Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 16/84/01. The contractual start date was in October 2017. The draft manuscript began editorial review in September 2021 and was accepted for publication in May 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Kyle et al. This work was produced by Kyle et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Kyle et al.
Chapter 1 Background to the research
This chapter uses material from an Open Access article previously published by the research team (see Kyle et al. 20201). This article is published under licence to BMJ. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
Insomnia disorder is characterised by persistent problems with sleep initiation and/or maintenance, which leads to impairment in daytime functioning and quality of life. 2–4 Insomnia affects approximately 10% of the adult population4 and is a risk factor for several mental and physical health problems, particularly depression and cardiometabolic disease. 5,6 Insomnia is also an expensive condition, associated with substantial direct and indirect costs, chiefly reflecting increased healthcare utilisation, work-related absenteeism, reduced work productivity and elevated accident risk. 7–9
Insomnia is treatable. Clinical guidelines10–13 recommend multicomponent cognitive–behavioural therapy (CBT) as the first-line treatment, but access remains extremely limited, particularly in primary care where insomnia is principally managed. Studies in multiple countries show that general practitioners (GPs) almost never offer CBT as a treatment for insomnia, either directly or via referral. 14,15 For example, in a study of primary care patients in Switzerland, just 1% of patients diagnosed with insomnia disorder received CBT. 16 Instead, patients are typically prescribed hypnotics (which are indicated for short-term use, and only if CBT is not available or ineffective), off-label sedative antidepressant medication, or self-help sleep hygiene (SH) advice. None of these treatment approaches are recommended or evidence-based for the treatment of chronic insomnia. GPs are frustrated by this situation. Barriers to wide-scale adoption of CBT in routine health care relate to limited training, expertise and funding. A major development in the insomnia field, therefore, has been the dismantling of multicomponent, multisession CBT into brief and focused treatment packages17 and the training of non-specialists to deliver such therapies. 18–22
Sleep restriction therapy (SRT) has emerged as one of the primary active components within multicomponent CBT. The therapy involves restricting and standardising a patient’s time in bed with the aim of increasing homeostatic sleep pressure, over-riding cognitive and physiological arousal and strengthening circadian regulation of sleep. 23–26 Tailored prescription of bed and rise times over several weeks leads to improved sleep consolidation and reduction in insomnia severity. We recently performed a meta-analysis of randomised trials (8 studies; 533 participants) comparing SRT to control and found medium-to-large effects on sleep continuity measures and large effects for reduction in insomnia severity (Hedges’ g = -0.93) at post treatment. 27
Trials were predominantly performed within specialist research settings, recruiting small samples from the community who were typically free from comorbidity and did not use hypnotic medication. One trial was performed in primary care and tested GP delivery of brief SRT relative to a SH control, and showed encouraging results on the Insomnia Severity Index (ISI) at 6 months follow-up. 28 Our view was that a pragmatic trial in primary care testing a scalable model of treatment delivery was required.
We developed a brief SRT protocol based on (1) our extensive research using multicomponent CBT18–20 and (2) systematic examination of the patient experience of SRT. 29 We aimed to test whether brief SRT (alongside SH advice) was both clinically and cost-effective, relative to SH advice on its own. We chose practice nurses (PNs) as sleep therapists because nurses are increasingly involved in supporting lifestyle change and self-management of chronic conditions in primary care, and with scalability and cost-effectiveness in mind. 30 While previous studies in UK primary care showed multicomponent CBT to be effective when delivered by nurses,18,19 counsellors31 or through self-help CBT booklets,32 there had been no large-scale evaluation of the clinical and cost-effectiveness of a brief and scalable behavioural intervention. 33
Objectives
The primary objective of the Health-professional Administered Brief Insomnia Therapy (HABIT) trial was to establish whether nurse-delivered SRT for insomnia disorder in primary care improves insomnia more than SH. We hypothesised that participants allocated to SRT would demonstrate lower insomnia severity at 6 months post randomisation compared with those allocated to SH.
Our secondary hypotheses were as follows:
-
Compared with SH, participants allocated to SRT would report improvements in health-related quality of life (HRQoL), sleep-related quality of life, depressive symptoms, work productivity, pre-sleep arousal and sleep effort (at 3, 6, and 12 months).
-
Compared with SH, participants allocated to SRT would demonstrate improvements in sleep parameters (diary and actigraphy-recorded) and report a reduction in use of sleep-promoting medication (6 and 12 months).
-
The effect of SRT on insomnia severity would be mediated via reduction in sleep effort and pre-sleep arousal.
Other objectives:
-
To establish whether nurse-delivered SRT for insomnia disorder in primary care is cost-effective compared with SH, from a NHS Personal Social Services (PSS) perspective, and from a societal perspective.
-
To undertake a process evaluation to understand intervention delivery, fidelity and acceptability.
-
To test whether insomnia phenotype moderates clinical benefit obtained from SRT. One prominent model posits that participants with objective short sleep duration are less likely to experience improvement in insomnia relative to those with normal sleep duration. 5 We will examine whether actigraphy-defined sleep duration (< 6 vs. ≥ 6 hours) at baseline moderates the effect of SRT on clinical outcomes (at 6 months).
-
To test whether SRT adherence is associated with degree of clinical change (ISI) from baseline to 3 months, and from baseline to 6 months.
Chapter 2 Methods
This chapter uses material from an Open Access article previously published by the research team (see Kyle et al. 2020, BMJ Open1). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
Study design
The HABIT trial was a pragmatic, multicentre, individually randomised, parallel-group, superiority trial. Participants were recruited from general practices across three regions in the UK (Thames Valley, Greater Manchester and Lincolnshire). Assessments took place at baseline and 3, 6 and 12 months post randomisation. The trial was prospectively registered with the ISRCTN (ISRCTN42499563).
Practice and participant recruitment
We identified interested practices in three regions of England (Thames Valley, Greater Manchester and Lincolnshire) through local clinical research networks (LCRNs). In collaboration with the lead CRN, we devised search criteria to identify potentially eligible individuals from practice records. Since insomnia is not commonly coded within practices, records were initially searched for broad sleep-related terms (e.g. cannot sleep, insomnia, non-organic sleep disorders), sleep-related medications (e.g. hypnotics, sedative antidepressants), and key conditions characterised by insomnia (e.g. depressive disorder, fatigue), while applying exclusion criteria (e.g. pregnancy, age, dementia). While this meant that we identified and invited a large number of participants per practice (see Appendix 1), it did increase the possibility of reaching a varied group of people with insomnia. Searches were performed by practice managers, and GPs were given the opportunity to review the list prior to study invitation. Practice managers mailed invitations to identified individuals using Docmail. We also identified potential participants through (1) direct face-to-face GP referral (participants were provided with an information sheet and contact details for the research team), (2) placing posters in practices (containing study contact details) and (3) posting study adverts on practice websites.
Alongside the study invitation letter and participant information sheet, participants were provided with three potential methods to engage with the eligibility process, depending on preference: (1) web-link to complete an online eligibility questionnaire, (2) a brief paper questionnaire with return reply slip (following which the research team contacted participants by phone to complete the remainder of the screening process) and (3) contact details for the research team to arrange completion of the eligibility questionnaire over the phone. Regardless of methods, all interested participants underwent the same eligibility screening questionnaire.
Eligibility criteria
The inclusion criteria were as follows: (1) participant is willing and able to give informed consent for participation, (2) screens positive for insomnia symptoms on the Sleep Condition Indicator34 and meets Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition35 (DSM-V) criteria for insomnia disorder, (3) self-reported sleep efficiency (SE) < 85% over the past month,36 (4) age ≥ 18 years and (5) able to attend appointments during baseline and 4-week intervention (both face-to-face at the practice and over the phone) and adhere to study procedures.
Exclusions were limited to conditions which may be contraindicated for SRT, or render SRT inappropriate or ineffective: (1) pregnant/pregnancy planning in the next 6 months; (2) additional sleep disorder diagnosis (e.g. restless legs syndrome, obstructive sleep apnoea, narcolepsy) or ‘positive’ screen on screening questionnaire;37 (3) dementia or mild cognitive impairment (MCI); (4) diagnosis of epilepsy, schizophrenia or bipolar disorder; (5) current suicidal ideation with intent or attempted suicide within past 2 months; (6) currently receiving cancer treatment or planned major surgery during treatment phase; (7) night, evening, early morning or rotating shift-work; (8) currently receiving psychological treatment for insomnia from a health professional or taking part in an online treatment programme for insomnia; (9) life expectancy of < 2 years; and (10) another person in the household already participates in this trial.
On completion of screening, eligible participants were invited to a baseline appointment with a member of the research team where they provided written informed consent, completed baseline questionnaires, and were provided with a sleep diary and actigraph watch for the following week. Participants subsequently returned the completed diary and actigraph watch to the research team via postal mail, and were then randomised.
Interventions
Sleep hygiene
While CBT is the guideline treatment, in practice treatment as usual comprises hypnotic or sedative medication, and SH guidance. 14 National Institute for Health and Care Excellence (NICE) recommends12 that patients should be provided with SH advice as part of the management pathway, although there is no evidence that SH is effective as a monotherapy. 11,38 GPs commonly provide advice on SH but there is little standardisation of such information, in terms of either delivery format or content. Assuming that some participants would have been exposed to such information in the past, and to avoid potential bias, participants in both trial arms were provided with the same SH information. We provided a booklet comprising standard behavioural guidance in relation to lifestyle and environmental factors associated with sleep and sleeplessness. 39 Participants randomised to the SH arm were sent their booklet via e-mail or post.
Consistent with the requirements of a pragmatic trial, there were no restrictions on usual care for both groups. In this way, the trial represents a comparison of SRT + SH plus treatment as usual versus SH plus treatment as usual, permitting clear judgement to be made regarding the relative clinical utility of SRT in routine clinical practice.
Sleep restriction therapy
Participants in the intervention arm were offered nurse-delivered insomnia therapy in the form of SRT, a manualised behavioural intervention (see Table 1 for a detailed description). SRT is hypothesised to treat insomnia symptoms by reducing and standardising a patient’s time in bed with the aim of increasing homeostatic sleep pressure, over-riding cognitive and physiological arousal, and strengthening circadian regulation of sleep. 23–25 It involves implementation of a prescribed and restricted sleep schedule, which is reviewed and adjusted each week by a therapist in order to optimise SE (the proportion of time spent in bed asleep). Time in bed is initially restricted to match reported total sleep time (TST) (with 5 hours set as the minimum sleep opportunity). PNs and research nurses from CRN were trained to deliver SRT. Nurses received a 4-hour training session on sleep, insomnia and the delivery of SRT as well as access to supporting resources (e.g. recorded video clips and a list of frequently asked questions and answers in relation to treatment delivery). Trained nurses delivered manualised SRT over four brief, weekly sessions (total contact time = approximately 1 hour 5 minutes). In session 1 the nurse introduced the rationale for SRT alongside a review of sleep diaries, selection of bed and rise times, management of daytime sleepiness (including implications for driving) and discussion of barriers/facilitators to implementation. Participants were provided with a booklet to read in their own time, which included information on theory underlying SRT and a list of SH guidelines (identical to those provided to the control arm). Participants were provided with diaries and SE calculation grids to support implementation of SRT instructions and permit weekly review of progress. Sessions 2, 3 and 4 consisted of brief sessions (10–15 minutes) to review progress, troubleshoot any difficulties and advise on adaptation of the sleep schedule. Sessions 1 and 3 took place in person at the practice while sessions 2 and 4 were conducted over the phone. Therapy materials were reviewed by our patient and public involvement (PPI) advisory group, which included people with lived experience of insomnia and SRT.
| Item | Description | |
|---|---|---|
| Name of intervention | SRT for insomnia disorder | |
| Why | Insomnia is assumed to be maintained, in part, by excessive amounts of TIB and irregular sleep–wake schedules, which serve to fragment sleep. TIB awake further contributes to insomnia because the bed/bedroom environment may become associated with wakefulness over time, subsequently acting as a trigger for arousal and sleep fragmentation. SRT aims to (1) restrict TIB (to enhance SE), (2) regularise the timing of the sleep–wake cycle and (3) recondition the bed–sleep association. | |
| What: materials | Materials for patients: patients were provided with a folder at the beginning of the intervention. This folder contained a copy of the slides used during session 1, worksheets to complete during sessions 1–4, sleep diaries and SE grids to enable recording/calculation of SE each day during the 4-week intervention period and a booklet which contained enhanced information on the background and implementation of SRT, including quotes from patients who had previously undergone SRT, as well as guidance on SH. This guidance briefly covered lifestyle behaviours (e.g. caffeine, alcohol use, exercise), environmental factors (e.g. light, temperature) and the sleep routine (e.g. napping, regular bed and rise times). Materials for nurses: nurses were provided with a training folder (as part of a 4-hour training session) which contained background information on sleep, insomnia (including its development and maintenance) and SRT. The folder also contained a list of frequently asked questions in relation to trouble-shooting and specific patient scenarios that may arise, with standardised guidance on how to navigate. Nurses were provided with access to two recorded videos that gave an overview of insomnia and SRT implementation. Nurses were provided with a PowerPoint slide set to work through with each patient during session 1. They also worked through a structured checklist (completed online) for each session to guide content and structure, and enable recording of session attendance and duration. |
|
| What: procedures | In session 1 the nurse worked through PowerPoint slides with the participant to introduce the rationale for SRT alongside a review of (baseline) sleep diaries, selection of bed and rise times (for the following seven nights), management of daytime sleepiness (including implications for driving) and discussion of barriers/facilitators to implementation. Participants were provided with diaries and SE calculation grids to support implementation of SRT instructions and permit weekly review of progress. Sessions 2, 3 and 4 were brief sessions to review progress, trouble-shoot any difficulties and advise upon titration of the sleep schedule. | |
| Who provided | Registered PNs in primary care and research nurses from local CRNs were trained to deliver SRT. | |
| How provided | Intervention was delivered one-to-one, involving both face-to-face (sessions 1 and 3) and over-the-phone contacts (sessions 2 and 4). | |
| Where | The face-to-face sessions took place in a consultation room within general practice. | |
| When and how much | Intervention was delivered over four sessions. Duration and format of sessions were as follows:
|
|
| Tailoring | The treatment was tailored to each individual’s sleep pattern but followed standardised instructions for setting and titrating TIB. | |
| Criterion | SRT | |
| Calculation of prescribed TIB | Based on average TST from baseline 7-day sleep diary. Minimum TIB = 5 hours | |
| Rise-time selection | Time that aligns with working schedule and can be adhered to 7 days a week | |
| Bedtime selection | Typically delayed in order to equal the prescribed TIB | |
| Weekly adjustments to TIB based on average SE for 7 days (SE) (sessions 2–4) |
Adjustments (advancing or delaying) are typically made to the prescribed bedtime |
|
| Napping | Recommendation to eliminate all napping | |
| Nurses were encouraged to adapt the TIB prescription in the following circumstances: patient is struggling to adhere, or cannot tolerate the restriction; patient is excessively sleepy; or change in health precludes full implementation. In these circumstances nurses were encouraged to agree a revised TIB (increasing in 15-minute blocks) until the patient is content. | ||
| On completion of nurse sessions participants were encouraged to continue self-implementing SRT on their own according to the standardised rules. Participants were provided with sleep diaries and grids to enable self-implementation at home. Once daytime functioning had improved, and SE remained high – and no further sleep was obtained with additional TIB – the participant had reached their optimal sleep schedule. | ||
| How well | Face-to-face sessions were audio-recorded (if consent was provided) and a sample was independently appraised for fidelity by a clinical psychologist experienced in CBT for insomnia. Nurses followed and ‘signed-off’ a checklist at the end of each session to capture duration of session and adherence to treatment instructions. | |
Outcomes
A list of outcomes and time points and corresponding objectives can be found in Table 2.
| Objectives | Outcome measures | Time point(s) of evaluation of this outcome measure |
|---|---|---|
| Primary objective: To compare the effect of SRT vs. SH on insomnia severity |
Self-rated insomnia severity using the ISI questionnaire | Baseline and 3, 6 and 12 months post randomisation. Primary outcome is at 6 months |
| Secondary objectives: To compare the effect of SRT vs. SH on HRQoL |
Self-rated HRQoL using the SF-36 questionnaire (total score, MCS, PCS) | Baseline and 3, 6 and 12 months post randomisation |
| To compare the effect of SRT vs. SH on subjective sleep | Subjective sleep recorded over 7 nights using the CSD (SOL; WASO; SE; TST; SQ) | Baseline and 6 and 12 months post randomisation |
| To compare the effect of SRT vs. SH on objective estimates of sleep | Actigraphy-defined sleep over 7 nights (SOL; WASO; SE; TST) | Baseline and 6 and 12 months post randomisation |
| To compare the effect of SRT vs. SH on (1) patient-generated quality of life; (2) depressive symptoms; (3) work productivity; (4) hypnotic medication use; (5) use of other prescribed sleep-promoting medications and (6) pre-sleep arousal and sleep effort |
|
Baseline and 3, 6 and 12 months post randomisation. Medication use will be quantified from diaries at baseline and 6 and 12 months post randomisation |
| To compare the incremental cost-effectiveness of SRT over SH, from both NHS and societal perspectives | Trial records (time and number of nurse-led appointments), practice records* (medications), CSRI, ISI, WPAI, EQ-5D-3L | Baseline and 3, 6 and 12 months postrandomisation. *Baseline and 12 months only |
| To undertake a process evaluation to explain trial results and understand intervention delivery, fidelity and acceptability | Semistructured interviews with (1) trial participants, (2) nurses, (3) GPs or practice managers | Throughout the trial |
| Moderator analysis: Test whether objective short sleep duration at baseline (< 6 vs. ≥ 6 hours) moderates the effect of SRT on clinical outcomes (at 6 months) |
Actigraphy, ISI, GSII, SF-36 | Baseline and 6 months |
| Mediator analysis: Test whether group difference on the ISI (6 months) is mediated by change in PSAS and sleep effort (GSES) assessed at month 3 Test whether SRT adherence mediates degree of clinical change on the ISI |
ISI, PSAS, GSES Sleep diary during intervention phase, ISI |
Baseline and 3 and 6 months |
| To compare the number of specified AEs between the groups | Questionnaire | Baseline and 3, 6 and 12 months |
Measures
Insomnia severity. Insomnia severity was measured with the ISI,40 a validated self-report questionnaire, at baseline and 3, 6 and 12 months post randomisation. The ISI is a seven-item self-report measure assessing both night-time and day-time symptoms of insomnia. The possible range on the scale is from 0 to 28, with higher scores indexing more severe insomnia symptoms. The internal consistency of the measure is high (α > 0.90) in both clinical and community samples. 41 An ISI score of ≥ 11 is sensitive for insomnia disorder while a ≥ 8-point reduction is associated with moderate improvement in insomnia as assessed by an independent rater. 41
Health-related quality of life. HRQoL was assessed with the Short Form questionnaire-36 items (SF-36)42 [mental component summary (MCS) score and physical component summary (PCS) score] at baseline and 3, 6 and 12 months post randomisation.
Sleep-related quality of life. Sleep-related quality of life was measured with the Glasgow Sleep Impact Index3 (GSII, ranks 1–3) at baseline and 3, 6 and 12 months post randomisation. At baseline, the GSII asks participants to generate, in their own words, three areas of sleep-related impairment. These areas are ranked in order of concern (1–3) and then rated on a visual analogue scale with respect to the previous 2 weeks (0–100, with lower scores indicating greater level of impairment). At follow-up, participants are asked to rate the same areas of impairment, enabling group-level analyses on the three patient-generated ranks.
Depressive symptoms. Depressive symptoms were assessed with the Patient Health Questionnaire-943 (PHQ-9) at baseline and 3, 6 and 12 months post randomisation.
Work productivity. Work productivity was assessed with the self-rated productivity and activity impairment questionnaire44 (WPAI) at baseline and 3, 6 and 12 months post randomisation. The WPAI yields three outcomes for those engaged in employment: absenteeism (% work time missed due to insomnia), presenteeism (% impairment while working due to insomnia) and work productivity loss (overall work impairment/absenteeism plus presenteeism due to insomnia). The final outcome relates to non-work activity impairment and can be completed by all participants.
Pre-sleep arousal. Pre-sleep arousal was measured with the pre-sleep arousal scale45 (PSAS) at baseline and 3, 6 and 12 months post randomisation.
Sleep effort. Sleep effort was assessed with the Glasgow Sleep Effort Scale46 (GSES) at baseline and 3, 6 and 12 months post randomisation.
Sleep parameters. Self-reported sleep parameters were derived from sleep diaries. Participants completed the consensus sleep diary47 for 7 days at baseline and 6 and 12 months post randomisation. Objective sleep-parameters were obtained from actigraphy. Participants wore an actigraph watch (MotionWatch 8, CamNtech Ltd., Cambridge, UK) for 7 days at baseline and 6 and 12 months post randomisationand were instructed to press a marker button on the watch when attempting sleep. These event markers were used to define sleep periods by an experienced scorer blinded to treatment allocation. In the absence of event markers, a decision was made based on bed and rise times from the sleep diary following a decision flow-chart developed at the Sleep and Circadian Neuroscience Institute, University of Oxford. Sleep variables of interest were calculated by the validated in-built algorithm of the MotionWare software 1.2.47. The following sleep parameters were derived from sleep diaries and actigraphy recordings: sleep onset latency (SOL), wake-time after sleep onset (WASO), SE, sleep quality (SQ, diary only) and TST.
Sleep-promoting medication. Medication use was quantified from sleep diaries at baseline and 6 and 12 months post randomisation. Use of prescribed hypnotics and other sleep-promoting medications (e.g. sedative antidepressants, antihistamines, antipsychotics, melatonin) was extracted in order to capture (1) proportion of nights of use per participant and (2) proportion of participants in each group at each time point who used sleep promoting medication at least once during the 7-day recording period.
Cost-effectiveness. Intervention records captured the number and duration of nurse-led sessions to quantify cost of delivery per trial participant. The Client Service Receipt Inventory48 (CSRI) captured self-reported service use, the WPAI was used to index productivity losses and utilities were measured with the EuroQol Questionnaire49 [EuroQol-5 Dimensions, three-level version (EQ-5D-3L)] to enable calculation of quality-adjusted life-years (QALYs). In addition to the EQ-5D-3L, participants completed two additional utility measures, the Short-Form-6 Dimensions (SF-6D)50 (derived from the SF-36) and the EQ-5D-3L + Sleep,51 at 3, 6 and 12 months. EQ-5D-3L + Sleep contains the same five dimensions as the original EQ-5D-3L questionnaire plus an extra dimension on sleep. A value set has been developed for EQ-5D-3L + Sleep enabling utility values to be obtained. 51 Utility values derived from the SF-6D and EQ-5D-3L + Sleep were used to estimate QALYs over the 12-month trial period so that we could assess, in pre-defined exploratory analyses, whether sensitivity to SRT could be improved with these measures (relative to the standard EQ-5D-3L).
Process evaluation. Semistructured interviews were conducted with trial participants, nurses, GPs or practice managers across the three study sites and throughout the trial. Number of appointments attended/received by participants, fidelity appraisal of recorded consultations, and adherence to the prescribed sleep window were also considered. Fidelity of sessions was assessed by a clinical psychologist for a subsample of recordings using a bespoke rating scale (range 0–26 for treatment session 1 and 0–16 for session 3) and converted to % score. Adherence to the prescribed sleep window (intervention group only) was quantified as the number of nights per week that the participant adhered (within 15 minutes) to the nurse-prescribed bed and rise times. Bed and rise times were derived from sleep diaries completed during the 4-week intervention phase and converted to a % score. Adherence was computed for participants with a minimum of 14 out of 28 diary days. Control group contamination (i.e. the possibility that participants in the SH arm access SRT via the trained PN) was assessed using an item from the CSRI and positive responses were followed up via phone interview to collect further information.
Serious adverse events (SAEs). We defined SAEs as any untoward medical occurrence that (1) results in death, (2) is life-threatening, (3) requires inpatient hospitalisation or prolongation of existing hospitalisation, (4) results in persistent or significant disability/incapacity or (5) consists of a congenital anomaly or birth defect. Nurse therapists and participants were prompted to self-report SAEs. Along with self-reporting of SAEs, we also used responses on the CSRI which includes questions on hospitalisations, to follow up participants who reported being hospitalised. We recorded planned hospital admissions at baseline and, when they occurred, these were not counted as SAEs. SAEs were assessed for severity, seriousness and relatedness to study procedures by a medically qualified member of the team. SAEs are reported after date of randomisation until either the date of trial withdrawal or 6-month follow-up completion, whichever was earlier.
Adverse events (AEs). We recorded incidences of falls, accidents (including road-traffic accidents and work-related injuries), near-miss driving incidents, and falling asleep while driving alongside outcomes at baseline and 3, 6 and 12 months post randomisation.
Sample size
It was estimated that 235 participants would be required in each group to detect a group difference of 1.35 points [standard deviation (SD) = 4.5] on the ISI with a power of 90% at 5% level of significance (two-sided). This equates to a standardised effect size of 0.3. The SD was chosen based on the results from the primary care evaluation of SRT. 28 Accounting for 20% attrition we aimed to recruit 588 participants (294 per group). During the trial, overall attrition initially appeared higher than expected, and therefore we made a protocol amendment to increase the sample size depending upon attrition. Research Ethics Committee (REC) approval for the change was obtained in February 2020. We sought a sample size of 628 participants if attrition was 25% or less, and 672 participants if it was between 25% and 30%. Attrition was estimated to be around 25% and therefore our revised target sample size was 628.
For the process evaluation interviews, we aimed to recruit up to 15 participants from each of the 3 stakeholder groups (trial participants, nurses, GPs or practice managers), consistent with our previous experience of framework analysis52 and ensuring a sufficient number of interviews to achieve theoretical data saturation. 53
Randomisation
Participants who completed baseline assessments (including having completed at least 4 days of sleep diary) were eligible for randomisation. Participants were randomised (1 : 1) to SRT or SH using a validated web-based randomisation programme (Sortition), with a non-deterministic minimisation algorithm to ensure site, use of prescribed sleep-promoting medication (yes/no), age (18–65 vs. > 65 years), sex, baseline insomnia severity (ISI score < 22 vs. 22–28) and depression symptom severity (PHQ-9 score < 10 vs. 10–27) were balanced across the two groups. Appropriate study members at each site had access to the web-based randomisation software to complete randomisation and subsequently informed participants of their allocation.
Blinding
This was an open-label study and therefore both participants and nurses were aware of allocation. The participant information sheet informed participants that the study compared two different sleep intervention programmes but did not reveal the study hypothesis. Treatment providers (nurses) were not involved in the collection of trial outcomes. Outcomes (questionnaires, diaries and actigraphy) were self-completed, remotely, by participants. Due to impracticalities associated with blinding of the research team, combined with minimal risk of bias due to use of self-report outcome measures, researchers at each site were aware of treatment allocation. Communication from the research team to participants, post randomisation, was limited to collection of outcome assessments and not therapeutic procedures. The statisticians remained blind to allocation. A full detailed statistical analysis plan (SAP) was prepared and finalised before data collection was complete.
Statistical methods
Descriptive statistics of recruitment, dropout and completeness of interventions were calculated. Baseline variables are presented by randomised group using frequencies (with percentages) for binary and categorical variables, and means (and SDs) or medians (with lower and upper quartiles) for continuous variables. There were no tests of statistical significance nor confidence intervals for differences between groups on any baseline variables. There was no planned interim analysis for efficacy or futility.
The primary analysis population included all eligible randomised participants who had at least one outcome measurement. Participants who withdrew from the trial were included in the analysis until the point at which they withdrew. Participants were analysed according to their allocated treatment group irrespective of what treatment they actually received. Every effort was made to follow up all participants.
