Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the Evidence Synthesis Programme on behalf of NICE as award number NIHR135478. The protocol was agreed in April 2022. The draft manuscript began editorial review in October 2022 and was accepted for publication in August 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Boyers et al. This work was produced by Boyers et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Boyers et al.
Chapter 1 Objectives
The overall objective of this assessment was to summarise the current evidence on the clinical and cost-effectiveness of automated devices to help identify peripheral arterial disease (PAD) in people with ulcers of the lower limb. PAD can lead to serious complications including critical limb ischaemia and amputation. The early identification of PAD is important to determine prompt and optimal patient management at the community and primary care levels.
The specific objectives of this assessment are the following:
-
to determine the diagnostic performance and clinical utility of automated devices available in UK clinical practice [BlueDop Vascular Expert (BlueDop Medical), boso ABI-system 100 (BOSCH + SOHN), WatchBP Office ABI (Microlife), WatchBP Office Vascular (Microlife)], MESI ankle–brachial pressure index (ABPI) MD (MESI), MESI mTABLET ABI (MESI), Dopplex Ability Automatic ABI System (Huntleigh Healthcare) for assessing the presence of PAD in people with leg ulcers;
-
to develop an economic model to assess the cost-effectiveness of the automated devices available in UK clinical practice for assessing the presence of PAD in people with leg ulcers.
Chapter 2 Background and definition of the decision problem
Description of the health problem
Peripheral artery disease
Peripheral artery disease is a common atherosclerotic condition caused by narrowing or blockage of the arteries by fatty deposits (known as atheroma), which results in a reduction of blood supply to the affected limb. PAD is associated with an increased risk of vascular complications such as myocardial infarction (MI) and stroke. Early treatment is known to reduce mortality and morbidity. 1 Although PAD is frequently asymptomatic, it can cause complications that can range from intermittent claudication (pain on walking which is relieved by rest) to critical limb ischaemia. Manifestations of critical limb ischaemia include ulceration and gangrene. People with critical limb ischaemia are at high risk of limb amputation and premature death. 2–4
Leg ulcers
Leg ulcers are defined as wounds that occur below the knee and either on or above the ankle (malleolus). Most leg ulcers (about 70%) are venous leg ulcers caused by blood accumulating in the legs due to problems in the veins, which tend to be chronic and recurring;5 about 10% of leg ulcers are caused by PAD and about 20% are mixed aetiology leg ulcers (both arterial and venous). 5–8 Outbreaks of ulceration can last from weeks to years, and ulcers can extend to a surface area > 25 cm2. 9–11
Compression therapy (bandages or stockings) has historically been used to treat venous leg ulcers, and there is a large evidence base to support its effectiveness. 12 However, using compression to treat ulcers may cause damage by impairing the arterial supply to the ulcerated leg. As compression therapy is unsuitable for people with PAD,13,14 it is recommended that people with leg ulcers are screened for arterial disease using the ABPI. 12,13
Incidence and/or prevalence
Peripheral artery disease
Global prevalence of PAD of 10–15% has been estimated3,15,16 and increases with age, especially in those aged in their 60s and 70s. 2,17–20 The incidence of PAD is similar between males and females and higher among black people compared to white people. 19 Hospital Episode Statistics for England for 2020–1 reported 4466 finished consultant episodes and 3220 admissions with a mean length of stay of 6.9 days for peripheral vascular disease (code I73.9).
Leg ulcers
It has been estimated that around 1 million or 2% of adults in the UK have leg ulcers. 21 Records from The Health Improvement Network (THIN) database show that in 2017–8, the annual number of people with an arterial leg ulcer was 1% while the annual number of people with a venous ulcer was 15%. The observed percentage of change (increase) in the annual number of venous ulcers between 2012–3 and 2017–8 was 101%. More recently, the Hospital Episode Statistics for England for the period 2020–1 have reported 20,555 finished consultant episodes and 11,423 admissions with a mean length of stay of 9.0 days for ‘ulcer of lower limb, not elsewhere classified’ (code L97.X). 22
Impact of health problem: significance for patients in terms of ill-health (burden of disease) and significance for the National Health Service
Peripheral artery disease
National Institute for Health and Care Excellence (NICE) guideline CG147 recommends that people are assessed for the presence of PAD if they:
-
have symptoms suggestive of PAD; or
-
have diabetes, non-healing wounds on the legs or feet or unexplained leg pain; or
-
are being considered for interventions to the leg or foot; or
-
need to use compression hosiery. 23
Leg ulcers
It has been shown that the quality of life in people with leg ulcers is affected negatively in terms of pain, impaired mobility, work and social life, anxiety and depression, activities of daily living, sleep disturbance and self-esteem. 24–26 Leg ulcers are also costly to healthcare providers. 12 It has been estimated that people with venous leg ulcers require a nursing visit/dressing change every 2–3 days and that all utilise general practitioner (GP) office visits. The total annual cost to the NHS of managing people with healed venous leg ulcers has been estimated at around £422,000,000 and unhealed venous leg ulcers at £2,781,000,000, with mean annual costs per patient as £2036 and £7886, respectively. 21
Purpose and description of the technologies under assessment
Measurement of the ABPI is widely used in clinical practice to help identify people with PAD who should not receive compression therapy. The current conventional method to measure ABPI consists of a sphygmomanometer and manual Doppler device. The procedure requires specific skills to be performed and can be protracted and unpleasant for those with leg ulcers. 13,23 Automated devices, which have the advantage to reduce the time of ABPI measurement and, therefore, any associated discomfort for the patient, have been proposed as a potential alternative to manual Doppler. Moreover, if automated devices demonstrated a better accuracy than the conventional manual method in detecting the presence of PAD, benefits such as reduced time to treatment and improved outcomes for people with leg ulcers could be conferred. 27 The technologies considered for this appraisal are devices that measure and calculate ABPI automatically, which are available to the NHS in England and have appropriate regulatory approval.
Characteristics of the technologies under assessment
These technologies include Doppler-, oscillometry- and plethysmography-based devices. Doppler-based devices use a Doppler probe and provide Doppler waveform signals as an output while oscillometry-based devices assess oscillations in the vessel wall and plethysmography-based devices assess blood volume changes. The signal measured by these methods is either directly used to estimate blood pressure or assist the measurement of this with a pressure cuff. Devices that do not provide Doppler waveform signals may provide information about the quality of arterial circulation in the ankles instead. However, it is unclear whether these alternative outputs can be considered equivalent to Doppler waveform signals. Current technologies comprise the BlueDop Vascular Expert (BlueDop Medical); the boso ABI-system 100 (BOSCH + SOHN), WatchBP Office ABI (Microlife) and WatchBP Office Vascular (Microlife) oscillometry-based devices; the MESI ABPI MD (MESI) and MESI mTABLET ABI (MESI) oscillometry and plethysmography-based devices; and the Dopplex Ability Automatic ABI System (Huntleigh Healthcare), which is a plethysmography-based device. Table 1 illustrates the characteristics and features of the relevant devices.
| Test name | BlueDop Vascular Expert (BlueDop Medical) | boso ABI-system 100 (BOSCH + SOHN) | WatchBP Office ABI (Microlife) | WatchBP Office Vascular (Microlife) | MESI ABPI MD (MESI) | MESI mTABLET ABI (MESI) | Dopplex Ability Automatic ABI System (Huntleigh Healthcare) |
|---|---|---|---|---|---|---|---|
| Components |
|
|
|
|
|
|
|
| How is the test done? |
|
|
|
|
|
|
|
|
|
|
|
||||
| Outputs |
|
|
|
|
|
|
|
| Time needed |
|
|
|
|
|
|
|
| Patient resting and position for the test |
|
|
|
|
|
|
|
| Indications for use |
|
|
|
|
|
|
|
|
|
Identification of important subgroups
The following subgroups were considered relevant to the scope of this assessment:
-
people with leg ulcers who require measurement of ABPI as part of their initial assessment
-
people with leg ulcers or healed leg ulcers who need reassessment of ABPI as part of monitoring
-
people with diabetes, rheumatoid arthritis, systemic vasculitis, atherosclerotic disease, advanced chronic renal failure or other conditions in which arterial calcification is common
-
people who have had lymph nodes removed or damaged, limb amputation or other conditions where blood pressure cannot be measured on both arms and legs
-
people with sickle cell disease who present with leg ulcers.
Comparators
In UK clinical practice, the current method for measuring ABPI as part of an initial clinical assessment for people with leg ulcers is a manual Doppler-based device: a hand-held Doppler ultrasound probe and a manually inflated blood pressure cuff (sphygmomanometer). The Doppler waveform output can identify health issues even if a person has an ABPI that does not indicate arterial disease. The procedure involves systolic pressure measurements on each limb and multiple measurements on the ankles. The Doppler probe is placed on the artery to assess the blood flow in the artery. The sound of the blood flow stops when the cuff is inflated around the artery and starts again when the cuff is deflated. The systolic blood pressure is then assessed by the sphygmomanometer for calculating the ABPI.
People are required to lie down and remain still before and during the test. The procedure may take between 30 minutes and 1 hour to be completed according to the expertise of the operator and may involve two operators. The assessment is typically carried out by district or community nurses at a person’s home, care home or a leg ulcer clinic, or by practice nurses at GP practices. The healthcare setting depends on the person’s ability to attend the assessment outside of their home and local service arrangements. Scarcity in the required skills and training to conduct ABPI assessments may necessitate onward referral to specialist services after immediate care for the ulcer.
The National Wound Care Strategy Programme (NWCSP) recommends a full clinical assessment of leg wounds within 14 days of initial presentation but there is variation in current clinical practice. 13
Care pathways
Assessment and treatment of leg ulcers in the NHS is conducted according to the recommendations of the NWCSP. 13 Recommended immediate care for leg ulcers consists of cleansing and emollient, simple, low-adherent dressing with sufficient absorbency and mild graduated compression. People should be supported to self-care, if appropriate. If any of the following are present, immediate referral to the relevant clinical specialist is recommended: acute infection, symptoms of sepsis, acute or chronic limb-threatening ischaemia, suspected deep vein thrombosis or suspected cancer. The NWCSP further recommends that assessment of leg wounds should take place within 14 days of original presentation. 13 The NWCSP and the NICE Guideline CG147 both recommend including vascular assessment of arterial supply by way of ABPI. 13,23 The guideline recommends measuring the ABPI by recording systolic blood pressure in both arms and in the posterior tibial, dorsalis pedis and, where possible, peroneal arteries. It is recommended that measurements are taken manually using a Doppler probe of suitable frequency in preference to an automated system. The guideline also recommends documenting the nature of the Doppler ultrasound signals in the foot arteries (pattern of the Doppler waveforms). The type of waveform can provide information about the quality of arterial circulation and might identify issues even if a person has an ABPI that does not indicate arterial disease (e.g. people with arterial calcification). The index in each leg is calculated by dividing the highest ankle pressure by the highest arm pressure.
Ankle–brachial pressure index values are usually interpreted as follows:
-
< 0.8 (or < 0.9 for most international guidelines) suggest arterial disease;
-
< 0.5 suggest severe arterial disease;
-
between 0.8/0.9 and 1.3 suggest no arterial disease; and
-
> 1.3 suggest arterial calcification.
Values above 1.5 indicate that the vessels are likely to be incompressible and the results are not reliable. Results may be misleadingly high in people with diabetes, rheumatoid arthritis, systemic vasculitis, atherosclerotic disease and advanced chronic renal failure and should be interpreted with caution. In addition, caution should be exercised in using compression therapy in people with diabetes due to potential arterial calcification and underlying sensory neuropathy. 28 The test can be uncomfortable for people with leg ulcers, due to both the need to lie still during the test and the placement and inflation of the blood pressure cuff near an ulcer.
Treatment of venous leg ulcers with an adequate arterial supply should include strong compression therapy that is intended to apply at least 40 mmHg compression, according to NWCSP recommendations. 13 The Scottish Intercollegiate Guidelines Network (SIGN) Guideline 120 for management of chronic venous leg ulcers also indicates that compression of at least 40 mmHg should be applied (this guideline was withdrawn in August 2020 as it was 10 years old and is currently being refreshed). 29 Strong multicomponent compression bandaging should be offered to people with chronic ankle/leg oedema not reduced by elevation, abnormal limb shape, copious exudate or very fragile skin. Cardiac clinicians should be consulted regarding the balance of the cardiac burden and using compression in people with advanced, unstable cardiac failure.
People with leg ulcers with signs of arterial disease should be referred for vascular surgical/endovenous interventions, and advice on compression and NICE clinical guideline CG147 on diagnosis and management of PAD should be followed. 23 While awaiting vascular expertise, mild graduated compression is appropriate in oedematous legs with no signs of arterial insufficiency.
People with leg ulcers of other or uncertain aetiology should be referred to a dermatologist and mild graduated compression used in the meantime if there are no signs of arterial insufficiency. For treating leg ulcers in people with lymphoedema, people with lymphoedema and ABPI < 0.5 should not receive compression. Those with ABPI of 0.5–0.8 should receive reduced compression of 15–25 mmHg. In addition, all should be referred to a vascular specialist. 30
People with mixed aetiology ulcers have both venous disease and arterial disease and, without intervention, the arterial disease will take priority in decision-making about treatments. There is currently no consensus on the appropriate level of compression for treating mixed leg ulcers and various criteria have been implemented. 31 The European Wound Management Association position document on compression therapy makes the following recommendations for treating people with mixed arterial and venous ulcers:
-
People with moderate arterial insufficiency with an ABPI 0.5–0.8: Reduced compression (15–25 mg) if there is access to expert bandagers and teams with immediate access to vascular services; refer to vascular specialist particularly if continuing rest pain.
-
People with severe arterial insufficiency with an ABPI < 0.5: Refer to vascular specialist. No compression. Many of these patients may benefit from either arterial surgery or interventional radiology. 32
Other recommendations for treatment of mixed ulcers include referral to tissue viability in the first instance. People with mixed aetiology ulcers will require close monitoring and reassessment of vascular status every 3 months, or sooner if the ulcer deteriorates. 33
Ongoing care of leg ulcers should continue with a review of the effectiveness of the treatment plan at each dressing change. Documentation by way of wound photography at least every 4 weeks is recommended, and escalation to the local specialist service is recommended if the ulcer does not show significant improvement or deteriorates. Additionally, at 12 weeks, the local specialist service should be consulted for the same reasons. Ulcers that have improved but not healed at this stage should be reassessed.
To prevent recurrence of leg ulcers, advice should be offered on skincare, footwear, exercise and mobility, rest and limb elevation, nutrition and self-care and, if appropriate, smoking cessation and weight loss. For people with healed venous leg ulcers, the NWCSP guidelines recommend the continuation of compression therapy and review every 6 months. Changes in symptoms or skin problems related to the compression hosiery should prompt a reassessment, including a vascular assessment of arterial supply.
The SIGN Guideline 120 for management of chronic venous leg ulcers indicated that patients should be offered the strongest compression that maintains patient concordance. The guideline was withdrawn in August 2020 and is currently being refreshed. 29
Chapter 3 Assessment of clinical effectiveness
This report contains reference to confidential information provided as part of the NICE Diagnostic Assessment process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Systematic review methods
An objective synthesis of the evidence of the clinical effectiveness of devices for automated assessment of ABPI as compared to a manual Doppler device for assessing ABPI and peripheral artery disease in people with leg ulcers. The evidence synthesis was conducted in accordance with the general principles of the Centre for Reviews and Dissemination (CRD) guidance for conducting reviews in healthcare and the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions. 34,35 The methods were pre-specified in a research protocol (www.crd.york.ac.uk/prospero/display_record.php?RecordID=327588).
Identification of studies
A sensitive literature search strategy was developed by an Information Specialist to identify published peer-reviewed studies. Major electronic databases were searched, including MEDLINE, EMBASE, Cochrane Library, Web of Science and CINAHL. The initial focus of the search was the list of approved devices in the NICE final scope. There were no restrictions on the date or language of publication at the time of the search. The reference lists of studies selected for full-text appraisal were screened for additional studies. Websites of manufacturers, professional organisations, regulatory bodies and Health Technology Assessment (HTA) organisations were searched to identify additional relevant reports. Any additional information on potentially relevant studies provided by the manufacturers of the devices of interest was also considered. All references were exported to EndNote for recording and deduplication. A draft MEDLINE search is detailed in Appendix 1.
Inclusion and exclusion criteria
Population
The NICE scope for this appraisal specified the population as people with leg ulcers who need assessment of ABPI. Initial screening of search results alongside material provided by the manufacturers of the respective devices suggested that there would be very few studies focusing on people with leg ulcers. Thus, the scope of this assessment was broadened to include studies with any population in which ABPI was measured using a suitable automated device, providing all other eligibility criteria were fulfilled.
Interventions
The interventions under investigation were the following automated devices for measuring ABPI:
-
BlueDop Vascular Expert (BlueDop Medical)
-
boso ABI-system 100 (BOSCH + SOHN)
-
WatchBP Office ABI (Microlife)
-
WatchBP Office Vascular (Microlife)
-
MESI mTABLET ABI (MESI)
-
MESI ABPI MD (MESI)
-
Dopplex Ability Automatic ABI System (Huntleigh Healthcare).
Comparator
The current method for measuring ABPI as part of an initial clinical assessment for people with leg ulcers and/or PAD is a manual Doppler-based device: a hand-held Doppler ultrasound probe and a manually inflated blood pressure cuff (sphygmomanometer). The Doppler waveform output can identify health issues even if a person has an ABPI that does not indicate arterial disease. The procedure involves systolic pressure measurements on each limb and multiple measurements on the ankles. The Doppler probe is placed on the artery to assess the blood flow in the artery. The sound of the blood flow stops when the cuff is inflated around the artery and starts again when the cuff is deflated. The systolic blood pressure is then assessed by the sphygmomanometer for calculating the ABPI.
People are required to lie down and remain still before and during the test. The procedure may take between 30 minutes and 1 hour to be completed according to the expertise of the operator and may involve two operators. The assessment is typically carried out by district or community nurses at a person’s home, care home or a leg ulcer clinic or by practice nurses at GP practices. The healthcare setting depends on the person’s ability to attend the assessment outside of their home and local service arrangements. Scarcity in the required skills/training to conduct ABPI assessments may necessitate onward referral to specialist services after immediate care for the ulcer.
Current methods for detecting the presence of PAD include Duplex ultrasound, angiography, computed tomography angiography (CTA) and magnetic resonance angiography (MRA).
Outcomes and study design
Relevant clinical outcomes and types of studies considered suitable for inclusion are reported in Tables 2 and 3.
| Population | People who require ABPI measurement |
|---|---|
| Devices under investigation | •BlueDop Vascular Expert (BlueDop Medical) •boso ABI-system 100 (BOSCH + SOHN) •WatchBP Office ABI (Microlife) •WatchBP Office Vascular (Microlife) •MESI mTABLET ABI (MESI) •MESI ABPI MD (MESI) •Dopplex Ability Automatic ABI System (Huntleigh Healthcare) |
| Current method for measuring ABPI and detecting PAD | Manual Doppler device: a hand-held Doppler ultrasound probe and a manually inflated blood pressure cuff |
| Reference standard for detecting PAD | Imaging technologies including Duplex ultrasound, angiography, CTA and MRA |
| Outcomes | Measures for consideration may include:
|
|
|
| Study design |
|
|
|
| Healthcare setting |
|
| Population | People who require ABPI measurement |
|---|---|
| Devices under investigation | BlueDop Vascular Expert (BlueDop Medical) boso ABI-system 100 (BOSCH + SOHN) WatchBP Office ABI (Microlife) |
Dopplex Ability Automatic ABI System (Huntleigh Healthcare) |
|
| Comparator | Measuring ABPI and assessing arterial circulation using a hand-held Doppler probe and manual blood pressure sphygmomanometer |
| Outcomes | Clinical outcomes for consideration may include:
|
| Mortality Patient-reported outcomes for consideration may include: |
|
|
|
| Intermediate measures for consideration may include: | |
|
|
|
|
| Study design | Randomised controlled trials
|
| Healthcare setting |
|
Study selection and data extraction
Two reviewers (MC, MB) independently screened the citations identified by the search strategies. This strategy differed from that detailed in the protocol, which specified that the results of the searches would be screened by one reviewer with a random sample of 20% of citations independently screened by a second reviewer. Potentially relevant articles were retrieved in full and independently screened by the same two reviewers for eligibility based on the pre-specified inclusion criteria. One reviewer (MC) screened all documents that had been submitted by the companies with an interest in the respective interventions using the same criteria as used on the results of the search strategies. Potentially relevant studies were selected and checked for relevance by a second reviewer (MB).
Disagreements were resolved by discussion. Multiple publications of the same studies were linked and considered together.
One reviewer (MC) extracted data from each eligible study using an annuitised form developed for the purpose of this assessment. A second reviewer (MB) cross-checked the extracted data. This strategy, which differs from that specified in the research protocol (i.e. two independent reviewers involved in data extraction), was adopted due to time constraints. Any disagreements were resolved by discussion or consultation with a third reviewer (LA).
The following information was recorded from each study:
-
Characteristics of studies: first author, year of publication, country, language, setting, objectives, inclusion and exclusion criteria, type of enrolment, source of funding and conflicts of interest.
-
Characteristics of study participants: age, sex, comorbidities, number of enrolled participants, numbers of limbs and participants included in the analysis, numbers and reasons for withdrawal.
-
Skills of the operator performing the measurement of ABPI using the devices under investigation or the reference device (i.e. years of experience).
-
Characteristics of the automated devices under investigation [BlueDop Vascular Expert (BlueDop Medical), boso ABI_system 100 (BOSCH + SOHN); WatchBP Office ABI (Microlife); WatchBP Office Vascular (Microlife); MESI ABPI MD (MESI); MESI mTABLET ABI (MESI); Dopplex Ability Automatic ABI System (Huntleigh Healthcare)].
-
Characteristics of the reference standard device (i.e. manual Doppler method, Duplex ultrasound, angiography, CTA, MRA).
-
The reported number of true positives (TPs), false positives (FPs), false negatives (FNs) and true negatives (TNs) and, when available, the area under the receiver-operating characteristic curve (AUC) for each device for each relevant outcome.
-
Measures assessing agreement between devices’ measurements (correlation and reliability measures).
-
Relevant patient-reported, clinical and intermediate outcome measures and information related to the use of the devices.
Assessment of risk of bias
Tools were used to assess the risk of bias of included studies according to their study design. Quality Assessment of Diagnostic Accuracy Studies – version 2 (QUADAS-2) criteria were used to assess the quality of included diagnostic studies. 36 QUADAS-2 consists of four domains: patient selection, index test, reference standard and flow and timing. Each domain is assessed in terms of ‘low’, ‘high’ or ‘unclear’ risk of bias and the first three in terms of concerns regarding ‘low’, ‘high’ or ‘unclear’ applicability. The QUADAS-C tool was used to assess the methodological quality of comparative diagnostic accuracy studies. 37 The following decision rules were applied to these assessments. The patient selection domain was judged to be at an ‘unclear’ risk of bias in studies that did not report the study exclusion criteria. For the purposes of this assessment, the results of the automated devices of interest (i.e. index tests) are considered to be objective measurements (as they are automatically calculated by the respective devices) and not subject to interpretation. Thus, in the ‘index test’ domain, the item ‘Were the index test results interpreted without knowledge of the results of the reference standard?’ was answered ‘yes’, indicating a low risk of bias. For the corresponding item regarding the reference standard, the response was ‘yes’ if the reference standard test had been conducted prior to the index test or was conducted after the index text but the operator was explicitly blinded to the automated test result. If both sets of measurements were conducted by the same operator, then the interpretation of the reference standard was classed as ‘high’ risk of bias if the automated device was utilised before the manual device or the order of devices was random, or ‘unclear’ risk of bias if the order of devices utilised was not reported, unless the operator had been explicitly blinded to results of the automated device. In the ‘flow and timing’ domain, the item ‘was there an appropriate interval between index test and reference standard’ was classed as ‘no’ if the resting period before testing was considered insufficient (i.e. < 10 minutes) or was not reported. The item ‘were all patients included in the analysis’ was assessed as a ‘no’ response where at least 10% of participants were not included in the analysis.
For assessing the quality of non-randomised evidence reporting quantitative data on the clinical utility of the devices, we used the checklist developed by the Health Services Research Unit (HSRU), University of Aberdeen, in partnership with the NICE Review Body for Interventional Procedures (ReBIP). The ReBIP checklist was adapted from several sources and comprises 17 items, which assess the following aspects: generalisability, sample definition and selection, description of the intervention, outcome assessment, adequacy of follow-up and performance of the analysis. 34,38–40 Individual items were rated as ‘yes’, ‘no’ or ‘unclear’. A rating of ‘yes’ indicated a low risk of bias.
One reviewer (MC) extracted the data, and a second reviewer (MB) checked the data extracted. Any disagreements were resolved by consensus.
Data synthesis and analysis
Analyses were performed using the methods recommended by Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. 41 For each automated device, we extracted data to populate 2 × 2 contingency tables (TP, FP, FN and TN) of test results cross-classified against those of the manual Doppler method or any other acceptable reference standard. If not reported in the included studies, we back-calculate the number of TP, FP, FN and TN cases using sensitivity and specificity estimates, the total number of patients and the prevalence of PAD. Back-calculation of data was not always precise due to the rounded of available published data. This also impacted on the precision of the confidence intervals (CIs) and where these were out with the plausible range they were truncated to be between 0 and 1.
Where appropriate, we used the hierarchical summary receiving operating characteristic (HSROC) with random-effects model implemented in Stata® (using the METANDI command) to assess the overall performance of each device. This statistical model provides summary estimates of sensitivity and specificity with their corresponding 95% confidence region and 95% prediction region. In accordance with the Stata requirements, we performed meta-analyses only when diagnostic data from four or more studies were available. 42
Heterogeneity was assessed by visual inspection of the forest plots of sensitivity and specificity and of the prediction region in the summary receiving operating characteristic (ROC) plots, when meta-analyses were performed. There were insufficient data, to allow investigation of sources of heterogeneity in estimates of test accuracy by adding covariates to the statistical model.
We initially planned to conduct sensitivity analysis to assess the impact of studies’ methodological quality on the meta-analyses results by restricting analyses to studies at low risk of bias; however, due to the limited number of studies available for each automated device, this proved unfeasible.
Measurements of agreement between the manual and automated devices or between measurements by different automated devices (e.g. Pearson’s correlation coefficient, intraclass correlation coefficient, Cohen’s kappa coefficient, Bland–Altman analysis) were tabulated and described narratively.
Statistical significance was considered for p values of < 0.05. Stata® software version 17.0 (StataCorp LP, College Station, TX, USA) was used for all statistical analyses. 43 Graphs were made using either Stata or Review Manager software version 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark).
Patient and public involvement
This assessment was conducted as part of the NICE Diagnostics Programme, in which a range of stakeholders such as members of the public and national groups representing patients or carers are involved in the interpretation of the identified evidence. Thus, it was not considered necessary to involve further patient representatives or lay people.
Results of the assessment of clinical effectiveness
Results of the literature searches
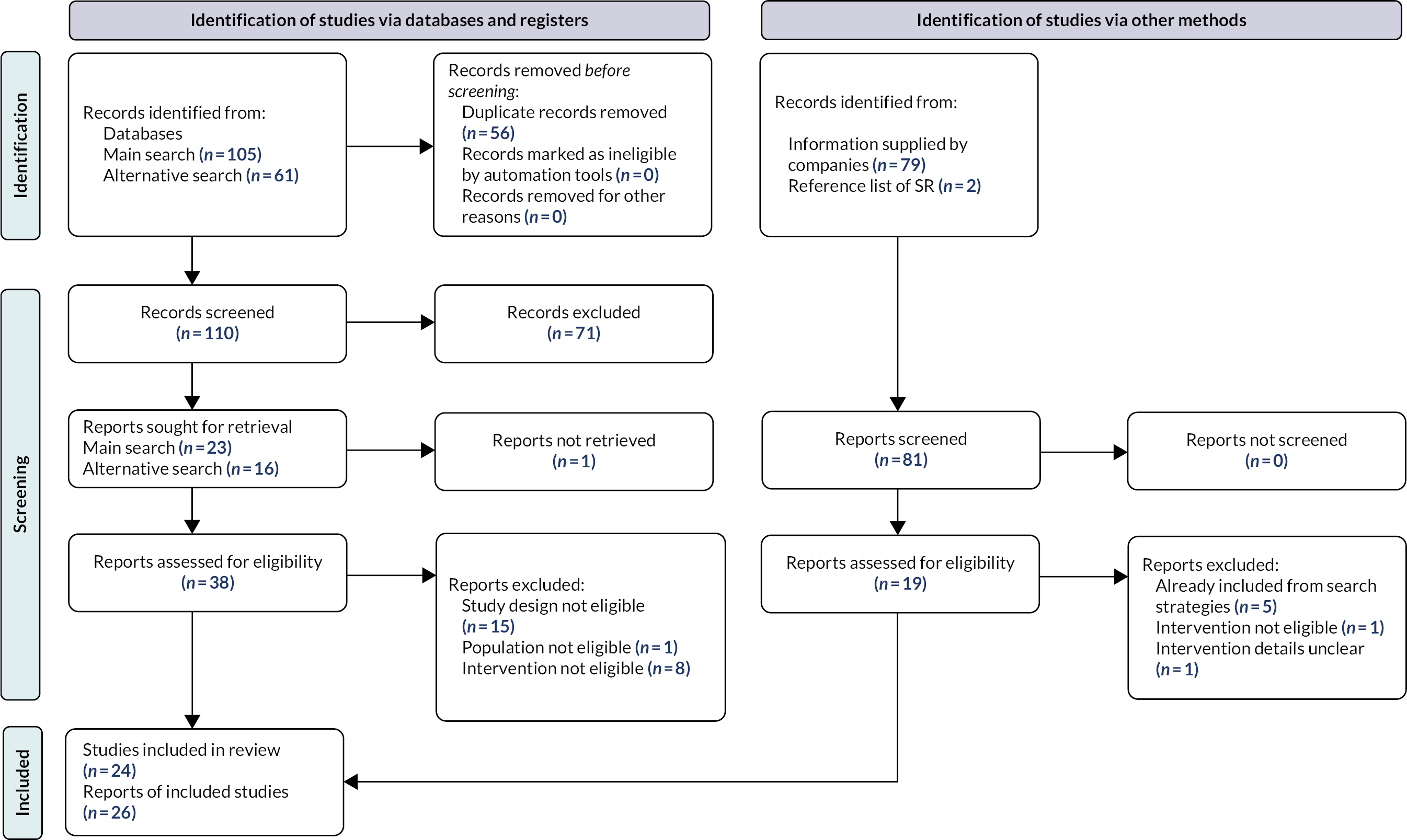
One hundred and sixty-six records were retrieved by the database searches. In addition, 79 records were supplied by the respective companies, giving a total of 245 records. After deduplication, 110 abstracts and all 79 records from the companies were screened for relevance. Two records were identified from the reference list of an existing systematic review. Of these, 57 reports were selected for full-text assessment from which 25 met our inclusion criteria, detailing a total of 23 studies. Reports detailing interim results of one further eligible study were submitted by the respective company after the screening process, resulting in a total of 24 studies reported in 26 publications (Figure 1).
FIGURE 1.
Preferred reporting items for systematic reviews and meta-analyses 2020 flow diagram. SR, systematic reviews.
