Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 12/67/12. The contractual start date was in June 2014. The draft manuscript began editorial review in September 2022 and was accepted for publication in May 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Bradshaw et al. This work was produced by Bradshaw et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Bradshaw et al.
Chapter 1 Introduction
Some text in the Scientific summary is reproduced with permission from Chalmers et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attrition (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build on this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/.
Background
Terminology and disease definition: In this monograph, we use the term ‘eczema’ throughout as a term commonly used in the UK to denote atopic eczema (AE) [also known as atopic dermatitis (AD) in the USA and other countries]. It could be argued that using the term ‘eczema’ is more precise, as around 50% of cases of ‘atopic’ eczema are not atopic2 [defined as immunoglobulin E (IgE) sensitivity to common environmental allergens determined by skin prick or blood tests]. The term ‘eczema’ is also the preferred term designated by the World Allergy Organization nomenclature committee. 3 A long list of major and minor clinical diagnostic criteria for eczema were suggested by Hanifin and Rajka in 1980 which were later refined in 1994 into a minimum list of reliable discriminators by a UK diagnostic criteria working party led by one of the authors (HW)4 (Table 1).
| In order to qualify as a case of AE with the UK diagnostic criteria, the child must have an itchy skin condition in the last 12 months. Plus three or more of: |
|---|
| Onset below age 2 yearsa |
| History of flexural involvement |
| History of a generally dry skin |
| Personal history of other atopic diseaseb |
| Visible flexural dermatitis as per photographic protocol5 |
The UK diagnostic criteria have been used for this study and are supplemented by a detailed training manual for users. 5
Defining a new/incident case of eczema for use in prospective studies has been challenging as most definitions rightly include an element of chronicity,6 which is important to separate true eczema from many unclassified transient irritant forms of dermatitis in early life. 7 A 1-year period prevalence is used in the UK diagnostic criteria to capture chronicity and overcome seasonal variation.
What is eczema? Eczema is a chronic inflammatory skin condition characterised by symptoms of itching, stinging and burning of the skin. Itching can lead to sleep loss, poor concentration and a reduction in quality of life. Eczema can affect any part of the body. Secondary infection of the skin with bacteria (most commonly Staphylococcus aureus) is common. Involvement of the cheeks and outer limbs and trunk is common in infancy, whereas in the older child, involvement of the skin creases such as behind the knees and elbow folds is common. The skin is inflamed, appearing red in lighter skin tones and a dark purple or brown colour in dark skin. Chronic scratching leads to leathery thickening of the skin (termed lichenification).
Eczema is caused by a combination of genetic and environmental factors. Genes controlling skin barrier formation and inflammatory responses are important. Epidemiological studies indicate that environmental factors are also critical for determining disease expression: eczema has increased in prevalence over the last 30 years; people migrating from low- to high-prevalence countries develop similarly high rates in the adopted country; and eczema prevalence increases with small family size and higher socioeconomic group. 8–10 Increased sensitivity to food and environmental allergens such as house dust mite is common in eczema. The role of gut and skin microbiota in driving eczema remains controversial. 11 Most cases of childhood eczema improve in childhood but around 5% persist into adulthood. 12 Although eczema is still considered as one disease for the purpose of scientific studies and clinical trials, recent studies suggest that it is composed of several distinct sub-phenotypes or endotypes with different disease trajectories. 13 The relationship between skin barrier dysfunction and underlying upregulation of type-2-mediated immune responses is summarised elsewhere. 14
Asthma, hay fever and food allergy (collectively called ‘atopic’ disorders) are also commoner in children with eczema and perhaps best considered as comorbidities rather than conditions that inevitably progress in the same individuals as part of the so-called allergic march (Figure 1). 16 Some children with eczema are also allergic to foods such as egg and nuts. Current thinking is that eczema in early life leads to food allergy rather than the other way around.
FIGURE 1.
Illustration of the typical onset of symptoms of allergic diseases during childhood. Reprinted from Davidsonet al. (2019),15 Copyright (2023), with permission from Elsevier.
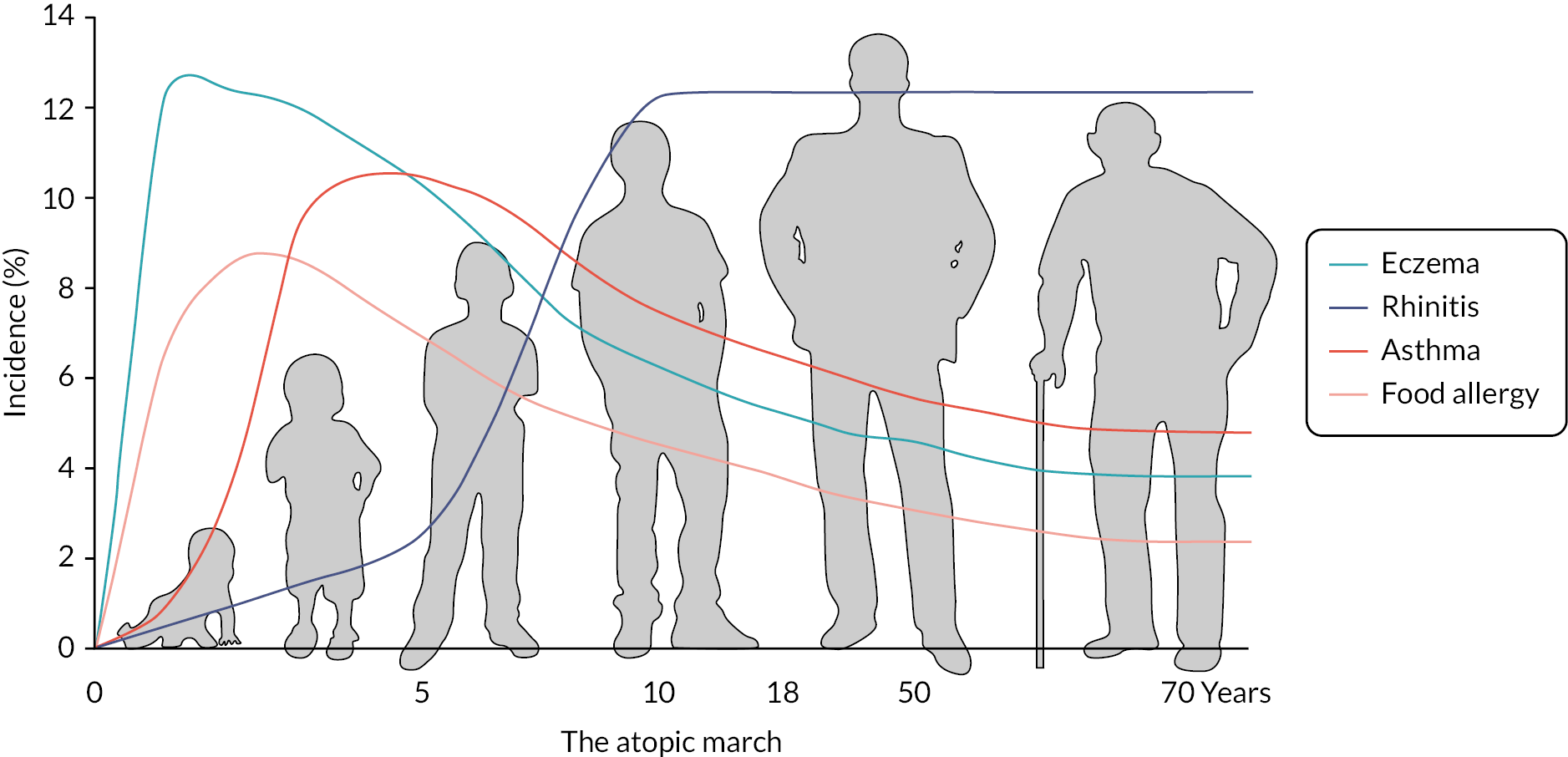
Eczema affects around 20% of children worldwide17 and around 5–10% of adults. 18 Black children in the UK seem to have an increased prevalence of eczema, the reasons for which are unclear. 19 Although around two-thirds of cases of childhood eczema are mild,20 moderate to severe eczema (Figure 2) can result in a significant quality-of-life impairment. 21 Having eczema or a child with eczema also confers high direct and indirect financial costs. 22
FIGURE 2.
Severe eczema is associated with poor quality of life in children.

Treatment of eczema. These are best summarised in the National Institute for Health and Care Excellence (NICE) eczema clinical knowledge summary. 23 Treatment depends to a large extent on eczema severity. In clinical practice, severity is commonly assessed using the Patient-Oriented Eczema Measure (POEM) which records eczema symptoms over the last week. 24 Mild eczema corresponds to a POEM score of 0–7, moderate 8–16 and severe/very severe ranging from 17 to 28. 25 In addition to avoiding irritants such as soap and rough clothing, mild eczema is generally treated in the community with topical application of creams and ointments such as short bursts of mild potency topical corticosteroids for inflammatory flares and emollients for restoring the skin barrier, flare prevention and treatment of dry skin symptoms. Moderate disease requires more potent topical corticosteroids and/or the addition of topical calcineurin inhibitors such as tacrolimus and pimecrolimus, often used proactively on weekends to prevent flares. More severe eczema requires specialist input and may require third-line treatments such as ultraviolet light or systemic immunomodulatory therapy such as ciclosporin, methotrexate or biologics such as dupilumab and baricitinib. 26 Three more biologics (abrocitinib and upadacitinib – both JAK1 inhibitors, and tralokinumab – a human monoclonal antibody that inhibits interleukin-13) have recently been approved by NICE for severe eczema27 and a large range of biologics are currently in development. There is a considerable unmet need for eczema care in the UK, with most treatment carried out at home with little contact with healthcare professionals. 14 Self-care informed by theory-based, evidence-informed online educational programmes have been shown to result in sustained benefit for managing eczema severity. 28
While good progress has been made with new topical and systemic treatments for established eczema,29 relatively little attention has been paid to the prevention of eczema – arguably a more desirable approach than the daily lifetime toil of applying greasy ointments or requiring expensive medicine that modify the body’s immune system in individuals in whom a long chain of pathological events have already occurred (Figure 3). 31
FIGURE 3.
Upstream prevention of eczema is a more desirable aim than treating sick individuals with costly drugs who present after a long chain of multistage events.

Rationale for the barrier enhancement for eczema prevention trial
A summary of the approaches that have been used to try and prevent eczema and the methodological considerations for such preventive studies have been summarised elsewhere. 32 In brief, systematic reviews of prevention strategies covering exclusive and prolonged breastfeeding, or those targeting reduction in food and/or airborne allergens in pregnancy and/or shortly after birth have not shown clear benefit. Maternal/infant supplements of vitamin D has also not shown any benefit. Some evidence supports the role of probiotics to prevent eczema, but the studies are quite variable and might benefit from individual patient data meta-analysis.
Interest in the use of emollients for preventing or reducing the severity of childhood eczema has been around for many years and was suggested by one of the authors (HW) at the 2001 International Symposium of Atopic Dermatitis in Portland. Although the idea gained some traction as it was already known that dry skin preceded the development of visible eczema, it was the discovery that mutations in the gene encoding filaggrin (FLG), a key skin barrier protein, that accelerated interest in emollients as a potential intervention for preventing eczema. 33 FLG mutations have since been shown to represent the strongest and most consistent genetic risk factor for eczema. Later studies showed that FLG-null mutations were also associated with related atopic conditions including asthma, hay fever and peanut allergy. 34 Intense interest grew in the concept of skin barrier dysfunction as the initial step in eczema development. In addition to FLG mutations, skin barrier defects are thought to arise as a result of immune dysregulation, low levels of antimicrobial peptides, a general disruption in skin flora (dysbiosis). 35 The genetic predisposition to skin barrier impairment led to the idea that babies at high risk of developing eczema are born with a skin barrier that allows irritants and allergens to initiate skin inflammation and hence set up a cascade of inflammatory events that could lead to chronic eczema driven by autoimmune mechanisms as a result of chronic scratching. 36
Emollients are used to improve the barrier function of skin by providing lipids to the outermost stratum corneum and by trapping water which in turn improves skin hydration. Emollients may prevent inflammation caused by external irritants as they are used to prevent irritant occupational hand eczema. 37 Emollients had also been shown to reduce skin inflammation in premature babies38 as well as preventing eczema flares in those with established eczema. 39 An earlier open-label pilot study of emollient therapy from birth showed that only 15% of high-risk infants developed eczema against an expected rate of 30–50%. 40 A case-control study conducted in Kenya published in 1991 also suggested that petroleum had protective effect against the development of eczema. 41
A workstream within a NIHR-funded programme grant conducted by several authors focused on exploring the feasibility of conducting a national trial of emollients to prevent eczema. At the time, there was considerable uncertainty of whether families with a strong history of atopic disease (eczema, asthma, hay fever) would agree to be randomised to normal care or daily application of an emollient to their newborn child. A series of pilot studies including qualitative work with families was carried out to explore emollient preferences. Full details of the pilot studies are described in the Programme Grant for Applied Research report. 42 Since some emollients such as aqueous cream had been shown to paradoxically damage the skin barrier,43 a mechanistic study by experts in skin barrier science was done to show that the two preferred emollients did not cause any such skin barrier disruption. 44 A feasibility randomised controlled trial (RCT) of 124 families was conducted and showed that the intervention was acceptable and that it was possible to conduct a national clinical trial. Although not powered to detect a difference in eczema prevention, infants in the emollient group in the feasibility study showed a reduced risk of developing eczema at 6 months of age compared to controls [22% vs. 43%, respectively, relative risk (RR) 0.50, 95% confidence interval (CI) 0.28 to 0.90; p-value = 0.017]. 42 Another small trial of 118 infants in Japan showed similar results, with 32% fewer neonates who received moisturiser developing eczema by week 32 when compared with control infants. 45
On the basis of the need to prevent eczema, empirical evidence on the role of a defective skin barrier as the initial event in eczema development, growing signals from observational and randomised pilot studies, a strong case was therefore made to the NIHR Health Technology Assessment Programme to fund a national trial of emollients to prevent eczema in babies at high risk of developing the condition. Timing was important as interest in the potential for emollients to prevent eczema was now becoming an entrenched belief following the two small pilot studies, and parents with a strong family history of atopic disease across the world were beginning to use emollients to prevent eczema in their newborns. Commercial companies were responding to demand by developing ‘designer’ emollients to enhance the skin barrier. The window of public/patient and healthcare professional equipoise was limited.
We therefore sought to test the hypothesis whether daily emollient application for 12 months after birth can reduce the development of eczema.
Chapter 2 Methods
Text in this chapter is reproduced with permission from Chalmers et al. 46 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attrition (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build on this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text. The full barrier enhancement for eczema prevention (BEEP) trial protocol (final version 7.0, 26 February 2021) is available on the NIHR Funding and Awards website47 and a summary protocol has been published. 46 The main changes to the protocol after the initial approval in June 2014 was the addition of secondary outcomes for confirmed diagnosis of food allergy and allergic sensitisation and the associated assessments after separate funding was obtained to complete these (added May 2016).
Trial objectives
Primary objective
To determine whether advising parents to apply emollient daily to the entire body surface area for the first year of life can prevent AE in high-risk children.
Secondary objectives
-
To determine whether emollients can delay the onset and/or reduce the severity in those who develop AE.
-
To determine whether emollients can prevent other allergic diseases developing.
-
To determine the safety and cost-effectiveness of the prevention strategy.
-
To determine whether any preventative effect is sustained into later childhood.
Trial design and setting
This was a randomised, controlled, two-arm (skin-care advice plus emollient vs. skin-care advice alone), parallel group, multicentre, assessor blind trial with 5-year follow-up and primary outcome assessed at 2 years (Figure 4). Recruitment took place in 12 secondary care sites and four primary care sites in England (see Appendix 1, Figure 12).
FIGURE 4.
Schematic diagram showing the trial design and duration for participating families. a, Screening took place during pregnancy or within 21 days of delivery. Reproduced from Chalmers et al. 46 under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
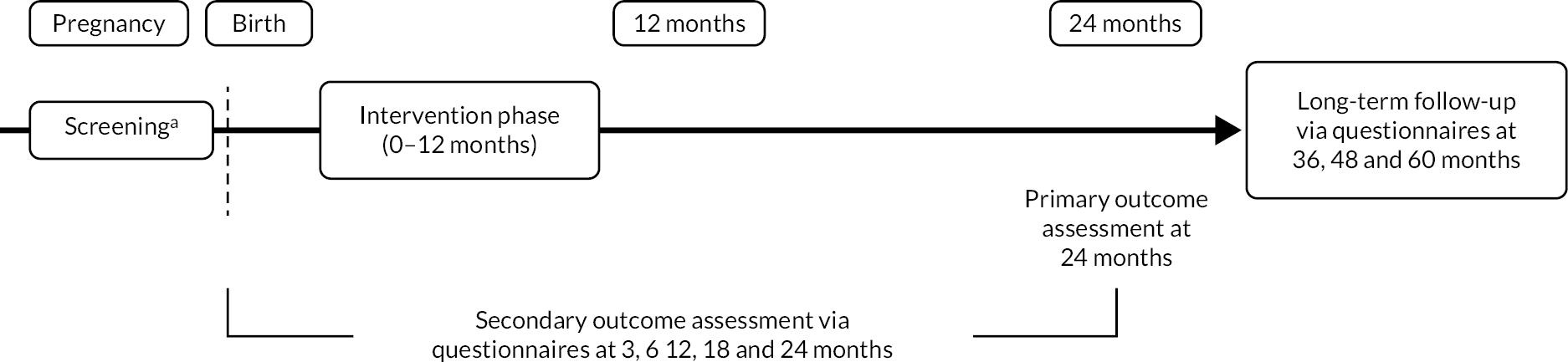
A methodological two-by-two factorial substudy was also nested within the trial to investigate the effectiveness of two interventions on the rates of follow-up data collection. The interventions were: (1) short message service (SMS) notification prior to sending questionnaires at 3, 6, 12 and 18 months versus no SMS notification and (2) sending the £10 voucher for the primary follow-up visit at 24 months with the invitation letter versus giving the voucher at the visit. Full details can be found in the studies within a trial (SWAT) registry (SWAT Repository Store ID 25). 48
Participants and eligibility
The eligibility criteria for the participants are shown below.
Inclusion criteria
-
Child had a first-degree relative with parental-reported doctor diagnosis of eczema, allergic rhinitis or asthma.
-
Child up to 21 days old.
-
Mothers must be at least 16 years old.
-
Consenting adult had the ability to understand English.
Exclusion criteria
-
Preterm birth (defined as birth prior to 37 weeks’ gestation).
-
Sibling (including twin) previously randomised to this trial. If multiple birth, the first-born child was randomised into the trial.
-
Child had a severe widespread skin condition that would make the detection and/or assessment of eczema difficult.
-
Child had a serious health issue which, at parent or investigator discretion, would make it difficult for the family to take part in the trial.
-
Any condition that would make the use of emollient inadvisable or not possible.
Recruitment to the trial came from a variety of sources, including primary and secondary care and advertising. Poster and flyers were displayed at secondary care and primary care sites. If interested in taking part, expectant mothers/parents of the newborn were asked to contact the research team directly. Expectant mothers were identified through antenatal and secondary care clinics in which case relevant healthcare professionals approached parents directly about the study or sent invitation letters. In addition to the general practitioner (GP) surgeries used as sites, GP surgeries in areas with a secondary care site were used as Participant Identification Centres (PICs). In the PICs, invitation letters and information sheets were sent to expectant parents. The trial was also promoted through local radio, television, newspapers, the Mumsnet website and the public display of posters and flyers at venues that families and expectant mothers frequent including nurseries, libraries, supermarkets and Sure Start centres.
Screening and consent
Pregnancy status and family history of atopic disease were checked for parents initially expressing an interest in taking part and, if eligible, the parents were sent the participant information leaflet and a screening visit was arranged. The screening visit took place either in the family home or at the recruiting site, depending on parent preference and was conducted either in the third trimester if the baby had not yet been born or as soon as possible after birth and within 21 days of being born. During the screening visit, the research nurse obtained informed consent and checked eligibility. Consent was also sought for the optional genetic component and for use of samples in potential future research. Parents who consented agreed to their child providing a saliva sample at the 24-month visit for FLG genotyping in order to conduct subgroup analysis to explore the effect of emollients on eczema prevention according to genetic risk of atopic disease (FLG null mutations).
Outcomes
Full details of definitions and derivation of outcomes are given in the statistical analysis plan (SAP). 47
Primary outcome
The primary outcome was a diagnosis of AE at 24 months defined as meeting the United Kingdom Working Party (UKWP) Diagnostic Criteria for Atopic Eczema,4 which assesses signs and symptoms present over the past year, assessed by a trained research nurse blinded to treatment allocation.
Applying the criteria at 24 months was chosen to ensure that any observed effect on reducing AE prevalence could be considered a true preventative effect rather than masking the emergence of mild eczema in the first year due to the use of the emollient.
Secondary outcomes
-
Presence of eczema between birth and 24 months:
-
Any parental report of a clinical diagnosis of eczema.
-
Completion by parents of UKWP Diagnostic Criteria for Atopic Dermatitis at 12 and 24 months.
-
-
Presence of visible eczema at 24 months (skin examination by researcher).
-
Time to onset of eczema:
-
First parental report of a clinical diagnosis of eczema.
-
First topical corticosteroid and/or immunosuppressant prescription for eczema.
-
-
Severity of eczema:
-
Presence of other allergic diseases:
-
Parental-reported wheezing and allergic rhinitis between 12 and 24 months.
-
Parental report of a clinical diagnosis of food allergy at 12 and 24 months.
-
Parental report of food allergy at 12 and 24 months. Parents were specifically questioned about cow’s milk, egg, peanuts and other nuts plus ‘any other food’.
-
Allergic sensitisation at 24 months to any of the following common allergens: milk, egg, peanut, cat, grass pollen, house dust mite (added in protocol version 4.0 after obtaining separate funding).
-
Confirmed diagnosis of food allergy at 24 months to milk, egg, peanut or ‘any of milk, egg or peanut’ (added in protocol version 4.0 after separate funding obtained). The diagnosis was derived from a combination of parental report, allergic sensitisation and food challenge.
-
-
Health-related quality of life:
-
Child Health Utility instrument-9 domains (CHU-9D)50 at 24 months in order to estimate quality-adjusted life-years (QALYs).
-
Parental quality of life measured using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L) at baseline and 24 months in order to estimate change in parental QALYs, if any.
-
-
Health economic outcomes:
-
Healthcare resource use at 3, 6, 12, 18 and 24 months.
-
Cost-effectiveness and cost–utility at 24 months (combining health resource use and health-related quality-of-life outcomes).
-
Safety outcomes
-
Number of skin infection events during the first year.
-
Number of infant slippage incidents (slippage in hand and slippages to the floor) that occur within an hour of applying emollient during the first year.
Tertiary outcomes (long-term follow-up)
-
Presence of eczema in the previous year at 36, 48 and 60 months based on parental report of a clinical diagnosis of eczema.
-
Any parental report that in their opinion their child has eczema at 3, 6, 12, 18, 24, 36, 48 and 60 months.
-
Presence of eczema at 36, 48 and 60 months based on completion by parents of UKWP Diagnostic Criteria for Atopic Dermatitis.
-
Severity of eczema at 36, 48 and 60 months as measured by POEM.
-
Presence of other atopic diseases:
-
Parental-reported wheezing, allergic rhinitis and food allergy symptoms at 36, 48 and 60 months.
-
Parental report of a clinical diagnosis of asthma or allergic rhinitis by 60 months.
-
Parental report of a clinical diagnosis of food allergy at 36, 48 and 60 months.
-
-
Health-related quality of life:
-
CHU-9D at 36, 48 and 60 months in order to estimate QALYs.
-
Parental quality of life: EQ-5D-5L at 36, 48 and 60 months in order to estimate parental QALYs.
-
-
Health economic outcomes:
-
Healthcare resource use at 36, 48 and 60 months.
-
Cost–utility and cost-effectiveness at 60 months (combining health resource use and health-related quality-of-life outcomes).
-
-
Cumulative incidence outcomes (not specified in protocol, see below for rationale for addition).
-
Parental report of clinical diagnosis of eczema from the age of 12 to 60 months.
-
Parental report of clinical diagnosis of food allergy by 60 months.
-
Exploratory outcomes (long-term follow-up, not specified in protocol)
-
Parental report of reaction to egg or nuts by 60 months.
-
Parental report of immediate reaction to egg or nuts by 60 months.
The protocol initially specified the paediatric quality of life (PedsQL)51 questionnaire to estimate QALYs for the child at 24, 36, 48 and 60 months. This was changed in May 2016 to the CHU-9D questionnaire to reflect the latest research in the area. The Infant Dermatitis Quality of Life questionnaire52 was also removed as an outcome in May 2016 to reduce the questionnaire burden and also due to BEEP being a prevention trial rather than a treatment trial.
Cumulative incidence tertiary outcomes and exploratory outcomes (not in the protocol) were specified in version 2.0 of the SAP, as a better measure of lifetime experience of eczema and food allergy than single sweeps of 1-year period prevalence. Eczema is a condition that undergoes relapses and remissions – both short term over the course of weeks or months that reflect seasonal influences such as temperature, pollen and humidity (which is adequately captured by enquiring about a one period prevalence) or over the course of years, with some children having eczema which then clears and some getting early eczema which then clears and then returns at different sites. Food allergy can cause only intermittent reactions, often less than once per annum; the condition can be immunologically present for many months or years before a clinical reaction is experienced, especially for allergy to foods which are not widespread components of everyday diets such as tree nuts. The first 12 months were not included in the tertiary outcome for cumulative incidence of eczema as transient eczematous rashes are common in the first year of life and often reported by parents as ‘eczema’ but are less likely to be true AE.
Randomisation and blinding
Within 21 days of the birth of the baby, a baseline visit was conducted (either on the phone, via e-mail or face to face) with the research nurse to confirm eligibility and collect baseline data. Babies meeting the eligibility criteria were then randomised by the research nurse via a web-based randomisation system developed and maintained by Nottingham Clinical Trials Unit (NCTU).
The randomisation schedule was based on a computer-generated pseudo-random code using random permuted blocks of randomly varying size created by NCTU and held on a secure University of Nottingham server. Infants were allocated in a 1 : 1 ratio to either emollient and standard skin-care advice (intervention group) or standard skin-care advice only (control group). Randomisation was stratified by recruiting centre and number of immediate family members (parents/siblings) with atopic disease (one, two, or more than two family members with either eczema, asthma or hay fever).
Parents were informed of their child’s allocation by staff at NCTU by letter. While it was not possible to blind parents to the treatment allocation, efforts were made to minimise expectation bias by emphasising that information on the effect of emollient in addition to standard skin-care advice was limited.
Research nurses were not informed of the allocation to maintain blinding for the follow-up visit for the primary outcome at 2 years. To reduce the chance of the research nurse being unblinded by the parents at the 2-year visit prior to the skin examination for eczema, the appointment letter reminded parents not to tell the nurse which group they had been assigned to in the first year and skin assessments were conducted first during the visit.
Researchers involved in the food allergy assessment process were also blinded to treatment allocation.
The trial statisticians and health economists remained blinded to treatment allocation until after the initial database lock for the analysis of primary outcome data at 2 years.
Interventions
Skin-care advice
Both groups received advice on general skin care in booklet and video format at the time of randomisation. 47 Skin advice was based on 2006 NICE guidance for postnatal care up to 8 weeks of birth available at the time (since replaced)53 and updated in 2016)54 and supplemented with expert opinion from dermatological nursing and skin barrier research expertise (SL and MC). The booklet and video advised to use mild cleansers and shampoos specifically formulated for infants, and to avoid soap, bubble bath and baby wipes. Parents were advised to seek medical advice from their GP if their baby developed any skin problems. Infants in the control group were allocated to skin-care advice alone.
Intervention group
Families in the intervention group were advised to apply emollient (Doublebase Gel® or Diprobase Cream®) at least once daily to the whole body (excluding the scalp) until the child reached 1 year, in addition to skin-care advice described above. These two emollients were chosen based on our pilot work with families from our preceding programme grant for applied research. 42 We were able to establish that the liquid-paraffin-based emollients, Doublebase Gel (Dermal Laboratories Ltd) and Diprobase Cream (Bayer Plc) are popular with parents and do not have any detrimental effects on skin barrier function. Parents in the pilot study also told us that having a choice of emollients was very important to them.
They were also advised to apply emollient after every bath, even if they had already applied the emollient that day. Daily application was advised in order to encourage regular use of emollient several times a week, but since the study was designed to reflect how the intervention might be delivered in normal practice, no prompts or reminders were sent to parents. The skin- care advice booklet and video for the intervention group additionally provided guidance on how to apply emollients correctly by dotting over the skin and using gentle downward strokes rather than rubbing in. The booklet also contained warnings about the skin being slippery after application and the need to clean up spillages from the floor to avoid slipping.
Upon randomisation, the central trial pharmacy sent the families a 500 g container of each emollient by post. Families reordered their preferred emollient during the intervention period (1 year) by contacting the NCTU. Parents were advised to stop applying emollients when their child reached 1 year of age and no further emollients were supplied after this point.
Trial assessments and procedures
Parents were initially contacted by telephone by the NCTU approximately 2 weeks after randomisation to check they have received their skin-care advice pack and web link for the video, and for infants randomised to the intervention group, to check the date that the family started applying the emollient. Parents were also reminded to contact the NCTU if they had any questions or problems (to protect the research nurses from becoming unblinded).
Parents were asked to complete web-based questionnaires at 3, 6, 12, 18, 36, 48 and 60 months to collect information on any skin problems, eczema and other allergy symptoms (including diagnosis, prescriptions and health resource use), feeding, skin-care practices (including emollient use during the intervention period), skin infections, infant slippage incidents within an hour of applying skin-care products, reactions to food and quality of life as detailed in Table 2 (all questionnaires available at47). Reminders were sent by e-mail after 2 and 3 weeks of non-completion and the questionnaires could be completed up to 4 weeks after the initial e-mail invitation was sent. Paper copies of the questionnaire were sent by post (with pre-paid envelopes) if parents did not wish to complete the questionnaires online. From May 2016, staff at the NCTU also telephoned and/or sent a text message to participants who had not completed the questionnaire after the first reminder and gave participants the opportunity to complete the questionnaire on the phone.
| Time point | Stuy period | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Screening/enrolment | Baseline/randomisation | Post randomisation | Final follow-up | ||||||
| During pregnancy or up to 21 days post delivery | Within 21 days of birth | Months | |||||||
| 3 | 6 | 12 | 18 | 24 | 36 and 48 | 60 | |||
| Enrolment | |||||||||
| Eligibility screen | X | ||||||||
| Informed consent | X | ||||||||
| Family demographic data including history of atopic disease | X | ||||||||
| Baby demographic data | X | ||||||||
| Randomisation | X | ||||||||
| Interventions | |||||||||
| Skin-care advice plus daily emollient | X | X | X | ||||||
| Skin-care advice alone | X | X | X | ||||||
| Assessments | |||||||||
| Parental-reported skin problems (including eczema) | X | X | X | X | X | X | X | ||
| Parental-reported clinical diagnosis of eczema | X | X | X | X | X | X | X | ||
| Parental completion of eczema diagnostic criteria (UKWP criteria) | X | X | X | X | |||||
| Blinded (researcher) assessment of eczema status | X | ||||||||
| Eczema severity (EASI, conducted by blinded researcher) | X | ||||||||
| Eczema severity (parent reported POEM) | X | X | X | X | |||||
| Parental-reported allergic rhinitis/wheezing symptoms | X | X | X | ||||||
| Parental-reported food allergy symptoms and diagnosis | X | X | X | X | |||||
| SPT ± oral food challenge if required | X | ||||||||
| Parental-reported skin infections and slippages | X | X | X | ||||||
| Adherence | X | X | X | ||||||
| Parent/carer health-related quality of life (EQ-5D-5L) | X | X | X | X | |||||
| Child health-related quality of life (CHU-9D) | X | X | X | ||||||
| Health resource use | X | X | X | X | X | X | X | ||
| Saliva sample collection | X | ||||||||
| Feeding and washing practices questionnaires | X | X | X | ||||||
| Parental-reported moisturiser use | X | X | X | X | |||||
| Parental-reported diagnosis of asthma/hay fever | X | ||||||||
At 24 months, a face-to-face visit with a research nurse took place either in the family home or in clinic according to parent preference. Prior to the first appointment, 28 research nurses were trained in all assessments either by attending an in-person training day or by watching a series of training videos. This included training in diagnosing eczema using the UK diagnostic criteria, the EASI, skin prick test (SPT) and anaphylaxis. After this initial training, nurses used the UKWP Diagnostic Criteria and performed an EASI assessment in clinic with three patients with the local Principal Investigator. Specialist allergy nurses accompanied research nurses at their initial 24 months visits to sign off on proficiency in SPT competency and using an EpiPen (Viatris UK HealthCare Ltd., Potters Bar, Hertfordshire, UK, for anaphylaxis).
