Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 17/72/02. The contractual start date was in November 2018. The draft report began editorial review in May 2021 and was accepted for publication in March 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2022 Coates et al. This work was produced by Coates et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Coates et al.
Chapter 1 Introduction
Ulcerative colitis
Epidemiology
Ulcerative colitis (UC) is a long-term condition of the colon with unknown cause. The incidence of UC varies, with the highest varying between 8 and 14 per 100,000 per year, and prevalence is 120–200 per 100,000 in North America, northern Europe and the UK. 1 The incidence is also increasing in areas where it has previously been low, including Asia, the Middle East and North Africa. 1 UC can occur at any age, with a peak incidence in the fourth and fifth decades, from 30 to 40 years. 1 At diagnosis, an estimated 15% of people are aged > 60 years. 2 A possible peak later than 60 years may occur, although this is reported in fewer studies. 3 There may be a slight predominance of UC in male patients. 1
Aetiology
The aetiology of UC is unknown and has remained elusive for decades, despite intensive investigation. A prominent mucosal immune reaction is evident, but a trigger for this has not been identified. A genetic predisposition is also clear, but this accounts for only a small proportion of the heritability risk. 4 Therefore, it has been suggested that an abnormal immune response to some components of the host intestinal microbiota occurs in genetically predisposed individuals in association with changes in epithelial integrity with stimulation of gut immune responses. 5
Genome-wide association studies have identified at least 260 genetic susceptibility loci across inflammatory bowel disease (IBD), mostly shared between UC and Crohn’s disease. 5 Specific associations with UC are mostly within the human leucocyte antigen region on chromosome 6. 6 Outside the human leucocyte antigen region, there is a strong association between UC and a missense variant in the adenylate cyclase 7 gene (ADCY7), with a twofold increase in the risk of UC. 7 Other UC-specific genes relate to epithelial integrity,8–10 and epigenetic factors may also play a role. 11,12
Pathology
Ulcerative colitis is characterised by inflammation of the colon, typically affecting the rectum, and to a variable proximal extent. The inflammation is continuously distributed. In about 50% of patients, the distal colon (i.e. rectum and sigmoid) is most commonly affected, and in the remaining patients the colonic involvement is greater, extending to the splenic flexure (i.e. left-sided colitis), to the hepatic flexure (i.e. subtotal colitis) or to the caecum (i.e. total or pancolitis).
On microscopy, overlap can be seen with other colitides, including those caused by infection, drugs and Crohn’s disease. UC is associated with acute and chronic inflammatory changes in the colonic mucosa and architectural changes including diffuse crypt abnormalities, cryptitis, crypt atrophy and abnormal crypt architecture. 13
Prognosis
Ulcerative colitis typically runs a relapsing and remitting course, and four main patterns of clinical course and disease evolution are described. 14 In approximately 90% of patients, UC has a typical relapsing and remitting course. Some patients experience just one attack followed by long-term remission (18% at 5 years and 10% at 25 years). Approximately 10% of patients have a severe presentation necessitating admission and emergency surgery within a year of diagnosis. A small percentage of patients (approximately 1% at 5 years and 0.1% at 25 years) have unremitting, continuous illness. Approximately 50% of patients have a relapse in any year, with an important minority experiencing more frequent rapidly relapsing or continuous disease. Thirty-five per cent of patients with pancolitis will undergo surgery. 14,15
The long-term survival of those with UC is not different from that of the general population, although the mortality rate from colorectal cancer and hepatobiliary complications is higher. 16
Economic burden
An estimated 620,000 patients in the UK have UC. 3 A UK national audit suggested that the costs of treating IBD (i.e. UC and Crohn’s disease) were more than £1B, with an average cost of £3000 per person per year. 17 A cost-of-care model for the UK has been reported, with estimated annual treatment costs per patient with UC of £3084. 18 Among patients in remission, patients in relapse with mild to moderately severe disease and patients with severe UC, the annual cost per patient is estimated to be £1693, £2903 and £10,760, respectively. 18
Humanistic burden
There may be societal costs to the economy, to individuals and to families and carers that are not captured in health economic analyses. These include costs of absence from work, caring responsibilities and psychological morbidity, which is widely recognised in the literature but may be under-recognised or assessed in clinical practice. These societal costs may occur as a result of the condition and also from the side effects of drugs, including steroid-related adverse effects. 19,20
Rationale
Ulcerative colitis runs a relapsing and remitting course, resulting in debilitating symptoms, impaired quality of life and severe attacks necessitating hospitalisation. For moderately active UC not requiring hospitalisation, oral corticosteroids may induce remission in those refractory to aminosalicylate therapy. 21 Corticosteroids are recommended as first-line therapy for a severe relapse of UC. 22 This programme of work applies to patients with moderately severe UC treated as outpatients.
Approximately 50% of patients with moderately severe flares do not respond fully to corticosteroids. 23,24 There are several subsequent treatment options in the National Institute for Health and Care Excellence (NICE) treatment pathway,21 and these have been the subject of randomised controlled trials (RCTs) comparing individual agents with placebo or steroid comparators. To the best of our knowledge, direct ‘head-to-head’ comparisons are not available, except in the VARSITY trial, which compares adalimumab and vedolizumab. 25 NICE has recommended that the risks and benefits of methotrexate, ciclosporin, tacrolimus, adalimumab and infliximab be assessed for the induction of remission in steroid-refractory UC. 21 Subsequently, vedolizumab and tofacitinib have demonstrated efficacy in RCTs and have been recommended for use in this population. 26,27 Ustekinumab has also been shown to be effective and has been recommended for use in UC, but the guidance on this was issued after the current study was under way. 28 Other options, including surgery and the use of intravenous (i.v. ) steroids, have not been the subject of RCTs. These options vary in cost, availability, mode of administration, patient acceptability and utilisation of health-care facilities, as well as clinician experience in their use, especially for newer agents. There is insufficient information to inform patients with steroid-resistant UC about the optimum choice of agent, concomitant immunosuppression and the timing of surgery.
There is no universally adopted definition of steroid-resistant UC. Current definitions might include clinical, endoscopic and quality-of-life dimensions. There is also an overlap between patients with ongoing symptoms or endoscopic inflammation despite corticosteroids (i.e. ‘steroid resistant’) and patients who initially respond and then relapse on reducing the steroid dose (i.e. ‘steroid dependent’). Both groups of patients have been included in clinical trials of agents used for steroid-resistant disease. Pivotal trials, particularly of biologic agents, have included patients in both groups, and the results of treatment in each group have been reported together. Both situations can lead to the prolonged use of systemic corticosteroids and the attendant consequences. The prolonged use of systemic corticosteroids is considered a disutility of care. 29 In addition, these patients may be taking concomitant immunomodulator drugs.
Therefore, although national and international guidance21,26,30 reflects this range of options, there remains a lack of clarity about:
-
the definition of steroid resistance
-
the specific applicability of current evidence to a population of patients with UC resistant to corticosteroids
-
the optimum choice of treatment for this group and the importance of factors such as patient and clinician preferences, concomitant immunosuppression and prior biological therapy.
Review of existing evidence
Ulcerative colitis is associated with disability, psychological morbidity and distress. 31–33 UC also has a significant socioeconomic impact arising from disrupted education and employment,31 with 20 days of household and recreational activities per year typically lost to illness. 34 In 2000, before the widespread use of biological agents, the costs of treating UC were estimated at £3021 per patient-year in the UK35 and at €1524 per patient-year in an European Collaborative inception cohort. 36 Substantial costs relate to hospitalisation and surgery, both of which are more common in those aged < 40 years, and to drug-refractory patients. 37,38 Costs may be reduced by more effective treatment, but it is unclear whether or not newer agents are cost-effective in this population. 37
Oral corticosteroids are associated with significant side effects, which preclude long-term treatment. Therefore, it is important to escalate treatment in patients who do not respond adequately to systemic steroids. A number of treatments are available for patients thought to be resistant to systemic steroids. The anti-tumour necrosis factor (TNF) agents infliximab, adalimumab and golimumab target the pro-inflammatory cytokine TNF-alpha. Infliximab is a human–murine chimeric monoclonal antibody and adalimumab and golimumab are humanised antibodies. Infliximab is most frequently administered intravenously, but a preparation for subcutaneous administration became available in 2021. Adalimumab and golimumab are administered by subcutaneous injection. Vedolizumab is a monoclonal antibody that targets α4β7 integrin, an adhesion molecule on the surface of T lymphocytes, preventing it from binding to endothelial MAdCAM-1 (mucosal addressin cell adhesion molecule-1) and preventing these lymphocytes from migrating into gut mucosa. 39 Therefore, it is suggested that this activity is gut specific. Vedolizumab is most often administered by i.v. infusion, but a preparation for subcutaneous injection became available in 2021. Tofacitinib is a ‘small-molecule’ agent that inhibits janus kinase (JAK1 and JAK3)-mediated transcription pathways, preventing inflammatory cytokine production. 40 Tofacitinib is administered orally.
Data about these agents are available from trials and also from meta-analyses. Clinical trials in patients who have not responded or responded inadequately to systemic corticosteroids show benefit of anti-TNF agents (e.g. infliximab,41 adalimumab42,43 and golimumab44,45), vedolizumab,46 tacrolimus, tofacitinib47 and ustekinumab. 48 Clinical benefit is seen in terms of inducing remission, inducing response (i.e. improvement), maintaining remission and healing the inflamed intestinal lining (i.e. mucosal healing).
In clinical practice, supported by guidance from NICE, the British Society of Gastroenterology (BSG) (London, UK) and the European Crohn’s and Colitis Organisation (ECCO) (Vienna, Austria), additional approaches are available, including inpatient i.v. steroids and surgery. 21,26,30,49,50 However, the preferred treatment or approach is not clear.
Guidance from NICE recommends the anti-TNF agents infliximab, adalimumab and golimumab as options for adults whose disease has responded inadequately to conventional therapy, including corticosteroids and mercaptopurine or azathioprine, because they either cannot tolerate them or have contraindications to them. Vedolizumab is also recommended for treating moderately to severely active UC. Tofacitinib is recommended when conventional therapy or a biological agent cannot be tolerated or when the disease has responded inadequately or lost response to treatment.
Definitions of steroid resistance also differ in the literature. Widely used guidelines define steroid resistance as a failure to aachieve symptomatic remission following treatment with systemic steroids (prednisolone at 0.75 mg or 1 mg/kg per day for 4 weeks). 26,51 Creed and Probert52 regard steroid-resistant UC as failure to respond to treatment with high-dose oral steroids within 30 days. However, consideration of escalation to additional treatment after 2 weeks is also discussed. 51 UK guidelines describe steroid dependence as the inability to wean systemic steroids below a prednisolone dose of 10 mg per day within 3 months without the development of active disease, or symptomatic relapse within 3 months of stopping steroids.
Prior steroid response or exposure is not uniform in pivotal trials. Not all patients included in these RCTs are steroid resistant, and results may not be reported separately for patents with steroid-resistant disease and those with steroid-dependent disease. For example, a minority of patients included in pivotal trials of infliximab in active UC were taking ≥ 20 mg of prednisolone per day at trial entry. 41 In addition, in trials of adalimumab and vedolizumab, remission rates were not significantly different from placebo in patients on corticosteroids42 or failing corticosteroids alone. 42,46
Side effects are also important when considering the use of drugs. The adverse effects of systemic steroids are well established, and additional treatments also have important side effects. The risk of infection, serious infection and cancer (including lymphoma) is related to the immunosuppressive properties of drugs. These risks may be low, and randomised trials involving small samples are often unable to detect risks of this size, resulting in the need for evidence from larger, longer-term studies. Therefore, clinicians and patients have to use the evidence in the forms available.
Although qualitative research highlights the diverse perspectives of medical and nursing staff,53 there are limited published patient perspectives, which are needed to inform the design of future clinical trials as well as clinical practice. Survey data suggest that patients’ ideal therapy would be an effective oral formulation that requires them to take few tablets infrequently, with minimal side effects. 54
The role of surgery also needs to be evaluated, as there is limited evidence to determine its ideal position in the treatment pathway. Emergency surgery is positioned in NICE guidance for acute severe colitis,55 but a position is not specifically defined for elective surgery for chronic relapsing disease. The NICE guidance indicates that surgery can be effective to remove symptoms of UC that recur rapidly, but does not describe its optimum timing. 55 The NICE guidance also does not take into account the differing impact of relapses of varying frequency and severity on an individual, nor the impact of drug administration regimes or side effects. Guidelines developed by the Association of Coloproctology of Great Britain and Ireland (London, UK) advocate surgery for relapsing and remitting disease but do not say where in the pathway this should go. 56 Optimum timing of surgery is, therefore, likely to be influenced by a number of disease factors, personal patient preferences and clinician perspectives and, therefore, will differ between patients. We have previously demonstrated that patients wish to undergo surgery when faced with severe restrictions on quality of life. 57 Clinician and patient preferences are, therefore, of central importance in understanding the position of surgery in the treatment pathway.
In a health economic assessment from our group58 (multitechnology appraisal TA329 of anti-TNF agents49) for patients in whom surgery is an option, colectomy was expected to dominate all medical treatment options. For patients in whom colectomy is not an option, infliximab and golimumab were ruled out because of dominance, with the incremental cost-effectiveness ratio for adalimumab compared with conventional treatment expected to be approximately £50,278 per quality-adjusted life-year gained. However, there remains debate with regard to whether surgery should be considered a comparator or an end point and as to when surgery would not be an option. Indeed, in NICE technology appraisal guidance TA342,27 neither surgery nor tacrolimus was thought to be a suitable comparator for vedolizumab.
These issues illustrate the pressing need for a trial with a clearly defined population of steroid-resistant patients to evaluate clinical effectiveness and cost-effectiveness. To develop such a trial, a detailed understanding of how best to describe steroid resistance, and of patients’ and clinicians’ views of treatment options and objectives, is required. This will allow appropriate identification of equipoise and acceptable invention and comparator arms in a trial.
Statement of the problem
In commissioning brief 17/72, the Health Technology Assessment programme indicated its interest in improving the understanding of how adults with steroids-resistant UC are managed, with a view to informing future commissioning briefs addressing which treatments require further evaluation in steroid-resistant UC.
Aims and objectives
The overarching research question for this study was ‘How are adults with steroid-resistant UC being managed in secondary care, and how does current practice compare with patient and clinician preferences?’.
To answer the research question, this mixed-methods study had five objectives:
-
to describe current practice in the management of adults with steroid-resistant UC and to describe how medical resistance to steroids is defined
-
to understand how treatment pathways and definitions of steroid resistance are operationalised in practice
-
to understand patient experiences of different treatment options and approaches to decision-making
-
to estimate the relative utility of different treatment options and to elicit patient and clinician preferences for these and their willingness to trade between them
-
to make recommendations about future research and treatment options.
The five objectives aligned with five corresponding work packages (WPs) delivered as part of this mixed-methods study. To understand the management and treatment preferences of patients, a survey of clinical practice (WP1) and qualitative interviews with adults with steroid-resistant UC (WP3) were carried out. A survey and qualitative interviews with a purposive sample of health-care professionals were designed to explore how health-care professionals define and treat steroid-resistant UC (WP2). A discrete choice experiment (DCE) with patients and health-care professionals was designed to quantify their preferences for, and estimate the relative utility of and willingness to trade between, different treatment options (WP4). Finally, the aim of a multidisciplinary workshop with patients and health-care professionals (WP5) was to present and discuss the study findings to generate recommendations about optimum treatment and future research.
Chapter 2 Methods
Overview of methodological approach
The PoPSTER (Patient preferences and current Practice in STERoid resistant ulcerative colitis) study was a mixed-methods study and was composed of five WPs, using a combination of qualitative and quantitative research methods to help achieve each of the five corresponding objectives. This included a cross-sectional survey of health-care professionals (objective 1, WP1), qualitative interviews with health-care professionals (objective 2, WP2), qualitative interviews with people with UC (objective 3, WP3), DCEs with health-care professionals and patients (objective 4, WP4) and a multistakeholder workshop (objective 5, WP5). The details of the study design, setting, eligibility criteria, sampling, recruitment, data collection and analysis for each of the WPs are described in this chapter in Health-care professional survey, Qualitative interviews, Discrete choice experiments and Multistakeholder workshop. This chapter also includes details of patient and public involvement (PPI) (see Patient and public involvement), ethics approval (see Ethics approval) and protocol management (see Protocol management and version history).
Health-care professional survey
Study design
This WP involved a cross-sectional survey of IBD health-care professionals in the UK. The aim of the WP was to describe the current management of patients with steroid-resistant UC. The survey looked at how health-care professionals define steroid resistance, health-care professionals’ preferences for different treatments and the factors that influence treatment offers.
Setting
Secondary care IBD services in the UK.
Eligibility criteria
Health-care professionals were eligible to participate in the survey if they were medical or nursing staff in an NHS trust in the UK and had specialist interest or expertise in working with patients with IBD, particularly UC. Membership of the IBD section of the BSG and the Royal College of Nursing (RCN) IBD Nurses Network was taken as an indication of interest in this clinical area.
Sampling
The survey was sent to approximately 1180 health-care professionals (IBD section of the BSG, n = 950; RCN IBD Nurses Network n = 230) and was conducted online using the Qualtrics platform (Qualtrics, Provo, UT, USA). We aimed for a 60% response rate, based on previous surveys of IBD health-care professionals,59–61 which represented an expected sample size of 700 participants.
Recruitment
Different routes were used to approach health-care professionals to participate in the survey during the 4-month recruitment period (20 March to 15 July 2019).
E-mail invitation
E-mail invitations from the chief investigator (AL) were sent to members of the IBD section of the BSG and the RCN IBD Nurses Network. The e-mail provided a detailed explanation of the study purpose, the participant information sheet and a direct link to the online questionnaire. Reminder e-mails were sent approximately every 4 weeks.
Advertising on social media and relevant websites
The survey was advertised on the dedicated Twitter page for the study (@PoPSTER_Study, URL: https://twitter.com/popster_study?lang=en-GB; Twitter, Inc., San Francisco, CA, USA), the Facebook (Meta Platforms, Inc., Menlo Park, CA, USA) page for the RCN IBD Nurses Network (URL: www.facebook.com/groups/RCNIBDNetwork) and the BSG website (URL: www.bsg.org.uk). Regular update tweets on survey recruitment were sent out to raise awareness of the survey and to encourage participation. All tweets contained a short summary about the survey’s purpose and a link to the online questionnaire.
Promotion at relevant national and regional meetings of inflammatory bowel disease health-care professionals
The survey was promoted at a BSG conference in June 2019. The research team had a poster presentation space. A member of the team (AB) was at the conference to raise awareness and distribute paper copies of the questionnaire to interested health-care professionals. In addition to this, the survey was presented by professional contacts of the study team at other relevant national and regional meetings of IBD health-care professionals to help boost recruitment to the survey.
Distribution via professional contacts of the study team
Finally, the survey was distributed to professional contacts of the study team via e-mail and, as a result, was distributed via other IBD professional networks, including Clinical Research Network leads for IBD and regional IBD nursing leads, as well as individual professional contacts. The approach here was to facilitate a ‘snowball sampling’ approach. As with the other recruitment methods, potential participants were provided with study information and a link to the online questionnaire.
Data collection
The survey was hosted online using the Qualtrics platform. The participant information sheet (see Report Supplementary Material 1) and consent form were also hosted online, and informed consent was sought from all participants prior to commencing the survey.
To understand current practice in the management of steroid-resistant UC, the questionnaire (see Report Supplementary Material 2) was divided into the following six sections:
-
demographic information (e.g. clinical role, year of appointment and personal experience of IBD)
-
centre and caseload information [e.g. hospital type, region, presence of a multidisciplinary team (MDT) and composition, proportion of time spent working on IBD and use of clinical guidelines/standards in practice]
-
definitions of steroid resistance (e.g. time frame over which incomplete response represents resistance to or dependence on steroids in UC)
-
treatment pathways (e.g. preferences for different treatments in four patient scenarios, i.e. whether resistant to or dependent on steroids and whether exposed or naive to thiopurines, factors influencing treatment choices, timing of survey referral and local treatment availability)
-
case scenarios (e.g. preferences for different treatments in six patient scenarios, i.e. resistant to or dependent on steroids, relapse at 5 mg or 25 mg, whether exposed or naive to thiopurines)
-
use of endoscopy (e.g. requirements for steroid-resistant and steroid-dependent patients).
A mix of question types was used in the questionnaire, including those that required binary response (i.e. yes or no), those that required a frequency response (i.e. always, sometimes or never), those in which responses were selected from a list and those to which open-ended responses could be given, as appropriate. The content of the questionnaire was developed by the study team, piloted with 13 local clinicians from collaborating centres and refined prior to distribution. 62 All data were collected between 20 March and 15 July 2019.
Data analysis
Quantitative data from the survey were mostly analysed using descriptive statistics and exploratory testing. Continuous outcomes are presented as means and standard deviations (SDs) or as medians and interquartile ranges, as appropriate. Categorical data are presented using frequencies and percentages per categories. Chi-squared tests between demographic groups (hospital type, job role, etc.) were conducted on outcomes of interest, and McNemar’s test was used to compare binary responses of participants between different scenarios. A statistical analysis plan was defined before all data had been collected to identify important outcomes to be analysed, and subsequent analyses were included after sight of the initial results that were deemed to be of interest. Correction for multiple testing was not included in the results, testing of hypotheses was restricted to those prespecified to be interest and limited post hoc testing was completed, depending on interesting and unexpected outcomes in the data. All testing was exploratory, and the results will be used to inform future work. All results were produced using R software (The R Foundation for Statistical Computing, Vienna, Austria).
Qualitative interviews
Health-care professionals
Study design
This WP involved qualitative interviews with a sample of health-care professionals with expertise in IBD. The aim of the WP was to understand in more depth how patients with steroid resistance are managed and how health-care professionals define steroid resistance in practice. This work is reported in accordance with the COREQ (consolidated criteria for reporting qualitative research) guidelines for qualitative research. 63
Setting
The WP was set in secondary care IBD services in the UK.
Eligibility criteria
A health-care professional was eligible to participate in the qualitative interviews if they were a member of medical or nursing staff with a specialist interest or expertise in working with patients with IBD, particularly UC.
Sampling
Health-care professionals were sampled purposively based on job role (i.e. medical or nursing), years of clinical experience, hospital status (i.e. secondary or tertiary referral centre) and location, and they were recruited from across the UK. In line with grounded theory, we used ‘theoretical sampling’ to allow us the flexibility to recruit health-care professionals with particular characteristics in line with emerging themes.
Recruitment
Health-care professionals were identified via the following four sources by the research team:
-
drawn from the subsample of participants in the WP1 survey who consented to being approached about a qualitative interview
-
IBD health-care professionals who opted in to the study through advertising via social media
-
IBD health-care professionals who opted in to the study through advertising via professional networks (e.g. the IBD section of the BSG e-mail)
-
IBD health-care professionals who opted in to the study following correspondence as professional contacts of the clinical members of the research team.
The initial contact e-mail invited all potential participants to take part in a telephone interview and gave a summary (reminder) of the study, and a copy of the participant information sheet was attached (see Report Supplementary Material 1). Potential participants who indicated their interest in the study were asked to provide further information on year of appointment and hospital type, to facilitate the purposive sampling strategy (note that location and gender were evident from information indicated by e-mail response). Availability for a telephone interview was also requested to help with scheduling.
Where appropriate, a member of the research team determined a mutually convenient time at which to carry out the interview. The interview date and time were then confirmed in an e-mail, and another copy of the participant information sheet and consent form, as well as the hypothetical patient case scenario, was attached. All participants were assigned a unique, anonymous study identifier at this point. Reasons for non-participation were recorded, where relevant.
Data collection
Verbal consent to participate in the interview and to this being audio-recorded was taken from all participants before data collection commenced, and participants were asked to refer to the copy of the consent form while the statements were read aloud by the researcher. Separate anonymous recordings of participants providing consent were stored securely for auditing purposes. The individual semistructured interviews were conducted over the telephone by Elizabeth Coates, Amy Barr and Nyantara Wickramasekera (all of whom are female non-clinical researchers educated to postgraduate level and have various levels of experience in qualitative research and seniority). For the duration of the interview, participants were asked to ensure that they were in a quiet and private location, in their workplace or home.
A semistructured interview schedule was used to guide the discussions (see Report Supplementary Material 2). This included questions on how health-care professionals operationalise definitions of steroid resistance, their current practice and their preferences for treatment options for patients with steroid-resistant UC. The interviews also included questions on the types of information that health-care professionals need to make decisions about the treatments that they offer. In addition, the interviews involved a case vignette of a hypothetical patient with steroid-resistant UC, which was developed by the clinical members of the research team and used to facilitate critical distance for the interviewee and as a mechanism to encourage the participant to think about different strategies for treating this patient group (i.e. to help participants explain their clinical practice). In addition to this, the qualitative interviews were used to inform the design (i.e. to identify attributes) of the DCE with health-care professionals.
All data were collected between 28 June and 24 October 2019. Data collection ceased at the point of data saturation. All interviews were recorded using a digitally encrypted device and transcribed verbatim for analysis.
Data analysis
Key themes arising from the data were summarised based on thematic analysis of transcriptions. 64 A thematic analysis was completed by Elizabeth Coates and Amy Barr in accordance with six recommended stages. First, familiarisation with the data, that is, the accuracy of all transcripts, was reviewed by the researchers and notes were generated to enable the second stage, generation of initial codes. A coding framework was developed and applied to the whole data set by Elizabeth Coates and Amy Barr, and NVivo software (QSR International, Warrington, UK) was used to help structure the coding and analysis process. This led to a further four stages: searching for themes, reviewing themes, defining and naming themes and, finally, writing the report.
Patients
Study design
This WP involved qualitative interviews with a sample of people living with UC. The aim of this WP was to explore people’s experiences of and decision-making about different treatments, as well as their treatment preferences. This work is reported in accordance with the COREQ guidelines for qualitative research. 63
Setting
The WP was set in three IBD services in secondary care in the north of England.
Eligibility criteria
Adults aged ≥ 18 years with UC extending beyond the rectum and who had active disease at the time of participation, or who had previously had active disease successfully treated with steroids, were eligible to participate. In addition, people with UC who may have been considered to have steroid-resistant disease and had already made the decision to have surgery were eligible on the grounds that they were able to reflect on decision-making processes for each treatment stage, albeit retrospectively.
Sampling
Patients with UC were sampled purposively based on age, gender, ethnicity, duration of disease and previous treatment, and they were recruited from three NHS IBD services in the north of England. In line with grounded theory, we used ‘theoretical sampling’ to allow us the flexibility to recruit patients with specific characteristics in line with emerging themes.
Recruitment
Staff from the participating IBD services identified and recruited patients on current caseloads and approached them during clinical appointments or by telephone. The study was also advertised in local gastroenterology departments using posters and leaflets. In two of the participating centres, potential participants were given verbal explanations of the study and, if interested, were provided with a copy of the participant information sheet (see Report Supplementary Material 1). Those patients who were interested in taking part then gave verbal consent for the clinical team to pass on their contact details to the research team. To inform the purposive sampling strategy, the research team collated summary clinical and demographic information about each patient during the initial telephone call. This call was also an opportunity for patients to ask questions about the research before they agreed to take part.
If a patient was eligible, and interested, a mutually convenient time for the telephone interview was agreed. In the third centre, written informed consent was taken from participants by the IBD research nurse, who then passed on the participant’s details to the research team. To facilitate the purposive sampling strategy, the research team regularly informed the clinical team of preferred patient characteristics. A lay version of the interview questions and a list of drugs for UC were sent to all participants along with confirmation of the interview date and time. Copies of the participant information sheet and consent form were also sent to all participants from centres 1 and 2 to inform them about verbal consent procedure undertaken prior to interview. All participants were assigned a unique, anonymous study identification at this point. Reasons for non-participation were recorded, where relevant.
Data collection
Verbal consent to participate in the interview and for the interview to be audio-recorded was taken from all participants from centres 1 and 2 before data collection commenced. Participants were asked to refer to the copy of the consent form while the statements were read aloud by the researcher. Separate anonymous recordings of participants providing consent was stored securely for audit purposes. As above, written informed consent was taken locally from participants from the third centre, but verbal consent to participate was confirmed before the interview commenced.
The individual semistructured interviews were conducted over the telephone by Elizabeth Coates, Amy Barr and Nyantara Wickramasekera. All participants were made aware prior to commencing data collection that the interviewers were non-clinical researchers and were advised to contact their IBD team if they had any clinical questions arising from the discussions. Participants were asked to ensure that they were in a quiet and private location for the duration of their interview and were in their homes or work offices during data collection.
A semistructured interview schedule was used to guide the data collection (see Report Supplementary Material 2). This was used to explore patients’ lived experience of UC and approaches to treatment decision-making, and so it was structured around the Coping in Deliberation (CODE) framework. 65 The CODE framework is a multilevel, theoretically informed framework that promotes an understanding of the complexity of decision-making in preference-sensitive health-care settings. 65 In the CODE framework, deliberation is classed as a six-stage process: (1) presentation of health threat, (2) choice, (3) options, (4) preference construction, (5) the decision itself and (6) consolidation. Coping, on the other hand, is presented in three stages: (1) threat, (2) primary and secondary appraisal, leading to (3) a coping effort. Therefore, the interview schedule was split into four sections to address (1) experiences of their UC, (2) treatment options considered at each stage/change of treatment and preference construction, (3) how treatment choices were made and (4) consolidation (i.e. how they currently feel about the treatment choices they made). The interviews were tailored to the patients’ treatment choices and experiences.
All data were collected between 4 June and 29 October 2019. Data collection stopped when data saturation was reached. All interviews were recorded using a digitally encrypted device and transcribed verbatim for analysis.
Data analysis
Key themes arising from the data were summarised based on a thematic analysis of transcriptions. 64 As with the health-care professional interviews, a thematic analysis was completed by Elizabeth Coates and Amy Barr in accordance with the six recommended stages (see Data analysis, above).
Discrete choice experiments
Health-care professionals
Study design
This WP involved an online DCE with health-care professionals in the UK with expertise in IBD. The DCE involves a series of tasks in which respondents select a preferred treatment option when presented with two alternative treatment profiles. These treatment profiles are constructed using a set number of attributes and levels that differ across the alternatives. An attribute is a treatment characteristic that is important to the treatment decision, and a level is a clinically plausible range for each attribute. 66
Identification of treatment characteristics
All relevant attributes and levels were identified using three approaches: (1) reviewing the literature, (2) conducting qualitative interviews and (3) consulting clinicians to select the most important attributes and levels for the DCE. As reported in WP2, qualitative interviews (n = 20) were conducted with health-care professionals with expertise in IBD to understand how patients with steroid-resistant UC are treated in the UK. From these interview transcripts, we identified 16 key themes that health-care professionals considered important when making treatment choices. To convert these themes to possible attributes and levels, we convened a panel of four IBD specialist clinicians (AL, ML, CP and SS). Using iterative rounds of discussion, the panel helped to consolidate and select the most important attributes and also refined the phrasing of attributes.
This process of selecting the key attributes is important for the structural reliability of a DCE, as evidence suggests that the DCE tasks can become cognitively challenging if attribute and level selection is not optimised. 67 When deciding the appropriate levels for each attribute, we carefully selected clinically meaningful values from the clinical trials literature that are sufficiently wide that respondents will be encouraged to trade treatment profiles. The final five attributes (each with three levels) focused on treatment efficacy and safety (Table 1).
| Attribute | Level |
|---|---|
| The likelihood of induction therapy successfully leading to a clinical response (i.e. significant improvement in clinical symptoms) |
|
| The likelihood of a treatment achieving mucosal healing (i.e. a Mayo endoscopic subscore of ≤ 1) |
|
| Remission: efficacy as a maintenance treatment – likelihood of achieving clinical response at 12 months |
|
| Risk of lymphoma |
|
| Risk of serious infection (the baseline risk in patients unexposed to immunosuppressive medication is approximately 1–2 per 100 patient-years) |
|
Questionnaire design
An online survey was developed that contained DCE questions, questions about respondent demographics and feedback questions about the survey. The DCE questions were generated using Ngene software (ChoiceMetrics, Sydney, NSW, Australia). Five attributes with three levels each produced 243 possible treatment profiles and, therefore, to create a manageable DCE task a fractional factorial design was used. The D-optimal design generated 12 choice questions, which followed the principles of orthogonality, minimum overlap and level balance. 68 Each DCE question contained two unlabelled treatment profiles (Figure 1). Opting out of treatment choices was not given as a response option because patients with steroid-resistant UC need treatment and doing nothing can be detrimental to their quality and length of life.
FIGURE 1.
Example of a DCE choice question from the health-care professional DCE.
