Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the Evidence Synthesis Programme on behalf of NICE as project number NIHR132153. The contractual start date was in July 2020. The draft report began editorial review in January 2021 and was accepted for publication in May 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Cruickshank et al. This work was produced by Cruickshank et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Cruickshank et al.
Chapter 1 Background and research question
Description of health problem
Status epilepticus is the most severe form of epilepsy; it is a life-threatening neurological condition that requires urgent treatment. 1–3 Status epilepticus can be convulsive (i.e. with limb stiffness, abnormal posturing and jerking, so-called tonic–clonic seizures, often with impaired awareness/consciousness) or non-convulsive (i.e. altered consciousness with little or no limb movements) and can be of focal or generalised onset. 2,4,5 The focus of the present study is generalised convulsive status epilepticus. Generalised convulsive status epilepticus is defined as ‘≥ 5 min of (a) continuous seizures or (b) two or more discrete seizures between which there is incomplete recovery of consciousness’. 6
Status epilepticus arises because of the failure of mechanisms that abort seizure activity, that is either the breakdown of the mechanisms that terminate seizures or the instigation of mechanisms responsible for abnormally sustained seizures. 3,7,8 There are various causes of status epilepticus. In adults, the most common causes of status epilepticus are low levels of antiepileptic drugs in people with existing epilepsy, cerebrovascular disease, metabolic abnormalities, alcohol-related causes and hypoxia. 9 Status epilepticus can also occur in people with no history of epilepsy or in people with established epilepsy. 10
The incidence of status epilepticus has been reported as 10–60 per 100,000 population per year, with half of these people having convulsive status epilepticus. 11–14 Mortality has been reported as 10–20% and is generally related to the underlying condition. 10–13 Those at the highest risk of seizures are children and people aged > 60 years. 10 The incidence of status epilepticus is equal in males and females. 3
Status epilepticus is a medical emergency with significant morbidity and mortality, which can worsen with suboptimal or delayed treatment. 15–19 The likelihood of status epilepticus spontaneously resolving is negatively related to its persistence, and treatment should be given as quickly as possible. 1,20 It has been shown that early treatment of status epilepticus is associated with better outcomes in terms of seizures terminated on arrival at the hospital emergency department (ED) and reduced morbidity and mortality. 21–23 Therefore, the main goal of treatment of status epilepticus is to promptly stop both clinical and electroencephalographic seizure activity. 4,24
First-line treatment of status epilepticus is currently benzodiazepines, a class of drugs that bind the gamma-aminobutyric acid (GABA) receptor complex that modulates GABA release in the central nervous system and causes down-regulation of neuronal excitation (i.e. neurons become less excitable). 25,26 In the community, buccal midazolam is recommended as first-line treatment in children, young people and adults with prolonged or repeated seizures. Rectal diazepam can be administered if preferred or if buccal midazolam is not available. Intravenous (i.v.) lorazepam can be administered if i.v. access is already established and resuscitation can be facilitated. In addition, care plans outlining the home use of buccal midazolam or rectal diazepam are recommended for children, young people and adults who have had a previous episode of prolonged or serial convulsive seizures. 25
The purpose of this assessment is to conduct a synthesis of the current evidence on the clinical effectiveness and cost-effectiveness of treatments for convulsive status epilepticus in pre-hospital settings (i.e. the community and ED) and to inform the design and conduct of any future randomised controlled trials (RCTs) in this clinical context.
Current service provision
Variation in services and/or uncertainty about best practice
There is considerable uncertainty and a limited evidence base on the effectiveness of the first-line treatment options available for treating status epilepticus. As a result, treatment guidelines do not recommend any specific treatment over another and treatment decisions are frequently based on expert opinion. 4,24,27
A recent retrospective chart review conducted for patients in the USA diagnosed with status epilepticus within the last 10 years concluded that treatment for status epilepticus does not consistently follow guidelines or recommendations. In particular, underdosing of first-line benzodiazepines (i.e. lorazepam and midazolam) was identified. 28
Relevant national guidelines
The National Institute for Health and Care Excellence (NICE) guideline on the diagnosis and management of epilepsies, which was published in 2012 and subsequently updated (last updated 12 May 2020),25 provides recommendations on first-line treatment for convulsive status epilepticus in people of all ages. 25
In particular, the NICE clinical guidance25 supports the following recommendations:
-
Give immediate emergency care and treatment to children, young people and adults who have prolonged (lasting ≥ 5 minutes) or repeated (three or more in an hour) convulsive seizures in the community.
-
Prescribe buccal midazolam or rectal diazepam for use in the community only for children, young people and adults who have had a previous episode of prolonged or serial convulsive seizures.
-
Administer buccal midazolam as first-line treatment in children, young people and adults with prolonged or repeated seizures in the community. Administer rectal diazepam if preferred or if buccal midazolam is not available. If i.v. access is already established and resuscitation facilities are available, administer i.v. lorazepam.
-
Care must be taken to secure the child, young person or adult’s airway and assess their respiratory and cardiac function.
-
Depending on response to treatment, the person’s situation and any personalised care plan, call an ambulance, particularly if:
-
the seizure is continuing 5 minutes after the emergency medication has been administered
-
the person has a history of frequent episodes of serial seizures or has convulsive status epilepticus, or this is the first episode requiring emergency treatment
-
there are concerns or difficulties monitoring the person’s airway, breathing, circulation or other vital signs.
-
Scottish Intercollegiate Guidelines Network (SIGN) 143 guideline for the diagnosis and management of epilepsy in adults, which was originally published in 2015 and subsequently revised in 2018,16 states that many status epilepticus seizures resolve themselves without treatment; emergency treatment is necessary if there are continuous seizures or serial seizures lasting for ≥ 5 minutes. The current recommended treatments are:
-
10 mg of midazolam administered either buccally or intranasally
-
4 mg of i.v. lorazepam if midazolam is unavailable
-
10 mg of diazepam administered either intravenously or rectally if both midazolam and lorazepam are not available.
The guidance further points out the need to treat status epilepticus as soon as possible as it can worsen if treatment is delayed.
In 2016, the American Epilepsy Society published an algorithm for the treatment of convulsive status epilepticus. 29 The use of a benzodiazepine is recommended for initial therapy, specifically intramuscular (i.m.) midazolam, i.v. lorazepam or i.v. diazepam. If none of these is available, then i.v. phenobarbital, rectal diazepam or intranasal midazolam is recommended as first-line treatment. Recommended second-line options are i.v. fosphenytoin, i.v. valproic acid or i.v. levetiracetam, with i.v. phenobarbital recommended if these are not available.
An older set of guidelines, published by the European Federation of Neurologists in 2010, recommends i.v. administration of 4–8 mg of lorazepam or 10 mg of diazepam directly followed by 18 mg/kg phenytoin. 30
Description of technologies under assessment
Benzodiazepines
Benzodiazepines are currently the first treatment for status epilepticus. 25 In UK clinical practice, the most common benzodiazepines used as first-line treatment for status epilepticus are midazolam, diazepam and lorazepam. Benzodiazepines vary in their potency, onset and duration of effect, uptake, distribution, metabolism and presence or absence of active metabolites. 31,32 Lorazepam is more potent than midazolam, which in turn is more potent than diazepam. Midazolam and diazepam are more lipid soluble than lorazepam and are quicker in crossing the blood–brain barrier, resulting in a more rapid onset of action (2–10 minutes) than lorazepam (5–20 minutes). 26,33 The half-life of midazolam is 3–11 hours, compared with 8–15 hours for lorazepam and 20–120 hours for diazepam. 33,34 Midazolam and diazepam metabolites are active and they tend to accumulate with prolonged administration, especially in patients with renal dysfunction. 26,35 Lorazepam metabolites are not active and, for this reason, it is the preferred benzodiazepine in patients with renal failure. 26 Clonazepam has been investigated in the treatment of status epilepticus but is rarely used in the USA, as it is not available as an i.v. formulation. 36,37 Adverse effects of benzodiazepines include hypotension, respiratory depression, paradoxical agitation and delirium. 31,33,38
Midazolam
Midazolam is a short-acting, water-soluble benzodiazepine that is available as 2.5-mg, 5-mg, 7.5-mg and 10-mg oromucosal solutions for buccal administration. Oromucosal midazolam is indicated for treatment of prolonged, active, convulsive seizures in infants, toddlers, children and adolescents (from 3 months to < 18 years). The NICE guideline25 on diagnosis and management of epilepsy notes the lack of a UK marketing authorisation for this indication and/or population (i.e. adults) and stipulates that informed consent should be obtained and documented in line with normal standards in emergency care. 25 In the community, a single dose of midazolam can be administered by a carer. If the seizure has not stopped within 10 minutes of administration of midazolam, emergency medical assistance must be sought. A second or repeat dose when seizures re-occur after an initial response should not be given without prior medical advice. 39 Midazolam is also occasionally given as an intranasal preparation, although this is not available in the UK. Buccal preparation can be given intranasally (5 mg in each nostril, 10 mg total dose) as unlicensed use.
Diazepam
Diazepam is a benzodiazepine available for rectal, i.v. and i.m. administration. The therapeutic indications for rectal administration (5 mg or 10 mg of rectal solution) include epileptic and febrile convulsions, and it may be used in circumstances where i.v. administration is not available but rapid effects are required. 40 Rectal administration may be particularly suitable for infants and children. 40 The posology for adults is specified as 0.5 mg/kg body weight. 40 Similarly, the therapeutic indications for i.v. and i.m. administration (5-mg/ml emulsion for injection) include control of convulsions and status epilepticus, with posology specified as an initial dose of 0.15–0.25 mg/kg body weight by i.v. injection repeated in 30–60 minutes if required and followed, if necessary, by infusion of up to 3 mg/kg body weight over 24 hours. The emulsion can also be administered by slow i.v. injection (1 ml/minute). 41
Lorazepam
Lorazepam is a benzodiazepine that is available as a 4-mg/ml solution for injection. Therapeutic indications include the control of status epilepticus. Lorazepam injections can be administered intravenously or intramuscularly, but the i.v. route is preferred. The dosage for adults in status epilepticus is 4 mg intravenously. 42
Inclusion in this assessment was not limited to specific benzodiazepines; all of those used as first-line pre-hospital treatment of status epilepticus were eligible.
Antiepileptic drugs
The NICE guideline25 on diagnosis and management of epilepsy recommends further research into three antiepileptic drugs for the initial treatment of status epilepticus: levetiracetam, sodium valproate and phenytoin. 25 This assessment includes any worldwide study assessing the effects of these drugs as a first-line treatment of status epilepticus in adults. At present, in the UK, these drugs are mainly used as a second-line treatment when benzodiazepines have failed to stop seizures.
Levetiracetam
Levetiracetam is an anticonvulsant drug that was the first antiepileptic drug to be licensed in oral and i.v. forms at the same time (in 2006) and has since been used widely to treat status epilepticus. 8 The recommended starting dose for non-emergency monotherapy use in adults and adolescents aged ≥ 16 years is 250 mg taken twice daily. This can be increased to an initial therapeutic dose of 500 mg taken twice daily after 2 weeks and further increased by 250 mg taken twice daily every 2 weeks up to the maximum dose of 1500 mg taken twice daily. 43 Emergency dosing for status epilepticus is not specified in the summary of product characteristics. The recent Established Status Epilepticus Treatment Trial (ESETT) of second-line treatment of patients with established status epilepticus in the emergency room specified weight-based infusion dosage of 60 mg/kg (maximum 4500 mg) levetiracetam. 44 However, first-line dosing is not specified in the literature.
Sodium valproate
Sodium valproate is an anticonvulsant drug that has been extensively used to treat primary generalised and partial-onset seizures; i.v. sodium valproate was approved by the US Food and Drug Administration (FDA) in 1996. 45 It is available as a 100-mg/ml solution for injection or infusion and is indicated for people with epilepsy who are normally maintained on oral sodium valproate when oral therapy is temporarily not possible. 46 Dosage requirements vary according to age and body weight. 46 Similarly, emergency first-line dosing of sodium valproate for status epilepticus is not specified in the summary of product characteristics or the literature in general. ESETT specified a fixed dose of 300 mg over 10 minutes, but first-line treatment may involve slightly lower doses. 44
Phenytoin
Phenytoin has long been used for the management of status epilepticus in circumstances when benzodiazepines are ineffective and is recommended as second-line treatment in hospital for ongoing status epilepticus. 25 However, there is insufficient evidence to support its efficacy as first-line treatment over other anticonvulsant drugs for status epilepticus in adults and children. 47 It is available as a 50-mg/ml solution for injection and is indicated for the control of status epilepticus of the tonic–clonic (grand mal) type and prevention and treatment of seizures occurring during or following neurosurgery and/or severe head injury. For patients showing continuous (rather than serial) seizure activity, i.v. diazepam or a short-acting barbiturate is recommended prior to the administration of phenytoin because of their faster onset of action. For the subsequent treatment of continuous seizures and the initial management of serial epilepsy, a loading dose of 10–15 mg/kg phenytoin should be administered intravenously, followed by a maintenance dose of 100 mg given orally or intravenously every 6–8 hours. 48
Phenobarbital
Phenobarbital is also recommended as second-line treatment in hospital for ongoing convulsive status epilepticus. 25 Phenobarbital was the first antiepileptic drug available and is still in use today, as it is considered to be both clinically effective and cost-effective. 49 Phenobarbital is available as a 30 mg/ml solution for injection and is indicated as an anticonvulsant for the treatment of all forms of epilepsy except absence seizures. A dose of 50–200 mg administered by i.m., subcutaneous or i.v. (after dilution) injection is recommended for the treatment of adults; this can be repeated after 6 hours if necessary. 50
Identification of important subgroups
Early treatment of convulsive status epilepticus has been found to be related to reduced morbidity and mortality and improved outcomes, such as shorter duration of seizures. 22,23,51 In addition, ongoing seizure activity may also render relevant drugs less effective. 52,53 It is common for patients with status epilepticus to be dealt with outside hospital settings, and delays in transfer to hospital may hamper prompt treatment. 22 Treatment of status epilepticus in the pre-hospital setting is potentially better than delaying it until arrival at the ED. Therefore, comparing outcomes of patients treated at the ED with those of patients treated in the pre-hospital setting was considered relevant to the scope of this assessment. 51
Current use
A recent US-based retrospective chart review of patients diagnosed with status epilepticus showed that lorazepam was the most frequently administered first-line treatment, a finding in accordance with an international survey of clinical experts on the treatment of status epilepticus. 27,28 The second-line treatments of choice were fosphenytoin and phenytoin, respectively. 27,28 In contrast, UK guidelines25 recommend i.v. phenobarbital or phenytoin as a second-line treatment in hospital for convulsive status epilepticus. The SIGN 143 guideline16 recommendation is for i.v. sodium valproate or i.v. phenytoin to be used as a second-line treatment in hospital (with the warning that sodium valproate is contraindicated in pregnancy and in people of childbearing potential). A further recommendation from the British Medical Journal Best Practice3 for second-line treatment is fosphenytoin/phenytoin, valproic acid or levetiracetam.
Overall aim and objectives of this assessment
The aim of this assessment was to synthesise current evidence on the effects of first-line antiepileptic drugs for the treatment of convulsive status epilepticus in adults either before arriving at the hospital or at arrival at the ED. Information on economic evaluations published in this clinical area was also collected and synthesised.
Chapter 2 Assessment of clinical effectiveness
This chapter reports the evidence for the clinical effectiveness of treatments for status epilepticus in pre-hospital and ED settings.
Systematic review methods
The systematic review was conducted in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses. 54,55 The methods for this assessment were pre-specified in a research protocol (PROSPERO registration CRD42020201953) (www.crd.york.ac.uk/prospero/display_record.php?RecordID=201953; accessed 4 August 2020).
Identification of studies (search strategy and information sources-dates)
A sensitive search strategy to identify all relevant literature, using database index terms and text words, was developed by an information specialist. The search encompassed the facets of status epilepticus, all benzodiazepines and antiepileptics in addition to named drugs, and the pre-hospital setting. The results were limited to RCTs in accordance with the review protocol. There were no date or language restrictions. The electronic databases searched included MEDLINE, EMBASE and PsycInfo® (all via the Ovid interface), and EBSCOhost, CINAHL and Cochrane CENTRAL. The searches were carried out in July 2020. The complete search strategies are reported in Appendix 1.
Inclusion and exclusion criteria
Types of studies
Evidence was considered from parallel-group RCTs and cluster RCTs assessing pharmacological interventions for the first-line treatment of convulsive status epilepticus in adults. Studies were included regardless of their publication status or language of publication. The following types of reports were excluded:
-
crossover trials
-
single-armed studies and observational studies
-
narrative reviews, editorials and opinions
-
case reports
-
conference abstracts for which a full publication or further methodological information could not be found
-
non-English-language reports for which a translation could not be organised.
Types of participants
Eligible participants for this assessment were:
-
adults (aged ≥ 16 years) with convulsive status epilepticus attended out of hospital by non-medical staff (e.g. caregiver)
-
adults (aged ≥ 16 years) with convulsive status epilepticus attended out of hospital by paramedics
-
adults (aged ≥ 16 years) experiencing convulsive status epileptics out of hospital who are attended (receive their first-line treatment) at arrival at the ED.
We did not restrict eligibility of participants to a specific definition of status epilepticus. Traditionally, status epilepticus was defined as a seizure lasting ≥ 30 minutes, but more recent definitions indicate ≥ 5 minutes of either continuous seizure activity or repetitive seizures with no recovery of consciousness in between. 3,7 It was anticipated that some studies may enrol participants with a metabolic cause of status epilepticus, for example hypoglycaemia or severe sodium or potassium imbalances. Some such metabolic disorders need to be corrected slowly (e.g. hyponatraemia or hypernatraemia) but the use of anticonvulsants is still appropriate to stop the seizure, while correcting the underlying metabolic disorder. Therefore, studies with a mixed population of patients with a known epilepsy syndrome and patients with a reversible metabolic cause of seizures were deemed eligible for inclusion. Studies recruiting a mix of other eligible and ineligible participants (e.g. studies including both children and adults) were deemed suitable for inclusion, providing that demographic and outcome data were reported separately for the group of interest or the proportion of ineligible participants was < 10% of the study participants. Studies that focused exclusively on children and young adults or on patients with non-convulsive status epilepticus were excluded.
Types of interventions
The interventions considered were any benzodiazepine offered as first-line treatment for the treatment of convulsive status epilepticus in adults administered either on site by paramedics or non-medical staff before or during transfer of patient to the ED or on arrival at the ED by ED staff.
Newer antiepileptic drugs (AEDs) including levetiracetam, sodium valproate and phenytoin, so far as they were used as first-line treatment in the pre-hospital setting or at arrival at the ED, were also considered. Pharmacological treatments were considered regardless of whether they were used as monotherapy or combination therapy and regardless of their routes of administration [i.e. i.v., i.m., intranasal, buccal, rectal or oromucosal administration].
Any second-line treatment of convulsive status epilepticus was excluded, as were antiepileptic drugs and anticonvulsants used in hospital or intensive care units (ICUs). We considered first-line treatment as any immediate pharmacological treatment that could be repeated once, and second-line treatment as any subsequent pharmacological treatment that involved the use of another class of drug, such as an anticonvulsant. Studies with a focus on in-hospital or second-line treatments of status epilepticus were excluded.
Types of comparators
The comparators considered included placebo or any of the active treatments eligible for this review. Comparisons of two or more active treatments or two or more treatment protocols of the same active treatment (e.g. different doses, dose frequencies or routes of administration) were eligible for inclusion.
Types of outcomes
The following primary outcomes were considered:
-
seizure cessation, measured in terms of –
-
the number of people with cessation of seizure activity within 5–15 minutes of study drug administration (or any designated period of time as specified by trial investigators)
-
the time to seizure cessation from the time of study drug administration
-
-
recurrence of seizure, measured in terms of –
-
the number of people with recurrence of seizures within a designated period (probably 12 hours)
-
the time from seizure cessation to recurrence
-
-
adverse events (AEs) –
-
respiratory depression
-
30-day mortality.
