Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/04/03. The contractual start date was in January 2015. The draft report began editorial review in March 2021 and was accepted for publication in August 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Lim et al. This work was produced by Lim et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Lim et al.
Chapter 1 Introduction
Material throughout this report has been reproduced from the trial protocol. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background and rationale
Lung cancer is a leading cause of cancer death worldwide and survival in the UK remains among the lowest in Europe. 2 In early-stage lung cancer, surgery is commonly undertaken through an open thoracotomy. However, minimal-access video-assisted thoracoscopic surgery (VATS) was introduced in the 1990s, and the evolution of the technique from minor procedures eventually led to its successful application in anatomic lung cancer resections, undertaken using a telescope and television screen, with small incisions in the chest. Since then, minimal access surgery has increased in popularity on the premise that smaller incisions without rib spreading may improve recovery after lung surgery. Data from the UK demonstrates exponential growth in popularity of this technique. In 2010, 14% of lobectomy procedures were performed using VATS access, and this increased to 40% in 2014. 3
To date, much of the evidence generated for VATS is based on non-randomised studies4,5 or small randomised controlled trials (RCTs) that focus on in-hospital outcomes. 6 These studies are underpowered to detect clinically meaningful differences in longer-term outcomes7 or have focused solely on operative technique. 8 Currently, the largest RCT, comparing VATS with open surgery in 206 participants followed for 1 year, reported shorter hospital stay and less pain in patients randomised to VATS lobectomy. 9 In this study,9 carried out in Denmark, all patients received epidural anaesthesia and anterior thoracotomy for open surgery, which is not the current practice for most thoracic surgery centres in the UK. In contrast, a recent trial10 in 425 patients recruited in China reported a similar hospital stay and rate of morbidity and mortality at 28 days in both the VATS and axillary thoracotomy groups. 10 To the best of our knowledge, there are no high-quality comparative data on physical function (as a global measure of recovery from surgery), hospital readmissions, the uptake and timing of chemotherapy nor cancer recurrence, and few high-quality RCT data on the cost-effectiveness of VATS compared with open surgery. The Danish investigators11 have compared VATS with open surgery from an economic societal cost perspective and there is an ongoing multicentre trial in (Lungsco01) France with a target sample size of 600 participants that plans a similar comparison. 12
A well-designed and well-conducted RCT comparing the clinical effectiveness and cost-effectiveness of VATS and open surgery is needed to inform current UK (NHS) practice, health policy and individual surgeon and patient decision-making.
Aims and objectives
The VIdeo assisted thoracoscopic lobectomy versus conventional Open LobEcTomy for lung cancer (VIOLET) study aimed to compare the clinical effectiveness, cost-effectiveness and acceptability of VATS lobectomy with open surgery for treatment of lung cancer.
Specific objectives were to estimate the differences in the primary outcome (i.e. self-reported physical function at 5 weeks) and a range of secondary outcomes, including efficacy, safety, oncological outcomes and survival, between participants allocated to VATS and participants allocated to open surgery, and to compare the cost-effectiveness of the two surgical strategies.
Chapter 2 Methods
Trial design
A multicentre, parallel-group, superiority RCT, with blinding of outcome assessors and participants (until hospital discharge after surgery) and active follow-up to 1 year. The trial included an internal pilot phase and a QuinteT Recruitment Intervention (QRI) to optimise recruitment (Figure 1).
FIGURE 1.
The trial schema for the VIOLET study.
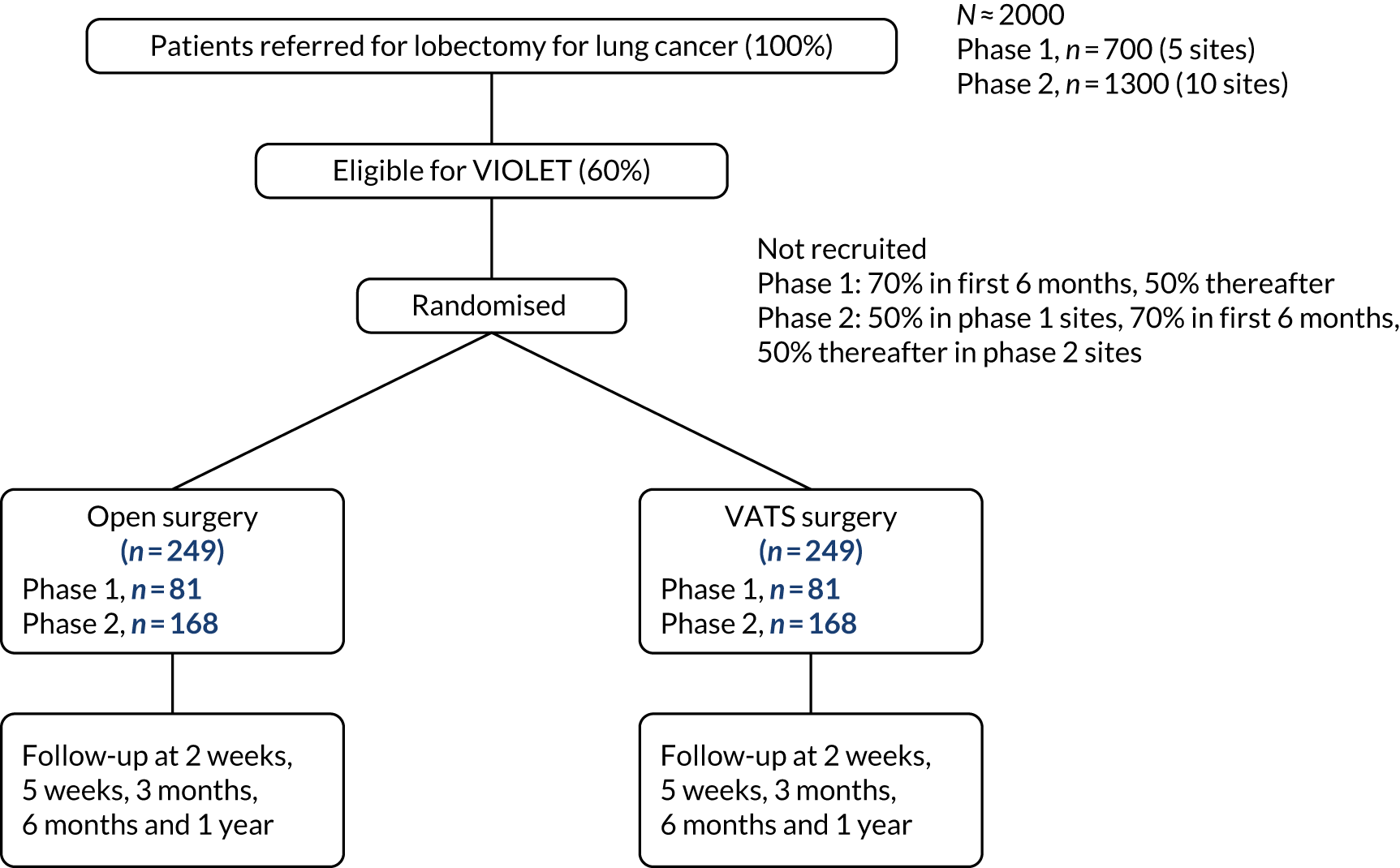
Criteria for progression from Phase I to Phase II
During the first phase, processes for trial conduct, including recruitment and consent, were established. Progression from the internal pilot (Phase I) to the full trial (Phase II) was dependent on the following criteria being met when assessed 18 months after the start of recruitment:
-
At least 60% of patients undergoing lobectomy are considered eligible for the trial (if necessary, by revising the eligibility criteria).
-
At least 50% of patients consent to randomisation after 6 months of recruitment.
-
Less than 5% of patients fail to receive their allocated treatment.
-
Less than 5% of patients are lost to follow-up (excluding deaths).
In Phase II, the number of study sites was increased and all sites used the optimum methods of recruitment established in Phase I.
Changes to trial design after commencement of the trial
There were several substantial amendments made to the study protocol throughout the course of the trial. The changes are summarised below. The protocol version in use when the trial started was version 2.0. The current full trial protocol can be found in the National Institute for Health and Care Research (NIHR) Journals Library [URL: www.journalslibrary.nihr.ac.uk/programmes/hta/130403/#/ (accessed 27 October 2021)].
First amendment (before recruitment started)
-
Definition of prolonged incision pain was changed from ‘need of analgesia for > 6 weeks after surgery’ to ‘need of analgesia for > 5 weeks after randomisation’.
-
Clarification that adverse health events would be collected to 1 year and graded in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) criteria, with postoperative complications classified in accordance with the Clavien–Dindo system. Surgical emphysema requiring intervention, reoperation for reasons other than recurrence or progression, and adverse events (AEs) associated with adjuvant chemotherapy and radiotherapy were added to the list of expected AEs.
-
Patient resource use questionnaires, collection of resource use at 2 weeks and computerised tomography (CT) of the pelvis were removed.
-
Clarification of measures to promote blinding of the trial participants.
Second amendment (approved 19 October 2015)
-
CT-based Response Evaluation Criteria in Solid Tumours criteria to assess recurrence/progression was replaced by specific objective criteria, as postoperative CT imaging is not routinely undertaken for patients following lobectomy. Therefore, there were no comparative images against which to reference the 12-month CT scan.
-
Addition of patient-reported pain scores at baseline and at 1 day and 2 days postoperatively.
-
Option for research team at sites to follow-up patients by telephone at 5 weeks and 1 year to facilitate data collection as some patients are referred back to tertiary or peripheral hospitals for follow-up.
-
Clarification of patient referral pathways, where potential participants may be identified and provided information, including the setup of patient identification centres.
Third amendment (approved 6 June 2017)
-
Inclusion criteria modified to include patients undergoing bi-lobectomy, and reflect the transition to TNM Classification of Malignant Tumours, Eighth Edition (TNM8)13 of the TNM staging system.
-
Clarification that elective surgery, interventions and treatments during follow-up that were planned prior to recruitment to the trial will not be reported as unexpected serious adverse events (SAEs).
-
Addition of pleural effusion, venous thromboembolism and other infection to the list of expected AEs.
Fourth amendment (approved 7 June 2018)
-
Addition of molecular residual disease substudy (not reported here, funded by industry).
-
Revision to archiving plan to remove scanning of documents.
-
Clarification of form of bleeding (e.g. in or around the operation site) considered an expected AE.
-
Addition of the option for research nurses (RNs) to obtain the questionnaire data directly from participants.
Fifth amendment (approved 21 January 2019)
-
CTCAE grade scheme changed from v4.0 to v5.0.
Sixth amendment (approved 17 April 2019)
-
Addition of the secondary outcome ‘pain scores in the first 2 days post surgery’, which had previously been missed from the protocol.
-
Clarification of exploratory analysis of pain scores.
-
Clarification of molecular residual disease substudy.
Seventh amendment (approved 4 December 2019)
-
Clarification of molecular residual disease substudy.
-
Reference corrected.
Eighth amendment (approved 3 February 2021)
-
Clarification of end-of-study definition.
Participants
Patient population
Adults referred for lung resection for known or suspected lung cancer to one of the participating centres.
Patient eligibility criteria
Patients were eligible to enter the study if all the following applied:
-
Aged ≥ 16 years.
-
Undergoing lobectomy or bi-lobectomy for treatment of known or suspected primary lung cancer beyond a lobar orifice, or undergoing frozen section biopsy with the intention to proceed with lobectomy or bi-lobectomy if primary lung cancer beyond a lobar orifice is confirmed. (Note that for bi-lobectomy, the distance for the lobar orifice is in reference to the bronchus intermedius.)
-
Cancer staging using TMN8:
-
clinical tumour stage 1–3 (cT1–3) [by size criteria, equivalent to TNM Classification of Malignant Tumours, Seventh Edition14 (TNM7) stage cT1a-2b] or cT3 (by virtue of two nodules in the same lobe)
-
node stage 0–1 (N0–1)
-
metastasis stage 0 (M0).
-
-
Multidisciplinary team (MDT) consider the disease is suitable for both VATS lobectomy and lobectomy via open surgery.
-
Ability to give written informed consent.
Patients were not eligible to enter the study if any of the following applied:
-
Previous malignancy that influences life expectancy.
-
Planned pneumonectomy, segmentectomy or non-anatomic resection (e.g. wedge resection).
-
Serious concomitant disorder that would compromise patient safety during surgery.
-
Planned robotic surgery.
Changes to trial eligibility criteria after commencement of the trial
In July 2015, planned segmentectomy was added as an exclusion.
In June 2017, the inclusion criteria were revised to allow for the inclusion of patients undergoing bi-lobectomy and to update the cancer staging from TNM7 to TMN8. The Trial Management Group (TMG) recommended widening the inclusion criteria to include patients scheduled for a bi-lobectomy (i.e. resection of two lobes rather than one), as this can be performed via VATS or open surgery and, therefore, the research question is equally applicable to patients having this procedure. The revisions to the TNM staging system necessitated a change to the eligibility criteria, which were widened to include patients with cT1–3 tumours (by size criteria, equivalent to TNM7 stage cT1a-2b) or cT3 by virtue of two nodules in the same lobe. The entry criteria for nodal and metastatic involvement remained unchanged at N0–1 and M0, respectively.
Settings
NHS trusts with an established and accredited lung cancer MDT, which included trusts from across the UK, were eligible to participate in the VIOLET trial if the site undertook at least 40 VATS lobectomies each year and employed at least one surgeon that had carried out ≥ 50 VATS lobectomies. Phase I was restricted to five sites and further sites were opened in Phase II.
Surgeons were eligible to participate if they had performed at least 40 VATS lobectomies. Lobectomy via open surgery is a standard procedure and, therefore, surgical ability and competence was assured by specialist General Medical Council registration.
Trial interventions
VATS lobectomy (experimental)
Surgeons were permitted to undertake the VATS lobectomy using between one and four keyhole incisions. The use of rib spreading was prohibited, as this intraoperative manoeuvre disrupts the intercostal nerves and is thought to be an important cause of pain (and is a key feature of open surgery). The procedure was to be performed with videoscopic visualisation without direct vision. The hilar structures (i.e. vein, artery and bronchus) were dissected, stapled and divided. Endoscopic ligation of pulmonary arterial branches was optional. The fissure was completed and the lobe of lung resected. The incisions were closed in layers and may have involved muscle, fat and skin layers. This definition of VATS lobectomy is a modification of CALGB 39802. 15
Open lobectomy (control)
Conventional open surgery was undertaken through a single incision. Rib spreading was mandated, but rib resection was optional. The operation was performed under direct vision, with isolation of the hilar structures (i.e. vein, artery and bronchus), which were dissected, ligated and divided in sequence, and the lobe of lung resected. Ligatures, over sewing or staplers could be used. The thoracotomy was closed in layers, starting from pericostal sutures over the ribs, muscle, fat and skin layers.
Aspects common to both groups
Participants without a confirmed tissue diagnosis at surgery
The surgeon could either take a confirmatory biopsy or proceed directly to surgery, as per MDT recommendation (see Identification of potential participants: referral and multidisciplinary team review).
Lymph node management
In both groups, lymph node management was undertaken in accordance with the International Association for the Study of Lung Cancer recommendations. The Association recommends that a minimum of six nodes/stations are removed, of which three are from the mediastinum that includes the subcarinal station.
Anaesthesia and postoperative pain management
All operations were undertaken with general anaesthesia and with the patient in the lateral decubitus position. This was a pragmatic trial and so adaptations and variations of both procedures were permitted at the discretion of the surgeon (intraoperative details were captured and monitored).
Standardising the use of analgesia across all participating sites was considered impractical and, if implementable, would be unrepresentative of clinical practice in the NHS. Each participating site prescribed analgesia in accordance with their local protocols. To minimise potential bias in pain outcomes, all sites were required to administer analgesia in accordance with a standard protocol applied, regardless of treatment allocation. Local protocols for the provision of analgesia were defined by the local principal investigator (PI) prior to the start of recruitment at the site. Details of the analgesia used throughout a participant’s hospital stay were recorded and compliance with the prespecified site-specific analgesia protocol was monitored.
Other aspects of postoperative care
As this was a pragmatic study, postoperative care and the criteria for drain removal were in accordance with local practice. The decision to discharge a patient home after surgery was at the surgeon’s discretion; however, to minimise the potential for bias, the criteria by which a patient was assessed as medically fit for discharge was prespecified and adherence to these criteria was monitored.
Outcomes
Primary outcome
The primary end point was self-reported physical function assessed using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30) at 5 weeks post randomisation. Physical function was chosen because it is a patient-centred outcome that would reflect the anticipated earlier recovery with VATS. It had also been used in other minimal access surgery trials. The 5-week primary end point (approximately 1-month post surgery) was chosen to capture the early benefits of minimal access surgery on recovery.
The EORTC QLQ-C30 is used to assess quality of life in cancer patients. The EORTC QLQ-C30 comprises 30 questions, which are used to derive an overall measure of global health, and a number of subscales, of which physical function is one. For all scales, higher scores indicate a higher level of functioning, symptoms or problems. The EORTC QLQ-C30 has been validated for use in European cohorts. Version 3 of the questionnaire was used.
Secondary outcomes
The following secondary outcomes were selected to assess the efficacy of the two approaches:
-
Time from surgery to hospital discharge.
-
Pain scores in the first 2 days post surgery.
-
Adverse health events in the period from randomisation to 1 year.
-
Uptake of adjuvant treatment (i.e. frequency and time from surgery).
-
Frequency of upstaging to pathologic node stage 2 (pN2) disease after the procedure.
-
Overall and disease-free survival to 1 year.
-
Frequency of complete resection during the procedure.
-
Frequency of prolonged incision pain (defined as the need of analgesia for > 5 weeks post randomisation).
-
Generic and disease-specific patient-reported health-related quality of life (HRQoL) measured using the EORTC QLQ-C30, Quality of Life Questionnaire Lung Cancer 13 (QLQ-LC13) and EQ-5D-5L questionnaires completed at 2 weeks, 5 weeks, 3 months, 6 months and 1-year post randomisation.
-
Resource use during the hospital stay and post discharge to 1 year after randomisation.
Changes to trial outcomes after commencement of the trial
In 2015, minor amendments were made to clarify some secondary outcomes prior to the study opening to recruitment (see Changes to trial design after commencement of the trial for details). In a further amendment in the same year, the collection of patient-reported pain scores at baseline and at 1 day and 2 days postoperatively was added to the table of assessments, but the list of secondary outcomes was inadvertently not updated in line with this addition. This error was corrected in 2019 when pain scores were added to the list of secondary outcomes in the protocol. Pain scores were added to allow further comparison between the two surgical techniques during the early postoperative period. Pain assessments are routinely undertaken by the clinical team to determine whether or not the analgesia provision is sufficient and so a patient verbal assessment of pain represented minimal additional burden to the patient.
Sample size
We hypothesised that self-reported physical function 5 weeks after randomisation for participants undergoing a VATS lobectomy would be superior to the physical function for participants having an open lobectomy, as derived from responses to the EORTC QLQ-C30 questionnaire. Data from the literature on minimal clinically important differences in HRQoL scores from the EORTC QLQ-C30 were used to inform the target effect size. 16
Although the primary end point was at 5 weeks, the questionnaire was also completed at other time points, namely at baseline, 2 weeks, 3 months, 6 months and 1 year. In estimating the sample size, the following assumptions were made:
-
One pre-surgery measure and five post-surgery measures.
-
A correlation between pre- and post-surgery measures of 0.3.
-
A correlation between repeated post-surgery measures of 0.6.
-
An effect size of 0.25 standard deviations (SDs) would be considered clinically important.
Under these assumptions, and allowing for up to 20% loss to follow-up at 1 year, the sample size was set at 498 patients (i.e. 249 patients per group), which provided 90% power to test the superiority hypothesis at the 5% significance level.
Interim analyses
There were no interim analyses planned nor undertaken for the VIOLET trial.
Randomisation
Participants were randomly allocated to either VATS lobectomy or open lobectomy in a 1 : 1 ratio. Randomisation took place through a secure internet-based randomisation system (Sealed Envelope Ltd, London, UK) approximately 1 week before the planned surgery, after eligibility had been confirmed and written informed consent given. This time frame was chosen to allow sufficient time for operating schedules to be arranged.
The randomisation was stratified by centre, and cohort minimisation (with a random element incorporated) was used to ensure balance across groups with respect to surgeon. Allocations were concealed until information to uniquely identify the participant and confirm eligibility was entered into the randomisation system, after which the randomised allocation was revealed. If there was a change in surgeon after randomisation, the analysis accounted for the surgeon responsible for the performing the operation and not the surgeon originally assigned to the patient.
Blinding
Research team
The surgical team, anaesthetist and other staff caring for the participant during the operation were not blinded to the patients’ treatment allocation. However, to minimise the risk of bias in the assessment of outcomes, the randomisation was performed by a member of the research team who was not responsible for the collection of outcome data.
Wound dressings
Efforts were made to minimise the risk of inadvertently unblinding the RN responsible for data collection during the patient’s postoperative stay by applying large adhesive dressings to the thorax of participants. These adhesive dressings were positioned similarly for all patients, regardless of their surgical allocation, to cover both real and potential incision/port locations. The initial adhesive dressings were applied in the operating room by the operating team. The dressings remained in place for 3 days unless the patient was discharged before day 3, when they were removed, or if the patient required replacing early because of soiling. After 3 days, dressings were changed by a nurse not involved in conducting follow-up assessments or data collection for the trial. Wound cleaning was performed on both actual and potential wounds to promote masking.
Fitness for discharge after surgery
To minimise bias in the decision-making around when a participant was discharged home, the following discharge suitability criteria were developed. Participants were evaluated against the following criteria to ensure that they are medically fit for discharge:
-
Patient has achieved satisfactory mobility with:
-
pain under control with analgesia
-
satisfactory serum haemoglobin and electrolytes (i.e. does not require intervention)
-
satisfactory chest-X-ray (which will be performed as part of routine clinical care)
-
no complications that require further/additional treatment.
-
Patients who were considered medically fit for discharge were not necessarily discharged immediately. In some instances, social and other factors may have necessitated extended hospitalisation. The time at which patients are considered medically fit for discharge and when they are physically discharged from hospital were both captured in the trial. For the two groups, the data were monitored for evidence of both early discharge before all the discharge criteria were met and delayed discharge.
Participants
To ensure that study participants remained blinded during the postoperative period to discharge home, participants were asked to turn their head away from the wound site(s) while wounds were being cleaned and dressed. Participants were advised of how best to care for their wounds when they were considered ‘fit for discharge’. Those participants who asked to know which treatment they had received were informed at this point.
Assessment of blinding
The success of blinding was assessed using Bang et al. ’s Blinding Index. 17 Participants were asked to complete the assessment 2 days postoperatively and at discharge, but before the treatment allocation was revealed. The RNs responsible for data collection and follow-up of participants completed the Blinding Index when the patient was ready for discharge, and after the 5-week and 1-year follow-up appointments.
Data collection
Overview
Data collection for the trial participants included the following elements:
-
A log of patients screened by the MDT for suitability for the trial and the date when patients were given or sent the patient information leaflet (PIL).
-
A log of patients assessed against the eligibility criteria and reason(s) if ineligible.
-
Audio-recording and transcription of consultations between surgeons and potential participants (see QuinteT Recruitment Intervention for further details).
-
Semistructured interviews with a sample of eligible patients, including patients who accept or decline to join the trial.
-
Approach and consent details, including reason(s) for non-approach or decline.
-
Baseline data, including the participant’s medical history, disease status and HRQoL prior to randomisation.
-
Operative details.
-
Histopathology of any samples (e.g. biopsies) taken intraoperatively.
-
Postoperative care, including analgesia and pain scores.
-
AEs and resource use in the period from randomisation to 1 year.
-
HRQoL during follow-up.
-
Results of scans taken to assess disease status.
An overview of the schedule of data collection is given in Table 1.
| Item | Study period | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post allocationa | Close-out | ||||||||
| –t1 | 0 | t1 | t2 | t3 | t4 | t5 | t6 | t7 | t8 | t9 | |
| Enrolment | |||||||||||
| Eligibility | ✓ | ||||||||||
| Informed consent | ✓ | ||||||||||
| Allocation | ✓ | ||||||||||
| Assessment | |||||||||||
| Imaging review (CT or PET-CT) | ✓ | ||||||||||
| Participant characteristics | ✓ | ||||||||||
| Audio-recorded consultation | ✓ | ||||||||||
| Lobectomy via VATS or open surgery | ✓ | ||||||||||
| Intraoperative details | ✓ | ||||||||||
| Histopathology staging | ✓ | ||||||||||
| Tumour sample for research | ✓ | ||||||||||
| QLQ-C30 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| QLQ-LC13 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| EQ-5D-5L | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Bang et al.’s Blinding Index17 | ✓ | ✓ | |||||||||
| Pain score | ✓ | ✓ | ✓ | ||||||||
| AEs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Resource use | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| CT of chest and abdomen | ✓ | ||||||||||
Collection of health-related quality-of-life data
Health-related quality-of-life data were collected on paper or online, according to participant preference. If data were not returned, the participant may have been telephoned by the local RN and the data collected over the telephone.
Collection of adverse event data
Serious adverse events and other AEs were recorded and reported in accordance with Good Clinical Practice guidelines. Data were collected from the time of consent until 1-year post randomisation. Events were graded in severity using the CTCAE, which is a standardised classification system used in cancer studies.
As lung resection surgery is a major surgical intervention, events related to the surgery were considered ‘expected’. Many participants would go on to receive adjuvant chemotherapy or radiotherapy after their lung resection surgery. Such treatments have a range of common serious side effects and toxicities, which were also considered ‘expected’ for participants undergoing adjuvant chemotherapy and/or radiotherapy. These expected events were listed in the study protocol. Events that occurred that were not listed in the protocol were considered unexpected.
Safety data were reviewed regularly by the study team and at least annually by the Data Monitoring and Safety Committee (DMSC). Reporting to the sponsor was required only if an AE was considered serious (i.e. resulted in a hospital admission, prolonged a hospital admission, was life-threatening, resulted in significant disability or death) and unexpected or expected and fatal. Reporting to the Research Ethics Committee and DMSC was required if an unexpected SAE was found to be causally related to the intervention.
Identification of potential participants: referral and multidisciplinary team review
Potential participants were identified from MDT meetings at each study site, where patient referrals from local and satellite lung cancer MDTs or from peripheral hospitals are considered. Several peripheral referring hospitals were set up as patient identification centres to allow potential participants to receive study information in a timely way and to allow participants time to consider the trial and discuss it with friends and family before their clinical appointment with a study surgeon.
Patients had undergone CT/CT plus positron emission tomography (PET) to assess the extent of their disease, but it is common for lung lesions to be of uncertain pathology before surgery: up to 25% of patients are listed without a preoperative tissue diagnosis. 18 Both patients with proven cancer and those without preoperative tissue diagnosis were eligible to participate in the VIOLET trial. Patients without a preoperative tissue diagnosis were considered eligible if the MDT either recommended lobectomy surgery or a biopsy with the option to proceed to lobectomy if cancer is confirmed, or if there was sufficient clinical certainty for direct lobectomy without biopsy. It was estimated that 75% of patients referred had a confirmed diagnosis. Of the remaining 25% of patients, the recommendation was a biopsy with the option to proceed in 20% of cases, and in 5% of patients the MDT recommended lobectomy without biopsy confirmation. This strategy could lead to a small proportion of participants (estimated 4% in total) finally being confirmed to have benign disease. These patients are included in the primary analyses. If the (real-time) results of the frozen section biopsy diagnosed primary lung cancer, surgery proceeded as allocated. Participants with a non-cancerous diagnosis received no further surgery.
QuinteT Recruitment Intervention
Overview and aims
A QRI19,20 was integrated throughout the recruitment period of the VIOLET trial because of anticipated recruitment challenges1 arising from the nature of the trial interventions [i.e. different approaches to undertaking lung resection (lobectomy) via VATS or open surgery]. The QRI methods, first developed in the ProtecT (Prostate testing for cancer and Treatment) study,21,22 have been refined and applied in nearly 70 RCTs, including other surgical RCTs. 23–25
The aim of the QRI in the VIOLET trial was to optimise and sustain recruitment and informed consent by preventing recruitment difficulties from arising, identifying new challenges as they arose and addressing those that did arise rapidly. The VIOLET trial QRI began with QRI-informed recruitment training workshops aimed at helping recruiters prepare for impending recruitment activities and preventing the development of recruitment barriers. Next, when recruitment commenced, we employed established QRI methods, which comprised two iterative components19,20 aimed at (1) understanding the recruitment issues in real time and identifying the clear obstacles and hidden challenges to recruitment,26,27 and (2) developing and implementing a plan of action comprising strategies28–31 to overcome the challenges, in collaboration with the chief investigator, TMG, Bristol Trials Centre and the recruiting sites. Evaluation of the QRI was carried out throughout the recruitment period by regularly monitoring recruitment figures [using the screened, eligible, approached, randomised (SEAR) framework]32 and recruitment practice. Each of these methods is described in detail below (for further context regarding the evolution of the QRI, see Appendix 1).
Preventing recruitment difficulties: training and guidance prior to recruitment
We aimed to prevent recruitment difficulties in the VIOLET trial by disseminating strategies to optimise recruitment and informed consent to recruiting surgeons, drawing from the QRI evidence base and using multiple avenues. These activities occurred prior to recruitment and involved study-wide, as well as site-specific, activities.
At the trial set-up phase, PIs and other recruiting surgeons from the five sites in the internal pilot phase were invited to attend a 1-day QRI-informed recruitment training workshop. 29 Surgeons from the new centres were invited to subsequent workshops. The evidence-based training was aimed at raising awareness of, and providing practical tips to manage, the clear obstacles27 (e.g. logistical issues) and hidden challenges to recruitment (e.g. conveying equipoise, addressing patient preferences). 26–28,30,33 The evidence that informed the above workshops was also presented to each site during site initiation visits (SIVs), summarised in a brief tips document circulated to all internal pilot sites and used to identify aspects of patient- and recruiter-facing study documentation (e.g. information leaflets, consent forms and trial protocol) that were potentially unclear, imbalanced or open to misinterpretation.
Understanding recruitment issues
We employed a range of methods, primarily qualitative, but also drawing on descriptive quantitative data obtained from the SEAR logs, to understand the recruitment processes in the VIOLET trial and to identify the challenges to optimal recruitment.
Sampling and recruitment
Our sampling frame consisted of the VIOLET trial sites (i.e. five sites in the internal pilot Phase I and four sites added in Phase II), all staff involved in recruitment at these sites and TMG members.
Sites
All sites in the VIOLET trial were approached for participation in the integrated QRI at the time of the SIV or subsequently. QRI researchers liaised with the PIs or RNs at the sites to explain the QRI purpose and methods.
In-depth interviews
We employed a combination of sampling strategies to ensure that a wide range of views were gathered. We purposefully selected and approached staff members who were involved in trial oversight, including TMG members, as well as clinical/research staff in different roles (e.g. surgeons, RNs) who were involved with recruitment, to ensure maximum variation in the views captured. We also selected participants who were likely to provide insights into recruitment challenges identified in previous interviewees or other sources of data (e.g. theoretical sampling). Participants were initially contacted via e-mail, with a follow-up reminder when necessary.
Audio-recordings of recruitment discussions
All sites were requested to routinely audio-record the discussions that recruitment staff had with patients regarding treatment options and the trial until a decision was made regarding trial participation. The sites were provided with digital audio-recording equipment and ‘recruiter packs’, which outlined the process of obtaining consent for the QRI and provided instructions on how to operate the audio-recorders and to name and upload the audio files in a safe and confidential manner. Prior to each feedback session, audio-recordings were sampled using strategies similar to those employed to select interviewees described above (i.e. purposive, maximum variation and theoretical sampling). We purposively selected recordings of randomised and declined patients, ensuring that they featured different recruiters and spanned across centres, with further selections being made based on the themes identified in previous feedback sessions.
Data collection
In-depth interviews
Staff members who agreed to participate were sent information sheets and their written consent was obtained prior to the interview. In-depth semistructured interviews were conducted at a mutually convenient time and place (face to face or via telephone) and were digitally audio-recorded. Topic guides drew from those used in previous QRIs and helped ensure consistency across interviews, but these were used flexibly to allow the exploration of issues of importance to participants. Topics covered in interviews included the development, purpose and design of the trial; potential participants’ pathway through eligibility and recruitment; views on equipoise in relation to the VIOLET trial; and how the trial and the interventions would be discussed with patients.
Audio-recordings of recruitment discussions
Staff and patient consent were obtained prior to audio-recording of recruitment discussions. Staff members were provided with an information sheet and one-off written consent was obtained (usually by RNs), which allowed the audio-recording of their subsequent VIOLET trial recruitment discussions. The PIL for the QRI was posted to patients before their first clinical consultation with the surgeons to ensure that they had sufficient time to consider QRI participation. When patients arrived for the consultation, research teams confirmed that the patients had read and understood the information and then obtained written consent if the patient was willing to participate in the QRI. Recruitment discussions of these patients were then audio-recorded.
Patient pathway through eligibility and recruitment
All study sites were asked to maintain detailed screening logs, capturing SEAR32 information (see section Overview). Sites entered screening and recruitment data to a study database designed by the trials centre. Information on the recruitment pathways (i.e. the pathway for patients from the time they were referred for treatment to the point at which a decision was made regarding trial participation) was gathered through the in-depth interviews described above.
Observations of study meetings
QuinteT Recruitment Intervention researchers also attended regular study meetings to gain an overview of trial conduct and overarching challenges. Meetings attended included TMG meetings that took place every few months (consisting of the chief investigator and all VIOLET trial co-applicants), investigators’ meetings (similar to TMG meetings, but also attended by the key recruiting teams from participating sites) and monthly study update meetings (for key members of the TMG and research team). These meetings provided insights into the recruitment concerns of key stakeholders that warranted further exploration.
Data analysis
In-depth interviews
Audio-recorded interviews were transcribed in full and verbatim. Transcripts were imported into NVivo version 10 (QSR International, Warrington, UK) and analysed using techniques of constant comparison, drawing from grounded theory. 34 This involved repeatedly moving within and across transcripts in the light of newly identified themes. We sought to develop a holistic understanding of recruitment challenges, as well as elements of good practice, and were attentive to shared, as well as disparate, views among staff members. Data collection and analysis were iterative and continued until we achieved data saturation (i.e. when we were no longer able to identify new themes).
Audio-recordings of recruitment discussions
Audio-recordings of recruitment discussions were transcribed verbatim and in a targeted manner, focusing on discussions of the trial and the operations. We employed similar methods of constant comparison as described for the interviews above. In addition, we used targeted conversation analytical techniques35 to delineate elements of good practice among recruiters for wider dissemination, as well as aspects of the discussion that could have contributed to misunderstandings among patients, precipitated patient preferences or adversely affected recruitment in other ways.
Patient pathways through eligibility and recruitment
Drawing from the interview data, we compiled recruitment pathways for each clinical centre. This comprised noting points at which patients received study information, underwent tests, had their eligibility determined and met clinical staff in different professional roles, and the timelines across these key points in the pathway. Recruitment pathways were compared across centres to identify good practice and bottlenecks that hindered recruitment.
Screened, eligible, approached, randomised data were collated and descriptively analysed by the trial statistician, with monthly summaries provided to the QRI team for each site (to aid group feedback) and individual surgeon (to inform individual feedback). QRI researchers carried out further analysis by designing a colour-coded spreadsheet for each recruiting site to facilitate easy identification of inconsistencies or missing data, as well as site-specific patterns in recruitment flow. These inconsistencies and patterns were discussed with the chief investigator/TMG during study update meetings. Data queries were often resolved by contacting site research teams or by alerting the trial manager. Patterns, such as lack of screening activity, patients not being approached or large numbers of patients declining to take part in the RCT, usually triggered further data collection and agreement on a plan of action to help sites address unhelpful patterns of recruitment.
The findings from the above data sets were brought together and detailed in a descriptive account that drew from all data sources to identify key challenges to recruitment, with brief update reports written throughout the recruitment period of the trial.
Plan of action: strategies to optimise recruitment and informed consent
Initial anonymised QRI findings that identified factors appearing to hinder recruitment were presented to the chief investigator/TMG (in February 2016, 8 months after recruitment to the VIOLET trial had commenced) so that they could agree on a plan of action to address factors impeding recruitment. This plan was implemented through the first set of group and individual feedback sessions covering all the internal pilot phase sites (May–June 2016), with the aim of optimising recruitment and informed consent. As recruitment progressed and the trial moved into the main phase, there was further QRI data collection, analysis and presentation of findings at TMG and investigator meetings and at VIOLET trial sites, which aimed to sustain the recruitment momentum gained early in the trial. The key findings were also summarised in succinct ‘tips’ documents circulated to all centres, especially during periods of low recruitment activity. Towards the end of the recruitment period, the QRI team, in collaboration with the chief investigator/TMG, developed a rapid communication strategy, which aimed to reiterate the most important QRI findings and strategies and disseminate them through monthly newsletters developed by the trials centre and e-mail communications with infographics on recruitment figures.
Evaluating the impact of the QRI
We evaluated the impact of the QRI in two ways through (1) monthly monitoring of recruitment figures (i.e. SEAR data) and (2) recruitment practice. First, SEAR data were monitored from the onset of recruitment until the achievement of the final recruitment target to check if recruitment commenced well following the training/guidance provided prior to recruitment, if any recruitment momentum gained was sustained thereafter and if there were periods of low recruitment activity that improved after rapid intervention. Second, we monitored recruitment practice by listening to the audio-recordings (and interviews where relevant) to document changes in practice following the provision of trial- and site-specific feedback and instances where recruitment challenges appeared to have been averted following the training/guidance prior to recruitment. A conventional pre- and post-intervention evaluation was not feasible in the VIOLET trial QRI, as it did not have a precise pre-QRI period of recruitment because of the preventative activities undertaken in advance of recruitment.
Statistical methods
All analyses were directed by a prespecified statistical analysis plan (SAP), which was finalised before the database was locked for analysis. The data are reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines. 36
Summary statistics and analysis population
Data were described using summary statistics, using mean and SD for continuous variables [or median and interquartile range (IQR) if distributions were skewed] and number and percentage for categorical variables. HRQoL questionnaires were scored in accordance with the developer’s scoring instructions and summary scales derived from the questionnaires are reported in summary tables.
Participants were grouped according to the randomised allocation (intention to treat). The analysis population consisted of all randomised participants, excluding those who withdrew and were unwilling for data already collected to be used. Data from any participant who withdrew and was unwilling for their data to be used were included in the study flow chart, but not in any subsequent data tables or figures.
Models used to compare primary and secondary outcomes
The models used to compare longitudinal HRQoL outcomes, including the primary outcome, are presented in Table 2. The adequacy of a model fit was assessed graphically.
| Outcome | Model | Time adjustment | Survival adjustment | Effect(s) reported |
|---|---|---|---|---|
|
QLQ-C30: physical functioning, global health status/quality of life, role functioning, social functioning and dyspnoea scores QLQ-LC13: dyspnoea, cough, pain in chest and pain in other parts scores |
Joint longitudinal survival model | Fixed or random, depending on model fit assessed using likelihood ratio tests | Survival time modelled jointly with HRQoL score | MD |
| QLQ-C30: fatigue, pain and insomnia scores | Linear mixed-effects model | Fixed: different variance/covariance structures assessed using likelihood ratio tests | None | MD |
|
QLQ-C30: emotional functioning and cognitive functioning scores EQ-5D-5L |
Two-part model, with logit first part and log-linear second part | Fixed |
No adjustment in emotional functioning model EQ-5D-5L score of 0 imputed after death |
OR and GMR |
|
QLQ-C30: appetite loss, diarrhoea and financial difficulties scores QLQ-LC13: sore mouth, dysphagia, peripheral neuropathy, alopecia and pain in shoulder or arm scores |
Ordinal logistic regression | Fixed | None | OR |
|
QLQ-C30: nausea and vomiting, and constipation scores QLQ-LC13: haemoptysis score |
Logistic regression | Fixed | None | OR |
The strategy for modelling longitudinal HRQoL outcomes, listed in order of preference, is as follows:
-
Joint longitudinal survival model.
-
Linear mixed-effects model (chosen if the joint longitudinal survival model did not provide an adequate fit to the data).
-
Mixed-effects ordinal logistic regression (chosen if the HRQoL score could take only four possible values, the proportional odds assumptions held and the previous models were not appropriate) or partial proportional odds model (chosen if proportional odds assumption did not hold for all variables to be included in the model).
-
Mixed-effects logistic regression (chosen if the ordinal logistic regression model did not converge or the proportional odds assumption did not hold for time or treatment variables).
If the distribution of the HRQoL score was non-monotonic, with a high proportion of participants scoring perfect functioning/health, a two-part model was used. Scores were dichotomised into perfect functioning/health (i.e. a QLQ-C30 score of 100 and an EQ-5D-5L utility score of 1) and less than perfect functioning/health. The first part of the model was an occurrence model, that is a mixed-effects logistic regression model comparing perfect functioning/health with less than perfect functioning/health. The second part was an intensity model, that is a log-linear mixed-effects model for the score, conditional on a less than perfect functioning/health score.
Time-by-treatment interactions were added to all longitudinal models. Overall treatment effects are presented unless the interaction reached 10% statistical significance, in which case treatment effects for each time point are reported. For the primary outcome, the treatment effect at 5 weeks is reported, estimated from the longitudinal model with a time-by-treatment interaction included.
Time-to-event outcomes were compared using Cox proportional hazards models and treatment estimates are presented as hazard ratios (HRs). Time to uptake of adjuvant treatment was analysed using competing-risks regression, with death modelled as a competing risk. Overall survival, progression-free survival (with progression defined as progression of lung cancer or new primary lung cancer or death from any cause, whichever occurred first) and uptake of adjuvant treatment were censored at last follow-up for those who had not experienced the event (or competing risk). For duration of hospital stay, in-hospital deaths were censored at the maximum observed time to discharge for survivors. The exact partial-likelihood method was used to account for tied times. Model assumptions were assessed graphically.
In-hospital pain scores were analysed using a linear mixed-effects model. Binary outcomes [i.e. complete R(0) resection and prolonged incision pain] were analysed using generalised linear models and multinomial outcomes (i.e. upstaging to pathologic node stage 1 (pN1) disease and upstaging to pN2 disease) using generalised structural equation models. Effect estimates are presented as relative risk (RR).
The mean daily dose of each analgesic, averaged over the hospital stay, was derived for each participant. Analgesic agents were combined into groups, where appropriate. The mean ratios were derived for each analgesic group and 95% confidence intervals (CIs) were estimated using bootstrapping.
Open surgery was the reference group in all analyses. Treatment estimates are reported with 95% CIs.
Adjustment in models
The plan was to adjust all models for centre and operating surgeon fitted as random effects (or as stratification variables in time-to-event outcomes). For binary outcomes, a clustered sandwich estimator was used to account for the clustering within surgeon, as random effects were not estimable. All longitudinal analyses were adjusted for baseline preoperative score as a fixed effect.
Subgroup analysis
A prespecified subgroup analysis, comparing in-hospital pain scores by type of analgesia (e.g. paravertebral block, intercostal block, both, neither) was performed. This was implemented by adding a treatment-by-analgesia interaction term into the model, comparing pain scores between groups.
Sensitivity analyses
A sensitivity analysis of the primary outcome, excluding participants with benign disease, was prespecified in the protocol. Sensitivity analyses of overall survival and progression-free survival were also performed, adjusting the model for participant’s disease stage based on pathological findings. These analyses were not in the protocol, but were added to the SAP on recommendation from the DMSC.
Exploratory analyses
Two exploratory analyses of pain scores were undertaken. The first was stated in the protocol and the second was requested by the DMSC:
-
An exploratory analysis comparing in-hospital pain scores by number of incisions (i.e. VATS with a single port site, VATS with multiple port sites and open surgery).
-
An exploratory analysis comparing in-hospital pain scores and QLQ-C30 pain scores by type of thoracotomy (i.e. anterior thoracotomy vs. posterolateral thoracotomy, muscle sparing vs. no muscle sparing, and rib resection vs. no rib resection).
An exploratory analysis comparing length of stay by incisions (i.e. single-port VATS vs. multiport VATS vs. open surgery) was not prespecified in the protocol, but was requested by the chief investigator before any comparative analyses had been undertaken.
Missing data
Missing data are described in footnotes to all tables. Rules for imputing missing data outlined in the SAP were dependent on the level of missing data. All HRQoL analyses and the in-hospital visual analogue scale (VAS) pain score analysis met the threshold for multiple imputation. For other outcomes, participants with missing data were excluded. For HRQoL analyses, each subscale was imputed separately. Imputation by chained equations was used to generate multiple complete data sets [using the Stata® version 16.1 (StataCorp LP, College Station, TX, USA) -ice- command] and results were combined using Rubin’s rules. Factors included in the imputation models used were baseline HRQoL score, treatment allocation, centre and operating surgeon.
Significance levels and adjustment for multiplicity
For hypothesis tests, two-tailed p-values of < 0.05 were considered statistically significant. Likelihood ratio tests were used in preference to Wald tests. For HRQoL outcomes derived from the QLQ-C30 and QLQ-LC13 questionnaires, significance levels were adjusted for multiplicity using the false discovery rate method proposed by Benjamini and Hochberg. 37 The adjustment was applied within each instrument (e.g. for QLQ-C30 functional scale scores, QLQ-C30 symptom scale scores and QLQ-LC13 scores). No formal adjustment for multiplicity was made for other outcomes. Formal statistical comparisons were not made for outcomes with low-event rates and only prespecified subgroup analyses were performed. The number of statistical tests performed should be considered when interpreting results.
All statistical analyses were performed with the use of Stata software.
Economic evaluation
Economic evaluation aim
The economic evaluation aimed to estimate the incremental cost-effectiveness of VATS lobectomy compared with open lobectomy for the treatment of lung cancer, in line with the VIOLET trial.
Economic evaluation overview
The perspective of the evaluation was that of the UK NHS and Personal Social Services, as recommended by the National Institute for Health and Care Excellence (NICE). 38 The perspective for outcomes comprised the patients undergoing treatment. The primary outcome measure for the cost-effectiveness analysis was quality-adjusted life-years (QALYs), estimated using the EQ-5D-5L. 39,40 Established guidelines on the conduct of economic evaluations set out by NICE were followed. 38 Table 3 summarises the key aspects of the economic evaluation, and further details are provided below.
| Aspect of methodology | Strategy used in base-case analysis |
|---|---|
| Form of economic evaluation | Cost-effectiveness analysis for comparison between VATS lobectomy and open surgery |
| Perspective | NHS and Personal Social Services |
| Time horizon | A within-trial analysis, taking a 1-year time horizon |
| Data set | All randomised participants were included (see Patient eligibility criteria for eligibility criteria) |
| Costs included in analysis | Index admission:
|
| Utility measurement | EQ-5D-5L administered at baseline (pre randomisation), 2 weeks, 5 weeks, 3 months, 6 months and 1 year post randomisation |
| QALY calculations | Assume that participants’ utility changes linearly between utility measurementsa |
| Adjustment for baseline utility | Regression used to adjust QALY calculations for differences in baseline utility |
| Missing data | Multiple imputation |
Form of analysis, primary outcome and cost-effectiveness decision rules
As advocated by NICE, a cost-effectiveness analysis (specifically a cost–utility analysis) was conducted, using QALYs as the primary outcome measure. 38 QALYs combine both quantity and quality of life into a single measure. Incremental costs (i.e. the difference in mean costs between the VATS and open lobectomy groups) were divided by incremental QALYs (i.e. the difference in mean QALYs between the groups) and presented as the incremental cost-effectiveness ratio (ICER), which quantifies the incremental cost per QALY gained by switching from using open surgery to VATS lobectomy. The economic evaluation analyses were performed on an intention-to-treat basis.
Video-assisted thoracoscopic surgery lobectomy was considered cost-effective if the ICER fell below £20,000, which is generally considered as the threshold that NICE adopts for considering an intervention to be cost-effective. 41
Time horizon
A within-trial analysis, taking a 1-year time horizon, was conducted. It was anticipated that all major resource use (i.e. surgery, complications relating to surgery and adjuvant therapy) would occur within this time frame and would, therefore, be captured.
The starting point for our analysis was from the point of surgery, rather than the point of randomisation, as was the case with the trial effectiveness analysis. Randomisation was performed within 1 week of the planned operation date. The time point for baseline costs and outcomes was not quite the same. The EQ-5D-5L data were collected preoperatively, whereas detailed resource use data collection began on the day of surgery. However, as no difference in resource use was expected between the groups until the time of surgery, we did not anticipate this being an issue. Our time horizon continued until 1-year postoperatively.
Collection of resource use and costs
Resource use data were collected on all significant health service resource inputs for the trial participants to the end of the 1-year follow-up period. Collection of detailed resource use data was integrated into the trial case report forms (CRFs) for the index admission, and captured from telephone calls with participants at 5 weeks, 3 months, 6 months and 1-year post randomisation. The main resource use categories that were captured and costed were initial thoracic surgery, hospital stay post surgery (by ward type), complications, adjuvant therapy, imaging, recurrence/progression of cancer, hospital readmissions, outpatient and emergency department (ED) attendances, and community health and social care contacts. Appendix 2, Table 25, details the sources of unit cost information for each of these categories. Costing decisions (e.g. resource use assumed for complications) were made without knowledge of the allocation of participants to trial groups.
Thoracic surgery
The key differences in resources required for VATS lobectomy and open surgery are the time in theatre and the number of staples required. These were captured on the trial CRFs and were used to cost the index surgery. Pathology costs associated with a biopsy and frozen section analysis are also included.
Treatment complications and serious adverse events
Trial CRFs captured postoperative complications that participants experienced, including pulmonary, cardiac, renal, gastrointestinal, infective and neurological complications, and the need for reoperations. We discussed with the research team the likely resource implications of complications that were not already being captured. Trial CRFs also captured resource use around SAEs. SAEs were individually reviewed and additional resources were costed, only if not already captured in complication costs to avoid double counting. Appendix 3, Tables 29–31 and 33–35, show all the complications, the corresponding diagnostic tests and treatments assumed and their unit costs.
Hospital readmissions and other post-discharge primary and secondary health and social care visits
The costs of hospital readmissions included all expected and unexpected thoracic surgery and chemotherapy/radiotherapy complications in terms of AEs and SAEs, but excluded all unexpected unrelated complications. For example, our analysis included the cost of readmissions for wound pain, but excluded the cost of readmissions for ophthalmology. Clinical opinion was sought to clarify whether unexpected complications were possibly related or were unrelated to the index surgery. Treatment relating to known pre-existing conditions (i.e. conditions known prior to randomisation) were excluded unless lung related. Similarly, resource use associated with lung cancer progression or recurrence was included, but resource use related to new non-lung cancer and totally unrelated cancer (e.g. prostate cancer) was excluded. The cost of an ED attendance was included if a participant was admitted via ED or referred by their general practitioner (GP) (and assumed to be admitted via ED).
We reviewed the reasons for outpatient attendance and discussed with the trial research team whether or not these were likely to be linked to the surgery to avoid costing any outpatient visits that were totally unlinked to the trial. Similarly, the reasons for ED visits and community health and social care contacts were reviewed, and any unrelated activity excluded.
Attaching unit costs to resource use
Unit costs for hospital and community health-care resource use were largely obtained from national sources, for example the National Schedule of Reference Costs42,43 for ward costs, scans and many complications, and Unit Costs of Health and Social Care44 for community costs. Resources were valued in 2018/19 GBP and any unit costs not in 2018/19 prices have been adjusted to 2018/19 prices using the NHS cost inflation index. 45 Where available, costs of drugs given in hospital were taken from the electronic marketing information tool (eMIT),46 which provides the reduced prices paid for generic drugs in hospital. Otherwise, costs were obtained from the British National Formulary. 47 For a summary of the sources of unit cost information, see Appendix 2, Table 25. For further details on all unit costs and their source, see Appendix 3, Tables 28–39.
Measurement of health-related quality of life and quality-adjusted life-years
Measurement of health-related quality of life
The EQ-5D-5L questionnaire, advocated for use in economic evaluations by NICE,38 was used to measure HRQoL. 39,40 The EQ-5D is a generic measure of health outcome, covering five dimensions: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort and (5) anxiety/depression. The EQ-5D-5L was completed by participants at six time points: (1) baseline (pre-randomisation), (2) 2 weeks, (3) 5 weeks, (4) 3 months, (5) 6 months and (6) 1-year post randomisation. Although data were gathered using the EQ-5D-5L (the five-level version, with five possible responses for each dimension), responses recorded on the instrument were converted into a single index value using the original three-level UK valuation set. 48 Scores were then used to facilitate the calculation of QALYs. Utility values were calculated by mapping the five-level descriptive system to the three-level valuation set using the crosswalk developed by van Hout et al. 49 in accordance with NICE recommendations at the time of analysis. 49,50
Calculation of quality-adjusted life-years
The QALY profile for each participant was estimated from surgery to 1 year, and the area under the curve of utility measurements was used to calculate the number of QALYs accrued by each participant. QALYs were calculated assuming that each participant’s utility changes linearly between each of the time points [i.e. that utility changes at a constant rate (in a straight line) between measurements]. For participants who died during the trial, their utility was assumed to change linearly between the preceding time point and the time of death, and to take the value of zero from death onwards.
Missing data
We first summarised the number of missing data for resource use and outcomes (EQ-5D scores) descriptively. Exploratory analyses were conducted to explore the possible mechanisms and patterns of missing data. 51 Logistic regressions were used to explore associations between missingness and baseline variables, and missingness and previously observed outcomes. If the number of missing data was small (< 1% of cases), then unconditional or conditional mean imputation was considered to be sufficient. However, we anticipated that it would be necessary to use multiple imputation to impute missing values. Multiple imputation is a flexible approach, which is valid if data are assumed to be missing at random (i.e. the probability that data are missing does not depend on the unobserved values; it is conditional on the observed data). 51,52 This assumption was assessed.
Multiple imputation uses regression to predict m values for each missing data cell, and enables all key variables used in the economic evaluation and demographic data (i.e. both complete and incomplete) to be used to predict the values of missing data cells. In accordance with guidelines,51,53 multiple imputation using chained equations was conducted and the number of imputations set to be at least equal to the percentage of incomplete cases. 53 Multiple imputation was performed separately for each treatment group.
Multiple imputation can be conducted at an aggregated level of total costs, for example, or at a disaggregated level of individual resource use items or EQ-5D domains. Given that imputing large numbers of variables may make the model difficult to estimate, a balance between the two is likely to be required. The patterns of missing data for resource use/costs and outcomes were used to determine the approach to multiple imputation. For example, data collected on a patient follow-up questionnaire may have similar patterns of missing data, in which case the total costs for that follow-up can be imputed rather than individual resource use items. For each variable with missing data, individual regressions were specified and tailored to the type of data being predicted. Linear regression with prediction mean matching was used, as it is particularly flexible.
Once multiple imputation had been conducted, tabulations and summaries of the observed and imputed data were compared to check the validity of the imputations. Rubin’s rule was then used to summarise data across the m data sets. 54 This approach accounts for the variability both within and between imputed data sets and takes uncertainty in the estimated mean into account.
Adjustment for baseline utility
Given that baseline utility directly contributes to QALY calculations, it is important to control for any potential imbalances in baseline utility in the estimation of the mean difference (MD) in QALYs between treatment groups to avoid introducing bias. 55 Regression adjustment also allows for regression to the mean and increases precision. Therefore, if there is an imbalance at baseline, we planned to adjust QALYs for baseline EQ-5D.
Within-trial statistical analysis of cost-effectiveness results
Analyses were conducted in Stata version 15 and Microsoft Excel® 2016 (Microsoft Corporation, Redmond, WA, USA).
Initially, descriptive summaries of resource use, costs and HRQoL were performed using means, SDs and standard errors around the means using the central limit theorem. Cost data are typically positively skewed; however, regardless of this, costs were summarised using the arithmetic mean, as it is this combined with the total number of patients that relates to the total budget impact of an intervention.
The ICER was derived from the average costs and QALYs gained in each trial group, producing an incremental cost per QALY gained of VATS lobectomy compared with open surgery. Non-parametric bootstrapping of costs and QALYs was used to quantify the degree of uncertainty around the ICER. Results are expressed in terms of a cost-effectiveness acceptability curve (CEAC), which indicates the likelihood that VATS lobectomy is cost-effective for different levels of willingness to pay for health gain. Although VATS lobectomy is considered cost-effective if the ICER falls below £20,000, the ICERs and CEACs presented allow decision-makers to assess cost-effectiveness at a willingness-to-pay threshold of their choice.
Discounting
Costs and effects were not discounted, as our time horizon was 12 months.
Sensitivity analysis
Univariate sensitivity analyses were used to investigate the impact on costs and cost-effectiveness results of variation in key parameters and major cost drivers, and to investigate the impact of uncertainty on the cost-effectiveness results.
Factors examined in the sensitivity analyses for costing were varying the unit costs for key cost drivers, including surgery and ward stays. The impact of any high-cost participants was also investigated. For outcomes, we examined not adjusting for baseline utility and the impact of any missing survival status.
For details of all sensitivity analyses, see Appendix 4.
Subgroup analysis
No subgroup analyses were pre-planned for the cost-effectiveness analyses; comparing pain scores by type of analgesia, as per the clinical analyses, would not be meaningful here.
Chapter 3 Results: trial cohort
Study sites
Five sites took part in Phase I of the trial and a further four were opened in Phase II. Sites were well spread geographically and represented a mix of university and NHS trusts that are representative of NHS practice. The study sites and the dates they opened to recruitment are given below.
Phase I: study sites and dates opened to recruitment
-
Royal Brompton Hospital and Royal Brompton and Harefield NHS Foundation Trust (29 July 2015).
-
Liverpool Heart and Chest Hospital NHS Foundation Trust (6 October 2015).
-
Bristol Royal Infirmary and University Hospitals Bristol NHS Foundation Trust (14 October 2015).
-
The James Cook University Hospital and South Tees Hospitals NHS Foundation Trust (29 October 2015).
-
Harefield Hospital and Royal Brompton and Harefield NHS Foundation Trust (18 December 2015).
Phase II: study sites and dates opened to recruitment
-
John Radcliffe Hospital and Oxford University Hospitals NHS Foundation Trust (25 September 2017).
-
Castle Hill Hospital and Hull University Teaching Hospitals NHS Trust (12 October 2017).
-
Birmingham Heartlands Hospital and University Hospitals Birmingham NHS Foundation Trust (28 September 2017).
-
Royal Infirmary of Edinburgh, NHS Lothian (20 September 2018).
Patients screened and recruited
Between 23 July 2015 and 14 February 2019, a total of 2109 patients were assessed for eligibility, of whom 1606 were excluded (1110 patients were ineligible, 147 patients were not approached by the local team, 315 patients were approached but declined to take part and 34 patients agreed to take part but then withdrew their consent prior to randomisation). Therefore, 503 patients (i.e. 50% of eligible patients and 59% of patients approached) were recruited and randomised. The main reasons for screened patients not being recruited, by study site, are shown in Appendix 5, Table 44. Participant flow through the trial is shown in Figure 2.
FIGURE 2.
The VIOLET trial CONSORT flow diagram. a, Reasons for exclusion are provided in Appendix 5, Table 44. Adapted with permission from NEJM Evidence, Lim E, Batchelor TJP, Dunning J, Shackcloth M, Anikin V, Naidu B, et al. , Video-assisted thorascopic or open lobectomy in early-stage lung cancer, Volume 1, Copyright © 2022 Massachusetts Medical Society. 56
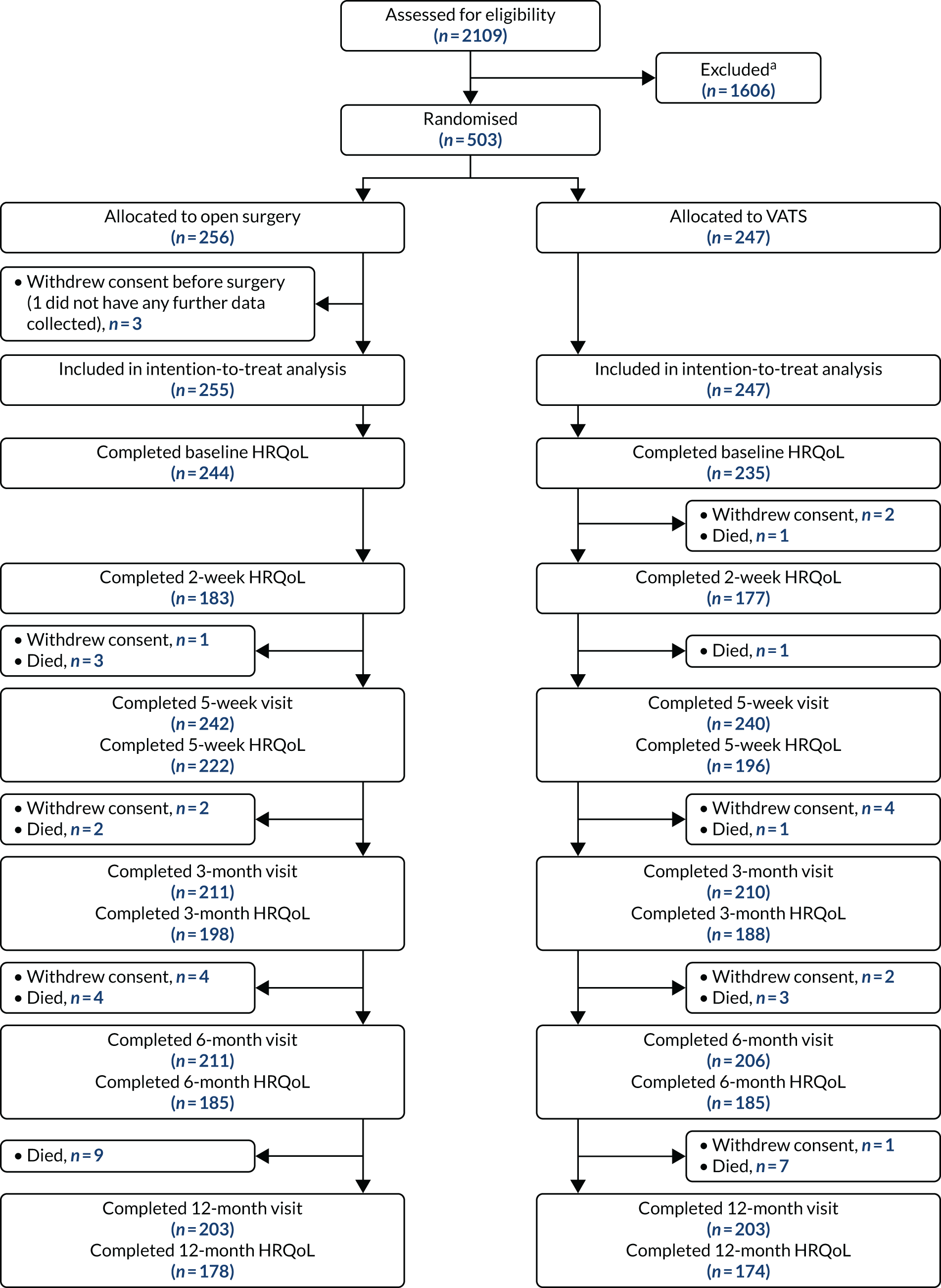
Recruitment
Between 30 July 2015 and 26 February 2019, 503 participants consented to take part and were randomised (VATS group, n = 247; open-surgery group, n = 256). One participant withdrew after randomisation and before surgery and no further data were collected (see Figure 2). The final follow-up for the last participant was completed on 23 March 2020.
Recruitment rate
When the study was designed, the estimated recruitment rate was expressed in terms of the proportion of eligible patients recruited, rather than as a recruitment per site per month. The proposed study sites were asked to estimate the number of lobectomies performed for early-stage lung cancer each year and from this the anticipated recruitment rate was derived, allowing for a staggered opening of study sites. It was estimated that 60% of patients would be eligible for the trial and that the participating surgical teams would initially recruit 30% of eligible patients, but that with training and feedback provided by the QRI team this might increase to 50% after 6 months. The anticipated recruitment rate for each of the participating centres is documented in Appendix 5, Table 45.