After arranging the 24-month visit, the research nurse sent parents an information leaflet and sheet to explain that their child would be offered an allergy test (SPT) at the visit. The nurse then discussed the SPT during the visit and took consent for this if the parents were willing for the child to have the optional allergy test.
During the visit, the research nurse conducted the skin examination for the UKWP Criteria and the EASI and for parents who had given the additional optional consents, collected a saliva sample and conducted a SPT. Parents were also asked to complete the POEM, EQ-5D and CHU-9D questionnaires and another questionnaire to collect information about skin problems, reactions to food, symptoms of allergic rhinitis and wheezing, skin care/washing and other characteristics potentially associated with the development of eczema (e.g. antibiotic use since birth, furry pets, dust mite reduction measures, number of other children in the household and whether the child attends nursery or playgroup), as detailed in Table 2 (case report form for 24-month visit available at NIHR Funding and Awards47). At the end of the visit, nurses recorded if they had become aware of which group the child had been randomised to and if so whether this happened before, during or after the skin examination.
Skin prick testing was carried out following a trial-specific procedure in line with the British Society for Allergy and Clinical Immunology procedures for SPT55 (Appendix 2 of protocol v7.0 26 February 2021, see NIHR Funding and Awards47). The following allergens were tested; grass pollen mix, dust mite and cat (Allergopharma GmBH & Co., Reinbek, Schleswig-Holstein, Germany), peanut (Inmunotek, Madrid, Spain), fresh skimmed cow’s milk and fresh chicken egg. Positive (1% histamine) and negative (0.9% saline) controls were used (Allergopharma GmBH & Co.). The research nurse measured the size of the reactions, and the results were reviewed by the BEEP food allergy team.
If it was not possible to complete a face-to-face visit, data collection was attempted remotely by telephone, text, e-mail or post. If no follow-up with the family was possible at 24 months, the NCTU attempted to collect key minimal data around diagnoses of eczema and food allergy (including number of prescriptions, primary and secondary care visits) from the child’s GP.
Children with a positive SPT or history suggestive of food allergy, where further information was needed to confirm a food allergy, were invited for a supervised oral food challenge (Figure 5) in an allergy clinic at Imperial College Healthcare National Health Service (NHS) Trust or Sheffield Children’s Hospital NHS Trust. A trial-specific food challenge information sheet was sent to parents of children invited to the food challenge and if needed the food allergy team contacted parents to gather more information about the child’s food allergy history. Parents who agreed to take part gave consent for their child to have a food challenge to confirm whether their child had a food allergy. Oral food challenges were conducted by experienced allergy nurses blinded to treatment allocation following a trial-specific standard procedure using incremental doses or a single dose of the relevant food (see Appendix 3 of protocol v7.0 26 February 2021, see NIHR Funding and Awards47). Allergy nurses were able to review SPT results and allergy history as needed to inform clinical decision-making. Trained nurses supported by a consultant paediatric allergist evaluated whether there had been a clinical reaction to the food using modified practical allergy (PRACTALL) and Integrated Approaches to Food Allergen and Allergy Risk Management criteria. 57,58 Clinical reactions during the food challenge were treated using standard clinical guidelines, with antihistamine, bronchodilator, intramuscular adrenaline or other medications, as needed.
FIGURE 5.
Food allergy assessment process.
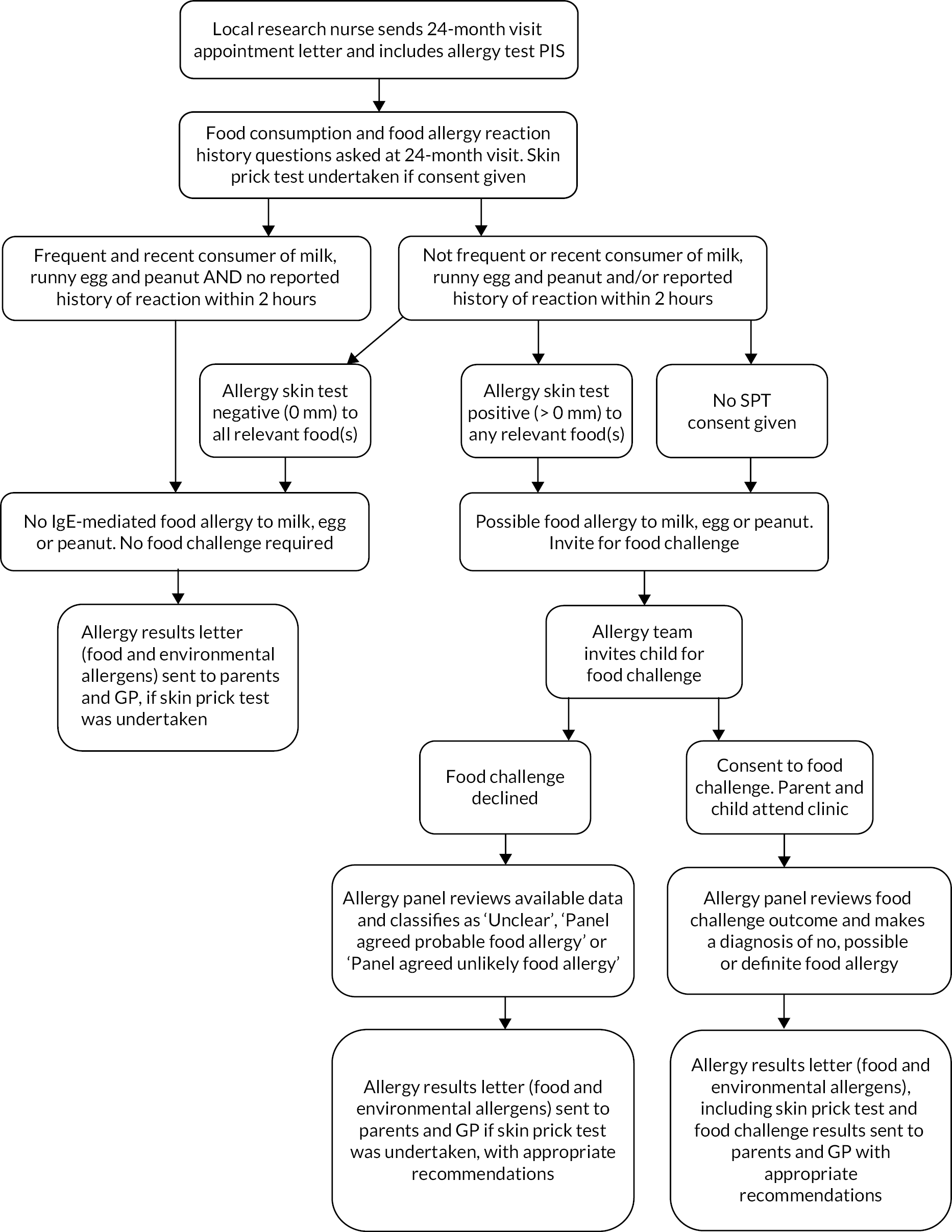
For participants invited to a food challenge where this did not take place, a panel of experienced paediatric allergists agreed allergy status (see Figure 5). The panel developed and validated an algorithm to make the food allergy diagnosis. 59 The panel were blinded to treatment allocation and used all available information from the questionnaires and assessments and, where necessary, from secondary care records and direct communication with participants’ parents. Following the SPT and food challenge (where applicable), parents and the child’s GP were sent a letter summarising the findings and any recommended action by the BEEP food allergy team.
Saliva samples were sent to the Centre for Dermatology and Genetic Medicine at the University of Dundee for deoxyribonucleic acid (DNA) extraction by standard techniques. DNA samples were genotyped for the four most common FLG null mutations in the white European population (2282del4, R501X, S3247X and R2447X). 60
Small tokens of appreciation were sent to all participating families by the NCTU throughout the trial including BEEP branded muslin or bib at randomisation, birthday card and BEEP branded plastic cutlery set or storybook at the child’s first birthday and BEEP branded cloth shoulder bag sent at 18 months. At 24 months parents received a £10 voucher either with the invitation letter for the primary follow-up visit or at the visit according to their allocation for the nested SWAT. A £10 voucher was also sent to parents on completion of the 48- and 60-month questionnaire. Parents were also sent trial newsletters every 6 months (from January 2016). The newsletter sent to parents in February 2020 summarised the results for the primary and secondary outcomes up to 24 months. 61
Trial oversight
An independent Trial Steering Committee (TSC) provided overall supervision of the trial. The TSC had an independent chair and four independent members (parent representative, statistician, consultant dermatologist and paediatric epidemiologist as detailed in Acknowledgements). The TSC met at least once a year during recruitment and follow-up for the primary outcome.
Due to the very low medical risk associated with the intervention, there was no separate data monitoring committee for the trial. Safety outcomes were monitored by the TSC during closed sessions of the meetings attended by only the independent members.
Sample size
The sample size was based on assuming 30% of children in the control group would have eczema between 1 and 2 years of age (based on previous epidemiological studies in this high-risk population) and a conservative relative reduction of 30% in the intervention group. This relative reduction was considered conservative as in the pilot study, a 50% reduction in eczema at 6 months was observed [43% developed eczema in the control group (n = 55) and 22% developed eczema in the emollient group (n = 53), 95% CI for RR 0.28 to 0.90]. The anticipated effect size was lower in the main trial due to the more pragmatic study design and the longer-term outcome assessment; however, such a reduction would still have important implications for families and health services.
A total of 1282 children were required to allow this difference to be detected (i.e. 30% of children in the control group, 30% relative reduction to –21% of children in the intervention group) at the 5% significance level (two-sided) with 90% power and allowing for 20% attrition at 24 months.
The protocol specified that the assumptions underpinning the sample size would be checked by independent members of the TSC after approximately 21 months of recruitment (i.e. by checking the percentage of children with eczema in the control group and percentage with follow-up data). However, the original target sample size of 1282 was exceeded prior to this point and the Chief Investigator requested that the sample size review therefore be brought forwards. At this point (July 2016), sites were told to stop consenting women to the trial however randomisation continued for women who consented before this date prior to the birth of their baby. In August 2016, independent members of the TSC were sent details of the follow-up questionnaire completion rate in each group and parental-reported medical diagnosis of eczema in the control group from the questionnaires by a NCTU statistician independent of BEEP. The TSC were asked to advise on whether consent and randomisation should continue. The TSC recommended that consent to the trial should be permanently terminated but randomisation should continue for any women who had consented but had not yet been randomised. The total number of children randomised was expected to be approximately 1400.
Stopping rules and discontinuation
There was no planned interim analysis of treatment efficacy for the primary outcomes. The criteria below (Box 1) were specified in the protocol in relation to recruitment and adherence to trigger discussion with the TSC and funder regarding the best course of action.
-
Recruitment: If recruitment (as documented in the recruitment plan) was < 50% of the expected rate by 15 months, and strategies to overcome the identified barriers to recruitment had not been successful.
-
Adherence to the intervention: If fewer than 90% of families in the intervention group had applied emollient over the majority of their child’s body at some stage and fewer than 70% were still using emollient at 6 months.
-
Emollient use by the control group: If emollient use in the control group exceeded 25% of families at 6 months. This excluded the use of emollients for the treatment of eczema and only applied to emollient use that closely reflected the intervention (i.e. regular widespread use in the first year of life, defined as widespread emollient use over the majority of the child’s body at least 3 or more days per week).
Statistical methods
Analyses are detailed in the SAP. Version 1.0 of the SAP was finalised prior to database lock and release of treatment allocation codes for analysis of the primary and secondary outcomes at 24 months. 47 Version 2.0 of the SAP was finalised prior to the database lock for the analysis of the tertiary outcomes at 60 months. 47 All analyses were carried out using Stata 15.0 or above (StataCorp LP, College Station, TX, USA), unless otherwise specified.
The main approach for analysis was to analyse participants (children) as randomised regardless of adherence with the allocated intervention using available data (i.e. without imputation for missing data) with sensitivity analysis for key outcomes using multiple imputation for missing data. Estimates of the intervention effect are presented with 95% CIs.
Descriptive analyses
Baseline characteristics were summarised by allocated group to describe the sample recruited and to examine balance between the two groups.
Follow-up completion was summarised in the two groups and baseline characteristics of children with and without primary outcome data in each group compared descriptively. Information collected on follow-up questionnaires and at the 24-month visit on characteristics which may be associated with the development of eczema and on the timing of introduction of allergenic foods as well as recent consumption of these foods were summarised by allocated group to inform the interpretation of the results.
Compliance and contamination
Compliance in the intervention group at each questionnaire time point was defined as widespread emollient use over the majority of the child’s body at least 3 or more days per week. The ‘majority of the child’s body’ was defined as at least two of the three body areas asked about on the questionnaire (face/neck, arms/legs or trunk). Contamination at each questionnaire time point in the control group was defined as use of a moisturiser or oil at least 3 days per week over most or all of the child’s body since the last questionnaire. Compliance in the intervention group and contamination in the control group at each questionnaire time point were tabulated.
Compliance and contamination over the first year of life were described using an ordered categorical variable, as defined in Table 3.
| Level of compliance in the intervention group/contamination in the control group | Criterion for compliance/contamination met at the following time points |
|---|---|
| Full | 3, 6 and 12 months |
| Early-onset application | 3 months (with neither or only one of 6 or 12 months) |
| Late-onset application | 6 and/or 12 months (but not at 3 months) |
| None | Compliance/contamination criterion not met at any of 3, 6 or 12 months |
Compliance/contamination was summarised for participants with complete data on compliance/contamination (i.e. completed questionnaires at 3, 6 and 12 months) and for all participants using the assumptions for missing data described below.
-
Participants with no reported data on emollient/moisturiser use were categorised as not compliant in the intervention arm and not contaminated in the control arm.
-
If participants missed a questionnaire(s) and went on to complete a subsequent questionnaire, missing emollient/moisturiser use was based on the next subsequent observation carried backwards. For example, if the 6-month questionnaire was missed for a participant in the intervention group and they reported being compliant at 12 months, then it was assumed that they were also compliant at the 6-month time point. The rationale for this was that it was assumed that compliance was likely to decrease over time so that this would be conservative for the intervention group.
-
If participants completed questionnaires initially and missed later questionnaires (e.g. completed at 3, did not complete 6 or 12 or completed at 3 and 6, did not complete 12), then it was assumed that there was no compliance (intervention)/contamination (control) for the later missed questionnaires.
-
Categorisation of compliance/contamination over the first 12 months following randomisation for participants with missing emollient/moisturiser use data then proceeded according to the above table.
Compliance/contamination was summarised according to (1) allocated group and (2) allocated group and whether there was a parental report of eczema diagnosis by a doctor or nurse in the first year (yes, no or unknown).
Primary outcome
The adjusted RR and difference in risk for the primary outcome were estimated using generalised estimating equations with the binomial family and log/identity link, respectively, with an exchangeable correlation matrix to account for randomisation being stratified by centre and number of immediate family members with atopic disease (one, two or more than two) included as a covariate.
Sensitivity analysis for the primary outcome
The following sensitivity analyses for the primary outcome were performed:
-
Analysis repeated including diagnosis of eczema data collected from GP records for participants with missing primary outcome data.
-
According to the method of collection of the primary outcome data: between-group estimates for the risk of eczema were calculated separately for outcomes collected during face-to-face visits and outcomes collected by telephone/e-mail/SMS using generalised estimating equations adjusting for stratification variables.
-
Analysis repeated replacing ‘visible flexural dermatitis’ in the UKWP criteria with any visible dermatitis (EASI score of > 0) in the derivation of eczema between 1 and 2 years of age.
-
Using multiple imputation for missing primary outcome data (further details described below).
-
Repeating the analysis assuming that all participants in the intervention group with missing primary outcome data were eczema free or had eczema and all participants in the control group with missing data had eczema or were eczema free (i.e. best-case and worst-case scenario for intervention).
A sensitivity analysis was also planned to further adjust for any baseline characteristics with an observed imbalance between groups. However, no important differences between groups in the baseline characteristics were observed so this sensitivity analysis was not conducted.
Multiple imputation was performed using chained equations. 62 The following variables were used in the imputation model:
-
Allocated group.
-
Randomisation stratification variables: centre, number of immediate family members with atopic disease (one, two or more than two).
-
Age of mother, number of other children in household at screening (none, one, two, three or more), any furry pets in the household at screening (yes/no) and baby sex (baseline variables identified as predictive of drop-out by examination only).
-
Variables used in subgroup analyses – number of FLG null mutations, the number of immediate family members with eczema, water hardness, season of birth and regular use of probiotic supplements during pregnancy.
-
Summary of compliance and contamination in the first year of life as per the definitions above (Table 3).
The following outcomes were imputed:
-
Diagnosis of eczema in the last year at age 2 years (primary outcome).
-
Parental report of a clinical diagnosis of eczema at 2 years.
-
Parental report of immediate allergy to cow’s milk, egg or peanut at 2 years.
-
Allergic sensitisation to cow’s milk, egg or peanut.
-
Food allergy to any of milk, egg or peanut at 2 years.
Forty data sets were imputed and the results of the analyses on the imputed data sets were combined using Rubin rules for multiply imputed data. This analysis assumed that unobserved outcomes are missing at random (MAR) and depend on observed characteristics but not the unobserved outcomes, since it was considered most plausible that missing outcomes would have been similar to observed outcomes for participants with similar characteristics.
Secondary analysis of the primary outcome
To explore the effect of application of emollient in the first year of life in parents who would comply with the allocated treatment, the complier-average causal effect (CACE) was estimated. 63 CACE models were implemented as latent growth mixture models64 in MPlus (Version 5.2), with compliance/contamination status included as a training variable for estimating class membership.
Two separate odds ratios (ORs) for the CACE were estimated based on the following definition of compliance:
-
full compliance over the first year of life as per Table 3 – participants in the control group who met the criteria for full contamination were considered as always-takers
-
compliance within the first 3 months (i.e. in the full compliance or early-onset application categories in Table 3) – participants in the control group who met the criteria for full or early-onset contamination were considered as always-takers.
For children with incomplete data on compliance/contamination, the assumptions described above were used to categorise their compliance/contamination.
Subgroup analysis of the primary outcome
Exploratory subgroup analyses were conducted by including appropriate interaction terms in the regression model for the following variables:
-
Number of FLG mutations (no FLG mutation vs. one or two FLG mutations).
-
Number of immediate family members with atopic disease (one, two or more than two).
-
Number of immediate family members with eczema (zero, one, two or more).
-
Season of birth (spring – born in March, April or May, summer, autumn, winter).
-
Water hardness (dichotomised into hard/very hard and moderate/soft).
-
Parental-reported regular use of probiotic supplements during pregnancy (yes/no).
Subgroup analyses one to three were specified in the protocol and subgroup analyses four to six were specified in the SAP prior to database lock for the primary outcome.
The FLG subgroup analysis included children whose mother and father were reported to be of white European ethnicity, since the mutations tested are most prevalent in the white European population. Children who were found to have at least one mutation (regardless of ethnicity) were also included in this subgroup analysis but children of other ethnicities without FLG mutations were excluded because the genotyping may have been a false-negative result.
Secondary outcomes
For binary secondary outcomes, the adjusted RR and difference in risk were estimated using the analysis model specified for the primary outcome.
Time to onset of eczema was presented descriptively by showing the cumulative percentage of children with eczema at 3, 6, 12, 18 and 24 months in a bar graph and table and presented separately according to: first parental report of a clinical diagnosis of eczema and first topical corticosteroid and/or immunosuppressant prescription for eczema.
Additional analyses were also performed for the main food allergy outcome of confirmed diagnosis of food allergy at 24 months to any of milk, egg or peanut. Sensitivity analyses were conducted using multiple imputation for missing outcomes as described above and repeating the analysis including panel decisions of ‘unclear – possible food allergy’ as allergic and ‘unclear – food allergy unlikely’ as not allergic. The CACE was also estimated for the confirmed food allergy outcome (using methods described above). Exploratory subgroup analyses were conducted by including appropriate interaction terms in the regression model for the following variables: number of FLG mutations, number of immediate family members with atopic disease and number of immediate family members with eczema.
Safety outcomes
The adjusted incidence rate ratio for the number of skin infections reported per child was estimated using generalised estimating equations with a negative binomial family and log link with an exchangeable correlation matrix to account for randomisation being stratified by centre and number of immediate family members with atopic disease (one, two or more than two) included as a covariate. Slippage incidents were compared between allocated groups as a binary variable (any slippages reported in the first year/none) using the same analysis model as specified for the primary outcome.
For each questionnaire time point, parental-reported skin infections and slippage incidents since the last questionnaire were also presented descriptively by allocated group and parental-reported emollient/moisturiser use (none/some/widespread over the majority of the child’s body at least 3 or more days per week).
Tertiary outcomes
The analysis of the tertiary outcomes was in keeping with the analysis of the primary and secondary outcomes. Analysis was according to randomised group, regardless of adherence with allocation and estimates of the intervention effect are presented with 95% CIs. The main analysis of the tertiary outcomes assumed that missing outcomes were MAR, that, it does not depend on the unobserved outcomes given the observed data.
Analysis of binary tertiary outcomes at 36, 48 and 60 months used mixed-effects logistic regression model, which gives valid inferences when data are assumed MAR. 65 The models included the outcome collected at earlier time points in the trial (i.e. 12 and 24 months where applicable) as dependent variables and adjusted for the randomisation stratification variables, using a fixed effect for the number of immediate family members with atopic disease (one, two, or more than two) and a random effect for the recruiting centre, and a random effect for participant. Models included an allocated treatment-by-time interaction to estimate the between-group difference at each follow-up time point and where technically possible an interaction between number of immediate family members with atopic disease and time. Adjusted risk differences and adjusted risk ratios along with corresponding 95% CIs were obtained using Stata’s margins command with standard errors computed using the delta method. 66
Multiple imputation using chained equations was used to impute missing outcomes collected at 60 months on parental report of a clinical diagnosis of asthma and parental report of a clinical diagnosis of allergic rhinitis and the derived outcomes of parental report of a clinical diagnosis of eczema from the age of 12 to 60 months and parental report of a clinical diagnosis of food allergy by 60 months. The following variables were used in the imputation model: allocated group, randomisation stratification variables (centre, number of immediate family members with atopic disease) and baseline variables identified as predictive of drop-out (by examination only: mothers age at randomisation, number of other children in the household at randomisation, decile of index of multiple deprivation). Fifty data sets were imputed. Between-group effects in each imputed data set were estimated using a mixed-effects logistic regression model including a fixed effect for randomisation stratification variable of number of immediate family members with atopic disease and a random effect for the recruiting centre. The adjusted risk differences and adjusted risk ratios were computed in each imputed data set (computed using the delta method described above) and combined using Rubin rules for multiply imputed data.
To explore the robustness of the results to the MAR assumption, sensitivity analysis was conducted for the tertiary outcomes of parental report of clinical diagnosis of eczema from the age of 12 to 60 months and parental report of clinical diagnosis of food allergy by 60 months under a missing not at random (MNAR) assumption using controlled multiple imputation. 67 Delta (δ)-based multiple imputation was used to modify the value imputed under a MAR assumption by a fixed amount to explore how the results change if participants with missing outcomes were more likely to have a worse outcome than predicted (based on the MAR assumption). A range of δ values were used in the sensitivity analysis.
Summary of changes to the protocol
A full list of all substantial amendments to the protocol can be found in Appendix 2. All amendments were reviewed by the Sponsor before submission to, and approval by, the Research Ethics Committee and/or the Health Research Authority.
Patient and public involvement
Patient and public involvement (PPI) members of the team provided a vital and valuable role throughout the trial. The aim of PPI was to enhance the design of the main trial.
We were fortunate in being able to conduct a thorough pilot trial as part of a programme grant42 in preparation for this definitive trial. The pilot RCT was similar in design to the main trial and therefore provided the opportunity for meaningful and significant input from parents who had direct experience of a similar eczema prevention trial. Their opinions and feedback during the design of the main trial were therefore largely based on experience rather than hypothetical scenarios and were key in reaching the final design of this main trial. The main areas of input from parents who had participated in the pilot were around the following issues:
-
Continue applying emollient until the child was 12 months old rather than the 6 months tested in the pilot. Parental feedback strongly suggested was that this was acceptable, and in fact, many had chosen to do so anyway in the pilot. Therefore, we felt confident that an extended intervention period of 12 months could be introduced to the main trial without high risk to intervention adherence and this was reflected in the continued high adherence rates during months 6–12 of the intervention period.
-
Restricting choice of emollient to cream/gel formulation only. Parents reported in the pilot that they liked having a choice of emollient, so it was important to retain this. However, because the cream/gel formulation was the most popular, the decision was made to offer a choice from within this emollient type. A group of parents who had participated in the pilot trial participated in a preference study which, along with mechanistic studies, supported the choice of emollient for the main trial.
-
Number of visits reduced to only screening and 24-month follow-up. The pilot trial involved multiple visits to the nurse to check the skin for signs of eczema. The feedback from parents was that although they appreciated the support, they often felt the visits were not necessary, especially when there was nothing wrong with their child’s skin. Taking this parental feedback into account and to design a large definitive trial that was more practical to deliver, the interim follow-up was conducted via online questionnaires and via contact with the co-ordinating centre over the telephone, coupled with advice to visit the GP if they had concerns about their child’s skin. This was a successful approach in terms of making it a viable trial but contacting the higher proportion of non-completers of online questionnaires than anticipated required more resource from the co-ordinating centre. Incentives to complete the questionnaires were introduced part way through the trial to help with completion rates.
-
Addition of allergic sensitisation (skin prick) tests at the 24-month visit. As these tests were not done as part of the pilot trial it was essential to get input from parents into the acceptability of these in the main trial. Parents were generally very keen for their child to receive these tests, but due to funding restrictions, SPTs were introduced part way through the trial recruitment period. As a result, and because of additional concerns raised by families participating in the main trial about the need to travel to the oral food challenge, the SPTs were introduced as optional and separate consent was sought.
Further details of how parental input shaped the main trial design are in the pilot study report. 42
In the early stages of the trial set-up, the Centre of Evidence Based Dermatology (CEBD) patient panel also provided input. The panel comprises of people with lived experience of a wide range of skin diseases but the input into this trial was sought mainly from members who are parents of children with eczema along with some adults with eczema. The panel reviewed and improved the BEEP patient-facing study documentation, including the participant information sheet (PIS), consent forms and the design and content of the online follow-up questionnaires. These were refined as a result of the feedback to ensure they were suitable. As the main trial progressed through the pilot phase, further feedback was sought from both parents and an eczema support group.
Patient and public involvement representatives were involved in promoting and publicising the trial (through local television and radio) alongside the Chief Investigator. In addition, the PPI team were consulted to help modify documentation to increase up-take of the SPT at the 24-month visit and subsequent food challenges. Their advice was sought to help disseminate the important primary outcome findings. We also decided to share the 2-year primary outcome results directly with participating families61 as our first audience, that is before the main publication in The Lancet. Additional PPI colleagues with lived experience of eczema also participated in our 5-year results reveal meeting and were helpful in contextualising the key results on lack of benefit and possible signals of increased minor skin infections and skin sensitisation.
A PPI representative sat on the TSC and attended the meetings throughout the trial and provided key advice on how to balance the key messages for parents and carers.
Chapter 3 Results
Recruitment
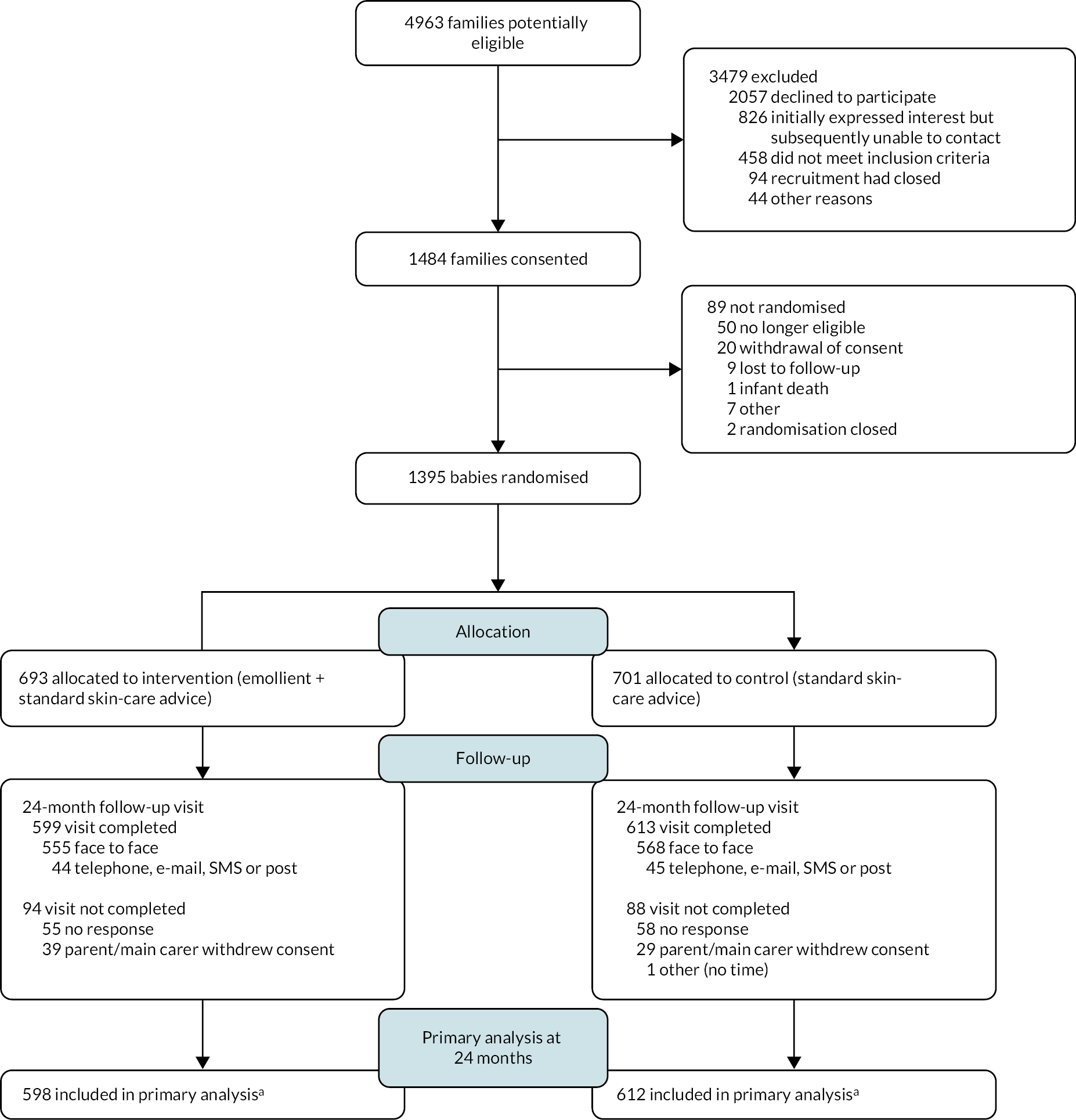
Recruitment to the trial took place between 19 November 2014 and 14 July 2016 and the last baby was randomised on 18 November 2016. During this time, 4963 families were assessed for eligibility, and of these 1484 families consented (30%) (Figure 6). The main reason families assessed for eligibility did not consent was due to declining (41% of those assessed). After the baby was born, 1395 babies were randomised (94%). Non-randomisation after consent was mainly as a result of no longer being eligible (n = 50), mostly as babies were born preterm (n = 41). One baby was randomly assigned in error 62 days after birth so was not included further. Of the 1394 babies, 693 were randomised to the emollient group and 701 to the control group (randomisation by site presented in Appendix 3, Table 28). The mean number of days between birth and randomisation was 5.8 [standard deviation (SD) 5.7]. Four babies were randomised more than 21 days after they were born.
FIGURE 6.
Participant flow diagram to 24 months. a, Insufficient data collected to derive primary outcome for two participants (one in each group) at 24 months.