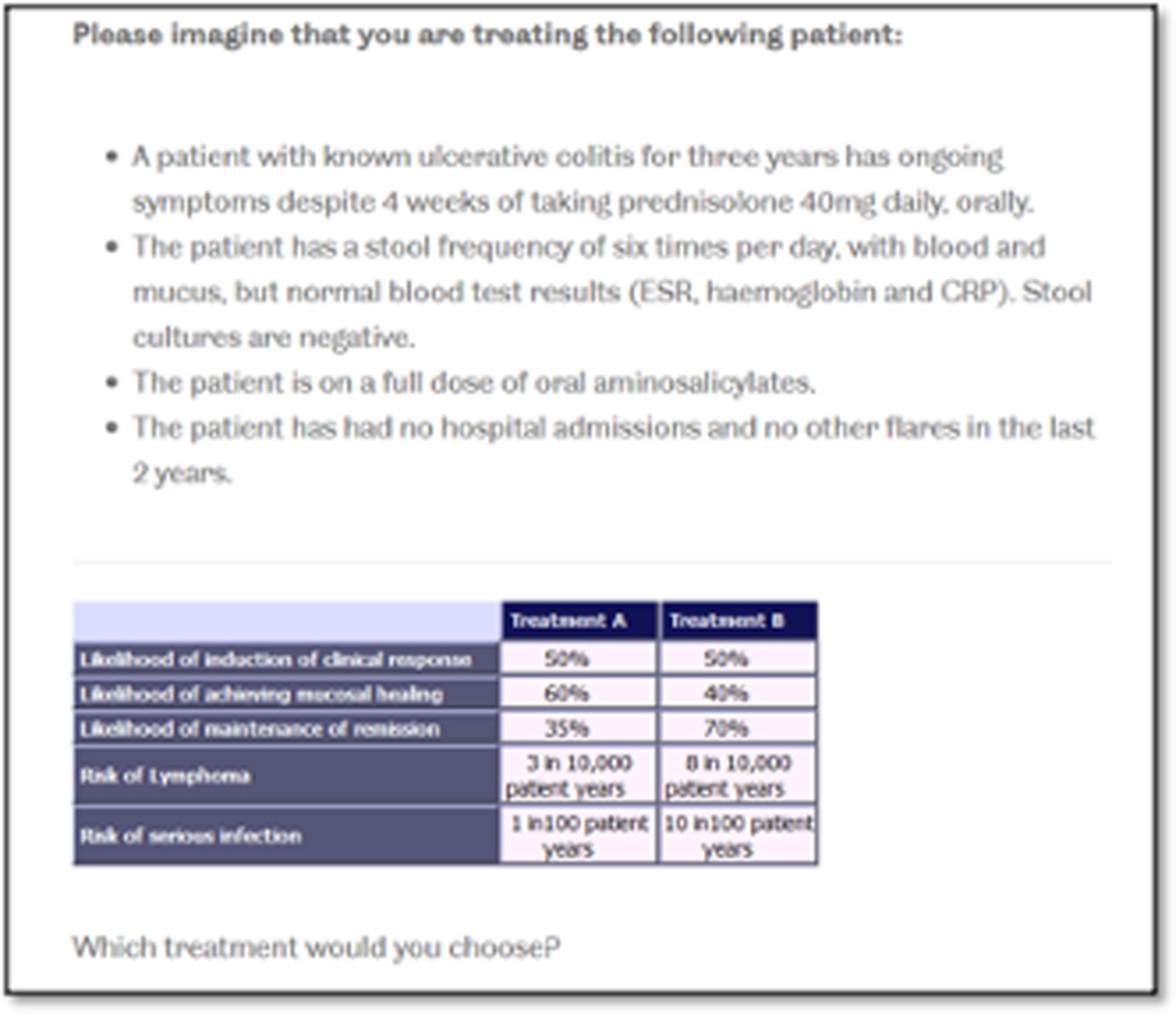
At the start of the survey, a series of screens displayed instructions, including detailed descriptions of the attributes, the levels and the choice context in which the respondents should address the DCE tasks. For each question, the respondents were asked to select the treatment they would choose to offer a patient with steroid-resistant UC (see Figure 1). In addition to the 12 choice questions, a dominant choice question, where one treatment was logically better, was included in the DCE section to test the internal validity of the survey. The final version of the survey contained 13 choice questions that were displayed in a random order to the respondents. A soft launch of the survey was undertaken (n = 50) to assess any problems, including comprehension and dropouts.
Sampling and recruitment
Clinicians and specialist nurses with experience of treating patients with UC in NHS trusts were invited to take part in the online survey through the Qualtrics platform. A link to the survey was sent to health-care professionals in an e-mail (from the IBD section of the BSG and the RCN IBD Nurses Network), via social media and in newsletters during the data collection period between June and August 2020. Participants provided informed consent to participate in the study and were not given any financial incentives to complete the survey. The target sample size was 100 survey respondents, which was based on the minimum sample size required (n ≥ 62.5) to estimate a model using the rule-of-thumb approach and also on the literature that reports the average sample of a DCE ranging between 100 and 300 participants. 69,70
Data analysis
Descriptive statistics were used to summarise respondents’ characteristics and survey feedback questions. Responses to the DCE questions were analysed using a conditional logit model. Attributes were first included as categorical variables using dummy coding; however, after the linear relationship was confirmed through visual inspection and model fit, all attributes were included as continuous variables in the models. 71 The models with the full sample report only main effects, and no interaction terms were included. All statistical analyses were conducted using Stata® v16 (StataCorp LP, College Station, TX, USA). The utility function for estimating the probability of choosing the preferred treatment profile takes the following form:
where V is a binary variable (1 = the preferred treatment profile is chosen, 0 = the treatment profile is not chosen), β is the estimated coefficient for each of the treatment attributes and ε is the unobserved error term.
Exploratory analyses were also conducted to evaluate preferences across subgroups according to hospital type. This was achieved by estimating a model with interactions for all main effect variables for health-care professionals working at secondary or tertiary hospitals to identify any significant differences in their preferences.
Using the results of the conditional logit model, marginal willingness to trade benefit and risk was calculated to find the rate at which health-care professionals are willing to trade levels of one attribute for the preferred levels of another attribute. Benefit–risk trade-offs were calculated by dividing the estimated parameter coefficient for benefit attributes (i.e. induction of response, mucosal healing or maintenance of remission) by the estimated parameter coefficient for the risk attributes (i.e. risk of lymphoma or serious infection).
Parameter estimates from the conditional model were also used to predict uptake rates of a selected number of drugs currently commonly prescribed to patients with steroid-resistant UC. 72 The probability of choosing alternative i is:
where Vi is the estimated utility associated with alternative i and Vj is the sum of utility of j alternatives (see example calculations in Appendix 5).
Moreover, parameter estimates from the conditional model were also used to calculate the change in probability of uptake from a baseline scenario where all attributes are set to their worst level and then improving each attribute one at a time (see example calculations in Appendix 5).
Patients
Study design
This WP involved an online DCE with adults living with UC in the UK.
Identification of treatment characteristics
The development of attributes and levels was informed by a combination of reviewing the literature, conducting qualitative interviews with patients and consulting Patient Advisory Group (PAG) members to find out what they considered the most important attributes. As reported in WP3, we conducted 33 interviews with patients to identify key characteristics that patients consider important when selecting a treatment. Thematic analysis of the qualitative interviews generated eight themes, which were ranked by the four PAG members using a dot-voting technique. 73 The patients were given 16 dots to distribute across the eight themes, with themes with the most dots indicating the most desirable treatment characteristics. Through this process the eight themes were converted and reduced to attributes. After discussions with the PAG members, one theme around the need for regular monitoring was dropped, as this was deemed the least important of the eight. Similar themes were merged; for example, route and frequency of administration were merged to create one single attribute, and quality of life and inducing a treatment response were merged to create another single attribute. The final five attributes focused on effectiveness, remission, speed of response, treatment administration and safety of treatment (Table 2).
| Attribute | Level |
|---|---|
| How effective the drug is at treating your symptoms: the drugs may improve or settle your symptoms (e.g. in reducing stool frequency and bleeding, or returning these to normal), improve your quality of life and make you feel better |
|
| Speed of response to treatment: some drugs take longer than others to take effect |
|
| Chance of your symptoms remaining improved after 12 months: after your initial symptoms improve, the drugs can help to control your symptoms over time; however, there is also a possibility that you may lose the improvement and develop a flare of your symptoms |
|
| Route and frequency of administration: how and where the medication would be taken is different according to which drug you take |
|
| Chance of experiencing side effects: drugs can cause unwanted side effects. Common side effects include nausea, headache, skin rashes and mild infections. These side effects often settle without treatment, can be easily treated or are reversed if the drug is stopped. In rare cases, the drugs may cause severe side effects over a longer period of time. These severe side effects include more severe infections (e.g. tuberculosis and viral infections, including the shingles virus), some cancers, including lymphoma (i.e. lymph gland cancer), blood clots in the leg (i.e. deep-vein thrombosis) or lung (pulmonary embolism) and nervous system problems. The chance of experiencing severe side effects is very low for all treatments |
|
The PAG and PPI co-applicants also helped to refine the language explaining the DCE task to respondents by piloting the draft questionnaire. For example, to aid understanding, we framed the risk attribute quantitatively in the DCE task (i.e. 60/100, 60%); however, in the introduction to the DCE, risks were framed qualitatively (i.e. ‘a drug that is 60% effective means that, if 100 people had the same drug for UC, for 60 people the treatment would be effective but for 40 people treatment would not be effective’). 71 The levels for the attributes were selected to reflect plausible values from the published clinical trials literature. For the side effects attribute, we expressed the levels modelled in accordance with the categories described in printed information leaflets included with drugs that patients read and are familiar with.
Questionnaire design
The first section of the survey contained the DCE tasks, where participants were shown a series of side-by-side comparisons of competing treatment profiles and were asked to select the preferred treatment profile (Figure 2). We used Ngene software to create 12 DCE tasks using the D-optimal method to maximise statistical efficiency, and followed the principles of orthogonality, minimum overlap and level balance. One additional dominant question that was logically better was also included to test whether or not participants understood the DCE task. 66 To make the choice realistic, participants were not given an opt-out option because treatment is necessary to improve their length and quality of life.
FIGURE 2.
Example of a DCE choice question from the patient DCE.
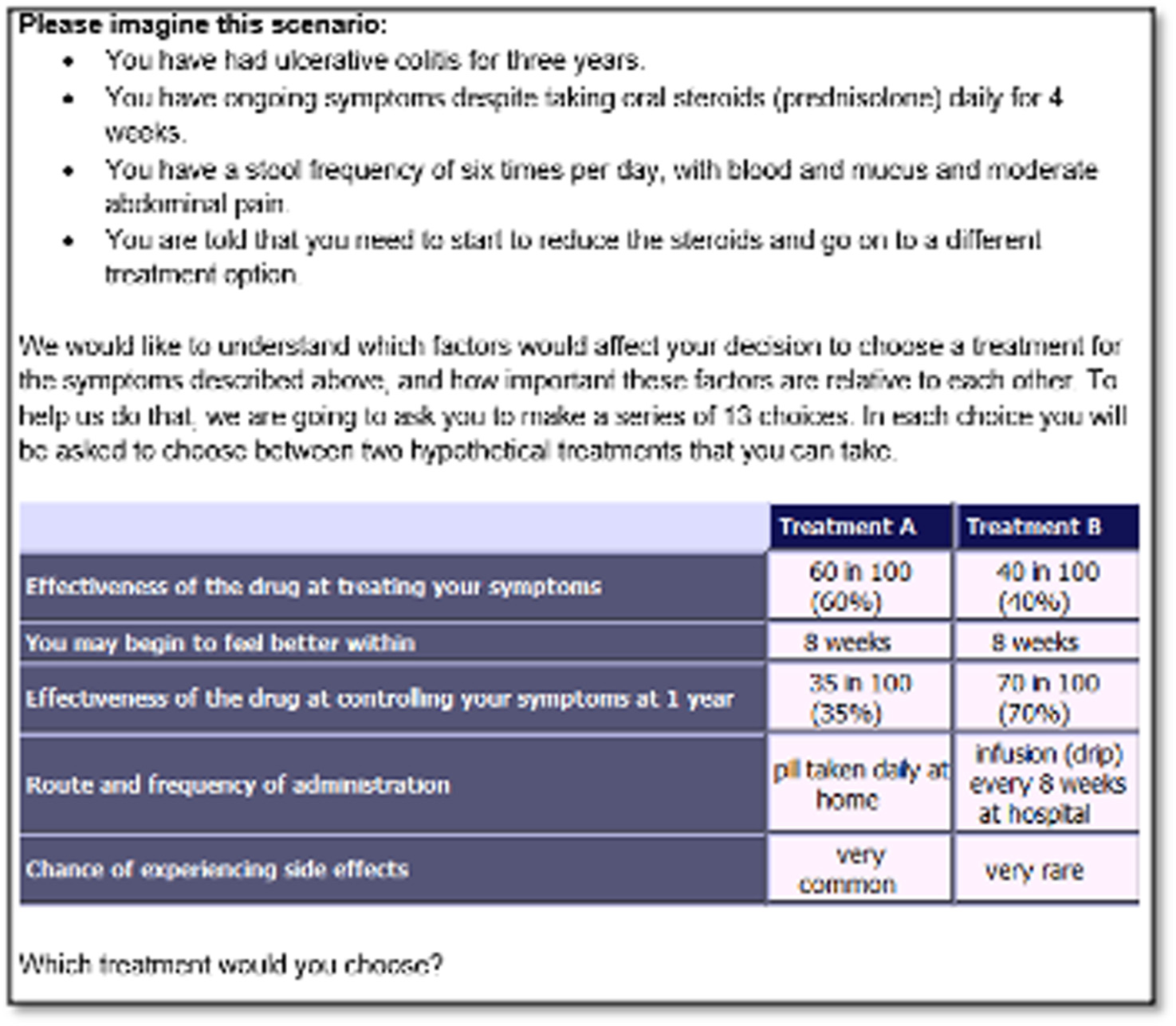
The second section of the survey involved a ranking exercise in which patients were asked to rank four commonly used treatments (i.e. adalimumab, infliximab, tofacitinib and vedolizumab) in order of preference from 1 to 4 (1 = best preferred treatment and 4 = least preferred treatment). To aid this task, we provided comprehensive details of the treatments, which included the effectiveness of the drug, speed of response to treatment, route of administration, side effects and whether or not concomitant medication is needed (see Report Supplementary Material 3). These treatment descriptions were developed using published literature, with clinical input from the study team, and were presented to participants in a randomised order to reduce question order bias.
In the third section of the survey, we gathered sociodemographic details and information on the respondents’ personal history and severity of UC. The survey included two validated instruments, the IBD Control-8 questionnaire and the EuroQol-5 Dimensions, five-level version (EQ-5D-5L). The IBD Control-8 questionnaire captures disease control and impact from the respondents’ perspective and generates a summary score, ranging from 0 (representing worst control of disease) to 16 (representing best control of disease). 75 The EQ-5D-5L instrument captures respondents’ overall quality of life, generating a summary score of between –0.59 and 1, where higher scores represent better quality of life (and 1 represents perfect health). 76 The four survey sections contained feedback questions about the survey. A soft launch of the survey was undertaken (n = 50) to assess any problems, including comprehension and dropouts.
Sampling and recruitment
The study population included adults aged ≥ 18 years who had a diagnosis of UC. Participants were recruited primarily through two NHS trusts where staff working in IBD services advertised the study by sending participants invitation letters. The study was also advertised on social media (via Twitter and Facebook) to recruit further participants from across the UK. If participants decided to take part, then they were able to access the online survey via the Qualtrics platform and complete the survey after providing informed consent. Respondents were not offered any financial incentives for completing the survey. We hoped to recruit up to 300 survey participants as the literature shows that DCE sample sizes can range from 100 to 300 participants. 70 However, the minimum sample size required was n ≥ 83.3 to estimate a model using the rule-of-thumb approach. 69
Data analysis
Descriptive analyses were performed to analyse the demographic data and IBD characteristics of the respondents and to rank medications in order of importance. We performed conditional logistic regression models to analyse the DCE task data. All of the models report main effects, and no interaction terms were included. Attributes were first included as categorical variables using dummy coding; however, after confirming the linear relationship through visual inspection and model fit, speed and remission attributes were treated as continuous variables and effectiveness, administration and side effects as categorical variables in the main model. All statistical analyses were conducted using Stata.
The utility function to estimate the probability of choosing the preferred treatment profile takes the following form:
where V is a binary variable (1 = the preferred treatment profile is chosen, 0 = the treatment profile is not chosen), β is the estimated coefficient for each of the treatment attributes and ε is the unobserved error term.
Parameter estimates from the conditional model were also used to calculate the change in probability of uptake from a baseline scenario in which all attributes are set to their worst level and then one attribute is improved at a time72 (see Appendix 6).
Multistakeholder workshop
Study design
This WP involved a multistakeholder workshop with people with UC and IBD health-care professionals. The workshop was conducted remotely in line with COVID-19 restrictions on face-to-face contact. The workshop involved direct knowledge mobilisation, using the findings to help generate realistic and meaningful recommendations from the PoPSTER study in collaboration with key stakeholders.
Setting
The workshop was conducted online via the Blackboard Collaborate platform (Blackboard Inc., Washington, DC, USA).
Eligibility criteria
Adults with UC and health-care professionals who were medical or nursing staff working with patients with IBD were eligible to participate in the workshop.
Sampling
Statements were included on the consent forms for the qualitative interviews in WPs 2 and 3 to ascertain whether or not participants were interested in attending this workshop (in principle) to generate the sampling frame. In addition to this, a small number of professional contacts of the study team were invited to participate in the workshop. Both groups were sampled purposively to achieve representation of various patient and professional groupings.
Recruitment
Invitation e-mails were sent to all potential participants reminding them about the research and the purpose of the workshop and providing the date and time, and the participant information sheet was attached (see Report Supplementary Material 1). People who were interested in attending the workshop were then asked to register with the research team. More information about the workshop (i.e. log-in details and the agenda) were sent to those people who were able to attend. Informed consent to participate in the online workshop and to have their contributions video- and audio-recorded for use for research purposes was taken from participants remotely (hosted on Qualtrics) prior to the start of the workshop to facilitate smoother running from the outset.
Data collection
At the workshop, the key research findings from WPs 1–4 were presented to participants by the research team. To encourage reflection, to provide a focus for discussion and to promote clearer decision-making, the workshop was structured around Borton’s77 reflective prompt questions, that is, ‘what?’, ‘so what?’ and ‘now what?’. The motivation for this was to enable workshop attendees to consider the research findings and to generate recommendations for future research and practice for steroid-resistant UC. Therefore, the workshop was structured as follows.
What?
The research team gave a presentation of key research findings from WPs 1–4 of the PoPSTER study.
So what?
Small discussion groups considered the implications of the findings for future research and practice.
Now what?
We sought agreement from participants about what needs to happen next and key recommendations.
The small discussion groups (each including patient representatives, medics and nurses, and two members of the PoPSTER team who acted as facilitators) considered and discussed the key findings and agreed recommendations. A final plenary session was used to share recommendations from each small group.
Data analysis
A summary descriptive report of the workshop discussions and decision points was generated, using the ‘what?’, ‘so what?’ and ‘now what?’ framework (see Chapter 8).
Patient and public involvement
The PoPSTER study was developed in collaboration with people with UC. PPI activity is reported here and in Chapter 9 in accordance with the GRIPP2 (Guidance for Reporting Involvement of Patients and the Public 2). 78
At the initial grant application stage, we invited Sue Blackwell to join the co-applicant team and, as part of this, she reviewed the stage 1 application (leading to changes to the design of WPs 2 and 3 and to the content of the Plain English summary). At the second stage of grant application, clarifications were made to the dissemination strategy based on Sue Blackwell’s feedback. Sue Blackwell has expertise in digital marketing, which was helpful to study promotion throughout the grant.
During the preparation of the second-stage application, a local group of patients from Sheffield Teaching Hospitals NHS Foundation Trust were convened to seek wider feedback on the design and scope of the study. The group of patients varied in age (ranging from 20 to 80 years), gender and ethnicity, but they shared a positive response to the research. The group provided encouraging feedback on the study aims and methods, highlighting that these were easy to understand and appropriate to the aims of the research call. This group also made some specific recommendations for improving the study design, including making explicit the maximum interview duration for patients (i.e. 60 minutes), offering breaks to encourage and support those people experiencing a UC flare and including a measure of health-related quality of life in WP4, all of which were introduced. Through this group, Hugh Bedford was identified and was invited to join the team of co-applicants on the grant.
In addition to this, Alan Lobo and Elizabeth Coates worked closely with Sue Blackwell to organise a remote feedback session with patient representatives across the UK. Sue Blackwell distributed an appeal for patient feedback via her Facebook networks and nine people with UC shared their views on the proposed research. Again, this group welcomed the focus on research addressing treatment for steroid-resistant UC. As with the Sheffield group of patients, this group also highlighted the importance of offering breaks during interviews and providing flexible timings for the patient interviews. This group also suggested that limited patient knowledge about the treatment options could make participation in the DCE challenging (highlighting the importance of ongoing PPI input to study documentation and data collection materials). Again, all of these suggestions were incorporated into the final grant application. In addition to this, Nicola Dames was identified and was invited to join the team as a third PPI co-applicant.
During the conduct of the PoPSTER study, the three patient co-applicants (SB, HB and ND) were integral members of the Study Management Group. Therefore, the three patient co-applicants were invited to all Study Management Group meetings and asked to provide input to relevant study documentation, outputs and key issues arising during the delivery of the research, alongside the clinical and methodologist co-applicants. This ensured that the patient perspective informed the design, delivery and reporting of each stage of the studies.
In parallel with this, Nicola Dames and Sue Blackwell helped the research team to establish a separate PAG of four people living with UC (please refer to the Acknowledgements for information on the membership of this group) to get a wider perspective. This group was convened three times to coincide with key stages of the study. At the first meeting (April 2019), the members gave detailed feedback on the content of the qualitative interview schedules for patients. This feedback included comments on the importance of mental health and trauma in the lived experience of UC, comments on the influence of health-care professionals on treatment choices and the significance of that relationship to informing those decisions, a number of helpful clarifications to question wording and practical suggestions, such as sending the list of questions to patients in advance. At the second meeting (November 2019), the PAG members were presented with the headline findings from the WP2 and WP3 interviews and were asked to help with prioritising the long list of attributes for the patient DCE. Subsequent to the meeting, the PAG and the patient co-applicants piloted the DCE questionnaire and gave helpful feedback to improve content and presentation. At the final meeting (March 2021), the headline findings from the patient DCE were presented and the group gave feedback on those, as also helped to interpret the results.
Other PPI activity included promotion of patient DCE on social media by SFB to help support recruitment and ongoing support for study recruitment on Twitter by Sue Blackwell and Nicola Dames. Sue Blackwell, Hugh Bedford and Nicola Dames also gave valuable input by reviewing the Plain English summary and PPI sections of this report.
Ethics approval
Ethics approval for the PoPSTER study was granted by the NHS East Midlands – Derby Research Ethics Committee on 10 January 2019 (reference 19/EM/0011) and governance approval was granted by the Health Research Authority on 17 January 2019 (Integrated Research Application System reference 255616).
Protocol management and version history
See study protocol version 5.0, which is available online at URL: www.fundingawards.nihr.ac.uk/award/17/72/02 (accessed 26 April 2022). A protocol version history is provided in Appendix 1.
Chapter 3 Results from the health-care professional survey
Participants
There were 387 unique visitors to the online survey, and 168 of these visitors consented to take part in the survey (i.e. a 43% participation rate). Of these 168 participants, 145 started the survey and 88 completed every survey question, giving an overall completion rate of 52%. The denominator for individual questions varied from 88 to 145, and is reported for each question to help facilitate interpretation of the results.
The characteristics of participants are summarised in Table 3 (see also Appendix 2, Table 24). The majority of survey respondents were medics (n = 99, 68%) and 44 (31%) were nurses. Fifty-five (38%) participants were working as consultant gastroenterologists with a special interest in IBD. On average, the participants were appointed 9.6 years ago. Eighty-one per cent of participants had no personal experience of IBD. Eighty-eight (62%) participants were from secondary referral centres, 48 (34%) from tertiary referral centres and five (3%) from quaternary referral centres. All UK regions were represented in the survey. Most participants stated that some (n = 59, 41%) or the majority (n = 53, 37%) of their clinical time was dedicated to working with IBD. The majority of participants were part of a MDT (n = 133, 93%) containing, on average, 14 health-care professionals. Most participants reported that they were using national and European guidelines for managing UC (77% and 87%, respectively), as well as NICE guidance for specific treatments (66–83%).
| Characteristic | Frequency (N = 145) |
|---|---|
| Profession, n (%) | |
| Doctor | 99 (68) |
| Nurse | 44 (30) |
| Other | 0 (0) |
| Missing | 2 (1) |
| Current job title, n (%) | |
| Consultant IBD specialist | 19 (13) |
| Consultant gastroenterologist with special interest in IBD | 55 (38) |
| Consultant gastroenterologist with special interest that is not IBD | 24 (17) |
| IBD specialist nurse | 44 (30) |
| Other | 0 (0) |
| Missing | 3 (2) |
| Years since appointment | |
| Mean (SD) | 9.6 (6.9) |
| Range | 1–27 |
| Median (IQR) | 7.5 (11) |
| Personal experience of IBD, n (%) | |
| Yes, I have IBD | 6 (4) |
| Yes, one of my family or friends has IBD | 20 (14) |
| No | 117 (81) |
| Prefer not to say | 0 (0) |
| Missing | 2 (1) |
Definitions of steroid resistance
As shown in Table 4, definitions of steroid-resistant UC varied, with 68% (92/135) of participants agreeing or strongly agreeing that this is indicated by an incomplete response to prednisolone at 40 mg per day (or equivalent) after 2 weeks. Of the remaining participants, 58% (25/43) agreed or strongly agreed that steroid resistance was indicated by an incomplete response after 4 weeks. If participants did not agree with either of the definitions presented (n = 13), then they were given the opportunity to design their own definition of steroid-resistant UC using pre-set response categories (see Appendix 2, Box 1).
| Time frame | Level of agreement with statement: corticosteroid resistance constitutes an incomplete response to prednisolone 40 mg/day (or equivalent) | |||||
|---|---|---|---|---|---|---|
| Strongly disagree | Disagree | Neither agree or disagree | Agree | Strongly disagree | Missing | |
| After 2 weeks (N = 135), n (%) | 4 (3) | 18 (13) | 18 (13) | 72 (53) | 20 (15) | 3 (2) |
| After 4 weeks (N = 43), n (%) | 0 (0) | 5 (12) | 8 (19) | 23 (53) | 2 (5) | 5 (12) |
Table 5 shows that 77 (57%) of participants agreed that steroid resistance did not include those patients who relapse after initial remission with corticosteroids (i.e. steroid dependent). A greater proportion of participants felt that treatments should be different for steroid-dependent and steroid-resistant disease at each time interval from 2 weeks to 3 months. Whereas, at 6 months, more participants felt that the treatment options should not differ. Only 13% of participants felt that steroid-dependent and steroid-resistant disease should be treated identically, regardless of the interval between remission and subsequent relapse.
| Definition of steroid resistance | Response (N = 135), n (%) | |||
|---|---|---|---|---|
| Yes | No | Unsure | Missing | |
| Does corticosteroid resistance include any patients who go into clinical remission after starting prednisolone treatment, but then relapse on corticosteroid reduction? | 32 (24) | 77 (57) | 12 (9) | 14 (10) |
| If no, after what period of remission would you consider a relapse to be classed as steroid dependent or resistant and, therefore, require different options for treatment? | ||||
| 2 weeks | 45 (58) | 17 (22) | 6 (8) | 9 (12) |
| 4 weeks | 57 (74) | 5 (6) | 6 (8) | 9 (12) |
| 3 months | 40 (52) | 18 (23) | 5 (6) | 14 (18) |
| 6 months | 20 (26) | 38 (49) | 7 (9) | 12 (16) |
| The situations should be managed identically after any interval | 10 (13) | 34 (44) | 6 (8) | 27 (35) |
Treatment options for steroid-resistant and steroid-dependent clinical scenarios
Clinical scenarios
To elicit their treatment preferences for patients with moderately severe UC, both steroid-resistant and steroid-dependent participants were presented with four typical clinical scenarios (Table 6). Participants were presented with six further typical clinical scenarios to elicit their treatment preferences for steroid-dependent disease that relapses at different levels of steroid reduction (Table 7).
| Patient | Scenario |
|---|---|
| Steroid resistant: on thiopurine | In treating a patient with UC, which additional treatment(s) (assuming that all of these are available in your centre) would you offer to someone with moderately severe disease and with no, or inadequate, response to systemic outpatient corticosteroid treatment? |
| Steroid resistant: thiopurine naive | As above, but patient is naive to thiopurine treatment |
| Steroid dependent: on thiopurine | In treating a patient with UC, what treatments (assuming that all of these are available in your centre) would you offer either alone or in combination for someone with moderately severe disease, who responds to steroids, but rapidly relapses when the dose is reduced? |
| Steroid dependent: thiopurine naive | As above, but patient is naive to thiopurine treatment |
| Patient | Scenario |
|---|---|
| Steroid resistant: thiopurine naive | Steroid resistant after 4 weeks of prednisolone 40 mg/day: thiopurine naive |
| Steroid resistant: on thiopurine | Steroid resistant after 4 weeks of prednisolone 40 mg/day: on thiopurine |
| Steroid dependent with relapse at 25 mg/day: thiopurine naive | Response to prednisolone 40 mg/day followed by relapse when dose reduced to 25 mg/day: thiopurine naive |
| Steroid dependent with relapse at 25 mg/day: on thiopurine | Response to prednisolone 40 mg/day followed by relapse when dose reduced to 25 mg/day: on thiopurine |
| Steroid dependent with relapse at 5 mg/day: thiopurine naive | Response to prednisolone 40 mg/day followed by relapse when dose reduced to 25 mg/day: thiopurine naive |
| Steroid dependent with relapse at 5 mg/day: on thiopurine | Response to prednisolone 40 mg/day followed by relapse when dose reduced to 25 mg/day: on thiopurine |
Treatment options for steroid-resistant and steroid-dependent clinical scenarios
The results are shown in Table 8 and Figure 3, with additional information on treatment preferences given in Appendix 2, Tables 25–28. In steroid-resistant patients, anti-TNF agents (i.e. infliximab, adalimumab and golimumab) were the most frequently suggested treatments for both those exposed to thiopurines and those who were thiopurine naive [95% (n = 114) and 87% (n = 104), respectively]. In steroid-dependent patients, anti-TNF agents remained the most frequently offered [88% (n = 105) in thiopurine-exposed patients and 75% (n = 90) in thiopurine-naive patients]. In all scenarios, infliximab was the most frequently suggested treatment, suggested by 94% (n = 113), 73% (n = 88), 86% (n = 103) and 67% (n = 80), respectively (and albeit with thiopurine or methotrexate in the thiopurine-naive groups).
| Treatment option | Number (%) of responses | |||
|---|---|---|---|---|
| Steroid resistant: thiopurine exposed | Steroid resistant: thiopurine naive | Steroid dependent: thiopurine exposed | Steroid dependent: thiopurine naive | |
| Anti-TNF agent | 114 (95) | 104 (87) | 105 (88) | 90 (75) |
| Admit for i.v. steroids | 78 (65) | 76 (63) | 23 (19) | 23 (19) |
| Thiopurine | 64 (53) | 70 (58) | ||
| Vedolizumab | 83 (69) | 71 (59) | 92 (77) | 73 (61) |
| Tofacitinib | 51 (42) | 34 (28) | 50 (42) | 33 (28) |
| Other | 87 (72) | 73 (61) | 74 (62) | 82 (68) |
FIGURE 3.
Treatment options for steroid-resistant and steroid-dependent patients.
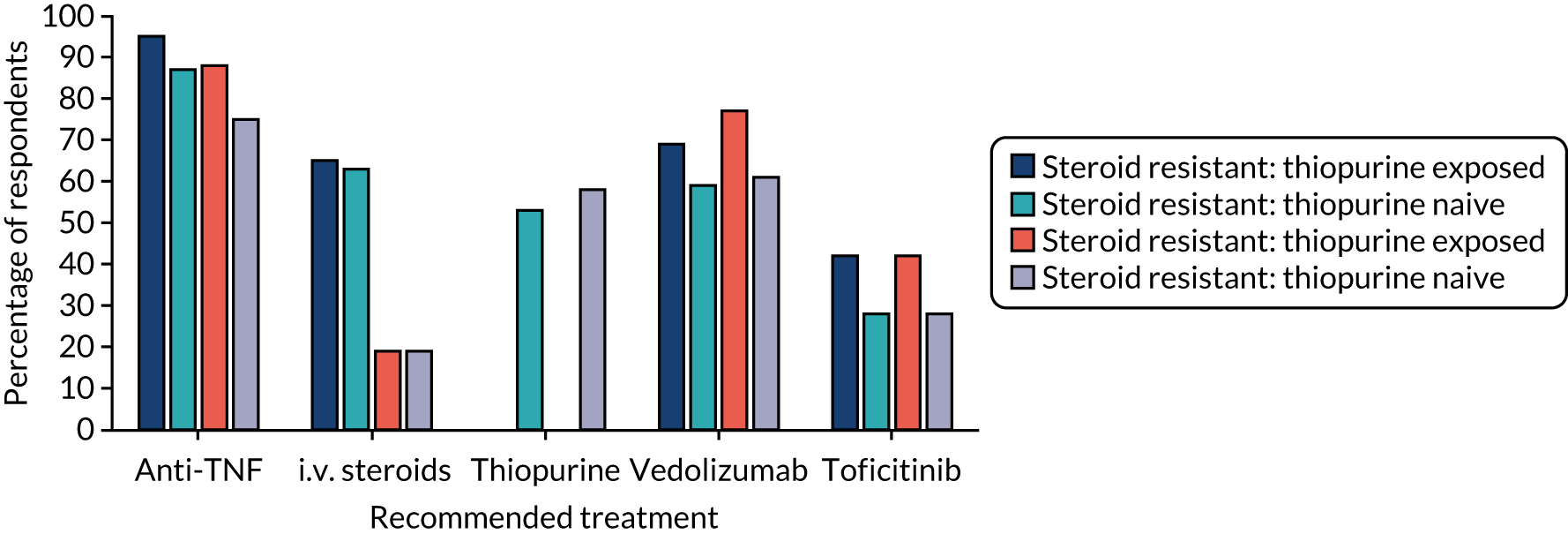
Vedolizumab was more frequently offered to steroid-dependent patients on thiopurines than to those with steroid-resistant disease on thiopurines, but this difference was not statistically significant [n = 92 (77%) vs. n = 83 (69%); p = 0.137]. As would be expected, admission for i.v. steroids was less frequently offered in steroid-dependent scenarios than in steroid-resistant scenarios [n = 23 (19%) vs. n = 76/78 (63–65%); p < 0.001]. Across all four scenarios, tofacitinib would be more likely to be offered in patients already on thiopurines than in patients naive to thiopurine [n = 101 (42%) vs. 67 (28%), χ2 = 10.586(1); p = 0.001].
