated in accordance with an agreed charter, based on the DAMOCLES (DAta MOnitoring Committees: Lessons, Ethics and Statistics) recommendations,32 and met six times during the trial.
Trial Steering Committee
A TSC was established to provide overall supervision of the trial. The TSC included an independent chairperson, two independent urological surgeons, an independent GP, two independent lay representatives and the chief investigator. Other members of the TMG attended as required. The committee met approximately every 6–12 months for the duration of the trial.
Data monitoring
Quality control was maintained by compliance with relevant standard operating procedures (sponsor and NCTU), local research policies, principles of GCP, the trial protocol, research governance and clinical trial regulations.
Monitoring of trial administration and data collection was performed using both central review and site monitoring visits conducted by the NCTU trial team. Monitoring included review of patient consent procedures, eligibility assessment, source data verification and completeness of investigator site files. Trial data were monitored centrally throughout the trial to ensure data completeness and accuracy; sites were contacted to help resolve queries when they arose.
An audit was conducted on data entered centrally by NCTU staff. As the UTI records relate directly to the primary outcome, a full audit of all such records was conducted to ensure accuracy. In addition, an audit of a random sample of 10% of the participant questionnaires and a further random sample of 10% of the uploaded laboratory sample data was conducted. These quality control checks resulted in an error rate below the threshold of 5%.
Ethics and governance
The Newcastle upon Tyne Hospitals NHS Foundation Trust Research and Development Directorate sponsored the trial (reference 06867). A favourable ethics opinion for the trial was obtained on 23 December 2015 from the NHS Research Ethics Service Committee North East – Tyne and Wear South (reference 15/NE/0381). Health Research Authority approval was issued for the trial on 20 June 2016 and subsequent local research and development approvals were sought for each participating site. Approval was sought and obtained for all substantive protocol amendments.
Trial registration and protocol availability
The trial was registered as International Standard Randomised Controlled Trial Number (ISRCTN) 70219762 on 31 May 2016 and in the UK NIHR portfolio (reference HTA 13/88/21). The latest version (version 3.0) of the full protocol is available at the NIHR Journals Library project web page (URL: www.journalslibrary.nihr.ac.uk/programmes/hta/138821/#/) and a published version is also available. 1
Serious adverse event reporting
The trial protocol provided guidance on AE and SAE reporting, as well as determining the degree of relatedness and assessment of causality for SAEs that may be related to trial participation. The reference safety information for assessment of expectedness of related events was contained in the summary of product characteristics for trimethoprim, nitrofurantoin, cefalexin and methenamine hippurate. SAEs excluded UTI, since this was the primary outcome collected and documented throughout the trial. All SAEs were reported for the duration of the trial and for 4 weeks after the trial intervention was stopped.
Chapter 3 Embedded qualitative study of the views of patients and recruiting staff on the ALTAR trial
Parts of this chapter have been reproduced with permission from Lie et al. 33 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
Understanding why patients agree or decline to participate in clinical trials is a crucial factor in the successful delivery of trials on time and to target. Embedded qualitative studies can provide in-depth information on the views and experiences of potential patient participants and recruiting staff that quantitative research cannot provide and highlight issues that may affect recruitment and retention. 34 To identify modifiable factors that could improve the conduct of the ALTAR trial, an embedded qualitative study was proposed. In the first 8 months that sites were open to recruitment, the plan was to conduct up to 15 in-depth interviews with patients approached about the ALTAR trial in each of three groups: (1) those who agreed to participate in the trial and continue on the trial until its end, (2) those who dropped out of the trial and (3) those who declined to participate. In addition, a focus group of eight recruiting staff participants was planned to explore views on recruiting to and delivering the trial.
Aim and objectives
The aim of this embedded qualitative study was to inform the trial team of potential barriers to recruitment and retention experienced by participants and trial staff in the first 8 months of recruitment to the ALTAR trial. The objective was to explore the views of patients approached to participate in, and those recruited to, the ALTAR trial, and collect opinions from staff involved in the study, to identify any factors of the processes and conduct that should be modified.
Methods
Participants and procedures
Potential participants for the qualitative study were women approached by the recruiting staff [site principal investigators (PIs) and research nurses (RNs)] to take part in the ALTAR trial. The team were interested in seeking the views of women whether they had agreed or declined to participate in the trial. Recruiting staff provided women willing to be approached about the qualitative study with a study pack. The pack contained a PIS, expression of interest (EOI) form and reply-paid envelope for NCTU. Those interested in discussing the study further completed the EOI form (with name and contact number) and returned it. NCTU informed the qualitative team when EOI forms were received and of any participants who withdrew from the trial. The aim was to conduct the interviews within 2 weeks of the patients being approached about the trial, to limit any recall bias.
Those who completed and returned an EOI form were contacted by telephone to answer any questions about the qualitative study and, if they were happy to participate, a date and time was arranged for the interview.
It was decided not to conduct a focus group with recruiting staff because of time constraints and logistics. In-depth telephone interviews were considered more appropriate, as they allowed greater flexibility about when they were conducted and meant that the views of staff from more than one site could be explored. RNs and PIs were approached via e-mail, 1 month after recruitment commenced at their site, to ask if they would be happy to participate in an interview.
Data collection
In-depth telephone interviews were conducted by a trained interviewer. A topic guide was used, which was developed with input from the study team and the Patient and Public Involvement Group. The topics, for both patients/participants and recruitment staff, included experiences of trial recruitment and conduct, and views on the trial interventions. Consent was obtained before the interview commenced. Interviews were digitally recorded with the permission of the interviewee. Two time points were selected for patient interviews: within 2 weeks of being approached to participate in the ALTAR trial and 3–6 months later, or earlier if they decided to withdraw within that period. Recruiting staff were interviewed on one occasion only.
Analysis
Any modifiable factors identified during the interviews were noted and quickly fed back to the Chief Investigator and NCTU. Interviews were transcribed verbatim. Transcripts were checked and anonymised. Data were analysed drawing on the constant comparative method and a thematic coding frame was agreed upon between the members of the qualitative team. NVivo version 12 (QSR International, Warrington, UK) was used as a tool to manage and code transcript data. 35 The overall headline results were made available to inform change in the trial procedures before the end of the first year of the recruitment phase.
Recruitment to the qualitative study
Despite the original plan to recruit up to 15 patients who had declined to take part in the ALTAR trial, only one person from this group returned an EOI form. As a total of 60 patients declined to take part in the trial, this was a cause for concern.
The low recruitment of trial decliners to the qualitative study was explored in some of the interviews with recruiting staff. At one site, one interviewee said that it tended to be characteristic of those who decline trial participation also to do so in response to other aspects of a study. At another site, an interviewee admitted that they had not been asking patients approached to participate in the ALTAR trial about the qualitative study because of the process of identification and recruitment, but promised to do so in future.
In addition to site staff overlooking the need to mention the qualitative study, another explanation could have been the design of the ALTAR trial PIS. The first three pages have information in bullet points about the trial, at the end of which it states, ‘If you are interested in taking part, please continue to read the rest of this leaflet’. Only those who chose to read on would learn about the qualitative study. It is likely that those who were not interested in participating in the trial would not have continued beyond that point. There was a separate information sheet about the qualitative study, but this meant that the burden was on site staff to supply this sheet with the trial information. In retrospect, if space permitted, a bullet point in the first three pages about the qualitative study, and the importance of seeking the views of those who do not participate, would have ensured that patients were aware of it.
Findings: patient interviewees
In the period the embedded qualitative study was conducted (July 2016 to March 2017), 77 patients agreed to participate in the trial and were randomised. For the qualitative study, 34 EOIs were returned via the NCTU and 29 interviews were conducted between July 2016 and March 2017. Despite several attempts, the remaining five patients were not contactable. One of the 29 patients interviewed had declined to participate in the trial; of the remainder, 16 were randomised to an antibiotic and 12 to methenamine hippurate. Ten patients participated in a second interview 3–6 months following the first. The ages of interviewees ranged from 19 to 80+ years.
Recurrent urinary tract infections: past experience
Interviewees talked about their experience of rUTIs. How long interviewees had experienced rUTIs ranged from 2 years to the whole of their lives. A few said that the infections began when they were children:
I was really young and I’ve been having them ever since and I’m 18 now, nearly 19.
Patient 1103
The inclusion criteria for the trial were to have experienced rUTI, defined as at least three episodes of symptomatic UTI in the last 12 months, two episodes in the last 6 months or one episode requiring hospitalisation. Some interviewees described an almost continuous state of having a UTI, although others said that there could be months in between and then they would suffer from a number of infections in a short period:
Since September of last year, I have had them pretty much constantly – one goes, and then I get another one.
Patient 1018
Sometimes I can go 9 months, a year and not have a problem and then perhaps I’ll get two or three in a year.
Patient 1104
The rUTIs were described as ‘terrible’ and ‘horrendous’. In terms of the impact, some said that the rUTIs affected their whole lives. One interviewee, who was experiencing rUTIs every couple of weeks, said that even in between episodes she felt unwell as if the infections had ‘not fully resolved’ (patient 1002). Another interviewee described how they affected her daily life:
A lot of people don’t understand a UTI, they just kind of think ‘waterworks’. But when it’s . . . basic things like walking, sleeping, sitting at work, having a conversation with people, when you’ve got that level of discomfort, it’s difficult to just carry on as normal, to be honest. So yes, it really does affect your quality of life.
Patient 1005
I teach, so (infection) has an impact on lifestyle. Sometimes I was just finishing one course and having to go straight onto another. So it was affecting lifestyles and . . . time off work to go and visit doctors, and put more samples in, and it was just becoming a bit of a burden, to be perfectly honest.
Patient 1011
Interviewees recounted that they had undergone investigation to find the cause of their rUTIs. For some there was a sense of frustration, particularly when no cause could be found and the view from their clinician was that they were ‘just one of these people that had recurring infections’ (patient 1101) or ‘sometimes it just happens’ (patient 1305). Interviewees talked about the numerous courses of antibiotics that they had previously been prescribed for their UTIs, with varied success:
So over the past maybe year or so, I’ve probably had about eight rounds of antibiotics.
Patient 1009
I’d been on sort of, a 3-day course of antibiotics which does clear it but then it keeps coming back.
Patient 1101
Last year when I was pregnant I had them every week. They kept giving me antibiotics and they weren’t responding and even after I had the baby they tried me on different tablets, even the doctors at the hospital and nothing worked.
Patient 1303
Trial processes: views and experiences
Approach to participate in the trial
Women presenting with rUTIs to the participating secondary care units were first checked for eligibility. The eligibility criteria were that their condition met the definition for a rUTI, there was no structural or functional urinary tract abnormality, and that they had the capacity to consent and complete the trial documentation. Those who were eligible were given, or sent, brief study information and, if they were interested, a trial information pack was provided.
Of those interviewed, it appeared that the majority were asked about the ALTAR trial by the consultant in the outpatient clinic. If interested, they then spoke with a RN who provided more comprehensive information and gave them trial documentation to take away and read. To convey their decision on whether or not they wished to participate, patients could call the RN directly. After a certain period, if the patient had not been in touch, the RN would contact them to ask if they had made a decision about participation. When asked about their views on how they were approached to participate in the trial, the majority of interviewees were happy with the approach, and a number mentioned, spontaneously, that they had not felt pressured. Similarly, most said that there was the opportunity to ask questions about the trial. Only one person said that they would have preferred a longer session with the RN, although this did not influence their decision to participate in the trial:
No, I think how [research nurse] approached me was . . . really professional; she was very well-mannered, very, very happy member of staff and when you have someone like that come in and ask you that kind of thing, it makes you feel better about thinking about it. Like, if I had someone miserable come and approach me I don’t think I’d be too happy.
Patient 1103
I was happy with it personally . . . maybe spend a little bit more time because the nurse on the first occasion . . . when I was attending my urology appointment it was sort of, ‘Oh, we recommend that you might want to go onto this trial’, and then I was put into a room with the nurse for about 5 or 10 minutes . . . . Maybe if you were sat with the nurse for half an hour or so going through the papers and sort of had more time to ask questions and the like, then that would be good. But I don’t think it put me off at all.
Patient 1013
Few of those interviewed could suggest ways in which the process could be improved. One person commented that they would have preferred to see information about the trial in the outpatient area, to raise awareness, rather than hear about it first from the consultant.
Trial information: verbal and written
Views on the verbal information participants received were mixed. On the one hand, it was said to be good and clear, particularly the process of receiving information from the consultant followed by a discussion with the RN:
The verbal was good with the consultant, s/he even put me in touch with the nurse and s/he went through everything again as well just to make sure that I had understood it correctly from the consultant. It’s then when they gave me the leaflet to take away and read.
Patient 1001
Conversely, others found the verbal information less helpful. This first patient wanted more information about the trial medications; although they later added that the written information had been helpful and had provided the reassurance that they needed:
I didn’t think the verbal information was very reassuring, to be honest. [. . .] when I was spoken to in person, I don’t know. I don’t think I was given as much information as I wanted in person, so I was a bit nervous at first.
Patient 1008
A second interviewee said that they had wanted further information about one of the trial medications, but the member of recruiting staff they spoke with was unable to help. Another reported that they had struggled to understand what the RN meant and felt the process was hurried:
The [person] who I saw seemed either a bit – I don’t know whether [s/he] was new on the team . . . but I think sometimes you felt that [s/he] was rushing a bit. I mean when I came out of the last meeting I still wasn’t, s/he was showing me what to do if I needed to send a sample in and all the rest of it and I still, I couldn’t really understand what [s/he] said to me.
Patient 1016
A few interviewees said that the risks had not been conveyed verbally.
Regarding written information, patients received a PIS, which they took away to read at their leisure. Interviewees’ views of the written information were all positive. It was said to be well written, clear and without jargon. Information was structured as questions, which was said to be helpful. In terms of content, the fact that the study information sheet explained what was required of participants over the 12 months of the trial appeared to be an important point, and this was mentioned by a number of interviewees:
Yes, it gave the different medication options, and it went through what exactly they did, what the possible side effects were, what would happen after the 12 months of taking whichever drug you were on, and when you came to the end of 12 months, what would happen then, etc.
Patient 1018
Really helpful because then I know what I am doing and what to do over the year.
Patient 1303
Clinical trial information is usually, by necessity, lengthy. Everyone interviewed found the length of the ALTAR trial information (14 pages) acceptable and justifiable:
Well, I think it needed to be (long) to fully explain and for the participant to understand what they were going into.
Patient 1105
It wasn’t too much to read, and it was easy to read as well. It was easy to digest and understand. It was very well written, and it definitely helped me understand as well.
Patient 1004
Some interviewees mentioned that the written information reiterated what was provided verbally when they met with the RN or consultant. However, this was expressed as neither a negative nor a positive factor:
It seemed to be repeating mostly what I’d already heard from the consultant. So I kind of quickly read through it and there was nothing particularly that stood out that I hadn’t already heard.
Patient 1005
The trial PIS had a ‘disadvantages and risks’ heading but there were no risks specified. When asked if from the information they had received about the trial, they understood the risks of participating, the majority of interviewees said that they did. However, one interviewee said that they were not aware of the risks from the written information (patient 1013). Another said that the risks were not clear and had found out themselves:
Not, if I’m honest, about the side effects, because I’ve gone and researched that later. I actually remember looking at the prescription and getting the name and thinking, ‘What exactly is this?’. So I went and did a little bit of research on it.
Patient 1005
When the remainder were asked what they understood the risks to be, some said that they believed they were negligible; one said that it was the very minor inconvenience of 3-monthly check-ups and another said that ‘it was a small price to pay if it’s going to work’ (patient 1011). Two interviewees mentioned the potential side effects of antibiotics. One expressed a concern that they were now immune to antibiotics, because of the number taken in the past, and thought it would be ‘more beneficial to try the lower dose’ (patient 1001). This interviewee and another mentioned the monitoring that was part of the trial, which was considered beneficial:
From a personal point, that level of monitoring, I’m equally benefiting from it. Do you know? So for me it was like, I would be getting medication anyway, so I can get the same medication and be slightly closer monitored for the problem I was having.
Patient 1009
Opinions of the ALTAR trial and outcome measures
When asked for their views of the ALTAR trial, one interviewee said that they were pleased someone was exploring a problem ‘that lots of women clearly have’ (patient 1005). Another commented that the trial ‘wasn’t too intrusive’ and wasn’t testing ‘some new drug’, which were positive and reassuring factors (patient 1401). A number of others similarly had no concerns about the trial. Of those who did, the responses were few and disparate. One interviewee was concerned about the length of time they would be taking antibiotics, and about the potential damage to their stomach lining. Another had a question about funding and whether or not money spent on conducting the trial could be detrimental to other key health services. Members of the research team addressed these concerns satisfactorily:
One of my main concerns was that a year was a very long time to do a treatment, so that was it really. The doctor told me that it was a year, just to be safe, I think. I can’t really remember. I think just to give it enough time to really work. I think he said if it was too short, then I would risk having everything come back again.
Patient 1008
Well I just wanted to know if they were taking money away from any urgent cases that people need, because you know they keep saying they are taking money from the elderly and the people in desperate need of cancer treatments and things. I’d hate to think I was taking 12 months of tablets when they could have maybes helped somebody live for another few months.
Patient 1019
Interviewees’ general views of antibiotics were explored. Several were aware of antibiotic resistance and mentioned this as a concern of longer-term use. Others raised the importance of antibiotics more widely without any mention of resistance. A smaller number talked about their long-term use of antibiotics, and the fact that they were no longer effective in treating their rUTIs. A few said that they disliked taking medication per se and commented that antibiotics were ‘bad’ for your body if taken on a long-term basis but did not expand on how:
There’s obviously a definite need for them. I have taken them, and would again take them happily if that’s what I need to cure whatever is wrong with me. [. . .] I realise that there can be drawbacks in terms of long-term use, and that the bugs can become resistant to a certain one.
Patient 1018
I have great faith in them, but they just don’t seem to work the same or if you do you’ve to take a lot of them. It shakes your confidence you know about it.
Patient 1201
While I have nothing against antibiotics, I just think the news that you hear that they’re not effective if you keep taking them all the time and they’re not good for your body.
Patient 1020
The fact that these interviewees may have to take antibiotics if they participated in the ALTAR trial did not impact negatively on their views of the trial.
Views on specific elements of the trial design were explored, specifically the 12-month period on antibiotics or the antiseptic, and the subsequent 6-month period when participants stop the prophylaxis and are monitored. In terms of the 12-month treatment period, apart from the interviewee mentioned above, most did not think that this was too long and understood the rationale:
One year? Mmm I mean, it’s a long time but . . . I’d rather be on some medication that potentially will stop the recurring UTI than not being on anything. I’m just happy to have treatment.
Patient 1013
There was a more varied response to the 6-month period when the prophylaxis stops. Although several interviewees were not worried and appreciated the need for this period to answer the research question, a few others had concerns:
I am just hoping that I don’t start with the recurring UTIs. That’s the only thing . . . the downside of it is that because I will not be on that any more, so, I might . . . hopefully I’ll not, but, I could end up back with the UTIs every 6 weeks.
Patient 1003
Scared. [. . .] I’ll see it through but I do feel a bit concerned about the 6 months where I’ll have no prevention or any kind of treatment whatsoever because I know exactly what’s going to happen.
Patient 1011
Trial participation involved the provision of blood and urine samples at baseline and then every 3 months until the end of the trial. Participants were asked for an additional urine sample if they suffered a UTI during the trial. The completion of a urinary symptom questionnaire, EQ-5D-5L and health resource use questionnaire was required at baseline and at 3, 6, 9, 12, 15 and 18 months. Diaries were supplied to record UTIs as they happened. Finally, at 12 and 18 months, participants were asked to complete the TSQM. The potential burden of the range of measures was explored. Although these interviews were conducted at the beginning of trial participation, most interviewees did not think that the range of measures and what they were expected to do was problematic. One interviewee thought that it was worth it because of the extra monitoring they would receive as part of the trial:
I knew I would be like an active participant in it anyway. I didn’t just expect to get the medication, then just to be left for 12 months. I know I’ve got to keep a diary, and if I do get another UTI, I’ve got to make a lot of notes about that, but I’m totally happy to do that, it’s totally fine.
Patient 1003
Well, I think it is just, you know, touch wood, if I don’t get a water infection I will not have anything to do apart from going back and forward every 3 months, which isn’t a bind really.
Patient 1019
There is stuff to do but I don’t mind. I just think if I’m being monitored and there’s information being provided then it’s surely better for the study. The more information the better, really.
Patient 1013
Although mentioned by a only few interviewees, the only part of the trial that was, at first, perceived as onerous was the submission of urine samples should the interviewee experience an infection:
It seems a bit daunting at first. Once it’s all explained to you, it’s quite simple. Once you realise that the little thing only has to be sent off if you get a urine infection that’s fine.
Patient 1401
I think if you’re fortunate enough not to get any infections the whole way through then you’re going to be sailing through it really. But if you get to the point where you are still getting them and you’re putting a sample in the doctors and sending one in to yourselves, then it gets a little bit more responsibility on you to do that sort of thing. But that’s what you sign up for, isn’t it. It’s part of the trial.
Patient 1016
Only one interviewee commented that the diary would be a burden. They appear to have misunderstood that they complete the diary only when they have symptoms of a UTI rather than daily, and the purpose of this was to assist trial participants to complete the UTI record questionnaire:
It’s this about, just about writing it up every day and all that, I mean you’re not at university, you’re at a hospital and you are a patient. But um I would still help in any way.
Patient 1201
Understanding of trial
Understanding of the ALTAR trial was explored with interviewees. Most understood and related that it was a comparison of two medications. A small number said that the aim of the trial was to explore the best treatment and that it would point to the cause of rUTIs. This latter point is probably from the PIS, which mentions that a benefit of participating is the opportunity to ‘learn more about your problem from the information we will give you’. Very few mentioned that the aim of the trial was to determine which medication was more effective in preventing rUTIs:
How I view it, it’s finding a different way of treating urinary tract infections. Just treating from a different angle and not having to rely solely on antibiotics.
Patient 1104
It’s to find out what’s causing bladder and urine infections and what antibiotics will work for me personally and what other antibiotics might work for other people. Everybody can be different and they can respond to tablets or medication in a different way. Does that make sense?
Patient 1303
One interviewee was unable to say anything about, and could not remember, the purpose of the trial; however, they understood every element of what was required of them as a participant, and which antibiotic they were taking as part of the trial.
Participating in the ALTAR trial
Reasons for participating
Only three of those interviewed had direct experience of participating in research previously. When interviewees were asked why they had agreed to participate in the trial, the majority did so in the hope of getting some answers to why they experienced repeated UTIs and obtaining therapeutic benefit or a cure. It was clear that a number of interviewees were desperate to find a solution to their rUTIs. The other reason given was to help others in the future or give something back to the NHS. The least mentioned reason was to support research. Some interviewees participated for both therapeutic and altruistic reasons:
I’m really willing to give it a right go and get rid of this, because it really pulls me down. I’m not in great health as it is, but it really pulls me down.
Patient 1105
You know, well, if I help someone else then that’s the main thing.
Patient 1006
In the time that I have been treated for cancer, treatments have changed slightly and new facts have come to light. And I appreciate that, to get to that point, you know, people have got to trial different things. So, from that point of view, I am really happy to do it.
Patient 1018
Only one interviewee declined to participate in the trial. The reasons given were strong objections to antibiotics and the fact that allocation was random. This person said that they had developed thrush every time they were prescribed antibiotics. They were reluctant to participate should they be randomised to the antibiotic arm. Interestingly, when interviewed, this person was unclear whether she was in the trial:
I was one of the people that specifically said I didn’t want to take antibiotics. I don’t know if that means I’m not actually part of the trial.
Patient 1005
Understanding of randomisation
The majority of interviewees said that they understood the process of randomisation. One interviewee understood that they were randomly selected for a particular treatment arm, but also thought other information from their medical records, such as antibiotic history, fed into that decision. Two interviewees commented that it was strange that a computer would decide, but this was not an issue or problematic for them. Although very much the minority view, one interviewee disliked the process and thought it could lead to problems with trial retention:
I don’t know . . . why a computer has to make these decisions, I think when it’s human input, and you’ve got an individual who might be going, ‘Yes, yes, I’ll take an antibiotic for 12 months’, and someone who doesn’t want to take an antibiotic, but is really keen to do the other trial, I don’t know why it’s then left to the computer to make a decision like that. Because you could then end up losing both candidates.
Patient 1014
Views of treatment arms
Most of the interviews with ALTAR trial participants were conducted after the participants had been randomised to a treatment arm. The majority were happy with the arm to which they were randomised, although some in the methenamine hippurate treatment arm expressed relief that they would not be taking antibiotics. Only one person said that they had some initial concerns about being randomised to the methenamine hippurate arm:
I was pleased that that’s the way it turned out. I mean, don’t get me wrong, I’m so fed up with the UTIs, I would have been pleased to get treatment in any form. But for me, personally, because I’m already allergic to penicillin, then the Hiprex [methenamine hippurate] – well, if it works, fingers crossed – it would be a better option, if it was something that would work for me.
Patient 1018
I was a bit apprehensive of whether it would be the antiseptic tablets because I’ve never had any . . . knowledge of them before. But on being given guidance at the hospital, and of course researching it myself, I was quite happy.
Patient 1401
The other reasons given for a preference for the antiseptic treatment were that antibiotics had not been effective in the past, and that they were keen to try something different, but also that there were concerns about antibiotic resistance:
I have to say I was more pleased to be on that (antiseptic) side than the other one. I would have taken the other one but I just – well just because I wanted to see if it would have any effect on what I’m – for the number of infections I’ve had, I was just wanting a bit of comfort . . . to be feeling better a bit. I thought if I get the antibacterial, which I would have gone through, but if it made life not much better, it would have seemed a long drag. The year would have been a long year you know?
Patient 1016
I’m kind of glad that I was put on the antiseptic rather than antibiotic just because I know that obviously prolonged use of antibiotics, you can build up resistance to it and obviously that’s the reason that this study is going on.
Patient 1013
Overwhelmingly, interviewees said that they would have accepted the medication that they were randomised to and completed the trial. However, one person who had concerns about taking antibiotics said that, had they been randomised to that arm and continued to suffer from rUTIs, this could have affected their seeing the trial through to the end:
I would have continued with it (if randomised to antibiotics) and given it a try but I think I would have been very open to, more open to saying, ‘Well, if this is not working I’m just coming off it,’ whereas with this one I want to participate until the time is up if I can.
Patient 1020
Two interviewees were disappointed that they were randomised to the antibiotic arm. The first interviewee said that they had previously experienced unpleasant side effects from antibiotics and that they had been ineffective in preventing their UTIs, and the second interviewee said that they had taken the antibiotic for a number of years with little effect. However, the former expressed a willingness to try:
It’s 8 years since I was last there. And I’ve been on it all that time. . . . it was a doctor at the chest clinic, who said, ‘I think you’ve been on these too long, and I’m going to get in touch with your GP’, and they stopped them. And then, when I got the tablets, to take one a day, with this scheme, it’s the same tablet. . . . So I don’t know why they’ve given us the same one, when I was taking that for all them years, and it didn’t stop them.
Patient 1015
Follow-up visits at 3 and 6 months and outcome measures
Interviews were conducted at 3 months, or 6 months if time permitted, to discuss aspects of the trial such as follow-up visits and the completion of the outcome measures. Ten follow-up interviews were conducted. One interviewee had dropped out of the trial and was unable to comment on trial processes. The majority of the nine interviewees had had no problems in terms of the trial processes. The questionnaires were described as ‘easy’ (patient 1101) and one interviewee said that they were ‘impressed with everything’ (patient 1010). The trial team at the sites were supportive, helpful and accessible:
Yes, the appointments have been fine, the questionnaires have been fine, and the trial seems to be working because I have gone 6 months, I got ear infections though, but I am quite happy with that one.
Patient 1003
No, I think it’s been quite smooth actually. I get a phone call every month, to check how things are going, and to be honest those are very quick and brief because I haven’t had any issues. When I am at the hospital for the check-ups on the bloods and the urine, it’s gone very smoothly, so nothing causing any concern or distress at all.
Patient 1009
Oh lovely, they are lovely people, do you know what I mean? Really nice, explain everything.
Patient 1002
Some members of the trial team made exceptional efforts to support the trial participants in attending for follow-up visits. One interviewee had found the hospital confusing to navigate and the RN offered to meet her and take her to the department:
The next time I go is in 2 months . . . So, she’ll probably meet me at the bus stop. . . . she’ll come and collect me because I’m getting lost going into all that, go down this way and that way and up lifts and everything else. [Also] She phones me asks me how I am.
Patient 1301
Only a few participants had issues with the written documentation, primarily some confusion over the wording. One participant sought help from a RN to complete one of the questionnaires. Another participant did not understand the instructions about completing specific sections of the documentation in the event of an incident requiring antibiotics; they submitted samples to the GP and trial team as they were experiencing symptoms but had not completed the paperwork as antibiotics were not prescribed. They later found out that they should have recorded this incident both in the diary and in the 3-month questionnaire. The same interviewee had also struggled with the form that is submitted with the sample and said, ‘a bit more explanation on that sheet might have helped’ (patient 1013). They said that they could have contacted the trial team at the site but was unsure whether the documentation was from the university or the hospital. Another issue raised was that submitting samples to the GP was inconvenient and problematic as it interfered with their working day: ‘the biggest inconvenience is getting it to the doctors’ (patient 1011). Finally, one interviewee had had to complete the 3-month questionnaires a second time, as they were not received by the trial team.
One interviewee had dropped out of the trial as they had suffered from repeat infections in the period since they had joined the trial. In addition, they had struggled to book appointments with their GP to change their medication and to submit urine samples:
I keep having to get new treatment and I can’t always get an appointment at my doctors, and I am supposed to be sending samples off, so I couldn’t get anything from the doctor, so it was a waste of time. They just said it was busy, there was like a 2-week waiting list. They classed it as not as urgent.
Patient 1007
When asked if they were disappointed to have to leave the trial they said:
Not really, because I don’t think it was working. I was still getting an infection every week.
Patient 1007
Experience of trial medication
There were very few comments on interviewees’ experiences of taking antibiotics as part of the ALTAR trial. Those randomised to the antiseptic (methenamine hippurate) arm had more to say about the experience of taking methenamine hippurate. Of those who were taking antibiotics, one asked for, and received, enteric-coated medication and another was suffering from nausea. The latter interviewee was planning to speak to the RN at site, as they were unsure if this was attributable to the antibiotics. For the antiseptic, although one or two reported no problems, the majority commented on the taste and some on the size of the antiseptic tablet:
Well, they’re very big tablets, so [laughter] – I’m trying to take those. . . . with a big gulp of water in the morning and the evening. Yeah, it can be a bit difficult sometimes psyching myself up to do it. Perhaps a smaller tablet certainly would have been better. But I think I’m going to look into the future about splitting them in half and trying to take them that way.
Patient 1105
The only thing I don’t like is when I first take them, I can taste it before I can swallow it. That’s the only thing. I shudder. [Laugh] It’s not a nice taste in my mouth before I swallow them. . . . That’s the only thing I’ve got against it. . . . But I’m getting used to it now. I drink plenty of water afterwards.
Patient 1106
As illustrated in the second quotation above, interviewees found ways to cope with taking the antiseptic. This was primarily by taking it with lots of water, and a few ate something sweet after taking it with food. None of the interviewees said that the size or taste would affect their continued participation in the trial.
Although mentioned by only a few interviewees, there were some issues with remembering to take the twice-daily antiseptic. One interviewee said that the fact that the doses must be taken 12 hours apart was a problem, but they were trying to ‘keep on top of it’ (patient 1103). Another had forgotten to take the antiseptic as they simply were not used to taking medication on a daily basis. They set reminders on their mobile phone to help them adhere to the twice-daily regimen.
Feedback to the Trial Management Group
As mentioned at the beginning of this chapter, the main reason for the embedded qualitative research was the early identification of any modifiable issues with trial processes and conduct that may affect recruitment and retention of participants. The factors identified in the 8 months of the qualitative study are listed in Table 5. In summary, these were clarification of the antiseptic, as one interviewee had searched for further information and discovered this was a medication that could eradicate bacteria; the fact that some interviewees expected the trial to identify the cause of and/or cure for their rUTIs; and potential AEs and SAEs reported during the interviews. Some of the other issues identified through the interviews were fed back to the NCTU directly by recruiting staff and were being addressed.
| Issue identified | Action |
|---|---|
| Interviewee had been hospitalised but no SAE reported | NCTU to speak to co-ordinator at site and chase possible SAE |
| Some interviewees unaware of risks and benefits of the trial | Reminder in the newsletter/bulletin to sites |
| Recruiting staff unfamiliar with the Child–Pugh score | Explanation of the Child–Pugh score provided in the newsletter/bulletin to sites |
| Patient expectation that trial will provide information on cause of and/or cure for rUTI | Feedback to recruiting staff to ensure participants understand purpose of trial |
Findings: recruiting staff
Seven RNs and two consultants were interviewed from four of the recruitment sites, and five trial staff members were non-contactable. Recruiting staff were extremely busy and the interviewer had some difficulty securing interviews. This was raised in one of the TMG meetings and the NCTU suggested adding an appeal in the newsletter/bulletin that was sent to sites asking recruiting staff to take part in an interview.
The ALTAR trial site initiation visit
The recruiting staff’s views of the site initiation visits (SIVs) were generally positive, although for most interviewees quite a bit of time had passed since it took place. Seven of the nine interviewees were at the SIV, one was on sick leave and another was unable to attend. The latter interviewee said that their manager conveyed the information from the SIV to them and they had no issues with the trial.
A number of interviewees said that the trial was straightforward and the information provided in the SIV was clear. One interviewee said that they were involved in the development stage and provided feedback on the logistics of running the trial at their own site. There were positive comments about the chief investigator for the ALTAR trial. In addition, a few interviewees mentioned that they had worked on a previous trial with NCTU and this was of benefit:
So I think there is the degree of familiarity with the staff who are involved with this study from the university side . . . helps. Obviously I know the study area, and the chief investigator, Mr Harding [. . .] his knowledge and enthusiasm for the studies was very obvious and helpful.
Recruiting staff 1003
The majority had found the SIV useful. There was one negative comment about the fact that the CRF was not ready at the SIV. Another interviewee commented that assumptions were made about how the trial would run, without acknowledging the differences across trial sites:
I did feel there were a lot of unanswered questions at the SIV that I probably would have liked to have more information about. There were things that weren’t quite set up. For example, the database wasn’t finalised so when they were trying to show the database, there were parts that were . . ., you know, ‘This bit hasn’t been completed yet so we can’t show you that’ or ‘This bit’s going to be getting changed’. It was more to do with actual electronic CRF.
Recruiting staff 1302
There were I think maybe a couple of queries where they presumed that we would do things a certain way which we had looked at different ways of doing things, possibly regarding recruitment and things like that. But generally overall very, very well presented.
Recruiting staff 1601
ALTAR patient identification and recruitment
For the recruiting staff interviewed from four sites, the pool of potential participants were those who attended the outpatient clinic. One site identified eligible patients in a variety of ways: through attendance at clinic, attendance for a cystoscopy and referrals from general practice. This was considered much less labour-intensive than other methods of identifying eligible patients:
So I’m not having to spend hours upon hours searching through lots of different clinics, trying to find people that are eligible for the trial. Because obviously, they’re people that are coming to see them anyway, and obviously they’re getting, they’re kind of first to know. So from primary care and things like that, they contact the hospital, which should be obviously urogyn [urogynaecology], and then refer them on. So it has been, I’ve had a lot of people that I’ve been able to contact.
Recruiting staff 1301
In most sites the clinicians explained the trial to eligible patients and provided them with the PIS and then referred them on to the RNs. The process of the clinician having the first conversation with the patient about the trial (primarily explaining the rationale for it) was considered of benefit in terms of recruitment and ensuring a good understanding. One thought it ‘endorsed’ the trial, particularly as patients have a level of trust in a clinician when they have been under their care for some time (recruiting staff 1302). Another commented that this early discussion ‘primed’ the patient for a more detailed discussion with the RN (recruiting staff 1303). One site also wrote to eligible patients and followed up with a telephone call from the RN to ask if they were interested and if they had any questions.
The majority found the trial information easy to convey, and the fact that there was no placebo, that patients were familiar with antibiotics and that everyone would be having treatment were positive factors. On the surface it could appear that patient participants were required to do a number of things, for example completing questionnaires and submitting samples. The recruiting staff mentioned this early in discussions so that there were no surprises for patients, and pointed out that when viewed over the full period of the trial it was not too burdensome:
So the earlier you introduce that, the better, really. So we do that both when we introduce the study to the patient, but we also reinforce that when they come back to give consent.
Recruiting staff 1003
I just try say, ‘Look, it’s at least every 3 months that we will ask you to provide this but in the meantime if you get an infection, you’ve got this bottle at home, this blue bottle that you can send to Newcastle and fill this in. Hopefully you won’t, but in essence you know it’s four times a year.’ I try and explain that it’s not . . . so time-consuming, if you think about coming in . . . to see [clinician] four times a year.
Recruiting staff 1601
In terms of patient understanding of the trial, the view from those interviewed was that patients were aware of antibiotic resistance and of the use of medication as a prophylactic:
The patients are usually . . . they’re expert patients, to be quite honest. [. . .] I think that the patients are very . . . well informed now, due to the general media, about antibiotic resistance. . . . a lot of them will say, ‘Oh, yes, because the bugs are resistant’, or ‘The bugs are getting stronger’ or something along those lines. [. . .] Many patients will understand what prophylaxis is, but I think we’re just reinforcing it as a preventative medication.
Recruiting staff 1003
Some women have heard about being on prophylactic, some of them have been on prophylactics already. But obviously, they understand it. I mean, you’re not having to teach them what prophylactic means and things like that, they all understand it.
Recruiting staff 1301
Use and views of the patient information sheet
Some recruiting staff sat with patients and explained the ALTAR trial using the PIS as a guide, and other recruiting staff asked patients who had received the PIS, often through the post with a letter, and had had an opportunity to read it, if they had any further questions:
And I think the information sheet lays it out . . . and I have the information sheet there, as we go through, whilst giving a verbal description of the study.
Recruiting staff 1003
When we do meet them face to . . . obviously I would say, ‘You’ve got the information sheet, did you understand it, are there any questions?’ And I think it says in the letter, if there are any queries about any of the information please ring and it has our numbers and things like that.
Recruiting staff 1601
Staff were very complimentary about the content, wording and layout of the PIS. One interviewer said that an ALTAR participant, who is an ex-RN and conscious of the need to make trial information clear and comprehensive, had commented specifically about how good the PIS was. One interviewee said that the PIS was not ‘any bigger or smaller than any of the other ones that we’re used to giving out’ (recruiting staff 1303). A few people agreed that it was long but did not think that this detracted from the quality:
It’s a lengthy PIS, I’ll say that, it’s 14 to 15 pages . . . But to be fair, sometimes I’ve come across PISs that are one page and it doesn’t really tell you a lot. So I think I would rather have overkill . . . I think people need to be completely understanding exactly what their involvement is. So I do like the way that it’s set out, I think it’s got everything, so that every question and every kind of possible query has been answered within that PIS. So no, I quite like the PIS.
Recruiting staff 1301
It’s long but . . . the writing is quite big. The typing is quite a big type. The font is good. Yes, I think it is fine. It’s good because it does give you chance to sit down with the patient and go through it with them properly.
Recruiting staff 1101
There was a general sense that, having read the PIS, patients understood the trial and what was required of them. Interviewees commented that, when they met face to face or talked on the telephone, they had not found patient participants to be unclear about the trial. There was only one criticism; it was felt that a step-by-step guide to submitting a sample for patients who have a urine infection would have been beneficial, and some recruiting staff mentioned another trial they had been involved in where this was provided. Until this issue was addressed, they were improvising with their own written instructions.
And I always try, when I send spare bottles out, to go through a few bullet points, and just handwrite it out and say, ‘Look, if you’ve any issues with all this or if you’ve misplaced these instructions, just please give me a ring, and we can go through it.’ It’s just about making yourself available sometimes myself, [name] and [name] who’s our clinical trials assistant we’ll go through it.
Recruiting staff 1601
Issues with trial processes
Apart from the issues mentioned in Use and views of the patient information sheet about clearer instructions for patients, one site identified another problem once the trial was up and running. RNs can be called on to work on trials in a different specialty, and this was the case for one team that participated in the ALTAR trial. This team’s main area of research was women’s health, and one interviewee did admit that the ALTAR trial had been a steep learning curve. However, the issue was with what they described as an oversight in the trial protocol (and SIV) around the need to calculate a Child–Pugh score before a patient could be randomised:
When you read the protocol . . . it didn’t quite explain what a Pugh score was. We weren’t familiar with it and what we had to do was Google it to find out. [Laugh] . . . and then we realised that as part of the Pugh score we had to have a blood sample, which wasn’t highlighted, in the protocol when we looked at it for clarification [. . .] We have a speciality in women’s health, but not necessarily gynaecology or urology, so I think the protocol has to make sure that it’s very prescriptive. You can’t just assume that that’s what everybody knows and that that’s what everybody does [. . .] when you look at what you have to do per visit, the blood sample for the Pugh score was not in that list.
Recruiting staff 1302
The team was unable to get a response from the NCTU and then discovered that their usual contact had left and the site staff had not been informed:
We would appreciate a bit prompt communication from the site team which we might now get because we appreciate now that [name] has left. That was unfortunate that nobody sent a generic e-mail out to all the sites . . . That never happened and that was a bit frustrating. Because we were sending all these e-mails and they were just not getting anywhere. I think that communication needs to be improved.
Recruiting staff 1302
None of the other interviewees highlighted any issues with the trial processes from the recruiting staff’s perspective.
Supporting ALTAR participants
It was acknowledged that trial participation could be a challenge for elderly patients and those who work and/or who have child care commitments, particularly attending the 3-monthly appointments. A number of interviewees tried to accommodate the needs of trial participants and provide flexibility and support whenever they could:
They work or they’re picking kids up . . . and I always try and say that we can be flexible enough you know to suit you. It’s not all written in tablets of stone that we need to meet on such a date, at such a time. It can be mutually convenient for us both, etc.
Recruiting staff 1601
At one site recruiting staff had identified two issues that could affect the successful delivery of the trial and ensuring that there was as little inconvenience to the participants as possible. First, patients who had to have bloods taken before randomisation could have to wait some time for the results. To reduce any inconvenience to participants, the recruiting staff had arranged for medication to be sent by courier or had delivered it personally:
A lady the other day . . . we had to do all her bloods from scratch before we could randomise her and obviously we send it to the labs as an urgent sample, but the lady could still be sitting there 2 and 3 hours later. So . . . we’ve been letting them go home and then we get the blood samples back, we randomise them, we get the prescription from the doctor, contact the lady and let her know which groups she’s been randomised to and then we drop the medication off to them.
Recruiting staff 1302
The second issue was participant understanding of what to do if they had a UTI during the trial. Recruiting staff also suspected that older participants might struggle with the process of submitting a urine sample if they had had an infection, particularly completing the paperwork. One interviewee described explaining the process to a participant, “I can see their faces just looking at, going like, ‘My goodness, this is an awful lot to remember’ ” (recruiting staff 1301). There were also concerns about older patients ensuring that the box was securely closed, ‘Even I struggle with closing it because it’s quite stiff to get it to shut’ (recruiting staff 1301). Another interviewee expressed potential health and safety risks if the sample is not secure (recruiting staff 1302). This team offered extra help, particularly for the first time a participant had to submit a sample:
We have got round people being unsure about sending back samples by reassuring them and asking them to phone us. In cases we go out to the house and we . . . show them how to pod their urine sample the first time in the hope that they’ll remember how to do it if they have a subsequent UTI [laugh] and I think that does help. And it also provides that reassurance that they’re valued and they’re not left floundering . . . and about being uncertain about how to do this appropriately.
Recruiting staff 1302
Why patients agree to or decline participation in the ALTAR trial
Recruiting staff were asked if, in their view or experience, there were any elements of the ALTAR trial that would encourage or discourage women from participating. One interviewee thought that many of the women at their site were keen to participate and are ‘desperate for something’ and ‘like that additional support that they get as part of this study’ (recruiting staff 1003). The opinion that participants were driven to participate because of the impact the rUTIs were having on their lives was reinforced by an interviewee at another site:
They’re kind of clutching at straws to try and get something to help them. Because they’ve tried everything else and now this is the last opportunity to try and see if there’s something that can help them.
Recruiting staff 1301
Other reasons that the trial was thought to be attractive to patients were that, unlike a placebo-controlled trial, everyone in the ALTAR trial received treatment, and patients are keen to have the chance to try something other than antibiotics that are in common use in the NHS:
I think they are reassured that it is used. It is not a new investigational drug, that it is used around the country. That reassures patients.
Recruiting staff 1102
One interviewee did acknowledge that as a participant progresses through the trial their views that the trial could be the answer to their problems could change, depending on their experience:
And I think, as they progress along their journey, their focus might change in the questions they ask in clinic. I suppose it depends to what degree it has made a difference in reducing the number of infections. You might see a change in their anxiety levels.
Recruiting staff 1003
The actual reasons patients gave for not participating varied. At one site the recruiting staff said that some people are too busy, or their concerns are with other health problems they have that take priority over their rUTIs. In contrast, other patients are well and do not ‘feel the need to do anything extra at the moment’ (recruiting staff 1301). Other patients did not like the idea of being part of a trial. The washout period was another factor that discouraged patients from participating, but one interviewee was surprised that this was not more of an issue:
If they’re already on treatment . . . they don’t want to not take anything for 3 months. [. . .] To be fair actually, they’ve been better than we thought. [Laughter] I thought I was going to have a big problem with that.
Recruiting staff 1303
Based on their experience with patients who have agreed to participate, another interviewee wondered if those who decline are overwhelmed with what is required of them. Others had direct experience of this being a barrier to participation:
I don’t know whether the people declining don’t want any additional hassle, additional visits, additional medication.
Recruiting staff 1302
We have had one lady already come off study, because she thought it would be a burden as well, after having initially being recruited.
Recruiting staff 1002
There is quite a lot for them to do within the trial, so that also puts patients off, but not many.
Recruiting staff 1102
Discussion
This embedded qualitative study achieved its objective of identifying modifiable issues in the ALTAR trial processes. The two main issues were participants’ understanding of the nature of the trial and the risks and benefits of participation. These were easily addressed by ensuring that recruiting staff checked patients’ understanding when they discussed the trial with them.
The majority of patient and recruiting staff interviewees were happy with the PIS, and there was praise for its structure and format. Patient interviewees were complimentary about the recruiting staff, most of whom made exceptional efforts to ensure that participants had a positive experience. The pragmatic nature of the trial, the absence of a placebo arm or experimental intervention facilitated recruitment. The periods on trial medication (12 months) and off trial medication (6 months) were acceptable to patient interviewees (although some were anxious about the latter), and this perhaps is indicative of how keen some were to find an answer to their rUTIs through the trial.
Ensuring trial participants have a positive experience is paramount, but the experiences of the recruiting staff are also important. This qualitative study highlighted some issues for the recruiting staff that were able to be addressed and will also inform the conduct of future trials, particularly where the teams are working in a specialty that is new to them.
The severity of the symptoms experienced by the patients interviewed was salient and was, for many, a motivating factor to enrol in the trial. There was concern about long-term antibiotic use, yet patients demonstrated commitment to the trial by stating that they would continue to participate even if they were randomised to the antibiotic arm. Although not a key focus of the qualitative study, a matrix of factors for trial participation (Figure 2) was developed from the patient and recruiting staff interview data and published before the final report. 33 This analysis of the conduct and processes in the ALTAR trial was beneficial to the current study and may also be utilised by researchers designing UTI trials in the future.
FIGURE 2.
Matrix of factors for trial participation (amended from Lie et al. 33). Reproduced with permission from Lie et al. 33 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
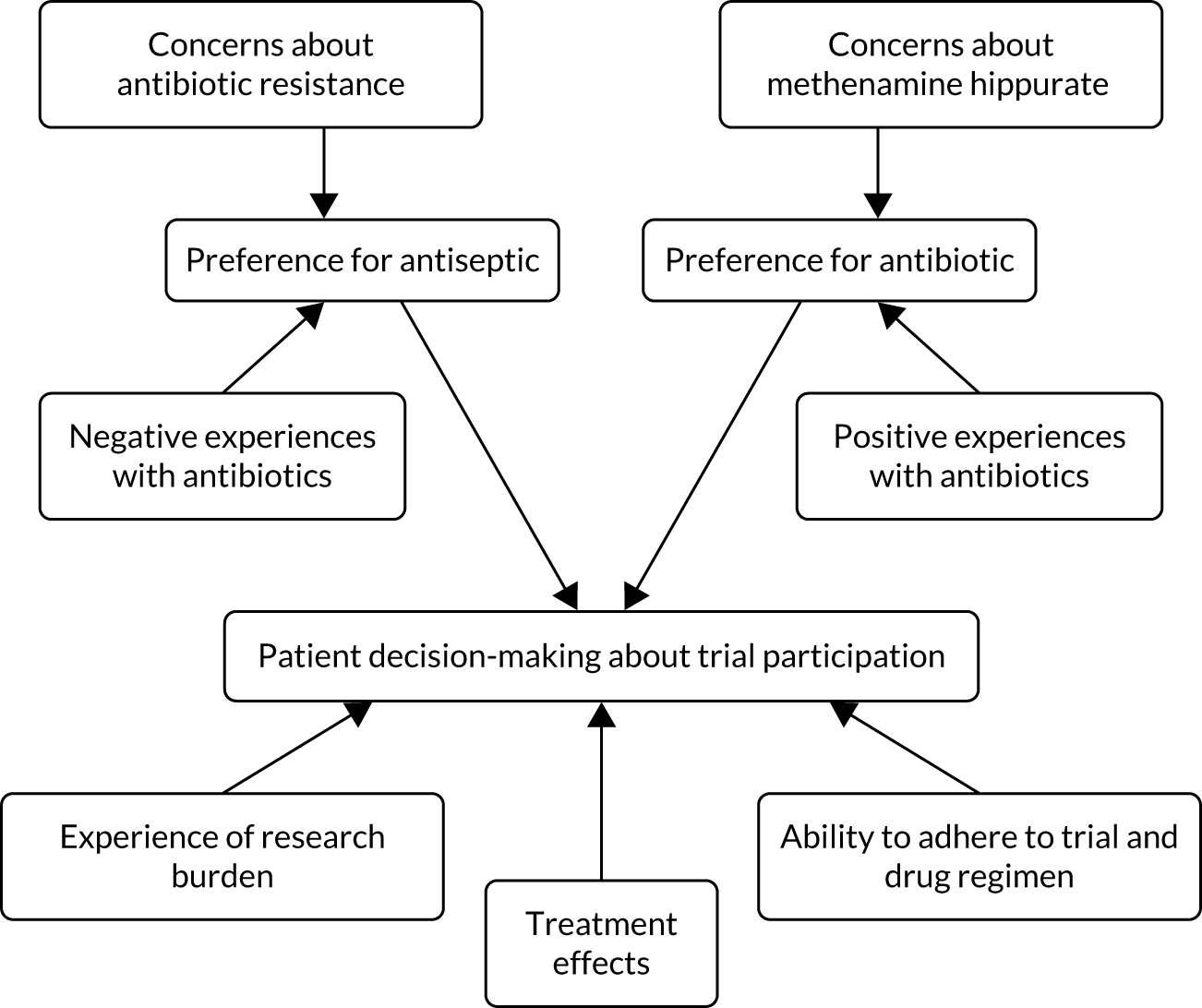
Limitations
It was unfortunate that the qualitative study was unable to achieve its target of interviewing up to 15 patients who had declined to participate in the trial and up to 15 who had dropped out. Although it was possible to identify some of the reasons that patients declined from the recruiting staff interviews and the screening log data, the opportunity to explore this with patients could have highlighted issues or misunderstandings about the trial. In hindsight, ensuring that the qualitative study was more prominent in the PIS for the trial may have been beneficial to recruitment. In addition, early contact between the interviewer and the site staff to establish rapport would have helped to raise the profile of the qualitative study. With regard to participants who dropped out of the trial, it could be that this number was small among those who took part in a first interview, but it is possible that the process of informing the interviewer of dropouts was flawed.
Conclusions
The short, embedded qualitative study conducted in the early stages of trial recruitment and the process of rapid feedback to the trial team meant that we were able to identify modifiable factors in trial processes. In future, where such embedded studies are planned, processes to enhance recruitment, particularly of those who decline participation or drop out of the trial, should be carefully thought through, possibly with the input of recruiting staff at sites. Finally, seeking the views of recruiting staff is crucial, and their agreement to participate in interviews could form part of the early scoping of sites to be part of future trials.
Chapter 4 Results
Parts of this chapter have been reproduced with permission from Harding et al. 36 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Recruitment
The ALTAR trial opened to recruitment on 23 June 2016, with the first participant recruited on 7 July 2016. In total, 240 participants were randomised from eight centres across the UK. The number of sites was increased from six to eight in June 2017 to aid participant recruitment. The last participant was recruited on 20 June 2018, with the final 18-month follow-up visit taking place on 27 January 2020. The recruitment period was originally planned to be 18 months; however, this was extended by 9 months in order to achieve the target sample size (Figure 3).
FIGURE 3.
Observed and predicted accrual.
In total, 480 patients were screened for eligibility. Reasons for non-participation are shown in Table 6. Out of those not recruited into the trial (n = 240), 151 (63%) patients decided not to participate after receiving further information; the main reasons for declining participation were unwillingness to adhere to the 18-month trial protocol (34; 14%) and unwillingness to undertake a 3-month washout of prophylactic treatment (22; 9%). A further 38 (16%) patients did not respond to an invitation letter about the trial or attend an appointment to discuss trial participation. Qualitative data on the willingness of patients to participate in the trial are discussed in Chapter 3. In total, 34 (14%) patients did not meet the eligibility criteria.
| Reasons for non-participation | Number of patients | Percentage of total |
|---|---|---|
| Not eligible | 34 | 14 |
| Did not meet the criteria for rUTI | 9 | 4 |
| (Potentially) correctable urinary tract abnormality that is contributory to rUTI | 8 | 3 |
| Using CISC, therefore classified as ‘complicated’ rUTI | 5 | 2 |
| Unable to take any trial prophylactic antibiotics | 3 | 1 |
| Aged < 18 years | 2 | 1 |
| Unable to take methenamine hippurate | 2 | 1 |
| Current pregnancy or breastfeeding/intending to become pregnant in the next 12 months | 2 | 1 |
| Unable to adhere to trial protocol | 2 | 1 |
| Reason unknown | 1 | 0 |
| Declined participation | 151 | 63 |
| Unable/unwilling to adhere to the 18-month trial protocol | 34 | 14 |
| Unwilling to complete 3-month washout/unable to tolerate washout | 22 | 9 |
| Not interested | 9 | 4 |
| Other health reasons | 8 | 3 |
| Does not want antibiotic prophylaxis/preference for methenamine hippurate | 5 | 2 |
| Too busy/does not wish to travel | 5 | 2 |
| Does not want to take medication | 4 | 2 |
| Feeling better | 4 | 2 |
| Unwilling to adhere to trial protocol | 4 | 2 |
| Unable to tolerate washout | 1 | 0 |
| No reason given | 55 | 23 |
| No response to invitation/did not attend appointment | 38 | 16 |
| Logistical | 5 | 2 |
| Unknown | 12 | 5 |
| Total | 240 | 100 |
Participant flow through the trial
Participant flow through the trial is presented in a Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 4). Participants were randomised (1 : 1) to receive 12 months of antibiotic prophylaxis (n = 120) or the urinary antiseptic methenamine hippurate (n = 120).
FIGURE 4.
Participant flow through the trial (CONSORT flow diagram). Reproduced with permission from Harding et al. 36 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
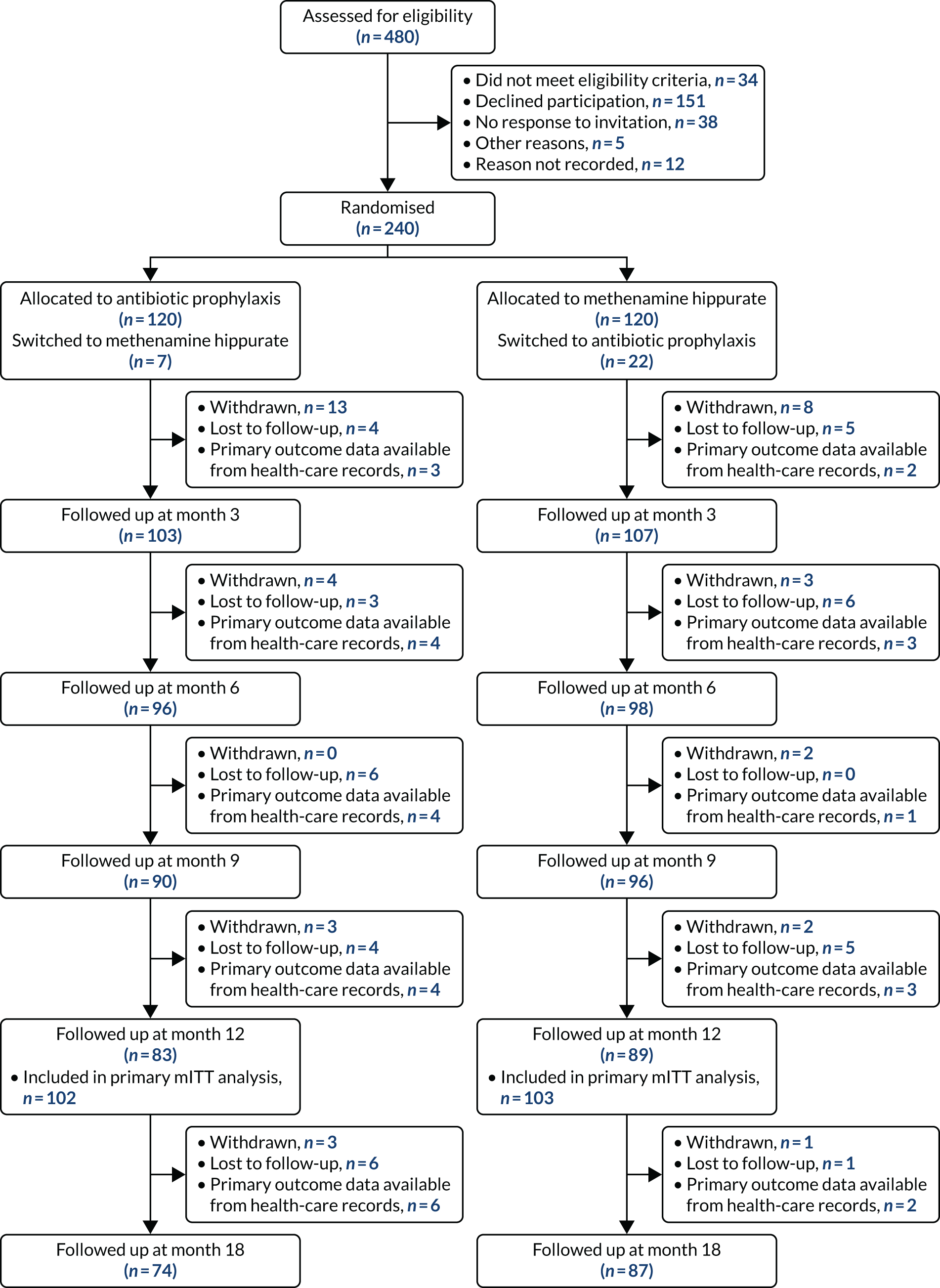
By 12 months, 172 participants remained in active follow-up (83 antibiotic prophylaxis, 89 methenamine hippurate). The primary analysis was conducted in the mITT population, which included all participants with at least 6 months’ follow-up, including data available from review of health-care records. In total, 205 (85%) participants (102 antibiotic prophylaxis, 103 methenamine hippurate) were evaluable for the primary analysis in the mITT population. This surpassed the 87 participants per group required by the sample size calculation.
Over the 18-month trial period, 79 (33%) participants withdrew from the trial or became lost to follow-up: 46 (38%) who were allocated to antibiotic prophylaxis and 33 (28%) who were allocated to methenamine hippurate. Of those, 32 (41%) participants had primary outcome data available from routine health-care records (21 in the antibiotic arm and 11 in the methenamine hippurate arm) (see Appendix 2, Table 27). Reasons for withdrawal, where available, are provided in Appendix 2, Table 28.
Baseline data
Participant demographics and clinical characteristics were generally well balanced across treatment groups (Table 7).
| Characteristic | Antibiotic prophylaxis arm (N = 120) | Methenamine hippurate arm (N = 120) | Total (N = 240) |
|---|---|---|---|
| Stratification factors, n (%) | |||
| Menopausal status | |||
| Pre | 49 (41) | 50 (42) | 99 (41) |
| Peri/post | 71 (59) | 70 (58) | 141 (59) |
| Self-reported UTI episodes in the last 12 months | |||
| < 4 | 14 (12) | 16 (13) | 30 (13) |
| ≥ 4 | 106 (88) | 104 (87) | 210 (88) |
| Demographics | |||
| Age at randomisation (years) | |||
| Mean (SD) | 50.3 (18.1) | 49.9 (19.1) | 50.1 (18.6) |
| Median (IQR) | 56 (36–64) | 52 (30–66) | 54 (32–65) |
| Minimum, maximum | 18, 88 | 18, 82 | 18, 88 |
| Weight (kg) | |||
| Mean (SD) | 70.1 (15.3) | 75.1 (18.5) | 72.6 (17.1) |
| UTI history | |||
| Self-reported UTI episodes in the last 12 months | |||
| Mean (SD) | 6.8 (3.8) | 7.0 (3.4) | 6.9 (3.6) |
| Median (IQR) | 6 (4–8) | 6 (4–8) | 6 (4–8) |
| Positive urine culture reports in last 12 months | |||
| Mean (SD), n | 2.6 (2.6), 117 | 3.6 (3.0), 117 | 3.1 (2.8), 234 |
| Median (IQR) | 2 (1–4) | 3 (1–5) | 3 (1–4) |
| Central laboratory urine culture at baseline, n (%) | |||
| No growth | 93 (78) | 98 (82) | 191 (80) |
| Growth of one or two isolates | 18 (15) | 13 (11) | 31 (13) |
| No sample, n (%) | 9 (8) | 9 (8) | 18 (8) |
| Prior prophylaxis | |||
| Previous use of antibiotic prophylaxis, n (%) | 28 (23) | 27 (23) | 55 (23) |
| If yes, type of antibiotic (not exclusive), n (%) | |||
| Nitrofurantoin | 20 (17) | 20 (17) | 40 (17) |
| Trimethoprim | 16 (13) | 11 (9) | 27 (11) |
| Cefalexin | 6 (5) | 13 (11) | 19 (8) |
| Co-amoxiclav | 2 (2) | 5 (4) | 7 (3) |
| Amoxicillin | 3 (3) | 3 (3) | 6 (3) |
| Ciprofloxacin | 1 (1) | 4 (3) | 5 (2) |
| Pivmecillinam | 1 (1) | 3 (3) | 4 (2) |
| Months of antibiotic prophylaxis in last 12 months | |||
| Mean (SD) | 1.6 (2.8) | 1.5 (2.7) | 1.6 (2.7) |
| Taking any antibiotic prophylaxis in last 6 months, n (%) | 17 (14) | 19 (16) | 36 (15) |
| 3-month washout period required prior to randomisation, n (%) | 16 (13) | 16 (13) | 32 (13) |
| Previously taken methenamine hippurate, n (%) | 2 (2) | 4 (3) | 6 (3) |
The majority of participants [210 (88%) out of 240] self-reported four or more UTI episodes in the 12 months prior to trial entry, with a median of six episodes [interquartile range (IQR) 4–8 episodes]. The number of positive urine cultures reported in the 12 months prior to trial entry was slightly higher in those allocated to methenamine hippurate (median 3, IQR 1–5 positive urine cultures) than in those allocated to antibiotic prophylaxis (median 2, IQR 1–4 positive urine cultures).
All participants with a previous history of taking antibiotic prophylaxis had to complete a 3-month washout period, without daily antibiotics, prior to randomisation. Overall, 55 (23%) participants had taken antibiotic prophylaxis previously as a preventative treatment for UTI. The completion of the advised washout period was required for only 32 (13%) participants prior to randomisation.
Treatment compliance
Participants were randomised (1 : 1) to receive daily antibiotic (nitrofurantoin, trimethoprim or cefalexin) or twice-daily antiseptic (methenamine hippurate) for 12 months. For those allocated to antibiotic prophylaxis, the majority of participants, 66 (55%), were initially prescribed nitrofurantoin, with 30 (25%) and 24 (20%) receiving trimethoprim and cefalexin, respectively.
Overall, 170 (71%) participants achieved at least 90% compliance with any trial preventative treatment. This was similar in both randomised treatment groups: 84 (70%) antibiotic prophylaxis and 86 (72%) methenamine hippurate.
Switching between the three trial prophylactic antibiotic agents and between treatment strategies (antibiotic or antiseptic) because of AEs or lack of efficacy was permitted as part of the protocol; however, no participant was permitted to use two preventative agents concurrently. In total, 39 (16%) participants received more than one preventative agent during the 12-month trial treatment period, 17 (14%) of whom were allocated to antibiotic prophylaxis and 22 (18%) of whom were allocated to methenamine hippurate. Switching between preventative treatment strategies was more common in those randomised to methenamine hippurate, with 22 (18%) participants switching to antibiotic prophylaxis, compared with seven (6%) participants switching from antibiotic prophylaxis to methenamine hippurate. Switching between preventative treatment strategies occurred slightly earlier in those allocated to methenamine hippurate (median 2.8 months post randomisation) than in those allocated to antibiotic prophylaxis (median 4.1 months), which may be attributed to those allocated to antibiotic prophylaxis first switching between antibiotic agents. A summary of compliance with preventative treatment is given in Table 8.
| Antibiotic prophylaxis arm (N = 120) | Methenamine hippurate arm (N = 120) | Total (N = 240) | |
|---|---|---|---|
| ≥ 90% compliance with any trial preventative treatment strategy, n (%) | 84 (70) | 86 (72) | 170 (71) |
| Initial prophylactic antibiotic agent prescribed, n (%) | |||
| Nitrofurantoin | 66 (55) | NA | NA |
| Trimethoprim | 30 (25) | ||
| Cefalexin | 24 (20) | ||
| Months on any trial preventative treatment, n (%) | |||
| 12 months | 84 (70) | 88 (73) | 172 (72) |
| ≥ 6 months, < 12 months | 11 (9) | 9 (8) | 20 (8) |
| < 6 months | 25 (21) | 23 (19) | 48 (20) |
| Mean (SD) | 9.7 (3.9) | 10.1 (3.6) | 9.9 (3.7) |
| Median (IQR) | 11.9 (8.8–12.0) | 12.0 (10.9–12.0) | 11.9 (10.4–12.0) |
| Number of preventative agents received, n (%) | |||
| 1 | 103 (86) | 98 (82) | 201 (84) |
| 2 | 14 (12) | 18 (15) | 32 (13) |
| 3 | 3 (3) | 4 (3) | 7 (3) |
| Changed allocated treatment strategy, n (%) | 7 (6) | 22 (18) | 29 (12) |
| If yes, months until change in strategy | |||
| Mean (SD) | 4.4 (2.8) | 2.9 (2.2) | 3.3 (2.4) |
| Median (IQR) | 4.1 (1.8–5.6) | 2.8 (1.2–3.7) | 3.0 (1.4–4.1) |
| Minimum, maximum | 1.6, 9.7 | 0.1, 9.0 | 0.1, 9.7 |
Numbers analysed
The primary mITT analysis included 205 (85%) randomised participants who had at least 6 months of follow-up data available, including data from routine health-care records. Pre-planned sensitivity analyses were also performed in a strict ITT population, comprising all 240 randomised participants, and a per-protocol population, comprising 170 (71%) participants achieving at least 90% compliance with any trial preventative treatment. A further post hoc, unplanned sensitivity analysis was also performed in a strict per-protocol population, including only those achieving 90% compliance with their allocated preventative treatment strategy (n = 153; 64%).
Analyses in the 6-month follow-up period (i.e. at 12–18 months) were conducted in all participants in active follow-up at 15 or 18 months, plus those with data available from health-care record review.
For all analyses, with the exception of AE data, participants were analysed according to their randomised treatment assignment. A summary of participants included in each analysis population is given in Table 9.
| 12-month treatment period | 12- to 18-month follow-up period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mITT | ITT | PP | Strict PP | |||||||
| Antibiotic prophylaxis arm | Methenamine hippurate arm | Antibiotic prophylaxis arm | Methenamine hippurate arm | Antibiotic prophylaxis arm | Methenamine hippurate arm | Antibiotic prophylaxis arm | Methenamine hippurate arm | Antibiotic prophylaxis arm | Methenamine hippurate arm | |
| Number analysed, n (%) | 102 (85) | 103 (86) | 120 (100) | 120 (100) | 84 (70) | 86 (72) | 82 (68) | 71 (59) | 97 (81) | 98 (82) |
| ≥ 90% compliance with any trial preventative treatment, n (%) | 84 (82) | 86 (83) | 84 (70) | 86 (72) | 84 (100) | 86 (100) | 82 (100) | 71 (100) | 82 (85) | 85 (87) |
| Received alternative treatment strategy, n (%) | 7 (7) | 19 (18) | 7 (6) | 22 (18) | 2 (2) | 15 (17) | 0 (0) | 0 (0) | 5 (5) | 15 (15) |
| Follow-up time (days, including health-care review data) | ||||||||||
| Mean (SD) | 362.6 (14.5) | 361.6 (18.0) | 319.4 (105.6) | 323.1 (97.8) | 364.7 (2.9) | 364.8 (1.7) | 365.0 (0.0) | 365.0 (0.0) | 179.9 (11.2) | 181.4 (7.0) |
| Minimum, maximum | 239, 365 | 218, 365 | 14, 365 | 35, 365 | 338, 365 | 349, 365 | 365, 365 | 365, 365 | 83, 183 | 120, 183 |
| UTI episodes in the 12 months prior to trial entry, n (%) | ||||||||||
| < 4 | 12 (12) | 16 (16) | 14 (12) | 16 (13) | 11 (13) | 14 (16) | 11 (13) | 12 (17) | 12 (12) | 16 (16) |
| ≥ 4 | 90 (88) | 87 (84) | 106 (88) | 104 (87) | 73 (87) | 72 (84) | 71 (87) | 59 (83) | 85 (88) | 82 (84) |
| Menopausal status, n (%) | ||||||||||
| Pre | 40 (39) | 41 (40) | 49 (41) | 50 (42) | 30 (36) | 34 (40) | 29 (35) | 32 (45) | 35 (36) | 39 (40) |
| Peri/post | 62 (61) | 62 (60) | 71 (59) | 70 (58) | 54 (64) | 52 (60) | 53 (65) | 39 (55) | 62 (64) | 59 (60) |
Clinical outcomes
This section reports the results of the clinical outcome measures. In all analyses of the primary outcome, methenamine hippurate was not found to be inferior to the standard daily low-dose prophylactic antibiotics. Results pertaining to cost-effectiveness outcomes are included in Chapter 5.
Primary clinical outcome
The primary clinical outcome was the absolute difference in the incidence rate of symptomatic, antibiotic-treated UTI episodes between the antibiotic prophylaxis and antiseptic (methenamine hippurate) arms over the 12-month treatment period. The primary analysis was performed in the mITT population, which contained participants with at least 6 months of follow-up data, including data obtained through health-care record review.
The frequency of symptomatic UTI episodes in the mITT population over the 12-month period is shown in Appendix 2, Figure 17, and summarised in Table 10.
| Frequency and incidence of UTI | Antibiotic prophylaxis arm (N = 102) | Methenamine hippurate arm (N = 103) |
|---|---|---|
| Episodes of symptomatic UTI, n (%) | ||
| 0 | 55 (54) | 44 (43) |
| 1 | 21 (21) | 27 (26) |
| 2 | 15 (15) | 7 (7) |
| 3 | 7 (7) | 10 (10) |
| 4 | 2 (2) | 7 (7) |
| 5 | 2 (2) | 6 (6) |
| 6 | 0 (0) | 2 (2) |
| Mean (SD) | 0.88 (1.20) | 1.37 (1.67) |
| Median (IQR) | 0 (0–2) | 1 (0–2) |
| Incidence rate | ||
| Total number of UTI episodes/total follow-up time (years) | 90/101.32 | 141/102.03 |
| Incidence rate (95% CI) | 0.89 (0.65 to 1.12) | 1.38 (1.05 to 1.72) |
| Difference (90% CI) | 0.49 (0.15 to 0.84) | |
| Unadjusted IRR (95% CI) | 1.56 (1.09 to 2.22) | |
| Adjusted IRR (95% CI) | 1.52 (1.16 to 1.98) | |
| Incidence density rate | ||
| Total number of UTI episodes/total follow-up time (years) | 90/99.23 | 141/98.59 |
| Incidence rate (95% CI) | 0.91 (0.66 to 1.15) | 1.43 (1.07 to 1.79) |
| Difference (90% CI) | 0.52 (0.16 to 0.89) | |
| Unadjusted IRR (95% CI) | 1.58 (1.10 to 2.27) | |
| Adjusted IRR (95% CI) | 1.55 (1.17 to 2.06) | |
| At least one episode of symptomatic UTI | ||
| ≥ 1 episode, n (%) | 47 (46) | 59 (57) |
| Unadjusted OR (95% CI) | 1.57 (0.90 to 2.72) | |
| Adjusted OR (95% CI) | 1.55 (0.88 to 2.74) | |
The incidence of symptomatic, antibiotic-treated UTIs drastically reduced from baseline in both groups and was 0.89 episodes per person-year (95% CI 0.65 to 1.12 episodes per person-year) in those allocated to antibiotic prophylaxis and 1.38 episodes per person-year (95% CI 1.05 to 1.72 episodes per person-year) in those randomised to methenamine hippurate (see Table 10). The absolute difference between the incidence rates showed that those allocated to methenamine hippurate had an excess of 0.49 episodes per person-year (90% CI 0.15 to 0.84 episodes per person-year) compared with those allocated to antibiotic prophylaxis. As the upper limit of the 90% CI for the difference between incidence rates does not exceed 1, we were able to conclude that methenamine hippurate is non-inferior to antibiotic prophylaxis as a preventative treatment for recurrent uncomplicated UTI in this setting. To facilitate comparison with other studies and meta-analyses, a 95% CI for the difference in incidence rates was estimated to be 0.49 (95% CI 0.08 to 0.90), also showing the upper limit of the CI to be below the non-inferiority limit of 1.
To provide a relative measure of effectiveness, the IRR was calculated as 1.56 (95% CI 1.09 to 2.22), indicating that those in the methenamine hippurate arm had 1.56 times the number of UTI episodes per person-year compared with those allocated to antibiotic prophylaxis. After adjusting for site and baseline stratification factors of menopausal status and prior UTI frequency (< 4 vs. ≥ 4), the IRR was estimated as 1.52 (95% CI 1.16 to 1.98).
The incidence density rate, which excluded time on therapeutic antibiotics for UTI from the per-person follow-up time at risk, showed similar results (see Table 10).
In the mITT population, 47 (46%) participants in the antibiotic prophylaxis arm reported at least one episode of symptomatic UTI in the 12-month treatment period, compared with 59 (57%) participants allocated to methenamine hippurate (OR 1.57, 95% CI 0.90 to 2.72). After adjusting for site and baseline stratification factors, the OR was estimated as 1.55 (95% CI 0.88 to 2.74). In all cases, mixed-effects logistic regression models showed no improvement in model fit compared with the unadjusted model.
A post hoc, unplanned analysis explored the number of participants meeting the definition of rUTI (two episodes in a 6-month period or three episodes in 1 year) over the 12-month treatment period. In the mITT population, 22 out of 102 (22%) participants allocated to antibiotic prophylaxis continued to experience rUTI, compared with 31 out of 103 (30%) participants allocated to methenamine hippurate (adjusted OR 1.61, 95% CI 0.84 to 3.11; p-value = 0.153).
Sensitivity analyses
Sensitivity analyses were performed, as prespecified in the analysis plan, in the ITT and per-protocol populations (Table 11). Analyses in the ITT population gave similar results, with the difference between incidence rates showing those allocated to methenamine hippurate had an excess of 0.53 episodes per person-year (90% CI 0.20 to 0.86 episodes per person-year) compared with those allocated to antibiotic prophylaxis. The difference in incidence rates was found to be smaller when the analysis was repeated in the per-protocol population (0.42 episodes per person-year, 90% CI 0.05 to 0.79 episodes per person year).
| Frequency and incidence of UTI | ITT analysis | Per-protocol analysis | ||
|---|---|---|---|---|
| Antibiotic prophylaxis arm (N = 120) | Methenamine hippurate arm (N = 120) | Antibiotic prophylaxis arm (N = 84) | Methenamine hippurate arm (N = 86) | |
| Incidence rate | ||||
| Total number of UTI episodes/total follow-up time (years) | 92/105.01 | 149/106.22 | 73/83.93 | 111/85.96 |
| Incidence rate (95% CI) | 0.88 (0.65 to 1.11) | 1.40 (1.08 to 1.73) | 0.87 (0.61 to 1.13) | 1.29 (0.93 to 1.66) |
| Difference (90% CI) | 0.53 (0.20 to 0.86) | 0.42 (0.05 to 0.79) | ||
| Unadjusted IRR (95% CI) | 1.60 (1.13 to 2.26) | 1.48 (0.99 to 2.23) | ||
| Adjusted IRR (95% CI) | 1.58 (1.24 to 2.03) | 1.44 (1.02 to 2.02) | ||
| Incidence density rate | ||||
| Total number of UTI episodes/total follow-up time (years) | 92/102.82 | 149/102.56 | 73/82.40 | 111/83.14 |
| Incidence rate (95% CI) | 0.89 (0.65 to 1.13) | 1.45 (1.11 to 1.80) | 0.89 (0.61 to 1.16) | 1.34 (0.95 to 1.72) |
| Difference (90% CI) | 0.56 (0.21 to 0.91) | 0.45 (0.05 to 0.84) | ||
| Unadjusted IRR (95% CI) | 1.62 (1.14 to 2.32) | 1.51 (0.99 to 2.28) | ||
| Adjusted IRR (95% CI) | 1.62 (1.26 to 2.09) | 1.48 (1.03 to 2.13) | ||
| At least one episode of symptomatic UTI | ||||
| ≥ 1 episode, n (%) | 49 (41) | 64 (53) | 39 (46) | 45 (52) |
| Unadjusted OR (95% CI) | 1.66 (0.99 to 2.76) | 1.27 (0.69 to 2.31) | ||
| Adjusted OR (95% CI) | 1.69 (1.00 to 2.87) | 1.29 (0.69 to 2.41) | ||
The per-protocol population was defined by compliance with any trial preventative treatment, not compliance with the treatment strategy allocated at randomisation (i.e. switching between methenamine hippurate and antibiotic prophylaxis and vice versa was considered ‘per protocol’). A further post hoc, unplanned, sensitivity analysis was performed in a strict per-protocol population, including only those achieving 90% compliance with their initially allocated preventative treatment strategy (see Appendix 2, Table 29). This showed a further reduction in the estimated difference between incidence rates (0.30 episodes per person year, 90% CI –0.08 to 0.67 episodes per person year).
