Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 11/114/01. The contractual start date was in December 2013. The draft manuscript began editorial review in June 2023 and was accepted for publication in November 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Cooper et al. This work was produced by Cooper et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Cooper et al.
Chapter 1 Introduction
Symptomology of endometriosis
Endometriosis is characterised by endometrial cells (tissue that normally lines the cavity of the uterus) growing outside the uterus, commonly on the pelvic peritoneum, ovaries, fallopian tubes, bladder and bowel. Endometriotic deposits undergo cyclical proliferation in response to ovarian hormones (mainly oestrogen), resulting in internal bleeding and inflammation, followed by scarring and adhesion formation. It is characterised by painful symptoms such as dysmenorrhoea, dyspareunia, dyschezia and non-cyclical persistent pelvic pain. Pain can be cyclical or constant and can range from mild to debilitating in terms of severity. In common with other chronic pain syndromes, fatigue and depression are often also reported.
Endometriosis can also impact on fertility; women with endometriosis are more than twice as likely to be infertile compared with those without the condition. 1 Other comorbidities include bladder pain syndrome,2,3 irritable bowel syndrome4–6 and adenomyosis. 7
Burden of disease
Endometriosis affects up to 1 in 10 women of reproductive age, that is potentially 190 million women worldwide. 8 It has a serious impact on the quality of life in affected women,9–11 and poses a considerable socioeconomic burden. In a 2012 multinational study,12 the average annual total health cost per woman was estimated to be €9579 [95% confidence intervals (CIs) €8559 to €10,599]. Loss of productivity was costed at €6298 per woman, while a further €3113 was incurred by direct healthcare costs including surgery (29%), monitoring tests (19%) and hospitalisation (18%), and physician visits (16%). On average, women experiencing endometriosis-associated symptoms had a quality-adjusted life-year (QALY) of 0.809 per year, representing a 19% decrease in quality of life compared with a woman who is in the best possible health condition. 12
Diagnosis of endometriosis
A stepwise approach to diagnosis is recommended by international guidelines. 13,14 Women presenting with the characteristic symptoms of pelvic pain, dysmenorrhoea and dyspareunia undergo an abdominal and pelvic examination, which can raise suspicion of an ovarian mass or deep endometriosis. In the absence of any validated serum or urinary biomarkers of endometriosis, 15–17 imaging, either using ultrasound or magnetic resonance imaging is the next step. While the absence of visible pathology does not rule out endometriosis, particularly superficial peritoneal disease,18 a working clinical diagnosis of probable endometriosis can be made and medical treatment, using hormonal contraceptives and analgesics started at this point.
For a confirmatory diagnosis, and where medical treatment is unable to provide adequate pain relief, a diagnostic laparoscopy is recommended. Under general anaesthetic, the pelvic and abdominal cavity is systematically examined, with some surgeons taking biopsies of endometrial lesions for histopathological confirmation. Superficial and uncomplicated endometriosis can be treated during the laparoscopy, while deep endometriosis and lesions on the bladder and bowel require more extensive excision at a subsequent procedure at dedicated endometriosis treatment centres. Despite pelvic pain symptoms and clinical suspicion of endometriosis, approximately 40% of diagnostic laparoscopies fail to reveal any pathology. 19
Three subtypes of endometriosis are defined13 in Table 1. Of the several staging systems currently in use, the most commonly used is the revised American Society for Reproductive Medicine (ASRM) classification, which grades the condition into four stages ranging from stages I (minimal) to IV (severe) disease. 20 Staging is undertaken during surgical inspection of the pelvis but has poor correlation with reported pain. 21
| Term | Definition |
|---|---|
| Peritoneal/superficial endometriosis | Endometrium-like tissue lesions involving the peritoneal surface. The lesions can have different appearances and colour |
| Ovarian endometriotic cyst/endometrioma | Endometrium-like tissue in the form of ovarian cysts. They may be either invagination cysts or true cysts with the cyst wall also containing endometrium-like tissue and dark blood-stained fluid (endometrioma or ‘chocolate cysts’) |
| Deep endometriosis | Endometrium-like tissue lesions in the abdomen, extending on or under the peritoneal surface. They are usually nodular, able to invade adjacent structures, and are associated with fibrosis and disruption of normal anatomy |
Management of endometriosis
Clinical practice guidelines13,14 recommend a combination of analgesic and hormonal medical treatments for endometriosis-related pain and surgical removal of endometriosis lesions for persistent pain and infertility. Recommendations are often contingent on the subtype of endometriosis and are frequently based on low-grade evidence.
Surgical treatment of endometriosis
Laparoscopic excisional/ablative surgery has been shown to improve endometriosis-associated pain compared with diagnostic laparoscopy alone at 6 months [odds ratio (OR) 6.58, 95% CI 3.31 to 13.10], based on evidence from only three randomised controlled trials (RCTs) with a total of 171 participants22 Only one small trial has follow-up data to 12 months showing benefit of surgery, and there are no high-quality outcome data over the medium (1–5 years) or long term (> 5 years). Furthermore, there is only low-quality evidence for specific surgical approaches. Laparoscopic ablation is not associated with reduction in dysmenorrhoea at 12 months compared with laparoscopic excision [on 0–10 visual analogue scale (VAS) −0.03, 95% CI −1.27 to 1.22, 2 trials, 251 participants]. 23
Surgery is unable to guarantee complete relief from symptoms and its impact has been shown to wane over time24 with reoperation rates due to pain reported to be as high as 54–58% after 5–7 years since the index intervention. 25,26 More recent data suggest that the chance of repeat surgery could be as high as 62%. 27 This has prompted surgeons to seek effective post-surgical hormonal treatment to reduce the risk of recurrence. 24–28
Evidence for hormonal prevention of recurrence
A number of hormonal treatments have been used to reduce circulating oestrogen levels and shrink residual endometriotic deposits as well as discourage any new areas of growth. 29 Gonadotropin-releasing hormone analogues (GnRHa), which reduce gonadotrophin and oestrogen secretion, have been found to be more effective than no treatment,30 but menopausal symptoms and loss of bone mineral density limited their use beyond 6 months unless combined with add-back hormone replacement therapy (HRT).
Progestogens, including depot medroxyprogesterone acetate (DMPA) and the levonorgestrel-releasing intrauterine system (LNG-IUS), have been shown to reduce recurrence of symptoms after endometriosis surgery; they possess the advantage of less frequent administration. 31,32 DMPA administered as 3-monthly injections is less reliant on patient adherence than the oral pill and LNG-IUS can be effective for up to 5 years. The combined oral contraceptive pill (COCP) has been used for many years and has been shown to be effective in reducing pain following surgery. 33–35
Despite a number of systematic reviews based on relatively small trials (with short periods of follow-up,36,37 there is no consensus on the most effective means of preventing recurrence. The Cochrane review of postoperative LNG-IUS found limited evidence in support of its use and called for more well-designed randomised trials,29,38 as did other reviews of progestogens. 39,40
Evidence on the cost-effectiveness of hormonal prevention of recurrence
Although previous research has explored the cost-effectiveness of different treatment strategies for endometriosis, none has specifically evaluated the role of long-acting reversible contraceptive (LARC) and COCP in the prevention of post-surgical recurrence of the condition. 41,42
Background to the PRE-EMPT trial
Given the lack of evidence on managing endometriosis following surgical treatment, the National Institute for Health and Care Research Health Technology Assessment Programme (NIHR HTA) released a commissioned call in November 2011 for trials evaluating ‘the clinical and cost-effectiveness of LARCs in preventing recurrence of endometriosis?’ The applicants were asked to justify the type of LARC they thought most appropriate, and which ‘usual treatment’ should be used as the control comparator. In the next chapter, we detail the rationale for how we made these decisions and provide further detail on how we designed the trial to answer the questions posed.
Chapter 2 Designing the PRE-EMPT trial informed by an internal pilot study incorporating a flexible entry design
This chapter is an abbreviated version of articles published elsewhere,43,44 detailing the methods and results of the internal pilot phase, which ultimately informed the design of the substantive phase of the trial.
Methods
Survey of practice
With the evidence base unable to guide the specifics of study design for a trial, we turned to a survey of national practice in December 2011. Members of the British Society for Gynaecological Endoscopy were sent an online questionnaire asking:
-
whether they prescribed postoperative hormonal treatments
-
their most commonly used hormonal treatment and
-
the most relevant comparison for any future trial.
Some 62 members responded, with 56 of them having experience of treating endometriosis. Of these members, 45 (80%) indicated that they prescribed hormonal treatments and 11 (20%) did not. GnRH analogues, LNG-IUS, COCP and DMPA were the most commonly used treatments but with none obviously preferred over the others (39, 38, 37 and 25 responses, respectively). Three comparisons of interest ranked higher than the others (40 responses): LNG-IUS versus no treatment (18, 45%); LNG-IUS versus COCP (17, 43%); and LNG-IUS versus DMPA (12, 30%), but again with no particular favourite. 45
Trial design considerations
With no clear LARC or comparator favoured, we needed to consider how two viable LARCs, the LNG-IUS and DMPA, and two non-LARC options, COCP and no treatment could be accommodated into a substantive trial. A four-arm trial was the obvious choice, but concerns were raised about whether patient views would prohibit recruitment to a design requiring consent to four different interventions – it was likely a large proportion of this population would have tried one or more of these treatments before as they are recommended by the National Institute for Health and Care Excellence (NICE) for the initial (presurgical) management of endometriosis. 46 Pragmatic designs,47 where the patient or clinician could select their choice of LARC or non-LARC, were considered, but concerns were raised about ultimately unsatisfying and underpowered comparisons. The non-LARC group was particularly problematic, being a mixture of active (COCP) and non-active (no treatment) options. Given these difficult design issues, we decided to use feasibility of recruitment to a particular randomisation scheme to guide the type of design which should be taken forward.
Internal pilot design
Our proposal was to include a flexible-entry design approach where participants could be randomised to two, three or four treatments provided one was a LARC and the other was a non-LARC. This meant that a patient could enter any one of nine randomisation schemes, shown in Figure 1. On completion of the pilot phase, a decision about the substantive study design based on feasibility of recruitment would be made. As no outcome assessment was proposed, inflation of type I error was not a concern. 48 The options for a definitive trial included continuing with a four-way randomisation design if acceptable to women, or alternatively to drop one or two treatment groups if randomisation proved difficult. The design would be fixed, together with an appropriate sample size target to ensure that we would have enough power to detect a minimally important difference. 49 Data collected from participants randomised in the pilot phase to designs that remain in the main phase would be taken forward and combined with subsequent data collected; however, all women would be followed up to study conclusion regardless of the randomisation options selected.
FIGURE 1.
Design of the internal pilot.

To ensure an appropriate design choice was made, a post-pilot phase report was scheduled to be prepared for a joint Trial Steering Committee (TSC) and Data Monitoring Committee (DMC) meeting to review at the end of the pilot phase. The pilot phase was intended to last for 1 year with a recruitment target of 100 participants. The TSC and DMC would have the final say on any proposed changes, which would also be communicated to the funding body for approval. The post-pilot report was to include data on which randomisation schemes had been selected by patients and the results of a qualitative assessment (see Qualitative assessment). A threshold of 10% of eligible participants was set for each of the four arms as a criterion for inclusion on the definitive trial. Any updated review of external evidence published since the grant application was submitted would also be taken into consideration. Apart from informing the design changes, the pilot phase also aimed to fine tune operational procedures, assess data capture forms and confirm initial assumptions around sample size.
Internal pilot general trial methods
The internal pilot phase followed the same methods detailed in Chapter 3 in terms of inclusion and exclusion criteria, recruitment, randomisation methods and intervention-specific procedures.
Qualitative assessment
A qualitative approach to the study was considered appropriate, as the aim of the study was to gain insight into women’s experiences and motivations and also to explore issues of importance to them rather than to adhere strictly to a script. Narratives are an important way for people to explain disruptive events in their lives, and the use of narrative interviews and a focus group in this study allowed women to reflect on living with endometriosis and to raise the issues that had greatest impact on their lives. 50 Three sites, Aberdeen Royal Infirmary, Birmingham Women’s Hospital and Edinburgh Royal Infirmary, all in the UK, took part, which comprised a focus group discussion and individual interviews (Appendix 1, Table 22). The focus group and semistructured interview topic guides were informed by available literature on women’s experiences of medical treatments for endometriosis symptoms, as well as the expertise of the PRE-EMPT Trial Management Group (TMG). As a means of establishing rapport, at the beginning of interviews and the focus group, some demographic data were collected. Favourable ethical approval for this study was obtained from the North of Scotland Research Ethics Committee and site-specific permission from the NHS trusts of each of the hospitals involved.
One focus group and 10 individual semistructured interviews were conducted to elicit women’s past experiences with the proposed treatments and to assess whether they constituted a barrier to participation. The focus group discussion took place in one of the centres and included four women. Three women were interviewed in their homes and seven were interviewed over the telephone. The focus group and interviews were recorded and transcribed verbatim. Content analysis51 was employed, with a qualitative lead and two assistants independently reading the transcripts and agreeing upon common themes. Dissident views were also considered. The Standards for Reporting Qualitative Research (SRQR) were adopted. 52 Women participating in both the focus group and individual interviews shared their views and experiences of medical treatments and their motivation for enrolling in the PRE-EMPT trial.
Results
Recruitment to the pilot phase
Six centres in the UK were involved with staggered starts from April 2014 to the end of March 2015 (recruiting on average for 10.5 months). During this period, 504 patients were assessed for eligibility and 77 were recruited. The most common reasons for ineligibility included a plan to conceive in the near future (42 patients, 10%); contraindications to one or more treatments (35 patients, 8%) and no endometriosis identified at diagnostic laparoscopy (33 patients, 8%). The main reason for not wishing to take part was a preference for a particular treatment [94 patients, (22%); the most common being LNG-IUS (30) and DMPA (30), with COCP less favoured (6)]. Details of randomised participants are given in Table 2.
| N = 77 | ||
|---|---|---|
| Age, years | Mean (SD) | 31 (7.5) |
| Age < 35, n (%) | Yes | 53 (69) |
| Missing | 0 | |
| BMI, kg/m2 | Mean (SD) | 27 (5.7) |
| Missing | 17 | |
| Ethnic group, n (%) | White British | 64 (86) |
| Black/Black British Caribbean | 2 (3) | |
| Asian/Asian British Indian | 3 (4) | |
| Asian/Asian British Pakistani | 1 (1) | |
| Mixed white/Black Caribbean | 2 (3) | |
| Mixed white/Asian | 1 (1) | |
| Other mixed background | 1 (1) | |
| Missing | 3 | |
| Stage of endometriosis, n (%) | I | 36 (47) |
| II | 20 (26) | |
| III | 11 (14) | |
| IV | 10 (13) | |
| Missing | 0 | |
| Ever smoked, n (%) | Yes | 34 (48) |
| Missing | 6 | |
| Extent of excision as judged by surgeon, n (%) | Complete | 71 (92) |
| Missing | 0 | |
| EHP-30 pain score | Mean (SD) | 58 (18.5) |
| Missing | 2 |
Randomisation options chosen in the pilot phase
Only 5 of the 77 participants (6%) were willing to be randomised to all four treatment options (Figure 2 and Table 3). Participants willing to be randomised to both LNG-IUS and DMPA were relatively low, with the vast majority (82%, 63/77) expressing a preference for one or the other in roughly even proportions (43% for LNG-IUS and 57% for DMPA). In a similar fashion, most (71%, 55/77) expressed a preference for their choice of comparator in even proportions (51% for COCP and 49% for no treatment). Forty-six of the participants (60%) expressed a preference for both a LARC and their comparator and hence opted for variations of two-way randomisations.
FIGURE 2.
Randomisation options chosen in the internal pilot phase – summary.

| Randomisation option chosen | Frequency, n (%) | Allocation | |||
|---|---|---|---|---|---|
| LNG-IUS (n) | DMPA (n) | COCP (n) | None (n) | ||
| All four treatments | 5 (6) | 1 | 1 | 2 | 1 |
| Three-way including both LARCs | |||||
| LNG-IUS vs. DMPA vs. none | 6 (8) | 1 | 3 | – | 2 |
| LNG-IUS vs. DMPA vs. COCP | 3 (4) | 1 | 1 | 1 | – |
| Three-way including both non-LARCs | |||||
| DMPA vs. COCP vs. none | 12 (16) | – | 4 | 4 | 4 |
| LNG-IUS vs. COCP vs. none | 5 (6) | 2 | – | 1 | 2 |
| Two-way | |||||
| DMPA vs. COCP | 14 (18) | – | 6 | 8 | – |
| LNG-IUS vs. COCP | 11 (14) | 5 | – | 6 | – |
| LNG-IUS vs. none | 11 (14) | 6 | – | – | 5 |
| DMPA vs. none | 10 (13) | – | 5 | – | 5 |
| Total | 77 | 16 | 20 | 22 | 19 |
Findings of the qualitative study
Women who agreed to participate represented a range of symptomology, treatment histories and allocated trial treatment groups (Appendix 1, Table 23). As no novel treatment was on offer, many women had previous experience of treatments available as part of the trial (either as endometriosis treatment or for contraceptive purposes), which strongly influenced their acceptability.
Women found flexible randomisation acceptable as they had an element of choice over which treatment groups to which to be randomised. Half of participants (n = 7) reported that without the option to opt out of a particular treatment group (or groups) they would have declined trial participation. No single treatment group was found more or less acceptable to women. Women made decisions regarding which treatment arms were acceptable based on their past negative or positive treatment experiences and the treatment experiences of significant others (female friends and family). Women chose to participate in the trial for reasons of altruism and self-interest and found the 3-year length of their participation acceptable.
New external evidence
In the period between the initial study proposal and completion of the pilot, two systematic reviews examining the use of COCP in this population were published. 53,54 The first identified 15 randomised trials including 850 patients. The combined odds of recurrence were noted to be lower in the COCP group compared with surgery alone (OR 0.31, 95% CI 0.22 to 0.45; p < 0.001). The second evaluated the use of prolonged (at least 2 years) postoperative COCP and endometrioma recurrence in a total of 965 women (726 in cohort studies and 239 in one RCT). Recurrence was lower with COCP compared with no treatment (OR 0.12, 95% CI 0.05 to 0.29; p < 0.001). The data from these two systematic reviews showed that COCP was beneficial in preventing recurrence of endometriosis and was instrumental in changing equipoise among clinicians as well as challenging the ethical justification for including a no-treatment arm in the definitive trial.
Revised trial design
Proposal
Four-way and three-way randomisation designs were ruled out due to low numbers selecting these randomisation options. We also ruled out a trial design involving solely the most commonly selected two-way randomisation option (DMPA vs. COCP) as this attracted only 14 participants (18% of all participants). Given the strong preferences noted (including in the results of the qualitative work), we decided to incorporate some elements of choice in the revised design (Figure 3). The main comparison proposed was LARC, considered as a class of treatments, versus COCP, with LARC selected before randomisation by the patient if a preference was apparent (or alternatively allocated randomly if there was no opinion). The choice of LARC would need to be decided prior to randomisation to enable unbiased stratified (subgroup) analyses of LNG-IUS versus COCP and DMPA versus COCP (e.g. only those selecting LNG-IUS pre randomisation would be included in the LNG-IUS vs. COCP comparison). COCP was chosen as the comparator over no treatment on the basis of the new external evidence. Participants randomised to combinations not taken forward through to the main phase (e.g. DMPA vs. no treatment) would still be followed up as per the main LARC versus COCP comparison.
FIGURE 3.
Revised trial design for the substantive phase.

Sample size considerations
The sample size in the definitive trial was revised to reflect a main two-arm comparison and took into consideration a revised estimate of the standard deviation (SD) of the primary outcome (from pooled baseline data). To detect an 8-point difference on the Endometriosis Health Profile-30 (EHP-30) pain domain with 90% power (p = 0.05) and assuming the SD is 22 points requires 160 participants per group, 320 in total (to account for any loss to follow-up – estimated 20% – this target was inflated to 400). Eight points is equivalent to 0.36 SD, which can be considered half-way between a small (0.2 SD) to moderate (0.5 SD) effect size. 55 This size of sample would have also given us good power (80%) to detect a 10-point difference in the two stratified analyses of LNG-IUS versus COCP and DMPA versus COCP provided the remaining recruits into the study have a roughly even split.
Health economic considerations
In terms of the health economic evaluation, we conducted a pretrial model-based economic evaluation (6 months at the beginning of the trial coinciding with the internal pilot). A decision analytic model based on the alternative treatment pathways outlined in the trial design was constructed and populated from a pragmatic review of the available evidence on resource use, associated costs, effectiveness of interventions and the health-related quality of life (HRQoL) for the resulting health states. This collated evidence was used to estimate a baseline decision model, which allowed identification of important elements of resource use, costs and issues and gaps relating to either the interventions or quality of life and the level of detail to be obtained from primary trial data.
Conclusions
In this internal pilot phase incorporating a flexible randomisation scheme, we found that few participants were willing to be randomised to all four treatment options on offer; indeed, most were only willing to be randomised to two treatments that did not include both LARCs. Qualitative assessment found that women favoured some element of control over which groups they were to be randomised to. No single treatment was preferred, and patient decisions were based on previous experiences with these treatments. Meanwhile, emerging evidence suggested that COCP was more effective for prevention of recurrence of pain following surgery for endometriosis than no treatment in this population. Given these findings, we revised our study to include a main comparison of LARC versus COCP, with LARC preselected ahead of randomisation to also enable stratified analysis of DMPA versus COCP and LNG-IUS versus COCP. This revised trial design was ratified by the external DMC and TSC in April 2015 and subsequently approved by the NIHR HTA.
The advantage of our approach was that it allowed the trial team to engage with and listen to patients faced with real-life decisions regarding randomisation (as opposed to data from a survey of potential participants). An easier choice would have been to plan a two-group trial, which could have limited the number of questions that could be potentially answered or, alternatively, to embark on a four-arm trial that was incapable of recruiting. The assessment of the randomisation data was preplanned and was overseen and approved by an external, independent committee including expert clinical and statistical advisors. We believed our revised design to be feasible while providing us with the opportunity to include three of the four options initially identified and at the same to incorporate some element of patient choice, which was very apparent.
We underestimated the potential for variation in the randomisation options chosen and were wrong in predicting that some would show obviously poorer recruitment than others, allowing some randomisation options to be confidently dropped, leading to a ‘neat’ substantive trial design. Reality was rather more complicated and gave us results of preference that were less straightforward. The independent TSC and DMC were important in this respect to make sure that we retained a study capable of changing practice rather than one that was just ‘easy to answer’. The decision to combine two different interventions (LNG-IUS and DMPA) into one LARC drug class for the main trial was a difficult one as, although similar pharmacologically (both deliver a progestogen), they have very different routes of administration. However, this was considered by the committee members to be a pragmatic response to the pilot evidence outlined above.
Finalised objectives of the PRE-EMPT trial
In light of these pilot phase findings, the objectives for the main phase trial were as follows:
Primary objective
To compare, in women undergoing conservative surgery for pain due to endometriosis, the effectiveness of LARCs compared with COCP in preventing the recurrence of endometriosis-related pain and improving quality of life.
Secondary clinical objectives
-
To compare LARCs versus COCP as per the primary objective in those that selected LNG-IUS as their method of delivery.
-
To compare LARCs versus COCP as per the primary objective in those that selected DMPA as their method of delivery.