Characteristics of included studies
A total of 24 studies published in 26 reports were included in the review of clinical effectiveness. Characteristics of the included studies are presented in Table 4. Two studies focused on assessment of ABPI in people with leg ulcers,44,45 while the populations of the remaining studies were either people with symptoms of PAD,46–53 people with risk factors for PAD,54–56 people otherwise requiring assessment of ABPI,57–65 or people taking part in an epidemiology study. 66,67 Six studies were conducted in the UK,44,45,48,49,51,57 two studies in each of the Czech Republic58,66 and France,52,62 three in Spain46,64,67 and one each in Hungary,59 Switzerland,47 Iran,54 New Zealand,60 Sweden,50 the Netherlands,61 Italy,63 Slovenia,53 India,55 Poland65 and Greece. 56 The studies by Boilley et al. and Catillon et al. appear to have been conducted by the same research group with overlapping recruitment periods. 52,62 While it is unclear whether the same participants may have been included in both studies, it is worth noting that the two studies report different outcome measures.
| Study ID, country (secondary study) | Study design/consecutive enrolment? | Automated device/reference device | Population | Main exclusion criteria | Funding source | N analysed: participants/limbs |
|---|---|---|---|---|---|---|
| People with leg ulcers | ||||||
| Welsh 2016, UK44 | Cross-sectional/N/R | Dopplex Ability/ Manual Doppler |
People with leg ulcers who need assessment of ABPI |
|
N/R | 22/N/R |
| Green 2020, UK45 (Boast 2019)68 |
Cross-sectional/N/R | MESI ABPI MD/ Manual Doppler |
People with leg ulcers who need assessment of ABPI | N/R | NHS Executive’s Estate and Technology Transformation Fund | 145/N/R |
| Not people with leg ulcers | ||||||
| NCT05073510 2022, Spain46 | Prospective cohort/N/R | BlueDop Vascular Expert/ Duplex ultrasound |
People with suspected or history of PAD |
|
BlueDop Medical Ltd | Confidential information has been removed |
| Kordzadeh 2018, UK57 | Prospective cohort/Yes | BlueDop Vascular Expert/ Duplex ultrasound |
People referred to vascular outpatient services (one-stop clinic) | N/R | N/R | 166/276 |
| Homza 2019, Czech Republic58 | Cross-sectional/Yes | BOSO ABI-System 100/ Duplex ultrasound |
People with diabetes presenting to a cardiovascular outpatient clinic |
|
N/R | 62/N/R |
| Jarai 2018, Hungary59 | Cross-sectional/Yes | BOSO ABI-System 100/ Manual Doppler |
People enrolled in the Hungarian Hypertension Society’s ERV Registration Program | N/R | N/R | 397/793 |
| Wohlfahrt 2011, Czech Republic66 | Cohort/ N/R |
BOSO ABI-System 100/ Manual Doppler |
General population (1% random sample of Czech population) | N/R | Internal Grant Agency of the Ministry of Health of the Czech Republic | 839/1678 |
| Diehm 2009, Switzerland47 | Cross-sectional/ Yes |
BOSO ABI-System 100/ Manual Doppler |
People with chronic symptomatic PAD |
|
N/R | 50/98 |
| Babaei 2020, Iran54 | Cross-sectional/N/R | Dopplex Ability/ Ultrasound Duplex scan |
People with diabetes and symptoms of PAD |
|
None | 303/606 |
| Millen 2018, New Zealand60 | Cross-sectional/N/R | Dopplex Ability/ Doppler air plethysmography-based Parks Flo-Lab system |
People attending for standard non-invasive vascular assessment |
|
None | 66/129 |
| Davies 2016, UK48 (Davies 2014)69 |
Prospective observational and cross-sectional/N/R | Dopplex Ability/ Doppler ultrasound |
People with CV risk factors but no known CV disease or diabetes | N/R | Huntleigh Healthcare and European Knowledge Economy Skills Scholarship | 380/724 |
| Lewis 2016, UK49 | Cross-sectional/Yes | Dopplex Ability/ Duplex ultrasound |
People referred for lower limb arterial assessment |
|
Health and Care Research Wales and Huntleigh Diagnostics | 189/109 |
| Lewis 2010, UK51 | RCT cross-over/N/R | Dopplex Ability/ Manual Doppler |
People with symptoms of PAD |
|
N/R | N/R/295 |
| Zebari 2022, Sweden50 | Prospective cohort/Yes | MESI ABPI MD/ Manual Doppler |
Patients attending a vascular surgery outpatient clinic |
|
Swedish state, ALF agreement, Swedish Heart-Lung Foundation and Hjart-Lungfonden | 153/306 |
| Hageman 2021, Netherlands61 | Cross-sectional/Yes | MESI ABPI MD/ Manual Doppler |
Patients referred to vascular laboratory for ABI measurement |
|
No relevant financial relationships | 201/402 |
|
||||||
| Boilley 2020, France52 | Cross-sectional/Yes | MESI ABPI MD/ Manual Doppler |
Patients referred to vascular medicine unit for suspected PAD based on exertional limb symptoms | N/R | None | 102/N/R |
| Catillon 2020, France62 | Cross-sectional/N/R | MESI ABPI MD/ Doppler ultrasound |
Patients with a scheduled Doppler ultrasound appointment assessed either by medical students or vascular specialists |
|
None | 43/N/R |
| Varetto 2019, Italy63 |
Cross-sectional/Yes | MESI ABPI MD/ Manual Doppler |
Patients undergoing vascular consultation | N/R | N/R | 185/370 |
| Span 2016, Slovenia53 | Cross-sectional/N/R | MESI ABPI MD/ Manual Doppler |
People with symptoms of PAD |
|
N/R | 136/N/R |
| Verma 2022, India55 | Cross-sectional/N/R | WatchBP Office ABI/ Vascular Doppler device |
Construction workers (described as a ‘high-risk population’) |
|
None | 200/N/R |
| Raya 2019, Spain64 | Cross-sectional/Yes | WatchBP Office, MESI ABPI MD/ Manual Doppler |
People attending a primary care centre (for any reason) |
|
Catalan Society of Family and Community Medicine | 202/404 |
| Rodriguez-Roca 2014, Spain67 | Cross-sectional/N/R | WatchBP Office ABI/ Manual Doppler |
People without PAD seen in primary care | N/R | Governmental grant from the Socio-Sanitary Foundation of Castile-La Mancha | 322/N/R |
| Sinski 2013, Poland65 | Cross-sectional/Yes | WatchBP Office ABI/ Ultrasound Doppler |
People with known coronary artery disease |
|
Medical University of Warsaw | 80/158 |
| Kollias 2011, Greece56 | Cross-sectional/N/R | WatchBP Office ABI/ Manual Doppler |
People with cardiovascular risk factors attending a hypertension or diabetes outpatient clinic |
|
Microlife, Widnau, Switzerland | 93/186 |
One study assessed the performance of two automated devices (WatchBP Office and MESI ABPI MD) for measuring ABPI while the remaining studies assessed only the performance of one automated device. 64 The BlueDop Vascular Expert device was assessed by a published study and an ongoing study for which the sponsor provided confidential interim results,46,57 the BOSO ABI-System 100 device by four studies,47,58,59,66 the Dopplex Ability by six studies,44,48,49,51,54,60 the MESI ABPI MD by seven studies,45,50,52,53,61–63 and the WatchBP Office ABI device by four studies. 55,56,65,67 We did not identify any study assessing the performance of WatchBP Office Vascular or MESI mTABLET ABI for measuring ABPI in people with leg ulcers or symptoms of PAD. The reference standard was manual Doppler in 20 studies44,45,47–53,55,56,59–67 and Duplex ultrasound in 4 studies. 46,54,57,58 Two of these four studies also assessed the performance of the manual Doppler. All studies were published in English except for the studies by Jarai et al. and Raya et al. that were published in Hungarian and Spanish, respectively. 59,64 Google Translate was used to facilitate screening and data extraction of these studies.
Characteristics of the automated devices under investigation and the reference standard device for each included study are reported in Appendix 2, Table 28. The healthcare professional assessing ABPI was reported in 17 studies: 6 studies reported the involvement of a vascular specialist,44,46,52,57,60–62 5 involved trained nurses,48,54,59,64,67 3 studies an experienced physician or technician,47,65,66 2 studies involved a podiatrist,49,51 and 1 study involved general practice staff. 45
Baseline characteristics of participants in the included studies are reported in Appendix 3, Table 29. Mean age of participants ranged from 27.555 to 72.5 years63 and the proportion of male participants ranged from 39.8%54 to 100%. 55
Risk-of-bias assessments
Twenty-one studies were assessed using the QUADAS-2 tool,46–63,65–67 one study using QUADAS-C64 and two using the ReBIP checklist. 44,45
Of the 21 studies assessed with the QUADAS-2 criteria, 5 studies were at unclear risk of bias for the patient selection domain due to lack of reporting exclusion criteria. 52,57,63,66,67 Risk of bias was low across all studies for the index test domain but unclear for nine studies in the reference standard domain (confidential information has been removed) 46,63,66 and/or whether the operator was blinded to the results of the automated device measurement. 55,56,58–61 In the study by Boilley et al. , all measurements were conducted by one operator, with the manual Doppler measurement taken after the automated device measurement. 52 Thus, risk of bias was assessed as high for the reference standard domain. The flow and timing domain was judged to be at high risk of bias in seven studies due to either insufficient resting time prior to testing54,66,67 or (confidential information has been removed). 46,48,53,63 The risk of bias in the flow and timing domain was assessed as unclear in six studies due to the lack of information about either the number of participants included in the analysis51 or the resting period before the actual testing procedure. 49,55,57,59,60 In general, applicability concerns were low across studies.
The one study assessed using QUADAS-C was assessed as having a low risk of bias across all domains except reference standard and flow and timing due to lack of information regarding the resting period before testing and the order of administration of the automated devices. 64
Two studies – Welsh et al. and Green et al. – were assessed using the ReBIP tool. 44,45 The study by Welsh included a representative sample, clearly defined inclusion and exclusion criteria and participants at a similar point in disease progression. The study by Green did not report inclusion and exclusion criteria and information on the representativeness of the sample was limited. Both studies involved prospective data collection, with clearly defined interventions delivered in an appropriate setting, and important and objective outcomes. Information on withdrawals was not reported by either study, and there was insufficient information to assess whether participants who dropped out were similar to those who completed the study or whether important prognostic factors had been identified. Across the five devices, overall patterns of risk of bias were generally similar with the majority of domains being assessed as low risk of bias. The exception to this was the flow and timing domain, which was often assessed as either high risk of bias due to insufficient resting time prior to testing or > 10% of participants not being included in the analysis or unclear risk of bias because these details were not reported. The reference standard domain also suffered due to lack of reporting of the order of devices used in testing or blinding of the operator.
Full details of risk-of-bias assessments are reported in Appendix 4 (see Figures 16 and 17 and Table 30).
Diagnostic outcomes
People with leg ulcers
A summary of key findings of the two studies involving people with leg ulcers is presented in Table 5. 44,45 These studies did not report the sensitivity and specificity of the automated devices for diagnosing PAD.
| Study ID | Automated device/ reference device |
Population setting | Patients analysed, n | Summary of findings |
|---|---|---|---|---|
| Welsh 2016 (UK)44 | Dopplex Ability/ Manual Doppler |
People with leg ulcers attending a community leg ulcer clinic for ABPI assessment | 22 |
|
| Green 2020 (UK)45 | MESI ABPI MD/ Manual Doppler |
People with leg ulcers who need assessment of ABPI in general practice | 145 |
|
Acceptability and experience of using the device
The two studies that assessed ABPI in people with leg ulcers both reported information on the acceptability of the respective automated devices and experience of their use. 44,45 The study by Welsh et al. reported that the Dopplex Ability was easier to use than the manual Doppler and more convenient in terms of testing time. 44 This study also reported that the majority of patients found the automated device to be acceptable, but some felt discomfort when the cuff was fully inflated. The study by Green et al. acknowledges some issues related to the use of the automated device including the length of time and complexity of the initial setting up of the software; the insufficient general practice personnel and the fact that GPs may not refer patients for ABPI assessments due to limited availability of appointments. 45 They also reported that several GP practices felt that wound care was not within the remit of their practice and would be better managed by leg ulcer services. 45 The reported benefits of the MESI ABPI MD include speed, simplicity of use, accuracy and printouts of the assessment, as well as enhanced patient management and appropriate referral to more specialised services. Half of the staff involved in the study expressed the intention to continue using the MESI ABPI MD device but pointed out that additional resources such as staff, time and funding would be needed.
People not with leg ulcers
Given the lack of studies enrolling people with leg ulcers and the fact that ABPI measurement in people with leg ulcers is used to identify patients with PAD who should not receive compression therapy, after consulting with key stakeholders, we considered it relevant to summarise the evidence on the ability of the automated devices to detect PAD in people with no leg ulcers who required ABPI measurement.
A summary of the key findings for the 22 studies assessing patients without leg ulcers is presented in Table 6. A total of 4258 people were analysed. Most people were referred to a vascular service or had cardiovascular risk factors. The prevalence of PAD was 100% in one study that focused on people with a previous diagnosis of PAD47 and ranged from 2%54,66 to 80%52 across the remaining studies. Seventeen studies reported sensitivity and specificity estimates of the automated device for detecting PAD with either manual Doppler or Duplex ultrasound used as the reference device. 46,48–50,52–61,64–66 Across studies, sensitivity estimates ranged from 20% for the Dopplex Ability54 to 95% for the BlueDop Vascular Expert57 device; specificity estimates ranged from 67%50 to 100%. 55
| Study ID | Automated device/reference device | Population setting |
Patients analysed, n | Age, years, mean (SD) Male sex (%) |
PAD prevalence according to Doppler (%) | Doppler ABI Automated ABI |
AUC (95% CI) |
Sensitivity (%) | Specificity (%) | Agreement between automated device and reference standard | Technical failure rate, % automated device/ reference device |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT05073510 Interim results to May 2022 (Spain)46 | BlueDop Vascular Expert/ Duplex ultrasound |
People referred for Duplex ultrasound of lower limb(s) with suspected or previous history of PAD | Confidential information has been removed | Confidential information has been removeda Confidential information has been removed Confidential information has been removeda | Confidential information has been removed | Confidential information has been removed N/R | N/R | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed N/R |
| Kordzadeh 2018 (UK)57 | BlueDop Vascular Expert/ Duplex ultrasound |
People referred to vascular outpatient services | 166 | Median (IQR) 73 (65–81) 62.0 |
N/R | DPA N/R |
0.92 (0.88 to 0.95) |
95 | 90 | N/R | N/R |
| Homza 2019 (Czech Republic)58 | BOSO ABI-System 100/ Duplex ultrasound |
People with diabetes at a cardiovascular outpatient clinic | 62 | 67.6 (min, max 41.8, 83.2) 74.2 |
N/R | N/R DPA or ATA |
N/R | 61 | 94 | N/R | N/R |
| Jarai 2018 (Hungary)59 | BOSO ABI-System 100/ Manual Doppler |
People enrolled in Hungarian Hypertension Society’s ERV Registration Program | 397 | 63.9 (11.5) 44.6 |
N/R | N/R N/R |
0.94 (0.92 to 0.95) |
77 | 94 | r = 0.689b Kappa statistics: 0.7 Bland–Altman plot (difference between ABI measurements): r = 0.01 (limits of agreement: −0.29, 0.32) |
7.7/ <1 |
| Wohlfahrt 2011 (Czech Republic)66 | BOSO ABI-System 100/ Manual Doppler |
General population (1% random sample of Czech population) | 839 | 54.3 (13.8) 46.8 |
2 | ↑DPA or PTA/R BA N/R |
N/R | 77 | 98 | Pearson’s correlation coefficient: r = 0.45 Bland–Altman plot (difference between ABI measurements): mean 0.1 (limits of agreement: −0.11, 0.30) |
N/R |
| Diehm 2009 (Switzerland)47 | BOSO ABI-System 100/ Manual Doppler |
People with chronic symptomatic PAD | 50 | 65 (6) 62.0 |
100 | DPA and PTA/↑BA N/R |
N/R | N/R | N/R | Pearson’s product-moment correlation: r = 0.76 | N/R |
| Bland–Altman plot (difference between ABI measurements; non-diabetic patients): Low Doppler ABI: r = 0.05 (95% CI −0.02 to 0.11), p > 0.1 | |||||||||||
| High Doppler ABI: r = −0.02 (95% CI −0.08 to 0.04), p > 0.1 | |||||||||||
| Babaei 2020 (Iran)54 | Dopplex Ability/ Duplex ultrasound |
People with diabetes and symptoms of PAD | 303 | 60.1 (0.3) 39.8 |
2 (according to Duplex ultrasound) | ↑PT or DP/↑BA N/R |
0.48 (0.44 to 0.52) |
20 | 96 | N/R | N/R |
| Millen 2018 (New Zealand)60 | Dopplex Ability/ Manual Doppler |
People attending for non-invasive vascular assessment | 66 | 69.5 (12) 77.3 |
43 | BA, PTA and DPA N/R |
N/R | 59 | 86 | Pearson’s correlation coefficient: R2 = 0.17 | 2.3/ N/R |
| Davies 2016 (UK)48 |
Dopplex Ability/ Manual Doppler |
People with CV risk factors but no known CV disease or diabetes | 380 | 64 (9) 57.0 |
6 | N/R N/R |
0.96 (0.94 to 0.98) |
70 | 96 | Bland–Altman plot (difference between ABI measurements): mean 0.016 ± 0.1 | 3.9/ 0.0 |
| Lewis 2016 (UK)49 |
Dopplex Ability/ Duplex ultrasound |
People referred for lower limb arterial assessment | 189 | 67 (12) 65.1 |
36 (according to Duplex ultrasound) | Distal CFA, SFA and PA (Duplex ultrasound) N/R |
0.88 (0.83 to 0.93) |
79 | 91 | N/R | N/R |
| Lewis 2010 (UK)51 | Dopplex Ability/ Manual Doppler |
People with symptoms of PAD | N/R | N/R N/R |
N/R | N/R N/R |
N/R | N/R | N/R | r = 0.89b | N/R |
| Zebari 2022 (Sweden)50 | MESI ABPI MD/ Manual Doppler |
Patients attending a vascular surgery outpatient clinic | 153 | 72 (10) 63.4 |
52 | N/R N/R |
N/R | 75 | 67 | Spearman rank correlation: r = 0.552 Bland–Altman plot (difference between ABI measurements): mean −0.067 (limits of agreement: −0.52, 0.38) |
n = 28 (%N/R)/ N/R |
| Hageman 2021 (Netherlands)61 | MESI ABPI MD/ Manual Doppler |
Patients referred to vascular laboratory for an ABI measurement | 201 | 67 (11) 55.7 |
31 | ↑ankle/↑BA ↑BA and ankle |
0.96 (0.93 to 1.00) | 74 | 97 | Pearson’s correlation coefficient: r = 0.87 Bland–Altman plot (difference between ABI measurements): mean 0.05 (limits of agreement: −0.20, 0.29) |
15.7/ 0.0 |
| Boilley 2020 (France)52 | MESI ABPI MD/ Manual Doppler |
Patients referred to vascular medicine unit for suspected PAD based on exertional limb symptoms | 102 | 63 (11) 84.3 |
80 | N/R N/R |
N/R | 66 | 85 | Kappa coefficient = 0.35 (0.15) Correlation coefficient = 0.63b Bland–Altman plot (difference between ABI measurements): mean 0.12 ± 0.26 |
N/R |
| Catillon 2020 (France)62 | MESI ABPI MD/ Manual Doppler |
Patients with a scheduled Doppler ultrasound appointment assessed either by medical students or vascular specialists | 43 | 66 (14.4) 67.4 |
11 | PTA and ATA/↑BA and ankle N/R |
N/R | N/R | N/R | Pearson’s correlation coefficient: r = 0.2 | N/R |
| Varetto 2019 (Italy)63 | MESI ABPI MD/ Manual Doppler |
Patient who underwent vascular consultation | 185 | 72.5 (13.6) | N/R | ↑TBA and PDA/↑BA ↑TBA and PDA/↑BA |
N/R | N/R | N/R | Kendall’s tau = 0.63 Bland–Altman plot (difference between ABI measurements): mean 0.07 (95% CI 0.05 to 0.09) |
19%/ 11% |
| Span 2016 (Slovenia)53 | MESI ABPI MD/ Manual Doppler |
People with symptoms of PAD | 136 | 64 (7.8) N/R |
10 | ↑DPA or PTA/↑BA N/R |
N/R | 57 | 99 | Pearson’s correlation coefficient: r = 0.61 Bland–Altman plot (difference between ABI measurements): mean 0.06 (limits of agreement: −0.21, 0.33) |
9.3 (total for automated and Doppler failures) |
| Verma 2022 (India)55 | WatchBP Office ABI/ Manual Doppler |
Construction workers (described as a ‘high-risk population’) People with ulceration in the lower limbs and marked oedema were excluded |
200 | 27.5 (4.1) 100.0 |
6 | BA and PTA ↑Arm and ankle |
0.98 (0.96 to 1.0) |
50 | 100 | Pearson’s correlation coefficient: r = 0.96 (95% CI 0.99 to 1.07) ICC (agreement between methods): 0.98 (95% CI 0.97 to 0.99) Bland–Altman plot (difference between ABI measurements): mean 0.07 (95% CI −0.03 to 0.12) |
N/R |
| Raya 2019 (Spain)64 | WatchBP Office, MESI ABPI MD/ Manual Doppler |
People attending a primary care centre (for any reason) | 202 | 63 (7) 44.1 |
6 | Pedal and tibial arteries N/R |
WatchBP: 0.80 MESI: 0.78 |
WatchBP: 44 MESI: 63 |
WatchBP: 98 MESI: 98 |
WatchBP ICC: 0.27 (95% CI 0.0 to 0.5) MESI ICC: 0.20 (95% CI 0.0 to 0.4) |
WatchBP: 13 MESI: 14/ 4 |
| Rodriguez-Roca 2014 (Spain)67 | WatchBP Office ABI/ Manual Doppler |
People without PAD seen in primary care | 322 | 47.7 45.7 |
17 | ↑PTA or pedal artery/↑SBP N/R |
N/R | N/R | N/R | Pearson’s correlation coefficient: r = 0.7 ICC: 0.7 (95% CI 0.6 to 0.8) Bland–Altman plot (difference between ABI measurements): mean −0.03 (limits of agreement: −0.21, 0.15) |
N/R |
| Sinski 2013 (Poland)65 | WatchBP Office ABI/ Manual Doppler |
People with known coronary artery disease | 80 | 70.1 (9.4) 66.3 |
40 | PTA/↑BA N/R |
N/R | 46 | 98 | Pearson’s correlation coefficient: r = 0.51 Bland–Altman plot (difference between ABI measurements): mean −0.15 (limits of agreement: −0.58 to 0.28) |
2.5/ N/R |
| Kollias 2011 (Greece)56 | WatchBP Office ABI/ Manual Doppler |
People with cardiovascular risk factors attending a hypertension or diabetes outpatient clinic | 93 | 62.5 (11.1) 62.4 |
17 | ↑DPA or PTA/↑BA N/R |
0.98 | 83 | 97 | Pearson’s correlation coefficient: r = 0.80, p < 0.001 Bland–Altman plot (difference between ABI measurements): mean 0.03 ± 0.11 |
1.6/ N/R |
Results according to the type of automated device are reported below. When sufficient data were available, diagnostic results across studies were pooled using recommended methods.
BlueDop vascular expert device
Data on the BlueDop Vascular Expert device were provided by a published study57 and an ongoing study46 for which the company provided confidential interim results. Both studies assessed people who were referred to a vascular service and compared the performance of the BlueDop Vascular Expert device with that of Duplex ultrasound (255 participants in total). The study by Kordzadeh et al. reported a sensitivity of 95% and a specificity of 90%. 57 The interim results of the ongoing study show a moderate sensitivity (confidential information has been removed) and good specificity (confidential information has been removed). 46 Prevalence of PAD was not reported.
BOSO ABI-System 100 device
The BOSO ABI-System 100 device was assessed by four. 47,58,59,66 The patient population varied across studies (see Table 6). Three studies (1298 participants in total) provided sensitivity and specificity estimates. 58,59,66 Sensitivity of the BOSO ABI-System 100 device ranged from 61% in a sample of diabetic people58 to 77% in the general population or people enrolled in a hypertension programme. 59,66 Specificity estimates were higher across studies and ranged from 94%58,59 to 98%. 66 Prevalence of PAD was not consistently reported across studies (see Table 6) and, as expected, varied according to the characteristics of the enrolled patient population (e.g. 2% among a random sample derived from the general population and 100% among a sample of patients with an established PAD diagnosis).
Dopplex Ability
The Dopplex Ability device was assessed by five studies that enrolled people with symptoms of PAD or at risk of cardiovascular events. 48,49,51,54,60 Four studies (938 participants in total) provided estimates of accuracy. 48,49,54,60 The reference device was either manual Doppler48,60 or Duplex ultrasound. 49,54 Sensitivity varied considerably across studies and ranged from 20% in a sample of diabetic people54 to 79% in people referred for lower limb arterial assessment;49 specificity ranged from 86%60 to 96%;48,54 prevalence of PAD ranged from 2%54 to 63%. 49
For the BlueDop Vascular Expert, BOSO ABI-System 100 and Dopplex Ability devices, there were too few studies to conduct a meaningful meta-analysis.
MESI ABPI MD device
The MESI ABPI MD device was assessed by seven studies enrolling people who had been referred to a vascular service or to a primary care centre, and people presenting with symptoms of PAD. 50,52,61–64,67 Five studies provided estimates of accuracy;50,52,53,61,64 sensitivity ranged from 57%53 to 75%50 and specificity estimates from 67%50 to 99%. 53 Prevalence of PAD ranged from 6%64 to 80%. 52 The sample size ranged from 102 to 202 participants.
We were able to combine the results of five studies [742 participants in total; 243 (33%) with PAD], which provided relevant diagnostic data. The pooled sensitivity of MESI ABPI MD for detection of PAD was 67% (95% CI 59% to 74%) and the pooled specificity was 94% (95% CI 83% to 98%). Figures 2 and 3 show the forest plot and summary ROC plot depicting the accuracy of MESI ABPI MD measurement versus manual Doppler measurement for detection of PAD.
FIGURE 2.
Forest plot of MESI ABPI MD measurement vs. manual Doppler measurement for PAD diagnosis.
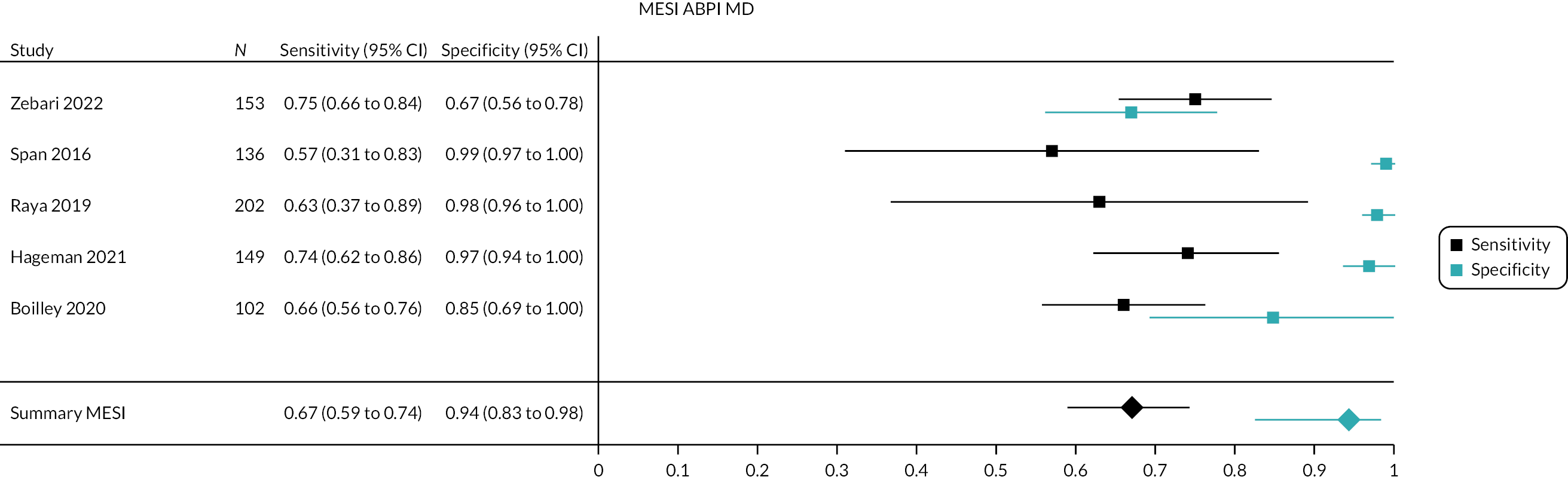
FIGURE 3.
Summary ROC plot for ABPI measurement using the MESI ABPI MD device.

WatchBP office ABI device
The WatchBP Office ABI device was assessed by five studies enrolling people with cardiovascular risk factors or established coronary artery disease, people seen in primary care and people sampled from the general population. 55,56,64,65,67 Four studies provided estimates of accuracy. 55,56,64,65 Sensitivity estimates ranged from 44%64 to 83%56 and specificity estimates from 97%56 to 100%. 55 Prevalence of PAD ranged from 6%55,64 to 40%. 65 The sample size ranged from 80 to 202 participants.
We were able to combine the results of four studies [575 participants in total; 73 (13%) with PAD], which provided relevant diagnostic data. The pooled sensitivity of WatchBP Office ABI for detection of PAD was 53% (95% CI 37% to 69%) and the pooled specificity was 98% (95% CI 96% to 99%). Figures 4 and 5 show the forest plot and summary ROC plot depicting the accuracy of WatchBP Office ABI versus manual Doppler ABPI for detection of PAD.
FIGURE 4.
Forest plot of WatchBP Office ABI measurement vs. manual Doppler measurement for PAD diagnosis.

FIGURE 5.
Summary ROC plot for ABPI measurement using the WatchBP Office ABI device.

Figures 6 and 7 show the forest plot and summary ROC plot for all studies, irrespective of the type of automated device, for which accuracy data to a construct 2 × 2 contingency table (i.e. TPs, FPs, FNs and TNs) were available or could be calculated from the information provided in the included studies (12 studies). Across included studies, the pooled sensitivity for the diagnosis of PAD using automated devices was 0.64% (95% CI 57% to 71%) and the pooled specificity 96% (95% CI 92% to 98%). The sample size ranged from 66 to 696 participants.
FIGURE 6.
Forest plot of automated ABPI measurement vs. manual Doppler measurement for PAD diagnosis.

FIGURE 7.
Summary ROC plot for ABPI measurement using automated devices. aThree studies used Dopplex Ability, five studies MESI ABPI MD and four studies WatchBP Office ABI.

Agreement between devices and threshold for diagnosing peripheral artery disease
Correlation coefficients for ABPI measurements varied across studies but in most cases showed a moderate or good relationship between the automated devices under investigation and the reference devices (see Table 6). However, the Bland–Altman plot was often suboptimal, and most studies reported a systematic tendency for the automated device to overestimate ABPI values with larger differences observed in the lower range of ABPI values. These differences could translate in a potential risk for the automated devices of underestimating the presence of PAD when the common 0.9 threshold is applied.