Treatment options for clinical scenarios with steroid-dependent disease and relapse at different doses
The results are shown in Table 9 and in Figures 4–6, and in full in Appendix 2, Tables 29–34. Anti-TNF drugs were, again, the most frequently suggested treatment for steroid-resistant disease, with significantly more patients on thiopurines being offered anti-TNF agents than patients who were thiopurine naive [n = 75 (81%) vs. n = 58 (62%); p < 0.001]. For patients receiving prednisolone at 25 mg per day, on relapse, 78% (n = 73) of clinicians would offer an anti-TNF agent to thiopurine-exposed patients and 49% (n = 46) of clinicians would offer an anti-TNF agent to thiopurine-naive patients (p < 0.001). Forty-four (47%) clinicians would offer thiopurine-naive patients a further increase in steroids to allow thiopurine or methotrexate introduction and 46 (49%) clinicians would introduce thiopurines alone. Sixty-seven (72%) clinicians would offer either of these options in thiopurine-naive patients. For patients receiving prednisolone at 5 mg per day, on relapse, 43 (46%) clinicians would offer anti-TNF agents for a thiopurine-naive patient and 65 (70%) clinicians would introduce a thiopurine. In an individual on thiopurine, 79 (85%) clinicians would offer an anti-TNF agent, 43 (46%) clinicians would offer vedolizumab, 23% (n = 21) clinicians would offer tofacitinib and 20 (22%) clinicians would increase the steroids alone.
| Treatment option | Number (%) of responses | |||||
|---|---|---|---|---|---|---|
| Steroid resistant | Steroid dependent with relapse at 25 mg/day | Steroid dependent with relapse at 5 mg/day | ||||
| Thiopurine naive | Thiopurine exposed | Thiopurine naive | Thiopurine exposed | Thiopurine naive | Thiopurine exposed | |
| Anti-TNF agent | 58 (62) | 75 (81)* | 46 (49) | 73 (78)** | 43 (46) | 79 (85)*** |
| Admit for i.v. steroids | 30 (32) | 36 (39) | 11 (12) | 15 (16) | 10 (11) | 7 (8) |
| Thiopurine (including increase in steroid dose and addition of thiopurine) | 39 (42) | 6 (6) | 67 (72) | 11 (12) | 65 (70) | |
| Vedolizumab | 27 (29) | 41 (44) | 25 (27) | 44 (47) | 36 (39) | 43 (46) |
| Tofacitinib | 20 (22) | 23 (25) | 19 (20) | 20 (22) | 14 (15) | 21 (23) |
| Other | 44 (47) | 50 (54) | 52 (56) | 45 (48) | 42 (45) | 49 (53) |
FIGURE 4.
Percentage of respondents who would offer an anti-TNF agent according to steroid dose at relapse (thiopurine naive and exposed).
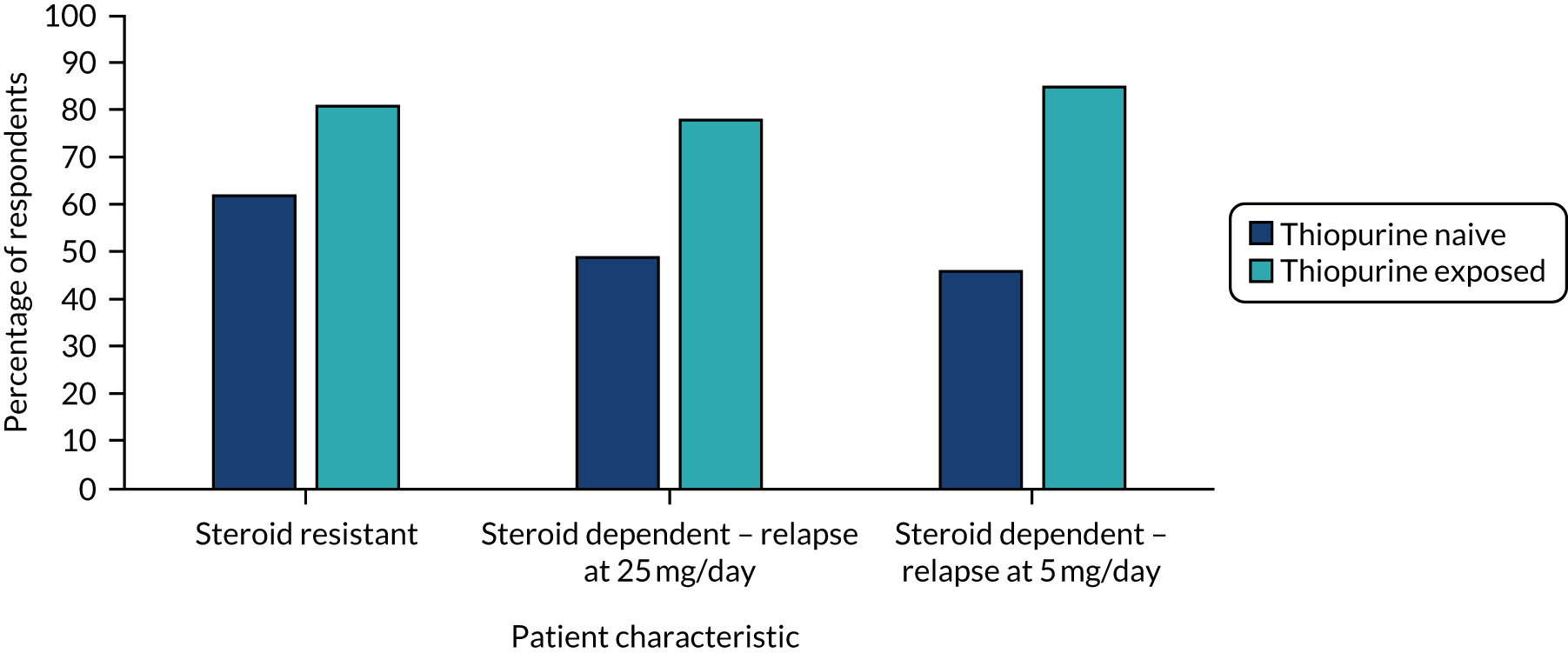
FIGURE 5.
Percentage of respondents who would offer vedolizumab according to steroid dose at relapse (thiopurine naive and exposed).
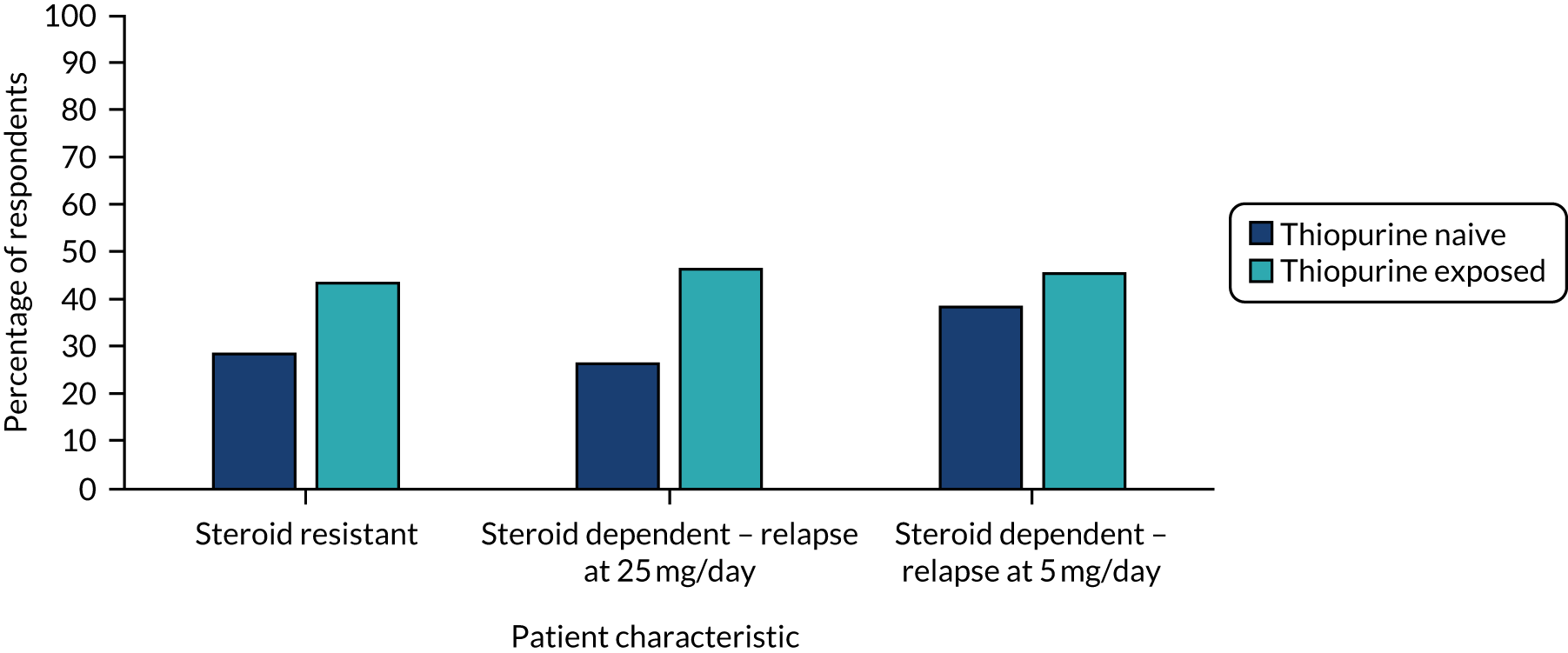
FIGURE 6.
Percentage of respondents who would offer tofacitinib according to steroid dose at relapse (thiopurine naive and exposed).
The difference in suggested use of anti-TNF agents, depending on thiopurine use and dose of steroid at relapse, is shown in Figure 4. In those patients who are naive to thiopurines, there is reducing likelihood that anti-TNF agents will be offered when steroid-resistant patients are compared with patients who relapse at a prednisolone dose of 25 mg and 5 mg [62% vs. 49% (p = 0.014) and 46% (p = 0.009), respectively]. Anti-TNF treatment would be used more frequently in patients on thiopurines, but with no difference at the differing prednisolone doses at relapse (78% vs. 85%; p = 0.077).
As shown in Appendix 2, Tables 29–34, across the scenarios, infliximab was the most frequently offered treatment (albeit with thiopurine or methotrexate in the thiopurine-naive steroid-resistant group), apart from in thiopurine-naive steroid-dependent patients who relapse at a prednisolone dose of 25 mg or 5 mg, where thiopurine was most preferred [at 49% (n = 46) and 70% (n = 65), respectively].
The frequency with which vedolizumab was suggested (see Figure 5) did not change with flares as a consequence of steroid dose reduction (range 44–47%) in thiopurine-exposed patients; however, vedolizumab was most frequently offered (albeit not statistically significant) in thiopurine-naive patients who flared at 5 mg, than when flares occurred at 25 mg daily (p = 0.003) or in a resistant scenario (p = 0.006). Offering tofacitinib occurred less frequently (at 15–23% across all scenarios) and the frequency did not change according to the steroid dose or with thiopurine use (see Figure 6).
Treatment availability
Participants were asked about the availability of treatments for UC at their local IBD centres. Treatment availability was good, with 52% (62/120) of respondents stating that all treatments listed were available in their IBD centre. A small number of participants were not able to access adalimumab (n = 1, 1%), infliximab (n = 1, 1%), vedolizumab (n = 2, 2%) or methotrexate (n = 3, 2%), and tacrolimus, golimumab, tofacitinib and ciclosporin were not available to 13% (n = 16), 10% (n = 12), 7% (n = 8) or 4% (n = 5) of respondents, respectively.
Factors influencing treatment choices
To understand the importance of a range of factors influencing treatment decisions for patients with steroid-resistant UC (both thiopurine naive and exposed), participants were asked to rate factors on a five-point Likert scale (i.e. very important, important, neutral, low importance and not at all important). These responses were converted from five to three categories (i.e. important, neutral and not important). The results for thiopurine-exposed patients are displayed in order of most importance in Table 10.
| Factor | Thiopurine exposed (N = 103), n (%) | Thiopurine naive (N = 103), n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Important | Neutral | Not important | Missing | Important | Neutral | Not important | Missing | |
| Efficacy | 99 (96) | 1 (1) | 0 (0) | 3 (3) | 91 (88) | 0 (0) | 0 (0) | 12 (12) |
| Effect on quality of life | 98 (95) | 2 (2) | 0 (0) | 3 (3) | 91 (88) | 0 (0) | 0 (0) | 12 (12) |
| Patient preference | 95 (92) | 6 (6) | 0 (0) | 2 (2) | 85 (83) | 5 (5) | 0 (0) | 13 (13) |
| Effect on mucosal healing | 93 (90) | 6 (6) | 1 (1) | 3 (3) | 82 (80) | 8 (8) | 1 (1) | 12 (12) |
| Previous cancer | 92 (89) | 6 (6) | 2 (2) | 3 (3) | 83 (81) | 5 (5) | 2 (2) | 13 (13) |
| Comorbidity | 92 (89) | 5 (5) | 1 (1) | 5 (5) | 84 (82) | 7 (7) | 0 (0) | 12 (12) |
| Safety: frequency of side effects | 92 (89) | 7 (7) | 0 (0) | 4 (4) | 87 (84) | 4 (4) | 0 (0) | 12 (12) |
| Effect on fertility/pregnancy | 91 (88) | 10 (10) | 0 (0) | 2 (2) | 79 (77) | 10 (10) | 1 (1) | 13 (13) |
| Safety: severity of drug side effects, if rare | 89 (86) | 9 (9) | 3 (3) | 2 (2) | 76 (74) | 9 (9) | 3 (3) | 15 (15) |
| Burden on patient | 89 (86) | 11 (11) | 1 (1) | 2 (2) | 74 (72) | 14 (14) | 3 (3) | 12 (12) |
| Potential impact of side effects | 88 (85) | 12 (12) | 0 (0) | 3 (3) | 85 (83) | 6 (6) | 0 (0) | 12 (12) |
| Patient age | 87 (84) | 13 (13) | 1 (1) | 2 (2) | 79 (77) | 9 (9) | 2 (2) | 13 (13) |
| Cancer risk from drugs | 84 (82) | 10 (10) | 7 (7) | 2 (2) | 76 (74) | 11 (11) | 4 (4) | 12 (12) |
| Availability of a treatment in your centre | 84 (82) | 5 (5) | 11 (11) | 3 (3) | 73 (71) | 9 (9) | 7 (7) | 14 (14) |
| Route of administration of medication | 81 (79) | 14 (14) | 6 (6) | 2 (2) | 72 (70) | 13 (13) | 5 (5) | 13 (13) |
| Disease-related risk of cancer | 81 (79) | 11 (11) | 8 (8) | 3 (3) | 76 (74) | 9 (9) | 6 (6) | 12 (12) |
| Your own familiarity with treatment option as a clinician | 80 (78) | 12 (12) | 8 (8) | 3 (3) | 69 (67) | 14 (14) | 8 (8) | 12 (12) |
| Effect on patient intimacy | 57 (55) | 32 (31) | 11 (11) | 3 (3) | 51 (50) | 31 (30) | 8 (8) | 13 (13) |
| Infusion bay or service capacity | 44 (43) | 24 (23) | 32 (31) | 3 (3) | 45 (44) | 21 (20) | 25 (24) | 12 (12) |
| Cost | 42 (41) | 29 (28) | 30 (29) | 2 (2) | 43 (42) | 25 (24) | 23 (22) | 12 (12) |
| Hospital inpatient bed use | 39 (38) | 28 (27) | 33 (32) | 3 (3) | 33 (32) | 29 (28) | 29 (28) | 12 (12) |
Whether or not patients had been exposed to thiopurines, treatment efficacy and effect on quality of life were most frequently rated as important, at 96% (n = 99), 95% (n = 98) and 88% (n = 91), respectively. Although the frequency of ratings was often lower for patients who are naive to thiopurines, more than two-thirds of respondents rated most of the factors as being important to treatment decisions for steroid-resistant patients (ranging from 67% to 92% positive ratings across both groups). Differences between the groups were statistically significant for effect on fertility/pregnancy only (p = 0.010).
Some factors were less frequently rated as important for both thiopurine-exposed and thiopurine-naive patients, that is, effect on intimacy, infusion bay or service capacity, cost and hospital inpatient bed use, with lower importance scores (32–55%) and greater neutral (20–31%) or negative ratings (8–31%) relative to the other factors. Taken together, these findings highlight the wide range of factors that potentially influence treatment decisions in this patient group.
Referral for surgery
Participants were asked ‘In general terms, in which situations would you consider referring a patient with corticosteroid-resistant UC, of moderate severity, for surgery?’. As shown in Table 11, the majority (n = 57, 48%) of participants stated that they would offer surgery ‘at any time’, whereas 12% (n = 14) would wait to offer surgery only once all medical options had been used. Only two respondents stated that they would refer a patient to surgery once they had been deemed resistant to steroids. Six per cent (n = 7) of participants would offer surgery after unsuccessful use of one biologic and 9% (n = 11) of participants would do so after unsuccessful use of two biologic therapies.
| Timing of surgery referral | Overall (N = 120), n (%) | Medics (N = 83), n (%) | Nurses (N = 35), n (%) | Tertiary and quaternary (n = 44), n (%) | Secondary (N = 74), n (%) | Majority/all of your time (N = 63), n (%) | Some/little of your time (N = 54), n (%) |
|---|---|---|---|---|---|---|---|
| Once the patient has been deemed to be resistant to systemic corticosteroids | 2 (2) | 0 (0) | 2 (6) | 0 (0) | 2 (3) | 2 (3) | 0 (0) |
| After the patient has tried one biologic and this was unsuccessful | 7 (6) | 5 (6) | 2 (6) | 5 (11) | 2 (3) | 3 (5) | 4 (7) |
| After the patient has tried two biologic therapies and this was unsuccessful | 11 (9) | 6 (7) | 5 (14) | 3 (7) | 8 (11) | 7 (11) | 4 (7) |
| Only when the patient asks about this | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Only after using all available medical options | 14 (12) | 10 (12) | 4 (11) | 5 (11) | 9 (12) | 6 (10) | 8 (15) |
| I would not consider offering surgery | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 4 (3) | 3 (4) | 1 (3) | 0 (0) | 4 (5) | 3 (5) | 1 (2) |
| At any time | 57 (48) | 42 (51) | 15 (43) | 26 (59) | 31 (42) | 27 (43) | 30 (56) |
| Missing | 25 (21) | 17 (20) | 6 (17) | 5 (11) | 18 (24) | 15 (24) | 7 (13) |
Participants who would offer surgery ‘at any time’ were asked to clarify which factors would influence their decision to refer patients. Sixty-one per cent (n = 35) of respondents cited patient preferences for treatment options as the reason to refer patients. Over half (n = 31, 54%) of respondents indicated that patients were jointly managed by surgeons in the local MDT and, therefore, surgery was presented early in the treatment pathway for UC. Both failure to respond to medical treatment options (n = 18, 32%) and disease severity (n = 14, 25%) were also important reasons. The four respondents who selected ‘other’ for the timing of surgery referral also gave similar reasons.
Differences in practice around surgery referral between key subgroups (i.e. profession, centre type and clinical time devoted to IBD care) were explored using chi-squared tests. However, there were no statistically significant differences in timing of surgery referral between professions (medics vs. nurses; p = 0.28), centre type (tertiary and quaternary vs. secondary; p = 0.14) or clinical time devoted to IBD care (majority/all of your time vs. some/little; p = 0.49).
Use of endoscopy
Participants were asked about how they use endoscopy with steroid-resistant and steroid-dependent patients (Tables 12 and 13). In a patient with ongoing symptoms, resistant to what the respondent felt was their maximum duration and dose of systemic steroids, 51% (n = 45) of participants would always carry out endoscopic assessment to confirm disease activity, whereas 43% (n = 38) of participants stated that they would never do so. For steroid-resistant patients, a moderate degree or more severe symptoms/unchanged from the last examination were most frequently reported as being indicative of a need for treatment change, at 42% (n = 37) and 31% (n = 27), respectively.
| Patient | Use of endoscopy (N = 88), n (%) | |||
|---|---|---|---|---|
| Always | Sometimes | Never | Missing | |
| Steroid resistant | 45 (51) | 1 (1) | 38 (43) | 4 (5) |
| Steroid dependent | 27 (31) | 5 (6) | 51 (58) | 5 (6) |
| Patient | Degree of endoscopic appearance indicative of change of treatment required (N = 88), n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Normal/inactive | Mild | Moderate | Severe | Any degree | More severe/unchanged | Missing | |
| Steroid resistant | 0 (0) | 8 (9) | 37 (42) | 0 (0) | 11 (13) | 27 (31) | 5 (6) |
| Steroid dependent | 0 (0) | 7 (8) | 33 (38) | 2 (2) | 13 (15) | 23 (26) | 10 (11) |
In contrast, in a patient whose symptoms rapidly recur on reduction of systemic steroid dose (at or before reaching 15 mg/day), only 31% (n = 27) of participants would always undertake endoscopic assessment and 58% (n = 51) of participants reported that they would never do so. As with steroid-resistant patients, a moderate degree (n = 33, 38%) or more severe symptoms/unchanged from the last examination (n = 23, 26%) were most frequently reported as being indicative of a need for treatment change.
Chapter 4 Results from qualitative interviews with health-care professionals
Participants
A total of 25 health-care professionals were approached for interview, but five health-care professionals did not reply (n = 3) or there was loss of contact, despite initial expression of interest (n = 2).
Twenty health-care professionals participated in the qualitative interviews (Table 14). Twelve (60%) participants were consultant gastroenterologists and eight (40%) were IBD nurses. The median time since appointment was 14 (minimum–maximum 2–21) years. There was an equal split in the gender of participants. Participants were based in all regions of England and Wales, and there was a 50 : 50 split between secondary and tertiary referral centres. The median length of individual interviews was 33 (minimum–maximum 18–60) minutes.
| Characteristic | Frequency |
|---|---|
| Job title, n (%) | |
| Consultant gastroenterologist | 12 (60) |
| IBD specialist nurse | 8 (40) |
| Gender, n (%) | |
| Male | 10 (50) |
| Female | 10 (50) |
| Median years since appointed (minimum–maximum) | 14 (2–21) |
| Region, n (%) | |
| East Anglia | 2 (10) |
| East Midlands | 1 (5) |
| London | 1 (5) |
| North East | 1 (5) |
| North West | 3 (15) |
| South West | 5 (25) |
| Wales | 1 (5) |
| West Midlands | 2 (10) |
| Yorkshire and the Humber | 4 (20) |
| Hospital type, n (%) | |
| Secondary referral service | 10 (50) |
| Tertiary referral service | 10 (50) |
Analysis
Through familiarisation with the interview transcripts, an initial list of codes was developed. After several iterations, it was possible to agree on a coding framework that included 32 codes, which were grouped under five categories (see Appendix 3, Table 35). The coding framework was then applied to the whole data set and summaries of each code were produced to help identify themes. The presentation of the findings in this chapter is structured around these themes and subthemes, as follows.
Definitions of steroid resistance
Steroid-resistant ulcerative colitis
Health-care professionals were asked about their understandings of steroid resistance in UC and how they operationalise these definitions in practice. In line with the findings from the health-care professional survey, the majority of health-care professionals explained that they define steroid resistance as a lack of response or absence of clinical improvement in symptoms after 2 weeks of 40 mg per day of prednisolone:
I would define that as failure to improve symptoms despite 2 weeks of adequate corticosteroid therapy.
Consultant gastroenterologist 6
I mean I would say if they haven’t responded after 2 weeks, I would call them resistant.
Consultant gastroenterologist 7
However, although the 2-week time frame was commonly used to delineate steroid resistance, this was not necessarily considered to be a ‘hard and fast’ definition. Therefore, several health-care professionals explained that this time frame operated more like a spectrum and they would consider an earlier lack of response over a 1- to 2-week period as indicative of steroid resistance. The shorter time frame was generally considered more meaningful when patients’ symptoms were more severe:
I mean it depends on the level of the symptoms, you know, if they’re very severe then I wouldn’t sit on them for more than 1 week, not even that long probably.
IBD nurse 3
I would expect to see the initial response to steroids within a week or two of starting them. It depends on what’s happened and if someone’s had no response within that time frame . . . then I would’ve counselled them when they first started them to let the IBD nurses know.
Consultant gastroenterologist 4
A few of the health-care professionals interviewed in this study defined steroid resistance as a lack of response to 40 mg per day of prednisolone over 4 weeks. These health-care professionals explained that a cohort of patients may need longer to respond to steroids and would be monitored over 1 month. Despite this, other health-care professionals suggested that the 4 weeks was a ‘historical definition’ and said that they would have concerns about waiting that long to see how patients respond:
So it’s the patients that have not responded to, or responded and relapsed to a course of steroid medication and usually I would define that as 4 weeks.
Consultant gastroenterologist 10
I mean we would normally now receive patients within 2 weeks to start a high dose of oral steroids and if they haven’t responded then actually they’re terribly ill. So we’re just mindful that the old definitions are I think somewhat redundant now.
Consultant gastroenterologist 9
To understand the response to steroid treatments, participants explained that they would typically rely on a review of symptoms (i.e. number of stools per day, bleeding and pain) and, if these do not improve, assess faecal calprotectin and C-reactive protein (CRP) levels. In addition, where necessary, participants would undertake a flexible sigmoidoscopy.
One participant said that they use alternative terminology to describe this group of patients, preferring ‘steroid non-responders’ because patients could see ‘resistance’ as pejorative. This participant explained that using the term ‘resistant’ suggests to patients that the problem lies with them whereas the term ‘non-responder’ is more balanced, as they explain here:
I call them steroid non-responders and that basically tells the patient that you’ll be started on or given a fair trial of steroids, which is our first line of treatment for active ulcerative colitis, and for whatever reason, you fall into a group where your disease is not responding to treatment. So that automatically gets the patient thinking through the fact that they will need something different, so it primes them to expect a change in therapy.
Consultant gastroenterologist 3
Steroid-dependent ulcerative colitis
In line with the survey findings, the majority of health-care professionals differentiated dependence from resistance on the basis that patients who are resistant do not respond at all, whereas those who are dependent may respond initially, but as the dose is tapered their symptoms return. Most participants defined this in general terms:
Well, steroid-dependent patients respond to steroids but they relapse as soon as you taper the dose.
Consultant gastroenterologist 8
I think steroid dependence is the steroids work but then when you reduce them, the symptoms come back, whereas steroid resistant is that they just don’t work at all.
IBD nurse 1
Steroid dependence is when patients have relapse of their symptoms of colitis, by way of increase in stool frequency and recurrence or increase in rectal bleeding below a certain dose of steroid.
Consultant gastroenterologist 3
A minority of participants reported that steroid dependence would be monitored over a 3-month period, in line with ECCO79 and BSG13 guidelines, and many participants suggested that steroid dependence would be indicated by a reduction to 10 mg of steroids; however, other doses were also mentioned (e.g. 15 mg and 20 mg). Two participants also reported another related group of patients whom they described as being reliant (dependent) on steroids to feel better generally, irrespective of the impact on UC symptoms.
Treatments for steroid-resistant ulcerative colitis
Health-care professionals were asked to describe the treatments that they use with patients whose UC is resistant to steroids. Overall, health-care professionals consistently explained that they followed a treatment pathway that started with optimisation of aminosalicylates for mild to moderate disease, before escalating to thiopurines (e.g. azathiopurine or mercaptopurine) for patients able to tolerate this treatment, followed by biologic therapies, such as anti-TNF treatments, vedolizumab or tofacitinib. For example, as this IBD nurse succinctly explained:
So we would offer them thiopurines, so azathioprine and mercaptopurine, we usually use azathioprine first line and then mercaptopurine as second line and then if patients fail on that, then we would go at biologics and our trust we generally use infliximab as first line depending on the reasons for any loss of response we’d then go to adalimumab. Obviously if it’s the loss of response because they’ve built up antibodies, then we would switch out of class and use vedolizumab but and then now we’d probably do tofacitinib, but that is more of like a third line at the moment, unless there’s a reason why we can’t use any of the others.
IBD nurse 1
Some health-care professionals explained that they may not always use thiopurines and prefer instead to move straight to biologic treatments as first-line agents when steroids are not working. When considering anti-TNF treatments, infliximab was commonly described as the first-line treatment option, with adalimumab or golimumab used to a lesser extent. During the interviews, it was clear that practice around the use of tofacitinib varied, which is not surprising, given the timing of this study. It was evident that some health-care professionals were already using this on a parity with other biologics, whereas other participants were more hesitant and have not yet tried this with any or many UC patients:
At the moment in most patients we probably still use an anti-TNF first line in that patient population because of familiarity and relatively straight-forward access. In some patients I would consider using tofacitinib, we’ve got reasonable access to that; locally we’ve treated 36 patients so far, some of whom were biologic naive, which is unusual. I think most UK sites are using tofacitinib and have mainly used it in patients who have been refractory to other biologic or to biologics.
Consultant gastroenterologist 4
. . . so the things that changed relatively recently, which is interesting, so for, so if we just start with steroid-refractory disease, so people who have got active disease that hasn’t responded to steroids, and you need an induction agent, we would probably use, here tofacitinib first line now.
Consultant gastroenterologist 5
But those would be our sort of two, the erm TNF-alpha blockers, plus the vedolizumab, we haven’t yet had to use tofacitinib acutely yet.
Consultant gastroenterologist 9
And we are looking into a new drug that’s out there, tofacitinib, but obviously there is quite a lot of things to consider when putting patients on, you know, using the tofacitinib.
IBD nurse 7
Health-care professionals also explained how their treatment decisions are made on a patient-by-patient basis and so their relationships with patients were paramount to their understanding of the most appropriate next treatment. More broadly, health-care professionals explained that their decisions are informed by their knowledge of different treatments, which is gathered from clinical guidelines, research, conferences and their IBD colleagues.
Factors influencing treatment choices by health-care professionals
Health-care professionals were also asked about the factors that they take into account when offering treatments to people with UC. As in the survey results, a broad range of inter-related issues were identified as important considerations, and are summarised below. It is also important to note that most interview participants were keen to clarify that they consider treatment choices on a case-by-case or patient-by-patient basis, taking into account different factors as necessary, and, as explained in Patient preference, patient preferences are key.
Treatment effectiveness
Perhaps unsurprisingly, all health-care professionals explained that the effectiveness of treatments for UC was a major factor in their treatment choices for steroid-resistant patients. Several different aspects of treatment effectiveness were discussed during the interviews. The capacity of treatments to alleviate symptoms and the speed of response to treatment were typically reported as most important to patients when choosing a treatment and, as a consequence, of great importance to health-care professionals. This was particularly the case for patients experiencing more severe symptoms, for whom quick-acting treatments would be preferred over treatments with a longer-term effectiveness profile (e.g. recommending infliximab or tofacitinib over vedolizumab or adalimumab). Symptom relief was, of course, described as important to patients in helping to improve their quality of life:
I think initially when you’re dealing with it at the beginning it, you’re talking about quality of life, lots of patients are reporting that they can’t sleep, they can’t go to work, they can’t go to school, you know, whatever it is that they can’t do. They’re not able to perform their usual activities of living, so initial thoughts really are for that, you know it’s more around getting the patient’s symptoms under control.
IBD nurse 3
Managing patient expectations around the likely speed of response was considered important, and health-care professionals were mindful of differential responses to treatments:
I think in an ideal world we’d all have magic potions that would work within hours, I think you know it does play a part but I think if we manage patient expectations about how long drugs might take to work, then that can be overcome. I think if you’ve got steroid-resistant disease, then you may be making a choice about something you know is gonna work pretty quickly because you want patients to get symptomatic relief.
IBD nurse 2
Several participants contrasted the patient focus on symptom relief with their professional concern with maintaining remission (and potentially achieving mucosal healing). Having an understanding of the likely remission rates for different treatments was another important factor in what is offered to patients:
I guess the other thing we haven’t said on either mine or patients’ reasons for choosing medications would be about longevity of response for remission for a given treatment, whether they’re going to still be well, you know, in 3, 5 years’ time on the same drug or whether the drug will have stopped working, because that does differ between drugs.
Consultant gastroenterologist 4
I think they are all important so you’ve got efficacy, so short-term efficacy and long-term efficacy I think are important things, you know whether it works in remission and whether it works to maintain remission.
Consultant gastroenterologist 6
Side effects and safety
All participants agreed that the safety of treatments and potential side effects are important factors influencing treatment choices for patients with steroid-resistant UC. A number of potential side effects were described by health-care professionals, from abnormal liver function tests, abdominal pain, pancreatitis and minor symptoms (e.g. nausea or vomiting, flu-like symptoms or tiredness) to monitoring for toxicity and cytomegalovirus count or concerns about thrombosis. Monitoring for infection is important for patients treated with biologics, as well as, in the longer term, monitoring for the risks of cancers and lymphomas. The safety of proposed treatments was critical:
Firstly, no harm. So the likelihood of harm is probably the most important thing . . . Side effects and adverse events in relative risks, in risk terms and probably an absolute risk actually.
Consultant gastroenterologist 2
. . . from our point of view, you’re going to be thinking about safety, what, you know, are their other considerations, things we want, certain drugs we want to avoid or other drugs they might be taking that might affect their choice of drug . . .
IBD nurse 4
Some health-care professionals also explained how they discuss side effects with patients when presenting them with different treatment options. Striking a balance between presenting the necessary information and not scaring patients was considered key. When talking about the use of azathiopurine, one medic explained:
We do mention the main side effects when we commence them on treatment. So, say when we commence them on azathioprine we tell them about flu-like illnesses, we tell them about the importance of having a test done to look for abnormal biopsy, we tell them about sort of risk of infection and how to be aware that you’re not getting an infection. We tend not to scare them too much with the risks of lymphoma and cancers out the outset and we let them read the information leaflet themselves to realise that there are potential risks. We do talk to people about exposure to sun, we talk a little bit about sort of risk of skin cancer and use of sunscreens, etc.
Consultant gastroenterologist 3
Many health-care professionals explained that consideration of patient characteristics, particularly age, was important in determining appropriate treatment options. Although some health-care professionals explained that frailty was a more appropriate indicator of treatment appropriateness than chronological age, the latter was described as a major factor in deciding on the use of immunosuppression and biologic treatments because of the associated risks for older people.
Comorbidities and burden on patient
Closely related to side effects, health-care professionals described how patients’ comorbidities are also taken into consideration in treatment suggestions and gave several examples of this, mainly related to safety. First, where patients currently or previously had cancer (e.g. skin cancers, lymphomas, bladder, breast or cervical cancers), health-care professionals generally preferred to avoid azathiopurines or anti-TNF treatment because of immunosuppression and further cancer risks. Second, several health-care professionals said that they would guide patients with a previous history of thrombosis or at risk of this (e.g. younger women who are on the combined pill) away from tofacitinib. Third, a reluctance to use anti-TNFs in patients with multiple sclerosis or heart disease was also mentioned by several health-care professionals. Finally, concerns were highlighted about the use of mesalazine for patients with chronic kidney disease or other renal problems.