Baseline characteristics
Family and baseline baby characteristics were well balanced across groups (Table 4). The mean age of the mothers at randomisation was 31 (SD 5.3), 32% of babies were delivered by caesarean section and 82% of babies had at least one first-degree relative with a history of eczema (parent report of doctor diagnosis).
| Intervention (n = 693) | Control (n = 701) | Total (n = 1394) | |
|---|---|---|---|
| Age of mother at randomisation | |||
| Mean (SD) | 31.7 (5.3) | 31.5 (5.2) | 31.6 (5.3) |
| Minimum, maximum | 16, 45 | 18, 46 | 16, 46 |
| Singleton pregnancy | 690 (100%) | 696 (99%) | 1386 (99%) |
| Ethnicity of mother | |||
| White | 589 (85%) | 601 (86%) | 1190 (85%) |
| Asian | 45 (6%) | 40 (6%) | 85 (6%) |
| Black | 31 (4%) | 22 (3%) | 53 (4%) |
| Other | 28 (4%) | 38 (5%) | 66 (5%) |
| Ethnicity of father | |||
| White | 583 (84%) | 606 (86%) | 1189 (85%) |
| Asian | 45 (6%) | 26 (4%) | 71 (5%) |
| Black | 30 (4%) | 31 (4%) | 61 (4%) |
| Other | 26 (4%) | 29 (4%) | 55 (4%) |
| Not given | 9 (1%) | 9 (1%) | 18 (1%) |
| Any furry pets living in house | 295 (43%) | 302 (43%) | 597 (43%) |
| Maternal antibiotics during pregnancy | 210 (30%) | 201 (29%) | 411 (29%) |
| Maternal probiotic supplements taken during pregnancy (collected at 6 months) | 33/511 (6%) | 32/505 (6%) | 65/1016 (6%) |
| Mother has/had a history of eczema (parent report of doctor diagnosis) | 348 (50%) | 372 (53%) | 720 (52%) |
| At least one first-degree relative with history of eczema (parent report of doctor diagnosis) | 563 (81%) | 580 (83%) | 1143 (82%) |
| Male infant | 374 (54%) | 359 (51%) | 733 (53%) |
| Gestation at birth (weeks) | |||
| Median (25th, 75th centile) | 40 (39.1, 40.9) | 40 (39, 40.9) | 40 (39, 40.9) |
| Delivery method | |||
| Vaginal delivery | 482 (70%) | 472 (67%) | 954 (68%) |
| Caesarean section | 211 (30%) | 229 (33%) | 440 (32%) |
| No other children living in household at screening | 275 (40%) | 293 (42%) | 568 (41%) |
| FLG genotype for children with both parents of white ethnicity or with a mutation detecteda | n = 402 | n = 414 | n = 816 |
| +/+ (no mutations) | 339 (84%) | 352 (85%) | 691 (85%) |
| +/− (one FLG null mutation) | 62 (15%) | 60 (14%) | 122 (15%) |
| −/− (two FLG null mutations) | 1 (< 0.5%) | 2 (< 0.5%) | 3 (< 0.5%) |
Follow-up to 24 months
Follow-up of the infants between 3 and 24 months took place between February 2015 and November 2018. The questionnaires at 3, 6, 12 and 18 months were completed for around 75% at each time point and completion was similar in both groups (Table 5). No questionnaires were completed for 14% of participants in both groups (see Table 5).
| Intervention (n = 693) | Control (n = 701) | Total (n = 1394) | |
|---|---|---|---|
| 3 months completed | 534 (77%) | 524 (75%) | 1058 (76%) |
| 6 months completed | 530 (76%) | 521 (74%) | 1051 (75%) |
| 12 months completed | 523 (75%) | 535 (76%) | 1058 (76%) |
| 18 months completed | 497 (72%) | 512 (73%) | 1009 (72%) |
| Total number of questionnaires completed between 3 and 18 months | |||
| None | 99 (14%) | 97 (14%) | 196 (14%) |
| One | 39 (6%) | 45 (6%) | 84 (6%) |
| Two | 49 (7%) | 51 (7%) | 100 (7%) |
| Three | 77 (11%) | 87 (12%) | 164 (12%) |
| Four | 429 (62%) | 421 (60%) | 850 (61%) |
At 24 months, follow-up was completed for 1212 randomised participants (87%, Figure 6) and completion was similar in both groups. Most visits (1123 in total) were completed face to face with a research nurse. For 89 participants, where a face-to-face visit was not possible, some data were collected via telephone, e-mail, SMS or post, although for two participants insufficient data were collected to be able to derive the primary outcome. Research nurses completing the skin examination reported becoming aware of which group the child was randomised to (or possibly becoming aware) for 30 participants in the intervention group and 11 participants in the control group. For 12 participants in the intervention group and 6 in the control group, the research nurses reported that this unblinding happened before the examination of the child’s skin. Families of infants where no primary outcome data were collected in both groups were more likely to have joined the study after the birth of their baby rather than consenting antenatally, had slightly younger mothers on average when they were born and were more likely to be in a household with other children (see Appendix 3, Table 31). A slightly greater proportion of girls had primary outcome data collected compared to boys in the intervention group with the opposite observed in the control group (see Appendix 3, Table 31). The number of family members with a history of atopic disease, delivery method and days between birth and randomisation were similar between infants with and without primary outcome data collected (see Appendix 3, Table 31).
Of the 1212 infants where the 24-month follow-up visit was completed, SPTs to assess allergic sensitisation were fully completed for 81% in both groups (Table 6). Five children were given antihistamines after the SPT. No children had a serious allergic reaction to the SPTs requiring the use of an auto adrenaline injector.
| Intervention | Control | Total | |
|---|---|---|---|
| Total number of follow-up visits completed | 599 | 613 | 1212 |
| SPT completion | |||
| Consent to SPT | |||
| No | 49 (8%) | 58 (9%) | 107 (9%) |
| Yes | 508 (85%) | 512 (84%) | 1020 (84%) |
| N/A – not face to facea | 42 (7%) | 43 (7%) | 85 (7%) |
| SPT completion | |||
| SPT not done | 11 (2%) | 7 (1%) | 18 (1%) |
| SPT done | 484 (81%) | 494 (81%) | 978 (81%) |
| SPT partially done | 13 (2%) | 11 (2%) | 24 (2%) |
| Food allergy assessment completion | |||
| Food challenge required | |||
| For peanut | 76 (13%) | 85 (14%) | 161 (13%) |
| For cow’s milk | 32 (5%) | 28 (5%) | 60 (5%) |
| For egg | 83 (14%) | 73 (12%) | 156 (13%) |
| For at least one of these three foods | 133 (22%) | 125 (20%) | 258 (21%) |
| Food challenge took place | |||
| For peanut | 22 (4%) | 19 (3%) | 41 (3%) |
| For cow’s milk | 5 (1%) | 1 (< 0.5%) | 6 (< 0.5%) |
| For egg | 24 (4%) | 11 (2%) | 35 (3%) |
| For at least one of these three foods | 41 (7%) | 28 (5%) | 69 (6%) |
| Diagnosis method where food challenge required | |||
| For peanut | |||
| By food challenge | 22 (29%) | 19 (22%) | 41 (25%) |
| Panel diagnosis – probable food allergy/no food allergyb | 33 (43%) | 40 (47%) | 73 (45%) |
| Panel diagnosis – possible food allergy/food allergy unlikely unclearc | 21 (28%) | 26 (31%) | 47 (29%) |
| n | 76 | 85 | 161 |
| For cow’s milk | |||
| By food challenge | 5 (16%) | 1 (4%) | 6 (10%) |
| Panel diagnosis – probable food allergy/no food allergyb | 22 (69%) | 22 (79%) | 44 (73%) |
| Panel diagnosis – possible food allergy/food allergy unlikely unclearc | 5 (16%) | 5 (18%) | 10 (17%) |
| n | 32 | 28 | 60 |
| For egg | |||
| By food challenge | 24 (29%) | 11 (15%) | 35 (22%) |
| Panel diagnosis – probable food allergy/no food allergyb | 43 (52%) | 45 (62%) | 88 (56%) |
| Panel diagnosis – possible food allergy/food allergy unlikely unclearc | 16 (19%) | 17 (23%) | 33 (21%) |
| n | 83 | 73 | 156 |
Based on the results of the SPTs and/or parent report of consumption and reaction to milk, egg and peanut, 258 children were invited to a food challenge for at least one of the three foods with similar numbers invited in the two groups (see Table 6). At least one food challenge took place for 69 children (27%). Similar numbers in each group had food challenges for peanut and cow’s milk. Twice as many children in the intervention group had a food challenge for egg compared to the control group (24 vs. 11). There were no incidents of the person completing the food challenge becoming unblinded to the group that the child was randomised to. The main reason that food challenges were not conducted was due to parents being unwilling to participate or due to the travelling distance to the two centres where the food challenges were conducted. For allergy to each food, more than 75% of diagnoses were made by panel consensus due to food challenges not being conducted. For some of these children, the panel made a diagnosis of possible food allergy or food allergy unlikely where there was insufficient information available to make a more definitive diagnosis; these diagnoses were included in a sensitivity analysis for the main food allergy outcome (see Table 6).
Adherence with the allocated intervention
For families in the intervention group, 509 (73%) responded to the telephone call at around 2 weeks to check whether they had received the skin-care pack and emollients, and to collect information on the date they started applying the emollient to the infant. Of those responding, the median age that families reported starting to apply the emollient was 11 days after birth [interquartile range (IQR) 7–17], and 452 (89%) reported starting to apply the emollient within 3 weeks of birth.
In the year after randomisation, around half of families in the intervention group reported usually applying the emollient every day (Table 7) and emollient was applied at least 3 days per week by 75%. Most families reported usually applying the emollient to the arms/legs and trunk and around three-quarters also reported usually applying it to the face/neck. Emollient was most commonly applied once a day and most families reported usually applying the emollient after bathing or showering their child. The small number of parents who said that they had not used the emollient at all at each time point reported several different reasons for this (see Table 7).
| 3 months | 6 months | 12 months | |
|---|---|---|---|
| Usual frequency of emollient use | |||
| Never | 20 (4%) | 33 (6%) | 58 (11%) |
| Once or twice a week | 34 (6%) | 53 (10%) | 68 (13%) |
| 3 or 4 days a week | 75 (14%) | 61 (12%) | 74 (15%) |
| 5 or 6 days a week | 104 (20%) | 84 (16%) | 59 (12%) |
| Every day | 299 (56%) | 289 (56%) | 248 (49%) |
| n | 532 | 520 | 507 |
| Emollient usually applied to | |||
| Face/neck | 398 (75%) | 397 (76%) | 351 (69%) |
| Arms/legs | 508 (95%) | 480 (92%) | 448 (88%) |
| Trunk | 483 (91%) | 464 (89%) | 427 (84%) |
| At least two of the areas above | 495 (93%) | 472 (91%) | 438 (86%) |
| n | 533 | 519 | 508 |
| Usual number of applications per day | |||
| None | 17 (3%) | 31 (6%) | 50 (10%) |
| Once | 422 (79%) | 382 (74%) | 362 (72%) |
| Twice | 63 (12%) | 68 (13%) | 69 (14%) |
| More than twice | 30 (6%) | 36 (7%) | 25 (5%) |
| n | 532 | 517 | 506 |
| Emollient use after bathing/showering child | |||
| Never | 21 (4%) | 37 (7%) | 58 (11%) |
| Sometimes | 40 (8%) | 38 (7%) | 44 (9%) |
| Usually | 471 (89%) | 441 (85%) | 406 (80%) |
| n | 532 | 516 | 508 |
| Reasons if emollient not used at all | |||
| Prescribed or advised to use a different emollient | 3 | 18 | 31 |
| Advised to stop applying emollient altogether | 1 | – | 2 |
| Not enough time to apply emollient | 4 | 2 | 4 |
| Baby didn’t like | – | 1 | 4 |
| Ran out of emollient | – | – | 2 |
| Other | 12 | 10 | 14 |
| na | 20 | 31 | 57 |
| Compliance with intervention (used emollient at least 3 days per week over the majority of the child’s bodyb) | |||
| No | 66 (12%) | 92 (18%) | 131 (26%) |
| Yes | 466 (88%) | 427 (82%) | 375 (74%) |
| n | 532 | 519 | 506 |
Of families with complete data on emollient use at each time point, 88% at 3 months, 82% at 6 months and 74% at 12 months reported using the emollient at least 3 days per week over the majority of the child’s body (compliance, Table 7). Complete data on adherence over the first year were collected for 442 infants (64%) and of these 311 (70%) infants were considered fully compliant with the intervention (as defined in Table 3). Using the conservative assumptions described in the Statistical methods section for participants with missing questionnaires, full compliance over the first year in the intervention group was estimated to be 51% (350/693) (see Appendix 3, Table 33).
In the control group, self-directed use of emollients at least 3 days per week to most of the body (contamination) was reported for 18% (82/457), 17% (62/372) and 15% (49/324) at 3, 6 and 12 months, respectively, for infants who did not have a parental report of a doctor diagnosis of eczema (see Appendix 3, Table 32).
Washing, feeding and other post-randomisation characteristics between birth and 24 months
Parent report of washing practices at 6, 12 and 24 months in terms of frequency, the products used to wash the child and use of oils in the child’s bath water were balanced across the two groups (see Appendix 3, Table 34). Between 12 and 24 months, just under half of the families in the intervention group and just under 30% in the control group reported using a moisturiser on their child at least 3 days per week to most of the body. This moisturiser use was more common in both groups in children with a parental report of a clinical diagnosis of eczema (see Appendix 3, Table 35).
At 6 months, parental report of the milk type used to feed their baby since birth and the introduction of solid foods was similar in the two groups (see Appendix 3, Table 36). Around 40% of babies in both groups had antibiotics in their first year and 50% in their second year of life (see Appendix 3, Table 36). Other characteristics such as additional children in the household, use of dust mite reduction measures, water softeners fitted in the house and whether the child regularly attended nursery or a playgroup were also similar in both groups (see Appendix 3, Table 36).
By 24 months, almost all children had eaten foods containing cow’s milk and egg and over three-quarters had eaten foods containing peanut (see Appendix 3, Table 37). The timing of the introduction of these foods was similar in the two groups. The percentage of children who had consumed these three foods recently and frequently at 24 months was also similar in the two groups (see Appendix 3, Table 37).
Primary outcome: diagnosis of eczema between 12 and 24 months of age
Primary analysis
A diagnosis of eczema between 12 and 24 months, defined as meeting the UKWP Criteria for Atopic Eczema, was present in 139 (23%) of 598 infants in the intervention group and in 150 (25%) of 612 in the control group. The adjusted RR of eczema in the intervention group compared to the control group was 0.95 (95% CI 0.78 to 1.16); p-value = 0.61 and adjusted risk difference –1.2% (95% CI –5.9% to 3.6%).
Sensitivity analysis
All sensitivity analyses conducted, including using multiple imputation for missing primary outcome data, were consistent with the primary analysis showing no difference between the two groups in the presence of eczema (Table 8).
| Intervention | Control | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) | |
|---|---|---|---|---|
| Primary analysis | ||||
| AE in the previous 12 months using the UKWP Diagnostic Criteria for children under 4 years | 139/598 (23%) | 150/612 (25%) | 0.95 (0.78 to 1.16) | −1.2% (−5.9% to 3.6%) |
| Sensitivity analyses | ||||
| Using data from GP records for participants with no primary outcome data | ||||
| AE in the previous 12 months using the UKWP Diagnostic Criteria for children under 4 years or eczema ascertained from GP records | 145/624 (23%) | 157/639 (25%) | 0.95 (0.78 to 1.15) | −1.2% (−5.9% to 3.5%) |
| According to method of data collection | ||||
| For infants with data collected at a face-to-face visit AE in the previous 12 months using the UKWP Diagnostic Criteria for children under 4 years |
131/555 (24%) | 142/568 (25%) | 0.95 (0.77 to 1.16) | −1.3% (−6.3% to 3.7%) |
| For infants with data collected via telephone, e-mail, SMS or post AE in the previous 12 months using the UKWP Diagnostic Criteria for children under 4 years |
8/43 (19%) | 8/44 (18%) | 1.02 (0.43 to 2.40) | −1.4% (−17.7% to 14.9%) |
| Replacing the UKWP criteria definition of visible dermatitis with visible dermatitis anywhere on the body | ||||
| AE in the previous 12 months | 147/598 (25%) | 161/612 (26%) | 0.93 (0.77 to 1.14) | −1.5% (−6.4% to 3.4%) |
| Using imputation for missing outcome data | ||||
| Using multiple imputation model as specified in SAPa | 23.3% (SE 1.7%) | 24.5% (SE 1.8%) | 0.96 (0.78 to 1.17) | −1.0% (−5.8% to 3.8%) |
| Using multiple imputation model including randomisation stratification variables onlyb | 23.0% (SE 1.7%) | 24.6% (SE 1.7%) | 0.94 (0.77 to 1.14) | −1.4% (−6.0% to 3.3%) |
Secondary analysis
The CACE estimate of the adjusted OR of eczema was 0.78 (95% CI 0.32 to 1.89; n = 1210) when compliance was defined as widespread emollient use over the child’s body at least 3 or more days per week over the first year of life (i.e. full compliance). The CACE estimate of the adjusted OR of eczema based in compliance in the first 3 months was 0.88 (95% CI 0.50 to 1.56; n = 1210).
Subgroup analysis
There was no evidence of an interaction effect with allocated group on the risk of eczema according to number of first-degree relatives with atopic disease, number of first-degree relatives with a history of eczema, FLG genotype, season of birth, water hardness or regular use of probiotics during pregnancy (see Figure 7, see also Appendix 3, Table 38).
FIGURE 7.
Adjusted RR with 95% CIs in each subgroup for primary outcome of diagnosis of eczema between 12 and 24 months.
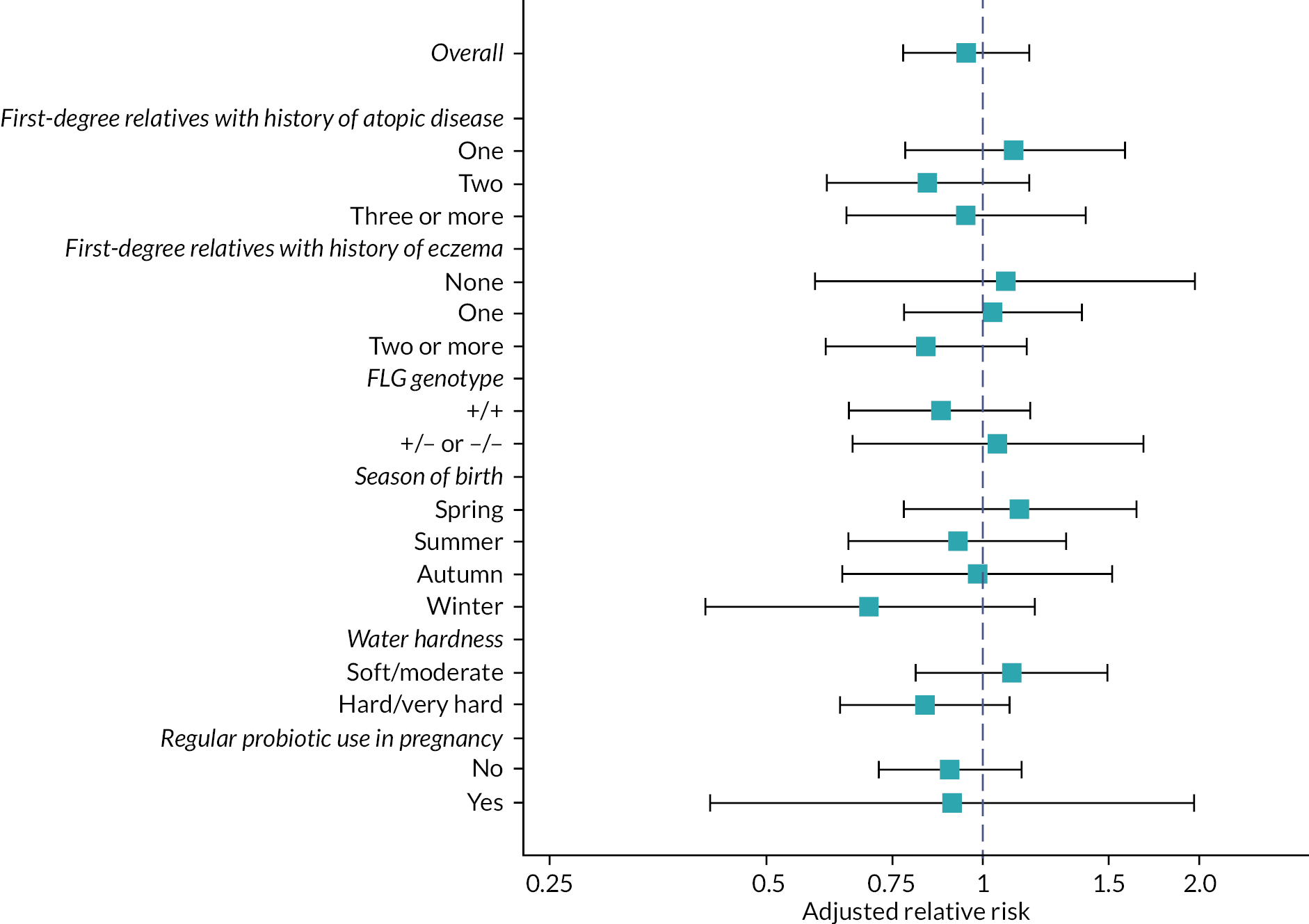
Secondary outcomes
Eczema-related secondary outcomes
There were no differences between the groups in any of the secondary outcomes for eczema, including different definitions of eczema diagnosis (Table 9), time to onset of eczema (see Figure 8, Appendix 3, Figure 13 and Table 39) and severity of eczema (see Table 9, Figure 9 and Appendix 3, Table 40).
| Intervention | Control | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) | |
|---|---|---|---|---|
| At 12 months | ||||
| Eczema according to UKWP Diagnostic Criteria (parent completion) | 103/516 (20%) | 107/527 (20%) | 0.98 (0.77 to 1.25) | −0.3% (−5.1% to 4.6%) |
| Moderate/severe/very severe on POEM | 52/512 (10%) | 49/522 (9%) | 1.09 (0.75 to 1.57) | 1.0% (−2.5% to 4.6%) |
| At 24 months | ||||
| Presence of visible eczema at 24 months | 151/555 (27%) | 149/568 (26%) | 1.05 (0.86 to 1.27) | 1.1% (−4.0% to 6.3%) |
| Parent report of a clinical diagnosis of eczema between birth and 24 months | 266/610 (44%) | 282/616 (46%) | 0.96 (0.85 to 1.08) | −2.0% (−7.5% to 3.6%) |
| Eczema according to UKWP Diagnostic Criteria (parent completiona) | 187/599 (31%) | 195/612 (32%) | 0.98 (0.83 to 1.16) | −0.5% (−5.7% to 4.8%) |
| Moderate/severe/very severe eczema on EASI | 9/553 (2%) | 10/567 (2%) | 0.93 (0.38 to 2.27) | 0.0% (−1.5% to 1.4%) |
| Moderate/severe/very severe on POEM | 58/576 (10%) | 51/595 (9%) | 1.18 (0.82 to 1.68) | 1.7% (−1.6% to 5.0%) |
FIGURE 8.
Time to onset of eczema based on first parental report of clinical diagnosis of eczema.
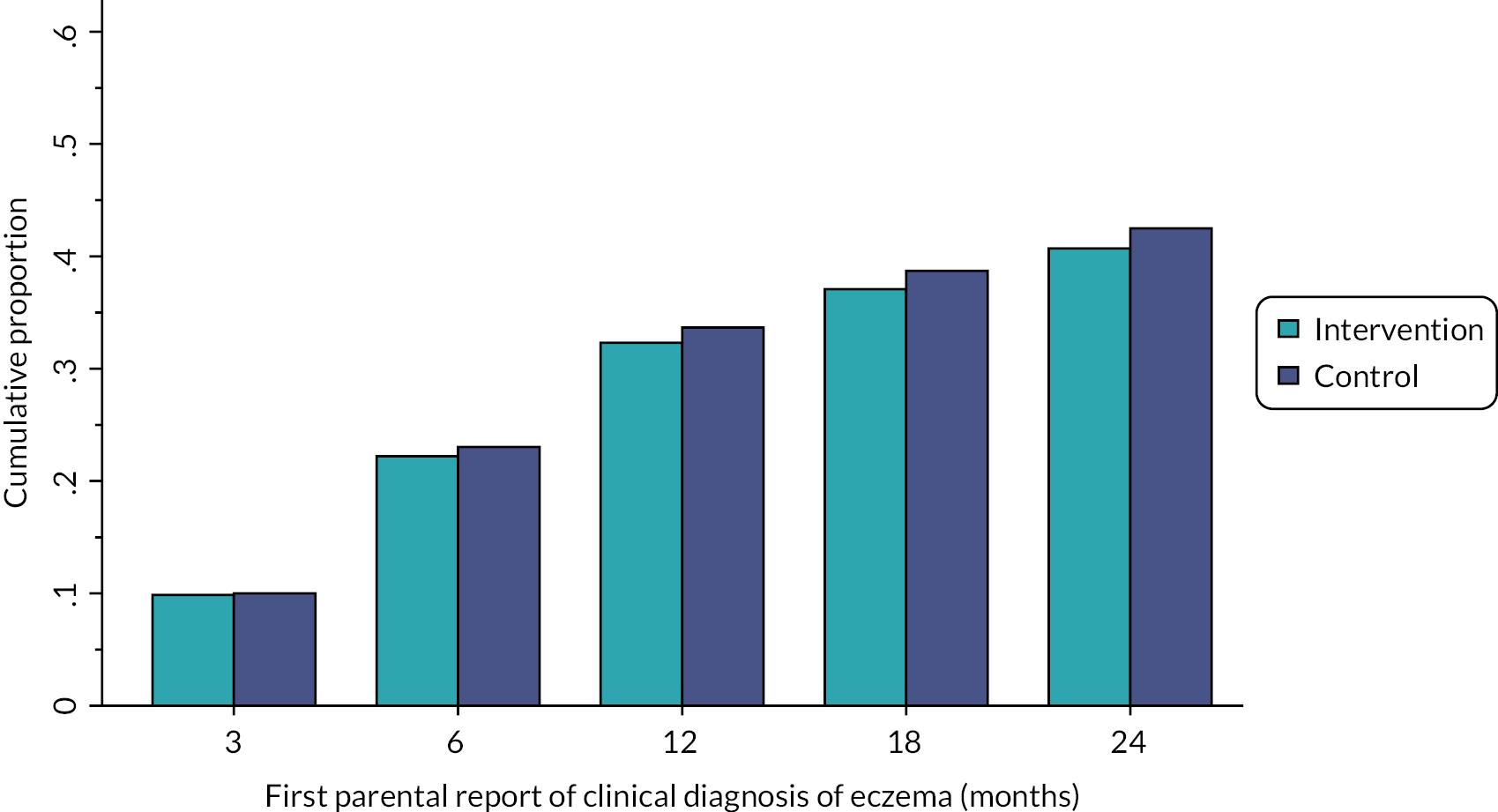
FIGURE 9.
Severity of eczema. (a) EASI at 24 months (blinded assessment of severity by research nurse) based on categories in Leshem et al. 56 (b) POEM at 12 months (parent-reported severity) based on categories in Charman et al 2013. (c) POEM at 24 months. Reproduced from Chalmers et al. 1 under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Allergic rhinitis and wheezing at 24 months
The number and percentage of infants with parental-reported allergic rhinitis and wheezing between 12 and 24 months were similar in the two groups (Table 10).
| Intervention (n = 572) | Control (n = 598) | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) | |
|---|---|---|---|---|
| Parental report of allergic rhinitis between 12 and 24 months | 174 (30%) | 188 (31%) | 0.97 (0.82 to 1.15) | −0.8% (−6.2% to 4.5%) |
| Itchy, watery eyes with the allergic rhinitis | 77 (13%) | 76 (13%) | ||
| Parental report of wheezing between 12 and 24 months | 197 (34%) | 191 (32%) | 1.07 (0.91 to 1.26) | 2.5% (−2.9% to 7.9%) |
| Number of attacks of wheezing | ||||
| None | 1 (< 0.5%) | 1 (< 0.5%) | ||
| 1–3 | 128 (22%) | 130 (22%) | ||
| 4–12 | 52 (9%) | 44 (7%) | ||
| More than 12 | 16 (3%) | 16 (3%) | ||
Allergic sensitisation at 24 months
There was no evidence of a difference between the two groups in the percentage of infants with allergic sensitisation on SPT overall or to inhalant allergens, and although allergic sensitisation to milk, egg or peanut was more common in the intervention group, the CI for the difference between groups was wide and included the possibility of no effect (adjusted RR 1.36, 95% CI 0.94 to 1.95, Table 11).
| SPT longest wheal diameter | Intervention | Control | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) |
|---|---|---|---|---|
| Cow’s milk | ||||
| 0 mm | 461 (94%) | 468 (94%) | ||
| 1–2 mm | 13 (3%) | 19 (4%) | ||
| 3–6 mm | 8 (2%) | 6 (1%) | ||
| 7 mm | 6 (1%) | 5 (1%) | ||
| n | 488 | 498 | ||
| Egg | ||||
| 0 mm | 435 (89%) | 450 (90%) | ||
| 1–2 mm | 12 (2%) | 16 (3%) | ||
| 3–6 mm | 20 (4%) | 17 (3%) | ||
| ≥ 7 mm | 23 (5%) | 16 (3%) | ||
| n | 490 | 499 | ||
| Peanut | ||||
| 0 mm | 457 (93%) | 468 (93%) | ||
| 1–2 mm | 15 (3%) | 18 (4%) | ||
| 3–6 mm | 17 (3%) | 15 (3%) | ||
| ≥ 7 mm | 1 (< 0.5%) | 1 (< 0.5%) | ||
| n | 490 | 502 | ||
| Grass pollen | ||||
| 0 mm | 464 (94%) | 471 (94%) | ||
| 1–2 mm | 18 (4%) | 18 (4%) | ||
| 3–6 mm | 10 (2%) | 13 (3%) | ||
| ≥ 7 mm | – | – | ||
| n | 492 | 502 | ||
| Cat | ||||
| 0 mm | 466 (95%) | 471 (94%) | ||
| 1–2 mm | 14 (3%) | 16 (3%) | ||
| 3–6 mm | 11 (2%) | 13 (3%) | ||
| ≥ 7 mm | 1 (< 0.5%) | – | ||
| n | 492 | 500 | ||
| Dust mite | ||||
| 0 mm | 432 (88%) | 444 (89%) | ||
| 1–2 mm | 27 (5%) | 26 (5%) | ||
| 3–6 mm | 29 (6%) | 26 (5%) | ||
| ≥7 mm | 5 (1%) | 4 (1%) | ||
| n | 493 | 500 | ||
| Allergic sensitisation to any allergen (SPT ≥ 3 mm) | 88/490 (18%) | 74/498 (15%) | 1.22 (0.92 to 1.62) | 3.1% (−1.5% to 7.7%) |
| Allergic sensitisation to cow’s milk, egg or peanut | 58/487 (12%) | 44/498 (9%) | 1.36 (0.94 to 1.95) | 2.9% (−0.9% to 6.8%) |
| Allergic sensitisation to grass pollen, cat or dust mite | 50/492 (10%) | 48/499 (10%) | 1.07 (0.74 to 1.55) | 0.9% (−2.8% to 4.5%) |
Parental report of food allergy
For most measures of parental-reported food allergy in the first 2 years, including parental report of a clinical diagnosis of food allergy, there were slightly increased numbers in the intervention group compared with the control group. However, CIs were wide and included the possibility of no difference between the two groups (Table 12).
| Intervention | Control | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) | |
|---|---|---|---|---|
| At 12 months | ||||
| Parental report of clinical diagnosis of food allergy at 12 months | 54/507 (11%) | 44/522 (8%) | 1.27 (0.87 to 1.85) | 2.4% (−1.1% to 6.0%) |
| Parental report of any food allergy at 12 months | 110/505 (22%) | 103/523 (20%) | 1.11 (0.87 to 1.40) | 2.1% (−2.8% to 7.1%) |
| Reaction to cow’s milk | 72/508 (14%) | 66/525 (13%) | ||
| Reaction to egg | 45/507 (9%) | 32/525 (6%) | ||
| Reaction to nuts | 14/503 (3%) | 8/522 (2%) | ||
| Reaction to other food | 40/507 (8%) | 40/525 (8%) | ||
| Parental report of allergy to cow’s milk, egg or nut at 12 months | 98/505 (19%) | 86/523 (16%) | 1.18 (0.91 to 1.53) | 3.1% (−1.6% to 7.8%) |
| At 24 months | ||||
| Parental report of clinical diagnosis of food allergy between 12 and 24 months | 51/573 (9%) | 41/598 (7%) | ||
| Parental report of clinical diagnosis of food allergy at 24 monthsa | ||||
| No | 421 (73%) | 436 (73%) | ||
| Yes | 72 (13%) | 66 (11%) | 1.12 (0.82 to 1.52) | 1.5% (−2.8% to 5.7%) |
| No diagnosis of food allergy reported between 12 and 24 months, not known between birth and 12 months | 82 (14%) | 97 (16%) | ||
| n | 575 | 599 | ||
| Parental report of any food allergy at 24 months | 208/574 (36%) | 197/597 (33%) | 1.10 (0.94 to 1.28) | 3.3% (–2.1% to 8.8%) |
| Reaction to cow’s milk | 86/575 (15%) | 77/598 (13%) | ||
| Reaction to egg | 45/575 (8%) | 45/598 (8%) | ||
| Reaction to peanut | 9/574 (2%) | 10/598 (2%) | ||
| Reaction to nuts other than peanut | 5/574 (1%) | 5/597 (1%) | ||
| Reaction to other food | 141/575 (25%) | 124/598 (21%) | ||
| Parental report of allergy to cow’s milk, egg or nut at 24 months | 121/574 (21%) | 116/597 (19%) | 1.09 (0.87 to 1.36) | 1.8% (−2.8% to 6.4%) |
| Parental report of immediate food allergy to common allergen at 24 monthsb,c | 118/574 (21%) | 96/597 (16%) | 1.28 (1.00 to 1.63) | 4.7% (0.2% to 9.1%) |
| Reaction to cow’s milk | 61/575 (11%) | 46/598 (8%) | ||
| Reaction to egg | 44/575 (8%) | 41/598 (7%) | ||
| Reaction to peanut | 8/574 (1%) | 10/598 (2%) | ||
| Reaction to nuts other than peanut | 4/574 (1%) | 5/597 (1%) | ||
| Reaction to other common food allergenb | 35/575 (6%) | 26/598 (4%) | ||
| Parental report of immediate allergy to cow’s milk, egg or peanut at 24 monthsc | 98/574 (17%) | 83/598 (14%) | 1.23 (0.94 to 1.61) | 3.3% (−0.9% to 7.4%) |
At 12 months, around 20% of parents in both groups reported that their child had had a reaction to at least one food since birth (see Table 12). At 24 months, just over 30% of parents reported that their child had had a reaction to at least one food since birth with more immediate reactions (within 2 hours of eating the food) in the intervention group compared to the control group (see Table 12). The number of children with a parental report of ever having any reaction to milk, egg or nuts and immediate reactions to milk, egg and peanuts were similar in the two groups at 24 months (see Table 12).