All sensitivity analyses of the primary clinical outcome found the upper limit of the 90% CI to be below the non-inferiority limit of 1, hence confirming methenamine hippurate to be non-inferior to antibiotic prophylaxis for the prevention of rUTI in this population.
Secondary outcomes
Symptomatic urinary tract infection in the 6-month follow-up period (12–18 months)
The frequency of symptomatic UTI episodes in the 6-month follow-up period is shown in Appendix 2, Figure 18. The frequency and incidence of symptomatic UTI over the 6-month follow-up period are summarised in Table 12. The incidence of symptomatic UTI episodes in the 6-month follow-up period was higher in both groups than in the 12-month treatment period, but did not return to anywhere close to baseline levels.
| Frequency and incidence of UTI | Antibiotic prophylaxis arm (N = 97) | Methenamine hippurate arm (N = 98) |
|---|---|---|
| Episodes of symptomatic UTI, n (%) | ||
| 0 | 55 (57) | 49 (50) |
| 1 | 31 (32) | 27 (28) |
| 2 | 8 (8) | 13 (13) |
| 3 | 2 (2) | 6 (6) |
| 4 | 1 (1) | 2 (2) |
| 5 | 0 (0) | 1 (1) |
| Mean (SD) | 0.59 (0.81) | 0.86 (1.10) |
| Median (IQR) | 0 (0–1) | 0.5 (0–1) |
| Incidence rate | ||
| Total number of UTI episodes/total follow-up time (years) | 57/47.80 | 84/48.71 |
| Incidence rate (95% CI) | 1.19 (0.86 to 1.52) | 1.72 (1.27 to 2.18) |
| Difference (95% CI) | 0.53 (–0.03 to 1.09) | |
| Unadjusted IRR (95% CI) | 1.45 (0.99 to 2.10) | |
| Adjusted IRR (95% CI) | 1.45 (1.16 to 1.81) | |
| Incidence density rate | ||
| Total number of UTI episodes/total follow-up time (years) | 57/46.25 | 84/46.59 |
| Incidence rate (95% CI) | 1.23 (0.88 to 1.59) | 1.80 (1.31 to 2.29) |
| Difference (95% CI) | 0.57 (–0.04 to 1.18) | |
| Unadjusted IRR (95% CI) | 1.46 (0.99 to 2.16) | |
| Adjusted IRR (95% CI) | 1.47 (1.14 to 1.89) | |
| At least one episode of symptomatic UTI | ||
| ≥ 1 episode, n (%) | 42 (43) | 49 (50) |
| Unadjusted OR (95% CI) | 1.31 (0.75 to 2.30) | |
| Adjusted OR (95% CI) | 1.32 (0.73 to 2.39) | |
The incidence rate was 1.19 (0.86–1.52) in those allocated to antibiotic prophylaxis and 1.72 (95% CI 1.27 to 2.18) in those allocated to methenamine hippurate, giving an absolute difference between incidence rates of 0.53 episodes per person-year (95% CI –0.03 to 1.09) in favour of antibiotic prophylaxis. The unadjusted IRR comparing methenamine hippurate with antibiotic prophylaxis was calculated as 1.45 (95% CI 0.99 to 2.10) and the adjusted IRR was estimated as 1.45 (95% CI 1.16 to 1.81).
Over the 6-month follow-up period, 42 (43%) participants in the antibiotic prophylaxis arm and 49 (50%) participants in the methenamine hippurate arm reported at least one episode of symptomatic UTI (unadjusted OR 1.31, 95% CI 0.75 to 2.30). Over half of all trial participants remained UTI free in the 6-month period following completion of preventative treatment.
A post hoc, unplanned analysis comparing the number of participants reporting two or more UTI episodes during the 6-month follow-up period (a definition of rUTI) showed that 11 of 97 (11%) participants in the antibiotic prophylaxis arm, compared with 22 of 98 (22%) participants in the methenamine hippurate arm (chi-squared p-value = 0.039), returned to suffering UTIs that could be classified as recurrent.
Microbiologically proven symptomatic urinary tract infections
In the mITT population, a total of 231 symptomatic UTI episodes were reported during the 12-month treatment period (90 in the antibiotic prophylaxis arm, 141 in the methenamine hippurate arm). Of these, 183 (79%) had a urine sample sent to the central or local laboratory at the time of their UTI episode: 72 (80%) allocated to antibiotic prophylaxis and 111 (79%) allocated to methenamine hippurate. Only 96 (42%) of the 231 symptomatic UTI episodes were confirmed with a positive urine culture: 42 of 90 (47%) in the antibiotic prophylaxis arm and 54 of 141 (38%) in the methenamine hippurate arm.
Consequently, incidence rates for microbiologically proven symptomatic UTI episodes were lower across both arms than at the primary end-point analysis: 0.41 (95% CI 0.27 to 0.56) in the antibiotic prophylaxis arm and 0.53 (95% CI 0.34 to 0.72) in the methenamine hippurate arm (Table 13). The absolute difference between incidence rates showed that those allocated to methenamine hippurate had an excess of 0.11 microbiologically proven UTI episodes per person-year (95% CI –0.12 to 0.35 microbiologically proven UTI episodes per person-year) compared with those allocated to antibiotic prophylaxis. The unadjusted IRR comparing methenamine hippurate with antibiotic prophylaxis was found to be 1.28 (95% CI 0.78 to 2.09). After adjusting for baseline stratification factors, the IRR was estimated as 1.28 (95% CI 1.05 to 1.49). Analyses of the incidence density ratio, excluding time on treatment antibiotics for UTI, gave similar results.
| Frequency and incidence of UTI | 12-month treatment period | 6-month follow-up period | ||
|---|---|---|---|---|
| Antibiotic prophylaxis arm (N = 102) | Methenamine hippurate arm (N = 103) | Antibiotic prophylaxis arm (N = 97) | Methenamine hippurate arm (N = 98) | |
| Episodes of symptomatic microbiologically confirmed UTI, n (%) | ||||
| 0 | 73 (72) | 70 (68) | 76 (78) | 65 (66) |
| 1 | 18 (18) | 21 (20) | 19 (20) | 25 (26) |
| 2 | 9 (9) | 6 (6) | 2 (2) | 7 (7) |
| 3 | 2 (2) | 3 (3) | 0 (0) | 1 (1) |
| 4 | 0 (0) | 3 (3) | 0 (0) | 0 (0) |
| Mean (SD) | 0.41 (0.74) | 0.52 (0.95) | 0.24 (0.47) | 0.43 (0.67) |
| Incidence rate | ||||
| Total number of UTI episodes/total follow-up time (years) | 42/101.32 | 54/102.03 | 23/47.80 | 42/48.71 |
| Incidence rate (95% CI) | 0.41 (0.27 to 0.56) | 0.53 (0.34 to 0.72) | 0.48 (0.28 to 0.68) | 0.86 (0.59 to 1.14) |
| Difference (95% CI) | 0.11 (–0.12 to 0.35) | 0.38 (0.04 to 0.72) | ||
| Unadjusted IRR (95% CI) | 1.28 (0.78 to 2.09) | 1.79 (1.08 to 2.96) | ||
| Adjusted IRR (95% CI) | 1.25 (1.05 to 1.49) | 1.86 (1.27 to 2.73) | ||
| Incidence density rate | ||||
| Total number of UTI episodes/total follow-up time (years) | 42/99.23 | 54/98.59 | 23/46.25 | 42/46.59 |
| Incidence rate (95% CI) | 0.42 (0.27 to 0.58) | 0.55 (0.35 to 0.75) | 0.50 (0.29 to 0.70) | 0.90 (0.61 to 1.20) |
| Difference (95% CI) | 0.12 (–0.13 to 0.37) | 0.40 (0.05 to 0.76) | ||
| Unadjusted IRR (95% CI) | 1.29 (0.79 to 2.13) | 1.81 (1.09 to 3.02) | ||
| Adjusted IRR (95% CI) | 1.29 (1.06 to 1.57) | 1.89 (1.28 to 2.78) | ||
| At least one episode | ||||
| ≥ 1 episode, n (%) | 29 (28) | 33 (32) | 21 (22) | 33 (34) |
| Unadjusted OR (95% CI) | 1.19 (0.65 to 2.16) | 1.84 (0.97 to 3.48) | ||
| Adjusted OR (95% CI) | 1.19 (0.65 to 2.17) | 2.00 (1.03 to 3.91) | ||
Analysis in the 6-month follow-up period (12–18 months) showed that 93 (66%) of 141 symptomatic UTI episodes were associated with a urine sample sent to the local or central laboratory: 37 (65%) in the antibiotic prophylaxis arm and 56 (67%) in the methenamine hippurate arm. Only 65 of 141 (46%) symptomatic UTI episodes were confirmed with a positive urine culture: 23 (40%) of 57 in the antibiotic prophylaxis arm and 42 (50%) of 84 in the methenamine hippurate arm. Incidence rates were found to be 0.48 (95% CI 0.28 to 0.68) in the antibiotic prophylaxis arm and 0.86 (95% CI 0.59 to 1.14) in the methenamine hippurate arm, giving a difference between incidence rates of 0.38 (95% CI 0.04 to 0.72) episodes per person-year (see Table 13).
Antibiotic use
During the 12-month treatment period, a total of 22 (18%) participants allocated to methenamine hippurate received prophylactic antibiotics (see Appendix 2, Table 30). During the 12-month treatment period the average proportion of time participants received prophylactic antibiotic treatment was 91% in the antibiotic prophylaxis arm and 12% in the methenamine hippurate arm. Therapeutic antibiotics for UTI were received by 51 (43%) participants allocated to antibiotic prophylaxis and 67 (56%) participants allocated to methenamine hippurate during the 12-month treatment period. The median total days of therapeutic antibiotic treatment was 13 days for those allocated to antibiotic prophylaxis and 16 days for those allocated to methenamine hippurate. Therapeutic antibiotics were prescribed for other reasons to 32 (27%) participants allocated to antibiotic prophylaxis and to 38 (32%) participants allocated to methenamine hippurate.
Only 13 participants were reported to have received prophylactic antibiotic treatment during the 6-month follow-up period (eight randomised to antibiotic prophylaxis and five to methenamine hippurate). During the 6-month follow-up period, therapeutic antibiotics for UTI were received by 48 (49%) and 52 (53%) participants allocated to antibiotic prophylaxis and methenamine hippurate, respectively; the median total days of treatment was 7.5 and 13.5 days, respectively. Therapeutic antibiotics prescribed for other reasons were taken by 15 (15%) participants allocated to antibiotic prophylaxis and 28 (29%) participants allocated to methenamine hippurate during the 6-month follow-up period.
Antimicrobial resistance
Perineal swabs
The availability of perineal swabs at each trial time point is shown in Table 14. In total, 89 participants (37%) had provided a sample at all time points (45 participants in the antibiotic prophylaxis arm and 44 participants in the methenamine hippurate arm) and 37 (15%) participants had E. coli isolated in all four samples (19 participants in the antibiotic prophylaxis arm and 18 participants in the methenamine hippurate arm).
| Antibiotic prophylaxis arm (N = 120) | Methenamine hippurate arm (N = 120) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Month 6 | Month 12 | Month 18 | Baseline | Month 6 | Month 12 | Month 18 | |
| Samples available, n (%) | 107 (89) | 75 (63) | 66 (55) | 59 (49) | 94 (78) | 79 (66) | 70 (58) | 62 (52) |
| E. coli isolated, n (%) | 76 (71) | 51 (68) | 43 (65) | 39 (66) | 64 (68) | 58 (73) | 47 (67) | 45 (73) |
| Antibiotic resistance in E. coli (number of antibiotics) | ||||||||
| 0, n (%) | 32 (42) | 19 (37) | 12 (29) | 24 (62) | 29 (45) | 27 (47) | 23 (49) | 26 (58) |
| 1 or 2, n (%) | 23 (30) | 21 (41) | 22 (52) | 12 (31) | 17 (27) | 18 (31) | 17 (36) | 10 (22) |
| ≥ 3, n (%) | 21 (28) | 11 (22) | 8 (19) | 3 (8) | 18 (28) | 13 (22) | 7 (15) | 9 (20) |
| Mean (SD) | 1.7 (2.1) | 1.5 (1.6) | 1.7 (1.8) | 0.8 (1.2) | 1.7 (2.2) | 1.3 (1.6) | 1.1 (1.6) | 1.2 (1.8) |
| Median (IQR); range | 1 (0–3); 0–8 | 1 (0–2); 0–6 | 1 (0–2); 0–7 | 0 (0–2); 0–4 | 1 (0–3); 0–9 | 1 (0–2); 0–6 | 1 (0–2); 0–6 | 0 (0–2); 0–8 |
| Antibiotic resistance in E. coli (number of antimicrobial categories) | ||||||||
| 0, n (%) | 32 (42) | 19 (37) | 12 (29) | 24 (62) | 29 (45) | 27 (47) | 23 (49) | 26 (58) |
| 1 or 2, n (%) | 28 (37) | 24 (47) | 22 (52) | 13 (33) | 22 (34) | 24 (41) | 18 (38) | 10 (22) |
| ≥ 3 (MDR), n (%) | 16 (21) | 8 (16) | 8 (19) | 2 (5) | 13 (20) | 7 (12) | 6 (13) | 9 (20) |
| Mean (SD) | 1.3 (1.5) | 1.2 (1.2) | 1.4 (1.3) | 0.7 (1.0) | 1.3 (1.6) | 1.1 (1.2) | 1.0 (1.2) | 1.0 (1.5) |
| Median (IQR); range | 1 (0–2); 0–6 | 1 (0–2); 0–4 | 1 (0–2); 0–5 | 0 (0–2); 0–3 | 1 (0–2); 0–6 | 1 (0–2); 0–4 | 1 (0–2); 0–5 | 0 (0–2); 0–6 |
The proportions of participants demonstrating resistance in E. coli to at least one antibiotic at baseline, during the preventative treatment period (at 6 or 12 months) and at 18 months are shown in Figure 5. Comparisons between groups were made using a chi-squared test. Similarly, the rates of MDR are shown in Figure 6 but with comparisons made using Fisher’s exact tests.
FIGURE 5.
Any resistance (per participant) in E. coli isolated from perineal swabs at baseline, 6 or 12 months, and at the 18-month follow-up (chi-squared p-values). Reproduced with permission from Harding et al. 36 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
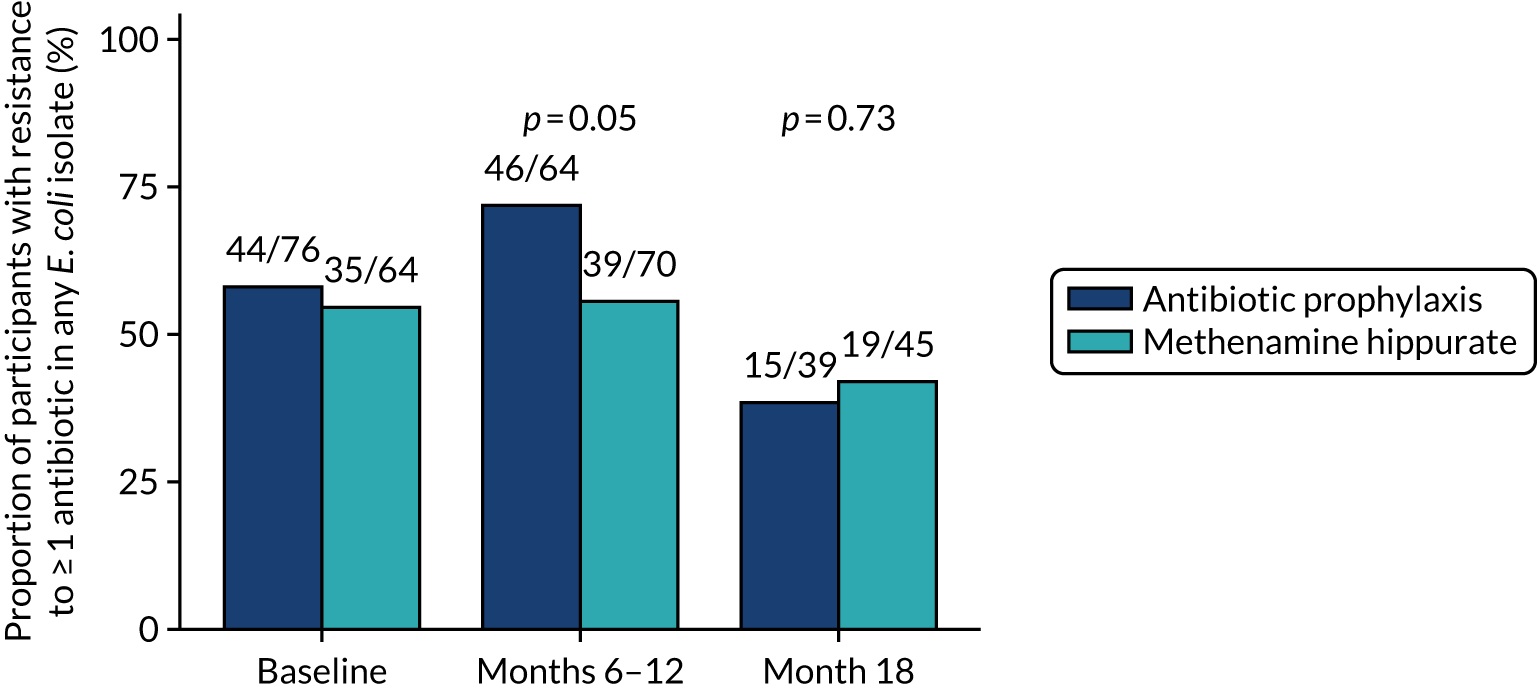
FIGURE 6.
Any MDR (per participant) in E. coli isolated from perineal swabs at baseline, 6 or 12 months, and at the 18-month follow-up (Fisher’s exact p-values). Reproduced with permission from Harding et al. 36 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
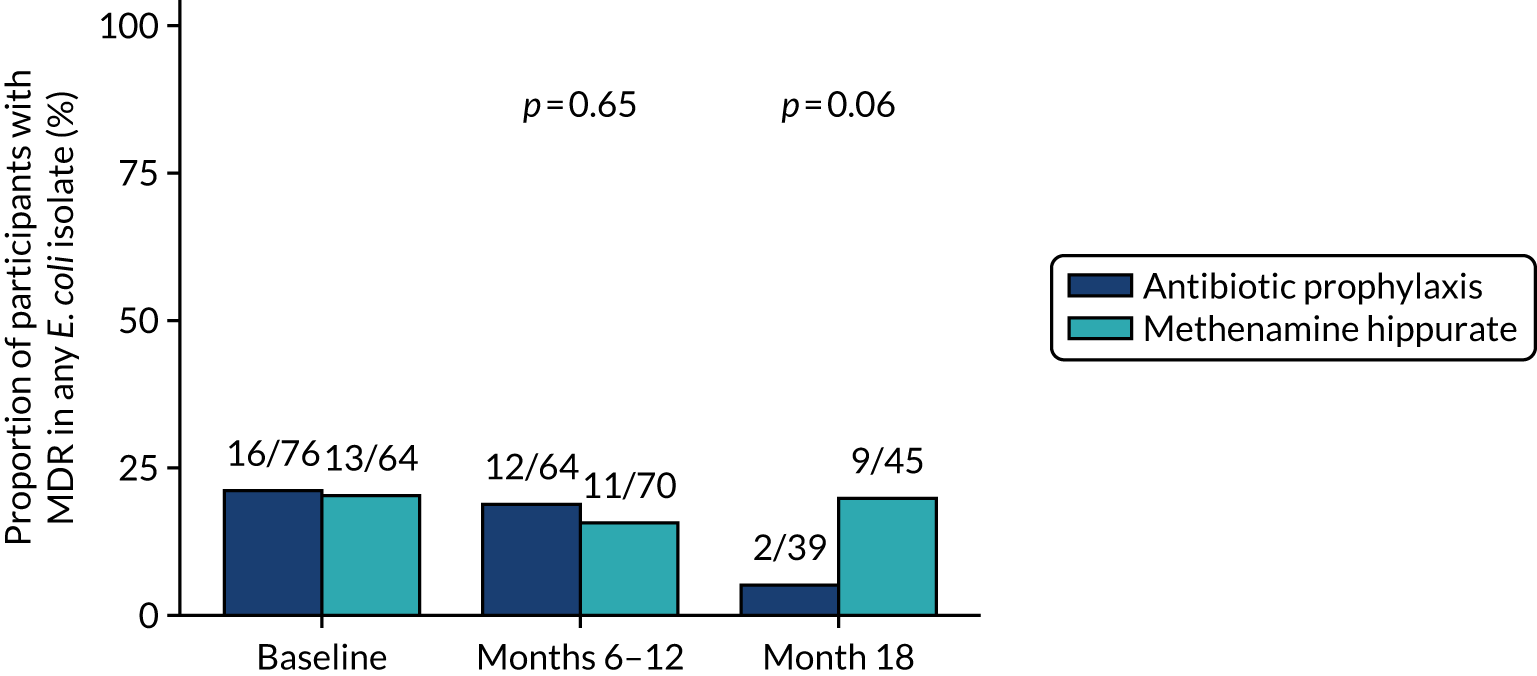
There were no apparent differences between groups in the antimicrobial susceptibility patterns of the baseline perineal E. coli isolates, as judged by the number of antibiotics to which the isolate was resistant or the percentage of multidrug-resistant isolates. During the preventative treatment period, a higher proportion of participants in the antibiotic prophylaxis arm were found to have at least one E. coli isolate demonstrating resistance to at least one antibiotic (46/64; 72%) than in the methenamine hippurate arm (39/70; 56%) (chi-squared p-value = 0.052). However, at the 18-month time point a higher proportion of participants allocated to methenamine hippurate were colonised with multidrug-resistant E. coli isolates (9/45; 20%) than were participants in the antibiotic prophylaxis arm (2/39; 5%) (Fisher’s exact p-value = 0.06).
Out of 105 participants with E. coli isolated at baseline and in at least one post-baseline sample, 46 (44%) participants developed resistance to at least one different antibiotic in a post-baseline sample; 27 out of 52 (52%) participants in the antibiotic prophylaxis arm and 19 out of 53 (36%) participants in the methenamine hippurate arm (chi-squared p-value = 0.10) (see Appendix 2, Figure 19). The number of participants known to have acquired MDR in E. coli since baseline was similar in both randomised treatment arms, with 7 out of 42 (17%) participants in the antibiotic prophylaxis arm and 6 out of 42 (14%) participants in the methenamine hippurate arm (chi-squared p-value = 0.76) (see Appendix 2, Figure 20).
For eight different antibiotic agents (the three trial prophylactic antibiotic agents, cefalexin, nitrofurantoin and trimethoprim, plus the five agents with the highest resistance rates in our study), the proportions of participants demonstrating ‘known resistance since baseline’ and ‘resistance since baseline’ in perineal isolates are shown in Appendix 2, Figure 21. For each antibiotic, ‘known resistance since baseline’ was defined as participants with at least one E. coli isolate resistant to at least one of the antimicrobial agents tested in a post-baseline perineal sample, out of those who demonstrated sensitivity to the antimicrobial panel in E. coli at baseline. ‘Resistance since baseline’ was defined for each antibiotic as participants with at least one E. coli isolate resistant to at least one of the antimicrobial agents tested in a post-baseline perineal sample out of those who demonstrated sensitivity to the antimicrobial panel in E. coli at baseline or did not have E. coli isolated in their baseline sample. There was a slightly higher proportion of participants allocated to antibiotic prophylaxis showing ‘known resistance since baseline’ to a number of antibiotics (amoxicillin, cefalexin, ciprofloxacin) than there was in those allocated to the methenamine hippurate arm; however, there were no statistically significant differences between treatment arms.
For each of the eight antibiotics, resistance rates in E. coli over time are plotted by antibiotic in Appendix 2, Figure 22. The same data are also provided in Appendix 2, Table 31.
Urine samples
The availability of routine urine samples sent to the central laboratory at baseline and at 3-monthly follow-up visits (3–18 months) is shown in Table 15, along with the number of samples with significant E. coli bacteriuria. E. coli was isolated from relatively small numbers of routine urine samples collected during the 12-month treatment period, particularly from the antibiotic prophylaxis cohort.
| Antibiotic prophylaxis arm (N = 120), n (%) | Methenamine hippurate arm (N = 120), n (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 3 | Month 6 | Month 9 | Month 12 | Month 15 | Month 18 | Baseline | Month 3 | Month 6 | Month 9 | Month 12 | Month 15 | Month 18 | |
| Samples available | 111 (93) | 95 (79) | 81 (68) | 79 (66) | 74 (62) | 64 (53) | 62 (52) | 111 (93) | 96 (80) | 89 (74) | 81 (68) | 78 (65) | 71 (59) | 71 (59) |
| E. coli isolated | 15 (14) | 2 (2) | 4 (5) | 3 (4) | 5 (7) | 11 (17) | 7 (11) | 7 (6) | 8 (8) | 9 (10) | 5 (6) | 12 (15) | 8 (11) | 8 (11) |
| Antibiotic resistance in E. coli (number of antibiotics) | ||||||||||||||
| 0 | 5 (33) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 3 (27) | 3 (43) | 2 (29) | 3 (38) | 4 (44) | 4 (80) | 6 (50) | 2 (25) | 5 (63) |
| 1 or 2 | 7 (47) | 1 (50) | 2 (50) | 3 (100) | 4 (80) | 7 (64) | 2 (29) | 2 (29) | 3 (38) | 0 (0) | 0 (0) | 3 (25) | 4 (50) | 2 (25) |
| ≥ 3 | 3 (20) | 0 (0) | 2 (50) | 0 (0) | 1 (20) | 1 (9) | 2 (29) | 3 (43) | 2 (25) | 5 (26) | 1 (20) | 3 (25) | 2 (25) | 1 (13) |
| Multidrug resistanta | 3 (20) | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 1 (9) | 1 (14) | 2 (29) | 2 (25) | 4 (44) | 1 (20) | 3 (25) | 2 (25) | 1 (13) |
In total, 124 positive urine cultures were submitted from 62 individual participants during periods of symptomatic UTI, 46 from 29 participants allocated to antibiotic prophylaxis and 78 from 33 participants allocated to methenamine hippurate. Significant E. coli bacteriuria was detected in 93 urine cultures from 51 individual participants: 36 samples from 25 participants allocated to antibiotic prophylaxis and 57 from 26 participants allocated to methenamine hippurate.
The proportions of participants demonstrating resistance in E. coli in any symptomatic urine sample submitted during the 12-month treatment and 6-month follow-up (12–18 months) periods are shown for eight different antibiotics (the three trial prophylactic antibiotic agents plus the five agents with the highest resistance rates in our study) in Figure 7. A higher proportion of participants allocated to antibiotic prophylaxis demonstrated resistance to co-trimoxazole in at least one urinary E. coli isolate during the 12-month preventative treatment period than did those allocated to methenamine hippurate: 6 out of 13 (46%) participants and 3 out of 14 (21%) participants, respectively. Similarly, six out of eight (75%) participants allocated to antibiotic prophylaxis demonstrated resistance to trimethoprim in at least one urinary E. coli isolate during the 12- to 18-month follow-up period, compared with 5 out of 13 (38%) participants allocated to methenamine hippurate. There were no significant differences in the proportion of participants showing resistance to at least one antibiotic in any urinary E. coli isolate or MDR in any E. coli isolate during the preventative treatment or follow-up periods (Figures 8 and 9).
FIGURE 7.
Resistance rates to individual antibiotics (per participant) in E. coli isolated from symptomatic urine samples in the 12-month treatment period and 6-month follow-up period. (a) Amoxicillin; (b) cefalexin; (c) cefuroxime; (d) ciprofloxacin; (e) co-amoxiclav; (f) co-trimoxazole; (g) nitrofurantoin; and (h) trimethoprim. Reproduced with permission from Harding et al. 36 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
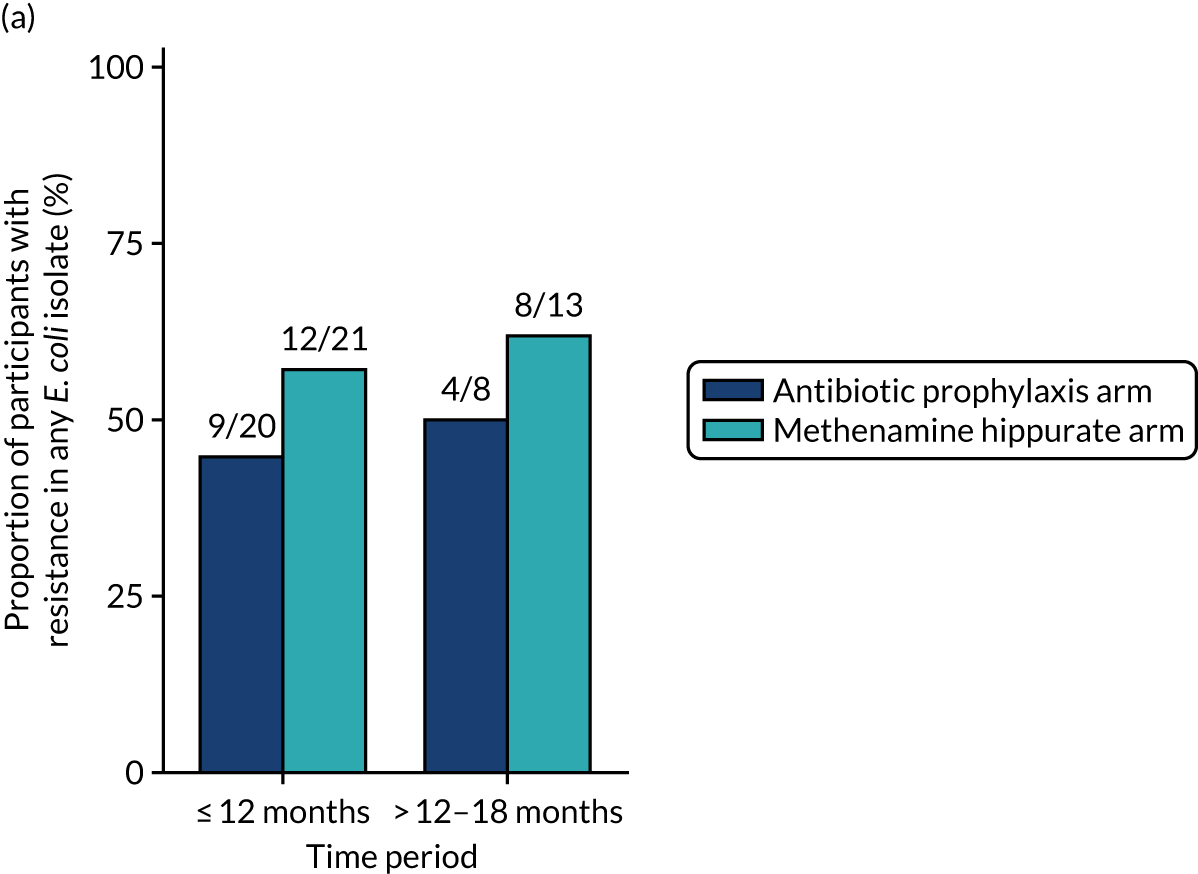
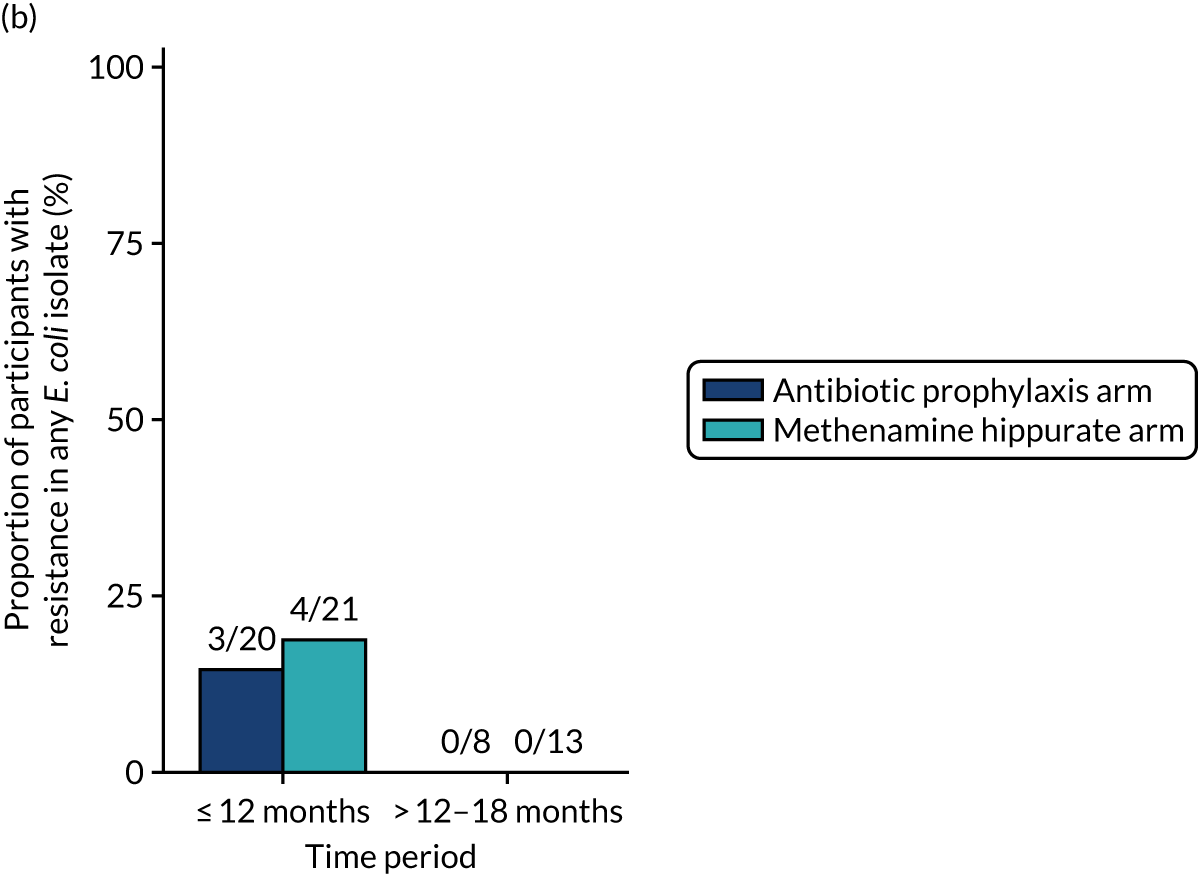
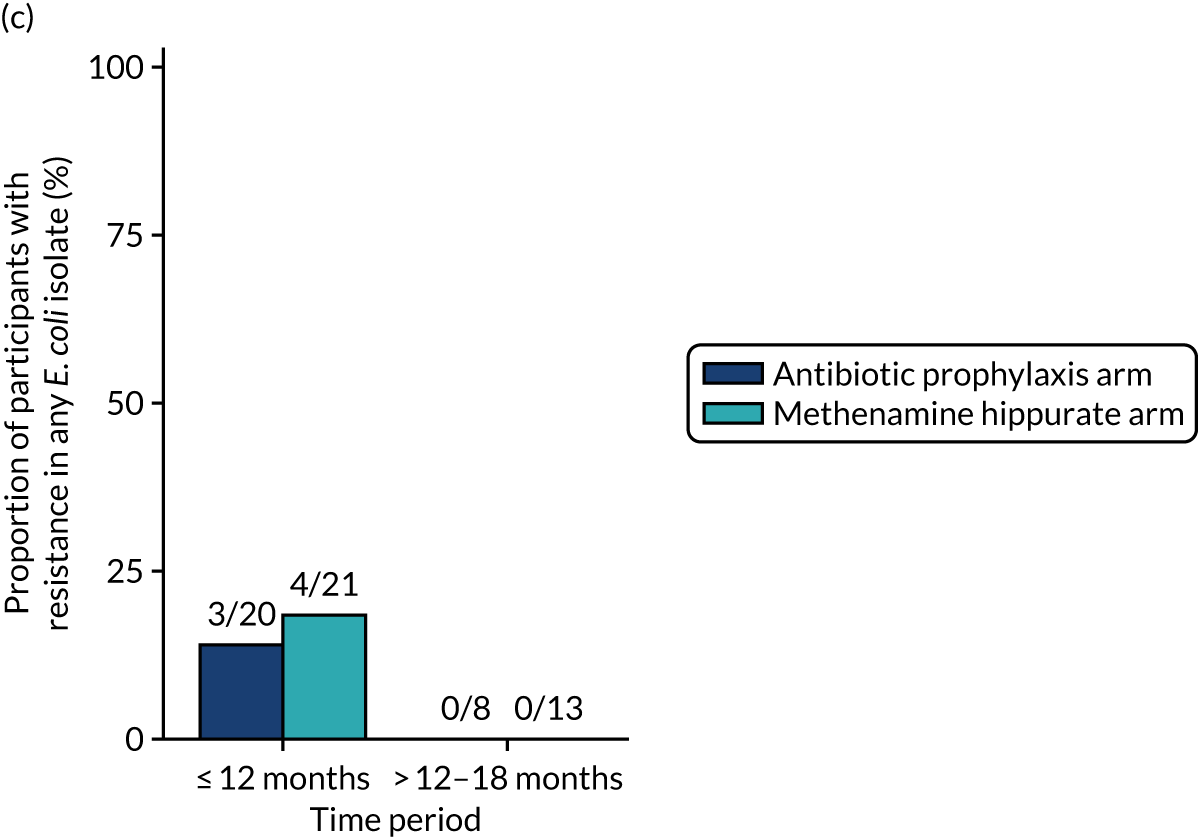
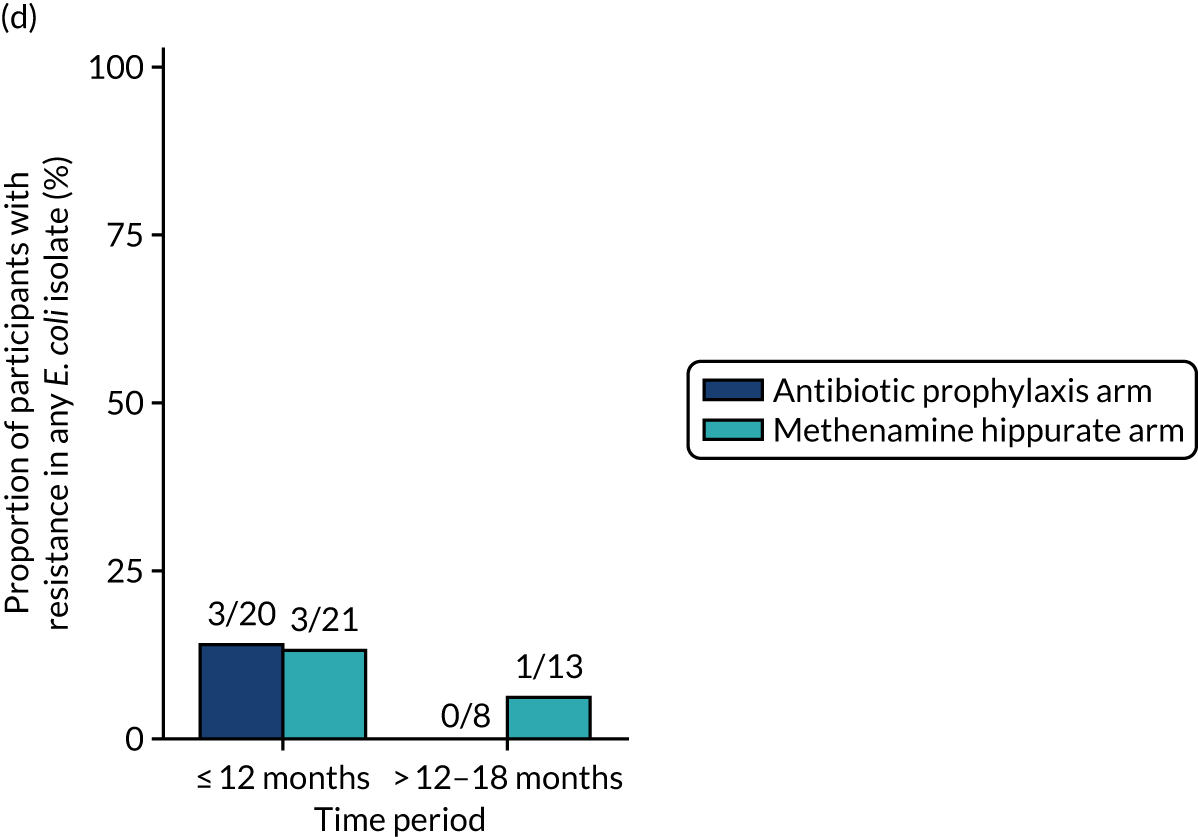
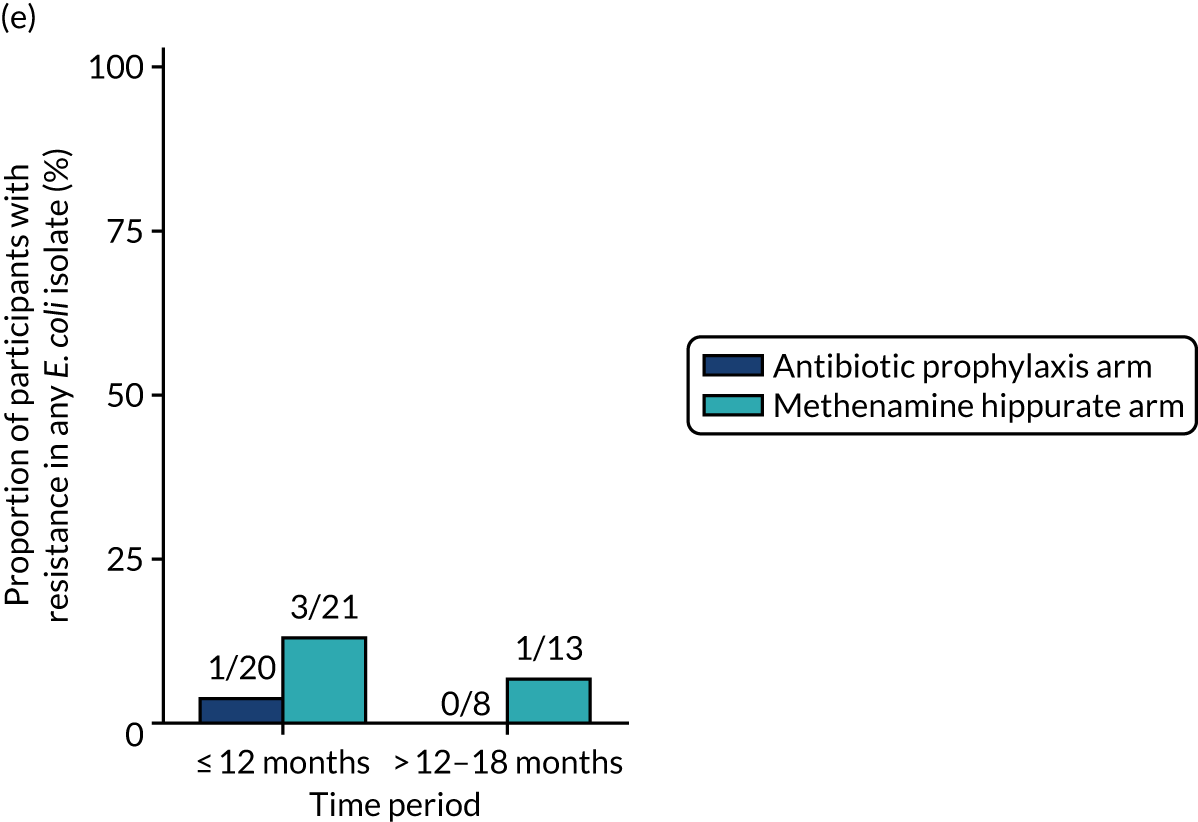
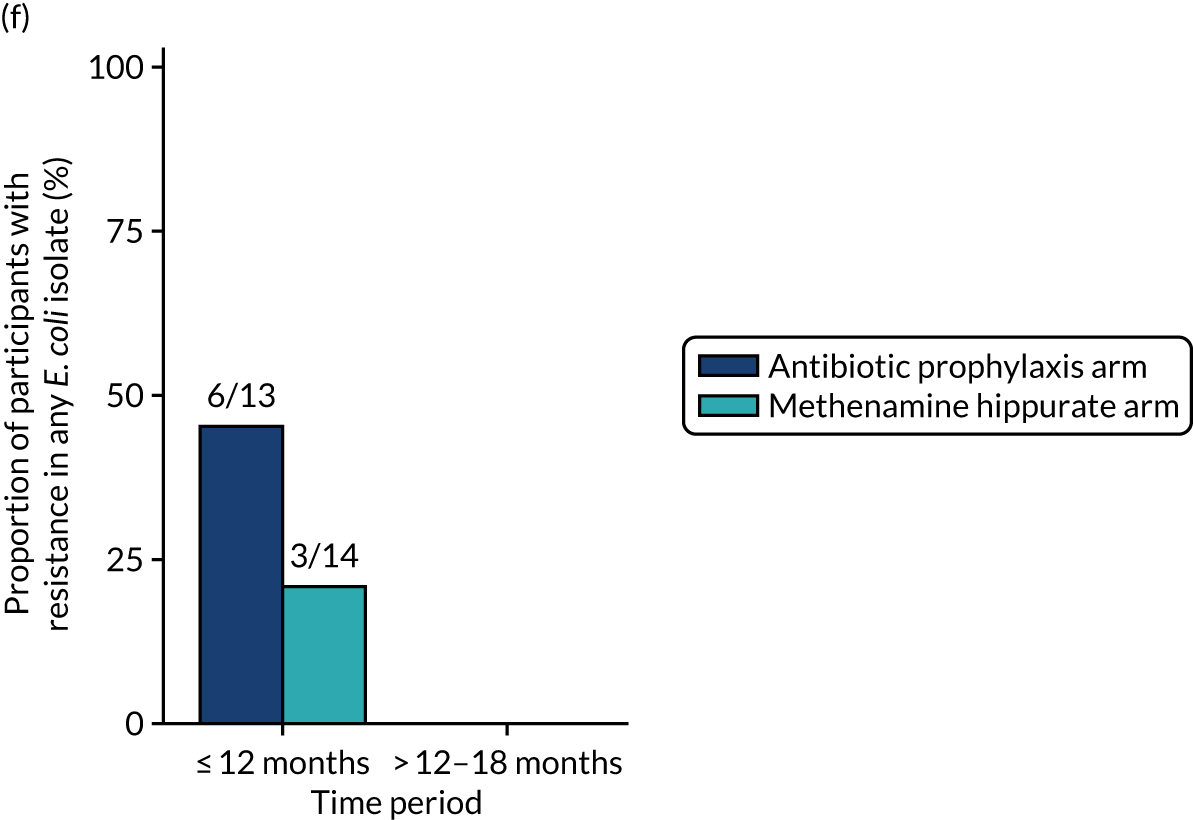
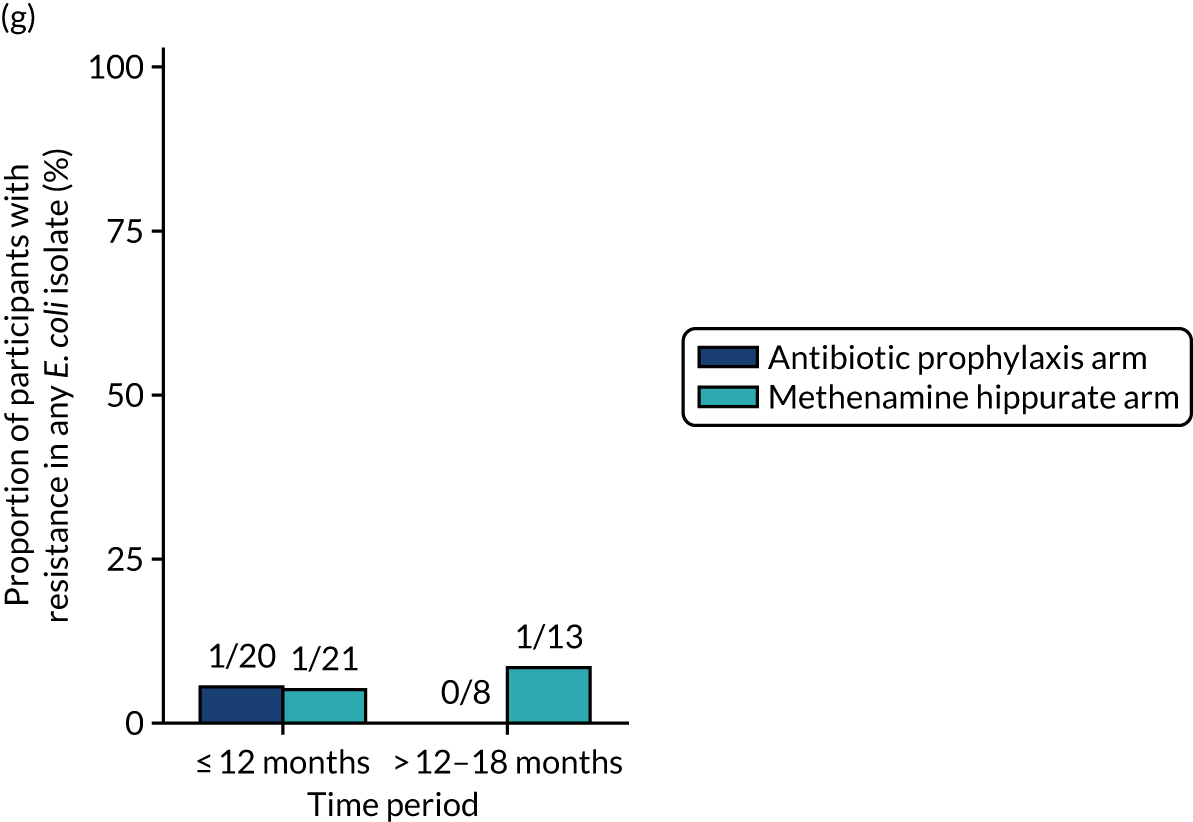
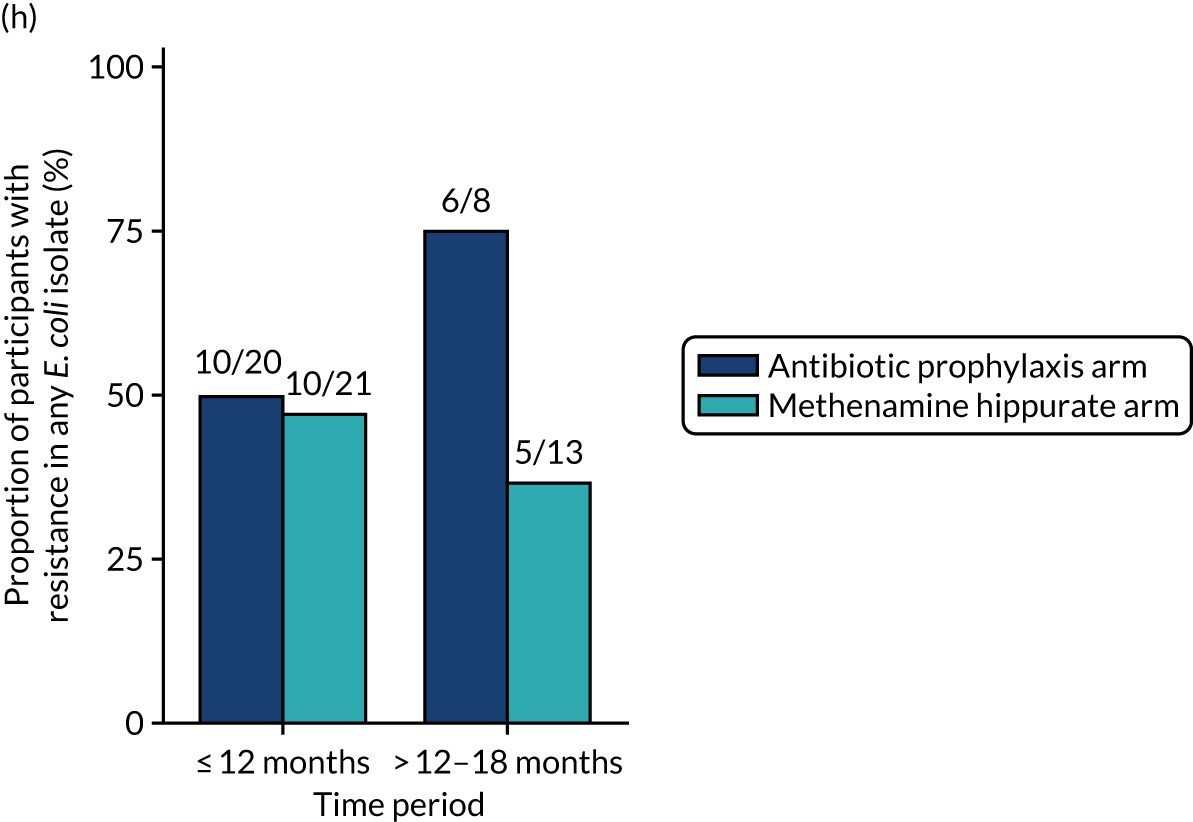
FIGURE 8.
Any resistance (per participant) in E. coli isolated from symptomatic urine samples in the 12-month treatment period and 6-month follow-up period. Fisher’s exact p-values.
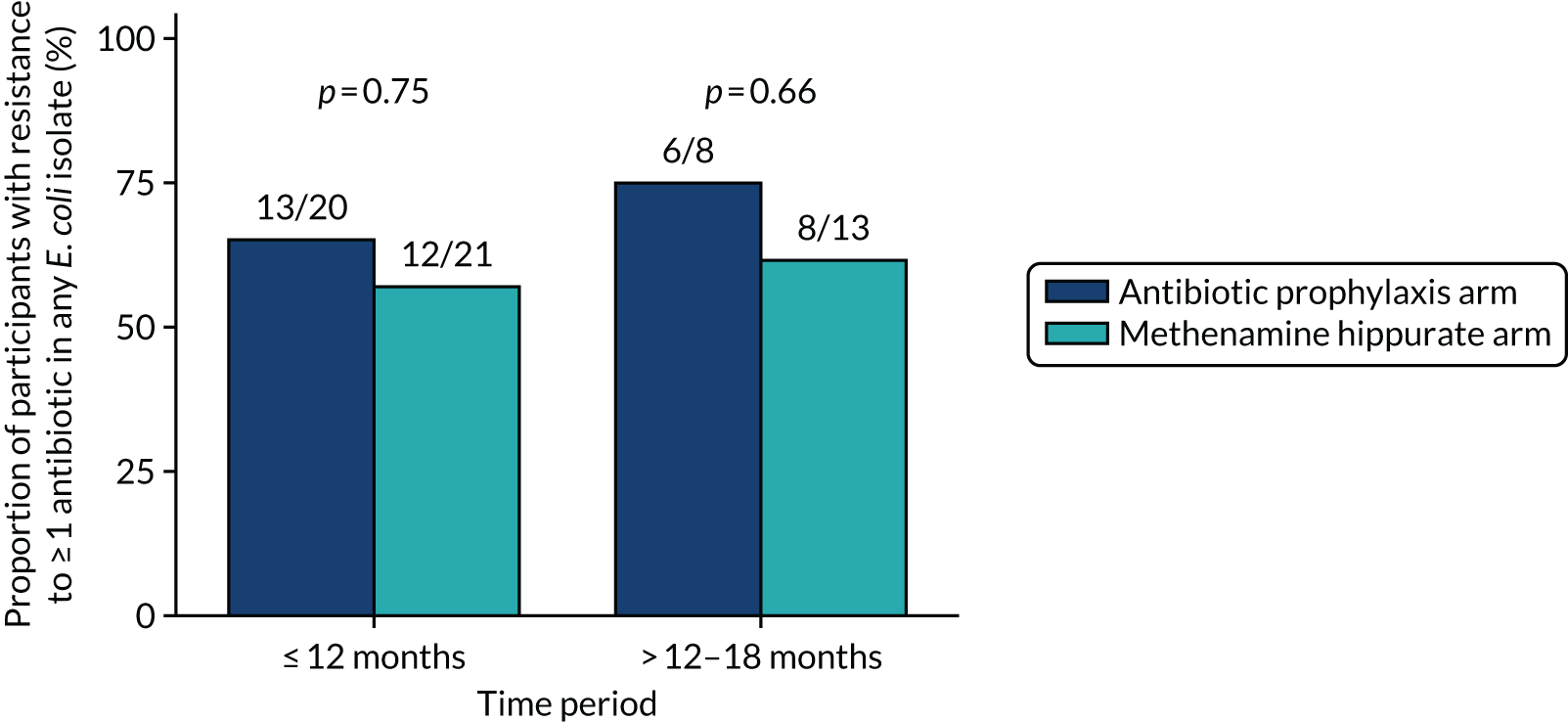
FIGURE 9.
Any MDR (per participant) in E. coli isolated from symptomatic urine samples in the 12-month treatment period and 6-month follow-up period. Fisher’s exact p-values.
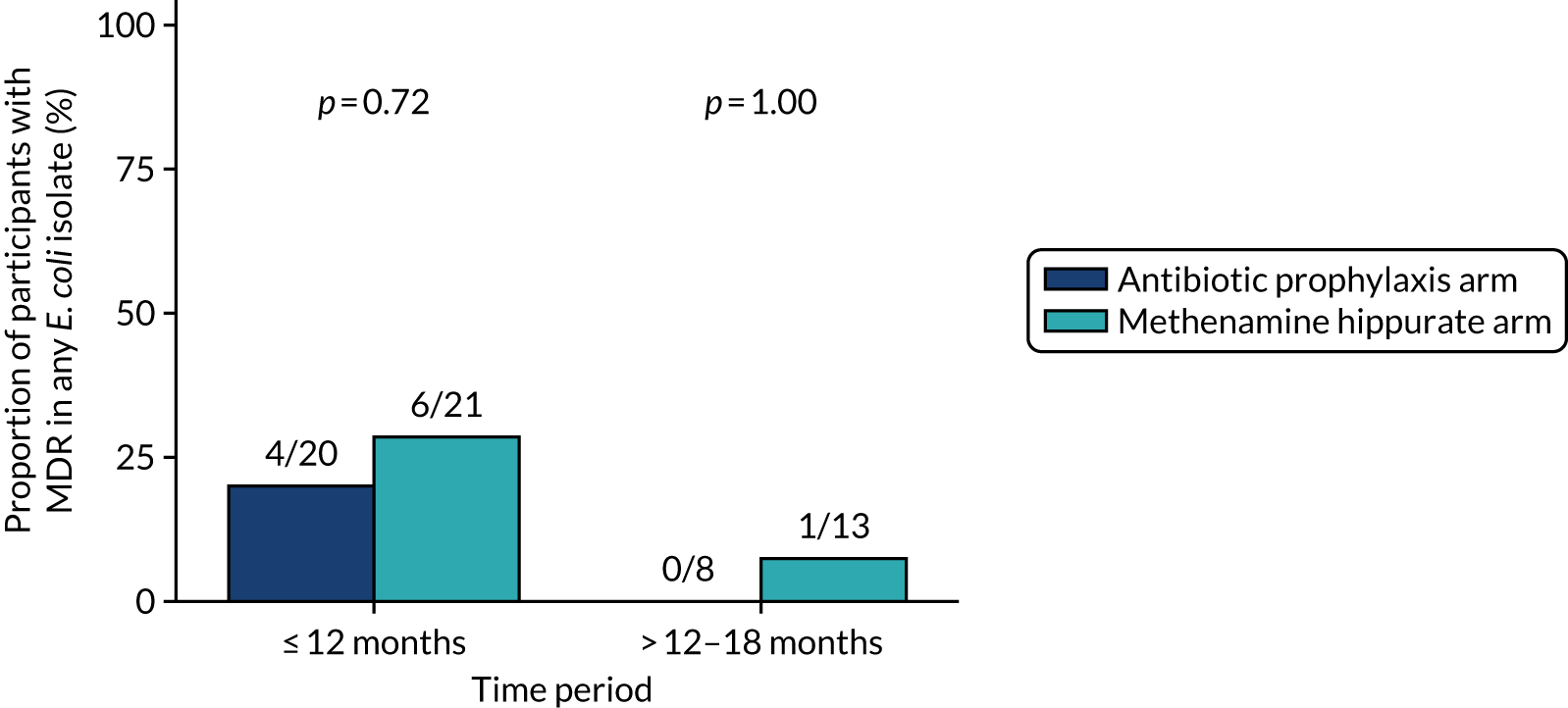
Similarly, the proportions of symptomatic urine samples demonstrating resistance in E. coli during the 12-month treatment period and 6-month follow-up period are shown by antibiotic in Figure 10. Data are also presented cumulatively over time in Appendix 2, Figure 23. There was a higher proportion of samples showing resistance to cephalosporins (cefalexin and cefuroxime) and folate pathway inhibitors (trimethoprim and co-trimoxazole) in E. coli isolates in those allocated to antibiotic prophylaxis than in those allocated to methenamine hippurate; however, these did not differ significantly. There was no significant difference in the proportion of samples showing resistance to at least one antibiotic in E. coli (see Appendix 2, Figure 24) or demonstrating MDR in E. coli (see Appendix 2, Figure 25).
FIGURE 10.
Resistance rates to individual antibiotics (per sample) in E. coli isolated from symptomatic urine samples in the 12-month treatment period and 6-month follow-up period. (a) Amoxicillin; (b) cefalexin; (c) cefuroxime; (d) ciprofloxacin; (e) co-amoxiclav; (f) co-trimoxazole; (g) nitrofurantoin; and (h) trimethoprim.
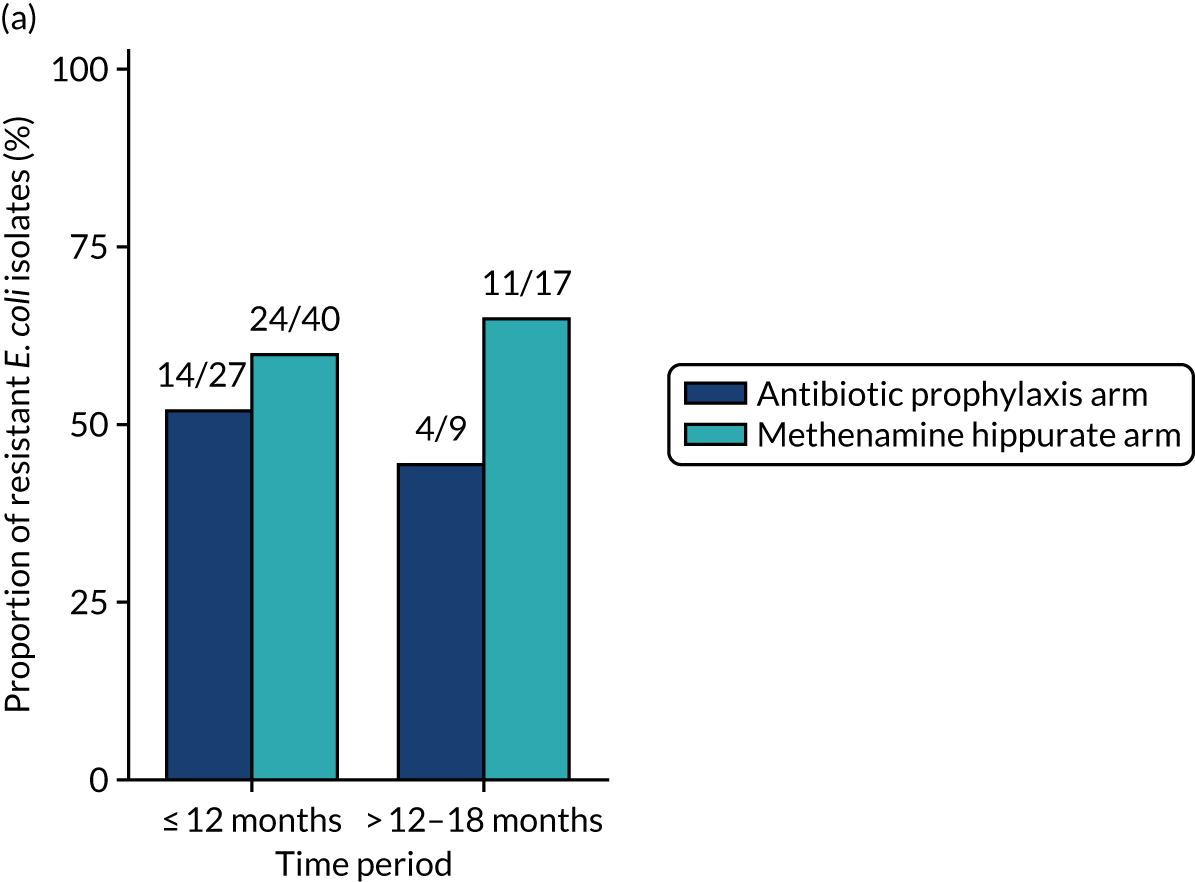
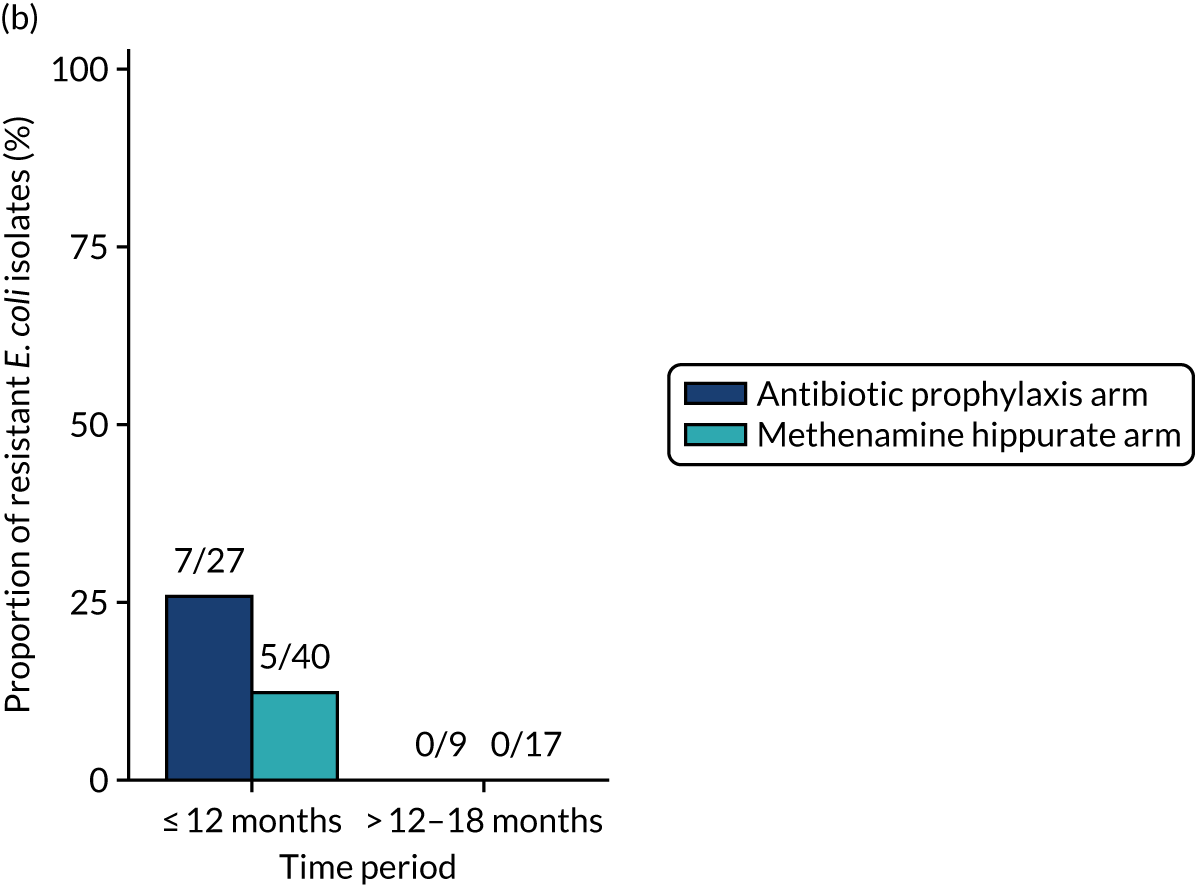
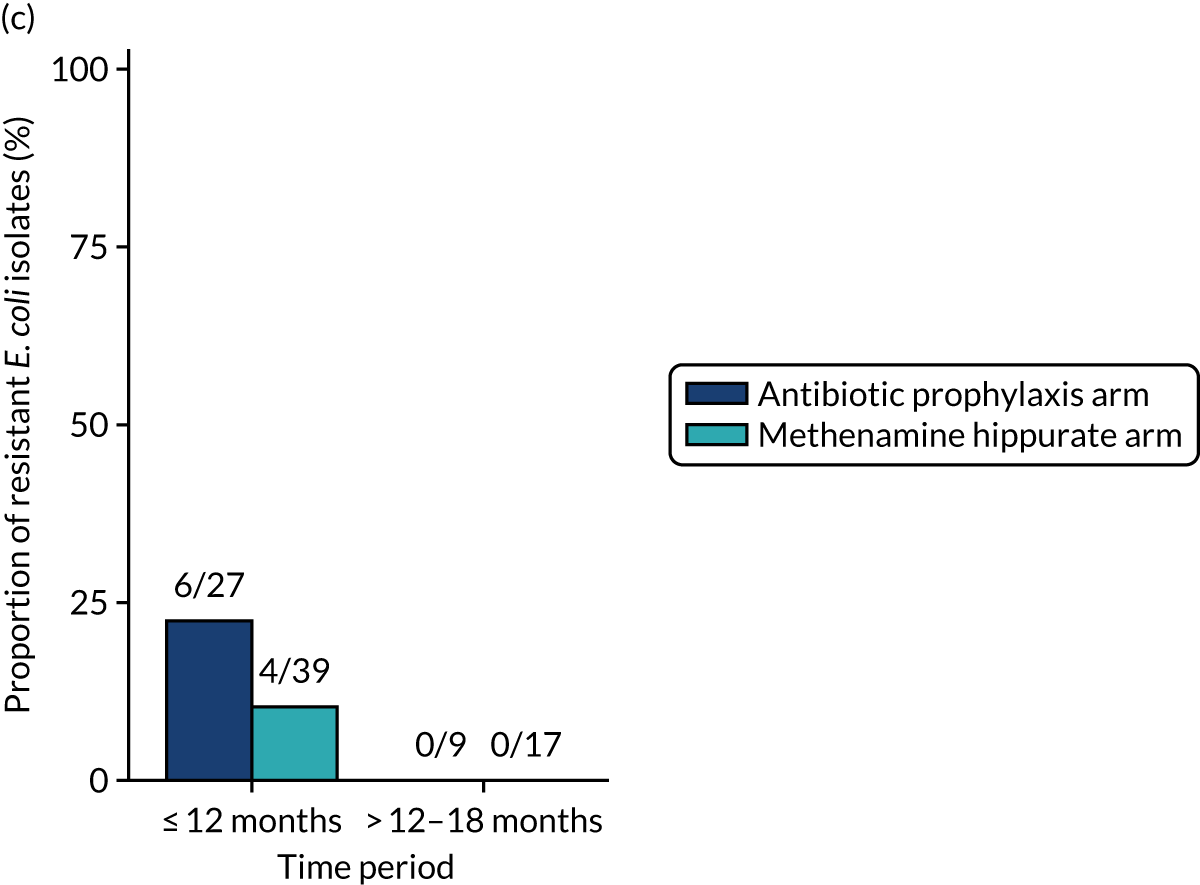
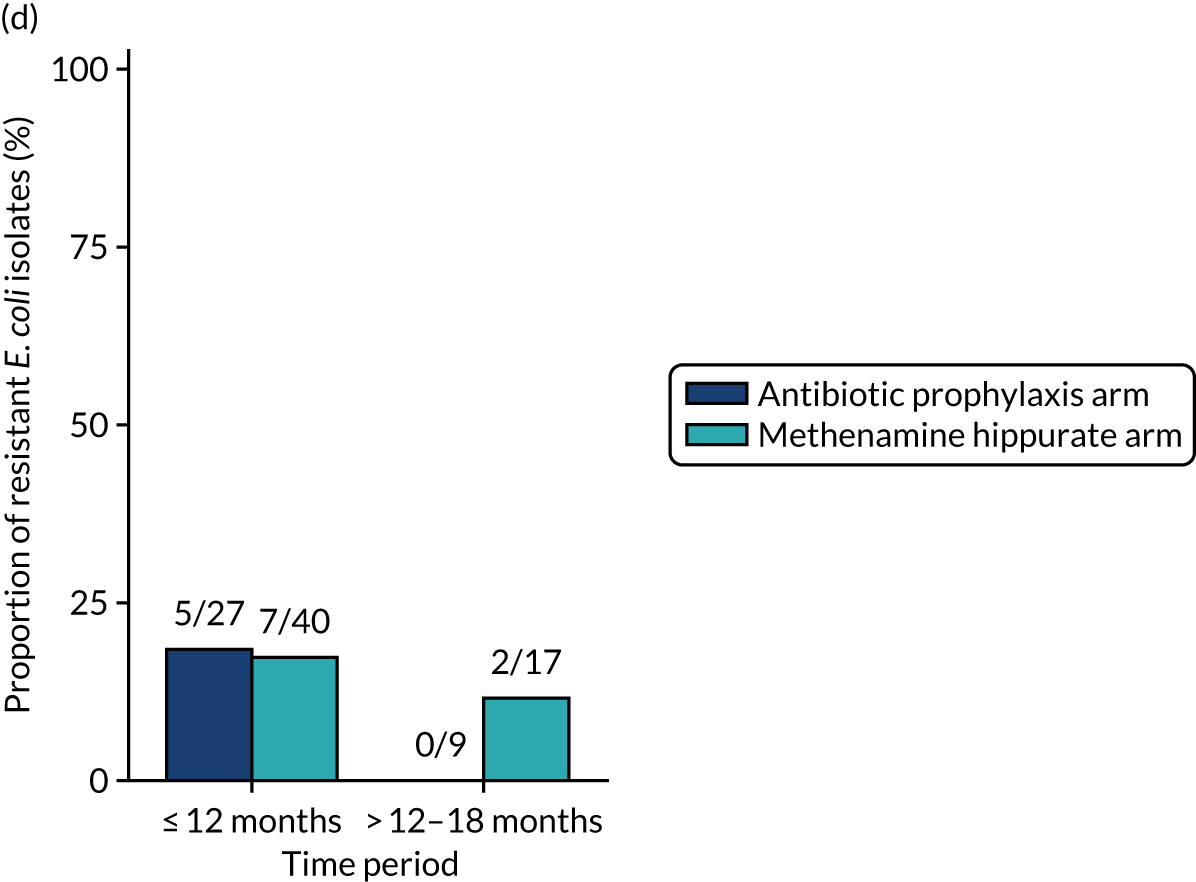
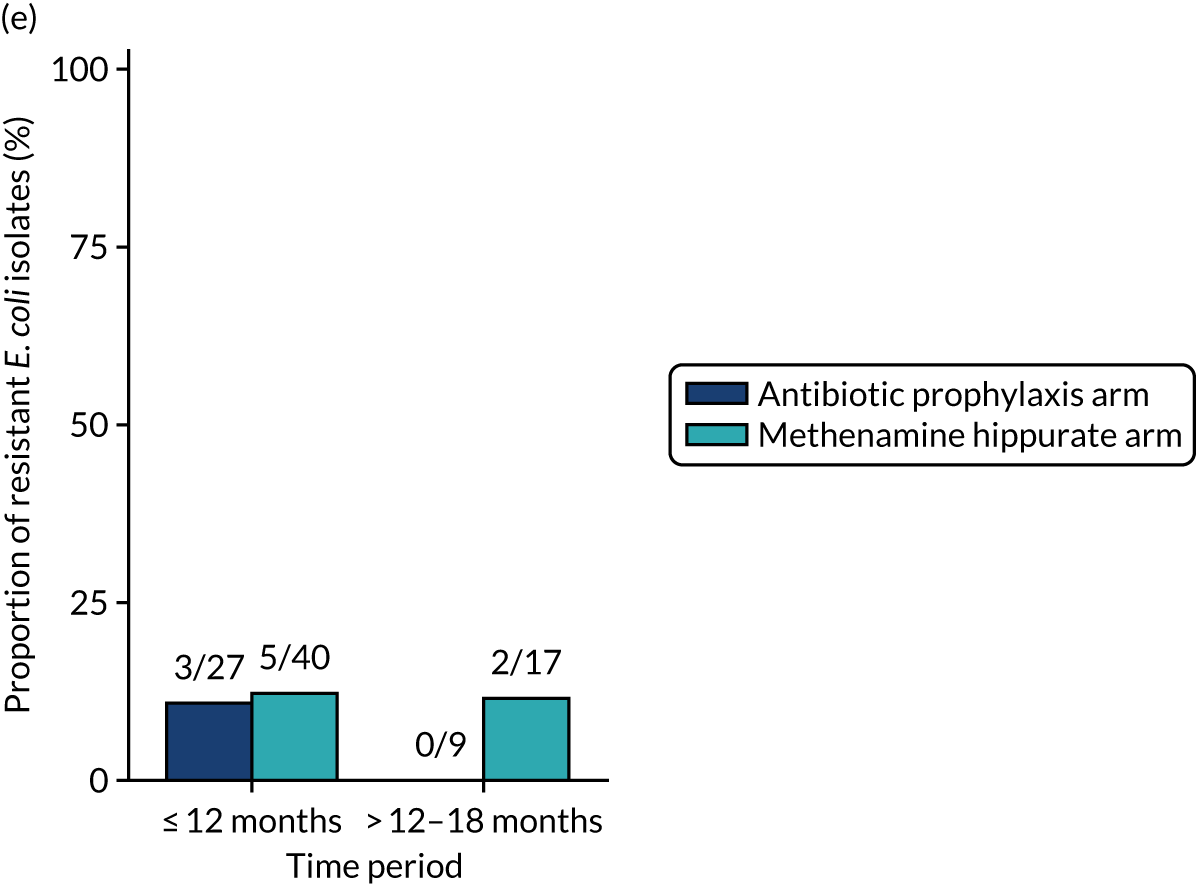
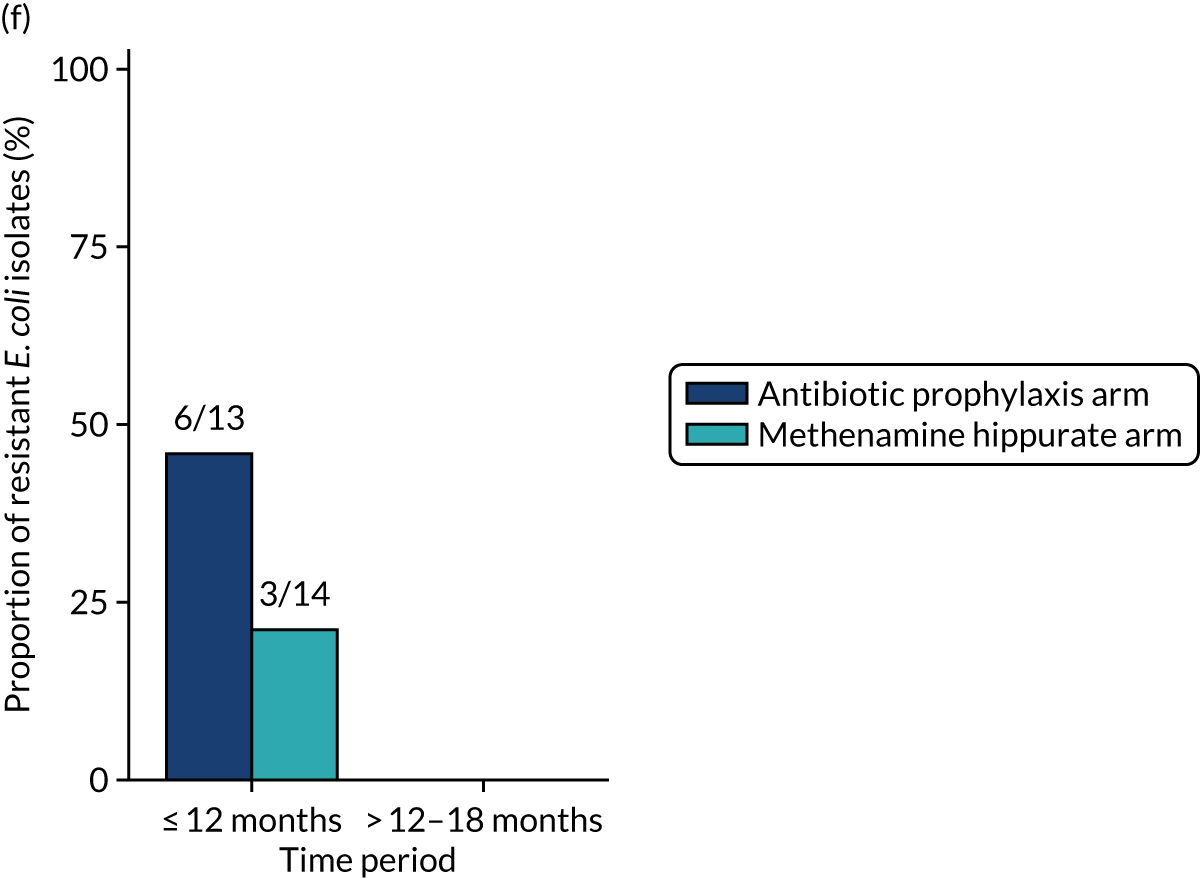
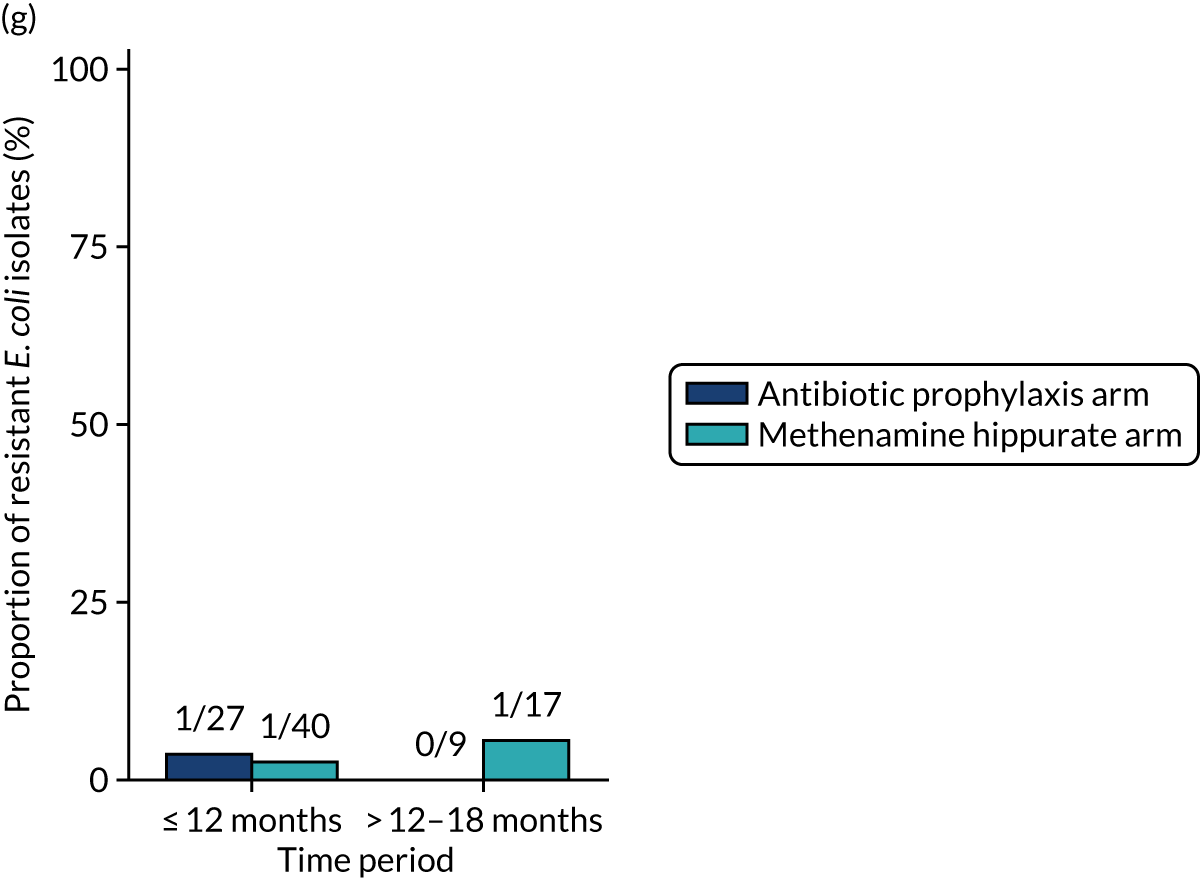
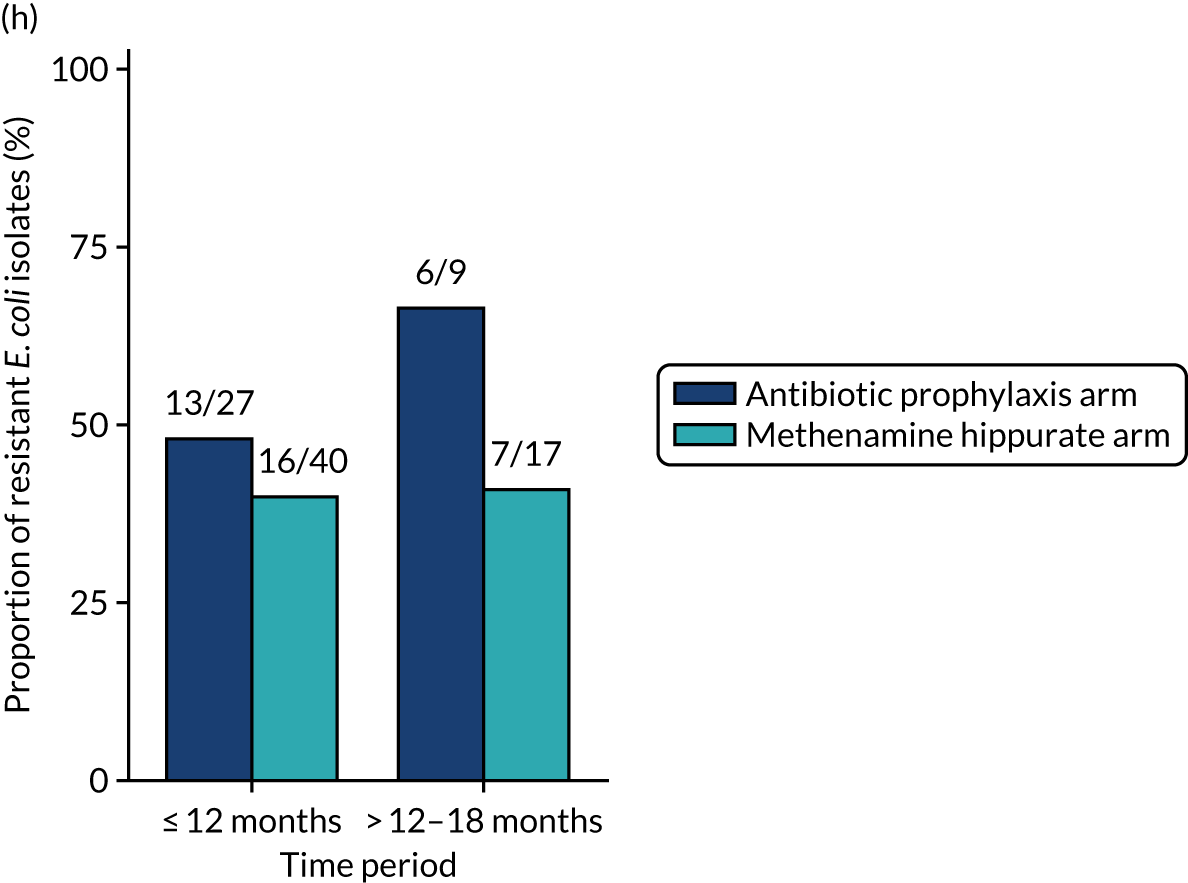
For each antibiotic, the proportions of participants demonstrating ‘resistance since baseline’ (participants with at least one urinary E. coli isolate resistant to at least one of the panel of antibiotics tested in a post-baseline sample who demonstrated sensitivity in E. coli at baseline or did not have E. coli isolated in their baseline sample) and ‘known resistance since baseline’ (participants with at least one urinary E. coli isolate resistant to at least one of the panel of antibiotics tested in a post-baseline sample who demonstrated sensitivity in E. coli at baseline) are shown in Appendix 2, Figure 26. Owing to the small number of positive urine cultures at baseline, the number of participants evaluable for the ‘known resistance since baseline’ analyses was very small. There was no difference between treatment groups in the proportion of participants developing MDR since baseline in E. coli isolates from urine samples.