Related to this, several health-care professionals described their concerns about the burden that could be placed on patients from recommending certain treatments, particularly for patients who had complex comorbidities. As this IBD nurse explained:
I think, you know, we have to think about what other patients’ medicines are they’re taking, what their pill burden is like already. If you’re asking someone to take azathiopurine, mercaptopurine and maybe in combinations, so you could be adding another three or four tablets a day to their pill burden, but also it’s about . . . optimising treatments.
IBD nurse 2
Route of administration
Inflammatory bowel disease health-care professionals also discussed the route of administration of treatments and patients’ preferences for oral medication, subcutaneous injection or infusion therapy. Overall, health-care professionals explained that they present the different treatment options and, generally, allow patients to choose the treatment in line with their preferences or lifestyle factors. For example, people with a needle phobia would be able to opt for tablets or regular visits to the infusion room for infliximab or vedolizumab:
Some people find injections very uncomfortable and given the concept of them giving themselves the injections is also uncomfortable, so that a personal choice really.
Consultant gastroenterologist 2
I think I mean mode of administration to certain patients would be a big thing, you know, we’ve got a couple of patients that are quite needle phobic, so that’s massively impacted what we would do.
IBD nurse 8
People with busy lifestyles or who are self-employed may struggle to attend hospital regularly for infusions or monitoring blood tests and so may prefer to be treated with adalimumab or tofacitinib, for example. Ensuring that patients are happy with the route of administration was also acknowledged as important in encouraging compliance (see Compliance):
Patient preference . . . if you know the patient and it’s about what treatment will fit in better with their life, which is gonna be the most easily complied with, you know. We talk a lot about compliance in and concordance in treatment in IBD but I think often the reason we don’t get it is because we don’t consider that before we start the treatments.
IBD nurse 2
Patient lifestyle
As suggested in the previous section, work, family and travel commitments were all important patient lifestyle factors taken into account by health-care professionals when considering treatments for UC:
Also just things like lifestyle because a lot of people will choose based on how it’s going to fit around work and home life, things like that.
IBD nurse 4
It may not be feasible to start some people on medication that requires frequent blood tests and monitoring, or visits to hospital to receive the treatment, when their work involves frequent travel outside their local area. It may also be challenging for self-employed people to take time off for treatment and, therefore, they may prefer self-administered treatments. Likewise, health-care professionals explained that they are mindful of patients with young families, who may have limited time for treatments.
Patient preferences
As described in Route of administration, patient preferences for different treatments, whether because of route of administration or lifestyle factors, as well as compliance, was explained by health-care professionals as an overarching factor that influences the treatments offered. Health-care professionals also explained how level of tolerance of UC symptoms is another aspect of patient preference that is considered when selecting a treatment because this was seen to influence a patient’s willingness to try a new medication (or surgical options). Many of the participants described a process of treatment choice that was guided by them as health-care professionals while also accommodating patient preferences:
Yeah a little bit about the patient characteristics, but largely it’s patient choice and what they would like to have.
Consultant gastroenterologist 3
I guess from previous perception with a patient you’ve got a perception as to what they’re likely to be inclined towards. Having said that . . . I think patients do sometimes surprise us by the choices they make between different therapies. So I do try and present any therapy which I think is sort of reasonably appropriate at a point in time, rather than trying to be too prescriptive about what, for the patients . . .
Consultant gastroenterologist 4
Compliance
The issue of patient compliance with recommended treatments is considered when offering new medications for UC. Compliance or adherence is linked to route of administration and patient lifestyle, that is, health-care professionals were keen to recommend infusions to patients with a poor record of attending outpatient clinic appointments, as bringing these patients into hospital for treatment ensures that treatment is administered and also serves as an opportunity to monitor their condition. Similarly, health-care professionals spoke about the limited value of offering self-administered subcutaneous treatments to patients with a record of forgetting to take oral medication. More generally, some health-care professionals highlighted the importance of patient ‘buy-in’ or acceptance of treatments in facilitating compliance with recommended regimens.
Disease severity
Another important factor influencing treatment offers to patients with steroid-resistant UC was, of course, disease severity. In line with health-care professionals’ explanations of the range of treatments that they typically recommend to these patients, health-care professionals clarified this by describing how this was is informed by the extent or severity of the disease. As might be expected, having a detailed understanding of the disease (i.e. symptoms, e.g. blood, stool frequency or pain), blood results and inflammatory markers was vital:
. . . it is really important to understand what you’re treating, because if you don’t fully understand the patient’s disease, it’s very hard to give coherent answers about where the risk benefit lies for someone. Because if you don’t understand whether they’ve got pancolitis or whether they’ve got you know chronic/active colitis or they’ve got acute/severe colitis, whatever, very, very hard to give them proper advice.
Consultant gastroenterologist 11
Again, as might be expected, health-care professionals also described how disease severity is a key consideration in whether they recommend admitting a patient to hospital for treatment or escalating to surgery:
So for steroid resistant if a patient was very symptomatic I would usually be, either offering them admission or if they don’t want admission I would be discussing about escalating to a biologic agent and if I’ve sort of assessed them in clinic and think that that’s what they need. Then I would usually always discuss surgery at that point because actually you do get the occasional patient who actually wants surgery or you know patients often have different reasons. So I like to present them with the options and try not to overwhelm them information, which is a bit more difficult now, we got more options.
Consultant gastroenterologist 10
Effect on fertility
Fertility and family planning was another issue influencing treatment offers for some particular patient subgroups, given the potential risks of some drugs, such as vedolizumab and tofacitinib. A couple of health-care professionals mentioned that they highlight this potential risk to patients, but this would not preclude them from offering these treatments to people of childbearing age, particularly those who are very unwell, as the need for health improvement would take precedence.
Familiarity with treatment
Many health-care professionals acknowledged that both their clinical experience and their familiarity with particular treatments play a part in how they choose which treatment to recommend to people with UC:
But it’s based on a number of things and sometimes it’s actually, number one it’s experience, I’ve been doing the job for a long time and number two it’s sort of experience of the patient as well.
IBD nurse 3
Yes I think you can’t help but be influenced by your successes or failures in the past.
Consultant gastroenterologist 8
Another facet of this was the impact on health-care professionals’ readiness to try newer drug treatments with patients. Tofacitinib was a frequently mentioned example of a new treatment that health-care professionals were beginning to use cautiously, partly because of the lack of experience and partly because of safety concerns (e.g. the risk of deep-vein thrombosis) issued around the time of the interviews. Tofacitinib was often compared with more established and familiar treatments, such as anti-TNF and vedolizumab:
So I might feel more confident with tofacitinib in a couple of years, when I’ve used it in more patients . . . but I guess because I have experience in using anti-TNF agents and we can optimise them and I guess I feel more confident with that.
Consultant gastroenterologist 10
Cost of treatment
Health-care professionals were also asked how the costs of treatments for UC influenced their practice. Overall, most participants explained that treatment cost was not actually an explicit consideration when choosing which treatment to recommend or offer to patients. Although health-care professionals acknowledged that their access to treatments is governed by NICE approval and local Clinical Commissioning Group (CCG) commissioning, this did not translate into an immediate consideration when working with individual patients:
Only that we need to be compliant with NICE guidance, I don’t really take that into a major part of my decision making, that’s somebody else’s problem, I give them the best drug for them.
Consultant gastroenterologist 8
We are quite lucky that the CCG don’t really put up many boundaries there, so it is about what is best for the patient really. We do think, obviously, about the bigger picture and the cost implications, because obviously we wouldn’t be very good, but also it is about what is best for the patient.
IBD nurse 7
The availability of biosimilar options for anti-TNF drugs, which are cheaper, was also described by health-care professionals as reducing the importance of cost considerations in treatment choices. There were exceptions to this, and some health-care professionals reported that they might limit the use of vedolizumab, adalimumab or golimumab as first-line agents because of cost concerns:
I mean we use biosimilars . . . for patients on infliximab. But I think if, if it wasn’t for costs, we might be offering more vedolizumab as a first line, you know maybe to patients who don’t need an immediate response. But because it is significantly more costly, we tend to only use it in certain circumstances or a second line.
Consultant gastroenterologist 7
Service capacity
Service capacity was the final factor identified by health-care professionals as a possible influence on treatment choices. Although service capacity was discussed during only a few of the interviews, and was not always problematised by participants (i.e. there were no perceived problems with this), it is useful to note that service capacity for some IBD services, in terms of outpatient hospital appointments or infusion room availability, was an important factor in which treatments professionals felt able to offer patients. For example, this consultant gastroenterologist, taking into account relative clinical effectiveness and costs of treatments, may prefer to offer tofacitinib over anti-TNF treatments to reduce the burden on the infusion room at their hospital:
The way in which we are using them is part driven by costs and in part driven by access to the infusion room, and so, and in part driven by their relative efficacies. So I think vedolizumab probably works a bit better than the anti-TNFs but they’re pretty expensive. And tofacitinib seems to work about as well as the, about as well as the anti-TNFs so broadly speaking we are choosing to go with tofacitinib because it’s cheaper and as effective and is an oral preparation and keeps people out of the infusion room.
Consultant gastroenterologist 5
Referral for surgery
Most health-care professionals explained that they typically introduce the possibility of surgery for UC to patients at an early stage, usually at, or shortly following, diagnosis. Although surgery was generally seen as a longer-term treatment option for UC, health-care professionals explained that it is important to present this as one of the available options alongside medical treatments from the outset:
We do use surgery as a long resort but we should be talking about it earlier so actually if it does come up it’s not quite as scary and because we do have more medications. But even still there will still be a certain amount of patients who will end up having surgery and it’s important that we prepare them for that.
IBD nurse 5
I talk about it really quite early on erm in fact when I see people in clinic for the first time at diagnosis, I will mention that surgery, so what I tend to do is I tend to talk to them about a therapeutic ladder where they are starting with 5-ASAs [5-aminosalicylic acids] then steroid then immunosuppressant and biologicals. But I say to them ‘but you can take two steps on the ladder or three steps on the ladder without you know don’t worry about that, and if you fall off the ladder that’s surgery’.
Consultant gastroenterologist 2
Central to this was the importance of carefully managing patients’ expectations about possible surgical options to help with acceptance in the longer term. Health-care professionals generally said that early discussions about surgery facilitated patient acceptance and helped to reduce fear. Providing verbal explanations or written information about the types of surgery available [e.g. Crohn's & Colitis UK (CCUK) leaflets or booklets developed locally in their NHS trust] were the typical approaches at this stage:
We give them all treatment options, we would even discuss surgery as well obviously, I haven’t mentioned that. But it’s always discussed with patient and the first-line treatment, if they want it, we always say that surgery should be done as a managed therapy basically rather than it being an acute reaction, it is better for patients, that’s what we try and do, doesn’t always work . . .
IBD nurse 2
I mean biologics has changed it an awful lot but you have to try and plant the seed that surgery might be required, quite early I think and so we do try and do that but I think if a patient is sort of heading in that direction I will sort of say that you know look this is a possibility, you need to be filing it in your head so yes.
IBD nurse 3
Health-care professionals also described situations in which surgery may become more appropriate or necessary, such as when patients had tried one or two biologic therapies and their symptoms had not improved, and this was often described as having exhausted all medical treatments or ‘running out of options’. Some health-care professionals also talked about presenting surgery as an option specifically for patients who are resistant to steroids. At this point, many health-care professionals explained that they would refer patients either to surgeons as part of a joint medical–surgical clinic or for more in-depth individual discussions with a local surgeon or stoma nurse. A minority of health-care professionals also explained that their IBD services maintain a list of patients who have previously had surgery for UC and are willing to discuss their experiences with other prospective candidates:
There’s always, there’s, you know, there’s nearly, I mean sometimes when you’re through everything, then there isn’t a choice, but I think we’re quite proactive about surgery, we offer surgery relatively early in addition to all of the other things.
Consultant gastroenterologist 5
We have a joint medical–surgical clinic that patients come to and they are also seen individually by the surgeon to see and talk them through the logistics of it, and they also get to see a stoma nurse and they also get to see a patient who’s had a similar operation, so they can speak to somebody who has had the experience of it.
Consultant gastroenterologist 8
In keeping with their perspectives on medical treatment options, health-care professionals also frequently mentioned the importance of respecting patient preferences and patient choice, and what patients are able to tolerate, in terms of their symptoms, possible toxicity from medication, quality of life and socially (i.e. post surgery). Patient age was another important factor influencing referral for surgery, given that younger people often have more concerns about living with a stoma. Likewise, for women who want to start a family, health-care professionals would encourage them to go through pregnancy before opting for surgery. Ensuring that patients have the necessary information about surgery and are comfortable with the decision they are making was important to health-care professionals:
I think one of the things that I would say is I wouldn’t, I can advise people to have an operation but I wouldn’t bully someone into an operation because if you twist somebodies arm into having an operation when it’s uncomfortable they will always regret it.
Consultant gastroenterologist 2
. . . their choice really, sometimes it depends, patients have got different threshold to what they will accept and some patients have really very mild symptoms but really want to go ahead for surgery. You know it’s kind of a combination really, it’s not really just one thing.
IBD nurse 1
Chapter 5 Results from qualitative interviews with patients
Participants
A total of 53 patients were approached for interview; however, it was not possible to establish contact with 11 people, contact was lost with three people (despite initial interest), four people failed to attend their prearranged interview, one person was ineligible at screening and one person was too busy to participate in an interview.
Therefore, a total of 33 people with UC participated in the qualitative interviews (Table 15). There was an equal split in the gender of participants (51.5% female) and the majority of participants were white British (81.8%). The median age of participants was 39 years and the median time since diagnosis of UC was 6 years. The participants were also sampled on self-reported current and previous treatments (in addition to steroids, which all patients had previously received). At the time of the interview, 13 patients were receiving more than one treatment, and the median number of previous treatments was 2 (minimum–maximum 0–5). The median length of individual interviews was 31 (minimum–maximum 16–74) minutes.
| Characteristic | Frequency |
|---|---|
| Gender, n (%) | |
| Female | 17 (51.5) |
| Male | 16 (48.5) |
| Ethnicity, n (%) | |
| White British | 27 (81.8) |
| Iranian | 2 (6.1) |
| Indian | 1 (3) |
| Lebanese | 1 (3) |
| Pakistani | 1 (3) |
| Not disclosed | 1 (3) |
| Median age (minimum–maximum) | 39 (19–73) |
| Median time (years) since diagnosis (minimum–maximum) | 6 (0.5–33) |
| Current treatment, n (%)a | |
| Vedolizumab | 14 (42) |
| Mesalazine | 8 (24) |
| Infliximab | 6 (18) |
| Azathiopurine | 4 (12) |
| Surgery | 4 (12) |
| Awaiting surgery | 4 (12) |
| Mercaptopurine | 3 (9) |
| Steroids | 3 (9) |
| Ciclosporin | 1 (3) |
| Sulfasalazine | 1 (3) |
| Tofacitinib | 1 (3) |
| Previous treatment, n (%)a | |
| Azathiopurine | 16 (48) |
| Mesalazine | 16 (48) |
| Adalimumab | 7 (21) |
| Infliximab | 6 (18) |
| Methotrexate | 4 (12) |
| Mercaptopurine | 3 (9) |
| Vedolizumab | 3 (9) |
| Golimumab | 2 (6) |
| Surgery | 2 (6) |
| Tofacitinib | 1 (3) |
Analysis
Through familiarisation with the interview transcripts, a preliminary list of codes was developed. After several iterations, it was possible to agree on the coding framework. The coding framework included 49 codes that were grouped under 10 categories (see Appendix 4, Table 36). The coding framework was applied to the whole data set and summaries of each code were produced to help identify themes. The presentation of the findings in this chapter is structured around these themes and subthemes, as follows.
Lived experiences of ulcerative colitis
Diagnosis
Participants had different experiences of being diagnosed with UC. For many, the diagnosis process for UC took longer than they would have liked. Some participants felt that their general practitioner (GP) ignored or disregarded their symptoms (including blood in stool, diarrhoea, sickness and weight loss) and, consequently, they needed to attend multiple GP appointments before being referred to gastroenterology. Some reported that they had been misdiagnosed with irritable bowel syndrome, food poisoning or haemorrhoids before UC was even mentioned as a possible diagnosis, and many had to push their GPs to refer them to secondary care. Some participants were frustrated by the time taken be diagnosed, but others were appreciative of the time frames and processes involved:
. . . the diagnosis I think just took a long time to get sorted really from the GP because there was putting it off, putting it off and then eventually when I told them, ‘look, I need something sorting, it’s getting onto me now’.
P5
I kept going back to my doctors and insisting on seeing a consultant and having tests done.
P29
Not really no, it maybe, it maybe took a bit longer than I would have liked, so I think that’s just routine of the doctors, the blood tests were abnormal and they just wanted to do more testing before referring me.
P9
However, other participants had a more positive and streamlined experience of UC diagnosis, including being quickly referred by their GP for further investigations:
I was quite fortunate in a way that I was diagnosed very quickly, I know a lot of people spend years being misdiagnosed, but I was fortunate enough to be diagnosed quite quickly.
P1
It was straightforward really. Soon as, soon as they knew what I was dealing with, you know, I just went, my doctors sent me to [hospital] and from there just went to [consultant] and then the camera then and everything was all right.
P10
Following this diagnosis, most participants explained that, initially, they were relieved because UC is a treatable condition, as opposed to something they saw as ‘worse’, such as bowel cancer. However, the diagnosis was still difficult for participants who had been told at a younger age, for example as a teenager or as a young adult, as they had struggled to relate UC to their age. A few participants also said that they had never heard of UC before their diagnosis and, therefore, had struggled to grasp the meaning of this at the time:
. . . in some ways it was, it was good to know what I had because then I had something, I could put a name on something, because up to that point no one knew what was up with me.
P17
I didn’t really know what it was at first you know, I think it were a bit of relief to know that there was actually some, there was a reason I was feeling as ill as I was, and having all the problems I were having.
P24
Yes, I did actually, yes ’cos I think my, sort of, thoughts were obviously you have all sorts of thoughts and I was like thinking, oh what if it’s like bowel cancer, or what if it’s or what if it’s that so I think when it was, when it was IBD I was kind of like relieved actually.
P9
Well in one way I was relieved that it wasn’t cancer. That was the first thing you think of – ‘oh, it’ll be cancer’, I was relieved it wasn’t that.
P14
The impact of ulcerative colitis on everyday life
Participants explained that living with UC could be difficult on a day-to-day basis. Participants had to learn how to manage the condition, as well as managing those things that they felt could trigger flares, such as stress or intake of specific foods. Living with UC also affected some participants’ work life, whether owing to illness or time off needed for medical appointments. The impact of this varied in line with types of job and employer attitudes; however, for some participants, UC meant that they had to reduce their hours to cope with the dual pressures of work and illness:
I used to work full time, I actually reduced my hours due to my colitis, I was always tired, in and out of hospital, and I just couldn’t cope with every day as well as work.
P31
. . . because I work in [shop name], you are sitting on a till and the till to the toilet it’s a very long time you know to walk, and now I’m at that point where, if I need that toilet, I’ve got to go there and then, I can’t wait, so obviously them 10 absences that I’ve had off. So it’s always like well you’ve took that into consideration but these days, well I’m actually going for a disciplinary because of my attendance.
P11
Participants also noted the impact that UC had on their social lives and emotional well-being. Some participants explained that during a flare they can be unwilling to leave their homes and every visit needs to be carefully planned around the location of the nearest toilets. Related to this, some participants spoke directly of the stigma and embarrassment of UC. Support from family, friends and other people with UC was important to help deal with the emotional impact of the disease:
I think the biggest problem is, is the uncertainty of what’s the matter, and it’s the embarrassment of what happens to you where you can’t control yourself a lot of the time . . . It’s, you haven’t no time to get to toilet sometimes you know what I mean? It’s the worst part about this disease I think.
P2
I get no warning and it will just come, I do become incontinent sometimes when it’s a bad flare-up, so you can understand how embarrassing it is, I mean I’m only in my 40s as well, you know it’s just horrendous.
P19
By contrast, one participant who had recently had stoma surgery said that this was the first time in a while that they had felt that it was not necessary to worry about the impact of UC on their everyday life:
I’m not frightened to go out because I know if I go out and eat I’m not going to be in pain, I can go out and drink hot drinks whereas before I couldn’t do that because I’m going to be in a lot of pain and have to rush to the toilet.
P32
Experiences of steroids
Most participants explained that they had become steroid resistant or dependent during their treatment, with steroids not relieving their symptoms at all or relieving them only at higher doses and becoming less effective as the dose was tapered off. Some participants also said that, even when they felt that the steroids ‘do work’ for them, their symptoms were only dulled, but still present:
I think they stopped, I felt like they’d stopped working because I was getting symptoms again and I wasn’t, my symptoms wasn’t improving, so when I had like low symptoms, I’ve had the diarrhoea and stuff, I expected to take steroids and it be OK again but it didn’t it just sort of carried on. So, I just sort of knew they wasn’t really working.
P1
I do appear to be quite steroid dependent; when the dose starts to reduce, the symptoms start to come back.
P3
It stopped working, just my symptoms came back so when I went on the lower dose the blood, so the mucous stools, they’d stopped on the higher dose but the second I’d start tapering down, so from, I think I started on eight tablets twice, second I get down to about six or five, I’d start seeing the symptoms coming back again, so the mucousy stools and stuff like that so.
P30
In addition to this, most participants reported the side effects or adverse events they experienced while taking steroids. These included increased hunger and weight gain (i.e. ‘moon face’), altered mood, anxiety, joint pain and sickness. A small number of participants also said that because of these side effects they felt that they would rather manage their UC without steroids:
Well, I suffer with anxiety and depression and I’m medicated for that and it [steroids] made me even worse.
P28
I was like I’m sick of being on these tablets cause for me the worse side effects came from steroids. I didn’t really feel it the other side but my hair was falling out a lot but steroids as soon as I went on steroids I knew I was going to sleep, I’m going to be achy, I’m going to be hungry, I’m going to have a fat face so they were like devil tablets in the end.
P32
I still have issues but because I don’t want to go to another medication, I’m happy to take those you know conditions you know, rather than to go on a steroid.
P13
Reasons for treatment changes
All participants had tried several different treatments in addition to, or subsequent to, steroids and so were able to reflect on those experiences and the main reasons why they had changed treatment. In general, the decision to move from steroids to other treatments was not sharply distinguished from other treatment changes during their disease course for participants in this study, and participants reported three main reasons for changing treatment: (1) reduced effectiveness, (2) lack of response and (3) side effects.
Reduced effectiveness
All participants had experience of at least one treatment losing effectiveness and, overall, many were frustrated that medications would suddenly just stop working for ‘no reason’ and they would often stay on treatments longer if they felt that their symptoms were still manageable, even if this did not mean that they were in complete remission. This experience was often made worse as their UC symptoms became more severe over time:
This has been the problem, each medication, it dulls it for a little bit but then it comes back and it comes back worse and it seems to grow with each treatment I’ve had.
P28
Right in the very beginning when I was first diagnosed I was on mesalazine and I was brilliant, I was in remission for years and years and then all of a sudden it stopped working. I don’t know why, nobody knows why.
P4
Several participants had tried infliximab and explained that this often worked well for a period of time (ranging from 6 months to 2 years within the sample) before the effectiveness reduced and their symptoms returned:
When I first started on all these drugs what they give me, infusions and that, I thought ‘hey presto, I’ve got my life back’, and then, it only lasted for so long and then it started deteriorating again.
P2
Lack of response
On a related note, around half of the participants said that they had changed treatments because these were ‘not working’. Participants defined ‘not working’ in a number of ways, including not improving symptoms, increasing CRP levels or not decreasing inflammation:
We tried infliximab so I’ve had five infusions of that, the last dose they gave me the maximum and as it turned out, I’m a primary non-responder there as well, so we’ve knocked that on the head.
P10
For one participant, even though some of their symptoms improved and their inflammation decreased, this was not thought to be sufficient and so was also classed as ‘not working’:
It’s actually gone from 35 to 18 cm so it has gone down, but is still acute and is still active.
P30
Side effects
More than half of participants had changed treatments because of side effects. In addition to the side effects from steroids (see Experiences of steroids), participants gave various other examples, including adverse reactions to azathiopurine in the form of increased CRP levels, headaches, sickness, low mood, constipation, hypersensitivity and muscle aches. Likewise, some participants had experienced hair loss, psoriasis and mouth ulcers after taking adalimumab.
Factors influencing treatment choices
Participants were able to identify and explain factors that influenced their choices of treatment for UC over time. With the exception of decisions about surgery, treatment choice was described in general terms and participants did not necessarily differentiate between the treatments following steroid resistance or dependence, relative to other decision points. The main factors influencing treatment choices reported by participants were treatment effectiveness, guidance from health-care professionals, route of administration and treatment convenience, safety and side effects, and ‘running out of options’. Participants also raised a number of other issues, which are summarised in this section.
Treatment effectiveness
The effectiveness of treatments was the main priority for all participants when choosing a new treatment. Participants explained that the potential of a treatment to alleviate symptoms and, therefore, improve their quality of life was the most important factor in making their treatment decisions. For some participants, treatment decisions were made based purely on the perceived effectiveness of treatments and many placed effectiveness higher up than the route of administration or potential side effects:
As long as I’m in remission, it makes no difference to me whatever the treatment is, as long as I feel better.
P18
Nothing really phases me in a way, in the sense of how you take the treatment or having to do for it, because I look at the bigger picture as in, is it gonna repair me?
P5
Some participants also explained that they sought information about other patients’ experiences of treatment effectiveness to help make decisions:
For me, I went on to forum and I asked other people what they experience on that drug was and if people came back to me with a lot of negatives or they came back with quite a positive suggestion that kind of eased my mind and made me think ‘oh it could work for me’.
P32
Will it work mostly, I mean obviously you don’t know until you try it, but I’ll always ask like, you know, ‘how do other patients deal with this?’.
P21
Some participants said that they were happy if a treatment worked quickly and kept them stable, even if they were not necessarily in remission, that is, if the treatment relieved symptoms (e.g. reducing the number of toilet visits, stopping bleeding and reducing pain) to allow them to live a normal lifestyle. However, other participants explained that they valued the possibility of long-term remission over the speed of effectiveness when making treatment decisions:
I guess it’s hopefully achieving remission quickly is nice, but at the moment, I’m more concerned with can be find that manageable long-term symptom control with I guess, minimal intervention.
P3
Guided by health-care professionals
Most participants said that their treatment decisions were guided by IBD health-care professionals to a greater or lesser extent. Some participants said that they considered treatments based only on the recommendations from health-care professionals, whereas other participants used those recommendations to inform decisions following further research and discussions with family and friends:
I’ve got a trust in the people that are looking after me, and if they say I need something, then I need it, you know, I believe that, and I believe them.
P25
I would say it was, it’s more down to the doctor and IBD nurses, I don’t, it’s not like my choice at the end of the day, I’m just a patient and they’re the experts in the field, aren’t they? So I go with what they think is best for me at the current time.
P18
It was evident that these participants trusted their IBD team with treatment decisions, valuing their clinical knowledge and expertise. Participants also reported that they valued their relationships with their IBD team, describing functioning and open discussions about treatment decisions. In addition, participants valued the available methods of contact for the IBD team (e.g. telephone, e-mail and nurse hotline) and felt that staff were readily accessible when they had questions about how to manage their UC:
If you need them like, you can e-mail, you can phone them, they will get you straight into clinic, you will see consultant, you will see the IBD nurse, they are always there at the end of the phone if you need them.
P11
Some participants contrasted the care they received from the IBD nurses and consultants with a greater focus on well-being and illness, respectively. However, this largely seemed to be what participants expected from their health-care professionals, and they were complimentary about the care they received overall:
. . . that part of the NHS just works exceptionally well, that you have specialist nurses who understand and really focus on the patient, whereas doctors, because of time sometimes, have to focus on the illness.
P16
There was, however, one clear exception to this among the patient participants. One patient explained that they had not received consistent care or advice from the consultants in their IBD service. This meant that the patient felt confused by the conflicting advice given about the appropriateness of available treatments and, overall, did not trust the medical professionals whose care they were under, preferring to see the IBD nurses, whom they perceived to be more knowledgeable and practical:
Yes, and one of the problems at the hospital, every time that you go to the hospital, you see a different consultant, you don’t see you know the same consultant, each time the consultant is different. This is, I think, another issue because your previous consultant tells you something and another consultant tells you something else and sometimes you can get confused which one is right . . .
P13
Route of administration and treatment convenience
Overall, as described above, participants explained that they prioritised perceived effectiveness over route of administration (e.g. tablets, self-administered injection or infusions) when considering a new treatment option. However, on the whole, participants suggested that where they had a choice they preferred convenient treatments, such as tablets or injections that can be taken at home, particularly when they were recently diagnosed or had busy lifestyles (e.g. had young children or were working away):
Initially, I actually picked to try Humira first because it was more convenient to me being an injection at home done by me. Whereas the infliximab you have to go up to the ward for a 3-hour infusion, which having a [young child], the hospital is at the other side of the city from where I live so it’s quite a journey for me.
P28
However, this diminished over time as participants tried more treatments or if participants preferred to keep trying medical options to avoid surgery. Similarly, route of administration was more important to some participants because of, for example, needle phobias or need for time off work or education, but, again, these participants were relatively recently diagnosed, had less severe disease or were currently on a treatment that was working effectively for them.
Safety and side effects
Some participants stated that, when deciding on a new treatment, they would weigh up the potential side effects and safety issues against perceived effectiveness. A number of participants were fairly comfortable with making this decision, being aware that most treatments come with potential side effects, and with reassurance from, and monitoring by, their health-care professionals, participants generally accepted this situation as part of all treatments:
I guess that depends on the side effects, if the side effects outweigh the benefits, then we’d have to look at something else.
P3
I don’t want any drugs in my body, God knows what the long-term effects of them are, what they could do to me but needs must I guess. Otherwise I’d probably be in a worse condition now than what I have been.
P18
However, some participants also explained that they had refused treatments previously because of concerns about the severity of the side effects. In addition, concerns about treatment side effects increased if participants had experienced a bad reaction from a previous treatment. For other participants, concerns about the long-term side effects of treatments were important.
‘Running out of options’
A number of participants suggested that because of the severity of their disease, and because of previous medical treatment failures, they were forced to choose drugs that were less convenient or had side effects that are more dangerous because they were ‘running out of options’. Similarly, participants who were even further on in their treatment journeys may have realised that surgery was their only available option, with varying degrees of acceptance (see Perspectives on surgery for more details on surgery):
So, unfortunately for me, there’s no other way forward but surgery, which I still, I’m not happy about, personally, I’m not happy about.
P4
I felt like, you know, I’m running out of options, I’m running out of medications available that are actually working for me, and it’s quite, it was quite frustrating.
P27
Other issues
A number of other specific issues were raised by a minority of patients when asked about the factors that influence their choice of treatment, including comorbidities, concerns about fertility and convenience of surgery timing. One patient said that they also had diabetes, which ruled out some treatments because of the effects on blood glucose levels and kidney and liver function. A few other patients explained how their previous treatment choices had been influenced by fertility concerns, when offered infliximab or an ileo-anal pouch. Finally, those patients who were currently considering surgery for UC explained that they would prefer to delay this until after the summer (most of the interviews were completed during summer 2019) because they had plans for this time and waiting would be more convenient.
Perspectives on surgery
Most participants referred to surgery as a ‘last resort’ treatment for UC, as they wanted to try other medical treatments first. Following diagnosis of UC, and often towards the beginning of their treatment journey, participants typically did not consider surgery a viable treatment option because they were aware that medical options were available. Some participants had negative views of surgery due to the stigma of having a stoma, as well as concerns about the cosmetic appearance of this. For this reason, some participants explained that they preferred the idea of an ileo-anal pouch, as opposed to a permanent stoma:
The last one was the colostomy bag, you see. So I wanted to try everything else before I go down that route and he [consultant] was quite happy with that but yeah.
P10
I just didn’t want to go down that road, I just didn’t want to feel that I was gonna be carrying my toilet around on the side all my life.
P4
Oh yes, I would probably jump off a building before I had a stoma bag. Like, my mental health would not cope with having a stoma bag . . .
P29
However, participants reported these concerns to be less important as surgical options became more likely (because of the severity of their condition), or indeed post surgery. Participants who had discussed the possibility of surgery with their IBD team or had already had surgery explained how they had become less resistant to and more accepting of surgery as their medical treatment options were ‘running out’. Talking to people who had already had surgery, discussing this with their health-care professionals and family and friends, and reading information from CCUK or NHS information leaflets were all helpful in facilitating the decision to have surgery:
As I say, looking back, you know, I think I’m the kind of person that was glad that we tried all the alternatives, you know, surgery is not something I ever want, but at the same time, you know, I can look back and say, there was no choice.
P16
Initially it was ‘oh no! you know I don’t want surgery!’ you know, that’s a bit drastic, and you know, now I would consider it, so yeah, it’s definitely changed.
P8
I had a fear of that [surgery] at one time and then I read up a lot of people that had had it done on the internet and everything, and also with the Crohn’s [and Colitis UK] leaflets and pamphlets about it all and I sort of got it in my head, I’d accepted it and I was quite happy with it.
P2
Participants explained that, when surgery had been mentioned as an option, their consultants had given explanations of the different types of surgery available and some had been referred for meetings with stoma nurses to find out more information about how to manage their UC post surgery. Participants also said that surgery was mentioned at different time points. Some participants had been made aware of surgery soon after their diagnosis and so they had always been aware of this option, whereas others had become aware of the surgical options only when the pharmaceutical options had been exhausted and still others (usually patients with less severe UC and early in the disease course) were not aware of the surgical options. This variety of experiences is in keeping with the variation in duration of disease and timing of diagnosis, as well as, presumably, divergent clinical practice even within the three IBD centres:
. . . she did mention it [surgery] a couple of times but what she would just say is, you know, ‘if your options run out, that’s what we’d do, but we’ll always try every options first’.
P6
They don’t just stick leaflets in your face; they do give you other areas to get advice and support.
P28
No, no as my nurse says, we’ll just take one step at a time, let’s try all options and then it’s just only ’cos I’ve read up on it, and that’s how I know it could be a possibility.