Confirmed diagnosis of food allergy
Seventy infants had a confirmed diagnosis of a food allergy to either milk, egg or peanut by either food challenge or panel consensus, which was the predefined main food allergy outcome. There were 41 participants with food allergy in the intervention group (7.5%) and 29 in the control group (5.1%) with adjusted RR of 1.47 (95% CI 0.93 to 2.33) (Table 13). A greater number of participants in the intervention group were confirmed allergic after a food challenge compared to the control group (15 vs. 6) due to more number of infants in the intervention group being confirmed allergic to egg at the food challenge (12 vs. 3) (see Appendix 3, Table 41). The number of infants diagnosed as allergic or possibly allergic by panel consensus was similar for each food (see Appendix 3, Table 41). Table 13 shows the between-group comparison of food allergy for milk, egg and peanut individually at 24 months as well as sensitivity analysis including infants with a panel consensus decision of food allergy possible or food allergy unlikely.
| Intervention | Control | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) | |
|---|---|---|---|---|
| Main analysis including diagnoses of allergic/not allergic from food challenges and panel consensus | ||||
| Allergic to cow’s milka | 9/571 (1.6%) | 8/593 (1.3%) | 1.17 (0.45 to 3.01) | 0.2% (−1.2% to 1.6%) |
| Allergic to egg | 33/560 (5.9%) | 22/581 (3.8%) | 1.56 (0.92 to 2.65) | 2.1% (−0.4% to 4.6%) |
| Allergic to peanut | 10/555 (1.8%) | 8/572 (1.4%) | 1.29 (0.51 to 3.25) | 0.4% (−1.1% to 1.8%) |
| Allergic to at least one of milk, egg or peanut | 41/547 (7.5%) | 29/568 (5.1%) | 1.47 (0.93 to 2.33) | 2.4% (−0.5% to 5.2%) |
| Sensitivity analysis also including panel consensus decision of food allergy possible or food allergy unlikely | n = 576 | n = 598 | ||
| Allergic to cow’s milka | 9 (1.6%) | 9 (1.5%) | 1.04 (0.42 to 2.60) | 0.1% (−1.3% to 1.5%) |
| Allergic to egg | 39 (6.8%) | 25 (4.2%) | 1.63 (1.00 to 2.65) | 2.5% (−0.1% to 5.1%) |
| Allergic to peanut | 13 (2.3%) | 12 (2.0%) | 1.12 (0.51 to 2.45) | 0.3% (−1.3% to 2.0%) |
| Allergic to at least one of milk, egg or peanut | 47 (8.2%) | 31 (5.2%) | 1.59 (1.02 to 2.46) | 2.9% (0.0% to 5.7%) |
Results from sensitivity analyses using multiple imputation for missing outcomes of confirmed diagnosis of food allergy were consistent with the main analysis (see Appendix 3, Table 42). Subgroup analyses for FLG genotype and number of first-degree relatives with atopic disease or eczema found no evidence of an interaction (see Appendix 3, Table 43). The CACE could not be estimated when compliance was defined as widespread emollient use over the child’s body at least 3 or more days per week over the first year of life due to a problem with the estimation procedure (thought due to the small number with confirmed diagnosis of food allergy). The CACE estimate of the adjusted OR of confirmed food allergy based in compliance in the first 3 months was 1.79 (95% CI 0.62 to 5.14; n = 1115).
Safety outcomes
Infant skin infections between birth and 12 months were reported by a slightly greater number of parents in the intervention group than in the control group: 89 in the intervention group (15%) and 67 parents in the control group (11%) with an incidence rate ratio of 1.55 (95% CI 1.15 to 2.09; Table 14). The types of skin infections reported by parents were diverse with impetigo and unspecified bacterial, viral or fungal skin infections the most common if known (see Table 14).
| Intervention | Control | Adjusted difference (95% CI) | |
|---|---|---|---|
| Skin infections | n = 585 | n = 585 | |
| Child had at least one skin infection | 89 (15%) | 67 (11%) | |
| Number of skin infections reported per child | Incidence rate ratio | ||
| Mean (SD) | 0.23 (0.68) | 0.15 (0.46) | 1.55 (1.15 to 2.09) |
| Median (25th, 75th centile) | 0 (0, 0) | 0 (0, 0) | |
| Minimum, maximum | 0, 9 | 0, 5 | |
| Type of skin infection | |||
| Child had at least one infection of: | |||
| Impetigo | 12 (2%) | 7 (1%) | |
| Folliculitis | 2 (< 0.5%) | 2 (< 0.5%) | |
| Boils | 1 (< 0.5%) | 2 (< 0.5%) | |
| Other bacterial infection | 10 (2%) | 12 (2%) | |
| Other viral infection | 19 (3%) | 9 (2%) | |
| Other fungal infection | 26 (4%) | 14 (2%) | |
| Other infection | 7 (1%) | 5 (1%) | |
| Didn’t know | 26 (4%) | 20 (3%) | |
| Did not specify | 1 (< 0.5%) | 1 (< 0.5%) | |
| Infant slippage incidents | n = 584 | n = 584 | |
| At least one infant slippage incident within an hour of applying skin-care products to the baby’s skin | 15 (2.6%) | 11 (1.9%) | RR: 1.37 (0.63 to 2.97) Difference in risk: 0.8% (−0.9% to 2.5%) |
| Number of questionnaires slippage incidents reported | |||
| One | 14 (2%) | 11 (2%) | |
| Two | 1 (< 0.5%) | – | |
The number of parents reporting infant slippage incidents within an hour of applying skin-care products to the baby’s skin was small (n = 26, < 3%) and similar in both groups (see Table 14). The trial management team rang parents of children reporting a slippage incident on the questionnaires to find out more about what happened. Of the 20 parents who responded, all confirmed with the trial team that the baby was okay, and that the slippage incident had not caused a serious injury.
Tables showing reported skin infections and slippages according to allocated group and reported emollient use are shown in Appendix 3, Table 44.
Tertiary outcomes
Follow-up for the tertiary outcomes
Follow-up for the tertiary outcomes at 36, 48 and 60 months took place between November 2017 and November 2021. Overall completion was 70% at each time point; however, completion was higher in the control group at all time points, particularly at 48 and 60 months (Table 15). At least one questionnaire was completed at 36, 48 or 60 months for 77% in the intervention group and 82% in the control group (see Table 15). Ninety-two per cent of participants had data for at least one follow-up time point between birth and 60 months (see Table 15).
| Intervention (n = 693) | Control (n = 701) | Total (n = 1394) | |
|---|---|---|---|
| 36 months completed | 478 (69%) | 503 (72%) | 981 (70%) |
| 48 months completed | 467 (67%) | 523 (75%) | 990 (71%) |
| 60 months completed | 467 (67%) | 509 (73%) | 976 (70%) |
| Withdrawal of consent after 24 months | 3 | 1 | 4 |
| Total number of questionnaires completed between 36 and 60 months | |||
| None | 156 (23%) | 126 (18%) | 282 (20%) |
| One | 61 (9%) | 52 (7%) | 113 (8%) |
| Two | 77 (11%) | 86 (12%) | 163 (12%) |
| Three | 399 (58%) | 437 (62%) | 836 (60%) |
| Total number of follow-ups completed between birth and 60 months | |||
| None | 58 (8%) | 54 (8%) | 112 (8%) |
| 1–3 | 94 (14%) | 82 (12%) | 176 (13%) |
| 4–7 | 198 (29%) | 203 (29%) | 401 (29%) |
| All (8) | 343 (49%) | 362 (52%) | 705 (51%) |
The baseline characteristics of infants where the 60-month questionnaire was completed were similar in the two groups (see Appendix 3, Table 45). Families of infants in both groups where the 60-month questionnaire was not completed were more likely to have joined the study after the birth of their baby rather than consenting antenatally, had slightly younger mothers on average, were more likely to be of non-white ethnicity, were more likely to be in a household with other children, lived in areas on average with lower deciles of the Index of Multiple Deprivation and were less likely to have a first-degree relative with a history of eczema at randomisation (Appendix 3, Table 45). Follow-up completion at 60 months in both groups was slightly higher for participants who did not have eczema based on the primary outcome at 24 months compared to those with eczema (see Appendix 3, Table 46). Completion rates were similar according to other secondary outcomes at 24 months (see Appendix 3, Table 46).
Moisturiser use after 2 years
The percentage of parents reporting frequent moisturiser use (at least three times per week) over all or most of their child’s body in the previous year remained higher in the intervention group than the control group through to 60 months. In the intervention group, this frequent all-over moisturiser use in the past year was reported by 31% at 36 months (139/449), 25% at 48 months (114/459) and 22% at 60 months (99/448) compared to 20% at 36 months (94/471), 18% at 48 months (90/500) and 16% at 60 months (76/471) in the control group. In both groups and at all time points, this frequent whole-body moisturiser use was more common in children with a parental report of a clinical diagnosis of eczema (see Appendix 3, Table 35).
Eczema outcomes
Outcomes for eczema in the previous year at 36, 48 and 60 months were slightly higher in the intervention group than the control group; however, adjusted differences were small, and none were statistically significant. This was consistent across the tertiary outcomes for eczema based on parental report of a clinical diagnosis, parental completion of the UKWP Diagnostic Criteria for eczema (see Table 16) and parental opinion of whether their child had eczema (see Appendix 3, Table 48). There was also no difference between groups in the severity of eczema as measured by parent-reported symptoms on the POEM (see Table 16 and Appendix 3, Table 40).
| Intervention | Control | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) | |
|---|---|---|---|---|
| Presence of eczema in the previous year based on parental report of a clinical diagnosis | ||||
| 36 months | 81/469 (17%) | 61/493 (12%) | 1.31 (0.97 to 1.76) | 4.1% (−0.4% to 8.6%) |
| 48 months | 50/462 (11%) | 46/509 (9%) | 1.20 (0.83 to 1.73) | 1.9% (−1.9% to 5.7%) |
| 60 months | 49/462 (11%) | 34/492 (7%) | 1.41 (0.94 to 2.12) | 3.1% (−0.5% to 6.6%) |
| Presence of eczema based on completion by parents of UKWP Diagnostic Criteria | ||||
| 36 months | 119/474 (25%) | 109/495 (22%) | 1.07 (0.87 to 1.33) | 1.7% (−3.4% to 6.8%) |
| 48 months | 122/458 (27%) | 134/511 (26%) | 1.01 (0.82 to 1.24) | 0.2% (−5.2% to 5.5%) |
| 60 months | 137/461 (30%) | 132/495 (27%) | 1.07 (0.88 to 1.30) | 1.9% (−3.7% to 7.5%) |
| Moderate, severe or very severe eczema according to POEM | ||||
| 36 months | 30/464 (6%) | 37/482 (8%) | 0.85 (0.55 to 1.31) | −1.2% (−4.4% to 2.0%) |
| 48 months | 32/453 (7%) | 45/505 (9%) | 0.82 (0.54 to 1.23) | −1.6% (−4.9% to 1.7%) |
| 60 months | 42/458 (9%) | 39/496 (8%) | 1.12 (0.76 to 1.66) | 1.0% (−2.4% to 4.5%) |
Food allergy outcomes
Longer-term food allergy outcomes were also more common in the intervention than control group. Again, CIs were wide and the estimates for most outcomes were compatible with the role of chance. Parental reports of a reaction to any food within the previous year were significantly higher in the intervention group than the control group at both 36 and 48 months (Table 17). However, CIs for the difference between groups in parental report of immediate reactions to foods containing cow’s milk, egg or nuts and of a clinical diagnosis of food allergy in the previous year at 36 and 48 months were inconclusive (see Table 17). At 60 months, all outcomes relating to food allergy were similar between the two groups including parental report of an immediate reaction to any common food allergen (see Table 17). Full details of the foods that parents reported their child reacted are presented in Appendix 3, Table 49.
| Intervention | Control | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) | |
|---|---|---|---|---|
| Parental report of reaction to any food within the previous year | ||||
| 36 months | 81/430 (19%) | 56/455 (12%) | 1.37 (1.02 to 1.85) | 5.0% (0.3% to 9.7%) |
| 48 months | 59/419 (14%) | 43/472 (9%) | 1.54 (1.08 to 2.20) | 5.0% (0.9% to 9.2%) |
| 60 months | 52/432 (12%) | 49/459 (11%) | 1.07 (0.75 to 1.51) | 0.8% (−3.4% to 4.9%) |
| Parental report of immediate reaction to milk, egg or nuts within the previous yeara | ||||
| 36 months | 40/437 (9%) | 26/468 (6%) | 1.44 (0.92 to 2.27) | 2.7% (−0.6% to 5.9%) |
| 48 months | 29/432 (7%) | 21/485 (4%) | 1.64 (0.97 to 2.76) | 2.8% (−0.2% to 5.7%) |
| 60 months | 21/429 (5%) | 21/453 (5%) | 1.05 (0.60 to 1.84) | 0.3% (−2.5% to 3.0%) |
| Parental report of immediate reaction to any common food allergen within the previous year at 60 monthsa,b | 30/424 (7%) | 23/447 (5%) | 1.36 (0.83 to 2.24) | 2.0% (−1.2% to 5.2%) |
| Parental report of a clinical diagnosis of food allergy within the previous year | ||||
| 36 months | 37/407 (9%) | 20/422 (5%) | 1.55 (0.96 to 2.49) | 3.0% (−0.3% to 6.2%) |
| 48 months | 26/453 (6%) | 17/498 (3%) | 1.54 (0.89 to 2.66) | 2.1% (−0.6% to 4.7%) |
| 60 months | 19/441 (4%) | 15/474 (3%) | 1.16 (0.64 to 2.11) | 0.6% (−1.8% to 3.0%) |
Wheezing and allergic rhinitis symptoms
At 36 months, fewer parents in the intervention group reported wheezing or whistling in their child’s chest in the previous year (adjusted RR 0.79, 95% CI 0.68 to 0.98; Table 18). The percentage of parents reporting that their child had been prescribed an inhaler for wheezing was also lower in the intervention group at 36 months (see Table 18). At 48 and 60 months, parental reporting wheezing or whistling in the previous year decreased in both groups with CIs including no difference between groups (see Table 18). The percentage of parents reporting that their child had been prescribed an inhaler for wheezing was also similar in the two groups at 48 and 60 months (see Table 18).
| Intervention | Control | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) | |
|---|---|---|---|---|
| Parental report of wheezing or whistling in the chest in previous yeara | ||||
| 36 months | 96/449 (21%) | 134/472 (28%) | 0.79 (0.64 to 0.98) | −6.0% (−11.4% to −0.5%) |
| 48 months | 81/456 (18%) | 115/501 (23%) | 0.84 (0.66 to 1.07) | −3.7% (−8.7% to 1.3%) |
| 60 months | 63/459 (14%) | 72/490 (15%) | 1.00 (0.74 to 1.35) | 0.0% (−4.4% to 4.4%) |
| Parental report of inhaler prescription for wheezing in the previous year | ||||
| 36 months | 52/445 (12%) | 79/461 (17%) | ||
| 48 months | 62/437 (14%) | 71/472 (15%) | ||
| 60 months | 45/446 (10%) | 57/467 (12%) | ||
| Parental report of allergic rhinitis symptoms in previous yearb | ||||
| 36 months | 120/455 (26%) | 123/477 (26%) | 1.02 (0.83 to 1.25) | 0.5% (−5.2% to 6.2%) |
| 48 months | 111/453 (25%) | 136/498 (27%) | 0.91 (0.74 to 1.12) | −2.5% (−8.1% to 3.1%) |
| 60 months | 120/457 (26%) | 116/485 (24%) | 1.10 (0.89 to 1.35) | 2.4% (−3.1% to 8.0%) |
| Parental report of itchy, watery eyes with the allergic rhinitis symptoms in the previous year | ||||
| 36 months | 56/458 (12%) | 54/480 (11%) | ||
| 48 months | 64/455 (14%) | 68/500 (14%) | ||
| 60 months | 72/457 (16%) | 65/484 (13%) | ||
At 36, 48 and 60 months, the percentage of parents reporting that their child had had symptoms of allergic rhinitis in the previous year were similar in the two groups, with approximately a quarter of parents in each group reporting such symptoms at each time point (see Table 18).
Cumulative incidence outcomes
By 60 months, the percentage of parents who had reported that their child had had a clinical diagnosis of eczema or food allergy was similar in the two groups (Table 19). Thirty per cent of parents had reported a clinical diagnosis of eczema between 12 and 60 months and 15% of parents reported that their child had a clinical diagnosis of food allergy by 60 months.
| Intervention | Control | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) | |
|---|---|---|---|---|
| Parental report of a clinical diagnosis of eczema between 12 and 60 monthsa | 188/608 (31%) | 178/631 (28%) | 1.10 (0.93 to 1.30) | 2.8% (−2.3% to 7.8%) |
| Parental report of clinical diagnosis of food allergy by 60 monthsa | 92/609 (15%) | 87/632 (14%) | 1.11 (0.84 to 1.45) | 1.5% (−2.5% to 5.6%) |
| Parental report that child ever had clinical diagnosis of asthma or allergic rhinitis by 60 monthsb | 63/431 (15%) | 60/454 (13%) | 1.06 (0.77 to 1.47) | 0.9% (−4.0% to 5.8%) |
| Parental report that child ever had clinical diagnosis of asthma | 38/431 (9%) | 36/456 (8%) | 1.08 (0.71 to 1.64) | 0.7% (−3.2% to 4.6%) |
| Parental report that child ever had clinical diagnosis of allergic rhinitis | 36/459 (8%) | 35/485 (7%) | 1.04 (0.67 to 1.63) | 0.3% (−3.4% to 4.1%) |
Similarly, there was no difference between the two groups in the percentage of parents reporting that their child had had a clinical diagnosis of asthma or allergic rhinitis at 60 months (see Table 19).
Sensitivity analyses for parental report of a clinical diagnosis of eczema between 12 and 60 months and food allergy by 60 months exploring the impact of a worse outcome in those with missing data were consistent with the main analysis presented in Table 19 (see Appendix 3, Table 50).
There was no evidence of an interaction effect with allocated group on the risk of a parental report of eczema between 12 and 60 months according to FLG genotype (Table 20).
| Intervention | Control | Adjusted interaction effect (RR) (95% CI) | Adjusted interaction effect (risk difference) (95% CI) | |
|---|---|---|---|---|
| FLG genotype for children with mother and father of white ethnicity and children of other ethnicity with mutation | n = 402 | n = 414 | ||
| +/+ (no mutations) | 104/339 (31%) | 100/352 (28%) | ||
| +/− (one FLG null mutation) | 26/62 (42%) | 20/60 (33%) | 1.15 (0.71 to 1.88) | 6.4% (−11.9% to 24.6%) |
| −/− (two FLG null mutations) | 1/1 (100%) | 1/2 (50%) |
In exploratory tertiary outcomes for parental-reported reactions to egg or nuts by 60 months, 17% (101/597) of parents reported that their child had had a reaction in the intervention group compared to 13% (80/622) in the control group. The percentage of parents reporting that their child had had an immediate reaction to egg or nuts was similar in the two groups (12% intervention, 10% control, see Appendix 3, Table 51).
Chapter 4 Economic evaluation
Introduction
As described elsewhere in this report, the BEEP trial sought to determine if advising parents to apply an all-over-body application of emollient to their child’s skin throughout the first year of life in addition to standard infant skin-care advice might prevent the onset of eczema in children at high risk of allergic disease. The trial took a pragmatic, randomised, controlled, parallel group, multicentre assessor-blind design in which parents were advised to follow the skin-care advice for their child at home with minimal clinical contact. One thousand three hundred and ninety-four newborns at high risk of developing eczema were randomly allocated to the intervention or control arm. Standard practice infant skin-care advice was provided to all parents and those randomised to the intervention arm were also given advice to apply emollient daily to the child’s entire body surface area for the first year of the child’s life. Families were given a choice of two emollients (Doublebase Gel and Diprobase Cream). Diagnosis of eczema between 12 and 24 months of age (defined as meeting the UKWP Diagnostic criteria) was the primary outcome. The methods and results have been published1 showing no evidence that daily emollient during the first year of life prevents eczema in high-risk children and some evidence to suggest there may be an increased risk of skin infections.
Data on health resource use and quality of life were captured alongside the trial in order to undertake a within trial economic evaluation. Though concerns have been raised in the literature about so-called paradoxical (not clinically effective but yet found cost effective) conclusions between clinical and economic analyses of the same studies,68 we report the economic results despite the main clinical findings for several reasons. Firstly, the intervention was preventative in nature and when designing the study, we considered it may be possible for emollients not to be found statistically clinically effective yet estimated to be cost-effective. This is because the intervention is relatively cheap and thus even a small insignificant improvement across a lot of people could potentially be deemed cost-effective. Cost-effectiveness, via changes in resource use and costs, may also have been driven by improved secondary outcomes, such as change in severity of eczema, were such differences found. Secondly, Xu et al. 69 published a decision tree model estimating the cost-effectiveness of seven moisturisers used in the first 6 months of life to prevent eczema in high-risk individuals. The study concluded that daily moisturisation is a cost-effective preventative strategy that can reduce the burden of eczema. It is therefore important to report the economic evaluation for this preventative strategy based on individual-level data collected alongside the definitive trial in order to add to the evidence base around whether this preventative strategy is cost effective or not. Thirdly, since the data were collected, it is important to analyse and report it so as to help inform any future or related research that might find this analysis relevant. The current level of economic evidence available for interventions aimed at preventing and treating eczema is limited. 70,71 Understanding resource use, costs and quality of life in early life in high-risk children is of value in its own right and is something this study adds to the literature.
The primary objective of this within-trial economic evaluation was to estimate the cost-effectiveness of advice to provide daily all-over-body application of emollient during the first year of life for preventing AE in high-risk children over a 2-year time horizon. A sensitivity analysis is undertaken to estimate the medium-term cost-effectiveness at 5 years using a within-trial analysis.
Methods
Overview of economic analysis
The primary analysis forms the base (or reference) case within-trial economic analysis using individual participant-level data collected over 2 years from the BEEP trial to compare the cost-effectiveness of providing parents with advice to apply an all-over-body application of emollient daily in addition to standard skin-care advice compared to standard skin-care advice alone. This comparison was chosen to enable us to estimate the additional costs and benefits over usual care. The time horizon was chosen to be consistent with the time points used for the clinical study. The base-case analysis undertakes a cost-effectiveness analysis (CEA) from an NHS perspective in terms of the difference in proportion of cases without eczema. A secondary analysis was undertaken using a cost–utility approach; this was chosen as the secondary analysis due to uncertainties about how best to capture child health utilities especially in the very young. 72–74
The evaluation was undertaken in line with published guidelines for the economic evaluation of health-care interventions as appropriate. 75–79 After analysis had started NICE published updated guidance,80 which changed the preferred mapping function to be used in reference case analyses from that published by van Hout et al. 81 to that published by Hernandez Alava et al. 82,83 Since the analysis started prior to the change in guidelines we have adhered to the earlier guidelines, which for the most part is unchanged, and given the study results is unlikely to be significant in the conclusions reached. 78
A health economic analysis plan was written and reviewed prior to the trial database being locked, the health economics analysis plan (HEAP) is publicly available. 47
Identifying and measuring resource use
In keeping with the trial being conducted in the UK, where the NHS provides publicly funded health care, the analysis takes a health service perspective which is also in line with the NICE reference case. 78 Disease-specific (eczema, asthma, and rhinitis) resource use was collected via online or postal paper questionnaires completed by participants at 3, 6, 12, 18 and 24 months. Personal social service (PSS) resource use was not captured explicitly, as it was anticipated that these types of services would not be accessed for the diseases of interest. Since the trial was light touch, in that after recruitment participants only had one face-to-face contact at 2 years, the resource use data collected were limited to NHS resource use relevant to the diseases of interest and did not collect any costs incurred by the family or wider society. This enabled us to keep the respondent burden low while still meeting the requirement of the base case to capture the intervention costs to the NHS and the participant’s wider disease-specific resource use of the NHS. Resource use related to food allergies was not collected as this aspect was added to the trial rather than included from the outset. Patient and public involvement members of the research team were involved in the design of the questionnaires.
Valuing costs
The cost of the intervention was estimated using data collected by the NCTU and costed using published unit costs for Doublebase Gel and Diprobase Cream in the prescription cost analysis (PCA). 84 In the trial, the emollients were posted to participants homes but in practice if these were rolled out, people would collect these via repeat prescription from their GP surgery/pharmacy. As a result, these postage costs were not included in the economic evaluation.
Resource use relevant to the NHS perspective was valued using UK unit costs (Great British pounds) for the most current price year available at the start of the analysis (2019–20). Unit costs were identified from published sources and are clearly reported in the Results section.
A mean cost (SD) per participant per arm was estimated for 2 years.
Identifying, measuring and valuing outcomes
The primary economic outcome measure was incremental cost per percentage decrease in risk of eczema, a CEA. Secondary analysis reports a cost–utility analysis (CUA) where QALYs are estimated using utility scores obtained from the parental proxy CHU-9D instrument at 24 months. The CHU-9D is a generic preference-based measure of health-related quality-of-life instrument that asks how a child is today on nine questions (worries, sad, pain, tired, annoyed, schoolwork/homework, sleep, daily routine, activities) each with five response levels (ranging from no difficulty through to a lot or cannot do). We used the additional guidance given to us by the developer of the CHU-9D. This guidance provided extra wording to help parents of younger children understand how to answer questions 6 (schoolwork/homework), 8 (daily routine) and 9 (join in activities) given their child was of pre-school age. Utility ranges from 0.33 (worst health-related quality of life) through to 1 (best health-related quality of life). 50 We also elicited utility scores for the main parent/carer using the EQ-5D-5L at baseline, and 24 months. The EQ-5D-5L is a generic preference-based measure of health-related quality-of-life instrument with five dimensions (mobility, pain/discomfort, usual activities, self-care, anxiety/depression) with five levels ranging from no problems through to unable to or extreme problems. Responses were converted to utility scores using the EQ-5D-5L Crosswalk UK preference weights as this was in line with recommendations at the point analysis started. Utility ranges from −0.594 to 1. 81
In the CUA, the responses received on the quality-of-life instruments were converted to utility scores using the Stevens, 2012 valuation set for the CHU-9D and UK preference weights published by van Hout et al. 81 for parental EQ-5D-5L. Utility values were used to estimate QALYs over 24 and 60 months, using both linear interpolation and area under the curve analysis with and without baseline adjustment. 85 Child utility was assumed to be 1 (perfect health) at baseline, where baseline represented birth, for all participants. This was assumed because such instruments are not appropriate to ask at such a young age and babies were not eligible for inclusion in the study if they had a serious health issue or severe widespread skin condition at birth. The health-related quality of life for the main carer was elicited using the EQ-5D-5L in order to explore whether, if significantly fewer infants developed eczema, there might be any health spillover effects. Parental QALYs were estimated in the same way using baseline, and 24-month utility derived from the EQ-5D-5L. Parental QALYs were not incorporated into the CUA because the appropriate methods to do this are unclear86 and changes to main parent/carers health resource use and productivity were not also collected.
Economic analysis at primary outcome end point (2 years)
A CEA was planned regardless of what the full clinical results showed in terms of change in eczema cases for the reasons highlighted in the Introduction section of Chapter 4.
The economic base-case analysis included all randomised participants with complete cost and outcome data available. As the time horizon was 24 months, costs and benefits in months 13–24 were discounted using recommended rates, 3.5% for both. 78
The main base-case analysis was a CEA, to estimate the incremental cost of preventing an additional case of eczema. Since it is unknown how much decision-makers would be willing to pay to prevent an additional case of eczema, a secondary analysis using the proxy reported CHU-9D to estimate QALYs in a CUA was undertaken and reports incremental cost per QALY as the outcome measure. A cost-effectiveness threshold (ʎ) of £20,000 (£30,000) per QALY is used in line with NICE guidance. 78
Mean (SD) resource use and mean (SD) cost per participant are estimated per randomised arm. Mean difference (95% CI) in cost per participant between arms is estimated unadjusted and adjusted [for centre and number of immediate family members with atopic disease (one, two or more than two)]. Mean (SD) utility and mean (SD) QALYs per participant per randomised arm are presented along with mean difference (95% CI) in QALYs between arms unadjusted and adjusted. QALYs for the main carer were also estimated using responses to the EQ-5D-5L and are presented separately.
The unadjusted CEA was analysed using the heabs command87 in STATA (for which explanatory variables cannot be added to the regression command), while the adjusted analysis used a generalised linear model (GLM) for continuous and binary outcomes assuming costs and outcomes are uncorrelated. The Gaussian distribution was used for the cost GLM model and the binomial for the outcome GLM model. The identity option is used for the link function for both GLM models. The CUA uses a regression-based approach (seemingly unrelated regression equations). 88
To determine the level of sampling uncertainty surrounding the mean incremental cost-effectiveness ratios (ICERs) non-parametric bootstrapping was undertaken generating 10,000 estimates of incremental costs and benefits. Cost-effectiveness acceptability curves (CEACs) were also produced, which show the probability that the intervention is cost effective at different values of willingness to pay. STATA MP version 17 was used to conduct the analysis. No subgroup analysis was undertaken as FLG mutation was not shown to be important in terms of the clinical effect. 1
Sensitivity analysis
In the 2-year analysis the cost of emollients was varied in a threshold analysis to explore what cost of emollients would switch the results from cost effective to cost ineffective or vice versa.