In addition to E. coli, other isolates were identified in significant growth from routine and symptomatic urine samples; these are tabulated in Appendix 2, Table 32. The analyses in this section have been repeated to explore antimicrobial resistance in any isolate rather than resistance only in E. coli (see Appendix 2, Table 33 and Figures 27–32).
Asymptomatic bacteriuria
The occurrence of asymptomatic bacteriuria over the 18-month trial period was similar in both randomised treatment arms (see Appendix 2, Table 34). Among those allocated to antibiotic prophylaxis, 64 of 557 (11%) routine samples submitted in the absence of symptoms were positive, compared with 79 of 571 (14%) in those allocated to methenamine hippurate (chi-squared p-value = 0.24). In a post hoc comparison of samples submitted during the trial treatment period (i.e. those submitted at the 3-, 6-, 9- and 12-month time points), the occurrence of asymptomatic bacteriuria was 7% in the antibiotic prophylaxis arm, compared with 14% in the methenamine hippurate arm (chi-squared p-value = 0.005).
Hospitalisation due to urinary tract infection and febrile urinary tract infection
Four participants were hospitalised due to UTI; all were allocated to receive methenamine hippurate. Three of the four participants were hospitalised within the 12-month treatment period and one was hospitalised in the 6-month follow-up period.
Six participants reported at least one UTI episode with a recorded fever of ≥ 38 °C. All six participants were allocated to receive methenamine hippurate. In total, 12 febrile UTI episodes were reported from those six participants, eight during the 12-month preventative treatment period and four during the 6-month follow-up period. One participant reported six febrile UTI episodes: three during the 12-month treatment period and three during the 6-month follow-up period.
Participant satisfaction with treatment
Among those completing the TSQM, overall participant satisfaction with preventative treatment was high across both randomised groups, with a mean global satisfaction score at 12 months of 80.6 (SD 22.4) in those randomised to antibiotic prophylaxis and 77.3 (SD 23.9) in those randomised to methenamine hippurate. At 12 months, participants allocated to antibiotic prophylaxis reported a higher score for convenience (mean 91.4; SD 12.7) than those allocated to methenamine hippurate (mean 82.2; SD 18.4); however, there was no evidence of a difference when asked again at 18 months. Scores were similar across treatment groups for all other domain scores (Table 16).
| Domain/time point | Antibiotic prophylaxis arm (N = 120) | Methenamine hippurate arm (N = 120) | Comparison between arms | |
|---|---|---|---|---|
| Mean (SD), n | Mean (SD), n | Mean difference (95% CI); two-sample t-test; p-value | ANCOVA,a p-value | |
| Month 12 | ||||
| Effectiveness | 80.0 (22.5), 63 | 77.5 (23.7), 74 | –2.5 (–10.4 to 5.4); 0.53 | 0.43 |
| Side effects | 92.9 (19.2), 62 | 95.8 (13.7), 72 | 2.9 (–2.7 to 8.5); 0.31 | 0.29 |
| Convenience | 91.4 (12.7), 64 | 82.2 (18.4), 73 | –9.2 (–14.6 to –3.8); 0.001 | 0.001 |
| Global satisfaction | 80.6 (22.4), 64 | 77.3 (23.9), 73 | –3.3 (–11.2 to 4.5); 0.40 | 0.34 |
| Month 18 | ||||
| Effectiveness | 74.4 (28.8), 57 | 75.0 (24.7), 69 | 0.7 (–8.8 to 10.1); 0.89 | 0.95 |
| Side effects | 93.2 (18.2), 55 | 94.9 (15.7), 70 | 1.7 (–4.3 to 7.7); 0.57 | 0.56 |
| Convenience | 85.7 (17.0), 59 | 84.6 (15.7), 72 | –1.1 (–6.8 to 4.5); 0.69 | 0.70 |
| Global satisfaction | 75.8 (25.5), 60 | 74.7 (27.1), 72 | –1.1 (–10.3 to 8.0); 0.81 | 0.70 |
Adverse events
In total, 38 SAEs were reported (23 in the antibiotic prophylaxis arm and 15 in the methenamine hippurate arm) from 28 participants (14 from each arm) (see Appendix 2, Table 35). Two SAEs were classified as being possibly, probably or definitely related to trial treatment. One participant allocated to antibiotic prophylaxis (trimethoprim) was admitted to hospital with severe abdominal pain. One further participant who was allocated to antibiotic prophylaxis reported an increase in ALT to 465 U/l on their month 9 blood test. This resolved to 25 U/l at month 12 following a change in prophylactic antibiotic treatment from nitrofurantoin to trimethoprim.
To account for switching between preventative treatment strategies, AEs are summarised according to the preventative treatment strategy in use at the time the AE started. The mean number of AEs and ARs reported over the 18-month trial period was found to be similar across both treatment groups (Table 17). This is also shown by randomised treatment group in Appendix 2, Table 36. The most commonly occurring AEs (affecting at least 3% of participants in either treatment group) are tabulated by treatment received in Table 18 and by randomised treatment group in Appendix 2, Table 37.
| Antibiotic prophylaxis arm (N = 142) | Methenamine hippurate arm (N = 127) | Total (N = 269) | |
|---|---|---|---|
| All AEs | |||
| Number of events per participant | |||
| Mean (SD) | 1.9 (2.8) | 1.8 (2.4) | 1.9 (2.7) |
| Median (IQR) | 1 (0–2) | 1 (0–3) | 1 (0–3) |
| Minimum, maximum | 0, 17 | 0, 13 | 0, 17 |
| Worst grade reported per participant, n (%) | |||
| None | 59 (42) | 45 (35) | 104 (39) |
| Mild | 41 (29) | 47 (37) | 88 (33) |
| Moderate | 34 (24) | 29 (23) | 63 (23) |
| Severe | 8 (6) | 6 (5) | 14 (5) |
| ARs (possibly, probably, definitely) | |||
| Number of ARs per participant | |||
| Mean (SD) | 0.4 (0.7) | 0.4 (0.7) | 0.4 (0.7) |
| Median (IQR) | 0 (0–0) | 0 (0–1) | 0 (0–1) |
| Minimum, maximum | 0, 3 | 0, 3 | 0, 3 |
| Worst grade AR reported per participant, n (%) | |||
| None | 108 (76) | 92 (72) | 200 (74) |
| Mild | 24 (17) | 26 (20) | 50 (19) |
| Moderate | 9 (6) | 9 (7) | 18 (7) |
| Severe | 1 (1) | 0 (0) | 1 (< 1) |
| Event term | Antibiotic prophylaxis arm (N = 142) | Methenamine hippurate arm (N = 127) | Total (N = 269) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Lower respiratory tract infection | 10 | 7 | 9 | 7 | 19 | 7 |
| Nausea | 12 | 8 | 5 | 4 | 17 | 6 |
| Abdominal pain | 7 | 5 | 9 | 7 | 16 | 6 |
| Diarrhoea | 8 | 6 | 4 | 3 | 12 | 4 |
| Alanine aminotransferase levels increased | 5 | 4 | 5 | 4 | 10 | 4 |
| Back pain | 7 | 5 | 3 | 2 | 10 | 4 |
| Headache | 3 | 2 | 7 | 6 | 10 | 4 |
| Candida infection | 4 | 3 | 5 | 4 | 9 | 3 |
| Dyspepsia | 5 | 4 | 4 | 3 | 9 | 3 |
| Rash | 3 | 2 | 5 | 4 | 8 | 3 |
| Abdominal discomfort | 3 | 2 | 4 | 3 | 7 | 3 |
| Dyspnoea | 5 | 4 | 2 | 2 | 7 | 3 |
| Fall | 3 | 2 | 4 | 3 | 7 | 3 |
| Vomiting | 3 | 2 | 4 | 3 | 7 | 3 |
| Depressed mood | 1 | 1 | 4 | 3 | 5 | 2 |
| Herpes zoster | 5 | 4 | 0 | 0 | 5 | 2 |
Kidney and liver function was assessed by measuring levels of creatinine eGFR, bilirubin, alkaline phosphatase and ALT at baseline and 3-monthly to month 18. For all measurements, there was little difference in distribution between randomised treatment groups, or over time (Figure 11).
FIGURE 11.
Box plots of creatinine, eGFR and LFTs over time, by randomised treatment group. (a) Creatinine (eGFR); (b) bilirubin; (c) alkaline phosphate; and (d) ALT.
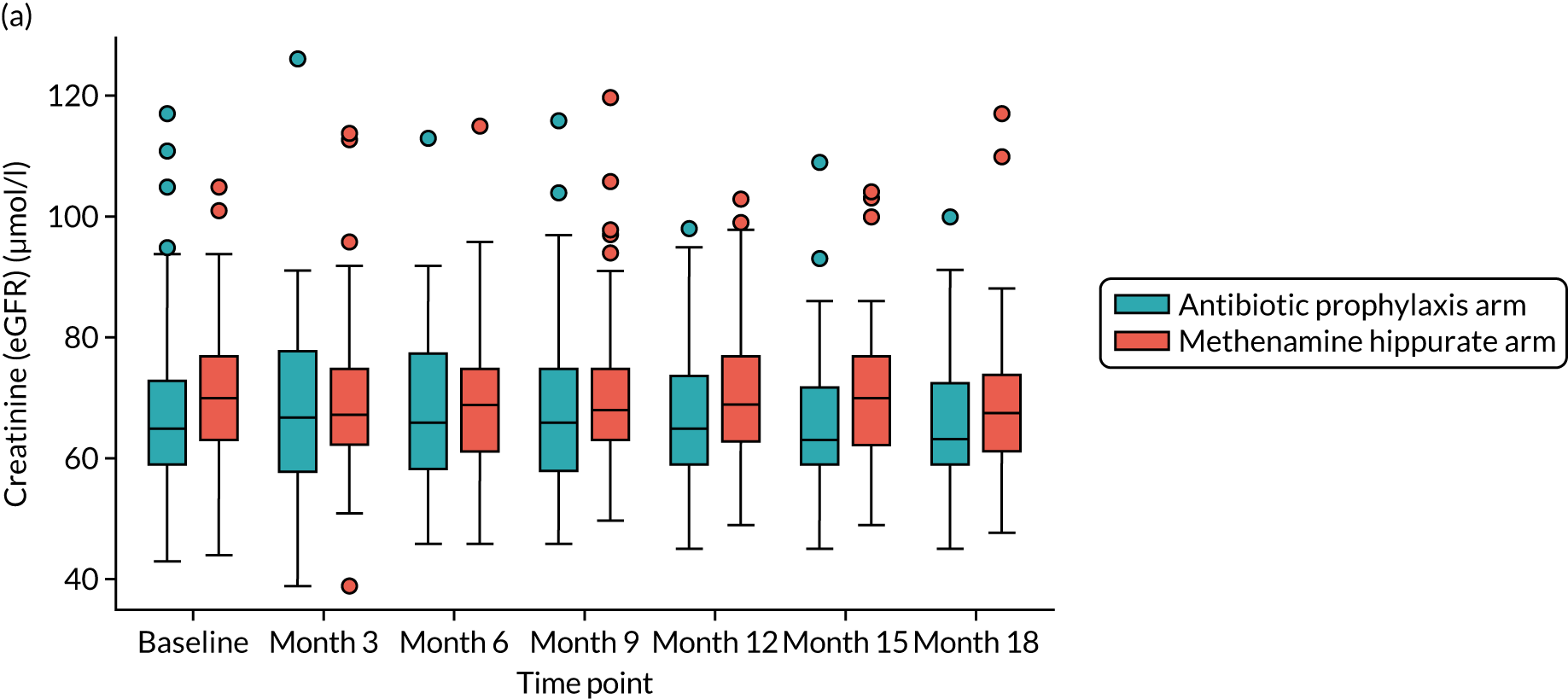
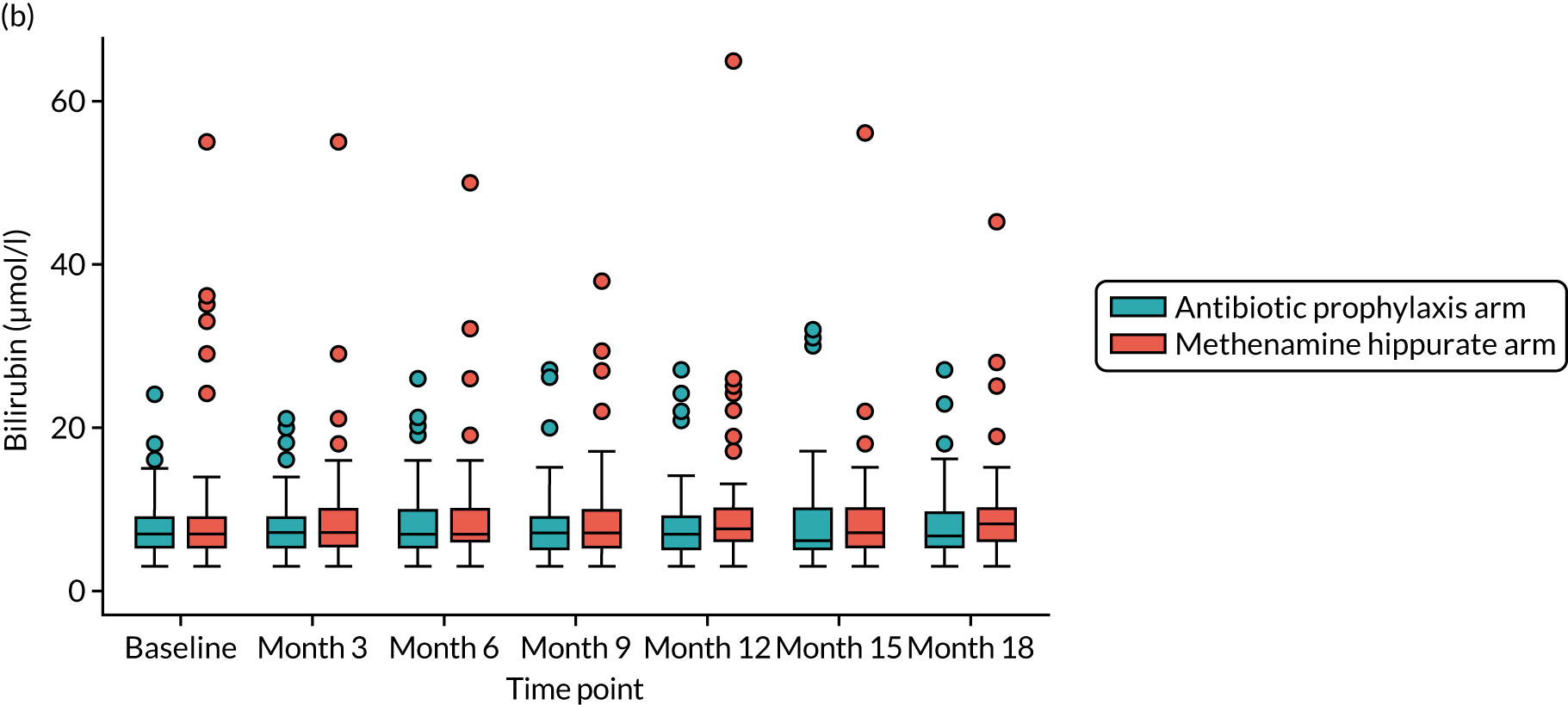
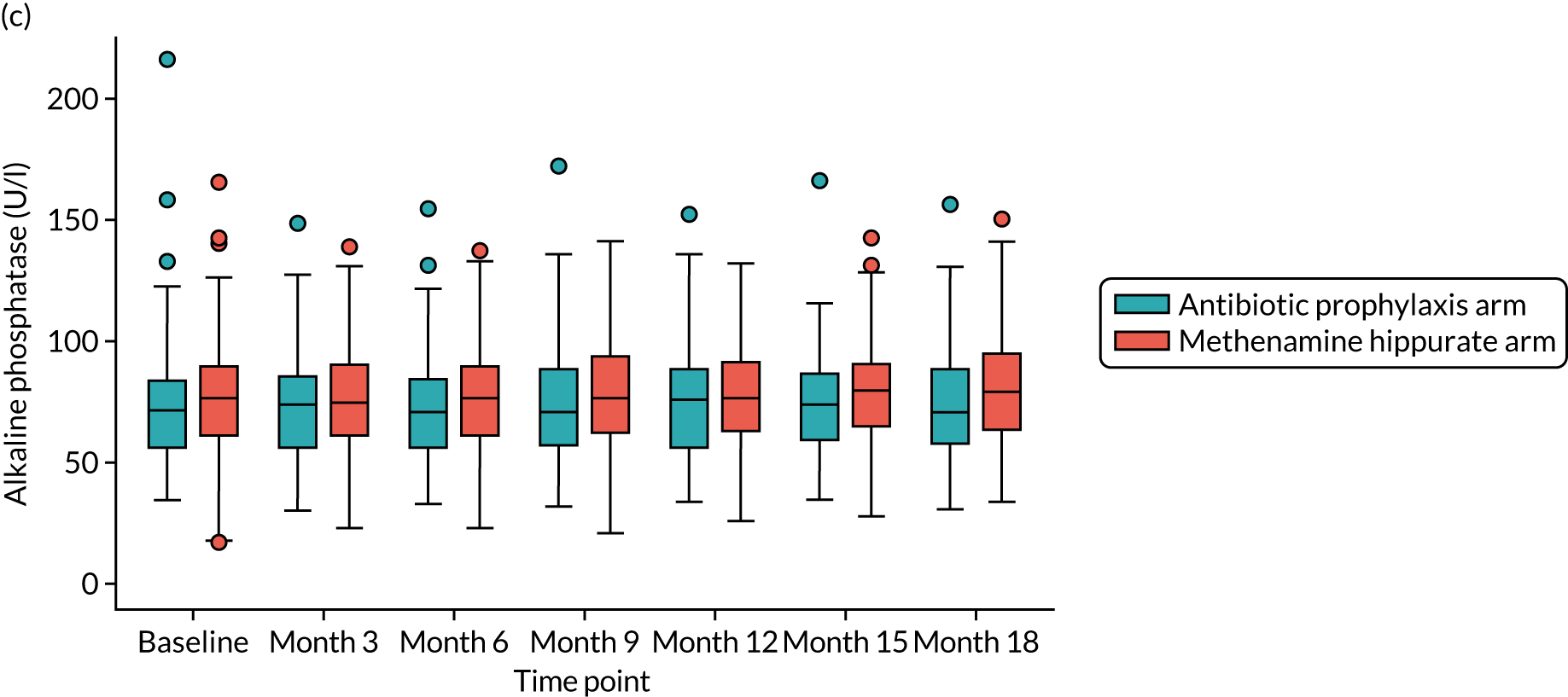
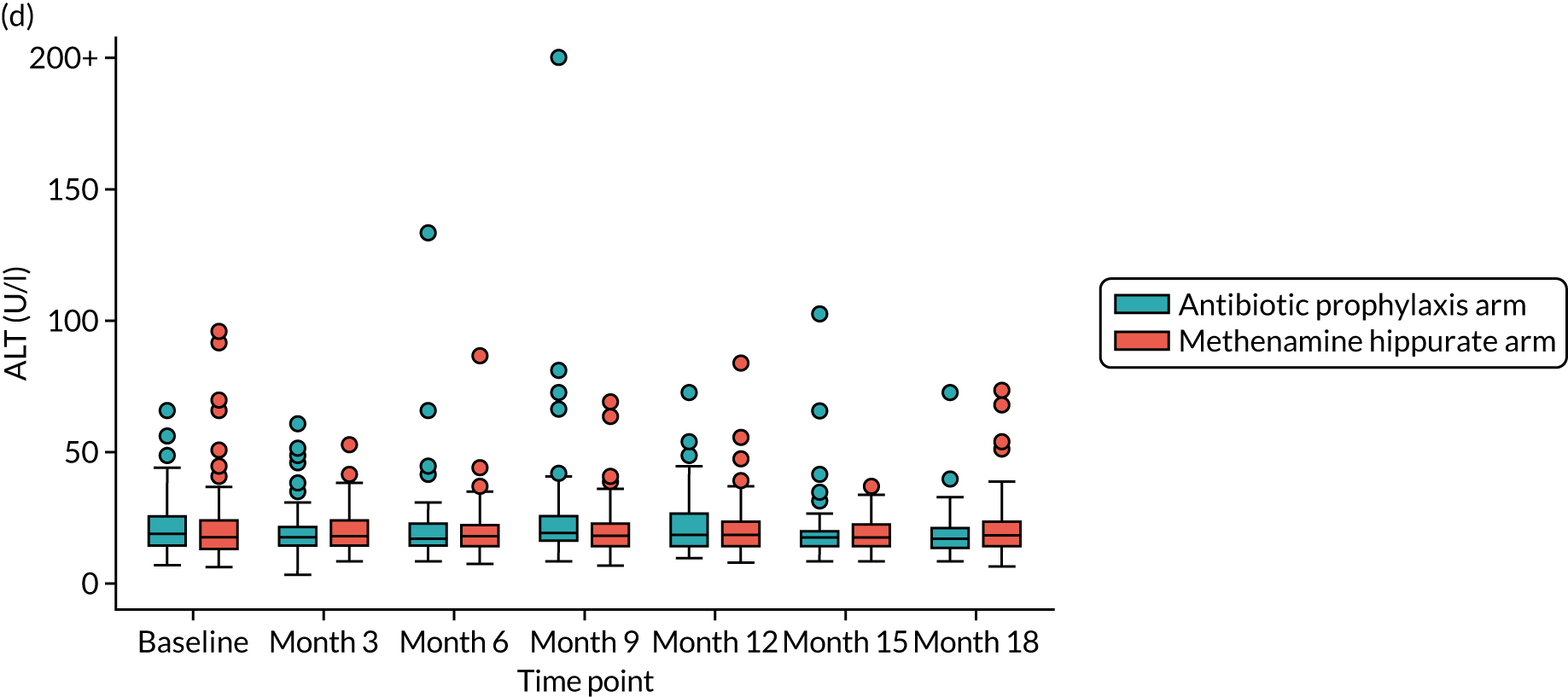
Chapter 5 Economic evaluation
Parts of this chapter have been reproduced with permission from Harding et al. 36 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The economic evaluation comprised both within-trial and model-based analyses. The primary aim of the economic evaluation was to determine the relative efficiency of methenamine hippurate compared with prophylactic antibiotics in women suffering from rUTIs. The within-trial analysis determined the relative cost-effectiveness of methenamine hippurate compared with prophylactic antibiotics over 18 months post randomisation. The model-based analysis extrapolated the findings from the within-trial analysis over the estimated lifetime of a woman suffering from rUTIs. Sensitivity analyses were undertaken to assess the robustness of both the within-trial and the model results.
The perspective chosen was that of the UK NHS and personal and social services. Costs and outcomes incurred after 12 months post randomisation were discounted at the UK recommended rate of 3.5%. 37 All economic analyses were based on a mITT principle (see Chapter 2) and were designed and conducted in accordance with best practice, conforming to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS). 38
In this chapter, the methods and results of the within-trial analysis are reported. These are followed by the methods and results of the model-based analysis. The chapter concludes with a short summary of key findings.
Within-trial analysis
The following outcomes were reported for the within-trial economic evaluation:
-
health-care costs to the NHS of managing rUTIs
-
participant costs
-
mean incidence rate of rUTIs
-
QALYs estimated based on responses to the EQ-5D-5L administered at baseline and at 3, 6, 9, 12, 15 and 18 months post randomisation
-
regression models which estimated the predictors of costs and effects to inform the calculation of incremental costs, effects and cost-effectiveness
-
incremental cost per incidence of rUTI avoided
-
incremental cost per QALY gained at the end of the follow-up period (18 months post randomisation).
Methods
Cost data collection
The costs collected as part of the economic evaluation were based on the cost of the treatments, the use of health-care services, the antibiotics to treat UTIs and the concomitant medications reported over the 18-month follow-up period.
Cost of prophylactic treatment
Prophylactic treatment costs were based on the medications being evaluated in each randomised arm: methenamine hippurate and prophylactic antibiotics. Information on these medications was collected from the CRF, which contained information on medication name, dose, frequency and duration. Trial estimates were combined with unit costs obtained from the BNF24 to estimate the total treatment cost per participant; these are summarised as the average total treatment cost per randomised arm.
Health-care resource costs
Health-care resource use was collected via a self-completed participant questionnaire administered at baseline and at 3, 6, 9, 12 and 18 months post randomisation. This questionnaire collected information on primary and secondary care resource use.
Primary care contacts included consultations with GPs and nurses. These primary care contacts could take place at the GP practice, the participant’s home, over the telephone or out of hours. Unit costs were obtained from the unit costs of community care and combined with the number of contacts reported by each participant to estimate the total cost of primary care resource use per participant. and summarised as the average total primary care cost per randomised arm. 39
Secondary care contacts included visits to an accident and emergency (A&E) department, outpatient visits, hospital admissions (either as a daycase or overnight) and consultations with a hospital doctor, which could be via telephone or out-of-hours consultations. Unit costs were collected from routine sources and multiplied by the number of contacts reported by each participant. The total cost of secondary care resource use was estimated for every participant and summarised as the average total secondary care cost per randomised arm. 40
Additional medications costs
Additional medications reported were summarised as (1) antibiotics for UTIs and (2) concomitant medications. Information on antibiotics prescribed as treatment for a UTI was collected from a number of sources (the health-care record review, participant UTI log, phone-reported UTI CRF, 3-monthly participant review CRF and 3-monthly self-reported participant questionnaire). The hierarchy of data to be used was assumed to be the same as that reported to define the primary outcome; therefore, only antibiotics related to a UTI, as defined in Chapter 2, were assigned a unit cost. Information on the name and duration of these antibiotics was provided but assumptions were made for dose and frequency based on the BNF guidelines24 and clinical advice. The additional concomitant medications reported were collected on the concomitant medication form. This form contained information on medication name, dose, frequency and duration. To prevent double counting, only medications and antibiotics not previously reported were included in the analysis; medication name and start dates were used to identify duplicate medications. All reported medications were combined with unit costs collated from the BNF24 to estimate the total medication cost per participant and average total medication cost per randomised arm.
Participant costs
Direct and indirect costs incurred by participants were considered in a sensitivity analysis. Direct health-care costs incurred by participants were collected in the self-reported participant questionnaire administered at baseline and at 3, 6, 9, 12 and 18 months post randomisation. This questionnaire collected information on what additional health-care was received by women because of UTIs and the total amount they had to pay. Self-reported out-of-pocket costs were totalled for each participant and summarised as the average total out-of-pocket cost per randomised arm.
Time and travel data were not collected as part of this trial, to reduce participant burden; however, that does not mean that these costs were not incurred. Experiences from a previous UK RCT, examining once-daily prophylactic antibiotic treatment for patients with rUTI performing intermittent bladder catheterisation (the AnTIC trial16), were used to guide the health economic analyses. It was assumed that the time and travel costs reported by participants in the AnTIC trial16 would be similar to those reported by participants in the ALTAR trial. Time and travel unit costs from the AnTIC trial16 were inflated to the same price year as other unit cost estimates. 39 Each unit cost was combined with each health-care contact reported by each participant to estimate the total time and travel cost per participant and the average total time and travel cost per randomised arm. The baseline characteristics of participants in both the ALTAR trial and the AnTIC trial16 were compared to ensure comparability.
Estimation of effects
Two effectiveness measures were used in this economic evaluation: incidence of UTIs and QALYs.
Incidence of urinary tract infections
Incidence of UTIs was estimated using the same approach for calculating the primary outcome: the number UTI episodes divided by total observation time. Further details of how the incidence of UTIs was calculated are provided in Chapters 2 and 4. The total incidence of UTIs was estimated for every participant; from this, the average total incidence of UTIs was estimated for each randomised arm.
Quality-adjusted life-years
The EQ-5D-5L was completed at scheduled and unscheduled time points throughout the trial period. The EQ-5D-5L was administered as part of the participant questionnaire at scheduled time points (baseline and at 3, 6, 9, 12, 15 and 18 months post randomisation) and was administered as part of the participant UTI record that was completed when participants reported having a UTI episode treated with antibiotics.
The responses to the EQ-5D-5L questionnaire were cross-walked onto the EQ-5D-5L value set to generate a health state utility score. 41 Utility values were estimated for every participant at each scheduled time point and are presented as the average total utility value per randomised arm at each time point. The area under the curve approach put a time weight onto each utility score, and the time-weighted average of the utility scores based on the responses to the EQ-5D-5L throughout the follow-up period allowed us to generate QALY values for each participant. 42 Equation 3 illustrates how QALYs were estimated based on responses to the EQ-5D-5L administered at scheduled visits only. QALYs were summarised as the average total QALY per randomised arm:
in which bl is baseline.
However, given the large number of data points for the EQ-5D-5L, it was anticipated that not all participants would have information available for each time point. To be included in the QALY calculation it was assumed that participants had to have utility data at the first assessment (baseline) and four other time points. This calculation assumed missing data were missing at random. Equation 4 illustrates how QALYs were calculated for a participant with missing utility values at two time points (6 and 15 months). If the last time point (18 months) was missing, then it was assumed that the QALY value estimated between 12 and 15 months was for a 6-month duration not 3 months. This assumption was consistent with the 6-month duration assumed for other calculations when a utility score is missing:
in which bl is baseline.
Sensitivity analysis incorporated the utility scores associated with UTIs into the QALY equation (Equation 5). It was assumed, based on clinical advice, that the duration of a UTI episode was 3 days. Equation 5 illustrates how the QALY equation was adapted for one participant with a UTI between baseline and 3 months:
in which bl is baseline and it was assumed that 3 months was equivalent to 91.5 days.
In Equation 5, the QALY value for baseline to 3 months was multiplied by 3 months minus the duration of the UTI (3 days per UTI). This value was then added to the utility associated with the UTI multiplied by the duration of the UTI to estimate the revised QALY for baseline to 3 months for this participant with one UTI during baseline and 3 months. If this participant provided utility values for two UTIs during baseline and 3 months, the duration of the first part of the equation would be changed to 85.5 and the other UTI utility weight would be added to the first part of the equation estimating QALYs between baseline and 3 months. This equation was adapted for all participants who had (1) a QALY value based on Equation 4 and (2) utility data for their UTI episode depending on when their UTI(s) occurred and the frequency of UTIs in each time period.
Comparative incremental analyses of costs and effects
Both unadjusted and adjusted analyses were performed to estimate the cost-effectiveness of methenamine hippurate compared with prophylactic antibiotics. Unadjusted analyses were used to estimate mean average total costs and average total effects between the two randomised arms. The results of the adjusted analyses (see Adjusted analysis: seemingly unrelated regression) were presented as point estimates of the mean incremental costs, effects and cost-effectiveness.
If one arm was found to be less costly and more effective, it was the dominant strategy and hence considered to be cost-effective. If methenamine hippurate was found to be more effective but more costly, consideration had to be given to whether or not it was cost-effective. Decisions were based on the incremental cost-effectiveness ratio (ICER) and society’s willingness to pay (WTP) for an additional unit of effect. The ICER is estimated by dividing the difference in costs between the two randomised arms by the difference in effects between the two randomised arms.
Incidence of urinary tract infections
This analysis was based on incremental cost per UTIs avoided. The average total cost and average incidence of UTIs were estimated for each arm. These were presented as point estimates of mean incremental costs, mean incidence of UTIs and the incremental cost per UTI avoided. If one strategy was not dominant (i.e. more costly and less effective) then consideration was given to how to interpret the ICER. There is no established threshold value for society’s WTP per UTI avoided with which the cost per UTI avoided could be compared. However, WTP to avoid a UTI was estimated as part of the AnTIC trial16 and this was used to interpret these results if neither treatment strategy was dominant in terms of costs and UTIs avoided.
Quality-adjusted life-years
This analysis estimated the incremental cost per QALY gained. The average total cost and average total QALYs were estimated for each arm and presented as point estimates of mean incremental costs and QALYs and the incremental cost per QALY gained. A WTP threshold of £20,000 for an additional QALY, as advocated by the National Institute for Health and Care Excellence,37 was used to interpret the ICER if one strategy was not dominant (i.e. less costly and more effective). If the ICER was within this £20,000 threshold, methenamine hippurate could still be considered cost-effective.
Adjusted analysis: seemingly unrelated regression
An adjusted analysis, using seemingly unrelated regression, estimated the point estimates of the mean incremental costs, effects and cost-effectiveness. 43 Seemingly unrelated regression estimates costs and effects simultaneously, which is required when using individual participant data, as there could be possible correlations between the two dependent variables. 44 The independent variables included in the regression model were baseline costs, baseline utility, baseline severity of disease (number of UTIs in the previous 6 months) and menopausal status.
Sensitivity analysis
Sensitivity analyses were conducted to assess the robustness of the results to realistic variations in the levels of underlying data. Deterministic sensitivity analyses were used to address any uncertainty in the assumptions used in our base-case analysis. Specific sensitivity analyses are described below.
Urinary tract infection utility values
As previously mentioned, the EQ-5D-5L was administered when participants reported a UTI. These utility values were incorporated into the QALY equation (see Equation 5) for participants who had utility data for at least five trial time points (including baseline). Assumptions were not made for those missing UTI utility data.
Participant costs
The base-case analysis estimating the cost per QALY gained over 18 months was replicated to incorporate costs falling on participants and their families.
Changing eligibility criteria
The economic analysis followed the mITT principle for the base-case analysis. In a sensitivity analysis, the cost per QALY gained was estimated for participants who were eligible if we used the per-protocol rules to define eligibility to identify what effect, if any, the inclusion of these participants would have on our overall conclusions.
Changing the time horizon
The primary outcome of this trial focused on the difference in incidence of UTIs over the treatment phase of the trial (i.e. 12 months). The economic analysis was replicated to identify any potential difference in our conclusions when costs and effects (incidence of UTIs and QALYs) were considered only for the treatment phase of the trial.
Antibiotic resistance
The cost of antibiotic resistance per prescription was estimated using the total cost of antibiotic resistance for the UK population,45 inflated to price year 2020,46 and divided by the total number of prescriptions issued in the UK in 2020 (£20,245,397,623/27,335,365 = £741). 47 We conservatively assigned this cost once to every participant randomised to the prophylactic antibiotic arm to estimate the cost of antibiotic resistance associated with the antibiotic prophylaxis. Furthermore, for every UTI that occurred, we also assumed that this cost was incurred. The antibiotic resistance cost per prescription (£741) was then assigned to the rate of UTIs reported in each arm (see Table 22). These costs were estimated for each arm of the trial to estimate the mean cost per arm of antibiotic resistance. The antibiotic resistance cost associated with antibiotic prophylaxis and antibiotic resistance costs associated with treating a UTI were then added to the other costs previously prescribed to estimate a total cost per participant that included a component for antibiotic resistance. These costs were used to estimate both the incremental cost per UTI avoided and the incremental cost per QALY gained.
Stochastic sensitivity analyses
The results from the adjusted analysis were bootstrapped to estimate the statistical imprecision surrounding estimates of costs, effects and cost-effectiveness, the results of which were presented on a cost-effectiveness plane. 48,49 Cost-effectiveness acceptability curves (CEACs) were also generated to illustrate which treatment option maximised net benefits at each WTP value for an additional unit of health effect (i.e. a reduction in incidence of UTIs and QALYs gained). 50 The results of the contingent valuation study undertaken as part of the AnTIC trial16 were used to inform the WTP thresholds to avoid a UTI.
Results
Data on 205 participants were used in the economic analysis.
Data validity and completeness
Table 19 summarises the response rate to the participant questionnaire and EQ-5D-5L at each time point. There was a progressive decline in the response rate to these questionnaires over the trial follow-up period. The pattern of missing data was similar across both randomised arms. Only 29% of participants (n = 59) responded to the participant questionnaire at all time points and 33% (n = 67) of participants responded to the EQ-5D-5L at all time points.
| Scheduled time points | Participant questionnaire, n (%) | EQ-5D-5L, n (%) | ||
|---|---|---|---|---|
| Prophylactic antibiotic arm (N = 102) | Methenamine hippurate arm (N = 103) | Prophylactic antibiotic arm (N = 102) | Methenamine hippurate arm (N = 103) | |
| Baseline | 97 (95.1) | 89 (86.4) | 95 (93.1) | 98 (95.1) |
| 3 months | 75 (73.5) | 71 (68.9) | 76 (74.5) | 76 (73.8) |
| 6 months | 59 (57.8) | 72 (69.9) | 64 (62.7) | 76 (73.8) |
| 9 months | 66 (64.7) | 65 (63.1) | 66 (64.7) | 76 (73.8) |
| 12 months | 59 (57.8) | 65 (63.1) | 66 (64.7) | 73 (70.9) |
| 15 months | N/A | N/A | 60 (58.8) | 69 (67.0) |
| 18 months | 55 (53.9) | 60 (58.3) | 59 (57.8) | 67 (65.0) |
| Data at all time points | 29 (28.4) | 30 (29.1) | 32 (31.1) | 35 (34.0) |
Resource use and costs
Over the 18-month follow-up period participants reported using a variety of different primary and secondary health-care services. On average, the health-care resources most frequently reported were outpatient visits, consultations with a GP at a GP practice and telephone consultations with a GP or nurse. The costs and pattern of resource use for primary and secondary health-care resources are presented in Appendix 3, Tables 38 and 39, and were similar across both randomised arms for the 18-month follow-up period. Appendix 3, Table 40, summarises the unit costs used in the estimation of average total costs.
Total NHS costs were estimated for all participants who had at least one completed participant questionnaire, excluding baseline (n = 183). Costs were assumed to be zero for missing items and missing time points. Missing data were assumed to be missing at random. There was no meaningful difference in baseline costs (mean difference £16; p = 0.8806) and QALYs (mean difference 0.04; p = 0.3024) between those with missing data and those with complete data. Table 20 summarises the average total costs for each health-care resource by randomised arm. On average, participants reported higher costs in the methenamine hippurate arm than in the antibiotic prophylaxis arm. The main difference in costs was due to the intervention medication costs. The daily cost associated with methenamine hippurate was slightly higher than the daily cost associated with the antibiotic. In addition, those in the methenamine hippurate arm received more therapeutic antibiotics to treat UTIs than those in the antibiotic prophylaxis arm.
| Health-care resources | Antibiotic prophylaxis arm (n = 89), mean (SD) (£) | Methenamine hippurate arm (n = 94), mean (SD) (£) |
|---|---|---|
| Intervention costs | 89 (76) | 188 (67) |
| Concomitant medication costs | 2 (8) | 1 (7) |
| Antibiotic costs (due to UTI) | 4 (5) | 8 (28) |
| Primary care costs | 201 (265) | 236 (236) |
| Secondary care costs | 636 (1814) | 580 (876) |
| Average total NHS costs per participant | 931 (2015) | 1013 (1024) |
| Private health-care costs | 14 (123) | 0 (0) |
| Time and travel costs | 331 (657) | 359 (430) |
| Average total NHS costs and participant costs per participant | 1276 (2775) | 1372 (1438) |
Two participants in the antibiotic prophylaxis arm reported incurring private health-care costs related to appointments with a physiotherapist and pain consultant. Both arms incurred similar time and travel costs. Total participant costs were combined with total NHS costs to estimate the average total NHS and participant cost per participant (see Table 20) that was used in a sensitivity analysis (see Appendix 3, Table 41).
Effectiveness outcomes
Incidence of urinary tract infection
On average, the incidence of UTIs was higher in the methenamine hippurate arm than in the antibiotic prophylaxis arm, although the absolute difference did not exceed the predefined non-inferiority margin. Further details on this outcome measure can be found in Chapter 4.
Quality-adjusted life-years
On average, participants reported their health status to be 75% of full health over the 18-month follow-up period. On average, participants in the antibiotic prophylaxis arm reported a higher baseline utility, but this difference was not statistically significant (mean difference 0.063, 95% CI –0.006 to 0.134; p-value = 0.077). Only 33% of participants (n = 67) completed the EQ-5D-5L at all seven time points. Using the assumption that utility data had to be available at five time points (excluding baseline) increased the sample in the base-case analysis to 66% (n = 129).
In total, 38 participants (antibiotic prophylaxis, n = 9; methenamine hippurate, n = 29) completed EQ-5D-5L questionnaires when they experienced a UTI. These women experienced a total of 98 UTIs but there were only utility data available for 86 of these UTIs. QALYs were recalculated incorporating utilities associated with a UTI. The inclusion of these data had no effect on the overall QALY values (Table 21).
| Antibiotic prophylaxis arm (N = 102) | Methenamine hippurate arm (N = 103) | |||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Baseline | 95 | 0.813 (0.21) | 98 | 0.750 (0.28) |
| 3 months | 76 | 0.792 (0.28) | 76 | 0.765 (0.27) |
| 6 months | 64 | 0.783 (0.26) | 76 | 0.791 (0.21) |
| 9 months | 66 | 0.780 (0.28) | 76 | 0.768 (0.24) |
| 12 months | 66 | 0.764 (0.27) | 73 | 0.743 (0.26) |
| 15 months | 60 | 0.783 (0.26) | 69 | 0.739 (0.28) |
| 18 months | 59 | 0.795 (0.18) | 67 | 0.746 (0.24) |
| QALYs (complete case) | 32 | 1.202 (0.33) | 35 | 1.141 (0.34) |
| QALYs | 58 | 1.182 (0.35) | 71 | 1.133 (0.34) |
| UTIs | ||||
| Utility value per reported UTIa | 20 | 0.720 (0.28) | 67 | 0.527 (0.32) |
| QALYs with UTI utilitiesb | 58 | 1.182 (0.35) | 71 | 1.133 (0.34) |
Incremental cost-effectiveness
Incremental cost per urinary tract infection avoided
The results of the cost-effectiveness analysis using UTI avoided as the outcome measure are presented in Table 22. In the unadjusted analysis, on average, antibiotic prophylaxis dominated methenamine hippurate, as it was less costly and more effective. In the adjusted analysis, our conclusions remained the same. However, in both analyses the 95% CI around the difference in costs is wide.
| Strategy | Cost (SD) (£) | Incremental cost (95% CI),a (£) | Incidence of UTI (SD) | Incremental incidence of UTIs avoided (95% CI)a | ICER | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP for avoiding a UTI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £100 | £150 | £200 | £250 | ||||||
| Antibiotic prophylaxis (costs, n = 89; outcomes, n = 102) | 931 (2015) | 0.99 (1.13) | 0.65 | 0.73 | 0.78 | 0.80 | 0.81 | |||
| Methenamine hippurate (costs, n = 94; outcomes, n = 103) | 1013 (1024) | 83 (–414 to 580) | 1.50 (1.62) | –0.64b (–1.0 to 0.2) | Antibiotic prophylaxis dominant | 0.35 | 0.27 | 0.22 | 0.20 | 0.19 |
These deterministic results alone are not sufficient to inform decision-making; we need to consider the imprecision around costs, effects and cost-effectiveness. If society is not willing to pay to avoid a UTI, then decisions are made on cost alone. In this circumstance, antibiotic prophylaxis had a 65% probability of being considered cost-effective compared with methenamine hippurate. As society’s WTP to avoid an episode of UTI increases, the probability that antibiotic prophylaxis would be considered cost-effective increased (Figures 12 and 13).
FIGURE 12.
Cost-effectiveness plane for methenamine hippurate compared with antibiotic prophylaxis using incidence of UTIs avoided as the measure of outcome.
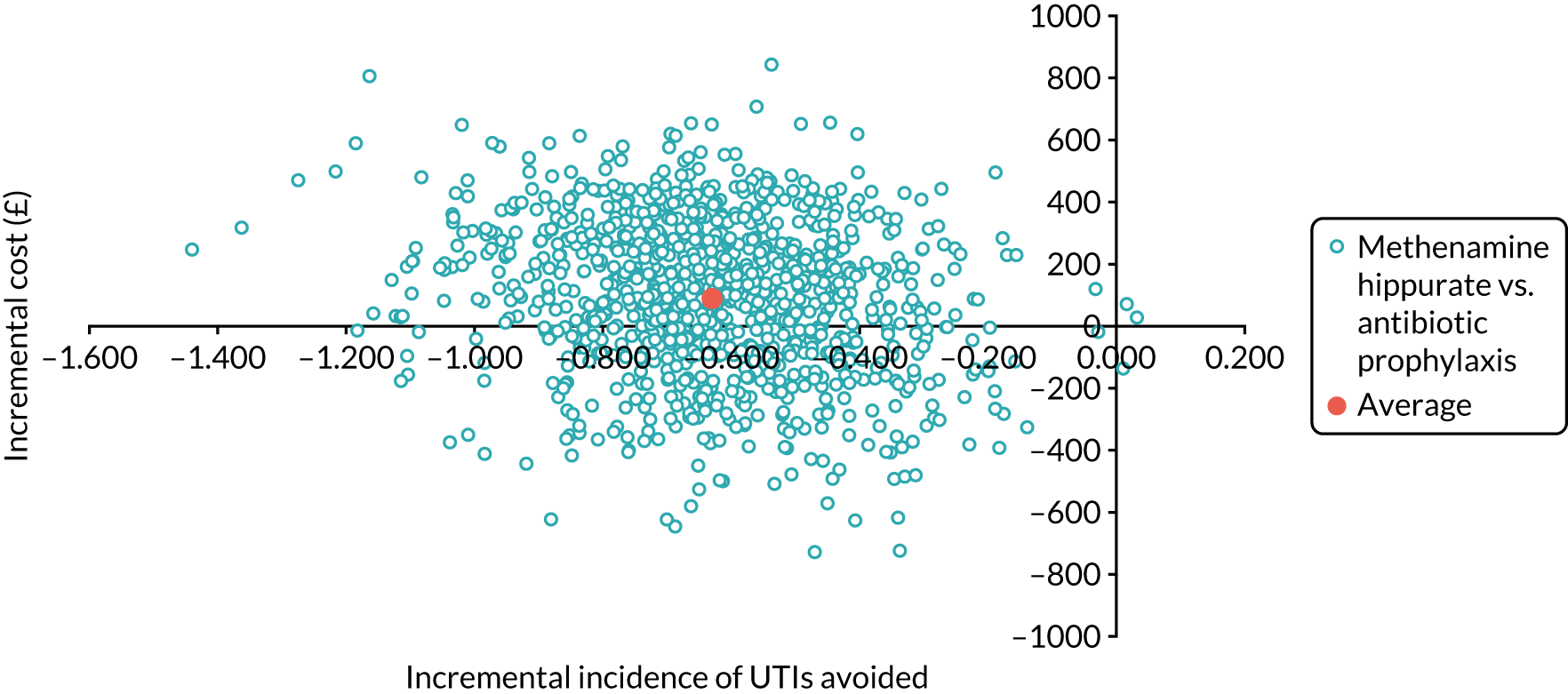
FIGURE 13.
Cost-effectiveness acceptability curve for methenamine hippurate compared with antibiotic prophylaxis using incidence avoided as the measure of outcome.
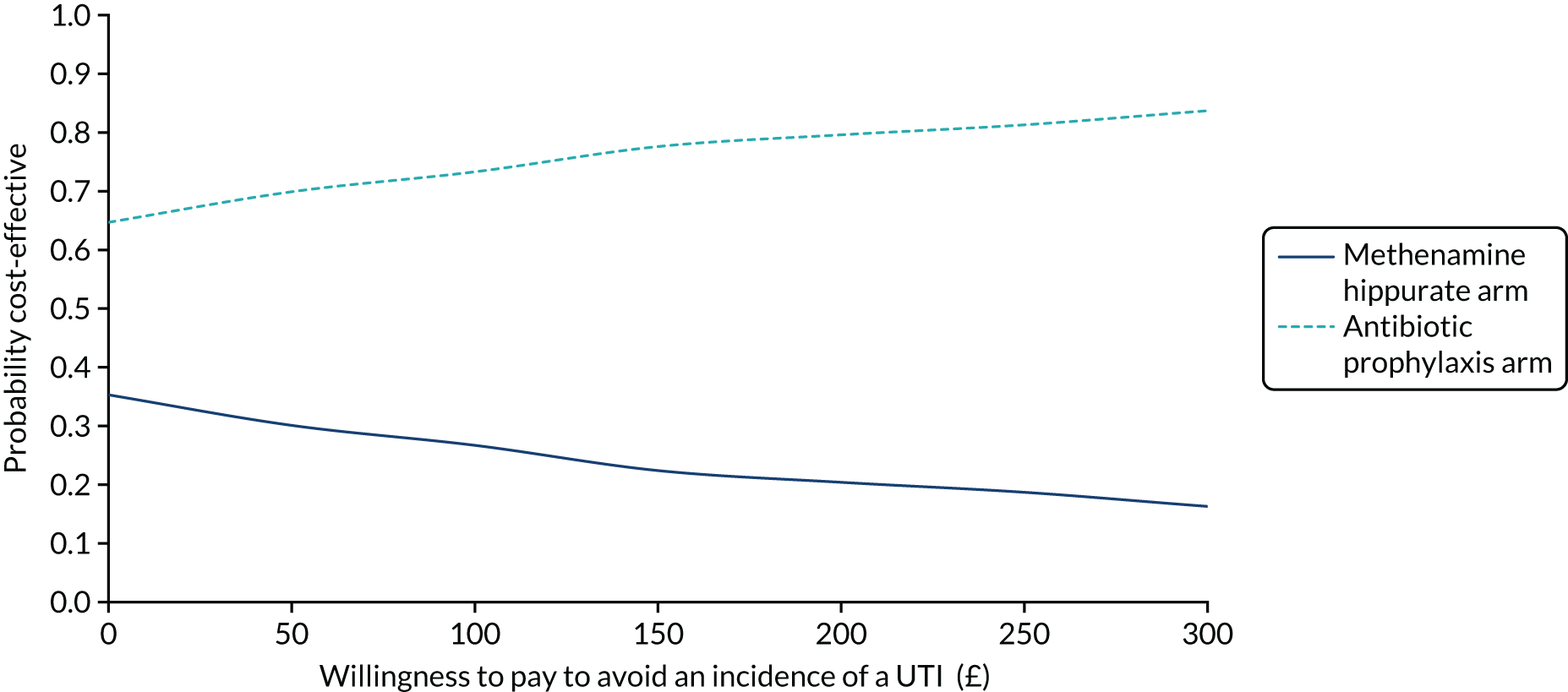
Incremental cost per quality-adjusted life-year gained
The results of the cost-effectiveness analysis using QALYs gained as the outcome measure is presented in Table 23. In the unadjusted analysis, antibiotic prophylaxis dominated methenamine hippurate, as it was, on average, less costly and more effective in terms of QALYs gained. However, in the adjusted analysis our conclusions changed and methenamine hippurate dominated antibiotic prophylaxis on average. However, in both circumstances the 95% CIs around the difference in costs and QALYs were wide.
| Strategy | Cost (SD) (£) | Incremental cost (95% CI)a (£) | QALYs (SD) | Incremental QALYs (95% CI)a | ICER | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP for a QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Antibiotic prophylaxis (costs n = 89; outcomes n = 58) | 931 (2015) | 1.182 (0.35) | 0.49 | 0.37 | 0.35 | 0.34 | 0.33 | |||
| Methenamine hippurate (costs n = 94; outcomes n = 71) | 1013 (1024) | –40 (–684 to 603) | 1.133 (0.35) | 0.014 (–0.05 to 0.07) | Methenamine hippurate dominant | 0.51 | 0.63 | 0.65 | 0.66 | 0.67 |
The bootstrapped results (Figures 14 and 15) illustrate the level of imprecision in our conclusions. If society was not willing to pay for a QALY, methenamine hippurate had a 51% probability of being considered cost-effective; however, this increased to 65% at the UK recommended threshold of £20,000 per QALY gained. 37
FIGURE 14.
Cost-effectiveness plane for methenamine hippurate compared with antibiotic prophylaxis using QALYs as the measure of outcome.
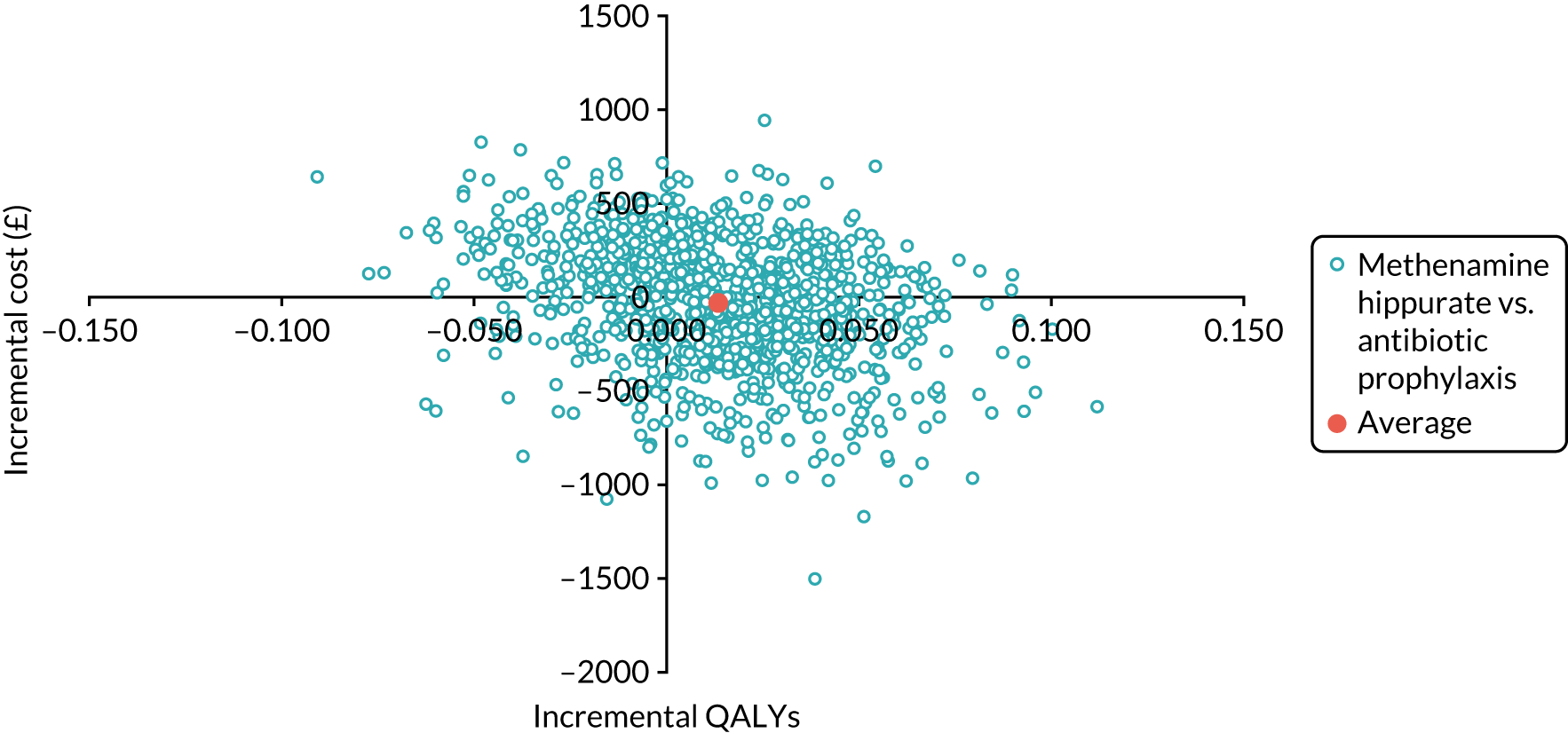
FIGURE 15.
Cost-effectiveness acceptability curve for methenamine hippurate compared with antibiotic prophylaxis using QALYs as the measure of outcome.
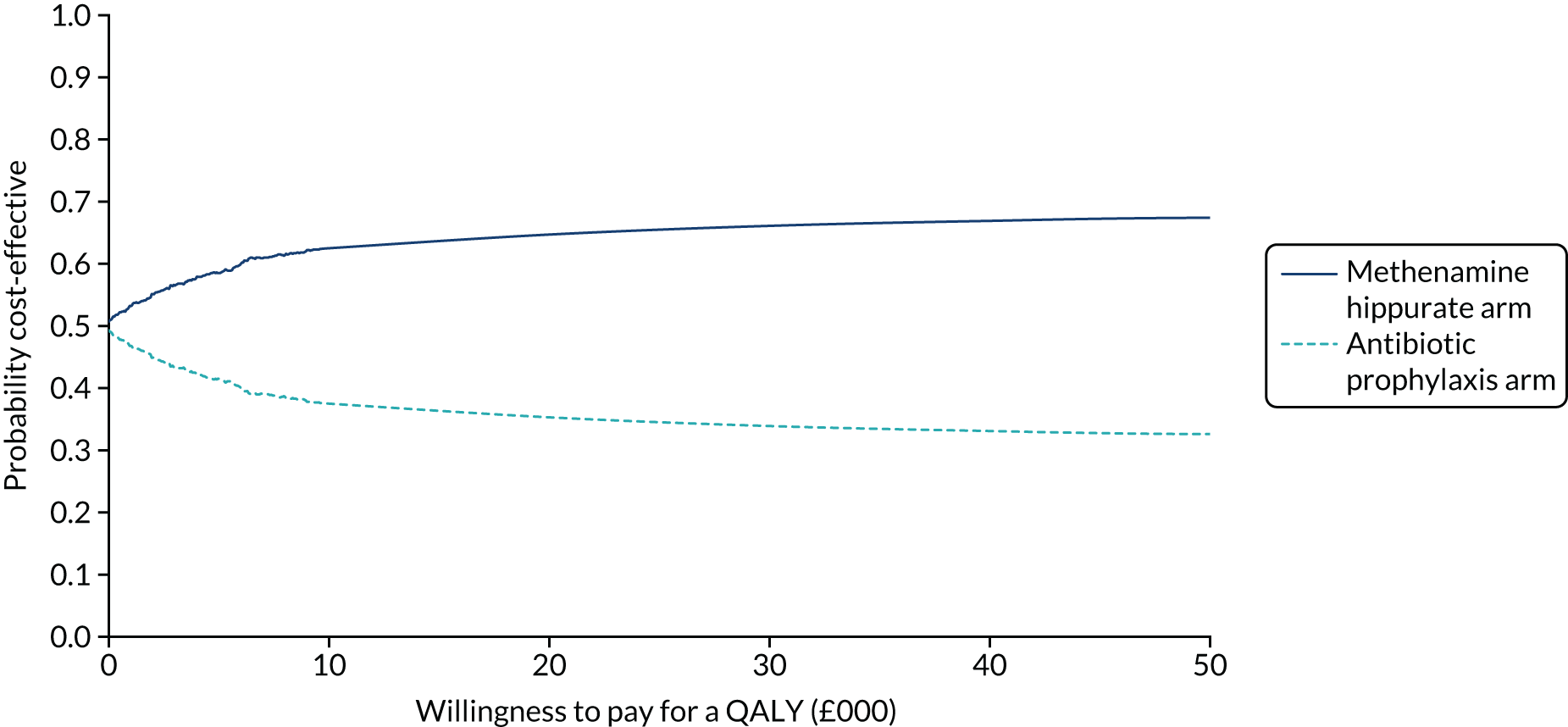
Sensitivity analysis
Urinary tract infection utility values
The results of the analysis incorporating utilities associated with UTIs in to the QALY equation are presented in Appendix 3, Table 40 and Figure 33. The result mirrors that of the primary analysis using QALYs as the outcome measure in that, on average in the unadjusted analysis, methenamine hippurate is dominated by antibiotic prophylaxis but this finding is reversed in the adjusted analysis. At current WTP thresholds for a QALY gained, methenamine hippurate has a 65% probability of being considered cost-effective.
Participant costs
The base-case analysis estimating the cost per QALY gained over 18 months was replicated to incorporate participant costs. The results of this analysis are presented in Appendix 3, Table 41 and Figure 34. The result mirrors that of the primary analysis; in the unadjusted analysis, methenamine hippurate was dominated by antibiotic prophylaxis but this reverses in the adjusted analysis. At current WTP thresholds for a QALY gained, methenamine hippurate has a 65% probability of being considered cost-effective.
Changing eligibility criteria
The results of the sensitivity analyses including participants eligible based on the per-protocol criteria are presented in Appendix 3, Table 42 and Figure 35. Again, the conclusions are similar to the base-case analysis using QALYs gained as the outcome measure.
Changing the time horizon
The results of the sensitivity analyses estimating costs and effects (incidence of UTIs and QALYs) over a 12-month time horizon are presented in Appendix 3, Tables 43 and 44 and Appendix 3, Figures 36 and 37. Our conclusions did not change in these analyses.
Antibiotic resistance
On average, when we considered the cost associated with antibiotic resistance, the antibiotic prophylaxis arm was more costly than the methenamine hippurate arm. In the cost-effectiveness analysis, methenamine hippurate was, on average, less costly and less effective at avoiding a UTI and had a 71% probability of being considered cost-effective if we were not willing to pay to avoid a UTI. As the value placed on avoiding a UTI increased so did the probability of methenamine hippurate being considered cost-effective (see Appendix 3, Table 45 and Figure 38). In the cost–utility analysis, methenamine hippurate remained the dominant strategy as it was, on average, less costly and more effective than antibiotic prophylaxis (see Appendix 3, Table 46 and Figure 39). If we were not willing to pay for an additional QALY, methenamine hippurate had a 69% probability of being considered cost-effective. Over the range of threshold values considered for an additional QALY, the probability of methenamine hippurate being considered cost-effective never exceeded 77%.
Model-based analysis
The aim of the economic model was to extrapolate the findings of the trial over the estimated lifetime of a woman suffering from rUTIs. The following outcomes were reported for the economic model:
-
health-care costs to the NHS of managing UTIs over the estimated lifetime of women with rUTIs
-
QALYs estimated over the estimated lifetime of women with rUTIs
-
incremental cost per QALY gained over the estimated lifetime of women with rUTIs.
Methods
An economic model was used to estimate the cost-effectiveness of methenamine hippurate compared with prophylactic antibiotics beyond the 18-month trial period. The model design and parameters estimated used are described in this section.
Economic model
Model structure
A Markov model was developed using R software [heemod (Health Economics Evaluation MODelling) package] (The R Foundation for Statistical Computing, Vienna, Austria) to extrapolate the results of the trial beyond the 18-month period. 51 The model was designed to simulate the pathway of women who experience rUTIs from initial treatment to end of life. Data from the trial alongside UK-relevant data were used to define the transition probabilities (probability of moving between the health states defined in the model) and other model parameter estimates (e.g. costs and effects).
Markov models comprise Markov states (or health states) that individuals stay in for a defined period of time. 51 Each state represents a stage in the pathway of women who experience rUTIs in a 6-month cycle. The model used in the trial, as illustrated in Figure 16, consisted of four health states: asymptomatic, mild symptoms (one UTI episode), moderate symptoms (two or more UTI episodes) and dead. In the model, each woman starts by receiving one of the two trial interventions (antibiotic prophylaxis or methenamine hippurate) and is followed through the model, which describes the process of care and incidence of rUTIs linked by logical and mathematical relationships (Tables 24 and 25).
FIGURE 16.
Model structure. mr = all-cause mortality rate; p_Asy_Mil = probability of transitioning from asymptomatic to mild; p_Asy_Mod = probability of transitioning from asymptomatic to moderate; p_Mil_Asy = probability of transitioning from mild to asymptomatic; p_Mil_Mod = probability of transitioning from mild to moderate; p_Mod_Asy = probability of transitioning from moderate to asymptomatic; and p_Mod_Mil = probability of transitioning from moderate to mild. C, cycle.
| Cycle | Health states | ||||||
|---|---|---|---|---|---|---|---|
| Asymptomatic | Mild | Moderate | Death | ||||
| Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean | |
| Costs (£) | |||||||
| 1 | 256 (370) | 124 | 659 (1430) | 46 | 472 (616) | 35 | 0 |
| 2 | 245 (268) | 141 | 278 (249) | 39 | 699 (1222) | 25 | 0 |
| 3–100 | 145 (37) | 114 | 233 (61) | 58 | 298 (108) | 33 | 0 |
| Utilities | |||||||
| 1 | 0.803 (0.24) | 124 | 0.750 (0.27) | 46 | 0.716 (0.237) | 35 | 0 |
| 2 | 0.796 (0.22) | 141 | 0.737 (0.256) | 39 | 0.666 (0.298) | 25 | 0 |
| 3–100 | 0.810 (0.21) | 114 | 0.695 (0.28) | 58 | 0.676 (0.27) | 33 | 0 |
| Strategy | Lifetime cost (£) (SD) | Incremental cost (£) (95% CI)a | Lifetime QALYs (SD) | Incremental QALYs (95% CI)a | ICER | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP for a QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Antibiotic prophylaxis | 7231 (10,096) | 15.24 (3.03) | 0.59 | 0.59 | 0.60 | 0.59 | 0.59 | |||
| Methenamine hippurate | 7876 (10,468) | 645 (359 to 931) | 14.96 (2.95) | –0.283 (–0.35 to –0.22) | Antibiotic prophylaxis dominant | 0.41 | 0.41 | 0.40 | 0.41 | 0.41 |
A model time horizon of 50 years was used, which was broken down into 6-monthly Markov cycles, the length of which have to be relevant to the condition being considered. The main reason for assuming a 6-month cycle was that the post-treatment follow-up period of the trial was 6 months. By using a 6-monthly cycle we could use the data from the trial to estimate the costs, effects and probability of women experiencing UTIs when they were no longer receiving the intervention medication on a regular basis. At the end of each 6-month cycle, women can either remain in the state in which they started the cycle or move to a different state. The rate at which women moved between states was dependent on the transition probabilities. An absorbing state, which is technically impossible to move out of, needs to be included in every model. 51 The death state was included as our absorbing state. The transition probability of a woman entering the death state was informed using UK all-cause mortality rates, given that there was no probability of mortality associated with experiencing a UTI cycle based on the trial data. The model was run for 100 cycles as it was assumed that after this time all of the cohort would be in the death state. A cost and utility weight were assigned to each of the health states. A total cost per participant was estimated depending on how long each individual spent in each health state. Similarly, QALYs were estimated for each woman by summing the utility values associated with the length of time they were in each health state and their length of time in the model.