-
To compare LARCs versus COCP in terms of pain relief, serious adverse effects and repeat surgery.
Economic objectives
To compare the relative cost-effectiveness of alternative hormonal interventions DMPA and LNG-IUS for the prevention of recurrent endometriosis. The main comparator will be COCP. The evaluation will have two principal components:
-
To collate the cost and effectiveness evidence available from existing research, systematic reviews and routine health administrative sources to provide data for a pretrial decision analysis model based on the design of the proposed trial.
-
To use prospectively collected resource use data associated with the alternative treatment pathways, outcomes in terms of quality of life [EuroQol-5 Dimensions, five-level version (EQ-5D-5L) and ICEpop CAPability measure for Adults (ICECAP-A)] and reported symptoms such as pain, and cost data collected alongside the trial where necessary, to evaluate the cost and cost-effectiveness of the alternative strategies in a model-based economic evaluation based on the trial.
Chapter 3 Methods for the randomised controlled trial
This chapter reports the methods used to conduct the PRE-EMPT trial.
Design
The PRE-EMPT trial was a randomised, open, pragmatic multicentre trial comparing the effectiveness of LARCs (LNG-IUS or DMPA) with the COCP in preventing recurrence of pain due to endometriosis. The trial initially received clinical trial authorisation (CTA 21583/0219/001-0001) from the Medicines and Healthcare products Regulatory Authority (MHRA) and ethical approval from the North of Scotland Research Ethics Committee (13/NS/0103) in August 2013, before transferring to the East of Scotland Ethics Committee (14/ES1004) in May 2014 for approval of the adapted substantive phase of the trial [Integrated Research Application System (IRAS) ID 101577].
Trial oversight
Study oversight and monitoring were provided by a TSC, chaired by Professor Mary Ann Lumsden (University of Glasgow) and a DMC, chaired by Professor Lucy Chappell (King’s College, London).
The TSC provided independent supervision for the trial, providing advice to the chief and co-investigators and the sponsor on all aspects of the trial throughout the trial. The DMC adopted the DAMOCLES (DAta MOnitoring Committees: Lessons, Ethics, Statistics) charter to define its terms of reference and operation in relation to oversight of the PRE-EMPT trial. 56 Both committees met annually during the period of recruitment and follow-up.
Eligibility and recruitment
Women with symptoms suggestive of endometriosis and referred to general or specialist gynaecological clinics for clinical assessment (sometimes involving an ultrasound or magnetic resonance imaging scan) were considered for participation. The target population was all women of reproductive age, where long-term medical treatment following ablation and or excision of endometriosis might be reasonably considered. Symptoms of endometriosis tend to resolve spontaneously as menopause approaches, hence the upper age of recruitment was set at 45 years to account for the 3-year follow-up, given average age of menopause being 51 years in the UK.
There was no restriction in terms of disease severity or staging: all women who had undergone conservative surgery, where the aim was to excise or ablate areas of endometriosis and dissect pelvic adhesions, were considered eligible. Women who were due to have radical surgical treatment, such as hysterectomy and or removal of both ovaries, were not approached for participation, while those for whom further surgery was considered necessary after the laparoscopy, for example, bowel resection, were excluded intraoperatively.
Specific eligibility criteria were as follows:
Inclusion criteria:
-
Women aged 16–45 years.
-
No immediate plans to conceive.
-
Scheduled for laparoscopic conservative surgery, or diagnostic laparoscopy with concurrent surgery if endometriosis is found, for pelvic pain associated with endometriosis.
-
Willing to be randomised to one long-acting progestogen (LNG-IUS or DMPA) and COCP.
The following women were also eligible for PRE-EMPT if they had recurrent pain and were to have conservative surgery for endometriosis:
-
Had one or more previous diagnostic laparoscopies.
-
Had previous laparoscopic conservative surgery for endometriosis, provided that this surgery did not involve rectovaginal dissection or bowel resection.
-
Used postoperative medical treatment, including the treatment options included in PRE-EMPT.
-
Previous use of treatment options included in PRE-EMPT as contraceptives.
-
Use of preoperative GnRHa, provided that this was stopped at least 4 weeks prior to laparoscopy.
Exclusion criteria:
-
No endometriosis identified at diagnostic laparoscopy.
-
Infertility.
-
Any plans for further elective endometriosis surgery (for deep disease or endometrioma).
-
Contraindications to the use of hormonal treatment with oestrogen or progestogens.
-
Suspicion of malignancy.
Recruitment was supported by dedicated research nurses, who worked with local principal investigators. In some units, a diagnostic laparoscopy was performed to establish the presence and extent of endometriosis before definitive surgery. Other gynaecologists used a ‘see and treat’ approach to laparoscopically diagnosed endometriosis. All potentially eligible women scheduled for laparoscopy were approached preoperatively with information regarding the PRE-EMPT trial and asked to consent for the trial. If participants expressed an interest, written informed consent was sought and eligibility was confirmed. All participants were told that participation in the trial was completely voluntary and that they could withdraw at any stage in the trial without any impact on their normal clinical care.
Randomisation
Randomisation took place in two stages to enable rapid intraoperative completion of the process where investigators intended to fit the LNG-IUS, if allocated, at the end of the conservative surgery. Randomisation notepads were provided to investigators and were used to collate the necessary demographic and historical information prior to randomisation.
Once preoperative eligibility criteria were confirmed and consent for the trial was obtained, the participant could be pre-registered for PRE-EMPT. Randomisation then occurred either intraoperatively or immediately postoperatively, according to the randomisation options and the intention of the investigator. A central internet randomisation service was provided by the Birmingham Clinical Trials Unit (BCTU). A central telephone back-up service was available.
A ‘minimisation’ procedure using a computer-based algorithm was used to avoid chance imbalances in important stratification variables. No allocation could be given until all participant entry criteria were confirmed by the local study team. The variables chosen were:
-
stage of endometriosis (using the ASRM classification): I (minimal), II (mild) versus III (moderate)/IV (severe)
-
extent of excision/ablation of endometriosis: complete versus incomplete, as judged by the surgeon at the time of conservative surgery
-
age in years: < 35 years versus ≥ 35 years
-
selection of LNG-IUS or DMPA if randomised to LARC
-
whether selection of LARC was due to patient preference or not
-
centre, to balance for experience of the gynaecologist.
If the participant had no preference for a particular LARC, the LARC needed to be randomly allocated prior to LARC versus COCP randomisation. This was completed using a random blocked list (variable length) incorporated into the computer-based algorithm.
Treatment allocations
The three hormonal treatments used in the trial are all licensed and commonly used as contraceptives and have a long and well-established adverse-effect profile57 While widely used for the prevention of recurrence of endometriosis, they are not specifically licensed for this purpose.
Depot medroxyprogesterone acetate injection
Depot medroxyprogesterone acetate injection (only available as Depo-Provera®, Pfizer, Walton Oaks, Surrey) is a long-acting reversible progestogen contraceptive that is administered at a dose of 150 mg in an aqueous suspension by intramuscular injection every 3 months.
Levonorgestrel-releasing intrauterine system
The LNG-IUS is a contraceptive system that slowly releases a daily dose of 20 μg levonorgestrel into the uterine cavity. Bayer Pharma AG market their LNG-IUS under the name of Mirena® and Gedeon Richter plc under the name of Levosert®: either was permitted. They are long-acting reversible preparations that require removal and reinsertion every 5 years for Mirena and 3 years for Levosert.
Comparator – combined oral contraceptive pill
Participants allocated the COCP were prescribed a formulation containing 30 μg ethinylestradiol and 150 μg levonorgestrel. For the management of endometriosis-related pain, it is unclear whether combined oral contraceptives should be taken conventionally, continuously or in tricycle regimen, so their intended regimen was according to the clinician or participant’s decision and recorded at randomisation.
Initiation and repeat prescription for trial treatment
The intention was to initiate the allocated treatment as soon as possible, ideally before discharge, to minimise non-compliance and for the convenience of the participant. The fitting of the LNG-IUS was ideally done by the treating gynaecologist during the conservative surgery, or before discharge. Similarly, the first DMPA injection was ideally given before discharge after surgery. If this was not possible, the participant was provided with a prescription and asked to attend their general practitioner (GP) practice or a sexual health clinic for fitting or injection, ideally within a month. Repeat prescription of the COCP and repeat DMPA injections were undertaken by the participants’ GP or sexual health clinic.
Blinding
The use of participant-reported outcome measures can cause biased responses, manifesting as over- or underestimation of any treatment effect. The solution is usually to mask the intervention and control, often by using placebo or dummy interventions, and we were challenged to consider the issue of masking very carefully by the funder. Following discussions with clinicians and patients, the conclusion was that masking would not be feasible, ethical or acceptable. Women at a support group meeting acknowledged the potential for a placebo effect, but thought that it came from receiving a definitive diagnosis, acknowledgment of the condition and having had the opportunity to talk about their symptoms rather than the knowledge of the treatment they were taking. They described their perceptive of the placebo effect more in terms of overall quality of life rather than specifically pain, which they thought was reported honestly. The interventions in this trial differ considerably with regards to routes of delivery, that is oral, intramuscular injection or an intrauterine system. To fully blind the study, a triple dummy design would be needed, which would be complex and place an unnecessary burden on participants. The idea of a placebo injection or coil was considered completely unacceptable to support group members and felt to be a serious barrier to recruitment. For these reasons, all possible treatment options in the trial were unblinded.
Adherence to treatment
If a woman decided, after randomisation, that she no longer wished to remain in the allocated treatment group, she was free to change, in consultation with her GP or gynaecologist. Change of treatment could be to another trial treatment or another non-trial hormonal treatment. Participants were similarly free to stop treatment, to conceive, to plan further endometriosis surgery or for any other reason. Adherence was primarily patient reported. In those allocated LNG-IUS, participants were considered compliant provided they had confirmed that the device was fitted and later reported that they were still taking this treatment on the follow-up questionnaires. For those allocated DMPA or COCP, participants were considered adherent provided that they had confirmed starting treatment and later confirmed they were still taking their treatment course. Where this was not the case, the reason for treatment change or cessation was captured where possible and categorised as either due to lack of perceived effect, due to adverse effects, to conceive, due to surgery or for other reasons.
Withdrawal from trial
Participants could voluntarily withdraw their consent to participation in PRE-EMPT at any time. Reasons for withdrawal were documented where possible. Participants who explicitly withdrew consent to have any further data collected, had their decision respected and noted on the electronic data capture system and in the patient’s medical notes. No further data were collected for that participant.
Outcomes and assessments
Timing of assessments
Women who agreed to enter the study completed a baseline participant booklet before randomisation, consisting of disease specific and generic quality of life questionnaires, pain scores and resource use questions (see Primary outcome measures and Secondary outcome measures sections for full details of the outcome measures used). Participants were then followed up for a period of 3 years with a similar questionnaire booklet, initially solicited by post, with consent given for additional methods of contact; telephone and e-mail. Over this period, the booklets were also sought at 6 months, then 1 and 2 years post randomisation. Women who did not return the questionnaire after two postal reminders, at 2- and 3-year follow-up, were contacted by a member of the clinical team, with the aim of completing a shorter questionnaire (containing the most pertinent information: primary outcome, generic quality of life required for the economic evaluation, treatment changes, relevant surgical interventions and pregnancy status) over the telephone. This was in response to lower-than-anticipated postal returns. Questionnaires were considered to have been completed on time if they were completed within 6 months for assessments prior to 3 years or by 4 years post randomisation for the final 3-year assessment.
Information on the results of the surgical procedure was taken from the surgical notes prior to discharge from hospital; pregnancies and repeat procedures were monitored throughout the period of follow-up by the clinical team at the trial centre. If there was a failure to obtain patient-reported returns pertaining to further hospital-based treatment (outpatient visit, pregnancy or further surgery, this was obtained directly from hospital records from the principal investigator of the participant’s recruiting centre.
The end of trial was determined to be when the final participant recruited completed their 3-year assessment. Owing to the long-term nature of some of the interventions, participants may remain on treatment beyond the end of the trial and will be cared for by the GP as they would outside of the trial.
Primary outcome measures
The primary outcome was the recurrence of symptoms as evaluated by the pain domain of the EHP-30 questionnaire at 3 years post randomisation.
The EHP-30 is a disease-specific questionnaire to measure the health status of women with endometriosis. It demonstrates good reliability, validity, acceptability and responsiveness,58 with low floor and ceiling effects for the core questions. 59 There are 30 core items with five scales and six modular parts of 23 questions, which are dependent on the woman’s circumstances (e.g. impact on work, sexual activity and fertility). The pain domain has possible scores: 0 (best outcome) – 100 pain score (worst score).
Traditionally, recurrence of endometriosis was diagnosed objectively by laparoscopy. Here, we have recognised the need to avoid unnecessary invasive surgery while acknowledging the impact of symptoms of pain on women’s lives. Objective evidence to determine prevention of recurrence was deemed unethical, as it would expose the participants to the risks of a repeat surgical procedure and general anaesthetic, when the most important outcome was maintained reduction of pain and improvement in quality of life.
Secondary outcome measures
Secondary clinical outcome measures were as follows:
-
The pain domain of the EHP-30 at the other assessment points.
-
The remaining four core domains of the EHP-30 questionnaire:
-
Control and powerlessness (0 = best outcome, 100 = worst outcome).
-
Emotional well-being (0 = best outcome, 100 = worst outcome).
-
Social support (0 = best outcome, 100 = worst outcome).
-
Self-image (0 = best outcome, 100 = worst outcome).
-
-
The six modular domains of the EHP-30 questionnaire:
-
Work (0 = best outcome, 100 = worst outcome).
-
Relationship with family (0 = best outcome, 100 = worst outcome).
-
Sexual relationship (0 = best outcome, 100 = worst outcome).
-
Feelings about medical profession (0 = best outcome, 100 = worst outcome).
-
Feelings about treatment (0 = best outcome, 100 = worst outcome).
-
Feelings about infertility (0 = best outcome, 100 = worst outcome).
-
-
Pelvic pain measured by VAS [three scales: pelvic pain during periods (0 = best outcome, 10 = worst outcome), pelvic pain during intercourse (0 = best outcome, 10 = worst outcome), pelvic pain at any times (0 = best outcome, 10 = worst outcome)].
-
Responses to the question ‘compared to 1 month ago, would you say your pelvic pain has “Got much better”, “Got a little better”, “Not changed much”, “Got worse”’.
-
Fatigue, as measured by Fatigue Severity Scale (FSS)60 score (7 = best outcome, 63 = worst outcome), which is the sum of the responses of the nine statements contained in the questionnaire.
-
Menstrual regularity. Patients will be asked whether they are still having periods and, if so, to rate how regular their cycle is in one of the four categories; ‘Regular’, ‘Fairly regular’, ‘Irregular’ and ‘I have bleeding on and off all the time’.
-
Generic quality of life (EQ-5D-5L):61,62
-
EQ-5D index score (patient completed; −0.59 = worst outcome, 1.0 = best outcome).
-
EQ-5D health thermometer (patient completed; 0 = worst outcome, 100 = best outcome).
-
-
Capabilities, as a measure of wellbeing (ICECAP-A: patient completed; 0 = worst outcome, 1.0 = best outcome). 61,63 A score will be calculated from the five ICECAP attributes (attachment, stability, achievement, enjoyment, autonomy).
-
Further therapeutic surgery or second-line treatment for endometriosis as a proxy for recurrence or ‘treatment failure’, defined as having undergone hysterectomy, ‘surgery for endometriosis’, laparoscopy or taking GnRHa treatment.
-
Discontinuation rates of randomised treatment (time to first treatment change – see Analysis section for further details), with reasons for change.
-
Serious adverse events (SAEs).
Adverse events and serious adverse events
Adverse event reporting was conducted primarily by the participant. Reports were captured in the routine questionnaires received during follow-up. Participants were instructed to contact the clinical research team (once randomised) if they had an event that required hospitalisation or an event that resulted in persistent or significant disability or incapacity. On receipt of the follow-up questionnaires, the trial team reviewed the completed data and raised any potential adverse events to the local site teams who then investigated the need to expedite reporting of SAEs.
All SAEs were recorded on a SAE form and e-mailed or faxed to BCTU within 24 hours of the research staff becoming aware of the event. The local principal investigator (or other nominated clinician) had to assign seriousness, severity, causality and expectedness (if deemed related) to the SAE before reporting. SAEs categorised by the local investigator as both suspected to be related to the trial drug and unexpected were classified as suspected unexpected serious adverse reactions (SUSARs) and were subject to expedited reporting to the sponsor, the Medicines and Healthcare Products Regulatory Authority and the Research Ethics Committee.
Statistical considerations
Sample size
The rationale for the revised sample of 400 is provided in Chapter 2; in brief, we planned to detect an 8-point difference on the EHP-30 pain domain in our main comparison (LARC vs. COCP) with 90% power (p = 0.05).
Statistical analysis
A comprehensive statistical analysis plan was reviewed by independent DMC and TSCs prior to any analysis. Full details of the statistical analysis can be found in the statistical analysis plan, which can be requested from bctudatashare@adf.bham.ac.uk.
Categorical baseline data were summarised with frequencies and percentages. Normally distributed continuous variables were summarised with means with SDs. Participants were analysed in the treatment group to which they were randomised (intention to treat), irrespective of adherence with the treatment protocol. All participants recruited under versions 2.0–7.0 of the protocol (from 23 October 2015) were included in the final analysis population, as these participants were randomised directly to ‘LARC’ or ‘COCP’ (with pre-randomisation choice of LNG-IUS or DMPA if allocated LARC). For those randomised under v1.0 of the protocol (pilot phase, participants were included in the final analysis population provided they were randomised to combinations of treatments that only included LARCs and COCP (see Chapter 2). Participants randomised to combinations that did not involve COCP (LNG-IUS vs. no treatment; DMPA vs. no treatment; LNG-IUS vs. DMPA vs. no treatment) were not included in the final analysis population (a summary of the primary outcome results comparing LARC vs. no treatment is provided in Chapter 4).
For the primary outcome (EHP-30 pain scores at 3 years), a mixed-effects linear regression model for repeated measures64 was used to calculate an adjusted difference between group means, along with 95% CIs. Parameters for participant, treatment group, time and time by treatment interaction were included as well as baseline response (as a continuous variable) and the minimisation variables (see Randomisation). Time was assumed to be a categorical variable and to allow for a varying treatment effect over time, a time-by-treatment interaction parameter was also included. Centre was included as a random intercept in the model, and all other factors as fixed effects. A compound symmetry covariance structure was assumed. An F-test was used to test statistical significance (p-value produced) of the estimated treatment group parameter generated from the maximum likelihood estimate.
Secondary outcomes measured on a continuous scale (remaining four core domains and the six modular domains of the EHP-30, VAS, FSS, EQ-5D index score, EQ-5D Health thermometer and ICECAP-A) were analysed in a similar manner as the primary outcome. Cycle regularity was analysed using a generalised estimating equation model with logit link that took into account all assessment times (correlated longitudinal data) and adjusting for the minimisation parameters. An independent covariance structure was assumed and ORs with 95% CIs for the treatment group parameter were produced. Change in pelvic pain was also analysed using a similar generalised estimating equation model, this time using cumulative logit link for ordered categorical data. Responses to ‘Are you still having periods?’ were tabulated. Time to further therapeutic surgery or second-line treatment for endometriosis (defined as having undergone hysterectomy, ‘surgery for endometriosis’, laparoscopy or taking GnRHa treatment) was analysed using a Cox regression model. Adjusted hazard ratios (and 95% CIs) were generated and a Kaplan–Meier plot produced. Centre was regarded as a fixed effect in the model. Discontinuation rates of randomised treatment (time to first treatment change) were summarised by group and presented using Kaplan–Meier plots but not formally analysed; reasons for treatment changes were tabulated. The number and percentage of participants experiencing any SAEs and were presented by intervention group. Statistical significance was determined by a chi-squared test. All estimates of differences between groups were presented with two-sided, 95% CIs.
Sensitivity analysis was performed on the primary outcome to investigate the assumption that missing data were missing at random. This incorporated a delta-based approach, which assumes missing data is missing not at random. 65 Missing data were imputed using multiple imputation with chained equations; 50 imputations were generated and all variables that were included in the analysis of this outcome were included to ensure compatibility of approach. The average increase of observed data at each time was calculated; a value ‘delta’, which is equivalent to a proportion of this average increase, was then subtracted from the imputed value in all of the imputed sets (i.e. missing data responses were assumed to be worse than those returned). The delta values were taken in turn as the following in separate investigations: 20% of average increase in both groups at each time point, 20% of average increase in the LARC group at each time point; 10% in the COCP treatment group and 10% of average increase in the LARC group at each time point; 20% in the COCP treatment group. For each of these investigations, analysis was then performed as per the original approach on the imputed and manipulated sets, with results combined using Rubin’s rules. Further sensitivity analysis was also performed removing a small number of late returned responses.
Preplanned subgroup analyses on the primary outcome were completed on the pre-randomisation selection of LARC (LNG-IUS or DMPA). This initially included participants who had their LARC randomly allocated (as they were happy to use either LARC), but this was repeated excluding these participants and then further excluding those where the pre-randomisation selection was specifically chosen by the participant (as opposed to the clinician). In addition, subgroup analyses were carried out on the minimisation variables: stage and extent of endometriosis and age. The effects of these subgroups were examined by adding the subgroup by treatment group interaction parameters to the linear model described above; statistical significance of these interaction parameters was determined by F-tests/Wald tests. Differences between treatment groups within subgroups and 95% CIs were generated.
Interim analyses of effectiveness and safety endpoints were performed on behalf of the DMC on an approximately annual basis during the period of recruitment. These analyses were performed with the use of the Haybittle–Peto principle,66,67 hence, no adjustment was made in the final p-values to determine significance.
Chapter 4 Results of the clinical trial
This chapter reports the results of the PRE-EMPT Trial.