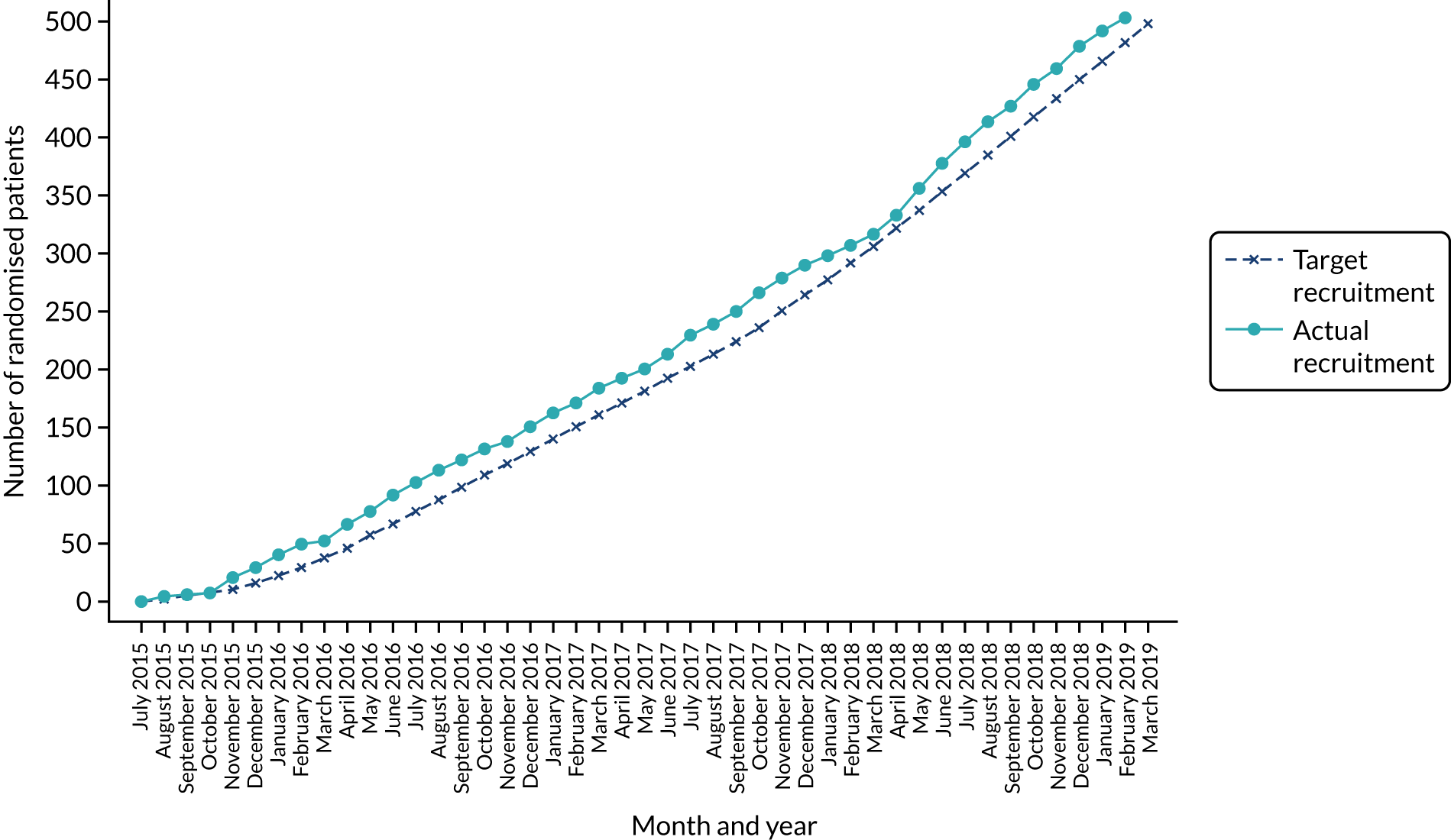
The actual recruitment rate is illustrated in Figure 3. The trial was assessed for progression 18 months after the start of recruitment. Recruitment was ahead of target at that time and remained so throughout the trial, completing 2 months ahead of schedule. The trial over-recruited by five participants. The number of patients recruited by site and the rate per month at each site is given in Appendix 5, Table 46.
FIGURE 3.
Predicted and actual recruitment.
Progression from Phase I to Phase II
Progress against the predefined progression criteria was assessed in December 2016. Performance against the prespecified criteria is shown in Appendix 5, Table 47. The Trial Steering Committee (TSC) recommended progression to Phase II. Following review of the screening data and reasons for ineligibility, the eligibility criteria were widened to include bi-lobectomies to increase the generalisability of the trial results.
Comparison of recruited and non-recruited patients
The age of trial participants was similar to those patients who were screened but did not join the trial because they were ineligible, not approached or did not wish to take part (see Appendix 5, Table 48).
Patient withdrawals
In total, 34 participants withdrew consent prior to randomisation. Nineteen participants withdrew after randomisation (before surgery, n = 3; after surgery, n = 16). The reasons for post-randomisation withdrawal are detailed in Table 4. The most cited reason was that the participant ‘changed their mind’ about trial participation.
| Withdrawal detail | Participant allocation | Overall (N = 503), n/N (%) | |
|---|---|---|---|
| Randomised to VATS (N = 247), n/N (%) | Randomised to open surgery (N = 256), n/N (%) | ||
| Any withdrawal | 9/247 (3.6) | 10/256 (3.9) | 19/503 (3.8) |
| Timing of withdrawal | |||
| Before surgerya | 0/9 (0.0) | 3/10 (30.0) | 3/19 (15.8) |
| After surgery | 9/9 (100.0) | 7/10 (70.0) | 16/19 (84.2) |
| Reason for withdrawal | |||
| Clinician’s advice | 1/9 (11.1) | 2/10 (20.0) | 3/19 (15.8) |
| Surgery no longer appropriate | 0/1 (0.0) | 1/2 (50.0) | 1/3 (33.3) |
| Patient no longer eligible | 1/1 (100.0) | 1/2 (50.0) | 2/3 (66.7) |
| Patient’s decision | 8/9 (88.9) | 8/10 (80.0) | 16/19 (84.2) |
| Patient changed their mind about the study | 6/8 (75.0) | 2/8 (25.0) | 8/16 (50.0) |
| Patient does not want to continue with follow-up | 2/8 (25.0) | 3/8 (37.5) | 5/16 (31.3) |
| Refused to give reason | 0/8 (0.0) | 1/8 (12.5) | 1/16 (6.3) |
| Other | 0/8 (0.0) | 2/8 (25.0) | 2/16 (12.5) |
| Further details | |||
| Withdrawn from follow-up | 9/9 (100.0) | 10/10 (100.0) | 19/19 (100.0) |
Protocol deviations
Eligibility and surgery
Overall, the number of protocol deviations were low at 13% (66/502) (Table 5). Forty-nine patients did not undergo a lobectomy (31 patients were found to have benign disease on frozen section, 11 patients underwent a wedge resection, three patients underwent a segmentectomy, two patients were found to have extensive malignancy and so no resection was performed, and two patients underwent a pneumonectomy). A further 17 participants who underwent a lobectomy received the other trial intervention to that they were allocated [15 participants randomised to VATS received open surgery (one participant decided preoperatively to have open surgery and the remaining 14 participants were intraoperative conversions necessitated by the surgeon) and two participants randomised to open surgery had a VATS procedure (both participants decided preoperatively to have VATS)]. Reasons for conversions from VATS to open surgery can be found in Table 6.
| Protocol deviation | Participant allocation | Overall (N = 502), n/N (%) | |
|---|---|---|---|
| Randomised to VATS (N = 247), n/N (%) | Randomised to open surgery (N = 255), n/N (%) | ||
| Protocol deviation | 41/247 (16.6) | 25/255 (9.8) | 66/502 (13.1) |
| Patient ineligible but treated in the study | 0/247 (0.0) | 0/255 (0.0) | 0/502 (0.0) |
| Patient did not undergo lobectomy | 26/247 (10.5) | 23/255 (9.0) | 49/502 (9.8) |
| Patient received the other trial intervention to that they were allocateda | 15/221 (6.8) | 2/232 (0.9) | 17/453 (3.8) |
| Reason for conversion | Total (N = 14), n (%) |
|---|---|
| Diffuse pleural adhesion | 4 (28.6) |
| Bleeding from vascular injury | 3 (21.4) |
| Poor visualisation | 1 (7.1) |
| Calcified periarterial nodes | 1 (7.1) |
| Absent or thick fissure | 1 (7.1) |
| Margin extension | 1 (7.1) |
| Invasion of the artery | 1 (7.1) |
| Bleeding from vascular injury and poor visualisation | 1 (7.1) |
| Invasion of the artery and discovery of N2 tumours | 1 (7.1) |
Adherence to the mandated and prohibited aspects of the surgical procedure (i.e. number of ports used for a VATS procedure and use of rib spreading) and to the criteria for fitness for discharge are presented in Baseline data and operative characteristics and Chapter 5, Exploratory analysis: length of stay, respectively.
Success of blinding
Bang et al. ’s Blinding Index13 asks for individuals to guess which treatment (i.e. method of surgical access) the participant received. Results of the assessment of blinding of participants and RNs responsible for outcome data collection are presented in Table 7. At day 2, 159 of 442 (36.0%) participants correctly identified the surgery they had received. At discharge, 202 of 417 (48.4%) participants correctly identified the surgery they had received. A greater proportion of RNs correctly identified the surgical approach at discharge (275/440, 62.5%). This had reduced by 12 months (208/414, 50.2%).
| Blinding assessment | Participant allocation | Total (N = 502), n/N (%) | |
|---|---|---|---|
| Randomised to VATS (N = 247), n/N (%) | Randomised to open surgery (N = 255), n/N (%) | ||
| Participant | |||
| Guessed correctly: 2 days post surgery | 87/220 (39.5) | 72/222 (32.4) | 159/442 (36.0) |
| Guessed correctly: discharge | 107/209 (51.2) | 95/208 (45.7) | 202/417 (48.4) |
| RN | |||
| Guessed correctly: discharge | 145/220 (65.9) | 130/220 (59.1) | 275/440 (62.5) |
| Guessed correctly: 5 weeks | 150/236 (63.6) | 142/239 (59.4) | 292/475 (61.5) |
| Guessed correctly: 12 months | 118/206 (57.3) | 90/208 (43.3) | 208/414 (50.2) |
Patient follow-up
The number of patients for whom follow-up data were available is presented in Figure 2. Follow-up data at 1 year was available for 81% of randomised participants. Of the 50 patients not followed to 1 year, 19 had withdrawn and 31 had died.
Numbers analysed
The analysis population consisted of 502 randomised participants. One randomised participant withdrew consent prior to surgery, at which point data collection stopped. This patient was excluded from the analysis population. The numbers of participants included in the analyses of each outcome are presented in Table 8.
| Outcome | Number (%) of participants included in analysis |
|---|---|
| QLQ-C30 physical functioning (primary) | 502 (100)a |
| Time from surgery to hospital discharge | 502 (100) |
| In-hospital pain scores | 502 (100)b |
| Lymph node upstaging | 496 (99) |
| Resection completeness | 439 (87) |
| Overall survival | 502 (100) |
| Progression-free survival | 502 (100) |
| Uptake of adjuvant treatment | 502 (100) |
| Prolonged incision pain | 482 (96) |
| QLQ-C30 questionnaire | 502 (100)c |
| QLQ-LC13 questionnaire | 502 (100)d |
| EQ-5D questionnaire | 502 (100)e |
| Any in-hospital AE | 502 (100) |
| Any post-discharge SAE | 493 (98) |
Baseline data and operative characteristics
The baseline characteristics were similar in the two groups (Table 9 and see Appendix 5, Table 49). The mean age of participants was 69 (SD 8.8) years and 249 (49.5%) participants were men. Most participants were white (96.4%), not obese [mean body mass index of 27 (SD 5) kg/m2] and were a past or current smoker (87.3%). Most participants were cT stage 1 (67.2%) and cN stage 0 (95.6%). Almost half (48.2%) of the participants did not have a tissue-confirmed diagnosis at recruitment. Comorbidities were common, with almost half (46.3%) of participants having a history of cardiovascular disease.
| Characteristic | Participant allocation | Total (N = 502) | |
|---|---|---|---|
| Randomised to VATS (N = 247) | Randomised to open surgery (N = 255) | ||
| Participant demography | |||
| Age (years), mean (SD) | 69 (8.7) | 69 (9.0) | 69 (8.8) |
| Male, n/N (%) | 119/247 (48.2) | 130/255 (51.0) | 249/502 (49.6) |
| Clinical characteristics | |||
| Clinical stage: cT, n/N (%) | |||
| 1a | 24/247 (9.7) | 17/255 (6.7) | 41/502 (8.2) |
| 1b | 77/247 (31.2) | 86/255 (33.7) | 163/502 (32.5) |
| 1c | 64/247 (25.9) | 70/255 (27.5) | 134/502 (26.7) |
| 2a | 50/247 (20.2) | 47/255 (18.4) | 97/502 (19.3) |
| 2b | 13/247 (5.3) | 16/255 (6.3) | 29/502 (5.8) |
| 3 | 19/247 (7.7) | 19/255 (7.5) | 38/502 (7.6) |
| Clinical stage: cN, n/N (%) | |||
| 0 | 232/247 (93.9) | 238/255 (93.3) | 470/502 (93.6) |
| 1 | 15/247 (6.1) | 17/255 (6.7) | 32/502 (6.4) |
| ECOG performance status, n/N (%) | |||
| 0 | 148/244 (60.7) | 172/252 (68.3) | 320/496 (64.5) |
| 1 | 84/244 (34.4) | 75/252 (29.8) | 159/496 (32.1) |
| 2 | 10/244 (4.1) | 5/252 (2.0) | 15/496 (3.0) |
| 3 | 2/244 (0.8) | 0/252 (0.0) | 2/496 (0.4) |
| Mean predicted lung function (%), mean (SD) | |||
| FEV1a | 82 (19.8) | 82 (21.2) | 82 (20.5) |
| FVCb | 95 (17.1) | 95 (18.3) | 95 (17.7) |
| TLcoc | 76 (26.3) | 72 (20.4) | 74 (23.5) |
| Histological type, n/N (%) | |||
| Adenocarcinoma | 80/247 (32.4) | 91/255 (35.7) | 171/502 (34.1) |
| Squamous carcinoma | 33/247 (13.4) | 26/255 (10.2) | 59/502 (11.8) |
| Other NSCLC | 7/247 (2.8) | 7/255 (2.7) | 14/502 (2.8) |
| SCLC | 0/247 (0.0) | 2/255 (0.8) | 2/502 (0.4) |
| Carcinoid | 6/247 (2.4) | 8/255 (3.1) | 14/502 (2.8) |
| Histology not confirmed | 121/247 (49.0) | 121/255 (47.5) | 242/502 (48.2) |
| HRQoL | |||
| QLQ-C30 physical functioning score, median (IQR)d | 87 (73.3–100.0) | 87 (73.3–100.0) | 87 (73.3–100.0) |
| Surgical details, n/N (%) | |||
| Biopsy outcome | |||
| Benign disease on frozen section | 15/247 (6.1) | 16/255 (6.3) | 31/502 (6.2) |
| Benign disease on frozen section and lobectomy | 1/247 (0.4) | 0/255 (0.0) | 1/502 (0.2) |
| Lobectomy | |||
| Lobectomy performed | 221/247 (89.5) | 232/255 (91.0) | 453/502 (90.2) |
| Rib spreadinge | 15/221 (6.8) | 228/230 (99.1) | 243/451 (53.9) |
| VATS | |||
| VATS performed | 206/221 (93.2) | 2/232 (0.9) | 208/453 (45.9) |
| Number of VATS ports | |||
| One port | 42/206 (20.4) | 0/2 (0.0) | 42/208 (20.2) |
| Two ports | 18/206 (8.7) | 0/2 (0.0) | 18/208 (8.7) |
| Three ports | 119/206 (57.8) | 1/2 (50.0) | 120/208 (57.7) |
| Four ports | 27/206 (13.1) | 1/2 (50.0) | 28/208 (13.5) |
| Thoracotomy | |||
| Thoracotomy performed | 15/221 (6.8) | 230/232 (99.1) | 245/453 (54.1) |
| Posterolateral thoracotomy | 12/15 (80.0) | 161/230 (70.0) | 173/245 (70.6) |
| Anterior thoracotomy | 3/15 (20.0) | 69/230 (30.0) | 72/245 (29.4) |
Most operations (83.6%) were consultant led. Of the 242 participants who did not have a confirmed histological diagnosis at randomisation and so underwent a biopsy first, 32 (13.2%) were confirmed benign. Of these 32 participants, 31 did not undergo a lobectomy. The team proceeded with a lobectomy for the other case because of a suspicion of cancer. All surgeons adhered to the protocol in terms of the number of ports used in a VATS procedure (between one and four ports allowed) and the use of rib spreading, which was mandated for open surgery and prohibited with VATS.
Chapter 4 Results: QuinteT Recruitment Intervention
Overview
The number of patients randomised and the QRI activities undertaken by month are presented in Figure 4 to provide the context for the QRI findings described below.
FIGURE 4.
Recruitment to the VIOLET trial and QRI actions by month. a, Facebook, Inc., Menlo Park, CA, USA.
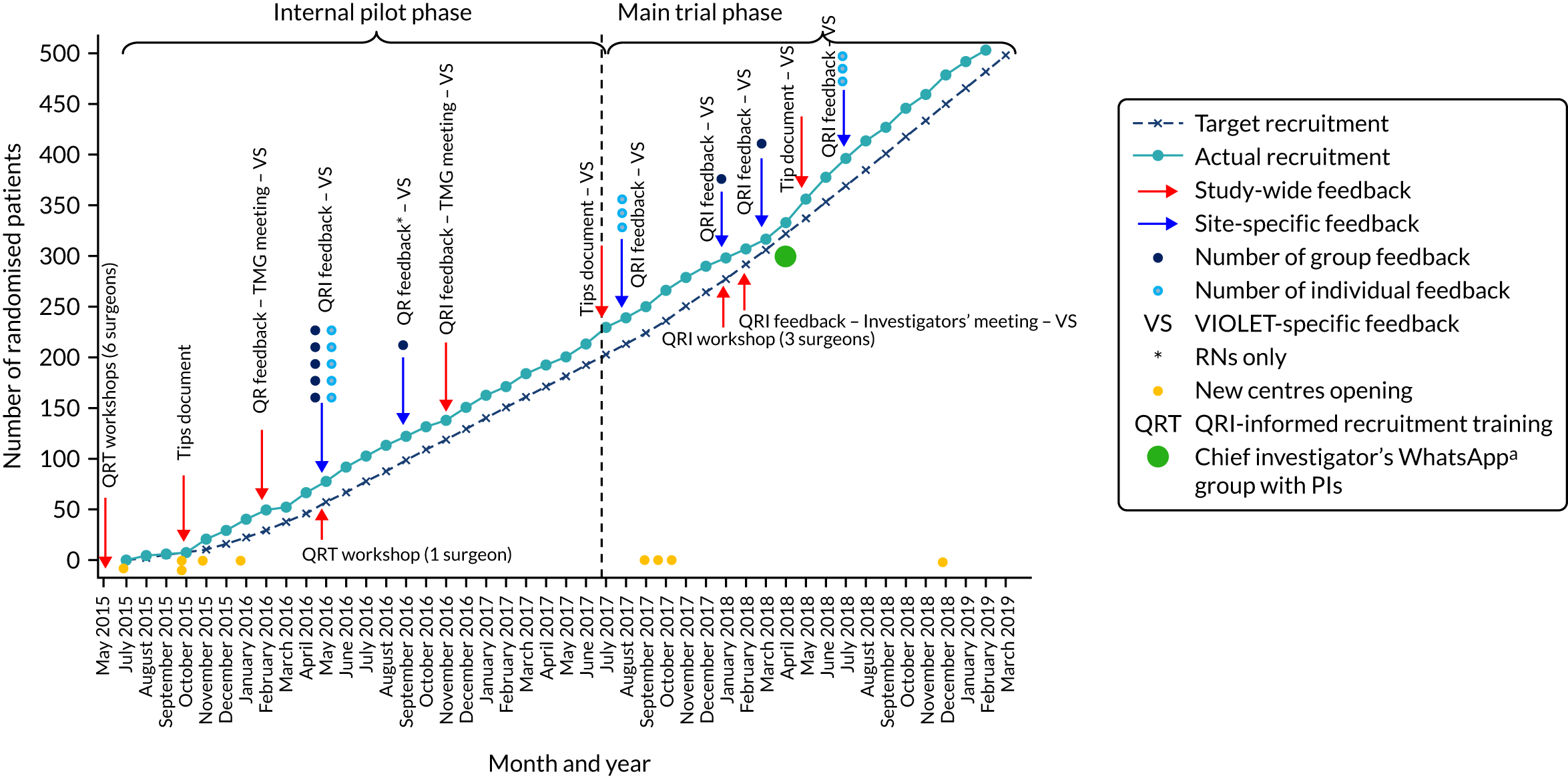
Following the initial activities aimed at averting recruitment challenges (May–October 2015), we undertook data collection/analysis to understand the VIOLET trial recruitment processes and to identify instances of good practice and opportunities to optimise recruitment (October 2015–February 2016). Next, we collaboratively developed and implemented strategies to overcome the identified recruitment challenges (March 2016–February 2019; note that the iterative model of the QRI meant that this period also involved new data collection/analysis).
Data set
Our data set comprised interview data from 15 staff (recruiting surgeons, n = 11; RNs, n = 3; TMG members, n = 1) involved in VIOLET trial oversight and recruitment. A total of 451 individual patients’ audio-recordings were available from six recruitment sites and a purposively selected sample of 304 individual patient recordings were analysed in detail.
Understanding recruitment processes and issues
We identified four overarching recruitment themes of importance in the VIOLET trial (strategies to address the recruitment issues are discussed in the next section). Elements of good practice and recruitment challenges in relation to each of these themes are presented below. The themes are (1) patient pathways through eligibility and recruitment, (2) recruiter equipoise, (3) patient preferences and (4) explaining the VIOLET trial. These key themes were not discrete or exclusive, and had overlapping threads that ran through them all. The findings are supported by anonymised quotations from interviews and audio-recordings, as appropriate (also see Appendix 6). In addition to the key recruitment themes, some findings that related to specific aspects of the trial design, such as challenges with the implementation of blinding, are summarised at the end of this section (with details further details provided in Appendix 7).
Patient pathways through eligibility and recruitment
A description of the standard patient pathway in VIOLET trial sites is presented, followed by pathway-related recruitment issues and recruiter perceptions regarding eligibility criteria in VIOLET (perceived to be clear and unambiguous, with some reservations expressed for specific groups of patients).
Patient pathways
The pathway for potentially eligible patients for the VIOLET trial was simple and consistent across centres. Recruiters felt that the VIOLET trial was easily integrated into standard clinical practice. Although there was some site variation, patients generally underwent similar processes to those illustrated in Figure 5.
FIGURE 5.
Patient pathway from referral for treatment to decision about trial participation.
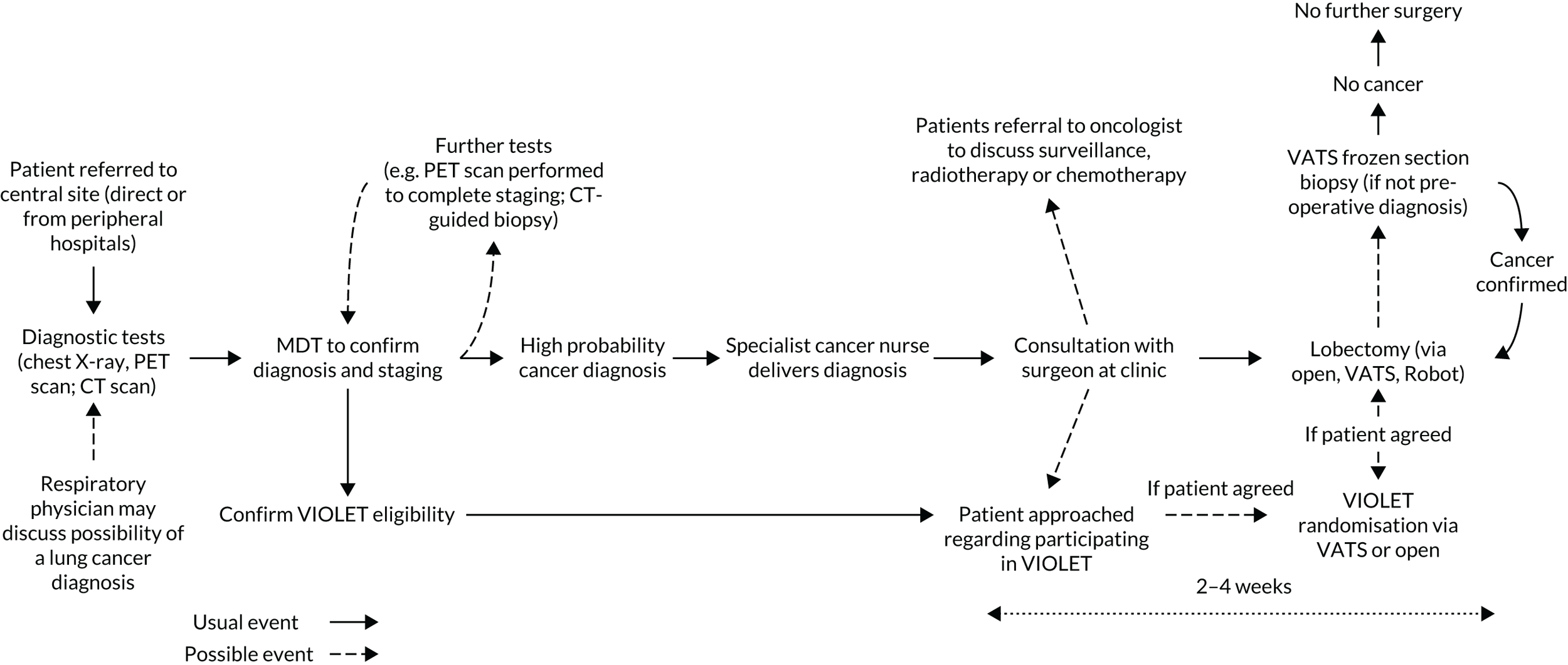
Patients referred to secondary care centres received diagnostic tests and their results were discussed in MDTs. PIs screened these patients and confirmed eligibility of potential trial participants. Approaches to invite patients to take part in research occurred during patient consultation with surgeons who would perform the surgery. Surgeons were the primary information providers and recruiters in the VIOLET trial. RNs facilitated study-related paperwork, such as written consent for audio-recording of consultations, trial baseline assessments and follow-up questionnaires.
Recommendation for VATS by staff early in the pathway
Despite the relatively simple pathway, it was quickly discovered that treatment recommendations were made by some health-care professionals early in the patient pathway (e.g. preoperative nurses, respirologists). The recommendations, reflecting routine clinical practice, were often in favour of VATS. This was evident in the consultations:
We had a problem where we obviously hadn’t given enough information to the preoperative nurses who came in and told a patient that they’re having a VATS lobectomy just because that’s what I did all the time.
Surgeon, interview
Well when I went to the [clears throat] hospital here last time and we saw the clinical nurse she said you’ll be having a keyhole and I said well we’re in a trial we don’t know which one we’re going to have. And she said, no you’ll be having keyhole.
Patient, consultation
Subsequently, two centres had informed any potential health-care professionals in the pathway about the VIOLET trial and the importance of not stating which type of procedure the patient would be undergoing:
We’ve had to make sure that they’re educated as well and everyone’s kept in the loop, so generally it now seems to be very, very good.
Surgeon, interview
However, there was some indication of reluctance to address treatment recommendations made by respirologists (i.e. chest physicians, as opposed to nurses):
There are quite a few respirologists that say ‘I believe this patient is high risk and I don’t think it’s fair for them to have a thoracotomy’, ’cause of their experience of seeing very sore thoracotomies and not seeing very many sore VATS [ . . . ]
That’s interesting. And what do you do in that circumstance?
If the respirologists want it by VATS, they’ll give it by VATS, because they are the people that send us the cases so I have to [listen] to them.
Eligibility criteria: pragmatic and straightforward, with some exceptions
Recruiters consistently described how patients had to be stage T1a–2b N0–1 M0 or be undergoing frozen section biopsy with the intention to proceed with lobectomy if T1a–2b N0–1 M0 was confirmed. Overall, there was agreement that the VIOLET trial eligibility criteria were ‘straightforward’ and ‘pragmatic’. There were, however, some differences in opinion in relation to specific groups of patients (e.g. older patients, patients with poor health/lung function and those with deeper or larger tumours) and their suitability to have VATS (screening logs showed that 29.2% of patients were ineligible because they were not suitable for both open surgery and VATS):
The higher risk are older people with worse lung function and comorbidity, in the past we would have turned down for surgery, but now we can do it by VATS, we’re sort of pushing the boat out a bit to say ‘well, OK I’m happy for you to have surgery but only just happy, I wouldn’t want to do a thoracotomy on this yet’.
Surgeon, interview
I would refer a patient with bad lungs to have VATS because of the pain issues and everything.
Surgeon, interview
Some recruiters, however, acknowledged that there was no evidence to support the view that VATS was more suitable for some groups of patients:
There is a feeling that a VATS approach was less traumatic, less stressful for a patient, so maybe you can get those older and less fit patients through a VATS lobectomy, you wouldn’t necessarily get them through an open lobectomy, when of course we don’t have any evidence for that at all . . . But we might find that as our natural prejudices come out as the trial progresses.
Surgeon, interview
Recruiters also described the location and size of the tumour to be important. For instance, some recruiters commented that if the tumour was ‘sitting too deep’, then the majority of thoracic surgeons would perform an open lobectomy. There was also a suggestion that surgeons who were less experienced with VATS may not feel comfortable to do the bigger resection through keyhole surgery. Although most surgeons commented that tumours < 7 cm were suitable for the VIOLET trial, two recruiters commented that this was < 5 cm.
Views on eligibility criteria were sometimes observed to influence whether or not patients were approached for the VIOLET trial and the way in which the trial was introduced:
I’m not 100% sure you are a perfect candidate for the study but if you’re interested, we’re here to talk about it.
Surgeon, consultation
Summary
Despite a simple patient pathway, there were some concerns around treatment recommendations for VATS made by some staff members who saw patients early in the patient pathway, with indication of sites addressing this issue prior to their first trial-specific feedback session. Similarly, eligibility criteria were generally considered pragmatic and easy to apply, but there were some groups of patients for whom eligibility was more contested, with some indication that views on eligibility criteria influenced how and whether or not the study was put forward to patients. These issues, once understood, were addressed in the QRI plan of actions (see Plan of action: strategies to optimise recruitment and informed consent).
Recruiter equipoise
In the interviews, recruiters expressed strong enthusiasm for the VIOLET trial, yet there were a number of discernible instances of recruiter biases evident in the interviews, usually in favour of VATS. In the consultations, many recruiters were adept at withholding their personal biases and carried out balanced information provision on the two operations, with an emphasis on uncertainty. There were some instances where these biases were unwittingly conveyed to patients. In addition, recruiter bias towards open lobectomy was observed in some consultations as the trial progressed (mainly from one centre). Some of these findings are detailed below.
Recruiter preference for VATS
Some surgeons questioned the need for a trial to persuade surgeons to do VATS, as ‘that ship has sailed already in this country’ (surgeon, interview). VATS was described as ‘promising’ and ‘exciting’, with the potential for patients to experience less pain and recover faster than with an open lobectomy. Several recruiters explicitly stated that they would opt for VATS if they were a patient:
If you were a patient would you be randomised to VIOLET?
No.
You wouldn’t. Why not?
I would want a keyhole operation.
Some recruiters commented that, although the oncological outcomes were likely to be similar across the two operation, patients who had VATS appeared to recover faster (which was considered particularly beneficial if adjuvant chemotherapy was needed).
Recruiter discomfort when patients were randomised to thoracotomy
Some surgeons described initial discomfort when a patient had been randomised to a thoracotomy when they would have had VATS outside the trial or noted instances where they felt their concerns regarding thoracotomy were validated when patients developed complications:
These days if it was straightforward, I would do a VATS, so [laugh], it was, yeah, [small pause], it was a bit strange doing an open.
Surgeon, interview
The patient was randomised to a thoracotomy and, uh, had complications. That wasn’t a good start.
No, I can imagine. How did that make you feel?
Mad, small . . . bad luck for the patient more than anything else. [ . . . ] Maybe it’s because of the thoracotomy, so that’s all I can say.
Acknowledgement of lack of evidence to support preference for VATS
Recruiters described how existing research that compared the two procedures comprised primarily observational studies and that the few randomised studies were of poor quality or had small sample sizes. Recruiters appeared well aware that their views in favour of VATS were not grounded in evidence and they exhibited signs of experiencing an intellectual struggle in relation to equipoise. Many recruiters had joined the VIOLET trial intuitively believing that VATS was better, but giving due consideration to the design and purpose of the trial had enabled them to take a step back and feel more comfortable with the concept of equipoise (also see Appendix 6):
There’s this implicit assumption, keyhole is just better . . . Actually, when you look critically at the world literature then we’ve got no evidence to show that any of these things are actually true, so it’s this bias, that assumption that keyhole must be better.
Surgeon, interview
It is possible that the QRI-informed recruitment training that a number of surgeons attended prior to commencing recruitment in the VIOLET trial, and prior to these interviews, played a role in their increased awareness of own biases and how they overcame these during discussions with patients, as described below.
Conveying equipoise in consultations: general patterns and concerns
Analysis of the consultations showed that recruiters were relatively skilled at conveying equipoise by communicating the uncertainty around the two operations early in the consultation, presenting them in a neutral manner, stating that there was variation across the country and explaining that both approaches were established (all of which were key aspects of the QRI-informed training sessions attended by PIs prior to recruitment):
We can use an open operation or a keyhole operation and they’re both standard approaches for lung cancer surgery. We don’t know which is better and that’s why we’re doing a study to compare them.
Surgeon, consultation
We don’t have a clear-cut top-quality evidence that tells us which is the best approach, so we are running a study.
Surgeon, consultation
However, some recruiters made statements later in the consultation that went against their previously expressed neutrality, reflecting some of their views in favour of VATS expressed in the interviews. For instance, imbalanced information was provided and loaded terminology was used to describe the treatments. VATS was described with/without the mention of cuts as keyhole, whereas cuts were mentioned for open lobectomy. In addition, where cuts were mentioned in VATS, they were described as small cuts in comparison to the big cut for open lobectomy. Please see Appendix 6, Table 68, for more examples.
Similarly, personal opinions or treatment recommendations were provided to patients, which also affected recruitment, and this was usually in favour of VATS (note that, on rare occasions, especially as the trial progressed into the main phase, a bias towards open lobectomy was observed, especially in one centre) (see Appendix 6):
We need to offer patients the type of treatment which is actually beneficial. At the moment we really don’t know. I’m comfortable with it, it’s not a problem . . . but I believe that VATS lobectomy patients have a quicker recovery.
Surgeon, consultation
Outside of the trial, I would perform a VATS.
Surgeon, consultation
If you say no, I don’t want the chance of having a cut on my chest, I would rather have a keyhole surgery, I will respect that, it’s up to you.
OK. That’s what I want then.
Keyhole surgery?
Yes please.
OK [recorder switched off].
Let’s just say you was having it done, which one would you prefer?
Um . . . [patient laughs].
Um personally if it was a small tumour I, I don’t know I may go for the keyhole but I don’t know which is better but uh I might go for the keyhole.
Crucially, lack of recruiter equipoise had an impact on whether or not the VIOLET trial was explained to patients. This is detailed in Explaining the VIOLET trial and related concepts.
Summary
There was widespread support for the VIOLET trial among recruiters. A number of recruiters appeared to have a personal (i.e. for themselves) and a professional (i.e. for their patients) preference for VATS lobectomy, and may have participated in the trial while believing that VATS was better. However, recruiters’ awareness of this bias and the intellectual challenges involved in overcoming this allowed for balanced discussions in many consultations, as recruiters were able to assume a position of equipoise. This may have been facilitated by the QRI-informed recruitment training received by a number of surgeons prior to recruitment commencing in the VIOLET trial. In some instances, however, these biases became evident in the consultations in the form of imbalanced treatment presentations, which involved loaded terminology, providing personal opinions and treatment recommendations, and assuming that patients would not be interested in the VIOLET study (see Explaining the VIOLET trial and related concepts). These issues were addressed in the QRI plan of actions (see Plan of action: strategies to optimise recruitment and informed consent).
Patient preferences
Responding to patient preferences
In interviews, there was a feeling among recruiters that, because the trial was not comparing two radically different interventions, most patients did not come with strong preferences as to how a lobectomy was performed. Some recruiters, however, did describe instances where patients attributed more positive connotations to keyhole surgery rather than open surgery:
I think in my experience 90%, 95, would want a keyhole operation, given the choice of both, ’cause it’s just intuitive for the patient, makes sense.
Surgeon, interview
It’s very emotive. Keyhole means minimal access means better recovery means better outcomes.
Surgeon, interview
As described in the previous section (see Recruiter equipoise), this may be a reflection of the recruiters’ own biases.
There were instances where recruiters responded well to patient preferences prior to trial-specific feedback:
Can I ask about visualisation, isn’t the issue with the camera that you . . . is it easier to miss something visually that’s going on that you might otherwise see?
Some people say that, some people say it’s easier to see all the way round the chest with a camera. Some people say that you can’t do as good an operation with the cameras so that’s why they do it open, other people feel they can do the operation exactly the same. There’s pros and cons and you can give arguments for one or the other hmm but the bottom line is we really don’t know if one is better than the other.
However, there were also instances where it appeared that patients’ preferences (often for a keyhole procedure) were readily accepted without exploration to check the patient’s understanding, introducing the VIOLET trial or stating uncertainty:
Have you had a think about things at all?
I would like to go for the keyhole.
If that’s what you prefer. No, that’s absolutely fine. [VIOLET not discussed.]
Can I just have the keyhole surgery?
You would rather have keyhole surgery. OK. So, I will put down that you would not like to participate in the study.
Summary
There was a feeling that the VIOLET trial’s interventions were not entirely different from each other and, therefore, did not generate entrenched preferences among patients (as might radiotherapy vs. surgery, for example). Although recruiters appeared to be addressing preferences to some extent, there was indication of preferences being accepted at face value and without further study information provision or exploration of patient’s understanding or reasons for preference. We addressed these issues in the QRI plan of actions (see Plan of action: strategies to optimise recruitment and informed consent).
Explaining the VIOLET trial and related concepts
In many instances, recruiters placed the study in the context of existing uncertainty and emphasised that the two operations being compared were established techniques. A number of recruiters also avoided using the word ‘trial’ in their consultations, opting for ‘study’ instead, as recommended in QRI-informed training accessed by VIOLET trial recruiters prior to recruitment (occasional use of ‘trial’ was noticed and was limited to specific centres or recruiters). However, recruiters sometimes presented the VIOLET trial in an apologetic manner and appeared reluctant to present the trial information when they assumed that patients would not want to consider participating in VIOLET trial and would have preferences in line with their own (i.e. a preference for VATS). These beliefs led to a tendency to close down, rather than open up, conversations and patients missing opportunities to hear about the VIOLET trial, with recruiters appearing surprised when patients were willing to consider trial participation (see Appendix 6):
If you don’t want to be part of the study, that is the other option, then you tell me which procedure you want and we will do it.
He was thinking he’d quite like to be randomised.
Like you say there’s no, it’s toss a coin really isn’t it, it’s, yeah, whatever, yeah. I’m all right, yeah.
Right, so you’ve agreed to have the lobotomy operation, what do you think about the VIOLET study? Do you want to be part of that?
Yeah.
You sure?
So, coming to the study, have you got any interest in this or you, just to step out and let . . . no one will force you and, that’s absolutely fine with me and then you say, OK, I’m not interested, and I . . .
No, I would like to do it [patient was subsequently randomised].
Explanations of randomisation were sometimes absent or not well explained in consultations, with recruiters emphasising ‘lack of choice’ or implying that treatment is ‘selected’ or ‘decided’ by a computer. Similarly, some recruiters struggled with explanations of blinding, leading to patient concerns about what the process meant for them (see Appendix 6):
Before that you don’t know if you had keyhole surgery or surgery by thoracotomy, that’s the main let’s say parameter for you.
I’m wary of that, sounds a bit like a lottery. I’m surprised that the experts don’t know which is the best, especially for somebody of my age perhaps.
You and the research nurse looking after you whilst you are an inpatient, the study attempts to blind you to what you had.
Summary
Although it appeared that recruiters explained uncertainty well and avoided ‘trial’ in favour of ‘study’, likely in response to the training received prior to recruitment, there were opportunities for improvement in other areas. There were noticeable instances of reluctance from recruiters in putting the trial forward to patients, likely stemming from their own preferences for VATS. Trial concepts, namely randomisation and blinding, were particularly difficult to explain to patients. These issues were addressed in the QRI plan of actions (see Plan of action: strategies to optimise recruitment and informed consent).
Trial design-related issues
In addition to the above four recruitment themes, during interviews recruiters mentioned challenges they faced that were linked to aspects of the trial design. Blinding (of outcome assessors, usually RNs and patients) was particularly discussed as difficult to implement in some centres because of existing processes, and some RNs and patients disliked the concept. However, this issue resolved over time. Other issues were in relation to the availability of treatment options outside the trial, the possibility of including segmentectomy in the VIOLET trial protocol as an intervention, the standardisation of analgesia, variations in surgical expertise and the trial not accounting for recent advancements in the way open lobectomies were carried out. These issues were highlighted to the trial team, as discussed in Plan of action: strategies to optimise recruitment and informed consent. Further details are in Appendix 7.
Summary of recruitment processes and issues
Recruiters expressed high levels of support for the VIOLET trial. The patient pathway and eligibility criteria were considered straightforward, with some concerns regarding treatment recommendations for VATS made early in the pathway, which recruiters had addressed before it became overly problematic (i.e. from the prior training they received). Eligibility criteria for specific groups of patients was felt to be debatable. It appeared that a number of recruiters in the VIOLET trial had a preference for VATS and yet, for the most part, were able to overcome this, assume a position of equipoise and present balanced information on the two operations to patients (likely as a result of prior training). There were instances, however, where this was more challenging for recruiters, as they offered treatment recommendations and conveyed their own biases to patients. Patient preferences were being addressed by recruiters to some extent in the VIOLET trial, but we observed instances where patient preferences were accepted without further discussion or information. In addition, although concepts, such as the uncertainty underpinning the need for the VIOLET trial, were well presented, there were some noticeable instances of reluctance from recruiters in putting the trial forward to patients, likely stemming from their own preferences for VATS. Recruiters struggled with explanations of randomisation and blinding. Recruiters were found to follow recruitment advice from the training received prior to recruitment to use ‘study’ instead of ‘trial’. Although the blinding component of the study had been challenging to implement at first, the process became easier in most centres over time. In summary, although upfront training helped overcome some recruitment challenges, it did not resolve all issues. A number of key recruitment issues identified through the QRI were addressed, as outlined below.
Plan of action: strategies to optimise recruitment and informed consent
The QRI identified a number of elements of good recruitment practice, as well as challenges to recruitment, in an iterative process that spanned the entire recruitment period. A plan of action that comprised strategies to optimise recruitment and informed consent was designed in collaboration with the TMG and chief investigator, and was delivered to centres as recruitment proceeded. Issues relating to the trial design that were identified through the QRI were passed on to the trial team and the QRI team focused on support and training for recruiters to address the recruitment challenges. Table 10 outlines the key QRI findings and corresponding QRI actions/strategies recommended to optimise recruitment.
| Key recruitment theme | Action and QRI strategya |
|---|---|
Patient pathway through eligibility and recruitment:
|
|
Recruiter equipoise:
|
|
Patient preferences:
|
|
Explaining the VIOLET trial:
|
|
Other:
|
|
Key QRI dissemination activities are outlined in Figure 4 and explained in detail below.
Preventing recruitment difficulties: training and guidance prior to recruitment
Six surgeons, including PIs of all five pilot sites, attended the QRI-informed recruitment training workshops in May 2015, prior to the internal pilot phase in July 2015. A further pilot site surgeon attended the same workshop in May 2016. After the VIOLET trial moved to the main phase (July 2017), PIs of the new centres were invited to the training workshop in January 2018, which was attended by three VIOLET trial surgeons, including two PIs from two of the four new sites. This occurred in the same month that VIOLET trial-specific feedback was provided to the main phase sites.
Individual feedback
Eleven individual feedback sessions were held with nine recruiting surgeons from five centres (two surgeons received feedback on two occasions each), either face to face or over the telephone. The QRI researchers analysed each surgeon’s audio-recordings of recruitment consultations and prepared a two-page report. The confidential feedback highlighted aspects of their communication that worked well in promoting informed consent and trial participation, and provided suggestions for improving aspects of communication that may benefit from using alternative approaches.
Group feedback
The QRI team conducted eight feedback sessions with six sites (internal pilot sites, n = 5; main phase site, n = 1). Note that one site had three feedback sessions, one of which was for RNs only. Three of the new sites in the main phase did not receive a group feedback session. One centre submitted only two audio-recordings, which were insufficient to provide feedback; one centre did not submit any audio-recordings and provided screening log data that were incomplete or delayed; and one centre opened 4 months before recruitment was completed. Group feedback sessions included a presentation of site-specific recruitment figures (i.e. SEAR data) and anonymised QRI findings, drawing from audio-recordings from the site and the wider findings across other centres. Interactive group discussions focused on elements of good practice and suggestions for improving recruitment practice that were developed in collaboration with recruiters at each site to ensure that strategies were tailored to address their particular recruitment issues.
Recruitment and informed consent guidance (tips) documents
The QRI team produced two tips documents. The first version, disseminated early in the internal pilot phase (October 2015), contained suggestions for recruiters on how to discuss the study purpose and procedures, including randomisation, drawing from evidence in previous QRIs and a few audio-recordings collected in the first months of recruitment. A revised version of the guidance was developed and disseminated (June 2017) when new sites came on board in the main phase of the trial. The revised version was based on VIOLET trial audio-recordings and provided more extensive communication suggestions, including tips to balance information provided about the two operations, exploring patient preferences and how best to close a recruitment appointment.
Trial Management Group, investigator and study update meetings
The QRI findings and updates were regularly discussed at all available opportunities in VIOLET trial meetings (as mentioned in Chapter 2). QRI researchers disseminated findings on recruitment challenges at three TMG meetings. These meetings helped shape the recruitment strategies that were then disseminated more widely to the sites. The presentations were based on the challenges faced by recruiters at the given time. For instance, the TMG organised a meeting (Birmingham; February 2018) with PIs and co-applicants mid-way through the main phase of the study, with the purpose of providing an update on the study’s progress and encouraging sites to continue recruiting to the VIOLET trial after declining randomisation rates in the preceding 3 months. The QRI team’s presentation, therefore, focused on strategies to ensure that the momentum gained in recruitment previously was sustained for the remainder of the recruitment period.
Rapid communication strategy
When recruitment dipped towards the target line (March 2019), the TMG and QRI teams made co-ordinated efforts to support and continue engaging with sites until recruitment target was achieved (February 2019). These activities included the following.
Monthly newsletter with tip of the month
Recruitment tips were added to the monthly update newsletter sent by the trial co-ordinator to all staff recruiting to the VIOLET trial.
‘New recruitment targets’ infographics
New recruitment targets’ infographics were meant to provide the final push to achieve the recruitment target by encouraging site teams to increase their monthly recruitment rates to complete the study on time and within budget.
Chief investigator’s virtual group with principal investigators
In response to the QRI team’s concerns in April 2018, regarding achieving the recruitment target on time (at this point, the VIOLET trial had nearly 40% of the recruitment target to achieve in < 1 year), the chief investigator led a WhatsApp (Facebook, Inc., Menlo Park, CA, USA) group to encourage and support recruiting surgeons in their efforts to recruit patients to the study.
QRI evaluation
We evaluated the QRI in the VIOLET trial in three ways. First, there were many examples where QRI concepts had already been taken up because of recruiters’ upfront training. A number of recruitment challenges reported in other RCTs21,25,27–30,33 with QRIs were of lesser intensity (e.g. patient preferences) or already addressed by recruiters in the VIOLET trial. For instance, recruiters managed a potential recruitment issue caused by staff who met patients early in the patient pathway by speaking to them and requesting them to not convey a treatment recommendation to patients. Recruiters also avoided the misunderstanding caused by the term ‘trial’ among patients, as reported in other RCTs,21,22 by using the term ‘study’. Similarly, despite being vocal about their preference for VATS in interviews, recruiters became skilled at not conveying them to potential participants in most instances. Given that these and other similar topics were part of the QRI-informed recruitment training workshops that surgeons attended, this could be considered the first indication of prevention of recruitment difficulties in a RCT.
Second, we monitored SEAR data at site and recruiter level on a monthly basis and intervened rapidly whenever it appeared to fall towards the target line (as the VIOLET trial consistently recruited above target). The trial achieved its target sample size on time. However, it is difficult to determine the exact impact of the QRI on recruitment figures in the VIOLET trial or attribute causality between recruitment success and the QRI for the following reasons (see also Appendix 1). The amount of support VIOLET trial recruiters received well in advance of recruitment precludes identification of a precise ‘pre-intervention’ period and it is difficult to assess how much this support contributed to the excellent start to recruitment in the VIOLET trial or the momentum for the consistently above-target recruitment line in the VIOLET trial. In the absence of evidence on factors that predict good or poor recruitment to RCTs, we cannot ascertain if recruitment to the VIOLET trial would have been different if the intervention had not taken place. If the VIOLET trial had a precise pre- and post-intervention period, a causal link would still not be established because of the large number of confounding factors that may also contribute to successful recruitment. The QRI, alongside the highly committed chief investigator and PIs and efficient and supportive trials centre, all played a role in the VIOLET trial’s success.
Third, we evaluated changes in recruitment practice. Figure 4 shows the monitoring of changes to recruitment based on QRI feedback. From listening to audio-recordings, we were able to note that recruiters advanced their recruitment skills after they received VIOLET trial-specific feedback. For instance, although recruiters addressed patient preferences from the early days of recruitment, their skills were further refined and nuanced with more examples of good practice after they received tailored feedback from the QRI team (see Appendix 8, Table 69).
Chapter 5 Results: primary and secondary outcomes
Primary outcome: EORTC QLQ-C30 physical function at 5 weeks
Using the QLQ-C30 physical functioning score at 5 weeks from randomisation as a global marker of recovery, with higher scores indicating higher levels of functioning, participants allocated to VATS had a median score of 73 (IQR 60.0–86.7) compared with median score of 67 (IQR 53.3–86.7) for participants allocated to open surgery [adjusted MD (i.e. VATS – open surgery) 4.65, 95% CI 1.69 to 7.61; p = 0.0089]. The sensitivity analysis, excluding participants with benign disease, was consistent with the primary analysis (Table 11).
| Outcome | Primary analysis | Analysis excluding benign patients | ||
|---|---|---|---|---|
| MD (95% CI) | p-valuea | MD (95% CI) | p-valuea | |
| QLQ-C30 physical function at 5 weeks | 4.65 (1.69 to 7.61) | 0.0089 | 4.66 (1.71 to 7.62) | 0.0089 |
The target treatment effect, unpinning our sample size calculation, was 0.25 SDs (see Chapter 2, Sample size). Our observed unadjusted treatment effect was slightly lower at 0.21 SDs (i.e. an unadjusted difference of 4.62, with a pooled SD of 22.4).
Secondary outcomes
EORTC QLQ-C30 physical function over time
The EORTC QLQ-C30 physical functioning scores over time from randomisation to 1 year are shown in Figure 6 and Appendix 5, Table 50. The improvement in physical function was more marked in the early discharge period and less pronounced after 6 months (see Figure 6). On average, the score was 4.22 points higher with VATS than with open surgery (95% CI 1.48 to 6.97 points; p = 0.009).
FIGURE 6.
QLQ-C30 physical function over time. Higher scores indicate better physical function. Adapted with permission from NEJM Evidence, Lim E, Batchelor TJP, Dunning J, Shackcloth M, Anikin V, Naidu B, et al. , Video-assisted thorascopic or open lobectomy in early-stage lung cancer, Volume 1, Copyright © 2022 Massachusetts Medical Society. 56
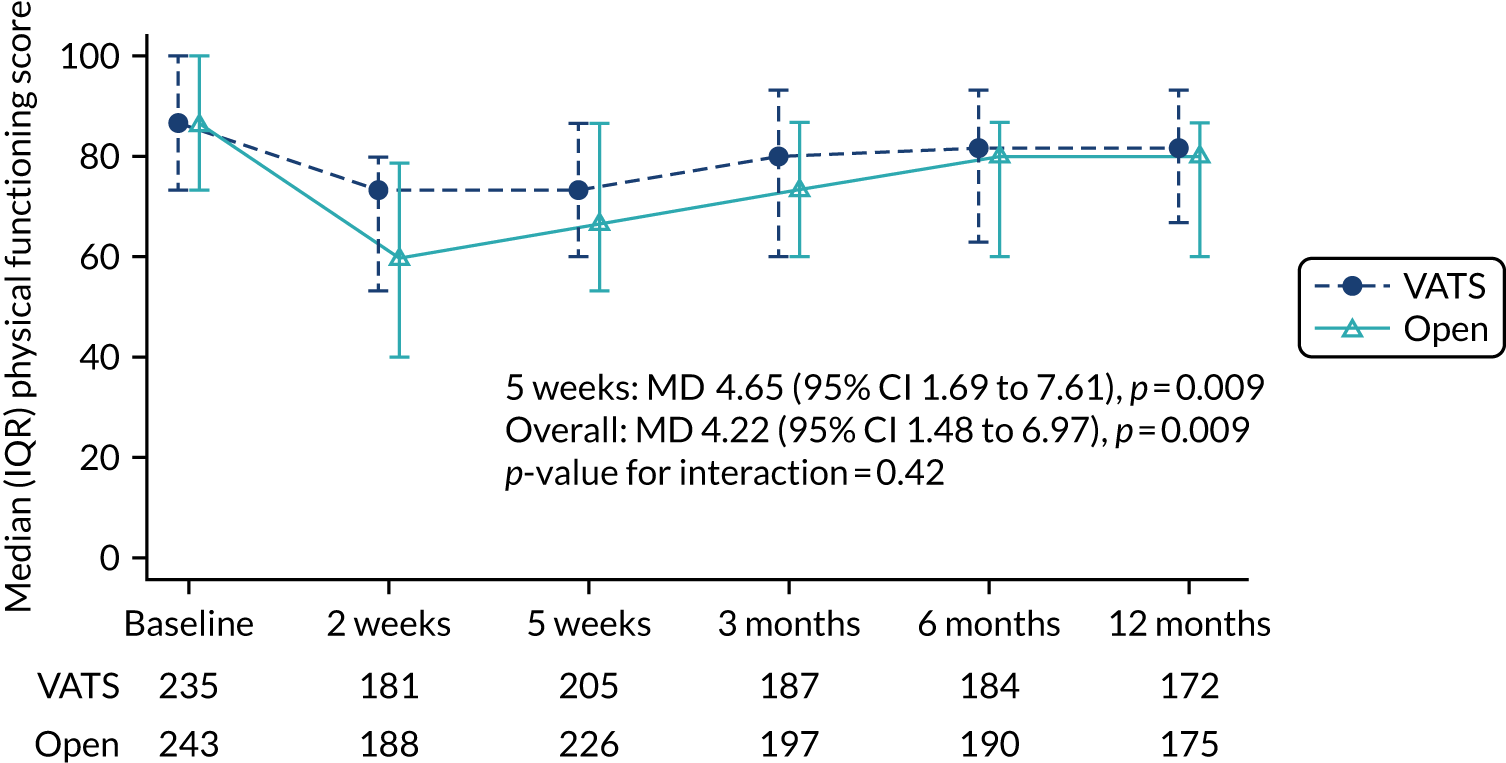
Complete resection
The total number of lymph node stations harvested (median, IQR 4–6) was very similar in both groups, as was the number of mediastinal nodes harvested (median 3, IQR 3–4) (Table 12). Complete R(0) resection was achieved in 429 of 439 (97.7%) participants and there was no difference between the groups (RR 0.999, 95% CI 0.97 to 1.26; p = 0.94) (see Appendix 5, Figure 27). All participants with residual disease had R1 disease.
| Outcome | Participant allocation | |
|---|---|---|
| Randomised to VATS (N = 247) | Randomised to open surgery (N = 255) | |
| Total number of lymph node stations harvested, median (IQR) | 5 (4.0–6.0) | 5 (4.0–6.0) |
| Mediastinal nodes harvested (stations 2 to 9), median (IQR) | 3 (3.0–4.0) | 3 (3.0–4.0) |
| Complete (R0) resection, n/N (%) | 210/215 (97.7) | 219/224 (97.8) |
| Site of residual (R1) disease, n/N (%) | ||
| Bronchial margin | 2/5 (40.0) | 3/5 (60.0) |
| Vascular margin | 0/5 (0.0) | 1/5 (20.0) |
| Lung parenchymal margin | 2/5 (40.0) | 0/5 (0.0) |
| Other | 1/5 (20.0) | 0/5 (0.0) |
| No data | 0/5 (0.0) | 1/5 (20.0) |
Lymph node upstaging
Lymph node upstaging is summarised in Table 13. Upstaging from clinical node stage 0 (cN0) to pN1 and from clinical node stage 0 or 1 (cN0/1) to pN2 was similar in the groups (see Appendix 5, Figure 28).
| Outcome | Participant allocation | RR (95% CI) | p-value | |
|---|---|---|---|---|
| Randomised to VATS (N = 247) | Randomised to open surgery (N = 255) | |||
| cN0 to pN1, n/N (%) | ||||
| Yes | 15/244 (6.2) | 13/252 (5.2) | 1.18 (0.54 to 2.58) | 0.68 |
| No | 211/244 (86.5) | 219/252 (86.9) | ||
| Not cancer | 18/244 (7.4) | 20/252 (7.9) | ||
| cN0/1 to pN2, n/N (%) | ||||
| Yes | 15/244 (6.2) | 12/252 (4.8) | 1.31 (0.60 to 2.86) | 0.50 |
| No | 211/244 (86.5) | 220/252 (87.3) | ||
| Not cancer | 18/244 (7.4) | 20/252 (7.9) | ||
Pain in the first 2 days post surgery
Visual analogue scale pain scores
In-hospital VAS pain scores in the first 2 days post surgery are shown in Figure 7 and in Appendix 5, Table 51. Pain scores were similar in the two groups on day 1, with a median score of 4 in both groups (MD –0.02, 95% CI –0.46 to 0.41; p = 0.913), but participants in the VATS group had significantly lower pain scores on day 2 than participants in the open-surgery group (median 3 vs. 4, MD –0.54, 95% CI –0.99 to –0.09; p = 0.018). The complete-case sensitivity analysis gave consistent results (see Appendix 5, Table 52).
FIGURE 7.
Pain scores over time. Adapted with permission from NEJM Evidence, Lim E, Batchelor TJP, Dunning J, Shackcloth M, Anikin V, Naidu B, et al. , Video-assisted thorascopic or open lobectomy in early-stage lung cancer, Volume 1, Copyright © 2022 Massachusetts Medical Society. 56
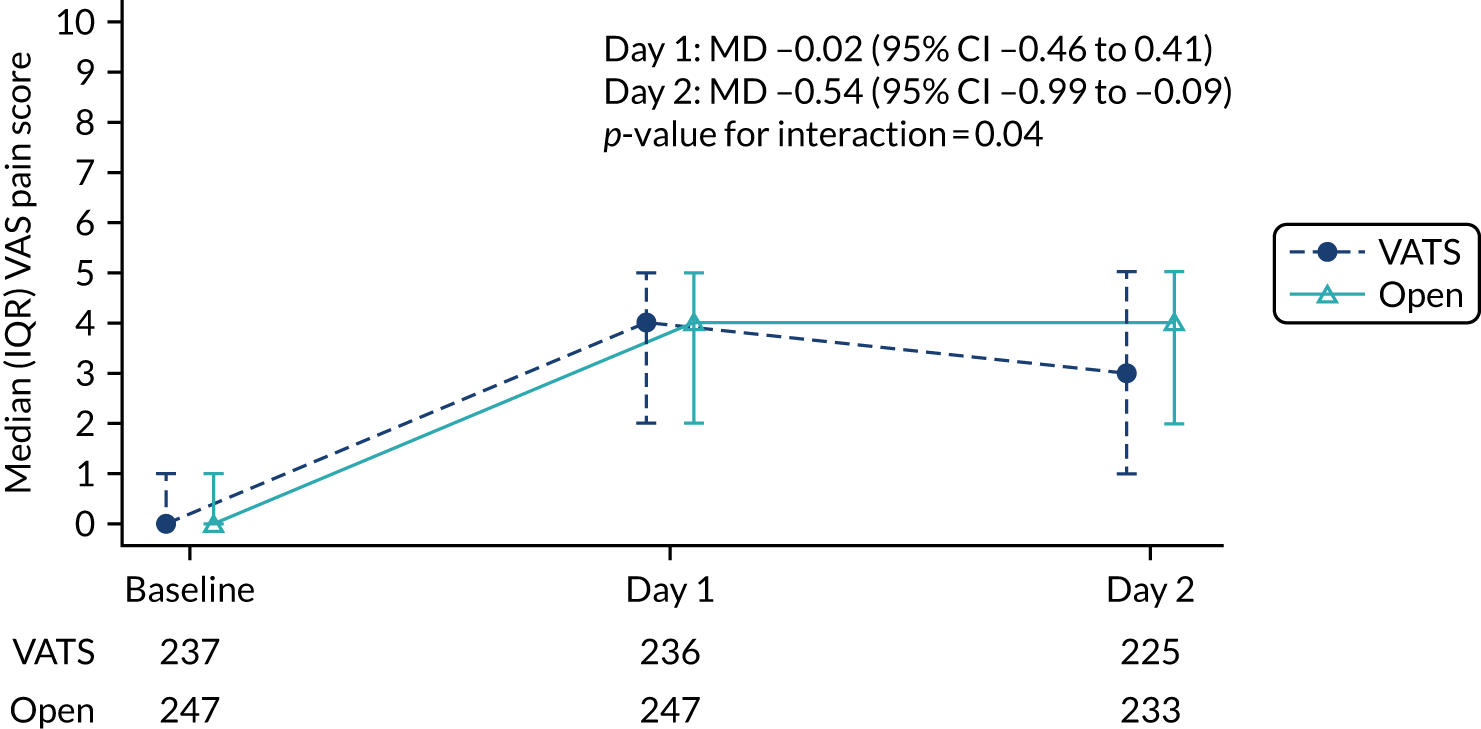
Postoperative analgesia
Analgesia is used to manage pain following surgery. The most commonly prescribed analgesia during the intraoperative and postoperative hospital stay, expressed as the ratio of mean daily dose, is shown in Figure 8. Overall, analgesic consumption was 10% lower in the VATS group (mean ratio 0.9, 95% CI 0.80 to 1.01). Additional analgesia prescribed, which are included in the overall estimate but not depicted in Figure 8, are given in Appendix 5, Table 53.
FIGURE 8.