Within the model, the mean age of the modelled cohort matches that of the trial participants at baseline [mean: 50 (SD 18.6) years].
Model assumptions
The following assumptions were made when developing the model:
-
Similarly to the within-trial analysis, the model-based analysis took the perspective of the UK NHS and personal and social services.
-
For extrapolation, the cost and utility values (by number of UTI episodes and treatment allocation) incurred beyond the 18-month trial period were assumed to be the same as those incurred in the last 6 months of the trial follow-up period (12–18 months).
-
All model parameters were defined as statistical distributions in the model.
-
Ranges and distributional assumptions for input parameters were based on the trial data. Gamma distributions were assumed for costs and beta distributions for utility data. Further details on parameter distributions are given in Model parameters.
-
Costs and QALYs incurred after 1 year (two cycles) were discounted at the recommended UK rate of 3.5%.
-
Cost and utility parameters for each state were homogeneous between the treatment arms, that is, for the same health state, the same values were used irrespective of the treatment received.
-
All individuals begin the model in the moderate state, as the inclusion criterion for the trial was individuals who have rUTIs (three episodes of infection in a 12-month period).
-
The absorbing state was assumed to be costless and have a utility value of zero.
Model parameters
The model parameters were informed using data from the trial and the distribution of each parameter was defined using the mean, standard error and shape of the distribution. Model parameters included NHS costs, utilities based on responses to the EQ-5D-5L, probabilities of a single or of multiple UTI episodes, and probability of death. Gamma distributions were used for cost parameters, beta distributions were used for utilities and the Dirichlet distribution was used for transition probabilities. Table 24 and Appendix 3, Table 47, describe the model parameters.
Costs
Costs were assigned to each stage in the model, reflecting the NHS costs incurred for each 6-month cycle. The costs considered were those associated with the intervention medications (methenamine hippurate and antibiotic prophylaxis) for the first two cycles only, health-care resource use and concomitant medications reported by those receiving each intervention medication during their time in the trial, and additional antibiotics received to treat UTIs. Regression techniques were applied to the data on total costs per woman obtained from the trial to identify potential differences in costs between each health state. The results of the regression analysis were used to identify the main drivers of costs for women who experience rUTIs over their lifetime. An ordinal least squares regression model was applied to estimate the difference in average total cost between each health state (Equation 6):
in which β = cost estimate, S1 = mild health state, S2 = moderate health state and ê = error term.
In Equation 6, we estimated the difference in health-care costs between the asymptomatic, mild and moderate states. As there are three states (not including the death state), two dummy variables for the mild and moderate states (S1 and S2) were included in the model. The cost estimate for the asymptomatic state was derived from the intercept (β0) in the regression model. Beta coefficients in the equation provide estimates of average total cost in each health state. State health-care costs were assumed to be time variant and, therefore, the regression was run for each cycle in the model. Cost values are presented in Table 24.
Utilities
Utility values were attached to each of the health states in the model, which were used to estimate QALYs. A utility value of zero was assigned to the death health state. The mean QALYs for each arm were estimated by multiplying the length of time in each health state by the health state utility value associated with that health state. The estimated utility data used in the model were based on the utility values estimated in the within-trial analysis, which were based on responses to the EQ-5D-5L.
Similarly to how the cost parameters were estimated, an OLS regression was used to estimate potential differences in utilities between health states (Equation 7):
in which β = utility estimate, S1 = mild health state, S2 = moderate health state and ê = error term.
Equation 7 describes how state utility values were derived. Dummy variables S1 and S2 estimated utility values for the mild and moderate states, while the intercept estimated a utility value for the asymptomatic state. State utility values were assumed to be time variant and, therefore, the regression was run for each cycle in the model. Utility values are presented in Table 24.
Transition probabilities
The structure of the model allowed for a cohort of women with rUTIs to enter the model and followed their pathway over time to eventual death. During each 6-month model cycle, a portion of the cohort entered each of the health states based on the probability of experiencing a single episode or multiple episodes of UTIs (trial data) or dying. Transition probabilities are presented in Appendix 3, Table 47.
Model validation
The model was validated by checking the model structure, calculations and data inputs. 52 To further establish model validity, the model was run for three cycles (18 months) to replicate the results of the within-trial analysis (see Appendix 3, Table 48 and Figure 42). Comparing the results for incremental cost per QALY gained between the within-trial and model-based analyses allowed us to verify the accuracy of the model and identify any potential issues in the parameters used to populate the model.
Sensitivity analysis
Probabilistic sensitivity analysis (PSA) was used to quantify any uncertainty in the results due to uncertainty in the parameters of the model. The PSA defined each parameter in the model as a statistical distribution; distributional assumptions were based on trial data. In the PSA, costs and effects were estimated using a set of parameters drawn using a Monte Carlo simulation from each specified distribution. This random sampling was repeated for 1000 iterations, the results of which were plotted onto the cost-effectiveness plane.
Results
Costs
As mentioned earlier in the chapter, state costs were assumed to be homogeneous between the treatment arms. This led to any difference in average total costs being driven by differences in transition probabilities and the Markov cohort trace. On average, costs were higher in the methenamine hippurate arm than in the antibiotic prophylaxis arm (mean difference £645.21, 95% CI £359.45 to £930.97). Average total costs are presented in Table 25.
Effectiveness
Utilities for each model state were also assumed to be homogeneous between the treatment arms. Therefore, any difference in QALYs was driven by differences in transition probabilities and the Markov cohort trace. On average, QALYs were lower in the methenamine hippurate arm than in the antibiotic prophylaxis (mean difference –2.828, 95% CI –2.938 to –2.718). Average total QALYs are presented in Table 25.
Incremental cost per quality-adjusted life-year gained
The incremental results are presented in Table 25 and Appendix 3, Figures 40 and 41. Antibiotic prophylaxis dominated methenamine hippurate, as it was, on average, less costly and more effective. However, the deterministic results alone are not sufficient to inform decision-making; we need to consider the imprecision around costs, effects and cost-effectiveness. As illustrated in the cost-effectiveness plane (see Appendix 3, Figure 40) and the CEAC (see Appendix 3, Figure 41), there is considerable uncertainty in this result. At current WTP thresholds, methenamine hippurate has a 40% probability of being considered cost-effective.
Chapter 6 Discussion
Statement and interpretation of results
The results of this randomised trial, which employed a pragmatic methodology in a routine NHS setting, have clearly demonstrated that, for women with recurrent uncomplicated UTIs, a non-antibiotic preventative treatment strategy in the form of methenamine hippurate is not inferior to the current standard of care of daily low-dose prophylactic antibiotics. Uncomplicated UTIs refers to UTIs occurring in patients with no structural or functional urinary tract abnormalities that could be contributory to their infective episodes. The observed numerical difference in clinically diagnosed UTI between the two arms, which favoured antibiotic prophylaxis, did not exceed the strict predefined non-inferiority margin of one UTI episode per year. This finding was consistent across the entire range of conducted analyses:
-
Modified ITT analysis – this was the primary analysis and included all patients with at least 6 months of follow-up data, analysed according to their original treatment allocation.
-
Strict ITT analysis – this included all patients who were randomised, analysed according to their original treatment allocation.
-
Per-protocol analysis – this included all patients with at least 6 months of follow-up data who achieved ≥ 90% compliance with any trial preventative treatment, analysed according to their original treatment allocation.
-
Post hoc modified per-protocol analysis – this included only those patients who achieved ≥ 90% compliance with their original allocated treatment, excluding those who changed treatment arm during the trial.
Similar substantial reductions in the primary outcome (rate of clinically diagnosed UTIs) were noted in both trial arms during the 12-month treatment period. These results confirm that both prophylactic daily antibiotics and twice-daily methenamine hippurate are very effective treatments for female patients with recurrent uncomplicated UTI. Overall, during the treatment phase of the trial, the mean number of antibiotic-treated UTIs decreased from a mean of 6.9 to a mean of 1.1 episodes per patient per year, equating to a RR of 0.16 (or an overall risk reduction of around 84%). This level of improvement is very much in line with previously published Cochrane systematic reviews of both trial treatments. 5,14 The review of prophylactic antibiotic treatment describes a RR of UTI of 0.21, whereas a similar review of methenamine hippurate describes a RR of uncomplicated UTI of 0.24. 5,14 Underlining the considerable beneficial effect of both treatments in this trial is the finding that around half of all participants were UTI free during the entire treatment period (UTI-free rates of 43% in the methenamine hippurate arm and 54% in the antibiotic prophylaxis arm).
Using an alternative (urine culture-based) definition of microbiologically confirmed UTI revealed an equivalent proportional change in UTI episodes. However, the use of a microbiological diagnosis of UTI alone would have resulted in failure to capture over half (58%) of all patient-reported, antibiotic-treated UTI episodes. Similarly, during the 6-month follow-up period, only 46% of all patient-reported, antibiotic-treated UTIs were confirmed by positive results from a microbiological culture. This supports previous findings from another recent RCT45 with a primary outcome of clinical (rather than microbiological) UTI, which reports that only 58% of patient-reported symptomatic UTIs were confirmed by a positive urine culture. The finding of such poor correlation between clinical and microbiological culture-based diagnoses of UTI suggests that the use of a clinical definition of UTI allows for greater detection and is better aligned with actual antibiotic-treated UTI episodes. Furthermore, the diagnostic accuracy of MSU samples for acute UTI was recently critically evaluated in a case series of 92 patients, in which the authors concluded that ‘routine MSU culture, adopting the UK interpretation criteria tailored to acute UTI, failed to detect a variety of bacterial species, including recognised uropathogens’. 53 This information will be useful to researchers in this topic area in the future.
Infections, when they occurred in this trial, were almost always not clinically severe, and only four (1.7%) participants (all allocated to the methenamine hippurate arm) required hospitalisation for UTI throughout the entire 18-month trial duration. Overall, the AE rate was low in both arms, as would be expected from a comparative trial involving two licensed treatments for the prevention of rUTI. Only two serious AEs (abdominal pain requiring hospital admission and severe derangement in LFTs) were classified as possibly or probably related to trial medication, and both occurred in the antibiotic arm. All other AEs occurred with a frequency of ≤ 8%. Kidney and liver function were assessed by regular blood tests and, overall, were largely unchanged during the 12 months of treatment in both arms.
In the 6-month follow-up period, the incidence rate of clinical UTI was seen to increase slightly but did not return to anywhere near pre-treatment levels. Over half of the patients (50% in the methenamine hippurate arm and 56% in the antibiotic prophylaxis arm) were UTI free during the entire follow-up period. This is in contrast to the findings from a Cochrane review5 on the subject of antibiotic prophylaxis that demonstrated that the relative risk of having one microbiologically diagnosed UTI after completion of prophylaxis was 0.82. This may be a function of the longer duration of prophylactic treatment in this trial (12 months) compared with those studies included in the Cochrane review, in which the majority of participants used antibiotic prophylaxis for 6 months.
Equivalent rates of overall treatment satisfaction were observed in those taking methenamine hippurate and daily prophylactic antibiotics at both 12 and 18 months. Both arms scored highly for treatment satisfaction, with the only significant difference reported in the convenience domain. Those allocated to once-daily antibiotics found the treatment more convenient than those allocated to methenamine hippurate, which had to be taken twice daily.
Antibiotic use was a secondary outcome in this trial, and the use of methenamine hippurate as a preventative treatment against recurrent uncomplicated UTI was associated with a significant reduction in overall antibiotic use, both prophylactic and therapeutic. First, in the methenamine hippurate arm, only 18% of participants switched to prophylactic antibiotics during the trial, indicating that first-line treatment with the non-antibiotic option of methenamine hippurate would be appropriate for the vast majority of female patients with recurrent uncomplicated UTI. Second, in terms of therapeutic antibiotics, of those patients allocated to methenamine hippurate, 44% did not receive any therapeutic antibiotics during the 12-month treatment period. This is in contrast to their self-reported average incidence of seven antibiotic-treated UTIs per year in the 12 months prior to trial enrolment. This reduction in antibiotic therapy associated with the use of methenamine hippurate as a first-line preventative treatment for women with a history of recurrent uncomplicated UTIs is directly aligned with current antibiotic stewardship strategies designed to reduce antimicrobial resistance. 54
In line with these national and international strategies, the main driver for conducting this research was the exploration of relative efficacy of a non-antibiotic option for the prevention of recurrent uncomplicated UTIs in women (in comparison with the current gold standard of daily antibiotic treatment). The theoretical risk of increased antimicrobial resistance associated with antibiotic treatment was explored through an assessment of bacteria cultured from both perineal swabs and urine samples serially throughout the trial. Significant rates of resistant bacteria were noted in both trial arms, which underlines the importance of simple, conservative measures at presentation prior to the use of pharmacological prophylaxis and emphasises the need for this information to be used when counselling patients regarding treatment options. There were no statistically significant differences between groups in terms of the number of E. coli isolates demonstrating resistance to antibiotics from perineal swabs or urine samples, or in the corresponding rates of MDR from those bacteria at baseline. However, during the treatment period, a higher proportion of patients allocated to daily prophylactic antibiotics exhibited resistance to at least one antibiotic in E. coli isolates from their perineal swabs. This suggests that, despite the observed higher incidence of antibiotic-treated UTIs in the methenamine hippurate arm, the use of continuous low-dose antibiotic prophylaxis was a more significant factor in the induction of antimicrobial resistance in the most common urinary tract pathogen for this trial population. This was similar to findings from a previous RCT1 exploring antibiotic prophylaxis and associated antimicrobial resistance in a population of patients with recurrent complicated UTIs. By the end of the follow-up period, the rate of MDR in E. coli isolates from perineal swabs was higher in the methenamine hippurate arm than in the antibiotic prophylaxis arm, perhaps because of the more prolonged effect of daily antibiotics on the faecal microbiome or greater incidence of antibiotic-treated acute UTIs in the methenamine hippurate arm after cessation of preventative treatment.
Interestingly, these differences in rates of antimicrobial resistance from bacteria isolated from perineal swabs were not always seen when the bacteria isolated in significant concentrations directly from urine samples were analysed. During the treatment period, a higher proportion of participants allocated to antibiotic prophylaxis demonstrated resistance to trimethoprim and co-trimoxazole in at least one E. coli isolate from urine when compared with those allocated to methenamine hippurate. In addition, there was a higher proportion of samples showing resistance to cephalosporins (cefalexin and cefuroxime) in E. coli isolates in those allocated to antibiotic prophylaxis than in those allocated to methenamine hippurate. Antimicrobial resistance occurring in the antibiotic prophylaxis arm since baseline was not confined to the antibiotics prescribed during the trial but covered a host of other commonly used antibiotics for UTI.
The co-primary objective of this trial was to examine the relative cost-effectiveness of methenamine hippurate relative to daily prophylactic antibiotics. The economic evaluation consisted of a within-trial analysis and an economic model that extrapolated the results of the trial beyond the 18-month follow-up period. The within-trial analysis estimated the incremental cost per UTI avoided and incremental cost per QALY gained. In both unadjusted analyses and the adjusted analysis estimating the difference in UTIs avoided, methenamine hippurate was dominated by antibiotic prophylaxis because, on average, it was more costly and less effective. However, in the adjusted analysis estimating the difference in QALYs gained, methenamine hippurate dominated antibiotics, as it was, on average, less costly and more effective in terms of QALYs gained and had a 65% probability of being considered cost-effective at a £20,000 WTP threshold. The change in our unadjusted and adjusted results in this analysis was unsurprising given that those randomised to antibiotic prophylaxis reported higher utility values, on average, at baseline than did those in the methenamine hippurate arm. However, there is a lot of uncertainty in these results, as illustrated on the cost-effectiveness planes for both adjusted analyses. In the longer-term economic model, methenamine hippurate was dominated by antibiotic prophylaxis, as it was, on average, more costly and less effective; however, the probability of antibiotic prophylaxis being considered cost-effective never exceeded 60%. This is because there is considerable uncertainty in these results as the difference in costs and QALYs is being driven by the number of UTIs reported during the 6-month follow-up period, which were higher in the methenamine hippurate arm than in the antibiotic prophylaxis arm.
Strengths and limitations
This trial was conducted in line with current best practice. A central web-based randomisation system using an algorithm written by an independent statistician ensured concealment of allocation. Randomisation was stratified based on important confounders such as previous UTI frequency and menopausal status. The patient characteristics at baseline were well balanced between arms for other possible confounders such as age, weight and previous use of either of the trial treatments. The median number of self-reported infections per year prior to trial enrolment was six in trial arms, reflecting a typical, representative population of female patients attending urology/urogynaecology units with the complaint of rUTI. Although representative, both trial arms contained a mix of pre- and postmenopausal women. It is highly likely that different aetiological factors are responsible for causing rUTIs in pre- compared with postmenopausal women. This trial was therefore unable to define whether the two tested preventative treatment strategies were more beneficial in specific patient groups.
The pragmatic nature of the trial did not allow for the blinding of participants or responsible clinicians to treatment allocation. A computerised adjudication process for attribution of primary outcome was included to avoid observer bias. The primary outcome of clinical UTI rather than reliance on a microbiological diagnosis purposefully aimed to ensure widespread capture of infection episodes. This reflects clinical practice accurately, given that most UTIs are treated prior to knowledge of microbiological culture results. Similar proportional reductions in both clinically and microbiologically confirmed UTIs highlighted the avoidance of detection bias. The trial’s data collection tools allowed for multiple different opportunities to capture the primary outcome. The design of a non-blinded comparative trial of two licensed preventative treatments for rUTI aimed to reduce the risk of differential reporting of outcomes between trial arms, which may occur in a non-blinded placebo-controlled trial. The allocation of primary outcome was also verified using medical records, and a representative sample of cases was given to an independent clinician who was blinded to treatment arm. The independent clinician agreed with the allocation of primary outcome in 100% of cases.
The three antibiotic options included in this trial are the most commonly used prophylactic antibiotic agents in routine clinical practice, and the inclusion of antibiotic choice in this trial reflects the significant prevalence of antimicrobial resistance in patients with rUTI. This degree of choice did, however, hamper the ability to analyse individual antibiotic agents according to benefit. Although the pragmatic nature of this trial allowed for crossover between treatment arms, a range of analyses were performed to ensure that key trial findings were valid. In addition, the compliance of participants with their allocated treatment was regularly assessed by research staff and was recorded in trial documentation. The results showed that the vast majority of participants were compliant with the allocated treatment more than 90% of the time.
An early within-trial qualitative study provided important insight into the recruitment and retention process. The opinions of trial participants, patients declining participation and clinical trial staff were very valuable both in terms of informing the current trial and highlighting important aspects for future UTI trial design.
The trial was conducted according to a pre-published protocol and all outcomes were clearly stated within that publication. 1 A realistic attrition rate was incorporated into the sample size calculation, and the number of withdrawals was in line with predicted rates. Despite the expected significant number of patients dropping out of the trial, which was largely due to an extended 18-month trial period/protocol, every effort was made to capture relevant data from participants after withdrawal. All pre-set thresholds regarding numbers of participants contributing to the primary analysis were met.
The early embedded qualitative study (see Chapter 3) was an important aspect of the trial that highlighted both facilitators of and barriers to trial recruitment and retention, and contributed to the overall success with regards to these fundamental metrics. One of the weaknesses of this qualitative work, however, was the poor recruitment of patients who had declined participation or had withdrawn to the qualitative work package included in the trial.
A major strength of the trial was the robust economic evaluation that was undertaken alongside the trial. The economic analysis incorporated changes in clinical benefits and also changes in quality of life (QoL) based on responses to the EQ-5D-5L, which is arguably more useful for decision-makers. The inclusion of both outcome measures illustrates the importance of looking at clinical and QoL outcomes in economic evaluations, because our conclusions changed depending on the outcome measure chosen; antibiotic prophylaxis, on average, led to a greater reduction in the number of UTIs reported, whereas methenamine hippurate, on average, gained more QALYs (albeit neither difference was statistically significant). Another strength of the economic evaluation was that the trial data were extrapolated over the expected lifetime of participants to estimate the lifetime costs and effects associated with antibiotic prophylaxis and methenamine hippurate.
One of the main challenges of the economic evaluation was the progressive loss of data over the 18-month follow-up period. However, assumptions were made to maximise the data available, which increased the number of participants who could be included in the economic evaluation. Another limitation of the economic analysis was that the perspective chosen in the base-case analysis did not consider the wider economic costs associated with antibiotic resistance. To address this limitation, as part of a sensitivity analysis we estimated the average total cost of antibiotic resistance for each antibiotic prescription and incorporated it into our within-trial results. Incorporating a conservative estimate of antibiotic resistance into the analysis strengthened our conclusions in that methenamine hippurate had a higher probability of being considered cost-effective in the management of rUTIs in both the cost-effectiveness and cost-utility analyses than antibiotic prophylaxis. However, given the crude nature of these cost estimates, it is important that they are interpreted with caution. Although widening our perspective to incorporate the costs associated with antibiotic resistance is important to policy-makers, further research is required to derive robust estimates that can be routinely incorporated into economic evaluations of antibiotic interventions. 55
There were some limitations associated with the economic model. First, the parameters of the model were informed using the trial data, so conservative assumptions had to be made for missing data (e.g. if a participant had partial cost data, missing data were assumed to be zero). Second, few participants were able to provide information to populate the parameters for the moderate model state, which meant that only the randomised variable and model states could be used as covariates in the regression models. 55 Third, the difficulty in estimating the economic impact of antimicrobial resistance meant that these costs were not considered in the model; this is a recognised limitation. Finally, the main difference in costs and effects over the longer term was driven by the difference in rUTIs experienced in the 6-month follow-up period of the trial. The uncertainty caused by the assumptions used to inform the model are illustrated on the cost-effectiveness plane, which shows that the difference in costs and effects over the longer term is uncertain as the spread of iteration is very wide.
To our knowledge, this is the first economic evaluation investigating the effects of urinary antiseptics compared with antibiotics in the management of rUTIs. Although the response rate to the data collection tools used in the economic evaluation was lower than anticipated, this was not surprising, as a similar trend was seen in the AnTIC trial,16 which had 3-monthly data collection points but a shorter trial period (i.e. 12 months). 45 The AnTIC trial16 was a previous UK RCT examining once-daily prophylactic antibiotic treatment for patients with rUTI who perform intermittent bladder catheterisation. Future studies evaluating UTI interventions should be considered while bearing in mind that, although more frequent data collection points are required to capture changes in costs and QoL (owing to the acute nature of rUTIs), the frequency of these data collection points increases participant burden and missing data. The effect of UTIs on QoL was also estimated as part of this trial. On average, participants who experienced a UTI reported a lower utility score, estimated based on responses to the EQ-5D-5L. However, when this was incorporated into the QALY analysis, there was a negligible difference in the results. This is consistent with the results of the AnTIC trial,16 which found no difference in QALYs when utility values associated with a UTI were incorporated into the QALY equation. It is likely that, although participants experience a decrease in QoL associated with their UTI, this has a minimal effect on overall QALYs given its short duration.
Generalisability
The pragmatic design of this trial has ensured widespread applicability and generalisability. The wide inclusion criteria and minimal exclusion criteria encompassed a broad range of eligible participants without introducing excessive heterogeneity into the trial. The included patients accurately represent those women with rUTI seen regularly in routine NHS practice. This is reflected in the baseline characteristics of participants. In addition, the trial recruited patients from a range of geographic and socioeconomic areas. The protocol allowed for shared decision-making between patients and clinicians regarding choice of antibiotic, which is in line with current clinical practice. Patients were allowed to cross over between trial arms, which is again reflective of usual care. The results of this trial will be of interest to guideline writers and policy-makers alike given the global initiative to reduce antimicrobial resistance, which uses antibiotic stewardship as one of its cornerstones. This trial has provided robust evidence of the effectiveness of a non-antibiotic option for the preventative treatment of rUTI that will allow patients and clinicians to avoid extended-course antibiotics in the future. The demonstration that methenamine hippurate is not inferior to daily low-dose prophylactic antibiotics may support a change in practice in terms of guideline-recommended treatments for rUTI prevention.
Chapter 7 Conclusions
The robust evaluation of a non-antibiotic preventative treatment for women with recurrent uncomplicated UTIs provided by this trial represents a high level of supportive evidence for the routine use of methenamine hippurate for this indication. This study fulfils the recommendations made by the Cochrane collaboration regarding methenamine hippurate that ‘large well conducted clinical trials involving this promising treatment particularly in the setting of prevention of recurrent infection’ should be conducted. 14 The benefit of this treatment from both a patient and a NHS perspective has been clearly defined. 14 Alternatives to antibiotic treatment are vital in the global mission to reduce the rate of antimicrobial resistance development. The demonstration that methenamine hippurate significantly reduces the number of UTI episodes to a level comparable to the current gold standard of daily prophylactic antibiotic treatment provides patients and clinicians alike with a viable first-line non-antibiotic alternative treatment option. Despite the observed numerical difference in UTI episodes (which did not exceed the non-inferiority margin) between daily antibiotics and methenamine hippurate, the documented increased levels of antimicrobial resistance associated with daily antibiotic treatment will undoubtedly play a part in the decision-making process when preventative treatment for rUTI is considered. From an economic perspective, on average, we found methenamine hippurate to be less costly and more effective in terms of QALYs gained; however, there is uncertainty over the longer-term costs and benefits when compared with prophylactic antibiotics.
The ALTAR trial will now ensure that guideline writers and policy-makers alike consider methenamine hippurate when treatment recommendations are made regarding UTI prevention. Current national and international guidelines do not recommend the use of methenamine hippurate because of the existing evidence base being of low quality. 11,12 The results from this trial have significant potential to change practice in the field of UTI preventative therapy.
Recommendations for research
-
Evaluation of both methenamine hippurate and other non-antibiotic preventative treatment options for rUTIs in specifically defined populations, such as exclusively pre- or postmenopausal cohorts or those with recurrent complicated UTIs.
-
Longer-term studies of UTI prevention treatments given the significant proportion of patients who relapse after cessation of treatment.
-
A specific and thorough evaluation of antimicrobial resistance in response to low-dose antibiotic prophylaxis against UTIs, especially in patients on this treatment long term.
-
Evaluation of non-antibiotic preventative treatment options in the context of prophylaxis against infections associated with urological or urogynaecological surgery.
-
Evaluation of novel diagnostic tests for UTI that may allow for more precise treatment and the avoidance of extended or repeated courses of antibiotics.
-
Determination of the longer-term costs and benefits associated with methenamine hippurate and antibiotic prophylaxis. In addition, the potential costs and effects associated with antibiotic resistance need to be considered when comparing the long-term effects of both management strategies.
Acknowledgements
The ALTAR trial was only possible owing to the generous support and enthusiasm of a large number of individuals.
The investigators would like to acknowledge the NIHR Clinical Research Networks that provided NHS research support to the trial.
We would like to thank all the staff involved at the Newcastle upon Tyne Hospitals NHS Foundation Trust who acted as sponsor for the trial.
Many clinicians and research staff from across the UK contributed to the trial at individual sites and we express our gratitude to all staff at sites in the UK for their support of trial recruitment and data collection. In particular, we would like to thank the following organisations and staff: Freeman Hospital and Royal Victoria Infirmary, the Newcastle upon Tyne Hospitals NHS Foundation Trust (PI: Chris Harding; RNs: Alex Hall, Dianne Wake, Nic Brown, Lynn D Langthorne and Peter Murphy); Addenbrookes Hospital, Cambridge (PI: Nikesh Thiruchelvam; RN: Kelly Leonard); Queen Elizabeth University Hospital, Glasgow (PI: Karen Guerrero; RNs Kirsteen Patterson and Therese McSorly); Pinderfields Hospital, Wakefield (PI: Ased Ali; RN: Stephen Littler); Leeds Teaching Hospital Trust, Leeds (PI: Ian Eardley; RN: Lorraine Wiseman); Liverpool University Hospitals NHS Foundation Trust, formerly The Royal Liverpool University Hospital, Liverpool (PI: Henry Lazarowicz; RNs: Nicola Bermingham and Sue Lancaster); The Royal Oldham Hospital, Oldham (PI: Zahid Hussain; RNs: Sue Dermody and Sarah Winnard); Manchester University Hospitals Foundation NHS Trust, Manchester (PI: Ian Pearce; RN: Houda Chea).
We would like to thank NCTU staff, in particular Jennifer Wilkinson (Senior Trial Manager) for support in securing funding for the trial and Trial Managers Catherine Brennand, Robbie Brown and Rosy Lampitt. We would like to thank Population Health Sciences Institute (formerly Institute of Health and Society) staff, in particular Nick Steen (statistician) who contributed to the original trial design, Valentina Mamasoula (statistician) and Heather Brown (health economist). We would also like to acknowledge the contribution of Mabel Lie in the collection and analysis of data for the qualitative embedded study and thank all patients and site staff who gave up their time to be interviewed.
We would like to thank NHS staff of the Freeman Hospital at Newcastle upon Tyne Hospitals NHS Foundation Trust for their help, in particular Michael Ford, Jennifer Collins, Amrijit Singh, Robert Oxley and Natalie Bell from the Department of Microbiology and Virology for microbiology support and careful husbandry of trial specimens, and Ian Campbell (Assistant Director, Pharmacy and Medicines Management) for his advice and pharmacy support.
We would like to thank the members of the TSC, Doreen McClurg (chairperson), Chris Betts, Jon Rees, Christine Francis and Jane Stapleford, and members of the DMC, Marcus Drake (chairperson), Tamsin Greenwell and Tim Pickles, for all their valuable guidance and for reviewing trial documentation and reports.
We would like to thank Jane Stapleford and Bladder Health UK for their help with the study protocol design. We would like to thank the Newcastle UTI Focus Group for their help with trial design and, in particular, for defining the non-inferiority margin from the patient perspective.
We would like to thank Richard Parkinson for critical review of the primary outcome data.
Finally, but most importantly, we would like to thank all the ALTAR trial participants for agreeing to take part in the ALTAR trial.
Contributions of authors
Chris Harding (https://orcid.org/0000-0002-9407-382X) (Chief Investigator) designed the trial, was the principal applicant for funding, co-wrote the study protocol, supervised the overall conduct of the study, interpreted trial data, and co-wrote and edited the final report.
Thomas Chadwick (https://orcid.org/0000-0002-7995-0302) (Clinical Trials Statistician) designed the statistical plan in the study protocol, led the final study statistical analysis and edited the final report.
Tara Homer (https://orcid.org/0000-0002-6664-0671) (Health Economist and Senior Research Associate) designed the health economics analysis plan, led the health economics analysis and co-wrote the final report.
Jan Lecouturier (https://orcid.org/0000-0002-5191-5804) (Senior Research Associate) contributed to the design of the study and protocol (including the embedded qualitative study), was a co-applicant for funding, led the embedded qualitative study and co-wrote the final report.
Helen Mossop (https://orcid.org/0000-0002-6260-3734) (Research Associate in Biostatistics) wrote the SAP, conducted the main study analysis and co-wrote the final report.
Sonya Carnell (https://orcid.org/0000-0002-7648-4297) (Senior Trial Manager) led the NCTU team’s involvement in the trial and co-wrote and edited the final report.
Will King (https://orcid.org/0000-0001-7040-9133) (Health Economist and Training Fellow) wrote the health economics analysis plan, conducted the main study health economics analysis and co-wrote part of the final report.
Alaa Abouhajar (https://orcid.org/0000-0001-7220-6380) (Database Manager) designed and set up trial-specific databases, managed central data processes, reviewed trial results and reviewed the final report.
Luke Vale (https://orcid.org/0000-0001-8574-8429) (Health Foundation Professor of Health Economics) was a co-applicant for funding, contributed to the design of the protocol, designed and supervised the health economics analysis, reviewed the trial results and edited the final report.
Gillian Watson (https://orcid.org/0000-0003-2466-0268) (Trial Manager) was the trial manager for the trial and reviewed the final report.
Rebecca Forbes (https://orcid.org/0000-0002-2391-1371) (Trial Manager) was the trial manager for the trial and reviewed the final report.
Stephanie Currer (https://orcid.org/0000-0002-7074-9370) (Clinical Trial Administrator) was the Clinical Trial Administrator for the trial and reviewed the final report.
Robert Pickard (https://orcid.org/0000-0001-6151-9031) (Professor of Urology) contributed to the design of the protocol and was a co-applicant for funding.
Ian Eardley (https://orcid.org/0000-0002-8411-2987) (Consultant Urologist) contributed to the design of the protocol, was a co-applicant for funding, acted as PI for the Leeds site and reviewed the final report.
Ian Pearce (https://orcid.org/0000-0002-7710-0531) (Consultant Urologist) contributed to the design of the protocol, was a co-applicant for funding, acted as PI for the Manchester site and reviewed the final report.
Nikesh Thiruchelvam (https://orcid.org/0000-0002-2910-2209) (Consultant Urologist) contributed to the design of the protocol, was a co-applicant for funding, acted as PI for the Cambridge site and reviewed the final report.
Karen Guerrero (https://orcid.org/0000-0001-9591-059X) (Consultant Urogynaecologist) contributed to the design of the protocol, was a co-applicant for funding, acted as PI for the Glasgow site and reviewed the final report.
Katherine Walton (https://orcid.org/0000-0002-3434-7753) (Consultant Clinical Microbiologist) contributed to the design of the protocol, was a co-applicant for funding, and led the reporting and interpretation of microbiological results and reviewed the final report.
Zahid Hussain (https://orcid.org/0000-0002-9852-6478) (Consultant Urologist), acted as PI for the Oldham site and reviewed the final report.
Henry Lazarowicz (https://orcid.org/0000-0003-0792-2983) (Consultant Urologist) acted as PI for the Liverpool site and reviewed the final report.
Ased Ali (https://orcid.org/0000-0001-9576-9047) (Consultant Urologist) contributed to the design of the protocol, was a co-applicant for funding, acted as PI for the Wakefield site and reviewed the final report.
Publications
Forbes R, Ali A, Abouhajar A, Brennand C, Brown H, Carnell S, et al. ALternatives To prophylactic Antibiotics for the treatment of Recurrent urinary tract infection in women (ALTAR): study protocol for a multicentre, pragmatic, patient-randomised, non-inferiority trial. Trials 2018;19:616.
Lie MLS, Lecouturier J, Harding C. Should I stay or should I go? A qualitative study exploring participation in a urology clinical trial. Int Urogynecol J 2019;30:9–16.
Harding C, Mossop H, Homer T, Chadwick T, King W, Carnell S, et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, non-inferiority trial. BMJ 2022;376:e068229.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Forbes R, Ali A, Abouhajar A, Brennand C, Brown H, Carnell S, et al. ALternatives To prophylactic Antibiotics for the treatment of Recurrent urinary tract infection in women (ALTAR): study protocol for a multicentre, pragmatic, patient-randomised, non-inferiority trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2998-4.
- Laupland KB, Ross T, Pitout JD, Church DL, Gregson DB. Community-onset urinary tract infections: a population-based assessment. Infection 2007;35:150-3. https://doi.org/10.1007/s15010-007-6180-2.
- Ikaheimo R, Sutonen A, Heiskanen T, Kärkkäinen U, Kuosmanen P, Lipponen P, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis 1996;22:91-9. https://doi.org/10.1093/clinids/22.1.91.
- Office for National Statistics . Census. Population Estimates for the United Kingdom 2011 n.d.
- Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev 2004;3. https://doi.org/10.1002/14651858.CD001209.pub2.
- Beerepoot MA, ter Riet G, Nys S, van der Wal WM, de Borgie CA, de Reijke TM, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med 2012;172:704-12. https://doi.org/10.1001/archinternmed.2012.777.
- Department of Health and Social Care (DHSC) . UK Antimicrobial Resistance Strategy and Action Plan 2000.
- Healthcare Associated Infection Task Force . The Scottish Management of Antimicrobial Resistance Action Plan [ScotMARAP] 2008. www.sehd.scot.nhs.uk/mels/CEL2008_30.pdf (accessed December 2020).
- Scottish Intercollegiate Guideline Network (SIGN) . Management Of Suspected Bacterial Urinary Tract Infection in Adults 2012. www.sign.ac.uk/media/1051/sign88.pdf (accessed December 2020).
- Grabe M, Bjerklund-Johansen T-E, Botto H, Çek M, Naber KG, Pickard RS, et al. EAU Guidelines on Urological Infections n.d.
- National Institute for Health and Care Excellence (NICE) . Urinary Tract Infection (Lower) – Women 2020. https://cks.nice.org.uk/topics/urinary-tract-infection-lower-women/ (accessed 13 November 2020).
- European Association of Urology (EAU) . Guidelines (Urological Infections) n.d.
- Barclay J, Veeratterapillay R, Harding C. Uncertainties: non antibiotic options for recurrent urinary tract infections in women. BMJ 2017;23. https://doi.org/10.1136/bmj.j5193.
- Lee BS, Bhuta T, Simpson JM, Craig JC. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev 2012;10. https://doi.org/10.1002/14651858.CD003265.pub3.
- Murray BE, Rensimer ER, DuPont HL. Emergence of high-level trimethoprim resistance in fecal Escherichia coli during oral administration of trimethoprim or trimethoprim-sulfamethoxazole. N Engl J Med 1982;306:130-5. https://doi.org/10.1056/NEJM198201213060302.
- Fisher H, Oluboyede Y, Chadwick T, Abdel-Fattah M, Brennand C, Fader M, et al. Continuous low-dose antibiotic prophylaxis for adults with repeated urinary tract infections (AnTIC): a randomised, open-label trial. Lancet Infect Dis 2018;18:957-68. https://doi.org/10.1016/S1473-3099(18)30279-2.
- Department of Health and Social Care (DHSC) . UK Antmicrobial Resistance Strategy 2013.
- Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011;52:e103-20. https://doi.org/10.1093/cid/ciq257.
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 2010;10:597-602. https://doi.org/10.1016/S1473-3099(10)70143-2.
- Scottish Medicines Consortium Scottish Antimicrobial Prescribing Group . Good Practice Recommendations for Hospital Antimicrobial Stewardship in NHS Scotland 2012.
- Health Protection Scotland (HPS) . Quarterly Report on the Surveillance of Clostridium Difficile Infection (CDI) in Scotland, October–December 2011 2012.
- Beerepoot MA, Geerlings SE, van Haarst EP, van Charante NM, ter Riet G. Nonantibiotic prophylaxis for recurrent urinary tract infections: a systematic review and meta-analysis of randomized controlled trials. J Urol 2013;190:1981-9. https://doi.org/10.1016/j.juro.2013.04.142.
- World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Joint Formulary Committee . British National Formulary (online) n.d. www.medicinescomplete.com/mc/bnf/current/ (accessed August 2014).
- Public Health England . Diagnosis of Urinary Tract Infections: Quick Reference Guide for Primary Care n.d. www.gov.uk/government/publications/urinary-tract-infection-diagnosis (accessed 26 October 2020).
- Public Health England . UK Standards for Microbiology Investigations: Investigation of Urine n.d. www.gov.uk/government/publications/smi-b-41-investigation-of-urine (accessed 26 October 2020).
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268-81. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
- Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004;2. https://doi.org/10.1186/1477-7525-2-12.
- European Commission . Commission Directive 2005 28 EC of 8 April 2005 Laying Down Principles and Detailed Guidelines for Good Clinical Practice As Regards Investigational Medicinal Products for Human Use, As Well As the Requirements for Authorisation of the Manufacturing or Importation of Such Products (Text With EEA Relevance) 2005.
- Great Britain . Data Protection Act 1998 1998.
- Council of the European Union . Regulation (EU) 2016 679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons With Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95 46 EC (General Data Protection Regulation) (Text With EEA Relevance) 2016.
- NHS Health Technology Assessment Programme . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)17965-3.
- Lie MLS, Lecouturier J, Harding C. Should I stay or should I go? A qualitative study exploring participation in a urology clinical trial. Int Urogynecol J 2019;30:9-16. https://doi.org/10.1007/s00192-018-3784-2.
- Hennessy M, Hunter A, Healy P, Galvin S, Houghton C. Improving trial recruitment processes: how qualitative methodologies can be used to address the top 10 research priorities identified within the PRioRiTy study. Trials 2018;19. https://doi.org/10.1186/s13063-018-2964-1.
- Fram SM. The constant comparative analysis method outside of grounded theory. Qual Rep 2013;18.
- Harding C, Mossop H, Homer T, Chadwick T, King W, Carnell S, et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, non-inferiority trial. BMJ 2022;376. https://doi.org/10.1136/bmj-2021-0068229.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2019. PSSRU, University of Kent: Canterbury; 2019.
- NHS . NHS Reference Costs 2018–19 n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed October 2019).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230-5. https://doi.org/10.1136/bmj.300.6719.230.
- Fiebig DG, Baltagi BH. A Companion to Theoretical Econometrics. Malden, MA: Blackwell Publishing Ltd; 2003.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Pickard R, Chadwick T, Oluboyede Y, Brennand C, von Wilamowitz-Moellendorff A, McClurg D, et al. Continuous low-dose antibiotic prophylaxis to prevent urinary tract infection in adults who perform clean intermittent self-catheterisation: the AnTIC RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22240.
- Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre) . CCEMG – EPPI-Centre Cost Converter n.d. https://eppi.ioe.ac.uk/costconversion/default.aspx (accessed December 2020).
- NHS Improvement . AMR NHS Oversight Framework 20-21, Antibiotic Dashboard December 2020 n.d. www.england.nhs.uk/ccg-out-tool/anti-dash (accessed December 2020).
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. https://doi.org/10.1002/1097-0258(20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-14. https://doi.org/10.1177/0272989X9001000308.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Eonomic Evaluation. New York, NY: Oxford University Press; 2006.
- Gray A, Clarke P, Wolstenholme J, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Health Care. Oxford: Oxford University Press; 2011.
- Sathiananthamoorthy S, Malone-Lee J, Gill K, Tymon A, Nguyen TK, Gurung S, et al. Reassessment of routine midstream culture in diagnosis of urinary tract infection. J Clin Microbiol 2019;57:e01452-18. https://doi.org/10.1128/JCM.01452-18.
- Department of Health and Social Care (DHSC) . Contained and Controlled: The UK’s 20-Year Vision for Antimicrobial Resistance 2019.
- Oppong R, Smith RD, Little P, Verheij T, Butler CC, Goossens H, et al. Cost effectiveness of amoxicillin for lower respiratory tract infections in primary care: an economic evaluation accounting for the cost of antimicrobial resistance. Br J Gen Pract 2016;66:e633-9. https://doi.org/10.3399/bjgp16X686533.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU, University of Kent; 2011.
- Briggs AH, Ades AE, Price MJ. Probabilistic sensitivity analysis for decision trees with multiple branches: use of the Dirichlet distribution in a Bayesian framework. Med Decis Making 2003;23:341-50. https://doi.org/10.1177/0272989X03255922.
Appendix 1 Child–Pugh classification
| T measure | 1 point | 2 points | 3 points |
|---|---|---|---|
| Total bilirubin, µmol/l (mg/dl) | < 34 (< 2) | 34–50 (2–3) | > 50 (> 3) |
| Serum albumin (g/dl) | > 3.5 | 2.8–3.5 | < 2.8 |
| Prothrombin time, prolongation (seconds) | < 4.0 | 4.0–6.0 | > 6.0 |
| Ascites | None | Mild | Moderate to severe |
| Hepatic encephalopathy | None | Grade I or II (or suppressed with medication) | Grade III or IV (or refractory) |
Appendix 2 Results supplementary information
| Antibiotic prophylaxis arm (N = 120) | Methenamine hippurate arm (N = 120) | Total (N = 240) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Overall | ||||||
| Total withdrawn/lost to follow-up | 46 | 38 | 33 | 28 | 79 | 33 |
| Withdrawn | 23 | 19 | 16 | 13 | 39 | 16 |
| Lost to follow-up | 23 | 19 | 17 | 14 | 40 | 17 |
| Primary outcome data available from health-care record review | 21 | 18 | 11 | 9 | 32 | 13 |
| Last follow-up visit < 6 months | ||||||
| Total withdrawn/lost to follow-up | 24 | 20 | 22 | 18 | 46 | 19 |
| Withdrawn | 17 | 14 | 11 | 9 | 28 | 12 |
| Lost to follow-up | 7 | 6 | 11 | 9 | 18 | 8 |
| Primary outcome data available from health-care record review | 7 | 6 | 5 | 4 | 12 | 5 |
| Last follow-up visit ≥ 6 and < 12 months | ||||||
| Total withdrawn/lost to follow-up | 13 | 11 | 9 | 8 | 22 | 9 |
| Withdrawn | 3 | 3 | 4 | 3 | 7 | 3 |
| Lost to follow-up | 10 | 8 | 5 | 4 | 15 | 6 |
| Primary outcome data available from health-care record review | 8 | 7 | 4 | 3 | 12 | 5 |
| Last follow-up visit ≥ 12 months | ||||||
| Total withdrawn/lost to follow-up | 9 | 8 | 2 | 2 | 11 | 5 |
| Withdrawn | 3 | 3 | 1 | 1 | 4 | 2 |
| Lost to follow-up | 6 | 5 | 1 | 1 | 7 | 3 |
| Primary outcome data available from health-care record review | 6 | 5 | 2 | 2 | 8 | 3 |
| Antibiotic prophylaxis arm (N = 120) | Methenamine hippurate arm (N = 120) | Total (N = 240) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Overall | ||||||
| Total withdrawn | 23 | 19 | 16 | 13 | 39 | 16 |
| Last follow-up visit < 6 months | ||||||
| Total withdrawn | 17 | 14 | 11 | 9 | 28 | 12 |
| AE/side effects of study medication | 4 | 3 | 3 | 3 | 7 | 3 |
| Other health problems/pregnancy | 5 | 4 | 3 | 3 | 8 | 3 |
| Too busy/personal circumstances | 3 | 3 | 1 | 1 | 4 | 2 |
| Lack of efficacy | 3 | 3 | 0 | 0 | 3 | 1 |
| Too many hospital visits | 0 | 0 | 1 | 1 | 1 | < 1 |
| Did not want to take study medication | 1 | 1 | 1 | 1 | 2 | 1 |
| Reason unknown | 1 | 1 | 2 | 2 | 3 | 1 |
| Last follow-up visit ≥ 6 and < 12 months | ||||||
| Total withdrawn | 3 | 3 | 4 | 3 | 7 | 3 |
| AE/side effects of study medication | 1 | 1 | 0 | 0 | 1 | 0 |
| Lack of efficacy | 0 | 0 | 2 | 2 | 2 | 1 |
| Clinician decision to withdraw due to non-compliance | 1 | 1 | 0 | 0 | 1 | < 1 |
| Too busy/personal circumstances | 0 | 0 | 2 | 2 | 2 | 1 |
| Reason unknown | 1 | 1 | 0 | 0 | 1 | < 1 |
| Last follow-up visit ≥ 12 months | ||||||
| Total withdrawn | 3 | 3 | 1 | 1 | 4 | 2 |
| Other health problems | 2 | 2 | 0 | 0 | 2 | 1 |
| Too busy/personal circumstances | 0 | 0 | 1 | 1 | 1 | < 1 |
| Frequency of UTIs since stopping trial treatment | 1 | 1 | 0 | 0 | 1 | < 1 |
FIGURE 17.
Frequency of symptomatic UTI episodes during the 12-month treatment period: mITT population.
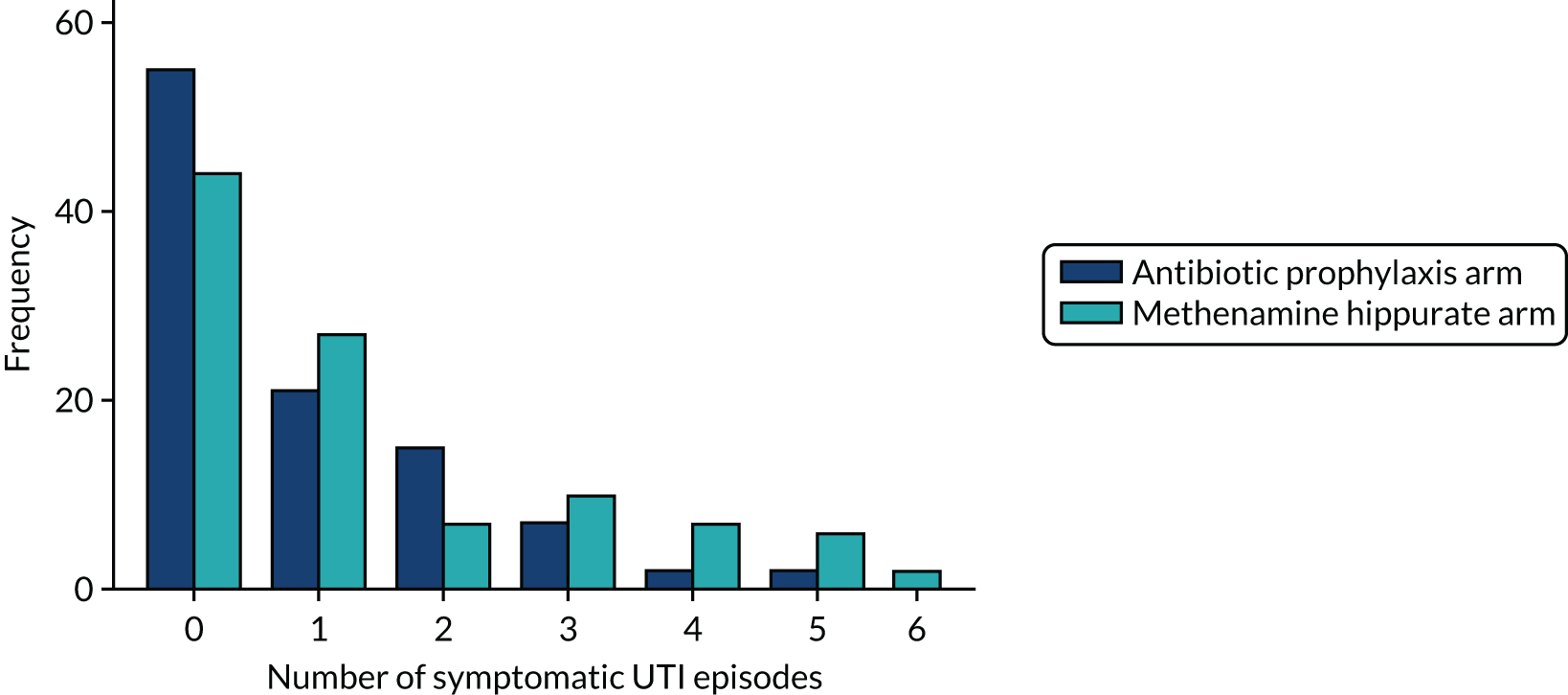
| Strict PP | ||
|---|---|---|
| Antibiotic prophylaxis arm (N = 82) | Methenamine hippurate arm (N = 71) | |
| Incidence rate | ||
| Total number of UTI episodes/total follow-up time (years) | 68/82.0 | 80/71.0 |
| Incidence rate (95% CI) | 0.83 (0.58 to 1.08) | 1.13 (0.76 to 1.50) |
| Difference (90% CI) | 0.30 (–0.08 to 0.67) | |
| Unadjusted IRR (95% CI) | 1.36 (0.88 to 2.10) | |
| Adjusted IRR (95% CI) | 1.35 (1.06 to 1.71) | |
| Incidence density rate | ||
| Total number of UTI episodes/total follow-up time (years) | 68/80.62 | 80/68.92 |
| Incidence rate (95% CI) | 0.84 (0.59 to 1.10) | 1.16 (0.77 to 1.55) |
| Difference (90% CI) | 0.32 (–0.08 to 0.71) | |
| Unadjusted IRR (95% CI) | 1.38 (0.88 to 2.15) | |
| Adjusted IRR (95% CI) | 1.38 (1.07 to 1.79) | |
| At least one episode of symptomatic UTI | ||
| ≥ 1 episode, n (%) | 38 (46) | 35 (49) |
| Unadjusted OR (95% CI) | 1.13 (0.60 to 2.13) | |
| Adjusted OR (95% CI) | 1.14 (0.59 to 2.21) | |
FIGURE 18.
Frequency of symptomatic UTI episodes in the 6-month follow-up period.
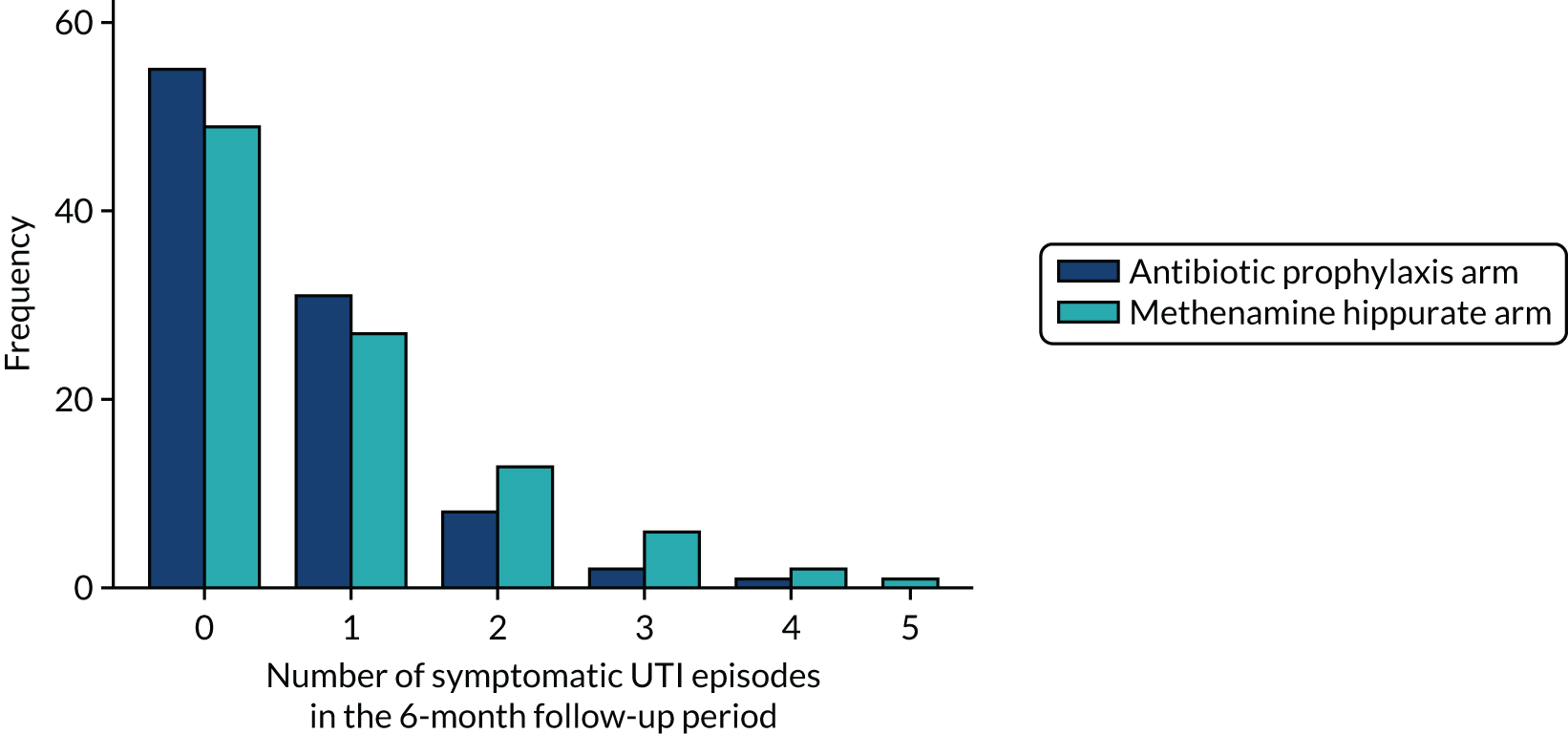
| Antibiotic use | Antibiotic prophylaxis arm (N = 120) | Methenamine hippurate arm (N = 120) |
|---|---|---|
| 12-month treatment period | ||
| Antibiotic prophylaxis | ||
| Number receiving ≥ 1 day of treatment, n (%) | 120 (100) | 22 (18) |
| Days of treatment, median (IQR); minimum, maximum | 362 (184–365); 13, 365 | 229 (120–321); 4, 362 |
| Rate of antibiotic use; mean (SD) | 0.91 (0.23) | 0.12 (0.28) |
| Treatment antibiotics for UTI | ||
| Number receiving ≥ 1 day of treatment, n (%) | 51 (43) | 67 (56) |
| Days of treatment, median (IQR); minimum, maximum | 13 (8–19); 1, 59 | 16 (7–25); 1, 162 |
| Rate of antibiotic use, mean (SD) | 0.025 (0.063) | 0.036 (0.065) |
| Treatment antibiotics for other infections | ||
| Number receiving ≥ 1 day of treatment, n (%) | 32 (27) | 38 (32) |
| Days of treatment, median (IQR); minimum, maximum | 10.5 (7–20.5); 3, 79 | 9 (6–19); 1, 84 |
| Rate of antibiotic use, mean (SD) | 0.017 (0.054) | 0.020 (0.058) |
| 6-month follow-up period | ||
| Antibiotic prophylaxis | ||
| Number evaluable, n (%) | 78 (65) | 87 (73) |
| Number receiving antibiotic prophylaxis, n (%) | 8 (10) | 5 (6) |
| Treatment antibiotics for UTI | ||
| Number evaluable, n (%) | 97 (81) | 98 (82) |
| Number receiving ≥ 1 day of treatment, n (%) | 48 (49) | 52 (53) |
| Days of treatment, median (IQR); minimum, maximum | 7.5 (4–15); 2, 56 | 13.5 (6.5–23); 3, 69 |
| Rate of antibiotic use, mean (SD) | 0.036 (0.081) | 0.043 (0.064) |
| Treatment antibiotics for other infections | ||
| Number evaluable, n (%) | 97 (81) | 98 (82) |
| Number receiving ≥ 1 day of treatment, n (%) | 15 (15) | 28 (29) |
| Days of treatment, median (IQR); minimum, maximum | 8 (7–12); 4, 49 | 8 (5.5–13); 4, 105 |
| Rate of antibiotic use, mean (SD) | 0.013 (0.062) | 0.021 (0.067) |
FIGURE 19.
Development of resistance to at least one additional antibiotic agent since baseline in E. coli isolated from perineal swabs. New resistance since baseline: participants shown to have resistance in a post-baseline perineal swab E.coli isolate to at least one antibiotic agent to which the E. coli was susceptible at baseline or where the participant did not have E. coli isolated in their baseline sample. Known new resistance since baseline: participants demonstrating the acquisition of resistance in a post-baseline perineal swab E. coli isolate to at least one antibiotic agent to which the baseline E. coli isolate was susceptible. Chi-squared p-values.
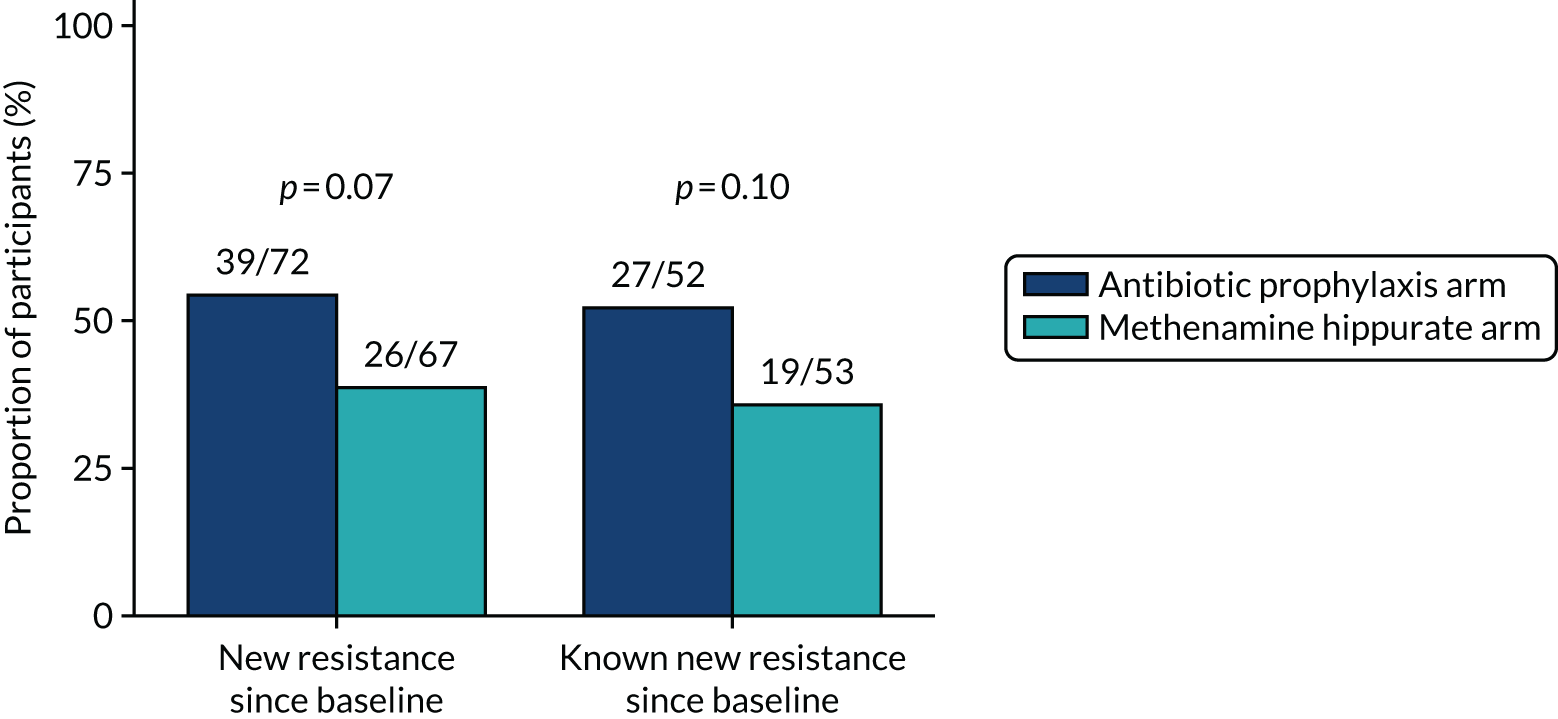
FIGURE 20.
Development of MDR since baseline in E. coli isolated from perineal swabs. MDR since baseline: participants with MDR in at least one post-baseline perineal swab E. coli isolate out of those who did not demonstrate MDR in E. coli at baseline or did not have E. coli isolated in their baseline sample. Known MDR since baseline: participants with MDR in at least one post-baseline perineal swab E. coli isolate out of those who did not demonstrate MDR in E. coli at baseline. Chi-squared p-values.
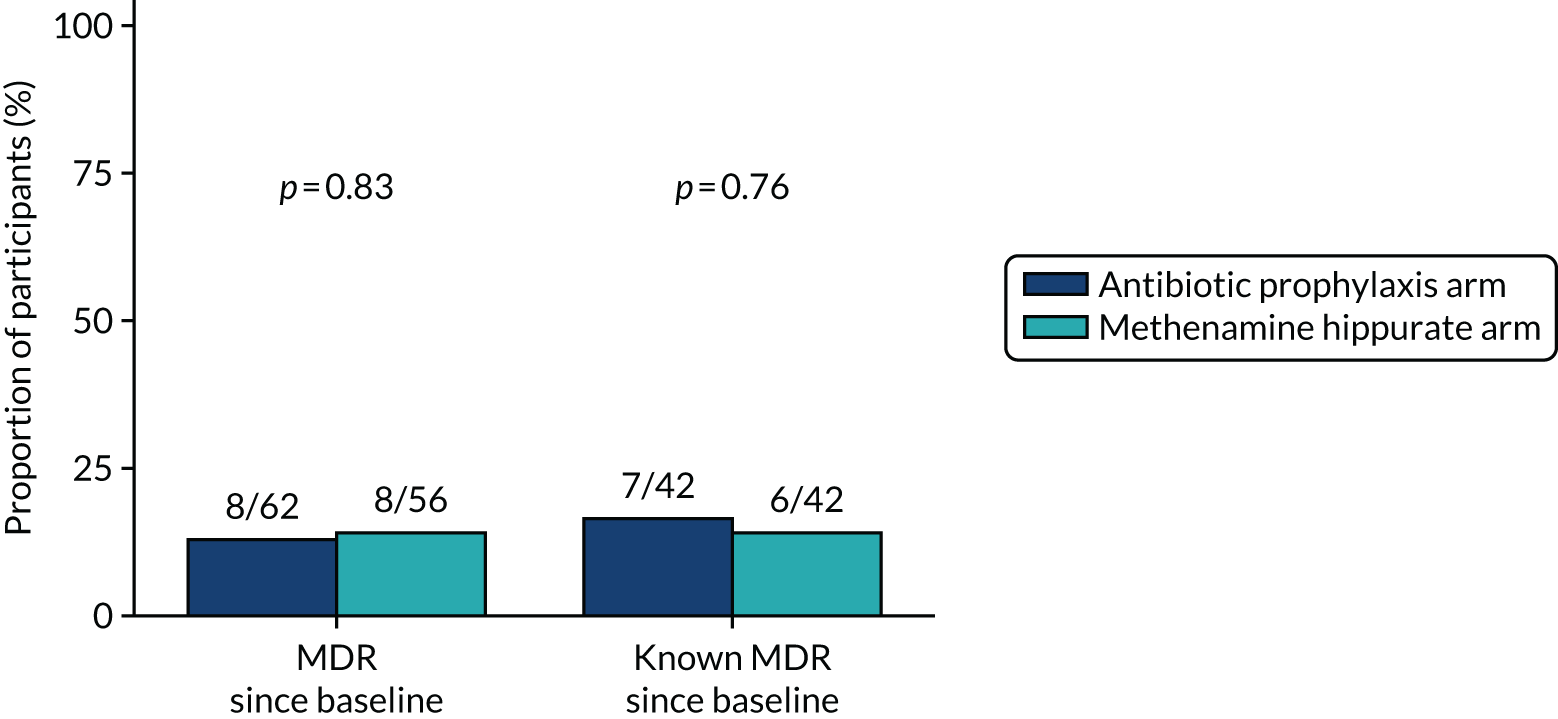
FIGURE 21.
Development of resistance since baseline in E. coli isolated from perineal swabs. (a) Amoxicillin; (b) cefalexin; (c) cefuroxime; (d) ciprofloxacin; (e) co-amoxiclav; (f) co-trimoxazole; (g) nitrofurantoin; and (h) trimethoprim. Resistance since baseline: participants with at least one resistant perineal swab E. coli isolate in a post-baseline sample out of those who demonstrated sensitivity in E. coli at baseline or did not have E. coli isolated in their baseline sample. Known resistance since baseline: participants with at least one resistant perineal swab E. coli isolate in a post-baseline sample out of those who demonstrated sensitivity in E. coli at baseline.