P19
Other issues
Patients raised several other issues during the qualitative interviews, including reflections on their relationships with their GP, how they involve their families in decision-making about treatments, use of alternative treatments and longer-term views on treatment choices.
Relationship with general practitioners
As discussed above, the majority of patients reflected positively on their relationships with, and the care they received from, the IBD services. However, in contrast with this, some patients were negative about their relationships with their GP with regard to the management and treatment of UC. These participants said that they had ‘lost faith’ in their GP and did not receive enough support from them and felt that GPs had insufficient knowledge about the symptoms and treatments for UC, particularly at the point of diagnosis:
You can’t go to your GP because my illness, it’s kind of specialised and GP doesn’t have enough knowledge to know how to deal with my condition.
P13
So my GP’s not very clued up on ulcerative colitis. He’s just not someone that I would trust at all with any of the decision-making or anything.
P29
Another participant experienced problems with securing timely appointments at their general practice for safety monitoring and is now solely under the care of their IBD team:
I’ve stopped going to my doctors for that [blood testing] now because it’s so hard to get an appointment to do it, it’s virtually impossible sometimes . . . Like I say, I just go to the blood room up in the hospital and they take my blood for me.
P5
Involvement of families and partners in decision-making
Patients were asked about the involvement of their families and partners in decision-making about treatments for UC. Although some participants were single or did not have close relationships with their family members, most were keen to discuss how they valued the support from their families and partners. Some patients said that their partners (or parents, for younger participants) would often attend clinic appointments with them to help with questions during the consultation or to help with recall by making notes or discussing the possibilities after the appointment:
It made a lot more sense whenever I saw a doctor to have my wife with me because she could then take notes, and she could then ask questions as well. ’Cos it’s difficult to do and to think when you’re not feeling well, especially dehydrated, so we kind of worked out very quickly that it was better to do this together.
P16
Although I’m an adult, and you know, I can make the decisions myself, it’s always nice to have family there for such big changes in your life.
P1
Other patients simply explained that their partners were supportive of whatever they decided to do regarding their UC treatment, as opposed to sharing the decision-making or being very involved:
My husband is 100% supportive of anything, he’s never actually said to me ‘I think you should have this operation’, never. It’s my decision. He’s just there to support me in whatever decision I make.
P4
Alternative treatments
A small number of patients explained that they had tried alternative remedies in addition to the medical treatments prescribed for their UC. These patients had experienced a variety of alternative approaches, including hypnotherapy, acupuncture, herbalists, Ayurveda practitioners and reflexology, and many had made changes to their diets in a bid to help control their UC symptoms. Patient experiences of non-medical approaches varied, with some more certain about the effectiveness of these than others. In addition, several patients were aware of cannabidiol oil, but were typically sceptical about its healing properties and explained that they had not yet tried this or were unwilling to do so.
Long-term views on treatment choices
Overall, most patients interviewed were satisfied with the treatment choices that they had made during the course of living with UC. Patients were generally happy with their experiences, which often required trying a large number of medical treatments, as they felt that this was the best way to work out the most effective treatment for them:
Until you try it and see how it affects different people, you don’t know, do you? So it’s a matter of just trial and error I guess, ’til you try it and then it works, good. If it doesn’t, what’s next?
P18
I’m just grateful that there is all this treatment available to me, I’ve been fortunate. But no, I wouldn’t change anything and they’ve all helped in their own way, even if it is just a short period of time.
P1
Some of the other patients experienced some decisional regret. These patients explained that they would have liked to have been given more information about the number of treatments available to them earlier on. With the benefit of hindsight, these patients would have liked to start the more potent treatments earlier, but were also mindful that finding the most effective treatment was a process:
I think I’ve been probably been given pretty much everything that I could, maybe that’s sort of, order of it but I’ve maybe like to have changed maybe, maybe would have liked some of the stronger treatments earlier on, like I said, and then maybe I wouldn’t be in the condition that I’m in now.
P9
I think the other treatment options, again with hindsight I should’ve started the level two treatments faster, the move to immunosuppressants and biologics, but that wasn’t really something I could’ve known. So I’m still happy that I now know for sure that the mesalazine wasn’t doing the job and I made the right decision to switch to biologics but I would’ve made it a few months earlier with hindsight I think.
P3
Similarly, some participants who had already had surgery said that they wished it had been presented as a more realistic option earlier in their treatment journey (see Perspectives on surgery).
Chapter 6 Results from the health-care professional discrete choice experiment
Sample characteristics
A total of 116 participants completed the survey. The response rate was 63.7%, based on the respondents who visited the survey platform and not on the number of who saw the e-mail or social media invitation. The median age of respondents was 46 years, and 57% of the respondents were male (Table 16). Health-care professionals had a median of 9 years of experience in practice. Approximately 60% of the respondents were consultant IBD specialists or gastroenterologists and 64% were primarily working in secondary referral NHS hospitals. All regions of the UK were represented in the DCE, except Northern Ireland.
| Characteristic | Frequency |
|---|---|
| Gender, n (%) | |
| Male | 64 (56.64) |
| Female | 47 (41.59) |
| Prefer not to say | 2 (1.77) |
| Age (years) by category, n (%) | |
| 30–39 | 33 (29.2) |
| 40–49 | 42 (37.17) |
| 50–59 | 32 (28.32) |
| 60–69 | 3 (2.65) |
| ≥ 70 | 3 (2.65) |
| Job, n (%) | |
| Consultant IBD specialist | 18 (15.93) |
| Consultant gastroenterologist with IBD interest | 35 (30.97) |
| Consultant gastroenterologist without IBD interest | 14 (12.39) |
| IBD specialist nurse | 29 (25.66) |
| Gastroenterologist trainee | 14 (12.39) |
| Clinical permanent posts | 3 (2.65) |
| Hospital, n (%) | |
| Secondary referral service | 72 (63.72) |
| Tertiary referral service | 39 (34.51) |
| Quaternary referral service | 2 (1.77) |
| Region, n (%) | |
| North East | 8 (7.08) |
| North West | 7 (6.19) |
| Yorkshire and the Humber | 30 (26.55) |
| East Midlands | 13 (11.5) |
| West Midlands | 8 (7.08) |
| East of England | 4 (3.54) |
| London | 17 (15.04) |
| South East | 8 (7.08) |
| South West | 11 (9.73) |
| Scotland | 5 (4.42) |
| Northern Ireland | 0 (0) |
| Wales | 2 (1.77) |
| Average age (years), median (range) | 46 (31–73) |
| Years qualified, median (range) | 9 (0–39) |
Understanding and engagement
All participants completed all the choice tasks, and for the remainder of the survey the number of missing data was small and did not exceed 5%. The median time taken to complete the survey was 8 minutes. All survey participants answered the dominant choice question correctly, and the majority (94.64%) reported that they agreed or strongly agreed that they understood the DCE tasks (Table 17). However, half of the sample said that they needed more information than was provided in the DCE tasks, for example further details about patient preferences, patient compliance, patient history and patient demographics.
| Feedback question | Frequency |
|---|---|
| I understood the questions about making choices between different treatment options, n (%) | |
| Strongly disagree | 1 (0.89) |
| Disagree | 0 (0) |
| Uncertain | 5 (4.46) |
| Agree | 67 (59.82) |
| Strongly agree | 39 (34.82) |
| When choosing options, I needed more information than was provided, n (%) | |
| Strongly disagree | 2 (1.79) |
| Disagree | 28 (25) |
| Uncertain | 25 (22.32) |
| Agree | 40 (35.71) |
| Strongly agree | 17 (15.18) |
| I found making a choice between different treatments confusing, n (%) | |
| Strongly disagree | 4 (3.6) |
| Disagree | 55 (49.55) |
| Uncertain | 26 (23.42) |
| Agree | 26 (23.42) |
| Strongly agree | 0 (0) |
| Survey duration in minutes, median (range) | 8 (3–297) |
Modelled preferences
We found that all five attributes in the model were statistically significant at predicting the probability of choosing a preferred treatment, implying that health-care professionals consider all five important when choosing a treatment to offer to patients with steroid-resistant UC. Figure 7 depicts the coefficients of the conditional logit model and shows that induction of response, mucosal healing and maintenance of remission have positive coefficients, which implies that higher levels of these attributes will increase the uptake of a treatment. By contrast, risk of lymphoma and risk of serious infection have negative coefficients, which implies that higher levels of these attributes dissuade health-care professionals from choosing a treatment. Figure 7 shows that a 1-unit increase in both lymphoma risk and serious infection risk attributes were strongly considered when health-care professionals were choosing a treatment compared with a 1-unit increase in the symptom improvement attributes. However, as the attributes were not measured in the same units (i.e. a 1-unit increase in lymphoma is measured in 10,000 patient-years, but a 1-unit increase in response is measured in percentages), the coefficients are not directly comparable.
FIGURE 7.
Model results of attributes for health-care professional DCE. The figure shows the coefficients and standard errors from the conditional logit model. All attributes are significant (p < 0.01). The raw coefficients can be found in Appendix 5, Table 37.
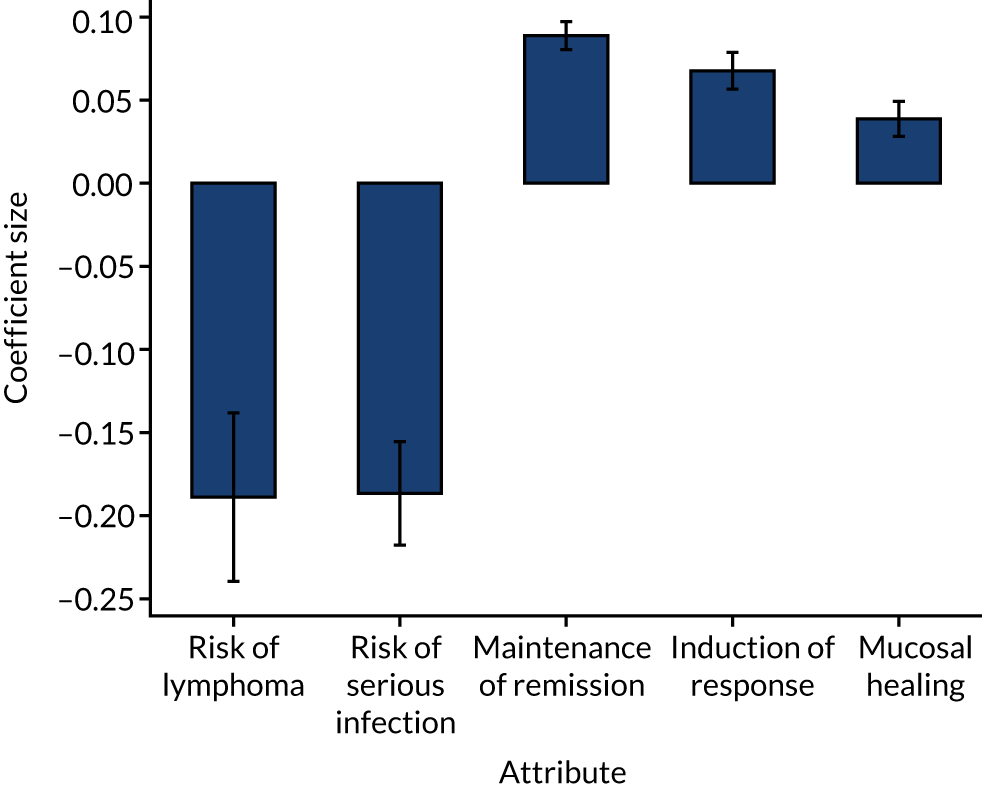
The willingness to trade benefit and risk
The benefit–risk trade-offs that health-care professionals were willing to make are presented in Table 18. The larger trade-offs in risks indicate that health-care professionals viewed that attribute as relatively more important than other attributes in their decision-making. Table 18 shows that maintenance of remission was the most important symptom improvement attribute, followed by induction of response and mucosal healing. On average, health-care professionals were willing to accept a lymphoma risk of 4 cases per 10,000 patient-years if the medication would increase patients’ induction of response by 10%. The lymphoma risks that health-care professionals are willing to accept for a 10% improvement in remission is 5 cases per 10,000 patient-years, whereas the lymphoma risk that health-care professionals are willing to accept for a similar increase in mucosal healing is 2 cases per 10,000 patient-years. This shows that the maintenance of remission is approximately twice as important as mucosal healing when deciding which treatment to offer to patients with steroid-resistant UC. Similar results were found with risk trade-offs calculated using serious infection risk, as the regression coefficients for lymphoma risk and serious infection risks were similar.
| Attribute | All health-care professionals | Health-care professionals working at secondary hospital | Health-care professionals working at tertiary hospital |
|---|---|---|---|
| Lymphoma risk: cases per 10,000 patient-yearsa | |||
| For a 10% increase in induction of response | 4 (0.6) | 3 (0.5) | 6 (0.3) |
| For 10% increase in mucosal healing | 2 (0.4) | 1 (0.4) | 4 (0.2) |
| For 10% increase in maintenance of remission | 5 (0.6) | 4 (0.5) | 7 (0.3) |
| Serious infection risk: cases per 100 patient-yearsb | |||
| For a 10% increase in induction of response | 4 (0.3) | 3 (0.4) | 4 (0.8) |
| For 10% increase in mucosal healing | 2 (0.4) | 1 (0.4) | 3 (0.7) |
| For 10% increase in maintenance of remission | 5 (0.3) | 4 (0.3) | 5 (0.7) |
Exploratory analyses were also conducted to evaluate preferences across subgroups, such as health-care professional type (consultants vs. specialist nurses) or place of work (secondary vs. tertiary hospitals) (see Appendix 5, Table 37). Similar to the full sample results, in the secondary and tertiary hospitals subgroup analyses we found that maintenance of remission was the most important symptom improvement attribute, followed by induction of remission and, finally, mucosal healing. Data also suggest that health-care professionals who work at tertiary hospitals have a higher risk tolerance than those who work at secondary hospitals. For example, for a 10% improvement in remission, the lymphoma risks that health-care professional at a secondary hospital are willing to accept is 4 cases per 10,000 patient-years, whereas health-care professionals at a tertiary hospital are willing to accept a higher risk of 7 cases per 10,000 patient-years. This suggests that health-care professionals working at tertiary centres are willing to accept higher risks in exchange for improvements in all three symptom improvement attributes.
Probability of uptake
When we applied the results from our model to predict how the respondents would prescribe four currently used treatments for patients with steroid-resistant UC, we found that uptake rates were highest for infliximab (62%), followed by tofacitinib (18%) and vedolizumab (15%). The probability of uptake was lowest for adalimumab (5%) (Table 19).
| Predicted uptake of treatment | Treatment | |||
|---|---|---|---|---|
| Infliximab | Adalimumab | Vedolizumab | Tofacitinib | |
| Induction of response, % | 65 | 54 | 53 | 64 |
| Mucosal healing, % | 57 | 47 | 49 | 38 |
| Maintenance of remission, % | 34 | 16 | 23 | 24 |
| Risk of lymphoma, na | 6 | 6 | 2 | 6 |
| Risk of serious infection, nb | 3.5 | 2.5 | 4 | 0.9 |
| Predicted probability of uptake, % | 62 | 5 | 15 | 18 |
Figure 8 shows the change in uptake rates of improved treatment profiles compared with a baseline treatment that contains the worst possible levels for each attribute. Larger changes in uptake rates indicate the attributes that health-care professionals prioritised more when choosing a treatment. For example, a treatment with 70% remission rate was ranked very favourably, with a change in uptake rate of 92%, followed by a treatment with a risk of infection of 1 case per 100 patient-years, with a change in uptake rate of 69%. When comparing the attribute levels, some were interspersed; for example, a 60% response rate was at the top end of the priority list, but a 50% response rate was at the bottom end. However, the data show that remission attribute and risk of serious infection attributes were prioritised over risk of lymphoma and mucosal healing attributes when health-care professionals were choosing a treatment.
FIGURE 8.
Uptake rates of a baseline and improved treatment profile. Note that each vertical line represents the change in uptake rate of moving from a baseline (worse) treatment profile to an improved treatment profile. The baseline treatment profile was selected to represent the worst possible treatment profile, where induction of response was 40%, mucosal healing was 40%, remission rate was 35%, risk of lymphoma was 8 cases per 10,000 patient-years and risk of serious infection was 10 cases per 100 patient-years.
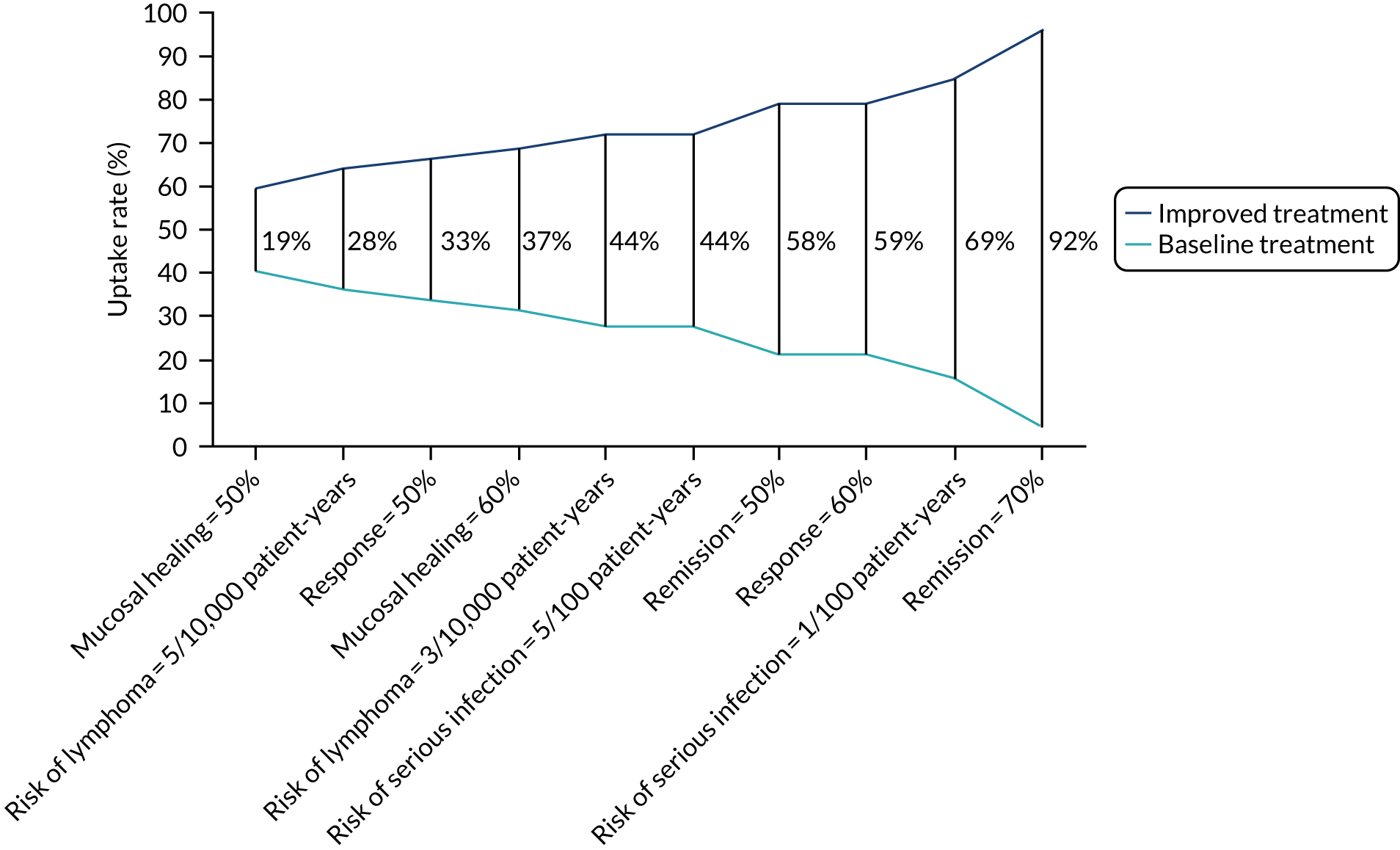
Chapter 7 Results from the patient discrete choice experiment
Sample characteristics
A total of 720 invitation letters were sent to eligible participants. Of the invited participants, 166 visited the Qualtrics survey platform, 115 completed the survey and 5 declined to complete the survey. Based on the respondents who visited the survey, the response rate was 69%. Table 20 presents the sociodemographic characteristics of the respondents. The median age of respondents was 41 years, and 52% of the responders were female. The majority of patients were white, employed and educated to secondary General Certificate of Secondary Education level or above.
| Characteristic | Frequency |
|---|---|
| Sex, n (%) | |
| Female | 60 (52) |
| Male | 55 (48) |
| Age (years) by category, n (%) | |
| 18–29 | 17 (15) |
| 30–39 | 32 (28) |
| 40–49 | 24 (21) |
| 50–59 | 18 (16) |
| 60–69 | 12 (10) |
| ≥ 70 | 12 (10) |
| Median age in years (range) | 41 (18–84) |
| Activity, n (%) | |
| Employed | 82 (71) |
| Retired | 17 (15) |
| Homemaker | 6 (5) |
| Unemployed | 5 (4) |
| Student | 4 (3) |
| Volunteer work | 1 (1) |
| Ethnicity, n (%) | |
| White | 109 (95) |
| Asian | 5 (4) |
| Mixed | 1 (1) |
| Black | 0 (0) |
| Education | |
| Primary | 1 (1) |
| GCSE | 21 (18) |
| A Level | 37 (32) |
| Degree | 53 (46) |
| Prefer not to say | 3 (3) |
| Marital status | |
| Married/partner | 89 (77) |
| Single | 21 (18) |
| Divorced/separated | 3 (3) |
| Widowed | 1 (1) |
| Prefer not to say | 1 (1) |
Table 21 presents the clinical characteristic of the sample, including current and previous medical treatments. Among the 115 patients, the median time since diagnosis was 10 years. The median IBD Control-8 score was 13, with 45% of the sample reporting poor control of the disease at the time of the survey. The median quality-of-life score was 0.77, which is lower than the UK general population mean quality-of-life score of 0.85. 80 The majority of patients had previously received steroids (93%), thiopurines (70%) and biological therapies or tofacitinib (70%). The most commonly used biological therapy was infliximab (51%). At the time of the survey, one-quarter of the sample was taking a combination of treatments [i.e. biologics/thiopurines/5-aminosalicylic acids (5-ASAs)].
| Characteristic | Frequency |
|---|---|
| UC severity | |
| Duration (years) with UC, median (range) | 10 (1–51) |
| Quality of life: EQ-5D-5L utility score, median (range) | 0.77 (–0.25 to 1) |
| IBD Control-8 score, median (range) | 13 (0–16) |
| Poor control of disease (IBD Control-8 score of ≤ 12), n (%) | 51 (44.7) |
| Good control of disease (IBD Control-8 score of ≥ 13), n (%) | 63 (55.3) |
| Treatment, n (%) | |
| Current use | |
| Adalimumab | 6 (5.3) |
| Infliximab | 11 (9.7) |
| Golimumab | 0 (0.0) |
| Steroids | 4 (3.5) |
| Tacrolimus | 1 (0.9) |
| Tofacitinib | 3 (2.7) |
| Thiopurines | 7 (6.2) |
| Ustekinumab | 1 (0.9) |
| Vedolizumab | 19 (16.8) |
| Combination of treatments | 29 (25.7) |
| 5-ASA | 24 (21.2) |
| None | 8 (7.1) |
| Ever usea | |
| Adalimumab | 32 (27.8) |
| Infliximab | 59 (51.3) |
| Golimumab | 4 (3.5) |
| Steroids | 107 (93.0) |
| Tacrolimus | 0 (0.0) |
| Tofacitinib | 8 (7.0) |
| Thiopurines | 81 (70.4) |
| Ustekinumab | 3 (2.6) |
| Vedolizumab | 34 (29.6) |
Understanding and engagement
All participants completed all choice tasks. For the remainder of the survey, missing data were few and did not exceed 5%. The median time taken to complete the survey was 21 minutes. Ninety-seven per cent of survey participants answered the dominant choice question correctly and only four participants failed this internal validity test. Overall, the majority of participants understood the DCE (92%) and ranking tasks (91%), and agreed that the information provided in the DCE task was appropriate (70%) (Table 22).
| Feedback question | Frequency |
|---|---|
| Dominant DCE choice question, n (%) | |
| Passed | 111 (97) |
| Failed | 4 (3) |
| I understood the questions about making choices between different pairs of treatment options, n (%) | |
| Strongly disagree | 4 (4) |
| Disagree | 1 (1) |
| Uncertain | 4 (4) |
| Agree | 44 (39) |
| Strongly agree | 59 (53) |
| I found that the more questions I answered the easier it was to make a choice between pairs of treatment options, n (%) | |
| Strongly disagree | 4 (4) |
| Disagree | 7 (6) |
| Uncertain | 20 (18) |
| Agree | 51 (46) |
| Strongly agree | 30 (27) |
| When choosing between the pairs of treatment options I needed more information than was provided, n (%) | |
| Strongly disagree | 10 (9) |
| Disagree | 68 (61) |
| Uncertain | 14 (13) |
| Agree | 15 (13) |
| Strongly agree | 5 (4) |
| I found making a choice between different pairs of treatment options confusing, n (%) | |
| Strongly disagree | 19 (17) |
| Disagree | 66 (59) |
| Uncertain | 14 (13) |
| Agree | 7 (6) |
| Strongly agree | 5 (5) |
| I found the ranking question made sense, n (%) | |
| Strongly disagree | 1 (1) |
| Disagree | 3 (3) |
| Uncertain | 7 (6) |
| Agree | 55 (50) |
| Strongly agree | 45 (41) |
| I found the ranking questions difficult to answer, n (%) | |
| Strongly disagree | 26 (24) |
| Disagree | 54 (49) |
| Uncertain | 11 (10) |
| Agree | 18 (16) |
| Strongly agree | 1 (1) |
| Duration (minutes), median (range) | 21 (6–119) |
Modelled preferences
All attributes have a significant influence on the choice of treatment patients preferred. Table 23 and Figure 9 show the relative changes that make respondents more likely (i.e. a positive coefficient) or less likely (i.e. a negative coefficient) to take a treatment. Higher levels of the remission attribute strongly influenced respondents’ choice of treatment (β 0.065; p < 0.01). Similarly, a treatment having a lower likelihood of side effects [i.e. a change from very common to very rare (β 2.937; p < 0.01) or from very common to uncommon (β 2.260; p < 0.01) or from very common to common (β 1.417; p < 0.01)] strongly increased the likelihood of the respondent choosing it. An increase in the induction of response from a lower level (40%) to a higher level (i.e. 50% or 60%) made respondents more likely to take the treatment. However, patients are less likely to choose a treatment that takes longer to improve their symptoms (β –0.145; p < 0.01). In addition, taking a pill daily at home (β 0.848; p < 0.01) and injections at home every 8 weeks (β 0.541; p < 0.01) were preferable to receiving infusions at hospital every 8 weeks. Notably, a change from infusion at hospital to injection every 2 weeks at home was not significant (β –0.029; p = 0.85), suggesting that patients found these two ways of administering the treatment comparable.
| Attribute | Coefficient (p-value) |
|---|---|
| Induction of response (relative to 40%) | |
| Response = 50% | 1.148 (0.000) |
| Response = 60% | 1.263 (0.000) |
| Speed | –0.145 (0.000) |
| Remission | 0.065 (0.000) |
| Administration (relative to infusion every 8 weeks at hospital) | |
| Injection every 2 weeks at home | –0.029 (0.850) |
| Injection every 8 weeks at home | 0.541 (0.001) |
| Pill taken daily at home | 0.848 (0.000) |
| Side effects (relative to very common) | |
| Common | 1.417 (0.000) |
| Uncommon | 2.260 (0.000) |
| Very rare | 2.937 (0.000) |
| Observations, n | 2760 |
| Log-likelihood | –811.6 |
| Pseudo-R2 | 0.406 |
FIGURE 9.
Conditional logit model results reproduced in a diagram. Note that the figure shows the coefficients and standard errors from the conditional logit model. All attributes are significant (p < 0.05), except injection every 2 weeks at home. Light-blue points represent the reference category of categorical variables. Speed of response and maintaining remission are continuous variables and represented by a single coefficient.
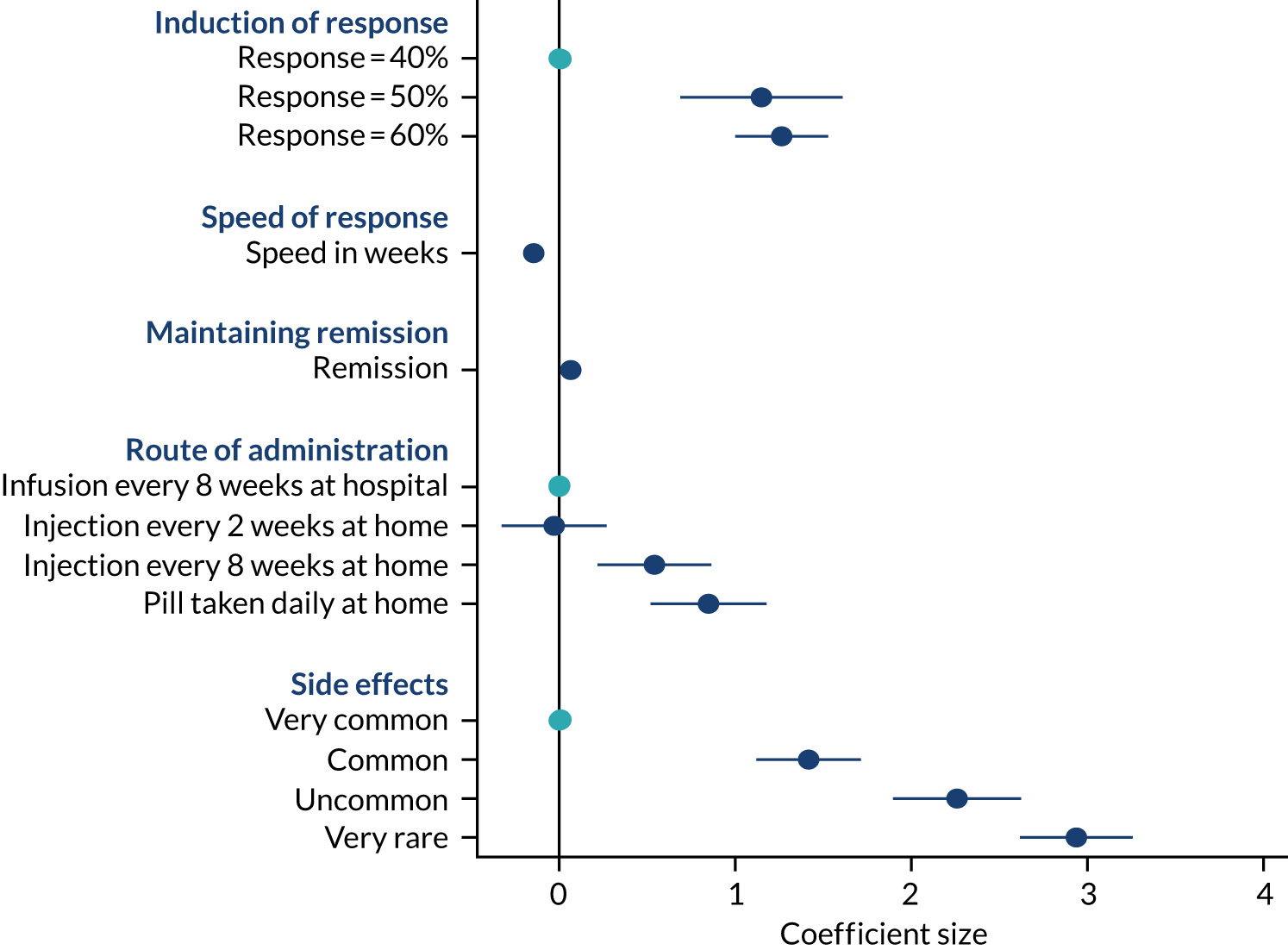
Predicted probabilities
To compare the relative importance of treatment attributes, we calculated the change in predicted probabilities in a baseline scenario compared with a new scenario. In the baseline scenario, the treatment profile consisted of the worst possible levels for all of the attributes and in each new scenario only one attribute level was improved at a time to find out how important that attribute is when selecting a treatment (see example calculation in Appendix 6). Figure 10 shows the change in uptake rates of the improved treatment profiles. Larger changes in uptake rates indicate more favourable treatment attributes. For example, a treatment with very rare side effects was ranked very favourably, with a change in uptake rate of 90%, followed by a treatment with a 70% remission rate that had a change in uptake rate of 81%. When comparing the change in probabilities across these scenarios, a treatment with very rare side effects was ranked very favourably, followed by a treatment with a remission rate of 70% and treatments with high induction of response rates. Conversely, route and frequency of administration were seen as less important.
FIGURE 10.
Uptake rates of a baseline and improved treatment profile. Note that each vertical line represents the change in uptake rate of moving from a baseline (worse) treatment profile to an improved treatment profile. The baseline treatment profile was selected to represent the worst possible treatment profile, where induction of response was 40%, speed of response was 14 weeks, remission rate was 35% and mode of administration was injection every 2 weeks at home, with very common side effects.