-
Secondary outcomes considered were:
-
need for additional drugs to stop seizure (within 12 hours)
-
need for hospital admission
-
length of stay in ICU
-
6-month mortality
-
return to baseline function (3–6 months)
-
health-related quality of life (e.g. psychosocial sequelae, depression, anxiety)
-
number of people requiring an emergency call out (among those attended out of hospital by non-medical staff).
Data extraction strategy
Two review authors (MC and MI) independently screened all citations identified by the search strategies. Full-text versions of potentially relevant articles were retrieved and independently assessed for eligibility by the same two reviewers. Disagreements were resolved by discussion or consultation with a third review author (MB, LA or CC). A data extraction spreadsheet was developed for the purpose of this assessment, piloted and amended as necessary. Two review authors (MC and MI) independently extracted information from each study on study characteristics, participant characteristics, flow of participants through the study, details of intervention/comparators, specified outcomes, potential confounding factors, funding and declarations of interest by study investigators. Disagreements were resolved by discussion.
Critical appraisal strategy
The risk of bias of included RCTs was assessed independently by two review authors (MC and MI) using a revised Cochrane Risk of Bias tool for randomised trials (RoB 2). 56 Disagreements were resolved by discussion. For the purposes of the risk-of-bias assessments only, we categorised the specified outcomes into two categories, with advice from the review team’s clinical expert (CC): subjective (i.e. requiring judgement by the observer) or objective (i.e. not requiring judgement by the observer). It was decided to split the subjective outcome ‘respiratory depression’ to include the specified objective outcome ‘respiratory depression requiring ventilation’ to take account of the objective nature of the ventilation component. The outcomes were categorised as follows:
-
subjective outcomes –
-
seizure cessation
-
recurrence of seizure
-
respiratory depression (all related outcomes with the exception of respiratory depression requiring ventilation)
-
return to baseline neurological function
-
quality of life.
-
-
objective outcomes –
-
mortality
-
need for additional treatment
-
need for hospital admission
-
length of stay in ICU
-
number of people requiring an emergency call out
-
respiratory depression requiring ventilation.
-
Methods of data synthesis
Random-effects meta-analyses and subgroup analyses had been specified in the protocol but were not carried out owing to the limited number of identified studies and their heterogeneity in terms of treatment comparisons and reported outcomes. The results of each included study were tabulated and summarised narratively for each outcome.
Results of the evidence synthesis
Quantity of the evidence (studies included and excluded)
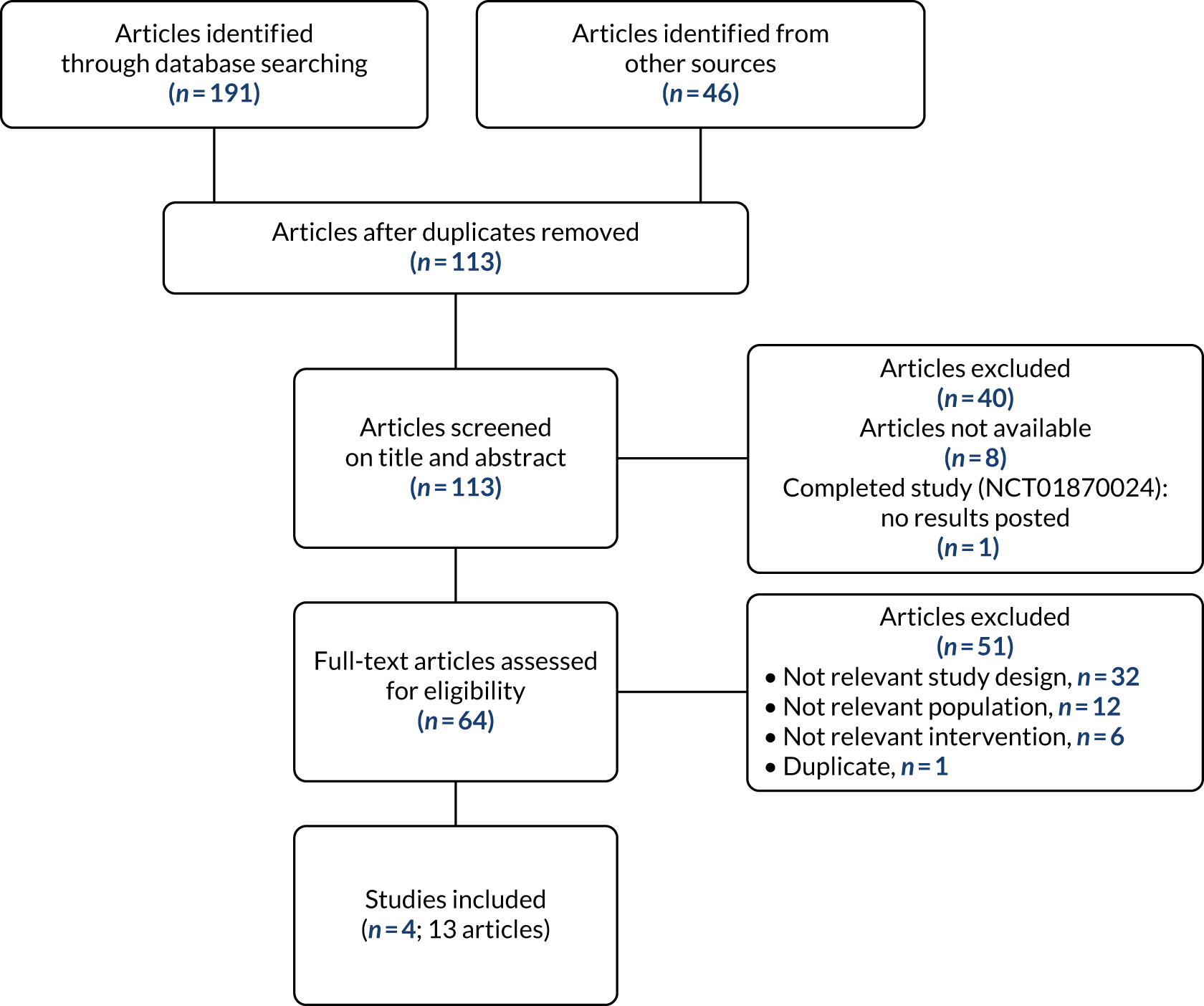
The literature searches identified 191 records, and 46 additional records were identified in the study brief for a total of 237 retrieved records. After deduplication, 113 records were screened for relevance. Of these, 73 were considered potentially relevant and selected for full-text assessment. Eight articles could not be obtained because of COVID-19 pandemic restrictions. The search also identified one ongoing study. Of the 64 articles retrieved and assessed in depth, 13 articles relating to four studies met the inclusion criteria, while 51 articles were not deemed suitable for inclusion.
A PRISMA flow diagram detailing the process of study selection is presented in Figure 1. Appendices 2–4 provide the bibliographic details of the included, excluded and ongoing studies, respectively.
FIGURE 1.
The PRISMA flow diagram of selected studies. Reproduced with permission from Cruickshank et al. 60 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Study characteristics
The study characteristics of the four included RCTs22,57–59 (total number of participants 1345, of whom 1234 were adults) are detailed in Appendix 5 and are summarised in Table 1. All four trials were published in full. Three trials enrolled only adults22,57,59 and one trial included a mixed population of adults (89%) and children (11%). 58 Each trial reported a different treatment comparison. The trial by Alldredge et al. ,22 with a total of 205 participants, was the only three-arm trial and compared 2 mg of i.v. lorazepam with 5 mg of i.v. diazepam and i.v. placebo; the trial by Navarro et al. ,57 with a total of 203 participants, compared 2.5 g of i.v. levetiracetam plus 1 mg of clonazepam with i.v. placebo plus 1 mg of clonazepam; the trial by Silbergleit et al. ,58 with a total of 893 participants, of whom 782 were adults, compared 10 mg of i.m. midazolam with 4 mg of i.v. lorazepam; and the remaining trial, by Shaner et al. ,59 with a total of 44 participants, assessed 100 mg/minute i.v. phenobarbital plus 40 mg/minute optional phenytoin compared with 2 mg/minute i.v. diazepam plus 40 mg/minute phenytoin.
| Study (first author and year of publication) | Geographical location | Type of comparison | Total number of participants randomised | Number of centres | Primary outcomes |
|---|---|---|---|---|---|
| Alldredge 200122 | USA | 2 mg of i.v. lorazepam vs. 5 mg of i.v. diazepam vs. i.v. placebo | 205 | 10a | Termination of status epilepticus by arrival at the ED |
| Navarro 201657 | France | 2.5 g of i.v. levetiracetam plus 1 mg of i.v. clonazepam vs. 1 mg of i.v. clonazepam plus i.v. placebo | 203 | 39b | Cessation of convulsions within 15 minutes of study drug administration |
| Shaner 198859 | USA | 100 mg/minute i.v. phenobarbital plus 40 mg/minute i.v. phenytoin vs. 2 mg/minute i.v. diazepam plus 40 mg/minute i.v. phenytoin | 44 | 1 | Cumulative convulsion time |
| Silbergleit 201258 | USA | 10 mg of i.m. midazolam vs. 4 mg of i.v. lorazepam | 893 (published data); 782 (adults’ data) | 79c | Seizures terminated without need for rescue therapy before arrival at the ED |
Three trials involved the administration of study drugs by paramedics22,57,58 and one study was based in an emergency room setting. 59
Three trials were conducted in the USA22,58,59 and one trial was conducted in France. 57 Shaner et al. ,59 recruited participants from a single centre, whereas the other three trials were multicentre: one physician-based hospital and nine destination hospitals;22 13 emergency medical centres and 26 hospital departments;57 and 4314 paramedics, 33 emergency medical centres and 79 receiving hospitals. 58
Participant characteristics
The eligible population in the trial by Silbergleit et al. ,58 known in the literature as the Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART), consisted of adults and children with body weight of at least 13 kg. Via personal correspondence with the principal investigator (Professor Robert Silbergleit, Department of Emergency Medicine, University of Michigan, MI, USA), it was established that 116 participants were aged < 16 years and that a publicly accessible data set of participant-level data was available. This data set was obtained, and relevant data for participants aged ≥ 16 years were extracted. Despite there being a comprehensive data dictionary alongside the data, it was not always possible to identify which variables were pertinent for the relevant outcomes. Therefore, only primary outcomes that were considered to be of clear origin in the data set are reported in this assessment.
Table 2 presents a summary of the characteristics of participants included in the four trials and Appendix 6 presents full details of participants’ characteristics.
| Study (first author and year of publication) | Study arm | Number analysed | Age (years), mean (SD) | Gender (M/F), n (%) | Ethnicity, % | Final diagnosis, n (%) | Time from onset of convulsive status epilepticus to study drug administration; minutes, mean (SD) or median [range] |
|---|---|---|---|---|---|---|---|
| Alldredge 200122 | i.v. LOR | 66 | 49.9 (20.1) | M 46 (70) | Black: 18.2 | NR | 34.0 (17.8) |
| F 20 (30) | White: 48.5 | ||||||
| Other:a 33.3 | |||||||
| i.v. DIZ | 68 | 50.4 (19.1) | M 41 (60) | Black: 16.2 | NR | 31.3 (14.5) | |
| F 27 (40) | White: 54.4 | ||||||
| Other:a 29.4 | |||||||
| i.v. PBO | 71 | 52.0 (18.2) | M 42 (59) | Black: 29.6 | NR | 46.7 (38.8) | |
| F 29 (41) | White: 46.5 | ||||||
| Other:a 23.9 | |||||||
| Navarro 201657 | i.v. LEV + CLZ | 68 | 55 (18) | M 49 (72) | NR | SE: 66 (97.1) | 58 [15–135] |
| F 19 (28) | Non-epileptic: 2 (2.9) | ||||||
| i.v. PBO + CLZ | 68 | 53 (18) | M 45 (66) | NR | SE: 64 (94.1) | 60 [20–258] | |
| F 23 (34) | Non-epileptic: 4 (5.9) | ||||||
| Shaner 198859 | i.v. PHB + PHT | 18 | 55.9 (19.4) | M 13 (72) | NR | GCSE:b 18 (100) | NR |
| F 5 (28) | Other:c 0 (0) | ||||||
| i.v. DIZ + PHT | 18 | 43.8 (16.5) | M 9 (50) | NR | GCSE:b 17 (94) | NR | |
| F 9 (50) | Other:c 1 (6) | ||||||
| Silbergleit 201258 (published data) | i.m. MDZ | 448 | 43 (22) | M 250 (56) | Black: 51.1 | SE: 404 (90) | NR |
| F 198 (44) | White: 36.8 | Non-epileptic: 31 (7) | |||||
| Other:a 12.1 | Undetermined: 13 (3) | ||||||
| i.v. LOR | 445 | 44 (22) | M 238 (53) | Black: 50.3 | SE: 399 (90) | NR | |
| F 207 (47) | White: 41.1 | Non-epileptic: 32 (7) | |||||
| Other:a 8.5 | Undetermined: 14 (3) | ||||||
| Silbergleit 201258 (adult population) | i.m. MDZ | 391 | 48 (17) | M 217 (56) | Black: 54.0 | SE: 352 (90) | NR |
| F 174 (44) | White: 35.3 | Non-epileptic: 28 (7) | |||||
| Other:a 10.7 | Undetermined: 11 (3) | ||||||
| i.v. LOR | 391 | 49 (18) | M 203 (52) | Black: 52.2 | SE: 348 (89) | NR | |
| F 188 (48) | White: 39.9 | Non-epileptic: 29 (7) | |||||
| Other:a 7.9 | Undetermined: 14 (4) |
The mean age was reported in all four trials and ranged from 55 years57 to 66 years22 in the active treatment arms and from 43.8 years59 to 71 years22 in the control arms. All four trials also reported the sex of participants and all involved more males than females, with around two-thirds of study populations tending to be male.
With regard to the discharge diagnosis, two trials distinguished between status epilepticus and non-epileptic psychogenic events or spells. Navarro et al. 57 reported a final diagnosis of status epilepticus for 97.1% of participants in the levetiracetam plus clonazepam group and 94.1% of participants in the placebo plus clonazepam group and a diagnosis of a non-epileptic psychogenic event for 2.9% and 5.9% of participants in each treatment group, respectively. For each treatment group (midazolam group and lorazepam group), Silbergleit et al. 58 reported a diagnosis of status epilepticus for 90% of participants, a diagnosis of a non-epileptic spell for 7% of participants and an undetermined diagnosis for 3% of participants. Shaner et al. 59 reported 55.6% and 50% participants with focal features in the phenobarbital and placebo groups, respectively.
Two trials reported time from onset of status epilepticus to drug administration. Alldredge et al. 22 reported means of 34 [standard deviation (SD) 17.8] minutes, 31.3 (SD 14.5) minutes and 46.7 (SD 38.8) minutes for the lorazepam, diazepam and placebo groups, respectively. Navarro et al. 57 reported a median of 58 (range 15–135) minutes for the levetiracetam plus clonazepam group and a median of 60 (range 20–258) minutes for the placebo plus clonazepam group.
With respect to the total dose of active drug administered (see Appendix 6), Alldredge et al. 22 reported the number of injections received by the study participants [lorazepam arm, 34 participants received one injection (2 mg) and 32 received two injections (4 mg); diazepam arm, 34 participants received one injection (2 mg) and 32 received two injections (4 mg); placebo arm, 29 participants received one injection and 42 received two injections]. Shaner et al. 59 reported a range of 5–23 mg/kg phenobarbital administered in the phenobarbital plus optional phenytoin arm. In addition, seven participants in this group received phenytoin in doses ranging from 6 to 23 mg/kg. The remaining two trials did not report the total dose of study drug. 57,58
All four included trials reported causes of status epilepticus (see Appendix 6). A subtherapeutic level of AEDs was the most common cause in three trials,22,58,59 while lesion was reported as the most frequent cause of status epilepticus in the Navarro et al. trial. 57 Other commonly reported causes were alcohol abuse22,57 or withdrawal,59 infections22,57,59 and metabolic factors. 22,57,59
None of the included trials reported the patients’ previous history of status epilepticus. The proportion of participants with a history of seizures ranged from 54.6% to 66.2% in the treatment arms of the Alldredge et al. trial22 and from 61% to 78% in the treatment arms of the Shaner et al. trial. 59 The proportion of participants with a history of epilepsy ranged from 59% to 70%57 in the treatment arms of the Navarro et al. trial57 and from 65% to 66% in the Silbergleit et al. trial. 58 None of the trials reported presence of known difficult-to-treat epilepsy, or functional dependence status at baseline.
All characteristics reported in the publications by Silbergleit et al. 58 (i.e. RAMPART) were comparable to those extracted for the adult population from the data set received by the trial investigators. 58
Risk-of-bias assessment of included studies
Each risk-of-bias domain was assessed separately for objective outcomes and for subjective outcomes for each of the four included trials. Therefore, a total of eight risk-of-bias assessments are reported. Figure 2 presents the summary of the risk-of-bias assessments for all included trials. Risk-of-bias assessments of individual trials are presented in Figure 3.
FIGURE 2.
Summary of risk of bias of all included trials.
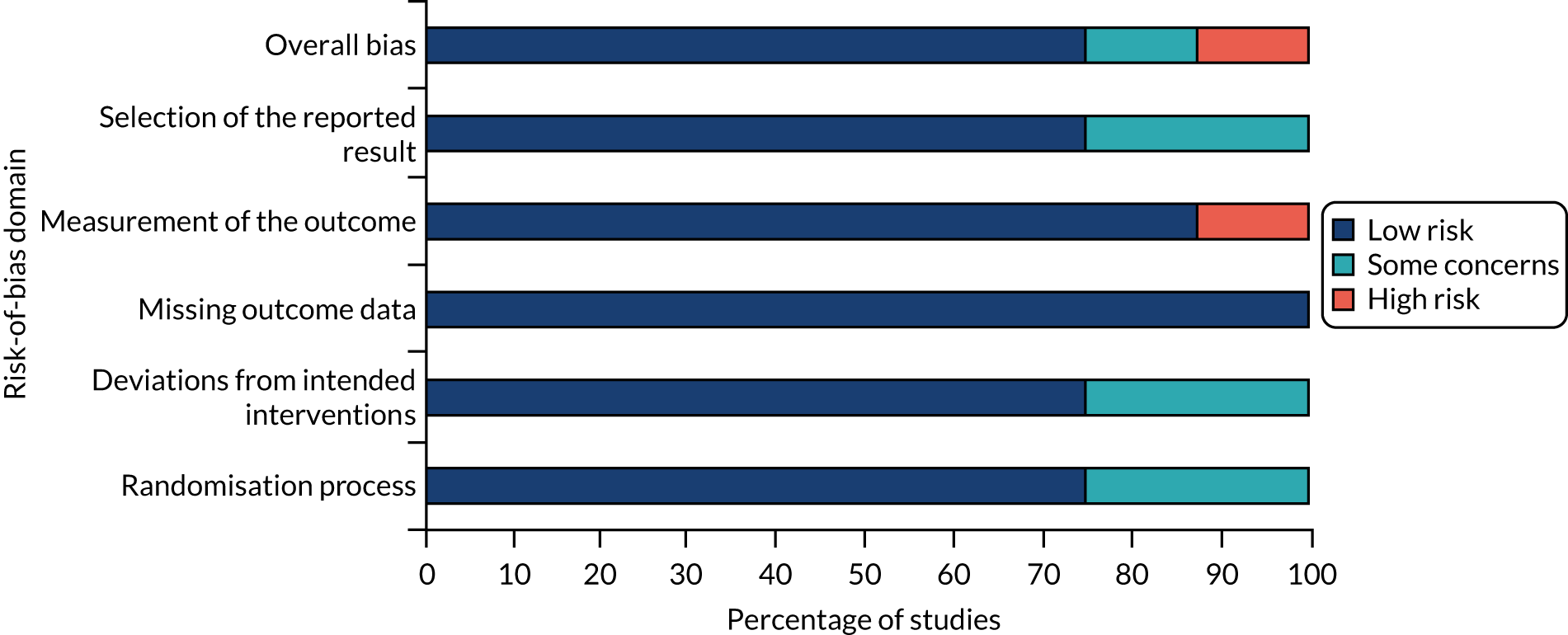
FIGURE 3.