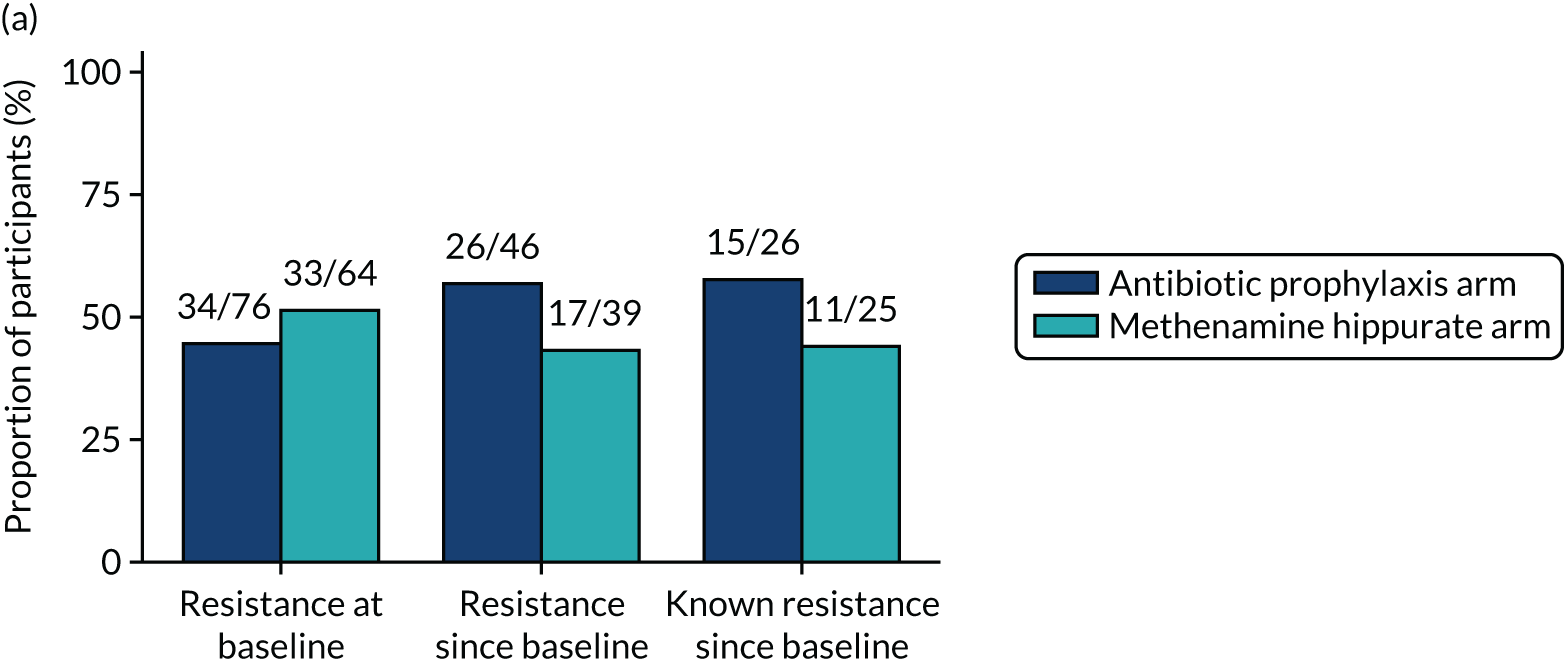
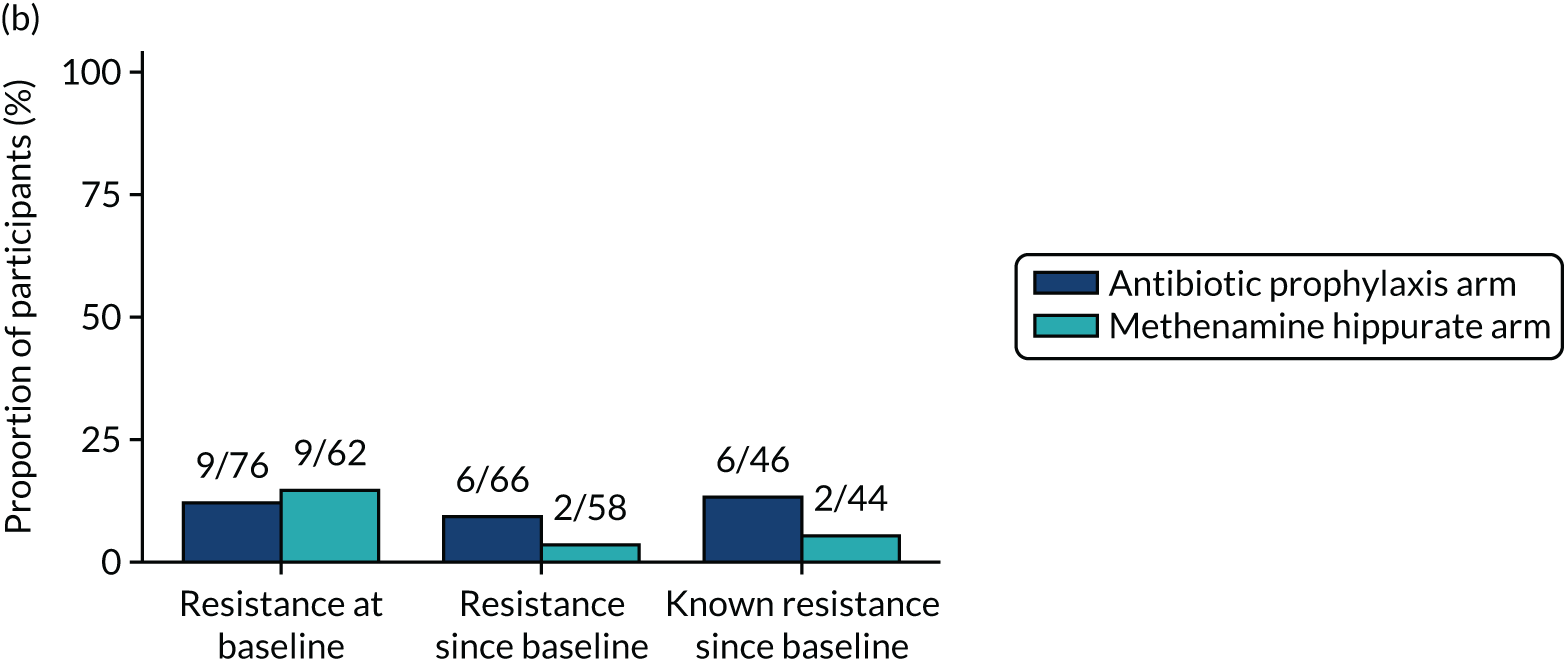
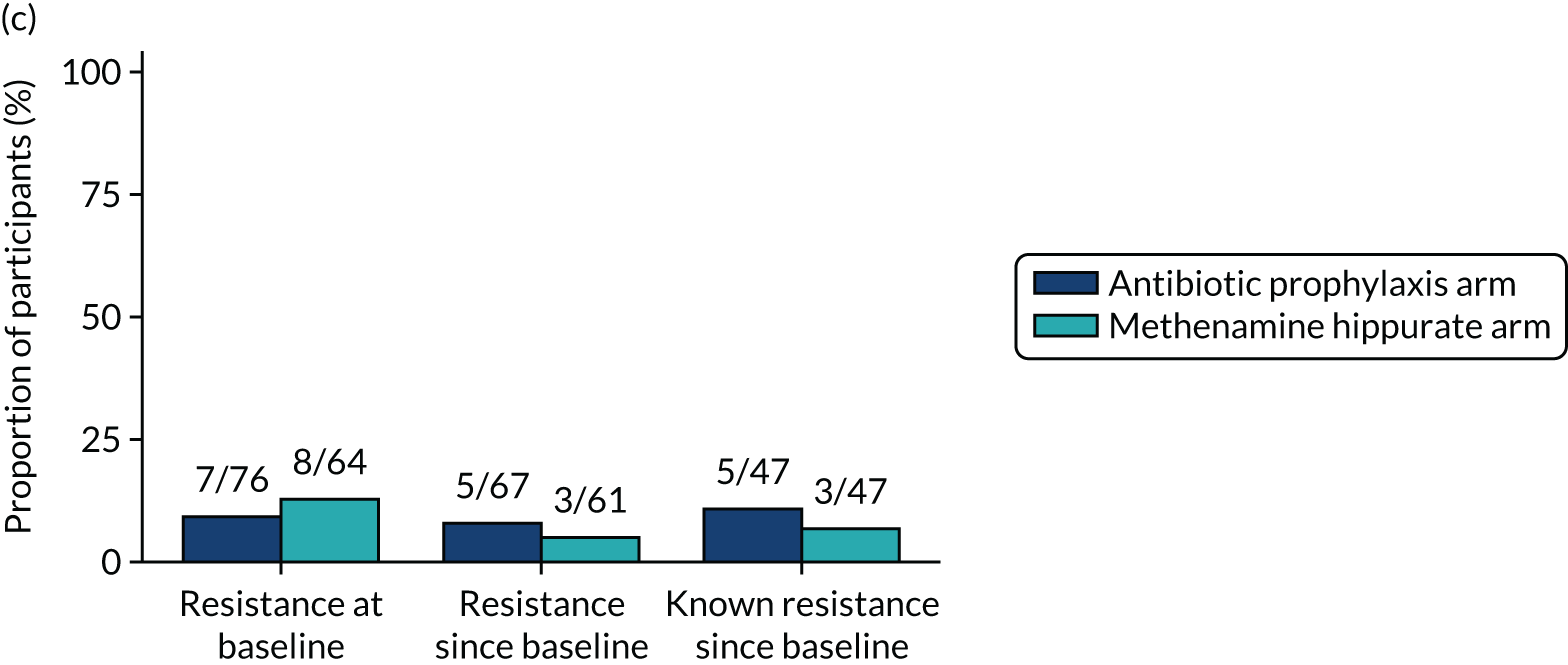
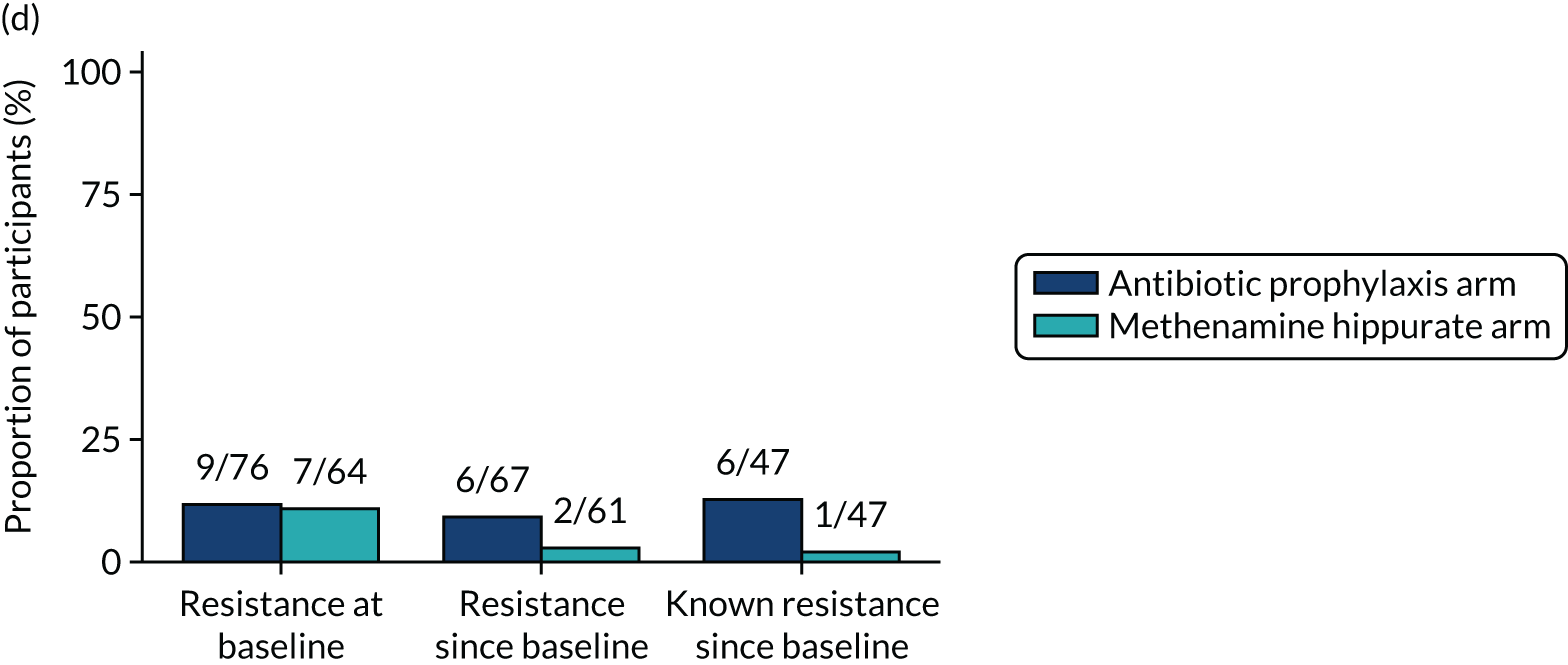
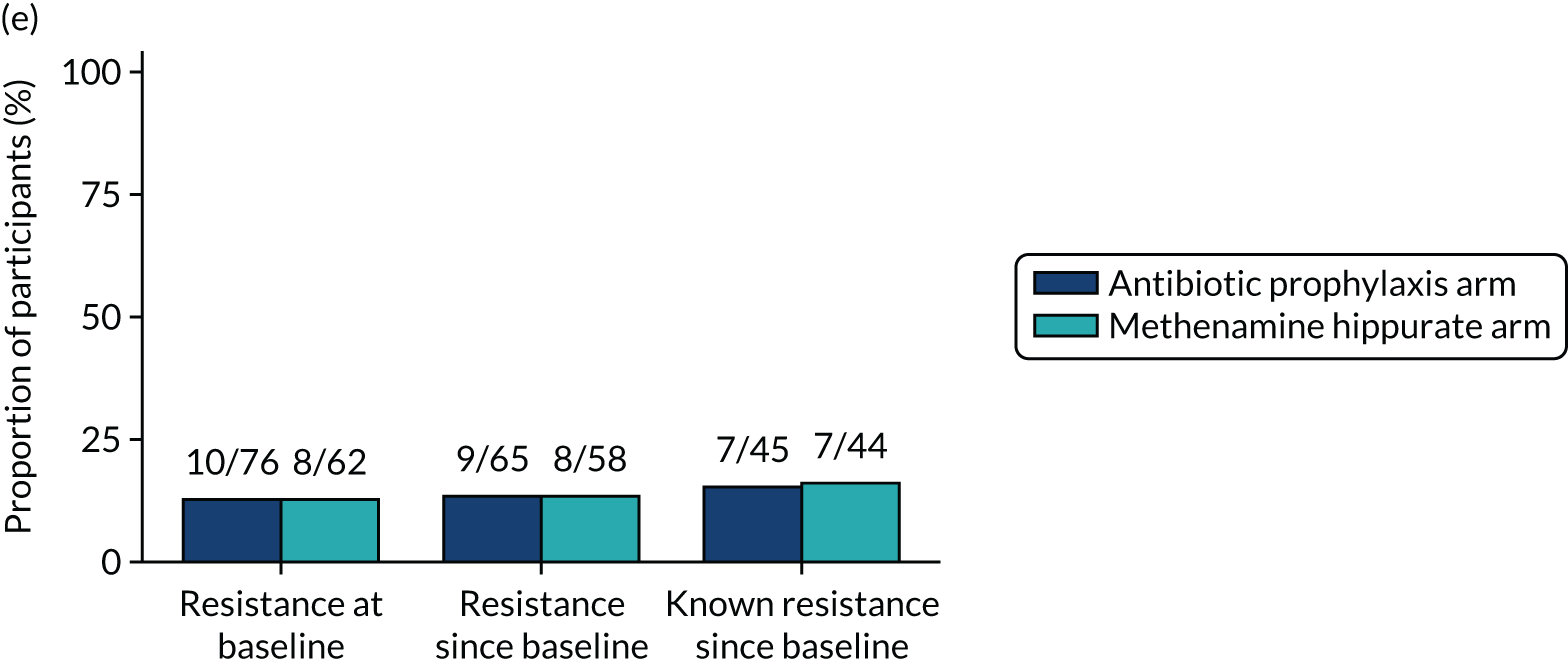
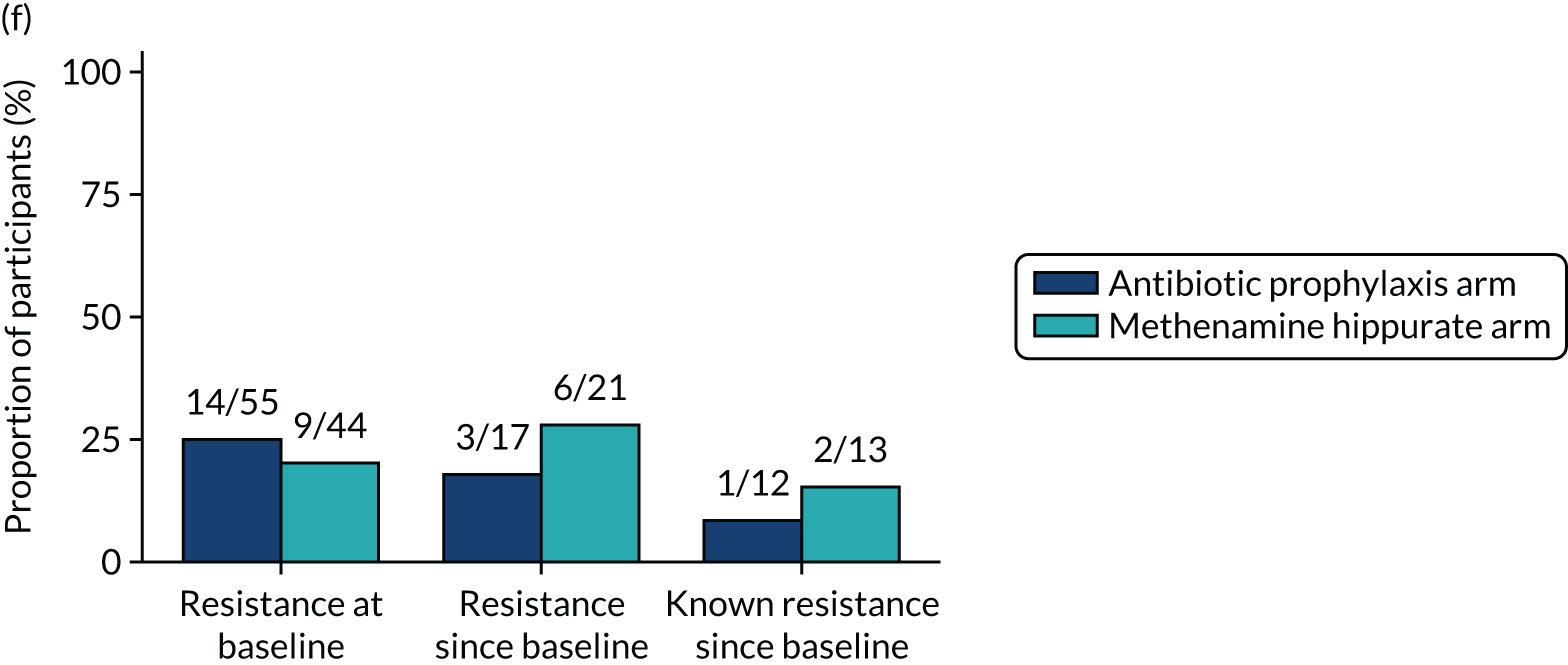
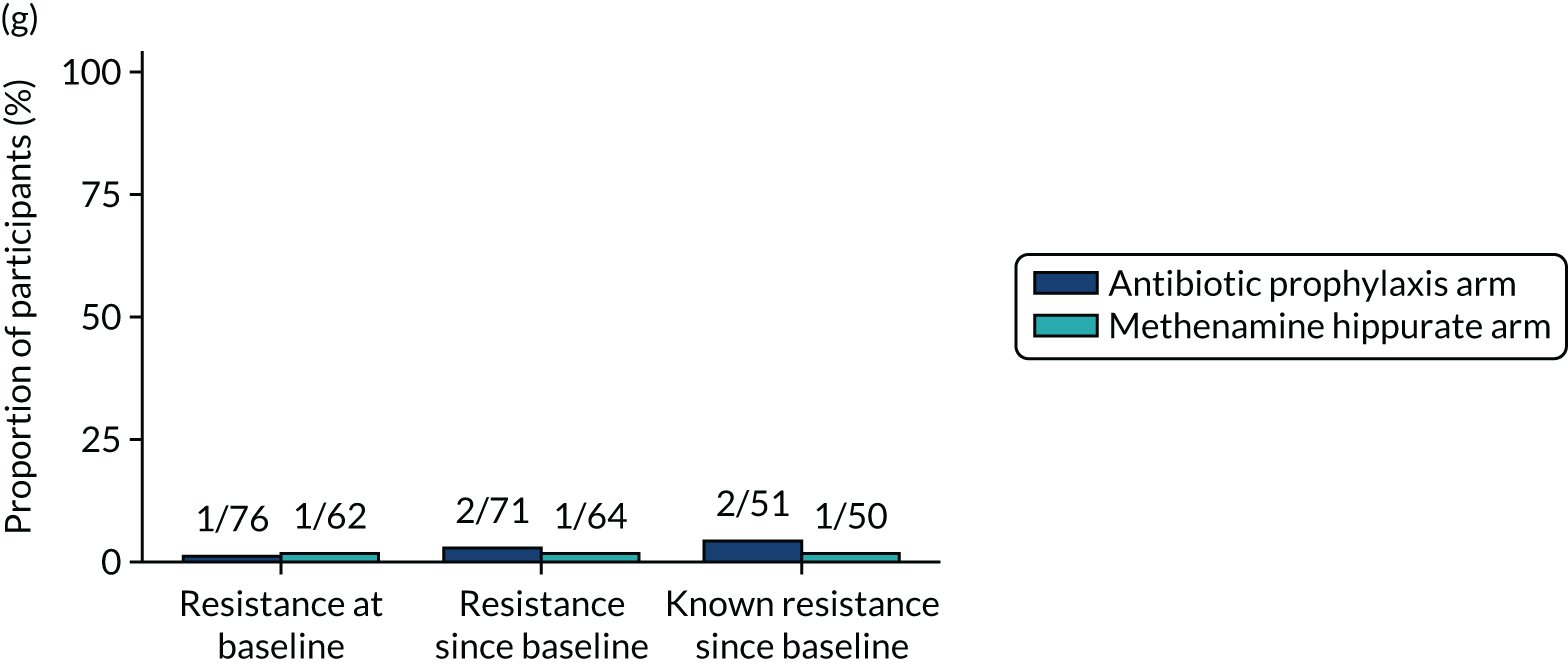
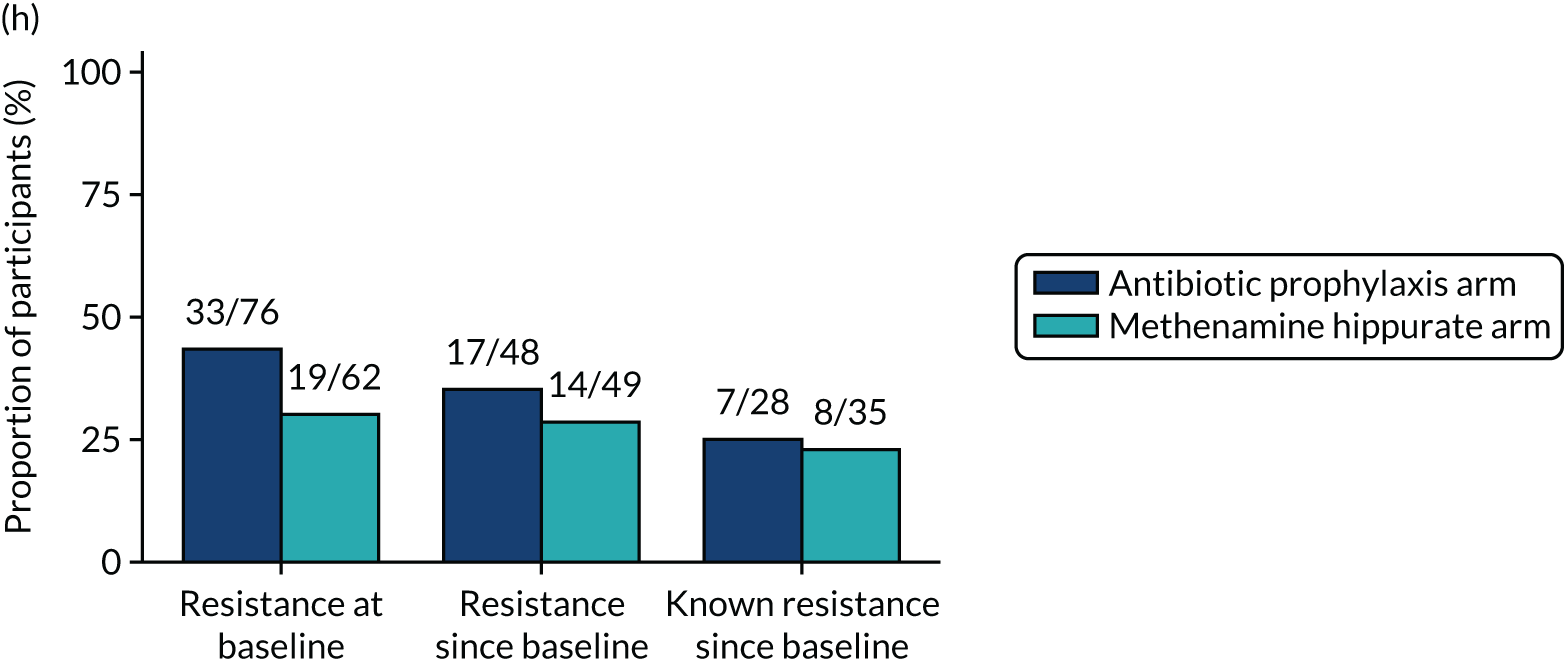
FIGURE 22.
Resistance rates (95% CI) in E. coli isolated from perineal swabs: plotted by antibiotic over the 18-month study period. (a) Amoxicillin; (b) cefalexin; (c) cefuroxime; (d) ciprofloxacin; (e) co-amoxiclav; (f) co-trimoxazole; (g) nitrofurantoin; and (h) trimethoprim.
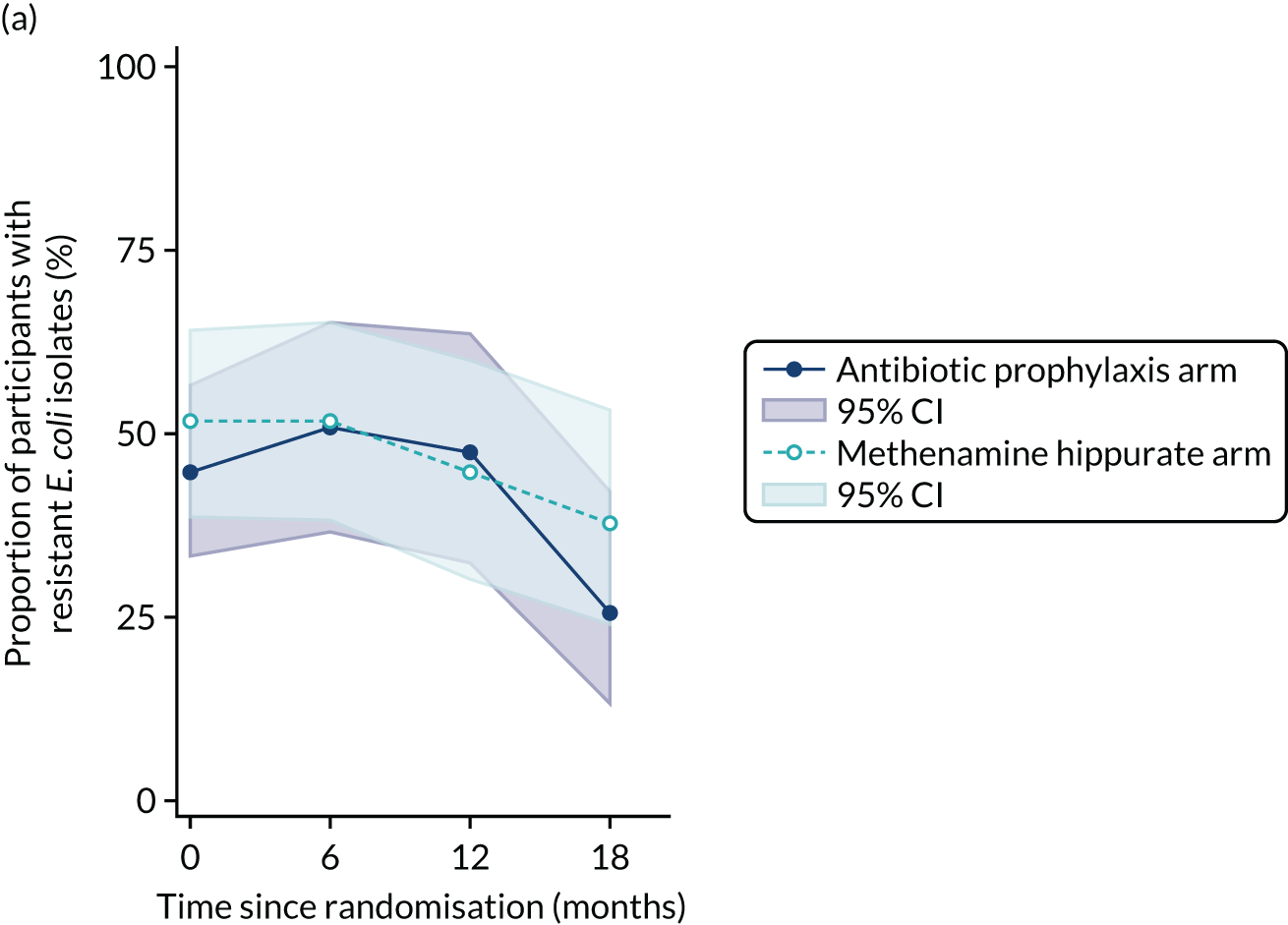
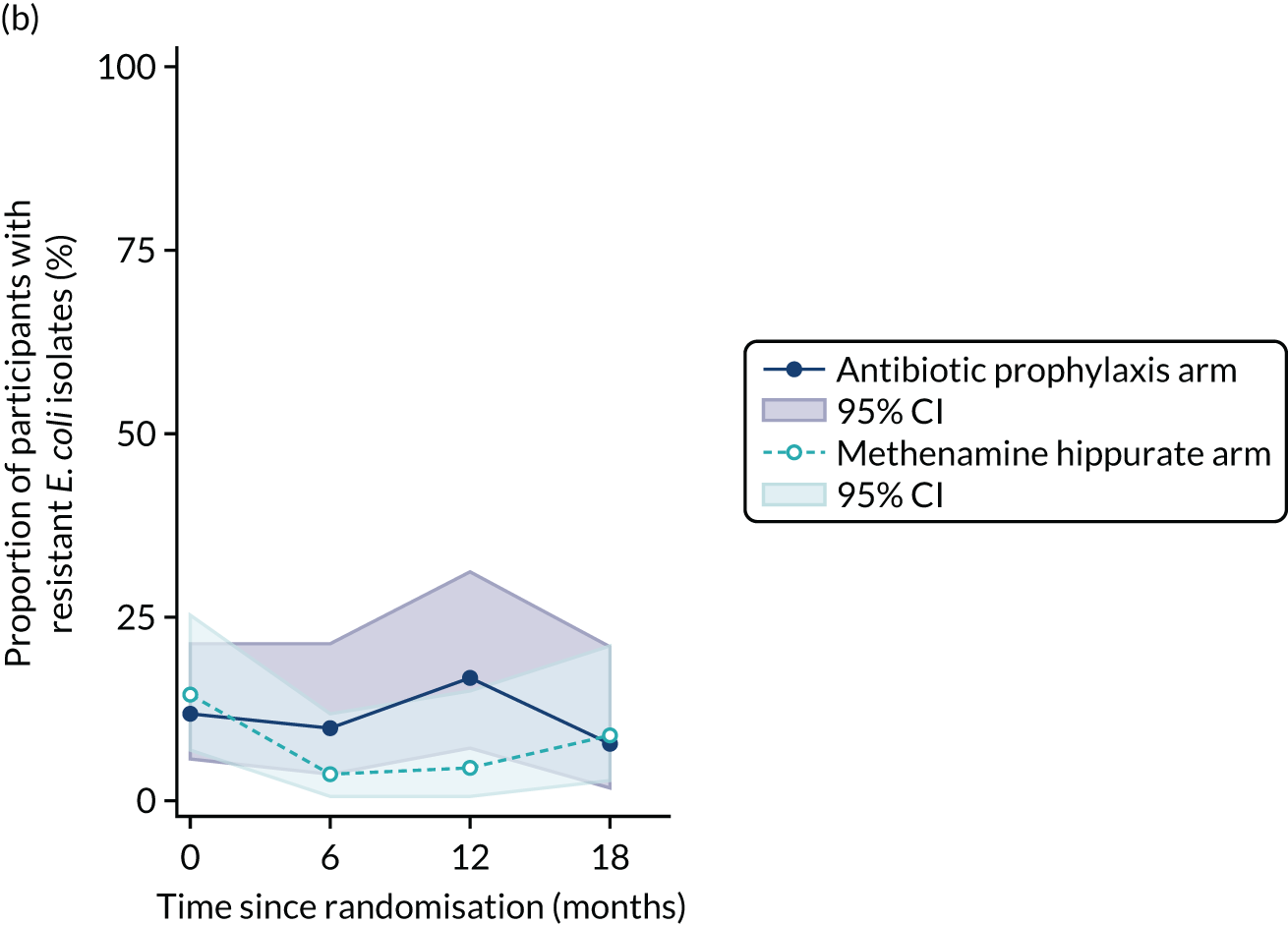
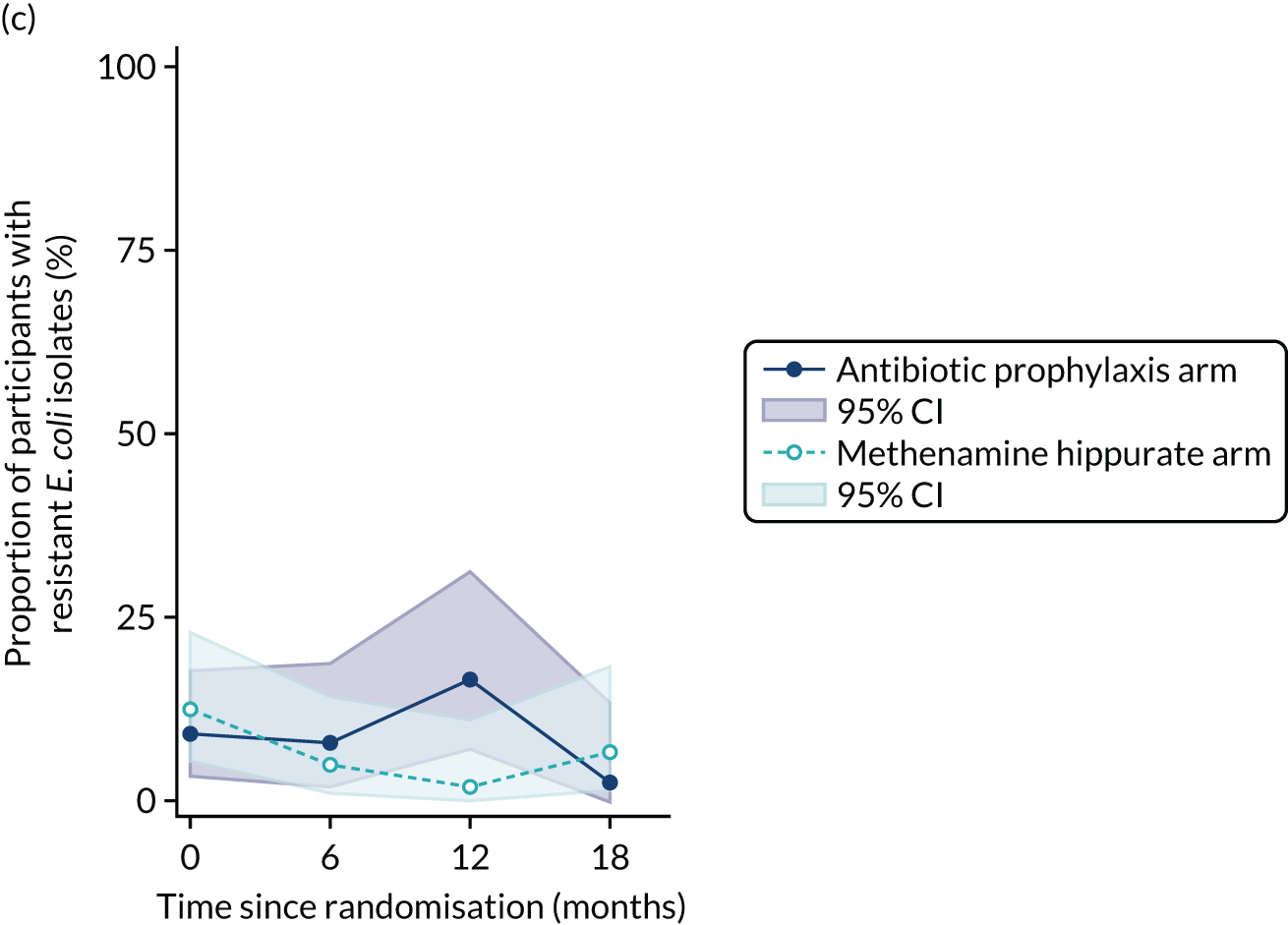
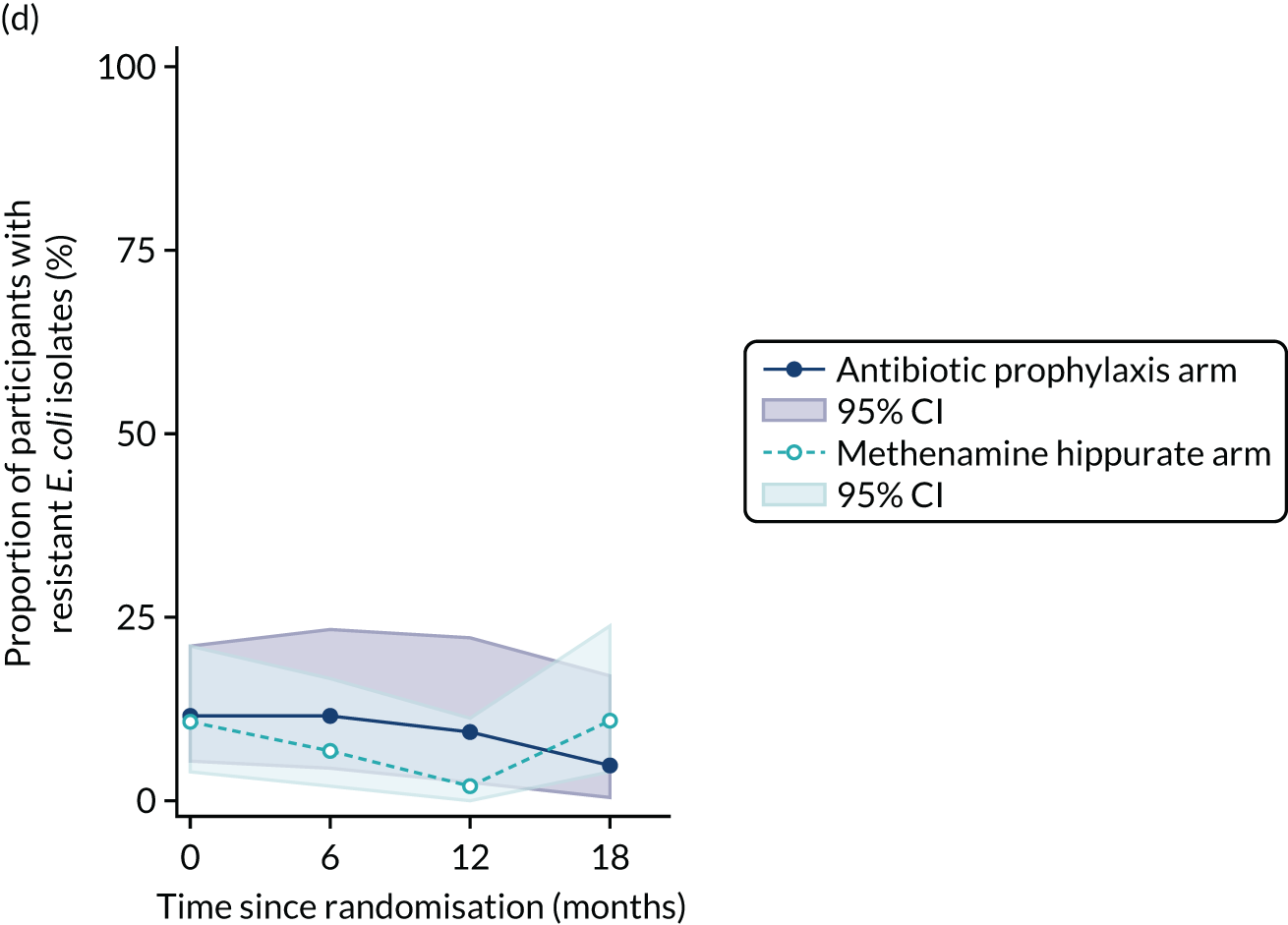
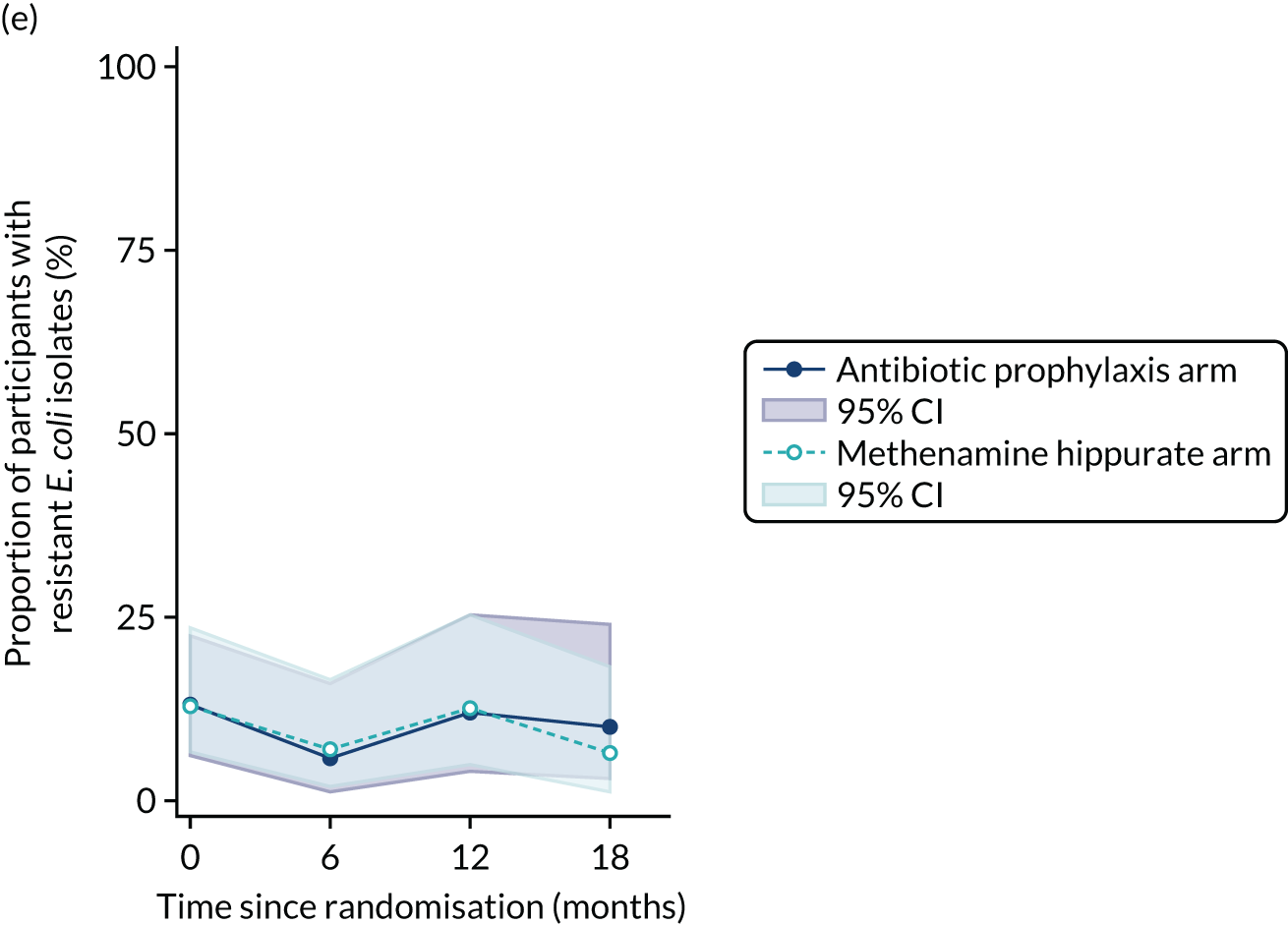
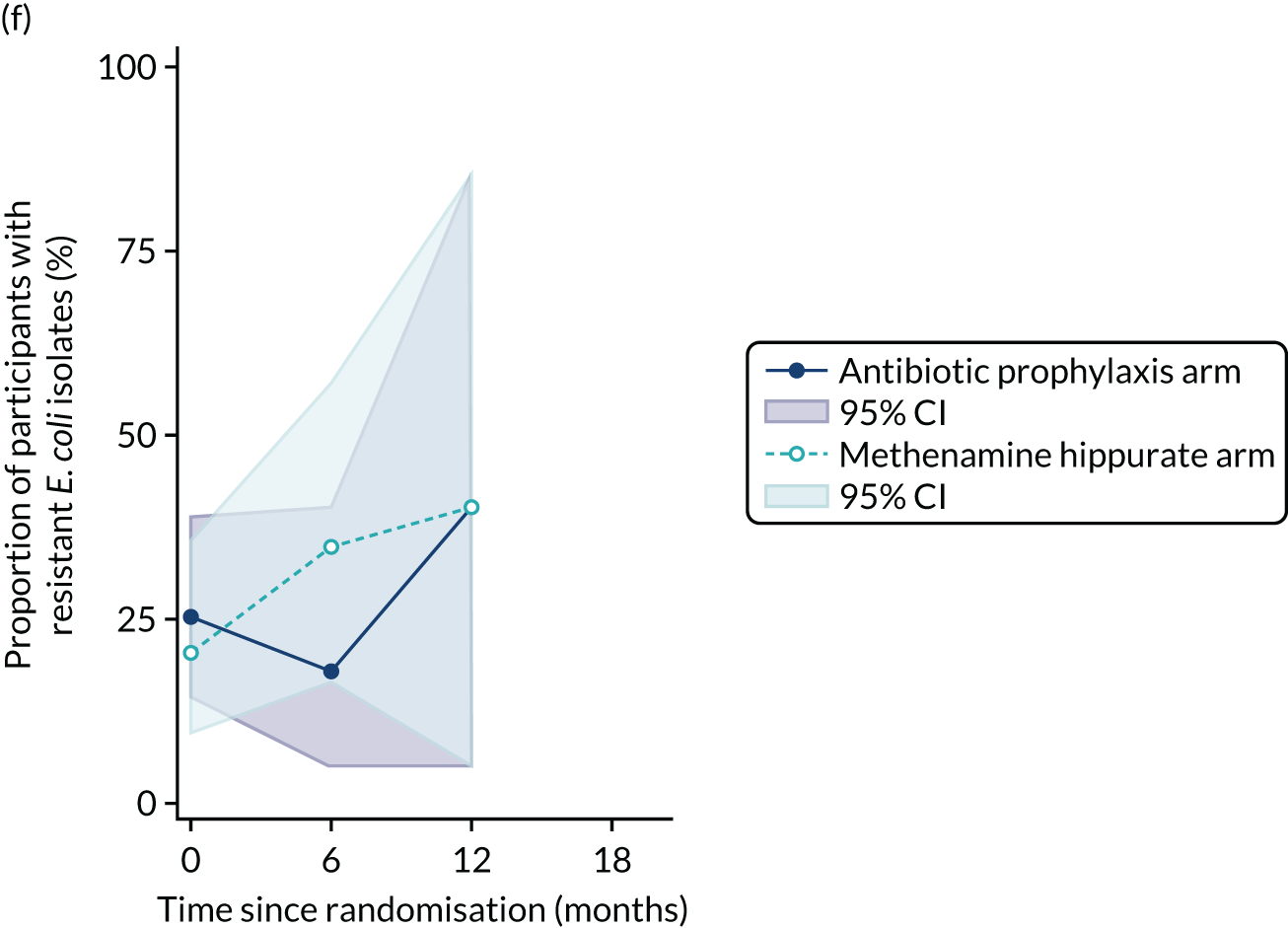
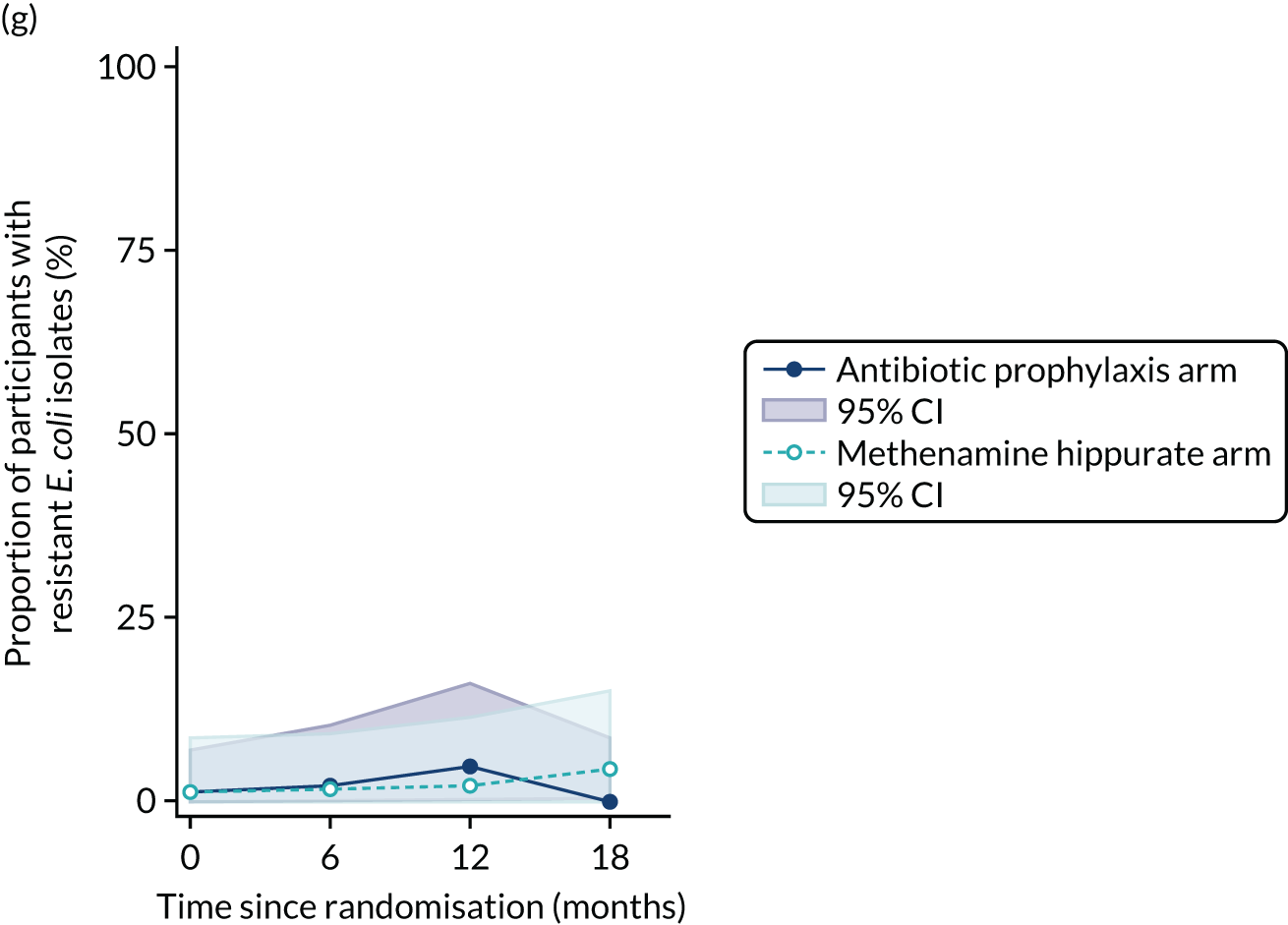
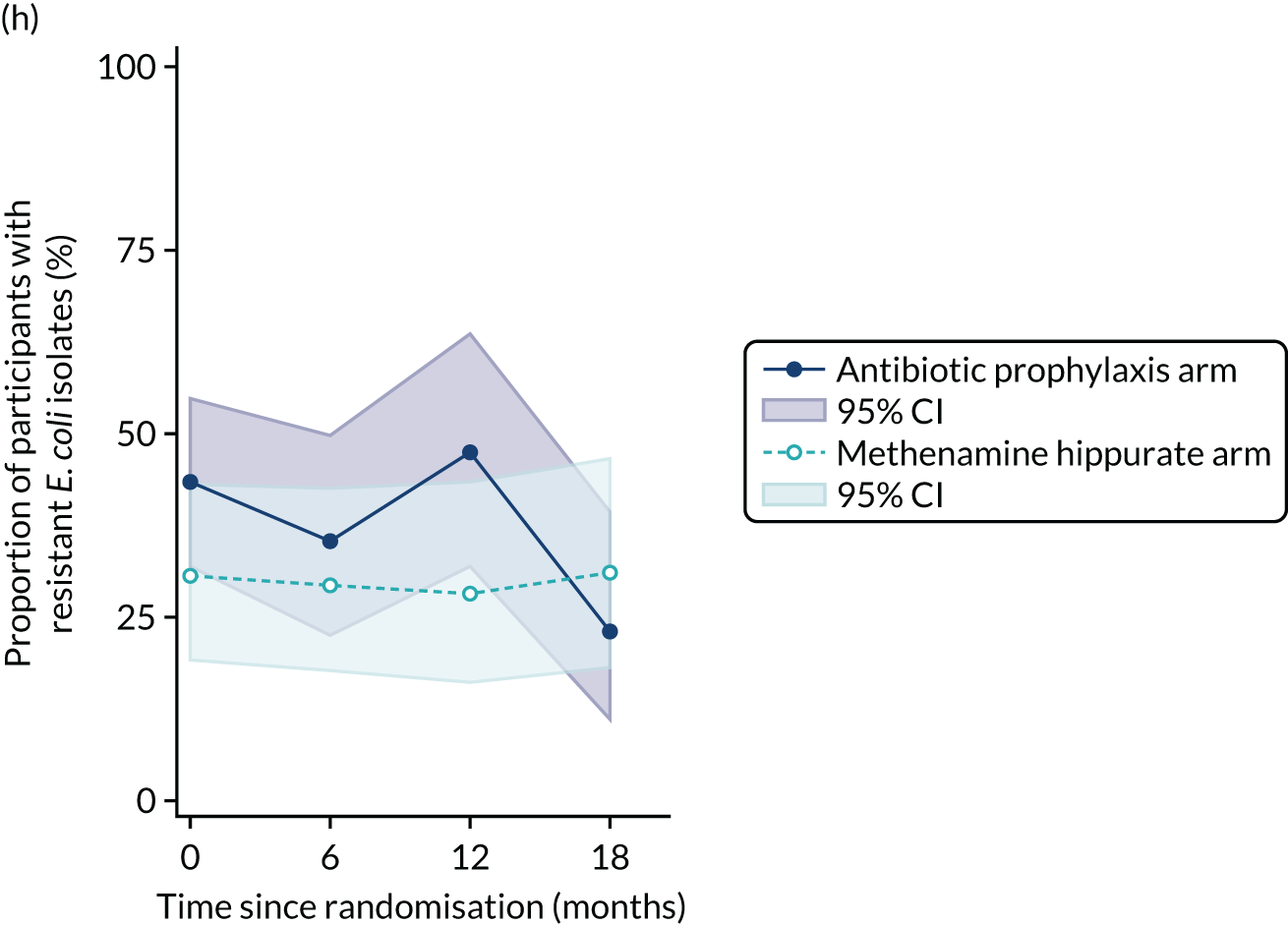
| Antibiotic prophylaxis arm (N = 120) | Methenamine hippurate arm (N = 120) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Month 6 | Month 12 | Month 18 | Baseline | Month 6 | Month 12 | Month 18 | |
| Amoxicillin | ||||||||
| Resistant/tested (%) | 34/76 (44.7) | 26/51 (51.0) | 20/42 (47.6) | 10/39 (25.6) | 33/64 (51.6) | 30/58 (51.7) | 21/47 (44.7) | 17/45 (37.8) |
| 95% CI (%) | 33.3 to 56.6 | 36.6 to 65.2 | 32.0 to 63.6 | 13.0 to 42.1 | 38.7 to 64.2 | 38.2 to 65.0 | 30.2 to 59.9 | 23.8 to 53.5 |
| Cefalexin | ||||||||
| Resistant/tested (%) | 9/76 (11.8) | 5/51 (9.8) | 7/42 (16.7) | 3/39 (7.7) | 9/62 (14.5) | 2/58 (3.4) | 2/46 (4.3) | 4/45 (8.9) |
| 95% CI (%) | 5.6 to 21.3 | 3.3 to 21.4 | 7.0 to 31.4 | 1.6 to 20.9 | 6.9 to 25.8 | 0.4 to 11.9 | 0.5 to 14.8 | 2.5 to 21.2 |
| Cefuroxime | ||||||||
| Resistant/tested (%) | 7/76 (9.2) | 4/51 (7.8) | 7/42 (16.7) | 1/39 (2.6) | 8/64 (12.5) | 3/57 (5.3) | 1/47 (2.1) | 3/45 (6.7) |
| 95% CI (%) | 3.8 to 18.1 | 2.2 to 18.9 | 7.0 to 31.4 | 0.1 to 13.5 | 5.6 to 23.2 | 1.1 to 14.6 | 0.1 to 11.3 | 1.4 to 18.3 |
| Ciprofloxacin | ||||||||
| Resistant/tested (%) | 9/76 (11.8) | 6/51 (11.8) | 4/42 (9.5) | 2/39 (5.1) | 7/64 (10.9) | 4/58 (6.9) | 1/47 (2.1) | 5/45 (11.1) |
| 95% CI (%) | 5.6 to 21.3 | 4.4 to 23.9 | 2.7 to 22.6 | 0.6 to 17.3 | 4.5 to 21.2 | 1.9 to 16.7 | 0.1 to 11.3 | 3.7 to 24.1 |
| Co-amoxiclav | ||||||||
| Resistant/tested (%) | 10/76 (13.2) | 3/51 (5.9) | 5/42 (11.9) | 4/39 (10.3) | 8/62 (12.9) | 4/58 (6.9) | 6/47 (12.8) | 3/45 (6.7) |
| 95% CI (%) | 6.5 to 22.9 | 1.2 to 16.2 | 4.0 to 25.6 | 2.9 to 24.2 | 5.7 to 23.9 | 1.9 to 16.7 | 4.8 to 25.7 | 1.4 to 18.3 |
| Co-trimoxazole | ||||||||
| Resistant/tested (%) | 14/55 (25.5) | 4/22 (18.2) | 2/5 (40.0) | 9/44 (20.5) | 8/23 (34.8) | 2/5 (40.0) | ||
| 95% CI (%) | 14.7 to 39.0 | 5.2 to 40.3 | 5.3 to 85.3 | 9.8 to 35.3 | 16.4 to 57.3 | 5.3 to 85.3 | ||
| Nitrofurantoin | ||||||||
| Resistant/tested (%) | 1/76 (1.3) | 1/51 (2.0) | 2/42 (4.8) | 0/39 (0.0) | 1/62 (1.6) | 1/58 (1.7) | 1/46 (2.2) | 2/45 (4.4) |
| 95% CI (%) | 0.0 to 7.1 | 0.0 to 10.4 | 0.6 to 16.2 | 0.0 to 9.0 | 0.0 to 8.7 | 0.0 to 9.2 | 0.1 to 11.5 | 0.5 to 15.1 |
| Trimethoprim | ||||||||
| Resistant/tested (%) | 33/76 (43.4) | 18/51 (35.3) | 20/42 (47.6) | 9/39 (23.1) | 19/62 (30.6) | 17/58 (29.3) | 13/46 (28.3) | 14/45 (31.1) |
| 95% CI (%) | 32.1 to 55.3 | 22.4 to 49.9 | 32.0 to 63.6 | 11.1 to 39.3 | 19.6 to 43.7 | 18.1 to 42.7 | 16.0 to 43.5 | 18.2 to 46.6 |
FIGURE 23.
Cumulative resistance rates to individual antibiotics (95% CI) over time in E. coli isolated from symptomatic urine samples. (a) Amoxicillin; (b) cefalexin; (c) cefuroxime; (d) ciprofloxacin; (e) co-amoxiclav; (f) co-trimoxazole; (g) nitrofurantoin; and (h) trimethoprim.
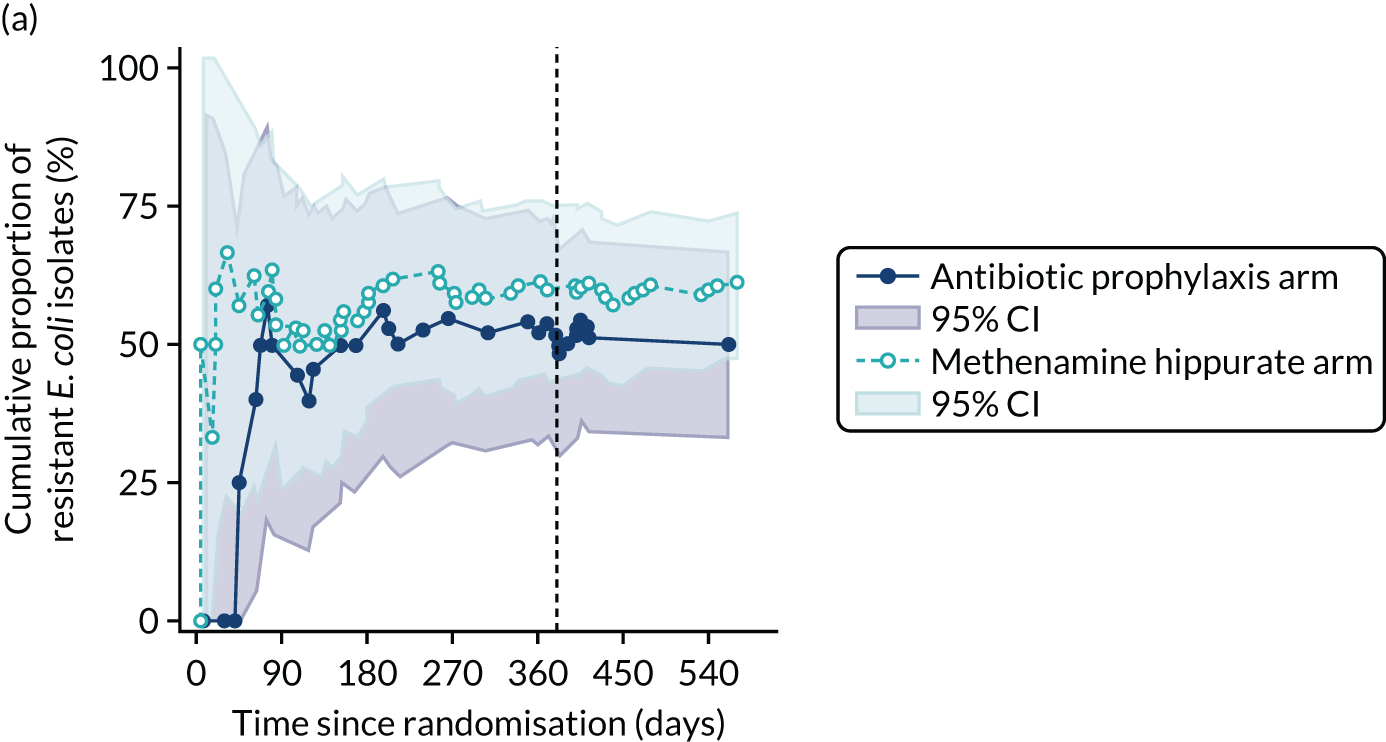
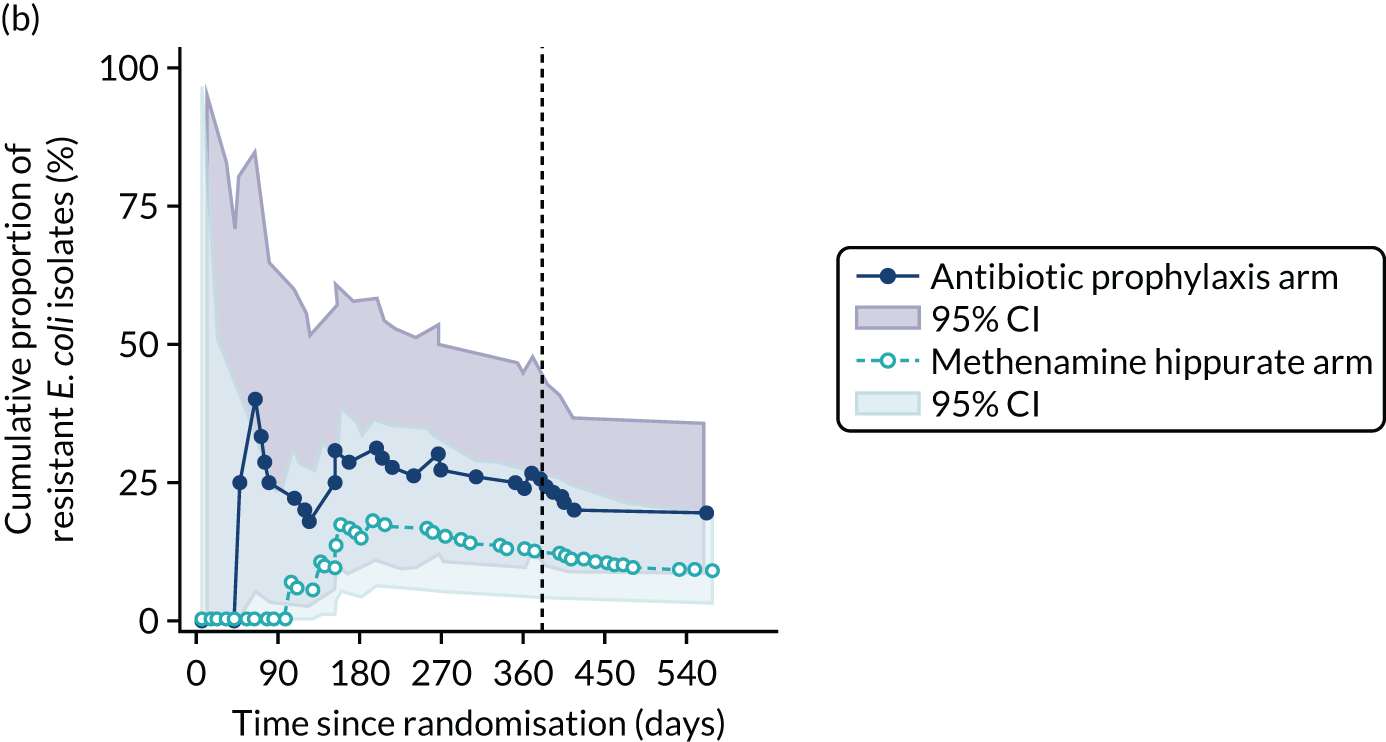
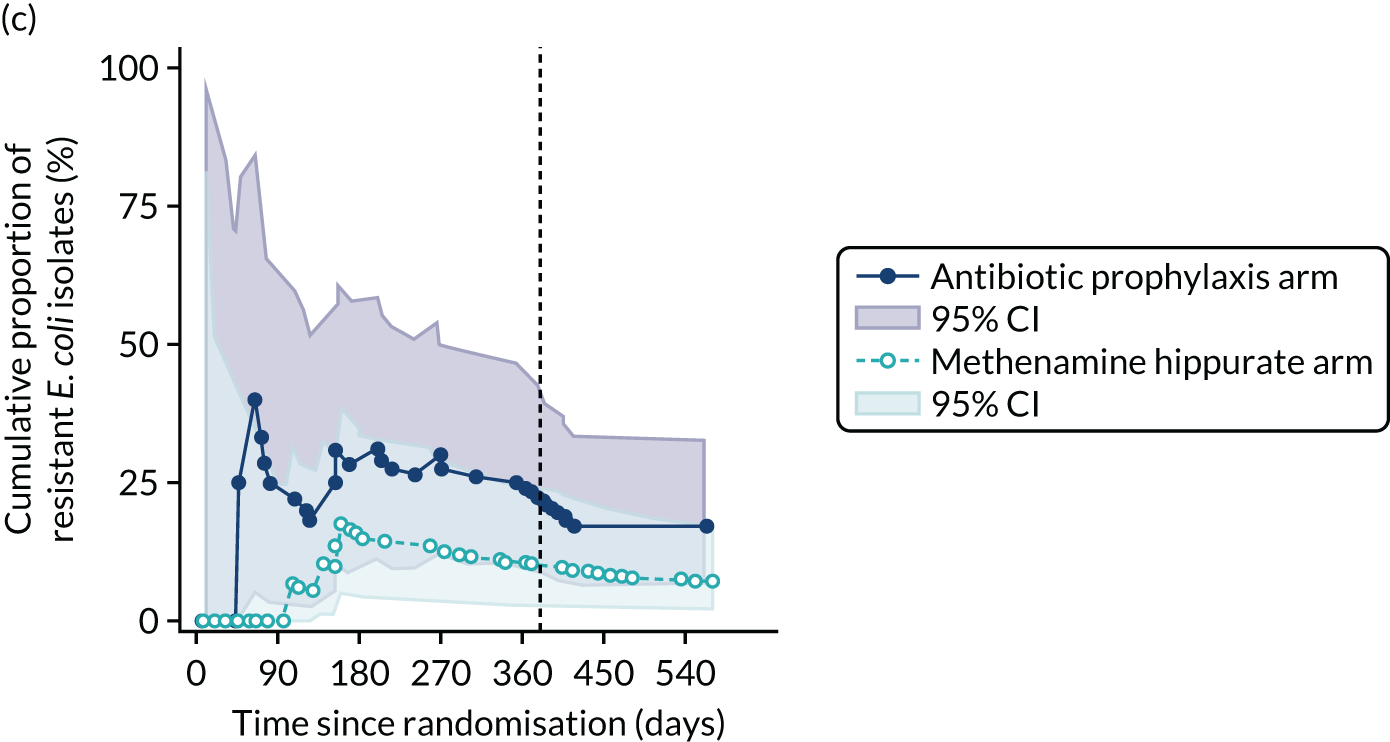
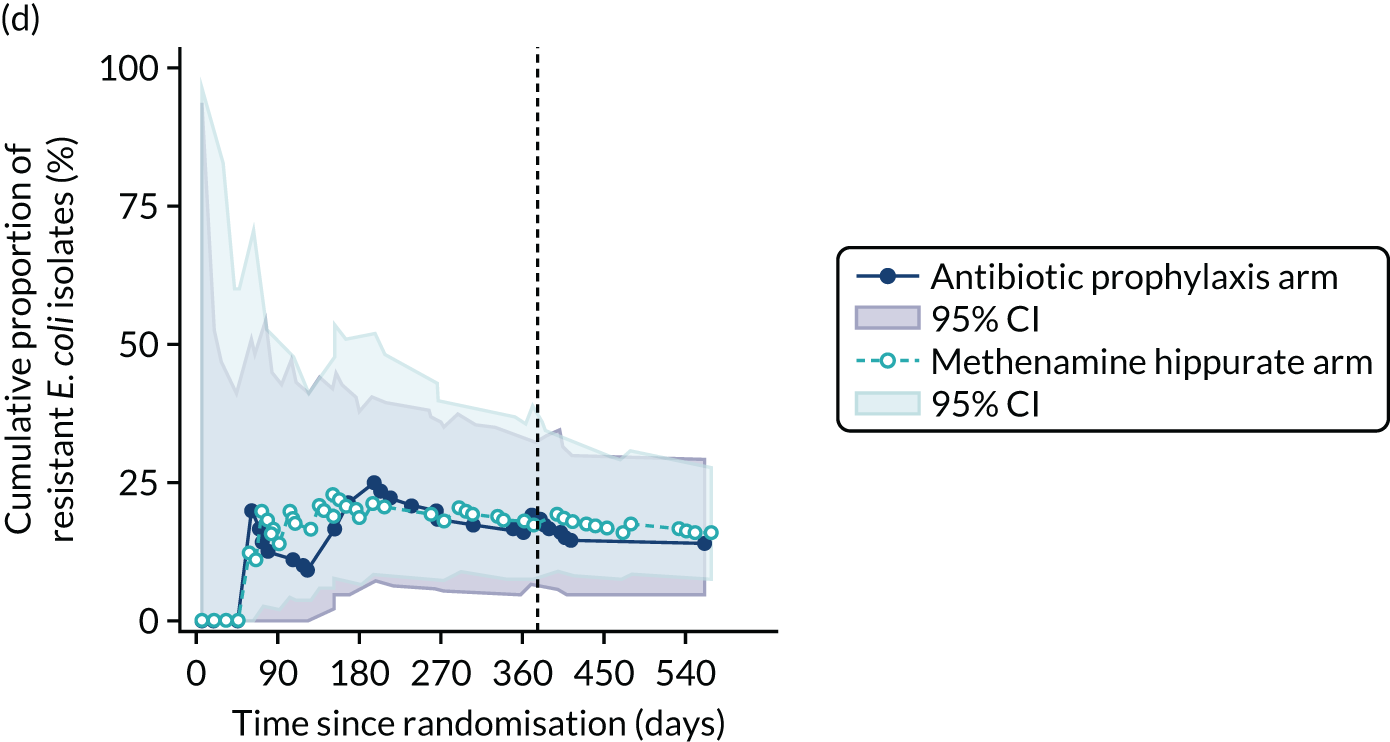
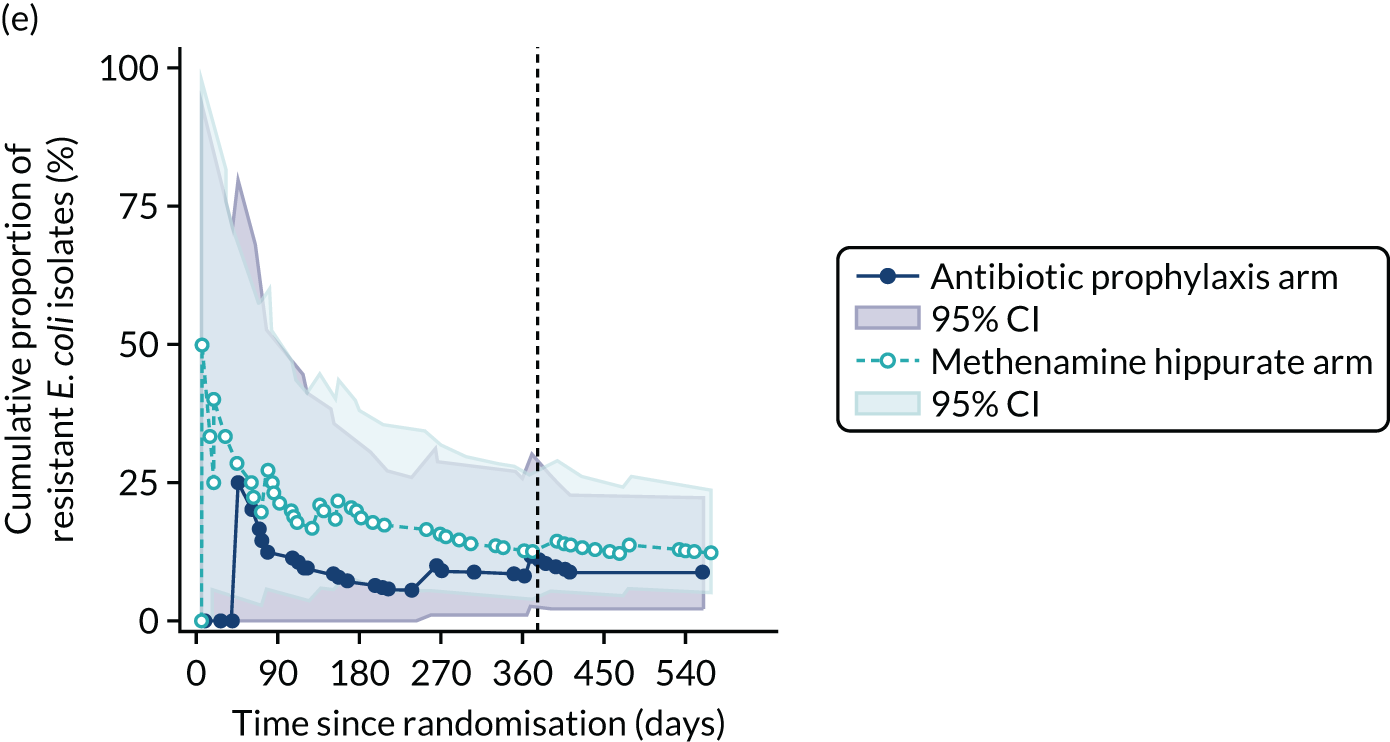
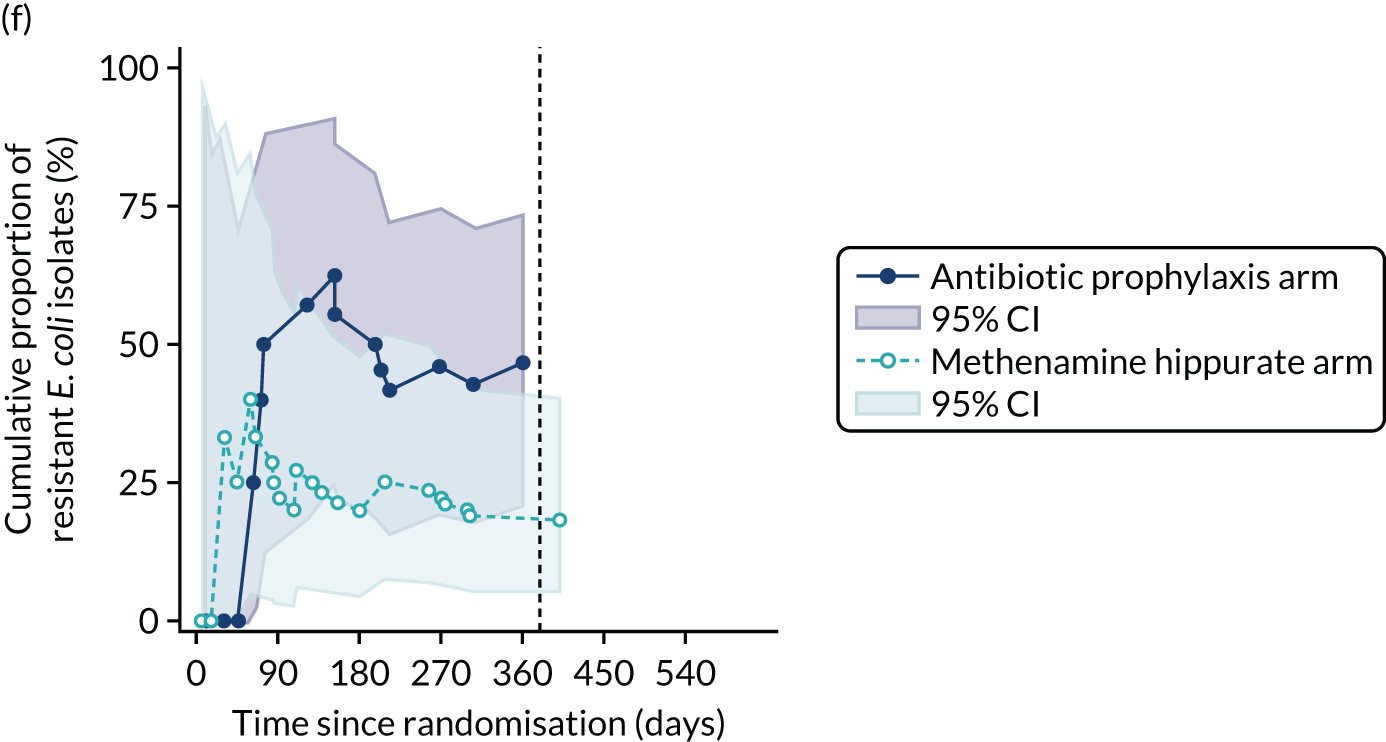
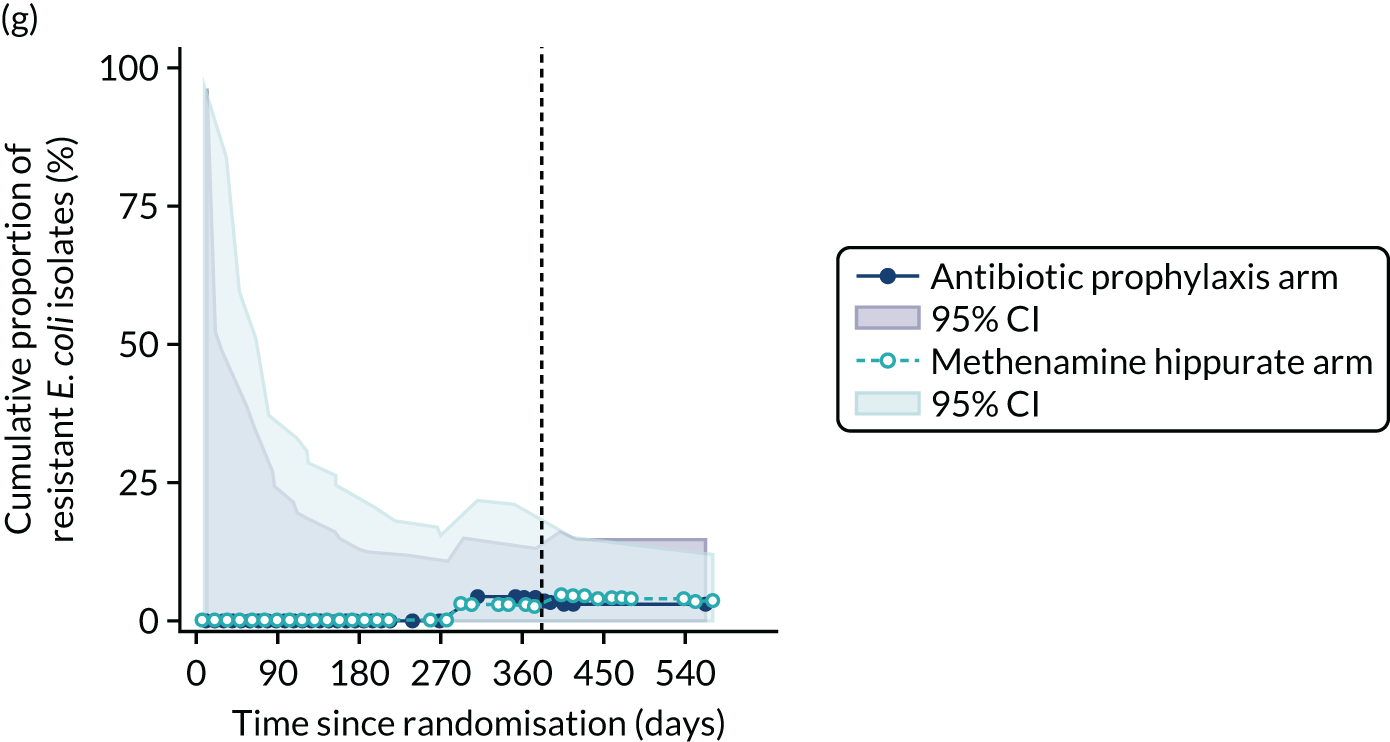
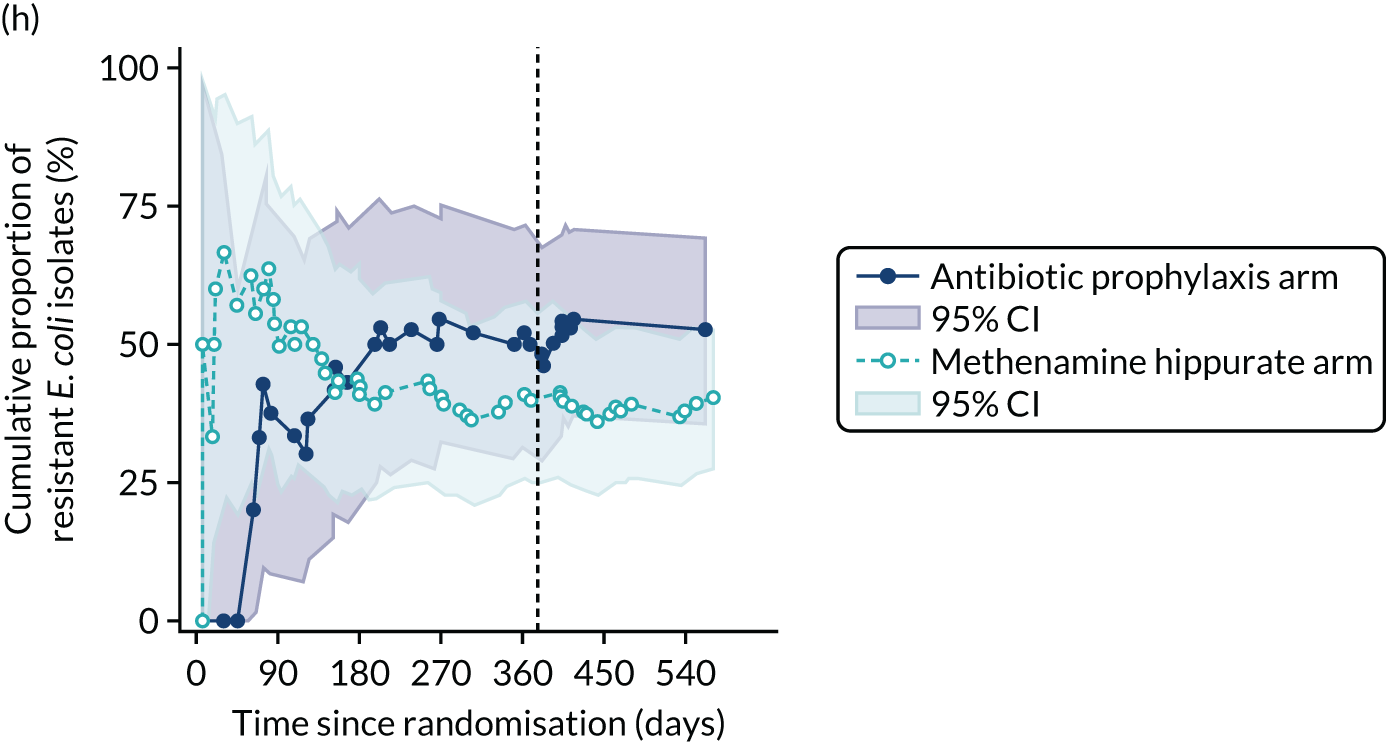
FIGURE 24.
Any resistance (per sample) in E. coli isolated from symptomatic urine samples in the 12-month treatment period and 6-month follow-up period. Fisher’s exact p-values.