Primary outcome. A three-level linear mixed-effect model was fitted to the ISI score assessed at 3, 6 and 12 months following randomisation. Practice and participant were included as random effects. The model specified an unstructured variance–covariance structure for the random effects. Fixed effects included randomised group, minimisation factors [baseline ISI score (continuous), site, age (continuous), use of prescribed sleep promoting medication (yes/no), sex and baseline PHQ-9 score (continuous)], time, and a time by randomised group interaction term to allow estimation of treatment effect at each time point. The estimated difference between arms at 6 months was extracted from the model by means of a linear contrast statement.
Secondary outcomes. Continuous secondary outcomes were analysed using the same method. Secondary outcomes that were binary were analysed using generalised linear mixed-effect models with appropriate link function. For continuous outcomes standardised effect sizes (Cohen’s d) were calculated as the adjusted treatment effect divided by the pooled SD at baseline.
The Mann–Whitney test was used for three of the secondary outcomes (WPAI absenteeism, proportion of days usage of prescribed hypnotic medication, and proportion of days usage of prescribed other medication) due to violation of model assumptions. The p-value for a difference is reported at each time point, and no treatment effect has been reported.
The two count outcomes of interest were the number of times falling asleep while driving and the number of falls. The SAP stated that these outcomes would be analysed using a Poisson model and if there were excess zeros and/or over-dispersion in the data, a zero-inflated Poisson model and/or a negative binomial model would be considered instead. Both outcomes had excess zeros; < 10% of participants had one or more times falling asleep while driving or number of falls. Due to the event rates being low, a simpler analysis was undertaken instead. These outcomes were defined as a binary outcome [no (0 events)/yes (1 + events)], and a logistic mixed-effect model was used.
Serious adverse events were analysed based on the number of participants who actually received the intervention and Fisher’s exact test was used to compare SRT and SH.
Missing data and sensitivity analyses. The following sensitivity analyses were pre-specified in the SAP to examine the robustness of the primary outcome results to different assumptions regarding missing data:
-
analysis adjusted for baseline covariates found to be predictive of missingness
-
exclusion of any self-rated insomnia severity scores from the analysis which were deemed to be outliers (none were observed)
-
analysis using pattern mixture model to examine the robustness of the missing at random assumption
-
analysis for missing data on the primary outcome at 6 months assuming plausible arm-specific differences between responders and non-responders.
Full details of the sensitivity analyses were specified in the SAP. Multiple imputation of the primary outcome analysis was also conducted as a post hoc sensitivity analysis.
Moderation analyses. We conducted pre-specified subgroup analysis of the primary outcome by baseline actigraphy-defined sleep duration (< 6 vs. ≥ 6 hours), sleep medication use, depression severity (PHQ-9), age, level of deprivation, and chronotype [assessed with the morningness–eveningness questionnaire, reduced version (MEQr)]. 54 The subgroup analyses were conducted using the same method above but adding a three-way interaction term between randomised group, assessment time point, and a subgroup indicator variable to allow the treatment effect to be estimated at each time point and in each level of the subgroups.
Mediation analyses. We proposed in the statistical analysis plan to use structural equation modelling for the mediation analyses; however, due to convergence problems, the analysis strategy was revised and conducted using the Baron and Kenny55 approach but adapted to make use of linear mixed-effect models (similar to Freeman et al. 56). A mixed effects model was fitted to estimate the mediator-outcome effect and another mixed effects model to estimate the treatment-mediator effect. The indirect effect was then calculated as the product of the effect of the mediator at 3 months on outcome at 6 months and the effect of treatment on mediator at 3 months. Confidence intervals and p-values were calculated using Sobel’s test. This allowed us to determine the extent to which the 3-month arousal and sleep effort outcomes (PSAS, GSES) mediated the 6-month ISI outcome. All models included baseline assessments of the mediator and ISI as covariates.
Compliance and adherence. A complier-average causal effect (CACE) analysis of the primary outcome was carried out to determine the impact of compliance with the allocated intervention on the treatment effect. Compliance was defined as attending at least one treatment session. CACE models were estimated using an instrumental variable approach where the outcome is total ISI score at 6 months adjusted for baseline ISI. Additionally, models were fitted adjusting for baseline characteristics that appeared to be associated with compliance. Sensitivity analyses were carried out which adjusted the definition of compliance to attending at least two, three or four sessions, and multiple imputation was carried out on the primary CACE analysis as a sensitivity analysis to assess the impact of missing data.
We also explored the effect of level of adherence to prescribed bed and rise times (captured by sleep diaries) on the primary outcome in those who received SRT. Percentage treatment adherence was categorised (≥ 0 to ≤ 40/> 40 to ≤ 60/> 60 to ≤ 80/> 80 to ≤ 100) and descriptive estimates for the ISI at 6 months (primary end point), change from baseline to 3 months, and change from baseline to 6 months are presented for each category. Treatment effects on the change scores for different levels of adherence were estimated by fitting a group by categorised adherence interaction in the model, with the reference category being the control group. The models are adjusted for baseline ISI score and a random effect is fitted for practice. Therefore, these estimated treatment effects reflect difference in the change in ISI from baseline for each adherence category as compared to control.
All analyses were conducted using Stata (version 16.1).
Economic evaluation
A within-trial economic evaluation was performed to estimate the incremental cost-effectiveness of SRT over SH. Full details are described in Chapter 4 and a brief summary is presented here for continuity.
The cost–utility analysis was conducted from the recommended NHS and PSS perspective. Individual patient data on the use of health services were collected at 3, 6 and 12 months post randomisation as part of the follow-up data-collection process. We calculated the cost of delivering the SRT intervention, including preparation and training of nurses, and the cost of sending SH information to the control group. HRQoL was captured through the EQ-5D-3L at baseline and 3, 6 and 12 months post randomisation, and was used to calculate QALYs. Cost and QALYs were combined to calculate the incremental cost-effectiveness ratio (ICER) and net monetary benefit (NMB) statistics.
Process evaluation
We used a Framework approach to data analysis supported by QSR NVivo (version 10), with the framework based on the main areas of implementation, mechanisms of impact, and contextual factors together with the more detailed issues that arise from these. 57 Full details of the methodology and analysis are provided in Chapter 5. Analysis began as soon as the initial interviews were transcribed, and interview schedules were applied flexibly so that qualitative data were collected iteratively, allowing themes that were identified in earlier interviews to be explored in later ones. We analysed qualitative process data prior to knowing trial outcomes to avoid biased interpretation. Analysis of quantitative data allowed us to ascertain the extent to which we sampled participants with differences in insomnia severity at baseline, and the integration of qualitative and quantitative data enabled us to link improvements in sleep (efficiency) to interview findings from patients and staff.
Patient and public involvement
Four people from the Healthier Ageing Public and Patient Involvement group, University of Lincoln, read and provided detailed comments on the original grant proposal, helping to shape key methodological choices. For example, the group recommended adding a patient-centred measure of quality of life and assessing long-term follow-up of sleep and daytime functioning outcomes. Two individuals, one with experience of insomnia and SRT, contributed during the conduct of the trial by reviewing the participant information sheet, consent form, therapy workbooks and questionnaire measures. They recommended amendments to improve the readability and accessibility of all participant-facing documents. They also advised on recruitment procedures and methods to engage prospective participants and retain enrolled participants, and were members of the Trial Steering Committee (TSC) who met every 6 months during the trial. They supported interpretation of findings and will advise on dissemination of findings once published.
Ethical approval
The trial received both Health Research Authority approval (IRAS: 238138) and ethical approval (Yorkshire and the Humber – Bradford Leeds REC, reference: 18/YH/0153).
Summary of changes to the project protocol
Table 3 summarises the key changes made to the protocol during the trial.
| Change | Justification |
|---|---|
| Sample size increased from 588 up to 672 based on attrition level. | To allow for higher than expected attrition. |
| Added 1 person per household as exclusion criterion. | To minimise risk of contamination between trials arms. |
| Removed SF-36 total score as an outcome during the trial (and therefore prior to data lock). | This was initially recorded in error. A total score cannot be generated from the questionnaire. |
| Treatment sessions to be completed via web-conferencing. | To adapt nurse treatment so it could be delivered during COVID-19. |
Chapter 3 Results: clinical effectiveness
Recruitment
This chapter uses material from an Open Access article previously published by the research team [see Kyle SD, Siriwardena AN, Espie CA, Yang Y, Petrou S, Ogburn E, et al. Clinical and cost-effectiveness of nurse-delivered sleep restriction therapy for insomnia in primary care (HABIT): a pragmatic, superiority, open-label, randomised controlled trial. Lancet 2023;402(10406):975–87. https://doi.org/10.1016/S0140-6736(23)00683-9. Epub
10 Aug 2023. PMID: 37573859]. This article is published under licence to The Lancet. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
We recruited participants from 35 practices (average patient list size = 11,802) across three sites (Thames Valley, Greater Manchester, Lincolnshire) between 29 August 2018 and 23 March 2020. A total of 31,464 invitation letters were sent out from practices; 3171 people entered the screening phase and 642 participants were randomised (321 to intervention and 321 to control; Figure 1). Main reasons for exclusion following eligibility assessment were not meeting insomnia criteria, shift work and suspected sleep disorder other than insomnia (see Appendix 1, Table 33).
FIGURE 1.
Participant flow chart.
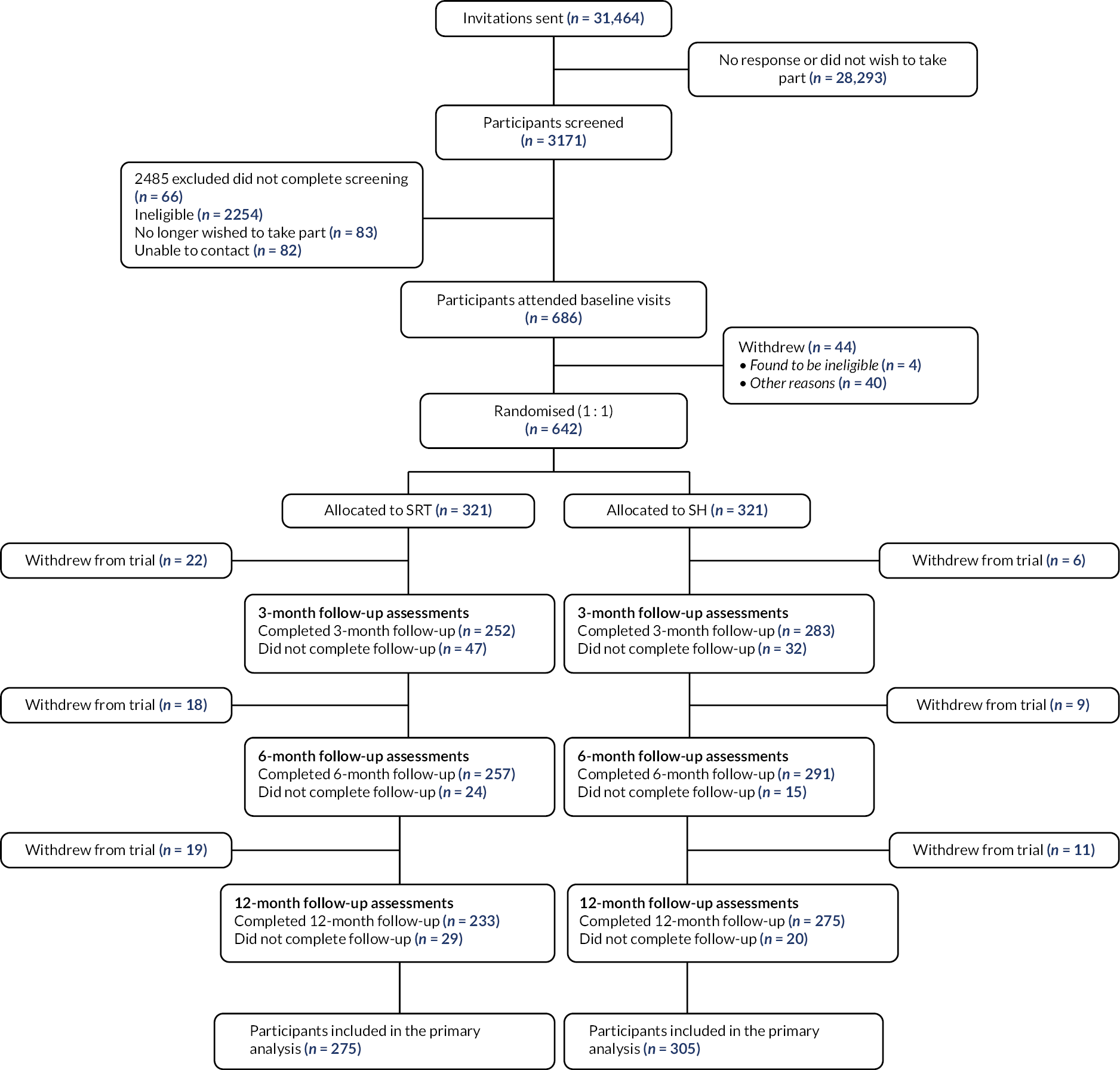
Baseline data
Baseline characteristics by randomised group are presented in Tables 4–6. Mean age (range) was approximately 55 (19–88) years old, 76% were female, 97% were from a white ethnic background, and nearly 50% had a university degree. Mean (SD) ISI scores were in the clinical range (17.5–4.1), median duration of insomnia was 10 years, 76% had previously consulted their doctor for insomnia, and 25% reported current use of prescribed sleep medication. The sample had a range of comorbid conditions. For example, 41% had a mental health problem, 30% had a musculoskeletal disorder and 20% had a respiratory illness. Seventy-one per cent had two or more medical conditions. Consistent with these data, mean SF-36 scores for mental health and physical health were lower than normative values58 and 49% met ‘caseness’ for depression on the PHQ-9 (score ≥ 10). Baseline characteristics were similar between the two groups, with a slightly higher percentage of participants in the SRT group having consulted for insomnia (78% vs. 74% for SH).
| SRT (N = 321) | SH (N = 321) | Overall (N = 642) | |
|---|---|---|---|
| Region, n (%) | |||
| Thames Valley | 156 (48.6) | 156 (48.6) | 312 (48.6) |
| Greater Manchester | 109 (34.0) | 111 (34.6) | 220 (34.3) |
| Lincolnshire | 56 (17.4) | 54 (16.8) | 110 (17.1) |
| Age, mean (SD) (min, max) | 55.7 (15.3) (19.0 to 88.0) |
55.2 (16.5) (19.0 to 87.0) |
55.4 (15.9) (19.0 to 88.0) |
| Sex, n (%) | |||
| Female | 245 (76.3) | 244 (76.0) | 489 (76.2) |
| Male | 76 (23.7) | 77 (24.0) | 153 (23.8) |
| Ethnicity, n (%) | |||
| White | 312 (97.2) | 312 (97.2) | 624 (97.2) |
| Asian/Asian British | 3 (0.9) | 6 (1.9) | 9 (1.4) |
| Black/African/Caribbean/Black British | 1 (0.3) | 1 (0.3) | 2 (0.3) |
| Mixed/multiple ethnic groups | 2 (0.6) | 1 (0.3) | 3 (0.5) |
| Other ethnic group | 2 (0.6) | 1 (0.3) | 3 (0.5) |
| Prefer not to say | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| Education level, n (%) | |||
| None | 16 (5.0) | 22 (6.9) | 38 (5.9) |
| GCSE or equivalent | 82 (25.5) | 70 (21.8) | 152 (23.7) |
| A-levels or equivalent | 50 (15.6) | 76 (23.7) | 126 (19.6) |
| University undergraduate | 80 (24.9) | 65 (20.2) | 145 (22.6) |
| University postgraduate | 90 (28.0) | 85 (26.5) | 175 (27.3) |
| Choose not to say | 3 (0.9) | 3 (0.9) | 6 (0.9) |
| Marital status, n (%) | |||
| Single | 48 (15.0) | 54 (16.8) | 102 (15.9) |
| Married, or in a domestic partnership | 220 (68.5) | 195 (60.7) | 415 (64.6) |
| Divorced | 21 (6.5) | 37 (11.5) | 58 (9.0) |
| Widowed | 24 (7.5) | 22 (6.9) | 46 (7.2) |
| Separated | 7 (2.2) | 10 (3.1) | 17 (2.6) |
| Choose not to say | 1 (0.3) | 3 (0.9) | 4 (0.6) |
| Index of multiple deprivation score (quintiles), n (%) | |||
| 1 (most deprived) | 10 (3.1) | 8 (2.5) | 18 (2.8) |
| 2 | 30 (9.3) | 36 (11.2) | 66 (10.3) |
| 3 | 52 (16.2) | 35 (10.9) | 87 (13.6) |
| 4 | 82 (25.5) | 93 (29.0) | 175 (27.3) |
| 5 (least deprived) | 144 (44.9) | 146 (45.5) | 290 (45.2) |
| Missing, n (%) | 3 (0.9) | 3 (0.9) | 6 (0.9) |
| BMI, mean (SD) (min, max) | 26.7 (5.5) (17.1 to 64.8) |
26.3 (5.3) (15.9 to 54.1) |
26.5 (5.4) (15.9 to 64.8) |
| Missing, n (%) | 18 (5.6) | 35 (10.9) | 53 (8.3) |
| Smoking status, n (%) | |||
| Non-smoker | 214 (66.7) | 202 (62.9) | 416 (64.8) |
| Ex-smoker | 84 (26.2) | 94 (29.3) | 178 (27.7) |
| Smoker | 23 (7.2) | 25 (7.8) | 48 (7.5) |
| Alcohol consumption, n (%) | |||
| Never | 62 (19.3) | 55 (17.1) | 117 (18.2) |
| Sometimes | 133 (41.4) | 151 (47.0) | 284 (44.2) |
| Every week | 126 (39.3) | 115 (35.8) | 241 (37.5) |
| Duration of insomnia (years), median (IQR) (min, max) | 10.0 (4.8–20.0) (0.4 to 66.0) |
10.0 (4.2–20.0) (0.3 to 80.0) |
10.0 (4.5–20.0) (0.3 to 80.0) |
| Consulted for insomnia, n (%) | 249 (77.6) | 237 (73.8) | 486 (75.7) |
| Work-related accident in last 3 months, n (%) | 4 (1.2) | 5 (1.6) | 9 (1.4) |
| Motor-vehicle accident in last 3 months, n (%) | 5 (1.6) | 3 (0.9) | 8 (1.2) |
| Near-miss driving incident in last 3 months, n (%) | 25 (7.8) | 24 (7.5) | 49 (7.6) |
| Times fallen asleep while driving in last 3 months, n (%) | |||
| None | 318 (99.1) | 313 (97.5) | 631 (98.3) |
| Once | 0 (0.0) | 6 (1.9) | 6 (0.9) |
| More than once | 3 (0.9) | 2 (0.6) | 5 (0.8) |
| Times had a fall in last 3 months, n (%) | |||
| None | 265 (82.6) | 263 (81.9) | 528 (82.2) |
| Once | 30 (9.3) | 30 (9.3) | 60 (9.3) |
| More than once | 26 (8.1) | 28 (8.7) | 54 (8.4) |
| Patient currently taking prescribed sleep medication, n (%) | 83 (25.9) | 80 (24.9) | 163 (25.4) |
| Number of medical conditions, n (%) | |||
| 0 | 38 (11.8) | 34 (10.6) | 72 (11.2) |
| 1 | 60 (18.7) | 52 (16.2) | 112 (17.4) |
| 2 | 73 (22.7) | 60 (18.7) | 133 (20.7) |
| 3 or more | 150 (46.7) | 175 (54.5) | 325 (50.6) |
| Category of medical condition, n (%) | |||
| Cardiovascular disease or chronic kidney disease, n (%) | 63 (19.6) | 67 (20.9) | 130 (20.2) |
| Neurological problems, n (%) | 29 (9.0) | 49 (15.3) | 78 (12.1) |
| Respiratory conditions, n (%) | 61 (19.0) | 66 (20.6) | 127 (19.8) |
| High cholesterol or taking cholesterol-lowering medication, n (%) | 51 (15.9) | 53 (16.5) | 104 (16.2) |
| Diabetes, n (%) | 22 (6.9) | 14 (4.4) | 36 (5.6) |
| Previous diagnosis of cancer, n (%) | 27 (8.4) | 23 (7.2) | 50 (7.8) |
| Atrial fibrillation or other heart rhythm problems, n (%) | 13 (4.0) | 27 (8.4) | 40 (6.2) |
| Musculoskeletal problems, n (%) | 94 (29.3) | 99 (30.8) | 193 (30.1) |
| Autoimmune diseases, n (%) | 16 (5.0) | 17 (5.3) | 33 (5.1) |
| Digestive disorders, n (%) | 72 (22.4) | 78 (24.3) | 150 (23.4) |
| Mental health problems, n (%) | 139 (43.3) | 126 (39.3) | 265 (41.3) |
| Neurodevelopment disorders, n (%) | 3 (0.9) | 5 (1.6) | 8 (1.2) |
| Pain conditions, n (%) | 86 (26.8) | 77 (24.0) | 163 (25.4) |
| Endocrine disorders, n (%) | 35 (10.9) | 31 (9.7) | 66 (10.3) |
| Other condition, n (%) | 89 (27.7) | 82 (25.5) | 171 (26.6) |
| Outcome | SRT (N = 321) | SH (N = 321) | Overall (N = 642) |
|---|---|---|---|
| ISI score, mean (SD) | 17.7 (4.0) | 17.4 (4.2) | 17.5 (4.1) |
| PHQ-9 score, mean (SD) | 10.4 (5.3) | 10.1 (5.3) | 10.2 (5.3) |
| SF-36 PCS, mean (SD) | 46.9 (10.9) | 47.3 (10.2) | 47.1 (10.5) |
| Missing, n (%) | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| SF-36 MCS, mean (SD) | 39.8 (12.0) | 39.3 (11.9) | 39.6 (11.9) |
| Missing, n (%) | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| GSII rank 1, mean (SD) | 17.9 (17.4) | 20.7 (18.1) | 19.3 (17.8) |
| GSII rank 2, mean (SD) | 27.5 (17.7) | 31.0 (20.7) | 29.2 (19.3) |
| GSII rank 3, mean (SD) | 40.4 (22.0) | 40.4 (21.5) | 40.4 (21.7) |
| WPAI absenteeism, mean (SD)a | 5.9 (16.9) | 7.5 (21.6) | 6.6 (19.2) |
| Missing, n (%) | 157 (48.9) | 186 (57.9) | 343 (53.4) |
| WPAI presenteeism, mean (SD)a | 44.2 (22.1) | 43.3 (22.5) | 43.8 (22.2) |
| Missing, n (%) | 160 (49.8) | 192 (59.8) | 352 (54.8) |
| WPAI work productivity loss, mean (SD)a | 45.9 (22.9) | 44.8 (23.2) | 45.4 (23.0) |
| Missing, n (%) | 160 (49.8) | 192 (59.8) | 352 (54.8) |
| WPAI activity impairment, mean (SD) | 53.2 (23.5) | 51.8 (23.4) | 52.5 (23.5) |
| PSAS cognitive arousal, mean (SD) | 25.4 (6.7) | 25.1 (6.5) | 25.3 (6.6) |
| PSAS somatic arousal, mean (SD) | 14.3 (6.4) | 14.4 (6.2) | 14.3 (6.3) |
| GSES, mean (SD) | 8.0 (2.9) | 7.8 (3.0) | 7.9 (2.9) |
| SRT (N = 321) | SH (N = 321) | Overall (N = 642) | |
|---|---|---|---|
| Sleep diary | |||
| SOL (minutes), mean (SD) | 45.0 (36.8) | 47.4 (39.5) | 46.2 (38.2) |
| Missing, n (%) | 2 (0.6) | 7 (2.2) | 9 (1.4) |
| WASO (minutes), mean (SD) | 104.1 (62.9) | 104.7 (60.6) | 104.4 (61.7) |
| Missing, n (%) | 17 (5.3) | 16 (5.0) | 33 (5.1) |
| SE (%), mean (SD) | 65.3 (13.1) | 64.5 (13.6) | 64.9 (13.4) |
| Missing, n (%) | 4 (1.2) | 2 (0.6) | 6 (0.9) |
| TST (minutes), mean (SD) | 351.1 (73.7) | 346.7 (75.6) | 348.9 (74.6) |
| Missing, n (%) | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| SQ, mean (SD) | 2.6 (0.6) | 2.5 (0.6) | 2.5 (0.6) |
| Missing, n (%) | 3 (0.9) | 3 (0.9) | 6 (0.9) |
| Actigraphy | |||
| SOL (minutes), mean (SD) | 12.5 (15.0) | 12.1 (12.7) | 12.3 (13.9) |
| Missing, n (%) | 12 (3.7) | 10 (3.1) | 22 (3.4) |
| WASO (minutes), mean (SD) | 73.8 (35.1) | 72.5 (28.7) | 73.1 (32.0) |
| Missing, n (%) | 12 (3.7) | 10 (3.1) | 22 (3.4) |
| SE (%), mean (SD) | 80.7 (7.3) | 80.8 (6.5) | 80.8 (6.9) |
| Missing, n (%) | 14 (4.4) | 11 (3.4) | 25 (3.9) |
| TST (minutes), mean (SD) | 436.4 (60.0) | 437.4 (52.5) | 436.9 (56.3) |
| Missing, n (%) | 12 (3.7) | 10 (3.1) | 22 (3.4) |
Treatment receipt and fidelity
Sleep hygiene
All participants in the SH group were sent their SH booklet by e-mail or postal mail. No participant in the SH group met criteria for contamination (i.e. receiving nurse-delivered SRT) at 3 months (0/265) or 6 months (0/285).
Sleep restriction therapy
Sleep restriction therapy sessions were provided by 40 nurses (31 PNs and 9 research nurses). The median number of participants treated per nurse was 10 (min = 1, max = 24). Median time between randomisation and first treatment session was 23 days (min = 2, max = 306).
Table 7 summarises the number of treatment sessions attended by participants in the SRT arm: 92% attended one or more nurse sessions, while 65% attended all four treatment sessions; 8% did not attend any SRT sessions.
| Number of sessions attended | Frequency (%) |
|---|---|
| 0 | 25 (7.8) |
| 1 | 296 (92.2) |
| 2 | 250 (77.9) |
| 3 | 219 (68.2) |
| 4 | 207 (64.5) |
Table 8 provides a breakdown of reasons for withdrawal from SRT. The most common reasons were (1) finding implementation of SRT challenging, (2) not finding SRT useful and (3) personal circumstances.
| Randomised Withdrawal from intervention |
321 n = 62 (19.3%) (%) |
|---|---|
| Reason | |
| SRT too challenging | 19 (5.9) |
| Did not find intervention useful | 16 (5.0) |
| Personal circumstances | 13 (4.0) |
| Medical circumstances changed | 5 (1.6) |
| No reason given | 4 (1.2) |
| Sleeping better | 2 (0.6) |
| Previously tried SRT with no benefit | 1 (0.3) |
| Conflict with existing TAU | 1 (0.3) |
| Appointments not accessible | 1 (0.3) |
Fidelity of sleep restriction therapy sessions
Seventy-nine audio recordings of therapy sessions (53 session 1, 26 session 3) were sampled and reviewed by a clinical psychologist experienced in sleep medicine. Fidelity ratings were high for session 1 [median % = 100, interquartile range (IQR) 96.2–100] and session 3 (median % = 87.5, IQR 75–100).
Numbers analysed
Table 9 summarises data on completion of follow-up assessments, withdrawals (and reasons) and analysis population. Five hundred and eighty participants (90.3%) provided data at a minimum of one follow-up time point.
| SRT (%) | SH (%) | Overall (%) | |
|---|---|---|---|
| Participants attended baseline visits | 686 | ||
| Withdrew between baseline and randomisation | 44 | ||
| Found to be ineligible | 4 | ||
| Assessments too demanding | 4 | ||
| Participant not contactable | 9 | ||
| Watch and/or diary not received within randomisation window | 4 | ||
| Personal reason | 13 | ||
| No reason given | 5 | ||
| Did not like wearing actiwatch | 2 | ||
| Participant no longer met eligibility criteria after rescreening | 1 | ||
| Previously taken part in CBT for insomnia and not found useful | 1 | ||
| Sleeping better | 1 | ||
| Randomised | 321 | 321 | 642 |
| Withdrew from trial between baseline and 3-month follow-up | 22 (6.9) | 6 (1.9) | 28 (4.4) |
| Moved location | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| Due to being in control group/did not find SH useful | 0 (0.0) | 3 (0.9) | 3 (0.5) |
| Personal reasons | 9 (2.8) | 0 (0.0) | 9 (1.4) |
| No reason given | 2 (0.6) | 0 (0.0) | 2 (0.3) |
| Did not find SRT useful or challenging to implement | 8 (2.5) | 0 (0.0) | 8 (1.2) |
| Died | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| Scheduling difficulties for SRT appointments | 2 (0.6) | 0 (0.0) | 2 (0.3) |
| Medical circumstances changed | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| Sleeping better | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| 3-month follow-up | 299 | 315 | 614 |
| Completed | 253 (84.6) | 287 (91.1) | 540 (87.9) |
| Did not complete | 46 (15.4) | 28 (8.9) | 74 (12.1) |
| Withdrew from trial between 3- and 6-months follow-up | 18 (6.0) | 9 (2.9) | 27 (4.4) |
| Moved location | 1 (0.3) | 1 (0.3) | 2 (0.3) |
| Due to being in control group/did not find SH useful | 0 (0.0) | 2 (0.6) | 2 (0.3) |
| Personal reasons | 10 (3.3) | 3 (1.0) | 13 (2.1) |
| No reason given | 2 (0.7) | 0 (0.0) | 2 (0.3) |
| Did not find SRT useful or challenging to implement | 2 (0.7) | 0 (0.0) | 2 (0.3) |
| Did not like monitoring sleep | 0 (0.0) | 2 (0.6) | 2 (0.3) |
| Died | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| Medical circumstances changed | 2 (0.7) | 0 (0.0) | 2 (0.3) |
| Sleeping better | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| 6-month follow-up | 281 | 306 | 587 |
| Completed | 257 (91.5) | 291 (95.1) | 548 (93.4) |
| Did not complete | 24 (8.5) | 15 (4.9) | 39 (6.6) |
| Withdrew from trial between 6- and 12-months follow-up | 19 (6.8) | 11 (3.6) | 30 (5.1) |
| Due to being in control group/did not find SH useful | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| Personal reasons | 14 (5.0) | 5 (1.6) | 19 (3.2) |
| No reason given | 1 (0.4) | 2 (0.7) | 3 (0.5) |
| Did not find SRT useful or challenging to implement | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Died | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| Medical circumstances changed | 1 (0.4) | 2 (0.7) | 3 (0.5) |
| Sleeping better | 2 (0.7) | 0 (0.0) | 2 (0.3) |
| 12-month follow-up | 262 | 295 | 557 |
| Completed | 234 (89.3) | 276 (93.6) | 510 (91.6) |
| Did not complete | 28 (10.7) | 19 (6.4) | 47 (8.4) |
Table 10 summarises the availability of data for the primary and secondary outcomes at each time point by randomised group and overall. Eighty-five per cent of participants provided data on the primary outcome (ISI) at 6 months post randomisation. Of note, data completion for sleep diaries and actigraphy at 6 and 12 months was low (≤ 41%), chiefly due to the pandemic, which precluded sending out watches and diaries. Data on absenteeism, presenteeism and work productivity loss (from the WPAI) were only available for those in employment.
| SRT | SH | Overall | |
|---|---|---|---|
| (N = 321) | (N = 321) | (N = 642) | |
| Primary outcome | |||
| Self-rated insomnia severity, n (%) | |||
| 3 months | 252 (78.5) | 283 (88.2) | 535 (83.3) |
| 6 monthsa | 257 (80.1) | 291 (90.7) | 548 (85.4) |
| 12 months | 233 (72.6) | 275 (85.7) | 508 (79.1) |
| Secondary outcomes | |||
| SF-36 PCS, n (%) | |||
| 3 months | 244 (76.0) | 285 (88.8) | 529 (82.4) |
| 6 months | 233 (72.6) | 280 (87.2) | 513 (79.9) |
| 12 months | 224 (69.8) | 265 (82.6) | 489 (76.2) |
| SF-36 MCS, n (%) | |||
| 3 months | 244 (76.0) | 285 (88.8) | 529 (82.4) |
| 6 months | 233 (72.6) | 280 (87.2) | 513 (79.9) |
| 12 months | 224 (69.8) | 265 (82.6) | 489 (76.2) |
| Diary-SOL, n (%) | |||
| 6 months | 111 (34.6) | 148 (46.1) | 259 (40.3) |
| 12 months | 92 (28.7) | 124 (38.6) | 216 (33.6) |
| Diary-WASO, n (%) | |||
| 6 months | 107 (33.3) | 146 (45.5) | 253 (39.4) |
| 12 months | 88 (27.4) | 122 (38.0) | 210 (32.7) |
| Diary-SE, n (%) | |||
| 6 months | 114 (35.5) | 150 (46.7) | 264 (41.1) |
| 12 months | 95 (29.6) | 125 (38.9) | 220 (34.3) |
| Diary-TST, n (%) | |||
| 6 months | 114 (35.5) | 150 (46.7) | 264 (41.1) |
| 12 months | 95 (29.6) | 126 (39.3) | 221 (34.4) |
| Diary-SQ, n (%) | |||
| 6 months | 114 (35.5) | 149 (46.4) | 263 (41.0) |
| 12 months | 95 (29.6) | 125 (38.9) | 220 (34.3) |
| Actigraphy-SOL, n (%) | |||
| 6 months | 97 (30.2) | 123 (38.3) | 220 (34.3) |
| 12 months | 91 (28.3) | 117 (36.4) | 208 (32.4) |
| Actigraphy-WASO, n (%) | |||
| 6 months | 97 (30.2) | 123 (38.3) | 220 (34.3) |
| 12 months | 91 (28.3) | 117 (36.4) | 208 (32.4) |
| Actigraphy-SE, n (%) | |||
| 6 months | 95 (29.6) | 122 (38.0) | 217 (33.8) |
| 12 months | 91 (28.3) | 117 (36.4) | 208 (32.4) |
| Actigraphy-TST, n (%) | |||
| 6 months | 97 (30.2) | 123 (38.3) | 220 (34.3) |
| 12 months | 91 (28.3) | 117 (36.4) | 208 (32.4) |
| GSII rank 1, n (%) | |||
| 3 months | 246 (76.6) | 282 (87.9) | 528 (82.2) |
| 6 months | 234 (72.9) | 278 (86.6) | 512 (79.8) |
| 12 months | 224 (69.8) | 266 (82.9) | 490 (76.3) |
| GSII rank 2, n (%) | |||
| 3 months | 246 (76.6) | 283 (88.2) | 529 (82.4) |
| 6 months | 234 (72.9) | 279 (86.9) | 513 (79.9) |
| 12 months | 224 (69.8) | 266 (82.9) | 490 (76.3) |
| GSII rank 3, n (%) | |||
| 3 months | 246 (76.6) | 283 (88.2) | 529 (82.4) |
| 6 months | 232 (72.3) | 279 (86.9) | 511 (79.6) |
| 12 months | 224 (69.8) | 266 (82.9) | 490 (76.3) |
| PHQ-9, n (%) | |||
| 3 months | 244 (76.0) | 284 (88.5) | 528 (82.2) |
| 6 months | 234 (72.9) | 278 (86.6) | 512 (79.8) |
| 12 months | 224 (69.8) | 264 (82.2) | 488 (76.0) |
| Absenteeism, n (%) | |||
| 3 months | 111 (34.6) | 117 (36.4) | 228 (35.5) |
| 6 months | 101 (31.5) | 113 (35.2) | 214 (33.3) |
| 12 months | 100 (31.2) | 111 (34.6) | 211 (32.9) |
| Presenteeism, n (%) | |||
| 3 months | 111 (34.6) | 113 (35.2) | 224 (34.9) |
| 6 months | 99 (30.8) | 111 (34.6) | 210 (32.7) |
| 12 months | 98 (30.5) | 107 (33.3) | 205 (31.9) |
| Work productivity loss, n (%) | |||
| 3 months | 111 (34.6) | 113 (35.2) | 224 (34.9) |
| 6 months | 99 (30.8) | 111 (34.6) | 210 (32.7) |
| 12 months | 98 (30.5) | 107 (33.3) | 205 (31.9) |
| Activity impairment, n (%) | |||
| 3 months | 247 (76.9) | 285 (88.8) | 532 (82.9) |
| 6 months | 234 (72.9) | 280 (87.2) | 514 (80.1) |
| 12 months | 222 (69.2) | 267 (83.2) | 489 (76.2) |
| Proportion of days usage of prescribed hypnotic sleep-promoting medication, n (%) | |||
| 6 months | 112 (34.9) | 146 (45.5) | 258 (40.2) |
| 12 months | 93 (29.0) | 116 (36.1) | 209 (32.6) |
| Proportion of days usage of prescribed other sleep-promoting medication, n (%) | |||
| 6 months | 112 (34.9) | 146 (45.5) | 258 (40.2) |
| 12 months | 93 (29.0) | 116 (36.1) | 209 (32.6) |
| Pre-sleep cognitive arousal, n (%) | |||
| 3 months | 246 (76.6) | 284 (88.5) | 530 (82.6) |
| 6 months | 235 (73.2) | 279 (86.9) | 514 (80.1) |
| 12 months | 224 (69.8) | 266 (82.9) | 490 (76.3) |
| Pre-sleep somatic arousal, n (%) | |||
| 3 months | 246 (76.6) | 283 (88.2) | 529 (82.4) |
| 6 months | 234 (72.9) | 280 (87.2) | 514 (80.1) |
| 12 months | 223 (69.5) | 267 (83.2) | 490 (76.3) |
| GSES, n(%) | |||
| 3 months | 246 (76.6) | 282 (87.9) | 528 (82.2) |
| 6 months | 235 (73.2) | 279 (86.9) | 514 (80.1) |
| 12 months | 223 (69.5) | 266 (82.9) | 489 (76.2) |
| Prescribed hypnotic sleep-promoting medication use over 7 days, n (%) | |||
| 6 months | 112 (34.9) | 146 (45.5) | 258 (40.2) |
| 12 months | 93 (29.0) | 116 (36.1) | 209 (32.6) |
| Prescribed other sleep-promoting medication use over 7 days, n (%) | |||
| 6 months | 112 (34.9) | 146 (45.5) | 258 (40.2) |
| 12 months | 93 (29.0) | 116 (36.1) | 209 (32.6) |
| Work-related accident resulting in injury, n (%) | |||
| 3 months | 245 (76.3) | 285 (88.8) | 530 (82.6) |
| 6 months | 235 (73.2) | 279 (86.9) | 514 (80.1) |
| 12 months | 224 (69.8) | 267 (83.2) | 491 (76.5) |
| Motor-vehicle accident, n (%) | |||
| 3 months | 245 (76.3) | 285 (88.8) | 530 (82.6) |
| 6 months | 235 (73.2) | 280 (87.2) | 515 (80.2) |
| 12 months | 224 (69.8) | 267 (83.2) | 491 (76.5) |
| Near-miss driving incident, n (%) | |||
| 3 months | 245 (76.3) | 285 (88.8) | 530 (82.6) |
| 6 months | 235 (73.2) | 280 (87.2) | 515 (80.2) |
| 12 months | 224 (69.8) | 267 (83.2) | 491 (76.5) |
| Number of times fallen asleep while driving, n (%) | |||
| 3 months | 244 (76.0) | 284 (88.5) | 528 (82.2) |
| 6 months | 234 (72.9) | 280 (87.2) | 514 (80.1) |
| 12 months | 224 (69.8) | 266 (82.9) | 490 (76.3) |
| Number of falls, n (%) | |||
| 3 months | 245 (76.3) | 285 (88.8) | 530 (82.6) |
| 6 months | 235 (73.2) | 280 (87.2) | 515 (80.2) |
| 12 months | 224 (69.8) | 267 (83.2) | 491 (76.5) |
Table 11 shows that randomised group was associated with missingness of the primary outcome, with the SRT more likely to have missing data at 3, 6 and 12 months post randomisation.
| SRT | SH | Odds ratio (95% CI)a |
p-valueb | |
|---|---|---|---|---|
| (N = 321) | (N = 321) | |||
| Primary outcome | ||||
| 3-month follow-up, n (%) | 2.04 (1.33 to 3.14) | 0.001 | ||
| Available | 252 (78.5) | 283 (88.2) | ||
| Missing | 69 (21.5) | 38 (11.8) | ||
| 6-month follow-up,c n (%) | 2.42 (1.52 to 3.85) | < 0.001 | ||
| Available | 257 (80.1) | 291 (90.7) | ||
| Missing | 64 (19.9) | 30 (9.3) | ||
| 12-month follow-up, n (%) | 2.26 (1.52 to 3.36) | < 0.001 | ||
| Available | 233 (72.6) | 275 (85.7) | ||
| Missing | 88 (27.4) | 46 (14.3) | ||
Outcomes and estimation
Primary outcome
The primary objective of the HABIT trial was to compare the effect of SRT versus SH on insomnia severity (assessed by the ISI) at baseline and 3, 6 and 12 months post randomisation. The primary end point was the 6-month time point.
Table 12 summarises the adjusted treatment effect at each time point from the linear mixed-effect model (Figure 2). At 6 months post randomisation, the estimated adjusted mean difference on the ISI was −3.05 (95% CI −3.83 to −2.28; p < 0.001, Cohen’s d = 0.74), indicating that participants in the SRT arm reported lower insomnia severity compared to the SH group. Treatment effects were also evident at 3 and 12 months. Mean differences between arms were reflected in the number of participants showing a treatment response (ISI change score reduction ≥ 8 points) and scoring in the non-clinical range (ISI absolute score < 11). At 6 months, 42% (108/257) of the SRT group met criteria for a clinically significant treatment response, while only 17% (49/291) of the SH arm did. Fifty per cent (128/257) of the SRT arm were in the non-clinical range at 6 months compared with 28% (80/291) in the SH arm.
| SRT (N = 321) |
SH (N = 321) |
Adjusted treatment difference (95% CI)a |
p-valueb | Cohen’s dc | |
|---|---|---|---|---|---|
| Primary analysis | |||||
| Self-rated insomnia severity, mean (SD) (n)d | |||||
| 3 months | 10.9 (5.47) (252) | 14.8 (5.11) (283) | −3.88 (−4.66 to −3.10) | < 0.001 | −0.95 |
| 6 monthse | 10.9 (5.51) (257) | 13.9 (5.23) (291) | −3.05 (−3.83 to −2.28) | < 0.001 | −0.74 |
| 12 months | 10.4 (5.89) (233) | 13.5 (5.52) (275) | −2.96 (−3.75 to −2.16) | < 0.001 | −0.72 |
FIGURE 2.
Changes in the primary outcome, insomnia severity, across groups and time points. Raw means (±SD) are presented for both groups at each time point.

