Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the Evidence Synthesis Programme on behalf of NICE as award number NIHR135636. The protocol was agreed in September 2022. The draft manuscript began editorial review in January 2023 and was accepted for publication in January 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Shabaninejad et al. This work was produced by Shabaninejad et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Shabaninejad et al.
Chapter 1 Background and definition of decision problem
Background to decision problem
Infection can develop into sepsis, which is the body’s potentially life-threatening response to an infection. Sepsis and bacterial infections are significant causes of mortality and morbidity in neonates (up to and including a corrected gestational age of 28 days). Expert opinion suggests that the incidence of culture-confirmed neonatal infection is around 1 in 2000 deliveries. But a larger proportion of babies will go on to receive precautionary antibiotic treatment for suspected infection. For example, of every 1000 blood culture samples taken in neonatal intensive care units (NICUs) 2020–2, approximately 30–60 were positive. 1
Prevalence of m.1555A>G variant and risk of aminoglycoside-induced hearing loss
Neonates with suspected infection are commonly treated with gentamicin, an antibiotic of the aminoglycoside family. These antibiotics are associated with a very high risk of damage to the ear (ototoxicity), including profound bilateral deafness, in people with the MT-RNR1 gene m.1555A>G mitochondrial variant. 2,3
Cohort studies in various countries suggest that the variant is rare. For example, in the UK, Rahman et al. have found similar prevalence rates of m.1555A>G in two representative samples of the UK population: the Avon Longitudinal Study of Parents And Children (ALSPAC) [0.19%, 95% confidence interval (CI) 0.10 to 0.28; 18/9371 participants]4 and the 1958 Birth Cohort study (0.26%, 95% CI 0.14% to 0.38%; 19/7350 participants). 5
Given these low prevalence rates, it is unsurprising that aminoglycoside-induced hearing loss (AIHL) has been investigated primarily in case–control studies, in families who have experienced hearing impairment due to maternal inheritance of the m.1555A>G variant. These studies have found that all people exposed to aminoglycosides experienced hearing loss. 2,3 However, these studies’ designs are likely to have overestimated the risk of aminoglycoside exposure. Cohort studies of hearing loss in people with the m.1555A>G genetic variant in broader populations (e.g. preterm infants, neonates in NICUs not selected on the basis of existing hearing impairment) have suggested greater uncertainty about the risk of AIHL.
A German study of preterm infants found that only 3 out of 10 infants with the m.1555A>G variant and exposed to aminoglycosides failed the newborn hearing screening test. 6 Two American studies conducted in NICUs also suggest that not all infants with the variant and exposed to aminoglycosides experienced hearing loss. Ealy et al. 20117 identified two infants with the m.1555A>G genetic variant who received aminoglycosides. Both passed the newborn hearing screening test. Johnson et al. 20108 identified three infants with the m.1555A>G genetic variant, all of whom were exposed to aminoglycosides. Only one of these infants failed the newborn hearing screening test.
However, these studies also have multiple limitations. For example, later hearing loss due to neonatal exposure to aminoglycosides cannot be ruled out in those infants who passed newborn hearing screening tests. In addition, these studies are based on very small samples of people with the m.1555A>G variant. Therefore, there is substantial uncertainty regarding how many neonates with the m.1555A>G variant and exposed to aminoglycosides are likely to experience hearing loss.
m.1555A>G variant and nonsyndromic hearing loss (without exposure to aminoglycosides)
The prevalence of nonsyndromic hearing loss in people with the m.1555A>G variant is a further uncertainty.
Case–control studies in people with the m.1555A>G genetic variant experiencing hearing impairment suggest that AIHL may not explain all hearing impairment in these populations. For example, one Spanish study2 found that 65% (45/69) of families who carried the variant experienced hearing impairment despite having no exposure to aminoglycosides. In another case–control study of 70 Spanish families, Estivill et al. 3 estimated that 39.9% of carriers of the variant who were not exposed to aminoglycosides still experienced hearing loss. However, these authors found a much lower median age for hearing loss among those treated with aminoglycosides (5 years) than among those not treated with aminoglycosides (20 years).
As indicated above, case–control studies may overestimate the risk of nonsyndromc hearing loss. For example, no evidence of hearing loss was found in people with the m.1555A>G variant in two UK population cohort studies conducted by Rahman et al. 4,5 However, no data on aminoglycoside use were available, and the sample of people with the variant was small in both studies. The Australian Blue Mountains Hearing Study had contrasting findings. Six participants (total sample size n = 2856 participants) identified with the m.1555A>G variant all experienced hearing loss, yet none reported aminoglycoside use. After statistical adjustment, three of six carriers of the m.1555A>G variant were found to have mean auditory thresholds higher than those of the general population.
Maternal inheritance of m.1555A>G variant
As it is a variant of mitochondrial deoxyribonucleic acid (mtDNA), the m.1555A>G variant is inherited maternally. Mitochondrial DNA variants are commonly heteroplasmic (when mtDNA varies widely within the same cell and mitochondrion). Therefore, most children have similar but not identical mtDNA to their mothers and other maternal relatives. However, some mitochondrial variants are homoplasmic (when all or most copies are identical throughout mtDNA), resulting in greater penetrance of the variant.
Most studies of this variant (e.g. Matsunaga et al. 9) have found that people are homoplasmic for the G allele. However, people with a heteroplasmic variant have been identified in several studies, including one in Spanish families with m.1555A>G and hearing impairment10 and a large genetic screening study (n = 24,349 neonates) in a Chinese hospital. 11 Del Castillo et al. 10 found that in six families there were 19 people had a heteroplasmic variant and 12 people had a homoplasmic variant. The proportion of variant copies differed widely in the heteroplasmic participants (3.75–96.60%). Although del Castillo et al. found correlations between variant load and hearing thresholds, the small sample size makes these data difficult to interpret. Luo et al. found that, of 46 neonates, most (n = 39) with m.1555A>G were homoplasmic and 7 were heteroplasmic. 11
Description of current practice
MT-RNR1 testing is more commonly conducted retrospectively, although prospective testing is currently used for people who have a predisposition to Gram-negative infections. Current genetic testing varies between different laboratories but may include techniques, such as restriction enzyme assay and sequence analysis. Laboratory testing is estimated to take 2–6 weeks. Such testing is unable to provide results within the time frame required to impact treatment for infection or sepsis, as antibiotics are recommended within 1 hour of the decision to treat. The company states that the Genedrive MT-RNR1 ID Kit has a run time of 26 minutes. Therefore, this technology has the potential to identify those at most risk of ototoxicity from aminoglycoside antibiotics and to inform treatment decisions within the time frame recommended by National Institute for Health and Care Excellence (NICE) guidance.
Description of interventions
This assessment evaluated whether the Genedrive MT-RNR1 ID Kit can be used to assess the presence of the m.1555A>G variant in neonates with suspected infection or sepsis or in mothers prior to giving birth. This technology aims to identify those with the m.1555A>G gene variant. The test requires a buccal swab sample. The test is reported to take 26 minutes to complete, fitting in the time frame of antibiotic prescribing within 1 hour of identification of possible infection or sepsis. There are no other tests of a similar nature that can accomplish this. The Genedrive MT-RNR1 ID Kit would therefore be the first of its kind to be used as a point-of-care test in practice, with the possibility of informing prescribing decisions.
Population and relevant subgroups
The population under consideration was neonates with suspected infection or sepsis who need antibiotics (i.e. a decision to start antibiotics has already been made) or who were anticipated to need antibiotics (i.e. a decision to start antibiotics has not already been made), as well as mothers of neonates who are at risk of sepsis prior to giving birth.
Where data permitted, the following subgroups were to be considered:
-
neonates who need antibiotic treatment (i.e. a decision to start antibiotics has already been made)
-
neonates who are anticipated to need antibiotics (i.e. a decision to start antibiotics has not already been made)
-
babies of different ethnicities
-
babies with early-onset neonatal infection
-
babies with late-onset neonatal infection.
However, there were insufficient data to consider any of these subgroups.
Place of intervention in current pathway: treatment for neonatal infections and sepsis
National Institute for Health and Care Excellence guidance (NG195) is available on the antibiotic treatment of suspected infections and sepsis for neonates. 12 Investigations prior to starting antibiotics include a blood culture to test for bacteria in the blood, the measurement of baseline C-reactive protein concentration and, if safe, a lumbar puncture when there is a strong clinical suspicion of early-onset neonatal infection and clinical symptoms or signs suggesting meningitis. If an infection or sepsis is suspected, antibiotics must be given within 1 hour of the decision to treat with antibiotics.
For the treatment of early-onset infection, intravenous benzylpenicillin with gentamicin is recommended as the first-choice antibiotic regimen. The starting dosage of gentamicin should be 5 mg/kg every 36 hours, administered in a single dose. If a second dose of gentamicin is given, this should be 36 hours after the first dose; however, a shorter interval can be used if clinical judgement suggests this is needed. NICE guidance also recommends that, in those receiving antibiotics because of risk factors for early-onset infection or clinical indicators of possible infection, stopping antibiotics at 36 hours be considered.
For babies with late-onset infection who are already in a neonatal unit, a combination of narrow-spectrum antibiotics, such as intravenous flucloxacillin plus gentamicin, is recommended as first-line treatment. Local antibiotic susceptibility and resistance data should be taken into account when deciding which antibiotics to use. NICE guidance recommends considering stopping antibiotics at 48 hours in those with suspected late-onset infection.
The document Clinical Pharmacogenetics Implementation Consortium Guideline for Aminoglycosides Based on MT-RNR1 Genotype13 recommends that aminoglycoside antibiotics be avoided in individuals with the MT-RNR1 m.1555A>G variant unless the high risk of permanent hearing loss is outweighed by the severity of infection and a lack of safe or effective alternative therapies.
Alternative antibiotic therapies may be used instead of aminoglycosides in cases of neonatal infection. However, clinical experts have advised that there are strong clinical concerns regarding antibiotic resistance to these. Alternative antibiotics include the following.
Cefotaxime is a third-generation cephalosporin effective against Gram-negative bacteria, but it is less effective against Gram-positive bacteria, such as Staphylococcus aureus.
Meropenem is a type of carbapenem. It is not licensed for children under 3 months of age, but its efficacy, safety and tolerability have been studied in this age group.
Imipenem with cilastatin may be used to treat aerobic and anaerobic Gram-positive and Gram-negative infections in neonates.
The Genedrive MT-RNR1 Kit could be used before antibiotic treatment to confirm the existence of the m.1555A>G variant. During the scoping workshop and assessment subgroup meeting, clinical experts raised the possibility that Genedrive MT-RNR1 Kit could also be used to test mothers of neonates at risk of sepsis, providing information on the likelihood of neonates inheriting the m.1555A>G variant. This could enable informed decisions regarding antibiotic prescription, specifically whether or not to prescribe an alternative to aminoglycosides.
Objectives
The overall aim of this early value assessment (EVA) was to summarise and critically appraise existing evidence on the clinical effectiveness and cost-effectiveness of the Genedrive MT-RNR1 ID Kit for identifying the m.1555A>G gene variant in neonates.
More specifically, this EVA had the following objectives.
The clinical effectiveness objectives were:
-
to undertake a rapid review and, if feasible, a meta-analysis of the usability and accuracy of the Genedrive MT-RNR1 ID Kit
-
to undertake a rapid review and, if feasible, a meta-analysis of the clinical impact of the device
-
to undertake a rapid review and narratively synthesise patient and physician experience on the ease of use and value of use
-
to identify evidence gaps to support further evidence generation.
The cost-effectiveness objectives were:
-
to conduct a rapid review of existing economic evaluations studies of the use of Genedrive MT-RNR1 ID Kit for detecting single nucleotide polymorphism m.1555A>G in neonates
-
to estimate the costs of Genedrive MT-RNR1 ID Kit for detecting single nucleotide polymorphism m.1555A>G in neonates
-
to develop an early economic model to identify key drivers, and identify evidence gaps, of the cost-effectiveness of Genedrive MT-RNR1 ID Kit for detecting single nucleotide polymorphism m.1555A>G in neonates.
Chapter 2 Methods for synthesising evidence of clinical effectiveness
The protocol for this review was published on PROSPERO (CRD42022364770). A rapid review of the available evidence was conducted based on Cochrane Rapid Review guidance. 14
Search strategy
An experienced information specialist designed the search in MEDLINE in collaboration with the project team, and a second information specialist reviewed it. The search used the following concepts:
-
point-of-care testing
-
gene of interest
-
antibiotic treatment
-
hearing loss.
We searched the following bibliographic databases on 13 October 2022:
-
MEDLINE® and Epub Ahead of Print, In-process, In-data-review and Other Non-indexed Citations, Daily and Versions 1946 to 12 October 2022 via Ovid
-
EMBASE (1974 to 12 October 2022) via Ovid
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1982 to October 2022) via EBSCOhost.
We designed the search using database thesaurus headings and keywords on MEDLINE and translated the strategy as appropriate to other databases. An example of the full search strategy can be found in Appendix 1.
We also searched the following resources.
Trial registries:
-
Clinicaltrials.gov
-
EudraCT (European Union Drug Regulating Authorities Clinical Trials Database)
-
WHO ICTRP (World Health Organization International Clinical Trials Registry Platform)
-
ISRCTN (International Clinical Trials Registry Platform) registry.
We restricted the search to 2010 onwards. All search results were downloaded to EndNote X9.0 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and deduplicated.
Eligibility criteria
Population
The population was all babies being considered for treatment with aminoglycosides. Possible subgroups of these patients included those who presented with early-onset (≤ 72 hours post birth) or late-onset (≥ 72 hours post birth) neonatal infection; neonates who needed antibiotic treatment (i.e. a decision to start antibiotics had already been made); neonates who were anticipated to need antibiotics (i.e. a decision to start antibiotics had not already been made); and neonates of different ethnicities. Additionally, we planned to include mothers tested for the variant pre-birth of the neonate. However, none of the subgroups could be examined due to the lack of data.
Intervention
The intervention was Genedrive MT-RNR1 ID Kit, used to determine a neonate’s MT-RNR1 m.1555A>G status, when used to test:
-
the neonate directly, or
-
their mother (pre-birth of the neonate).
Comparator
The comparator was no testing to determine a neonate’s MT-RNR1 m.1555A>G variant status prior to them receiving aminoglycosides.
Outcomes
The outcomes of interest were divided into intermediate measures of the usage of the equipment and its effects on antibiotic treatment plans, clinical outcomes, patient-reported outcomes and patient experience (Table 1).
| Outcome type | Outcome(s) assessed |
|---|---|
| Intermediate | Number or proportion of neonates successfully tested |
| Number or proportion of mothers successfully tested (evidence not available) | |
| Test failure rate | |
| Test accuracy | |
| Impact of test result on decisions about care (e.g. antibiotic use) | |
| Impact of test implementation and use on healthcare resources (e.g. time taken to do and interpret test) | |
| Time to obtaining a sample for testing | |
| Time to results | |
| Time to antibiotic treatment | |
| Number of neonates identified with m.1555A>G | |
| Usability of the test (evidence not available) | |
| Clinical | Morbidity (e.g. hearing loss) (evidence not available) |
| Mortality (evidence not available) | |
| Patient-reported | Health-related quality of life (evidence not available) |
| Patient experience (evidence not available) | |
| Physician-reported | Physician experience (evidence not available) |
Timing
Antibiotic treatment for neonates is recommended within 1 hour of the decision to treat. Therefore, the test is time sensitive.
Reference standard (for test accuracy data)
The reference standard was laboratory-based confirmatory genetic testing. Approaches may differ across genetic laboratory testing centres, including techniques, such as restriction enzyme assay and sequence analysis (such as Sanger sequencing).
Study design(s)
We considered all study designs that provide relevant outcome data, as listed in Table 1.
Setting(s)
The setting was secondary care (hospital, neonatal unit).
Study selection
The deduplicated citations in EndNote were exported to Rayyan, an online tool used to speed up the review process, for title and abstract screening. 15 We planned to screen 20% of citations in duplicate, by two reviewers independently, with conflict resolution before moving on to a single screener approach. However, owing to the small number of records, all titles and abstracts were screened by two reviewers independently. Full-text copies of studies included at title and abstract screening stage were obtained and their eligibility further assessed by two independent reviewers. Disagreements at either stage were resolved through discussion.
Data extraction
A data extraction form was designed, piloted and finalised to facilitate standardised data extraction. Basic study information (e.g. author, year), study design, patient characteristics, recruitment method, analysis information, results and interpretation were extracted. One reviewer extracted the data, and a second reviewer checked the extracted data for accuracy. Any disagreements were resolved through discussion.
Quality assessment
Consistent with Cochrane Rapid Review guidance, we conducted quality assessment only on key outcomes: test accuracy, test failure rate and impact of test result on decisions about care.
The risk of bias for diagnostic accuracy outcomes was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. 16
For all other outcomes reported in non-randomised studies, the risk of bias was assessed using the Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I) tool. 17
The risk-of-bias assessment was completed by one reviewer and independently checked by a second reviewer. Any disagreements were resolved through discussion and, where necessary, in consultation with a third reviewer.
Method of analysis/synthesis
Where possible, we planned to present the results in structured tables and to pool data using appropriate meta-analytic techniques. However, owing to a lack of evidence, all the outcomes were summarised narratively.
Chapter 3 Clinical effectiveness review results
Results of the search
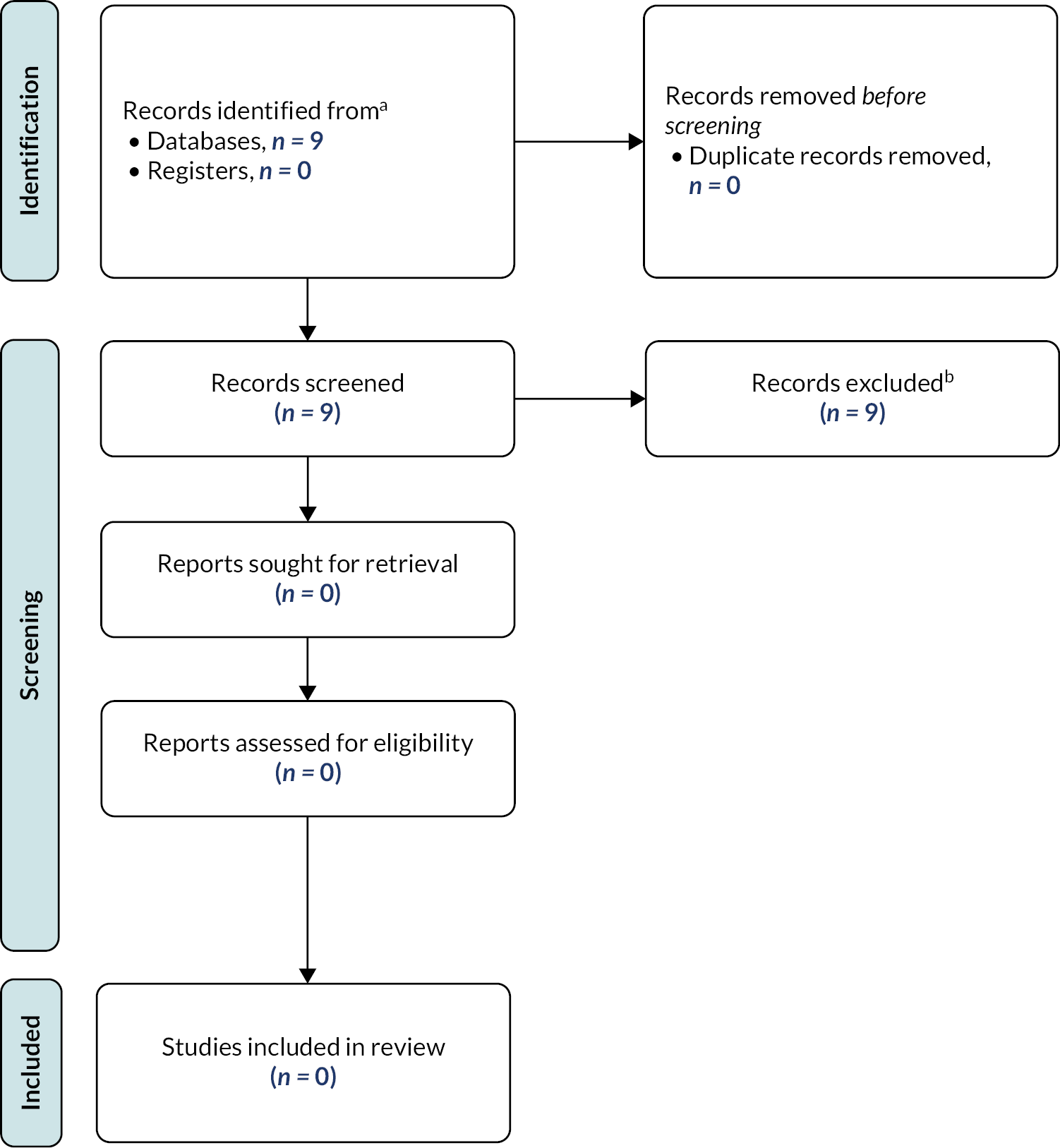
Overall, database searching retrieved 179 records (after deduplication) for title and abstract screening. Of these, 13 were sought for full-text assessment. Two records were included, one of which was a linked conference abstract19 meaning that one study, with two associated records, was included in the review. 18,19 The data were extracted from only the McDermott et al. 19 record as it provided more information and so that study participants were not double-counted.
Studies were excluded for the following reasons: wrong publication type (n = 7), wrong population (n = 3) and wrong index test (n = 1). A list of excluded records is available in Appendix 3. Figure 1 shows the flow of the studies through the selection process.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses flow chart of the clinical effectiveness review.
Overview of the included study
A single study met the eligibility criteria. 18,19 The study assessed neonates who were admitted to two NICUs between January and November 2020. However, one NICU paused recruitment and did not recommence owing to the SARS-CoV-2 outbreak. The Genedrive MT-RNR1 ID Kit was used as the index text, while Sanger sequencing was the reference standard. The study recruited 749 neonates, 526 of whom needed treatment with antibiotics. Owing to failed tests or not testing eligible patients, 424 neonates were genotyped and were prescribed antibiotics; 416 did not have the m.1555G variant and three were confirmed to possess the variant.
Data on ethnicity and gender were not provided. Participants’ median (range) age was 2.5 (0–198) days at the time of recruitment. Mean (standard deviation) gestational age at time of delivery was 37 (4) weeks.
Study quality
Study quality of the included study was evaluated per outcome. To accomplish this, we utilised the QUADAS-216 for diagnostic test accuracy. For other clinical outcomes, the ROBINS-I was completed. 17
Diagnostic test accuracy
For patient selection, McDermott et al. 18,19 was rated as being at low risk of bias. This was based on the assumption that consecutive sampling was used, although this is not explicitly stated. Additionally, while a case–control design was used for the preclinical trial, a prospective study design was used for the implementation, from which the diagnostic accuracy results are presented.
The index test was also rated as being at low risk of bias, even though the test was modified at a point during the study (due to high failure rate) as all samples were retested with the updated version of the device. The question regarding thresholds was not considered for this assessment as it is a genetic variant that is either present or not. The conduct and interpretation of the test was reported in adequate detail. Details regarding the reference standard are unclear, with no information reported on whether those interpreting the test had knowledge of the index test result. Therefore, the reference standard is rated as being at unclear risk of bias. The final domain of flow and timing was rated as being at high risk of bias. This was due to the reported variation in numbers who underwent the test, compared with those not included in the analysis.
Other clinical outcomes
All outcomes except one (test failure rate, which was rated as being at low risk of bias) were rated as being at moderate risk of bias. This is because the failure rate, which was 17.1%, was not included in the analyses of the outcomes illustrated in Figure 2. Consequently, not including failure rate could affect the outcome results. All of the other risk-of-bias domains seemed to be reported adequately. See Figure 2 for a risk-of-bias visualisation using the ROBINS-I tool. 17
FIGURE 2.
Risk Of Bias In Non-randomized Studies – of Interventions tool visualisation by outcomes.