It is worth noting that even though all included studies used the common ABPI threshold of 0.9 for the detection of PAD, some studies calculated a ROC curve to determine the optimal threshold for PAD diagnosis. 48–50,54,58,59,61 Table 7 presents the sensitivity and specificity of automated ABPI measurement according to the best-identified threshold for diagnosing PAD. As expected, modification of the ABPI threshold resulted in higher sensitivity estimates and slightly lower specificity estimates.
| Study ID | Automated device/ reference device |
Patients analysed, n | Optimal ABPI threshold for PAD diagnosis using the automated device | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Homza 201958 | BOSO ABI-System 100/ Duplex ultrasound |
62 | 1.0a | 84 | 75 |
| Jarai 201859 | BOSO ABI-System 100/ Manual Doppler |
397 | 0.96 | N/R | N/R |
| Babaei 202054 | Dopplex Ability/ Duplex ultrasound |
303 | 1.2 | 40 | 80 |
| Davies 201648 | Dopplex Ability/ Doppler ultrasound |
380 | 1.04 | 98 | 75 |
| Lewis 201649 | Dopplex Ability/ Duplex ultrasound |
189 | 0.98 | 87 | 80 |
| Zebari 202250 | MESI ABPI MD/ Manual Doppler |
153 | 1.0a | 77 | 62 |
| Hageman 202161 | MESI ABPI MD/ Manual Doppler |
201 | Diabetic patients: 1.00 | 96 | 91 |
| Non-diabetic patients: 1.02 | 91 | 90 | |||
| Span 201653 | MESI ABPI MD/ Manual Doppler |
136 | 1.0a | 85 | 96 |
| Raya 201964 | WatchBP Office, MESI ABPI MD/ Manual Doppler |
202 | WatchBP: 1.12 | 84 | 86 |
| MESI: 1.16 | 88 | 70 | |||
| Kollias 201156 | WatchBP Office ABI/ Manual Doppler |
93 | 0.97 | 92 | 92 |
People with diabetes
Table 8 presents key diagnostic outcomes of people with diabetes in studies that enrolled solely people with diabetes54,58 or those that reported results separately for diabetic and non-diabetic patients. 46,56,60,61 Apart from Babaei et al. who reported a very low sensitivity (20%) for automated ABPI measurement in people with diabetes, there was not a clear indication that the accuracy of the automated devices was much different in diabetic patients. 54
| Study ID (geographical location) |
Patients analysed | Automated device/reference device | People with diabetes | People not with diabetes | ||
|---|---|---|---|---|---|---|
| NCT05073510 (Spain)46 | Confidential information has been removed Confidential information has been removeda diabetic patients |
BlueDop Vascular Expert/ Duplex ultrasound |
Sensitivity Confidential information has been removed |
Specificity Confidential information has been removed |
Sensitivity Confidential information has been removed |
Specificity Confidential information has been removed |
| Homza 2019 (Czech Republic)58 | 62 diabetic patients | BOSO ABI-System 100/Duplex ultrasound | Sensitivity 61% |
Specificity 94% |
N/A | N/A |
| Babaei 2020 (Iran)54 | 303 diabetic patients |
Dopplex Ability/Duplex ultrasound | Sensitivity 20% |
Specificity 96% |
N/A | N/A |
| Millen 2018 (New Zealand)60 | 66 18 diabetic patients |
Dopplex Ability/Manual Doppler | The presence of diabetes had no significant effect on the ABI accuracy | |||
| Hageman 2021 (Netherlands)61 | 201 61 diabetic patients |
MESI ABPI MD/Manual Doppler | Sensitivity 68% |
Specificity 95% |
Sensitivity 76% |
Specificity 97% |
| Kollias 2011 (Greece)56 | 93 42 diabetic patients |
WatchBP Office ABI/Manual Doppler | The mean difference between the manual Doppler and automated ABI measurements was similar in diabetics and non-diabetics | |||
Time of ankle–brachial pressure index measurement using the automated device and the reference device
The time required to assess ABPI using the respective devices was not consistently reported across studies. Often it was not clearly reported what ‘timing’ entailed (e.g. resting period, fitting of cuffs, resting plus testing period) making it challenging to compare findings across studies. Apart from the study by Raya et al. , in all remaining studies that reported this information, the assessment with the automated device required less time than that with the manual Doppler, mainly due to shorter resting time before starting the automated measurement. 64 In the study by Raya et al. , measurement of ABPI with the WatchBP Office ABI device required longer time (mean 14.4 minutes) than that with the MESI ABPI MD device (mean 10.7 minutes) or the manual Doppler (mean 12.1 minutes) because of the time needed to identify the arm with the highest systolic blood pressure. 64
Technical failures
Some studies reported the occurrence of technical failures in the measurement of ABPI. Davies et al. and Millen et al. reported failed Dopplex Ability measurements in 2.3% and 3.9% of limbs, respectively. 48,60 Davies et al. further explained that the failures were caused by hypertension in the limbs and that there were no failed manual Doppler measurements. Hageman et al. reported measurement errors relating to the use of the MESI ABPI MD device in a total of 15.7% of limbs – with a higher proportion in limbs with PAD (28%) compared with limbs without PAD (7%). 61 Similarly, Varetto et al. reported a higher proportion of measurement failures related to the use of the MESI ABPI MD (19%) compared with the manual Doppler (11% of failures). 63 Zebari et al. reported 28 error codes produced by the MESI ABPI MD device in 306 legs, with 6/28 error codes being considered technical failures. 50 In general, across studies assessing the use of the MESI ABPI MD device, failed measurements occurred in people with critical limb ischaemia/incompressible arteries (9.3%),53 arterial calcifications (4.8%),61 values consistent with PAD (71.4%),61 or normal values (23.8%). 61 ABPI measurement failures after the use of the WatchBP Office ABI device were reported in a low number of participants (2.5% of patients65 and 1.6% of limbs56). More errors were observed in limbs with PAD (35.2%) than in limbs without PAD (5.7%). 56 One study reported that zero values were returned by the BOSO ABI-System 100 in 7.7% of limbs and by the manual Doppler in 3.3% of limbs. 59 The confidential interim results of the ongoing study assessing the use of the BlueDop Vascular Expert device show that ‘device deficiencies’ incidents (confidential information has been removed). 46 Additional information related to the use of the automated devices is reported in Appendix 5, Table 31.
Chapter 4 Assessment of cost-effectiveness
This report contains reference to confidential information provided as part of the NICE Diagnostic Assessment process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Systematic review of existing cost-effectiveness evidence
Objective
The objective of the cost-effectiveness review was to identify, summarise and critically appraise existing economic evaluations of devices for automated assessment of ABPI for diagnosing PAD in people with leg ulcers.
Search strategies
A per-protocol search was carried out, first using Ovid MEDLINE® (see Appendix 1), which generated zero results for economic evaluations of the different approaches to measuring ABPI in people with leg ulcers. The search was broadened to include any economic evaluations of the candidate tests, and similarly no results were identified.
Inclusion and exclusion criteria
Inclusion and exclusion criteria with regard to population, intervention and comparators were as per those included and described in Systematic review methods. With regard to study type, we sought full economic evaluations, defined as comparative analyses of costs and outcomes in the framework of cost-utility, cost-effectiveness, cost-benefit or cost-minimisation analyses. Economic evaluations conducted alongside single effectiveness studies [e.g. randomised controlled trials (RCTs) or cohort studies], or decision analysis models were deemed eligible for inclusion.
Quality assessment of included studies
It was anticipated that included studies would be appraised against the NICE reference case for the assessment of the cost-effectiveness of diagnostic tests. 70
Evidence synthesis of cost-effectiveness studies
It was intended that detailed summary tables of study methods and results would be provided alongside a narrative assessment of cost-effectiveness results across studies.
Results
The per-protocol search identified no studies of the cost-effectiveness of the candidate tests for the assessment of PAD in people with leg ulcers. We therefore conducted further literature searches with the aim of informing the development of a de novo decision analysis model for the assessment.
Additional literature searches
Methods for additional literature searches
Two supplementary, sensitive literature searches using database index terms and free text were carried out by an Information Specialist to find additional peer-reviewed literature relevant to development of the model structure and/or population of the economic model. Search 1 focused on the cost-effectiveness of decision analysis models evaluating any method for the diagnosis/detection of PAD. Search 2 focused on identifying cost-effectiveness decision analysis models for either the diagnosis or treatment of leg ulcers. The resources included in both searches were MEDLINE, EMBASE, EconPapers, EconLit and the journal Value in Health. There were no restrictions on date or language of publication at the time of the search. The reference lists of studies selected for full-text appraisal were screened for additional studies. All references were exported to EndNote for recording and deduplication. A draft MEDLINE search is provided in Appendix 6.
Cost-effectiveness models for the diagnosis of peripheral arterial disease
The PAD diagnostic model search identified 239 possibly relevant titles and abstracts after deduplication. For the PAD search, a systematic review (Moloney et al. ) was identified which summarised the literature up until 2018. 71 Therefore, further detailed screening of the literature was undertaken for the period 2018–22 only, to identify additional studies not already captured in the Moloney et al. systematic review. 71 The search identified 80 potentially relevant titles and abstracts of papers published between 2018 and 2022, of which 6 full texts were retrieved and read and only 1 included as it was a decision analysis model for the diagnosis of PAD. The methods of the identified decision analysis model are summarised briefly in Table 9. The single identified study, Itoga et al. is of limited relevance to the current assessment as it relates to the use of ABI for general population screening, and the setting in the USA may not be directly transferrable to the UK setting in terms of model parameterisation. 72
| Study | EE type | Intervention, comparator | Population | Country, perspective | Currency, price year | Model type (cycle length, time horizon) | Model states | Outcome measures | Sensitivity analysis | Results | Relevance for this assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Itoga 201872 | CUA | ABI screening/no screening | 65-year-olds general population, asymptomatic of PAD | USA, healthcare system perspective | USD; 2016–7 | Markov cohort (1 month; 35 years) | Symptomatic PAD; Asymptomatic PAD ± meds; amputation; post amputation; stroke; MI; death | Cost, QALY, Cost per QALY | Varied starting PAD prevalence, medication costs, adherence to medications | Inc. Cost: $338; Inc QALY: 0.0038; ICER: $88,758 | Partial: Earlier stage than current scope, general population screening. Downstream health states, including amputation potentially relevant but parameters outside UK setting |
Cost-effectiveness models for the treatment and management of leg ulcers
The leg ulcers search identified 520 studies after removal of non-English language studies and duplicates. The search identified a recent systematic review (Layer et al. ) which summarised the leg ulcer modelling literature up until 2018. 73 Therefore, assessment of studies was undertaken only for the period between 2018 and 2022 to identify any studies not captured in that review which may be useful for the current assessment. This process included screening of 114 abstracts, of which 21 full texts were retrieved and read and 8 included as they were decision analysis models for the treatment or management of leg ulcer patients. These studies are summarised briefly in Table 10.
| Study | EE type | Intervention, comparator | Population | Country. Perspective | Currency, price year | Model type (cycle length, time horizon) | Model states | Outcome measures | Sensitivity analysis | Results | Relevant parameters for this assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cheng et al. 201874 | CUA | Electric stimulation therapy (Accel-Heal) plus dressings and compression bandaging, dressings and compression bandaging alone | Venous leg ulcers | Australia, payer | AUD ($), 2015 | Markov (2 weeks, 5 years) | No VLU, unhealed VLU, healed, complicated VLU, death | Total costs, total QALYs, expected cost of compression therapy | Univariate deterministic, probabilistic | Total cost intervention/comparator: $68,78,106/$37,875,018 Total QALY intervention/comparator: 504,431/476,090 Expected cost of compression over 5 years: $270,000,000 |
QALY source: Iglesias et al.75 Probability of healing – usual care (3 months): 0.228176 Probability of recurrence – usual care (annual): 0.557477 |
| Guest et al. 201878 | CUA | Collagen-containing dressings plus compression therapy followed by SoC, SoC | Venous leg ulcers | UK, NHS | GBP (£), 2015–6 | Decision tree (1 month, 6 months) | Healed or unhealed at 6 months | Total QALY, total cost, probability of healing | Univariate deterministic, probabilistic | Total QALY intervention/comparator: 0.373/0.331 Total management costs per patient intervention/comparator: £3789/£6328 Probability of healing – intervention/comparator: 0.49/0.11 |
Utility soured from Clegg and Guest79 All healing rate and cost parameters were sourced from Guest et al.80 |
| Health Quality Ontario 201981 | CUA | Compression stockings, usual care (no compression stockings) | Healed venous leg ulcers | Canada, payer | CAD ($), 2018 | Markov (1 month, 5 years) | Healed ulcer, recurred ulcer, infected ulcer, dead | Average total cost, average total effects (QALYs), ICER | Univariate deterministic, probabilistic | Average total cost intervention/comparator: $1518/$971 Average total QALY intervention/comparator: 4.060/4.040 ICER: £27,300 |
Utility parameters – recurred/healed: 0.77/0.87 Pham et al. 82 Probability of recurrence (monthly) – compression bandages: 0.16382 |
| Rognoni et al. 202083 | CUA | Stenting, standard medical treatment | Deep venous outflow obstruction and leg ulcers | Italy, payer | EUR (€), 2019 | Markov (1 month, 3 years) | Active ulcer, healed ulcer, recurred ulcer | ICER | Univariate deterministic, probabilistic | ICER: €2388 | Utility parameters – active/recurred/healed: 0.73/0.64/1.0079 Metanalysis of 8 studies from China, Poland, Canada, UK and USA for healing and recurrence estimates. Healing rate (3 months) = 0.62 (95% CI: 0.49 to 0.74) Ulcers recurred (1 year) = 0.10 (95% CI: 0.07 to 0.13) |
| Veličković et al. 202084 | CUA | Super absorbent wound dressing (Zetuvit plus silicone), standard of care dressing mix as defined by Atkin et al.85 | Moderate-to-highly exuding leg ulcers | UK, NHS | GBP (£), 2019 | Time invariant state-transition microsimulation model (1 week, 24 weeks) | HS1 (healed- skin intact) HS2 (unhealed grade 1 – progressing) HS3 (Unhealed grade 1: static) HS4 (Unhealed grade 1 – deteriorating) HS5 (Unhealed grade 2 – severe) |
Total costs, total QALWs, ICER, NMB | Univariate deterministic, probabilistic | Total costs – intervention/comparator: £2887/£3109 Total QALWs – intervention/comparator: 15.933/15.852 ICER: Intervention dominant NMB: £1841 |
Utility soured from Clegg and Guest79 Costs sourced from Harding et al.86 |
| Ontario Health (Quality) 202187 | CUA, CEA | Skin substitute dressings as an adjunct to standard care, standard care alone | Uninfected, difficult-to-heal neuropathic diabetic foot ulcers or uninfected difficult-to-heal venous leg ulcers | Canada, payer | CAD ($), 2020 | Markov (1 week, 26 weeks) | Healed, Unhealed, Dead | Mean cost, mean QALY, mean ulcer-free weeks, ICER, cost per ulcer-free week |
Univariate deterministic, probabilistic | Mean cost – intervention/comparator: $19,415/$7148 Mean QALYs – intervention/comparator: 0.330/0.324 Mean ulcer-free weeks – intervention/comparator: 10.12/6.33 ICER: $1,868,850 Cost per ulcer-free week: $3235 |
Utility soured from Clegg and Guest79 |
| Guest et al. 202188 | CUA | Thigh annuitised IPC in addition to standard care, standard care | Hard-to-heal venous leg ulcers | UK, NHS | GBP (£), 2019–20 | Markov (1 week, 24 weeks) | Uninfected ulcer, infected ulcer, improved ulcer, healed ulcer | Total costs, total QALY, probability of healing, ICER | Univariate deterministic, probabilistic | Total cost (if IPC stopped after 6 weeks) – intervention/comparator: £3020/£3037 Total QALY – intervention/comparator: 0.34/0.32 Probability healing – intervention/comparator: 0.38/0.24 ICER: Intervention dominant |
Utility soured from Clegg and Guest79 Costs sourced from Guest et al.89 and Guest et al.80 |
| Veličković et al. 202290 | CUA | Super absorbent wound dressing, standard of care dressing mix as defined by Atkin et al.85 | Moderate-to-highly exuding leg ulcers | Germany, payer | EUR (€), 2020 | Microsimulation state-transition model (1 week, 6 months) | HS1 (healed- skin intact) HS2 (unhealed grade 1 – progressing) HS3 (unhealed grade 1: static) HS4 (unhealed grade 1 – deteriorating) HS5 (unhealed grade 2 – severe) |
Total cost, total QALW, healing rate. | Univariate deterministic, probabilistic | Total cost – intervention/comparator: €4528/€5299 Total QALW – intervention/comparator: 17.229/17.077 Healing rate – intervention/comparator: 34.27%/31.70% |
Independent assessment of cost-effectiveness
The systematic review did not identify any cost-effectiveness studies involving the candidate tests for this assessment. The external assessment group (EAG) has therefore developed a de novo decision analysis model to assess the cost-effectiveness, measured as incremental cost per quality-adjusted life-year (QALY) gained, of automated ABPI devices, compared to standard care (manual Doppler testing) to assess PAD among adults with leg ulcers.
Modelled population
The population for the model base-case analysis is adults with leg ulcers presenting to healthcare professionals in the community setting, in whom ABPI testing is required to identify or rule out PAD. In community setting, the base-case analysis refers to a leg ulcer clinic setting, where sufficient skills to assess the patient’s condition are available. It does not refer to screening in a GP/nurse setting. Scenario analyses explore the potential for different costs and time to ulcer healing parameters in other settings, for example, where the skills to complete manual Doppler testing are not accessible in the community. We model a cohort of adults, average age 70, 30.46% female, in accordance with the data reported by Callam et al. 6 [quoted in SIGN and NICE clinical knowledge summary (CKS) guidelines]. 29,91 We use data from Callam et al. because, to our knowledge, it is the largest study that reports prevalence of arterial insufficiency, alongside the age and sex profile of leg ulcer patients in UK clinical practice. Despite the study being over 30 years old, the EAG’s clinical expert confirms that the demographics of the modelled cohort are consistent with those that would be seen in current UK clinical practice.
Interventions and comparators
The model compares the cost-effectiveness of seven automated ABPI measurement devices, compared to standard care (manual Doppler testing). Full details and characteristics of each test are provided in Chapter 3. Briefly, the tests under consideration for this assessment are:
-
BlueDop Vascular Expert (BlueDop Medical)
-
Boso ABI-system 100 (Bosch and Sohn)
-
watch BP Office ABI (Microlife)
-
watch BP Office Vascular (Microlife)
-
MESI ABPI MD (Mesi)
-
MESI mTABLET ABI (Mesi)
-
Dopplex Ability Automatic ABI system (Huntleigh).
Where a company has more than one test included in the scope for this assessment (e.g. Mesi), we include both in the model as separate strategies. Where parameters for one of a company’s tests are missing (e.g. where no diagnostic accuracy data are available), it is assumed that the model parameters for the other test can be imputed directly. The assumption was deemed reasonable following discussion with the EAG’s clinical expert and is consistent with submissions from the relevant companies (Microlife and Mesi).
Modelling methods
A two-stage model (decision tree followed by Markov cohort state transition) was developed to evaluate the cost–utility of the candidate tests. The model was developed using Treeage Pro 2021. 92 Model development, parameterisation and reporting were conducted in accordance with the NICE reference case for diagnostic test evaluations. 70
As outlined in the assessment of clinical effectiveness (see Chapter 3), there is no direct evidence to inform the consequences of the tests for clinical or patient outcomes (e.g. ulcer healing rates/time). The model therefore uses a linked-evidence approach to quantify a range of potential consequences of test accuracy for ulcer healing times, risk of requiring invasive pad treatment and subsequent outcomes that might be observed in UK clinical practice. The implications for the treatment pathway of inaccurate test results were highly uncertain and based on clinical expert opinion. Clinical experts indicated that the implications of FN test results for the treatment pathway were increased risks of more invasive treatments for arterial disease (angioplasty, bypass, amputation), whereas for FP test results, the implication of delayed compression was delayed ulcer healing time. This guidance informed the model structure. Full details of the expert opinions used in the model are described in subsequent sections.
The model structure was informed by an assessment of existing leg ulcer economic evaluation models (see Table 10) and was developed to be consistent with the recommendations of national guidance on the management of leg ulcers (NCWSP), NICE guidance on PAD – CG147 and SIGN guidance (SIGN 2010)13,23,29 NICE specialist committee members (SCMs) provided feedback on how inaccurate test results (FP or FN) would be identified in clinical practice and the associated consequences for patient outcomes. The final model structure was adapted following SCM feedback and EAG clinical expert advice.
Model structure and assumptions
Decision tree phase
The initial decision tree (diagnostic) phase of the model implements the linked-evidence approach to capture the costs and consequences (advantages and disadvantages in terms of clinical and patient outcomes) of the diagnostic accuracy (sensitivity and specificity) of automated ABPI measurement compared to manual Doppler testing.
The initial time horizon for the decision tree phase of the model was chosen as 24 weeks post initial presentation to healthcare services with a leg ulcer. Twenty-four weeks was chosen to be consistent with the primary outcome of existing leg ulcer RCTs in this field [e.g. Early venous reflux ablation (EVRA) trial93]. Discussion with several clinical experts confirmed that 24 weeks would be sufficient to identify FP and FN testing errors in clinical practice and assign the patient to correct treatment pathways, which is consistent with guidelines for treating arterial disease and management of arterial ulcers. 13,23,29 It would also be sufficient to capture urgent PAD referrals from the community and to initiate appropriate surgical management in secondary care if appropriate. It is therefore assumed that after 24 weeks, all patients would be allocated to the correct diagnosis, and the surviving cohort would then enter the appropriate Markov model to assess long-term costs and outcomes.
The cohort are initially assigned to the venous or arterial disease pathway, according to the underlying PAD prevalence (i.e. ABPI < 0.9) among leg ulcer patients. The PAD proportion of the cohort is then further split into the proportion with purely arterial, and the proportion with mixed (arterial/venous) aetiology. Splitting the cohort into purely arterial and mixed aetiology allows for the application of different parameters and treatment pathways in the model, based on the severity of the underlying arterial component of the disease (defined using Fontaine stage). Costs and outcomes (QALYs) are then accrued depending on the sensitivity and specificity of the test result, depending on whether the underlying disease is arterial (including mixed) or venous.
Figure 8 illustrates the decision tree phase of the model, up to the point where the surviving cohort are allocated to appropriate starting states in the Markov model.
FIGURE 8.
Diagnostic phase, simplified decision tree model pathway. Simplified decision tree structure: Blue, salmon and grey highlighted boxes reflect the venous, arterial and mixed pathways, respectively. Angio, angioplasty; F2, F3, F4, Fontaine stages 2, 3 and 4, respectively.
Arterial ulcers: For the proportion of the cohort where the automated test accurately reports an ABPI output indicative of PAD (i.e. a TP test – sensitivity), the cohort enter the arterial disease pathway, where they are referred to vascular services for further assessment and treatment of the arterial ulcer in accordance with NWSCP recommendations. 13 Using PAD classification (Fontaine system), ulcerated PAD patients are classed as Fontaine stage 4 (F4), because of the presence of the ulcer in a patient with arterial disease. All F4 patients with purely arterial disease are therefore assumed to have critical limb ischaemia (CLI) meaning that arterial ulcers will not heal with conservative or medical management alone and thus require surgical treatment to restore blood flow (e.g. angioplasty or surgical bypass) to enable successful healing. A proportion may require primary amputation, but this would generally be avoided where possible. The cohort receive their first arterial treatment within the decision tree phase of the model, because a positive ABI test in the presence of other symptoms will trigger an urgent referral to vascular services. Based on the EAG’s clinical expert opinion, it is assumed that in UK clinical practice, urgent referrals will receive treatment within approximately 6 weeks. Patients receiving intervention for CLI are assumed to be at an increased risk of mortality, dependent on the treatment received. The surviving cohort are then allocated to the appropriate Markov model health state depending on whether their procedure was successful (enter the healed post-CLI state, assumed similar in terms of costs and outcomes to intermittent claudication) or unsuccessful (enter the CLI state where repeat treatments are provided, and an increased risk of amputation and mortality). Success of the initial treatment is defined as no further procedure required within the hospital admission.
It is assumed that purely arterial ulcers (F4) will have multiple signs of arterial disease and that, in the context of a FN automated test result, a comprehensive and holistic assessment of the patient’s condition would identify the FN result promptly and inappropriate compression would not be applied. This is captured in the decision tree through the parameter ‘act on test result’. The base-case analysis therefore assumes that a FN test result in an arterial-only patient would not be acted upon in clinical practice and that a FN test alone would be unlikely to lead to long-term negative patient consequence, regardless of the setting in which the patent® was seen (community or secondary care).
Mixed aetiology ulcers
For the proportion of the cohort with mixed aetiology ulcers, impact on patient outcomes is likely to depend on the severity of the underlying arterial disease. Unlike purely arterial disease, a patient with mixed ulceration could have an ulcer primarily caused by venous disease. The mixed ulcer proportion of the cohort is therefore assumed to include Fontaine stages 2, 3 and 4. Patients with mixed aetiology disease would not be classified as Fontaine stage 1 (asymptomatic) because F1 patients would typically have a higher ABPI > 0.9. Table 11 summarises Fontaine stages, descriptions and an approximate map of Rutherford stages (which is used for some model parameters). 94 It should be noted that while the Fontaine system may not be adopted universally in UK clinical practice for patient management, it is a useful approach to categorise the underlying severity of arterial disease that can be used to explore disease severity-specific implications of FN results.
| Fontaine stage | Description | Rutherford approximation |
|---|---|---|
| Stage I | Asymptomatic | Stage 0 |
| Stage II (IIA and IIB) | Intermittent claudication (IIA pain after > 200 m walking; IIB: pain after < 200 m walking) | Stages 1 and 2 |
| Stage III | Rest pain/severe claudication | Stages 3 and 4 |
| Stage IV | Ischaemic ulceration/gangrene | Stages 5 and 6 |
While a mixed ulcer may heal within 24 weeks using conservative treatment (e.g. modified compression), under close monitoring to ensure no adverse events, in a manner similar to those for purely venous disease, this is unlikely to represent the management of mixed ulcers in UK clinical practice. The EAG clinical expert view is that, in UK practice, the arterial component of the ulcer takes priority for clinical management because the health gain forgone associated with non-treatment or delayed treatment of arterial disease is substantially greater than for venous disease. Therefore, in most cases, strong compression would not be applied to a PAD patient (ABPI < 0.9), regardless of the Fontaine staging, even for moderate F2 disease. The EAG’s clinical expert notes that it is more difficult to detect arterial disease among patients with mixed aetiology ulcers, especially for those with less severe arterial disease, as the underlying arterial disease may be less symptomatic, and the ulcer may present primarily with its venous component. Therefore, FN test mistakes may be more likely to be missed in clinical practice for mixed aetiology compared to solely arterial disease.
We conducted a survey with NICE SCMs to better understand the implications of FN test results that are acted upon (i.e. where an ABPI test indicates that an ulcer is venous, when it has an arterial component, and inappropriate compression is applied). Five clinical experts responded. The majority felt that a FN test, leading to inappropriate compression of an arterial ulcer, could feasibly lead to delayed ulcer healing, increased risks of requiring invasive treatment (angioplasty or bypass) and potentially an increased risk of ultimately requiring limb amputation. These outcomes and risks are all explicitly included within the model structure.
It was generally felt however that a FN would not directly lead to MI, stroke or intracranial haemorrhage (ICH), hence these PAD outcomes have not been explicitly included in the model. One respondent felt that there would be an increased mortality risk. The additional risk of mortality due to a FN test result is captured indirectly in the model through increasing mortality following more invasive surgery due to the FN test result. Figure 9 summarises the implications of FN test results that are acted upon (i.e. strong compression inappropriately applied to an ulcer with arterial disease) as stated by the NICE SCMs.
FIGURE 9.
Implications of FN test results.
Two clinical experts (one NICE SCM and the EAG’s expert) commented that the extent to which these additional risks may be realised in clinical practice depends on the severity of the underlying arterial disease and the extent to which a full and complete holistic assessment of the patient’s condition was undertaken to identify a test result error (i.e. whether the test result is acted upon). If a FN is identified at the initial comprehensive patient assessment, implications would be minimal because the FN would not be acted upon.
For the base-case analysis, we therefore assume that the implications of acting on a FN test result are Fontaine stage dependent. Based on the EAG’s clinical expert advice, FN test results that are acted upon are modelled to affect patient outcomes through the need for more invasive treatments on a spectrum of treatment options for PAD ranging from mild (medical management), moderate (mix of angioplasty and bypass) to highly invasive (bypass and amputation) for patients in whom a mixed aetiology ulcer is inappropriately treated with strong compression. The increased risks applied in the model base-case analysis are detailed in the ‘parameters’ section of the report. Whether the additional risks of treatment escalation are realised is dependent on whether the test is acted up on in the first place. The base-case analysis assumes that all FN tests in mixed ulcer patients would be acted upon, but uncertainty surrounding this is explored in scenario analyses. FN, mixed aetiology ulcers may also be subject to consequences of delayed healing time due to a lack of clinical certainty around wound treatment, though the exact delay is unclear, with substantial variability across UK clinical practice.
Venous ulcers: Where a test is highly sensitive and accurately identifies absence of arterial disease (i.e. a TN test result), the cohort enter the venous pathway, with the ulcer treated using strong compression and follow-up patient management in accordance with the NWCSP and SIGN guidance for venous ulcers. 13,23,29
With regard to FP (1 – specificity) automated ABPI test results, clinical experts (N = 4 NICE SCMs) explained that a FP test result (i.e. an ABI that indicates arterial disease, compression withheld) would be identified in UK clinical practice primarily due to a failure of the ulcer to progress towards healing. Re-examination/review might identify a lack of history of claudication, previous venous disease or varicose veins or previous venous ulcers healed with compression. One expert noted that again, an effective holistic assessment should identify the absence of PAD, for example, due to identifying the signs associated with chronic venous disease, and they note that the absence of PAD on Doppler/ABPI alone does not lead to a diagnosis of venous disease. There was broad agreement that the main consequence of a FP test result that was acted upon would be delayed ulcer healing time for the venous, due to unnecessarily withholding compression. All SCMs agreed that a FP would not lead to amputation of a venous ulcer as amputation in modern clinical practice is extremely rare.
Expected costs and benefits (utilities) are accrued within the decision tree phase of the model, dependent on the average duration of time that the cohort spend with healed/unhealed ulcers over the first 24 weeks. This allows the model to flexibly incorporate time advantages due to earlier testing in clinical practice if feasible, as well as time delays to ulcer healing due to compression treatment decisions driven by a test result. The base-case analysis assumes that a FP test result would be acted upon, and initiation of appropriate compression therapy would be delayed until a definitive diagnosis was reached, thereby increasing ulcer healing times for the venous ulcer.
Markov model pathways – arterial
The surviving cohort at the end of the decision tree phase of the model (24 weeks) enter the Markov model in either the ‘healed post-CLI’ state (if initial surgery from the decision tree phase was successful), the ‘CLI’ state (if initial surgery was unsuccessful and further treatment required) or the ‘amputation’ state (if primary amputation was required or if bypass surgery was not successful). The arterial Markov model is described in Figure 10.
FIGURE 10.
Markov model structure (arterial ulcers).
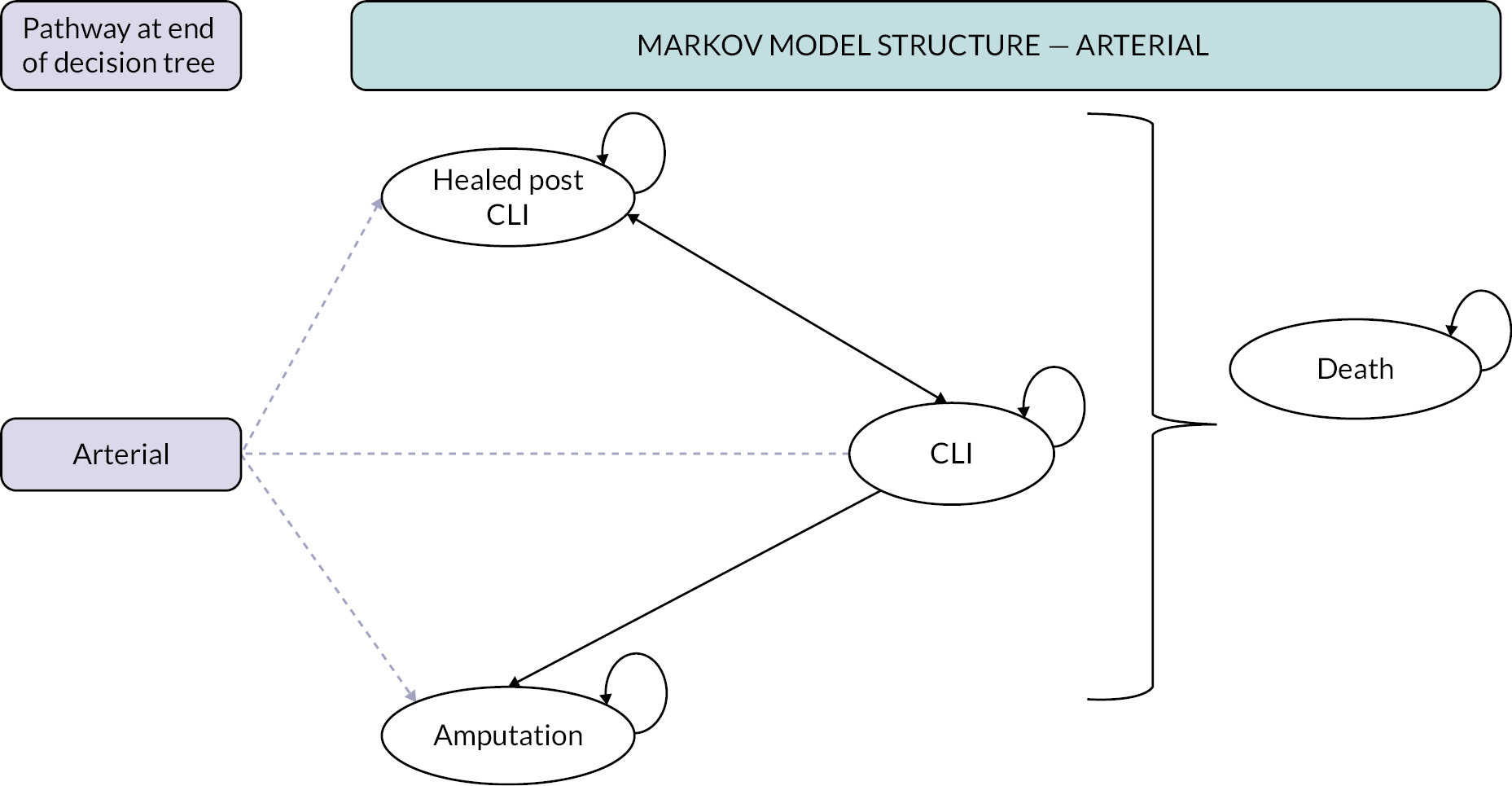
For the proportion entering the CLI state (those whose initial surgery was unsuccessful), the cohort receive either a repeat angioplasty or bypass procedure for limb salvage or may require amputation. Those who have successful treatment (obtained from National Vascular Registry data, defined as no further procedure required) enter a ‘healed post-CLI’ state, where they are exposed to a risk of recurrence, after which it is assumed that they re-enter the CLI state and start the treatment cycle again. Any further recurrences are thus assumed to be CLI. Patients whose initial treatment was unsuccessful at restoring blood flow within a single model cycle (6 months) recycle through the CLI state and receive subsequent treatments, increasing in intensity up to bypass and amputation. CLI patients with unhealing ulcers are subject to a risk of amputation that increases following each subsequent round of surgical treatment (e.g. those with a failed bypass are all assumed to enter the amputation state).
