Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the Evidence Synthesis Programme on behalf of NICE as award number NIHR134985. The contractual start date was in November 2021. The draft manuscript began editorial review in June 2022 and was accepted for publication in February 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Fleeman et al. This work was produced by Fleeman et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Fleeman et al.
Chapter 1 Background
This chapter is reproduced from the assessment group (AG) study protocol www.nice.org.uk/guidance/gid-ta10629/documents/final-protocol. The protocol is registered with PROSPERO (registration number: CRD42021285879), an international database of prospectively registered systematic reviews in health and social care.
Introduction
This systematic review and cost-effectiveness analysis (CEA) has been conducted to inform the following National Institute for Health and Care Excellence (NICE) multiple technology appraisal (MTA): lenvatinib with pembrolizumab for untreated advanced renal cell carcinoma (aRCC) (ID3760). The clinical and cost-effectiveness evidence to inform NICE’s final guidance has been submitted by the companies of both lenvatinib (Eisai1) and pembrolizumab [Merck Sharp & Dohme (MSD)2] as well as by the AG [Liverpool Reviews and Implementation Group (LRiG)]. The evidence presented by the AG is presented in this report, in addition to the AG’s consideration of analyses presented by the companies in their submissions. 1,2 Additional sensitivity analyses were presented by the AG during the appraisal and are also included in this report. Final NICE guidance on whether to recommend lenvatinib plus pembrolizumab as a treatment option for patients in NHS clinical practice was published in January 2023. 3
Description of the health problem
Renal cell carcinoma (RCC) is the most common type of kidney cancer, comprising approximately 85% of all renal malignancies. 4,5 Risk factors for RCC include smoking, obesity, hypertension and acquired cystic kidney disease. 4,6,7
There are a number of RCC histological subtypes,8 the most common being clear cell RCC, which accounts for between 70% and 90% of all cases of RCC. 4–7 Non-clear cell RCC is a heterogeneous group of kidney cancers with distinct histologies, diverse biologic behaviours and different clinical outcomes. 9,10
Patients with RCC are often asymptomatic and > 50% of cases are diagnosed incidentally. 6,7 At diagnosis, RCC can be categorised into four disease stages. Patients with Stage 1 and Stage 2 RCC are considered to have early-stage disease, and those with Stage 3 and Stage 4 RCC are considered to have aRCC. 6,7,11 In Stage 1 and Stage 2 RCC, the tumour is confined to the kidney. 6,7,11 The difference between the two early stages is the size of the tumour. A diagnosis of Stage 3 (locally advanced) disease is made when the tumour is growing into a major vein or has spread to regional lymph nodes. 6,7,11 A diagnosis of Stage 4 (metastatic) disease is made when the tumour is growing into one of the adrenal glands (these are situated on top of the kidneys) or has spread to distant lymph nodes and/or other organs. 6,7,11
Patients with Stage 3 or Stage 4 aRCC are the focus of this NICE appraisal and, therefore, of this report.
Epidemiology
Incidence of disease
Between 2015 and 2017, there were 13,055 new cases of kidney cancer in the UK (England: 10,759; Wales: 631). 12 Worldwide, kidney cancer is twice as common in men than in women. 4 In the UK, between 2015 and 2017, there were 1.7 times more new cases in men (62.8%) than in women (37.2%);12 a quarter (25.5%) of cases were diagnosed in people aged 60–69 years, with nearly half of the cases (49.2%) diagnosed in people aged ≥ 70 years. 12
Incidence and death rates by stage of disease
In England, between 2013 and 2017, 43.0% of all cases of kidney cancer with a known stage at diagnosis were classified as being advanced, that is Stage 3 or Stage 4 (see Table 1). During this period, the 5-year relative survival rates by stage of disease were markedly lower for patients with Stage 4 (metastatic) disease than for patients with the other stages of kidney cancer, including Stage 3 (locally advanced) disease (see Table 1).
| Disease stage | Number diagnosed | Proportion with a known diagnosis, % | Proportion alive ≥ 5 years, % |
|---|---|---|---|
| Stage 1 | 17,708 | 48.0 | 86.8 |
| Stage 2 | 3346 | 9.1 | 76.6 |
| Stage 3 | 6829 | 18.5 | 74.2 |
| Stage 4 | 9024 | 24.5 | 12.4 |
| All | 36,907a | 100.0 | 63.8 |
Incidence and death rates by disease risk status
Two models commonly used to classify risk status are the Memorial Sloan-Kettering Cancer Center (MSKCC) risk stratification model13,14 and the International Metastatic Renal Cell Carcinoma Database Consortium (IDMC) model. 15,16 As highlighted in the Eisai company submission (CS),1 the former ‘was originally the gold standard method for assessing risks associated with targeted treatment in metastatic RCC, and is still considered relevant by UK clinicians today to estimate patient prognosis’ and the latter ‘was developed to extend the MSKCC criteria to increase concordance, and is primarily applied in UK clinical practice’.
Both models13–16 calculate patients’ risk of progression taking into consideration a number of specific prognostic risk factors. The following risk factors are common to both models:13–16 time from diagnosis to treatment, haemoglobin levels, calcium levels and Karnofsky Performance Status (KPS). The MSKCC model also includes lactate dehydrogenase concentration, and the IMDC model also considers absolute neutrophil count and platelet count. 13–16 Both models13–16 classify risk as favourable (no adverse prognostic risk factors), intermediate (one or two adverse prognostic risk factors) or poor (three or more adverse prognostic risk factors). In a study to validate the IMDC, Heng et al. 16 reported that 83% of patients were classified into the same risk subgroup by both models.
The proportions of patients with metastatic RCC who belong to each risk subgroup in eight population-based studies16–23 are presented in Table 2.
| Study authors | Study type | Risk model na |
Favourable risk | Intermediate risk | Poor risk | |
|---|---|---|---|---|---|---|
| Heng et al. 201316 | International study validating IMDC, 2004–10 | IMDC n = 849 |
18% | 52% | 30% | |
| Gore et al. 201520 | Global expanded access programme of sunitinib, 2005–7 | IMDC n = 4065 |
24% | 54% | 22% | |
| Kubackova et al. 201517 | Czech Republic population-based study, 2006–13 | IMDCb n = 495 |
22% | 62% | 16% | |
| Schwab et al. 201822 | Germany single-centre study, 2006–13 | IMDC n = 104 |
14% | 63% | 23% | |
| Savard et al. 202021 | International population-based study, 2010–3 | IMDC n = 1769 |
18% | 58% | 24% | |
| I1: 26%c | I2: 24%c | |||||
| de Groot et al. 201618 | Netherlands population-based study, 2008–10 | MSKCC n = 645 (n = 210)d |
0 | 42% (69%)d |
58% (31%)d |
|
| de Groot et al. 201618 | Netherlands population-based study, 2011–3 | MSKCC n = 233 (n = 181)d |
58% (76%)d |
42% (24%)d |
||
| Fiala et al. 202019 | Czech Republic registry, 2006–18 | MSKCC n = 2390 |
34% | 61% | 6% | |
| I1: 41% | I2: 21% | |||||
| Tamada et al. 201823 | Consecutively treated patients in Japan | MSKCC n = 225e |
22% | 56% | 22% | |
| I1: 28% | I2: 28% | |||||
| Kubackova et al. 201517 | Czech Republic population-based study, 2006–13 | Modified MSKCCb,f n = 495 |
12% | 61% | 27% | |
The OS estimates are reported by risk subgroup in six population-based studies16–21 of patients with metastatic RCC who received sunitinib as a first-line treatment (see Table 3). Three19,21,23 of the four most recently published studies included in Table 2 also considered prognosis based on whether patients with intermediate-risk status had one or two prognostic factors.
| Study authors | Study type | Median OS, months (95% CI) | |||
|---|---|---|---|---|---|
| Risk model, na |
Favourable risk | Intermediate risk | Poor risk | ||
| Gore et al. 201520 | International study validating IMDC, 2004–10 | IMDC n = 4065 |
45.5b | 18.9b | 6.2b |
| Heng et al. 201316 | Global expanded access programme of sunitinib, 2005–07 | IMDC n = 849 |
43.2 (31.4 to 50.1) | 22.5 (18.7 to 25.1) | 7.8 (6.5 to 9.7) |
| Kubackova et al. 201517 | Czech Republic population-based study, 2006–13 | IMDC n = 495 |
44.3 (31.6 to 56.9) |
24.8 (19.8 to 29.8) |
9.3 (5.1 to 13.5) |
| Savard et al. 202021 | International population-based study, 2010–3 | IMDC n = 1769 |
52.1 (43.4 to 61.2) |
31.5 (28.9 to 33.9)c |
9.8 (8.3 to 11.4) |
| de Groot et al. 201618 | Netherlands population-based study, 2008–10 | MSKCC n = 210 |
NA | 14.6 (11.5 to 16.0) |
6.1 (4.9 to 7.7) |
| Netherlands population-based study, 2011–3 | MSKCC n = 181 |
16.6 (10.1 to NR) |
6.5 (3.4 to 10.0) |
||
| Fiala et al. 202019 | Czech Republic registry, 2006–18 | MSKCC n = 2390 |
44.7 (40.9 to 50.5) |
24.1 (21.9 to 26.0)d |
9.5 (7.2 to 14.1) |
| Kubackova et al. 201517 | Czech Republic population-based study, 2006–13 | Modified MSKCCe n = 495 |
39.5 (23.9 to 55.2) |
28.5 (20.1 to 36.8) |
10.6 (6.3 to 14.8) |
Some drugs are recommended only by NICE24,25 for patients with IMDC intermediate or poor (intermediate/poor) risk. Only one of the population studies (Savard et al. 21) listed in Table 3 reported OS for the combined IMDC intermediate-/poor-risk subgroup. The reported median OS for this subgroup was 23.2 [95% confidence interval (CI) 21.0 to 25.8] months. In the total (all-risk) population, median OS was 28.6 (95% CI 25.9 to 31.0) months, whereas median OS for the IMDC favourable-risk population was 52.1 (95% CI 43.4 to 61.2) months. Information on treatment options for patients in different IMDC risk subgroups is provided in Current service provision.
Current service provision
Surgery
Surgery is usually possible, and is the preferred treatment, for patients with early RCC and patients with locally aRCC29 and is usually curative. However, results from two studies30,31 that have explored disease progression following surgery suggest that approximately 30% of patients who have received surgery subsequently develop metastatic RCC. Surgery is rarely a treatment option for patients with metastatic RCC.
National Institute for Health and Care Excellence guidance for first-line drug treatment
At the time of this appraisal, the NICE-recommended treatments (see Table 4) are systemic vascular endothelial growth factor receptor (VEGFR)-targeted tyrosine-kinase inhibitor (TKI) agents (sunitinib,32 pazopanib,33 tivozanib34 and cabozantinib24). Two-drug combination treatments have been made available to patients via the Cancer Drugs Fund (CDF): avelumab plus axitinib35 [a programmed-death ligand 1 (PD-L1) checkpoint inhibitor in combination with a VEGFR-TKI] and nivolumab plus ipilimumab25 [a programmed death cell protein 1 (PD-1) inhibitor and a cytotoxic T-lymphocyte antigen 4 (CTLA-4) checkpoint inhibitor]. Nivolumab plus ipilimumab was subsequently recommended by NICE as a routine treatment option for patients with intermediate-/poor-risk aRCC (TA78036) on 24 March 2022. Although licensed for treating patients with aRCC, pembrolizumab plus axitinib is not recommended by NICE37 and so is not used in NHS clinical practice. Treatment options that are now rarely used due to their associated toxicities6 are cytokines (interferon alpha and high-dose interleukin-2).
| NICE TA | Intervention(s) | NICE recommendation |
|---|---|---|
| Recommended for use as a first-line treatment | ||
| TA169 (2009)32 | Sunitinib | Sunitinib is recommended as a first-line treatment option for people with advanced and/or metastatic RCC who are suitable for immunotherapy and have an ECOG PS of 0 or 1. |
| TA215 (2011–3)33 | Pazopanib | Pazopanib is recommended as a first-line treatment option for people with aRCC who have not received prior cytokine therapy and have an ECOG PS of 0 or 1. |
| TA512 (2018)34 | Tivozanib | Tivozanib is recommended for treating aRCC in adults who have had no previous treatment and only if the company provides tivozanib with the discount stated in the PAS agreement. |
| TA542 (2018)24 | Cabozantinib | Cabozantinib is recommended, within its marketing authorisation, for adults with untreated aRCC, i.e. intermediate/poor risk as defined in the IMDC criteria. It is recommended only if the company provides cabozantinib according to the commercial arrangement. |
| TA780 (2022)36 | Nivolumab plus ipilimumab | Nivolumab with ipilimumab is recommended, within its marketing authorisation, as an option for untreated aRCC in adults whose disease is intermediate or poor risk as defined in the IMDC criteria and only if the company provides nivolumab with ipilimumab according to the commercial arrangement. |
| Recommended for use as a first-line treatment within the CDF | ||
| TA581 (2019)25 superseded by TA78036 | Nivolumab plus ipilimumab | The following recommendation has been superseded by the NICE recommendation in TA780: Nivolumab with ipilimumab is recommended for use within the CDF as an option for adults with untreated aRCC, i.e. intermediate/poor risk as defined in the IMDC criteria. It is recommended only if the conditions in the managed access agreement for nivolumab with ipilimumab are followed. |
| TA645 (2020)35 | Avelumab plus axitinib | Avelumab with axitinib is recommended for use within the CDF as an option for untreated aRCC in adults. It is recommended only if the conditions in the managed access agreement for avelumab with axitinib are followed. |
| Not recommended for use as a first-line treatment | ||
| TA178 (2009)40,a | Bevacizumab Sorafenib Temsirolimus |
Bevacizumab, sorafenib and temsirolimus are not recommended as first-line treatment options for people with advanced and/or metastatic RCC. |
| TA650 (2020)37 | Pembrolizumab plus axitinib | Pembrolizumab with axitinib is not recommended, within its marketing authorisation, for untreated aRCC in adults. |
European clinical guidelines for first-line drug treatment
Clinical practice guidelines published in 2021 by the European Association of Urology38 and the European Society for Medical Oncology (ESMO)39 recommend four combination treatments for the first-line treatment of metastatic clear cell RCC: lenvatinib plus pembrolizumab, pembrolizumab plus axitinib and nivolumab plus cabozantinib for intermediate-/poor-risk or favourable-risk disease and nivolumab plus ipilimumab for intermediate-/poor-risk disease only. For patients who cannot tolerate immune checkpoint inhibitors, the European Association of Urology38 recommend sunitinib, pazopanib and cabozantinib for intermediate-/poor-risk disease and sunitinib and pazopanib for favourable-risk disease. The AG highlights that pembrolizumab plus axitinib is not recommended by NICE37 and nivolumab plus cabozantinib has not been appraised by NICE, the planned single technology appraisal (STA) being suspended. 41
NHS first-line treatment options
Clinical advice to the AG is that in NHS clinical practice, patients with aRCC receive the treatments recommended in NICE guidance24,25,32–35 (see Table 4) and that treatment decisions are made based on histological subtype, IMDC disease risk category, patient age and comorbidities, patient fitness, disease aggressiveness/biology and patient preference.
In line with recommendations in NICE guidance,24,36 at the time of this appraisal, the clinical advice to the AG is that, in general, nivolumab plus ipilimumab is the preferred first-line treatment option for patients with intermediate-/poor-risk disease and that cabozantinib is the preferred treatment option for fitter patients in this subgroup who have rapidly progressing disease (approximately 20%). The clinical advice to the AG is also that patients unable to tolerate either of these treatments receive sunitinib, pazopanib or tivozanib.
The treatment options available in NHS clinical practice to patients with favourable-risk disease at the time of this appraisal are sunitinib, pazopanib or tivozanib and, via the CDF, avelumab plus axitinib. 35 The clinical advice to the AG is that, where available, avelumab plus axitinib is the preferred first-line treatment option for patients with favourable-risk disease who can tolerate this combination, and tivozanib is the favoured treatment option for patients who are able to tolerate only VEGFR-TKI monotherapy.
Subsequent lines of drug treatment
The NICE has recommended five treatment options24,25,32–34 for previously treated patients with aRCC (Table 5). All of these subsequent treatments are recommended for patients regardless of their risk status. The clinical advice to the AG is that cabozantinib and nivolumab monotherapy are the most commonly used second-line treatments; lenvatinib plus everolimus is not a treatment option for patients who have previously received lenvatinib.
| NICE TA | Drug(s) | Type of drug(s) | Specified previous treatments |
|---|---|---|---|
| TA333 (2015)42 | Axitinib | VEGFR-TKI | VEGFR-TKI or cytokine |
| TA417 (2016)47 | Nivolumab | PD-1 inhibitor | None specified |
| TA432 (2017)48 | Everolimus | mTOR inhibitor | VEGFR-TKI |
| TA463 (2017)43 | Cabozantinib | VEGFR-TKI | VEGFR-TKI |
| TA498 (2018)44,a | Lenvatinib plus everolimus | Multiple receptor TKI plus mTOR inhibitor | VEGFR-TKI |
The ESMO39 recommends axitinib, cabozantinib and lenvatinib plus everolimus, which are all recommended by NICE,42,43,44 and sunitinib, pazopanib and tivozanib.
Description of technology under assessment
The technology under assessment in this appraisal is lenvatinib plus pembrolizumab. In November 2021, the Medicines and Healthcare Products Regulatory Agency (MHRA) granted UK marketing authorisation for the use of lenvatinib plus pembrolizumab for untreated aRCC. 45,46 Information regarding lenvatinib plus pembrolizumab is provided in Table 6.
| Feature | Lenvatinib | Pembrolizumab |
|---|---|---|
| Brand name | Kisplyx | Keytruda |
| Manufacturer | Eisai Ltd | MSD |
| Class of drug | Multiple receptor TKI | Monoclonal antibody |
| Mechanism of action | Inhibits the activity of VEGFR | Blocks the interaction between PD-1 and its ligands, i.e. PD-L1 and PD-L2 |
| Dose information for treating aRCC | 20 mg (oral) once daily until disease progression or unacceptable toxicity | 200 mg every 3 weeks or 400 mg every 6 weeks administered as an i.v. infusion over 30 minutes Maximum duration of 2 years |
| List price per pack | 30 capsules (4 mg) = £1437 30 capsules (10 mg) = £1437 |
100 mg vial = £2630 A single administration of 200 mg = £5260 A single administration of 400 mg = £10,520 |
| PAS | Simple discount PAS | Simple discount PAS |
As noted in the Eisai CS1 (p. 18):
It has been proposed that combining an immune checkpoint inhibitor (pembrolizumab) with the simultaneous inhibition of angiogenesis and VEGF-mediated immune suppression (lenvatinib), i.e., co-inhibition of PD-1 and VEGF, may offer complimentary modulation of different aspects of tumour immunobiology and potentially improve survival in patients with aRCC.
Eisai also highlights that lenvatinib plus pembrolizumab may be a more convenient treatment for patients than the alternative combination therapies currently recommended by NICE25,35 because lenvatinib can be taken with or without food and the capsules can be swallowed whole or ingested by dissolving in water or apple juice (although using the dissolving route to administer the drugs is not a straightforward process), and pembrolizumab requires only a 30-minute infusion once every 3 or 6 weeks. In contrast, both cabozantinib49 and axitinib50 must be swallowed whole (and cabozantinib must be administered after a ≥ 2-hour fast49) and other checkpoint inhibitors51,52 require longer infusions, for example, treatment with avelumab requires a 60-minute infusion every 2 weeks. 51
Systematic reviews of lenvatinib plus pembrolizumab for advanced renal cell carcinoma
A substantial number of systematic reviews that compare the clinical effectiveness of first-line treatments for aRCC have been published; however, the AG has identified only seven reviews53–59 that include patients treated with lenvatinib plus pembrolizumab. The focus and results of these reviews are summarised in Focus of the systematic reviews of lenvatinib plus pembrolizumab and Results from the systematic reviews of lenvatinib plus pembrolizumab, respectively (for further details see Table 49 in Appendix 1).
Focus of the systematic reviews of lenvatinib plus pembrolizumab
Six of the reviews53–57,59 focused on the efficacy and safety of treatment, and one review58 focused only on safety. One review56 compared lenvatinib plus pembrolizumab versus other combination therapies and versus sunitinib. Six other reviews53–55,57–59 assessed the evidence for lenvatinib plus pembrolizumab and other combination therapies versus sunitinib; three reviews54,55,59 presented only pooled results and two reviews57,58 compared lenvatinib plus pembrolizumab versus other combination therapies by ranking the probability of maximal efficacy.
The therapies included in the seven reviews53–59 were a combination of PD-1 and CTLA-4 checkpoint inhibitors (nivolumab plus ipilimumab),54,56–59 a PD-L1 checkpoint inhibitor in combination with an angiogenesis inhibitor (atezolizumab plus bevacizumab54,55,57–59), a PD-L1 checkpoint inhibitor in combination with VEGFR-TKI (avelumab plus axitinib53–55,57–59) or a PD-1 checkpoint inhibitor in combination with VEGFR-TKI (pembrolizumab plus axitinib53–59 or nivolumab plus cabozantinib53–59). Three reviews55,57,59 included subgroup analyses by risk subgroup and one review53 included only favourable-risk patients.
Results from the systematic reviews of lenvatinib plus pembrolizumab
All-risk population results
Five reviews54–57,59 showed that combination therapies (including lenvatinib plus pembrolizumab) statistically significantly improved progression-free survival (PFS) and objective response rate (ORR) in comparison with sunitinib. Massari et al. 54 also showed that combination therapies statistically significantly improved OS in comparison with sunitinib; however, Mori et al. 55 showed that this finding was applicable only to PD-1 checkpoint inhibitors (including lenvatinib plus pembrolizumab) and not to PD-L1 checkpoint inhibitors.
Four reviews54–56,59 showed that lenvatinib plus pembrolizumab statistically significantly improved OS in comparison to sunitinib, and one review57 showed that OS may favour lenvatinib plus pembrolizumab, but the result was not statistically significant. In the two reviews56,57 that ranked the probability of most effective treatment, lenvatinib plus pembrolizumab ranked highest for PFS and ORR56,57 and second highest for OS,56,57 while nivolumab plus cabozantinib ranked highest for OS. 56,57
Compared with other combination therapies, lenvatinib plus pembrolizumab was less well tolerated; patients receiving lenvatinib plus pembrolizumab experienced the highest proportion of Grade ≥ 3 adverse events (AEs) and treatment discontinuations due to AEs. Treatment with lenvatinib plus pembrolizumab was also shown to have the highest likelihood of all-grade adrenal insufficiency and the highest likelihood of high-grade aspartate aminotransferase increase. 58
Intermediate-/poor-risk subgroup results
Three reviews55,57,59 compared PFS and OS for combination therapies versus sunitinib and reported statistically significant evidence that combination therapies improved efficacy. The two reviews55,57 that also compared ORR for combination therapies versus sunitinib found statistically significant evidence that combination therapies improved this outcome.
Favourable-risk subgroup results
Three reviews53,55,59 identified statistically significant evidence that, compared to sunitinib, combination therapies improved PFS but not OS. A fourth review57 identified statistically significant evidence that, compared to sunitinib, four out of six combination therapies studied (including lenvatinib plus pembrolizumab) improved PFS. Only two of the six combination therapies (nivolumab plus ipilimumab and pembrolizumab plus axitinib), compared to sunitinib, resulted in statistically significantly improved OS. The two reviews55,57 that also compared ORR for combination therapies versus sunitinib found statistically significant evidence that combination therapies improved this outcome [the exception being atezolizumab plus bevacizumab in the network meta-analysis (NMA)57].
Chapter 2 Definition of the decision problem
Decision problem
The key elements of the decision problem for this appraisal, as defined in the final scope29 issued by NICE, are presented in Table 7 (for further information, see Patient population, Comparators and Subgroup analyses).
| Parameter | Final scope issued by NICE | Addressed by AG |
|---|---|---|
| Intervention | Lenvatinib plus pembrolizumab | As per scope |
| Patient population | Adults with untreated aRCC | Most patients considered in the AG analyses had clear cell aRCC |
The AG considered the following groups of patients:
|
||
| Comparators |
|
Direct evidence is available only for sunitinib (CLEAR trial) Some indirect evidence is available for all relevant comparators from Eisai, MSD and AG NMAs |
| Outcomes |
|
As per scope for the comparison of lenvatinib plus pembrolizumab with sunitinib Some indirect evidence was available for some outcomes for some subgroups |
| Economic analysis | The reference case stipulates that:
|
As per scope |
| Costs should be considered from an NHS and personal and social services perspective. The availability of any commercial arrangements for the interventions, comparators and subsequent treatments should be taken into account. The availability of any managed access arrangement for the intervention should be taken into account |
||
| Other considerations | If the evidence allows, the following subgroups should be considered: people with aRCC, i.e. intermediate/poor risk as defined in IMDC criteria. Guidance will be issued only in accordance with the marketing authorisations. |
As per scope |
Patient population
In previous NICE appraisals of treatments for untreated aRCC,25,35 NICE appraisal committees (ACs) noted that there was a lack of evidence to guide treatment decisions for patients with non-clear cell RCC. This is primarily due to non-clear cell RCC being (1) heterogeneous (up to 15 different subtypes are listed in the most recent World Health Organization classification of RCC9) and (2) less common9,10 than clear cell RCC. The AG made no attempt to provide evidence separately for patients with clear cell and non-clear cell histologies.
As noted in Current service provision, decisions about the most appropriate first-line treatments for patients with aRCC are now typically made based on patient risk subgroup. Therefore, the AG conducted subgroup analyses for intermediate-/poor-risk and favourable-risk subgroups.
Unless otherwise stated, risk subgroup within this report refers to IMDC model risk stratification subgroups.
Comparators
Four of the five comparators listed in the final scope29 issued by NICE (sunitinib, pazopanib, tivozanib, and cabozantinib for patients with intermediate-/poor-risk aRCC) are all used in current NHS clinical practice. Nivolumab plus ipilimumab is also listed as a comparator; however, at the time of writing this AG report, nivolumab plus ipilimumab was subject to an ongoing CDF review25 and was not available for routine use in the NHS. Following advice from the NICE technical team, the AG has included nivolumab plus ipilimumab as a relevant comparator. Nivolumab plus ipilimumab was subsequently recommended by NICE as a routine treatment option for patients with intermediate-/poor-risk aRCC (TA78036) on 24 March 2022.
Subgroup analyses
In line with the final scope29 issued by NICE, the AG carried out clinical and cost-effectiveness analyses of lenvatinib plus pembrolizumab for the subgroup of patients with intermediate-/poor-risk disease. While it is stated in the AG protocol that analyses would be undertaken separately for the two subgroups, the AG has carried out analyses only for the combined intermediate-/poor-risk subgroup; clinical advice to the AG is that, in line with NICE guidance,24,36 treatment decisions are based on the combined intermediate-/poor-risk disease category (one category, not two categories). If a patient does not have intermediate-/poor-risk disease then, by definition, the patient has favourable-risk disease; hence the AG has carried out subgroup analysis for the subgroup of patients with favourable risk.
Intermediate/poor risk
The clinical advice to the AG is that, in line with NICE guidance,24,36 cabozantinib and nivolumab plus ipilimumab are first-line treatment options for patients with intermediate-/poor-risk aRCC; in the first-line setting, sunitinib, pazopanib or tivozanib are considered only for those individuals in this subgroup who are unable to tolerate cabozantinib or nivolumab plus ipilimumab. The clinical advice to the AG is that patients unable to tolerate cabozantinib or nivolumab plus ipilimumab would be unlikely to tolerate lenvatinib plus pembrolizumab. Therefore, the AG does not consider that sunitinib, pazopanib and tivozanib are relevant comparators to lenvatinib plus pembrolizumab for patients with intermediate-/poor-risk disease.
Avelumab plus axitinib is also an option for patients with all-risk disease and, therefore, intermediate-/poor-risk disease. As this treatment is currently available only via the CDF, it was not considered by NICE to be a relevant comparator because it could not be said to represent standard practice.
Favourable risk
Sunitinib, pazopanib and tivozanib are NICE-recommended treatment options32–34 for patients who are not specifically categorised as having intermediate-/poor-risk aRCC, that is those with favourable-risk disease. The AG has, therefore, carried out subgroup analyses to compare lenvatinib plus pembrolizumab versus sunitinib, versus pazopanib and versus tivozanib for the subgroup of patients with favourable-risk disease.
Avelumab plus axitinib is also an option for patients with all-risk disease and, therefore, favourable-risk disease. As this treatment is currently available only via the CDF, it was not considered by NICE to be a relevant comparator because it could not be said to represent standard practice.
Overall aims and objectives of assessment
The overall aim of this appraisal is to appraise the clinical effectiveness and cost-effectiveness of lenvatinib plus pembrolizumab within its MHRA marketing authorisation45,46 for patients with untreated aRCC.
Lenvatinib plus pembrolizumab is licensed to treat all patients with aRCC irrespective of risk status. However, two of the comparators listed in the final scope29 issued by NICE (cabozantinib and nivolumab plus ipilimumab) are recommended only for patients with intermediate-/poor-risk disease. Therefore, the objectives of this assessment are to appraise the clinical effectiveness and cost-effectiveness of lenvatinib plus pembrolizumab versus:
-
cabozantinib or nivolumab plus ipilimumab for the intermediate-/poor-risk subgroup
-
sunitinib, pazopanib and tivozanib for the favourable-risk subgroup
-
sunitinib, pazopanib and tivozanib for the all-risk population.
Chapter 3 Assessment of clinical effectiveness: direct evidence
This manuscript contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Methods for reviewing effectiveness
The AG carried out a systematic review of clinical effectiveness evidence following the general principles outlined by the Centre for Reviews and Dissemination (CRD). 60 The review is reported using the criteria recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 61 Searches were conducted in accordance with the general principles recommended by the European Network for Health Technology Assessment. 62
Search strategies
The clinical effectiveness search strategy was designed to identify randomised controlled trials (RCTs) that met the inclusion criteria for the review of direct clinical effectiveness evidence and to identify RCTs that could potentially be used to populate the AG NMAs. The AG identified clinical effectiveness studies by searching relevant major medical databases, trial registries, conference abstracts, the NICE technology appraisal (TA) website listed in Appendix 2, Table 50. and grey literature websites. The search terms used to search the database are given in Appendix 2.
As part of the MTA process, companies were invited to submit evidence to NICE to inform this appraisal. Two companies provided direct and indirect evidence: Eisai,1 the manufacturer of lenvatinib, and MSD,2 the manufacturer of pembrolizumab. The AG screened the reference lists of the Eisai CS1 and the MSD CS2 alongside all other included reports for relevant studies and consulted the AG clinical experts to identify any relevant studies that may have been missed.
A database of identified published literature was compiled. MEDLINE, EMBASE, PubMed, CENTRAL, International Health Technology Assessment (INAHTA), ClinicalTrials.gov and International Clinical Trials Registry Platform (ICTRP) data were collated in a bibliographic database (Endnote X9 software package63) and exported to a specialist systematic review management system (Covidence Systematic Review software64). Conference abstracts results were screened on organisations’ websites. The search terms used to search each of the databases and the websites are given in Appendix 2.
Inclusion and exclusion criteria: direct evidence
The eligibility criteria used to identify studies for the review of direct clinical effectiveness are listed in Table 8.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Limits |
|
|
| Population |
|
|
| Study design |
|
|
| Intervention |
|
|
| Comparators |
|
|
| Outcomes |
|
|
Titles and abstracts identified through electronic searches were uploaded to Covidence and screened by two reviewers (NF and either JG or KE). Full-text articles of any titles and abstracts that were considered potentially eligible for inclusion were obtained via online resources, or through the University of Liverpool libraries, and uploaded to Covidence. These full-text articles were assessed for inclusion by two reviewers (NF and either JG or KE). Discrepancies at each stage of screening were resolved via discussion between the three reviewers. Full-text articles that did not meet the inclusion criteria were excluded with reasons for exclusion noted.
In addition to screening the articles exported to Covidence, two out of three reviewers (RH, JG and KE) screened the conference proceedings independently following the eligibility criteria shown in Table 8.
Data extraction and quality assessment strategy: direct evidence
Data relating to study characteristics, population characteristics and outcomes were extracted by one reviewer (NF) into tables and independently checked for accuracy by a second reviewer (SN or KE). Data from multiple publications of the same study were extracted and reported as a single study.
Study quality was assessed independently by two reviewers (JG and KE) using the criteria published in the CRD Guidance for Undertaking Reviews in Healthcare. 60 Disagreements were resolved through discussion and, when necessary, a third reviewer (SN) was consulted.
Statistical approaches for the conduct and analysis of randomised controlled trials: direct evidence
The AG assessed the prespecified statistical approach of the only included RCT. 66 This assessment considered:
-
analysis populations
-
trial design and sample size
-
amendments to the protocol and statistical analysis plan
-
definition and analysis approach for primary and secondary efficacy outcomes
-
definition and analysis approach for patient reported outcomes (PROs)
-
definition and analysis approach for safety outcomes and AEs
-
validity of modelling assumptions [e.g. proportional hazards (PH)]
-
approach to handling missing data
-
subgroup and sensitivity analyses.
The AG also performed an assessment of specific statistical approaches, where appropriate for any relevant study (e.g. analyses to adjust for treatment switching).
Data analysis/synthesis: direct evidence
Meta-analysis
Only one RCT66 was identified for inclusion in the review and, therefore, a meta-analysis was not required.
Presentation of results
The results of the data extraction, quality assessment and statistical assessment from the included RCT66 were summarised in tables and described in text.
Direct treatment effect estimates are presented as hazard ratios (HRs) for time-to-event data (i.e. OS and PFS), as odds ratios (ORs) for dichotomous data (i.e. ORR and AEs) or as mean differences for continuous data [i.e. health-related quality-of-life (HRQoL) outcomes]. All treatment effect estimates are presented with 95% CIs.
Results of search for direct evidence: included and excluded studies
The AG study selection process is shown in Appendix 2 (see Sources searched, Figure 2).
At the title and abstract stage, the AG included any study report that appeared to be a RCT that considered a relevant intervention or comparator. Such a broad approach to inclusion was carried out to aid the identification and selection of studies that provided data that could be used in AG NMAs. This approach resulted in the retrieval of 694 reports (577 via searches of databases and registries and 117 via other searches). After applying inclusion/exclusion criteria, a total of 20 reports1,2,66–83 describing one RCT [CLEAR/KEYNOTE-581 trial (NCT02811861) and hereafter referred to as the CLEAR trial] were included in the review.
Sources of CLEAR trial data
The AG review of direct evidence included one RCT, the CLEAR trial; this trial was jointly sponsored by Eisai and MSD. Although 20 study reports1,2,66–83 were included in the review, data were extracted only from the sources listed in Table 9. After reviewing the companies’ submissions, the AG requested additional information via the NICE appraisal clarification process and used companies’ responses to the clarification letters as sources of evidence.
| Source | Note |
|---|---|
| Motzer et al. 202166 | Published paper, including the online appendix and protocol |
| Motzer et al. 202181 | HRQoL data reported in conference abstract |
| Eisai CS1 and response to AG clarification letter | CS received 15 November 2021; response to the AG clarification letter received 20 December 2021 |
| MSD CS2 and responses to AG clarification letters | CS received 15 November 2021; initial response to the AG clarification letter received 20 December 2021; additional response to the AG clarification letter received 11 January 2022 |
| Protocol v773 | Final protocol (Amendment 7), 6 August 2020 |
| TSAP, v3.0 | 14 August 2020, available online as appendix to published paper66 |
| CSR70 | 28 August 2020, provided by both companies |
| Updated OS report71 | 20 May 2021, provided by both companies |
| HRQoL analysis plan, v2.168 and HRQoL report72 | Additional source of HRQoL data (13 February 2021 and 28 August 2020, respectively) provided by Eisai (with Eisai response to the AG clarification letter) |
The AG employed a hierarchical approach to data extraction. The initial source of data for the results of clinical effectiveness and safety analyses was the published paper of Motzer et al. ,66 including the online appendix and accompanying trial statistical analysis plan (TSAP). 74 The initial source of data for HRQoL was the conference abstract by Motzer et al. 81 Additional data were extracted first from the Eisai CS1 and then cross-checked with data in the MSD CS. 2 Finally, the Clinical Study Report (CSR)70 and other CLEAR trial documents provided as part of the companies’ submissions to NICE68–73 were consulted and additional data extracted.
CLEAR trial design and characteristics
The CLEAR trial was a phase III, multicentre, open-label RCT (with an ongoing extension phase) that was designed to compare the efficacy of lenvatinib plus pembrolizumab versus sunitinib and of lenvatinib plus everolimus versus sunitinib. Patients (n = 1069) were randomised 1 : 1 : 1 to the treatment arms. Randomisation was stratified according to geographic region (Western Europe and North America, or the rest of the world) and MSKCC prognostic risk subgroup (favourable, intermediate or poor risk). The treatment combination of lenvatinib plus everolimus is not relevant to this appraisal and is not discussed further in this AG report.
A summary of CLEAR trial design and conduct details is provided in Table 10.
| Parameter | CLEAR trial |
|---|---|
| Key eligibility criteria | Inclusion:
|
| Patients with CNS metastasis were excluded unless they had completed local therapy and discontinued corticosteroids for this indication for ≥ 4 weeks before study treatment | |
| Recruitment period | 13 October 2016 to 24 July 2019 |
| Number of centres (patients) | All: 181 sites in 20 countries, including 93 sites in Europe (407 patients) UK: 8 sites (26 patients) |
| Drug doses and schedule | Lenvatinib plus pembrolizumab:
|
Sunitinib:
|
|
| In both arms, patients continued to receive study treatment until disease progression was confirmed by BIRC, development of unacceptable toxicity, patient request, withdrawal of consent, completion of 35 treatments (2 years) for pembrolizumab or study termination by the sponsor. All patients could continue treatment beyond initial RECIST v1.1-defined progression at the investigator’s discretion |
|
| Dose modifications | Dose interruptions were permitted for all study drugs Dose reductions were not permitted for pembrolizumab If one drug in the combination treatment arm was discontinued (e.g. due to toxicity), the other drug could be continued |
The CLEAR trial primary outcome was PFS assessed by Blinded Independent Review Committee (BIRC), using the censoring method preferred by the US Food and Drug Administration (FDA). All other outcomes relevant to the decision problem were reported (OS, ORR, AEs and HRQoL). Prespecified subgroup analyses, by IMDC and MSKCC risk subgroups, were:
-
age (< 65 years, ≥ 65 years)
-
sex (male, female)
-
race (white, Asian)
-
geographic region (Western Europe or North America, rest of the world)
-
MSKCC risk subgroup (favourable, intermediate, poor)
-
IMDC risk subgroup (favourable, intermediate, poor)
-
baseline KPS score (100 to 90, 80 to 70)
-
number of organs with metastases (1, 2, ≥ 3)
-
baseline bone, liver and lung metastasis (yes, no)
-
PD-L1 combined positive score (≥ 1, < 1)
-
prior nephrectomy (yes, no)
-
clear cell histology with sarcomatoid features (yes, no).
Analyses of MSKCC intermediate-/poor-risk subgroup PFS, OS and ORR data were also presented in the Eisai CS. 1
The CLEAR trial had an ongoing OS extension phase with the final prespecified OS analysis planned to occur after approximately 304 OS events had occurred; the final OS analysis was therefore conducted after this appraisal had concluded (data cut-off: 31 July 2022 with a median OS follow-up time of approximately 4 years). 84 At the time of this appraisal, OS had only been reported at two different time points: (1) at the time of the third interim analysis (IA3 data cut-off), which was also the final data cut-off for PFS and the time at which all other outcomes were reported, and (2) at the time of the updated OS analysis (see Table 11 for details). As patients could receive subsequent anticancer treatment on disease progression, company post hoc analyses were also performed excluding patients who received subsequent treatment from the analysis and by adjusting for subsequent anticancer treatment using the two-stage estimation method85 (see also Table 55 in Appendix 3).
| Parameter | IA3 data cut-off | Updated OS analysis |
|---|---|---|
| Data cut-off date | 28 August 2020 | 31 March 2021 |
| Duration of follow-up | Median OS follow-up: 26.6 months All efficacy, safety and PROs were reported at this time point |
Median OS follow-up: ~33 months Only OS was assessed at this follow-up |
| Number (%) of patients still on study treatment | Lenvatinib plus pembrolizumab: 142 (40.0%) Sunitinib: 67 (18.8%) |
Lenvatinib plus pembrolizumab: 114 (32.1%) Sunitinib: 49 (13.7%) |
Analyses of efficacy outcomes were undertaken using data from the full analysis set (FAS) population, which is also the intention-to-treat (ITT) population and the all-risk population. Safety analyses were undertaken using data from the randomised population who received at least one dose of a study drug and who had at least one post-baseline safety evaluation (safety population).
CLEAR trial participant characteristics
A summary of baseline characteristics is presented in Table 12. There were 2.9 times as many men as women. The lenvatinib plus pembrolizumab arm included a higher proportion of patients aged ≥ 65 years; the median age of patients in this arm was higher than the median age of patients in the sunitinib arm (64 vs. 61 years).
| Characteristic | Lenvatinib + pembrolizumab (N = 355) |
Sunitinib (N = 357) |
|---|---|---|
| Mean (SD) age, years | 62.3 (10.23) | 60.8 (9.96) |
| Median (range) age, years | 64 (34–88) | 61 (29–82) |
| < 65 years, n (%) | 194 (54.6) | 225 (63.0) |
| Male, n (%) | 255 (71.8) | 275 (77.0) |
| Region, n (%) | ||
| Western Europe or North America | 198 (55.8) | 199 (55.7) |
| Rest of the world | 157 (44.2) | 158 (44.3) |
| Race/ethnicity, n (%) | ||
| White | 263 (74.1) | 270 (75.6) |
| Black or African American | 2 (0.6) | 3 (0.8) |
| Asian | 81 (22.8) | 67 (18.8) |
| KPS, n (%) | ||
| 90–100 | 295 (83.1) | 294 (82.4) |
| 70–80 | 60 (16.9) | 62 (17.4) |
| Missing | 0 | 1 (0.3) |
| MSKCC risk subgroup, n (%) | ||
| Favourable | 96 (27.0) | 97 (27.2) |
| Intermediate | 227 (63.9) | 228 (63.9) |
| Poor | 32 (9.0) | 32 (9.0) |
| IMDC risk subgroup, n (%) | ||
| Favourable | 110 (31.0) | 124 (34.7) |
| Intermediate | 210 (59.2) | 192 (53.8) |
| Poor | 33 (9.3) | 37 (10.4) |
| Could not be evaluated | 2 (0.6) | 4 (1.1) |
| Sarcomatoid features, n (%) | 28 (7.9) | 21 (5.9) |
| Number of metastatic organs or sitesa | ||
| 1 | 97 (27.3) | 108 (30.3) |
| ≥ 2 | 254 (71.5) | 246 (68.9) |
| Prior nephrectomy, n (%) | 262 (73.8) | 275 (77.0) |
In both the trial arms, more patients were categorised as having favourable-risk disease when using the IMDC classification than using the MSKCC classification, and fewer patients were categorised as having intermediate-risk disease when using the IMDC classification than using the MSKCC classification. Six patients were not assigned a risk category according to the IMDC classification.
Generally, the baseline characteristics of patients included in the CLEAR trial were balanced between treatment arms. However, while the proportions of patients classified in each MSKCC risk subgroup were the same across the trial arms, there were slight imbalances between arms in terms of IMDC risk status.
Quality assessment of the CLEAR trial
The AG conducted a quality assessment of the CLEAR trial using the criteria published in the CRD’s guidance for undertaking reviews in healthcare. 60 The results of the assessment are presented in Appendix 3, Table 53 (see Quality assessment of the CLEAR trial). The AG considers that the CLEAR trial is a good-quality trial.
Statistical approach followed to analyse the CLEAR trial data
A summary of the AG’s checks of the CLEAR trial preplanned statistical approach is provided in Appendix 3 (see Table 54). The AG was satisfied with the statistical approach taken by the companies. However, the AG highlights that in cases where the PH assumption is violated, the estimated HR is not applicable to all time points across the observed CLEAR trial follow-up period. In the context of a single trial, where violations of the PH assumption are demonstrated, visual inspection of the Kaplan–Meier (K-M) data may provide some insight into the likely direction of relative effect at different time points and changes in the direction or magnitude of relative effect over the time period of the trial (i.e. where K-M curves cross or diverge).
Eisai assessed the PH assumption for BICR-assessed PFS and OS by plotting the log cumulative hazard versus log(time), using the Grambsch–Therneau test86 of Schoenfeld’s residuals [see Eisai CS1 (sections 5.3.1 and 5.3.2) and Eisai response to the AG clarification letter, questions A1 and A2].
On the basis of these assessments, Eisai considered that over the observed period, the assumption of PH was not violated for BICR-assessed PFS but was violated for the updated analyses of OS (unadjusted for treatment crossover).
CLEAR trial results
Progression-free survival results from the CLEAR trial
Key PFS results from the CLEAR trial are summarised in Table 13.
| Characteristic/outcome | All-risk (FAS) | Intermediate/poor risk | Favourable risk | |||
|---|---|---|---|---|---|---|
| Lenvatinib + pembrolizumab (N = 355) | Sunitinib (N = 357) |
Lenvatinib + pembrolizumab (N = 243) |
Sunitinib (N = 229) |
Lenvatinib + pembrolizumab (N = 110) |
Sunitinib (N = 124) |
|
| Number of events (%) | 160 (45.1) | 205 (57.4) | 115 (47.3) | 136 (59.4) | 43 (45.1) | 67 (54.0) |
| Death from PFS (%) | 15 (4.2) | 9 (2.5) | Not reported | Not reported | Not reported | Not reported |
| Median PFS in months (95% CI) | 23.9 (20.8 to 27.7) | 9.2 (6.0 to 11.0) | Confidential information has been removed | Confidential information has been removed | 28.1 (Confidential information has been removed) | 12.9 (Confidential information has been removed) |
| Stratified HR (95% CI) p-value |
0.39 (0.32 to 0.49) p < 0.001 |
Confidential information has been removeda Confidential information has been removed |
0.41 (0.28 to 0.62) p < 0.001 |
|||
| PFS rates (%) (95% CI) at: | ||||||
| 6 months | 84.9 (80.6 to 88.3) | 57.0 (51.1 to 62.5) | Not reported | Not reported | Not reported | Not reported |
| 12 months | 70.6 (65.3 to 75.2) | 38.4 (32.4 to 44.3) | Not reported | Not reported | Not reported | Not reported |
| 18 months | 57.4 (51.5 to 62.8) | 31.2 (25.4 to 37.2) | Not reported | Not reported | Not reported | Not reported |
| 24 months | 48.9 (42.7 to 54.9) | 20.7 (15.0 to 26.9) | Not reported | Not reported | Not reported | Not reported |
Progression-free survival: full analysis set population (intention-to-treat population, all-risk population)
In the CLEAR trial, median PFS was statistically significantly longer in the lenvatinib plus pembrolizumab arm than in the sunitinib arm [median 23.9 months, 95% CI 20.8 to 27.7 months vs. 9.2 months, 95% CI 6.0 to 11.0; HR = 0.39 (95% CI 0.32 to 0.49); p < 0.001]. In addition, PFS rates were higher in the lenvatinib plus pembrolizumab arm than in the sunitinib arm at 6, 12, 18 and 24 months.
Exploratory subgroup analyses of progression-free survival assessed by Blinded Independent Review Committee
All results from CLEAR trial PFS subgroup analyses for the comparison of lenvatinib plus pembrolizumab versus sunitinib were statistically significantly in favour of lenvatinib plus pembrolizumab (Motzer et al. 2021,66 figure 1B). The AG highlights that these subgroup analyses were not powered to detect statistically significant differences between the two treatment arms.
FIGURE 1.
Structure of MSD and MSD/AG company model. PD = progressed disease.
Subgroup results by MSKCC and IMDC risk subgroups for PFS assessed by BIRC, using both the FDA and European Medicines Agency (EMA) preferred censoring methods, were provided by Eisai and MSD in their CSs (appendices D2.4.21 and D1.1, respectively). The AG highlights that these subgroup analyses were not powered to detect statistically significant differences between the two treatment arms. The data are marked as academic-in-confidence and cannot be presented here.
Overall survival results from the CLEAR trial
Key OS results from the CLEAR trial are presented in Table 14.
| Characteristic/outcome | All-risk (FAS) | Intermediate/poor risk | Favourable risk | |||
|---|---|---|---|---|---|---|
| Lenvatinib + pembrolizumab (N = 355) | Sunitinib (N = 357) |
Lenvatinib + pembrolizumab (N = 243) |
Sunitinib (N = 229) |
Lenvatinib + pembrolizumab (N = 110) |
Sunitinib (N = 124) |
|
| OS – IA3 data cut-off | ||||||
| Number of deaths (%) | 80 (22.5) | 101 (28.3) | 66 (27.2) | 85 (37.1) | 14 (12.7) | 15 (12.1) |
| Median OS in months (95% CI) | NE (33.6 to NE) | NE (NE to NE) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Stratified HR (95% CI) | 0.66 (0.49 to 0.88)a | Confidential information has been removed | Confidential information has been removed | |||
| p-value | p = 0.005a | Confidential information has been removed | Confidential information has been removed | |||
| OS rate (%) (95% CI) at: | ||||||
| 12 months | 91.4 (87.9 to 93.9) | 80.2 (75.5 to 84.1) | Not reported | Not reported | Not reported | Not reported |
| 18 months | 87.1 (83.1 to 90.3) | 74.4 (69.3 to 78.8) | Not reported | Not reported | Not reported | Not reported |
| 24 months | 79.2 (74.1 to 83.3) | 70.4 (65.0 to 75.2) | Not reported | Not reported | Not reported | Not reported |
| OS – updated OS analysis | ||||||
| Number of deaths (%) | 105 (29.6) | 122 (34.2) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Median OS in months (95% CI) | NE (41.5 to NE) | NE (38.4 to NE) | Not reported | Not reported | NE (NE to NE) | NE (NE to NE) |
| Stratified HR (95% CI) | 0.72 (0.55 to 0.93)a | Confidential | 1.22 (0.66 to 2.26) | |||
| p-value | Not reporteda | Not reported | Not reported | |||
| OS rate (%) (95% CI) at: | ||||||
| 12 months | 91.4 (87.9 to 93.9) | 80.2 (75.5 to 84.1) | Not reported | Not reported | Not reported | Not reported |
| 18 months | 86.9 (82.9 to 90.1) | 73.8 (68.7 to 78.2) | Not reported | Not reported | Not reported | Not reported |
| 24 months | 80.2 (75.5 to 84.1) | 69.7 (64.4 to 74.3) | Not reported | Not reported | Not reported | Not reported |
| 36 months | 65.5 (59.4 to 71.0) | 61.8 (55.8 to 67.1) | Not reported | Not reported | Not reported | Not reported |
Full analysis set (intention-to-treat population, all-risk population)
Median OS had not been reached in either CLEAR trial arm at the time of the IA3 data cut-off or at the time of the updated OS analysis (Table 14). As the PH assumption is violated, the HR should not be used to infer statistical significance or the magnitude of treatment effect from the HR. However, MSD OS K-M data [MSD CS2 (figures 5 and 6)] show early survival differences between patients treated with lenvatinib plus pembrolizumab and those treated with sunitinib; OS rates at 12, 18, 24 and 36 months were consistently higher for patients treated with lenvatinib plus pembrolizumab compared with patients treated with sunitinib.