We performed sensitivity analyses to assess missingness of the primary outcome (ISI). Table 13 shows the results for the primary outcome when (1) adjusting for characteristics associated with non-completion of the ISI at 6 months and (2) performing multiple imputation. Both models yielded similar estimates as the primary analysis, demonstrating superiority of SRT over SH. A pattern mixture model was also conducted where missing ISI outcome values were imputed by up to five points either side of the observed average, both overall and in the SRT and SH arms separately. Even under these conservative assumptions the treatment effect and 95% CI would still not include 0 (see Appendix 2, Figure 21). Analyses assuming informative missingness of insomnia severity scores at 6 months [i.e. data missing not at random (MNAR)] indicated that even with asymmetrical differences between responders and non-responders conclusions are similar to the primary analysis (Appendix 2, Table 34).
| SRT | SH | Adjusted treatment difference (95% CI)a | p-valueb | |
|---|---|---|---|---|
| (N = 321) | (N = 321) | |||
| Self-rated insomnia severity, mean (SD) (N) | ||||
| Adjusting for characteristics associated with non-completion of ISI | ||||
| 3 months | 10.9 (5.47) (252) | 14.8 (5.11) (283) | −3.64 (−4.42 to −2.85) | < 0.001 |
| 6 monthsc | 10.9 (5.51) (257) | 13.9 (5.23) (291) | −2.83 (−3.61 to −2.05) | < 0.001 |
| 12 months | 10.4 (5.89) (233) | 13.5 (5.52) (275) | −2.71 (−3.50 to −1.91) | < 0.001 |
| Multiple imputation | ||||
| 3 months | 11.4 (5.86) (321) | 15.0 (5.25) (321) | −3.86 (−4.63 to −3.08) | < 0.001 |
| 6 monthsc | 11.3 (5.88) (321) | 14.1 (5.40) (321) | −3.03 (−3.78 to −2.29) | < 0.001 |
| 12 months | 11.1 (6.86) (321) | 13.7 (5.75) (321) | −2.84 (−3.66 to −2.01) | < 0.001 |
Secondary outcomes
Adjusted treatment effects are presented for secondary outcomes in Tables 14 and 15.
| SRT (N = 321) |
SH (N = 321)) |
Adjusted treatment difference (95% CI)a | p-valueb | Cohen’s dc | |
|---|---|---|---|---|---|
| Secondary outcomes | |||||
| SF-36 PCS, mean (SD) (n)d | |||||
| 3 months | 48.4 (10.78) (244) | 46.1 (10.80) (285) | 1.87 (0.76 to 2.98) | 0.001 | 0.18 |
| 6 months | 48.1 (10.90) (233) | 47.2 (10.28) (280) | 0.77 (−0.35 to 1.89) | 0.179 | 0.07 |
| 12 months | 48.6 (10.26) (224) | 47.4 (10.47) (265) | 0.94 (−0.20 to 2.09) | 0.105 | 0.09 |
| SF-36 MCS, mean (SD) (n)d | |||||
| 3 months | 44.6 (11.27) (244) | 41.2 (11.79) (285) | 2.80 (1.37 to 4.23) | < 0.001 | 0.24 |
| 6 months | 44.7 (11.88) (233) | 42.2 (11.79) (280) | 1.97 (0.52 to 3.43) | 0.008 | 0.17 |
| 12 months | 44.7 (11.29) (224) | 42.3 (11.29) (265) | 2.01 (0.53 to 3.49) | 0.008 | 0.17 |
| GSII rank 1, mean (SD) (n)d | |||||
| 3 months | 48.2 (28.39) (246) | 35.4 (21.63) (282) | 12.82 (8.71 to 16.93) | < 0.001 | 0.72 |
| 6 months | 50.6 (28.00) (234) | 37.7 (23.42) (278) | 12.80 (8.63 to 16.96) | < 0.001 | 0.72 |
| 12 months | 52.1 (29.42) (224) | 40.3 (24.79) (266) | 11.77 (7.54 to 16.00) | < 0.001 | 0.66 |
| GSII rank 2, mean (SD) (n)d | |||||
| 3 months | 51.5 (26.78) (246) | 38.6 (22.23) (283) | 12.78 (8.79 to 16.77) | < 0.001 | 0.66 |
| 6 months | 53.2 (27.74) (234) | 40.7 (23.66) (279) | 12.45 (8.40 to 16.49) | < 0.001 | 0.65 |
| 12 months | 54.9 (28.63) (224) | 41.5 (24.55) (266) | 13.72 (9.60 to 17.84) | < 0.001 | 0.71 |
| GSII rank 3, mean (SD) (n)d | |||||
| 3 months | 51.6 (27.01) (246) | 41.1 (23.14) (283) | 10.06 (6.02 to 14.10) | < 0.001 | 0.46 |
| 6 months | 54.2 (27.11) (232) | 43.0 (23.90) (279) | 10.93 (6.82 to 15.03) | < 0.001 | 0.50 |
| 12 months | 57.1 (28.97) (224) | 45.1 (24.11) (266) | 11.70 (7.53 to 15.87) | < 0.001 | 0.54 |
| PHQ-9, mean (SD) (n)d | |||||
| 3 months | 7.2 (5.72) (244) | 9.1 (5.62) (284) | −1.86 (−2.56 to −1.16) | < 0.001 | −0.35 |
| 6 months | 7.2 (5.77) (234) | 8.8 (5.75) (278) | −1.60 (−2.31 to −0.90) | < 0.001 | −0.30 |
| 12 months | 7.0 (5.82) (224) | 8.6 (5.51) (264) | −1.61 (−2.32 to −0.89) | < 0.001 | −0.30 |
| Absenteeism, median (IQR) (n)e | |||||
| 3 months | 0.0 (0.0−0.0) (111) | 0.0 (0.0−0.0) (117) | − | 0.095 | − |
| 6 months | 0.0 (0.0−0.0) (101) | 0.0 (0.0−0.0) (113) | − | 0.014 | − |
| 12 months | 0.0 (0.0−0.0) (100) | 0.0 (0.0−0.0) (111) | − | 0.005 | − |
| Proportion of participants who missed work because of insomnia (absenteeism > 0), n/N (%) | |||||
| 3 months | 14/111 (12.6) | 23/117 (19.7) | − | − | − |
| 6 months | 7/101 (6.9) | 21/113 (18.6) | − | − | − |
| 12 months | 5/100 (5.0%) | 20/111 (18.0) | − | − | − |
| Presenteeism, mean (SD) (n)d | |||||
| 3 months | 29.6 (23.66) (111) | 41.4 (21.91) (113) | −10.56 (−16.25 to −4.87) | < 0.001 | −0.48 |
| 6 months | 24.6 (22.01) (99) | 34.5 (23.38) (111) | −10.69 (−16.56 to −4.81) | < 0.001 | −0.48 |
| 12 months | 22.4 (22.62) (98) | 33.8 (24.37) (107) | −11.76 (−17.73 to −5.79) | < 0.001 | −0.53 |
| Work productivity loss, mean (SD) (n)d | |||||
| 3 months | 30.6 (24.71) (111) | 42.7 (22.93) (113) | −10.90 (−16.80 to −5.01) | < 0.001 | −0.47 |
| 6 months | 25.0 (22.39) (99) | 35.9 (24.71) (111) | −11.96 (−18.04 to −5.87) | < 0.001 | −0.52 |
| 12 months | 22.7 (22.98) (98) | 35.1 (25.34) (107) | −12.96 (−19.14 to −6.77) | < 0.001 | −0.56 |
| Activity impairment, mean (SD) (n)d | |||||
| 3 months | 33.5 (25.07) (247) | 46.7 (23.37) (285) | −13.23 (−16.79 to −9.68) | < 0.001 | −0.56 |
| 6 months | 31.0 (25.05) (234) | 42.9 (24.03) (280) | −11.99 (−15.60 to −8.38) | < 0.001 | −0.51 |
| 12 months | 31.0 (26.44) (222) | 40.1 (24.42) (267) | −9.11 (−12.80 to −5.43) | < 0.001 | −0.39 |
| Pre-sleep cognitive arousal, mean (SD) (n)d | |||||
| 3 months | 19.6 (6.88) (246) | 23.0 (6.77) (284) | −3.30 (−4.24 to −2.35) | < 0.001 | −0.50 |
| 6 months | 19.6 (7.14) (235) | 22.1 (6.91) (279) | −2.36 (−3.32 to −1.41) | < 0.001 | −0.36 |
| 12 months | 19.2 (7.04) (224) | 22.2 (6.83) (266) | −2.99 (−3.96 to −2.02) | < 0.001 | −0.45 |
| Pre-sleep somatic arousal, mean (SD) (n)d | |||||
| 3 months | 12.0 (5.41) (246) | 13.6 (5.64) (283) | −1.30 (−1.99 to −0.60) | < 0.001 | −0.21 |
| 6 months | 12.2 (5.38) (234) | 13.3 (5.39) (280) | −1.24 (−1.94 to −0.54) | 0.001 | −0.20 |
| 12 months | 12.1 (5.27) (223) | 13.4 (5.80) (267) | −1.27 (−1.99 to −0.56) | < 0.001 | −0.20 |
| GSES, mean (SD) (n)d | |||||
| 3 months | 5.6 (3.25) (246) | 7.0 (3.11) (282) | −1.49 (−1.93 to −1.05) | < 0.001 | −0.51 |
| 6 months | 5.4 (3.15) (235) | 6.7 (3.12) (279) | −1.27 (−1.71 to −0.83) | < 0.001 | −0.44 |
| 12 months | 4.9 (3.08) (223) | 6.6 (3.23) (266) | −1.64 (−2.09 to −1.20) | < 0.001 | −0.57 |
| SRT (N = 321) |
SH (N = 321) |
Adjusted treatment difference (95% CI)a | p-valueb | Cohen’s dc | |
|---|---|---|---|---|---|
| Diary | |||||
| SOL (minutes), mean (SD) (n)d | |||||
| 6 months | 30.4 (30.54) (111) | 41.2 (40.82) (148) | −7.30 (−13.90 to −0.70) | 0.030 | −0.19 |
| 12 months | 32.1 (33.12) (92) | 38.5 (34.79) (124) | −3.28 (−10.51 to 3.95) | 0.374 | −0.09 |
| WASO (minutes), mean (SD) (n)d | |||||
| 6 months | 61.4 (43.54) (107) | 92.5 (61.87) (146) | −31.04 (−41.14 to−20.95) | < 0.001 | –0.50 |
| 12 months | 57.8 (36.09) (88) |
87.2 (52.67) (122) | −31.21 (−42.14 to −20.27) | < 0.001 | –0.51 |
| SE (%), mean (SD) (n)d | |||||
| 6 months | 77.5 (10.86) (114) | 68.8 (13.04) (150) | 7.95 (5.77 to 10.13) | < 0.001 | 0.59 |
| 12 months | 77.0 (11.26) (95) | 69.9 (13.92) (125) | 7.43 (5.07 to 9.78) | < 0.001 | 0.55 |
| TST (minutes), mean (SD) (n)d | |||||
| 6 months | 391.7 (63.61) (114) | 374.0 (74.36) (150) |
17.50 (5.95 to 29.05) | 0.003 | 0.23 |
| 12 months | 399.3 (57.91) (95) | 379.9 (76.27) (126) | 23.97 (11.47 to 36.47) | < 0.001 | 0.32 |
| SQ, mean (SD) (n)d | |||||
| 6 months | 3.0 (0.77) (114) |
2.8 (0.60) (149) |
0.23 (0.08 to 0.37) | 0.002 | 0.38 |
| 12 months | 3.1 (0.74) (95) |
2.9 (0.67) (125) |
0.20 (0.05 to 0.36) | 0.010 | 0.33 |
| Actigraphy | |||||
| SOL (minutes), mean (SD) (n)d | |||||
| 6 months | 11.5 (14.48) (97) |
11.7 (13.70) (123) | −0.39 (−3.73 to 2.94) | 0.818 | −0.03 |
| 12 months | 11.4 (19.96) (91) |
10.5 (14.92) (117) | 1.95 (−1.47 to 5.37) | 0.265 | 0.14 |
| WASO (minutes), mean (SD) (n)d | |||||
| 6 months | 61.7 (28.58) (97) |
70.1 (27.07) (123) | −6.80 (−11.43 to −2.16) | 0.004 | −0.21 |
| 12 months | 63.0 (26.21) (91) |
66.6 (25.07) (117) | −3.30 (−8.04 to 1.43) | 0.172 | −0.10 |
| SE (%), mean (SD) (n)d | |||||
| 6 months | 83.4 (6.97) (95) |
81.4 (5.71) (122) |
1.64 (0.54 to 2.74) | 0.004 | 0.24 |
| 12 months | 83.4 (7.36) (91) |
82.7 (5.89) (117) |
0.57 (−0.55 to 1.69) | 0.317 | 0.08 |
| TST (minutes), mean (SD) (n)d | |||||
| 6 months | 422.2 (48.00) (97) | 442.9 (59.70) (123) |
−15.15 (−24.79 to −5.50) | 0.002 | −0.27 |
| 12 months | 435.0 (53.70) (91) | 451.6 (52.10) (117) |
−13.44 (−23.32 to −3.56) | 0.008 | −0.24 |
| Sleep medication use (diary) | |||||
| Proportion of days usage of prescribed hypnotic sleep-promoting medication, median (IQR) (n)e | |||||
| 6 months | 0.0 (0.0 to 0.0) (112) | 0.0 (0.0 to 0.0) (146) | − | 0.809 | − |
| 12 months | 0.0 (0.0 to 0.0) (93) | 0.0 (0.0 to 0.0) (116) | − | 0.658 | − |
| Proportion of days usage of prescribed other sleep-promoting medication, median (IQR) (n)e | |||||
| 6 months | 0.0 (0.0 to 0.0) (112) | 0.0 (0.0 to 0.0) (146) | − | 0.548 | − |
| 12 months | 0.0 (0.0 to 0.0) (93) | 0.0 (0.0 to 0.0) (116) | − | 0.754 | − |
| Prescribed hypnotic sleep-promoting medication use over 7 days, n/N (%)f | |||||
| 6 months | 15/112 (13.4) | 18/146 (12.3) | 1.35 (0.39 to 4.68) | 0.639 | − |
| 12 months | 10/93 (10.8) | 13/116 (11.2) | 0.94 (0.23 to 3.91) | 0.932 | − |
| Prescribed other sleep-promoting medication use over 7 days, n/N (%)f | |||||
| 6 months | 7/112 (6.3) | 12/146 (8.2) | 0.21 (0.02 to 2.35) | 0.206 | − |
| 12 months | 9/93 (9.7) | 13/116 (11.2) | 0.36 (0.04 to 2.99) | 0.344 | − |
At 6 months, the SRT group relative to SH reported better mental HRQoL (SF-36 MCS) and sleep-related quality of life (GSII, patient-generated ranks 1–3), as well as lower depressive symptoms (PHQ-9) and activity impairment (WPAI). For employed participants, those in the SRT arm reported less absenteeism, presenteeism and work productivity loss (WPAI). Group effects on these measures were observed at all follow-up time points. Physical HRQoL (SF-36 PCS) was higher for the SRT group at 3 months but there was no evidence of group differences at 6 or 12 months. The SRT group also reported lower levels of cognitive and somatic arousal, and sleep effort, at 3, 6 and 12 months post randomisation.
All sleep diary metrics (SOL, WASO, SE, TST, SQ) were improve compared to control at 6 months (Table 15) and these effects were largely maintained at 12 months (except for SOL). Actigraphy-defined SE and WASO were improved, while TST was reduced, in the SRT group compared to control at 6 months. The only group difference at 12 months for actigraphy was lower TST for the SRT group relative to control. There was no evidence of group differences for use of prescribed sleep-promoting medication at 6 or 12 months.
Complier-average causal effects analyses
Baseline characteristics are presented for compliers and non-compliers in the treatment arm (Table 16). Compliance was defined as attending at least one SRT session.
| Non-compliers (N = 25) | Compliers (N = 296) | |
|---|---|---|
| Region, n (%) | ||
| Thames Valley | 9 (36.0) | 147 (49.7) |
| Greater Manchester | 10 (40.0) | 99 (33.4) |
| Lincolnshire | 6 (24.0) | 50 (16.9) |
| Age, mean (SD) (min, max) | 50.6 (18.6) (20.0 to 83.0) | 56.1 (15.0) (19.0 to 88.0) |
| Sex, n (%) | ||
| Female | 19 (76.0) | 226 (76.4) |
| Male | 6 (24.0) | 70 (23.6) |
| Ethnicity, n (%) | ||
| White | 24 (96.0) | 288 (97.3) |
| Other | 0 (0.0) | 8 (2.7) |
| Prefer not to say | 1 (4.0) | 0 (0.0) |
| Education level, n (%) | ||
| None | 3 (12.0) | 13 (4.4) |
| GCSE or equivalent | 4 (16.0) | 78 (26.4) |
| A-levels or equivalent | 6 (24.0) | 44 (14.9) |
| University undergraduate | 2 (8.0) | 78 (26.4) |
| University postgraduate | 9 (36.0) | 81 (27.4) |
| Choose not to say | 1 (4.0) | 2 (0.7) |
| Marital status, n (%) | ||
| Single | 7 (28.0) | 41 (13.9) |
| Married, or in a domestic partnership | 14 (56.0) | 206 (69.6) |
| Divorced | 1 (4.0) | 20 (6.8) |
| Widowed | 3 (12.0) | 21 (7.1) |
| Separated | 0 (0.0) | 7 (2.4) |
| Choose not to say | 0 (0.0) | 1 (0.3) |
| Index of multiple deprivation score (quintiles), n (%) | ||
| 1 | 1 (4.0) | 9 (3.0) |
| 2 | 1 (4.0) | 29 (9.8) |
| 3 | 4 (16.0) | 48 (16.2) |
| 4 | 7 (28.0) | 75 (25.3) |
| 5 | 10 (40.0) | 134 (45.3) |
| Missing, n (%) | 2 (8.0) | 1 (0.3) |
| BMI, mean (SD) (min, max) | 28.0 (5.4) (19.7 to 40.8) | 26.6 (5.5) (17.1 to 64.8) |
| Missing, n (%) | 2 (8.0) | 16 (5.4) |
| Smoking status, n (%) | ||
| Non-smoker | 18 (72.0) | 196 (66.2) |
| Ex-smoker | 5 (20.0) | 79 (26.7) |
| Smoker | 2 (8.0) | 21 (7.1) |
| Alcohol consumption, n (%) | ||
| Never | 5 (20.0) | 57 (19.3) |
| Sometimes | 8 (32.0) | 125 (42.2) |
| Every week | 12 (48.0) | 114 (38.5) |
| Duration of insomnia in yrs, median (IQR) (min, max) | 6.3 (4.0–15.0) (1.0 to 60.0) | 10.0 (5.0–20.0) (0.4 to 66.0) |
| Consulted for insomnia, n (%) | 23 (92.0) | 226 (76.4) |
| Work-related accident in last 3 months, n (%) | 0 (0.0) | 4 (1.4) |
| Motor vehicle accident in last 3 months, n (%) | 1 (4.0) | 4 (1.4) |
| Near-miss driving incident in last 3 months, n (%) | 1 (4.0) | 24 (8.1) |
| Times fallen asleep while driving in last 3 months, n (%) | ||
| None | 25 (100.0) | 293 (99.0) |
| Once | 0 (0.0) | 0 (0.0) |
| More than once | 0 (0.0) | 3 (1.0) |
| Times had a fall in last 3 months, n (%) | ||
| None | 24 (96.0) | 241 (81.4) |
| Once | 0 (0.0) | 30 (10.1) |
| More than once | 1 (4.0) | 25 (8.4) |
| Patient reported use of sleep medication, n (%) | 9 (36.0) | 74 (25.0) |
| Cardiovascular disease or chronic kidney disease, n (%) | 4 (16.0) | 59 (19.9) |
| Neurological problems, n (%) | 2 (8.0) | 27 (9.1) |
| Respiratory conditions, n (%) | 3 (12.0) | 58 (19.6) |
| High cholesterol or taking cholesterol lowering medication, n (%) | 3 (12.0) | 48 (16.2) |
| Diabetes, n (%) | 2 (8.0) | 20 (6.8) |
| Previous diagnosis of cancer, n (%) | 2 (8.0) | 25 (8.4) |
| Atrial fibrillation or other heart rhythm problems, n (%) | 1 (4.0) | 12 (4.1) |
| Musculoskeletal problems, n (%) | 5 (20.0) | 89 (30.1) |
| Autoimmune diseases, n (%) | 2 (8.0) | 14 (4.7) |
| Digestive disorders, n (%) | 5 (20.0) | 67 (22.6) |
| Mental health problems, n (%) | 10 (40.0) | 129 (43.6) |
| Neurodevelopment disorders, n (%) | 0 (0.0) | 3 (1.0) |
| Pain conditions, n (%) | 3 (12.0) | 83 (28.0) |
| Endocrine disorders, n (%) | 2 (8.0) | 33 (11.1) |
| Other condition, n (%) | 6 (24.0) | 83 (28.0) |
| Objective SOL, mean (SD) (min, max) | 11.4 (12.9) (0.0 to 57.0) | 12.6 (15.2) (0.0 to 126.0) |
| Missing, n (%) | 2 (8.0%) | 10 (3.4%) |
| Objective SE (%), median (IQR) (min, max) | 80.0 (76.0 to 86.8) (62.2 to 90.6) | 82.0 (77.0 to 85.8) (44.6 to 92.4) |
| Missing, n (%) | 3 (12.0) | 11 (3.7) |
| Subjective SQ, mean (SD) (min, max) | 2.7 (0.5) (1.4 to 3.7) | 2.6 (0.6) (1.0 to 4.3) |
| Missing, n (%) | 2 (8.0) | 1 (0.3) |
| ISI score, mean (SD) (min, max) | 19.0 (3.8) (12.0 to 27.0) | 17.6 (4.0) (5.0 to 28.0) |
| PHQ-9 score, median (IQR) (min, max) | 12.0 (7.0–17.0) (4.0 to 23.0) | 9.0 (6.0–14.0) (1.0 to 27.0) |
| SF-36 PCS, mean (SD) (min, max) | 49.3 (9.8) (26.3 to 67.4) | 46.7 (10.9) (12.0 to 71.3) |
| Missing, n (%) | 0 (0.0) | 1 (0.3) |
| SF-36 MCS, mean (SD) (min, max) | 35.6 (13.3) (4.7 to 55.0) | 40.2 (11.9) (5.9 to 64.3) |
| Missing, n (%) | 0 (0.0) | 1 (0.3) |
| GSES, mean (SD) (min, max) | 9.5 (3.2) (1.0 to 14.0) | 7.8 (2.9) (1.0 to 14.0) |
| MEQr, mean (SD) (min, max) | 14.3 (3.3) (6.0 to 19.0) | 15.6 (3.6) (6.0 to 24.0) |
Table 17 summarises complier average causal effects for those attending a minimum of one, two, three, and four treatment sessions, respectively. Results show that attending more treatment sessions was associated with a larger treatment effect, relative to the primary analysis. For example, there is a > 1-point difference in the treatment effect on the ISI for those attending all four sessions (−4.10, 95% CI −5.06 to −3.14) versus the primary analysis (−3.05, 95% CI −3.83 to −2.28).
| Model | Estimate (95% CI) | p-value |
|---|---|---|
| Primary analysis | ||
| 6 months, adjusted | −3.05 (−3.83 to −2.28) | < 0.001 |
| Estimates of CACE-defined as attending at least 1 session | ||
| 6 months, adjusted for predictors of compliancea | −3.24 (−4.01 to −2.47) | < 0.0001 |
| Estimates of CACE-sensitivity defined as at least 2 sessions | ||
| 6 months, adjusted for predictors of compliancea | −3.59 (−4.43 to −2.75) | < 0.0001 |
| Estimates of CACE-sensitivity defined as at least 3 sessions | ||
| 6 months, adjusted for predictors of compliancea | −3.94 (−4.86 to −3.02) | < 0.0001 |
| Estimates of CACE-sensitivity defined as at least 4 sessions | ||
| 6 months, adjusted for predictors of compliancea | −4.10 (−5.06 to −3.14) | < 0.0001 |
Adherence to sleep restriction therapy
Implementation of SRT was indexed using self-reported bed and rise times from sleep diaries completed during the 4-week intervention. A percentage score was calculated for each participant, reflecting the number of bed and rise times adhered to within 15 minutes of the nurse prescription. One hundred and fifty-seven participants (49%) returned intervention diaries; 164 participants did not return diaries or returned incomplete diaries (i.e. < 50% of days with relevant questions completed). Mean adherence for returned diaries was 76.4% (SD = 21.6), with the majority of participants categorised as 60–100% adherent (Table 18).
| % adherence |
ISI at 6 months | Change in ISI at 3 months from baseline | Change in ISI at 6 months from baseline | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Mean (SD) | Estimated treatment effect (95% CI) |
p-value | Mean (SD) | Estimated treatment effect (95% CI) |
p-value | |
| ≥ 0–≤ 40 | 13 | 12.82 (6.76) | −4.82 (6.88) | −2.07 (−4.64 to 0.51) |
0.12 | −5.64 (5.57) | −1.955 (−4.559 to 0.649) |
0.14 |
| > 40–≤ 60 | 26 | 11.96 (6.04) | −8.65 (4.74) | −5.97 (−7.69 to −4.26) |
< 0.001 | −6.19 (5.55) | −2.584 (−4.318 to −0.851) |
0.004 |
| > 60–≤ 80 | 32 | 10.19 (5.37) | −7.97 (5.49) | −5.47 (−7.05 to −3.88) |
< 0.001 | −7.48 (4.57) | −4.018 (−5.615 to −2.420) |
< 0.001 |
| > 80–≤ 100 | 86 | 9.10 (4.65) | −7.14 (5.09) | −4.96 (−5.987 to −3.924) |
< 0.0001 | −7.51 (5.29) | −4.350 (−5.399 to −3.301) |
< 0.001 |
Treatment effects on the change scores for different levels of adherence were estimated by fitting a group by adherence interaction in the model, with the reference category being the control group. The models are adjusted for baseline ISI score and a random effect was fitted for GP practice. Estimated treatment effects therefore reflect the difference in the change in ISI scores from baseline for each adherence category as compared to control.
At 6 months, those with higher diary-defined SRT adherence tended to display greater change from baseline and stronger estimated treatment effects. At 3 months the pattern appeared non-linear, with adherence categories > 40–≤ 60/> 60–≤ 80/> 80–≤ 100 separating and exhibiting stronger treatments relative to the 0–40% category.
Mediation and moderation analyses
Our proposed mediators, pre-sleep arousal (PSAS) and sleep effort (GSES), were significantly reduced in the SRT group relative to control at 3 months post randomisation (see Table 14). The extent to which these variables causally mediated 6-month ISI was investigated using the approach of Baron and Kenny adapted for linear mixed-effect models. Tables 19–21 summarise the direct and indirect effects for each mediator separately. There were statistically significant indirect effects for sleep effort, pre-sleep cognitive arousal and somatic arousal, which mediated between 15% and 36% of the total treatment effect at 6 months.
| Estimate | 95% CI | p-value | Percentage mediated | |
|---|---|---|---|---|
| Total effect | 3.05 | 2.28 to 3.83 | < 0.0001 | |
| Direct effect | 2.03 | 1.28 to 2.78 | < 0.0001 | |
| Indirect effect | 1.12 | 0.75 to 1.49 | < 0.0001 | 35.6 |
| Estimate | 95% CI | p-value | Percentage mediated | |
|---|---|---|---|---|
| Total effect | 3.05 | 2.28 to 3.83 | < 0.0001 | |
| Direct effect | 2.08 | 1.32 to 2.84 | < 0.0001 | |
| Indirect effect | 1.10 | 0.74 to 1.47 | < 0.0001 | 34.6 |
| Estimate | 95% CI | p-value | Percentage mediated | |
|---|---|---|---|---|
| Total effect | 3.05 | 2.28 to 3.82 | < 0.0001 | |
| Direct effect | 2.72 | 1.94 to 3.49 | < 0.0001 | |
| Indirect effect | 0.46 | 0.19 to 0.73 | 0.0008 | 14.5 |
We performed exploratory moderation analyses on the following subgroups at baseline for the ISI at 6 months:
-
actigraphy-defined TST at baseline, categorised as either < 6 or ≥ 6 hours
-
chronotype (morning, intermediate or evening) defined by the MEQr at baseline
-
age (18–65 years vs. > 65 years)
-
patient-reported prescribed sleep medication use at baseline (Yes vs. No)
-
depression ‘caseness’ (PHQ-9 score < 10 vs. ≥ 10)
-
socialeconomic deprivation [Index of Multiple Deprivation (IMD) score: National quartiles 1 and 2 vs. 3 and 4].
Figure 3 summarises the adjusted mean differences between the randomised groups at 6 months for each level of the subgroup and the test of interaction. There were no significant subgroup differences for TST, chronotype, depression severity, age, sleep medication use, or level of deprivation.
FIGURE 3.
Forest plot of the results from the subgroup analyses.