The cohort can enter the ‘death’ state from all other model health states, with an excess risk of mortality applied for underlying arterial disease in all model states. Further additional risks of mortality are applied in the CLI state reflecting the excess mortality risk compared to less severe stages of arterial disease.
Model pathways – mixed aetiology
The mixed pathway is assumed to be very similar to that of the arterial pathway, given that in clinical management, arterial disease is the primary focus of treatment, even when ulcers are of mixed aetiology. The model pathway for mixed ulceration is described in Figure 11.
FIGURE 11.
Markov model structure (mixed venous/arterial ulcers).

Prior to entering the Markov model, the mixed arterial ulcer proportion of the cohort is split according to severity of the arterial component of disease (Fontaine stage 2, 3 or 4). This staging and the success of initial treatment processes is used to assign the cohort to the initial ‘healed’ state (assumed equivalent in terms of costs and consequences to the ‘healed post-CLI’ state). This simplifying assumption was made due to a lack of alternative evidence to assign separate costs and utilities to ‘healed’ and ‘healed post-CLI’ states for patients with mixed ulceration.
The proportion of the mixed cohort entering each Markov state depends on the Fontaine staging of the arterial component of disease, distribution of initial treatments (medical management for F2/angioplasty or bypass for F3 and F4) and the success of those initial procedures. Those with successful treatment enter in the ‘healed’ state, failed treatment in the ‘CLI’ state and those who have a primary amputation or amputation following failed bypass surgery enter the ‘amputation’ state. It is assumed that primary amputation would be very rare for mixed ulcer patients and would only ever occur for F4 disease.
Markov model pathways – venous
The proportion of the cohort with venous ulcers enters the pathway in the healed or unhealed ulcer states, depending on the surviving proportion of the cohort with healed/unhealed ulcers, respectively, at the end of the decision tree phase of the model (24 weeks). Figure 12 illustrates the model pathway for venous ulcers.
FIGURE 12.
Markov model structure (venous ulcers).
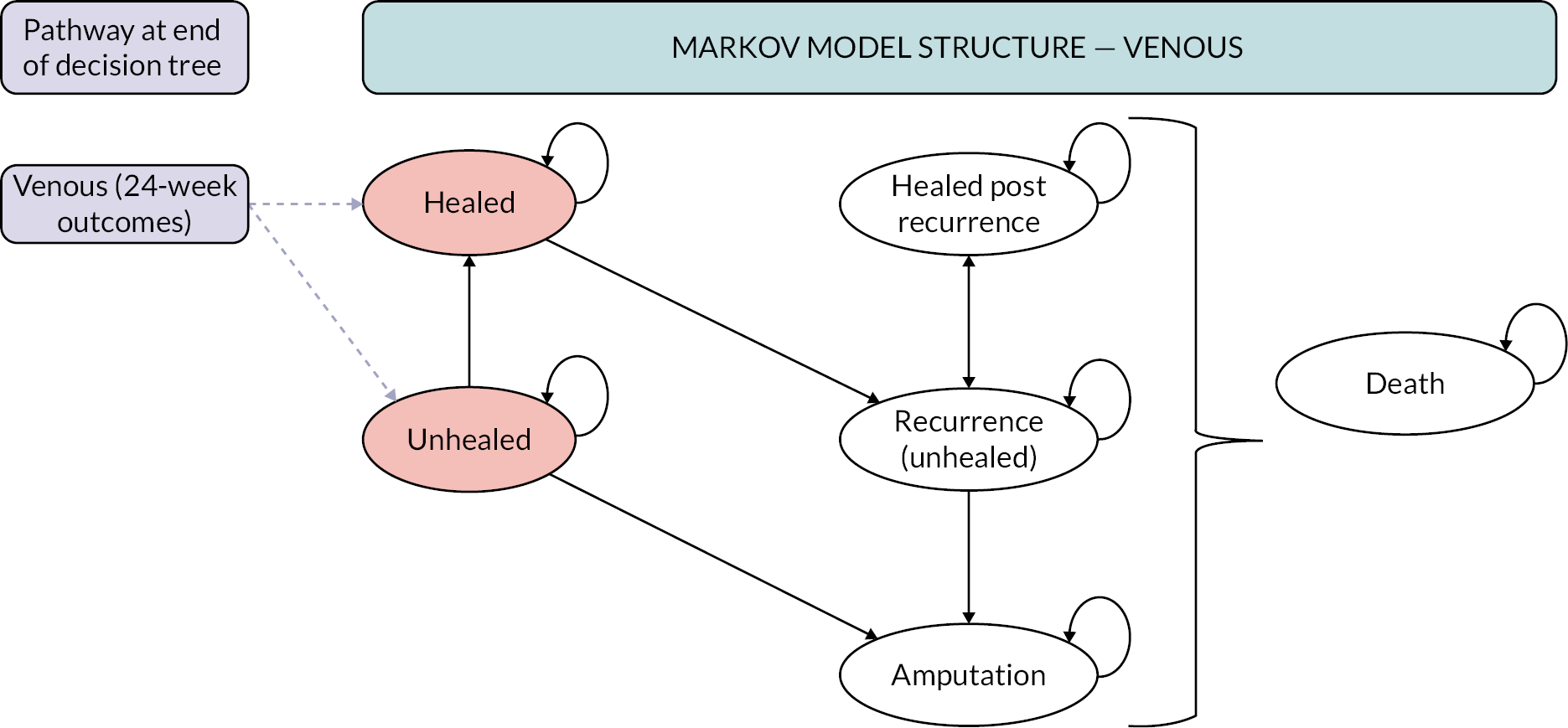
Unhealed ulcers can continue to heal in subsequent model cycles, and a small proportion may remain unhealed longer term. Once an ulcer heals, it can remain healed or experience a recurrence (assumed equivalent in terms of costs and utilities to the ‘unhealed’ state). Multiple rounds of healing and recurrence are allowed within the model accounting for the chronic recurrent nature of venous ulcers. The model structure also allows for a risk of amputation for venous ulcers, but this parameter is set to zero in the base case, reflecting clinical expert advice sought by the EAG that in modern clinical practice, amputation is extremely rare in a patient with purely venous disease. The cohort are exposed to a risk of mortality in all model cycles, assumed equal to that of the UK age- and sex-adjusted general population mortality risks. Mortality is not dependent on whether a venous ulcer heals or not.
Model parameters – prevalence and diagnostic accuracy
Prevalence parameters
To maintain consistency with the modelled cohort age and sex profile, the underlying prevalence of PAD (including purely arterial and mixed aetiology disease) of 22% was obtained from Callam et al. ,6 quoted in both SIGN guidance and NICE CKS. 29,91 The study was based on an assessment of 600 participants with leg ulcers in Scotland. It is assumed that each patient has one leg ulcer only. For the purposes of our model, the prevalence of arterial disease encompasses both patients with solely arterial involvement and mixed aetiology (i.e. predominantly venous ulcers, but with some evidence of arterial insufficiency, such as an ABPI < 0.9). This prevalence estimate was used in preference to an average prevalence from the diagnostic accuracy studies included in the review, because none of those studies were conducted specifically in patients with leg ulceration. Prevalence from the diagnostic accuracy studies is considered in a scenario analysis. Our prevalence estimate is also consistent with Guest et al. who conducted an analysis of the THIN database of over 2.4 million UK adult patients in 2017–8 (mean age 57.9; proportion female: 56%), of which 174,569 had ulcers identified within that year. 21 Of the 174,569 ulcers identified, 15% (scaled 79%; N = 26,185) were venous, 3% mixed venous/arterial (scaled: 16%; N = 5237) and 1% (scaled 5%; N = 1746) arterial only. 21 The THIN database was thus used to parameterise the proportion of arterial ulcers that were of mixed aetiology, as 5237/6983 (75%). Prevalence parameters are entered in the model probabilistically using beta distributions as described in Table 12.
Diagnostic accuracy parameters (sensitivity and specificity)
Diagnostic accuracy (i.e. sensitivity and specificity) parameters for automated compared to manual Doppler tests were obtained from the diagnostic accuracy review (see Results of the assessment of clinical effectiveness). Data were incorporated into the decision tree phase of the model to determine the proportion of PAD patients with a TP (sensitivity) or FN (1 – sensitivity) test result, and the proportion of venous patients with a TN (specificity) or FP (1 – specificity) result.
The base-case analysis considers manual Doppler to be the reference standard in primary care and, therefore, implicitly assumes perfect diagnostic accuracy (sensitivity and specificity equal to 1) for the base-case analysis. The EAG acknowledges that the manual Doppler test is an imperfect reference standard and that better existing techniques such as angiography, CTA or magnetic resonance imaging angiography would be required to generate a definitive diagnosis of PAD. However, it was not possible to adjust diagnostic accuracy estimates for the automated tests for several reasons. First, none of the studies included in this assessment compared automated versus manual readings against an acceptable reference standard that would enable direct estimation of diagnostic accuracy parameters. Second, the evidence base comparing manual Doppler with an acceptable reference standard was sparse. A recent systematic review95 identified only one such study that compared manual Doppler testing with CTA,96 and any comparisons about accuracy against automated tests would be purely naïve and across heterogeneous studies. Finally, none of the included studies provided any further investigation or arbitration of disagreements between manual and automated devices, meaning that the correlation in testing errors is unknown. Any analyses that attempt to correct the estimates without knowledge of the correlation in testing errors may introduce further bias of unknown direction and magnitude.
It should also be noted that all but three of the included studies in the clinical effectiveness systematic review treated manual Doppler as the reference standard, with the remaining studies using Duplex ultrasound as the reference device. Where available, we have used data for a comparison against manual Doppler in our base-case analyses. There were no comparisons of diagnostic accuracy of the BlueDop Vascular Expert device versus manual Doppler. To include the BlueDop Vascular Expert device within the economic model, it was therefore required to assume that the diagnostic accuracies of manual Doppler testing and Duplex ultrasound were equivalent. This assumption clearly adds substantial further uncertainty to the cost-effectiveness estimates but was a requirement given a lack of information/studies comparing Duplex ultrasound and manual Doppler in the literature.
As noted in the section Assessment of clinical effectiveness, study populations were highly heterogeneous, raising serious concerns about the validity of pooling diagnostic accuracy studies. Our base-case analysis therefore uses single studies for each test, with the most appropriate study selected based on similarity of population to the scope (more similar preferred), country (UK preferred) and sample size (larger studies preferred). The approach was taken to apply data from a population as close as possible to that of the assessment scope but acknowledging that there remain no diagnostic accuracy studies that report sensitivity and specificity in a population with leg ulcers. While this base-case approach chooses the most appropriate evidence available, it does not account for the overall uncertainty in the evidence base. Therefore, we apply several scenario analyses which vary sensitivity and specificity parameters as follows:
-
Scenario 1: uses data from the study with the lowest diagnostic accuracy (average of sensitivity and specificity) for each test.
-
Scenario 2: uses data from the study with the highest diagnostic accuracy (average of sensitivity and specificity) for each test.
-
Scenario 3: uses data pooled across all available studies for each test, where there are at least four studies to enable a meta-analysis to be conducted. This was applicable only for MESI ABPI MD and WatchBP Office ABI. For this scenario, it was not possible to obtain pooled estimates for BlueDop, BOSO ABI-System 100 and Dopplex Ability. While this approach has the limitation of pooling data where there is substantial heterogeneity, it has the advantage of capturing the uncertainty in the evidence base across all available studies for MESI ABPI MD and WatchBP Office ABI.
-
Scenario 4: several studies reported an optimal cut-off value for automated test results to generate the best diagnostic accuracy compared to manual Doppler testing. This scenario applies the sensitivity and specificity parameters derived from studies that reported optimal cut-offs. None of the studies assessing the BlueDop Vascular Expert device reported optimal cut-off thresholds.
-
Scenario 5: is a subgroup analysis, applying diagnostic accuracy data from studies that provided information for diabetes patients separately either because a subgroup analysis was available or where the study was conducted in a diabetic population.
For scenarios 1 and 2, where we vary the source of a parameter between maximum and minimum available values across studies, we assume the base-case value is retained where the min/max is equal to the base-case parameters. For scenarios 3–5, where the scenario-specific diagnostic accuracy parameters are not available for a test, we exclude that test from the cost-effectiveness calculations. For example, pooled meta-analysis estimates are only available for MESI ABPI MD and WatchBP Office ABI devices; therefore, the other automated tests are excluded from this scenario.
Sensitivity and specificity parameters are incorporated in the model using multinormal distributions, with correlations between sensitivity and specificity obtained from the meta-analysis for MESI ABPI MD and WatchBP Office ABI studies. For the remaining studies, the correlation was assumed to be equal to the average correlation across all studies where this information could be calculated. The resultant correlations for MESI ABPI MD and WatchBP Office ABI were −1.00 and −0.99, respectively, whereas the correlation across all available studies (including available Dopplex studies) was −0.84. The value of −0.84 was therefore applied to obtain correlated draws of sensitivity and specificity for all studies assessing the BlueDop Vascular Expert and BOSO ABI-System 100 devices, where this information could not be derived. Table 13 summarises the diagnostic accuracy parameters used in the model for the base case and each of the described scenario analyses.
| Sensitivity | Specificity | Corr.a | Notes/source | ||||
|---|---|---|---|---|---|---|---|
| Scenariob | Mean | SEc | Mean | SEc | |||
| Manual | Base case | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | Assumes perfect reference standard |
| BlueDop | Base case | 0.95 | 0.155 | 0.9 | 0.021 | −0.84 | Kordzadeh et al.57 |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | (confidential information has been removed)46 | |
| High value | 0.95 | 0.155 | 0.9 | 0.021 | −0.84 | dKordzadeh et al.57 | |
| Pooled estimate | – | – | – | – | – | Insufficient number of studies to conduct meta-analysis | |
| Optimal cut-off | – | – | – | – | – | No studies reporting optimal threshold | |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | (confidential information has been removed)46 | |
| Boso | Base case | 0.77 | 0.125 | 0.94 | 0.022 | −0.84 | Jarai et al.59 |
| Low value | 0.61 | 0.099 | 0.94 | 0.022 | −0.84 | Homza et al.58 | |
| High value | 0.77 | 0.102 | 0.98 | 0.005 | −0.84 | Wohlfahrt et al.66 | |
| Pooled estimate | – | – | – | – | – | Insufficient number of studies to conduct meta-analysis | |
| Optimal cut-off | 0.84 | 0.137 | 0.75 | 0.017 | −0.84 | eHomza et al. – optimal threshold = 1.058 | |
| Diabetes subgroup | 0.61 | 0.099 | 0.94 | 0.022 | −0.84 | Homza et al.58 | |
| Dopplex | Base case | 0.7 | 0.073 | 0.96 | 0.008 | −0.84 | Davies et al.48 |
| Low value | 0.59 | 0.093 | 0.86 | 0.056 | −0.84 | fMillen et al.60 | |
| High value | 0.79 | 0.049 | 0.91 | 0.026 | −0.84 | Lewis et al.49 | |
| Pooled estimate | – | – | – | – | – | Insufficient number of studies to conduct meta-analysis | |
| Optimal cut-off | 0.98 | 0.022 | 0.75 | 0.017 | −0.84 | Davies et al. (optimal = 1.04)48 | |
| Diabetes subgroup | 0.2 | 0.033 | 0.96 | 0.022 | −0.84 | Babaei et al.54 | |
| MESI g | Base case | 0.74 | 0.06 | 0.97 | 0.018 | −1.00 | Hageman et al.61 |
| Low value | 0.75 | 0.048 | 0.67 | 0.055 | −1.00 | Zebari et al.50 | |
| High value | 0.74 | 0.06 | 0.97 | 0.018 | −1.00 | dHageman et al.61 | |
| Pooled estimate | 0.67 | 0.038 | 0.94 | 0.038 | −1.00 | Pooled across N = 5 studies | |
| Optimal cut-off | 0.91 | 0.039 | 0.9 | 0.031 | −1.00 | Hageman et al. (optimal = 1.02, non-diabetic)61 | |
| Diabetes subgroup | 0.68 | 0.063 | 0.95 | 0.082 | −1.00 | Hageman et al.61 | |
| WatchBP h | Base case | 0.83 | 0.094 | 0.97 | 0.019 | −0.99 | Kollias et al.56 |
| Low value | 0.44 | 0.138 | 0.98 | 0.01 | −0.99 | Raya et al.64 | |
| High value | 0.83 | 0.094 | 0.97 | 0.019 | −0.99 | dKollias et al.56 | |
| Pooled estimate | 0.53 | 0.082 | 0.98 | 0.008 | −0.99 | Pooled across N = 4 studies | |
| Optimal cut-off | 0.92 | 0.068 | 0.92 | 0.031 | −0.99 | Kollias et al. (optimal = 0.97)56 | |
| Diabetes subgroup | 0.83 | 0.135 | 0.97 | 0.022 | −0.99 | Kollias et al.56 | |
Model parameters – decision tree treatments and outcomes for arterial (and mixed aetiology) disease
Characterisation of disease
Due to the presence of an ulcer, the proportion of the cohort with solely arterial disease is classified as Fontaine stage 4. The proportion of mixed ulcer patients across the disease stages is uncertain, and there is limited published evidence on the disease categorisation for mixed ulceration. We identified one study which is a retrospective analysis of 180 patients with suspected or known PAD and chronic venous insufficiency, median age 69 at a single centre in Germany between 2012 and 2018. 97 Assessment of clinical notes was used to determine Fontaine stage, and the distribution is used to categorise disease stage for mixed ulceration in the model. We assume that there are no Fontaine stage I cases among prevalent arterial disease due to this proportion of the cohort having an ABPI reading < 0.9.
Treatment for peripheral artery disease
The economic model assumes that most PAD patients will first be treated with limb salvage. This reflects clinical practice where clinical teams would always attempt to save the limb first and attempt to avoid primary amputation where possible. Clinical expert opinion sought from the EAG’s expert and one NICE SCM indicates that primary amputation is rare and would only ever occur in patients with Fontaine stage 4 arterial disease. Approximately 90–95% of limbs will be amenable to some form of revascularisation/surgical bypass, and this would be the preferred option in clinical practice. The base-case model includes the costs and outcomes of angioplasty and/or bypass for treating F3 and F4 disease and the costs of medical management for F2 disease (intermittent claudication). Information on initial treatment probabilities is sourced from the National Vascular Registry data where possible and includes both elective and non-elective procedures to calculate the proportion of treatments in each Fontaine stage that are endovascular (angioplasty), with the remainder assumed to be bypassed. While the data show that angioplasty may also be conducted for F2 disease, the EAG’s clinical expert opinion was that, for a mixed ulcer, this would usually be managed with medical management initially. Therefore, the decision tree proportion of the model assumes that all stage 2 mixed ulcer patients are initially managed medically.
For those in whom limb salvage is not possible, primary amputation may be indicated. This may occur, for example, where there is a non-functional lower extremity, arteries that cannot be reconstructed, where there is massive tissue loss and bone exposure, meaning bypass is not possible, and where there are significant comorbidities. The base-case analysis applies a conservative estimate of the proportion of F4 arterial disease patients requiring primary amputation of 5%. Clinical experts explain that primary amputation in people with mixed ulceration is less likely, and the probability is therefore assumed to be 0%. It is assumed that F2 and F3 disease would never proceed directly to primary amputation.
The probability of treatment success for endovascular treatment and bypass is also obtained from the National Vascular Registry Annual Report and is defined as no further unplanned lower limb procedure prior to hospital discharge. The probability of treatment success is not available specifically for each Fontaine stage of disease. However, information is available according to whether the procedure is elective or non-elective, with a lower probability of success, a higher probability of amputation and death following non-elective procedures compared to elective. Because non-elective procedures are more commonly required for more extensive arterial disease (higher Fontaine stage), the model indirectly calculates the probability of success in each Fontaine stage by weighting the outcomes by the proportion of procedures in each stage that were elective/non-elective.
Detailed probability parameters applied in the decision tree phase of the model for arterial and mixed aetiology disease are summarised in Table 14. These include the disease characterisation (Fontaine staging), treatment options by Fontaine stage where data allow this to be derived and treatment success probabilities. Probability data are primarily sourced from the National Vascular Registry, 2021 Annual Report and supplemented with clinical expert opinion for primary amputation. 98 All probabilities are incorporated as beta distributions with alpha and beta parameters obtained from published event counts.
| Parameter | n/N (%) | Alpha | Beta | Dis. | Source/notes/assumptions |
|---|---|---|---|---|---|
| Disease classification | |||||
| Mixed – F2 | 77/152 (50.7%) | – | – | Remainder | Ammerman et al.97 |
| Mixed – F3 | 15/152 (9.9%) | 15 | 137 | Beta | |
| Mixed – F4 | 60/152 (39.5%) | 60 | 92 | Beta | |
| Proportion of stage-specific surgeries that are angioplasty vs. bypass (remainder) | |||||
| Stage F2 | 4204/6160 (68.3%) | 4204 | 1956 | Beta | National Vascular Registry Annual Report98 |
| Stage F3 | 2128/4926 (43.2%) | 2128 | 2798 | Beta | |
| Stage F4 | 6821/10,963 (62.2%) | 6821 | 4142 | Beta | |
| Elective vs. non-elective procedures (remainder non-elective) | |||||
| Proportion angio-elective (F2) | 4009/4204 (95.4%) | 4009 | 195 | Beta | Table A3.3, National Vascular Registry Annual Report98 |
| Proportion angio-elective (F3) | 1599/2128 (75.1%) | 1599 | 529 | Beta | |
| Proportion angio-elective (F4) | 3552/6821 (52.1%) | 3552 | 3269 | Beta | |
| Proportion bypass elective (F2) | 1860/1956 (95.1%) | 1860 | 96 | Beta | Table 5.4, National Vascular Registry Annual Report98 |
| Proportion bypass elective (F3) | 1939/2798 (69.3%) | 1939 | 859 | Beta | |
| Proportion bypass elective (F4) | 1714/4142 (41.4%) | 1714 | 2428 | Beta | |
| Treatment outcomes | |||||
| P success angioplasty (elective) | 4061/4221 (96.2%) | 4061 | 160 | Beta | Table 5.5A and 5.5B, National Vascular Registry Annual Report98 |
| P success angioplasty (emergency) | 1805/2169 (83.2%) | 1805 | 364 | Beta | |
| P success bypass (elective) | 2428/2642 (91.9%) | 2428 | 214 | Beta | |
| P success bypass (emergency) | 1980/2429 (81.5%) | 1980 | 449 | Beta | |
| P die angioplasty (elective) | 34/4221 (0.8%) | 34 | 4187 | Beta | |
| P die angioplasty (emergency) | 104/2169 (4.8%) | 104 | 2065 | Beta | |
| P die bypass (elective) | 42/2642 (1.6%) | 42 | 2600 | Beta | |
| P die bypass (emergency) | 119/2429 (4.9%) | 119 | 2310 | Beta | |
Implications of false-negative results for treatment of arterial and mixed aetiology disease
The model structure has been developed to incorporate the implications of FN test results through an increased requirement for treatment escalation to more invasive procedures to treat arterial ulcers. The base case assumes that those with purely arterial disease will not be acted upon because the test error will be identified through other patient symptoms. The additional risks of FN tests impacting on treatment escalation are only applied to mixed aetiology ulcers and are modelled to be dependent on disease severity (Fontaine stage).
The base-case modelled consequences for a mixed aetiology ulcer where a FN test result is acted upon are as follows:
-
Fontaine stage II, relatively mild disease, a patient with strong compression would experience extreme pain and would usually return within 1 week at which point the mistake would be identified. Most people at this stage would have no long-lasting negative consequences once the compression was removed and would continue to have their arterial disease managed medically, with monitoring and eventual reflux surgery for the venous disease.
-
Fontaine stage III patients are more likely to require escalation of treatment to more invasive procedures and would be unlikely to be managed medically. While highly uncertain, the EAG’s clinical expert’s best guess estimate is that approximately 70% (sampled probabilistically to account for uncertainty) of stage III patients (managed with angioplasty in the absence of a FN result) would require escalation to bypass, with the remaining having no longer-term implications and retaining the initial procedure distribution.
-
Fontaine stage IV: A FN test for a Fontaine stage IV patient would require escalation in all patients, directly to a bypass procedure. We assume an additional 5% would require a primary amputation [i.e. relative risk (RR) = 2, total 10%] if a purely arterial ulcer (F4) was inappropriately treated with strong compression (although proportion acted upon assumed 0 in base case, some scenarios vary this assumption). Inappropriate compression of a F4 mixed ulcer case could increase the risk of requiring primary amputation in a patient who would otherwise have been suitable for revascularisation. We therefore assume that a FN result that is acted upon by applying strong compression to a F4 mixed aetiology ulcer would require primary amputation in 2.5% of cases. We assume there are no primary amputations for F2 or F3 disease, regardless of whether an arterial ulcer was compressed or not. Scenario analyses are explored where all risks of primary amputation are removed from the model, and it is assumed that all initially receive limb salvage.
Model parameters – ulcer healing probabilities and healing times
Targeted literature reviews were conducted to identify existing data for the population of the baseline model. Where more than one source of evidence was available, we used data from the largest study population that was deemed to most closely reflect UK clinical practice by the EAG’s clinical expert advisor. Evidence was scarce, particularly for mixed aetiology disease and significant uncertainty remains regarding baseline mixed aetiology disease progression.
Probability of achieving successful ulcer healing
Baseline venous ulcer healing probabilities were obtained from the delayed ablation arm of the UK EVRA RCT, per-protocol analysis, showing a 24-week healing probability of 0.826 (0.768 to 0.876), sampled from a beta distribution. 93 For arterial ulcers, it was assumed that all ulcers remained unhealed at 24 weeks. For mixed aetiology ulcers, the analysis not only focuses on the arterial pathway but also accounts for the utility gains and losses associated with different ulcer healing times over the first 24 weeks. Average healing times for a mixed aetiology ulcer were obtained from Humphreys et al. , which was a prospective study of leg ulcer patients, treated with modified compression and assessed for revascularisation at 3 months if no improvement or worsening symptoms. 8 The sample consisted of participants who had a mean age of 81 at baseline and were treated at a specialist centre in Cheltenham from 1998 to 2003. A total of 193 patients with moderate arterial insufficiency (i.e. ABPI 0.5–0.85) achieved a 36-week ulcer healing probability of 0.676. The Humphreys et al. study was chosen because it was a large UK study, provided parameters suitable for the model (healing probabilities and times in a single source), and was one of the few that categorised and defined mixed arterial ulcers in a manner that appeared somewhat transferrable to the parameterisation of the model. 8 Given that measures of uncertainty were not reported in the study, it was assumed that the SE was 20% of the mean and probabilities were parameterised using a beta distribution and converted to a 24-week probability for application in the decision tree phase of the model, assuming a constant rate over time.
Ulcer healing times
The average duration of time to healing for an ulcer that ultimately heals by week 24 is calculated in the model as a function of the baseline healing time (assumed for manual Doppler testing, obtained from the literature8,93), adjusted for time gains due to potential early diagnoses, and time delays due to inaccurate diagnoses associated with the automated tests. The following equation is used to calculate healing time:
Baseline healing times
Baseline ulcer healing times were obtained from the EVRA trial, with a median, [interquartile range (IQR)] healing time of 82 (69 to 92) days. 93 The median healing time was converted to a mean for use in the economic model as 82/ln(2) = 118.30 days. A log-normal (LN) distribution was then parameterised using the median and the approximated mean value. The median baseline healing time for mixed aetiology ulcers was obtained from the Kaplan–Meier (KM) curve in Humphries et al. and was approximately 13 weeks (91 days), converted to a mean of 91/ln(2) = 131 days and parameterised using a LN distribution. The detailed model parameters derived from the EVRA and Humphries studies are provided in Table 16.
Time gains due to early disease detection or improved referral pathways
It is unclear whether automated tests could lead to tangible reductions in ulcer healing time. For this to be the case, automated tests would be required to enable more efficient referrals to appropriate community or vascular services. For any improvements in referral pathways to be tangible, it would require automated tests to be used in settings where staff do not have the skill sets required to complete manual Doppler testing. This would not be the case in community leg ulcer clinics or vascular services. The EAG’s clinical expert believed that most GP practices would also have access to ABPI measurements in the community, either from a nurse at the GP practice or within a group of practices, and that referrals to vascular services purely for an ABPI assessment to be completed would be unusual. This would suggest that it is unlikely automated tests could lead to more efficient triage of patients to community or vascular services in most settings. The EAG base-case analysis therefore assumed there are no time gains from the use of automated tests.
There may however be a very small number of settings where automated devices could lead to more efficient referrals where access to healthcare professionals with the skills to complete manual Doppler assessment are not readily accessible in the primary care setting. This might include, for example, some small rural GP practices or district nurses who may not have been trained in manual Doppler assessment. In such scenarios, a TN automated test may lead to referrals directly to community leg ulcer services rather than to outpatient vascular clinics. Any time gains might then be approximated as the difference in waiting times for vascular services compared to community leg ulcer clinics. These too are uncertain parameters. Waiting time information for community leg ulcer clinics is not readily available, but one clinical expert suggested waiting times for community leg ulcer clinics may be as low as 2 weeks (Kate Donovan, personal communication). Patients in England are guaranteed to receive an outpatient consultation within 18 weeks (non-urgent), so this could be considered the usual maximum waiting time, though some trusts may struggle to meet these targets post pandemic. Applying these assumptions would suggest a maximum possible time saving to initiation of strong compression for a venous ulcer of 16 weeks, in a setting where manual Doppler assessment is inaccessible. This optimistic scenario analysis is provided for the committee’s information. For arterial ulcers, it is even less likely that time gains could be realised, because the patient should present with other indications of arterial disease that would prompt urgent referral to vascular services.
Delayed healing times due to inaccurate results
Delays in healing time are modelled to depend on the diagnostic accuracy of the test, and whether the underlying cause of the ulcer is arterial, mixed or venous. Time delays due to inaccurate test results are plausible but are likely to be highly variable in UK clinical practice and will depend on the setting, the expertise of the staff conducting the test, whether the test is used as a screening tool or conducted as part of a holistic patient assessment (i.e. whether a false reading is acted upon), how long it takes to identify the error, to what extent best practice guidelines are followed in clinical practice and how patients present to clinical services when ulcers do not heal adequately. The uncertainty associated with multiple different assumptions makes it difficult to select a single base-case parameter value that adequately reflects variability in clinical practice across and within settings.