Recruitment and participant flow
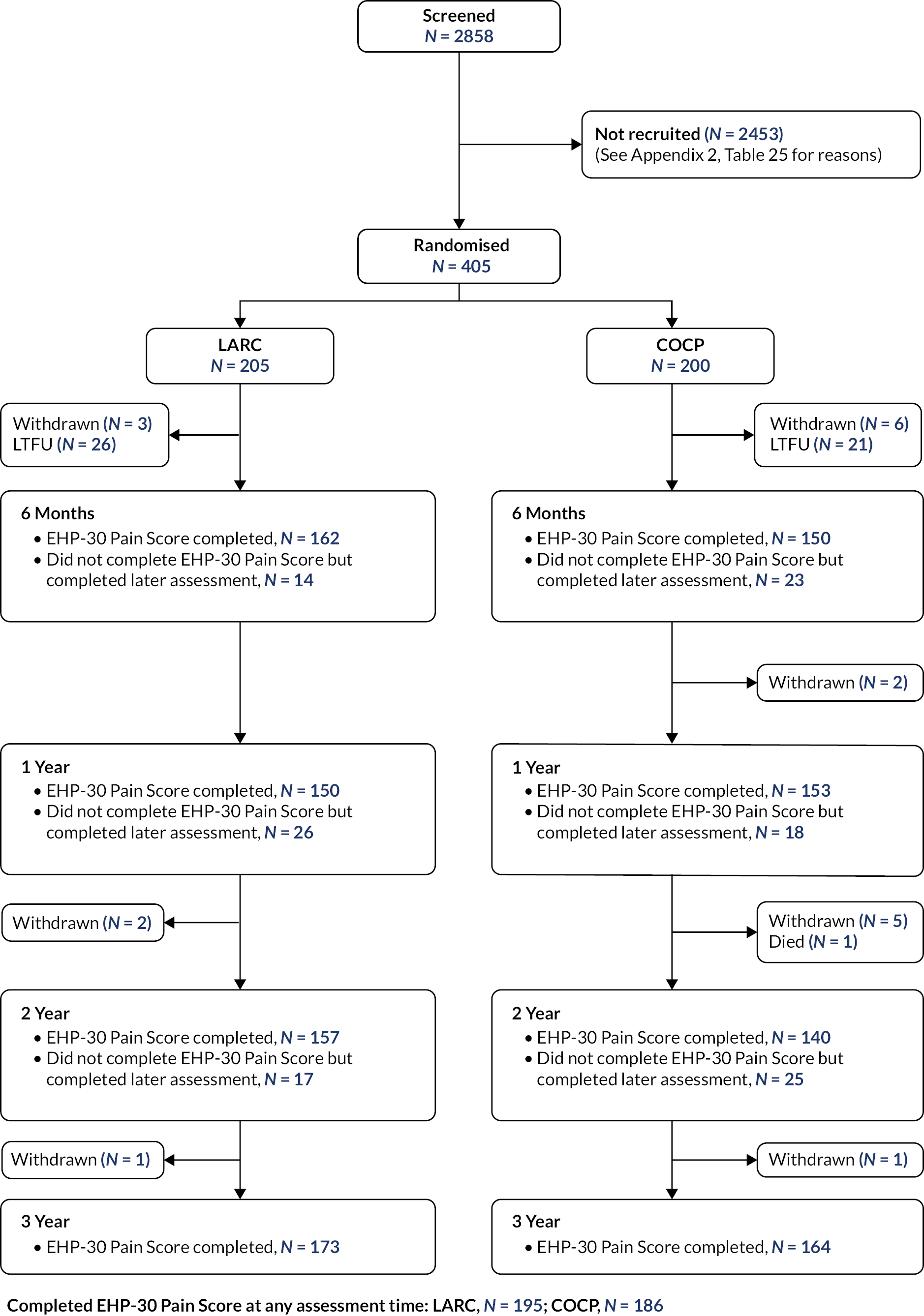
Recruitment under version 1.0 of the protocol (pilot phase design) continued until November 2015, with 92 participants ultimately taken forward into the main trial phase comparison of LARC versus COCP. Recruitment then commenced under the revised design from 23 November 2015 until 25 March 2019 when the sample size target of 400 was achieved (405 ultimately randomised). A total of 34 centres in the UK took part (Table 24, Appendix 2) for recruitment breakdown by centre) and 2858 women were screened for eligibility (Figure 4). Reasons for ineligibility or declining consent are provided in Table 25, Appendix 2. The follow-up rate for the primary outcome was 337 of 405 (83%) at 3 years; 381 women (94%) provided an EHP-30 pain score at least at one of the assessment times (6 months to 3 years).
FIGURE 4.
Consolidated Standards of Reporting Trials diagram for PRE-EMPT.
Participant characteristics
Participating women had a mean age of 29 years (SD 6.6 years) and most (91%) were white (Table 4). Most cases (79%) were graded by the surgeon as either stage I or stage 2 (ASRM classification of minimal or mild) and endometrial tissue was deemed to have been completely excised at operation in 91% of cases. The minimisation algorithm ensured balance between groups in terms of age, stage of endometriosis, LARC selection and centre; the groups were also well balanced for the other baseline characteristics.
| LARC (N = 205) | COCP (N = 200) | ||
|---|---|---|---|
| Age (years)a | < 35 years, n (%) | 161 (79) | 158 (79) |
| ≥ 35 years, n (%) | 44 (21) | 42 (21) | |
| Mean (SD) | 29.6 (6.7) | 29.3 (6.6) | |
| BMI | Mean (SD) | 27.0 (10.6) | 26.3 (5.5) |
| Missing, n | 12 | 12 | |
| Systolic blood pressure (mm/Hg) | Mean (SD) | 119.0 (11.6) | 118.3 (11.6) |
| Missing, n | 22 | 17 | |
| Diastolic blood pressure (mm/Hg) | Mean (SD) | 73.9 (9.3) | 74.2 (9.1) |
| Missing, n | 22 | 17 | |
| Ever smokers | Yes, n (%) | 38 (26) | 39 (26) |
| No, n (%) | 110 (74) | 112 (74) | |
| Missing, n | 57 | 49 | |
| Extent of excision as judged by surgeona | Complete, n (%) | 188 (92) | 181 (90) |
| Incomplete, n (%) | 17 (8) | 19 (10) | |
| Stage of endometriosisa | I, n (%) | 88 (43) | 82 (41) |
| II, n (%) | 73 (36) | 76 (38) | |
| III, n (%) | 25 (12) | 23 (12) | |
| IV, n (%) | 19 (9) | 19 (10) | |
| Self-declared ethnicity | White, n (%) | 186 (91) | 183 (92) |
| Mixed, n (%) | 3 (1) | 2 (1) | |
| Asian, n (%) | 5 (2) | 3 (1) | |
| Black, n (%) | 2 (1) | 3 (1) | |
| Other ethnic group, n (%) | 0 (–) | 1 (< 1%) | |
| Not stated, n (%) | 0 (–) | 0 (–) | |
| Missing, n | 9 | 8 | |
| LARC selection if randomised to LARC (pilot phase recruits n = 92)a | LNG-IUS, n (%) | 17 (35) | 16 (36) |
| DMPA, n (%) | 21 (44) | 23 (52) | |
| Either, n (%) | 10 (21) | 5 (12) | |
| LARC selection if randomised to LARC (main phase recruits n = 313)b | LNG-IUS, n (%) | 59 (38) | 55 (35) |
| DMPA, n (%) | 77 (49) | 81 (52) | |
| Randomly allocated, n (%) | 21 (13) | 20 (13) | |
| Mode of LARC selectionc (main phase recruits n = 313)b | Patient’s preference, n (%) | 126 (80) | 128 (82) |
| Clinician advice, n (%) | 10 (6) | 8 (5) | |
| Neither, n (%) | 21 (13) | 20 (13) | |
| Previous treatment (more than one modality possible)d | LNG-IUS, n (%) | 27 (7) | 21 (5) |
| DMPA, n (%) | 31 (8) | 28 (7) | |
| COCP, n (%) | 48 (12) | 44 (11) | |
| None reported, n | 157 | 156 |
Adherence
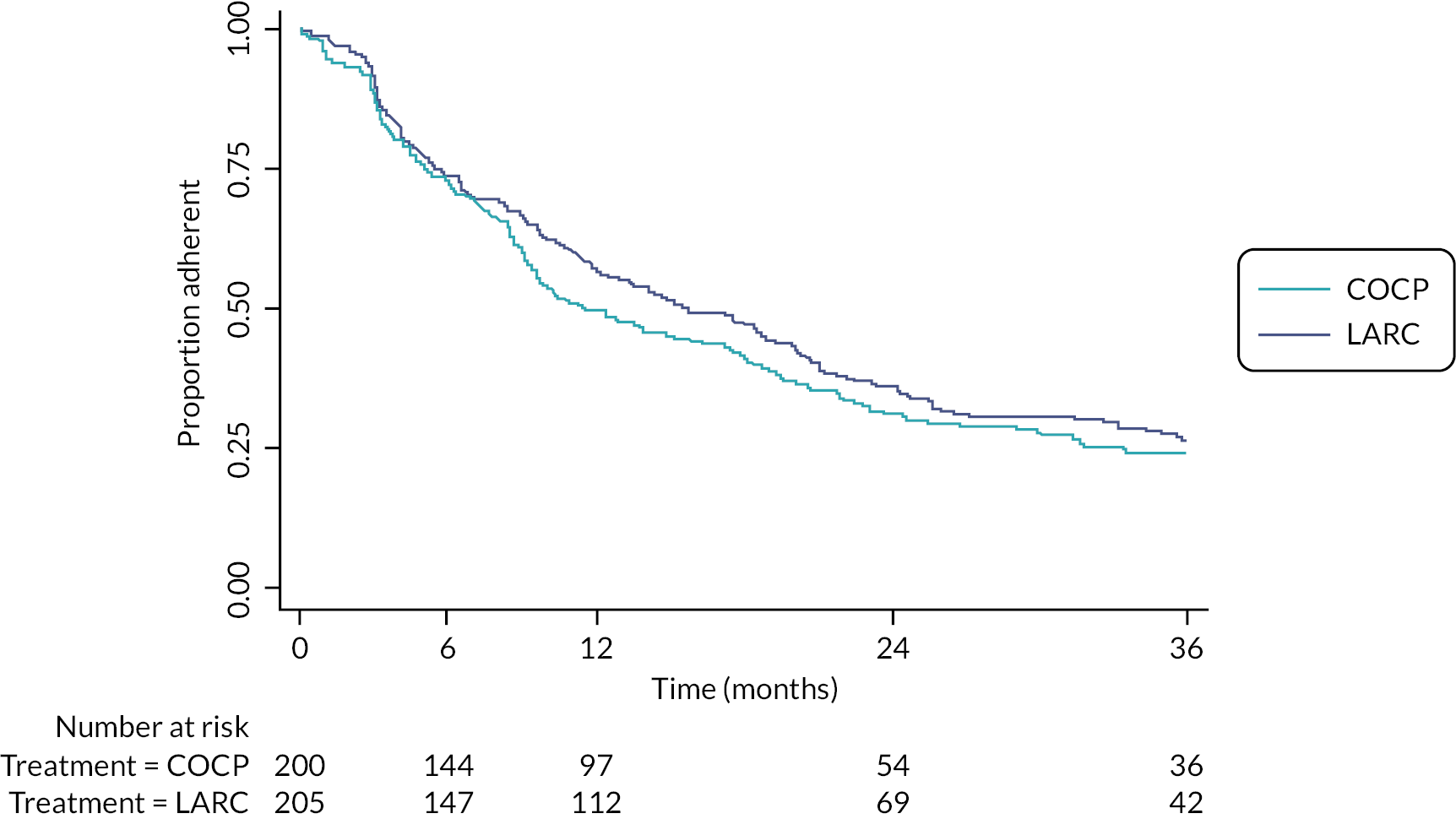
Of the 205 women randomised to LARC, slightly more were offered treatment with DMPA compared with LNG-IUS [114 (56%) vs. 91 (44%)]. Approximately four-fifths [81% (254/313); see Table 4] of these treatment options were driven by patient preference. Of those offered LNG-IUS, 85% (77/91) had the system fitted at the time of their laparoscopic treatment to endometriosis and, of those offered DMPA, 51% (58/114) had the first dose administered before discharge (Table 26). Of the 200 women that were allocated to COCP, 52% (103/200) had this prescribed while in hospital (Appendix 2, Table 27). Approximately 65% of participants allocated LARC were still using a LARC treatment (either LNG-IUS or DMPA) at 1 year, reducing to 37% by 3 years (Figure 5). The equivalent figures in the COCP group were lower, at 53% and 25%. Self-reported reasons for treatment change included perceived lack of effectiveness and commonly known adverse effects of these treatments. Over half the treatment changes in both groups involved cessation of any treatment; some of these were due to a desire for pregnancy (see Secondary outcomes – further therapeutic surgery or second-line treatment for endometriosis) or had undergone further surgery for endometriosis or hysterectomy (Appendix 2, Tables 28 and 29). Switching from one LARC treatment to another (i.e. from LNG-IUS to DMPA or vice versa) or supplementation of (related) non-trial drug was also a relatively common occurrence. Adherence to the allocated intervention (without any treatment change at all) occurred in 56% and 48% of participants at 1 year and 26% and 24% at 3 years, in the LARC and COCP groups, respectively (Figure 6; details in Appendix 2, Tables 28 and 29).
FIGURE 5.
Time to first treatment change (no longer on assigned treatment – changes from one LARC to another, e.g. LNG-IUS to DMPA, are not classified as a change; see Appendix 2, Tables 28 and 29 for full details of changes).
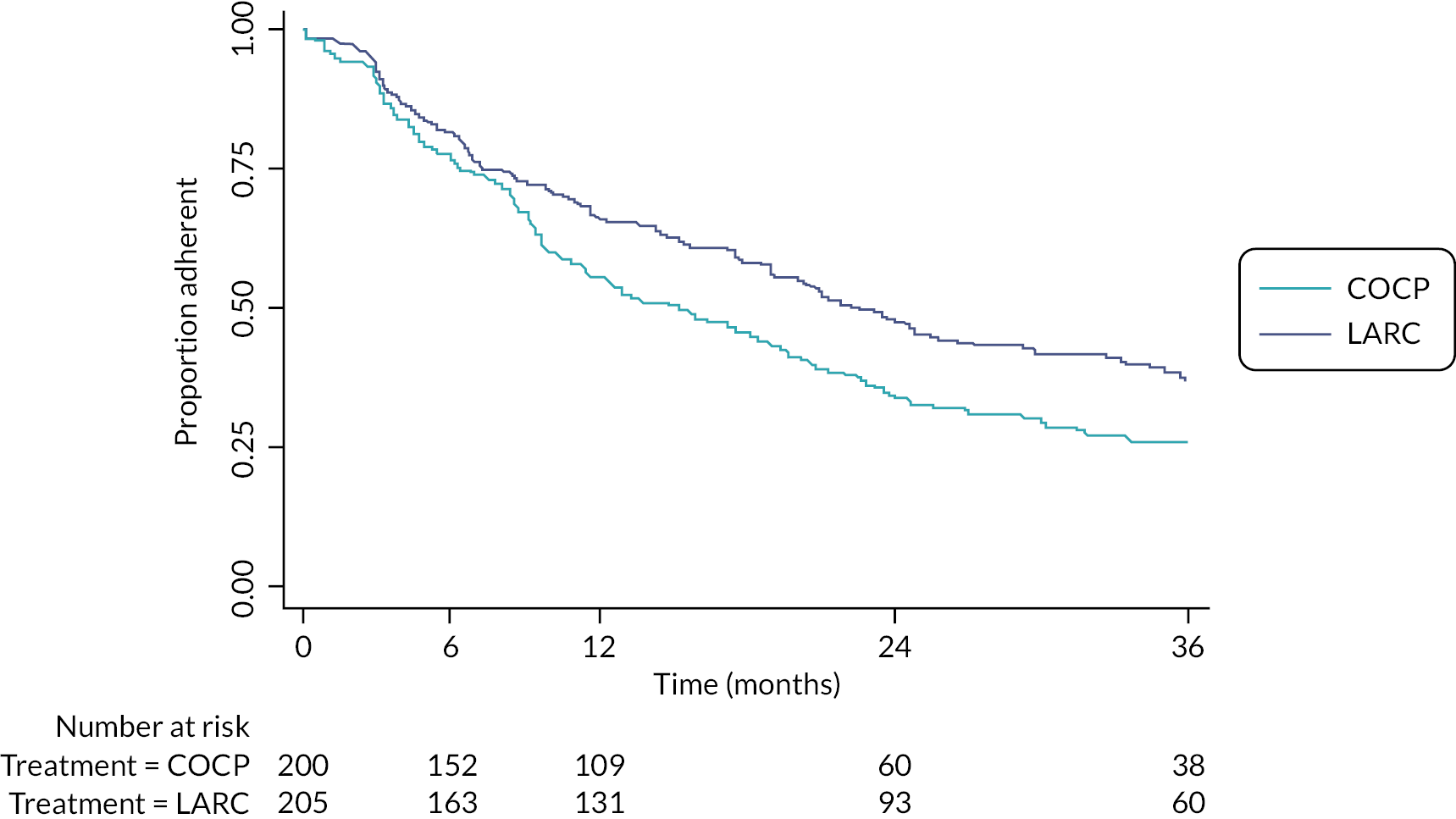
FIGURE 6.
Time to first treatment change (any treatment change – includes any relevant treatment change, i.e. includes the addition of a trial or related non-trial treatment; see Appendix 2, Tables 28 and 29 for full details of changes).
Primary outcome: Endometriosis Health Profile-30 pain score at 3 years
On average, both groups maintained improved pain scores at all follow-up intervals compared with their preoperative scores, but there was no evidence of a statistically significant difference between groups at 3 years (adjusted mean difference: −0.8, 95% CI −5.7 to 4.2; p = 0.76). No differences between the two randomised groups were apparent at the other time points (Table 5 and Figure 7).
| LARC Mean (SD), n |
COCP Mean (SD), n |
Adjusted mean difference (95% CI)b | p-value | |
|---|---|---|---|---|
| Baseline | 56.6 (17.3), 197 | 55.8 (19.9), 192 | ||
| 6 months | 35.0 (25.6), 162 | 38.0 (26.4), 150 | −1.9 (−7.0 to 3.2) | |
| 1 year | 35.1 (26.4), 150 | 37.5 (25.4), 153 | −2.3 (−7.5 to 2.9) | |
| 2 years | 32.1 (26.2), 157 | 33.6 (26.5), 140 | −0.4 (−5.6 to 4.9) | |
| 3 yearsc | 32.9 (25.0), 173 | 32.9 (27.6), 164 | −0.8 (−5.7 to 4.2) | 0.76d |
FIGURE 7.
Longitudinal plot for Endometriosis Health Profile-30 pain scores at all time points by group.
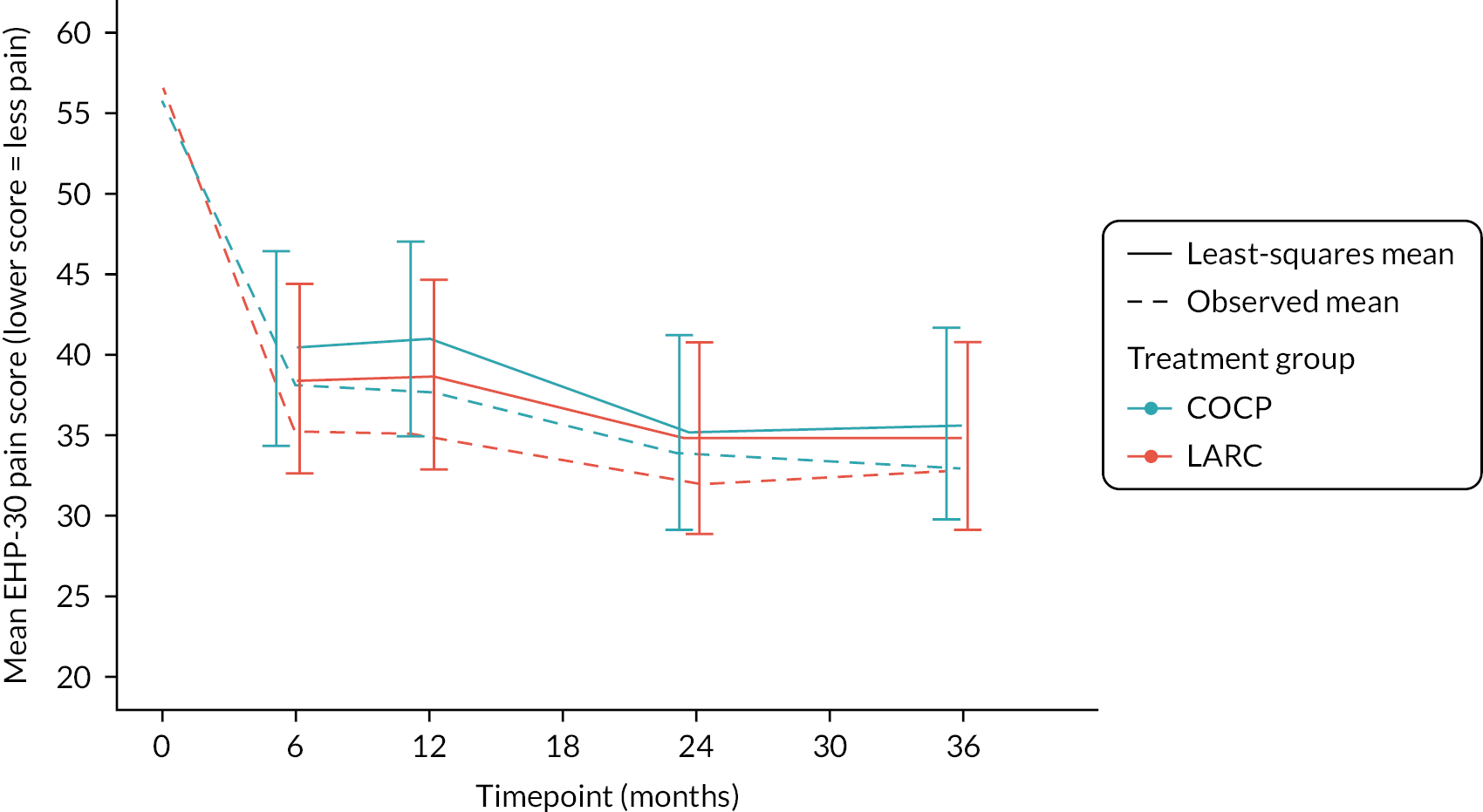
Improvements from baseline were 24 and 23 points on average in the LARC and COCP groups, respectively, equating to moderate to large effect sizes (approximately 0.9 SD).
Subgroup, sensitivity and supportive analyses
There was no evidence of any differential effect in any of the prespecified subgroups in relation to the primary outcome (Table 6).
| LARC, mean (SD), n | COCP, mean (SD), n | Adjusted mean difference (95% CI)b | Interaction, p-value | |
|---|---|---|---|---|
| Pre-randomisation selection of LNG-IUS or DMPA including all methods of allocation | ||||
| LNG-IUS | 32.1 (24.8), 71 | 37.2 (30.1), 67 | −1.9 (−9.7 to 5.9) | 0.95 |
| DMPA | 33.2 (24.9), 92 | 29.5 (25.4), 93 | 0.1 (−6.8 to 6.9) | |
| Pre-randomisation selection of LNG-IUS or DMPA excluding random allocation | ||||
| LNG-IUS | 32.5 (25.0), 64 | 38.0 (30.4), 56 | −1.7 (−9.9 to 6.5) | 0.96 |
| DMPA | 34.3 (24.6), 83 | 29.5 (25.1), 87 | 1.3 (−5.6 to 8.3) | |
| Pre-randomisation selection of LNG-IUS or DMPA including only those participants where the LARC was specifically chosen by the patient | ||||
| LNG-IUS | 31.3 (25.1), 46 | 35.9 (32.6), 37 | −3.4 (−13.2 to 6.4) | 0.84 |
| DMPA | 34.2 (22.1), 60 | 31.8 (25.0), 67 | −1.5 (−9.6 to 6.5) | |
| Stage of endometriosis | ||||
| I/II | 32.2 (24.6), 134 | 32.0 (26.5), 129 | −1.6 (−7.3 to 4.0) | 0.67 |
| III/IV | 35.5 (26.4), 39 | 35.9 (31.4), 35 | 2.2 (−8.6 to 13.0) | |
| Extent of excision | ||||
| Complete | 33.2 (24.5), 160 | 32.5 (27.6), 150 | 0.3 (−13.2 to 13.7) | 0.94 |
| Incomplete | 29.5 (30.8), 13 | 36.9 (27.3), 14 | −3.3 (−21.3 to 14.7) | |
| Age ≥ 35 years | ||||
| Yes | 25.1 (18.9), 36 | 24.6 (27.8), 35 | 3.6 (−7.1 to 14.4) | 0.14 |
| No | 35.0 (26.0), 137 | 35.1 (27.2), 129 | −2.0 (−7.6 to 3.6) | |
Sensitivity analysis conducted to investigate missing data assumptions produced results that did not alter the initial interpretation (Table 30, Appendix 2).