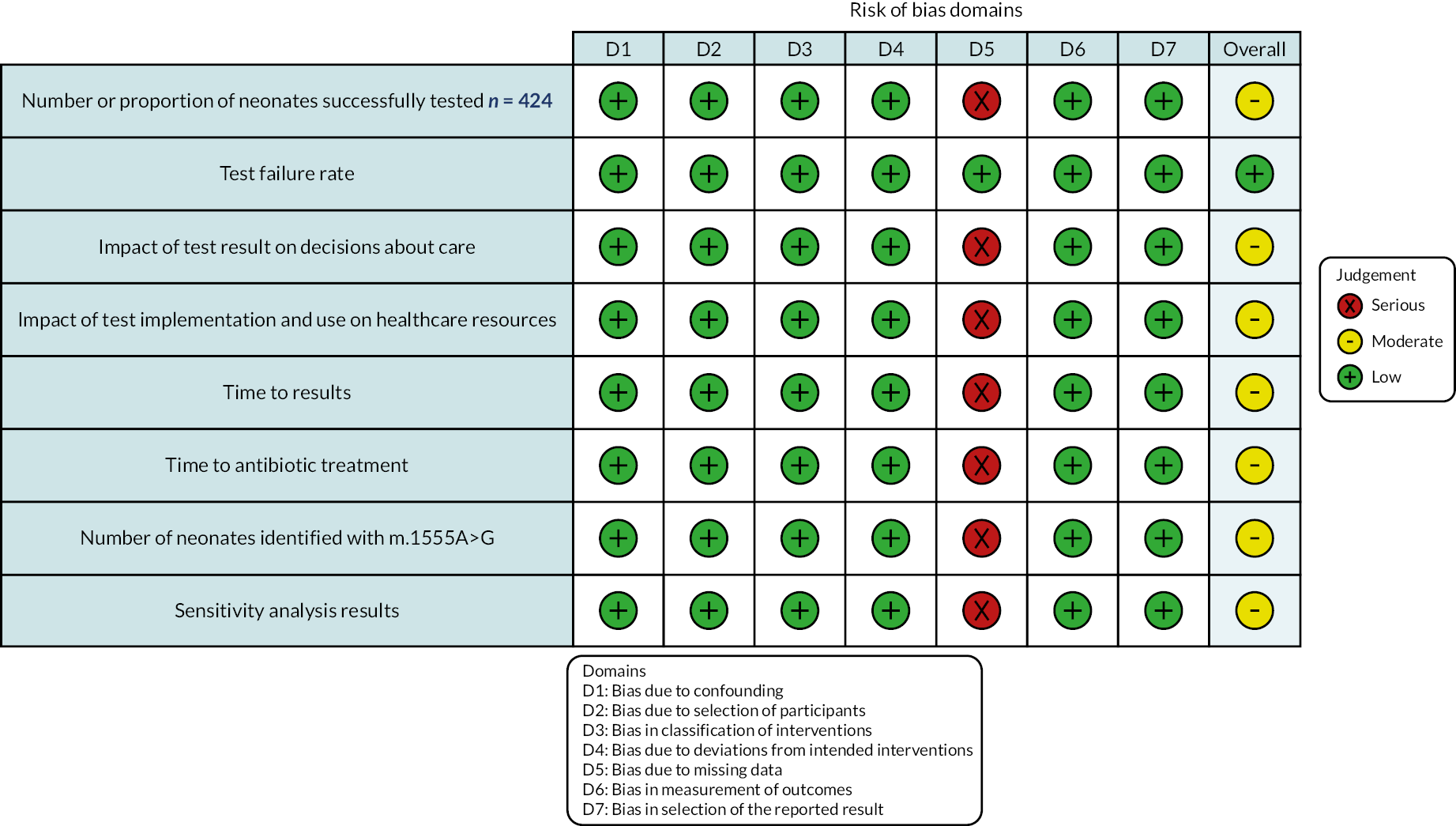
Bias due to confounding was rated low as there was a lack of apparent confounding effect in the causal relationship between the outcomes. Bias in selection of participants into the study was rated as low because all participants who would have been eligible were included in the study. Bias in classification of interventions was rated low as the intervention group was described in detail, and, although the intervention was most likely known before implementation, this is unlikely to have negatively impacted the outcomes. Bias due to deviations from intended interventions was rated as low as there were no apparent deviations. Bias in the selection of reported results was considered low as the method used appears to be the only way of measuring the outcomes.
Intermediate outcome results
Diagnostic test accuracy
In the preclinical trial, buccal samples were collected and genotyped from 159 participants, with 304 samples. The controls were split into two groups. The first was people who had received confirmation that they did not carry the m.1555A>G genetic variant (assessed via normal clinical laboratory processes; n = 74). Second, children on the NICU were recruited (n = 55, 110 individual specimens) to ensure that there were no factors specific to neonatal swab sampling that would impair the assay. The cases were individuals who previously had received confirmation that they carried the m.1555A>G genetic variant (n = 32, 62 individual specimens). The Genedrive MT-RNR1 ID Kit was validated for both adults and neonatal populations in this case–control study. The sensitivity was reported as 100% (95% CI 93.9% to 100%) and the specificity was reported as 100% (95% CI 98.5% to 100%). This part of the study was not assessed in the quality appraisal above.
In the prospective study, 424 of the 526 (80.6%) neonates who received antibiotic treatment were included in the analysis. Three neonates were identified to have the m.1555A>G variant and this was confirmed by Sanger sequencing. There were five false positives and no false negatives. The assay produced a sensitivity of 100% (95% CI 29.2% to 100%), a specificity of 99.2% (95% CI 98% to 99.7%), and an accuracy of 99.2% (95% CI 98% to 99.7%). Throughout the trial, the MT-RNR1 assay was updated to improve efficiency, a process that led to the identification of an issue with the buffer and cartridge, which was linked to the false-positive rates. The issue was resolved with an updated buffer and cartridge design.
Number successfully tested
Only neonates were assessed in this included study, with 424 successful tests of 526 admissions. Of the 526 admissions, 12 did not have an index test (no further information was provided regarding the reasons). The remaining tests failed (unsuccessful genotyping).
No mothers were tested.
Test failure rate
Among the 526 admissions who received antibiotics, 90 (17.1%) failed tests were reported. Among the whole cohort (n = 749), the failure rate was 128 (17.1%). The failure rate was determined to be caused by low signal intensity during the melting phase, which was resolved after the recruitment period by modifying the assay buffer and using a redesigned cartridge. Repeated testing of samples in which genotyping had previously failed led to a reduced failure rate of 5.7% in a clinical setting and 0% when performed in the laboratory. 19
Impact of test result on decisions about care
The study reports that ‘in all cases where a m.1555A>G genotype was identified, aminoglycoside antibiotics were avoided and alternative cephalosporin-based regimens were used’. 19
Impact of test implementation and use on healthcare resources
The MT-RNR1 point-of-care test analysis is automated without any user interpretation, providing the user with a ‘detected’ or ‘not detected’ actionable result in 26 minutes of initiating the analysis. The authors suggest an approximate 30 minutes from collection to an actionable result. 19
No further data regarding the impact of the test implementation and use on healthcare resources are reported.
Time to obtaining a sample for testing
The median time to swab throughout the study was 6 minutes (interquartile range 3–16 minutes).
Time to results
The MT-RNR1 point-of-care test was able to genotype the m.1555A>G variant in 26 minutes.
Time to antibiotic treatment
The study authors report that, prior to implementation, the mean time to antibiotic therapy was 55.87 (standard deviation 22.56) minutes based on 95 consecutive acute admissions over 1 month. During the study, the corresponding mean time to antibiotic therapy was 55.18 (standard deviation 23.82) minutes. The difference in mean time to antibiotic therapy (–0.87 minutes, 95% CI −5.96 to 4.23 minutes), before and after implementation of the MT-RNR1 assay, was not statistically significant. The 95% CI was within the prespecified boundaries of statistical equivalence.
Number of neonates identified with m.1555A>G
There were three neonates identified with the variant, five false positives and no false negatives.
Usability of the test
The study did not report on this outcome.
Clinical outcome results
Mortality
The study did not report on this outcome.
Morbidity
The study did not report on this outcome.
Patient-reported outcome results
Health-related quality of life
The study did not report on this outcome.
Patient experience
The study did not report on this outcome.
Physician-reported outcome results
No evidence was identified on this outcome.
Chapter 4 Methods for synthesising evidence of cost-effectiveness
Decision problem
The economic evaluation assessed the cost-effectiveness of Genedrive MT-RNR1 ID Kit for detecting single nucleotide polymorphism m.1555A>G in neonates compared with current clinical standard (no testing). The decision problem for the economic evaluation is summarised in Table 2.
| Item | Description |
|---|---|
| Populations | Neonates who need antibiotic treatment or who are anticipated to need antibiotic treatment, and who are being considered for treatment with aminoglycosides |
| Intervention | Genedrive MT-RNR1 ID Kit used to test for single nucleotide polymorphism m.1555A>G variant status, when used to test the neonate directly, or their mother (pre-birth of the neonate) |
| Comparators | No point-of-care testing for single nucleotide polymorphism m.1555A>G prior to them receiving aminoglycosides |
| Perspective | NHS and Personal Social Services |
| Time horizon | Lifetime |
| Outcomes | Cost per Genedrive MT-RNR1 ID Kit Incremental cost per hearing loss case prevented Incremental cost per QALY gained |
The decision problem consists of neonates in need of antibiotic treatment (both early-onset and late-onset infection) and who are being considered for treatment with aminoglycosides. The economic assessment was undertaken from the perspective of the NHS and Personal Social Services. The main economic questions to be addressed were:
-
What existing, published cost-effectiveness studies are available about Genedrive MT-RNR1 ID Kit, for detecting single nucleotide polymorphism m.1555A>G in neonates?
-
What are the costs, from a NHS and Personal Social Services perspective, of Genedrive MT-RNR1 ID Kit, for detecting single nucleotide polymorphism m.1555A>G in neonates?
-
What are the key drivers of the cost and effectiveness of Genedrive MT-RNR1 ID Kit roll-out for detecting single nucleotide polymorphism m.1555A>G in neonates?
Rapid review of cost-effectiveness studies
We utilised the search from the clinical effectiveness review and combined it with an economics filter (see Appendix 2 for a list of the economic filters used). We searched the following bibliographic databases on 3 November 2022:
-
MEDLINE® and Epub Ahead of Print, In-process, In-data-review and Other Non-indexed Citations, Daily and Versions 1946 to 2 November 2022 via Ovid
-
EMBASE (1974 to 2 November 2022) via Ovid
-
CINAHL (1982 to November 2022) via EBSCOhost
-
Cochrane (via Wiley).
We also searched the following resources:
-
RePEc-IDEAS (https://ideas.repec.org/).
In both cases, we restricted the search to 2010 onwards. All search results were downloaded to EndNote X9.09 and deduplicated.
The above sources were also searched using the clinical effectiveness search with health-related quality of life (HRQoL) and hearing loss filter terms in a targeted search to inform the utility values to be used in the early economic model (see Appendix 2 for a list of HRQoL and hearing loss filter terms). No restrictions were made in relation to year of publication. Once more, all search results were downloaded to EndNote X9.09 and deduplicated.
Development of an early health economic model
To identify the key drivers of cost and effectiveness of the Genedrive MT-RNR1 ID Kit for detecting single nucleotide polymorphism m.1555A>G in neonates, the Evidence Assessment Group (EAG) developed an economic model. This economic model reflected the pathways of care that individuals follow under standard practice in the UK NHS and how the use of the Genedrive MT-RNR1 ID Kit might change those pathways of care. The purpose of the model was threefold. First, to outline the structure and parameter requirements for a model. Second to use that model to help define the utilities, costs and probabilities needed to populate that model. Third, to use the available data, accepting that there would be insufficient information to complete a full economic evaluation, to conduct an early economic evaluation modelling exercise. The purpose of this model was to provide an early indication as to whether the use Genedrive MT-RNR1 ID Kit could potentially be cost-effective and to identify key drivers of cost-effectiveness.
In line with the decision problem set out in Table 2, outcomes included the lifetime impact on costs for the NHS and Personal Social Services of AIHL in neonates, impact on number of cases of AIHL avoided and the lifetime impact on quality-adjusted life-years (QALYs) of AIHL in neonates.
The full model incorporated the risk of ototoxicity/hearing loss for people with and without the m.1555A>G variant who have (1) aminoglycoside and (2) non-aminoglycoside alternatives; the likely prevalence of MT-RNR1 gene m.1555A>G variant in neonates (and how this varies across different groups); and diagnostic failure as well as diagnostic accuracy. The capacity to explore the time to antibiotic delivery using the Genedrive MT-RNR1 ID Kit was incorporated in the full model. Within the early economic model, however, it was assumed that all neonates will receive antibiotics in 1 hour, irrespective of a successful or failed test. For those with successful test results, it was assumed that neonates identified with the m.1555A>G variant would receive non-aminoglycoside alternatives, and those neonates identified without the m.1555A>G variant would receive aminoglycoside. If the 1st test (and 2nd test) failed, it was assumed (after consulting with clinical experts) that the neonates could receive non-aminoglycoside alternatives in order to ‘play it safe’. However, as described in Chapter 5, the early economic evaluation model was much simplified due to the limited data available to explore some issues including some of the ones noted in this paragraph, for example how prevalence of MT-RNR1 gene m.1555A>G variant varies across groups.
Cost data relating to the Genedrive system (Genedrive MT-RNR1 ID Kit to detect m.1555A>G variant and Genedrive system software), the medical management of people with suspected/diagnosed hearing loss and the need for Cochlear implants in the long-term were included. To identify cost and resource use evidence, the EAG searched the same sources identified for the economic evidence supplied by the test manufacturers together with NHS reference costs, the unit costs of health and social care and the British National Formulary (BNF). All costs were updated to the price year 2021–2. Data on HRQoL were extracted from the rapid review of cost-effectiveness studies and the targeted literature search for publications reporting HRQoL or health state utilities for the populations of interest.
The early economic model was developed according to standard modelling guidelines. 20 The model structure was reviewed by clinical and methodological experts for appropriateness to the current NHS clinical and diagnostic pathway and the face validity of the model was checked by clinical experts.
Chapter 5 Cost-effectiveness
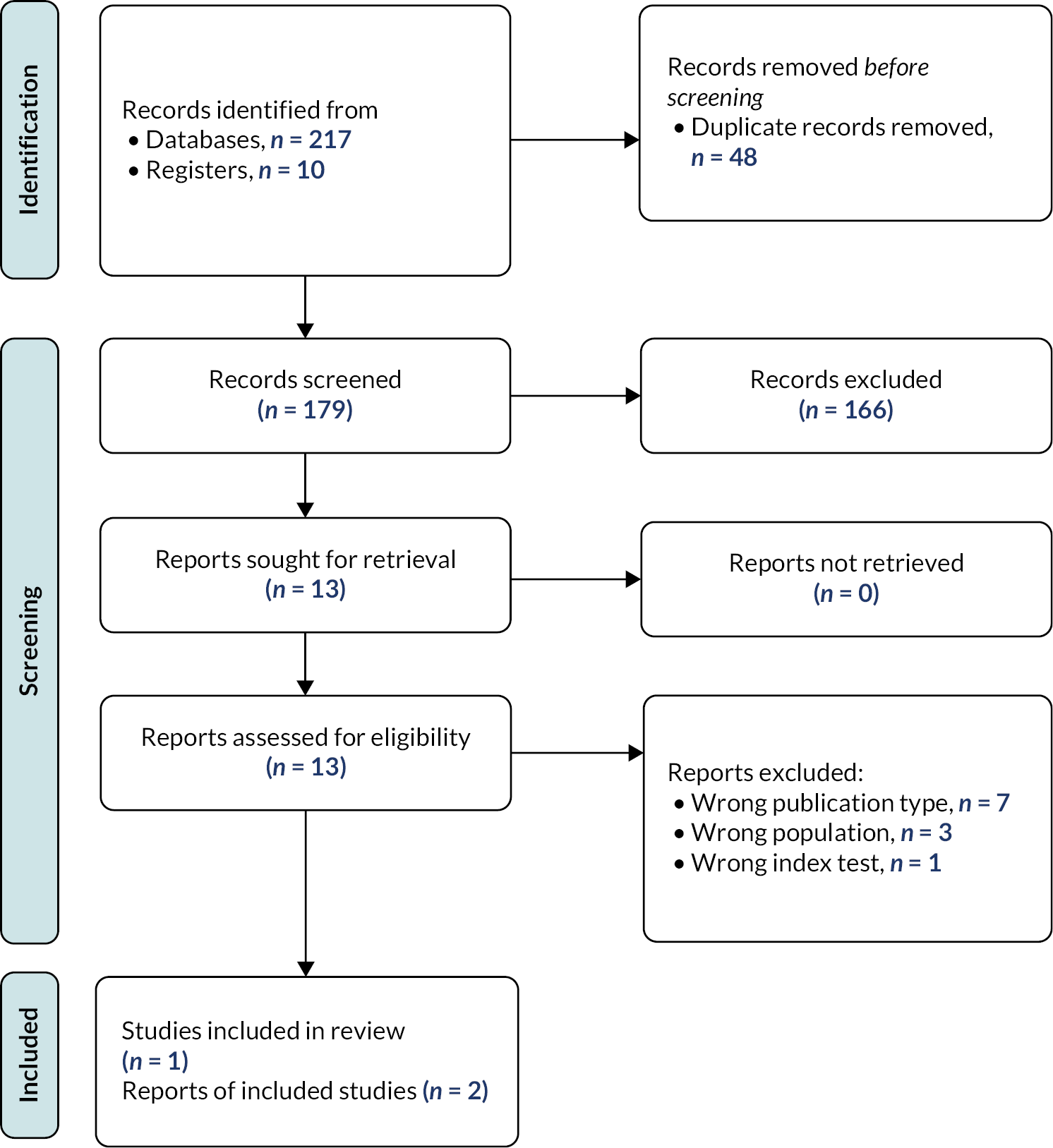
Results of the cost-effectiveness studies search
Overall, database screening retrieved nine records for title and abstract screening. No studies were sought for full-text assessment as no records were judged relevant (Figure 3).
Developing a clinical pathway and economic model
Given the lack of economic evaluations, the EAG went on to consider how an economic evaluation model might be structured to identify the information needs for this model, the availability of these data and, from that, the information gaps that exist. Given the anticipated information gaps, an early economic evaluation model was developed to provide an indication as to whether the use of the Genedrive MT-RNR1 ID Kit could plausibly be cost-effective and to explore the impact of key uncertainties on estimates of cost-effectiveness.
The first stage in developing the economic evaluation model was to develop conceptual models of the clinical pathways for situations representing the current standard of care and instances when the Genedrive MT-RNR1 ID Kit is used.
Developing a clinical pathway
To develop the clinical pathway [using GitMind (Wangxu Technology Co. Limited, Hong Kong)] for using the Genedrive MT-RNR1 ID Kit to detect m.1555A>G in neonates, we reviewed related documents to map out the treatment pathway in the NHS for the target population. This clinical pathway was checked with clinical experts consulted by the EAG and revised following their comments. The main documents that we initially used to develop clinical pathway are as follows:
-
NICE advice: Genedrive MT-RNR1 ID Kit For Detecting Single Nucleotide Polymorphism m.1555A>G in Newborn Babies21
-
NICE guideline 195: Neonatal Infection: Antibiotics for Prevention and Treatment12
-
‘Clinical Pharmacogenetics Implementation Consortium Guideline for the use of aminoglycosides based on MT-RNR1 genotype’13
-
‘Pharmacogenetics to Avoid Loss of Hearing (PALOH) trial: a protocol for a prospective observational implementation trial’22
-
World Health Organization report: Childhood Hearing Loss: Strategies for Prevention and Care23
-
Public Health England guidance: Newborn Hearing Screening Programme (NHSP): Care Pathways for Babies in Neonatal Intensive Care Units (NICU). 24
Pathway for the current standard of care
A simple structure of clinical pathway for the current standard of care is shown in Figure 4. In the current standard pathway, neonates with suspected infection or sepsis will receive an aminoglycoside, such as gentamicin, irrespective of whether they have the MT-RNR1 gene m.1555A>G mitochondrial genetic variant. The current pathway also considered the administration of antibiotics to women during labour who are at risk of early-onset neonatal infection. The risk factors for early-onset neonatal infection in women in labour are set out in box 1 in NICE guideline 195.
FIGURE 4.
Clinical pathway for the normal standard of care.
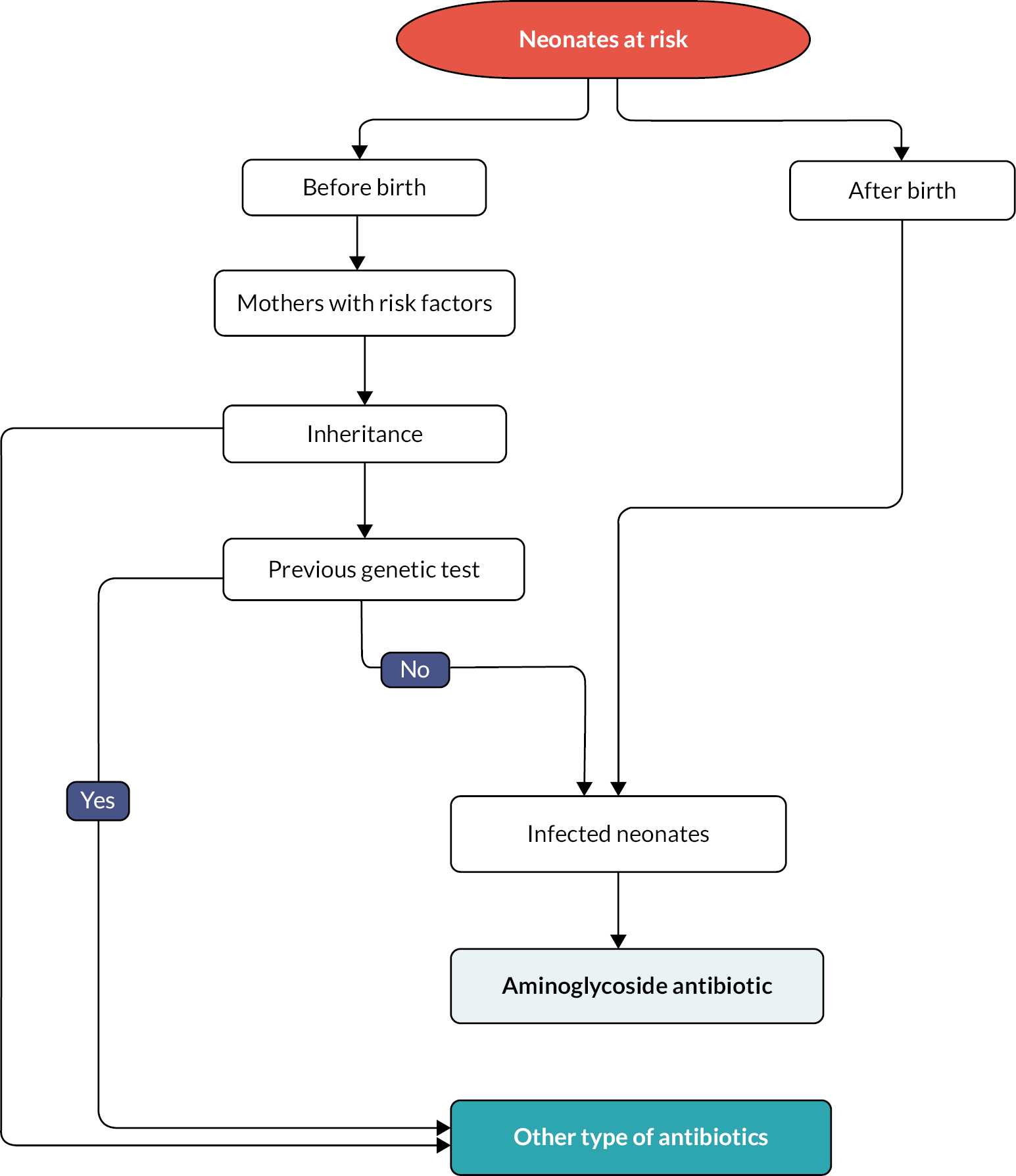
Owing to the inheritance pattern of MT-RNR1, the current pathway included mitochondrial mutation screening for neonates with a mitochondrial mutation, maternal history of deafness, or both, who need aminoglycoside prescription. The current pathway also considered the findings of any previous genetic test to determine an antibiotic prescription. For example, children with cystic fibrosis are tested for the variant once they are identified as having cystic fibrosis as it is expected that these individuals will require aminoglycoside antibiotics at some stage in their lives.
Pathway when using the Genedrive MT-RNR1 ID Kit
A simple structure of clinical pathway for the integration of the Genedrive MT-RNR1 ID Kit into the clinical pathway is shown in Figure 5. As with the current standard of care, inheritance data and previous genetic tests for mothers with relevant risk factors are considered when deciding whether or not to prescribe aminoglycoside to neonates with suspected infection or sepsis. Although the time taken to administer the Genedrive MT-RNR1 ID Kit is short (26 minutes), for some of the neonates who present with suspected infection or sepsis there is insufficient time to use the Genedrive MT-RNR1 ID Kit as they are in immediate need. This issue was discussed by clinical experts consulted by the EAG. Their view was that using the Genedrive MT-RNR1 ID Kit may cause a delay for some neonates; however, ˂ 5% of neonates will need immediate antibiotics.
FIGURE 5.
Clinical pathway when using the Genedrive MT-RNR1 ID Kit.