The main sensitivity analysis was focused on taking a longer time horizon. It focused on the 5-year economic evaluation, where the primary 2-year CEA and secondary 2-year CUA [see Economic analysis at primary outcome end point (2 years)] were repeated using the 5-year data. Disease-specific (eczema, wheezing and rhinitis) resource use was collected via online or postal paper questionnaires completed by participants at 36, 48 and 60 months. The costs are not broken down and reported by individual year (e.g. year 3, 4 and 5) because secondary inpatient stays were collected in a format that did not enable us to see which year they pertained to. As the time horizon was 60 months, costs and benefits in months 13–60 were discounted using recommended rates, 3.5% for both. 89 As part of these analyses, we re-ran the 5-year analysis after removing inpatient hospital costs incurred for wheezing, as it seems unlikely these would have been incurred as a result of having the intervention or not. We did this in order to explore how influential these costs were on the results of the 5-year analysis. In addition, we report the mean utility at 36, 48 and 60 months alongside the mean QALYs at 5 years per participant using the CHU-9D and the EQ-5D-5L for the main carer.
In the base-case economic evaluation, we did not impute missing data; instead, a complete-case analysis was undertaken in line with the clinical analysis. To evaluate the impact of missing data on the cost-effectiveness estimates, multiple imputation was employed for the cost–utility analyses, assuming that the data were MAR and using chained equations to handle the missing cost and outcome data to assess the impact on the conclusions reached. 90 We did not undertake multiple imputation for the cost-effectiveness analyses as we had no baseline costs or effects, and all children were the same age.
There were plans to model the longer-term (i.e. beyond 5 years) costs and benefits of preventing or reducing the severity of eczema if the intervention had been found effective as detailed in the HEAP but this was not undertaken due to the clinical results.
We did not plan to look at the distributional effects, describing how costs and outcomes were distributed across different individuals, as this was not part of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) criteria at the time of writing the HEAP, but given the findings of the clinical study such analysis is unlikely to add much to this study.
This economic evaluation is reported in line with the CHEERS guidance. 77
Results
The full trial paper1 provides a detailed description of the final sample size and characteristics at 24 months. Of the 1394 babies randomly assigned to the emollient or control arm at the start of the study, 184 infants and their families either dropped out or were lost to follow-up by the 24-month period. This resulted in a sample of 1210 infants at 24 months: 598 allocated the emollient intervention alongside standard skin-care advice and 612 allocated standard skin-care advice only. Of these, 598 had complete cost and outcome data in the emollient intervention arm and 610 had this in the control arm (less than % missing) and it is this sample (n = 1208) that is analysed in the base-case CEA at 2 years.
Resource use and costs
Unit costs, together with their source, are presented in Table 21.
| Resource item | Unit cost (£) | Source (notes) |
|---|---|---|
| Intervention | ||
| Doublebase Gel | 6.63059 | PCA 202084 |
| Diprobase Cream | 6.67394 | PCA 202084 |
| NHS care | ||
| GP | 39.23 | PSSRU 202091 (9.22 minutes) |
| Practice nurse | 21 | PSSRU 202091 (0.33 of an hour per patient) |
| Hospital doctor | 85 | PSSRU 202091 (per half hour) |
| Hospital nurse | 19.8 | PSSRU 202091 (per 20 minutes) |
| Health visitor | 15.84 | PSSRU 202091(0.33 of an hour per patient) |
| Dietician | 92 | PSSRU 202091 (1 hour) |
| Physiotherapy | 82 | PSSRU 202091 (1 hour) |
| Pharmacist | 12 | PSSRU 202091 (1 hour) |
| Paediatric endocrinologist | 244 | NHS unit costs 2019/202089 (1 hour) |
| Paediatric dermatologist | 170 | NHS unit costs 2019/202089 (1 hour) |
| Paediatric respiratory medicine | 229 | NHS unit costs 2019/202089 (1 hour) |
| Paediatric ear nose and throat | 124 | NHS unit costs 2019/202089 (1 hour) |
| Paediatric clinical immunology and allergy service | 247 | NHS unit costs 2019/202089 (1 hour) |
| Paediatric ophthalmologist | 103 | NHS unit costs 2019/202089 (1 hour) |
| Paediatric dentistry | 152 | NHS unit costs 2019/202089 (1 hour) |
| Hospital admission/overnight stay | 1889 | NHS unit costs 2019/202089 |
| Medication | Range from 0.74 to 583 | PCA 202084 |
Intervention resource use and costs
In the 12-month intervention period, the mean number of pots issued per participant in the emollient arm was 4.21 (SD 2.20) (Table 22). The associated mean cost of emollients was £28.00 (SD £14.65) per participant in the base case (Table 23).
| Emollient arm | Control arm | Mean difference (95% CI) | |
|---|---|---|---|
| Mean ± SD (n) | Mean ± SD (n) | ||
| Intervention | 4.21 ± 2.20 (693) | 0.00 ± 0.00 (637) | 4.21 (4.04 to 4.38) |
| Doublebase Gel | 2.52 ± 2.05 (693) | 0.00 ± 0.00 (637) | 2.52 (2.36 to 2.68) |
| Diprobase Cream | 1.70 ± 1.54 (693) | 0.00 ± 0.00 (637) | 1.70 (1.58 to 1.81) |
| Wider NHS resource use | |||
| GP | 1.73 ± 3.31 (693) | 1.73 ± 2.81 (637) | 0.0003 (−0.33 to 0.33) |
| Practice nurse | 0.12 ± 0.53 (693) | 0.17 ± 0.80 (637) | −0.06 (−0.13 to 0.02) |
| Hospital doctor | 0.35 ± 1.27 (693) | 0.38 ± 1.48 (637) | −0.03 (−0.18 to 0.12) |
| Hospital nurse | 0.02 ± 0.18 (693) | 0.03 ± 0.27 (637) | −0.01 (−0.03 to 0.02) |
| Other health professional | 0.14 ± 0.68 (693) | 0.18 ± 0.75 (637) | −0.04 (−0.12 to 0.03) |
| Hospital episode | 0.17 ± 1.50 (693) | 0.19 ± 1.81 (637) | −0.02 (−0.19 to 0.16) |
| Medication | 3.74 ± 9.84 (693) | 3.50 ± 7.71 (637) | 0.24 (−0.72 to 1.20) |
| Emollient arm | Control arm | Mean difference (95% CI) | |
|---|---|---|---|
| Mean ± SD (n) | Mean ± SD (n) | ||
| Total intervention | 28.00 ± 14.65 (693) | 0.00 ± 0.00 (637) | 28.00 (26.86 to 29.14) |
| Doublebase Gel | 16.69 ± 13.59 (693) | 0.00 ± 0.00 (637) | 16.69 (15.63 to 17.74) |
| Diprobase Cream | 11.32 ± 10.25 (693) | 0.00 ± 0.00 (637) | 11.32 (10.52 to 12.11) |
| Wider NHS cost | |||
| GP | 67.33 ± 129.01 (693) | 67.30 ± 109.54 (637) | 0.03 (−12.90 to 12.96) |
| Practice nurse | 2.40 ± 10.89 (693) | 3.59 ± 16.77 (637) | −1.19 (−2.70 to 0.32) |
| Hospital doctor | 50.14 ± 207.69 (693) | 51.17 ± 226.93 (637) | −1.03 (−24.40 to 22.35) |
| Hospital nurse | 0.40 ± 3.31 (693) | 0.56 ± 5.18 (637) | −0.16 (−0.63 to 0.30) |
| Other health professional (eczema) | 6.67 ± 32.99 (693) | 10.46 ± 47.42 (637) | −3.79 (−8.15 to 0.58) |
| Hospital episode | 170.32 ± 1106.85 (693) | 145.29 ± 950.98 (637) | 25.03 (−86.43 to 136.50) |
| Medication | 24.06 ± 83.95 (693) | 23.58 ± 79.39 (637) | 0.48 (−8.33 to 9.28) |
| Mean total cost (Int+NHS) | 349.32 ± 1314.29 (693) | 301.94 ± 1083.61 (637) | 47.37 (−82.84 to 177.59) |
Other health resource use and costs
Resource use between the emollient and control arms was not significantly different (see Table 22). Table 23 reports the disaggregated mean discounted costs per infant for both arms using available case data. In the complete-case analysis, mean cost was £398.23 (SD 1408.39) per child in the emollient arm (n = 598) and £312.16 (SD 1105.04) in the control arm (n = 610). When intervention use was combined with other health resource use, the unadjusted mean incremental cost per infant was £86.07 (95% CI £ −57.77 to 229.90; Table 23). The difference in total costs between groups largely reflects the cost of the intervention and greater hospital inpatient stays; other NHS costs were not significantly different between groups. The largest component of cost was overnight hospital stays, especially for those infants reporting wheezing.
Outcome measures
Table 24 presents the outcomes for both arms unadjusted on the available-case sample at 2 years. In the complete-case analysis, the proportion of cases without eczema according to the UKWP-AD definition for the emollient arm was 0.7676 (SD 0.4227) (n = 598) and 0.7557 (SD 0.4300) for the control arm (n = 610) over the 24-month period. In the complete-case analysis, the adjusted incremental difference in proportion of cases without eczema at 2 years was 0.0164 (95% −0.0329 to 0.0656) adjusted or 0.0118 (−0.0359 to 0.0595) unadjusted. Complete responses to the CHU-9D were received from 75.6% (77.3%) of all participants in the intervention (control) arm at 2 years (or 88% of those completing and returning the study questionnaire booklet). Complete responses to the EQ-5D-5L were received from 71.6% to 82.7% (72.5–84.2%) of participants in the intervention (control) arm at baseline and 2 years. The outcomes for the CHU-9D and the EQ-5D-5L are not too dissimilar between the two arms.
| Intervention (n = 693) | Control (n = 701) | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| Mean ± SD (n) | Missing | Mean ± SD (n) | Missing | ||
| Child participants | |||||
| Proportion without eczema at 24 months (based on UKWP-AD) | 0.7676 ± 0.4227 (598) | 99 | 0.7549 ± 0.4305 (612) | 89 | 0.0127 (−0.0355 to 0.0608) |
| CHU-9D 24 months | 0.9349 ± 0.0690 (524) | 169 | 0.9338 ± 0.0685 (541) | 160 | 0.0010 (−0.0071 to 0.0091) |
| QALYs at 24 months | 1.9030 ± 0.0672 (524) | 169 | 1.9020 ± 0.0642 (541) | 160 | 0.0010 (−0.0069 to 0.0089) |
| Main carer | |||||
| EQ-5D-5L at baseline | 0.8560 ± 0.1513 (496) | 198 | 0.8520 ± 0.1580 (508) | 193 | 0.0040 (−0.0152 to 0.0232) |
| EQ-5D-5L at 24 months | 0.9212 ± 0.1417 (573) | 120 | 0.9187 ± 0.1303 (592) | 109 | 0.0024 (−0.0132 to 0.0181) |
| QALYs – EQ-5D-5L (parent) (24 months) | 1.7441 ± 0.2279 (457) | 236 | 1.7453 ± 0. 2314 (467) | 234 | −0.0012 (−0.0308 to 0.0285) |
Base-case cost-effectiveness analysis at 2 years
Table 25 presents the unadjusted and adjusted results of the CEA in terms of the number of eczema cases diagnosed together with an estimate of the ICER and separately the CEAC for the adjusted analysis (Figure 10). The incremental difference in cost for the emollient arm (n = 598) compared to the control arm (n = 610) was £87.45 (95% CI −54.31 to 229.27) (unadjusted this was £86.07, 95% CI £ −57.77 to 229.90). The adjusted incremental difference in proportion without eczema for the emollient arm compared with the control arm was 0.0164 (95% −0.0329 to 0.0656) (unadjusted was 0.012, 95% CI −0.0359 to 0.0600), meaning that the emollient arm had less cases (by a very small margin) of eczema at 24 months. The ICER was £5337 (£7281 unadjusted) per percentage decrease in risk of eczema. The amount decision-makers would be willing to pay per percentage decrease in risk of eczema is unknown, but it can be seen from the CEAC that there is a lot of uncertainty around the probability of the intervention being cost effective, at willingness-to -pay values over £10,000 per percentage decrease in risk of eczema the probability of cost-effectiveness is always < 60 to 72%.
| Analysis (N e, N c) | Incremental cost (UK£) (95% CI) | Incremental effect (95% CI) | ICER | % Cost effective at £20k (£30K) |
|---|---|---|---|---|
| CEA base case (CCA, unadjusted) (598, 610) |
86.07 (−57.77 to 229.90) | 0.012 (−0.0359 to 0.0600) | £7281 per percentage decrease in risk of eczema | Willingness-to-pay threshold per percentage decrease in risk of eczema unknown. |
| CEA base case (CCA, adjusted) (598, 610) |
87.45 (−54.31 to 229.27) | 0.0164 (−0.0329, 0.0656) | £5337 per percentage decrease in risk of eczema | |
| CUA (CCA, CHU-9D, unadjusted) (524, 542) |
81.47 (−80.21 to 243.14) | 0.0010 (−0.0069 to 0.0089) | £82,250 per QALY | 30% (36%) |
| CUA (CCA, CHU-9D, adjusted) (524, 542) |
84.28 (−78.36 to 246.93) | 0.0010 (−0.0068 to 0.0089) | £82,580 per QALY | 29% (36%) |
FIGURE 10.
Cost-effectiveness acceptability curve for the complete-case adjusted CEA analysis (UKWP-AD) at 2 years.

Secondary cost–utility analysis at 2 years
Proxy-reported Child Health Utility instrument-9 domains for the infant
The adjusted incremental difference in cost per infant for the emollient arm (n = 524) compared to the control arm (n = 542) was £82.28 (95% CI −78.36 to 246.93). The adjusted mean QALYs for infants was very slightly more in the emollient arm, incremental mean difference of 0.0010 (95% CI of −0.0068 to 0.0089), see Table 25. This means that the emollient arm was more expensive and slightly more effective than the control arm. The adjusted ICER was £82,580 which, when using a threshold value of £20,000 (£30,000), would not be considered cost-effective. 78 The probability of the intervention being cost-effective at 2 years was 29% (36%) for a threshold value of £20,000 (£30,000) (Figure 11).
FIGURE 11.
Cost-effectiveness acceptability curve for the complete-case adjusted CUA (CHU-9D) at 2 years.

EuroQol-5 Dimensions, five-level version for the main carer
The adjusted mean difference in the QALYs was close to zero, −0.0012 (95% CI −0.0308 to 0.0285) (see Table 24). We did not combine these results into a CUA as we only collected NHS resource use for the infants. However, on average main carers in the emollient arm had slightly lower QALYs than main carers in the control arm.
Sensitivity analyses
Cost of emollients
Given we do not know what a decision-maker’s willingness to pay per percentage decrease in risk of eczema is, the cost of the emollient was explored in the 2-year adjusted CUA by undertaking a threshold analysis (i.e. the cost of emollients that would switch the intervention from cost-ineffective to cost effective at a willingness to pay of £30,000 per QALY). Doing this, we observed that even at a cost of £0 per emollient item it would not be enough to make this intervention cost-effective unless reduced healthcare use/NHS cost savings could also be found elsewhere.
Cost-effectiveness analysis over 5 years
The complete-case analysis for the CEA at 5 years consisted of 383 (55.3%) children in the intervention arm and 411 (58.6%) children in the control arm and it is this sample that is analysed in the 5-year CEA analysis. In the CUA analysis, this was 354 (36.7%) and 263 (37.5), respectively. Questionnaire response rates declined significantly in years 3–5.
Table 26 reports the mean outcomes per child for both arms for years 3–5 for available case data. The proportion without eczema is slightly higher at 5 years in the control arm, 0.8939 (SD 0.3082) in the intervention arm versus 0.9315 (SD 0.2529) in the control arm [incremental effect −0.0375 (95% CI −0.0732 to 0.0019)], though it should be noted this is based on parental report of a clinical diagnosis of eczema in the previous year unlike at 2 years. The adjusted incremental difference in QALYs (based on proxy report for the CHU-9D) at 5 years for the emollient arm compared with the control was 0.0166 (95% −0.0135 to 0.0467) (unadjusted was 0.0171, 95% CI −0.0138 to 0.0480). Mean QALYs for parental health-related quality of life using the EQ-5D-5L at 5 years was 4.2063 (SD 0.4707) in the intervention arm and 4.1684 (SD 0.5135) in the control arm, with an incremental difference of 0.0379 (95% CI −0.0319 to 0.1077) QALYs (available case). These incremental effects are small, just as they were at 2 years.
| Intervention (n = 693) | Control (n = 701) | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| Mean ± SD (n) | Missing | Mean ± SD (n) | Missing | ||
| Child participants | |||||
| Proportion without eczema at 60 months (Parent report of a clinical diagnosis of eczema in the previous year at 60 months) | 0.8939 ± 0.3082 (462) | 231 | 0.9315 ± 0.2529 (496) | 205 | −0.0375 (−0.0732 to 0.0019) |
| CHU-9D 36 months | 0.9283 ± 0.0713 (335) | 358 | 0.9235 ± 0.0671 (350) | 351 | 0.0048 (−0.0056 to 0.0152) |
| CHU-9D 48 months | 0.9337 ± 0.0718 (445) | 248 | 0.9296 ± 0.0754 (480) | 221 | 0.0048 (−0.0056 to 0.0152) |
| CHU-9D 60 months | 0.9398 ± 0.0660 (451) | 242 | 0.9349 ± 0.0696 (472) | 229 | 0.0049 (−0.0039 to 0.0137) |
|
QALYs
(CHU-9D) at 60 months |
4.424 ± 0.1820 (255) | 438 | 4.4053 ± 0.1740 (263) | 438 | 0.0181 (−0.0126 to 0.0488) |
| Main carer | |||||
| EQ-5D-5L at 36 months | 0.8957 ± 0.1436 (506) | 187 | 0.8931 ± 0.1404 (539) | 162 | 0.0026 (−0.0148 to 0.0200) |
| EQ-5D-5L at 48 months | 0.8813 ± 0.1605 (452) | 241 | 0.8764 ± 0.1666 (491) | 210 | 0.0049 (−0.0160 to 0.0259) |
| EQ-5D-5L at 60 months | 0.8953 ± 0.1504 (452) | 241 | 0.8771 ± 0.1635 (477) | 224 | 0.0181 (−0.0021 to 0.0384) |
|
QALYs (EQ-5D-5L)
at 60 months |
4.2063 ± 0.4707 (379) | 314 | 4.1684 ± 0.5135 (390) | 311 | 0.0379 (−0.0319 to 0.1077) |
The results of the 5-year analysis are shown in Table 27. The adjusted incremental cost at 5 years for the emollient arm (n = 383) compared to control (n = 411) was £−106.89 (95% CI −354.66 to 140.88) [unadjusted this was £−123.74 (95% CI £−382.55 to 127.08)]. The adjusted incremental difference in proportion without eczema at 5 years for the emollient arm compared with the control was –0.0329 (95% −0.0659 to 0.0002) (unadjusted –0.0386, 95% CI –0.0776 to 0.0004), meaning that the intervention was cost saving but less effective (i.e. associated with slightly more cases of eczema) at 5 years. The adjusted ICER was £3201 (£3312 unadjusted) per percentage decrease in risk of eczema. The amount decision-makers would be willing to pay per percentage decrease in risk of eczema is unknown. It should be noted, however, that the driver for the intervention to be cost saving was the number and cost of inpatient hospital stays incurred as a result of wheezing which were higher in the control group. The control arm had a greater number of participants reporting such costs at 5 years than the intervention arm (22 compared to 7 in the intervention arm) and the control arm had a higher range on the cost incurred than the intervention arm, thus leading the incremental cost to look cost saving for the intervention. When these costs were removed from the analysis, incremental cost became positive (i.e. those in the intervention arm cost more on average) such that since the proportion without eczema was lower in the intervention arm, we would conclude that the intervention is dominated by the control (i.e. we would not recommend the intervention). Such a finding is possible because the costs observed are small and as such a single large cost item can sway the result. It seems quite implausible that the intervention could impact asthma inpatient stays given it was not significantly effective at preventing eczema which would be the first change required in the mechanism of action for it to impact resource use for other atopic diseases.
| Analysis (N e, N c) | Incremental cost (UK£) (95% CI) | Incremental effect (95% CI) | ICER |
|---|---|---|---|
| 5-year CEA (CCA, unadjusted) (383, 411) |
−123.74 (−382.55 to 127.08) | −0.0386 (−0.0776 to 0.0004) | £3312 per percentage decrease in risk of eczema |
| 5-year CEA (CCA, adjusted)a (383, 411) |
−106.89 (−354.66 to 140.88) | −0.0329 (−0.0659 to 0.0002) | £3201 per percentage decrease in risk of eczema |
| 5-year CEA (CCA, adjusted)a without inpatient costs due to wheezing (383, 411) |
100.34 (−30.09 to 230.83) | −0.0329 (−0.0658 to 0.0001) | Dominated |
| 5-year CUA (CCA, CHU-9D, unadjusted) (254, 263) |
−336.02 (−670.22 to −1.82) | 0.0171 (−0.0138 to 0.0480) | Dominant |
| 5-year CUA (CCA, CHU-9D, adjusted) (254, 263) |
−312.68 (−645.17 to 19.82) | 0.0166 (−0.0135 to 0.0467) | Dominant |
| 5-year CUA (MI, CHU-9D, adjusted) (693, 701) |
−49.80 (−306.83 to 207.22) | 0.0174 (−0.0045 to 0.0394) | Dominant |
| 5-year CUA (MI, CHU-9D, adjusted) without inpatient costs due to wheezing (693, 701) | 90.92 (−17.06 to 198.90) | 0.0173 (−0.0051 to 0.0396) | £5268 per QALY |
Table 27 also shows the cost–utility results at 5 years, despite more cases having eczema in the emollient group the intervention was dominant (cheaper and slightly more effective). Dealing with the missing data using multiple imputation did not change this finding. However, incremental costs were higher for the emollient group when high-cost wheezing inpatient stays were excluded, although the ICER was lower than NICE’s implicit threshold. It seems likely the CEA is more plausible, although all results reflect the very small values involved.
Discussion
Main findings
In our economic analysis of the main multicentre, pragmatic RCT of high-risk infants, we find no evidence that regular emollient use for the first year of life is cost-effective at 2 years of age. Incremental costs and effects were very small. These results support the findings of the original clinical study. 1 We find that the intervention is more expensive, with potential to prevent only marginally more cases of eczema and generates very slightly more QALYs as measured using the proxy version of the CHU-9D. When the results were adjusted for covariates, the same conclusion held for the primary and secondary outcome measures.
At 5 years almost half the sample had missing cost data. The complete-case CEA found the intervention to be cost saving at 5 years but to have a slightly lower proportion without eczema. However, when inpatient hospital costs for wheezing were removed from the analysis (because < 4% of the sample incurred them and they were associated with high cost), it was found the cost saving of the intervention at 5 years disappeared, and that the CEA was dominated by control (as the intervention was both more costly and less effective).
In the 5-year CUA (as complete case or using multiple imputation) the intervention was found to dominate; the incremental cost was negative indicating the intervention was cost saving and the incremental effect was higher (better quality of life) in the intervention arm. Given the intervention was not clinically effective, the plausibility of these results needs to be questioned. In part, the cost savings found in the 5-year analyses were driven by differences in number and cost of inpatient hospital stays for wheezing between study arms in years 3–5; when these were removed as part of sensitivity analysis, the incremental cost was very small in the CUA. Given there is no evidence the intervention was clinically effective at preventing eczema, it seems highly unlikely it will have had any impact on wheezing resource use such that the 5-year CUA results are likely to be spurious. That we find this seemingly ‘paradoxical’68 finding at 5 years (particularly in the CUA) is indicative of there being non-significant small incremental costs and effects.
Our results, alongside those of the full trial, have important implications for the existing evidence on whether to use emollients prophylactically in the early years of a high-risk infant’s life. This is important to report as a previous economic analysis of emollient use in infants prophylactically69 reached a different conclusion, that daily emollient use was a cost-effective strategy. Their study was a secondary analysis based on pilot evidence about the effectiveness of emollients, primarily relying on a very large RR estimate of preventing 50% of new eczema cases (n = 108 in complete-case analysis) reported in Simpson et al (2014). 92 Using this estimate along with assumptions over the amount of emollient that would be used and equivalent effectiveness across the seven products compared, the decision tree was reportedly analysed using a CUA approach for a 6-month time horizon. Using data from a larger, definitive study, our results suggest that emollient use as a preventative measure early in life for high-risk infants is not effective or cost-effective taking a 2-year time horizon and this conclusion holds at 5 years given the caveats discussed.
Strengths and limitations
Issues about how to best capture utility for infants and young children for use in economic evaluations are well known. 72–74 In this study, we used the CHU-9D by parental proxy for infants at ages 2, 3, 4 and 5 years old. The CHU-9D is currently validated for children 7 years and upwards50 and for children aged 5–7 years by parental proxy. 93 We have been unable to find any published studies using the CHU-9D in children under 5 years of age, although the MAGIC trial94 plans to use the CHU-9D with children as young as 3 years old (Sheffield Clinical Trials Research Unit, 2020). While it is a strength to explore a new approach to add to the growing academic discussion on appropriate HR-QoL measures for young children and infants, it is important to interpret the results tentatively until the use of this instrument in this context is less experimental. Other potential issues such as using parental proxies to value child health also need to be acknowledged. 95
Response rates to the 36-month questionnaire was comparatively low due to issues distributing this to those first randomised into the study (i.e. the CHU-9D and EQ-5D was inadvertently left out of the questionnaire initially). Completion rates for the questionnaires declined in years 3–5. Together, these may affect the quality and conclusions that can be taken away from the 5-year sensitivity analysis.
The study collected disease-specific resource use but did not capture resource use related to food allergy as this component was not included at the outset of the study. Given the small number of patients who experience clinically diagnosed food allergy and the outcome of the study, this is unlikely to be important. However, it may be appropriate to include resource use related to food allergy in future studies seeking to explore interventions to prevent eczema and other allergic disease.
If a favourable difference in risk had been found at 24 or 60 months and costs and outcomes had not converged at 60 months, a longer-term CEA from birth to 16 years using a model-based analysis would have been undertaken. Given the results, a decision was taken not to develop a longer-term model for this intervention, but future preventative strategies might well benefit from such an approach and from considering a longer time horizon.
Further research
This study is the first we know to have published results using the proxy version of the CHU-9D in children aged as young as 2 years. We did this using the additional guidance developed by the developer of the CHU-9D. We plan to analyse these data further to help inform the design of future studies of very young children, but in terms of practicality it can be seen in the response rate reported in this chapter that most parents did not have difficulties completing this instrument at 2 years (84% provided complete responses which was not too dissimilar to the completion rate for the main clinical outcome, see Outcome measures for further details). There is a need for further research to confirm the validity of the CHU-9D for children and infants aged under 5 years.
Given the results of the clinical and economic study, there is a need for further research looking for potentially preventative strategies that are both effective and cost-effective in order to help reduce the burden of AE.
Conclusions
The daily use of all-over-body application of emollient during the first year of life as described in this study is unlikely to be a cost-effective intervention in preventing AE in high-risk children under 2 years of age if a 2-year time horizon is taken. At 5 years the intervention appears to be cost-effective, but this seemed to reflect the high cost of inpatient hospital stays due to wheezing amongst a small proportion (< 4%) of the sample in a study that shows no clinical benefit for wheezing prevention at 5 years. The 5-year results in this study illustrate what Raftery et al. 68 call ‘doubly null’ results, that is no significant difference in both primary outcome and cost per patient. We seek to avoid ‘paradoxical’ conclusions at 5 years by exploring and acknowledging the issue (small incremental costs and effects) and factors (such as the differentially high wheezing inpatient costs between arms over years 3–5) that might explain the findings68 regarding not clinically effective but potentially cost-effective results. Resource allocation decisions should be based on the totality of evidence established by systematic reviews and meta-analysis. Our results add an important contribution to the evidence available to inform any future decisions in this clinical area. Our results also indicate that in terms of practicality it is possible to use the proxy CHU-9D with children as young as 2 years old and further research to explore the validity of this would be useful.
Chapter 5 Discussion
Main findings and interpretation
The overriding finding from BEEP is a lack of any clear benefit from emollients for the prevention of eczema in high-risk children. The findings are remarkably consistent across the range of eczema outcomes used and time points. Results for the main outcomes are robust to sensitivity analyses that account for missing data. In subgroup analysis, there was also no evidence of a difference in the effect of emollients on eczema prevention according to genetic risk of atopic disease, for example, presence of filaggrin gene mutations. No benefit was seen for eczema severity (which could still have been useful in terms of reducing health burden), time to onset of eczema or associated allergic diseases including food allergy, asthma or hay fever. While disappointing, the findings from this study are very important in that they indicate that perhaps skin barrier enhancement using emollients is not an effective pragmatic strategy to prevent eczema and associated diseases, despite earlier interest in this approach. The null result is important because it releases carers from the inconvenience and financial cost of daily emollient application.
Data beyond 2 years
The BEEP study is perhaps a little unusual in that the primary outcome for the 5 years study was elicited at 2 years. The original idea of the 5-year outcome was mainly to collect outcomes on other allergic diseases such as asthma that are potentially difficult to diagnose under the age of 5 years. We also sought to see whether any protective benefits of eczema prevention were transient or sustained as it is unclear from studies on probiotics to prevent eczema whether the benefits diminish over time. 96 The findings of no clinically useful eczema prevention at our primary outcome time point when study children were aged 2 years were upheld in parent-reported 3-, 4- and 5-year data of doctor-diagnosed eczema and other measures. We did not find any evidence of benefit for asthma or hay fever prevention, although we cannot exclude small degrees of benefit. We did not measure additional emollient-related safety outcomes beyond 2 years. None were reported spontaneously from participants for this period. Interestingly, moisturiser use over the body continued at a higher level in the intervention group than in the control group after the 12-month intervention period ceased. Such a ‘legacy’ effect might be due to routine or a genuine perceived benefit from parents, while some usage would have inevitably reflected the need to use emollients for recently diagnosed eczema.
Health economics
The economic evaluation results are consistent with the clinical findings of the study and finds that at 2 years the use of emollients to prevent eczema is unlikely to be cost-effective. The results at 5 years, if read uncritically, could be interpreted as suggesting the intervention is cost-effective over this longer period. However, incremental costs and effects were small in both the 2- and 5-year analyses. In addition, the costs in the 5-year analysis were largely driven by the small number of participants who incurred inpatient hospital costs due to wheezing and which were unequally distributed between the two study arms. Given the findings elsewhere in this report do not find evidence of benefit for asthma, or indeed hay fever, it seems unlikely these costs were related to the intervention. When they were excluded in a sensitivity analysis the CEA results suggest that the intervention is dominated by control (i.e. the intervention is both more costly and less effective than control).
Possible signals of harms?
Given that the combination of greasy emollients and water in the bathroom is a recipe for very slippery surfaces, one of our main concerns with the use of emollients was slippages. Thankfully, slippages were not common, and none were serious. More importantly, they were similar in intervention and control group and mainly due to non-study emollients for eczema or general skin-care use.
The finding of increased parental report of doctor-diagnosed skin infections in the intervention group is an area of concern. Although none were serious, they nevertheless represent a nuisance to the infant and parents and may involve healthcare contact. Interpretation of the skin infection data is challenging though as the range of skin infections was quite wide and not coherent with the sort of specific infections such as folliculitis due to S. aureus that one would normally associate with eczema or occlusion of the skin with emollients. 39 Although we recorded parental report of doctor-diagnosed skin infection, we did not verify these reports or diagnoses with the doctors concerned and whether such infections were confirmed by laboratory investigations. Ideally, more specific investigation of skin infections should be undertaken in ongoing and future emollient prevention studies.
Skin sensitisation and food allergy outcomes; although funded separately, the results of this aspect of the BEEP warrant some brief discussion as food allergy and eczema are so inextricably linked. 97 Our hope was that successful prevention or postponement, of eczema onset might lead to prevention of food allergy, since early-onset IgE-mediated food allergy is thought to be directly caused by early-onset eczema. 98 BEEP trial findings showed no prevention or postponement of eczema, and a possible increase in IgE-mediated food allergy. Food allergy is less common than eczema, so the trial was not adequately powered to detect changes in food allergy prevalence. This means there is significant uncertainty about food allergy outcomes. While most food allergy outcomes, including the a priori defined main food allergy outcome, showed increased food allergy or sensitisation in the intervention group, CIs were wide and, for most analyses, included the possibility of no effect. If emollient application increases risk of IgE-mediated food allergy, then this may be an important mediating factor in the relationship between eczema and food allergy. Although no statistically significant increase in food allergy was seen across most estimates provided by the BEEP study (whether reported at 2, 3, 4 and 5 years or cumulative incidence), there is some uncertainty which ideally needs to be resolved by combining results with other studies. Stimulated by concerns of emollients possibly increasing sensitisation to food allergens and causing food allergy, some members of this team investigated the relationship between frequency of emollient use and food allergy in the enquiring about tolerance study population and found a dose–response relationship between frequency of emollient application and subsequent food allergy. 99 Although this finding was based on observational data, the relationship between emollient use and transcutaneous sensitisation to foods clearly requires further investigation. Other work supports the concept of transcutaneous sensitisation to foods, and this process could potentially be enhanced by the use of lipid-rich emollient, by rubbing the skin and by environmental traces of food allergens on parent hands or infant skin. 100 A European Union project called TRANS-FOODS, led by BEEP co-investigator Carsten Flohr, is building on these BEEP findings to try to understand how transcutaneous sensitisation to foods might be prevented. 101
Although the possibility of harms as a result of emollients used in early life seems low, small degrees of harm affecting a large population of healthy children can add up to a considerable absolute burden and attention to such concerns become a significant issue for public health intervention studies on otherwise healthy children.