Endometriosis Health Profile-30 pain scores summaries for the small number of participants randomised to combinations that did not include COCP (LARC vs. no treatment) are provided in Table 31, Appendix 2.
Secondary outcomes: other domains of the Endometriosis Health Profile-30
Most of the other domains of the EHP-30 were improved in both groups at all time points compared with preoperative scores, but there was no consistent evidence of any difference between groups when estimates of uncertainty were considered (Table 7).
| LARC, mean (SD), n | COCP, mean (SD), n | Adjusted mean difference (95% CI) | |
|---|---|---|---|
| Core domain: control and powerlessness | |||
| Baseline | 69.1 (19.7), 198 | 66.6 (23.4), 193 | |
| 6 months | 46.1 (30.2), 160 | 49.3 (31.7), 148 | −3.0 (−9.4 to 3.4) |
| 1 year | 47.9 (32.6), 150 | 48.7 (31.5), 150 | −1.2 (−7.6 to 5.3) |
| 2 years | 42.1 (31.6), 127 | 43.1 (32.8), 110 | −2.6 (−9.7 to 4.5) |
| 3 years | 40.9 (28.5), 103 | 45.4 (34.2), 99 | −2.4 (−10.0 to 5.2) |
| Core domain: social support | |||
| Baseline | 56.8 (23.5), 198 | 56.5 (26.5), 193 | |
| 6 months | 47.9 (31.2), 161 | 50.1 (33.6), 152 | −0.9 (−7.3 to 5.4) |
| 1 year | 48.9 (31.9), 152 | 48.8 (31.5), 152 | 1.5 (−4.9 to 7.9) |
| 2 years | 43.8 (33.2), 127 | 46.8 (34.0), 111 | −3.6 (−10.7 to 3.5) |
| 3 years | 40.7 (31.5), 102 | 48.4 (36.1), 99 | −5.1 (−12.6 to 2.5) |
| Core domain: emotional well-being | |||
| Baseline | 53.0 (20.3), 198 | 52.4 (23.2), 193 | |
| 6 months | 42.3 (27.2), 160 | 39.1 (27.2), 150 | 2.5 (−2.9 to 8.0) |
| 1 year | 42.2 (27.2), 152 | 40.1 (26.7), 151 | 1.9 (−3.5 to 7.4) |
| 2 years | 36.6 (27.4), 127 | 36.7 (29.6), 111 | −0.9 (−6.9 to 5.1) |
| 3 years | 35.6 (26.6), 103 | 38.6 (31.1), 99 | −1.8 (−8.2 to 4.7) |
| Core domain: self-image | |||
| Baseline | 54.3 (28.4), 198 | 52.6 (29.0), 194 | |
| 6 months | 47.4 (33.2), 161 | 48.2 (33.4), 152 | −1.0 (−7.6 to 5.7) |
| 1 year | 47.6 (33.8), 152 | 45.7 (33.2), 152 | 1.9 (−4.8 to 8.6) |
| 2 years | 40.6 (32.9), 127 | 43.0 (37.0), 111 | −4.1 (−11.5 to 3.3) |
| 3 years | 43.7 (34.4), 103 | 48.1 (36.7), 99 | −1.7 (−9.6 to 6.2) |
| Modular domain: work life | |||
| Baseline | 51.2 (25.9), 165 | 50.2 (28.0), 168 | |
| 6 months | 29.9 (29.9), 136 | 32.9 (30.8), 126 | −1.0 (−7.6 to 5.7) |
| 1 year | 33.8 (30.2), 126 | 29.5 (29.7), 121 | 6.0 (−0.8 to 12.9) |
| 2 years | 28.1 (29.1), 108 | 25.3 (29.3), 86 | 0.5 (−7.0 to 8.1) |
| 3 years | 23.5 (25.4), 94 | 23.2 (27.4), 79 | −0.7 (−8.5 to 7.1) |
| Modular domain: relationship with children | |||
| Baseline | 40.5 (29.9), 107 | 33.5 (26.6), 87 | |
| 6 months | 27.3 (28.1), 71 | 23.3 (26.7), 51 | 0.7 (−7.9 to 9.4) |
| 1 year | 27.4 (28.8), 68 | 26.8 (29.3), 55 | −4.1 (−12.5 to 4.3) |
| 2 years | 20.5 (25.1), 53 | 22.2 (27.2), 40 | −9.4 (−19.3 to 0.6) |
| 3 years | 19.9 (25.8), 47 | 19.3 (28.7), 42 | −4.2 (−14.7 to 6.4) |
| Modular domain: sexual relationship | |||
| Baseline | 68.4 (26.0), 173 | 69.6 (24.3), 169 | |
| 6 months | 56.9 (30.6), 138 | 53.9 (31.8), 130 | 2.5 (−4.4 to 9.5) |
| 1 year | 55.5 (33.8), 116 | 58.3 (31.3), 122 | 0.8 (−6.5 to 8.0) |
| 2 years | 52.6 (32.6), 104 | 54.1 (32.2), 92 | 1.9 (−6.0 to 9.8) |
| 3 years | 53.4 (31.7), 87 | 55.9 (32.5), 87 | −0.0 (−8.4 to 8.4) |
| Modular domain: feelings about medical profession | |||
| Baseline | 36.0 (29.0), 169 | 31.2 (27.9), 162 | |
| 6 months | 37.7 (30.4), 109 | 42.6 (30.5), 101 | −5.7 (−14.4 to 3.0) |
| 1 year | 38.5 (33.0), 88 | 38.2 (31.7), 102 | 0.3 (−8.8 to 9.4) |
| 2 years | 37.6 (32.8), 83 | 37.0 (33.4), 67 | 2.4 (−7.7 to 12.5) |
| 3 years | 41.3 (33.0), 53 | 43.1 (34.1), 58 | −3.4 (−15.0 to 8.1) |
| Modular domain: feelings about treatment | |||
| Baseline | 48.3 (26.1), 121 | 46.4 (27.5), 115 | |
| 6 months | 44.5 (29.2), 137 | 44.7 (30.2), 130 | −4.4 (−13.2 to 4.5) |
| 1 year | 51.6 (29.8), 102 | 44.9 (29.9), 124 | 6.5 (−2.9 to 15.9) |
| 2 years | 43.2 (31.6), 92 | 44.1 (32.8), 79 | −0.3 (−10.9 to 10.2) |
| 3 years | 40.4 (27.2), 65 | 39.1 (32.7), 67 | 2.5 (−8.7 to 13.7) |
| Modular domain: feelings about infertility | |||
| Baseline | 49.9 (32.5), 110 | 48.5 (33.7), 110 | |
| 6 months | 58.5 (32.6), 75 | 48.7 (31.1), 70 | 11.7 (0.9 to 22.5) |
| 1 year | 58.1 (33.1), 66 | 44.3 (33.9), 68 | 9.1 (−2.2 to 20.4) |
| 2 years | 51.6 (35.7), 51 | 43.9 (33.2), 50 | 16.1 (2.7 to 29.5) |
| 3 years | 55.9 (31.2), 35 | 44.9 (36.3), 45 | 4.3 (−9.8 to 18.4) |
Secondary outcomes: other patient-reported outcome measures
Pain scores (as measured by VAS) and generic quality of life scores were marginally improved at all time points compared with preoperative scores, but there was no consistent evidence of any difference between groups (Table 8). Fatigue Severity Scale and capability (ICECAP-A) scores were similar to baseline scores throughout in both groups.
| LARC, mean (SD), n | COCP, mean (SD), n | Adjusted mean difference (95% CI) | |
|---|---|---|---|
| Visual analogue scale a | |||
| Pain during periods | |||
| Baseline | 7.8 (1.4), 158 | 7.9 (1.5), 152 | |
| 6 months | 6.5 (2.6), 80 | 6.8 (2.3), 110 | −0.2 (−0.8 to 0.4) |
| 1 year | 6.8 (2.3), 76 | 6.9 (1.9), 106 | 0.2 (−0.4 to 0.9) |
| 2 years | 6.4 (2.2), 61 | 6.5 (2.2), 64 | −0.1 (−0.8 to 0.7) |
| 3 years | 7.0 (1.7), 44 | 7.0 (2.1), 53 | −0.4 (−1.2 to 0.4) |
| Pain during intercourse | |||
| Baseline | 6.4 (2.4), 150 | 6.4 (2.6), 159 | |
| 6 months | 5.1 (2.8), 119 | 4.9 (2.6), 109 | 0.0 (−0.7 to 0.7) |
| 1 year | 5.4 (2.8), 104 | 5.7 (2.5), 103 | 0.3 (−0.4 to 1.0) |
| 2 years | 5.3 (2.8), 82 | 5.0 (2.7), 75 | −0.2 (−1.0 to 0.6) |
| 3 years | 5.4 (3.0), 63 | 5.6 (2.8), 74 | 0.2 (−0.6 to 1.1) |
| Pain at any other time | |||
| Baseline | 6.4 (2.0), 180 | 5.8 (2.1), 175 | |
| 6 months | 5.1 (2.7), 140 | 4.9 (2.7), 134 | 0.0 (−0.5 to 0.6) |
| 1 year | 5.5 (2.5), 131 | 5.2 (2.4), 134 | 0.0 (−0.5 to 0.6) |
| 2 years | 5.1 (2.4), 107 | 5.2 (2.5), 95 | 0.2 (−0.4 to 0.8) |
| 3 years | 5.3 (2.3), 81 | 5.4 (2.5), 78 | 0.3 (−0.4 to 1.0) |
| Fatigue Severity Scale b | |||
| Summary score | |||
| Baseline | 43.6 (14.1), 197 | 42.3 (13.4), 191 | |
| 6 months | 41.9 (15.0), 160 | 40.6 (15.6), 150 | 1.5 (−1.6 to 4.5) |
| 1 years | 44.0 (15.7), 151 | 40.7 (15.2), 152 | 3.2 (0.2 to 6.3) |
| 2 years | 43.4 (13.9), 125 | 41.4 (16.4), 109 | 1.7 (−1.7 to 5.0) |
| 3 years | 43.0 (15.1), 102 | 42.0 (17.1), 98 | 1.5 (−2.0 to 5.1) |
| Generic quality of life | |||
| EQ-5D-5Lc | |||
| Baseline | 0.63 (0.24), 198 | 0.63 (0.24), 190 | |
| 6 months | 0.68 (0.24), 160 | 0.67 (0.28), 149 | −0.01 (−0.06 to 0.04) |
| 1 year | 0.67 (0.28), 151 | 0.67 (0.25), 152 | 0.01 (−0.04 to 0.07) |
| 2 years | 0.67 (0.28), 157 | 0.69 (0.27), 141 | 0.01 (−0.05 to 0.06) |
| 3 years | 0.69 (0.27), 176 | 0.69 (0.29), 167 | −0.01 (−0.06 to 0.04) |
| Health thermometer d | |||
| Baseline | 60.4 (20.0), 195 | 61.2 (19.7), 191 | |
| 6 months | 63.0 (20.9), 162 | 61.5 (21.7), 150 | 1.0 (−3.5 to 5.5) |
| 1 year | 60.8 (22.4), 150 | 61.2 (21.8), 153 | −2.4 (−6.9 to 2.1) |
| 2 years | 64.5 (20.7), 158 | 61.4 (22.1), 139 | 3.2 (−1.4 to 7.8) |
| 3 years | 67.1 (20.8), 176 | 63.2 (23.6), 166 | 3.3 (−1.0 to 7.7) |
| ICECAP-A e | |||
| Capabilities | |||
| Baseline | 0.80 (0.17), 195 | 0.80 (0.17), 192 | |
| 6 months | 0.79 (0.18), 162 | 0.80 (0.18), 151 | 0.01 (−0.03 to 0.04) |
| 1 year | 0.81 (0.18), 152 | 0.79 (0.21), 152 | −0.01 (−0.05 to 0.02) |
| 2 years | 0.81 (0.19), 124 | 0.82 (0.19), 110 | 0.01 (−0.03 to 0.05) |
| 3 years | 0.83 (0.16), 100 | 0.77 (0.22), 97 | −0.02 (−0.1 to 0.02) |
Changes in pelvic pain (as measured by Likert scale) appeared consistent throughout, with most women reporting that their pelvic pain had not changed much or had become worse (Table 9) over the past month. There was no evidence of consistent differences between the groups.
| LARC, N (%) | COCP, N (%) | Adjusted ORa (95% CI) | |
|---|---|---|---|
| 6 months | |||
| Got much better | 27 (18) | 19 (14) | |
| Got a little better | 24 (16) | 22 (16) | |
| Not changed much | 71 (49) | 64 (46) | |
| Got worse | 24 (16) | 35 (25) | |
| Total | N = 146 | N = 140 | 1.56 (0.97 to 2.51) |
| 1 year | |||
| Got much better | 14 (10) | 8 (6) | |
| Got a little better | 18 (13) | 15 (11) | |
| Not changed much | 57 (41) | 74 (54) | |
| Got worse | 49 (36) | 39 (29) | |
| Total | N = 138 | N = 136 | 1.02 (0.64 to 1.64) |
| 2 years | |||
| Got much better | 8 (7) | 11 (11) | |
| Got a little better | 10 (9) | 8 (8) | |
| Not changed much | 56 (51) | 48 (50) | |
| Got worse | 35 (32) | 29 (30) | |
| Total | N = 109 | N = 96 | 0.83 (0.48 to 1.43) |
| 3 years | |||
| Got much better | 5 (6) | 5 (6) | |
| Got a little better | 4 (5) | 6 (8) | |
| Not changed much | 42 (50) | 38 (48) | |
| Got worse | 33 (39) | 30 (38) | |
| Total | N = 84 | N = 79 | 0.83 (0.44 to 1.57) |
The number of participants reporting menstrual periods remained relatively consistent throughout and appeared lower in the LARC group (43–54%) compared with the COCP group (63–76%) (Appendix 2, Table 32); these periods appeared less regular in the LARC group during the early stages of follow-up (Appendix 2, Table 33).
The number of recorded pregnancies was 17 in the LARC group and 24 in the COCP group (Appendix 2, Table 34).
Secondary outcomes: further therapeutic surgery or second-line treatment for endometriosis
The number of further therapeutic operations or second-line treatments was lower in the LARC group compared with COCP (73 vs. 97 events, occurring in 50 vs. 61 women due to repeat interventions); translating to a 33% reduction in time to treatment (operative) failure (HR 0.67, 95% CI 0.44 to 1.00) (Table 10 and Figure 8).
| LARC, N | COCP, N | |
|---|---|---|
| Treatment failures | ||
| Hysterectomy | 6 | 14 |
| Surgery for endometriosis | 21 | 30 |
| Laparoscopy | 22 | 28 |
| GnRHa treatment | 24 | 25 |
| Totala | 73 | 97 |
| Total number of women experiencing treatment failure | 50 | 61 |
| Other surgeries | ||
| Removal of polyps | 1 | 3 |
| Removal of fibroids | 1 | 1 |
| Endometrial ablation | 7 | 4 |
| Totala | 9 | 8 |
| Total number of women who have had other types of surgery | 9 | 8 |
FIGURE 8.
Kaplan–Meier plot: time to further therapeutic surgery or second-line treatment.

Inclusion of return to pre-randomisation EHP-30 pain score as a marker of treatment failure demonstrated 11% fewer failures in the LARC arm compared to the COCP arm (HR 0.89, 95% CI 0.66 to 1.19) (Figure 9). Using this definition, by 3 years, around half the women had experienced treatment failure.
FIGURE 9.
Kaplan–Meier plot: time to further therapeutic surgery or second-line treatment (including those that returned to their pre-randomisation EHP-30 scores).

Safety: serious adverse events
The number of SAEs were similar in each group: 21 in the LARC group versus 17 in the COCP group (repeat events meant this occurred in 14 vs. 15 women, respectively; p = 0.79).
There was one death from pancreatic cancer in the COCP group, which occurred 2 years following recruitment. The single SUSAR in the LARC group involved readmission of a women for abdominal pelvic pain 2 weeks following endometriosis surgery and insertion of LNG-IUS. A diagnosis of urinary tract infection was made which was deemed to be unrelated to the trial medication. Three SAEs pertained to prolonged admission following the index surgery, one for inadvertent uterine perforation and two for post-operative pain.
All other submitted SAE forms were for hospital readmission. Seven of these reports (four LARC and three COCP) were linked to planned pregnancy and birth and eight were associated with recurrent pain, presumed to be from endometriosis (four in each arm). Colonic carcinoma was diagnosed in a woman in the COCP arm 1 year following index surgery. One woman in the LARC arm had to stop DMPA after requiring admission for depression. All other events were considered to be unrelated to trial medication.
Chapter 5 Economic evaluation
Pretrial health economic analysis
This section is a summary of an article published elsewhere68 detailing the methods and results of the pretrial economic analysis which ultimately informed the design of the substantive economic analysis of the PRE-EMPT trial data.
Background
We incorporated a pretrial model into the design of the economic analysis for this project, in accordance with best practice. A model-based analysis has the advantage of collating data from a number of diverse sources to enable an economic evaluation in the absence or presence of robust data. Typically, the objective of a pretrial model-based analysis is to identify and anticipate areas of ambiguity in the decision recommendation that could arise in the full trial analysis and thus identify key areas of focus with respect to data collection in order to minimise anticipated uncertainty caused by inadequate data. In general, if the data available at this early stage are robust, but new intervention options become available, the model can provide some indication of the practice recommended while awaiting the trial results. If we deem the available data non-robust, the pretrial analysis can support the case for a trial.
Of course, ambiguity in the decision recommendation can still exist even when the most robust data are collected. This typically occurs when the options compared have strengths and weaknesses with respect to different outcomes to which available instruments for capturing outcomes may have some limited sensitivity.
Summary
Aims and objectives
We carried out a preliminary (pretrial) economic evaluation based on best currently available evidence that existed prior to the trial being undertaken. We compared alternative treatments LNG-IUS, DMPA, COCP and ‘no treatment’ to prevent recurrence of endometriosis after conservative surgery in primary care.
The RCT was designed to collect data to explore the most effective and cost-effective treatment for preventing endometriosis following surgery. Given that the RCT was not due to report for at least 4 years, the pretrial economic analysis was carried out to support immediate decisions, explore the likely decision uncertainty and particularly to inform areas of particular focus for data collection in the full RCT to aid the subsequent full trial-based economic evaluation.
Methods of data collection and analysis
The evaluation took the form of a cost–utility analysis, based on an outcome of cost per QALY. We used a UK NHS perspective in a primary care setting. We developed a state transition (Markov) model in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA). The model structure was based on the NIHR-funded (PRE-EMPT study) RCT design, which was informed by a review of existing evidence and clinical input. We refined the model structure through iterative discussion with expert clinical colleagues.
We carried out a pragmatic literature search to identify evidence on the effectiveness of the treatment strategies to inform parameter inputs and their distributions for the transition probabilities of the decision model. We used estimates for resource use from the literature and some were proxy estimated for the anticipated resource use for the trial. In the analyses, we compared the four treatment strategies. A probabilistic sensitivity analysis, which simultaneously changes all relevant parameters in the model and is repeated 1000 times, was carried out. The analysis provides an assessment of the difference in costs and outcomes in terms of QALYs between the potential interventions over a 36-month time horizon.
Limitations
The limitations of this model were that there were no reliable data available to populate it and give confidence that the model-based analysis could help determine treatment recommendation. We did not test the structural assumptions in a sensitivity analysis, as this analysis would not have improved the precision of our findings. However, these limitations do not undermine the purpose of the pretrial analysis to inform data collection. We assigned wide distributions to test the extent to which uncertainty in the parameters altered the results and whether a trial is required. We used some illustrative data given that the impact of the treatment on this particular condition is subjective, wide distributions around values seemed most suitable.
Key findings
The main findings from the pretrial analysis are that there is considerable uncertainty about the cost-effectiveness of the existing treatments to prevent endometriosis following conservative surgery. There is little difference between the probability of all existing treatment strategies (DMPA, COCP, LNG-IUS) being the most cost-effective, and none can yet be singled out as a potential clear contender. In terms of the direction of results, COCP had the greatest probability of being cost-effective when compared with DMPA and LNG-IUS. DMPA had the highest probability of being cost-effective when compared with LNG-IUS.
Interrelation with other parts of the project
We carried out the model-based analysis for the sole purpose of aiding the design and focus of the full trial-based analysis. We report the trial-based economic analysis in full in next section.
Trial-based health economics analysis
Introduction
This section reports the main economic evaluation conducted alongside the PRE-EMPT trial. The objective was to compare the relative cost-effectiveness of LARC with the COCP in preventing recurrence of endometriosis in women undergoing conservative surgery.
Methods
The primary evaluation was a within-trial cost–utility analysis, with the results expressed in terms of additional cost per QALY gained over 36 months. The base-case analysis was performed from the UK NHS perspective as per recommended practice,69 with an additional sensitivity analysis for the partial societal perspective incorporating productivity costs. Subgroup analyses were also conducted comparing LNG-IUS with COCP and DMPA injection with COCP. Secondary analyses took the form of cost-effectiveness analysis, with secondary endpoints of cost per year’s full capability, cost per EHP-30 pain score reduction and cost per treatment failure avoided, respectively. All statistical analyses were performed using Stata® version 17 (StataCorp LP, College Station, TX, USA).
Measurement and valuation of outcomes
All the outcome measures were collected at baseline, 6, 12, 24 and 36 months after randomisation and the results were presented as means and SD. Differences between the groups were calculated using bootstrapped mean differences. The measurement and valuation of outcome measures is described below.
Instruments and outcomes
EuroQol-5 Dimensions, five-level version
The primary economic analysis is based on the outcome of the QALY gained over 36 months. In this study, the QALY scores were generated through the EQ-5D-5L questionnaire. The EQ-5D-5L questionnaire is a tool that allows people to report on their own perception of their health and quality of life. It measures five different aspects of health (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and each of these aspects has five levels of functioning, ranging from ‘no problem’ to ‘unable to’. 61 The participants’ responses were converted to index scores using a cross-walk value set to map from the EQ-5D-5L to the EuroQol-5 Dimensions, three-level version. 62 QALYs were then calculated for each participant using the approach of area under the utility curve assuming linear interpolation between the five utility measurements,70 where the utility score associated with certain health state was multiplied by the duration of time spent in that health state. To minimise potential bias from an imbalance in baseline utilities, adjustments were made for any differences between the groups in their initial EQ-5D-5L scores using a multiple linear regression method. 71 The health utility values and QALYs obtained during the 36-month follow-up were analysed by trial groups and time point.
ICEpop CAPability for Adults
A secondary analysis was carried out based on the outcome of years of full capability (YFC). YFC refers to the number of years that an individual can expect to live in a state of full capability or ability to perform the activities that are important to them. The YFCs were generated from the ICECAP-A questionnaire. The ICECAP-A is a validated capability measure for adult population, focusing on wellbeing in a broader sense. 63 It comprises five attributes (attachment, stability, achievement, enjoyment and autonomy), where each item has four levels of responses. The score is anchored at 1 (full capability) and 0 (no capability). To generate the YFC, the ICECAP-A score was combined with time, representing the total amount of capability that is available over time using an approach similar to the area-under-the-curve method used to calculate the QALYs. Multiple linear regression method was used to make adjustments for potential differences between groups in their initial ICECAP-A scores to minimise bias.