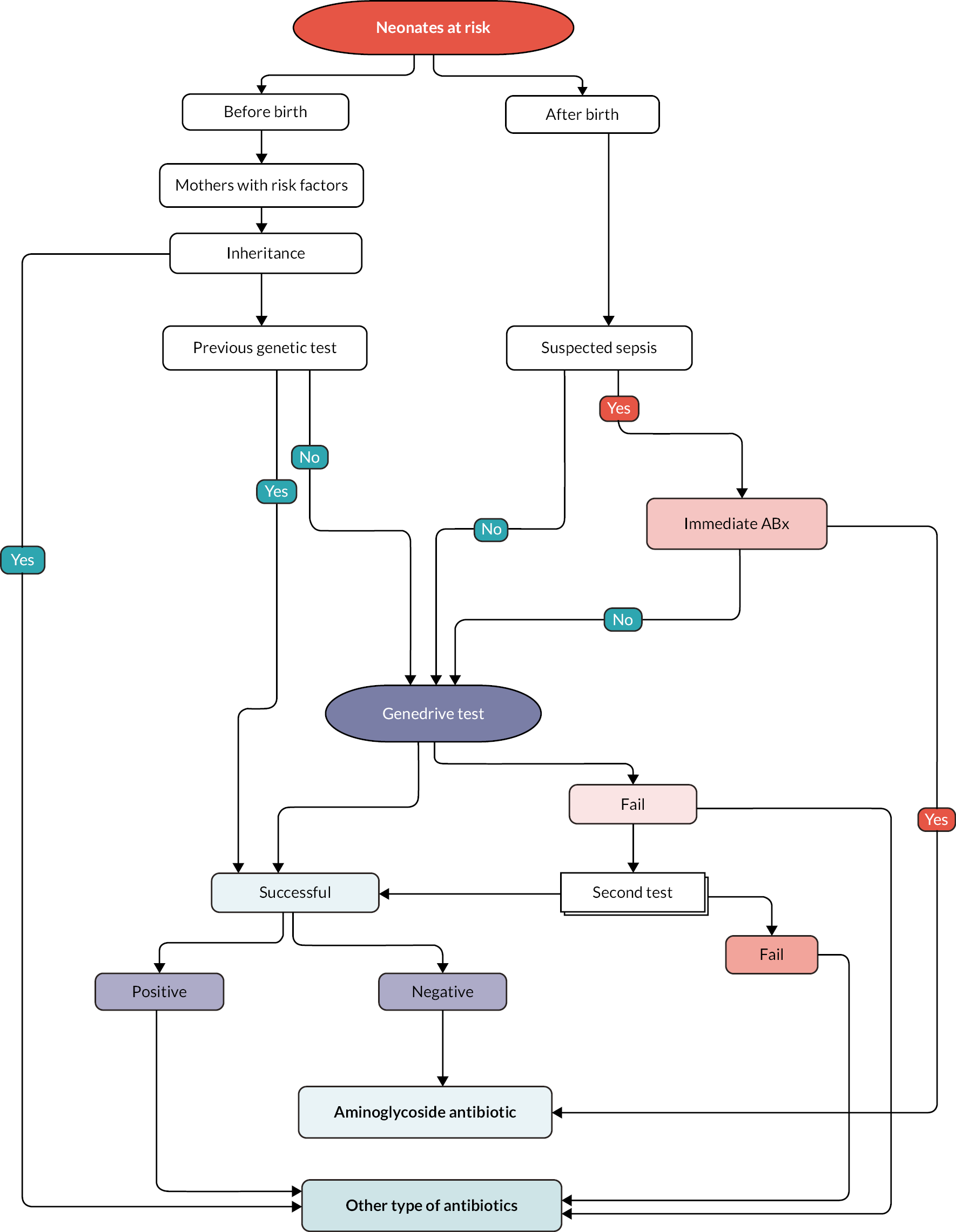
As shown in Figure 5, antibiotic prescription for neonates will be based on Genedrive MT-RNR1 ID Kit results, with aminoglycosides being prescribed only if the test results are negative. As also shown in Figure 5, there is the possibility that the Genedrive MT-RNR1 ID Kit will be conducted for a second time if the first test fails. If both the first and the second test were to fail, then there would be no more time for any extra tests within the ‘golden hour’ for the administration of the antibiotics. Clinical experts consulted by the EAG noted that, in this situation, neonates with suspected infection or sepsis would almost certainly be provided with other alternative antibiotics owing to the need to ‘play it safe’.
Developing an economic evaluation model
In the following subsection, we outline the structure and key assumptions for a full economic model. The proposed economic model seeks to capture the components of the care pathways described above and also to consider the long-term implications of preventing AIHL for a child presenting with suspected infection or sepsis who has the m.1555A>G variant. Figure 6 provides a schematic (albeit simplified) representation of this model. In this model the key long-term implications considered are those that follow AIHL.
FIGURE 6.
Schematic outline of the Markov model.
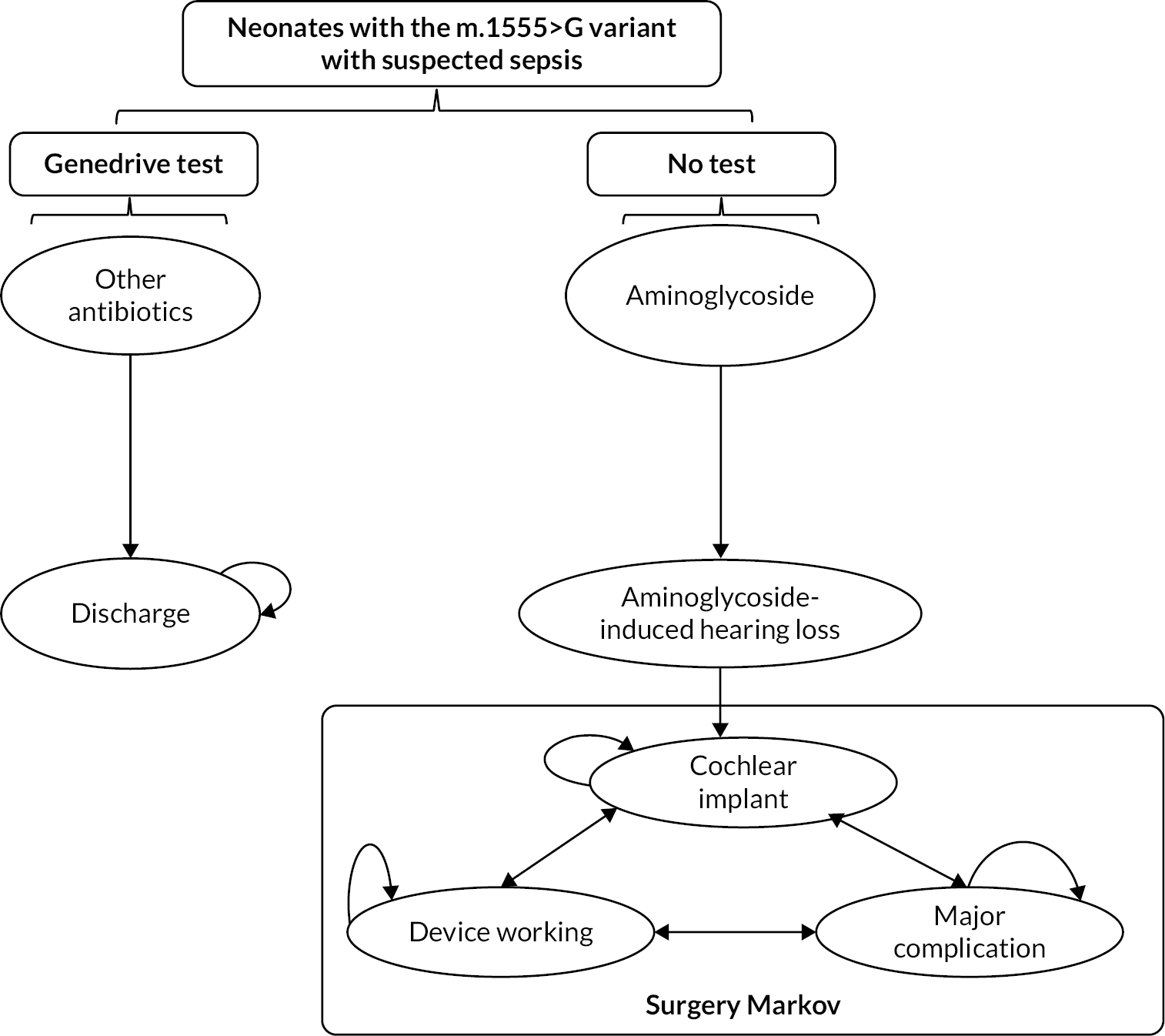
The model was developed in accordance with standard modelling guidelines. 25,26 The face validity of the economic model structure was checked by our clinical experts and methodological experts for appropriateness to the current NHS clinical and diagnostic pathways. The model’s calculations and proposed data inputs were also checked for technical correctness.
The model simulates the patient pathway from the initial diagnosis of neonates with the m.1555A>G gene variant to treatment for AIHL (e.g. a cochlear implantation) for a patient’s lifetime. As per NICE scope, the population defined in the model is neonates with suspected infection or sepsis. The patient pathway described by the Markov model involves a series of mutually exclusive health states between which a patient may move over time (Figure 6). Once someone is in a state, they stay in that state for a defined period of time called the cycle length. We have defined a 1-year cycle length, as it was thought that year would be sufficient to capture both cost and effectiveness impacts in the model.
Each Markov model includes at least one absorbing state. This is a state that a person can enter but cannot leave. In the context of a chronic disease, the absorbing state might be death. In our model, the probability of movement to death was informed by the UK National Life Tables. 27 All programming for the model was implemented in TreeAge Pro 2022 (Williamstown, MA, USA).
Set out below are some key features of the proposed economic model:
-
The population modelled is neonates with early-onset and late-onset infection who need antibiotic treatment and are being considered for treatment with aminoglycosides.
-
Some neonates will require antibiotic administration immediately (i.e. there is no time for the Genedrive MT-RNR1 ID Kit to be used before antibiotics must be started).
-
Increased time to antibiotics will increase the risk of death for neonates with sepsis.
-
The clinical pathways for neonates with early-onset and late-onset infection are different (in terms of how long antibiotics are prescribed for).
-
There is a chance the Genedrive MT-RNR1 ID Kit will give a false-negative result.
-
There is a chance the Genedrive MT-RNR1 ID Kit will give a false-positive result.
-
There is a chance that the Genedrive MT-RNR1 ID Kit will fail to give a result.
-
If the first Genedrive MT-RNR1 ID Kit fails to give a result, there is time for a second test.
-
If both the first Genedrive MT-RNR1 ID Kit and the second Genedrive MT-RNR1 ID Kit fail, there will be insufficient time for further testing and neonates with suspected infection will not be treated with aminoglycosides and receive other antibiotics (such as cefotaxime).
-
An increased time to antibiotics will increase the risk of death for neonates from sepsis.
-
Where neonates are identified as not having the m.1555A>G variant using the Genedrive test, aminoglycosides (e.g. gentamicin) will be used.
-
Where neonates are identified as having the m.1555A>G variant using the Genedrive test, alternative antibiotics (e.g. cefotaxime) will be used.
-
Different antibiotics will have different adverse event profiles.
-
For neonates with the m.1555A>G variant treated with aminoglycosides, there is a risk of AIHL.
-
For neonates with the m.1555A>G variant not treated with aminoglycosides, there is a risk of nonsyndromic hearing loss.
-
For neonates who experience hearing loss, the severity of this may vary.
-
Women with risk factors (for sepsis) are eligible for the Genedrive MT-RNR1 ID Kit, but antibiotic prescription will be for neonates (after birth).
-
Maternal inheritance may be considered before testing.
-
The use of the Genedrive MT-RNR1 ID Kit, other than affecting time to administration and the type of antibiotic used, does not affect normal standard of care for neonates presenting with suspected infection or sepsis.
-
There will be training costs for staff to carry out the test, which will vary by the size of type of hospital ward.
-
Staff time is required to carry out the test, which will vary by the size and type of hospital ward.
-
Additional audiological monitoring will be required for infants with AIHL.
-
AIHL has associated adverse events.
-
If AIHL occurs, neonates will require hearing aids, unilateral cochlear implants or bilateral cochlear implants.
-
HRQoL will vary by age, level of hearing loss, type of cochlear implant and time since cochlear implant has been implanted.
-
To demonstrate no adequate benefit from hearing aids, children need to have had a valid trial of an acoustic hearing aid for at least 3 months.
-
There are pre-procedure, procedure and post-procedure costs associated with both unilateral and bilateral cochlear implants.
-
There is a chance that the cochlear implant surgery will not be successful.
-
It is possible to upgrade cochlear implants after they have been fitted.
-
There may be complications associated with the implantation of cochlear implants (i.e. internal or external device failure, death).
-
There are short-term and long-term adverse events associated with cochlear implants, such as dysgeusia and vertigo, that will impact both costs and utilities.
In the next sections, information on the health state utilities and costs required to populate the model is set out. As is described below, not all of these are used in the early economic model.
Results of the targeted search for health-related quality-of-life studies
Overall, database screening retrieved 465 records (after deduplication) for title and abstract screening. Of these studies, 46 were sought for full-text assessment. Following discussion with the project team it was decided that only utility data from studies based in the UK would be considered for inclusion as these were most relevant to the decision problem. Eight studies were therefore initially identified as having utility data that could potentially be used in the early economic model. On review of citations of these identified studies, three additional studies were identified. These 11 studies are briefly summarised in Table 3.
| Study | Population | Description | Utility measure(s) used |
|---|---|---|---|
| Summerfield et al.28 | Adults | Cost–utility modelling study of unilateral cochlear implantation | HUI2,36 TTO |
| UKCISG36 | Adults | Prospective cohort study of unilateral cochlear implantation | HUI338 |
| Barton et al.39 | Adults | Study comparing utility in hearing-impaired adults before and after being provided with a hearing aid | EQ-5D-3L,40 HUI3,38 SF-6D41 |
| Summerfield et al.35 | Adults | Randomised controlled trial of benefits of successive bilateral cochlear implants | HUI3,38 VAS |
| Barton et al.29 | Children | Cost–utility analysis of paediatric cochlear implantation | HUI338 |
| Petrou et al.42 | Children | Study looking at the impact of bilateral heating impairment on HRQoL | HUI2,36 HUI338 |
| Bond et al.30 | Children and adults | Cost–utility analysis of cochlear implants for severe to profound deafness | HUI338 – taken from UKCISG and Barton et al. |
| Lovett et al.33 | Children | Study looking at the impact of cochlear implants for deaf children | HUI3,38 VAS |
| Summerfield et al.31 | Children | Cost–utility analysis of paediatric bilateral cochlear implantation | TTO, VAS |
| Petrou et al.34 | Children | Study looking at the impact of permanent bilateral hearing loss of HRQoL | HUI2,36 HUI338 |
| Cutler et al.32 | Adults | Cost–utility analysis of unilateral cochlear implants | HUI338 – taken from UKCISG |
The studies identified in the targeted review and gathered through a review of citations were mainly a mix of cost-effectiveness analyses (Summerfield et al.,28 Barton et al.,29 Bond et al.,30 Summerfield et al.,31 Cutler et al. 32) and standalone studies with the objective of measuring the HRQoL associated with different levels of hearing impairment and/or the implementation of different types of cochlear implant in either children or adults. 30,31,33,34 Summerfield et al. 35 was a randomised controlled trial of the effects of successive cochlear implants.
All of those standalone studies used parent proxy-reported outcomes. The most common HRQoL questionnaire used to measure utility was the health utilities index mark 3 (HUI3) and its predecessor the health utilities index mark 2 (HUI2). Barton et al. 39 additionally used the EuroQol-5 Dimensions, three-level version (EQ-5D-3L) and Short Form 6 Dimensions (SF-6D); however, neither of these measures includes a question specifically related to hearing and therefore may not be sensitive to changes in utility related to hearing loss [the EAG notes that a hearing bolt-on for the EuroQol-5 Dimensions (EQ-5D) is in development]. 43 Summerfield et al. 31 and Lovett et al. 33 additionally used the Visual Analogue Scale, an assessment of general health scored between 0 and 100.
For cost-effectiveness studies, the utility values were gathered from several sources. Summerfield et al. 28 collected HUI2 and time trade-off (TTO) data from a sample of adults. Barton et al. 29 collected HUI3 data from the parents of children with hearing loss with and without cochlear implants. Bond et al. 30 used the child utility values from Barton et al. 29 and the adult utility values from the UK Cochlear Implant Study Group. 37 Summerfield et al. 31 used the TTO (a choice-based method of eliciting health state utility commonly used in health economic studies) and the Visual Analogue Scale. Cutler et al. 32 used the utility values from the UK Cochlear Implant Study Group. 37
Health-related quality of life
Utility values
The utility values used in the early economic model are based on those used in Bond et al.,30 a highly cited NIHR Health Technology Assessment investigating the effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in both children and adults. The study by Bond et al. 30 was the health economic evaluation submitted as part of TA166 (‘Cochlear implants for children and adults with severe to profound deafness’),44 which was subsequently updated in TA566. 45 These utility values are shown in Table 4 and further described below.
| Parameter | Value | Source |
|---|---|---|
| Children (aged < 18 years) | ||
| No hearing loss (population norm) | 0.908 | Pogany et al.46 |
| Profound/significant hearing loss | 0.421 | Barton et al.29 |
| Unilateral cochlear implant (˂ 2 years since implant) | 0.487 | Barton et al.29 |
| Unilateral cochlear implant (2–4 years since implant) | 0.633 | Barton et al.29 |
| Unilateral cochlear implant (over 4 years since implant) | 0.653 | Barton et al.29 |
| Bilateral cochlear implant (˂ 2 years since implant) | 0.490 | Barton et al.,29 Bond et al.30 |
| Bilateral cochlear implant (2–4 years since implant) | 0.636 | Barton et al.,29 Bond et al.30 |
| Bilateral cochlear implant (over 4 years since implant) | 0.656 | Barton et al.,29 Bond et al.30 |
| Adults (aged ≥ 18 years) | ||
| No hearing loss (population norm) | 0.850 | Pogany et al.46 |
| Profound/significant hearing loss | 0.433 | UKCISG37 |
| Unilateral cochlear implant | 0.630 | UKCISG37 |
| Bilateral cochlear implant | 0.633 | Summerfield et al.35 |
The utility values for profound hearing loss, unilateral cochlear implants and bilateral cochlear implants for children used in Bond et al. 30 are taken from Barton et al.,29 a cross-sectional study in which the parents of a representative sample of hearing-impaired children assessed the HRQoL of their children using the HUI3. The HUI3 is the HRQoL measure considered to be the most sensitive to the effects of hearing treatment on overall health status. 37 As reported in Bond et al.,30 the utility increment from cochlear implants in childhood will vary by time since implantation and whether the child has a unilateral or a bilateral cochlear implant, and therefore different utility values are provided for ‘less than 2 years since implant’, ‘2–4 years since implant’ and ‘over 4 years since implant’.
The utility value for no hearing loss in childhood is taken from Pogany et al.,46 which is the source of the HUI3 population norms reported on the website of the HRQoL tool. 47 Pogany et al. 46 report the HUI3 population norms for the Canadian general population by age band. 46 The value of 0.908 is a weighted average of the 5–12, 13–15 and 16–19 age bands. As this value is taken from the Canadian value set, there are likely to be small differences between the health preferences from Canada and those from the UK, impacting the generalisability of this utility value. However, the HUI3 was the measure used in the Barton et al. 29 study and there is no UK value set for the HUI3. It is worth noting that for all child utility values used in the early economic model it is assumed that the values for those aged ≥ 5 years generalise to those < 5 years. This is clearly a strong assumption.
The adult utility values for profound hearing loss, unilateral cochlear implants and bilateral cochlear implants used in Bond et al. 30 are taken from a UK Cochlear Implant Study Group study37 that estimated the cost-effectiveness of unilateral cochlear implants for deaf adults using the HUI3. It is worth noting that these utility values were also used in the recent Cutler et al. 32 study, which investigated the cost-effectiveness of unilateral cochlear implants in UK adults. The utility value of being profoundly deaf was estimated to be 0.433. There are utility increments associated with both unilateral (0.630) and bilateral (0.633) cochlear implants. It is worth noting that a recent network meta-analysis of both UK and non-UK studies estimated the utility increment of bilateral cochlear implants compared with unilateral cochlear implants to be 0.08,48 slightly higher than the 0.03 increment reported in Bond et al. 30 and used in the early economic model.
The utility value for no hearing loss (the adult population norm) was estimated to be 0.850, the HUI3 population norm value for adults reported in Pogany et al. 46 Once more, as this value is taken from the Canadian HUI3 value set it is unlikely to be fully representative of the UK population given the differences in health preferences between countries. However, the HUI3 is the HRQoL measure used in the UK Cochlear Implant Study Group study and there is no UK value set for the HUI3.
It has previously been shown that HRQoL decreases with age. 49 As argued in Bond et al.,30 using a single age-independent value for the utility increment associated with cochlear implants may result in a counterintuitive position whereby the utility of a cochlear implant recipient may be higher than that of their normal-hearing peers. As this is an EVA, aside from varying the utility values by time of implementation in childhood, age-adjustment has not been considered in the early economic model. In a definitive study, age-adjustment should be implemented in line with modelling good practice guidelines and NICE guidance. 20,25,26
Adverse event disutility values
As noted in Cutler et al.,32 there are adverse events associated with the implementation of cochlear implants that may be included in an economic model. The disutility values associated with these adverse events and the probability of these adverse events are shown in Tables 5 and 6. The disutility values used in Cutler et al. 32 and their duration are sourced from a number of previously published health preference studies,50–52 The probabilities of the adverse events used in Cutler et al. were sourced from a series of clinical studies reporting complications associated with cochlear implants. 53–56
| Adverse event | Value | Duration | Source |
|---|---|---|---|
| Dysgeusia | 0.020 | 6 months | Cutler et al.32 |
| Vertigo (short term) | 0.033 | 6 months | Cutler et al.,32 originally sourced from Swan et al.50 |
| Tinnitus | 0.050 | 6 months | Cutler et al.,32 originally sourced from Happich et al.51 |
| Wound infection | 0.042 | 6 months | Cutler et al.,32 originally sourced from Prosser et al.52 |
| Vertigo (long term) | 0.033 | Lifetime | Cutler et al.,32 originally sourced from Swan et al.50 |
| Adverse event | Probability | Source |
|---|---|---|
| Dysgeusia | 0.065 | Cutler et al.,32 originally sourced from Hansen et al.,53 Jeppesen et al.,54 Farinetti et al.55 |
| Vertigo (short term) | 0.194 | Cutler et al.,32 originally sourced from Hansen et al.,53 Jeppesen et al.,54 Farinetti et al.,55 Venail et al.,56 Stamatiou et al.57 |
| Tinnitus | 0.036 | Cutler et al.,32 originally sourced from Jeppesen et al.,54 Farinetti et al.,55 Venail et al.56 |
| Wound infection | 0.015 | Cutler et al.,32 originally sourced from Hansen et al.,53 Jeppesen et al.,54 Stamatiou et al.,57 Farinetti et al.,55 Venail et al.56 |
| Vertigo (long term) | 0.014 | Cutler et al.,32 originally sourced from Hansen et al.,53 Jeppesen et al.54 |
Given the relatively short duration of many of these events (with the exception of long-term vertigo), the relatively low probability of occurrence (Table 6) and the relatively low cost of these adverse events (as shown in Table 7), the disutilities and costs associated with adverse events are not included in the early economic model. In a definitive study, the disutilities and costs associated with adverse events should be included in line with standard methods guidelines. 20,25,26 The data in Table 5 on utilities and probabilities in Table 6 suggest that adverse effects that may be included in a definitive economic model may have only a negligible impact on the overall conclusions regarding cost-effectiveness.
| Adverse event | Cost (£) | Source |
|---|---|---|
| Dysgeusia | 31 | Unit Costs of Health and Social Care 2018 58 |
| Vertigo (short term) | 31 | Unit Costs of Health and Social Care 2018 58 |
| Tinnitus | 31 | Unit Costs of Health and Social Care 2018 58 |
| Wound infection | 41 | Unit Costs of Health and Social Care 2018,58 NHS Prescription Charges from April 201759 |
| Vertigo (long term) | 31 | Unit Costs of Health and Social Care 2018 58 |
Health resource use
Following a request for information by NICE, the test manufacturer provided the costs related to the Genedrive MT-RNR1 ID Kit to the EAG, including the cost of purchasing the Genedrive MT-RNR1 ID Kit itself, the cost of the other equipment required to carry out the diagnostic test and the annual warranty fee. In addition, a ‘health economic utility’ paper was provided to NICE by the manufacturer, which reported on the implementation of the test and the potential impact on routine clinical care in terms of the prescribed ‘golden hour’ for the administration of an antibiotic. As mentioned in the NICE Medtech Innovation Briefing (MIB) document,21 estimating the resource consequences from adopting the technology will vary depending on the NHS trust and how much the technology is used. Several pragmatic assumptions have been made in this analysis related to test use and staff costs. Therefore, the costs presented are unlikely to be generalisable to all sites.