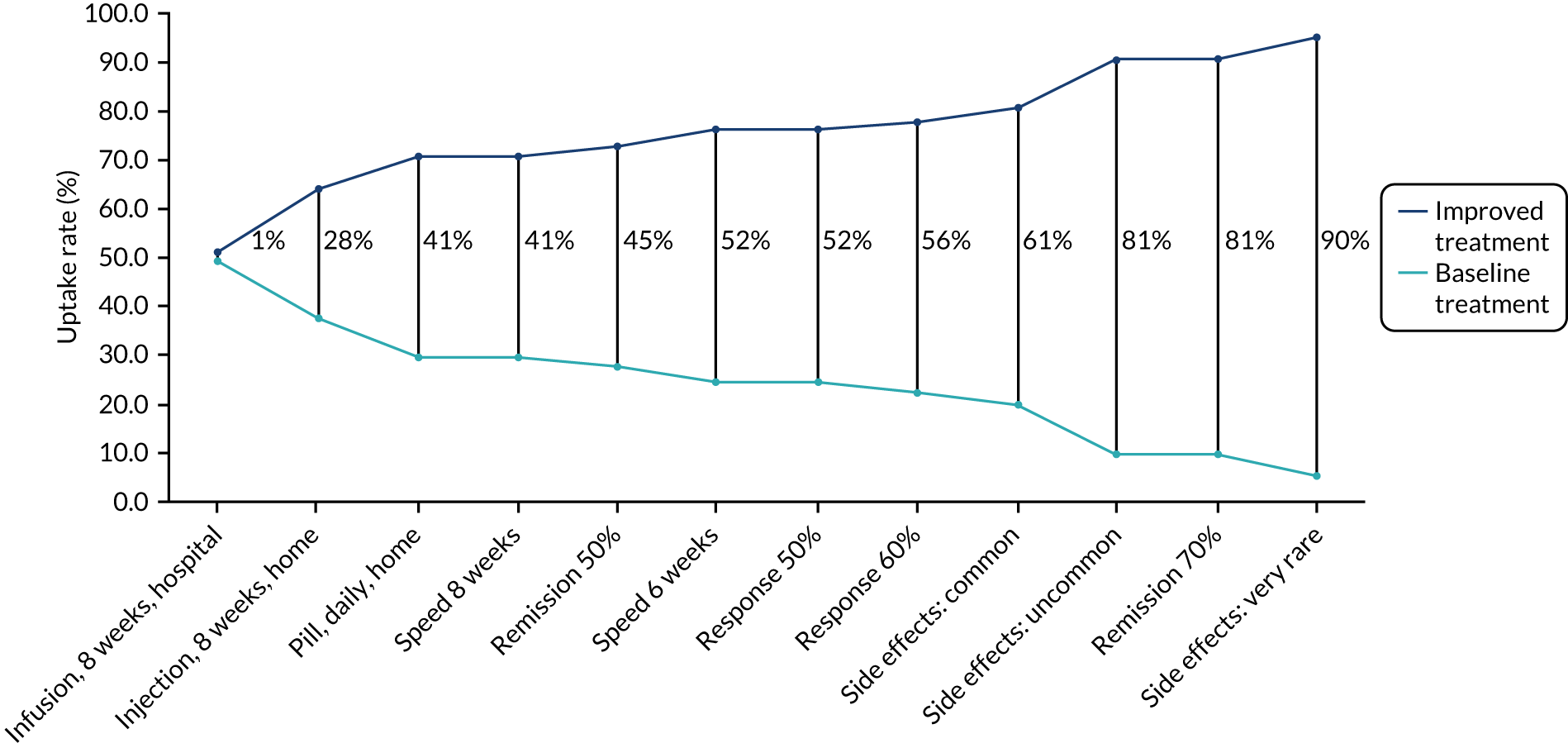
Ranking treatments
When participants were asked to rank the four biological treatments in order of preference, where ‘1’ equated to most preferred and ‘4’ equated to least preferred, the most preferred treatments were infliximab (38%) and tofacitinib (38%), followed by vedolizumab (17%) and adalimumab (6%) (Figure 11). The least preferred medication was adalimumab (54%), followed by vedolizumab (26%), infliximab (11%) and tofacitinib (9%). When respondents explained their choices, the most common reasons were the effectiveness of the drug at inducing and maintaining remission, the side effects, convenience and speed of response. Further analyses also showed that if participants have prior experience of taking any one of the four treatments, then they are more likely to rank that treatments more favourably than those who have no experience of taking the treatment (see Appendix 6). Nevertheless, a subgroup (n = 35) of biologic-naive patients were analysed separately to see if their ranking differed from that of those who were taking biologics. Among the biologic-naive subgroup, the most preferred treatment ordering changed slightly, with tofacitinib ranked first (46%), followed by infliximab (37%), vedolizumab (11%) and adalimumab (6%). The least preferred treatments were adalimumab (60%), vedolizumab (26%), infliximab (9%) and tofacitinib (6%), and this ordering was similar to the full sample.
FIGURE 11.
Biologic therapies ranked in order of preference.
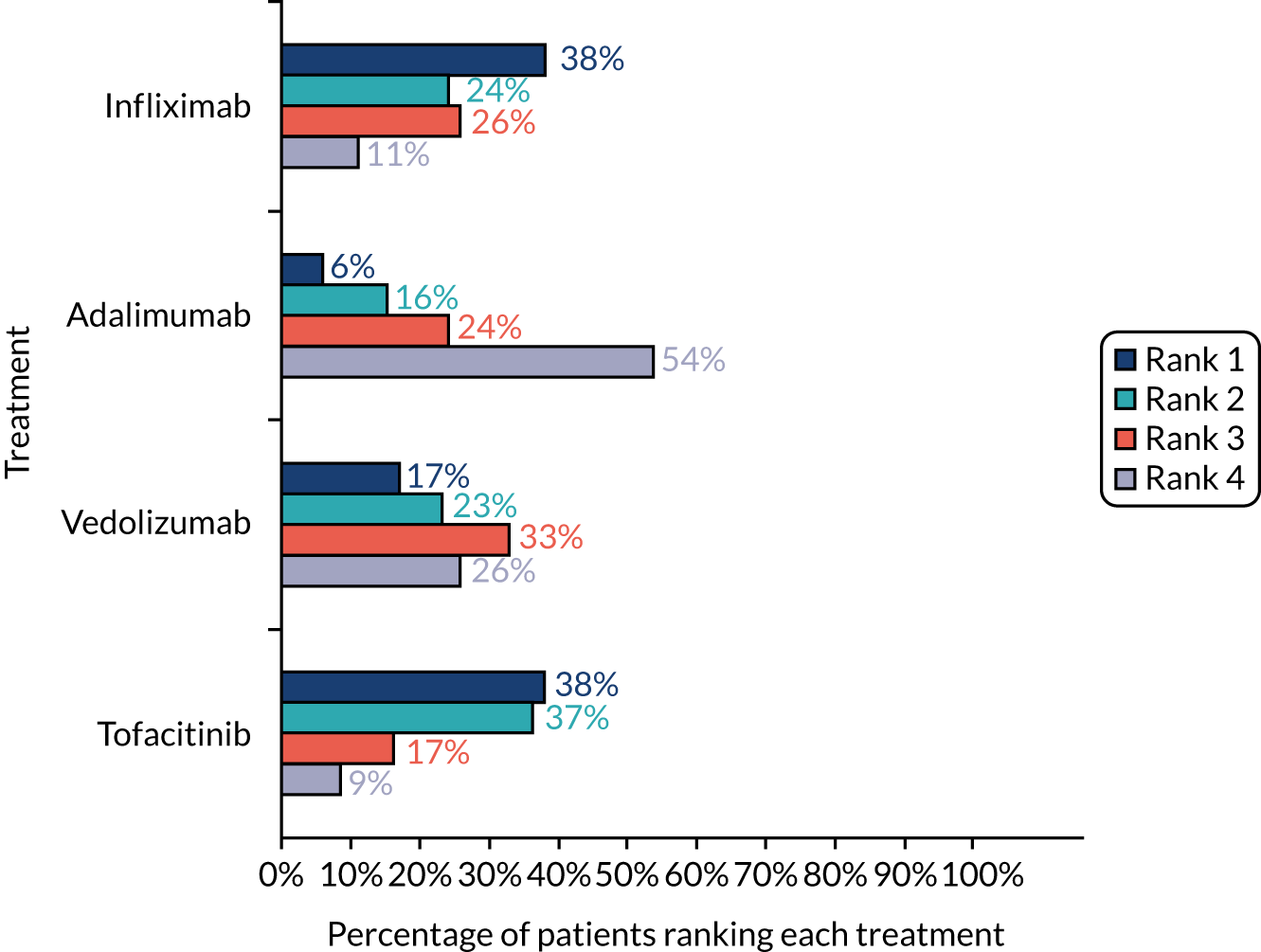
Chapter 8 Results from the multistakeholder workshop
Participants
A total of 36 people (medics, n = 16; nurses, n = 10; patients, n = 10) were invited to take part in the multistakeholder workshop. It was not possible to make contact with 15 people and seven confirmed that they were unable to attend because they were too busy with work commitments (whether clinical for health-care professionals or otherwise for patients), and five health-care professionals confirmed their places at the workshop but were unable to attend on the day because of urgent clinical pressures.
Nine people (two people with UC, three consultant gastroenterologists and four IBD nurses) participated in the multistakeholder online workshop on 11 March 2021. Further demographic information was not collated from workshop participants, as the focus was on sharing and discussing the research findings, as opposed to further understanding their experiences as health-care professionals or as patients.
Summary findings
The purpose of the online multistakeholder workshop was to bring together patient representatives and IBD health-care professionals to present them with the key findings from the PoPSTER study, to seek their feedback on the results and to work with them to generate recommendations for research and practice about steroid-resistant UC. To that end, a presentation summarising the methods and results from WPs 1–4 was generated by the team and shown to participants at the beginning of the workshop to orient them to the key findings. In addition to this, prior to the workshop, the key findings from each WP were reviewed by Elizabeth Coates, Nyantara Wickramasekera and Alan Lobo to identify the main areas of interest and convergence between the different studies. Triangulating the findings in this manner helped to provide a focus to the overall findings from the PoPSTER study and these findings were converted into summary statements that could then be considered by participants during the small group discussions session of the workshop. The findings were summarised as bullet points under four headings, as follows, and as shown in the first column of Table 39 in Appendix 6.
Definitions of steroid resistance
-
The majority of health-care professionals in the survey (92/135, 68%) agreed that steroid resistance is an incomplete response to 40 mg of prednisolone per day after 2 weeks.
-
This viewpoint was reflected in the health-care professional interview study, where the majority of health-care professionals agreed that a 2-week time frame was most appropriate.
-
However, of the remainder, 25 of 43 (58%) health-care professionals stated that they would judge steroid resistance over a 4-week period. Similarly, in the qualitative study, some health-care professionals highlighted that, for some patients, 4 weeks would give a clearer indication of the response to steroids.
-
Most health-care professionals interviewed distinguished steroid-dependent UC from steroid-resistant UC on the basis that the former represents an initial response but relapse on reduction of steroids (albeit at varied doses).
-
Only 13% of health-care professionals in the survey agreed that steroid-resistant and steroid-dependent disease should be treated identically.
Factors influencing treatment choices in steroid-resistant ulcerative colitis
The DCE with patients found that:
-
Higher levels of remission strongly influenced choice of treatment by patients.
-
A lower chance of side effects strongly increased the likelihood of a treatment being chosen.
-
Patients are more likely to choose treatments with higher rates of induction of response and patients are less likely to choose a treatment that takes longer to improve symptoms.
-
Taking a daily pill or injection at home every 8 weeks was preferable to infusions at hospital every 8 weeks; however, a change from infusion at hospital to injection at home every 2 weeks was not important to patients’ choices.
Correspondingly, the DCE with health-care professionals found that:
-
Health-care professionals are more likely to choose treatments with higher levels of induction of response, maintenance of remission and mucosal healing.
-
A treatment with lower risks of side effects (e.g. lymphoma or serious infection) is more likely to be chosen.
-
Health-care professionals are willing to make difficult trade-offs by tolerating greater safety risks in exchange for therapeutic benefits.
Use of surgery in steroid-resistant ulcerative colitis
-
In the survey, the majority (48%) of health-care professionals stated that they would refer patients with steroid-resistant UC for surgery ‘at any time’.
-
Other respondents preferred to wait to refer to surgery after all medical options had been tried (12%), after trying one (6%) or two (9%) biologics unsuccessfully or when patient was deemed steroid resistant (2%).
-
Health-care professionals who would offer surgery ‘at any time’ explained that patient preferences for treatments (61%) or joint management with surgeons in the MDT (54%) influenced this. Both failed response to medical treatment options (32%) and disease severity (25%) were also important.
-
Health-care professionals in the interview study identified situations where surgery may become necessary for patients (e.g. when they have tried all available medical treatments or were ‘running out of options’).
-
Concerns regarding surgery were also reflected in the patient interviews. Most patients described surgery as a ‘last resort’, preferring to try medical treatments first. Many patients had negative views about surgery because of the stigma or cosmetic appearance.
-
However, these concerns were less important to patients as surgery became more likely. Patients explained that they became less resistant to, and more accepting of, surgery as their medical options were ‘running out’.
Preferred treatments in steroid-resistant ulcerative colitis
-
In the survey, anti-TNF agents were most widely offered in all patient scenarios (steroid resistant: thiopurine exposed, 95%; thiopurine naive 87%; steroid dependent: thiopurine exposed, 88%; thiopurine naive, 74%).
-
Infliximab was most frequently suggested treatment in all scenarios, apart from steroid-dependent patients who are naive to thiopurines and who flare at either 25 mg or 5 mg per day.
-
Thiopurine use affects treatment preferences.
-
Anti-TNFs are offered less frequently:
-
in thiopurine-naive patients with steroid-resistant and dependent steroid-disease
-
with relapse at lower doses of the steroid than in resistant disease in thiopurine-naive patients but not in thiopurine-exposed patients.
-
-
Vedolizumab and tofacitinib would be offered more frequently in thiopurine-exposed than thiopurine-naive patients.
-
Patients were asked to rank four treatments in the DCE and the results suggested that the most preferred treatments were infliximab (38%) and tofacitinib (38%), followed by vedolizumab (17%) and adalimumab (6%).
Outputs from the workshop
Group 1 was tasked with considering the findings on topics 1 and 2 (i.e. definitions of steroid resistance and factors influencing treatment choices).
Definitions of steroid resistance
What is the optimal definition of steroid-resistant ulcerative colitis?
Implications
The group agreed that having a clear definition of steroid resistance in UC is important to avoid excessive use of steroids and that 2 weeks would be the optimal threshold. Although the group acknowledged that some clinicians who follow ECCO79 guidelines would use the 4-week duration, they also cautioned about the risks of being on a high dose of steroids for this length of time.
Recommendation
Consensus work to establish an agreed definition of steroid resistance in UC is needed. The group recommended that 2 weeks be used as the threshold, but agreement is also required on how a shorter interval might be implemented in practice (in the case of severe or worsening symptoms).
How should the response to steroids be monitored in practice?
Implications
The group suggested that, in clinical practice, it may simply be assumed that the steroids will be effective; however, a better mechanism is needed for assessing patients’ responses. The group also agreed that early identification of non-response would be helpful, given that 2 weeks is the recommended threshold, while being mindful that a lack of response may present earlier in some patients with more severe UC.
Recommendation
Inflammatory bowel disease services should implement a mechanism for assessing response by 2 weeks. This might include formal review by the service or tasking patients to proactively alert the service to their response (or non-response), for example via an IBD helpline or e-mail service.
How should the wider use of steroids be monitored in practice?
Implications
The group highlighted that the use of steroids beyond the IBD service is also important, as steroids can be started in accident and emergency or primary care settings or by patients themselves. As a result, there will also be variation in starting dose or duration (e.g. courses of 30 mg/day for 5 days). This creates variation in care provision, and without the correct dosage it is more difficult to monitor or assess response.
Recommendation
More work is needed to educate health-care professionals in primary and emergency care about the correct dosage and regime for steroids in UC (i.e. a full dose should be prednisolone 40 mg daily, which is then tapered, e.g. by 5 mg/day each week). Raising health-care professional awareness of existing resources, such as the Royal College of General Practitioners IBD toolkit or CCUK information leaflets, could be a helpful route to achieving this. In the longer term, better integration between primary and secondary/tertiary care records would help provide a mechanism for alerting IBD services to new courses of steroids being commenced elsewhere in the health economy.
How can we involve patients in monitoring their responses to steroids?
Implications
The group highlighted that educating patients, raising their expectations and empowering them to monitor their response (or lack thereof) to steroids was also important in improving care for patients with steroid-resistant UC.
Recommendation
The group recommended that helping patients to recognise non-response to steroids should be a priority, and this can be linked to the improved use of personalised ‘flare plans’ to inform patients’ discussions with their GP or at accident and emergency departments.
How might steroid-resistant and steroid-dependent disease be appropriately treated?
Implications
The group agreed that steroid-resistant and steroid-dependent UC should be considered separately, and that this distinction may be important in the choice of further treatments.
Recommendation
Despite this, the group suggested that further understanding of the possible circumstances in which resistant and dependent disease may be treated the same might be helpful.
How can biologic treatments be most effectively used in steroid-resistant disease?
Implications
The group agreed that the choice of biologic treatments may depend on issues such as prior use of anti-TNF, duration of interval until flare, speed of onset of agent and severity of symptoms, all of which differ from the scenario presented in the health-care professional survey.
Recommendation
Further understanding of how biologic agents can be used effectively to treat steroid-resistant disease is needed. The group suggested a related research question: ‘Can we use certain treatments to treat flares without the need for steroids?’. For example, are there newer biological agents, such as tofacitinib, that are effective induction agents, and which can be used alone and without the need for concomitant steroids? Can tofacitinib be used in the treatment paradigm where azathiopurine currently sits?
Factors influencing treatment choices in steroid-resistant ulcerative colitis
How can the risks of treatment be effectively presented to patients?
Implications
The group acknowledged the importance of discussing treatment risks, including serious risks, with patients during consultations, particularly younger patients. The group also reported differences between relative and absolute risk, and how these apply to individual patients. For example, the health-care professional DCE used lymphoma risk, which is a population risk, but the risk of lymphoma for an 80-year-old man is different from that for a 30-year-old man. Similarly, the patient DCE did not have sufficiently granularity to allow us to distinguish between small risks. Overall, the DCEs help understanding of the relative importance of treatment characteristics; however, in practice, these are applied to the individual, including previous treatments, and this may influence their next choice of treatment.
Recommendation
When treatments are selected, a more personalised approach needs to be used, framing risk in terms of the individual patient. To help effectively communicate risks to patients, clinicians could use pictures or information leaflets. The group also recommended a related research question: ‘What are the risk approaches of clinicians in different settings?’.
What are patients’ preferences for surgery compared with medical treatments?
Implications
The group agreed that more work is needed to understand patient preferences for medical treatment compared with surgery for steroid-resistant UC. It is important to remember the risks of systemic steroids and remember the role of surgery. In particular, patient representatives said that they wished that they had been offered the operation earlier.
Recommendation
The group agreed that surgery should be presented as a treatment option throughout the patient journey.
Use of surgery in steroid-resistant ulcerative colitis
Group 2 was tasked with considering the findings on topics 1 and 2 (i.e. definitions of steroid resistance and factors influencing treatment choices).
Is there an optimal time to refer ulcerative colitis patients for surgery?
Implications
The group agreed that decisions about when to refer for surgery are based on several considerations, including age, fitness for surgery, attitudes towards surgery and understanding of implications, as well as severity of disease. The group also agreed that it is difficult to be specific about exactly when a patient would be referred for surgery and that there is a need for stratification according to the above criteria. Health-care professionals said that they would refer a patient who was becoming steroid resistant because this would give them more time to prepare for surgery.
Recommendations
Patients who meet the steroid-resistant criteria should be referred to the surgical team at an early stage for initial discussion of options. Surgery should be presented as a treatment option in the same way as biologics. Greater access to experienced peer support for patients considering surgery is also needed.
What information about surgery in ulcerative colitis do patients need and what information about surgery do health-care professionals provide?
Implications
The group said that IBD nurses would ‘put out feelers’ to find out whether or not patients would like more information about surgery. The group recognised that patients find it harder to deal with surgery if they have not discussed this earlier. A patient representative was adamant that they would not want surgery in the future, but said that they would be happier talking about it with the IBD nurse than with a gastroenterologist or surgeon.
Recommendations
The group generated several research questions:
-
Do patients have better outcomes if they have earlier discussions with the surgical team?
-
What are the decision support needs of patients considering surgery?
-
What are patients’ perceptions of surgery in UC?
-
Do gastroenterologists and IBD nurses feel comfortable talking about surgery, and where do they get their surgical education?
Preferred treatments in steroid-resistant ulcerative colitis
What are the other factors that influence treatment preferences?
Implications
The group reported several other factors that influence treatment preferences. The preferences of the gastroenterologist play a part in the choice of medical treatments, as some gastroenterologists are happier than others to treat patients with new drugs. The group also said that CCGs and the costs of treatment influence which treatments are offered and when.
Recommendations
There were no direct recommendations from this part of the discussion. The other factors identified by the group were documented in the survey and qualitative study with health-care professionals.
Are patients getting the support they need to make treatment decisions?
Implications
There was recognition by the group that patients should be more involved in choosing treatment, but that current NHS practice does not allow the time in clinic for this. Supportive decision-making and deliberation are important. Time is needed for health-care professionals to have proper discussions with patients about treatment options, side effects and outcomes. It was suggested that the way that the treatment characteristics had been presented in the DCE would be a good way to present treatment options to patients in practice.
Recommendations
The group recommended that IBD services should encourage patients to be more involved in choosing their treatments, based on their preferences, lifestyle, etc. The group also made the following research recommendations:
-
Develop a tool [i.e. a BuzzFeed-style quiz (BuzzFeed, Inc., New York, NY, USA)] for patients that sets out treatments in the same way as in the DCE so that both patients and clinicians can make more informed treatment choices.
-
Qualitative research is required into whether or not patients do better if they have more choice about their treatment options.
What are the possible randomised controlled trials in steroid-resistant ulcerative colitis?
Implications
The group acknowledged that the findings related to preferred treatments and also highlighted the potential of tofacitinib (i.e. tofacitinib works quickly and can be prescribed directly from clinic so treatment can be started immediately).
Recommendations
Based on the discussions about the use of surgery and preferred treatments in steroid-resistant UC, the group made recommendations for two possible RCTs:
-
a RCT to compare infliximab and tofacitinib for treatment for steroid-resistant UC (with or without a surgery arm)
-
a RCT of supported decision-making compared with standard care and outcomes, with a focus on mental health, sexual function and other key patient outcomes.
Chapter 9 Discussion and conclusion
Discussion
Taken as a whole, the PoPSTER study provides a number of helpful insights into practice and patient and health-care professional preferences for treatments for steroid-resistant UC. The findings of this programme of work have a number of important implications for practice. We have demonstrated variation among health-care professionals in terms of definitions of steroid-resistance in UC, the importance of differentiating steroid resistance from steroid dependence, and preferences for different treatments for steroid-resistant disease. Current literature indicates either 2 or 4 weeks as the treatment duration required to determine steroid resistance. 51,52 The majority of respondents in the survey indicated that they considered 2 weeks the required duration, but a minority felt that treatment should continue for 2 further weeks before a patient is considered steroid resistant. The efficacy of a therapeutic intervention, both in practice and in clinical trials, may depend on this timing. Efficacy and safety in those resistant at 2 weeks may be different from efficacy and safety in those who have received 4 weeks of steroids, and greater steroid use is considered a disutility of care and should be avoided. 29
However, the qualitative interviews with health-care professionals also provided insight into how such guidelines might be interpreted at the level of the individual patient, for example when time thresholds might be extended or reduced based on the clinical picture. This form of variation can be captured in guidance, and the findings from this research help to inform this from the perspectives of both clinicians and patients. More generally, research shows that clinicians often deviate from practice guidelines for legitimate biomedical, patient-centred and contextually specific reasons. 81,82 Understanding these reasons for deviation can inform the development of guidelines that are better able to accommodate tailoring of management.
Anti-TNF agents were the most frequently preferred agent for steroid-resistant and steroid-dependent scenarios. However, there are few data to compare agents ‘head to head’. It is not clear whether this is a result of how long anti-TNF agents have been available and, therefore, whether health-care professionals are familiar with them or perceive benefits over other agents. However, in an assessment of the uptake probability of individual drugs in which drug attributes were matched to clinician preferences as part of the DCE, infliximab was the most preferred drug, followed by tofacitinib. Whether or not this pattern of use represents best practice is unclear, and the role that agents such as vedolizumab or tofacitinib should play needs to be further explored through additional direct comparisons in the steroid-resistant cohort as a whole or through clarification of when these agents might be the preferred option (e.g. in higher-risk subgroups, including elderly people, those with previous cancer or those with concomitant immunosuppression with thiopurines, who would normally be treated with anti-TNF agents). Again, the qualitative study provided insight into this, with some clinicians advocating treatment with biologics before the use of thiopurines, suggesting that they perceive tofacitinib and biologic treatments to be equivalent.
In the DCE, patients also selected infliximab as the preferred option based on its attributes, alongside tofacitinib. This may be because infliximab is the agent that has been available the longest and, therefore, the agent of which most patients have had experience. Among biologic-naive patients, tofacitinib was most preferred option. Clinicians should combine this evidence on patient and professional preferences with trial and ‘real-life’ data on these agents when choosing treatment for an individual.
Steroid dependence (i.e. the recurrence of symptoms on steroid dose reduction) was differentiated from steroid resistance by health-care professionals in both the survey and the qualitative studies. The distinction may not change treatment in some circumstances, with anti-TNF therapy being the most frequently chosen treatment option in both steroid-resistant and steroid-dependent disease. For those patients on thiopurines, during tapering the steroid dose at which symptoms recur did not affect the preference for anti-TNF, suggesting that, in this situation, the distinction did not affect treatment choice. However, among those patients who were naive to thiopurines, the uptake was less at lower steroid doses at the time of symptom flare, and this is presumably because clinicians would increase the dose of steroids again so that a thiopurine could be introduced. The potential for increased steroid use needs to be considered in this approach.
In addition, we have demonstrated that prior immunosuppression has an impact on the frequency with which biologics and tofacitinib are used, indicating that clinicians seem likely to want to have tried conventional immunosuppression with thiopurines before using biologics (i.e. a ‘step-up approach’ to treatment). The earlier use of biologics, without prior use of thiopurine immunosuppression, might reduce side effects relating to thiopurines and reduce long-term tissue damage. An approach in which previously thiopurine-naive patients receive thiopurines before receiving a biologic or tofacitinib may also encourage greater steroid use, that is, as a bridge to cover the 6 weeks until thiopurines are effective. Recognising this influence is, therefore, important in considering whether or not a paradigm shift away from thiopurine use is needed. Clinical guidelines might address the optimum approach, but evidence to support recommendations may be missing at this time.
Detailed understanding of patients’ preferences in their treatment and their decision-making has also been obtained. In the qualitative study, treatment effectiveness was cited as the primary concern of all participants when choosing a new treatment. The participants explained that the capacity of a treatment to alleviate symptoms and improve their quality of life was the most important driver of their preferences. Although participants explained that the route of administration and side effects were important influences on their treatment preferences, they generally placed limited value on this relative to treatment effectiveness when describing their experiences. It was also evident that patients’ preferences change over time and there was an increased willingness to try alternative treatments and, eventually, surgical options for UC according to the severity and duration of symptoms and, crucially, as medical treatment options were exhausted. This is the first qualitative study to explore patient preference for treatments in steroid-resistant UC and so the findings are unique in their qualitative perspective on this, but are in keeping with a recent DCE. 83 More broadly than this, the qualitative study also highlighted the wider concerns of the patients and the stigma and challenges they face in daily life with UC, which were very much in keeping with previous qualitative research in this area. 84–86
In contrast to findings from a recent systematic review,84 the importance of the relationship with the IBD team was clear in the patient qualitative study, with participants explaining how IBD health-care professionals guided their treatment discussions and choices. Most participants in the qualitative study described valuable relationships with their IBD nurses and medics, and said that they trust and respect the clinical expertise of these professionals. This finding accords with recommendations and standards regarding the role of multidisciplinary care, emphasising the need for such teams to supervise care and for clinicians to be part of these teams. 51,55
Several previous studies have quantified patient preferences for treatment through the use of DCEs with IBD patients. 87 The results of different studies, however, are not always comparable because different DCEs include diverse attributes for different severities of UC (e.g. 5-ASA vs. biologics vs. surgery). 87–91 To the best of our knowledge, our patient DCE is the only study that focuses on a steroid-resistant scenario conducted in the UK, but close comparative scenarios have evaluated treatments for moderate to severe UC. Almario et al. 83 conducted a DCE of patients with IBD selecting biologics as a treatment option in the USA and found that efficacy was the most important attribute for patients with UC and that side effects were the key priority factor for patients with Crohn’s disease. 83 In a DCE study by Boeri et al. conducted in the USA,92 for patients with moderate to severe disease, the authors reported that symptom improvement and risk of malignancy were the two most important attributes. Similar to our study, Boeri et al. 92 also reported that patients preferred oral route of administration to subcutaneous or i.v. administration.
The DCE studies also gave important information about the trade-offs that both clinicians and patients are prepared to make when choosing treatments. The importance that both groups placed on treatment risks should be taken into account and is perhaps greater than expected. Even low absolute risks and small differences in risk have been found to be important. Health-care professionals, therefore, need to be clear in communicating these risks to patients.
Health-care professionals expressed the importance of considering surgery as an elective option at any point in the treatment pathway. In the multistakeholder workshop, all participants supported this view and highlighted the importance of having clear information available to help inform the decision about whether or not to have surgery and its timing, and it might be helpful if this was in the form of an evidence-based patient decision aid. 93
The decision about treatment of steroid-resistant UC is necessarily preceded by an assessment of response to steroids. In addition to using a uniform and accepted definition, the process needs a mechanism to ensure that patients receive an assessment at an appropriate time point and agree about the nature of the assessment.
Route of treatment administration was also addressed in each of the PoPSTER studies. Unsurprisingly, oral administration was preferred by most patients in the DCE. However, perhaps surprisingly, the DCE showed that self-administered subcutaneous injections every 2 weeks were as acceptable as i.v. infusions every 8 weeks at hospital. The PAG explained that the injections, even at home, can be problematic for some people with UC, acting as a more frequent reminder of their illness.
Health-care professionals and patients at the multistakeholder workshop discussed a process for assessment. Services need to ensure that patients are assessed 2 weeks after starting a course of systemic steroids. This might be arranged by the IBD service, but there was also discussion of the role patients could have in self-reporting response. The process is also made more complex by the need to capture the responses of patients started on steroids in other parts of the health service (e.g. in primary care or emergency departments).
Assessment might also require objective evidence of ongoing inflammation beyond patients’ symptoms. This may be regarded as particularly important prior to escalating treatment and introducing biologic agents or further immunosuppression. Endoscopy provides direct and accurate assessment but is invasive. Measurement of faecal calprotectin is non-invasive but less sensitive and less specific. Therefore, guidance is needed on (1) the circumstances under which treatment can be escalated and a definition of steroid resistance given based on symptoms alone, (2) when additional non-invasive assessment by faecal calprotectin would suffice and (3) when endoscopic assessment is required.
The use of a multidisciplinary workshop using the ‘so what/now what’ approach was a useful method to synthesise questions. By the end of the study, the research team were quite familiar with the findings and were able to distil these into key points for discussion, which meant that preparation was not onerous. Breaking down the topics into manageable sections meant that we were able to hold focused discussions and could complete these in the time allocated. In addition, by ensuring a mix of health-care professionals and patient representatives in the group, the recommendations were kept relevant to all stakeholders. We believe that this method could be used in similar work to set priorities.
Strengths and limitations
Strengths and limitations of the health-care professional survey
To our knowledge, the survey of health-care professionals is the first to address and describe current clinical practice in steroid-resistant UC, including an exploration of how resistance to steroids is defined. The survey sample included good representation from across all regions of the UK and had a good balance of respondents from both secondary and tertiary referral centres.
However, the survey does have some limitations. First, despite several efforts to boost recruitment to the survey (e.g. extending the recruitment time frame and targeting promotion at relevant regional and national IBD meetings), the resulting sample was still relatively small. As a result, this limits the conclusions that can be drawn from the research and the opportunity to accurately compare practice between subgroups (e.g. between medics and nurses, between secondary and tertiary referral centres, or between geographical areas), which may be important in further understanding variations in practice. Despite these concerns, the sample size is still equivalent to that in other recently published surveys of IBD health-care professionals. 59,60,94 Second, and related to this, it may be that the survey captures the views of only those IBD health-care professionals with a substantial interest in the management of steroid-resistant UC. Third, the survey was limited to UK health-care professionals only, and views in other parts of the world may well differ. Similarly, the survey was restricted to established health-care professionals (i.e. consultant gastroenterologists and IBD specialist nurses), and it may be that the views of other staff within the IBD MDT and, indeed, the wider health economy may differ in ways that influence the care of patients who are resistant to steroids. Finally, the survey was conducted in 2019 prior to the COVID-19 pandemic and before the widespread availability of ustekinumab in the UK, both of which are likely to influence practice in the short and longer term. Despite all of this, the survey findings are useful for identifying potential ways to reduce the excessive use of steroids in routine clinical practice, especially when combined with the results of the other studies within PoPSTER.
Strengths and limitations of the qualitative interview studies
The strengths and limitations of the qualitative studies with IBD health-care professionals and patients can be considered together. Each study provides a rich account of the factors that influence decision-making around treatments for UC from both the patient and the health-care professional perspectives. In particular, the patient accounts give insight into the complex treatment journeys that people experience when living with UC, and the findings are generally in keeping with those of other recent qualitative research with this patient group. 84–86 In addition, the health-care study is one of only a very small number of other qualitative explorations of working in IBD care53 and is the only study, to our knowledge, to explore the issues related to the treatment of patients with steroid-resistant UC.
Despite this, the qualitative studies do have some limitations. First, although the individual interviews provide rich accounts of treatment decision-making in steroid-resistant UC, these data were collected separately and there may be gaps between patient and health-care professional perspectives that are not adequately accounted for in these studies. 95 Interviewing separately also means that the accounts lack the context of specific real-time decisions about which treatments would be offered and taken up, which could be observed, for example, during an outpatient clinic appointment. Although this alternative approach to exploring treatment decision-making by observing patient–health-care professional interactions may better capture this complexity, delivering it would be more time-consuming and resource intensive than using interviews to discuss retrospective accounts of treatment decision-making, which is considerably more practicable and feasible.
Second, as with the survey, the health-care professional study focused on the experiences of consultant gastroenterologists and IBD nurses; however, to understand the full picture of how treatments are used in the management of steroid-resistant UC, it would be useful to include other members of the MDT and wider health economy, such as GPs. 96
Third, both interview studies are subject to selection bias, as participants were recruited through volunteer samples. This is perhaps most marked in the patient study where the participants spoke about their relationships with health-care professionals because, with only one exception, the participants were positive about the support and advice given about their UC. However, other previous research95 has reported the gaps between patient and health-care professional experiences and perceptions of treatment. As the study recruited via IBD services, it is likely that only those patients with better relationships with health-care professionals were approached to participate and, subsequently, agreed to take part. As with the health-care professional survey, there is a possibility that the qualitative study may have captured the views of only those health-care professionals most concerned with the treatment of steroid-resistant UC.