Exploratory subgroup analyses of OS
Results from most of the OS subgroup analyses generated using data from the IA3 data cut-off favoured lenvatinib plus pembrolizumab versus sunitinib, except for favourable-risk subgroup results which favoured sunitinib [Motzer et al. 202166 (figure S4)]. The AG highlights that these subgroup analyses were not powered to detect statistically significant differences between the two treatment arms. Neither Eisai nor MSD submitted OS subgroup results, other than by risk subgroup, using data from the updated OS analysis.
Subgroup analyses carried out using updated OS analysis data by risk subgroup were provided by Eisai and MSD in their CSs (appendices D2.4.2 and D1.1). The AG highlights that these subgroup analyses were not powered to detect statistically significant differences between the two treatment arms. The data are marked as academic-in-confidence and cannot be presented here.
Treatment on disease progression and impact on overall survival in the CLEAR trial
In addition to the effect of the study drug, OS results may be influenced by subsequent anticancer treatment(s) received on disease progression. Just under half of all patients in the CLEAR trial received subsequent treatment [IA3 data cut-off (45.4%) and updated OS analysis (49.6%)]. Compared with patients in the lenvatinib plus pembrolizumab arm, at the IA3 data cut-off, 1.7 times as many patients in the sunitinib arm (57.1%) than in the lenvatinib plus pembrolizumab arm (33.0%) received subsequent treatment (71.0% and 54.9%, respectively, of patients who discontinued treatment). At the updated data cut off, the proportion of patients receiving subsequent treatment was 61.9% of all sunitinab arm patients and 37.2% of all lenvatinib plus pembrolizumab patients.
Eisai1 presented analyses of updated OS to attempt to take into account additional treatments received for the all-risk population. Eisai presented a comparison of OS data in each treatment arm for patients who received subsequent treatment, and a comparison of OS data in each treatment arm for patients who did not receive subsequent treatment. All the results are academic-in-confidence and so cannot be presented here. However, the AG highlights that the PH assumption was violated for the analysis of OS data from patients who received subsequent treatment and so the OS HR should not be used to infer magnitude of treatment effect or statistical significance for this comparison. Nonetheless, for patients who did not receive subsequent treatment, the K-M data suggested an OS benefit for patients treated with lenvatinib plus pembrolizumab. However, for patients who did receive subsequent treatment, the K-M data suggested an OS benefit for patients treated with lenvatinib plus pembrolizumab up to approximately 33 months, at which point the curves cross. Eisai also conducted prespecified analyses to adjust OS for the effect of any subsequent anticancer treatment (FAS population, updated OS analysis). These analyses were conducted using the two-stage estimation method with different models [log-normal acceleration factor (AF) with and without re-censoring; log-logistic AF with and without re-censoring; Weibull AF with and without re-censoring]. A summary of the AG checks of the treatment-switching analysis methods used by Eisai is provided in Appendix 3 (see Table 55). The results derived from the analysis were marked as academic-in-confidence.
Objective tumour response results from the CLEAR trial
Key tumour response results, including ORR results, from the CLEAR trial all-risk population are presented in Table 15. All subgroup data were marked as academic-in-confidence and cannot be presented.
| Characteristic/outcome | All-risk (FAS) | Intermediate/poor risk | Favourable risk | |||
|---|---|---|---|---|---|---|
| Lenvatinib + pembrolizumab (N = 355) | Sunitinib (N = 357) |
Lenvatinib + pembrolizumab (N = 243) |
Sunitinib (N = 229) |
Lenvatinib + pembrolizumab (N = 110) |
Sunitinib (N = 124) |
|
| ORR (CR + PR) by BIRC, % (95% CI) | 71.0 (66.3 to 75.7) |
36.1 (31.2 to 41.1) |
Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Difference, % (95% CI) | 34.9 (28.0 to 41.7) | Confidential information has been removed | Confidential information has been removed | |||
| OR (95% CI) | 4.35 (3.16 to 5.97) | Confidential information has been removed | Confidential information has been removed | |||
| p-value | Nominal p ≤ 0.0001a | Confidential information has been removed | Confidential information has been removed | |||
| Best objective response: | ||||||
| CR, n (%) | 57 (16.1) | 15 (4.2) | Not reported | Not reported | Not reported | Not reported |
| PR, n (%) | 195 (54.9) | 114 (31.9) | Not reported | Not reported | Not reported | Not reported |
| Stable disease, n (%) | 68 (19.2) | 136 (38.1) | Not reported | Not reported | Not reported | Not reported |
| Progressive disease, n (%) | 19 (5.4) | 50 (14.0) | Not reported | Not reported | Not reported | Not reported |
| Unevaluable for response/ not known, n (%) | 16 (4.5) | 42 (11.8) | Not reported | Not reported | Not reported | Not reported |
| No post-baseline tumour assessment | 12 (3.4) | 38 (10.6) | Not reported | Not reported | Not reported | Not reported |
| ≥ 1 Lesion NE | 1 (0.3) | 2 (0.6) | Not reported | Not reported | Not reported | Not reported |
| Early stable disease (< 7 weeks) | 3 (0.8) | 1 (0.3) | Not reported | Not reported | Not reported | Not reported |
| Median time to response, months (range) |
1.94 (1.41–18.50) |
1.94 (1.61–16.62) |
Not reported | Not reported | Not reported | Not reported |
| Median duration of response, months (95% CI) |
25.8 (22.1 to 27.9) |
14.6 (9.4 to 16.7) |
Not reported | Not reported | Not reported | Not reported |
Full analysis set population
CLEAR trial ORR assessed by BIRC was statistically significantly higher in the lenvatinib plus pembrolizumab arm than in the sunitinib arm [71.0% (95% CI 66.3% to 75.7%) vs. 36.0% (95% CI 31.2% to 41.1%); OR = 4.35 (95% CI 3.16 to 5.97)]. While time to response was 1.94 months in both arms, the duration of response was nearly twice as long for patients treated with lenvatinib plus pembrolizumab (25.8 months) than for patients treated with sunitinib (14.6 months).
Exploratory subgroup analyses of objective response rate
CLEAR trial ORR subgroup analyses results were presented in the CSR for the CLEAR trial, section 11.4.1.6.3. 70 The analyses have not been published and so are marked as academic-in-confidence. The AG highlights that these subgroup analyses were not powered to detect statistically significant differences between the two treatment arms.
Objective response rate results by risk subgroup are summarised by Eisai and MSD in their CS (appendices D2.4.2 and D1.1, respectively). The AG highlights that these subgroup analyses were not powered to detect statistically significant differences between the two treatment arms. The data are marked as academic-in-confidence and cannot be presented here.
Safety results
Safety data from the CLEAR trial were reported (IA3 data cut-off). The AEs were graded using common terminology criteria for adverse event (CTCAE) version 4.03. 87 The safety population included all patients who received at least one dose of either study drug.
The median duration of treatment was longer in the lenvatinib plus pembrolizumab arm than in the sunitinib arm (17.0 vs. 7.8 months).
A summary of treatment emergent adverse events (TEAEs) is presented in Table 16. Patients in the lenvatinib plus pembrolizumab arm experienced more AEs (of any type) than patients in the sunitinib arm. While 37.2% of patients discontinued either lenvatinib and/or pembrolizumab due to TEAEs, 13.4% of patients discontinued both lenvatinib and pembrolizumab and 14.4% of patients discontinued sunitinib due to TEAEs.
| Type of AE, n (%) | Lenvatinib + pembrolizumab (N = 352) |
Sunitinib (N = 340) |
|---|---|---|
| Any TEAE | 351 (99.7) | 335 (98.5) |
| TRAE | 341 (96.9) | 313 (92.1) |
| Any grade ≥ 3 TEAE | 290 (82.4) | 244 (71.8) |
| Non-fatal serious TEAE | 178 (50.6) | 113 (33.2) |
| Non-fatal serious treatment-related TEAE | 119 (33.8) | 51 (15.0) |
| TEAE leading to treatment interruption | 276 (78.4) | 183 (53.8) |
| Interruption of lenvatinib | 257 (73.0) | NA |
| Interruption of pembrolizumab | 194 (55.1) | NA |
| Interruption of both lenvatinib and pembrolizumab | 138 (39.2) | NA |
| TEAE leading to dose reduction | 242 (68.8) | 171 (50.3) |
| TEAEs leading to study drug discontinuation | 131 (37.2) | 49 (14.4) |
| Discontinuation of lenvatinib | 90 (25.6) | NA |
| Discontinuation of pembrolizumab | 101 (28.7) | NA |
| Discontinuation of both lenvatinib and pembrolizumab | 47 (13.4) | NA |
| Fatal TEAE | 15 (4.3) | 11 (3.2) |
| Fatal TRAE | 4 (1.1) | 1 (0.3) |
The AEs of any cause (any grade in ≥ 25% of patients and Grade ≥ 3 in ≥ 5% of patients) that emerged or worsened during the CLEAR are summarised in Tables 17 and 18, respectively. Nearly all patients in both arms experienced at least one all-grade AE with more Grade ≥ 3 AEs reported in the lenvatinib plus pembrolizumab arm (82.4%) than in the sunitinib arm (71.8%).
| AE | Lenvatinib + pembrolizumab (N = 352) | Sunitinib (N = 340) |
|---|---|---|
| n (%) | n (%) | |
| Any AE | 351 (99.7) | 335 (98.5) |
| Diarrhoea | 216 (61.4) | 168 (49.4) |
| Hypertension | 195 (55.4) | 141 (41.5) |
| Hypothyroidism | 166 (47.2) | 90 (26.5) |
| Decreased appetite | 142 (40.3) | 105 (30.9) |
| Fatigue | 141 (40.1) | 125 (36.8) |
| Nausea | 126 (35.8) | 113 (33.2) |
| Stomatitis | 122 (34.7) | 131 (38.5) |
| Dysphonia | 105 (29.8) | 14 (4.1) |
| Weight decrease | 105 (29.8) | 31 (9.1) |
| Proteinuria | 104 (29.5) | 43 (12.6) |
| PPE | 101 (28.7) | 127 (37.4) |
| Arthralgia | 99 (28.1) | 52 (15.3) |
| Rash | 96 (27.3) | 47 (13.8) |
| Vomiting | 92 (26.1) | 68 (20.0) |
| Constipation | 89 (25.3) | 64 (18.8) |
| Dysgeusia | 43 (12.2) | 95 (27.9) |
| AE | Lenvatinib + pembrolizumab (N = 352) |
Sunitinib (N = 340) |
|---|---|---|
| n (%) | n (%) | |
| Any grade ≥ 3 TEAE | 290 (82.4) | 244 (71.8) |
| Hypertension | 97 (27.6) | 64 (18.8) |
| Lipase increased | 45 (12.8) | 30 (8.8) |
| Diarrhoea | 34 (9.7) | 18 (5.3) |
| Amylase increased | 32 (9.1) | 10 (2.9) |
| Weight decreased | 28 (8.0) | 1 (0.3) |
| Proteinuria | 27 (7.7) | 10 (2.9) |
| Asthenia | 19 (5.4) | 15 (4.4) |
| Hypertriglyceridaemia | 17 (4.8) | 22 (6.5) |
| Hyponatraemia | 17 (4.8) | 17 (5.0) |
| Anaemia | 7 (2.0) | 18 (5.3) |
| Neutrophil count decreased | 6 (1.7) | 19 (5.6) |
| Platelet cell count decreased | 4 (1.1) | 31 (6.2) |
| Thrombocytopenia | 2 (0.6) | 19 (5.6) |
| Neutropenia | 2 (0.6) | 20 (5.9) |
The most commonly occurring all-grade AEs in both arms were diarrhoea (61.4% vs. 49.4%) and hypertension (55.4% vs. 41.5%). Hypertension was also the most common Grade ≥ 3 AE in both arms (27.6% vs. 18.8%). The other most common Grade ≥ 3 AEs in the lenvatinib plus pembrolizumab arm were increased lipase (12.8% vs. 8.8%), diarrhoea (9.7% vs. 5.3%), increased amylase (9.1% vs. 2.9%), decreased weight (8.0% vs. 0.3%), proteinuria (7.7% vs. 2.9%) and asthenia (5.4% vs. 4.4%).
MSD2 (p. 69) reported a ‘higher than expected’ incidence of Grade ≥ 3 hepatic AEs. From data presented by the companies [Eisai CS1 (table 20) and MSD CS2 (appendix F, see table 3)], incidences of Grade ≥ 3 alanine aminotransferase increased and Grade ≥ 3 aspartate aminotransferase increased were 4.3% and 3.1%, respectively, in the lenvatinib plus pembrolizumab arm versus 2.4% and 0.9%, respectively, in the sunitinib arm. Grade ≥ 3 blood bilirubin increased in 1.1% of patients treated with lenvatinib plus pembrolizumab and in 0.6% of patients treated with sunitinib. It is reported in the summary of product characteristics (SmPC) for lenvatinib that Grade 3 liver-related reactions occurred in 9.9% of patients in the lenvatinib plus pembrolizumab arm and in 5.3% of patients in the sunitinib arm. 45
MSD2 reported that the most common non-fatal serious adverse events (SAEs) in the lenvatinib plus pembrolizumab arm were diarrhoea (3.4%), vomiting (2.8%), pneumonitis (2.6%), acute kidney injury (2.3%) and hypertension (2.3%), each of which occurred with an incidence ≤ 1.2% in the sunitinib arm [MSD CS2 (appendix F and table 3)]. Pyrexia was the most common SAE in the sunitinib arm (2.1% vs. 1.7% in the lenvatinib plus pembrolizumab arm).
Eisai1 reported that adverse events of special interest (AEOSI) for pembrolizumab were experienced by 60.8% of patients in the lenvatinib plus pembrolizumab arm and 30.9% of patients in the sunitinib arm [Eisai CS1 (appendix F3.2)]. According to the CSR,70 for the comparison of lenvatinib plus pembrolizumab versus sunitinib, the most common AEOSI was hypothyroidism; other AEOSIs reported by ≥5% of patients in the lenvatinib plus pembrolizumab arm were hyperthyroidism, pneumonitis, adrenal insufficiency and severe skin reactions. 70
Health-related quality of life results from the CLEAR trial
In the CLEAR trial, HRQoL was assessed as a secondary end point using the following validated questionnaires: (1) the Functional Assessment of Cancer Therapy Kidney Symptom Index-Disease-Related Symptoms (FKSI-DRS), (2) the European Organisation for the Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30) and (3) the European Quality of Life-5 Dimensions-3 Levels Version (EuroQoL EQ-5D-3L). In summary:
-
The FKSI-DRS consists of nine items designed to assess the frequency/severity of symptoms specific to advanced kidney cancer, including fatigue, pain, bone pain, lack of energy, shortness of breath, fevers, weight loss, coughing and blood in the urine. Scores are measured using a 5-point Likert scale, and higher total scores correspond to better HRQoL.
-
The EORTC is a cancer-specific questionnaire consisting of function and symptom scales, which are scored from 0 to 100. Higher scores on the functional scales reflect better HRQoL, and higher scores on the symptom scales reflect worse symptoms.
-
The EQ-5D-3L is used to assess general HRQoL in five domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) with three levels of response. Responses are used to generate health state index scores, with higher scores indicating better health. The second part of this questionnaire consists of the visual analogue scale, where patients rate their perceived health on a scale of 0 (worst imaginable health) to 100 (best imaginable health).
Health-related quality of life assessments were performed at baseline, day 1 of each subsequent treatment cycle and at the off-treatment visit (30 days after final dose of study drug). As stated in the Eisai HRQoL outcomes study report,72 completion rates (at least one complete score; FAS population) for all HRQoL instruments were notably different for the two trial arms. The completion rates for any instrument declined below 50% at Cycle 26 for patients treated with lenvatinib plus pembrolizumab and at Cycle 12 for patients treated with sunitinib. The completion rates at the off-treatment visit were 40.0% for patients treated with lenvatinib plus pembrolizumab and 55.7% for patients treated with sunitinib. Compliance was generally greater than 90% in both trial arms during early cycles of treatment; however, at the off-treatment visit, compliance had dropped to approximately 80%.
Change from baseline in FKSI-DRS, EORTC QLQ-C30 and EQ-5D-3L score
For each CLEAR trial arm, the overall least squares (LS) mean change was calculated as an average of the change between baseline and each of the time points up until the mean follow-up time (Cycle 15). The difference between the arms in the overall LS mean change was interpreted as clinically meaningful if it exceeded the predefined minimally important difference (MID) for that outcome. As reported by Motzer et al. 202181 and in the MSD CS,2 only a few statistically significant differences were identified between treatment arms for the overall LS mean change in the EORTC QLQ-C30. Lenvatinib and pembrolizumab resulted in higher physical functioning scores and lower fatigue, dyspnoea and constipation scores than sunitinib; none of these differences exceeded the predefined MID. No statistically significant differences were identified between treatment arms for the overall LS mean change in the FKSI-DRS or EQ-5D-3L.
Time to first deterioration and time to definitive deterioration analyses
A deterioration event was defined as a detrimental change in HRQoL score from baseline that exceeded the MID value for that outcome. Two time points were assessed: time to first deterioration [time to treatment discontinuation (TTD)], as the earliest deterioration event during treatment, and time until definitive deterioration (TuDD), as the earliest deterioration event during treatment where there was no subsequent recovery above the deterioration threshold or no subsequent HRQoL data. As reported by Motzer et al. 202181 and in the Eisai CS1 (appendix M3.1), statistically significant differences were identified in the median TTD in favour of lenvatinib plus pembrolizumab versus sunitinib for the following EORTC QLQ-C30 scales: physical functioning, appetite loss and dyspnoea, and the EQ-5D-VAS score. As reported in the Eisai CS1 (appendix M3.2), statistically significant differences were also found in the median TuDD in favour of lenvatinib plus pembrolizumab versus sunitinib for all scales, except for the cognitive domain and financial difficulties symptom scales. It was not possible to compare the values for the cognitive domain, or constipation and financial difficulties symptom scales, due to no estimable values in one or both of the treatment arms.
Summary of response status during treatment
The proportions of participants in each treatment arm who, relative to baseline, had improved or deteriorated, or who were stable on treatment, were assessed. As reported in the Eisai CS1 (appendix M3.3), for all HRQoL scales, except for the EORTC QLQ-C30 financial difficulties, deterioration (not stable outcome or improvement) was the most frequently reported outcome for patients treated with lenvatinib plus pembrolizumab and for sunitinib.
Interpretation of evidence from the CLEAR trial
The CLEAR trial is a well-designed trial and results are generalisable to NHS clinical practice. However, the trial only provided evidence for the comparison of treatment with lenvatinib plus pembrolizumab versus one of the relevant comparators (sunitinib) identified in the final scope29 issued by NICE. Clinical effectiveness data were available from two data cuts: IA3 (PFS, OS, ORR and AEs) and an updated OS analysis (OS).
CLEAR trial efficacy results suggested that PFS and ORR were statistically significantly improved for patients treated with lenvatinib plus pembrolizumab compared with patients treated with sunitinib (all-risk population, intermediate-/poor-risk subgroup and favourable-risk subgroup). For the intermediate-/poor-risk and favourable-risk subgroups, PFS and ORR differences favoured patients in the lenvatinib plus pembrolizumab arm; all PFS and ORR results were statistically significant, and clinical advice to the AG was that they were also clinically meaningful.
For the all-risk population, OS results were difficult to interpret as the PH assumption was violated over the CLEAR trial follow-up period. Therefore, results should not be used to infer any statistically significant difference (or lack of statistically significant difference) for the comparison between treatment with lenvatinib plus pembrolizumab and treatment with sunitinib. However, the CLEAR trial OS survival rates at 12, 18, 24 and 36 months all favour lenvatinib plus pembrolizumab versus sunitinib.
The CLEAR trial OS PH assumption was not violated for the intermediate-/poor-risk and favourable-risk subgroups. The HR results from the updated OS analysis showed a statistically significant improvement for patients treated with lenvatinib plus pembrolizumab versus patients treated with sunitinib for the intermediate-/poor-risk subgroup; there were too few events in the favourable-risk subgroup for robust OS conclusions to be drawn.
Overall survival results can be influenced by subsequent anticancer treatments received by patients on disease progression. Eisai1 carried out a treatment-switching analysis to test whether adjusting for the effect of subsequent treatments affected OS results. Results were generated only for the all-risk population and were marked as academic-in-confidence. In addition to a treatment-switching analysis to test whether adjusting for the effect of subsequent treatment affected OS results, Eisai1 also conducted post hoc analyses that examined OS for patients who did and did not receive subsequent treatment separately. The PH assumption was violated for patients who received subsequent treatments making it difficult to interpret the results from this analysis. Clinical advice to the AG is that patients who do not receive subsequent treatments are a heterogeneous group and, therefore, the results from this post hoc analysis are also difficult to interpret.
More patients treated with lenvatinib plus pembrolizumab experienced Grade ≥ 3 AEs than patients treated with sunitinib. 1,2,66 Nonetheless, both companies1,2 highlighted that evidence from the CLEAR trial showed that, in general, lenvatinib plus pembrolizumab was well tolerated in patients with aRCC; generally, the AEs experienced by patients were consistent with the known safety profile of each drug. However, both companies1,2 highlighted that there was a higher than expected incidence of Grade 1 and Grade 2 hypothyroidism, a known AE associated with both lenvatinib and pembrolizumab. 2 MSD2 also highlighted there was a higher than expected incidence of Grade ≥ 3 hepatic AEs.
When compared to treatment with sunitinib, treatment with lenvatinib plus pembrolizumab appeared to neither improve or worsen HRQoL, as measured by the FKSI-DRS, EORTC QLQ-C30 and EQ-5D-3L instruments. 1,2,81
As the CLEAR trial provided only clinical effectiveness evidence for the comparison between lenvatinib plus pembrolizumab and sunitinib, it was necessary to generate indirect evidence to compare lenvatinib plus pembrolizumab with other relevant comparators (see Chapter 4, Assessment of clinical effectiveness: indirect evidence).
Chapter 4 Assessment of clinical effectiveness: indirect evidence
Limited direct clinical effectiveness evidence
The only direct clinical effectiveness evidence available for the comparison between lenvatinib plus pembrolizumab for patients with untreated aRCC and any comparator listed in the final scope29 issued by NICE is from the CLEAR trial (vs. sunitinib). To allow comparisons between lenvatinib plus pembrolizumab and other relevant comparators, indirect comparisons were required.
Eisai and Merck Sharp & Dohme indirect comparisons
A summary and AG critique of the Eisai and MSD NMA statistical approaches is provided in Appendix 3 (see Tables 56 and 57, respectively). The AG considered that the NMA statistical approaches used by Eisai and MSD were appropriate and appeared to be correctly implemented. However, neither company presented comparative evidence for the comparison of lenvatinib plus pembrolizumab with nivolumab plus ipilimumab for the intermediate-/poor-risk subgroup.
The two companies presented results from two different approaches to carrying out NMAs [Bayesian HR and fractional polynomial (FP)] for PFS and OS (see appendix D.4 in Eisai CS1 and appendix M in MSD CS2).
Assessment group methodological approach to network meta-analysis: feasibility assessment
Studies assessed by the assessment group for potential inclusion in network meta-analysis
Any study identified by the AG searches for direct evidence that appeared to be designed as a RCT of any drug used to treat adults with untreated aRCC was tagged as ‘RCT’ within Covidence (n = 1129 records). These records were then examined by SN to confirm that the study design and the study population were of interest (i.e. RCTs of adults with untreated aRCC) and to identify the drug treatments included in the studies.
In addition, any study previously identified by the AG searches that appeared to be a NMA of RCTs of drugs used to treat adults with untreated aRCC was tagged as a ‘NMA’ within Covidence (n = 36, published from 2009 to 2021). The AG examined the reference lists and network structures of recently published NMAs (n = 1057,88–96), that is, those published since 2020, to assess the feasibility of constructing suitable networks for each outcome listed in the final scope29 issued by NICE.
In total, the AG identified 10 RCTs27,66,97–104 of drug treatments for adults with untreated aRCC that were potentially eligible for inclusion in the AG NMAs.
Assessment group consideration of specific networks
The AG’s assessment of the feasibility of constructing specific networks considered the following:
-
the feasibility of constructing a ‘suitable connected network’ of relevant treatments for each outcome and for each risk subgroup
-
the clinical and methodological heterogeneity of the included studies in terms of (1) study population, (2) interventions and comparators, (3) outcome measures (OS, PFS, ORR, safety and HRQoL) and (4) study quality.
For each outcome listed in the final scope29 issued by NICE, the AG initially considered a ‘suitable connected network’ to be a network that included only RCTs of comparators listed in the final scope29 issued by NICE for the following risk groups, as defined in the IMDC criteria:15
-
intermediate-/poor-risk subgroup (network nodes: lenvatinib plus pembrolizumab, cabozantinib and nivolumab plus ipilimumab)
-
favourable-risk subgroup (network nodes: lenvatinib plus pembrolizumab, sunitinib, pazopanib and tivozanib)
-
the all-risk population (network nodes: lenvatinib plus pembrolizumab, sunitinib, pazopanib and tivozanib).
However, where it was not possible to construct a connected network using only the comparators listed in the final scope29 issued by NICE, the AG considered introducing additional treatments (i.e. nodes), such as interferon-alpha and sorafenib to form connections. The AG considered that it was not appropriate to attempt to connect comparators listed in the final scope29 issued by NICE via two or more non-relevant treatments as more uncertainty is introduced with the addition of each irrelevant node.
Following assessment of suitable network structures and consideration of the availability of outcome data from each of the 10 RCTs,27,66,97–104 the AG excluded two trials27,99 (reasons are listed in Table 19) in at least one of the AG NMAs.
| RCT | Randomised treatments | Notes |
|---|---|---|
| RCTs included in the AG NMAS | ||
| CABOSUN97 |
|
|
| CheckMate 214100 |
|
|
| CLEAR trial |
|
|
| COMPARZ101 |
|
|
| CROSS-J-RCC104 |
|
|
| SWITCH98 |
|
|
| SWITCH II103 |
|
|
| TIVO-1102 |
|
|
| RCTs not included in the AG NMAs | ||
| Escudier 200999 |
|
|
| Motzer 200727 |
|
|
Details about the comparators and a list of the RCTs that provided information to inform the AG PFS, OS and ORR NMAs for the intermediate-/poor-risk and favourable-risk subgroups and all-risk population are presented in Table 20. The AG PFS, OS and ORR network diagrams are presented in Figures 4–7 and the outcome data used to populate the AG PFS, OS and ORR NMAs are presented in Tables 58–60 (see Appendix 4).
| Outcome | Risk group | Comparatorsa | Trials | Notesb |
|---|---|---|---|---|
| PFS | Intermediate/poor |
|
|
|
| Favourable |
|
|
|
|
| All-risk |
|
|
||
| OS | Intermediate/poor |
|
IMDC risk subgroup data used for all trials | |
| Favourable |
|
|
Separate NMAs conducted using:
|
|
| All-risk |
|
|
|
|
| ORR | Intermediate/poor |
|
|
|
| All-risk |
|
|
BIRC assessed ORR data used for both trials | |
| Grade ≥ 3 AEs | Intermediate/poor |
|
|
|
| All-risk |
|
|
The AG considered that the different definitions of AEs reported within the trials (i.e. treatment-emergent, treatment-related or all-cause AEs for Grade ≥ 3 AEs and discontinuations due to AEs) made it difficult to interpret any relative differences between treatments. Furthermore, safety data were not reported separately for subgroups of interest, most notably for the intermediate-/poor-risk subgroup in the CheckMate 214 trial,100 and for the favourable-risk subgroup in any trials other than the CLEAR trial. AE data were unavailable for the previously untreated patients in the TIVO-1 trial. 102
Nonetheless, the AG performed NMAs for Grade ≥ 3 AEs where either treatment-emergent or all-cause AEs were reported (see Appendix 4, Figures 5 and 7 for network diagrams and Table 61 for outcome data used to populate these NMAs). The AG also considered performing NMAs for discontinuations due to AEs comparing (1) discontinuations of both lenvatinib and pembrolizumab and (2) discontinuations of either lenvatinib or pembrolizumab with relevant comparators. However, it appeared that only data for the latter were available from the CLEAR trial for risk subgroups. Further, when summing the total of AEs from the two subgroups, there were still many AEs in the all-risk population that appeared to be unaccounted for according to subgroup, that is summing the numbers of discontinuations due to AEs in the intermediate/poor and favourable-risk subgroups from Eisai CS,1 (Appendix F, see tables 64 and 65) did not sum to the total reported for the all-risk population in Table 16. Therefore, the AG considered it inappropriate to conduct NMAs for discontinuations due to AEs.
It was not possible for the AG to perform any HRQoL NMAs due to the heterogeneity of the HRQoL outcome scales used in the included trials and the sparsity of reported data (i.e. 95% CIs not reported and data not reported separately for subgroups of interest).
Assessment group methodological approach: intermediate-/poor-risk subgroup
The AG was able to construct a suitable network for PFS, OS and ORR including the two relevant comparators for this subgroup (cabozantinib and nivolumab plus ipilimumab); these networks also included sunitinib, a comparator common to the three included RCTs66,97,100 (see Figure 4 in Appendix 4). Safety data were not reported for the intermediate-/poor-risk subgroup in the CheckMate 214 trial,100 therefore the AG networks for Grade ≥3 AEs due to AEs for this subgroup included only cabozantinib (and sunitinib) as comparators (see Figure 5 in Appendix 4).
Assessment group methodological approach: International Metastatic Renal Cell Carcinoma Database Consortium/Memorial Sloan-Kettering Cancer Center favourable-risk subgroup
The AG PFS and OS networks included only sunitinib and pazopanib as comparators (see Figure 6). It was not possible to connect tivozanib to the PFS and OS networks as the only identified trial of tivozanib (TIVO-1 trial102) recruited a mixed population of untreated and previously treated patients with metastatic RCC and did not report PFS and OS data separately for the subgroup of untreated patients.
Only the CLEAR trial reported ORR and safety data for the favourable-risk subgroup; therefore, it was not possible to carry out NMAs of ORR or safety outcomes for lenvatinib plus pembrolizumab versus pazopanib or tivozanib.
Assessment group methodological approach: all-risk population
The AG PFS all-risk population network included all relevant comparators (sunitinib, pazopanib and tivozanib). This network was constructed by including sorafenib as a node and by using PFS data relating to first-line treatment from two trials (CROSS-J-RCC104 and SWITCH98) of sunitinib versus sorafenib that used a sequential design to connect tivozanib to the network (see Figure 7 in Appendix 4).
It was not possible to connect tivozanib to the OS network as OS data from patients receiving first-line treatment were not available from the CROSS-J-RCC104 and SWITCH98 trials and no trials were identified that allowed tivozanib to be included in the OS network via a single additional treatment node. The AG did not consider that it was appropriate to attempt to connect tivozanib to the OS network via two or more non-relevant treatments, which were not relevant comparators because of the increased level of uncertainty.
The AG was also unable to connect tivozanib to the ORR network as the only identified tivozanib trial (TIVO-1 trial102) recruited a mixed population of untreated and previously treated patients with metastatic RCC and did not report ORR data separately for the subgroup of untreated patients.
Therefore, for the all-risk population, the AG OS, ORR, Grade ≥3 AEs networks included only sunitinib and pazopanib as comparators (see Figure 6 in Appendix 4). The AG was not able to indirectly compare the clinical effectiveness of lenvatinib plus pembrolizumab with tivozanib for OS, ORR or Grade ≥ 3 AEs for patients in the all-risk population.
Quality assessment of the trials included in assessment group network meta-analysis
The quality assessment of the CLEAR trial and the seven other RCTs97,98,100–104 included in the AG NMAs is presented in Appendix 4 (see Table 62).
The AG considers that most of the trials included in the AG NMAs were of good methodological quality. However, due to insufficient information available, the AG was unable to assess the robustness of the randomisation procedures and whether robust procedures were in place to prevent patients or investigators predicting allocation to treatment in one trial. 103 All of the trials were open-label; however, the CLEAR trial and four other trials97,100–102 reported the use of blinded independent review of radiologic outcomes.
Assessment group summary of patient and trial characteristics and assessment of heterogeneity
Summaries of the design, demographic characteristics and the IMDC and MSKCC risk subgroups of patients enrolled in the CLEAR trial and other seven RCTs97,98,100–104 included in the AG NMAs are provided in Appendix 4 (see Tables 63 and 64).
In addition to the CLEAR trial, five of the trials were also phase III RCTs98,100,101,103,104 and two were phase II RCTs. 97,102 Three trials98,103,104 used a sequential design in which patients were randomised to first-line treatment, and patients who discontinued first-line treatment due to disease progression or toxicity received the alternative trial treatment as a second-line therapy; data from only these trials relating to first-line treatment were extracted. All of the RCTs were designed as open-label trials (see Table 62 in Appendix 4); the CLEAR trial and four other RCTs97,100–102 used blinded independent review for radiologic outcomes (i.e. PFS and ORR), two RCTs98,104 used unblinded investigator assessment, and the authors of one RCT103 did not report method of radiologic outcome assessment.
Two trials102,104 recruited patients with metastatic RCC only. The CLEAR trial and six other RCTs97,98,100,101,103,104 recruited untreated patients only, while one trial (TIVO-1102) recruited a mix of untreated patients (70.0%) and patients who had received previous systemic therapy (29.8%); data were extracted from the TIVO-1102 trial for the untreated subgroup only.
The CLEAR trial and five other RCTs97,100,102–104 recruited patients with clear cell RCC only, while 12.9% and 13.0% of recruited patients in the other two trials98,103 had non-clear cell histology. Results were not reported separately in the SWITCH trials98,103 for patients with clear cell histology.
The ages of recruited patients were similar across the RCTs (see Tables 63 and 64 in Appendix 4); across trial arms, the median age ranged from 61 years (in the CLEAR trial and two other trials100,101) to 68 years. 103 All trials recruited a majority of male patients (72.4%103 to 82.5%104).
In addition to the CLEAR trial, three RCTs100–102 recruited patients irrespective of disease risk according to IMDC or MSKCC criteria. However, data from the CheckMate 214 trial100 (nivolumab plus ipilimumab vs. sunitinib) were available for the intermediate-/poor-risk population and were used in the AG NMAs. The cabozantinib RCT97 recruited patients only with intermediate or poor-risk disease. Three RCTs98,103,104 were designed to recruit patients only with favourable- or intermediate-risk disease by MSKCC criteria.
Only the CLEAR trial reported disease risk classifications according to both IMDC and MSKCC risk criteria (see Table 64 in Appendix 4). Two other RCTs97,100 reported the proportion of patients classified by IMDC risk subgroup and four other RCTs98,101,103,104 reported the proportion of patients classified by MSKCC risk subgroup. The remaining RCT (TIVO-1102) did not report risk of disease according to IMDC or MSKCC criteria for the subgroup of untreated patients. The proportions of patients classified within each disease risk subgroup according to either IMDC or MSKCC criteria varied across RCTs (see Table 64 in Appendix 4).
The following differences between RCTs may have introduced heterogeneity into the AG NMAs:
-
population characteristics (see Table 63 and Table 64 in Appendix 4)
-
PFS and ORR assessment methods (BIRC, investigator or not reported) and types of AEs (all-cause AE or TEAE)
-
differences in median PFS, OS, ORR and Grade ≥ 3 follow-up times (see Tables 58–61 in Appendix 4).
The AG is not aware of any statistical methods that can be used to adjust for these differences in patient baseline characteristics and trial design.
Assessment group assessment of proportional hazards assumptions
For time-to-event outcomes presented as HRs (i.e. PFS and OS), the AG assessed the validity of the within-trial PFS and OS PH assumptions for each of the groups (intermediate-/poor-risk and favourable-risk subgroups and all-risk population). The AG PH assessments were carried out by examining the figures (Schoenfeld residuals plots or log cumulative hazard plots) and statistical test results (e.g. Grambsch–Therneau test86) presented in the Eisai CS1 (sections 5.3.1 and 5.3.2) and in the Eisai response to clarification (questions A1 and A2).
Data from the CheckMate214 trial100 (nivolumab plus ipilimumab vs. sunitinib) were not included in the company NMAs. The AG, therefore, digitised the published intermediate-/poor-risk subgroup PFS and OS 42-month K-M data100 and assessed proportionality by plotting Schoenfeld residuals and performing a Grambsch–Therneau test. 86 The AG OS and PFS PH assessments are presented in Appendix 4 (Proportional hazards assessments for trials included in the assessment group network meta-analysis). Violations of the PH assumption within the studies included in the AG NMAs are listed in Table 21.
| Risk group | PFS | OS |
|---|---|---|
| Intermediate/poor risk subgroup | CheckMate 214 trial100 | NA |
| Favourable risk subgroup | PH could not be assessed within the COMPARZ trial101 for PFS, or OS105 (pazopanib vs. sunitinib) as no K-M data were presented | |
| All-risk population | TIVO-1 trial102 | CLEAR trial |
If the PH assumption holds, a HR represents an average of the relative treatment effect during the trial follow-up period106 (or trials, in the context of a NMA) and the HR is proportional over time. 107 When the PH assumption is violated, this means that the HR (whether from a trial or from a NMA including data from one or more trials with PH violations) is not applicable to all time points across the trial follow-up periods. If the PH assumption holds, then it may not be unreasonable to assume that the estimated HRs is valid beyond the trial follow-up periods. However, when the PH assumption is violated, estimated HRs may not produce accurate projections of relative survival between treatment arms beyond the observed trial follow-up periods.
Some PH test results showed (see Table 21) that PFS and OS outcome hazards were not proportional. Within any network, if any within-trial hazards are not proportional, then Bayesian HR NMA results [i.e. the HRs and 95% credible intervals (CrIs)] should not be used to infer statistically significant differences (or lack of statistically significant difference) between treatments.
Where violations of the PH assumption are demonstrated, alternative flexible modelling approaches for NMA, which relax the PH assumption, including FP NMAs, have been proposed to aid decision-making. 108,109 However, interpretation of the estimates provided by these complex modelling techniques can be difficult and often are not intuitive. 108,109
The ‘best-fitting’ FP model (or alternative flexible model) for a NMA, which is defined according to model fit statistics, such as the deviance information criterion (DIC), reflects the model that most closely captures the shape of the observed data. However model fit statistics do not provide information about whether a model is a good fit to the data or whether the estimates generated by the model, including projections of results beyond the follow-up times of trials included in the NMA, are clinically plausible. 109 Furthermore, flexible models that appear similar according to model fit (i.e. according to DIC statistics) may generate very different long-term survival estimates; advice from the Medical Research Council Biostatistics Unit110 is that, ‘if the difference in DIC is, say, less than 5 and the models make very different inferences, then it could be misleading just to report the model with the lowest DIC.’ Because of these limitations, the AG does not consider that it is appropriate to use the results of FP NMAs for clinical decision-making.
The AG considers that the limitations associated with the interpretation of results from FP NMAs are greater than the limitations of interpretation of the Bayesian HR NMA results when the PH assumption is violated. In addition, for the intermediate-/poor-risk subgroup (the largest of the two risk subgroups considered), there was no violation of the OS PH assumption within any of the trials included in the AG OS network.
The AG carried out PFS, OS and ORR NMAs for the intermediate-/poor-risk and the favourable-risk subgroups and all-risk population. However, the AG emphasises that where violations of the PH assumption were demonstrated, HRs and 95% CrIs should not be used to infer any statistically significant difference (or lack of statistically significant difference) for the treatment comparisons.
Assessment group statistical approach to Bayesian hazard ratio network meta-analysis
The AG performed PFS, OS and ORR NMAs using a Bayesian framework. These were carried out using the multinma R package. 111 This approach is in line with Decision Support Unit (DSU) guidance (documents 2, 3 and 4112–114). All results were generated using 100,000 iterations on 3 chains after a burn-in of 100,000 and vague prior distributions were used for intercept, treatment and heterogeneity [for random-effects (RE) models only] parameters.
The AG performed NMAs using fixed-effects (FE) and RE models. As convergence issues occurred due to sparse data, RE NMA results were unusable. Because of the lack of published information to select informative prior distributions to improve convergence of RE models, the AG has only presented results from FE models in the main body of this report. The AG has described where important clinical or statistical heterogeneity between RCTs included in the NMA may have had an impact on how NMA results can be interpreted.
For PFS, the only outcome with a closed loop present within the network, the AG assessed inconsistency in the NMAs by applying an unrelated mean effects model114 and by comparing model fit statistics of inconsistency models with consistency models.
Treatment effect estimates for direct and indirect clinical effectiveness evidence are presented as HRs for time-to-event data (i.e. PFS and OS) and ORs for dichotomous data (i.e. ORR). All treatment effect estimates are presented with 95% CrIs.
An example of the statistical code used by the AG to perform PFS, OS, ORR and safety NMAs is provided in Appendix 4 (see Example statistical code for assessment group network meta-analysis).
Results of the assessment group network meta-analyses
Results of the AG FE NMAs are presented in this section and results of the AG RE NMAs are presented in Appendix 4 (see Tables 65–67 for PFS, OS and ORR, respectively, and Table 68 for Grade ≥ 3 AEs). The AG RE NMAs were associated with convergence issues; it is likely that these issues arose due to sparse networks (i.e. a small number of included trials). Because of the convergence issues, 95% CrIs around the HRs are very wide and unstable, these RE NMA results should not be used to inform clinical decision-making.
When interpreting AG FE NMA results, it should be noted that the results do not account for the observed heterogeneity between the trials (see Assessment group summary of patient and trial characteristics and assessment of heterogeneity).
Progression-free survival: assessment group fixed-effects network meta-analysis
The AG PFS NMA results for all pairs of treatments for the intermediate-/poor-risk subgroup and the IMDC/MSKCC favourable-risk subgroup and all-risk population are presented in Table 22.
| Treatment | Comparator | FEs HR (95% CrI)a |
|---|---|---|
| Intermediate-/poor-risk subgroup | ||
| Lenvatinib + pembrolizumab | Sunitinib | 0.36 (0.28 to 0.46) |
| Lenvatinib + pembrolizumab | Cabozantinib | 0.75 (0.45 to 1.25) |
| Lenvatinib + pembrolizumab | Nivolumab + ipilimumab | 0.48 (0.35 to 0.66) |
| Cabozantinib | Sunitinib | 0.48 (0.31 to 0.74) |
| Nivolumab + ipilimumab | Sunitinib | 0.75 (0.62 to 0.90) |
| Nivolumab + ipilimumab | Cabozantinib | 1.57 (0.97 to 2.51) |
| IMDC/MSKCC favourable-risk subgroupb | ||
| Lenvatinib + pembrolizumab | Sunitinib | 0.41 (0.28 to 0.60) |
| Lenvatinib + pembrolizumab | Pazopanib | 0.40 (0.21 to 0.75) |
| Pazopanib | Sunitinib | 1.02 (0.63 to 1.68) |
| All-risk population | ||
| Lenvatinib + pembrolizumab | Sunitinib | 0.39 (0.32 to 0.48) |
| Lenvatinib + pembrolizumab | Pazopanib | 0.34 (0.26 to 0.43) |
| Lenvatinib + pembrolizumab | Tivozanib | 0.50 (0.34 to 0.73) |
| Lenvatinib + pembrolizumab | Sorafenib | 0.38 (0.29 to 0.50) |
| Pazopanib | Sunitinib | 1.16 (1.01 to 1.34) |
| Tivozanib | Sunitinib | 0.78 (0.57 to 1.07) |
| Sorafenib | Sunitinib | 1.03 (0.86 to 1.22) |
| Pazopanib | Tivozanib | 1.49 (1.07 to 2.05) |
| Pazopanib | Sorafenib | 1.13 (0.94 to 1.35) |
| Tivozanib | Sorafenib | 0.76 (0.58 to 1.00) |
The AG NMAs included PFS data that were assessed using FDA censoring rules. The AG PFS NMA sensitivity analysis included CLEAR trial PFS data assessed using the EMA censoring rules and data from all other included trials using FDA censoring rules (see Table 69 in Appendix 4). Results from the two AG PFS NMAs were similar.
Because of PH violations or uncertainty regarding the validity of the PH assumption, the HRs and 95% CrIs shown in Table 22 cannot be used to infer any statistically significant difference (or lack of statistically significant difference) for any of the treatment comparisons (see Assessment group assessment of proportional hazards assumptions).
Overall survival: assessment group fixed-effects network meta-analysis
The OS FE NMA results for all pairs of treatments for the intermediate-/poor-risk subgroup and the IMDC/MSKCC favourable-risk subgroup and all-risk population are presented in Table 23.
| Treatment | Comparator | FEs HR (95% CrI)a |
|---|---|---|
| Intermediate-/poor-risk subgroup | ||
| Lenvatinib + pembrolizumab | Sunitinib | 0.62 (0.46 to 0.83) |
| Lenvatinib + pembrolizumab | Cabozantinib | 0.78 (0.47 to 1.28) |
| Lenvatinib + pembrolizumab | Nivolumab + ipilimumab | 0.94 (0.66 to 1.32) |
| Cabozantinib | Sunitinib | 0.80 (0.53 to 1.21) |
| Nivolumab + ipilimumab | Sunitinib | 0.66 (0.55 to 0.79) |
| Nivolumab + ipilimumab | Cabozantinib | 0.83 (0.53 to 1.30) |
| IMDC/MSKCC favourable-risk subgroupb | ||
| Lenvatinib + pembrolizumab | Sunitinib | 1.22 (0.66 to 2.25) |
| Lenvatinib + pembrolizumab | Pazopanib | 1.38 (0.69 to 2.80) |
| Pazopanib | Sunitinib | 0.88 (0.63 to 1.23) |
| All-risk population | ||
| Lenvatinib + pembrolizumab | Sunitinib | 0.72 (0.55 to 0.94) |
| Lenvatinib + pembrolizumab | Pazopanib | 0.79 (0.58 to 1.06) |
| Pazopanib | Sunitinib | 0.92 (0.79 to 1.07) |
In the intermediate-/poor-risk subgroup, a numerical advantage in terms of OS was shown for lenvatinib plus pembrolizumab versus cabozantinib (HR = 0.78, 95% CrI 0.47 to 1.28) and versus nivolumab plus ipilimumab (HR = 0.94, 95% CrI 0.66 to 1.32). However, neither of these numerical advantages was statistically significant. No violations of the PH assumption were observed for OS in this subgroup (see Assessment group assessment of proportional hazards assumptions).
Because of PH violations or uncertainty regarding the validity of the PH assumption, the AG OS NMA HRs and 95% CrIs for the IMDC/MSKCC favourable-risk subgroup and all-risk population (see Table 23) cannot be used to infer any statistically significant difference (or lack of statistically significant difference) for any of the treatment comparisons (see Assessment group assessment of proportional hazards assumptions).
Objective response rate: assessment group fixed-effects network meta-analysis
The AG ORR NMA results for all pairs of treatments for the intermediate-/poor-risk subgroup and all-risk population and are presented in Appendix 4 (see Table 67).
In the intermediate-/poor-risk subgroup, ORR was statistically significantly higher for lenvatinib plus pembrolizumab compared to nivolumab plus ipilimumab (OR = 3.19, 95% CrI 1.95 to 5.26); however, no statistically significant difference was shown between lenvatinib plus pembrolizumab and cabozantinib (OR = 2.46, 95% CrI 0.84 to 6.82). In the all-risk population, ORR was statistically significantly higher for lenvatinib plus pembrolizumab compared to sunitinib (OR = 4.35, 95% CrI 3.16 to 5.99) and compared to pazopanib (OR = 3.22, 95% CrI 2.14 to 4.85).
Grade ≥ 3 adverse events: assessment group fixed-effects network meta-analysis
The AG Grade ≥ 3 FE NMA results for all pairs of treatments for the intermediate-/poor-risk subgroup and the all-risk population are presented in Appendix 4 (see Table 70).
In the intermediate-/poor-risk subgroup, for the comparison of lenvatinib plus pembrolizumab with cabozantinib, there were no statistically significant differences in Grade ≥ 3 AEs (OR = 1.80, 95% CrI 0.79 to 4.10). In the all-risk population, there were statistically significantly more Grade ≥ 3 AEs for lenvatinib plus pembrolizumab compared to sunitinib (OR = 1.84, 95% CrI 1.28 to 2.66) and compared to pazopanib (OR = 1.86, 95% CrI 1.17 to 2.94).
Assessment group sensitivity analysis network meta-analysis: favourable-risk subgroup
The COMPARZ trial101 reported PFS and OS results (including a separately reported final OS analysis105) for the MSKCC favourable-risk subgroup (not for the IMDC favourable-risk subgroup). Therefore, the AG performed sensitivity analyses including MSKCC favourable-risk subgroup data from the CLEAR trial and the COMPARZ trial101 for the PFS (FDA and EMA censoring rules) and the OS NMAs (using COMPARZ trial final OS analysis105). Results of the MSKCC/MSKCC favourable-risk subgroup PFS and OS NMAs are presented in Appendix 4 (see Table 68). Numerical results (i.e. HRs and 95% CrIs) from the sensitivity analyses of PFS and OS (Appendix 4, Table 70) were similar to the results presented in Table 23 and Table 24, respectively.
Assessment of inconsistency for overall survival, progression-free survival and objective response rate network meta-analysi
The AG assessments of inconsistency for PFS in the all-risk population, the only NMA with a closed loop present within the network, are presented in Appendix 4 (see Assessment group assessment of inconsistency in the network meta-analysis). Although a model which accounts for inconsistency in the NMA provides a better statistical model fit compared to a model which assumes consistency, results of AG FE NMAs which assumed consistency or accounted for inconsistency were very similar. Therefore, any inconsistency present between direct and indirect evidence for PFS in the all-risk population does not seem to have had an important impact on AG PFS NMA results.
Because of the lack of closed loops in any of the other AG networks, the consistency of indirect estimates of OS, ORR and AEs are unknown.
Additional assessment group network meta-analysis sensitivity analysis
Additional sensitivity analyses were conducted for reasons described in Appendix 4 (see Additional assessment group network meta-analysis sensitivity analyses). In summary, updated results from the CheckMate 214 trial115,116 were incorporated into the intermediate-/poor-risk subgroup NMA. The AG found that, as with the original NMAs, PH is violated for PFS data, but not for OS data. Including the updated data from the CheckMate 214 trial had little impact on the results.
Interpretation of the indirect evidence from assessment group network meta-analysis
The CLEAR trial only provided evidence for the comparison of lenvatinib plus pembrolizumab with one of the relevant comparators (sunitinib). Therefore, indirect treatment comparisons were carried out to provide evidence for the comparison of lenvatinib plus pembrolizumab with cabozantinib, nivolumab plus ipilimumab, pazopanib and tivozanib. The AG was unable to consider the impact of observed heterogeneity between the trials when carrying out NMAs.
Because of limited data availability and within-trial PFS and OS PH violations (or uncertainty regarding the validity of the PH assumption), AG NMA HRs and 95% CrIs can only be used to infer a statistically significant OS difference for the comparison of lenvatinib plus pembrolizumab with cabozantinib and with nivolumab plus ipilimumab for patients in intermediate-/poor-risk subgroup. Results demonstrated a numerical advantage for lenvatinib plus pembrolizumab versus cabozantinib and versus nivolumab plus ipilimumab; these results were not statistically significant.
For any treatment comparisons that include sunitinib, pazopanib and tivozanib, where it is not possible to draw conclusions from NMA results about statistical significance, the AG highlights that previous NICE ACs24,25,34,35 have concluded that sunitinib and pazopanib are of equivalent clinical effectiveness in the all-risk population and that: ‘At best, tivozanib may have a similar effect to sunitinib or pazopanib’. 34
The AG ORR NMA results for the intermediate-/poor-risk subgroup suggested that treatment with lenvatinib plus pembrolizumab led to only a statistically significant improvement in ORR versus treatment with nivolumab plus ipilimumab. It was not possible to generate results for the IMDC/MSKCC favourable-risk subgroup due to data limitations. The AG ORR NMA results for the all-risk population suggested that treatment with lenvatinib plus pembrolizumab led to a statistically significant improvement in ORR versus treatments with sunitinib and with pazopanib.