Equality, diversity and inclusion
The majority of participants were recruited via secondary care sites, with the remainder via GP surgeries. While sites were mainly in Central/Northern England and London, it is unlikely that any population were systematically excluded from taking part by virtue of where they lived in the country or how they were approached.
The majority of children had mothers of white ethnicity (see Table 4, 85%), which reflects the overall mix of ethnicity the UK. 102 The high proportion of mothers (52%) or other first-degree relatives (82%) with a history of eczema reflect the inclusion criterion of ‘first degree relative with parental-reported doctor diagnosis of eczema, allergic rhinitis or asthma’.
No information was collected directly from mothers on their educational or economic status, but deprivation indices derived from participant’s home postcodes included a reasonable range (from 3 to 9).
We were conscious of the fact that severity assessment of eczema involves scoring the intensity of erythema (redness) which is traditionally underestimated in darker skin tones. 103 During our face-to-face training that included tests images, we took measures to ensure that all research nurses were aware of the need to upgrade erythema scores by 1 point as per the EASI guidance by including several images of darker skins in the training material. 104 The quality control test105 for determining the presence of visible flexural dermatitis which the nurses had to pass in order to undertake the physical assessments also included images of darker skin.
The interpretation of genetic test results is affected by the prevalence of genetic variants in different populations. The most prevalent FLG null mutations have been established in the white European population and other ethnicities have population-specific mutations. 34,106,107 To maximise inclusivity in this study we welcomed DNA collection from all participants and all samples were genotyped.
The composition of the BEEP study team was diverse with a range of content expertise from medical (dermatology, paediatrics and primary care) and nursing backgrounds. The team ethos was to always include the important contribution of trial managers, co-ordinators and administrators in emergent publications, and allow opportunities for methodologists such as statisticians (who are rarely afforded an opportunity to be first author) to lead on some publications such as this monograph. The study also allowed the development of early career investigators (such as Maeve Kelleher) who then led on funded parallel projects such as the Cochrane Individual Patient Data meta-analysis of similar studies and also Stella Lartey, who is new to the UK, who benefited from having the opportunity to gain skills in undertaking an economic evaluation in a UK context.
Patient and public involvement
Our trial was unusual for a NIHR Health Technology Assessment (HTA) Programme trial as it was the only study that successfully applied to the specific HTA call at the time to undertake definitive trials as a result of feasibility work carried out in a NIHR Programme Grants for Applied Research (PGfAR) project. Most of the critically important PPI work for the BEEP trial was undertaken in a specific eczema prevention workstream in our PGfAR award entitled ‘Setting priorities and reducing uncertainties in the prevention and treatment of people with skin diseases’. PPI in this eczema prevention workstream defined the choice of intervention, duration of the intervention period and nature of follow-up and contact and is described briefly in the methods section of this report and in full elsewhere. 42
Additional PPI work throughout the trial was critical in informing the way in which the add-on food allergy study and genetics substudy could be offered to participants resulting in both being offered as optional studies with a short additional consent procedure that resulted in excellent uptake. In addition, PPI colleagues helped to design, advertise and promote the study and to comment on and disseminate the study results as described in our methods section. It was also very beneficial to have a PPI member in the TSC as they provided an important and varied perspective throughout the trial, and in particular when disseminating the results to parents at 24 and 60 months in order to balance the positive aspects of the key study message (one less thing to do) versus the signal of possible harm (increased minor skin infections). There were no negative experiences of working with parents, carers and patients as PPI colleagues before and during the BEEP study. Although the whole team was disappointed that emollients did not prevent eczema in the BEEP study, our PPI colleagues were remarkably accepting and objective about the finding and enthusiastic about the need to disseminate our important findings.
Throughout the trial we have received positive feedback from the families and the level of engagement and enthusiasm is evidenced by the high retention rate, particularly for the longer-term follow-up at 36, 48 and 60 months. There were no negative experiences communicated to us from parents and carers who participated in the BEEP study which we suspect is due to the thorough PPI work and preparation undertaken in the proceeding Programme Grant for Applied Research.
Findings in context
The BEEP trial results contrast with the BEEP feasibility study92 where a signal on a preventive effect on eczema was found. It should be pointed out that the BEEP feasibility study was designed to test feasibility issues and was one-tenth of the size of the main BEEP trial. Reasons for the difference are unclear but could be due to chance or the fact that the outcome in the BEEP feasibility study was measured at 6 months when irritant dermatitis is more common.
Another large independent eczema prevention trial was conducted in Scandinavia (Preventing Atopic Dermatitis and ALLergies in children – PreventADALL) around the same time as BEEP and its primary outcome results for eczema were published alongside the BEEP trial in The Lancet in 2020. PreventADALL108 was a factorial cluster trial of 2397 newborns that included a skin-care intervention (bath additives and facial cream) and a food intervention (early complementary feeding of peanut, cow’s milk, wheat and egg) as well as both or neither with two primary outcomes: eczema at 12 months and food allergy at 36 months. That study found that neither the skin intervention nor food intervention reduced eczema development, with a risk difference of 3.1% (95% CI –0.3% to 6.5%) for the skin intervention and 1.0% (95% CI −2.1% to 4.1%) for food intervention, in favour of control. No safety concerns were identified.
The most important overall summary of contextual evidence to frame the BEEP study comes from the Cochrane living review of skin-care interventions to prevent eczema that includes traditional aggregate-based data and individual participant data (IPD), funded by the NIHR through the Research for Patient Benefit programme and a personal fellowship award to MK. 109 Up to July 2020, the review identified 33 trials that included 25,827 participants. Information on one or more of the review’s specified outcomes was available in 17 studies that included 5823 participants. Eleven trials with 5217 participants, 10 of which provided IPD, were included in one or more meta-analysis. The review found that risk of eczema by 1–2 years of age was not decreased by interventions for skin barrier enhancement in infancy, with a pooled risk ratio from 7 trials and 3075 participants of 1.03, 95% CI 0.81 to 1.31 (classified as moderate-certainty evidence). Time to onset of eczema was also not earlier for those receiving the intervention, with a pooled hazard ratio from 9 trials and 3349 participants of 0.86, 95% CI 0.65 to 1.14 (classified as moderate-certainty evidence). Interestingly, the skin infections signal from BEEP was also found in other studies, with a pooled increased relative risk of skin infection over the intervention period from 6 trials and 2728 participants of 1.34, 95% CI 1.02 to 1.77 (classified as moderate-certainty evidence). Only one trial (the BEEP study) provided data on food allergy outcomes.
The review failed to find any evidence that specific subgroups based on age, intervention duration, hereditary risk, FLG mutation, or classification of intervention type for risk of developing eczema are more likely to benefit from the skin-care interventions. The study also undertook a trial sequential analysis that indicates that further studies of similar interventions are unlikely to change the conclusion that emollients do not reduce eczema risk by more than 30%, although the sequential analysis was inconclusive for a RR reduction of 20%. The review also included studies which used more sophisticated emollients containing ceramides. The review concluded that skin-care interventions in early life are probably not effective for preventing eczema, and probably increase risk of skin infection, whereas the effects of such interventions on food allergy risk remain uncertain. The review also compared findings from aggregate studies versus those obtained from IPD and found that, although IPD did not significantly change the overall primary outcome risk estimates, certainty of evidence and safety outcomes were significantly different using IPD compared to aggregate data. In addition, subgroup and adherence analyses are only possible with IPD demonstrating the added value of using an IPD approach to meta-analysis. 110
An update of the review has recently been accepted for publication in the Cochrane Library. This update was planned in order to include the food allergy outcome data from the largest similar trial, PreventADALL. At the timing of the updated review, no new relevant studies with eczema outcomes were published in time to be included, so the eczema outcome at 1–3 years did not change. For food allergy, skin-care interventions during infancy may increase the risk of IgE-mediated food allergy by 1–3 years of age (RR 2.53, 95% CI 0.99 to 6.49; low-certainty evidence; 976 participants, 1 trial) but may not change risk of allergic sensitisation to a food allergen by age 1–3 years (RR 1.05, 95% CI 0.64 to 1.71; low-certainty evidence; 1794 participants, 3 trials). Pre-planned sensitivity analysis assessing food allergy by oral food challenge or investigator assessment showed similar findings (RR 1.45, 95% CI 0.98 to 2.15; low certainty evidence; 2081 participants, 2 trials). The additional results from in this Cochrane review analysis consolidate the results for food allergy diagnosed in this way that were shown in the BEEP study.
Another systematic review on the same topic using aggregate data concluded that emollients do prevent eczema in high-risk children and that the significant protective benefit is only evident where emollients were used continuously to the point of eczema outcome assessment but not when treatment was stopped before eczema assessment. 111 However, that review has been criticised for registering the review retrospectively with limited public record of pre-planned eligibility criteria, outcomes and analysis, including subgroup analyses. 112 That review also failed to include three eligible trials that were included in the Cochrane review rendering it an unreliable source of evidence for informing clinical practice.
Following the update of the Cochrane IPD meta-analysis, the 3-year results for the Scandinavian PreventADALL study has been published. 113 These showed that neither the skin-care intervention nor early complementary feeding of peanut, cow’s milk, wheat and egg from age 3 months had any influence on AD (eczema) diagnosis at 36 months. The study found early complementary feeding reduced food allergy (risk difference −1.6%, 95% CI −2.7% to −0.5%; OR 0.4, 95% CI 0.2 to 0.8) but not in the skin intervention group (risk difference 0.4%, 95% CI −0.6% to 1.5%; OR 1.3, 95% CI 0.7 to 2.3), with no evidence of an interaction effect (p = 0.98).
Study strengths and limitations
The strengths of the BEEP trial can be considered using the population, intervention, comparator, outcome framework. Participants were representative of babies born across the UK who would normally be considered at high risk of developing eczema and associated atopic disorders. The intervention was chosen to be acceptable in a previous feasibility study and was shown in pre-study experiments not to paradoxically damage the skin barrier as some emollients have been shown to do. 114 The intervention was simple and cheap and adherence to the intervention was good, especially for study with only two face-to-face contacts with the study team (baseline and at 2 years). Participants in the control group followed standard best advice for infant skin care, and contamination rates with emollients were low (< 20% as specified in our stop/go criteria for study progression). Outcomes were robust and included family reported as well as objective outcomes on the presence of eczema and eczema severity with objective outcomes measured by trained assessors blinded to allocation status. Unblinding of intervention status prior to skin examination was minimal (12 in the intervention group and 6 in the control group). As some debate exists over the validity of diagnostic criteria for eczema in early life,115 a range of eczema outcomes were used that showed consistent lack of a preventive effect. Timing of the outcome was also conducted well away from the emollient intervention period in order to avoid any potential inflation of emollient benefit due to the mild anti-inflammatory effect of the emollient concealing mild eczema. The long duration of follow-up meant that any later benefits of emollient prevention could be picked up. Additional data from the add-on genetic study provided useful insights into whether filaggrin gene mutations are an important consideration for disease stratification in such studies. The add-on food allergy study also provided useful data on the feasibility and prevalence of SPT sensitivity to common food allergens in the UK community setting and resulted in a novel pragmatic method of confirming food allergy for those not able to travel to regional test centres for oral food challenges. 59
In terms of study design, BEEP conforms to a relatively pragmatic design when considered using the PRECIS-2 (PRagmatic Explanatory Continuum Indicator Summary) tool. 116 The large sample size had sufficient power to exclude a moderate reduction of eczema incidence. Various sensitivity analyses have confirmed the stability of the main conclusions. The effects of missing data on the study conclusions have also been thoroughly explored. The independence of the BEEP study from any commercial influence from emollient manufacturers is also a study strength. Risk of bias for the BEEP study has been rated independently in a Cochrane review109 as low risk of bias for randomisation process, deviations from intended interventions, missing outcome data, outcome measurement and selection of reported results for the eczema outcomes.
The BEEP study has also generated several useful spin-off publications that have contributed to thinking about the design and conduct of eczema prevention trials including how to define an incident case,6 an algorithm for diagnosing IgE-medicated food allergy,59 a SWAT to explore strategies for enhancing follow-up in RCTs,117 an overview of methodological considerations for eczema prevention studies,32 and the value of hyperlinear palms in evaluating FLG mutations. 118
Study limitations include the risk that BEEP used the ‘wrong’ emollients. Not all emollients are the same. Some exhibit different physiological effects on skin barrier function in adults with eczema in terms of epidermal water loss, hydration, natural moisturising factor levels and response to irritant challenges. 119 Alternatively, it may be that it is critically important to start emollients immediately after birth. Although we were pleased that families started reporting using emollient at a median of 11 days after birth (IQR 7–17 days), one single-centre industry-funded study120 has suggested preventive benefit of eczema at 12 months as a result of twice-daily specially formulated emollients applied for 8 weeks of life when started early (63% within 2 weeks). Perhaps the intervention was not intense enough and rather than an ‘advice to use’ study, an efficacy study whereby emollient application was supervised and measured accurately might have shown benefit. However, such a study would not have been practical and unlikely to be translated into everyday practice. It is also worth pointing out that based on strong user feedback from our BEEP feasibility study, parents wished to have a choice of emollients for the BEEP main study. This meant that the BEEP study used two emollients which might have had slightly different barrier enhancement properties. 44 We were however unable to explore this in a meaningful sensitivity analysis as parents initially received both types of emollients and then chose which emollient they preferred when reordering. Perhaps we were wrong to ignore various forms of transient irritant eczemas that emerge in the first year of life during the intervention period, although other studies that have measured such skin findings at 3, 6 and 12 months have not found any benefit from emollients. 108 As mentioned in the introduction, it is likely that eczema represents a range of distinct phenotypes with different disease trajectories and severity, but such variation is unlikely to affect the interpretation of this study which evaluates the prevention of new onset eczema that corresponds to a well-defined phenotype depicted by the UK diagnostic criteria for eczema. The strongest predictors of eczema onset and subsequent severity are family history of atopic disease and filaggrin gene mutations. Both were explored in subgroup analyses in this study with neither showing evidence of benefit in such subgroups. Although contamination in our control was considered low (< 20%), perhaps such a degree of contamination was sufficient to mask a small preventive effect. The results of the CACE analysis (with compliance defined as widespread emollient use at least 3 days per week) were consistent with the main analysis of no difference; however, the CIs for the CACE estimate were wider. Due to the pragmatic low-contact efficient study design, we inevitably used parental report for many of the outcomes such as skin infections and allergic diseases at 3, 4 and 5 years. Such unmasked outcome assessment is perhaps a potential source of information bias. We mitigated such bias by mainly asking for parental report of doctor-diagnosed skin infections or allergic disease. We would also argue that capturing parental report of such illnesses are important as not all families who are used to dealing with eczema will necessarily take a child with mild disease to a healthcare professional. Studies comparing parental report of the UK diagnostic criteria for eczema have also shown them to be equally valid when compared with research nurses’ assessed findings. 121 The findings from parental reports were also consistent with findings from the research nurse assessors for eczema presence and eczema severity who were masked from intervention or control status at the 2-year primary outcome assessment. Information bias as a result of enthusiasm for the intervention is also perhaps more likely to have resulted in a positive benefit for eczema prevention, whereas the study showed consistent null effects regardless of the timing and choice of outcome. The trial was not powered to detect interaction effects in subgroup analysis meaning that there is considerable uncertainty in the interaction effects (shown through wide 95% CIs) between subgroups and the effect of the intervention including for presence of FLG mutations.
Although the spread of our centres was reasonable representative of English cities and towns, we could have been more inclusive by considering translation of written materials at the study outset. Although the proportion of mothers of white ethnicity in BEEP is broadly reflective of the UK, we could have done more to reach out to under-represented groups using such frameworks as INCLUDE that appeared towards the end of this study. 122
Recommendations for clinical practice
Based on the findings of the BEEP study and the emerging evidence from other similar studies, summarised in an ongoing living systematic review that uses individual patient data meta-analysis, emollients cannot be recommended for primary prevention of eczema. Small benefits cannot be excluded, but the burden of applying whole-body emollients daily for the first year of life is considerable, and uncertainty exists about possible signals of harm in terms of skin infections and food allergy.
The use of emollients for eczema prevention should not be confused with use of emollients for eczema treatment. Emollients should continue to be used as part of standard treatment for eczema along with topical corticosteroids and calcineurin inhibitors and systemic therapies for more severe disease. In addition to treating the symptoms of dry skin associated with eczema, emollients may contribute to reducing flares (secondary prevention). 123 A recent NIHR HTA study suggests that there is little difference between the efficacy of emollient types (creams, ointments, gels or lotions) for eczema treatment in the community but patient and carer preference for each formulation varies. 124
Recommendations for future research – specific and general
Apart from the PreventADALL study, emollient prevention studies to date have focused on barrier enhancement in early life to prevent eczema in high-risk children on the basis that the preventive fraction is likely to be higher in this group when compared to others. The assumption that potential preventive benefits are likely to be higher in high-risk babies may be untrue, as it is possible that babies born to high-risk families inherit such a strong deviation in immunological responses that any potential benefit of barrier enhancement is overcome by the inherited prevailing immunological dysfunction. Studies evaluating emollients for the prevention of eczema in all (including lower risk children) may be useful to provide a complete the picture. Such a study (the CASCADE trial)125 is underway in the USA recruiting around 1250 infant/parent dyads from community settings in Oregon, Colorado, Wisconsin and North Carolina. Future studies should describe and define the phenotype of eczema that develops in the infants participating in prevention studies using well-established diagnostic criteria for defining incident cases6 and by describing the severity and trajectory of such cases once they develop. Many transient irritant forms of eczema may develop in the first year of life most of which probably do not develop into eczema,7 so care should be taken in following up such cases to see if they evolve into persistent eczema. Candidate emollients tested in future studies should ideally be tested for their skin barrier properties on infant skin. Measurement of eczema should also be done well away from the intervention period, for example, 1 year after the intervention has ceased as in the BEEP study, in order to avoid masking any potential mild eczema by the use of emollients.
With around 33 similar studies published or in progress, we suggest that enough studies on emollients for eczema prevention in high-risk families have now been set up and/or completed. Large independent high-quality studies are always welcome, but the addition of a lot of smaller studies is unlikely to help as indicated by the trial sequential analysis illustrated in the ongoing Cochrane IPD review. The priority must be to try and include all existing eczema prevention studies in that Cochrane review in some form – preferably individual patient data rather than aggregate data. We predict that there could be a degree of publication bias from some studies funded by companies with a vested interest in showing a positive outcome that claim that specially formulated emollients containing ceramides or natural moisturising factors are useful in preventing eczema. Although such emollients have not appeared to work so far, we cannot exclude the possibility that such newer specialised formulations could work, and we look forward to seeing the authors share their individual patient data of all registered and non-registered trials with the living Cochrane IPD review on this topic. Some degree of heterogeneity of effects between studies is expected since not all emollients are the same. Other studies should also link their choice of emollient to functional studies of the skin barrier. Attention to measuring and pooling potential harms of daily emollients during infancy should be encouraged in all similar future studies, with more refined collection and verification of the types and severity of skin infection. Experimental studies on the role of whole-body emollients as a vehicle for inducing skin sensitisation should also be considered.
In general terms, investing in research to prevent eczema and associated diseases is important given the burden of disease in the UK and worldwide and concerns about increasing prevalence. The variation in eczema prevalence between different environmental settings and variable genetic penetrance indicates that there are important environmental determinants, which may be modifiable. Given the disappointing results of BEEP and other trials on the strategy of skin barrier enhancement in early life using moisturisers, future eczema prevention research might concentrate on strategies to reduce potentially harmful interventions such as frequent bathing, exposure to hard water and/or use of wash products126 or future molecular mechanisms led by personalised medicine to improve skin barrier formation and function. 127 Other areas warranting further investigation are interventions which might influence early immune development, including nutritional and microbial interventions during pre or postnatal life. 128 There is already some supportive evidence for probiotics during pregnancy/lactation – however the data are heterogeneous and potentially selectively reported, so the probiotic evidence may potentially be clarified through a high-quality individual patient data meta-analysis. 128
Chapter 6 Conclusions
Despite a compelling rationale and promising early indications of a possible benefit of emollients as a means of preventing eczema from developing in babies born to families with allergic disease, the BEEP study found no evidence to support a clinically useful benefit for emollients when used in this way. The BEEP study found no evidence that advice to use daily emollients for the first year of life can prevent eczema when defined in a range of ways, nor did emollients reduce the severity distribution of eczema or time to onset. The study did not show any other benefits in terms of prevention of associated conditions including food allergy, asthma and hay fever up to the age of 5 years. The intervention delivered in this study is unlikely to be considered cost effective.
Some uncertainty exists around possible harms of emollients used in the first year of life including a possible increase in food allergy and increased parental report of doctor-confirmed skin infections; these uncertainties need to be explored in future studies. The findings of the BEEP study are consistent with other studies that form part of an ongoing living systematic review and meta-analysis. 129
Based on the BEEP study and other similar studies, advice to use daily emollients for the first year of life cannot be recommended as a method to prevent eczema and associated atopic diseases. This is a useful scientific finding as the burden of applying daily emollients for the first year of life is considerable, so it is one less thing for busy parents to do when coping with allergic disease in the family. Parents often feel guilty for not doing all they can to prevent ‘passing on’ eczema to their newborn child, so not applying emollients from birth is one less thing for parents to feel they should have done. Apart from testing earlier initiation of emollients and/or more potent skin barrier enhancers as a means of primary prevention, the addition of a lot more ‘me too’ studies is unlikely to be helpful. Enough research has been set up and conducted on emollients for preventing eczema. The emphasis should be on encouraging those leading existing skin barrier prevention studies to measure potential harms as well as benefits, and to share their individual patient data with the living systematic review in order to add precision to the outcome estimates and to explore the full range of interventions in different populations over the world. Future prevention of eczema should concentrate more on preventing potentially harmful skin-care practices during infancy and on identifying interventions that can influence the course of early skin barrier and immune development.
Additional information
Contributions of authors
Lucy E Bradshaw (https://orcid.org/0000-0001-8382-6040) (Medical Statistician) contributed to the conception and design of the trial and the acquisition, analysis and interpretation of the data; was responsible for statistical analysis; drafted, reviewed and edited the final report; and was a member of the Trial Management Group (TMG).
Laura A Wyatt (https://orcid.org/0000-0002-9817-5356) (Trial Manager) supported the conduct of the trial; drafted, reviewed and edited the final report; and was a member of the TMG.
Sara J Brown (https://orcid.org/0000-0002-3232-5251) (Professor of Dermatology, and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis, or interpretation of the data, and was responsible for the genetic analysis. Sara also contributed clinical experience of eczema or eczema trials, or both; reviewed and edited the final report and was a member of the TMG.
Rachel H Haines (https://orcid.org/0000-0001-7924-0602) (Senior Trial Manager) contributed to the conception or design of the trial and the acquisition, analysis, or interpretation of the data and supported the design and conduct of the trial; reviewed and edited the final report and was a member of the TMG.
Alan A Montgomery (https://orcid.org/0000-0003-0450-1606) (Professor of Medical Statistics and Clinical Trials, Senior Statistician and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data and was responsible for statistical analysis; reviewed and edited the final report and was a member of the TMG.
Michael R Perkin (https://orcid.org/0000-0001-9272-2585) (Consultant in Paediatric Allergy and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data; supported the food allergy assessment and reviewed and edited the final report.
Tracey H Sach (https://orcid.org/0000-0002-8098-9220) (Professor of Health Economics, and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data, and was responsible for the health economic design and analysis; drafted, reviewed and edited the final report; and was a member of the TMG.
Sandra Lawton (https://orcid.org/0000-0002-6163-5822) (Nurse Consultant Dermatology and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data, and contributed clinical experience of eczema or eczema trials, or both; reviewed and edited the final report; and was a member of the TMG.
Carsten Flohr (https://orcid.org/0000-0003-4884-6286) (Professor of Dermatology) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data, and contributed clinical experience of eczema or eczema trials, or both; reviewed and edited the final report.
Matthew J Ridd (https://orcid.org/0000-0002-7954-8823) (NIHR Clinical Trials Fellow and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data, and contributed clinical experience of eczema or eczema trials, or both; reviewed and edited the final report.
Joanne R Chalmers (https://orcid.org/0000-0002-2281-7367) (Senior Research Fellow and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data, and supported the design and conduct of the trial; reviewed and edited the final report; and was a member of the TMG.
Joanne Brooks (https://orcid.org/0000-0003-2456-0160) (Trial Coordinator) contributed to the conduct of the trial (data collection and management).
Richard Swinden (https://orcid.org/0000-0001-9877-8301) (Trial Coordinator) contributed to the conduct of the trial (data collection and management).
Eleanor J Mitchell (https://orcid.org/0000-0002-6998-4533) (Associate Professor) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data and supported the design and conduct of the trial; reviewed and edited the final report.
Stella Tarr (https://orcid.org/0000-0001-8990-4425) (Data Manager) contributed to the conduct of the trial (data collection and management).
Nicola Jay (https://orcid.org/0000-0003-1388-192X) (Consultant Paediatrician Respiratory and Allergy and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data, and supported the food allergy assessment; reviewed and edited the final report.
Kim S Thomas (https://orcid.org/0000-0001-7785-7465) (Professor of Applied Dermatology Research and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data and supported the design and conduct of the trial, and contributed clinical experience of eczema or eczema trials, or both; reviewed and edited the final report.
Hilary Allen (https://orcid.org/0000-0002-7013-0308) (General Practitioner) contributed to additional analysis of the food allergy data; reviewed and edited the final report.
Michael J Cork (https://orcid.org/0000-0003-4428-2428) (Professor of Dermatology and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data, and provided expertise in emollients and the skin barrier; reviewed and edited the final report.
Maeve Kelleher (https://orcid.org/0000-0002-3764-0461) (Honorary Consultant in Paediatric Allergy) supported the food allergy assessment, reviewed and edited the final report.
Eric L Simpson (https://orcid.org/0000-0003-0853-0252) (Associate Professor in Dermatology, and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis or interpretation of the data, and contributed clinical experience of eczema or eczema trials, or both; reviewed and edited the final report.
Stella T Lartey (https://orcid.org/0000-0001-9519-7886) (Lecturer) contributed to the health economic analysis and reviewed and edited the final report.
Susan Davies-Jones (Research Nurse) contributed to the conception or design of the trial and the acquisition, analysis, or interpretation of the data, and contributed clinical experience of eczema or eczema trials, or both; reviewed and edited the final report.
Robert J Boyle (https://orcid.org/0000-0002-4913-7580) (Consultant in Paediatric Allergy and co-applicant) contributed to the conception or design of the trial and the acquisition, analysis, and interpretation of the data, and led the food allergy assessments; reviewed and edited the final report; and was a member of the TMG.
Hywel C Williams (https://orcid.org/0000-0002-5646-3093) (Professor of Dermato-Epidemiology and Chief Investigator). Hywel conceived the trial, contributed to the design of the trial and the acquisition, analysis and interpretation of the data; drafted, reviewed and edited the final report; and was a member of the TMG.
Acknowledgements
Additional funding for the food allergy and sensitisation tests was obtained from Goldman Sachs Gives and the Sheffield Children’s Hospital Research Fund (reference CA15008). Research nurse support was provided by the NIHR Clinical Research Networks.
The trial was developed with and supported by the UK Dermatology Clinical Trials Network (UK DCTN). The UK DCTN is grateful to the British Association of Dermatologists and the University of Nottingham for financial support of the Network.
We would like to thank the parents and infants who took time to participate in this trial, and the patients who contributed to trial design by providing helpful feedback at different stages of trial development.
We would like to thank the independent members of the Trial Steering Committee: Sarah Meredith, (Chair, Medical Research Council Clinical Trials Unit at University College London), Angela Crook (Statistician, Medical Research Council Clinical Trials Unit at University College London), Paula Beattie (Dermatologist, Royal Hospital for Children, Glasgow), Kirsty Logan (Paediatric Epidemiologist, King’s College London) and Emma Thomas (patient representative). Michael Perkin (St George’s, London) was previously an independent member of the TSC prior to becoming part of the team involved in the food allergy assessment.
We would also like to thank the following for their work on BEEP at the Nottingham Clinical Trials Unit:
-
Jim Thornton for work on the grant application during his time as deputy director of the NCTU.
-
Clinical Research Facilitator: Trish Hepburn.
-
Senior Trial Manager: Rebecca Haydock.
-
Trial Managers: Amy Moody, Angela Pushpa-Rajah, Sandip Stapleton.
-
Trial Co-ordinators: Margherita Carucci, Louisa Gray, Juliet Jackson.
-
Trial Administrators: Jennifer White, Andrew Jadowski, Gurmit Dhanjal, Aneesa Mehmood.
-
Database Programmers: Daniel Simpkins, Keith Whitaker, Chris Rumsey.
-
Data Management: Sarah Walker, Jessica Haywood, Lisa Evans.
-
Statisticians: Reuben Ogollah (assistance with CACE analysis), Chris Partlett (independent validation of primary outcome, main food allergy outcome and skin infection outcome) and Grace Holt (independent validation of the UKWP AD tertiary outcome).
We would like to acknowledge and thank Dr Charlotte Davies (RAND corporation) who contributed to earlier versions of the health economic evaluation analysis undertaken using the 24-month data.
The genetic analysis work was co-ordinated by SJB at the Skin Research Group, School of Medicine, University of Dundee. During this time SJB also worked clinically at the Ninewells Hospital and Medical School, Dundee. We would like to thank Sheila C Wright (Skin Research Group, Division of Molecular and Clinical Medicine, University of Dundee, UK) for cataloguing and processing the saliva samples for DNA analysis.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
The study protocol, SAP and HEAP are available on the trial website and the NIHR journals library. All other related documents are available on request to Prof. Hywel C Williams as chief investigator of the BEEP trial, at any point.
Ethics statement
The trial was approved by the NRES Committee West Midlands – Solihull (REC reference 14/WM/0162) on 9 June 2014 prior to the start of recruitment.
Information governance statement
The University of Nottingham is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, the University of Nottingham is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://www.nottingham.ac.uk/governance/records-and-information-management/contact.aspx.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the report publication page of NIHR Journals Library at https://doi.org/10.3310/RHDN9613.