Non-staff costs of diagnostic test
Using the information provided by the test manufacturer and information gathered from various other sources (including NHS reference costs and the Unit Costs of Health and Social Care), the cost of implementing the Genedrive MT-RNR1 ID Kit was micro-costed (Tables 8 and 9). The work reported in this subsection addresses the first objective for the cost-effectiveness set out in Objectives.
| Item | Cost (£) |
|---|---|
| Purchase costs | |
| Genedrive MT-RNR1 ID System (GS-002) | 4995 |
| Bluetooth printer + charging cradle | 400 |
| Annual warranty fee for equipment (year 2–year 6) | 750 |
| Genedrive MT-RNR1 ID Kit (per test) | 100 |
| Genedrive MT-RNR1 control kit (one kit per system per month) | 35 |
| Custom labels (200 per pack) | 40 |
| Capital costs | |
| Opportunity cost of Genedrive MT-RNR1 ID System (assume 6 years’ equipment life) | 5624.42 |
| Annual cost of Genedrive MT-RNR1 ID System (assume 6 years’ equipment life) | 937.61 |
| Cost per test of Genedrive MT-RNR1 ID System (assume three tests per site per day) | 0.86 |
| Opportunity cost of Bluetooth printer + charging cable (assume 6 years’ equipment life) | 450.40 |
| Annual cost of Bluetooth printer + charging cable (assume 6 years’ equipment life) | 75.07 |
| Cost per test of Bluetooth printer + charging cable (assume three tests per day) | 0.07 |
| Other costs | |
| Cost of Genedrive MT-RNR1 control kit per test (assume three tests per day) | 0.38 |
| Cost of custom label (one per test) | 0.20 |
| Cost of warranty per test (assume three tests per day) | 0.57 |
| Estimated total non-staff cost per test | 102.08 |
| Item | Minutes | Hourly cost (£) | Total cost (£) | Source |
|---|---|---|---|---|
| Staff costs | ||||
| Nurse (band 5) | 30 | 50 | 25 | Unit Costs of Health and Social Care 2021 61 |
| Nurse (band 6) | 30 | 62 | 31 | Unit Costs of Health and Social Care 2021 61 |
| Total staff cost per test | 28 | |||
The costs of the diagnostic test were assumed to include:
-
cost of the Genedrive MT-RNR1 ID System (GS-002)
-
cost of the Genedrive MT-RNR1 ID Kit
-
cost of the Genedrive MT-RNR1 control kit
-
cost of a Bluetooth printer
-
cost of custom labels
-
annual warranty fee for the Genedrive equipment.
Capital costs of the Genedrive MT-RNR1 ID System and Bluetooth printer were calculated using the equivalent annual cost methodology. 60 This method converts the initial capital cost into an annual sum that equals the resources and investment plus their opportunity cost. The equivalent annual cost of implementing the Genedrive MT-RNR1 ID Kit was calculated under the following assumptions:
-
lifespan of the Genedrive MT-RNR1 ID System and Bluetooth printer: 6 years
-
capital costs spread over its lifespan (6 years)
-
weeks per year in use: 52 weeks
-
Genedrive MT-RNR1 ID Kit usage: three times per day
-
annual warranty fee
-
discount factor of 3.5% (in line with NICE reference case).
Following a request for information from the manufacturer, the lifespan of the Genedrive MT-RNR1 ID System was assumed to be 6 years (Table 8). In documentation provided by the manufacturer, the company recommends running a positive and negative control (both contained in a single Genedrive MT-RNR1 control kit) once per month to confirm that the Genedrive MT-RNR1 ID System is working correctly. It was therefore assumed that each site would undertake the recommended quality control using the control kit once per month. It was also assumed that each site would purchase a Bluetooth printer to print labels (together with a charging cradle) and that custom labels provided by the company would also be purchased. It was further assumed that lifespan of the Bluetooth printer would also be 6 years, in line with the lifespan of the Genedrive MT-RNR1 ID System. As specified by the manufacturer, the Genedrive System has been designed to be easily integrated into a NICU and does not need special storage for either the Genedrive MT-RNR1 ID System itself or the Genedrive MT-RNR1 ID Kit, and therefore it was assumed that no costs were associated with modifying existing infrastructure to accommodate the system.
It was assumed that the Genedrive MT-RNR1 ID Kit would be in use throughout the year. However, estimating the test usage at a site level is complicated by the fact that usage will be determined by the size, type and geographical location of each site. In the NICE MIB document for Genedrive it was assumed there are approximately 90,000 annual admissions to NICUs for neonates with suspected infection in the UK. 21 Given that there are currently estimated to be 72 level 3 NICUs in the UK,61 this indicates that the average number of eligible admissions per NICU per day may be between three and four. In the PALOH study,19 751 neonates were recruited from two centres in an 11-month period (January–November 2020). Owing the COVID-19 pandemic, the majority (n = 713, 94.9%) of these admissions were from a single centre, giving an average number of admissions to the participating site per day of between two and three.
Given the information from both the MIB document and the PALOH study,19,21 in the early economic model, it was assumed that the Genedrive MT-RNR1 ID Kit was used three times per site per day. It is worth emphasising that this estimate is subject to a significant level of uncertainty, given that the use of the equipment per site could vary markedly. However, it is also worth emphasising that because the Genedrive MT-RNR1 ID System itself is a relatively inexpensive medical device, the cost per neonate tested of the Genedrive MT-RNR1 ID System would be negligible over the lifetime of its use even in very small sites, and therefore should not materially impact the cost-effectiveness results.
Staff costs
There are significant staffing requirements in NICUs, with NICE quality standards stating that the minimum standard should be 1 : 1 nursing for all neonates. 62 Additional time for nursing staff to be trained and undertake the diagnostic testing will have cost implications.
Training costs
In terms of training, in the protocol for the PALOH study22 it was stated that a minimum of 80% of all relevant nursing and medical staff within the two NICUs involved in the PALOH study would be trained with this training including practical use and interpretation of the assay, with standard operating procedures for use integrated into the standard admission procedure. It was also stated that a ‘train the trainer’ approach will be adopted, whereby a number of experienced NICU research nurses plus additional clinical nursing staff identified as ‘super-users’ will receive training directly from representatives of the device manufacturer, who will then cascade training to the remaining nursing and medical staff. The Genedrive MIB document22 states that the manufacturer would provide training free and that this training would last between 15 minutes and 1 hour. 21 In the Genedrive MIB document, two of the three experts consulted stated that minimal training would be needed for staff using the technology as it is similar to other point-of-care testing currently used in practice. Estimating the training costs at a site level is difficult to determine, given the different size, type and structures of the different sites. As this is an EVA, training costs were not considered for inclusion in the early economic model. However, given the estimated relatively short time for training and the high potential for use of the Genedrive MT-RNR1 ID Kit, it is likely that the training costs per neonate tested would be negligible, even in smaller sites.
Staff costs of implementing the test
In terms of staff time to implement the diagnostic tests, in the ‘health economic utility’ paper provided by the test manufacturer to NICE, the manufacturer stated that no increase in nursing time was required to implement the assay into practice, pointing to evidence from the PALOH study. 18 However, the sites used in the PALOH trial were large academic teaching hospitals with extensive experience of implementing new technologies. Therefore, clinical experts consulted by the EAG considered it unlikely that staffing requirements for these hospitals would be generalisable to smaller sites where there is less experience of research activity. The Genedrive MIB document21 reported differing views of clinical experts regarding the impact of Genedrive on staffing levels. One expert noted that although the technology itself was relatively simple, its implementation may be hindered by the need to communicate the findings across the healthcare system. One of the clinical experts consulted by the EAG stated that the assumption of no increase in nursing time was very strong, given that a member of staff would need to physically implement the test. In the final scope for Genedrive, experts commented that while the Genedrive MT-RNR1 ID Kit may be intended to be used in a near patient setting, in some hospitals this may not be possible, for example because of a lack of space on neonatal units. If the Genedrive system was instead housed in a laboratory rather than a near-patient setting, this could increase the staff time required to implement the test.
In the early economic model (Table 9), it was assumed that 30 minutes of nurse time would be required to implement each diagnostic test, inclusive of collecting the buccal swab from the neonates, entering the assay into the Genedrive MT-RNR1 ID System, reporting the results and communicating the findings to the other members of the team. In the ‘health economics utility’ paper provided by the test manufacturers, this was the average analysis time reported from sample collection to result. In the early economic model, it was assumed that either band 5 or band 6 nurses would be responsible for carrying out the diagnostic test. Owing to uncertainties regarding the proportion of different bands of nurses working at different sites, it was pragmatically assumed that an equal proportion of band 5 and band 6 nurses would undertake the test, and therefore the hourly cost used is the mid-point of the two cost bandings.
Cost of standard of care
Although there is no current standard care for MT-RNR1 testing in neonatal sepsis, expert opinion and company information suggests that pyrosequencing and Sanger sequencing are the two closest comparators in the NHS. 21 The estimated total costs of pyrosequencing and Sanger sequencing are shown in Table 10. These are retrospective investigations of the cause of hearing loss. Given the uncertainty regarding current standard care, in the economic model it was pragmatically assumed that Sanger sequencing was used, as this was the sequencing method used to confirm the results from the Genedrive MT-RNR1 ID Kit in the PALOH study. 19 Given the relatively small difference in the costs between pyrosequencing and Sanger sequencing, this assumption is likely to have little impact on the results from the early economic model. As well as being used retrospectively in standard care to confirm the cause of hearing loss, it was also assumed that an investigation of hearing loss would also be used retrospective to confirm any positive results from the Genedrive MT-RNR1 ID Kit.
| Cost (£) | Source | |
|---|---|---|
| Diagnostic testing (standard care) | ||
| Pyrosequencing | 212 | MIB Genedrive document21 |
| Sanger sequencing | 191 | MIB Genedrive document21 |
Costs of antibiotics
The implementation of the Genedrive MT-RNR1 ID Kit will have an impact on the antibiotics given to neonates. The first-choice antibiotic regime for the empirical treatment of suspected early-onset infection (˂ 72 hours) is intravenous benzylpenicillin with gentamicin. The starting dosage of this antibiotic regime is 5 mg/kg every 36 hours administered in a single dose. A second dose may be given after 36 hours. A shorter interval can be used if clinical judgement suggests that this is needed. According to the BNF,63 the price of a single vial of benzylpenicillin is between £3 and £4 and the price of a single vial or ampoule of gentamicin is between £1 and £3 depending on the specific brand. For late-onset infection, the first-choice antibiotic regime is a narrow-spectrum antibiotic, such as intravenous flucloxacillin with gentamicin. The starting dose of this antibiotic regime is 50 mg/kg every 6–12 hours. According to the BNF,61 the price of a single vial is between £1 and £4 depending on the specific brand.
If m.1555A>G were to be detected, Clinical Pharmacogenetics Implementation Consortium guidance13 recommends that the use of aminoglycosides be avoided unless the infection is very severe and there is a lack of safe or effective alternative therapies. Therefore, alternative antibiotic therapies would be administered. Alternative antibiotic therapies include cefotaxime and amoxicillin, with the exact antibiotic regime used depending on local antimicrobial guidelines. In the PALOH study, when an infant was identified as carrying m.1555A>G, they were prescribed cefotaxime, which is considered to have comparable antimicrobial coverage to benzylpenicillin with gentamicin. 19 The starting dosage of cefotaxime is 50 mg/kg administered in a single dose. According to the BNF, the price of a single vial is between £2 and £4 depending on the specific brand. 63
As this is an EVA and the various antibiotics that may be used are relatively inexpensive, the antibiotic costs were not included in the early economic model, as their impact on the cost-effectiveness was predicted to be negligible. In a definitive study these costs should be included.
Costs of testing for hearing loss
As part of the NHS newborn screening programme, all neonates should be screened within 26 days of birth for possible hearing loss. 24 An automated otoacoustic emissions test is commonly used in the first instance. If the results are not clear, a second automated otoacoustic emissions test may be conducted, or an auditory brainstem response test may be used. Clinical experts commented that babies with AIHL may have discordant results and that, therefore, all those with a known m.1555A>G variant should be referred for immediate follow-up and additional audiological monitoring. The exact health resource requirements for this additional audiological monitoring are unclear. As all neonates are assumed to be screened as part of the NHS newborn screening programme, the costs of attending the newborn screening programme and the associated automated otoacoustic emissions and auditory brainstem response tests are not included in the economic analysis. Moreover, as the additional monitoring of those with a known m.1555A>G variant is not predicted to differ between current standard care and the proposed care pathway with the Genedrive MT-RNR1 ID Kit, these costs are also not included in the economic analysis.
Costs of hearing aids and cochlear implants
Hearing aids
For those children with severe to profound deafness who do not benefit from acoustic hearing aids, NICE guidelines recommend bilateral hearing aids. 45 The age at cochlear implant surgery was assumed to be 1 year; in order to demonstrate no adequate benefit from hearing aids, children need to have had a valid trial of an acoustic hearing aid for at least 3 months. 64 It was therefore assumed that all neonates with AIHL would be fitted with two acoustic hearing aids for a trial period. The cost of a pair of hearing aids was estimated to be £396 (£198 per individual hearing aid), together with a fitting cost of £249 (Table 11). It was assumed that hearing aids have a lifetime of 5 years, and therefore only one pair would be needed per neonate. 30,32
| Item | Cost (£) | Source |
|---|---|---|
| Pair of hearing aids | 396 | Cutler et al.32 |
| Fitting of hearing aids | 249 | Cutler et al.32 |
Cochlear implants
For cochlear implants, cost estimates were gathered for pre-procedure health resource use, the cost of the procedure itself and post-procedure resource use. Estimates of pre-implant resource use were taken from Cutler et al.,32 a cost-effectiveness analysis of unilateral cochlear implants in UK adults. These estimates were based on clinical expert judgement sought within the development of the clinical pathway for that study. The unit costs used in Cutler et al. 32 were derived from clinical expert opinion, literature reviews, NHS National Schedule of Reference Costs,65 NHS National Tariffs,66 and the Unit Costs of Health and Social Care publication. 58
As outlined by Cutler et al.,32 the cost of fitting a cochlear implant can be split into a number of stages. These include an initial assessment with an audiologist, testing, electrophysiologic assessments, surgeon and general practitioner consultation and a pre-procedural assessment. Although these estimates were gathered specifically in relation to adult testing, the costs are estimated to be broadly similar to those for children. A previously published budget impact assessment of cochlear implants in children in Scotland estimated the total costs of pre-surgery assessments to be £1575 (inflated to 2022 prices). This estimate is broadly in line with the costs presented in Table 12. 67
| Item | Cost (£) | Source |
|---|---|---|
| Stage 1: initial assessment | ||
| Audiologist initial assessment | 100 | NHS National Schedule of Reference Costs65 |
| Speech and language therapist | 114 | NHS National Schedule of Reference Costs65 |
| Stage 2: testing | ||
| Vestibular assessment and tests | 100 | NHS National Schedule of Reference Costs65 |
| Radiologist | 105 | Unit Costs of Health and Social Care58 |
| MRI scan | 164 | NHS National Schedule of Reference Costs65 |
| CT scan | 105 | NHS National Schedule of Reference Costs65 |
| Stage 3: electrophysiology | ||
| Audio scientist | 100 | NHS National Schedule of Reference Costs65 |
| Electrophysiology assessment | 84 | Unit Costs of Health and Social Care58 |
| Stage 4: medical assessment | ||
| Audiologist preoperative assessment | 100 | NHS National Schedule of Reference Costs65 |
| ENT surgeon consultation | 124 | NHS National Schedule of Reference Costs65 |
| Anaesthetist consultation | 155 | NHS National Schedule of Reference Costs65 |
| Multidisciplinary team meeting (audiology, SLT, ENT) | 338 | NHS National Schedule of Reference Costs65 |
| GP consultation | 37 | Unit Costs of Health and Social Care58 |
| Meningitis vaccination | 71 | NHS Vaccine Price List |
| Stage 5: pre-procedural assessment outcome discussion | ||
| Cochlear implant surgery co-ordinator | 52 | Unit Costs of Health and Social Care58 |
| Total pre-surgery costs | 1749 | |
The procedure and post-procedure costs were taken from TA56645 (a partial review of TA166) and originally based on the assumptions made in Bond et al. 30 regarding the long-term cost implications of cochlear implants. These include the costs of the procedures themselves and multiple hearing assessments in the first year post procedure, as well as post-procedure, annual maintenance and rehabilitation costs. It should be noted that the resource use associated with cochlear implant surgery, and subsequently used in TA566 and this report (Table 13), is lower than that used in Barton et al. 68 when considering inflation and higher than that used in Cutler et al. 32 The cost estimates in these studies were also based on clinical expert opinion.
| Item | Cost (£) | Source |
|---|---|---|
| Unilateral cochlear implant | ||
| Procedure (assuming Children’s Services Surgery Multiplier of 34.38%) | 36,049 | TA566 resource impact template45 |
| Audiometry or hearing assessment | 1650 | TA566 resource impact template45 |
| Cochlear implant maintenance and programming: year 1 | 3290 | TA566 resource impact template45 |
| Cochlear implant maintenance and programming: year 2 (ongoing) | 823 | TA566 resource impact template45 |
| One-to-one rehabilitative audiology service: year 1 | 993 | TA566 resource impact template45 |
| One-to-one rehabilitative audiology service: year 2 (ongoing) | 124 | TA566 resource impact template45 |
| Procedure + assessment total | 37,699 | |
| Costs in first year post procedure | 4283 | |
| Ongoing yearly costs after year 1 | 947 | |
| Bilateral cochlear implant | ||
| Procedure (assuming Children’s Services Surgery Multiplier of 34.38%) | 59,618 | TA566 resource impact template45 |
| Audiometry or hearing assessment | 1650 | TA566 resource impact template45 |
| Cochlear implant maintenance and programming: year 1 | 3290 | TA566 resource impact template45 |
| Cochlear implant maintenance and programming: year 2 (ongoing) | 823 | TA566 resource impact template45 |
| One-to-one rehabilitative audiology service: year 1 | 993 | TA566 resource impact template45 |
| One-to-one rehabilitative audiology service: year 2 (ongoing) | 124 | TA566 resource impact template45 |
| Procedure + assessment total | 61,268 | |
| Costs in first year post procedure | 4283 | |
| Ongoing yearly costs after year 1 | 947 | |
Early economic modelling
In Developing an economic evaluation model, the key features of the economic model required for the full economic evaluation were outlined. For some of these, data are sparse or lacking altogether. Table 14 outlines the key parameters for which good-quality evidence is needed for a full economic evaluation but currently not available.
| Evidence gaps |
|---|
| Proportion of neonates who require antibiotics immediately and therefore will not be tested using the Genedrive MT-RNR1 ID Kit |
| Proportion of neonates with the m.1555A>G variant treated with aminoglycosides who experience AIHL |
| Proportion of neonates with the m.1555A>G variant treated with aminoglycosides who experience mild/moderate/severe/profound AIHL |
| Proportion of neonates with the m.1555A>G variant not treated with aminoglycosides who experience nonsyndromic hearing loss |
| Proportion of neonates with the m.1555A>G variant not treated with aminoglycosides who experience mild/moderate/severe/profound nonsyndromic hearing loss |
| The proportion of neonates with either AIHL or nonsyndromic hearing loss who require hearing aids, unilateral cochlear implants and bilateral cochlear implants |
| Valid utility values for children under 5 years with different degrees of hearing loss (either AIHL or nonsyndromic) and different types of cochlear implant |
| The impact of adverse events related to AIHL on costs and utilities |
| Proportion of neonates who are considered for treatment with aminoglycosides |
| Proportion of women in labour who are identifiable with risk factors (for infection or sepsis of the neonate) |
| Proportion of women in labour for whom maternal inheritance data exist |
| Proportion of neonates for whom maternal inheritance data exist |
Given the evidence gaps shown in Table 14, an early economic model was used rather than attempting to conduct a full economic model. Early economic evaluation provides an initial assessment of whether a technology has the potential to be cost-effective (and under what conditions) and can help prioritise further required research and the evidence needed to populate a full economic model.
The early economic model used in this assessment is a simplification of the proposed economic model set out in Developing an economic evaluation model and illustrated in Figure 6. It follows the same fundamental structure set out in Figure 6 but makes a series of simplifying assumptions. These are set out in Table 15, in which we describe some key features of the proposed full economic model and how these have been adapted for the early economic model. These simplifications have been made following consideration of when inclusion of a given model feature would be unlikely to change estimates of cost-effectiveness or where there is an evidence gap (Table 14). As already noted, these evidence gaps would need to be addressed before a full economic model could be conducted.
| Key features of the full economic model | Changes in early economic model |
|---|---|
| The population modelled are neonates with early-onset and late-onset infection who need antibiotic treatment and are being considered for treatment with aminoglycosides | No change |
| Some neonates will require antibiotic administration immediately (i.e. there is no time for the test before an antibiotic must be started) | No neonates required antibiotic administration immediately – it was assumed that all neonates were tested |
| Increased time to antibiotics will increase the risk of death for neonates with sepsis | Time to antibiotics was not included as part of the early economic model |
| There is a chance the Genedrive MT-RNR1 ID Kit will give a false-negative result | In the base case, the Genedrive MT-RNR1 ID Kit was assumed to have perfect accuracy. This assumption was tested in the deterministic sensitivity analysis |
| There is a chance the Genedrive MT-RNR1 ID Kit will give a false-positive result | No change |
| There is a chance that the Genedrive MT-RNR1 ID Kit will fail to give a result | No change |
| If the first Genedrive MT-RNR1 ID Kit fails to give a result, there is time for a second test | No change |
| The clinical pathway for neonates with early-onset and late-onset infection are different (in terms of duration of antibiotic prescription) | We assumed the same clinical pathway for neonates with early-onset and late-onset infection |
| If both the first Genedrive MT-RNR1 ID test kit and the second Genedrive MT-RNR1 ID test kit fail, there will be insufficient time for further testing, and neonates with suspected infection will not be treated with aminoglycosides and will receive other antibiotics | The second Genedrive MT-RNR1 ID test was assumed to never fail |
| Where neonates are identified as not having the m.1555A>G variant using the Genedrive MT-RNR1 ID Kit, aminoglycosides will be used | No change |
| Where neonates are identified as having the m.1555A>G variant using the Genedrive MT-RNR1 ID Kit, alternative antibiotics will be used | No change |
| Different aminoglycosides will have different adverse event profiles | All classes of aminoglycoside (gentamicin, amikacin, tobramycin and neomycin) were assumed to have the same adverse reaction profile |
| For neonates with the m.1555A>G variant treated with aminoglycosides, there is a risk of AIHL | In the base case it was assumed that all neonates with the m.1555A>G variant treated with aminoglycosides would experience AIHL. This assumption was tested in the deterministic sensitivity analysis |
| For neonates with the m.1555A>G variant not treated with aminoglycosides, there is a risk of nonsyndromic hearing loss | In the base case it was assumed that no neonates with the m.1555A>G variant not treated with aminoglycosides would experience hearing loss. This assumption was tested in the deterministic sensitivity analysis |
| For neonates with AIHL, the severity of the hearing loss may vary | In the base case it was assumed that if AIHL occurs it will result in severe/profound irreversible deafness |
| Women with risk factors (for sepsis) are eligible for Genedrive MT-RNR1 ID Kit, but antibiotic prescription will be for neonates (after birth) | We assume the same prevalence of disease (suspected to sepsis) and gene mutation in mothers and neonates |
| Maternal inheritance may be considered before testing | Maternal inheritance will not be considered before testing |
| An increased time to antibiotics will increase the risk of death for neonates | Time to antibiotics was not included in the early economic model |
| The use of the Genedrive MT-RNR1 ID Kit, other than affecting time to administration and the type of antibiotic used, does not affect normal standard of care for neonates presenting with suspected infection or sepsis | No change |
| There will be training costs for staff to carry out the test | Training costs were excluded. It was assumed that training costs would not have a large impact on the cost-effectiveness results from the model |
| Staff time is required to carry out the test | No change |
| Additional audiological monitoring will be required for infants with AIHL | The costs of this additional monitoring were not included |
| There are pre-procedure, procedure and post-procedure costs associated with both unilateral and bilateral cochlear implants | No change |
| AIHL has several associated adverse events | It was assumed the adverse events of AIHL would not have a large impact on the cost-effectiveness results from the model |
| If AIHL occurs, neonates will require hearing aids, unilateral cochlear implants or bilateral cochlear implants | In the base case it was assumed that if AIHL occurs all neonates will require bilateral cochlear implants. This assumption was tested in the deterministic sensitivity analysis |
| HRQoL will vary by age, level of hearing loss, type of cochlear implant and time since cochlear implant has been implanted | It was assumed that hearing loss could vary by type of cochlear implant and time since the cochlear implant was implanted. It was assumed that all neonates with AIHL would experience profound hearing loss. Utility values for children aged ≥5 years were used as proxies for children aged < 5 years. Different utility values were used for those aged < 18 years and ≥ 18 years; however, no further age-adjustment was used |
| To demonstrate no adequate benefit from hearing aids, children need to have had a valid trial of an acoustic hearing aid for at least 3 months | No change |
| There is a chance that the cochlear implant surgery will not be successful | No change |
| It is possible to upgrade cochlear implants after they have been fitted | It was assumed it was not possible to upgrade cochlear implants |
| There may be complications associated with the implantation of cochlear implants (i.e. internal or external device failure, death) | It was assumed there are no complications associated with the implantation of cochlear implants |
| There are short-term and long-term adverse events associated with cochlear implants | Adverse events related to cochlear implants were not included |
The early economic Markov model was informed by the key features described in Table 15. The model starts with the presentation of a neonate with suspected infection or sepsis. For the Genedrive pathway in the model the neonate receives the Genedrive test (Figure 7). All the events described in Figure 7 are assumed to occur in the first cycle (i.e. year 1) of the model. Although not shown in Figure 7, neonates may also receive an additional test to confirm AIHL (Sanger sequencing). In terms of cost, we excluded costs associated with the different antibiotics that may be prescribed to the neonate with suspected infection or sepsis, as the impact of these on the cost-effectiveness was predicted to be negligible.