The AG Grade ≥ 3 AE NMA results for the intermediate-/poor-risk subgroup suggested that treatment with lenvatinib plus pembrolizumab led to statistically significantly more Grade ≥ 3 AEs versus treatment with cabozantinib. It was not possible to generate results for the IMDC/MSKCC favourable-risk subgroup. AG Grade ≥ 3 AE NMA results for the all-risk population suggested that treatment with lenvatinib led to statistically significantly more Grade ≥ 3 AEs versus treatments with sunitinib and with pazopanib.
The AG NMAs incorporated data from subgroups of patients defined by their risk status. An efficacy estimate calculated for a specific subgroup of patients from a RCT may be subject to imbalances in prognostic factors between treatment arms, if randomisation in the trial was not stratified by the subgroup variable of interest. In all but one101 of the RCTs included in the AG NMAs, randomisation was stratified by risk status, and so the AG considers that the impact of imbalanced prognostic factors across treatment arms in the NMAs is likely to be very small.
Chapter 5 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
The AG conducted a systematic review of the economic literature to identify the existing evidence base assessing the cost-effectiveness of treatment with lenvatinib plus pembrolizumab for patients with untreated aRCC versus five different treatments (sunitinib, pazopanib, tivozanib, cabozantinib and nivolumab plus ipilimumab).
The AG critiqued the companies’ systematic reviews (see Assessment group assessment of the companies’ systematic review of cost-effectiveness evidence) and the companies’ economic analyses (see Assessment group summary and critique of companies’ economic analyses). The companies’ cost-effectiveness results are presented and discussed by the AG in section Eisai and Merck Sharp & Dohme cost-effectiveness results.
Assessment group review of cost-effectiveness evidence
Assessment group search strategy
The AG searched the electronic sources listed in Appendix 2, Table 51. Full search strategies are presented in Appendix 2. As lenvatinib was first approved for the treatment of aRCC by the FDA in 2016, the AG considered that searching databases from 2006 onwards would allow all relevant economic evidence to be identified. In addition, the reference lists of all included publications were assessed for relevance. The results of the searches were entered into an Endnote (X9 software package63) library, de-duplicated, and then exported into Covidence Systematic Review software. 64
Assessment group study selection and inclusion criteria
Records were selected for inclusion in the review on the basis of the criteria shown in Table 24. The criteria were developed to ensure that the included studies would provide information to help address the AG decision problem which aligns to the final scope29 issued by NICE, that is to assess the cost-effectiveness of treatment with lenvatinib plus pembrolizumab for patients with untreated aRCC versus treatment with sunitinib, pazopanib, tivozanib, cabozantinib and nivolumab plus ipilimumab.
| Criteria | Inclusion criteria |
|---|---|
| Limits | Studies published from 2006 to present; English language only |
| Population | Adults with untreated aRCC |
| Study design | Full economic evaluations that consider both costs and consequences (CEA, cost–utility analysis, cost-minimisation analysis and cost–benefit analysis) |
| Intervention | Lenvatinib + pembrolizumab |
| Comparators | Sunitinib Pazopanib Tivozanib Cabozantinib (only for intermediate-/poor-risk disease as defined in IMDC criteria) Nivolumab with ipilimumab (only for intermediate-/poor-risk disease as defined by IMDC criteria) |
| Costs | Direct healthcare costs |
| Outcomes | Incremental cost per life-year gained and/or incremental cost per quality-adjusted life-years gained |
Two reviewers (RH/DB) independently screened the titles and abstracts of all records identified by the searches. Full-text versions of all studies considered potentially relevant were obtained. The same two reviewers then independently assessed the relevance of these full-text publications and reasons for exclusion were assigned based on the hierarchical order as shown in Table 24. Disagreements about inclusion were resolved through discussion and, in all cases, a consensus was reached.
Quantity of cost-effectiveness evidence
The AG searches identified 3127 records. Of these, 2742 records were obtained from the database searches and 385 records were identified from other sources, that is from conference proceedings (n = 129) and website searches (n = 256). After duplicates were removed, 1899 records remained. Following screening of titles and abstracts, 47 full-text publications were retrieved (one potentially relevant report could not be retrieved) and checked for eligibility using prespecified inclusion criteria. The AG study selection process is shown in the section Sources searched of Appendix 2, Figure 3.
Included study
Only one cost-effectiveness study117 was included in the AG review. Using this study, forward citation searches were carried out; however, no additional studies were identified. As the included study was published in 2021, this was to be expected.
Excluded studies
In total, 46 reports were excluded from the review at the full-text stage. Reasons for exclusion were wrong population (n = 4), wrong study design (n = 15), wrong intervention (n = 25) and duplicate publications (n = 2).
Assessment group data extraction
A data extraction form was designed in MS Excel. Extracted data included bibliographic information (e.g. authors and title) and details of the type of analyses conducted. Details about the economic model were also extracted (e.g. parameters used and their sources, results of the analyses, authors’ conclusions and limitations reported by the authors). Information from the included study was extracted independently by two reviewers (RH/DB).
Quality of cost-effectiveness evidence
The AG assessed the quality of the included cost-effectiveness study (i.e. Li et al. 2021117) using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist118 (see Table 75 in Appendix 5). Two reviewers (RH/DB) independently carried out the quality assessment. The reviewers agreed that, except for resource use items, the included study117 had transparently reported the methods used to conduct their CEA.
Key information from the included cost-effectiveness study
The data extracted by the AG from the included cost-effectiveness study117 are provided in Table 74 in Appendix 5.
The cost-effectiveness results generated by Li et al. 2021117 showed that lenvatinib plus pembrolizumab generated more life-years (LYs) and more quality-adjusted life-years (QALYs) in comparison to sunitinib. However, incremental costs were high and the base-case incremental cost-effectiveness ratio (ICER) for this comparison was more than US$100,000 per QALY – a level that the authors report is an acceptable willingness to pay (WTP) threshold.
Assessment group systematic review conclusions
The Li et al. 2021117 cost-effectiveness study included estimates of the comparative cost-effectiveness of lenvatinib plus pembrolizumab versus sunitinib. However, the study was undertaken from the perspective of the US healthcare system and, therefore, the extent to which resource use and results are generalisable to the NHS is unclear. Further, the study was limited to the all-risk population and included comparators that are not recommended by NICE for patients with untreated aRCC.
Assessment group assessment of the companies’ systematic review of cost-effectiveness evidence
The searches for cost-effectiveness studies carried out by Eisai and MSD were very similar. The AG appraisal of the review methods described by the authors was based on information provided in the Eisai1 and MSD2 CSs.
The date span for both of the companies’ searches was from the inception of relevant databases to the date on which the searches were conducted. Both first searches were carried out in March 2019 and both companies conducted an updated search in January 2021. No relevant studies were identified. As the companies’ searches were last updated in January 2021, the only cost-effectiveness study included in the AG review was not identified.
The AG assessed the companies’ literature review using the LRiG in-house systematic review checklist, and details of this assessment are provided in Appendix 6, Table 76.
The AG considers that the companies used appropriate methods to identify potentially relevant cost-effectiveness studies for inclusion in their systematic reviews. However, the final searches were undertaken in January 2021, and therefore the cost-effectiveness study117 included in the AG systematic review was not identified.
Assessment group summary and critique of companies’ economic analyses
Assessment group summary of companies’ economic models
Key information about the models submitted by the companies is presented in Table 25.
| Parameter | Eisai CS | MSD CS |
|---|---|---|
| Type of economic evaluation | Cost–utility analysis | Cost–utility analysis |
| Population | People with untreated aRCC Subgroups: intermediate/poor risk |
People with untreated aRCC Subgroups: intermediate/poor risk and favourable riska |
| Intervention(s) and comparator(s) | Pembrolizumab in combination with: Lenvatinib Sunitinib Pazopanib Tivozanib Cabozantinib (only for intermediate- or poor‑risk disease as defined in the IMDC criteria) |
Pembrolizumab in combination with: Lenvatinib Sunitinib Pazopanib Tivozanib Cabozantinib (only for intermediate‑ or poor-risk disease as defined in the IMDC criteria) |
| Model structure | Partitioned survival model | Partitioned survival model |
| Health states | PFS, PPS, OS | PFS (on and off treatment), PPS (on and off treatment), OS |
| Time horizon | 40 years | 40 years |
| Cycle length | 7 days | 7 days |
| Discount rates for costs and benefits | 3.5% | 3.5% |
| Perspective used (country, healthcare system, societal) | NHS and Personal Social Services perspective | NHS and Personal Social Services perspective |
| Sources of clinical evidence | CLEAR trial data and Eisai NMA results | CLEAR trial data and MSD NMA results |
| Sources of utilities evidence | CLEAR trial EQ-5D-3L data | CLEAR trial EQ-5D-3L data |
| Sources of costs evidence | Resource use was based on current clinical practice, previous HTAs in advanced/metastatic RCC and published literature; unit costs were informed by recognised national databases | Resource use was based on current clinical practice, previous HTAs in advanced/metastatic RCC and published literature; unit costs were informed by recognised national databases |
| Currency used | GBP 2019–20 | GBP 2019–20 |
Critical appraisal of the companies’ economic analyses
The AG critical appraisal of the companies’ economic analyses was carried out using the Drummond checklist (see Appendix 6, Table 77) and the NICE Reference Case checklist (see Appendix 6, Table 78).
Eisai and Merck Sharp & Dohme cost-effectiveness results
Because of the differences in the companies’ modelling approaches, there are differences between the Eisai and MSD cost-effectiveness results. Eisai and MSD pairwise cost-effectiveness results for the intermediate-/poor-risk subgroup are presented in Table 26. MSD pairwise base-case and fully incremental cost-effectiveness results for the favourable-risk subgroup are presented in Tables 27 and 28, respectively. Eisai did not present any cost-effectiveness results for the favourable-risk subgroup.
| Treatment | ICER per QALY gained |
|---|---|
| Eisai | |
| Lenvatinib + pembrolizumab vs. cabozantinib | £118,571 |
| MSD | |
| Lenvatinib + pembrolizumab vs. cabozantinib | £77,730 |
| Treatment | ICER per QALY gained |
|---|---|
| Gamma distribution for comparator OS | |
| Lenvatinib + pembrolizumab vs. sunitinib | £354,839 |
| Lenvatinib + pembrolizumab vs. pazopanib | £359,052 |
| Lenvatinib + pembrolizumab vs. tivozanib | £350,580 |
| Weibull distribution for comparator OS | |
| Lenvatinib + pembrolizumab vs. sunitinib | £225,227 |
| Lenvatinib + pembrolizumab vs. pazopanib | £227,898 |
| Lenvatinib + pembrolizumab vs. tivozanib | £222,527 |
| Treatment | ICER per QALY gained |
|---|---|
| Gamma distribution for comparator OS | |
| Pazopanib | |
| Sunitinib | Sunitinib dominated by pazopanib |
| Tivozanib | Tivozanib dominated by pazopanib |
| Lenvatinib + pembrolizumab | £357,332 |
| Weibull distribution for comparator OS | |
| Pazopanib | |
| Sunitinib | Sunitinib dominated by pazopanib |
| Tivozanib | Tivozanib dominated by pazopanib |
| Lenvatinib + pembrolizumab | £229,186 |
Assessment group economic evaluation and description of company models
The Eisai and MSD CSs to NICE included economic models built in Microsoft Excel. The AG considers that results from both models can be used to inform decision-making; however, in some instances, the companies could have made more appropriate assumptions and parameter choices. The AG has not developed a de novo economic model; instead, the AG has modified the model provided by MSD (referred to in this report from now on as the MSD/AG model). The AG adapted the MSD model to reflect what the AG considered to be the most appropriate assumptions and parameters on the basis of the economic models submitted by both companies (MSD and Eisai). The main reason for modifying the MSD model rather than the Eisai model was that MSD provided cost-effectiveness analyses for the favourable-risk subgroup and, therefore, fewer modifications to this model were needed. Neither of the companies produced cost-effectiveness results for the comparison of lenvatinib plus pembrolizumab versus nivolumab plus ipilimumab (intermediate-/poor-risk subgroup), despite both models having the functionality for this comparison. Furthermore, Eisai did not generate any cost-effectiveness results for the favourable-risk subgroup.
Overview of clinical effectiveness evidence used to populate the models
Direct clinical evidence from the CLEAR trial is available for the comparison of lenvatinib plus pembrolizumab with sunitinib and is the primary source of clinical effectiveness data used to populate the Eisai, MSD and MSD/AG models. The CLEAR trial is a good-quality, phase III, multicentre, open-label RCT. The final analysis of PFS was carried out using data from the IA3 data cut-off (28 August 2020); EQ-5D-3L and TTD data were also reported at this time point. OS data are available from an updated OS analysis (31 March 2021) at which point median OS follow-up was approximately 33 months. At the time of the updated OS analysis, 114 (32.1%) and 49 (13.7%) patients in the lenvatinib plus pembrolizumab and sunitinib arms, respectively, were still receiving their randomised treatment.
For the comparison of lenvatinib plus pembrolizumab with comparator treatments, the AG considered the following three approaches to generate model inputs:
(1) Use direct clinical evidence
Direct clinical evidence is available from the CLEAR trial to allow comparison of the efficacy of lenvatinib plus pembrolizumab with sunitinib.
(2) Use results from NMAs
The PFS and OS NMA results were generated by Eisai, MSD and the AG for the comparison of lenvatinib plus pembrolizumab with sunitinib, pazopanib and tivozanib. However, violations of the PH assumption within some of the studies included within the AG NMAs were observed (see Table 29). As previously stated (see Assessment group assessment of proportional hazards assumptions), when the PH assumption is violated, NMA results (HRs and 95% CrIs) cannot be used to infer any statistically significant difference (or lack of statistically significant difference).
| Risk group | PFS | OS |
|---|---|---|
| Intermediate/poor subgroup | CheckMate 214 trial100 (nivolumab plus ipilimumab vs. sunitinib) | Nonea |
| Favourable subgroup | Unclear if HRs were proportional COMPARZ trial101 information (including final OS analysis105 information) did not include K-M data for this subgroup (pazopanib vs. sunitinib) |
|
| All-risk population | TIVO-1 trial102 (tivozanib vs. sorafenib) |
CLEAR trial (lenvatinib plus pembrolizumab vs. sunitinib) |
(3) Assume clinical equivalence/similarity
Assume that sunitinib, pazopanib and tivozanib are clinically similar and use CLEAR trial sunitinib data to reflect the effectiveness of pazopanib and tivozanib. The assumption that pazopanib and tivozanib have equivalent efficacy to sunitinib is supported by the conclusions reached by NICE ACs,24,25,34,35 namely that sunitinib and pazopanib are of equivalent clinical effectiveness and that ‘At best, tivozanib may have a similar effect to sunitinib or pazopanib’. 34 No robust evidence to dispute these conclusions was generated by the Eisai, MSD or AG NMAs. This assumption was made based on all-risk population data; the AG has, however, assumed that it also holds for the intermediate-/poor-risk and favourable-risk subgroups.
Model structure
The Eisai and MSD economic models are partitioned survival models with the same three health states: preprogression, postprogression and death. The preprogression and postprogression health states in the MSD model also include on-treatment and off-treatment substates. These models use the same structure as models previously submitted to inform NICE appraisals of treatments for untreated aRCC (Figure 1).
The cycle length used in both company models was 1 week. Eisai implemented a half-cycle correction but neither MSD nor the AG considered that this was necessary due to the short cycle length and therefore did not implement a half-cycle correction.
Population characteristics
In the Eisai model, the mean age (61.2 years) and proportion of males (74.5%) reflect the characteristics of all patients recruited to the CLEAR trial (Eisai CS,1 section 5.2.1). In the MSD (and MSD/AG) model, the mean age, proportion of males and weight of patients vary by subgroup and reflect the baseline age, proportion of males, and mean weight of patients in the CLEAR trial who were recruited only from European sites (Table 30).
| Risk groups | Mean age | Proportion males | Weight |
|---|---|---|---|
| Intermediate/poor risk | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Favourable risk | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| All-risk | 61.7 | 74.5% | 81.1 kg |
Prognostic risk subgroups
The IMDC prognostic risk subgroup data are available from the CLEAR trial:
-
intermediate/poor risk (n = 472, 66.3%)
-
intermediate risk (n = 402, 56.5%)
-
poor risk (n = 70, 9.8%)
-
favourable risk (n = 234, 32.9%).
Previous NICE TAs24,25,32–34,36 have produced treatment recommendations for patients with untreated aRCC for the combined intermediate/poor-risk subgroup (TA542,24 TA58125 and TA64535 for use within the CDF; TA78036 which superseded TA58125 for use in routine practice) and all-risk population (TA169,32 TA21533 and TA51234). As some treatments are only available for the intermediate/poor-risk subgroup, the AG considers that cost-effectiveness results for the all-risk population (CLEAR trial FAS/ITT population) are not relevant to this appraisal. The AG has therefore conducted separate CEAs for the intermediate-/poor-risk and favourable subgroups by using relevant comparator data for each subgroup (i.e. intermediate/poor risk: cabozantinib or nivolumab plus ipilimumab; favourable risk: sunitinib, pazopanib or tivozanib). For completeness, cost-effectiveness results for the all-risk population are provided in Appendix 7. As cabozantinib and nivolumab plus ipilimumab are only recommended by NICE for treating patients with intermediate-/poor-risk disease, the AG does not consider that cost-effectiveness results for the poor-risk subgroup only are relevant and so has not generated any cost-effectiveness results for this subgroup.
Intervention and comparator treatments
The intervention is lenvatinib plus pembrolizumab. The comparators listed in the final scope3 issued by NICE are shown in Table 31. For patients with intermediate-/poor-risk disease, clinical advice to the AG is that sunitinib, pazopanib and tivozanib are treatments that are generally reserved for use as later lines of treatment and would only be offered as first-line treatments to patients who were unable to tolerate cabozantinib, nivolumab plus ipilimumab or lenvatinib plus pembrolizumab (if recommended by NICE). Therefore, the AG considers that sunitinib, pazopanib and tivozanib are not relevant comparators for the intermediate-/poor-risk subgroup.
| Subgroup | Comparators |
|---|---|
| Intermediate/poor risk | Cabozantinib Nivolumab plus ipilimumab |
| Favourable risk | Sunitinib Pazopanib Tivozanib |
Eisai and MSD did not include nivolumab plus ipilimumab as a comparator (Eisai CS,1 table 1; MSD CS,2 table 1). However, as nivolumab plus ipilimumab is a comparator listed in the final scope29 issued by NICE, the AG has included it as a comparator in the MSD/AG model.
Discounting, time horizon and perspective
In line with the NICE Reference Case,119 in the Eisai and MSD (and MSD/AG) models, costs and benefits were discounted at a rate of 3.5%. In the MSD model, discounting was incorrectly applied from the first cycle; in the MSD/AG model, this error was corrected and discounting now starts at the beginning of the second year. Scenario analyses were performed by the AG using annual discount rates of 0% and 6% for costs and benefits.
The time horizon used in the Eisai, MSD and MSD/AG models is 40 years. The AG considers that this is sufficient to capture all relevant costs and benefits. The perspective of all three models is the NHS and personal and social services (PSS).
Populating the model with clinical effectiveness data: general methods
Direct clinical effectiveness evidence (PFS, OS and TTD) is only available from the CLEAR trial for the comparison of the efficacy of lenvatinib plus pembrolizumab with sunitinib.
In line with DSU guidance,120 Eisai, MSD and the AG assessed the goodness-of-fit to PFS, OS and TTD K-M data of standard distributions (exponential, gamma, generalised gamma, Gompertz, log-logistic, log-normal, Weibull) using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) statistics. The distribution producing the lowest AIC and BIC statistics is considered the best fitting (i.e. highest ranking); however, Eisai suggests that other distributions may be as good as the highest-ranking distribution. The AG highlights that, for PFS and OS, Eisai only provided AIC and BIC statistics for the all-risk population.
As well as the visual fit of the seven distributions to the K-M data, the AG assessed the:
-
clinical plausibility of long-term projections (i.e. whether the mortality rate rapidly fell below background mortality)
-
whether the distribution used to model PFS led to higher mortality than the distribution chosen to model OS
-
whether survival projections for the intermediate-/poor-risk subgroup were more/less optimistic than those for the favourable-risk subgroup.
Populating the Merck Sharp & Dohme/assessment group model: progression-free survival
Eisai and MSD fitted distributions to CLEAR trial BICR assessed PFS data (FDA censoring rules). The PFS distributions chosen by Eisai, MSD and the AG are shown in Table 32. The PFS distributions chosen by the AG cannot be shown graphically as these data are confidential.
| Treatment | Eisai | MSD | AG |
|---|---|---|---|
| Modelling | |||
| Intermediate-/poor-risk subgroup | |||
| Lenvatinib plus pembrolizumab | Exponential | ||
| Cabozantinib | Eisai NMA result: LEN + PEM vs. cabozantinib HR = Confidential information has been removed |
MSD NMA result: first-order FP model | AG NMA result: LEN + PEM vs. cabozantinib HR = Confidential information has been removed |
| Nivolumab plus ipilimumab | No results generated | AG NMA result: LEN + PEM vs. nivolumab plus ipilimumab HR = Confidential information has been removed | |
| Favourable-risk subgroup | |||
| Lenvatinib plus pembrolizumab | No results generated | Generalised gamma | |
| Sunitinib | Log-normal | ||
| Pazopanib/tivozanib | Equal to sunitinib | ||
Intermediate-/poor-risk subgroup (progression-free survival)
Lenvatinib plus pembrolizumab
All the MSD AIC statistics for the distributions fitted to CLEAR trial lenvatinib plus pembrolizumab data lie within five AIC points of each other. The AIC statistics and distributions fitted to the CLEAR trial cannot be shown as these data are confidential. Eisai and MSD chose to model PFS using similar exponential distributions. The AG considered that it was appropriate to use the exponential distribution with the parameters estimated by MSD.
Cabozantinib and nivolumab plus ipilimumab
Eisai and MSD used results from their respective PFS NMAs and applied these to their chosen lenvatinib plus pembrolizumab distribution to generate results for lenvatinib plus pembrolizumab versus cabozantinib. No NMA results were presented by Eisai or MSD for the comparison of lenvatinib plus pembrolizumab with nivolumab plus ipilimumab.
For the comparison of lenvatinib plus pembrolizumab with cabozantinib, the AG adopted the same approach as Eisai and MSD and applied the HR generated by the AG PFS NMA (lenvatinib plus pembrolizumab vs. cabozantinib) to the distribution chosen for lenvatinib plus pembrolizumab. For the comparison of lenvatinib plus pembrolizumab with nivolumab plus ipilimumab, the AG applied the HR generated by the AG PFS NMA (lenvatinib plus pembrolizumab vs. nivolumab plus ipilimumab) to the distribution chosen for lenvatinib plus pembrolizumab.
Eisai NMAs did not include data from the CheckMate 214 trial;100 nevertheless, the Eisai and AG NMA results were very similar for the comparison of lenvatinib plus pembrolizumab with cabozantinib. This suggests that the AG PFS NMA results (lenvatinib plus pembrolizumab vs. cabozantinib) are not substantially affected by the inclusion of data from the CheckMate 214 trial. 100 As shown in Table 29 the CheckMate 214 trial100 PFS PH assumption is violated; this means that the CheckMate 214 trial100 PFS HR is not applicable to all time points across the observed follow-up period. Therefore, the AG PFS NMA HRs are not applicable to all time points across the observed follow-up of the trials included in the NMAs.
Favourable-risk subgroup (progression-free survival)
Eisai did not generate any cost-effectiveness estimates for the favourable-risk subgroup.
Lenvatinib plus pembrolizumab
MSD chose the generalised gamma distribution to model PFS for patients receiving lenvatinib plus pembrolizumab (ranked 5/7 using AIC statistics). The distributions cannot be shown as these data are confidential. The generalised gamma distribution’s AIC statistic lies within five points of the AIC statistic for the highest-ranking distribution. The AG agrees with MSD that the higher-ranking distributions are either a poor visual fit to the PFS K-M data for patients receiving lenvatinib plus pembrolizumab or produce unrealistic long-term extrapolations, that is patients either progress very rapidly or experience very little progression. The generalised gamma distribution, on visual inspection, seemed to offer long-term projections that were clinically plausible; the AG therefore considered that the generalised gamma distribution was an appropriate choice of distribution to use in the base-case analysis.
Sunitinib (pazopanib and tivozanib)
MSD chose the distribution with the lowest AIC statistic (log-normal) to model PFS for patients in the favourable-risk subgroup receiving sunitinib, pazopanib and tivozanib. On the basis of the AIC statistic, there is little to choose between the alternative distributions. The distributions fitted to the CLEAR trial cannot be shown as these data are confidential. The AG considered that because the log-normal distribution was the highest-ranking distribution based on AIC and BIC statistics and was a good visual fit to sunitinib CLEAR trial PFS K-M data as well as the long-term projections appeared clinically plausible, the log-normal distribution was an appropriate choice to use in the base-case analysis.
Assessment group scenario analyses: intermediate-/poor-risk and favourable-risk subgroups (progression-free survival)
Intermediate-/poor-risk subgroup
The AG explored the effect on cost-effectiveness results of using the parametric distributions that had AIC statistics that were within five points of the AIC statistic for the distribution used to model PFS for patients treated with lenvatinib plus pembrolizumab; distributions for cabozantinib and nivolumab plus ipilimumab changed automatically.
The AG also explored the effect on cost-effectiveness results of using the MSD FP NMA results to model PFS for patients treated with cabozantinib PFS.
Favourable-risk subgroup
The AG explored the effect on cost-effectiveness results of using the parametric distributions that had AIC statistics that were within five points of the AIC statistic for the distribution used to model PFS for patients treated with lenvatinib plus pembrolizumab; distributions for sunitinib, pazopanib and tivozanib were unchanged.
The AG explored the effect on cost-effectiveness results of using the parametric distributions that had AIC statistics that were within five points of the AIC statistic for the distribution used to model PFS for patients treated with sunitinib (pazopanib and tivozanib); distributions for lenvatinib plus pembrolizumab were unchanged.
Populating the Merck Sharp & Dohme/assessment group model: overall survival
The distributions chosen by Eisai, MSD and the AG for OS are shown in Table 33. The OS distributions chosen by the AG cannot be shown graphically as these data are confidential.
| Treatment | Eisai | MSD | AG |
|---|---|---|---|
| Intermediate/poor risk | |||
| Lenvatinib plus pembrolizumab | Exponential | Exponential | K-M + exponential |
| Cabozantinib | Eisai NMA: LEN + PEM vs. cabozantinib HR = Confidential information has been removed | MSD NMA: first-order FP model |
AG NMA: LEN + PEM vs. cabozantinib HR = Confidential information has been removed |
| Nivolumab plus ipilimumab | No results presented | AG NMA: LEN + PEM vs. nivolumab plus ipilimumab HR = Confidential information has been removed |
|
| Favourable risk | |||
| Lenvatinib plus pembrolizumab | No results presented | Exponential | Log-logistic |
| Sunitinib | Gamma or Weibulla | Gamma | |
| Pazopanib | Equal to sunitinib | Equal to sunitinib | |
| Tivozanib | Equal to sunitinib | Equal to sunitinib | |
Intermediate-/poor-risk subgroup (overall survival)
Lenvatinib plus pembrolizumab
Both companies chose the exponential distribution (ranked 6/7 using AIC statistics) to estimate OS for patients in the intermediate/poor risk subgroup receiving lenvatinib plus pembrolizumab despite this not being the highest-ranking distribution based on AIC statistics or within five points of the highest-ranking distribution. Their choice was based on good visual fit to CLEAR trial OS K-M data and the fact that higher ranking distributions generated implausible long-term OS estimates.
Although the AG was satisfied that the companies followed DSU guidance,120 the AG did not consider that any of the distributions considered by Eisai or MSD provided a good visual fit to the available CLEAR trial OS K-M data. The AG examined the CLEAR trial OS K-M data received during the NICE appraisal clarification process and observed that the lenvatinib plus pembrolizumab OS hazard was constant beyond 50 weeks. The AG therefore considered that the companies’ choice of an exponential distribution was appropriate, but that K-M data should be used up to the point that censoring and small numbers of events rendered the data too uncertain (the AG considered that this occurred at 120 weeks). The AG appended the exponential distribution (based on the hazard between 50 and 150 weeks) to the CLEAR trial OS K-M data from 120 weeks onwards.
Cabozantinib and nivolumab plus ipilimumab
For the comparison of lenvatinib plus pembrolizumab with cabozantinib, Eisai and MSD applied the HRs generated by their OS NMAs (lenvatinib plus pembrolizumab vs. cabozantinib) to the OS distributions chosen for lenvatinib plus pembrolizumab.
For the comparison of lenvatinib plus pembrolizumab with cabozantinib, the AG applied the HR generated by the AG OS NMA (lenvatinib plus pembrolizumab vs. cabozantinib) to the OS distribution chosen for lenvatinib plus pembrolizumab.
No NMA results were presented by Eisai or MSD for the comparison between lenvatinib plus pembrolizumab and nivolumab plus ipilimumab.
For the comparison of lenvatinib plus pembrolizumab with nivolumab plus ipilimumab, the AG applied the HR generated by the AG OS NMA (lenvatinib plus pembrolizumab vs. nivolumab plus ipilimumab) to the distribution chosen for lenvatinib plus pembrolizumab.
As described in Overall survival: assessment group fixed-effects network meta-analysis, the AG concluded that, for the intermediate-/poor-risk subgroup, the OS PH assumption was not violated in the CLEAR trial or either of the two other trials97,100 included in the AG OS NMA.
Favourable-risk subgroup (overall survival)
For patients in the favourable-risk subgroup, there was considerable uncertainty around the validity of the CLEAR trial OS estimates due to the low number of events experienced by these patients.
Lenvatinib plus pembrolizumab
MSD chose the exponential distribution (ranked 7/7 using AIC statistics) to model OS for patients treated with lenvatinib plus pembrolizumab. The AG considered that the exponential distribution generated OS estimates that were too optimistic and was a poor fit to the CLEAR trial OS K-M data. The AG considered that survival in the favourable-risk subgroup should be no worse than survival in the intermediate-/poor-risk subgroup. Four of the seven distributions considered by MSD (i.e. Gompertz, generalised gamma, Weibull and gamma) produced 10-year survival estimates that were above the AG 10-year survival estimates for the intermediate-/poor-risk subgroup. The AG therefore chose the Log-logistic distribution which was the highest-ranking, based on AIC and BIC statistics, of the four distributions that the AG considered clinically plausible.
Sunitinib (pazopanib and tivozanib)
To model OS for patients in the favourable-risk subgroup who received sunitinib, MSD used two distributions (gamma and Weibull) that they considered were equally plausible.
During the NICE appraisal clarification process, MSD provided CLEAR trial OS K-M and HR data that suggested improved survival for patients in the sunitinib arm versus patients in the lenvatinib plus pembrolizumab arm. Similarly, AG OS NMA results suggested improved survival for patients treated with sunitinib versus patients treated with lenvatinib plus pembrolizumab (although the difference was not statistically significant). The MSD model predicted a survival benefit that was greater for patients treated with lenvatinib plus pembrolizumab than for patients treated with sunitinib. As the CLEAR trial evidence does not support such a benefit, a benefit should not be modelled.
Given the AG’s chosen survival distribution for lenvatinib plus pembrolizumab, the AG considered that the gamma distribution was the appropriate distribution to use to model OS for patients treated with sunitinib (and therefore also for patients treated with pazopanib and tivozanib). The gamma distribution was the highest-ranking distribution, based on AIC and BIC statistics, that produced survival estimates that were consistent with a sustained survival benefit for patients treated with sunitinib versus patients treated with lenvatinib plus pembrolizumab while not producing implausibly long survival estimates.
Assessment group scenario analyses: intermediate-/poor-risk and favourable-risk subgroups (overall survival)
Intermediate-/poor-risk subgroup
The AG carried out scenario analyses that employed Eisai and MSD base-case approaches to modelling OS:
-
Use the exponential distribution (Eisai and MSD preferred distribution) to model OS for lenvatinib plus pembrolizumab.
-
Apply Eisai and MSD OS NMA HRs to the AG lenvatinib plus pembrolizumab distribution to generate cabozantinib OS estimates.
-
Apply the MSD FP NMA HR to the AG lenvatinib plus pembrolizumab distribution to generate cabozantinib OS estimates.
The AG OS NMA HRs for the comparison of lenvatinib plus pembrolizumab with nivolumab plus ipilimumab and for the comparison of lenvatinib plus pembrolizumab with cabozantinib were not statistically significantly different from 1. The AG, therefore, carried out a scenario analysis using a HR equal to 1 for the comparison of lenvatinib plus pembrolizumab with nivolumab plus ipilimumab and for the comparison of lenvatinib plus pembrolizumab with cabozantinib (i.e. the OS distributions for nivolumab plus ipilimumab and for cabozantinib were assumed to be the same as that for lenvatinib plus pembrolizumab).
Favourable-risk subgroup
The AG carried out a scenario analysis using the AG OS NMA HR for the comparison of lenvatinib plus pembrolizumab with sunitinib applied to the log-logistic distribution used to represent OS for patients treated with lenvatinib plus pembrolizumab in the AG base case.
As the AG NMA OS HR for the comparison of lenvatinib plus pembrolizumab with sunitinib was not statistically significantly different from 1, the AG carried out a scenario analysis using an OS HR = 1 (i.e. the OS distribution for sunitinib was assumed to be the same as that for lenvatinib plus pembrolizumab).
In two other scenarios, the AG used an OS HR = 1 for the comparison of lenvatinib plus pembrolizumab with pazopanib and with tivozanib.
Populating the model: time to treatment discontinuation
The parametric distributions chosen by Eisai, MSD and the AG to model TTD for all treatments are shown in Table 34. The TTD distributions chosen by the AG cannot be shown graphically as these data are confidential.
| Treatment | Eisai | MSD | AG |
|---|---|---|---|
| Intermediate-/poor-risk subgroup | |||
| Lenvatinib | Generalised gamma | Generalised gamma | Generalised gamma (Eisai) |
| Pembrolizumab | Weibull | K-M data (CLEAR trial data are complete) | |
| Cabozantinib | Generalised gamma | MSD NMA: first-order FP model | Log-logistic (Eisai) |
| Nivolumab plus ipilimumab | Not estimated | Set equal to lenvatinib | |
| Favourable-risk subgroup | |||
| Lenvatinib | Not estimated | Exponential | |
| Pembrolizumab | K-M data (CLEAR trial data are complete) | ||
| Sunitinib | Exponential | ||
| Pazopanib | Equal to sunitinib | ||
| Tivozanib | Equal to sunitinib | ||
Intermediate-/poor-risk subgroups (time to treatment discontinuation)
The AG considered that TTD for patients receiving lenvatinib should be modelled by fitting a distribution to CLEAR trial TTD K-M data, and for patients receiving pembrolizumab, the CLEAR trial TTD K-M data should be used directly.
Lenvatinib
Eisai and MSD provided CLEAR trial lenvatinib TTD K-M data during the NICE appraisal clarification process. However, the two data sets differed slightly (within 24 months there was a clear gap between the two data sets). The AG concluded that as safety data from the CLEAR trial suggested a lower level of treatment discontinuation due to lenvatinib than due to pembrolizumab (25.6% vs. 28.7%66), the Eisai lenvatinib TTD K-M data were likely to be the most accurate as they followed a trajectory that was consistently above the pembrolizumab TTD K-M data until 24 months, that is until the time when the pembrolizumab stopping rule was activated. In contrast, the MSD lenvatinib TTD K-M data crossed the pembrolizumab TTD K-M data at 20 months.
Both companies chose to use generalised gamma distributions to model TTD for patients treated with lenvatinib [this was the highest-ranking distribution using AIC statistics (MSD CS2)]. The distributions considered by MSD and the AG cannot be shown visually against the CLEAR trial PFS-K-M data as these data are confidential. The AG considered that the Eisai generalised gamma distribution provided a good visual fit to lenvatinib TTD K-M data and did not cross the pembrolizumab TTD K-M data until 24 months. The AG therefore chose to use Eisai’s generalised gamma distribution to model lenvatinib K-M TTD data.
Pembrolizumab
The MSD modelled pembrolizumab TTD by directly using the K-M data from the CLEAR trial and applied a 2-year stopping rule in line with the CLEAR trial protocol. Eisai modelled pembrolizumab TTD by fitting a Weibull distribution to the CLEAR trial K-M data; it is clear from the Eisai model outputs that a stopping rule for pembrolizumab at 2 years had been applied. The CLEAR trial pembrolizumab TTD K-M data are almost complete and so the AG used the TTD K-M data directly to estimate the cost of treatment with pembrolizumab for patients in the intermediate-/poor-risk subgroup. As the AG used the K-M data directly, an enforced 2-year stopping rule was not implemented; however, this did mean that some patients remained on pembrolizumab for a short period of time beyond 2 years.
Cabozantinib
The MSD modelled cabozantinib TTD using results from their FP TTD NMA. Eisai digitised the (intermediate-/poor-risk subgroup) cabozantinib TTD K-M data used to inform NICE TA54224 and selected a distribution based on AIC and BIC statistics, visual fit and clinical plausibility. The distributions considered by Eisai and the AG cannot be shown visually as these data are confidential. The generalised gamma distribution was not the highest-ranking distribution based on AIC statistics or BIC statistics. However, the generalised gamma distribution based on AIC statistics was within five points of the lowest AIC statistics (log-logistic distribution). In addition, the generalised gamma distribution was the same distribution as the one Eisai used to model TTD for patients receiving lenvatinib, which has a similar mode of action as cabozantinib.
The AG considered that the Eisai approach to modelling cabozantinib TTD was more robust than the MSD approach. While the Eisai approach was essentially a naïve between-trial analysis, the AG considered that Eisai’s transparent approach was preferable to the largely arbitrary parameterisation of MSD’s FP TTD model. All six distributions assessed by Eisai had AIC statistics that were within five points of each other, were broadly similar in terms of visual fit and generated similar long-term estimates. The AG chose to use the log-logistic distribution as this was the distribution with the lowest AIC statistic.
Nivolumab plus ipilimumab
Nivolumab plus ipilimumab TTD K-M data from the CheckMate 214 trial100 are not in the public domain. The AG considered using pembrolizumab CLEAR trial TTD K-M data to model TTD for patients treated with nivolumab plus ipilimumab as both treatments are immunotherapies. However, the effect of the pembrolizumab 2-year stopping rule on TTD data is unclear. Therefore, in the absence of an alternative data source, the AG used the approach that was used to model TTD for patients treated with lenvatinib (generalised gamma distribution) to model TTD for patients treated with nivolumab plus ipilimumab.
In the MSD/AG model, treatment with ipilimumab was restricted to four cycles, that is, it was stopped at 12 weeks (in line with information provided in the nivolumab plus ipilimumab appraisal52).
Favourable-risk subgroup
Of the two companies, only MSD provided cost-effectiveness results for the favourable-risk subgroup.
Pembrolizumab
The CLEAR trial pembrolizumab TTD K-M data are complete. Therefore, MSD and the AG used pembrolizumab TTD K-M data directly in the MSD and MSD/AG models to estimate the cost of treatment with pembrolizumab for the favourable-risk subgroup. MSD applied a 2-year stopping rule in line with the CLEAR trial protocol. The AG used the TTD K-M data directly to estimate the cost of treatment with pembrolizumab for patients in the favourable-risk subgroup. As the AG used the K-M data directly, an enforced 2-year stopping rule was not fully implemented; some patients remained on pembrolizumab for a short period of time beyond 2 years.
Lenvatinib, sunitinib, pazopanib and tivozanib
MSD fitted exponential distributions to the lenvatinib and sunitinib CLEAR trial TTD K-M data; these were the highest-ranking distributions based on AIC statistics and BIC statistics. The distributions considered by MSD and the AG cannot be shown visually against the CLEAR trial TTD-K-M data as these data are confidential. MSD and the AG used these distributions to model TTD for patients treated with lenvatinib and sunitinib as they were also a good visual fit to the CLEAR trial TTD K-M data. MSD and the AG assumed that TTD for patients treated with pazopanib and tivozanib was the same as TTD for patients treated with sunitinib.
Assessment group scenario analyses: intermediate-/poor-risk and favourable-risk subgroups (time to treatment discontinuation)
Intermediate-/poor-risk subgroup
The AG explored the effect on cost-effectiveness results of using the parametric distributions that had AIC statistics that were within five points of the AIC statistic for the distribution used to model TTD for patients receiving lenvatinib. The cabozantinib distribution was unchanged and the nivolumab plus ipilimumab distribution automatically updated as it was the same as the lenvatinib TTD distribution.
The AG explored the effect on cost-effectiveness results of using alternative parametric distributions (i.e. the five distributions that had not been used in the AG base-case analysis) to model TTD for patients treated with cabozantinib. The distribution for lenvatinib, and consequently for nivolumab plus ipilimumab, was unchanged.
The AG explored the effect on cost-effectiveness results of using the MSD TTD FP NMA results applied to the AG TTD lenvatinib distribution to model TTD for patients treated with cabozantinib.
The AG explored the effect on cost-effectiveness results of using the distribution used in the base case to model TTD for patients treated with pembrolizumab (Weibull) to model TTD for patients treated with nivolumab plus ipilimumab.
Favourable-risk subgroup
The AG explored the effect on cost-effectiveness results of using the parametric distributions that had AIC statistics that were within five points of the AIC statistic for the distribution used to model TTD for patients treated with lenvatinib; distributions for sunitinib, pazopanib and tivozanib were unchanged.
The AG explored the effect on cost-effectiveness results of using the parametric distributions that had AIC statistics that were within five points of the AIC statistic for the distribution used to model TTD for patients treated with sunitinib and consequently for patients treated with pazopanib and tivozanib. The distribution for lenvatinib was unchanged.
Utility values
Eisai and MSD used EQ-5D-3L data (IA3 data cut-off) collected as part of the CLEAR trial to estimate utility values. In the CLEAR trial, the EQ-5D-3L questionnaire was administered at baseline (prior to first dose) on day 1 of each subsequent cycle until treatment discontinuation, at the discontinuation visit, at time of withdrawal and at the off-treatment visit (i.e. within 30 days of the final dose of study treatment). Thus, the data used to inform postprogression utility values were limited. The UK scoring functions were developed based on the time trade-off technique. Values were calculated using safety population data, but were not calculated for the different risk subgroups.
Eisai used the health state utility value approach, with treatment-specific utilities in the progression-free health state; CLEAR trial data showed that the utility values for patients treated with lenvatinib plus pembrolizumab and patients treated with sunitinib utility were statistically significantly different.
MSD used a time-to-death approach in their base case and carried out a scenario that explored the impact on cost-effectiveness results of using the health state utility approach. In the scenario analysis, utility values varied depending on whether the patient was on- or off-treatment.
The AG considered that the MSD time-to-death approach provided the best reflection of the HRQoL of long-term survivors and used this approach in the MSD/AG model. The utility values are confidential and therefore cannot be reported.
Assessment group scenario analyses (utility values)
The AG carried out two scenario analyses. One scenario analysis used the Eisai treatment dependent health state utility values and the other used the MSD treatment independent health state utility values. The utility values are confidential and therefore cannot be reported.
Health state resource use and unit costs
Levels of health state resource use (outpatient consultations, CT scans and blood tests) modelled by Eisai and MSD differed. Eisai implemented the resource use estimates that were used to inform the NICE appraisal of pembrolizumab plus axitinib for untreated aRCC (TA65037), and MSD used the resource estimates that were used to inform the NICE appraisal of cabozantinib for untreated aRCC (TA54224).
Clinical advice to the AG was as follows:
-
An initial CT scan was not necessary as scans would have previously been conducted to determine whether the RCC needed treatment and the disease stage.
-
All patients would have an initial appointment with a consultant, which would include blood tests.
-
Patients would subsequently be seen monthly by a consultant, although, in the longer-term, some patients might be seen less frequently.
-
It was appropriate for resource use to be the same for patients in the preprogression health sate (after the first visit) and patients in the postprogression health state as monitoring remained broadly the same regardless of treatment.
-
The resource use estimates in the MSD economic model appeared too low.
Clinical advice to the AG was that the estimates used by Eisai were a better reflection of clinical practice than the estimates used by MSD; however, all patients would receive a blood test as part of the initial outpatient consultation (Table 35).
| Health state | Resource | Eisai, % | MSD, % | AG, % |
|---|---|---|---|---|
| Progression-free: first week | Outpatient consultation | 100 | 100 | 100 |
| Computed tomography | 0 | 3 | 0 | |
| Blood tests | 0 | 8 | 100 | |
| Progression-free: subsequent weeks | Outpatient | 25 | 8 | 25 |
| Computed tomography | 8 | 3 | 8 | |
| Blood tests | 25 | 8 | 25 | |
| Postprogression | Outpatient | 25 | 8 | 25 |
| Computed tomography | 8 | 3 | 8 | |
| Blood tests | 25 | 8 | 25 |
Eisai, MSD and the AG sourced unit costs for all modelled health state resources from the National Schedule of NHS Costs 2019–20121 (Table 36).
| Resource | Unit cost, £ | HRG | Type of visit | |
|---|---|---|---|---|
| Consultation | First visit | 253.20 | WF01B (service code 370) |
Non-admitted face-to-face attendance First |
| Subsequent visits | 200.20 | WF01A | Non-admitted face-to-face attendance Follow-up |
|
| Computed tomography | 120.55 | RD22Z | Outpatient | |
| Blood test | 1.81 | DAPS03 | Integrated blood services | |
Drug costs
Lenvatinib
Eisai and MSD estimated drug acquisition costs for lenvatinib and pembrolizumab on the basis of dosing schedules for each drug as described in the CLEAR trial protocol. Eisai calculated the cost of lenvatinib using a weighted average cost per mg on the basis of average dose received by CLEAR trial patients, and MSD used weekly CLEAR trial dosing data. These data were provided for the all-risk population and not separately by risk subgroups. Clinical advice to the AG was that dosing was unlikely to vary by risk subgroup.
Lenvatinib tablets are available in two strengths (4 and 10 mg); the cost of a 30-tablet pack is the same irrespective of dose. Clinical advice to the AG was that, in NHS clinical practice, a patient’s dose of lenvatinib varies in line with the CLEAR trial protocol descriptions, that is a patient will start on a dose of 20 mg per day and then their dose will be reduced to 14 mg, then to 10 mg and finally to 8 mg, with reductions ceasing once a level that the patient can tolerate has been reached. Further, clinical advice to the AG was as follows:
-
A dose of 8 mg per day was quite rare as patients unable to tolerate a 10 mg per day dose were unlikely to be able to tolerate an 8 mg per day dose.
-
In the short term, 14 mg per day was the dose that most patients were titrated to from 20 mg.
-
In the longer term, approximately 25% of patients were prescribed a 10 mg per day dose.
As the cost per pack of lenvatinib is the same for a 20 mg per day dose and a 14 mg per day dose, the proportion of people prescribed a 10 mg dose (i.e. one capsule) is important.
The AG has used the weekly lenvatinib CLEAR trial dosing data (available from the MSD model). The AG highlights that after 120 weeks, patient CLEAR trial data are limited and, therefore, are unreliable. The AG estimated the cost of lenvatinib using CLEAR trial data (tablets per week) over the first 120 weeks and, for the remainder of the model timeframe, used the average weekly number of lenvatinib tablets patients received between weeks 94 and 120 (i.e. 6 months prior to the end of the reliable data). This approach meant that use of a relative dose intensity (RDI) multiplier was not relevant.
Pembrolizumab
In the CLEAR trial, treatment with pembrolizumab was available for a maximum of 2 years. On the basis of CLEAR trial data, Eisai and MSD used a RDI multiplier (based on all-risk population data) to account for ‘delays in drug administration’. Eisai and MSD used the same methods to estimate RDI values and therefore it is unclear why the values presented by Eisai and MSD differ. Eisai did not provide the values used in their calculation; however, MSD did provide this detail and the AG was able to verify the MSD RDI value. Therefore, the AG used the MSD value in the MSD/AG model.
Sunitinib
Eisai, MSD and the AG estimated the cost of sunitinib using the CLEAR trial dosing schedule. Eisai and MSD used a RDI multiplier (estimated using CLEAR trial data) to adjust the cost of sunitinib. Eisai used a mean value (confidential information has been removed) and MSD used the published median value of 83.2%. 66 The AG has used the Eisai mean value.
Pazopanib, tivozanib, cabozantinib and nivolumab plus ipilimumab
Eisai and MSD estimated the costs of treatment with pazopanib, tivozanib and cabozantinib using dosing schedules published in the relevant SmPCs (Table 37). Eisai and MSD used RDI multipliers published in previous NICE TAs to adjust the costs of pazopanib (86%), tivozanib (94%) and cabozantinib (94%). The AG considered that the approach followed by the companies were appropriate and used the same dosing schedules and RDI values in the MSD/AG model.
| Regimen | Treatment | Dose per administration | Frequency | Administration method |
|---|---|---|---|---|
| Pembrolizumab plus lenvatinib | Pembrolizumab | 200 mg | Every 3 weeks | Intravenous |
| Lenvatinib | Varies | Once daily | Oral | |
| Sunitinib | Sunitinib | 50 mg | Once daily (4 weeks on, 2 weeks off) | Oral |
| Pazopanib | Pazopanib | 800 mg | Once daily | Oral |
| Tivozanib | Tivozanib | 1.34 mg | Once daily (3 weeks on, 1 week off) | Oral |
| Cabozantinib | Cabozantinib | 60 mg | Once daily | Oral |
| Nivolumab plus ipilimumab | Nivolumab | 3 mg/kg | Every 3 weeks (4 doses) | Intravenous |
| Ipilimumab | 1 mg/kg | Every 3 weeks (4 doses) | Intravenous | |
| Nivolumab (monotherapy) | 480 mg | Every 4 weeks | Intravenous |
The AG used the published dosing schedule for nivolumab plus ipilimumab52 (Table 37). No RDI multiplier information was available for nivolumab plus ipilimumab and therefore the AG used the MSD pembrolizumab RDI multiplier (confidential information has been removed), based on CLEAR trial data, to adjust the cost of nivolumab plus ipilimumab.