Analgesia prescribed intraoperatively and postoperatively. Data show the mean ratio (95% CI). PCA, patient-controlled analgesia. Adapted with permission from NEJM Evidence, Lim E, Batchelor TJP, Dunning J, Shackcloth M, Anikin V, Naidu B, et al. , Video-assisted thorascopic or open lobectomy in early-stage lung cancer, Volume 1, Copyright © 2022 Massachusetts Medical Society. 56
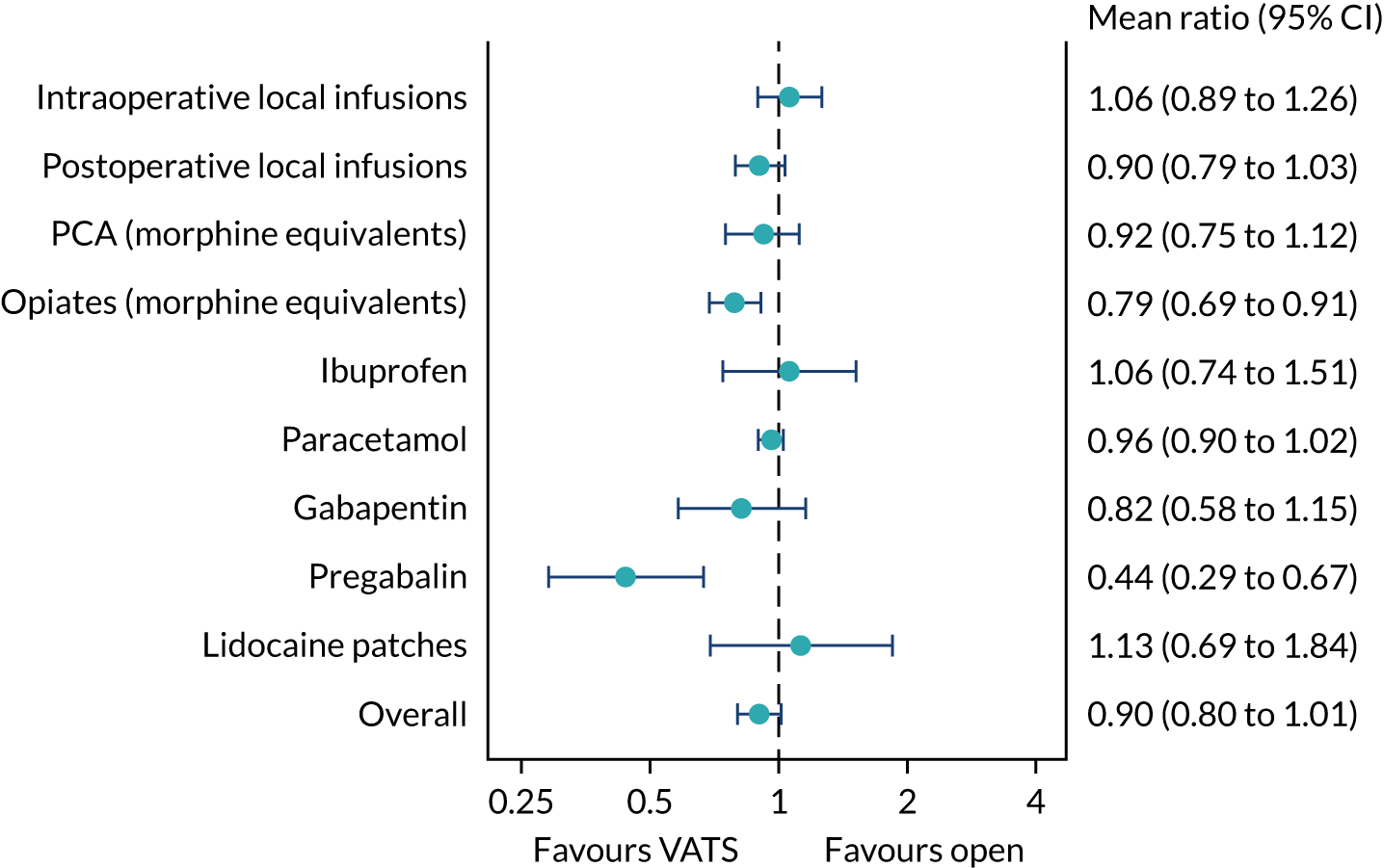
Subgroup analysis: impact of type of analgesia received during surgery
Most participants received an intercostal block (315/502, 63%), with a similar proportion of participants receiving a paravertebral block (96/502, 19%) or neither (85/502, 17%). There was no evidence to suggest the difference in pain scores between VATS and open surgery differed by the type of analgesia received (test for treatment by analgesia interaction p = 0.19) (Figure 9).
FIGURE 9.
Subgroup analysis of pain scores by analgesia received.
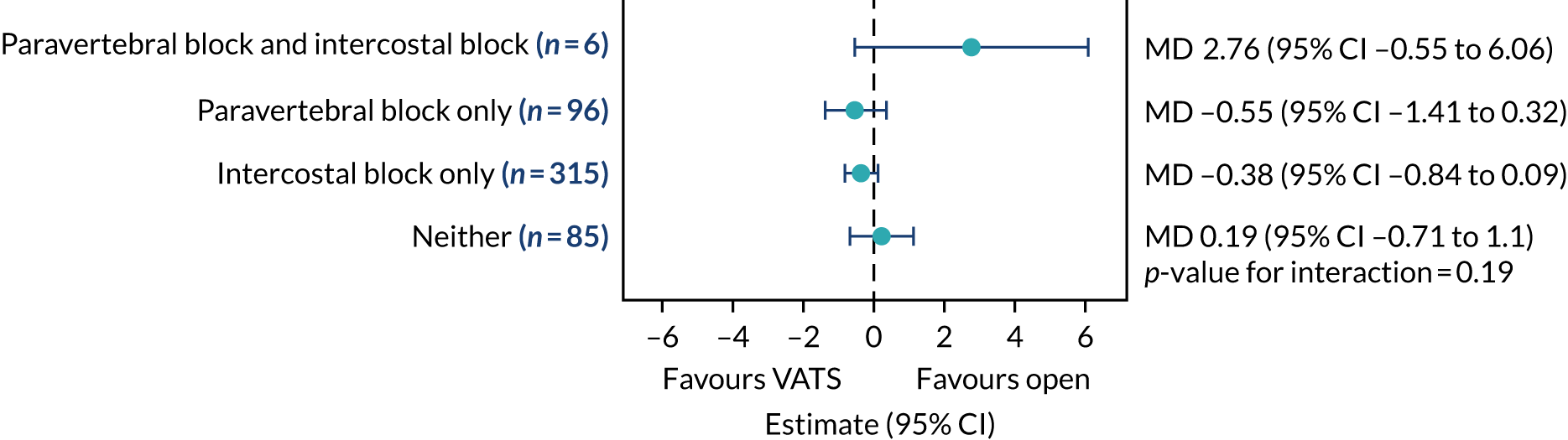
Exploratory analyses: pain scores
Impact of type of thoracotomy, use of muscle sparing and rib resection
Differences in pain scores by type of thoracotomy performed, use of muscle sparing and rib resection are shown in Figure 10. In the first 2 days, pain scores did not vary significantly by type of thoracotomy, the use of muscle sparing or with rib resection.
FIGURE 10.
Exploratory analysis of the impact of type of thoracotomy on pain scores.
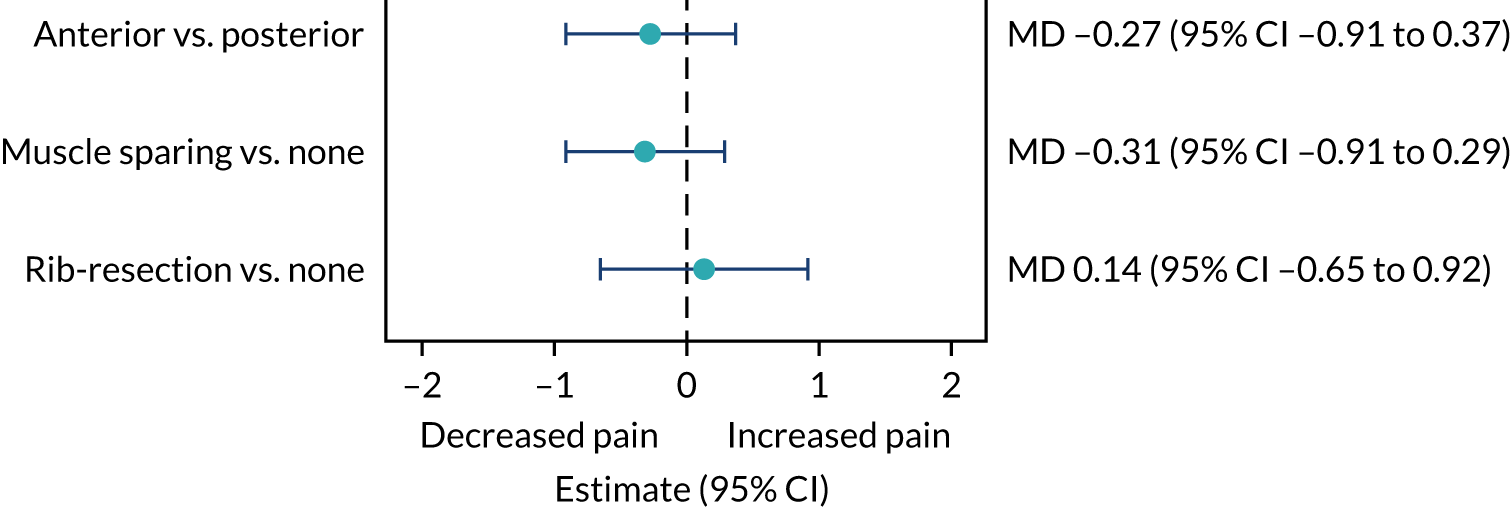
Impact of number of port sites
Summaries of pain scores by number of port sites are presented in Table 14. Lower pain scores on day 1 were reported in participants receiving single-port VATS than in participants receiving multiport VATS and open surgery (median score 3 vs. 4 vs. 4, respectively). On day 2, the median pain score report by participants was the same for single-port VATS and multiport VATS (i.e. a median score of 3) and lower than open surgery (i.e. a median score of 4). The exploratory analysis comparing pain scores found no significant differences (see Appendix 5, Table 54).
| Time point | Patients receiving VATS (N = 208) | Patients receiving open surgery (N = 245) | |
|---|---|---|---|
| Single-port VATS (n = 42) | Multiport VATS (n = 166) | ||
| Baselinea | 0 (0.0–1.0) | 0 (0.0–2.0) | 0 (0.0–1.0) |
| Day 1b | 3 (2.0–5.0) | 4 (2.0–6.0) | 4 (2.0–6.0) |
| Day 2c | 3 (1.0–5.0) | 3 (0.0–5.0) | 4 (2.0–5.0) |
Prolonged incision pain beyond 5 weeks
The proportion of patients with prolonged incision pain (defined as the need for analgesia after 5 weeks post randomisation) is shown in Table 15. A total of 143 (59.6%) patients in the VATS group experienced prolonged incision pain compared with a total of 175 (72.3%) patients in the open-surgery group. This difference was significant (RR 0.82, 95% CI 0.72 to 0.94; p = 0.003) (see Appendix 5, Figure 29).
| Outcome | Participant allocation | RR (95% CI) | p-value | |
|---|---|---|---|---|
| Randomised to VATS (N = 247), n/N (%) | Randomised to open surgery (N = 255), n/N (%) | |||
| Prolonged incision paina | 143/240 (59.6) | 175/242 (72.3) | 0.82 (0.72 to 0.94) | 0.0033 |
Time from surgery to hospital discharge: postoperative length of hospital stay
Time from surgery to hospital discharge is presented in Figure 11 and in Appendix 5, Table 55. Median length of stay was lower for patients in the VATS group than for patients in the open-surgery group (at 4 and 5 days, respectively). This difference was statistically significant (HR 1.34, 95% CI 1.09 to 1.65; p = 0.006).
FIGURE 11.
Time from surgery to hospital discharge. Adapted with permission from NEJM Evidence, Lim E, Batchelor TJP, Dunning J, Shackcloth M, Anikin V, Naidu B, et al. , Video-assisted thorascopic or open lobectomy in early-stage lung cancer, Volume 1, Copyright © 2022 Massachusetts Medical Society. 56
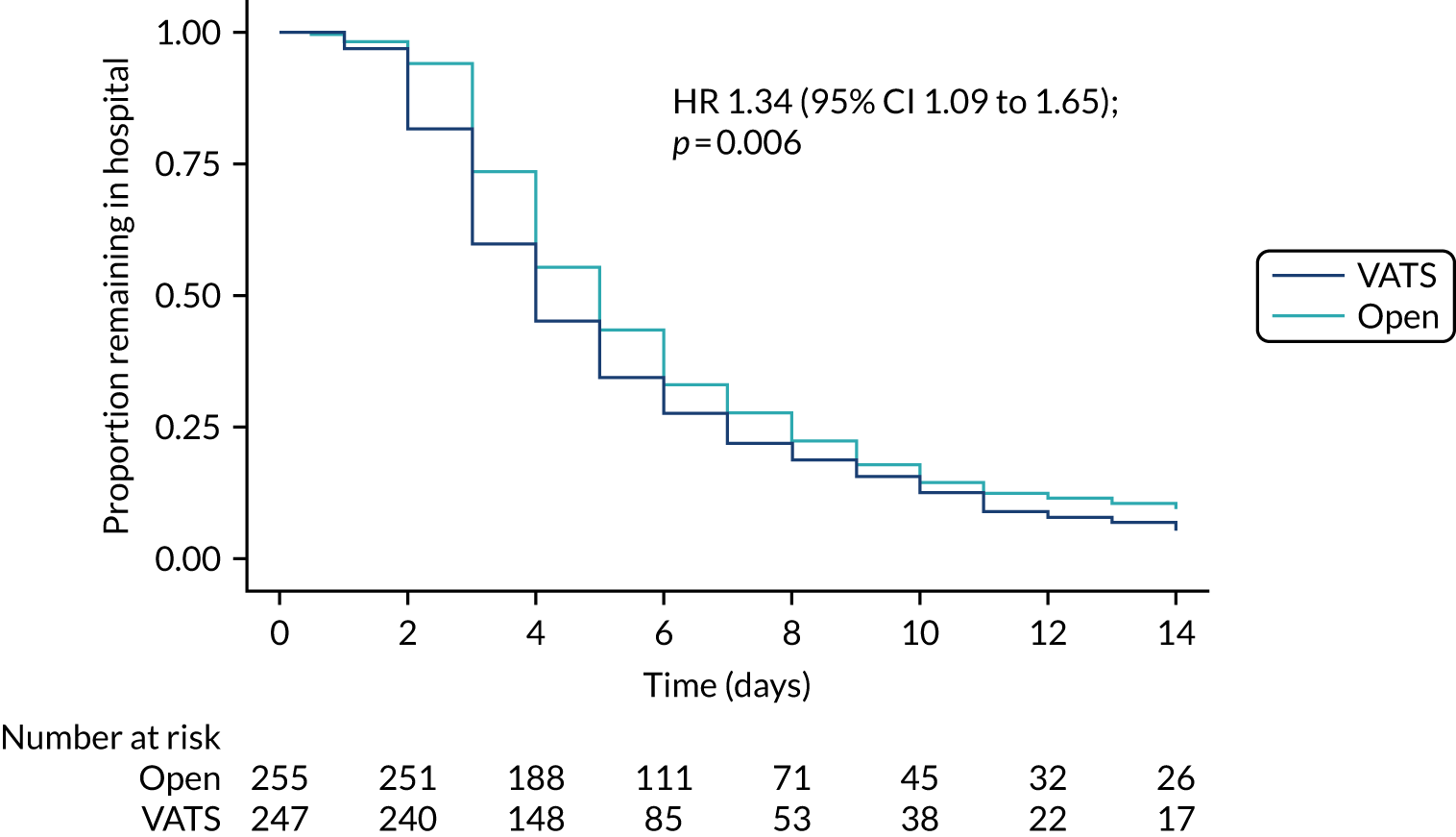
Exploratory analysis: length of stay
Impact of number of port sites on length of stay
Summaries of length of hospital stay by number of port sites are presented in Table 16. The median length of stay was the same in patients who received single-port VATS and in patients who received multiport VATS (i.e. 4 days), but differed from patients who received open surgery (i.e. 5 days).
| Length of stay | Patients receiving VATS (N = 208) | Patients receiving open surgery (N = 245) | |
|---|---|---|---|
| Single-port VATS (n = 42) | Multiport VATS (n = 166) | ||
| Time to discharge (days), median (IQR) | 4 (3–8) | 4 (3–7) | 5 (4–8) |
The exploratory analysis comparing length of stay confirmed this difference as statistically significant (p = 0.017) (see Appendix 5, Table 56).
Fitness for hospital discharge
All participants were assessed against predefined ‘fit-for-discharge’ criteria (see Chapter 2, Fitness for discharge after surgery for the definition). The time in days when first considered fit, and the numbers discharged before the criteria were first met, when they were first met and after they were first met are shown in Table 17. The median time to first meeting the fitness criteria was 1 day before the median length of stay in both groups. The proportion of patients discharged ‘early’ was similar in the two groups (i.e. 8.4% overall). Discharge was ‘delayed’ in one-quarter of participants.
| Fitness for discharge | Participant allocation | Overall (N = 492) | |
|---|---|---|---|
| Randomised to VATS (N = 240) | Randomised to open surgery (N = 252) | ||
| Time until fitness criteria first met (days), median (IQR) | 3 (2.0–5.0) | 4 (3.0–6.5) | 4 (2.0–6.0) |
| Patient discharged, n/N (%) | |||
| On first day fit | 161/235 (68.5) | 162/251 (64.5) | 323/486 (66.5) |
| After first day fit | 54/235 (23.0) | 68/251 (27.1) | 122/486 (25.1) |
| Before first day fit | 20/235 (8.5) | 21/251 (8.4) | 41/486 (8.4) |
Overall survival and progression-free survival to 1 year
There were 31 deaths within 1 year of randomisation, 18 in the open-surgery group and 13 in the VATS group (Table 18). Overall, 94.6% of participants were alive at 1 year in the VATS group compared with 92.6% of participants in the open-surgery group (HR for death 0.67, 95% CI 0.32 to 1.40; p = 0.28). Overall survival by group is shown in Appendix 5, Figure 30.
| Cause of death | Participant allocation | |
|---|---|---|
| Randomised to VATS (N = 11), n | Randomised to open surgery (N = 13), n | |
| Bronchopneumonia | 0 | 1 |
| Cardiac arrest | 0 | 1 |
| Disease progression | 7 | 5 |
| Infective exacerbation of COPD | 1 | 0 |
| Ischaemic brain injury, cardiac arrest, myocardial ischaemia | 0 | 1 |
| Pneumonia | 2 | 0 |
| Pseudomonas, respiratory failure | 0 | 1 |
| Pulmonary embolism | 0 | 1 |
| Respiratory/cardiac failure | 0 | 1 |
| Stroke, myocardial infarction | 1 | 0 |
| Subarachnoid haemorrhage | 0 | 1 |
| Unknown | 0 | 1 |
Sixteen VATS participants and 17 open-surgery participants experienced disease recurrence/progression within 1 year. Overall, 90.4% of participants were alive and disease free at 1 year in the VATS group compared with 88.0% of participants in the open group (HR for disease progression 0.73, 95% CI 0.42 to 1.27; p = 0.26) (Table 19). Progression-free survival by group is shown in Appendix 5, Figure 31.
| Outcome | Primary analysis | Analysis adjusting for pathological disease stage | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Survival | 0.67 (0.32 to 1.40) | 0.283 | 0.71 (0.34 to 1.50) | 0.366 |
| Progression-free survival | 0.73 (0.42 to 1.27) | 0.262 | 0.75 (0.42 to 1.32) | 0.312 |
Sensitivity analyses adjusting for pathological disease stage were carried out for survival and progression-free survival outcomes. See Table 19 for data contrasting the results of the primary analyses for these outcomes and the results of the sensitivity analyses, which were very similar.
The locations of recurrence and new cancers are shown in Table 20. There were 24 cases of locoregional recurrence (VATS, n = 11; open surgery, n = 13), 17 cases of distant recurrence (VATS, n = 7; open surgery, n = 10) and 10 cases of new cancer (VATS, n = 4; open surgery, n = 6).
| Type/location | Participant allocation | |
|---|---|---|
| Randomised to VATS (N = 18) | Randomised to open surgery (N = 21) | |
| Locoregional recurrence | ||
| Lung | 3/3 (16.7) | 7/6 (28.6) |
| Mediastinal | 4/4 (22.2) | 1/1 (4.8) |
| Bronchus | 0 | 1/1 (4.8) |
| Pleura and lymph nodes | 1/1 (5.6) | 0 |
| Not collecteda | 3/2 (11.1) | 4/4 (19) |
| Distant recurrence | ||
| Adrenal gland | 0 | 3/2 (9.5) |
| Adrenal gland and liver | 0 | 1/1 (4.8) |
| Brain | 1/1 (5.6) | 2/2 (9.5) |
| Brain/spine | 1/1 (5.6) | 0 |
| Liver | 2/2 (11.1) | 0 |
| Liver, adrenal glands, intra-abdominal lymph nodes | 1/1 (5.6) | 0 |
| Thoracic and lumbar spine | 1/1 (5.6) | 0 |
| Not collecteda | 1/1 (5.6) | 4/4 (19) |
| New cancer | ||
| Prostate | 1/1 (5.6) | 2/2 (9.5) |
| Lung | 1/1 (5.6) | 1/1 (4.8) |
| Acute myeloid leukaemia | 0 | 1/1 (4.8) |
| Bowel | 1/1 (5.6) | 0 |
| Cholangiocarcinoma | 1/1 (5.6) | 0 |
| Sarcoma | 0 | 1/1 (4.8) |
| Not collecteda | 0 | 1/1 (4.8) |
Uptake of adjuvant treatment
Time to uptake of adjuvant treatment was analysed in the primary intention-to-treat population and, for the subset of participants eligible for adjuvant treatment according to NICE guidelines,57 namely participants with a postoperative disease stage of (1) N1–2 and M0 or (2) T2b to 4, N0 and M0 were deemed eligible. Overall, 73 participants had adjuvant treatment, 56 of whom met the eligibility criteria defined by NICE (see Appendix 5, Table 57). The time to uptake of adjuvant treatment was similar between the two treatment groups, both for the primary intention-to-treat population and the NICE-eligible subset of participants (Figures 12 and 13, see also Appendix 5, Table 57).
FIGURE 12.
Uptake of adjuvant treatment in the primary analysis population.
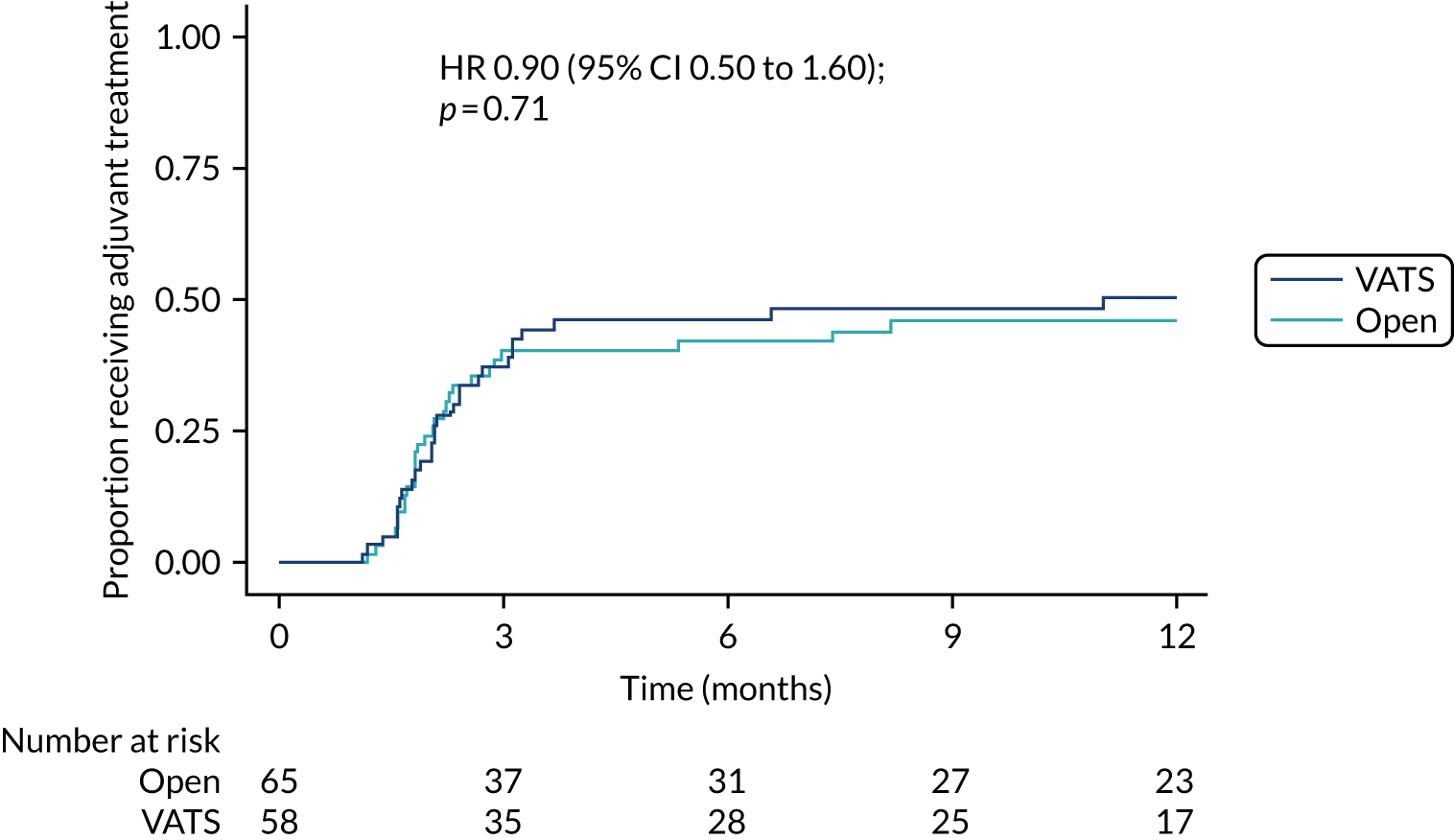
FIGURE 13.
Uptake of adjuvant treatment in the NICE-eligible subset of participants.
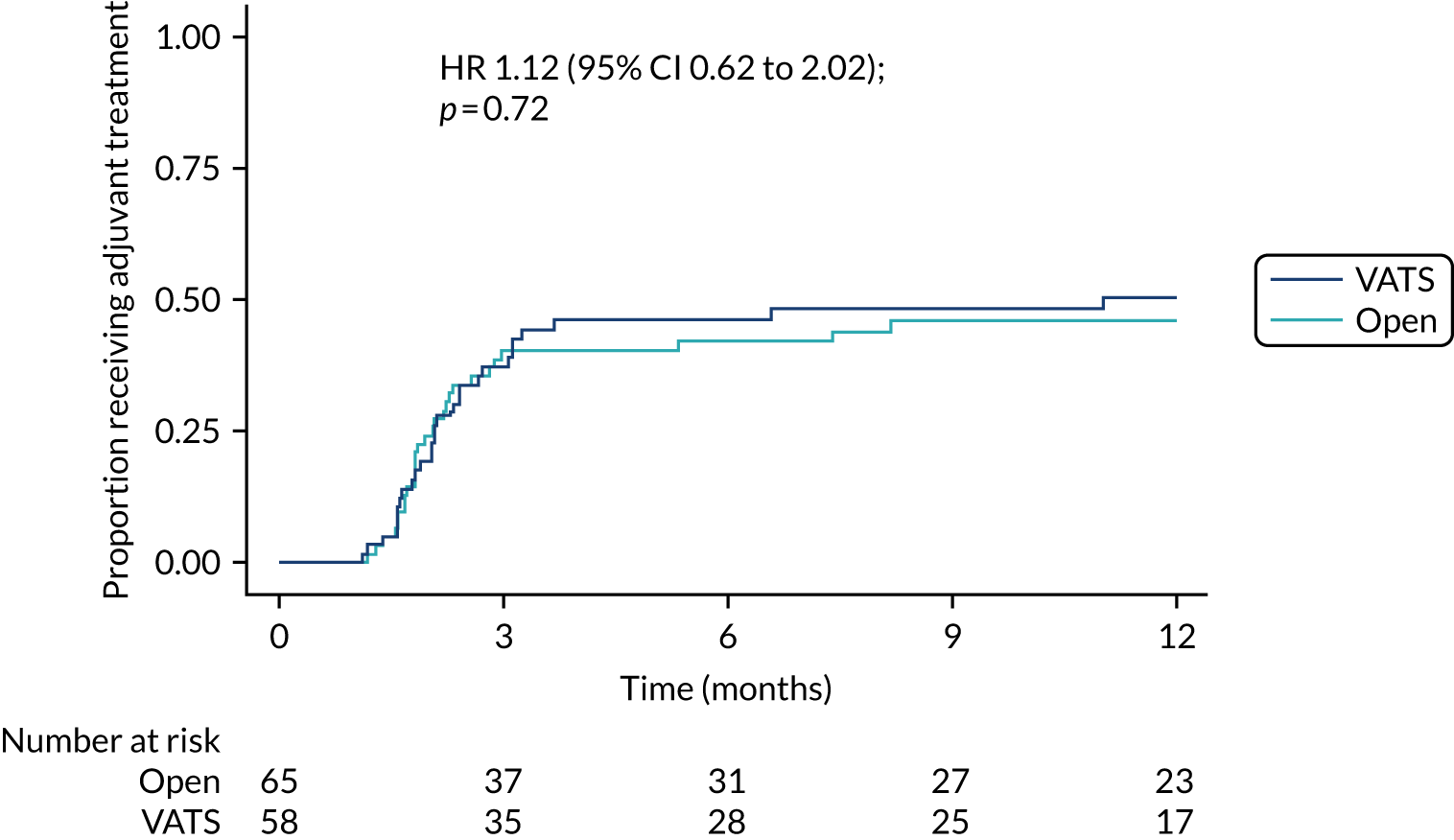
EORTC QLQ-C30 quality-of-life questionnaire
The EORTC QLQ-C30 comprises an overall measure of global health and a number of subscales. For all scales, higher scores indicate a higher level of functioning or symptoms or problems.
Global health status and subscales assessing functioning
In addition to the global health status, the questionnaire measures physical, role, social, cognitive and emotional functioning. The physical function subscale was the primary outcome for the VIOLET trial and is reported in Primary outcome: EORTC QLQ-C30 physical function at 5 weeks and EORTC QLQ-C30 physical function over time. Scores for the other scales over time for participants randomised to VATS or open surgery are shown in Appendix 5, Figures 32–34. Global health status, role and social functioning were all significantly higher in the VATS group (Figure 14) than in the open-surgery group, and where cognitive function was impaired, the impairment was less in the VATS group than in the open-surgery group (Figure 15). The effect of surgery on emotional function varied over time. At 2 weeks, fewer participants in the VATS group than in the open-surgery group reported some impaired emotional function, but thereafter the results were similar in the two groups (Figure 16). Summary data and the estimated treatment effects for each scale are given in Appendix 5, Tables 58 and 59.
FIGURE 14.
QLQ-C30 global health status and role and social functioning: treatment effects.
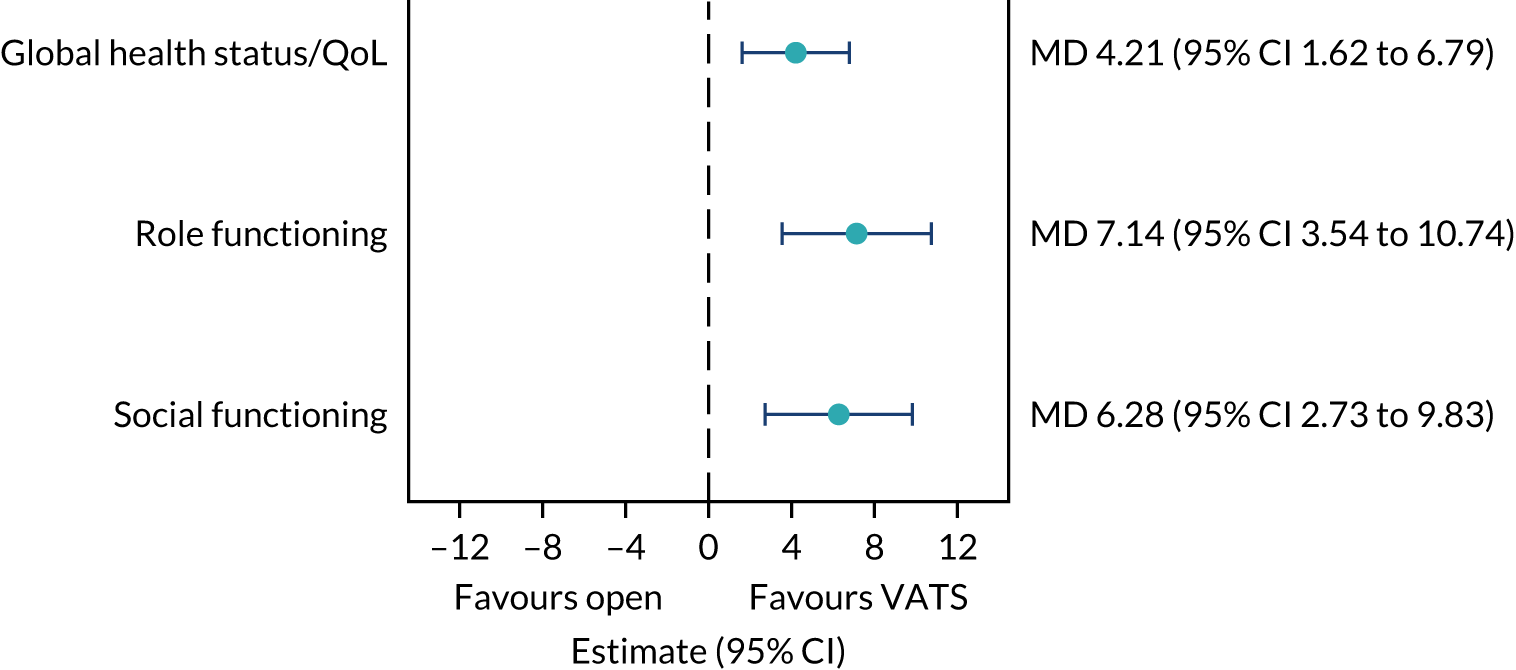
FIGURE 15.
QLQ-C30 cognitive functioning over time. Higher scores indicate better health.
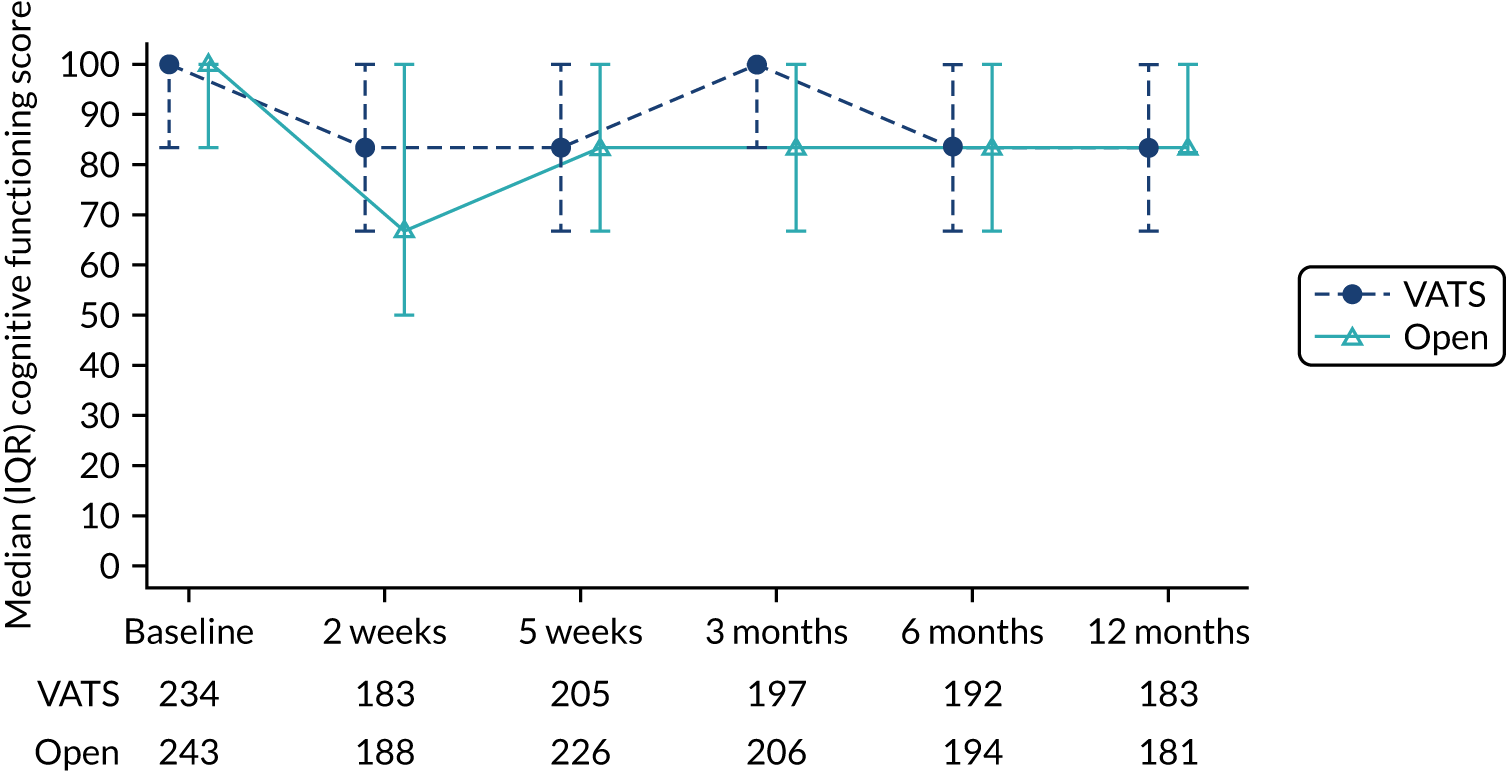
FIGURE 16.
QLQ-C30 emotional functioning over time. Higher scores indicate better health.
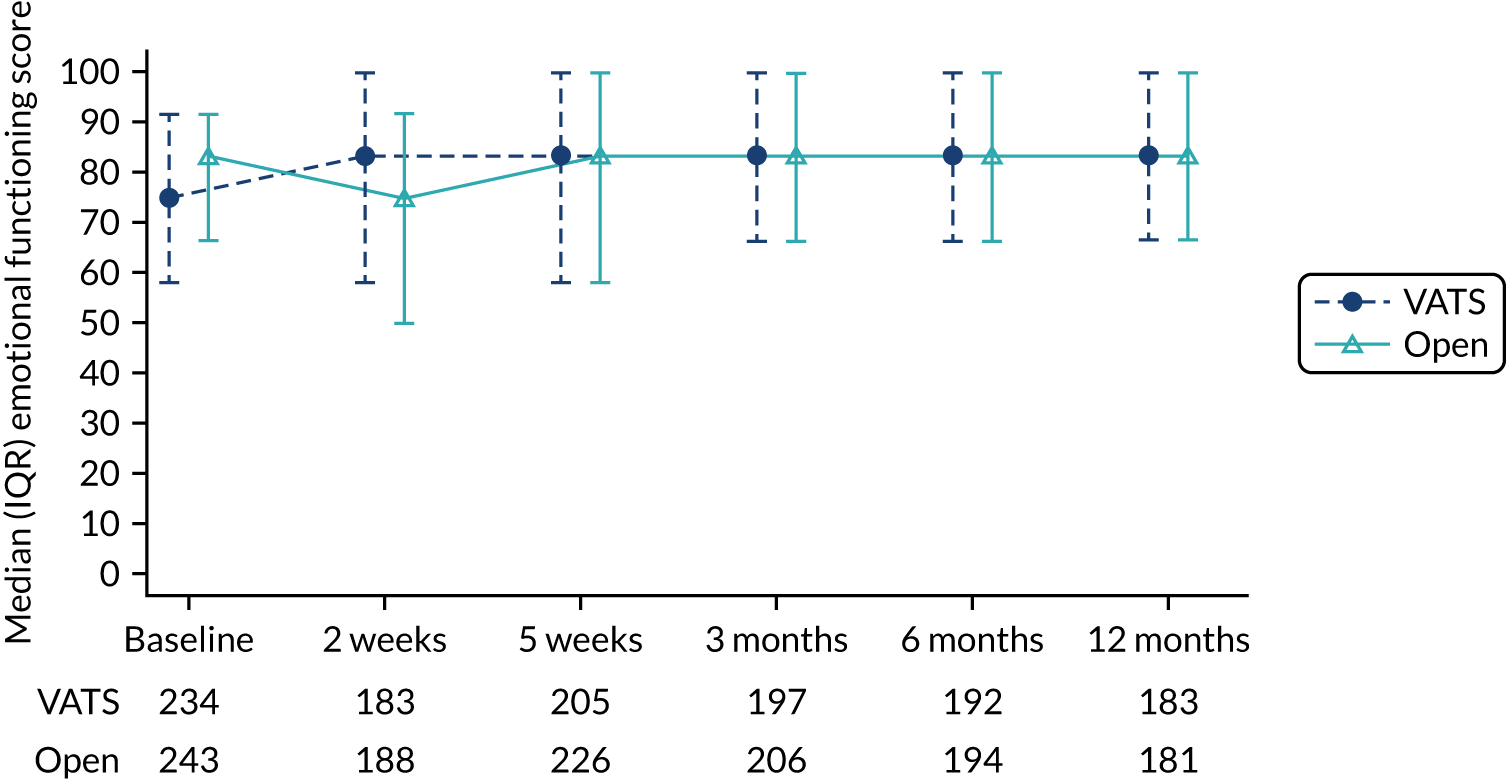
Subscales assessing symptoms and problems
Scores for scales measuring symptoms and problems over time for participants randomised to VATS or open surgery are shown in Appendix 5, Figures 35–43. Participants randomised to VATS experienced less pain and fatigue and had less difficulty sleeping in the first 2 weeks than participants randomised to open surgery. These participants were also less likely to experience appetite loss and nausea, and constipation in the early period post surgery. Other measures were similar in the two groups (Figure 17). Summary data and the estimated treatment effects for each subscale are given in Appendix 5, Tables 60 and 61.
FIGURE 17.
QLQ-C30 symptoms and problems subscales: treatment effects.
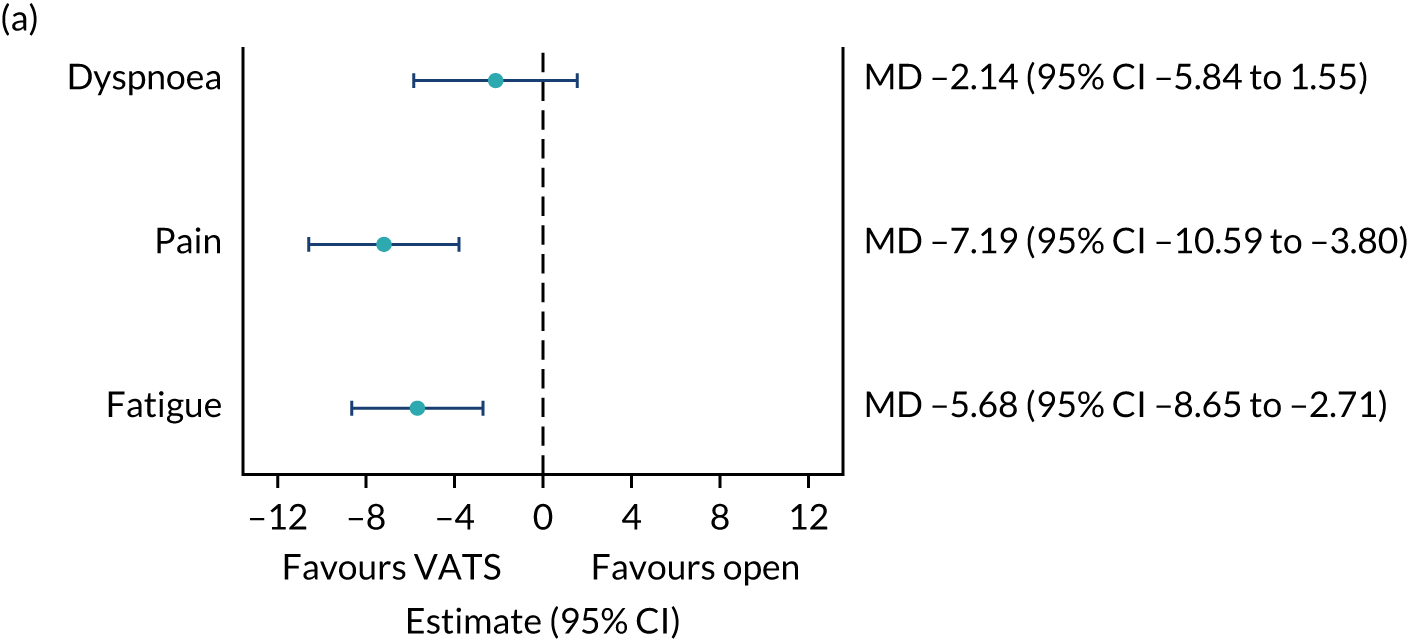
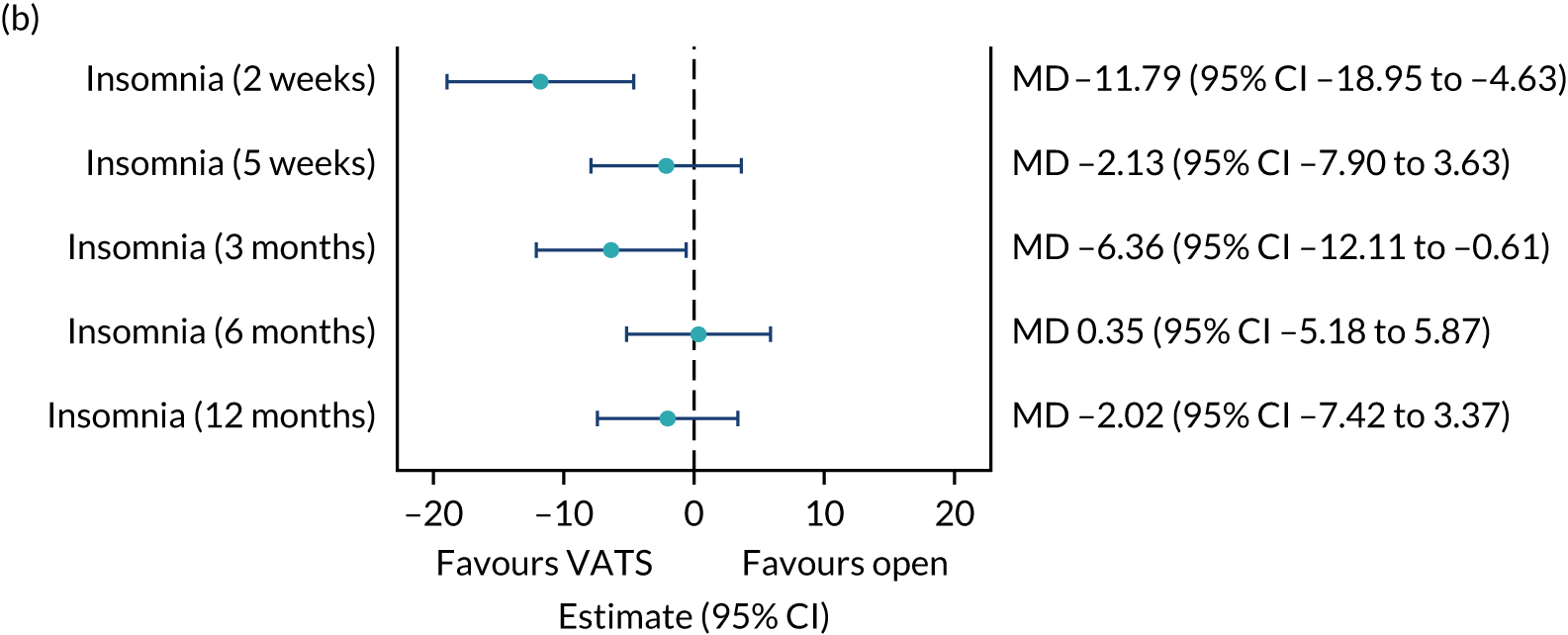
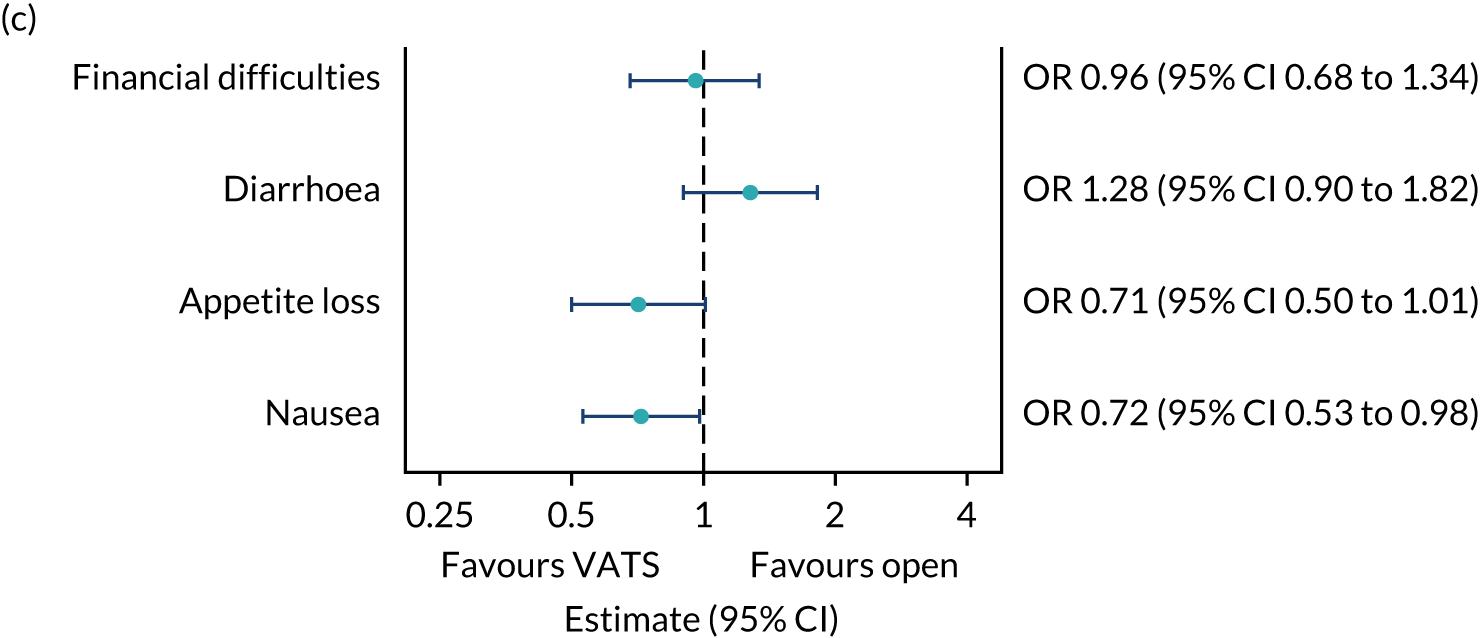
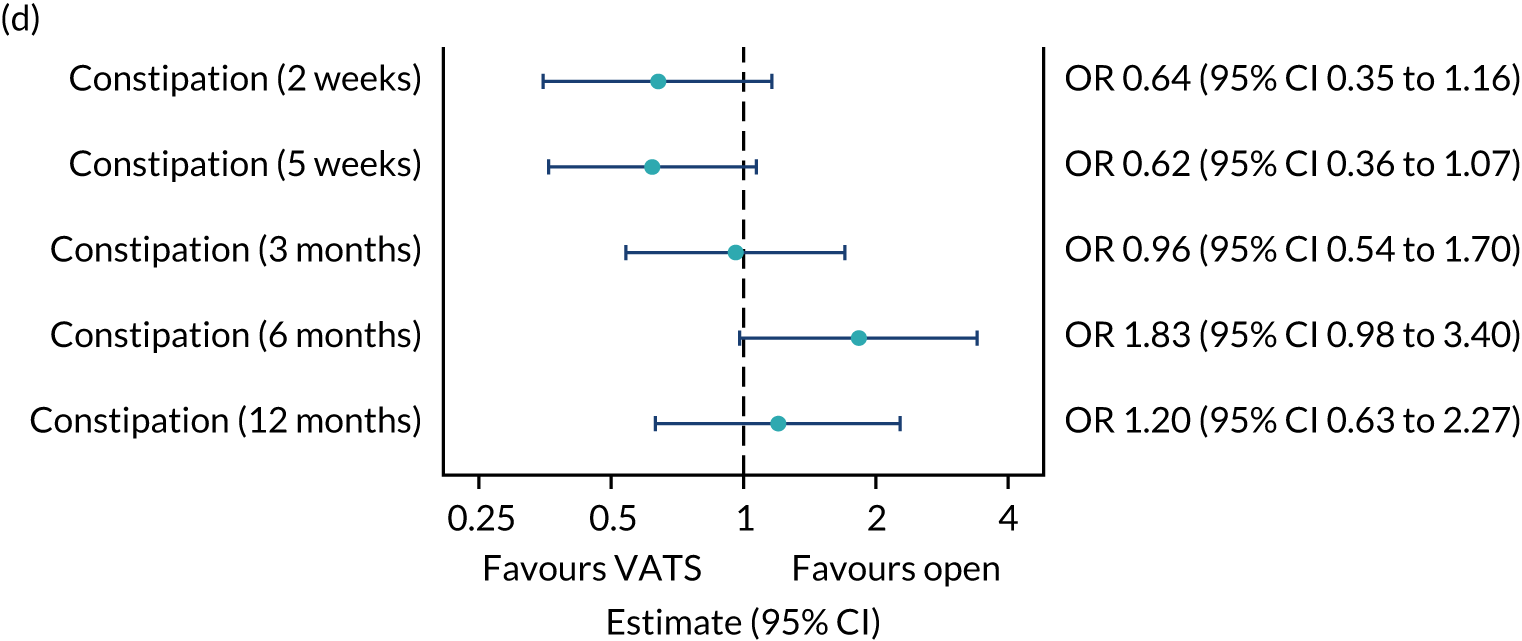
QLQ-C30 exploratory analysis: pain scores
Differences in pain scores to 1 year by type of thoracotomy performed, use of muscle sparing and rib resection are shown in Figure 18. The data show the same trend as was observed in the pain scores in the first 2 days after surgery (see Figure 10), with pain scores in those who had received an anterior thoracotomy being lower, on average, than those who received a posterior thoracotomy, and there being less pain with muscle sparing and significantly more pain with rib resection (MD 9.8, 95% CI 2.07 to 17.52).
FIGURE 18.
Impact of type of thoracotomy on QLQ-C30 pain scores.
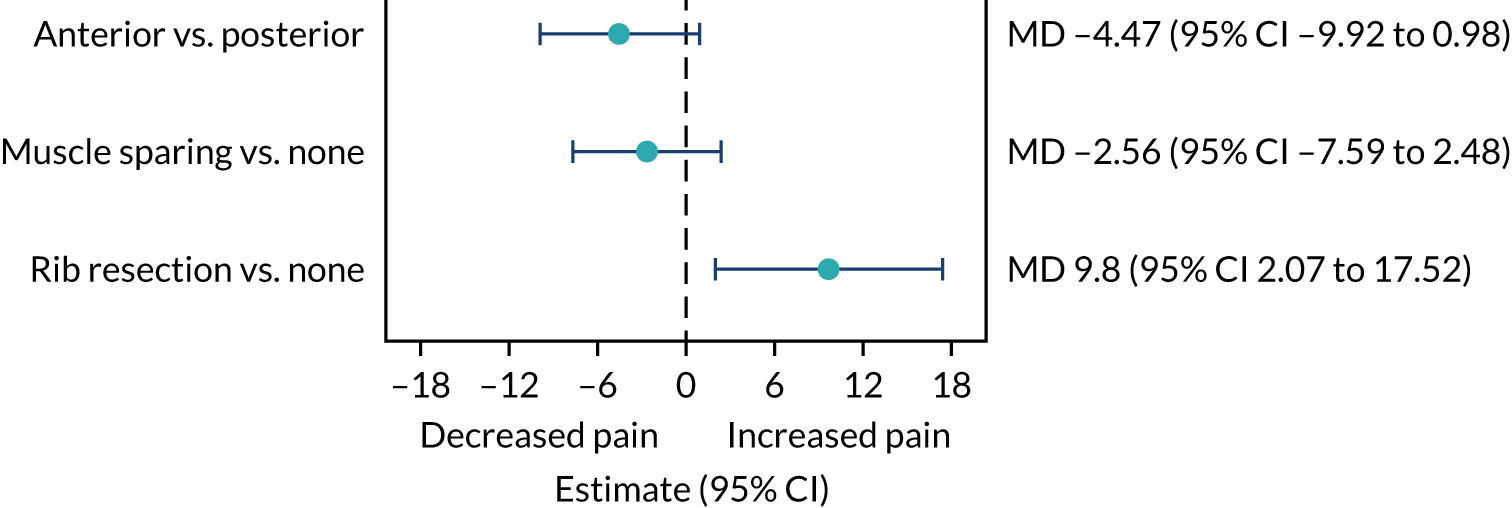
EORTC QLQ-LC13 quality-of-life questionnaire
The QLQ-LC13 measures a range of cancer-related symptoms and problems. As for the QLQ-C30, higher scores indicate a higher level of symptoms or problems. Scores over time for participants randomised to VATS or open surgery are shown in Appendix 5, Figures 44–53. Participants randomised to VATS experienced significantly less pain in the chest and less pain in the arm at 5 weeks than participants randomised to open surgery, but other outcomes were similar in the two groups (Figure 19). Summary data and the estimated treatment effects for each subscale are given in Appendix 5, Tables 62 and 63.
FIGURE 19.
QLQ-LC13 symptoms and problems subscales: treatment effects.
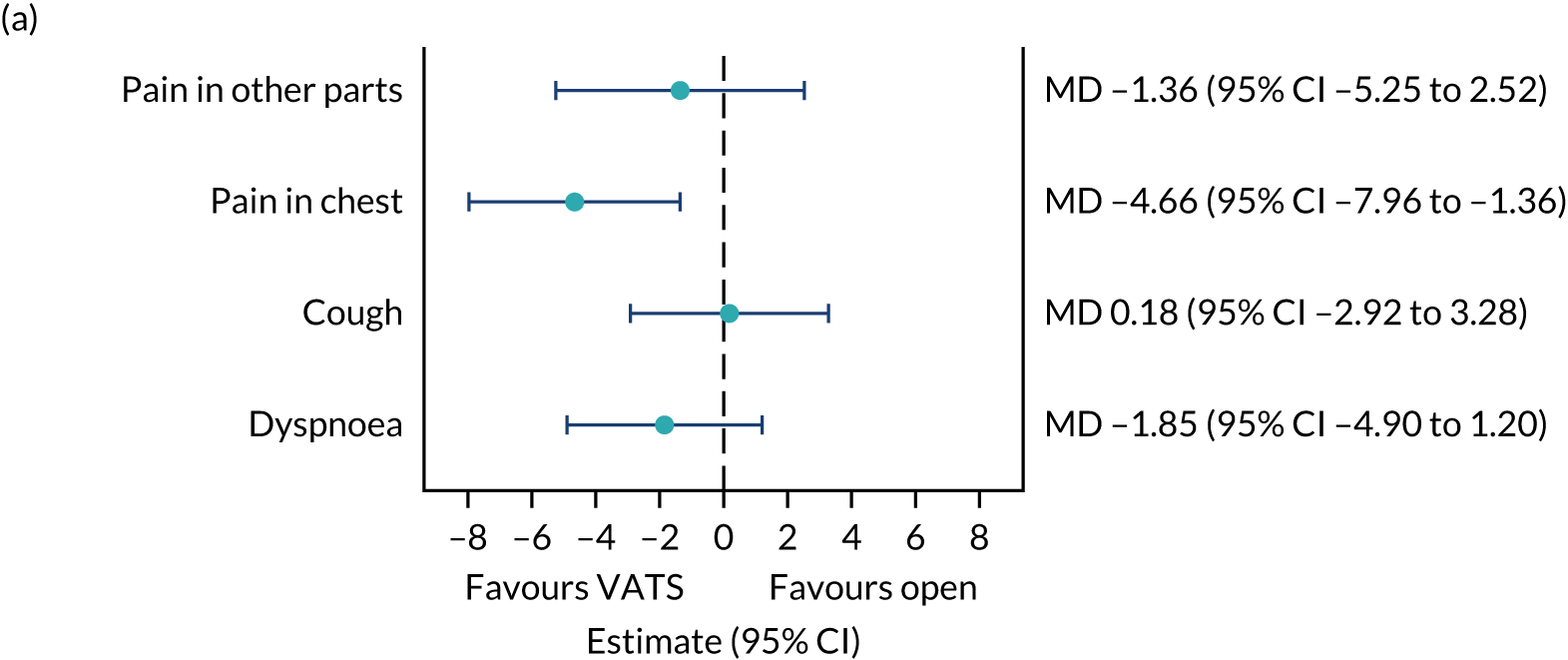
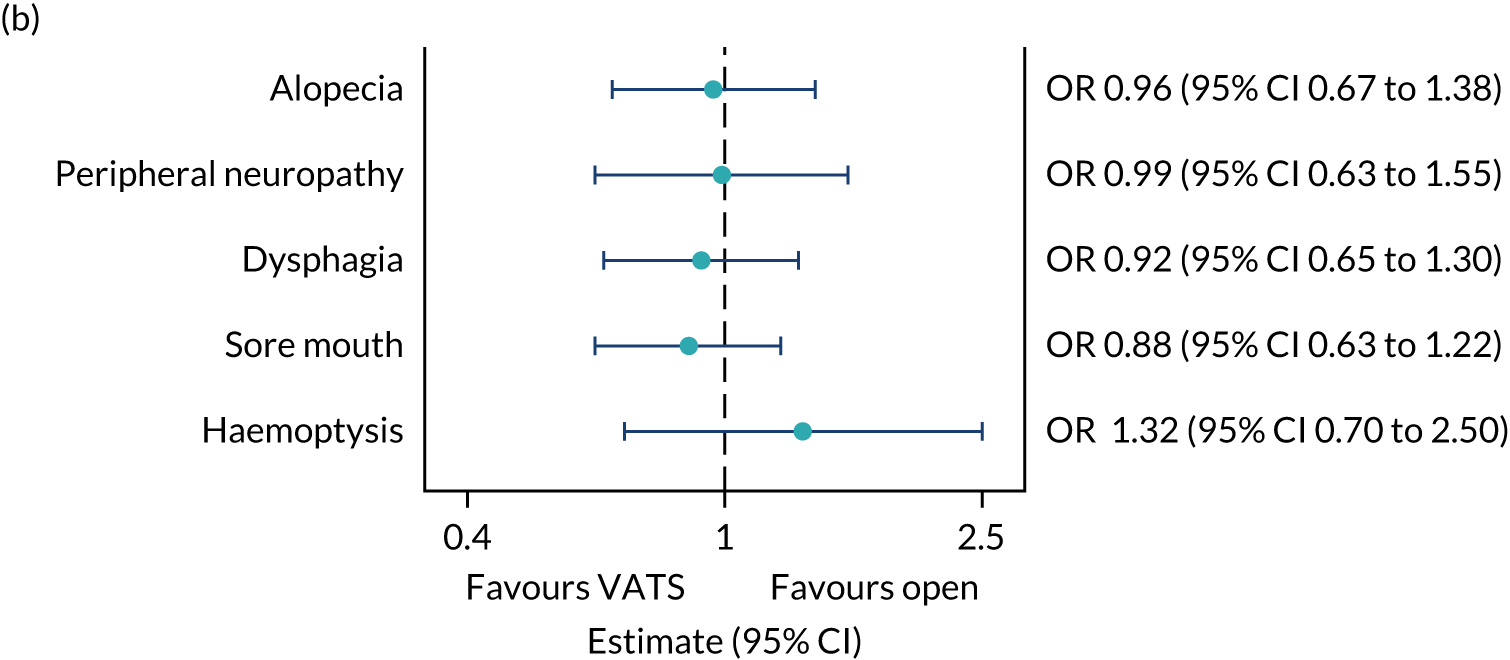
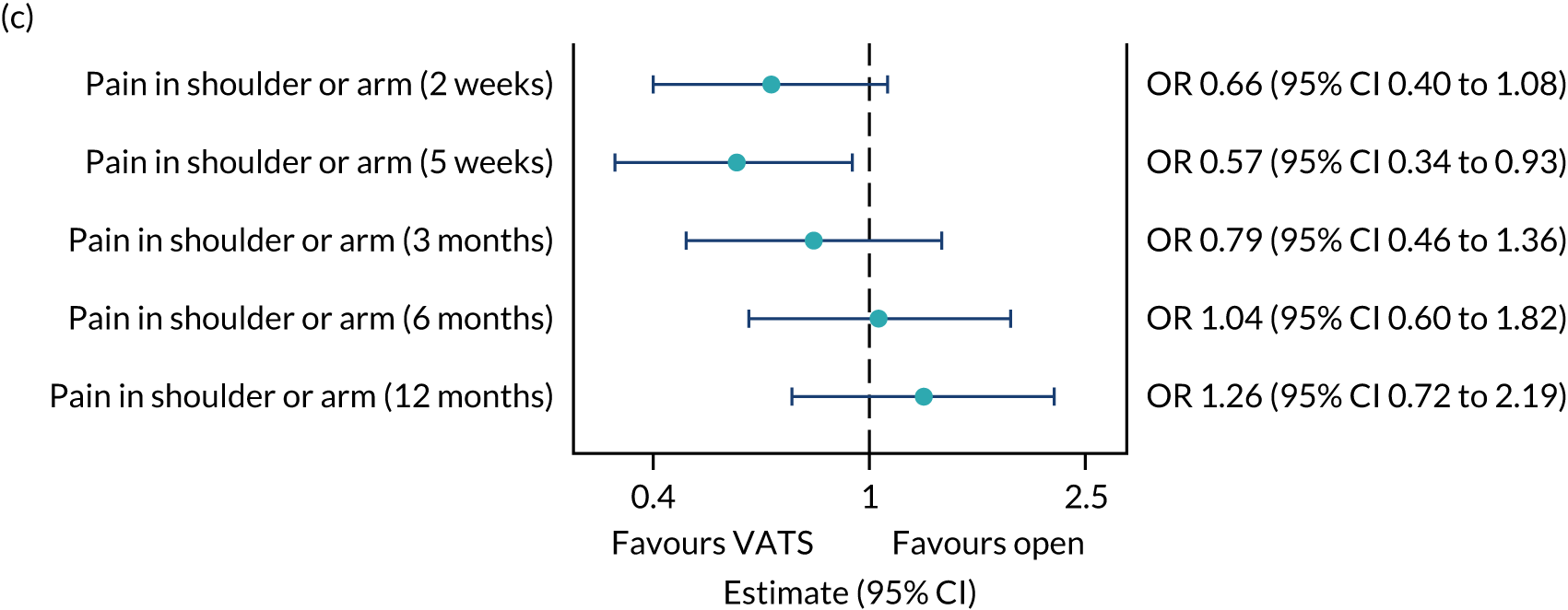
EQ-5D-5L utility score
The EQ-5D-5L utility scores over time are shown in Figure 20. The median utility scores were higher in the VATS group than in the open-surgery group at all post-baseline time points. Participants in the VATS group were less likely to have less than perfect health (i.e. a score < 1) than participants in the open-surgery group [odds ratio (OR) 0.57, 95% CI 0.38 to 0.86; p = 0.007]. Of those participants with less than perfect health, participants in the VATS group had, on average, a higher score (representing better health) than those in the open-surgery group (geometric mean ratio 0.90, 95% CI 0.84 to 0.96; p = 0.003) (see Appendix 5, Table 64, for summary data).