Adverse events
Pre-defined AEs (work-related accidents, falls, motor-vehicle accidents, near-miss driving incidents, falling asleep while driving) were assessed at baseline, 3, 6 and 12 months. Logistic mixed-effect models revealed no differences between groups for any outcome at any time point (Table 22).
| SRT (N = 321) |
SH (N = 321) |
Adjusted treatment difference (95% CI)a |
p-valuea | |
|---|---|---|---|---|
| Work-related accident resulting in injury, n/N (%)b | ||||
| 3 months | 1/245 (0.4) | 7/285 (2.5) | 0.14 (0.01 to 1.43) | 0.099 |
| 6 months | 1/235 (0.4) | 6/279 (2.2) | 0.19 (0.02 to 1.90) | 0.158 |
| 12 months | 1/224 (0.4) | 1/267 (0.4) | 1.37 (0.07 to 26.33) | 0.835 |
| Motor-vehicle accident, n/N (%)b | ||||
| 3 months | 5/245 (2.0) | 3/285 (1.1) | 2.42 (0.42 to 14.06) | 0.325 |
| 6 months | 5/235 (2.1) | 5/280 (1.8) | 1.43 (0.30 to 6.84) | 0.655 |
| 12 months | 4/224 (1.8) | 0/267 (0.0) | – | – |
| Near-miss driving incident, n/N (%)b | ||||
| 3 months | 12/245 (4.9) | 21/285 (7.4) | 0.56 (0.21 to 1.49) | 0.244 |
| 6 months | 9/235 (3.8) | 21/280 (7.5) | 0.40 (0.14 to 1.14) | 0.087 |
| 12 months | 11/224 (4.9) | 14/267 (5.2) | 0.92 (0.32 to 2.69) | 0.884 |
| Number of times fallen asleep while driving, mean (SD) (n) | ||||
| 3 months | 0.1 (1.28) (244) | 0.0 (0.18) (284) | – | – |
| 6 months | 0.1 (1.97) (234) | 0.0 (0.10) (280) | – | – |
| 12 months | 0.1 (1.00) (224) | 0.0 (0.06) (266) | – | – |
| Fallen asleep while driving, n/N (%)b | ||||
| 3 months | 1/244 (0.4) | 3/284 (1.1) | 0.09 (0.00 to 7.55) | 0.284 |
| 6 months | 2/234 (0.9) | 3/280 (1.1) | 0.33 (0.01 to 16.19) | 0.576 |
| 12 months | 1/224 (0.4) | 1/266 (0.4) | 0.36 (0.00 to 44.85) | 0.680 |
| Number of falls, mean (SD) (n) | ||||
| 3 months | 0.4 (3.25) (245) | 0.3 (0.94) (285) | – | – |
| 6 months | 0.4 (2.13) (235) | 0.4 (1.46) (280) | – | – |
| 12 months | 0.3 (0.81) (224) | 0.4 (1.83) (267) | – | – |
| Falls, n/N (%)b | ||||
| 3 months | 31/245 (12.7) | 43/285 (15.1) | 0.77 (0.39 to 1.53) | 0.452 |
| 6 months | 32/235 (13.6) | 49/280 (17.5) | 0.68 (0.35 to 1.34) | 0.265 |
| 12 months | 33/224 (14.7) | 47/267 (17.6) | 0.83 (0.42 to 1.62) | 0.579 |
Serious adverse events
The number of SAEs is presented in Table 23. In total, 16 participants (8 in each arm) experienced at least one SAE. There was one death per group [one due to major haemorrhage (SH) and one due to pneumonia (SRT group)]. None of the SAEs were deemed to be related to the intervention or study.
| SRT | SH | p-valuea | |
|---|---|---|---|
| (N = 296) | (N = 321) | ||
| SAEs | |||
| Experienced SAE, n/N (%) | |||
| None | 288/296 (97.3) | 313/321 (97.5) | |
| One | 8/296 (2.7) | 7/321 (2.2) | |
| Two | 0/296 (0.0) | 1/321 (0.3) | |
| At least one | 8/296 (2.7) | 8/321 (2.5) | > 0.999 |
Impact of COVID-19
The final participant was randomised on 23 March 2020, which was the start date for the national UK lockdown due to COVID-19. The trial was able to continue with remote data collection for most outcomes during the pandemic. Sleep diaries and actigraphy watches were not sent out during lockdown because the research team could not access university buildings; this led to low completion rates for sleep diary, actigraphy and medication use outcomes. A small number of participants in the SRT arm were directly affected by the pandemic (n = 13) such that treatment sessions were adjusted so that they could be completed remotely. Because the lockdown and pandemic may have adversely affected sleep, a sensitivity analysis was conducted to explore whether there was a difference in treatment effect on the ISI between participants who completed the 6-month follow-up before the pandemic (< 23 March 2020) compared with participants whose follow-up was completed during the pandemic (≥ 23 March 2020). There was no evidence that treatment effects differed pre versus during the pandemic (see Appendix 3, Table 35).
Chapter 4 Economic evaluation
Introduction
This chapter uses material from an Open Access article previously published by the research team [see Kyle SD, Siriwardena AN, Espie CA, Yang Y, Petrou S, Ogburn E, et al. Clinical and cost-effectiveness of nurse-delivered sleep restriction therapy for insomnia in primary care (HABIT): a pragmatic, superiority, open-label, randomised controlled trial. Lancet 2023;402(10406):975–87. https://doi.org/10.1016/S0140-6736(23)00683-9. Epub 10 Aug 2023. PMID: 37573859]. This article is published under licence to The Lancet. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
This chapter presents the health economic evaluation conducted as part of the HABIT trial. The base-case analysis took an NHS and PSS perspective and assessed the cost-effectiveness of nurse-delivered SRT relative to SH alone for insomnia in primary care. Health care and PSS resource utilisation data and EQ-5D-3L utility data were collected alongside clinical data. These data were used to conduct a cost–utility analysis, calculating the incremental cost per QALY gained during the 12-month trial period as the primary outcome of the economic analysis. Sensitivity analyses were conducted to explore how the result was affected by altering several key features of the economic evaluation, including (1) complete-case analysis without data imputation, (2) adopting a societal perspective and (3) adjusting the nurse training cost for SRT. Pre-specified secondary exploratory analyses were also conducted: (1) using two other utility measures (SF-6D and EQ-5D + Sleep) to calculate QALYs, (2) only including participants who attended at least one SRT session in the intervention arm for the per-protocol analysis, (3) using only NHS and PSS costs and EQ-5D-3L data over the first 6 months of follow-up and (4) using improvement of ISI scores and treatment response measured by ISI change score as health outcomes.
We followed current guidelines59,60 for conducting and reporting economic evaluations within clinical trials, including in relation to the design, conduct, data analysis and reporting.
Methods
Aim
The primary aim of the health economics component of the HABIT study was to address the question of whether nurse-delivered SRT is cost-effective compared with SH among patients with insomnia in primary care.
The within-trial economic analysis was performed using individual patient-level data collected from the HABIT trial. The analysis used data from the HABIT trial only and did not combine this with any external data or evidence. The primary analytical approach took the form of a cost–utility analysis, which uses QALYs as the main measure of health outcome. The economic analysis compared the costs and outcomes of each intervention group over the 12-month period following randomisation, with no extrapolation beyond the study period as pre-specified by the study protocol. The time horizon of the evaluation was 1 year and so no discounting of costs and QALYs was applied.
Measurement of resource use and costs
Resource use and costs during the trial follow-up period were estimated using an adapted version of the self-reported CSRI, which all participants were asked to complete. Data were collected at four time points: baseline, 3 and 6 months after randomisation (with a recall period of ‘in the last 3 months’) and 12 months after randomisation (with a recall period of ‘in the last 6 months’). The CSRI collected individual patient use of NHS and PSS services due to (1) their insomnia and (2) health-related reasons other than their insomnia. We followed current guidelines61 and included insomnia-related NHS and PSS resource use and costs in our analysis because these were deemed to be important and relevant to the intervention and the underlying condition.
The adapted version of the CSRI captured both NHS- and PSS-related resource use and costs, and insomnia-related resource use and costs borne by trial participants. This included: frequency of use of hospital care (including accident and emergency visits, hospital outpatient appointments, overnight hospital admissions), community-based health and social care (including consultations with GPs, consultations with PNs), mental health services (including consultations with psychiatrists, psychologists, mental health nurses, and counsellors) and prescribed insomnia-related medications. These items were identified as relevant and important to reflect the clinical care that patients with insomnia are provided in the NHS based on discussion with clinical experts in the team. For inpatient admissions, the trial participants were also asked to record how many nights of hospital stays they experienced. For contacts with various community-based health and social care professionals, in addition to frequency, the trial participants were also asked to record how many minutes, on average, each contact lasted. The trial participants were additionally asked to record the name and dose of their prescribed insomnia-related medications.
The trial participants also documented their purchases of over-the-counter remedies (name) and frequency of use of complementary therapies (such as homeopathy and acupuncture) at each of the four time points. These were treated as participants’ out-of-pocket spending on their insomnia because they are not normally provided by the NHS, and the associated costs were included in the sensitivity analysis with the societal perspective.
We made some assumptions when cleaning, analysing, or costing the health and social care resource use data. The assumptions included: (1) if a patient answered ‘No’ to a prompt question about resource utilisation, ‘have you used any of the services below for help with your insomnia?’, then we assumed that the frequency of service use for that particular item was equal to zero; (2) if a patient answered ‘Yes’ to a prompt question about resource utilisation, ‘have you used any of the services below for help with your insomnia?’ but did not report the frequency of service use for that particular item, then we assumed the data were missing; (3) where patients were asked to record the names and doses for prescribed insomnia medications and over-the-counter (OTC) remedies, we assumed one monthly pack (28-tablet pack) per 3-month period, so two monthly packs per 6-month period for short-acting hypnotics and sedative antihistamines; and nightly use (so three monthly pack per 3-month period and six monthly pack per 6 months) for other medications and (4) we assumed that patients purchased one item of over-the-counter remedies for their insomnia over the recalled period. The assumptions of prescribed and over-the-counter remedies use were based on clinical expert opinion.
Costing of the sleep restriction therapy intervention and sleep hygiene
The SRT intervention introduced and tested by the HABIT trial includes two main components: (1) SRT training and (2) nurse-delivered SRT sessions. Both arms provided SH advice, which is typically NHS usual care for patients who seek help in community care.
Training
We included the cost of training the community nurses in how to deliver the SRT intervention as it is not part of standard NHS practice. We assumed SH to be implicitly known without any training as it was delivered as standard NHS practice.
Two members of the research team (SK and NS) delivered a total of 17 training sessions (14 by SK and 3 by NS) to a total of 56 community nurses. Each nurse spent approximately 4 hours on their session. SK spent 4 hours to deliver each session and NS spent 3 hours to deliver each session. We assumed that SK and NS spent 5 minutes for the preparation of each training session. SK also spent 8 hours developing training materials. Among the 56 community nurses trained, only 40 delivered SRT sessions to participants. The other 16 trained nurses did not see any patients, or the practices they were based at did not open for the HABIT trial, and therefore their costs were not included in the analysis after discussions with the clinical team.
The total cost of SRT training within the HABIT trial was calculated mainly based on the time the 2 trainers and 40 community nurses spent on training sessions, multiplied by the trainers and community nurse cost per hour, which were obtained from the staff salaries from the project budget (SDK and ANS) and from the Unit Costs of Health and Social Care [Personal Social Services Research Unit (PSSRU)] 2019 compendium. This reflected the NHS cost of training as it was delivered in the HABIT trial and included the time costs for the trainers and the community nurses. The cost of venue hire, trainer’s and trainees’ travel costs to training sites and NHS parking charges were not included in the analysis. We applied and averaged the total cost of SRT training for the SRT intervention group across all participants randomised to obtain the mean training cost per patient, regardless of how many SRT sessions they attended.
In a sensitivity analysis we adjusted the per-patient training cost to reflect how many patients a nurse in the NHS may see over a 1-year period, if SRT were introduced into primary care. That is, in practice, the trained nurses would see many more patients than those involved in the trial and therefore the cost of training would be averaged across a larger number of patients. We assumed that a PN would hold a weekly sleep clinic lasting for 3 hours (i.e. 12 hours per month). We calculated how many patients each nurse would be able to see each month using the mean time to deliver four SRT sessions in the trial, and calculated how many patients each nurse would be able to see for 1 year. We divided the total SRT training cost in the trial by the number of patients the 40 nurses would be able to see.
Delivery of sleep restriction therapy sessions and sleep hygiene
Patients randomised to the SRT intervention arm were provided a total of four SRT sessions with the community nurse. We recorded whether individual patients attended each of the four SRT sessions, whether the SRT sessions were delivered in person or via telephone, and associated start and end time of each SRT session attended. The duration of each SRT session delivered was calculated and used to indicate associated community nurse time spent on the delivery of the session. If the participant did not attend the planned SRT session or withdrew from the study, the time duration of that session was assumed to be 0 with 0 costs incurred.
The total and mean nurse time spent on delivering each of the four SRT sessions was calculated. The total and mean cost of community nurse time spent on delivering each of the four SRT sessions was calculated by multiplying the cost per hour [£41.5 per hour, or £0.7 per minute, Unit Costs of Health and Social Care (PSSRU) 2019] by the duration, regardless of whether they were in person or via telephone. The total time and associated cost of the 40 community nurses’ time spent on delivering the four SRT sessions to all the patients in the SRT arm were calculated as the sum of each session delivered. We calculated the mean intervention cost per participant by dividing the total SRT costs by the total number of the patients in the SRT arm regardless of whether they attended no session or only some of the four sessions. The SH leaflet was contained within the SRT patient guide and given at the end of the first SRT session, so no extra nurse time was calculated or included for patients in the SRT intervention arm.
In the SH arm, we consulted the clinical team and estimated staff time spent on e-mailing or posting the SH booklet, and postage cost for posting paper copies of the SH booklet. We estimated the mean cost of the SH per patient by multiplying average staff time on SH with their cost per minute.
Measuring and valuing productivity loss due to insomnia
Time off work and productivity loss due to insomnia for those in employment were captured and quantified using the WPAI44 questionnaire at baseline and 3, 6 and 12 months. Questions 1, 2, 4 and 5 of the WPAI were used to quantify productivity loss due to insomnia. Patients were asked whether they were currently employed (Q1). If they were employed, the participants were asked about hours of missed work due to insomnia (Q2) and hours actually worked (Q4). The degree to which insomnia affected productivity while working was recorded on a scale from 0 to 10, with a higher score indicating worse impairment (Q5).
Participants’ productivity losses had two components: absenteeism (work time missed) and presenteeism for employment (impairment at work). Participants’ time absence from work due to insomnia was taken directly from question 2 of the WPAI for those who were working at the time of data collection (captured by Q1). Presenteeism was calculated as the total working time (Q4) multiplied by the extent (converted from Q5) to which insomnia affected productivity while working. The 0–10 scale in question 5 was converted into a percentage score from 0% to 100%. 9 The total productivity loss was calculated as the sum of time lost due to both absenteeism and presenteeism.
Work productivity and activity impairment questions asked about patients’ productivity loss over the past 7 days. In order to keep a consistent recall time period with other cost categories, the productivity loss over the previous 3-month period (i.e. baseline and the time points of 3 and 6 months after randomisation) and 6-month period (at the time point of 12 months after randomisation) were extrapolated by multiplying values by 12 for 3-month periods and by 24 for the 6-month period, assuming each month has 4 weeks.
Economic values associated with productivity losses were estimated by multiplying total working hours lost by average hourly salaries based on gender and age groups obtained from Office of National Statistics 2019. Productivity losses were valued for participants who were in employment. For the participants who were not in employment, we assumed 0 productivity loss. We then obtained average values of productivity losses due to insomnia across all participants randomised into the SRT and SH arms, regardless of whether they were in employment or not. Economic values associated with productivity losses are regarded as falling outside of the perspective of NHS and PSS, so these values were only included in the sensitivity analysis that adopted a societal perspective.
Valuation of resource use
The unit costs for clinical staff time to develop training materials, deliver and receive the SRT training, and deliver the SRT sessions were obtained from national standard sources [Unit Costs of Health and Social Care (PSSRU)62].
Unit costs of community health and social service inputs were based on PSSRU national cost compendia. The costs of medications were estimated from the British National Formulary. 63 NHS references costs were assigned to use of alternative categories of hospital services. 61 Complementary services were assigned an average cost according to clinical opinion. The costs of OTC remedies were obtained from the PAGB OTC directory (www.pagb.co.uk/product/pagbs-otc-directory/) and from a search of the websites of large pharmacies in the UK, including Boots and Lloyds pharmacies.
The cost of each resource item was calculated by multiplying the number of resource units used by the relevant unit cost. The total cost for each individual trial participant was estimated as the sum of the costs of resource use items consumed during the specific time period. For example, total cost at 12-month follow-up was the sum of total costs at 3-, 6- and 12-month follow-ups, as the data were collected for the previous 3 months at the 3- and 6-month follow-up time points, and for the previous 6 at the 12-month follow-up point.
All costs were reported in 2018–9 Great British pounds. Given that the trial follow-up period was 12 months, no discounting was applied to cost estimates.
Calculation of utilities and quality-adjusted life-years
Trial participants completed the EQ-5D-3L questionnaire at baseline and 3, 6 and 12 months post randomisation. The EQ-5D-3L questionnaire facilitates the generation of a utility score from the measure’s health status classification system. A utility score reflects the preference of the general population for any particular set of health states. The EQ-5D-3L has been recommended by NICE64 for the measurement and valuation of health outcomes in economic evaluations. Effectiveness was estimated in terms of QALYs, calculated as the baseline-adjusted utility curve of EQ-5D-3L utility scores across the baseline and 3-, 6-, and 12-month intervals, using the trapezoidal rule.
We understand that follow-ups may not have fallen exactly at the expected time points (e.g. 3, 6 and 12 months post randomisation). However, we made the assumption that the time points were exact to simplify the calculation of QALYs.
In addition to the EQ-5D-3L, participants completed two additional utility measures, the SF-6D (derived from the SF-36) and the EQ-5D-3L + sleep, at baseline, 3, 6 and 12 months. EQ-5D-3L + Sleep contains the same five dimensions of the original EQ-5D-3L questionnaire plus an extra dimension on sleep. A value set has been developed for EQ-5D-3L + Sleep. Utility values derived from the SF-6D and EQ-5D-3L + Sleep were used to estimate QALYs over the 12-month trial period using the same method described for the EQ-5D-3L above, and the QALYs derived were used for further secondary exploratory analysis. Given that the trial follow-up period was 12 months, no discounting was applied to QALY estimates.
Missing data
Many sources of information on patient characteristics, treatments, utilities and resource use are used to conduct the economic evaluation within a clinical trial. Therefore, missing data are a frequent and particularly challenging issue that requires careful consideration. Costs and outcomes for individuals with missing data may differ systematically from those individuals with observed data. We followed current method guidance65,66 on handling missing data in cost-effectiveness analysis conducted alongside clinical trials. We examined missing data status at the trial time points, and estimated logistic regressions to investigate association between missingness of NHS and PSS costs and QALYs with key baseline covariates including age, sex, region, EQ-5D utility score, PHQ-9 score, ISI score, and use of prescribed sleep medication, and treatment group.
Consequently, we decided to impute missing data for use in our base-case analysis. Both chained equations and predictive mean matching (PMM with knn = 8) were used for multiple imputation using the Stata command ‘mi impute chained’. The imputed variables included EQ-5D-3L, EQ-5D-3L + Sleep and SF-6D utility values, and ISI scores at 3-, 6- and 12-month follow-ups, and NHS and PSS costs at 3-, 6- and 12-month follow-ups, and non-NHS and PSS costs at 3-, 6- and 12-month follow-ups, and the value of productivity loss at 12 months. We used the same set of baseline covariates as predictor variables for multiple imputation and regression models to estimate incremental costs and incremental QALYs, which included age, sex, site, baseline ISI scores, baseline EQ-5D-3L utility scores, baseline PHQ-9 scores, prescribed sleep medication at baseline, and NHS and PSS costs at baseline. The imputation was conducted for cost and utilities for the two treatment arms separately within a single command. The chained equation method means that the costs and EQ-5D-3L utility scores at each time point contributed to the multiple imputation as both predictors and imputed variables, which made efficient use of the data.
We used multiple imputation to generate 50 data sets using PMM, which provides plausible values when costs and utility values are not normally distributed. The number of imputations was run following the rule of thumb. The imputation models were validated by comparing the distributions of the imputed data with the observed data.
Cost-effectiveness analysis
The base-case analysis was conducted using the full data set with missing data imputed as described above, comparing the two arms as randomised and including all patients in the analysis where practical. The purpose of the economic analysis was not to test statistical hypotheses such as whether there are significant differences between costs or/and health outcome such as QALYs. The fundamental aim of the economic analysis was to estimate the ICER associated with SRT, to quantify the uncertainty surrounding the ICER estimate, and to examine whether and to what extent the intervention is cost-effective by comparing the ICER with conventional cost-effectiveness thresholds for an extra unit of health outcome (i.e. QALY).
Reporting the cost and health outcomes
We report means and SDs (or standard errors) and medians and IQRs for EQ-5D-3L utilities and associated QALYs for the two arms at the different follow-up time points based on individual patient data. We similarly report means and SDs (or standard errors) and medians and IQRs for EQ-5D-3L + Sleep and SF-6D utilities and associated QALYs. We report intervention costs, including training and delivery of SRT sessions to participants in the SRT arm, and SH to participants in the SH arm. We report mean costs of key NHS and PSS services in relation to insomnia, including hospital services, community health and social care, and prescribed medications, as well as non-NHS out-of-pocket healthcare costs, costs due to productivity losses, and total societal costs for both arms of the trial at the different follow-up time points. We performed parametric t-tests (bootstrapped 95% CIs, 1000 samples) to compare mean costs of cost categories in relation to insomnia, and QALYs based on the EQ-5D-3L by treatment group at each assessment time point.
Regression analysis and bootstrapping
In the base case, bivariate regression using seemingly unrelated regression was used to estimate incremental NHS and PSS costs and incremental QALYs between the SRT and SH arms over the 12-month follow-up period on each of the imputed samples controlling for baseline covariates [ISI score, region, age, prescribed sleep medication use, sex, and PHQ-9 score, and either baseline EQ-5D-3L utility scores (for incremental QALYs) or baseline NHS and PSS costs (for incremental costs)]. The mean estimate of the ICER was calculated by dividing incremental costs by incremental QALYs.
Non-parametric bootstrapping was used to quantify uncertainty surrounding the mean ICER estimate by resampling 1000 times from incremental costs and incremental QALYs obtained from the seemingly unrelated regression. This method addressed the effects of missing data and sampling uncertainty using the MI Boot approach suggested by Schomaker and Heumann. 67 This approach is simpler to implement and less demanding of computing capacity, and it has been shown to produce valid inference and to be equivalent to nesting bootstraps within imputations and combining results using Rubin’s rule. The outputs were displayed graphically on a cost-effectiveness plane to determine the uncertainty surrounding cost-effectiveness, enabling investigation of the joint distribution of both incremental costs and incremental QALYs by scatter-plotting the incremental cost-QALY pairs in the plane and exploring the joint density of the plots. NMBs were estimated from the incremental costs and incremental QALYs at alternative cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY gained in order to reflect the overall resource gain or loss associated with SRT. By calculating NMBs for each of these 1000 simulated ICER values at alternative levels of the cost-effectiveness threshold, the probability of cost-effectiveness of SRT (defined as the proportion of positive NMBs at a given threshold level) was calculated, and plotted as a cost-effectiveness acceptability curve (CEAC).
Sensitivity analysis and exploratory analysis