For all first-line treatments (intervention and comparators), costs per cycle were calculated using published British National Formulary prices (online database) (Table 38).
| Treatment | Milligrams (mg) per unit | Pack size | Cost per pack (£) |
|---|---|---|---|
| Lenvatinib | 10 mg/4 mg | 30 | 1437.00 |
| Pembrolizumab | 100 mg | 1 vial | 2630.00 |
| Sunitinib | 12.5 mg | 28 | 784.70 |
| Pazopanib | 200 mg | 30 | 560.50 |
| Tivozanib | 1.3 mg | 21 | 2052.00 |
| Cabozantinib | 60 mg | 30 | 5143.00 |
| Nivolumab | 240 mg | 1 | 2633.00 |
| Ipilimumab | 50 mg | 1 | 3750.00 |
Drug administration costs
Drug administration costs are presented in Table 39. Eisai and MSD estimated chemotherapy administration costs using the National Schedule of NHS Costs 2019–20 (SB12Z Simple parenteral chemotherapy at first attendance). 121 However, the costs associated with this code differ as Eisai has assumed that administration is an outpatient appointment (£221.35) and MSD has assumed that administration is a day case appointment (£299.61). Clinical advice to the AG is that chemotherapy infusions are delivered as part of an outpatient appointment and, therefore, the AG has used the same administration cost as Eisai (£221.35) for first attendance and SB15Z Deliver Subsequent Elements of a Chemotherapy Cycle for all other attendances (£253.77).
| Drug | Eisai | MSD | AG |
|---|---|---|---|
| Lenvatinib | Assume no administration costs for oral treatments | Deliver exclusively oral chemotherapy (SB11Z) – day case and regular day/night £226.45 Hospital-based staff – pharmacist [Band 6 radiographer – £55 per hour (assumed 12 minutes)] £11.00a |
|
| Pembrolizumab | Deliver simple parenteral chemotherapy at first attendance – outpatient (SB12Z) £221.35 | Simple parenteral chemotherapy at first attendance – day case (SB12Z) £299.61 | Deliver simple parenteral chemotherapy at first attendance (SB12Z) – outpatient £221.35 |
| Sunitinib | Assume no administration costs for oral treatments | Deliver exclusively oral chemotherapy (SB11Z) – day case and regular day/night £226.45 – first cycle only Hospital-based staff – pharmacist [Band 6 radiographer – £55 per hour (assumed 12 minutes)] £11.00a |
|
| Pazopanib | Assume no administration costs for oral treatments | Same as sunitinib | |
| Tivozanib | |||
| Cabozantinib | |||
| Nivolumab | NAb | Deliver complex chemotherapy at first attendance (SB14Z) – outpatient £352.24 (for first 4 cycles when NIV + IPI are delivered jointly) Deliver simple parenteral chemotherapy at first attendance (SB12Z) – outpatient £221.35 (from the fifth cycle – nivolumab maintenance) |
|
| Ipilimumab | |||
Eisai and MSD assumed that the cost of administering oral drugs was zero. The AG considered that this was a conservative assumption and therefore included the cost of the delivery of oral chemotherapy for the first cycle and the cost of a hospital-based pharmacist dispensing the drugs for the subsequent cycles. These assumptions are the same as the assumptions used in TA64535 (Table 39).
As nivolumab and ipilimumab are both intravenous drugs, the AG assumed that for the period patients received both drugs (first four cycles), the most appropriate administration cost was Deliver Complex Chemotherapy at First Attendance (SB14Z) – outpatient. For the subsequent cycles, when patients received only nivolumab, the administration cost used was Deliver Simple Parenteral Chemotherapy at First Attendance (SB12Z) – outpatient.
End-of-life costs
Eisai and MSD models included a fixed cost to cover end-of-life care (applied at death). Both companies used a published cost (inflated to 201920 prices) associated with delivering end-of-life care in hospital (Nuffield Trust report122). MSD also included costs for local authority funded social care, district nursing and GP visits (Nuffield Trust report122); these additional costs were considered relevant during NICE TA54224 and TA650. 37 The AG considered that it was appropriate to include the additional costs associated with end-of-life care and has, therefore, used the MSD end-of-life costs in the MSD/AG model (£8442.02).
Adverse events
Eisai and MSD assumed that the frequency of AEs did not vary by risk subgroup and used all-risk population AE rates for all-risk groups. Clinical advice to the AG was that this approach was appropriate.
Eisai, MSD and the AG estimated the cost of Grade ≥ 3 AEs that occurred in ≥ 5% of patients in either of the CLEAR trial treatment arms. Eisai, MSD and the AG used CLEAR trial AE rates for patients treated with lenvatinib plus pembrolizumab and sunitinib and rates used to inform NICE TAs for patients treated with sunitinib, pazopanib, tivozanib and cabozantinib. For patients treated with nivolumab plus ipilimumab, the AG used CheckMate 214 trial100 AE data.
Eisai carried out a detailed process to estimate AE treatment costs; the approach followed by MSD was much simpler and was largely based on assumptions. The AG was satisfied that the simpler approach followed by MSD was appropriate and has used the MSD AE treatment costs in the MSD/AG model.
Assessment group scenario analysis (adverse events)
The AG carried out two scenario analyses: one in which AE costs were set to zero and one in which AE costs were doubled.
Subsequent treatments
Eisai and MSD relied on expert advice to forecast the specific subsequent treatments that patients would receive and the proportions of patients receiving each of these specific treatments. Eisai estimates of subsequent treatment duration were based on data from the CLEAR trial; MSD relied on expert advice to estimate durations of treatment.
The AG considered that for patients treated with lenvatinib plus pembrolizumab and sunitinib (pazopanib and tivozanib), modelled subsequent treatments should be based on the treatments received by patients in the CLEAR trial. The AG estimated subsequent treatments, for each risk subgroup, separately using IA3 data presented by MSD (CS and response to clarification letter, question B5). Eisai also provided subsequent treatment data in their response to clarification letter, question B5 (updated OS analysis); however, the MSD data were more detailed than the Eisai data and the AG was able to use the MSD data to estimate subsequent treatment costs using a microcosting approach.
On the basis of clinical advice, the AG assumed that 60% of patients treated with cabozantinib would receive subsequent treatment with nivolumab and 40% of patients would receive a VEGFR-TKI, that is sunitinib, pazopanib or tivozanib. The AG assumed that the split between sunitinib, pazopanib and tivozanib was the same as the split for CLEAR trial patients randomised to treatment with lenvatinib plus pembrolizumab who were subsequently treated with a VEGFR-TKI. The duration of treatment with nivolumab was set equal to the average length of time that patients in the sunitinib arm of the CLEAR trial received nivolumab as a subsequent treatment, and the duration of VEGFR-TKI treatment was set equal to the average length of time that patients in the sunitinib arm received a VEGFR-TKI as a subsequent therapy.
For patients treated with nivolumab plus ipilimumab, the AG assumed that subsequent treatments (and the duration of these treatments) were the same as those for CLEAR trial patients randomised to treatment with lenvatinib plus pembrolizumab.
The AG estimated the cost of two lines of subsequent treatment on the basis of treatments received by at least five patients in each arm of the CLEAR trial. Treatments received by fewer than five patients or in the third-line setting were not considered as they were often used off-licence or were only available as part of a clinical trial. The total costs of subsequent treatments were reweighted to account for the cost of treatments received by fewer than five patients. The AG did not consider any subsequent treatments received after the end of the trial period. The AG considers that MSD/AG subsequent treatment costs are likely to be underestimates.
Assessment group sensitivity analyses (subsequent treatment costs)
The AG carried out sensitivity analyses that varied the costs of subsequent treatments by ± 20%.
Assessment group cost-effectiveness results
As the treatment options for the intermediate-/poor-risk and favourable-risk subgroups differ, the cost-effectiveness results for these subgroups should be considered separately. The AG considers that the all-risk population results are not relevant to NHS patients; these results are presented in Appendix 7.
The AG cost-effectiveness results for the intermediate-/poor-risk and favourable-risk subgroups have been estimated using the list prices for the intervention, comparators and subsequent treatment drugs. AG cost-effectiveness results generated using confidential discounted prices are presented in a confidential appendix.
A list of the AG scenarios can be found in Appendix 8. All of the parameters that were varied in the AG sensitivity analysis and probabilistic sensitivity analysis (PSA) are listed in Appendix 9.
Intermediate-/poor-risk subgroup
For the intermediate-/poor-risk subgroup, the AG base-case cost-effectiveness results suggest that treatment with lenvatinib plus pembrolizumab generates more QALYs than treatment with cabozantinib or nivolumab plus ipilimumab but at a greater overall cost (list prices for all drugs). For the comparison of lenvatinib plus pembrolizumab with cabozantinib, the ICER per QALY gained is £133,362, and for the comparison of lenvatinib plus pembrolizumab with nivolumab plus ipilimumab, the ICER per QALY gained is £166,249. Detailed results are presented in Tables 40 and 41.
| Drug | ICER per QALY gained (£) |
|---|---|
| Lenvatinib plus pembrolizumab | – |
| Cabozantinib | 166,249 |
| Nivolumab plus ipilimumab | 133,362 |
| Drug | ICER per QALY gained |
|---|---|
| Cabozantinib | – |
| Nivolumab plus ipilimumab | Extendedly dominated by LEN + PEM |
| Lenvatinib plus pembrolizumab | £166,249 |
Favourable-risk subgroup
For the favourable-risk subgroup, the AG OS NMA results and the CLEAR trial data suggest that treatment with sunitinib generates improved OS compared to treatment with lenvatinib plus pembrolizumab. The AG base-case cost-effectiveness results suggest that treatment with sunitinib generates more QALYs than treatment with lenvatinib plus pembrolizumab at a lower overall cost (list prices for all drugs), that is treatment with lenvatinib plus pembrolizumab is dominated by treatment with sunitinib. Detailed results are presented in Tables 42 and 43.
| Drug | ICER per QALY gained |
|---|---|
| LEM + PEM | – |
| Sunitinib | LEN + PEM is dominated |
| Pazopanib | |
| Tivozanib |
| Drug | ICER per QALY gained |
|---|---|
| Sunitinib | – |
| Pazopanib | Pazopanib is dominated by sunitinib |
| Tivozanib | Tivozanib is dominated by sunitinib |
| LEN + PEM | LEN + PEM is dominated by sunitinib |
Assessment group probabilistic sensitivity analysis results
The AG undertook PSAs using the parameter values and distributions detailed in Appendix 9. For both the intermediate-/poor-risk and favourable-risk subgroups, as the MSD/AG model mean results [ICERs per QALY gained and incremental net monetary benefits (INMBs)] converged by 1000 iterations, the AG calculated cost-effectiveness results generated using 1000 iterations.
Intermediate-/poor-risk subgroup
The mean probabilistic ICERs per QALY gained for the comparison of lenvatinib plus pembrolizumab with cabozantinib (£169,019) and with nivolumab plus ipilimumab (£134,253) are slightly higher than the deterministic cost-effectiveness results. In all iterations, lenvatinib plus pembrolizumab was the most expensive treatment option and generated the most QALYs. At a WTP threshold of £50,000 per QALY gained, in 100% of iterations cabozantinib was the most cost-effective treatment option. At a WTP threshold of £100,000 per QALY gained, in 0.8% of iterations lenvatinib plus pembrolizumab was the most cost-effective treatment option.
Favourable-risk subgroup
The mean probabilistic results were almost identical to the deterministic cost-effectiveness results. Lenvatinib plus pembrolizumab was dominated by sunitinib, pazopanib and tivozanib, and sunitinib was the most cost-effective treatment option. In all iterations, lenvatinib plus pembrolizumab was the most expensive treatment option and generated the fewest QALYs. As the QALYs generated for sunitinib, pazopanib and tivozanib are always the same in each iteration, the cost effectiveness acceptability curve shows horizontal lines for these, that is the probability of any of these three treatments being cost-effective does not vary with the WTP for a QALY threshold. For the majority (85.9%) of iterations, sunitinib was the cheapest option and therefore also the most cost-effective option. In 14.1% of iterations, pazopanib was the cheapest option and therefore the most cost-effective option. Lenvatinib plus pembrolizumab or tivozanib were not the most cost-effective options at any WTP threshold.
Sensitivity and scenario analyses
The AG performed one-way deterministic sensitivity analysis using the upper and lower bounds for all parameter values reported in Appendix 9.
Assessment group one-way deterministic sensitivity analysis results
Intermediate-/poor-risk subgroup
The AG produced tornado diagrams for the comparison of lenvatinib plus pembrolizumab with cabozantinib and with nivolumab plus ipilimumab. The tornado diagrams showed that the INMBs were insensitive across the ranges of input values considered for most model parameters. Cost-effectiveness results were most sensitive to the OS HRs for lenvatinib plus pembrolizumab versus cabozantinib and versus nivolumab plus ipilimumab.
Favourable-risk subgroup
The AG produced tornado diagrams for lenvatinib plus pembrolizumab versus sunitinib, versus pazopanib and versus tivozanib. The tornado diagrams showed that the INMBs were insensitive across the range of input values considered for model parameters; the INMB values never change by more or less than 2%.
Assessment group deterministic scenario analysis results (intermediate-/poor-risk subgroup)
Intermediate-/poor-risk subgroup
The AG has presented deterministic scenario results for the comparison of lenvatinib plus pembrolizumab with cabozantinib (Table 44) and with nivolumab plus ipilimumab (Table 45) for the intermediate-/poor-risk subgroup. The ICERs per QALY gained did not change substantially for most of the scenarios considered. This suggests that the results of the AG analyses were robust over most of the assumptions that were required to construct the MSD/AG model. The ICERs per QALY gained were sensitive to the magnitude of the discount rate but as there are no grounds to move away from using the annual base-case value of 3.5% for costs and benefits, these results are not relevant. The AG considered that the following scenario results were particularly important when determining the cost-effectiveness of lenvatinib plus pembrolizumab versus cabozantinib and versus nivolumab plus ipilimumab:
| AG scenarios Intermediate-/poor-risk subgroup | ICER per QALY gained |
|---|---|
| AG base case | £166,249 |
| Discount rate 6% | £199,613 |
| Discount rate 0% | £122,771 |
| LEN + PEM PFS (gamma) | £166,313 |
| LEN + PEM PFS (generalised gamma) | £166,139 |
| LEN + PEM PFS (Gompertz) | £166,377 |
| LEN + PEM PFS (log-logistic) | £165,725 |
| LEN + PEM PFS (log-normal) | £165,665 |
| LEN + PEM PFS (Weibull) | £166,330 |
| CAB MSD FP PFS HR | £166,248 |
| LEN + PEM OS (exponential) | £143,746 |
| Eisai CABO OS HR | £158,945 |
| MSD CABO FP OS HR | £145,823 |
| CABO OS = LEN + PEM OS | LEN + PEM is dominated |
| LEN + PEM TTD (exponential) | £175,417 |
| LEN + PEM TTD (Gompertz) | £169,392 |
| LEN + PEM TTD (Weibull) | £175,541 |
| MSD LEN + PEM TTD (generalised gamma) | £155,332 |
| Eisai CABO TTD (Weibull) | £186,377 |
| Eisai CABO TTD (log-normal) | £172,583 |
| Eisai CABO TTD (exponential) | £185,941 |
| Eisai CABO TTD (generalised gamma) | £178,656 |
| Eisai CABO TTD (Gompertz) | £181,077 |
| MSD CABO FP TTD HR | £166,249 |
| MSD health state utilities | £174,341 |
| Eisai health state utilities | £170,260 |
| AE costs doubled | £168,187 |
| AE costs set to zero | £163,967 |
| Subsequent treatment costs increased by 20% | £165,702 |
| Subsequent treatment costs decreased by 20% | £167,141 |
| AG scenarios intermediate-/poor-risk subgroup | ICER, £/QALY |
|---|---|
| AG base case | £133,362 |
| Discount rate 6% | £161,647 |
| Discount rate 0% | £98,200 |
| LEN + PEM PFS (gamma) | £133,926 |
| LEN + PEM PFS (generalised gamma) | £132,574 |
| LEN + PEM PFS (Gompertz) | £134,380 |
| LEN + PEM PFS (log-logistic) | £129,201 |
| LEN + PEM PFS (log-normal) | £128,425 |
| LEN + PEM PFS (Weibull) | £134,052 |
| LEN + PEM OS (exponential) | £116,331 |
| LEN + PEM TTD (exponential) | £85,146 |
| LEN + PEM TTD (Gompertz) | £116,143 |
| LEN + PEM TTD (Weibull) | £84,529 |
| MSD LEM + PEM TTD (generalised gamma) | £190,334 |
| MSD health state utilities | £119,761 |
| Eisai health state utilities | £136,597 |
| AE costs doubled | £140,673 |
| AE costs set to zero | £125,817 |
| Subsequent treatment costs increased by 20% | £132,004 |
| Subsequent treatment costs decreased by 20% | £134,954 |
| NIV + IPI = Eisai PEM TTD (Weibull) | LEN + PEM is dominant |
| OS LEM + PEM = OS NIV + IPI | LEN + PEM is dominated |
-
Uncertainty around the choice of PFS distribution or uncertainty around subsequent treatment costs did not noticeably affect cost-effectiveness results for lenvatinib plus pembrolizumab versus cabozantinib or versus nivolumab plus ipilimumab.
-
With the exception of using the MSD FP TTD approach to model TTD for cabozantinib, all the other AG alternative scenarios used to model TTD for lenvatinib plus pembrolizumab or cabozantinib increased the size of the ICER per QALY gained for this comparison.
-
All the AG alternative scenarios used to model TTD for nivolumab plus ipilimumab or for lenvatinib plus pembrolizumab decreased the ICERs per QALY gained for this comparison.
-
Using Eisai or MSD approaches to modelling OS for patients treated with cabozantinib lowers the ICER per QALY gained for lenvatinib plus pembrolizumab versus cabozantinib by 4.4% and 12.3%, respectively; however, the resulting ICERs per QALY gained are still above £145,000. If the OS for patients treated with cabozantinib was the same as the OS for patients treated with lenvatinib plus pembrolizumab, then cabozantinib would dominate lenvatinib plus pembrolizumab.
Assessment group deterministic scenario analysis results (favourable-risk subgroup)
The AG has presented deterministic scenario results for the comparison of lenvatinib plus pembrolizumab with sunitinib (see Table 46), with pazopanib (see Table 47) and with tivozanib (see Table 48) for the favourable-risk subgroup. Lenvatinib plus pembrolizumab was dominated by sunitinib, pazopanib and tivozanib across all scenarios considered.
| AG scenario favourable-risk subgroup | ICER per QALY |
|---|---|
| AG base case | LEN + PEM is dominated by sunitinib |
| Discount rate 6% | LEN + PEM is dominated by sunitinib |
| Discount rate 0% | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (exponential) | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (gamma) | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (Gompertz) | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (log-logistic) | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (log-normal) | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (Weibull) | LEN + PEM is dominated by sunitinib |
| Sunitinib PFS (gamma) | LEN + PEM is dominated by sunitinib |
| Sunitinib PFS (generalised gamma) | LEN + PEM is dominated by sunitinib |
| Sunitinib PFS (log-logistic) | LEN + PEM is dominated by sunitinib |
| Sunitinib PFS (Weibull) | LEN + PEM is dominated by sunitinib |
| AG OS NMA HR for sunitinib | LEN + PEM is dominated by sunitinib |
| OS LEN + PEM = OS sunitinib | LEN + PEM is dominated by sunitinib |
| MSD LEN + PEM TTD (generalised gamma) | LEN + PEM is dominated by sunitinib |
| MSD LEN + PEM TTD (gamma) | LEN + PEM is dominated by sunitinib |
| MSD LEN + PEM TTD (Gompertz) | LEN + PEM is dominated by sunitinib |
| MSD LEN + PEM TTD (log-logistic) | LEN + PEM is dominated by sunitinib |
| MSD LEN + PEM TTD (Weibull) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (gamma) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (generalised gamma) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (Gompertz) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (log-logistic) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (log-normal) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (Weibull) | LEN + PEM is dominated by sunitinib |
| MSD health state utilities | LEN + PEM is dominated by sunitinib |
| AE costs doubled | LEN + PEM is dominated by sunitinib |
| AE costs set to zero | LEN + PEM is dominated by sunitinib |
| Subsequent treatment costs increased by 20% | LEN + PEM is dominated by sunitinib |
| Subsequent treatment costs decreased by 20% | LEN + PEM is dominated by sunitinib |
| AG scenario favourable-risk subgroup | ICER per QALY |
|---|---|
| AG base case | LEN + PEM is dominated by pazopanib |
| Discount rate 6% | LEN + PEM is dominated by pazopanib |
| Discount rate 0% | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (exponential) | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (gamma) | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (Gompertz) | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (log-logistic) | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (log-normal) | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (Weibull) | LEN + PEM is dominated by pazopanib |
| Pazopanib PFS (gamma) | LEN + PEM is dominated by pazopanib |
| Pazopanib PFS (generalised gamma) | LEN + PEM is dominated by pazopanib |
| Pazopanib PFS (log-logistic) | LEN + PEM is dominated by pazopanib |
| Pazopanib PFS (Weibull) | LEN + PEM is dominated by pazopanib |
| AG OS NMA HR for pazopanib | LEN + PEM is dominated by pazopanib |
| OS LEN + PEM = OS pazopanib | LEN + PEM is dominated by pazopanib |
| MSD LEN + PEM TTD (generalised gamma) | LEN + PEM is dominated by pazopanib |
| MSD LEN + PEM TTD (gamma) | LEN + PEM is dominated by pazopanib |
| MSD LEN + PEM TTD (Gompertz) | LEN + PEM is dominated by pazopanib |
| MSD LEN + PEM TTD (log-logistic) | LEN + PEM is dominated by pazopanib |
| MSD LEN + PEM TTD (Weibull) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (gamma) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (generalised gamma) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (Gompertz) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (log-logistic) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (log-normal) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (Weibull) | LEN + PEM is dominated by pazopanib |
| MSD health state utilities | LEN + PEM is dominated by pazopanib |
| AE costs doubled | LEN + PEM is dominated by pazopanib |
| AE costs set to zero | LEN + PEM is dominated by pazopanib |
| Subsequent treatment costs increased by 20% | LEN + PEM is dominated by pazopanib |
| Subsequent treatment costs decreased by 20% | LEN + PEM is dominated by pazopanib |
| AG scenario favourable-risk subgroup | ICER per QALY |
|---|---|
| AG base case | LEN + PEM is dominated by tivozanib |
| Discount rate 6% | LEN + PEM is dominated by tivozanib |
| Discount rate 0% | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (exponential) | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (gamma) | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (Gompertz) | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (log-logistic) | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (log-normal) | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (Weibull) | LEN + PEM is dominated by tivozanib |
| Tivozanib PFS (gamma) | LEN + PEM is dominated by tivozanib |
| Tivozanib PFS (generalised gamma) | LEN + PEM is dominated by tivozanib |
| Tivozanib PFS (log-logistic) | LEN + PEM is dominated by tivozanib |
| Tivozanib PFS (Weibull) | LEN + PEM is dominated by tivozanib |
| AG OS NMA HR for tivozanib | LEN + PEM is dominated by tivozanib |
| OS LEN + PEM = OS tivozanib | LEN + PEM is dominated by tivozanib |
| MSD LEN + PEM TTD (generalised gamma) | LEN + PEM is dominated by tivozanib |
| MSD LEN + PEM TTD (gamma) | LEN + PEM is dominated by tivozanib |
| MSD LEN + PEM TTD (Gompertz) | LEN + PEM is dominated by tivozanib |
| MSD LEN + PEM TTD (log-logistic) | LEN + PEM is dominated by tivozanib |
| MSD LEN + PEM TTD (Weibull) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (gamma) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (generalised gamma) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (Gompertz) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (log-logistic) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (log-normal) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (Weibull) | LEN + PEM is dominated by tivozanib |
| MSD health state utilities | LEN + PEM is dominated by tivozanib |
| AE costs doubled | LEN + PEM is dominated by tivozanib |
| AE costs set to zero | LEN + PEM is dominated by tivozanib |
| Subsequent treatment costs increased by 20% | LEN + PEM is dominated by tivozanib |
| Subsequent treatment costs decreased by 20% | LEN + PEM is dominated by tivozanib |
Additional assessment group sensitivity analyses
In response to errors identified during the NICE appraisal consultation comments, and to incorporate the results from the updated intermediate-/poor-risk subgroup NMA, the AG produced additional sensitivity analyses to address two modelling errors. The details of the errors and the results are presented in Appendix 10. In summary, using the updated costs and results from the updated intermediate-/poor-risk subgroup NMA in the model had little impact on results (Appendix 10, Tables 91–98) and the same conclusions could be drawn.
Assessment group consideration of the cost-effectiveness analysis
The data (clinical effectiveness and cost-effectiveness) used to populate the MSD/AG model are relevant to NHS clinical practice and can be used to inform NICE decision-making.
The AG considered the cost-effectiveness of lenvatinib plus pembrolizumab versus relevant comparators for the two distinct risk subgroups that comprise the all-risk population: patients with intermediate-/poor-risk disease and patients with favourable-risk disease. For the largest risk subgroup (intermediate-/poor-risk disease), OS data from the CLEAR trial were used in the MSD/AG model (via the AG OS NMAs) to generate cost-effectiveness results for the comparison of lenvatinib plus pembrolizumab with cabozantinib and with nivolumab plus ipilimumab.
An area of uncertainty that could not be resolved was around TTD for patients in the intermediate-/poor-risk subgroup who were treated with nivolumab plus ipilimumab. In the base-case analysis, the AG assumed that nivolumab plus ipilimumab TTD data could be represented by lenvatinib TTD data (CLEAR trial). However, this assumption may not be valid as, compared to lenvatinib, both nivolumab and ipilimumab have different mechanisms of action, means of administration and dosing schedules. An alternative approach considered by the AG as a scenario analysis was to use the CLEAR trial MSD pembrolizumab TTD estimates (generalised gamma distribution) to represent TTD for patients treated with nivolumab plus ipilimumab. However, such an approach results in an implausibly long tail and generates higher costs for nivolumab plus ipilimumab than for lenvatinib plus pembrolizumab. While the AG considers that the approach in the AG base case to model TTD for patients treated with nivolumab plus ipilimumab was reasonable (CLEAR trial lenvatinib TTD data) and was preferable to using CLEAR trial MSD pembrolizumab TTD, the AG cannot reject the possibility that nivolumab plus ipilimumab is more costly than lenvatinib plus pembrolizumab at list prices.
For the favourable-risk subgroup, due to limited comparator RCT data, the AG assumed that the clinical effectiveness of pazopanib and tivozanib was equal to that of sunitinib. This assumption aligns with the view of previous NICE ACs. 24,25,34,35 Evidence from the CLEAR trial was incorporated into the MSD/AG model and generated cost-effectiveness results that suggested that lenvatinib plus pembrolizumab was dominated by sunitinib, pazopanib and tivozanib. This finding was robust for all analysis of uncertainty undertaken by the AG.
Chapter 6 Discussion
Statement of principal findings
The NICE, the European Association of Urology38 and the ESMO39 have recommended treatments for patients with untreated aRCC with different levels of disease risk. In the main body of the report, the AG has presented clinical effectiveness results for the three risk groups and has presented cost-effectiveness results for patients in the intermediate-/poor-risk and favourable-risk subgroups; cost-effectiveness results for the all-risk population are presented in Appendix 7.
Direct clinical effectiveness results
The AG systematic review of clinical effectiveness evidence identified only one RCT of lenvatinib plus pembrolizumab versus sunitinib for patients with untreated aRCC, the CLEAR trial. Results from this trial demonstrated improved PFS and ORR for lenvatinib plus pembrolizumab in the intermediate/poor and favourable-risk subgroups and all-risk population. CLEAR trial results from the updated OS analysis showed a statistically significant improvement for patients treated with lenvatinib plus pembrolizumab versus patients treated with sunitinib for the intermediate-/poor-risk subgroup and the all-risk population; there were too few events in the favourable-risk subgroup for robust OS conclusions to be drawn. Generally, the AEs experienced by patients treated with lenvatinib plus pembrolizumab were consistent with the known safety profile of the two drugs. When compared to treatment with sunitinib, treatment with lenvatinib plus pembrolizumab appears to neither improve nor worsen HRQoL.
Indirect clinical effectiveness results
The AG carried out Bayesian HR NMAs for the three patient disease risk groups. However, due to limited data availability, it was not possible to carry out NMAs for all outcomes for all three patient risk groups. Further, as networks were sparse, it was possible to generate meaningful results only using FE NMAs.
The AG PFS NMA results for the intermediate-/poor-risk subgroup, the favourable-risk subgroup and the all-risk population should not be used to infer any statistically significant difference (or lack of statistically significant difference) for any of the treatment comparisons owing to within-trial PH violations or uncertainty regarding the validity of the PH assumption.
The AG OS NMA results for the intermediate-/poor-risk subgroup suggested that there was a numerical, but not a statistically significant, improvement in OS for patients treated with lenvatinib plus pembrolizumab compared with patients treated with cabozantinib or nivolumab plus ipilimumab. Because of within-trial PH violations or uncertainty regarding the validity of the PH assumption, the AG OS NMA results for the favourable-risk subgroup and the all-risk population should not be used to infer any statistically significant difference (or lack of statistically significant difference) for any of the treatment comparisons.
The AG ORR NMA showed a statistically significantly improved ORR for lenvatinib plus pembrolizumab versus nivolumab plus ipilimumab and a non-statistically significant numerical advantage for lenvatinib plus pembrolizumab versus cabozantinib in the intermediate-/poor-risk subgroup. Lenvatinib plus pembrolizumab also resulted in statistically significant improvements versus sunitinib and pazopanib in the all-risk population. Evidence was unavailable for lenvatinib plus pembrolizumab versus tivozanib in the all-risk population or versus any relevant comparator in the favourable-risk population.
Results from the AG AE NMAs in the intermediate-/poor-risk subgroup showed non-statistically significant evidence that lenvatinib plus pembrolizumab resulted in an increase in Grade ≥ 3 AEs versus cabozantinib. In the all-risk population, there were statistically significantly more Grade ≥ 3 AEs for patients treated with lenvatinib plus pembrolizumab versus sunitinib and versus pazopanib.
It was not possible for the AG to perform any HRQoL NMAs due to the heterogeneity of the HRQoL outcome scales used in the included trials and limited reported data (i.e. 95% CIs not reported, data not reported separately for risk subgroups).
Cost-effectiveness results
For the intermediate-/poor-risk subgroup, AG base-case cost-effectiveness results (list prices) suggested that treatment with lenvatinib plus pembrolizumab generated more QALYs than cabozantinib and more QALYs than nivolumab plus ipilimumab, but at a greater overall cost than either of these two treatments. Using list prices, the ICERs per QALY gained for the comparison of lenvatinib plus pembrolizumab versus cabozantinib and versus nivolumab plus ipilimumab exceeded £100,000.
For the favourable-risk subgroup, AG base-case cost-effectiveness results (list prices) suggested that treatment with sunitinib generated more QALYs than lenvatinib plus pembrolizumab at a lower overall cost, that is treatment with lenvatinib plus pembrolizumab was dominated by treatment with sunitinib (and, using the assumption of equivalent effectiveness, by pazopanib and tivozanib).
The AG base-case cost-effectiveness results for the intermediate-/poor-risk and favourable-risk subgroups were robust over most of the assumptions used in the AG PSA, sensitivity and scenario analyses.
Patient and public involvement
There was no PPI regarding the production of the protocol or report for this systematic review and CEA. However, as the analyses were conducted to inform a NICE appraisal, NICE received input from experts and stakeholders in addition to the evidence presented by the AG and companies. All stakeholders and the public were able to comment on the preliminary guidance issued by NICE. For this appraisal, NICE received a written submission from the following patient organisations: Action Kidney Cancer and Kidney Cancer Support Network. In addition, patient experts attended the NICE AC meeting and offered valuable insight into living with the disease.
Equality, diversity and inclusion
Participant representation
The AG is unaware of published data regarding the characteristics of patients with Stage 3 or Stage 4 aRCC, that is for the patients who are the main focus of this report. However, it is known that in the UK, 62.8% of new cases of kidney cancer occurring between 2015 and 2017 were in men. 12 Men may have been slightly over-represented in the trials discussed in this report as the proportion of men included in the trials ranged from 72.4%103 to 82.5%. 104
Older patients may have been under-represented in the trials discussed in this report. Data by age range were reported only in the CLEAR trial; in this trial, only 41.2% of patients were aged ≥ 65 years. 12 In the UK, between 2015 and 2017, 64.0% of new cases of kidney cancer occurred in patients aged ≥ 65 years. 12 Older patients are commonly under-represented in clinical trials in all disease areas. This is largely due to trial eligibility criteria, which commonly excludes patients with comorbidities that often arise as people age. 123–125
Data regarding ethnicity were reported only in the CLEAR trial and in three other trials. 97,100,102 Compared to England and Wales 2011 UK Census data,126 people identifying as white or black may have been under-represented in the CLEAR trial (74.9% vs. 86.0% and 0.7% vs. 3.3%, respectively), while people identifying as Asian appear to be over-represented in the CLEAR trial (20.0% vs. 7.5%). However, in the other (non-CLEAR) trials, the proportion who identified as white ranged from 88.4%100 to 92.4%,97 the proportion who identified as Asian ranged from 0.6%97 to 8.5%100 and the proportion who identified as black ranged from 0.2%102 to 3.2%. 97 These differences in ethnicity across trials are to be expected as the CLEAR trial and five other trials98,101–104 were all reported to be international trials.
Reflections on research team and wider involvement
The research team was made up of academic researchers and healthcare professionals (including clinicians and a senior medicines information pharmacist) with a wide range of experience and expertise. The team worked well together and met regularly to discuss key issues related to the topic area (RCC) and to develop the methods employed in the appraisal (systematic review and CEA). Not everyone in the team had carried out an MTA before and it was important that the less experienced staff were encouraged to participate and were supported by the more experienced members of the team.
Strengths, limitations and uncertainties of the assessment
Strengths
Use of CLEAR trial data
The CLEAR trial is a well-designed trial and clinical advice to the AG is that efficacy and safety results are generalisable to NHS clinical practice for patients with untreated aRCC. This trial provided reliable evidence for the AG direct and indirect comparisons of lenvatinib plus pembrolizumab with all relevant treatments listed in the final scope29 issued by NICE.
Comparators
The AG included nivolumab plus ipilimumab as a comparator (intermediate-/poor-risk subgroup). Evidence for this comparison was missing from the Eisai1 and MSD2 submissions to NICE.
Cost-effectiveness results
The MSD/AG model was populated with data provided by Eisai1 and data provided by MSD2 and generated base-case ICERs per QALY gained that can be used to inform decision-making. The AG carried out extensive one-way sensitivity analyses, scenario analyses and PSA. Results from these analyses demonstrate that AG base-case cost-effectiveness results are robust.
Weaknesses
Lack of direct evidence
Direct efficacy and safety evidence is only available for the comparison of lenvatinib plus pembrolizumab with sunitinib from a single RCT. However, previous NICE ACs24,25,34,35 have concluded that it may be appropriate to assume that sunitinib, pazopanib and tivozanib are similarly effective in clinical practice.
Proportional hazards assumption
The PH assumption is violated for the data used in five of the six time-to-event (PFS and OS) NMAs, the exception being the intermediate-/poor-risk subgroup OS NMAs. This means that the HRs estimated from these NMAs are not applicable to all time points across the observed follow-up of the trials included in the NMAs. Further, the AG has confidence only in the FE NMA results. The RE NMA results are presented in Appendix 4, Tables 65–70; these are considered unusable because of convergence issues that have occurred due the small number of included trials and sparse data.
Uncertainties
CLEAR trial subsequent treatments
In addition to a treatment-switching analysis to test whether adjusting for the effect of subsequent treatment affected OS results, Eisai1 also conducted post hoc analyses that examined OS for patients who did and did not receive subsequent treatment separately. The PH assumption was violated for patients who received subsequent treatments. Clinical advice to the AG is that patients who do not receive subsequent treatments are a heterogeneous group. Therefore, the results from these analyses are difficult to interpret.
Assessment group network meta-analysis results
The main area of uncertainty affecting interpretation of AG HR NMA results was the effect of PH assumption violations; this was an issue for five of the six time-to-event (PFS and OS) NMAs.
There were limited data to inform some indirect comparisons. For the IMDC/MSKCC favourable-risk subgroup, there were no ORR data for any of the comparators, and for the all-risk population, there were no ORR data for tivozanib. Similarly, there were no AE outcomes available for nivolumab plus ipilimumab for the intermediate-/poor-risk subgroup, all comparators for the IMDC/MSKCC favourable-risk subgroup and tivozanib for the all-risk population.
A total of 13% of patients included in the SWITCH trials98,103 had non-clear cell aRCC. Results were not reported separately for patients with clear cell and non-clear cell histology. However, the AG considers that the inclusion of this proportion of patients with non-clear cell histology would not have a substantial impact on NMA results.
NICE ACs24,25,34,35 have concluded that sunitinib, pazopanib and tivozanib can be considered to deliver similar efficacy outcomes. This means that CLEAR trial sunitinib results could be used as a proxy for the efficacy of pazopanib and tivozanib for the all-risk population and for the favourable-risk subgroup. Thus, conclusions regarding the relative efficacy of lenvatinib plus pembrolizumab versus pazopanib and versus tivozanib may be generated from the CLEAR trial.
Since the OS PH assumptions for the data used to populate the AG OS NMAs were not violated for patients in the intermediate-/poor-risk subgroup, the AG OS NMA results are robust. However, the PFS PH assumptions for data used to populate the AG PFS NMAs were violated in some cases and, therefore, these results should not be used to infer any statistically significant difference (or lack of statistically significant difference) between treatments. However, a naïve comparison shows that CLEAR trial median PFS for patients treated with lenvatinib plus pembrolizumab (Condifential information has been removed) is longer than the PFS for patients treated with cabozantinib (8.6 months97) or nivolumab plus ipilimumab (11.6 months100). This is, potentially, the area of relative clinical effectiveness for patients with untreated aRCC where there is most uncertainty.
Adverse events
While it was not possible for the AG to present AE evidence for the comparison of lenvatinib plus pembrolizumab versus nivolumab plus ipilimumab, previously published reviews have compared the relative effectiveness of combination therapies to treat aRCC. The Mori et al. 55 meta-analysis results showed that lenvatinib plus pembrolizumab was less well tolerated (any AE, Grade ≥ 3 AEs and discontinuation due to AEs) than nivolumab plus cabozantinib or pembrolizumab plus axitinib. Three other NMAs56–58 also reported that patients who received lenvatinib plus pembrolizumab were more likely to experience Grade ≥ 3 AEs and treatment discontinuations (due to AEs) when compared with other combination therapies, including nivolumab plus ipilimumab.
Cost-effectiveness
The AG OS NMA results for the intermediate/poor and favourable-risk subgroups showed that there were no statistically significant differences between treatments. As AG cost-effectiveness results are driven by differences in OS between treatments, if there is no OS gain for patients treated with lenvatinib plus pembrolizumab versus comparators, then the higher costs associated with lenvatinib plus pembrolizumab (list prices) means that it is unlikely to be a cost-effective treatment.
An area of uncertainty that could not be resolved was around TTD for the intermediate-/poor-risk subgroup who were treated with nivolumab plus ipilimumab. The AG base-case assumption that nivolumab plus ipilimumab TTD data would equal CLEAR trial lenvatinib TTD data may not be valid as both nivolumab and ipilimumab have different mechanisms of action, means of administration and dosing schedules compared to lenvatinib.
Other relevant factors
Favourable-risk population
NICE24,36 has recommended aRCC treatments for the all-risk population and for the intermediate-/poor-risk subgroup. If a patient does not have intermediate-/poor-risk disease, then, by definition, the patient has favourable-risk disease. The AG has, therefore, carried out clinical and cost-effectiveness analyses for the favourable-risk subgroup. Efficacy results from a recent population-based study21 showed that median OS for the all-risk population was approximately half the length of that for the favourable-risk subgroup [all-risk population: 28.6 (95% CI 25.9 to 31.0) months; favourable-risk subgroup: 52.1 (95% CI 43.4 to 61.2) months]. These results suggest that it is informative to consider the favourable-risk subgroup separately alongside results for the intermediate-/poor-risk subgroup.
While there were few events, favourable-risk subgroup CLEAR trial results show no statistically significant OS benefit for lenvatinib plus pembrolizumab versus sunitinib; these results are consistent with previously published reviews53,55,59 of combination therapies, including lenvatinib plus pembrolizumab.
It was beyond the scope of this appraisal to compare lenvatinib plus pembrolizumab with avelumab plus axitinib. Clinical advice to the AG is that treatment with avelumab plus axitinib is the preferred option for patients with favourable-risk aRCC.
Issues identified during the National Institute for Health and Care Excellence appraisal
After the NICE AC Meeting, the AG conducted additional clinical and cost-effectiveness sensitivity analyses. The additional clinical effectiveness sensitivity analyses were PFS and OS NMAs for the intermediate-/poor-risk subgroup using updated information from the CheckMate 214 trial. 100 The additional cost-effectiveness analyses were to correct for two modelling errors identified in the tivozanib engine for AE costs and application of oral administration costs. Using the revised costs and updated NMA data had relatively little impact on the clinical and cost-effectiveness results and the same conclusions could be drawn as from the original analyses.
The AG considers that it is important to reiterate that the cost-effectiveness analyses presented in this appraisal are based on list prices only. As patient access scheme (PAS) discount prices are in place for lenvatinib, pembrolizumab, sunitinib, pazopanib, tivozanib, cabozantinib, nivolumab, ipilimumab, everolimus and axitinib, the cost-effectiveness comparisons presented using list prices in this report cannot be used as the basis for NHS decision-making. The AG provided cost-effectiveness results generated using the discounted prices for lenvatinib and pembrolizumab in a confidential appendix presented to NICE. The NICE AC concluded that, when using PAS prices for all drugs, in the intermediate-/poor-risk subgroup:
-
the cost-effectiveness estimates were above the range that NICE considers an acceptable use of NHS resources when lenvatinib plus pembrolizumab was compared with cabozantinib
-
the cost-effectiveness estimates were within the range that NICE considers acceptable when lenvatinib plus pembrolizumab was compared with nivolumab plus ipilimumab.
The NICE AC concluded that, when using PAS prices for all drugs, in the favourable-risk subgroup, all the cost-effectiveness estimates were above the range that NICE considers an acceptable use of NHS resources. 3
Chapter 7 Conclusions
Good-quality efficacy and safety evidence for the comparison of lenvatinib plus pembrolizumab with sunitinib was available from the CLEAR trial. For most of the AG Bayesian HR NMA comparisons, it was difficult to reach conclusions due to within-trial PH violations or uncertainty regarding the validity of the PH assumption. However, the data (clinical effectiveness and cost-effectiveness) used to populate the MSD/AG model are relevant to clinical practice and could be used to inform decision-making. The all-risk population comprises patients with intermediate-/poor-risk and patients with favourable-risk disease. The AG cost-effectiveness analyses have focused on the two subgroups. The AG cost-effectiveness results, generated using list prices for all drugs, show that lenvatinib plus pembrolizumab is less cost-effective than all other treatment options. Within the NHS, PAS discount prices are in place for lenvatinib, pembrolizumab, sunitinib, pazopanib, tivozanib, cabozantinib, nivolumab, ipilimumab, everolimus and axitinib. Therefore, the cost-effectiveness comparisons presented in this AG report were not used as the basis for decision-making by NICE. Rather, NICE considered cost-effectiveness evidence where all relevant discount prices were applied. 3
Implications for service provision
Clinical advice to the AG is that if NICE were to recommend lenvatinib plus pembrolizumab as a treatment option for patients with aRCC, there would be minimal impact on current NHS staffing and infrastructure.
Final NICE guidance on whether to recommend lenvatinib plus pembrolizumab as a treatment option for patients in NHS clinical practice was published in January 2023. 3 NICE recommended lenvatinib plus pembrolizumab as a routine treatment option for patients with IMDC intermediate-/poor-risk aRCC if:
-
nivolumab plus ipilimumab would otherwise be offered
-
the companies provide lenvatinib and pembrolizumab according to the confidential commercial arrangements.
Suggested research priorities
Clinical advice to the AG is that avelumab plus axitinib is the preferred first-line treatment option for patients with favourable-risk disease and who can tolerate this combination. As avelumab plus axitinib is currently only available to NHS patients via the CDF, it was not a relevant comparator for this appraisal. If NICE were to recommend routine treatment with avelumab plus axitinib, clinical and cost-effectiveness comparisons of this treatment combination with lenvatinib plus pembrolizumab, sunitinib, pazopanib and tivozanib would generate useful information for clinicians and patients. NMAs may be useful for generating this evidence.
Clinical advice to the AG is that the likelihood of future RCTs versus established treatments is low. Therefore, it is important that real-world evidence is monitored to check that results seen in clinical practice reflect RCT results for patients with untreated aRCC.
Additional information
Acknowledgements
The authors thank Dr Naveen Vasudev (Clinical Associate Professor/Honorary Consultant in Medical Oncology, The Leeds Teaching Hospitals NHS Trust) for providing feedback on the final draft version of this report. Within the last 3 years, Naveen Vasudev has received consultancy fees from Bristol-Myers Squibb, MSD and Pfizer, reimbursement for attending symposiums organised by Bristol-Myers Squibb and EUSA Pharma, fees for speaking from Bristol-Myers Squibb, EUSA Pharma and Ipsen, and funds for research from Bristol-Myers Squibb.
Contributions of authors
Nigel Fleeman (https://orcid.org/0000-0002-4637-9779) was the project lead and reviewed clinical effectiveness evidence, including study selection, data extraction, synthesis and interpretation.
Rachel Houten (https://orcid.org/0000-0002-4315-7732) reviewed cost-effectiveness evidence, including study selection, data extraction, synthesis and interpretation; clinical effectiveness review support; and development of the economic model.
Sarah Nevitt (https://orcid.org/0000-0001-9988-2709) reviewed statistical clinical effectiveness evidence, including study selection, data extraction, synthesis and interpretation and also carried out indirect network analyses.
James Mahon (https://orcid.org/0000-0002-2187-1003) contributed to the development of the economic model.
Sophie Beale (https://orcid.org/0000-0003-0164-103X) critiqued clinical and economic evidence.
Angela Boland (https://orcid.org/0000-0002-5435-8644) critiqued clinical and economic evidence.
Janette Greenhalgh (https://orcid.org/0000-0003-4812-1904) reviewed clinical effectiveness evidence, including study selection, data extraction, synthesis and interpretation.
Katherine Edwards (https://orcid.org/0000-0002-1092-0092) reviewed clinical effectiveness evidence, including study selection, data extraction, synthesis and interpretation.
Michelle Maden (https://orcid.org/0000-0003-4419-6343) carried out literature searches.
Devarshi Bhattacharyya (https://orcid.org/0000-0003-1694-8106) applied cost-effectiveness review inclusion criteria.
Marty Chaplin (https://orcid.org/0000-0002-7097-8704) carried out clinical quality assessment, data extraction and statistical support.
Joanne McEntee (https://orcid.org/0000-0002-7486-4018) provided pharmacy advice and critical appraisal of clinical effectiveness evidence.
Shien Chow (https://orcid.org/0000-0001-8609-1297) provided clinical advice and critical appraisal of clinical effectiveness evidence.
Tom Waddell (https://orcid.org/0000-0001-5652-3829) provided clinical advice and critical appraisal of clinical effectiveness evidence.
All authors contributed to the writing of the report.
Data-sharing statement
This systematic review and cost-effectiveness analysis includes published and confidential data supplied by the participating companies. Data that are not in the public domain cannot be shared further owing to the nature of this study. All queries should be submitted to the corresponding author.
Ethics statement
This is a systematic review and cost-effectiveness analysis. No primary data were created for this report. Ethical approval was therefore not required.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/TRRM4238.
Primary conflicts of interest: During the last 3 years, Shien Chow has received reimbursement for attending symposiums organised by EUSA Pharma, Ipsen, Novartis and Pfizer, received fees for speaking from EUSA Pharma, Novartis and Pfizer, and received funds for research from Novartis. During the last 3 years, Tom Waddell has received reimbursement for attending symposiums organised by EUSA Pharma, Bristol-Myers Squibb and Ipsen, acted in a consultancy or advisory role for Roche, Pfizer, Ipsen, Bristol-Myers Squibb, Merck Sharp & Dohme (MSD) and Eisai Europe, received fees for speaking from Pfizer, Ipsen, Bristol-Myers Squibb and EUSA Pharma, and received research funding from Bristol-Myers Squibb, Pfizer, Ipsen, MSD, Roche and Eisai.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Eisai . Lenvatinib With Pembrolizumab for Untreated Advanced Renal Cell Carcinoma [ID3760]. Company Evidence Submission 2021. www.nice.org.uk/guidance/ta858/evidence/appraisal-consultation-committee-papers-pdf-11317402909 (accessed 24 April 2024).
- MSD . Lenvatinib With Pembrolizumab for Untreated Advanced Renal Cell Carcinoma [ID3760]. Document B. Company Evidence Submission 2021. www.nice.org.uk/guidance/ta858/evidence/appraisal-consultation-committee-papers-pdf-11317402909 (accessed 24 April 2024).
- National Institute for Health and Care Excellence . Lenvatinib With Pembrolizumab for Untreated Advanced Renal Cell Carcinoma. Technology Appraisal Guidance [TA858] 2023. www.nice.org.uk/guidance/ta858 (accessed 18 January 2023).
- Kabaria R, Klaassen Z, Terris MK. Renal cell carcinoma: links and risks. Int J Nephrol Renovasc Dis 2016;9:45-52.
- Cancer Research UK . Types and Grades. Kidney Cancer 2020. www.cancerresearchuk.org/about-cancer/kidney-cancer/stages-types-grades/types-grades (accessed 10 September 2021).
- BMJ Best Practice . Renal Cell Carcinoma n.d. https://bestpractice.bmj.com/topics/en-gb/261 (accessed 10 September 2021).
- Gray RE, Harris GT. Renal cell carcinoma: diagnosis and management. Am Fam Physician 2019;99:179-84.
- Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol 2016;70:93-105.
- Ahrens M, Scheich S, Hartmann A, Bergmann L;. IAG-N Interdisciplinary Working Group Kidney Cancer of the German Cancer Society . Non-clear cell renal cell carcinoma – pathology and treatment options. Oncol Res Treat 2019;42:128-35.
- Valenca LB, Hirsch MS, Choueiri TK, Harshman LC. Non-clear cell renal cell carcinoma, part 1: histology. Clin Adv Hematol Oncol 2015;13:308-13.
- American Cancer Society . Kidney Cancer Stages. Renal Cell Carcinoma Staging 2020. www.cancer.org/cancer/kidney-cancer/detection-diagnosis-staging/staging.html (accessed 10 September 2021).
- Cancer Research UK . Kidney Cancer Incidence Statistics 2020. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer/incidence#heading-Zero (accessed 10 September 2021).
- Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289-96.
- Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794-9.
- Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530-40.
- Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 2013;14:141-8.
- Kubackova K, Melichar B, Bortlicek Z, Pavlik T, Poprach A, Svoboda M, et al. Czech Renal Cancer Cooperative Group . Comparison of two prognostic models in patients with metastatic renal cancer treated with sunitinib: a retrospective, registry-based study. Target Oncol 2015;10:557-63.
- de Groot S, Sleijfer S, Redekop WK, Oosterwijk E, Haanen JBAG, Kiemeney LALM, et al. Variation in use of targeted therapies for metastatic renal cell carcinoma: results from a Dutch population-based registry. BMC Cancer 2016;16.
- Fiala O, Finek J, Poprach A, Melichar B, Kopecký J, Zemanova M, et al. Outcomes according to MSKCC risk score with focus on the intermediate-risk group in metastatic renal cell carcinoma patients treated with first-line sunitinib: a retrospective analysis of 2390 patients. Cancers (Basel) 2020;12.
- Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br J Cancer 2015;113:12-9.
- Savard MF, Wells JC, Graham J, Dudani S, Steinharter JA, McGregor BA, et al. Real-world assessment of clinical outcomes among first-line sunitinib patients with clear cell metastatic renal cell carcinoma (mRCC) by the International mRCC Database Consortium Risk Group. Oncologist 2020;25:422-30.
- Schwab M, Hofmann R, Heers H, Hegele A. mRCC outcome in the treatment of metastatic renal cell carcinoma – a German single-center real-world experience. In Vivo 2018;32:1617-22.
- Tamada S, Iguchi T, Yasuda S, Kato M, Yamasaki T, Nakatani T. The difference in the survival rate of patients with metastatic renal cell carcinoma in the intermediate-risk group of the Memorial Sloan Kettering Cancer Center criteria. Oncotarget 2018;9:27752-9.
- National Institute for Health and Care Excellence . Cabozantinib for Untreated Advanced Renal Cell Carcinoma. Technology Appraisal Guidance [TA542] 2018. www.nice.org.uk/guidance/ta542 (accessed 20 August 2019).