FIGURE 20.
EQ-5D-5L utility scores over time.
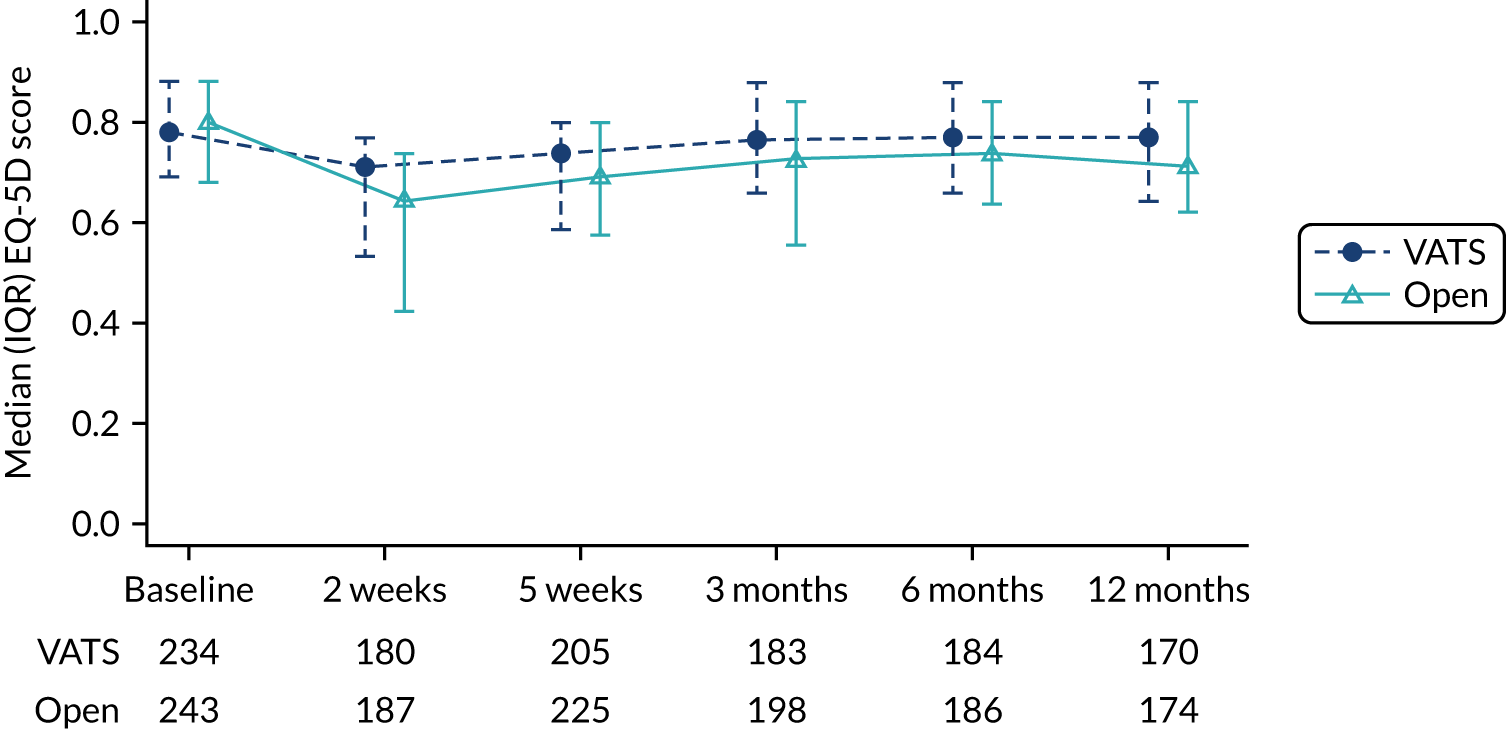
Adverse events
Adverse events in the period from surgery to discharge from hospital following surgery
Eighty-one (32.8%) participants allocated to VATS and 113 (44.3%) participants allocated to open surgery experienced at least one AE in the period from surgery to discharge from hospital (RR 0.74, 95% CI 0.66 to 0.84; p < 0.001), but the number of SAEs was similar in the two groups (8.1% in the VATS group vs. 8.2% in the open group) (Table 21). AE and SAEs summarised by Medical Dictionary for Regulatory Activities (MedDRA) system organ class are presented in Figure 21. Details of the events within each system organ class are given in Appendix 5, Table 65.
| Outcome | Participant allocation | RR (95% CI) | p-value | |
|---|---|---|---|---|
| Randomised to VATS (N = 247), n/N (%) | Randomised to open surgery (N = 255), n/N (%) | |||
| In hospital before discharge | ||||
| Any in-hospital AE | 81/247 (32.8) | 113/255 (44.3) | 0.74 (0.66 to 0.84) | < 0.001 |
| Any in-hospital SAE | 20/247 (8.1) | 21/255 (8.2) | 0.98 (0.59 to 1.63) | 0.948 |
| After discharge following surgery (events/patients) | ||||
| Readmissions | 117/70 (29.0) | 141/88 (35.9) | ||
| SAE | 142/75 (30.7) | 207/94 (37.8) | 0.81 (0.66 to 1.00) | 0.053 |
FIGURE 21.
In-hospital AEs summarised by MedDRA system organ class.
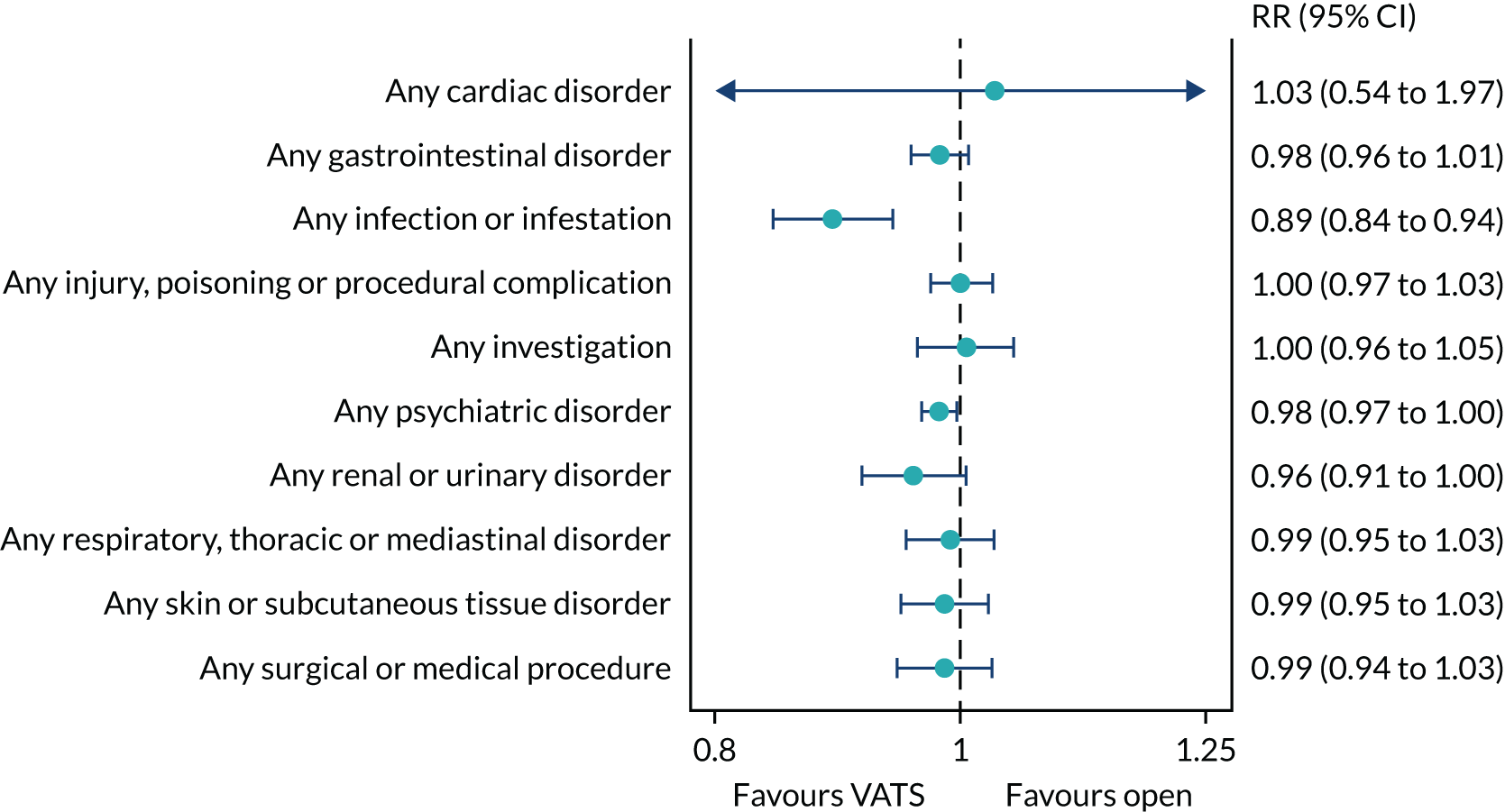
Infective complications were the most common in both groups, the majority of which were pneumonia or lower respiratory tract infections (see Appendix 5, Table 65). Comparing the two groups, participants in the VATS group had fewer infective (RR 0.89, 95% CI 0.84 to 0.94), psychiatric (RR 0.98, 95% CI 0.97 to 1.00) and renal (RR 0.96, 95% CI 0.91 to 1.00) complications than participants in the open-surgery group (see Figure 21). There were seven deaths prior to discharge from hospital (VATS group, n = 2; open-surgery group, n = 5). Causes of death for these seven participants are summarised in Table 18.
Serious adverse events in the period after discharge from hospital to 1 year
Seventy-five (30.7%) participants allocated to VATS and 94 (37.8%) participants allocated to open surgery experienced at least one SAE in the period after discharge from hospital to 1 year (RR 0.81, 95% CI 0.66 to 1.00; p = 0.053), of which 158 (93.5%) resulted in an admission to hospital (VATS group, n = 70; open-surgery group, n = 88) (see Table 21). SAEs summarised by MedDRA system organ class are presented in Figure 22. Details of the events within each system organ class are given in Appendix 5, Table 66.
FIGURE 22.
Post-discharge SAEs summarised by MedDRA system organ class.
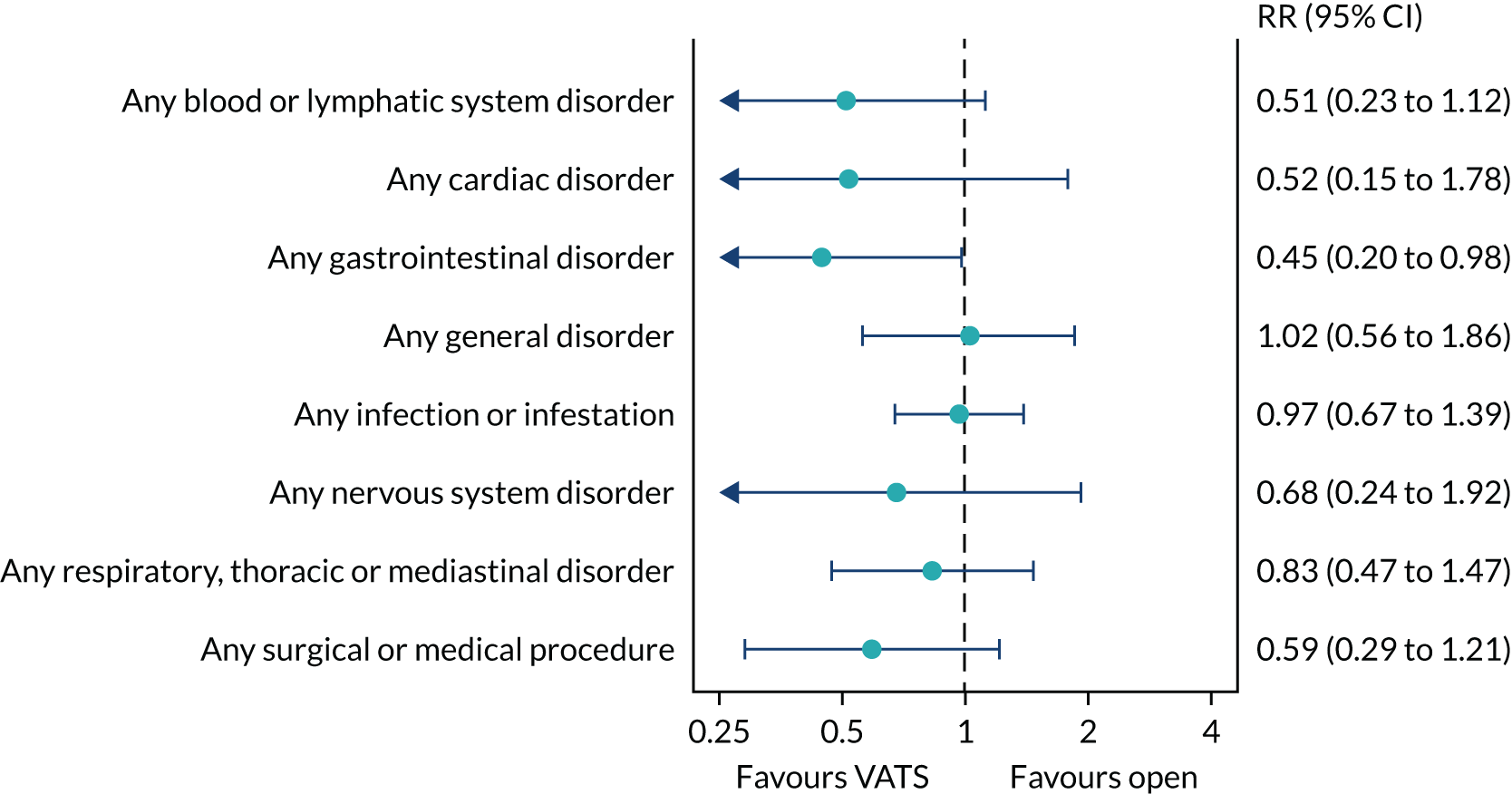
As with the early postoperative period, infective complications were the most common in both groups, the majority of which were pneumonia or lower respiratory tract infections, followed by respiratory, thoracic and mediastinal disorders (see Appendix 5, Table 66). The events that resulted in admission to hospital are summarised in Appendix 5, Table 67. There were 24 deaths after discharge from hospital (VATS group, n = 11; open-surgery group, n = 13). Causes of death for these 24 participants are summarised in Table 18. Half of the deaths (12/24) were due to disease progression.
Chapter 6 Results: economic evaluation
Analysis data set
All randomised participants were included in the health economic evaluation, except one participant who withdrew before surgery and for whom no further data were collected, and 38 participants who were found to have benign disease and who were not intended to be followed up beyond 5 weeks. Therefore, a total of 464 participants (VATS surgery, n = 229; open surgery, n = 235) were included in our analyses.
Missing data
The number of complete data for resource use and outcomes (i.e. individual EQ-5D-5L scores and QALYs) for patients in each trial group is detailed in Appendix 2, Table 26. In summary, 40% of participants in both trial groups had complete resource use data, and 49% overall (VATS, 48%; open surgery, 51%) had complete EQ-5D-5L scores across the six time points. Data collection on information related to staples used in surgery and two complications (pleural effusion and prolonged air leak) began part way through the study and were, therefore, not available for around 50% of participants. Aside from information on staples and these two complications, 460 (99%) participants had complete data for their index admission. Missing data were non-monotonic, as individuals with missing resource use or EQ-5D-5L data at 3 months, for example, may have complete resource use or EQ-5D-5L data at 6 months. Multiple imputation can handle non-monotonic missing data. There were 29 (7%) participants who had died by 12 months. Survival status was unknown for 23 (5%) participants. These participants were assumed to be alive and we examined this assumption in a sensitivity analysis.
When associations between missing total costs and QALY data and key baseline variables (i.e. age, sex, hospital site and treatment group) were assessed, hospital site was found to be a significant predictor of missing costs, and hospital site and age were significant predictors of missing QALYs, at a 5% significance level. This suggests that data were not missing completely at random. Hospital site was a significant predictor of costs and QALYs, and treatment group also predicted costs. Associations were found between missingness and previously observed EQ-5D-5L outcomes, which suggests that missing data are dependent on more than just observed baseline covariates.
These findings support a missing-at-random assumption and, therefore, multiple imputation was appropriate to be used, as this is a flexible approach for handling the missing data. Cost components for the initial index admission (i.e. time in theatre; staples; days in intensive care, high dependency and on a ward; and the complications pleural effusion and prolonged air leak combined) were imputed along with total primary and secondary care costs for hospital discharge to 5 weeks, 5 weeks to 3 months, 3 months to 6 months, and 6 months to 12 months, and the six EQ-5D-5L scores, separately by treatment group. When conducting the multiple imputation, the complete variables, costs of in-hospital complications (excluding pleural effusion and prolonged air leak), SAEs and pathology for frozen section, and an indicator variable for whether or not the patient survived to 12 months were included in regression models, as were the baseline variables of age and hospital site, as missingness may depend on them. Prediction mean matching with 10 nearest neighbours was used (i.e. based on the variables included, the 10 most similar patients were identified and the costs for one randomly selected patient assigned to the patient with missing data). Given that 80% of cases were incomplete (82% and 77% in the VATS and open-surgery arms, respectively), multiple imputation with m = 85 imputations was conducted.
Quality-adjusted life-years
A summary of the mean EQ-5D-5L scores at each of the follow-up time points is shown in Appendix 2, Figure 26, for participants who completed the questionnaire (or who had died by that follow-up time point and were given a score of zero). Although the mean score at baseline was slightly higher in the open-surgery group than in the VATS group at each follow-up time point, thereafter mean scores were higher in the VATS group than in the open-surgery group.
Table 22 reports EQ-5D-5L scores at each of the time points and QALYs for all participants, with missing data imputed. As in Appendix 2, Figure 26, mean EQ-5D-5L scores at baseline were slightly higher in the open-surgery group than in the VATS group, but at all other time points were higher in the VATS group than in the open-surgery group. This results in a greater gain in QALYs in the VATS group than in the open-surgery group, and this difference is statistically significant. Participants in the VATS group enjoy greater combined quantity and quality of life than participants in the open-surgery group.
| Outcome | Participant allocation | VATS vs. open surgery, MD (95% CI) | |
|---|---|---|---|
| Randomised to VATS (N = 229), mean (SE) | Randomised to open surgery (N = 235), mean (SE) | ||
| EQ-5D-5La | |||
| Baseline | 0.746 (0.014) | 0.762 (0.014) | –0.016 (–0.056 to 0.023) |
| 2 weeks | 0.608 (0.018) | 0.544 (0.018) | 0.064 (0.013 to 0.115) |
| 5 weeks | 0.658 (0.017) | 0.619 (0.017) | 0.039 (–0.008 to 0.086) |
| 3 months | 0.721 (0.017) | 0.643 (0.017) | 0.078 (0.031 to 0.126) |
| 6 months | 0.708 (0.018) | 0.672 (0.018) | 0.036 (–0.013 to 0.086) |
| 12 months | 0.693 (0.019) | 0.637 (0.019) | 0.057 (0.004 to 0.109) |
| QALYs to 12 months (adjusted for baseline EQ-5D-5L) | 0.841 (0.017) | 0.780 (0.016) | 0.060 (0.025 to 0.095) |
Resource use and costs
Table 23 reports information on the main resource use items for the trial groups to 12 months. In the index admission, this includes time in theatre and the number of staples used in surgery, length of hospital stay by ward type and complications. Post hospital discharge, this includes hospital readmissions and other primary and secondary care resource use over the 12 months’ follow-up, including hospital visits for chemotherapy and radiotherapy.
| Resource use | Participant allocation | VATS vs. open surgery, MD (95% CI) | |||
|---|---|---|---|---|---|
| Randomised to VATS (N = 229) | Randomised to open surgery (N = 235) | ||||
| n (%) | Mean (SD) | n (%) | Mean (SD) | ||
| Surgery/ward stays | |||||
| Time in theatre (hours) | 228 (100) | 2.7 (0.9) | 235 (100) | 2.3 (0.8) | 0.3 (0.2 to` 0.5) |
| Number of staples | 120 (52) | 8.1 (3.6) | 129 (55) | 7.6 (3.4) | 0.5 (–0.4 to 1.4) |
| Intensive care unit stay (days)a | 227 (99) | 0.5 (3.0) | 234 (100) | 1.0 (5.9) | –0.4 (–1.3 to 0.4) |
| High-dependency unit stay (days) | 227 (99) | 1.0 (1.4) | 234 (100) | 1.4 (3.5) | –0.4 (–0.9 to 0.1) |
| Ward stay (days)a | 227 (99) | 4.1 (5.3) | 234 (100) | 4.8 (4.1) | –0.7 (–1.6 to 0.2) |
| Total stay (days)a | 227 (99) | 5.6 (6.7) | 234 (100) | 7.2 (9.4) | –1.5 (–3.0 to –0.0) |
| Selected complicationsd | |||||
| Pulmonary collapse | 229 (100) | 10 (4)b | 235 (100) | 8 (3)b | 1c |
| Surgical emphysema | 229 (100) | 9 (4)b | 235 (100) | 13 (6)b | –4c |
| Bronchoscopy | 229 (100) | 11 (5)b | 235 (100) | 9 (4)b | 2c |
| Infection | 229 (100) | 38 (17)b | 235 (100) | 69 (29)b | –12c |
| Acute psychosis | 229 (100) | 6 (3)b | 235 (100) | 12 (5)b | –2c |
| Reoperation | 229 (100) | 3 (1)b | 235 (100) | 6 (3)b | –2c |
| Post hospital discharge | |||||
| Further inpatient days | 187 (82) | 2.4 (6.4) | 192 (82) | 4.2 (12.1) | –1.8 (–3.7 to 0.2) |
| Hospital visits | 188 (82) | 7.7 (7.6) | 190 (81) | 8.6 (7.6) | –0.9 (–2.4 to 0.6) |
| Community visits | 187 (82) | 7.2 (6.2) | 191 (81) | 7.3 (6.3) | –0.1 (–1.4 to 1.1) |
Surgery took, on average, 2.7 and 2.3 hours for participants in the VATS and open-surgery groups, respectively. Participants in the VATS group spent, on average, 19 minutes longer in theatre than those in the open-surgery group (MD in hours 0.3, 95% CI 0.2 to 0.5 hours). A mean of eight staples was used in each arm of the trial, although use was slightly less in the open-surgery group than in the VATS group.
Participants spent a mean of 5.6 and 7.2 days in hospital after surgery in the VATS and open-surgery groups, respectively. Participants in the VATS group spent less time in intensive care, in high dependency and on a ward than participants in the open-surgery group. The overall difference in length of stay was statistically significantly lower in the VATS group than in the open-surgery group. Only a few selected complications are shown here (those with high associated costs and those most frequently occurring), but overall complications were lower in the VATS group than in the open-surgery group. Resource use post hospital discharge follows a similar pattern. The mean number of days readmitted to hospital, and the number of hospital and community visits, are lower in the VATS group than in the open-surgery group.
A breakdown of total costs for all participants is provided in Figure 23 (with missing data imputed). The key cost drivers are surgery, time in critical care and on a ward, and costs post discharge. Costs are clearly lower in the VATS group than in the open-surgery group. Greater detail on these costs is provided in Appendix 2, Table 27.
FIGURE 23.
Total costs to 12 months for all participants.
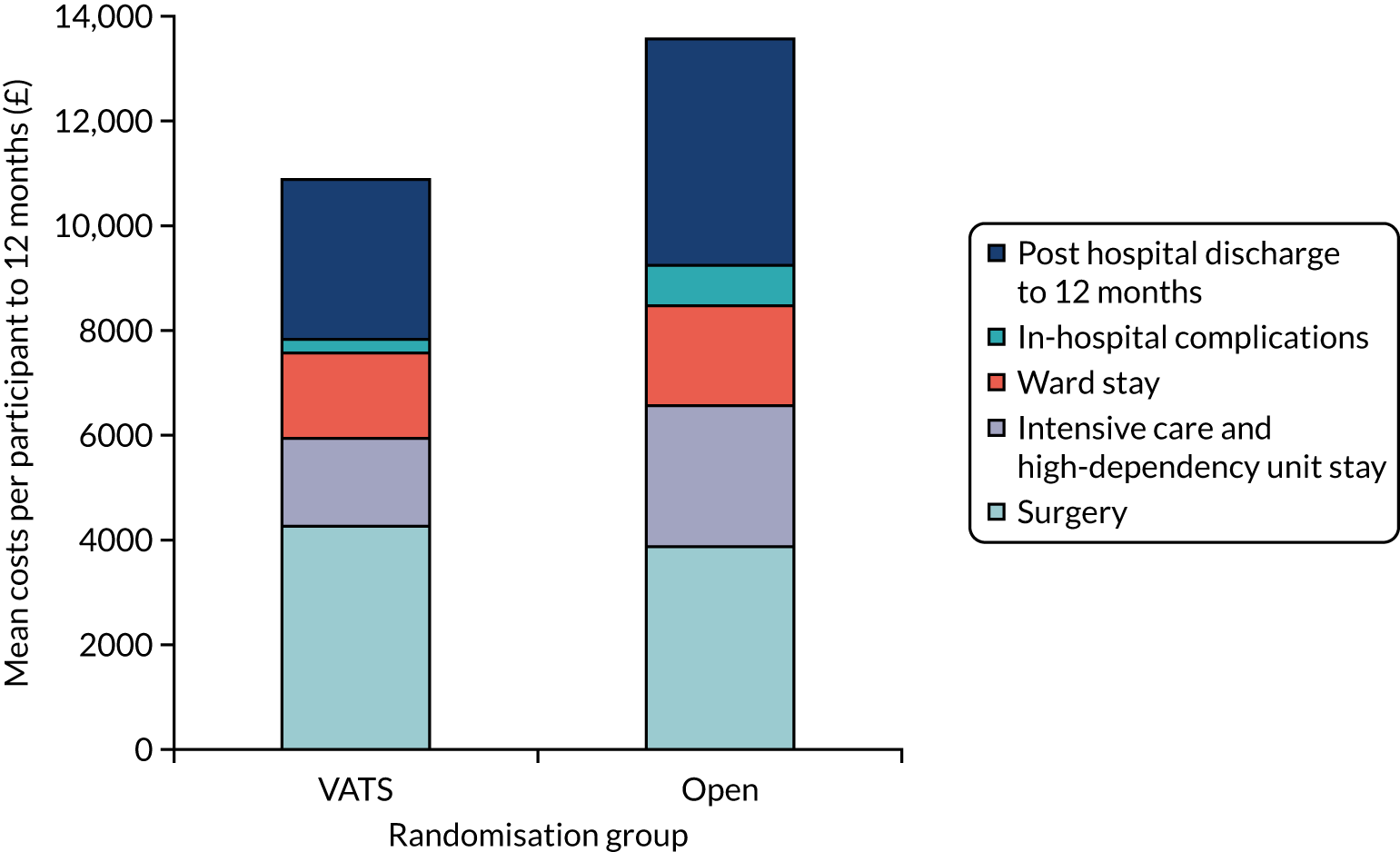
Although costs associated with time in theatre are statistically significantly higher in the VATS group than in the open-surgery group, costs associated with length of stay are statistically significantly lower, and these cost savings more than outweigh the higher costs associated with surgery. Costs post hospital discharge are also statistically significantly lower in the VATS group than in the open-surgery group, driven by smaller numbers of days readmitted to hospital and fewer hospital visits. Discharge to 5-week costs do not follow this trend because of a couple of high-cost outliers for this time point in the VATS group. Total costs to 12 months are, on average, £10,879 in the VATS group and £13,581 in the open-surgery group (MD –£2702, 95% CI –£5624 to £221).
Base-case cost-effectiveness results
Table 24 combines the cost and outcome results and presents the cost-effectiveness. The difference in costs favours the VATS group and is close to statistical significance. The difference in QALYs favours the VATS group and is statistically significant. Based on the point estimates of the cost and QALY differences and on the point estimate of the ICER (–£44,908), VATS is considered cost-effective. VATS surgery is dominant over open surgery, as it is both more effective (more QALYs) and less costly. However, it is important to consider the uncertainty around this result. Figure 24 shows the cost-effectiveness plane, with the bootstrap replicates of the cost and QALY differences. The black dot is the point estimate of the cost and QALY difference. Virtually all the bootstrap replicates are in the south-east quadrant where costs are lower and QALYs are higher in the VATS group, indicating that we can be very certain that VATS is cost-effective.
| Cost-effectiveness element | Participant allocation | VATS vs. open surgery, MD (95% CI)a | |
|---|---|---|---|
| Randomised to VATS (N = 229), mean (95% CI)a | Randomised to open surgery (N = 235), mean (95% CI)a | ||
| Total costs (£) | 10,879 (10,021 to 11,738) | 13,581 (10,793 to 16,369) | –2702 (–5632 to 228) |
| QALYs | 0.841 (0.811 to 0.870) | 0.780 (0.746 to 0.815) | 0.060 (0.029 to 0.092) |
| ICER (£) (cost/QALY) | VATS dominant (–£44,908) | ||
FIGURE 24.
Cost-effectiveness plane. For points to be seen, a random sample of 1000 of the 5100 bootstrap replicates were plotted.
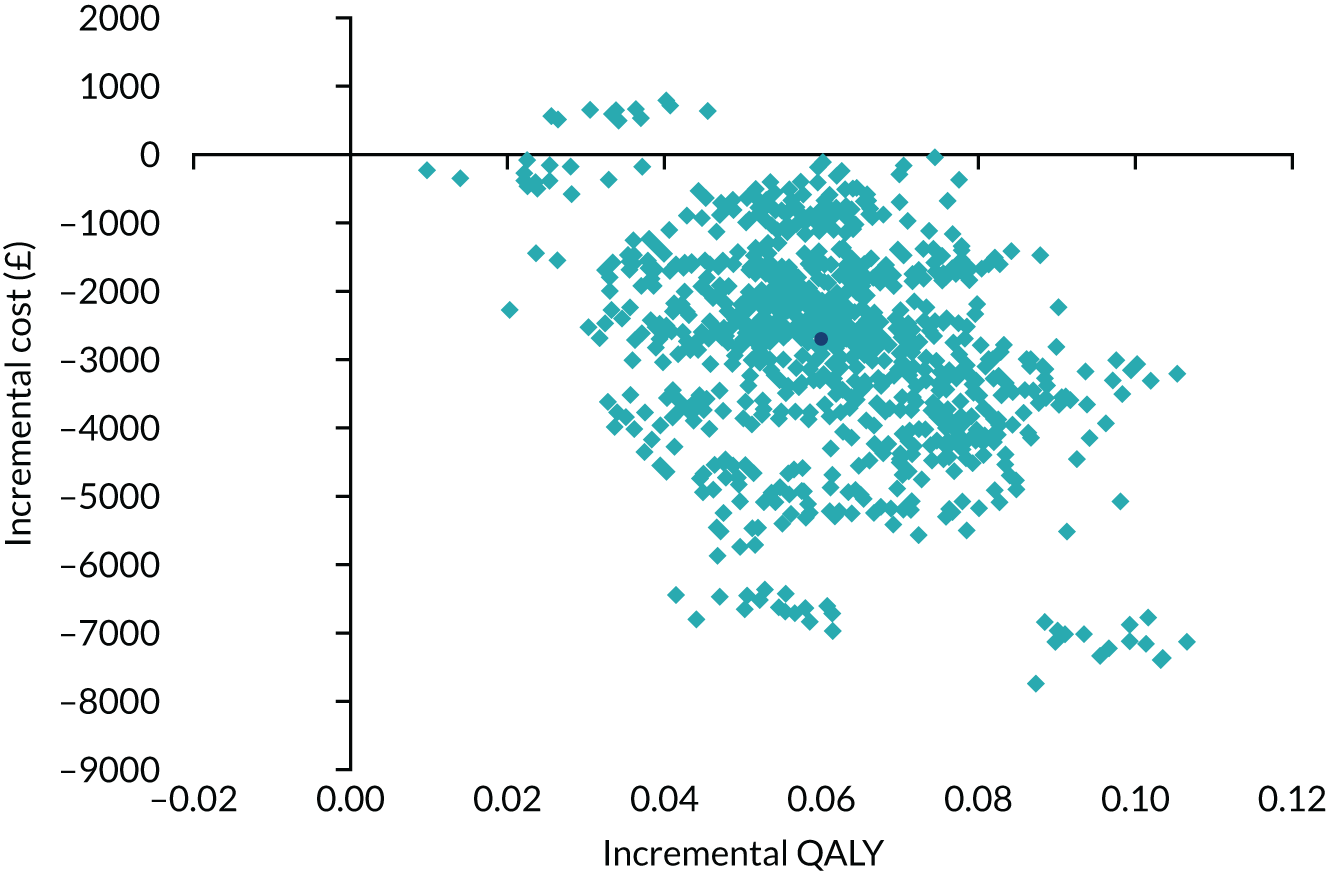
The CEAC in Figure 25 shows the probability that VATS surgery is cost-effective for a range of willingness-to-pay thresholds. Even at a willingness-to-pay threshold of £0, the probability that VATS is cost-effective is 0.98 (equivalent to the probability that VATS is less costly than open surgery). At a willingness-to-pay threshold of £20,000 per QALY, which is generally considered as the threshold that NICE adopts for considering an intervention to be cost-effective, the probability that VATS surgery is cost-effective is 1 (0.996). Indeed, at any willingness-to-pay threshold, VATS surgery is considered cost-effective and there is negligible uncertainty around this finding.
FIGURE 25.
Cost-effectiveness acceptability curve.
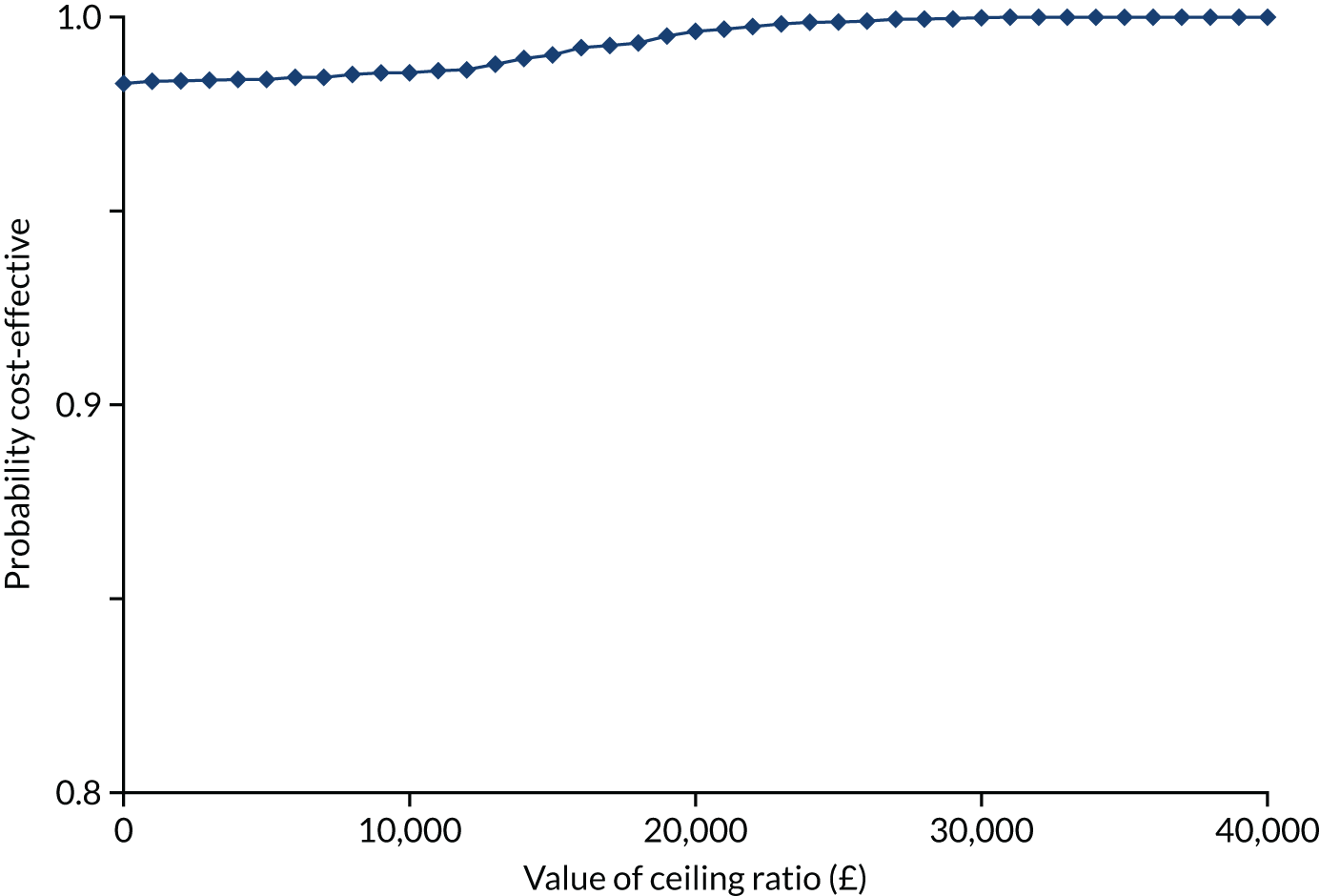
Sensitivity analyses
The results of the sensitivity analyses conducted around costs and outcomes are provided in full in Appendix 4, Tables 40–43, and key findings are summarised here. None of the sensitivity analyses varying unit costs had a great impact on the cost difference between the groups. Several high-cost participants, who exert a significant impact on the cost results but do not alter conclusions, were identified.
Sensitivity analyses around outcomes (assuming patients with missing survival data died at 12 months, and no adjustment for baseline utility) did not impact on the differences between the groups, nor on the cost-effectiveness conclusions.
Summary
There were differences in costs and QALYs in favour of the VATS group and, when combined, the VATS group was clearly cost-effective. The mean QALYs to 12 months were 0.841 and 0.780 in the VATS and open-surgery groups, respectively, resulting in a statistically significant MD of 0.060 (95% CI 0.029 to 0.092). The total costs of care from surgery to 12 months were £10,879 in the VATS group and £13,581 in the open-surgery group, creating a MD of –£2702 (95% CI –£5632 to £228). When combined, the cost-effectiveness results clearly indicated that VATS is cost-effective across all willingness-to-pay thresholds we examined. Results were robust to all the sensitivity analyses performed.
Chapter 7 Discussion
Main findings
Trial conduct
With the support of the QRI to optimise recruitment, the trial successfully recruited to time and target. Average recruitment rates at study sites ranged from 0.5 participants randomised per month in Hull to 3.4 participants randomised per month in Bristol. The lead study site (Brompton) was the second highest recruiting site, with an average of 2.5 participants randomised per month.
Recruitment was paused in Bristol for 3 months in early 2016 (i.e. 5 January 2016 to 29 March 2016) because of issues in the postoperative recruitment pathway that were unblinding the researcher responsible for data collection, which had the potential to bias the cost-effectiveness analysis. During late 2015, it became apparent that the Bristol research team were experiencing difficulties integrating the VIOLET trial into their local practice. These difficulties stemmed from historical hospital guidelines that specified that patients undergoing thoracotomy should recover in a high-dependency unit, whereas patients undergoing VATS should go to a general ward for their recovery. Recruitment was paused while the Bristol PI and colleagues liaised with the policy-makers to encourage the hospital to adopt a more patient-focused, risk-based approach to patient management. These discussions were protracted, but, on 29 March 2016, the hospital agreed to change the guidelines and adopt a risk-based approach to patient management, allowing recruitment to restart immediately. Following this, recruitment at the Bristol site progressed well. Bristol was the highest recruiting site in the VIOLET trial, with 136 participants.
The study sites were engaged with the trial throughout and data completion rates were good. There was one change that related to the transition to TNM813 of the TNM staging system, which had a significant impact on study sites and the trials centre. Following approval of an amendment to the protocol to reflect this change in June 2017, the screening log and CRFs were revised to collect the clinical staging in accordance with TNM8. 13 Subsequent to this, the trials centre became aware that TMN813 was not mandated for clinical use until January 2018, and that at least one study site was still using TMN7,14 which meant that there was not a clear transition from TMN714 to TNM813 in line with the change to the CRF. In addition, there was not a clear mapping from TNM714 to TNM8. 13 Following discussion at a study investigator meeting, it was agreed that all participants recruited before January 2018 would be restaged using TNM813 to ensure that we had consistent data for the full trial. A ‘restaging’ CRF and associated page in the study database were created to facilitate this data collection exercise.
During a regular review of the study data, it also became apparent that the CRF for collecting data on ‘ward movements’ (i.e. length of stay in the intensive care unit/high-dependency unit/ward) was being misinterpreted by some sites. The CRF was redesigned to maximise the accuracy of the data and sites were asked to reconfirm the data submitted for participants recruited before the change. To minimise the burden for study sites, this was carried out at the same time the participant staging was reviewed.
QRI
We applied a range of QRI methods to optimise recruitment and informed consent in the VIOLET trial, focusing on prevention of recruitment challenges and the identification and resolution of potential recruitment issues. Recruitment to the VIOLET trial commenced and progressed well, completing to target and on time. Although examples of good practice were seen (e.g. expressing uncertainty and avoiding the term ‘trial’ in favour of the term ‘study’), the QRI also identified and addressed a number of recruitment challenges (e.g. recruiters recommending a treatment, using imbalanced/loaded terminology to explain the two operations and explaining the study apologetically). QRI actions included upfront training, followed by trial-specific group and individual feedback sessions, tips documents and a rapid communication strategy. These actions were aimed at helping recruiters present the study to all eligible patients, balance information provision on the two operations, provide clearer explanations of randomisation and blinding, and avoid loaded terminology, treatment recommendations or assumptions regarding patients’ views on the trial.
The recruitment issues reported in the VIOLET trial have been previously identified in other RCTs with QRIs. 25–27,30,33 However, the VIOLET trial recruited above target from the very beginning and throughout the recruitment period. Although it is difficult to prove cause and effect, it is plausible that the QRI training contributed towards the good start to recruitment. It is also plausible that the QRI training sustained the momentum thereafter, including when new centres joined the study, and helped recruitment pick up when it dipped towards the target line in the last year of recruitment. An observational study58 that evaluated five QRIs showed that randomisation improved in three of the five RCTs in the post-intervention period. The two RCTs that showed no difference in recruitment after the QRI were similar to the VIOLET trial in that it had completed recruitment on target following training and support before recruitment began and then throughout the recruitment period. 58
It is important to note, however, that the prior training and support received by recruiters did not resolve all challenges in advance of recruitment, with many being identified and addressed later with the help of the QRI, which contributed towards sustaining the recruitment momentum gained early on. The most crucial aspect of QRI evaluation is the change in recruitment practice observed qualitatively. Despite some healthy recruitment practices, there was scope for improvement, and we observed noticeable changes in how recruiters addressed patient preferences, expressed uncertainty or explained the trial following VIOLET trial-specific training.
Trial results
The results of the VIOLET trial suggest that for patients with early-stage lung cancer a VATS approach to lobectomy was associated with less pain, fewer complications and a shorter length of hospital stay, without any compromise to oncologic outcome. The benefits of a VATS approach extended beyond the in-hospital period. The primary outcome of physical function was significantly improved at the 5-week point and improved recovery was consistent for all secondary measures of quality-of-life outcomes up to 1 year. In addition, there were fewer post-discharge readmissions for care and no difference in the measures of oncologic outcome of recurrence-free and overall survival up to 1 year.
Prior to the conduct of the VIOLET trial, the benefit of VATS lobectomy was widely considered to be ‘better recovery’ (i.e. a readily understandable but nebulous term with multifactorial and time-dependent components). When challenged to consider one single most relevant outcome that encompasses all the potential benefits of better ‘recovery’ at a single most relevant time point, physical function at 5 weeks was chosen by our patient and public involvement (PPI) group and unanimously agreed by the Trial Management Committee. With this global composite measure, our results revealed a striking 13-point improvement between the observed median scores in favour of VATS at 2 weeks, which reduced to a modelled difference of approximately 5-point difference at 5 weeks. It took a further 6 months for participants in the open-surgery group to reach similar levels of physical function as the participants in the VATS group. The results shed light on the duration of functional recovery provided by a VATS approach, as a 9-point difference has been proposed to correspond to a 1-point difference in performance status. 16 On a World Health Organization performance scale, 1 unit of change would correspond to a difference from performance status 0 (i.e. ‘able to carry out all normal activity without restriction’) to performance status 1 (i.e. ‘restricted in strenuous activity but ambulatory and able to carry out light work’). Our earliest assessment was undertaken at 2 weeks and, therefore, we may not have captured the full effects, which we now know to be most prominent within the first 2 weeks.
Pain is a near universal trade-off for surgery and one of the most important considerations for improvement. The open-surgery approach requires rib spreaders (metal retractors) to splay the ribs apart. The result is inevitable compression of the intercostal nerves and is considered the main source of pain after open lobectomy. Results from a Danish trial9 reported a lower proportion of patients randomised to VATS with severe pain (38% vs. 63%), but the trial did not report a direct comparison of pain scores between the two groups. 9 On a linear scale, we noted similar pain scores on day 1 and less pain (by 1 point in the VAS) on day 2. The lack of difference on day 1 can be attributed to the effective local anaesthetic blocks that were administered (often taken down on days 1 or 2 to allow patients to mobilise). Having adjusted for total analgesic use, patients randomised to VATS had approximately 10% lower composite analgesic use, with an adjusted estimate of a half-point lower pain score, surmising that a VATS approach is (modestly) less painful. We analysed the different forms of local anaesthetic block (i.e. paravertebral and intercostal block), subtypes of thoracotomy (i.e. posterior and anterior), use of rib resection and number of port sites, and found no evidence of interaction with the surgical approach (similarly effective). After discharge, however, we noted that prolonged incision pain, defined as the need for analgesia after 5 weeks, was lower in the VATS group than in the open-surgery group (59.6% vs. 72.3%), suggesting better recovery with VATS when measured by the cessation for the need of analgesics (at 5 weeks). Pain scores using QLQ-C30 and QLQ-LC13 were consistent in the direction of the estimates in favour of VATS out to 1 year. In addition, we noted that patients who underwent rib resection as part of a thoracotomy experienced significantly more pain, with a 9.8-point difference out to 1 year (an observation that has not been previously described).
Another important consideration when evaluating a new procedure is a demonstration of safety (i.e. if the new procedure can be performed without increasing harm). When we observed the in-hospital AEs, we noted that the RR of harm was in fact lower with VATS (i.e. a 26% reduction in AEs with no difference in SAEs). One specific complication that was noted was that the proportion of patients experiencing intraoperative bleeding (often attributed to blood vessel injury when using a keyhole approach) was similar in the two groups (VATS group, 6.2%; open-surgery group, 3.9%). The main benefits for VATS during the in-hospital phase was a notable reduction in kidney and infective complications. There are a number of hypotheses to be offered for this, including less analgesic use, which may reduce renal complications and improve mobility for lower chest infections, and smaller incisions, resulting in fewer wound infections.
The benefits of a less painful and safer operation culminated in a shorter length of hospital stay, an additional global outcome for ‘better recovery’. The results were consistent in both fitness for hospital discharge (based on discharge criteria) and actual measured time to discharge in favour of VATS by 1 day. The benefits of a better recovery persisted in the year after discharge, with benefits from global scales (e.g. QLQ-C30 and EQ-5D-5L) and individual scales (e.g. dyspnoea, fatigue, appetite loss) broadly consistent in direction in favour of VATS or no difference between the two groups. There was no single measure that was consistently, or was of a clinically important magnitude, in favour of open surgery. AEs after discharge continued in favour of VATS, with fewer readmissions (29.0% vs. 35.9%) and a 19% reduction in the number of SAEs (p = 0.053) up to 1 year.
Perhaps the most important contribution of the VIOLET trial is the study of oncologic outcomes, which, to date, and to the best of our knowledge, has not been comprehensively reported in any RCT. One concern for keyhole surgery has been the ability to perform a cancer operation without the surgeon’s hands entering the chest through small incisions (without direct tactile feedback), using a video camera and television monitor (without direct visualisation) through fixed positions in the skin (without a full range of movement of the instruments). We assessed the quality of the lymph node harvesting both in terms of the number and position of the nodes assessed, and found no difference in between the two groups (five stations harvested and three mediastinal stations for both arms), indicating that the VATS techniques and instruments used were able to effectively access the same extent of lymph node harvesting. Lymph node upstaging is often considered to be a more discriminating assessment of quality of lymph node dissection, with the premise that a more thorough dissection would yield more positive lymph nodes (upstaging). When we assessed lymph node upstaging, we found 6.2% in the VATS group compared with 5.2% in the open-surgery group (cN0 to pN1) and 6.2% in the VATS group compared with 4.8% in the open-surgery group (cN0/1 to pN2). In addition, there was no difference in the ability to achieve complete pathologic resection (97.7% vs. 97.8%) between the VATS and open-group groups, respectively, confirming that there was no difference in the completeness of the cancer operation. After discharge, the uptake of chemotherapy was similar in both groups, at 50.9% in the VATS group and 45.9% in the open-surgery group, indicating that the improvement in time to recovery with VATS did not translate to an important difference in uptake of adjuvant chemotherapy. After discharge to 1 year, the HR for disease-free survival and overall survival was 0.67 (p = 0.366) and 0.74 (p = 0.312), respectively, in favour of VATS, giving assurance on the longer-term oncologic safety of a VATS approach. Our trial was not powered for long-term survival and this is an important area for further research clarification, as systematic review of (mainly) non-randomised studies indicated the possibility of a VATS approach leading to better overall survival. 4 Given that the main oncologic outcomes are similar in both groups in the VIOLET trial, we hypothesise any difference in survival to be more likely associated with secondary improvement in quality of life, rather than technical oncologic surgery.
Health economic evaluation
In the economic evaluation, differences in costs and QALYs favoured the VATS group, and when combined VATS proved to be a cost-effective option for the NHS. The mean QALYs to 12 months were 0.841 and 0.780 in the VATS and open-surgery groups, respectively, resulting in a statistically significant MD of 0.060. The mean total cost from surgery to 12 months was £10,879 in the VATS group and £13,581 in the open-surgery group (i.e. a MD of –£2702, although this was not statistically significant). This cost difference is largely driven by less time in critical care in the index admission and fewer days readmitted to hospital in the VATS group. Results were robust to all the sensitivity analyses performed.
The cost-effectiveness results clearly indicate that VATS is cost-effective across any willingness-to-pay threshold. Based on the point estimates of the cost and QALY differences and on the point estimate of the ICER (–£44,908), VATS is considered cost-effective. VATS surgery is dominant over open surgery as it is both more effective and less costly. The probability that VATS is cost-effective at a willingness-to-pay threshold of £20,000 per QALY, which is generally considered as the threshold that NICE adopts for considering an intervention to be cost-effective, is 1. Indeed, at any willingness-to-pay threshold, VATS surgery is considered cost-effective, and there is negligible uncertainty around this finding so we can be confident of this result.
Our cost-effectiveness results are consistent with the Danish study,11 in which 103 patients were randomised to VATS and 103 patients were randomised to thoracotomy, which concluded that VATS was cost-effective compared with thoracotomy following lobectomy for stage 1 lung cancer. VATS provided a larger number of QALYs and generated lower costs. Specifically, the mean cost per patient for VATS was 103,108 kr (£11,857) and the mean cost per patient for thoracotomy was 134,945 kr (£15,518), making the costs for VATS 31,837 kr (£3661) lower than thoracotomy (p < 0.001). In terms of HRQoL, the difference between the two surgery types for QALYs gained over 1 year of follow-up was 0.021 (p = 0.048). The CEAC presented clearly showed that VATS was superior to thoracotomy more or less regardless of the willingness-to-pay threshold for a QALY, just as was shown here. Results of a French study by Pagès et al. 12 were not available at the time that our report was written.
Patient and public involvement
The Royal Brompton Hospital Cancer Consortia PPI group was fully engaged in the initial trial design and during the set-up phase of the trial. The PPI group comprised four patients who had undergone surgery for cancer and one carer. The PPI group advised the study team on trial design and identification of the choice and timing of the primary outcome, and secondary outcomes that were considered to be important. The PPI group was consulted between August 2012 and September 2013. One member of the PPI group remained a member of the TSC throughout the trial.
The PPI group was also consulted before the study commenced in 2014. The group was asked for its feedback on the patient documents, and it advised that the PIL should be shortened and suggested edits on how to do this. The PPI group also encouraged the use of a flow chart to show how the main study and information study by the QRI interact. All of the PPI group’s feedback was incorporated into the trial documents and the group’s feedback was invaluable in producing a clear and understandable PIL for patients.
We consulted Chris Hall (the TSC PPI member) for feedback on the Plain English summary of this report, who commented that it was presented simply, was comprehensive and clearly worded. Furthermore, Chris Hall provided further comments about his involvement in the study:
I felt I was an integral part of the team whose opinions were respected, and I was fully able to contribute to the study and protocol as it applied to patients. Meeting face to face with the authors and participants enabled me to understand the many problems involved. The conclusion of the study gives an opportunity no doubt for publicity and perhaps provision of a further patient information leaflet.
We also plan to consult the PPI group for advice on the dissemination of the research and its findings to the public.
Strengths and limitations
Strengths
Trial conduct
One of the strengths of the VIOLET trial was the ability to minimise by surgeon to ensure that the same surgeon would perform approximately equal numbers of VATS and open-surgery operations. This is an important consideration to negate surgeon effect, as VATS surgeons are usually considered to be better skilled and more forward-thinking regarding postoperative patient management. The ability to successfully mask the procedure (using large dressings) to patients and researchers in the early postoperative phase allowed a more unbiased assessment of outcome. An important secondary strength of the VIOLET trial was the ability to train research-active thoracic surgeons in the communication skills required to successfully randomise patients into a clinical trial. Strong camaraderie was also instilled among the participating surgeons and trial centres. We achieved worldwide acknowledgement and accolade, earning the UK a newfound reputation regarding the ability to conduct and deliver thoracic surgery RCTs.
QRI
Qualitative research methods and its applied nature are the main strengths of the QRI. A key strength of the QRI in the format that it was applied in the VIOLET trial is that it adopted a multifaceted approach to optimising recruitment and began by aiming to prevent recruitment challenges with upfront training, followed by investigations and actions to identify and address new challenges. This holistic approach to optimising recruitment, especially the use of a preventative component, needs to be further refined and formalised in future QRIs.
Limitations
Trial participants
Although there was good coverage across England in terms of the study sites and there was a site in Scotland, the study did not recruit from Wales or Northern Ireland. At the last census, in 2011, 14% of the population of England and Wales59 were classified as non-white (i.e. from an ethnic minority group); however, in the VIOLET trial, < 5% of participants were from the ethnic minority community, which is a limitation. Patient information was provided in only English and it is unknown if providing the information in other languages would have increased participation and resulted in a study more reflective of the diversity of the UK population. Nevertheless, the proportion of non-white participants is reflective of people diagnosed with lung cancer in the UK. 60
Blinding
Blinding of participants and research staff was successful in most sites. In some sites, blinding of research staff was less successful because of the limited pool of research staff available to support the study. There were insufficient numbers of available to separate the elements of the study that necessitated unblinding and data collection that was intended to be conducted by a blinded member of the team.
QRI
The QRI could not be applied to the main trial phase in the same way as in the internal pilot. Only one of the four sites in the main trial phase engaged with the QRI and received feedback. We received minimal audio-recordings from two of the sites and one site opened towards the close of recruitment. In addition, we did not conduct interviews with the new centres in the main trial phase because saturation had been achieved in the internal pilot phase and recruitment rates remained high. Given that the QRI interviews in the internal pilot phase in VIOLET had helped with the PIs and site staff engaging with the QRI, it is possible that interviews with new site staff would have been similarly beneficial.
Patient and public involvement
The PPI group’s input into the VIOLET trial was effective, insofar as patient-facing documentation was greatly improved with their input; however, we had limited engagement with the PPI group during the conduct of the trial. In addition, although there were few participant withdrawals and 88% of survivors attended the 1-year follow-up, HRQoL response rates at 1 year were lower at 76%. It is possible that this response rate, although good, could have been improved with more engagement with the PPI group. Wider engagement with the PPI group may also have facilitated wider uptake of the trial among the ethnic minority community.
Missing data for the economic evaluation
There were high levels of data completeness for resource use items and EQ-5D-5L data [aside from information on staples used in surgery and two complications (i.e. pleural effusion and prolonged air leak), for which data collection began part way through the study]. Despite this, overall, 80% of participants had some missing data, which is a limitation, but does reflect the large number of variables included in analyses. For each variable with missing data that required some imputation, on average, only 13% of cases were missing.
Future research
An important outcome that needs further clarification is the effect of a VATS approach on overall survival. All existing trials do not have sufficient power to detect any meaningful difference in overall survival, and the chief investigators of the Danish,9 Chinese10 and French12 (ongoing) trials have agreed, in principle, to conduct an individual patient data meta-analysis of approximately 1800 randomised participants on the completion of the French trial. 12
Currently, there is a world-wide movement towards robotic surgery, which (in essence) is VATS surgery undertaken with robotic arms. There is a current moratorium of NHS funding for robotic surgery, considering the huge expense, lack of any high-quality evidence of clinical efficacy and documentation of harm. Despite this, many hospitals across the UK are currently offering thoracic surgery undertaken with robotic assistance and a randomised trial comparing outcomes to VATS is likely to be useful in determining future management.
Chapter 8 Conclusion
For patients with early-stage lung cancer in whom a lobectomy is proposed, the results of the VIOLET trial suggest that VATS should be the approach of choice. The clinical benefits include less pain and fewer complications, leading to better recovery when measured by shorter hospital stay, and better physical function at 5 weeks. The benefits of a VATS approach extended well into the first year, with continuing better recovery, fewer AEs and improved general quality of life. This was achieved without any compromise to important oncologic markers of complete resection, lymph node upstaging, disease-free survival and overall survival up to 1 year. VATS was found to be cost-effective and to provide excellent value for money for the NHS. Prior to this study, to the best of our knowledge, no high-quality comparative data on physical function, hospital readmissions, uptake and timing of chemotherapy, nor cancer recurrence, were available. Therefore, the VIOLET trial makes a substantial contribution to the evidence base. Although the majority of surgery for UK patients with lung cancer is performed by VATS access, this has been a result of a trend, rather than a concerted effort to increase uptake of minimal invasive lobectomy.
In the light of the clinical effectiveness and cost-effectiveness results from the VIOLET trial, we recommend that patients with lung cancer requiring lobectomy should have access to VATS and that the UK provides appropriate training for the existing and next generation of thoracic surgeons in minimal access techniques.
Acknowledgements
At Bristol Trials Centre, we are grateful to Dr Lucy Culliford for the mentoring and guidance she provided to the VIOLET trial managers and assistants who have worked tirelessly to deliver the trial. In addition, we are grateful to the information technology team, led by David Hutton, Heike Cappel-Porter and Samir Bellini, for developing and maintaining the VIOLET trial database and providing unwavering support when changes were requested. We also acknowledge Jade Salter Hewitt for supporting the study and arranging room hire and travel for investigator meetings, and Dr Manuela Antognozzi for overseeing and managing the staffing budget.
We are grateful to Professor Jenny Donovan (QuinteT team lead and QRI pioneer) for her guidance and support at crucial junctures in the VIOLET trial’s recruitment journey. Jenny Donovan’s comments on the QRI sections of this report have been valuable and helped shape these sections.
We would like to acknowledge the support of the UK Thoracic Surgery Research Collaborative for its support of the trial.