To characterise the uncertainty around the impact of test results on healing times, the EAG survey asked NICE SCMs to provide a ‘best guess’ estimate alongside a range of plausible values for the time to detect FN and FP results and the associated related delay in ulcer healing times for arterial and venous ulcers. These ‘best guess’ estimates were used as the base-case values for Equation 1. The time to recognition and delayed healing time for FN/FP results provided by each clinical expert are provided in Table 15.
| Expert 1; best guess (min–max) | Expert 2; best guess (min–max) | Expert 3; best guess (min–max) | Expert 4; best guess (min–max) | Expert 5; best guess (min–max) | |
|---|---|---|---|---|---|
| Time to recognise FP result | 120 (N/R) days | N/R | 28 (14–180 days) | 42 (7–84 days) | N/R |
| Delay in healing due to FP result | 180 (N/R) days | N/R | 90 (60–180 days) | 84 (N/R) | N/R |
| Time to recognise FN result | 42 (7–112 days) | 14 (1–30 days) | 3(1–7 days) | N/R | 7 (3–7 days) |
| Delay in healing due to FN result | 84 (42-N/R) days | N/R | 84 (14–360 days) | N/R | 180 (90–180) |
Uncertainty in each of the modelled time parameters is incorporated into the model probabilistically, assuming a gamma distribution to allow for a left-skewed distribution. Mean and standard deviation (SD) parameters for the distribution are calculated using the clinical expert’s best guess estimates to each timing question, converted to days. All available data from each question posed to the clinical experts are used to parameterise the distribution, and we attach equal weight to each expert’s response. All timing parameters were incorporated into the model probabilistically using gamma distributions to account for left-skewed data. Table 16 describes all timing parameters in the model.
| Parameter | Mean value | SEa | Alpha | Beta/lambdab | Dist. | Notes/source |
|---|---|---|---|---|---|---|
| Venous ulcers | ||||||
| 24 weeks healing rate c | 0.826 | 0.028 | 150.6 | 31.72 | Beta | Deferred ablation arm of EVRA RCT (per-protocol analysis)93 |
| Baseline healing time (days) | Mean: 118.30d Median: 82 |
– | Mean of logs: 4.41 | SD logs: 0.86 | LN | EVRA RCT93 |
| Time gains due to early testing | 0 days | – | – | – | Fixed | Base-case assumption, varied in scenario analyses |
| Time delay to recognise FP results | 63 days | 29 days | 4.72 | 0.075 | Gamma | Clinical expert survey (see Table 15) |
| Time delay to ulcer healing due to FP results | 118 days | 31 days | 14.49 | 0.123 | Gamma | Clinical expert survey (see Table 15) |
| Arterial ulcer | ||||||
| Probability of healing by 24 weeks (n/N) | 0 | – | – | – | Fixed | Assumption that purely arterial ulcers will not heal with conservative management alone |
| Baseline healing time | > 24 weeks | – | – | – | Fixed | Assumption that purely arterial ulcers will not heal within the 24-week decision tree phase |
| Time gains due to early testing | 0 days | – | – | – | Fixed | Base-case assumption, varied in scenario analyses |
| Time delay to recognise FN results | 0 days | 0 days | Fixed | Assumes that a purely arterial ulcer would be unlikely to be missed in the context of a holistic patient assessment | ||
| Arterial ulcers (with mixed aetiology) | ||||||
| 36-week ulcer healing probabilityc | 0.676 | 0.135 | 7.45 | 3.57 | Beta | Humphreys et al.8 |
| Baseline healing time | Mean: 91d Median: 131 |
– | Mean of logs: 4.51 | SD logs: 0.851 | LN | Humphreys et al.8 |
| Time gains due to early testing | 0 days | – | – | – | Fixed | Base-case assumption, varied in scenario analyses |
| Time delay to recognise FN results | 17 days | 9 days | 3.57 | 0.210 | Gamma | Assumption based on clinical expert survey (see Table 15) |
| Time delay to ulcer healing due to FN results | 116 days | 32 days | 13.14 | 0.113 | Gamma | Assumption based on clinical expert survey (see Table 15) |
As described in this section, potential time delays due to inaccurate results and potential time gains due to more appropriate referrals of TN cases are highly uncertain, and the potential consequences (positive and negative) of the automated diagnostic tests are unclear. The EAG has conducted a range of scenario analyses to explore the impact of uncertainty in test timings on cost-effectiveness results, ranging from optimistic (where tests provide time gains, but no delays because inaccurate results are not acted upon) to pessimistic (where tests provide no time gains, but inaccurate results lead to ulcer healing delays and negative consequences of inappropriate compression). The key base-case assumptions around the impact of test results on healing times and a range of optimistic and pessimistic assumptions are detailed in Table 17.
| Parameter | Base case | Scenario analyses |
|---|---|---|
| Timing consequences for venous ulcers | ||
| Reduced time to applying compression to a venous ulcer due to automated tests. | Assume that in a community leg ulcer setting, there would be no gains in time to compression because all required skills to provide automated or manual Doppler testing and to apply compression would be available in this setting. | It could be argued that automated testing could improve referral pathways if easier to deliver as a screening tool in settings without access to manual Doppler testing, to inform prompt referral to leg ulcer services. We therefore explore the impact of an optimistic scenario where the difference in waiting times between leg ulcer clinics and vascular outpatient clinics could be considered a reduction in ulcer healing time through ensuring prompt compression applied to the venous ulcer. |
| Automated Doppler may reduce test times, but would not consistently shorten appointments (Kate Donovan, personal communication). We have therefore not modelled any time gains for the base-case scenario. | While this time saving is speculative and based on the assumption that an automated test could feasibly alter primary care referral behaviours, the optimistic scenario assumes a 16-week time saving might be achievable based on assumed wait times for community leg ulcer services = 2 weeks and for vascular services = 18 weeks (NHS guaranteed time to consultation). | |
| Delay in recognition of a FP result and thus delayed time to ulcer healing. | Delayed ulcer healing time based on clinical expert opinion survey. | Optimistic scenario: Assume no delay as mistake would be picked up in a holistic assessment of the patient, especially in leg ulcer clinics. Pessimistic scenario: Assume no FP test results are healed by 24 weeks (broadly consistent with upper end of clinical expert delay in ulcer healing times). |
| Implications for mixed and arterial ulcers | ||
| Probability that a FN test result is acted upon (and inappropriate compression applied). | Arterial = 0% (fixed) Mixed = 100% (fixed). |
Optimistic: 0% arterial; 0% mixed Pessimistic: 100% arterial; 100% mixed. |
| Additional risk of requiring treatment escalation, among those in whom a FN test is acted upon. | Highly uncertain parameter, based on escalation of treatment according to underlying disease severity (approximated using Fontaine stage), implications based on EAG clinical expert opinion, include increased risk of requiring bypass surgery (F3/4) and increased risk of primary amputation (F4). | Optimistic: Remove the risk of primary amputation and assume no consequences because all FNs identified in routine clinical practice through a holistic clinical assessment. Pessimistic: Assume all FNs that are acted upon require non-elective surgery. |
Model parameters – transition probabilities for Markov model
At the end of the decision tree phase of the model, the surviving cohort enter the Markov model. Patients with venous ulcers enter the healed/unhealed states depending on whether their ulcer healed by week 24. Patients with arterial disease are assumed to have CLI, due to the presence of an arterial ulcer (Fontaine stage 4), and enter the CLI state where they receive invasive treatment (angioplasty or bypass surgery). Patients with mixed ulcers follow a pathway according to disease severity defined using the Fontaine stages of disease.
Venous ulcers
Those who are unhealed at 24 weeks entered the ‘unhealed’ model state but can continue to transition to the healed state over the longer term, following long-term follow-up data from the EVRA RCT. 99 Data from the per-protocol analysis for deferred ablation show that 87.2% (170/195) of venous ulcer patients have achieved healing of the primary index ulcer by 1 year.
The proportion of the cohort with a healed ulcer is then subject to an ongoing risk of recurrence. Two sources were deemed potentially relevant for parameterising venous ulcer recurrence risk, with long-term data available from both the ESCHAR and EVRA long-term follow-up studies. The ESCHAR study showed a recurrence rate of 56% for the compression-only arm of the trial at 4 years follow-up. 100 Data from the deferred intervention arm of the EVRA study showed that 38/154 (24.7%) of those followed up at 1 year had a recurrence of the primary ulcer. Ninety-three long-term follow-up data showed that 2- and 3-year cumulative recurrence rates were 0.239 (95% CI 0.1852 to 0.3053) and 0.2995 (95% CI 0.2392 to 0.3710), respectively, converting to 6-monthly probabilities of additional ulcer recurrence of 3.5% and 5.5% in years 2 and 3, respectively. 99 Four-year data were also available, but the numbers at risk were small (N = 32) and considered insufficient to populate the model. Three-year recurrence probabilities are then extrapolated over the remaining lifetime horizon of the model. The data from EVRA are applied in the base-case analysis because (1) it provides a consistent source to populate multiple model parameters, (2) data are obtained from a large UK sample and (3) granular data across multiple time points, including CIs to derive distributions for the probabilistic analysis, are available for several parameters.
The probability of healing for a recurrent ulcer was also obtained from the EVRA long-term follow-up data. The probability of healing and subsequent recurrence risks for second and subsequent recurrences were assumed to be equal to the first given a lack of data beyond the first venous ulcer recurrence. This is likely to be a conservative estimate of future long-term recurrence risk, based on clinical expert opinion that recurrence risk is likely to be higher for greater number of previous ulcer healing cycles. While the model allows for a transition to amputation, EAG clinical expert advice was that amputation for venous leg ulcers in the UK is extremely rare; therefore, the base case assumes a transition probability equal to 0. Venous ulcers are not assumed to be fatal, and, therefore, transition to the death state is assumed equal to UK age- and sex-adjusted all-cause mortality. The transition probabilities for the venous ulcer pathway are summarised in Table 18.
| Transition from | To | Source time | n/N (%) | Mean (SE) | 6-monthy cycle prob | Alpha | Beta | Dist. | Source/notes |
|---|---|---|---|---|---|---|---|---|---|
| Unhealeda | Healed | 1 year prob | 170/195 (87.2%) | 64.2% | 170 | 25 | Beta | EVRA study93 | |
| 2 year prob | 0.954 (0.047)a | 53.7% | 18.0 | 0.868 | Beta | EVRA long-term follow-up99 | |||
| 3 year prob | 0.962 (0.048)a | 42.0% | 14.3 | 0.565 | Beta | EVRA long-term follow-up99 | |||
| Unhealed | Amputation | – | – | 0 | – | – | Fixed | Assumption | |
| Unhealed | Death | – | – | ACM | – | – | Fixed | Assumption | |
| Healed | Recurrence | 1 year prob | 38/154 (24.7%) | 13.2% | 38 | 116 | Beta | EVRA long-term follow-up99 | |
| 2 year prob | 9/132 (6.8%) | 3.5% | 9 | 123 | Beta | EVRA long-term follow-up99 | |||
| 3 year prob | 10/93 (10.8%) | 5.5% | 10 | 83 | Beta | EVRA long-term follow-up99 | |||
| Healed | Amputation | – | – | 0 | – | – | Fixed | Assumption | |
| Healed | Death | – | – | ACM | – | – | Fixed | Assumption | |
| Recurrence | Healed | 1 year prob | 0.884 (0.044)a | 0.659 | 45.94 | 6.03 | Beta | EVRA long-term follow-up99 | |
| 2 year prob + | 0.927 (0.046)a | 0.480 | 28.72 | 2.26 | Beta | EVRA long-term follow-up99 | |||
| Recurrence | Amputation | – | – | 0 | – | – | Fixed | Assumption | |
| Recurrence | Death | – | – | ACM | – | – | Fixed | Assumption |
Mixed and arterial ulcer transition probabilities
Table 19 describes the transition probabilities applied in the arterial (and mixed) disease model. The arterial and mixed proportion of the cohort enter the model in the CLI, healed or amputation state, depending on the outcomes achieved during CLI treatment (angioplasty or bypass surgery) in the decision tree phase of the model. Mixed (venous/arterial) and arterial ulcers follow similar pathways. For mixed ulcers, where Fontaine stage-dependent transitions are available in the literature, these are applied in the model. However, for several parameters, it has not been possible to source stage-dependent transition probabilities. Where this is the case, we assume that transition probabilities are independent of the underlying stage of disease severity.
| Transition from | To | Parameter type | Source time frame (e.g. over 2 years) | Parameter: n/N (%) HR/RR (CI) |
6-monthly cycle prob | Alpha | Beta/Lambda | Dist. | Source/notes |
|---|---|---|---|---|---|---|---|---|---|
| CLI | Healed post CLI | Probability | Post-treatment success | Multiple | Multiple | Multiple | Multiple | Multiple | 6-monthly transition probability-based treatment-specific success probabilities following angioplasty and bypass, weighed according to procedure type (elective/emergency). See Table 14 for further details. Data obtained from the National Vascular Audit Report98 |
| CLI | Death | HR | HR of F4 vs. F2 (background arterial) | 3.026 (average of Rutherford 5 and 6) | Age-dependent (0.044 in cycle 1 for age 70) | R5: 0.900 R6: 1.278 |
R5: 0.030 R6: 0.026 |
LN | HR of mortality for a population with a low ABPI, background excess mortality risk for a population with arterial disease,101 multiplied by HR of CLI (average of Rutherford stages 5 and 6 [F4] vs. R1-3 [F2]), obtained from Luders et al.105 |
| CLI | Amp | Probability | 3-month | 6.9% (6.3 to 7.6%) | 0.1340 | 372.17 | 4990.92 | Beta | Obtained from CG147, based on ACC/AHA 2005 practice guidelines23 |
| Healed post CLI | CLI | Rate | 1 year | 0.0466 (0.0260 to 0.0672) | 0.023 | 18.73 | 383.25 | Beta | Assumed equal to the transition between symptomatic PAD (e.g. IC) to CLI, in the absence of any information on long-term recurrence post healing of arterial ulcers; Sigvant et al. meta-analysis101 |
| Healed post CLI | Death | HR applied to all-cause mortality rates | Median 5-year follow-up | HR: 1.98 (symptomatic PAD vs. normal ABI) | Age-dependent (0.015 in cycle 1 for age 70) | 0.683 | 0.149 | LN | HR obtained from Sigvant et al. meta-analysis,101 symptomatic PAD vs. normal ABI. Normal ABI assumed to be equal to UK general population ACM |
| Amputation | Death | Probability | Cycle 1 Cycle 2+ |
0.047 0.047/2 |
Tunnel 1: 0.046; Tunnel 2+: 0.023 | −3.06 | 0.06 | LN | Age-adjusted risk of mortality following amputation, based on HR obtained from National Vascular Registry98 |
The proportion of the cohort that enters the CLI state is treated with angioplasty or surgical bypass as it is assumed that medical management will be unsuccessful. The cohort are exposed to the same probabilities of success as defined for the decision tree pathways. For the proportion of patients who have a successful CLI procedure (no further procedure required), they enter the ‘healed post-CLI’ state. Healed arterial and mixed ulcers (post CLI) then remain at risk of recurrence. The transition probability from healed post-CLI back to the CLI state was assumed equal to the transition from symptomatic PAD to CLI as reported in a meta-analysis of N = 7 studies of symptomatic PAD, conducted by Sigvant et al. 101 The study published a recurrence rate at 1 year of 0.046, converted to a probability of 2.3% per cycle for application in the model, leading to a cumulative probability of CLI recurrence at 5 years equal to 21%. Each cycle of recurrence of CLI is assumed to carry the same event probabilities given a lack of evidence on the treatments and outcomes for recurrent CLI.
The proportion of the cohort who do not have a successful outcome from CLI surgery require further surgery or amputation. For the proportion who do not receive amputation, tunnel states are used to increase the level of invasiveness of treatment in subsequent rounds of treatment, assuming one round of treatment per cycle. Those who have failed angioplasty are assumed to require bypass, and those who have failed bypass require a repeat surgery. All of those who have failed to achieve successful outcomes in the first four cycles of the tunnel state are assumed to require amputation. The risk of progressing directly to amputation post first or second treatment failure with angioplasty/bypass is obtained from CG147 as the 3-monthly transition from CLI to amputation and converted to a 6-monthly probability for use in our model.
Long-term mortality risks in the arterial and mixed disease models
Transitions to the model death state for those with arterial or mixed disease are uncertain and likely to be patient and risk-factor dependent. For example, those with diabetes and other cardiovascular risk factors are at significantly increased mortality risk. 102 Mortality risks from PAD health states include all-cause general population mortality, excess risks for PAD patients generally, excess risk for CLI and in-hospital mortality risks for CLI-related procedures (angioplasty, bypass and amputation). Age- and sex-adjusted UK general population all-cause mortality risk is obtained from UK life tables. 103
The background hazard ratio (HR) of death for asymptomatic PAD, assumed equivalent to Fontaine stage 1, compared to age- and sex-adjusted UK population all-cause mortality rates was obtained from a meta-analysis of four studies, reporting a pooled HR of all-cause mortality of 1.53 (1.18 to 1.99) for asymptomatic PAD (i.e. low ABPI, but no symptoms reported) compared to normal ABI. 101 Sigvant et al. 100 also estimate a HR of 1.98 (1.48 to 2.65) for symptomatic PAD compared to normal ABI, based on a meta-analysis that included five studies. As our base-case analysis assumes all patients with arterial or mixed disease have at least mild claudication, they are at least a Fontaine stage II (equivalent to Rutherford stage 1+). The HR of 1.98 is therefore applied to the general population’s all-cause mortality rates and converted to a 6-month cycle-specific probability for transition to the model death state. This transition is applied as a background transition to death in all stages of the model.
Patients with CLI are at an even greater risk of death, with mortality rates increasing with more severe CLI disease. A systematic review identified two studies that reported mortality risks according to Rutherford disease classification stage. 104–106 For example, Luders et al. report an analysis of German health insurance data for patients with CLI over a median of 2.1 years of follow-up. 105 The study found that mortality HRs, obtained from a Cox regression model controlling for comorbidities, were:
-
Rutherford stage 4 (F3) versus Rutherford stages 1–3: 2.01 (1.88 to 2.14);
-
Rutherford stage 5 (F4) versus Rutherford stages 1–3: 2.46 (2.32 to 2.61); and
-
Rutherford stage 6 (F4) versus Rutherford stages 1–3: 3.59 (3.40 to 3.78).
Assuming the HR for Fontaine 4 CLI is the midpoint of Rutherford stages 5 and 6, then a HR of (2.46 + 3.59)/2 = 3.025 could be applied to CLI-related mortality in the model. The second study conducted a similar study, also using German insurance claims data, drawing very similar conclusions. 106 The data from Luders et al. are therefore applied in the base-case model.
The additional risk of mortality for more extensive CLI disease will, by definition, include mortality in the immediate aftermath of surgery, such as post-angioplasty and post-bypass. The base-case analysis therefore does not apply any further risk of ‘in-hospital’ mortality following surgery to avoid the risk of double counting the risk of death in the model. However, the mortality impact of a FN test result that leads to inappropriate compression of the arterial ulcer may be better captured in our model structure by accounting for the additional mortality risk associated with more invasive/intensive procedures associated with the requirement for escalated care. National audit data (from the National Vascular Registry) show that patients requiring bypass surgery are at increased risk of ‘in-hospital’ mortality compared to those requiring angioplasty, though this additional risk is not reported according to a measurement of arterial disease severity. 98 As a scenario analysis, we therefore obtain the probability of in-hospital mortality for angioplasty, bypass and amputation from the national audit report and apply a treatment-specific post-treatment mortality instead of applying the same mortality risk across all treatments in the CLI state. The mortality risk is obtained as a weighted average of mortality post elective and emergency procedures for angioplasty and bypass, respectively. 98 Given that the model structure calculates the expected impact of FN on requirement for escalated treatment, this approach may better capture any long-term mortality impact of escalated treatment due to more invasive procedures for those who receive inappropriate compression of an arterial ulcer. However, this approach likely underestimates overall mortality in the arterial cohort.
Model parameters – resource use and costs
Diagnostic test costs
The costs of manual and automated Doppler measurements have been calculated using a microcosting approach. Resource usage included in the test cost calculations are:
-
Staff costs: Time to conduct the tests in clinical practice. The base-case analysis assumes one band 5 community nurse is required to complete both the manual and automated tests, with test times derived from the review of diagnostic accuracy studies (see Results of the assessment of clinical effectiveness for details of test times). Some studies and ‘how to’ guides suggest that two nurses may be required to complete the manual Doppler test and the impact of this on costs is considered in scenario analyses. 107 Further scenario analyses explore the impact of different levels of staff completing the test, including band 7 advanced nurses, for example, at a leg ulcer clinic, or tests completed by a consultant at a vascular surgery clinic. A final scenario applies the resource use times as reported by the companies in their response to NICE’s information request, or from the relevant product manuals. Unit costs per minute of staff time were obtained from Personal Social Services Research Unit (PSSRU) average cost per hour, including qualification costs. 108
-
Equipment costs: These include the costs of measurement devices, additional purchase of a range of cuff sizes to ensure all patients can receive a measurement, software where appropriate and replacement cuffs. Unit costs of equipment are provided by the companies or sourced from online suppliers where data are unavailable. All costs are provided exclusive of VAT. Per-patient costs are allocated based on the tests’ useful lifetime horizon and expected throughput per year. The useful lifetime horizon was obtained directly from the companies or conservatively assumed to be equal to 2 years where this information was unavailable to the EAG. Expected device throughput is uncertain and driven by the time taken to complete the respective tests and the setting in which the test may be used (e.g. at a leg ulcer clinic, at a GP practice, by a district nurse or in a vascular surgery consultation). The base-case analysis assumes that, on average, eight tests per day might be completed. Scenario analysis explores varying this between a minimum value of one test per day and a maximum value equal to the maximum number of tests that could be conducted given the time taken to conduct each test over a 7-day, 40-hour week, 52-week year. The latter analysis would allow shorter tests to have greater throughput and a lower average equipment cost allocated per patient tested.
-
Consumables: Based on the company response to information requests, where consumable costs have been provided, these are included in the evaluation. These include the costs of printed results where these are available and the costs of ultrasound gel for the manual Doppler test. Consumables and equipment for the manual Doppler test are obtained from a manual Doppler technical guide available from Wounds International. 107
-
Repeat test costs: The proportion of tests that deliver an error message, zero reading or other technical failure may have implications for costs. The base-case analysis assumes that where a manual or an automated test fails to deliver a reading, the patient would be retested once only using the same method. This assumption was validated with clinical expert opinion. The definition of a technical failure is however incompletely and inconsistently defined across the diagnostic accuracy review studies; therefore, differences in rates should be interpreted cautiously. The base case assumes that all failures would be retested once using the same method and implicitly that the device will then provide a correct reading. However, this may be an underestimate of retesting costs because some technical failures may require referral onwards to vascular services to obtain a more definitive estimate using Duplex ultrasound or CTA. The implication is that the base case may be biased in favour of automated testing, though the magnitude of any bias is unclear because it is unknown what proportion of technical failures would provide an accurate reading upon retest. This proportion is varied in two scenario analyses assuming that 50% and 100% of technical failures would require referral onwards to vascular services where they incur the costs of an outpatient consultation and Duplex ultrasound test.
Table 20 provides a breakdown of resource use and cost for each test in the base-case analysis. Table 21 details the costs of each test under a range of scenario analyses. All test costs are incorporated into the model as fixed parameters.
| Manual Doppler | BlueDOP Medical | Bosch and Sohn | Dopplex Ability, Huntleigh | Mesi ABPI MD | Mesi M Tablet ABI | WATCH BP ABI Microlife | WATCH BP Vascular Microlife | |
|---|---|---|---|---|---|---|---|---|
| Staff costs | ||||||||
| Staff grade | Nurse (B5) | Nurse (B5) | Nurse (B5) | Nurse (B5) | Nurse (B5) | Nurse (B5) | Nurse (B5) | Nurse (B5) |
| Number of staff to conduct test | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Staff unit cost (per minute) | £0.73 | £0.73 | £0.73 | £0.73 | £0.73 | £0.73 | £0.73 | £0.73 |
| Rest/preparation time (minutes)a | 10.00 | 3.00 | 5.00 | 2.50 | 2.00 | 5.00 | 10.00 | 10.00 |
| Test time (minutes)a | 11.92 | 1.00 | 3.00 | 4.30 | 5.24 | 5.24 | 10.10 | 10.10 |
| Interpretation time (minutes)a | 5.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total time (minutes) | 26.92 | 5.00 | 9.00 | 7.80 | 8.24 | 11.24 | 21.10 | 21.10 |
| Staff cost (£) | 19.74 | 3.67 | 6.60 | 5.72 | 6.04 | 8.24 | 15.47 | 15.47 |
| Device costs | ||||||||
| Device equipment fixed costb (£) | 252.81c | 4995.00 | 3150.00 | 2749.00 | 2325.00 | 2700.00 | 1695.00 | 1995.00 |
| Initial purchase of additional cuffs to complete setb (£) | 87.96c | 0.00 | 37.10 | 304.00 | 174.00 | 174.00 | 250.00 | 250.00 |
| Software (£) | 0.00 | 0.00 | 0.00 | 454.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Other fixed costs (£) | 0.00 | 0.00 | 0.00 | 430.00 | 0.00 | 0.00 | 200.00 | 200.00 |
| Total fixed costs per unit (£) | 340.77 | 4995.00 | 3187.10 | 3937.00 | 2499.00 | 2874.00 | 2145.00 | 2445.00 |
| Number of cuffs | 1 | 0 | 4 | 4 | 3 | 4 | 2 | 2 |
| Replacement cuffs per yeard | 5.84 | 0 | 5.84 | 5.84 | 5.84 | 5.84 | 5.84 | 5.84 |
| Unit cost per replacement cuff (£)b | 21.99 | 0.00 | 18.50 | 73.50 | 46.67 | 35.00 | 55.00 | 55.00 |
| Useful life (years) | 2e | 3 | 2e | 7 | 5 | 5 | 5 | 5 |
| Max possible throughput per yearf | 2920 | 2920 | 2920 | 2920 | 2920 | 2920 | 2920 | 2920 |
| Replacement cuff costs: (£) | 0.04 | 0 | 0.15 | 0.59 | 0.28 | 0.28 | 0.22 | 0.22 |
| Device cost per test (£) | 0.10 | 0.57 | 0.69 | 0.78 | 0.45 | 0.48 | 0.37 | 0.39 |
| Consumable 1 (number required) | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Consumable 1 (unit cost) (£) | 0.16g | 0.00 | 0.00 | 0.37 | 0.00 | 0.00 | 0.00 | 0.00 |
| Consumable 1 costs per test(£) | 0.16 | 0.00 | 0.00 | 0.37 | 0.00 | 0.00 | 0.00 | 0.00 |
| Technical failure probabilityh | 0.02 | Confidential information has been removed | 0.08 | 0.04 | 0.15 | 0.15 | 0.08 | 0.08 |
| Additional time between tests | 0.00 | 0.00 | 0.00 | 5.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Proportion of technical failures referred to vascular services | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cost per referral to vascular clinic + ultrasound (£)i | 418.44 | 418.44 | 418.44 | 418.44 | 418.44 | 418.44 | 418.44 | 418.44 |
| Retest costs per test (£) | 0.48 | Confidential information has been removed | 0.56 | 3.92 | 0.96 | 1.29 | 1.25 | 1.25 |
| Total cost per test (£) | 20.48 | Confidential information has been removed | 7.86 | 10.79 | 7.45 | 10.01 | 17.09 | 17.11 |
| Scenario description | Manual Doppler (£) | BlueDOP Medical (£) | Bosch and Sohn (£) | Dopplex Ability (£) | Mesi MD (£) | Mesi M Tablet (£) | WATCH BP ABI (£) | WATCH BP vascular (£) |
|---|---|---|---|---|---|---|---|---|
| Base case total cost per test | 20.48 | Confidential information has been removed | 7.86 | 7.26 | 7.45 | 10.01 | 17.09 | 17.11 |
| Scenario 1: Assume a grade 7 nurse conducts tests | 30.59 | Confidential information has been removed | 11.41 | 10.30 | 10.92 | 14.74 | 25.44 | 25.46 |
| Scenario 2: Assume a consultant conducts tests | 56.32 | Confidential information has been removed | 20.46 | 18.02 | 19.75 | 26.78 | 46.69 | 46.71 |
| Scenario 3: Test times from the companies | 20.48 | Confidential information has been removed | 6.28 | 5.89 | 3.89 | 6.44 | 15.03 | 15.06 |
| Scenario 4: Assume two nurses required for manual Doppler | 40.70 | Confidential information has been removed | 7.86 | 7.26 | 7.45 | 10.01 | 17.09 | 17.11 |
| Scenario 5: Apply low test throughput | 20.90 | Confidential information has been removed | 11.97 | 8.66 | 8.83 | 11.59 | 18.20 | 18.38 |
| Scenario 6: Apply high test throughput | 20.45 | Confidential information has been removed | 7.36 | 7.09 | 7.29 | 9.83 | 16.99 | 17.00 |
| Scenario 7: 50% of technical failures require referral to vascular services + Duplex ultrasound | 25.26 | Confidential information has been removed | 23.68 | 14.81 | 37.94 | 40.33 | 32.99 | £33.02 |
| Scenario 8: 100% of technical failures require referral to vascular services + Duplex ultrasound | 30.05 | Confidential information has been removed | 39.51 | 22.36 | 68.42 | 70.65 | 48.90 | 48.92 |
Model parameters – costs
Costs are evaluated from a UK NHS perspective and reported in 2020–1 GBP. Data to inform health state costs were obtained from targeted searches of the literature with preference given to studies conducted in the UK for model parameterisation. Where possible, resource use from published studies has been recosted using the appropriate national average unit costs for 2020–1, including PSSRU for primary care and hospital staff time, NHS reference costs for procedures and the British National Formulary (BNF) for drug treatments. 108–110 Where resource use data are unavailable, source cost study estimates have been inflated from their study reported year to 2020–1 values. 108 Costs were incorporated into the model probabilistically by sampling from gamma distributions. Where a measure of spread was unavailable, we assumed a SD of 20% of the mean.
Arterial and mixed pathways
All patients with a test result indicative of arterial disease are referred to vascular services for further investigation and initiation of preventative treatment for their arterial disease. The cost of referral for a first attendance at a consultant-led vascular surgery outpatient clinic (£307.70) was obtained from NHS reference costs 2020–1. 109 This referral cost is applied to all positive test result cases, including FPs. The base-case analysis assumes that a FP result would then be recognised at the point of referral to a vascular surgery clinic and patients would then appropriately incur the venous pathway costs. Scenario analysis explores the impact of an additional Duplex ultrasound investigation for all initial test-positive patients who are referred to vascular services (£110.74). 109 It is assumed that no preventative treatment for arterial disease would be initiated because of a FP result.
The decision tree phase of the model also includes a nominal cost applied universally across all arterial (including mixed) pathways and is informed by resource use ascribed to the mild arterial disease state within Ezeofor et al. supplemented with costs sourced from CG147 and the NHS reference costs 2020–1. 109,111 This equates to a cost of £709.06 per year, which includes: cholesterol testing, medication, 3-month supervised exercise programme and vascular nurse specialist visits (one per quarter). 111 Additional intervention costs are then applied depending on whether the patient is modelled to undergo an angioplasty, surgical bypass or amputation. All arterial patients will receive some intervention within the decision tree phase because clinical expert opinion indicates that arterial ulcers are unlikely to heal with conservative management alone. Within the Markov component of the model, this cost is then applied within the healed post-CLI state, or equivalently, Fontaine stage 2.
The Markov health state cost of arterial or mixed ulcers is dependent upon the progression of their condition. All patients with an arterial ulcer would be classified as Fontaine 4 which is synonymous with CLI. Due to a lack of healthcare resource use data for mixed ulcer patients, it is assumed that the focus of treatment is on the arterial aetiology of their disease. However, given mixed aetiology patients have a higher ABPI (0.5–0.85) than their purely arterial counterparts, we assume a distribution of severity from Fontaine stages 2 to 4. Within the model structure, all patients of Fontaine stage 3 or 4 are assumed to undergo an invasive intervention (e.g. angioplasty/bypass) within the decision tree phase. Following successful intervention, patients would then move to the healed post-CLI state. For simplicity, we have assumed that this is synonymous with intermittent claudication (or Fontaine stage 2) where the resource use is based on the mild arterial disease state within Ezeofor et al. similar to the nominal cost applied in the decision tree phase of the model. 111 Should the ulcer not heal, or through recurrence, the patient would move to the CLI state. The CLI state consists of a consultant-led appointment, angiogram and wound management resulting in an annual cost of £1885.07. The surgery required to treat CLI is accounted for through the tunnel state, angioplasty and bypass, which carry respective mortality risks and resource use.