Risk-of-bias assessments of individual trials apart from Shaner et al. ,59 which was judged to be at a high risk of bias (due to the high risk-of-bias assessment of measurement of subjective outcomes). All other trials were considered to have a low risk of bias. 22,57,58 CLZ, clonazepam; DIZ, diazepam; LEV, levetiracetam; LOR, lorazepam; MDZ, midazolam; PBO, placebo; PHB, phenobarbital; PHT, phenytoin. Reproduced with permission from Cruickshank et al. 60 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
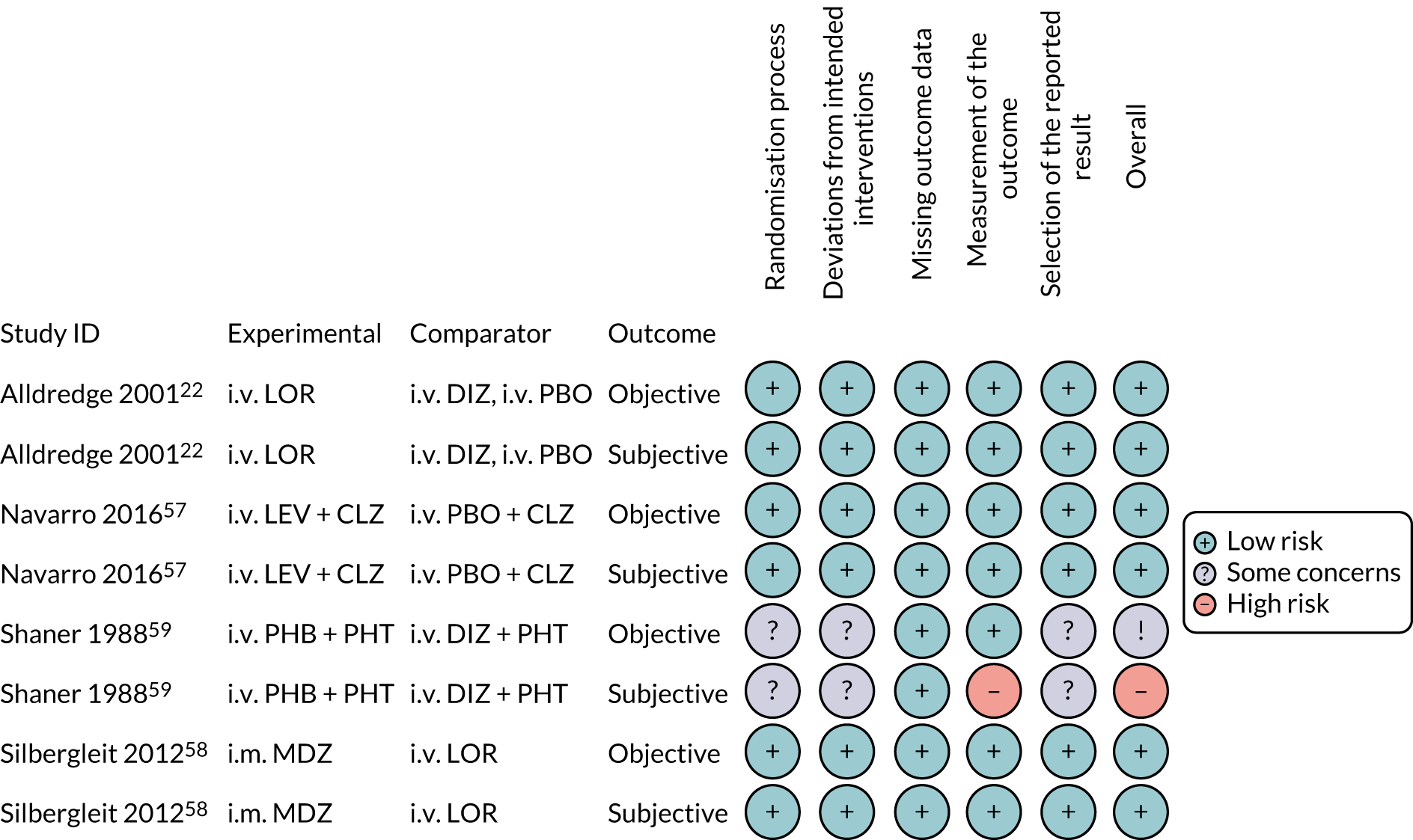
For the ‘randomisation process’ domain, three trials were judged to be at a low risk of bias,22,57,58 but in the Shaner et al. 59 trial there was insufficient information on which to make a definitive judgement.
Regarding the ‘deviations from intended interventions’ domain, three trials were properly blinded and judged to be at a low risk of bias;22,57,58 the trial by Shaner et al. 59 was described as ‘non-blinded’, indicating that those delivering the intervention would have been aware of the allocated intervention. Nevertheless, it was considered unlikely that participants would have been aware of the intervention received because of the nature of the clinical condition (i.e. status epilepticus). There was insufficient information on which to make a judgement regarding deviations from the intended intervention due to the experimental context. The combination of these factors led to a judgement of ‘some concerns’ for this trial. 59
All four trials were judged to be at a low risk of bias for ‘missing outcome data’ because of the low withdrawal/discontinuation rates, which were balanced across treatment groups. 22,57–59
The ‘measurement of the outcome’ domain was judged to be at a low risk of bias for three trials for both objective and subjective outcomes. 22,57,58 As the Shaner et al. 59 trial was not blinded, the measurement of the subjective outcomes was judged to be at a high risk of bias, but objective outcomes were less likely to be affected by this bias and, therefore, were judged to be at a low risk of bias.
For the Shaner et al. 59 trial, there was insufficient information on which to make a robust judgement about the ‘selection of the reported result’. There was no evidence of selective reporting in the remaining three trials, which were considered to be at a low risk of bias for this domain. 22,57,58
Overall, three trials were assessed to be at a low risk of bias for both the subjective and the objective outcomes. 22,57,58 The trial by Shaner et al. ,59 which was published in 1988 and was not blinded, was assessed as being at a high risk of bias for subjective outcomes and with some concerns for objective outcomes.
Results of the individual studies and synthesis: primary outcomes
A summary of the primary clinical outcomes reported in the four included trials is presented in Table 3. Full details of these outcomes, including outcome definitions, are presented in Appendices 7 and 8.
| Study (first author and year of publication) | Arm | Seizure cessation | Recurrence of seizures | |||
|---|---|---|---|---|---|---|
| Number of people with cessation of seizure activity, n/N (%) | Effect estimate | Time to seizure cessation from administration of study drug (minutes) | Number of people with recurrence of seizures, n/N (%) | Time from seizure cessation to recurrence (minutes), mean (SD) | ||
| Alldredge 200122 | i.v. LOR (n = 66) | 39/66 (59.1) | LOR vs. PBO: OR 4.8 (95% CI 1.9 to 13.0)a | LOR vs. PBO: HR 2.94 (95% CI 1.41 to 5.88)b | NR | NR |
| i.v. DIZ (n = 68) | 29/69 (42.0) | LOR vs. DIZ: OR 1.9 (95% CI 0.8 to 4.4)a | LOR vs. DIZ: HR 1.54 (95% CI 0.85 to 2.77)b | NR | NR | |
| i.v. PBO (n = 71) | 15/71 (14.3) | DIZ vs. PBO: OR 2.3 (95% CI 1.0 to 5.9)a | NR | NR | ||
| Navarro 201657 | i.v. LEV + CLZ (n = 68) | 50/68 (73.5) | RR 0.88 (95% CI 0.74 to 1.05) | Median 3 (range 0–50) | 7/67 (10.4)c | NR |
| i.v. PBO + CLZ (n = 68) | 57/68 (83.8) | Median 5 (range 0–41) | 13/68 (19.1)c | NR | ||
| Shaner 198859 | i.v. PHB + PHT (n = 18) | 13/18 (72.2) | NR | Median 5.5 | NR | NR |
| i.v. DIZ + PHT (n = 18) | 6/18 (33.3) | Median 15 | NR | NR | ||
| Silbergleit 201258 (published data) | i.m. MDZ (n = 448) | 329/448 (73.4) | AD 10 percentage points (95% CI 4.0 to 16.1 percentage points); p < 0.001 for non-inferiority and p < 0.001 for superiority | Median 3.3 | 51/448 (11.4)d | NR |
| i.v. LOR (n = 445) | 282/445 (63.4) | Median 1.6 | 47/445 (10.6)d | NR | ||
| Silbergleit 201258 (adult data) | i.m. MDZ (n = 391) | 289/391 (73.9) | NR | Median 3 (IQR 2–6.3) | 47/391 (12.0)d | NR |
| i.v. LOR (n = 391) | 244/391 (62.4) | Median 2 (IQR 1–4.4) | 42/391 (10.7)d | NR | ||
All four included trials reported the number of people with cessation of seizure activity, albeit the definitions of the outcome varied across studies. Two trials22,58 defined the outcome as termination of seizures before arrival at the ED, with one of these also specifying that no rescue therapy was needed. 58 These two trials both involved i.v. administration of lorazepam in either the intervention group22 or the control group,58 with the proportion of participants in the lorazepam groups with seizure cessation being similar (59.1% and 63.4%, respectively). The trial by Alldredge et al. 22 showed that, compared with placebo, both 2 mg of i.v. lorazepam and 5 mg of i.v. diazepam were effective in terminating the episodes of status epilepticus (the proportions of participants with seizure cessation were 59.1%, 42.0% and 21.1%, in the lorazepam, diazepam and placebo groups, respectively). 22 In RAMPART, by Silbergleit et al. ,58 assessing 10 mg of i.m. midazolam versus 4 mg of i.v. lorazepam, the proportion of adults achieving seizure cessation at the time of arrival in the hospital ED was similar between intervention groups (73.9% and 62.4% in the i.m. midazolam and i.v. lorazepam groups, respectively) and akin to those reported for the overall mixed population (73.4% and 63.4% in the i.m. midazolam and i.v. lorazepam groups, respectively). 58
The remaining two trials specified that seizures had to stop within either 15 minutes57 or 10 minutes59 from onset of treatment. In general, the proportion of participants with seizure cessation was similar across these trials and across treatment groups: 73.2% of participants in the 2.5 g of i.v. levetiracetam plus 1 mg of clonazepam group and 83.8% of participants in the i.v. placebo plus 1 mg of clonazepam group of the Navarro et al. trial,57 and 72.2% of participants in the 100 mg/minute i.v. phenobarbital plus 40 mg/minute phenytoin group in the Shaner et al. 59 trial. The exception was the 2 mg/minute i.v. diazepam plus 40 mg/minute phenytoin control group in the Shaner et al. 59 trial, which reported only 33.3% of participants with seizure cessation.
All four trials reported time to seizure cessation from administration of study drug using broadly similar definitions. The median time for i.v. administration ranged from 1.6 (i.v. lorazepam group)58 to 15 minutes (i.v. diazepam plus phenytoin group). 59 The Navarro et al. 57 trial reported administration times of up to 50 minutes. For time to seizure cessation, Alldredge et al. 22 reported a HR of 2.94 [95% confidence interval (CI) 1.41 to 5.88] for the lorazepam versus the placebo comparison and a HR of 1.54 (95% CI 0.85 to 2.77) for the lorazepam versus the diazepam comparison.
Two trials reported the number of participants with recurrence of seizures. Navarro et al. 57 reported that 10.4% of the i.v. levetiracetam plus clonazepam group and 19.1% of the placebo plus clonazepam group experienced seizure recurrence during their hospital stay, whereas Silbergleit et al. 58 reported that 11.4% of participants in the i.m. midazolam group and 10.6% of participants in the i.v. lorazepam group experienced recurrence of seizures within 12 hours of arrival at the ED.
None of the four included trials reported time from seizure cessation to recurrence. Silbergleit et al. 58 reported that the mean time of seizure recurrence since ED arrival for the adult population was 4.3 (SD 9.4) minutes in the i.m. midazolam group (n = 53) and 4.5 (SD 13.6) minutes in the i.v. lorazepam group (n = 50).
Table 4 presents a summary of the primary safety outcomes reported by the four trials. Full details of these outcomes, including outcome definitions, are presented in Appendix 9.
| Study (first author and year of publication) | Arm | Adverse events, n/N (%) | |
|---|---|---|---|
| Respiratory depression | Mortality | ||
| Alldredge 200122 | i.v. LOR (n = 66) | 7/66 (10.6) | 5/66 (7.6)a |
| i.v. DIZ (n = 68) | 6/68 (8.8) | 3/68 (4.4)a | |
| i.v. PBO (n = 71) | 11/71 (15.5) | 11/71 (15.5)a | |
| Navarro 201657 | i.v. LEV + CLZ (n = 68) | 7/68 (10.3)b | 3/66 (4.5)c |
| i.v. PBO + CLZ (n = 68) | 3/66 (4.5)b | 4/65 (6.2)c | |
| Shaner 198859 | i.v. PHB + PHT (n = 18) | NR | NR |
| i.v. DIZ + PHT (n = 18) | NR | NR | |
| Silbergleit 201258 | i.m. MDZ (n = 514)d | 33/514 (6.4)e | 11/391 (2.8)e |
| i.v. LOR (n = 509)d | 51/509 (10)e | 8/391 (2.0)e | |
Respiratory depression was reported in three of the four trials, with varying definitions across trials. 22,57,58 Silbergleit et al. 58 reported ‘respiratory depression’ as a serious adverse event (SAE) in 6.4% of total enrolments in the i.m. midazolam group and 10% in the i.v. lorazepam group. Alldredge et al. 22 reported ‘change in respiratory status requiring ventilation assistance by bag–valve–mask or an attempt at ventilation’ in 10.6% of the i.v. lorazepam group, 8.8% of the i.v. diazepam group and 15.5% of the placebo group. Navarro et al. 57 reported several outcomes relating to respiratory depression; these included ‘prehospital health failures: respiratory’ in 10.3% of the i.v. levetiracetam plus clonazepam group and 4.5% of the placebo plus clonazepam groups and ‘need for prehospital assistance: respiratory’ in 42.6% of the i.v. levetiracetam plus clonazepam group and 34.8% of the placebo plus clonazepam group. In addition, a small number of participants was reported to experience ‘respiratory, thoracic and mediastinal’ AEs or SAEs [i.e. hypoxia (SAE), acidosis respiratory (AE), hypoxaemia (AE), respiratory distress (AE), respiratory failure (AE)], with a maximum of two participants (2.9%) in any category. 57
The protocol for this assessment specified 30-day mortality as a primary outcome and 6-month mortality as a secondary outcome. However, none of the included studies reported mortality at these specified time points. Two trials22,57 reported the proportion of participants who died between enrolment and discharge from hospital. 22,61 The differences in mortality rates among treatment groups in each individual trial were not significant. In the trial by Alldredge et al. ,22 mortality rates ranged from 4.4% to 7.6% in the i.v. diazepam and i.v. lorazepam group, respectively, whereas the proportion of deaths in the placebo group was 15.5%. 22 In the Navarro et al. 57 trial, the proportion of people who died was 4.5% in the levetiracetam plus clonazepam group and 6.2% in the placebo plus clonazepam group. 57 Silbergleit et al. 58 reported similar mortality rates among adults treated with i.m. midazolam and those treated with i.v. lorazepam (2.8% vs. 2%, respectively).
Results of the individual studies and synthesis: secondary outcomes
A summary of the secondary outcomes reported in the four included studies is presented in Table 5. Full details of these outcomes, including outcome definitions, are presented in Appendix 10.
| Study (first author and year of publication) | Arm (n analysed) | Need for additional drugs to stop seizure, n/N (%) | Need for hospital admission, n/N (%) | Length of stay in ICU, days, mean (SD) | Return to baseline function, n/N (%) |
|---|---|---|---|---|---|
| Alldredge 200122 | i.v. LOR (n = 66) | NR | 37/65 (56.9) | NR | 49/65 (75.4) |
| i.v. DIZ (n = 68) | NR | 32/67 (47.8) | NR | 52/67 (77.6) | |
| i.v. PBO (n = 71) | NR | 45/71 (63.4) | NR | 49/70 (70) | |
| Navarro 201657 | i.v. LEV + CLZ (n = 68) | 28/67 (41.8)a | NR | Median 3 (range 0–15)b | 1/66 (1.5)c |
| i.v. PBO + CLZ (n = 68) | 28/65 (43.1)a | NR | Median 3 (range 1–15)b | 8/65 (12.3)c | |
| Shaner 198859 | i.v. PHB + PHT (n = 18) | 0/18 (0) | NR | NR | NR |
| i.v. DIZ + PHT (n = 18) | 1/18 (5.6) | NR | NR | NR | |
| Silbergleit 201258 (published data) | i.m. MDZ (n = 448) | Seizures not terminated:d 22/448 (4.9) | 258/448 (57.6)e | 5.7 (9.5);f n = 123 | NR |
| Seizures terminated:d 47/448 (10.5) | |||||
| i.v. LOR (n = 445) | Seizures not terminated:d 42/445 (9.4) | 292/445 (65.6)e | 4.1 (4.7);f n = 155 | NR | |
| Seizures terminated:d 57/445 (12.8) |
The need for additional drugs to stop a seizure within 12 hours was specified in the protocol for this assessment. None of the studies reporting this outcome specified a 12-hour limit, so relevant outcomes are reported here as defined by the trials’ investigators. Outcome definitions varied across studies.
Navarro et al. 57 reported that similar numbers of participants in the levetiracetam plus clonazepam group and in the placebo plus clonazepam groups required a second clonazepam injection after 5 minutes (41.8% and 43.1%, respectively) and an injection of antiepileptic drugs after 15 minutes (28.4% and 23.1%, respectively). 57 In the trial by Shaner et al. ,59 anaesthesia was needed in one participant (5.6%) who received i.v. diazepam plus phenytoin but in none of those treated with i.v. phenobarbital plus phenytoin. RAMPART, by Silbergleit et al. ,58 reported the number of participants who required rescue therapy and the number who were still having seizures on arrival at the ED. 22 The proportion of participants who received rescue therapy and whose seizures were terminated was 10.5% in the i.m. midazolam group and 12.8% in the i.v. lorazepam group; the proportion of those who received rescue therapy and whose seizures were not terminated was 4.9% in the i.m. midazolam group and 9.4% in the i.v. lorazepam group; the proportion of participants whose seizures were not terminated but did not receive rescue therapy was 11.2% in the i.m. midazolam group and 14.4 in the i.v. lorazepam group. In general, those in the i.m. midazolam group were less likely to have seizures at arrival at the ED than those in the i.v. lorazepam group (proportion of participants without seizures: 83.9% vs. 76.2% in the i.m. and i.v. groups, respectively).
Two trials reported the numbers of participants who were admitted to hospital. Alldredge et al. 22 reported that a higher proportion of participants were admitted to ICU (56.9% in the i.v. lorazepam arm, 47.8% in the i.v. diazepam arm and 63.4% in the placebo arm) than to a hospital ward (29.2%, 26.9%, 23.9%, respectively). In the Silbergleit et al. 58 trial, more participants were admitted to a hospital ward (57.6% in the i.m. midazolam group and 65.6% in the i.v. lorazepam group) than to ICU (28.6% and 36.2%, respectively).
Navarro et al. 57 reported that, in each treatment group, the median length of stay in ICU was 3 days, whereas Silbergleit et al. 58 reported a mean of 5.7 days for the i.m. midazolam group and of 4.1 days for the i.v. lorazepam group.
None of the identified trials reported 6-month mortality, health-related quality of life or the number of people requiring an emergency call-out.
Summary of clinical effectiveness
Findings from four included trials showed that the benzodiazepines were effective for the pre-hospital treatment of status epilepticus in adults, albeit the definitions of status epilepticus and outcome measures varied across trials. In particular, more of the participants receiving either 2 mg of i.v. lorazepam or 5 mg of i.v. diazepam had no seizures at arrival to the ED than those treated with placebo. A dose of 10 mg of i.m. midazolam was as effective as 4 mg of i.v. lorazepam in controlling status epilepticus. Of some note is that participants treated with i.m. midazolam had a higher rate of discharge from the hospital ED than those treated with i.v. lorazepam. Across the trials, levels of seizure recurrence was generally low, as was the number of AEs in terms of respiratory depression and mortality.