Members of the VIOLET investigators
Project management team members
Professor Eric Lim, Chief Investigator; Professor Chris A Rogers, Methodological Lead and Statistician; Timothy Brush, Clinical Trial Co-ordinator; Lucy Dabner, Clinical Trial Co-ordinator; Dawn Phillips, Clinical Trial Co-ordinator; Holly McKeon, Clinical Trial Co-ordinator; Chloe Beard, Assistant Trial Co-ordinator; Surinder Kaur, Assistant Trial Co-ordinator; Rosie A Harris, Medical Statistician; Dr Daisy Elliott, Research Fellow, QRI; Dr Sangeetha Paramasivan, Research Fellow, QRI; Dr Alba Realpe, Senior Research Associate, QRI; Professor Sarah Wordsworth, Lead Health Economist; Dr Elizabeth Stokes, Health Economist; Professor Jane Blazeby, Methodologist and Surgeon; Professor Andrew G Nicholson, Pathologist; and David Hutton, Database Manager.
Participating sites members: Phase I
Royal Brompton Hospital
Professor Eric Lim, PI, Consultant Thoracic Surgeon; Ms Sofina Begum, Consultant Thoracic Surgeon; Mr Simon Jordan, Consultant Thoracic Surgeon; Paulo De Sousa, Senior RN; Monica Tavares Barbosa, RN; Jessica E Wallen, RN; Sarah Ann Booth, Surgical Care Practitioner; Hilgardt Raubenheimer, Specialist Registrar; Chiara Proli, Specialist Registrar; Aleksander Mani, Consultant Thoracic Surgeon; Silviu I Buderi, Consultant Thoracic Surgeon; and Hemangi Chavan, Advanced Nurse Practitioner.
Bristol Royal Infirmary
Mr Tim JP Batchelor, PI, Consultant Thoracic Surgeon; Ms Eveline Internullo, Consultant Thoracic Surgeon; Mr Rakesh Krishnadas, Consultant Thoracic Surgeon; Mr Gianluca Casali, Consultant Thoracic Surgeon; Mr Doug West, Consultant Thoracic Surgeon; Karen Bobruk, RN; Catherine O’Donovan, RN; Louise Flintoff, RN; Amelia Lowe, Trial Coordinator; Joanna Nicklin, RN; Emma Heron, RN; Jo Chambers, RN; Becky Houlihan, RN; Laura Beacham, RN; Heather Hudson, RN; Katy Tucker, Trial Co-ordinator; Toni Farmery, Trial Co-ordinator; and Danielle Davis, Trial Co-ordinator.
Liverpool Heart and Chest Hospital
Mr Michael Shackcloth, PI, Consultant Thoracic Surgeon; Mr Julius Asante-Siaw, Consultant Thoracic Surgeon; Miss Susannah Love, Consultant Thoracic Surgeon; Sarah Feeney, RN; Lindsey Murphy, RN; Almudena Duran Rosas, RN; Andrea Young, RN; and Magenta Black, RN.
The James Cook University Hospital
Mr Joel Dunning, PI, Consultant Thoracic Surgeon; Mr Ian Paul, Consultant Thoracic Surgeon; Hyder Latif, Clinical Trial Co-ordinator; Charlotte Jacobs, Clinical Trial Co-ordinator; Alison Chilvers, Clinical Trial Co-ordinator; Edward Stephenson, Research Data Assistant; Martyn Cain, Research Data Assistant; Nazalie Iqbal, Research Data Assistant; and Emma Mahmoud, Clinical Trial Co-ordinator Assistant.
Harefield Hospital
Mr Vladimir Anikin, PI, Consultant Thoracic Surgeon; Mr Niall McGonigle, previous PI, Consultant Thoracic Surgeon; Claire Prendergast, RN; Matthew Johnson, Lead Nurse for Lung Cancer; Lisa Jones, RN; and Paula Rogers, RN Manager.
Participating sites members: Phase II
Birmingham Heartlands Hospital
Mr Babu Naidu, PI, Consultant Thoracic Surgeon; Mr Hazem Fallouh, Consultant Thoracic Surgeon; Mr Ehab Bishay, Consultant Thoracic Surgeon; Mr Luis Hernandez, Consultant Thoracic Surgeon; Mr Maninder Kalkat, Consultant Thoracic Surgeon; Mr Richard Steyn, Consultant Thoracic Surgeon; Nicola Oswald, Thoracic Research Fellow; Amy Kerr, Senior RN; Charlotte Ferris, RN; Jo Webb, RN; Joanne Taylor, RN; Hollie Bancroft, Research and Development Biomedical Scientist; Salma Kadiri, Research Practitioner; Zara Jalal Senior, Thoracic Research Data Manager; Samantha Caddick, RN; and Helen Shackleford, RN.
John Radcliffe Hospital
Miss Elizabeth Belcher, PI, Consultant Thoracic Surgeon; Mr Dionisios Stavroulias, Consultant Thoracic Surgeon; Mr Francesco Di Chiara, Consultant Thoracic Surgeon; Kathryn Saunders, RN; May Havinden-Williams, RN; Mark Ainsworth, RN; Penny Carter, RN; and Angela Bloss, RN.
Castle Hill Hospital
Professor Mahmoud Loubani, PI, Consultant Cardiothoracic Surgeon; Mr Syed Qadri, Consultant Thoracic Surgeon; Karen Dobbs, RN; Paul Atkin, RN; Dominic Fellowes, RN; and Leanne Cox, Clinical Trials Assistant.
Edinburgh Royal Infirmary
Mr Vipin Zamvar, PI, Consultant Cardiothoracic Surgeon; Lucy Marshall, RN; Fiona Strachan, RN; Manager Stacey Stewart, RN; and Nicola Rea, Senior RN.
Acknowledgement of non-author contributions
We would like to acknowledge the teams at the VIOLET trial sites, without whom the trial would not have been possible. We also acknowledge the advice and support provided by the members of the VIOLET TSC and DMSC (see Appendix 9).
Funding statement
This project is funded by the NIHR Health Technology Assessment (HTA) programme (reference 13/04/03). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Jane Blazeby is a NIHR senior investigator. Jane Blazeby, Daisy Elliott and Sangeetha Paramasivan are supported by the Medical Research Council’s (MRC’s) ConDuCT (Collaboration and Innovation in Difficult and complex randomised controlled Trials) II hub (reference MR/K025643/1). Jane Blazeby and Daisy Elliott are also supported by the NIHR Bristol and Weston Biomedical Research Centre. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Chris A Rogers was supported by the British Heart Foundation until April 2016.
This study was designed and delivered in collaboration with the Clinical Trials and Evaluation Unit, Bristol Trials Centre, a UKCRC registered clinical trials unit that, as part of the Bristol Trials Centre, is in receipt of NIHR Clinical Trials Unit support funding.
The NIHR, MRC and British Heart Foundation were not involved in the study management.
Contributions of authors
Eric Lim (https://orcid.org/0000-0002-9078-3226) (Chief Investigator, Professor of Thoracic Surgery) had full access to all data in the trial and takes responsibility for the integrity of the data and the accuracy of the data analysis; conceived the trial; was involved in obtaining funding and designing the trial; managed the trial with the TMG; interpreted the data; and co-authored the first draft of report.
Rosie A Harris (https://orcid.org/0000-0002-2405-667X) (Medical Statistician) was responsible for data management and preparation of reports for the study team and independent oversight committees during the trial; wrote the SAP; analysed the data; and co-authored the first draft of the report.
Holly E McKeon (https://orcid.org/0000-0002-0220-503X) (Trial Manager) was responsible for trial management and close down; drafted and critically revised this manuscript; and provided technical and project support.
Timothy JP Batchelor (https://orcid.org/0000-0003-4103-6061) (PI, Consultant Thoracic Surgeon) provided expert input on the trial; interpreted the data; and critically revised this report.
Joel Dunning (https://orcid.org/0000-0001-8792-5089) (PI, Consultant Thoracic Surgeon) provided expert input on the trial; interpreted the data; and critically revised this report.
Michael Shackcloth (https://orcid.org/0000-0002-6494-9907) (PI, Consultant Thoracic Surgeon) provided expert input on the trial; interpreted the data; and critically revised this report.
Vladimir Anikin (https://orcid.org/0000-0001-5634-9306) (PI, Consultant Thoracic Surgeon) provided expert input on the trial; interpreted the data; and critically revised this report.
Babu Naidu (https://orcid.org/0000-0003-1576-230X) (PI, Consultant Thoracic Surgeon) provided expert input on the trial; interpreted the data; and critically revised this report.
Elizabeth Belcher (https://orcid.org/0000-0002-8408-0452) (PI, Consultant Thoracic Surgeon) provided expert input on the trial; interpreted the data; and critically revised this report.
Mahmoud Loubani (https://orcid.org/0000-0003-1826-6686) (PI, Consultant Cardiothoracic Surgeon) provided expert input on the trial; interpreted the data; and critically revised this report.
Vipin Zamvar (https://orcid.org/0000-0003-2447-828X) (PI, Consultant Cardiothoracic Surgeon) provided expert input on the trial; interpreted the data; and critically revised this report.
Lucy Dabner (https://orcid.org/0000-0001-7269-1945) (Trial Manager) was responsible for trial management; critically revised this report; and provided technical and project support.
Timothy Brush (https://orcid.org/0000-0002-9266-6867) (Trial Manager) was responsible for trial management during trial set-up; critically revised the report; and provided technical and project support.
Elizabeth A Stokes (https://orcid.org/0000-0002-4179-1369) (Senior Researcher) was involved in trial concept and design; interpreted the data; critically revised this report; and undertook the health economic analysis.
Sarah Wordsworth (https://orcid.org/0000-0002-2361-3040) (Professor of Health Economics) was involved in trial concept and design; interpreted the data; and critically revised this report.
Sangeetha Paramasivan (https://orcid.org/0000-0001-7329-9574) (Research Fellow) was the QRI lead and contributed towards the study design, funding application, ethics approval, study documentation and initial study site set-up; analysed audio-recordings and provided feedback to centres/recruiters; and wrote the QRI sections of this report, informed by descriptive accounts from Alba Realpe and Daisy Elliott, and from other extensive QRI documentation maintained by Daisy Elliott, Alba Realpe and Sangeetha Paramasivan.
Alba Realpe (https://orcid.org/0000-0001-9502-3907) (Senior Research Associate) carried out the QRI in the last year of the VIOLET trial’s recruitment; analysed audio-recordings; monitored SEAR data; contributed to the rapid communication strategy developed in the final year of recruitment; wrote sections of the first draft of this report; drew together previous descriptive accounts to inform this report; and contributed towards the final QRI sections.
Daisy Elliott (https://orcid.org/0000-0001-8143-9549) (Research Fellow) led and carried out the QRI (in Sangeetha Paramasivan’s absence) in the initial year of the study; carried out all the interviews; analysed interviews and audio-recordings; monitored SEAR data and provided feedback to centres/recruiters; wrote an initial descriptive account of the QRI findings in the internal pilot phase; and contributed towards the final QRI sections.
Jane Blazeby (https://orcid.org/0000-0002-3354-3330) (Professor of Surgery) was involved in trial concept and design and in obtaining funding; provided expert input on the trial; interpreted the data; and critically revised this manuscript.
Chris A Rogers (https://orcid.org/0000-0002-9624-2615) (Professor of Medical Statistics and Clinical Trials, Director of Bristol Trials Centre) had full access to all data in the trial and takes responsibility for the integrity of the data and the accuracy of the data analysis; was involved in trial concept and design; obtained funding; interpreted the data; co-authored the first draft of the report; and oversaw the statistical analysis and reporting of the trial.
Publications
Peer-reviewed publications
Lim E, Batchelor T, Shackcloth M, Dunning J, McGonigle N, Brush T, et al. Study protocol for VIdeo assisted thoracoscopic lobectomy versus conventional Open LobEcTomy for lung cancer, a UK multicentre randomised controlled trial with an internal pilot (the VIOLET study). BMJ Open 2019;9:e029507.
Lim E, Batchelor T, Dunning J, Shackcloth M, Anikin V, Naidu B, et al. Video-assisted thoracoscopic or open lobectomy in early-stage lung cancer. NJEM Evid 2022;1.
Conference abstracts
Lim E, Brush T, Rogers C. Video Assisted Thoracoscopic Lobectomy Versus Conventional Open Lobectomy for Lung Cancer, a Multi-Centre Randomised Controlled Trial with an Internal Pilot: the VIOLET Study. 14th Annual British Thoracic Oncology Group Conference, Dublin, Ireland, 27–29 January 2016.
Rogers CA, Paramasivan S, Elliott D, Whybrow P, Kanavou S, Harris RA, et al. Audio-Recording Recruitment Consultations – An Exploratory Study in Two RCTs to Investigate the Impact on Randomisation Rates. 4th International Clinical Trials Methodology Conference and the 38th Annual Meeting of the Society for Clinical Trials, Liverpool, UK, 7–10 May 2017.
Lim E, Batchelor T, Dunning J, Shackcloth M, Anikin V, Naidu B, et al. PL02.06 in hospital clinical efficacy, safety and oncologic outcomes from violet: a UK multi-centre RCT of VATS versus open lobectomy for lung cancer. J Thorac Oncol 2019;14:S6.
Lim E, Begum S, Batchelor T, Krishnadas R, Shackcloth M, Dunning J. S23 optimum diagnostic pathway and pathologic confirmation rate of early stage lung cancer: results from VIOLET. Thorax 2019;74:A15.
Press release
International Association for the Study of Lung Cancer. Video Assisted Lung Surgery Reduces Complications and Hospital Stays Compared to Open Surgery. Press release, 9 September 2019.
Data-sharing statement
Following publication, anonymised individual patient data will be made available on request to the corresponding author for secondary research, conditional on assurance from the secondary researcher that the proposed use of the data is compliant with the MRC Policy on Data Sharing regarding scientific quality, ethics requirements and value for money. A minimum requirement with respect to scientific quality will be a publicly available prespecified protocol describing the purpose, methods and analysis of the secondary research (e.g. a protocol for a Cochrane systematic review, approved by a UK Research Ethics Committee or other similar approved ethics review body). Patient identifiers will not be passed on to any third party.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Lim E, Batchelor T, Shackcloth M, Dunning J, McGonigle N, Brush T, et al. Study protocol for VIdeo assisted thoracoscopic lobectomy versus conventional Open LobEcTomy for lung cancer, a UK multicentre randomised controlled trial with an internal pilot (the VIOLET study). BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-029507.
- Francisci S, Minicozzi P, Pierannunzio D, Ardanaz E, Eberle A, Grimsrud TK, et al. Survival patterns in lung and pleural cancer in Europe 1999–2007: results from the EUROCARE-5 study. Eur J Cancer 2015;51:2242-53. https://doi.org/10.1016/j.ejca.2015.07.033.
- The SCTS. Thoracic Surgery Audit Group, Society for Cardiothoracic Surgery in Great Britain and Ireland . The Thoracic Surgery Registry Brief Report Audit Years 2011–12 to 2013–14 n.d. https://scts.org/_userfiles/pages/files/thoracic/3_year_data_summary_20151.pdf (accessed 26 January 2022).
- Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. https://doi.org/10.1200/JCO.2008.18.2733.
- Cao C, Manganas C, Ang SC, Yan TD. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. https://doi.org/10.3978/j.issn.2225-319X.2012.04.18.
- Kirby TJ, Mack MJ, Landreneau RJ, Rice TW. Lobectomy – video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995;109:997-1001. https://doi.org/10.1016/S0022-5223(95)70326-8.
- Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg 2000;24:27-30. https://doi.org/10.1007/s002689910006.
- Sugiura H, Morikawa T, Kaji M, Sasamura Y, Kondo S, Katoh H. Long-term benefits for the quality of life after video-assisted thoracoscopic lobectomy in patients with lung cancer. Surg Laparosc Endosc Percutan Tech 1999;9:403-8. https://doi.org/10.1097/00129689-199912000-00007.
- Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. https://doi.org/10.1016/S1470-2045(16)00173-X.
- Long H, Tan Q, Luo Q, Wang Z, Jiang G, Situ D, et al. Thoracoscopic surgery versus thoracotomy for lung cancer: short-term outcomes of a randomized trial. Ann Thorac Surg 2018;105:386-92. https://doi.org/10.1016/j.athoracsur.2017.08.045.
- Bendixen M, Kronborg C, Jørgensen OD, Andersen C, Licht PB. Cost-utility analysis of minimally invasive surgery for lung cancer: a randomized controlled trial. Eur J Cardiothorac Surg 2019;56:754-61. https://doi.org/10.1093/ejcts/ezz064.
- Pagès PB, Abou Hanna H, Bertaux AC, Serge Aho LS, Magdaleinat P, Baste JM, et al. Medicoeconomic analysis of lobectomy using thoracoscopy versus thoracotomy for lung cancer: a study protocol for a multicentre randomised controlled trial (Lungsco01). BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-012963.
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours, Eighth Edition. Chichester: John Wiley & Sons; 2016.
- Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours, Seventh Edition. Chichester: John Wiley & Sons; 2009.
- Swanson SJ, Herndon JE, D’Amico TA, Demmy TL, McKenna RJ, Green MR, et al. Video-assisted thoracic surgery lobectomy: report on CALGB 39802 – a prospective, multi-institution feasibility study. J Clin Oncol 2007;31:4993-7.
- Maringwa JT, Quinten C, King M, Ringash J, Osoba D, Coens C, et al. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support Care Cancer 2011;19:1753-60. https://doi.org/10.1007/s00520-010-1016-5.
- Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Control Clin Trials 2004;25:143-56. https://doi.org/10.1016/j.cct.2003.10.016.
- Edwards SL, Roberts C, McKean ME, Cockburn JS, Jeffrey RR, Kerr KM. Preoperative histological classification of primary lung cancer: accuracy of diagnosis and use of the non-small cell category. J Clin Pathol 2000;53:537-40. https://doi.org/10.1136/jcp.53.7.537.
- Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials 2016;17. https://doi.org/10.1186/s13063-016-1391-4.
- Rooshenas L, Paramasivan S, Jepson M, Donovan JL. Intensive triangulation of qualitative research and quantitative data to improve recruitment to randomized trials: the QuinteT approach. Qual Health Res 2019;29:672-9. https://doi.org/10.1177/1049732319828693.
- Donovan JL, Lane JA, Peters TJ, Brindle L, Salter E, Gillatt D, et al. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. J Clin Epidemiol 2009;62:29-36. https://doi.org/10.1016/j.jclinepi.2008.02.010.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70. https://doi.org/10.1136/bmj.325.7367.766.
- Beard D, Rees P, Cook J, Rombach I, Cooper C, Merritt N, et al. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): a multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet 2017;391:329-38. https://doi.org/10.1016/S0140-6736(17)32457-1.
- Elliott D, Husbands S, Hamdy FC, Holmberg L, Donovan JL. Understanding and improving recruitment to randomised controlled trials: qualitative research approaches. Eur Urol 2017;72:789-98. https://doi.org/10.1016/j.eururo.2017.04.036.
- Paramasivan S, Rogers CA, Welbourn R, Byrne JP, Salter N, Mahon D, et al. Enabling recruitment success in bariatric surgical trials: pilot phase of the By-Band-Sleeve study. Int J Obes 2017;41:1654-61. https://doi.org/10.1038/ijo.2017.153.
- Donovan JL, de Salis I, Toerien M, Paramasivan S, Hamdy FC, Blazeby JM. The intellectual challenges and emotional consequences of equipoise contributed to the fragility of recruitment in six randomized controlled trials. J Clin Epidemiol 2014;67:912-20. https://doi.org/10.1016/j.jclinepi.2014.03.010.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-5.
- Mills N, Blazeby JM, Hamdy FC, Neal DE, Campbell B, Wilson C, et al. Training recruiters to randomized trials to facilitate recruitment and informed consent by exploring patients’ treatment preferences. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-323.
- Mills N, Gaunt D, Blazeby JM, Elliott D, Husbands S, Holding P, et al. Training health professionals to recruit into challenging randomized controlled trials improved confidence: the development of the QuinteT randomized controlled trial recruitment training intervention. J Clin Epidemiol 2018;95:34-4. https://doi.org/10.1016/j.jclinepi.2017.11.015.
- Rooshenas L, Elliott D, Wade J, Jepson M, Paramasivan S, Strong S, et al. Conveying equipoise during recruitment for clinical trials: qualitative synthesis of clinicians’ practices across six randomised controlled trials. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002147.
- Jepson M, Elliott D, Conefrey C, Wade J, Rooshenas L, Wilson C, et al. An observational study showed that explaining randomization using gambling-related metaphors and computer-agency descriptions impeded randomized clinical trial recruitment. J Clin Epidemiol 2018;99:75-83. https://doi.org/10.1016/j.jclinepi.2018.02.018.
- Wilson C, Rooshenas L, Paramasivan S, Elliott D, Jepson M, Strong S, et al. Development of a framework to improve the process of recruitment to randomised controlled trials (RCTs): the SEAR (Screened, Eligible, Approached, Randomised) framework. Trials 2018;19. https://doi.org/10.1186/s13063-017-2413-6.
- Mills N, Donovan JL, Wade J, Hamdy FC, Neal DE, Lane JA. Exploring treatment preferences facilitated recruitment to randomized controlled trials. J Clin Epidemiol 2011;64:1127-36. https://doi.org/10.1016/j.jclinepi.2010.12.017.
- Glaser B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. Piscataway, NJ: Transaction Publishers; 1967.
- Wade J, Donovan JL, Athene Lane J, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018-28. https://doi.org/10.1016/j.socscimed.2009.02.023.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c869.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Method 1995;57:289-300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- National Institute for Health and Care Excellence (NICE) . Social Value Judgments: Principles for the Development of NICE Guidance 2008.
- NHS Improvement . National Schedule of Reference Costs 2018–19 2019. www.england.nhs.uk/national-cost-collection/ (accessed 28 January 2021).
- NHS Improvement . National Schedule of Reference Costs 2017–18 2018. https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed 26 January 2022).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: Personal Social Services Research Unit, University of Kent; 2020.
- GOV.UK . Drugs and Pharmaceutical Electronic Market Information Tool (eMIT) n.d. www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit (accessed 29 November 2020).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2020.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019). 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 28 January 2021).
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing . . . presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. https://doi.org/10.1002/hec.766.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Rubin DB. Multiple Imputation for Non-Response in Surveys. New York, NY: John Wiley & Sons; 1987.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Lim E, Batchelor T, Dunning J, Shackcloth M, Anikin V, Naidu B, et al. Video-assisted thoracoscopic or open lobectomy in early-stage lung cancer. NJEM Evid 2022;1. https://doi.org/10.1056/EVIDoa2100016.
- National Institute for Health and Care Excellence . Lung Cancer: Diagnosis and Management NICE Guideline [NG122] 2019. www.nice.org.uk/guidance/ng122/chapter/Recommendations (accessed 15 February 2021).
- Rooshenas L, Scott LJ, Blazeby JM, Rogers CA, Tilling KM, Husbands S, et al. The QuinteT Recruitment Intervention supported five randomized trials to recruit to target: a mixed-methods evaluation. J Clin Epidemiol 2019;106:108-20. https://doi.org/10.1016/j.jclinepi.2018.10.004.
- Gov.UK . Population of England and Wales 2020. www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/national-and-regional-populations/population-of-england-and-wales/latest (accessed 17 February 2021).
- Jack RH, Davies EA, Møller H. Lung cancer incidence and survival in different ethnic groups in South East England. Br J Cancer 2011;105:1049-53. https://doi.org/10.1038/bjc.2011.282.
- ISD Scotland . Theatre Services. R142X_2019 Average Theatre Running Costs, and Usage by Specialty, by Board 2019. www.isdscotland.org/Health-Topics/Finance/Costs/Detailed-Tables/Theatres.asp (accessed 3 August 2020).
- Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13330.
- National Institute for Health and Clinical Excellence (NICE) . Nutrition Support in Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition. Costing Report. Implementing NICE Guidance in England. Clinical Guideline No. 32 2006.
- NHS Blood and Transplant . NHS Blood and Transplant Price List 2018–2019 2018. https://hospital.blood.co.uk/components/portfolio-and-prices/ (accessed 26 January 2022).
- Krishnamoorthy B, Mehta V, Critchley W, Callan P, Shaw S, Venkateswaran R. Financial implications of using extracorporeal membrane oxygenation following heart transplantation. Interact Cardiovasc Thorac Surg 2021;32:625-31. https://doi.org/10.1093/icvts/ivaa307.
- Dretzke J, Blissett D, Dave C, Mukherjee R, Price M, Bayliss S, et al. The cost-effectiveness of domiciliary non-invasive ventilation in patients with end-stage chronic obstructive pulmonary disease: a systematic review and economic evaluation. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19810.
- Georghiou T, Bardsley M. Exploring the Cost of Care at the End of Life 2014. www.nuffieldtrust.org.uk/files/2017-01/end-of-life-care-web-final.pdf (accessed 3 April 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Pope C, Turnbull J, Jones J, Prichard J, Rowsell A, Halford S. Has the NHS 111 urgent care telephone service been a success? Case study and secondary data analysis in England. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014815.
- Snee MP, McParland L, Collinson F, Lowe CM, Striha A, Baldwin DR, et al. The SABRTooth feasibility trial protocol: a study to determine the feasibility and acceptability of conducting a phase III randomised controlled trial comparing stereotactic ablative radiotherapy (SABR) with surgery in patients with peripheral stage I non-small cell lung cancer (NSCLC) considered to be at higher risk of complications from surgical resection. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0046-2.
Appendix 1 Evolution of the QRI
The QRI protocol published in 201619 describes how its primary aim of optimising recruitment and informed consent in RCTs can be achieved through a flexible two-phased iterative study design to understand recruitment challenges (Phase I) and collaboratively develop and implement strategies to overcome the challenges (Phase II), followed by an evaluation component. Since then, and throughout the recruitment period of the VIOLET trial, the QRI has continued to evolve and adapt to the requirements of each RCT, guided by two broader advancements. First, a substantial body of empirical research from QRIs across multiple RCTs now exists on clear, hidden27 and specific recruitment challenges26,28,30–33 (e.g. conveying equipoise) and strategies to overcome them. Second, this evidence base led to the development of QRI-informed recruitment training workshops to address generic and trial- and site-specific recruitment issues, tailored to recruiters from different disciplines, including surgeons, across the UK,28 and delivered at the University of Bristol [partly funded by the MRC’s Hubs for Trials Methodology Research ConDuCT II hub]. These advancements have meant that the QRI team have been able to provide upfront training and guidance prior to recruitment commencing for recruiters in RCTs with integrated QRIs. This training included activities such as QRI-informed recruitment training workshops, training during trial launch events and SIVs, and recruitment tips documents circulated to sites to share good practice and to guide revisions to patient-facing documentation. In this way, the boundaries between Phase I and Phase II of the QRI are now blurred in many RCTs, including the VIOLET trial, bringing recruiter training/guidance upfront rather than delaying it until Phase II.
Appendix 2 Additional tables and figures for the economic evaluation
| Resource | CRFa | Sources of unit cost information |
|---|---|---|
| Initial thoracic surgery | CRFs C1, C2, C4 | ISD Scotland;61 Medtronic plc (Medtronic plc, 2020, personal communication); Johnson & Johnson (Johnson & Johnson, 2020, personal communication) |
| Initial stay in hospital post surgery by ward type | CRF D6 | National Schedule of Reference Costs 2018–19 42 |
| Complications, including reoperations and SAEs | CRFs C3, D1–D3, E5, S0–S4 | National Schedule of Reference Costs 2018–19;42 eMIT46 |
| Adjuvant therapy | CRFs E4 | National Schedule of Reference Costs 2018–19 42 |
| Imaging | CRFs F1 and I1 | National Schedule of Reference Costs 2018–19 42 |
| Recurrence/progression of cancer | CRF G1 | National Schedule of Reference Costs 2018–19 42 |
| Hospital readmissions | CRFs E1 and E5 | National Schedule of Reference Costs 2018–19 42 |
| Outpatient and ED attendances | CRFs E1 and E6 | National Schedule of Reference Costs 2018–19 42 |
| Community health and social care contacts | CRFs E1 and E7 | Unit Costs of Health and Social Care 2019 44 |
| Category | Participant allocation | |
|---|---|---|
| Randomised to VATS (N = 229), n (%) | Randomised to open surgery (N = 235), n (%) | |
| Resource use | ||
| Index admission | ||
| Time in theatre | 228 (100) | 235 (100) |
| Staples | 120 (52) | 129 (55) |
| Pathology for biopsy/frozen section | 228 (100) | 235 (100) |
| Intensive care unit stay | 227 (99) | 234 (100) |
| High-dependency unit stay | 227 (99) | 234 (100) |
| Ward stay | 227 (99) | 234 (100) |
| In-hospital complications and SAEs (excluding pleural effusion and prolonged air leak) | 229 (100) | 235 (100) |
| Pleural effusion and prolonged air leak | 131 (57) | 134 (57) |
| Index admission total | 119 (52) | 123 (52) |
| Primary and secondary care post-hospital discharge | ||
| Hospital discharge to 5 weeks | 222 (97) | 225 (96) |
| 5 weeks to 3 months | 208 (91) | 216 (92) |
| 3–6 months | 206 (90) | 212 (90) |
| 6–12 months | 206 (90) | 210 (89) |
| Post-hospital discharge total | 189 (83) | 195 (83) |
| All | 91 (40) | 94 (40) |
| Outcomes | ||
| EQ-5D-5L | ||
| Baseline | 217 (95) | 225 (96) |
| 2 weeks | 173 (76) | 180 (77) |
| 5 weeks | 191 (83) | 211 (90) |
| 3 months | 186 (81) | 203 (86) |
| 6 months | 190 (83) | 195 (83) |
| 12 months | 182 (79) | 191 (81) |
| QALYs | 109 (48) | 119 (51) |
| All costs and QALYs | 42 (18) | 53 (23) |
FIGURE 26.
Observed mean (95% CI) EQ-5D-5L scores at each time point.
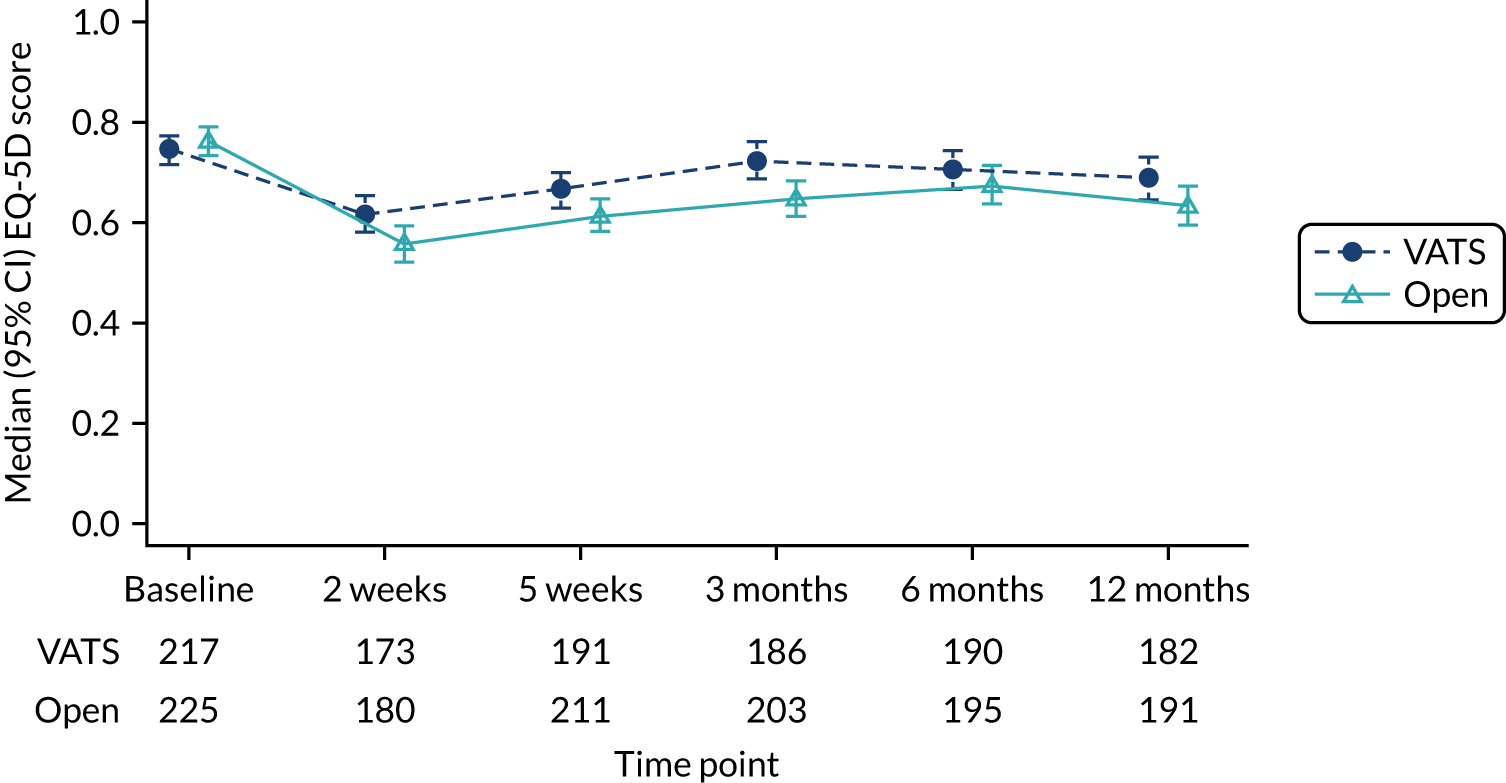
| Resource | Participant allocation | VATS vs. open surgery, mean cost difference (£) (95% CI) | |
|---|---|---|---|
| Randomised to VATS (n = 229), mean (SE) costs (£) | Randomised to open surgery (n = 235), mean (SE) costs (£) | ||
| Index admission | |||
| Surgery | |||
| Time in theatre | 2328 (48) | 2051 (47) | 277 (146 to 409) |
| Staples | 1921 (49) | 1816 (47) | 104 (–31 to 240) |
| Pathology for biopsy/frozen section | 21 (2) | 21 (2) | –0 (–7 to 7) |
| Hospital staya | |||
| Intensive care unit | 764 (448) | 1400 (444) | –636 (–1877 to 605) |
| High-dependency unit | 922 (164) | 1300 (166) | –379 (–838 to 81) |
| Ward | 1614 (121) | 1905 (120) | –291 (–626 to 43) |
| Total | 3299 (546) | 4605 (542) | –1305 (–2818 to 207) |
| In-hospital complications and SAEs | 259 (272) | 739 (269) | –480 (–1232 to 273) |
| Index admission total | 7829 (771) | 9232 (763) | –1403 (–3536 to 729) |
| Post-discharge care (primary and secondary care) | |||
| Discharge to 5 weeks | 875 (120) | 740 (117) | 135 (–195 to 465) |
| 5 weeks to 3 months | 595 (317) | 1378 (314) | –783 (–1659 to 92) |
| 3–6 months | 601 (139) | 1002 (143) | –401 (–791 to –11) |
| 6–12 months | 980 (181) | 1229 (178) | –248 (–745 to 248) |
| Post-discharge total | 3051 (443) | 4349 (438) | –1298 (–2522 to –75) |
| Total costs | 10,879 (1057) | 13,581 (1046) | –2702 (–5624 to 221) |
Appendix 3 Unit costs used in the economic evaluation
The majority of hospital unit cost estimates were sourced from National Schedule of Reference Costs 2018–19. 42 However, National Schedule of Reference Costs 2017–1843 was used when it was necessary to make use of ‘excess bed-day costs’, as these are not available in the 2018/19 data. 42 ‘Excess bed-day costs’ are estimates of the daily cost for patients who stay in hospital beyond a nationally set length of stay, and were used as a proxy for the ‘hotel’ costs associated with admissions. Costs of additional treatments were added separately. These are the best national estimates of ward costs available in England.
For complications, reference costs include the cost of treating the complication and time on a ward. As time on a ward was collected for each patient and costed separately, ‘excess bed-day costs’ were used to strip out the average cost of time on a ward to provide an estimate of the cost of treating the complication only. This prevented double counting.
Note that unit costs not in 2018/19 prices have been adjusted to 2018/19 prices using the NHS Cost Inflation Index (Tables 28–39). 45
| Resource | Unit cost (£) | Source |
|---|---|---|
| Surgery | ||
| Theatre (per hour) | 876 | ISD Scotland61 |
| Staplers per surgery | 533 | Average of the cost for a standard handle and vascular handle from Johnson & Johnson, and a handler from Medtronic plc; NHS Supply Chain catalogue costs provided by the companies for 2018/19 |
| Staple | 168 | Average of the cost for relevant staples from Johnson & Johnson and Medtronic plc; NHS Supply Chain catalogue costs provided by the companies for 2018/19 |
| Pathologist time per frozen section (15 minutes)a | 27 | Unit Costs of Health and Social Care 2019:44 14. Hospital-based doctors. Consultant: medical/surgical. Cost per working hour: £109 |
| Biomedical scientist time per frozen section (70 minutes)a | 57 | Unit Costs of Health and Social Care 2019:44 12. Hospital-based scientific and professional staff. Band 6. Cost per working hour: £49 |
| Inpatient stay | ||
| Ward day (thoracic) | 396 | National Schedule of Reference Costs 2017–18:43 weighted average of elective inpatient excess bed-day costs for relevant HRGs (DZ02H, DZ02J, DZ02K complex thoracic procedures, 19 years and over) |
| High-dependency unit day | 917 | National Schedule of Reference Costs 2018–19:42 critical care. CCU07 thoracic surgical adult patients predominate. XC07Z adult critical care, 0 organs supported |
| Intensive care day | 1445 | National Schedule of Reference Costs 2018–19:42 critical care. CCU07 thoracic surgical adult patients predominate. Weighted average of XC01Z–XC06Z, adult critical care, 1–6 organs supported |
| Ward day at another hospital | 354 | National Schedule of Reference Costs 2017–18:43 weighted average of elective and non-elective inpatient excess bed-day costs across all activities |
| Complication | Treatment/action | Cost (£) | Assumptions |
|---|---|---|---|
| Pulmonary complications | |||
| Acute respiratory failure | No additional treatment | 0 | Captured in intensive care and ward length of stay |
| Pulmonary collapse (requiring intervention: CPAP) | CPAP; chest X-ray | 570 | |
| Empyema (requiring antibiotics or drainage) | Average of chest X-ray and antibiotics (piperacillin/tazobactam, 4.5 g i.v. three times per day for 5 days) and chest X-ray and chest drain | 423 | Return to theatre captured separately |
| Surgical emphysema (requiring intervention) | Chest X-ray; chest drain reinsertion | 786 | |
| Bronchopleural fistula | No additional treatment | 0 | Captured in return to theatre |
| Post-drain pneumothorax requiring intervention | Chest X-ray; chest drain | 786 | |
| Chylothorax | No additional treatment | 0 | Captured in return to theatre or increased length of stay |
| Acute respiratory distress syndrome | No additional treatment | 0 | Captured in intensive care unit admission |
| Acute lung injury | No additional treatment | 0 | Captured in intensive care unit admission |
| Pulmonary embolus | Transthoracic echocardiogram, CT chest, i.v. heparin for 5 days (initial 5000 units, then 15,000 units every 12 hours for 5 days) | 210.81 | |
| Insertion of a mini-tracheostomy tube | Mini-tracheostomy | 1122 | |
| Bronchoscopy | Bronchoscopy | 540 | |
| Pleural effusion | Chest X-ray; chest drain | 786 | |
| Prolonged air leak | Chest X-ray | 31 | |
| Cardiac complications | |||
| Myocardial infarction | No additional treatment | 0 | Captured in increased length of stay |
| Arrhythmia (requirement treatment) | Two ECGs, amiodarone (1.2 g i.v., then oral 200 mg three times per day for 1 week, then twice per day for 1 week) | 101.49 | |
| Renal complications | |||
| Acute kidney injury | i.v. fluids | 4.59 | |
| Haemofiltration (per day) | Haemofiltration | 214 | |
| Gastrointestinal complications | |||
| Peptic ulcer/gastrointestinal bleed/perforation | No additional treatment | 0 | Reoperations captured separately |
| Pancreatitis | CT; parenteral nutrition for 5 days; i.v. fluids (1500 ml) | 328.59 | Reoperations captured separately |
| Other: abdominal pain | CT | 108 | |
| Other: constipation | Laxatives; enemas (bisacodyl, 5 mg; sodium citrate, assume for 5 days) | 1.99 | |
| Other: ileus/paralytic ileus | CT | 108 | |
| Other: melena/upper gastrointestinal haemorrhage | Endoscopy; omeprazole (i.v. omeprazole 40 mg for 3 days, then 40 mg oral daily for 5 days) | 311.78 | |
| Other: bowel ischaemia | CT | 108 | Reoperations captured separately |
| Other: small bowel infection | Antibiotics (cefuroxime and metronidazole for 3 days) | 6.25 | |
| Other: small bowel obstruction | CT | 108 | Reoperations captured separately |
| Infective complications | |||
| Infection (requiring antibiotic treatment for suspected infection) | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)] | 28.13 | |
| Site: pneumonia/chest infection | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)]; chest X-ray; CT | 167.13 | |
| Site: wound infection | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)]; chest X-ray | 59.13 | |
| Site: other infection – drain site infection | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)]; chest X-ray; CT | 167.13 | |
| Site: other infection – epidural site | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)]; chest X-ray; CT | 167.13 | |
| Site: other infection – pleural fluid growth group B streptococcus | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)]; chest X-ray; CT | 167.13 | |
| Site: other infection – haemophilus influenza in sputum | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)]; chest X-ray | 59.13 | |
| Site: other infection – respiratory tract infection | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)]; chest X-ray | 59.13 | |
| Site: other infection – sepsis (of unknown origin) | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)]; CT | 136.13 | |
| Site: other infection – urinary tract infection | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)]; urine test | 36.13 | |
| Site: other infection – non-specific high inflammatory markers | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)] | 28.13 | |
| Site: other infection – kidney | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)] | 28.13 | |
| Site: other infection – pancreatitis | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)] | 28.13 | |
| Site: other infection – superadded infection | Antibiotics [assume piperacillin/tazobactam (4.5 g i.v. three times per day for 5 days)] | 28.13 | |
| Neurological complications | |||
| Transient ischaemic attack | CT | 108 | |
| Stroke | Rehabilitation; CT | 531 | |
| Acute psychosis | CT | 108 | |
| Other complications | |||
| Wound dehiscence requiring dressing | 151 | ||
| Laryngeal nerve damage | Review by ear, nose and throat for vocal cord medialisation procedure after hospital discharge | 107 | |
| Deep-vein thrombosis | Duplex scan of leg veins, i.v. heparin (initial 5000 units, then 15,000 units every 12 hours for 5 days) | 131.81 | |
| Haematoma | No additional treatment | 0 | |
| Reoperation for | |||
| Bleeding | 3627 | ||
| Pleural effusion | 1308 | ||
| Right VATS drainage of empyema | 1571 | ||
| Sputum retention | 2082 | ||
| Treatment/action | Unit cost (£) | Source |
|---|---|---|
| Amiodarone (1.2 g i.v., then oral 200 mg three times per day for 1 week, then twice per day for 1 week) | 3.49 | eMIT46 |
| Antibiotics (piperacillin/tazobactam, 4.5 g i.v. three times per day for 5 days) | 28.13 | eMIT46 |
| Antibiotics (cefuroxime and metronidazole for 3 days) | 6.25 | eMIT46 |
| Bronchoscopy | 540 | National Schedule of Reference Costs 2018–19:42 outpatient procedures. Service code 340 respiratory medicine. DZ69A Diagnostic bronchoscopy, 19 years and over |
| Chest drain | 755 | National Schedule of Reference Costs 2017–18:43 average of the costs of two codes –
|
| Chest X-ray | 31 | National Schedule of Reference Costs 2018–19:42 directly accessed diagnostic services. Direct access plain film |
| CPAP | 539 | Gray et al.62 |
| CT | 108 | National Schedule of Reference Costs 2018–19:42 Diagnostic imaging – outpatient. RD21A computerised tomography scan of one area, with post-contrast only, 19 years and over |
| Duplex scan of leg veins | 105 | National Schedule of Reference Costs 2018–19:42 diagnostic imaging – outpatient. RD22Z computerised tomography scan of one area, with pre and post contrast |
| ECG | 49 | National Schedule of Reference Costs 2018–19:42 directly accessed diagnostic services. EY51Z electrocardiogram monitoring or stress testing |
| Echocardiogram: transthoracic | 76 | National Schedule of Reference Costs 2018–19:42 diagnostic imaging – outpatient. RD51A simple echocardiogram, 19 years and over |
| Endoscopy | 308 | National Schedule of Reference Costs 2018–19:42 outpatient procedure. FE22Z Diagnostic endoscopic upper gastrointestinal tract procedures, 19 years and over for service code 301 gastroenterology |
| Ear, nose and throat review | 107 | As outpatient cost |
| Haemofiltration | 214 | National Schedule of Reference Costs 2018–19:42 renal dialysis. LE01A haemodialysis for acute kidney injury, 19 years and over |
| i.v. fluids (sodium chloride 0.9%, 1500 ml) | 4.59 | British National Formulary 47 |
| i.v. heparin (initial 5000 units, then 15,000 units every 12 hours for 5 days) | 26.81 | eMIT46 |
| Laxatives; enemas (bisacodyl, 5 mg; sodium citrate, assume for 5 days) | 1.99 | eMIT46 |
| Mini-tracheostomy | 1122 | NHS Reference National Schedule of Reference Costs 2017–18:43 day case. CA63Z tracheostomy, with bed-day cost excluded by subtracting the overall elective inpatients excess bed-day cost |
| Minor treatment for wound dehiscence | 151 | National Schedule of Reference Costs 2018–19:42 outpatient procedures. Service code 330 dermatology. JC43C minor skin procedures, 19 years and over |
| Omeprazole (i.v. 40 mg for 3 days, then 40 mg oral daily for 5 days) | 3.78 | eMIT46 |
| Parenteral nutrition (assume 5 days) | 216 | NICE63 |
| Rehabilitation for stroke | 423 | National Schedule of Reference Costs 2018–19:42 rehabilitation. REHABL3. Non-specialist rehabilitation services level 3. Admitted patient care. VC04Z. Rehabilitation for stroke |
| Reoperation for bleeding | 3627 | National Schedule of Reference Costs 2017–18:43 non-elective long stay. DZ63A/B/C major thoracic procedures, 19 years and over, with CC score 0–6+, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Reoperation for pleural effusion | 1308 | National Schedule of Reference Costs 2017–18:43 non-elective long stay. DZ16H/J, Pleural Effusion with multiple interventions, with CC score 6–11+, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Reoperation for right VATS drainage of empyema | 1571 | National Schedule of Reference Costs 2017–18:43 non-elective long stay. DZ10H/J/K lung abscess-empyema with interventions, with CC score 0–9+, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Reoperation for sputum retention | 2082 | National Schedule of Reference Costs 2017–18:43 elective inpatients. DZ67Z major therapeutic bronchoscopy, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Urine test | 8 | National Schedule of Reference Costs 2018–19:42 directly accessed pathology services. DAPS07 microbiology |
| Treatment/action | Unit cost (£) | Source |
|---|---|---|
| Anaphylactic reaction [500 µg adrenaline 1 : 1000 solution (0.5 ml)] | 6.56 | eMIT46 |
| Blood transfusion (red blood cells) | 128.99 | NHS Blood and Transplant64 |
| Cardiac arrest | 574 | National Schedule of Reference Costs 2018–19:42 non-elective short stay. EB05C cardiac arrest with CC score 0–4 |
| Cardioversion | 696 | National Schedule of Reference Costs 2018–19:42 day case. EB07E. Arrhythmia or conduction disorders with CC score 0–3 |
| Extracorporeal membrane oxygenation per day | 3112 | Krishnamoorthy et al.65 |
| Fibrin patch used in theatre | 93.13 | HEMOPATCH® Bicarb Medium (Baxter Healthcare). 2018/19 product list prices confirmed by Baxter Healthcare (Baxter Healthcare, 2021, personal communication) |
| Inotropes (noradrenaline 1 mg/hour for 2 days) | 18.78 | eMIT46 |
| Laparotomy for bleeding gastric ulcer | 3590 | National Schedule of Reference Costs 2017–18:43 elective inpatient. FF04A/B/C/D, major oesophageal, stomach, or duodenum procedures, 19 years and over, with CC score 0–7+, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Ultrasound scan | 52 | National Schedule of Reference Costs 2018–19:42 imaging: direct access RD40Z ultrasound scan with duration of less than 20 minutes, without contrast |
| Resource | Unit cost (£) | Source |
|---|---|---|
| Ward day for readmissions | 354 | National Schedule of Reference Costs 2017–18:43 weighted average of elective and non-elective inpatient excess bed-day costs across all activities |
| Intensive care day for readmissions | 1436 | National Schedule of Reference Costs 2018–19:42 critical care. CCU07 thoracic surgical adult patients predominate. Weighted average of XC01Z–XC07Z, 0–6 organs supported |
| Accident and emergency attendance, leading to admission | 261 | National Schedule of Reference Costs 2018–19:42 accident and emergency. Weighted average of all admitted codes |
| Accident and emergency attendance, not leading to admission | 144 | National Schedule of Reference Costs 2018–19:42 accident and emergency. Weighted average of all non-admitted codes |
| Ambulance to hospital | 257 | National Schedule of Reference Costs 2018–19:42 ambulance. ASS02 see and treat and convey |
| Complication | Treatment/action | Cost (£) |
|---|---|---|
| Atelectasis | Chest X-ray; chest physiotherapy | 167 |
| Bleeding | Chest X-ray; blood transfusion (reoperations already captured) | 159.99 |
| Sepsis | CT; antibiotics (piperacillin/tazobactam) | 136.13 |
| Infection (other) | ||
| Cellulitis | Antibiotics (piperacillin/tazobactam) | 28.13 |
| Removal of PICC line | Antibiotics (piperacillin/tazobactam) | 28.13 |
| Bronchoscopy (reason: stent insertion) | Therapeutic bronchoscopy | 887 |
| Recurrence/progression/new cancer | Specific treatments captured and costed in hospital admissions and visits as occurred | Various |
| Anaemia | Blood transfusion (readmission length of stay already captured) | 128.99 |
| Neutropenia/febrile neutropenia | Antibiotics (teicloplanin and piperacillin/tazobactam) | 66.12 |
| Nausea | Antinausea medication (ondanestron) | 1.01 |
| Vomiting | Antinausea medication | 1.01 |
| Vomiting (ileus specified) | Antinausea medication; CT | 109.01 |
| Diarrhoea | i.v. fluids | 4.59 |
| Headaches | No additional treatment | 0 |
| Reoperation: left VATS mediastinal lymphadenectomy | 2557 | |
| Treatment/action | Unit cost (£) | Source |
|---|---|---|
| Surgery and procedures | ||
| Right VATS segmentectomy | 6060 | National Schedule of Reference Costs 2017–18:43 elective inpatient. DZ02H/J/K complex thoracic procedures, 19 years and over, with CC score 0–6+, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Lung wedge resection/surgical lung biopsy/left VATS mediastinal lymphadenectomy | 2557 | National Schedule of Reference Costs 2017–18:43 elective inpatient. DZ64A/B/C intermediate thoracic procedures, 19 years and over, with CC score 0–6+, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Surgery for brain metastases | 7868 | National Schedule of Reference Costs 2017–18:43 elective inpatient. AA53A/B/C/D major intracranial procedures, 19 years and over, with CC score 0–12+, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Therapeutic bronchoscopy | 887 | National Schedule of Reference Costs 2018–19:42 day case. DZ68Z therapeutic bronchoscopy |
| Haemorrhoidectomy | 1375 | National Schedule of Reference Costs 2017–18:43 elective inpatient. FF41A/B/C, intermediate anal procedures, 19 years and over, with CC score 0–3+, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Hysterectomy | 3517 | National Schedule of Reference Costs 2017–18:43 elective inpatient. MA06A/BC major, open or laparoscopic, upper or lower genital tract procedures for malignancy, with CC score 0–4+, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Ileostomy reversal | 3366 | National Schedule of Reference Costs 2017–18:43 elective inpatient. FF22A/B/C/D major small intestine procedures, 19 years and over, with CC score 0–7+, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Laparoscopic resection with ileostomy formation | 6374 | National Schedule of Reference Costs 2017–18:43 elective inpatient. FF31A/B/C/D complex large intestine procedures, 19 years and over, with CC score 0–9+, with costs of average length of stay reported subtracted at the corresponding excess bed-day cost |
| Parathyroidectomy | 2796 | National Schedule of Reference Costs 2017–18:43 elective inpatient. KA03C/D parathyroid procedures with CC score 0–2+, with costs of average length of stay reported subtracted at the corresponding excess bed-day cost |
| Polypectomy | 642 | National Schedule of Reference Costs 2018–19:42 day case. FE30Z therapeutic colonoscopy, 19 years and over |
| Excision of anterior abdominal wall necrosis | 1068 | National Schedule of Reference Costs 2018–19:42 day case. JC42C intermediate skin procedures, 19 years and over |
| Intercostal nerve block for post thoracotomy pain | 721 | National Schedule of Reference Costs 2018–19:42 day case. DZ71Z minor thoracic procedures |
| Other | ||
| Antibiotics (teicoplanin, i.v. 400 mg twice per day first day, then 400 mg once per day for 2 days) | 37.99 | eMIT46 |
| Antinausea medication (ondanestron, 4 mg i.v. for 5 days) | 1.01 | eMIT46 |
| Chest physiotherapy | 136 | National Schedule of Reference Costs 2018–19:42 outpatient procedures. DZ30Z chest physiotherapy. 340 respiratory medicine |
| Colonoscopy | 521 | National Schedule of Reference Costs 2018–19:42 day case. FE32Z diagnostic colonoscopy, 19 years and over |
| MRI | 143 | National Schedule of Reference Costs 2018–19:42 imaging: outpatient RD01A magnetic resonance imaging scan of one area, without contrast, 19 years and over |
| Physiotherapy rehabilitation and ongoing care | 351 | National Schedule of Reference Costs 2018–19:42 rehabilitation. non-specialist rehabilitation services level 3. Admitted patient care. VC40Z rehabilitation for respiratory disorders |
| Treatment/action | Unit cost (£) | Source |
|---|---|---|
| Surgery | ||
| Bowel resection for diverticulitis | 4316 | National Schedule of Reference Costs 2017–18:43 elective inpatient. FF33A/B distal colon procedures, 19 years and over, with CC score 0–3, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Traumatic hip fracture repair | 1786 | National Schedule of Reference Costs 2017–18:43 non-elective long stay. HE11C/D hip fracture with single intervention, with CC score 0–8, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Surgery for lower leg ischaemia | 4885 | National Schedule of Reference Costs 2017–18:43 elective inpatient. YQ12D. Single open procedure on blood vessel of lower limb with CC score 0–3, with costs of average length of stay reported, subtracted at the corresponding excess bed-day cost |
| Other | ||
| Atrial fibrillation (amiodarone) | 3.49 | As reported in Table 30 |
| Bone marrow aspirate carried out to investigate immune suppression | 587 | National Schedule of Reference Costs 2018–19:42 day case. SA33Z diagnostic bone marrow extraction |
| Coronary angiogram | 1005 | National Schedule of Reference Costs 2018–19:42 day case. EY43F standard cardiac catheterisation with CC score 0–1 |
| Discharged home on ambulatory oxygen | 463 for first month, then 73 per month | Dretzke et al.:66 non-invasive ventilation |
| Gastroscopy | 308 | As reported in Table 30 (endoscopy) |
| Nasogastric tube | 160 | National Schedule of Reference Costs 2018–19:42 outpatient procedure. FF05Z. Intermediate upper gastrointestinal tract procedures, 19 years and over for 301 gastroenterology |
| Psychiatric liaison nurse | 70 | National Schedule of Reference Costs 2017–18:43 community health services. Nursing N29AF other specialist nursing, adult, face to face |
| Specialty | Service code | Unit cost (£) |
|---|---|---|
| General surgery | 100 | 134 |
| Urology | 101 | 108 |
| Breast surgery | 103 | 147 |
| Colorectal surgery | 104 | 121 |
| Hepatobiliary and pancreatic surgery | 105 | 209 |
| Vascular surgery | 107 | 145 |
| Trauma and orthopaedics | 110 | 120 |
| Ear, nose and throat | 120 | 107 |
| Neurosurgery | 150 | 183 |
| Plastic surgery | 160 | 107 |
| Cardiac surgery | 172 | 259 |
| Thoracic surgery | 173 | 206 |
| Anaesthetics | 190 | 141 |
| Pain management | 191 | 157 |
| General medicine | 300 | 167 |
| Gastroenterology | 301 | 141 |
| Endocrinology | 302 | 161 |
| Clinical haematology | 303 | 167 |
| Hepatology | 306 | 196 |
| Rehabilitation service | 314 | 156 |
| Palliative medicine | 315 | 176 |
| Cardiology | 320 | 139 |
| Anticoagulant service | 324 | 37 |
| Stroke medicine | 328 | 197 |
| Transient ischaemic attack | 329 | 197 |
| Respiratory medicine | 340 | 157 |
| Respiratory physiology | 341 | 120 |
| Nephrology | 361 | 164 |
| Medical oncology | 370 | 187 |
| Neurology | 400 | 177 |
| Clinical neurophysiology | 401 | 235 |
| Rheumatology | 410 | 147 |
| Geriatric medicine | 430 | 253 |
| Gynaecology | 502 | 141 |
| Gynaecological oncology | 503 | 127 |
| Physiotherapy | 650 | 58 |
| Occupational therapy | 651 | 71 |
| Speech and language therapy | 652 | 100 |
| Clinical psychology | 656 | 199 |
| Clinical oncology (previously radiotherapy) | 800 | 143 |
| Interventional radiology | 811 | 93 |
| Resource | Unit cost (£) | Source |
|---|---|---|
| Chemotherapy | ||
| First chemotherapy administration | 385 | National Schedule of Reference Costs 2018–19:42 chemotherapy. Day case. SB14Z deliver complex chemotherapy, including prolonged infusional treatment, at first attendance |
| Subsequent chemotherapy administration | 223 | National Schedule of Reference Costs 2018–19:42 chemotherapy. Outpatient. SB15Z deliver subsequent elements of a chemotherapy cycle |
| Chemotherapy drugs (average per visit, assuming cisplatin 145 mg on day 1 of cycle and vinorelbine 55 mg on days 1 and 8 of cycle) | 21.91 | eMIT46 |
| Radiotherapy | ||
| Define volume for radiation therapy | 380 | National Schedule of Reference Costs 2018–19:42 radiotherapy. Outpatient. SC45Z preparation for simple radiotherapy with imaging and dosimetry |
| First radiotherapy administration | 127 | National Schedule of Reference Costs 2018–19:42 radiotherapy. Outpatient. SC23Z deliver a fraction of complex treatment on a megavoltage machine |
| Subsequent radiotherapy administration | 109 | National Schedule of Reference Costs 2018–19:42 radiotherapy. Outpatient. SC22Z deliver a fraction of treatment on a megavoltage machine |
| Resource | Unit cost (£) | Source |
|---|---|---|
| Day case unit | 752 | National Schedule of Reference Costs 2018–19:42 average across all day case activity |
| Biopsy | 659 | National Schedule of Reference Costs 2018–19:42 day case. YD03Z Percutaneous biopsy of lesion of lung or mediastinum |
| Cardiac MRI | 332 | National Schedule of Reference Costs 2018–19:42 diagnostic imaging. Weighted average of outpatient costs for RD08Z/09Z/10Z cardiac magnetic resonance imaging scan |
| CT biopsy | 742 | National Schedule of Reference Costs 2018–19:42 day case. YD03Z Percutaneous biopsy of lesion of lung or mediastinum; and diagnostic imaging. RD20A computerised tomography scan of one area, without contrast, 19 years and over |
| Direct access blood test | 4 | National Schedule of Reference Costs 2018–19:42 directly accessed pathology services. DAPS08 phlebotomy |
| Electroencephalogram monitor | 204 | National Schedule of Reference Costs 2018–19:42 outpatient procedure. AA33C conventional EEG, EMG or nerve conduction studies, 19 years and over for 401 clinical neurophysiology |
| Endobronchial ultrasound | 728 | National Schedule of Reference Costs 2018–19:42 Day Case. DZ70Z Endobronchial Ultrasound Examination of Mediastinum |
| Full pulmonary function testing | 164 | National Schedule of Reference Costs 2018–19:42 outpatient procedure. DZ52Z full pulmonary function testing for 340 respiratory medicine |
| PET with CT scan | 549 | National Schedule of Reference Costs 2018–19:42 nuclear medicine. Imaging: outpatient. RN01A Positron emission tomography with computed tomography (PET-CT) of one area, 19 years and over |
| Vascular ultrasound scan | 66 | National Schedule of Reference Costs 2018–19:42 diagnostic imaging. RD47Z vascular ultrasound scan |
| Resource | Unit cost (£) | Source |
|---|---|---|
| Residential home (1 week) | 620 | Unit Costs of Health and Social Care 2019:44 1.2 private sector residential care for older people (age 65 + years). Mean per person weekly PSS contributions to residential care |
| Hospice (per day) | 144 | Cost per day in hospice and estimate of proportion paid for by government (one-third) sourced from Georghiou and Bardsley67 |
| GP or out-of-hours GP at surgery or walk-in centre | 28 | Unit Costs of Health and Social Care 2019:44 10.3b, general practitioner – unit costs. Per surgery consultation contact lasting 9.22 minutes. Excluding qualification costs and direct care staff costs |
| GP at home | 50 | Unit Costs of Health and Social Care 2019:44 10.3b, general practitioner – unit costs. Assumes 9.22 minutes of patient contact and 12 minutes of travel time. Excluding qualification costs and direct care staff costs |
| GP by telephone | 28 | Unit Costs of Health and Social Care 2019:44 10.3b, general practitioner – unit costs. Assumes 9.22 minutes of patient contact. Excluding qualification costs and direct care staff costs |
| Nurse at GP surgery or walk-in centre | 12.43 | Unit Costs of Health and Social Care 2019:44 10.6, nurse (general practice). £37 per hour, excluding qualification costs. (Assumes average contact of 15.5 minutes and a ratio of direct : indirect time of 1 : 0.3, from previous edition) |
| Nurse at home | 40 | National Schedule of Reference Costs 2018–19:42 community health services. N02AF district nurse, adult, face to face |
| Nurse by telephone | 16 | National Schedule of Reference Costs 2018–19:42 community health services. N02AN district nurse, adult, non face to face |
| Health-care assistant | 7.64 | Unit Costs of Health and Social Care 2017:68 14. hospital-based nurses. Band 2. Cost per working hour £22. (Assume average contact of 15.5 minutes and a ratio of direct : indirect time 1 : 0.3, as nurse above) |
| Respiratory nurse | 91 | National Schedule of Reference Costs 2018–19:42 community health services. Nursing N08AF specialist nursing, asthma and respiratory nursing/liaison, adult, face to face |
| Cardiac clinical nurse specialist by telephone | 59 | National Schedule of Reference Costs 2018–19:42 community health services. Nursing. N11AN specialist nursing, cardiac nursing/liaison, adult, non-face to face |
| Clinical nurse specialist by telephone | 38 | National Schedule of Reference Costs 2018–19:42 community health services. Nursing N29AN other specialist nursing, adult, non-face to face |
| Doctor at a community hospital | 83 | National Schedule of Reference Costs 2018–19:42 non-consultant led. WF01A/B non-admitted face-to-face attendance, weighted average of first and follow-up for all service codes except those for paediatrics |
| Hospital doctor by telephone | 66 | National Schedule of Reference Costs 2018–19:42 non-consultant led. WF01C/D non-admitted non-face-to-face attendance, weighted average of first and follow-up for all service codes except those for paediatrics |
| Dietitian | 90 | National Schedule of Reference Costs 2018–19:42 community health services. Allied health professionals. A03 dietitian |
| Dietitian by telephone | 48 | National Schedule of Reference Costs 2018–19:42 non-consultant led. 654 dietetics WF01C/D non-admitted non-face-to-face attendance, weighted average of first and follow-up |
| Occupational therapy | 83 | National Schedule of Reference Costs 2018–19:42 community health services. Allied health professionals. A06A1 occupational therapist, adult, one to one |
| Physiotherapist | 63 | National Schedule of Reference Costs 2018–19:42 community health services. Allied health professionals. A08A1 physiotherapist, adult, one to one |
| Physiotherapist: pulmonary rehabilitation class | 54 | National Schedule of Reference Costs 2018–19:42 community health services. Allied health professionals. A08AG physiotherapist, adult, group |
| Physiotherapist by telephone | 42 | National Schedule of Reference Costs 2018–19:42 non-consultant led. 650 physiotherapy WF01C/D non-admitted non-face-to-face attendance, weighted average of first and follow-up |
| Speech and language therapist | 107 | National Schedule of Reference Costs 2018–19:42 community health services. Allied health professionals. A13A1 speech and language therapist, adult, one to one |
| Pharmacist | 15 | Unit Costs of Health and Social Care 2019:44 9 scientific and professional staff. Band 6. Cost per working hour: £45. Assume 20 minutes |
| Cognitive–behavioural therapist | 96 | Unit Costs of Health and Social Care 2019:44 2.1 NHS reference costs for mental health services; mental health specialist teams (per care contact); improving access to psychological therapies (IAPT), adult and elderly |
| Paramedic | 209 | National Schedule of Reference Costs 2018–19:42 ambulance ASS01, see and treat or refer |
| Call to 111 | 13.77 | Pope et al.69 |
Appendix 4 Sensitivity analyses for the economic evaluation
Sensitivity analyses for costing were conducted to examine the impact of varying key unit costs and the impact of any high-cost participants on overall costing results. Sensitivity analyses around outcomes explored the robustness of results to the missing survival data and the impact of not adjusting for baseline EQ-5D-5L. Each of these sensitivity analyses is considered in turn.