- National Institute for Health and Care Excellence . Nivolumab With Ipilimumab for Untreated Advanced Renal Cell Carcinoma. Technology Appraisal Guidance [TA581] 2019. www.nice.org.uk/guidance/ta581 (accessed 23 September 2021).
- Office for National Statistics . Cancer Survival in England: Adult, Stage at Diagnosis and Childhood – Patients Followed up to 2018 n.d. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancersurvivalinengland/stageatdiagnosisandchildhoodpatientsfollowedupto2018#cancer-survival-by-stage-for-less-common-cancers (accessed 10 September 2021).
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24.
- Mekhail TM, Abou-Jawde RM, Boumerhi G, Malhi S, Wood L, Elson P, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005;23:832-41.
- National Institute for Health and Care Excellence . Final Scope for the Appraisal of Lenvatinib With Pembrolizumab for Untreated Advanced Renal Cell Carcinoma 2021. www.nice.org.uk/guidance/ta858/documents/final-scope (accessed 28 May 2024).
- Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 2003;97:1663-71.
- Ljungberg B, Alamdari FI, Rasmuson T, Roos G. Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int 1999;84:405-11.
- National Institute for Health and Care Excellence . Sunitinib for the First-Line Treatment of Advanced and Or Metastatic Renal Cell Carcinoma. Technology Appraisal Guidance [TA169] 2009. www.nice.org.uk/guidance/ta169 (accessed 20 August 2019).
- National Institute for Health and Care Excellence . Pazopanib for the First-Line Treatment of Advanced Renal Cell Carcinoma. Technology Appraisal Guidance [TA215] 2011. www.nice.org.uk/guidance/ta215 (accessed 20 August 2019).
- National Institute for Health and Care Excellence . Tivozanib for Treating Advanced Renal Cell Carcinoma. Technology Appraisal Guidance [TA512] 2018. www.nice.org.uk/guidance/ta512 (accessed 20 August 2019).
- National Institute for Health and Care Excellence . Avelumab With Axitinib for Untreated Advanced Renal Cell Carcinoma. Technology Appraisal Guidance [TA645] 2020. www.nice.org.uk/guidance/ta645 (accessed 27 September 2021).
- National Institute for Health and Care Excellence . Nivolumab With Ipilimumab for Untreated Advanced Renal Cell Carcinoma. Technology Appraisal Guidance [TA780] 2022. www.nice.org.uk/guidance/TA780 (accessed 18 January 2023).
- National Institute for Health and Care Excellence . Pembrolizumab With Axitinib for Untreated Advanced Renal Cell Carcinoma. Technology Appraisal Guidance [TA650] 2020. www.nice.org.uk/guidance/ta650 (accessed 24 September 2021).
- Bedke J, Albiges L, Capitanio U, Giles RH, Hora M, Lam TB, et al. The 2021 updated European Association of Urology guidelines on renal cell carcinoma: immune checkpoint inhibitor–based combination therapies for treatment-naive metastatic clear-cell renal cell carcinoma are standard of care. Eur Urol 2021;80:393-7.
- Powles T, Albiges L, Bex A, Grünwald V, Porta C, Procopio G, et al. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann Oncol 2021;32:1511-9.
- National Institute for Health and Care Excellence . Bevacizumab (First-Line), Sorafenib (First- and Second-Line), Sunitinib (Second-Line) and Temsirolimus (First-Line) for the Treatment of Advanced and Or Metastatic Renal Cell Carcinoma. Technology Appraisal Guidance [TA178] 2009. www.nice.org.uk/guidance/ta178 (accessed 24 September 2021).
- National Institute for Health and Care Excellence . Nivolumab With Cabozantinib for Untreated Advanced or Metastatic Renal Cell Carcinoma [TA785] 2022. www.nice.org.uk/guidance/TA785 (accessed 24 April 2024).
- National Institute for Health and Care Excellence . Axitinib for Treating Advanced Renal Cell Carcinoma After Failure of Prior Systemic Treatment. Technology Appraisal Guidance [TA333] 2015. www.nice.org.uk/guidance/ta333 (accessed 28 January 2022).
- National Institute for Health and Care Excellence . Cabozantinib for Previously Treated Advanced Renal Cell Carcinoma. Technology Appraisal Guidance [TA463] 2017. www.nice.org.uk/guidance/ta463 (accessed 28 January 2022).
- National Institute for Health and Care Excellence . Lenvatinib With Everolimus for Previously Treated Advanced Renal Cell Carcinoma. Technology Appraisal Guidance [TA498] 2018. www.nice.org.uk/guidance/ta498 (accessed 28 January 2022).
- Eisai Ltd . Summary of Product Characteristics: Kisplyx 4 mg Hard Capsules n.d. https://mhraproducts4853.blob.core.windows.net/docs/b093e43d81241ff07e64dcd06bd91b8af037cf57 (accessed 9 February 2022).
- Merck Sharp & Dohme (UK) Limited . Summary of Product Characteristics: KEYTRUDA® 25 mg ML Concentrate for Solution for Infusion n.d. https://mhraproducts4853.blob.core.windows.net/docs/e3ba892a68b7cc3cb7add2c4a906a985e6134d27 (accessed 9 February 2022).
- National Institute for Health and Care Excellence . Nivolumab for Previously Treated Advanced Renal Cell Carcinoma. Technology Appraisal Guidance [TA417] 2016. www.nice.org.uk/guidance/ta417 (accessed 28 January 2022).
- National Institute for Health and Care Excellence . Everolimus for Advanced Renal Cell Carcinoma After Previous Treatment. Technology Appraisal Guidance [TA432] 2017. www.nice.org.uk/guidance/ta432 (accessed 28 January 2022).
- Ipsen Ltd . Summary of Product Characteristics: CABOMETYX 20 Mg Film-Coated Tablets, CABOMETYX 40 Mg Film-Coated Tablets, CABOMETYX 60 Mg Film-Coated Tablets n.d. www.medicines.org.uk/emc/product/4331#gref (accessed 9 February 2022).
- Pfizer Limited . Summary of Product Characteristics: Inlyta 1 mg Film-Coated Tablets n.d. www.medicines.org.uk/emc/product/4325/smpc#gref (accessed 9 February 2022).
- Merck Sharp & Dohme (UK) Limited . Summary of Product Characteristics: Bavencio 20 mg ML Concentrate for Solution for Infusion n.d. www.medicines.org.uk/emc/product/8453/smpc#gref (accessed 9 February 2022).
- Bristol-Myers Squibb Pharmaceuticals Limited . Summary of Product Characteristics: OPDIVO 10 mg ML Concentrate for Solution for Infusion n.d. www.medicines.org.uk/emc/product/6888/smpc#gref (accessed 9 February 2022).
- Ciccarese C, Iacovelli R, Porta C, Procopio G, Bria E, Astore S, et al. Efficacy of VEGFR-TKIs plus immune checkpoint inhibitors in metastatic renal cell carcinoma patients with favorable IMDC prognosis. Cancer Treat Rev 2021;100.
- Massari F, Rizzo A, Mollica V, Rosellini M, Marchetti A, Ardizzoni A, et al. Immune-based combinations for the treatment of metastatic renal cell carcinoma: a meta-analysis of randomised clinical trials. Eur J Cancer 2021;154:120-7.
- Mori K, Pradere B, Quhal F, Katayama S, Mostafaei H, Laukhtina E, et al. Differences in oncological and toxicity outcomes between programmed cell death-1 and programmed cell death ligand-1 inhibitors in metastatic renal cell carcinoma: a systematic review and meta-analysis. Cancer Treat Rev 2021;99.
- Nocera L, Karakiewicz PI, Wenzel M, Tian Z, Shariat SF, Saad F, et al. Clinical outcomes and adverse events after first-line treatment in metastatic renal cell carcinoma: a systematic review and network meta-analysis. J Urology 2021;207:16-24. https://doi.org/10.1097/JU.0000000000002252.
- Quhal F, Mori K, Bruchbacher A, Resch I, Mostafaei H, Pradere B, et al. First-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Eur Urol Oncol 2021;4:755-65.
- Quhal F, Mori K, Remzi M, Fajkovic H, Shariat SF, Schmidinger M. Adverse events of systemic immune-based combination therapies in the first-line treatment of patients with metastatic renal cell carcinoma: systematic review and network meta-analysis. Curr Opin Urol 2021;31:332-9.
- Shpilsky J, Catalano PJ, McDermott DF. First-line immunotherapy combinations in advanced renal cell carcinoma: a rapid review and meta-analysis. Kidney Cancer 2021;5:153-63.
- Centre for Reviews and Dissemination . CRD’s Guidance for Undertaking Reviews in Health Care 2009. www.york.ac.uk/crd/guidance/ (accessed 20 September 2021).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6.
- European network for Health Technology Assessment (EUnetHTA) . Process of Information Retrieval for Systematic Reviews and Health Technology Assessments on Clinical Effectiveness 2019. www.eunethta.eu/wp-content/uploads/2020/01/EUnetHTA_Guideline_Information_Retrieval_v2-0.pdf (accessed 21 September 2021).
- The EndNote Team . EndNote X9 2013.
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia n.d. www.covidence.org (accessed 24 September 2021).
- National Institute for Health and Care Excellence . Position Statement: Consideration of Products Recommended for Use in the Cancer Drugs Fund As Comparators, or in a Treatment Sequence, in the Appraisal of a New Cancer Product 2019.
- Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. New Engl J Med 2021;384:1289-300.
- Choueiri TK, Eto M, Kopyltsov E, Rha SY, Porta CG, Motzer R, et al. Phase III CLEAR trial in advanced renal cell carcinoma (aRCC): outcomes in subgroups and toxicity update. Ann Oncol 2021;32:S683-5.
- Eisai Inc . Analysis of Health-related Quality of Life Outcomes for Eisai Study E7080-G000-307 (CLEAR) in Renal Cell Carcinoma. Statistical analysis plan, version 2.1 2020.
- Eisai Inc . Statistical Report: Overall Survival of Lenvatinib Plus Pembrolizumab Versus Sunitinib Adjusted for Subsequent Anticancer Medication Using 2-Stage Estimation and IPCW Approach. Study Protocol Number: E7080-g000-307 Keynote 581. Study Protocol Title: A Multicenter, Open-Label, Randomized, Phase 3 Trial to Compare the Efficacy and Safety of Lenvatinib in Combination With Everolimus or Pembrolizumab Versus Sunitinib Alone in First-Line Treatment of Subjects With Advanced Renal Cell Carcinoma (Clear) n.d.
- Eisai Inc, Eisai Ltd, Eisai Co, Ltd . Clinical Study Report. Study Protocol Number: E7080-G000-307 KEYNOTE 581. Study Protocol Title: A Multicenter, Open-Label, Randomized, Phase 3 Trial to Compare the Efficacy and Safety of Lenvatinib in Combination With Everolimus or Pembrolizumab Versus Sunitinib Alone in First-Line Treatment of Subjects With Advanced Renal Cell Carcinoma (CLEAR) n.d.
- Eisai Inc, Eisai Ltd, Eisai Co, Ltd . Study E7080-G000-307/KN 581. Overall survival follow-up as of 31 March 2021. Study protocol number: E7080-G000-307/ KEYNOTE 581. Study Protocol Title: A Multicenter, Open-label, Randomized, Phase 3 Trial to Compare the Efficacy and Safety of Lenvatinib in Combination with Everolimus or Pembrolizumab versus Sunitinib alone in First-line Treatment of Subjects with Advanced Renal Cell Carcinoma (CLEAR). Report Date: 20 May 2021 2021.
- Eisai Inc, Eisai Ltd, Eisai Co, Ltd . A Multicenter, Open-label, Randomized, Phase 3 Trial to Compare the Efficacy and Safety of Lenvatinib in Combination with Everolimus or Pembrolizumab versus Sunitinib alone in First-line Treatment of Subjects with Advanced Renal Cell Carcinoma (CLEAR). Health-related quality of life outcomes study report. Version 1 n.d.
- Eisai Inc, Eisai Ltd, Eisai Co, Ltd . Clinical study protocol. Study protocol number: E7080-G000-307. Study Protocol Title: A Multicenter, Open-label, Randomized, Phase 3 Trial to Compare the Efficacy and Safety of Lenvatinib in Combination with Everolimus or Pembrolizumab versus Sunitinib alone in First-line Treatment of Subjects with Advanced Renal Cell Carcinoma. Protocol amendment 07 n.d.
- Eisai Inc, Eisai Ltd, Eisai Co, Ltd . Statistical analysis plan. Study protocol number: E7080-G000-307/KEYNOTE-581. Study Protocol Title: A Multicenter, Open-label, Randomized, Phase 3 Trial to Compare the Efficacy and Safety of Lenvatinib in Combination with Everolimus or Pembrolizumab versus Sunitinib alone in First-line Treatment of Subjects with Advanced Renal Cell Carcinoma (CLEAR). Version: 3.0 n.d.
- Grunwald V, Powles T, Choueiri TK, Hutson TE, Porta C, Eto M, et al. Lenvatinib plus everolimus or pembrolizumab versus sunitinib in advanced renal cell carcinoma: study design and rationale. Future Oncol 2019;15:929-41.
- Grunwald V, Powles T, Kopyltsov E, Kozlov V, Gordoa TA, Eto M, et al. Analysis of the CLEAR study in patients (pts) with advanced renal cell carcinoma (RCC): depth of response and efficacy for selected subgroups in the lenvatinib (LEN) + pembrolizumab (PEMBRO) and sunitinib (SUN) treatment arms. J Clin Oncol 2021;39.
- JapicCTI . Lenvatinib/Everolimus/or/Lenvatinib/Pembrolizumab/Versus/Sunitinib/Alone/As/Treatment/of/Advanced/Renal/Cell/Carcinoma n.d. www.clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI-173807 (accessed 8 March 2022).
- Motzer R, Grunwald V, Hutson TE, Porta C, Powles T, Eto M, et al. A phase III trial to compare efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab vs sunitinib alone in first-line treatment of patients (Pts) with metastatic renal cell carcinoma (RCC). J Clin Oncol 2017;35.
- Motzer RJ, Grunwald V, Hutson TE, Porta C, Powles T, Eto M, et al. A phase III trial to compare efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab vs sunitinib alone in first-line treatment of patients with metastatic renal cell carcinoma (RCC). Asia Pac J Clin Oncol 2017;13.
- Motzer RJ, Grunwald V, Hutson TE, Porta C, Powles T, Eto M, et al. A phase III trial to compare efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab vs sunitinib alone in first-line treatment of patients (Pts) with metastatic renal cell carcinoma (RCC). J Clin Oncol 2017;35.
- Motzer RJ, Porta C, Alekseev B, Rha SY, Choueiri TK, Mendez-Vidal MJ, et al. Health-related quality-of-life (HRQoL) analysis from the phase 3 CLEAR trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) for patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol 2021;39.
- Motzer RJ, Porta C, Eto M, Powles T, Grunwald V, Hutson TE, et al. Phase 3 trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) monotherapy as a first-line treatment for patients (pts) with advanced renal cell carcinoma (RCC) (CLEAR study). J Clin Oncol 2021;39.
- Nct . Lenvatinib/Everolimus/or/Lenvatinib/Pembrolizumab/Versus/Sunitinib/Alone/As/Treatment/of/Advanced/Renal/Cell/Carcinoma 2016. https://clinicaltrials.gov/show/NCT02811861 (accessed 8 March 2022).
- Motzer RJ, Porta C, Eto M, Powles T, Grünwald V, Hutson TE, et al. Lenvatinib plus pembrolizumab versus sunitinib in first-line treatment of advanced renal cell carcinoma: final prespecified overall survival analysis of CLEAR, a Phase III Study. J Clin Oncol 2024;42:1222-8. https://doi.org/10.1200/JCO.23.01569.
- Latimer NR, Abrams KR. NICE DSU Technical Support Document 16. Adjusting Survival Time Estimates in the Presence of Treatment Switching. [Commissioned Report] 2014. http://nicedsu.org.uk/wp-content/uploads/2016/03/TSD16_Treatment_Switching.pdf (accessed July 2021).
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515-26.
- US Department of Health and Human Services, National Institutes of Health National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009. www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf (accessed 8 March 2022).
- Heo JH, Park C, Ghosh S, Park SK, Zivkovic M, Rascati KL. A network meta-analysis of efficacy and safety of first-line and second-line therapies for the management of metastatic renal cell carcinoma. J Clin Pharm Ther 2021;46:35-49.
- Motzer R, Grunwald V, Hutson TE, Porta C, Powles T, Eto M, et al. A phase 3 trial to compare efficacy and safety of Lenvatinib in combination with Everolimus or Pembrolizumab vs Sunitinib alone in first-line treatment of patients with metastatic renal cell carcinoma. Kidney Cancer 2018;2:S2-3.
- Manz KM, Fenchel K, Eilers A, Morgan J, Wittling K, Dempke WCM. Efficacy and safety of approved first-line tyrosine kinase inhibitor treatments in metastatic renal cell carcinoma: a network meta-analysis. Adv Ther 2020;37:730-44.
- Liu Z, Chen Y, Wei Z, He Y, Wang J, Mu X, et al. Comparative efficacy and safety of immunotherapy in the first-line treatment of metastatic renal cell carcinoma: a systematic review and network meta-analysis. Ann Palliat Med 2021;10:2805-14.
- Elaidi R, Phan L, Borchiellini D, Barthelemy P, Ravaud A, Oudard S, et al. Comparative efficacy of first-line immune-based combination therapies in metastatic renal cell carcinoma: a systematic review and network meta-analysis. Cancers 2020;12.
- Alam MU, Jazayeri SB, Gautam S, Norez D, Kumar J, Tanneru K, et al. Combination therapy for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Am J Clin Oncol 2020;43:477-83.
- Cao G, Wu X, Wang Z, Tian X, Zhang C, Wu X, et al. What is the optimum systemic treatment for advanced/metastatic renal cell carcinoma of favourable, intermediate and poor risk, respectively? A systematic review and network meta-analysis. BMJ Open 2020;10.
- Su Y, Fu J, Du J, Wu B. First-line treatments for advanced renal-cell carcinoma with immune checkpoint inhibitors: systematic review, network meta-analysis and cost-effectiveness analysis. Ther Adv Med Oncol 2020;12.
- Riaz IB, He H, Ryu AJ, Siddiqi R, Naqvi SAA, Yao Y, et al. A living, interactive systematic review and network meta-analysis of first-line treatment of metastatic renal cell carcinoma. Eur Urol 2021;80:712-23.
- Choueiri TK, Hessel C, Halabi S, Sanford B, Michaelson MD, Hahn O, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer 2018;94:115-25.
- Eichelberg C, Vervenne WL, De Santis M, von Weikersthal LF, Goebell PJ, Lerchenmuller C, et al. SWITCH: a randomised, sequential, open-label study to evaluate the efficacy and safety of sorafenib-sunitinib versus sunitinib-sorafenib in the treatment of metastatic renal cell cancer. Eur Urol 2015;68:837-47.
- Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:1280-9.
- Motzer RJ, Escudier B, McDermott DF, Aren Frontera O, Melichar B, Powles T, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer 2020;8.
- Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722-31.
- Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 2013;31:3791-9.
- Retz M, Bedke J, Bogemann M, Grimm M-O, Zimmermann U, Muller L, et al. SWITCH II: phase III randomized, sequential, open-label study to evaluate the efficacy and safety of sorafenib-pazopanib versus pazopanib-sorafenib in the treatment of advanced or metastatic renal cell carcinoma (AUO AN 33/11). Eur J Cancer 2019;107:37-45.
- Tomita Y, Naito S, Sassa N, Takahashi A, Kondo T, Koie T, et al. Sunitinib versus sorafenib as initial targeted therapy for mCC-RCC with favorable/intermediate risk: multicenter randomized trial CROSS-J-RCC. Clin Genitourin Cancer 2020;18:e374-85.
- Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med 2014;370:1769-70.
- Royston P, Parmar MK. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med 2011;30:2409-21.
- Cox DR. Regression models and life-tables. J R Stat Soc B Stat Methodol 1972;34:187-220.
- Freeman SC, Cooper NJ, Sutton AJ, Crowther MJ, Carpenter JR, Hawkins N. Challenges of modelling approaches for network meta-analysis of time-to-event outcomes in the presence of non-proportional hazards to aid decision making: application to a melanoma network. Stat Methods Med Res 2022;31:839-61. https://doi.org/10.1177/09622802211070253.
- Le Moine J, Hawe E, Abeysinghe S. PRM221 – network meta-analysis in the presence of non-proportionality: a review of NICE submissions. Value Health 2018;21.
- Medical Research Council (MRC) Biostatistics Unit (BSU) . DIC: Deviance Information Criteria n.d. www.mrc-bsu.cam.ac.uk/software/bugs/the-bugs-project-dic/ (accessed 23 March 2022).
- Phillippo DM. Multinma: Network Meta-Analysis of Individual and Aggregate Data in Stan 2021. https://dmphillippo.github.io/multinma/ (accessed 23 September 2021).
- Dias S, Welton NJ, Sutton AJ, Ades A. NICE DSU Technical Support Document 2. A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials 2011. http://nicedsu.org.uk/technical-support-documents/evidence-synthesis-tsd-series/ (accessed 23 September 2021).
- Dias S, Sutton AJ, Welton NJ, Ades A. NICE DSU Technical Support Document 3. Heterogeneity: Subgroups, Meta-Regression, Bias and Bias-Adjustment n.d. http://nicedsu.org.uk/technical-support-documents/evidence-synthesis-tsd-series/ (accessed 23 September 2021).
- Dias S, Welton NJ, Sutton AJ, Caldwell D, Lu G, Ades AE. NICE DSU Technical Support Document 4. Inconsistency in Networks of Evidence Based on Randomised Controlled Trials 2011. http://nicedsu.org.uk/technical-support-documents/evidence-synthesis-tsd-series/ (accessed 23 September 2021).
- Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthelemy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020;5.
- Hammers HJ, Motzer RJ, Tannir NM, McDermott DF, Burotto M, Choueiri TK, et al. Conditional survival and 5-year follow-up in CheckMate 214: first-line nivolumab plus ipilimumab versus sunitinib in advanced renal cell carcinoma 2021. www.kidneycancer.org/wp-content/uploads/2022/11/IKCSNA21_E39_Hammers.pdf (accessed 24 April 2024).
- Li S, Li J, Peng L, Li Y, Wan X. Cost-effectiveness of frontline treatment for advanced renal cell carcinoma in the era of immunotherapies. Front Pharmacol 2021;12.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9] 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 22 March 2022).
- Woods BS, Sideris E, Palmer SJ, Latimer N, Soares MFO. NICE DSU Technical Support Document 19: Partitioned Survival Analysis for Decision Modelling in Health Care: A Critical Review 2017. www.sheffield.ac.uk/sites/default/files/2022-02/TSD19-Partitioned-Survival-Analysis-final-report.pdf (accessed 24 April 2024).
- NHS . National Schedule of Reference Costs 2019 20 2018. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/ (accessed 22 August 2021).
- Georghiou T, Bardsley M. Exploring the Cost of Care at the End of Life. Nuffield Trust; 2014.
- Pitkala KH, Strandberg TE. Clinical trials in older people. Age Ageing 2022;51. https://www.nuffieldtrust.org.uk/research/exploring-the-cost-of-care-at-the-end-of-life.
- Sedrak MS, Freedman RA, Cohen HJ, Muss HB, Jatoi A, Klepin HD, et al. Cancer and Aging Research Group (CARG) . Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J Clin 2021;71:78-92.
- van Marum RJ. Underrepresentation of the elderly in clinical trials, time for action. Br J Clin Pharmacol 2020;86:2014-6.
- GOV.UK . Population of England and Wales: GOV.UK Ethnicity Facts and Figures 2022. www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/national-and-regional-populations/population-of-england-and-wales/latest#by-ethnicity (accessed 18 January 2023).
- National Institute for Health and Care Excellence . Lenvatinib With Everolimus or Pembrolizumab for Untreated Advanced Renal Cell Carcinoma [ID3760]. In Development [GID-TA10629] 2023. www.nice.org.uk/guidance/indevelopment/gid-ta10629 (accessed 24 September 2021).
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985;4:213-26.
- Jansen JP. Network meta-analysis of survival data with fractional polynomials. BMC Med Res Methodol 2011;11:1-14.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91.
- Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021;384:829-41.
- Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116-27.
- Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1103-15.
- Cella D, Escudier B, Tannir NM, Powles T, Donskov F, Peltola K, et al. Quality of life outcomes for cabozantinib versus everolimus in patients with metastatic renal cell carcinoma: METEOR phase III randomized trial. J Clin Oncol 2018;36:757-64.
- de Groot S, Redekop WK, Versteegh MM, Sleijfer S, Oosterwijk E, Kiemeney LALM, et al. Health-related quality of life and its determinants in patients with metastatic renal cell carcinoma. Qual Life Res 2018;27:115-24.
- Wan X, Zhang Y, Tan C, Zeng X, Peng L. First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma: a cost-effectiveness analysis. JAMA Oncol 2019;5:491-6.
- Patel KK, Giri S, Parker TL, Bar N, Neparidze N, Huntington SF. Cost-effectiveness of first-line versus second-line use of daratumumab in older, transplant-ineligible patients with multiple myeloma. J Clin Oncol 2021;39:1119-28.
- Centers for Medicare & Medicaid Services . Physician Fee Schedule 2021. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched (accessed 4 March 2021).
- Agency for Healthcare Research and Quality, US Department of Health & Human Services . Healthcare Cost and Utilization Project 2021. https://hcupnet.ahrq.gov (accessed 26 February 2021).
- Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277-90.
- Perrin A, Sherman S, Pal S, Chua A, Gorritz M, Liu Z, et al. Lifetime cost of everolimus vs axitinib in patients with advanced renal cell carcinoma who failed prior sunitinib therapy in the US. J Med Econ 2015;18:200-9.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83.
Appendix 1 Systematic reviews including patients treated with lenvatinib plus pembrolizumab
| Author (year) | Title | Population (n = total patients) |
Stated purpose and included studies | Main results/conclusions |
|---|---|---|---|---|
| Ciccarese et al. (2021)53 |
Efficacy of VEGFR-TKIs plus immune checkpoint inhibitors in mRCC for patients with favourable IMDC prognosis. | First-line mRCC patients with favourable IMDC prognosis (n = 839) |
Meta-analysis evaluating whether the combinations of VEGFR-TKI + ICI compared to VEGFR-TKIs alone improve the outcome of mRCC patients with favourable IMDC prognosis. Included four RCTs of VEGFR-TKI + ICI therapies (pembrolizumab plus axitinib, nivolumab plus cabozantinib, avelumab plus axitinib, lenvatinib plus pembrolizumab) vs. sunitinib. |
Combination therapies improved PFS, but did not significantly prolong OS compared to sunitinib. Combination therapies resulted in a higher rate of treatment discontinuation compared to sunitinib. |
| Massari et al. (2021)54 |
Immune-based combinations for the treatment of mRCC. | Treatment-naïve mRCC patients (n = 5175) |
Meta-analysis of phase III clinical trials of immune-based combinations in mRCC patients. Included six RCTs of immune-based combination therapies (pembrolizumab plus axitinib, nivolumab plus cabozantinib, avelumab plus axitinib, pembrolizumab plus bevacizumab, nivolumab plus ipilimumab) vs. sunitinib. |
Compared with sunitinib, combination therapy resulted in statistically significant improvements in PFS, OS and ORR. Some combination therapies resulted in more all-Grade and Grade ≥ 3 AEs and others less all-Grade and Grade ≥ 3 AEs than treatment with sunitinib. |
| Mori et al. (2021)55 |
Differences in oncological and toxicity outcomes between PD-L1 and PD-1 inhibitors in mRCC. | First-line mRCC patients (n = 4025) |
Systematic review, meta-analysis and NMA assessing the differences between anti-PD-1 and anti-PD-L1 therapies in RCTs of combination therapies. Included five RCTs total. Three RCTs for PD-1 meta-analysis of combination therapies (pembrolizumab plus axitinib, nivolumab plus cabozantinib, lenvatinib plus pembrolizumab) vs. sunitinib. |
Anti-PD-1 type combination therapy (including lenvatinib plus pembrolizumab) had statistically significantly longer PFS, OS and ORR than sunitinib in the all-risk population and intermediate-/poor-risk subgroup. However, there was no statistically significant difference for OS in the favourable-risk subgroup. There was no difference vs. sunitinib for any grade AEs, but combination therapy had significantly worse grade ≥ 3 AEs. Lenvatinib plus pembrolizumab was less tolerated than other PD-1 combination therapies. |
| Nocera et al. (2021)56 |
Clinical outcomes and AEs after first-line treatment in metastatic RCC: A systematic review and meta-analysis. | First-line mRCC patients (n = 3320) |
NMA of first-line trials comparing immune-based combination therapies. Only phase III RCTs with proven OS benefit relative to sunitinib were included, four in total. Interventions were: pembrolizumab plus axitinib, nivolumab plus cabozantinib, lenvatinib plus pembrolizumab, nivolumab plus ipilimumab |
In NMA-derived ranking, against other combination therapies and sunitinib, lenvatinib plus pembrolizumab ranked first for PFS and ORR, and second for OS for providing maximal benefit. Lenvatinib plus pembrolizumab resulted in statistically significantly more grade ≥ 3 AEs than sunitinib and was ranked lower (i.e. considered to be least tolerated) than all other combination therapies. |
| Quhal et al. (2021)57 |
First-line immunotherapy-based combinations for mRCC. | First-line mRCC patients (n = 5121) |
NMA of the efficacy and safety of first-line ICI-based combination therapies. Included six RCTs of immune-based combination therapies (pembrolizumab plus axitinib, nivolumab plus cabozantinib, avelumab plus axitinib, lenvatinib plus pembrolizumab, atezolizumab plus bevacizumab, nivolumab plus ipilimumab). |
Immune-based combination therapies had higher likelihood of providing better PFS, OS and ORR than sunitinib. Lenvatinib plus pembrolizumab resulted in statistically significantly improved PFS and ORR vs. sunitinib. Compared with other immune-based combination therapies, lenvatinib plus pembrolizumab had highest likelihood of providing maximal PFS benefit and highest ORR. In the intermediate-/poor-risk subgroup, lenvatinib plus pembrolizumab had the highest likelihood of providing maximal PFS and OS and the highest probability of maximal PFS benefit in the favourable-risk subgroup. The highest likelihood of grade ≥ 3 AEs and AE-related treatment discontinuation was associated with lenvatinib plus pembrolizumab. |
| Quhal et al. (2021)58 |
AEs of systemic immune-based combination therapies in the first-line treatment of patients with mRCC. | First-line mRCC patients (n = 5121) |
Comparison of the safety profiles of systemic immune checkpoint inhibitor-based combination therapies that were evaluated in the first-line setting of the management of patients with aRCC or mRCC. Included six RCTs of immune-based combination therapies (pembrolizumab plus axitinib, nivolumab plus cabozantinib, avelumab plus axitinib, lenvatinib plus pembrolizumab, atezolizumab plus bevacizumab, nivolumab plus ipilimumab). |
Low treatment-related mortality was found from all combination therapies with no statistically significant differences vs. sunitinib. Lenvatinib plus pembrolizumab had highest likelihood of grade ≥ 3 AEs, and treatment discontinuation due to AEs. Lenvatinib plus pembrolizumab had the highest likelihood of all-grade adrenal insufficiency and high-grade AST increase. All combinations had low likelihood of thrombocytopenia and neutropenia than sunitinib. |
| Shpilsky et al. (2021)59 |
First-line immunotherapy combinations in aRCC: a rapid review and meta-analysis. | First-line aRCC patients (n = 5121) |
Meta-analysis to combine the evidence of available first-line combination therapies compared to sunitinib monotherapy in aRCC. Included six RCTs of combination therapies (pembrolizumab plus axitinib, nivolumab plus cabozantinib, avelumab plus axitinib, lenvatinib plus pembrolizumab, atezolizumab plus bevacizumab, nivolumab plus ipilimumab). |
Combination therapies resulted in statistically significantly improved PFS and OS compared to sunitinib in the all-risk population and intermediate-/poor-risk subgroup. ORR and AEs were only reported for the all-risk population. ORR was statistically significantly improved vs. sunitinib. The incidence of grade ≥ 3 AEs was comparable between combination therapies and sunitinib. There were no statistically significant differences between combination therapies and sunitinib for PFS or OS in the favourable-risk subgroup. |
Appendix 2 Assessment group searches for clinical effectiveness and cost-effectiveness
Sources searched
| Search type | Sources | Dates searched |
|---|---|---|
| Databases | MEDLINE, EMBASE, PubMed, CENTRAL, INAHTA | From inception to 11 October 2021 |
| Trial registries | ClinicalTrials.gov, ICTRP | From inception to 11 October 2021 |
| Conference proceedings | ASCO, ASCO-GU, ESMO, HTAi | From 1 January 2019 to 19 November 2021 |
| NICE TAs | TA169,32 TA178,40 TA215,33 TA512,34 TA542,24 TA581,25 TA650,37 TA64535 | From inception to 18 November 2021 |
| Grey literature websites | EMA, CADTH, HAS, FDA, MHRA, PBAC, SMC | Searched on 22 November 2021 |
| Other | CSs1,2 for this appraisal127 | Received 16 November 2021 |
| Search type | Sources | Dates |
|---|---|---|
| Databases | MEDLINE, EMBASE, PubMed, CENTRAL, INAHTA, NHS EED, EconLit, CEA Registry | From 1 January 2006 to 11 October 2021 |
| Trial registries | ClinicalTrials.gov, ICTRP | From 1 January 2006 to 11 October 2021 |
| Conference proceedings | ASCO, ASCO-GU, ESMO and HTAi, ISPOR | From 2019 to 22 November 2021 |
| Websites | SMC, CADTH, HAS, PBAC | Searched on 22 November 2021 |
Clinical effectiveness searches
MEDLINE (via Ovid)
Ovid MEDLINE(R) ALL <1946–7 October 2021>
| 1 | exp Carcinoma, Renal Cell/ |
| 2 | exp Kidney Neoplasms/ |
| 3 | (renal adj2 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 4 | (kidney adj1 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 5 | (clear?cell adj3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 6 | (non?clear?cell adj3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 7 | hypernephroma.tw,kw. |
| 8 | hypernephroid carcinoma*.tw,kw. |
| 9 | grawitz tumo?r$.tw,kw. |
| 10 | rcc.tw,kw. |
| 11 | or/1–10 |
| 12 | (advanced or metastatic or mRCC or m-RCC or aRCC or a-RCC or ‘first-line’ or ‘first line’ or metastasize or metastasis or metastases or ‘stage iii’ or ‘stage 3’ or ‘stage 4’ or ‘stage iv’ or recurrent or ‘non resectable’ or inoperable or ‘non operable’ or unresectable).tw,kw. or Neoplasm Metastasis/ |
| 13 | 11 and 12 |
| 14 | (mrcc or arcc).tw,kw. |
| 15 | 13 or 14 |
| 16 | randomized controlled trial.pt. |
| 17 | controlled clinical trial.pt. |
| 18 | (randomized or randomised).ab. |
| 19 | placebo.ab. |
| 20 | clinical trials as topic.sh. |
| 21 | randomly.ab. |
| 22 | trial.ti. |
| 23 | (randomised or randomized or RCT).ti. |
| 24 | or/16–23 |
| 25 | exp animals/ not humans.sh. |
| 26 | 24 not 25 |
| 27 | 15 and 26 |
| 28 | limit 27 to english language |
The Cochrane Library (CENTRAL)
Cochrane Central Register of Controlled Trials
Issue 10 of 12 October 2021
| #1 | MeSH descriptor: [Carcinoma, Renal Cell] explode all trees |
| #2 | MeSH descriptor: [Kidney Neoplasms] explode all trees |
| #3 | ((renal NEAR/2 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*))):ti,ab,kw |
| #4 | ((kidney NEAR/1 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*))):ti,ab,kw |
| #5 | ((clear-cell NEAR/3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*))):ti,ab,kw |
| #6 | ((‘non-clear cell’ NEAR/3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*))):ti,ab,kw |
| #7 | (hypernephroma):ti,ab,kw |
| #8 | (hypernephroid carcinoma*):ti,ab,kw |
| #9 | (grawitz tumo?r*):ti,ab,kw |
| #10 | (rcc):ti,ab,kw |
| #11 | {OR #1-#10} |
| #12 | (advanced or metastatic or mRCC or m-RCC or aRCC or a-RCC or ‘first-line’ or ‘first line’ or metastasize or metastasis or metastases or ‘stage iii’ or ‘stage 3’ or ‘stage 4’ or ‘stage iv’ or recurrent or ‘non resectable’ or inoperable or ‘non operable’ or unresectable):ti,ab,kw |
| #13 | MeSH descriptor: [Neoplasm Metastasis] this term only |
| #14 | #12 OR #13 |
| #15 | #11 AND #14 |
| #16 | (mrcc or arcc):ti,ab,kw |
| #17 | #15 OR #16 |
Searches terms with and without hyphen, that is same results for clear-cell as for ‘clear cell’.
EMBASE (via Ovid)
EMBASE <1974–7 October 2021>
| 1 | exp renal cell carcinoma/ |
| 2 | exp kidney tumor/ or exp kidney carcinoma/ |
| 3 | (renal adj2 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 4 | (kidney adj1 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 5 | (clear?cell adj3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 6 | (non?clear?cell adj3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 7 | hypernephroma.tw,kw. |
| 8 | hypernephroid carcinoma*.tw,kw. |
| 9 | grawitz tumo?r$.tw,kw. |
| 10 | rcc.tw,kw. |
| 11 | or/1–10 |
| 12 | (advanced or metastatic or mRCC or m-RCC or aRCC or a-RCC or ‘first-line’ or metastasize or metastasis or metastases or ‘stage iii’ or ‘stage 3’ or ‘stage 4’ or ‘stage iv’ or recurrent or ‘non resectable’ or inoperable or ‘non operable’ or unresectable).tw,kw. |
| 13 | metastasis/ |
| 14 | 12 or 13 |
| 15 | 11 and 14 |
| 16 | (mrcc or arcc).tw,kw. |
| 17 | 15 or 16 |
| 18 | randomized controlled trial.sh. |
| 19 | controlled clinical trial.sh. |
| 20 | (randomized or randomised).ab. |
| 21 | placebo.ab. |
| 22 | ‘clinical trial (topic)’/ |
| 23 | randomly.ab. |
| 24 | trial.ti. |
| 25 | (randomised or randomized or RCT).ti. |
| 26 | or/18–25 |
| 27 | (random$ adj sampl$ adj7 (cross section$ or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) |
| 28 | Cross-sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) |
| 29 | (((case adj control$) and random$) not randomi?ed controlled).ti,ab. |
| 30 | (Systematic review not (trial or study)).ti. |
| 31 | (nonrandom$ not random$).ti,ab. |
| 32 | Random field$.ti,ab. |
| 33 | (random cluster adj3 sampl$).ti,ab. |
| 34 | (review.ab. and review.pt.) not trial.ti. |
| 35 | we searched.ab. and (review.ti. or review.pt.) |
| 36 | update review.ab. |
| 37 | (databases adj4 searched).ab. |
| 38 | (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ |
| 39 | Animal experiment/ not (human experiment/ or human/) |
| 40 | or/27–39 |
| 41 | 26 not 40 |
| 42 | 17 and 41 |
| 43 | limit 42 to embase |
| 44 | limit 42 to (conference abstracts and yr=‘2019 -Current’) |
| 45 | 43 or 44 |
| 46 | limit 45 to english language |
PubMed
https://pubmed.ncbi.nlm.nih.gov/
((((‘Carcinoma, Renal Cell’[Mesh]) OR (‘Kidney Neoplasms’[Mesh]) OR (‘renal cancer*’[Text Word] OR ‘renal carcinoma*’[Text Word] OR ‘renal adenocarcinoma*’[Text Word] OR ‘renal tumor*’[Text Word] OR ‘renal tumour*’[Text Word] OR ‘renal malignanc*’[Text Word]) OR (‘kidney cancer*’[Text Word] OR ‘kidney carcinoma*’[Text Word] OR ‘kidney adenocarcinoma*’[Text Word] OR ‘kidney tumor*’[Text Word] OR ‘kidney tumour*’[Text Word] OR ‘kidney malignanc*’[Text Word]) OR (‘clear-cell cancer*’[Text Word] OR ‘clear-cell carcinoma*’[Text Word] OR ‘clear-cell adenocarcinoma*’[Text Word] OR ‘clear-cell tumor*’[Text Word] OR ‘clear-cell tumour*’[Text Word] OR ‘clear-cell malignanc*’[Text Word]) OR (‘non-clear cell cancer*’[Text Word] OR ‘non-clear cell carcinoma*’[Text Word] OR ‘non-clear cell adenocarcinoma*’[Text Word] OR ‘non-clear cell tumor*’[Text Word] OR ‘non-clear cell tumour*’[Text Word] OR ‘non-clear cell malignanc*’[Text Word]) OR (hypernephroma[Text Word]) OR (hypernephroid carcinoma*[Text Word]) OR (grawitz tumor*[Text Word] OR grawitz tumour*[Text Word]) OR (rcc[Text Word])) AND ((advanced[Text Word] OR metastatic[Text Word] OR mRCC[Text Word] OR m-RCC[Text Word] OR aRCC[Text Word] OR a-RCC[Text Word] OR ‘first-line’[Text Word] OR ‘first line’[Text Word] OR metastasize[Text Word] OR metastasis[Text Word] OR metastases[Text Word] OR ‘stage iii’[Text Word] OR ‘stage 3’[Text Word] OR ‘stage 4’[Text Word] OR ‘stage iv’[Text Word] OR recurrent[Text Word] OR ‘non resectable’[Text Word] OR inoperable[Text Word] OR ‘non operable’[Text Word] OR unresectable[Text Word]) OR (‘Neoplasm Metastasis’[Mesh]))) OR (mrcc[Text Word] OR arcc[Text Word])) AND ((((randomized controlled trial [pt] OR ‘controlled clinical trial’[Publication Type] OR ‘randomized’[Title/Abstract] OR ‘randomised’ [Title/Abstract] OR ‘placebo’[Title/Abstract]) OR (‘clinical trials as topic’ [mesh: noexp]) OR (randomly [tiab] OR trial [ti] OR RCT [ti])) NOT (animals [mh] NOT humans [mh]))) Filters: English
Note: Cannot search in abstract only field in PubMed [RCT filter].
ClinicalTrials.gov
((advanced OR metastatic OR secondary OR EXPAND[Concept] ‘first-line’ OR EXPAND[Concept] ‘first line’ OR metastasis or mRCC or m-RCC OR aRCC OR a-RCC OR metastasize OR metastasis OR metastases OR EXPAND[Concept] ‘stage iii’ OR EXPAND[Concept] ‘stage 3’ OR EXPAND[Concept] ‘stage 4’ OR EXPAND[Concept] ‘stage iv’ OR recurrent OR EXPAND[Concept] ‘non resectable’ OR EXPAND[Concept] ‘non-resectable’ OR inoperable OR EXPAND[Concept] ‘non operable’ OR EXPAND[Concept] ‘non-operable’ OR unresectable) AND AREA[ConditionSearch] (EXPAND[Concept] ‘Renal cell’ OR EXPAND[Concept] ‘renal clear cell’ OR EXPAND[Concept] ‘renal clear-cell’ OR EXPAND[Concept] ‘renal non-clear cell’ OR EXPAND[Concept] ‘renal non clear cell’ OR RCC OR EXPAND[Concept] ‘renal carcinoma’ OR EXPAND[Concept] ‘renal cancer’ OR EXPAND[Concept] ‘renal tumor’ OR EXPAND[Concept] ‘renal tumour’ OR EXPAND[Concept] ‘renal adenocarcinoma’ OR EXPAND[Concept] ‘renal malignancy’ OR EXPAND[Concept] ‘kidney cancer’ OR EXPAND[Concept] ‘kidney carcinoma’ OR EXPAND[Concept] ‘kidney adenocarcinoma’ OR EXPAND[Concept] ‘kidney tumor’ OR EXPAND[Concept] ‘kidney tumour’ OR EXPAND[Concept] ‘kidney malignancy’ OR EXPAND[Concept] ‘clear-cell cancer’ OR EXPAND[Concept] ‘clear cell cancer’ OR EXPAND[Concept] ‘clear-cell carcinoma’ OR EXPAND[Concept] ‘clear cell carcinoma’ OR EXPAND[Concept] ‘clear-cell adenocarcinoma’ OR EXPAND[Concept] ‘clear cell adenocarcinoma’ OR EXPAND[Concept] ‘clear-cell tumor’ OR EXPAND[Concept] ‘clear cell tumor’ OR EXPAND[Concept] ‘clear-cell tumour’ OR EXPAND[Concept] ‘clear cell tumour’ OR EXPAND[Concept] ‘clear-cell malignancy’ OR EXPAND[Concept] ‘clear cell malignancy’ OR EXPAND[Concept] ‘non-clear cell cancer’ OR EXPAND[Concept] ‘non clear cell cancer’ OR EXPAND[Concept] ‘non-clear cell carcinoma’ OR EXPAND[Concept] ‘non clear cell carcinoma’ OR EXPAND[Concept] ‘non-clear cell adenocarcinoma’ OR EXPAND[Concept] ‘non clear cell adenocarcinoma’ OR EXPAND[Concept] ‘non-clear cell tumor’ OR EXPAND[Concept] ‘non clear cell tumor’ OR EXPAND[Concept] ‘non-clear cell tumour’ OR EXPAND[Concept] ‘non clear cell tumour’ OR EXPAND[Concept] ‘non-clear cell malignancy’ OR EXPAND[Concept] ‘non clear cell malignancy’ OR hypernephroma OR EXPAND[Concept] ‘hypernephroid carcinoma’ OR grawitz)) OR (aRCC OR mRCC or a-RCC OR m-RCC)
International Clinical Trials Registry Platform
Search 1:
TITLE: advanced OR metastatic OR metastasis OR metastasize OR secondary OR ‘first line’ OR ‘first-line’ recurrent OR non-resectable OR ‘non resectable’ OR ‘stage 3’ OR ‘stage 4’ OR ‘stage iii’ OR ‘stage iv’ OR mRCC OR aRCC OR inoperable OR ‘non operable’ OR unresectable
AND
CONDITION: ‘renal cell’ OR ‘clear-cell’ OR ‘non-clear cell’ OR RCC OR ‘kidney cancer*’ OR ‘renal cancer*’ OR ‘renal carcinoma*’ OR ‘renal adenocarcinoma’ OR ‘renal tumor*’ OR ‘renal tumour*’ OR hypernephroma OR ‘hypernephroid carcinoma’ OR grawitz
Search 2:
aRCC OR mRCC or a-RCC OR m-RCC
Note: Parentheses (brackets) cannot be used to determine the order in which terms are combined.
Searches automatically include synonyms generated using the UMLS metathesaurus.
Searches are restricted to 256 character spaces, truncated search strategies used.
With/without hyphen retrieves same numbers.