All patient and health-care professional interviews were conducted by female researchers, which could be seen as a limitation. It is sometimes said that research participants frame responses to avoid sharing socially undesirable opinions with interviewers, and that interviewees assume different shared experiences with male and female researchers. For example, in some contexts, men may open up to women more than to other men. 97 However, in interviews where body image is an issue, most male research participants state no preference in terms of interviewer gender. 98 Other researchers state that, although researcher gender is a factor in the success of an interview, it is subsidiary to how the interviewer and interviewees negotiate social norms about openness, power and professionalism. 99 Finally, our studies were completed prior to the COVID-19 pandemic, and it may be that this has altered perspectives on treatment decision-making in ways that we have been unable to capture.
Strengths and limitations of the discrete choice experiments
The strengths and limitations of the two DCEs can also be considered collectively. The strengths of both DCEs include the use of a robust methodology and the following of best practice (as the design of both was informed by extensive qualitative interviews with health-care professionals and UC patients), as well as utilising evidence from systematic reviews. 100–102 In addition, there was careful selection of the attributes and levels through consultation with clinical experts, our PAG and patient co-applicants. The surveys also contained additional questions to ensure that respondents understood and interpreted the DCE tasks, and the results of these internal validity checks suggest good comprehension of the tasks, which increases confidence in the findings of both studies. Moreover, to the best of our knowledge, these are the first DCEs to quantify both health-care professional and patient preferences in steroid-resistant UC.
In addition, we were able to recruit 116 health-care professionals and 115 patients during the COVID-19 pandemic, which was a sufficiently large sample to estimate the modelled preferences. Our initial target sample size for the patient DCE was 300 participants; however, owing to the pandemic, we were unable to achieve this target. If recruitment numbers had been larger, then this would have allowed for more conclusive results from the subgroup analyses.
Nevertheless, the DCEs also have some limitations. The findings from the survey and the health-care professional interviews show variation in the definition of steroid-resistant UC, and so to ensure that all health-care professionals answered the DCE task using the same context we created a scenario detailing a typical patient with steroid-resistant UC, which included information on the symptoms of the disease and co-medication. However, this scenario did not include details about the patient’s demographic characteristics, such as gender, age and employment, because this would have necessitated different scenarios with different patient profiles. We did not do this in this study for feasibility reasons because it would necessitate undertaking the DCE with a larger sample of health-care professionals to estimate the models. We are unable to say whether or not the scenario we presented was too hypothetical and could have affected the choices health-care professionals made and if (and how) they might respond in real-life clinical settings. Despite these concerns, it is interesting to look at the findings from another recent DCE from the USA. 92 Boeri et al. 92 conducted a DCE using three different patient profiles (i.e. young patient without children, patient aged 30 years with children, and older patient) and found no statistically significant differences in treatment preferences across the three profiles and, therefore, conducted a pooled analysis of the data. 92
Furthermore, only a selection of factors that health-care professionals and patients considered during treatment decision-making in practice could be included in the DCE. For example, for feasibility reasons, factors such as speed of response to treatment, drug formulation, patient acceptability, cost and availability of infusion facilities were not included in the health-care professional DCE. All characteristics that were important to the treatment decision in the real world could not be included in the DCE design because this would require a longer more complex survey, with a high cognitive burden for participants, as well as a larger sample size, which would likely be infeasible within the constraints of the current study.
Although every effort was made to objectively describe the four treatments used in the patient survey, interpretation of the ranking of treatments should take into consideration the uncertainty in the evidence base. Although there are a significant number of data assessing treatment safety and effectiveness, there are several uncertainties in the evidence base. 103 The majority of the evidence comes from placebo-controlled efficacy trials, with, to the best of our knowledge, only one head-to-head trial and some real-world evidence from registries. 25,100,101 This is further complicated by different definitions of outcomes, which are not always measured with the same instrument or scale. Therefore, developing an accurate treatment profile was challenging. Nevertheless, to ensure rigour and objectivity, the descriptions were compiled with key statistics, which were calculated using systematic reviews and with input from experienced consultant gastroenterologists. Owing to the significant heterogeneity in the literature, it is possible that different teams could use different treatment profiles, and, unfortunately, until the gaps in the evidence base are met by well-conducted head-to-head trials, this problem is likely to persist.
Interpretation of the survey results should take into consideration that data collection was undertaken during the COVID-19 pandemic. Respondents’ attitudes towards treatment, in particular risks and side effects, may have been affected by the pandemic; however, it has not been possible to eliminate this impact, as the pandemic is still ongoing at the time of writing.
Recommendations for future research
Following discussions with health-care professionals and patients at the multistakeholder workshop, several areas of further research are recommended as follows:
-
A parallel-group individually RCT comparing infliximab and tofacitinib for people with steroid-resistant UC would be acceptable to both patients and health-care professionals. The primary outcome of interest would likely be the presence of clinical remission, as used in previous similar research. 41,44,47 Other outcomes, such as the IBD-specific health-related quality of life (e.g. the IBD Control-8 or IBD Questionnaire),75,104 would also be included.
-
Further work to reach a consensus on the clinically meaningful definition of steroid resistance in UC is recommended to build on the findings from this study. A nominal group technique or Delphi study with health-care professionals would be advisable to address the uncertainty and to clearly define steroid resistance.
-
Greater understanding of how tofacitinib can be used optimally to treat patients with steroid-resistant UC is still needed. There are several unanswered questions: can tofacitinib be used alone without the need for concomitant steroids? Can tofacitinib be used in the treatment paradigm where azathiopurine currently sits? A detailed practice survey of health-care professionals would provide a useful first step to exploring these questions.
-
Further understanding of the optimal position of surgery within a pathway for steroid-resistant UC is needed. The multistakeholder workshop recommended several related research questions: do patients have better outcomes if they have earlier discussions with the surgical team? What are the decision support needs of patients considering surgery? What are patients’ perceptions of surgery in UC? Do gastroenterologists and IBD nurses feel comfortable in talking about surgery and where do they get their surgical education?
-
During the multistakeholder workshop, there was also a desire for a RCT comparing surgery and medical therapy for steroid-resistant UC; however, patient attitudes and experience might make this difficult to deliver. Therefore, a cohort-based assessment may be more useful and feasible initially.
Conclusions
To the best of our knowledge, this is the largest programme of work to address both clinician and patient preferences and attitudes to steroid-resistant UC. This study has been carried out using quantitative and qualitative methods to provide a detailed and nuanced picture to inform practice and research.
Variation in the very definitions used for steroid-resistant and steroid-dependent disease have been highlighted, as well as the variations in drugs used, the influence of previous treatment, methods of assessment and the role of surgery. These findings have been set in the context of individual health-care professionals’ and patients’ experience and values in qualitative work to better understand how principles and guidance are best operationalised. The consistent influence of prior thiopurine immunosuppression on treatment choices is of particular importance in terms of future treatment paradigms. More general principles that enhance quality of care and patient experience are also evident, including information, support and access to a MDT.
This research has also gone further to use, to our knowledge for the first time, DCEs to understand the trade-offs that health-care professionals and patients are prepared to make in choosing treatment. These DCEs have highlighted the importance that both groups place on risks of treatment and perhaps more than is widely appreciated, even when absolute risks are low and differences in risk are low. The responses have allowed extrapolation to specific drugs that might be preferred and, although there are clear factors that confound those results, the information can usefully form the basis for choosing treatment in clinical practice and for designing trials, both in terms of choice of intervention and outcomes.
Future research and clinical practice developments in the management of steroid-resistant UC can, therefore, utilise the methods used in this study, as well as its findings.
Acknowledgements
We would like to extend special thanks to all of the people with UC and the health-care professionals working in IBD who participated in this research.
Co-applicants
Professor Alan Lobo (chief investigator, Consultant Gastroenterologist and Professor of Gastroenterology, Sheffield Teaching Hospitals NHS Foundation Trust), Professor Christopher Probert (Professor of Gastroenterology, University of Liverpool), Professor Shaji Sebastian (Professor of Gastroenterology, Hull University Teaching Hospitals NHS Trust), Dr Elizabeth Coates (Research Fellow, University of Sheffield), Dr Aoife Howard (Postdoctoral Researcher, National University of Ireland, Galway), Professor Daniel Hind (Professor of Evaluation and Assistant Director, University of Sheffield), Dr Phil Shackley (Reader in Health Economics, University of Sheffield), Mr Matthew Lee (National Institute for Health and Care Research Clinical Lecturer in General Surgery, Sheffield Teaching Hospitals NHS Foundation Trust and University of Sheffield), Nicola Totton (Medical Statistician, University of Sheffield), Sue Blackwell (patient representative), Nicola Dames (patient representative) and Hugh Bedford (patient representative).
Study Management Group
Professor Alan Lobo (chief investigator, Consultant Gastroenterologist and Professor of Gastroenterology, Sheffield Teaching Hospitals NHS Foundation Trust), Professor Christopher Probert (Professor of Gastroenterology, University of Liverpool), Professor Shaji Sebastian (Professor of Gastroenterology, Hull University Teaching Hospitals NHS Trust), Dr Elizabeth Coates (Research Fellow, University of Sheffield), Amy Barr (Research Assistant, University of Sheffield), Nyantara Wickramasekera (Research Associate, University of Sheffield), Professor Daniel Hind (Professor of Evaluation and Assistant Director, University of Sheffield), Dr Phil Shackley (Reader in Health Economics, University of Sheffield), Mr Matthew Lee (National Institute for Health and Care Research Clinical Lecturer in General Surgery, Sheffield Teaching Hospitals NHS Foundation Trust and University of Sheffield), Nicola Totton (Medical Statistician, University of Sheffield), Sue Blackwell (patient representative), Nicola Dames (patient representative), Hugh Bedford (patient representative) and Zoe Furniss (Trial Support Officer, University of Sheffield).
Staff in participating centres
Jemima Clarke (Research Co-ordinator, Sheffield Teaching Hospitals NHS Foundation Trust), Luke Barron (Directorate Research Co-ordinator for the Academic Directorate of Gastroenterology, Sheffield Teaching Hospitals NHS Foundation Trust), Rebecca Bolton (Medical Secretary, Sheffield Teaching Hospitals NHS Foundation Trust), Sally Myers (Senior IBD Clinical Research Nurse, Hull University Teaching Hospitals NHS Trust) and Martina Lofthouse (Gastroenterology and Hepatology Research Nurse, Royal Liverpool and Broadgreen University Hospitals NHS Trust).
Patient Advisory Group
Sahara Fleetwood-Beresford, Joseph Greenly, Emma Greenwood and Stephie-Jayne Simpson.
Study Steering Committee
Professor Tariq Iqbal (chairperson of committee and Consultant Gastroenterologist, University Hospitals Birmingham NHS Foundation Trust), Jayne Kranat (public representative), Cath Stansfield (Nurse Consultant Gastroenterology, Salford Royal Hospital NHS Foundation Trust), Dr Laura Ternent (Senior Lecturer in Health Economics, Newcastle University) and Gabrielle Thorpe (Lecturer, University of East Anglia).
Contributions of authors
Elizabeth Coates (https://orcid.org/0000-0002-2388-6102) (Research Fellow) contributed to the conception, design and delivery of the overall project, the acquisition and management of data in all WPs, analysis and interpretation of data in WPs 1–3 and 5, and drafted the report.
Nyantara Wickramasekera (https://orcid.org/0000-0002-6552-5153) (Research Associate) contributed to the acquisition of data in WPs 2–4, with particular responsibility for design, delivery and analysis of the DCEs, drafted the DCE sections of the report and commented on drafts of the report.
Amy Barr (https://orcid.org/0000-0002-7990-7451) (Research Assistant) contributed to the delivery of WPs 1–3, to the acquisition and management of data in WPs 1–3 and to the analysis and interpretation of data in WPs 2 and 3, and commented on drafts of the report.
Phil Shackley (https://orcid.org/0000-0002-1862-0596) (Reader in Health Economics) contributed to the conception, design and delivery of the overall project, supervised the DCEs and commented on drafts of the report.
Matthew Lee (https://orcid.org/0000-0001-9971-1635) (National Institute for Health and Care Research Clinical Lecturer in General Surgery) contributed to the conception, design and delivery of the overall project, supported analysis and interpretation of data, and commented on drafts of the report.
Daniel Hind (https://orcid.org/0000-0002-6409-4793) (Professor of Evaluation) contributed to the conception, design and delivery of the overall project, supervised analysis and interpretation of data, and commented on drafts of the report.
Christopher Probert (https://orcid.org/0000-0003-4550-0239) (Professor of Gastroenterology) contributed to the conception, design and delivery of the overall project, acted as local principal investigator for the NHS components of WPs 3 and 4, and commented on drafts of the report.
Shaji Sebastian (https://orcid.org/0000-0002-3670-6545) (Professor of Gastroenterology) contributed to the conception, design and delivery of the overall project, acted as local principal investigator for the NHS components of WPs 3 and 4, and commented on drafts of the report.
Nikki Totton (https://orcid.org/0000-0002-1900-2773) (Medical Statistician) contributed to the conception, design and delivery of the overall project, contributed to analysis and interpretation of data in WP 1, and commented on drafts of the report.
Sue Blackwell (https://orcid.org/0000-0002-2819-3727) (Patient Representative) contributed to the conception, design and delivery of the overall project, and commented on drafts of the report.
Hugh Bedford (Patient Representative) contributed to the conception, design and delivery of the overall project, and commented on drafts of the report.
Nicola Dames (Patient Representative) contributed to the conception, design and delivery of the overall project, and commented on drafts of the report.
Alan Lobo (https://orcid.org/0000-0001-8037-793X) (Professor of Gastroenterology) contributed to the conception, design and delivery of the overall project, supervised the project as chief investigator and acted as local principal investigator for the NHS components of WPs 3 and 4, and drafted the report.
Publication
Wickramasekera N, Coates E, Barr A, Lee MJ, Blackwell S, Bedford H, et al. Patient preferences for treatment in steroid resistant ulcerative colitis – a discrete choice experiment. Scand J Gastroenterol 2022;57:797–806.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Cosnes J, Gowerrousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785-94.e4. https://doi.org/10.1053/j.gastro.2011.01.055.
- Jeuring SFG, van den Heuvel TRA, Zeegers MP, Hameeteman WH, Romberg-Camps MJL, Oostenbrug LE, et al. Epidemiology and long-term outcome of inflammatory bowel disease diagnosed at elderly age – an increasing distinct entity?. Inflamm Bowel Dis 2016;22:1425-34. https://doi.org/10.1097/MIB.0000000000000738.
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46-54.e42. https://doi.org/10.1053/j.gastro.2011.10.001.
- Chen GB, Lee SH, Brion MJ, Montgomery GW, Wray NR, Radford-Smith GL, et al. Estimation and partitioning of (co)heritability of inflammatory bowel disease from GWAS and immunochip data. Hum Mol Genet 2014;23:4710-20. https://doi.org/10.1093/hmg/ddu174.
- Porter RJ, Kalla R, Ho GT. Ulcerative colitis: recent advances in the understanding of disease pathogenesis. F1000Res 2020;9. https://doi.org/10.12688/f1000research.20805.1.
- Goyette P, Boucher G, Mallon D, Ellinghaus E, Jostins L, Huang H, et al. High-density mapping of the MHC identifies a shared role for HLA-DRB1*01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat Genet 2015;47:172-9. https://doi.org/10.1038/ng.3176.
- Luo Y, de Lange KM, Jostins L, Moutsianas L, Randall J, Kennedy NA, et al. Exploring the genetic architecture of inflammatory bowel disease by whole-genome sequencing identifies association at ADCY7. Nat Genet 2017;49:186-92. https://doi.org/10.1038/ng.3761.
- Cattin AL, Le Beyec J, Barreau F, Saint-Just S, Houllier A, Gonzalez FJ, et al. Hepatocyte nuclear factor 4alpha, a key factor for homeostasis, cell architecture, and barrier function of the adult intestinal epithelium. Mol Cell Biol 2009;29:6294-308. https://doi.org/10.1128/MCB.00939-09.
- Asano K, Matsushita T, Umeno J, Hosono N, Takahashi A, Kawaguchi T, et al. A genome-wide association study identifies three new susceptibility loci for ulcerative colitis in the Japanese population. Nat Genet 2009;41:1325-9. https://doi.org/10.1038/ng.482.
- McGovern DP, Gardet A, Törkvist L, Goyette P, Essers J, Taylor KD, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet 2010;42:332-7. https://doi.org/10.1038/ng.549.
- Ventham NT, Kennedy NA, Nimmo ER, Satsangi J. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology 2013;145:293-308. https://doi.org/10.1053/j.gastro.2013.05.050.
- Kalla R, Ventham NT, Kennedy NA, Quintana JF, Nimmo ER, Buck AH, et al. MicroRNAs: new players in IBD. Gut 2015;64:504-17. https://doi.org/10.1136/gutjnl-2014-307891.
- Feakins RM. British Society of Gastroenterology . Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. J Clin Pathol 2013;66:1005-26. https://doi.org/10.1136/jclinpath-2013-201885.
- Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology 1994;107:3-11. https://doi.org/10.1016/0016-5085(94)90054-X.
- Leijonmarck CE, Persson PG, Hellers G. Factors affecting colectomy rate in ulcerative colitis: an epidemiologic study. Gut 1990;31:329-33. https://doi.org/10.1136/gut.31.3.329.
- Rioux K. What is the prognosis of ulcerative colitis?. Inflamm Bowel Dis 2008;14:S52-3. https://doi.org/10.1002/ibd.20574.
- Royal College of Physicians . Report of the Results for the National Clinical Audit of Adult Inflammatory Bowel Disease Inpatient Care in the UK. UK IBD Audit 2012.
- Ghosh N, Premchand P. A UK cost of care model for inflammatory bowel disease. Frontline Gastroenterol 2015;6:169-74. https://doi.org/10.1136/flgastro-2014-100514.
- Nurmi E, Haapamäki J, Paavilainen E, Rantanen A, Hillilä M, Arkkila P. The burden of inflammatory bowel disease on health care utilization and quality of life. Scand J Gastroenterol 2013;48:51-7. https://doi.org/10.3109/00365521.2012.685750.
- Burisch J, Jess T, Martinato M, Lakatos PL. ECCO-EpiCom . The burden of inflammatory bowel disease in Europe. J Crohns Colitis 2013;7:322-37. https://doi.org/10.1016/j.crohns.2013.01.010.
- National Institute for Health and Care Excellence (NICE) . Ulcerative Colitis: Management 2013.
- European Medicines Agency . Guideline on the Development of New Medicinal Products for the Treatment of Crohn’s Disease 2016.
- Faubion WA, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology 2001;121:255-60. https://doi.org/10.1053/gast.2001.26279.
- Ho GT, Chiam P, Drummond H, Loane J, Arnott ID, Satsangi J. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther 2006;24:319-30. https://doi.org/10.1111/j.1365-2036.2006.02974.x.
- Sands BE, Peyrin-Biroulet L, Loftus EV, Danese S, Colombel JF, Törüner M, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019;381:1215-26. https://doi.org/10.1056/NEJMoa1905725.
- Stange EF, Travis SP, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, et al. European evidence-based consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis 2008;2:1-23. https://doi.org/10.1016/j.crohns.2007.11.001.
- National Institute for Health and Care Excellence (NICE) . Vedolizumab for Treating Moderately to Severely Active Ulcerative Colitis 2015.
- National Institute for Health and Care Excellence (NICE) . Ustekinumab for Treating Moderately to Severely Active Ulcerative Colitis 2020.
- Melmed GY, Siegel CA, Spiegel BM, Allen JI, Cima R, Colombel J-F, et al. Quality indicators for inflammatory bowel disease. Inflamm Bowel Dis 2013;19:662-8. https://doi.org/10.1097/mib.0b013e31828278a2.
- Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011;60:571-607. https://doi.org/10.1136/gut.2010.224154.
- Brooks AJ, Rowse G, Ryder A, Peach EJ, Corfe BM, Lobo AJ. Systematic review: psychological morbidity in young people with inflammatory bowel disease – risk factors and impacts. Aliment Pharmacol Ther 2016;44:3-15. https://doi.org/10.1111/apt.13645.
- Høivik ML, Moum B, Solberg IC, Henriksen M, Cvancarova M, Bernklev T. IBSEN Group . Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN Study. Gut 2013;62:368-75. https://doi.org/10.1136/gutjnl-2012-302311.
- Woodward S, Dibley L, Coombes S, Bellamy A, Clark C, Czuber-Dochan W, et al. Identifying disease-specific distress in patients with inflammatory bowel disease. Br J Nurs 2016;25:649-60. https://doi.org/10.12968/bjon.2016.25.12.649.
- Sonnenberg A. Disability from inflammatory bowel disease among employees in West Germany. Gut 1989;30:367-70. https://doi.org/10.1136/gut.30.3.367.
- Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut 2004;53:1471-8. https://doi.org/10.1136/gut.2004.041616.
- Odes S, Vardi H, Friger M, Wolters F, Russel MG, Riis L, et al. Cost analysis and cost determinants in a European inflammatory bowel disease inception cohort with 10 years of follow-up evaluation. Gastroenterology 2006;131:719-28. https://doi.org/10.1053/j.gastro.2006.05.052.
- van der Valk ME, Mangen MJ, Severs M, van der Have M, Dijkstra G, van Bodegraven AA, et al. Evolution of costs of inflammatory bowel disease over two years of follow-up. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0142481.
- Odes S, Vardi H, Friger M, Esser D, Wolters F, Moum B, et al. Clinical and economic outcomes in a population-based European cohort of 948 ulcerative colitis and Crohn’s disease patients by Markov analysis. Aliment Pharmacol Ther 2010;31:735-44. https://doi.org/10.1111/j.1365-2036.2009.04228.x.
- Rogler G. Mechanism of action of vedolizumab: do we really understand it?. Gut 2019;68:4-5. https://doi.org/10.1136/gutjnl-2018-316777.
- Nwaogu A, Bond A, Smith PJ. Guideline review: tofacitinib for adults with moderately to severely active ulcerative colitis – NICE guidance Colorectal. Gastroenterology 2021;12:133-6. https://doi.org/10.1136/flgastro-2020-101502.
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462-76. https://doi.org/10.1056/NEJMoa050516.
- Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011;60:780-7. https://doi.org/10.1136/gut.2010.221127.
- Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257-65.e1. https://doi.org/10.1053/j.gastro.2011.10.032.
- Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85-9. https://doi.org/10.1053/j.gastro.2013.05.048.
- Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:96-109.e1. https://doi.org/10.1053/j.gastro.2013.06.010.
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699-710. https://doi.org/10.1056/NEJMoa1215734.
- Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723-36. https://doi.org/10.1056/NEJMoa1606910.
- Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201-14. https://doi.org/10.1056/NEJMoa1900750.
- National Institute for Health Care and Excellence (NICE) . Infliximab, Adalimumab and Golimumab for Treating Moderately to Severely Active Ulcerative Colitis After the Failure of Conventional Therapy 2015.
- Carbonnel F, Colombel JF, Filippi J, Katsanos KH, Peyrin-Biroulet L, Allez M, et al. Methotrexate is not superior to placebo for inducing steroid-free remission, but induces steroid-free clinical remission in a larger proportion of patients with ulcerative colitis. Gastroenterology 2016;150:380-8.e4. https://doi.org/10.1053/j.gastro.2015.10.050.
- Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1-s106. https://doi.org/10.1136/gutjnl-2019-318484.
- Creed TJ, Probert CS. Review article: steroid resistance in inflammatory bowel disease – mechanisms and therapeutic strategies. Aliment Pharmacol Ther 2007;25:111-22. https://doi.org/10.1111/j.1365-2036.2006.03156.x.
- Clement C, Rapport F, Seagrove A, Alrubaiy L, Williams J. Healthcare professionals’ views of the use and administration of two salvage therapy drugs for acute ulcerative colitis: a nested qualitative study within the CONSTRUCT trial. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014512.
- Loftus EV. A practical perspective on ulcerative colitis: patients’ needs from aminosalicylate therapies. Inflamm Bowel Dis 2006;12:1107-13. https://doi.org/10.1097/01.mib.0000235831.01682.8d.
- National Institute for Health and Care Excellence (NICE) . Ulcerative Colitis: Management 2019.
- Brown SR, Fearnhead NS, Faiz OD, Abercrombie JF, Acheson AG, Arnott RG, et al. The Association of Coloproctology of Great Britain and Ireland consensus guidelines in surgery for inflammatory bowel disease. Colorectal Dis 2018;20:3-117. https://doi.org/10.1111/codi.14448.
- Baker DM, Lee MJ, Jones GL, Brown SR, Lobo AJ. The informational needs and preferences of patients considering surgery for ulcerative colitis: results of a qualitative study. Inflamm Bowel Dis 2017;24:179-90. https://doi.org/10.1093/ibd/izx026.
- Archer R, Tappenden P, Ren S, Martyn-St James M, Harvey R, Basarir H, et al. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20390.
- Lee MJ, Brown SR, Fearnhead NS, Hart A, Lobo AJ. PCD Collaborators . How are we managing fistulating perianal Crohn’s disease? Results of a national survey of consultant gastroenterologists. Frontline Gastroenterol 2018;9:16-22. https://doi.org/10.1136/flgastro-2017-100866.
- Lee MJ, Heywood N, Sagar PM, Brown SR, Fearnhead NS. pCD Collaborators . Surgical management of fistulating perianal Crohn’s disease: a UK survey. Colorectal Dis 2017;19:266-73. https://doi.org/10.1111/codi.13462.
- Lee MJ, Sayers AE, Wilson TR, Acheson AG, Anderson ID, Fearnhead NS. NASBO Steering Group . Current management of small bowel obstruction in the UK: results from the national audit of small bowel obstruction clinical practice survey. Colorectal Dis 2018;20:623-30. https://doi.org/10.1111/codi.14016.
- Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care 2003;15:261-6. https://doi.org/10.1093/intqhc/mzg031.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. https://doi.org/10.1093/intqhc/mzm042.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3. https://doi.org/10.1191/1478088706qp063oa.
- Witt J, Elwyn G, Wood F, Brain K. Decision making and coping in healthcare: the Coping in Deliberation (CODE) framework. Patient Educ Couns 2012;88:256-61. https://doi.org/10.1016/j.pec.2012.03.002.
- Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. PharmacoEconomics 2008;26:661-77. https://doi.org/10.2165/00019053-200826080-00004.
- Ratcliffe J, Longworth L. Investigating the structural reliability of a discrete choice experiment within health technology assessment. Int J Technol Assess Health Care 2002;18:139-44.
- Carlsson F, Martinsson P. Design techniques for stated preference methods in health economics. Health Econ 2003;12:281-94. https://doi.org/10.1002/hec.729.
- Orme B. Getting Started with Conjoint Analysis: Strategies for Product Design and Pricing Research. Madison, WI: Research Publishers LLC; 2010.
- de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient 2015;8:373-84. https://doi.org/10.1007/s40271-015-0118-z.
- Ryan M, Gerard K, Amaya-Amaya M. Using Discrete Choice Experiments to Value Health and Health Care. New York, NY: Springer; 2008.
- Lancsar E, Louviere J, Flynn T. Several methods to investigate relative attribute impact in stated preference experiments. Soc Sci Med 2007;64:1738-53. https://doi.org/10.1016/j.socscimed.2006.12.007.
- Dalton J, Dalton J. Great Big Agile. New York, NY: Springer; 2019.
- Wickramasekera N, Coates E, Barr A, Lee MJ, Blackwell S, Bedford H, et al. Patient preferences for treatment in steroid resistant ulcerative colitis – a discrete choice experiment. Scand J Gastroenterol 2022;57:797-806. https://doi.org/10.1080/00365521.2022.2036808.
- Ormerod C. The IBD Control Questionnaire: the Development and Psychometric Validation of a Questionnaire for Measuring Inflammatory Bowel Disease Control from the Patient’s Perspective. Liverpool: University of Liverpool; 2019.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Borton T. Reach, Touch, and Teach: Student Concerns and Process Education. New York, NY: McGraw-Hill Paperbacks; 1970.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- Magro F, Gionchetti P, Eliakim R, . Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohn’s Colitis 2017;11:649-70. https://doi.org/10.1093/ecco-jcc/jjx008.
- Janssen MF, Szende A, Cabases J, Ramos-Goñi JM, Vilagut G, König HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ 2019;20:205-16. https://doi.org/10.1007/s10198-018-0955-5.
- Cohen-Stavi CJ, Key C, Molcho T, Yacobi M, Balicer RD, Shadmi E. Mixed methods evaluation of reasons why care deviates from clinical guidelines among patients with multimorbidity. Med Care Res Rev 2022;79:102-13. https://doi.org/10.1177/1077558720975543.
- Mercuri M. How do we know if a clinical practice guideline is good? A response to Djulbegovic and colleagues’ use of fast-and-frugal decision trees to improve clinical care strategies. J Eval Clin Pract 2018;24:1255-8. https://doi.org/10.1111/jep.12928.
- Almario CV, Keller MS, Chen M, Lasch K, Ursos L, Shklovskaya J, et al. Optimizing selection of biologics in inflammatory bowel disease: development of an online patient decision aid using conjoint analysis. Am J Gastroenterol 2018;113:58-71. https://doi.org/10.1038/ajg.2017.470.
- Fourie S, Jackson D, Aveyard H. Living with inflammatory bowel disease: a review of qualitative research studies. Int J Nurs Stud 2018;87:149-56. https://doi.org/10.1016/j.ijnurstu.2018.07.017.
- Rapport F, Clement C, Seagrove AC, Alrubaiy L, Hutchings HA, Williams JG. Patient views about the impact of ulcerative colitis and its management with drug treatment and surgery: a nested qualitative study within the CONSTRUCT trial. BMC Gastroenterol 2019;19. https://doi.org/10.1186/s12876-019-1085-y.
- McMullan C, Pinkney TD, Jones LL, Magill L, Nepogodiev D, Pathmakanthan S, et al. Adapting to ulcerative colitis to try to live a ‘normal’ life: a qualitative study of patients’ experiences in the Midlands region of England. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-017544.
- Bewtra M, Kilambi V, Fairchild AO, Siegel CA, Lewis JD, Johnson FR. Patient preferences for surgical versus medical therapy for ulcerative colitis. Inflamm Bowel Dis 2014;20:103-14. https://doi.org/10.1097/01.MIB.0000437498.14804.50.
- Hodgkins P, Swinburn P, Solomon D, Yen L, Dewilde S, Lloyd A. Patient preferences for first-line oral treatment for mild-to-moderate ulcerative colitis: a discrete-choice experiment. Patient 2012;5:33-44. https://doi.org/10.2165/11595390-000000000-00000.
- MacKenzie-Smith L, Marchi P, Thorne H, Timeus S, Young R, Le Calvé P. Patient preference and physician perceptions of patient preference for oral pharmaceutical formulations: results from a real-life survey. Inflamm Intest Dis 2018;3:43-51. https://doi.org/10.1159/000493346.
- Hagelund LM, Elkjær Stallknecht S, Jensen HH. Quality of life and patient preferences among Danish patients with ulcerative colitis – results from a survey study. Curr Med Res Opin 2020;36:771-9. https://doi.org/10.1080/03007995.2020.1716704.
- Gregor JC, Williamson M, Dajnowiec D, Sattin B, Sabot E, Salh B. Inflammatory bowel disease patients prioritize mucosal healing, symptom control, and pain when choosing therapies: results of a prospective cross-sectional willingness-to-pay study. Patient Prefer Adherence 2018;12:505-13. https://doi.org/10.2147/PPA.S152872.
- Boeri M, Myers K, Ervin C, Marren A, DiBonaventura M, Cappelleri JC, et al. Patient and physician preferences for ulcerative colitis treatments in the United States. Clin Exp Gastroenterol 2019;12:263-78. https://doi.org/10.2147/CEG.S206970.
- Baker DM, Lee MJ, Folan AM, Blackwell S, Robinson K, Wootton R, et al. Development and evaluation of a patient decision aid for patients considering ongoing medical or surgical treatment options for ulcerative colitis using a mixed-methods approach: protocol for DISCUSS study. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-031845.
- Sebastian S, Lisle J, Subramanian S, Dhar A, Shenoy A, Limdi J, et al. Practice pattern variability in the management of acute severe colitis: a UK provider survey. Frontline Gastroenterol 2020;11:272-9. https://doi.org/10.1136/flgastro-2019-101277.
- Schreiber S, Panés J, Louis E, Holley D, Buch M, Paridaens K. Perception gaps between patients with ulcerative colitis and healthcare professionals: an online survey. BMC Gastroenterol 2012;12. https://doi.org/10.1186/1471-230X-12-108.
- Prasad SS, Potter M, Keely S, Talley NJ, Walker MM, Kairuz T. Roles of healthcare professionals in the management of chronic gastrointestinal diseases with a focus on primary care: a systematic review. JGH Open 2020;4:221-9. https://doi.org/10.1002/jgh3.12235.
- Williams CL, Heikes EJ. The importance of researcher’s gender in the in-depth interview: evidence from two case studies of male nurses. Gend Soc 1993;7:280-91. https://doi.org/10.1177/089124393007002008.
- Yager Z, Diedrichs PC, Drummond M. Understanding the role of gender in body image research settings: participant gender preferences for researchers and co-participants in interviews, focus groups and interventions. Body Image 2013;10:574-82. https://doi.org/10.1016/j.bodyim.2013.06.004.
- Brown S. What makes men talk about health?. J Gend Stud 2001;10:187-95. https://doi.org/10.1080/09589230120053300.
- Bonovas S, Nikolopoulos GK, Lytras T, Fiorino G, Peyrin-Biroulet L, Danese S. Comparative safety of systemic and low-bioavailability steroids in inflammatory bowel disease: systematic review and network meta-analysis. Br J Clin Pharmacol 2018;84:239-51. https://doi.org/10.1111/bcp.13456.
- Bonovas S, Lytras T, Nikolopoulos G, Peyrin-Biroulet L, Danese S. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther 2018;47:454-65. https://doi.org/10.1111/apt.14449.
- Bonovas S, Fiorino G, Allocca M, Lytras T, Nikolopoulos GK, Peyrin-Biroulet L, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol 2016;14:1385-97.e10. https://doi.org/10.1016/j.cgh.2016.04.039.