Sensitivity analyses around unit costs
Table 40 describes the unit costs around time in theatre, staples and inpatient stay, which were varied in sensitivity analyses. Variables investigated were high-cost items used by many (or all) patients. Table 41 reports the results. Although these resources were all key cost drivers, varying these costs by ± 50% did not have a great impact on the cost difference between groups. The cost differences across the sensitivity analyses ranged from –£3210 to –£2194, bracketing and not substantially different from the base-case cost difference of –£2702.
| Sensitivity analysis | Resource | Unit costs used in base-case analysis | Alternative strategy for sensitivity analysis |
|---|---|---|---|
| 1 | Time in theatre | £876 per hour | ± 50% |
| 2 | Staples | £533 per stapler and £168 per staple | ± 50% |
| 3 | Intensive care and high-dependency bed-days in index admission | £1445 for intensive care and £917 for high dependency | ± 50% |
| 4 | Ward stay in index admission | £396 | ± 50% |
| Sensitivity analysis | Participant allocation | VATS vs. open surgery, mean cost (£) difference (95% CI) | |
|---|---|---|---|
| Randomised to VATS (n = 229), mean cost (£) (SE) | Randomised to open surgery (n = 235), mean cost (£) (SE) | ||
| Base case | 10,879 (1057) | 13,581 (1046) | –2702 (–5624 to 221) |
| 1: theatre | |||
| +50% | 12,043 (1059) | 14,606 (1048) | –2563 (–5491 to 365) |
| –50% | 9715 (1055) | 12,556 (1044) | –2840 (–5758 to 77) |
| 2: staples | |||
| +50% | 11,840 (1058) | 14,489 (1047) | –2649 (–5576 to 278) |
| –50% | 9919 (1056) | 12,673 (1045) | –2754 (–5673 to 165) |
| 3: critical care | |||
| +50% | 11,720 (1286) | 14,931 (1273) | –3210 (–6767 to 347) |
| –50% | 10,037 (845) | 12,231 (836) | –2194 (–4530 to 141) |
| 4: ward stay | |||
| +50% | 11,686 (1072) | 14,533 (1060) | –2848 (–5811 to 116) |
| –50% | 10,072 (1045) | 12,628 (1034) | –2556 (–5446 to 334) |
Sensitivity analyses around high-cost participants
The distribution of total costs per participant is positively skewed in both surgery groups. Therefore, it is possible that a few high-cost outliers are exerting significant influence on the overall findings. Accordingly, we examined the existence of outliers and their effects. There were five participants with costs > £60,000 (VATS group, n =2; open-surgery group, n =3). Three of these participants had costs in the order of £60,000–70,000, but two open-surgery participants had costs of £113,000 and £294,000. These participants had long stays in hospital, with significant time spent in intensive care, and one participant had 3 weeks of extracorporeal membrane oxygenation. There are no grounds for excluding these participants from the analyses. Nevertheless, it is instructive to investigate the impact these participants are having on the cost results, as an imbalance across groups of these outliers could easily have arisen by chance.
Table 42 shows the effects on costs in each treatment group of excluding the two highest-cost participants and of excluding the five highest-cost participants. In both cases, the mean cost difference between groups halves and uncertainty reduces, resulting in a significant difference in costs when the five highest-cost participants are excluded. Although these participants exert a significant impact on the cost results, they do not alter conclusions.
| Sensitivity analysis | Participant allocation | VATS vs. open surgery, mean cost (£) difference (95% CI) | |
|---|---|---|---|
| Randomised to VATS (n = 229), mean cost (£) (SE) | Randomised to open surgery (n = 235), mean cost (£) (SE) | ||
| Base case (all participants) | 10,879 (1057) | 13,581 (1046) | –2702 (–5624 to 221) |
| Excludes two highest-cost participants (> £100,000) | 10,879 (525) | 11,950 (524) | –1071 (–2530 to 388) |
| Excludes five highest-cost participants (> £60,000) | 10,435 (445) | 11,701 (445) | –1266 (–2502 to –29) |
Sensitivity analyses around outcomes
Two sensitivity analyses were conducted around outcomes. In the base-case analysis, participants missing survival status were assumed to be alive at 12 months. In a sensitivity analysis, we have assumed that these participants died at 12 months. A second sensitivity analysis compares results without adjustment for baseline utility. Results are shown in Table 43. Neither sensitivity analysis alters conclusions and under each scenario QALYs are statistically significantly higher in the VATS group than in the open-surgery group.
| Sensitivity analysis: QALYs to 12 months | Participant allocation | VATS vs. open surgery, MD (95% CI) | |
|---|---|---|---|
| Randomised to VATS (N = 229), mean (SE) | Randomised to open surgery (N = 235), mean (SE) | ||
| Base case | 0.841 (0.017) | 0.780 (0.016) | 0.060 (0.025 to 0.095) |
| Unknown survivors died | 0.827 (0.017) | 0.769 (0.017) | 0.057 (0.021 to 0.094) |
| No adjustment for baseline utility | 0.695 (0.015) | 0.644 (0.015) | 0.051 (0.009 to 0.092) |
Appendix 5 Additional tables and figures
| Exclusion reason | Site | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Brompton | Liverpool | Bristol | Middlesbrough | Harefield | Oxford | Hull | Birmingham | Edinburgh | |
| Excluded, n | 182 | 311 | 662 | 85 | 183 | 121 | 41 | 21 | 0 |
| Ineligible, n | 120 | 219 | 484 | 62 | 121 | 68 | 35 | 1 | 0 |
| Age < 16 years | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unable to give informed consent | 2 | 3 | 8 | 2 | 4 | 1 | 1 | 0 | 0 |
| Disease not suitable for VATS and open surgery | 34 | 61 | 95 | 25 | 53 | 28 | 28 | 0 | 0 |
| Not undergoing lobectomy/bi-lobectomy or frozen section biopsy with option to proceed to lobectomy/bi-lobectomy | 45 | 21 | 117 | 22 | 73 | 15 | 2 | 0 | 0 |
| Not known or suspected primary lung cancer beyond lobar orifice | 3 | 100 | 45 | 16 | 18 | 7 | 5 | 0 | 0 |
| Not TNM813 stage cT1–3, N0–1, M0 | 68 | 149 | 143 | 27 | 58 | 16 | 9 | 1 | 0 |
| Planned wedge resection | 19 | 23 | 72 | 5 | 34 | 7 | 11 | 0 | 0 |
| Planned segmentectomy | 15 | 9 | 64 | 6 | 10 | 3 | 1 | 0 | 0 |
| Planned pneumonectomy | 9 | 14 | 23 | 4 | 4 | 4 | 2 | 0 | 0 |
| Planned robotic surgery | 0 | 18 | 3 | 9 | 1 | 1 | 1 | 0 | 0 |
| Previous malignancy that influences life expectancy | 3 | 97 | 166 | 4 | 34 | 8 | 1 | 0 | 0 |
| Serious concomitant disorder that would compromise patient safety during surgery | 3 | 15 | 82 | 5 | 20 | 15 | 3 | 0 | 0 |
| Not approached, n | 13 | 59 | 36 | 7 | 14 | 13 | 3 | 2 | 0 |
| Not sent PIL | 2 | 45 | 11 | 3 | 12 | 3 | 3 | 1 | 0 |
| Clinical reason | 3 | 3 | 15 | 2 | 0 | 1 | 0 | 1 | 0 |
| Declined surgery/wants alternative treatment | 8 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Logistics | 0 | 1 | 4 | 1 | 1 | 6 | 0 | 0 | 0 |
| Declined/no reason given | 0 | 2 | 1 | 1 | 0 | 2 | 0 | 0 | 0 |
| Patient preference | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Personal reasons | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Other | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Did not consent, n | 46 | 33 | 122 | 12 | 44 | 37 | 3 | 18 | 0 |
| Patient preference | 25 | 17 | 30 | 4 | 20 | 5 | 1 | 1 | 0 |
| Declined | 4 | 10 | 31 | 8 | 1 | 20 | 2 | 9 | 0 |
| Personal reasons | 1 | 3 | 31 | 0 | 3 | 9 | 0 | 0 | 0 |
| Unwilling to be randomised/wants to know surgery | 2 | 1 | 8 | 0 | 17 | 2 | 0 | 2 | 0 |
| Logistics | 3 | 2 | 5 | 0 | 3 | 1 | 0 | 3 | 0 |
| Clinical reason | 4 | 0 | 9 | 0 | 0 | 0 | 0 | 3 | 0 |
| Wants alternative treatment | 5 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery cancelled | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other reason, n | 3 | 0 | 20 | 4 | 4 | 3 | 0 | 0 | 0 |
| Patient withdrew post consent, but pre randomisation | 3 | 0 | 20 | 4 | 4 | 3 | 0 | 0 | 0 |
| Site | Lobectomies/year | Recruitment rate/month in first 6 monthsa | Recruitment rate/month from month 7 onwards patients recruitedb | |||
|---|---|---|---|---|---|---|
| 2015 | 2016 onwards | 2015 | 2016 onwards | 2015 | 2016 onwards | |
| Phase I sites | ||||||
| Brompton | 120 | 150 | 1.8 | 2.25 | 3 | 3.75 |
| Liverpool | 100 | 100 | 1.5 | 1.5 | 2.5 | 2.5 |
| Bristol | 50 | 70 | 0.75 | 1.05 | 1.25 | 1.75 |
| Middlesbrough | 70 | 90 | 1.05 | 1.35 | 1.75 | 2.25 |
| Harefield | 40 | 50 | 0.6 | 0.75 | 1 | 1.25 |
| Phase II sites | ||||||
| Birmingham | 80 | 1.2 | 2 | |||
| Hull | 101 | 1.5 | 2.5 | |||
| Oxford | 67 | 1 | 1.67 | |||
| Edinburgh | Not provided | |||||
| Site | Total randomised, n | Randomisation rate per month |
|---|---|---|
| Brompton | 106 | 2.5 |
| Liverpool | 59 | 1.4 |
| Bristol | 136 | 3.4 |
| Middlesbrough | 98 | 2.5 |
| Harefield | 34 | 0.9 |
| Oxford | 25 | 1.5 |
| Hull | 9 | 0.5 |
| Birmingham | 30 | 1.8 |
| Edinburgh | 6 | 1.1 |
| Criterion | Target | Achieved, n/N (%) [95% CI] |
|---|---|---|
| Eligible | At least 60% | 281/513 (54.8%) [50.3% to 59.2%] |
| Consented | 50% after 6 monthsa | 149/281 (53.0%) [47.0% to 59.0%] |
| Failure to receive allocated treatment | < 5% | 3/119 (2.5%) [1.4% to 7.2%] |
| Lost to follow-up | < 5% | 0 |
| Confirmed benign disease | < 5% | 1/115 (0.8%) [0.02% to 4.7%] |
| Characteristic | Non-randomised patients | |||
|---|---|---|---|---|
| Ineligible (n = 1110) | Not approached (n = 147) | Did not consent (n = 315) | Randomised (n = 503) | |
| Age (years), median (IQR)a | 70.3 (63.1–75.7) | 71.6 (65.6–77.0) | 69.3 (62.7–75.9) | 69.9 (63.5–75.6) |
| Characteristic | Participant allocation | Total (N = 502) | |
|---|---|---|---|
| Randomised to VATS (N = 247) | Randomised to open surgery (N = 255) | ||
| Participant demography | |||
| Ethnicity, n/N (%) | |||
| White | 239/247 (96.8) | 245/255 (96.1) | 484/502 (96.4) |
| Black | 2/247 (0.8) | 3/255 (1.2) | 5/502 (1.0) |
| Mixed | 1/247 (0.4) | 1/255 (0.4) | 2/502 (0.4) |
| Asian | 4/247 (1.6) | 3/255 (1.2) | 7/502 (1.4) |
| Other | 1/247 (0.4) | 3/255 (1.2) | 4/502 (0.8) |
| BMI (kg/m2), mean (SD) | 27 (5.1) | 27 (4.8) | 27 (5.0) |
| Smoking status (ever smoked), n/N (%) | 212/247 (85.8) | 226/255 (88.6) | 438/502 (87.3) |
| Comorbidities, n/N (%) | |||
| Respiratorya | 87/247 (35.2) | 88/255 (34.5) | 175/502 (34.9) |
| Neurological dysfunctionb | 9/247 (3.6) | 9/255 (3.5) | 18/502 (3.6) |
| Diabetes mellitus | 29/247 (11.7) | 32/255 (12.5) | 61/502 (12.2) |
| Alcoholismc | 19/247 (7.7) | 15/255 (5.9) | 34/502 (6.8) |
| Previous lung surgery | 4/247 (1.6) | 5/255 (2.0) | 9/502 (1.8) |
| CVA and/or TIA | 24/247 (9.7) | 21/255 (8.2) | 45/502 (9.0) |
| Cardiovasculard | 109/247 (44.1) | 124/255 (48.6) | 233/502 (46.4) |
| Chronic pain syndromee | 31/247 (12.6) | 28/255 (11.0) | 59/502 (11.8) |
| Deep-vein thrombosis | 11/247 (4.5) | 9/255 (3.5) | 20/502 (4.0) |
| Previously treated malignancy | 26/247 (10.5) | 35/255 (13.7) | 61/502 (12.2) |
| Surgical details | |||
| First operator classification, n/N (%)f | |||
| Consultant surgeon | 194/220 (88.2) | 177/224 (79.0) | 371/444 (83.6) |
| Trainee surgeon | 26/220 (11.8) | 47/224 (21.0) | 73/444 (16.4) |
| Resection extent, n/N (%) | |||
| Lobectomy | 216/247 (87.4) | 225/255 (88.2) | 441/502 (87.8) |
| Lobectomy and wedge resection | 3/247 (1.2) | 7/255 (2.7) | 10/502 (2.0) |
| Lobectomy and resection of airway | 1/247 (0.4) | 0/255 (0.0) | 1/502 (0.2) |
| Segmentectomy | 1/247 (0.4) | 2/255 (0.8) | 3/502 (0.6) |
| Pneumonectomy | 2/247 (0.8) | 0/255 (0.0) | 2/502 (0.4) |
| Wedge resection | 8/247 (3.2) | 3/255 (1.2) | 11/502 (2.2) |
| Open and close (inoperable/extensive malignancy) | 0/247 (0.0) | 2/255 (0.8) | 2/502 (0.4) |
| Lobectomy | |||
| Location of resection, n/N (%) | |||
| Right-upper lobe | 76/221 (34.4) | 90/232 (38.8) | 166/453 (36.6) |
| Right-middle lobe | 14/221 (6.3) | 9/232 (3.9) | 23/453 (5.1) |
| Right-lower lobe | 39/221 (17.6) | 40/232 (17.2) | 79/453 (17.4) |
| Left-upper lobe | 54/221 (24.4) | 52/232 (22.4) | 106/453 (23.4) |
| Left-lower lobe | 34/221 (15.4) | 39/232 (16.8) | 73/453 (16.1) |
| Right-upper and right-middle lobe | 1/221 (0.5) | 0/232 (0.0) | 1/453 (0.2) |
| Right-middle and right-lower lobe | 3/221 (1.4) | 2/232 (0.9) | 5/453 (1.1) |
| Muscle-sparing approach used, n/N (%) | 6/15 (40.0) | 124/229 (54.1) | 130/244 (53.3) |
| Serratus sparingb | 4/4 (100.0) | 56/81 (69.1) | 60/85 (70.6) |
| Latissimus sparingb | 0/4 (0.0) | 34/81 (42.0) | 34/85 (40.0) |
| Time point | QLQ-C30 physical functioning score, median IQR | |
|---|---|---|
| Randomised to VATS (n = 247) | Randomised to open surgery (n = 255) | |
| Baselinea | 87 (73.3–100.0) | 87 (73.3–100.0) |
| 2 weeksb | 73 (53.3–80.0) | 60 (40.0–78.9) |
| 5 weeksc | 73 (60.0–86.7) | 67 (53.3–86.7) |
| 3 monthsd | 80 (60.0–93.3) | 73 (60.0–86.7) |
| 6 monthse | 82 (63.3–93.3) | 80 (60.0–86.7) |
| 12 monthsf | 82 (66.7–93.3) | 80 (60.0–86.7) |
FIGURE 27.
Relative risk for complete R(0) resection.
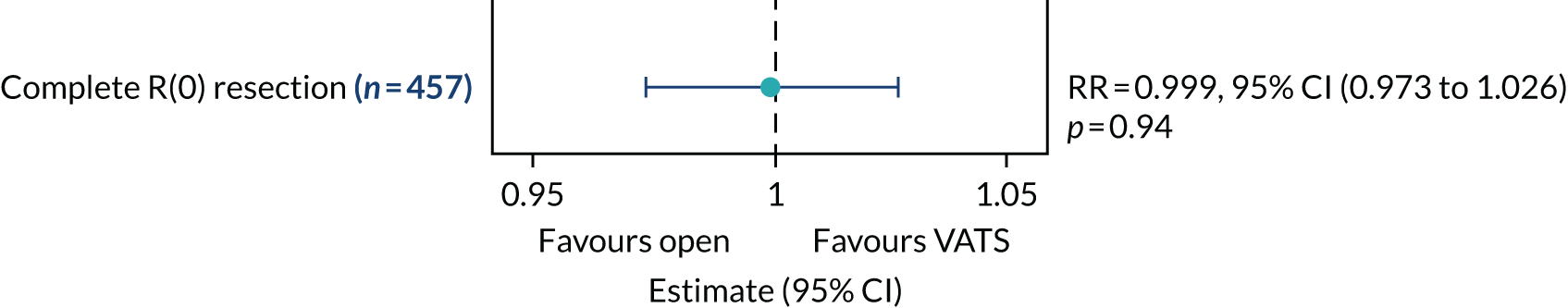
FIGURE 28.
Relative risks for upstaging to N1 and upstaging to N2.
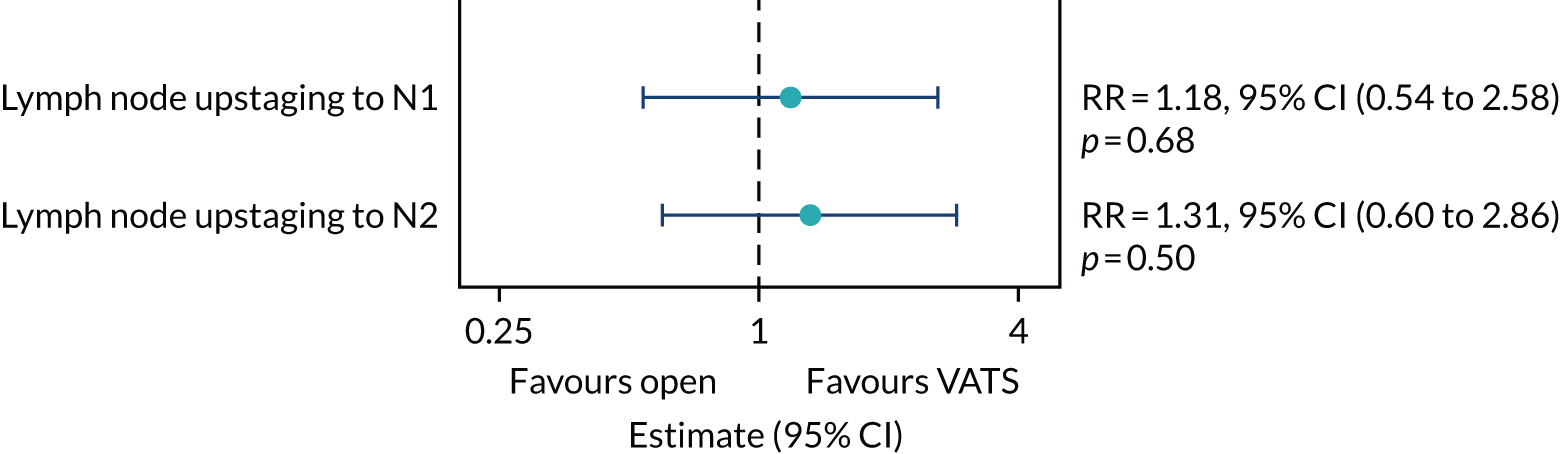
| Outcome | Participant allocation | MD (95% CI) | p-value | |
|---|---|---|---|---|
| Randomised to VATS (n = 247) | Randomised to open surgery (n = 255) | |||
| VAS pain score, median (IQR) | ||||
| Baselinea | 0 (0.0–1.0) | 0 (0.0–1.0) | ||
| Day 1b | 4 (2.0–5.0) | 4 (2.0–5.0) | –0.024 (–0.463 to 0.414) | 0.913 |
| Day 2c | 3 (1.0–5.0) | 4 (2.0–5.0) | –0.539 (–0.986 to –0.092) | 0.018 |
| Test for treatment-by-time interaction | 0.044 | |||
| Outcome | Participant allocation | MD (95% CI) | p-value | |
|---|---|---|---|---|
| Randomised to VATS (n = 218) | Randomised to open surgery (n = 221) | |||
| VAS pain score, median (IQR) | ||||
| Baselinea | 0 (0.0–1.0) | 0 (0.0–1.0) | ||
| Day 1b | 4 (2.0–5.0) | 4 (2.0–5.0) | –0.12 (–0.56 to 0.32) | 0.591 |
| Day 2c | 3 (1.0–5.0) | 4 (2.0–5.0) | –0.55 (–1.01 to –0.10) | 0.017 |
| Test for treatment-by-time interaction | 0.086 | |||
| Analgesia | Participant allocation | |
|---|---|---|
| Randomised to VATS (N = 247), n/N (%) | Randomised to open surgery (N = 254), n/N (%) | |
| Epidural | 2/247 (0.8) | 6/254 (2.4) |
| Diclofenac | 6/247 (2.4) | 11/254 (4.3) |
| Alfentanil | 4/247 (1.6) | 6/254 (2.4) |
| Clonidinea | 12/247 (4.9) | 16/254 (6.3) |
| Ketamine | 2/247 (0.8) | 6/254 (2.4) |
| Parecoxib | 6/247 (2.4) | 12/254 (4.7) |
| Magnesium | 9/247 (3.6) | 13/254 (5.1) |
| Outcome | MD (95% CI) | p-value |
|---|---|---|
| Single-port VATS vs multiport VATS | –0.25 (–1.07 to 0.56) | 0.524 |
| Single-port VATS vs. open surgery | –0.71 (–1.94 to 0.51) | |
| Multiport VATS vs. open surgery | –0.46 (–1.50 to 0.58) |
FIGURE 29.
Relative risk for prolonged incision pain.
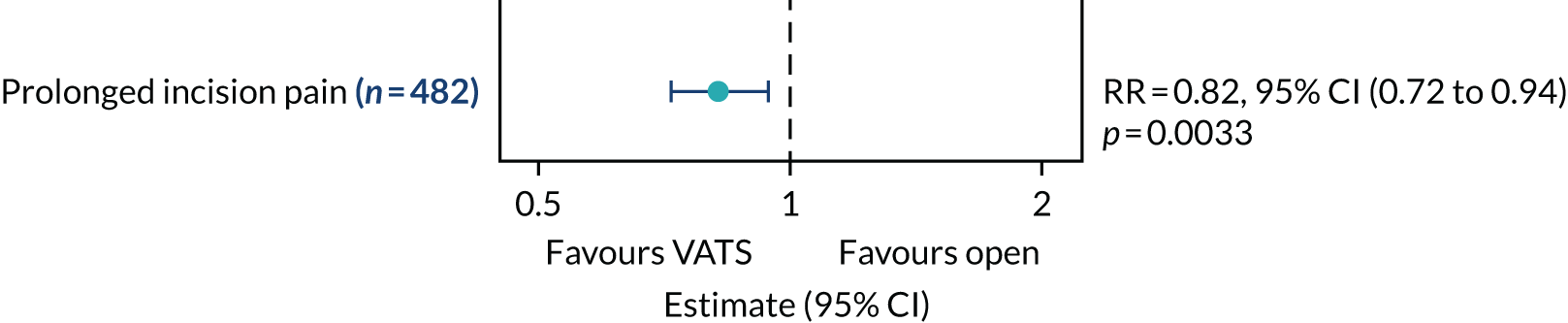
| Outcome | Participant allocation | HR (95% CI) | p-value | |
|---|---|---|---|---|
| Randomised to VATS (n = 247) | Randomised to open surgery (n = 255) | |||
| Time to discharge (days), median (IQR) | 4 (3–7) | 5 (3–8) | 1.34 (1.09 to 1.65) | 0.0059 |
| Outcome | HR (95% CI) | p-value |
|---|---|---|
| Single-port VATS vs. multiport VATS | 0.91 (0.56 to 1.49) | 0.017 |
| Single-port VATS vs. open surgery | 2.00 (1.02 to 3.91) | |
| Multiport VATS vs. open surgery | 2.19 (1.25 to 3.85) |
FIGURE 30.
Overall survival.
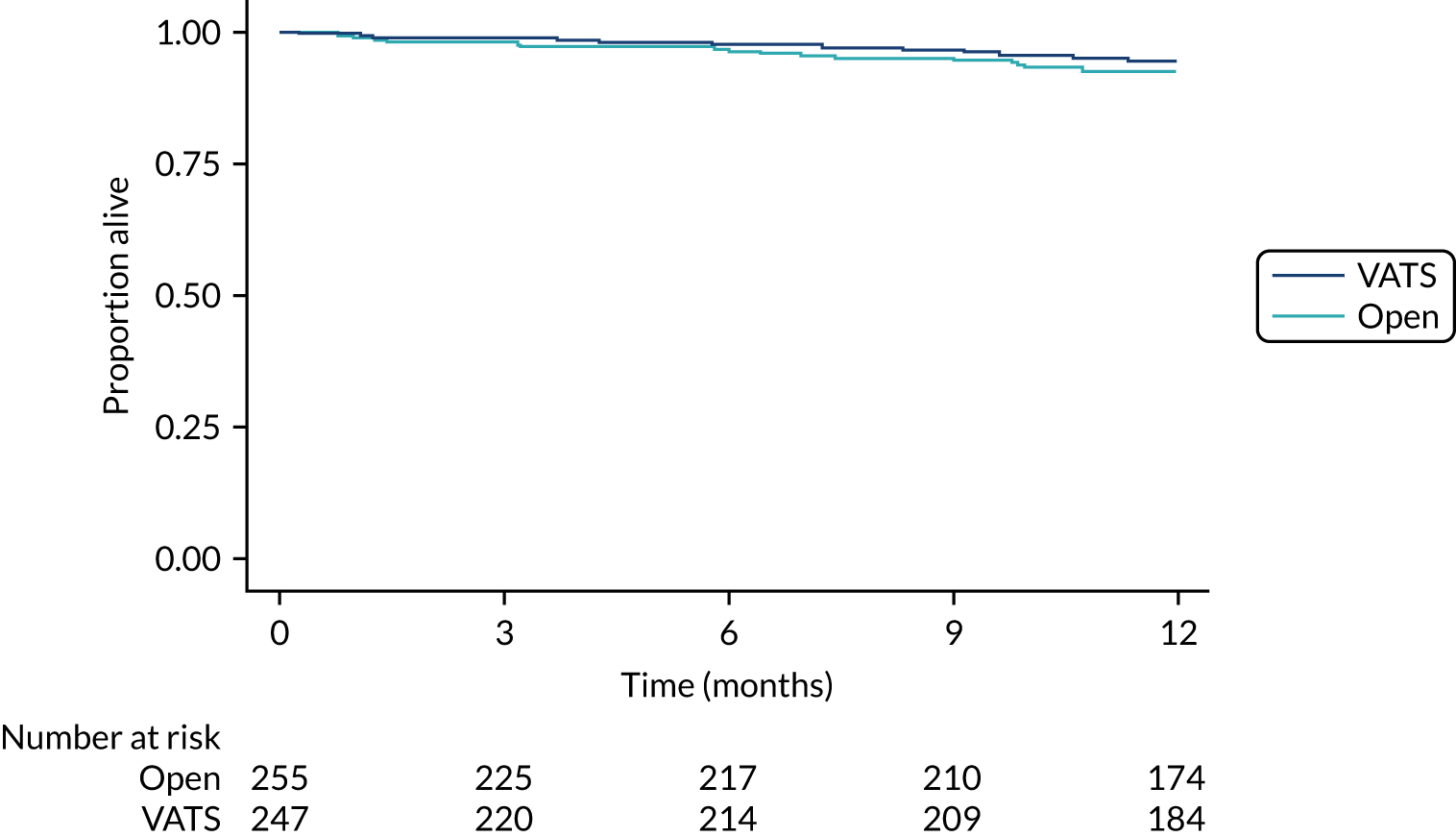
FIGURE 31.
Progression-free survival.
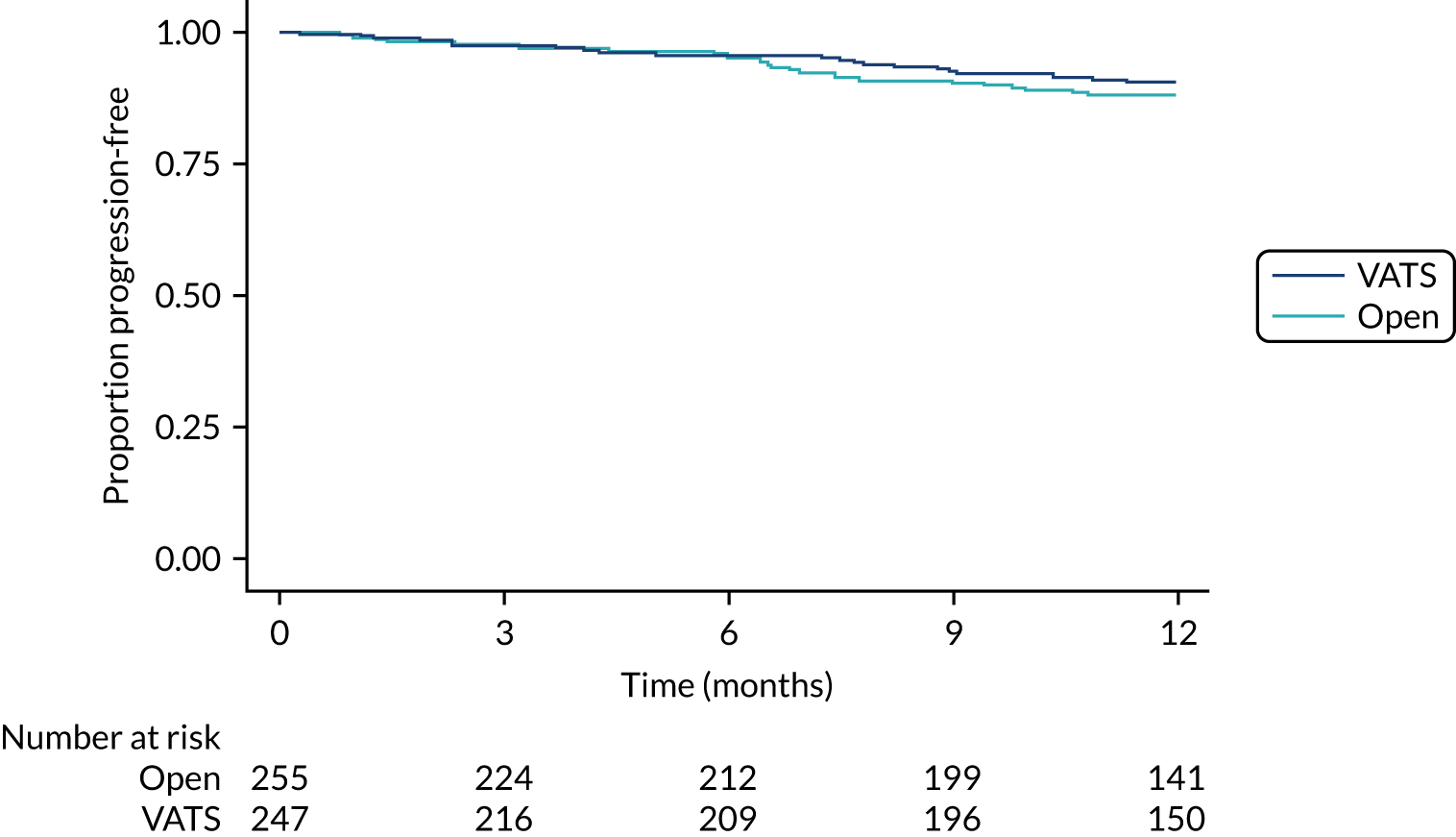
| Outcome | Participant allocation | HR (95% CI) | p-value | |
|---|---|---|---|---|
| Randomised to VATS (N = 247) | Randomised to open surgery (N = 255) | |||
| Received adjuvant treatment, n/N (%) | 34/216 (15.7) | 39/216 (18.1) | ||
| Received adjuvant treatment (eligible subseta), n/N (%) | 28/55 (50.9) | 28/61 (45.9) | ||
| Time to uptake of adjuvant treatment (months) | 0.90 (0.50 to 1.61) | 0.716 | ||
| Time to uptake of adjuvant treatment (eligible subseta) (months) | 11.0 (2.1, –) | – (2.0, –) | 1.12 (0.62 to 2.02) | 0.716 |
FIGURE 32.
QLQ-C30 global health status over time. Higher scores indicate better health.
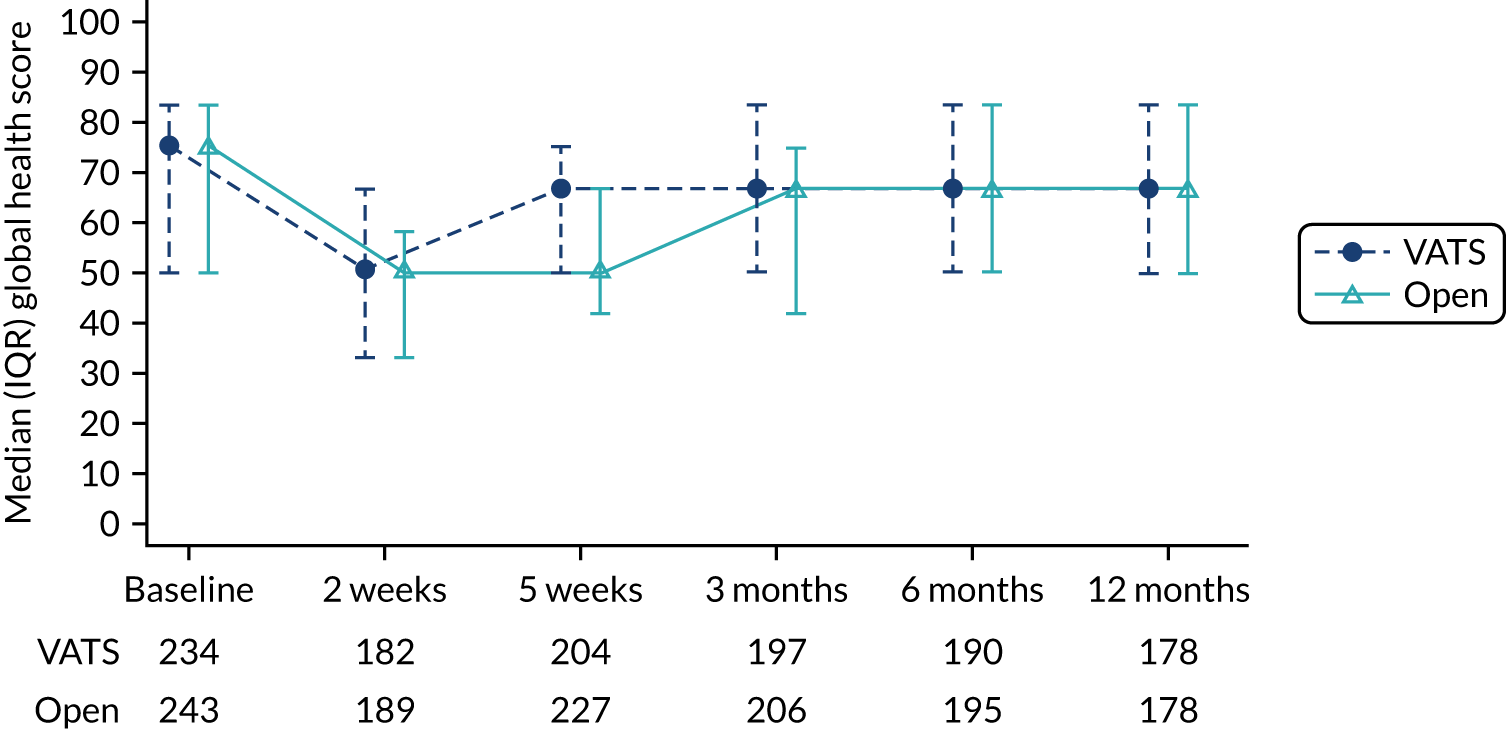
FIGURE 33.
QLQ-C30 role functioning over time. Higher scores indicate better health.
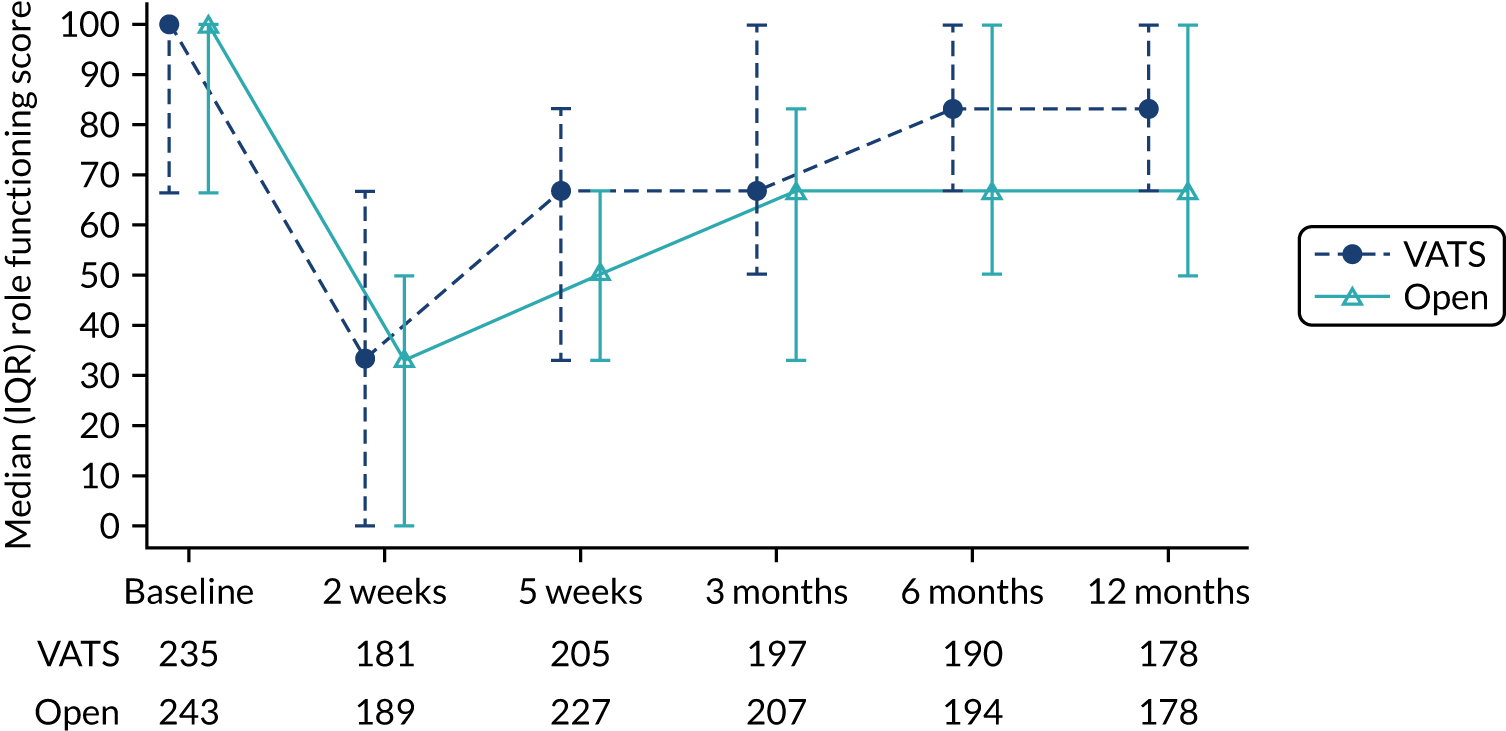
FIGURE 34.
QLQ-C30 social functioning over time. Higher scores indicate better health.
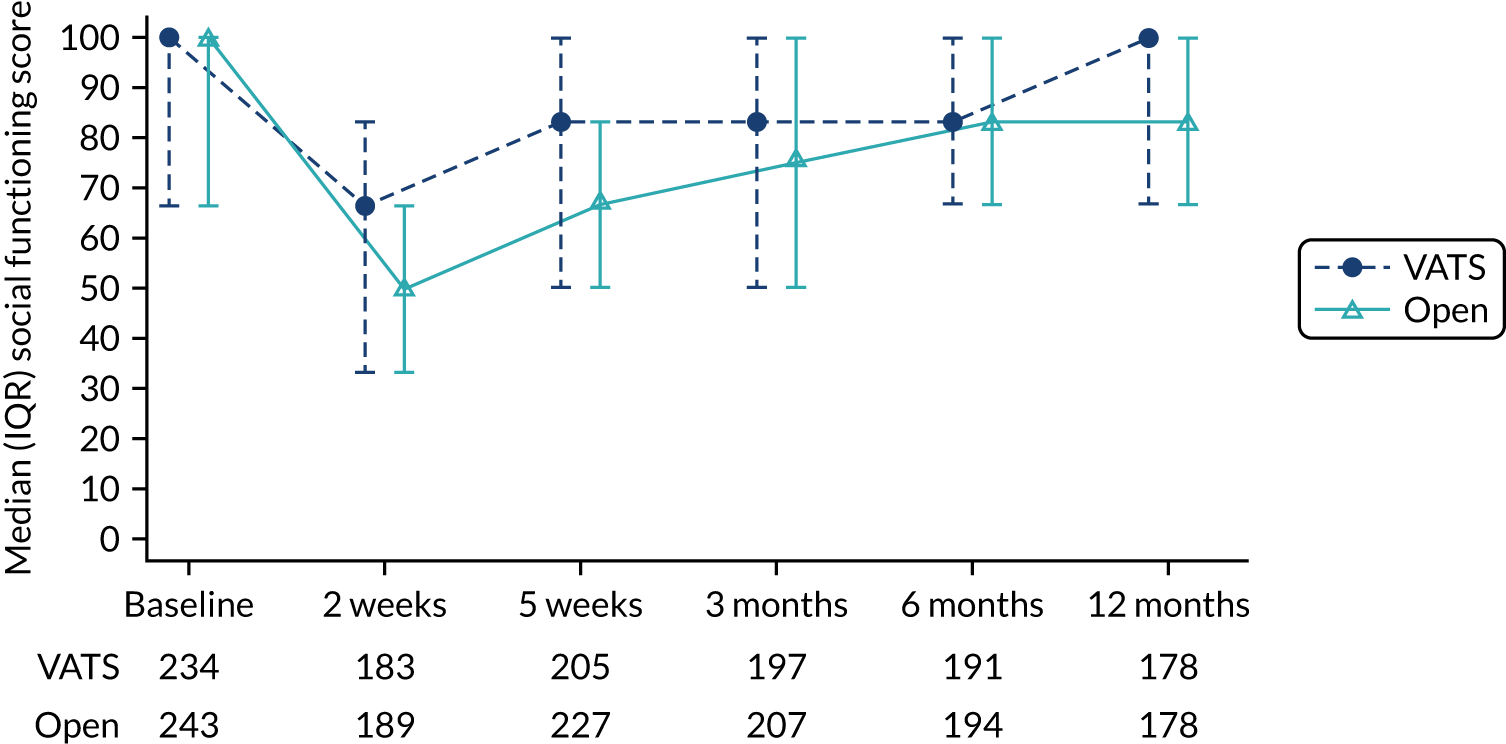
| Outcome | Participant allocation | MD (95% CI) | p-value | |
|---|---|---|---|---|
| Randomised to VATS (n = 247), median (IQR) | Randomised to open surgery (n = 255), median (IQR) | |||
| Global health status/quality of life | ||||
| Time point | ||||
| Baselinea | 75.0 (50.0–83.3) | 75.0 (50.0–83.3) | ||
| 2 weeksb | 50.0 (33.3–66.7) | 50.0 (33.3–58.3) | ||
| 5 weeksc | 66.7 (50.0–75.0) | 50.0 (41.7–66.7) | ||
| 3 monthsd | 66.7 (50.0–83.3) | 66.7 (41.7–75.0) | ||
| 6 monthse | 66.7 (50.0–83.3) | 66.7 (50.0–83.3) | ||
| 12 monthsf | 66.7 (50.0–83.3) | 66.7 (50.0–83.3) | ||
| Test for time-by-treatment interaction | 0.29 | |||
| Overall treatment effect | 4.21 (1.62 to 6.79) | 0.0088g | ||
| Role functioning | ||||
| Time point | ||||
| Baselineh | 100.0 (66.7–100.0) | 100.0 (66.7–100.0) | ||
| 2 weeksi | 33.3 (0.0–66.7) | 33.3 (0.0–50.0) | ||
| 5 weeksj | 66.7 (33.3–83.3) | 50.0 (33.3–66.7) | ||
| 3 monthsk | 66.7 (50.0–100.0) | 66.7 (33.3–83.3) | ||
| 6 monthsl | 83.3 (66.7–100.0) | 66.7 (50.0–100.0) | ||
| 12 monthsf | 83.3 (66.7–100.0) | 66.7 (50.0–100.0) | ||
| Test for time-by-treatment interaction | 0.18 | |||
| Overall treatment effect | 7.14 (3.54 to 10.74) | 0.0019g | ||
| Social functioning | ||||
| Time point | ||||
| Baselinea | 100.0 (66.7–100.0) | 100.0 (66.7–100.0) | ||
| 2 weeksm | 66.7 (33.3–83.3) | 50.0 (33.3–66.7) | ||
| 5 weeksj | 83.3 (50.0–100.0) | 66.7 (50.0–83.3) | ||
| 3 monthsk | 83.3 (66.7–100.0) | 75.0 (50.0–100.0) | ||
| 6 monthsn | 83.3 (66.7–100.0) | 83.3 (66.7–100.0) | ||
| 12 monthsf | 100.0 (66.7–100.0) | 83.3 (66.7–100.0) | ||
| Test for time-by-treatment interaction | 0.32 | |||
| Overall treatment effect | 6.28 (2.73 to 9.83) | 0.0049g | ||
| Outcome | Participant allocation | Occurrence model | Intensity model | Test for time-by-treatment interactiond | |||
|---|---|---|---|---|---|---|---|
| Randomised to VATS (n = 247), median (IQR) | Randomised to open surgery (n = 255), median (IQR) | ORa (95% CI) | p-valueb | GMRc (95% CI) | p-valueb | ||
| Emotional functioning | |||||||
| Time point | |||||||
| Baselinee | 75.0 (58.3–91.7) | 83.3 (66.7–91.7) | |||||
| 2 weeksf | 83.3 (58.3–100.0) | 75.0 (50.0–91.7) | 0.51 (0.31 to 0.85) | 0.023 | 0.93 (0.81 to 1.07) | 0.441 | |
| 5 weeksg | 83.3 (58.3–100.0) | 83.3 (58.3–100.0) | 0.76 (0.49 to 1.19) | 0.343 | 1.05 (0.90 to 1.22) | 0.618 | |
| 3 monthsh | 83.3 (66.7–100.0) | 83.3 (66.7–100.0) | 0.67 (0.42 to 1.07) | 0.185 | 0.98 (0.84 to 1.15) | 0.804 | |
| 6 monthsi | 83.3 (66.7–100.0) | 83.3 (66.7–100.0) | 0.91 (0.56 to 1.47) | 0.736 | 0.96 (0.82 to 1.13) | 0.706 | |
| 12 monthsj | 83.3 (66.7–100.0) | 83.3 (66.7–100.0) | 1.48 (0.91 to 2.42) | 0.208 | 0.88 (0.75 to 1.05) | 0.246 | 0.016 |
| Cognitive functioning | |||||||
| Time point | |||||||
| Baselinee | 100.0 (83.3–100.0) | 100.0 (83.3–100.0) | |||||
| 2 weeksf | 83.3 (66.7–100.0) | 66.7 (50.0–100.0) | |||||
| 5 weeksg | 83.3 (66.7–100.0) | 83.3 (66.7–100.0) | |||||
| 3 monthsh | 100.0 (83.3–100.0) | 83.3 (66.7–100.0) | |||||
| 6 monthsi | 83.3 (66.7–100.0) | 83.3 (66.7–100.0) | |||||
| 12 monthsj | 83.3 (66.7–100.0) | 83.3 (83.3–100.0) | |||||
| Overall treatment effect | 0.87 (0.65 to 1.17) | 0.452 | 0.91 (0.84 to 0.99) | 0.047 | 0.352 | ||
FIGURE 35.
QLQ-C30 pain scores over time. Higher scores indicate more symptoms.
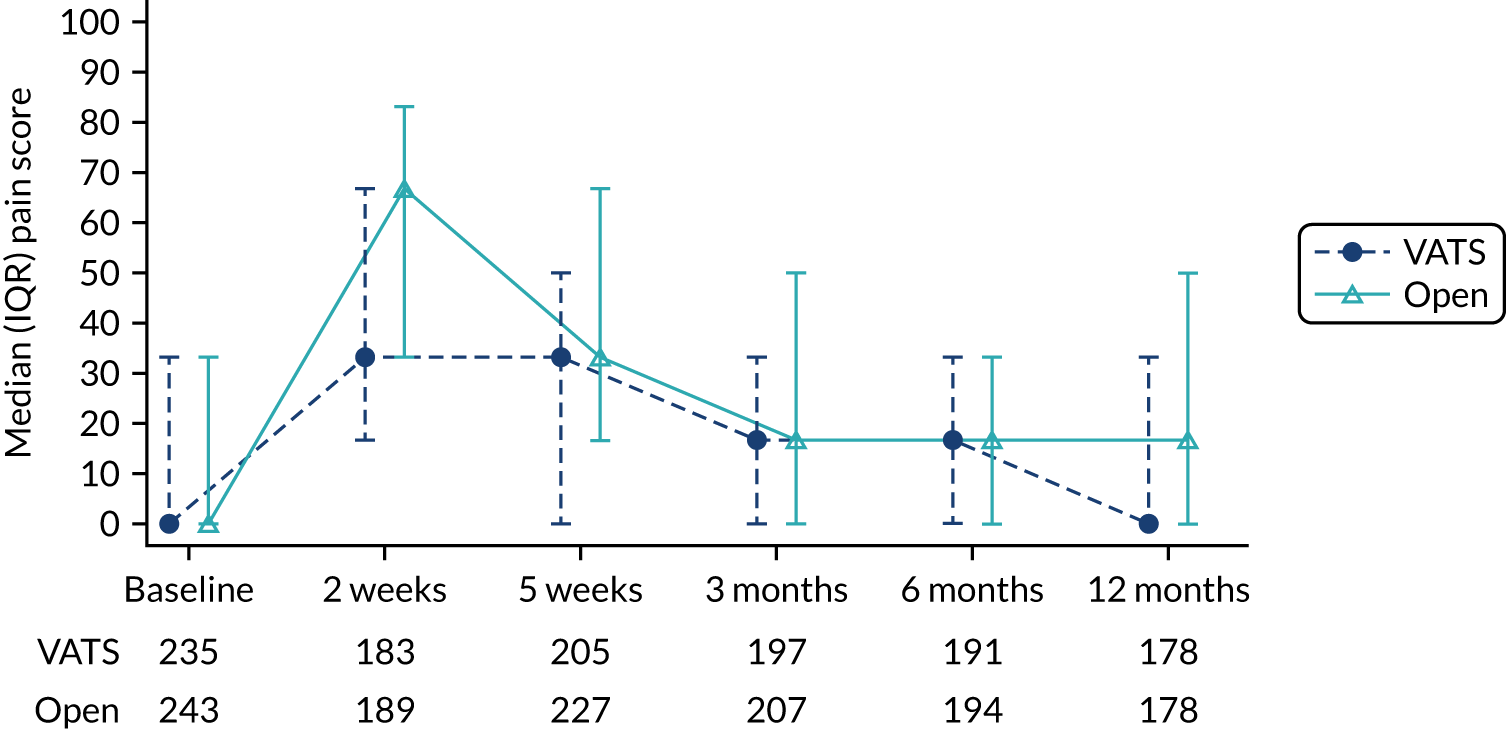
FIGURE 36.
QLQ-C30 fatigue scores over time. Higher scores indicate more symptoms.
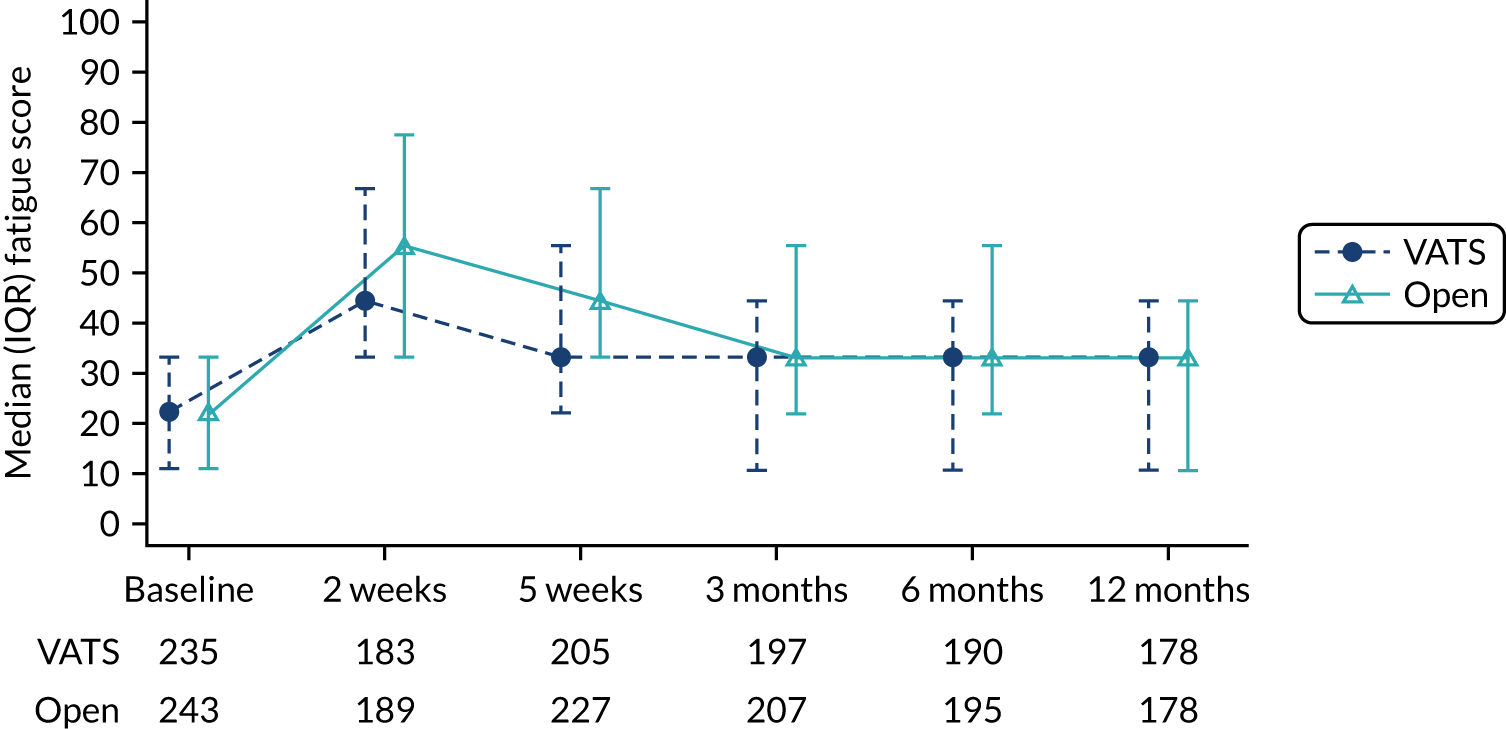
FIGURE 37.
QLQ-C30 nausea symptoms over time. Higher scores indicate more symptoms.
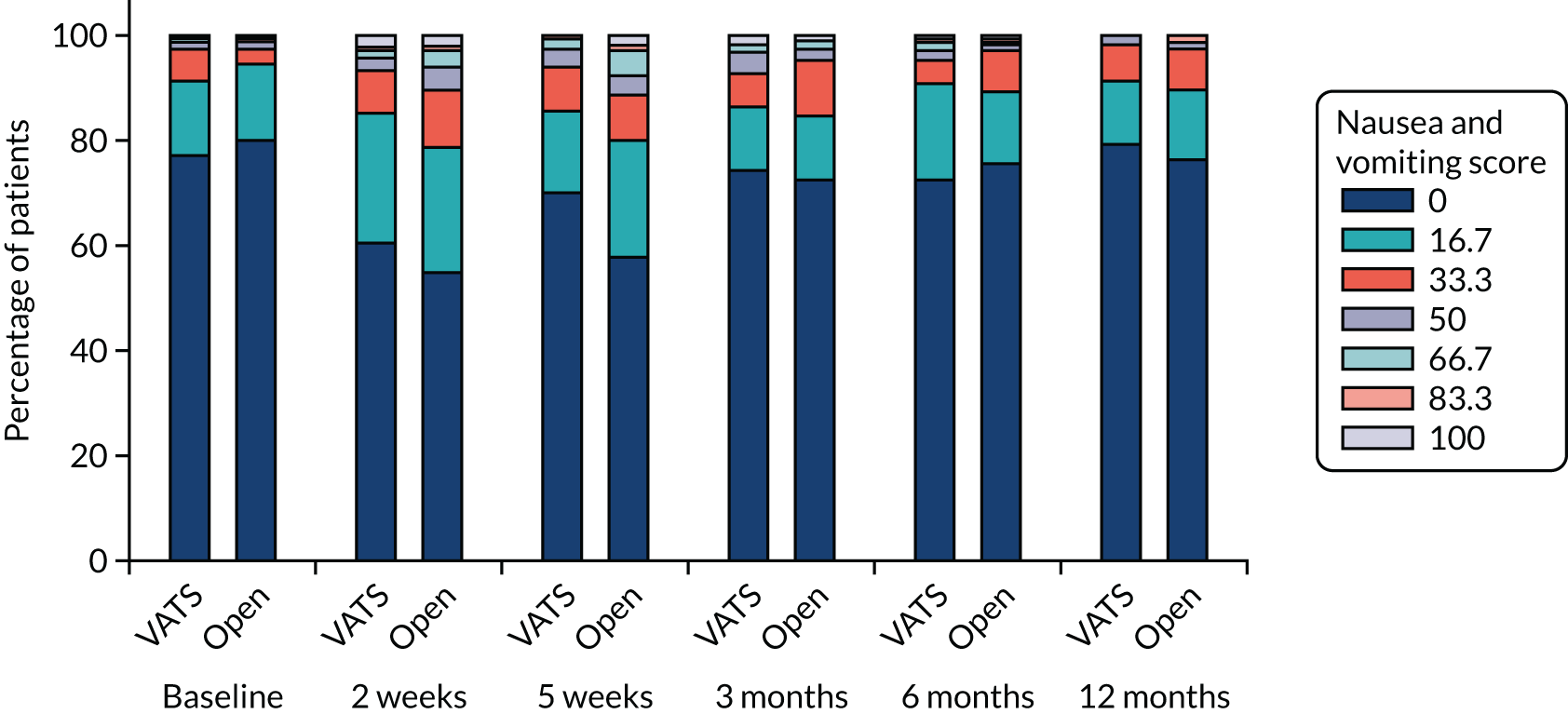
FIGURE 38.
QLQ-C30 dyspnoea symptoms over time. Higher scores indicate more symptoms.
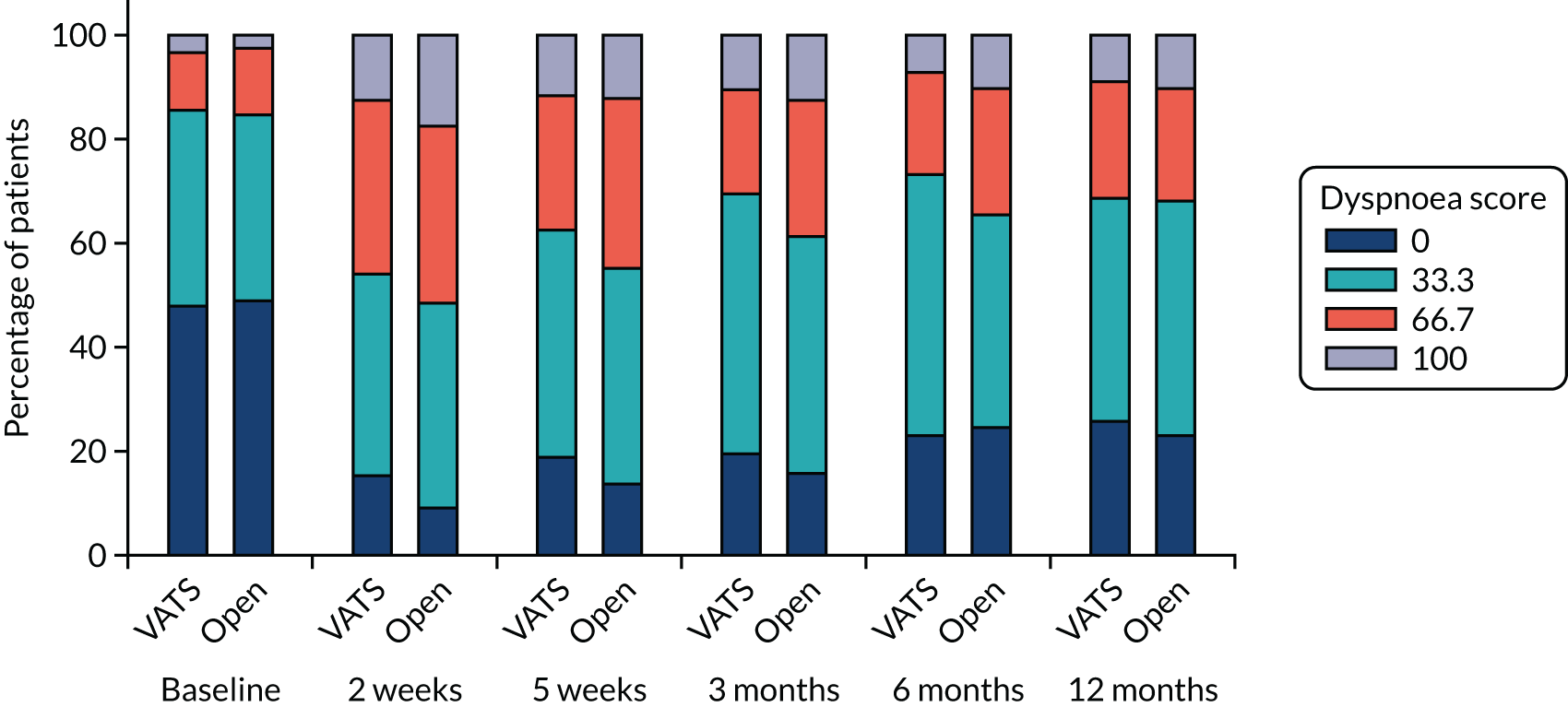
FIGURE 39.