Chapter 3 Economic evaluation
The health economic objectives for this assessment were to:
-
review and critically appraise published economic evaluations of first-line pre-hospital or ED treatments for adults with status epilepticus, with a focus on the type and structure of decision models used to address the decision problem
-
identify the key elements of a suitable economic model for assessing the cost-effectiveness of pre-hospital or ED treatments from the perspective of the UK NHS, based on the review of published economic evaluations and findings from the clinical effectiveness review.
Methods
Search strategies were developed to identify economic evaluations of pre-hospital or first-line ED treatments for adults with status epilepticus (see Appendix 11 for more details). The following databases were searched, with no date, language, or publication type restrictions: MEDLINE, EMBASE, NHS Economic Evaluation Database (NHS EED), Health Technology Assessment (HTA) Database, Research Papers in Economics (RePEc), and the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) Scientific Presentations Database.
Any identified full economic evaluations matching the scope of this assessment were included. Full economic evaluations were defined as comparative analyses of costs and outcomes in the framework of cost–utility, cost-effectiveness, cost–benefit or cost minimisation analyses. Economic evaluations conducted alongside single effectiveness studies (e.g. RCTs) or decision-analysis models were also deemed eligible for inclusion.
The publications identified through the literature search as potentially meeting the scope of the cost-effectiveness assessment were reviewed for eligibility. Publications were screened intially at abstract level, with eligible abstracts undergoing detailed screening at full-text level.
Results
Quantity of the evidence
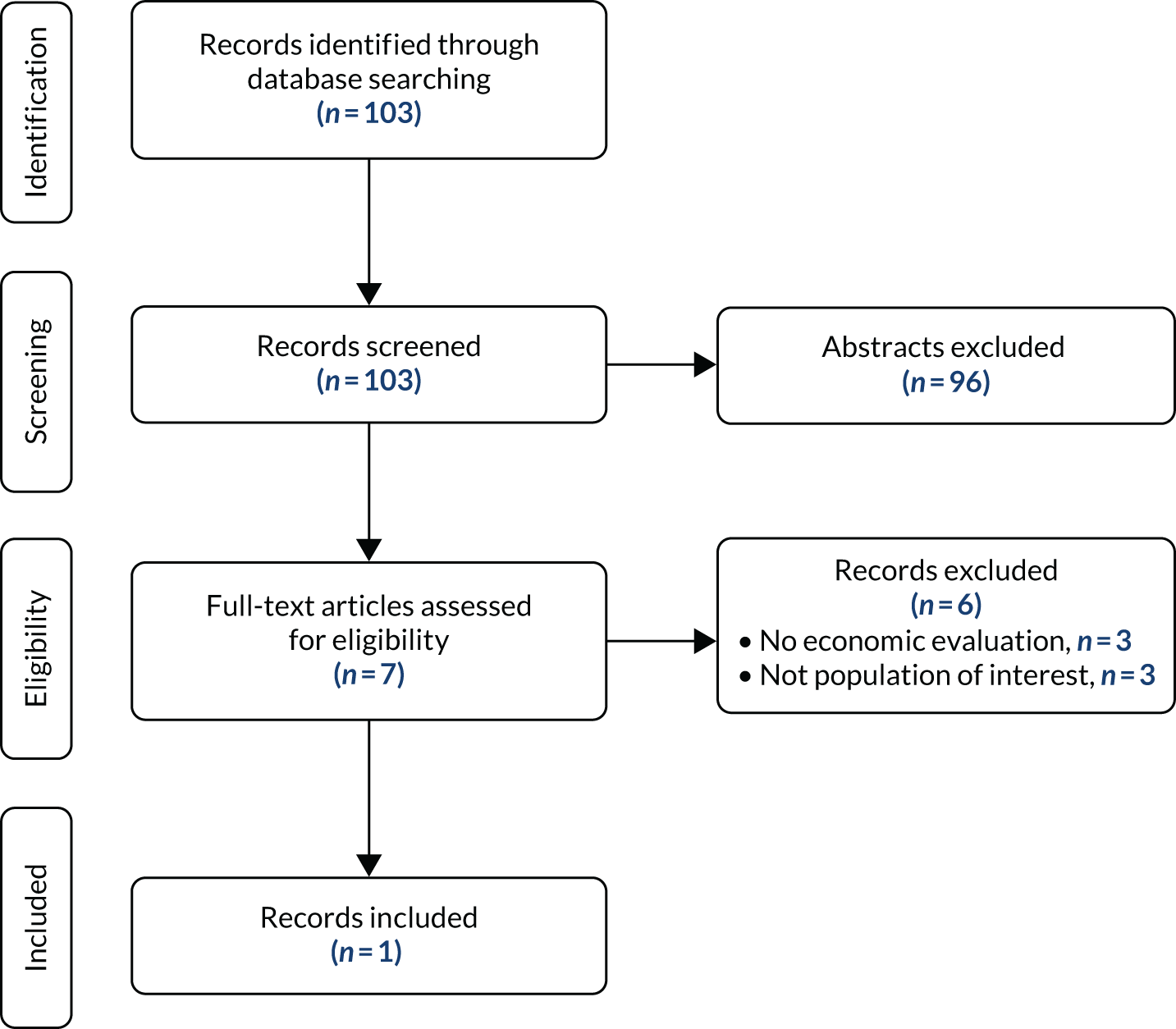
In total, 103 results were identified through database searching. Abstract screening excluded 96 records, with the main reason for exclusion being the lack of economic evaluation. The remaining seven full-text articles were assessed for eligibility, and six were excluded because of either inappropriate study design (no economic evaluations, n = 3) or patient population (n = 3). Of the three articles excluded because of the lack of economic evaluation, two reported only costs and one was a review of evidence on the use of fosphenytoin. One record matched the inclusion criteria and was included in this review. The PRISMA flow diagram for study selection is provided in Figure 4.
FIGURE 4.
The PRISMA flow diagram for the selection of cost-effectiveness evidence.
Results of the individual studies
The included study is a retrospective case note audit comparing the efficacy, safety and cost of lorazepam (4 mg i.v., repeated up to two times) compared with diazepam (10 mg i.v., repeated up to three times) in adults with convulsive status epilepticus who received treatment in a teaching hosptial in London. 62 Cases of convulsive status epilepticus were retrospectively reviewed over two 18-month periods before and after the introduction of a new management protocol that recommended lorazepam in place of diazepam for first-line treatment of convulsive status epilepticus. Phenobarbitone was also recommended instead of phenytoin for second-line therapy. A total of 720 episodes were identified and 590 medical records were retrieved (82%). These records were examined and 90 episodes of convulsive status epilepticus were identified. Of these, 13 were excluded as no benzodiazepine was received, and a further five were excluded as neither lorazepam nor diazepam was received as part of first-line treatment. The remaining 72 episodes were used to provide a comparison of lorazepam and diazepam. The results of the analysis showed that, when given as the first benzodiazepine, lorazepam was associated with a higher likelihood of treatment success than diazepam (9/17 doses lorazepam vs. 14/55 doses diazepam; p = 0.042). The cost of lorazepam was higher (£0.78 for 4 mg compared with £0.45 for 10 mg) (cost year not specified) but, when used as the first benzodiazepine, lorazepam was significantly more likely to achieve seizure control and patients treated with lorazepam had a lower likelihood of recurrence. The cost per successful outcome was not different between the two treatments (£1.47 for lorazepam vs. £1.46 for diazepam). It was concluded that, where venous access is possible, lorazepam should be the first-line treatment for premonitory and established status epilepticus in place of diazepam in both pre-hospital and in-hospital settings.
A strength of the study is that it is UK based. However, there are some important limitations, notably the retrospective case note design and challenges with note retrieval potentially introducing bias. There were also some differences between the two groups including more patients with premonitory convulsive status epilepticus in the lorazepam group and a shorter time to first treatment, although neither was significant. The number of episodes included in the analysis is also relatively small to draw robust conclusions. The economic analysis presented in the study was a simple cost-effectiveness analysis using the outcome of cost per successful outcome, which was defined as seizure cessation without recurrence over 12 hours. No other outcomes were included in the economic analysis and costs were also limited to first-line medicine acquisition costs with no details provided on the impact on resource use or other medicine costs. Given the narrow focus and limitations identified, it is not possible to draw any robust conclusions on cost-effectiveness.
Other relevant studies
The literature search showed that there is limited evidence available on the cost-effectiveness of first-line pre-hospital or ED treatments for adults with status epilepticus. Given this lack of evidence, other studies (e.g. cost-of-illness studies) were considered with the aim of informing the design of future economic evaluations in this area. With this aim, two costing studies initially excluded during the full-text review were identified in the relevant patient population and these studies were reviewed in detail, as summarised below.
The first study is a retrospective cost-of-illness study that was conducted using data on patients with status epilepticus at three hospitals in Germany. 63 The aim of the study was to identify and characterise direct hospital costs (2014 EUR) and cost-driving factors for inpatients with status epilepticus from a German health-care perspective. In total, data from 341 admissions in 316 adult patients between 2013 and 2014 were analysed for costs and cost-driving factors. For each inpatient admission due to status epilepticus, average costs, length of stay and ventilation time were calculated. Patients were categorised according to the aetiology and onset of status epilepticus as follows: acute symptomatic status epilepticus due to acute brain injury (26.3%), new-onset remote symptomatic status epilepticus with no previous history of epilepsy or status epilepticus (28.8%), remote symptomatic status epilepticus with previous history of epilepsy or status epilepticus (38.9%), and other aetiologies (e.g. idiopathic generalised epilepsy) (6.0%). Patients were defined as refractory if they had recurrent seizures despite two appropriately selected and dosed AEDs including benzodiazepine. Super-refractory status epilepticus was defined as status epilepticus that continues or recurs ≥ 24 hours after initiation of treatments with anaesthetic AEDs including cases where seizure control is attained after induction of anaesthesia but recurs on weaning the patient off the anaesthetic agent.
The results of the analysis showed that benzodiazepines were used as first-line treatment in 137 admissions (40.2%) pre-hospital and in 308 admissions (90.3%) prior to AED treatment. Nearly all admissions required the use of electroencephalography (EEG) and cerebral imaging [computerised tomography (CT) and/or magnetic resonance imaging (MRI)]. The mean costs of hospital treatment of status epilepticus were €14,946 per patient per admission with a mean length of stay of 19 days (mean cost per day of €787). Significant cost differences were identified according to the aetiology of status epilepticus, with significantly higher mean costs in patients with acute symptomatic aetiology (€25,269) than in those with new-onset status epilepticus (€12,511), remote symptomatic status epilepticus (€11,204) or status epilepticus of other aetiologies (€10,380). Severity of status epilepticus also had a significant impact on costs, with the largest cost per admission associated with super-refractory status epilepticus (€50,488). Data on length of stay showed that severity was again a significant factor, with durations ranging from 12.1 days in non-refractory patients to 21.0 days in refractory status epilepticus patients and 37.0 days in super-refractory patients.
To identify cost-driving factors, univariate analyses of inpatient treatment costs and length of stay were conducted. This showed increased costs associated with newly diagnosed patients, acute aetiology, unfavourable status epilepticus severity score (STESS) of 4–6 and length of stay of > 14 days. Significantly higher costs and significantly longer hospitalisations were linked to no acute treatment with benzodiazepine before admission, high number of AEDs in hospital, refractory status epilepticus and super-refractory status epilepticus, anaesthesia, requirement for ventilation and unfavourable outcome on discharge (modified Rankin Scale 4–6). Multivariate analysis showed that super-refractory status epilepticus, ventilation and length of stay > 14 days were independent predictors of costs. Limitations of the study include potential lack of generalisability of results to other health-care systems, difficulty in distinguishing costs of status epilepticus from costs due to other acute illnesses (e.g. stroke) and the exclusion of potentially relevant costs, such as rehabilitiation and outpatient costs.
The second study is a retrospective analysis conducted in two hospitals in Spain in which data on patients with status epilepticus were analysed from December 2012 to July 2017. 64 The aim of the study was to identify factors that had an impact on the high cost of care in patients with status epilepticus, with focus on the timing of treatment and duration of status epilepticus. Data on seizure history were recorded, as well as whether the status epilepticus episode occurred out of hospital (64%) or in hospital (36%). Aetiology of status epilepticus was categorised into acute symptomatic (55.1%), remote symptomatic (36.4%), progressive symptomatic (7.9%) and cryptogenic (9.5%). The severity of status epilepticus was also evaluated using the modified STESS. Data were collected on the time from onset of status epilepticus to arrival at hospital, time to administration of first-line treatment, time to second-line treatment received (AEDs) and the number of AEDs used, and time to third-line treatment (anaesthetics). Length of stay data were also collected. Duration of status epilepticus was defined as the recorded time from symptom onset until EEG showed a seizure suppression pattern following treatments.
In total, 305 patients were included in the analysis that showed several factors influencing the cost of treatment. These include no previous history of epilepsy, lower level of consciousnessness, the presence of a potentially fatal aetiology, major complications, the presence of lateralised periodic discharges in EEG, a higher epidemiology-based mortality score, being refractory or super-refractory, and the duration of status epilepticus (all p-values ≤ 0.005). The study found that the cost of management is higher for patients in whom onset of status epilepticus occurs in the hospital, rather than out of hospital, meaning that the results for these groups were analysed separately. The total mean cost of in-hospital status epilepticus was €15,174, compared with €6559 for out-of-hospital status epilepticus (p < 0.001) (cost year not specified). Patients in the in-hospital group tended to have more severe status epilepticus symptoms, greater comorbidity, a higher proportion of super-refractory status epilepticus and a higher risk of complications, which together result in an increased length of stay and higher associated costs. The results also showed that the cost of out-of-hospital onset of status epilepticus is higher when the duration of the episode is > 24 hours. In the out-of-hospital group, the duration of the status epilepticus episode was a key factor in the length of hospitalisation and the cost of disease management, with the analysis showing a clear relationship between duration of episode and the time to treatment. For episodes lasting < 24 hours the median cost was €5005, compared with €8733 for episodes lasting ≥ 24 hours (p = 0.005). The authors concluded that the speed at which treatment is initiated is an important factor; therefore, early detection and prompt establishment of treatment could have an impact on both patients’ outcomes and health-care costs. However, there are some limitations with the study, including the retrospective design, meaning that it is difficult to distinguish between costs due to status epilepticus and those associated with other conditions.
Discussion
Limited evidence on the cost-effectiveness of first-line treatments for status epilepticus was identified within the scope of the review. A single retrospective case note audit reporting a cost per successful outcome was the only study meeting the inclusion criteria for the review. This showed that lorazepam was significantly more likely to achieve seizure control and patients treated with lorazepam had a lower likelihood of recurrence, but there was no difference between lorazepam and diazepam in terms of the cost per successful outcome. Owing to limitations with the study, it is not possible to draw firm conclusions. Clinical guidelines show that there is clinical evidence to support benzodiazepines for first-line treatment for convulsive status epilepticus, for which the usual treatments are lorazepam, diazepam and midazolam. As these treatments are associated with broadly similar efficacy in terms of seizure cessation and the cost of treatment is very low (< £1 per dose), they are likely to be considered cost-effective at conventional cost-effectiveness thresholds. However, further research is needed to show which is more cost-effective and which mode of administration is preferable.
When comparing treatments with similar low costs, it is plausible that the more effective treatment will dominate less effective treatments over the course of a treatment episode if it can be shown to lower costs by reducing length of stay in hospital, as is suggested in some of the observational studies. This would preclude the need to estimate quality-adjusted life-years (QALYs) in this scenario. However, from the evidence identified it is clear that, although status epilepticus is an acute condition requiring rapid emergency treatment, to fully assess the cost-effectiveness of status epilepticus treatments an economic model should aim to capture all relevant costs and outcomes associated with the whole episode of status epilepticus. In the event that any higher-cost first-line treatments were to become available in the future, an economic model using the outcome of cost per QALY would be preferable; however, acknowledging the acute nature of status epilepticus may provide some challenges in assessing patients’ quality of life. This approach would allow for the value of higher-cost treatments to be assessed where short-term cost-savings driven by reductions in length of hospital stay are not sufficient to fully offset increased medicine acquisition costs. In addition, the model should aim to capture the potential impact of more effective treatments on reducing longer term health-related quality-of-life loss and account for the recurrent nature of the condition for some patients.
The two costing studies described above provide an indication of the key resource use implications associated with status epilepticus and showed that time to effective first-line treatment of benzodiazepine is key in determining the duration of the status epilepticus episode, the clinical outcomes from treatment, the duration of length of stay and associated treatment costs. In terms of relevant clinical outcomes for inclusion in the economic model, based on the clinical effectiveness review the key clinical outcomes relevant to the economic analysis include cessation of seizure activity, time to seizure cessation, recurrence of seizures, length of stay and ICU admissions. Evidence considered also suggests that different subgroups of status epilepticus have different episode costs, which may warrant further consideration in any future economic evaluation. The costs and quality-of-life loss due to AEs associated with treatment should also be captured, as highlighted by the occurrence of respiratory depression in the included clinical studies. Longer-term complications associated with status epilepticus should also be considered to capture all relevant costs and outcomes. Both costing studies also collected resource use data for patients requiring EEG, CT/MRI scans, AED treatments and requirements for ventilation and anaesthesia.
Summary of cost-effectiveness
Overall, there is a lack of robust data from which to draw firm conclusions on the cost-effectiveness of first-line treatments for status epilepticus, suggesting that further research is required to address this. Evidence from the reviewed costing studies showed the potential for good pre-hospital care to improve clinical outcomes and significantly reduce the burden of hospital resource use.
Chapter 4 Discussion and conclusions
Discussion
Statement of principal findings
Clinical effectiveness
The purpose of this assessment was to systematically review the current evidence on the effects of the use of benzodiazepines and other antiepileptic drugs for the treatment of adults with convulsive status epilepticus in the pre-hospital setting. We included evidence from four published RCTs (total number of participants 1345, of whom 1234 were adults) comparing i.v. lorazepam with i.v. diazepam and i.v. placebo; i.m. midazolam with i.v. lorazepam; i.v. levetiracetam plus i.v. clonazepam with i.v. placebo plus i.v. clonazepam; and i.v. phenobarbital plus i.v. phenytoin with i.v. diazepam plus i.v. phenytoin. 22,57,58 We considered the following primary outcomes: proportion of people with termination of seizure activity, time to seizure cessation from the time of study drug administration, recurrence of seizure, and AEs (respiratory depression and mortality). We considered the following secondary outcomes: need for additional drugs to stop seizure (within 12 hours), need for hospital admission, length of stay in ICU, return to baseline function, health-related quality of life and number of people requiring an emergency call-out. Not all trials provided data for the assessment of the prespecified primary and secondary outcomes. Differences across the four trials in terms of treatment comparisons and definitions of outcome measures precluded the possibility of combining findings in a meta-analysis. Three trials were at a low risk of bias22,57,58 and the remaining trial, published in 1988, was at a high risk of bias. 59
On the whole, the evidence from the four identified trials indicates that i.v. and i.m. benzodiazepines administered by paramedics or ED personnel are safe and effective for the pre-hospital treatment of convulsive status epilepticus in adults.
In particular, evidence from Alldredge et al. ’s22 trial assessing i.v. administration of lorazepam, diazepam and placebo indicates that the proportion of adults with termination of seizure is higher in the lorazepam group and in the diazepam group than in the placebo group, but with no significant differences between the two benzodiazepine groups. 22 Evidence from RAMPART by Silbergleit et al. 58 shows a higher rate of seizure cessation and of discharge from the ED among people treated with i.m. midazolam than among those treated with i.v. lorazepam.
Furthermore, evidence from Navarro et al. ’s57 trial shows that the addition of the AED levetiracetam to the benzodiazepine clonazepam is safe for the treatment of adults with status epilepticus but does not result in a higher rate of seizure cessation.