Primary conflicts of interest: Sara Brown holds a Wellcome Trust Senior Research Fellowship in Clinical Science (reference 106865/Z/15/Z and 220875/Z/20/Z). Alan Montgomery was a member of NIHR HTA Clinical Trials and Evaluations Funding Committee 2015–21 but had no part in the decision-making for funding this study. Tracey Sach was a member of NIHR HTA Efficient Study Designs – 2, HTA Efficient Study Designs Board, HTA End of Life Care and Add-on-Studies, HTA Primary Care Themed Call Board and the HTA Commissioning Board between 2013 and December 2019. She is a steering committee member of the UK Dermatology Clinical Trials Network and Chair of the NIHR Research for Patient Benefit Regional Advisory Panel for the East of England. Tracey had no part in the decision-making for funding this study. Carsten Flohr is Chief Investigator of the UK NIHR-funded TREAT (ISRCTN15837754) and SOFTER (ClinicalTrials.gov: NCT03270566) trials as well as the UK-Irish Atopic Eczema Systemic Therapy Register (A-STAR; ISRCTN11210918) and the Principal Investigator in the European Union (EU) Horizon 2020-funded BIOMAP Consortium (www.biomap-imi.eu/). He also leads the EU Joint Program Initiative Trans-Foods and the UK Medical Research Foundation-funded consortia. His department has received funding from Sanofi-Genzyme and Pfizer for skin microbiome work. Carsten received a grant from the EU IMI grant scheme (Horizon 2020), outside of the submitted work. Maeve Keller holds an NIHR Transitional Research Fellowship (TRF-2017–10–003). Matthew Ridd holds a Post-Doctoral Research Fellowship from NIHR (PDF-2014–07–013). Matthew Ridd was a member of HTA General Committee and ESP Evidence Synthesis Programme Advisory Group. Michael Cork received institutional research grants and personal consulting fees from Hyphens Pharma, L’Oréal and Perrigo (ACO Nordic). Michael is on the advisory board for L’Oréal and was a member of the Board of the European Academy of Dermatology and Venereology (EADV) and is a voluntary medical advisor to the National Eczema Society, UK. Eric Simpson reports personal fees from AbbVie, Amgen, Arena Pharmaceuticals, Aslan Pharma, Boston Consulting Group, Collective Acumen LLC (CA), Dermira, Eli Lilly, Evidera, ExcerptaMedica, Forte Bio RX, Galderma, GlaxoSmithKline, Incyte, Janssen, Kyowa Kirin Pharmaceutical Development, Leo Pharm, Medscape LLC, Merck, Pfizer, Physicians World LLC, Regeneron, Roivant, Sanofi-Genzyme, Trevi therapeutics, Valeant, WebMD. Eric Simpson also reports grants (or Principal investigator role) from AbbVie, Amgen, Arcutis, Aslan, CorEvitas, Dermavant, Dermira, Eli Lilly, Incyte, Kymab, Kyowa Hakko Kirin, Leo Pharmaceuticals, Pfizer, Regeneron, Sanofi, and TARGET-DERM. These potential conflicts of interest have been reviewed and managed by OHSU. Robert Boyle received personal fees from Cochrane, Wiley, British Society of Allergy and Clinical Immunology for editorial work and fees for expert witness work, outside of the submitted work. Robert’s employing institution Imperial College London has a formal research and innovation partnership with Nestlé, who manufactures and markets nutritional products for managing food allergy and sponsor infant nutrition research related to eczema and food allergy. Hywel Williams was director of the NIHR Health Technology Assessment Programme from 2015 to 2020. Hywel Williams was a member of the HTA Antimicrobial Resistance Themed Call Board, HTA Commissioning Sub-Board, HTA Efficient Study Designs – 2, HTA Efficient Study Designs Board, HTA Funding Teleconference Member, HTA Surgery Themed Call Board, NIHR CTU Standing Advisory Committee, NIHR Journals Library Board, PGfAR EOIs – HTA projects Remit meeting, Pre-Exposure Prophylaxis Impact Review Panel, HTA Remit and Competitiveness Group, HTA General Committee, HTA Post-Funding Committee teleconference, HTA Funding Committee Policy Group and HTA Commissioning Committee. Hywel had no part in the decision-making for funding this study. All other authors declare no competing interests.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Publications
Bradshaw LE, Haines RH, Thomas KS, Chalmers JR, Irvine AD, Williams HC, Brown SJ. Clinical examination for hyperlinear palms to determine filaggrin genotype: a diagnostic test accuracy study. Clin Exp Allergy 2021;51:1421–28. https://doi.org/10.1111/cea.14025. Epub 18 October 2021. PMID: 34608691.
Bradshaw LE, Montgomery AA, Williams HC, Chalmers JR, Haines RH. Two-by-two factorial randomised study within a trial (SWAT) to evaluate strategies for follow-up in a randomised prevention trial. Trials 2020;21:529. https://doi.org/10.1186/s13063-020-04373-4. PMID: 32546180; PMCID: PMC7296963.
Bradshaw LE, Wyatt LA, Brown SJ, Haines RH, Montgomery AA, Perkin MR, et al. Emollients for prevention of atopic dermatitis: 5-year findings from the BEEP randomized trial. Allergy 2023;78:995–1006. https://doi.org/10.1111/all.15555. Epub 3 November 2022. PMID: 36263451.
Chalmers JR, Haines RH, Bradshaw LE, Montgomery AA, Thomas, KS, Brown SJ, et al. Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial. Lancet 2020;395:962–72. https://doi.org/10.1016/S0140-6736(19)32984-8
Chalmers JR, Haines RH, Mitchell EJ, Thomas KS, Brown SJ, Ridd M, et al. Effectiveness and cost-effectiveness of daily all-over-body application of emollient during the first year of life for preventing atopic eczema in high-risk children (The BEEP trial): protocol for a randomised controlled trial. BMC Trials 2017;18:343. https://doi.org/10.1186/s13063-017-2031-3
Kelleher MM, Cro S, Van Vogt E, Cornelius V, Lodrup Carlsen KC, Ove Skjerven H, et al. Skincare interventions in infants for preventing eczema and food allergy: a cochrane systematic review and individual participant data meta-analysis. Clin Exp Allergy 2021;51:402–18. https://doi.org/10.1111/cea.13847. Epub 25 February 2021. PMID: 33550675.
Overgaard LEK, Main KM, Frederiksen H, Stender S, Szecsi PB, Williams HC, Thyssen JP. Children with atopic dermatitis and frequent emollient use have increased urinary levels of low-molecular-weight phthalate metabolites and parabens. Allergy 2017;72:1768–77. https://doi.org/10.1111/all.13157. Epub 4 April 2017. PMID: 28281298.
Perkin MR, Logan K, Marrs T, Radulovic S, Craven J, Boyle RJ, et al.; EAT Study Team. Association of frequent moisturizer use in early infancy with the development of food allergy. J Allergy Clin Immunol 2021;147:967–76.e1. https://doi.org/10.1016/j.jaci.2020.10.044. PMID: 33678253.
Van Vogt E, Cro S, Cornelius VR, Williams HC, Askie LM, Phillips R, et al. Individual participant data meta-analysis versus aggregate data meta-analysis: a case study in eczema and food allergy prevention. Clin Exp Allergy 2021. https://doi.org/10.1111/cea.14085. Epub ahead of print. PMID: 34939249.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Chalmers JR, Haines RH, Bradshaw LE, Montgomery AA, Thomas KS, Brown SJ, et al. BEEP study team . Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial. Lancet 2020;395:962-72.
- Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis?. J Allergy Clin Immunol 2004;114:150-8.
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004;113:832-6.
- Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol 1994;131:406-16.
- Williams H. UK Diagnostic Criteria for Atopic Dermatitis – Training Manual n.d. https://nottingham.ac.uk/research/groups/cebd/resources/uk-diagnostic-criteria-for-atopic-dermatitis.aspx (accessed 1 September 2022).
- Simpson EL, Keck LE, Chalmers JR, Williams HC. How should an incident case of atopic dermatitis be defined? A systematic review of primary prevention studies. J Allergy Clin Immunol 2012;130:137-44.
- Endre KMA, Landrø L, LeBlanc M, Gjersvik P, Lødrup Carlsen KC, Haugen G, et al. Diagnosing atopic dermatitis in infancy using established diagnostic criteria: a cohort study. Br J Dermatol 2022;186:50-8.
- Williams HC. Atopic eczema – we should look to the environment. BMJ 1995;311.
- Williams HC, Strachan DP, Hay RJ. Childhood eczema: disease of the advantaged?. BMJ 1994;308:1132-5.
- Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups . Is eczema really on the increase worldwide?. J Allergy Clin Immunol 2008;121:947-54.e15.
- Petersen EBM, Skov L, Thyssen JP, Jensen P. Role of the gut microbiota in atopic dermatitis: a systematic review. Acta Derm Venereol 2019;99:5-11.
- Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol 2016;75:681-87.e11.
- Bosma AL, Ascott A, Iskandar R, Farquhar K, Matthewman J, Langendam MW, et al. Classifying atopic dermatitis: a systematic review of phenotypes and associated characteristics. J Eur Acad Dermatol Venereol 2022;36:807-19.
- Cork MJ, Danby SG, Ogg GS. Atopic dermatitis epidemiology and unmet need in the United Kingdom. J Dermatolog Treat 2020;31:801-9.
- Davidson WF, Leung DYM, Beck LA, Berin CM, Boguniewicz M, Busse WW, et al. Report from the National Institute of Allergy and Infectious Diseases workshop on ‘atopic dermatitis and the atopic march: mechanisms and interventions’. J Allergy Clin Immunol 2019;143:894-913.
- Yaneva M, Darlenski R. The link between atopic dermatitis and asthma-immunological imbalance and beyond. Asthma Res Pract 2021;7.
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. ISAAC Phase Three Study Group . Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009;124:1251-8.e23.
- Kan T, Tanaka A, Kanamoto M, Morioke S, Takahagi S, Hide M. Longitudinal prevalence of atopic dermatitis among freshmen at Hiroshima University between 2002 and 2019. J Dermatol 2022;49:724-8.
- Williams HC, Pembroke AC, Forsdyke H, Boodoo G, Hay RJ, Burney PG. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol 1995;32:212-7.
- Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis 2014;25:107-14.
- Blome C, Radtke MA, Eissing L, Augustin M. Quality of life in patients with atopic dermatitis: disease burden, measurement, and treatment benefit. Am J Clin Dermatol 2016;17:163-9.
- Hebert AA, Stingl G, Ho LK, Lynde C, Cappelleri JC, Tallman AM, et al. Patient impact and economic burden of mild-to-moderate atopic dermatitis. Curr Med Res Opin 2018;34:2177-85.
- National Institute for Health and Care Excellence (NICE) Eczema Clinical Knowledge Summary n.d. https://cks.nice.org.uk/topics/eczema-atopic/ (accessed 1 August 2022).
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004;140:1513-9.
- Charman CR, Venn AJ, Ravenscroft JC, Williams HC. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol 2013;169:1326-32.
- Drucker AM, Morra DE, Prieto-Merino D, Ellis AG, Yiu ZZN, Rochwerg B, et al. Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatology 2022;158:523-32.
- Wise J. NICE recommends three more treatments for atopic dermatitis. BMJ 2022;377.
- EczemaCareOnline . Eczema Care Online Tool Kit n.d. https://eczemacareonline.org.uk/en?language_set=1 (accessed 31 July 2022).
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020;396:345-60.
- Williams HC. Textbook of Pediatric Dermatology. Oxford: Blackwell Scientific; 2000.
- Williams H. Rook’s Textbook of Dermatology. Oxford: Wiley Blackwell; 2016.
- Williams HC, Chalmers J. Prevention of atopic dermatitis. Acta Derm Venereol 2020;100.
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006;38:441-6.
- Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol 2012;132:751-62.
- Kim BE, Leung DYM. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol Res 2018;10:207-15.
- Tang TS, Bieber T, Williams HC. Does ‘autoreactivity’ play a role in atopic dermatitis?. J Allergy Clin Immunol 2012;129:1209-1215.e2.
- Madan I, Parsons V, Ntani G, Coggon D, Wright A, English J, et al. A behaviour change package to prevent hand dermatitis in nurses working in the National Health Service: results of a cluster randomized controlled trial. Br J Dermatol 2020;183:462-70.
- Edwards WH, Conner JM, Soll RF. Vermont Oxford Network Neonatal Skin Care Study Group . The effect of prophylactic ointment therapy on nosocomial sepsis rates and skin integrity in infants with birth weights of 501 to 1000 g. Pediatrics 2004;113:1195-203.
- van Zuuren EJ, Fedorowicz Z, Christensen R, Lavrijsen A, Arents BWM. Emollients and moisturisers for eczema. Cochrane Database Syst Rev 2017;2.
- Simpson EL, Berry TM, Brown PA, Hanifin JM. A pilot study of emollient therapy for the primary prevention of atopic dermatitis. J Am Acad Dermatol 2010;63:587-93.
- Macharia WM, Anabwani GM, Owili DM. Effects of skin contactants on evolution of atopic dermatitis in children: a case control study. Trop Doct 1991;21:104-6.
- Thomas KS, Batchelor JM, Bath-Hextall F, Chalmers JR, Clarke T, Crowe S, et al. A Programme of Research to Set Priorities and Reduce Uncertainties for the Prevention and Treatment of Skin Disease. Southampton (UK): NIHR Journals Library; 2016.
- Cork MJ, Danby S. Aqueous cream damages the skin barrier. Br J Dermatol 2011;164:1179-80.
- Danby SG, Chalmers J, Brown K, Williams HC, Cork MJ. A functional mechanistic study of the effect of emollients on the structure and function of the skin barrier. Br J Dermatol 2016;175:1011-9.
- Horimukai K, Morita K, Narita M, Kondo M, Kitazawa H, Nozaki M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol 2014;134:824-30.e6.
- Chalmers JR, Haines RH, Mitchell EJ, Thomas KS, Brown SJ, Ridd M, et al. Effectiveness and cost-effectiveness of daily all-over-body application of emollient during the first year of life for preventing atopic eczema in high-risk children (The BEEP trial): protocol for a randomised controlled trial. Trials 2017;18.
- NIHR Funding and Awards . A Randomised Controlled Trial to Determine Whether Skin Barrier Enhancement With Emollients Can Prevent Eczema in High Risk Children (12 67 12) n.d. https://fundingawards.nihr.ac.uk/award/12/67/12 (accessed 1 September 2022).
- SWAT 25 . Two-by-Two Factorial Randomised Trial to Evaluate Strategies to Improve Follow-up in a Randomised Prevention Trial n.d. https://qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,976688,en.pdf (accessed 5 September 2022).
- Barbier N, Paul C, Luger T, Allen R, De Prost Y, Papp K, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol 2004;150:96-102.
- Stevens K. Valuation of the Child Health Utility 9D Index. PharmacoEconomics 2012;30:729-47.
- Khan KA, Petrou S, Rivero-Arias O, Walters SJ, Boyle SE. Mapping EQ-5D utility scores from the PedsQL™ generic core scales. PharmacoEconomics 2014;32:693-706.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol 2001;144:104-10.
- Postnatal Care n.d. www.nice.org.uk/guidance/ng194 (accessed 3 January 2024).
- Holme N, Boullier L, Harrison C. Postnatal care: a neonatal perspective. Arch Dis Child Educ Pract Ed. 2016;101:136-8.
- BSACI Standard Operating Procedure: Paediatric Allergy Skin Prick Testing n.d. https://bsaci.org/wp-content/uploads/2019/12/paedSPTnew.pdf (accessed 5 September 2022).
- Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol 2015;172:1353-7.
- Sampson HA, van Wijk RG, Bindslev-Jensen C, Sicherer S, Teuber SS, Burks AW, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma and Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol 2012;130:1260-74.
- Grabenhenrich LB, Reich A, Bellach J, Trendelenburg V, Sprikkelman AB, Roberts G, et al. A new framework for the documentation and interpretation of oral food challenges in population-based and clinical research. Allergy 2017;72:453-61.
- Kelleher MM, Jay N, Perkin MR, Haines RH, Batt R, Bradshaw LE, et al. An algorithm for diagnosing IgE-mediated food allergy in study participants who do not undergo food challenge. Clin Exp Allergy 2020;50:334-42.
- Paternoster L, Savenije OEM, Heron J, Evans DM, Vonk JM, Brunekreef B, et al. Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J Allergy Clin Immunol 2018;141:964-71.
- The BEEP Study, Participant Newsletter – Results Edition 2019. https://nottingham.ac.uk/research/groups/cebd/documents/researchdocs/beep-parentsresultsnewsletterweb.pdf (accessed 17 May 2022).
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99.
- Shrier I, Steele RJ, Verhagen E, Herbert R, Riddell CA, Kaufman JS. Beyond intention to treat: what is the right question?. Clin Trials 2014;11:28-37.
- Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res 2005;14:369-95.
- Carpenter JR, Kenward MG. Missing Data in Randomised Controlled Trials: A Practical Guide. Health Technology Assessment Methodology Programme. Birmingham; 2007.
- Norton EC, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. Stata J 2013;13:492-509.
- Cro S, Morris TP, Kenward MG, Carpenter JR. Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: a practical guide. Stat Med 2020;39:2815-42.
- Raftery J, Williams H, Clarke A, Thornton J, Norrie J, Snooks H, et al. ‘Not clinically effective but cost-effective’ – paradoxical conclusions in randomised controlled trials with ‘doubly null’ results: a cross-sectional study. BMJ Open 2020;10.
- Xu S, Immaneni S, Hazen GB, Silverberg JI, Paller AS, Lio PA. Cost-effectiveness of prophylactic moisturization for atopic dermatitis. JAMA Pediatrics 2017;171:e163909-09.
- Sach TH, McManus E, Levell NJ. Economic evidence for the prevention and treatment of atopic eczema. Br J Dermatol 2019;181.
- McManus E, Sach T, Levell N. The use of decision-analytic models in atopic eczema: a systematic review and critical appraisal. PharmacoEconomics 2018;36:51-66.
- Griebsch I, Coast J, Brown J. Quality-adjusted life-years lack quality in pediatric care: a critical review of published cost-utility studies in child health. Pediatrics 2005;115:e600-14.
- Petrou S. Methodological issues raised by preference-based approaches to measuring the health status of children. Health Econ 2003;12:697-702.
- Petrou S, Gray R. Methodological challenges posed by economic evaluations of early childhood intervention programmes. Appl Health Econ Health Policy 2005;4:175-81.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR good research practices task force report. Value Health 2015;18:161-72.
- Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. Handbooks in Health Economic Evaluation. Oxford: Oxford University Press; 2014.
- Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value Health 2022;25:10-31.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/chapter/the-reference-case (accessed 26 July 2018).
- Drummond M, Sculpher M, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- NICE Health Technology Evaluations: The Manual 2022. www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation (accessed 5 September 2022).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15.
- Hernandez Alava M, Pudney S, Wailoo A. The EQ-5D-5L value set for England: findings of a quality assurance program. Value Health 2020;23:642-8.
- Hernandez Alava M, Wailoo A, Pudney S. Methods for Mapping Between the EQ 5D 5L and the 3L. NICE Decision Support Unit report. 2017.
- NHS Business Services Authority. Prescription Cost Analysis. https://opendata.nhsbsa.net/dataset/prescription-cost-analysis-pca-annual-statistics (accessed 19 July 2022).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96.
- Scope A, Bhadhuri A, Pennington B. Systematic review of cost-utility analyses that have included carer and family member health-related quality of life. Value Health 2022;25:1644-53. https://doi.org/10.1016/j.jval.2022.02.008.
- Gallacher D. HEABS: Stata Module to Calculate the ICER and Net Benefit for up to Two Datasets n.d. https://EconPapers.repec.org/RePEc:boc:bocode:s458438 (accessed 2 September 2022).
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75.
- NHS England . NHS Schedule of Reference Costs: 2019–20 2021. https://england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/ (accessed 1 September 2022).
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70.
- Curtis L, Burns A. Personal Social Services Research Unit, in Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit, University of Kent; 2020.
- Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014;134:818-23.
- Canaway AG, Frew EJ. Measuring preference-based quality of life in children aged 6–7 years: a comparison of the performance of the CHU-9D and EQ-5D-Y – the WAVES pilot study. Qual Life Res 2013;22:173-83.
- Sheffield Clinical Trials Research Unit, Study Protocol, The MAGIC Trial (Melatonin for Anxiety Prior to General Anaesthesia In Children): A Multicentre, Parallel Randomised Controlled Trial of Melatonin Versus Midazolam in the Premedication of Anxious Children Attending for Elective Surgery Under General Anaesthesia, National Institute for Health Research, Version 4.1 n.d. https://www.sheffield.ac.uk/scharr/research/centres/ctru/magic (accessed 25 July 2022).
- Bray N, Noyes J, Harris N, Edwards RT. Measuring the health-related quality of life of children with impaired mobility: examining correlation and agreement between children and parent proxies. BMC Res Notes 2017;10.
- Kalliomäki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol 2007;119:1019-21.
- Papapostolou N, Xepapadaki P, Gregoriou S, Makris M. Atopic dermatitis and food allergy: a complex interplay what we know and what we would like to learn. J Clin Med 2022;11.
- Tsakok T, Marrs T, Mohsin M, Baron S, du Toit G, Till S, et al. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol 2016;137:1071-8.
- Perkin MR, Logan K, Marrs T, Radulovic S, Craven J, Boyle RJ, et al. EAT Study Team . Association of frequent moisturizer use in early infancy with the development of food allergy. J Allergy Clin Immunol 2021;147:967-76.e1.
- Walker MT, Green JE, Ferrie RP, Queener AM, Kaplan MH, Cook-Mills JM. Mechanism for initiation of food allergy: dependence on skin barrier mutations and environmental allergen costimulation. J Allergy Clin Immunol 2018;141:1711-25.e9.
- Flohr C. TRANS-FOODS: Reducing the risk of developing peanut allergy through the skin by improved understanding, modifying peanut snack production, and adjusting skincare practices. ISRCTN 2023. www.isrctn.com/ISRCTN12492694 (accessed 25 September 2023).
- Office for National Statistics . Population Estimates by Ethnic Group and Religion, England and Wales 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/populationestimatesbyethnicgroupandreligionenglandandwales/2019 (accessed 5 September 2022).
- Finlay AY, Griffiths TW, Belmo S, Chowdhury MMU. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol 2021;185:1240-1.
- Hanifin JM, Baghoomian W, Grinich E, Leshem YA, Jacobson M, Simpson EL. The Eczema Area and Severity Index – a practical guide. Dermatitis 2022;33:187-92.
- Centre of Evidence Based Dermatology, University of Nottingham, Test of Your Ability to Diagnose Visible Flexural Dermatitis n.d. https://nottingham.ac.uk/~mzzfaq/dermatology/eczema/TestFotos.html (accessed 8 Jan 2023).
- Thawer-Esmail F, Jakasa I, Todd G, Wen Y, Brown SJ, Kroboth K, et al. South African amaXhosa patients with atopic dermatitis have decreased levels of filaggrin breakdown products but no loss-of-function mutations in filaggrin. J Allergy Clin Immunol 2014;133:280-2.e1.e1.
- Chen H, Common JE, Haines RL, Balakrishnan A, Brown SJ, Goh CS, et al. Wide spectrum of filaggrin-null mutations in atopic dermatitis highlights differences between Singaporean Chinese and European populations. Br J Dermatol 2011;165:106-14.
- Skjerven HO, Rehbinder EM, Vettukattil R, LeBlanc M, Granum B, Haugen G, et al. Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): a factorial, multicentre, cluster-randomised trial. Lancet 2020;395:951-61.
- Kelleher MM, Cro S, Cornelius V, Lodrup Carlsen KC, Skjerven HO, Rehbinder EM, et al. Skin care interventions in infants for preventing eczema and food allergy. Cochrane Database Syst Rev 2021;2.
- Van Vogt E, Cro S, Cornelius VR, Williams HC, Askie LM, Phillips R, et al. Individual participant data meta-analysis versus aggregate data meta-analysis: a case study in eczema and food allergy prevention. Clin Exp Allergy 2022;52:628-45.
- Zhong Y, Samuel M, van Bever H, Tham EH. Emollients in infancy to prevent atopic dermatitis: a systematic review and meta-analysis. Allergy 2022;77:1685-99.
- Kelleher MM, Cro S, Phillips R, Williams HC, Lowe AJ, Boyle RJ. Correspondence to ‘Emollients in infancy to prevent atopic dermatitis: a systematic review and meta-analysis’. Allergy 2022;77:1931-3.
- Skjerven HO, Lie A, Vettukattil R, Rehbinder EM, LeBlanc M, Asarnoj A, et al. Early food intervention and skin emollients to prevent food allergy in young children (PreventADALL): a factorial, multicentre, cluster-randomised trial. Lancet 2022;399:2398-411.
- Danby SG, Al-Enezi T, Sultan A, Chittock J, Kennedy K, Cork MJ. The effect of aqueous cream BP on the skin barrier in volunteers with a previous history of atopic dermatitis. Br J Dermatol 2011;165:329-34.
- Pittet LF, Messina NL, Gardiner K, Freyne B, Abruzzo V, Morrison C, et al. Discordance between diagnosis tools for assessing eczema in infants: a challenge for intervention trials. Dermatitis 2022;33:207-14.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350.
- Bradshaw LE, Montgomery AA, Williams HC, Chalmers JR, Haines RH. Two-by-two factorial randomised study within a trial (SWAT) to evaluate strategies for follow-up in a randomised prevention trial. Trials 2020;21.
- Bradshaw LE, Haines RH, Thomas KS, Chalmers JR, Irvine AD, Williams HC, et al. Clinical examination for hyperlinear palms to determine filaggrin genotype: a diagnostic test accuracy study. Clin Exp Allergy 2021;51:1421-8.
- Danby SG, Andrew PV, Taylor RN, Kay LJ, Chittock J, Pinnock A, et al. Different types of emollient cream exhibit diverse physiological effects on the skin barrier in adults with atopic dermatitis. Clin Exp Dermatol 2022;47:1154-64. https://doi.org/10.1111/ced.15141.
- Chaoimh CN, Lad D, Nico C, Puppels GJ, Wong XFCC, Common JE, et al. Early initiation of short-term emollient use for the prevention of atopic dermatitis in high-risk infants – The STOP-AD randomised controlled trial. Allergy 2023;78:984-94.
- Fleming S, Bodner C, Devereux G, Russell G, Campbell D, Godden D, et al. An application of the United Kingdom Working Party diagnostic criteria for atopic dermatitis in Scottish infants. J Invest Dermatol 2001;117:1526-30.
- Treweek S, Banister K, Bower P, Cotton S, Devane D, Gardner HR, et al. Developing the INCLUDE Ethnicity Framework – a tool to help trialists design trials that better reflect the communities they serve. Trials 2021;22.
- van Zuuren EJ, Fedorowicz Z, Arents BWM. Emollients and moisturizers for eczema: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol 2017;177:1256-71.
- Ridd MJ, Santer M, MacNeill SJ, Sanderson E, Wells S, Webb D, et al. Effectiveness and safety of lotion, cream, gel, and ointment emollients for childhood eczema: a pragmatic, randomised, phase 4, superiority trial. Lancet Child Adolesc Health 2022;6:522-32.
- Eichner B, Michaels LAC, Branca K, Ramsey K, Mitchell J, Morris CD, et al. A community-based assessment of skin care, allergies, and eczema (CASCADE): an atopic dermatitis primary prevention study using emollients-protocol for a randomized controlled trial. Trials 2020;21.
- Jabbar-Lopez ZK, Ezzamouri B, Briley A, Greenblatt D, Gurung N, Chalmers JR, et al. Randomized controlled pilot trial with ion-exchange water softeners to prevent eczema (SOFTER trial). Clin Exp Allergy 2022;52:405-15.
- Brown SJ. Atopic eczema: how genetic studies can contribute to the understanding of this complex trait. J Invest Dermatol 2022;142:1015-9.
- Garcia-Larsen V, Ierodiakonou D, Jarrold K, Cunha S, Chivinge J, Robinson Z, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PLOS Med 2018;15.
- Kelleher MM, Cro S, Van Vogt E, Cornelius V, Lodrup Carlsen KC, Ove Skjerven H, et al. Skincare interventions in infants for preventing eczema and food allergy: a cochrane systematic review and individual participant data meta-analysis. Clin Exp Allergy 2021;51:402-18.
Appendix 1 Distribution of primary and secondary care recruitment sites
| Secondary care recruiting sites | Principal investigator | Recruiting staff |
|---|---|---|
| Nottingham University Hospitals NHS Trusta | Prof. Hywel Williams | Susan Davies-Jones, Jim Thornton, Barbara Maston, Victoria Maddox, Faye Shelton, Catherine Thorne |
| Portsmouth Hospital NHS Trust | Dr Bronwyn Hughes | Andrew Gribbin, Sharon McCready, Zoe Garner, Amanda Hungate, Emma Glasspool, Rachel Watson |
| Harrogate and District NHS Foundation Trust | Dr Alison Layton | Louise Wills, Elizabeth Marshall, Joyce Guy, Christine Morgan |
| Sherwood Forest Hospitals NHS Foundationa | Dr Michael Yanney | Caroline Moulds, Lisa Foster, Yvette Girvan, Victoria Moore, Andrea Palfreman |
| Burton Hospitals NHS Foundation Trust | Dr Mansoor Ahmed | Stephanie Boswell, Claire Prince, Jane Radford, Clare Mewies, Claire Backhouse, Elizabeth Kemp |
| Derby Hospitals NHS Foundation Trusta | Dr Adam Ferguson | Elaine Coulborn, Melody McGregor, Coral Smith, Vanessa Unsworth, SallyAnn Bell, Jill Smith, Liane Hufton |
| University Hospitals of Leicester NHS Trusta | Dr Karen Harman | Dr Ingrid Helbling, Suzanne Foxon, Simal Patel, Jackie Philps, Esther Rook |
| York Teaching Hospital NHS Foundation Trust | Dr Calum Lyon | Anna Clayton, Jill Green, Jessica Scott, Richard Furnival, Samantha Roche, Holly Alcock, Sian Sturdy |
| Sheffield Teaching Hospitals NHS Foundation Trusta | Prof. Michael Cork | Heather Chisem, Hilary Rosser, Alyson Barber, Sarah Besley, Emma Steel, Sarah Senbeto, Pauline Bayliss, Carolyn Clark |
| Imperial College Healthcare NHS Trusta | Dr Robert Boyle | Anna Bosanquet, Batia Gourin |
| Guy’s and St Thomas’s NHS Foundation Trust | Dr Carsten Flohr | Nikeeta Gurung, Annette Briley, Claire Singh, Rebecca Williams, Shelley Carter, Elodie Lawley |
| University of Bristola | Dr Matthew Ridd | Kingsley Powell, Lyn Liddiard, Anna Gilberston |
| Primary care recruiting sites | ||
| Francis Grove Surgery | Dr Katharine Broad | Nina Walters, Sarah Buttinger, Rachel Joy |
| Streatham Common Group Practice | Dr Kirsty Rankin | Dr Ruth Robinson, Ellen Trendell |
| Clapham Park Group Practice | Dr Mydhili Chellappah | Dr Dina Saleh |
| The Park Group Practice | Dr Mita Patel | Jayshireen Singh |
FIGURE 12.
Geographical distribution of recruitment sites within the UK.