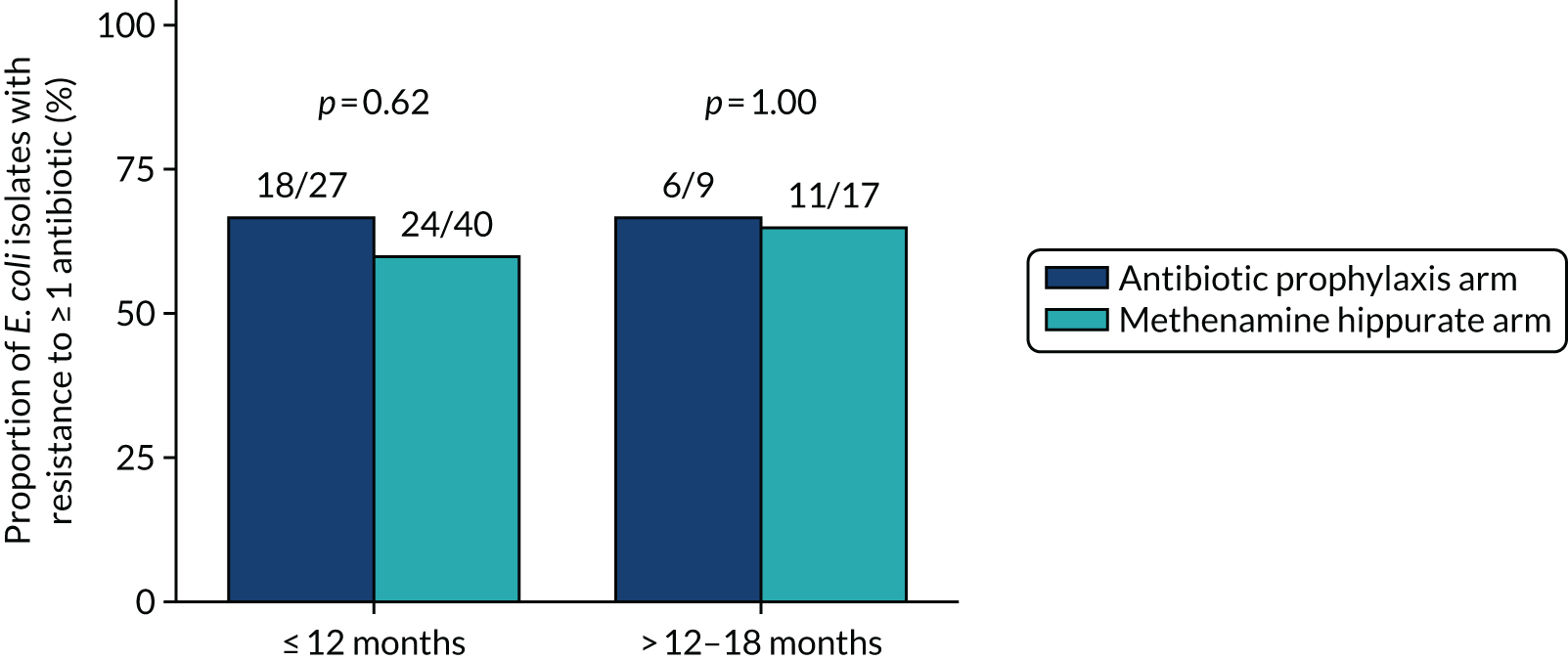
FIGURE 25.
Any MDR (per sample) in E. coli isolated from symptomatic urine samples in the 12-month treatment period and 6-month follow-up period. Fisher’s exact p-values.
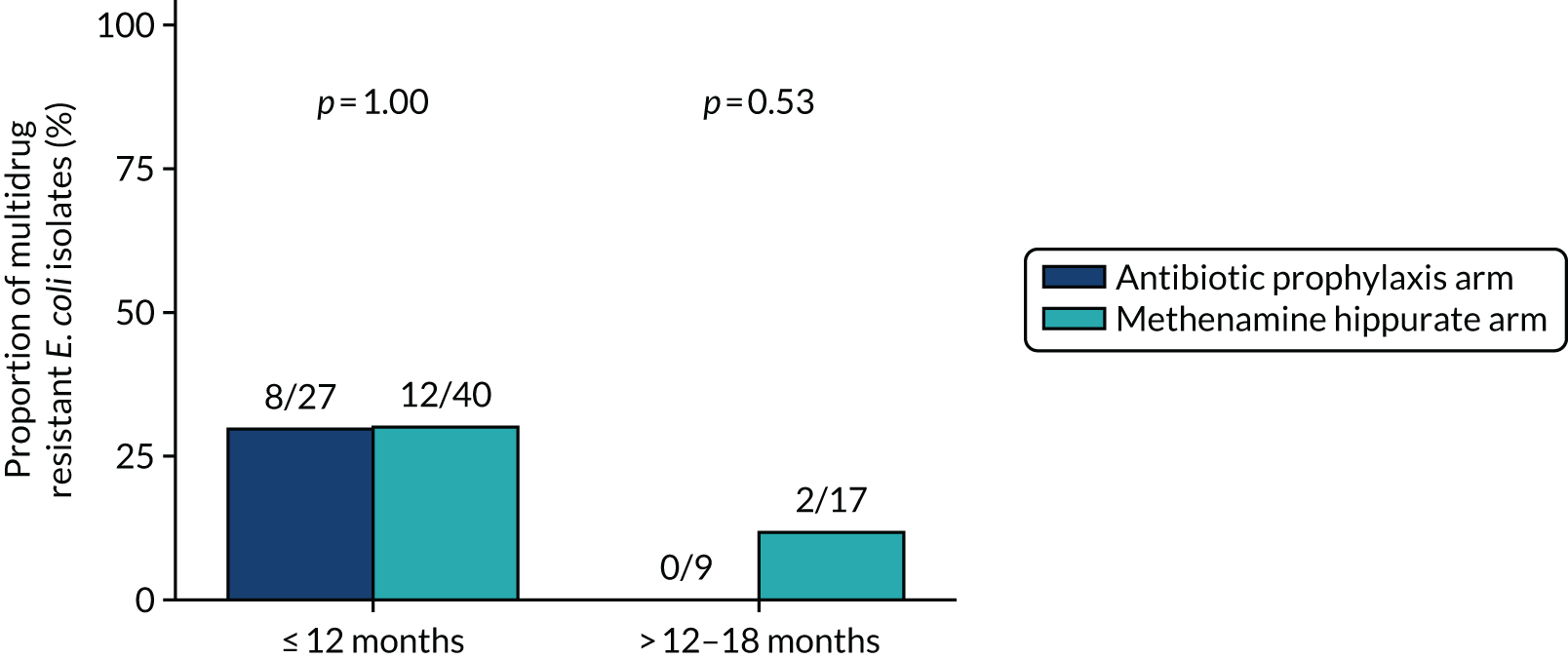
FIGURE 26.
Development of resistance since baseline in E. coli isolated from any urine sample. (a) Amoxicillin; (b) cefalexin; (c) cefuroxime; (d) ciprofloxacin; (e) co-amoxiclav; (f) co-trimoxazole; (g) nitrofurantoin; (h) trimethoprim; and (i) MDR. Resistance since baseline: participants with at least one resistant E. coli isolate in a post-baseline urine sample out of those who demonstrated sensitivity in E. coli isolated from their baseline urine sample or did not have E. coli isolated in their baseline urine sample. Known resistance since baseline: participants with at least one resistant E. coli isolate in a post-baseline urine sample out of those who demonstrated sensitivity in E. coli isolated from their baseline urine sample.
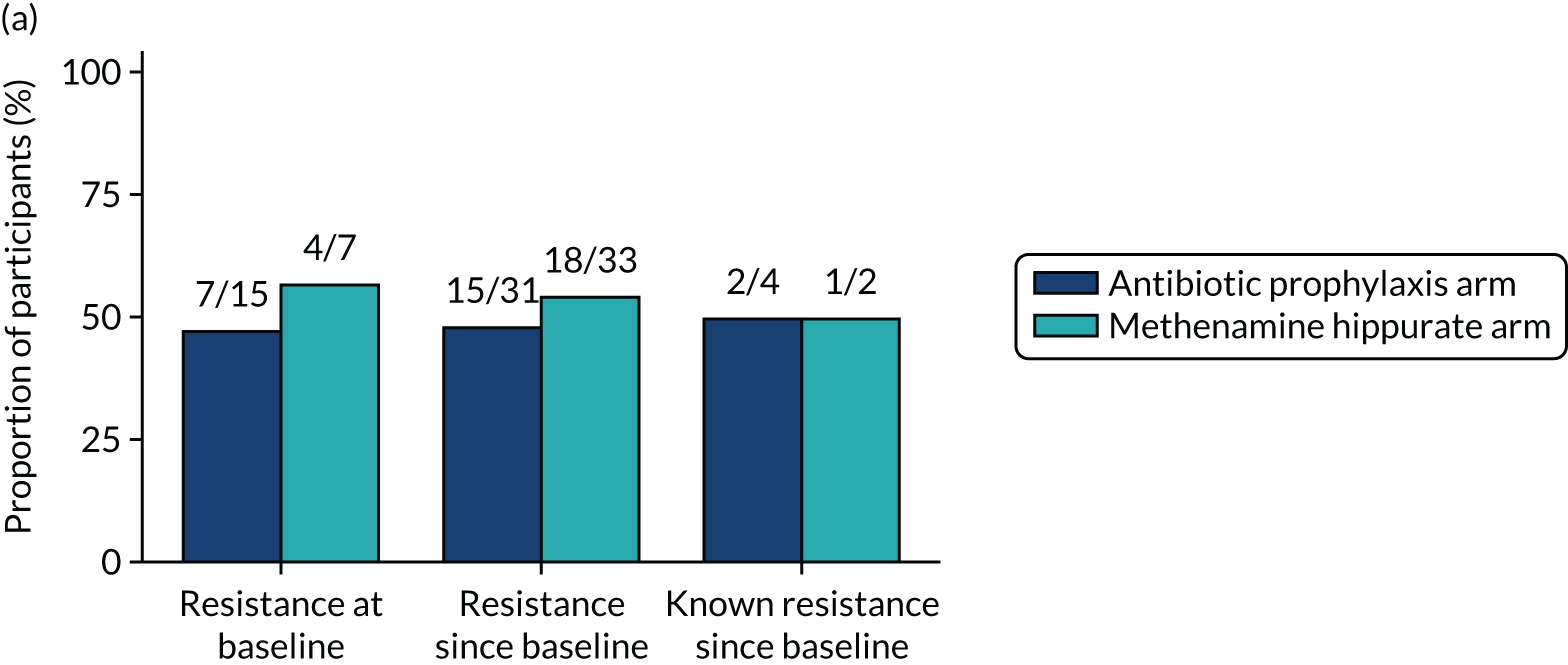
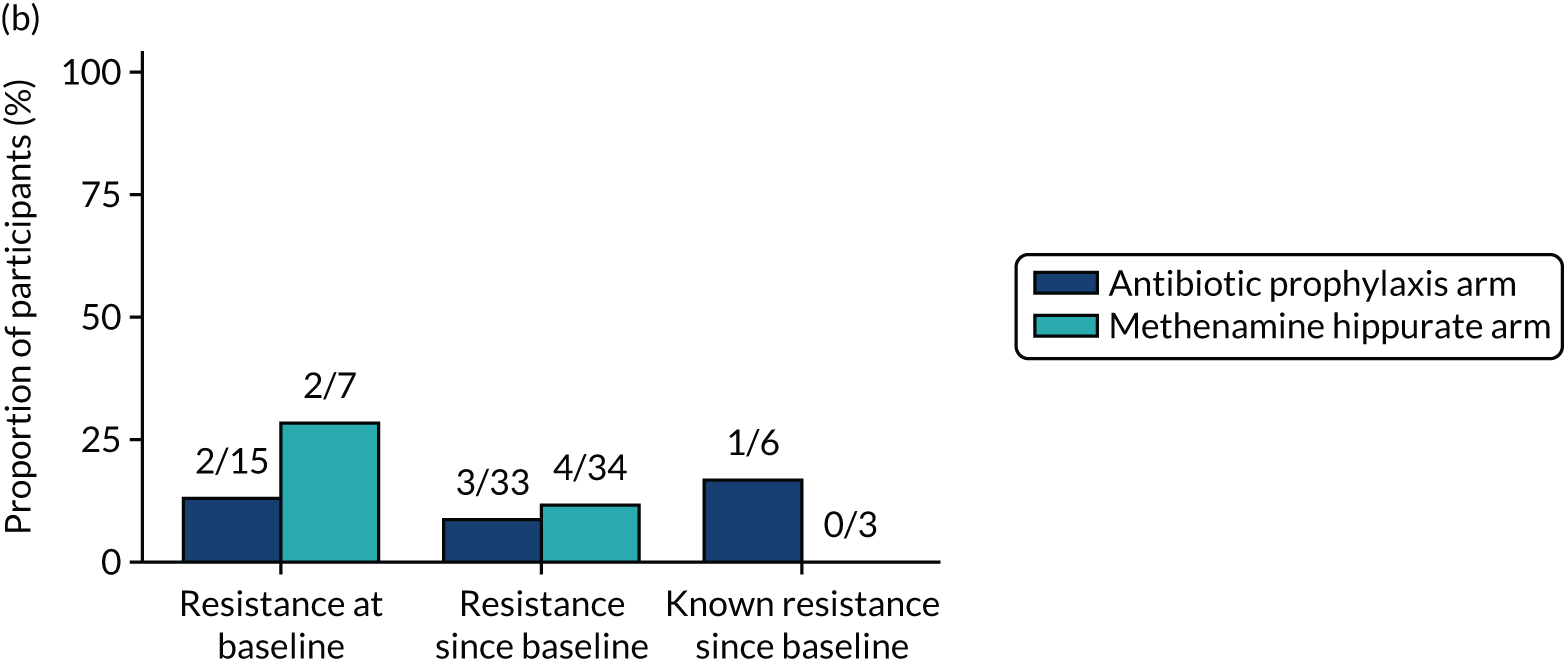
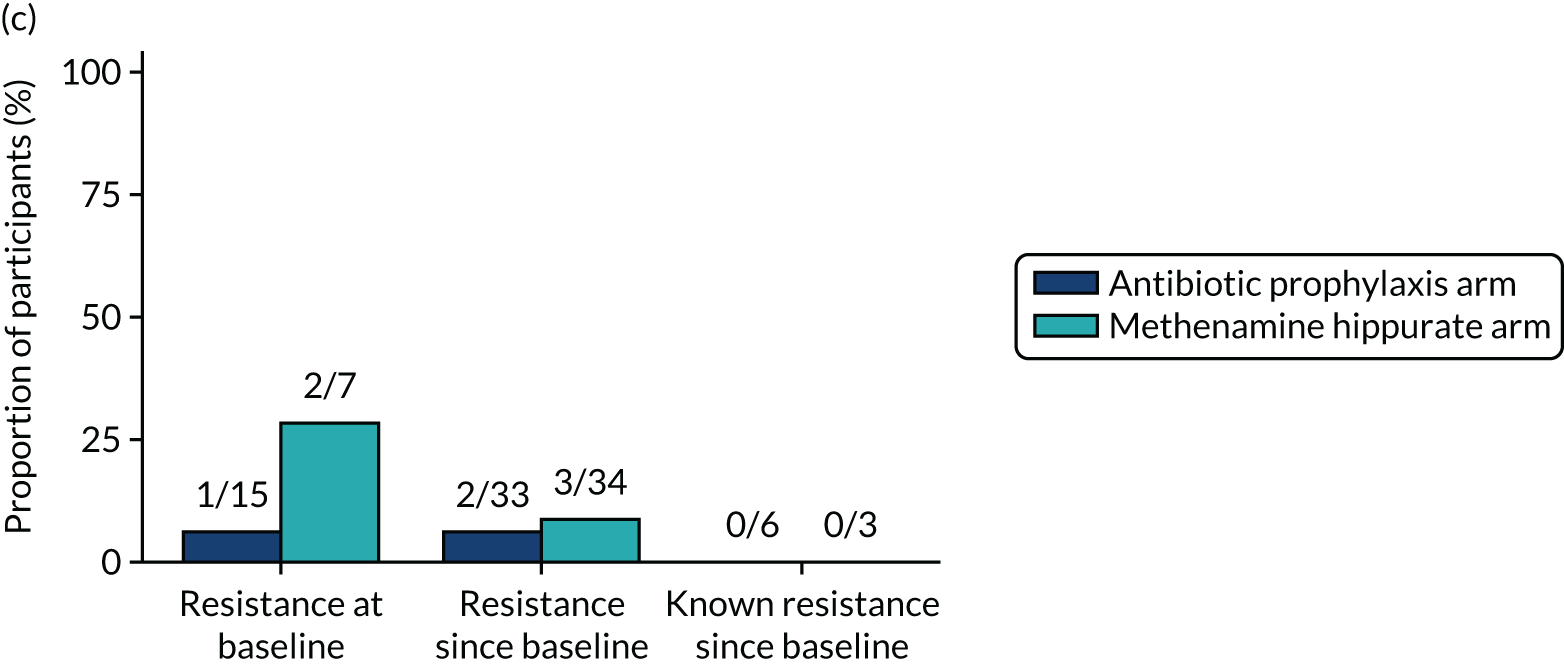
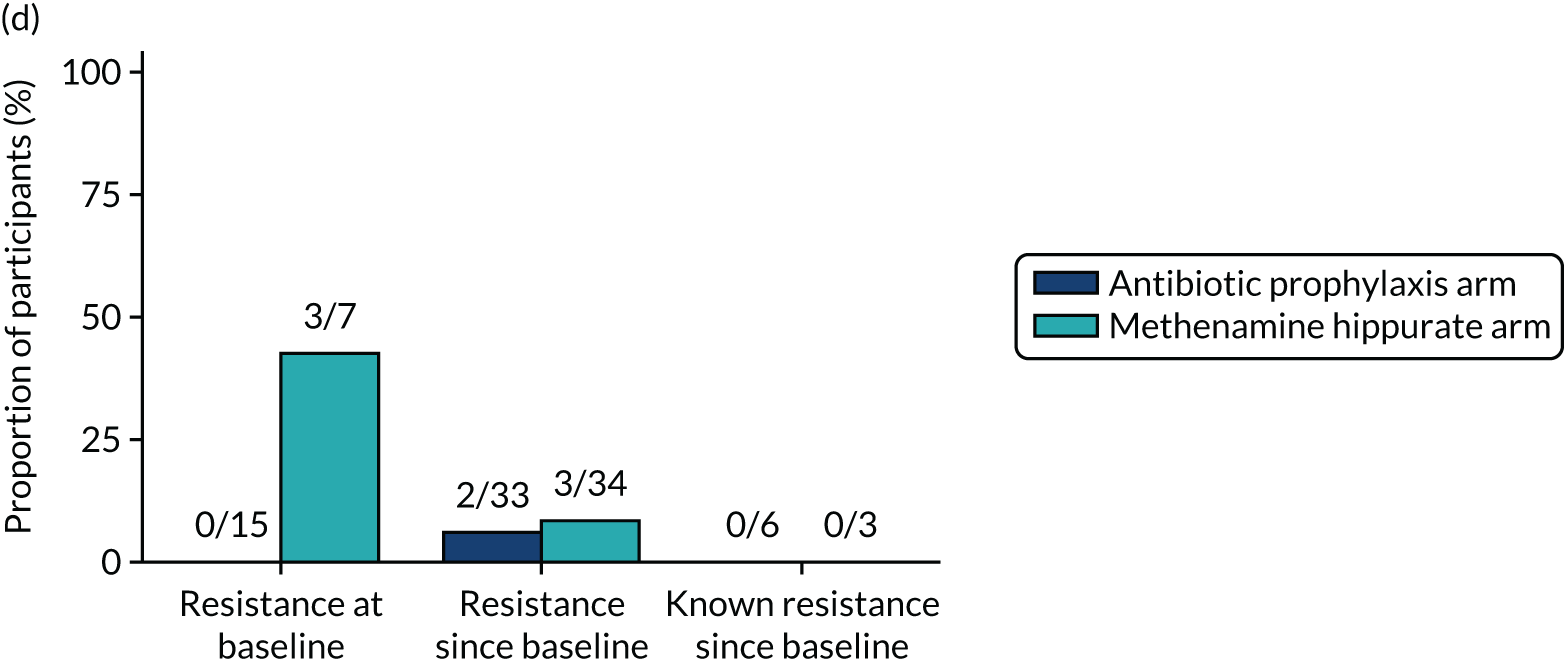
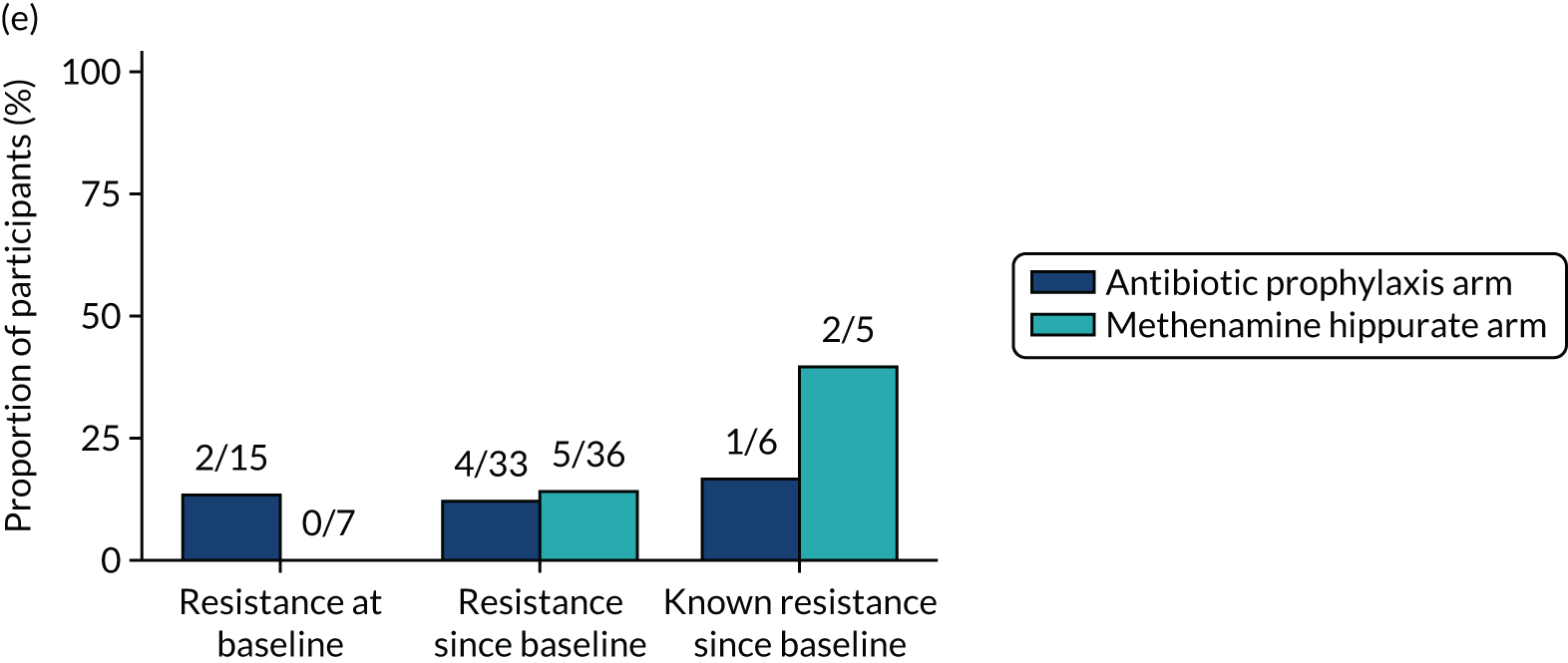
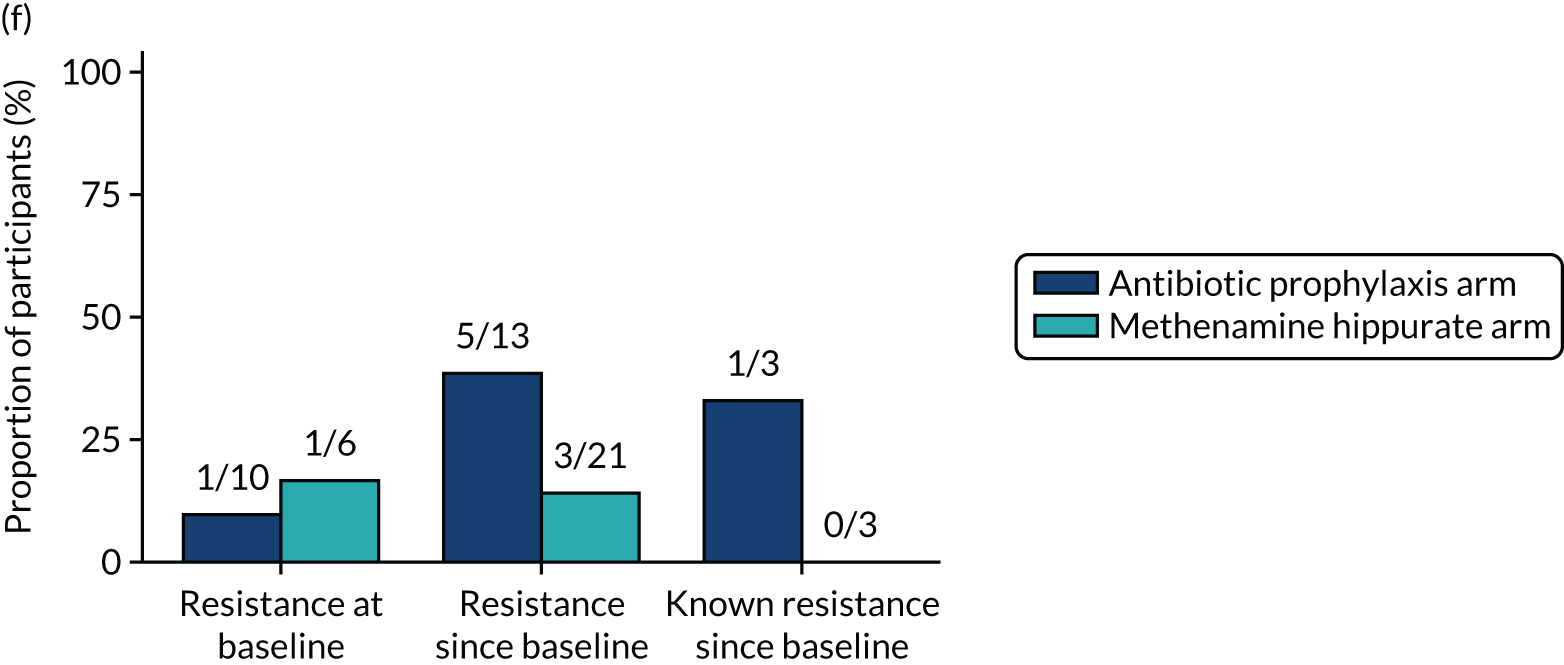
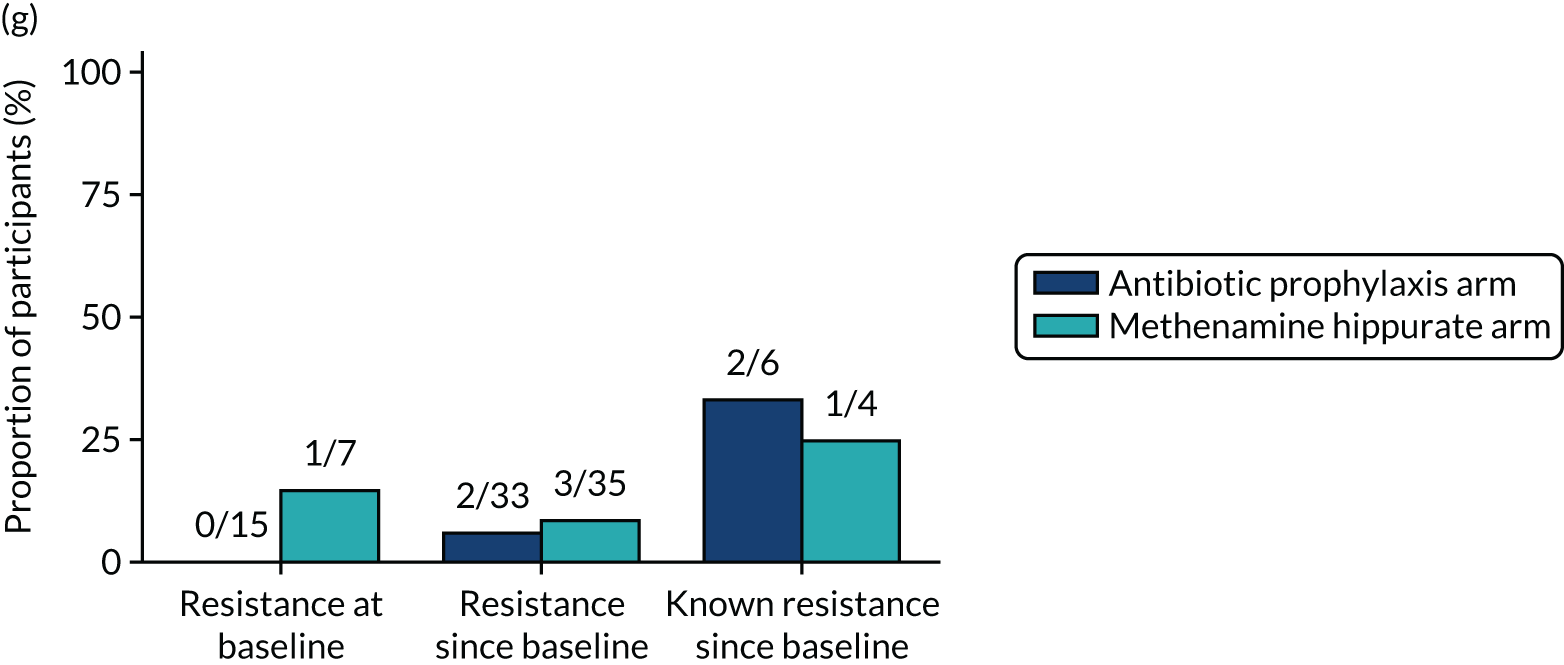
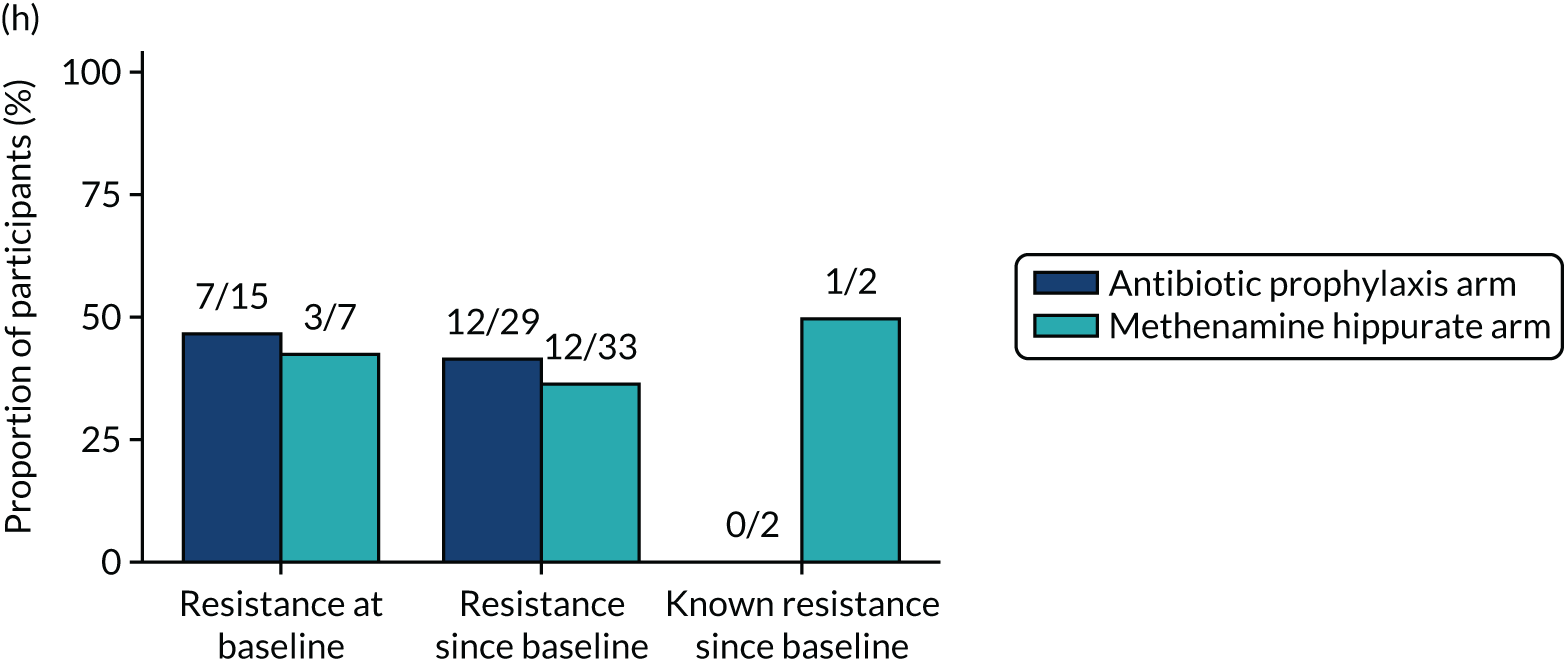
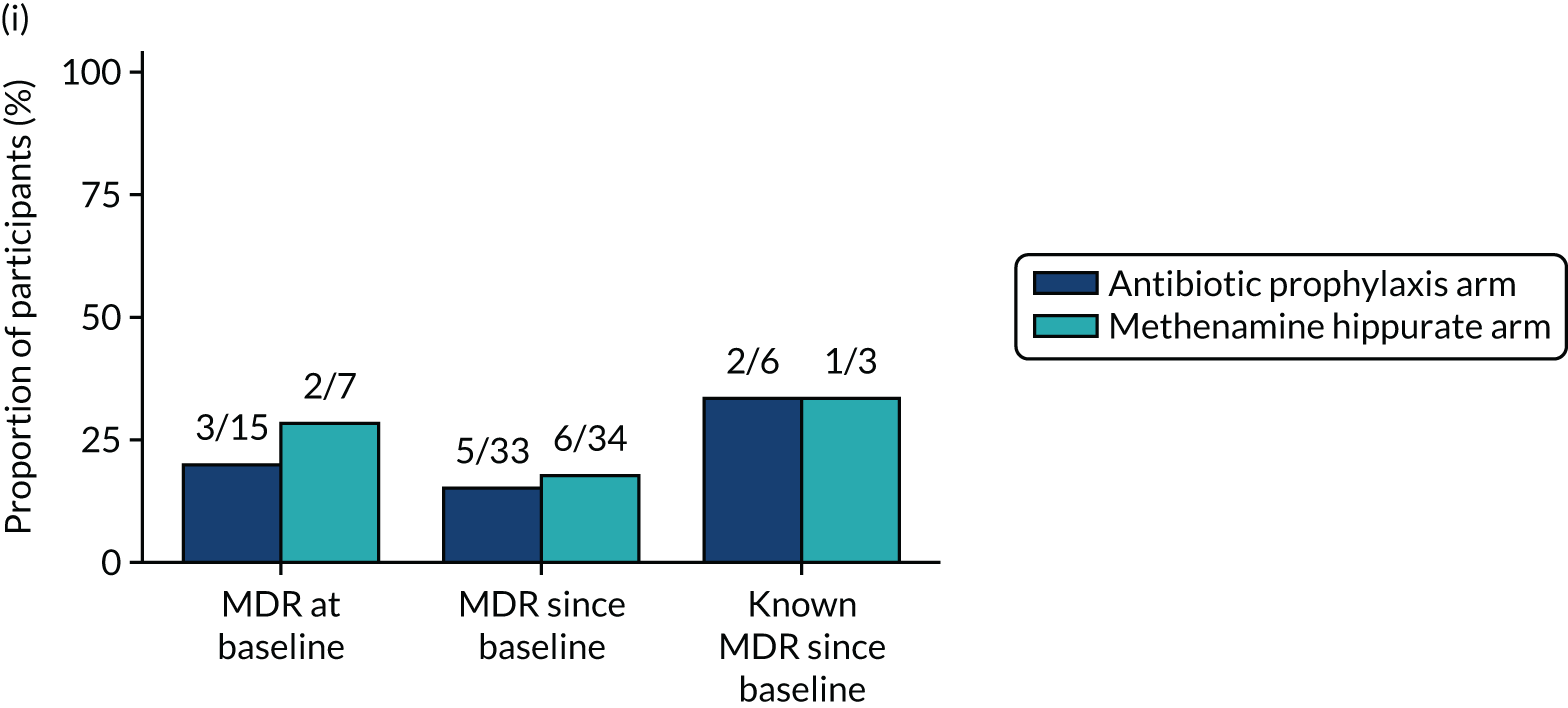
| Isolate | Number of urine samples | Percentage of the total |
|---|---|---|
| E. coli | 198 | 68 |
| Coliform other | 21 | 7 |
| Enterococcus faecalis | 19 | 7 |
| Klebsiella pneumoniae | 15 | 5 |
| Pseudomonas aeruginosa | 9 | 3 |
| Streptococcus agalactiae | 7 | 2 |
| Acinetobacter spp. | 5 | 2 |
| Staphylococcus aureus | 3 | 1 |
| Proteus spp. | 2 | 1 |
| Proteus mirabilis | 2 | 1 |
| Klebsiella oxytoca | 2 | 1 |
| Enterobacter cloacae group | 1 | < 1 |
| Citrobacter freundii group | 1 | < 1 |
| Pseudomonas spp. | 1 | < 1 |
| Enterococcus faecium | 1 | < 1 |
| Streptococcus spp. | 1 | < 1 |
| Streptococcus bovis | 1 | < 1 |
| Candida albicans | 1 | < 1 |
| Total | 290 | 100 |
| Antibiotic prophylaxis arm (N = 120), n (%) | Methenamine hippurate arm (N = 120), n (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 3 | Month 6 | Month 9 | Month 12 | Month 15 | Month 18 | Baseline | Month 3 | Month 6 | Month 9 | Month 12 | Month 15 | Month 18 | |
| Samples available | 111 (93) | 95 (79) | 81 (68) | 79 (66) | 74 (62) | 64 (53) | 62 (52) | 111 (93) | 96 (80) | 89 (74) | 81 (68) | 78 (65) | 71 (59) | 71 (59) |
| Any isolate | 18 (16) | 2 (2) | 7 (9) | 7 (9) | 7 (9) | 15 (23) | 10 (16) | 13 (12) | 10 (10) | 14 (16) | 9 (11) | 16 (21) | 14 (20) | 13 (18) |
| Antibiotic resistance (number of antibiotics) | ||||||||||||||
| 0 | 6 (33) | 1 (50) | 2 (29) | 1 (14) | 1 (14) | 5 (33) | 4 (40) | 6 (46) | 4 (40) | 7 (50) | 6 (67) | 6 (38) | 3 (21) | 6 (46) |
| 1 or 2 | 7 (39) | 1 (50) | 3 (43) | 4 (57) | 4 (57) | 9 (60) | 4 (40) | 3 (23) | 3 (30) | 2 (14) | 2 (22) | 6 (38) | 9 (64) | 5 (38) |
| ≥ 3 | 5 (28) | 0 (0) | 2 (29) | 2 (29) | 2 (29) | 1 (7) | 2 (20) | 4 (31) | 3 (30) | 5 (36) | 1 (11) | 4 (25) | 2 (14) | 2 (15) |
FIGURE 27.
Resistance rates (per participant) in any significant isolate from symptomatic urine samples in the 12-month treatment period and 6-month follow-up period. (a) Amoxicillin; (b) cefalexin; (c) cefuroxime; (d) ciprofloxacin; (e) co-amoxiclav; (f) co-trimoxazole; (g) nitrofurantoin; and (h) trimethoprim.
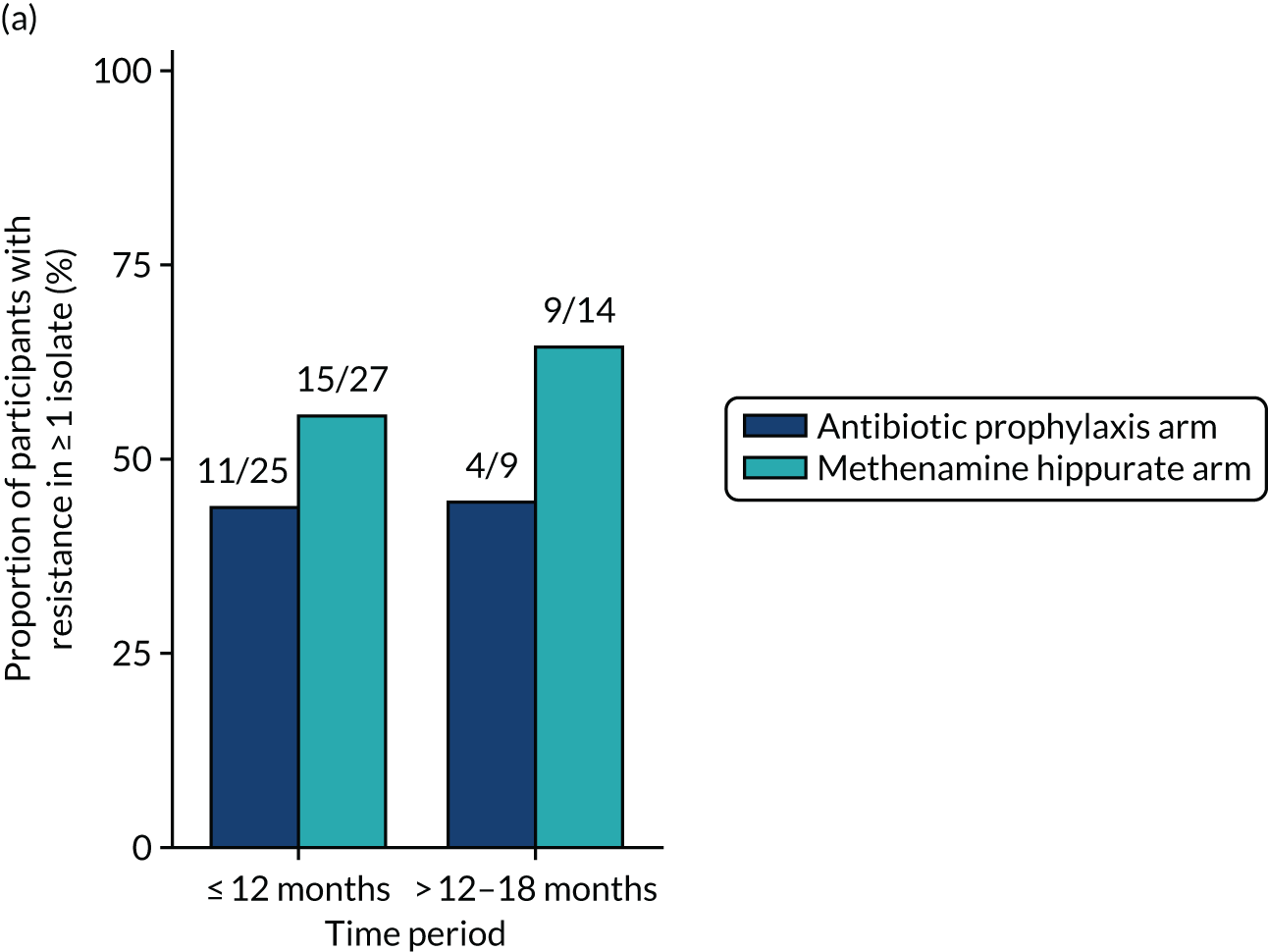
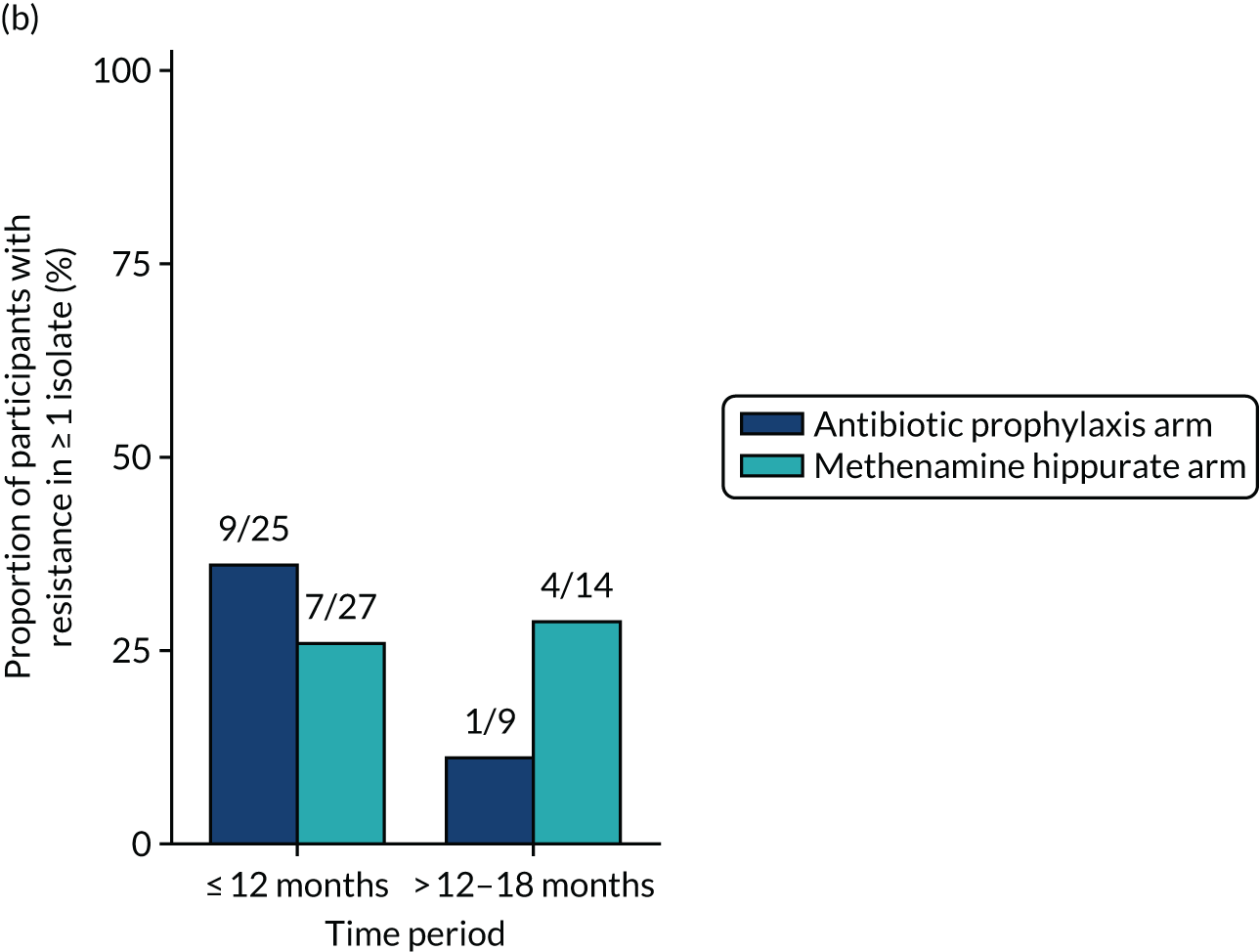
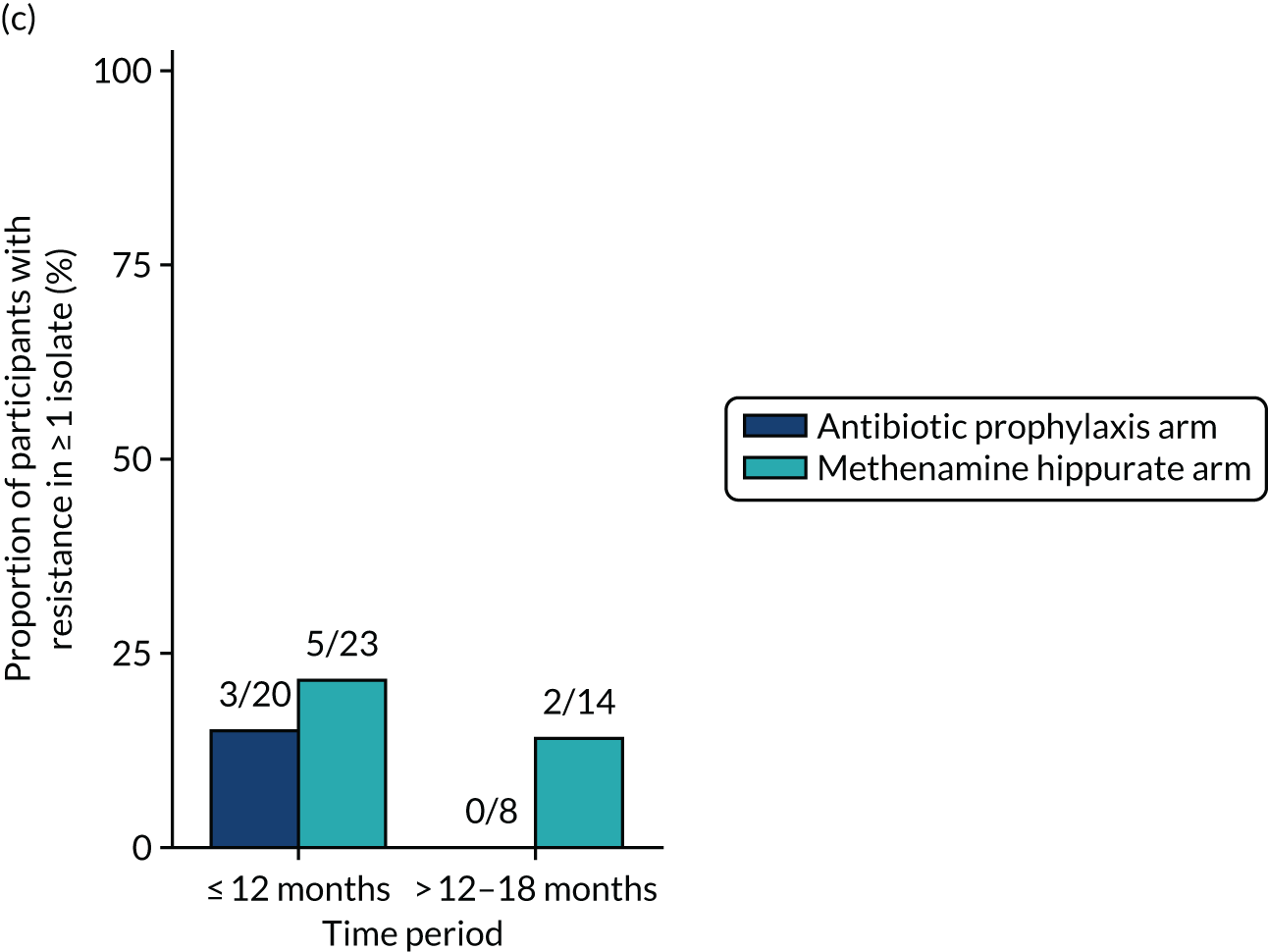
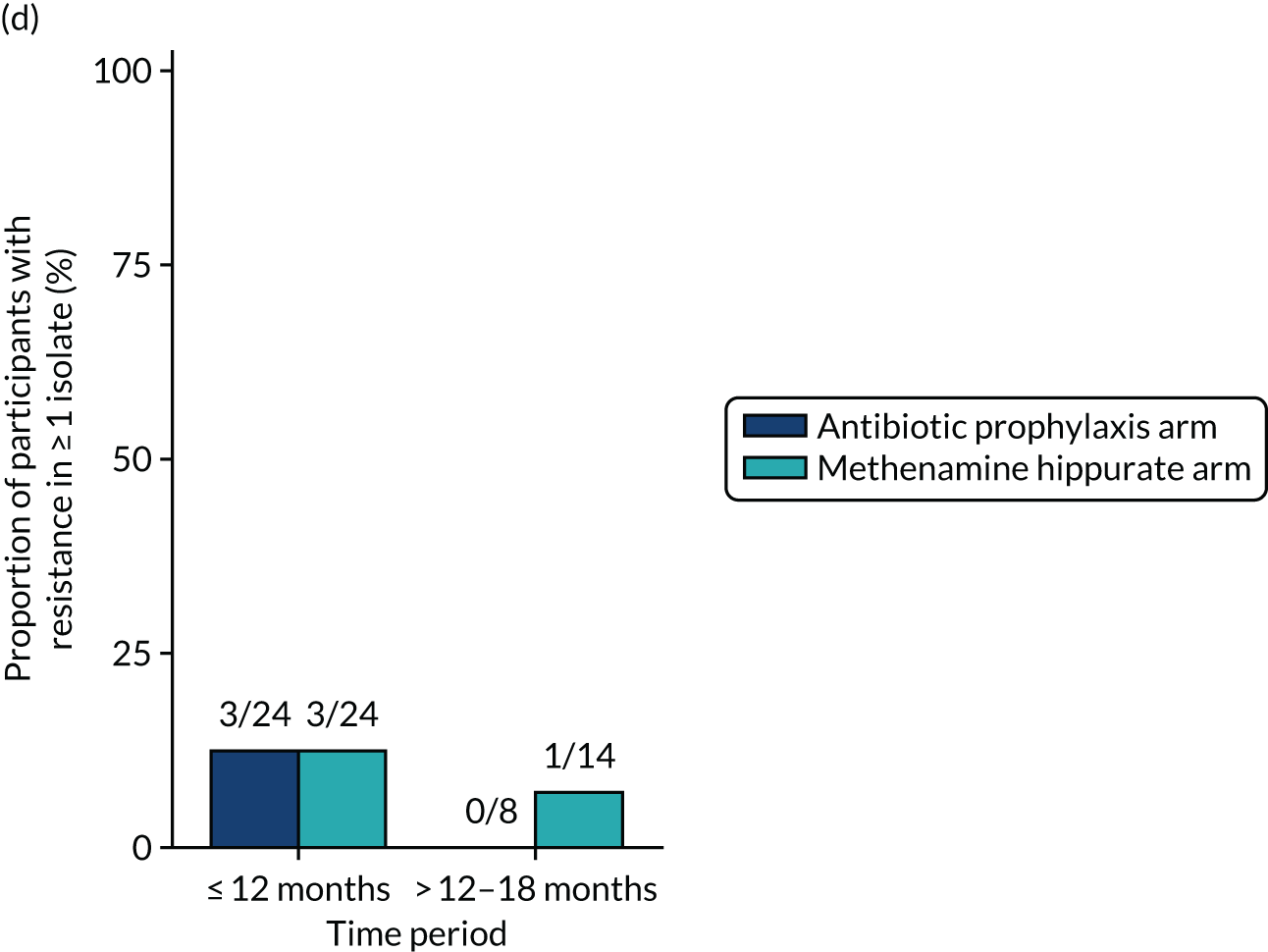
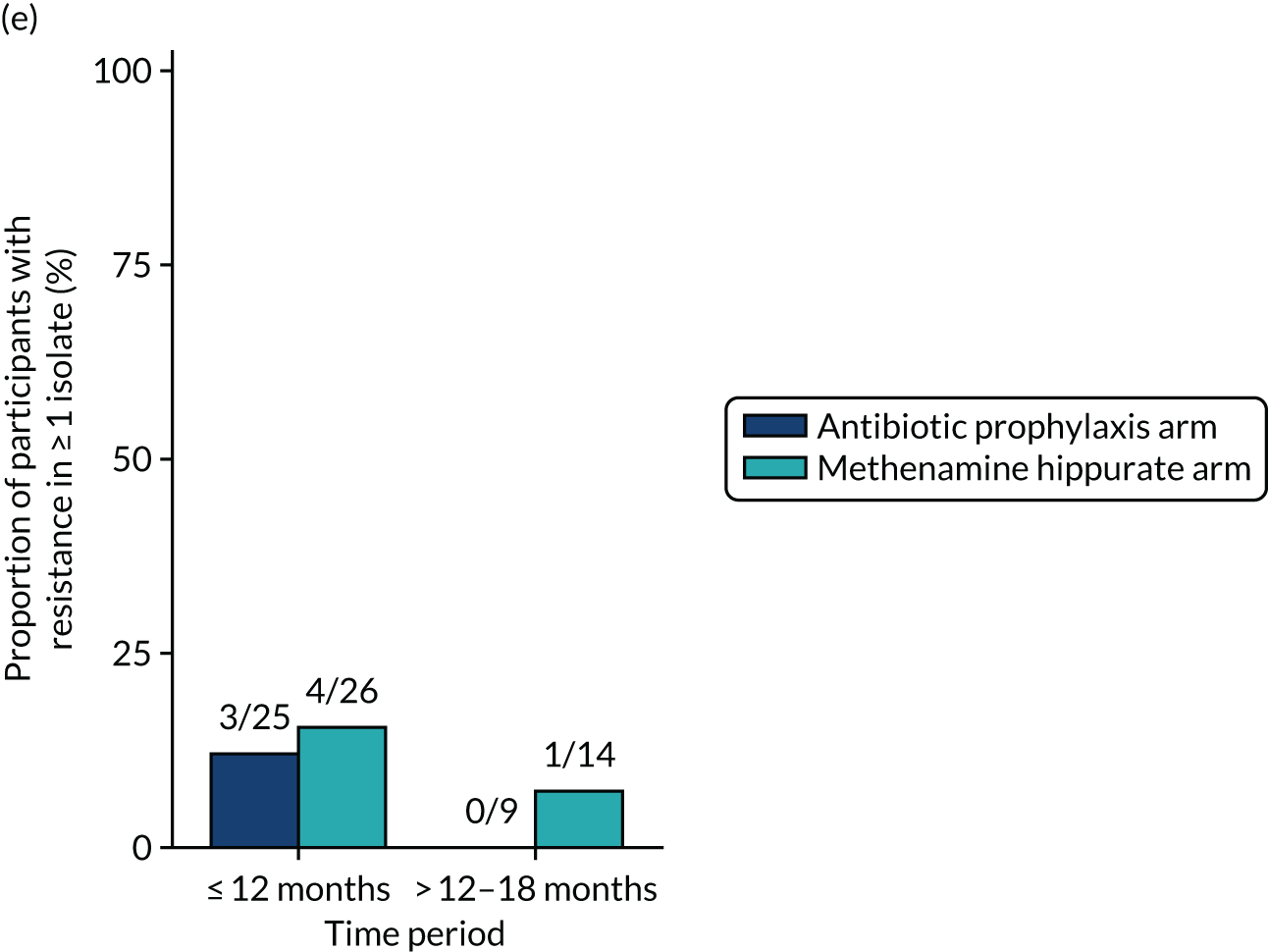
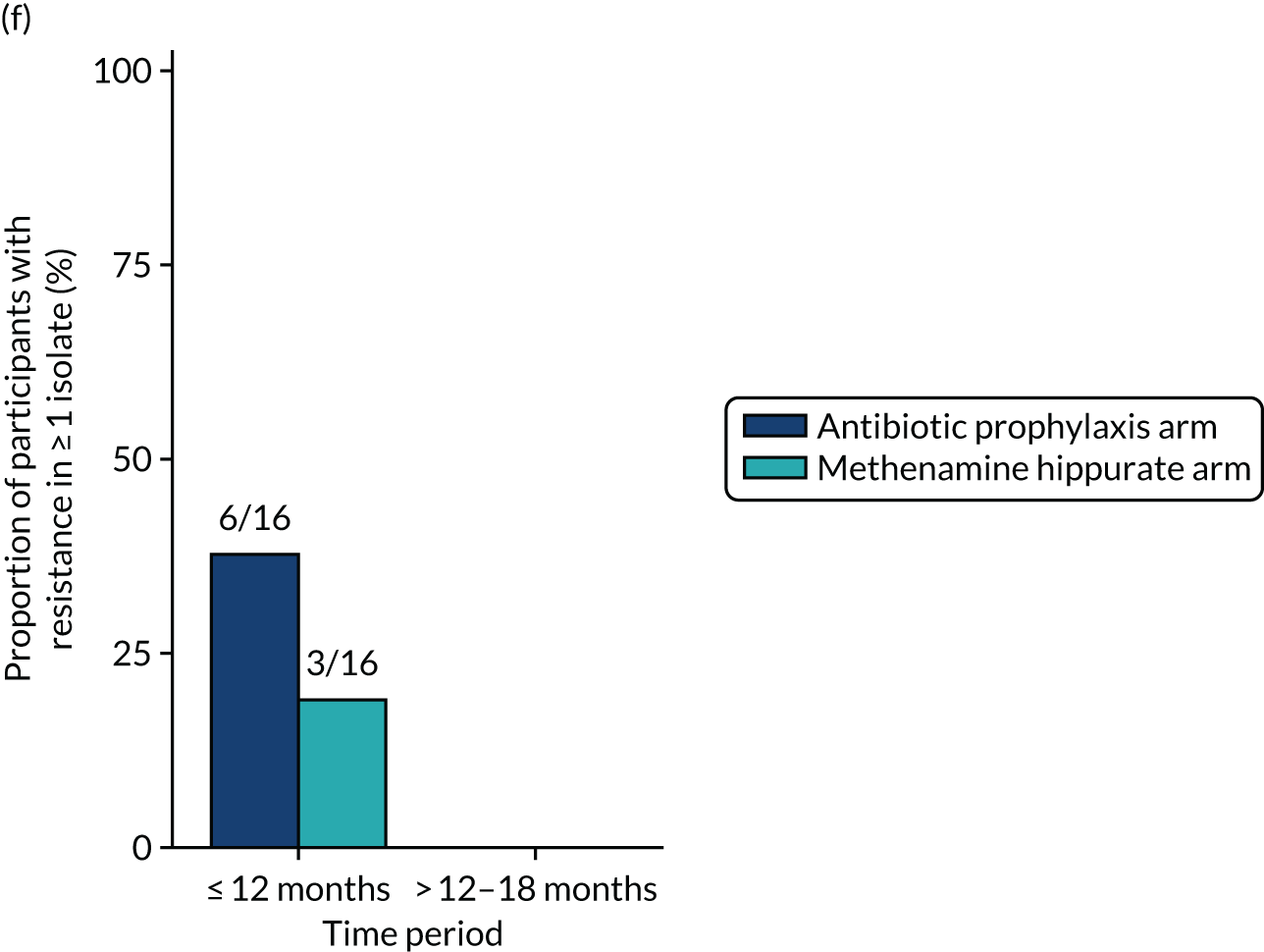
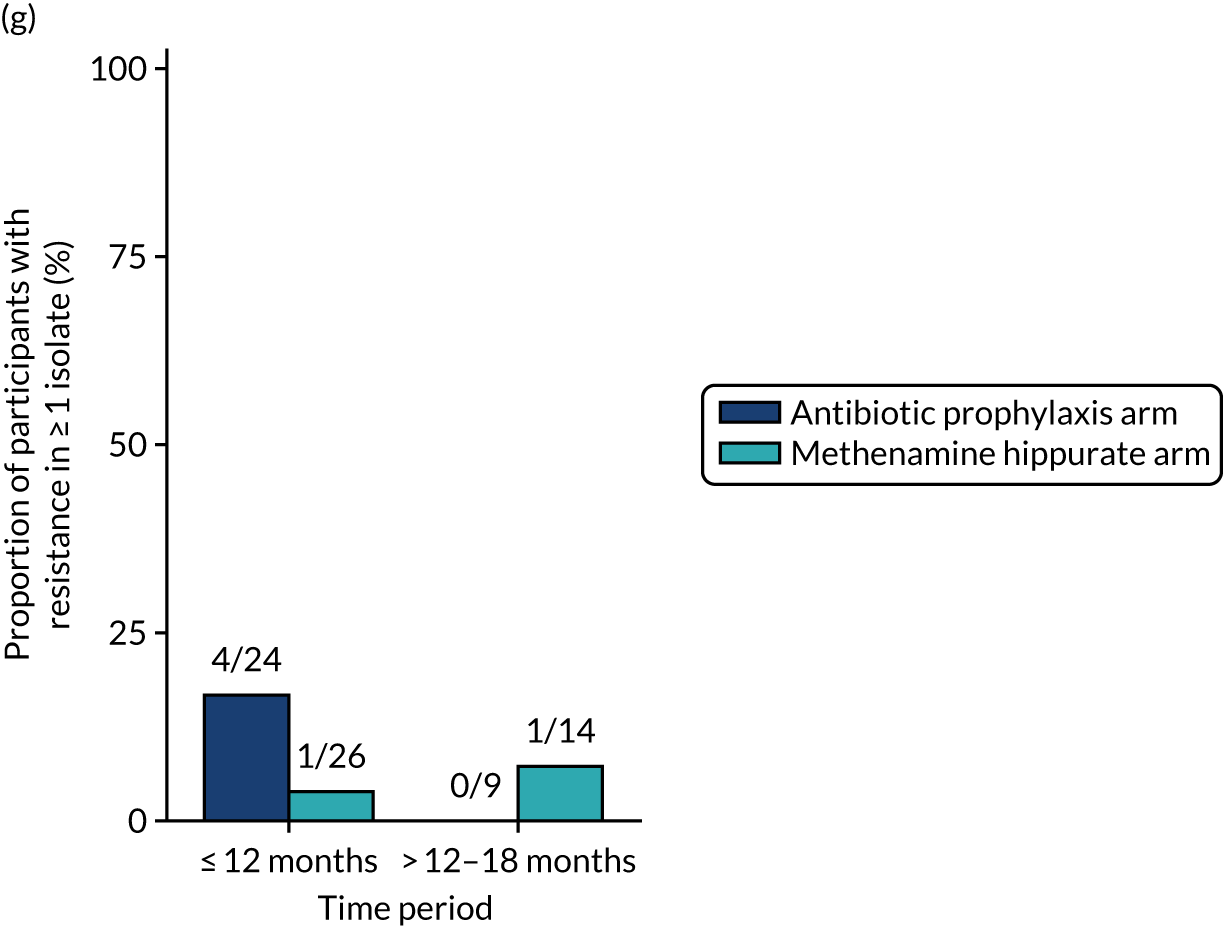
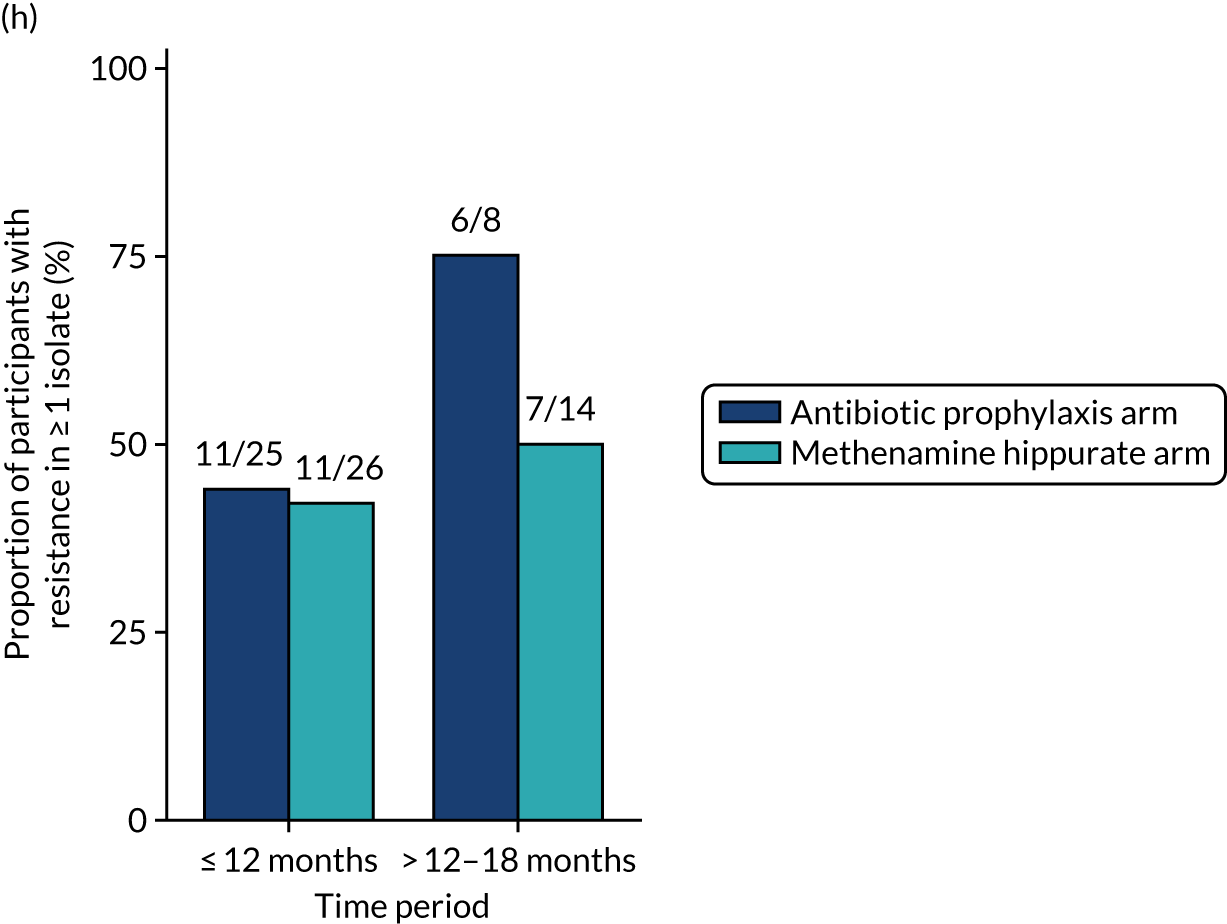
FIGURE 28.
Any resistance (per participant) in any significant isolate from symptomatic urine samples in the 12-month treatment period and 6-month follow-up period. Fisher’s exact p-values.
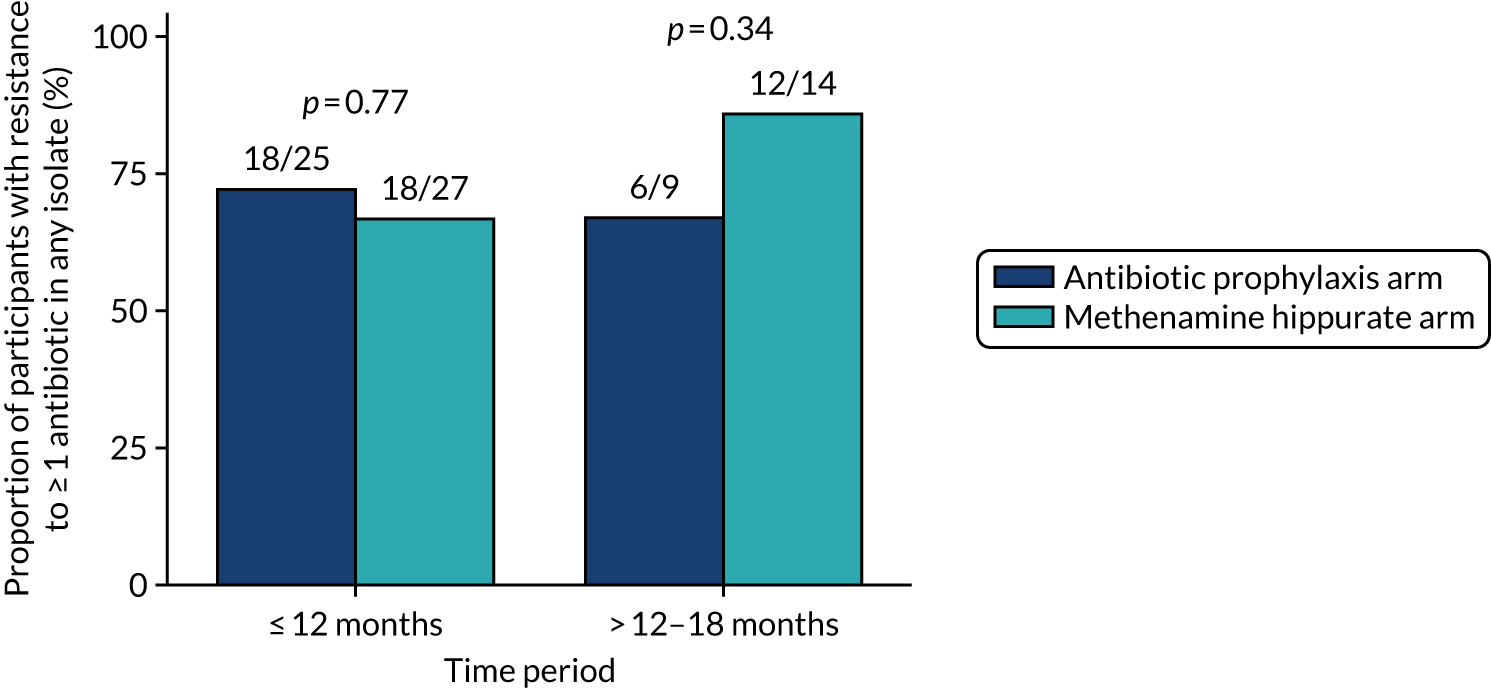
FIGURE 29.
Resistance rates (per sample) in any significant isolate from symptomatic urine samples in the 12-month treatment period and 6-month follow-up period. (a) Amoxicillin; (b) cefalexin; (c) cefuroxime; (d) ciprofloxacin; (e) co-amoxiclav; (f) co-trimoxazole; (g) nitrofurantoin; and (h) trimethoprim.