FIGURE 7.
Decision tree of using Genedrive MT-RNR1 ID Kit in the Markov model.
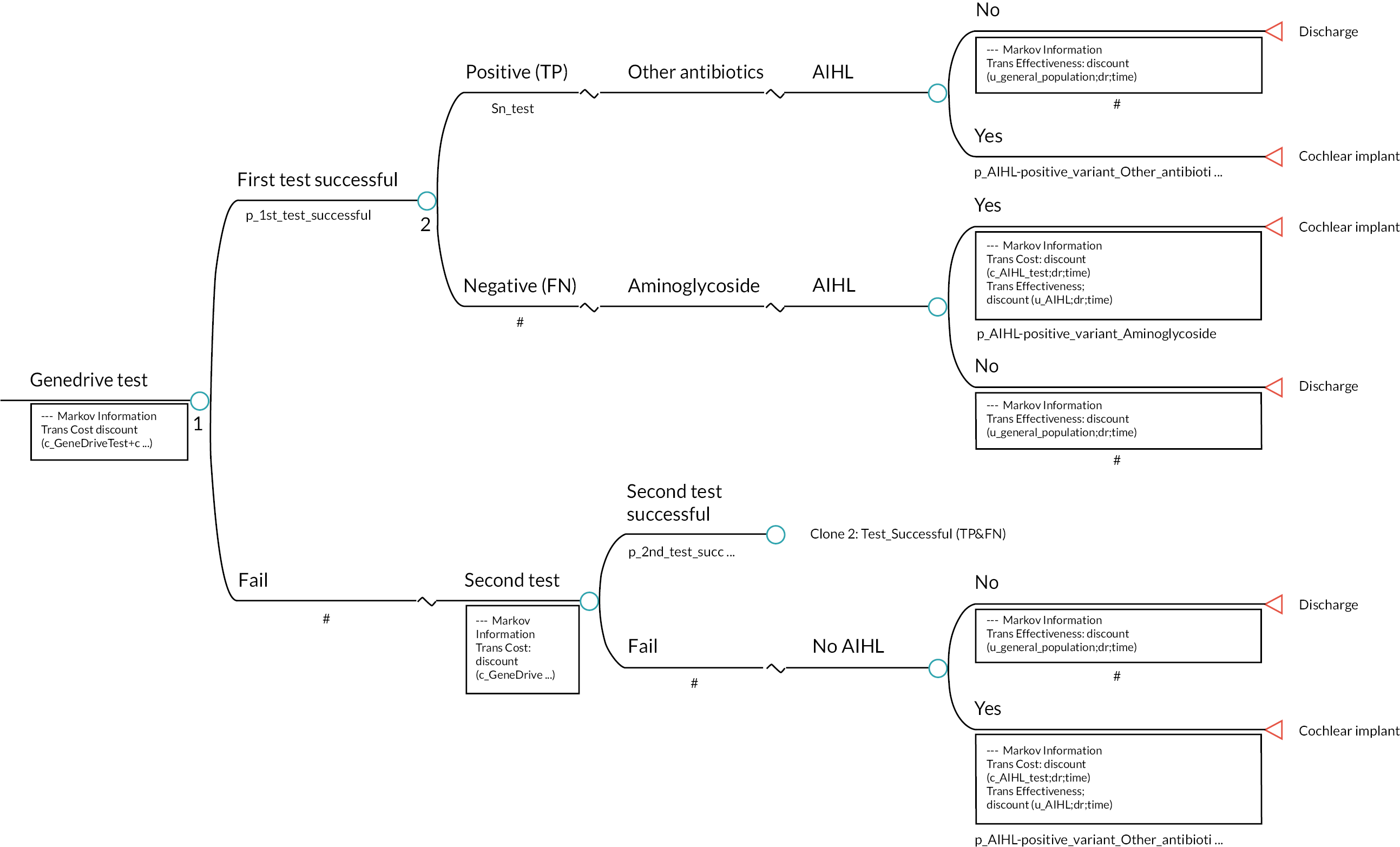
As shown in Figure 7, the destination of the neonate in terms of which Markov state they transition to is based on the results of the Genedrive test. For (true) positive cases, alternative antibiotic therapies (e.g. cefotaxime) are prescribed instead of aminoglycosides, and neonates move to the ‘discharge’ state. These neonates will not experience AIHL. The model structure allows the possibility that some neonates with gene mutation will suffer hearing loss even if they receive other types of antibiotics. These neonates would move to the to the cochlear implant state in the next cycle. However, in the base-case analysis the probability of this occurring is set to zero.
For (false) negative cases, neonates will be prescribed aminoglycosides. This may result in AIHL, and these neonates also move to the cochlear implant state in the next cycle. Again, the model allows the possibility that some neonates with gene mutation will not experience AIHL even if they have received aminoglycosides. In the base-case analysis we assumed that the Genedrive test has near-perfect accuracy. We also assumed that all neonates with the gene mutation who received aminoglycosides will experience AIHL and that none of neonates who were prescribed with other antibiotics will experience hearing loss.
As part of the early economic model, we also modelled what would happen if the first test failed to provide a result. Here we allowed the possibility that a second test could be conducted. If the second test was successful, the neonate followed the same pathway they would have done had the first test been successful. If the second test failed, we assumed that there would be no time to conduct a third test and that neonates would not be prescribed aminoglycosides and instead would be prescribed alternative antibiotics, such as cefotaxime.
For neonates who follow the standard pathway, the Genedrive MT-RNR1 ID Kit is not used, and those with the m.1555A>G variant receive an aminoglycoside and experience AIHL. Those without the variant likewise receive an antibiotic but do not experience AIHL and are discharged from care. Thus, the pathway here is a simplification of that described in Figure 7.
After the sequence of events described in Figure 7 occurs, the individual modelled can move to one of two states: discharge or cochlear implant. Those who move to the discharge state stay there for the rest of their life. Those who move to the cochlear implant state (i.e. infants with AIHL) receive a unilateral/bilateral cochlear implantation. In either case the implant has a probability of failing. In this situation other forms of hearing support are used. This process of care is described in Figure 8 and is assumed to all take place in a single cycle of the model. At the end of the cycle, individuals move to states where either the cochlear implant is working or other forms of hearing support are needed. In the base-case analysis, individuals stay in these states for the rest of their lives.
FIGURE 8.
Decision tree for the cochlear implant state with the Markov model.
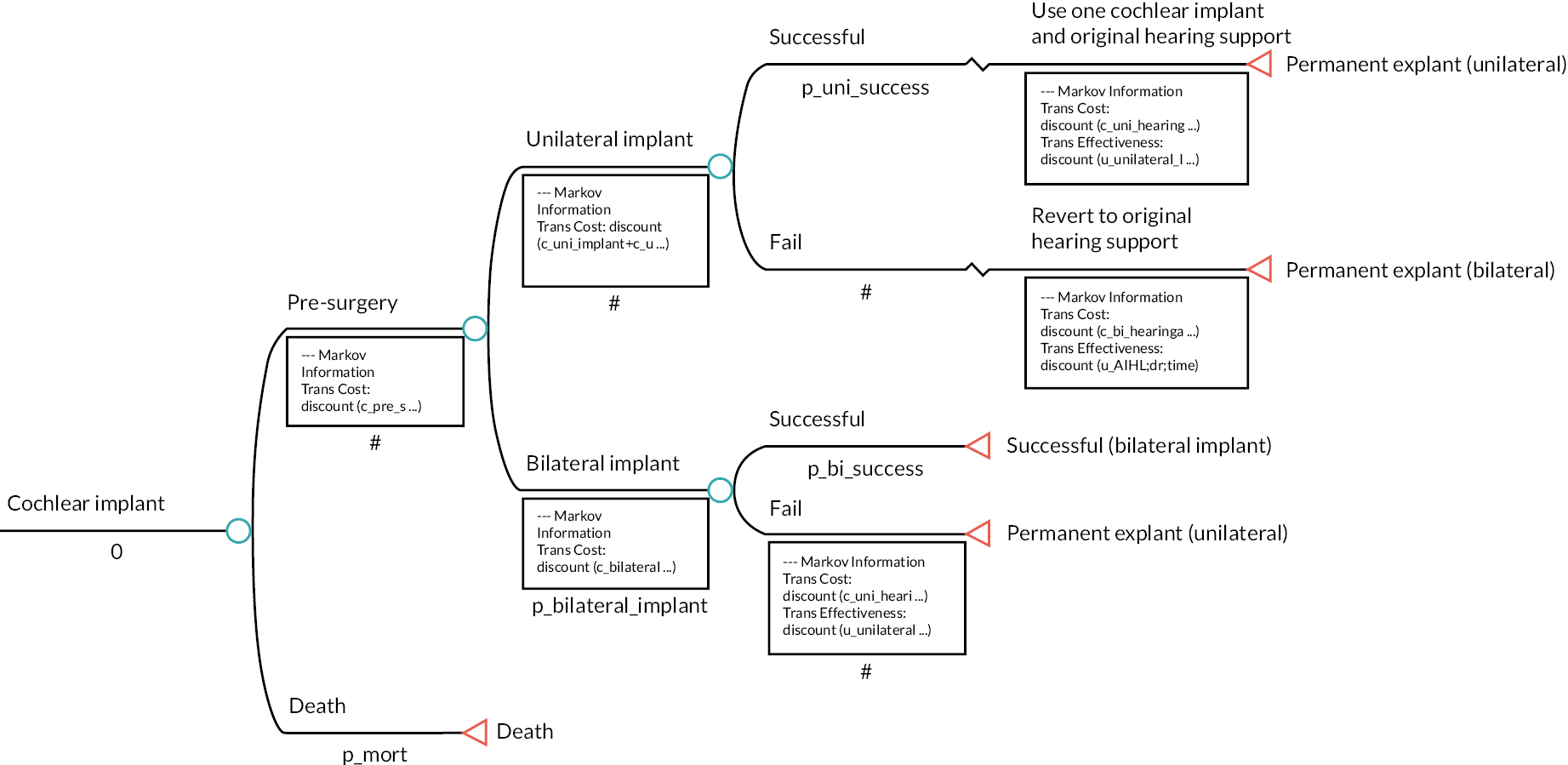
Model parameters
The parameters used in the early economic model are shown in Tables 16–19.
| Parameter | Base-case value | Sensitivity analysis values (low–high) | Source |
|---|---|---|---|
| Time horizon (years) | 100 | 1–100 | Model assumption |
| Starting age (years) | 0 | – | Model assumption |
| Discount rate (%) | 0.035 | 0.015–0.05 | Model assumption |
| Prevalence of MT-RNR1 variant m.1555A>G in the UK population | 0.0019 | 0.0010–0.0028 | Bitner-Glindzicz et al.4 |
| Probability of first Genedrive MT-RNR1 ID test being successful | 0.943 | 0.5–1 | PALOH study19 |
| Probability of second Genedrive MT-RNR1 ID test being successful | 1 | 0.8–1 | Model assumption |
| Probability of AIHL for neonates with the m.1555A>G variant prescribed with aminoglycoside | 1 | 0.3–1 | Model assumption, Göpel et al.6 |
| Probability of AIHL for neonates with the m.1555A>G variant prescribed with other antibiotics | 0 | 0–0.30 | Model assumption, Ballana et al.,2 Estivill et al.3 |
| Proportion of cases with bilateral cochlear implant | 1 | 0.5–1 | Model assumption |
| Probability of unilateral or bilateral cochlear implant being successful | 0.97 | 0.8–1 | Wang et al.69 |
| Probability of death for the model cohort | UK life table mortality 2022 | – | National Life Tables UK 2018 to 202027 |
| Parameter | Base-case value | Sensitivity analysis values (low–high) | Source |
|---|---|---|---|
| Genedrive MT-RNR1 ID Kit price (per test) | £102 | £50–150 | Model assumption |
| Genedrive MT-RNR1 ID Kit accuracy (sensitivity) | 1 | 0.292–1 | PALOH study19 |
| Genedrive MT-RNR1 ID Kit accuracy (specificity) | 0.992 | 0.980–997 | PALOH study19 |
| Parameter | Base-case value (£) | Sensitivity analysis values (low–high) (£) | Source |
|---|---|---|---|
| Cost of Sanger sequencing | 191 | 150–250 | MIB document21 |
| Cost of unilateral hearing aids | 447 | 400–500 | Cutler et al.32 |
| Cost of bilateral hearing aids | 645 | 600–700 | Cutler et al.32 |
| Cost of unilateral cochlear implant (procedure + assessment total) | 37,699 | 20,000–50,000 | TA56645 |
| Cost of bilateral cochlear implant (procedure + assessment total) | 61,268 | 40,000–80,000 | TA56645 |
| Cost of unilateral cochlear implant (first year post procedure) | 4283 | 2000–6000 | TA56645 |
| Cost of bilateral cochlear implant (first year post procedure) | 4283 | 2000–6000 | TA56645 |
| Annual ongoing cost of unilateral or bilateral cochlear implant | 947 | 500–1500 | TA56645 |
| Cost for the staff (nurse) doing the test | 28 | 15–40 | Unit Costs of Health and Social Care 2021 61 |
| Aggregated pre-surgery costs associated with unilateral or bilateral cochlear implants | 1749 | 1500–2000 | Cutler et al.32 |
| Parameter | Base-case value | Sensitivity analysis values (low–high) | Source |
|---|---|---|---|
| Children (aged < 18 years) | |||
| No hearing loss (population norm) | 0.908 | 0.899–0.917 | Pogany et al.46 |
| Profound/significant hearing loss | 0.421 | 0.398–0.452 | Barton et al.29 |
| Unilateral cochlear implant (˂ 2 years since implant) | 0.487 | 0.408–0.565 | Barton et al.29 |
| Unilateral cochlear implant (2–4 years since implant) | 0.633 | 0.582–0.684 | Barton et al.29 |
| Unilateral cochlear implant (over 4 years since implant) | 0.653 | 0.605–0.701 | Barton et al.29 |
| Bilateral cochlear implant (˂ 2 years since implant) | 0.490 | 0.411–0.568 | Barton et al.,29 Bond et al.30 |
| Bilateral cochlear implant (2–4 years since implant) | 0.636 | 0.585–0.687 | Barton et al.,29 Bond et al.30 |
| Bilateral cochlear implant (over 4 years since implant) | 0.656 | 0.608–0.704 | Barton et al.,29 Bond et al.30 |
| Adults (aged ≥ 18 years) | |||
| No hearing loss (population norm) | 0.850 | 0.841–0.859 | Pogany et al.46 |
| Profound/significant hearing loss | 0.433 | 0.407–0.468 | UKCISG37 |
| Unilateral cochlear implant | 0.630 | 0.609–0.651 | UKCISG37 |
| Bilateral cochlear implant | 0.633 | 0.585–0.734 | Summerfield et al.35 |
Estimation of cost-effectiveness and sensitivity analysis
Estimation of cost-effectiveness
Using the data set out in Tables 16–19, two estimates of cost-effectiveness were produced:
-
incremental cost per case of AIHL avoided
-
incremental cost per QALY gained.
For each point, costs and effects for neonates that either follow current standard of care or are tested with the Genedrive MT-RNR1 ID Kit for detecting single nucleotide polymorphism m.1555A>G were estimated. From these incremental costs, effects and incremental cost-effectiveness were calculated.
Deterministic sensitivity analysis
We also conducted a deterministic sensitivity analysis to explore the uncertainty regarding key parameters in the early economic model, using the sensitivity analysis values shown in Tables 16–19. This sensitivity analysis focused on costs, QALYs and incremental cost per QALY only. Owing to the uncertainty regarding the majority of the parameters in the early economic model, a probabilistic sensitivity analysis was not implemented.
For the prevalence of the m.1555A>G variant, the high and low values used in the sensitivity analysis were the 95% CIs from Bitner-Glindzicz et al. 4 For the sensitivity and specificity values, the high and low values used in the sensitivity analysis were the 95% CIs reported in the PALOH study. 19 For the utility values, the high and low values for the sensitivity analysis were the 95% CIs from the original studies from which the values were sourced.
For the parameter related to the probability of AIHL for neonates with the m.1555A>G variant prescribed with aminoglycosides, the lower bound estimate (0.3) was taken from Göpel et al., a prospective cohort study in a German population. 6 For the other parameters (including all of the cost parameters), reasonable high and low values were chosen to explore the potential uncertainty related to these parameters.
Model results
Base-case results
Using the parameters shown in Model parameters, the base-case results from the early economic model are shown in Table 20 for the cases of AIHL avoided and Table 21 for QALYs. In terms of AIHL, the results show that using the Genedrive MT-RNR1 ID Kit is estimated to be cost saving over the lifetime of the neonate tested for the m.1555A>G genetic variant with the Genedrive MT-RNR1 ID Kit.
| Strategy | Total costs (£) | Cases of AIHL | Incremental cost (£) | Incremental AIHL avoided | ICER (£) |
|---|---|---|---|---|---|
| Genedrive MT-RNR1 ID Kit | 151.45 | 0 | –58.48 | 0.002 | Dominant |
| Normal standard of care | 209.93 | 0.002 |
| Strategy | Total costs (£) | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| Genedrive MT-RNR1 ID Kit | 151.45 | 23.12 | –58.48 | 0.01 | Dominant |
| Normal standard of care | 209.93 | 23.11 |
In terms of cost of per QALY, the results show that the Genedrive MT-RNR1 ID Kit dominates the current standard of care over the lifetime, as it is less costly and more effective (Table 21).
Results of deterministic sensitivity analysis
The results of the deterministic sensitivity analysis are presented in a tornado plot (Figure 9). The tornado shows the impact of the high and low parameter values specified in Tables 14–18 on the estimated incremental cost-effectiveness ratio (ICER).
FIGURE 9.
Tornado plot of Genedrive MT-RNR1 ID Kit pathway vs. standard pathway. EV, expected value.
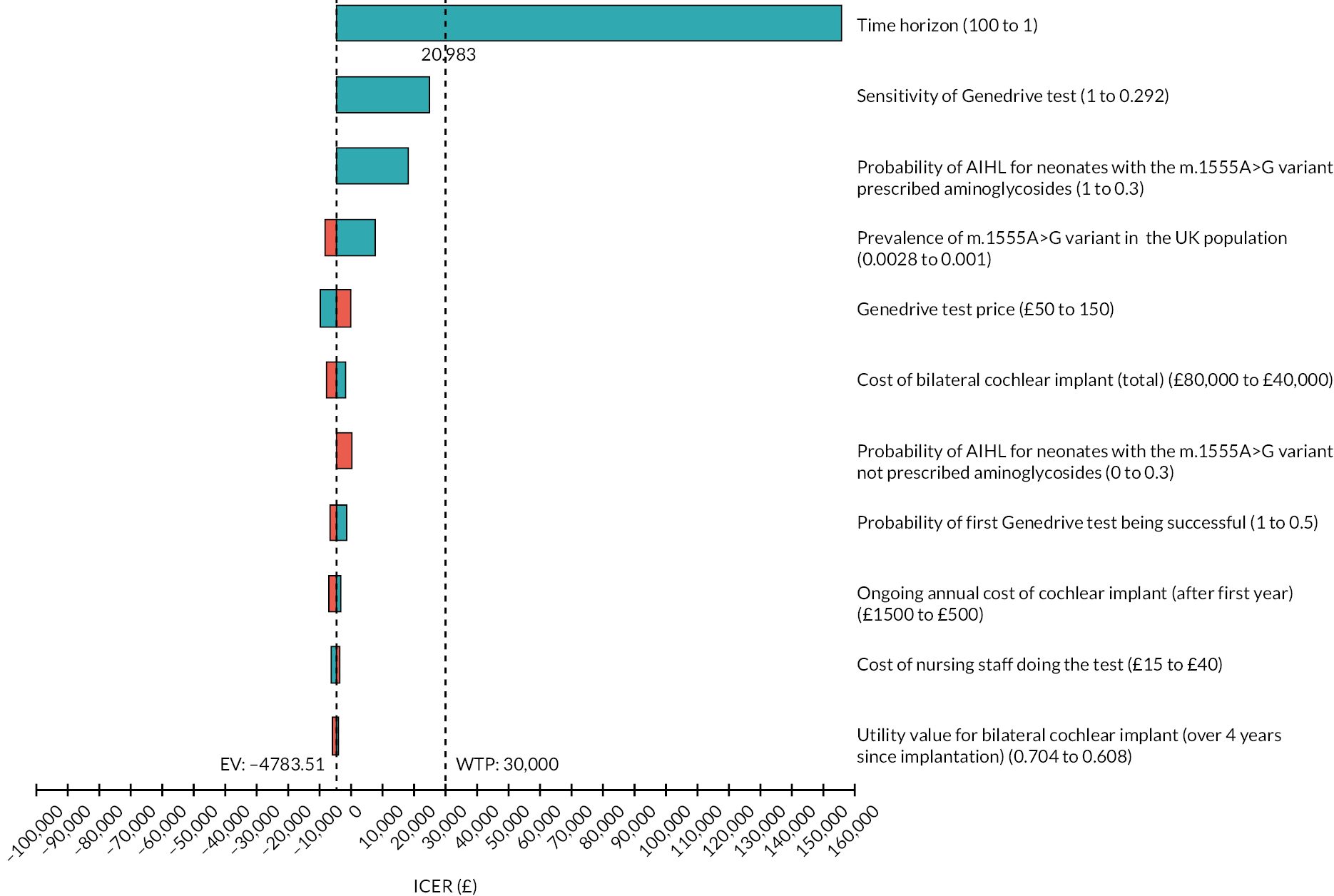
As shown in Figure 9, the parameter values that have the largest impact on the ICER are the time horizon of the model, the sensitivity of the Genedrive MT-RNR1 ID Kit, the probability of neonates with the m.1555A>G variant prescribed with aminoglycosides suffering from AIHL, the prevalence of the m.1555A>G variant across the population and the cost of the Genedrive MT-RNR1 ID Kit. As Figure 9 shows, varying other parameter values (e.g. the utility values associated with bilateral cochlear implants and the probability of cochlear implants being successful) did not appear to materially impact the incremental cost per QALY.
The sensitivity of the results to the time horizon reflects the fact that from an NHS resource use perspective, significant costs are required to identify one neonate with the m.1555A>G variant, while the benefits (specifically cost savings related to cochlear implants avoided and utility gains from avoiding AIHL) are likely to only be felt in the medium to long term. Table 22 illustrates this by showing the impact on cost-effectiveness of varying the time horizon between 1, 10 and 50 years. As shown in Table 22, although the Genedrive MT-RNR1 ID Kit has a very large ICER when compared with normal standard of care for a 1-year time horizon, the kit has an incremental cost per QALY of just over £100 when using a 10-year time horizon and dominates the current normal standard of care when using a 50-year time horizon.
| Time horizon | Strategy | Total costs (£) | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 1-year time horizon | Genedrive MT-RNR1 ID Kit | 151.45 | 0.90 | 151.07 | 0.00 | 155,767 |
| Normal standard of care | 0.38 | 0.90 | ||||
| 10-year time horizon | Genedrive MT-RNR1 ID Kit | 151.45 | 7.78 | 0.62 | 0.01 | 103 |
| Normal standard of care | 150.83 | 7.77 | ||||
| 50-year time horizon | Genedrive MT-RNR1 ID Kit | 151.45 | 20.42 | –48.43 | 0.01 | Dominant |
| Normal standard of care | 199.88 | 20.41 |
The impact of the sensitivity of Genedrive MT-RNR1 ID Kit on the cost-effectiveness results reflects the fact that the real-world sensitivity of the Genedrive test (as reported in the PALOH study19) is highly uncertain due to the very small number of positive cases. This uncertainty was reflected in the reported wide CIs used as the high and low values in the deterministic sensitivity analysis.
The sensitivity of the results to the proportion of neonates with m.1555A>G variant experiencing AIHL after being exposed to aminoglycosides reflects the inherent uncertainty related to this parameter. As discussed in Prevalence of m.1555A>G variant and risk of aminoglycoside-induced hearing loss, although there is clear evidence that m.1555A>G variant is a risk factor for AIHL, most evidence comes from case–control studies that may overestimate this risk, and, therefore, the precise level of this risk is unknown.