The most severe cases of CLI result in amputation. This represents a substantial burden to the health service in the short and long term as patients who were once independent often require full-time care. Therefore, we have included an amputation pathway and health state within the decision tree and Markov components of our model. Within our analysis, we attribute costs to the first year and subsequent years post procedure. The first-year costs are substantially higher to account for recovery through rehabilitation and wound care (£35,813.44). For subsequent years, we apply the cost of any additional care related to lifestyle changes after amputation (£14,294.65). A detailed breakdown of how these costs were calculated is provided in Appendix 7, Tables 32–35.
Venous
Venous ulcer treatment costs obtained from Urwin et al. are applied during the unhealed time in the decision tree. Urwin et al. reports a mean cost of £166.39 (95% CI £157.78 to £175.00) for 2 weeks of treatment. 112 An average daily cost of £11.89 (or £14.61 in 2020–1 prices) is applied. For ulcers that heal, a daily cost of medical management and prevention is incurred which equates to a daily cost of £0.32. This assumes two visits with a nurse [cost per visit of £53.33 (mean of bands 5–7)]108 and four pairs of knee-high graduated compression stockings (£4.31 each) per year. 113
Venous ulcer treatment costs, applied to the unhealed time in the decision tree and the unhealed health state within the Markov model, are also obtained from Urwin et al. 112 The corresponding 6-monthly costs for the unhealed Markov state are therefore £2163.07 (or £2667.93 in 2020–1 prices). Patients with a healed venous ulcer are assumed to require monitoring and preventative treatment for the first 2 years post healing and none thereafter. We accept that this may be conservative; however, under limited resources within the NHS, it is not reasonable that healed patients would be monitored indefinitely. We applied two visits with a nurse in the first year and one visit in the second. This results in an annual cost of £115.28 and £61.95 in years 1 and 2, respectively. It is assumed that the cost of treating a healed or unhealed venous ulcer does not depend on whether it is the primary or a recurrent ulcer. Table 22 summarises the health state costs included in the model.
| Health state | Dist. | Study year | Mean | Std error | Mean 2020–1 values |
SE 2020–1 values | Alpha | Beta/lambda | Notes | References/sources |
|---|---|---|---|---|---|---|---|---|---|---|
| Healed venous | Gamma | 2013 and 2021 | Year 1. £115.28 Year 2. £61.95 Year 3. £0 |
£23.06 £12.39 |
Year 1. £116.49 Year 2. £63.16 Year 3. £0 |
£23.30 £12.63 |
24.99 25.01 |
0.2416 0.3959 |
Includes the costs of compression stockings (2 per year at £4.31) and nurse visits (bands 5–7) for monitoring (Y1: 2 visits; Y2: 1 visit; Y3: 0 visits) | PSSRU108 Wade et al.113 |
| Healed post-recurrence venous | Gamma | 2015 | Year 1. £115.28 Year 2. £61.95 Year 3. £0 |
£23.06 £12.39 |
Year 1. £116.49 Year 2. £63.16 Year 3. £0 |
£23.30 £12.63 |
24.99 25.01 |
0.2416 0.3959 |
Assumed equal to the costs of a healed ulcer | PSSRU108 Wade et al.113 |
| Unhealed venous | Gamma | 2019 | £166.39/2 weeks | £104.69 | £175.31/2 weeks | £109.98 | 2.84 | 0.0162 | Converted to daily costs for DT and 6-month costs for Markov state | Urwin et al.112 |
| Recurrence venous | Gamma | 2019 | £166.39/2 weeks | £104.69 | £175.31/2 weeks | £109.98 | 2.84 | 0.0162 | Assumed equal to unhealed ulcer | Urwin et al.112 |
| CLI (base cost excl. surgery) | Gamma | 2021 | – | – | £1885.07/year | £377.01 | 25.00 | 0.0133 | Resource use assumptions sourced from severe health state of Ezeofor et al. 2021, includes: consultant-led clinic appointment, weekly nurse visits and dressings, angiogram111 | Ezeofor et al.111 NHS reference costs 2020–1109 PSSRU 2021108 |
| Angioplasty | Gamma | 2021 | – | – | £4796.36 | £959.27 | 25.00 | 0.0052 | Weighted average of HRG codes YR11 and YR15 | NHS reference costs 2020–1109 |
| Bypass (elective) | Gamma | 2021 | – | – | £15,281.37 | £3056.27 | 25.00 | 0.0016 | Weighted average of elective long and short stay HRG codes YQ05, YQ12 and YQ13 | NHS reference costs 2020–1109 |
| Bypass (non-elective) | Gamma | 2021 | – | – | £15,859.06 | £3171.81 | 25.00 | 0.0016 | Weighted average of non-elective long and short stay HRG codes YQ05, YQ12 and YQ13 | NHS reference costs 2020–1109 |
| Healed post CLI | Gamma | 2021 | – | – | £354.53/6 months | £70.90 | 25.00 | 0.071 | Resource use assumptions sourced from Ezeofor et al.. Two visits with nurse at non-consultant-led clinic. Exercise programme cost uplifted from CG147 (assumed once per year). Cholesterol tests and medication uplifted from Ezeofor et al. 111 | NHS reference costs 2020–1109 Ezeofor et al.111 CG14723 |
| Amputation (initial procedures) | Gamma | 2021 | – | – | £11,392.48 | £2278.50 | 25.00 | 0.002 | Unit cost includes procedure only weighted by the proportion of below to above-knee amputation procedures within the National Vascular Registry 2021 (YQ22/YQ26) | NHS reference costs 2020–1109 |
| First year following amputation | Gamma | 2021 | – | – | £35,804.46 /year |
£7160.89 | 25.00 | 6.981 | See Appendix 7 | NICE Clinical Guideline. CG14723 Davie-Smith et al.114 Taylor et al.115 NHS reference costs. 2020–1109 PSSRU 2019–20116 PSSRU 2020–1108 |
| Second year plus, following amputation | Gamma | 2021 | – | – | £14,293.65 /year |
£2858.73 | 24.83 | 0.0017 | See Appendix 7 | NICE Clinical Guideline CG14723 Davie-Smith et al.114 Taylor et al.115 NHS reference costs. 2020–1109 PSSRU 2019–20116 PSSRU 2020–1108 |
| Death | Fixed | 0 | – | – | – | Assumption | – |
Model parameters – utilities
Venous ulcers
Utilities for healed and unhealed venous ulcers were obtained from Iglesias et al. , a large UK study, reporting an economic evaluation of the VenUS1 trial. 75 The study is preferred over alternative sources as it reports EuroQol-5 Dimensions (EQ-5D) utility data classified by healed/unhealed status. Due to a lack of data, it is assumed that the utility of recurrent and healed post-recurrence venous ulcers are equivalent to healing and recurrence of the primary venous ulcer. Similarly, for mixed venous/arterial ulcers, it is assumed that, where the ulcer is treated with compression (i.e. primarily venous), then the utility of the healed, unhealed and recurrence states is equivalent to those of venous ulcers. Mixed ulcers, where the cohort enter the arterial pathway, are assigned the utilities of the arterial pathway as described below.
Arterial ulcers
A recent review of the literature, conducted by Duff et al. ,104 identified two studies that reported EQ-5D utilities for patients with CLI. 117,118 Forbes et al. report baseline EQ-5D data from the Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial prior to randomisation to angioplasty or surgery, reflecting a population of UK CLI patients (N = 417; angioplasty: 214; surgery: 203) requiring active treatment. 117 UK value set utilities were mean (SD): 0.26 (0.32) and 0.28 (0.34) for those randomised to angioplasty and surgery, respectively. We apply the angioplasty utility for the base-case analysis. Pisa et al. was an international study of 200 CLI patients, 50 of whom were from the UK. 118 UK value sets for CLI (defined as Fontaine stage 3 or 4) were mean (SD): 0.474 (0.303). These higher values are considered for scenario analyses; however, they are not used in the base case due to the smaller UK sample and because Forbes et al. allow exploration of utilities following different procedures (angioplasty and bypass). Utility in the amputation is 0.564 based on Ernsston et al. , based on EQ-5D utility (UK value set) for a below-the-knee amputation. 119
Summary of utility values applied in the model
Table 23 provides a summary of the utilities applied in the economic model. All utilities are incorporated using beta distributions. All utility input parameters are age and sex adjusted to the starting age and sex distribution of the modelled cohort. Utility inputs are then further adjusted in each subsequent model cycle by a multiplier (utility of general population at model cycle age/utility of general population at model start age) to account for reducing quality of life as the cohort ages over time. Utility of the death state is zero.
| Health state | Dist. | Mean | Std error | Alpha | Beta | Notes | References/sources |
|---|---|---|---|---|---|---|---|
| Venous ulcers (including mixed aetiology treated as venous) | |||||||
| Healed | Beta | 0.75 | 0.03 | 155.50 | 51.83 | EQ-5D, UK value set | Iglesias et al.75 |
| Unhealed | Beta | 0.64 | 0.02 | 368.00 | 207.00 | EQ-5D, UK value set | Iglesias et al.75 |
| Recurrence | Beta | 0.64 | 0.02 | 368.00 | 207.00 | Assume equal to unhealed venous ulcer | Iglesias et al.75 |
| Healed post recurrence | Beta | 0.75 | 0.03 | 155.50 | 51.83 | Assume equal to healed venous ulcer | Iglesias et al.75 |
| Arterial ulcers (including mixed aetiology in arterial health states) | |||||||
| CLI (on entry to state) | Beta | 0.2967 | 0.0053 | 2203.77 | 5223.82 | Pooled SD across randomised arms at baseline, EQ-5D, UK population | Forbes et al.117 |
| Healed post CLI | Beta | 0.70 | 0.14 | 6.80 | 2.91 | EQ-5D study with 280 respondents | Holler et al.120 Simpson et al.121 |
| Amputation | Beta | 0.564 | 0.0171 | 473.74 | 366.22 | Below-knee amputation, EQ-5D value set | Ernstsson et al.119 |
| Death | Fixed | 0 | – | – | – | Assumption | – |
Time horizon and discounting
The decision tree phase of the model describes ulcer healing probabilities over 24 weeks, before the cohort then enter a Markov cohort model, with 6-monthly cycles, up until age 100, reflecting a lifetime horizon. With a starting age of 70, the entire model therefore runs for a maximum of 30.46 years. Scenario analyses explore the impact of shorter time horizons. Costs and QALYs occurring beyond year 1 are discounted at 3.5% per annum, with scenario analyses varying the discount rate between 0% and 6%.
Analyses
The model is constructed to be fully probabilistic, sampling a selected number of Monte Carlo draws from distributions applied to model input parameters as described in the parameter tables. Base-case results for three alternative plausible base-case assumptions are reported probabilistically. Parameter uncertainty is described using cost-effectiveness acceptability curves (CEACs). Due to computation run time challenges, it was not possible to provide probabilistic analyses for all scenario analyses considered, and these have instead been presented deterministically.
Results are reported as incremental cost per QALY gained from a UK NHS perspective. Incremental cost-effectiveness ratios (ICERs) were first calculated for all tests compared to the standard care, manual Doppler test. A fully incremental analysis was also undertaken, where all test strategies were ranked in ascending order of QALYs gained. Tests that were more costly and less effective than an alternative test were excluded based on strict dominance, with ICERs calculated relative to the next best non-dominated strategy. Tests that provided more QALYs at a lower ICER were further excluded on the grounds of extended dominance, and ICERs again recalculated versus the next best, non-dominated alternative. The test strategy that generated the highest ICER, under the threshold value can be considered the optimal testing strategy. ICERs are also presented for pairwise comparisons of each candidate test versus manual Doppler testing.
Model validation
Several ‘black-box’ error checks were undertaken, following the approach suggested by published black-box verification checklists. 122,123 Verification checks were conducted on estimation of costs and QALYs, varying parameters between extreme value scenarios to identify any modelling errors. Distributions were examined for plausibility, and expected values of total cost, QALY and cumulative amputation probability, at different points of the tree pathway, were examined for face validity and coherence with intended modelled pathways. Several issues were identified in early model drafts and corrected for the final base-case model. No remaining issues were identified following a recheck of the final base-case analysis.
Modelling results
The economic model assessed the potential cost-effectiveness of six different automated ABPI tests compared to manual Doppler testing for the detection of PAD in people with leg ulcers. There were no diagnostic accuracy studies that provided sensitivity and specificity data for people with leg ulcers and no studies in any population that assessed the impact of different tests on health outcomes such as ulcer healing or need for invasive arterial procedures, such as angioplasty or bypass.
Overview of key assumptions
Due to the lack of available data, the economic model implicitly assumes that the diagnostic accuracy data from a broadly defined heterogeneous population are transferrable to people with leg ulcers. It further assumes that the reference standard (manual Doppler testing) provides accurate results. While it is known that manual Doppler testing is not the best method for PAD diagnosis, there is insufficient information to appropriately adjust estimates for the model to account for an imperfect reference standard. These assumptions should be considered when evaluating the appropriateness of the diagnostic accuracy data to populate the model.
The model uses a linked-evidence approach, informed heavily by clinical expert opinion to describe the impact of tests on health outcomes, including the impact of inaccurate test results on delayed ulcer healing time and need for invasive surgery due to inappropriate compression of arterial (and mixed Fontaine 3 or 4) ulcers.
Importantly, it is unknown what proportion of FN or FP test results would be identified by healthcare professionals at the time of testing and, thus, what proportion of patients would incur negative consequences of inaccurate tests. The extent to which inaccurate test results could lead to delayed ulcer healing (FP) or increased risk of invasive arterial procedures (FN) is likely to be dependent on several factors but may be higher in settings where there is a scarcity of healthcare professionals who are skilled in the assessment of leg ulcers and PAD. Conversely, these may also be the same settings in which an accurate automated test may lead to time savings or reduced unnecessary referrals to secondary care of TN venous ulcer patients.
The model base-case analyses are built around the following key assumptions:
-
The modelled cohort reflects a population of leg ulcer patients, rather than from the diagnostic accuracy studies that contribute sensitivity and specificity data.
-
The PAD cohort are split between the proportion who have arterial versus mixed ulcers. The arterial component of disease is described in the model according to Fontaine stage (2, 3, 4) to allow the application of different treatments, costs, quality of life and mortality risk according to the severity of the underlying PAD. While Fontaine staging may not be used universally in clinical practice, it is a useful approach to describe the severity of disease and, in particular, the implication of inappropriate compression of arterial ulcers, the consequences of which are dependent on the underlying disease severity.
-
It is assumed that primary amputation is rare and that limb salvage is attempted using bypass and/or revascularisation wherever possible, though it is more likely to be required for an inappropriately compressed arterial ulcer.
-
For the Markov models, it is assumed that any test errors would be identified within 24 weeks with patients being allocated to the correct disease pathway (venous, mixed, arterial) and receiving appropriate treatment for their condition within this time (i.e. compression applied to a patient with an initial FP result and appropriate surgical management of an inappropriately compressed patients due to a FN result).
-
It is assumed that the proportion of the cohort with arterial (or mixed) disease who have a recurrence after 6 months incur the same costs and utilities regardless of the number of previous cycles in the CLI state.
-
It is assumed that venous ulcers experience healing post recurrence incur the same costs and utilities as those who achieve healing of the index ulcer. It is also assumed that the risk of recurrence does not depend on the number of previous recurrences due to a lack of data to determine RR parameters.
-
The model assumes that for the proportion of the cohort with mixed ulceration, clinical management prioritises the arterial component of disease first.
-
It is assumed that people with venous ulcer disease have similar mortality risks to the general population and that amputation does not take place in modern clinical practice for venous disease.
-
The model is run for a lifetime horizon up to death or age 100 years, whichever comes first, with costs and QALYs discounted at an annual rate of 3.5% per annum.
Base-case analyses
There are no data to populate the model with regard to (1) the proportion of inaccurate test results (FP or FN) that would be acted upon in clinical practice; (2) the implications of inappropriately applying compression to an arterial ulcer, though this may include a requirement for more invasive surgery with more serious consequences for more advanced arterial disease (e.g. F3 or F4); (3) the delay in ulcer healing associated with delayed compression of a venous ulcer due to a FP test result or (4) the extent to which automated tests could generate reductions in ulcer healing time for TN cases in scenarios with limited access to manual Doppler testing and compression. These are important drivers of cost-effectiveness results but are highly uncertain parameters. It has therefore not been possible for the EAG to determine a preferred ‘base-case’ set of assumptions. We have instead reported results for three possible alternative base cases, according to moderate, optimistic and pessimistic assumptions for automated testing. The moderate set of assumptions could be considered the EAG’s best guess at a set of plausible base-case assumptions, but further evidence is required on several key parameters before a definitive base-case analysis could be determined. The assumptions for each base-case analysis are outlined in Table 24 and the corresponding results from probabilistic runs of the model (1000 simulations) are reported in Table 25. Figures 13–15 illustrate the CEACs for the base case, pessimistic and optimistic scenarios, respectively.
| Parameter/assumption | Pessimistic | Moderate | Optimistic |
|---|---|---|---|
| Time gains from early testing (TN) | None | None | 16 weeks (reflecting potential to refer TN cases directly to community leg ulcer clinics for compression). Assumes that average waiting time for a non-urgent consultation at vascular outpatient clinic = 18 weeks and wait times for community leg ulcer clinics are 2 weeks |
| Delayed healing time for venous ulcers with a FP test | Healing time of at least 168 days (all venous ulcers with a FP test result remain unhealed at 24 weeks), consistent with pessimistic end of range of clinical expert opinion | As per mean of clinical expert opinion | No delay (all FP tests correctly identified as such during holistic patient assessment, compression not delayed) |
| Proportion of FN tests acted upon | Arterial: 100% Mixed: 100% |
Arterial: 0% Mixed: 100% |
Arterial: 0% Mixed: 0% |
| Proportion of FP tests acted upon | 100% | 100% | 0% |
| Test | Total cost (£) | Incremental cost (£) | Total QALY | Incremental QALY | ICER (ranked) | Probability cost-effective at £20K | ICER (vs. Manual Doppler) | Prob (C/E vs. Manual Doppler) |
|---|---|---|---|---|---|---|---|---|
| Moderate assumptions (see Table 24) | ||||||||
| Manual Doppler | 11,713 | 8.046 | 0.999 | |||||
| BlueDop Vascular Expert | 11,930 | 217 | 8.043 | −0.003 | Dominated | 0.001 | Dominated | 0.001 |
| WatchBP Office ABI | 12,037 | 325 | 8.042 | −0.004 | Dominated | 0.000 | Dominated | 0.000 |
| WatchBP Office Vascular | 12,037 | 325 | 8.042 | −0.004 | Dominated | 0.000 | Dominated | 0.000 |
| boso ABI-system 100 | 12,149 | 436 | 8.041 | −0.005 | Dominated | 0.000 | Dominated | 0.000 |
| MESI ABPI MD | 12,189 | 476 | 8.040 | −0.005 | Dominated | 0.000 | Dominated | 0.000 |
| MESI mTABLET ABI | 12,191 | 478 | 8.040 | −0.005 | Dominated | 0.000 | Dominated | 0.000 |
| Dopplex Ability | 12,262 | 549 | 8.040 | −0.006 | Dominated | 0.000 | Dominated | 0.000 |
| Pessimistic assumptions (see Table 24) | ||||||||
| Manual Doppler | 11,680 | 8.042 | 1.000 | |||||
| BlueDop Vascular Expert | 12,136 | 456 | 8.035 | −0.007 | Dominated | 0.000 | Dominated | 0.000 |
| WatchBP Office ABI | 12,216 | 535 | 8.035 | −0.007 | Dominated | 0.000 | Dominated | 0.000 |
| WatchBP Office Vascular | 12,216 | 535 | 8.035 | −0.007 | Dominated | 0.000 | Dominated | 0.000 |
| boso ABI-system 100 | 12,449 | 769 | 8.032 | −0.010 | Dominated | 0.000 | Dominated | 0.000 |
| MESI ABPI MD | 12,462 | 782 | 8.033 | −0.009 | Dominated | 0.000 | Dominated | 0.000 |
| MESI mTABLET ABI | 12,465 | 785 | 8.033 | −0.009 | Dominated | 0.000 | Dominated | 0.000 |
| Dopplex Ability | 12,591 | 910 | 8.031 | −0.011 | Dominated | 0.000 | Dominated | 0.000 |
| Optimistic assumptions (Table 24) | ||||||||
| MESI ABPI MD | 10,977 | 8.072 | 0.509 | Dominant | 0.994 | |||
| MESI mTABLET ABI | 10,980 | 3 | 8.072 | 0.000 | Dominated | 0.000 | Dominant | 0.000 |
| Dopplex Ability | 10,987 | 10 | 8.071 | 0.000 | Dominated | 0.153 | Dominant | 0.981 |
| WatchBP Office ABI | 10,988 | 11 | 8.072 | 0.000 | Dominated | 0.270 | Dominant | 0.989 |
| WatchBP Office Vascular | 10,988 | 11 | 8.072 | 0.000 | Dominated | 0.000 | Dominant | 0.000 |
| boso ABI-system 100 | 11,008 | 31 | 8.071 | −0.001 | Dominated | 0.065 | Dominant | 0.956 |
| BlueDop Vascular Expert | 11,048 | 71 | 8.070 | −0.001 | Dominated | 0.000 | Dominant | 0.000 |
| Manual Doppler | 11,779 | 802 | 8.053 | −0.018 | Dominated | 0.003 | ||
FIGURE 13.
Cost-effectiveness acceptability curves for moderate base case.
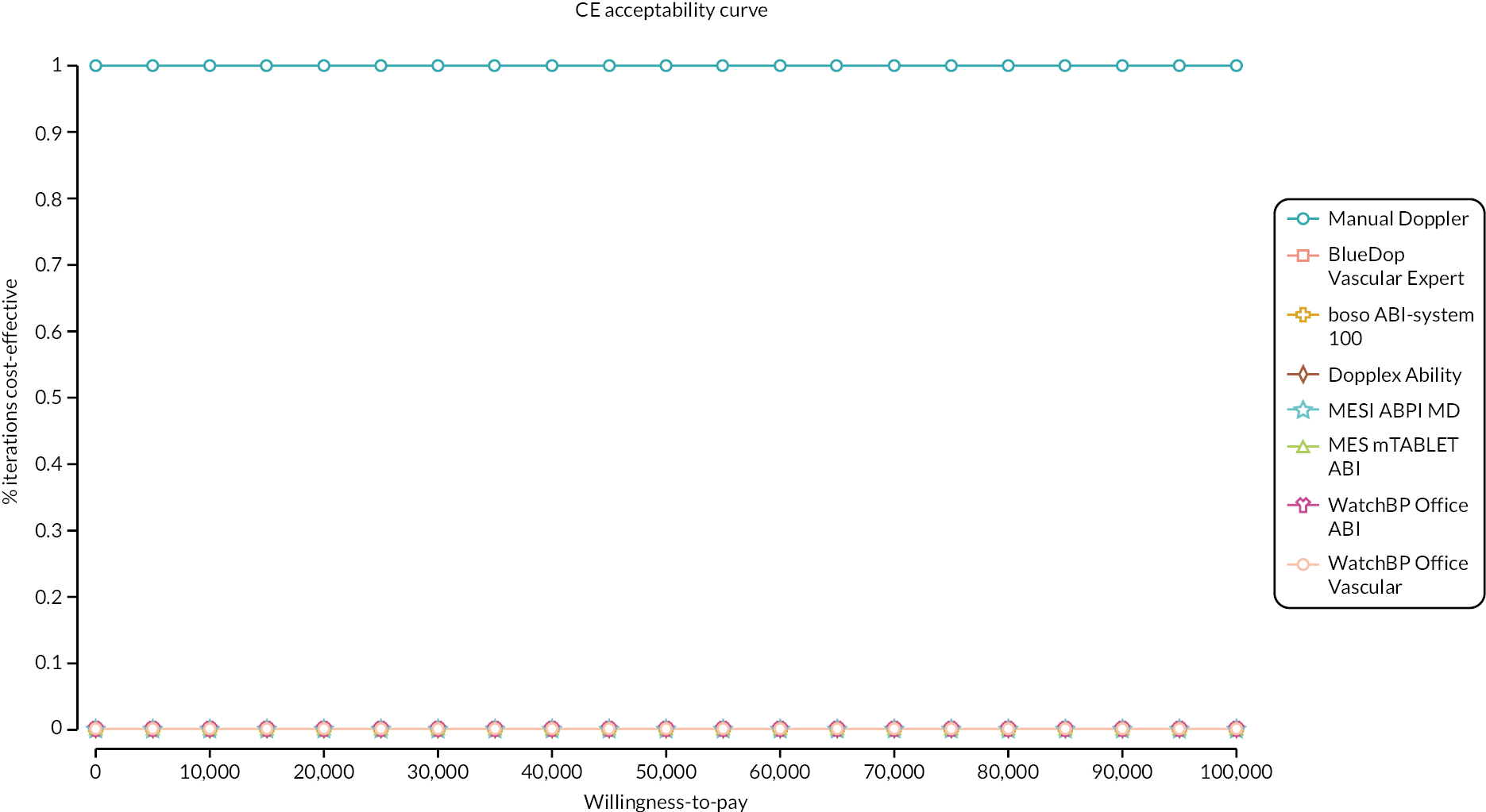
FIGURE 14.
Cost-effectiveness acceptability curves for pessimistic base case.

FIGURE 15.
Cost-effectiveness acceptability curves for optimistic base case.
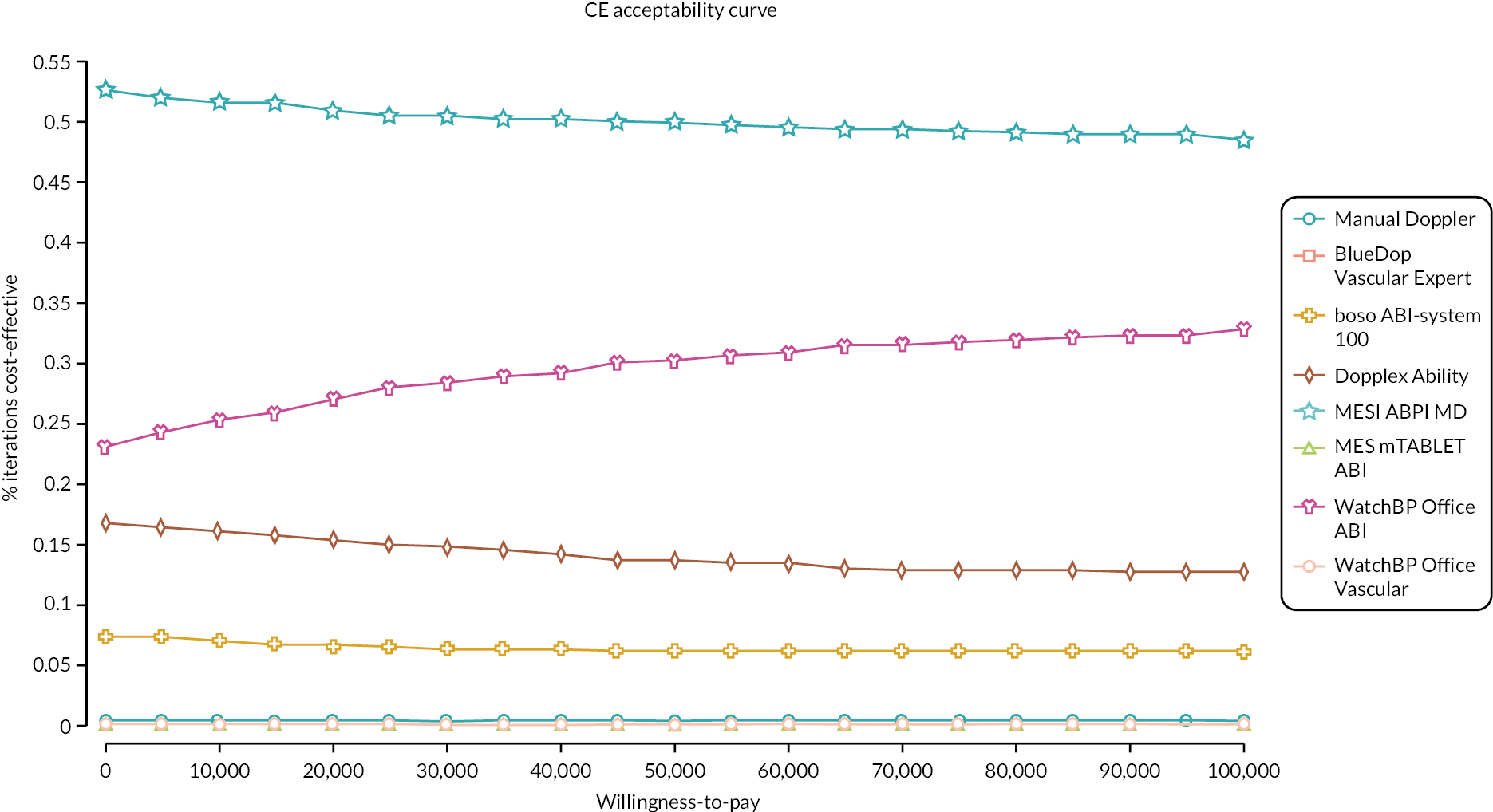
Deterministic scenario analyses
A total of 28 deterministic scenario analyses were conducted to explore and illustrate the impact of various assumptions and alternative parameter sources on results. Scenario analyses include:
-
Scenarios 1–7 describe the impact of different assumptions about the impact of the test’s diagnostic accuracy on initial patient management, varying assumptions about the proportion of inaccurate tests that are acted upon in clinical practice and exploring potential reductions in ulcer healing time if automated tests can contribute to more appropriate referrals to community leg ulcer services. Scenarios 1–7 describe the one-way changes to parameters that contribute to the pessimistic and optimistic base cases described in Table 25.
Scenarios 8–12 vary the diagnostic accuracy data used to populate the model for each test, using worst-case studies, best-case studies, pooled data from meta-analysis where available, data from studies that calculate optimal thresholds, and for studies reporting solely in a subgroup of diabetes (Scenarios 1–5 based on parameter inputs from Table 13). Scenario 13 explores the impact of applying the PAD prevalence from the diagnostic accuracy studies (weighted average prevalence, weighted by the number of patients in each study).
-
Scenarios 14–21 varying the diagnostic test costs according to different assumptions outlined in Table 21. These scenarios explore the impact of different healthcare professionals conducting the test, the time taken to complete each test, high and low estimates of test throughput and the cost implications of technical failures in terms of retesting or referral. (Scenario analyses 6–13 based on test cost scenario descriptions in Table 21.) Scenario 22 explores the impact of all positive test patients requiring a Duplex ultrasound in addition to an outpatient consultation with vascular services to confirm the positive ABPI result.
-
Scenarios 23 and 24 vary the sources of mortality parameters used for arterial disease in the model, relying on published literature associated with health state mortality rather than applying procedure-specific mortality risks.
-
Scenario 25 removes the possibility for primary amputation in the model, assuming all patients first receive an attempt at revascularisation through angioplasty or bypass surgery.
-
Scenario 26 explores the impact of assuming that all arterial procedures require non-elective intervention in the presence of a FN test result, utilising data from the National Vascular Registry which shows poorer outcomes post non-elective treatment. 98
-
Scenarios 27 and 28 reduce the time horizon to 5 years and provide undiscounted ICERs, respectively.