International Health Technology Assessment Database
((‘Neoplasm Metastasis’[mhe]) OR (advanced OR metastatic OR mRCC OR m-RCC OR aRCC OR a-RCC OR ‘first-line’ OR ‘first line’ OR metastasize OR metastasis OR metastases OR ‘stage iii’ OR ‘stage 3’ OR ‘stage 4’ OR ‘stage iv’ OR recurrent OR ‘non resectable’ OR inoperable OR ‘non operable’ OR unresectable)) AND ((‘renal cancer*’ OR ‘renal carcinoma*’ OR ‘renal adenocarcinoma*’ OR ‘renal tumor*’ OR ‘renal tumour*’ OR ‘renal malignanc*’ OR ‘kidney cancer*’ OR ‘kidney carcinoma*’ OR ‘kidney adenocarcinoma*’ OR ‘kidney tumor*’ OR ‘kidney tumour*’ OR ‘kidney malignanc*’ OR ‘clear cell cancer*’ OR ‘clear cell carcinoma*’ OR ‘clear cell adenocarcinoma*’ OR ‘clear cell tumor*’ OR ‘clear cell tumour*’ OR ‘clear cell malignanc*’ OR ‘non clear cell cancer*’ OR ‘non clear cell carcinoma*’ OR ‘non clear cell adenocarcinoma*’ OR ‘non clear cell tumor*’ OR ‘non clear cell tumour*’ OR ‘hypernephroma’ OR ‘hypernephroid carcinoma*’ OR ‘grawitz tumor*’ OR ‘grawitz tumour*’ OR ‘rcc’) OR (‘Kidney Neoplasms’[mhe]) OR (‘Carcinoma, Renal Cell’[mhe])) OR mRCC OR m-RCC or aRCC or a-RCC
Cost-effectiveness searches
MEDLINE (via Ovid)
Ovid MEDLINE(R) ALL <1946–7 October 2021>
| 1 | exp Carcinoma, Renal Cell/ |
| 2 | exp Kidney Neoplasms/ |
| 3 | (renal adj2 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 4 | (kidney adj1 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 5 | (clear?cell adj3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 6 | (non?clear?cell adj3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 7 | hypernephroma.tw,kw. |
| 8 | hypernephroid carcinoma*.tw,kw. |
| 9 | grawitz tumo?r$.tw,kw. |
| 10 | rcc.tw,kw. |
| 11 | or/1–10 |
| 12 | (advanced or metastatic or mRCC or m-RCC or aRCC or a-RCC or ‘first-line’ or ‘first line’ or metastasize or metastasis or metastases or ‘stage iii’ or ‘stage 3’ or ‘stage 4’ or ‘stage iv’ or recurrent or ‘non resectable’ or inoperable or ‘non operable’ or unresectable).tw,kw. or Neoplasm Metastasis/ |
| 13 | 11 and 12 |
| 14 | (mrcc or arcc).tw,kw. |
| 15 | 13 or 14 |
| 16 | Economics/ |
| 17 | exp ‘Costs and Cost Analysis’/ |
| 18 | Economics, Nursing/ |
| 19 | Economics, Medical/ |
| 20 | Economics, Pharmaceutical/ |
| 21 | exp Economics, Hospital/ |
| 22 | Economics, Dental/ |
| 23 | exp ‘Fees and Charges’/ |
| 24 | exp Budgets/ |
| 25 | budget*.ti,ab,kf. |
| 26 | (economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kf. |
| 27 | (economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab. |
| 28 | (cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf. |
| 29 | (value adj2 (money or monetary)).ti,ab,kf. |
| 30 | exp models, economic/ |
| 31 | economic model*.ab,kf. |
| 32 | markov chains/ |
| 33 | markov.ti,ab,kf. |
| 34 | monte carlo method/ |
| 35 | monte carlo.ti,ab,kf. |
| 36 | exp Decision Theory/ |
| 37 | (decision* adj2 (tree* or analy* or model*)).ti,ab,kf. |
| 38 | or/16–37 |
| 39 | 15 and 38 |
| 40 | limit 39 to yr=‘2006 -Current’ |
| 41 | limit 40 to english language |
The Cochrane Library (CENTRAL)
Cochrane Central Register of Controlled Trials
Issue 10 of 12 October 2021
| #1 | MeSH descriptor: [Carcinoma, Renal Cell] explode all trees |
| #2 | MeSH descriptor: [Kidney Neoplasms] explode all trees |
| #3 | ((renal NEAR/2 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*))):ti,ab,kw |
| #4 | ((kidney NEAR/1 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*))):ti,ab,kw |
| #5 | ((clear-cell NEAR/3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*))):ti,ab,kw |
| #6 | ((‘non-clear cell’ NEAR/3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*))):ti,ab,kw |
| #7 | (hypernephroma):ti,ab,kw |
| #8 | (hypernephroid carcinoma*):ti,ab,kw |
| #9 | (grawitz tumo?r*):ti,ab,kw |
| #10 | (rcc):ti,ab,kw |
| #11 | {OR #1-#10} |
| #12 | (advanced or metastatic or mRCC or m-RCC or aRCC or a-RCC or ‘first-line’ or ‘first line’ or metastasize or metastasis or metastases or ‘stage iii’ or ‘stage 3’ or ‘stage 4’ or ‘stage iv’ or recurrent or ‘non resectable’ or inoperable or ‘non operable’ or unresectable):ti,ab,kw |
| #13 | MeSH descriptor: [Neoplasm Metastasis] this term only |
| #14 | #12 OR #13 |
| #15 | #11 AND #14 |
| #16 | (mrcc or arcc):ti,ab,kw |
| #17 | #15 OR #16 |
| #18 | MeSH descriptor: [Economics] this term only |
| #19 | MeSH descriptor: [Costs and Cost Analysis] explode all trees |
| #20 | MeSH descriptor: [Economics, Nursing] this term only |
| #21 | MeSH descriptor: [Economics, Medical] this term only |
| #22 | MeSH descriptor: [Economics, Pharmaceutical] this term only |
| #23 | MeSH descriptor: [Economics, Hospital] explode all trees |
| #24 | MeSH descriptor: [Economics, Dental] this term only |
| #25 | MeSH descriptor: [Fees and Charges] explode all trees |
| #26 | MeSH descriptor: [Budgets] explode all trees |
| #27 | (budget*):ti,ab,kw |
| #28 | (economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed):ti,kw |
| #29 | (economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed):ab |
| #30 | (cost* NEAR/2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)):ab,kw |
| #31 | ((value NEAR/2 (money or monetary))):ti,ab,kw |
| #32 | MeSH descriptor: [Models, Economic] explode all trees |
| #33 | (economic model*):ti,ab,kw |
| #34 | MeSH descriptor: [Markov Chains] this term only |
| #35 | (markov):ti,ab,kw |
| #36 | MeSH descriptor: [Monte Carlo Method] this term only |
| #37 | (monte carlo):ti,ab,kw |
| #38 | MeSH descriptor: [Decision Theory] explode all trees |
| #39 | ((decision* NEAR/2 (tree* or analy* or model*))):ti,ab,kw |
| #40 | {OR #18-#39} |
| #41 | #17 AND #40 |
EMBASE (via Ovid)
EMBASE <1974–7 October 2021>
| 1 | exp renal cell carcinoma/ |
| 2 | exp kidney tumor/ or exp kidney carcinoma/ |
| 3 | (renal adj2 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 4 | (kidney adj1 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 5 | (clear?cell adj3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 6 | (non?clear?cell adj3 (cancer* or carcinoma* or adenocarcinoma* or tumo?r* or malignanc*)).tw,kw. |
| 7 | hypernephroma.tw,kw. |
| 8 | hypernephroid carcinoma*.tw,kw. |
| 9 | grawitz tumo?r$.tw,kw. |
| 10 | rcc.tw,kw. |
| 11 | or/1–10 |
| 12 | (advanced or metastatic or mRCC or m-RCC or aRCC or a-RCC or ‘first-line’ or ‘first line’ or metastasize or metastasis or metastases or ‘stage iii’ or ‘stage 3’ or ‘stage 4’ or ‘stage iv’ or recurrent or ‘non resectable’ or inoperable or ‘non operable’ or unresectable).tw,kw. |
| 13 | metastasis/ |
| 14 | 12 or 13 |
| 15 | 11 and 14 |
| 16 | (mrcc or arcc).tw,kw. |
| 17 | 15 or 16 |
| 18 | Economics/ |
| 19 | Cost/ |
| 20 | exp Health Economics/ |
| 21 | Budget/ |
| 22 | budget*.ti,ab,kw. |
| 23 | (economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kw. |
| 24 | (economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab. |
| 25 | (cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kw. |
| 26 | (value adj2 (money or monetary)).ti,ab,kw. |
| 27 | Statistical Model/ |
| 28 | economic model*.ab,kw. |
| 29 | Probability/ |
| 30 | markov.ti,ab,kw. |
| 31 | monte carlo method/ |
| 32 | monte carlo.ti,ab,kw. |
| 33 | Decision Theory/ |
| 34 | Decision Tree/ |
| 35 | (decision* adj2 (tree* or analy* or model*)).ti,ab,kw. |
| 36 | or/18–35 |
| 37 | 15 and 36 |
| 38 | limit 37 to embase |
| 39 | limit 37 to (conference abstract status and yr=‘2019 -Current’) |
| 40 | 38 or 39 |
| 41 | limit 40 to yr=‘2006 -Current’ |
| 42 | limit 41 to english language |
PubMed
https://pubmed.ncbi.nlm.nih.gov/
((((‘carcinoma, renal cell’[MeSH Terms] OR ‘Kidney Neoplasms’[MeSH Terms] OR (‘renal cancer*’[Text Word] OR ‘renal carcinoma*’[Text Word] OR ‘renal adenocarcinoma*’[Text Word] OR ‘renal tumor*’[Text Word] OR ‘renal tumour*’[Text Word] OR ‘renal malignanc*’[Text Word]) OR (‘kidney cancer*’[Text Word] OR ‘kidney carcinoma*’[Text Word] OR ‘kidney adenocarcinoma*’[Text Word] OR ‘kidney tumor*’[Text Word] OR ‘kidney tumour*’[Text Word] OR ‘kidney malignanc*’[Text Word]) OR (‘clear cell cancer*’[Text Word] OR ‘clear cell carcinoma*’[Text Word] OR ‘clear cell adenocarcinoma*’[Text Word] OR ‘clear cell tumor*’[Text Word] OR ‘clear cell tumour*’[Text Word] OR ‘clear cell malignanc*’[Text Word]) OR (‘non clear cell cancer*’[Text Word] OR ‘non clear cell carcinoma*’[Text Word] OR ‘non clear cell adenocarcinoma*’[Text Word] OR ‘non clear cell tumor*’[Text Word] OR ‘non clear cell tumour*’[Text Word]) OR ‘hypernephroma’[Text Word] OR ‘hypernephroid carcinoma*’[Text Word] OR (‘grawitz tumor*’[Text Word] OR ‘grawitz tumour*’[Text Word]) OR ‘rcc’[Text Word]) AND (‘advanced’[Text Word] OR ‘metastatic’[Text Word] OR ‘mRCC’[Text Word] OR ‘m-RCC’[Text Word] OR ‘aRCC’[Text Word] OR ‘a-RCC’[Text Word] OR ‘first-line’[Text Word] OR ‘first line’[Text Word] OR ‘metastasize’[Text Word] OR ‘metastasis’[Text Word] OR ‘metastases’[Text Word] OR ‘stage iii’[Text Word] OR ‘stage 3’[Text Word] OR ‘stage 4’[Text Word] OR ‘stage iv’[Text Word] OR ‘recurrent’[Text Word] OR ‘non resectable’[Text Word] OR ‘inoperable’[Text Word] OR ‘non operable’[Text Word] OR ‘unresectable’[Text Word] OR ‘Neoplasm Metastasis’[MeSH Terms])) AND (‘Economics’ OR ‘Costs and Cost Analysis’[mh] OR ‘Economics, Nursing’[mh] OR ‘Economics, Medical’[mh] OR ‘Economics, Pharmaceutical’[mh] OR ‘Economics, Hospital’[mh] OR ‘Economics, Dental’[mh] OR ‘Fees and Charges’[mh] OR ‘Budgets’[mh] OR budget*[tiab] OR economic*[tiab] OR cost[tiab] OR costs[tiab] OR costly[tiab] OR costing[tiab] OR price[tiab] OR prices[tiab] OR pricing[tiab] OR pharmacoeconomic*[tiab] OR pharmaco-economic*[tiab] OR expenditure[tiab] OR expenditures[tiab] OR expense[tiab] OR expenses[tiab] OR financial[tiab] OR finance[tiab] OR finances[tiab] OR financed[tiab] OR value for money[tiab] OR monetary value*[tiab] OR ‘models, economic’[mh] OR economic model*[tiab] OR ‘markov chains’[mh] OR markov[tiab] OR ‘monte carlo method’[mh] OR monte carlo[tiab] OR ‘Decision Theory’[mh] OR decision tree*[tiab] OR decision analy*[tiab] OR decision model*[tiab])) AND ((english[Filter]) AND (2006:2021[pdat])))
NHS Economic Evaluation Database via Centre for Reviews and Dissemination
-
MeSH DESCRIPTOR Carcinoma, Renal Cell EXPLODE ALL TREES
-
MeSH DESCRIPTOR Kidney Neoplasms EXPLODE ALL TREES
-
(‘renal cancer*’)
-
(‘renal carcinoma*’)
-
(‘renal adenocarcinoma*’)
-
(‘renal tumor*’)
-
(‘renal tumour*’)
-
(‘renal malignanc*’)
-
(‘kidney cancer*’)
-
(‘kidney carcinoma*’)
-
(‘kidney adenocarcinoma*’)
-
(‘kidney tumor*’)
-
(‘kidney tumour*’)
-
(‘kidney malignanc*’)
-
(‘clear-cell cancer*’)
-
(‘clear-cell carcinoma*’)
-
(‘clear-cell adenocarcinoma*’)
-
(‘clear-cell tumor*’)
-
(‘clear-cell tumour*’)
-
(‘clear-cell malignanc*’)
-
(‘non-clear cell cancer*’)
-
(‘non-clear cell carcinoma*’)
-
(‘non-clear cell adenocarcinoma*’)
-
(‘non-clear cell tumor*’)
-
(‘non-clear cell tumour*’)
-
(‘non-clear cell malignanc*’)
-
(hypernephroma)
-
(hypernephroid carcinoma*)
-
(grawitz tumor*)
-
(grawitz tumour*)
-
(rcc)
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31
-
(advanced)
-
(metastatic)
-
(mRCC)
-
(m-RCC)
-
(aRCC)
-
(a-RCC)
-
(‘first-line’ or ‘first line’)
-
(metastasize)
-
(metastasis)
-
(metastases)
-
(‘stage iii’)
-
(‘stage 3’)
-
(‘stage 4’)
-
(‘stage iv’)
-
(recurrent)
-
(‘non resectable’)
-
(inoperable)
-
(‘non operable’)
-
(unresectable)
-
MeSH DESCRIPTOR Neoplasm Metastasis EXPLODE ALL TREES
-
#33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52
-
#32 AND #53
-
(mrcc)
-
(m-rcc)
-
(arcc)
-
(a-rcc)
-
#55 OR #56 OR #57 OR #58
-
#54 OR #59
EconLit (via EBSCOhost)
-
S1 TI ((renal N2 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*))) OR AB ((renal N2 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*))) OR SU ((renal N2 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*)))
-
S2 TI ((kidney N1 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*)) OR AB ((kidney N1 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*)) OR SU ((kidney N1 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*))
-
S3 TI ((clear-cell N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*))) OR AB ((clear-cell N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*))) OR SU ((clear-cell N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*)))
-
S4 TI ((‘clear cell’ N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*))) OR AB ((‘clear cell’ N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*))) OR SU ((‘clear cell’ N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*)))
-
S5 TI ((non-clear-cell N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*))) OR AB ((non-clear-cell N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*))) OR SU ((non-clear-cell N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*)))
-
S6 TI ((‘non clear cell’ N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*))) OR AB ((‘non clear cell’ N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*))) OR SU ((‘non clear cell’ N3 (cancer* or carcinoma* or adenocarcinoma* or tumo#r* or malignanc*)))
-
S7 TI hypernephroma OR AB hypernephroma OR SU hypernephroma
-
S8 TI ‘hypernephroid carcinoma*’ OR AB ‘hypernephroid carcinoma*’ OR SU ‘hypernephroid carcinoma*’
-
S9 TI grawitz tumo#r* OR AB grawitz tumo#r* OR SU grawitz tumo#r*
-
S10 TI rcc OR AB rcc OR SU rcc
-
S11 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10
-
S12 TI (advanced or metastatic or mRCC or m-RCC or aRCC or a-RCC or ‘first-line’ or ‘first line’ or metastasize or metastasis or metastases or ‘stage iii’ or ‘stage 3’ or ‘stage 4’ or ‘stage iv’ or recurrent or ‘non resectable’ or inoperable or ‘non operable’ or unresectable) OR AB (advanced or metastatic or mRCC or m-RCC or aRCC or a-RCC or ‘first-line’ or ‘first line’ or metastasize or metastasis or metastases or ‘stage iii’ or ‘stage 3’ or ‘stage 4’ or ‘stage iv’ or recurrent or ‘non resectable’ or inoperable or ‘non operable’ or unresectable) OR SU (advanced or metastatic or mRCC or m-RCC or aRCC or a-RCC or ‘first-line’ or ‘first line’ or metastasize or metastasis or metastases or ‘stage iii’ or ‘stage 3’ or ‘stage 4’ or ‘stage iv’ or recurrent or ‘non resectable’ or inoperable or ‘non operable’ or unresectable)
-
S13 S11 AND S12
-
S14 TI (mRCC OR m-RCC or aRCC or a-RCC) OR AB (mRCC OR m-RCC or aRCC or a-RCC) OR SU (mRCC OR m-RCC or aRCC or a-RCC)
-
S15 S13 OR S14
-
S16 S13 OR S14
Narrow by Language: - English, Published: 20060101-20211231
CEA Registry
https://cevr.tuftsmedicalcenter.org/databases/cea-registry
advanced renal cell
metastatic renal cell
advanced kidney
metastatic kidney
mRCC
aRCC
first-line renal cell
first-line kidney
first line renal cell
first line kidney
lenvatinib
sunitinib
pazopanib
tivozanib
cabozantinib
nivolumab
Note: Basic search only with free version of CEA Registry. No Boolean. No download function. Screened on website
ClinicalTrials.gov
(((advanced OR metastatic OR secondary OR EXPAND[Concept] ‘first-line’ OR EXPAND[Concept] ‘first line’ OR metastasis or mRCC or m-RCC OR aRCC OR a-RCC OR metastasize OR metastasis OR metastases OR EXPAND[Concept] ‘stage iii’ OR EXPAND[Concept] ‘stage 3’ OR EXPAND[Concept] ‘stage 4’ OR EXPAND[Concept] ‘stage iv’ OR recurrent OR EXPAND[Concept] ‘non resectable’ OR EXPAND[Concept] ‘non-resectable’ OR inoperable OR EXPAND[Concept] ‘non operable’ OR EXPAND[Concept] ‘non-operable’ OR unresectable) AND AREA[ConditionSearch] (EXPAND[Concept] ‘Renal cell’ OR EXPAND[Concept] ‘renal clear cell’ OR EXPAND[Concept] ‘renal clear-cell’ OR EXPAND[Concept] ‘renal non-clear cell’ OR EXPAND[Concept] ‘renal non clear cell’ OR RCC OR EXPAND[Concept] ‘renal carcinoma’ OR EXPAND[Concept] ‘renal cancer’ OR EXPAND[Concept] ‘renal tumor’ OR EXPAND[Concept] ‘renal tumour’ OR EXPAND[Concept] ‘renal adenocarcinoma’ OR EXPAND[Concept] ‘renal malignancy’ OR EXPAND[Concept] ‘kidney cancer’ OR EXPAND[Concept] ‘kidney carcinoma’ OR EXPAND[Concept] ‘kidney adenocarcinoma’ OR EXPAND[Concept] ‘kidney tumor’ OR EXPAND[Concept] ‘kidney tumour’ OR EXPAND[Concept] ‘kidney malignancy’ OR EXPAND[Concept] ‘clear-cell cancer’ OR EXPAND[Concept] ‘clear cell cancer’ OR EXPAND[Concept] ‘clear-cell carcinoma’ OR EXPAND[Concept] ‘clear cell carcinoma’ OR EXPAND[Concept] ‘clear-cell adenocarcinoma’ OR EXPAND[Concept] ‘clear cell adenocarcinoma’ OR EXPAND[Concept] ‘clear-cell tumor’ OR EXPAND[Concept] ‘clear cell tumor’ OR EXPAND[Concept] ‘clear-cell tumour’ OR EXPAND[Concept] ‘clear cell tumour’ OR EXPAND[Concept] ‘clear-cell malignancy’ OR EXPAND[Concept] ‘clear cell malignancy’ OR EXPAND[Concept] ‘non-clear cell cancer’ OR EXPAND[Concept] ‘non clear cell cancer’ OR EXPAND[Concept] ‘non-clear cell carcinoma’ OR EXPAND[Concept] ‘non clear cell carcinoma’ OR EXPAND[Concept] ‘non-clear cell adenocarcinoma’ OR EXPAND[Concept] ‘non clear cell adenocarcinoma’ OR EXPAND[Concept] ‘non-clear cell tumor’ OR EXPAND[Concept] ‘non clear cell tumor’ OR EXPAND[Concept] ‘non-clear cell tumour’ OR EXPAND[Concept] ‘non clear cell tumour’ OR EXPAND[Concept] ‘non-clear cell malignancy’ OR EXPAND[Concept] ‘non clear cell malignancy’ OR hypernephroma OR EXPAND[Concept] ‘hypernephroid carcinoma’ OR grawitz)) OR (aRCC OR mRCC or a-RCC OR m-RCC)) AND (economic OR economics OR cost OR costs OR costly OR costing OR budget OR price OR prices OR pricing OR pharmacoeconomics OR pharmaco-economics OR expenditure OR expenditures OR expense OR expenses OR financial OR finance OR finances OR financed OR EXPAND[Concept] ‘value for money’ OR EXPAND[Concept] ‘monetary value’ OR EXPAND[Concept] ‘economic model’ OR EXPAND[Concept] ‘economic models’ OR markov OR monte carlo OR EXPAND[Concept] ‘Decision Theory’ OR EXPAND[Concept] ‘decision tree’ OR EXPAND[Concept] ‘decision analysis’ OR EXPAND[Concept] ‘decision model’)
International Clinical Trials Registry Platform
Search 1:
TITLE: (economic OR economics OR cost OR costs OR costly OR costing OR budget OR price OR prices OR pricing OR pharmacoeconomics OR pharmaco-economics OR expenditure OR expenditures OR expense OR expenses OR financial OR finance OR finances OR financed OR ‘value for money’ OR ‘monetary value’ OR ‘economic model’ OR ‘ economic models’ OR markov OR monte carlo OR ‘Decision Theory’ OR decision tree OR decision analysis OR decision model)
AND
CONDITION: ‘renal cell’ OR ‘clear-cell’ OR ‘clear cell’ OR RCC OR ‘kidney cancer*’ OR ‘renal cancer*’ OR ‘renal carcinoma*’ OR ‘renal adenocarcinoma’ OR ‘renal tumor*’ OR ‘renal tumour*’ OR hypernephroma OR ‘hypernephroid carcinoma’ OR grawitz
Search 2:
TITLE: (economic OR economics OR cost OR costs OR costly OR costing OR budget OR price OR prices OR pricing OR pharmacoeconomics OR pharmaco-economics OR expenditure OR expenditures OR expense OR expenses OR financial OR finance OR finances OR financed OR ‘value for money’ OR ‘monetary value’ OR ‘economic model’ OR ‘ economic models’ OR markov OR monte carlo OR ‘Decision Theory’ OR decision tree OR decision analysis OR decision model)
AND
CONDITION: (aRCC OR mRCC or a-RCC OR m-RCC)
Note: Limited to 2006 onwards
Parentheses (brackets) cannot be used to determine the order in which terms are combined.
Searches automatically include synonyms generated using the UMLS metathesaurus.
Searches are restricted to 256 character spaces per line – truncated strategies used
International Health Technology Assessment Database
((‘Neoplasm Metastasis’[mhe]) OR (advanced OR metastatic OR mRCC OR m-RCC OR aRCC OR a-RCC OR ‘first-line’ OR ‘first line’ OR metastasize OR metastasis OR metastases OR ‘stage iii’ OR ‘stage 3’ OR ‘stage 4’ OR ‘stage iv’ OR recurrent OR ‘non resectable’ OR inoperable OR ‘non operable’ OR unresectable)) AND ((‘renal cancer*’ OR ‘renal carcinoma*’ OR ‘renal adenocarcinoma*’ OR ‘renal tumor*’ OR ‘renal tumour*’ OR ‘renal malignanc*’ OR ‘kidney cancer*’ OR ‘kidney carcinoma*’ OR ‘kidney adenocarcinoma*’ OR ‘kidney tumor*’ OR ‘kidney tumour*’ OR ‘kidney malignanc*’ OR ‘clear cell cancer*’ OR ‘clear cell carcinoma*’ OR ‘clear cell adenocarcinoma*’ OR ‘clear cell tumor*’ OR ‘clear cell tumour*’ OR ‘clear cell malignanc*’ OR ‘non clear cell cancer*’ OR ‘non clear cell carcinoma*’ OR ‘non clear cell adenocarcinoma*’ OR ‘non clear cell tumor*’ OR ‘non clear cell tumour*’ OR ‘hypernephroma’ OR ‘hypernephroid carcinoma*’ OR ‘grawitz tumor*’ OR ‘grawitz tumour*’ OR ‘rcc’) OR (‘Kidney Neoplasms’[mhe]) OR (‘Carcinoma, Renal Cell’[mhe])) OR mRCC OR m-RCC or aRCC or a-RCC
Summary of search results
A summary of the results from the AG searches is presented in Table 52.
| Database | Date | Clinical No date (+ English language) |
Economics 2006- (+ English language) |
|---|---|---|---|
| MEDLINE | 11 October 2021 | 2565 | 449 |
| EMBASE | 11 October 2021 | 3163 | 1625 |
| PubMed | 11 October 2021 | 2628 | 387 |
| Cochrane (CENTRAL)a | 11 October 2021 | 2937 | 109 |
| ClinicalTrials.gova,b | 11 October 2021 | 1770 | 54 |
| ICTRP | 11 October 2021 | 1383 | 9 |
| NHS EED | 11 October 2021 | – | 44 |
| EconLit | 11 October 2021 | – | 26 |
| International Health Technology Assessment Database | 11 October 2021 | 58 | 43 |
| Total in Endnote (excluding EU-CTR, CEA, confs) | 14,504 | 2746 | |
| Duplicates removed in Endnote | 6168 | 843 | |
| Total uploaded to Covidence | 8336 | 1903 | |
| Duplicates in removed in Covidence | 50 | 4 | |
| Total to screen in Covidence | 8286 | 1899 |
FIGURE 2.
PRISMA flow diagram: direct clinical effectiveness evidence. (Reports exclude information provided by Eisai and MSD as part of the NICE appraisal clarification process).
FIGURE 3.
PRISMA 2020 flow diagram for cost-effectiveness systematic review.

Appendix 3 Assessment group quality assessment and assessment of company statistical approaches for deriving clinical effectiveness evidence
Quality assessment of CLEAR trial
| Quality assessment item | AG assessment |
|---|---|
| Was the method used to assign participants to treatment arms really random? | ✓ |
| Was the allocation of treatment concealed? | ✓ |
| Was the number of participants randomised stated? | ✓ |
| Were details of baseline comparability presented in terms of prognostic factors? | ✓ |
| Was baseline comparability achieved in terms of prognostic factors? | ✓ |
| Were the eligibility criteria for study entry specified? | ✓ |
| Were any co-interventions identified that may influence the outcomes for each group? | ✕ |
| Were the outcome assessors blinded to the treatment allocation? | ✓ |
| Were the individuals administering the intervention blinded to treatment allocation? | ✕ |
| Were the participants receiving the intervention blinded to treatment allocation? | ✕a |
| Was the success of the blinding procedure assessed? | NA |
| Were at least 80% of the participants included in the randomisation process followed up in the final analysis? | ✓ |
| Were the reasons for patient withdrawals stated? | ✓ |
| Was an intention to treat analysis included? | ✓ |
| Is there any evidence that more outcomes were measured than were reported? | ✕ |
Statistical approach followed for analysis of CLEAR trial data
Information about the statistical approach followed by the company to analyse the CLEAR trial data has been extracted from the Eisai CS,1 the CSR of the IA3 data cut-off,70 the HRQoL outcomes study report (version 1, dated 13 February 2021)72 and the HRQoL outcomes statistical analysis plan (HRQoL SAP version 2.1, dated 5 October 2020),68 the trial protocol (Amendment 7, dated 6 August 2020)73 and the TSAP (version 3, dated 14 August 2020),74 which was available as online supplementary documents to the published paper of the CLEAR trial. 66 A summary of the AG checks of the preplanned statistical approach for the CLEAR trial is provided in Table 54.
| Item | AG assessment | Statistical approach | AG comments |
|---|---|---|---|
| Were all analysis populations clearly defined and prespecified? | Yes | Analysis populations of the CLEAR trial are the ITT population (FAS), PP analysis set and the safety analysis set [Eisai CS1 (see section 4.4)]. | The AG is satisfied that the CLEAR trial analysis populations are clearly defined and prespecified (TSAP, section 5.2.1). |
| Was an appropriate trial design and sample size calculation prespecified? | Yes | The CLEAR trial sample size and power calculations are prespecified (TSAP, section 4). Five interim analyses (IA1–IA5) were preplanned with a Lan-DeMets O’Brien-Fleming alpha spending function used to determine the threshold for statistical significance for each analysis (TSAP, section 6). Multiplicity adjustments for testing the superiority of both lenvatinib plus pembrolizumab and lenvatinib plus everolimus compared to sunitinib are also prespecified (TSAP, section 5.3.3). |
The AG is satisfied that the CLEAR trial prespecified sample size calculation and statistical power calculations are appropriate and were correctly implemented. |
| Results of preplanned IA3 data cut-off (28 August 2020) are presented in the Eisai CS1 (section 4.6). The IA3 data cut-off is the final planned analysis of PFS and served as the primary analysis of OS as the superiority of lenvatinib plus pembrolizumab over sunitinib was demonstrated.71 Updated OS analyses requested by the EMA (data cut-off date 31 March 2021) are also presented [Eisai CS1 (see section 4.6.2.2)]. | |||
| Were all protocol amendments made prior to analysis? | Yes | A summary of the ‘Revision History’ is provided in the latest version of the protocol (Amendment 7, 6 August 2020). Most amendments relate to administrative changes or minor clarifications of wording. Amendments 4 and 6 include modifications to the sample size and power calculations, interim analyses and multiplicity adjustments following IA1 and IA2. |
The AG is satisfied that all protocol amendments were made prior to the IA3 data cut-off and were appropriate. |
| Were all primary and secondary efficacy outcomes predefined and analysed appropriately? | Yes | The CLEAR trial primary efficacy outcome is BICR-assessed PFS using FDA censoring rules. Key secondary efficacy outcomes are BICR-assessed PFS using EMA censoring rules, OS and BICR-assessed ORR. Definitions and statistical analysis approaches for primary and secondary efficacy outcomes are outlined in the Eisai CS1 (appendix L3 and table 99) and clinical effectiveness results are presented for the ITT population [Eisai CS1 (see section 4.6 and appendices M3, M4 and M6)]. |
The AG is satisfied that efficacy outcomes were clearly defined, prespecified, analysed appropriately, and that relevant primary and secondary efficacy outcomes are presented. |
| A complete list of primary, secondary and exploratory end points and statistical analysis approaches is prespecified [TSAP (section 5.1 and section 5.4)]. | |||
| Was the analysis approach for PROs appropriate and prespecified? | Yes | PROs presented in the Eisai CS1 (appendix M3) and in the HRQoL study report were assessed in the HRQoL analysis set (i.e. all patients who had any HRQoL data and received at least one dose of study treatment). PROs measured were changes from baseline FKSI-DRS, EORTC QLQ-C30 and EQ-5D-3L scores, analysed using an MMRM approach and time to deterioration analysed using K-M methods and Cox PH models. |
The AG is satisfied that the PRO outcome definitions and analysis approaches were prespecified (HRQoL SAP sections 2–3) and are appropriate. |
| Was the analysis approach for AEs appropriate and prespecified? | Partly | AEs were assessed and graded using the NCI CTCAE version 4.03 classification system (Protocol, section 9.5.1.4) within the safety analysis population [all randomised patients who received at least one dose of study medication (TSAP, section 5.2.1)]. AEs are presented as numbers and percentages of patients experiencing events. An overview of AEs, SAEs, AEs leading to study drug discontinuation, dose modification or death, TEAEs by NCI CTCAE grade and AESIs occurring in the CLEAR trial are presented in the Eisai CS1 (section 4.8 and appendix F). |
The AG is satisfied that the analysis approach for AEs was prespecified (TSAP, section 5.6.2) and is appropriate. The AG notes that the comparative analyses of AEs were not prespecified in the TSAP and is uncertain why these comparisons are not computed for all AE summaries. |
| RDs and 95% CIs are presented comparing lenvatinib plus pembrolizumab and sunitinib for some of the AE summaries in the Eisai CS1 (section 4.8), computed using the Miettinen and Nurminen method.128 Additional summary tables of safety data in the CLEAR trial are provided in the CSR (section 12.2 and section 12.3). |
|||
| Were modelling assumptions (e.g. PHs) assessed? | Yes | The PH assumption for BICR-assessed PFS and OS were assessed by plotting the log cumulative hazard vs. log(time), by using the Grambsch–Therneau test86 of Schoenfeld’s residuals [Eisai CS1 (section 5.3.1 and 5.3.2) and Eisai response to the AG clarification letter (questions A1 and A2)]. On the basis of these assessments, Eisai consider that over the observed period, the assumption of PH was not violated for BICR-assessed PFS but was violated for the updated analyses of OS (unadjusted for treatment crossover). |
The AG agrees with the Eisai assessments of the PH assumption. |
| Was a suitable approach employed for handling missing data? | Yes | Missing data were handled with censoring rules for time-to-event outcomes (TSAP, section 5.4.1 and table 4) or general rules for handling other missing data (TSAP, section 5.3.5). | The AG is satisfied that all prespecified methods for handling missing data are appropriate. |
| Were all subgroup and sensitivity analyses prespecified? | Yes | Subgroup analyses were prespecified for BICR-assessed PFS, OS and BICR-assessed ORR in the ITT population (TSAP, section 5.3.4) and presented in the Eisai CS1 (appendix E). Sensitivity analyses were prespecified for BICR-assessed PFS in the ITT population (TSAP, section 5.4.1) and BICR-assessed PFS results in the PP analysis set are presented as a sensitivity analysis [Eisai CS1 (see appendices M1 and M2)]. |
The AG is satisfied that all relevant, prespecified subgroup analyses and sensitivity analyses are presented. |
Statistical approach followed for treatment-switching analyses of OS in CLEAR trial
CLEAR trial OS data were confounded due to patients in both the lenvatinib plus pembrolizumab arm and the sunitinib arm receiving subsequent systemic anticancer medication during OS follow-up. Therefore, Eisai performed treatment-switching analyses. A summary and AG critique of the Eisai approach to the treatment-switching analyses used to assess OS in the CLEAR trial are provided in Table 55.
| Item | AG assessment | Statistical approach | AG comments |
|---|---|---|---|
| Were treatment switchers clearly defined? | Yes | Treatment-switching analyses were conducted to adjust for receiving any subsequent anticancer therapy in the CLEAR trial; 132 (37.2%) of 355 patients in the lenvatinib plus pembrolizumab arm and 221 (61.9%) of 357 patients in the sunitinib arm had received any subsequent systemic anticancer medication up to the data cut-off date (31 March 2021) of the updated OS analyses [Eisai CS1 (see table 15)]. | The AG considers that the company has clearly defined which patients were included in the treatment-switching analyses. |
| Was an appropriate method used? | Yes | Eisai used two different adjustment methods, as described in DSU TSD 16:85 the two-stage estimation method and the IPCW method. Eisai preferred the two-stage estimation method over the IPCW method due to the ‘capability of the two-stage approach to generate two counterfactual scenarios where (1) no patients receive subsequent treatment and (2) all patients receive subsequent treatment and combine both of these estimates to generate additional scenarios with varying proportions of patients receiving subsequent treatment to more closely reflect real-world practice’ [Eisai CS1 (see section 4.6.3.2)]. |
The AG agrees that the two-stage method is appropriate and that the company has implemented the two-stage method correctly [Eisai CS1 (section 4.6.3.2)]. The AG also considers that methods to select an accelerated failure time model in the first stage and adjustment factors considered within the two-stage estimation are appropriate. The AG also considers that it was appropriate for the company to present adjusted OS HRs from all models considered. |
| In the first stage of the two-stage estimation method, Eisai used log-normal, log-logistic and Weibull models to estimate the acceleration factor (i.e. the effect of subsequent anticancer medication on OS in the lenvatinib plus pembrolizumab and sunitinib arms). The company selected the log-normal model as the best fitting model according to AIC and BIC statistics, but presented adjusted OS results for all three accelerated failure time models [Eisai CS1 (see table 16)]. Eisai implemented the two-stage method with and without re-censoring, and adjusting for treatment arm and (1) stratification factors of the CLEAR trial (geographic region and MSKCC prognostic groups) or (2) selected baseline covariates (IMDC prognostic risk subgroup, number of metastatic organs/sites involved, and prior nephrectomy). Eisai presented adjusted OS results with and without re-censoring and for both sets of adjustment factors [Eisai CS1 (see table 16)]. |
Given the limited OS data available from the CLEAR trial, the AG considers that the two-stage method adjusted OS HRs without re-censoring are the most appropriate for decision making. However, the AG notes that two-stage adjusted OS HRs without re-censoring may be at risk of bias due to informative censoring if any prognostic factors in the CLEAR trial are related to the censoring mechanism. | ||
| Were modelling assumptions assessed and shown to be valid? | Yes | Assessment of the ‘no unmeasured confounders’ for the two-stage method and the IPCW method were presented in an additional report of the OS treatment switching analyses (see Assessment group study selection and inclusion criteria).71 The two-stage method requires the identification of a ‘secondary baseline’, defined by the company as the date of study treatment discontinuation for the CLEAR trial,71 and requires the assumption that all patients are in a similar clinical condition (e.g. disease stage) at the time of secondary baseline. Patients discontinued study treatments due to disease progression, AEs and patient choice/withdrawal of consent (CSR, table 2). |
The AG agrees with the company that assumption of no unmeasured confounders may not be met fully but the impact of any violation of this assumption is likely to be small. The AG considers that patients who have discontinued treatment due to disease progression cannot be considered to be in a similar clinical condition to patients who have discontinued treatment due to AEs or due to personal choice. However, the impact of the violation of this assumption on the adjusted OS HRs is unknown. Due to the similarity in the durations of time on treatment and time from randomisation to first subsequent anticancer therapy in the CLEAR trial, the AG considers that it is unlikely that any time-dependent confounding could have occurred. |
| The two-stage method also requires the strong assumption that there is no time-dependent confounding between the time of secondary baseline and the time of treatment switch (i.e. the date that a subsequent anticancer therapy was started). The median (range) duration of treatment in the CLEAR trial is 17.0 (0.1–39.1) months in the lenvatinib plus pembrolizumab arm and 7.8 (0.1–40.0) months in the sunitinib arm and the median (range) time from randomisation to first subsequent anticancer therapy in the CLEAR trial also differed by treatment [Eisai CS1 (see table 16)]. The assumptions that patients are in a similar condition at the time of secondary baseline and no time-dependent confounding were not assessed by the company within the CS or the additional report of the OS treatment-switching analyses.71 |
|||
| Were results presented appropriately? | Yes | Numbers of OS events and adjusted OS HRs with 95% CIs are presented for lenvatinib plus pembrolizumab vs. sunitinib for the CLEAR trial ITT population for all treatment-switching analyses conducted: no treatment-switching adjustment (i.e. unadjusted), and two-stage estimation method with log-normal, log-logistic and Weibull AFs, with and without re-censoring and with adjustment for stratification factors only or with adjustment for selected baseline covariates [Eisai CS1 (see table 16)]. 95% CIs of adjusted median OS and HRs were estimated using bootstrapping to account for uncertainty introduced into the OS estimates following treatment-switching adjustments. Results of the IPCW adjustment method are presented in an additional report of the OS treatment-switching analyses71 (see Assessment group summary and critique of companies’ economic analyses). |
The AG considers that all relevant results are presented appropriately. |
AG assessment of the statistical approach to the companies’ NMA
Summaries and AG critiques of the Eisai and MSD NMA statistical approaches are provided in Tables 56 and 57 respectively.
| Item | AG assessment | Statistical approach | AG comments |
|---|---|---|---|
| Were NMAs conducted for all relevant outcomes? | Yes | Eisai presented NMAs for PFS (according to FDA and EMA censoring rules), OS, ORR, CR, all-cause Grade ≥ 3 AEs and treatment discontinuation due to AEs for the intermediate-/poor-risk subgroup and separately by IMDC or MSKCC risk subgroups where data were available and the all-risk population [Eisai CS1 (section 4.7 and appendices D 3.1–D 3.7)]. | Indirect evidence is presented for all relevant outcomes for all relevant patient populations and subgroups. |
| Were the networks of comparators appropriate? | Partly | The Eisai search process identified 36 trials that met the SLR inclusion criteria. Following a feasibility assessment, Eisai excluded 27 trials [Eisai CS1 (appendix D.2.1.2)] and included nine trials27,66,97–99,101–104 in at least one of their NMAs. Eisai NMAs of PFS included [Eisai CS1 (appendix D.3.2)]: |
No comparative evidence is presented in the Eisai CS1 for lenvatinib plus pembrolizumab vs. nivolumab plus ipilimumab in the intermediate-/poor-risk subgroup. Therefore, the AG has performed NMAs of PFS, OS and ORR to include all relevant comparators by IMDC risk subgroup (see Results of the assessment group network meta-analyses). The AG acknowledges that as it is not possible to connect tivozanib to the network of comparators for the all-risk population for OS, ORR or Grade ≥ 3 AEs, no indirect comparisons of lenvatinib plus pembrolizumab with tivozanib can be made for OS, ORR or Grade ≥ 3 AEs. |
|
|||
Eisai NMAs of OS included [Eisai CS1 (appendix D.3.1)]:
|
|||
Eisai NMAs of ORR, CR, all-cause Grade ≥ 3 AEs and treatment discontinuation due to AEs included [Eisai CS1 (appendices D.3.3–D.3.7)]:
|
|||
| Were NMA methods appropriate? | Yes | The methods used in the Eisai NMAs are described in the Eisai CS1 (appendices D.2.2 and D.2.3) and Eisai response to the AG clarification letter (question A3). | The AG considers that the Bayesian HR NMAs for all outcomes as described in appendix D.2.2 and that the FP NMAs for PFS and OS using the methods described by Jansen129 have been correctly implemented. |
| Eisai performed NMAs in a Bayesian framework using both FE and RE models. For PFS and OS, the company conducted NMAs estimating constant HRs, as well as first-order and second-order FP NMAs (with first- and second-order parameter values ranging from −3 to 3) according to the methods of Jansen,129 to estimate time-varying HRs due to PH assumption violation within the included trials. Model fit was assessed according to the DIC statistic and clinical plausibility of estimates. | The AG agrees with Eisai that due to the heterogeneity in the evidence base, RE models are more clinically plausible than FE models (see Assessment group summary of patient and trial characteristics and assessment of heterogeneity) but acknowledges the instability of results of RE NMAs, due to the small number of included trials and sparse data. However, it should be noted when interpreting FE NMA results that FE NMAs do not take account of observed heterogeneity between the trials. | ||
| Although Eisai considered that due to heterogeneity of the evidence base, RE models would be more clinically plausible, as a small number of trials were included in the NMAs with few or no data present to estimate heterogeneity variance (appendix D.2.2), FE models were presented and selected as the base case for all NMAs. | |||
| Was inconsistency appropriately assessed in the NMAs? | Yes | Eisai assessed inconsistency ‘locally’ within the closed loops including sunitinib, sorafenib, pazopanib, tivozanib, interferon-alpha and sorafenib in the all-risk population networks of PFS, ORR, CR, all-cause Grade ≥ 3 AEs and treatment discontinuation due to AEs using methods described by Bucher130 to compare direct and indirect evidence. Statistically significant inconsistency between the studies providing direct and indirect comparisons between sunitinib and sorafenib was observed for PFS and treatment discontinuation due to AEs. Inconsistency could not be statistically assessed within the OS NMAs or the NMAs within IMDC or MSKCC risk subgroups due to lack of closed loops within the networks. |
The local assessments of inconsistency performed by Eisai are appropriate. The AG has performed a ‘global’ assessment of inconsistency in the AG PFS NMA in the all-risk population by applying an unrelated mean effects NMA model114 and by comparing model fit statistics of inconsistency models with consistency models (see Assessment group statistical approach to Bayesian hazard ratio network meta-analysis). The AG acknowledges that the consistency of indirect estimates of OS and indirect estimates for all outcomes within the IMDC and MSKCC risk subgroups is unknown. |
| Was the PH assumption appropriately assessed within the NMAs of PFS and OS? | Yes | Eisai assessed the PH assumption for PFS and OS in the included trials by plotting the log cumulative hazard vs. log(time) and by using the Grambsch–Therneau test86 of PH [Eisai CS1 (sections 5.3.1 and 5.3.2) and Eisai response to the AG clarification letter (questions A1 and A2)]. | The AG agrees with the Eisai assessments of PH violation and agrees that estimating time-varying HRs for the PFS and OS NMAs is appropriate. |
| On the basis of these assessments, Eisai considers that over the observed periods of the trials, the assumption of PH was violated for at least one of the trials for PFS and for OS. Due to these PH violations, in addition to PFS and OS NMAs estimating constant HRs, Eisai also used FP models to estimate time-varying HRs in their PFS and OS NMAs. | The AG considers that due to the limitations of FP NMAs for decision-making [Eisai CS1 (appendix D.2.3) and section Assessment group assessment of proportional hazards assumptions of this report)], it is appropriate to also present NMAs estimating constant HRs for PFS and OS. | ||
| Was the presentation of NMA results appropriate? | Yes | Eisai presented FE NMA results for lenvatinib plus pembrolizumab vs. each comparator included in the network for the intermediate-/poor-risk subgroup and by IMDC/MSKCC risk subgroups and all-risk population [(Eisai CS1 (section 4.7 and appendices D3.1–D3.7)]. Constant HRs and time-varying HRs (with 95% CrIs) are presented for PFS and OS NMAs [(Eisai CS1 and appendices D.3.1–D.3.3, D.4.1 and D4.2)]. ORs (with 95% CrIs) are presented for ORR, CR, all-cause Grade ≥ 3 AEs and treatment discontinuation due to AEs NMAs. | The presentation of Eisai NMA results for all outcomes is appropriate. In addition to results for lenvatinib plus pembrolizumab vs. each comparator, the AG presents FE NMA results for all pairs of comparators included within each network (see Results of the assessment group network meta-analyses). |
| The probability that lenvatinib plus pembrolizumab is better than the comparator is also presented for NMAs of all outcomes [Eisai CS1 and appendices D.3.1–D.3.7)]. Eisai also present subgroup, scenario and sensitivity analyses where data are available to examine NMA results for IMDC or MSKCC risk subgroups and to examine the robustness of NMA results to assumptions and to the exclusion of trials from the NMAs [Eisai CS1 (appendices D.2.2.2.3 and D.3.1–D.3.7)]. |
| Item | AG assessment | Statistical approach | AG comments |
|---|---|---|---|
| Were NMAs conducted for all relevant outcomes? | Yes | MSD presented NMAs for PFS and OS (according to FDA censoring rules) for the intermediate-/poor-risk subgroup and all-risk population (section 2.9.3 and appendix M). | Indirect evidence is presented for the key efficacy outcomes for the relevant populations listed within the final scope.29 No indirect evidence is presented for response outcomes or safety outcomes, or separately for IMDC or MSKCC risk subgroups. |
| Were the networks of comparators appropriate? | Partly | Following a feasibility assessment of trials identified in the SLR (appendix D.1.1), MSD included six trials66,97,98,101,102,104 in at least one of their NMAs. | No comparative evidence is presented in the MSD CS2 for lenvatinib plus pembrolizumab vs. nivolumab plus ipilimumab in the intermediate-/poor-risk subgroup. Therefore, the AG has performed NMAs of PFS, OS and ORR to include all relevant comparators by IMDC risk subgroup (see Results of the assessment group network meta-analyses). The AG acknowledges that as it is not possible to connect tivozanib to the network of comparators for the all-risk population for OS, no indirect comparisons of lenvatinib plus pembrolizumab with tivozanib can be made for OS. |
MSD NMAs of PFS included (section 2.9.3, figure 13 and appendix M):
|
|||
| Were NMA methods appropriate? | Yes | The methods used for the MSD NMAs are described in the MSD CS2 (appendix D.1.1 and MSD response to the AG clarification letter, question A2). | The AG considers that the Bayesian HR NMAs for all outcomes as described in appendix D.1.1 and that the FP NMAs for PFS and OS using the methods described by Jansen129 have been correctly implemented. |
| MSD performed NMAs in a Bayesian framework using both FE and RE models. For PFS and OS, the company conducted NMAs estimating constant HRs, as well as first-order and second-order FP NMAs (with first-and second-order parameter values of −1, 0 and 1) according to the methods of Jansen,129 to estimate time-varying HRs due to PH assumption violation within the included trials. Model fit was assessed according to the DIC statistic and clinical plausibility of estimates. Although MSD considered that RE models would be more clinically plausible due to heterogeneity of the evidence base, as a small number of trials were included in the NMAs with most treatment comparisons informed by one trial, only FE models were presented (section 2.9 and appendices D.1.1 and M). |
The AG agrees with MSD that RE models are more clinically plausible than FE models due to the heterogeneity in the evidence base (see Assessment group summary of patient and trial characteristics and assessment of heterogeneity) but acknowledges the instability of the results of RE NMAs due to the small number of included trials and sparse data. However, it should be noted when interpreting FE NMA results that FE NMAs do not take account of observed heterogeneity between the trials. | ||
| Was inconsistency appropriately assessed in the NMAs? | Not assessed | MSD did not undertake any assessments of inconsistency in the NMAs. | The AG has performed a ‘global’ assessment of inconsistency for PFS by applying an unrelated mean effects NMA model114 and by comparing model fit statistics of inconsistency models with consistency models (see Assessment group statistical approach to Bayesian hazard ratio network meta-analysis). Due to lack of closed loops within the network for OS, inconsistency cannot be formally assessed. Therefore, the consistency of indirect estimates of OS is unknown. |
| Was the PH assumption appropriately assessed within the NMAs of PFS and OS? | Partly | MSD assessed the PH assumption for PFS and OS in the CLEAR trial by plotting the log cumulative hazard vs. log(time), by plotting Schoenfeld residuals vs. time and by using the Grambsch–Therneau test86 of PH (MSD CS:2 section 3.3 and MSD response to the AG clarification letter, question A1). MSD did not present assessments of the PH assumption for PFS and OS in the other trials included in the NMAs. In order to relax the PH assumption for the NMAs, in addition to PFS and OS NMAs estimating constant HRs, MSD also used FP models to estimate time-varying HRs in their PFS and OS NMAs. |
The AG agrees that estimating time-varying HRs for the PFS and OS NMAs is appropriate to relax the PH assumption. The AG considers that due to the limitations of FP NMAs for decision-making [Eisai CS1 (appendix D.2.3) and section Assessment group assessment of proportional hazards assumptions of this report], it is appropriate to also present NMAs estimating constant HRs for PFS and OS. |
| Was the presentation of NMA results appropriate? | Yes | MSD presented FE NMA results for all pairs of comparators included in each network for the intermediate-/poor-risk subgroup and by IMDC or MSKCC risk subgroups and all-risk population. Constant HRs and time-varying HRs (with 95% CrIs) are presented for PFS and OS NMAs (section 2.9 and appendix M). | The presentation of MSD PFS and OS NMA results is appropriate. |
Appendix 4 Assessment group network meta-analyses
This appendix contains additional information about the methods used by the AG to conduct its NMAs in sections Network diagrams for assessment group network meta-analyses, Outcome data included in assessment group network meta-analyses, Assessment group quality assessment of the trials included in the network meta-analysis, Trial design and patient characteristics in the trials included in the assessment group network meta-analysis, Proportional hazards assessments for trials included in the assessment group network meta-analysis and Example statistical code for assessment group network meta-analysis, including the AG assessment of the methodological quality of the included trials (see Assessment group quality assessment of the trials included in the network meta-analysis). Additional results are presented in sections Additional network meta-analysis results tables, Assessment group assessment of inconsistency in the network meta-analysis and Additional AG NMA analyses, including the AG assessment of inconsistency in the NMAs (see Assessment group assessment of inconsistency in the network meta-analysis, Additional assessment group network meta-analysis sensitivity analyses).
Network diagrams for assessment group network meta-analyses
FIGURE 4.
Network diagram for the AG NMAs for the intermediate-/poor-risk subgroup (PFS, OS and ORR).
FIGURE 5.
Network diagram for the AG NMAs for the intermediate-/poor-risk subgroup (Grade ≥ 3 AEs).

FIGURE 6.
Network diagram for the AG NMAs for the all-risk population (OS, ORR and Grade ≥ 3 AEs) and for the favourable-risk subgroup (PFS and OS).

FIGURE 7.
Network diagram for the AG NMAs for the favourable-risk subgroup (PFS and OS) and for the all-risk population (OS, ORR and Grade ≥ 3 AEs). a, The CROSS-J-RCC,104 SWITCH98 and SWITCH II103 had a sequential design (patients received first-line therapy with the treatment they were randomised to, and patients who discontinued first-line therapy due to disease progression or toxicity received the other trial treatment, i.e. second line). PFS data for first-line treatment used in the NMAs; and b, The TIVO-1 trial recruited patients with untreated mRCC and patients who had received prior systematic therapy for mRCC. PFS data for the untreated subgroup are used in the NMAs. mRCC, metastatic renal cell carcinoma.