With respect to the sensitivity of the results to the prevalence of the m.1555A>G variant among the population, this parameter affects how many neonates need to be tested to detect a single neonate with the m.1555A>G variant. As the probability increases, fewer neonates need to incur the cost of testing to detect a neonate with the variant, and hence the cost-effectiveness of using the Genedrive MT-RNR1 ID Kit improves. Although no data were available to consider how cost-effectiveness varied by different subgroups, this sensitivity analysis helps illustrate how cost-effectiveness might vary if testing were focused on subgroups in whom the m.1555A>G variant is more prevalent.
For the cost of the Genedrive MT-RNR1 ID Kit, the analysis made some simplifying assumptions about what costs were included. Although there is some variation in the incremental cost per QALY, the sensitivity analysis shows that including these costs would not substantially alter the cost-effectiveness results over the lifetime horizon.
Chapter 6 Public involvement
Methods
Public involvement took place at a single time point, at an influencing level as described within the ACTIVE continuum of involvement, using two different approaches. 70
The first approach was to scan data held on social media forums. A total of 40,346 individual posts (39,374 from the children’s health section of Mumsnet and 972 from the National Deaf Children’s Society forum) were collected using a custom web-scraping script written in Python (Python Language Reference, version 2.7). Posts were then filtered using search terms generated by an information specialist (concepts as follows: newborn; infection; antibiotics; hearing loss) applied with gestalt pattern matching, with a match of ≥ 80% considered relevant (Python, Difflib). 71 A second filter was applied using two regular expression searches (‘(\d+) year[s]? old’ and ‘(\d+) month[s]? old’) to identify posts mentioning children under 1 year old. Less relevant posts identified using broader terms reflective of only two concepts, ‘antibiotics’ and ‘baby’, were removed. This left 92 individual posts, which a single researcher manually screened and thematically analysed using a reflexive, inductive approach. 72
The second approach was a focus group. Recruitment was facilitated through contact with organisations and individuals relevant to the EVA-scope population. Present at the focus group were mothers of newborns (n = 1), mother of toddlers (n = 3), professionals who care for newborns and their families (n = 2) and an effectiveness reviewer (n = 1). Participants consented to recording via Zoom (Zoom Video Communications, San Jose, CA, USA), and handwritten notes were also taken, and Zoom chat messages were monitored and saved. Introductions were given to establish a climate of trust among the attendees and to begin active listening. To orientate participants, they were given a high-level overview of the NICE technology appraisal and EVA; diagnostic test accuracy review; bacterial infection, and sepsis and its treatment; the MT-RNR1 gene variant m.1555A>G; and the proposition of testing for this variant within the infection or sepsis care pathway. Participants were given regular opportunities to ask questions about the information given. Participants were then asked to share their thoughts and feelings about testing for m.1555A>G during the infection or sepsis care pathway. Facilitation was neutral to allow for open discussion. However, discussions of how participants would make decisions on test use, treatment before or after testing and outcomes of importance were probed. Recordings were transcribed by a single researcher using rapid intelligent verbatim transcription. 73 One researcher then thematically analysed the data, abstracting and organising concepts into broad themes and then seeking cross-cutting commonalities using a reflexive, inductive approach. The identified codes and themes were reviewed, and their relevance was agreed on by other researchers in the team.
Findings
Social media posts
The 92 social media posts centre around three descriptive themes: neonatal sepsis experiences, infection causing hearing loss, and hearing loss from gentamycin use.
Neonatal sepsis experiences
Families of infants who have had sepsis are using social media to:
-
share experiences of neonatal sepsis, connect with other parents with shared experiences –
After a load of tests … sepsis, a week on two broad spectrum antibiotics …
… other mums of sepsis babies …?
-
understand and express fear of perceived long-term consequences of sepsis
-
future infection rate and impact –
-
… still suffers a lot of viral infections and bacterial infections now aged 3 …
-
potential side effects of antibiotic treatment on feeding and digestion –
… what is wrong with Lo and his feeding … One of the things that I’ve read can cause it is antibiotics in early life …
Infection (sepsis or meningitis) as a cause of hearing loss
Families of infants who have had sepsis or meningitis and now have hearing loss are using social media to:
-
explore the aetiology of their child’s hearing loss
-
share experience of difficulties finding child care –
… childminders are not getting back to me …
-
share experience of difficulties using hearing aids –
… hair is now brushing the back of the aids I wonder if that is causing distress …
-
express a need for earlier hearing loss testing after infection –
… trying to arrange a hearing test but … audiology department is not being particularly forthcoming …
Hearing loss and gentamicin use
Families of infants with hearing loss potentially due to gentamicin:
-
express a preference for treatment with gentamicin, with a perceived trade-off between side effects and effective treatment –
… antibiotics are given with best intentions at the time, and … was very poorly so I would rather … had the antibiotics …
-
have a lack of clarity on alternate treatment options and comparative effectiveness –
… I’m not sure if there are options for other antibiotics which are strong enough …
-
want use of treatments with ototoxic potential to be after informed consent given –
… doctor should have advised and obtained informed consent …
-
indicate a lack of clarity on safe dosing –
doctor told us that … didn’t receive ‘too much’ however we don’t know the impact of what … did receive
-
indicate the utility of test to inform future treatment for individual and offspring –
have it (variant) … this done as could have issues if needed … these drugs again … could pass this to … children
-
desire clarity on the aetiology of hearing loss
-
emotional support dealing with a lack of clarity about aetiology of hearing loss and moving on from search for a cause –
-
I haven’t thought about it for a long time … move on from finding a cause … is what it is …
-
identify that testing for the variant may not give this –
tested negative so we were no further forward
Focus group discussion
Two overarching analytical themes emerged from focus group discussion: information need to inform parents decision to test and/or treat; and testing desirability depending on context.
Information need to inform parents decision to test and/or treat.
Information was wanted on:
-
The prevalence of the variant (see Prevalence of m.1555A>G variant and risk of aminoglycoside-induced hearing loss, evidence available) –
what is the chance of having the variant where you might be predisposed to losing your hearing.
-
The test’s accuracy and safety both in children (see Diagnostic test accuracy, limited evidence) and in mothers (Table 1, no evidence).
So I suppose I’m just asking how … safe is this test
… it would be a non-starter for me if there was any question on the accuracy of the test …
… how accurate it is when they test the mother …
-
The chance of hearing loss developing after aminoglycosides are taken when the variant is not present (see Prevalence of m.1555A>G variant and risk of aminoglycoside-induced hearing loss, limited evidence) and when the variant is present (evidence not sought) –
… the risk of taking the initial drug and losing your hearing …
-
The chance of spontaneous hearing loss with the variant (see m.1555A>G variant and nonsyndromic hearing loss (without exposure to aminoglycosides), limited evidence) and without the variant (evidence not sought). This links to social media comments on a desire for clarity about hearing loss aetiology –
… I didn’t have the numbers of what the chance of them having hearing loss was, I would want to know that chance …
-
The chance of morbidity and mortality from infection and from sepsis, as well as if and how this changes based on time to treatment initiation –
… I’m presuming that within that hour the quicker, the better it is even within that hour …
What other risks are there then? What other repercussions for your child?
… so, chances of like the long-term health effects increase the longer you wait?
-
The longer-term health risks of infection and sepsis –
What are the other long-term or like health risks of having?
-
The comparative effectiveness and tolerability of antibiotics (see Place of intervention in current pathway: treatment for neonatal infections and sepsis, limited evidence). This links with the lack of clarity expressed about treatment options and comparative effectiveness in social media posts –
I’d want to know the differences between the two drugs … taking the second one, does that increase the chance of mortality?
… why wouldn’t you just use the second antibiotic, whatever that might be, if that was equal …
You want to know which outweighs the other …
-
The certainty in aetiology of hearing loss after testing. This links with social media data on the lack of clarity that testing for the variant may give about hearing loss aetiology –
Obviously they do a hearing test on newborn babies not long after they’ve been born. How do they know that it’s a drug that caused the hearing loss …?
Testing desirability dependent on context
Testing is undesirable if:
-
Variant prevalence is low (with evidence showing this is the case; see Prevalence of m.1555A>G variant and risk of aminoglycoside-induced hearing loss), test is not very accurate, chance of test failure and poor safety –
… because if that’s (prevalence) incredibly low … the majority of people would not want to go down that route (testing) if the chance of actually having the genetic variant is incredibly low.
So do we know what the failure of it is, what is it?
-
Within the care pathway for infection and sepsis and parents unable to make truly informed choices about testing due to stress of the situation. Parents wanted to make the decision about whether testing was undertaken and to then be guided by clinical expertise as to how or if this should inform treatment. This links to the desire for a discussion of potential side effects of treatment and seeking of consent in social media data –
… is there somewhere that this test is offered to before giving birth, to determine whether this gene might be there on the newborn prior to any possible infection? So things can be looked at in advance and thought about, rather than in a panic …
Is it, you know, this is what we could treat your baby with, but the risks are of them potentially having hearing loss …?
… if I was in that position I would want to make a decision, but I think I would be guided by the other people around …
-
If there is a decline in outcomes from infection or sepsis over time to treatment initiation, parents did not want any treatment delay even if this led to adverse events. A second test was not deemed acceptable. This links with the preference for treatment regardless of trade-off against side effects expressed in social media posts –
… save that 26 min, or the 52, whatever it might be to stop the risk of the whole situation getting worse …
Testing is desirable if:
-
It is undertaken upstream of infection among neonates at high risk of infection or mothers at risk of giving birth to a neonate at high risk of infection. There is evidence on testing at this upstream point in the pathway, although not with the Genedrive MT-RNR1 ID Kit. However, evidence indicates that this mtDNA variant could potentially be heteroplasmic, which would affect the accuracy of testing mothers as a proxy for newborns (see Maternal inheritance of m.1555A>G variant) –
… why this test isn’t given to pregnant mothers, that’s my personal point of view … if it was down to the cost-effectiveness of that test when the mother is pregnant that would make me so mad … and that hearing loss is avoidable … obviously you have your babies here. But if you could have avoided hearing loss …
Comes back to what Z said earlier … It’s maybe not the time when parents would want that test to be happening.
Chapter 7 Discussion
Statement of principal findings
Clinical effectiveness
Only one study (reported in two publications) met the eligibility criteria for our rapid review. 18,19 The study reported on the following outcomes of interest: diagnostic test accuracy, number of successful tests for neonates, test failure rate, impact of the results on care decisions, impact of test implementation and use on healthcare resources (e.g. the time taken to do and interpret the test), time to obtaining a sample for a test, time to results, time to antibiotic treatment, and number of neonates identified with the m.1555A>G genetic variant. However, it did not report on the following outcomes of interest: successful test of mothers, usability of the test, mortality and morbidity.
The diagnostic test accuracy of the Genedrive MT-RNR1 Kit was high, with no false negatives reported. However, estimates of real-world sensitivity of the test lacked precision, as only three participants with the m.1555A>G variant were identified in the study. 19
There were five false positives, which was suggested to have been rectified by updating the cartridge used in the machine. 19 Similarly, the Genedrive MT-RNR1 ID Kit was adapted to reduce the test failure rate. After this correction, in a laboratory-based setting there were no failed tests; however, with the intended point-of-care a failure rate of 5.7% was still observed. 19 Three neonates were successfully identified as carrying the genetic variant, leading to aminoglycoside antibiotics being avoided and alternative cephalosporin-based regimens being provided. 19 The time to results for the Genedrive MT-RNR1 ID Kit was consistent with predefined boundaries of statistical equivalence with standard care (mean difference –0.87 minutes, 95% CI –5.96 to 4.23 minutes). This finding justified the simplification of the early economic evaluation model, which did not address the impact of time to antibiotics on cost-effectiveness.
Regarding the usability of the test, the analysis provides an actionable result in 26 minutes, with an estimated time of approximately 30 minutes from the collection of the buccal swab to an actionable result (i.e. genetic variant detected or not detected). However, the time to obtaining a sample can vary (median 6 minutes, IQR 3–16 minutes).
Overall, these results suggest that the Genedrive MT-RNR1 ID Kit has promise as an accurate point-of-care diagnostic test. In addition, it has the potential to provide rapid identification within the time-sensitive period required to impact treatment decisions about neonates with the m.1555A>G genetic variant.
Cost-effectiveness
No existing economic evaluations were identified that addressed the topic of this study.
The costs of the Genedrive MT-RNR1 ID Kit were estimated using information provided by the company and assumptions made by the EAG. Considering the equipment needed to carry out the test, it was estimated that it would cost approximately £102 per diagnostic test inclusive of capital costs. It was also estimated that 30 minutes of nurse time would be needed to carry out each diagnostic test, raising the estimated total cost per diagnostic test to approximately £130. This estimate is subject to considerable uncertainty given the different types of hospital wards in which the Genedrive MT-RNR1 ID Kit could potentially be used. Although estimated to be relatively inexpensive at an individual level, the rarity of the m.1555A>G genetic variant means that the costs of identifying one neonate with this variant are more substantial. For example, under the strong assumptions of a test with perfect diagnostic accuracy, an estimated prevalence of the m.1555A>G genetic variant of 0.002 and an estimated cost of £130 per test, the cost to identify one neonate with the variant would be £65,000.
If the Genedrive MT-RNR1 ID Kit were to be recommended for use in clinical practice while further data are being collected (potentially to address some of the evidence gaps identified as part of this EVA), the sunk costs to the NHS would include the Genedrive System itself (£4995) and a Bluetooth printer (£200) for each site, the bulk purchasing of the Genedrive MT-RNR1 ID Kits and any accumulated training costs for healthcare professionals to carry out the test. As discussed previously, the training costs even for large sites with large numbers of nursing staff are likely to be relatively minor given the predicted short time of training and the fact that this could be provided free by the manufacturer. Estimating the total sunk costs at a national level is very difficult given the uncertainty regarding the type and numbers of sites that would potentially use the Genedrive MT-RNR1 ID Kit System. However, if each of the reported 55 level 3 NICUs in England was to purchase the Genedrive MT-RNR1 ID System and a Bluetooth printer, the total costs would be approximately £280,000. 61
The EAG developed an early economic model to identify the key drivers of the cost-effectiveness of the Genedrive MT-RNR1 ID Kit for detecting single nucleotide polymorphism m.1555A>G in neonates, and also to identify evidence gaps. A targeted literature review was undertaken to identify utility values relevant to the specific population. A detailed care pathway and model were constructed, and the evidence requirements were defined. There are several key evidence gaps that exist, including the magnitude of risk of AIHL in neonates with m.1555A>G, the risk of hearing loss for neonates with m.1555A>G genetic variant without exposure to aminoglycosides, the proportion of neonates potentially requiring different types of cochlear implants, and how data regarding maternal inheritance may potentially be used in the clinical pathway.
The early economic model was constructed to explore introducing the Genedrive diagnostic test into NHS services. This model focused on the likely key determinants of costs and QALYs. These key areas were arrived at by considering the likely impact on average costs or QALYs for the two clinical pathways (with Genedrive MT-RNR1 ID Kit and current standard care) and focusing on these parameters that might have the greatest impact on cost-effectiveness. This model was then subject to one-way deterministic sensitivity analysis to explore the impact of changes in these key parameter values. Of note is that some of the changes explored indirectly assessed whether the omission of an element could affect the model result. For example, in the early economic evaluation model the costs of training have been omitted, but the cost of the test has been varied in the sensitivity analysis over a range that would include the cost of testing had the training costs been included.
Overall, the base-case results from the early economic model suggest that the use of the Genedrive MT-RNR1 ID Kit could potentially be cost-effective, mainly driven by the high diagnostic accuracy reported in the PALOH study, the estimated relatively low cost per test and the avoidance of high future healthcare costs associated with the fitting of cochlear implants in those infants experiencing AIHL. From a deterministic sensitivity analysis conducted as part of the early economic model, the results were most sensitive to the time horizon of the model (which allows more time for the health benefits that flow from avoiding the accrual of hearing impairment), the sensitivity of the Genedrive MT-RNR1 ID Kit, the probability of neonates with the m.1555A>G variant experiencing AIHL after being exposed to aminoglycosides, and the prevalence of the genetic variant among the population. This suggests that research to identify more robust data on the sensitivity of the Genedrive MT-RNR1 ID Kit, the risk of AIHL for those with the m.1555A>G variant exposed to aminoglycosides and the prevalence of the m.1555A>G variant in the UK would be particularly useful. Given the limitations of the early economic model, these results are not sufficient to enable decisions about adoption, but they are suggestive that the generation of new data may be useful.
Limitations
Clinical effectiveness
There are several limitations to the current evidence base. First, there has been only one study of the use of Genedrive MT-RNR1 ID Kit. This study was conducted in two specialised large NICUs, although one NICU did drop out of the study, and therefore it is unclear if these findings can be generalised to smaller neonatal units, especially as there is limited evidence on the implementation and use of healthcare resources associated with the test kit. Additionally, some infants are born and evaluated for infection in other venues that may not have access to this technology. 74
Second, the test was refined during the PALOH study (e.g. to reduce failure rate). Although there is preliminary evidence to suggest the benefits of these more recent iterations, further studies are needed to confirm that the reduced failure rate for the test can be replicated in different settings.
Third, our rapid review identified no studies investigating the clinical effectiveness or cost-effectiveness of the Genedrive MT-RNR1 Test Kit in mothers (pre birth of the neonates). Therefore, we have no evidence to inform the use of the test kit in this population. Mahmood and Leung75 have argued that it would be theoretically possible to pre-test expectant mothers with the Genedrive MT-RNR1 ID Kit. Clinical advice received by NICE also highlighted this potential application for the technology. This could be especially important when families are anticipating neonatal antibiotics based on a peripartum diagnosis, meaning that those neonates who were excluded from the PALOH study because they required antibiotics immediately19 could be included. 75
Fourth, prior to conducting the PALOH study19 there were some concerns regarding ethical challenges, such as testing prior to informed consent, and the burden of responsibility placed on the practitioner and the wider societal impacts of technology, such as the Genedrive MT-RNR1 ID Kit. 76 However, the test is relatively simple, needing only a buccal swab,77 and would only be used in an intended point-of-care healthcare setting. 78 Additionally, the point-of-care test could be considered integral to the broad package of care offered to the unwell neonate, for which broad parental consent is provided, or, if this is unavailable, is done in the best interest of the child. 79
Fifth, some have raised concerns regarding the antibiotics being chosen as a result of the point-of-care test. Specifically, gentamicin is suggested to be the gold-standard treatment for neonates with suspected/confirmed sepsis, and giving second-line care could increase the risk of death. 80 This concern was also raised in our focus group with parents (see Chapter 6). Therefore, there are alternative views on whether using the Genedrive MT-RNR1 ID Kit to identify the m.1555A>G genetic variant is required. However, our clinical advisors suggest that this risk of death due to the antibiotics given is low.
Finally, other variants in other genes that result in the same risk phenotype are currently not assessed by the current test. 74
Cost-effectiveness
Further limitations can be highlighted for the economics analyses. Foremost among these is that, given the lack of data, the results generated from the early economic evaluation are highly uncertain. For example, in the base-case analysis of the early economic model, the sensitivity and specificity of the test are assumed to be 100% and 99.2%, respectively, despite the significant uncertainty associated with the sensitivity value. This assumption was explored in the deterministic sensitivity analysis, which showed that getting more accurate data is likely to be highly important to the cost-effectiveness results. Other relevant data that are highly uncertain include the precise proportion of neonates with the m.1555A>G genetic variant who will suffer from hearing loss both with and without the prescription of aminoglycosides, the staffing requirements in different sizes and types of hospital wards where the Genedrive MT-RNR1 ID Kit may be implemented and the how the kit could be used to test mothers rather than neonates.
Second, several parameters have effectively been omitted from the early economic evaluation model. These include training costs, the costs of antibiotics, and the impact (in terms of both costs and utilities) of long-term adverse events related to cochlear implants. The justification for this is that it was felt that the cost per patient of adding these costs would have a minimal impact on the cost-effectiveness results; however, this may not be the case for all parameters. For example, there is uncertainty surrounding the proportions of neonates who experience some degree of hearing impairment who go on to use normal hearing aids or require unilateral/bilateral cochlear implants. The results of the sensitivity analysis show that the cost-effectiveness results could be sensitive to changes in the costs of these, and, by extension, the results will be sensitive to the proportions receiving each form of hearing aid. These omissions would need to be addressed in a full economic evaluation.
Third, other data used in the economic model may not be strictly relevant to the NICE reference case. For example, although the long-term utility data related to deafness and cochlear implants used in the early economic model are sourced from a pivotal study, these are based on data from the HUI3 rather than the EQ-5D. 30 This is potentially justifiable as the HUI3 directly captures the impact on hearing that the EQ-5D does not. A bolt-on for hearing for the EQ-5D is under development but not yet available and of course would not relate to NICE’s preferred source of utility values. The data for the HUI3 were taken from a study that was completed around 20 years ago, and technology related to cochlear implants has improved significantly since then. The utility values for the HUI3 are also approaching 30 years old and are derived from a Canadian population. It is also unclear how applicable they are to children under 5 years of age, as the HUI3 is validated only for those ≥ 5 years old. There is a scarcity of validated HRQoL instruments suitable for infants. Although the Infant health-related Quality of life Instrument (IQI) has recently been developed, none of the seven attributes is related to hearing, and, again, the tool is not compatible with the NICE reference case. 81,82
Fourth, related to the cost perspective, the economic model has thus far considered NHS costs only. There are likely to be costs needed that fall within Personal Social Services relating to other aids and adaptations. Furthermore, there may be broader societal impacts on children and families (e.g. increased caring responsibilities; possible impacts on the speech development and educational attainment of the child) that also need to be considered.
Finally, the scope of the economic evaluation may be too narrow in terms of capturing the broader implications of integrating the test into the clinical pathway. These are likely to vary substantially according to the centre. In addition, there may be some further impacts on laboratory testing. This is uncertain as the number of neonates presenting with suspected infection or sepsis is itself uncertain. The model also has not considered the impacts on antibiotic resistance. Clinical experts have advised that there are strong clinical concerns regarding resistance to alternative antibiotic therapies than may be prescribed instead of aminoglycosides, such as cefotaxime. Therefore, the incidence of antimicrobial resistance in the healthcare setting could be improved if testing reduces the use of these alternative antibiotics. 12 How this latter impact could be captured in a definitive model is, however, unclear.
Equality, diversity and inclusion
Epidemiological evidence on the risk, incidence and prevalence of AIHL is very limited (see Background to decision problem). These studies suggest that the prevalence of m.1555A>G may vary by ethnicity. However, given the substantial limitations of these studies, including very small sample sizes, it is difficult to confirm.
The NICE scope also points out that early-onset neonatal infection, a key factor prompting antibiotic treatment, varies both by socioeconomic position and ethnicity. Mothers with a lower socioeconomic position and/or with a minority ethnic family background were more likely to have babies with early-onset neonatal infection.
As the Genedrive MT-RNR1 ID Kit is a genetic test, the NICE scope suggested that acceptability and consent for testing may vary according to personal or religious beliefs.
Evidence gaps
Diagnostic accuracy and failure rate of test
There are several uncertainties regarding the accuracy of the Genedrive MT-RNR1 ID Kit. The NICE scope included testing mothers of neonates who may require antibiotics. We found no studies using the Genedrive MT-RNR1 ID Kit in this population.
Although the PALOH study provides data on the diagnostic accuracy of the Genedrive MT-RNR1 ID Kit, estimates of the test’s sensitivity are severely limited by the small number of neonates (only three) identified with the m.1555A>G genetic variant. 19 Therefore, although the test may be potentially very sensitive, the 95% CI was wide (mean sensitivity 100%, 95% CI 29.2% to 100.00%), indicating very high imprecision. As the early economic evaluation shows, estimates of cost-effectiveness are very sensitive to this imprecision.
The failure rate was originally 17.1% (90 of 514 neonates), but after modifications to the assay buffer and a redesigned cartridge consumable this was reduced. In a laboratory setting the failure was zero, while in a clinical setting this was reduced to 5.1%, when repeated testing was conducted. 19 This suggests that when the kit is used as the point-of-care test there is still a failure rate, and more than one test may be required. As the test is reported to take 26 minutes, this could cause issues with antibiotic prescribing that is required to be within the hour. Therefore, further work is required to ascertain the number of test failures that may be expected and the impact of this on prescribing. The early economic modelling suggested that this evidence gap would have only a modest effect on cost-effectiveness.
Clinical outcomes
We did not identify any studies assessing mortality or morbidity. Future studies should look to ascertain the effects of using the Genedrive MT-RNR1 ID Kit and the implications of these effects on these clinical outcomes.
Generalisability of results
The NICE scope included NICU and hospital wards. The PALOH study provided evidence that the Genedrive MT-RNR1 ID Kit did not substantially impact on time to antibiotics compared with standard care,19 a key outcome given the need to provide antibiotics within 1 hour of the decision to treat. However, the PALOH study was conducted in two large and well-resourced NICUs; therefore, some of our clinical experts indicated that it was unclear whether data from time to antibiotics in this study generalise to smaller NICUs and other hospital wards.