Endometriosis Health Profile-30 pain domain
Another secondary analysis was based on the changes in the EHP-30 pain domain score. The EHP-30 questionnaire is a patient-reported outcome measure to assess HRQoL in endometriosis. 72 The core instruments cover pain, control and powerlessness, social support, emotional well-being and self-image scale scores. Only the pain-domain score was considered for the secondary analysis outcome. The pain domain consists of 11 questions, with overall 0 as the best outcome to 100 pain score as worst score. The pain score changing was the difference between the pain score at the baseline and at the 36 months follow-up.
Treatment failure avoided
The final outcome in a secondary cost-effectiveness analysis centred on treatment failure avoided. Treatment failure was classified as:
-
further surgery for endometriosis, hysterectomy, laparoscopy
-
use of GnRHa for symptom control.
Resource use and costs
From the UK NHS perspective, only the direct cost that the health service provider had incurred within the time horizon of the trial were included in analysis. Healthcare resource use data were collected alongside the trial at baseline, 6, 12, 24, 36 months after randomisation using the PRE-EMPT follow-up questionnaires. Information was obtained for medications including type of hormonal treatment and painkiller used, hospital and primary consultations, investigation procedures (laparoscopy, hysteroscopy, ultrasound scan) and further surgical management (surgery for endometriosis, hysterectomy). For the societal perspective analysis, the questionnaires also captured indirect non-medical costs, referring to income or productivity loss. This included paid and unpaid time off work due to endometriosis. This was valued by using the human capital approach, where the time lost (measured in days) because of endometriosis symptoms was multiplied by the average gross wage estimates. 73 This approach was considered appropriate, given that the majority of work absences were of relatively shorter duration.
Assumptions related to resource use
A number of assumptions were required in the measurement of the healthcare resource use and costs within the trial:
-
All healthcare visits, including those to a GP or hospital, were counted, although it is possible that some of them might not have been related to endometriosis symptoms.
-
When a participant reported having ‘surgery for endometriosis’ or ‘hysterectomy’ and specified the use of ‘laparoscopy’ as the same time, this was counted as a single procedure (i.e. surgery for endometriosis with laparoscopy or laparoscopic hysterectomy, respectively).
-
If a participant switched treatment, it was assumed that the switch occurred midway between follow-up points for costing purposes.
Valuation of resource use
Relevant unit costs were identified from the established national sources, including the NHS Reference Costs 2020–1,74 the Personal Social Services Research Unit costs,75 and the British National Formulary. 76 Unit costs related to productivity loss due to time-off work were obtained from the Office for National Statistics in 2022. The relevant unit costs were then multiplied by resource use data to calculate the total treatment costs. Table 11 presents the relevant items of resource use, their associated unit costs and the source from which these costs were obtained. All costs were reported in 2021–2 Great British pounds. Costs were inflated where necessary, using the Hospital and Community Health Services Pay and Prices Index. 75
| Resource use items | Unit cost (£) | HRG code/details | Source |
|---|---|---|---|
| Medication | |||
| COCP – (Microgynon®, Bayer plc, Reading, UK) Ethinylestradiol 30 µg, Levonorgestrel 150 µg | 0.94 | Per pack | BNF 84 |
| LNG-IUS device (Mirena®) 20 µg/24 hours | 88 | Per device | BNF 84 |
| Medroxyprogesterone acetate (Depo-Provera®) 150 mg/1 ml suspension for injection vials | 6.01 | Per vial | BNF 84 |
| Triptorelin (Decapeptyl SR®, Ipsen Pharma Biotech, Signes, France) 3 mg (GnRHa) | 69 | Per vial | BNF 84 |
| Pain relief medication | 0.97 | Weighted average of participant pain relief medication | BNF 84 |
| Primary care visit | |||
| GP consultation (10 minutes) | 45.17 | PSSRU 2022 | |
| Further surgery | |||
| Removal of polyps | 4369.71 | MA09B | NHS Reference cost 2020/21 |
| Removal of fibroids | 4369.71 | MA09B | NHS Reference cost 2020/21 |
| Endometrial ablation | 1416.51 | MA12Z | NHS Reference cost 2020/21 |
| Laparoscopic hysterectomy | 5935.16 | MA08B | NHS Reference cost 2020/21 |
| Laparoscopic surgery for endometriosis | 4369.71 | MA09B | NHS Reference cost 2020/21 |
| Test/investigations | |||
| Laparoscopy | 3280.88 | MA10Z | NHS Reference cost 2020/21 |
| Ultrasound | 71.90 | RD40Z | NHS Reference cost 2020/21 |
| Hysteroscopy | 521.82 | MA31Z | NHS Reference cost 2020/21 |
| Follow-up after surgery | 235.39 | WF01A | NHS Reference cost 2020/21 |
| Productivity loss | |||
| Full-time employee work absence | 640b | per weekc | ONS 2022 |
| Part-time employee work absence | 228b | per weekc | ONS 2022 |
Analysis
Missing data
Multiple imputation techniques were used to handle missing costs and missing EQ-5D-5L, ICECAP-A and EHP-30 pain domain data at each follow-up time point. 67,77 Costs were imputed at the total cost level for each cost category. Resource use and therefore cost data were considered missing if participants did not complete and return their follow-up questionnaire.
A multiple imputation with chained equation technique was performed, together with predictive mean matching method to the closest neighbour across 20 imputations to account for the non-normality of the distribution of costs and the outcome values for missing total costs and missing outcomes;78 and Rubin’s rule was used to combine the imputed data sets into one final imputed variable. 77 Subsequently, Rubin’s rules were applied to pool the estimates obtained from the multiple imputed datasets. Rubin’s rules are a statistical method used to appropriately pool parameter estimates and their variances from multiple imputed datasets, enhancing the accuracy and reliability of the overall statistical analysis. This helps to account for the uncertainty introduced by the imputation process and provides a more accurate estimate of the missing values. 67,77 The model for imputing missing EQ-5D-5L, ICECAP-A and EHP-30 pain domain data included the trial arm and score baseline. The imputed data were used to inform the base-case and sensitivity analyses unless specified otherwise.
Some assumptions were also made to deal with some missing data in the trial,
-
For partially completed questionnaires:
-
At any particular time point, if participants had not reported a change to their hormonal treatment, they were assumed to still be using the last one they previously reported.
-
Women who did not state that they visited their GP were assumed to have not done so.
-
In the absence of any specific mention of surgery or investigations, it was assumed that participants did not undergo those procedures.
-
-
If participants reported a healthcare visit (such as a GP visit or gynaecologist follow-up after surgery) without specifying the number of visits, a conservative assumption was made that they had one visit.
-
If participants did not report any healthcare visits, it was assumed that they had one additional visit to GP if they changed treatment, stopped using LNG-IUS (due to removal) or received DMPA (since a GP visit is required every 3 months for injection).
Economic evaluation
The first step was to undertake a cost–consequence analysis. This describes all the important disaggregated results relating to resource use, costs, and outcomes. 79 The analyses were performed according to the intention-to-treat principle. The primary base-case economic analysis took the form of a cost–utility analysis from the perspective of the UK NHS. This method assesses the gains in QALY relative to cost of different interventions. The secondary economic evaluation was the cost-effectiveness analysis, where the health consequences were measured in a natural unit as YFCs, pain score reduction from baseline to 36 months and treatment failure avoided. An incremental cost–utility and effectiveness analyses were undertaken to calculate the incremental cost-effectiveness ratio (ICER) as the cost per outcome. This is the ratio of the mean difference in the cost and mean difference in outcome between the two trial arms.
Both cost and QALY were discounted by 3.5% per annum as recommended by NICE. 69 Because cost and QALY data were skewed, all estimates were presented as means with bootstrapped 95% CIs, each with 5000 replications.
Sensitivity analysis
Probabilistic sensitivity analysis
To represent the overall uncertainty in the trial cost and outcome data, a probabilistic sensitivity analysis was undertaken for the base-case analysis only, by jointly bootstrapping mean cost and outcome differences to generate 5000 paired ICER estimates. The 5000 paired bootstrap estimate pairs of the mean costs against mean outcomes (paired differences) provided a graphical display of a cost-effectiveness plane. 80 The plane is divided into four quadrants, each representing a different cost-effectiveness scenario. The north-east quadrant represented situations where the intervention is both more effective and more costly than the comparator, indicating that the intervention is cost-effective if the decision-maker is willing to pay more for the additional benefit. The south-east quadrant represented situations where the intervention is both more effective and cheaper than the comparator, indicating that the intervention is dominant. This meant that the intervention was the preferred option, as it provided better outcomes at a lower cost compared with the alternative. The south-west quadrant represented situations where the intervention is less effective and less costly than the comparator while the north-west quadrant represented situations where the intervention is less effective and more costly than the comparator, indicating that the intervention is dominated by comparator. This meant that the intervention is deemed to be not cost-effective and should not be chosen unless there are other compelling and important factors that justify the additional costs. 55,79
Uncertainty was also estimated by constructing cost-effectiveness acceptability curves (CEACs). 81 For the primary economic analysis, the CEAC shows the probability for COCP and/or LARC being cost-effective at different cost-per-QALY thresholds. In the UK, interventions were deemed cost-effective if the cost per QALY gained is ≤ £20,000. 69
Deterministic sensitivity analysis and subgroup analysis
To assess the robustness of the base-case results, additional deterministic sensitivity analyses were also carried out.
-
Complete-case analysis – the analysis was rerun using only observations with complete cost and outcome data.
-
Undiscounted analysis – this analysis presented the undiscounted costs and outcomes.
-
Partial societal perspective analysis – this analysis assessed the impact of including work-related costs of patients.
-
Additional analysis incorporating costs of other types of surgery mentioned by participants (removal of fibroids, removal of polyps and endometrial ablation).
-
Subgroup analysis – this analysis assessed the cost-effectiveness of the COCP with each of the LARC’s subgroups:
-
COCP versus LNG-IUS
-
COCP versus DMPA.
-
Results
Participants
In the total of 405 participants included in the trial, 200 were randomised to COCP. In the trial 205 women were randomised to LARC, of which 91 were either allocated based on their preference or randomised to LNG-IUS and 114 to DMPA. Follow-up rate at 36 months was 86% of all groups.
Primary economic analysis
Utility and quality-adjusted life-years
The response rates for the health economics participant-completed outcome using the EQ-5D-5L questionnaire at each follow-up time point are presented in Table 12. The level of missingness of the EQ-5D-5L data shows that, at end of the study (month 36), complete data were available for approximately 85% of women in both groups. Complete economic data from baseline to month 36 from EQ-5D-5L questionnaires were available for 214 (52.84%) participants.
| Time point | LARC group (N = 205), n (%) | COCP group (N = 200), n (%) | Total (n) | Missing(n) |
|---|---|---|---|---|
| EQ-5D-5L questionnaire | ||||
| Baseline | 198 (96.58) | 190 (95) | 388 | 17 |
| Month 6 | 160 (78.05) | 149 (74.5) | 309 | 96 |
| Month 12 | 151 (73.66) | 152 (76) | 303 | 102 |
| Month 24 | 157 (76.59) | 141 (70.5) | 298 | 107 |
| Month 36 | 176 (85.85) | 167 (83.5) | 343 | 62 |
| Complete case baseline to month 36 | 115 (56.09) | 99 (49.5) | 214 | 191 |
The utility score at the baseline and each follow-up time points along with the QALY for the complete data and for the imputed data set are presented in Table 13. At the baseline, the participants in the LARC group had a slightly lower average starting EQ-5D-5L score than those in COCP group (0.626 and 0.634, respectively), but they had slightly higher score at 36 months than those in COCP arm (0.693 and 0.686, respectively). The mean adjusted imputed QALY difference between two arms was 0.043 (95% CI −0.069 to 0.152) in favour of COCP, where participants in LARC group had lower QALY than the COCP one (1.937 and 1.976, respectively). This result was inconsistent with the complete case QALY analysis, which suggested that the mean adjusted QALY difference between the two arms was 0.032 (95% CI −1.634 to 0.093) in favour of LARC, but the complete case analysis had a higher amount of missing data. More details of complete and imputed EQ-5D-5L score can be found in Appendix 3, Table 35.
| Time point | LARC group (N = 205) | COCP group (N = 200) | Bootstrap adjusted mean difference (95% CI) | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Baseline EQ-5D-5L score | 198 | 0.626 (0.240) | 190 | 0.634 (0.237) | 0.008 (−0.039 to 0.057) |
| Month 6 EQ-5D-5L score | 160 | 0.678 (0.236) | 149 | 0.674 (0.276) | −0.009 (−0.059 to 0.041) |
| Month 12 EQ-5D-5L score | 151 | 0.671 (0.280) | 152 | 0.674 (0.248) | 0.010 (−0.042 to 0.072) |
| Month 24 EQ-5D-5L score | 157 | 0.673 (0.280) | 141 | 0.687 (0.270) | 0.011 (−0.047 to 0.068) |
| Month 36 EQ-5D-5L score | 176 | 0.693 (0.266) | 167 | 0.686 (0.287) | −0.008 (−0.062 to 0.044) |
| Total complete case QALYs | 115 | 1.975 (0.602) | 99 | 1.945 (0.645) | −0.032 (−1.634 to 0.093) |
| Total imputed QALYs | 205 | 1.937 (0.550) | 200 | 1.976 (0.576) | 0.043 (−0.069 to 0.152) |
Resource use
Average resource use per trial participant is presented in Table 14. There were few variations in mean resource use between groups. On average, participants in the LARC group had fewer surgical procedures (and therefore fewer follow-up episodes); fewer instances of GnRHa and analgesic use and fewer ultrasound scans than the COCP group. The LARC group reported more primary care (GP) visits (which may reflect the fact that DMPA needs to be injected every 3 months) and an increased number of diagnostic procedures (laparoscopy and hysteroscopy) compared with the COCP arm. In terms of productivity loss, the LARC group reported fewer days off work due to endometriosis.
| Resource item | LARC group (N = 205) | COCP group (N = 200) | Bootstrap difference, mean difference (95% CI) | ||
|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | ||
| Healthcare visit | |||||
| GP visit | 7.28 (4.88) | 121 | 6.64 (6.36) | 110 | −0.64 (−1.97 to 0.96) |
| Gynaecology follow-up after surgery visit | 0.62 (1.20) | 121 | 1.35 (2.42) | 110 | 0.73 (0.29 to 1.23) |
| Medication | |||||
| COCP | 3.92 (7.32) | 121 | 18.63 (12.07) | 110 | 14.71 (12.14 to 17.52) |
| LARC | 4.44 (4.21) | 121 | 0.96 (2.14) | 110 | −3.47 (−4.33 to −2.64) |
| LNG-IUS | 0.63 (0.57) | 121 | 0.21 (0.41) | 110 | −0.42 (−0.55 to −0.29) |
| DMPA | 3.81 (4.52) | 121 | 0.76 (2.14) | 110 | −3.06 (−3.94 to −2.18) |
| GnRHa | 0.94 (2.57) | 121 | 0.98 (2.99) | 110 | 0.04 (−0.62 to 0.85) |
| Painkiller | 16.10 (13.67) | 121 | 23.07 (19.59) | 110 | 6.97 (2.81 to 11.82) |
| Further procedures | |||||
| Surgery for endometriosis | 0.16 (0.47) | 121 | 0.22 (0.42) | 110 | 0.06 (−0.06 to 0.17) |
| Hysterectomy combined | 0.07 (0.26) | 121 | 0.10 (0.30) | 110 | 0.03 (−0.04 to 0.10) |
| Test/investigations | |||||
| Laparoscopy | 0.07 (0.28) | 121 | 0.06 (0.23) | 110 | −0.01 (−0.08 to 0.04) |
| Ultrasound scan | 0.04 (0.20) | 121 | 0.07 (0.32) | 110 | 0.03 (−0.03 to 0.11) |
| Hysteroscopy | 0.02 (0.18) | 121 | 0 (0) | 110 | −0.02 (−0.06 to 0) |
| Productivity loss | |||||
| Days taken off-paid work | 13.38 (20.19) | 109 | 14.89 (25.56) | 96 | 1.52 (−4.81 to 7.85) |
| Days taken off-unpaid work | 11.19 (16.75) | 36 | 17.47 (30.56) | 36 | 6.28 (−4.48 to 18.23) |
Costs
In order to derive the health service costs accruing from each intervention in Table 15, the mean resource use was then combined with the unit costs (Table 11). Average costs of healthcare service use differed between the two groups, given that difference in mean resource use. The most significant cost difference between the two groups was in the cost of further/extra surgeries. Specifically, the COCP group had to undergo more surgeries for endometriosis and hysterectomy for which the associated additional costs compared with the LARC group was approximately £328 and £177, respectively. Furthermore, the required follow-up visits after these additional surgeries cost an another additional £160 compared with the LARC group. In terms of the non-health service cost, LARC was associated with lower cost of productivity loss in terms of cost of days-off paid-work due to endometriosis. While the non-health-service costs were not incorporated into the base-case results, they were included in the sensitivity analysis to assess the CE from a broader perspective.
| Resource item | LARC group (N = 205) | COCP group (N = 200) | Bootstrap difference, mean cost difference (95% CI) | ||
|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | ||
| Healthcare visit | |||||
| GP visit | 317.16 (168.13) | 205 | 290.88 (212.03) | 200 | −26.29 (−63.51 to 9.89) |
| Follow-up visit after surgery | 141.33 (213.19) | 205 | 300.48 (414.22) | 200 | 159.14 (98 to 223.91) |
| Medication | |||||
| COCP | 3.56 (5.16) | 205 | 17.06 (8.24) | 200 | 13.50 (12.09 to 14.77) |
| LARC | 76.71 (30.43) | 205 | 21.65 (27.05) | 200 | −55.05 (−60.72 to −49.20) |
| LNG-IUS | 54.79 (38.23) | 205 | 17.39 (26.17) | 200 | −37.40 (−44.31 to −31.39) |
| DMPA | 21.92 (20.47) | 205 | 4.26 (9.28) | 200 | −17.66 (−20.71 to −14.51) |
| GnRHa | 63.82 (135.54) | 205 | 64.98 (150.94) | 200 | 1.16 (−25.33 to 30.03) |
| Painkiller | 15.11 (10.01) | 205 | 21.79 (13.99) | 200 | 6.89 (4.31 to 9.07) |
| Further procedures | |||||
| Conservative surgery for endometriosis | 660.70 (1528.89) | 205 | 988.68 (1344.43) | 200 | 327.98 (27.98 to 585.83) |
| Hysterectomy | 420.66 (1173.08) | 205 | 597.24 (1314.11) | 200 | 176.58 (−47.59 to 437.96) |
| Test/investigations | |||||
| Laparoscopy | 226.28 (701.81) | 205 | 162.73 (550.69) | 200 | −63.56 (−195.26 to 63.58) |
| Ultrasound scan | 3.07 (10.91) | 205 | 5.15 (17.08) | 200 | 2.08 (−0.28 to 5.25) |
| Hysteroscopy | 9.01 (73.05) | 205 | 0 (0) | 200 | −9.01 (−22.80 to −2.74) |
| Productivity loss | |||||
| Days taken off-paid work | 2608.16 (1388.07) | 205 | 2848.04 (1225.99) | 200 | −239.88 (−468.36 to 10.78) |
Mean total costs
Mean total costs for each group are presented in Table 16. Cost of healthcare visit, further surgical procedures were almost £133 and £505 less per woman, respectively, in LARC group, and almost £34 and £71 higher in terms of medication and investigation cost, respectively. Overall, the intervention of LARC was less costly.
| Resource item | LARC group (N = 205) | COCP group (N = 200) | Bootstrap difference, mean cost difference (95% CI) |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Cost of healthcare visit | 458.49 (278.35) | 591.35 (513.71) | 132.86 (53.55 to 213.16) |
| Cost of medications | 159.20 (139.52) | 125.49 (153.59) | −33.70 (−60.96 to −0.27) |
| Cost of further procedures | 1081.36 (2024.50) | 1585.92 (2111.36) | 504.56 (108.25 to 931.66) |
| Cost of test/investigations | 238.36 (707.14) | 167.87 (550.19) | −70.49 (−190.48 to 47.91) |
| Total costs of health service use | 1937.41 (2375.22) | 2470.64 (2358.75) | 533.23 (42.17 to 1008.77) |
Cost–utility analysis
Table 17 provides a summary of the cost–utility analysis using data from the base-case UK NHS perspective. In the base-case analysis, the COCP group was estimated to be £533 (95% CI 52 to 983) per woman more costly but offered slightly higher QALYs by 0.043 (95% CI −0.069 to 0.152) compared with the LARC group, over the 36 months of follow-up. This resulted in an ICER of almost £12,280 per QALY, which is within the £20,000 threshold recommended by NICE. 69 The cost-effectiveness plane in Figure 10 shows the majority of the points are in the north-east quadrant, indicating that COCP was costlier, but more effective than LARC. The CEAC shows the probability that the both interventions are cost-effective at different levels of willingness-to-pay for a QALY. At the £20,000 and £30,000 per QALY, the probability that the COCP intervention is cost-effective was 61% and 66%, respectively. This means that the probability of COCP being cost-effective is higher than the LARC group at these thresholds.
| Treatment arms | Mean cost (£) | Mean effect | Mean incremental cost difference (95% CI) | Mean incremental effect difference (95% CI) | ICER |
|---|---|---|---|---|---|
| LARC | 1937.41 | 1.933 | 533.23 (52.26 to 983.46) | 0.043 (−0.069 to 0.152) | 12,279.574 |
| COCP | 2470.64 | 1.976 |
FIGURE 10.
Cost–utility analysis from the UK NHS perspective. (a) Cost-effectiveness plane showing 5000 bootstrapped replicates of the ICER. (b) cost-effectiveness acceptability curve for COCP compared with LARC.