-
Scenario analyses are applied deterministically to the moderate base-case configuration described above and are reported in Table 26. Further additional scenario analysis results around the likely reductions in ulcer healing time that would be required for automated devices to be cost-effective are reported in Appendix 8 (see Tables 36–41).
| Test | Total cost (£) | Incremental cost (£) | Total QALY | Incremental QALY | ICER (ranked) | ICER (vs. manual) |
|---|---|---|---|---|---|---|
| Base case (moderate) – deterministic | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,099 | 138 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,392 | 431 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,424 | 463 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,427 | 466 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,501 | 540 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario 1 – Time gains for TN tests of 16 weeks (112 days) | ||||||
| BlueDop Vascular Expert | 11,255 | 8.051 | Dominant | |||
| WatchBP Office ABI | 11,357 | 102 | 8.053 | 0.002 | £46,934 | Dominant |
| WatchBP Office Vascular | 11,357 | 0 | 8.053 | 0.000 | Dominated | Dominant |
| boso ABI-system 100 | 11,495 | 139 | 8.052 | −0.001 | Dominated | Dominant |
| MESI ABPI MD | 11,498 | 141 | 8.053 | 0.000 | Dominated | Dominant |
| MESI mTABLET ABI | 11,500 | 144 | 8.053 | 0.000 | Dominated | Dominant |
| Dopplex Ability | 11,580 | 224 | 8.053 | 0.000 | Dominated | Dominant |
| Manual Doppler | 11,961 | 605 | 8.032 | −0.021 | Dominated | Dominant |
| Scenario 2 – Assume FP test results are not acted upon | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,058 | 97 | 8.031 | −0.001 | Dominated | Dominated |
| WatchBP Office ABI | 12,263 | 302 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,263 | 302 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,367 | 406 | 8.028 | −0.004 | Dominated | Dominated |
| MESI ABPI MD | 12,412 | 451 | 8.028 | −0.004 | Dominated | Dominated |
| MESI mTABLET ABI | 12,414 | 453 | 8.028 | −0.004 | Dominated | Dominated |
| Dopplex Ability | 12,484 | 523 | 8.027 | −0.005 | Dominated | Dominated |
| Scenario 3 – Assume mixed FN test results are not acted upon | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| MESI ABPI MD | 11,968 | 7 | 8.032 | 0.000 | Dominated | Dominated |
| MESI mTABLET ABI | 11,970 | 9 | 8.032 | 0.000 | Dominated | Dominated |
| Dopplex Ability | 11,974 | 13 | 8.032 | 0.000 | Dominated | Dominated |
| WatchBP Office ABI | 11,977 | 16 | 8.032 | 0.000 | Dominated | Dominated |
| WatchBP Office Vascular | 11,977 | 16 | 8.032 | 0.000 | Dominated | Dominated |
| boso ABI-system 100 | 11,988 | 27 | 8.032 | −0.001 | Dominated | Dominated |
| BlueDop Vascular Expert | 12,011 | 50 | 8.031 | −0.001 | Dominated | Dominated |
| Scenario 4 – Base-case, optimistic combination of assumptions as described in Table 24 (Scenario 1 + 2 + 3) | ||||||
| MESI ABPI MD | 11,050 | 8.053 | Dominant | |||
| MESI mTABLET ABI | 11,052 | 3 | 8.053 | 0.000 | Dominated | Dominant |
| WatchBP Office ABI | 11,060 | 10 | 8.053 | 0.000 | Dominated | Dominant |
| WatchBP Office Vascular | 11,060 | 10 | 8.053 | 0.000 | Dominated | Dominant |
| Dopplex Ability | 11,061 | 12 | 8.053 | 0.000 | Dominated | Dominant |
| boso ABI-system 100 | 11,086 | 36 | 8.053 | −0.001 | Dominated | Dominant |
| BlueDop Vascular Expert | 11,130 | 80 | 8.052 | −0.002 | Dominated | Dominant |
| Manual Doppler | 11,961 | 911 | 8.032 | −0.021 | Dominated | |
| Scenario 5 – Assume all FP test results remain unhealed at 24 weeks | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,248 | 287 | 8.027 | −0.005 | Dominated | Dominated |
| WatchBP Office ABI | 12,320 | 359 | 8.028 | −0.004 | Dominated | Dominated |
| WatchBP Office Vascular | 12,320 | 359 | 8.028 | −0.004 | Dominated | Dominated |
| MESI ABPI MD | 12,469 | 508 | 8.026 | −0.006 | Dominated | Dominated |
| MESI mTABLET ABI | 12,471 | 510 | 8.026 | −0.006 | Dominated | Dominated |
| boso ABI-system 100 | 12,481 | 520 | 8.026 | −0.007 | Dominated | Dominated |
| Dopplex Ability | 12,560 | 599 | 8.025 | −0.007 | Dominated | Dominated |
| Scenario 6 – Assume all FN arterial ulcers are acted upon | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,150 | 189 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,449 | 488 | 8.027 | −0.005 | Dominated | Dominated |
| WatchBP Office Vascular | 12,449 | 488 | 8.027 | −0.005 | Dominated | Dominated |
| boso ABI-system 100 | 12,626 | 665 | 8.025 | −0.007 | Dominated | Dominated |
| MESI ABPI MD | 12,689 | 728 | 8.024 | −0.008 | Dominated | Dominated |
| MESI mTABLET ABI | 12,692 | 731 | 8.024 | −0.008 | Dominated | Dominated |
| Dopplex Ability | 12,806 | 845 | 8.023 | −0.009 | Dominated | Dominated |
| Scenario 7 – Base-case pessimistic combination of assumptions as described in Table 24 (Scenario 5 + 6) | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,299 | 338 | 8.026 | −0.006 | Dominated | Dominated |
| WatchBP Office ABI | 12,494 | 533 | 8.026 | −0.006 | Dominated | Dominated |
| WatchBP Office Vascular | 12,494 | 533 | 8.026 | −0.006 | Dominated | Dominated |
| boso ABI-system 100 | 12,715 | 754 | 8.023 | −0.009 | Dominated | Dominated |
| MESI ABPI MD | 12,734 | 773 | 8.023 | −0.009 | Dominated | Dominated |
| MESI mTABLET ABI | 12,736 | 775 | 8.023 | −0.009 | Dominated | Dominated |
| Dopplex Ability | 12,866 | 905 | 8.022 | −0.011 | Dominated | Dominated |
| Scenario 8 – Diagnostic accuracy scenario 1 (low) | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,582 | 621 | 8.026 | −0.006 | Dominated | Dominated |
| MESI ABPI MD | 12,604 | 642 | 8.025 | −0.008 | Dominated | Dominated |
| MESI mTABLET ABI | 12,606 | 645 | 8.025 | −0.008 | Dominated | Dominated |
| boso ABI-system 100 | 12,672 | 711 | 8.025 | −0.007 | Dominated | Dominated |
| Dopplex Ability | 12,759 | 798 | 8.024 | −0.008 | Dominated | Dominated |
| WatchBP Office ABI | 12,954 | 993 | 8.022 | −0.010 | Dominated | Dominated |
| WatchBP Office Vascular | 12,954 | 993 | 8.022 | −0.010 | Dominated | Dominated |
| Scenario 9 – Diagnostic accuracy scenario 2 (high) | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,099 | 138 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,365 | 404 | 8.028 | −0.004 | Dominated | Dominated |
| Dopplex Ability | 12,376 | 415 | 8.028 | −0.004 | Dominated | Dominated |
| MESI ABPI MD | 12,424 | 463 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,427 | 466 | 8.027 | −0.005 | Dominated | Dominated |
| Scenario 10 – Diagnostic accuracy scenario 3 (pooled; exclude BlueDop Vascular Expert, boso ABI – system 100 and Dopplex Ability) | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| MESI ABPI MD | 12,567 | 606 | 8.026 | −0.006 | Dominated | Dominated |
| MESI mTABLET ABI | 12,569 | 608 | 8.026 | −0.006 | Dominated | Dominated |
| WatchBP Office ABI | 12,796 | 835 | 8.024 | −0.008 | Dominated | Dominated |
| WatchBP Office Vascular | 12,796 | 835 | 8.024 | −0.008 | Dominated | Dominated |
| Scenario 11 – Diagnostic accuracy scenario 4 (optimal threshold; exclude BlueDop Vascular Expert) | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| WatchBP Office ABI | 12,147 | 186 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,151 | 190 | 8.030 | −0.002 | Dominated | Dominated |
| MESI ABPI MD | 12,151 | 190 | 8.030 | −0.002 | Dominated | Dominated |
| MESI mTABLET ABI | 12,172 | 211 | 8.030 | −0.003 | Dominated | Dominated |
| Dopplex Ability | 12,174 | 213 | 8.030 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,393 | 432 | 8.027 | −0.005 | Dominated | Dominated |
| Scenario 12 – Diagnostic accuracy scenario 5 (diabetes subgroup) | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| WatchBP Office ABI | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| BlueDop Vascular Expert | 12,503 | 542 | 8.026 | −0.006 | Dominated | Dominated |
| MESI ABPI MD | 12,543 | 582 | 8.026 | −0.006 | Dominated | Dominated |
| MESI mTABLET ABI | 12,545 | 584 | 8.026 | −0.006 | Dominated | Dominated |
| boso ABI-system 100 | 12,672 | 711 | 8.025 | −0.007 | Dominated | Dominated |
| Dopplex Ability | 13,378 | 1417 | 8.018 | −0.014 | Dominated | Dominated |
| Scenario 13 – PAD prevalence obtained as weighted average of diagnostic accuracy studies | ||||||
| Manual Doppler | 10,533 | 8.192 | ||||
| BlueDop Vascular Expert | 10,651 | 118 | 8.190 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 10,762 | 230 | 8.190 | −0.002 | Dominated | Dominated |
| WatchBP Office Vascular | 10,762 | 230 | 8.190 | −0.002 | Dominated | Dominated |
| boso ABI-system 100 | 10,849 | 316 | 8.189 | −0.003 | Dominated | Dominated |
| MESI ABPI MD | 10,865 | 332 | 8.189 | −0.003 | Dominated | Dominated |
| MESI mTABLET ABI | 10,867 | 335 | 8.189 | −0.003 | Dominated | Dominated |
| Dopplex Ability | 10,922 | 389 | 8.188 | −0.004 | Dominated | Dominated |
| Scenario 14 – Test cost scenario 1: Assume a grade 7 nurse conducts tests | ||||||
| Manual Doppler | 11,971 | 8.032 | ||||
| BlueDop Vascular Expert | 12,101 | 130 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,284 | 313 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,284 | 313 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,395 | 424 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,428 | 456 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,431 | 460 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,504 | 533 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario 15 – Test cost scenario 2: Assume a consultant conducts tests | ||||||
| Manual Doppler | 11,997 | 8.032 | ||||
| BlueDop Vascular Expert | 12,107 | 110 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,305 | 308 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,305 | 309 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,404 | 407 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,436 | 440 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,443 | 447 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,512 | 515 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario 16 – Test cost scenario 3: Test times from the companies | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,099 | 138 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,274 | 313 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,274 | 313 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,390 | 429 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,421 | 460 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,423 | 462 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,499 | 538 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario 17 – Test cost scenario 4: Assume 2 nurses required for manual Doppler | ||||||
| Manual Doppler | 11,981 | 8.032 | ||||
| BlueDop Vascular Expert | 12,099 | 118 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,276 | 295 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,276 | 295 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,392 | 410 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,424 | 443 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,427 | 445 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,501 | 519 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario 18 – Test cost scenario 5: Apply low test throughput | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,104 | 143 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,277 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,277 | 316 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,396 | 434 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,426 | 464 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,428 | 467 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,502 | 541 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario 19 – Test cost scenario 6: Apply high test throughput | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,099 | 137 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,391 | 430 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,424 | 463 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,427 | 465 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,501 | 540 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario 20 – Test cost scenario 7: 50% of technical failures require referral to vascular services + Duplex ultrasound | ||||||
| Manual Doppler | 11,966 | 8.032 | ||||
| BlueDop Vascular Expert | 12,145 | 179 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,292 | 326 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,292 | 326 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,407 | 442 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,455 | 489 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,457 | 491 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,508 | 542 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario 21 – Test cost scenario 8: 100% of technical failures require referral to vascular services + Duplex ultrasound | ||||||
| Manual Doppler | 11,971 | 8.032 | ||||
| BlueDop Vascular Expert | 12,190 | 220 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,308 | 337 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,308 | 337 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,423 | 453 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,485 | 514 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,487 | 517 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,516 | 545 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario 22 – Increased costs of referral post a positive test result (to include the costs of a Duplex ultrasound in secondary care) | ||||||
| Manual Doppler | 11,985 | 8.032 | ||||
| BlueDop Vascular Expert | 12,131 | 147 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,302 | 317 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,302 | 317 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,420 | 436 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,450 | 466 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,453 | 468 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,528 | 543 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario 23 – Apply mortality parameters for arterial disease according to disease state rather than procedure specific | ||||||
| Manual Doppler | 12,220 | 8.055 | ||||
| BlueDop Vascular Expert | 12,360 | 140 | 8.054 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,541 | 321 | 8.052 | −0.004 | Dominated | Dominated |
| WatchBP Office Vascular | 12,541 | 321 | 8.052 | −0.004 | Dominated | Dominated |
| boso ABI-system 100 | 12,659 | 439 | 8.050 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,692 | 473 | 8.050 | −0.006 | Dominated | Dominated |
| MESI mTABLET ABI | 12,695 | 475 | 8.050 | −0.006 | Dominated | Dominated |
| Dopplex Ability | 12,770 | 551 | 8.049 | −0.006 | Dominated | Dominated |
| Scenario 24 – Probability of mortality post amputation sourced from external literature | ||||||
| Manual Doppler | 11,497 | 8.014 | ||||
| BlueDop Vascular Expert | 11,617 | 120 | 8.012 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 11,752 | 255 | 8.009 | −0.005 | Dominated | Dominated |
| WatchBP Office Vascular | 11,752 | 255 | 8.009 | −0.005 | Dominated | Dominated |
| boso ABI-system 100 | 11,846 | 349 | 8.007 | −0.008 | Dominated | Dominated |
| MESI ABPI MD | 11,868 | 371 | 8.006 | −0.008 | Dominated | Dominated |
| MESI mTABLET ABI | 11,871 | 374 | 8.006 | −0.008 | Dominated | Dominated |
| Dopplex Ability | 11,931 | 434 | 8.005 | −0.010 | Dominated | Dominated |
| Scenario 25 – Remove potential for primary amputation from the model | ||||||
| Manual Doppler | 11,712 | 8.038 | ||||
| BlueDop Vascular Expert | 11,840 | 128 | 8.036 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 11,994 | 282 | 8.035 | −0.002 | Dominated | Dominated |
| WatchBP Office Vascular | 11,994 | 282 | 8.035 | −0.002 | Dominated | Dominated |
| boso ABI-system 100 | 12,098 | 386 | 8.034 | −0.003 | Dominated | Dominated |
| MESI ABPI MD | 12,125 | 413 | 8.034 | −0.004 | Dominated | Dominated |
| MESI mTABLET ABI | 12,127 | 415 | 8.034 | −0.004 | Dominated | Dominated |
| Dopplex Ability | 12,194 | 482 | 8.034 | −0.004 | Dominated | Dominated |
| Scenario 26 – In the presence of a FN test, assume all surgical procedures become non-elective | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,127 | 166 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,370 | 409 | 8.027 | −0.005 | Dominated | Dominated |
| WatchBP Office Vascular | 12,370 | 409 | 8.027 | −0.005 | Dominated | Dominated |
| boso ABI-system 100 | 12,519 | 558 | 8.025 | −0.007 | Dominated | Dominated |
| MESI ABPI MD | 12,568 | 607 | 8.024 | −0.008 | Dominated | Dominated |
| MESI mTABLET ABI | 12,571 | 610 | 8.024 | −0.008 | Dominated | Dominated |
| Dopplex Ability | 12,667 | 706 | 8.023 | −0.009 | Dominated | Dominated |
| Scenario 27– 5-year time horizon | ||||||
| Manual Doppler | 6726 | 3.159 | ||||
| BlueDop Vascular Expert | 6836 | 110 | 3.158 | −0.001 | Dominated | Dominated |
| WatchBP Office ABI | 6946 | 220 | 3.158 | −0.001 | Dominated | Dominated |
| WatchBP Office Vascular | 6946 | 220 | 3.158 | −0.001 | Dominated | Dominated |
| boso ABI-system 100 | 7029 | 303 | 3.158 | −0.002 | Dominated | Dominated |
| MESI ABPI MD | 7045 | 319 | 3.158 | −0.001 | Dominated | Dominated |
| MESI mTABLET ABI | 7047 | 321 | 3.158 | −0.001 | Dominated | Dominated |
| Dopplex Ability | 7099 | 373 | 3.158 | −0.002 | Dominated | Dominated |
| Scenario 28 – 0% discount rate on costs and QALYs | ||||||
| Manual Doppler | 14,178 | 10.431 | ||||
| BlueDop Vascular Expert | 14,326 | 147 | 10.428 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 14,524 | 345 | 10.426 | −0.004 | Dominated | Dominated |
| WatchBP Office Vascular | 14,524 | 345 | 10.426 | −0.004 | Dominated | Dominated |
| boso ABI-system 100 | 14,651 | 472 | 10.425 | −0.006 | Dominated | Dominated |
| MESI ABPI MD | 14,689 | 510 | 10.424 | −0.006 | Dominated | Dominated |
| MESI mTABLET ABI | 14,691 | 513 | 10.424 | −0.006 | Dominated | Dominated |
| Dopplex Ability | 14,772 | 594 | 10.423 | −0.007 | Dominated | Dominated |
For most scenarios, the results remain consistent when applied to the ‘moderate’ base-case analysis, with manual Doppler testing being the least costly and also achieving small QALY gains over all automated tests. The magnitude of additional costs and QALY losses for automated tests is largely dependent on the sensitivity of the automated test. That is because low-sensitivity tests lead to an increased risk of invasive and costly arterial procedures, if strong compression is applied to an arterial ulcer due to a FN test result. For a small proportion of the cohort, this could ultimately lead to an increased risk of amputation, with substantial lifelong costs of health and social care. These additional risks quickly offset any cost savings due to shorter test times for automated tests. Conversely, the ‘optimistic’ scenario shows that there may be potential for automated tests with a high specificity (TN) to generate reductions in venous ulcer healing time, reducing costs and improving quality of life in the initial decision tree phase of the model. The extent to which these hypothetical time gains could be realised in clinical practice is unclear. However, in a scenario where healthcare professionals do not have access to the skills to conduct manual Doppler tests, some efficiencies in referral pathways may be plausible if the tests are highly accurate.
Expected costs and outcomes across different branches in the model
The complexity of the model structure prevents easily categorising each test strategy within a single Markov trace, due to the cloning of model arms in the structure. Hence, instead of presenting cohort traces for each test, and for the pathway at each branch of the model separately, we detail the expected outcomes at different key points in the pathway. Table 27 details the expected costs, QALYs and probability of amputation for different branches in the model for arterial, mixed and venous disease, respectively, categorised depending on whether inaccurate tests are acted upon or not.
| Model pathway | Expected value | ||||
|---|---|---|---|---|---|
| Disease | Test categorisation | Act upon inaccurate result? | Cost (£) | QALY | Proportion with amputation |
| Arterial disease (F4) | TP | – | 35,792 | 5.82 | 0.16 |
| Arterial disease (F4) | FN | Yes | 54,944 | 5.58 | 0.26 |
| Arterial disease (F4) | FN | No | 35,792 | 5.82 | 0.16 |
| Mixed disease (F2) | TP | – | 19,366 | 6.28 | 0.06 |
| Mixed disease (F3) | TP | – | 34,694 | 6.09 | 0.13 |
| Mixed disease (F4) | TP | – | 31,225 | 5.95 | 0.12 |
| Mixed disease (all stages) | TP | – | 28,266 | 6.06 | 0.10 |
| Mixed disease (all stages) | FN | Yes | 39,265 | 5.95 | 0.16 |
| Mixed disease (all stages) | FN | No | 28,266 | 6.06 | 0.10 |
| Venous disease | TN | – | 7044 | 8.58 | 0.00 |
| Venous disease | FP | Yes | 7878 | 8.57 | 0.00 |
| Venous disease | FP | No | 7352 | 8.58 | 0.00 |
Subgroup analyses
There were insufficient data to explore the impact of subgroup analyses on results.
Interpretation of the results
In summary, the results are highly uncertain, and it is impossible to ascertain the most likely ICER given the available evidence. The range of variation in incremental costs across different plausible sets of assumptions is substantial, and the probabilistic analyses indicate substantial uncertainties regarding the optimal test strategy, particularly in scenarios where a set of optimistic assumptions for automated tests are applied. The moderate and pessimistic base-case analyses show that, while automated tests may be slightly less costly to use in clinical practice, due to a theoretical time saving in patient appointment duration, these cost savings are quickly offset by the potential for substantial additional costs associated with inaccurate test results and, in particular, inappropriate compression of an arterial ulcer that could, in a small percentage of cases, lead to amputation. For this reason and given that the base-case analysis is generated using information from studies showing quite a low sensitivity of the automated tests, unless a high proportion of FP and FN tests could be reliably identified in clinical practice through holistic patient assessment, it is unlikely that the automated tests would be a cost-effective use of resource.
Importantly, this conclusion assumes that automated tests cannot out-perform the imperfect reference standard (manual Doppler testing). It also assumes that there are tangible consequences of inaccurate test results and that inaccurate test results (FP and FN) would lead to changes in patient care and outcomes. The extent to which these negative consequences would be realised in clinical practice is unclear and dependent on whether testing errors would be identified during holistic patient assessment. Similarly, the extent to which automated tests could deliver reductions in time to compression of venous ulcers without referral to secondary care is unclear, and scenarios around this parameter are speculative.
It is feasible that any of the scenarios explored might be plausible in specific settings or circumstances, and so it is important to consider the cost-effectiveness results with caution, and in light of the substantial uncertainty underlying the impact of the tests on ulcer outcomes, the potential for inaccurate results to be identified through holistic patient assessment, and whether improvements in referral of TN venous ulcer patients could offset the risk of further invasive management of arterial disease due to inappropriate compression of arterial or mixed ulcers in the presence of a FN test result.
Chapter 5 Discussion
Statement of principal findings
The primary focus of this assessment was to evaluate the performance of automatic devices to measure ABPI and detect the presence of PAD in people with leg ulcers and to assess their cost-effectiveness compared to manual Doppler testing. Five different automated devices were evaluated; these include the BlueDop Vascular Expert, BOSO ABI-System 100, Dopplex Ability, MESI ABPI MD and WatchBP Office ABI devices. Current evidence related to people with leg ulcers is limited to two studies assessing two different devices (Dopplex Ability and MESI ABPI MD); both these studies do not assess the accuracy of the automated devices for the diagnosis of PAD but report the concordance of their readings compared with those of manual Doppler as the reference device. 44,45 In general, automated devices were found to give different ABPI readings than the manual Doppler (higher readings in most cases) with only a proportion of the automated readings considered equal or similar to the manual Doppler readings (34% in one study and 17% in the other). The authors of these studies conclude that while the use of automated devices in general practice may have the potential to improve access to treatment, cut down costs and reduce delays for patients, the manual Doppler is still the preferable measurement tool, especially in people with symptoms of PAD, until more robust evidence on the efficacy of the automated devices becomes available.
The 22 studies assessing people without leg ulcers showed variable results. Methodological quality varied across studies. In general, automated devices demonstrated good specificity but only moderate sensitivity. Sensitivity estimates varied considerably across studies and devices. The results of our meta-analysis including 12 studies with a total of 2004 participants showed a pooled sensitivity of 0.64% (95% CI 57% to 71%) and a pooled specificity of 96% (95% CI 92% to 98%) for the detection of PAD using an automated ABPI measurement. Most patients were from vascular or cardiovascular risk clinics. When we considered each specific type of device separately, we observed a pooled sensitivity of 67% and a pooled specificity of 94% for detection of PAD using the MESI ABPI MD device and a pooled sensitivity of 53% and a pooled specificity of 98% using the WatchBP Office ABI device. Not enough studies were available for conducting meta-analyses assessing the accuracy of the other devices under investigation. Our meta-analysis results are in line with the findings of recent systematic reviews and meta-analyses assessing the accuracy of automated ABPI measurement against a reference device. A meta-analysis published in 2012 by Verberk et al. showed that the average sensitivity and specificity estimates of the automated ABPI measurement for PAD diagnosis were 69% and 96%, respectively. 124 Another meta-analysis published by Herráiz-Adillo et al. in 2017 showed an overall sensitivity of 0.65% (95% CI 57% to 74%) and specificity of 96% (95% CI 93% to 99%). 125 It is worth noting, however, that the existing published meta-analyses included various types of automated devices with only limited overlapping with those included in our meta-analysis.
The uncertainties in the diagnostic accuracy evidence base mean it is difficult to draw any firm conclusions on cost-effectiveness. A lack of evidence on the impact of the tests on important patient outcomes, the extent to which inaccurate test results would be identified in practice and the implications of acting of inaccurate test results contribute further uncertainty to the assessment of cost-effectiveness. For most scenarios, automated tests may appear to be slightly cheaper to deliver in clinical practice but are quickly offset by any risks and costs associated with withholding compression (FP) or inappropriately applying compression (FN). Given the current evidence base, it is therefore unlikely that the automated tests would generate QALY gains or cost savings, unless a high proportion of FP and FN tests could be reliably identified in clinical practice through holistic patient assessment, and automated tests could deliver improvements in patient referral over manual Doppler testing.
Strength and limitations of the assessment
The methods used to conduct this assessment were detailed and thorough. The main weakness of the systematic review of clinical effectiveness evidence was the limited evidence available for each automated device, especially in people with leg ulcers. Moreover, due to the heterogeneity of the included studies in terms of the characteristics of the patient population, setting, prevalence of PAD and testing protocols, it is hard to draw any firm conclusions on the performance of the automated devices in clinical practice and the findings of this assessment should be interpreted with caution. In addition, there was insufficient information reported in the two studies in people with leg ulcers to fully ascertain their risk of bias in some of the specified domains.
Most studies used manual Doppler as the reference device. Although manual Doppler is commonly used in clinical practice, it is not the best available method to detect the presence of PAD. More reliable methods for the diagnosis of PAD include Duplex ultrasound, angiography, CTA and MRA. Therefore, the results of our included studies should be considered with caution because the direction of bias introduced by the use of an imperfect reference standard is not straightforward.
While diabetes is known to influence the accuracy of ABPI measurement, only a few studies assessed diabetic patients. The presence of diabetics does not seem to have a significant effect on the accuracy of ABPI measurements in studies that provide subgroup analyses for diabetic and non-diabetic patients. However, it is worth pointing out that the proportion of diabetic patients in these studies was generally low. On the contrary, the study by Babaei et al. , which focused exclusively on a large sample of diabetes patients, reported the lowest sensitivity estimate (20%). 54 This low diagnostic performance could be explained by the presence of calcified, incompressible arteries in diabetes patients resulting in higher, less diagnostic, ABPI levels.
Uncertainties
The moderate sensitivity of the automated ABPI measurement and consequent high FNs rate also raises the question of whether these devices should be used as screening tools to rule out the presence of PAD in non-specialised settings (general practice, community setting). It is worth pointing out that we were not able to assess the impact that the routine use of the automated devices may have on clinical outcomes; in particular, we did not identify any study assessing the consequences of FN results (delayed diagnosis of PAD) in clinical practice.
Most of the included studies found a relatively good correlation between the readings of the automated device and those of the reference device (manual Doppler in most cases); however, the use of correlation coefficients may be inadequate or misleading for assessing agreement between diagnostic methods because they evaluate only the linear association between sets of observations. The Bland–Altman plot is considered a better method to describe the level of agreement between two measurements. 126 Across studies, the analysis of the Bland–Altman plot showed a systematic trend towards higher automated ABPI readings, which according to the current threshold of 0.9, would underestimate the presence of PAD. While clinicians in specialised settings may adopt different strategies to determine whether the peripheral arterial status in patients with higher ABPI measurements is compromised, it is unclear whether less specialised professionals such as community or general practice nurses would be able to convey the same clinical judgement. It is worth noting that some of the studies included in this assessment found that the best threshold for detection of PAD using automated ABPI was 1.0 (or close to 1.0). 48–50,53,54,56,58,59,61,64 However, optimised criteria for automated ABPI measurement need to be prospectively validated in non-specialised settings.
Rates of erroneous automated measurements varied across studies but often they were not negligible indicating the need for further assessment, especially in less specialised settings. Some investigators also found that the frequency of errors with automated devices was higher in patients with PAD compared with those without PAD. 56
In general, the automated devices required less time to measure ABPI compared with the manual Doppler device mainly because of the shorter duration of the resting period. However, considering the need for consultation time before starting ABPI measurement in primary or community care, it is questionable whether this represents a real advantage.
In all included studies, the automated and reference ABPI measurements were performed by experienced or trained professionals. It is unclear whether measurements performed by less skilled professionals would produce the same findings.
Conclusions and implications for future research
There is an increasing interest in the use of automated devices to measure ABPI in non-specialised settings such as primary care and community settings. These devices do not require extensive training and are less time-consuming than the current manual Doppler device. However, information on the performance of automated devices in people with leg ulcers was too scant to draw any meaningful conclusions. We found that automated devices for ABPI measurement have good specificity but only modest sensitivity for diagnosing PAD in people without leg ulcers, but the current evidence base is considerably heterogeneous in terms of patient populations, settings, prevalence of PAD and testing procedures.
Additional research is required to clarify whether these devices can play a role in the management of both people with leg ulcers and people without leg ulcers who require ABPI measurement in non-specialised settings (community care, primary care). In particular, more robust evidence is needed to establish whether automated devices should be used for the general screening of clinical populations with any vascular concerns or considered an acceptable alternative or adjunct to manual Doppler in people with symptoms of PAD. In addition, the use of a different threshold, from the current recommended threshold of 0.9, should require prospective validation. Consideration should also be given to relevant subgroup of patients (i.e. people with diabetes, rheumatoid arthritis, systemic vasculitis, atherosclerotic disease, advanced chronic renal failure or other conditions in which arterial calcification is common, and people with limb amputation or other conditions where blood pressure cannot be measured on both arms and legs).
Ideally, future research should consider comparing each automated device with manual Doppler against an acceptable reference standard (i.e. Duplex ultrasound). When designing future studies, it would be useful if the experience of professionals using the devices to measure ABPI could mirror those of the professionals who are expected to use the device in clinical practice including community and general practice nurse. Studies should endeavour where possible to also assess the impact of the tests on patient outcomes such as ulcer healing times, risk of CLI and treatment requirements.
The current evidence base is also inadequate to fully appraise the economic value of the use of the automated devices under investigation to provide cost-effective improvement in the clinical management of people with leg ulcers who require ABPI measurement. As such we have provided a large number of scenarios to illustrate the range of possible cost and QALY implications under different assumptions about how the tests may influence patient care. In most scenarios, the risks of providing inappropriate care on the basis of inaccurate test results offset the cost savings of faster testing, leading to overall additional costs to the NHS and potential for QALY losses. Unless a high proportion of inaccurate test results could be identified during a holistic patient assessment, and substantial gains in efficiency could be achieved in the care pathway reducing time to compression of venous ulcers, it is unlikely, given the current evidence base, that the tests could be considered cost-effective.
Future economic evaluations and modelling would also benefit from more robust information on the implications of inaccurate test results on patient outcomes (such as ulcer healing and risks of requiring escalated care due to inappropriate compression) and robust evidence regarding the costs and quality-of-life outcomes for patients specifically with mixed aetiology disease.
Reporting equality, diversity and inclusion
This is an evidence synthesis and economic analysis. The scope of the project was defined by NICE, which is committed to promoting equality of opportunity, eliminating unlawful discrimination and fostering good relations between people with particular protected characteristics and others.
People with sickle cell disease are prone to leg ulcers. Sickle cell disease is more common in people with an African or Caribbean family background. The risk of cardiovascular disease, including PAD, is greater in men, people from South Asian family background and areas of socioeconomic deprivation. The risk increases with age. People with diabetes have an increased risk of cardiovascular disease, including PAD. Some people with leg ulcers may find it difficult to lie flat for the length of time needed to complete a manual Doppler test. Swelling of the leg, obesity or complex ulceration may make it difficult or painful to wear blood pressure cuffs. Automated and manual devices may not be suitable or work accurately for people who have had lymph nodes removed or damaged (and are at risk of lymphoedema), limb amputation or other conditions where blood pressure cannot be measured on both arms or legs.
The team involved in this research project included people with a range of expertise and background.
Additional information
Contributions of authors
Dwayne Boyers (https://orcid.org/0000-0002-9786-8118) (Senior Health Economist) developed the economic model, conducted cost-effectiveness analyses and interpreted their results.