Estimating the magnitude of risk for aminoglycoside-induced hearing loss in neonates with m.1555A>G
There is clear evidence that the m.1555A>G genetic variant is a risk factor for AIHL. 13 However, data on the magnitude of this risk are uncertain. Case–control studies usually find that all people with the variant experience hearing loss. 3 However, selecting participants for hearing loss may overestimate the risk associated with aminoglycosides. Studies that do not select for hearing loss suggest that people with the m.1555A>G variant may not always experience hearing loss when exposed to aminoglycosides. 6 The early economic evaluation suggests that cost-effectiveness estimates are likely to be sensitive to the magnitude of this risk.
To understand the long-term benefits (on clinical outcomes and cost-effectiveness) of avoiding aminoglycoside use in neonates with the m.1555A>G genetic variant, more precise estimates on the magnitude of risk for AIHL in this population are required. It is also worth noting that there is an evidence gap related to hearing loss in those without exposure to aminoglycosides. However, the results from the sensitivity analysis in the early economic model indicate that this may not be important.
Maternal inheritance and use of point-of-care testing in mothers
The m.1555A>G variant is inherited maternally, and therefore identifying a mother’s m.1555A>G status may be another way of identifying a child’s m.1555A>G status. However, there are several uncertainties and evidence gaps related to this. First, there in uncertainty about how well a mother’s m.1555A>G status can indicate the risk of AIHL in her child. Although there are studies related to the variant load and hearing thresholds, these studies have small sample sizes. Second, there is uncertainty about the proportion of mothers in whom a clinically relevant genotype has previously been identified. Finally, in relation to using point-of-care testing for the mother, it is unclear what proportion of women are likely to be given aminoglycosides during labour or in other clinical settings.
Resource implications
The PALOH study provided evidence that no increase in nursing time was required to implement the assay into practice. 19 As stated previously, the PALOH study was conducted in two large and well-resourced NICUs that have significant experience of conducting research. There is, therefore, a clear lack of evidence related to the resource implications in different-sized NICUs and different hospital wards where the Genedrive MT-RNR1 ID Kit may potentially be used in the future.
Estimating the severity of aminoglycoside-induced hearing loss in neonates with m.1555A>G and quantifying the proportion of neonates requiring different types of cochlear implants in the long term
Alongside the uncertainty related to the risk of AIHL following exposure to aminoglycosides, there is uncertainty related to the severity of AIHL in those who experience it. Consequently, there is uncertainty about the proportion of neonates with AIHL who would require different cochlear implants of different types over the long term. This is important, as cochlear implants have significant NHS resource implications, with substantial costs related to surgery and annual maintenance and programming. Current NICE guidance states that cochlear implants are recommended for children (and adults) with severe to profound deafness who do not gain adequate benefit from acoustic hearing aids. 45 Severe to profound deafness in this case is defined as hearing only sounds that are louder than 80 decibels hearing level. In children, adequate benefit is defined as speech, language and listening skills appropriate to age, developmental stage and cognitive ability. It is currently unclear what proportion of neonates would require acoustic hearing aids only, unilateral cochlear implants and bilateral cochlear implants, making estimates of the long-term savings associated with preventing cochlear implants highly uncertain.
Utility values for health states related to hearing loss and cochlear implants that conform to the National Institute for Health and Care Excellence reference case for use in an economic model
As noted in Table 3 (see Results of the targeted search for health-related quality-of-life studies), the majority of previous economic evaluations related to hearing loss and/or cochlear implants in the UK population identified in this EVA used the HUI3 HRQoL tool to measure health state utilities. The main reason for this is that the HUI3 has a specific dimension related to hearing, which other commonly used HRQoL tools, such as the EQ-5D and SF-6D do not. Indeed, the HUI3 has been shown to have better validity and responsiveness than the EQ-5D and SF-6D in previous studies of patients with hearing impairments. 83
However, although the HUI3 may have some advantages over the EQ-5D in respect to validity and responsiveness in this clinical area, the EQ-5D is NICE’s preferred measure in the reference case. Furthermore, the value set used for the HUI3 is from a Canadian adult sample and is over 20 years old. Research is ongoing regarding the development of a hearing ‘bolt-on’ for the EQ-5D; however, the measurement properties of this are yet to be established. 43
Chapter 8 Conclusion
Implications for service provision
This rapid review shows that the Genedrive MT-RNR1 ID Kit has the potential to identify the m.1555A>G variant and to be cost-effective. However, as anticipated, there is insufficient evidence to conduct a full diagnostic assessment of the clinical effectiveness and cost-effectiveness of the Genedrive MT-RNR1 ID Kit in neonates for the neonate directly or for their mother.
The evidence to inform this EVA was limited, based on only one study that included only three neonates with the m.1555A>G variant. In addition, the study was conducted in two large specialist NICUs, so it is unclear whether the benefits of the technology generalise to smaller units. Too few data are available to derive robust estimates of cost-effectiveness, and, although the Genedrive MT-RNR1 ID Kit has the potential to be cost-effective, the early economic evaluation model is subject to considerable uncertainties.
Suggested research priorities
The following studies may reduce uncertainty in the clinical effectiveness and cost-effectiveness of the Genedrive MT-RNR1 ID Kit identified in this EVA.
Risk and severity of aminoglycoside-induced hearing loss in people with m.1555A>G variant
Proposed eligibility criteria
Population: people with m.1555A>G variant.
Exposure: aminoglycosides (either directly or by exposure through mother).
Comparator: no exposure to aminoglycosides.
Outcomes: prevalence of AIHL, severity of AIHL, HRQoL, costs to the NHS and Personal Social Services.
Study design: cohort studies.
Why this is important
There are several uncertainties regarding the risk of hearing loss in people with the m.1555A>G variant:
-
risk of AIHL in people with m.1555A>G variant exposed to aminoglycosides
-
severity of AIHL in people with m.1555A>G variant exposed to aminoglycosides.
The risk of AIHL in people with the m.1555A>G variant was identified as an important uncertainty in the economic model (Figure 9). Cohort studies on the risk of AIHL in people with the m.1555A>G variant have identified a small number of people who meet the above criteria. 6 However, substantial uncertainty regarding the magnitude of these risks remains.
Existing cohort studies in the UK and beyond, such as the Born in Bradford study, are potentially important sources of data for identifying people who meet these eligibility criteria. However, given the rarity of the variant, present in approximately 0.3% of the UK population, it is unlikely that one single cohort study will provide a large enough sample size. Therefore, it is likely that meta-analyses of future cohort studies will be required for sufficiently precise estimates.
Further validation of Genedrive MT-RNR1 ID Kit
Proposed eligibility criteria
Population: population of the NICE scope (neonates needing or expected to need aminoglycosides and mothers with risk factors for sepsis).
Intervention: Genedrive MT-RNR1 ID Kit.
Comparator: usual care.
Outcomes: outcomes identified in NICE scope that are particularly important for informing uncertainties in the economic model: diagnostic accuracy (sensitivity of test); failure rate; time to antibiotic use; and health resource use implications (including a detailed micro-costing). Qualitative data on the barriers to and facilitators of implementing the test (including obtaining informed consent for parents).
Study design: mixed methods (quantitative and qualitative).
Why this is important
The sensitivity of the Genedrive MT-RNR1 ID Kit for identifying neonates with the m.1555A>G variant was identified as a key uncertainty in the economic model. The PALOH study, the only study identified for inclusion in the rapid review of clinical effectiveness data, identified only three participants with the variant. Therefore, although the estimated sensitivity of the test was very high, the 95% CI was also very uncertain (mean 1.00, 95% CI 0.29 to 1.00).
In addition, the PALOH study was conducted in two large NICUs (94.9% of participants were recruited from one centre, with the other centre dropping out part way through the study). Therefore, it is unclear whether these findings generalise to smaller NICUs or other hospital wards where service configuration may differ.
Conducting further investigations in other NICUs and hospitals of varying sizes would provide more detailed evidence of the real-world application for the point-of-care test. There are also uncertainties surrounding the test failure rate. Although it was reduced after edits to the Genedrive MT-RNR1 ID Kit, there was still some failure rate in the intended clinical setting. This could impact time to treatment with antibiotics, and further research could allow for reduced uncertainty in the true test failure rate in practice.
In addition, PPIE (see Chapter 6) identified concerns from parents about the risks and benefits of the Genedrive MT-RNR1 ID Kit. Some parents were reluctant to give consent for their baby to take the test, particularly when the baby was at high risk of infection. Therefore, a qualitative study alongside further evaluation of the technology would help to identify barriers to implementing it and obtaining informed consent.
Assessment of clinical outcomes
Proposed eligibility criteria
Population: people with the m.1555A>G variant.
Intervention: Genedrive MT-RNR1 ID Kit.
Comparator: usual care.
Outcomes: mortality and morbidity.
Study design: cohort study.
Why this is important
It is important to understand whether the change in antibiotic treatment affects the mortality rate of the neonates receiving non-aminoglycoside regimens. Morbidity is important to assess in case there are risks of developing other conditions. Additionally, it may be that those with the m.1555A>G variant are more predisposed to hearing loss. It is therefore critical to provide information on such morbidities.
Measurement of utilities associated with hearing loss, hearing aids and cochlear implants using preference elicitation techniques to validate existing values and use in economic model
Proposed eligibility criteria
Population: adult general population sample.
Outcomes: utility values for different levels of hearing loss, different types of hearing aid and different types of cochlear implant.
Study design: patient preference study where a general population sample measure the HRQoL associated with hearing loss, hearing aids and cochlear implants using either the standard gamble or the TTO technique.
Why this is important
The majority of previous economic evaluations related to hearing loss and/or cochlear implants in the UK population identified in this EVA used the HUI3 HRQoL tool to measure utility; however, the EQ-5D is NICE’s preferred method of measuring utility in the reference case. Furthermore, the value set used for the HUI3 is from a Canadian adult sample and is over 30 years old. Although research is ongoing regarding a hearing ‘bolt-on’ for the EQ-5D, the measurement properties of this bolt-on are yet to be established. 43
Given that the EQ-5D is unlikely to be an appropriate measure of utility for this condition, one approach that could generate alternative utility values for use in a future economic model could be to use either the TTO or standard gamble, two choice-based methods of eliciting health state utilities commonly used in health economics. These methods of measuring utility are seen as acceptable alternatives to NICE in the absence of good-quality EQ-5D data. Summerfield et al. 28 previously used the TTO in relation to unilateral cochlear implantations in adults; however, the general population sample was relatively small (n = 70) and valued only four health states. Summerfield et al. 31 also used the TTO in relation to bilateral cochlear implantation in children; however, the sample gathered was a convenience sample composed of clinicians/researchers, students, and parents of children with hearing problems (n = 180), and, once more, they valued only four health states.
A larger study with a representative general population sample (in line with the NICE reference case) and a larger range of health states to be valued (related to hearing loss, hearing aids and different sorts of cochlear implants) could potentially provide health state utility values. Such values would be more appropriate for use in a future economic model in the absence of EQ-5D data, or could be used to validate the existing utility values from the literature used in economic models that have been generated using the HUI3.
Additional information
Contributions of authors
Hosein Shabaninejad (https://orcid.org/0000-0001-9512-1398) (Senior Research Associate, Health Economics) led the cost-effectiveness analyses and writing of the cost-effectiveness section of the report.
Ryan PW Kenny (https://orcid.org/0000-0001-9743-4259) (Research Associate, Evidence Synthesis and Statistics) led the clinical effectiveness analyses and writing of the clinical-effectiveness section of the report.
Tomos Robinson (https://orcid.org/0000-0001-8695-9738) (Senior Research Associate, Health Economics) conducted the cost-effectiveness analyses and wrote the cost-effectiveness section of the report.
Akvile Stoniute (https://orcid.org/0000-0001-8279-5123) (Research Assistant, Evidence Synthesis) conducted the clinical-effectiveness analyses and wrote the clinical effectiveness section of the report.
Hannah O’Keefe (https://orcid.org/0000-0002-0107-711X) (Research Associate, Information Science) conducted the electronic search and managed the EndNote Library, and wrote the search section of the report.
Madeleine Still (https://orcid.org/0000-0003-0625-6325) (Research Associate, Evidence Synthesis) contributed to the PPIE focus group and wrote the PPIE section of the report.
Christopher Thornton (https://orcid.org/0000-0003-0055-5500) (Research Associate, Data Science) conducted the scan of social media forums and wrote the PPIE section of the report.
Fiona Pearson (https://orcid.org/0000-0003-1626-0862) (Principal Research Associate, Evidence Synthesis and PPIE) led the PPIE for this project and writing of the PPIE section of the report.
Fiona Beyer (https://orcid.org/0000-0002-6396-3467) (Senior Research Associate, Information Science) led the electronic search and the writing up of the search section of the report.
Nick Meader (https://orcid.org/0000-0001-9332-6605) (Principal Research Associate) had overall responsibility for the project and led the writing-up of the report.
Acknowledgements
We gratefully acknowledge the expert advice from members of the Specialist Assessment Subgroup (in particular Professor Sam Oddie, Dr Ruth Gottstein, Dr Jim Gray, Hayley Wickens), the NICE Diagnostic Programme team, Professor Nick Embleton (Professor of Neonatal Medicine, Newcastle University), from the maternity PPIE group, Janine Smith (Lay Facilitator) and Alison Farnworth (Research Midwife, Newcastle University) and Professor Luke Vale (Professor of Health Economics).
Data-sharing statement
All available data can be obtained by contacting the corresponding author.
Ethics statement
No ethical approval was required for this report as we conducted a systematic review and early economic model.
Information governance statement
This study did not handle any personal information.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/TGAC4201.
Primary conflicts of interest: none.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- UK Health Security Agency . NICU Aggregate Report (July 2020–March 2022) 2022.
- Ballana E, Morales E, Rabionet R, Montserrat B, Ventayol M, Bravo O, et al. Mitochondrial 12S rRNA gene mutations affect RNA secondary structure and lead to variable penetrance in hearing impairment. Biochem Biophys Res Commun 2006;341:950-7.
- Estivill X, Govea N, Barceló E, Badenas C, Romero E, Moral L, et al. Familial progressive sensorineural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment of aminoglycosides. Am J Hum Genet 1998;62:27-35.
- Bitner-Glindzicz M, Pembrey M, Duncan A, Heron J, Ring SM, Hall A, et al. Prevalence of mitochondrial 1555A>G mutation in European children. N Engl J Med 2009;360:640-2.
- Rahman S, Ecob R, Costello H, Sweeney MG, Duncan AJ, Pearce K, et al. Hearing in 44–45 year olds with m.1555A>G, a genetic mutation predisposing to aminoglycoside-induced deafness: a population based cohort study. BMJ Open 2012;2.
- Göpel W, Berkowski S, Preuss M, Ziegler A, Küster H, Felderhoff-Müser U, et al. Mitochondrial mutation m.1555A>G as a risk factor for failed newborn hearing screening in a large cohort of preterm infants. BMC Pediatr 2014;14.
- Ealy M, Lynch KA, Meyer NC, Smith RJ. The prevalence of mitochondrial mutations associated with aminoglycoside-induced sensorineural hearing loss in an NICU population. Laryngoscope 2011;121:1184-6. https://doi.org/10.1002/lary.21778.
- Johnson RF, Cohen AP, Guo Y, Schibler K, Greinwald JH. Genetic mutations and aminoglycoside-induced ototoxicity in neonates. Otolaryngol Head Neck Surg 2010;142:704-7. https://doi.org/10.1016/j.otohns.2010.01.030.
- Matsunaga T, Kumanomido H, Shiroma M, Ohtsuka A, Asamura K, Usami S-I. Deafness due to A1555G mitochondrial mutation without use of aminoglycoside. Laryngoscope 2004;114:1085-91.
- del Castillo FJ, Rodríguez-Ballesteros M, Martín Y, Arellano B, Gallo-Terán J, Morales-Angulo C, et al. Heteroplasmy for the 1555A>G mutation in the mitochondrial 12S rRNA gene in six Spanish families with non-syndromic hearing loss. J Med Genet 2003;40:632-6.
- Luo H, Yang Y, Wang X, Xu F, Huang C, Liu D, et al. Concurrent newborn hearing and genetic screening of common hearing loss variants with bloodspot-based targeted next generation sequencing in Jiangxi province. Front Pediatr 2022;10.
- National Institute for Health and Care Excellence (NICE) . Neonatal Infection: Antibiotics for Prevention and Treatment 2021.
- McDermott JH, Wolf J, Hoshitsuki K, Huddart R, Caudle KE, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium guideline for the use of aminoglycosides based on MT-RNR1 genotype. Clin Pharmacol Ther 2022;111:366-72.
- Garritty C, Gartlehner G, Nussbaumer-Streit B, King VJ, Hamel C, Kamel C, et al. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol 2021;130:13-22.
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan: a web and mobile app for systematic reviews. Syst Rev 2016;5.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;55. https://doi.org/10.1136/bmj.i4919:i4919.
- McDermott JH, Mahaveer A, James RA, Booth N, Turner M, Harvey KE, et al. PALOH Study Team . Rapid point-of-care genotyping to avoid aminoglycoside-induced ototoxicity in neonatal intensive care. JAMA Pediatr 2022;176:486-92.
- McDermott JH, Mahood R, Stoddard D, Ainsworth S, Miele G, Bruce I, et al. Pharmacogenetics to Avoid Loss of Hearing (PALOH): a prospective observational trial to assess the implementation of rapid genotyping to avoid aminoglycoside induced ototoxicity in newborns. Eur J Hum Genet 2022;30.
- National Institute for Health and Care Excellence (NICE) . NICE Health Technology Evaluations: The Manual Process and Methods [PMG36] 2022.
- National Institute for Health and Care Excellence (NICE) . Medtech Innovation Briefing [MIB290] Genedrive MT-RNR1 ID System for Detecting Single Nucleotide Polymorphism m.1555A≫G in Newborn Babies 2022.
- McDermott JH, Mahood R, Stoddard D, Mahaveer A, Turner MA, Corry R, et al. Pharmacogenetics to Avoid Loss of Hearing (PALOH) trial: a protocol for a prospective observational implementation trial. BMJ Open 2021;11.
- World Health Organization . Childhood Hearing Loss: Strategies for Prevention and Care 2016.
- UK Government . Guidance: Newborn Hearing Screening Programme (NHSP): Care Pathways for Babies in Neonatal Intensive Care Units (NICU) 2020.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. PharmacoEconomics 2006;24:355-71.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Office for National Statistics . Interim Life Tables, National Life Tables UK 2018 to 2020 2018.
- Summerfield AQ, Marshall DH, Barton GR, Bloor KE. A cost–utility scenario analysis of bilateral cochlear implantation. Arch Otolaryngol Head Neck Surg 2002;128:1255-62.
- Barton GR, Stacey PC, Fortnum HM, Summerfield AQ. Hearing-impaired children in the United Kingdom, IV: cost-effectiveness of pediatric cochlear implantation. Ear Hear 2006;27:575-88.
- Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, et al. The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model. Health Technol Assess 2009;13.
- Summerfield AQ, Lovett RE, Bellenger H, Batten G. Estimates of the cost-effectiveness of pediatric bilateral cochlear implantation. Ear Hear 2010;31:611-24.
- Cutler H, Gumbie M, Olin E, Parkinson B, Bowman R, Quadri H, et al. The cost-effectiveness of unilateral cochlear implants in UK adults. Eur J Health Econ 2022;23:763-79.
- Lovett RE, Kitterick PT, Hewitt CE, Summerfield AQ. Bilateral or unilateral cochlear implantation for deaf children: an observational study. Arch Dis Child 2010;95:107-12.
- Petrou S, Khan K, Kennedy C. Bilateral permanent childhood hearing loss and health-related quality of life in adolescence. Children 2021;8.
- Summerfield A, Barton GR, Toner J, McAnallen C, Proops D, Harries C, et al. Self-reported benefits from successive bilateral cochlear implantation in post-lingually deafened adults: randomised controlled trial. Int J Audiol 2006;45:S99-107.
- Torrance GW, Feeny DH, Furlong WJ, Barr RD, Zhang Y, Wang Q. Multiattribute utility function for a comprehensive health status classification system. Health Utilities Index Mark 2. Med Care 1996;34:702-22.
- UK Cochlear Implant Study Group . Criteria of candidacy for unilateral cochlear implantation in postlingually deafened adults I: theory and measures of effectiveness. Ear Hear 2004;25:310-35.
- Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies. Ann Med 2001;33:375-84.
- Barton GR, Bankart J, Davis AC, Summerfield QA. Comparing utility scores before and after hearing-aid provision. Appl Health Econ Health Policy 2004;3:103-5.
- EuroQol G. EuroQol – a new facility for the measurement of health-related quality of life. Health Pol 1990;16:199-208.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92.
- Petrou S, McCann D, Law CM, Watkin PM, Worsfold S, Kennedy CR. Health status and health-related quality of life preference-based outcomes of children who are aged 7 to 9 years and have bilateral permanent childhood hearing impairment. Pediatrics 2007;120:1044-52.
- Besley S, Henderson N, Sampson C. OHE is Leading Research to Develop an EQ-5D ‘Bolt-on’ for Hearing. London: Office of Health Economics; 2022.
- National Institute for Health and Care Excellence (NICE) . Cochlear Implants for Children and Adults With Severe to Profound Deafness 2009.
- National Institute for Health and Care Excellence (NICE) . Technology Appraisal Guidance [TA566] Cochlear Implants for Children and Adults With Severe to Profound Deafness 2019.
- Pogany L, Barr RD, Shaw A, Speechley KN, Barrera M, Maunsell E. Health status in survivors of cancer in childhood and adolescence. Qual Life Res 2006;15:143-57.
- Horsman J, Gauld M. Health Utilities Inc Health-Related Quality-of-Life 2018. www.healthutilities.com/ (accessed 25 April 2024).
- Dixon PR, Shapiro J, Tomlinson G, Cottrell J, Lui JT, Falk L, et al. Health state utility values associated with cochlear implants in adults: a systematic review and network meta-analysis. Ear Hearing 2023;44:244-53.
- Paul K, Geoffrey H, Susan M. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Swan IR, Guy FH, Akeroyd MA. Health-related quality of life before and after management in adults referred to otolaryngology: a prospective national study. Clin Otolaryngol 2012;37:35-43.
- Happich M, Moock J, von Lengerke T. Health state valuation methods and reference points: the case of tinnitus. Value Health 2009;12:88-95.
- Prosser LA, Ray GT, O’Brien M, Kleinman K, Santoli J, Lieu TA. Preferences and willingness to pay for health states prevented by pneumococcal conjugate vaccine. Pediatrics 2004;113:283-90.
- Hansen S, Anthonsen K, Stangerup SE, Jensen JH, Thomsen J, Cayé-Thomasen P. Unexpected findings and surgical complications in 505 consecutive cochlear implantations: a proposal for reporting consensus. Acta Otolaryngol 2010;130:540-9.
- Jeppesen J, Faber CE. Surgical complications following cochlear implantation in adults based on a proposed reporting consensus. Acta Otolaryngol 2013;133:1012-21.
- Farinetti A, Ben Gharbia D, Mancini J, Roman S, Nicollas R, Triglia JM. Cochlear implant complications in 403 patients: comparative study of adults and children and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis 2014;131:177-82.
- Venail F, Sicard M, Piron JP, Levi A, Artieres F, Uziel A, et al. Reliability and complications of 500 consecutive cochlear implantations. Arch Otolaryngol Head Neck Surg 2008;134:1276-81.
- Stamatiou GA, Kyrodimos E, Sismanis A. Complications of cochlear implantation in adults. Ann Otol Rhinol Laryngol 2011;120:428-32.
- Curtis L, Burns AY. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- Dunne P. NHS Prescription Charges from April 2017. London: Department of Health and Social Care; 2017.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Jones K, Burns A. Unit Costs of Health and Social Care 2021. Canterbury: Personal Social Services Research Unit, University of Kent; 2021.
- National Institute for Health and Care Excellence (NICE) . Neonatal Specialist Care Quality Standard [QS4] 2010.
- Joint Formulary Committee . British National Formulary 83 2022.
- Mathew R, Bajo FR, Hatton N, Buttfield L, Gowrishankar S, Vickers D, et al. Assessment of the cochlear implant pathway for newborn hearing screening referrals. Cochlear Implants Int 2021;22:345-52.
- NHS Improvement . National Schedule of Reference Costs Year: 2017–18 2017.
- NHS England . National Tariff Payment System 2018 19 2017.
- Healthcare Improvement Scotland . A Budget Impact Analysis of Implementing Changes to the Eligibility Criteria for Cochlear Implants in NHS Scotland 2019.
- Barton GR, Bloor KE, Marshall DH, Summerfield AQ. Health-service costs of pediatric cochlear implantation: multi-center analysis. Int J Pediatr Otorhinolaryngol 2003;67:141-9.
- Wang JT, Wang AY, Psarros C, Da Cruz M. Rates of revision and device failure in cochlear implant surgery: a 30-year experience. Laryngoscope 2014;124:2393-9.
- Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Development of the ACTIVE framework to describe stakeholder involvement in systematic reviews. J Health Serv Res Pol 2019;24:245-55.
- Ratcliff J, Metzener D. Pattern matching: the gestalt approach. Dr Dobbs 1988;13:68-72.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101.
- Halcomb EJ, Davidson PM. Is verbatim transcription of interview data always necessary?. Appl Nurs Res 2006;19:38-42.
- Pillers DM. Genetic testing in newborns moves from rare to routine application. JAMA Pediatr 2022;176:448-9.
- Muñoz Mahmood M, Leung RK. Options for detecting risk of aminoglycoside-induced ototoxicity in neonates. JAMA Pediatr 2022;176.
- McDermott JH. Genetic testing in the acute setting: a round table discussion. J Med Ethics 2020;46:531-2.
- Brazier MR. Great idea: what a fuss about a swab. J Med Ethics 2020;46:534-5.
- Newman WG. Genetic testing in the acute setting: a round table discussion. J Med Ethics 2020;46.
- Coulson-Smith P, Lucassen A. Using biomarkers in acute medicine to prevent hearing loss: should this require specific consent?. J Med Ethics 2020;46:536-7.
- Parker J, Wright D. Terrible choices in the septic child: a response to the PALOH trial round table authors. J Med Ethics 2021;47:114-6.
- Jabrayilov R, van Asselt ADI, Vermeulen KM, Volger S, Detzel P, Dainelli L, et al. Pediatrics Expert Group . A descriptive system for the Infant health-related Quality of life Instrument (IQI): measuring health with a mobile app. PLOS ONE 2018;13.
- Krabbe PFM, Jabrayilov R, Detzel P, Dainelli L, Vermeulen KM, van Asselt ADI. A two-step procedure to generate utilities for the Infant health-related Quality of life Instrument (IQI). PLOS ONE 2020;15.
- Yang Y, Longworth L, Brazier J. An assessment of validity and responsiveness of generic measures of health-related quality of life in hearing impairment. Qual Life Res 2013;22:2813-28.
Appendix 1 Clinical effectiveness searches
MEDLINE
-
Point-of-Care Systems/
-
Point-of-Care Testing/
-
Genetic Testing/
-
(POCT or “point of care” or “point-of-care”).ti,ab,kw.
-
genedrive*.af.
-
MIB?290.ti,ab,kw.
-
PALoH.ti,ab,kw.
-
((pharmacogenetics or pharmacogenomics or genetic* or geno* or gene* or hear* or pyrosequenc* or sequenc*) adj4 (test* or assay* or system* or screen* or sequenc*)).ti,ab,kw.
-
or/1-8
-
“mt.1555A>G”.ti,ab,kw.
-
“1555A>G”.ti,ab,kw.
-
“m.1555A>G”.ti,ab,kw.
-
“A1555G”.ti,ab,kw.
-
“1555 A to G”.ti,ab,kw.
-
MT?RNR?1.ti,ab,kw.
-
((penetrance or snp or polymorphism or mutation) adj3 “1555”).ti,ab,kw.
-
or/10-16
-
exp Aminoglycosides/
-
(Gentamicin or paromomycin or amikacin or plazomicin or tobramycin or neomycin or kanamycin or streptomycin or netilmicin).ti,ab,kw.
-
aminoglycoside*.ti,ab,kw.
-
(anti?biotic* or anti?bacterial* or anti?infective*).ti,ab,kw.
-
exp Anti-Bacterial Agents/ae, to [Adverse Effects, Toxicity]
-
or/18-22
-
induc*.ti,ab,kw.
-
ototoxicity.ti,ab,kw.
-
exp Hearing Loss/
-
exp Hair Cells, Auditory/
-
Ototoxicity/
-
Deaf*.ti,ab,kw.
-
(hear* adj2 (loss or impair*)).ti,ab,kw.
-
or/24-30
-
9 and 17 and 23 and 31
-
“Genedrive MT-RNR1 ID”.af.
-
32 or 33
-
exp Animals/
-
exp Humans/
-
35 not 36
-
34 not 37
-
limit 38 to dt=20100101-20220929
-
limit 39 to english language
EMBASE
-
“point of care system”/
-
“point of care testing”/
-
genetic screening/
-
(POCT or “point of care” or “point-of-care”).ti,ab.
-
genedrive*.af.
-
MIB?290.ti,ab.
-
PALoH.ti,ab.
-
((pharmacogenetics or pharmacogenomics or genetic* or geno* or gene* or hear* or pyrosequenc* or sequenc*) adj4 (test* or assay* or system* or screen* or sequenc*)).ti,ab.
-
or/1-8
-
“mt.1555A>G”.ti,ab.
-
“1555A>G”.ti,ab.
-
“m.1555A>G”.ti,ab.
-
“A1555G”.ti,ab.
-
“1555 A to G”.ti,ab.
-
MT?RNR?1.ti,ab.
-
((penetrance or snp or polymorphism or mutation) adj3 “1555”).ti,ab.
-
or/10-16
-
exp aminoglycoside antibiotic agent/
-
(Gentamicin or paromomycin or amikacin or plazomicin or tobramycin or neomycin or kanamycin or streptomycin or netilmicin).ti,ab.
-
aminoglycoside*.ti,ab.
-
(anti?biotic* or anti?bacterial* or anti?infective*).ti,ab.
-
exp antiinfective agent/to [Drug Toxicity]
-
or/18-22
-
induc*.ti,ab.
-
ototoxicity.ti,ab.
-
exp hearing impairment/
-
exp “hair cell (inner ear)”/
-
exp ototoxicity/
-
deaf*.ti,ab.
-
(hear* adj2 (loss or impair*)).ti,ab.
-
or/24-30
-
9 and 17 and 23 and 31
-
“Genedrive MT-RNR1 ID”.af.
-
32 or 33
-
exp animal/
-
exp human/
-
35 not 36
-
34 not 37
-
limit 38 to dc=20100101-20221004
-
limit 39 to english language
Cochrane
-
MeSH descriptor: [Point-of-Care Systems] this term only
-
MeSH descriptor: [Point-of-Care Testing] this term only
-
MeSH descriptor: [Genetic Testing] this term only
-
(POCT or “point of care” or “point-of-care”):ti,ab,kw
-
(genedrive*)
-
(MIB?290):ti,ab,kw
-
(PALoH):ti,ab,kw
-
((pharmacogenetics or pharmacogenomics or genetic* or geno* or gene* or hear* or pyrosequenc* or sequenc*) NEAR/4 (test* or assay* or system* or screen* or sequenc*)):ti,ab,kw
-
{OR #1-#8}
-
(“mt.1555A>G”):ti,ab,kw
-
(“1555A>G”):ti,ab,kw
-
(“m.1555A>G”):ti,ab,kw
-
(“A1555G”):ti,ab,kw
-
(“1555 A to G”):ti,ab,kw
-
(MT?RNR?1):ti,ab,kw
-
((penetrance or snp or polymorphism or mutation) NEAR/3 “1555”):ti,ab,kw
-
{OR #10-#16}
-
MeSH descriptor: [Aminoglycosides] explode all trees
-
(Gentamicin or paromomycin or amikacin or plazomicin or tobramycin or neomycin or kanamycin or streptomycin or netilmicin):ti,ab,kw
-
(aminoglycoside*):ti,ab,kw
-
(anti?biotic* or anti?bacterial* or anti?infective*):ti,ab,kw
-
MeSH descriptor: [Anti-Bacterial Agents] explode all trees
-
{OR #18-#22}
-
(induc*):ti,ab,kw
-
(ototoxicity):ti,ab,kw
-
MeSH descriptor: [Hearing Loss] explode all trees
-
MeSH descriptor: [Hair Cells, Auditory] explode all trees
-
MeSH descriptor: [Ototoxicity] this term only
-
(deaf*):ti,ab,kw
-
(hear* NEAR/2 (loss or impair*)):ti,ab,kw
-
{OR #24-#30}
-
#9 AND #17 AND #23 AND #31
-
(“Genedrive MT-RNR1 ID”)
-
#32 OR #33
CINAHL
-
S35 S33 AND S34
-
S34 Limiters: Publication Date: 20100101-20221231
-
S33 S31 OR S32
-
S32 TX “Genedrive MT-RNR1 ID”
-
S31 S9 AND S13 AND S19 AND S30
-
S30 S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29
-
S29 TI (AEP or BAER or BAEP) OR AB (AEP or BAER or BAEP)
-
S28 TI (audit* N4 (respons* or evok* or potential*)) OR AB (audit* N4 (respons* or evok* or potential*))
-
S27 (MH “Evoked Potentials, Auditory, Brainstem”)
-
S26 TI (hear* N2 (loss or impair*)) OR AB (hear* N2 (loss or impair*))
-
S25 TI deaf* OR AB deaf*
-
S24 (MH “Ototoxicity”)
-
S23 (MH “Hair Cells”)
-
S22 (MH “Hearing Disorders+”) OR (MH “Deafness+”)
-
S21 TI ototoxicity OR AB ototoxicity
-
S20 TI induc* OR AB induc*
-
S19 S14 OR S15 OR S16 OR S17 OR S18
-
S18 (MH “Antibiotics+/AE”)
-
S17 TI (anti?biotic* or anti?bacterial* or anti?infective*) OR AB (anti?biotic* or anti?bacterial* or anti?infective*)
-
S16 TI aminoglycoside* OR AB aminoglycoside*
-
S15 TI (Gentamicin or paromomycin or amikacin or plazomicin or tobramycin or neomycin or kanamycin or streptomycin or netilmicin) OR AB (Gentamicin or paromomycin or amikacin or plazomicin or tobramycin or neomycin or kanamycin or streptomycin or netilmicin)
-
S14 (MH “Aminoglycosides+”)
-
S13 S10 OR S11 OR S12
-
S12 TI (((penetrance or snp or polymorphism or mutation) N3 “1555”)) OR AB (((penetrance or snp or polymorphism or mutation) N3 “1555”))
-
S11 TI MT#RNR#1 OR AB MT#RNR#1
-
S10 TI (“mt.1555A>G” OR “1555A>G” OR “m.1555A>G” OR “A1555G” OR “1555 A to G”) OR AB (“mt.1555A>G” OR “1555A>G” OR “m.1555A>G” OR “A1555G” OR “1555 A to G”)
-
S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8
-
S8 TI (((pharmacogenetics or pharmacogenomics or genetic* or geno* or gene* or hear* or pyrosequenc* or sequenc*) N4 (test* or assay* or system* or screen* or sequenc*))) OR AB (((pharmacogenetics or pharmacogenomics or genetic* or geno* or gene* or hear* or pyrosequenc* or sequenc*) N4 (test* or assay* or system* or screen* or sequenc*)))
-
S7 TI PALoH OR AB PALoH
-
S6 TI (MIB290 OR “MIB 290”) OR AB (MIB290 OR “MIB 290”)
-
S5 TX genedrive*
-
S4 TI (POCT OR “point-of-care” OR “point of care”) OR AB (POCT OR “point-of-care” OR “point of care”)
-
S3 (MH “Genetic Screening”)
-
S2 TI point-of-care systems OR AB point-of-care systems
-
S1 (MH “Point-of-Care Testing”)
Appendix 2 Economic evaluation searches
# MEDLINE
-
Point-of-Care Systems/
-
Point-of-Care Testing/
-
Genetic Testing/
-
(POCT or “point of care” or “point-of-care”).ti,ab,kw.
-
genedrive*.af.
-
MIB?290.ti,ab,kw.
-
PALoH.ti,ab,kw.
-
((pharmacogenetics or pharmacogenomics or genetic* or geno* or gene* or hear* or pyrosequenc* or sequenc*) adj4 (test* or assay* or system* or screen* or sequenc*)).ti,ab,kw.
-
or/1-8
-
“mt.1555A>G”.ti,ab,kw.
-
“1555A>G”.ti,ab,kw.
-
“m.1555A>G”.ti,ab,kw.
-
“A1555G”.ti,ab,kw.
-
“1555 A to G”.ti,ab,kw.
-
MT?RNR?1.ti,ab,kw.
-
((penetrance or snp or polymorphism or mutation) adj3 “1555”).ti,ab,kw.
-
or/10-16
-
exp Aminoglycosides/
-
(Gentamicin or paromomycin or amikacin or plazomicin or tobramycin or neomycin or kanamycin or streptomycin or netilmicin).ti,ab,kw.
-
aminoglycoside*.ti,ab,kw.
-
(anti?biotic* or anti?bacterial* or anti?infective*).ti,ab,kw.
-
exp Anti-Bacterial Agents/ae, to [Adverse Effects, Toxicity]
-
or/18-22
-
induc*.ti,ab,kw.
-
ototoxicity.ti,ab,kw.
-
exp Hearing Loss/
-
exp Hair Cells, Auditory/
-
Ototoxicity/
-
Deaf*.ti,ab,kw.
-
(hear* adj2 (loss or impair*)).ti,ab,kw.
-
or/24-30
-
9 and 17 and 23 and 31
-
“Genedrive MT-RNR1 ID”.af.
-
32 or 33
-
exp Animals/
-
exp Humans/
-
35 not 36
-
34 not 37
-
limit 38 to dt=20100101-20220929
-
limit 39 to english language
# EMBASE
-
socioeconomics/
-
“cost benefit analysis”/
-
“cost effectiveness analysis”/
-
“cost of illness”/
-
“cost control”/
-
economic aspect/
-
financial management/
-
“health care cost”/
-
health care financing/
-
health economics/
-
“hospital cost”/
-
(fiscal or financial or finance or funding).tw.
-
“cost minimization analysis”/
-
(cost adj estimate$).mp.
-
(cost adj variable$).mp.
-
(unit adj cost$).mp.
-
or/1-16
-
“point of care system”/
-
“point of care testing”/
-
genetic screening/
-
(POCT or “point of care” or “point-of-care”).ti,ab.
-
genedrive*.af.
-
MIB?290.ti,ab.
-
PALoH.ti,ab.
-
((pharmacogenetics or pharmacogenomics or genetic* or geno* or gene* or hear* or pyrosequenc* or sequenc*) adj4 (test* or assay* or system* or screen* or sequenc*)).ti,ab.
-
or/18-25
-
“mt.1555A>G”.ti,ab.
-
“1555A>G”.ti,ab.
-
“m.1555A>G”.ti,ab.
-
“A1555G”.ti,ab.
-
“1555 A to G”.ti,ab.
-
MT?RNR?1.ti,ab.
-
((penetrance or snp or polymorphism or mutation) adj3 “1555”).ti,ab.
-
or/27-33
-
exp aminoglycoside antibiotic agent/
-
(Gentamicin or paromomycin or amikacin or plazomicin or tobramycin or neomycin or kanamycin or streptomycin or netilmicin).ti,ab.
-
aminoglycoside*.ti,ab.
-
(anti?biotic* or anti?bacterial* or anti?infective*).ti,ab.
-
exp antiinfective agent/to [Drug Toxicity]
-
or/35-39
-
induc*.ti,ab.
-
ototoxicity.ti,ab.
-
exp hearing impairment/
-
exp “hair cell (inner ear)”/
-
exp ototoxicity/
-
deaf*.ti,ab.
-
(hear* adj2 (loss or impair*)).ti,ab.
-
or/41-47
-
26 and 34 and 40 and 48
-
“Genedrive MT-RNR1 ID”.af.
-
49 or 50
-
exp animal/
-
exp human/
-
52 not 53
-
51 not 54
-
limit 55 to dc=20100101-20221004
-
limit 56 to english language
-
17 and 57
# CINAHL
-
S46 S13 AND S45
-
S45 S43 AND S42
-
S44 Limiters – Publication date: 20100101-20221231
-
S43 S41 OR S42
-
S42 S22 AND S32 AND S40
-
S41 TX “Genedrive MT-RNR1 ID”
-
S40 S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39
-
S39 TI (hear* N2 (loss or impair*)) OR AB (hear* N2 (loss or impair*))
-
S38 TI deaf* OR AB deaf*
-
S37 (MH “Ototoxicity”)
-
S36 (MH “Hair Cells”)
-
S35 (MH “Hearing Disorders+”) OR (MH “Deafness+”)
-
S34 TI ototoxicity OR AB ototoxicity
-
S33 TI induc* OR AB induc*
-
S32 S27 OR S28 OR S29 OR S30 OR S31
-
S31 (MH “Antibiotics+/AE”)
-
S30 TI (anti?biotic* or anti?bacterial* or anti?infective*) OR AB (anti?biotic* or anti?bacterial* or anti?infective*)
-
S29 TI aminoglycoside* OR AB aminoglycoside*
-
S28 TI (Gentamicin or paromomycin or amikacin or plazomicin or tobramycin or neomycin or kanamycin or streptomycin or netilmicin) OR AB (Gentamicin or paromomycin or amikacin or plazomicin or tobramycin or neomycin or kanamycin or streptomycin or netilmicin)
-
S27 (MH “Aminoglycosides+”)
-
S26 S23 OR S24 OR S25
-
S25 TI (((penetrance or snp or polymorphism or mutation) N3 “1555”)) OR AB (((penetrance or snp or polymorphism or mutation) N3 “1555”))
-
S24 TI MT#RNR#1 OR AB MT#RNR#1
-
S23 TI (“mt.1555A>G” OR “1555A>G” OR “m.1555A>G” OR “A1555G” OR “1555 A to G”) OR AB (“mt.1555A>G” OR “1555A>G” OR “m.1555A>G” OR “A1555G” OR “1555 A to G”)
-
S22 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S27 OR S28 OR S29
-
S21 TI (((pharmacogenetics or pharmacogenomics or genetic* or geno* or gene* or hear* or pyrosequenc* or sequenc*) N4 (test* or assay* or system* or screen* or sequenc*))) OR AB (((pharmacogenetics or pharmacogenomics or genetic* or geno* or gene* or hear* or pyrosequenc* or sequenc*) N4 (test* or assay* or system* or screen* or sequenc*)))
-
S20 TI PALoH OR AB PALoH
-
S19 TI (MIB290 OR “MIB 290”) OR AB (MIB290 OR “MIB 290”)
-
S18 TX genedrive*
-
S17 TI (POCT OR “point-of-care” OR “point of care”) OR AB (POCT OR “point-of-care” OR “point of care”)
-
S16 (MH “Genetic Screening”)
-
S15 TI point-of-care systems OR AB point-of-care systems
-
S14 (MH “Point-of-Care Testing”)
-
S13 S11 NOT S12
-
S12 PT news OR PT Letter OR PT Editorial
-
S11 S9 OR S10
-
S10 TX (cost or costs or economic$ or pharmacoeconomic$ or price$ or pricing$)
-
S9 S7 OR S8
-
S8 MW Health resource utilization OR MW Health resource allocation
-
S7 S1 NOT S6
-
S6 S2 OR S3 OR S4 OR S5
-
S5 (MH “Business+”)
-
S4 (MH “Financing, Organized+”)
-
S3 (MH “Financial Support+”)
-
S2 (MH “Financial Management+”)
-
S1 (MH “Economics+”)
#COCHRANE
-
MeSH descriptor: [Point-of-Care Systems] this term only 477
-
MeSH descriptor: [Point-of-Care Testing] this term only 102
-
MeSH descriptor: [Genetic Testing] this term only 423
-
(POCT or “point of care” or “point-of-care”):ti,ab,kw 2583
-
(genedrive*) 0
-
(MIB?290):ti,ab,kw 0
-
(PALoH):ti,ab,kw 0
-
((pharmacogenetics or pharmacogenomics or genetic* or geno* or gene* or hear* or pyrosequenc* or sequenc*) NEAR/4 (test* or assay* or system* or screen* or sequenc*)):ti,ab,kw 50,919
-
{OR #1-#8} 53,314
-
(“mt.1555A>G”):ti,ab,kw 0
-
(“1555A>G”):ti,ab,kw 1
-
(“m.1555A>G”):ti,ab,kw 1
-
(“A1555G”):ti,ab,kw 3
-
(“1555 A to G”):ti,ab,kw 0
-
(MT?RNR?1):ti,ab,kw 0
-
((penetrance or snp or polymorphism or mutation) NEAR/3 “1555”):ti,ab,kw 1
-
16-#16 5
-
MeSH descriptor: [Aminoglycosides] explode all trees 9189
-
(Gentamicin or paromomycin or amikacin or plazomicin or tobramycin or neomycin or kanamycin or streptomycin or netilmicin):ti,ab,kw 5794
-
(aminoglycoside*):ti,ab,kw 986
-
(anti?biotic* or anti?bacterial* or anti?infective*):ti,ab,kw 45,868
-
MeSH descriptor: [Anti-Bacterial Agents] explode all trees 13,127
-
{OR #18-#22} 55,411
-
(induc*):ti,ab,kw 187,979
-
(ototoxicity):ti,ab,kw 576
-
MeSH descriptor: [Hearing Loss] explode all trees 1357
-
MeSH descriptor: [Hair Cells, Auditory] explode all trees 7
-
MeSH descriptor: [Ototoxicity] this term only 6
-
(deaf*):ti,ab,kw 1577
-
(hear* NEAR/2 (loss or impair*)):ti,ab,kw 4297
-
{OR #24-#30} 192804
-
#9 AND #17 AND #23 AND #31 2
-
(“Genedrive MT-RNR1 ID”) 0
-
#32 OR #33 2
#REPEC
-
“m.1555” | “mt.1555” | “MTRNR1” | “MT-RNR-1” | “MT RNR 1” | Genedrive | PAHoL | “MIB290” | “MIB-290” | “MIB 290”
Appendix 3 List of excluded records
-
Fan W, Zhu Y, Tang X, Xue L. Noninvasive test for mitochondrial DNA A1555G mutation associated with deafness. Clin Lab 2017;63:127–31. (Exclusion reason: wrong population.)
-
Fischer PR. Aminoglycoside-induced ototoxicity: test before you treat?. Infect Dis Alert 2022;41. (Exclusion reason: wrong publication type.)
-
Huang S, Xiang G, Kang D, Wang C, Kong Y, Zhang X, et al. Rapid identification of aminoglycoside-induced deafness gene mutations using multiplex real-time polymerase chain reaction. Int J Pediatr Otorhinolaryngol 2015;79:1067–72. (Exclusion reason: wrong population.)
-
Kato T, Nishigaki Y, Noguchi Y, Ueno H, Hosoya H, Ito T, et al. Extensive and rapid screening for major mitochondrial DNA point mutations in patients with hereditary hearing loss. J Hum Genet 2010;55:147–54. (Exclusion reason: wrong population.)
-
Parker J, Wright D. Terrible choices in the septic child: a response to the PALOH trial round table authors. J Med Ethics 2021;47:114–16. (Exclusion reason: wrong publication type.)
-
Phillips LL, Glindzicz MB, Lench N, Steel KP, Langford C, Dawson SJ, et al. The future role of genetic screening to detect newborns at risk of childhood-onset hearing loss. Int J Audiol 2013;52:124–33. (Exclusion reason: wrong publication type.)
-
Pillers DM. Genetic testing in newborns moves from rare to routine application. JAMA Pediatr 2022;176:448–9. (Exclusion reason: wrong publication type.)
-
McDermott JH. Genetic testing in the acute setting: a round table discussion. J Med Ethics 2020;46:531–2. Exclusion reason: wrong publication type.)
-
McDermott JH, Mahood R, Stoddard D, Mahaveer A, Turner MA, Corry R, et al. Pharmacogenetics to Avoid Loss of Hearing (PALOH) trial: a protocol for a prospective observational implementation trial. BMJ Open 2021;11:e044457. (Exclusion reason: wrong publication type.)
-
The Hearing Review. Genedrive Pediatric Hearing Screening Test Receives CE Marking. 2019. URL: https://hearingreview.com/hearing-products/testing-equipment/pediatric-testing/genedrive-pediatric-hearing-screening-test-receives-ce-marking (accessed 1 December 2022). (Exclusion reason: wrong publication type.)
-
Zhu Q, Li M, Zhuang X, Chen K, Xu W-Q, Jiang Y-H, Qin G. Assessment of hearing screening combined with limited and expanded genetic screening for newborns in Nantong, China. JAMA Netw Open 2021;4:e2125544. (Exclusion reason: wrong index test.)
List of abbreviations
- AIHL
- aminoglycoside-induced hearing loss
- BNF
- British National Formulary
- DNA
- deoxyribonucleic acid
- EAG
- Evidence Assessment Group
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EVA
- early value assessment
- HRQoL
- health-related quality of life
- HUI2
- Health Utilities Index Mark 2
- HUI3
- Health Utilities Index Mark 3
- ICER
- incremental cost-effectiveness ratio
- MIB
- Medtech Innovation Briefing
- mtDNA
- mitochondrial DNA
- NICE
- National Institute for Health and Care Excellence
- NICU
- neonatal intensive care unit
- PALOH
- Pharmacogenetics to Avoid Loss of Hearing
- QALY
- quality-adjusted life-year
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies-2
- ROBINS-I
- Risk Of Bias In Non-randomized Studies – of Interventions
- SF-6D
- Short Form 6 Dimensions
- TTO
- time trade-off