Sensitivity analyses were performed to explore how the ICER was affected by altering several key features of the economic evaluation. The sensitivity analyses were conducted with the intention of providing evidence on whether the results from the base-case analysis remained robust. Our sensitivity analyses included: (1) using complete-case analysis rather than imputed data to explore any potential effects due to data imputation; (2) adopting a societal perspective where extra costs beyond NHS and PSS costs were included in the analysis (these included non-NHS out-of-pocket spending on complementary therapies, OTC remedies, and the value of productivity losses due to insomnia); and (3) adjusting costs associated with SRT training from the overall cost of SRT intervention. We also conducted several pre-defined secondary exploratory analyses, including (1) using two other utility measures (SF-6D and EQ-5D + Sleep) to calculate QALYs. We compared cost-effectiveness results for these measures with those obtained from the EQ-5D-3L. (2) Including participants in the SRT arm who attended at least one SRT session for a per-protocol analysis. (3) Restricting the analysis period to the first 6 months post randomisation using NHS and PSS costs and using QALYs estimated from utilities obtained from the EQ-5D-3L up to the 6-month follow-up. The rationale was to explore short-term cost-effectiveness of the SRT given the primary outcome of the HABIT trial was insomnia severity at 6 months. (4) Performing a cost-effectiveness analysis using improvement of ISI scores between the baseline and 12-month follow-up as the health outcome, and expressed in terms of incremental cost per unit reduction in ISI score. In this analysis, hypothetical cost-effectiveness thresholds were used to estimate the probability of cost-effectiveness and net economic benefit of the SRT intervention. Other hypothetical cost-effectiveness thresholds were also used for a further exploration. (5) Defining treatment responders as those exhibiting a reduction of ISI score ≥ 8 points between baseline and 12 months, we estimated the incremental cost per additional treatment responder.
Results
Descriptive analysis and quality-of-life measures
Six hundred and forty-two participants were randomised in the HABIT trial, half of them (321) to the SRT arm and the other half (321) to the SH arm. Table 24 presents mean (SD) and median IQR values for the key health outcomes between the two arms at different time points using the available cases without imputation.
| SRT | SH | |||||
|---|---|---|---|---|---|---|
| N | Mean (SD) | Median (IQR) |
N | Mean (SD) | Median (IQR) |
|
| EQ-5D-3L utility | ||||||
| Baseline | 321 | 0.70 (0.26) | 0.73 (0.66–0.85) | 321 | 0.72 (0.24) | 0.76 (0.69–0.85) |
| 3-month | 245 | 0.72 (0.29) | 0.80 (0.69–1) | 284 | 0.68 (0.28) | 0.73 (0.62–0.85) |
| 6-month | 233 | 0.72 (0.27) | 0.80 (0.69–0.85) | 281 | 0.72 (0.25) | 0.76 (0.69–0.85) |
| 12-month | 223 | 0.72 (0.27) | 0.80 (0.69–0.85) | 266 | 0.72 (0.23) | 0.75 (0.69–0.85) |
| QALYs (by EQ-5D-3L) | ||||||
| 3-month | 245 | 0.18 (0.06) | 0.19 (0.17–0.22) | 284 | 0.18 (0.06) | 0.19 (0.16–0.22) |
| 6-month | 218 | 0.36 (0.13) | 0.39 (0.34–0.45) | 267 | 0.36 (0.12) | 0.38 (0.32–0.43) |
| 12-month | 202 | 0.73 (0.24) | 0.79 (0.66–0.88) | 249 | 0.72 (0.21) | 0.77 (0.66–0.85) |
| SF-6D | ||||||
| Baseline | 321 | 0.63 (0.11) | 0.64 (0.57–0.70) | 321 | 0.63 (0.10) | 0.62 (0.56–0.69) |
| 3-month | 243 | 0.68 (0.13) | 0.66 (0.61–0.79) | 283 | 0.63 (0.11) | 0.64 (0.56–0.70) |
| 6-month | 230 | 0.67 (0.13) | 0.67 (0.58–0.76) | 282 | 0.65 (0.11) | 0.64 (0.58–0.7) |
| 12-month | 222 | 0.68 (0.13) | 0.67 (0.6–0.76) | 262 | 0.65 (0.10) | 0.64 (0.59–0.71) |
| QALYs (by SF-6D) | ||||||
| 3-month | 243 | 0.17 (0.03) | 0.16 (0.15–0.18) | 283 | 0.16 (0.02) | 0.16 (0.14–0.17) |
| 6-month | 215 | 0.34 (0.06) | 0.33 (0.3–0.38) | 267 | 0.32 (0.05) | 0.32 (0.29–0.35) |
| 12-month | 200 | 0.68 (0.11) | 0.67 (0.60–0.76) | 245 | 0.65 (0.09) | 0.64 (0.59–0.7) |
| EQ-5D-3L + Sleep | ||||||
| Baseline | 321 | 0.76 (0.15) | 0.79 (0.71–0.87) | 321 | 0.77 (0.14) | 0.79 (0.72–0.87) |
| 3-month | 245 | 0.79 (0.16) | 0.86 (0.73–0.9) | 284 | 0.76 (0.16) | 0.79 (0.7–0.87) |
| 6-month | 233 | 0.79 (0.16) | 0.86 (0.73–0.9) | 282 | 0.78 (0.14) | 0.80 (0.72–0.87) |
| 12-month | 221 | 0.79 (0.16) | 0.86 (0.73–0.9) | 266 | 0.78 (0.13) | 0.82 (0.72–0.87) |
| QALYs (by EQ-5D-3L + Sleep) | ||||||
| 3-month | 245 | 0.19 (0.03) | 0.21 (0.18–0.22) | 284 | 0.19 (0.03) | 0.2 (0.17–0.22) |
| 6-month | 218 | 0.39 (0.07) | 0.42 (0.38–0.44) | 268 | 0.39 (0.07) | 0.4 (0.35–0.43) |
| 12-month | 201 | 0.79 (0.14) | 0.85 (0.76–0.88) | 249 | 0.78 (0.12) | 0.81 (0.72–0.87) |
In general, more data were missing at follow-up for the SRT intervention group than for the SH group. The mean and median differences of EQ-5D-3L utilities and QALYs between the two arms at different time points were very small. The difference in mean QALYs derived from the EQ-5D-3L over 12 months post randomisation was 0.01. We also present utilities at baseline to assess for any imbalance of health states between the two arms. On average, the SH group had a slightly better HRQoL at baseline.
The EQ-5D-3L + Sleep utilities and QALYs were higher than those for the EQ-5D-3L but the mean and median differences were similarly small. The difference in mean QALYs derived from the EQ-5D-3L + Sleep over 12 months post randomisation was also 0.01.
The SF-6D utilities and QALYs were the lowest among the HRQoL measures. Interestingly, the mean and median differences for SF-6D utilities at different time points and QALYs between the two arms seem to be slightly larger than those for the EQ-5D-3L and EQ-5D-3L + Sleep, although they were also very small. The difference in mean QALYs derived from SF-6D over 12 months post randomisation was 0.03.
Missing data analysis
Table 25 summarises the proportion of individuals with missing health economic data by treatment group over time. There were very few missing data at baseline, and more data were missing at subsequent time points. We explored the patterns of missing data, which indicated that patients with missing data included those lost to follow-up or who withdrew from the trial, as well as those who had missing data at one time point but not at the next. We estimated logistic regressions to investigate the association between missingness of NHS and PSS costs and QALYs with key baseline covariates, including age, sex, region, EQ-5D utility score, PHQ-9 score, prescribed sleep medication use and ISI score. A significant association was found between missingness of NHS and PSS costs and QALYs over 12 months and baseline ISI scores (p < 0.05), with worse ISI scores (greater insomnia severity) being associated with missingness. Region and intervention group were also significantly associated with missingness of NHS costs and QALYs over 12 months (p < 0.05).
| Missing values | ||||||
|---|---|---|---|---|---|---|
| SRT (N = 321) | SH (N = 321) | Total (N = 642) | ||||
| N | % | N | % | N | % | |
| EQ-5D index at baseline | 0 | 0 | 0 | 0 | 0 | 0 |
| EQ-5D index at 3 months | 76 | 24 | 37 | 12 | 113 | 18 |
| EQ-5D index at 6 months | 88 | 27 | 40 | 12 | 128 | 20 |
| EQ-5D index at 12 months | 98 | 31 | 55 | 17 | 153 | 24 |
| QALYs at 12 months generated from EQ-5D utility scores | 119 | 37 | 72 | 22 | 191 | 30 |
| Total NHS cost at baseline (over previous 3 months) | 6 | 2 | 2 | 1 | 8 | 1 |
| Total NHS cost at 3-month follow-up | 80 | 25 | 49 | 15 | 129 | 20 |
| Total NHS cost at 6-month follow-up | 91 | 28 | 48 | 15 | 139 | 22 |
| Total NHS cost at 12-month follow-up | 103 | 32 | 65 | 20 | 168 | 26 |
| Total NHS cost over 12-month trial period | 129 | 40 | 92 | 29 | 221 | 34 |
| Hospital cost at baseline (over previous 3 months) | 0 | 0 | 0 | 0 | 0 | 0 |
| Hospital cost at 3-month follow-up | 73 | 23 | 34 | 11 | 107 | 17 |
| Hospital cost at 6-month follow-up | 69 | 21 | 28 | 9 | 97 | 15 |
| Hospital cost at 12-month follow-up | 92 | 29 | 51 | 16 | 143 | 22 |
| Total hospital cost over 12-month trial period | 111 | 35 | 66 | 21 | 177 | 28 |
| Primary care cost at baseline (over previous 3 months) | 0 | 0 | 0 | 0 | 0 | 0 |
| Primary care cost at 3-month follow-up | 75 | 23 | 39 | 12 | 114 | 18 |
| Primary care cost at 6-month follow-up | 81 | 25 | 39 | 12 | 120 | 19 |
| Primary care cost at 12-month follow-up | 96 | 30 | 55 | 17 | 151 | 24 |
| Total primary care cost over 12-month trial period | 119 | 37 | 77 | 24 | 196 | 31 |
| Mental Health service cost at baseline (over previous 3 months) | 0 | 0 | 0 | 0 | 0 | 0 |
| Mental health service cost at 3-month follow-up | 75 | 23 | 39 | 12 | 114 | 18 |
| Mental health service cost at 6-month follow-up | 83 | 26 | 38 | 12 | 121 | 19 |
| Mental health service cost at 12-month follow-up | 95 | 30 | 57 | 18 | 152 | 24 |
| Total mental health service cost over 12-month trial period | 117 | 36 | 77 | 24 | 194 | 30 |
| Prescribed insomnia medications cost at baseline (over previous 3 months) | 6 | 2 | 2 | 1 | 8 | 1 |
| Prescribed insomnia medications cost at 3-month follow-up | 73 | 23 | 42 | 13 | 115 | 18 |
| Prescribed insomnia medications cost at 6-month follow-up | 86 | 27 | 41 | 13 | 127 | 20 |
| Prescribed insomnia medications cost at 12-month follow-up | 98 | 31 | 55 | 17 | 153 | 24 |
| Total prescribed insomnia medication cost over 12-month trial period | 119 | 37 | 80 | 25 | 199 | 31 |
Intervention-related training costs
Table 26 summarises the time that the 2 trainers (SK and NS) and 40 community nurses spent on preparing, delivering and receiving the SRT training. A total cost of £10,182 and an average of £31.7 per participant was estimated and included the training cost of SRT. The training costs would be likely to reduce after scaling up.
| Items | Number | Number of hours | Time (hours) | Unit cost (£ per hour) | Total cost (£) |
|---|---|---|---|---|---|
| SRT training | |||||
| Nurse SRT training | 40 | 4 | 160 | 41.5 | 6,640 |
| Trainer 1 delivery | 14 | 4 | 56 | 44.16 | 2473 |
| Trainer 1 preparation | 14 | 0.08 | 1.2 | 44.16 | 52 |
| Trainer 2 delivery | 3 | 3 | 9 | 71.78 | 646 |
| Trainer 2 preparation | 3 | 0.08 | 0.25 | 71.78 | 18 |
| Others | |||||
| Time for trainer 1 to generate SRT training materials | 1 | 8 | 8 | 44.16 | 353 |
| Total training cost | – | 226.45 | – | 10,182 | |
An adjusted average of £2.52 per participant for the training cost of SRT was estimated and used in sensitivity analysis, reflecting that the trained nurse would likely see more patients than the 321 patients in the SRT arm in the trial. This assumed a PN will hold a weekly sleep clinic that lasted for 3 hours each week (12 hours per month). The mean time to complete four treatment sessions was 85.5 minutes in the trial, and so assuming 8.4 patients a month, a PN would see 101 (12 × 8.4) patients a year in routine practice. There were 40 nurses in the trial so 4040 patients would be seen, generating an average SRT training cost of £2.52.
Nurse-led sleep restriction therapy sessions, average duration and associated costs
Details of the nurse-led SRT sessions, including number attending, duration and associated costs, are presented in Table 27. The unit cost of community nurse time was identified as £0.7 per minute (£41.5 per hour).
| Session 1 | Session 2 | Session 3 | Session 4 | |
|---|---|---|---|---|
| Attendance | 296 | 250 | 217 | 209 |
| Mean (SD) duration (minutes) | 39.63 (12.70) | 15.07 (7.14) | 17.82 (9.36) | 13.76 (6.57) |
| Range duration (minutes) | 8–98 | 1–55 | 5–75 | 4–49 |
| Mean cost (£) | 27.74 (8.89) | 8.87 (6.03) | 9.14 (7.87) | 6.80 (5.85) |
The first session lasted the longest and hence cost the most. The average cost of the nurse-led SRT sessions was £52.6 for participants in the intervention group. Adding training cost and delivery cost together, the average SRT cost was £84.3 (31.7 + 52.6).
For the SH group, the cost of sending out the leaflet was estimated as £1.7 per participant.
Insomnia-related healthcare utilisation and associated costs
Table 28 shows the number of participants (n) who had data for various insomnia-related healthcare contacts at 3, 6 and 12 months post randomisation by group. It also summarises mean (SD) frequencies of those services at the three time points between the two groups. Very few participants used hospital-based services for their insomnia. They tended to go to their GP, request repeat prescriptions for medications, and purchase OTC remedies for their insomnia. It is worth noting that whether the health resource utilisation was associated with insomnia relied on participant’s judgement and attribution. A few participants also used alcohol and internet apps for their insomnia, although we do not have sufficiently detailed data to convert these into costs.
| SRT (N = 321) | SH (N = 321) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) 3 months | n | Mean (SD) 6 months | n | Mean (SD) 12 months | n | Mean (SD) 3 months | n | Mean (SD) 6 months | n | Mean (SD) 12 months | |
| NHS services | ||||||||||||
| Hospital service | ||||||||||||
| Accident and Emergency visits | 251 | 0.01 (0.11) | 255 | 0.008 (0.09) | 230 | 0 | 287 | 0.02 (0.13) | 293 | 0.007 (0.08) | 273 | 0.01 (0.15) |
| Hospital admission | 251 | 0.02 (1.26) | 255 | 0.008 (0.13) | 230 | 0.004 (0.07) | 287 | 0.01 (0.13) | 293 | 0.003 (0.06) | 274 | 0.05 (0.79) |
| Outpatient | 250 | 0.21 (1.04) | 252 | 0.10 (0.65) | 229 | 0.14 (0.51) | 287 | 0.19 (0.83) | 293 | 0.08 (0.37) | 272 | 0.36 (2.37) |
| Primary care service | ||||||||||||
| GP | 249 | 0.25 (0.89) | 245 | 0.20 (0.62) | 228 | 0.35 (1.06) | 284 | 0.38 (0.90) | 288 | 0.20 (0.60) | 273 | 0.37 (0.96) |
| PN | 247 | 0.38 (1.01) | 247 | 0.04 (0.26) | 228 | 0.10 (0.43) | 287 | 0.11 (0.42) | 291 | 0.02 (0.17) | 273 | 0.10 (0.39) |
| Repeat prescription | 248 | 0.62 (1.71) | 241 | 0.69 (1.36) | 225 | 1.41 (3.18) | 283 | 0.78 (1.35) | 282 | 0.70 (1.54) | 266 | 1.42 (2.66) |
| Mental health service | ||||||||||||
| Psychiatrist | 248 | 0.008 (0.09) | 240 | 0.008 (0.09) | 227 | 0.04 (0.30) | 284 | 0.007 (0.08) | 284 | 0.007 (0.08) | 265 | 0.02 (0.21) |
| Psychologist | 247 | 0.008 (0.13) | 240 | 0 | 227 | 0.02 (0.21) | 284 | 0.10 (0.83) | 284 | 0.05 (0.63) | 264 | 0.01 (0.11) |
| Mental health nurse | 248 | 0.008 (0.13) | 240 | 0.04 (0.43) | 227 | 0.12 (1.37) | 284 | 0.08 (1.19) | 284 | 0.02 (0.24) | 265 | 0.003 (0.06) |
| Counsellor | 247 | 0.07 (0.54) | 239 | 0.08 (0.63) | 227 | 0.16 (1.11) | 284 | 0.11 (0.74) | 283 | 0.14 (1.05) | 264 | 0.06 (0.75) |
| Other mental health professional | 247 | 0.02 (0.18) | 240 | 0.004 (0.06) | 227 | 0.11 (1.35) | 282 | 0.01 (0.19) | 284 | 0.02 (0.26) | 265 | 0.03 (0.23) |
| % participants prescribed insomnia medication | 249 | 50 (20.1) | 236 | 37 (15.6) | 226 | 55 (24.3) | 285 | 66 (23.2) | 282 | 70 (24.8) | 267 | 67 (25.1) |
| Non-NHS services | ||||||||||||
| Homeopathy | 246 | 0.05 (0.47) | 237 | 0.03 (0.24) | 227 | 0.01 (0.15) | 285 | 0.05 (0.39) | 280 | 0.03 (0.26) | 265 | 0.07 (0.67) |
| Other complementary therapies | 247 | 0.13 (0.52) | 237 | 0.10 (0.54) | 227 | 0.10 (0.59) | 283 | 0.13 (0.98) | 278 | 0.13 (0.80) | 265 | 0.14 (0.80) |
| Alcohol use (%) | ||||||||||||
| Not at all | 249 | 201 (80.7) | 235 | 184 (78.3) | 226 | 182 (80.5) | 285 | 211 (74.0) | 281 | 205 (73.0) | 266 | 198 (74.4) |
| Less than once a week | 249 | 17 (6.8) | 235 | 21 (8.9) | 226 | 24 (10.6) | 285 | 37 (13.0) | 281 | 35 (12.5) | 266 | 34 (12.8) |
| Once or twice a week | 249 | 17 (6.8) | 235 | 20 (8.5) | 226 | 9 (4.0) | 285 | 18 (6.3) | 281 | 26 (9.3) | 266 | 25 (9.4) |
| Three or more times a week | 249 | 14 (5.6) | 235 | 10 (4.3) | 226 | 11 (4.9) | 285 | 19 (6.7) | 281 | 15 (5.3) | 266 | 9 (3.4) |
| Proportion who used internet/apps (%) | ||||||||||||
| 249 | 22 (8.8) | 235 | 22 (9.4) | 226 | 27 (12.0) | 285 | 29 (10.2) | 281 | 31 (11.0) | 266 | 30 (11.3) | |
Table 29 summarises unit cost estimates for calculation of NHS and PSS services and broader categories of service use and costs, obtained from various national sources.
| Unit cost (£) | Source (2018–9) | |
|---|---|---|
| Accident and emergency (per visit) | 166 | PSSRU |
| Hospital admission (per visit) | 1311.2 | Reference costa |
| Hospital extra days beyond trim point (per day) | 276.6 | Reference costb |
| Outpatient/day case (per consultation) | 224.8 | Reference cost |
| GP (per consultation) | 33 | PSSRU |
| PN (per hour) | 41.5 | PSSRU |
| Repeat prescription (per service) | 6 | PSSRU |
| Psychiatrist (per hour) | 111 | PSSRU |
| Psychologist (per hour) | 56.3 | PSSRU |
| Mental health nurse (per hour) | 37 | PSSRU |
| Counsellor (per hour) | 44.3 | PSSRU |
| Other mental health professional (per hour) | 34 | PSSRU |
| Homeopathy (per visit) | 40 | Expert opinion |
| Acupuncture (per visit) | 40 | Expert opinion |
| Other complementary therapies (per visit) | 40 | Expert opinion |
| Average hourly salary | National Office of Statistics (2019) | |
| Age (18–21) and male | 8.6 | National Office of Statistics (2019) |
| Age (18–21) and female | 8.5 | National Office of Statistics (2019) |
| Age (22–29) and male | 12.3 | National Office of Statistics (2019) |
| Age (22–29) and female | 11.43 | National Office of Statistics (2019) |
| Age (30–39) and male | 15.7 | National Office of Statistics (2019) |
| Age (30–39) and female | 13.7 | National Office of Statistics (2019) |
| Age (40–49) and male | 17.5 | National Office of Statistics (2019) |
| Age (40–49) and female | 13.5 | National Office of Statistics (2019) |
| Age (50–59) and male | 16.4 | National Office of Statistics (2019) |
| Age (50–59) and female | 12.2 | National Office of Statistics (2019) |
| Age (60 and over) and male | 13.6 | National Office of Statistics (2019) |
| Age (60 and over) and female | 10.8 | National Office of Statistics (2019) |
Average costs for various cost categories, EQ-5D-3L utility values and associated QALYs by study time points are presented in Table 30 and compared across the study arms.
| Treatment group, cost (£) | Mean difference | Bootstrap 95% CI | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SRT | SH | ||||||||
| N | Mean | SE | N | Mean | SE | ||||
| Cost categories by period | |||||||||
| Baseline to 3 months | |||||||||
| Primary care services | 246 | 17.80 | 2.76 | 282 | 17.88 | 2.07 | −0.08 | −7.04 to 6.89 | 0.982 |
| Hospital services | 248 | 48.22 | 15.96 | 287 | 50.54 | 12.52 | −2.32 | −41.96 to 37.32 | 0.909 |
| Mental health services | 246 | 7.30 | 3.85 | 282 | 8.09 | 2.67 | −0.79 | −10.04 to 8.46 | 0.866 |
| Prescribed insomnia medications | 248 | 0.85 | 0.19 | 279 | 1.25 | 0.37 | −0.40 | −1.23 to 0.43 | 0.336 |
| NHS and PSS | 241 | 69.53 | 16.37 | 272 | 76.93 | 14.46 | −7.40 | −50.40 to 35.61 | 0.735 |
| Non-NHS and PSS | 246 | 9.02 | 2.55 | 279 | 9.11 | 1.87 | −0.09 | −6.44 to 6.26 | 0.978 |
| Productivity losses | 237 | 619.74 | 76.74 | 268 | 823.49 | 83.77 | −203.75 | −434.17 to 26.68 | 0.073 |
| 3–6 months | |||||||||
| Primary care service | 240 | 10.93 | 1.55 | 282 | 11.07 | 1.47 | −0.14 | −4.16 to 3.88 | 0.948 |
| Hospital services | 252 | 27.98 | 9.89 | 293 | 21.04 | 5.11 | 6.94 | −14.52 to 28.41 | 0.533 |
| Mental health services | 238 | 4.03 | 1.74 | 283 | 20.14 | 12.57 | −16.11 | −41.30 to 9.09 | 0.205 |
| Prescribed insomnia medications | 235 | 0.99 | 0.41 | 280 | 1.02 | 0.23 | −0.03 | −0.95 to 0.90 | 0.956 |
| NHS and PSS | 230 | 46.55 | 11.22 | 273 | 41.05 | 6.47 | 5.50 | −19.90 to 30.91 | 0.671 |
| Non-NHS and PSS | 233 | 6.47 | 1.62 | 276 | 7.76 | 2.04 | −1.29 | −6.37 to 3.78 | 0.620 |
| Productivity losses | 217 | 553.64 | 83.81 | 265 | 639.51 | 70.06 | −85.87 | −292.26 to 120.53 | 0.432 |
| 6–12 months | |||||||||
| Primary care services | 225 | 20.74 | 3.25 | 266 | 20.80 | 2.56 | −0.06 | −7.93 to 7.81 | 0.988 |
| Hospital services | 229 | 33.32 | 9.89 | 270 | 56.48 | 5.12 | −23.16 | −61.63 to 15.30 | 0.230 |
| Mental health services | 226 | 13.55 | 5.32 | 264 | 7.52 | 3.76 | 6.03 | −6.74 to 18.80 | 0.355 |
| Prescribed insomnia medications | 223 | 3.21 | 0.94 | 266 | 1.73 | 0.36 | 1.48 | −0.45 to 3.41 | 0.136 |
| NHS and PSS | 218 | 69.69 | 11.45 | 256 | 88.95 | 19.70 | −19.26 | −64.18 to 25.66 | 0.398 |
| Non-NHS and PSS | 205 | 5.44 | 1.74 | 243 | 8.94 | 2.63 | −3.50 | −9.62 to 2.62 | 0.268 |
| Productivity losses | 212 | 970.81 | 136.01 | 250 | 1372.84 | 155.00 | −402.03 | −800.02 to −4.04 | 0.052 |
| Total cost over 12-month period (complete case for entire period) | |||||||||
| Total NHS and PSS cost | 192 | 182.13 | 26.56 | 229 | 189.16 | 28.90 | −7.04 | −82.29 to 68.22 | 0.858 |
| Total NHS and PSS cost and intervention cost | 186 | 268.26 | 27.17 | 229 | 190.86 | 28.90 | 77.39 | 2.91 to 151.87 | 0.047 |
| Total NHS and PSS cost and intervention cost with reduced SRT training cost to £2.52 | 186 | 236.56 | 27.17 | 229 | 190.86 | 28.90 | 45.69 | −30.53 to 121.92 | 0.250 |
| Total societal costa | 142 | 2176.35 | 298.83 | 180 | 2676.03 | 306.89 | −501.38 | −1312.76 to 313.40 | 0.244 |
| EQ-5D at follow-up points | |||||||||
| EQ-5D-3L value at baseline | 321 | 0.704 | 0.015 | 321 | 0.723 | 0.014 | − | − | - |
| EQ-5D-3L value at 3-month follow-up | 245 | 0.724 | 0.018 | 284 | 0.684 | 0.016 | 0.040 | −0.009 to 0.089 | 0.105 |
| EQ-5D-3L value at 6-month follow-up | 233 | 0.718 | 0.018 | 281 | 0.722 | 0.015 | −0.004 | −0.049 to 0.041 | 0.857 |
| EQ-5D-3L value at 12-month follow-up | 223 | 0.722 | 0.018 | 266 | 0.721 | 0.014 | 0.001 | −0.045 to 0.047 | 0.969 |
| QALYs for different periods | |||||||||
| QALYs (baseline to 3 months) | 245 | 0.180 | 0.004 | 284 | 0.176 | 0.004 | 0.004 | −0.007 to 0.015 | 0.471 |
| QALYs (3–6 months) | 218 | 0.181 | 0.008 | 267 | 0.177 | 0.007 | 0.004 | −0.007 to 0.015 | 0.487 |
| QALYs (6–12 months) | 209 | 0.363 | 0.007 | 258 | 0.361 | 0.009 | 0.007 | −0.020 to 0.024 | 0.85 |
| QALY (baseline – 12 months based on available EQ-5D-3L data at all time points) | 202 | 0.729 | 0.017 | 249 | 0.719 | 0.013 | 0.01 | −0.033 to 0.054 | 0.642 |
Mean NHS and PSS costs (excluding intervention costs) for the 12-month period were similar between the SRT and SH groups (£189.16 vs. £182.13). After including intervention costs, mean NHS and PSS costs for the SRT arm were significantly higher than the SH arm (£268.26 vs. £190.86).
Cost-effectiveness and cost–utility analysis
Table 31 reports results from the base-case cost–utility analysis, sensitivity analyses and secondary exploratory analyses. The base-case analysis was conducted from an NHS and PSS perspective and used NHS and PSS costs and QALYs (obtained from the EQ-5D-3L) over the 12-month follow-up, applying multiple imputation for missing data and controlling for baseline characteristics. It generated incremental costs of £43.59 (95% CI −18.41 to 105.59) and incremental QALYs of 0.021 (95% CI 0.0002 to 0.042) associated with SRT relative to SH. This resulted in a mean ICER of £2076 per QALY gained. The probability that the SRT is cost-effective at the NICE cost-effectiveness threshold of £20,000 per QALY gained was 95.3%, with a mean NMB of £377.84. The probabilities that SRT is cost-effective at the NICE cost-effectiveness threshold of £15,000 and £30,000 per QALY were 94.4% and 96.2%, with respective mean NMBs of £272.12 and £589.28. The cost-effectiveness plane (Figure 4) displays graphically the uncertainty surrounding the mean ICER estimate, while the CEAC (Figure 5) summarises the effects of uncertainty surrounding the value of the cost-effectiveness threshold.
FIGURE 4.
Cost-effectiveness plane representing bootstrapped mean differences in costs and QALYs for SRT compared with SH (NHS and PSS costs and QALYs based on EQ-5D-3L).
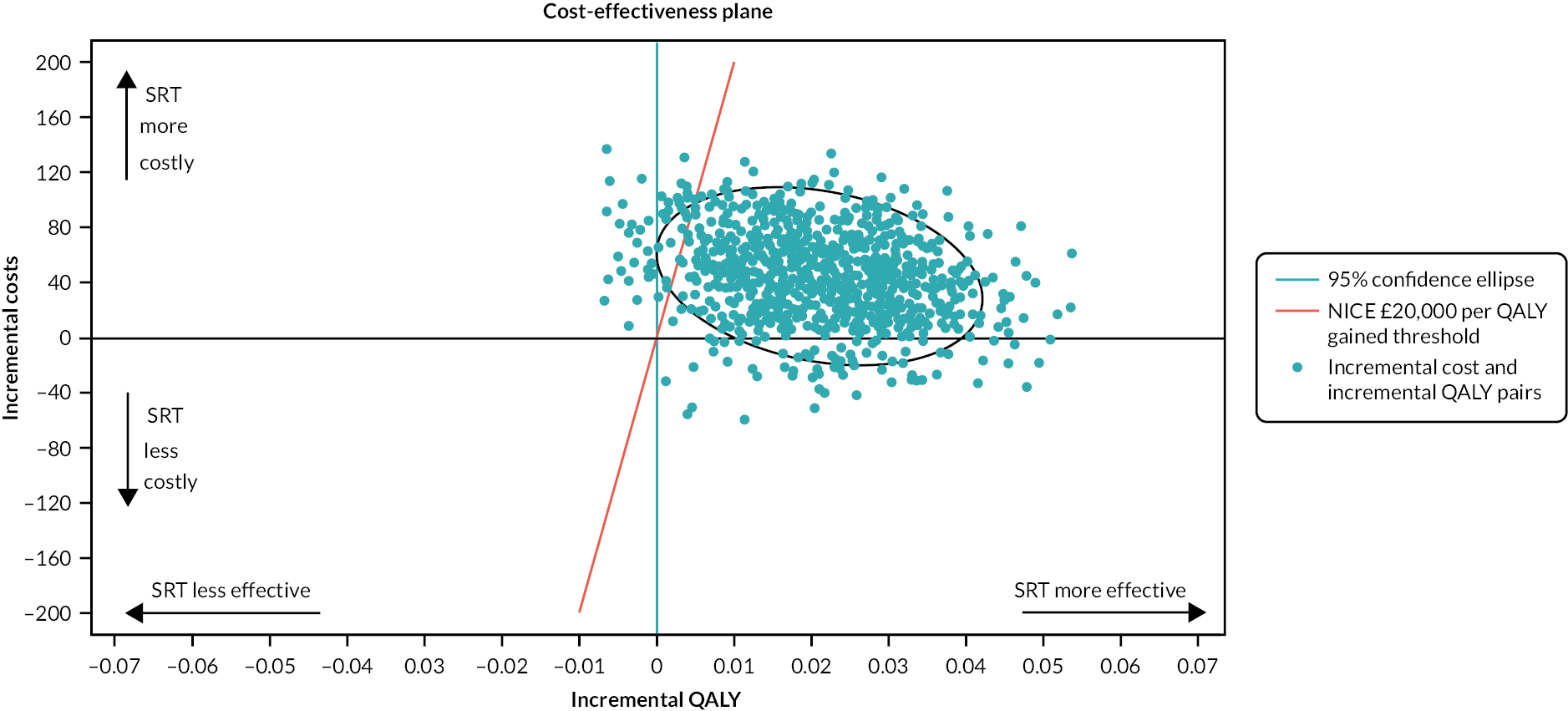
FIGURE 5.
Cost-effectiveness acceptability curve for the base-case analysis (NHS and PSS costs and QALYs based on EQ-5D-3L).
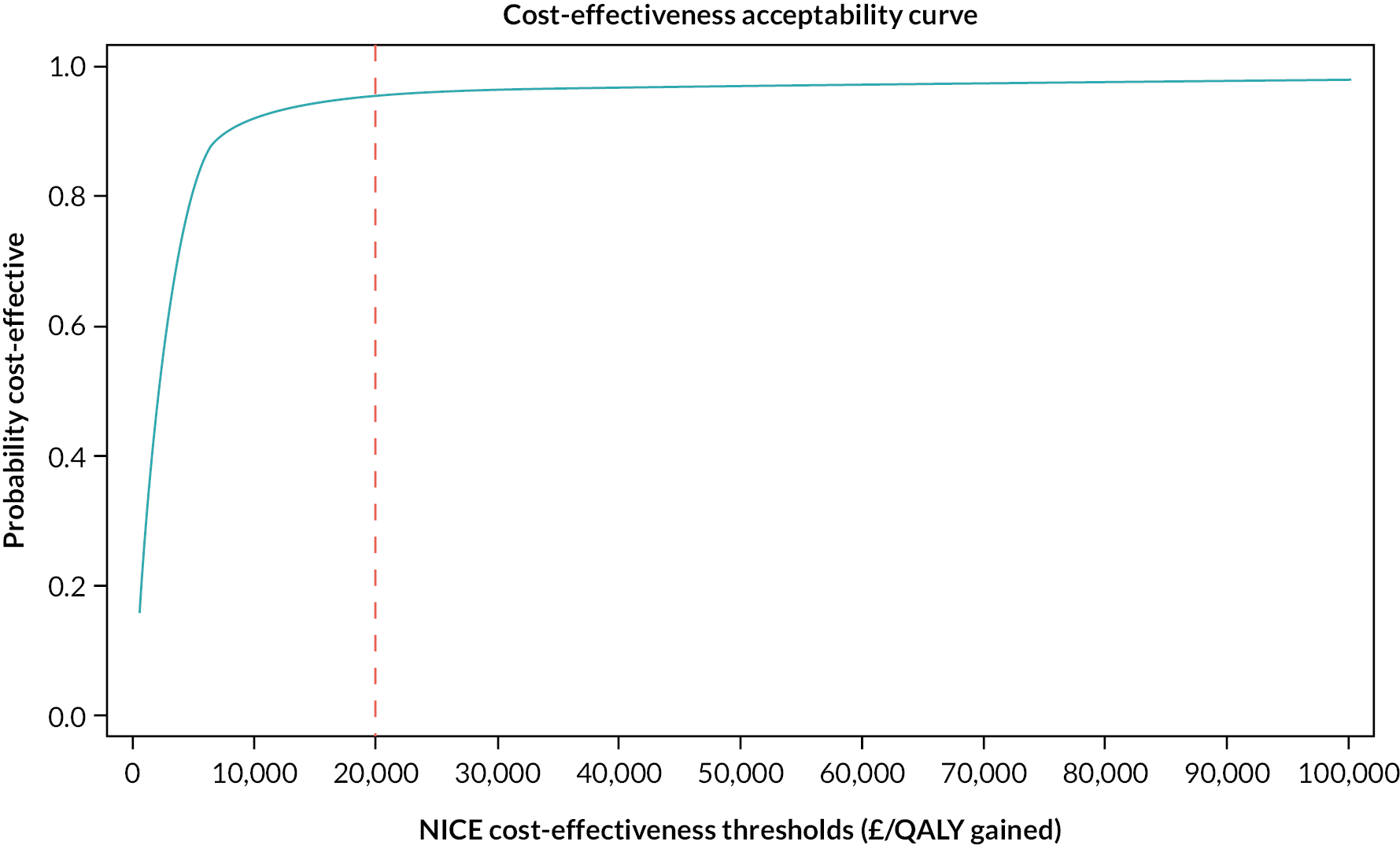
| Scenario | Mean cost (£) (SE) | Mean QALY (SE) | ICER (£) | Probability that SRT is cost-effective at NICE cost-effectiveness thresholds | Mean NMB (95% CI) at NICE cost-effectiveness threshold | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SRT | SH | Incremental cost (bootstrap 95% CI) | SRT | SH | Incremental QALYs (bootstrap 95% CI) | £15,000 (%) | £20,000 (%) | £30,000 (%) | £15,000 | £20,000 | £30,000 | ||
| Scenario | Mean cost (£) (SE) | Mean ISI score (SE) | ICER (£) | Probability that SRT is cost-effective at arbitrary cost-effectiveness thresholds | Mean NMB (95% CI) at arbitrary cost-effectiveness threshold | ||||||||
| SRT | SH | Incremental cost (bootstrap 95% CI) | SRT | SH | Incremental ISI score (bootstrap 95% CI) | £15 | £30 | £50 | £15 | £30 | £50 | ||
| Base-case analysis | |||||||||||||
| NHS and PSS cost and QALYs based on EQ-5D-3L [multiple imputation (n = 50); covariates adjusted]a | 266.00 (25.55) |
222.41 (26.24) |
43.59 (−18.41 to 105.59) |
0.723 (0.008) |
0.702 (0.008) |
0.021 (0.0002 to 0.042) |
2076 | 94.4 | 95.3 | 96.2 | 272.12 (261.59 to 282.65) |
377.84 (364.06 to 391.62) |
589.28 (568.96 to 609.60) |
| Sensitivity analyses | |||||||||||||
| NHS and PSS cost and QALYs based on EQ-5D-3L (complete-case analysis; covariates adjusted) | 252.35 (28.41) |
199.23 (25.45) |
53.12 (−20.00 to 126.23) |
0.742 (0.009) |
0.726 (0.008) |
0.017 (−0.007 to 0.041) |
3125 | 85.8 | 88.0 | 89.7 | 207.96 (195.93 to 219.98) |
295.02 (279.23 to 310.81) |
469.15 (445.77 to 492.52) |
| Societal cost and QALYs using EQ-5D-3L [multiple imputation (n = 50); covariates adjusted] | 2340.62 (189.56) |
3426.75 (186.21) |
−1086.13 (−1485.59 to −686.67) |
0.723 (0.008) |
0.702 (0.008) |
0.021 (0.0003 to 0.042) |
Dominates | 100 | 100 | 100 | 1404.30 (1387.82 to 1420.77) | 1510.54 (1491.78 to 1529.30) | 1723.03 (1698.99 to 1747.06) |
| NHS and PSS cost using £2.52 as SRT training cost and QALYs using EQ-5D-3L [multiple imputation (n = 50); covariates adjusted] | 236.82 (25.55) |
222.41 (26.24) |
14.41 (−47.59 to 76.41) |
0.723 (0.008) |
0.702 (0.008) |
0.021 (0.0002 to 0.042) |
686 | 96 | 96.30 | 97 | 301.30 (290.77 to 311.83) |
407.02 (393.24 to 420.80) |
618.46 (598.14 to 638.78) |
| Secondary analyses | |||||||||||||
| NHS and PSS cost and QALYs based on EQ-5D + Sleep [multiple imputation (n = 50); covariates adjusted]b | 266.22 (25.55) |
222.20 (26.23) |
44.02 (−17.93 to 105.97) |
0.789 (0.005) |
0.767 (0.005) |
0.022 (0.010 to 0.034) |
2001 | 99.6 | 99.9 | 100 | 281.58 (275.32 to 287.84) |
390.58 (382.51 to 398.64) |
608.57 (596.83 to 620.31) |
| NHS and PSS cost and QALYs based on SF-6D [multiple imputation (n = 50); covariates adjusted]c | 266.19 (25.55) |
222.23 (26.23) |
43.95 (−17.88 to 105.78) |
0.666 (0.004) |
0.642 (0.004) |
0.025 (0.015 to 0.034) |
1758 | 100 | 100 | 100 | 324.36 (319.35 to 329.37) |
447.58 (441.19 to 453.97) |
694.03 (684.81 to 703.25) |
| NHS and PSS cost and QALYs based on EQ-5D-3L (per-protocol analysis) [multiple imputation (n = 50); covariates adjusted] | 257.25 (25.67) |
220.86 (26.17) |
36.40 (−27.72 to 100.52) |
0.722 (0.009) |
0.703 (0.008) |
0.019 (0.00009 to 0.038) |
1916 | 92.3 | 94.9 | 95.9 | 256.72 (247.01 to 266.43) |
353.97 (341.28 to 366.67) |
548.47 (529.76 to 567.19) |
| NHS and PSS cost and QALYs based on EQ-5D-3L (6-month follow-up) [multiple imputation (n = 50); covariates adjusted] | 198.37 (18.36) |
126.60 (17.01) |
71.76 (31.94 to 111.59) |
0.362 (0.004) |
0.347 (0.004) |
0.015 (0.006 to 0.025) |
4784 | 97.6 | 98.7 | 99.8 | 158.38 (153.47 to 163.28) |
235.13 (228.74 to 241.53) |
388.65 (379.24 to 398.05) |
| NHS and PSS cost and ISI improvement between baseline and 12-month follow-up [multiple imputation (n = 50); covariates adjusted] | 270.68 (29.41) |
226.24 (27.91) |
44.43 (−28.93 to 117.80) |
−6.90 (0.32) |
−3.74 (0.29) |
−3.16 (−4.04 to −2.28) |
14 | 52.1 | 88.1 | 99.7 | £1.95 | £49.51 | 112.92 |
All sensitivity analyses confirmed the robustness of the result that SRT is likely to be cost-effective at a cost-effectiveness threshold of £20,000 per QALY (see Table 31 and Figures 6–11). Indeed, when the societal perspective was used for the analysis, SRT cost less and generated more QALYs, on average, and so dominates SH in health economic terms. When the SRT training cost was reduced to £2.52 from £31.7, the ICER reduced to £686 per QALY gained (from £2076 in the base-case analysis).
FIGURE 6.
Cost-effectiveness plane: bootstrapped mean differences in costs and QALYs (NHS and PSS costs and QALYs based on EQ-5D-3L, complete-case analysis).