Outcome data included in assessment group network meta-analyses
| Trial | Intervention | Analysis methods | Median follow-up months (95% CI) | N | Median PFS months (95% CI)a |
HR (95% CI)a |
|---|---|---|---|---|---|---|
| Intermediate-/poor-risk subgroup | ||||||
| CLEAR | Lenvatinib + pembrolizumab |
|
22.3 (21.1 to 25.6) | 243 | FDA: Confidential information has been removed EMA: Confidential information has been removed |
FDA: Confidential information has been removed EMA: Confidential information has been removed |
| Sunitinib | 16.6 (13.1 to 18.5) | 229 | FDA: Confidential information has been removed EMA: Confidential information has been removed |
|||
| Lenvatinib + pembrolizumab |
|
22.3 (21.1 to 25.6) | 259 | FDA: Confidential information has been removed EMA: Confidential information has been removed |
FDA: Confidential information has been removed EMA: Confidential information has been removed |
|
| Sunitinib | 16.6 (13.1 to 18.5) | 260 | FDA: Confidential information has been removed EMA: Confidential information has been removed |
|||
| CABOSUN97 | Cabozantinib |
|
25 (IQR: 21.9–30.9) | 79 | 8.6 (6.8 to 14.0) | 0.48 (0.31 to 0.74) |
| Sunitinib | 25 (IQR: 21.9–30.9) | 78 | 5.3 (3.0 to 8.2) | |||
| CheckMate 214100 | Nivolumab + ipilimumab |
|
NRb | 425 | 11.6 (8.4 to 15.5) | 0.75 (0.62 to 0.90) |
| Sunitinib | NRb | 422 | 8.3 (7.0 to 10.8) | |||
| Favourable-risk subgroup | ||||||
| CLEAR | Lenvatinib + pembrolizumab |
|
22.3 (21.1 to 25.6) | 110 | FDA: 28.1 (NR to NR) EMA: Confidential information has been removed |
FDA: 0.41 (0.28 to 0.62) |
| Sunitinib | 16.6 (13.1 to 18.5) | 124 | FDA: 12.9 (NR to NR) Confidential information has been removed |
EMA: Confidential information has been removed | ||
| Lenvatinib + pembrolizumab |
|
22.3 (21.1 to 25.6) | 96 | FDA: Confidential EMA: Confidential information has been removed |
FDA: 0.36 (0.23 to 0.54) | |
| Sunitinib | 16.6 (13.1 to 18.5) | 97 | FDA: Confidential information has been removed EMA: Confidential information has been removed |
EMA: Confidential | ||
| COMPARZ101 | Pazopanib |
|
NR | 151 | NR | 1.02 (0.62 to 1.42)c |
| Sunitinib | NR | 152 | NR | |||
| Pazopanib |
|
NR | 151 | NR | 1.01 (0.63 to 1.39)c | |
| Sunitinib | NR | 152 | NR | |||
| CROSS-J-RCC104,d | Sunitinib |
|
NR | 12 | NR | 0.25 (0.08 to 0.73)e |
| Sorafenib | NR | 14 | NR | |||
| SWITCH98,d | Sorafenib |
|
NR | 71 | NR | 1.30 (0.87 to 1.94)e |
| Sunitinib | NR | 82 | NR | |||
| SWITCH II103,d | Sorafenib |
|
NR | NR | NR | NR |
| Pazopanib | NR | NR | NR | |||
| TIVO-1102 | Tivozanib |
|
NR | NR | NR | NR |
| Sorafenib | NR | NR | NR | |||
| All-risk population | ||||||
| CLEAR | Lenvatinib + pembrolizumab |
|
22.3 (21.1 to 25.6) | 355 | FDA: 23.9 (20.8 to 27.7) EMA: 22.1 (18.4 to 25.9) |
FDA: 0.39 (0.32 to 0.49) EMA: 0.41 (0.33 to 0.50) |
| Sunitinib | 16.6 (13.1 to 18.5) | 357 | FDA: 9.2 (6.0 to 11.0) EMA: 9.2 (7.0 to 11.0) |
|||
| COMPARZ101 | Pazopanib |
|
NR | 557 | 8.4 (8.3 to 10.9) | 1.05 (0.90 to 1.22) |
| Sunitinib | NR | 553 | 9.5 (8.3 to 11.1) | |||
| CROSS-J-RCC104,d | Sunitinib |
|
NR | 57 | 8.7 (5.5 to 21.1) | 0.67 (0.42 to 1.08) |
| Sorafenib | NR | 63 | 7.0 (6.1 to 12.2) | |||
| SWITCH98,d | Sorafenib |
|
Mean: 10.3 | 182 | 5.9 (90% CI 5.5 to 7.9) | 1.19 (0.93 to 1.45)f |
| Sunitinib | Mean: 10.3 | 183 | 8.5 (90% CI 7.1 to 11.2) | |||
| SWITCH II103,d | Sorafenib |
|
NR | 189 | 5.6 (4.7 to 6.3) | 0.69 (0.54 to 0.87) |
| Pazopanib | NR | 188 | 9.3 (7.4 to 10.6) | |||
| TIVO-1102 | Tivozanib |
|
NR | 181 | 12.7 (9.1 to 15.0) | 0.76 (0.58 to 0.99) |
| Sorafenib | NR | 181 | 9.1 (7.3 to 10.8) | |||
| Trial | Intervention | Analysis methods | Median follow-up months (95% CI) | N | Median OS months (95% CI) |
HR (95% CI) |
|---|---|---|---|---|---|---|
| Intermediate-/poor-risk subgroup | ||||||
| CLEAR | Lenvatinib + pembrolizumab |
|
33.7 (32.8 to 34.4) | 243 | Confidential information has been removed | Confidential information has been removed |
| Sunitinib | 33.4 (32.5 to 34.1) | 229 | Confidential information has been removed | |||
| Lenvatinib + pembrolizumab |
|
33.7 (32.8 to 34.4) | 259 | Confidential information has been removed | Confidential information has been removed | |
| Sunitinib | 33.4 (32.5 to 34.1) | 260 | Confidential information has been removed | |||
| CABOSUN97 | Cabozantinib |
|
35.4 (IQR 31.4–40.4) | 79 | 26.6 (14.6 to NE) | 0.80 (0.53 to 1.21) |
| Sunitinib | 35.4 (IQR 31.4–40.4) | 78 | 21.2 (16.3 to 27.4) | |||
| CheckMate 214100 | Nivolumab + ipilimumab |
|
43.6 (NR to NR) | 425 | 47.0 (35.6 to NE) | 0.66 (0.55 to 0.80) |
| Sunitinib | 32.3 (NR to NR) | 422 | 26.6 (22.1 to 33.5) | |||
| Favourable-risk subgroup | ||||||
| CLEAR | Lenvatinib + pembrolizumab |
|
33.7 (32.8 to 34.4) | 110 | NE | 1.22 (0.66 to 2.26) |
| Sunitinib | 33.4 (32.5 to 34.1) | 124 | NE | |||
| Lenvatinib + pembrolizumab |
|
33.7 (32.8 to 34.4) | 96 | NE | 1.00 (0.51 to 1.96) | |
| Sunitinib | 33.4 (32.5 to 34.1) | 97 | NE | |||
| COMPARZ101 | Pazopanib |
|
NR | 151 | 42.5 (37.9 to NR) | 0.88 (0.63 to 1.21) |
| Sunitinib | NR | 152 | 43.6 (37.1 to 47.4) | |||
| TIVO-1102 | Tivozanib |
|
NR | NR | NR | NR |
| Sorafenib | NR | NR | NR | |||
| All-risk population | ||||||
| CLEAR | Lenvatinib + pembrolizumab |
|
33.7 (32.8 to 34.4) | 355 | NE (41.5 to NE) | 0.72 (0.55 to 0.93) |
| Sunitinib | 33.4 (32.5 to 34.1) | 357 | NE (38.4 to NE) | |||
| COMPARZ101 | Pazopanib |
|
NR | 557 | 28.3 (26.0 to 35.5) | 0.92 (0.79 to 1.06) |
| Sunitinib | NR | 553 | 29.1 (25.4 to 33.1) | |||
| TIVO-1102 | Tivozanib |
|
NR | 181 | NR | 1.23 (0.67 to 1.55)c |
| Sorafenib | NR | 181 | NR | |||
| Trial | Intervention | Analysis methods | Median follow-up months (95% CI) |
N | ORR (n) | ORR (%) |
|---|---|---|---|---|---|---|
| Intermediate-/poor-risk subgroup | ||||||
| CLEAR | Lenvatinib + pembrolizumab |
|
NRa | 243 | Confidential information has beeb removed | Confidential information has been removed |
| Sunitinib | NRa | 229 | Confidential information has been removed | Confidential information has been removed | ||
| Lenvatinib + pembrolizumab |
|
NRa | 259 | Confidential information has been removed | Confidential information has been removed | |
| Sunitinib | NRa | 260 | Confidential information has been removed | Confidential information has been removed | ||
| CABOSUN97 | Cabozantinib |
|
NR | 79 | 16 | 20 |
| Sunitinib | NR | 78 | 7 | 9 | ||
| CheckMate 214100 | Nivolumab + ipilimumab |
|
NRb | 425 | 179 | 42.1 |
| Sunitinib | NRb | 422 | 111 | 26.3 | ||
| All-risk population | ||||||
| CLEAR | Lenvatinib + pembrolizumab |
|
NR | 355 | 252 | 71 |
| Sunitinib | NR | 357 | 129 | 36.1 | ||
| COMPARZ101 | Pazopanib |
|
NR | 557 | 3 | 31 |
| Sunitinib | NR | 553 | 137 | 25 | ||
| CROSS-J-RCC104,c | Sunitinib |
|
NR | 57 | 14d | 29.8d |
| Sorafenib | NR | 63 | 10d | 21.2d | ||
| SWITCH98,c | Sorafenib |
|
NR | 177 | 55d | 31d |
| Sunitinib | NR | 176 | 51d | 29d | ||
| SWITCH II103,c | Sorafenib |
|
NR | 189 | 54d | 28.6d |
| Pazopanib | NR | 188 | 87d | 46.3d | ||
| TIVO-1102 | Tivozanib |
|
NR | NR | NR | NR |
| Sorafenib | NR | NR | NR | NR | ||
| Trial | Intervention | Analysis methods | Median follow-up months (95% CI) |
N | Grade ≥ 3 AE (n) | Grade ≥ 3 AE (%) |
|---|---|---|---|---|---|---|
| Intermediate-/poor-risk subgroup | ||||||
| CLEAR | Lenvatinib + pembrolizumab | Grade ≥ 3 TEAE, NCI CTCAE v4.03 (IMDC) | NRa | 241 | Confidential information has been removed | Confidential information has been removed |
| Sunitinib | NRa | 220 | Confidential information has been removed | Confidential information has been removed | ||
| Lenvatinib + pembrolizumab | Grade ≥ 3 TEAE, NCI CTCAE v4.03 (MSKCC) | NRa | 256 | Confidential information has been removed | Confidential information has been removed | |
| Sunitinib | NRa | 247 | Confidential information has been removed | Confidential information has been removed | ||
| CABOSUN97 | Cabozantinib | All cause AEs, NCI CTCAE v4 (IMDC) | NR | 78 | 53 | 68 |
| Sunitinib | NR | 72 | 47 | 65 | ||
| CheckMate 214100 | Nivolumab + ipilimumab | NR | NR | NR | NR | NR |
| Sunitinib | NR | NR | NR | NR | ||
| All-risk population | ||||||
| CLEAR | Lenvatinib + pembrolizumab | Grade ≥ 3 TEAE, NCI CTCAE v4.03 | NRa | 352 | 290 | 82.4 |
| Sunitinib | NRa | 340 | 244 | 71.8 | ||
| COMPARZ101 | Pazopanib | Grade 3 + TEAEs, NCI CTCAE v3 | NR | 554 | 423 | 76 |
| Sunitinib | NR | 548 | 419 | 77 | ||
| CROSS-J-RCC104,b | Sunitinib | Interim analysis, first-line treatment, Grade ≥ 3 all-cause AEs, NCI CTCAE v3 | NR | 57 | 48c | 84.2c |
| Sorafenib | NR | 63 | 50c | 79.4c | ||
| SWITCH98,b | Sorafenib | Grade 3/4 TEAEs, NCI CTCAE v3 | NR | 177 | 117c | 66c |
| Sunitinib | NR | 176 | 118c | 67c | ||
| SWITCH II103,b | Sorafenib | Grade 3/4 TEAEs, NCI CTCAE v4.03 | NR | 183 | 108c | 59c |
| Pazopanib | NR | 183 | 117c | 64c | ||
| TIVO-1102 | Tivozanib | NR | NR | NR | NR | NR |
| Sorafenib | NR | NR | NR | NR | ||
Assessment group quality assessment of the trials included in the network meta-analysis
The AG assessed quality of the RCTs in accordance with suggested criteria published in the CRD’s Guidance for undertaking reviews in health care. 60 The results of the AG’s quality assessment of the eight RCTs66,97,98,100–104 included in the AG NMAs are presented in Table 62.
| Quality assessment item60 | CABOSUN97 | CheckMate 214100 | CLEAR | COMPARZ101 | CROSS-J-RCC104 | TIVO-1102 | SWITCH98 | SWITCH II103 |
|---|---|---|---|---|---|---|---|---|
| Was the method used to assign participants to treatment arms really random? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓a | ✓ | Unclear |
| Was the allocation of treatment concealed? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓a | ✓ | Unclear |
| Was the number of participants randomised stated? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Were details of baseline comparability presented? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Was baseline comparability achieved? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Were the study eligibility criteria specified? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Were any co-interventions identified that may influence the outcomes for each group? | ✕ | × | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| Were the outcome assessors blinded to treatment allocation? | ✓ | ✓ | ✓ | ✓ | ✕ | ✓a | ✕ | ✕ |
| Were the individuals administering the intervention blinded to treatment allocation? | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| Were the participants receiving the intervention blinded to treatment allocation? | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| Was the success of the blinding procedure assessed? | NA | NA | NA | NA | NA | NA | NA | NA |
| Were at least 80% of the participants originally included in the randomisation process followed up in the final analysis? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Were the reasons for patient withdrawals stated? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Was an intention to treat analysis included? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Is there any evidence that more outcomes were measured than were reported? | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
Trial design and patient characteristics in the trials included in the assessment group network meta-analysis
| Trial | Trial design and location | Population | Treatments | N | Median age (range) years | Male: n (%) by trial arm | Male: n/N overall |
|---|---|---|---|---|---|---|---|
| CABOSUN97 | Phase II, open label, USA | Untreated advanced or metastatic clear cell RCC; intermediate- or poor-risk disease by IMDC criteria | Cabozantinib | 79 | 63 (IQR 56–69) | 66 (83.5%) | 123/157 (78.3%) |
| Sunitinib | 78 | 64 (IQR 57–71) | 57 (73.1%) | ||||
| CheckMate 214100 | Phase III, open label, international | Untreated advanced clear cell RCC | Nivolumab + ipilimumab | 425a | 62 (26–85) | 314 (73.9%) | 615/847 (72.6%) |
| Sunitinib | 422a | 61 (2185) | 301 (71.3%) | ||||
| CLEAR | Phase III, open label, international | Untreated advanced clear cell RCC | Lenvatinib + pembrolizumab | 355 | 64 (34–88) | 255 (71.8%) | 530/712 (74.4%) |
| Sunitinib | 357 | 61 (29–82) | 275 (77.0%) | ||||
| COMPARZ101 | Phase III, open label, international | Untreated advanced or metastatic clear cell RCC | Pazopanib | 557 | 61 (18–88) | 398 (71.5%) | 813/1110 (73.2%) |
| Sunitinib | 553 | 62 (23–86) | 415 (75.0%) | ||||
| CROSS-J-RCC104 | Phase III sequential design, open label, Japan | Untreated metastatic clear cell RCC; favourable or intermediate-risk disease by MSKCC criteria | Sunitinib | 57 | 67 (41–79) | 46 (80.7%) | 99/120 (82.5%) |
| Sorafenib | 63 | 66 (44–79) | 53 (84.1%) | ||||
| SWITCH98 | Phase III sequential design, open label, Europe | Untreated advanced or metastatic RCC; 87% with clear cell histology; favourable or intermediate-risk disease by MSKCC criteria | Sunitinib | 182 | 65 (40–83) | 135 (74.2%) | 274/365 (75.1%) |
| Sorafenib | 183 | 64 (39–84) | 139 (76.0%) | ||||
| SWITCH II103 | Phase III sequential design, open label, Europe | Untreated advanced or metastatic RCC; 87% with clear cell histology; favourable or intermediate-risk disease by MSKCC criteria | Pazopanib | 188 | 68 (26–86) | 137 (72.9%) | 273/377 (72.4%) |
| Sorafenib | 189 | 68 (31–84) | 136 (72.0%) | ||||
| TIVO-1102 | Phase II, open label, international | Metastatic clear cell RCC; untreated patients (70%) and patients who had received previous systematic therapy (30%) | Tivozanib | 181b | NR | NR | NR |
| Sorafenib | 181b | NR | NR |
| Trial | Treatments | N | IMDC risk subgroups: n (% of N) | MSKCC risk subgroups: n (% of N) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Favourable | Intermediate | Poor | Intermediate/Poor | Not evaluated | Favourable | Intermediate | Poor | Intermediate/Poor | Unknown | |||
| CABOSUN97 | Cabozantinib | 79 | NA | 64 (81.0%) | 15 (19.0%) | 79 (100%) | NA | NR | NR | NR | NR | NR |
| Sunitinib | 78 | NA | 63 (80.8%) | 15 (19.2%) | 78 (100%) | NA | NR | NR | NR | NR | NR | |
| CheckMate 214100 | Nivolumab + ipilimumab | 425a | NAa | 334 (78.6%) | 91 (21.4%) | 425 (100%) | NA | NR | NR | NR | NR | NR |
| Sunitinib | 422a | NAa | 333 (78.9%) | 89 (21.1%) | 422 (100%) | NA | NR | NR | NR | NR | NR | |
| CLEAR | Lenvatinib + pembrolizumab | 355 | 110 (31.0%) | 210 (59.2%) | 33 (9.3%) | 243 (68.5%) | 2 (0.6%) | 96 (27.0%) | 227 (63.9%) | 32 (9.0%) | 259 (73.0%) | NA |
| Sunitinib | 357 | 124 (34.7%) | 192 (54.1%) | 37 (10.4%) | 229 (64.1%) | 4 (1.1%) | 97 (27.2%) | 228 (63.9%) | 32 (9.0%) | 260 (72.8%) | NA | |
| COMPARZ101 | Pazopanib | 557 | NR | NR | NR | NR | NR | 151 (27.1%) | 322 (57.8%) | 67 (12.0%) | 389 (69.8%) | 17 (3.1%) |
| Sunitinib | 553 | NR | NR | NR | NR | NR | 152 (27.5%) | 328 (59.3%) | 52 (9.4%) | 380 (68.7%) | 21 (3.8%) | |
| CROSS-J-RCC104 | Sunitinib | 57 | NR | NR | NR | NR | NR | 12 (21.1%) | 45 (78.9%) | NA | NA | NA |
| Sorafenib | 63 | NR | NR | NR | NR | NR | 14 (22.2%) | 49 (77.8%) | NA | NA | NA | |
| SWITCH98 | Sunitinib | 182 | NR | NR | NR | NR | NR | 71 (39.0%) | 108 (59.3%) | 1 (0.5%) | 109 (59.9%) | 2 (1.1%) |
| Sorafenib | 183 | NR | NR | NR | NR | NR | 82 (44.8%) | 94 (51.4%) | 1 (0.5%) | 95 (51.9%) | 6 (3.3%) | |
| SWITCH II103 | Pazopanib | 188 | NR | NR | NR | NR | NR | 91 (48.4%) | 89 (47.3%) | 5 (2.7%) | 94 (50.0%) | 3 (1.6%) |
| Sorafenib | 189 | NR | NR | NR | NR | NR | 95 (50.3%) | 90 (47.6%) | 4 (2.1%) | 94 (49.7%) | 0 (0%) | |
| TIVO-1102 | Tivozanib | 181b | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sorafenib | 181b | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
Proportional hazards assessments for trials included in the assessment group network meta-analysis
The AG assessed the validity of the PH assumption for RCTs included in the AG NMAs using figures (i.e. Schoenfeld residuals plots or log cumulative hazard plots) and statistical tests (i.e. Grambsch–Therneau test86) presented in the Eisai CS1 (sections 5.3.1 and 5.3.2), the Eisai response to question A1 and A2 of the AG clarification letter, and in the MSD response to additional clarification questions. The AG is unable to present the results of these tests or the plots due to their confidential nature. The AG also digitised K-M data presented in the publication of the 42-month follow-up of the CheckMate 214 trial100 (this RCT was not included in the Eisai or MSD NMAs), and assessed the PH assumption for OS and PFS in the intermediate-/poor-risk subgroup by plotting Schoenfeld residuals and performing a Grambsch–Therneau test. 86
Results of the tests of Schoenfeld residuals conducted by the AG for the IMDC intermediate-/poor-risk subgroup in the CheckMate 214 trial are p = 0.0002 for PFS and p = 0.4055 for OS. Plots of Schoenfeld residuals against time for the intermediate-/poor-risk subgroup in the CheckMate 214 trial100 for PFS and OS are presented in Figures 8 and 9.
FIGURE 8.
Schoenfeld residuals plot for PFS (CheckMate 214 trial, intermediate-/poor-risk subgroup).

FIGURE 9.
Schoenfeld residuals plot for OS (CheckMate 214 trial, intermediate-/poor-risk subgroup).
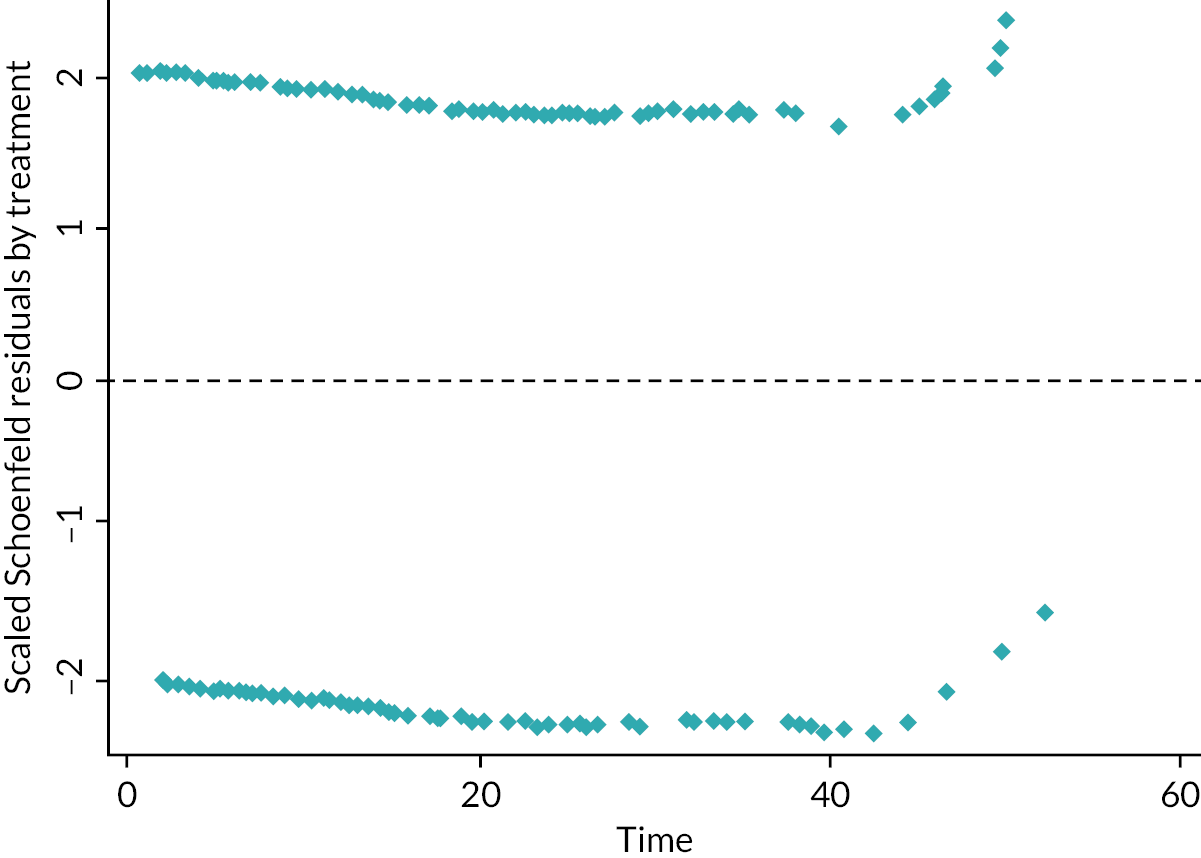
Example statistical code for assessment group network meta-analysis
Fixed and REs NMAs of contrast-based time-to-event data (PFS and OS)
### Install and run multinma to conduct Bayesian network meta-analysis ###
if (!require(‘multinma’)) install.package(‘multinma’)
library(‘multinma’)
options(mc.cores = parallel::detectCores())
### Load datasets ###
os_1 <- read.csv(‘OS all-risk.csv’)
os_2 <- read.csv(‘OS intermediate poor IMDC.csv’)
os_3 <- read.csv(‘OS favourable IMDC.csv’)
os_4 <- read.csv(‘OS favourable MSKCC.csv’)
### Setting up networks and network plots ###
os_1_network <- set_agd_contrast(os_1,
study = studyc,
trt = trtc_1,
y = loghr,
se = seloghr,
sample_size = n,
trt_ref = ‘Sunitinib’)
plot(os_1_network, weight_edges = TRUE, weight_nodes = TRUE)
os_2_network <- set_agd_contrast(os_2,
study = studyc,
trt = trtc_1,
y = loghr,
se = seloghr,
sample_size = n,
trt_ref = ‘Sunitinib’)
plot(os_2_network, weight_edges = TRUE, weight_nodes = TRUE)
os_3_network <- set_agd_contrast(os_3,
study = studyc,
trt = trtc_1,
y = loghr,
se = seloghr,
sample_size = n,
trt_ref = ‘Sunitinib’)
plot(os_3_network, weight_edges = TRUE, weight_nodes = TRUE)
os_4_network <- set_agd_contrast(os_4,
study = studyc,
trt = trtc_1,
y = loghr,
se = seloghr,
sample_size = n,
trt_ref = ‘Sunitinib’)
plot(os_4_network, weight_edges = TRUE, weight_nodes = TRUE)
### Fixed-effect NMA ###
FE_os_1 <- nma(os_1_network,
trt_effects = ‘fixed’,
consistency = ‘consistency’,
link=‘log’,
chains = 3,
iter = 2e5,
warmup = 1e5,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10))
FE_os_2 <- nma(os_2_network,
trt_effects = ‘fixed’,
consistency = ‘consistency’,
link=‘log’,
chains = 3,
iter = 2e5,
warmup = 1e5,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10))
FE_os_3 <- nma(os_3_network,
trt_effects = ‘fixed’,
consistency = ‘consistency’,
link=‘log’,
chains = 3,
iter = 2e5,
warmup = 1e5,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10))
FE_os_4 <- nma(os_4_network,
trt_effects = ‘fixed’,
consistency = ‘consistency’,
link=‘log’,
chains = 3,
iter = 2e5,
warmup = 1e5,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10))
### Random-effects NMA ###
RE_os_1 <- nma(os_1_network,
trt_effects = ‘random’,
consistency = ‘consistency’,
link=‘log’,
chains = 3,
iter = 2e5,
warmup = 1e5,
adapt_delta = 0.99,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10),
prior_het = half_normal(scale = 5))
RE_os_2 <- nma(os_2_network,
trt_effects = ‘random’,
consistency = ‘consistency’,
link=‘log’,
chains = 3,
iter = 2e5,
warmup = 1e5,
adapt_delta = 0.99,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10),
prior_het = half_normal(scale = 5))
RE_os_3 <- nma(os_3_network,
trt_effects = ‘random’,
consistency = ‘consistency’,
link=‘log’,
chains = 3,
iter = 2e5,
warmup = 1e5,
adapt_delta = 0.99,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10),
prior_het = half_normal(scale = 5))
RE_os_4 <- nma(os_4_network,
trt_effects = ‘random’,
consistency = ‘consistency’,
link=‘log’,
chains = 3,
iter = 2e5,
warmup = 1e5,
adapt_delta = 0.99,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10),
prior_het = half_normal(scale = 5))
### Generate all pairwise contrasts between treatments ###
### All-risk ###
FE_all_os1 <- relative_effects(FE_os_1, all_contrasts = TRUE)
RE_all_os1 <- relative_effects(RE_os_1, all_contrasts = TRUE)
### Intermediate poor IMDC ###
FE_all_os2 <- relative_effects(FE_os_2, all_contrasts = TRUE)
RE_all_os2 <- relative_effects(RE_os_2, all_contrasts = TRUE)
### NMA favourable IMDC ###
FE_all_os3 <- relative_effects(FE_os_3, all_contrasts = TRUE)
RE_all_os3 <- relative_effects(RE_os_3, all_contrasts = TRUE)
### OS NMA favourable MSKCC ###
FE_all_os4 <- relative_effects(FE_os_4, all_contrasts = TRUE)
RE_all_os4 <- relative_effects(RE_os_4, all_contrasts = TRUE)
### Inconsistency models - all-risk only ###
FE_pfs_1_inc <- nma(pfs_1_network,
trt_effects = ‘fixed’,
consistency = ‘ume’,
link = ‘log’,
chains = 3,
iter = 2e5,
warmup = 1e5,
control = list(max_treedepth = 15),
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10))
FE_pfs_1_sens_inc <- nma(pfs_sens1_network,
trt_effects = ‘fixed’,
consistency = ‘ume’,
link = ‘log’,
chains = 3,
iter = 2e5,
warmup = 1e5,
control = list(max_treedepth = 15),
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10))
### Model fit statistics ####
dic_FE_pfs1 <- dic(FE_pfs_1)
dic_FE_pfs1_inc <- dic(FE_pfs_1_inc)
dic_FE_pfs_sens1 <- dic(FE_pfs_sens1)
dic_FE_pfs_sens1_inc <- dic(FE_pfs_1_sens_inc)
Fixed and random effects NMAs of arm-based binary data (ORR)
### Install and run multinma to conduct Bayesian network meta-analysis ###
if (!require(‘multinma’)) install.package(‘multinma’)
library(‘multinma’)
options(mc.cores = parallel::detectCores())
### Load datasets ###
orr_1 <- read.csv(‘ORR all-risk.csv’)
orr_2 <- read.csv(‘ORR intermediate poor IMDC.csv’)
### Setting up networks and network plots ###
orr_1_network <- set_agd_arm(orr_1,
study = study.c,
trt = trtc,
r = r1,
n = n1,
trt_ref = ‘Sunitinib’)
plot(orr_1_network, weight_edges = TRUE, weight_nodes = TRUE)
orr_2_network <- set_agd_arm(orr_2,
study = study.c,
trt = trtc,
r = r1,
n = n1,
trt_ref = ‘Sunitinib’)
plot(orr_2_network, weight_edges = TRUE, weight_nodes = TRUE)
### Fixed effects NMA ###
FE_orr_1 <- nma(orr_1_network,
trt_effects = ‘fixed’,
consistency = ‘consistency’,
link=‘logit’,
chains = 3,
iter = 2e5,
warmup = 1e5,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10))
FE_orr_2 <- nma(orr_2_network,
trt_effects = ‘fixed’,
consistency = ‘consistency’,
link=‘logit’,
chains = 3,
iter = 2e5,
warmup = 1e5,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10))
### Random effects NMA ###
RE_orr_1 <- nma(orr_1_network,
trt_effects = ‘random’,
consistency = ‘consistency’,
link=‘logit’,
chains = 3,
iter = 2e5,
warmup = 1e5,
adapt_delta = 0.99,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10),
prior_het = half_normal(scale = 5))
RE_orr_2 <- nma(orr_2_network,
trt_effects = ‘random’,
consistency = ‘consistency’,
link=‘logit’,
chains = 3,
iter = 2e5,
warmup = 1e5,
adapt_delta = 0.99,
prior_intercept = normal(scale = 10),
prior_trt = normal(scale = 10),
prior_het = half_normal(scale = 5))
### Generate all pairwise contrasts between treatments ###
### All-risk ###
FE_all_orr1 <- relative_effects(FE_orr_1, all_contrasts = TRUE)
RE_all_orr1 <- relative_effects(RE_orr_1, all_contrasts = TRUE)
## Intermediate poor IMDC ###
FE_all_orr2 <- relative_effects(FE_orr_2, all_contrasts = TRUE)
RE_all_orr2 <- relative_effects(RE_orr_2, all_contrasts = TRUE)
Additional network meta-analysis results tables
| Treatment | Comparator | REs HR (95% CrI)a |
|---|---|---|
| Intermediate-/poor-risk subgroup | ||
| Lenvatinib + pembrolizumab | Sunitinib | 0.40 (0 to 773) |
| Lenvatinib + pembrolizumab | Cabozantinib | 0.76 (0 to 25,591) |
| Lenvatinib + pembrolizumab | Nivolumab + ipilimumab | 0.53 (0 to 21,807) |
| Cabozantinib | Sunitinib | 0.53 (0 to 953) |
| Nivolumab + ipilimumab | Sunitinib | 0.76 (0 to 1339) |
| Nivolumab + ipilimumab | Cabozantinib | 1.46 (0 to 48,050) |
| IMDC/MSKCC favourable-risk subgroup | ||
| Lenvatinib + pembrolizumab | Sunitinib | 0.45 (0 to 1249) |
| Lenvatinib + pembrolizumab | Pazopanib | 0.44 (0 to 34,201) |
| Pazopanib | Sunitinib | 1.02 (0 to 2592) |
| All-risk population | ||
| Lenvatinib + pembrolizumab | Sunitinib | 0.39 (0.04 to 3.49) |
| Lenvatinib + pembrolizumab | Pazopanib | 0.30 (0.02 to 4.85) |
| Lenvatinib + pembrolizumab | Tivozanib | 0.45 (0.02 to 12.43) |
| Lenvatinib + pembrolizumab | Sorafenib | 0.34 (0.02 to 4.57) |
| Pazopanib | Sunitinib | 1.31 (0.24 to 7.17) |
| Tivozanib | Sunitinib | 0.88 (0.07 to 11.59) |
| Sorafenib | Sunitinib | 1.15 (0.29 to 4.71) |
| Pazopanib | Tivozanib | 1.49 (0.09 to 23.1) |
| Pazopanib | Sorafenib | 1.14 (0.20 to 6.05) |
| Tivozanib | Sorafenib | 0.76 (0.09 to 7.03) |
| Treatment | Comparator | REs HR (95% CrI)a |
|---|---|---|
| Intermediate-/poor-risk subgroup | ||
| Lenvatinib + pembrolizumab | Sunitinib | 0.66 (0 to 1200) |
| Lenvatinib + pembrolizumab | Cabozantinib | 0.80 (0 to 32,209) |
| Lenvatinib + pembrolizumab | Nivolumab + ipilimumab | 0.95 (0 to 36,680) |
| Cabozantinib | Sunitinib | 0.83 (0 to 1525) |
| Nivolumab + ipilimumab | Sunitinib | 0.69 (0 to 1274) |
| Nivolumab + ipilimumab | Cabozantinib | 0.84 (0 to 30,031) |
| IMDC/MSKCC favourable-risk subgroup | ||
| Lenvatinib + pembrolizumab | Sunitinib | 1.19 (0 to 2981) |
| Lenvatinib + pembrolizumab | Pazopanib | 1.30 (0 to 74,608) |
| Pazopanib | Sunitinib | 0.92 (0 to 2465) |
| All-risk population | ||
| Lenvatinib + pembrolizumab | Sunitinib | 0.74 (0 to 1959) |
| Lenvatinib + pembrolizumab | Pazopanib | 0.81 (0 to 57,526) |
| Pazopanib | Sunitinib | 0.91 (0 to 2345) |
| Treatment | Comparator | OR (95% CrI)a | |
|---|---|---|---|
| FEs | REs | ||
| Intermediate-/poor-risk subgroup | |||
| Lenvatinib + pembrolizumab | Sunitinib | 6.55 (4.39 to 9.87) | 5.37 (0 to 7259) |
| Lenvatinib + pembrolizumab | Cabozantinib | 2.46 (0.84 to 6.82) | 2.25 (0 to 72,403) |
| Lenvatinib + pembrolizumab | Nivolumab + ipilimumab | 3.19 (1.95 to 5.26) | 2.83 (0 to 86,682) |
| Cabozantinib | Sunitinib | 2.66 (1.05 to 7.32) | 2.36 (0 to 3533) |
| Nivolumab + ipilimumab | Sunitinib | 2.03 (1.52 to 2.75) | 1.90 (0 to 3072) |
| Nivolumab + ipilimumab | Cabozantinib | 0.76 (0.27 to 2.03) | 0.80 (0 to 30,638) |
| All-risk population | |||
| Lenvatinib + pembrolizumab | Sunitinib | 4.35 (3.16 to 5.99) | 3.56 (0 to 7044) |
| Lenvatinib + pembrolizumab | Pazopanib | 3.22 (2.14 to 4.85) | 2.77 (0 to 130,614) |
| Pazopanib | Sunitinib | 1.35 (1.03 to 1.75) | 1.30 (0 to 3072) |
| Treatment | Comparator | OR (95% CrI)b | |
|---|---|---|---|
| FEs | REs | ||
| IMDC intermediate-/poor-risk subgroupc | |||
| Lenvatinib + pembrolizumab | Sunitinib | 2.03 (1.30 to 3.19) | 1.88 (0 to 4188) |
| Lenvatinib + pembrolizumab | Cabozantinib | 1.80 (0.79 to 4.10) | 1.68 (0 to 100,710) |
| Cabozantinib | Sunitinib | 1.13 (0.57 to 2.25) | 1.12 (0 to 2670) |
| All-risk population | |||
| Lenvatinib + pembrolizumab | Sunitinib | 1.84 (1.28 to 2.66) | 1.70 (0 to 4230) |
| Lenvatinib + pembrolizumab | Cabozantinib | 1.86 (1.17 to 2.94) | 1.70 (0 to 115,844) |
| Cabozantinib | Sunitinib | 0.99 (0.76 to 1.31) | 0.99 (0 to 2566) |
| Treatment | Comparator | HR (95% CrI)a | |
|---|---|---|---|
| FEs | REs | ||
| Intermediate/poor-risk subgroup | |||
| Lenvatinib + pembrolizumab | Sunitinib | 0.45 (0.36 to 0.56) | 0.49 (0 to 953) |
| Lenvatinib + pembrolizumab | Cabozantinib | 0.93 (0.57 to 1.52) | 0.92 (0 to 33,190) |
| Lenvatinib + pembrolizumab | Nivolumab + ipilimumab | 0.60 (0.45 to 0.80) | 0.63 (0 to 24,343) |
| Cabozantinib | Sunitinib | 0.48 (0.31 to 0.74) | 0.53 (0 to 973) |
| Nivolumab + ipilimumab | Sunitinib | 0.75 (0.62 to 0.90) | 0.77 (0 to 1313) |
| Nivolumab + ipilimumab | Cabozantinib | 1.57 (0.97 to 2.51) | 1.46 (0 to 45,707) |
| IMDC/MSKCC favourable-risk subgroup | |||
| Lenvatinib + pembrolizumab | Sunitinib | 0.42 (0.28 to 0.63) | 0.47 (0 to 1495) |
| Lenvatinib + pembrolizumab | Pazopanib | 0.41 (0.22 to 0.78) | 0.46 (0 to 36,316) |
| Pazopanib | Sunitinib | 1.02 (0.62 to 1.68) | 1.03 (0 to 2592) |
| All-risk population | |||
| Lenvatinib + pembrolizumab | Sunitinib | 0.41 (0.33 to 0.51) | 0.42 (0.04 to 4.48) |
| Lenvatinib + pembrolizumab | Pazopanib | 0.35 (0.27 to 0.46) | 0.32 (0.02 to 5.99) |
| Lenvatinib + pembrolizumab | Tivozanib | 0.53 (0.36 to 0.78) | 0.48 (0.01 to 18.17) |
| Lenvatinib + pembrolizumab | Sorafenib | 0.40 (0.30 to 0.53) | 0.36 (0.02 to 6.05) |
| Pazopanib | Sunitinib | 1.16 (1.01 to 1.34) | 1.31 (0.23 to 8.00) |
| Tivozanib | Sunitinib | 0.78 (0.57 to 1.07) | 0.88 (0.06 to 13.2) |
| Sorafenib | Sunitinib | 1.03 (0.86 to 1.22) | 1.15 (0.26 to 5.1) |
| Pazopanib | Tivozanib | 1.49 (1.07 to 2.05) | 1.51 (0.08 to 27.94) |
| Pazopanib | Sorafenib | 1.13 (0.94 to 1.35) | 1.15 (0.19 to 6.96) |
| Tivozanib | Sorafenib | 0.76 (0.58 to 1.00) | 0.76 (0.08 to 7.61) |
| Treatment | Comparator | HR (95% CrI)a | |
|---|---|---|---|
| FEs | REs | ||
| PFS by FDA censoring rule | |||
| Lenvatinib + pembrolizumab | Sunitinib | 0.36 (0.23 to 0.57) | 0.41 (0 to 1261) |
| Lenvatinib + pembrolizumab | Pazopanib | 0.36 (0.18 to 0.68) | 0.40 (0 to 30,946) |
| Pazopanib | Sunitinib | 1.01 (0.63 to 1.62) | 1.01 (0 to 2592) |
| PFS by EMA censoring rule | |||
| Lenvatinib + pembrolizumab | Sunitinib | 0.36 (0.24 to 0.54) | 0.41 (0 to 1176) |
| Lenvatinib + pembrolizumab | Pazopanib | 0.36 (0.19 to 0.66) | 0.41 (0 to 34,544) |
| Pazopanib | Sunitinib | 1.01 (0.63 to 1.62) | 1.00 (0 to 2441) |
| OS | |||
| Lenvatinib + pembrolizumab | Sunitinib | 1.00 (0.51 to 1.95) | 1.03 (0 to 2490) |
| Lenvatinib + pembrolizumab | Pazopanib | 1.14 (0.54 to 2.41) | 1.16 (0 to 72,403) |
| Pazopanib | Sunitinib | 0.88 (0.63 to 1.23) | 0.88 (0 to 2345) |
Assessment group assessment of inconsistency in the network meta-analysis
For PFS in the all-risk population, the only NMA with a closed loop present within the network, the AG assessed inconsistency by applying an unrelated mean effects model114 and by comparing model fit statistics and results of this inconsistency model with the results of the AG PFS NMAs presented in Table 22 and Table 69 which assume consistency.
Inconsistency models such as the unrelated mean effects model114 are more complex than NMA models which assume consistency. Therefore, due to the small number of trials included in the network and instability of REs NMA results (see Additional network meta-analysis results tables), only FE inconsistency models were applied.
Model fit statistics of FE AG PFS NMA models assuming consistency and inconsistency are presented in Table 71.
| Model | Posterior mean residual deviance | Number of data points | pD | DIC |
|---|---|---|---|---|
| Consistency model using FDA censoring rule | 13.4 | 6 | 4 | 17.4 |
| Inconsistency modela using FDA censoring rule | 5.7 | 6 | 5 | 10.7 |
| Consistency model using EMA censoring rule | 13.4 | 6 | 4 | 17.4 |
| Inconsistency modela using EMA censoring rule | 5.7 | 6 | 5 | 10.7 |
| Treatment | Comparator | FEs HR (95% CrI)a | |
|---|---|---|---|
| FDA censoring rule | EMA censoring rule | ||
| Lenvatinib + pembrolizumab | Sunitinib | 0.39 (0.32 to 0.48) | 0.41 (0.33 to 0.51) |
| Lenvatinib + pembrolizumab | Pazopanib | 0.34 (0.26 to 0.43) | 0.35 (0.27 to 0.46) |
| Lenvatinib + pembrolizumab | Tivozanib | 0.50 (0.34 to 0.73) | 0.53 (0.36 to 0.78) |
| Lenvatinib + pembrolizumab | Sorafenib | 0.38 (0.29 to 0.50) | 0.40 (0.30 to 0.53) |
| Pazopanib | Sunitinib | 1.05 (0.90 to 1.22) | 1.05 (0.90 to 1.22) |
| Tivozanib | Sunitinib | 0.78 (0.57 to 1.07) | 0.78 (0.57 to 1.07) |
| Sorafenib | Sunitinib | 1.25 (1.01 to 1.55) | 1.25 (1.00 to 1.55) |
| Pazopanib | Tivozanib | 1.49 (1.07 to 2.05) | 1.49 (1.07 to 2.05) |
| Pazopanib | Sorafenib | 1.45 (1.14 to 1.86) | 1.45 (1.14 to 1.86) |
| Tivozanib | Sorafenib | 0.76 (0.58 to 1.00) | 0.76 (0.58 to 1.00) |
Model fit statistics demonstrate that inconsistency models seem to provide a better fit (lower posterior mean residual deviance and DIC statistic) but a higher level of complexity (in terms of effective number of model parameters). However, despite the better model fit of the inconsistency models, AG FEs PFS NMA results from the unrelated mean effects model were very similar (Table 72) to the results of the AG FEs PFS NMA results assuming consistency [see Table 22 and Table 69] and conclusions are unchanged.
Therefore, any inconsistency present between direct and indirect evidence for PFS in the all-risk population does not seem to have had an important impact on the PFS NMA results.
Due to the lack of closed loops within the OS and ORR NMAs, and within all NMAs conducted in the intermediate-/poor-risk and favourable-risk subgroups, inconsistency cannot be statistically assessed within these networks. Therefore, the consistency of indirect estimates of OS is unknown.
Additional assessment group network meta-analysis sensitivity analyses
During the NICE appraisal, Bristol-Myers Squibb (BMS), the company that manufactures nivolumab plus ipilimumab, noted that the AG NMAs for the intermediate-/poor-risk subgroup incorporate CheckMate 214 trial100 PFS data according to the primary definition in the trial. The primary definition included censoring for subsequent anticancer therapy. This censoring definition was consistent with the primary definition of PFS in the CLEAR trial (using the censoring method preferred by the FDA) and the definition of PFS in the CABOSUN trial97 BMS highlighted that in both the original submission for nivolumab plus ipilimumab25 and the CDF review,36 both the ERG and the NICE AC preferred the analysis that used the secondary definition of PFS from the CheckMate 214 trial. 100 This secondary definition did not apply censoring for subsequent anticancer therapy. This definition is consistent with the secondary definition of PFS in the CLEAR trial (using the censoring method preferred by the EMA).
The PFS and OS data from the CheckMate 214 trial100 used in the NMAs presented by the AG were based on a minimum study follow-up time of 42 months (median follow-up time reported for OS was 39.3 months; 43.6 months for nivolumab plus ipilimumab and 32.3 months; for sunitinib). Only results using the primary definition of PFS were available in the publication of the CheckMate 214 trial100 that reported 42-month minimum follow-up data.
BMS highlighted that there were two sources of data in the public domain115,116 that report PFS and OS data from the CheckMate 214 trial100 which were more up to date than the data sources used in the AG NMAs. A published paper reported 48-month minimum follow-up PFS and OS data, and a conference poster reported 60-month minimum follow-up PFS and OS data. Both sources reported PFS data according to the primary definition. In its ACD response, BMS provided 60-month PFS results according to the secondary definition of PFS. These results were not previously in the public domain (and are considered to be academic-in-confidence).
Results from the updated intermediate-/poor-risk group NMAs including the most recent PFS and OS data from the CheckMate 214 trial (60-month minimum follow-up) are presented alongside results from the AG original NMAs in Appendix 4 (Table 73). In all three trials that contributed data to the updated NMAs, the primary definition of PFS included censoring on receipt of subsequent anticancer therapy. Therefore, the AG has used these primary definitions for its primary sensitivity analysis. Additional sensitivity analyses have also been conducted using the secondary definitions of PFS from the CLEAR trial and CheckMate 214 trial. 100
| Treatment | Comparator | FEs HR (95% CrI)a | REs HR (95% CrI)a | ||
|---|---|---|---|---|---|
| Original | Updated | Original | Updated | ||
| PFS – Primary analysisb | |||||
| Lenvatinib + pembrolizumab | Sunitinib | 0.36 (0.28 to 0.46) | 0.36 (0.28 to 0.46) | 0.40 (0 to 773) | 0.40 (0 to 812) |
| Lenvatinib + pembrolizumab | Cabozantinib | 0.75 (0.45 to 1.25) | 0.75 (0.45 to 1.25) | 0.76 (0 to 25,591) | 0.76 (0 to 28,283) |
| Lenvatinib + pembrolizumab | Nivolumab + ipilimumab | 0.48 (0.35 to 0.66) | 0.49 (0.36 to 0.67) | 0.53 (0 to 21,807) | 0.53 (0 to 22,471) |
| Cabozantinib | Sunitinib | 0.48 (0.31 to 0.74) | 0.48 (0.31 to 0.74) | 0.53 (0 to 953) | 0.52 (0 to 944) |
| Nivolumab plus ipilimumab | Sunitinib | 0.75 (0.62 to 0.90) | 0.73 (0.61 to 0.87) | 0.76 (0 to 1339) | 0.75 (0 to 1394) |
| Nivolumab plus ipilimumab | Cabozantinib | 1.57 (0.97 to 2.51) | 1.52 (0.95 to 2.44) | 1.46 (0 to 48,050) | 1.43 (0 to 54,176) |
| PFS – Sensitivity analysisc | |||||
| Lenvatinib + pembrolizumab | Sunitinib | 0.45 (0.36 to 0.56) | 0.45 (0.36 to 0.56) | 0.49 (0 to 953) | 0.49 (0 to 880) |
| Lenvatinib + pembrolizumab | Cabozantinib | 0.93 (0.57 to 1.52) | 0.93 (0.57 to 1.54) | 0.92 (0 to 33,190) | 0.94 (0 to 33,860) |
| Lenvatinib + pembrolizumab | Nivolumab + ipilimumab | 0.60 (0.45 to 0.80) | 0.69 (0.53 to 0.91) | 0.63 (0 to 24,343) | 0.72 (0 to 26,108) |
| Cabozantinib | Sunitinib | 0.48 (0.31 to 0.74) | 0.48 (0.31 to 0.74) | 0.53 (0 to 973) | 0.52 (0 to 1033) |
| Nivolumab + ipilimumab | Sunitinib | 0.75 (0.62 to 0.90) | 0.65 (0.55 to 0.76) | 0.77 (0 to 1313) | 0.68 (0 to 1236) |
| Nivolumab + ipilimumab | Cabozantinib | 1.57 (0.97 to 2.51) | 1.35 (0.85 to 2.16) | 1.46 (0 to 45,707) | 1.31 (0 to 52,052) |
| OS | |||||
| Lenvatinib + pembrolizumab | Sunitinib | 0.62 (0.46 to 0.83) | 0.62 (0.46 to 0.83) | 0.66 (0 to 1200) | 0.65 (0 to 1200) |
| Lenvatinib + pembrolizumab | Cabozantinib | 0.78 (0.47 to 1.28) | 0.78 (0.47 to 1.28) | 0.80 (0 to 32,209) | 0.78 (0 to 28,854) |
| Lenvatinib + pembrolizumab | Nivolumab + ipilimumab | 0.94 (0.66 to 1.32) | 0.91 (0.65 to 1.27) | 0.95 (0 to 36,680) | 0.9 (0 to 31,571) |
| Cabozantinib | Sunitinib | 0.80 (0.53 to 1.21) | 0.80 (0.53 to 1.21) | 0.83 (0 to 1525) | 0.84 (0 to 1510) |
| Nivolumab + ipilimumab | Sunitinib | 0.66 (0.55 to 0.79) | 0.68 (0.58 to 0.81) | 0.69 (0 to 1274) | 0.72 (0 to 1326) |
| Nivolumab + ipilimumab | Cabozantinib | 0.83 (0.53 to 1.30) | 0.85 (0.55 to 1.32) | 0.84 (0 to 30,031) | 0.87 (0 to 35,596) |
For the sensitivity analyses, the AG again assessed the PH assumption for PFS and OS data from the CheckMate 214 trial. The AG’s original conclusions (that PH is violated for PFS data, but not for OS data) remain valid.