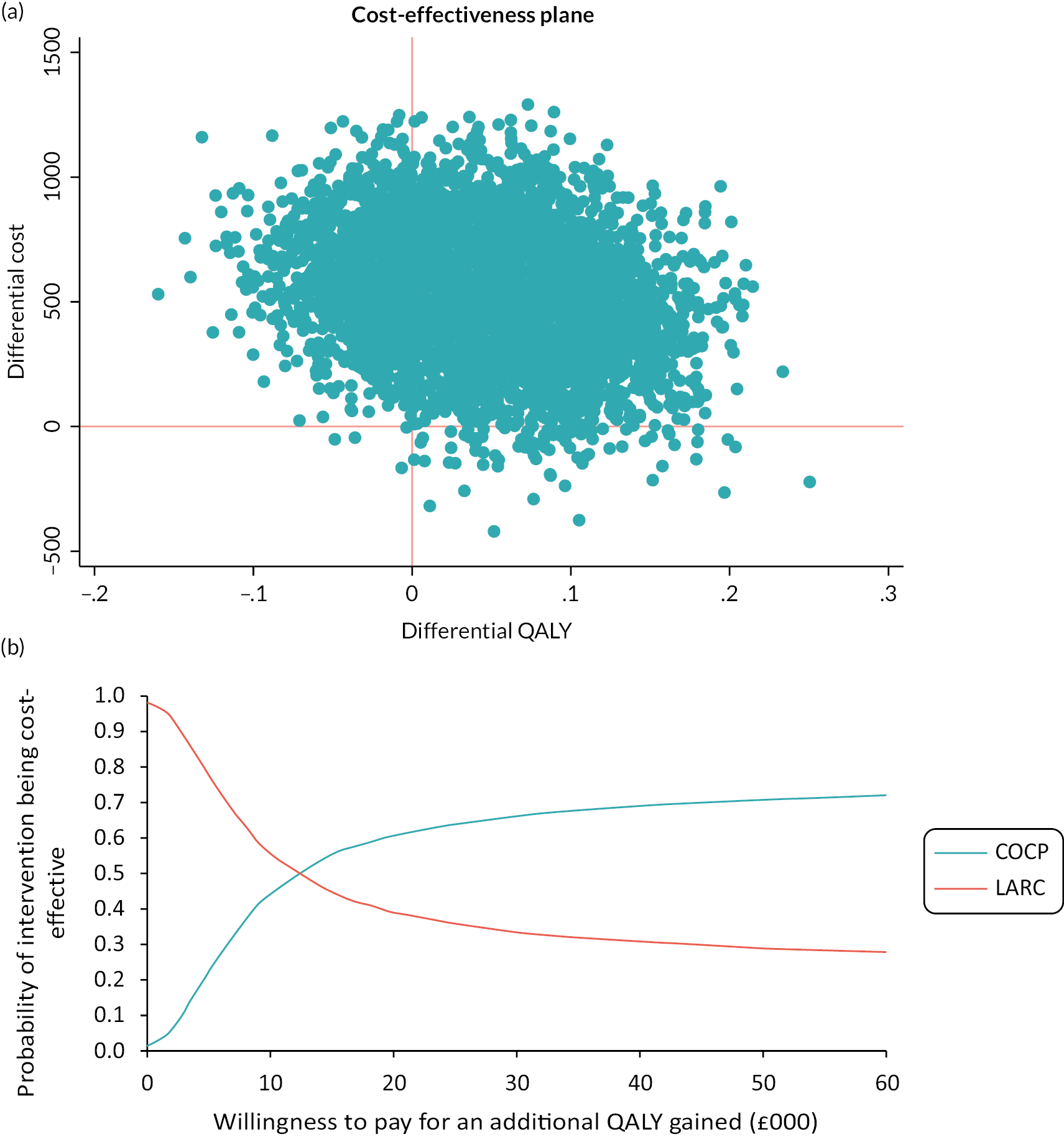
Sensitivity analyses
Details of deterministic sensitivity analyses and subgroup analyses are presented in Table 18. In complete case-analysis scenarios, the COCP intervention was shown to be less effective and more costly in terms of QALYs gained. However, it is important to note that the complete case analysis was only based on 212 cases (n = 113 in the LARC arm and n = 99 in the COCP arm), and the missing QALY values in the COCP arm tended to be higher than those in the LARC arm. This explains why the mean QALYs for the COCP arm in the complete case analysis were lower than those of the LARC arm. In other deterministic sensitivity analysis (undiscounted analysis, partial societal perspective analysis, additional analysis incorporating the cost of other type of surgery), the use of COCP was consistently more costly but also more effective in terms of QALYs gained compared with LARC. These findings were also consistent with the subgroup analysis comparing the COCP group with the LARC subgroups. Specifically, when compared with the DMPA subgroup, the COCP group incurred an additional cost of £851 (95% CI 391.075 to 1269.53) and yielded 0.052 (95% CI −0.091 to 0.171) more QALYs. Similarly, when compared with the LNG-IUS subgroup, the COCP group was associated with an additional cost of almost £135 (95% CI −708.614 to 769.042) and yielded 0.032 (95% CI −0.090 to 0.164) more QALYs.
| Mean cost (£) | Mean effect (QALY) | Bootstrap difference, mean incremental cost (95% CI) | Bootstrap difference, mean incremental effect (95% CI) | ICER | |
|---|---|---|---|---|---|
| Base case analysis | |||||
| LARC | 1937.41 | 1.933 | 533.23 (52.26 to 983.46) | 0.043 (−0.069 to 0.152) | 12,279.57 |
| COCP | 2470.64 | 1.976 | |||
| 1. Complete case analysis | |||||
| LARC | 1936.901 | 1.979 | 535.16 (−301.23 to 1317.32) | −0.035 (−0.198 to 0.121) | Dominated |
| COCP | 2472.059 | 1.945 | |||
| 2. Undiscounted cost and outcome | |||||
| LARC | 2011.537 | 2.0005 | 465.71 (−50.98 to 948.70) | 0.045 (−0.056 to 0.165) | 10,277.62 |
| COCP | 2477.242 | 2.0457 | |||
| 3. Partial societal perspective | |||||
| LARC | 4545.571 | 1.933 | 773.11 (229.25 to 1296.59) | 0.043 (−0.069 to 0.152) | 17,803.78 |
| COCP | 5318.681 | 1.976 | |||
| 4. Including cost of other types of surgery mentioned by participants (removal of fibroids, removal of polyps and endometrial ablation) | |||||
| LARC | 2006.138 | 1.933 | 630.58 (117.46 to 1128.01) | 0.043 (−0.069 to 0.152) | 14,521.60 |
| COCP | 2636.723 | 1.976 | |||
| 5a. Subgroup analysis: LNG-IUS vs. COCP | |||||
| LNG-IUS | 2336.09 | 1.944 | 134.55 (−708.61 to 769.04) | 0.032 (−0.090 to 0.164) | 4145.65 |
| COCP | 2470.64 | 1.976 | |||
| 5b. Subgroup analysis: DMPA vs. COCP | |||||
| DMPA | 1619.17 | 1.924 | 851.47 (391.08 to 1269.53) | 0.052 (−0.091 to 0.171) | 16,318.07 |
| COCP | 2470.64 | 1.976 | |||
Secondary economic analysis
Secondary outcomes
Table 19 displays the response rates for the analysis based on secondary outcomes, which included the ICECAP-A and EHP-30 pain domain questionnaires, at each follow-up time point. However, missing data were significant for the secondary outcomes. Of the total number of participants, complete economic data from baseline to month 36 were available for 147 (36.23%) and 212 (52.34%) participants for the ICECAP-A and EHP-30 pain domain questionnaires, respectively.
| Time point | LARC group (N = 205), n (%) | COCP group (N = 200), n (%) | Total (n) | Missing (n) |
|---|---|---|---|---|
| ICECAP-A questionnaire | ||||
| Baseline | 195 (95.12) | 192 (96) | 387 | 18 |
| Month 6 | 162 (79.02) | 151 (75.5) | 313 | 92 |
| Month 12 | 152 (74.15) | 152 (76) | 304 | 101 |
| Month 24 | 124 (60.49) | 110 (55) | 234 | 171 |
| Month 36 | 100 (48.78) | 97 (48.5) | 197 | 208 |
| Complete case baseline to month 36 | 76 (37.07) | 71 (35.5) | 147 | 258 |
| EHP-30 pain domain questionnaire | ||||
| Baseline | 197 (96.09) | 192 (96) | 389 | 16 |
| Month 6 | 162 (79.02) | 150 (75) | 312 | 93 |
| Month 12 | 150 (73.17) | 153 (76.5) | 303 | 102 |
| Month 24 | 157 (76.59) | 140 (70) | 297 | 108 |
| Month 36 | 173 (84.39) | 164 (82) | 337 | 68 |
| Complete case baseline to month 36 | 113 (55.12) | 99 (49.5) | 212 | 193 |
The scores of each outcome at each follow-up time point are presented in Table 20. Participants in LARC group had a slightly higher ICECAP-A and EHP-30 pain domain score compared with the COCP arm both at the baseline and at month 36. This resulted in mean adjusted years of full capabilities difference for imputed data of 0.0034 (95% CI −0.0562 to 0.0525) and 0.0254 (95% CI −0.116 to 0.0648) for the complete case, both favouring the LARC group. The reduction in pain score between baseline and month 36 of these two groups was also in favour of LARC, where the mean difference of LARC and COCP arm for the imputed case was 0.145 (−4.509 to 4.182) and 0.969 (95% CI −6.276 to 3.635) for the complete case. Furthermore, fewer treatment failure was observed in LARC group than the COCP group (16.59% and 20.5%, respectively). This resulted in mean difference of 0.039 (95% CI −0.121 to 0.034).
| Time point | LARC group (N = 205) | COCP group (N = 200) | Bootstrap adjusted mean difference (95% CI) | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Years of full capacity estimates: ICECAP-A scores | |||||
| Baseline ICECAP-A score | 195 | 0.802 (0.172) | 192 | 0.800 (0.175) | −0.002 (−0.038 to 0.032) |
| Month 6-ICECAP-A score | 162 | 0.792 (0.181) | 151 | 0.802 (0.176) | 0.011 (−0.019 to 0.044) |
| Month 12-ICECAP-A score | 152 | 0.811 (0.179) | 152 | 0.791 (0.213) | −0.017 (−0.054 to 0.021) |
| Month 24-ICECAP-A score | 124 | 0.810 (0.193) | 110 | 0.816 (0.186) | 0.0101 (−0.033 to 0.051) |
| Month 36-ICECAP-A score | 100 | 0.830 (0.161) | 97 | 0.774 (0.225) | −0.0275 (−0.067 to 0.016) |
| Total complete case YFCs | 76 | 2.419 (0.353) | 71 | 2.309 (0.495) | −0.0254 (−0.116 to 0.0648) |
| Total imputed YFCs | 205 | 2.326 (0.389) | 200 | 2.320 (0.454) | −0.0034 (−0.0562 to 0.0525) |
| Pain score estimates: EHP-30 pain domain scores | |||||
| Baseline | 197 | 56.587 (17.330) | 192 | 55.753 (19.941) | −0.835 (−4.6601 to 2.671) |
| Month 6 | 162 | 35.045 (25.580) | 150 | 38 (26.376) | 2.165 (−2.804 to 7.129) |
| Month 12 | 150 | 35.061 (26.353) | 153 | 37.493 (25.358) | 2.697 (−2.166 to 7.811) |
| Month 24 | 157 | 32.121 (26.171) | 140 | 33.555 (26.487) | 1.477 (−4.034 to 6.932) |
| Month 36 | 173 | 32.948 (24.950) | 164 | 32.858 (27.552) | 0.018 (−4.794 to 5.023) |
| Complete case pain score reduction from baseline to month 36 | 113 | 26.106 (23.910) | 99 | 22.062 (24.381) | −0.969 (−6.276 to 3.635) |
| Imputed pain score reduction from baseline to month 36 | 205 | 23.549 (22.060) | 200 | 23.403 (23.673) | −0.145 (−4.509 to 4.182) |
Cost-effectiveness analysis
The cost-effectiveness analysis of secondary outcomes, such as YFC, EHP-30 pain score reductions from baseline to 36 months and treatment failure avoided, is presented in Table 21. The results suggested that the COCP intervention was dominated by the LARC intervention (i.e. the LARC intervention was both cheaper and more effective). Probabilistic sensitivity analyses were carried out but are not reported in terms of the cost-effectiveness plane and CEAC in this report. This is because the analysis based on the secondary outcomes produced findings that were less reliable due to the higher number of missing data observed in secondary outcomes. For instance, the ICECAP-A questionnaire, had ˂ 50% completeness at 36 months.
| Treatment arms | Mean cost (£) | Mean effect | Bootstrap difference, mean incremental cost (95% CI) | Bootstrap difference, mean incremental effect (95% CI) | ICER |
|---|---|---|---|---|---|
| Years of full capacity | |||||
| LARC | 1937.41 | 2.326 | 533.23 (52.26 to 983.46) | −0.006 (−0.092 to 0.0762) | Dominated |
| COCP | 2470.64 | 2.32 | |||
| EHP-30 pain domain score reduction | |||||
| LARC | 1937.41 | 23.549 | 533.23 (52.26 to 983.46) | −0.145 (−4.509 to 4.182) | Dominated |
| COCP | 2470.64 | 23.403 | |||
| Treatment failure avoided | |||||
| LARC | 1937.41 | 0.166 | 533.23 (52.26 to 983.46) | 0.039 (−0.035 to 0.113) | Dominated |
| COCP | 2470.64 | 0.205 | |||
Discussion
Principal findings
Our results suggest that post-surgical use of COCP is more costly, with an average cost per participant of £2470 per woman compared with £1937 for the LARC. The difference in costs (£533, 95% CI £52 to £983) was mainly attributable to the cost of further surgery to treat endometriosis or hysterectomy. The COCP resulted in a small increment in QALYs of 0.04 (95% CI −0.069 to 0.152) over 36 months. The estimated ICER for COCP compared with LARC is £12,280 per QALY, which is within the acceptable threshold of £20,000 per QALY recommended by NICE. 69 Thus, treatment with COCP is likely to be considered the most cost-effective treatment according to standard thresholds.
The results of almost all the (cost–utility) sensitivity analyses bar one, and the subgroup analyses, supported these base case results. They consistently suggest that COCP is more expensive but more effective in terms of QALYs gained compared with the LARC. The only sensitivity analysis carried out that did not support the base case findings was the complete case scenario, which indicated that the LARC was cheaper and more effective compared with COCP in terms of QALYs gained, but this analysis is challenged by the much smaller sample size and the missing data.
It is noteworthy that the QALY improvement with the COCP compared with the LARC for the primary base case analysis was very small at 0.04. In contrast, LARC dominated COCP for all of the secondary outcomes, which included YFC; EHP-30 pain domain score reduction and treatment failure avoided. For all these secondary outcome measures, the results suggest that the LARC group had better outcomes compared with the COCP group, but all are challenged in terms of a robust interpretation because of the extent of missing data.
Comparison with other studies
This study is unique in its focus on the cost-effectiveness of COCP versus LARC for preventing the recurrence of endometriosis in women who have undergone surgery. To the best of our knowledge, no previous study has directly compared these two options. While prior research has examined the cost-effectiveness of different treatment strategies for endometriosis, none has specifically evaluated LARC and COCP in this manner. 41,42
Strengths and limitations of the study
A strength of this study is that it is the first economic evaluation conducted alongside a RCT comparing the relative cost-effectiveness of COCP and LARC to prevent the recurrence of endometriosis in women who have undergone surgery for endometriosis.
In addition, the analysis is that the economic evaluation is based on the outcome of QALYs using the EQ-5D-5L, which had a relatively high degree of completeness of 83% at 36 months. A range of sensitivity analyses were also conducted to test the robustness of our findings and to explore the impact of different assumptions and inputs on the cost-effectiveness results.
A potential limitation is the duration of follow up of 3 years, given that endometriosis is a chronic condition that can recur until menopause82 and the average age of women in the study was 29 years. Also, healthcare resource use information was based on self-reported data, which can potentially be affected by under-reporting. 83
A further potential weakness is that the QALY may not capture all the outcomes that are important to women and could further inform their treatment pathway. Although we undertook analyses based on other secondary outcomes, such as pain score reductions using the EHP-30 questionnaire (which is specific to endometriosis), YFC gained from the ICECAP-A questionnaire and the treatment failure avoided that requires further and more invasive interventions; the high rate of missing data, for these other outcomes, only served to undermine attempts at robust interpretation.
Finally, the high rates of discontinuation and treatment switching among participants posed challenges when attributing outcomes to specific treatments. Nevertheless, an intention-to-treat analysis was employed, sensitivity analyses were carried out and data collection at various time points to address these challenges. This is the most pragmatic approach that can be adopted and provides insight into real-world clinical scenarios, reflecting patient preferences and responses that influence treatment decisions.
Implications for policy
This trial-based economic evaluation suggests that COCP has a 61% probability of being cost-effective compared with the LARC. Confidence in such a probability is likely to be subjective and other factors can impact this. For instance, we found that COCP is associated with a higher risk of further surgery compared with LARCs. In terms of outcomes, both LARC and COCP offer similar benefits in terms of QALYs, with only marginal differences between the two. Therefore, we suggest that both options should be discussed with women. For some, LARCs may become the preferred option, informed by past experiences, acceptability of their invasiveness balanced by lower risks of future surgery.
Recommendations for future research
The complexity of treating endometriosis and the various factors such as patient adherence, variability in clinical practice and comorbidities that can influence outcomes make it challenging to provide a single recommendation with respect to the economic impact of LARC and COCP.
To improve the understanding of the cost-effectiveness of COCP, further exploration of the potential for treatment failure is necessary. However, crucially the emphasis should be placed on improving patient outcomes and alleviating the overall burden of endometriosis on both patients and healthcare systems. Therefore, it is essential to continue researching and identifying opportunities for more effective and cost-effective treatments.
Chapter 6 Discussion
Main findings
Recurrence of pain following surgical treatment, resulting in repeat procedures, has been a long-standing problem in the management of endometriosis. The PRE-EMPT trial sought to compare two commonly used hormonal treatments, LARCs and the COCP, in terms of their effectiveness in reducing pain symptoms as the primary outcome, with overall quality of life and repeat treatments as secondary outcomes.
At 36 months post randomisation, we found comparable levels of pain in women allocated to either LARCs or COCP, with both groups achieving an improvement of around 40% from presurgical levels. We did not find any evidence that the pre-randomisation choice of LARC (LNG-IUS or DMPA) altered these findings. Although COCP likely to be considered more cost-effective at a threshold of £20,000 per QALY, the difference between the two is marginal and LARCs may be preferred by some women as it is associated with lower rates of second-line medical treatment and further surgery.
Clinical interpretation
Our results show that the two LARCs (LNG–IUS and DMPA), as well as the combined oral pill, are similar in terms of prevention of recurrence of pain symptoms at each follow-up interval up to 3 years following endometriosis surgery, irrespective of stage of disease. Both are associated with sustained and significant improvements in pain scores of around 40% compared with presurgical values. Although the primary outcome was no different, women randomised to LARCs underwent fewer repeat surgical procedures (particularly laparoscopic treatment of recurrent endometriosis and hysterectomy) and treatment with GnRH analogues – a fact that reduced the overall costs of treatment in this group. At the same time, COCPs had a marginal advantage in terms of generating QALYs, suggesting that there may be some room for flexibility in decision-making according to women’s choice. The fact that pregnancies occurred in participants in both randomised groups reflects the complex nature of endometriosis, where, alongside symptom relief, fertility is a desired outcome for many women.
After 3 years, 37% of those in the LARC arm were still using their allocated treatment, while 25% of those initially randomised to COCP continued on this treatment. Reasons for this attrition included desire for pregnancy, further surgery for endometriosis or hysterectomy and perceived ineffectiveness or adverse effects prompting a change in medication.
The outcomes of the trial, both clinical and economic, are supportive of instituting a policy of preventing recurrence of endometriosis symptoms after conservative surgery using either LARC or COCP.
Strengths and limitations
Major strengths of this trial include its focus on patient-centred outcomes, a follow-up period of 3 years and the availability of outcome data on over 80% of participants. The pragmatic nature of the trial is more likely to enhance the generalisability of our findings although the predominance of white women in the recruited sample limits our ability to be confident about how our results might apply to women from other ethnic backgrounds. The ability to corroborate self-reported episodes of further treatment through direct interrogation of hospital records was an added strength.
The 3-year follow-up period and the pragmatic design meant that relatively few women continued with their allocated medication and changed to other treatments or stopped altogether, depending on their clinical circumstances. Our strategy of using telephone calls to collect to obtain primary outcome data from those who did not return questionnaires at 3 years, meant that follow-up data on secondary outcomes were available on fewer women.
The prolonged time horizon of this trial, which started recruitment in 2014, means that some aspects of clinical practice have changed, and newer treatments such as modern oral progestogens (such as dienogest) and GnRH antagonist tablets containing add-back HRT were not available for evaluation.
The commissioning brief requested that hormonal treatments were compared, which would be expected to target oestrogen and progestogen-driven mechanisms of pain and suppress recurrence from residual endometriosis lesions. As a pragmatic trial, we did not restrict the use of any analgesics such as non-steroidal anti-inflammatory drugs or neuromodulator drugs, and did not record their use. Investigation of coexistent pain syndromes (e.g. irritable bowel syndrome or bladder pain) was not mandated by the protocol and could confound the results, although should be balanced by randomisation.
The broad nature of the commissioning brief prompted us to opt for an initial four-arm pilot in the first instance, before considerations of equipoise, choice and emerging evidence shaped our design for the definitive study; that is, a two-arm trial comparing two types of LARCs with the combined oral pill, with preplanned subgroup analyses to investigate the mode of delivery of LARC (LNG-IUS or DMPA). In retrospect, this plan seems justified in the light of subsequent clinical guidance recommending both treatments for preventing recurrence of endometriosis following conservative surgery. At the same time, the lack of a no-treatment arm has made it impossible to provide any conclusive data on the sustained impact of surgery alone while treating the two LARC preparations as a single group in any comparisons with COCP has limited the power needed for meaningful comparisons between LNG-IUS and DMPA and COCP.
The open-label nature of the hormonal treatments can cause biased responses to our participant-reported outcome measures. We explored the possibility of dummy interventions but could not ethically proceed with masking the allocation. Any bias could be considered to impact both randomised groups equally. We also dismissed a repeat laparoscopy as an objective outcome as it would involve unnecessary surgical risk and would be unlikely to be acceptable to eligible women.
While all patients were recruited prior to the COVID-19 pandemic, as the follow-up period was for 3 years, the number of women who required further surgery may be underestimated given the negative impact of COVID-19 on elective surgery throughout the UK. It is possible that this may also have led to an increase in the use of GnRHa treatment if recourse to surgical treatment was affected.
Patient and public involvement
Input from patients and the public was crucial in shaping the design of the pilot, as well as the main trial, and in the choice of the primary and secondary outcomes. The fatigue scale was added at the request of patient and public involvement (PPI) feedback. As co-applicant, our lead PPI representative was instrumental in providing a patient-centred perspective to all discussions and decisions on recruitment, follow-up and the use of language within documents aimed at participants. The appreciation that flexibility of LARC choice was critical to recruitment and that certain types of dummy interventions, like an inert intrauterine device, would be unacceptable to women informed our decision to opt for a pragmatic approach to the trial. The transition from the pilot phase to the main trial also involved several decisions which involved close interaction with PPI partners.
Patient and public involvement colleagues also influenced our recruitment and follow-up strategies, especially the decision to opt for telephone follow-up for participants after two unsuccessful attempts to contact them by mail. Finally, input from PPI colleagues has been invaluable in interpreting trial results. As we enter the dissemination phase of this project, we continue to work closely with PPI groups, including Endometriosis UK, and to ensure that we use several complementary routes of communication to engage with patients from all backgrounds and ensure that the key messages from this trial are available to all those with endometriosis, their families and all those who care for them.
Equality, diversity and inclusion
Although we worked closely with our PPI partners in initiating and designing this trial, our hospital-based recruitment policy did not include any specific measures targeted at hard-to-reach populations or racialised groups. This remains a weakness of the trial, along with the fact that we did not include patient information leaflets in languages other than English and relied on normal translation facilities available within the NHS.
Generalisability
As a multicentre trial recruiting from hospitals across the UK, the results of this trial are generalisable across the NHS. This is helped by its pragmatic approach, flexible design to accommodate preferences and the use of medications which are familiar to women and clinicians. These preparations have been used by millions of women worldwide as contraceptives and have an excellent safety record. The trial is unable to comment on drugs which have come into clinical use more recently like dienogest or oral GnRH antagonists containing add-back HRT.