- Gordon JP, McEwan PC, Maguire A, Sugrue DM, Puelles J. Characterizing unmet medical need and the potential role of new biologic treatment options in patients with ulcerative colitis and Crohn’s disease: a systematic review and clinician surveys. Eur J Gastroenterol Hepatol 2015;27:804-12. https://doi.org/10.1097/MEG.0000000000000378.
- Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989;96:804-10. https://doi.org/10.1016/S0016-5085(89)80080-0.
- National Institute for Health and Care Excellence (NICE) . Tofacitinib for Moderately to Severely Active Ulcerative Colitis 2018.
- Suzuki Y, Motoya S, Hanai H, Matsumoto T, Hibi T, Robinson AM, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol 2014;49:283-94. https://doi.org/10.1007/s00535-013-0922-y.
- Kobayashi T, Suzuki Y, Motoya S, Hirai F, Ogata H, Ito H, et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol 2016;51:241-51. https://doi.org/10.1007/s00535-015-1102-z.
- Jiang XL, Cui HF, Gao J, Fan H. Low-dose infliximab for induction and maintenance treatment in Chinese patients with moderate to severe active ulcerative colitis. J Clin Gastroenterol 2015;49:582-8. https://doi.org/10.1097/MCG.0000000000000319.
- Siegel CA. Risk of lymphoma in inflammatory bowel disease. Gastroenterol Hepatol 2009;5.
- Lichtenstein GR, Rogler G, Ciorba MA, Su C, Chan G, Pedersen RD, et al. Tofacitinib, an oral janus kinase inhibitor: analysis of malignancy (excluding nonmelanoma skin cancer) events across the ulcerative colitis clinical program. Inflamm Bowel Dis 2020;27:816-25. https://doi.org/10.1093/ibd/izaa199.
- Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D’Haens G, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839-51. https://doi.org/10.1136/gutjnl-2015-311079.
- Sandborn WJ, Panés J, D’Haens GR, Sands BE, Su C, Moscariello M, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019;17:1541-50. https://doi.org/10.1016/j.cgh.2018.11.035.
Appendix 1 Protocol version history
| Version | Date | Comments |
|---|---|---|
| 1.0 | 13 December 2018 | Study commenced with version 1.0 |
| 2.0 | 20 February 2019 |
Minor changes to the protocol to broaden the recruitment strategy for the health-care professional survey so that this more accurately reflected the intention to recruit through social media and through professional contacts and networks of clinical members of the research team. In addition, clarification of the ‘theoretical sampling’ approach for the qualitative interviews with health-care professionals and patients to provide flexibility to recruit participants in line with emerging themes Approvals for this amendment also covered the updated questionnaire for the health-care professional survey, invitation e-mail and participant information sheet Amendment number: substantial amendment 1 Amendment date: 20 February 2019 Approval date: 18 March 2019 |
| 3.0 | 12 April 2019 |
Minor changes to the protocol to broaden the recruitment strategy for the health-care professional qualitative interviews so that this includes the possibility of recruitment via social media advertising or professional networks. In addition, the protocol was updated to extend the recruitment period for the health-care professional survey and to allow promotion of the survey at forthcoming national IBD meetings Approvals for this amendment also covered the updated invitation e-mail and participant information sheet for the health-care professional qualitative interviews Amendment number: substantial amendment 2 Amendment date: 12 April 2019 Approval date: 22 May 2019 |
| 4.0 | 5 November 2019 |
Minor changes to the protocol to change the sample sizes for the DCEs (i.e. decrease from 200 to 100 health-care professionals and increase from 180 to 300 patients), based on previous challenges in health-care professional recruitment and advice of the PAG, respectively Approvals for this amendment also covered the replacement of the participant information sheet with a plain language statement and updated versions of the DCE questionnaires Amendment number: substantial amendment 3 Amendment date: 28 January 2020 Approval date: 5 March 2020 |
| 5.0 | 10 November 2020 |
Minor change to the protocol to change the delivery format of the multistakeholder workshop to be delivered online (in line with COVID-19 restrictions) This amendment also covered the updated study documents for this workshop (i.e. the participant information sheet, consent form and invitation e-mail), as well as extending the study timelines by 6 months because of delays caused by COVID-19 Amendment number: non-substantial amendment 1 Amendment date: 18 November 2020 Approval date: 26 November 2020 |
Appendix 2 Supporting information for the health-care professional survey results
| Characteristic | Frequency (N = 143) |
|---|---|
| IBD service, n (%) | |
| Secondary | 88 (62) |
| Tertiary | 48 (34) |
| Quaternary | 5 (3) |
| Missing | 2 (1) |
| Region, n (%) | |
| North East | 7 (5) |
| North West | 25 (17) |
| Yorkshire and the Humber | 24 (17) |
| East Midlands | 10 (7) |
| West Midlands | 4 (3) |
| East of England | 7 (5) |
| London | 15 (10) |
| South East | 13 (9) |
| South West | 23 (16) |
| Scotland | 2 (1) |
| Northern Ireland | 3 (2) |
| Wales | 7 (5) |
| Missing | 3 (2) |
| How much clinical time is devoted to IBD?, n (%) | |
| None of your time | 0 (0) |
| Very little of your time | 5 (3) |
| Some of your time | 59 (41) |
| The majority of your time | 53 (37) |
| All of your time | 20 (14) |
| Missing | 6 (4) |
| Is there a MDT?, n (%) | |
| Yes | 133 (93) |
| No | 7 (5) |
| Unsure | 1 (1) |
| Missing | 2 (1) |
| Mean number (SD) of MDT members | 14 (5) |
| Role, n (%) | |
| Consultant gastroenterologist | 129 (90) |
| Nurse | 126 (88) |
| Consultant radiologist | 110 (77) |
| Consultant histopathologist | 91 (64) |
| Consultant colorectal surgeon | 113 (79) |
| Dietitian | 72 (50) |
| Pharmacist | 64 (45) |
| Psychologist | 45 (31) |
| Trainee gastroenterologist | 110 (77) |
| Trainee radiologist | 58 (41) |
| Trainee colorectal surgeon | 66 (46) |
| Other | 25 (17) |
| Use of guidelines, n (%) | |
| Locally developed NHS trust guidelines | 74 (52) |
| NICE clinical guideline for UC (CG166)21 | 106 (74) |
| NICE Technology Appraisal Guidance (TA329)49 | 114 (80) |
| NICE Technology Appraisal Guidance (TA342)27 | 113 (80) |
| NICE Technology Appraisal Guidance (TA547)105 | 91 (64) |
| BSG: Mowat et al.30 | 97 (68) |
| ECCO 201779 | 119 (83) |
| I do not refer to any guidelines | 0 (0) |
| Other | 16 (11) |
Incomplete improvement to equivalent treatment of at least 40 mg per day after 1 week.
Absent response to prednisolone of at least 40 mg per day after 2 weeks.
Incomplete response to prednisolone of at least 30 mg per day after 2 weeks.
Incomplete response to prednisolone of at least 40 mg per day after 4 weeks.
Partial improvement to equivalent treatment of at least 40 mg per day after 4 weeks.
Absent improvement to equivalent treatment of at least 40 mg per day after 4 weeks.
Absent improvement to equivalent treatment of at least 40 mg per day after 2 weeks.
Absent response to prednisolone of at least 40 mg per day after 2 weeks.
Absent improvement to prednisolone of at least 40 mg per day after 2 weeks.
Incomplete response to prednisolone of at least 20 mg per day after 4 weeks.
Partial improvement to prednisolone of at least 40 mg per day after 2 weeks.
Missing.
| Treatment | Frequency (N = 120), n (%) |
|---|---|
| Infliximab | 113 (94) |
| Vedolizumab | 83 (69) |
| Adalimumab | 82 (68) |
| Admit for i.v. steroids | 78 (65) |
| Surgery | 55 (46) |
| 5-ASA (aminosalicylate) | 53 (44) |
| Tofacitinib | 51 (42) |
| Golimumab | 36 (30) |
| Methotrexate | 19 (16) |
| Tacrolimus | 19 (16) |
| Other | 11 (9) |
| Treatment | Frequency (N = 120), n (%) |
|---|---|
| Infliximab plus thiopurinea or methotrexate | 88 (73) |
| Admit for i.v. steroids | 76 (63) |
| Infliximab | 66 (55) |
| Thiopurinea | 64 (53) |
| Adalimumab plus thiopurinea or methotrexate | 63 (52) |
| Vedolizumab | 63 (52) |
| Adalimumab | 62 (52) |
| 5-ASA (aminosalicylate) | 51 (42) |
| Surgery | 47 (39) |
| Tofacitinib | 34 (28) |
| Vedolizumab plus thiopurinea or methotrexate | 31 (26) |
| Golimumab | 25 (21) |
| Golimumab plus thiopurinea or methotrexate | 25 (21) |
| Methotrexate | 12 (10) |
| Tacrolimus | 12 (10) |
| Other | 7 (6) |
| Treatment | Frequency (N = 120), n (%) |
|---|---|
| Infliximab | 103 (86) |
| Adalimumab | 88 (73) |
| Vedolizumab | 81 (68) |
| Vedolizumab and a further course of prednisolone | 59 (49) |
| Tofacitinib | 50 (42) |
| 5-ASA (aminosalicylate) | 41 (34) |
| Surgery | 39 (32) |
| Golimumab | 32 (27) |
| Admit for i.v. steroids | 23 (19) |
| Methotrexate | 16 (13) |
| Tacrolimus | 8 (7) |
| Other | 7 (6) |
| Ciclosporin (oral) | 6 (5) |
| Treatment | Frequency (N = 120), n (%) |
|---|---|
| Infliximab plus thiopurinea or methotrexate | 80 (67) |
| Thiopurinea | 70 (58) |
| Adalimumab plus thiopurinea or methotrexate | 59 (49) |
| Vedolizumab | 52 (43) |
| Infliximab | 51 (42) |
| Adalimumab | 49 (41) |
| A further course of steroids and add thiopurinea or methotrexate | 45 (38) |
| Vedolizumab and a further course of prednisolone | 44 (37) |
| 5-ASA (aminosalicylate) | 43 (36) |
| Vedolizumab plus thiopurinea or methotrexate | 37 (31) |
| Tofacitinib | 33 (28) |
| Surgery | 32 (27) |
| Admit for i.v. steroids | 23 (19) |
| Golimumab | 23 (19) |
| Golimumab plus thiopurinea or methotrexate | 22 (18) |
| Methotrexate | 15 (12) |
| Ciclosporin (oral) | 11 (9) |
| Tacrolimus | 9 (8) |
| Other | 4 (3) |
| Treatment | Frequency (N = 93), n (%) |
|---|---|
| Infliximab | 55 (59) |
| Adalimumab | 42 (45) |
| Vedolizumab | 40 (43) |
| Infliximab plus thiopurinea or methotrexate | 38 (41) |
| 5-ASA (aminosalicylate): oral or combined oral/topical | 37 (40) |
| Admit for i.v. steroids | 36 (39) |
| Adalimumab plus thiopurinea or methotrexate | 29 (31) |
| Tofacitinib | 23 (25) |
| Vedolizumab plus thiopurinea or methotrexate | 19 (20) |
| Surgery | 14 (15) |
| Golimumab | 13 (14) |
| Golimumab plus thiopurinea or methotrexate | 9 (10) |
| Methotrexate | 6 (6) |
| Thiopurine alonea | 6 (6) |
| Other | 6 (6) |
| Ciclosporin | 3 (3) |
| Tacrolimus | 3 (3) |
| Treatment | Frequency (N = 93), n (%) |
|---|---|
| Infliximab plus thiopurinea or methotrexate | 40 (43) |
| Thiopurine alonea | 39 (42) |
| 5-ASA (aminosalicylate): oral or combined oral/topical | 37 (40) |
| Infliximab | 32 (34) |
| Admit for i.v. steroids | 30 (32) |
| Adalimumab plus thiopurinea or methotrexate | 27 (29) |
| Vedolizumab | 23 (25) |
| Adalimumab | 22 (24) |
| Tofacitinib | 20 (22) |
| Vedolizumab plus thiopurinea or methotrexate | 19 (20) |
| Ciclosporin | 10 (11) |
| Golimumab | 10 (11) |
| Golimumab plus thiopurinea or methotrexate | 10 (11) |
| Methotrexate | 7 (8) |
| Surgery | 7 (8) |
| Tacrolimus | 2 (2) |
| Other | 1 (1) |
| Treatment | Frequency (N = 93), n (%) |
|---|---|
| Infliximab | 54 (58) |
| Adalimumab | 46 (49) |
| Vedolizumab | 39 (42) |
| Infliximab plus thiopurinea or methotrexate | 34 (37) |
| 5-ASA (aminosalicylate): oral or combined oral/topical | 27 (29) |
| Vedolizumab plus thiopurinea or methotrexate | 24 (26) |
| Adalimumab plus thiopurinea or methotrexate | 22 (24) |
| Tofacitinib | 20 (22) |
| Admit for i.v. steroids | 15 (16) |
| Surgery | 14 (15) |
| Golimumab | 14 (15) |
| Increase the steroids back to 40 mg/day and add thiopurinea or methotrexate | 11 (12) |
| Golimumab plus thiopurinea or methotrexate | 7 (8) |
| Tacrolimus | 4 (4) |
| Thiopurine alonea | 4 (4) |
| Other | 4 (4) |
| Methotrexate | 3 (3) |
| Ciclosporin | 2 (2) |
| Treatment | Frequency (N = 93), n (%) |
|---|---|
| Thiopurine alonea | 46 (49) |
| Increase the steroids back to 40 mg/day and add thiopurinea or methotrexate | 44 (47) |
| Infliximab plus thiopurinea or methotrexate | 38 (41) |
| Adalimumab plus thiopurinea or methotrexate | 27 (29) |
| Infliximab | 26 (28) |
| Adalimumab | 24 (26) |
| 5-ASA (aminosalicylate): oral or combined oral/topical | 23 (25) |
| Vedolizumab | 21 (23) |
| Tofacitinib | 19 (20) |
| Vedolizumab plus thiopurinea or methotrexate | 15 (16) |
| Admit for i.v. steroids | 11 (12) |
| Golimumab | 9 (10) |
| Golimumab plus thiopurinea or methotrexate | 8 (9) |
| Surgery | 8 (9) |
| Ciclosporin | 4 (4) |
| Methotrexate | 4 (4) |
| Tacrolimus | 3 (3) |
| Other | 0 (0) |
| Treatment | Frequency (N = 93), n (%) |
|---|---|
| Infliximab | 69 (74) |
| Adalimumab | 56 (60) |
| Vedolizumab | 43 (46) |
| 5-ASA (aminosalicylate) | 27 (29) |
| Tofacitinib | 21 (23) |
| Increase the corticosteroids to 40 mg/day | 20 (22) |
| Surgery | 15 (16) |
| Golimumab | 14 (15) |
| Admit for i.v. steroids | 7 (8) |
| Methotrexate | 5 (5) |
| Other | 3 (3) |
| Tacrolimus/ciclosporin | 1 (1) |
| Treatment | Frequency (N = 93), n (%) |
|---|---|
| Thiopurinea | 65 (70) |
| Infliximab plus thiopurinea or methotrexate | 40 (43) |
| 5-ASA (aminosalicylate) | 33 (35) |
| Infliximab | 30 (32) |
| Adalimumab | 24 (26) |
| Vedolizumab | 21 (23) |
| Vedolizumab and increase prednisolone | 21 (23) |
| Adalimumab plus thiopurinea or methotrexate | 20 (22) |
| Tofacitinib | 14 (15) |
| Vedolizumab plus thiopurinea or methotrexate | 13 (14) |
| Admit for i.v. steroids | 10 (11) |
| Golimumab | 9 (10) |
| Golimumab plus thiopurinea or methotrexate | 8 (9) |
| Surgery | 8 (9) |
| Other | 5 (5) |
| Methotrexate | 5 (5) |
| Tacrolimus | 2 (2) |
| Ciclosporin | 1 (1) |
Appendix 3 Supporting information for the health-care professional qualitative interviews
| Category | Code | |
|---|---|---|
| 1. Job role and hospital information |
1.1 Job role and experience 1.2 Caseload 1.3 MDT 1.4 Patient pathway |
|
| 2. Steroid-resistant UC |
2.1 Definition 2.2 Process/monitoring 2.3 Steroid resistance vs. dependence |
|
| 3. Treatments for steroid-resistant UC | 3.1 Go-to treatment |
3.1.1 Outpatient 3.1.2 Inpatient 3.1.3 Clinical trials |
| 3.2 Treatment discussions |
3.2.1 MDT working 3.2.2 Relationships with patients |
|
| 3.3 Knowledge |
3.3.1 Clinical research 3.3.2 Guidelines 3.3.3 Conferences 3.3.4 Pharma 3.3.5 IBD networks 3.3.6 Colleagues |
|
| 3.4 Opinions and preferences |
3.4.1 Efficacy 3.4.2 Availability 3.4.3 Changed over time 3.4.4 Importance of topical treatment |
|
| 3.5 Monitoring |
3.5.1 Physical tests (e.g. faecal calprotectin, scope, blood) 3.5.2 Follow-up appointments 3.5.3 Patient led (e.g. nurse helpline) |
|
| 4. Factors influencing treatment decisions | 4.1 Efficacy |
4.1.1 Speed of response 4.1.2 Mucosal healing 4.1.3 Histological healing 4.1.4 Biomarkers 4.1.5 Symptom relief 4.1.6 Quality of life |
| 4.2 Treatment variations and patient characteristics |
4.2.1 Gender 4.2.2 Age 4.2.3 BMI 4.2.4 Location of disease |
|
|
4.3 Drug availability 4.4 Professional experience 4.5 Patient preference 4.6 Severity of disease 4.7 Patient lifestyle 4.8 Route of administration 4.9 Fertility 4.10 Side effects 4.11 Comorbidities 4.12 Cost 4.13 Guidelines 4.14 Infusion room availability 4.15 Compliance 4.16 Miscellaneous |
||
| 5. Surgery |
5.1 Timing of conversation 5.2 Factors influencing referral 5.3 Information available 5.4 Opinions/perspectives |
|
Appendix 4 Supporting information for the patient qualitative interviews
| Category | Code |
|---|---|
| 1. Diagnosis |
1.1 Time since diagnosis 1.2 Comorbidity 1.3 Experiences of diagnosis 1.4 Information-seeking post diagnosis |
| 2. Perspectives on UC |
2.1 Future 2.2 Current views |
| 3. Current treatment regimen |
3.5 Adalimumab 3.6 Azathioprine 3.7 Ciclosporin 3.8 Infliximab 3.9 Mercaptopurine 3.10 Mesalazine 3.11 Prednisolone 3.12 Surgery 3.13 Toficitnib 3.14 Vedolizumab |
| 4. Previous treatments |
4.1 Adalimumab 4.2 Azathioprine 4.3 Golimumab 4.4 Infliximab 4.5 Mercaptopurine 4.6 Mesalazine 4.7 Methotrexate 4.8 Prednisolone 4.9 Surgery 4.10 Toficitnib 4.11 Vedolizumab |
| 5. Experiences of steroids | |
| 6. Reasons for changing treatments |
6.1 Reduced effectiveness 6.2 Side effects 6.3 Lack of response |
| 7. Surgery |
7.1 Views on surgery 7.2 Changes in views on surgery over time 7.3 Information provided by health-care professionals on surgery |
| 8. Factors influencing treatment decisions |
8.1 Trust in health-care professional 8.2 Effectiveness 8.3 Route of administration 8.4 Safety/side effects 8.5 Convenience of treatment 8.6 ‘Running out of options’ 8.7 Miscellaneous |
| 9. Long-term views on treatment choices | |
| 10. Other issues |
10.1 Greater involvement as adult patients vs. paediatric patient 10.2 Impact of UC on everyday life 10.3 Stigma and embarrassment of UC 10.4 Use of alternative treatments 10.5 Relationship with GP 10.6 Relationship with IBD team 10.7 Relationship and involvement of family |
Appendix 5 Supporting information for the discrete choice experiment with health-care professionals
| Attribute | Main model including all health-care professionals (p-value) | Model with place of work interaction (p-value) | Model with only health-care professionals at secondary hospitals (p-value) | Model with only health-care professionals at tertiary hospitals (p-value) |
|---|---|---|---|---|
| Induction of response | 0.068*** (0.000) | 0.068*** (0.000) | 0.061*** (0.000) | 0.055*** (0.000) |
| Mucosal healing | 0.039*** (0.000) | 0.033*** (0.000) | 0.030*** (0.000) | 0.038*** (0.000) |
| Maintenance of remission | 0.089*** (0.000) | 0.106*** (0.000) | 0.093*** (0.000) | 0.059*** (0.000) |
| Lymphoma risk | –0.189*** (0.000) | –0.274*** (0.000) | –0.235*** (0.000) | –0.091** (0.010) |
| Serious infection risk | –0.187*** (0.000) | –0.242*** (0.000) | –0.213*** (0.000) | –0.126*** (0.000) |
| Tertiary_hospital*response | –0.002 (0.831) | |||
| Tertiary_hospital *mucosal | 0.012 (0.170) | |||
| Tertiary_hospital*remission | –0.038*** (0.000) | |||
| Tertiary_hospital*lymphoma | 0.172*** (0.002) | |||
| Tertiary_hospital *infection | 0.106*** (0.002) | |||
| Observations | 2784 | 2664 | 1728 | 936 |
| n | 116 | 111 | 72 | 39 |
| Log-likelihood | –817.8 | –767.8 | –431.6 | –250.3 |
| Pseudo-R2 | 0.407 | 0.413 | 0.438 | 0.338 |
| Reference | Induction of response | Maintenance of remission | Mucosal healing | Serious infection | Lymphoma risk | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | N | % | n | N | % | n | N | % | n | N | % | Reference | n a | |
| Adalimumab | ||||||||||||||
| Reinisch et al.42 (ULTRA1-ITT-A3) | 71 | 130 | 55 | 24 | 130 | 19 | 61 | 130 | 47 | 2 | 353 | 0.57 | Siegel et al.109 | 4 |
| Reinisch et al.42 (ULTRA1-ITT-E) | 45 | 93 | 48 | 11 | 93 | 12 | 38 | 93 | 41 | Baseline risk | 2 | |||
| Sandborn et al.43 (ULTRA 2) | 89 | 150 | 59 | 32 | 150 | 21 | 74 | 150 | 49 | 4 | 258 | 1.55 | ||
| Suzuki et al.106 2014 | 45 | 90 | 50 | 9 | 90 | 10 | 40 | 90 | 44 | 8 | 178 | 4.49 | ||
| Overall | 250 | 463 | 54 | 76 | 463 | 16 | 217 | 463 | 47 | 14 | 789 | 1.8 | 6 | |
| Infliximab | ||||||||||||||
| Rutgeerts et al.41 (ACT 1) | 84 | 121 | 69 | 47 | 121 | 39 | 75 | 121 | 62 | 11 | 243 | 4.53 | Siegel et al.109 | 4 |
| Rutgeerts et al.41 (ACT 2) | 78 | 121 | 65 | 41 | 121 | 34 | 73 | 121 | 60 | 5 | 241 | 2.07 | Baseline risk | 2 |
| Kobayashi et al.107 2016 (Japic) | 57 | 104 | 50 | 21 | 104 | 20 | 48 | 104 | 46 | |||||
| Jiang et al.108 2015 | 32 | 41 | 78 | 22 | 41 | 54 | 24 | 41 | 59 | |||||
| Overall | 251 | 387 | 65 | 131 | 387 | 34 | 220 | 387 | 57 | 16 | 484 | 3.3 | 6 | |
| Tofacitinib | ||||||||||||||
| Sandborn et al.47 (OCTAVE Induction 1) | 267 | 417 | 64 | 56 | 222 | 25 | 88 | 222 | 38 | 8 | 938 | 0.9 | Lichtenstein et al.110 | 1/2576 (converted = 3.9/10,000) |
| Sandborn et al.47 (OCTAVE Induction 2) | 43 | 195 | 22 | 71 | 195 | 36 | Baseline risk | 2 | ||||||
| Overall | 267 | 417 | 64 | 99 | 417 | 24 | 159 | 417 | 38 | 8 | 938 | 0.9 | 6 | |
| Vedolizumab | ||||||||||||||
| Feagan et al.46 (GEMINI 1) | 69 | 130 | 53 | 30 | 130 | 23 | 64 | 130 | 49 | 1 | 225 | Colombel et al.111 | No additional risk | |
| Feagan et al.46 (GEMINI 1 maintenance) | 5 | 247 | Baseline risk | 2 | ||||||||||
| Overall | 69 | 130 | 53 | 30 | 130 | 23 | 64 | 130 | 49 | 6 | 472 | 1.3 | 2 | |
Examples of predicted treatment uptake calculations
Examples of change in predicted uptake rate calculations
Appendix 6 Supporting information for the discrete choice experiment with patients
Examples of change in predicted uptake rate calculations
Appendix 7 Supporting information for the multistakeholder workshop
| What are the key findings from the PoPSTER study?a | So what are the implications?b | Now what do we recommend?c |
|---|---|---|
| Definitions of steroid resistance | ||
|
The majority of health-care professionals in the survey (92/135, 68%) agreed that steroid resistance is an incomplete response to 40 mg/day prednisolone after 2 weeks This viewpoint was reflected in the health-care professional interview study, in which the majority of health-care professionals agreed that a 2-week time frame was most appropriate However, among the remaining health-care professionals, 25 of 43 (58%) stated that they would judge steroid resistance over a 4-week period. Similarly, in the qualitative study, some health-care professionals reported that, for some patients, 4 weeks would give a clearer indication of the response to steroids |
A clear definition is important to avoid prolonged steroid use In clinical practice, it may be assumed that steroids will work; however, to detect resistance, there needs to be a mechanism for assessing response. Early identification of non-response would be helpful It is important to remember that systemic steroids are not always started from the IBD service and may be started in A&E, in primary care or by the patients themselves. Courses may differ from standard or recommended regimes in terms of starting dose or duration (e.g. use of courses of 30 mg of prednisolone for 5 days). The correct dosing is important to provide uniform care and to help assessment of response Patients felt that accessing the IBD team through the helpline was better rather than going to their GP (if the service is available) Clinicians think that 4 weeks is used by some because of ECCO79 guidelines, but the respondents and this group felt that 4 weeks was too long to be on a high dose of steroids |
Having an agreed definition is important, and this is an area on which clinicians should reach consensus. According to this group, 2 weeks should be the threshold; however, in practice, a shorter interval might be implemented in the presence of worsening or severe symptoms Services should implement a mechanism for assessing response by 2 weeks. This might include formal review by the service or patients proactively alerting the service to their response (or non-response), for example via a help-line or e-mail service (i.e. ‘Raise patients’ expectations’) Work should be carried out to educate GPs or staff in A&E about administering steroid use and regimes. A full dose should be 40 mg of prednisolone daily, which is then tapered (e.g. by 5 mg/day each week) The GPs can also refer to the Royal College of General Practitioners IBD toolkit as guideline or CCUK information leaflet, as these are validated and from reputable sources, but this may be part of the educational need Educating and empowering patients was also important A mechanism for alerting IBD teams to new courses of steroids should be considered. GPs could share discharge summaries via e-mail with IBD teams. Better integrated health records or registries could help with this as a longer-term aim The recognition of non-response to steroids and education can be linked to the use of a ‘flare plan’ to inform their discussions with GP or at A&E |
|
Most health-care professionals interviewed distinguished steroid-dependent UC from steroid-resistant UC on the basis that the former represents an initial response but relapse on reduction of steroids (albeit at varied doses) Only 13% of health-care professionals in the survey agreed that steroid-resistant and steroid-dependent disease should be treated identically |
One clinician said that the terms resistance and refractory are synonyms. Therefore, it is helpful to clarify that everyone is on the same page The majority of health-care professionals thought that resistant and dependent diseases should be considered separately, as the distinction may be important in the choice of further treatment However, in this context, there is a need to understand further the 13% of health-care professionals who think that resistant and dependent may be treated the same way and there should be a discussion of circumstances in which that might be the same Choice of agent might depend on, for example, prior use of anti-TNF. Discussion that this is a different scenario from that in the survey |
Choice of treatment in steroid-dependent disease might depend on a few factors:
|
| Factors influencing treatment choices in steroid-resistant UC | ||
The DCE with patients found that:
|
Clinicians can distinguish between small risks (or appear to do so); however, we do not have sufficient granularity in the patient DCE to be able to assess this How health-care professionals select treatments overall is captured well by the DCE, but the DCE misses patients’ personal characteristics The DCE helps to unpick treatment characteristics, but it needs to be applied to the individual, including previous treatment experience, which might influence their next treatment choice It is important to recognise the influence of CCGs on health-care professional decisions and the potential for postcode variation More work can be carried out to understand patient preference for medical treatment vs. surgery. Some patient representatives said that they wished the operation had been offered sooner It is important to remember the risks of systemic steroids and to remember the role of surgery |
Therefore, when treatments are selected a more personalised approach needs to be used, framing risk in terms of the patient It is important to ensure that surgery is part of the discussion of treatment options throughout patient journey |
Correspondingly, the DCE with health-care professionals found that:
|
It is important to consider the difference between relative and absolute risks and these should be applied to individual patients, for example the DCE used lymphoma risk, which is a population risk, but the risk of lymphoma for an 80-year-old man is different from that for a 30-year-old man |
It is important to ensure that risks are discussed during consultations, including serious risks, especially for younger patients Clinicians could use pictorial representation to communicate risks or information leaflets to help communicate risks Research questions: |
| Use of surgery in steroid-resistant UC | ||
|
In the survey, the majority (48%) of health-care professionals stated that they would refer patients with steroid-resistant UC for surgery ‘at any time’ Other respondents preferred to wait to refer to surgery after all medical options had been tried (12%), after trying one (6%) or two (9%) biologics unsuccessfully or after the patient was deemed steroid resistant (2%) Health-care professionals who would offer surgery ‘at any time’ explained that patient preferences for treatments (61%) or joint management with surgeons in the MDT (54%) influenced this. Both failed response to medical treatment options (32%) and disease severity (25%) were also important |
Decisions on when to refer for surgery are based on considerations such as age, fitness for surgery, attitude towards surgery and understanding of implications, as well as severity of disease It is difficult to be absolute on when you would refer. There is a need for stratification based on the above considerations Health-care professionals would refer if the patient was becoming steroid resistant, as then the patient would have more time to prepare for surgery |
Patients who meet the criteria of steroid resistant should be referred at an early stage to the surgical team for initial discussion of options There is a need to understand patients’ perceptions of surgery in more detail There is a need for decision support for patients considering surgery and for experienced peer support for patients Research questions: |
|
Health-care professionals in the interview study identified situations where surgery may become necessary for patients (e.g. when patients have tried all available medical treatments or were ‘running out of options’) This was also reflected in the patient interviews. Most patients described surgery as a ‘last resort’, preferring to try medical treatments first. Many patients had negative views about surgery because of the stigma or cosmetic appearance. However, these concerns were less important to patients as surgery became more likely. Patients explained that they became less resistant to and more accepting of surgery as their medical options were ‘running out’ |
IBD nurses would ‘put out feelers’ with patients about whether or not they would like further information on surgery Health-care professionals should aim to position surgery as a treatment option the same way in which biologics are presented There is recognition that patients find it harder to deal with surgery if they have not had previous discussion A patient representative was adamant that they would not want surgery, but would be happier talking about it to the IBD nurse rather than gastroenterologist or surgeon |
|
| Preferred treatments in steroid resistant UC | ||
|
In the survey, anti-TNF agents were most widely offered in all patient scenarios (steroid resistant: thiopurine exposed, 95%; thiopurine naive 87%; steroid dependent: thiopurine exposed, 88%; thiopurine naive, 74%) Infliximab was the most frequently suggested treatment in all scenarios, apart from in steroid-dependent patients who were naive to thiopurines and who flare at 5 mg/day Thiopurine use affects treatment preferences Anti-TNFs are offered less frequently:Vedolizumab and tofacitinib would be offered more frequently in thiopurine-exposed patients than in thiopurine-naive patients Patients were asked to rank four treatments in the DCE and this found that the most preferred treatments were infliximab (38%) and tofacitinib (38%), followed by vedolizumab (17%) and adalimumab (6%) The predicted uptake of four treatments was estimated via the health-care professional DCE and this found that uptake rates were highest for infliximab (62%), followed by tofacitinib (18%) and vedolizumab (15%). The probability of uptake was lowest for adalimumab (5%) |
The personality of the gastroenterologist plays a part in the choice of medical treatment. Some gastroenterologists are happier to treat with new drugs than others CCGs and the costs of treatment also play a part in what options are offered and when Tofacitinib works quickly and can be prescribed direct from clinic. In addition, with tofacitinib, there is no need to wait to start treatment It was felt that the way in which the treatment characteristics were presented in the DCE would be a good way to present treatment options to patients in practice There was also a recognition that patients should be more involved in choosing a treatment option, but that current NHS practice does not allow the time in clinic for this to be carried out |
It is important to encourage the involvement of patients when choosing a treatment option (based on their preferences and lifestyle, etc.) It is important to have supportive decision-making and deliberation (i.e. health-care professionals need time to have proper discussions with patients about treatment options, side effects and outcomes) Research question:Recommendations for two possible RCTs: |
List of abbreviations
- 5-ASA
- 5-aminosalicylic acid
- BSG
- British Society of Gastroenterology
- CCG
- Clinical Commissioning Group
- CCUK
- Crohn's & Colitis UK
- CODE
- Coping in Deliberation
- COREQ
- consolidated criteria for reporting qualitative research
- CRP
- C-reactive protein
- DCE
- discrete choice experiment
- ECCO
- European Crohn’s and Colitis Organisation
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- IBD
- inflammatory bowel disease
- i.v.
- intravenous
- MDT
- multidisciplinary team
- NICE
- National Institute for Health and Care Excellence
- PAG
- Patient Advisory Group
- PoPSTER
- Patient preferences and current Practice in STERoid resistant ulcerative colitis
- PPI
- patient and public involvement
- RCN
- Royal College of Nursing
- RCT
- randomised controlled trial
- SD
- standard deviation
- TNF
- tumour necrosis factor
- UC
- ulcerative colitis
- WP
- work package
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/RHXR5192).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