QLQ-C30 insomnia symptoms over time. Higher scores indicate more symptoms.
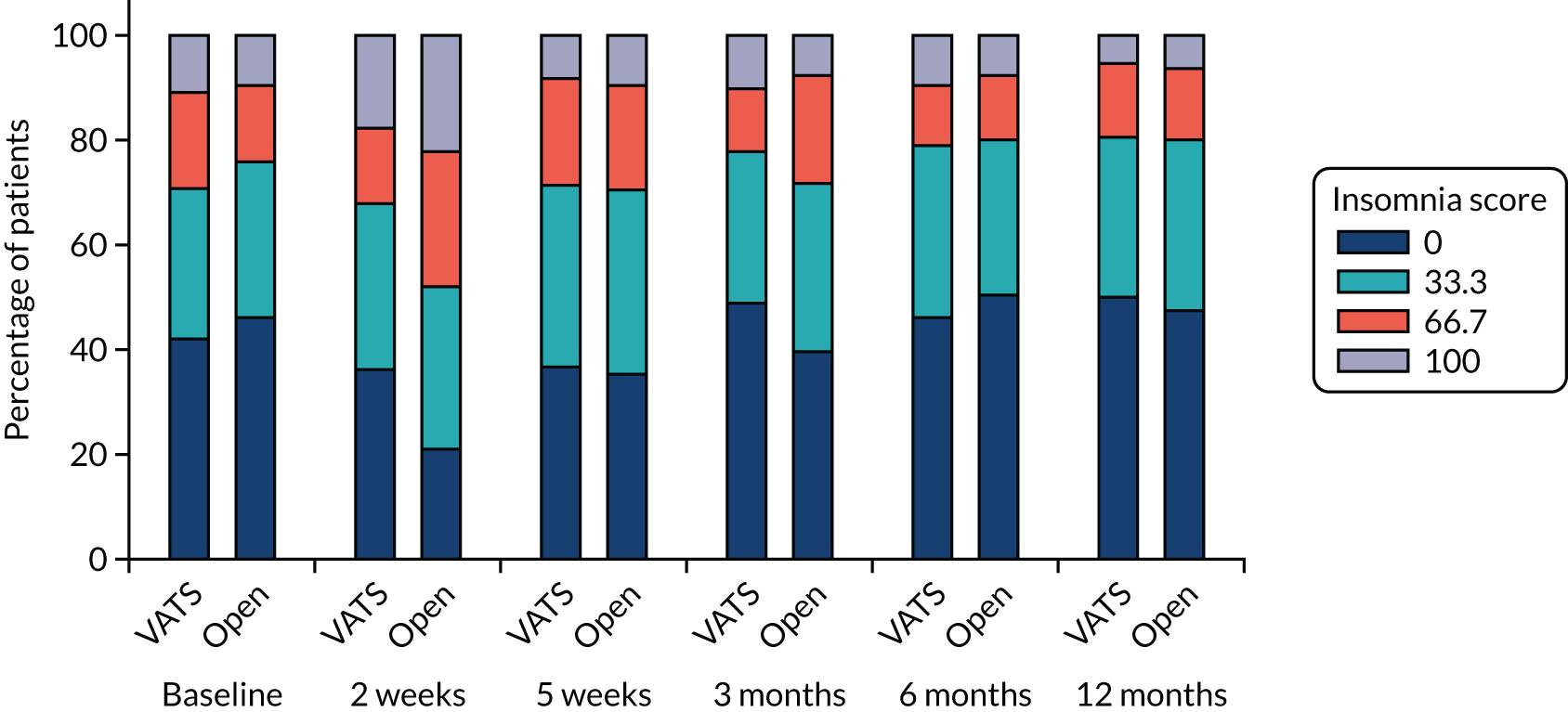
FIGURE 40.
QLQ-C30 constipation symptoms over time. Higher scores indicate more symptoms.
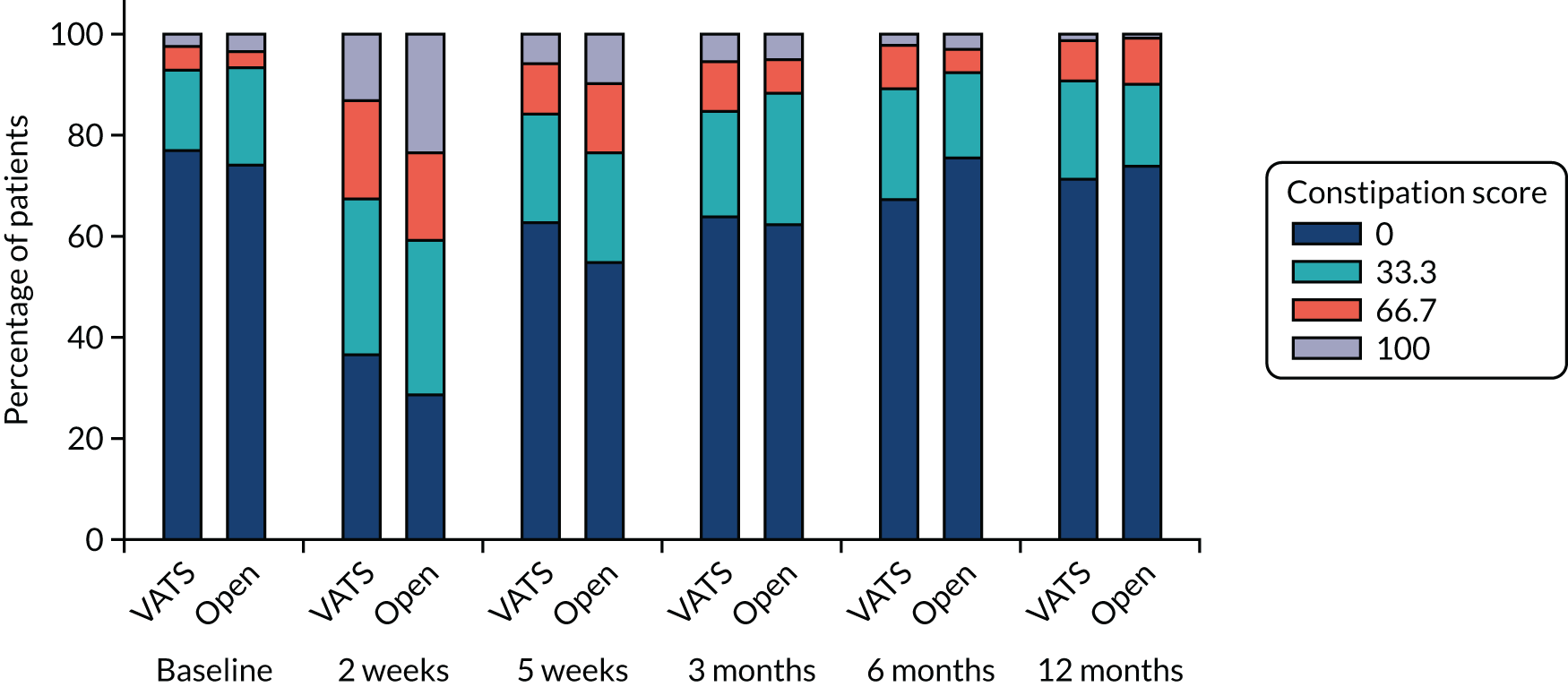
FIGURE 41.
QLQ-C30 loss of appetite symptoms over time. Higher scores indicate more symptoms.
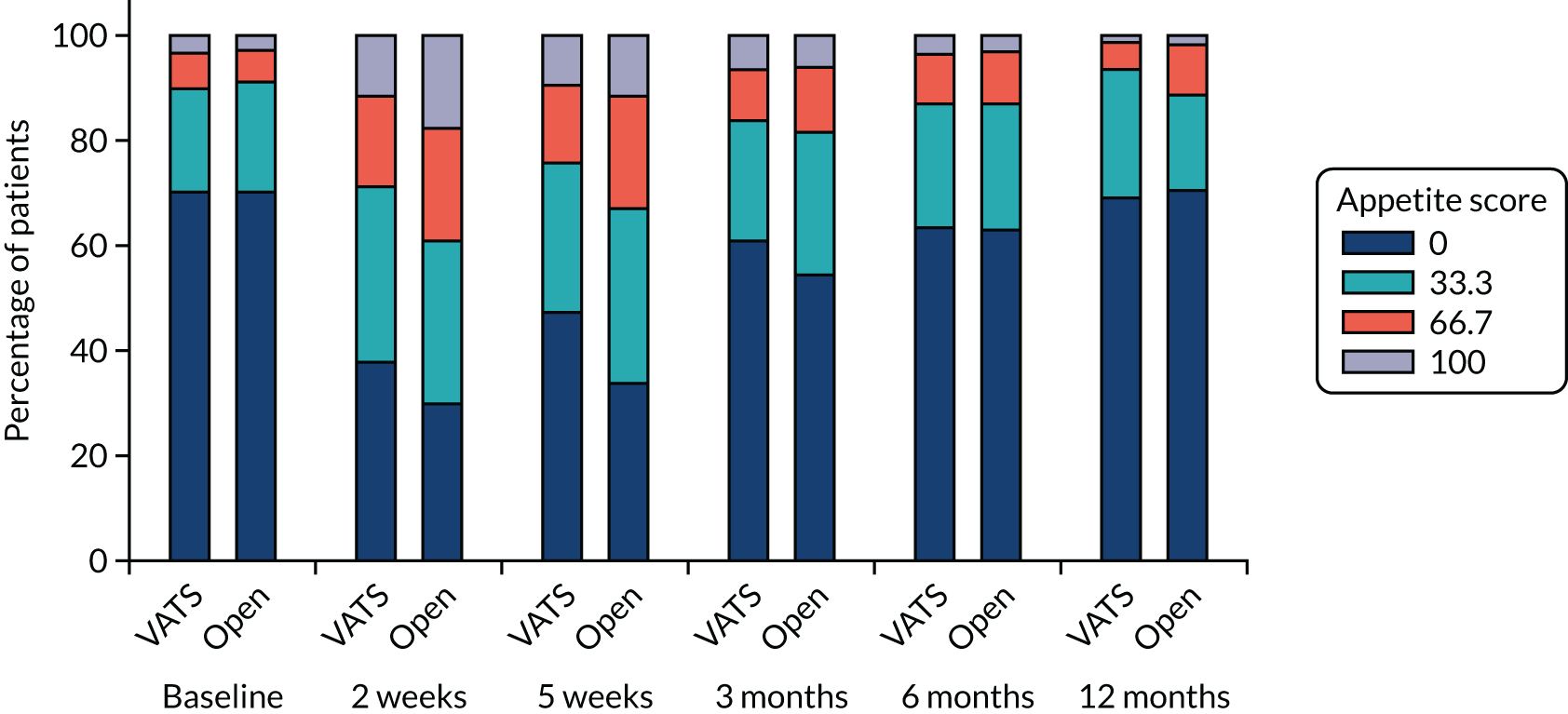
FIGURE 42.
QLQ-C30 diarrhoea symptoms over time. Higher scores indicate more symptoms.
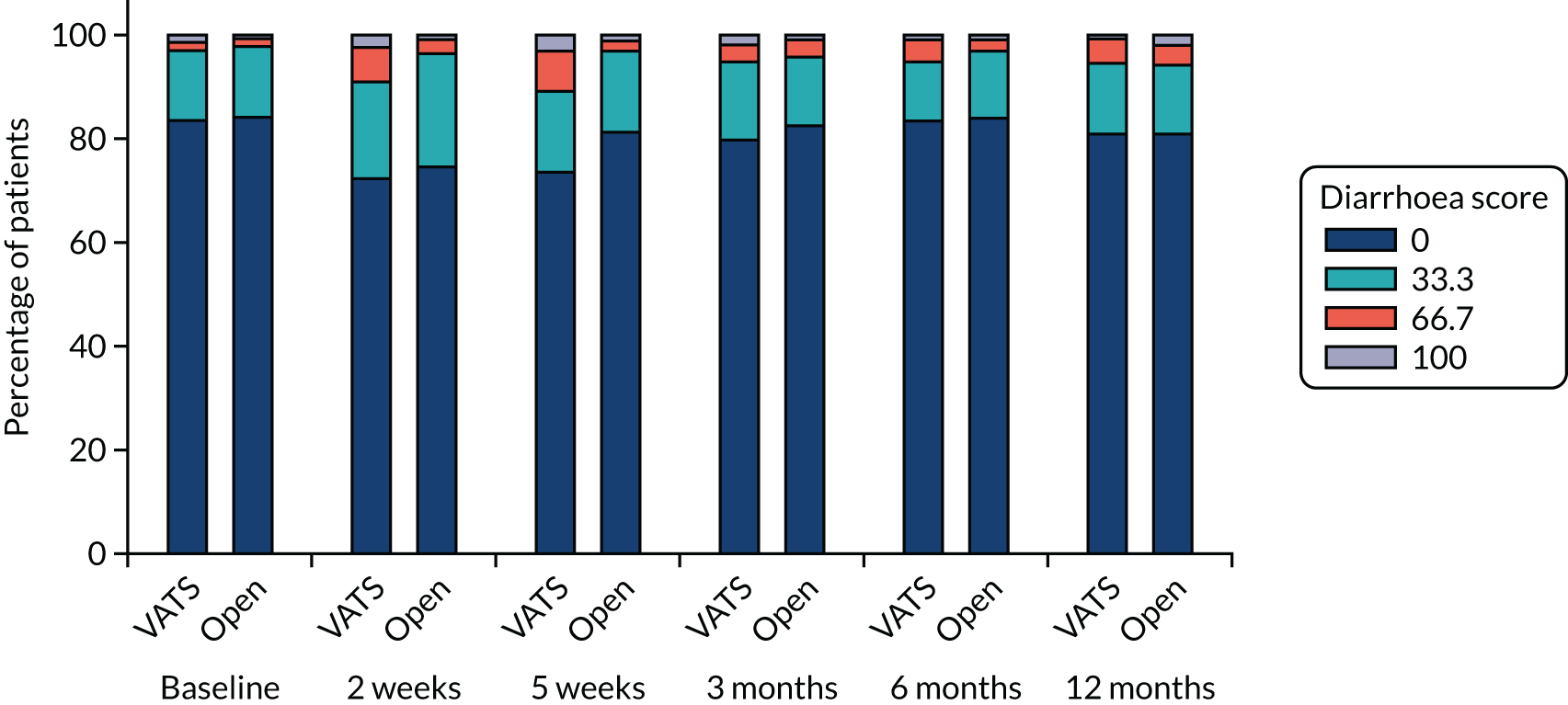
FIGURE 43.
QLQ-C30 financial difficulties over time. Higher scores indicate more symptoms.
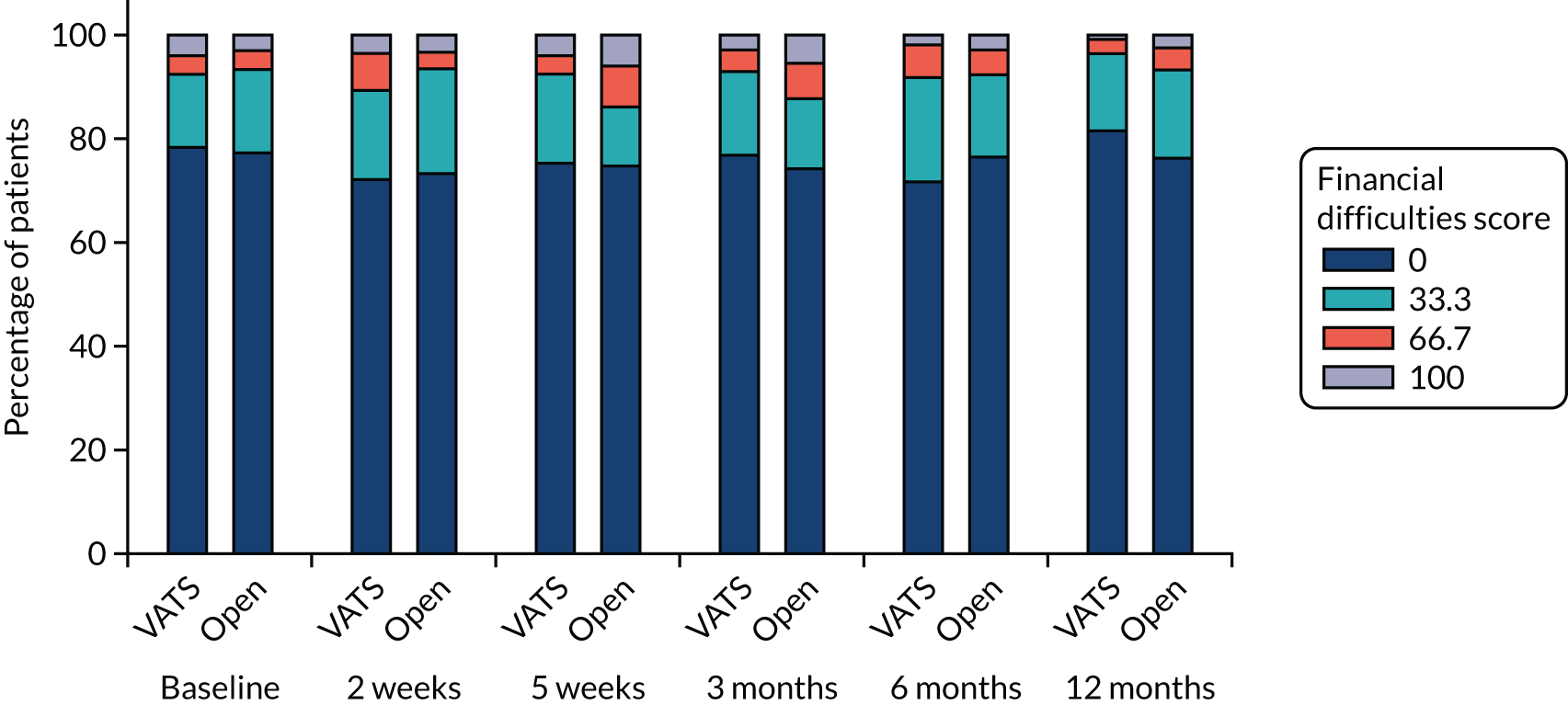
| Outcome | Participant allocation | MD/OR (95% CI) | p-value | |
|---|---|---|---|---|
| Randomised to VATS (n = 247), median (IQR) or n/N (%) | Randomised to open surgery (n = 255), median (IQR) or n/N (%) | |||
| Fatigue | ||||
| Time point | ||||
| Baselinea | 22.2 (11.1–33.3) | 22.2 (11.1–33.3) | ||
| 2 weeksb | 44.4 (33.3–66.7) | 55.6 (33.3–77.8) | ||
| 5 weeksc | 33.3 (22.2–55.6) | 44.4 (33.3–66.7) | ||
| 3 monthsd | 33.3 (11.1–44.4) | 33.3 (22.2–55.6) | ||
| 6 monthse | 33.3 (11.1–44.4) | 33.3 (22.2–55.6) | ||
| 12 monthsf | 33.3 (11.1–44.4) | 33.3 (11.1–44.4) | ||
| Test for time-by-treatment interaction | 0.41 | |||
| Overall treatment effect | MD –5.68 (–8.65 to –2.71) | 0.0015g | ||
| Nausea and vomiting | ||||
| Time point | ||||
| Baseline | 54/235 (23.0) | 48/243 (19.8) | ||
| 2 weeks | 72/183 (39.3) | 84/187 (44.9) | ||
| 5 weeks | 61/205 (29.8) | 96/226 (42.5) | ||
| 3 months | 47/187 (25.1) | 54/198 (27.3) | ||
| 6 months | 50/184 (27.2) | 46/190 (24.2) | ||
| 12 months | 35/173 (20.2) | 41/175 (23.4) | ||
| Test for time-by-treatment interactionh | 0.20 | |||
| Overall treatment effect | OR 0.72 (0.53 to 0.98) | 0.128g | ||
| Pain | ||||
| Time point | ||||
| Baselinea | 0.0 (0.0–33.3) | 0.0 (0.0–33.3) | ||
| 2 weeksb | 33.3 (16.7–66.7) | 66.7 (33.3–83.3) | ||
| 5 weeksc | 33.3 (0.0–50.0) | 33.3 (16.7–66.7) | ||
| 3 monthsd | 16.7 (0.0–33.3) | 16.7 (0.0–50.0) | ||
| 6 monthsi | 16.7 (0.0–33.3) | 16.7 (0.0–33.3) | ||
| 12 monthsf | 0.0 (0.0–33.3) | 16.7 (0.0–50.0) | ||
| Test for time-by-treatment interaction | 0.12 | |||
| Overall treatment effect | MD –7.19 (–10.59 to –3.80) | 0.0006g | ||
| Dyspnoea | ||||
| Time point | ||||
| Baselinej | 33.3 (0.0–33.3) | 33.3 (0.0–33.3) | ||
| 2 weeksb | 33.3 (33.3–66.7) | 66.7 (33.3–66.7) | ||
| 5 weeksc | 33.3 (33.3–66.7) | 33.3 (33.3–66.7) | ||
| 3 monthsk | 33.3 (33.3–66.7) | 33.3 (33.3–66.7) | ||
| 6 monthsl | 33.3 (33.3–66.7) | 33.3 (33.3–66.7) | ||
| 12 monthsm | 33.3 (0.0–66.7) | 33.3 (33.3–66.7) | ||
| Test for time-by-treatment interaction | 0.16 | |||
| Overall treatment effect | MD –2.14 (–5.84 to 1.55) | 0.40g | ||
| Insomnia | ||||
| Time point | ||||
| Baselinea | 33.3 (0.0–66.7) | 33.3 (0.0–33.3) | ||
| 2 weeksb | 33.3 (0.0–66.7) | 33.3 (33.3–66.7) | MD –11.79 (–18.95 to –4.63) | 0.007g |
| 5 weeksc | 33.3 (0.0–66.7) | 33.3 (0.0–66.7) | MD –2.13 (–7.90 to 3.63) | 0.66g |
| 3 monthsn | 33.3 (0.0–33.3) | 33.3 (0.0–66.7) | MD –6.36 (–12.11 to –0.61) | 0.13g |
| 6 monthso | 33.3 (0.0–33.3) | 0.0 (0.0–33.3) | MD 0.35 (–5.18 to 5.87) | 0.95g |
| 12 monthsf | 33.3 (0.0–33.3) | 33.3 (0.0–33.3) | MD –2.02 (–7.42 to 3.37) | 0.66g |
| Test for time-by-treatment interaction | 0.0059 | |||
| Constipation | ||||
| Time point | ||||
| Baseline | 54/235 (23.0) | 63/243 (25.9) | ||
| 2 weeks | 115/182 (63.2) | 133/187 (71.1) | OR 0.64 (0.35 to 1.16) | 0.27g |
| 5 weeks | 76/205 (37.1) | 102/226 (45.1) | OR 0.62 (0.36 to 1.07) | 0.18g |
| 3 months | 67/186 (36.0) | 74/197 (37.6) | OR 0.96 (0.54 to 1.70) | 0.95g |
| 6 months | 60/183 (32.8) | 46/189 (24.3) | OR 1.83 (0.98 to 3.40) | 0.15g |
| 12 months | 49/173 (28.3) | 45/173 (26.0) | OR 1.20 (0.63 to 2.27) | 0.70g |
| Test for time-by-treatment interactionh | 0.012 | |||
| Outcome | Time point | Score | Participant allocation | OR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Randomised to VATS (N = 247), n/N (%) | Randomised to open surgery (N = 255), n/N (%) | |||||
| Appetite loss | Baseline | 0 | 163/233 (70.0) | 170/243 (70.0) | ||
| 33.3 | 47/233 (20.2) | 51/243 (21.0) | ||||
| 66.7 | 15/233 (6.4) | 15/243 (6.2) | ||||
| 100 | 8/233 (3.4) | 7/243 (2.9) | ||||
| 2 weeks | 0 | 69/182 (37.9) | 56/187 (29.9) | |||
| 33.3 | 61/182 (33.5) | 58/187 (31.0) | ||||
| 66.7 | 31/182 (17.0) | 40/187 (21.4) | ||||
| 100 | 21/182 (11.5) | 33/187 (17.6) | ||||
| 5 weeks | 0 | 97/205 (47.3) | 77/226 (34.1) | |||
| 33.3 | 59/205 (28.8) | 75/226 (33.2) | ||||
| 66.7 | 29/205 (14.1) | 47/226 (20.8) | ||||
| 100 | 20/205 (9.8) | 27/226 (11.9) | ||||
| 3 months | 0 | 113/186 (60.8) | 108/198 (54.5) | |||
| 33.3 | 43/186 (23.1) | 54/198 (27.3) | ||||
| 66.7 | 18/186 (9.7) | 24/198 (12.1) | ||||
| 100 | 12/186 (6.5) | 12/198 (6.1) | ||||
| 6 months | 0 | 117/184 (63.6) | 120/190 (63.2) | |||
| 33.3 | 43/184 (23.4) | 46/190 (24.2) | ||||
| 66.7 | 18/184 (9.8) | 18/190 (9.5) | ||||
| 100 | 6/184 (3.3) | 6/190 (3.2) | ||||
| 12 months | 0 | 119/172 (69.2) | 124/175 (70.9) | |||
| 33.3 | 42/172 (24.4) | 31/175 (17.7) | ||||
| 66.7 | 9/172 (5.2) | 17/175 (9.7) | ||||
| 100 | 2/172 (1.2) | 3/175 (1.7) | ||||
| Test for time-by-treatment interactiona | 0.22 | |||||
| Overall treatment effect | 0.71 (0.50 to 1.01) | 0.15b | ||||
| Diarrhoea | Baseline | 0 | 196/234 (83.8) | 205/243 (84.4) | ||
| 33.3 | 31/234 (13.2) | 33/243 (13.6) | ||||
| 66.7 | 4/234 (1.7) | 4/243 (1.6) | ||||
| 100 | 3/234 (1.3) | 1/243 (0.4) | ||||
| 2 weeks | 0 | 132/182 (72.5) | 141/188 (75.0) | |||
| 33.3 | 34/182 (18.7) | 40/188 (21.3) | ||||
| 66.7 | 12/182 (6.6) | 6/188 (3.2) | ||||
| 100 | 4/182 (2.2) | 1/188 (0.5) | ||||
| 5 weeks | 0 | 151/205 (73.7) | 184/226 (81.4) | |||
| 33.3 | 32/205 (15.6) | 35/226 (15.5) | ||||
| 66.7 | 16/205 (7.8) | 5/226 (2.2) | ||||
| 100 | 6/205 (2.9) | 2/226 (0.9) | ||||
| 3 months | 0 | 147/184 (79.9) | 161/195 (82.6) | |||
| 33.3 | 28/184 (15.2) | 26/195 (13.3) | ||||
| 66.7 | 6/184 (3.3) | 7/195 (3.6) | ||||
| 100 | 3/184 (1.6) | 1/195 (0.5) | ||||
| 6 months | 0 | 152/182 (83.5) | 160/190 (84.2) | |||
| 33.3 | 21/182 (11.5) | 24/190 (12.6) | ||||
| 66.7 | 8/182 (4.4) | 5/190 (2.6) | ||||
| 100 | 1/182 (0.5) | 1/190 (0.5) | ||||
| 12 months | 0 | 140/173 (80.9) | 142/175 (81.1) | |||
| 33.3 | 24/173 (13.9) | 23/175 (13.1) | ||||
| 66.7 | 8/173 (4.6) | 7/175 (4.0) | ||||
| 100 | 1/173 (0.6) | 3/175 (1.7) | ||||
| Test for time-by-treatment interactiona | 0.35 | |||||
| Overall treatment effect | 1.28 (0.90 to 1.82) | 0.29b | ||||
| Financial difficulties | Baseline | 0 | 184/234 (78.6) | 189/243 (77.8) | ||
| 33.3 | 32/234 (13.7) | 38/243 (15.6) | ||||
| 66.7 | 9/234 (3.8) | 9/243 (3.7) | ||||
| 100 | 9/234 (3.8) | 7/243 (2.9) | ||||
| 2 weeks | 0 | 132/183 (72.1) | 138/188 (73.4) | |||
| 33.3 | 32/183 (17.5) | 38/188 (20.2) | ||||
| 66.7 | 13/183 (7.1) | 6/188 (3.2) | ||||
| 100 | 6/183 (3.3) | 6/188 (3.2) | ||||
| 5 weeks | 0 | 154/205 (75.1) | 169/226 (74.8) | |||
| 33.3 | 36/205 (17.6) | 26/226 (11.5) | ||||
| 66.7 | 7/205 (3.4) | 17/226 (7.5) | ||||
| 100 | 8/205 (3.9) | 14/226 (6.2) | ||||
| 3 months | 0 | 143/186 (76.9) | 147/198 (74.2) | |||
| 33.3 | 30/186 (16.1) | 27/198 (13.6) | ||||
| 66.7 | 8/186 (4.3) | 13/198 (6.6) | ||||
| 100 | 5/186 (2.7) | 11/198 (5.6) | ||||
| 6 months | 0 | 133/185 (71.9) | 145/189 (76.7) | |||
| 33.3 | 37/185 (20.0) | 30/189 (15.9) | ||||
| 66.7 | 12/185 (6.5) | 8/189 (4.2) | ||||
| 100 | 3/185 (1.6) | 6/189 (3.2) | ||||
| 12 months | 0 | 141/173 (81.5) | 134/175 (76.6) | |||
| 33.3 | 26/173 (15.0) | 30/175 (17.1) | ||||
| 66.7 | 5/173 (2.9) | 7/175 (4.0) | ||||
| 100 | 1/173 (0.6) | 4/175 (2.3) | ||||
| Test for time-by-treatment interactiona | 0.35 | |||||
| Overall treatment effect | 0.96 (0.68 to 1.34) | 0.91b | ||||
FIGURE 44.
QLQ-LC13 dyspnoea symptoms over time. Higher scores indicate more symptoms.
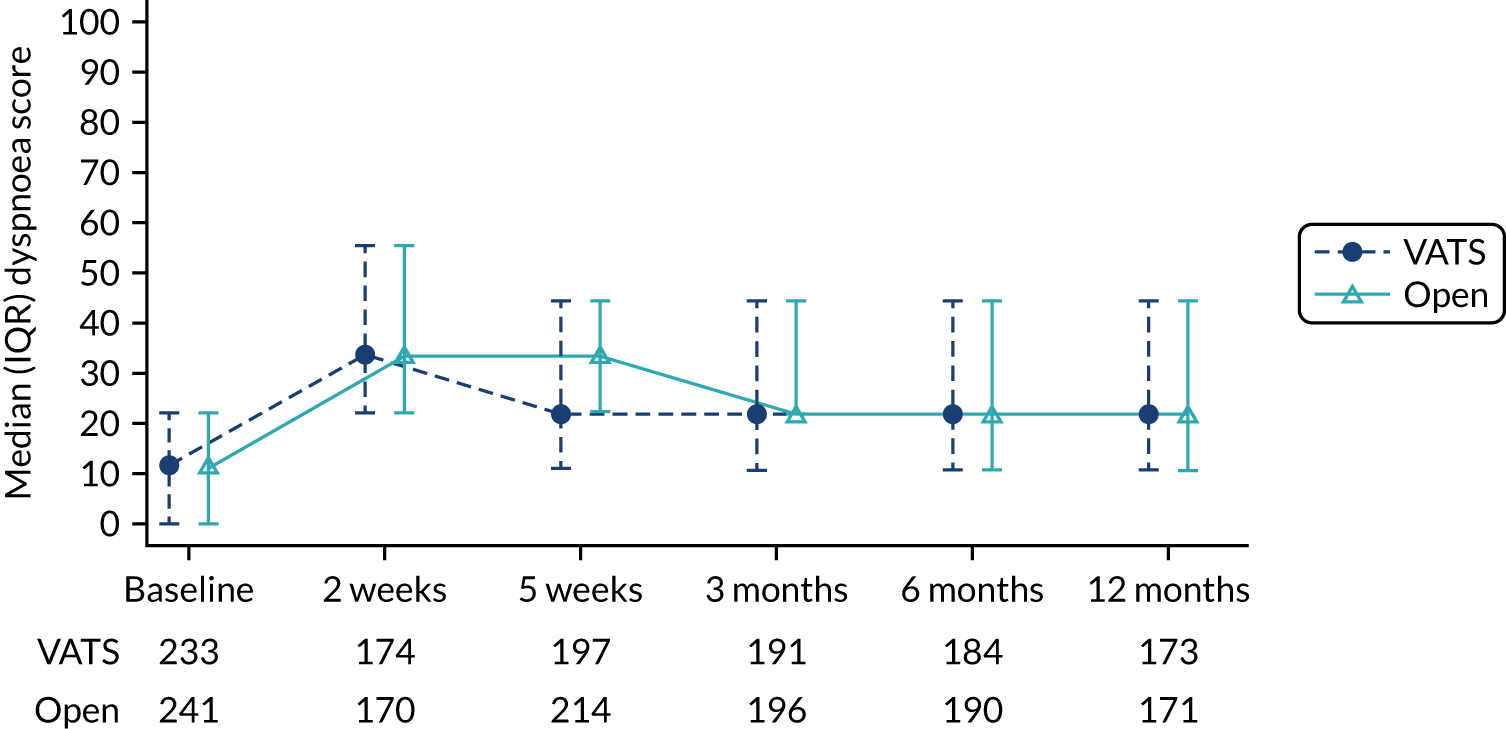
FIGURE 45.
QLQ-LC13 scores for cough over time. Higher scores indicate more symptoms.
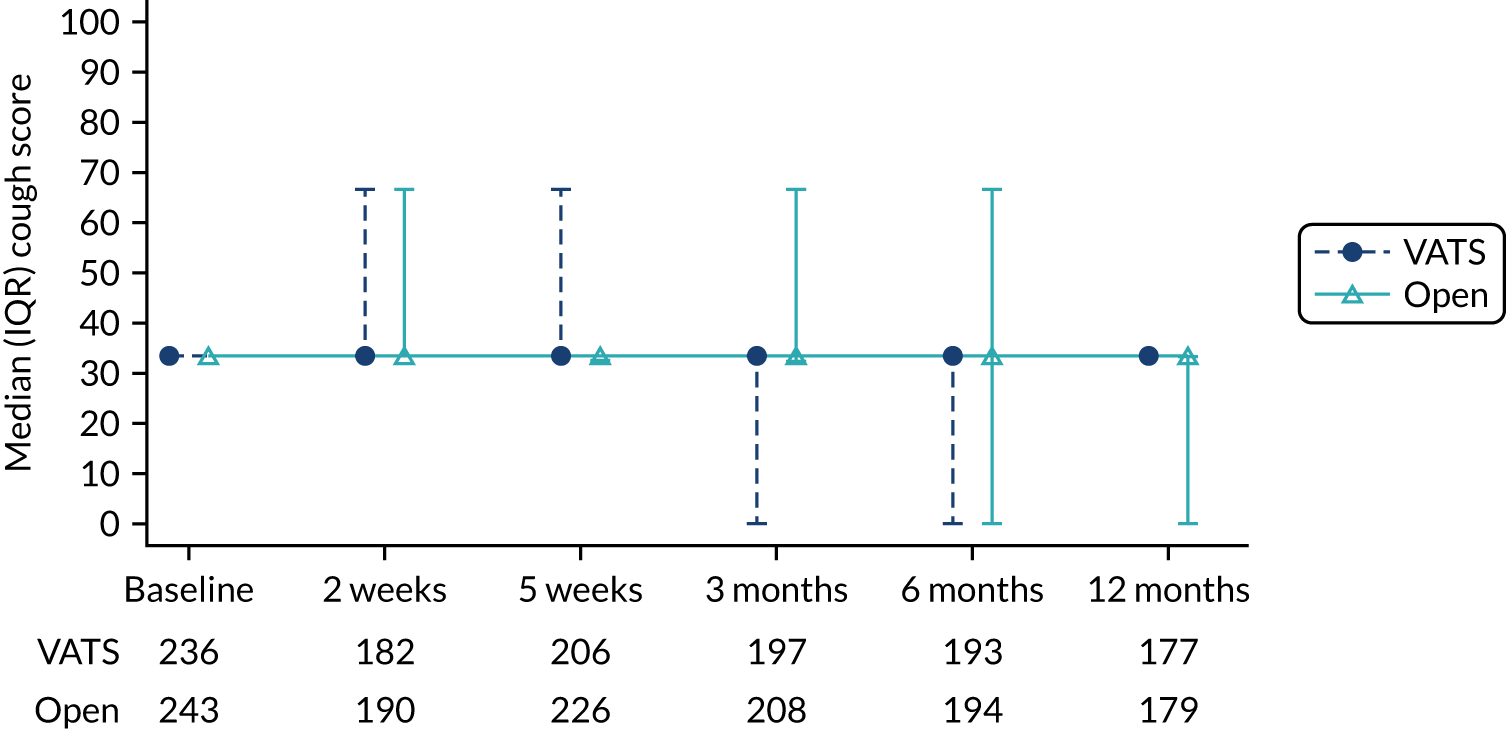
FIGURE 46.
QLQ-LC13 scores for haemoptysis over time. Higher scores indicate more symptoms.
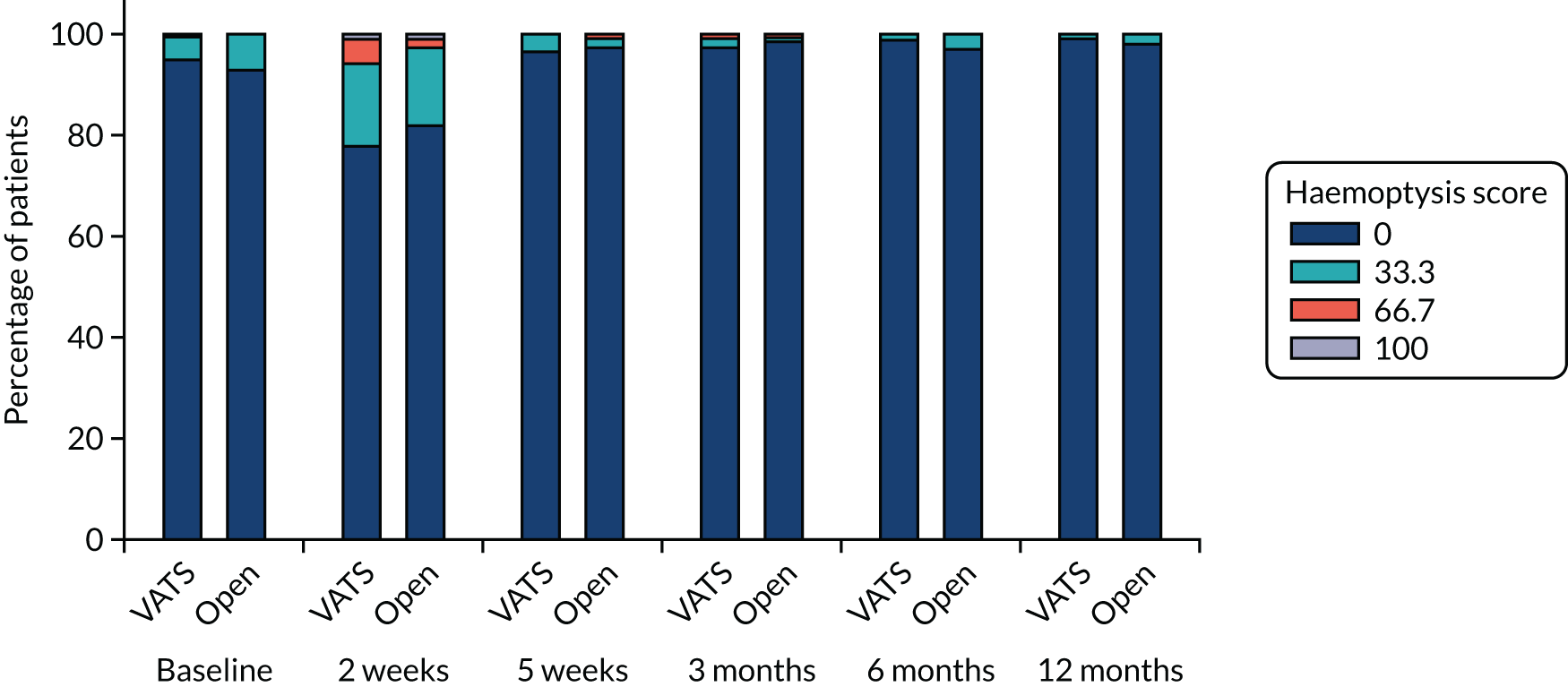
FIGURE 47.
QLQ-LC13 scores for pain in the chest over time. Higher scores indicate more symptoms.
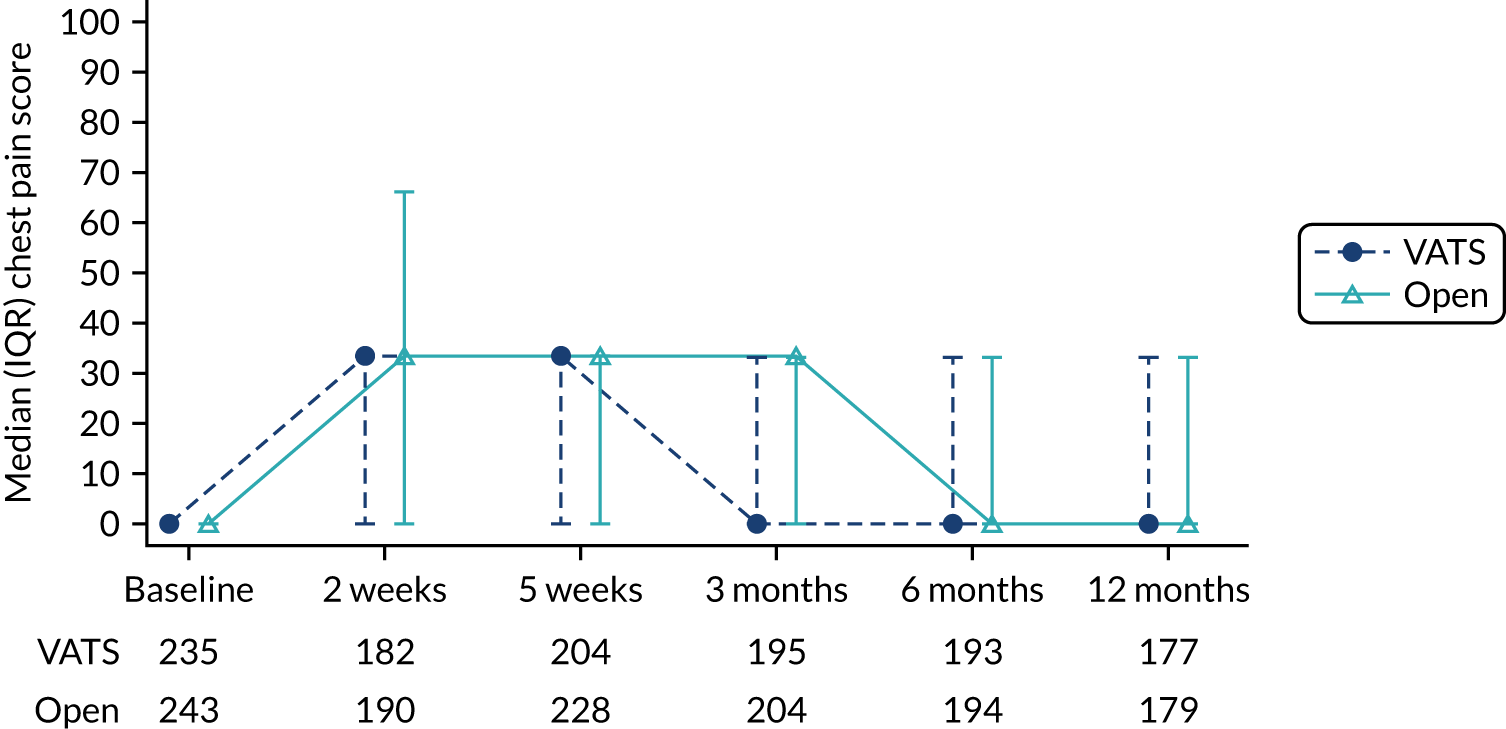
FIGURE 48.
QLQ-LC13 scores for pain in other parts (not chest, arm or shoulder) over time. Higher scores indicate more symptoms.
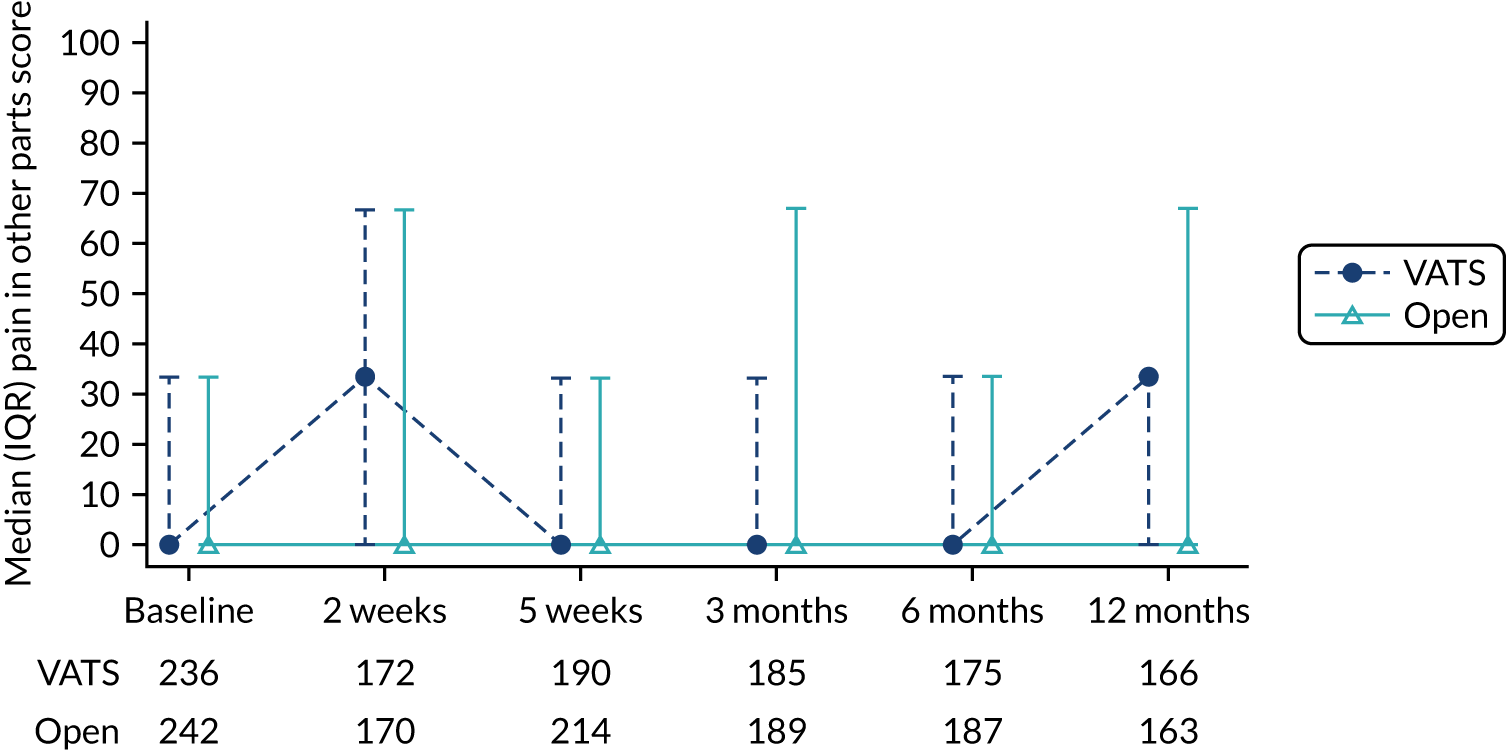
FIGURE 49.
QLQ-LC13 scores for sore mouth over time. Higher scores indicate more symptoms.
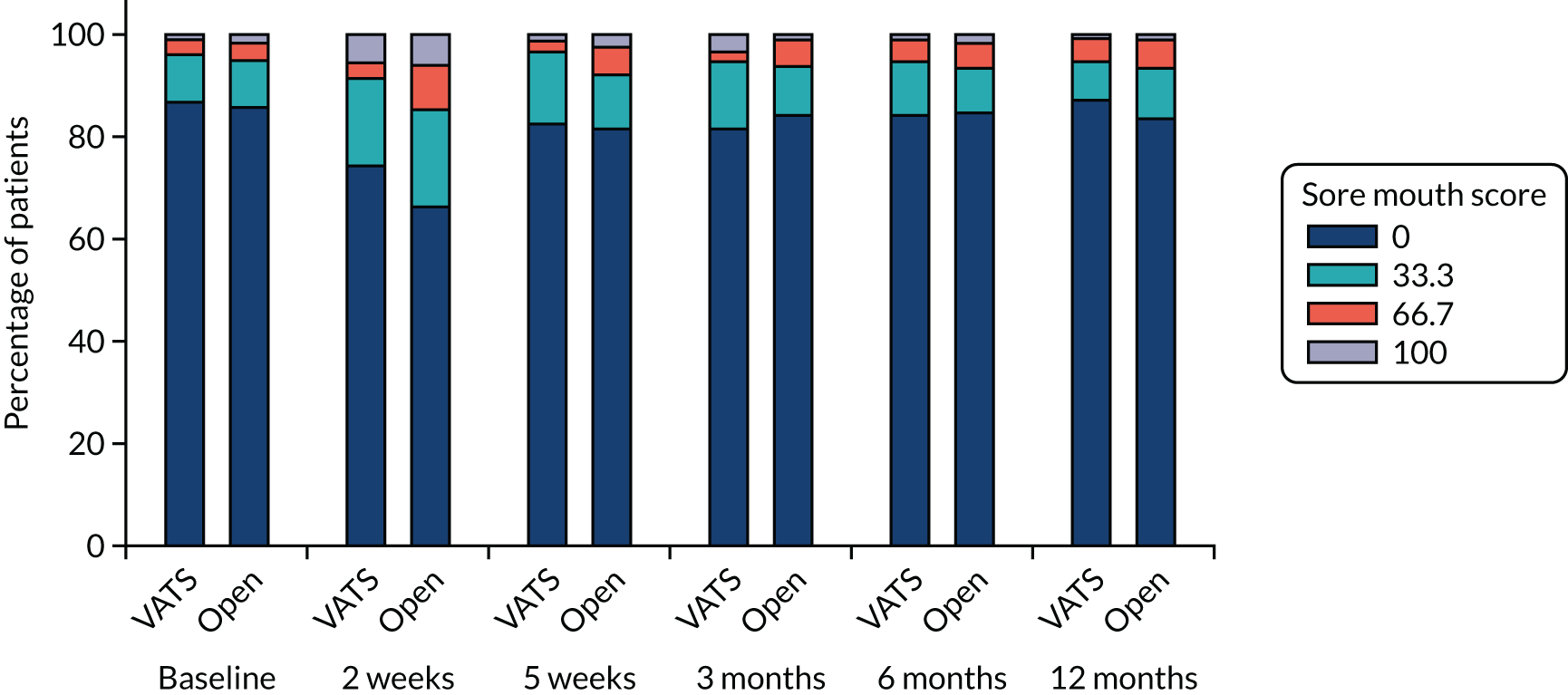
FIGURE 50.
QLQ-LC13 scores for dysphagia over time. Higher scores indicate more symptoms.
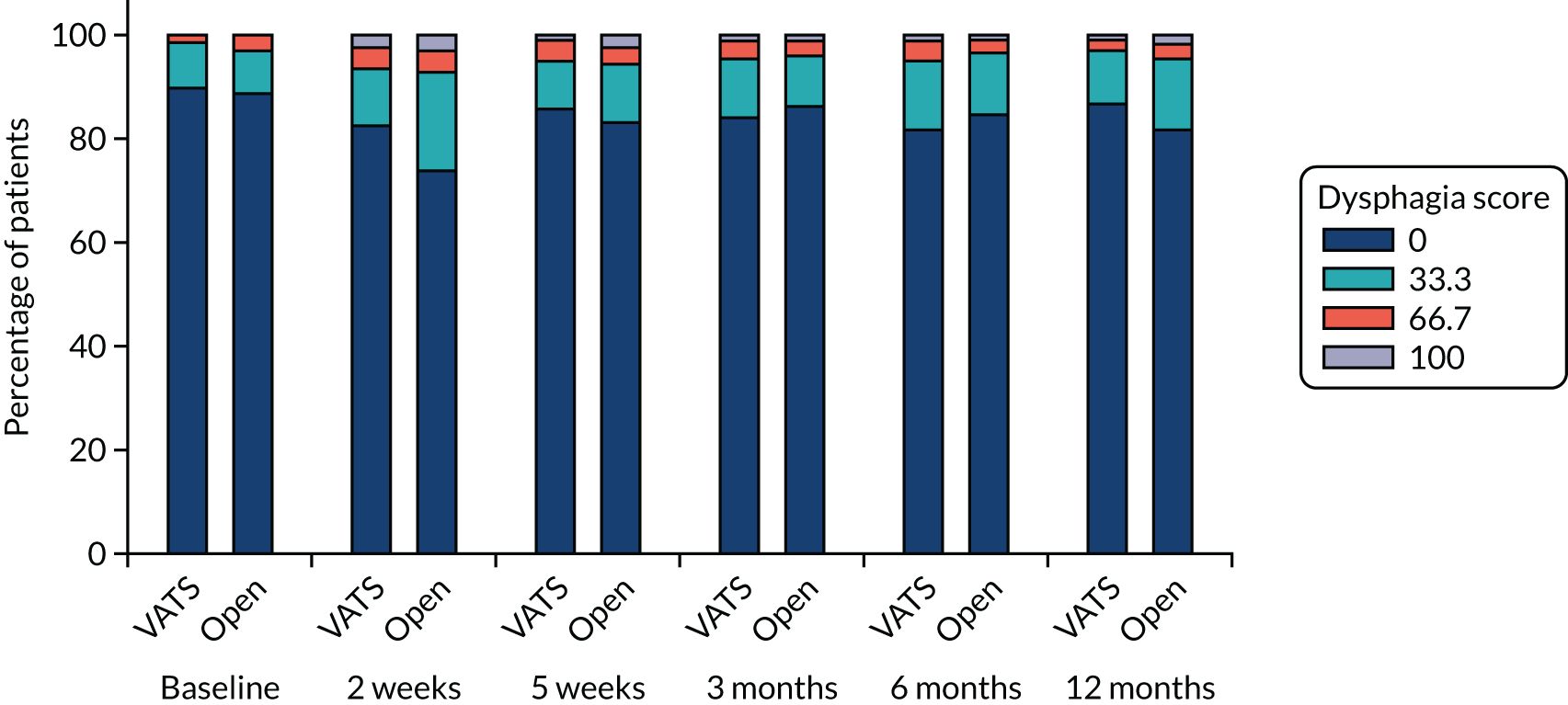
FIGURE 51.
QLQ-LC13 scores for peripheral neuropathy over time. Higher scores indicate more symptoms.
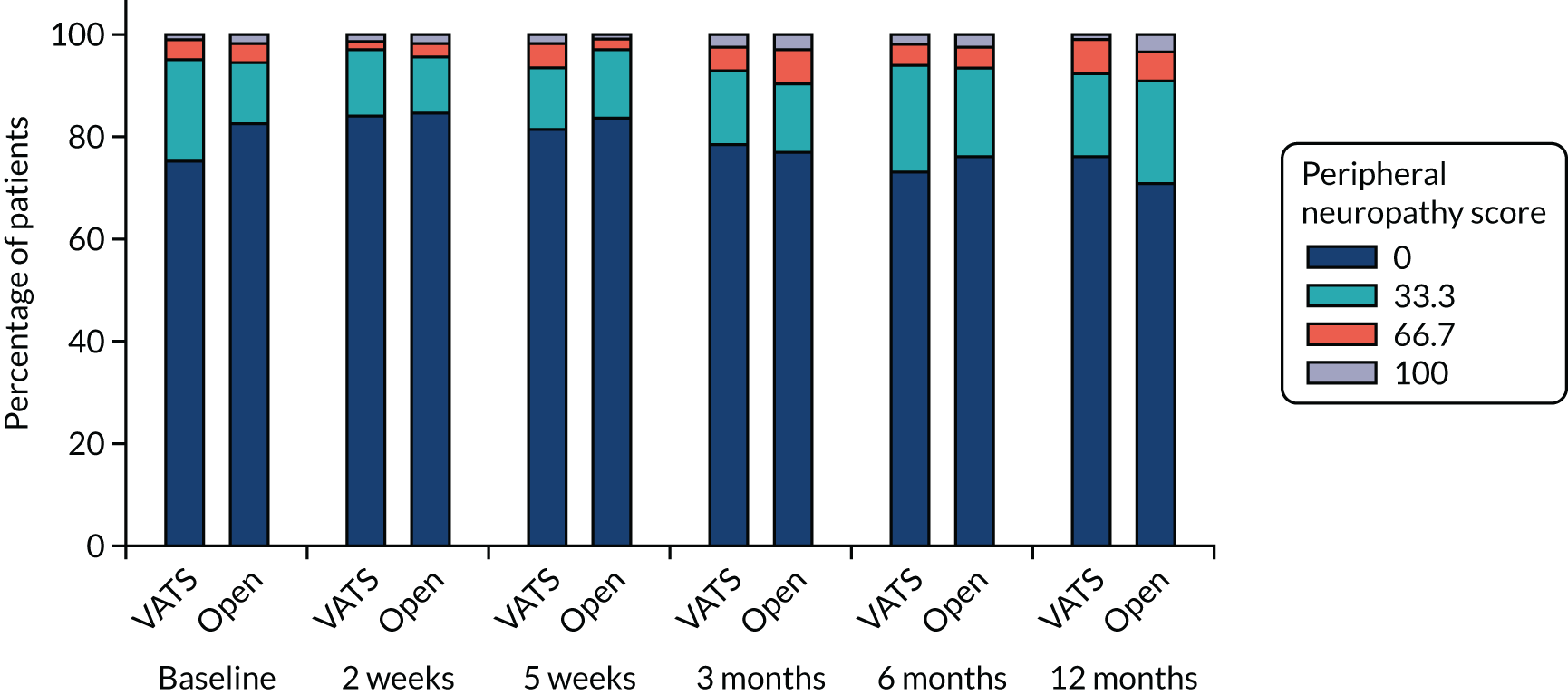
FIGURE 52.
QLQ-LC13 scores for alopecia over time. Higher scores indicate more symptoms.
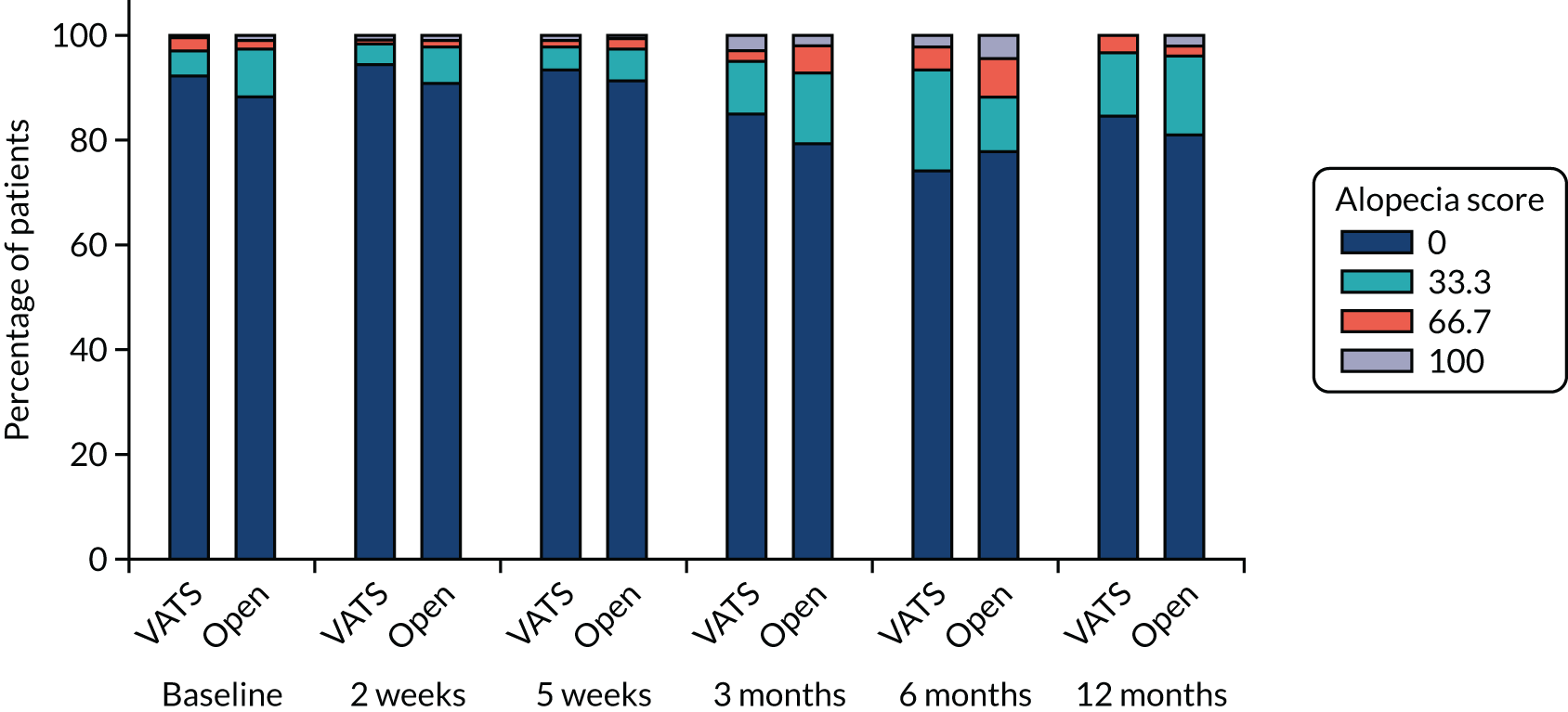
FIGURE 53.
QLQ-LC13 scores for pain in the arm over time. Higher scores indicate more symptoms.