Median time to seizure cessation from administration of i.v. medications ranged from 1.6 minutes (i.v. lorazepam) to 15 minutes (i.v. diazepam plus phenytoin) across trials. In the trial by Silbergleit et al. 58 that assessed i.v. lorazepam versus i.m. midazolam, the reported median time from drug administration to termination of seizure was shorter with i.v. lorazepam (1.6 minutes vs. 3.3 minutes). However, this does not take into account the time from paramedic arrival to drug administration, which was longer in the i.v. group than in the i.m. group (4.8 minutes vs. 1.2 minutes, respectively), reflecting the longer time needed to establish i.v. access. Thus, there was little difference in total time from paramedic arrival to seizure termination in the two benzodiazepine groups. 58
The frequency of cardiorespiratory complications and the need for respiratory assistance were generally low across trials, with no significant differences between treatment arms of individual trials. Recurrent seizures, need for additional drugs to stop seizures, need for hospital admission and length of stay in ICU were also similar between treatment arms of the individual trials that assessed these outcomes.
It is worth noting that, despite the beneficial outcomes associated with the use of benzodiazepines in the pre-hospital setting, a proportion of people who received the active treatment were still experiencing seizures at the time of arrival to the ED (from 16% to 67% of people across trials).
Overall, our results are in line with current clinical recommendations. There is a general agreement that benzodiazepines should be used for the initial treatment of status epilepticus. 4,24,65 The 2016 evidence-based guideline of the American Epilepsy Society,24 for example, recommends the administration of a benzodiazepine (specifically i.m. midazolam, i.v. lorazepam or i.v. diazepam) as the initial therapy of choice.
Cost-effectiveness
Despite comprehensive literature searches, we identified only one economic evaluation of first-line treatments for status epilepticus that matched our predefined inclusion criteria. The findings of this economic evaluation indicate that there is potential for good pre-hospital care to improve outcomes and significantly reduce the burden of hospital resource use.
Strength and limitations of the assessment
This assessment was conducted in accordance with current methodological standards, and its methods were prespecified in a peer-reviewed research protocol (PROSPERO registration CRD42020201953) (www.crd.york.ac.uk/prospero/display_record.php?RecordID=201953; accessed 4 August 2020). In particular, we conducted comprehensive literature searches of major electronic databases and relevant websites and used transparent methods for data selection and extraction.
There are, however, some limitations to take into consideration when interpreting the findings of this assessment:
-
We identified only a limited number of trials for the pre-hospital treatment of convulsive status epilepticus in the adult population.
-
The included studies differed in terms of treatment comparisons and choice and definition of outcome measures. This hampered the possibility of conducting any meaningful meta-analysis.
-
None of the included trials was conducted in the UK, with three of the four included trials conducted in the USA.
-
Only one study the met the inclusion criteria for economic evaluation, and the study in question was limited by its reliance on retrospective observational data from a small number of cases, and a narrow focus on treatment acquisition costs in the relation to outcomes.
Uncertainties from the assessment
Clinical effectiveness
In the pre-hospital setting, the optimal pharmacological treatment for adults with convulsive status epilepticus or prolonged seizures is still unclear. The trials identified for this assessment focused exclusively on i.m. or i.v. treatment that needed to be given by paramedics.
Current NICE clinical guidance on the diagnosis and management of epilepsies recommends buccal midazolam as first-line pre-hospital treatment in children, young people and adults with prolonged or repeated seizures in the community and rectal diazepam as an alternative option or when buccal midazolam is not available. 25 These have the advantage that they can be given immediately by trained caregivers in those at risk, rather than waiting for paramedics to attend. Administration of rectal diazepam requires, however, the removal of clothes and adequate positioning of the patient66 and may be regarded as a less practicable and socially acceptable option for adult patients than benzodiazepines administered via the mouth or nose. In children, buccal and intranasal midazolam have been reported to have similar efficacy for the early treatment of status epilepticus and the use of midazolam by non-i.v. routes has been proposed as a favourable alternative to diazepam. 66,67 Nevertheless, in the adult population, we have not identified any RCTs assessing the use of buccal midazolam or rectal diazepam, or indeed intranasal midazolam, which, even though unlicensed, can also be used in clinical practice. Although it would not be acceptable to perform placebo-controlled trials of such interventions, given the recognised effectiveness of benzodiazepines in stopping prolonged seizures, head-to-head randomised clinical trials, which compare different routes of benzodiazepines administration, would be particularly useful to inform clinical practice. Comparisons of interest include rectal diazepam versus nasal or buccal midazolam administered by trained caregivers in people with known epilepsy at high risk of status epilepticus; i.m. midazolam versus buccal midazolam versus intranasal midazolam administered by paramedics; and i.v. lorazepam versus i.m. midazolam administered by paramedics, which could also include patients in whom caregiver administration of rectal, buccal or nasal benzodiazepines had failed to stop the seizure. Observational studies using existing routinely collected data may provide some information on effectiveness and safety of acute rescue therapy (whatever route of administration) versus no rescue therapy, but they are more susceptible to methodological bias.
The doses of the benzodiazepines used in the identified studies were effective, but it is unclear whether other doses may have similar or different efficacy and safety.
The level of training paramedics should receive to recognise status epilepticus and treat people in the community setting is unknown. Similarly, the acceptability and ease of administration of different pharmacological treatments in the pre-hospital setting have not been properly evaluated but rather reported anecdotally.
Cost-effectiveness
Overall, there is a lack of robust data from which to draw firm conclusions on the cost-effectiveness of first-line treatments of status epilepticus, suggesting that further research is required. While no firm conclusions can be drawn from the available studies, cost-of-illness studies do suggest that the duration of the status epilepticus episode is a key determinant of the length of hospitalisation and the management cost, with the analysis also showing a clear relationship between duration of episode and time to treatment. This suggests that there is potential for more effective first-line treatments that can reduce the time to seizure control, to deliver significant cost-savings that can offset increases in the initial treatment cost.
Conclusions
Implications for health care
Both 2 mg of i.v. lorazepam and 5 mg of i.v. diazepam administered by paramedics are more effective than placebo for the pre-hospital treatment of convulsive status epilepticus in adults; 10 mg of i.m. midazolam is non-inferior to 4 mg of i.v. lorazepam in the pre-hospital setting. The addition of levetiracetam to clonazepam does not offer clear advantages over clonazepam alone.
Recommendations for research
Harmonisation of outcome measures would be useful to facilitate future clinical research.
Identification of the optimal pre-hospital pharmacological treatment of adults with status epilepticus requires further RCTs assessing different benzodiazepines (and potentially other anticonvulsant medications) as well as their route of administration and acceptability.
This assessment indicates that large well-designed clinical trials are needed to assess the use of i.v. lorazepam versus i.v. diazepam and to confirm the efficacy and safety of i.m. midazolam compared with i.v. lorazepam. In the pre-hospital setting, i.m. midazolam may have the advantage of a rapid and easy administration, which counterbalances the faster effect of i.v. medications.
Given the current clinical recommendations about the use of benzodiazepines, which can be administered rectally or via the mouth by caregivers in the pre-hospital setting, and the absence of evidence in the adult population, direct head-to-head trials comparing buccal midazolam and rectal diazepam are required to establish their efficacy, safety and acceptability.
It is also necessary to establish which midazolam formulation is the best option for the first-line treatment of adults with convulsive status epilepticus. Future clinical trials comparing i.m. midazolam versus buccal midazolam and versus intranasal midazolam would provide useful information to inform the pre-hospital management of patients, especially when i.v. access is not feasible. Further trials aiming to conclusively establish whether or not i.m. midazolam is more effective than i.v. lorazepam, as suggested by the single trial in the review, would be helpful.
Future clinical studies could also aim to establish optimal doses of benzodiazepines used as first-line treatments in the pre-hospital setting.
It would be important to improve adherence to clinical guidelines on the use of currently available benzodiazepines in the pre-hospital setting. For example, a recent large trial of second-line hospital-based treatment of status epilepticus in the USA found that only 57% of patients had received benzodiazepines before arrival at the hospital and 7% breached the protocol by not receiving a predefined minimally adequate benzodiazepine dose. 44
As there is still a proportion of patients who do not respond to treatment, further research aiming to understand the underlying pathophysiology of treatment response would be useful to inform future treatment development.
High-quality economic evaluations are required to determine the value for money of different treatments for convulsive status epilepticus and their modes of administration. It would be useful if future economic evaluations could capture the full cost of managing the convulsive episode up to the time of discharge from hospital. In cases where higher-cost first-line treatments are not fully offset by subsequent management cost savings, it may be necessary to model the impact of improved short-term outcomes on longer-term health-related quality of life and future resources use.
Acknowledgements
The authors are grateful to Robert Silbergleit, Department of Emergency Medicine, University of Michigan, MI, USA, for providing the individual participant data set of RAMPART.
Patient and public involvement
The Plain English summary was shared with the Health Services Research Unit (HSRU) Public Partnership Group at the University of Aberdeen, which consists of 11 patient and public involvement partners (seven men, four women; three working age, eight retired). Communication with the Public Partnership Group was facilitated by the HSRU patient and public involvement co-ordinator. The Group consists of members of the public, who meet regularly to discuss aspects of HSRU research and provide a public perspective. Six members of the group provided comments on the language and general meaning of the summary.
Contributions of authors
Moira Cruickshank (https://orcid.org/0000-0002-5182-884X) (Research Fellow) and Mari Imamura (https://orcid.org/0000-0003-4871-0354) (Research Fellow) led the day-to-day running of the assessment, reviewed the evidence on the clinical effectiveness and drafted the first version of this report.
Corinne Booth (https://orcid.org/0000-0002-5437-6789) (Independent Consultant, Health Economist) conducted the review of economic evaluation with help from Graham Scotland (https://orcid.org/0000-0001-5539-8819) (Senior Research Fellow).
Lorna Aucott (https://orcid.org/0000-0001-6277-7972) (Senior Statistician) checked data from all included studies, including the adult data we received from RAMPART, and interpreted results.
Carl Counsell (https://orcid.org/0000-0001-6622-7839) (Clinical Reader) provided expert advice, contributed to the interpretation of results and commented on the draft version of this report.
Paul Manson (https://orcid.org/0000-0002-1405-1795) (Information Officer) was responsible for running the literature searches, obtaining full-text papers and compiling the reference list of the report.
Miriam Brazzelli (https://orcid.org/0000-0002-7576-6751) (Reader) oversaw and co-ordinated all aspects of the assessment, interpreted data, contributed to draft the first version of this report and completed its final revision.
All authors approved the final version of this report.
Publication
Cruickshank M, Imamura M, Counsell C, Aucott L, Manson P, Booth C, et al. Management of the first stage of convulsive status epilepticus in adults: a systematic review of current randomised evidence. J Neurol 2022.
Data-sharing statement
All technical data are included within the main text or as appendices to this report. All queries should be submitted to the corresponding author for consideration.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Falco-Walter JJ, Bleck T. Treatment of established status epilepticus. J Clin Med 2016;5. https://doi.org/10.3390/jcm5050049.
- Prasad M, Krishnan PR, Sequeira R, Al-Roomi K. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev 2014;9. https://doi.org/10.1002/14651858.CD003723.pub3.
- Lesser RP, Johnson E. Status Epilepticus: BMJ Best Practice 2018. https://bestpractice.bmj.com/topics/en-gb/3000127 (accessed 24 June 2020).
- Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3-23. https://doi.org/10.1007/s12028-012-9695-z.
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:522-30. https://doi.org/10.1111/epi.13670.
- Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia 1999;40:120-2. https://doi.org/10.1111/j.1528-1157.1999.tb02000.x.
- Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med 1998;338:970-6. https://doi.org/10.1056/NEJM199804023381407.
- Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus – report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015;56:1515-23. https://doi.org/10.1111/epi.13121.
- Pellock JM. Overview: definitions and classifications of seizure emergencies. J Child Neurol 2007;22:9-13. https://doi.org/10.1177/0883073807303064.
- Hauser WA. Status epilepticus: epidemiologic considerations. Neurology 1990;40:9-13.
- Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French-speaking Switzerland: (EPISTAR). Neurology 2000;55:693-7. https://doi.org/10.1212/wnl.55.5.693.
- Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology 1998;50:735-41. https://doi.org/10.1212/wnl.50.3.735.
- Knake S, Rosenow F, Vescovi M, Oertel WH, Mueller HH, Wirbatz A, et al. Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia 2001;42:714-18. https://doi.org/10.1046/j.1528-1157.2001.01101.x.
- Walker M. Status epilepticus: an evidence based guide. BMJ 2005;331:673-7. https://doi.org/10.1136/bmj.331.7518.673.
- Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia 2010;51:251-6. https://doi.org/10.1111/j.1528-1167.2009.02323.x.
- Scottish Intercollegiate Guidelines Network (SIGN) . Diagnosis and Management of Epilepsy in Adults: SIGN 143 2015. www.sign.ac.uk/sign-143-diagnosis-and-management-of-epilepsy-in-adults (accessed July 2020).
- Walker MC, Smith SJ, Shorvon SD. The intensive care treatment of convulsive status epilepticus in the UK. Results of a national survey and recommendations. Anaesthesia 1995;50:130-5. https://doi.org/10.1111/j.1365-2044.1995.tb15095.x.
- Walker MC, Howard RS, Smith SJ, Miller DH, Shorvon SD, Hirsch NP. Diagnosis and treatment of status epilepticus on a neurological intensive care unit. QJM 1996;89:913-20. https://doi.org/10.1093/qjmed/89.12.913.
- Scholtes FB, Renier WO, Meinardi H. Generalized convulsive status epilepticus: causes, therapy, and outcome in 346 patients. Epilepsia 1994;35:1104-12. https://doi.org/10.1111/j.1528-1157.1994.tb02562.x.
- Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med 1998;339:792-8. https://doi.org/10.1056/NEJM199809173391202.
- Jagoda A, Riggio S. Refractory status epilepticus in adults. Ann Emerg Med 1993;22:1337-48. https://doi.org/10.1016/S0196-0644(05)80120-9.
- Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med 2001;345:631-7. https://doi.org/10.1056/NEJMoa002141.
- Silbergleit R, Lowenstein D, Durkalski V, Conwit R. NETT Investigators . Lessons from the RAMPART study – and which is the best route of administration of benzodiazepines in status epilepticus. Epilepsia 2013;54:74-7. https://doi.org/10.1111/epi.12284.
- Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr 2016;16:48-61. https://doi.org/10.5698/1535-7597-16.1.48.
- National Institute for Health and Care Excellence (NICE) . Epilepsies: Diagnosis and Management. Clinical Guideline [CG137] 2012. www.nice.org.uk/guidance/cg137 (accessed July 2020).
- Patel SB, Kress JP. Sedation and analgesia in the mechanically ventilated patient. Am J Respir Crit Care Med 2012;185:486-97. https://doi.org/10.1164/rccm.201102-0273CI.
- Riviello JJ, Claassen J, LaRoche SM, Sperling MR, Alldredge B, Bleck TP, et al. Treatment of status epilepticus: an international survey of experts. Neurocrit Care 2013;18:193-200. https://doi.org/10.1007/s12028-012-9790-1.
- Parsons AM, Patel AA, Crepeau AZ. Treatment of status epilepticus: what are the guidelines and are we following them?. Neurologist 2020;25:89-92. https://doi.org/10.1097/NRL.0000000000000281.
- American Epilepsy Society . Proposed Algorithm for Convulsive Status Epilepticus 2016 n.d. www.aesnet.org/sites/default/files/file_attach/PressReleases/2016/CSE%20Treatment%20chart-final_rerelease%20%282%29.jpg (accessed 30 June 2020).
- Meierkord H, Boon P, Engelsen B, Göcke K, Shorvon S, Tinuper P, et al. European Federation of Neurological Societies. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol 2010;17:348-55. https://doi.org/10.1111/j.1468-1331.2009.02917.x.
- Roberts DJ, Haroon B, Hall RI. Sedation for critically ill or injured adults in the intensive care unit: a shifting paradigm. Drugs 2012;72:1881-916. https://doi.org/10.2165/11636220-000000000-00000.
- Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 2002;30:119-41. https://doi.org/10.1097/00003246-200201000-00020.
- Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med 2014;370:444-54. https://doi.org/10.1056/NEJMra1208705.
- Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263-306. https://doi.org/10.1097/CCM.0b013e3182783b72.
- Bauer TM, Ritz R, Haberthür C, Ha HR, Hunkeler W, Sleight AJ, et al. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet 1995;346:145-7. https://doi.org/10.1016/S0140-6736(95)91209-6.
- Alvarez N, Besag F, Iivanainen M. Use of antiepileptic drugs in the treatment of epilepsy in people with intellectual disability. J Intellect Disabil Res 1998;42:1-15.
- Troester MM, Hastriter EV, Ng YT. Dissolving oral clonazepam wafers in the acute treatment of prolonged seizures. J Child Neurol 2010;25:1468-72. https://doi.org/10.1177/0883073810368312.
- Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298:2644-53. https://doi.org/10.1001/jama.298.22.2644.
- Electronic Medicines Compendium . BUCCOLAM 2.5mg Oromucosal Solution 2020. www.medicines.org.uk/emc/product/2768/smpc (accessed 30 June 2020).
- Electronic Medicines Compendium . Diazepam RecTubes 10mg Rectal Solution 2020. www.medicines.org.uk/emc/product/6799/smpc (accessed 30 June 2020).
- Electronic Medicines Compendium . Diazemuls Emulsion 2020. www.medicines.org.uk/emc/product/3770/smpc#gref (accessed 30 June 2020).
- Electronic Medicines Compendium . Ativan Injection 2020. www.medicines.org.uk/emc/medicine/2196/SPC/ativan+injection (accessed 30 June 2020).
- Electronic Medicines Compendium . Keppra 100 Mg Ml Concentrate for Solution for Infusion 2020. www.medicines.org.uk/emc/product/2296/smpc (accessed 30 June 2020).
- Kapur J, Elm J, Chamberlain JM, Barsan W, Cloyd J, Lowenstein D, et al. Randomized trial of three anticonvulsant medications for status epilepticus. N Engl J Med 2019;381:2103-13. https://doi.org/10.1056/NEJMoa1905795.
- Hodges BH, Mazur JE. Intravenous valproate in status epilepticus. Ann Pharmacother 2001;35:1465-70. https://doi.org/10.1345/aph.10387.
- Electronic Medicines Compendium . Sodium Valproate 100mg Ml Solution for Injection or Infusion 2020. www.medicines.org.uk/emc/product/1209/smpc (accessed 30 June 2020).
- Hall EA, Wheless JW, Phelps SJ. Status epilepticus: the slow and agonizing death of phenytoin. J Pediatr Pharmacol Ther 2020;25:4-6. https://doi.org/10.5863/1551-6776-25.1.4.
- Electronic Medicines Compendium . Phenytoin Hospira 50 Mg Ml Injection BP. 2019. www.medicines.org.uk/emc/product/3794/smpc (accessed 30 June 2020).
- Jamali A, Ali W, Ur Rehman F. Comparison of phenobarbital and sodium valproate for the treatment of status epilepticus in children. Professional Med 2020;27. https://doi.org/10.29309/TPMJ/2020.27.07.3543.
- Electronic Medicines Compendium . Phenobarbital Sodium 30mg Ml Injection. 2018. www.medicines.org.uk/emc/product/3604/smpc#gref (accessed 30 June 2020).
- Brigo F, Nardone R, Tezzon F, Trinka E. Nonintravenous midazolam versus intravenous or rectal diazepam for the treatment of early status epilepticus: a systematic review with meta-analysis. Epilepsy Behav 2015;49:325-36. https://doi.org/10.1016/j.yebeh.2015.02.030.
- Niquet J, Baldwin R, Suchomelova L, Lumley L, Naylor D, Eavey R, et al. Benzodiazepine-refractory status epilepticus: pathophysiology and principles of treatment. Ann N Y Acad Sci 2016;1378:166-73. https://doi.org/10.1111/nyas.13147.
- Zaccara G, Giannasi G, Oggioni R, Rosati E, Tramacere L, Palumbo P. Convulsive Status Epilepticus Study Group of the Uslcentro Toscana, Italy. Challenges in the treatment of convulsive status epilepticus. Seizure 2017;47:17-24. https://doi.org/10.1016/j.seizure.2017.02.015.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2011. http://handbook-5-1.cochrane.org/ (accessed July 2020).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123-30. https://doi.org/10.1371/journal.pmed.1000097.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366. https://doi.org/10.1136/bmj.l4898.