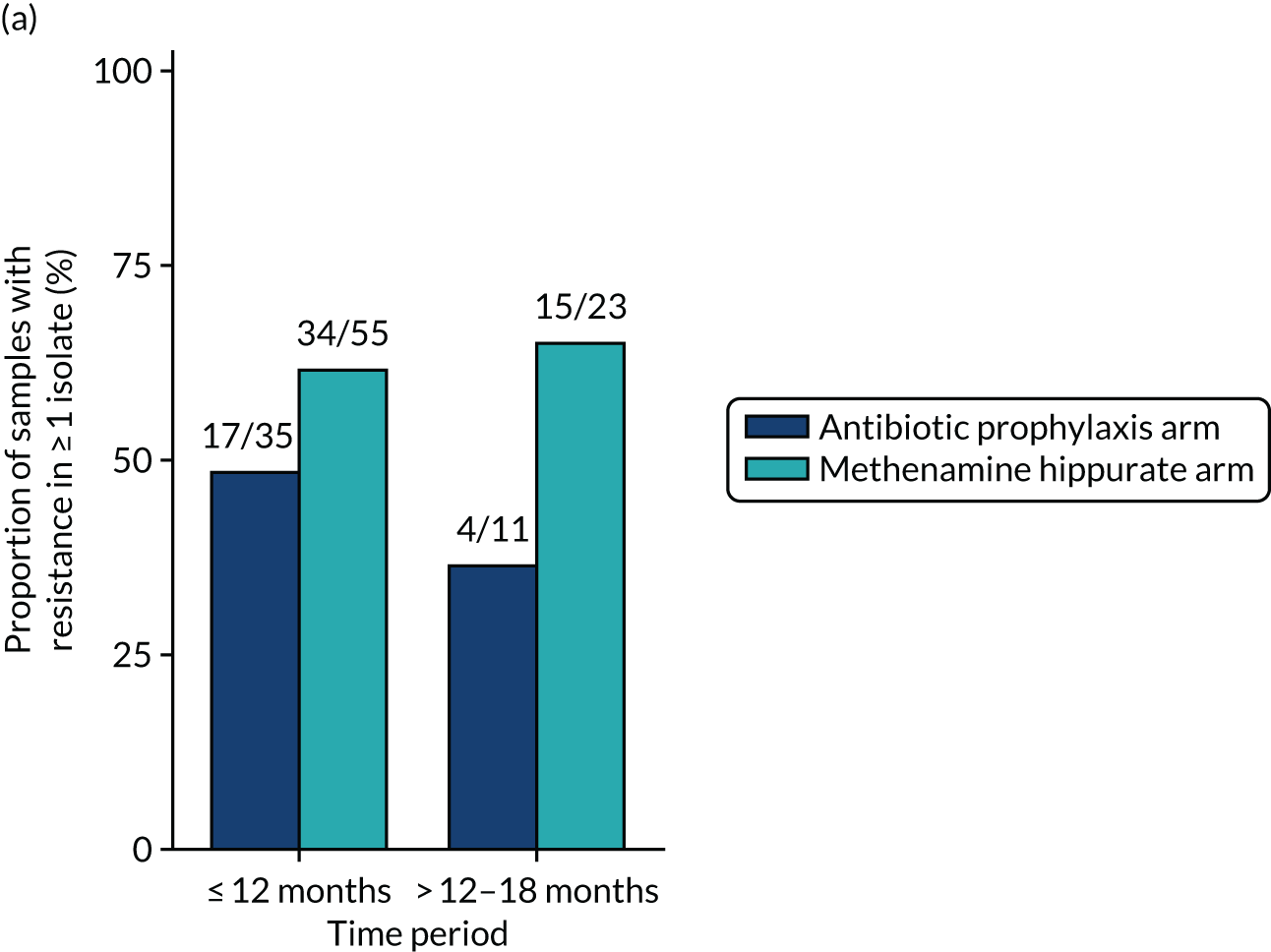
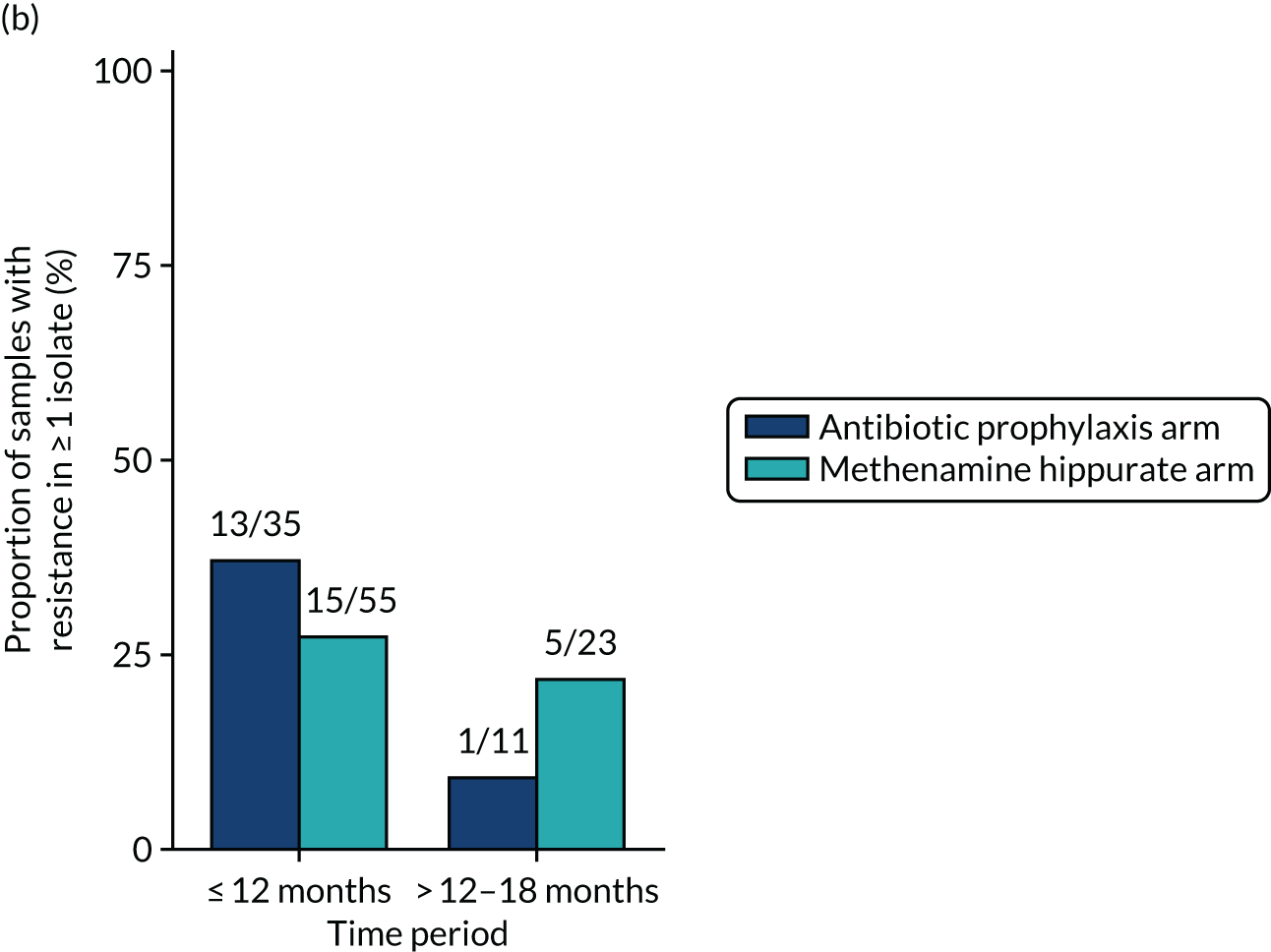
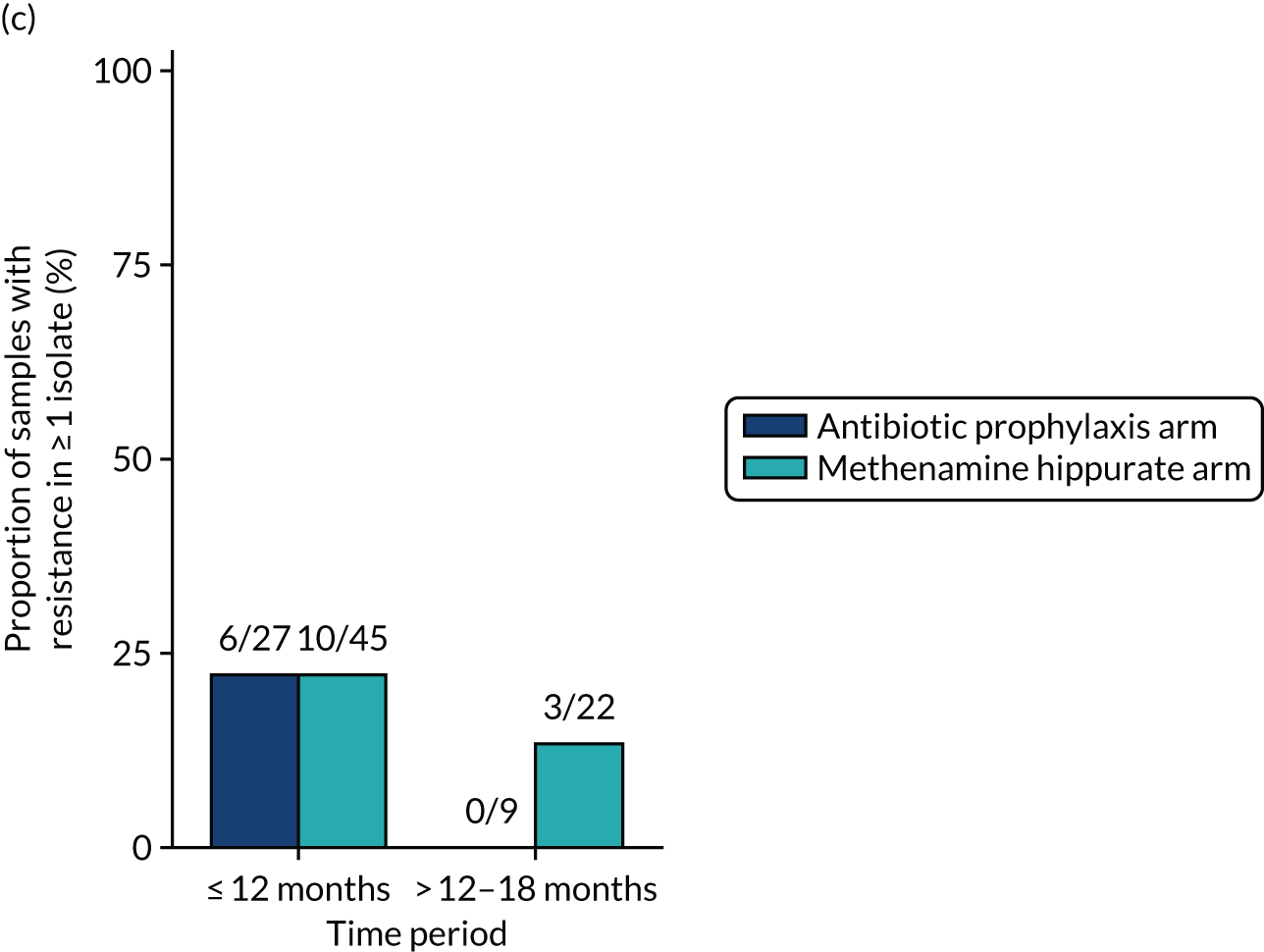
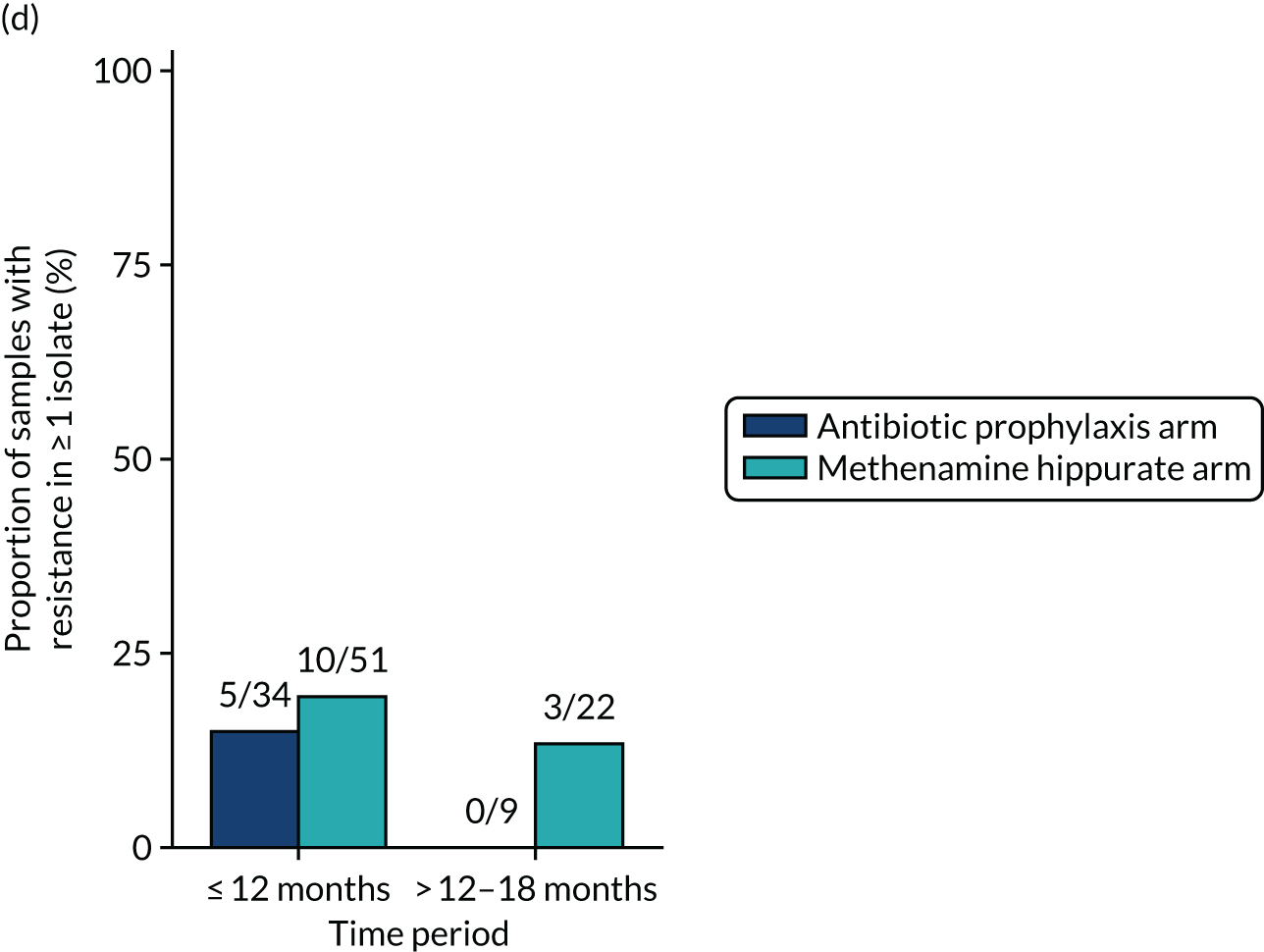
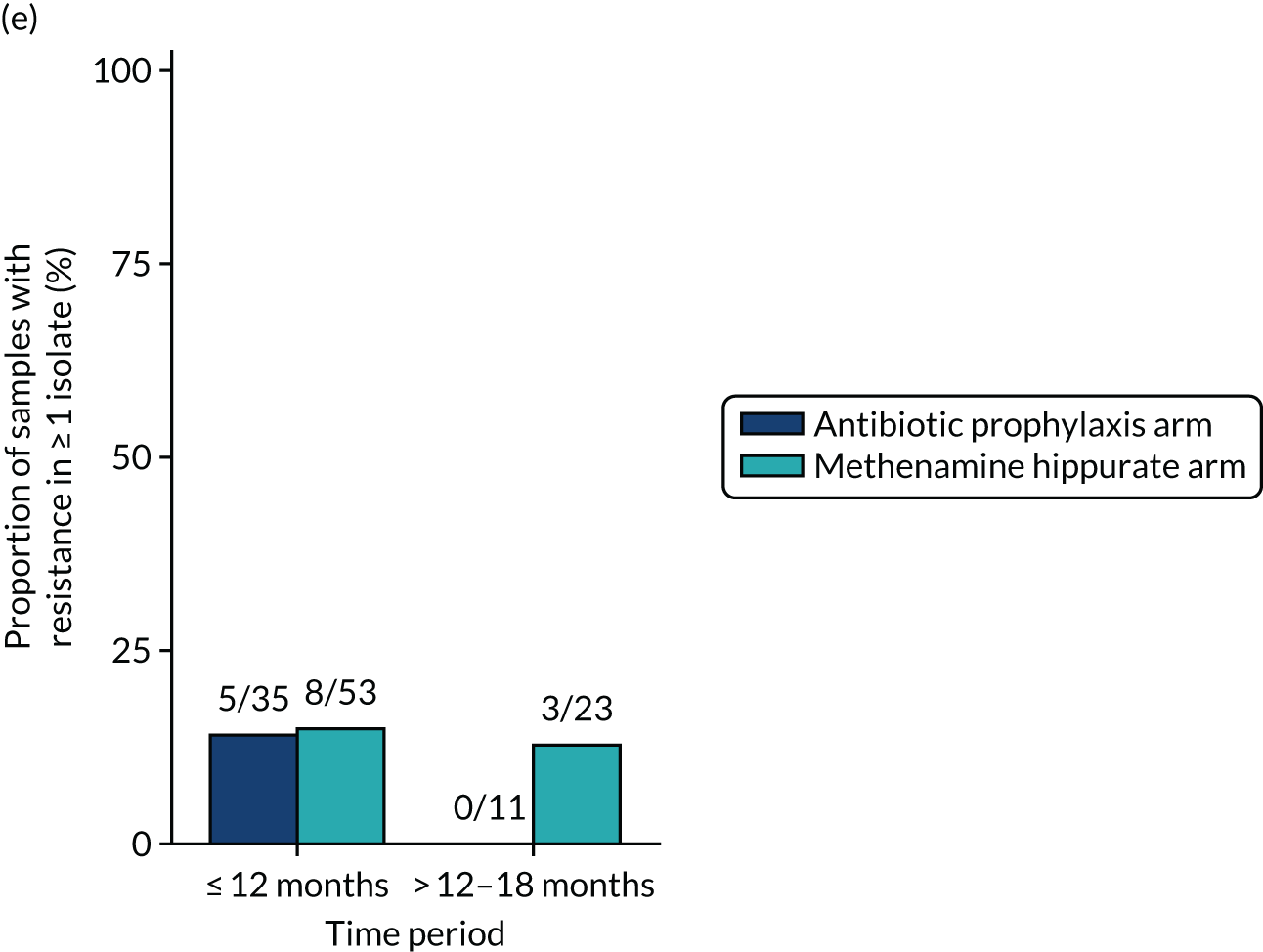
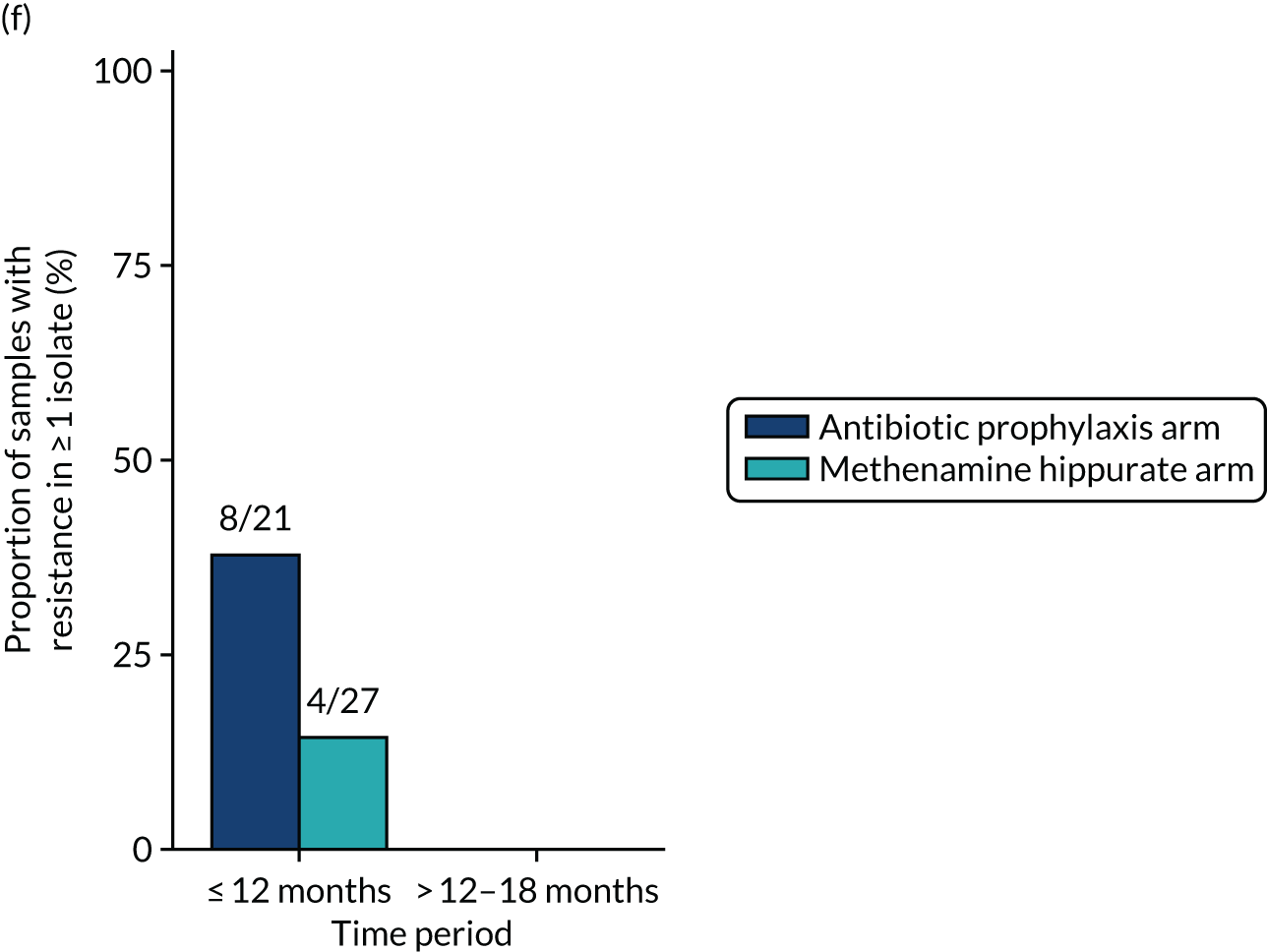
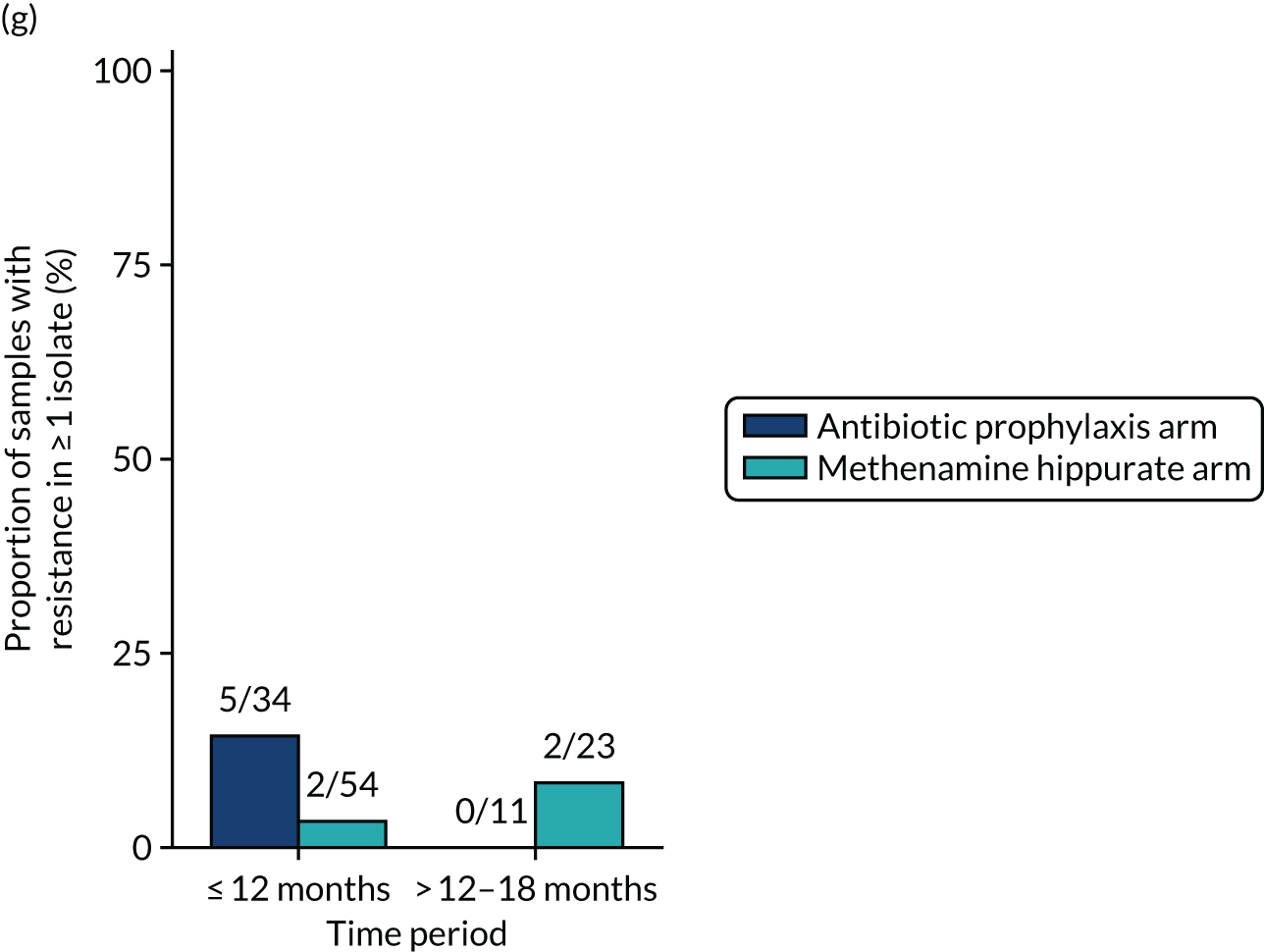
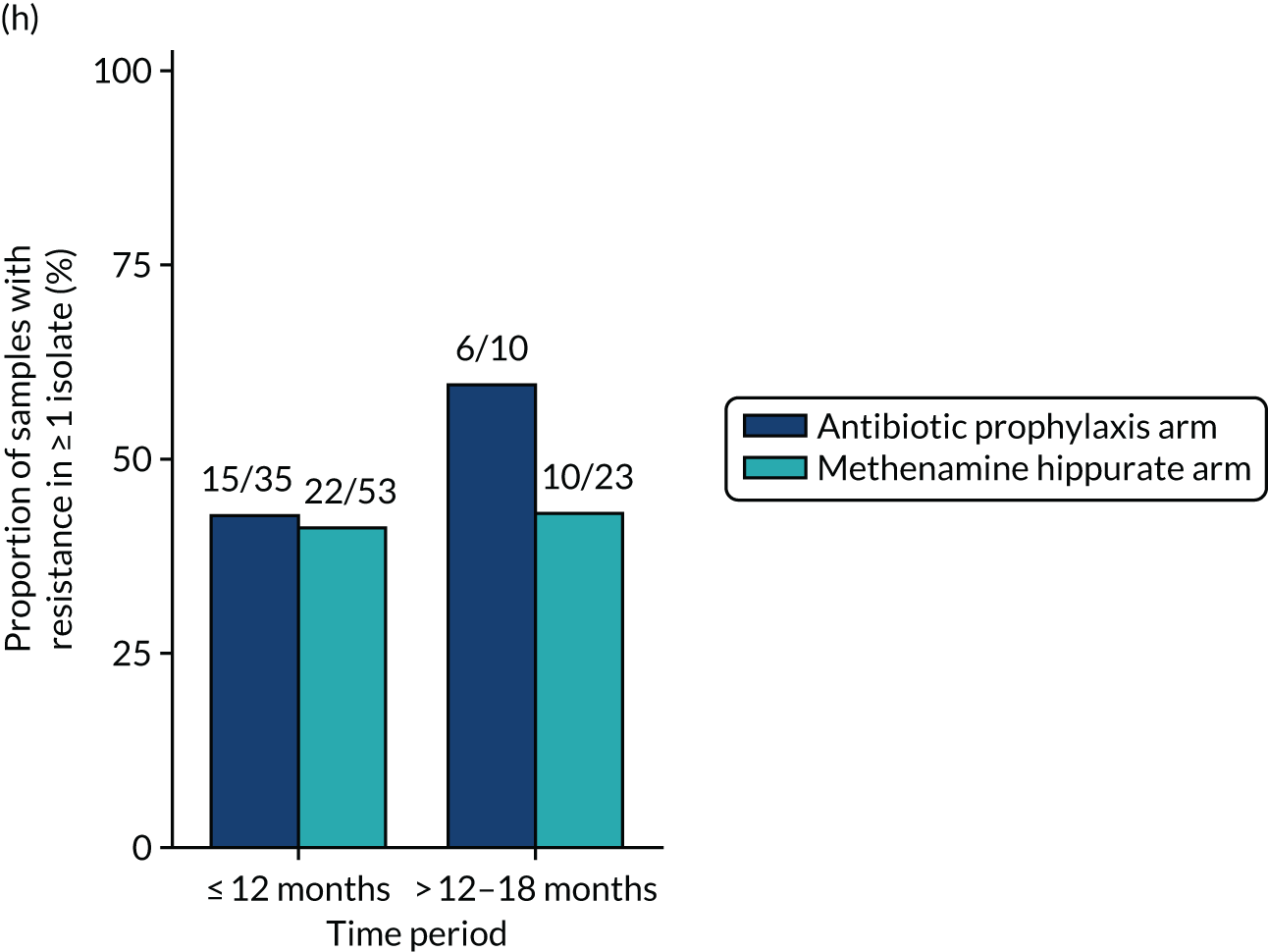
FIGURE 30.
Cumulative resistance rates (95% CI) over time in any significant isolate from symptomatic urine samples. (a) Amoxicillin; (b) cefalexin; (c) cefuroxime; (d) ciprofloxacin; (e) co-amoxiclav; (f) co-trimoxazole; (g) nitrofurantoin; and (h) trimethoprim.
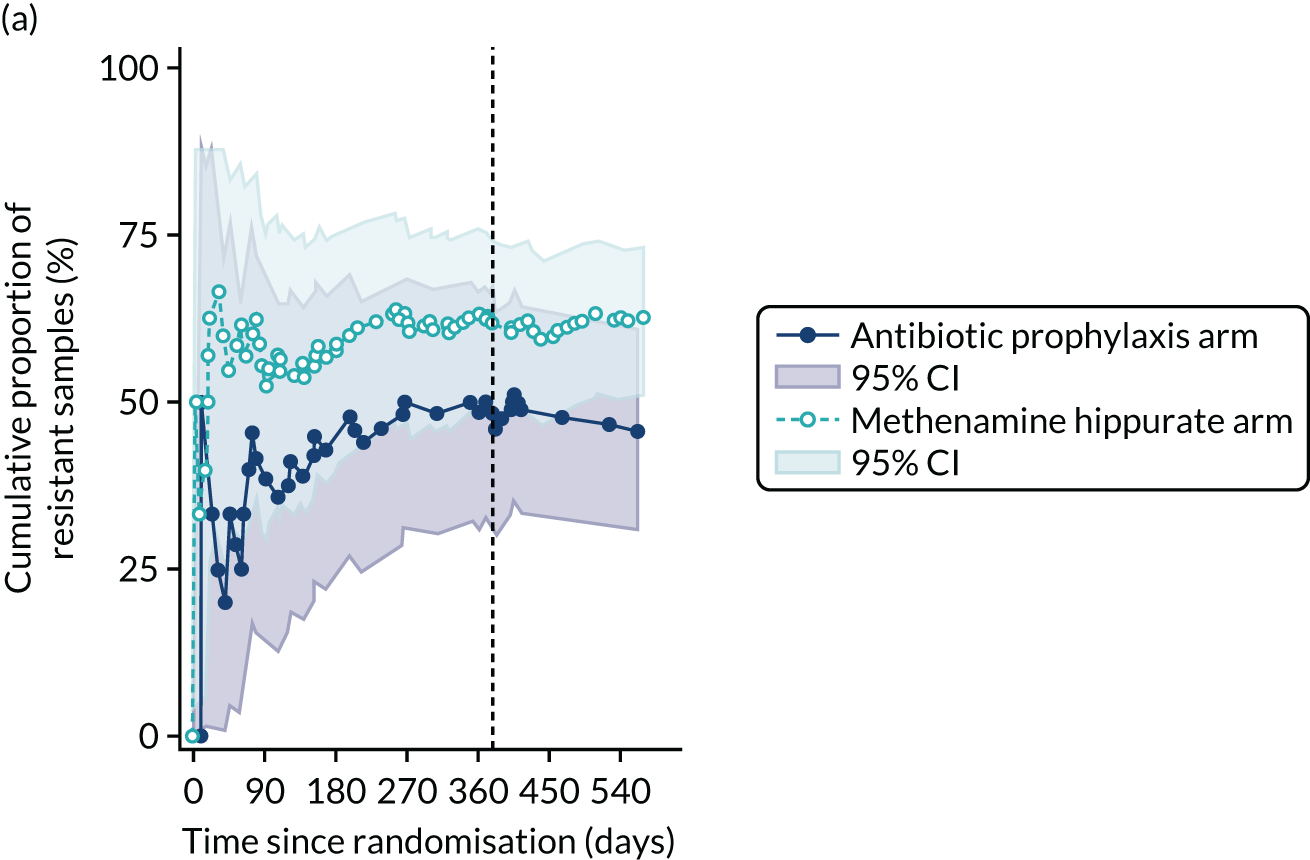
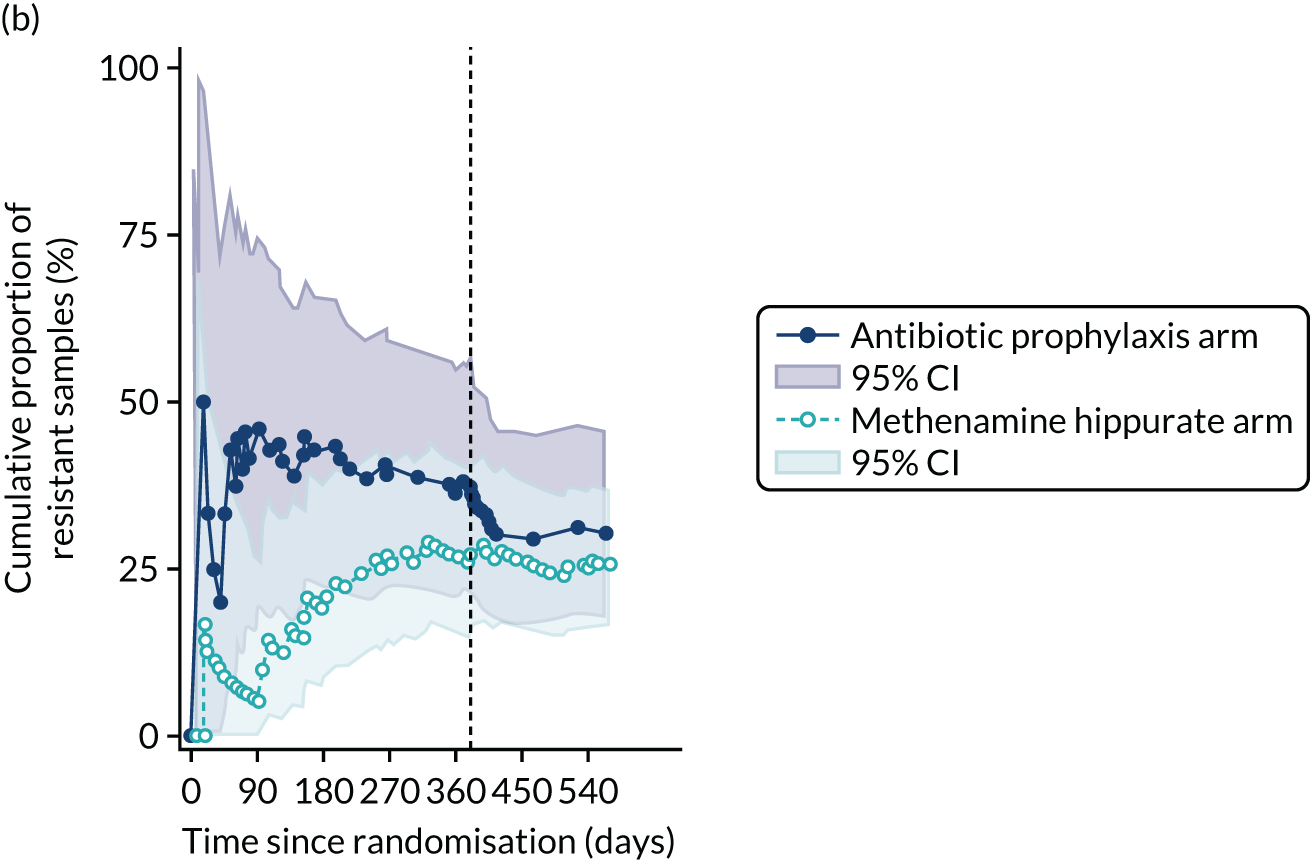
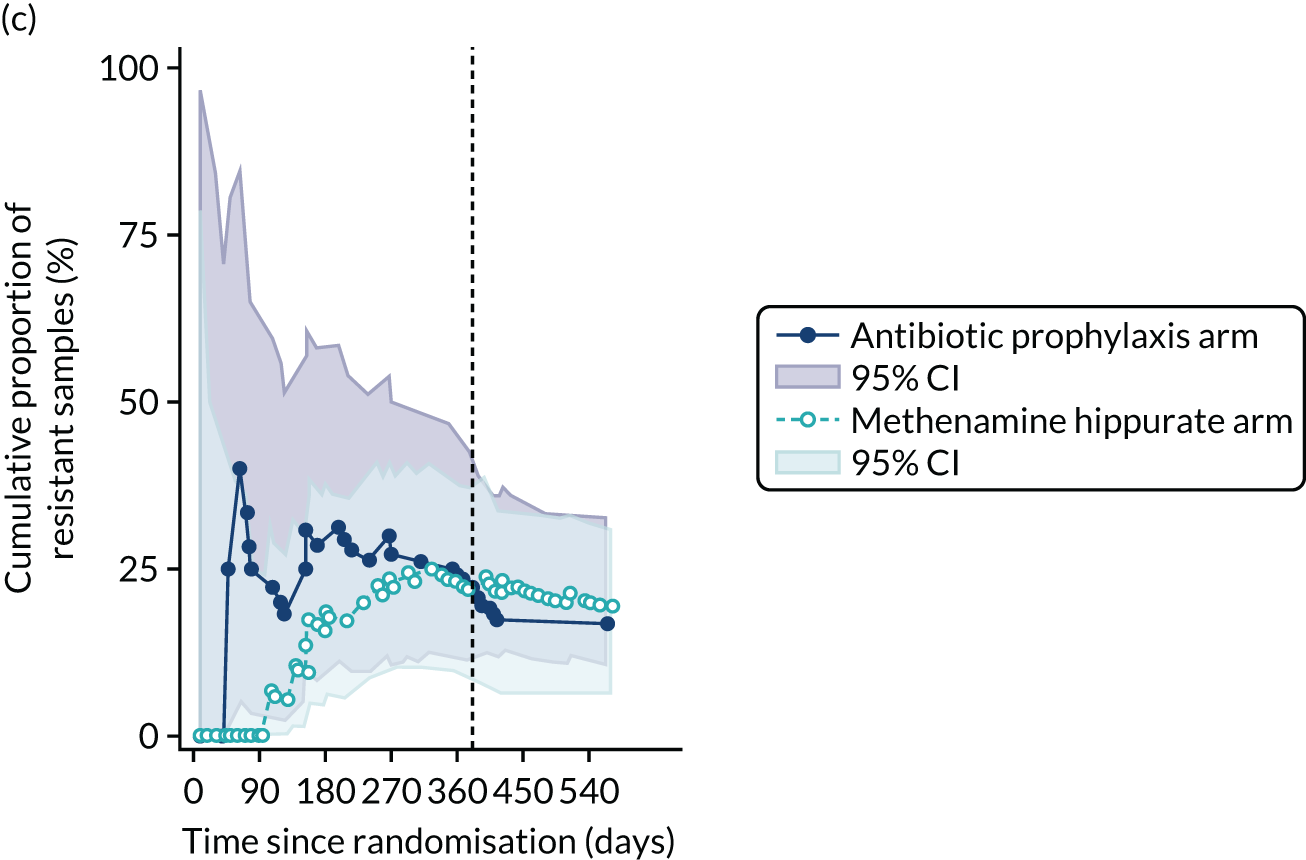
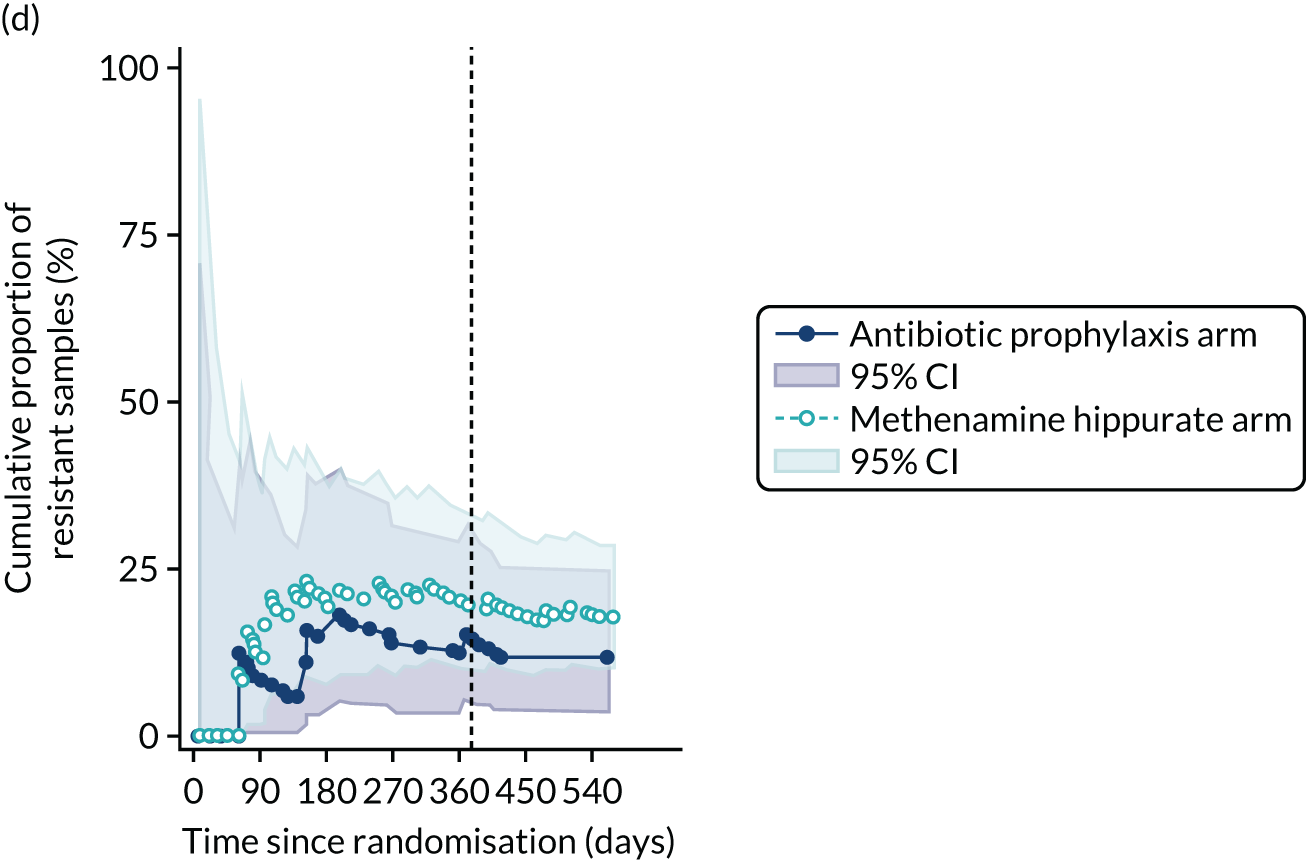
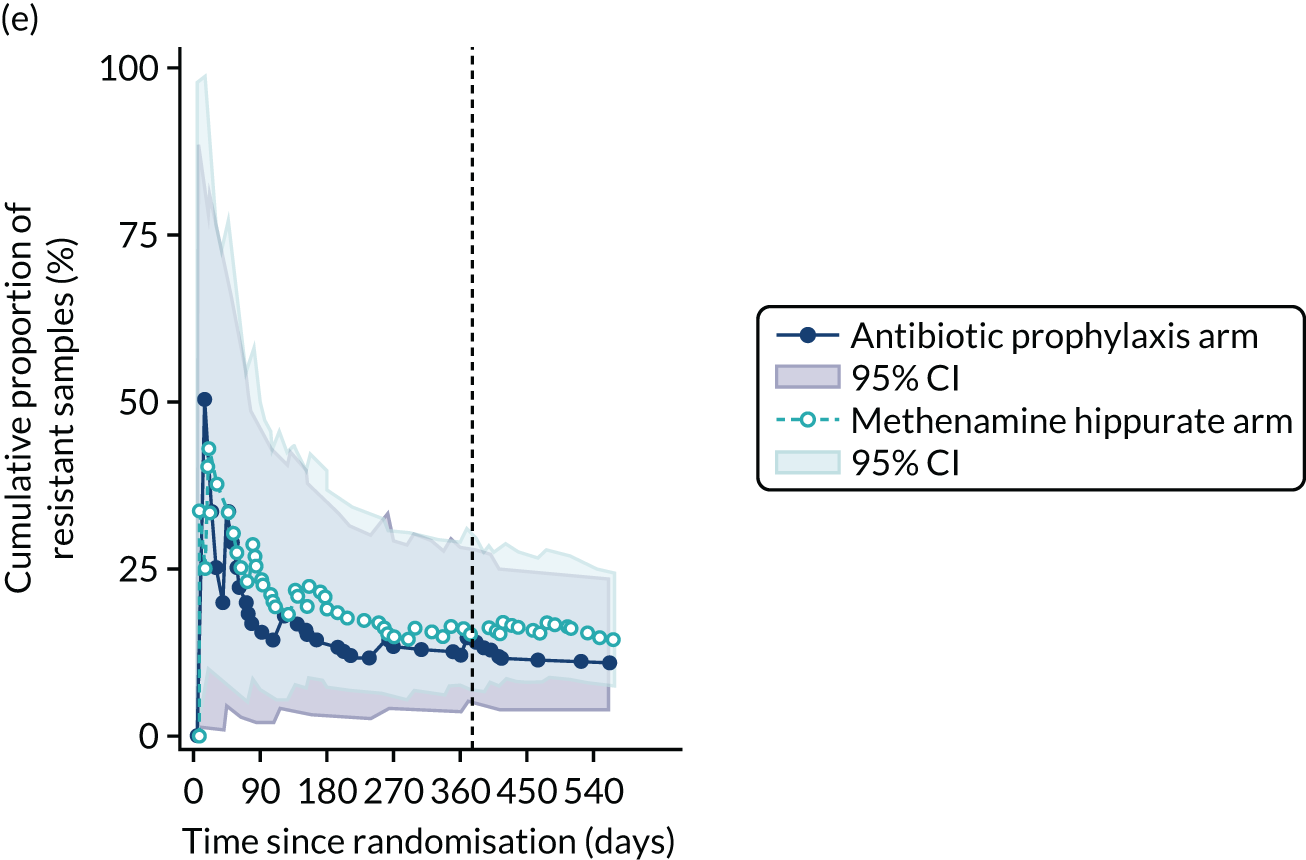
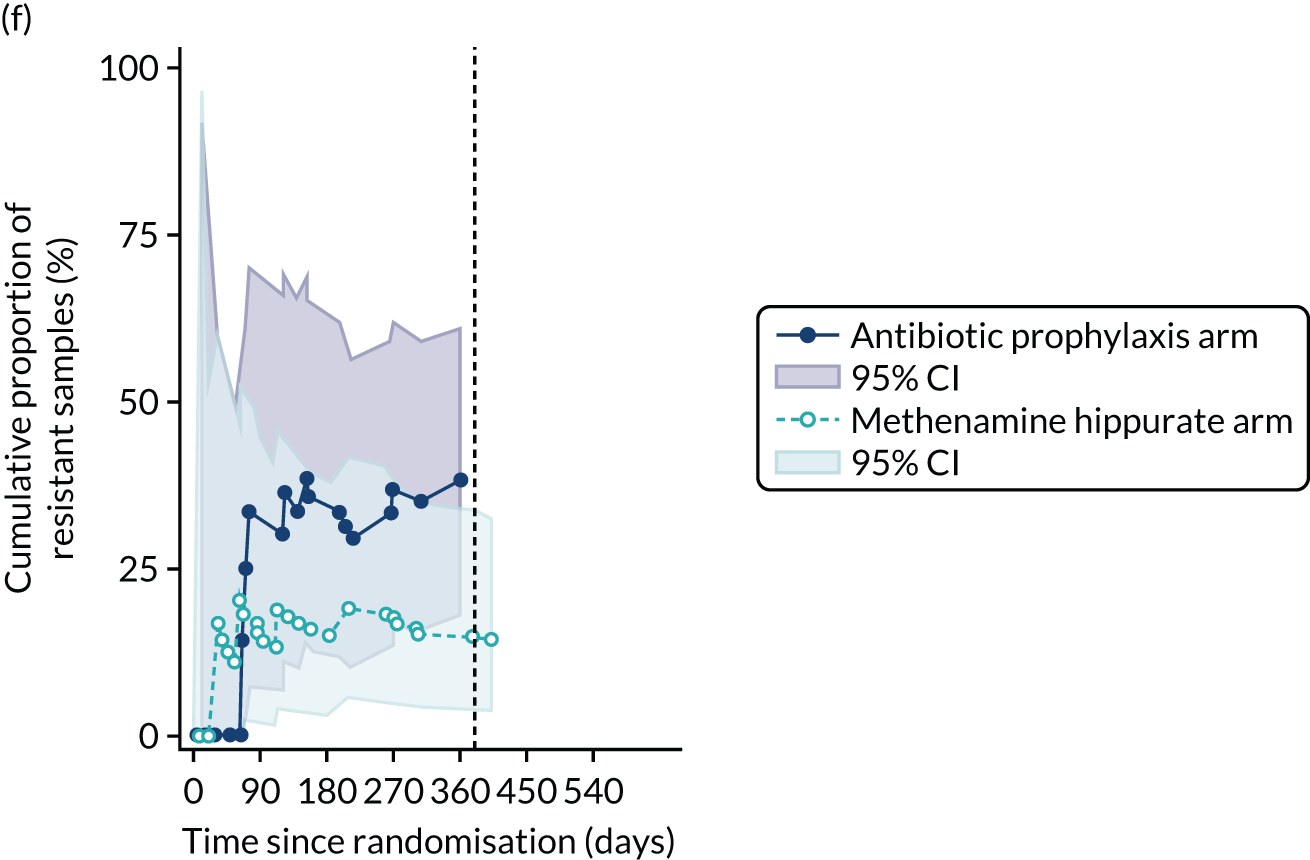
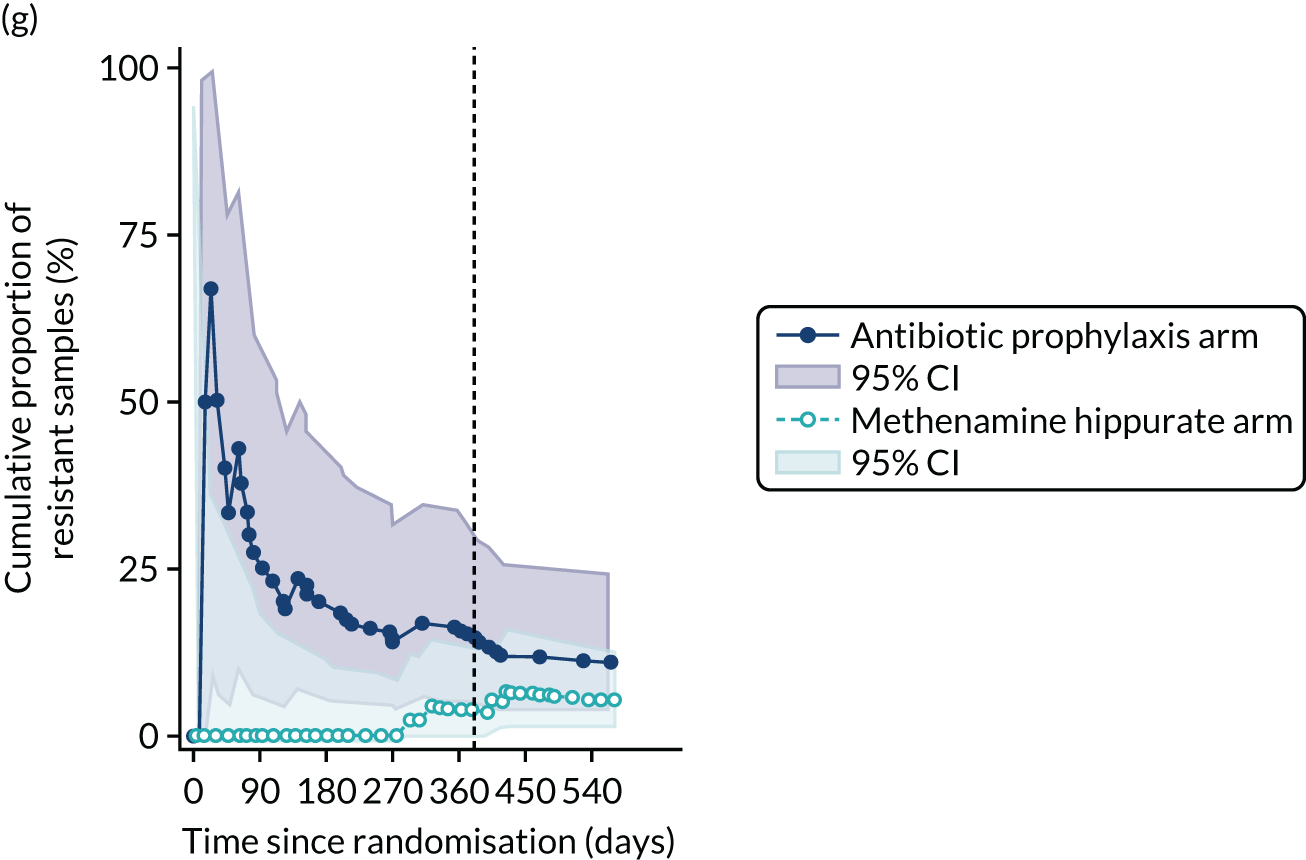
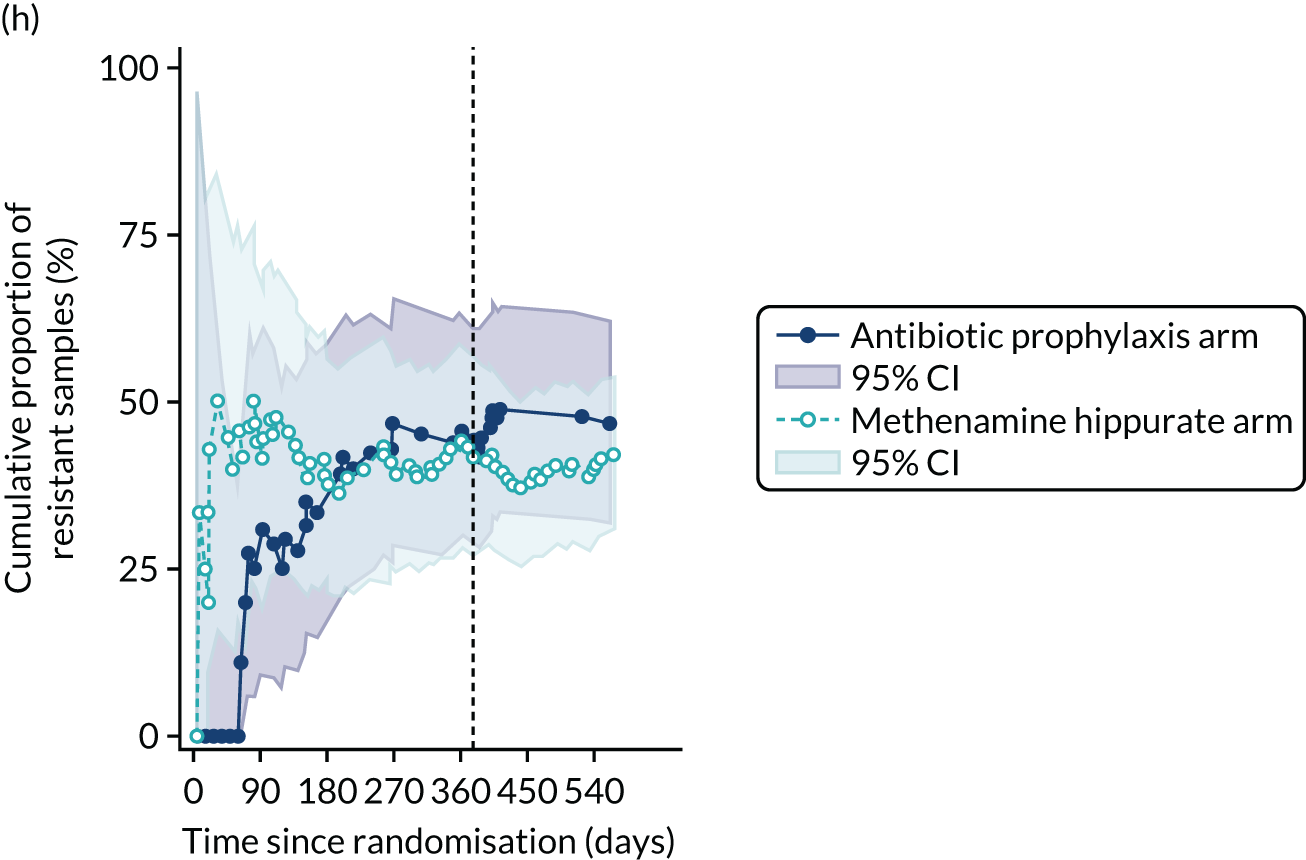
FIGURE 31.
Any resistance (per sample) in any significant isolate from symptomatic urine samples in the 12-month treatment period and 6-month follow-up period. Fisher’s exact p-values.
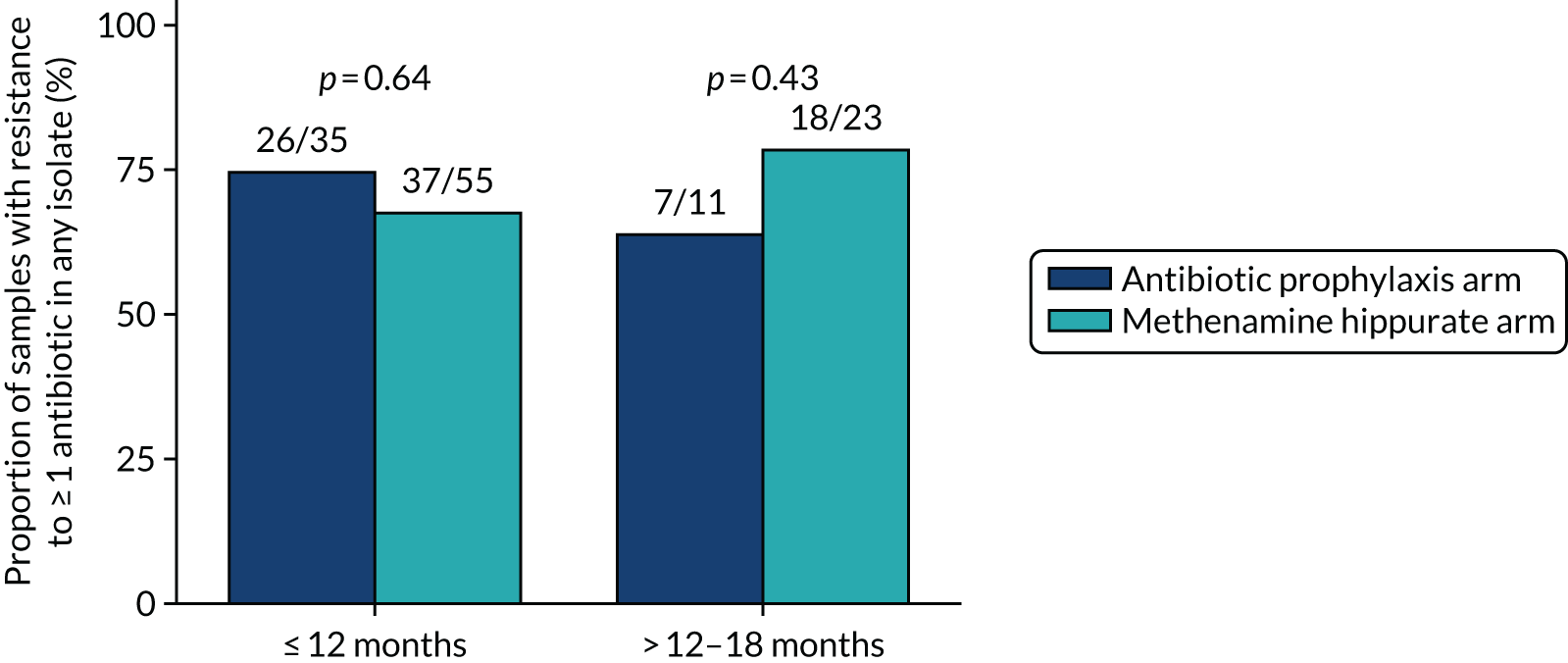
FIGURE 32.
Development of resistance since baseline in any significant isolate from any urine sample. (a) Amoxicillin; (b) cefalexin; (c) cefuroxime; (d) ciprofloxacin; (e) co-amoxiclav; (f) co-trimoxazole; (g) nitrofurantoin; and (h) trimethoprim. Resistance since baseline: participants with at least one resistant isolate in a post-baseline urine sample out of those who demonstrated sensitivity in isolates from their baseline urine sample or did not have any isolates in their baseline urine sample. Known resistance since baseline: participants with at least one resistant isolate in a post-baseline urine sample out of those who demonstrated sensitivity in isolates from their baseline urine sample.