Appendix 5 Included cost-effectiveness study
| Li et al. 2021117 | |
|---|---|
| Title | Yes, p. 1 |
| Abstract | Yes, p. 1 |
| Background and objectives | Yes, p. 2 |
| Target population and subgroup | Yes, p. 2 (Methods: Analytics Overview) |
| Setting and location | Yes, p. 2 (Introduction) |
| Study perspective | Yes, p. 2 (Introduction) |
| Comparators | Yes, p. 2 (Methods: Analytics Overview) |
| Time horizon | Yes, p. 2 (Methods: Analytics Overview) |
| Discount rate | Yes, p. 2 (Methods: Analytics Overview) |
| Choice of health outcomes | Yes, p. 3 (Transition Probability and Costs and Utilities) |
| Measurement of effectiveness | Yes, p. 2 and p. 3 (Transition Probability) |
| Measurement and valuation of preference-based outcomes | Yes, p. 3 (Costs and Utilities) |
| Estimating resources and costs | Individual resource use was reported for drug costs in the supplementary material but not for AEs |
| Currency, price date, and conversion | Costs were adjusted to 2021 US$, p. 2 |
| Choice of model | Yes, p. 2 |
| Assumptions | Yes, p. 2 and p. 3 |
| Analytical methods | Yes, p. 2 and p. 3 |
| Study parameters | Yes, p. 4 and p. 5 |
| Incremental costs and outcomes | Yes, p. 6 |
| Characterising uncertainty | Yes, one-way sensitivity, probabilistic sensitivity and scenario analyses were undertaken (p. 7 and supplementary material) |
| Characterising heterogeneity | NA |
| Study findings, limitations, generalisability, and current knowledge | Yes, p. 7 and p. 8 |
| Source of funding | Yes, p. 8 |
| Conflicts of interest | Yes, p. 10 |
| Parameter | Li et al. 2021117 |
|---|---|
| Year | 2021 |
| Type of economic evaluation | Cost–utility analysis |
| Population | Adults aged 62 years with aRCC, all-risk population |
| Intervention(s) and comparator(s) | Sunitinib, avelumab + axitinib,a nivolumab + ipilimumab,a lenvatinib + pembrolizumab, pembrolizumab + axitinib,a nivolumab + cabozantiniba |
| Model structure | Microsimulation |
| Health states | First-line treatment, second-line treatment, third-line treatment, discontinued treatment due to AEs, BSC, dead |
| Time horizon | Lifetime |
| Cycle length | 42 days |
| Discount rates for costs and benefits | 3% for costs and benefits |
| Perspective used (country, healthcare system, societal) | US payer (direct costs only) |
| Sources of clinical evidence | K-M data from the key trials (the CLEAR trial, CheckMate 9ER trial,131 CheckMate 214 trial,100 KEYNOTE-426 trial,132 and JAVELIN Renal 101 trial133) |
| Sources of utilities evidence | Published sources: Cella et al. 2018;134 de Groot et al. 2018;135 Wan et al. 2019;136 Patel et al. 202137 |
| Sources of costs evidence | Published sources include Centres for Medicare and Medicaid Services 2021;138 Agency for Healthcare Research and Quality US Dept of Health and Human Services 2021;139 Motzer et al. 2018;140 Perrin et al. 2015141 |
| Currency used | US $ |
| Year to which costs apply | 2021 |
| Total costs | LEN + PEM = $562,080.09 SUN = $239,257.68 |
| Total QALYs | LEN + PEM = 2.61 SUN = 2.42 |
| Total LYs | LEN + PEM = 3.44 SUN = 3.21 |
| Incremental costs | LEN + PEM vs. SUN=$322,822.41 |
| Incremental QALYs | LEN + PEM vs. SUN = 0.19 |
| Incremental LYs | LEN + PEM vs. SUN = 0.23 |
| ICER per LY gained | LEN + PEM vs. SUN=$1,403,575.70 |
| ICER per QALY gained | LEN + PEM vs. SUN=$172,749.53 |
| Sensitivity analysis results | The time horizon varied to 5, 10 and 20 years. A time horizon of 5 years significantly increased the ICER per QALY gained as most of the costs occurred in the first 5 years but the period over which benefits accrued exceeded 5 years. |
| Conclusions of cost-effectiveness results | Pembrolizumab plus axitiniba is the best option at a WTP threshold of $100,000. |
| Limitations | Indirect comparisons include bias of different patient characteristics, lack of long-term OS data for patients treated with immune checkpoint inhibitors to validate model estimates, estimates of treatment discontinuation do not extend beyond the trial periods studied and the utility estimates come from a range of sources that may not accurately reflect clinical reality. The model is designed to represent the US health system so estimates may not be transferable to other healthcare systems. |
Appendix 6 Assessment group quality assessment and assessment of company approaches for deriving cost-effectiveness evidence
| Review process | AG response |
|---|---|
| Was the review question clearly defined in terms of population, interventions, comparators, outcomes and study designs? | Yes |
| Were appropriate sources searched? | Yes |
| Was the timespan of the searches appropriate? | Partially |
| Were appropriate search terms used? | Yes |
| Were the eligibility criteria appropriate to the decision problem? | Yes |
| Was study selection applied by two or more reviewers independently? | Yes |
| Were data extracted by two or more reviewers independently? | NA |
| Were appropriate criteria used to assess the risk of bias and/or quality of the primary studies? | NA |
| Was the quality assessment conducted by two or more reviewers independently? | NA |
| Were attempts to synthesise evidence appropriate? | NA |
| Question | Eisai model | MSD model |
|---|---|---|
| Was a well-defined question posed in answerable form? | ✓ | ✓ |
| Was a comprehensive description of the competing alternatives given? | ✓ | ✓ |
| Was the effectiveness of the programme or services established? | ✓ | ✓ |
| Where all the important and relevant costs and consequences for each alternative identified? | ✓ | ✓ |
| Were costs and consequences measured accurately in appropriate physical units? | ✓ | ✓ |
| Were the cost and consequences valued credibly? | ✓ | ✓ |
| Were costs and consequences adjusted for differential timing? | ✓ | ✓ |
| Was an incremental analysis of costs and consequences of alternatives performed? | ✓ | ✓ |
| Was allowance made for uncertainty in the estimates of costs and consequences? | ✓ | ✓ |
| Did the presentation and discussion of study results include all issues of concern to users? | ✓/✕ | ✓/✕ |
| Element of health technology assessment | Reference Case | MSD and Eisai models |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | Yes |
| Comparators | As listed in the scope developed by NICE | Partly – nivolumab + ipilimumab was not included as a comparator |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, carers | Yes |
| Perspective on costs | NHS and PSS | Yes |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes |
| Synthesis of evidence on health effects | On the basis of systematic review and NMA | Yes |
| Measuring and valuing health effects | Health effects should be expressed in QALYs; the EQ-5D is the preferred measure of HRQoL in adults | Yes |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | Yes |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | Yes |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | Yes |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | Yes |
Appendix 7 Assessment of cost-effectiveness (all-risk population)
Unless described in this section, all parameters used in the all-risk population model are the same as were used in the intermediate-/poor-risk and favourable-risk subgroup models (see main body of the report).
Intervention and comparator treatments
The intervention is lenvatinib plus pembrolizumab. The comparators listed in the final scope3 issued by NICE are sunitinib, pazopanib and tivozanib.
Populating the Merck Sharp & Dohme/assessment group model: progression-free survival
Eisai and MSD fitted distributions to CLEAR trial BIRC PFS data (FDA censoring rules). The PFS distributions chosen by Eisai, MSD and the AG for the all-risk population are shown in Table 79. The PFS distributions chosen by the AG for lenvatinib plus pembrolizumab and sunitinib/pazopanib/tivozanib cannot be shown graphically as the data are confidential.
| Treatment | Eisai | MSD | AG |
|---|---|---|---|
| Lenvatinib plus pembrolizumab | Log-normal | Exponential | Gamma |
| Sunitinib | Log-normal | Gamma | Log-normal |
| Pazopanib/tivozanib | Equal to sunitinib | Equal to sunitinib | Equal to sunitinib |
Lenvatinib plus pembrolizumab
All the MSD AIC statistics for the distributions fitted to CLEAR trial lenvatinib plus pembrolizumab data lie within five AIC points of each other. Eisai chose to model lenvatinib and pembrolizumab PFS using a log-normal distribution and MSD chose to model lenvatinib and pembrolizumab PFS using an exponential distribution. The AG considered that the gamma distribution, which has the lowest AIC statistic (highest ranking), and on visual inspection, seemed to offer long-term projections that were clinically plausible, was an appropriate option in the base case.
Sunitinib (pazopanib and tivozanib)
Eisai chose to model sunitinib (pazopanib and tivozanib) PFS using a log-normal distribution. MSD chose to model sunitinib (pazopanib and tivozanib) PFS using a gamma distribution. Although the gamma distribution only ranked 4/7 using AIC statistics, MSD considered the gamma distribution generated the most plausible long-term survival estimates.
The AG considered the distribution with the lowest AIC statistic (generalised gamma distribution) generated PFS estimates that were too optimistic. The AG considered that the log-normal distribution (ranked 2/7 using AIC statistics) produced long-term PFS projections that were clinically plausible and therefore considered that this was an appropriate option to use in the base case.
Assessment group scenario analyses: all-risk population (progression-free survival)
The AG explored the effect on cost-effectiveness results of using the distributions that were within five points of the AIC statistic for the chosen distribution to model PFS for lenvatinib plus pembrolizumab. The distributions for sunitinib, pazopanib and tivozanib were unchanged.
The AG explored the effect on cost-effectiveness results of using the MSD preferred gamma distribution to model PFS for sunitinib, pazopanib and tivozanib. The distribution for lenvatinib plus pembrolizumab was unchanged.
Populating the Merck Sharp & Dohme/assessment group model: overall survival
The distributions chosen by Eisai, MSD and the AG for OS in the all-risk population are shown in Table 80.
| Treatment | Eisai | MSD | AG |
|---|---|---|---|
| Lenvatinib plus pembrolizumab | Exponential | Exponential | K-M + exponential |
| Sunitinib | Exponential | Gamma | K-M + exponential |
| Pazopanib/tivozanib | Equal to sunitinib | Equal to sunitinib | Equal to sunitinib |
Lenvatinib plus pembrolizumab
Both companies chose the exponential distribution (ranked 6/7 using AIC and BIC statistics) to estimate OS for patients receiving lenvatinib plus pembrolizumab. This distribution was not within 5 points of the distribution with the lowest AIC statistic. The companies’ choice was based on good visual fit to the CLEAR trial OS K-M data and because the higher-ranking distributions appeared to generate implausible long-term OS estimates. Although the AG was satisfied that the companies followed DSU guidance,120 the AG did not consider that any of the distributions considered by Eisai or MSD provided a good visual fit to the available CLEAR trial OS K-M data available.
The AG examined the CLEAR trial OS K-M data received during the NICE appraisal clarification process and observed that the lenvatinib plus pembrolizumab OS hazard was constant beyond 80 weeks. The AG therefore considered that the companies’ choice of an exponential distribution was appropriate, but that K-M data should be used up to the point that censoring and small numbers of events rendered the data too uncertain (the AG considered that this occurred at 120 weeks). The AG observed that between 80 and 120 weeks the OS hazard was constant. The AG appended the exponential distribution (based on the hazard between 80 and 120 weeks) to the CLEAR trial OS K-M data from 120 weeks onwards.
Sunitinib (pazopanib and tivozanib)
To model OS for patients treated with sunitinib, Eisai chose the exponential distribution as it did not cross the lenvatinib plus pembrolizumab OS distribution. MSD chose the gamma distribution as they considered distributions with higher ranking AIC statistics generated implausible long-term OS projections. Although the AG was satisfied that the companies followed DSU guidance,120 it did not consider that any of the distributions considered by Eisai or MSD provided a good visual fit to the available CLEAR trial OS K-M data.
The AG examined the CLEAR trial OS K-M data received during the NICE appraisal clarification process and observed that the sunitinib OS hazard was constant beyond 50 weeks. The AG therefore considered that the MSD choice of an exponential distribution was appropriate, but that K-M data should be used up to the point that censoring and small numbers of events rendered the data too uncertain (the AG considered that this occurred at 120 weeks). The AG observed that between 50 and 120 weeks the OS hazard was constant. The AG appended the exponential distribution (based on the hazard between 50 and 120 weeks) to the CLEAR trial OS K-M data from 120 weeks onwards.
Assessment group scenario analyses: all-risk population (overall survival)
The AG carried out the following scenario analyses using company base approaches to modelling:
-
Use the exponential distribution (Eisai and MSD preferred distribution) instead of the AG K-M + exponential distribution to model OS for lenvatinib plus pembrolizumab.
-
Use the exponential distribution (Eisai preferred distribution) instead of the AG K-M + exponential distribution to model OS for sunitinib.
-
Use the gamma distribution (MSD preferred distribution) instead of the AG K-M + exponential distribution to model OS for sunitinib.
Populating the model: time to treatment discontinuation
The AG considered that TTD for patients receiving lenvatinib and sunitinib should be modelled by fitting a distribution to CLEAR trial TTD K-M data and, for patients receiving pembrolizumab, the CLEAR trial TTD K-M data should be used directly. The parametric distributions chosen by Eisai, MSD and the AG to model TTD for all treatments are shown in Table 81. The TTD distributions chosen by the AG cannot be shown graphically for the all-risk population as the data are confidential.
| Treatment | Eisai | MSD | AG |
|---|---|---|---|
| Lenvatinib | Generalised gamma | Generalised gamma | Generalised gamma (Eisai) |
| Pembrolizumab | Weibull | K-M data (CLEAR trial data are complete) | |
| Sunitinib | Generalised gamma | Log-logistic | |
| Pembrolizumab/tivozanib | Equal to sunitinib | Equal to sunitinib | Equal to sunitinib |
Lenvatinib
Eisai and MSD provided CLEAR trial lenvatinib TTD K-M data during the NICE appraisal clarification process. However, the two data sets differed slightly (within 24 months there was a clear gap between the two data sets). The AG concluded that as the safety data from the CLEAR trial suggested a lower level of treatment discontinuation for lenvatinib than for pembrolizumab (25.6% vs. 28.7%66), the Eisai TTD K-M lenvatinib data were likely to be the most accurate as they followed a trajectory that was consistently above the TTD K-M pembrolizumab data until 24 months, that is until the time when the pembrolizumab stopping rule was activated. In contrast, the MSD TTD lenvatinib K-M data crossed the pembrolizumab TTD K-M data at 20 months.
Both companies chose to use generalised gamma distributions to model TTD for patients treated with lenvatinib (in the MSD CS,2 this was the highest-ranking distribution using AIC statistics). The AG considered that the Eisai generalised gamma distribution provided a good visual fit to the TTD K-M data and did not cross the pembrolizumab TTD K-M data until 24 months. The AG therefore chose to use the Eisai generalised gamma distribution to model lenvatinib K-M TTD data.
Pembrolizumab
The MSD modelled pembrolizumab TTD by directly using the K-M data from the CLEAR trial and applied a 2-year stopping rule in line with the CLEAR trial protocol. Eisai modelled pembrolizumab TTD by fitting a Weibull distribution to the CLEAR trial K-M data; it is clear from the Eisai model outputs that a stopping rule for pembrolizumab at 2 years had been applied. The CLEAR trial pembrolizumab TTD K-M data are almost complete and so the AG used the TTD K-M data directly to estimate the cost of treatment with pembrolizumab for patients in the all-risk population. The AG did not include an enforced stopping rule at 2 years but used the K-M data directly, which means that some patients remained on pembrolizumab for a short period of time beyond 2 years.
Sunitinib
Eisai used the generalised gamma distribution to model sunitinib TTD. The company considered this distribution to have good statistical and visual fit to the tail of the sunitinib TTD K-M data. The AG and MSD used the log-logistic distribution as this has the lowest AIC and was a good visual fit to the sunitinib TTD K-M data.
Assessment group scenario analyses: all-risk population (time to treatment discontinuation)
The AG explored the effect on cost-effectiveness results of using the distributions that were within five points of the AIC statistic for the distribution used to model TTD for patients treated with lenvatinib. The distributions for sunitinib, pazopanib and tivozanib were unchanged.
The AG explored the effect on cost-effectiveness results of using the distributions that were within five points of the AIC statistic for the distribution used to model TTD for patients treated with sunitinib. The distribution for lenvatinib plus pembrolizumab was unchanged.
Utility values
The AG considers that the MSD time-to-death approach provided the best reflection of the HRQoL of long-term survivors and used this approach in the MSD/AG model. The values used cannot be reported as they are confidential.
Assessment group scenario analyses (utility values)
The AG has carried out two scenario analyses. One scenario analysis used the Eisai treatment dependent health state utility values and the other used the MSD treatment independent health state utility values. The values used cannot be reported as they are confidential.
Assessment group scenario analysis (adverse events)
The AG has carried out two scenario analyses: one in which AE costs were set to zero and one in which AE costs were doubled.
Assessment group sensitivity analyses (subsequent treatment costs)
The AG carried out sensitivity analyses that varied the costs of subsequent treatments by ± 20%.
Assessment group cost-effectiveness results
The all-risk population cost-effectiveness results are presented here for completeness. The AG cost-effectiveness results were estimated using the list prices for the intervention, comparators and subsequent treatments (Tables 82 and 83). AG cost-effectiveness results generated using confidential discounted prices are presented in a confidential appendix. Results from all AG probabilistic and sensitivity analyses are confidential. Results from AG scenario analyses are presented in Tables 84–86.
Deterministic results
| Drug | Incremental: LEM + PEM vs. comparator |
|---|---|
| ICER per QALY gained | |
| LEN + PEM | – |
| Sunitinib | £4,205,044 |
| Pazopanib | £4,167,492 |
| Tivozanib | £4,048,514 |
| Drug | ICER per QALY gained |
|---|---|
| Sunitinib | – |
| Pazopanib | Pazopanib is dominated by sunitinib |
| Tivozanib | Tivozanib is dominated by sunitinib |
| LEN + PEM | £4,205,044 |
Assessment group deterministic scenario analysis results (all-risk population)
| AG scenarios All-risk population |
ICER per QALY gained |
|---|---|
| AG base case | £4,205,044 |
| Discount rate 6% | £1,498,809 |
| Discount rate 0% | LEN + PEM is dominated |
| LEN + PEM PFS (exponential) | £4,197,889 |
| LEN + PEM PFS (generalised gamma) | £4,197,048 |
| LEN + PEM PFS (Gompertz) | £4,211,511 |
| LEN + PEM PFS (log-logistic) | £4,169,615 |
| MSD sunitinib PFS (gamma) | £4,191,672 |
| LEN + PEM OS (exponential) | £263,613 |
| Eisai sunitinib OS (exponential) | LEN + PEM is dominated |
| MSD sunitinib OS (gamma) | £241,564 |
| Eisai LEN + PEM TTD (exponential) | £4,356,024 |
| Eisai LEN + PEM TTD (Gompertz) | £4,281,938 |
| Eisai LEN + PEM TTD (Weibull) | £4,381,303 |
| MSD LEN + PEM TTD (generalised gamma) | £4,157,860 |
| Eisai sunitinib TTD (generalised gamma) | £4,364,812 |
| Eisai sunitinib TTD (Gompertz) | £4,050,501 |
| Eisai sunitinib TTD (log-normal) | £4,256,635 |
| MSD health state utilities | £1,871,468 |
| Eisai health state utilities | £859,692 |
| AE costs doubled | £4,203,370 |
| AE costs set to zero | £4,206,717 |
| Subsequent treatment costs increased by 20% | £4,128,236 |
| Subsequent treatment costs decreased by 20% | £4,281,851 |
| AG scenarios All-risk population |
ICER per QALY gained |
|---|---|
| AG base case | £4,167,492 |
| Discount rate 6% | £1,487,254 |
| Discount rate 0% | LEN + PEM is dominated |
| LEN + PEM PFS (exponential) | £4,160,337 |
| LEN + PEM PFS (generalised gamma) | £4,159,496 |
| LEN + PEM PFS (Gompertz) | £4,173,960 |
| LEN + PEM PFS (log-logistic) | £4,132,063 |
| MSD sunitinib PFS (gamma) | £4,158,249 |
| LEN + PEM OS (exponential) | £261,289 |
| Eisai sunitinib OS (exponential) | LEN + PEM is dominated |
| MSD sunitinib OS (gamma) | £239,468 |
| Eisai LEN + PEM TTD (exponential) | £4,318,472 |
| Eisai LEN + PEM TTD (Gompertz) | £4,244,386 |
| Eisai LEN + PEM TTD (Weibull) | £4,343,751 |
| MSD LEN + PEM TTD (generalised gamma) | £4,120,308 |
| Eisai sunitinib TTD (generalised gamma) | £4,336,576 |
| Eisai sunitinib TTD (Gompertz) | £4,004,184 |
| Eisai sunitinib TTD (log-normal) | £4,221,966 |
| MSD health state utilities | £1,854,755 |
| Eisai health state utilities | £852,015 |
| AE costs doubled | £4,191,262 |
| AE costs set to zero | £4,143,721 |
| Subsequent treatment costs increased by 20% | £4,090,684 |
| Subsequent treatment costs decreased by 20% | £4,244,299 |
| AG scenarios All-risk population |
ICER per QALY gained |
|---|---|
| AG base case | £4,048,514 |
| Discount rate 6% | £1,041,860 |
| Discount rate 0% | LEN + PEM is dominated |
| LEN + PEM PFS (exponential) | £1,630,398 |
| LEN + PEM PFS (generalised gamma) | £1,604,639 |
| LEN + PEM PFS (Gompertz) | £2,003,596 |
| LEN + PEM PFS (log-logistic) | £1,168,137 |
| MSD sunitinib PFS (gamma) | £1,742,343 |
| LEN + PEM OS (exponential) | £253,739 |
| Eisai sunitinib OS (exponential) | LEN + PEM is dominated |
| MSD sunitinib OS (gamma) | £233,603 |
| Eisai LEN + PEM TTD (exponential) | £1,839,917 |
| Eisai LEN + PEM TTD (Gompertz) | £1,821,429 |
| Eisai LEN + PEM TTD (Weibull) | £1,845,753 |
| MSD LEN + PEM TTD (generalised gamma) | £1,788,521 |
| Eisai sunitinib TTD (generalised gamma) | £1,711,271 |
| Eisai sunitinib TTD (Gompertz) | £1,904,812 |
| Eisai sunitinib TTD (log-normal) | £1,773,649 |
| MSD health state utilities | £1,801,804 |
| Eisai health state utilities | £827,691 |
| AE costs doubled | £4,058,317 |
| AE costs set to zero | £4,038,712 |
| Subsequent treatment costs increased by 20% | £3,971,707 |
| Subsequent treatment costs decreased by 20% | £4,125,322 |
Appendix 8 Assessment group table of cost-effectiveness scenario analyses
A summary of the AG’s scenario analyses conducted is presented in Table 87.
| Scenario analysis | Intermediate/poor risk | Favourable risk | All-risk population |
|---|---|---|---|
| Discounting | 6% | 6% | 6% |
| 0% | 0% | 0% | |
| PFS | LEN + PEM distributions within 5 AIC points | LEN + PEM distributions within 5 AIC points | LEN + PEM distributions within 5 AIC points |
| Gamma | Exponential | Exponential | |
| Generalised gamma | Gamma | Generalised gamma | |
| Gompertz | Gompertz | Gompertz | |
| Log-logistic | Log-logistic | Log-logistic | |
| Log-normal | Log-normal | MSD gamma distribution for SUN | |
| Weibull | Weibull | – | |
| CABO MSD FP PFS NMA HR | SUN distributions within five AIC points | Eisai/MSD exponential distribution for LEN + PEM | |
| – | Gamma | Eisai exponential distribution for SUN | |
| – | Generalised gamma | MSD gamma distribution for SUN | |
| – | Log-logistic | – | |
| – | Weibull | – | |
| OS | Eisai/MSD exponential distribution for LEN + PEM | AG OS NMA HR for SUN | LEN + PEM distributions within five AIC points (exponential) |
| Eisai CABO OS | SUN OS = LEN + PEM OS | Eisai SUN OS exponential | |
| MSD CABO FP OS | – | MSD SUN OS gamma | |
| CABO OS = LEN + PEM OS | – | – | |
| NIV + IP OS = LEN + PEM OS | – | – | |
| TTD | LEN + PEM distributions within five AIC points | LEN + PEM distributions within five AIC points | LEN + PEM distributions within five AIC points |
| Exponential | Generalised gamma | Eisai exponential | |
| Gompertz | Gamma | Eisai Gompertz | |
| Weibull | Gompertz | Eisai Weibull | |
| MSD generalised gamma | Log-logistic | MSD generalised gamma | |
| Eisai CABO TTD within five AIC points | Weibull | Eisai SUN generalised gamma | |
| Weibull | SUN distributions within five AIC points | Eisai SUN generalised gamma | |
| Log-normal | Gamma | Eisai SUN Gompertz | |
| Exponential | Generalised gamma | Eisai SUN log-normal | |
| Generalised gamma | Gompertz | – | |
| Gompertz | Log-logistic | – | |
| MSD CABO FP TTD | Log-normal | – | |
| NIV + IPI = Eisai PEM TTD (Weibull) | Weibull | – | |
| Utility values | MSD treatment independent health state utility values | MSD treatment independent health state utility values | MSD treatment independent health state utility values |
| Eisai treatment dependent health state utility values | – | Eisai treatment dependent health state utility values | |
| AEs | Double AE costs | Double AE costs | Double AE costs |
| Set AE costs to zero | Set AE costs to zero | Set AE costs to zero | |
| Subsequent treatments | Increase costs by 20% | Increase costs by 20% | Increase costs by 20% |
| Decrease costs by 20% | Decrease costs by 20% | Decrease costs by 20% |
Appendix 9 Assessment group one-way sensitivity analysis and probabilistic sensitivity analysis parameters
A summary of the parameter values used for the intermediate-/poor-risk subgroup sensitivity analyses is presented in Table 88.
| Parameter | Base-case value | Lower bound | Upper bound | Distribution | Distribution parameters |
|---|---|---|---|---|---|
| Age at model start | 61 | 55.21 | 67.48 | Normal | SE = 0.405 |
| Percentage of males | 74.61% | 0.67 | 0.82 | Normal | α = 529 β = 180 |
| Patient weight | 79.40 | 71.46 | 87.34 | Normal | SE = 0.693 |
| OS HR CABO | 1.28a | 1.05 | 1.56 | Log-normal | SE = 0.128 |
| OS HR, NIV + IPI | 1.06a | 0.87 | 1.29 | Log-normal | SE = 0.106 |
| PFS HR (constant), CABO | 1.33a | 1.10 | 1.62 | Log-normal | SE = 0.133 |
| PFS HR (constant), NIV + IPI | 2.08a | 1.71 | 2.53 | Log-normal | SE = 0.208 |
| RDI – PEM | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Beta | Confidential information has been removed |
| RDI – CABO | 0.94 | 0.91 | 0.97 | Beta | α = 229.149 β = 13.851 |
| Drug costs: admin costs, oral prescription cost | £11.00 | 8.84 | 13.16 | Normal | SE = 1.100 |
| Drug costs: admin costs, i.v. – simple, first | £221.35 | 177.97 | 264.73 | Normal | SE = 22.135 |
| Drug costs: admin costs, i.v. – simple, subsequent | £365.91 | 294.19 | 437.62 | Normal | SE = 36.591 |
| Drug costs: admin costs, i.v. – complex, first | £352.24 | 283.20 | 421.28 | Normal | SE = 35.224 |
| Drug costs: admin costs, oral chemo admin, first | £226.45 | 182.07 | 270.83 | Normal | SE = 22.645 |
| EOL cost: NICE ID1426 (ERG) | 8073.00 | 6490.72 | 9655.28 | Normal | SE = 807.300 |
| Subsequent treatment costs – LEN + PEM | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| Subsequent treatment costs – CABO | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| Subsequent treatment costs – NIV + IPI | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| AE costs – LEN + PEM | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| AE costs – CABO | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| AE costs – NIV + IPI | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| Resource use: health state cost, progression-free (first cycle) | £255.01 | £205.03 | £305.00 | Normal | SE = 25.501 |
| Resource use: health state cost, progression-free (subsequent cycles) | £59.89 | £48.15 | £71.63 | Normal | SE = 5.989 |
| Resource use: health state cost, disease progression | £59.89 | £48.15 | £71.63 | Normal | SE = 5.989 |
| Resource use: frequency – PF first cycle – outpatient consultation | 1.00 | 0.80 | 1.20 | Normal | SE = 0.100 |
| Resource use: frequency – PF first cycle – blood test | 1.00 | 0.80 | 1.20 | Normal | SE = 0.100 |
| Resource use: frequency – PF subsequent cycle – outpatient consultation | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Resource use: frequency – PF subsequent cycle – CT scan | 0.08 | 0.06 | 0.10 | Normal | SE = 0.008 |
| Resource use: frequency – PF subsequent cycle – blood test | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Resource use: frequency – PD – Outpatient consultation | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Resource use: frequency – PD – CT scan | 0.08 | 0.06 | 0.10 | Normal | SE = 0.008 |
| Resource use: frequency – PD – blood test | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Time-to-death utilitiesb | See description in text | ||||
A summary of the parameter values used for the favourable-risk subgroup sensitivity analyses is presented in Table 89.
| Parameter | Base-case value | Lower bound | Upper bound | Distribution | Distribution parameters |
|---|---|---|---|---|---|
| Age at model start | 62.18 | 55.96 | 68.40 | Normal | SE = 0.501 |
| Percentage of males | 74.71% | 0.67 | 0.82 | Normal | α = 260 β = 88 |
| Patient weight (kg) | 84.32 | 75.89 | 92.75 | Normal | SE = 0.993 |
| RDI – PEM | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Beta | Confidential information has been removed |
| RDI – SUN | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Beta | Confidential information has been removed |
| RDI – PAZO | 0.86 | 0.81 | 0.90 | Beta | α = 208.980 β = 34.020 |
| RDI – TIVO | 0.94 | 0.91 | 0.97 | Beta | α = 228.420 β = 14.580 |
| Drug costs: admin costs, oral prescription cost | £11.00 | £8.84 | £13.16 | Normal | SE = 1.100 |
| Drug costs: admin costs, i.v. – simple, first | £221.35 | £177.97 | £264.73 | Normal | SE = 22.135 |
| Drug costs: admin costs, i.v. – simple, subsequent | £365.91 | £294.19 | £437.62 | Normal | SE = 36.591 |
| Drug costs: admin costs, i.v. – complex, first | £352.24 | £283.20 | £421.28 | Normal | SE = 35.224 |
| Drug costs: admin costs, oral chemo admin, first | £226.45 | £182.07 | £270.83 | Normal | SE = 22.645 |
| EOL cost: NICE ID1426 (ERG) | £8073.00 | £6490.72 | £9655.28 | Normal | SE = 807.300 |
| Subsequent treatment costs – LEN + PEM | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| Subsequent treatment costs – SUN/PAZO/TIVO | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| AE costs – LEN + PEM | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| AE costs – SUN | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| AE costs – PAZO | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| AE costs – TIVO | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | |
| Resource use: health state cost, progression-free (first cycle) | £255.01 | £205.03 | £305.00 | Normal | SE = 25.501 |
| Resource use: health state cost, progression-free (subsequent cycles) | £59.89 | £48.15 | £71.63 | Normal | SE = 5.989 |
| Resource use: health state cost, disease progression | £59.89 | £48.15 | £71.63 | Normal | SE = 5.989 |
| Resource use: frequency – PF first cycle – outpatient consultation | 1.00 | 0.80 | 1.20 | Normal | SE = 0.100 |
| Resource use: frequency – PF first cycle – blood test | 1.00 | 0.80 | 1.20 | Normal | SE = 0.100 |
| Resource use: frequency – PF subsequent cycle – outpatient consultation | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Resource use: frequency – PF subsequent cycle – CT scan | 0.08 | 0.06 | 0.10 | Normal | SE = 0.008 |
| Resource use: frequency – PF subsequent cycle – blood test | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Resource use: frequency – PD – outpatient consultation | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Resource use: frequency – PD – CT scan | 0.08 | 0.06 | 0.10 | Normal | SE = 0.008 |
| Resource use: frequency – PD – blood test | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Time-to-death utilitiesa | See description in text | ||||
A summary of the parameter values used for the all-risk population sensitivity analyses is presented in Table 90.
| Parameter | Base-case value | Lower bound | Upper bound | Distribution | Distribution parameters |
|---|---|---|---|---|---|
| Age at model start | 62.18 | 55.96 | 68.40 | Normal | SE = 0.501 |
| Percentage of males | 74.71% | 0.67 | 0.82 | Normal | α = 260 β = 88 |
| Patient weight (kg) | 84.32 | 75.89 | 92.75 | Normal | SE = 0.993 |
| RDI – PEM | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Beta | Confidential information has been removed |
| RDI – SUN | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Beta | Confidential information has been removed |
| RDI – PAZO | 0.86 | 0.81 | 0.90 | Beta | α = 208.980 β = 34.020 |
| RDI – TIVO | 0.94 | 0.91 | 0.97 | Beta | α = 228.420 β = 14.580 |
| Drug costs: admin costs, oral prescription cost | £11.00 | £8.84 | £13.16 | Normal | SE = 1.100 |
| Drug costs: admin costs, i.v. – simple, first | £221.35 | £177.97 | £264.73 | Normal | SE = 22.135 |
| Drug costs: admin costs, i.v. – simple, subsequent | £365.91 | £294.19 | £437.62 | Normal | SE = 36.591 |
| Drug costs: admin costs, i.v. – complex, first | £352.24 | £283.20 | £421.28 | Normal | SE = 35.224 |
| Drug costs: admin costs, oral chemo admin, first | £226.45 | £182.07 | £270.83 | Normal | SE = 22.645 |
| EOL cost: NICE ID1426 (ERG) | £8073.00 | £6490.72 | £9655.28 | Normal | SE = 807.300 |
| Subsequent treatment costs – LEN + PEM | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| Subsequent treatment costs – SUN/PAZO/TIVO | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| AE costs – LEN + PEM | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| AE costs – SUN | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| AE costs – PAZO | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | – |
| AE costs – TIVO | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Uniform | |
| Resource use: health state cost, progression-free (first cycle) | £255.01 | £205.03 | £305.00 | Normal | SE = 25.501 |
| Resource use: health state cost, progression-free (subsequent cycles) | £59.89 | £48.15 | £71.63 | Normal | SE = 5.989 |
| Resource use: health state cost, disease progression | £59.89 | £48.15 | £71.63 | Normal | SE = 5.989 |
| Resource use: frequency – PF first cycle – outpatient consultation | 1.00 | 0.80 | 1.20 | Normal | SE = 0.100 |
| Resource use: frequency – PF first cycle – blood test | 1.00 | 0.80 | 1.20 | Normal | SE = 0.100 |
| Resource use: frequency – PF subsequent cycle – outpatient consultation | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Resource use: frequency – PF subsequent cycle – CT scan | 0.08 | 0.06 | 0.10 | Normal | SE = 0.008 |
| Resource use: frequency – PF subsequent cycle – blood test | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Resource use: frequency – PD – outpatient consultation | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Resource use: frequency – PD – CT scan | 0.08 | 0.06 | 0.10 | Normal | SE = 0.008 |
| Resource use: frequency – PD – blood test | 0.25 | 0.20 | 0.30 | Normal | SE = 0.025 |
| Time-to-death utilitiesa | See description in text | ||||
Appendix 10 Assessment group additional cost-effectiveness sensitivity analyses
During the appraisal, in response to consultation comments, the AG produced additional sensitivity analyses to correct two modelling errors and to update the cost-effectiveness results for the intermediate-/poor-risk subgroup using the updated NMA results with the addition of the CheckMate 214 trial 60-month minimum follow-up data. The two modelling errors were:
-
error in tivozanib engine for AE costs
-
error in application of oral administration costs.
Using the updated costs and NMA data in the model had relatively little impact on results (Tables 91–98) and the same conclusions could be drawn.
Assessment group deterministic scenario analysis results (intermediate-/poor-risk population)
| AG scenarios intermediate/poor-risk subgroup | ICER per QALY gained |
|---|---|
| AG base case | £161,714 |
| Discount rate 6% | £194,420 |
| Discount rate 0% | £119,138 |
| LEN + PEM PFS (gamma) | £161,757 |
| LEN + PEM PFS (generalised gamma) | £161,633 |
| LEN + PEM PFS (Gompertz) | £161,805 |
| LEN + PEM PFS (log-logistic) | £161,344 |
| LEN + PEM PFS (log-normal) | £161,317 |
| LEN + PEM PFS (Weibull) | £161,770 |
| CAB MSD FP PFS HR | £145,178 |
| LEN + PEM OS (exponential) | £139,828 |
| Eisai CABO OS HR | £154,615 |
| MSD CABO FP OS HR | £141,851 |
| CABO OS = LEN + PEM OS | Dominated |
| LEN + PEM TTD (exponential) | £170,839 |
| LEN + PEM TTD (Gompertz) | £164,842 |
| LEN + PEM TTD (Weibull) | £170,962 |
| MSD LEN + PEM TTD (generalised gamma) | £150,849 |
| Eisai CABO TTD (Weibull) | £181,794 |
| Eisai CABO TTD (log-normal) | £168,033 |
| Eisai CABO TTD (exponential) | £181,358 |
| Eisai CABO TTD (generalised gamma) | £174,091 |
| Eisai CABO TTD (Gompertz) | £176,507 |
| MSD CABO FP TTD HR | £155,158 |
| MSD health state utilities | £169,585 |
| Eisai health state utilities | £157,279 |
| AE costs doubled | £163,652 |
| AE costs set to zero | £159,432 |
| Subsequent treatment costs increased by 20% | £160,291 |
| Subsequent treatment costs decreased by 20% | £163,482 |
| AG scenarios intermediate-/poor-risk subgroup | ICER per QALY gained |
|---|---|
| AG base case | £89,524 |
| Discount rate 6% | £108,525 |
| Discount rate 0% | £66,007 |
| LEN + PEM PFS (gamma) | £89,839 |
| LEN + PEM PFS (generalised gamma) | £89,081 |
| LEN + PEM PFS (Gompertz) | £90,095 |
| LEN + PEM PFS (log-logistic) | £87,191 |
| LEN + PEM PFS (log-normal) | £86,762 |
| LEN + PEM PFS (Weibull) | £89,910 |
| LEN + PEM OS (exponential) | £78,171 |
| LEN + PEM TTD (exponential) | £57,441 |
| LEN + PEM TTD (Gompertz) | £78,069 |
| LEN + PEM TTD (Weibull) | £57,030 |
| MSD LEM + PEM TTD (generalised gamma) | £127,435 |
| MSD health state utilities | £85,712 |
| Eisai health state utilities | £63,626 |
| AE costs doubled | £94,372 |
| AE costs set to zero | £84,523 |
| Subsequent treatment costs increased by 20% | £88,791 |
| Subsequent treatment costs decreased by 20% | £90,411 |
| NIV + IPI = Eisai PEM TTD (Weibull) | LEN + PEM is dominant |
| OS LEM + PEM = OS NIV + IPI | LEN + PEM is dominated |
Assessment group deterministic scenario analysis results (favourable-risk subgroup)
| AG scenario favourable-risk subgroup | ICER per QALY gained |
|---|---|
| AG base case | LEN + PEM is dominated by sunitinib |
| Discount rate 6% | LEN + PEM is dominated by sunitinib |
| Discount rate 0% | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (exponential) | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (gamma) | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (Gompertz) | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (log-logistic) | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (log-normal) | LEN + PEM is dominated by sunitinib |
| LEN + PEM PFS (Weibull) | LEN + PEM is dominated by sunitinib |
| Sunitinib PFS (gamma) | LEN + PEM is dominated by sunitinib |
| Sunitinib PFS (generalised gamma) | LEN + PEM is dominated by sunitinib |
| Sunitinib PFS (log-logistic) | LEN + PEM is dominated by sunitinib |
| Sunitinib PFS (Weibull) | LEN + PEM is dominated by sunitinib |
| AG OS NMA HR for sunitinib | LEN + PEM is dominated by sunitinib |
| OS LEN + PEM = OS sunitinib | LEN + PEM is dominated by sunitinib |
| MSD LEN + PEM TTD (generalised gamma) | LEN + PEM is dominated by sunitinib |
| MSD LEN + PEM TTD (gamma) | LEN + PEM is dominated by sunitinib |
| MSD LEN + PEM TTD (Gompertz) | LEN + PEM is dominated by sunitinib |
| MSD LEN + PEM TTD (log-logistic) | LEN + PEM is dominated by sunitinib |
| MSD LEN + PEM TTD (Weibull) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (gamma) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (generalised gamma) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (Gompertz) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (log-logistic) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (log-normal) | LEN + PEM is dominated by sunitinib |
| MSD sunitinib TTD (Weibull) | LEN + PEM is dominated by sunitinib |
| MSD health state utilities | LEN + PEM is dominated by sunitinib |
| AE costs doubled | LEN + PEM is dominated by sunitinib |
| AE costs set to zero | LEN + PEM is dominated by sunitinib |
| Subsequent treatment costs increased by 20% | LEN + PEM is dominated by sunitinib |
| Subsequent treatment costs decreased by 20% | LEN + PEM is dominated by sunitinib |
| AG scenario favourable-risk subgroup | ICER per QALY gained |
|---|---|
| AG base case | LEN + PEM is dominated by pazopanib |
| Discount rate 6% | LEN + PEM is dominated by pazopanib |
| Discount rate 0% | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (exponential) | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (gamma) | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (Gompertz) | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (log-logistic) | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (log-normal) | LEN + PEM is dominated by pazopanib |
| LEN + PEM PFS (Weibull) | LEN + PEM is dominated by pazopanib |
| Pazopanib PFS (gamma) | LEN + PEM is dominated by pazopanib |
| Pazopanib PFS (generalised gamma) | LEN + PEM is dominated by pazopanib |
| Pazopanib PFS (log-logistic) | LEN + PEM is dominated by pazopanib |
| Pazopanib PFS (Weibull) | LEN + PEM is dominated by pazopanib |
| AG OS NMA HR for pazopanib | LEN + PEM is dominated by pazopanib |
| OS LEN + PEM = OS pazopanib | LEN + PEM is dominated by pazopanib |
| MSD LEN + PEM TTD (generalised gamma) | LEN + PEM is dominated by pazopanib |
| MSD LEN + PEM TTD (gamma) | LEN + PEM is dominated by pazopanib |
| MSD LEN + PEM TTD (Gompertz) | LEN + PEM is dominated by pazopanib |
| MSD LEN + PEM TTD (log-logistic) | LEN + PEM is dominated by pazopanib |
| MSD LEN + PEM TTD (Weibull) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (gamma) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (generalised gamma) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (Gompertz) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (log-logistic) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (log-normal) | LEN + PEM is dominated by pazopanib |
| MSD pazopanib TTD (Weibull) | LEN + PEM is dominated by pazopanib |
| MSD health state utilities | LEN + PEM is dominated by pazopanib |
| AE costs doubled | LEN + PEM is dominated by pazopanib |
| AE costs set to zero | LEN + PEM is dominated by pazopanib |
| Subsequent treatment costs increased by 20% | LEN + PEM is dominated by pazopanib |
| Subsequent treatment costs decreased by 20% | LEN + PEM is dominated by pazopanib |
| AG scenario favourable-risk subgroup | ICER per QALY gained |
|---|---|
| AG base case | LEN + PEM is dominated by tivozanib |
| Discount rate 6% | LEN + PEM is dominated by tivozanib |
| Discount rate 0% | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (exponential) | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (gamma) | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (Gompertz) | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (log-logistic) | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (log-normal) | LEN + PEM is dominated by tivozanib |
| LEN + PEM PFS (Weibull) | LEN + PEM is dominated by tivozanib |
| Tivozanib PFS (gamma) | LEN + PEM is dominated by tivozanib |
| Tivozanib PFS (generalised gamma) | LEN + PEM is dominated by tivozanib |
| Tivozanib PFS (log-logistic) | LEN + PEM is dominated by tivozanib |
| Tivozanib PFS (Weibull) | LEN + PEM is dominated by tivozanib |
| AG OS NMA HR for tivozanib | LEN + PEM is dominated by tivozanib |
| OS LEN + PEM = OS tivozanib | LEN + PEM is dominated by tivozanib |
| MSD LEN + PEM TTD (generalised gamma) | LEN + PEM is dominated by tivozanib |
| MSD LEN + PEM TTD (gamma) | LEN + PEM is dominated by tivozanib |
| MSD LEN + PEM TTD (Gompertz) | LEN + PEM is dominated by tivozanib |
| MSD LEN + PEM TTD (log-logistic) | LEN + PEM is dominated by tivozanib |
| MSD LEN + PEM TTD (Weibull) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (gamma) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (generalised gamma) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (Gompertz) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (log-logistic) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (log-normal) | LEN + PEM is dominated by tivozanib |
| MSD tivozanib TTD (Weibull) | LEN + PEM is dominated by tivozanib |
| MSD health state utilities | LEN + PEM is dominated by tivozanib |
| AE costs doubled | LEN + PEM is dominated by tivozanib |
| AE costs set to zero | LEN + PEM is dominated by tivozanib |
| Subsequent treatment costs increased by 20% | LEN + PEM is dominated by tivozanib |
| Subsequent treatment costs decreased by 20% | LEN + PEM is dominated by tivozanib |
Assessment group deterministic scenario analysis results (all-risk population)
| AG scenarios All-risk population |
ICER per QALY gained |
|---|---|
| AG base case | £4,151,860 |
| Discount rate 6% | £1,481,454 |
| Discount rate 0% | LEN + PEM is dominated |
| LEN + PEM PFS (exponential) | £4,145,679 |
| LEN + PEM PFS (generalised gamma) | £4,144,952 |
| LEN + PEM PFS (Gompertz) | £4,157,450 |
| LEN + PEM PFS (log-logistic) | £4,121,252 |
| MSD sunitinib PFS (gamma) | £4,139,053 |
| LEN + PEM OS (exponential) | £260,322 |
| Eisai sunitinib OS (exponential) | LEN + PEM is dominated |
| MSD sunitinib OS (gamma) | £238,557 |
| Eisai LEN + PEM TTD (exponential) | £4,302,116 |
| Eisai LEN + PEM TTD (Gompertz) | £4,228,387 |
| Eisai LEN + PEM TTD (Weibull) | £4,327,271 |
| MSD LEN + PEM TTD (generalised gamma) | £3,877,720 |
| Eisai sunitinib TTD (generalised gamma) | £4,310,945 |
| Eisai sunitinib TTD (Gompertz) | £3,997,979 |
| Eisai sunitinib TTD (log-normal) | £4,203,231 |
| MSD health state utilities | £1,847,799 |
| Eisai health state utilities | £848,819 |
| AE costs doubled | £4,150,186 |
| AE costs set to zero | £4,153,534 |
| Subsequent treatment costs increased by 20% | £4,065,229 |
| Subsequent treatment costs decreased by 20% | £4,238,491 |
| AG scenarios All-risk population |
ICER per QALY gained |
|---|---|
| AG base case | £4,116,623 |
| Discount rate 6% | £1,470,672 |
| Discount rate 0% | LEN + PEM is dominated |
| LEN + PEM PFS (exponential) | £4,110,442 |
| LEN + PEM PFS (generalised gamma) | £4,109,715 |
| LEN + PEM PFS (Gompertz) | £4,122,213 |
| LEN + PEM PFS (log-logistic) | £4,086,014 |
| MSD sunitinib PFS (gamma) | £4,107,944 |
| LEN + PEM OS (exponential) | £258,142 |
| Eisai sunitinib OS (exponential) | LEN + PEM is dominated |
| MSD sunitinib OS (gamma) | £236,590 |
| Eisai LEN + PEM TTD (exponential) | £4,266,878 |
| Eisai LEN + PEM TTD (Gompertz) | £4,193,150 |
| Eisai LEN + PEM TTD (Weibull) | £4,292,034 |
| MSD LEN + PEM TTD (generalised gamma) | £3,842,483 |
| Eisai sunitinib TTD (generalised gamma) | £4,284,682 |
| Eisai sunitinib TTD (Gompertz) | £3,954,305 |
| Eisai sunitinib TTD (log-normal) | £4,170,766 |
| MSD health state utilities | £1,832,116 |
| Eisai health state utilities | £841,615 |
| AE costs doubled | £4,140,393 |
| AE costs set to zero | £4,092,852 |
| Subsequent treatment costs increased by 20% | £4,282,780 |
| Subsequent treatment costs decreased by 20% | £3,950,466 |
| AG scenarios All-risk population |
ICER per QALY gained |
|---|---|
| AG base case | £4,000,330 |
| Discount rate 6% | £1,431,073 |
| Discount rate 0% | LEN + PEM is dominated |
| LEN + PEM PFS (exponential) | £3,994,149 |
| LEN + PEM PFS (generalised gamma) | £3,993,422 |
| LEN + PEM PFS (Gompertz) | £4,005,920 |
| LEN + PEM PFS (log-logistic) | £3,969,721 |
| MSD sunitinib PFS (gamma) | £3,989,820 |
| LEN + PEM OS (exponential) | £250,946 |
| Eisai sunitinib OS (exponential) | LEN + PEM is dominated |
| MSD sunitinib OS (gamma) | £230,057 |
| Eisai LEN + PEM TTD (exponential) | £4,150,585 |
| Eisai LEN + PEM TTD (Gompertz) | £4,076,857 |
| Eisai LEN + PEM TTD (Weibull) | £4,175,741 |
| MSD LEN + PEM TTD (generalised gamma) | £3,726,190 |
| Eisai sunitinib TTD (generalised gamma) | £4,182,510 |
| Eisai sunitinib TTD (Gompertz) | £3,824,226 |
| Eisai sunitinib TTD (log-normal) | £4,059,095 |
| MSD health state utilities | £1,780,359 |
| Eisai health state utilities | £817,840 |
| AE costs doubled | £4,012,817 |
| AE costs set to zero | £3,987,843 |
| Subsequent treatment costs increased by 20% | £4,166,487 |
| Subsequent treatment costs decreased by 20% | £3,834,173 |
List of abbreviations
- AE
- adverse event
- AEOSI
- adverse event of special interest
- AIC
- Akaike information criterion
- AG
- assessment group
- aRCC
- advanced renal cell carcinoma
- BIC
- Bayesian information criterion
- BIRC
- Blinded Independent Review Committee
- CDF
- Cancer Drugs Fund
- CEA
- cost-effectiveness analysis
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CS
- company submission
- CSR
- clinical study report
- CTCAE
- common terminology criteria for adverse event
- CTLA-4
- cytotoxic T-lymphocyte antigen 4
- DIC
- deviance information criterion
- EED
- Economic Evaluation Database
- EMA
- European Medicines Agency
- EORTC
- European Organisation for Research and Treatment of Cancer
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- ESMO
- European Society for Medical Oncology
- EuroQOL
- European Quality of Life
- FAS
- full analysis set
- FDA
- US Food and Drug Administration
- FE
- fixed effect
- FKSI-DRS
- Functional Assessment of Cancer Therapy Kidney Symptom Index-Disease-Related Symptoms
- FP
- fractional polynomial
- HRQoL
- health-related quality of life
- IA3
- third interim analysis (final data cut-off for PFS)
- ICER
- incremental cost-effectiveness ratios
- ICTRP
- International Clinical Trials Registry Platform
- IMDC
- International Metastatic Renal Cell Carcinoma Database Consortium
- INAHTA
- International Health Technology Assessment
- INMB
- incremental net monetary benefit
- ITT
- intention to treat
- K-M
- Kaplan–Meier
- KPS
- Karnofsky Performance Status
- LRiG
- Liverpool Reviews and Implementation Group
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MSKCC
- Memorial Sloan-Kettering Cancer Center
- MSD
- Merck Sharp & Dohme
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- ORR
- objective response rate
- OS
- overall survival
- PD-1
- programmed cell death protein 1
- PD-L1
- programmed death-ligand 1
- PFS
- progression-free survival
- PH
- proportional hazards
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PRO
- patient reported outcome
- PSA
- probabilistic sensitivity analysis
- PSS
- personal and social services
- QALY
- quality-adjusted life-years
- QLQ-C30
- quality of life questionnaire
- RCC
- renal cell carcinoma
- RCT
- randomised controlled trial
- RDI
- relative dose intensity
- SAE
- serious adverse event
- SmPC
- summary of product characteristics
- TA
- technology appraisal
- TEAE
- treatment-emergent adverse event
- TKI
- tyrosine kinase inhibitor
- TSAP
- trial statistical analysis plan
- TTD
- time to treatment discontinuation
- TuDD
- time until definitive deterioration
- VEGFR
- vascular endothelial growth factor receptor
- WTP
- willingness to pay
Note
This manuscript is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk. The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