- Navarro V, Dagron C, Elie C, Lamhaut L, Demeret S, Urien S, et al. Prehospital treatment with levetiracetam plus clonazepam or placebo plus clonazepam in status epilepticus (SAMUKeppra): a randomised, double-blind, phase 3 trial. Lancet Neurol 2016;15:47-55. https://doi.org/10.1016/S1474-4422(15)00296-3.
- Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, et al. NETT Investigators. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med 2012;366:591-600. https://doi.org/10.1056/NEJMoa1107494.
- Shaner DM, McCurdy SA, Herring MO, Gabor AJ. Treatment of status epilepticus: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology 1988;38:202-7. https://doi.org/10.1212/wnl.38.2.202.
- Cruickshank M, Imamura M, Counsell C, Aucott L, Manson P, Booth C, et al. Management of the first stage of convulsive status epilepticus in adults: a systematic review of current randomised evidence. J Neurol 2022. https://doi.org/10.1007/s00415-022-10979-2.
- Claassen J. Dr No: double drug fails to eliminate status epilepticus. Lancet Neurol 2016;15:23-4. https://doi.org/10.1016/S1474-4422(15)00333-6.
- Cock HR, Schapira AH. A comparison of lorazepam and diazepam as initial therapy in convulsive status epilepticus. QJM 2002;95:225-31. https://doi.org/10.1093/qjmed/95.4.225.
- Kortland LM, Alfter A, Bähr O, Carl B, Dodel R, Freiman TM, et al. Costs and cost-driving factors for acute treatment of adults with status epilepticus: a multicenter cohort study from Germany. Epilepsia 2016;57:2056-66. https://doi.org/10.1111/epi.13584.
- Santamarina E, Parejo B, Abraira L, Gutiérrez-Viedma Á, Alpuente A, Abarrategui B, . Cost of status epilepticus (SE): effects of delayed treatment and SE duration. Epilepsy Behav 2018;89:8-14. https://doi.org/10.1016/j.yebeh.2018.09.046.
- Shorvon S, Baulac M, Cross H, Trinka E, Walker M. TaskForce on Status Epilepticus of the ILAE Commission for European Affairs . The drug treatment of status epilepticus in Europe: consensus document from a workshop at the first London Colloquium on Status Epilepticus. Epilepsia 2008;49:1277-85. https://doi.org/10.1111/j.1528-1167.2008.01706_3.x.
- Brigo F, Nardone R, Tezzon F, Trinka E. A common reference-based indirect comparison meta-analysis of buccal versus intranasal midazolam for early status epilepticus. CNS Drugs 2015;29:741-57. https://doi.org/10.1007/s40263-015-0271-x.
- McMullan J, Sasson C, Pancioli A, Silbergleit R. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta-analysis. Acad Emerg Med 2010;17:575-82. https://doi.org/10.1111/j.1553-2712.2010.00751.x.
- Lowenstein DH, Alldredge BK, Allen F, Neuhaus J, Corry M, Gottwald M, et al. The PreHospital Treatment of Status Epilepticus (PHTSE) study: design and methodology. Control Clin Trials 2001;22:290-309. https://doi.org/10.1016/S0197-2456(01)00120-9.
- Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, et al. Correction: a comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. New Engl J Med 2001;345:631-7. https://doi.org/10.1056/NEJM200112203452521.
- Navarro V, Dagron C, Demeret S, An K, Lamhaut L, Bolgert F, et al. A prehospital randomized trial in convulsive status epilepticus. Epilepsia 2011;52:48-9. https://doi.org/10.1111/j.1528-1167.2011.03236.x.
- ClinicalTrials.gov . Study of Antiepileptic Drug in Generalised Convulsive Status Epilepticus n.d. www.cochranelibrary.com/central/doi/10.1002/central/CN-02025411/full (accessed 21 July 2020).
- Silbergleit R. Correction: intramuscular versus intravenous therapy for prehospital status epilepticus. New Engl J Med 2012;366:591-600. https://doi.org/10.1056/NEJMx120016.
- Silbergleit R, Lowenstein D, Durkalski V, Conwit R. Neurological Emergency Treatment Trials (NETT) Investigators . RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia 2011;52:45-7. https://doi.org/10.1111/j.1528-1167.2011.03235.x.
- Vohra TT, Miller JB, Nicholas KS, Varelas PN, Harsh DM, Durkalski V, et al. Endotracheal intubation in patients treated for prehospital status epilepticus. Neurocrit Care 2015;23:33-4. https://doi.org/10.1007/s12028-014-0106-5.
Appendix 1 Clinical literature search strategies
This appendix is reproduced with permission from Cruickshank et al. 60 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Database
EMBASE <1974 to 2020 Week 29>, Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions(R) <1946 to July 17, 2020>, the American Psychological Association (APA)’s PsycInfo <1987 to July Week 2 2020>.
Search date: 21 July 2020.
Search strategy
1 Emergency Medical Services/use ppezv
2 emergency health service/or emergency care/use oemez
3 Emergency Services/use psyf
4 (accident adj2 emergency).tw.
5 (“emergency room” or “emergency department” or ED).tw.
6 (pre-hospital or prehospital or “out of hospital” or community).tw.
7 Allied Health Personnel/use ppezv,psyf
8 paramedical personnel/use oemez
9 (paramedic* or ambulance).tw.
10 or/1-9
11 Status Epilepticus/use ppezv,psyf
12 epileptic state/use oemez
13 Status Epilepticus.tw.
14 11 or 12 or 13
15 exp Benzodiazepines/use ppezv,psyf
16 exp benzodiazepine derivative/use oemez
17 (midazolam or diazepam or lorazepam).tw.
18 exp Anticonvulsants/use ppezv
19 exp anticonvulsive agent/use oemez
20 exp Anticonvulsive Drugs/use psyf
21 (levetiracetam or “sodium valproate” or phenytoin).tw.
22 or/15-21
23 randomized controlled trial.pt. use ppezv
24 controlled clinical trial.pt. use ppezv
25 “randomized controlled trial”/use oemez
26 “controlled clinical trial”/use oemez
27 ((randomi#ed or controlled or clinical) adj2 (trial or study)).tw.
28 or/23-27
29 10 and 14 and 22 and 28
30 remove duplicates from 29
Database
CINAHL.
Search date: 21 July 2020.
Search strategy
S1 (MH “Emergency Medical Services”)
S2 TX accident N2 emergency
S3 TX “emergency room” or “emergency department” or ED
S4 TX pre-hospital or prehospital or “out of hospital” or community
S5 (MH “Allied Health Personnel”)
S6 TX paramedic* or ambulance
S7 (MH “Emergency Medical Technicians”)
S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7
S9 (MH “Status Epilepticus+”)
S10 TX Status Epilepticus
S11 S9 OR S10
S12 (MH “Antianxiety Agents, Benzodiazepine+”)
S13 TX midazolam or diazepam or lorazepam
S14 (MH “Anticonvulsants+”)
S15 TX levetiracetam or “sodium valproate” or phenytoin
S16 S12 OR S13 OR S14 OR S15
S17 TX (randomi#ed or controlled or clinical) N2 (trial or study)
S15 S8 AND S11 AND S16 AND S17
Database
CENTRAL.
Search date: 21 July 2020.
Search strategy
#1 MeSH descriptor: [Emergency Medical Services] explode all trees
#2 (accident Near/2 emergency):ti,ab,kw (Word variations have been searched)
#3 “emergency room” or “emergency department” or ED
#4 pre-hospital or prehospital or “out of hospital” or community
#5 MeSH descriptor: [Allied Health Personnel] explode all trees
#6 paramedic* or ambulance
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Status Epilepticus] explode all trees
#9 Status Epilepticus
#10 #8 or #9
#11 MeSH descriptor: [Benzodiazepines] explode all trees
#12 midazolam or diazepam or lorazepam
#13 #11 or #12
#14 #7 and #10 and #13
#15 MeSH descriptor: [Anticonvulsants] explode all trees
#16 (levetiracetam or “sodium valproate” or phenytoin):ti,ab,kw (Word variations have been searched)
#17 #11 or #12 or #15 or #16
#18 #7 and #10 and #17
Appendix 2 References to studies included in the clinical effectiveness review
*Denotes primary studies.
Alldredge 2001 (PHTSE)
*Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med 2001;345:631–7. https://doi.org/10.1056/NEJMoa00214122
Lowenstein DH, Alldredge BK, Allen F, Neuhaus J, Corry M, Gottwald M, et al. The PreHospital Treatment of Status Epilepticus (PHTSE) study: design and methodology. Control Clin Trials 2001;22:290–309. 68
Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, et al. Correction: a comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. New Engl J Med 2001;345:631–7. [Corrigendum published in New Engl J Med 2001;345:1860.]69
Navarro 2016 (SAMU-keppra)
*Navarro V, Dagron C, Elie C, Lamhaut L, Demeret S, Urien S, et al. Prehospital treatment with levetiracetam plus clonazepam or placebo plus clonazepam in status epilepticus (SAMUKeppra): a randomised, double-blind, phase 3 trial. Lancet Neurol 2016;15:47–55. https://doi.org/10.1016/S1474-4422(15)00296-357
Navarro V, Dagron C, Demeret S, An K, Lamhaut L, Bolgert F, et al. A prehospital randomized trial in convulsive status epilepticus. Epilepsia 2011;52(Suppl. 8):48–9. https://doi.org/10.1111/j.1528-1167.2011.03236.x70
ClinicalTrials.gov. Study of Antiepileptic Drug in Generalised Convulsive Status Epilepticus. Bethesda, MD: National Library of Medicine; 2010. URL: www.cochranelibrary.com/central/doi/10.1002/central/CN-02025411/full (accessed 21 July 2020). 71
Shaner 1988
Shaner DM, McCurdy SA, Herring MO, Gabor AJ. Treatment of status epilepticus: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology 1988;38:202–7. https://doi.org/10.1212/wnl.38.2.20259
Silbergleit 2012 (RAMPART)
*Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, Barsan W, NETT Investigators. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med 2012;366:591–600. https://doi.org/10.1056/NEJMoa110749458
Silbergleit R. Correction: intramuscular versus intravenous therapy for prehospital status epilepticus. New Engl J Med 2012;366:591–600. [Corrigendum published in N Engl J Med 2012;366:1261.]72
Silbergleit R, Lowenstein D, Durkalski V, Conwit R, NETT Investigators. Lessons from the RAMPART study – and which is the best route of administration of benzodiazepines in status epilepticus. Epilepsia 2013;54(Suppl. 6):74–7. https://doi.org/10.1111/epi.1228423
Silbergleit R, Lowenstein D, Durkalski V, Conwit R, Neurological Emergency Treatment Trials (NETT) Investigators. RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia 2011;52(Suppl. 8):45–7. https://doi.org/10.1111/j.1528-1167.2011.03235.x73
Vohra TT, Miller JB, Nicholas KS, Varelas PN, Harsh DM, Durkalski V, et al. Endotracheal intubation in patients treated for prehospital status epilepticus. Neurocrit Care 2015;23:33–43. https://doi.org/10.1007/s12028-014-0106-574
ClinicalTrials.gov. Paramedic Treatment of Prolonged Seizures by Intramuscular Versus Intravenous Anticonvulsant Medications. Bethesda, MD: National Library of Medicine; 2008. URL: www.cochranelibrary.com/central/doi/10.1002/central/CN-02031817/full (accessed 21 July 2020).
Appendix 3 Examples of excluded studies with reasons for exclusion
| Study ID | First author and year of publication | Reason for exclusion | Reference |
|---|---|---|---|
| Excluded: selected for full-text screening, but articles not available because of COVID-19 situations | |||
| 1 | Pourzitaki 2008 | Not relevant study design. The available abstract indicates this is a systematic review | Pourzitaki C, Tzellos T, Sardeli C, Papazisis G, Amaniti E, Kouvelas D. Evidence-based evaluation of emergency care treatment algorithms: 15 dominant myths. Rev Clin Pharmacol Pharmacokinet Int Ed 2008;22:302–3 |
| 2 | Chamberlain 1997 | Not relevant population. The available abstract suggests that the study focuses on children. It is not clear from the abstract if eligible adults were also among study participants | Chamberlain JM, Altieri MA, Futterman C, Young GM, Ochsenschlager DW, Waisman Y. A prospective, randomized study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatr Emerg Care 1997;13:92–4. https://doi.org/10.1097/00006565-199704000-00002 |
| Excluded references related to studies included in the review | |||
| 3 | Claassen 2016 | Not relevant study design. Comment on the SAMUKeppra study by Navarro et al.57 included in the review | Claassen J. Dr No: double drug fails to eliminate status epilepticus. Lancet Neurol 2016;15:23–4. https://doi.org/10.1016/S1474-4422(15)00333-6 |
| 4 | Durkalski 2011 | Not relevant study design. A case study related to RAMPART (by Silbergleit et al.58 included in the review) highlighting challenges of a trial conducted under the US FDA | Durkalski V, Silbergleit R, Lowenstein D. Challenges in the design and analysis of non-inferiority trials: a case study. Clin Trials 2011;8:601–8. https://doi.org/10.1177/1740774511418848 |
| 5 | Knopp 2001 | Not relevant study design. Letter to the editor regarding the PHTSE study by Alldredge et al.22 included in the review, and the authors’ reply | Knopp RK. Treatment of out-of-hospital status epilepticus. N Engl J Med 2001;345:1913. https://doi.org/10.1056/NEJM200112273452611 |
| 6 | Kossoff 2012 | Not relevant study design. Comment on RAMPART by Silbergleit et al.58 included in the review | Kossoff EH. A shot in the arm for prehospital status epilepticus: the RAMPART study. Epilepsy Curr 2012;12:103–4. https://doi.org/10.5698/1535-7511-12.3.103 |
| 7 | Lee 2016 | Not relevant study design. Comment on the SAMUKeppra study by Navarro et al.57 included in the review | Lee JW. Fruitful futility: what we learned from a failed clinical trial of out-of-hospital status epilepticus trial. Epilepsy Curr 2016;16:147–9. https://doi.org/10.5698/1535-7511-16.3.147 |
| 8 | Meurer 2013 | Not relevant study design. Secondary analysis of RAMPART by Silbergleit et al.58 included in the review. Conference abstract | Meurer W, Silbergleit R, Durkalski V. Handling repeat enrollments during an emergency clinical trial: the rapid anticonvulsant medications prior to arrival trial (RAMPART). Acad Emerg Med 2013;20(Suppl. 1):S108–S9 |
| 9 | Meurer 2015 | Not relevant study design. Secondary analysis of RAMPART by Silbergleit et al.58 included in the review | Meurer WJ, Silbergleit R, Nicholas KS, Burke JF, Durkalski V. Accounting for repeat enrollments during an emergency clinical trial: the Rapid Anticonvulsant Medications Prior to Arrival Trial (RAMPART). Acad Emerg Med 2015;22:373–7. https://doi.org/10.1111/acem.12596 |
| 10 | Miller 2013 | Not relevant study design. Secondary analysis of RAMPART by Silbergleit et al.58 included in the review. Poster | Miller JB, Vohra T, Nicholas K, Durkalski V, Silbergleit R, Wang H. Characteristics of prehospital status epilepticus patients receiving endotracheal intubation. Neurocrit Care 2013;19(Suppl. 1):S220 |
| 11 | Roy Moulik 2013 | Not relevant study design. Comment (journal club) on RAMPART by Silbergleit et al.58 included in the review | Roy Moulik N. Critical appraisal of “Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, Barsan W, NETT Investigators. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012 Feb 16;366(7): 591–600”. Clin Epidemiol Glob Health 2013;1:129–30 |
| 12 | Schomer 2016 | Not relevant study design. Comment on the SAMUKeppra study by Navarro et al.57 included in the review | Schomer AC, Kapur J. The SAMUKeppra study in prehospital status epilepticus: lessons for future study. Ann Transl Med 2016;4:468. https://doi.org/10.21037/atm.2016.11.67 |
| 13 | Schwartz 2012 | Not relevant study design. Comment on RAMPART by Silbergleit et al.58 included in the review | Schwartz A. Novel approach to neurologic emergency research yields results. Ann Neurol 2012;72:A8–A10. https://doi.org/10.1002/ana.23751 |
| Excluded references retained as background information | |||
| 14 | Brigo 2015 | Not relevant study design. Systematic review on status epilepticus | Brigo F, Nardone R, Tezzon F, Trinka E. Nonintravenous midazolam versus intravenous or rectal diazepam for the treatment of early status epilepticus: a systematic review with meta-analysis. Epilepsy Behav 2015;49:325–36. https://doi.org/10.1016/j.yebeh.2015.02.030 |
| 15 | Jain 2016 | Not relevant study design. Meta-analysis of antiepileptic drugs | Jain P, Sharma S, Dua T, Barbui C, Das RR, Aneja S. Efficacy and safety of anti-epileptic drugs in patients with active convulsive seizures when no i.v. access is available: systematic review and meta-analysis. Epilepsy Res 2016;122:47–55. https://doi.org/10.1016/j.eplepsyres.2016.02.006 |
| 16 | Kriel 1991 | Not relevant study design. Cost analysis | Kriel RL, Cloyd JC, Hadsall RS, Carlson AM, Floren KL, Jones-Saete CM. Home use of rectal diazepam for cluster and prolonged seizures: efficacy, adverse reactions, quality of life, and cost analysis. Pediatr Neurol 1991;7:13–17 |
| 17 | Lesser 2018 | Not relevant study design. BMJ best practice guidance | Lesser RP, Johnson E. Status Epilepticus: BMJ Best Practice. 2018. URL: https://bestpractice.bmj.com/topics/en-gb/3000127 (accessed 24 June 2020) |
| 18 | Lowenstein 1998 | Not relevant study design. Current concept of status epilepticus | Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med 1998;338:970–6 |
| 19 | Lowenstein 1999 | Not relevant study design. Key paper on status epilepticus definition | Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia 1999;40:120–2. https://doi.org/10.1111/j.1528-1157.1999.tb02000.x |
| 20 | Marshall 2007 | Not relevant study design. Systematic review | Marshall T. A systematic review of the use of buccal midazolam in the emergency treatment of prolonged seizures in adults with learning disabilities. Br J Learn Disabil 2007;35:99–101 |
| 21 | McTague 2018 | Not relevant study design. Cochrane review of convulsive status epilepticus in children | McTague A, Martland T, Appleton R. Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Syst Rev 2018;1:CD001905. https://doi.org/10.1002/14651858.CD001905.pub3 |
| 22 | NICE 2012 | Not relevant study design. NICE guidelines | National Institute for Health and Care Excellence (NICE). Epilepsies: Diagnosis and Management. Clinical Guideline [CG137]. London: NICE; 2012 |
| 23 | Prasad 2014 | Not relevant study design. Cochrane review on adults with status epilepticus | Prasad M, Krishnan PR, Sequeira R, Al-Roomi K. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev 2014;9:CD003723. https://doi.org/10.1002/14651858.CD003723.pub3 |