FIGURE 7.
Cost-effectiveness acceptability curve (NHS and PSS costs and QALYs based on EQ-5D-3L, complete-case analysis).

FIGURE 8.
Cost-effectiveness plane: bootstrapped mean differences in costs and QALYs (societal costs and QALYs based on EQ-5D-3L).

FIGURE 9.
Cost-effectiveness acceptability curve (societal costs and QALYs based on EQ-5D-3L).

FIGURE 10.
Cost-effectiveness plane: bootstrapped mean differences in costs and QALYs (NHS and PSS costs using £2.52 as SRT training costs, and QALYs based on EQ-5D-3L).

FIGURE 11.
Cost-effectiveness acceptability curve (NHS and PSS costs using £2.52 as SRT training costs, and QALYs based on EQ-5D-3L).

Secondary analyses (see Table 31 and Figures 12–20) also demonstrated that SRT is likely to be cost-effective when other utility measures were used, applying per-protocol analysis (those attending at least one treatment session), and using data for 6 months follow-up only. When the cost–utility analysis was repeated for the SF-6D and EQ-5D-3L, the point estimates of cost-effectiveness were very similar to those using the EQ-5D-3L, although the 95% CIs were smaller. After taking account of uncertainties, the SF-6D produced a stronger conclusion; that SRT has a 100% probability of being cost-effective at the cost-effectiveness threshold of £20,000 per QALY. The probability of cost-effectiveness when using the EQ-5D-3L + Sleep was also higher than that of the EQ-5D-3L (99.9% probability of being cost-effective at a cost-effectiveness threshold of £20,000 per QALY).
FIGURE 12.
Cost-effectiveness plane: bootstrapped mean differences in costs and QALYs (NHS and PSS costs and QALYs based on EQ-5D-3L + Sleep ‘bolt on’).

FIGURE 13.
Cost-effectiveness acceptability curve (NHS and PSS costs and QALYs based on EQ-5D-3L + Sleep ‘bolt on’).

FIGURE 14.
Cost-effectiveness plane: bootstrapped mean differences in costs and QALYs (NHS and PSS costs and QALYs based on SF-6D).

FIGURE 15.
Cost-effectiveness acceptability curve (NHS and PSS costs and QALYs based on SF-6D).
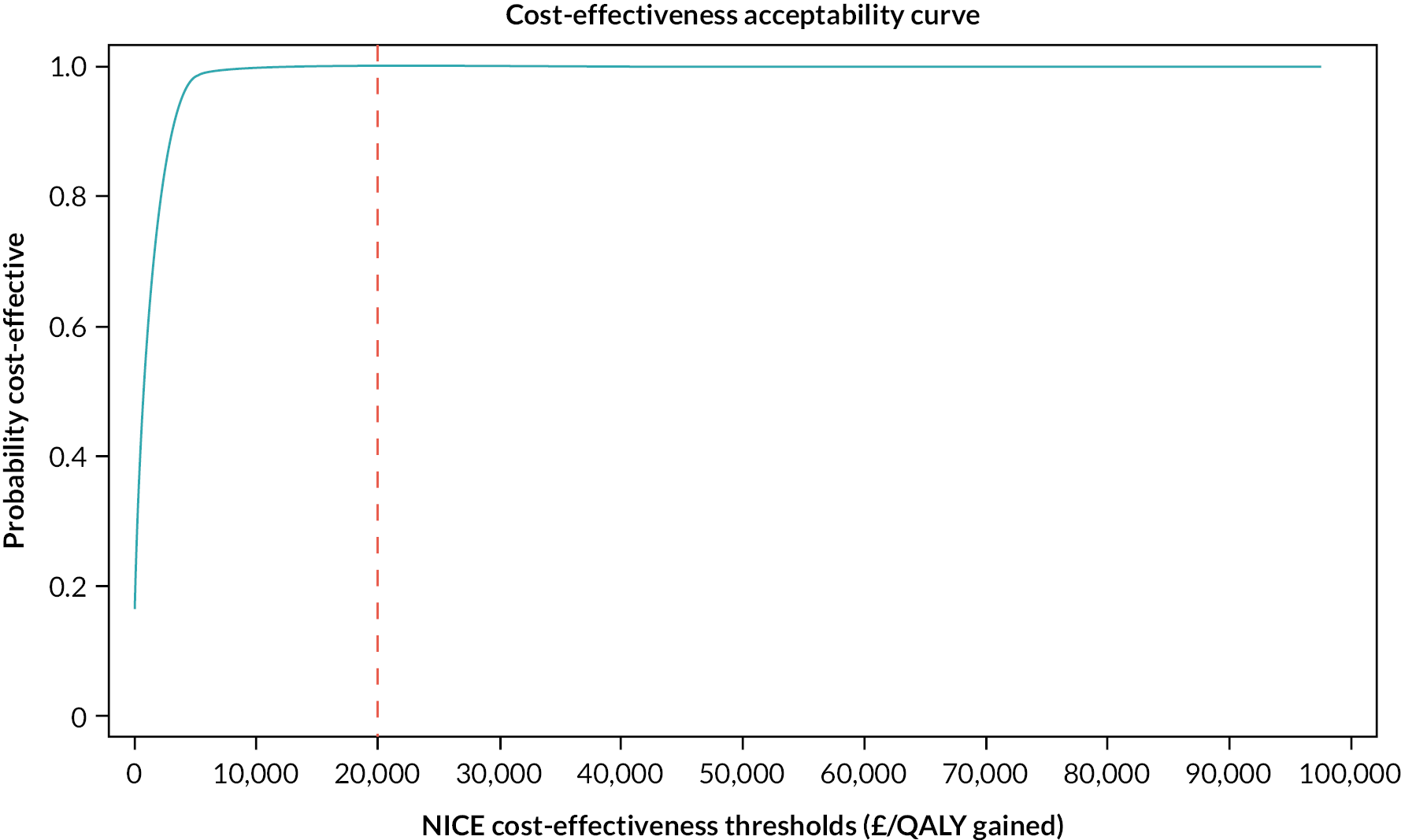
FIGURE 16.
Cost-effectiveness plane: bootstrapped mean differences in costs and QALYs (NHS and PSS costs and QALYs per-protocol analysis).
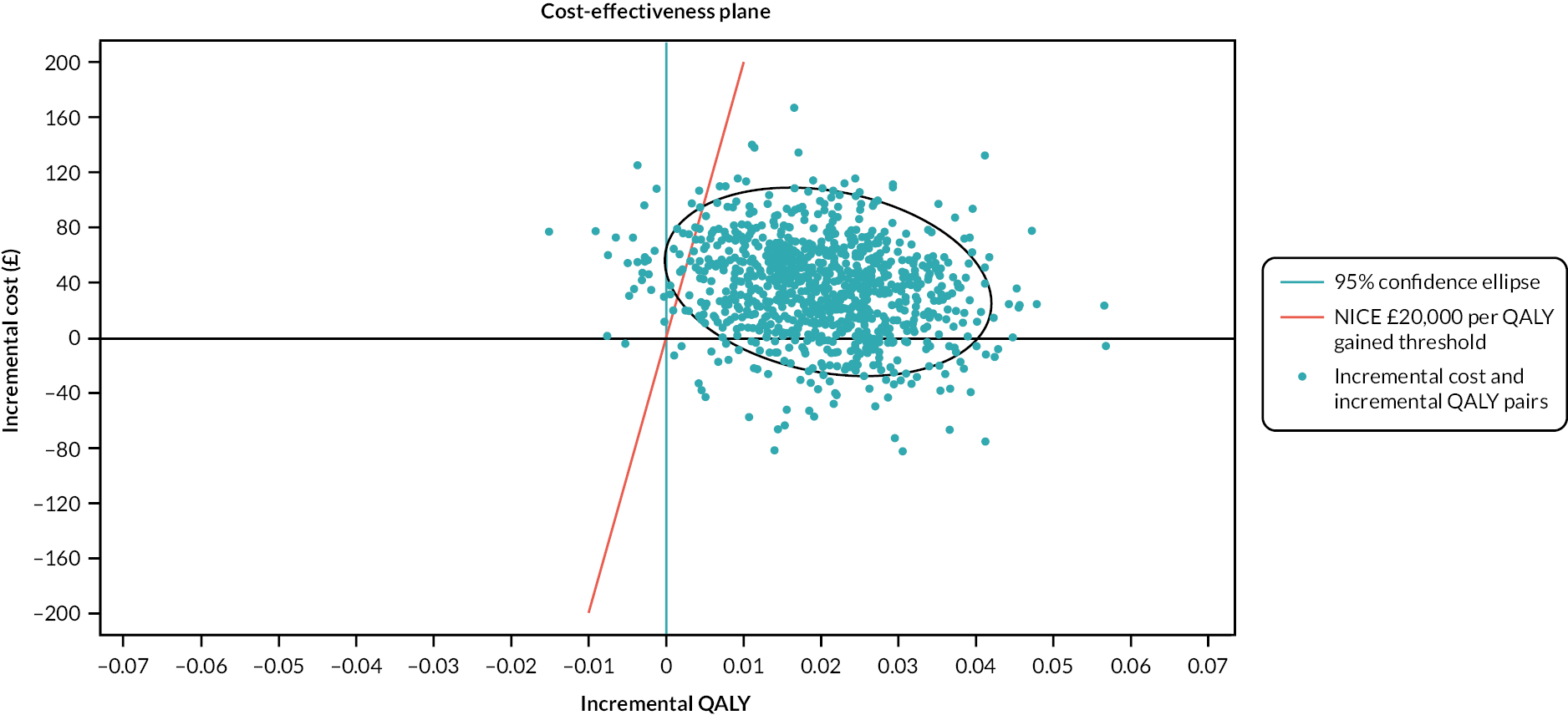
FIGURE 17.
Cost-effectiveness acceptability curve (NHS and PSS costs and QALYs per-protocol analysis).

FIGURE 18.
Cost-effectiveness plane: bootstrapped mean differences in costs and QALYs (NHS and PSS costs and QALYs at 6-month follow-up).

FIGURE 19.
Cost-effectiveness acceptability curve (NHS and PSS costs and QALYs at 6-month follow-up).

FIGURE 20.
Cost-effectiveness plane: bootstrapped mean differences in costs and QALYs (NHS and PSS costs and reduction of ISI scores between baseline and 12-month follow-up).