Comparison with the literature
Our findings are consistent with the findings of a recent systematic review and meta-analysis,84 which is supportive of hormonal treatment in preventing post-surgical recurrence of endometriosis in women for whom fertility is not a priority. The literature has progressed significantly since a Cochrane review in 2004,40 which was unable to find any conclusive evidence of benefit of postoperative hormonal suppression compared to surgery alone, based on short duration of follow-up of around 3 months. The role of COCP preparations for post-surgical prevention of recurrence of endometriosis has since been extensively studied85 and COCP has been recommended as the first-line treatments in clinical practice in recent guidelines. Long-acting progestogens like DMPA or LNG-IUS have also been considered to be viable options for women keen to avoid recurrence of endometriosis, while more recent oral progestogen preparations are used more widely. One such is dienogest, a fourth-generation nortestosterone-derived oral progestogen, with anti-oestrogenic, antiproliferative, anti-inflammatory and anti-angiogenic effects, which make it particularly suited to treating endometriosis. 86
The clinical trials register indicates that a phase 3 trial evaluating the use dienogest for treatment of endometriosis is actively recruiting at the time of writing (NCT04256200).
Gonadotropin-releasing hormone analogues, which were used as a second-line medical treatment for endometriosis, have found greater acceptance following regimens involving the addition of add-back HRT. Relugolix, an oral GnRH antagonist that includes add-back HRT, is licensed for treatment of fibroids and offers another possibility for endometriosis treatment. A recently published large, randomised trial has confirmed its effectiveness and acceptability for managing pain from endometriosis. 87 Two other GnRH antagonists with add-back HRT are in phase 3 clinical trials for treatment of endometriosis according to the clinical trials.gov register: linzagolix (NCT03992846) has completed recruitment and elagolix (NCT04333576) is actively recruiting at the time of writing.
The efficacy of hormonal treatments in prevention of endometrioma recurrence has been assessed by two meta-analyses. The first54 demonstrated the superiority of long-term (> 12 months) use of either cyclic or continuous COCPs compared with no treatment. The second88 pooled evidence from three RCTs and one cohort study and showed a lower recurrence rate for dysmenorrhoea following continuous COCP use compared with cyclic regimens. Although these systematic reviews showed a possible benefit of COCPs on recurrence, evidence was based on small sample sizes and the focus was specifically on endometriomas rather recurrence of pain and other hormonal regimens such as dienogest, LNG-IUS and GnRHa were not considered. A more recent systematic review and network meta-analysis that explored the efficacy of hormonal regimens preventing the recurrence of endometrioma ranked LNG-IUS highest, followed by dienogest and GnRHa with LNG-IUS but also found that long-term COCP superior to expectant treatment.
The most recent systematic review84 of post-surgical hormonal suppression for endometriosis, focused on recurrence, either based on imaging or recurrence of symptoms. Follow-up data (median duration 18 months) from 2137 women (13 randomised trials and 4 cohort studies) show a significantly reduced risk of post-surgical recurrence in patients receiving any type of hormonal suppression compared to no treatment or placebo (relative risk 0.41, 95% CI 0.26 to 0.65). This difference was also apparent in subgroup analyses of women treated with COCP or LNG-IUS as well as sensitivity analyses limited to randomised trials.
Implications for health care
Our results support current guidance which recommends hormonal treatment in women who have undergone surgery for endometriosis. While the effects of surgery alone have not been demonstrated, a policy of prescribing either LARCs or the COCP after surgery results in reduced pain scores, maintained over a 3-year period. LARCs resulted in lower rates of further surgery, in particular, surgery for recurrence of endometriosis and hysterectomy equating to lower costs on average, although COCP achieved marginally higher rates of QALYs.
Recommendations for research
-
Assessment of newer medications including dienogest, combination GnRH antagonists with HRT in adequately powered trials with meaningful length of follow-up.
-
Investigating non-hormonal approaches to symptom suppression and management of endometriosis using alternative supportive therapies, such as cannabidiol or fish oil supplements, in conjunction with traditional hormonal and analgesic medication.
-
Identification of biomarkers to allow non-invasive diagnosis of endometriosis and its recurrence to reduce the need for laparoscopy.
-
Finding ways of including a more diverse study population, that is representative of all individuals with endometriosis-associated pain into trials evaluating treatment modalities.
Additional information
CRediT contribution statement
Kevin G Cooper (https://orcid.org/0000-0003-1486-0071) (Chief Investigator, Consultant Gynaecologist): Conceptualisation, Funding acquisition, Investigation, Methodology, Supervision, Writing – Original draft, Writing – reviewing and editing.
Siladitya Bhattacharya (https://orcid.org/0000-0002-4588-356X) (Professor in Obstetrics and Gynaecology): Conceptualisation, Funding acquisition, Investigation, Methodology, Supervision, Writing – Original draft, Writing – reviewing and editing.
Jane P Daniels (https://orcid.org/0000-0003-3324-6771) (Professor of Clinical Trials): Conceptualisation, Investigation, Methodology, Supervision, Writing – Original draft, Writing – reviewing and editing.
Versha Cheed (https://orcid.org/0000-0002-6713-0913) (Statistician): Data curation, Formal analysis, Visualisation.
Laura Gennard (https://orcid.org/0009-0002-6061-041X) (Trial Manager): Investigation, Project administration.
Lisa Leighton (https://orcid.org/0009-0001-2398-6816) (Trial Manager): Investigation, Project administration, Writing – reviewing and editing.
Danielle Pirie (https://orcid.org/0009-0005-6785-4371) (Research Nurse): Investigation, Project administration, Writing – reviewing and editing.
Melyda Melyda (https://orcid.org/0000-0002-6740-4910) (Research Fellow, Health Economics): Data curation, Investigation, Visualisation, Writing – reviewing and editing.
Mark Monahan (https://orcid.org/0000-0002-1175-9421) (Lecturer in Health Economics): Data curation, Investigation, Writing – reviewing and editing.
Annalise Weckesser (https://orcid.org/0000-0002-1468-5464) (Research Fellow): Investigation, Writing – reviewing and editing.
Tracy Roberts (https://orcid.org/0000-0002-0624-0537) (Professor of Health Economics): Conceptualisation, Funding acquisition, Investigation, Methodology, Supervision, Writing – Original draft, Writing – reviewing and editing.
Elaine Denny (https://orcid.org/0000-0002-8118-4301) (Professor of Health Sociology): Conceptualisation, Funding acquisition, Investigation, Methodology, Supervision, Writing – Original draft, Writing – reviewing and editing.
Laura Ocansey (https://orcid.org/0009-0007-7139-7008) (Trial Manager): Investigation, Writing – reviewing and editing.
Clive Stubbs (https://orcid.org/0000-0003-2690-6583) (Trial Management Team Leader, Clinical Trials): Investigation, Project administration, Writing – reviewing and editing.
Emma Cox (https://orcid.org/0009-0000-5106-378X) (Patient and Public Involvement): Investigation, Writing – reviewing and editing.
Georgina Jones (https://orcid.org/0000-0002-5267-1776) (Professor of Health Psychology): Funding acquisition, Validation, Writing – reviewing and editing.
T Justin Clark (https://orcid.org/0000-0002-5943-1062) (Professor of Obstetrics and Gynaecology): Funding acquisition, Investigation, Writing – reviewing and editing.
Ertan Saridogan (https://orcid.org/0000-0001-9736-4107) (Professor of Gynaecological Surgery and Consultant Gynaecologist): Funding acquisition, Investigation, Writing – reviewing and editing.
Janesh K Gupta (https://orcid.org/0000-0003-3052-8423) (Honorary Professor of Obstetrics and Gynaecology): Funding acquisition, Writing – reviewing and editing.
Hilary OM Critchley (https://orcid.org/0000-0003-1913-4044) (Professor of Reproductive Medicine): Funding acquisition, Writing – reviewing and editing.
Andrew Horne (https://orcid.org/0000-0002-9656-493X) (Professor of Gynaecology and Reproductive Sciences): Funding acquisition, Investigation, Writing – reviewing and editing.
Lee J Middleton (https://orcid.org/0000-0003-4621-1922) (Reader in Clinical Trials, Senior Statistician): Conceptualisation, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – Original draft, Writing – reviewing and editing.
Acknowledgements
Members of the PRE-EMPT collaborative group
Kevin G Cooper, Aberdeen Royal Infirmary (Chief Investigator).
Co-investigators
Siladitya Bhattacharya, University of Aberdeen (former Chief Investigator until 30 April 2018); Justin Clark, University of Birmingham; Jane Daniels, University of Nottingham; Lee Middleton, University of Birmingham; Versha Cheed, University of Birmingham; Tracy Roberts, University of Birmingham; Andrew Horne, University of Edinburgh; Janesh Gupta, University of Birmingham; Christian Becker, University of Oxford; Georgina Jones, Leeds Beckett University;
Elaine Denny, Birmingham City University; Hilary Critchley, University of Edinburgh; Catherine Whittall, Robert Jones and Agnes Hunt Orthopaedic and District Hospital NHS Trust; Andrew Prentice, University of Cambridge; Ertan Saridogan, University College London Hospitals NHS Foundation Trust.
Trial Management Group
Kevin G Cooper, Aberdeen Royal Infirmary; Siladitya Bhattacharya, University of Aberdeen; Jane Daniels, University of Nottingham; Jamie Godsall, University of Birmingham; Clive Stubbs, University of Birmingham; Danielle Pirie, Aberdeen Royal Infirmary; Lee Middleton, University of Birmingham; Versha Cheed, University of Birmingham; Laura Ocansey, University of Birmingham; Lisa Leighton, University of Birmingham; Laura Gennard, University of Birmingham; Kirandeep Sunner, University of Birmingham; Mark Monahan, University of Birmingham; Konstantinos Tryposkiadis, University of Birmingham; Max Feltham, University of Birmingham; Rebecca Amos-Hirst, University of Birmingham; Leanne Fulcher, University of Birmingham.
Additional Birmingham Clinical Trials Unit staff
Annika Feilbach, BCTU Programmer.
Adrian Wilcockson, BCTU Programmer.
Recruiting site principal investigators (PI) and support staff
Aberdeen Royal Infirmary: Kevin Cooper (PI), Danielle Pirie, Minimol Paulose.
Addenbrookes Hospital: Andrew Prentice (PI), Amy Sutton.
Arrowe Park Hospital: Thomas Aust (PI), Julie Grindey.
Bedford Hospital: Montasser Mahran (PI), Marina Laverdino.
Birmingham Women’s Hospital: Janesh Gupta (PI), Shanteela McCooty, Fiona Beale, Virginia Iqbal.
Chesterfield Royal Hospital: Jennifer Parratt (PI), Louise Underwood, Mary Kelly Baxter.
City Hospitals Sunderland: Nicholas Matthews (PI), Jane Scollen, Lesley Hewitt.
Crosshouse Hospital: Santanu Acharya (PI), Cheryl Gibson, Debbie Callaghan.
Doncaster Royal Infirmary: Manju Singh (PI), Jane Dumville.
Forth Valley Royal Hospital: Shahzya Huda (PI), Anne Todd, Joanne Donnachie, Shoshana Morecroft.
John Radcliffe Hospital: Christian Becker (PI), Fenella Roseman, Sarah Collins.
Kings Mill Hospital: Jyothi Rajeswary (PI), Caro Moulds, Katie Slack, Rebecca Boulton.
Leicester Royal Infirmary: Tarek Gelbaya (PI), Rupa Modi.
Liverpool Women’s Hospital: George Botros (PI), Elizabeth Kane, Gillian Smith, Kathie Cooke, Pamela Corlett.
Milton Keynes General Hospital: Premila Thampi (PI), Cheryl Padilla, Edel Clare.
Peterborough City Hospital: Bruce Ramsay (PI), Coralie Huson, Jodi Carpenter.
Queens Medical Centre: Martin Powell (PI), Lucinda Wilson, Sophie Crowder.
Royal Albert Edward Infirmary: Philip Harris (PI), Claire Fairhurst, Tracy Taylor.
Royal Infirmary of Edinburgh: Andrew Horne (PI), Ann Doust, Helen Dewart.
Royal Preston Hospital: Brice Rodriquez (PI), Anne Gardner.
Royal Victoria Infirmary: Tony Chalhoub (PI), Alison Kimber.
Southend Hospital: Sanjaya Kalkur (PI), Joanne Galliford, Prisca Gondo, Eunis Mshengu.
St Marys Hospital: Kingshuk Majumder (PI), Christina Pritchard, Clare Waters, Lisa Cornwall, Louise Winter.
St Richards Hospital: Bronwyn Middleton (PI), Emma Meadows, Sally Moore.
Stepping Hill Hospital: Suku George (PI), Jayne Budd.
Stoke Mandeville Hospital: Christopher Wayne (PI), Julie Tebbutt.
The James Cook University Hospital: Pinky Khatri (PI), Mary Hodgers, Marrina Harrison, Helen Harwood, Hazel Alexander.
University College Hospital: Dimitrios Mavrelos (PI), Sarah Ekladios.
University Hospital of North Durham: Seema Sen (PI), Jean Dent, Vicki Atkinson.
University Hospital of North Tees: Dolonchampa Basu (PI), Sharon Gowans, Wendy Cheadle.
West Cumberland Hospital: Ajith Wijesiriwardana (PI), Rachel McCarthy, Toni Wilson, Una Poultney.
Yeovil District Hospital: Ahmar Shah (PI), Dianne Wood, Kerry Rennie.
York Hospital: Fawzia Sanaullah (PI), Deborah Phillips, Holly Alcock, Holly Hancock, Samantha Roche.
Trial Steering Committee
Mary Ann Lumsden (Chair), Glasgow University.
Sian Jones (until 18 June 2018), Bradford Teaching Hospitals NHS Trust.
Ying Cheong, University of Southampton.
Jayne Tullett, Public Member.
Sanjay Vyas, University of Bristol.
Data Monitoring Committee
Patrick Chien, University of Dundee.
Peter Brocklehurst (Chair until 26 September 2016), University of Oxford.
Pollyanna Hardy (until 28 June 2017), University of Oxford.
Lucy Chappell, King College London.
Sally Kerry, Queen Mary University of London.
Sponsor
Grampian Research Office: NHS Grampian and the University of Aberdeen. Monitoring and audit teams led by Juliette Snow, Louise King and Richard Cowie. The authors wish to thank Aisha Khan, Birmingham Clinical Trials Unit, University of Birmingham, for assistance with finalisation of manuscript preparation and submission.
Acknowledging the use of artificial intelligence tools
No artificial intelligence tools were used as part of this submission.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure to protect everyone’s privacy, and it is important that there are safeguards to make sure that data are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted after review.
Ethics statement
The trial initially received clinical trial authorisation (CTA 21583/0219/001-0001) from the Medicines and Healthcare products Regulatory Authority (MHRA) and ethical approval from the North of Scotland Research Ethics Committee (13/NS/0103) in August 2013, before transferring to the East of Scotland Ethics Committee (14/ES1004) in May 2014 for approval of the adapted substantive phase of the trial (IRAS ID 101577).
Information governance statement
The University of Birmingham is committed to handling all personal information in line with the UK Data Protection Act 2018 and the General Data Protection Regulation 2016/679. Under the data protection legislation, the University of Birmingham is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: www.birmingham.ac.uk/privacy.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/SQWY6998.
Primary disclosure of interests: Siladitya Bhattacharya receives royalties from Cambridge University Press and payment (to University of Aberdeen) for speaking at the 11th Singapore International Congress on Obstetrics and Gynaecology, and invited lectures to Merck, Organon and Ferring. He is a non-executive member of NHS Grampian Health Board for which University of Aberdeen receives payment. He is special Senior Editor of Cochrane Gynaecology and Infertility (no honorarium). Jane P Daniels is a member of the NIHR CTU standing advisory committee (May 2016–May 2023). T Justin Clark has received honoraria from Bayer AG for attending one advisory board meeting (2015) and hands-on training of three clinicians in Essure sterilisation (made by Bayer; 2016) and from Gedeon Richter as part of the Orbis educational programme in women’s health (sponsored by Gedeon Richter since 2016) and for travel and accommodation expenses to attend the International Federation of Gynecology and Obstetrics (FIGO) meeting in Rome (2012). He was President of the British Society for Gynaecological Endoscopy (2019–2022). He was a member of the HTA Prioritisation Committee B, in-hospital care (May 2017–July 2022) and HTA Prioritisation Committee C (mental health, women and children’s health) May 2017–July 2022. Hilary OM Critchley has received grant funding from Biotechnology and Biological Sciences Research Council, grants from Medical Research Council/NIHR to support salaries for research staff and study consumables and a research collaboration grant from Bayer AG, Berlin, with salaries for research staff and study consumables. She has personal receipt of royalties from ‘Up-To-Date’ for an article on abnormal uterine bleeding. She has received consulting fees paid to her institution from Bayer AG (consultancy and scientific advisory board advice; no personal remuneration received), Gedeon Richter (consultancy advice; no personal remuneration received) and Myovant Sciences GmbH (consultancy and scientific advisory board advice; with no personal remuneration received). She has received speaker fees from Vifor Pharma UK Ltd (with no personal remuneration received) and travel expenses for attendance(s) at SAB (scientific advisory board). She is Chair (from 2022) of the Committee for Menstrual Disorders and Related Health Impacts of FIGO (no payment received). All other authors do not disclose any interests.
Publications
Middleton LJ, Daniels JP, Weckesser A, et al. Preventing recurrence of endometriosis by means of long-acting progestogen therapy (PRE-EMPT): report of an internal pilot, multi-arm, RCT incorporating flexible entry design and adaption of design based on feasibility of recruitment. Trials 2017;18:121.
Denny E, Weckesser A, Jones G, Bibila S, Daniels J, Bhattacharya S; PRE-EMPT Team. Women’s experiences of medical treatment for endometriosis and its impact on PRE-EMPT trial participation: a qualitative study. Pilot Feasibility Stud 2018;4:168.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Prescott J, Farland LV, Tobias DK, Gaskins AJ, Spiegelman D, Chavarro JE, et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod 2016;31:1475-82.
- Rodriguez MA, Afari N, Buchwald DS. National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol 2009;182:2123-31.
- Tirlapur SA, Kuhrt K, Chaliha C, Ball E, Meads C, Khan KS. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg 2013;11:233-7.
- Chiaffarino F, Cipriani S, Ricci E, Roncella E, Mauri PA, Parazzini F, et al. Endometriosis and inflammatory bowel disease: a systematic review of the literature. Eur J Obstet Gynecol Reprod Biol 2020;252:246-51.
- DiVasta AD, Zimmerman LA, Vitonis AF, Fadayomi AB, Missmer SA. Overlap between irritable bowel syndrome diagnosis and endometriosis in adolescents. Clin Gastroenterol Hepatol 2021;19:528-37.e1.
- Singh SS, Missmer SA, Tu FF. Endometriosis and pelvic pain for the gastroenterologist. Gastroenterol Clin North Am 2022;51:195-211.
- Upson K, Missmer SA. Epidemiology of adenomyosis. Semin Reprod Med 2020;38:89-107.
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med 2020;382:1244-56.
- Eltabbakh GH, Bower NA. Laparoscopic surgery in endometriosis. Minerva Ginecol 2008;60:323-30.
- Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco Nardone F, de Cicco Nardone C, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96:366-73.e8.
- Jones GL, Kennedy SH, Jenkinson C. Health-related quality of life measurement in women with common benign gynecologic conditions: a systematic review. Am J Obstet Gynecol 2002;187:501-11.
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27:1292-9.
- Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, et al. ESHRE Endometriosis Guideline Group . ESHRE guideline: endometriosis. Hum Reprod Open 2022;2022.
- Kuznetsov L, Dworzynski K, Davies M, Overton C, Guideline C. Diagnosis and management of endometriosis: summary of NICE guidance. BMJ 2017;358.
- Gupta D, Hull ML, Fraser I, Miller L, Bossuyt PM, Johnson N, et al. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;2016.
- Nisenblat V, Bossuyt PM, Shaikh R, Farquhar C, Jordan V, Scheffers CS, et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;2016.
- Liu E, Nisenblat V, Farquhar C, Fraser I, Bossuyt PM, Johnson N, et al. Urinary biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2015;2015.
- Khan KS, Tryposkiadis K, Tirlapur SA, Middleton LJ, Sutton AJ, Priest L, et al. MRI versus laparoscopy to diagnose the main causes of chronic pelvic pain in women: a test-accuracy study and economic evaluation. Health Technol Assess 2018;22:1-92.
- Lamvu G, Carrillo J, Ouyang C, Rapkin A. Chronic pelvic pain in women: a review. JAMA 2021;325:2381-91.
- American Society for Reproductive Medicine . Revised American Society for reproductive medicine classification of endometriosis. Fertil Steril 1997;67:817-21.
- Schliep KC, Mumford SL, Peterson CM, Chen Z, Johnstone EB, Sharp HT, et al. Pain typology and incident endometriosis. Hum Reprod 2015;30:2427-38.
- Duffy JMN, Arambage K, Correa FJS, Olive D, Farquhar C, Garry R, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev 2014;4.
- Burks C, Lee M, DeSarno M, Findley J, Flyckt R. Excision versus ablation for management of minimal to mild endometriosis: a systematic review and meta-analysis. J Minim Invasive Gynecol 2021;28:587-97.
- Vercellini P, Crosignani PG, Abbiati A, Somigliana E, Viganò P, Fedele L. The effect of surgery for symptomatic endometriosis: the other side of the story. Hum Reprod Update 2009;15:177-88.
- Cheong Y, Tay P, Luk F, Gan HC, Li TC, Cooke I. Laparoscopic surgery for endometriosis: how often do we need to re-operate?. J Obstet Gynaecol 2008;28:82-5.
- Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol 2008;111:1285-92.
- Saraswat L, Ayansina D, Cooper KG, Bhattacharya S, Horne AW, Bhattacharya S. Impact of endometriosis on risk of further gynaecological surgery and cancer: a national cohort study. BJOG 2018;125:64-72.
- Chiu CC, Hsu TF, Jiang LY, Chan IS, Shih YC, Chang YH, et al. Maintenance therapy for preventing endometrioma recurrence after endometriosis resection surgery: a systematic review and network meta-analysis. J Minim Invasive Gynecol 2022;29:602-12.
- Valle RF, Sciarra JJ. Endometriosis: treatment strategies. Ann N Y Acad Sci 2003;997:229-39.
- Vercellini P, Crosignani PG, Fadini R, Radici E, Belloni C, Sismondi P. A gonadotrophin-releasing hormone agonist compared with expectant management after conservative surgery for symptomatic endometriosis. Br J Obstet Gynaecol 1999;106:672-7.
- Crosignani PG, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod 2006;21:248-56.
- Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril 2003;80:305-9.
- Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril 2008;90:1583-8.
- Koga K, Takemura Y, Osuga Y, Yoshino O, Hirota Y, Hirata T, et al. Recurrence of ovarian endometrioma after laparoscopic excision. Hum Reprod 2006;21:2171-4.
- Vercellini P, Somigliana E, Daguati R, Vigano P, Meroni F, Crosignani PG. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol 2008;198.
- Seracchioli R, Mabrouk M, Manuzzi L, Vicenzi C, Frasca C, Elmakky A, et al. Post-operative use of oral contraceptive pills for prevention of anatomical relapse or symptom-recurrence after conservative surgery for endometriosis. Hum Reprod 2009;24:2729-35.