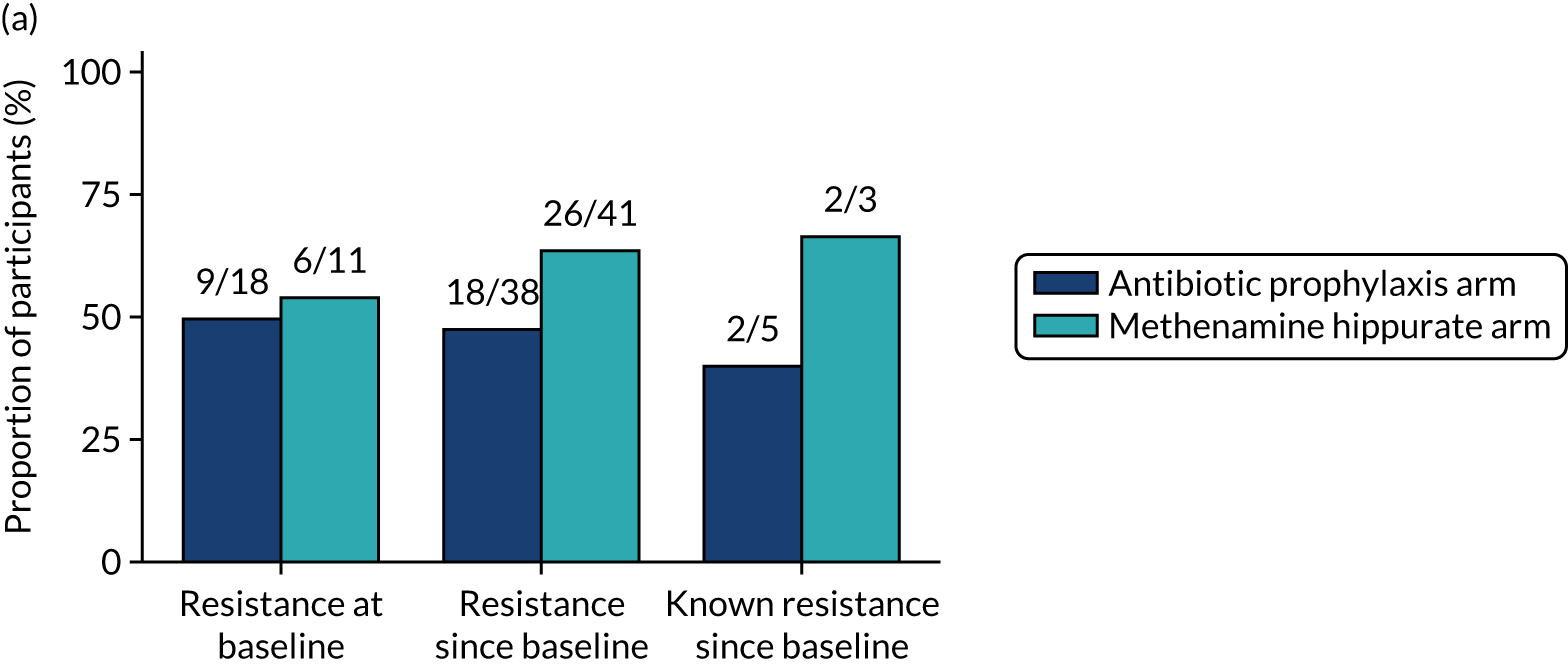
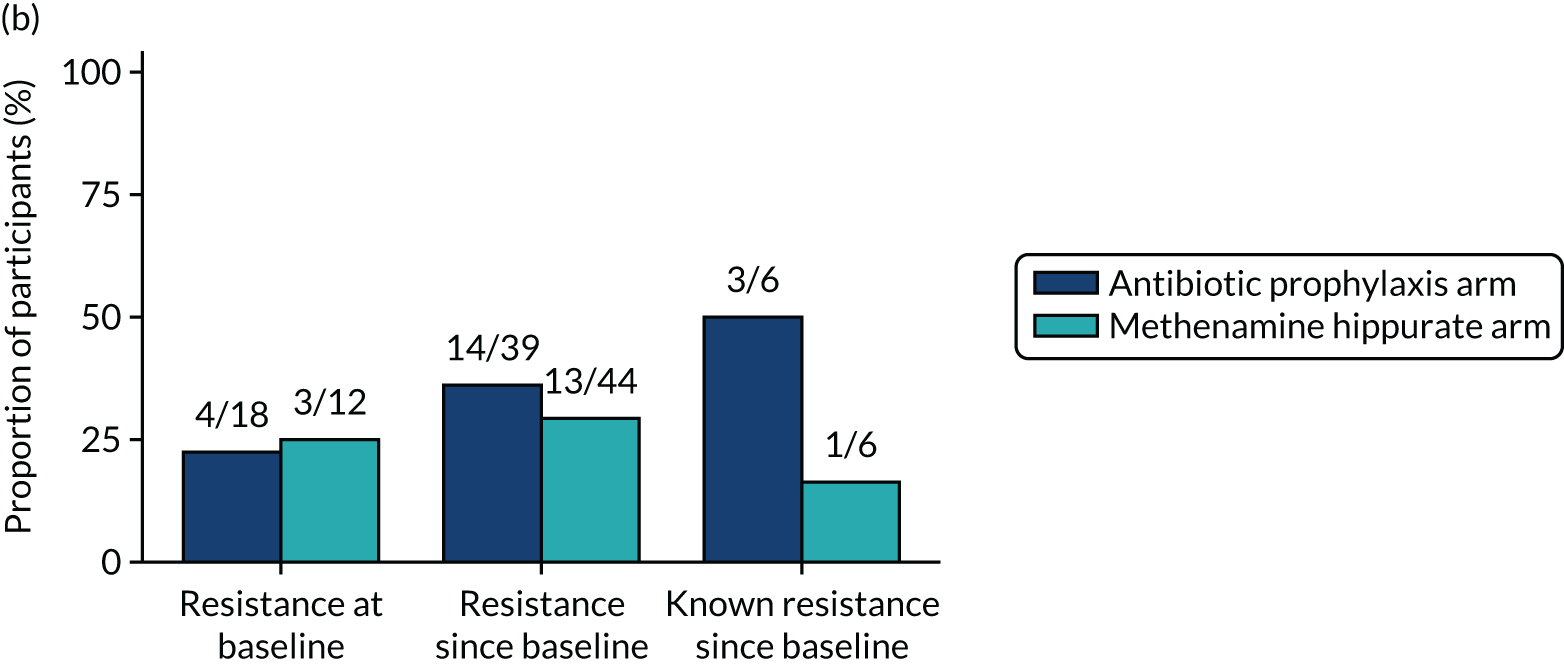
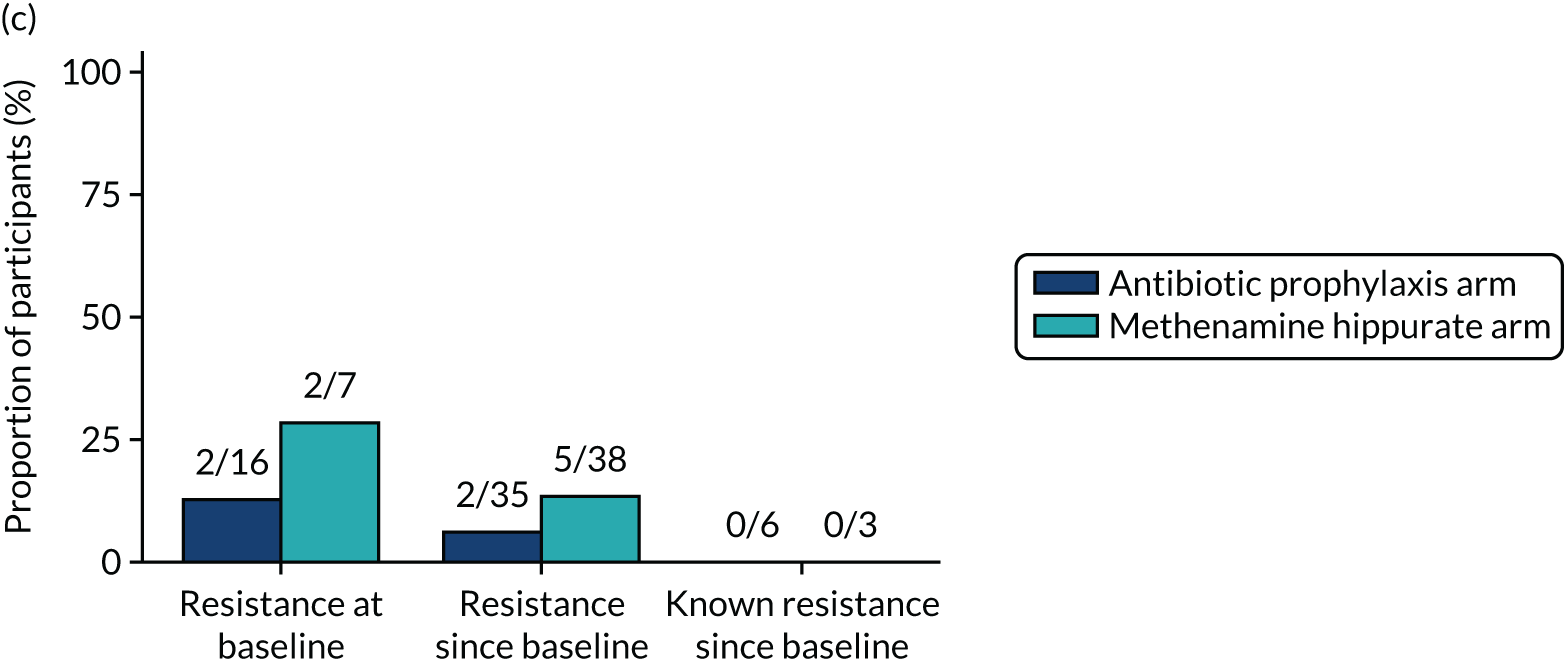
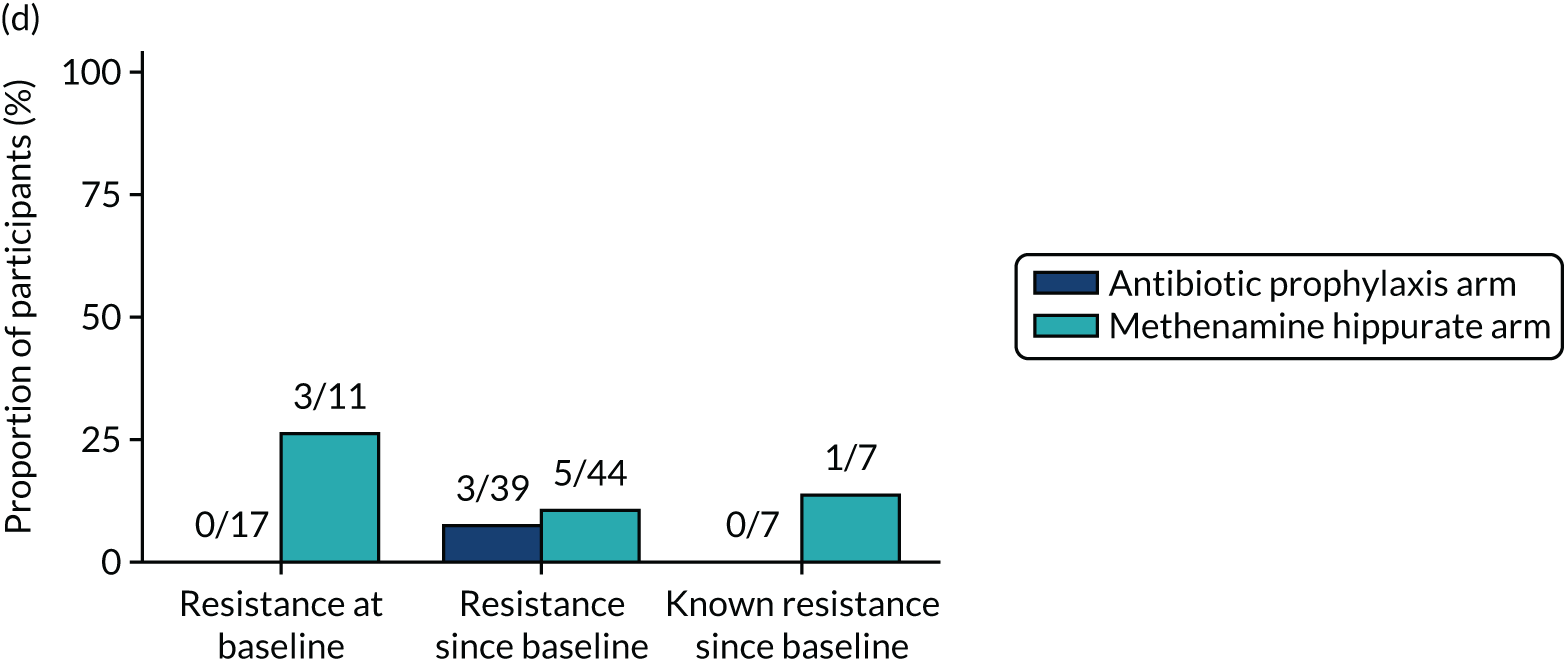
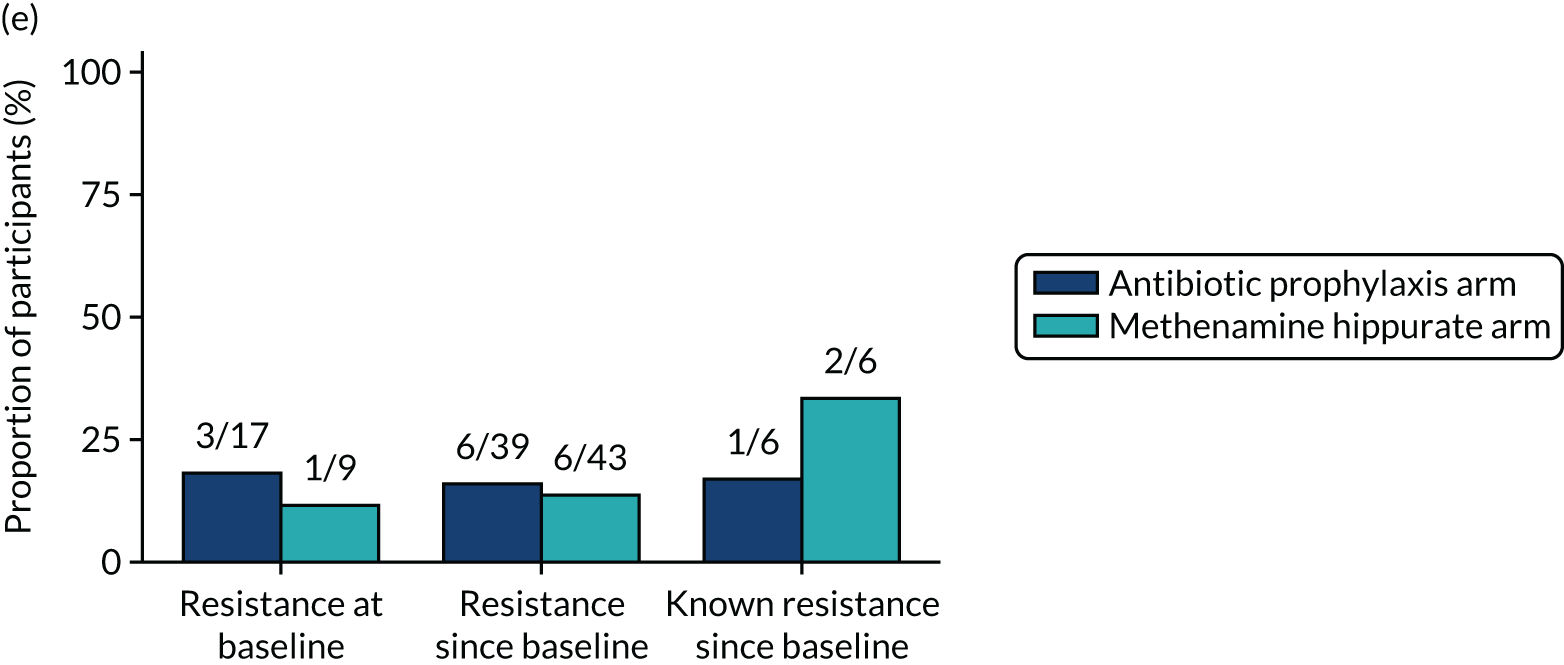
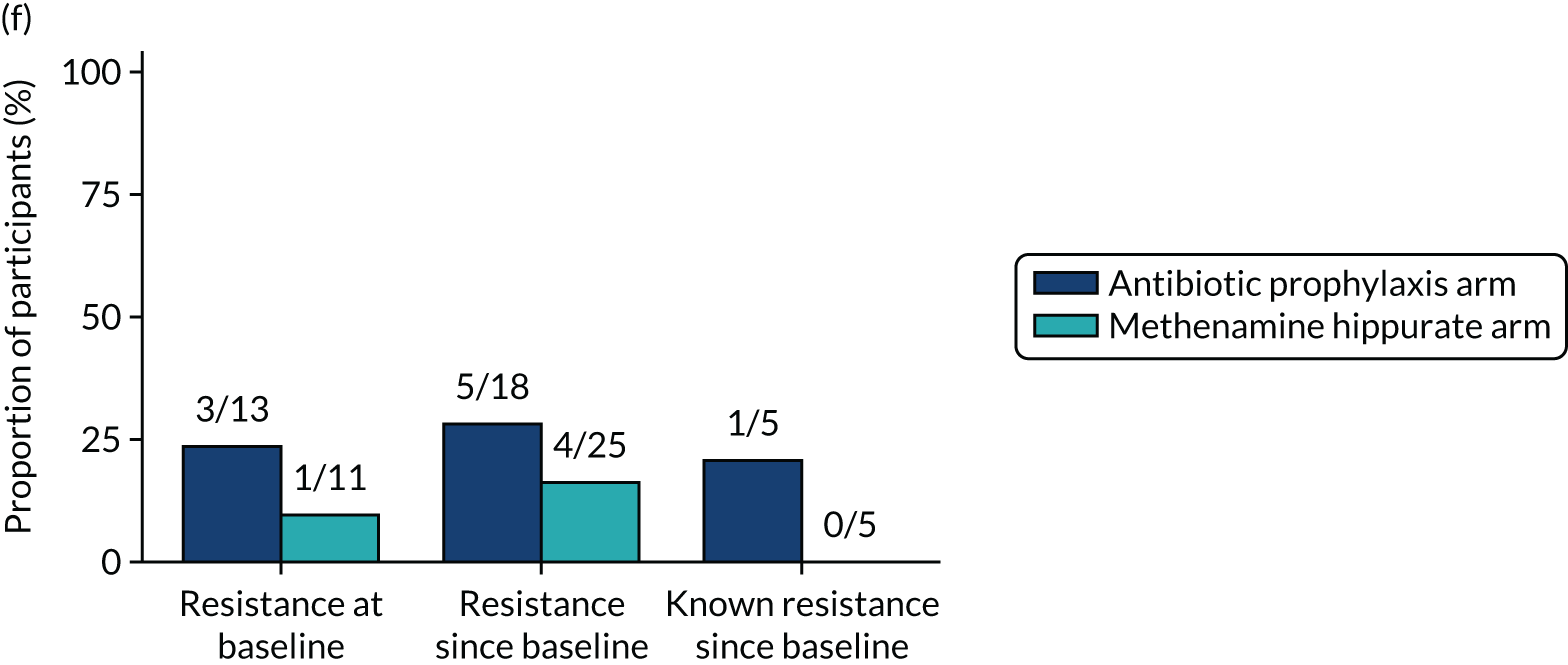
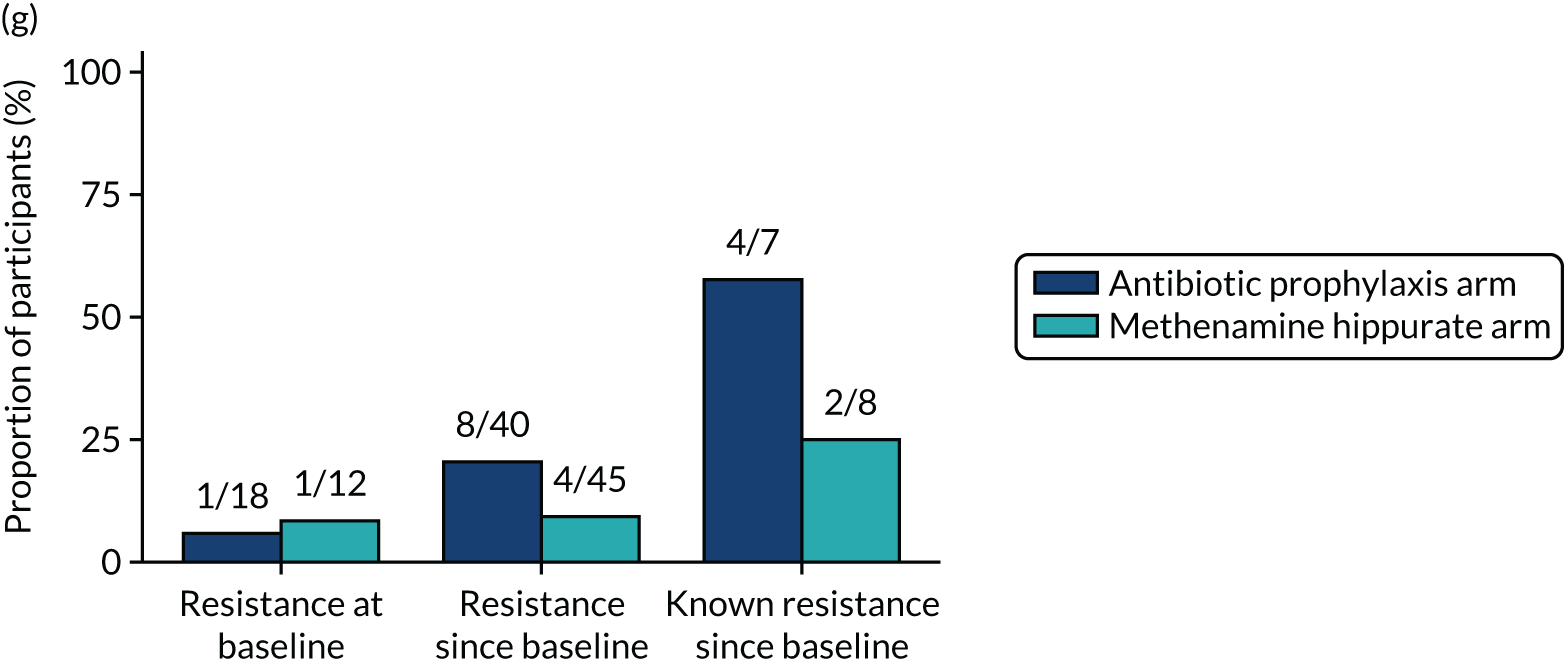
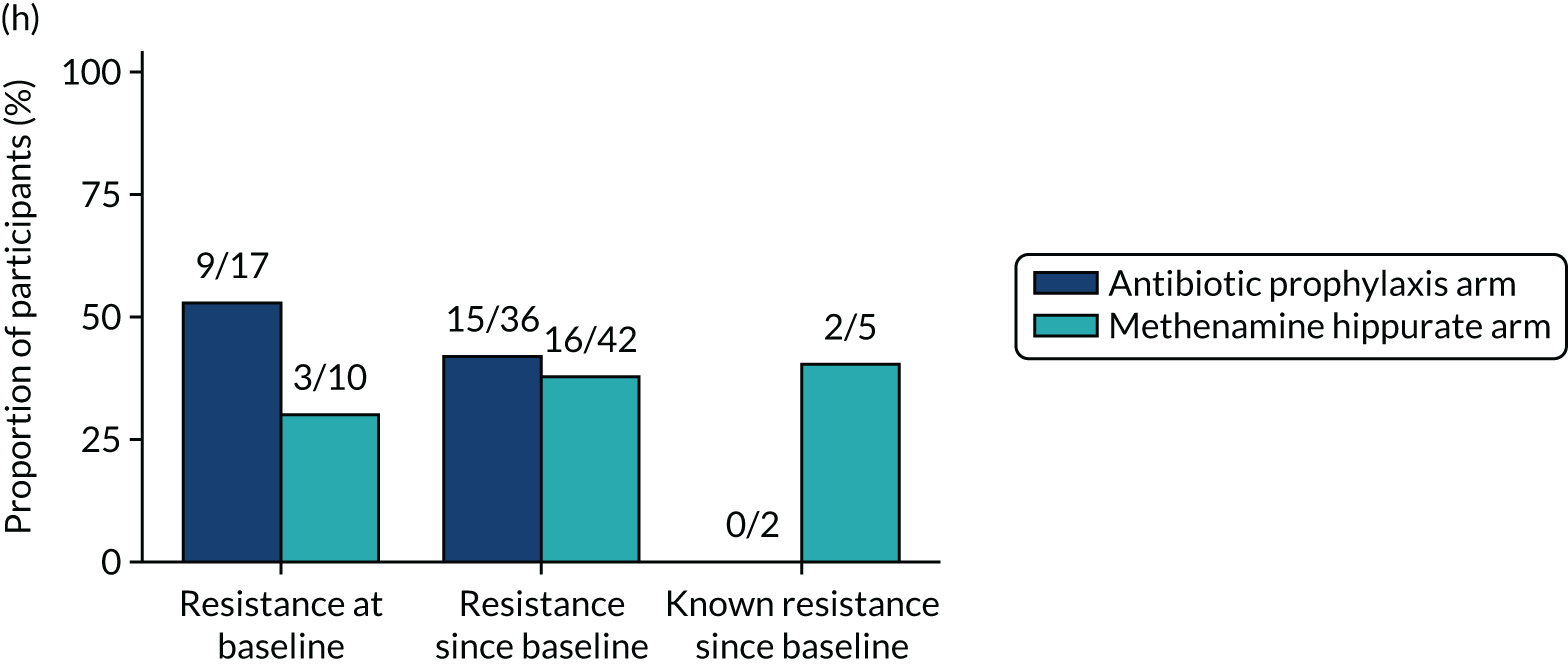
| Time point | Antibiotic prophylaxis arm (N = 120), n/N (%) | Methenamine hippurate arm (N = 120), n/N (%) | χ2 p-value |
|---|---|---|---|
| Overall | 64/557 (11) | 79/571 (14) | 0.24 |
| Baseline | 18/111 (16) | 13/111 (12) | 0.33 |
| Months 3–12 | 22/323 (7) | 44/326 (14) | 0.005 |
| Months 15–18 | 24/123 (20) | 22/134 (16) | 0.52 |
| Identifier | Randomised arm | Changed treatment strategy | Days to change in strategy | Event description | Severity | Relationship to IMP | Days from trial entry to SAE start |
|---|---|---|---|---|---|---|---|
| 10–0003 | Methenamine hippurate | No | Anaphylactic reaction | Moderate | Unrelated | 17 | |
| 10–0006 | Methenamine hippurate | No | Pneumonia | Moderate | Unrelated | 153 | |
| 10–0041 | Antibiotic prophylaxis | Yes | 294 | Asthma | Moderate | Unrelated | 48 |
| 10–0041 | Antibiotic prophylaxis | Yes | 294 | Abdominal pain | Severe | Possible | 253 |
| 10–0051 | Antibiotic prophylaxis | No | Caesarean section | Mild | Unrelated | 161 | |
| 10–0074 | Antibiotic prophylaxis | No | Tonsillitis | Moderate | Unrelated | 463 | |
| 10–0265 | Antibiotic prophylaxis | No | Alanine aminotransferase levels increased | Moderate | Probable | 271 | |
| 11–0048 | Methenamine hippurate | Yes | 105 | Ear infection | Moderate | Unrelated | 388 |
| 11–0085 | Methenamine hippurate | No | Procedural pain | Mild | Unrelated | 506 | |
| 11–0308 | Antibiotic prophylaxis | No | Pathological fracture | Severe | Unrelated | 47 | |
| 11–0308 | Antibiotic prophylaxis | No | Pneumonia | Severe | Unrelated | 166 | |
| 11–0320 | Methenamine hippurate | No | Seizure | Moderate | Unrelated | 357 | |
| 11–0508 | Antibiotic prophylaxis | No | Neurogenic shock | Mild | Unrelated | 428 | |
| 12–0035 | Antibiotic prophylaxis | Yes | 170 | Thyroid mass | Moderate | Unrelated | 194 |
| 12–0035 | Antibiotic prophylaxis | Yes | 170 | Angina pectoris | Moderate | Unrelated | 314 |
| 12–0080 | Methenamine hippurate | No | Abdominal pain | Moderate | Unrelated | 41 | |
| 12–0080 | Methenamine hippurate | No | Renal colic | Moderate | Unlikely | 175 | |
| 12–0306 | Methenamine hippurate | No | Angina unstable | Moderate | Unrelated | 88 | |
| 12–0319 | Antibiotic prophylaxis | No | Pneumonia | Severe | Unrelated | 409 | |
| 13–0276 | Antibiotic prophylaxis | No | Constipation | Mild | Unrelated | 471 | |
| 13–0276 | Antibiotic prophylaxis | No | Small intestinal obstruction | Severe | Unrelated | 473 | |
| 13–0294 | Methenamine hippurate | No | Fall | Severe | Unrelated | 456 | |
| 13–0295 | Antibiotic prophylaxis | No | Atrial tachycardia | Severe | Unrelated | 22 | |
| 13–0295 | Antibiotic prophylaxis | No | Lacunar stroke | Severe | Unrelated | 126 | |
| 13–0298 | Antibiotic prophylaxis | No | Infection | Mild | Unrelated | 297 | |
| 13–0304 | Methenamine hippurate | No | Dyspepsia | Moderate | Unrelated | 267 | |
| 13–0762 | Methenamine hippurate | No | Knee arthroplasty | Severe | Unrelated | 160 | |
| 13–0763 | Antibiotic prophylaxis | No | Vaginal prolapse repair | Severe | Unrelated | 49 | |
| 13–0763 | Antibiotic prophylaxis | No | Ovarian cyst | Mild | Unrelated | 300 | |
| 13–0763 | Antibiotic prophylaxis | No | Dyspnoea | Mild | Unrelated | 329 | |
| 13–0773 | Antibiotic prophylaxis | No | Large intestinal obstruction | Severe | Unrelated | 176 | |
| 14–0286 | Methenamine hippurate | No | Atrial fibrillation | Severe | Unrelated | 555 | |
| 14–0316 | Methenamine hippurate | No | Haemorrhage | Severe | Unrelated | 57 | |
| 14–0509 | Antibiotic prophylaxis | No | Breast cancer stage I | Severe | Unrelated | 198 | |
| 14–0509 | Antibiotic prophylaxis | No | Wound infection | Severe | Unrelated | 283 | |
| 14–0509 | Antibiotic prophylaxis | No | Diarrhoea | Severe | Unrelated | 349 | |
| 17–0772 | Methenamine hippurate | No | Lung lobectomy | Severe | Unrelated | 267 | |
| 18–0326 | Methenamine hippurate | No | Oedema peripheral | Mild | Unrelated | 286 |
| Antibiotic prophylaxis arm (N = 120) | Methenamine hippurate arm (N = 120) | Total (N = 240) | |
|---|---|---|---|
| All AEs | |||
| Number of events per participant | |||
| Mean (SD) | 2.1 (3.0) | 2.1 (2.6) | 2.1 (2.8) |
| Median (IQR) | 1 (0–3) | 1 (0–3) | 1 (0–3) |
| Minimum, maximum | 0, 17 | 0, 13 | 0, 17 |
| Worst grade reported per participant, n (%) | |||
| None | 48 (40) | 39 (33) | 87 (36) |
| Mild | 33 (28) | 45 (38) | 78 (33) |
| Moderate | 31 (26) | 30 (25) | 61 (25) |
| Severe | 8 (7) | 6 (5) | 14 (6) |
| ARs (possibly, probably, definitely) | |||
| Number of ARs per participant | |||
| Mean (SD) | 0.4 (0.9) | 0.4 (0.7) | 0.4 (0.8) |
| Median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| Minimum, maximum | 0, 5 | 0, 3 | 0, 5 |
| Worst grade AR reported per participant, n (%) | |||
| None | 88 (73) | 87 (73) | 175 (73) |
| Mild | 21 (18) | 25 (21) | 46 (19) |
| Moderate | 10 (8) | 8 (7) | 18 (8) |
| Severe | 1 (1) | 0 (0) | 1 (0) |
| Event term | Antibiotic prophylaxis arm (N = 120), n (%) | Methenamine hippurate arm (N = 120), n (%) | Total (N = 240), n (%) |
|---|---|---|---|
| Lower respiratory tract infection | 6 (5) | 13 (11) | 19 (8) |
| Nausea | 12 (10) | 5 (4) | 17 (7) |
| Abdominal pain | 5 (4) | 11 (9) | 16 (7) |
| Diarrhoea | 7 (6) | 5 (4) | 12 (5) |
| Alanine aminotransferase levels increased | 5 (4) | 5 (4) | 10 (4) |
| Back pain | 6 (5) | 4 (3) | 10 (4) |
| Headache | 4 (3) | 6 (5) | 10 (4) |
| Candida infection | 3 (3) | 6 (5) | 9 (4) |
| Dyspepsia | 6 (5) | 3 (3) | 9 (4) |
| Rash | 3 (3) | 5 (4) | 8 (3) |
| Abdominal discomfort | 4 (3) | 3 (3) | 7 (3) |
| Dyspnoea | 5 (4) | 2 (2) | 7 (3) |
| Fall | 1 (1) | 6 (5) | 7 (3) |
| Vomiting | 3 (3) | 4 (3) | 7 (3) |
| Depressed mood | 1 (1) | 4 (3) | 5 (2) |
| Herpes zoster | 4 (3) | 1 (1) | 5 (2) |
| Vulvovaginal discomfort | 4 (3) | 1 (1) | 5 (2) |
Appendix 3 Economic evaluation supplementary information
| Resource use | |||||
|---|---|---|---|---|---|
| Item | Cost (£) | Unit | Notes | ||
| Inpatient | 387.00 | Per night |
Non-elective: short stay (NES) CC score of 0 or 1 NHS Reference Costs 2018–19 40 |
||
| Outpatient appointment | 108.00 | Per appointment |
Total outpatient attendances: urology Total cost column (i.e. not the consultant-led or non-consultant-led cost) NHS Reference Costs 2018–19 40 |
||
| A&E/casualty attendance | 168.00 | Per visit |
Total outpatient attendances: A&E Total cost column (i.e. not the consultant-led or non-consultant-led cost) NHS Reference Costs 2018–19 40 |
||
| GP practice visit | 39.23 | Per appointment |
PSSRU 201939 Per 9.22 minutes including direct care staff costs (with qualification costs) |
||
| GP home visit | 49.02 | Per appointment |
PSSRU 201556 Time spent in the patient’s home: 11.4 minutes, as PSSRU 201939 did not include it (travel time has been allowed for in the estimation of the ratio of direct to indirect time spent on home visits) PSSRU 201939 Per minute of contact: £4.30. Calculation: 11.4 × 4.30 = £49.02 |
||
| Nurse (GP practice) | 10.50 | Per consultation |
PSSRU 201556 Time: advanced nurse 15 minutes as PSSRU 201939 did not include it PSSRU 201939 £42 per hour costs including qualifications Cost per minute: 42/60 = £0.70. Calculation: 15 × 0.70 = £10.50 |
||
| Nurse home visit | 17.50 | Per consultation |
PSSRU 201556 Time spent in the patient’s home: advanced nurse 25 minutes as PSSRU 201939 did not include it PSSRU 201939 £42 per hour costs including qualifications Cost per minute: 42/60 = £0.70. Calculation: 25 × 0.70 = £17.50 |
||
| Telephone consultation with GP | 30.53 | Per consultation |
PSSRU 201556 Average telephone consultation length: 7.1 minutes as PSSRU 201939 did not include it PSRU 201939 Per minute of contact: £4.30. Calculation: 7.1 × 4.30 = £30.53 |
||
| Telephone consultation with hospital doctor | 30.53 | Per consultation |
Apply same cost as GP telephone consultation (7.1 minutes) |
||
| Telephone consultation with nurse | 4.20 | Per consultation |
PSSRU 201556 Time: advanced nurse 6 minutes as PSSRU 201939 did not include it PSSRU 201939 Cost per minute: £0.70. Calculation 6 × 0.70 = £4.20 |
||
| Telephone consultation with other health professional (this was assumed to be a 111 call) | 15.05 | Per consultation | NHS 111 call costs £12.26 per call in PSSRU 201157 price year. This estimate has been inflated to the 2019 price year using the PSSRU pay and prices index39 | ||
| Out-of-hours consultation with GP | 73.96 | Per consultation |
PSSRU 201556 Clinic time 17.2 minutes as not recorded in PSSRU 201939 PSSRU 201939 Cost per minute = £4.30 Calculation 17.2 × 4.30 = £73.96 |
||
| Out-of-hours consultation with hospital doctor | 13.47 | Per consultation |
PSSRU 201556 Clinic time 17.2 minutes as not recorded in PSSRU 201939 PSSRU 201939 Cost per hour: £47. Calculation (47/60) × 17.2 = £13.47 |
||
| Out-of-hours consultation with other health professional | 108.00 | Per consultation |
Apply same cost as outpatient appointment NHS Reference Costs 2018–19 40 Total outpatient attendances: urology |
||
| Out-of-hours consultation with nurse | 10.50 | 15 minutes per consultation |
PSSRU 201556 Time: advanced nurse 15 minutes as PSSRU 201939 did not include it PSSRU 201939 £42 per hour costs including qualifications Cost per minute: 42/60 = £0.70. Calculation 15 × 0.70 = £10.50 |
||
| Time and travel unit costs | |||||
| Inpatient visit | 110.87 | Per visit | AnTIC trial supplementary material table 21:45 £100.10 inflated to 2019 price year using PSSRU 201939 | ||
| GP visit | 17.35 | Per visit | AnTIC trial supplementary material table 21:45 £15.66 inflated to 2019 price year using PSSRU 201939 | ||
| Outpatient visit | 42.38 | Per visit | AnTIC trial supplementary material table 21:45 £38.26 inflated to 2019 price year using PSSRU 201939 | ||
| Medication name | Dosage | Frequency per day | Unit cost | Cost per day | Source |
| Intervention medications | |||||
| Cefalexin | 250 mg daily | 1 | 2.25/28 = £0.08 | 2.25/28 = £0.08 | https://bnf.nice.org.uk/drug/cefalexin.html (accessed November 2020) |
| Nitrofurantoin | 50 mg | 1 | 8/28 = £0.29 | 8/28 = £0.29 | https://bnf.nice.org.uk/medicinal-forms/nitrofurantoin.html (accessed November 2020) |
| Nitrofurantoin | 100 mg | 1 | 12.75/28 = £0.46 | 12.75/28 = £0.46 | https://bnf.nice.org.uk/medicinal-forms/nitrofurantoin.html (accessed November 2020) |
| Trimethoprim | 100 mg | 1 | 0.95/28 = £0.03 | 0.95/28 = £0.03 | https://bnf.nice.org.uk/medicinal-forms/trimethoprim.html (accessed November 2020) |
| Methenamine hippurate | 1 g | 2 | 19.74/60 = £0.33 | (19.74/60) × 2 = £0.66 | https://bnf.nice.org.uk/drug/methenamine-hippurate.html (accessed November 2020) |
| UTI antibiotics | |||||
| Amoxicillin | 500 mg | 3 | 7.00/100 = £0.07 | (7.00/100) × 3 = £0.21 | https://bnf.nice.org.uk/medicinal-forms/amoxicillin.html (accessed November 2020) |
| Cefalexin | 250 mg | 4 | 1.74/28 = £0.06 | (1.74/28) × 4 = £0.24 | https://bnf.nice.org.uk/medicinal-forms/cefalexin.html (accessed November 2020) |
| Ciprofloxacin | 500 mg | 2 | 9.1/100 = £0.09 | (9.1/100) × 2 = £0.18 | https://bnf.nice.org.uk/medicinal-forms/ciprofloxacin.html#PHP74800 (accessed November 2020) |
| Clarithromycin | 500 mg | 2 | 1.97/14 = £0.14 | (1.97/14) × 2 = £0.28 | https://bnf.nice.org.uk/medicinal-forms/clarithromycin.html (accessed November 2020) |
| Co-amoxiclav | 250 mg/125 mg | 3 | 1.88/21 = £0.09 | (1.88/21) × 3 = £0.27 | https://bnf.nice.org.uk/medicinal-forms/co-amoxiclav.html (accessed November 2020) |
| Gentamicin | 360 mg/120 ml | 1 | 174.07/20 = £8.70 | (174.07/20) × 1 = £8.70 | https://bnf.nice.org.uk/medicinal-forms/gentamicin.html (accessed November 2020) |
| Nitrofurantoin | 100 mg | 2 | 9.5/14 = £0.68 | (9.5/14) × 2 = £1.36 | https://bnf.nice.org.uk/medicinal-forms/nitrofurantoin.html#PHP74629 (accessed November 2020) |
| Trimethoprim | 200 mg | 2 | 0.74/14 = £0.05 | (0.74/14) × 2 = £0.10 | https://bnf.nice.org.uk/medicinal-forms/trimethoprim.html (accessed November 2020) |
| Pivmecillinam | 200 mg | 3.333 | 5.4/10 = £0.54 | (5.4/10) × 3.33 = £1.80 | https://bnf.nice.org.uk/medicinal-forms/pivmecillinam-hydrochloride.html (accessed November 2020) |
| Health-care resource use | Antibiotic prophylaxis arm (N = 102) | Methenamine hippurate arm (N = 103) | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Baseline | ||||
| Number of inpatient nights | 99 | 0.263 (1.258) | 98 | 0.347 (1.277) |
| Number of outpatient appointments | 99 | 1.808 (1.589) | 100 | 2.130 (3.240) |
| Number of casualty attendances | 98 | 0.214 (0.722) | 96 | 0.167 (0.474) |
| Number of GP consultations | 98 | 2.133 (2.003) | 98 | 2.265 (2.161) |
| Number of GP consultations at home | 98 | 0.051 (0.333) | 96 | 0.031 (0.306) |
| Number of nurse consultations | 99 | 0.495 (0.862) | 97 | 0.660 (1.030) |
| Number of nurse consultations at home | 99 | 0.020 (0.201) | 96 | 0.000 (0.000) |
| Number of GP telephone consultations | 99 | 0.455 (0.848) | 98 | 0.837 (2.133) |
| Number of hospital doctor telephone consultations | 99 | 0.010 (0.101) | 98 | 0.041 (0.284) |
| Number of nurse telephone consultations | 99 | 0.131 (0.528) | 98 | 0.286 (0.963) |
| Number of other health professional consultations | 99 | 0.000 (0.000) | 98 | 0.020 (0.202) |
| Number of out-of-hours GP consultations | 98 | 0.071 (0.329) | 95 | 0.084 (0.453) |
| Number of out-of-hours hospital doctor consultations | 98 | 0.020 (0.202) | 95 | 0.074 (0.467) |
| Number of out-of-hours nurse consultations | 98 | 0.041 (0.404) | 95 | 0.074 (0.393) |
| Number of out-of-hours other health professional consultations | 98 | 0.010 (0.101) | 95 | 0.021 (0.144) |
| 3 months | ||||
| Number of inpatient nights | 80 | 0.163 (0.920) | 83 | 0.060 (0.361) |
| Number of outpatient appointments | 79 | 0.810 (1.641) | 83 | 0.904 (1.470) |
| Number of casualty attendances | 80 | 0.075 (0.265) | 83 | 0.060 (0.286) |
| Number of GP consultations | 80 | 0.812 (1.170) | 81 | 1.037 (1.453) |
| Number of GP consultations at home | 81 | 0.062 (0.398) | 81 | 0.000 (0.000) |
| Number of nurse consultations | 80 | 0.388 (0.771) | 82 | 0.500 (0.820) |
| Number of nurse consultations at home | 80 | 0.013 (0.112) | 82 | 0.000 (0.000) |
| Number of GP telephone consultations | 80 | 0.150 (0.677) | 80 | 0.237 (0.621) |
| Number of hospital doctor telephone consultations | 80 | 0.087 (0.427) | 80 | 0.037 (0.191) |
| Number of nurse telephone consultations | 80 | 0.050 (0.314) | 80 | 0.237 (0.750) |
| Number of other health professional consultations | 80 | 0.000 (0.000) | 80 | 0.000 (0.000) |
| Number of out-of-hours GP consultations | 79 | 0.013 (0.113) | 80 | 0.025 (0.157) |
| Number of out-of-hours hospital doctor consultations | 79 | 0.013 (0.113) | 80 | 0.025 (0.157) |
| Number of out-of-hours nurse consultations | 79 | 0.000 (0.000) | 80 | 0.000 (0.000) |
| Number of out-of-hours other health professional consultations | 79 | 0.000 (0.000) | 80 | 0.087 (0.679) |
| 6 months | ||||
| Number of inpatient nights | 69 | 0.087 (0.612) | 80 | 0.125 (0.718) |
| Number of outpatient appointments | 68 | 0.985 (2.919) | 79 | 0.962 (2.060) |
| Number of casualty attendances | 68 | 0.118 (0.368) | 80 | 0.050 (0.219) |
| Number of GP consultations | 72 | 0.819 (0.939) | 79 | 0.823 (1.071) |
| Number of GP consultations at home | 68 | 0.044 (0.270) | 79 | 0.025 (0.225) |
| Number of nurse consultations | 70 | 0.386 (0.708) | 78 | 0.449 (0.784) |
| Number of nurse consultations at home | 68 | 0.029 (0.243) | 79 | 0.038 (0.250) |
| Number of GP telephone consultations | 67 | 0.239 (0.818) | 78 | 0.192 (0.428) |
| Number of hospital doctor telephone consultations | 67 | 0.000 (0.000) | 78 | 0.026 (0.159) |
| Number of nurse telephone consultations | 67 | 0.045 (0.208) | 78 | 0.167 (0.590) |
| Number of other health professional consultations | 67 | 0.000 (0.000) | 78 | 0.000 (0.000) |
| Number of out-of-hours GP consultations | 67 | 0.000 (0.000) | 77 | 0.039 (0.253) |
| Number of out-of-hours hospital doctor consultations | 67 | 0.000 (0.000) | 77 | 0.000 (0.000) |
| Number of out-of-hours nurse consultations | 67 | 0.000 (0.000) | 77 | 0.000 (0.000) |
| Number of out-of-hours other health professional consultations | 67 | 0.015 (0.122) | 77 | 0.000 (0.000) |
| 9 months | ||||
| Number of inpatient nights | 71 | 0.085 (0.603) | 75 | 0.080 (0.693) |
| Number of outpatient appointments | 72 | 0.653 (1.531) | 73 | 0.726 (1.158) |
| Number of casualty attendances | 71 | 0.056 (0.232) | 71 | 0.113 (0.361) |
| Number of GP consultations | 70 | 0.886 (1.325) | 77 | 0.870 (1.116) |
| Number of GP consultations at home | 71 | 0.042 (0.356) | 74 | 0.000 (0.000) |
| Number of nurse consultations | 71 | 0.296 (0.571) | 77 | 0.455 (1.153) |
| Number of nurse consultations at home | 70 | 0.000 (0.000) | 74 | 0.014 (0.116) |
| Number of GP telephone consultations | 70 | 0.229 (0.745) | 74 | 0.108 (0.354) |
| Number of hospital doctor telephone consultations | 70 | 0.029 (0.168) | 74 | 0.000 (0.000) |
| Number of nurse telephone consultations | 70 | 0.114 (0.468) | 74 | 0.189 (0.541) |
| Number of other health professional consultations | 70 | 0.000 (0.000) | 74 | 0.000 (0.000) |
| Number of out-of-hours GP consultations | 72 | 0.000 (0.000) | 73 | 0.000 (0.000) |
| Number of out-of-hours hospital doctor consultations | 72 | 0.028 (0.236) | 73 | 0.000 (0.000) |
| Number of out-of-hours nurse consultations | 72 | 0.000 (0.000) | 73 | 0.000 (0.000) |
| Number of out-of-hours other health professional consultations | 72 | 0.000 (0.000) | 73 | 0.000 (0.000) |
| 12 months | ||||
| Number of inpatient nights | 67 | 0.000 (0.000) | 72 | 0.056 (0.285) |
| Number of outpatient appointments | 65 | 0.954 (2.154) | 73 | 0.822 (1.206) |
| Number of casualty attendances | 64 | 0.062 (0.244) | 72 | 0.069 (0.306) |
| Number of GP consultations | 65 | 1.185 (1.368) | 74 | 1.149 (1.246) |
| Number of GP consultations at home | 65 | 0.000 (0.000) | 74 | 0.000 (0.000) |
| Number of nurse consultations | 66 | 0.333 (0.664) | 73 | 0.342 (0.628) |
| Number of nurse consultations at home | 64 | 0.000 (0.000) | 71 | 0.000 (0.000) |
| Number of GP telephone consultations | 65 | 0.262 (0.644) | 71 | 0.197 (0.551) |
| Number of hospital doctor telephone consultations | 65 | 0.000 (0.000) | 71 | 0.014 (0.119) |
| Number of nurse telephone consultations | 65 | 0.000 (0.000) | 71 | 0.070 (0.308) |
| Number of other health professional consultations | 65 | 0.000 (0.000) | 71 | 0.000 (0.000) |
| Number of out-of-hours GP consultations | 66 | 0.015 (0.123) | 74 | 0.027 (0.163) |
| Number of out-of-hours hospital doctor consultations | 66 | 0.000 (0.000) | 74 | 0.000 (0.000) |
| Number of out-of-hours nurse consultations | 66 | 0.000 (0.000) | 74 | 0.000 (0.000) |
| Number of out-of-hours other health professional consultations | 66 | 0.000 (0.000) | 74 | 0.000 (0.000) |
| 18 months | ||||
| Number of inpatient nights | 61 | 0.328 (1.491) | 72 | 0.097 (0.417) |
| Number of outpatient appointments | 61 | 1.180 (3.238) | 70 | 1.114 (1.611) |
| Number of casualty attendances | 59 | 0.153 (0.611) | 70 | 0.129 (0.414) |
| Number of GP consultations | 59 | 1.322 (1.624) | 71 | 1.606 (1.626) |
| Number of GP consultations at home | 61 | 0.000 (0.000) | 71 | 0.000 (0.000) |
| Number of nurse consultations | 61 | 0.393 (0.640) | 67 | 1.060 (2.656) |
| Number of nurse consultations at home | 60 | 0.000 (0.000) | 69 | 0.029 (0.169) |
| Number of GP telephone consultations | 59 | 0.305 (0.895) | 68 | 0.279 (0.709) |
| Number of hospital doctor telephone consultations | 59 | 0.034 (0.260) | 68 | 0.015 (0.121) |
| Number of nurse telephone consultations | 59 | 0.017 (0.130) | 68 | 0.044 (0.207) |
| Number of other health professional consultations | 59 | 0.000 (0.000) | 68 | 0.015 (0.121) |
| Number of out-of-hours GP consultations | 60 | 0.017 (0.129) | 68 | 0.000 (0.000) |
| Number of out-of-hours hospital doctor consultations | 60 | 0.033 (0.258) | 68 | 0.000 (0.000) |
| Number of out-of-hours nurse consultations | 60 | 0.000 (0.000) | 68 | 0.000 (0.000) |
| Number of out-of-hours other health professional consultations | 60 | 0.000 (0.000) | 68 | 0.000 (0.000) |
| Strategy | Cost (£) (SD) | Incremental cost (£) (95% CI)a | QALYs (SD) | Incremental QALYs (95% CI)a | ICER | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP for a QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Antibiotic prophylaxis (costs, n = 89; outcomes, n = 58) | 931 (2015) | 1.182 (0.345) | 0.49 | 0.38 | 0.35 | 0.34 | 0.33 | |||
| Methenamine hippurate (costs, n = 94; outcomes, n = 71) | 1013 (1024) | –40 (–684 to 603) | 1.133 (0.345) | 0.014 (–0.05 to 0.07) | Methenamine hippurate dominant | 0.51 | 0.62 | 0.65 | 0.66 | 0.67 |
FIGURE 33.
Cost-effectiveness plane for methenamine hippurate compared with antibiotic prophylaxis using QALYs (including UTI utilities) gained as the measure of outcome.
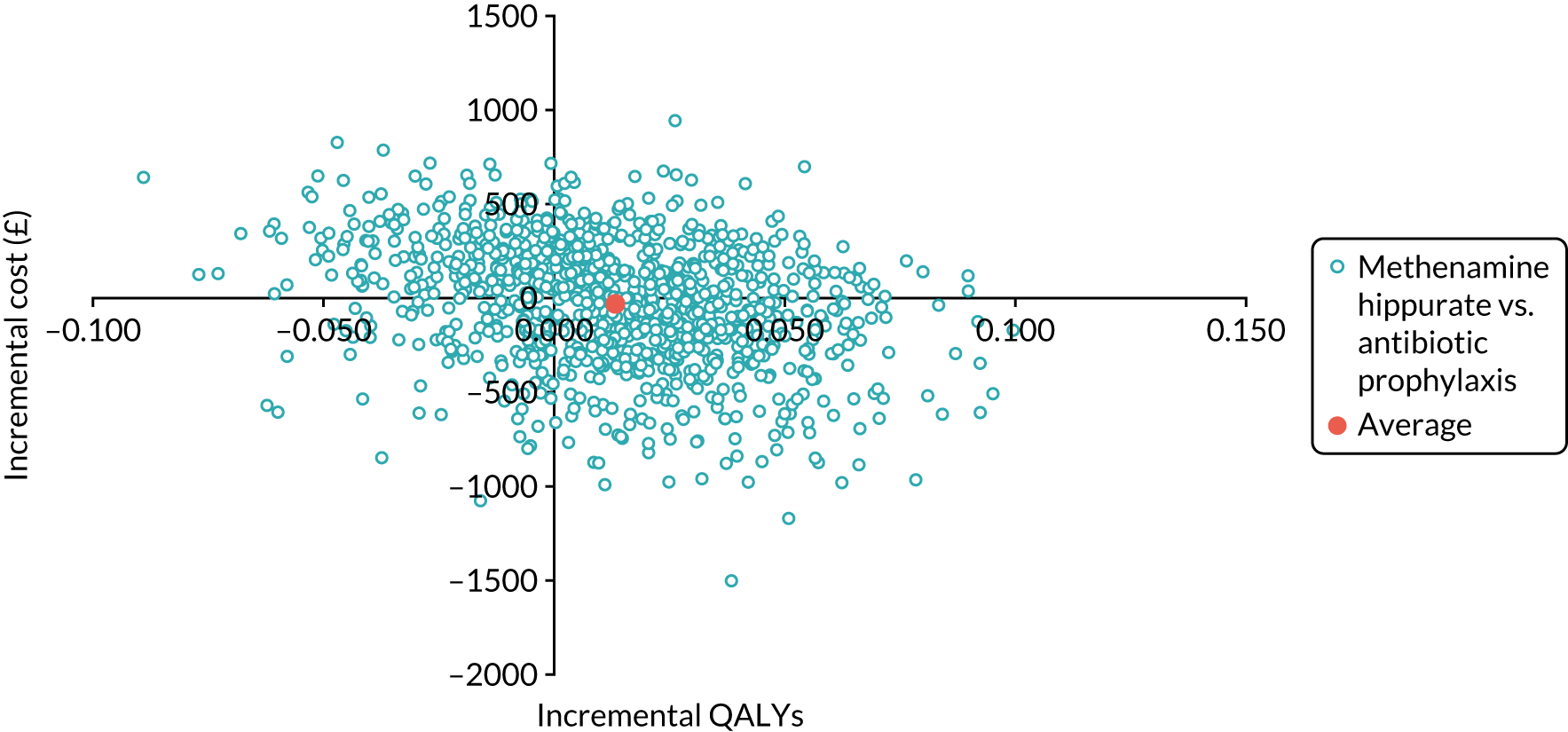
| Strategy | Cost (£) (SD) | Incremental cost (£) (95% CI)a | QALYs (SD) | Incremental QALYs (95% CI)a | ICER | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP for a QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Antibiotic prophylaxis (costs, n = 89; outcomes, n = 58) | 1276 (2775) | 1.182 (0.35) | 0.42 | 0.33 | 0.32 | 0.31 | 0.32 | |||
| Methenamine hippurate (costs, n = 94; outcomes, n = 71) | 1372 (1438) | –62.75 (–951 to 825) | 1.133 (0.35) | 0.014 (–0.05 to 0.07) | Methenamine hippurate dominant | 0.58 | 0.66 | 0.68 | 0.69 | 0.68 |
FIGURE 34.
Cost-effectiveness plane for methenamine hippurate compared with antibiotic prophylaxis (including participant costs).
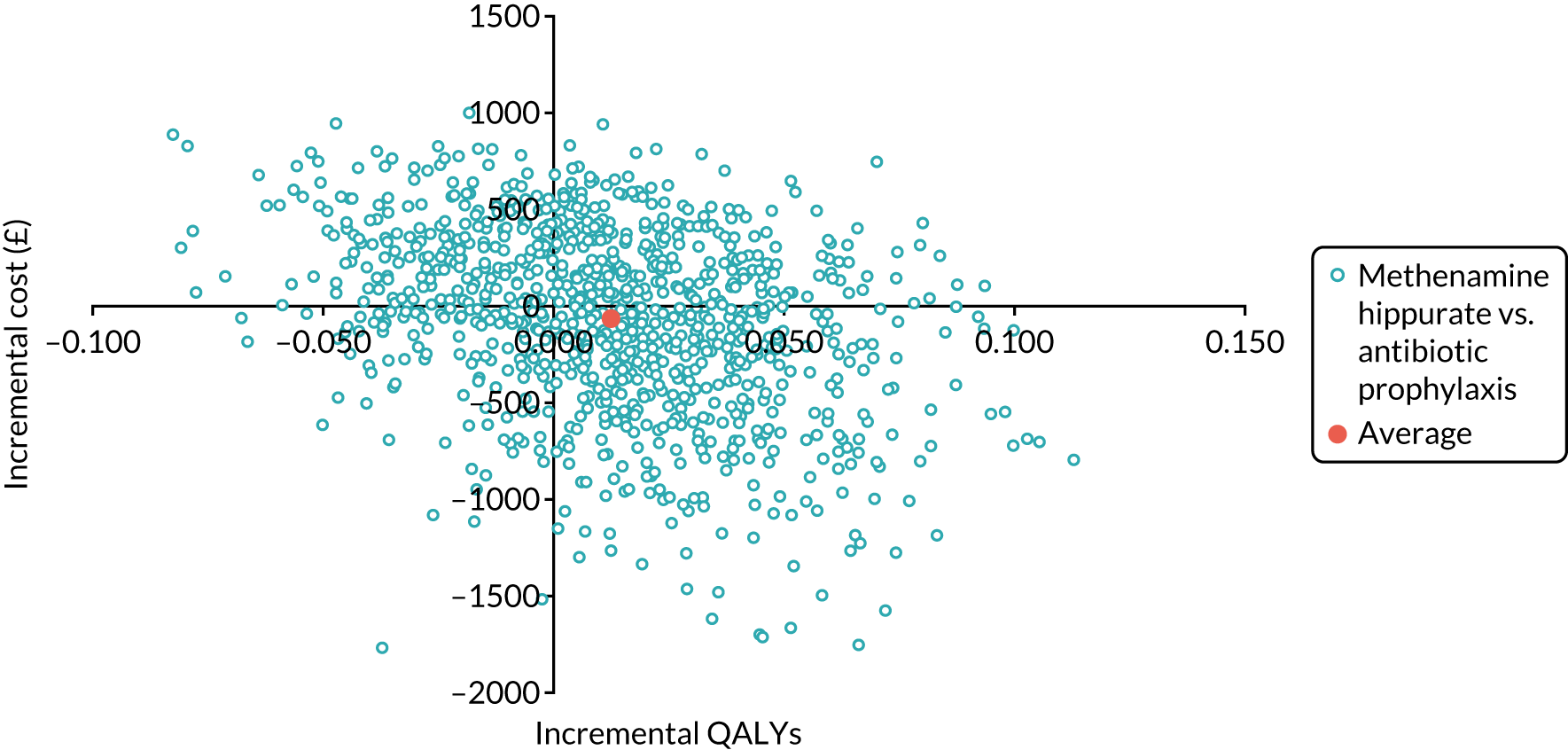
| Strategy | Cost (£) (SD) | Incremental cost (£) (95% CI)a | QALYs (SD) | Incremental QALYs (95% CI)a | ICER | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP for a QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Antibiotic prophylaxis (costs n = 81; outcomes n = 58) | 951 (2112) | 1.181 (0.35) | 0.51 | 0.42 | 0.40 | 0.38 | 0.38 | |||
| Methenamine hippurate (costs n = 86; outcomes n = 65) | 1045 (1066) | –14 (–701 to 674) | 1.117 (0.35) | 0.011 (–0.05 to 0.07) | Methenamine hippurate dominant | 0.49 | 0.58 | 0.60 | 0.62 | 0.62 |
FIGURE 35.
Cost-effectiveness plane for methenamine hippurate compared with antibiotic prophylaxis using QALYs gained as the measure of outcome.
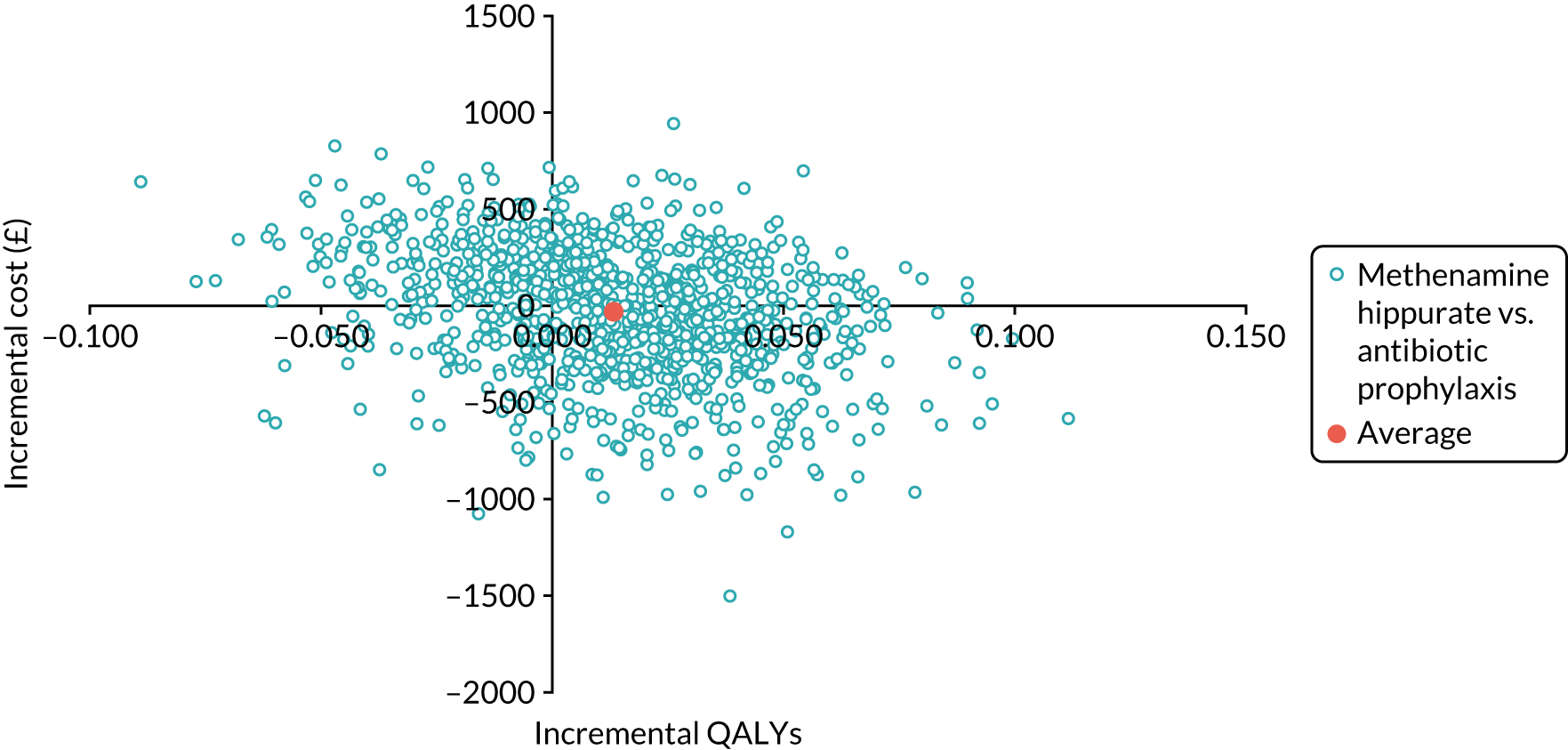
| Strategy | Cost (£) (SD) | Incremental cost (£) (95% CI)a | Incidence of UTI (SD) | Incremental incidence of UTIs avoided (95% CI)a | ICER | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid a UTI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £100 | £150 | £200 | £250 | ||||||
| Antibiotic prophylaxis (costs, n = 89; outcomes, n = 102) | 700 (1617) | 0.888 (1.22) | 0.65 | 0.73 | 0.78 | 0.80 | 0.81 | |||
| Methenamine hippurate (costs, n = 94; outcomes, n = 103) | 822 (848) | 83 (–414 to 580) | 1.398 (1.72) | –0.547 (–0.97 to –0.13) | Prophylaxis dominant | 0.35 | 0.27 | 0.22 | 0.20 | 0.19 |
FIGURE 36.
Cost-effectiveness plane for methenamine hippurate compared with antibiotic prophylaxis using number of UTIs avoided as the measure of outcome over treatment phase (12 months).
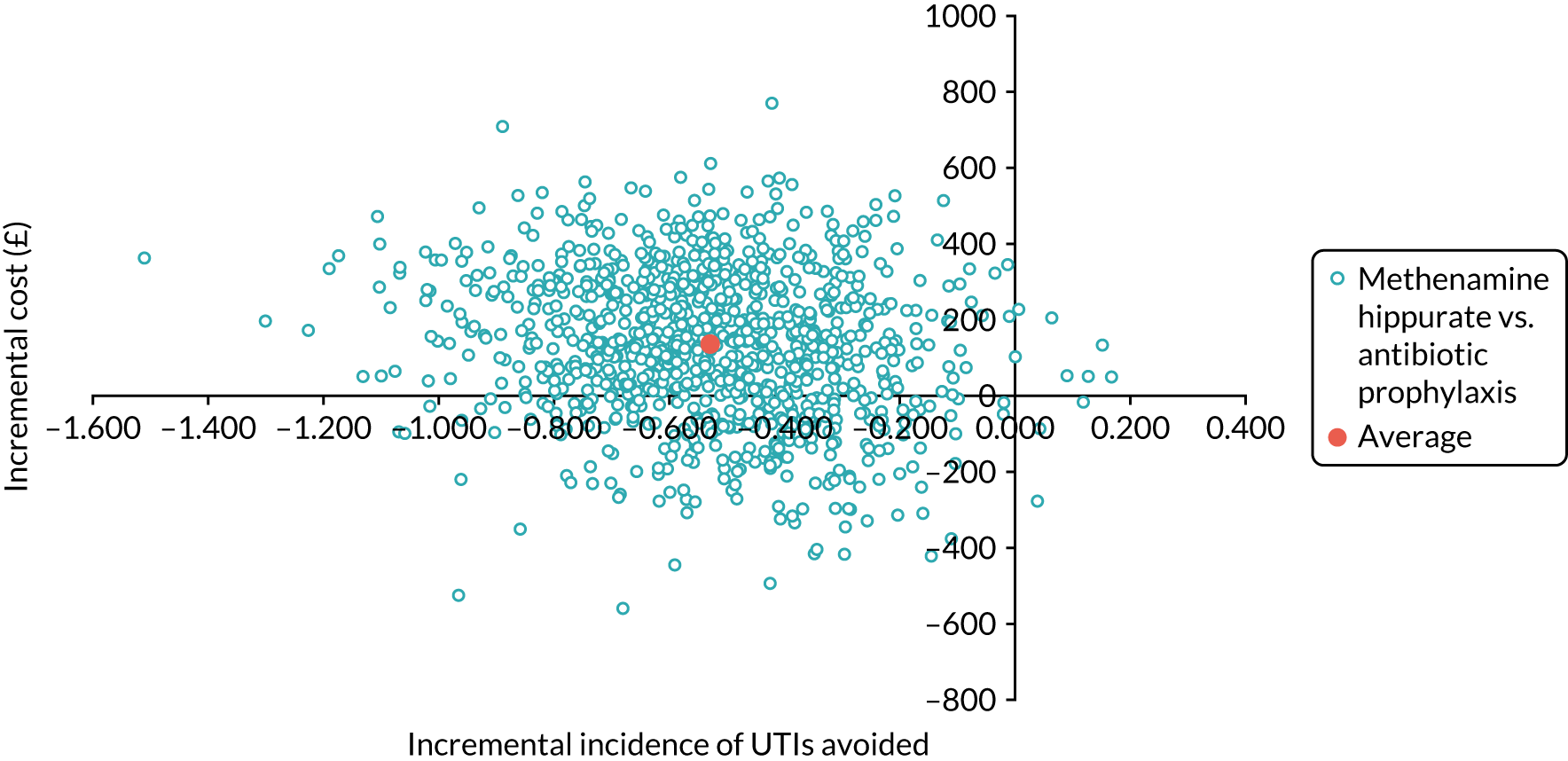
| Strategy | Cost (£) (SD) | Incremental cost (£) (95% CI)a | QALYs (SD) | Incremental QALYs (95% CI)a | ICER | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP for a QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Antibiotic prophylaxis (costs, n = 89; outcomes, n = 73) | 700 (1617) | 0.779 (0.26) | 0.70 | 0.40 | 0.27 | 0.23 | 0.21 | |||
| Methenamine hippurate (costs, n = 94; outcomes, n = 84) | 822 (848) | 89 (–358 to 535) | 0.765 (0.24) | 0.021 (–0.02 to 0.06) | 4332 | 0.30 | 0.60 | 0.73 | 0.77 | 0.79 |
FIGURE 37.
Cost-effectiveness plane for methenamine hippurate compared with antibiotic prophylaxis using QALYs as the measure of outcome over the treatment phase (12 months).
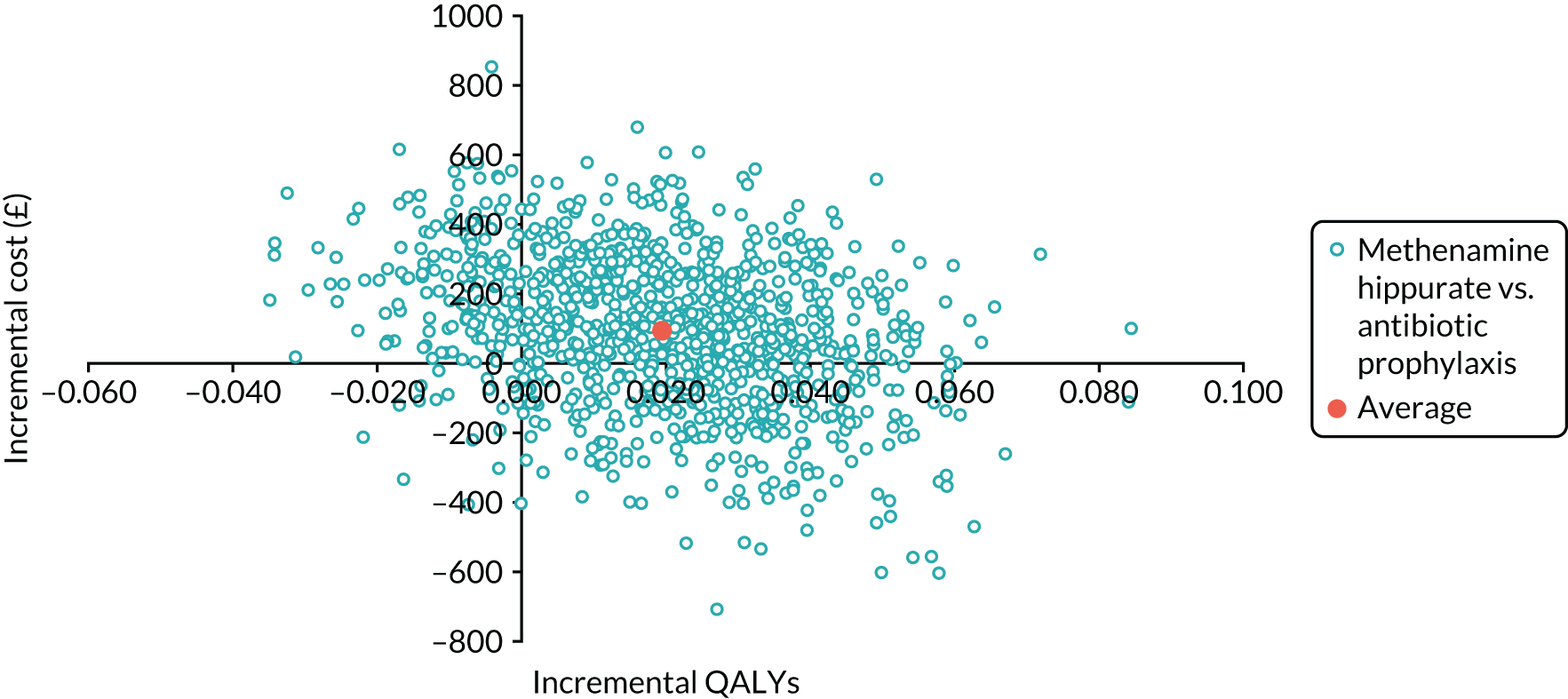
| Strategy | Cost (£) (SD) | Incremental cost (£) (95% CI)a | Incidence of UTI (SD) | Incremental incidence of UTIs avoided (95% CI)a | ICER | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP to avoid a UTI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £100 | £150 | £200 | £250 | ||||||
| Antibiotic prophylaxis (costs, n = 89; outcomes, n = 102) | 2411 (2383) | 0.99 (1.13) | 0.29 | 0.33 | 0.40 | 0.43 | 0.47 | |||
| Methenamine hippurate (costs, n = 94; outcomes, n = 103) | 2121 (1787) | –188 (–809 to 434) | 1.50 (1.62) | –0.64 (–1.03 to –0.24) | 295 | 0.71 | 0.67 | 0.60 | 0.57 | 0.53 |
FIGURE 38.
Cost-effectiveness plane for methenamine hippurate compared with antibiotic prophylaxis using number of UTIs avoided as the measure of outcome and costs including the cost of antimicrobial resistance.
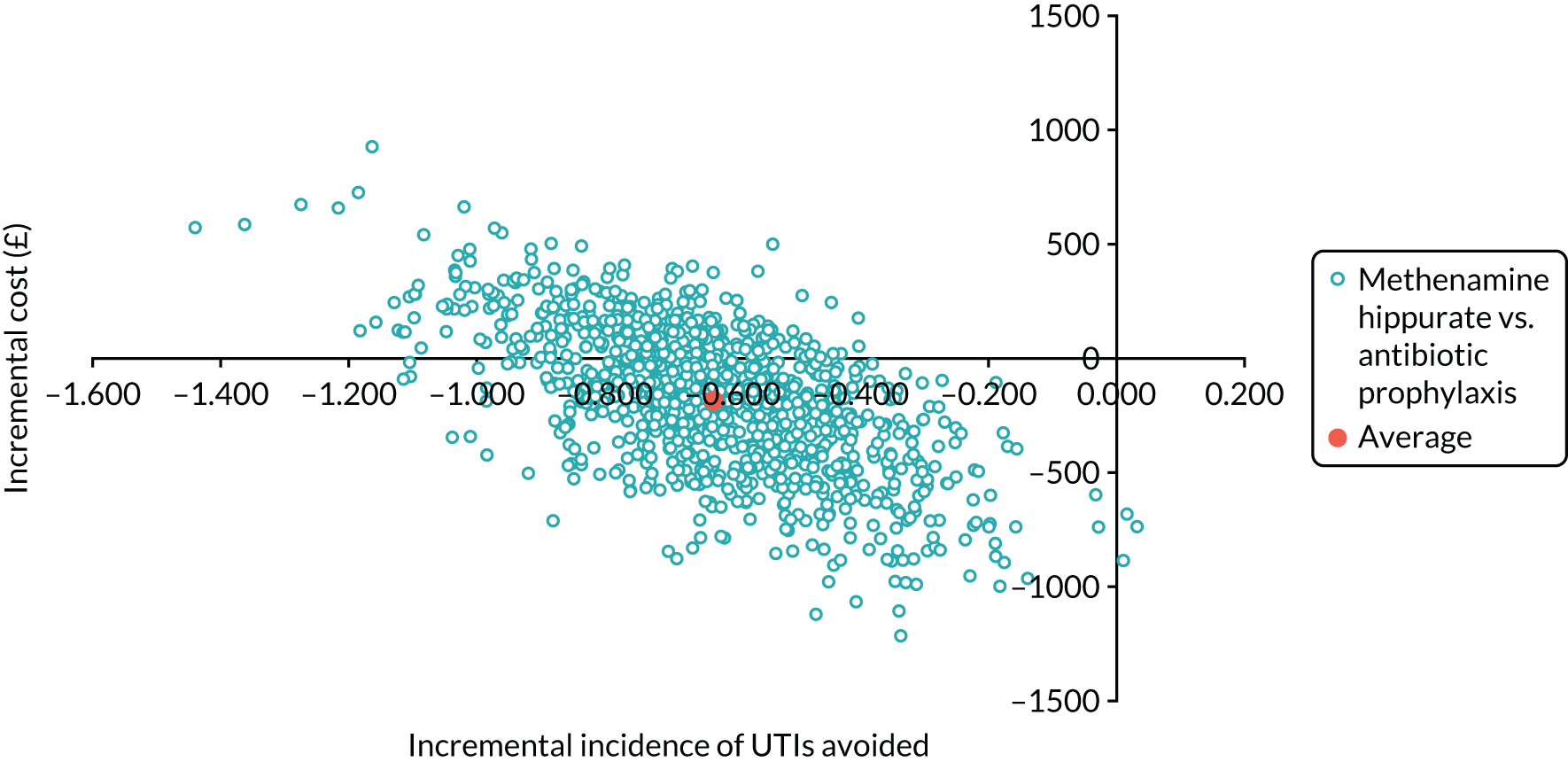
| Strategy | Cost (£) (SD) | Incremental cost (£) (95% CI)a | QALYs (SD) | Incremental QALYs (95% CI)a | ICER | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP for a QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Antibiotic prophylaxis (costs, n = 89; outcomes, n = 58) | 2411 (2383) | 1.182 (0.35) | 0.31 | 0.23 | 0.24 | 0.26 | 0.28 | |||
| Methenamine hippurate (costs, n = 94; outcomes, n = 71) | 2121 (1787) | –307 (–1105 to 491) | 1.133 (0.35) | 0.014 (–0.04 to 0.07) | Methenamine hippurate dominant | 0.69 | 0.77 | 0.76 | 0.74 | 0.72 |
FIGURE 39.
Cost-effectiveness plane for methenamine hippurate compared with antibiotic prophylaxis using QALYs gained as the measure of outcome and costs including the cost of antimicrobial resistance.
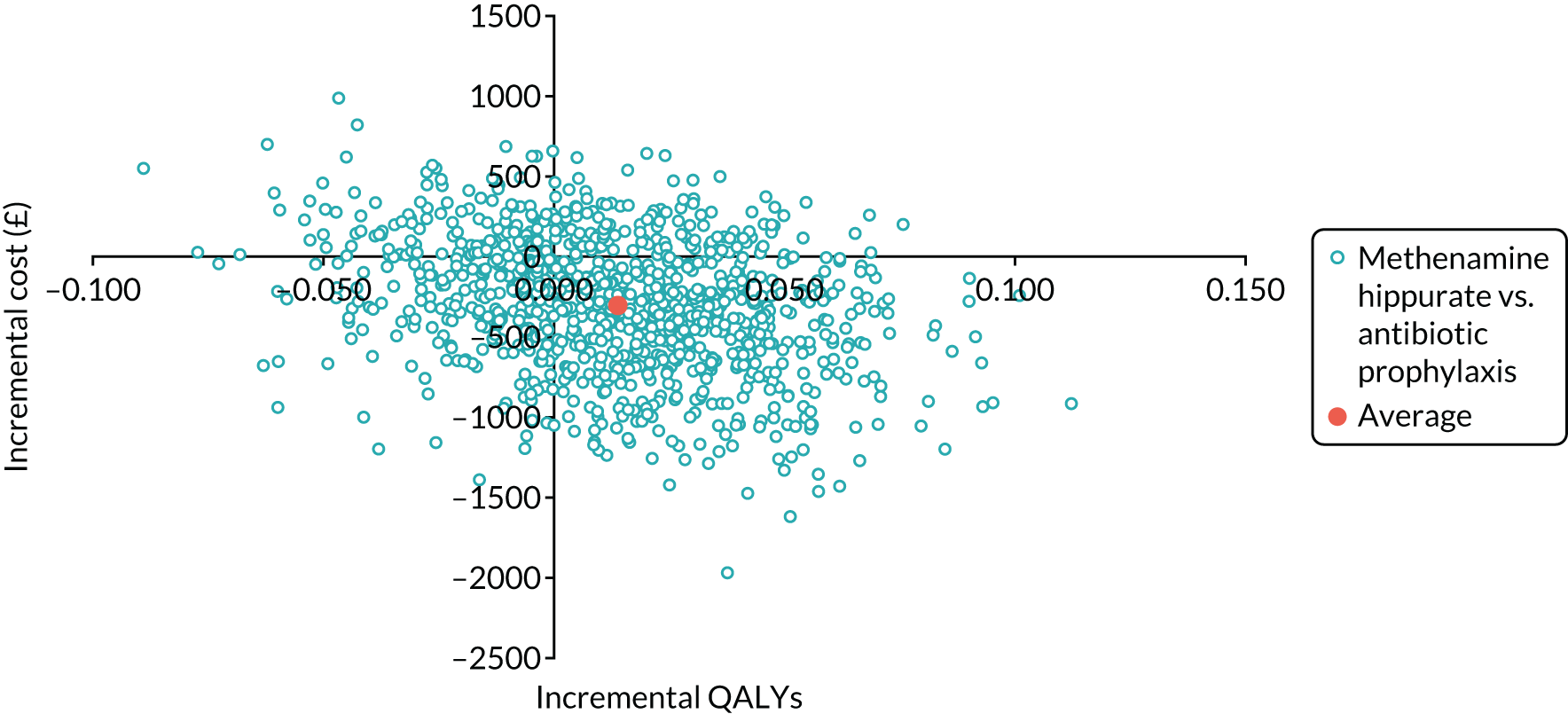
| Cycle | p_Asy_Mil | p_Asy_Mod | p_Mil_Asy | p_Mil_Mod | p_Mod_Asy | p_Mod_Mil | mr |
|---|---|---|---|---|---|---|---|
| Antibiotic prophylaxis | |||||||
| 1 | 0 | 0 | 0 | 0 | 0.69 | 0.23 | 0.001 |
| 2 | 0.10 | 0.05 | 0.56 | 0.22 | 0.60 | 0.30 | 0.001 |
| 3–100 | 0.24 | 0.04 | 0.53 | 0.18 | 0.27 | 0.36 | 0.001a |
| Methenamine hippurate | |||||||
| 1 | 0 | 0 | 0 | 0 | 0.58 | 0.19 | 0.001 |
| 2 | 0.15 | 0.03 | 0.74 | 0.13 | 0.36 | 0.32 | 0.001 |
| 3–100 | 0.19 | 0.10 | 0.32 | 0.23 | 0.29 | 0.07 | 0.001a |
FIGURE 40.
Cost-effectiveness plane for methenamine hippurate compared with antibiotic prophylaxis using QALYs estimated over participants’ lifetime as the measure of outcome.
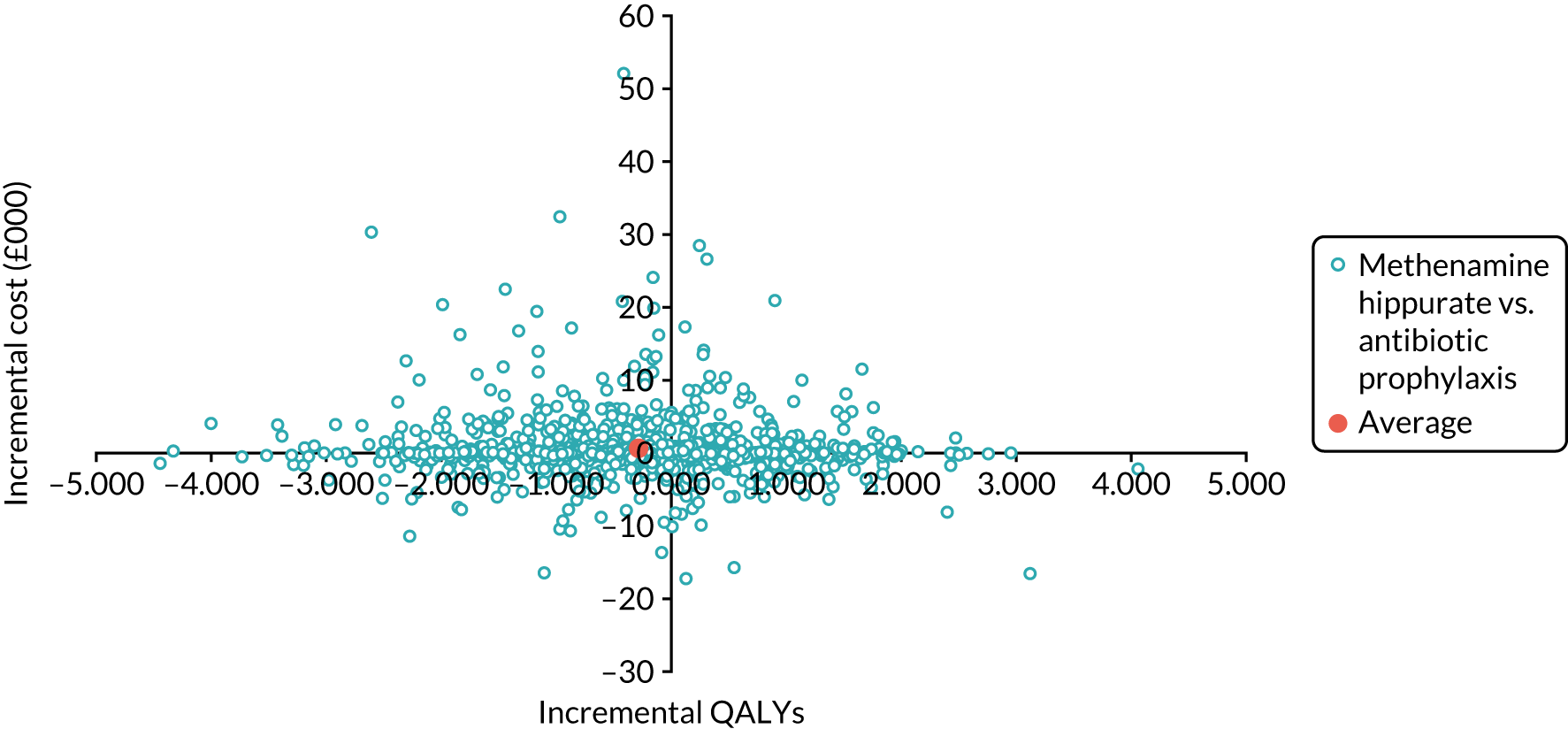
FIGURE 41.
Model-based CEAC for methenamine hippurate compared with antibiotic prophylaxis using QALYs as the measure of outcome.
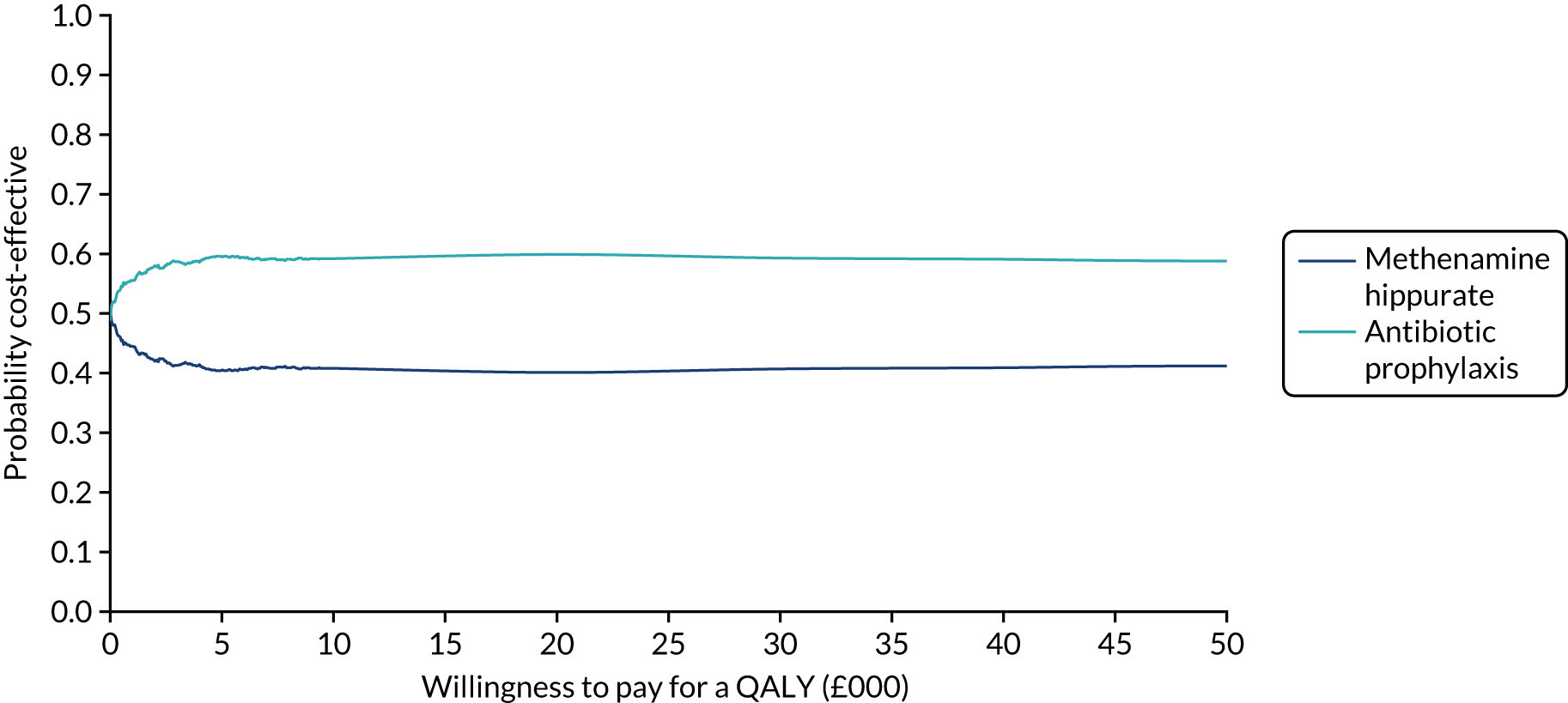
| Strategy | Cost (£) (SD) | Incremental cost (£) (95% CI)a | QALYs (SD) | Incremental QALYs (95% CI)a | ICER | Probability of each treatment strategy being considered cost-effective at different threshold values for society’s WTP for a QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Antibiotic prophylaxis | 879 (67) | 1.134 (0.11) | 0.55 | 0.53 | 0.54 | 0.54 | 0.54 | |||
| Methenamine hippurate | 937 (72) | 33 (6 to 60) | 1.122 (0.11) | –0.012 (–0.02 to –0.01) | Antibiotic prophylaxis dominant | 0.45 | 0.47 | 0.46 | 0.46 | 0.46 |
FIGURE 42.
Model validation: cost-effectiveness plane for methenamine hippurate compared with antibiotic prophylaxis.
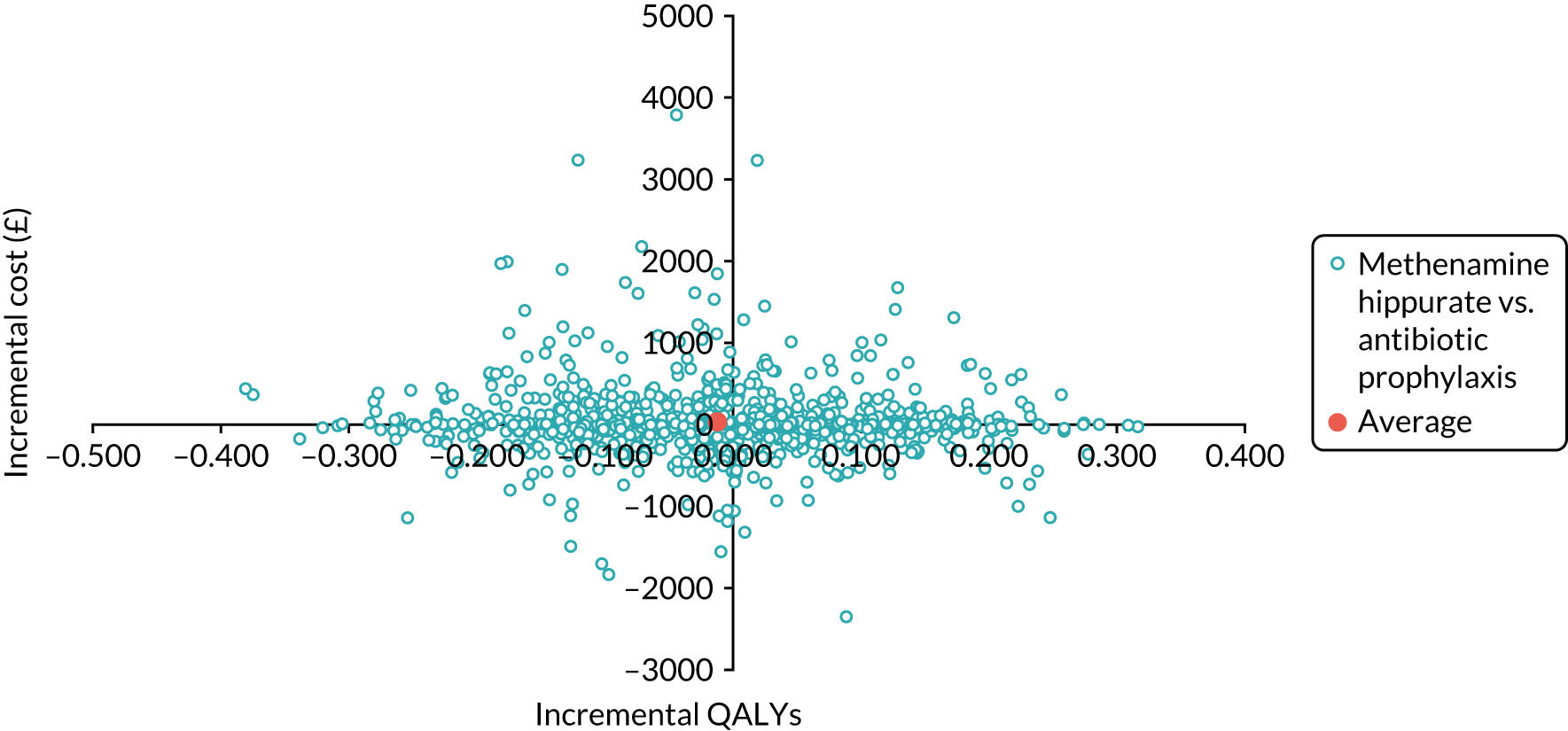
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AIC
- Akaike information criterion
- ALT
- alanine transaminase
- ALTAR
- ALternatives To prophylactic Antibiotics for the treatment of Recurrent urinary tract infection in women
- AR
- adverse reaction
- BIC
- Bayesian information criterion
- BNF
- British National Formulary
- CDI
- Clostridium difficile infection
- CEAC
- cost-effectiveness acceptability curve
- CFU
- colony-forming unit
- CI
- confidence interval
- COB
- Cystitis and Overactive Bladder
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DMC
- Data Monitoring Committee
- eGFR
- estimated glomerular filtration rate
- EOI
- expression of interest
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ESBL
- extended-spectrum beta-lactamase
- GCP
- good clinical practice
- GP
- general practitioner
- HAI
- health-care-associated infection
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- IRR
- incidence rate ratio
- ITT
- intention to treat
- LFT
- liver function test
- MDR
- multidrug resistance
- mITT
- modified intention to treat
- MRSA
- methicillin-resistant Staphylococcus aureus
- MSU
- mid-stream urine
- NCTU
- Newcastle Clinical Trials Unit
- NIHR
- National Institute for Health and Care Research
- OR
- odds ratio
- PI
- principal investigator
- PIS
- patient information sheet
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RN
- research nurse
- RR
- risk ratio
- rUTI
- recurrent urinary tract infection
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SIV
- site initiation visit
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TSQM
- Treatment Satisfaction Questionnaire for Medication
- UTI
- urinary tract infection
- WTP
- willingness to pay