| 24 | Raspall-Chaure 2014 | Not relevant study design. Cost-effectiveness | Raspall-Chaure M, Martinez-Bermejo A, Sanchez-Carpintero R, Ruiz-Falco Rojas ML, Verdu-Perez A, Smeyers-Dura P, et al. [Cost-effectiveness of buccal midazolam in the treatment of prolonged convulsive seizures in the outpatient setting in Spain.] Rev Neurol 2014;58:481–6 |
| 25 | Rogalski 2015 | Not relevant study design. Literature review | Rogalski R, Rogalski A. Benzodiazepine selection in the management of status epilepticus: a review. Adv Emerg Nurs J 2015;37:83–94. https://doi.org/10.1097/TME.0000000000000064 |
| 26 | SIGN 2015 | Not relevant study design. SIGN guidelines | Scottish Intercollegiate Guidelines Network (SIGN). Diagnosis and Management of Epilepsy in Adults. Edinburgh: SIGN; 2015 |
| 27 | Shtull-Leber 2016 | Not relevant study design. Conference abstract of Shtull-Leber 2017, Ref ID 96, excluded from the review | Shtull-Leber E, Silbergleit R, Meurer W. Pre-hospital midazolam for treatment of status epilepticus before and after RAMPART: a national observational cohort study. Acad Emerg Med 2016;23(Suppl. 1):S19 |
| 28 | Shtull-Leber 2017 | Not relevant study design. Retrospective, observational cohort study before and after the RAMPART study | Shtull-Leber E, Silbergleit R, Meurer WJ. Pre-hospital midazolam for benzodiazepine-treated seizures before and after the Rapid Anticonvulsant Medication Prior to Arrival Trial: a national observational cohort study. PLOS ONE 2017;12:e0173539. https://doi.org/10.1371/journal.pone.0173539 |
| 29 | Touchette 2000 | Not relevant study design. Cost-minimisation analysis | Touchette DR, Rhoney DH. Cost-minimization analysis of phenytoin and fosphenytoin in the emergency department. Pharmacotherapy 2000;20:908–16. https://doi.org/10.1592/phco.20.11.908.35269 |
| 30 | Zaccara 2017 | Not relevant study design. Review of convulsive status epilepticus (CSE) treatment | Zaccara G, Giannasi G, Oggioni R, Rosati E, Tramacere L, Palumbo P, Convulsive Status Epilepticus Study Group of the Uslcentro Toscana, Italy. Challenges in the treatment of convulsive status epilepticus. Seizure 2017;47:17–24 |
| Other excluded references | |||
| 31 | Agarwal 2007 |
Not relevant intervention. Probably second-line treatment: |
Agarwal P, Kumar N, Chandra R, Gupta G, Antony AR, Garg N. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure 2007;16:527–32 |
| 32 | Akinbi 1999 | Not relevant study design. Review | Akinbi MS, Welty TE. Benzodiazepines in the home treatment of acute seizures. Ann Pharmacother 1999;33:99–102. https://doi.org/10.1345/aph.17306 |
| 33 | Appleton 1995 | Not relevant population. Children. Quasi-RCT (odd and even dates) | Appleton R, Sweeney A, Choonara I, Robson J, Molyneux E. Lorazepam versus diazepam in the acute treatment of epileptic seizures and status epilepticus. Dev Med Child Neurol 1995;37:682–8. https://doi.org/10.1111/j.1469-8749.1995.tb15014.x |
| 34 | Banta-Banzali 2013 | Not relevant population. Status epilepticus patients 2–18 years old admitted at the ED, ICU, service and pay wards of a tertiary paediatric government hospital. Abstract only | Banta-Banzali LKF, Obligar PD, Panlilio JR, Pasco PM. The efficacy of intravenous valproate compared to intravenous phenobarbital in controlling seizures among pediatric patients with benzodiazepine-refractory status epilepticus: a randomized controlled trial. Epilepsia 2013;54:226–7 |
| 35 | Cereghino 1998 | Not relevant population. ARS (acute repetitive seizure). It is unclear if all participants had status epilepticus | Cereghino JJ, Mitchell WG, Murphy J, Kriel RL, Rosenfeld WE, Trevathan E. Treating repetitive seizures with a rectal diazepam formulation: a randomized study. The North American Diastat Study Group. Neurology 1998;51:1274–82. https://doi.org/10.1212/wnl.51.5.1274 |
| 36 | Cereghino 2002 | Not relevant population. ARS (acute repetitive seizure). Unclear if all participants had status epilepticus. This paper reports on a subset of participants from Dreifuss 1998 (Ref ID 720) and Cereghino 1998 (Ref ID 721) | Cereghino JJ, Cloyd JC, Kuzniecky RI, North American Diastat Study Group. Rectal diazepam gel for treatment of acute repetitive seizures in adults. Arch Neurol 2002;59:1915–20 |
| 37 | Connor 2013 | Not relevant study design. Describes Bayesian adaptive designs for ESETT, as part of the Adaptive Designs Accelerating Promising Trials Into Treatments (ADAPT-IT) project | Connor JT, Elm JJ, Broglio KR, ESETT and ADAPT-IT Investigators. Bayesian adaptive trials offer advantages in comparative effectiveness trials: an example in status epilepticus. J Clin Epidemiol 2013;66(Suppl. 8):130–7. https://doi.org/10.1016/j.jclinepi.2013.02.015 |
| 38 | de Haan 2010 | Not relevant study design. Participants were not fully randomised: each person was given six packages, with the first package randomly and the remaining packages alternating study drugs. Includes 21 adults with 124 seizure exacerbations. Results are reported for 124 events | de Haan GJ, van der Geest P, Doelman G, Bertram E, Edelbroek P. A comparison of midazolam nasal spray and diazepam rectal solution for the residential treatment of seizure exacerbations. Epilepsia 2010;51:478–82. https://doi.org/10.1111/j.1528-1167.2009.02333.x |
| 39 | Dreifuss 1998 | Not relevant population. ARS (acute repetitive seizure). Seizure pregressing to status epilepticus was excluded | Dreifuss FE, Rosman NP, Cloyd JC, Pellock JM, Kuzniecky RI, Lo WD, et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures. N Engl J Med 1998;338:1869–75. https://doi.org/10.1056/NEJM199806253382602 |
| 40 | Fitzgerald 2003 |
Not relevant intervention. Secondary treatment: |
Fitzgerald BJ, Okos AJ, Miller JW. Treatment of out-of-hospital status epilepticus with diazepam rectal gel. Seizure 2003;12:52–5 |
| 41 | ISRCTN 2014 | Not relevant population. Emergency treatment with Levetiracetam or Phenytoin in Status Epilepticus in children (EcLiPSE), an open-label RCT registered as ISRCTN22567894 | ISRCTN Registry. Emergency Treatment with Levetiracetam or Phenytoin in Status Epilepticus. London: BioMed Central; 2014 |
| 42 | jRCTs 2019 | Not relevant intervention. Secondary treatment – treated with diazepam before study drug. JPRN-jRCTs031190160 | Japan Primary Registries Network. Ibaraki ER Network Epilepsy Control Trial with LevetIracetam vs. FosphEnytoine. Saitama: National Institute of Public Health; 2019 |
| 43 | Leppik 1983 |
Not relevant population. Unclear if the first treatment was given in out-of-hospital setting: |
Leppik IE, Derivan AT, Homan RW, Walker J, Ramsay RE, Patrick B. Double-blind study of lorazepam and diazepam in status epilepticus. JAMA 1983;249:1452–4 |
| 44 | Misra 2012 | Not relevant population. Excluded as it is unclear where the first treatment was administered and whether or not it was out-of-hospital setting. Author contacted for clarification – no reply | Misra UK, Kalita J, Maurya PK. Levetiracetam versus lorazepam in status epilepticus: a randomized, open labeled pilot study. J Neurol 2012;259:645–8. https://doi.org/10.1007/s00415-011-6227-2 |
| 45 | Misra 2017 | Not relevant intervention. Focus on second-line antiepileptic drug. Retracted study | Misra UK, Dubey D, Kalita J. A randomized controlled trial of lacosamide versus sodium valproate in status epilepticus [published online ahead of print February 18 2017]. Epilepsia 2017. https://doi.org/10.1111/epi.13706 |
| 46 | NCT 2013 | Not relevant intervention. Secondary treatment – participant treated with benzodiazepines before study drugs. ESETT | ClinicalTrials.gov. Established Status Epilepticus Treatment Trial. Bethesda, MD: National Library of Medicine; 2013 |
| 47 | NCT 2013 | Not relevant intervention. Duplicate copy of NCT 2013 [Ref ID 118] excluded from the review | As above |
| 48 | Pellock 1998 | Not relevant population. ARS (acute repetitive seizure). Unclear if all participants had status epilepticus | Pellock J, Mitchell WG, Cloyd J. Diastat (diazepam rectal gel) in the treatment of acute repetitive seizures in adults. Annual Meeting of the American Epilepsy Society, San Diego, CA, 6–9 December 1998 |
| 49 | Rossetti 2011 | Not relevant intervention. Not first treatment – given at least one-first-line (benzodiazepines) and one-second-line (phenytoin, phenobar bital, or valproic acid) AED before study drug. Participants had refractory status epilepticus | Rossetti AO, Milligan TA, Vulliémoz S, Michaelides C, Bertschi M, Lee JW. A randomized trial for the treatment of refractory status epilepticus. Neurocrit Care 2011;14:4–10. https://doi.org/10.1007/s12028-010-9445-z |
| 50 | Scott 1999 | Not relevant population. Excluded as only around 50% of participants were over 16 years old. Author contacted to request data for adult participants – no reply. The unit of randomisation was the seizure, not person | Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet 1999;353:623–6 |
| 51 | Treiman 1998 | Not relevant population. Excluded as it is unclear whether treatment was given in out-of-hospital setting; it appears that study participants include in-patients. Author contacted for clarification – no reply | Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med 1998;339:792–8. https://doi.org/10.1056/NEJM199809173391202 |
Appendix 4 Identified ongoing study
| Study name | Comparison Between Lorazepam, Clonazepam and Clonazepam + Fosphenytoin for the Treatment of Out-of-hospital Generalised Status Epilepticus (LORACLOFT) |
| Methods | Multicenter, randomised, double-blind, Phase III trial with three arms |
| Participants | Estimated enrolment: 522 patients; 174 patients by group |
| Actual enrolment: 434 | |
Inclusion criteria:
|
|
Exclusion criteria:
|
|
| Interventions | 1. Lorazepam + placebo: lorazepam 0.1 mg/kg by i.v. injection over a period of 2–3 minutes + placebo 20 mg/kg by i.v. infusion over a period of 15 minutes |
| 2. Clonazepam + placebo: clonazepam 0.015 mg/kg by i.v. injection over a period of 2–3 minutes + placebo 20 mg/kg by i.v. infusion over a period of 15 minutes | |
| 3. Clonazepam + fosphenytoin: clonazepam 0.015 mg/kg by i.v. injection over a period of 2–3 minutes + fosphenytoin 20 mg/kg equivalent phenytoin by i.v. infusion over a period of 15 minutes | |
| Outcomes | Primary outcome:
|
Secondary outcomes:
|
|
| Starting date | 26 June 2013 |
| Contact information | Principal investigators:
|
| End date | 23 February 2018 |
| Notes |
|
| Reference | ClinicalTrials.gov. Comparison Between Lorazepam, Clonazepam and Clonazepam + Fosphenytoin for the Treatment of Out-of-hospital Generalized Status Epilepticus. Bethesda, MD: National Library of Medicine; 2013. URL: www.cochranelibrary.com/central/doi/10.1002/central/CN-01597356/full (accessed 21 July 2020) |
Appendix 5 Study characteristics of the four included trials
| Study details | Study characteristics | Intervention characteristics |
|---|---|---|
| First author and year: Alldredge 200122 | Study design: prospective double-blind RCT | Interventions:
|
| Secondary reports: Alldredge 2001,69 Lowenstein 200168 | Study setting: paramedics | Route/dose/frequency:
|
| Language: English | Number of centres: NR | Other information: all were administered by i.v. injection over a 1- to 2-minute period. If seizures recurred or continued ≥ 4 minutes after first injection, an identical second injection was administered. Open-label diazepam was immediately available if needed |
| Publication type: full text | Country: USA | |
| Study name: PHTSE (PreHospital Treatment of Status Epilepticus) | Recruitment period: 4 January 1994 to 31 January 1999 | |
| Duration of follow-up: NR | ||
Inclusion criteria:
|
||
Exclusion criteria:
|
||
| First author and year: Navarro 201657 | Study design: randomised, parallel-group, double-blind, Phase III, placebo-controlled, superiority trial | Interventions:
|
| Secondary reports: Navarro 201170 | Study setting: paramedics (‘mobile ICUs’, including a senior emergency physician, a nurse and paramedics) | Route/dose/frequency:
|
| Language: English | Number of centres: 13 emergency medical service centres and 26 hospital departments (including ICU, neurology and internal medicine) | Other information: if the status epilepticus continued after 5 minutes, a second dose of 1 mg of clonazepam was injected. If convulsions persisted at 15 minutes after the first injection, an AED (phenytoin, fosphenytoin, or phenobarbital) was given following standard procedure. If the status epilepticus had not stopped by 35 minutes, general anaesthesia was induced with propofol, thiopental, or midazolam in rapid sequence after an endotracheal intubation |
| Publication type: full text | Country: France | |
| Study name: SAMUKeppra | Recruitment period: 20 July 2009 to 15 December 2012 | |
| Duration of follow-up: 15 days after onset of status epilepticus | ||
| Inclusion criteria: Patients aged ≥ 18 years, with GCSE duration of > 5 minutes or generalised convulsions with no recovery of consciousness between seizures | ||
| Exclusion criteria: women with known or clinically detected pregnancy; patients with known allergies to clonazepam, levetiracetam, or pyrrolidone; and patients whose status epilepticus was linked to a pathological condition, such as trauma, who needed immediate surgery. Patients with post-anoxic or subtle status epilepticus (defined by minor and erratic myoclonic movements in patients with severely impaired consciousness) were also excluded, as were those whose parent, guardian, or other reliable person refused permission and those not covered by the French medical insurance system, who should not be included in clinical trials according to the French Public Health Code. In addition, patients with definite non-epileptic psychogenic pseudo-status, patients who previously received another drug for the ongoing status epilepticus, or those who had been included in the study previously for an episode of status epilepticus, were excluded | ||
| First author and year: Shaner 198859 | Study design: non-blinded RCT | Interventions:
|
| Secondary reports: N/A | Study setting: ED | Route/dose/frequency:
|
| Language: English | Number of centres: 1 | |
| Publication type: full text | Country: USA | |
| Study name: N/A | Recruitment period: 20 November 1983 to 10 March 1985 | If the patient continued to convulse after delivery of the intial 20 mg of diazepam dose, a continuous i.v. infusion of diazepam at 40 ml/hour (8 mg/hour) was started. General anaesthesia was considered if seizure activity persisted after completion of the phenytoin loading dose |
| Duration of follow-up: NR | Other information: N/A | |
| Inclusion criteria: patients aged > 15 years presenting to the emergency room with status epilepticus | ||
| Exclusion criteria: anticonvulsants given for the presenting status epilepticus episode before arrival in the emergency room | ||
| First author and year: Silbergleit 201258 | Study design: double-blind, randomised, non-inferiority trial | Interventions:
|
| Secondary reports: Silbergleit 2011,73 Silbergleit 2012,72 Silbergleit 2013,23 Vohra 201574 | Study setting: paramedics | |
| Language: English | Number of centres: NR (4314 paramedics, 33 emergency medical services and 79 receiving hospitals) | Route/dose/frequency:
|
| Publication type: full text | Country: USA | |
| Study name: RAMPART | Recruitment period: 15 June 2009 to 14 January 2011 | Other information: all participants were treated with the i.m. autoinjector, after which venous access was immediately achieved and treatment was administered by means of i.v. syringe |
| Duration of follow-up: NR (participants were followed for duration of hospital stay, an average of 6 days) | ||
Inclusion criteria:
|
||
Exclusion criteria:
|
Appendix 6 Summary of participants’ characteristics of the four included trials
| Study details | Participant characteristics |
|---|---|
| First author and year: Alldredge 200122 | Type of participant: adult patients with potential status epilepticus for whom a paramedic ambulance is despatched |
| Secondary reports: Alldredge 2001,69 Lowenstein 200168 |
Randomised, n: A: 66, B: 68, C: 71 Analysed, n: A: 66, B: 68, C: 71 |
| Language: English | |
| Publication type: full text | Age, years, mean (SD): A: 49.9 (20.1), B: 50.4 (19.1), C: 52.0 (18.2) |
| Study name: PHTSE | Sex, n (%): A: 46 M (69.7%)/20 F (30.3%), B: 41 M (60.3%)/27 F (39.7%), C: 42 M (59.2%)/29 F (40.8%) |
Interventions:
|
Ethnicity, %: i. American Indian or Alaskan, ii. Asian or Pacific Islander, iii. black, iv. Hispanic, v. white, vi. other, vii. unknown A: i. 1.5, ii. 21.2, iii. 18.2, iv. 9.1, v. 48.5, vi. 1.5, vii. 0 B: i. 1.5, ii. 7.4, iii. 16.2, iv. 20.6, v. 54.4, vi. 0, vii. 0 C: i. 4.2, ii. 9.9, iii. 29.6, iv. 8.5, v. 46.5, vi. 0, vii. 1.4 |
| Time from onset of status epilepticus to drug administration, minutes, mean (SD): A: 34.0 (14.5), B: 31.2 (14.5), C: 46.7 (38.8) | |
| Final diagnosis, n (%): NR | |
|
Cause of status epilepticus, n (%): i. low levels of AED, ii. refractory epilepsy, iii. alcohol abuse, iv. metabolic derangement, v. toxic effects of drugs, vi. anoxia or cardiopulmonary arrest, vii. CNS infection, viii. trauma, ix. CNS tumour, x. stroke, xi. non-epileptic seizures, xii. other, xiii. unknown A: i. 11 (16.7%), ii. 9 (13.6%), iii. 6 (9.1%), iv. 2 (3.0%), v. 7 (10.6%), vi. 1 (1.5%), vii. 5 (7.6%), viii. 4 (6.1%), ix. 4 (6.1%), x. 11 (16.7%), xi. 2 (3.0%); xii. 0, xiii. 4 (6.1%) B: i. 17 (25.0%), ii. 9 (13.2%), iii. 8 (11.8%), iv. 2 (2.9%), v. 5 (7.4%), vi. 0, vii. 5 (7.4%), viii. 6 (8.8%), ix. 3 (4.4%), x. 9 (13.2%), xi. 3 (4.4%), xii. 0, xiii. 1 (1.5%) C: i. 17 (25.0%), ii. 9 (13.2%), iii. 8 (11.8%), iv. 2 (2.9%), v. 5 (7.4%), vi. 0, vii. 5 (7.4%), viii. 6 (8.8%), ix. 3 (4.4%), x. 9 (13.2%), xi. 3 (4.4%), xii. 0, xiii. 1 (1.5%) |
|
| First author and year: Navarro 201657 | Type of participant: adults with convulsions lasting > 5 minutes |
| Secondary reports: Navarro 201170 | Randomised, n: A: 96, B: 107 |