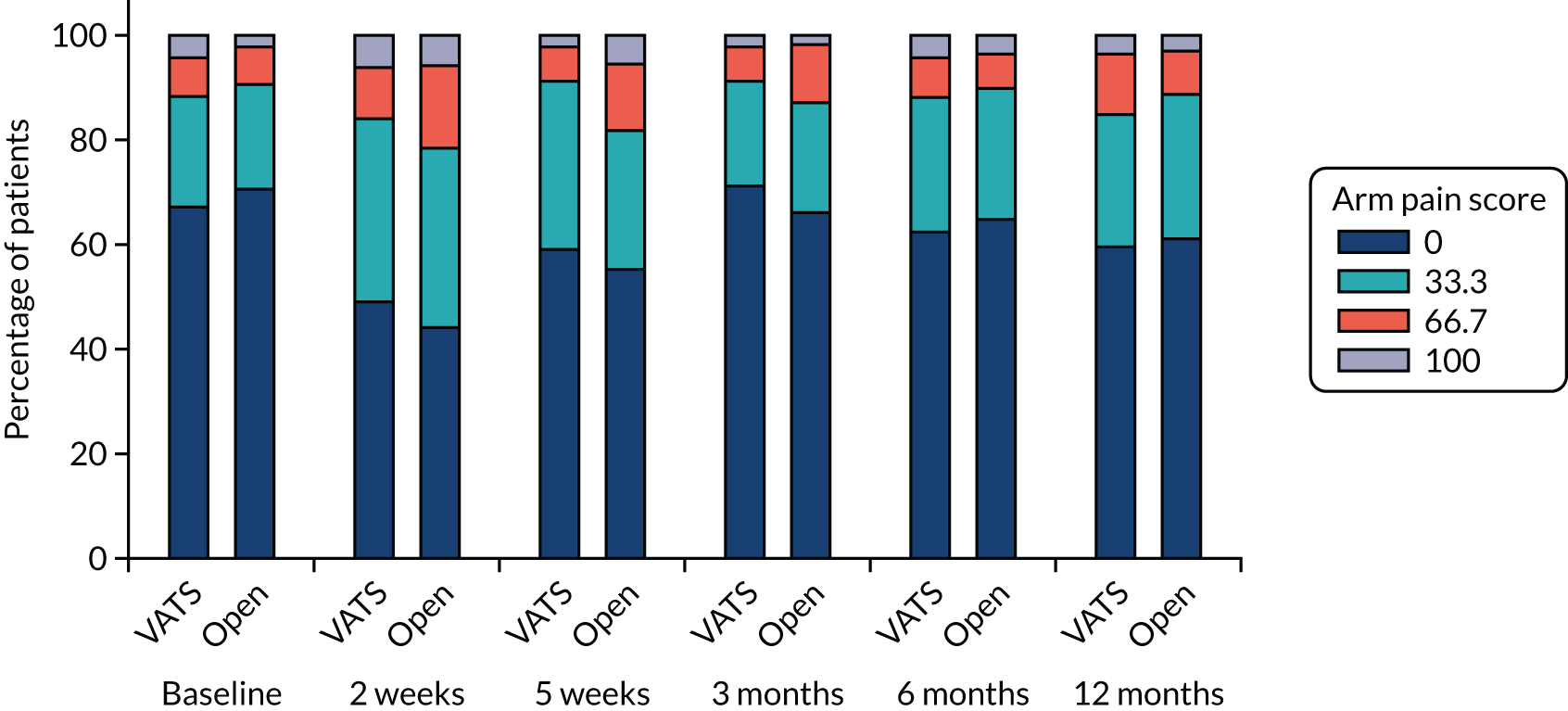
| Outcome | Participant allocation | MD/OR (95% CI) | p-value | |
|---|---|---|---|---|
| Randomised to VATS (n = 247), median (IQR) or n/N (%) | Randomised to open surgery (n = 255), median (IQR) or n/N (%) | |||
| Dyspnoea | ||||
| Time point | ||||
| Baselinea | 11 (0.0–22.2) | 11 (0.0–22.2) | ||
| 2 weeksb | 33 (22.2–55.6) | 33 (22.2–55.6) | ||
| 5 weeksc | 22 (11.1–44.4) | 33 (22.2–44.4) | ||
| 3 monthsd | 22 (11.1–44.4) | 22 (22.2–44.4) | ||
| 6 monthse | 22 (11.1–44.4) | 22 (11.1–44.4) | ||
| 12 monthsf | 22 (11.1–44.4) | 22 (11.1–44.4) | ||
| Test for time-by-treatment interaction | 0.69 | |||
| Overall treatment effect | MD –1.85 (–4.90 to 1.20) | 0.82g | ||
| Cough | ||||
| Time point | ||||
| Baselineh | 33 (33.3–33.3) | 33 (33.3–33.3) | ||
| 2 weeksi | 33 (33.3–66.7) | 33 (33.3–66.7) | ||
| 5 weeksj | 33 (33.3–66.7) | 33 (33.3–33.3) | ||
| 3 monthsk | 33 (0.0–33.3) | 33 (33.3–66.7) | ||
| 6 monthsl | 33 (0.0–33.3) | 33 (0.0–66.7) | ||
| 12 monthsm | 33 (33.3–33.3) | 33 (0.0–33.3) | ||
| Test for time-by-treatment interaction | 0.99 | |||
| Overall treatment effect | MD 0.18 (–2.92 to 3.28) | 1.00g | ||
| Haemoptysis | ||||
| Time point | ||||
| Baseline | 12/236 (5.1) | 17/243 (7.0) | ||
| 2 weeks | 40/181 (22.1) | 34/190 (17.9) | ||
| 5 weeks | 7/205 (3.4) | 6/227 (2.6) | ||
| 3 months | 4/187 (2.1) | 3/199 (1.5) | ||
| 6 months | 2/187 (1.1) | 6/189 (3.2) | ||
| 12 months | 1/172 (0.6) | 3/176 (1.7) | ||
| Test for time-by-treatment interaction | 0.23 | |||
| Overall treatment effect | OR 1.32 (0.70 to 2.50) | 1.00g | ||
| Pain in chest | ||||
| Time point | ||||
| Baselinen | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | ||
| 2 weeksi | 33.33 (0.00–33.33) | 33.33 (0.00–66.67) | ||
| 5 weekso | 33.33 (0.00–33.33) | 33.33 (0.00–33.33) | ||
| 3 monthsp | 0.00 (0.00–33.33) | 33.33 (0.00–33.33) | ||
| 6 monthsl | 0.00 (0.00–33.33) | 0.00 (0.00–33.33) | ||
| 12 monthsm | 0.00 (0.00–33.33) | 0.00 (0.00–33.33) | ||
| Test for time-by-treatment interaction | 0.97 | |||
| Overall treatment effect | MD –4.66 (–7.96 to –1.36) | 0.08g | ||
| Pain in other parts (not chest, arm of shoulder) | ||||
| Time point | ||||
| Baselineq | 0 (0.0–33.3) | 0 (0.0–33.3) | ||
| 2 weeksr | 33 (0.0–66.7) | 0 (0.0–66.7) | ||
| 5 weekss | 0 (0.0–33.3) | 0 (0.0–33.3) | ||
| 3 monthst | 0 (0.0–33.3) | 0 (0.0–66.7) | ||
| 6 monthsu | 0 (0.0–33.3) | 0 (0.0–33.3) | ||
| 12 monthsv | 33 (0.0–33.3) | 0 (0.0–66.7) | ||
| Test for time-by-treatment interaction | 0.93 | |||
| Overall treatment effect | MD –1.36 (–5.25 to 2.52) | 1.00g | ||
| Outcome | Time point | Score | Participant allocation | OR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Randomised to VATS (N = 247), n/N (%) | Randomised to open surgery (N = 255), n/N (%) | |||||
| Sore mouth | Baseline | 0 | 205/236 (86.9) | 209/243 (86.0) | ||
| 33.3 | 22/236 (9.3) | 22/243 (9.1) | ||||
| 66.7 | 7/236 (3.0) | 8/243 (3.3) | ||||
| 100 | 2/236 (0.8) | 4/243 (1.6) | ||||
| 2 weeks | 0 | 136/183 (74.3) | 124/187 (66.3) | |||
| 33.3 | 31/183 (16.9) | 35/187 (18.7) | ||||
| 66.7 | 6/183 (3.3) | 17/187 (9.1) | ||||
| 100 | 10/183 (5.5) | 11/187 (5.9) | ||||
| 5 weeks | 0 | 170/206 (82.5) | 184/226 (81.4) | |||
| 33.3 | 29/206 (14.1) | 24/226 (10.6) | ||||
| 66.7 | 4/206 (1.9) | 13/226 (5.8) | ||||
| 100 | 3/206 (1.5) | 5/226 (2.2) | ||||
| 3 months | 0 | 153/187 (81.8) | 167/198 (84.3) | |||
| 33.3 | 24/187 (12.8) | 19/198 (9.6) | ||||
| 66.7 | 3/187 (1.6) | 10/198 (5.1) | ||||
| 100 | 7/187 (3.7) | 2/198 (1.0) | ||||
| 6 months | 0 | 157/187 (84.0) | 160/189 (84.7) | |||
| 33.3 | 20/187 (10.7) | 17/189 (9.0) | ||||
| 66.7 | 8/187 (4.3) | 9/189 (4.8) | ||||
| 100 | 2/187 (1.1) | 3/189 (1.6) | ||||
| 12 months | 0 | 151/173 (87.3) | 147/176 (83.5) | |||
| 33.3 | 13/173 (7.5) | 18/176 (10.2) | ||||
| 66.7 | 8/173 (4.6) | 9/176 (5.1) | ||||
| 100 | 1/173 (0.6) | 2/176 (1.1) | ||||
| Test for time-by-treatment interactiona | 0.48 | |||||
| Overall treatment effect | OR 0.88 (0.63 to 1.22) | 1.00b | ||||
| Dysphagia | Baseline | 0 | 212/236 (89.8) | 216/243 (88.9) | ||
| 33.3 | 21/236 (8.9) | 20/243 (8.2) | ||||
| 66.7 | 3/236 (1.3) | 7/243 (2.9) | ||||
| 100 | 151/183 (82.5) | 139/188 (73.9) | ||||
| 2 weeks | 0 | 20/183 (10.9) | 36/188 (19.1) | |||
| 33.3 | 8/183 (4.4) | 7/188 (3.7) | ||||
| 66.7 | 4/183 (2.2) | 6/188 (3.2) | ||||
| 100 | 177/206 (85.9) | 188/226 (83.2) | ||||
| 5 weeks | 0 | 19/206 (9.2) | 26/226 (11.5) | |||
| 33.3 | 8/206 (3.9) | 7/226 (3.1) | ||||
| 66.7 | 2/206 (1.0) | 5/226 (2.2) | ||||
| 100 | 156/185 (84.3) | 172/199 (86.4) | ||||
| 3 months | 0 | 21/185 (11.4) | 19/199 (9.5) | |||
| 33.3 | 6/185 (3.2) | 6/199 (3.0) | ||||
| 66.7 | 2/185 (1.1) | 2/199 (1.0) | ||||
| 100 | 152/186 (81.7) | 161/190 (84.7) | ||||
| 6 months | 0 | 25/186 (13.4) | 22/190 (11.6) | |||
| 33.3 | 7/186 (3.8) | 6/190 (3.2) | ||||
| 66.7 | 2/186 (1.1) | 1/190 (0.5) | ||||
| 100 | 149/172 (86.6) | 144/176 (81.8) | ||||
| 12 months | 0 | 18/172 (10.5) | 24/176 (13.6) | |||
| 33.3 | 4/172 (2.3) | 5/176 (2.8) | ||||
| 66.7 | 1/172 (0.6) | 3/176 (1.7) | ||||
| 100 | 212/236 (89.8) | 216/243 (88.9) | ||||
| Test for time-by-treatment interactiona | 0.14 | |||||
| Overall treatment effect | OR 0.92 (0.65 to 1.30) | 1.00b | ||||
| Peripheral neuropathy | Baseline | 0 | 178/236 (75.4) | 201/243 (82.7) | ||
| 33.3 | 47/236 (19.9) | 29/243 (11.9) | ||||
| 66.7 | 9/236 (3.8) | 9/243 (3.7) | ||||
| 100 | 2/236 (0.8) | 4/243 (1.6) | ||||
| 2 weeks | 0 | 152/181 (84.0) | 160/189 (84.7) | |||
| 33.3 | 24/181 (13.3) | 21/189 (11.1) | ||||
| 66.7 | 3/181 (1.7) | 5/189 (2.6) | ||||
| 100 | 2/181 (1.1) | 3/189 (1.6) | ||||
| 5 weeks | 0 | 167/205 (81.5) | 187/224 (83.5) | |||
| 33.3 | 25/205 (12.2) | 31/224 (13.8) | ||||
| 66.7 | 9/205 (4.4) | 4/224 (1.8) | ||||
| 100 | 4/205 (2.0) | 2/224 (0.9) | ||||
| 3 months | 0 | 146/186 (78.5) | 153/199 (76.9) | |||
| 33.3 | 27/186 (14.5) | 27/199 (13.6) | ||||
| 66.7 | 9/186 (4.8) | 13/199 (6.5) | ||||
| 100 | 4/186 (2.2) | 6/199 (3.0) | ||||
| 6 months | 0 | 136/186 (73.1) | 144/189 (76.2) | |||
| 33.3 | 39/186 (21.0) | 33/189 (17.5) | ||||
| 66.7 | 8/186 (4.3) | 7/189 (3.7) | ||||
| 100 | 3/186 (1.6) | 5/189 (2.6) | ||||
| 12 months | 0 | 131/172 (76.2) | 124/175 (70.9) | |||
| 33.3 | 28/172 (16.3) | 35/175 (20.0) | ||||
| 66.7 | 12/172 (7.0) | 10/175 (5.7) | ||||
| 100 | 1/172 (0.6) | 6/175 (3.4) | ||||
| Test for time-by-treatment interactiona | 0.41 | |||||
| Overall treatment effect | OR 0.99 (0.63 to 1.55) | 1.00b | ||||
| Alopecia | Baseline | 0 | 217/235 (92.3) | 215/243 (88.5) | ||
| 33.3 | 11/235 (4.7) | 22/243 (9.1) | ||||
| 66.7 | 6/235 (2.6) | 4/243 (1.6) | ||||
| 100 | 1/235 (0.4) | 2/243 (0.8) | ||||
| 2 weeks | 0 | 172/182 (94.5) | 172/189 (91.0) | |||
| 33.3 | 7/182 (3.8) | 13/189 (6.9) | ||||
| 66.7 | 2/182 (1.1) | 3/189 (1.6) | ||||
| 100 | 1/182 (0.5) | 1/189 (0.5) | ||||
| 5 weeks | 0 | 192/205 (93.7) | 208/227 (91.6) | |||
| 33.3 | 9/205 (4.4) | 14/227 (6.2) | ||||
| 66.7 | 2/205 (1.0) | 4/227 (1.8) | ||||
| 100 | 2/205 (1.0) | 1/227 (0.4) | ||||
| 3 months | 0 | 159/187 (85.0) | 157/197 (79.7) | |||
| 33.3 | 19/187 (10.2) | 26/197 (13.2) | ||||
| 66.7 | 4/187 (2.1) | 10/197 (5.1) | ||||
| 100 | 5/187 (2.7) | 4/197 (2.0) | ||||
| 6 months | 0 | 139/187 (74.3) | 148/190 (77.9) | |||
| 33.3 | 36/187 (19.3) | 20/190 (10.5) | ||||
| 66.7 | 8/187 (4.3) | 14/190 (7.4) | ||||
| 100 | 4/187 (2.1) | 8/190 (4.2) | ||||
| 12 months | 0 | 146/172 (84.9) | 143/176 (81.3) | |||
| 33.3 | 20/172 (11.6) | 26/176 (14.8) | ||||
| 66.7 | 6/172 (3.5) | 4/176 (2.3) | ||||
| 100 | 0/172 (0.0) | 3/176 (1.7) | ||||
| Test for time-by-treatment interactiona | 0.73 | |||||
| Overall treatment effect | OR 0.96 (0.67 to 1.38) | 1.00b | ||||
| Pain in shoulder or arm | Baseline | 0 | 159/236 (67.4) | 172/243 (70.8) | ||
| 33.3 | 49/236 (20.8) | 48/243 (19.8) | ||||
| 66.7 | 18/236 (7.6) | 18/243 (7.4) | ||||
| 100 | 10/236 (4.2) | 5/243 (2.1) | ||||
| 2 weeks | 0 | 89/182 (48.9) | 84/189 (44.4) | |||
| 33.3 | 64/182 (35.2) | 64/189 (33.9) | ||||
| 66.7 | 18/182 (9.9) | 30/189 (15.9) | ||||
| 100 | 11/182 (6.0) | 11/189 (5.8) | OR 0.66 (0.40 to 1.08) | 0.452 | ||
| 5 weeks | 0 | 121/205 (59.0) | 125/226 (55.3) | |||
| 33.3 | 66/205 (32.2) | 60/226 (26.5) | ||||
| 66.7 | 14/205 (6.8) | 29/226 (12.8) | ||||
| 100 | 4/205 (2.0) | 12/226 (5.3) | OR 0.56 (0.34 to 0.93) | 0.172 | ||
| 3 months | 0 | 131/184 (71.2) | 129/195 (66.2) | |||
| 33.3 | 37/184 (20.1) | 41/195 (21.0) | ||||
| 66.7 | 12/184 (6.5) | 21/195 (10.8) | ||||
| 100 | 4/184 (2.2) | 4/195 (2.1) | OR 0.79 (0.46 to 1.36) | 1.002 | ||
| 6 months | 0 | 116/185 (62.7) | 123/189 (65.1) | |||
| 33.3 | 47/185 (25.4) | 47/189 (24.9) | ||||
| 66.7 | 14/185 (7.6) | 12/189 (6.3) | ||||
| 100 | 8/185 (4.3) | 7/189 (3.7) | OR 1.04 (0.60 to 1.82) | 1.002 | ||
| 12 months | 0 | 103/172 (59.9) | 106/173 (61.3) | |||
| 33.3 | 43/172 (25.0) | 48/173 (27.7) | ||||
| 66.7 | 20/172 (11.6) | 14/173 (8.1) | ||||
| 100 | 6/172 (3.5) | 5/173 (2.9) | OR 1.26 (0.72 to 2.19) | 1.00b | ||
| Test for time-by-treatment interactiona | 0.09 | |||||
| Time point | Participant allocation | Occurrence model | Intensity model | Test for time-by-treatment interactionc | |||
|---|---|---|---|---|---|---|---|
| Randomised to VATS (N = 247), median (IQR) | Randomised to open surgery (N = 255), median (IQR) | ORa (95% CI) | p-value | GMRb (95% CI) | p-value | ||
| Baselined | 0.78 (0.69–0.88) | 0.80 (0.68–0.88) | |||||
| 2 weekse | 0.71 (0.53–0.77) | 0.64 (0.42–0.74) | |||||
| 5 weeksf | 0.74 (0.58–0.80) | 0.69 (0.57–0.80) | |||||
| 3 monthsg | 0.76 (0.65–0.88) | 0.72 (0.55–0.84) | |||||
| 6 monthsh | 0.77 (0.65–0.88) | 0.74 (0.63–0.84) | |||||
| 12 monthsi | 0.77 (0.64–0.88) | 0.71 (0.62–0.84) | |||||
| Overall treatment effect | 0.57 (0.38 to 0.86) | 0.0071 | 0.90 (0.84 to 0.96) | 0.0027 | 0.20 | ||
| Event | Participant allocation | |||
|---|---|---|---|---|
| Randomised to VATS (N = 247) | Randomised to open surgery (N = 255) | |||
| All events, n/N (%) | SAE,a n/N (%) | All events, n/N (%) | SAE,b n/N (%) | |
| Any event | 81/247 (32.8) | 20/247 (8.1) | 113/255 (44.3) | 21/255 (8.2) |
| Cardiac disorders | 24/247 (9.7) | 4/247 (1.6) | 24/255 (9.4) | 3/255 (1.2) |
| Arrhythmia | 23/247 (9.3) | 3/247 (1.2) | 22/255 (8.6) | 1/255 (0.4) |
| Atrial fibrillation | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Bradycardia | 1/247 (0.4) | 1/247 (0.4) | 0/255 (0.0) | 0/255 (0.0) |
| Cardiac arrest | 2/247 (0.8) | 2/247 (0.8) | 2/255 (0.8) | 2/255 (0.8) |
| Myocardial infarction | 1/247 (0.4) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| Gastrointestinal disorders | 4/247 (1.6) | 1/247 (0.4) | 9/255 (3.5) | 4/255 (1.6) |
| Abdominal pain | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| Constipation | 1/247 (0.4) | 0/247 (0.0) | 0/255 (0.0) | 0/255 (0.0) |
| Ileus | 1/247 (0.4) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Intra-abdominal bleeding | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Melaena | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| Pancreatitis | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| Pancreatitis necrotising | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Peptic ulcer/gastrointestinal haemorrhage/gastrointestinal perforation | 2/247 (0.8) | 1/247 (0.4) | 3/255 (1.2) | 1/255 (0.4) |
| Small intestinal obstruction | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| Upper gastrointestinal haemorrhage | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| General disorders and administration site conditions | 1/247 (0.4) | 1/247 (0.4) | 2/255 (0.8) | 2/255 (0.8) |
| Organ failure | 1/247 (0.4) | 1/247 (0.4) | 1/255 (0.4) | 1/255 (0.4) |
| Systemic inflammatory response syndrome | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Immune system disorders | 1/247 (0.4) | 1/247 (0.4) | 0/255 (0.0) | 0/255 (0.0) |
| Anaphylactic reaction | 1/247 (0.4) | 1/247 (0.4) | 0/255 (0.0) | 0/255 (0.0) |
| Infections and infestations | 40/247 (16.2) | 9/247 (3.6) | 71/255 (27.8) | 5/255 (2.0) |
| Empyema | 1/247 (0.4) | 0/247 (0.0) | 2/255 (0.8) | 0/255 (0.0) |
| Gastrointestinal infection | 1/247 (0.4) | 0/247 (0.0) | 0/255 (0.0) | 0/255 (0.0) |
| Haemophilus infection | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| Kidney infection | 1/247 (0.4) | 1/247 (0.4) | 0/255 (0.0) | 0/255 (0.0) |
| Pneumonia/lower respiratory tract infection | 37/247 (15.0) | 9/247 (3.6) | 53/255 (20.8) | 5/255 (2.0) |
| Sepsis | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| Superinfection | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| Urinary tract infection | 4/247 (1.6) | 1/247 (0.4) | 6/255 (2.4) | 0/255 (0.0) |
| Wound infection | 4/247 (1.6) | 0/247 (0.0) | 9/255 (3.5) | 0/255 (0.0) |
| Unknown infection | 0/247 (0.0) | 0/247 (0.0) | 6/255 (2.4) | 0/255 (0.0) |
| Injury, poisoning and procedural complications | 10/247 (4.0) | 7/247 (2.8) | 10/255 (3.9) | 4/255 (1.6) |
| Bleeding from vascular injuryb | 8/129 (6.2) | 6/129 (4.7) | 5/127 (3.9) | 1/127 (0.8) |
| Fall | 1/247 (0.4) | 1/247 (0.4) | 0/255 (0.0) | 0/255 (0.0) |
| Laryngeal nerve dysfunction | 0/247 (0.0) | 0/247 (0.0) | 2/255 (0.8) | 0/255 (0.0) |
| Overdose | 0/247 (0.0) | 0/247 (0.0) | 2/255 (0.8) | 2/255 (0.8) |
| Tracheal injury | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Wound dehiscence | 1/247 (0.4) | 0/247 (0.0) | 0/255 (0.0) | 0/255 (0.0) |
| Investigations | 11/247 (4.5) | 6/247 (2.4) | 10/255 (3.9) | 2/255 (0.8) |
| Bronchoscopy | 11/247 (4.5) | 6/247 (2.4) | 9/255 (3.5) | 1/255 (0.4) |
| Endoscopy upper gastrointestinal tract | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Metabolism and nutrition disorders | 1/247 (0.4) | 1/247 (0.4) | 1/255 (0.4) | 1/255 (0.4) |
| Hypokalaemia | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Hyponatremia | 1/247 (0.4) | 1/247 (0.4) | 1/255 (0.4) | 1/255 (0.4) |
| Nervous system disorders | 0/247 (0.0) | 0/247 (0.0) | 2/255 (0.8) | 2/255 (0.8) |
| Syncope | 0/247 (0.0) | 0/247 (0.0) | 2/255 (0.8) | 2/255 (0.8) |
| Psychiatric disorders | 7/247 (2.8) | 0/247 (0.0) | 12/255 (4.7) | 0/255 (0.0) |
| Acute psychosis | 7/247 (2.8) | 0/247 (0.0) | 12/255 (4.7) | 0/255 (0.0) |
| Renal and urinary disorders | 5/247 (2.0) | 1/247 (0.4) | 16/255 (6.3) | 2/255 (0.8) |
| Acute kidney injury | 5/247 (2.0) | 1/247 (0.4) | 15/255 (5.9) | 1/255 (0.4) |
| Oliguria | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Respiratory, thoracic and mediastinal disorders | 18/247 (7.3) | 6/247 (2.4) | 21/255 (8.2) | 7/255 (2.7) |
| Acute lung injury | 2/247 (0.8) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Acute respiratory distress syndrome | 0/247 (0.0) | 0/247 (0.0) | 3/255 (1.2) | 2/255 (0.8) |
| Acute respiratory failure | 12/247 (4.9) | 6/247 (2.4) | 12/255 (4.7) | 6/255 (2.4) |
| Atelectasis | 11/247 (4.5) | 5/247 (2.0) | 8/255 (3.1) | 2/255 (0.8) |
| Chylothorax | 0/247 (0.0) | 0/247 (0.0) | 3/255 (1.2) | 1/255 (0.4) |
| Hypoxia | 1/247 (0.4) | 1/247 (0.4) | 2/255 (0.8) | 2/255 (0.8) |
| Pleural effusionb | 3/135 (2.2) | 0/135 (0.0) | 4/146 (2.7) | 1/146 (0.7) |
| Pneumothorax | 4/247 (1.6) | 0/247 (0.0) | 9/255 (3.5) | 2/255 (0.8) |
| Pulmonary air leakageb | 20/135 (14.8) | 0/135 (0.0) | 11/146 (7.5) | 0/146 (0.0) |
| Skin and subcutaneous tissue disorders | 9/247 (3.6) | 1/247 (0.4) | 13/255 (5.1) | 1/255 (0.4) |
| Subcutaneous emphysema | 9/247 (3.6) | 1/247 (0.4) | 13/255 (5.1) | 1/255 (0.4) |
| Surgical and medical procedures | 9/247 (3.6) | 4/247 (1.6) | 12/255 (4.7) | 4/255 (1.6) |
| Central venous catheterisation | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Extracorporeal membrane oxygenation | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Hemofiltration | 2/247 (0.8) | 1/247 (0.4) | 3/255 (1.2) | 1/255 (0.4) |
| Laparotomy | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Mini-tracheostomy | 5/247 (2.0) | 3/247 (1.2) | 6/255 (2.4) | 0/255 (0.0) |
| Reoperation for bleeding | 2/247 (0.8) | 1/247 (0.4) | 2/255 (0.8) | 1/255 (0.4) |
| Reoperation for pleural effusion | 1/247 (0.4) | 0/247 (0.0) | 0/255 (0.0) | 0/255 (0.0) |
| Reoperation for drainage of empyema | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| Reoperation for haemothorax | 0/247 (0.0) | 0/247 (0.0) | 2/255 (0.8) | 0/255 (0.0) |
| Reoperation for sputum retention | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| Tracheostomy | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Transfusion | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 1/255 (0.4) |
| Vascular disorders | 2/247 (0.8) | 2/247 (0.8) | 4/255 (1.6) | 3/255 (1.2) |
| Deep-vein thrombosis | 0/247 (0.0) | 0/247 (0.0) | 1/255 (0.4) | 0/255 (0.0) |
| Haematomab | 0/129 (0.0) | 0/129 (0.0) | 3/141 (2.1) | 0/141 (0.0) |
| Hypotension | 1/247 (0.4) | 1/247 (0.4) | 2/255 (0.8) | 2/255 (0.8) |
| Ischaemia | 1/247 (0.4) | 1/247 (0.4) | 1/255 (0.4) | 1/255 (0.4) |
| Event | Participant allocation | |
|---|---|---|
| Randomised to VATS (n = 244)a | Randomised to open surgery (n = 249)a | |
| Total events | 142/75 (30.7) | 207/94 (37.8) |
| Blood and lymphatic system disorders | 10/5 (2.0) | 10/10 (4.0) |
| Anaemia | 1/1 (0.4) | 1/1 (0.4) |
| Neutropenia | 9/5 (2.0) | 8/8 (3.2) |
| Pancytopenia | 0/0 (0.0) | 1/1 (0.4) |
| Cardiac disorders | 5/4 (1.6) | 9/8 (3.2) |
| Atrial fibrillation | 2/2 (0.8) | 3/3 (1.2) |
| Atrial flutter | 0/0 (0.0) | 1/1 (0.4) |
| Cardiac arrest | 0/0 (0.0) | 2/2 (0.8) |
| Cardiac failure | 0/0 (0.0) | 2/2 (0.8) |
| Intracardiac thrombus | 1/1 (0.4) | 0/0 (0.0) |
| Myocardial infarction | 2/2 (0.8) | 0/0 (0.0) |
| Myocardial ischaemia | 0/0 (0.0) | 1/1 (0.4) |
| Eye disorders | 1/1 (0.4) | 0/0 (0.0) |
| Vision blurred | 1/1 (0.4) | 0/0 (0.0) |
| Gastrointestinal disorders | 9/7 (2.9) | 27/16 (6.4) |
| Abdominal pain | 1/1 (0.4) | 0/0 (0.0) |
| Constipation | 0/0 (0.0) | 2/2 (0.8) |
| Diarrhoea | 1/1 (0.4) | 5/4 (1.6) |
| Dyschezia | 0/0 (0.0) | 1/1 (0.4) |
| Gastro-oesophageal reflux disease | 0/0 (0.0) | 1/1 (0.4) |
| Gastrointestinal haemorrhage | 1/1 (0.4) | 2/2 (0.8) |
| Ileus | 0/0 (0.0) | 2/2 (0.8) |
| Nausea | 1/1 (0.4) | 4/3 (1.2) |
| Oesophageal obstruction | 1/1 (0.4) | 0/0 (0.0) |
| Oesophagitis | 0/0 (0.0) | 1/1 (0.4) |
| Pancreatitis | 1/1 (0.4) | 0/0 (0.0) |
| Small intestinal obstruction | 0/0 (0.0) | 1/1 (0.4) |
| Vomiting | 3/3 (1.2) | 8/6 (2.4) |
| General disorders | 12/12 (4.9) | 14/12 (4.8) |
| Chest pain (unknown cause) | 0/0 (0.0) | 2/2 (0.8) |
| Death (unknown cause) | 0/0 (0.0) | 1/1 (0.4) |
| Disease recurrence/disease progression | 11/11 (4.5) | 9/7 (2.8) |
| Organ failure | 1/1 (0.4) | 1/1 (0.4) |
| Pain | 0/0 (0.0) | 1/1 (0.4) |
| Hepatobiliary disorders | 1/1 (0.4) | 0/0 (0.0) |
| Cholecystitis | 1/1 (0.4) | 0/0 (0.0) |
| Immune system disorders | 1/1 (0.4) | 0/0 (0.0) |
| Anaphylaxis/hypersensitivity reaction | 1/1 (0.4) | 0/0 (0.0) |
| Infections and infestations | 51/35 (14.3) | 48/37 (14.9) |
| Cellulitis | 1/1 (0.4) | 0/0 (0.0) |
| Diverticulitis | 1/1 (0.4) | 0/0 (0.0) |
| Empyema | 6/6 (2.5) | 5/5 (2.0) |
| Encephalitis | 0/0 (0.0) | 1/1 (0.4) |
| Respiratory tract infection/pneumonia | 29/24 (9.8) | 34/27 (10.8) |
| Sepsis | 4/4 (1.6) | 1/1 (0.4) |
| Unknown infection | 1/1 (0.4) | 1/1 (0.4) |
| Urinary tract infection | 8/7 (2.9) | 5/4 (1.6) |
| Wound infection | 1/1 (0.4) | 1/1 (0.4) |
| Injury poisoning and procedural complications | 2/2 (0.8) | 2/2 (0.8) |
| Fall | 1/1 (0.4) | 1/1 (0.4) |
| Hip fracture | 1/1 (0.4) | 0/0 (0.0) |
| Wound dehiscence | 0/0 (0.0) | 1/1 (0.4) |
| Investigations | 1/1 (0.4) | 5/4 (1.6) |
| Biopsy lung | 0/0 (0.0) | 1/1 (0.4) |
| Bronchoscopy | 0/0 (0.0) | 3/2 (0.8) |
| Colonoscopy | 0/0 (0.0) | 1/1 (0.4) |
| Cystoscopy | 1/1 (0.4) | 0/0 (0.0) |
| Metabolism and nutrition disorders | 3/3 (1.2) | 3/2 (0.8) |
| Decreased appetite | 0/0 (0.0) | 2/1 (0.4) |
| Hyperglycaemia | 1/1 (0.4) | 0/0 (0.0) |
| Hypoglycaemia | 1/1 (0.4) | 0/0 (0.0) |
| Hyponatraemia | 1/1 (0.4) | 1/1 (0.4) |
| Musculoskeletal and connective tissue disorders | 0/0 (0.0) | 4/3 (1.2) |
| Arthralgia | 0/0 (0.0) | 2/1 (0.4) |
| Musculoskeletal chest pain | 0/0 (0.0) | 2/2 (0.8) |
| Neoplasms benign, malignant and unspecified | 3/3 (1.2) | 3/3 (1.2) |
| Adenocarcinoma | 1/1 (0.4) | 0/0 (0.0) |
| Brain neoplasm | 0/0 (0.0) | 1/1 (0.4) |
| Endometrial cancer | 1/1 (0.4) | 0/0 (0.0) |
| Gastrointestinal carcinoma | 1/1 (0.4) | 0/0 (0.0) |
| Lung neoplasm malignant | 0/0 (0.0) | 1/1 (0.4) |
| Sarcoma | 0/0 (0.0) | 1/1 (0.4) |
| Nervous system disorders | 4/4 (1.6) | 6/6 (2.4) |
| Cerebral ischaemia | 0/0 (0.0) | 1/1 (0.4) |
| Cerebrovascular accident | 1/1 (0.4) | 2/2 (0.8) |
| Headache | 1/1 (0.4) | 0/0 (0.0) |
| Subarachnoid haemorrhage | 0/0 (0.0) | 1/1 (0.4) |
| Transient ischaemic attack | 1/1 (0.4) | 2/2 (0.8) |
| White matter ischaemia | 1/1 (0.4) | 0/0 (0.0) |
| Psychiatric disorders | 0/0 (0.0) | 1/1 (0.4) |
| Alcohol withdrawal syndrome | 0/0 (0.0) | 1/1 (0.4) |
| Renal and urinary disorders | 0/0 (0.0) | 5/4 (1.6) |
| Acute kidney injury | 0/0 (0.0) | 5/4 (1.6) |
| Respiratory, thoracic and mediastinal disorders | 25/22 (9.0) | 39/27 (10.8) |
| Acute lung injury | 2/2 (0.8) | 1/1 (0.4) |
| Atelectasis/pulmonary collapse | 1/1 (0.4) | 5/4 (1.6) |
| Bronchopleural fistula | 0/0 (0.0) | 2/2 (0.8) |
| Chronic obstructive pulmonary disease | 2/2 (0.8) | 3/2 (0.8) |
| Chylothorax | 0/0 (0.0) | 1/1 (0.4) |
| Dyspnoea | 1/1 (0.4) | 2/2 (0.8) |
| Haemothorax | 1/1 (0.4) | 0/0 (0.0) |
| Interstitial lung disease | 0/0 (0.0) | 1/1 (0.4) |
| Pleural effusion | 9/8 (3.3) | 8/7 (2.8) |
| Pulmonary air leakage/pneumothorax | 7/6 (2.5) | 9/7 (2.8) |
| Pulmonary embolism | 2/2 (0.8) | 6/6 (2.4) |
| Respiratory arrest | 0/0 (0.0) | 1/1 (0.4) |
| Surgical and medical procedures | 12/11 (4.5) | 25/19 (7.6) |
| Colectomy | 1/1 (0.4) | 0/0 (0.0) |
| Empyema drainage | 0/0 (0.0) | 2/1 (0.4) |
| Femoral hernia repair | 0/0 (0.0) | 1/1 (0.4) |
| Haemorrhoid operation | 0/0 (0.0) | 1/1 (0.4) |
| Haematoma evacuation | 0/0 (0.0) | 1/1 (0.4) |
| Hip arthroplasty | 1/1 (0.4) | 2/2 (0.8) |
| Hysterectomy | 2/2 (0.8) | 0/0 (0.0) |
| Ileostomy | 0/0 (0.0) | 1/1 (0.4) |
| Ileostomy closure | 0/0 (0.0) | 1/1 (0.4) |
| Intestinal resection | 0/0 (0.0) | 1/1 (0.4) |
| Knee arthroplasty | 0/0 (0.0) | 2/2 (0.8) |
| Limb operation | 0/0 (0.0) | 1/1 (0.4) |
| Lymphadenectomy | 1/1 (0.4) | 0/0 (0.0) |
| Parathyroidectomy | 1/1 (0.4) | 0/0 (0.0) |
| Peripheral artery bypass | 1/1 (0.4) | 0/0 (0.0) |
| Polypectomy | 1/1 (0.4) | 0/0 (0.0) |
| Proctectomy | 0/0 (0.0) | 1/1 (0.4) |
| Prostatic operation | 0/0 (0.0) | 1/1 (0.4) |
| Pulmonary resection | 1/1 (0.4) | 1/1 (0.4) |
| Radioactive iodine therapy | 0/0 (0.0) | 1/1 (0.4) |
| Rehabilitation therapy | 2/1 (0.4) | 2/2 (0.8) |
| Removal of foreign body | 0/0 (0.0) | 1/1 (0.4) |
| Salpingo-oophorectomy | 0/0 (0.0) | 1/1 (0.4) |
| Stent placement | 0/0 (0.0) | 2/2 (0.8) |
| Stent removal | 0/0 (0.0) | 1/1 (0.4) |
| Thyroidectomy | 1/1 (0.4) | 1/1 (0.4) |
| Vascular disorders | 2/1 (0.4) | 6/5 (2.0) |
| Deep-vein thrombosis | 1/1 (0.4) | 1/1 (0.4) |
| Haematoma | 0/0 (0.0) | 1/1 (0.4) |
| Haemorrhage | 0/0 (0.0) | 2/1 (0.4) |
| Hypotension | 0/0 (0.0) | 1/1 (0.4) |
| Peripheral ischaemia | 0/0 (0.0) | 1/1 (0.4) |
| Phlebitis | 1/1 (0.4) | 0/0 (0.0) |
| Reason | Participant allocation | |
|---|---|---|
| Randomised to VATS (N = 70), n/N (%) | Randomised to open surgery (N = 88), n/N (%) | |
| Infection | 32/22 (31.4) | 29/25 (28.4) |
| Medical procedure | 21/20 (28.6) | 30/25 (28.4) |
| Chemotherapy toxicities | 11/7 (10) | 11/10 (11.4) |
| Shortness of breath | 16/15 (21.4) | 12/11 (12.5) |
| Gastrointestinal disorder | 4/4 (5.7) | 13/11 (12.5) |
| Pain | 8/8 (11.4) | 9/8 (9.1) |
| Pneumothorax/surgical emphysema | 6/5 (7.1) | 4/3 (3.4) |
| Physiotherapy/rehabilitation/recovery | 5/4 (5.7) | 4/4 (4.5) |
| Cardiovascular | 1/1 (1.4) | 6/4 (4.5) |
| Neurological | 3/3 (4.3) | 5/5 (5.7) |
| Bleeding | 1/1 (1.4) | 5/4 (4.5) |
| Thromboembolism | 3/3 (4.3) | 4/4 (4.5) |
| Monitoring | 0 | 3/3 (3.4) |
| Pleural effusion | 2/2 (2.9) | 0 |
| Pyrexia | 1/1 (1.4) | 1/1 (1.1) |
| Metabolism and nutrition disorder | 2/2 (2.9) | 0 |
| Other | 1/1 (1.4) | 5/5 (5.7) |
Appendix 6 Additional QRI quotations and table demonstrating imbalanced information provision/loaded terminology
Additional QRI quotations
Acknowledgement of lack of evidence to support bias towards VATS
If you go to, if you’ve been to the big conferences there’s so much about VATS lobectomy, why it’s better, and so there’s been this incredible lack of equipoise, and I think a study like this is required to try and readdress the balance a bit, yeah. You can get carried away by the evangelists.
Surgeon, interview
Conveying equipoise in consultations: general patterns and concerns – expressing uncertainty
Of course we don’t know what the best way, it may well be that keyhole is better, it may well be that open is better, it may well be that they’re exactly the same and that’s what was found in a similar study with bowel cancer that it’s been very similar.
Surgeon, consultation
Conveying equipoise in consultations: general patterns and concerns – statements later in the consultation that went against their previously expressed neutrality
In favour of VATS
There are two ways of doing this, 70% of these operations in the country are done through big cuts round the side of your chest and spreading your ribs apart. Nowadays more of, some people such as myself are doing more of these with keyhole surgery. Because we think it might be better, we don’t know for certain that it’s better, but we think it may be better than making a bigger cut.
Be better wouldn’t it? [Conversation continues along similar lines. Patient declines study and opts for VATS.]
In favour of open surgery
So that’s [open lobectomy] the way we’re all taught how to do it and it’s the that’s the standard against which everything else is measured, so if you develop a new way of doing something it has to be measured against that.
And you can see what you’re doing presumably.
Yes so with an open operation you’re seeing things with your own eyes and you got conventional instruments, and if you need to then you’ve got your fingers and thumbs to get in there as well so a lot of people are very comfortable doing it that way so it’s a straightforward operation to do.
Explaining the VIOLET study and related concepts: apologetic study presentation, making assumptions regarding patient’s willingness to consider study, closing down conversation and not presenting study to all potentially eligible patients
So, if you say you are interested to hear more about this then I will go ahead. If you say well not I’ve mind up my mind I want this, or I want that then we just stop that, and we carry on with the consultation.
I’m quite happy for you to go on and explain.
We are running a nationwide study. You can say yes, I’m interested, or say no. And then if you say no we just turn that off and we just carry on with your consultation, or you can even say well I want more time to think about it, tell me more about it and I’ll make up my mind later. What is your answer?
Well . . . I didn’t really mind this part [recording], but I didn’t really want to carry on with it.
You don’t?
No.
OK. That’s fine, we can turn this off and just carry on with the consultation. OK, thank you.
Blinding
Where, whenever the patient was to participate, goes in the study and basically what happens is that we don’t know until the very last minute if you will have the thoracotomy or the keyhole surgery, it’s going to be decided the very day in order for the whole procedure and the postoperative recovery to be exactly the same for the two. So, is this something you would be interested to participate in?
Surgeon, consultation
Table demonstrating imbalanced information provision and loaded terminology used in consultations to describe the two operations
| VATS lobectomya | Open lobectomya |
|---|---|
| . . . keyhole [sometimes no mention of cuts] | . . . open cut |
| . . . small cuts or small incisions | . . . big cut or big incision |
| . . . we don’t know for certain that it’s better, but we think it may be better than making a bigger cut | . . . these operations are done through big cuts round the side of your chest and spreading your ribs apart |
| . . . we are big advocates of keyhole surgery ’cause we enjoy doing keyhole surgery | . . . doing it the traditional way [ . . . ] the way that we were originally trained to do; most common way; gold standard; extremely well established |
| . . . faster, early recovery | . . . better at taking lymph nodes out |
Appendix 7 Summary of issues related to trial design identified through QRI
Treatment options outside the trial
In the consultations, most surgeons described how there were several options for early-stage lung cancer. These options included the ‘main’ and ‘gold-standard’ option, that is, a lobectomy (via thoracotomy, VATS or robot). Two recruiters told patients that a lobectomy would ‘cure’ them and few recruiters discussed the possibility of patients needing chemotherapy after a lobectomy. This may suggest that patients might not be expecting any potential adjuvant treatment:
[Describes procedure] . . . we’re doing it to give you a long-term cure of cancer.
Surgeon, consultation
In addition, recruiters also described how patients could have active surveillance, radiotherapy, radiofrequency ablation and/or chemotherapy. The audio-recorded consultations demonstrated that there was recruiter variation within this. Many patients expressed a preference to have surgery and ‘get the cancer out’ (patient, consultation).
Robot-assisted lobectomy
Other than via VATS and a thoracotomy, some recruiters described how a lobectomy could also be conducted by robotic-assisted surgery (note that, in the interviews, this was not discussed in the consultations provided). Middlesbrough was the only recruiting centre that had access to a robot. There were mixed feelings as to whether or not robotic surgeries would increase in the future. One recruiter described how NHS England were carrying out a review into robotic thoracic surgery, but stated that this had been delayed. Several likened this to a prostatectomy for prostate cancer, whereby robotic surgery had become the ‘gold standard’. Three recruiters commented that the robot was the ‘future’ for lobectomies:
The next step from keyhole is the robot, and people instinctively think that the robot is better, and if you talk to robotic surgeons, and they say I can’t believe you’re still doing keyhole surgery, the robot is so much better, my patients go home on day 1 or day 2, so there’s this incredible bias now.
Surgeon, interview
Other recruiters were less convinced and it was described as being a ‘steep learning curve’ for surgeons in comparison with VATS, and substantially more costly:
[Sighs] A lot of expense is involved, they’ll be about five or six cuts in the chest . . . I’m not convinced with this yet. It’s an expensive learning curve, I wouldn’t get too excited with this.
Surgeon, interview
Radiotherapy
There were discrepancies if and in how radiotherapy was presented to patients. The quotes below demonstrate this:
As I say alternative treatment would be to treat this with some radiotherapy but that, although it has lower risks it doesn’t give you as good a chance of cure as the surgery.
Surgeon, consultation
There are two small studies which suggest that the outcomes of radiotherapy is better, has better overall survival, but those two studies were stopped because they couldn’t complete, and the results are considered to be not very conclusive.
Surgeon, consultation
In the interviews, several recruiters also described an ongoing randomised study in Leeds (SABRTooth;70 13029788). The feasibility study is currently recruiting and aims to randomise 54 patients to either surgery or stereotactic ablative radiotherapy. Middlesbrough was the only centre in the VIOLET trial to also recruit to the SABRTooth trial,70 although three other PIs described it as a ‘promising’ and ‘emerging’ procedure:
SABRE [stereotactic ablative radiotherapy] is very promising and might even be as good as surgery in some cases, whereas radical radiotherapy is far inferior to surgery.
Surgeon, interview
Two (4%) of the patients in the recordings patients opted for radiotherapy. In these recordings, patients commented that they were concerned about their lung function if they were to have a lobectomy. These patients tended to be in poorer health:
I think if my breathing gets any worse going uphill I won’t be able to go uphill at all, you know . . . I think I shall go for the radiotherapy.
Patient, consultation
Active surveillance
Patients were informed that they could also choose not to undergo any intervention, and some patients appeared anxious at the potential risks of surgery. From the recordings provided, 6% of patients opted for active surveillance:
You said you would take the top third of my lung away which frightened me. I’m sorry I’m a wimp [ . . . ] I don’t know, I’m, [–] I’m a bit scared of the big operation, I’m not a spring chicken anymore I’m quite . . . I’m getting on, hmm . . .
Patient, consultation
Segmentectomy
Recruiters described how a segmentectomy could be performed via VATS and open surgery. Although a lobectomy was described as the more effective procedure, there was a feeling that this tended to be more suitable for a small number of patients who were too high risk for a lobectomy:
There’s a group of patients who, if you did a lobectomy on them and that’s taken away too much lung so it would affect their quality of life, would they be breathless afterwards, or they would be at risk of death in hospital, death because it was just too much to take.
Surgeon, interview
The screening logs showed that 6.3% of patients underwent a segmentectomy. Following on from a TMG meeting where there was a discussion regarding whether or not a segmentectomy should be included in the protocol, the QRI researcher asked the recruiters their perspective on the trial design. Two recruiters felt that it should be included in the study:
I think it should be included, I don’t see really how come we’ve excluded that. I think that was a weird decision. I understand the reasoning for excluding it, it’s a lot cleaner to just go for lobectomies. I would have put it in myself really.
Do you see many patients?
Yeah, you see quite a few, that’s the thing, ’cause impaired lung function patients with a small tumour, you want to preserve as much lung tissue as possible, so we’re probably losing 20% of patients for people that you’d want to reserve the right to do a segmentectomy on. If you don’t want to take absolutely everybody to the study then that’s probably not too much of a problem.
Two recruiters also described how it would be considerably more difficult to perform a segmentectomy via VATS. Overall, there was a feeling that including the procedure would ‘overcomplicate’ a simple study and the potential patient numbers would only be small:
VIOLET is a lobectomy study. A segmentectomy to me is possible an inferior operation to lobectomy, and also it’s very small numbers as well. So I didn’t think there was much to gain, it just confuses, confuses the study, so it’s much cleaner if we just keep it to one operation which is the gold standard operation for lung cancer.
Surgeon, interview
In addition, one recruiter described how it would be technically difficult to distinguish between a segmentectomy and a wedge resection:
So segmentectomy is just taking a bit of the lobe, but you’re still disconnecting blood vessels, and I suspect one little issue may have had is that some people cheat at doing a segmentectomy. What some people call a segmentectomy, others would call something called a wedge resectomy, just get your stapler out and cut round it, with no identification of the vessels. And you can do little cheat ways of doing it where, ‘cause there’s an artery vein and a bronchus to every lobe and there’s an artery vein and a bronchus to every segment, so a segment you should be taking the artery vein and bronchus for that segment. You can cheat a bit, just take the artery and then use a staple gun to just chop through the rest without identifying the rest, though, but then the line becomes a bit blurred between a wedge resection, which everybody thinks is a very bad operation ‘cause the recurrence rate’s a lot higher, and a segmentectomy which people think is a pretty good operation. So there is a bit of blurring where there’s no blurring in a lobectomy. A lobectomy’s a lobectomy, there is no blurred lines.
Surgeon, interview
Completion of outcome measures
In some circumstances, patients’ planned surgery had been cancelled because of lack of available beds. One recruiter described how the cancellation of scheduled procedures had implications for data collection:
We randomise the day before surgery normally, and then get everybody up on the notes ready for surgery to come, and all your file is done from date of randomisation, so, yeah, so your 5-week follow-up, if they then don’t get operated on from that date for 2 weeks, then you’re stuck, you’re sort of, your 5 weeks date won’t be a 5-week post-surgery, it will be 3 weeks. So someone, yes, which will be very different data that you’re collecting at 3 weeks, post surgery, just because they were randomised. ‘cause their pain will be very different, all the questions that you’re asking them will be very different from someone that’s actually got to the 5-week post-surgery. ’Cause your day 1 and your day 2, obviously you do post-surgery date, but your actual visits are all deemed from the randomisation.
Surgeon, interview
Double blinding
One recruiting site (Bristol) reported difficulties in the blinding component in that patients who usually had a thoracotomy went to a high-dependency unit, whereas VATS patients went to the general ward. Although this made blinding impossible, the alternatives were to send all patients to the general ward (therefore breaching trust guidelines) or all patients to the high-dependency unit (consequently placing a burden on the high-dependency unit’s capacity). Recruitment was suspended from December 2015 to January 2016. Most other recruiters described the process of ensuring that the RNs remained blinded as challenging, and one that had been a learning curve. At the beginning, there were several instances where the nurses had quickly become unblinded. This was mostly due to electronic records, patients’ notes and handover processes:
I just went to look, erm, after the first patient had been in I went to see where he was, whether he was in ITU [intensive care unit] or on the ward, so on the system you’ve got a thing called e-handover, where all the patients are put on each of the wards, and there’s a little spiel about them when they came in, when they were admitted and what was wrong with them, and it just said, really nicely, ‘this patient has had an open lobectomy, entered the VIOLET study’ and in brackets it said ‘the patient is unaware of the approach’, so they were OK with that side of it but forgot that we weren’t meant to know [laughter].
RN, interview
At the beginning of the study it was quite difficult for the research nurse to be blinded, you know, if I go on our electronic system to check which drugs patients have during the surgery for intercostal block, the name of the surgery is there straight away, so it takes me 10 seconds to be unblinded.
Surgeon, interview
We’ve had a slight issue with the fact that we have to put what the operation is on the operation list, because theatres don’t want us to, they’re desperate to know and they can’t accept me just telling them in an e-mail, and want to know in advance. So we’re kind of putting it on the op list and so one of the nurses who was going to be in the perioperative management, sort of just saw it on the list by accident looking for another patient.
Surgeon, interview
Most recruiters felt that, having overcome these initial issues, the double blinding process was now ‘working well’:
Apart from that, erm, the others have all managed to stay blinded at the moment, yeah.
Surgeon, interview
It’s much easier now.
RN, interview
Patients were thought to have been successfully blinded, although surgeons at one site commented that patients became unblinded after having a shower and feeling the size of the incision:
Well the patients I think are relatively blinded, they can’t see the site of the operation.
Surgeon, interview
They say, you gonna tell me what I’ve had [laugh] and they keep changing their mind what they think they’ve had. Initially they all think they’ve had open so far. Because they feel pain they think they must have had it.
Surgeon, interview
The patients have, they’ve done fairly well in staying blinded.
Surgeon, interview
Some nurses stated that, although they sometimes had ‘gut instincts’ as to what patients had really had, they did not truly know:
You think you do, but you . . . It’s one of those things isn’t it, I suppose you know because you have a gut feeling that, what they’ve had done, but you don’t really know, so it’s a guess.
RN, interview
I would say that you can try to, you can try to guess . . . But if everything is done according to the protocol it’s a bit hard to know which kind of surgery they have. The thoracic is there in the same way, you know, the drain is there in the same way, so, only for looking, no, you would not say definitely which kind of surgery they had.
RN, interview
There were, however, two nurses who commented that it created unnecessary work to an otherwise straightforward study:
What’s the point of it, really? It just creates extra work. It serves no function. If I were a patient, I would have a lot to say about why I’m not allowed to know.
RN, interview
One surgeon also said that a patient had declined study because they had wanted to know which procedure they were having.
Standardisation of analgesia
A key component of the VIOLET trial protocol was that patients would be prescribed the same analgesia, regardless of their treatment allocation. When asked about this, the recruiters discussed that this was working well in practice. One centre had also added recovery drinks and mobilisation aids to ensure that everything was standardised between the two groups:
We’ve been doing the same for both procedures for the last few years already, so it’s not going to be an issue for us, it was a very straightforward thing to do.
Surgeon, interview
. . . it’s worked brilliantly for us.
Surgeon, interview
Several surgeons commented that there had initially been some reluctance from a minority of anaesthetists:
There have been . . . a couple of old-school people who are still not very happy.
Surgeon, interview
Yes, it’s been fine. Everyone’s very happy. With the exception of a few. But that’s been sorted now.
Surgeon, interview
Level of expertise of surgeon performing the procedure
One recruiter expressed concerns that as VATS were more technically difficult, consultants – rather than registrars – would perform them. This was perceived to have implications in how the findings of the study were interpreted:
What’s going to happen is that 80% of the opens, which is a very easy operation, are going to be thrown to the registrars, and so only 20% of the VATS, which are difficult, are going to the registrars, ’cause you just have to be a lot better to do it by VATS, and you have to be a VATS trained, or you have to be in a VATS training programme. So I don’t really let anybody do a VATS lobectomy who isn’t a thoracic trainee, and we get cardiac trainees quite a lot of the time, whereas an open lobe, anyone can do that, piece of cake. So I do have a worry that if I was an open surgeon, and the outcome, and finally I look at the lobectomy studies, and I go ‘Wait a second, 80% of registrars did open and only 10% of registrars did VATS, this is a study of registrars versus consultants’. So I think that’s a big problem.
Surgeon, interview
The development of VATS and open lobectomies
All of the PIs performed both VATS and open lobectomies. As PIs described the history of VATS, they discussed how the technique had evolved:
There was probably a lot of variability for what was called lobectomy in that people were using big spreaders and putting their hands inside the chest for smaller incision or just peeking through a smaller wound rather than looking into the screen so I think it took a while to kind of understand what actually was VATS lobectomy as compared to a small thoracotomy open procedure.
Surgeon, interview
One recruiter voiced concerns that a thoracotomy had also developed in recent years, and that there was a possibility that this had not been captured in the study:
I think quite a big thing in the study, is that sort of we haven’t thought enough about the open group, and that at the moment I think we’re all the VATS evangelists in the study, and we all thought initially we were just going to do 1980s huge thoracotomies, because that’s what maybe we were taught a bit as registrars. But actually talking to some American open surgeons they said basically ‘God, that’s not what a modern thoracotomy surgeon does’, and what a modern thoracotomy surgeon does is the best thoracotomy they can do as well. So not all thoracotomies are the same is my discovery, and what most of the study’s probably going to end up doing is very large thoracotomies, cutting all the muscles on the chest wall, when actually you can do quite a bit smaller thoracotomy and not cut any of the muscles on the chest wall, and it is, I think, considerably less painful for a patient. I kind of have a, a view that we should actually be trying to do the 2015 most modern thoracotomies we can do as well as the most modern VATS, rather than us all doing the massive posterolateral thoracotomies that they did in the eighties.
Surgeon, interview
Appendix 8 Good practice in addressing preferences as the trial progressed (quotations from the main phase)
| Patient preference or concern | Examples of good practice in addressing patient preferences and concerns |
|---|---|
Patient’s (possible) preference for keyhole because:
|
Patient:‘Cause I always feel that the keyhole is less invasive than the open surgerySurgeon:So some people believe that the keyhole surgery as you say is less invasive because there’s smaller scars, and we’re not spreading the ribs and looking directly in, and some people believe that that operation you might recover from more quickly. Or maybe [it] has advantages in terms of pain. Other people believe that the open surgery is better because you are not limited by the angles of your ports, you are free to move and maybe you take more of the lymph glands and maybe that’s better in cancer operations. We don’t know the answer to that, I don’t know the answer to that. I’m not sure, that’s why I’m happy to enter the trial as a surgeon |
| Patient:Well everybody seems to think that keyhole surgery is the best don’t they? I’m no expertSurgeon:At the moment the majority of these operations in this country are going through openPatient:YeahSurgeon:Erm, and only about a third of them are done by keyhole surgery. So the majority of people in this country prefer to do it through open. Erm, some people say that they can do a better operation through open. Some people say they can do it better through keyhole. So we don’t know | |
| Patient:Am I right in thinking that um [city] does slightly more keyhole operations than open?Surgeon:We are one of the centres which do a lot of bothPatient:It’s hard for you maybe to answer this question but I’ll ask it anyway do you feel that in [name of hospital] there would be a preference amongst the surgeons?Surgeon:I don’t think thatPatient:NoSurgeon:We wouldn’t take part in the study | |
Patient’s concern about lobectomy because:
|
Patient:OK . . . So I imagine that’s [open lobectomy] quite painfulSurgeon:Well, we don’t know, I mean sometimes I have patients, in fact not unusually I have patients who need an operation on both sides. And sometimes because of the way I need to perform the operation I’ve had to do it open on one side and keyhole on another, and I mean in one patient in particular actually he’d insisted the keyhole surgery was more painful than the open, but pain is a very individual response in all patients. We don’t know is the answerPatient:Yeah. I don’t know either [laughter] |
Patient’s (possible) preference for open lobectomy because:
|
Surgeon:We discussed in clinic the two ways of doing it, and I think you’ve got a preference. OK, how would you prefer to . . .Patient:The openSurgeon:Why’s that?Patient:Why? Because it’s tried and tested, right? That’s what you’ve been doing for a long time. The other one hasn’t been going for that long, maybe 6 years or somethingSurgeon:It’s been, was done 20 years agoPatient:Was it? See I read up that it had only been, 2010 was the first time it was done. But also, you know you’re going in with a camera in one bit and something else in the other bit and, how can, you might miss something, you know? This way you can see what you’re doing, and I prefer you to see what you’re doing [ . . . ]Surgeon:You also mentioned about it’s better if you have a look down through the cuts, ’cuz you can see everything inside. Well I would argue that through a small cut I’m seeing sort of that part of your chest, I can’t see properly right up the top, or I struggle to see up the top, and I struggle to see right down the bottom. When I put a camera in, I get a good panoramic view of all the, the whole of your chest. So you may actually be able to see better inside, and that’s something that we don’t know, and that’s why we’re doing the trial [ . . . ]Patient:No you’re giving me the information now that I didn’t haveSurgeon:I want to just give you the information that you didn’t have beforehandPatient:Yeah I didn’t have that you see |
| Patient:OK but I’m a lay person obviously one of the things that I, kind of, it was sorted of reflected in some of the notes and when I read that afterwards but I sort of instinctively feel that if you open it up you really can see that little rascal completely, you can see its contexts, you can see where to get the knife and really make sure you get the rascal . . .Surgeon:The keyhole gives you a more magnified visionPatient:RightSurgeon:So it’s all on the screen and that’s like, like a small problem glove will [unclear] not detect because it’s all –Patient:Right so you get it magnifiedSurgeon:Magnified, magnified yes |
Appendix 9 Trial committee membership
Independent Trial Steering Committee members
Professor Ruth Langley (chairperson), Professor of Oncology and Clinical Trials; Professor Joy Adamson, Professor of Applied Health Research and Ageing; Mr Ian Hunt, Consultant Thoracic Surgeon; Professor Peter Licht, Professor of Cardiothoracic Surgery; Dr Arjun Nair, Consultant Radiologist; Mr Chris Hall, patient representative; and Mr Mike Cowen, Consultant Cardiothoracic Surgeon (from study start to January 2017).
Independent Data Monitoring and Safety Committee members
Ms Susan J Dutton (chairperson since May 2017, previously a member of the committee), University Research Lecturer and Oxford Clinical Trials Research Unit Lead Statistician; Mr Alan Kirk, Consultant Thoracic Surgeon; Professor Keith Kerr, Professor of Pulmonary Pathology; Mr Rajesh Shah, Consultant Thoracic Surgeon; Dr Nagmi Qureshi, Consultant Radiologist; and Professor Tom Treasure, Professor of Cardiothoracic Surgery (chairperson from study start to March 2017).
Glossary
- Adverse event
- Any undesirable event in a subject receiving treatment in accordance with the protocol, including occurrences that are not necessarily caused by, or related to, administration of the research procedures.
- Serious adverse event
- Events that result in death, are life-threatening, require hospitalisation or prolongation of hospitalisation, or result in persistent or significant disability or incapacity.
List of abbreviations
- AE
- adverse event
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- cN0
- clinical node stage 0
- cN0/1
- clinical node stage 0 or 1
- ConDuCT
- Collaboration and Innovation in Difficult and complex randomised controlled Trials
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CT
- computerised tomography
- CTCAE
- Common Terminology Criteria for Adverse Events
- DMSC
- Data Monitoring and Safety Committee
- ED
- emergency department
- eMIT
- electronic marketing information tool
- EORTC
- European Organisation for Research and Treatment of Cancer
- GP
- general practitioner
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- M0
- metastasis stage 0
- MD
- mean difference
- MDT
- multidisciplinary team
- MedDRA
- Medical Dictionary for Regulatory Activities
- MRC
- Medical Research Council
- N0–1
- node stage 0–1
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- OR
- odds ratio
- PET
- positron emission tomography
- PI
- principal investigator
- PIL
- patient information leaflet
- pN1
- pathologic node stage 1
- pN2
- pathologic node stage 2
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QLQ-C30
- Quality of Life Questionnaire Core 30
- QLQ-LC13
- Quality of Life Questionnaire Lung Cancer 13
- QRI
- QuinteT Recruitment Intervention
- RCT
- randomised controlled trial
- RN
- research nurse
- RR
- relative risk
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SEAR
- screened, eligible, approached, randomised
- SIV
- site initiation visit
- TMG
- Trial Management Group
- TNM7
- TNM Classification of Malignant Tumours, Seventh Edition
- TNM8
- TNM Classification of Malignant Tumours, Eighth Edition
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- VATS
- video-assisted thoracoscopic surgery
- VIOLET
- VIdeo assisted thoracoscopic lobectomy versus conventional Open LobEcTomy for lung cancer