Furthermore, restricting the time horizon of the economic evaluation suggested that SRT remains cost-effective over 6 months post randomisation (ICER of £4784), but not as cost-effective as over 12 months post randomisation.
The ICER was estimated at £14 per unit reduction in ISI when reduction of ISI score was used as the measure of effectiveness. At a hypothetical cost-effectiveness threshold of £30 per unit reduction in ISI, SRT had a probability of cost-effectiveness of 88.1%.
For treatment response (defined as ISI reduction of ≥ 8 points), the mean incremental cost was £44.23 and the mean incremental probability of a treatment response was 0.26 with an ICER of £170, indicating a mean incremental cost of SRT of £170 is required to achieve a clinically relevant treatment response.
Conclusion
The economic analysis within the HABIT trial evaluated the cost–utility of SRT compared with SH in the NHS primary care setting. The analysis quantified the mean cost of SRT as £84, although the initial training costs are likely to reduce following scaling-up of the intervention, or delivery via alternative methods. Further implementation research is needed to consider how nurse-delivered SRT would operate in practice. For example, nurses were trained by experienced members of the research team but in practice we envisage that training and ongoing support could be provided by a clinical psychologist, or mental health professional with experience in sleep disorders and cognitive–behavioural approaches.
The primary cost–utility analysis used EQ-5D-3L-derived QALYs and NHS and PSS costs over the 12-month follow-up. Based on available data, the SRT arm produced a mean QALY of 0.73 versus 0.72 for the SH arm. After data imputation and adjustment for baseline covariates, the mean difference in QALYs between the two arms over the 12-month follow-up period was estimated at 0.02. Although the mean QALY gain is not large, the ICER was estimated at £2076 per QALY gained, which suggests great potential for SRT to be cost-effective at the NICE £20,000 per QALY cost-effectiveness threshold. Further exploration of the decision uncertainty around the estimate of mean ICER showed that SRT has a 95.3% probability of being cost-effective at a £20,000 cost-effectiveness threshold, and indeed a 94.4% probability of being cost-effective at a £15,000 cost-effectiveness threshold. Sensitivity analysis using the available sample with no imputation also confirmed the cost-effectiveness of SRT, although the mean ICER was larger relative to the baseline analysis that applied multiple imputation. When adjusting the SRT training cost, the mean ICER decreased from £2076 to £686. SRT had the effect of reducing productivity-related losses, which was reflected in the sensitivity analysis that adopted a societal perspective where SRT dominates SH.
The exploratory analysis using QALYs derived from the EQ-5D-3L and the SF-6D confirmed the conclusion that SRT is highly likely to be cost-effective, and the probabilities of SRT being cost-effective are higher using the EQ-5D-3L + Sleep and the SF-6D than using the EQ-5D-3L. Restricting the economic evaluation to a 6-month time horizon also confirms that SRT is highly likely to be cost-effective, but not as cost-effective as when a longer time horizon is adopted.
Chapter 5 Results process evaluation
This chapter uses material from an Open Access article previously published by the research team (see Armstrong et al., Br J Gen Pract 2024;74:e34–40. https://doi.org/10.3399/BJGP.2023.0162 https://doi.org/10.3399/BJGP.2023.0162). This article is published under licence to British Journal of General Practice. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
We conducted a process evaluation of the HABIT SRT intervention in line with the UK Medical Research Council (MRC) process evaluation framework in order to understand intervention delivery, fidelity, and acceptability from the perspective of patients, PNs and GPs or practice managers. 57 Process evaluations are recommended in trials of complex interventions and, in this study, we aimed to explore how nurse-administered SRT in primary care worked, by examining implementation, mechanisms of impact, and contextual factors. Implementation explores how the intervention is delivered and what is delivered. It includes the training and resources available to the intervention team as well as the fidelity of delivery and any adaptations to delivery. Mechanisms of impact explore participants’ reactions to the intervention, including perceived benefits as well as unintended or adverse effects. Finally, contextual factors can affect implementation and help us to understand the potential for sustaining and scaling the intervention more widely.
Methods
Design
The process evaluation used a mixed methods design, integrating data from qualitative interviews and quantitative data collected from intervention participants.
Qualitative interviews
Semistructured interviews were undertaken with patients who had received SRT, the nurses delivering the intervention and the practice managers or GPs at the practices involved. The interview schedules can be found on the NIHR project page. The interview schedules were developed using the three key themes of the MRC process evaluation framework, namely (1) implementation – did the patient understand what was being asked of them; (2) mechanism of impact – how did the patient feel about the intervention and (3) context – how easy was it for individuals to integrate the intervention and how sustainable was it. Similarly, the interview schedules for the nurses and practice managers/GPs sought to understand how well the intervention met their needs and could be integrated into practice.
We aimed to interview 15 patients, five per region in the three areas, Thames Valley, Greater Manchester and Lincolnshire, where the trial took place. Patients were asked during their baseline assessment appointment for consent to be interviewed. Interviewees were selected from the list of patients who consented and completed the SRT intervention < 6 months prior to the interview. In addition, participant sex and age were considered to ensure that a wide range of participants were selected for interview. We also interviewed some participants who were close to their 6-month outcome assessment, which was important as it allowed us to determine how they felt about longer-term adherence to the intervention. Nurses from all three regions were also interviewed. Finally, practice managers or GPs from each participating practice were invited for interview, and those who consented were asked about their perceptions of impacts on the practice and the sustainability and scalability of the intervention. All interviews took place by telephone and were digitally recorded and transcribed. Interviews were conducted by two trained and experienced non-clinical academic qualitative researchers (JP, SA).
Quantitative data
We compared patient interviewees’ qualitative perceptions of the intervention with two quantitative measures. These were baseline ISI and the SE recorded at baseline with a 7-day sleep diary, and during each week of the nurse-delivered intervention. ISI is a seven-item self-reported questionnaire, scoring between 0 and 28, which assesses the severity, nature and impact of insomnia, while SE is the time asleep divided by time in bed multiplied by 100 (to give a % value), which generally increases in participants for whom SRT is successful.
Data analysis and integration
Qualitative interview data were examined using Framework analysis supported by NVivo 12. Two members of the research team (SA and JP) undertook the interviews and checked the transcripts, which were transcribed by an independent service. Through familiarisation with the transcripts, examination of the interview schedules and the three key domains of the MRC Framework, an a priori set of categories was developed to form the basis of the framework.
Transcripts were then coded independently (by SA and JP) and codes categorised using NVivo 10. The interviews proved to be a rich source of data and therefore an ‘other’ category was included in the framework to ensure that relevant data that did not readily fit into the framework would not be lost. While the categories were applicable to each of the groups interviewed (nurse, patient and PM/GP), the codes were specific to each group as outlined in A. Three members of the research team (SA, JP and NS), one of whom was independent of the initial analysis, agreed the final themes presented in the results.
Joint display
Relationships between qualitative findings, notes that nurses made during treatment sessions, and quantitative measures were explored and presented using a joint display. This table allowed us to directly compare the patients’ perceptions of SRT with any noted changes to their sleep as measured by changes in their SE and nurse reflections following treatment sessions (Table 32).
| Area | Baseline ISI | Baseline SE | SE at sessions 2, 3, 4 | Nurse record summary | Patient perspectives |
|---|---|---|---|---|---|
| A | 16 | 64 | 90, 92, 96 | Largely positive participant has coped well with intervention and has shown marked improvement. | I think I noticed it really quickly within a couple of days. Because my sleep is always broken, I would go to sleep, and I would continually wake up; but I was going to sleep and then waking up with my alarm clock. I think that happened from day 3 onwards. And to me that hadn’t happened in years. I’ve never been woken by my alarm clock. |
| 22 | 57 | 91, 95, 95 | Participant found the intervention hard to start with but showed improved sleep patterns by session 4. | Although it took down the amount of times I was waking up, I don’t really think it helped my quality of sleep that much. I was still feeling tired during the day, so I never napped during the day; it’s something I don’t do. I’m still feeling tired by about mid-afternoon. But I soldiered on through and kept going with it. So, it basically got down into the fourth week when I’d gain an hour and a half; was going to bed earlier, but I was still getting up, still waking up, one or two times a night. It wasn’t normally for very long. | |
| 16 | 54 | 59, 57, 57 | Participant found the intervention very difficult to maintain, especially the ‘going to bed’ time. | I’m obviously quite a bad sleeper anyway. But by the third week, I could understand what the therapy was all about. It was obvious to me that this was going to work for some people. I can’t say it was working for me. It did some nights, but some it didn’t. | |
| 21 | 76 | 89, 90, 82 | Patient took sleep medication that interfered with later ‘going to bed’ time, leaving them feeling drowsy and tired. | The going to bed was fine, and I stuck to that really well. And then the getting up, I succeeded most of the time, because I’m on trazadone, that sedates me for about a 9-hour duration, from the dose I’m on; therefore, if I’ve only got 6 hours in bed, that sedated effect is going to continue longer than that, and that made it very difficult. I did succeed in waking up at that 6 o’clock time, almost every time though. | |
| 16 | 72 | 84, 82, 82 | No notes available. | It didn’t particularly work well for me, because I think my sleeping problems were menopause-related, and I don’t think it worked particularly well. I think that was the cause. So, the nurse was great; very positive; the meetings were good; so, I was clear what was happening. | |
| B | 25 | 66 | 79, 82, 82 | Participant struggled with new wake-up times but showed some improvement. | My hardest bit was the getting up at the time she wanted me to get up; and I couldn’t do that. I was getting up way too early; way too early. And then all of a sudden, bang, it stopped, and I reverted back. If I’m honest, I think it was a waste of time for me because it was only 4 weeks. If it had been a lot longer, then I think going around my head, I said it was all psychological, I think I would have been able to get my mind really in a mindset; but after 4 weeks, no, that was it. |
| 8 | 79 | 84, 86, 86 | No notes available. | I think if you look at the whole 4 weeks, it’s quite … explaining I’m a light sleeper etc., yeah, I was getting longer periods of sleep. If it was only an hour and a half before I woke up; on occasions 2 or 3 hours; that was good for me because generally speaking, if I was to take an average, a normal week without doing the programme, it is at least six or seven times a night that I wake up, and then I have a difficulty getting back to sleep. So that element of it certainly worked, yes. | |
| 13 | 78 | 92, 96, 93 | No notes available. | Like I said, the first week, I was struggling, and I was getting anxious because I wanted to go to bed. I felt – No come on, do something. I was quite strict on myself because I thought – It’s going to be for the better for you. So, I did shout at myself. | |
| 18 | 75 | 88, 81, 87 | Participant continued to wake multiple times at night but overall showed an improvement. | … initially the restriction was quite severe wasn’t it? We start at 6 hours of sleep. Or I don’t think I was tired. I do like to nap. I didn’t nap during the whole of the 4-week period, and actually beyond. And yet, I didn’t feel as though I was going to need to. | |
| 17 | 53 | 82, 82, 86 | Participant initially had increased tiredness but gradually this improved. | I think I got something out of it: sometimes I find it easier to go back to sleep when I’ve woken up. Other times I haven’t, I’ve found it quite hard. | |
| C | 21 | 66 | 52, nr, 83 | Sleep being disturbed by external influence; however, overall showed some improvement. | Certainly, from my perspective, it has improved my sleep quality, whether we can extend the sleeping hours a little bit, I don’t know; food for thought for the future, but regarding other people, I would certainly recommend that they try something because you can’t continue being sleepless; It’s to your detriment in the long term. |
| 16 | Missing | 100, 89, 86 | Participant struggled with later ‘going to bed’ time as felt too tired to stay up but some improvement in quality of sleep mentioned. | Well, I don’t know if it was just catching up with my sleep, you know. The first week I did sleep really well because I don’t think I was given enough sleep. So, I don’t know, it’s really difficult to say, but the second week and third week, I felt exhausted. Really exhausted, so. Then felt alright again this week. | |
| 18 | 62 | 93, 96, 96 | Participant struggled with not napping in the afternoon. Reduced nap time to 20 minutes and has shown some improvement overall. | I’ve done as requested. You know, gone to bed when I should, got up when I should, but I still fall asleep in an evening, sometimes I’ve just gone, do you know what I mean? And my head, in the beginning, was feeling terribly woolly: sometimes didn’t think it belonged to me. | |
| 22 | 50 | 95, 92, 94 | Found first week difficult but once settled in routine slept better and had more energy during the day. | I mean the first week, getting up, the alarm used to go off and I’d think – oh god, now I’ve got to get out of bed. But I’ve sort of forced myself to do it. Because I thought – If this is going to work, I’m going to have to stick to it. And I did. I mean after you get over that initial first week, you start to feel the benefits of it. | |
| 5 | 77 | This participant did not complete all four SRT sessions due to an underlying health condition that was diagnosed during the intervention. | It wasn’t actually my, well it was my decision as such, but it was the nurse actually who said – I really don’t think you should be on this. Because we had met up, I think it was for the last time, and we were talking about sleep routine, and looking again at my sleep diary, and she said I really don’t think it’s anything to do with how much sleep you are getting, it must be something else. And I was taking to her about how I feel in the morning, what time I was going to bed, the routine that she had told me to follow, and that is when she said I should get a blood test, rather than do the sleep clinic. | ||
Results
The initial aim was to recruit five practices per region with equal numbers of interviews of patients, nurses and practice managers or GPs from each. We interviewed 16 patients, 13 nurses and 7 practice managers or GPs. Interviews were conducted by telephone and were 30–60 minutes in duration. Patients ranged in age from 19 to 74 (mean 56) years, including 7 male and 9 female interviewees, all of whom identified as White British. Patients are designated in the results by region (A, B, C), gender (M, F) and age, for example Patient AF57. Nurses are designated by region and whether they were a Clinical Research Network (CRN) nurse or a practice nurse (PN).
Due to lack of availability of nurses at specific practices two regions utilised research nurses (employed by their LCRN) rather than practice nurses. ICRN research nurses covered more than one practice and therefore 13 nurse participants were interviewed. Finally, in two regions practices formed consortia, with several practices falling under one management group, so seven interviews were undertaken in the practice manager (six interviews) or GP (one GP) category.
Themes are listed under implementation, mechanisms of impact and contextual factors.
Implementation of sleep restriction therapy
Patients lacking experience of behavioural therapy did not know what to expect
Patients did not know what to expect from SRT. Most had no previous experience of behavioural therapy and they had not been offered this type of therapy for insomnia before.
No, it was the only sort of formal treatment I’ve had. I’ve tried things like relaxation, and things like that, but this was the only sort of scientific treatment I’ve had.
Patient: AM57
All the patients hoped for improvements in their sleep pattern and daytime symptoms. They expressed how uncomfortable they felt if they had not slept well.
The only thing I really hoped that would come out of it would be improvement in my sleeping patterns; sometimes, if I have not slept very well, I wake up in the morning and I am really quite dizzy which is very uncomfortable and is horrible.
Patient: CM65
Overall, patients hoped that their SRT would make them feel less tired and more refreshed in the mornings:
I had the hope, rather than the expectation, that it would make me feel better in the morning; I would feel fresh, less tired.
Patient: CF19
Appointment preparation and preferences
Nurses felt prepared and were supported with adequate training and tools enabling them to deliver SRT effectively:
It was quite straightforward, and obviously we were provided with a PowerPoint presentation to go through; so that first initial consultation with them; so that was really helpful.
PN
Both nurses and patients highlighted flexibility in appointments as important, particularly where the patient worked full time.
Yeah, I’ve just had one chap who missed his appointments because it had totally gone out of his head, and I just re-booked him for the next week; and he came to that one.
… they [SRT nurse] were really good and arranged a time to suit me because I work full time.
Patient: CF51
The benefits of face-to-face appointments for nurses and patients highlighted the relevance of non-verbal cues and patients maintaining motivation.
I think the face to face is probably better because you can see a reaction from someone.
Patient: BM74
So, I had a one-to-one meeting with a nurse, and I felt that those are really beneficial for me in terms of maintaining that treatment. For me personally, I don’t think I would have done it without the one-to-one.
Patient: AM57
You absolutely can’t do the first one on the phone. [Although] From a patient perspective, it’s very convenient I guess, because they don’t have to come back to the practice. It’s not the first time I’ve done phone stuff. I don’t mind it. I’ve got to say, maybe I prefer seeing patients, but I think it works fine.
CRN Nurse
Accommodating and tailoring therapy
When patients had difficulty implementing SRT, and particularly where their routines impacted on intervention delivery, nurses were able to modify SRT to the patient.
There’s only one really out of the three, where I think there was a bit more tweaking of the times, if you like, and changing; purely because their routine was different.
CRN Nurse
We tried to come up with a bit of a solution to it because not everybody is the same, so I felt it would be easier for me if I could knock it off in the morning. So, I didn’t mind getting up at 5.30 a.m. rather than staying up.
Patient: AF63
The SRT required individuals to calculate their SE. For some participants, understanding the calculations involved was challenging. Nurses found that they would need to tailor the sessions to individuals, with some sessions being significantly shorter or longer than expected due to the patient’s ability to comprehend the process. However, this did not represent a major deviation from protocol and did not appear to indicate poor implementation.
For someone who isn’t as bright or able to take on information, you then have to amend the way that you are giving that information. I did have to change some of the terminology.
PN
It varied definitely. Some patients were able to engage very quickly, and the sessions could be done within 20–25 minutes because the patients were well engaged, able to understand the maths, able to understand what we wanted from them, how it was going to influence their sleep. Other patients, however, were very surprised about what they were expected to do, finding the concept very difficult.
PN
Negotiating sleep timings
Interviews also highlighted a level of negotiation between the nurse and patient particularly around bed and rise times. Nurses sometimes allowed an extra 15 minutes of time in bed, but the protocol allowed for minor amendments to SRT to support a patient-centred approach, so this flexibility did not compromise fidelity.
And I said I was really struggling to get up at 5 a.m. in the morning at the moment. So, we moved that to 5:15 last week.
Patient: CF51
I want to go to bed! So, we negotiated that way around.
Patient: AF63
I have actually played around with it (flexibility in sleep times) if they had been over 85%; particularly as I have got more used to it. I think initially when you start something; you get worried about how strict you have to be.
PN
Learning to deliver despite complexity of sleep restriction therapy
Initially delivery and understanding of SRT involved a learning curve for both patient and nurse, who often adopted a collaborative approach to learning:
It was a learning curve for both the nurse and myself; between us we worked out what was needed.
Patient: CF65
Nurses felt that the intervention, although quite complex, was easy to deliver with practice.
I think I was probably quite nervous to start with; but I think that is probably like most things, something new, and you do have teething problems when you start anything new, and I don’t know about pressure. I think it was just being very honest with the patients when they first came, and just said that this was at the very beginning, we are going to go slowly, sort of thing, and to just bear with me; and I think everyone is very understanding really, if you are open with them.
PN
Patients indicated when they were able to calculate SE but suggested that simplifying this might help retain people on the intervention.
She took me through the calculations, on what we filled in on the form; so I could have an idea how to work them, but at home it took me a long time to do all the calculations.
Patient: AM57
I expect other people would drop out because it took them a lot of time doing the calculations. It’s quite fiddly. So, if there is a lookup table, or something like that that you could provide, it would make life a bit easier because that was the biggest challenge for me, was doing the calculation.
Patient: AF55
Two of the SRT sessions were delivered over the phone and for some the challenge of the calculations was compounded:
It is very difficult to explain maths over the phone to a patient if they really struggle to understand it.
PN
Challenge of delays
For some nurses there were delays between training and seeing their first SRT patient, which increased the challenge of delivery:
I think that was difficult because to do training and then wait, like quite a long time, till you are actually, physically seeing patients.
PN
In one case, nurses paired up to deliver SRT for their first patient to boost confidence. This was a divergence from the delivery protocol (but agreed by the team in advance) and would only have been problematic if subsequent sessions were delivered by different nurses:
I think myself and S doing it together, we seem to work quite well at this point, but as we get more patients, I think both of us will feel confident enough to do it on our own.
PN
Mechanisms of impact of sleep restriction therapy
We explored causal mechanisms, specifically how the delivered intervention produced change. We were interested in how participants interacted with nurses and responded to SRT and its effects. This was crucial to understanding how the intervention worked.
Self-motivation and effort
Nurses observed that self-motivated patients were more likely to continue with SRT at home and those who put in the effort were more likely to succeed.
The patients that have made it to the end of the study [end of intervention delivery] have taken it upon themselves to continue that process at home. I think it is because the patients that have made it through are self-motivated patients.
CRN Nurse
I did succeed in waking up at that 6 o’clock time, almost every time though; because I was so keen to see those results. I put the effort in, and the will power wasn’t too difficult.
Patient: AM33
Difficulty changing sleep habits
Some patients tried hard to adhere with their SRT but changing their existing sleep habit was challenging.
I used to do 12-hour shifts on a brain injury unit, so I used to come straight home at half eight, get a shower and go straight to bed, because I’d be up for 12 hours probably, the next day. So that’s carried on, now I’m retired, I still like to get into bed half past eight, read … and of course the lady has explained to me that I had to stay up till midnight, and I thought – I’m never going to be able to do this. And I tried my hardest, but that was very difficult for me actually.
Patient: AF63
Experiencing anticipated benefits
Most patients reported that the initial week could be hard but after that they started to feel the benefits, they felt more refreshed, their SE increased, and they were able to fall asleep more quickly and stay asleep.
I mean after you get over that initial first week, you start to feel the benefits of it. I mean physically it hasn’t helped, because my condition, there’s not a cure for, but mentally I’m so much better for it, and it’s worth sticking with and seeing it through.
Patient: CF64
[Sleep efficiency was] [a]bout 70% at the start, and the last sort of eight weeks or so, it has been around 85% mark; so that must be a good sign.
Patient: CM65
Patients noted they fell asleep more quickly than prior to SRT and so spent less time in bed awake. Nurses observed that patients receiving SRT perceived bedtime as a more positive experience and there were changes in perception of sleep:
Frequently could be anything up to an hour or an hour and a half previously, but now down to 15, 20, 25 minutes maximum, most nights before I drop off.
Patient: CM65
She wasn’t having a nap, it was becoming a positive thing because she was looking forward to going to bed; and knowing that when she went to bed, she’d sleep. And even if she woke up, she said, she might wake up once or twice in the night, but she was able to get straight back off to sleep again. So that was good.
CRN Nurse
Patients also noticed a change in their perception of sleep:
Even though I didn’t think my sleeping patterns had changed an awful lot, because of restriction, my perception of it had changed.
Patient: BF60
Continuing support for adverse effects
Patients did report some adverse effects during the initial phases of SRT.
But the second week and third week, I felt exhausted. Really exhausted, so. Then felt alright again this week.
Patient: CF51
I feel I could do with going to bed a bit earlier. I know in the booklet it suggests that you do things, but when you are so tired, you just can’t function.
CF73
Several patients and nurses highlighted the need for continued support following the end of the 4-week therapy.
The way it was expressed to me, and I certainly did, was to keep going, you know, and they had some spare sheets to fill out, and keep that diary going; and to be quite honest, it’s like anything: you start with good intentions, and then it slides off; so I think having more ongoing support over a longer period, probably would have helped me better, than it just coming to an end after 4 weeks.
Patient: AM57
Well for me anyway, 4 weeks wasn’t long enough for me at all … What’s the point? Nobody is going to see it (Sleep diary). And the first thing I did that day was have a nap – I’ve finished now, I can have a nap. So, I have reverted back to having naps now in the day. So, my insomnia at night has got worse.
Patient: BF60
They were a bit like – ‘Where do we go from now?’ … The chap I think was a little bit – ‘Oh!’; a little bit lost, if anything – ‘What do I do now?’; because he has not got anyone to report to at the end of the week. So just reassuring that he would get follow-up at 3 months, 6x months. So, it felt a little bit odd, if I am honest; that that’s it then and we are done.
PN
I sort of did say to her, you know, don’t feel that we are abandoning you completely, there will be follow-ups, and if you’re at any stage really struggling and you want to have a chat about it, then come back to me.
PN
Difficulties maintaining sleep restriction therapy
Patients expressed difficulties with very early rise times and the ability to maintain SRT every day.
Yeah. To do it overall, completely, yes, you know 365 days of the year. For me it is impractical, impossible.
Patient: BM74
My hardest bit was the getting up at the time she wanted me to get up; and I couldn’t do that. I was getting up way too early, way too early.
Patient: BF60
Reasons for withdrawal
Nurses shared opinions of why patients were likely to withdraw from the intervention, which was related to conflicting commitments, tiredness, negative attitudes (in particular, where other commitments were perceived to be impacted) and lack of self-efficacy.
The patients that are kind of like – Oh, yeah. And they go along. And it’s – Well I can’t do it on at Saturday because of this, and I can’t do this or that … or I’m not sure that will work. And I say – Well you know just try. This is your sleep efficiency now, and if we can improve on that, then anything above that number is an improvement, sort of thing. But sometimes when patients withdraw, you are not always surprised.
CRN
So, having the min of 5 hours in bed, and he just said – I can’t go to bed at 1 a.m. He just refused to do it. We talked about setting it back earlier … The first patient was younger, but she had very similar reasons in terms of she always went to bed at a set time.
PN
That was the biggest complaint that he just felt far too tired and didn’t feel he could go about his daily routines and things because of the tiredness. And another lady, said she had been doing this for so many years, ‘I don’t think I can manage with how I am’.
PN
Contextual factors in providing sleep restriction therapy in primary care
Contextual factors include those ‘external to the intervention that may act as a barrier or facilitator to its implementation or its effects’. Practice managers, GPs and nurses all commented on contextual factors, relating to the practicalities of delivery within practices and the facilitators and challenges of sustaining and scaling-up the intervention more widely.
Time constraints and conflicting priorities for nurses
Practice managers were aware that nurses had concerns about time constraints. These included the difficulties of fitting in extended SRT appointments into existing consultation times which were generally shorter. There were also concerns about pre-booking the appointments in advance, again due to lack of time.
The nurse practitioners who are doing the study, they are enjoying doing it, but they are worried about time constraints; and in particular trying to get those four appointments booked in on a weekly basis. And in general practice, that’s very difficult for us.
Practice Manager
But it looks like yeah, nurses can deliver this, it is my perception, if they feel confident and competent to. The only question I’d guess I’d have is how long their appointment slots are because they are going to have to factor that into clinics and stuff. Because if they are set up for 10-minute slots then obviously they need more time than that.
CRN Nurse
Freeing-up general practitioner time
Sleep restriction therapy could free up GP time, because it was an intervention that might stop patients calling into the surgery for sleep medication or to discuss their sleep problems.
Actually, what your argument is, if this works, is that actually I think this is something that GPs would take on board quite readily because actually it’s taking work away from GPs and it’s giving an intervention that will actually free up time, I think, actually free up GP time. So if we can avoid patients phoning in for sleeping tablets, or coming to discuss sleep problems, and sort of following up these patients that goes on and on, if they’ve got an intervention they can do early on, then I think that’s something that GPs would think is a worthwhile thing to do.
GP
Alternative delivery options
Practice staff felt it would be helpful to designate specific times and days for the SRT clinic to be held. This would help staff organise clinics, book patients for appointments and free time for nurses to complete additional administrative tasks associated with SRT delivery. One suggestion was to consider treating SRT like other behaviour-change clinics, including using set weekly times.
The way I see it running is, if we treat it like a behaviour change intervention, just like our weight management courses.
Practice Manager
… even if we did it with our nurses, we really should have said – right, these are the days that we are going to offer it.
Practice Manager
Several practice managers wondered about using other staff members such as healthcare assistants.
We have a very capable HCA, who would be more than capable of actually sitting and going through this with someone; and obviously that would be a lot more cost-effective.
Practice Manager
Small group therapy sessions were also suggested as a means of delivery and a way of optimising nurse time.
If they saw maybe four or five in a group; not to make it too big a group; because then you can’t personalise it, so much. I think it probably be good for the individual patients as well because as a group meeting for that education and going through it, there’s like a bit of a support group there for them as well.
CRN
Don’t know. But for me I think a group environment with a nurse would have been just as effective as the one to one.
Patient: AF57
Practice staff, including GPs, were supportive, but they did have some reservations about time constraints, availability and having set days for clinics. To ensure the intervention could be delivered in routine general practice suggestions were made that SRT is delivered in the format of other behavioural interventions (e.g. smoking cessation and weight-management courses).
Quantitative results and joint display
Most patients interviewed either had an improvement or at least no deterioration in SE. Table 32 displays baseline ISI and SE, and weekly SE during the intervention nurse session together with summary extracts from any notes made by the nurses during the SRT sessions and a ‘representative’ quote from each patient regarding the SRT process. This indicated, not unexpectedly, that participants who found SRT a positive process showed improvements in SE, while those who struggled with SRT did not.
Discussion
The aim of the process evaluation was to establish the experiences and perceptions of patients, nurses, and GPs or practice managers of SRT as part of the HABIT trial and to investigate how SRT was received and delivered, understand why it worked or did not, and explore facilitators or barriers to implementation that may affect wider use of this treatment in primary care, should the intervention prove effective.
Both patients and nurses reported that they were able to quickly grasp the purpose of SRT and the related processes. Patients preferred face-to-face consultations and felt that these helped maintain motivation. Although face-to-face interactions have been found to be preferred in some studies, overall the evidence is lacking that therapeutic alliance, disclosure, empathy, attentiveness or participation differs in face-to-face compared with telephone delivery of psychological interventions. 68 Some patients found calculating SE difficult and felt that they needed help from the nurse, while nurses pointed out that helping someone with maths over the telephone was harder than in person.
All patients interviewed found the first week of therapy difficult, with reduced time in bed and strict bedtime and rising times. This is consistent with previous evaluations of SRT, where participants reported worsening of daytime functioning in the first week, with improvements felt after a period of adjustment. 69,70 Additionally, it has been found that restriction of time in bed, which leads to transient daytime sleepiness and related side effects, outperforms regular bed and rise times without restriction. 25,26 This suggests that while the initial increase in side effects is challenging it may also be a necessary part of the therapy.
In this study there was negotiation between the nurses and the patients regarding sleep times and the need for flexibility, which was supported to some extent by the protocol. 1 Changing ingrained behaviours, in this case fixed night-time (or daytime nap) routines, was challenging and the flexibility on the part of the nurses allowed patients to feel some level of control. The flexibility built into the protocol meant that these did not affect the fidelity of delivery. Fidelity was found to be high by the independent reviewer. One nurse interviewed did mention sharing delivery of the intervention with a colleague for one patient, which would only be problematic if inconsistent advice was given.
Participants reported adverse effects such as increased tiredness, ‘exhaustion’ and worries about driving, which have been found in other studies. 69,70 For some, these experiences led them to discontinue SRT (Table 8). Other reported confounding factors, external to the intervention, included sleep disturbance due to menopause symptoms and the use of sleep aids (such as sedatives) which extended the allocated sleep time. These factors should be considered in the future rollout of SRT.
Patients who experienced improved SE also reported concerns, most commonly that 4 weeks of SRT was not long enough. All participants found the first week of the intervention very difficult as their body adjusted to limited time in bed. By the third week some were seeing significant benefits. For example, one participant spoke of being woken by their alarm for the first time in years. Others only started to see benefits by the final week and as such felt the loss of support at the end of the intervention had a direct impact on their motivation to continue. Those who saw improvements earlier tended to be more likely to continue after the final therapy session, while those that felt the benefit later were more likely to revert to previous habits. One patient reported taking a nap in the afternoon the day after the final session and that they quickly reverted to their previous habits as there was no-one ‘watching over them’ any more. This is reflected in the joint display (see Table 32), where comparisons between recorded improvements in sleep and the qualitative data suggest that participants who felt they were better able to apply the SRT intervention showed more improvement than those who struggled or needed more time and support. This is a significant finding that indicates the importance of individual/personalised delivery with regular check-ins continuing for some until the new habits and sleep patterns have been reinforced.
Previous research suggested it was possible for a single GP to deliver a modified version of SRT in general practice to patients without comorbidity,28 and this study confirmed that it was possible for nurses to deliver the intervention in a consistent manner across multiple practices. Practice managers and GPs also agreed that the intervention could be successfully delivered by nurses in this setting, which they considered may free up time for GPs. Several suggestions were put forward regarding how the intervention could be rolled out more widely. Practice managers suggested setting up specific clinics at set times in the week that could be run by healthcare assistants rather than practice nurses. It was also suggested that sessions could be run in small groups in a similar way to other behaviour-change clinics such as smoking cessation or weight-loss clinics. This is something that could be further explored in subsequent research.
Limitations
A limitation of this study was that we did not interview participants who withdrew or did not start the intervention. Reasons for withdrawal from intervention were systematically recorded as part of the trial, indicating that a key reason for discontinuation was finding the intervention too challenging, with respect to both adherence and the acute effects of restricted sleep opportunity. This type of behavioural therapy may not suit every patient, but a better understanding of why people discontinued SRT might inform changes to the intervention and ongoing support, leading to better retention. This is reflected by findings of this study where those who found the intervention hard, or did not see benefits until later, were less likely to maintain SRT and more quickly reverted to previous sleep habits.
Conclusions
We found that SRT can be successfully delivered by nurses in general practice and was generally well received by patients. Ongoing support after the initial intervention period could be assessed to determine whether this leads to improved adherence.
Chapter 6 Discussion
The vast majority of people with insomnia in the UK cannot access the first-line treatment (CBT). The HABIT trial sought to test whether brief nurse-delivered behavioural treatment for insomnia in primary care is clinically and cost-effective. The trial shows that nurses without prior clinical experience of sleep disorders or sleep intervention can be successfully trained to deliver SRT in a brief and manualised manner, and with high levels of fidelity. Results indicate superiority of nurse-delivered SRT over SH in reducing insomnia symptoms at all time points. Cost–utility analysis suggests that the intervention is likely to be cost-effective at established willingness-to-pay thresholds. Below we consider trial results in relation to the broader literature, and reflect on the generalisability of findings and potential implications for the management of insomnia in the UK (and beyond).
Clinical effectiveness
To our knowledge, HABIT is the largest randomised trial to date of a psychological treatment for insomnia delivered in a clinical setting. It is also one of the few controlled studies to follow up patients for 12 months. 71 Standardised effect sizes for the ISI were in the medium-to-large range at all time points, and multiple sensitivity analyses of the primary outcome suggest robustness to a range of assumptions regarding missingness. Descriptive data on treatment response (defined as ≥ 8 points on the ISI) parallel these changes (42% for SRT vs. 17% for SH at 6 months). Treatment effects exceed clinically significant thresholds defined by the American Academy of Sleep Medicine (AASM) Task Force on Behavioural and Psychological Treatments for Insomnia,38 as well as estimates from a recent meta-analysis of CBT-I trials performed within primary care (g = 0.40). 21 Moreover, pre-specified moderation analyses of the primary outcome revealed no significant effects for age, depression severity, chronotype, actigraphy-defined sleep duration, sleep-medication use, or level of deprivation, which is broadly consistent with meta-analyses looking at variability in effect sizes across trials. 72
While sleep diary data were available for only a minority of HABIT participants at follow-up, small-to-medium effects were found for sleep continuity variables (WASO, SE, SQ and TST) at both 6 and 12 months relative to control. Actigraphy-defined WASO and SE were also improved in the SRT group at 6 months, but not 12 months, and TST was reduced (by 13–15 minutes) at both time points. Results are broadly consistent with previous work showing that diary-recorded sleep is more sensitive to change following CBT relative to actigraphy. 73
In addition to improvements in insomnia we also observed treatment effects at all time points on several important secondary outcomes, including mental health-related and sleep-related quality of life, depressive symptoms, and work productivity and activity impairment. Effect sizes were in the small-to-medium range, consistent with meta-analysis of CBT for insomnia. 74 Treatment effects tended to be greater for patient-generated quality of life (GSII) relative to standardised measures (SF-36), presumably because the GSII is an idiographic measure, which increases signal-to-noise by measuring life domains important to each individual patient. 3,39 These results are important because the daytime consequences of insomnia are distressing for patients and the most common reasons for seeking treatment in primary care. 3,75 Effects on mental health outcomes are particularly noteworthy given the strong association between insomnia and psychiatric disorder. 76 For example, approximately 40% of the sample had a mental health condition at baseline and 49% met criteria for depression on the PHQ-9. Results suggest that targeting insomnia leads to a small and sustained reduction in depressive symptoms, which was also reflected in a reduction in depression ‘caseness’ (defined as PHQ-9 ≥ 10) between groups at 6 months (SRT = 29% vs. SH = 39%). While we did not specifically recruit a sample with depression, nor target depression during treatment, it is interesting that effect sizes appear similar in magnitude to those observed in trials of CBT for depression in primary care (assessing various delivery formats). 77 Given that insomnia is almost characteristic of depression, the specific management of insomnia through SRT may lead to improved mental health outcomes.
No group differences were found for number of nights of use, or proportion of participants using prescribed hypnotic or sedative medication at 6 or 12 months. Missing diary data due to the pandemic may have limited power to detect group effects for medication use; however, exploratory analysis of prescription data collected from practice records at 12-month follow-up also revealed similar proportions of participants in each arm being prescribed sleep-promoting hypnotic medication (SRT = 25.5% vs. SH = 25.1%). Conflicting findings have been observed in the CBT treatment literature,78 particularly for studies where hypnotic use was not an inclusion criterion, or where the intervention did not specifically have a focus on withdrawal or tapering of medication (both apply to the HABIT trial). It would be interesting to specifically test the effects of nurse-delivered SRT on long-term users of hypnotics, or alternatively investigate those first presenting to primary care with insomnia to ascertain whether offering SRT lessens prescriptions and use of sedative hypnotics.
HABIT is the first trial in the CBT field to rigorously measure SAEs and pre-defined AEs. 79 This was considered important because previous work has documented increased sleepiness, reduced psychomotor vigilance, and potential driving concerns during implementation of SRT,24,29,70 owing to the acute effects of restricted sleep opportunity. While participants reported challenges with sleepiness and fatigue during qualitative interviews (consistent with previous work29), we found no evidence that falls, accidents (including road traffic accidents and near misses) or sleepiness while driving were increased at any post-randomisation assessment. This was also the case for SAEs, which were infrequent, similar between arms, and not judged to be related to the intervention. Our nurse-delivered protocol emphasised to patients the importance of avoiding driving if sleepy. Moreover, nurses were able to modify the sleep window if participants reported concerns with excessive daytime sleepiness or experienced difficulties with adherence. Such flexibility in the delivery of SRT is important, particularly in routine clinical practice where patients have a range of comorbidities. Nevertheless, findings from the process evaluation suggested that participants still found the treatment challenging, prompting some participants to discontinue with the intervention. Descriptive data on reasons for withdrawal from intervention showed that 35 participants (11% of those randomised to SRT) discontinued due to lack of benefit or finding the intervention too challenging to implement.