| Language: English | Analysed, n: A: 68, B: 68 |
| Publication type: full text | Age, years, mean (SD): A: 55 (18), B: 53 (18) |
| Study name: SAMUKeppra | Sex, n (%): A: 49 M (72.1%)/19 F (27.9%), B: 45 M (66.2%)/23 F (33.8%) |
Interventions:
|
Ethnicity: NR |
| Time from onset of status epilepticus to drug administration, minutes, median (range): A: 58 (15–135), B: 60 (20–258) | |
|
Final diagnosis, n (%): i. status epilepticus, ii. non-epileptic psychogenic events A: i. 66/68 (97.1%), ii. 2/68 (2.9%) B: 64/68 (94.1%), ii. 4/68 (5.9%) |
|
|
Cause of status epilepticus, n (%): i. lesion, ia. tumour, ib. vascular, ic. trauma, id. inflammation, i.e. degenerative; ii. metabolic; iii. cerebral or extracerebral infection; iv. toxic (alcohol misuse or withdrawal, toxic effects of recreational or prescribed drugs); v. other; vi. undetermined A: i. 42/65 (65%), ia. 8/42 (19%), ib. 16/42 (38%), ic. 13/42 (31%), id. 0/42, i.e. 3/42 (7%), ii. 7/66 (11%), iii. 3/66 (5%), iv. 25/65 (38%), v. 7/66, (11%), vi. 3/65 (5%) B: i. 41/63 (65%), ia. 15/41 (37%), ib. 13/41 (32%), ic. 11/41 (27%), id. 1/41 (2%), i.e. 1/41 (2%), ii. 4/63 (6%), iii. 6/63 (10%), iv. 15/64 (23%), v. 6/64 (9%), vi. 6/64 (9%) |
|
| First author and year: Shaner 198859 | Type of participant: patients presenting to the emergency room with status epilepticus |
| Secondary reports: N/A | Randomised, n: A + B: 44 |
| Language: English | Analysed, n: A: 18, B: 18 |
| Publication type: full text | Age, years, mean (SD): A: 55.9 (19.4), B: 43.8 (16.5) |
| Study name: N/A | Sex, n (%): A: 13 M (72.2%)/5 F (27.8%), B: 9 M (50%)/9 F (50%) |
Interventions:
|
Ethnicity: NR |
| Time from onset of status epilepticus to drug administration, minutes, mean (SD): NR | |
|
Final diagnosis, n (%): i. focal features; ii. GCSE diagnostic criterion for ENTRANCE into study; iia. a history of 30 minutes of continuous GCSE, and witnessed generalised seizures in the emergency room; iib. a history of 30 minutes of recurrent GCSE but failure to attain baseline mental status between seizures, and witnessed generalised seizures in the emergency room; iic. a history of three or more GCSE in 1 hour in patients with obtundation prior to the onset of status epilepticus, and witnessed generalised convulsive seizures in the emergency room; iid. uncertain history of seizures but generalised convulsive seizures continuously for more than 5 minutes as witnessed in the emergency room A: i. 10/18 (55.6%), iia. 4/18 (22.2%), iib. 14/18 (77.8%), iic. 0/18, iid. 0/18 B: i. 9/18 (50%), iia. 6/18 (33.3%), iib. 11/18 (61.1%), iic. 0/18, iid. 1/18 (5.6%) |
|
|
Cause of status epilepticus, n (%): i. alcohol withdrawal, ii. subtherapeutic anticonvulsants, iii. infections, iv. structural lesions, v. toxic/metabolic A: i. 5/18 (27.8%), ii. 7/18 (38.9%), iii. 0/18, iv. 7/18 (38.9%), v. 2/18 (11.1%) B: i. 5/18 (27.8%), ii. 11/18 (61.1%), iii. 2/18 (11.1%), iv. 5/18 (27.8%), v. 2/18 (11.1%) |
|
| First author and year: Silbergleit 201258 (published data) | Type of participant: patients requiring treatment with benzodiazepines for status epilepticus in the pre-hospital setting |
|
Secondary reports: Silbergleit 2011,73 Silbergleit 2012,72 Silbergleit 2013,23 Vohra 201574 Language: English |
Randomised, n: A: 448, B: 445 Analysed, n: A: 448, B: 445 |
| Publication type: full text | Age, years, mean (SD): A: 43 (22), B: 44 (22) |
| Study name: RAMPART | Sex, n (%): A: 250 M (55.8%)/198 F (44.2%), B: 238 M (53.5%)/207 F (46.5%) |
Interventions:
|
Ethnicity, n (%): i. black, ii. white, iii. other, mixed or unknown A: i. 229 (51.1), ii. 165 (36.8), iii. 54 (12.1) B: i. 224 (50.3), ii. 183 (41.1), iii. 38 (8.5) |
| Time from onset of status epilepticus to drug administration, minutes, mean (SD): NR | |
|
Final diagnosis, n (%): i. status epilepticus, ii. non-epileptic spell, iii. undetermined A: i. 404 (90%), ii. 31 (7%), iii. 13 (3%) B: i. 399 (90%), ii. 32 (7%), iii. 14 (3%) |
|
|
Cause of status epilepticus, n (%): i. non-compliance with or discontinuation of anticonvulsant therapy, ii. idiopathic or breakthrough status epilepticus, iii. coexisting condition that lowered seizure threshold A: i. 137 (31%), ii. 121 (27%), iii. 33 (7%) B: i. 141 (32%), ii. 121 (27%), iii. 29 (7%) |
|
| First author and year: Silbergleit 201258 (adult data) | Type of participant: patients requiring treatment with benzodiazepines for status epilepticus in the pre-hospital setting |
|
Secondary reports: Silbergleit 2011,73 Silbergleit 2012,72 Silbergleit 2013,23 Vohra 201574 Language: English |
Randomised, n: A: 391, B: 391 Analysed, n: A: 391, B: 391 |
| Publication type: full text | Age, years, mean (SD): A: 48 (17), B: 49 (18) |
| Study name: RAMPART | Sex, n (%): A: 217 M (55.5%)/174 F (44.5%), B: 203 M (51.9%)/188 F (48.1%) |
Interventions:
|
Ethnicity, n (%): i. black, ii. white, iii. other, mixed or unknown A: i. 211 (54.0), ii. 138 (35.3), iii. 42 (10.7) B: i. 204 (52.2), ii. 156 (39.9), iii. 31 (7.9) |
| Time from onset of status epilepticus to drug administration, minutes, mean (SD): NR | |
|
Final diagnosis, n (%): i. status epilepticus, ii. non-epileptic spell, iii. undetermined A: i. 352 (90%), ii. 28 (7.2%), iii. 11 (2.8%) B: i. 348 (89%), ii. 29 (7.4%), iii. 14 (3.6%) |
|
|
Cause of status epilepticus, n (%): i. non-compliance with or discontinuation of anticonvulsant therapy, ii. idiopathic or breakthrough status epilepticus, iii. coexisting condition that lowered seizure threshold A: i. 129 (33%), ii. 101 (25.8%), iii. 28 (7.2%) B: i. 138 (35.3%), ii. 97 (24.8%), iii. 24 (6.%) |
Appendix 7 Full details of primary seizure cessation outcomes reported by the four included studies
| Study (first author and year of publication) | Intervention 1 | Intervention 2 | Number of people with seizure cessation | Time to seizure cessation | |||||
|---|---|---|---|---|---|---|---|---|---|
| Definition | Intervention 1 | Intervention 2 | Effect estimate | ||||||
| n/N | % | n/N | % | ||||||
| Alldredge 200122 (PHTSE) | i.v. LOR | i.v. PBO | Termination of SE before arrival in ED | 39/66 | 59.1 | 15/71 | 14.3 | ORa 4.8 (95% CI 1.9 to 13.0) | HRb 0.34 (95% CI 0.17 to 0.71) |
| i.v. LOR | i.v. DIZ | Termination of SE before arrival in ED | 39/66 | 59.1 | 29/69 | 42.0 | ORa 1.9 (95% CI 0.8 to 4.4) | HRb 0.65 (95% CI 0.36 to 1.17) | |
| i.v. DIZ | i.v. PBO | Termination of SE before arrival in ED | 29/69 | 42.0 | 15/71 | 14.3 | OR 2.3 (95% CI 1.0 to 5.9) | NR | |
| Navarro 201657 (SAMUKeppra) | i.v. LEV + CLZ | i.v. PBO + CLZ | Seizure cessation within 15 minutes of onset of treatment | 50/68 | 73.5 | 57/68 | 83.8 | RR 0.88 (95% CI 0.74 to 1.05) | Minute, median (range): LEV 3 (0–50) vs. PBO 5 (0–41) |
| Shaner 198859 | i.v. PHB + PHT | i.v. DIZ + PHT | Convulsions controlled within 10 minutes of initiation of therapy | 13/18 | 72.2 | 6/18 | 33.3 | NR | Minute, median: PHB 5.5 vs. DIZ 15.0, p < 0.10 |
| Silbergleit 201258 (RAMPART) (published data) | i.m. MDZ | i.v. LOR | Seizures terminated before arrival in ED without the need for rescue therapy | 329/448 | 73.4 | 282/445 | 63.4 | AD 10 percentage points (95% CI 4.0 to 16.1 percentage points); p < 0.001 for non-inferiority and p < 0.001 for superiority | Time from active treatment to cessation of convulsions (minutes, median): MDZ 3.3 vs. LOR 1.6 |
| Time from box opening to active treatment (minutes, median): MDZ 1.2 vs. LOR 4.8 | |||||||||
| Silbergleit 201258 (RAMPART) (adult data) | i.m. MDZ | i.v. LOR | Seizures terminated before arrival in ED without the need for rescue therapy | 289/391 | 73.9 | 244/391 | 62.4 | NR | Treatment to termination of seizure (minutes, median): MDZ 4.7 vs. LOR 2.7 |
Appendix 8 Full details of primary seizure recurrence outcomes reported by the four included trials
| Study (first author and year of publication) | Arm | Recurrence of seizures | |
|---|---|---|---|
| Number of people with recurrence of seizures, n/N (%) | Time from seizure cessation to recurrence, minutes, mean (SD) | ||
| Alldredge 200122 (PHTSE) | Outcome definition | NR | NR |
| i.v. LOR (n = 66) | NR | NR | |
| i.v. DIZ (n = 68) | NR | NR | |
| i.v. PBO (n = 71) | NR | NR | |
| Summary statistic | NR | NR | |
| Navarro 201657 (SAMUKeppra) | Outcome definition | Seizure recurrence during stay in hospital | NR |
| i.v. LEV + CLZ (n = 68) | 7/67 (10.4) | NR | |
| i.v. PBO + CLZ (n = 68) | 13/68 (19.1) | NR | |
| Summary statistic | RR 0.55 (95% CI 0.23 to 1.28); p = 0.16 | NR | |
| Shaner 198859 | Outcome definition | NR | NR |
| i.v. PHB + PHT (n = 18) | NR | NR | |
| i.v. DIZ + PHT (n = 18) | NR | NR | |
| Summary statistic | NR | NR | |
| Silbergleit 201258 (RAMPART) (published data) | Outcome definition | Recurrent seizure within 12 hours after ED arrival | NR |
| i.m. MDZ (n = 448) | 51/448 (11.4) | NR | |
| i.v. LOR (n = 445) | 47/445 (10.6) | NR | |
| Summary statistic | RR 1.08 (95% CI 0.74 to 1.56) | NR | |
Appendix 9 Full details of primary safety outcomes reported by the four included trials
| Study (first author and year of publication) | Arm | AEs | ||
|---|---|---|---|---|
| Respiratory depression, n/N (%) | 30-day mortality, n (%) | |||
| Alldredge 200122 (PHTSE) | Outcome definition | Change in respiratory status requiring ventilation assistance by bag–valve–mask or an attempt at ventilation | Death between enrolment and discharge from hospital | |
| i.v. LOR (n = 66) | 7/66 (10.6) | 5/66 (7.6) | ||
| i.v. DIZ (n = 68) | 6/68 (8.8) | 3/68 (4.4) | ||
| i.v. PBO (n = 71) | 11/71 (15.5) | 11/71 (15.5) | ||
| Summary statistic | NR | p = 0.08 | ||
| Navarro 201657 (SAMUKeppra) | Outcome definition | Pre-hospital health failures: respiratory | Need for pre-hospital assistance: respiratory | Death (time point NR) |
| i.v. LEV + CLZ (n = 68) | 7/68 (10.3) | 29/68 (42.6) | 3/66 (4.5) | |
| i.v. PBO + CLZ (n = 68) | 3/66 (4.5) | 23/66 (34.8) | 4/65 (6.2) | |
| Summary statistic | p = 0.33 | p = 0.35 | RR 0.74 (95% CI 0.17 to 3.17); p = 0.72 | |
| Outcome definition | Respiratory, thoracic and mediastinal disorders: hypoxia (SAE) | |||
| i.v. LEV + CLZ (n = 68) | 1/68 (1.5%) | |||
| i.v. PBO + CLZ (n = 68) | 1/68 (1.5%) | |||
| Summary statistic | NR | |||
| Outcome definition | Respiratory, thoracic and mediastinal disorders: acidosis respiratory (non-serious AE) | Respiratory, thoracic and mediastinal disorders: hypoxaemia (non-serious AE) | ||
| i.v. LEV + CLZ (n = 68) | 1/68 (1.5) | 0/68 (0) | ||
| i.v. PBO + CLZ (n = 68) | 0/68 (0) | 1/68 (1.5) | ||
| Summary statistic | NR | NR | ||
| Outcome definition | Respiratory, thoracic and mediastinal disorders: respiratory distress (non-serious AE) | Respiratory, thoracic and mediastinal disorders: respiratory failure (non-serious AE) | ||
| i.v. LEV + CLZ (n = 68) | 1/68 (1.5) | 0/68 (0) | ||
| i.v. PBO + CLZ (n = 68) | 0/68 (0) | 2/68 (2.9) | ||
| Summary statistic | NR | |||
| Shaner 198859 | Outcome definition | NR | NR | |
| i.v. PHB + PHT (n = 18) | NR | NR | ||
| i.v. DIZ + PHT (n = 18) | NR | NR | ||
| Summary statistic | NR | NR | ||
| Silbergleit 201258 (RAMPART) (published data) | Outcome definition | Respiratory depression | NR | |
| i.m. MDZ (n = 514) | 33/514 (6.4) | NR | ||
| i.v. LOR (n = 509) | 51/509 (10) | NR | ||
| Summary statistic | NR | NR | ||
Appendix 10 Full details of secondary outcomes reported by the four included studies
| Study (first author and year of publicaiton) | Arm | Need for additional drug to stop seizure, n/N (%) | Need for hospital admission, n/N (%) | Length of stay in ICU (days), mean (SD) | 6-month mortality, n (%) | Return to baseline function, n/N (%) | HRQoL, mean (SD) | Number of people requiring emergency call-out, n (%) |
|---|---|---|---|---|---|---|---|---|
| Alldredge 200122 (PHTSE) | Outcome definition | NR |
|
NR | NR | Neurological outcome at hospital discharge – returned to base-line neurological condition | NR | NR |
| i.v. LOR (n = 66) | NR |
|
NR | NR | 49/65 (75.4) | NR | NR | |
| i.v. DIZ (n = 68) | NR |
|
NR | NR | 52/67 (77.6) | NR | NR | |
| i.v. PBO (n = 71) | NR |
|
NR | NR | 49/70 (70) | NR | NR | |
| Summary statistic | NR | NR | NR | NR | NR | NR | NR | |
| Navarro 201657 (SAMUKeppra) | Outcome definition | Need for a second clonazepam injection after 5 minutes | NR | Length of hospital stay in ICU | NR | Neurological state at 15 days after admission to hospital, or earlier if discharged from hospital – new neurological deficit (in alive patients) | NR | NR |
| i.v. LEV + CLZ (n = 68) | 28/67 (41.8) | NR | Median 3 (range 0–15) | NR | 1/66 (1.5) | NR | NR | |
| i.v. PBO + CLZ (n = 68) | 28/65 (43.1) | NR | Median 3 (range 1–15) | NR | 8/65 (12.3) | NR | NR | |
| Summary statistic | RR 0.97 (95% CI 0.65 to 1.44); p = 0.88 | NR | p = 0.74 | NR | RR 0.12 (95% CI 0.02 to 0.94); p = 0.016 | NR | NR | |
| Outcome definition | Need for injection of AED after 15 minutes | NR | NR | NR | NR | NR | NR | |
| i.v. LEV + CLZ (n = 68) | 19/67 (28.4) | NR | NR | NR | NR | NR | NR | |
| i.v. PBO + CLZ (n = 68) | 15/65 (23.1) | NR | NR | NR | NR | NR | NR | |
| Summary statistic | RR 1.23 (95% CI 0.68 to 2.21); p = 0.49 | NR | NR | NR | NR | NR | NR | |
| Shaner 198859 | Outcome definition | Need for anaesthesia | NR | NR | NR | NR | NR | NR |
| i.v. PHB + PHT (n = 18) | 0/18 (0) | NR | NR | NR | NR | NR | NR | |
| i.v. DIZ + PHT (n = 18) | 1/18 (5.6) | NR | NR | NR | NR | NR | NR | |
| Summary statistic | NR | NR | NR | NR | NR | NR | NR | |
| Silbergleit 201258 (RAMPART) (published data) | Outcome definition | Treatment failed: seizures terminated, rescue therapy given | Hospitalisation, n/N (%) | Length of ICU stay, days | NR | NR | NR | NR |
| i.m. MDZ (n = 448) | 22/448 (4.9) | 258/448 (57.6) | 5.7 (9.5) n = 123 | NR | NR | NR | NR | |
| i.v. LOR (n = 445) | 42/445 (9.4) | 292/445 (65.6) | 4.1 (4.7) n = 155 | NR | NR | NR | NR | |
| Summary statistic | NR | RR 0.88 (95% CI 0.79 to 0.98) | t-test p = 0.09 | NR | NR | NR | NR | |
| Outcome definition | Treatment failed: seizures not terminated, rescue therapy given | ICU admission, n/N (%) | ||||||
| i.m. MDZ (n = 448) | 47/448 (10.5) | 128/448 (28.6) | ||||||
| i.v. LOR (n = 445) | 57/445 (12.8) | 161/445 (36.2) | ||||||
| Summary statistic | NR | RR 0.79 (95% CI 0.65 to 0.95) |
Appendix 11 Economics literature search strategies
Database
EMBASE <1974 to 2020 Week 30>, Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions(R) <1946 to July 27, 2020>, APA’s PsycInfo <1987 to July Week 3 2020>
Search date: 29 July 2020.
Search strategy
1 Emergency Medical Services/use ppezv
2 emergency health service/or emergency care/use oemez
3 Emergency Services/use psyf
4 (accident adj2 emergency).tw.
5 (“emergency room” or “emergency department” or ED).tw.
6 (pre-hospital or prehospital or “out of hospital” or community).tw.
7 Allied Health Personnel/use ppezv,psyf
8 paramedical personnel/use oemez
9 (paramedic* or ambulance).tw.
10 or/1-9
11 Status Epilepticus/use ppezv,psyf
12 epileptic state/use oemez
13 Status Epilepticus.tw.
14 11 or 12 or 13
15 exp Benzodiazepines/use ppezv,psyf
16 exp benzodiazepine derivative/use oemez
17 (midazolam or diazepam or lorazepam).tw.
18 exp Anticonvulsants/use ppezv
19 exp anticonvulsive agent/use oemez
20 exp Anticonvulsive Drugs/use psyf
21 (levetiracetam or “sodium valproate” or phenytoin).tw.
22 or/15-21
23 exp “costs and cost analysis”/use ppezv,psyf
24 exp economic evaluation/use oemez
25 *economics/
26 health economics/use oemez
27 exp health care cost/use oemez
28 exp Health Care Economics/use psyf
29 exp Health Care Costs/use psyf
30 economics, hospital/use ppezv
31 exp economics,medical/use ppezv
32 economics,pharmaceutical/use ppezv
33 pharmacoeconomics/use oemez,psyf
34 exp models, economic/use ppezv
35 exp Treatment Effectiveness Evaluation/use psyf
36 exp decision theory/
37 monte carlo method/
38 markov chains/
39 exp technology assessment, biomedical/
40 (cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab.
41 economics model$.tw.
42 (economic$ or pharmacoeconomic$).tw.
43 (price or prices or pricing).tw.
44 budget$.tw.
45 (value adj1 money).tw.
46 (expenditure$ not energy).tw.
47 markov$.tw.
48 monte carlo.tw.
49 (decision$ adj2 (tree? or analy$ or model$)).tw.
50 ec.fs. use ppezv
51 pe.fs. use oemez
52 or/23-51 (2216202)
53 10 and 14 and 22 and 52
54 remove duplicates from 53
Due to the limited number of results, the search was extended to include any therapy administered out-of-hospital
55 10 and 14 and 52
The following sources of economics literature were searched, using the broad text word ‘epilepticus’:
CEA Registry (http://healtheconomicsdev.tuftsmedicalcenter.org/cear2/search/search.aspx; accessed 29 July 2020)
RePEc (EconPapers) (https://econpapers.repec.org/; accessed 29 July 2020)
ICER (https://icer.org/; accessed 29 July 2020)
ISPOR (www.ispor.org/heor-resources/presentations-database/search; accessed 29 July 2020)
NHS EED (www.crd.york.ac.uk/CRDWeb/; accessed 29 July 2020)
List of abbreviations
- AE
- adverse event
- AED
- antiepileptic drug
- APA
- American Psychological Association
- CI
- confidence interval
- CT
- computerised tomography
- ED
- emergency department
- EEG
- electroencephalography
- ESETT
- Established Status Epilepticus Treatment Trial
- FDA
- Food and Drug Administration
- GABA
- gamma-aminobutyric acid
- i.m.
- intramuscular
- i.v.
- intravenous
- ICU
- intensive care unit
- ISPOR
- International Society of Pharmacoeconomics and Outcomes Research
- MRI
- magnetic resonance imaging
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- PHTSE
- PreHospital Treatment of Status Epilepticus
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RAMPART
- Rapid Anticonvulsant Medication Prior to Arrival Trial
- RCT
- randomised controlled trial
- RePEc
- Research Papers in Economics
- SAE
- serious adverse event
- SD
- standard deviation
- SIGN
- Scottish Intercollegiate Guidelines Network
- STESS
- status epilepticus severity score