- Koga K, Takamura M, Fujii T, Osuga Y. Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil Steril 2015;104:793-801.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst Rev 2013;1.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev 2012;2012.
- Yap C, Furness S, Farquhar C. Pre and post operative medical therapy for endometriosis surgery. Cochrane Database Syst Rev 2004;2004.
- Darba J, Marsa A. Economic implications of endometriosis: a review. Pharmacoeconomics 2022;40:1143-58.
- Grand TS, Basarir H, Jackson LJ. The cost-effectiveness of oral contraceptives compared to ‘no hormonal treatment’ for endometriosis-related pain: an economic evaluation. PLOS ONE 2019;14.
- Middleton LJ, Daniels JP, Weckesser A, Bhattacharya S. PRE-EMPT Trial Collaborative Group . Preventing recurrence of endometriosis by means of long-acting progestogen therapy (PRE-EMPT): report of an internal pilot, multi-arm, randomised controlled trial incorporating flexible entry design and adaption of design based on feasibility of recruitment. Trials 2017;18.
- Denny E, Weckesser A, Jones G, Bibila S, Daniels J, Bhattacharya S, et al. Women’s experiences of medical treatment for endometriosis and its impact on PRE-EMPT trial participation: a qualitative study. Pilot Feasibility Stud 2018;4.
- BSGE Survey of Current Post-operative Hormonal Treatment for Endometriosis 2012.
- National Institute for Health and Care Excellence . NICE Clinical Knowledge Summaries: Endometriosis 2014.
- Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci 2011;13:217-24.
- DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med 1994;13:1341-52.
- Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008;61:102-9.
- Riessman CK. Narrative Methods for the Human Sciences. Newbury Park, CA: SAGE Publications Ltd; 2008.
- Bryman A. Social Research Methods. Oxford: Oxford University Press; 2016.
- Noble H, Smith J. Issues of validity and reliability in qualitative research. Evid Based Nurs 2015;18:34-5.
- Wu L, Wu Q, Liu L. Oral contraceptive pills for endometriosis after conservative surgery: a systematic review and meta-analysis. Gynecol Endocrinol 2013;29:883-90.
- Vercellini P, De Matteis S, Somigliana E, Buggio L, Frattaruolo MP, Fedele L. Long-term adjuvant therapy for the prevention of postoperative endometrioma recurrence: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2013;92:8-16.
- Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA 1996;276:1253-8.
- Damocles Study Group NHSHTAP . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22.
- Joint Formulary Committee . British National Formulary 78 2019.
- Jones G, Jenkinson C, Kennedy S. Evaluating the responsiveness of the Endometriosis Health Profile Questionnaire: the EHP-30. Qual Life Res 2004;13:705-13.
- Jones G, Jenkinson C, Taylor N, Mills A, Kennedy S. Measuring quality of life in women with endometriosis: tests of data quality, score reliability, response rate and scaling assumptions of the Endometriosis Health Profile Questionnaire. Hum Reprod 2006;21:2686-93.
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121-3.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15.
- Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res 2012;21:167-76.
- Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester: Wiley; 2006.
- Cro S, Morris TP, Kenward MG, Carpenter JR. Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: a practical guide. Stat Med 2020;39:2815-42.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinicaltrials requiring prolonged observation of each patient. 1. Introduction and design. Br J Cancer 1976;34:585-612.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342.
- Sanghera S, Barton P, Bhattacharya S, Horne AW, Roberts TE;. PRE-EMPT Research Group . Pharmaceutical treatments to prevent recurrence of endometriosis following surgery: a model-based economic evaluation. BMJ Open 2016;6.
- National Institute for Health and Care Excellence . NICE Health Technology Evaluations: The Manual 2022.
- Economic Analysis in Health Care. Chichester: Wiley; 2007.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96.
- Jones G, Kennedy S, Barnard A, Wong J, Jenkinson C. Development of an endometriosis quality-of-life instrument: the Endometriosis Health Profile-30. Obstet Gynecol 2001;98:258-64.
- Office for National Statistics . Annual Survey of Hours and Earnings 2022.
- NHS England . National Cost Collection: National Schedule of NHS Costs - Year 2020-21 - NHS Trust and NHS Foundation Trusts 2021. https://www.england.nhs.uk/publication/2020-21-national-cost-collection-data-publication/ (accessed July 2024).
- Jones K, Weatherly HLA, Birch S, Castelli A, Chalkley MJ, Dargan A, et al. Unit Costs of Health and Social Care Manual. Canterbury: Personal Social Services Research Unit; 2022.
- Joint Formulary Committee . British National Formulary 84 2022.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Chichester: Wiley; 2004.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics 2014;32:1157-70.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-4.
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8.
- NHS . Overview: Endometriosis n.d. www.nhs.uk/conditions/endometriosis (accessed 20 March 2023).
- Petrou S, Murray L, Cooper P, Davidson LL. The accuracy of self-reported healthcare resource utilization in health economic studies. Int J Technol Assess Health Care 2002;18:705-10.
- Zakhari A, Delpero E, McKeown S, Tomlinson G, Bougie O, Murji A. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update 2021;27:96-107.
- Grandi G, Barra F, Ferrero S, Sileo FG, Bertucci E, Napolitano A, et al. Hormonal contraception in women with endometriosis: a systematic review. Eur J Contracept Reprod Health Care 2019;24:61-70.
- Barra F, Scala C, Ferrero S. Current understanding on pharmacokinetics, clinical efficacy and safety of progestins for treating pain associated to endometriosis. Expert Opin Drug Metab Toxicol 2018;14:399-415.
- Giudice LC, As-Sanie S, Arjona Ferreira JC, Becker CM, Abrao MS, Lessey BA, et al. Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: two replicate phase 3, randomised, double-blind, studies (SPIRIT 1 and 2). Lancet 2022;399:2267-79.
- Muzii L, Di Tucci C, Achilli C, Di Donato V, Musella A, Palaia I, et al. Continuous versus cyclic oral contraceptives after laparoscopic excision of ovarian endometriomas: a systematic review and metaanalysis. Am J Obstet Gynecol 2016;214:203-11.
Appendix 1
1. Past medical treatment experiences
|
| 2. Views on medical treatments offered in PRE-EMPT |
After undergoing surgery there are four possible treatments – how do you feel about:
|
3. Views on medical trials (General)
|
4. Views on participation in PRE-EMPT trial
|
5. Concluding questions
|
| (n = 14) | ||
|---|---|---|
| Age, years | Mean (SD) | 27.9 (5.7) |
| Ethnic group, n (%) | White British | 12 (86) |
| Black/Black British Caribbean | 1 (7) | |
| Asian/Asian British Pakistani | 1 (7) | |
| Parity, n (%) | 0 | 12 (86) |
| 1 | 1 (7) | |
| 2 | 1 (7) | |
| Employment status, n (%) | Full-time | 10 (72) |
| Part-time | 1 (7) | |
| Unemployed | 1 (7) | |
| Student | 2 (14) | |
| Previous treatment experiences with LNG-IUS, DMPA or COCP, n (%) | All | 2 (14) |
| LNG-IUS and COCP | 1 (7) | |
| DMPA and COCP | 5 (36) | |
| COCP | 6 (43) | |
| Stage of endometriosis, n (%) | I | 5 (35) |
| II | 4 (29) | |
| III | 4 (29) | |
| IV | 1 (7) | |
| Number of previous laparoscopies, n (%) | 0 | 7 (50) |
| 1 | 5 (36) | |
| 2 | 2 (14) | |
| Extent of excision as judged by surgeon, n (%) | Complete | 11 (79) |
| EHP-30 pain score at baseline | Mean (SD) | 59.7 (9.7) |
Appendix 2
| LARC (N = 205), n (%) | COCP (N = 200), n (%) | |
|---|---|---|
| Aberdeen Royal Infirmary | 33 (16) | 32 (16) |
| Milton Keynes General Hospital | 23 (11) | 20 (10) |
| Royal Infirmary of Edinburgh | 22 (11) | 21 (11) |
| James Cook University Hospital | 19 (9) | 15 (8) |
| John Radcliffe Hospital | 15 (7) | 14 (7) |
| Liverpool Women’s Hospital | 14 (7) | 14 (7) |
| Birmingham Women’s Hospital | 12 (6) | 16 (8) |
| University College Hospital, London | 7 (3) | 6 (3) |
| University Hospital of North Tees | 5 (2) | 7 (4) |
| Yeovil District Hospital | 4 (2) | 6 (3) |
| Chesterfield Royal Hospital | 5 (2) | 5 (3) |
| Royal Victoria Infirmary | 4 (2) | 5 (3) |
| St Mary’s Hospital, Manchester | 4 (2) | 5 (3) |
| Southend Hospital | 4 (2) | 3 (1) |
| Peterborough City Hospital | 4 (2) | 3 (1) |
| Stoke Mandeville Hospital | 1 (< 1) | 5 (3) |
| Bedford Hospital | 3 (1) | 3 (1) |
| Arrowe Park Hospital | 3 (1) | 2 (1) |
| Kings Mill Hospital | 4 (2) | 1 (< 1) |
| City Hospitals Sunderland | 1 (< 1) | 4 (2) |
| Forth Valley Royal Hospital | 4 (2) | 0 (–) |
| Royal Albert Edward Infirmary | 1 (< 1) | 2 (1) |
| Crosshouse Hospital | 1 (< 1) | 2 (1) |
| University Hospital of North Durham | 3 (1) | 0 (–) |
| Royal Preston Hospital | 2 (1) | 1 (< 1) |
| Queens Medical Centre | 1 (< 1) | 2 (1) |
| Addenbrooke’s Hospital | 1 (< 1) | 1 (< 1) |
| Leicester Royal Infirmary | 1 (< 1) | 1 (< 1) |
| Stepping Hill Hospital | 0 (–) | 2 (1) |
| St Richard’s Hospital | 0 (–) | 2 (1) |
| York Hospital | 1 (< 1) | 0 (–) |
| Cumberland Infirmary | 1 (< 1) | 0 (–) |
| West Cumberland Hospital | 1 (< 1) | 0 (–) |
| Doncaster Royal Infirmary | 1 (< 1) | 0 (–) |
| Reasons | N |
|---|---|
| Eligible but declined consent | 288 |
| Declined due to treatment preference | 326 |
| Preference for LNG-IUS | 130 |
| Preference for COCP | 55 |
| Had LNG-IUS and DMPA before, does not wish it again | 38 |
| Does not want any medical treatment | 36 |
| Preference for DMPA | 25 |
| Not willing to have any LARC | 22 |
| Does not like any of the treatment options | 20 |
| Ineligible | 1839 |
| Plans to conceive in the immediate future | 355 |
| Contraindications to the use of hormonal treatment with oestrogen or progestogens | 221 |
| No endometriosis identified at diagnostic laparoscopy | 180 |
| Deep infiltrating endometriosis requiring additional surgery | 145 |
| Age outside range of 16–46 years | 121 |
| Undergoing infertility treatment | 111 |
| History of drug sensitivity to COCP | 89 |
| Drug sensitivity to contraceptive pill | 68 |
| Patient did not attend | 45 |
| Other gynaecological treatment offered | 28 |
| Not able to understand written and spoken English | 12 |
| Contradiction to DMPA | 3 |
| Any other reason | 461 |
| Total | 2453 |
| LNG-IUS, n (%) | DMPA, n (%) | |
|---|---|---|
| N (total) | 91 | 114 |
| During surgery | 77 (85) | 11 (10) |
| Before discharge | 0 (–) | 58 (51) |
| Referred to GP/sexual health clinic | 5 (5) | 28 (25) |
| Failure to fit/administer | 2 (2) | 0 (–) |
| Declined | 0 (–) | 2 (2) |
| Missing | 7 | 15 |
| COCP, n (%) | |
|---|---|
| N (Total) | 200 |
| First cycle of tablets given/prescription dispensed in hospital/Prescription dispensed in hospital | 103 (52) |
| Referred to GP/sexual health clinic | 66 (33) |
| Declined | 2 (1) |
| Missing | 29 |
| Not on assigned treatment | On assigned treatment | ||||||
|---|---|---|---|---|---|---|---|
| Taking any other trial treatment (N = 44)a (n) | Taking any other non-trial treatment (N = 5)b (n) | Taking any other trial and non-trial treatment (N = 10)c (n) | Plus any other trial treatment (N = 12)d (n) | Plus any other non-trial treatment (N = 4)e (n) | Plus any other trial and non-trial treatment (N = 1)f (n) | Not taking any treatment (N = 71) (n) | |
| Lack of effectiveness | 7 | 1 | 4 | 3 | 0 | 1 | 17 |
| Did not control my bleeding | 4 | 1 | 3 | 2 | 0 | 0 | 13 |
| Irregular bleeding | 5 | 1 | 2 | 3 | 0 | 0 | 8 |
| Prolonged bleeding | 5 | 1 | 3 | 2 | 0 | 0 | 7 |
| Coil expulsion | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pelvic infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Disliked treatment | 7 | 2 | 3 | 1 | 1 | 0 | 10 |
| Tummy upset | 3 | 1 | 3 | 1 | 0 | 0 | 3 |
| Disliked taking tablets | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Vomiting/diarrhoea | 2 | 1 | 0 | 0 | 0 | 0 | 1 |
| Skin allergy | 3 | 1 | 1 | 1 | 0 | 0 | 6 |
| Depression/mood swings | 10 | 1 | 5 | 2 | 1 | 0 | 18 |
| Weight gain | 11 | 3 | 2 | 2 | 1 | 0 | 14 |
| Thread problems | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Headaches/migraine | 6 | 1 | 1 | 2 | 1 | 0 | 10 |
| Dizziness | 2 | 1 | 0 | 2 | 0 | 0 | 7 |
| Hypertension/increased blood pressure | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pelvic pain | 8 | 1 | 4 | 3 | 0 | 0 | 17 |
| Trying to conceive | 2 | 0 | 1 | 0 | 0 | 0 | 9 |
| Pregnant | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Other reasons | 13 | 3 | 2 | 2 | 0 | 1 | 16 |
| Not on assigned treatment | On assigned treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taking any other trial treatment (N = 37)a (n) | Taking any other non-trial treatment (N = 8)b (n) | Taking any other trial and non-trial treatment (N = 1)c (n) | Plus any other trial treatment (N = 4)d (n) | Plus taking any other non-trial treatment (N = 8)e (n) | Plus any other trial and non-trial treatment (N = 0) (n) | Not taking any treatment (N = 75) (n) | ||||
| Lack of effectiveness | 18 | 0 | 1 | 0 | 4 | 0 | 21 | |||
| Did not control my bleeding | 12 | 0 | 1 | 1 | 1 | 0 | 10 | |||
| Irregular bleeding | 13 | 0 | 1 | 1 | 1 | 0 | 7 | |||
| Prolonged bleeding | 8 | 0 | 1 | 1 | 2 | 0 | 11 | |||
| Coil expulsion | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Pelvic infection | 2 | 0 | 0 | 0 | 0 | 0 | 2 | |||
| Disliked treatment | 2 | 0 | 0 | 0 | 1 | 0 | 10 | |||
| Tummy upset | 3 | 0 | 0 | 0 | 0 | 0 | 10 | |||
| Disliked taking tablets | 0 | 0 | 0 | 0 | 0 | 0 | 5 | |||
| Vomiting/diarrhoea | 4 | 0 | 0 | 0 | 0 | 0 | 3 | |||
| Skin allergy | 2 | 0 | 0 | 0 | 1 | 0 | 1 | |||
| Depression/mood swings | 10 | 1 | 1 | 0 | 3 | 0 | 22 | |||
| Weight gain | 5 | 0 | 1 | 0 | 1 | 0 | 7 | |||
| Thread problems | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Headaches/migraine | 7 | 0 | 1 | 0 | 1 | 0 | 14 | |||
| Dizziness | 3 | 0 | 0 | 0 | 1 | 0 | 5 | |||
| Hypertension/increased blood pressure | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |||
| Pelvic pain | 12 | 0 | 0 | 0 | 1 | 0 | 11 | |||
| Trying to conceive | 1 | 1 | 0 | 0 | 0 | 0 | 7 | |||
| Pregnant | 2 | 0 | 0 | 0 | 0 | 0 | 2 | |||
| Other reasons | 7 | 2 | 0 | 1 | 1 | 0 | 16 | |||
| Adjusted mean difference (95% CI)b | |
|---|---|
| Multiple imputation: with delta value of 20% of average increase in both groups at each time pointa | −1.0 (−6.0 to 4.0) |
| Multiple imputation: MAR with delta value of 20% of average increase in the LARC group at each time point; 10% in the COCP treatment groupa | −1.1 (−6.0 to 3.9) |
| Multiple imputation: MAR with delta value of 10% of average increase in the LARC group at each time point; 20% in the COCP treatment groupa | −0.9 (−5.9 to 4.1) |
| Analysis removing any late responsesa | −2.2 (−7.4 to 3.0) |
| LARC, Mean (SD), n | No treatment, Mean (SD), n | |
|---|---|---|
| Baseline | 58.6 (16.0), 31 | 52.0 (18.7), 29 |
| 6 months | 33.1 (20.0), 25 | 36.7 (23.8), 26 |
| 1 year | 29.7 (23.7), 23 | 38.7 (28.2), 27 |
| 2 years | 28.0 (25.4), 21 | 36.5 (33.5), 24 |
| 3 years | 30.7 (23.5), 17 | 31.5 (33.0), 21 |
| LARC, N (%) | COCP, N (%) | |
|---|---|---|
| 6 months | ||
| Yes | 87 (54) | 116 (76) |
| No | 74 (46) | 36 (24) |
| Total | N = 161 | N = 152 |
| 1 year | ||
| Yes | 81 (54) | 108 (70) |
| No | 70 (46) | 46 (30) |
| Total | N = 151 | N = 154 |
| 2 years | ||
| Yes | 54 (43) | 72 (65) |
| No | 72 (57) | 38 (35) |
| Total | N = 126 | N = 110 |
| 3 years | ||
| Yes | 51 (51) | 62 (63) |
| No | 50 (50) | 36 (37) |
| Total | N = 101 | N = 98 |
| LARC, N (%) | COCP, N (%) | Adjusted ORa (95% CI) | |
|---|---|---|---|
| Baseline | |||
| Regular | 23 (20) | 23 (18) | |
| Fairly regular | 37 (32) | 36 (28) | |
| Irregular | 34 (29) | 39 (31) | |
| Bleeding on and off | 23 (20) | 29 (23) | |
| Total | N = 117 | N = 127 | |
| 6 months | |||
| Regular | 6 (7) | 26 (23) | |
| Fairly regular | 23 (26) | 39 (34) | |
| Irregular | 35 (40) | 31 (27) | |
| Bleeding on and off | 23 (23) | 18 (16) | |
| Total | N = 87 | N = 114 | 0.32 (0.16 to 0.63)b |
| 1 year | |||
| Regular | 12 (15) | 25 (23) | |
| Fairly regular | 27 (34) | 39 (36) | |
| Irregular | 28 (35) | 29 (27) | |
| Bleeding on and off | 13 (16) | 14 (13) | |
| Total | N = 80 | N = 107 | 0.73 (0.39 to 1.39)b |
| 2 years | |||
| Regular | 9 (17) | 17 (24) | |
| Fairly regular | 22 (42) | 27 (38) | |
| Irregular | 19 (36) | 18 (25) | |
| Bleeding on and off | 3 (6) | 9 (13) | |
| Total | N = 53 | N = 71 | 0.94 (0.39 to 2.23)b |
| 3 years | |||
| Regular | 12 (25) | 13 (21) | |
| Fairly regular | 15 (31) | 16 (26) | |
| Irregular | 15 (31) | 24 (39) | |
| Bleeding on and off | 6 (13) | 8 (13) | |
| Total | N = 48 | N = 61 | 1.43 (0.63 to 3.24)b |
| LARC, N | COCP, N | |
|---|---|---|
| No. of pregnancies | 17 | 24 |
| No. of deliveries | 12 | 18 |
| Normal | 12 | 18 |
| Abnormal | 0 | 0 |
| Stillbirth | 0 | 0 |
| Mode of delivery | 12 | 18 |
| Normal | 5 | 11 |
| Forceps/Ventouse | 2 | 3 |
| Caesarean | 5 | 4 |
| No. of terminations/miscarriages | 4 | 5 |
| Termination: therapeutic | 1 | 0 |
| Termination: planned | 2 | 2 |
| Miscarriage | 1 | 3 |
Appendix 3
| Time point | LARC group (N = 205) | COCP group (N = 200) | Bootstrap adjusted mean difference (95% CI) | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Complete case | |||||
| Baseline EQ-5D-5L score | 198 | 0.626 (0.240) | 190 | 0.634 (0.237) | 0.008 (−0.039 to 0.057) |
| Month 6 EQ-5D-5L score | 160 | 0.678 (0.236) | 149 | 0.674 (0.276) | −0.009 (−0.059 to 0.041) |
| Month 12 EQ-5D-5L score | 151 | 0.671 (0.280) | 152 | 0.674 (0.248) | 0.010 (−0.042 to 0.072) |
| Month 24 EQ-5D-5L score | 157 | 0.673 (0.280) | 141 | 0.687 (0.270) | 0.011 (−0.047 to 0.068) |
| Month 36 EQ-5D-5L score | 176 | 0.693 (0.266) | 167 | 0.686 (0.287) | −0.008 (−0.062 to 0.044) |
| Total complete case QALYs | 115 | 1.975 (0.602) | 99 | 1.945 (0.645) | −0.032 (−1.634 to 0.093) |
| Imputed case | |||||
| Baseline EQ-5D-5L score | 205 | 0.625 (0.237) | 200 | 0.636 (0.232) | −0.011 (−0.057 to 0.031) |
| Month 6 EQ-5D-5L score | 205 | 0.670 (0.221) | 200 | 0.668 (0.256) | 0.007 (−0.034 to 0.052) |
| Month 12 EQ-5D-5L score | 205 | 0.664 (0.252) | 200 | 0.678 (0.222) | −0.009 (−0.050 to 0.032) |
| Month 24 EQ-5D-5L score | 205 | 0.668 (0.254) | 200 | 0.701 (0.238) | −0.028 (−0.071 to 0.016) |
| Month 36 EQ-5D-5L score | 205 | 0.694 (0.251) | 200 | 0.691 (0.265) | 0.007 (−0.041 to 0.049) |
| Total imputed QALYs | 205 | 1.937 (0.550) | 200 | 1.976 (0.576) | 0.043 (−0.069 to 0.152) |
List of abbreviations
- BCTU
- Birmingham Clinical Trials Unit
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COCP
- combined oral contraceptive pill
- DMC
- Data Monitoring Committee
- DMPA
- depot medroxyprogesterone acetate
- EHP-30
- Endometriosis Health Profile-30
- EQ-5D-5L
- EuroQol-5 Dimensions, five levels
- FSS
- Fatigue Severity Scale
- GnRH
- gonadotropin-releasing hormone
- GnRHa
- gonadotropin-releasing hormone analogues
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HRT
- hormone replacement therapy
- ICECAP-A
- ICEpop CAPability for Adults
- ICER
- incremental cost-effectiveness ratio
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LARC
- long-acting reversible contraceptive
- LNG-IUS
- levonorgestrel-releasing intra-uterine system
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- OR
- odds ratio
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- SAE
- serious adverse event
- SD
- standard deviation
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- YFC
- years of full capability