Appendix 2 Summary of changes to the protocol after the start of the trial
| Protocol | Date | Summary of changes |
|---|---|---|
| V2.1a | 15 October 2014 | Clarification on inclusion and exclusion. |
| V2.2 | 13 November 2014 | Updates include eligibility, both inclusion and exclusion criteria, details about the Skin Care Video, information to be collected 2 weeks post randomisation, questionnaire collection and timing. |
| V3.0 | 12 October 2015 | Recruiting sites and PICs send text message to potential participants informing them of the trial and where to get further information. At the 2-week follow-up, the co-ordinating centre would like to text participants that have not responded to phone calls and/or e-mails. Addition to the inclusion criteria which states that mothers must be aged ≥ 16 years. |
| V4.0 | 20 May 2016 | Addition of food allergy outcome and tests, including SPT at 24 months’ visit and the option of a Food Challenge after 24-month visit |
| V5.0 | 26 October 2016 | Sample size revision proposed by TSC. Protocol and Information sheet updates to clarify procedures and safety around food allergy testing, and an additional letter for nurses to inform GP’s that the child was seen for a 24-month visit. |
| V6.0 | 2 August 2017 | Documents amended to attempt to increase retention rate at 24 months’ visit |
| V6.1 | 8 November 2017 | Update to the BEEP Trial SPT Working Practice Document and clarification and added completion instructions on 36-, 48- and 60-month questionnaires. |
| V6.2 | 8 October 2019 | Update to wording in the long-term follow-up questionnaire reminder e-mails and letters, now that the results of the main BEEP study are known. Wording has been amended to reflect the results and other minor wording updates. Addition of tertiary end point that will be collected in the 60-month follow-up questionnaire. |
| V7.0 | 26 February 2021 | Update to tertiary outcomes to clarify that eczema diagnosis will be analysed at 36, 48 and 60 months. Addition of a further verification of medical records: parental report of food allergy data highlighted discrepancies requiring further verification of the medical records. Two new letters created to give parents the option to opt out of this further level of data verification, and a second to request the records from the GP, if the parent does not opt out. Update to the location of the long-term storage of the collected saliva samples: Professor Sarah Brown changed affiliation, which required the samples to be moved to her new lab, in order to ensure their continued safe storage. Update to the letters and e-mails that parents receive with the last questionnaire at 60 months: in an attempt to further increase completion rates. |
Appendix 3 Additional results tables
| Site | Intervention (n = 693) | Control (n = 701) | Total (n = 1394) |
|---|---|---|---|
| Sheffield | 103 (15%) | 102 (15%) | 205 (15%) |
| Imperial College | 97 (14%) | 97 (14%) | 194 (14%) |
| Nottingham | 95 (14%) | 94 (13%) | 189 (14%) |
| Burton | 93 (13%) | 95 (14%) | 188 (13%) |
| Derby | 54 (8%) | 54 (8%) | 108 (8%) |
| Portsmouth | 47 (7%) | 48 (7%) | 95 (7%) |
| King’s Mill | 43 (6%) | 46 (7%) | 89 (6%) |
| Guy’s and St Thomas | 38 (5%) | 39 (6%) | 77 (6%) |
| Leicester | 36 (5%) | 36 (5%) | 72 (5%) |
| Harrogate | 34 (5%) | 34 (5%) | 68 (5%) |
| Bristol GP surgeries | 27 (4%) | 27 (4%) | 54 (4%) |
| York | 19 (3%) | 19 (3%) | 38 (3%) |
| London GP surgeries | 7 (1%) | 10 (1%) | 17 (1%) |
| Mother’s ethnicity | Father’s ethnicity | Total number | ||
|---|---|---|---|---|
| White | Black Caribbean | Other Asiana | ||
| White | 0 | 2 | 1 | 3 |
| Indian | 1 | 0 | 0 | 1 |
| Other Asiana | 0 | 0 | 1 | 1 |
| Mixed race | 1 | 0 | 0 | 1 |
| Total number | 2 | 2 | 2 | 6 |
| Mother’s ethnicity | n (%) |
|---|---|
| Whitea | 36 (23%) |
| Indian | 20 (13%) |
| Pakistani | 12 (8%) |
| Bangladeshi | 1 (1%) |
| Black Caribbean | 9 (6%) |
| Black African | 13 (8%) |
| Black (other) | 3 (2%) |
| Chinese | 12 (8%) |
| Other Asian (non-Chinese) | 11 (7%) |
| Mixed race | 22 (14%) |
| Other | 16 (10%) |
| Total | 155 |
| Intervention – no primary outcome (n = 95) | Intervention – primary outcome collected (n = 598) | Control – no primary outcome (n = 89) | Control – primary outcome collected (n = 612) | |
|---|---|---|---|---|
| Screening visit | ||||
| Prior to birth | 47 (49%) | 382 (64%) | 41 (46%) | 398 (65%) |
| After birth | 48 (51%) | 216 (36%) | 48 (54%) | 214 (35%) |
| Age of mother at infant randomisation | ||||
| Mean (SD) | 29.6 (6.6) | 32.1 (5.0) | 28.8 (5.6) | 31.9 (5.0) |
| Minimum, maximum | 16, 43 | 18, 45 | 20, 39 | 18, 46 |
| Ethnicity of mother | ||||
| White | 78 (82%) | 511 (85%) | 77 (87%) | 524 (86%) |
| Asian | 7 (7%) | 38 (6%) | 4 (4%) | 36 (6%) |
| Black | 4 (4%) | 27 (5%) | 5 (6%) | 17 (3%) |
| Other | 6 (6%) | 22 (4%) | 3 (3%) | 35 (6%) |
| Mother took oral antibiotics during pregnancy | ||||
| No | 56 (59%) | 418 (70%) | 62 (70%) | 435 (71%) |
| Yes | 37 (39%) | 173 (29%) | 27 (30%) | 174 (28%) |
| Not known | 2 (2%) | 7 (1%) | – | 3 (< 0.5%) |
| Total number of first-degree relatives with atopic disease | ||||
| 1 | 31 (33%) | 223 (37%) | 30 (34%) | 223 (36%) |
| 2 | 48 (51%) | 252 (42%) | 37 (42%) | 259 (42%) |
| 3 or more | 16 (17%) | 123 (21%) | 22 (25%) | 130 (21%) |
| Total number of first-degree relatives with history of eczema | ||||
| 0 | 20 (21%) | 110 (18%) | 21 (24%) | 100 (16%) |
| 1 | 35 (37%) | 279 (47%) | 42 (47%) | 310 (51%) |
| 2 or more | 40 (42%) | 209 (35%) | 26 (29%) | 202 (33%) |
| Boy | 58 (61%) | 316 (53%) | 39 (44%) | 320 (52%) |
| Girl | 37 (39%) | 282 (47%) | 50 (56%) | 292 (48%) |
| Number of other children in household at screening (including non-full blood siblings) | ||||
| 0 | 33 (35%) | 242 (40%) | 28 (31%) | 265 (43%) |
| 1 | 34 (36%) | 252 (42%) | 35 (39%) | 236 (39%) |
| 2 | 20 (21%) | 75 (13%) | 15 (17%) | 81 (13%) |
| 3 or more | 8 (8%) | 29 (5%) | 11 (12%) | 30 (5%) |
| Delivery method | ||||
| Vaginal delivery | 62 (65%) | 420 (70%) | 63 (71%) | 409 (67%) |
| Caesarean section | 33 (35%) | 178 (30%) | 26 (29%) | 203 (33%) |
| Days between birth and randomisation | ||||
| Median (25th, 75th centile) | 2 (1, 8) | 4 (1, 9) | 4 (1, 9) | 3 (1, 9) |
| Note that the questionnaires asked about applying moisturisers to the baby’s skin or used oil for baby massage | |
| a. All children | |
| Control | |
| 3 months | |
| Applied moisturiser to baby’s skin or used oil for baby massage at least 3 days per week over most or all of the child’s body during the past 3 months | |
| No | 407 (79%) |
| Yes | 110 (21%) |
| n | 517 |
| 6 months | |
| Applied moisturiser to baby’s skin or used oil for baby massage at least 3 days per week over most or all of the child’s body during the past 3 months | |
| No | 370 (72%) |
| Yes | 143 (28%) |
| n | 513 |
| 12 months | |
| Applied moisturiser to baby’s skin or used oil for baby massage at least 3 days per week over most or all of the child’s body during the past 6 months | |
| No | 376 (72%) |
| Yes | 144 (28%) |
| n | 520 |
| b. Excluding children with eczema (i.e. those that may have been using emollients for treating eczema) | |
| 3-month questionnaire and no reported eczema | n = 457 |
| Applied moisturiser to baby’s skin or used oil for baby massage at least 3 days per week over most or all of the child’s body during the past 3 months | |
| No | 375 (82%) |
| Yes | 82 (18%) |
| 6-month questionnaire and no reported eczema at 3 or 6 months | n = 372 |
| Applied moisturiser to baby’s skin or used oil for baby massage at least 3 days per week over most or all of the child’s body during the past 3 months | |
| No | 310 (83%) |
| Yes | 62 (17%) |
| 12-month questionnaire and no reported eczema at 3, 6 or 12 months | n = 324 |
| Applied moisturiser to baby’s skin or used oil for baby massage at least 3 days per week over most or all of the child’s body during the past 6 months | |
| No | 275 (85%) |
| Yes | 49 (15%) |
| Intervention | Control | |
|---|---|---|
| Child ever eaten food containing cow’s milk | ||
| No | 6 (1%) | 5 (1%) |
| Yes | 569 (99%) | 593 (99%) |
| n | 575 | 598 |
| Had at least 60 ml of cow’s milk in last 3 months | ||
| No | 40 (7%) | 33 (6%) |
| Yes | 512 (93%) | 539 (94%) |
| na | 552 | 572 |
| Had at least 60 ml of cow’s milk more than 3 times | ||
| No | 22 (4%) | 23 (4%) |
| Yes | 530 (96%) | 549 (96%) |
| na | 552 | 572 |
| Age in months the first time they had food containing cow’s milk | ||
| Median (25th, 75th centile) | 6 (1, 7) | 6 (1, 7) |
| Minimum, maximum | 0, 22 | 0, 22 |
| n | 566 | 591 |
| Before 4 months | 189 (33%) | 212 (36%) |
| Between 4 and 6 months | 233 (41%) | 224 (38%) |
| Between 7 and 12 months | 129 (23%) | 144 (24%) |
| Between 13 and 24 months | 15 (3%) | 11 (2%) |
| After 24 months/never eaten | 6 (1%) | 5 (1%) |
| n | 572 | 596 |
| Child ever eaten food containing egg | ||
| No | 10 (2%) | 8 (1%) |
| Yes | 565 (98%) | 590 (99%) |
| n | 575 | 598 |
| Had at least four teaspoons of runny egg in last 3 months | ||
| No | 240 (44%) | 238 (42%) |
| Yes | 307 (56%) | 333 (58%) |
| na | 547 | 571 |
| Had at least four teaspoons of runny egg more than three times | ||
| No | 223 (41%) | 213 (37%) |
| Yes | 325 (59%) | 358 (63%) |
| na | 548 | 571 |
| Age in months the first time they had food containing egg | ||
| Median (25th, 75th centile) | 7 (6, 9) | 7 (6, 9) |
| Minimum, maximum | 4, 21 | 1, 24 |
| n | 562 | 587 |
| Before 4 months | – | 1 (< 0.5%) |
| Between 4 and 6 months | 251 (44%) | 244 (41%) |
| Between 7 and 12 months | 284 (50%) | 319 (54%) |
| Between 13 and 24 months | 27 (5%) | 23 (4%) |
| After 24 months/never eaten | 10 (2%) | 8 (1%) |
| n | 572 | 595 |
| Child ever eaten food containing peanut | ||
| No | 138 (24%) | 146 (24%) |
| Yes | 436 (76%) | 452 (76%) |
| n | 574 | 598 |
| Had equivalent of one teaspoon of peanut butter in last month | ||
| No | 345 (62%) | 363 (63%) |
| Yes | 213 (38%) | 215 (37%) |
| na | 558 | 578 |
| Had equivalent of one teaspoon of peanut butter more than three times | ||
| No | 246 (44%) | 271 (47%) |
| Yes | 312 (56%) | 307 (53%) |
| na | 558 | 578 |
| Age in months the first time they had food containing peanut | ||
| Median (25th, 75th centile) | 12 (8, 14) | 12 (8, 14) |
| Minimum, maximum | 3, 24 | 3, 25 |
| n | 434 | 450 |
| Before 4 months | 1 (< 0.5%) | 1 (< 0.5%) |
| Between 4 and 6 months | 62 (11%) | 63 (11%) |
| Between 7 and 12 months | 245 (43%) | 251 (42%) |
| Between 13 and 24 months | 126 (22%) | 134 (22%) |
| After 24 months/never eaten | 138 (24%) | 147 (25%) |
| n | 572 | 596 |
| Child ever eaten food containing nuts other than peanut | ||
| No | 109 (19%) | 112 (19%) |
| Yes | 465 (81%) | 485 (81%) |
| n | 574 | 597 |
| Age in months the first time they had food containing nuts other than peanut | ||
| Median (25th, 75th centile) | 12 (10, 18) | 12 (12, 18) |
| Minimum, maximum | 4, 26 | 4, 24 |
| n | 462 | 484 |
| Before 4 months | – | – |
| Between 4 and 6 months | 36 (6%) | 35 (6%) |
| Between 7 and 12 months | 278 (49%) | 261 (44%) |
| Between 13 and 24 months | 147 (26%) | 188 (32%) |
| After 24 months/never eaten | 110 (19%) | 112 (19%) |
| n | 571 | 596 |
| Intervention | Control | Adjusted interaction effect (RR) (95% CI) | Adjusted interaction effect (risk difference) (95% CI) | |
|---|---|---|---|---|
| Number of first-degree relatives with atopic disease | ||||
| 1 | 51/223 (23%) | 46/223 (21%) | ||
| 2 | 53/252 (21%) | 65/259 (25%) | 0.76 (0.47 to 1.22) | −6.3% (−16.9% to 4.3%) |
| 3 or more | 35/123 (28%) | 39/130 (30%) | 0.86 (0.51 to 1.44) | −3.8% (−17.4% to 9.8%) |
| Number of first-degree relatives with history of eczema | ||||
| 0 | 19/110 (17%) | 16/100 (16%) | ||
| 1 | 69/279 (25%) | 75/310 (24%) | 0.96 (0.49 to 1.88) | −0.5% (−12.7% to 11.7%) |
| 2 or more | 51/209 (24%) | 59/202 (29%) | 0.78 (0.39 to 1.55) | −5.9% (−19.0% to 7.3%) |
| FLG genotype for children with mother and father of white ethnicity and children of other ethnicity with mutation a | ||||
| +/+ (no mutations) | 66/339 (19%) | 79/352 (22%) | ||
| +/− (one FLG null mutation) | 22/62 (35%) | 20/60 (33%) | 1.20 (0.70 to 2.09) | 5.1% (−12.6% to 22.9%) |
| −/− (two FLG null mutations) | 1/1 (100%) | 1/2 (50%) | ||
| Season of birth | ||||
| Spring | 47/183 (26%) | 38/169 (22%) | ||
| Summer | 43/172 (25%) | 51/185 (28%) | 0.82 (0.49 to 1.37) | −6.1% (−18.8% to 6.6%) |
| Autumn | 30/137 (22%) | 35/156 (22%) | 0.87 (0.49 to 1.54) | −4.0% (−17.0% to 9.0%) |
| Winter | 19/106 (18%) | 26/102 (25%) | 0.62 (0.32 to 1.18) | −11.0% (−25.2% to 3.1%) |
| Water hardness | ||||
| Soft/moderate | 66/270 (24%) | 60/274 (22%) | ||
| Hard/very hard | 71/323 (22%) | 90/334 (27%) | 0.76 (0.50 to 1.14) | −7.0% (−16.6% to 2.6%) |
| Parental report of regular use of probiotic supplements during pregnancy | ||||
| No | 107/457 (23%) | 121/463 (26%) | ||
| Yes | 9/31 (29%) | 9/30 (30%) | 1.01 (0.45 to 2.27) | 0% (−23.6% to 23.5%) |
FIGURE 13.
Time to onset of eczema based on first parental report of a topical corticosteroid and/or immunosuppressant prescription for eczema AND a parental report of a clinical diagnosis of eczema.

| Intervention | Control | |
|---|---|---|
| Cow’s milk | ||
| Not allergic based on parental report and/or SPT | 544 (94%) | 570 (95%) |
| Not allergic confirmed by OFC | 4 (1%) | 1 (< 0.5%) |
| Allergic confirmed by OFC | 1 (< 0.5%) | – |
| Allergic by panel consensus | 8 (1%) | 8 (1%) |
| Not allergic by panel consensus | 14 (2%) | 14 (2%) |
| Unclear – possible food allergy | – | 1 (< 0.5%) |
| Unclear – food allergy unlikely | 5 (1%) | 4 (1%) |
| n | 576 | 598 |
| Egg | ||
| Not allergic based on parental report and/or SPT | 493 (86%) | 525 (88%) |
| Not allergic confirmed by OFC | 12 (2%) | 8 (1%) |
| Allergic confirmed by OFC | 12 (2%) | 3 (1%) |
| Allergic by panel consensus | 21 (4%) | 19 (3%) |
| Not allergic by panel consensus | 22 (4%) | 26 (4%) |
| Unclear – possible food allergy | 6 (1%) | 3 (1%) |
| Unclear – food allergy unlikely | 10 (2%) | 14 (2%) |
| n | 576 | 598 |
| Peanut | ||
| Not allergic based on parental report and/or SPT | 500 (87%) | 513 (86%) |
| Not allergic confirmed by OFC | 16 (3%) | 16 (3%) |
| Allergic confirmed by OFC | 6 (1%) | 3 (1%) |
| Allergic by panel consensus | 4 (1%) | 5 (1%) |
| Not allergic by panel consensus | 29 (5%) | 35 (6%) |
| Unclear – possible food allergy | 3 (1%) | 4 (1%) |
| Unclear – food allergy unlikely | 18 (3%) | 22 (4%) |
| n | 576 | 598 |
| Overall classification | ||
| OFC allergic | 15 (3%) | 6 (1%) |
| Allergic by panel consensus | 26 (5%) | 23 (4%) |
| Panel consensus possible allergy | 6 (1%) | 2 (< 0.5%) |
| Passed OFC | 24 (4%) | 15 (3%) |
| Panel consensus allergy unlikely | 23 (4%) | 28 (5%) |
| Not allergic by panel consensus | 39 (7%) | 51 (9%) |
| Not allergic based on parental report and/or SPT | 443 (77%) | 473 (79%) |
| n | 576 | 598 |
| Intervention (n = 693) | Control (n = 701) | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) | |
|---|---|---|---|---|
| Allergic to at least one of milk, egg or peanut | ||||
| Using multiple imputation model as specified in SAPa | 9.6% (SE 1.4%) | 6.5% (SE 1.2%) | 1.49 (0.94 to 2.36) | 3.1% (−0.5% to 6.7%) |
| Using multiple imputation model with randomisation stratification variables onlyb | 7.6% (SE 1.1%) | 5.1% (SE 1.0%) | 1.49 (0.93 to 2.36) | 2.5% (−0.3% to 5.3%) |
| Intervention | Control | Adjusted interaction effect (RR) (95% CI) | Adjusted interaction effect (risk difference) (95% CI) | |
|---|---|---|---|---|
| Number of first-degree relatives with atopic disease | ||||
| 1 | 14/207 (7%) | 9/208 (4%) | ||
| 2 | 17/234 (7%) | 14/244 (6%) | 0.81 (0.28 to 2.35) | −0.9% (−7.2% to 5.3%) |
| 3 or more | 10/106 (9%) | 6/116 (5%) | 1.16 (0.32 to 4.12) | 1.8% (−6.4% to 10.0%) |
| Number of first-degree relatives with history of eczema | ||||
| 0 | 6/103 (6%) | 2/95 (2%) | ||
| 1 | 21/255 (8%) | 12/289 (4%) | 0.73 (0.13 to 4.06) | 0.4% (−6.3% to 7.1%) |
| 2 or more | 14/189 (7%) | 15/184 (8%) | 0.33 (0.06 to 1.86) | −4.3% (−12.0% to 3.3%) |
| FLG genotype for children with mother and father of white ethnicity and children of other ethnicity with mutation a , b | ||||
| +/+ (no mutations) | 14/325 (4%) | 12/344 (3%) | ||
| +/− (one FLG null mutation) | 8/59 (14%) | 4/56 (7%) | 1.57 (0.40 to 6.17) | 5.6% (−5.6% to 16.8%) |
| −/− (two FLG null mutations) | 0/1 (0%) | 0/2 (0%) | ||
| Intervention – no emollient use | Intervention – some emollient use | Intervention – widespread regulara emollient use | Control – no emollient/moisturiser use | Control – some emollient/moisturiser use | Control – widespread regulara emollient/moisturiser use | |
|---|---|---|---|---|---|---|
| 3 months | n = 20 | n = 46 | n = 466 | n = 298 | n = 109 | n = 110 |
| At least one skin infection | 0 | 2/46 (4%) | 20/466 (4%) | 6/298 (2%) | 9/107 (8%) | 5/110 (5%) |
| Number of skin infections | ||||||
| Median (25th, 75th centile) | – | 2 (1, 3) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) |
| Minimum, maximum | – | 1, 3 | 1, 2 | 1, 2 | 1, 1 | 1, 2 |
| Slippage incident involving infant within an hour of applying skin care products to the baby’s skin | 0 | 2/46 (4%) | 3/464 (1%) | 1/289 (< 0.5%) | 0 | 0 |
| 6 months | n = 33 | n = 59 | n = 427 | n = 264 | n = 106 | n = 143 |
| At least one skin infection | 2/33 (6%) | 4/59 (7%) | 28/426 (7%) | 7/262 (3%) | 1/105 (1%) | 14/140 (10%) |
| Number of skin infections | ||||||
| Median (25th, 75th centile) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1.5) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) |
| Minimum, maximum | 1, 1 | 1, 1 | 1, 7 | 1, 2 | 1, 1 | 1, 3 |
| Slippage incident involving infant within an hour of applying skin care products to the baby’s skin | 1/33 (3%) | 1/57 (2%) | 1/422 (< 0.5%) | 1/260 (< 0.5%) | 1/105 (1%) | 0 |
| 12 months | n = 58 | n = 73 | n = 375 | n = 268 | n = 108 | n = 144 |
| At least one skin infection | 9/58 (16%) | 4/73 (5%) | 32/375 (9%) | 12/268 (4%) | 6/108 (6%) | 15/144 (10%) |
| Number of skin infections | ||||||
| Median (25th, 75th centile) | 1 (1, 1) | 1 (1, 1.5) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 2) |
| Minimum, maximum | 1, 4 | 1, 2 | 1, 3 | 1, 2 | 1, 1 | 1, 2 |
| Slippage incident involving infant within an hour of applying skin care products to the baby’s skin | 1/57 (2%) | 0 | 7/368 (2%) | 2/262 (1%) | 0 | 5/144 (3%) |
| Intervention – did not complete 60-month questionnaire (n = 226) | Intervention –completed 60-month questionnaire (n = 467) | Control – did not complete 60-month questionnaire (n = 192) | Control – completed 60-month questionnaire (n = 509) | |
|---|---|---|---|---|
| Screening visit | ||||
| Prior to birth | 125 (55%) | 304 (65%) | 103 (54%) | 336 (66%) |
| After birth | 101 (45%) | 163 (35%) | 89 (46%) | 173 (34%) |
| Age of mother at infant randomisation | ||||
| Mean (SD) | 30.2 (6.2) | 32.5 (4.6) | 29.7 (5.6) | 32.2 (4.9) |
| Minimum, maximum | 16, 45 | 20, 44 | 18, 43 | 18, 46 |
| Ethnicity of mother | ||||
| White | 176 (78%) | 413 (88%) | 152 (79%) | 449 (88%) |
| Asian | 17 (8%) | 28 (6%) | 11 (6%) | 29 (6%) |
| Black | 20 (9%) | 11 (2%) | 15 (8%) | 7 (1%) |
| Other | 13 (6%) | 15 (3%) | 14 (7%) | 24 (5%) |
| Number of first-degree relatives with atopic disease | ||||
| 1 | 75 (33%) | 179 (38%) | 62 (32%) | 191 (38%) |
| 2 | 108 (48%) | 192 (41%) | 82 (43%) | 214 (42%) |
| 3 or more | 43 (19%) | 96 (21%) | 48 (25%) | 104 (20%) |
| Number of first-degree relatives with history of eczema | ||||
| 0 | 51 (23%) | 79 (17%) | 40 (21%) | 81 (16%) |
| 1 | 87 (38%) | 227 (49%) | 88 (46%) | 264 (52%) |
| 2 or more | 88 (39%) | 161 (34%) | 64 (33%) | 164 (32%) |
| Boy | 125 (55%) | 249 (53%) | 96 (50%) | 263 (52%) |
| Girl | 101 (45%) | 218 (47%) | 96 (50%) | 246 (48%) |
| Number of other children in household at screening (including non-full blood siblings) | ||||
| 0 | 76 (34%) | 199 (43%) | 61 (32%) | 232 (46%) |
| 1 | 91 (40%) | 195 (42%) | 71 (37%) | 200 (39%) |
| 2 | 40 (18%) | 55 (12%) | 40 (21%) | 56 (11%) |
| 3 or more | 19 (8%) | 18 (4%) | 20 (10%) | 21 (4%) |
| Delivery method | ||||
| Vaginal | 154 (68%) | 328 (70%) | 128 (67%) | 344 (68%) |
| Caesarean | 72 (32%) | 139 (30%) | 64 (33%) | 165 (32%) |
| Days between birth and randomisation | ||||
| Mean (SD) | 5.7 (6) | 5.8 (5.6) | 5.5 (5.8) | 5.8 (5.6) |
| Median (25th, 75th centile) | 3 (1, 9) | 4 (1, 9) | 3 (1, 8.5) | 4 (1, 9) |
| Decile of English Index of Multiple Deprivation 2015 (1 = most deprived) | ||||
| Mean (SD) | 4.9 (2.8) | 6.3 (2.7) | 4.6 (2.9) | 6.3 (2.6) |
| Median (25th, 75th centile) | 4 (3, 7) | 7 (4, 9) | 4 (2, 7) | 6 (4, 9) |
| Intervention | Control | |
|---|---|---|
| Diagnosis of eczema between 12 and 24 months of age (defined as meeting the UKWP Diagnostic criteria, primary outcome) | ||
| No | 357/459 (78%) | 385/462 (83%) |
| Yes | 102/139 (73%) | 115/150 (77%) |
| Parental report of clinical diagnosis of eczema between birth and 2 years | ||
| No | 259/344 (75%) | 277/334 (83%) |
| Yes | 203/266 (76%) | 225/282 (80%) |
| Parental report of any food allergy at 24 monthsa | ||
| No | 292/366 (80%) | 329/400 (82%) |
| Yes | 159/208 (76%) | 161/197 (82%) |
| Parental report of immediate food allergy to common allergen at 24 monthsb | ||
| No | 359/456 (79%) | 414/501 (83%) |
| Yes | 92/118 (78%) | 76/96 (79%) |
| Confirmed food allergy to at least one of milk, egg or peanut | ||
| No | 402/506 (79%) | 453/539 (84%) |
| Yes | 33/41 (80%) | 18/29 (62%) |
| Intervention (n = 693) | Control (n = 701) | |
|---|---|---|
| Table 16 | ||
| Presence of eczema in the previous year based on parental report of a clinical diagnosis | 4052 observations 632 participants |
4171 observations 643 participants |
| Presence of eczema based on completion by parents of UKWP Diagnostic Criteria | 2507 observations 619 participants |
2640 observations 637 participants |
| Moderate, severe or very severe eczema according to POEM | 2463 observations 612 participants |
2600 observations 633 participants |
| Table 17 | ||
| Parental report of reaction to any food within the previous year | 1855 observations 596 participants |
1983 observations 622 participants |
| Parental report of immediate reaction to milk, egg or nuts within the previous year | 1872 observations 596 participants |
2004 observations 622 participants |
| Parental report of immediate reaction to any common food allergen within the previous year at 60 months | 1867 observations 596 participants |
1997 observations 622 participants |
| Parental report of a clinical diagnosis of food allergy within the previous year | 2381 observations 609 participants |
2514 observations 632 participants |
| Table 18 | ||
| Parental report of wheezing or whistling in the chest in previous year | 1936 observations 596 participants |
2061 observations 623 participants |
| Parental report of allergic rhinitis symptoms in previous year | 1937 observations 595 participants |
2058 observations 623 participants |
| Selected ‘eczema’ in response to ‘In the last xx months (with xx = 3 months on the 3 and 6 month questionnaires and 6 months on the 12 month questionnaire), has your baby/child suffered from any of the following skin problems?’a | Emollient | Control | Adjusted RR (95% CI) | Adjusted difference in risk (95% CI) |
|---|---|---|---|---|
| 3 months | 91/530 (17%) | 107/523 (20%) | 0.83 (0.65 to 1.06) | −3.5% (−8.0% to 1.0%) |
| 6 months | 160/523 (31%) | 155/517 (30%) | 1.01 (0.85 to 1.21) | 0.4% (−4.9% to 5.8%) |
| 12 months | 189/514 (37%) | 186/528 (35%) | 1.03 (0.88 to 1.20) | 0.9% (−4.7% to 6.6%) |
| 18 months | 173/489 (35%) | 183/506 (36%) | 0.99 (0.84 to 1.16) | −0.3% (−6.1% to 5.4%) |
| 24 months | 189/591 (32%) | 193/607 (32%) | 1.01 (0.86 to 1.19) | 0.2% (−5.0% to 5.4%) |
| 36 months | 169/474 (36%) | 168/493 (34%) | 1.03 (0.87 to 1.21) | 1.0% (−4.8% to 6.8%) |
| 48 months | 154/459 (34%) | 162/513 (32%) | 1.06 (0.89 to 1.26) | 1.9% (−3.8% to 7.5%) |
| 60 months | 168/460 (37%) | 151/499 (30%) | 1.18 (0.99 to 1.39) | 5.4% (−0.3% to 11.1%) |
| Intervention | Control | |
|---|---|---|
| 36 months | ||
| Parental report of reaction within the previous year | ||
| Reaction to cow’s milk | 35/460 (8%) | 26/487 (5%) |
| Reaction to egg | 27/447 (6%) | 14/476 (3%) |
| Reaction to nuts | 13/451 (3%) | 11/480 (2%) |
| Reaction to other food | 33/441 (7%) | 25/469 (5%) |
| Parental report of immediate reaction within the previous yeara | ||
| Reaction to cow’s milk | 19/460 (4%) | 13/486 (3%) |
| Reaction to egg | 18/445 (4%) | 12/476 (3%) |
| Reaction to nuts | 11/449 (2%) | 10/480 (2%) |
| 48 months | ||
| Parental report of reaction within the previous year | ||
| Reaction to cow’s milk | 21/453 (5%) | 17/502 (3%) |
| Reaction to egg | 16/443 (4%) | 12/496 (2%) |
| Reaction to nuts | 14/451 (3%) | 8/496 (2%) |
| Reaction to other food | 22/430 (5%) | 17/477 (4%) |
| Parental report of immediate reaction within the previous yeara | ||
| Reaction to cow’s milk | 9/453 (2%) | 9/501 (2%) |
| Reaction to egg | 12/442 (3%) | 10/495 (2%) |
| Reaction to nuts | 12/449 (3%) | 5/493 (1%) |
| 60 months | ||
| Parental report of reaction within the previous year | ||
| Reaction to cow’s milk | 18/455 (4%) | 16/482 (3%) |
| Reaction to egg | 14/440 (3%) | 8/463 (2%) |
| Reaction to nuts | 6/452 (1%) | 14/479 (3%) |
| Reaction to other food | 26/452 (6%) | 21/480 (4%) |
| Parental report of immediate reaction within the previous yeara | ||
| Reaction to cow’s milk | 9/454 (2%) | 7/480 (1%) |
| Reaction to egg | 11/438 (3%) | 7/462 (2%) |
| Reaction to nuts | 5/451 (1%) | 11/476 (2%) |
| Reaction to other common food allergen | 14/448 (3%) | 4/475 (1%) |
| Total number of times child reacted to any food in the previous year | ||
| None | 387 (89%) | 417 (91%) |
| One | 10 (2%) | 15 (3%) |
| Two | 8 (2%) | 11 (2%) |
| More than two | 29 (7%) | 15 (3%) |
| n | 434 | 458 |
| Unusually runny or frequent poos or blood/slime in poo for a month or longer within the previous year | ||
| No | 420 (95%) | 450 (95%) |
| Yes | 22 (5%) | 23 (5%) |
| n | 442 | 473 |
| Stomach pains or been unusually irritable for more than a month within the previous year | ||
| No | 421 (95%) | 457 (97%) |
| Yes | 22 (5%) | 15 (3%) |
| n | 443 | 472 |
| Prescribed low-allergy formula milk within the previous year | ||
| No | 441 (100%) | 474 (100%) |
| Yes | 2 (< 0.5%) | 0 |
| n | 443 | 474 |
| Intervention | Control | |
|---|---|---|
| Parental report of reaction to egg or nuts | ||
| No | 496 (83%) | 542 (87%) |
| Yes | 101 (17%) | 80 (13%) |
| n | 597 | 622 |
| If yes, reaction reported to a : | ||
| Egg only | 68 (67%) | 46 (57%) |
| Nuts only | 19 (19%) | 21 (26%) |
| Egg and nuts | 14 (14%) | 13 (16%) |
| Parental report of immediate reaction to egg or nuts | ||
| No | 522 (88%) | 559 (90%) |
| Yes | 74 (12%) | 63 (10%) |
| n | 596 | 622 |
| If yes, reaction reported to a : | ||
| Egg only | 52 (70%) | 37 (59%) |
| Nuts only | 14 (19%) | 15 (24%) |
| Egg and nuts | 8 (11%) | 11 (17%) |
List of abbreviations
- AE
- atopic eczema
- AD
- atopic dermatitis
- BEEP
- barrier enhancement for eczema prevention
- CACE
- complier-average causal effect
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CHU-9D
- child health utility instrument-9 domains
- CUA
- cost–utility analysis
- DNA
- deoxyribonucleic acid
- EASI
- Eczema Area and Severity Index
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FLG
- gene encoding filaggrin
- GLM
- generalised linear model
- GP
- general practitioner
- HEAP
- health economics analysis plan
- ICER
- incremental cost-effectiveness ratio
- IgE
- immunoglobulin E
- IPD
- individual participant data
- MAR
- missing at random
- MNAR
- missing not at random
- NCTU
- Nottingham Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- PCA
- prescription cost analysis
- PIC
- Participant Identification Centre
- POEM
- Patient-Oriented Eczema Measure
- PPI
- patient and public involvement
- PreventADALL
- Preventing Atopic Dermatitis and ALLergies
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAP
- statistical analysis plan
- SMS
- short message service
- SWAT
- study within a trial
- TSC
- Trial Steering Committee
- UKWP
- United Kingdom Working Party