It is known that SRT is the most challenging component of CBT for insomnia80–83 – yet potentially the most active. Restricted sleep opportunity is central to driving clinical outcomes,25,26 and HABIT data show stronger treatment effects for participants who attend more treatment sessions and more closely adhere to prescribed bed and rise times. It would be prudent, therefore, for future studies to test strategies that may improve treatment engagement and adherence. For example, one strategy that could be tested is the combination of light therapy and SRT. Bright light is known to have alerting properties84,85 and has been shown to reduce the impact of experimental sleep restriction on sleepiness and vigilance when administered during the day. 86–88 Moreover, light acts as the main zeitgeber for the synchronisation of the circadian rhythm. Regular and enhanced light exposure alongside a prescribed sleep opportunity may strengthen the circadian rhythm and help align homeostatic and circadian drives for sleep, a proposed mechanism of SRT. 23 Other potential refinements could include involving family members in treatment to support behaviour change and reduce obstacles to implementation,89 or prescription of a more gradual reduction of time in bed (sleep compression) for those who find SRT challenging.
While 92% of participants attended at least one out of four treatment sessions, 65% completed all four. Our numbers are consistent with or higher than other primary care trials of in-person CBT for insomnia20,31,90 and exceed rates of engagement found for other low-intensity interventions, such as digital CBT. 39,52,91–93 Qualitative interviews with nurses and patients also generated areas of potential refinement that could support treatment engagement. For example, digital technology (app and/or wearable device) could be blended with nurse-delivered SRT to automate recording and calculation of SE to reduce participant burden. Additional follow-up sessions with the nurse were suggested as a way to help maintain sleep behaviour change beyond the acute intervention phase. There is suggestive evidence from meta-analysis that > 4 sessions may yield enhanced treatment effect sizes72 but this must be balanced against cost and scalability in primary care, particularly when considered within a stepped care framework. Such refinements could be explored in future research, but it is worth noting that the proportion of participants achieving a treatment response in the present study (42%) is similar to a high-quality trial that assessed eight weekly sessions (45–60 minutes in duration) of behavioural therapy delivered by licensed or trainee clinical psychologists (44%). 94
The HABIT trial was not designed to evaluate treatment mechanisms, but we performed mediation analyses to enhance understanding of how SRT may exert its effects. Drawing on a theoretical model of SRT mechanism of action,23 we examined the mediating role of pre-sleep arousal and sleep effort on insomnia severity. SRT led to reductions in pre-sleep arousal and sleep effort at 3 months, which significantly (though modestly) mediated the treatment effect on the ISI at 6 months. Proportion mediated was larger for cognitive measures [sleep effort (36%), cognitive arousal (35%)] vs. self-reported somatic arousal (15%). Excessive pre-sleep arousal and sleep effort are reliable features of insomnia and may be involved in the maintenance of poor sleep via effects on autonomic and cortical arousal prior to and during the sleep period, which ultimately degrades sleep quality. Integrating previous experimental work25,29 with HABIT findings we hypothesise that enhancing sleep pressure and regularising time in bed reduce arousal and obviate sleep effort, leading to improved sleep consolidation. Improved sleep consolidation and quality then positively influence cognitive processes that operate during daytime periods (e.g. sleep-related worry and monitoring), which further lessens pre-sleep arousal and sleep effort in the evening. While this sequence and feedback loop needs to be appraised in dedicated studies – alongside other putative causal mechanisms – HABIT suggests that addressing arousal (especially cognitive arousal) and sleep effort may be important in lessening insomnia severity.
Cost-effectiveness
The HABIT trial was designed to test a scalable and potentially cost-effective treatment for insomnia in primary care. Health economic analysis showed that the cost of brief SRT was modest at £52.60 per trial participant and mean NHS and PSS costs (excluding intervention-related costs) were similar between arms over the 12-month period. In the primary analysis, mean NHS and PSS costs (including intervention-related costs) were just £43.59 (95% CI −18.41 to 105.59) higher in the SRT arm compared to control. Adjusting the SRT training cost to reflect what may happen in clinical practice (trained nurses seeing a much larger number of patients) led to a small difference of just £14.41 (−47.59 to 76.41). In terms of utility, the EQ-5D-3L showed a small difference of 0.021 in QALYs in favour of SRT. Nevertheless, small differences in both QALYs and costs produced an incremental cost-effective ratio of just £2075.71 per QALY with a high probability (95%) that the intervention is cost-effective at a cost-effectiveness threshold of £20,000 per QALY (NMB = £377.84). This was supported by a range of sensitivity analyses. Indeed, a probability of 94.4% of cost-effectiveness was estimated at a cost-effectiveness threshold of just £15,000. Poor sensitivity of the EQ-5D-3L to insomnia interventions has been reported in several trials,95–97 contrasting with effects for insomnia-specific outcomes like the ISI. In exploratory analyses we also performed cost–utility analyses using the SF-6D and EQ-5D-3L + Sleep. Both measures showed a small advantage in QALYs relative to control, and with slightly higher levels of decision certainty than the EQ-5D-3L (96.3% and 100% probability of being cost-effective at the £20,000 cost-effectiveness threshold).
HABIT is the largest trial to date to assess cost-effectiveness of a psychological treatment for insomnia and the only trial to assess costs and effectiveness over a 12-month horizon. Results provide robust support for the cost-effectiveness of nurse-delivered SRT. Our trial compares favourably to smaller studies adopting a similar approach but over a shorter time-frame, where probability of cost-effectiveness was 67% (at a £20,000 cost-effectiveness threshold) for guided digital CBT-I96 and just 34% (at a £30,000 cost-effectiveness threshold) for community-based CBT workshop delivery. 95 HABIT intervention costs are also lower than other low-intensity interventions that have been trialled in primary care [e.g. £148 for community-delivered workshops,95 £191 for counsellor-delivered CBT31 and £85 pounds (99 euros) for nurse-guided digital CBT96]. While SRT does not appear to be cost-saving for the NHS over a 12-month horizon (that is, resource use was broadly similar between arms), we did find that SRT dominated SH from a societal perspective with societal costs being reduced, on average, by £1086.13 (−1485.59 to −686.67) in the SRT arm compared to control. These differences principally reflected reduced productivity loss in the SRT arm. 98,99
We focused on self-reported health and social care resource use for insomnia and, as a consequence, there was a high degree of missing data compared to data extracted from practice records. Nevertheless, sensitivity analyses across both imputed and complete data sets led to the same conclusion: SRT is highly likely to be cost-effective. However, given that SRT was not cost-saving for the NHS over 12 months, future implementation research is needed to assess incentives for practices to implement SRT, as well as capacity considerations in relation to nurse delivery.
Strengths and limitations
The HABIT trial is the largest trial of SRT to date and one of the largest trials of psychological treatment for insomnia, yielding precise estimates of effect. It is the only trial to perform cost-effective analysis over a 12-month follow-up period. We conducted the trial across multiple general practices, across different regions of England, and trained nurses without formal experience of sleep intervention or psychological therapy to effectively deliver brief SRT with high levels of fidelity. This supports the generalisability of our intervention, while the brief training and delivery model speaks to scalability. We initially sought to only train practice nurses but due to availability issues at some practices we also trained additional research nurses to deliver treatment; however, they represented a minority (22.5%), and none of them had prior experience delivering sleep or behavioural treatment.
Retention was 85% overall at 6 months and 79% at 12 months, which is higher than previous primary care studies in the UK19,32 and broadly consistent with CBT-I studies over shorter follow-up periods. 72 Participants in the treatment group were less likely to complete the primary outcome at all time points. We attribute this difference to the greater demands placed on participants in the SRT arm relative to SH with respect to scheduling of treatment sessions, recording of sleep diaries during the 4-week intervention phase, and (for some) the challenge and difficulties of following SRT instructions. Sensitivity analyses involving multiple imputation and covarying for baseline predictors of missingness yielded similar findings to the primary analysis. Indeed, even under conservative assumptions (i.e. models assuming high score differences between those with missing and non-missing ISI outcome), the conclusion remained the same. Although the pandemic affected the last 12 months of the trial, treatment adaptation was required for just 13 participants, and exploratory analysis of the 6-month primary outcome revealed no difference for pre versus during the pandemic. The pandemic also adversely affected our ability to collect data on sleep diary parameters, medication use, and actigraphy-defined sleep, and thus such analyses should be interpreted with caution given the low levels of outcome completion.
Our sample reflects the clinical reality of insomnia in that the majority of participants were female, had experienced insomnia for a long time (approximately 10 years), and had a range of comorbid conditions (89% had at least one comorbidity and 51% had three or more). Moreover, the majority had consulted their GP in relation to insomnia and 25% were taking prescribed sleep medication at baseline. However, our sample and results may not generalise to the entire UK insomnia population because participants tended to be well-educated (50% had a university degree), were more likely to be from a white ethnic background (97% of the sample), and live in areas with low levels of deprivation. These sample characteristics may, in part, be driven by greater than anticipated recruitment in Oxfordshire (which was unexpected but necessary to compensate for under-recruitment). There was, however, no evidence that the treatment effect was lower in people from more-deprived circumstances, but the analyses lacked power to detect such moderation. It was not possible to conduct such moderation analyses by ethnic group. Future trials should be informed by INCLUDE guidance and roadmap100 in order to improve representation of under-served groups and increase diversity of recruited participants.
Participants were not blind to treatment group and the primary outcome was self-reported insomnia severity; therefore, there is potential for bias in reporting. However, we did not reveal the hypothesis to participants (the study was set up as a test of two different sleep improvement programmes) and nurses were not involved in the collection of trial outcomes. We therefore believe that bias is unlikely to explain the results. In support of this, previous work has tested, and demonstrated superiority of, SRT against an active control condition matched for therapist time, support and implementation of behavioural sleep advice. 24,25 A related point is that while SRT clearly out-performed SH, the SH group showed a reduction in ISI scores of approximately 3.5 points from baseline to 6 months. It is not clear what explains this reduction, but it may reflect regression to the mean, the natural course of insomnia over time, the effect of taking part in a study and/or the effect of SH.
Our approach to screening was automated and based on responses to questionnaires in order to assess for and exclude conditions that may not be suitable for SRT. We took this approach because it simulates a potentially scalable method that could be implemented in clinical practice, since primary care staff are not experts in sleep medicine. Nevertheless, without clinical interview or polysomnographic evaluation it is possible that some patients in the trial had undiagnosed sleep disorders, which plausibly could lead to a marginal dilution of the treatment effect.
Implications for health care
Our trial shows that nurses can be trained to deliver a focused and manualised behavioural insomnia treatment, leading to patient benefit, and without safety concerns. Moreover, the intervention is very likely to be cost-effective. Nurse-delivered SRT could therefore become part of primary care management of insomnia. At present, patients are typically provided with SH advice or sedative medication. NICE guidelines recommend that patients with insomnia are offered CBT-I as the first-line treatment, but there is limited access to psychological treatment for insomnia, with the exception of a few specialist clinics or services, or digital CBT implementation projects. The European Academy of CBT for insomnia articulates a vision ‘to develop services in such a way that CBT-I becomes available at a scale equivalent to medication’ and emphasises GP intervention and digital CBT. 101 There are practice nurses in every GP surgery (approximately 23,000 practice nurses across England) who may have the capacity to support people with insomnia using behavioural therapy, consistent with other clinical activities like weight management and smoking cessation. This is likely to result in a shift of consultation from GPs to practice nurses. Nurses and practice managers in the HABIT trial considered it to be feasible and had additional suggestions for implementation, including group sessions and enhanced flexibility in scheduling appointments. Nurse-delivered treatment could complement initiatives to increase access to digital therapies and cater for those who prefer face-to-face contact with a health professional – indeed qualitative interviews emphasised the importance of face-to-face sessions for SRT engagement. Those who do not achieve sufficient response to nurse treatment could then be reviewed by the GP and, if appropriate, referred to a specialist in sleep.
It is also possible that this treatment with its brief training and delivery model could be incorporated into the Improving Access to Psychological Therapies (IAPT) service in England. Most people with depression and anxiety, the main conditions treated within IAPT, experience insomnia symptoms and our data show improvement in PHQ-9 and SF-36 MCS scores. Thus, our sleep treatment package may improve outcomes for many patients in IAPT programmes. Finally, beyond UK health care, brief nurse-delivered behavioural treatment could widen access to evidence-based intervention in developing countries where there are limited dedicated mental health provision and barriers to digital engagement.
Recommendations for future research
Below we summarise specific research areas that should be followed up in future studies to build on the findings of the HABIT trial:
-
Formally investigate the integration of nurse-delivered SRT into the insomnia management pathway in primary care, for example as part of a stepped-care framework.
-
Assess generalisability of results across diverse primary care patients with insomnia.
-
Investigate additional methods to support patient engagement with treatment.
-
From a health economics perspective, investigate practice incentives for adopting SRT, including practice nurse capacity.
-
Investigate the effects of nurse-delivered SRT in specific subgroups, for example long-term hypnotic users, people with mental health problems, those presenting with insomnia for the first time.
Conclusions
Brief nurse-delivered SRT in primary care is clinically effective for insomnia disorder, safe, and likely to be cost-effective. SRT could become part of a stepped care approach to insomnia treatment, helping to facilitate the implementation of NICE guidelines and increase access to evidence-based intervention.
Additional information
Contributions of authors
Simon D Kyle (https://orcid.org/0000-0002-9581-5311) (Professor of Clinical Neurosciences) was Chief Investigator, developed the original idea for the study and funding application with co-investigators, oversaw the delivery of the trial, led intervention design, training and delivery and led the writing of the final report.
Peter Bower (https://orcid.org/0000-0001-9558-3349) (Professor of Health Services Research) was a co-investigator on the funding application, designed the study, was responsible for its conduct and contributed to the writing of the report.
Ly-Mee Yu (https://orcid.org/0000-0003-0331-7364) (Associate Professor) was a co-investigator on the funding application, designed the study, was responsible for its conduct, led statistical analysis and contributed to the writing of the report.
Aloysius Niroshan Siriwardena (https://orcid.org/0000-0003-2484-8201) (Professor of Primary and Pre-Hospital Health Care) was a co-investigator on the funding application, designed the study, was responsible for its conduct, led the process evaluation and contributed to the writing of the report.
Yaling Yang (https://orcid.org/0000-0002-9529-1685) (Senior Researcher in Health Economics) performed the health economic evaluation and contributed to the writing of the report.
Stavros Petrou (https://orcid.org/0000-0003-3121-6050) (Professor of Health Economics) provided oversight of the health economic evaluation and contributed to the writing of the report.
Emma Ogburn (https://orcid.org/0000-0001-7643-572X) (CTU Director of Operations) was a co-investigator on the funding application, designed the study, was responsible for its conduct and contributed to the writing of the report.
Nargis Begum (https://orcid.org/0000-0002-8628-0319) (Clinical Trial Manager) was the trial manager, contributed to data collection and contributed to the writing and formatting of the report.
Leonie Maurer (https://orcid.org/0000-0001-7335-2320) (Post-Doctoral Research Associate) contributed to data collection, led actigraphy analysis, and contributed to the writing and formatting of the report.
Barbara Robinson (https://orcid.org/0000-0002-1721-7682) (Clinical Trial Facilitator) contributed to data collection and the writing and formatting of the report.
Caroline Gardner (https://orcid.org/0000-0003-0487-3979) (Post-Doctoral Research Associate) contributed to data collection and the writing and formatting of the report.
Stephanie Armstrong (https://orcid.org/0000-0002-2599-4844) (Senior Lecturer in Health Quality Improvement) contributed to data collection and analysis as part of the process evaluation, and contributed to the writing and formatting of the report.
Julie Pattinson (https://orcid.org/0000-0002-9824-3400) (Post-Doctoral Research Associate) contributed to data collection and the process evaluation, and contributed to the writing and formatting of the report.
Colin A Espie (https://orcid.org/0000-0002-1294-8734) (Professor of Sleep Medicine) was a co-investigator on the funding application, designed the study, was responsible for its conduct and contributed to the writing of the report.
Paul Aveyard (https://orcid.org/0000-0002-1802-4217) (Professor of Behavioural Medicine) was a co-investigator on the funding application, designed the study, was responsible for its conduct, was medical lead for the trial and contributed to the writing of the report.
Acknowledgements
We would like to thank all participants, nurses and practices who took part in the trial. We would like to acknowledge the research teams and administrators at sites in Thames Valley, Manchester and Lincoln, and the NIHR CRN. We would like to thank the TSC and DMEC, and our PPI Advisors (Danny Axford and Amanda Brewster) for supporting the trial.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
The trial received both Health Research Authority approval (IRAS: 238138) and ethical approval (Yorkshire and the Humber – Bradford Leeds REC, reference: 18/YH/0153).
Information governance statement
The University of Oxford is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, the University of Oxford is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights, and the contact details for our Data Protection Officer here (https://compliance.admin.ox.ac.uk/individual-rights).
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/RJYT4275.
Primary conflicts of interest: Simon D Kyle declares research funding from NIHR HTA (16/84/01 and 12/87/61), EME (NIHR131789) and Oxford Biomedical Research Centre and the Oxford Health Biomedical Research Centre, and non-financial support from Big Health Ltd. in the form of no-cost access to the digital sleep improvement programme, Sleepio, for use in clinical research (outside the submitted work). Paul Aveyard is NIHR Senior Investigator and declares research funding from NIHR HTA, NIHR Oxford Biomedical Research Centre, and NIHR Oxford and Thames Valley Applied Research Collaboration. Aloysius Niroshan Siriwardena declares research funding from Wellcome trust and NIHR HTA, RFPB and HS&DR. Ly-Mee Yu declares research funding from NIHR HTA. Peter Bower declares research funding from NIHR HTA. Leonie Maurer declares funding from NIHR Oxford BRC and consultancy fees from Mementor DE GmbH, outside the submitted work. Colin A Espie declares research funding from NIHR HTA, EME and Oxford Biomedical Research Centre, and is co-founder of and shareholder in Big Health Ltd., a company which specialises in the digital delivery of cognitive–behavioural therapy for sleep improvement (the Sleepio programme), outside the submitted work. All other authors declare no competing interests. Yaling Yang declares research funding from NIHR HTA, NIHR MIC and NIHR ARC Oxford and Thames Valley. Stavros Petrou receives support as a UK National Institute for Health and Care Research (NIHR) Senior Investigator (NF-SI-0616-10103) and from the NIHR Applied Research Collaboration Oxford and Thames Valley.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Kyle SD, Madigan C, Begum N, Abel L, Armstrong S, Aveyard P, et al. Primary care treatment of insomnia: study protocol for a pragmatic, multicentre, randomised controlled trial comparing nurse-delivered sleep restriction therapy to sleep hygiene (the HABIT trial). BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-036248.
- Kyle SD, Espie CA, Morgan K. ‘… Not just a minor thing, it is something major, which stops you from functioning daily’: quality of life and daytime functioning in insomnia. Behav Sleep Med 2010;8:123-40. https://doi.org/10.1080/15402002.2010.487450.
- Kyle SD, Crawford MR, Morgan K, Spiegelhalder K, Clark AA, Espie CA. The Glasgow Sleep Impact Index (GSII): a novel patient-centred measure for assessing sleep-related quality of life impairment in insomnia disorder. Sleep Med 2013;14:493-501. https://doi.org/10.1016/j.sleep.2012.10.023.
- Morin CM, Benca R. Chronic insomnia. Lancet 2012;379:1129-41. https://doi.org/10.1016/S0140-6736(11)60750-2.
- Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev 2013;17:241-54. https://doi.org/10.1016/j.smrv.2012.09.005.
- Taddei-Allen P. Economic burden and managed care considerations for the treatment of insomnia. Am J Manag Care 2020;26:S91-6. https://doi.org/10.37765/ajmc.2020.43008.
- Wickwire EM, Tom SE, Scharf SM, Vadlamani A, Bulatao IG, Albrecht JS. Untreated insomnia increases all-cause health care utilization and costs among Medicare beneficiaries. Sleep 2019;42. https://doi.org/10.1093/sleep/zsz007.
- Wickwire EM, Shaya FT, Scharf SM. Health economics of insomnia treatments: the return on investment for a good night’s sleep. Sleep Med Rev 2016;30:72-8. https://doi.org/10.1016/j.smrv.2015.11.004.
- Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep 2009;32:55-64. https://doi.org/10.5665/sleep/32.1.55.
- Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Clinical Guidelines Committee of the American College of Physicians . Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2016;165:125-33. https://doi.org/10.7326/M15-2175.
- Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res 2017;26:675-700. https://doi.org/10.1111/jsr.12594.
- National Institute for Health and Care Excellence (NICE) . CKS Is Only Available in the UK n.d. http://cks.nice.org.uk/insomnia#!scenario:1 (accessed accessed March 2022).
- Wilson S, Anderson K, Baldwin D, Dijk DJ, Espie A, Espie C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: an update. J Psychopharmacol 2019;33:923-47. https://doi.org/10.1177/0269881119855343.
- Everitt H, McDermott L, Leydon G, Yules H, Baldwin D, Little P. GPs’ management strategies for patients with insomnia: a survey and qualitative interview study. Br J Gen Pract 2014;64:e112-9. https://doi.org/10.1016/j.smrv.2018.09.004.
- Dyas JV, Apekey TA, Tilling M, Ørner R, Middleton H, Siriwardena AN. Patients’ and clinicians’ experiences of consultations in primary care for sleep problems and insomnia: a focus group study. Br J Gen Pract 2010;60:e180-200. https://doi.org/10.3399/bjgp10X484183.
- Maire M, Linder S, Dvorak C, Merlo C, Essig S, Tal K, et al. Prevalence and management of chronic insomnia in Swiss primary care: cross-sectional data from the ‘Sentinella’ practice-based research network. J Sleep Res 2020;29. https://doi.org/10.1111/jsr.13121.
- Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med 2011;171:887-95. https://doi.org/10.1001/archinternmed.2010.535.
- Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther 2001;39:45-60. https://doi.org/10.1016/s0005-7967(99)00157-6.
- Espie CA, MacMahon KM, Kelly HL, Broomfield NM, Douglas NJ, Engleman HM, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep 2007;30:574-84. https://doi.org/10.1093/sleep/30.5.574.
- Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol 2008;26:4651-8. https://doi.org/10.1200/jco.2007.13.9006.
- Cheung JMY, Jarrin DC, Ballot O, Bharwani A, Morin CM. A systematic review of adaptive cognitive behavioral therapy for insomnia implemented in primary care settings. Sleep Med 2017;40. https://doi.org/10.1016/j.sleep.2017.11.165.
- Davidson J, Dickson C, Han H. Cognitive behavioural treatment for insomnia in primary care: a systematic review of sleep outcomes. Br J Gen Pract 2019;69:e657-64. https://doi.org/10.3399/bjgp19X705065.
- Maurer LF, Espie CA, Kyle SD. How does sleep restriction therapy for insomnia work? A systematic review of mechanistic evidence and the introduction of the Triple-R model. Sleep Med Rev 2018;42:127-38. https://doi.org/10.1016/j.jad.2017.04.003.
- Maurer LF, Ftouni S, Espie CA, Bisdounis L, Kyle SD. The acute effects of sleep restriction therapy for insomnia on circadian timing and vigilance. J Sleep Res 2021;30. https://doi.org/10.1111/jsr.13260.
- Maurer LF, Espie CA, Omlin X, Kyle SD. The effect of sleep restriction therapy for insomnia on multidimensional assessments of sleep pressure and arousal: results from a randomised, controlled, mechanistic trial. Sleep 2022;45. https://doi.org/10.1093/sleep/zsab223.
- Maurer LF, Espie CA, Omlin X, Reid MJ, Sharman R, Gavriloff D, et al. Isolating the role of time in bed restriction in the treatment of insomnia: a randomized, controlled, dismantling trial comparing sleep restriction therapy with time in bed regularization. Sleep 2020;43. https://doi.org/10.1093/sleep/zsaa096.
- Maurer LF, Schneider J, Miller CB, Espie CA, Kyle SD. The clinical effects of sleep restriction therapy for insomnia: a meta-analysis of randomised controlled trials. Sleep Med Rev 2021;58. https://doi.org/10.1016/j.smrv.2021.101493.
- Falloon K, Elley CR, Fernando A, Lee AC, Arroll B. Simplified sleep restriction for insomnia in general practice: a randomised controlled trial. Br J Gen Pract 2015;65:e508-15. https://doi.org/10.3399/bjgp15X686137.
- Kyle SD, Morgan K, Spiegelhalder K, Espie CA. No pain, no gain: an exploratory within-subjects mixed-methods evaluation of the patient experience of sleep restriction therapy (SRT) for insomnia. Sleep Med 2011;12:735-47. https://doi.org/10.1016/j.sleep.2011.03.016.
- Sargent GM, Forrest LE, Parker RM. Nurse delivered lifestyle interventions in primary health care to treat chronic disease risk factors associated with obesity: a systematic review. Obes Rev 2012;13:1148-71. https://doi.org/10.1111/j.1467-789X.2012.01029.x.
- Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M. Psychological treatment for insomnia in the management of long-term hypnotic drug use: a pragmatic randomised controlled trial. Br J Gen Pract 2003;53.
- Morgan K, Gregory P, Tomeny M, David BM, Gascoigne C. Self-help treatment for insomnia symptoms associated with chronic conditions in older adults: a randomized controlled trial. J Am Geriatr Soc 2012;60:1803-10. https://doi.org/10.1111/j.1532-5415.2012.04175.x.
- Natsky AN, Vakulin A, Chai-Coetzer CL, Lack L, McEvoy RD, Kaambwa B. Economic evaluation of cognitive behavioural therapy for insomnia (CBT-I) for improving health outcomes in adult population: a systematic review. Sleep Med Rev 2020;54. https://doi.org/10.1016/j.smrv.2020.101351.
- Espie CA, Kyle SD, Hames P, Gardani M, Fleming L, Cape J. The Sleep Condition Indicator: a clinical screening tool to evaluate insomnia disorder. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004183.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders 2013.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193-21. https://doi.org/10.1016/0165-1781(89)90047-4.
- Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol 2010;24:1577-601. https://doi.org/10.1177/0269881110379307.
- Edinger JD, Arnedt JT, Bertisch SM, Carney CE, Harrington JJ, Lichstein KL, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med 2021;17:263-98. https://doi.org/10.5664/jcsm.8988.
- Espie CA, Emsley R, Kyle SD, Gordon C, Drake CL, Siriwardena AN, et al. Effect of digital cognitive behavioral therapy for insomnia on health, psychological well-being, and sleep-related quality of life: a randomized clinical trial. JAMA Psychiatry 2019;76:21-30. https://doi.org/10.1001/jamapsychiatry.2018.2745.
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2:297-30. https://doi.org/10.1016/S1389-9457(00)00065-4.
- Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601-8. https://doi.org/10.1093/sleep/34.5.601.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics 1993;4:353-65. https://doi.org/10.2165/00019053-199304050-00006.
- Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther 1985;23:263-71. https://doi.org/10.1016/0005-7967(85)90004-X.
- Broomfield NM, Espie CA. Towards a valid, reliable measure of sleep effort. J Sleep Res 2005;14:401-7. https://doi.org/10.1111/j.1365-2869.2005.00481.x.
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep 2012;35:287-302. https://doi.org/10.5665/sleep.1642.
- Beecham J, Knapp M. Measuring Mental Health Needs. London: Gaskell/Royal College of Psychiatrists; 1992.
- Rabin R, Charro F. EQ-SD: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Yang Y, Brazier J, Tsuchiya A. Effect of adding a sleep dimension to the EQ-5D descriptive system: a ‘bolt-on’ experiment. Med Decis Making 2013;34:42-53. https://doi.org/10.1177/0272989X13480428.
- Dyas JV, Togher F, Siriwardena AN. Intervention fidelity in primary care complex intervention trials: qualitative study using telephone interviews of patients and practitioners. Qual Prim Care 2014;22:25-34.
- Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods 2006;18:59-82.
- Adan A, Almirall H. Horne & Östberg morningness-eveningness questionnaire: a reduced scale. Pers Individ Dif 1991;12:241-53. https://doi.org/10.1016/0191-8869(91)90110-W.
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51. https://doi.org/10.1037//0022-3514.51.6.1173.
- Freeman D, Sheaves B, Goodwin GM, Yu LM, Nickless A, Harrison PJ, et al. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry 2017;4:749-58. https://doi.org/10.1016/S2215-0366(17)30328-0.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Jenkinson C, Stewart-Brown S, Petersen S, Paice C. Assessment of the SF-36 version 2 in the United Kingdom. J Epidemiol Community Health 1999;53. https://doi.org/10.1136/jech.53.1.46.
- Petrou S, Gray A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ 2011;342. https://doi.org/10.1136/bmj.d1548.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. CHEERS Task Force . Consolidated health economic evaluation reporting standards (cheers) statement. Int J Technol Assess Health Care 2013;29:117-22. https://doi.org/10.1017/S0266462313000160.
- NHS Improvement . Archived Reference Costs n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed March 2022).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Personal Social Services Research Unit. Canterbury: University of Kent; n.d.
- National Institute for Health and Care Excellence (NICE) . British National Formulary n.d. https://bnfc.nice.org.uk/ (accessed March 2022).
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed March 2022).
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med 2018;37:2252-66. https://doi.org/10.1002/sim.7654.
- Irvine A, Drew P, Bower P, Brooks H, Gellatly J, Armitage CJ, et al. Are there interactional differences between telephone and face-to-face psychological therapy? A systematic review of comparative studies. J Affect Disord 2020;265:120-31. https://doi.org/10.1016/j.jad.2020.01.057.
- Miller CB, Kyle SD, Marshall NS, Espie CA. Ecological momentary assessment of daytime symptoms during sleep restriction therapy for insomnia. J Sleep Res 2013;22:266-72. https://doi.org/10.1111/jsr.12024.
- Kyle SD, Miller CB, Rogers Z, Siriwardena AN, Macmahon KM, Espie CA. Sleep restriction therapy for insomnia is associated with reduced objective total sleep time, increased daytime somnolence, and objectively impaired vigilance: implications for the clinical management of insomnia disorder. Sleep 2014;37:229-37. https://doi.org/10.5665/sleep.3386.
- van der Zweerde T, Bisdounis L, Kyle SD, Lancee J, van Straten A. Cognitive behavioral therapy for insomnia: a meta-analysis of long-term effects in controlled studies. Sleep Med Rev 2019;48. https://doi.org/10.1016/j.smrv.2019.08.002.
- Van Straten A, Van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev 2018;38:3-16. https://doi.org/10.1016/j.smrv.2017.02.001.
- Mitchell LJ, Bisdounis L, Ballesio A, Omlin X, Kyle SD. The impact of cognitive behavioural therapy for insomnia on objective sleep parameters: a meta-analysis and systematic review. Sleep Med Rev 2019;47:90-102. https://doi.org/10.1016/j.smrv.2019.06.002.
- Benz F, Knoop T, Ballesio A, Bacaro V, Johann AF, Rücker G, et al. The efficacy of cognitive and behavior therapies for insomnia on daytime symptoms: a systematic review and network meta-analysis. Clin Psychol Rev 2020;80. https://doi.org/10.1016/j.cpr.2020.101873:101873.
- Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med 2006;7:123-30. https://doi.org/10.1016/j.sleep.2005.08.008.
- Hertenstein E, Feige B, Gmeiner T, Kienzler C, Spiegelhalder K, Johann A, et al. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev 2019;43:96-105. https://doi.org/10.1016/j.smrv.2018.10.006.
- Santoft F, Axelsson E, Öst LG, Hedman-Lagerlöf M, Fust J, Hedman-Lagerlöf E. Cognitive behaviour therapy for depression in primary care: systematic review and meta-analysis. Psychol Med 2019;49:1266-74. https://doi.org/10.1017/s0033291718004208.
- Sweetman A, Putland S, Lack L, McEvoy RD, Adams R, Grunstein R, et al. The effect of cognitive behavioural therapy for insomnia on sedative-hypnotic use: a narrative review. Sleep Med Rev 2020;56. https://doi.org/10.1016/j.smrv.2020.101404.
- Condon H, Maurer L, Kyle S. Reporting of adverse events in cognitive behavioural therapy for insomnia: a systematic examination of randomised controlled trials. Sleep Med Rev 2021;56. https://doi.org/10.1016/j.smrv.2020.101412.
- Kaldo V, Jernelöv S, Blom K, Ljótsson B, Brodin M, Jörgensen M, et al. Guided internet cognitive behavioral therapy for insomnia compared to a control treatment – a randomized trial. Behav Res Ther 2015;71:90-100. https://doi.org/10.1016/j.brat.2015.06.001.
- Krieger T, Urech A, Duss SB, Blattler L, Schmitt W, Gast H, et al. A randomized controlled trial comparing guided internet-based multi-component treatment and internet-based guided sleep restriction treatment to care as usual in insomnia. Sleep Med 2019;62:43-52. https://doi.org/10.1016/j.sleep.2019.01.045.
- Chan C, West S, Glozier N. Commencing and persisting with a web-based cognitive behavioral intervention for insomnia: a qualitative study of treatment completers. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.5639.
- Vincent N, Lewycky S, Finnegan H. Barriers to engagement in sleep restriction and stimulus control in chronic insomnia. J Consult Clin Psychol 2008;76. https://doi.org/10.1037/0022-006X.76.5.820.
- Souman JL, Tinga AM, te Pas SF, van Ee R, Vlaskamp BNS. Acute alerting effects of light: a systematic literature review. Behav Brain Res 2018;337:228-39. https://doi.org/10.1016/j.bbr.2017.09.016.
- Chellappa SL, Steiner R, Blattner P, Oelhafen P, Götz T, Cajochen C. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert?. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0016429.
- Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SMW. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep 2003;26:695-700. https://doi.org/10.1093/sleep/26.6.695.
- Gabel V, Maire M, Reichert CF, Chellappa SL, Schmidt C, Hommes V, et al. Dawn simulation light impacts on different cognitive domains under sleep restriction. Behav Brain Res 2015;281:258-66. https://doi.org/10.1016/j.bbr.2014.12.043.
- Comtet H, Geoffroy PA, Kobayashi Frisk M, Hubbard J, Robin-Choteau L, Calvel L, et al. Light therapy with boxes or glasses to counteract effects of acute sleep deprivation. Sci Rep 2019;9. https://doi.org/10.1038/s41598-019-54311-x.
- Mellor A, Hamill K, Jenkins MM, Baucom DH, Norton PJ, Drummond SPA. Partner-assisted cognitive behavioural therapy for insomnia versus cognitive behavioural therapy for insomnia: a randomised controlled trial. Trials 2019;20. https://doi.org/10.1186/s13063-019-3334-3.
- Cape J, Leibowitz J, Whittington C, Espie CA, Pilling S. Group cognitive behavioural treatment for insomnia in primary care: a randomized controlled trial. Psychol Med 2016;46:1015-25. https://doi.org/10.1017/S0033291715002561.
- Vedaa O, Kallestad H, Scott J, Smith OR, Pallesen S, Morken G, et al. Effects of digital cognitive behavioural therapy for insomnia on insomnia severity: a large-scale randomised controlled trial. Lancet Digit Health 2020;2:e397-406. https://doi.org/10.1016/S2589-7500(20)30135-7.
- Christensen H, Batterham PJ, Gosling JA, Ritterband LM, Griffiths KM, Thorndike FP, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry 2016;3:333-41. https://doi.org/10.1016/S2215-0366(15)00536-2.
- Stott R, Pimm J, Emsley R, Miller CB, Espie CA. Does adjunctive digital CBT for insomnia improve clinical outcomes in an improving access to psychological therapies service?. Behav Res Ther 2021;144. https://doi.org/10.1016/j.brat.2021.103922.
- Harvey AG, Bélanger L, Talbot L, Eidelman P, Beaulieu-Bonneau S, Fortier-Brochu E, et al. Comparative efficacy of behavior therapy, cognitive therapy, and cognitive behavior therapy for chronic insomnia: a randomized controlled trial. J Consult Clin Psychol 2014;82:670-83. https://doi.org/10.1037/a0036606.
- Bonin EM, Beecham J, Swift N, Raikundalia S, Brown JS. Psycho-educational CBT-Insomnia workshops in the community. A cost-effectiveness analysis alongside a randomised controlled trial. Behav Res Ther 2014;55:40-7. https://doi.org/10.1016/j.brat.2014.01.005.
- Baka A, van der Zweerde T, Lancee J, Bosmans JE, van Straten A. Cost-effectiveness of guided internet-delivered cognitive behavioral therapy in comparison with care-as-usual for patients with insomnia in general practice. Behav Sleep Med 2021;20:188-203. https://doi.org/10.1080/15402002.2021.1901708.
- Yeung K, Zhu W, McCurry SM, . Cost-effectiveness of telephone cognitive behavioral therapy for osteoarthritis-related insomnia. J Am Geriatr Soc 2022;70:188-99. https://doi.org/10.1111/jgs.17469.
- Thiart H, Ebert DD, Lehr D, Nobis S, Buntrock C, Berking M, et al. Internet-based cognitive behavioral therapy for insomnia: a health economic evaluation. Sleep 2016;39:1769-78. https://doi.org/10.5665/sleep.6152.
- Buntrock C, Lehr D, Smit F, Horvath H, Berking M, Spiegelhalder K, et al. Guided internet-based cognitive behavioral therapy for insomnia: health-economic evaluation from the societal and public health care perspective alongside a randomized controlled trial. J Med Internet Res 2021;23. https://doi.org/10.2196/25609.
- Witham MD, Anderson E, Carroll C, Dark PM, Down K, Hall AS, et al. INCLUDE Writing Group . Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process. Trials 2020;21. https://doi.org/10.1186/s13063-020-04613-7.
- Baglioni C, Altena E, Bjorvatn B, Blom K, Bothelius K, Devoto A, et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: an initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J Sleep Res 2020;29. https://doi.org/10.1111/jsr.12967.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trial with missing data. BMJ 2011;342. https://doi.org/10.1136/bmj.d40.
Appendix 1 Reasons for ineligibility from screening questionnaire
| Characteristic | Oxford | Manchester | Lincoln | Total |
|---|---|---|---|---|
| Total participants screened | 1940 | 648 | 583 | 3171 |
| SCI screened negative | 905 | 187 | 212 | 1304 |
| Night, evening, early morning or rotating shift work | 124 | 38 | 50 | 212 |
| Did not have difficulty falling asleep, staying asleep or wake up and return to sleep | 1 | 0 | 0 | 1 |
| Sleep problem due to caring, child care responsibility or noisy environment | 57 | 15 | 11 | 83 |
| SE ≥ 85% over the past month | 52 | 17 | 33 | 102 |
| Screened positive for possible narcolepsy | 116 | 23 | 31 | 170 |
| Screened positive for possible sleep apnoea | 75 | 14 | 9 | 98 |
| Screened positive for possible restless leg syndrome/periodic limb movements of sleep | 110 | 37 | 30 | 177 |
| Screened positive for possible circadian rhythm sleep–wake disorders | 13 | 3 | 9 | 25 |
| Screened positive for possible parasomnias | 9 | 9 | 2 | 20 |
| Have a diagnosis of, or are currently being treated for: | 13 | 7 | 6 | 26 |
| Dementia or MCI | 4 | 0 | 1 | 5 |
| Psychosis (schizophrenia) | 0 | 3 | 1 | 4 |
| Bipolar disorder | 2 | 1 | 0 | 3 |
| Epilepsy | 1 | 1 | 0 | 2 |
| Narcolepsy | 0 | 0 | 0 | 0 |
| Obstructive sleep apnoea | 3 | 0 | 0 | 3 |
| Restless leg syndrome | 5 | 3 | 4 | 12 |
| Currently receiving treatment for cancer | 8 | 1 | 1 | 10 |
| Currently receiving psychological treatment | 3 | 2 | 2 | 7 |
| Currently pregnant | 2 | 0 | 0 | 2 |
| Planning pregnancy in the next 6 months | 2 | 0 | 0 | 2 |
| Current suicidal ideation with intent | 3 | 1 | 1 | 5 |
| Attempted suicide in past 2 months | 0 | 0 | 3 | 3 |
| Planned major surgery within next 2 months | 2 | 2 | 2 | 6 |
| Life expectancy < 2 years | 3 | 0 | 2 | 5 |
Appendix 2 Results of sensitivity analyses for the primary outcome
Pattern mixture model results
Assumptions of the missing data mechanism were explored by imputing missing ISI outcome values that were up to five points either side of the observed average, both overall and in the SRT and SH arms separately. The results are displayed in Figure 21. If all participants with a missing ISI outcome at 6 months had an average ISI total score of 5 higher or 5 lower than those who were not missing, the estimated treatment effect and 95% confidence interval would still not include zero. If all participants with a missing 6-month ISI total score in the SRT group had an average ISI total score of 5 points higher than those who were not missing, and if all participants with a missing ISI total score in the SH group had an average ISI total score of 5 points lower than those who were not missing, the treatment effect and 95% CI would still not include zero.
FIGURE 21.
Results from pattern mixture model for the primary outcome at 6 months.
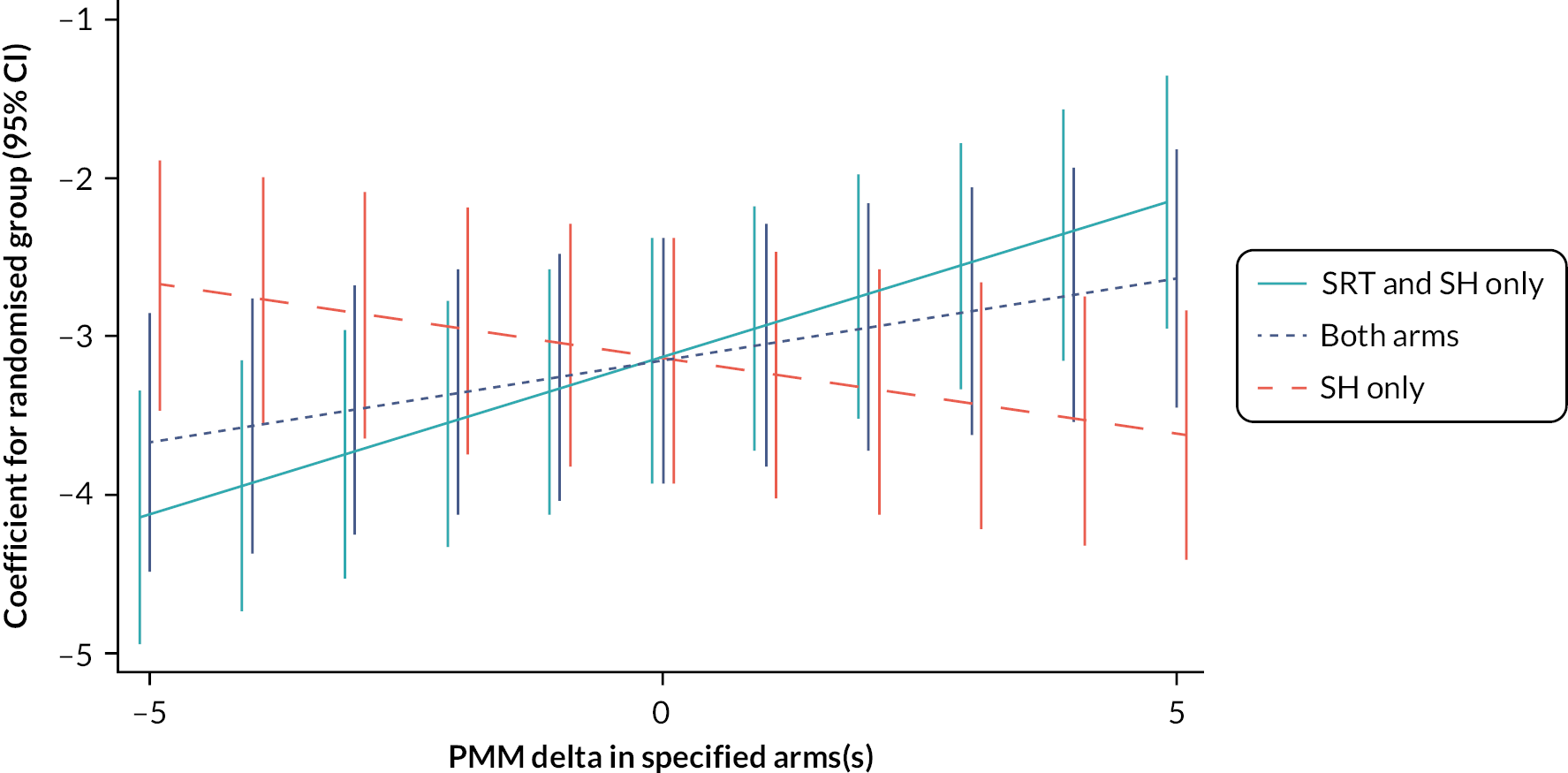
Assuming plausible arm-specific differences
We used the approach by White et al. 2011102 to carry out sensitivity analyses to investigate informative missingness of insomnia severity outcome data at 6 months. The following assumptions of differences between responders and non-responder were carried out:
-
when the proportions of missing ISI score at 6 months are assumed to be the same in both arms (i.e. both arms equally), assume the mean unobserved responses for ISI score at 6 months could be as much as 75% more or 50% less (i.e. −50%) than the mean of observed responses;
-
when the data are assumed to be informatively missing only in the SRT arm, assume the mean of unobserved responses for ISI score at 6 months could be as much as 50% more or 50% less (i.e. −50%) than the mean of observed responses;
-
when the data are assumed to be informatively missing only in the SH arm, assume the mean of unobserved responses for ISI score at 6 months could be as much as 50% more or 50% less (i.e. −50%) than the mean of observed responses;
-
additionally, more moderate sensitivity analyses include:
-
data are informatively missing in both arms, assume 50%
-
data are informatively missing in the SRT arm, assume as much as 25% more
-
data are informatively missing in SH arm, assume as much as 25% more.
-
Table 34 shows results when we assume plausible arm-specific differences of missing ISI score at 6 months between responders and non-responders. The results indicate that even with asymmetrical differences between responders and non-responders conclusions remain similar to the primary analysis.
| Non-responders differ in | Assumed difference between non-responders and responders | Adjusted mean difference (95% CI)a | p-valueb |
|---|---|---|---|
| Both arms equally | −50 | −3.23 (−4.00 to −2.45) | < 0.001 |
| 50c | −3.11 (−3.88 to −2.34) | < 0.001 | |
| 75 | −3.10 (−3.87 to −2.33) | < 0.001 | |
| Only SRT arm | −50 | −3.29 (−4.06 to −2.52) | < 0.001 |
| 25c | −3.11 (−3.88 to −2.34) | < 0.001 | |
| 50 | −3.07 (−3.85 to −2.30) | < 0.001 | |
| Only SH arm | −50 | −3.09 (−3.86 to −2.32) | < 0.001 |
| 25c | −3.18 (−3.95 to −2.40) | < 0.001 | |
| 50 | −3.19 (−3.96 to −2.42) | < 0.001 |
Appendix 3 Results for pre versus during pandemic
| SRT (N = 321) |
SH (N = 321) |
Adjusted treatment difference (95% CI)a | Test of interaction (p-value)b |
|
|---|---|---|---|---|
| ISI at 6 months, mean (SD) (N) | ||||
| 6-month follow-up assessment completion c | 0.420 | |||
| Pre-pandemic | 10.9 (5.82) (155) | 14.2 (5.15) (180) | −3.31 (−4.30 to −2.32) | |
| During pandemic | 10.9 (5.04) (102) | 13.4 (5.34) (111) | −2.65 (−3.90 to −1.41) | |
Appendix 4 Process evaluation framework categories and codes
| Process evaluation key theme | Category | Nurse codes | Patient codes | PM/GP codes |
|---|---|---|---|---|
| Implementation | Delivery of intervention | Consultations | Delivery as expected | Logistics |
| Modification to delivery | Well explained | Staff attitudes | ||
| Planned delivery | What could be improved | Wider implementation | ||
| Scaling of intervention | Positive | |||
| Worksheet paperwork | Post consultation | |||
| Understanding | ||||
| HABIT trial training | Improvement | GP experience of treating insomnia | ||
| Positives | GP understanding of intervention | |||
| Quality | Overall experience of the trial | |||
| Refresher training | ||||
| Patient expectations | Concerns | |||
| Expectations of SRT | ||||
| Previous experiences | ||||
| Mechanisms of impact | Response of patient | Barriers | Comparison to other treatments | |
| End of therapy | Effects | |||
| Facilitators | Feelings | |||
| Initial response | Improvements in insomnia | |||
| Logistics | Maintain SRT after trial | |||
| Patient attitude | ||||
| Withdrawal | ||||
| Context | Contextual factors | Previous experience | Challenges to SRT | Other |
| Other | Face-to-face appointments | |||
| Interactions with nurse | ||||
| Telephone appointments | ||||
| Other |
List of abbreviations
- AE
- adverse event
- CACE
- complier-average causal effect
- CBT
- cognitive–behavioural therapy
- CEAC
- cost-effectiveness acceptability curve
- CRN
- clinical research network
- CSRI
- Client Service Receipt Inventory
- DSM-V
- Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GP
- general practitioner
- GSES
- Glasgow Sleep Effort Scale
- GSII
- Glasgow Sleep Impact Index
- HABIT
- Health-professional Administered Brief Insomnia Therapy
- HRQoL
- health-related quality of life
- IAPT
- Improving Access to Psychological Therapies
- ICER
- incremental cost-effectiveness ratio
- ISI
- Insomnia Severity Index
- LCRN
- local clinical research network
- MCI
- mild cognitive impairment
- MCS
- mental component summary
- MEQr
- morningness–eveningness questionnaire reduced version
- MNAR
- missing not at random
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- OTC
- over the counter
- PCS
- physical component summary
- PHQ-9
- Patient Health Questionnaire-9 items
- PN
- practice nurse
- PSAS
- pre-sleep arousal scale
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SE
- sleep efficiency
- SF-36
- Short Form questionnaire-36 items
- SH
- sleep hygiene
- SOL
- sleep onset latency
- SQ
- sleep quality
- SRT
- sleep restriction therapy
- TST
- total sleep time
- WASO
- wake-time after sleep onset
- WPAI
- work productivity and activity impairment questionnaire