Moira Cruickshank (https://orcid.org/0000-0002-5182-884X) (Research Fellow) selected relevant papers from the literature, performed data extraction and risk-of-bias assessment of all studies included in the review of clinical effectiveness evidence and synthesised their results.
Lorna Aucott (https://orcid.org/0000-0001-6277-7972) (Senior Statistician) conducted all statistical analyses for the review of clinical effectiveness evidence.
Charlotte Kennedy (https://orcid.org/0000-0002-1974-6318) (Research Assistant) reviewed the identified cost-effectiveness evidence and contributed to the acquisition of input data for the economic model under the supervision of Dwayne Boyers.
Paul Manson (https://orcid.org/0000-0002-1405-1795) (Information Specialist) developed and ran the literature searches, retrieved full-text copies of the selected papers and provided information support throughout the project.
Paul Bachoo (https://orcid.org/0000-0002-1306-2572) (Consultant in Vascular Surgeon and Medical Director Acute Sector NHS Grampian) provided expert advice and guidance on the clinical aspects of this assessment.
Miriam Brazzelli (https://orcid.org/0000-0002-7576-6751) (Reader on Research) planned the systematic review of the clinical evidence, contributed to interpreting the test accuracy results and co-ordinated all aspects of this assessment. All authors contributed to the writing of this draft report.
Acknowledgements
The authors are grateful to Bev Smith for her secretarial support.
Data-sharing statement
This is an evidence synthesis; all technical data are presented in the text or contained within tables, figures and appendices. No new data, suitable for data sharing, have been created in the preparation of this synthesis. All queries should be submitted to the corresponding author.
Ethics statement
This is a synthesis of published or publicly available evidence and no primary research data were collected as part of this project. Ethics approval was not needed.
Information governance statement
There were no personal data involved in the preparation of this report.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/TWCG3912. Primary conflicts of interest: Lorna Aucott is a member of the National Institute for Health Research Public Health Research funding committee (February 2017–present); she was also a member of the ‘COVID-19 Reviewing’ committee from 1 June 2020 to 30 September 2020. The remaining authors have no competing interests to disclose.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Aung PP, Maxwell HG, Jepson RG, Price JF, Leng GC. Lipid-lowering for peripheral arterial disease of the lower limb. Cochrane Database Syst Rev 2007;2007.
- Morley RL, Sharma A, Horsch AD, Hinchliffe RJ. Peripheral artery disease. BMJ 2018;360.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. TASC II Working Group . Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45:S5-67.
- Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol 1996;25:1172-81.
- Nelson EA, Bell-Syer SE. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev 2014;2014.
- Callam MJ, Harper DR, Dale JJ, Ruckley CV. Chronic ulcer of the leg: clinical history. Br Med J (Clin Res Ed) 1987;294:1389-91.
- Nelson EA, Adderley U. Venous leg ulcers. BMJ Clin Evid 2016;2016.
- Humphreys ML, Stewart AH, Gohel MS, Taylor M, Whyman MR, Poskitt KR. Management of mixed arterial and venous leg ulcers. Br J Surg 2007;94:1104-7.
- Lorimer KR, Harrison MB, Graham ID, Friedberg E, Davies B. Assessing venous ulcer population characteristics and practices in a home care community. Ostomy Wound Manage 2003;49:32-4.
- Vowden KR, Vowden P. The prevalence, management and outcome for patients with lower limb ulceration identified in a wound care survey within one English health care district. J Tissue Viability 2009;18:13-9.
- Callam MJ, Ruckley CV, Harper DR, Dale JJ. Chronic ulceration of the leg: extent of the problem and provision of care. Br Med J (Clin Res Ed) 1985;290:1855-6.
- O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012;11.
- National Wound Care Strategy Programme . Recommendations for Lower Limb Ulcers 2020. www.nationalwoundcarestrategy.net/wp-content/uploads/2021/04/Lower-Limb-Recommendations-WEB-25Feb21.pdf (accessed 14 March 2022).
- Callam MJ, Ruckley CV, Dale JJ, Harper DR. Hazards of compression treatment of the leg: an estimate from Scottish surgeons. Br Med J (Clin Res Ed) 1987;295.
- Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286:1317-24.
- Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol 2017;14:156-70.
- Pabon M, Cheng S, Altin SE, Sethi SS, Nelson MD, Moreau KL, et al. Sex differences in peripheral artery disease. Circ Res 2022;130:496-511.
- Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509-26.
- Kalbaugh CA, Kucharska-Newton A, Wruck L, Lund JL, Selvin E, Matsushita K, et al. Peripheral artery disease prevalence and incidence estimated from both outpatient and inpatient settings among Medicare Fee-for-Service beneficiaries in the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc 2017;6.
- Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health 2019;7:e1020-30.
- Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ Open 2020;10.
- NHS . Hospital Admitted Patient Care Activity 2020–21 2021. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity (accessed 8 August 2022).
- National Institute for Health and Care Excellence . Peripheral Arterial Disease: Diagnosis and Management [CG147] 2012. www.nice.org.uk/guidance/cg147 (accessed 14 March 2022).
- Persoon A, Heinen MM, van der Vleuten CJ, de Rooij MJ, van de Kerkhof PC, van Achterberg T. Leg ulcers: a review of their impact on daily life. J Clin Nurs 2004;13:341-54.
- Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients’ quality of life. Health Qual Life Outcomes 2007;5.
- Qiu Y, Team V, Osadnik CR, Weller CD. Barriers and enablers to physical activity in people with venous leg ulcers: a systematic review of qualitative studies. Int J Nurs Stud 2022;135.
- Danieluk A, Chlabicz S. Automated measurements of ankle–brachial index: a narrative review. J Clin Med 2021;10.
- National Institute for Health and Care Excellence . How Should I Interpret Ankle Brachial Pressure Index (ABPI) Results? 2021. https://cks.nice.org.uk/topics/leg-ulcer-venous/diagnosis/interpretation-of-abpi/ (accessed 14 March 2022).
- Healthcare Improvement Scotland, Scottish Intercollegiate Guidelines Network . Management of Chronic Venous Leg Ulcers [SIGN 120]. 2010. www.sign.ac.uk/media/1058/sign120.pdf (accessed 9 August 2022).
- The Lymphoedema Framework . Best Practice for the Management of Lymphoedema. International Consensus 2006. www.lympho.org/wp-content/uploads/2021/09/Best_practice.pdf (accessed 9 August 2022).
- Mixed Aetiology Leg Ulcers 2006. www.nursingtimes.net/archive/mixed-aetiology-leg-ulcers-18-04-2006/ (accessed 31 March 2022).
- European Wound Management Association . Understanding Compression Therapy 2003. https://ewma.org/fileadmin/user_upload/EWMA.org/Position_documents_2002-2008/Compression.pdf (accessed 14 March 2022).
- Grothier L. Leg Ulcer Management Guidelines. Colchester, UK: Provide; 2015.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care 2009. www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm (accessed 4 March 2020).
- Higgins J, Green S, Thomas J, Chandler J, Cumpston M, Li T, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 2021. https://training.cochrane.org/handbook (accessed 14 March 2022).
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36.
- Yang B, Mallett S, Takwoingi Y, Davenport CF, Hyde CJ, Whiting PF, et al. QUADAS-C Group . QUADAS-C: a tool for assessing risk of bias in comparative diagnostic accuracy studies. Ann Intern Med 2021;174:1592-9.
- Verhagen AP, De Vet HCW, De Bie RA, Kessels AGH, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235-41.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84.
- Jackson R, Ameratunga S, Broad J, Connor J, Lethaby A, Robb G, et al. The GATE frame: critical appraisal with pictures. Evid Based Med 2006;11:35-8.
- Deeks J, Bossuyt P, Leeflang M, Takwoingi Y, Flemyng E. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 2.0 2022. https://training.cochrane.org/handbook-diagnostic-test-accuracy (accessed 14 March 2022).
- Harbord R, Whiting P. Metandi: meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J 2009;9:211-29.
- StataCorp . Stata Statistical Software: Release 17 2019.
- Welsh L, Robinson L, Stephenson J. Evaluation of an automated ankle brachial pressure index calculator in a nurse-led leg ulcer clinic. Wounds UK 2016;12:80-7.
- Green J, Boast G, Calderwood R, Chambers R. Optimising the assessment and management of lower limb wounds in general practice. Primary Health Care 2020. https://doi.org/10.7748/phc.2020.e1673.
- National Library of Medicine . Clinical Evaluation of the BlueDop Vascular Expert for Assessing Peripheral Arterial Disease 2021. https://clinicaltrials.gov/ct2/show/NCT05073510 (accessed 14 March 2022).
- Diehm N, Dick F, Czuprin C, Lawall H, Baumgartner I, Diehm C. Oscillometric measurement of ankle–brachial index in patients with suspected peripheral disease: comparison with Doppler method. Swiss Med Wkly 2009;139:357-63.
- Davies JH, Williams EM. Automated plethysmographic measurement of the ankle–brachial index: a comparison with the doppler ultrasound method. Hypertens Res 2016;39:100-6.
- Lewis JE, Williams P, Davies JH. Non-invasive assessment of peripheral arterial disease: automated ankle–brachial index measurement and pulse volume analysis compared to duplex scan. SAGE Open Med 2016;4.
- Zebari F, Amlani V, Langenskiold M, Nordanstig J. Validation of an automated measurement method for determination of the ankle–brachial index. Scand Cardiovasc J 2022;56:73-8.
- Lewis J, Hawkins M, Baree P, Cawley S, Dayananda S. A Comparison Between Dopplex Ability and the Doppler Method for Obtaining Ankle Brachial Pressures. Canada: Primary Care; 2010.
- Boilley PY, Howlett J, Tollenaere Q, Miossec A, Guilcher A, Lanéelle D, et al. Comparison of ankle–brachial index measured with an automatic oscillometric method with the standard continuous Doppler method and effect of rest time before the measure in patients with exertional limb symptoms. Hypertens Res 2020;43:585-7.
- Špan M, Geršak G, Millasseau SC, Meža M, Košir A. Detection of peripheral arterial disease with an improved automated device: comparison of a new oscillometric device and the standard Doppler method. Vasc Health Risk Manag 2016;12:305-11.
- Babaei MR, Malek M, Rostami FT, Emami Z, Madani NH, Khamseh ME. Non-invasive vascular assessment in people with type 2 diabetes: diagnostic performance of plethysmographic-and-Doppler derived ankle brachial index, toe brachial index, and pulse volume wave analysis for detection of peripheral arterial disease. Prim Care Diabetes 2020;14:282-9.
- Verma MK, Gangwar V, Jasrotia RB, John NA. Assessment of peripheral artery disease risk in building construction workers by ankle–brachial index measurement with automated oscillometric and hand-held Doppler device. J Family Med Prim Care 2022;11:139-43.
- Kollias A, Xilomenos A, Protogerou A, Dimakakos E, Stergiou GS. Automated determination of the ankle–brachial index using an oscillometric blood pressure monitor: validation vs. Doppler measurement and cardiovascular risk factor profile. Hypertens Res 2011;34:825-30.
- Kordzadeh A, Hoff M, Tokidis E, King DH, Browne T, Prionidis I. Novel assessment (BlueDop) device for detection of lower limb arterial disease: a prospective comparative study. J Ultrasound Med 2018;37:763-8.
- Homza M, Machaczka O, Porzer M, Kozak M, Plasek J, Sipula D. Comparison of different methods of ABI acquisition for detection of peripheral artery disease in diabetic patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2019;163:227-32.
- Jarai Z, Kolossvary E, Szabo I, Kiss I, Farsang C, Farkas K. The potential role of oscillometric devices for ankle–brachial index measurements in clinical practice. Orv Hetil 2018;159:176-82.
- Millen RN, Thomas KN, Majumder A, Hill BG, Van Rij AM, Krysa J. Accuracy and repeatability of the Dopplex Ability. Expert Rev Med Devices 2018;15:247-51.
- Hageman D, van den Houten MML, Pesser N, Gommans LNM, Scheltinga MRM, Teijink JAW. Diagnostic accuracy of automated oscillometric determination of the ankle–brachial index in peripheral artery disease. J Vasc Surg 2021;73:652-60.
- Catillon F, Tuffier S, Guilcher A, Tollenaere Q, Metairie A, Miossec A, et al. Proficiency of medical students at obtaining pressure measurement readings using automated ankle and toe measuring devices for diagnosis of lower extremity peripheral artery disease. Ann Vasc Surg 2020;65:183-9.
- Varetto G, Magnoni F, Aluigi L, Antignani P, Benevento D, . Comparison of ankle brachial index ABI measurement between a new oscillometric device MESI ABPI Md and the standard Doppler method. Non Invasive Vascular Investig 2019;4.
- Raya R, Martinez N, Cayuelas F, Pera G, Garcia Y. Comparison of two automatic oscillometers vs the traditional method with Doppler probe in the determination of the ankle brachial index. Atencion Primaria Practica 2019;1:3-8.
- Sinski M, Styczynski G, Szmigielski C. Automated oscillometric measurement of the ankle–brachial index in patients with coronary artery disease. Hypertens Res 2013;36:25-8.
- Wohlfahrt P, Ingrischova M, Krajcoviechova A, Palous D, Dolejsova M, Seidlerova J, et al. A novel oscillometric device for peripheral arterial disease screening in everyday practice. The Czech-post MONICA study. Int Angiol 2011;30:256-61.
- Rodriguez-Roca GC, Villarin-Castro A, Carrasco-Flores J, Artigao-Rodenas LM, Carbayo-Herencia JA, Escobar-Cervantes C, et al. RICARTO (Riesgo Cardiovascular Y Eventos Cardiovasculares En La Población General del Área Sanitaria De Toledo) Project Working Group . Concordance between automated oscillometric measurement of ankle–brachial index and traditional measurement by eco-Doppler in patients without peripheral artery disease. Blood Press 2014;23:270-5.
- Boast G, Green J, Chambers R, Calderwood R. Improving assessment and management of lower limb wounds. J Community Nurs 2019;33:34-8.
- Davies JH, Kenkre J, Williams EM. Current utility of the ankle–brachial index (ABI) in general practice: implications for its use in cardiovascular disease screening. BMC Fam Pract 2014;15.
- National Institute for Health Care Excellence . NICE Diagnostic Assessment Programme Manual 2011. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-diagnostics-guidance/Diagnostics-assessment-programme-manual.pdf (accessed 4 March 2020).
- Moloney E, O’Connor J, Craig D, Robalino S, Chrysos A, Javanbakht M, et al. Systematic review of economic models used to compare techniques for detecting peripheral arterial disease. PharmacoEcon Open 2019;3:21-30.
- Itoga NK, Minami HR, Chelvakumar M, Pearson K, Mell MM, Bendavid E, et al. Cost-effectiveness analysis of asymptomatic peripheral artery disease screening with the ABI test. Vasc Med 2018;23:97-106.
- Layer A, McManus E, Levell NJ. A systematic review of model-based economic evaluations of treatments for venous leg ulcers. PharmacoEcon Open 2020;4:211-22.
- Cheng Q, Gibb M, Graves N, Finlayson K, Pacella RE. Cost-effectiveness analysis of guideline-based optimal care for venous leg ulcers in Australia. BMC Health Serv Res 2018;18.
- Iglesias CP, Birks Y, Nelson EA, Scanlon E, Cullum NA. Quality of life of people with venous leg ulcers: a comparison of the discriminative and responsive characteristics of two generic and a disease specific instruments. Qual Life Res 2005;14:1705-18.
- Harrison MB, Graham ID, Lorimer K, Friedberg E, Pierscianowski T, Brandys T. Leg-ulcer care in the community, before and after implementation of an evidence-based service. CMAJ 2005;172:1447-52.
- Finlayson K, Edwards H, Courtney M. Factors associated with recurrence of venous leg ulcers: a survey and retrospective chart review. Int J Nurs Stud 2009;46:1071-8.
- Guest JF, Rana K, Singh H, Vowden P. Cost-effectiveness of using a collagen-containing dressing plus compression therapy in non-healing venous leg ulcers. J Wound Care 2018;27:68-7.
- Clegg JP, Guest JF. Modelling the cost-utility of bio-electric stimulation therapy compared to standard care in the treatment of elderly patients with chronic non-healing wounds in the UK. Curr Med Res Opin 2007;23:871-83.
- Guest JF, Fuller GW, Vowden P. Venous leg ulcer management in clinical practice in the UK: costs and outcomes. Int Wound J 2018;15:29-37.
- Health Quality Ontario . Compression stockings for the prevention of venous leg ulcer recurrence: a Health Technology Assessment. Ontario Health Technol Assess Ser 2019;19:1-86.
- Pham B, Harrison MB, Chen MH, Carley ME. Canadian Bandaging Trial Group . Cost-effectiveness of compression technologies for evidence-informed leg ulcer care: results from the Canadian Bandaging Trial. BMC Health Serv Res 2012;12.
- Rognoni C, Lugli M, Maleti O, Tarricone R. Venous stenting for patients with outflow obstruction and leg ulcers: cost-effectiveness and budget impact analyses. J Comp Eff Res 2020;9:705-20.
- Velickovic VM, Chadwick P, Rippon MG, Ilic I, McGlone ER, Gebreslassie M, et al. Cost-effectiveness of superabsorbent wound dressing versus standard of care in patients with moderate-to-highly exuding leg ulcers. J Wound Care 2020;29:235-46.
- Atkin L, Barrett S, Chadwick P, Callaghan R, Rippon MG, Rogers AA, et al. Evaluation of a superabsorbent wound dressing, patient and clinician perspective: a case series. J Wound Care 2020;29:174-82.
- Harding K, Posnett J, Vowden K. A new methodology for costing wound care. Int Wound J 2013;10:623-9.
- Health Quality Ontario . Skin substitutes for adults with diabetic foot ulcers and venous leg ulcers: a health technology assessment. Ontario Health Technol Assess Ser 2021;21:1-165.
- Guest JF, Staines K, Murphy N. Cost-effectiveness of using intermittent pneumatic compression to manage hard-to-heal venous leg ulcers in the UK. J Wound Care 2021;30:544-52.
- Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, et al. Health economic burden that different wound types impose on the UK’s National Health Service. Int Wound J 2017;14:322-30.
- Velickovic VM, Szilcz M, Milosevic Z, Godfrey T, Siebert U. Cost-effectiveness analysis of superabsorbent wound dressings in patients with moderate-to-highly exuding leg ulcers in Germany. Int Wound J 2022;19:447-59.
- National Institute for Health and Care Excellence . Leg Ulcer: Venous 2021. https://cks.nice.org.uk/topics/leg-ulcer-venous/ (accessed 14 March 2022).
- TreeAge Software . Treeage Pro 2021 2021.
- Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, Cullum N, et al. Early versus deferred endovenous ablation of superficial venous reflux in patients with venous ulceration: the EVRA RCT. Health Technol Assess 2019;23:1-96.
- Diaconu C, Horodinschi R, Belciu D. Clinical presentation of lower extremity arterial disease (LEAD). E-J Cardiol Pract 2018;16. www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-16/Clinical-presentation-of-lower-extremity-arterial-disease-LEAD (accessed 17 August 2022).
- Kashetsky N, Sachdeva M, Lu JD, Mufti A, Kim P, Bagit A, et al. Diagnostic accuracy of ankle–brachial pressure index compared with Doppler arterial waveforms for detecting peripheral arterial disease: a systematic review. Adv Skin Wound Care 2022;35:195-201.
- Ro du H, Moon HJ, Kim JH, Lee KM, Kim SJ, Lee DY. Photoplethysmography and continuous-wave Doppler ultrasound as a complementary test to ankle–brachial index in detection of stenotic peripheral arterial disease. Angiology 2013;64:314-20.
- Ammermann F, Meinel FG, Beller E, Busse A, Streckenbach F, Teichert C, et al. Concomitant chronic venous insufficiency in patients with peripheral artery disease: insights from MR angiography. Eur Radiol 2020;30:3908-14.
- Vascular Services Quality Improvement Programme . National Vascular Registry: 2021 Annual Report 2021. www.vsqip.org.uk/reports/2021-annual-report/ (accessed 17 August 2022).
- Gohel MS, Mora MJ, Szigeti M, Epstein DM, Heatley F, Bradbury A, et al. Long-term clinical and cost-effectiveness of early endovenous ablation in venous ulceration: a randomized clinical trial. JAMA Surg 2020;155:1113-21.
- Gohel MS, Barwell JR, Taylor M, Chant T, Foy C, Earnshaw JJ, et al. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ 2007;335.
- Sigvant B, Lundin F, Wahlberg E. The risk of disease progression in peripheral arterial disease is higher than expected: a meta-analysis of mortality and disease progression in peripheral arterial disease. Eur J Vasc Endovasc Surg 2016;51:395-403.
- Howard DP, Banerjee A, Fairhead JF, Handa A, Silver LE, Rothwell PM. Oxford Vascular Study . Population-based study of incidence of acute abdominal aortic aneurysms with projected impact of screening strategy. J Am Heart Assoc 2015;4.
- Office for National Statistics . National Life Tables: UK 2021. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 28 March 2022).
- Duff S, Mafilios MS, Bhounsule P, Hasegawa JT. The burden of critical limb ischemia: a review of recent literature. Vasc Health Risk Manag 2019;15:187-208.
- Lüders F, Bunzemeier H, Engelbertz C, Malyar NM, Meyborg M, Roeder N, et al. CKD and acute and long-term outcome of patients with peripheral artery disease and critical limb ischemia. Clin J Am Soc Nephrol 2016;11:216-22.
- Reinecke H, Unrath M, Freisinger E, Bunzemeier H, Meyborg M, Lüders F, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J 2015;36:932-8.
- Beldon P. Performing a Doppler assessment. Wound Essentials 2010;5:87-90.
- Jones K, Burns A. Unit Costs of Health and Social Care 2021. Canterbury: Personal Social Services Research Unit; 2021.
- NHS . National Cost Collection for the NHS 2021. www.england.nhs.uk/costing-in-the-nhs/national-cost-collection/#ncc1819 (accessed 28 March 2022).
- Medicines Complete . British National Formulary 2018. www.medicinescomplete.com/mc/bnf/current/index.htm (accessed 4 March 2020).
- Ezeofor VS, Bray N, Bryning L, Hashmi F, Hoel H, Parker D, et al. Economic model to examine the cost-effectiveness of FlowOx home therapy compared to standard care in patients with peripheral artery disease. PLOS ONE 2021;16.
- Urwin S, Dumville JC, Sutton M, Cullum N. Health service costs of treating venous leg ulcers in the UK: evidence from a cross-sectional survey based in the north west of England. BMJ Open 2022;12.
- Wade R, Sideris E, Paton F, Rice S, Palmer S, Fox D, et al. Graduated compression stockings for the prevention of deep-vein thrombosis in postoperative surgical patients: a systematic review and economic model with a value of information analysis. Health Technol Assess 2015;19:1-220.
- Davie-Smith F, Kennon B, Wyke S, Paul L. The demographic and clinical characteristics of those with and without diabetes that undergo a lower extremity amputation in Glasgow, UK. Physiotherapy 2015;101.
- Taylor SM, Kalbaugh CA, Blackhurst DW, Hamontree SE, Cull DL, Messich HS, et al. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. J Vasc Surg 2005;42:227-35.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019/20. Canterbury: Personal Social Services Research Unit; 2019.
- Forbes JF, Adam DJ, Bell J, Fowkes FG, Gillespie I, Raab GM, et al. BASIL Trial Participants . Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: health-related quality of life outcomes, resource utilization, and cost-effectiveness analysis. J Vasc Surg 2010;51:43S-51S.
- Pisa G, Reinhold T, Obi-Tabot E, Bodoria M, Brüggenjürgen B. Critical limb ischemia and its impact on patient health preferences and quality of life – an international study. Int J Angiol 2012;21:139-46.
- Ernstsson O, Hagberg K, Janssen MF, Bonsel GJ, Korkmaz S, Zethraeus N, et al. Health-related quality of life in patients with lower limb amputation – an assessment of the measurement properties of EQ-5D-3L and EQ-5D-5L using data from the Swedish Amputation and Prosthetics Registry. Disabil Rehabil 2021;44:8471-9.
- Holler D, Claes C. von der Schulenburg JMG. Cost–utility analysis of treating severe peripheral arterial occlusive disease. Int J Angiol 2006;15:25-33.
- Simpson EL, Kearns B, Stevenson MD, Cantrell AJ, Littlewood C, Michaels JA. Enhancements to angioplasty for peripheral arterial occlusive disease: systematic review, cost-effectiveness assessment and expected value of information analysis. Health Technol Assess 2014;18:1-252.
- Büyükkaramikli NC, Rutten-van Mölken M, Severens JL, Al M. TECH-VER: a verification checklist to reduce errors in models and improve their credibility. PharmacoEcon 2019;37:1391-408.
- Tappenden P, Chilcott JB. Avoiding and identifying errors and other threats to the credibility of health economic models. PharmacoEcon 2014;32:967-79.
- Verberk WJ, Kollias A, Stergiou GS. Automated oscillometric determination of the ankle–brachial index: a systematic review and meta-analysis. Hypertens Res 2012;35:883-91.
- Herráiz-Adillo A, Cavero-Redondo I, Álvarez-Bueno C, Martínez-Vizcaíno V, Pozuelo-Carrascosa DP, Notario-Pacheco B. The accuracy of an oscillometric ankle–brachial index in the diagnosis of lower limb peripheral arterial disease: a systematic review and meta-analysis. Int J Clin Pract 2017;71.
- Giavarina D. Understanding Bland Altman analysis. Biochem Med (Zagreb) 2015;25:141-51.
- National Institute for Health and Care Excellence . Lower Limb Peripheral Arterial: Disease Diagnosis and Management [CG147] Appendices 2012. www.nice.org.uk/guidance/cg147/evidence/appendices-an-pdf-186865022 (accessed 14 March 2022).
Appendix 1 Literature search strategies
Ovid MEDLINE and Epub Ahead of Print, In-process, In-data-review and Other Non-indexed Citations, Daily and Versions <1946–22 April 2022>
-
Peripheral Arterial Disease/ 10083
-
((“Peripheral Arter*’ adj3 Disease?) or PAD).tw,kw. 39214
-
Intermittent Claudication/ 8273
-
(Intermittent adj3 Claudication).tw. 5364 5(“lower extremity arter* disease’‘or “lower limb arter* disease’).tw,kw. 563
-
or/1-5 50938
-
Ankle Brachial Index/ 3769
-
((brachial or ankle or arm) adj4 (index or pressure)).tw,kw. 13191
-
(ABPI or ABI or AAI).tw,kw. 10130
-
7 or 8 or 9 20809
-
Oscillometry/ 10010
-
plethysmography/ or photoplethysmography/ or plethysmography, impedance/ 18419
-
(Oscillometr* or plethysmograph* or photoplethysmography*).tw,kw. 22043
-
Ultrasonography, Doppler/ 16902
-
doppler.tw,kw. 108996
-
automat*.tw,kw. 266127
-
or/11-16 416542
-
Leg Ulcer/ 8779
-
((leg or lower) adj3 ulcer*).tw,kw. 9555
-
18 or 19 13995
-
6 and 10 and 17 and 20 19
EMBASE <1974–2022 Week 16>
-
peripheral occlusive artery disease/ 42000
-
((“Peripheral Arter*’ adj3 Disease?) or PAD).tw,kw. 60412
-
intermittent claudication/ 10179
-
(Intermittent adj3 Claudication).tw. 6708 5(“lower extremity arter* disease’‘or “lower limb arter* disease’).tw,kw. 792
-
or/1-5 91191
-
ankle brachial index/ 12512
-
((brachial or ankle or arm) adj4 (index or pressure)).tw,kw. 19814
-
(ABPI or ABI or AAI).tw,kw. 19076
-
7 or 8 or 9 37102
-
oscillometry/ 7563
-
plethysmography/ or photoelectric plethysmography/ or impedance plethysmography/ 22337
-
(Oscillometr* or plethysmograph* or photoplethysmograph *).tw,kw. 29312
-
exp Doppler flowmetry/ 60632
-
doppler.tw,kw. 161681
-
automat*.tw,kw. 359026
-
or/11-16 575469
-
leg ulcer/ 14386
-
((leg or lower) adj3 ulcer*).tw,kw. 12231
-
18 or 19 18371
-
6 and 10 and 17 and 20 44
-
conference abstract.pt. 4374598
-
21 and 22 6
-
21 not 23 38
CINAHL
-
‘MH “Peripheral Vascular Diseases’) 7116
-
‘X (“Peripheral Arter*’ N3 Disease?) OR PAD 11305
-
‘MH “Intermittent Claudication’) 1521
-
TX Intermittent N3 Claudication 1930
-
‘TX “lower extremity arter* disease’‘OR “lower limb arter* disease’ 128
-
S1 OR S2 OR S3 OR S4 OR S5 16256
-
‘MH “Ankle Brachial Index’) 2260
-
TX (brachial OR ankle OR arm) N4 (index OR pressure) 5951
-
TX ABPI OR ABI OR AAI 4691
-
s7 OR s8 OR s9 9556
-
‘MH “Plethysmogr”phy’) 2642
-
TX Oscillometr* OR plethysmograph* or photoplethysmograph* 4853
-
‘MH “Ultrasonography, Doppler, Pulsed’) OR ‘MH “Ultrasonography, Doppler, Duplex+’) OR ‘MH “Ultrasonography, Dopller’) 11167
-
TX doppler 31651
-
TX automat* 53479
-
S11 OR S12 OR S13 OR S14 OR S15 88958
-
‘MH “Leg Ulcer’) 4072
-
TX (leg OR lower) N3 ulcer* 6611
-
S17 OR S18 6611
-
S6 AND S10 AND S16 AND S19 27
Cochrane Library
-
MeSH descriptor: [Peripheral Arterial Disease] this term only 1197
-
(“Peripheral Arter*’ Near/3 Disease?) or PAD 5812
-
MeSH descriptor: [Intermittent Claudication] this term only 981
-
Intermittent Near/3 Claudication 2104 #5lower extremity arter* disease”‘or “lower limb arter* disease’ 0
-
#1 or #2 or #3 or #4 or #5 8017
-
MeSH descriptor: [Ankle Brachial Index] this term only 234
-
(brachial or ankle or arm) Near/4 (index or pressure) 4552
-
ABPI or ABI or AAI 2140
-
#7 or #8 or #9 5910
-
MeSH descriptor: [Oscillometry] this term only 149
-
MeSH descriptor: [Plethysmography] this term only 691
-
MeSH descriptor: [Plethysmography, Impedance] this term only 101
-
MeSH descriptor: [Photoplethysmography] this term only 96
-
Oscillometr* or plethysmograph* photoplethysmography* 4508
-
MeSH descriptor: [Ultrasonography, Doppler] this term only 608
-
doppler 12406
-
automat* 17556
-
#11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 33636
-
MeSH descriptor: [Leg Ulcer] this term only 507
-
(leg or lower) Near/3 ulcer* 2391
-
#20 or #21 2391
-
#6 and #10 and #19 and #22 18
Web of Sscience (SCI-Expanded 1900–present)“
-
‘lower extremity arter* dise’ase”‘or “lower limb arter* disease’ (Topic) 560
-
Intermittent Near/3 Claudication (Topic) 6338
-
3(“Peripheral Arter*’ Near/3 Disease?) or PAD (Topic) 54,662
-
#1 OR #2 OR #3 60,068
-
(brachial or ankle or arm) Near/4 (index or pressure) (Topic) 15,090
-
ABPI or ABI or AAI (Topic) 10,094
-
#5 OR #6 21,953
-
Oscillometr* or plethysmograph* photoplethysmograph* (Topic) 19,747
-
doppler (Topic) 170,240
-
automat* (Topic) 571,715
-
#8 OR #9 OR #10 756,945
-
(leg or lower) Near/3 ulcer* (Topic) 9908
-
#4 AND #7 AND #11 AND #12 6
Health Technology Assessment organisations
Canada’s Drug and Health Technology Agency (CADTH) (www.cadth.ca/)
Text search: brachial, ABPI – no relevant results
Agency for Healthcare Research and Quality (AHRQ) (https://effectivehealthcare.ahrq.gov/products)
Text search: brachial pressure, ABPI – no relevant results
McGill University Health Technology Assessment Unit (https://muhc.ca/tau/tau-reports)
Browsable list – no relevant results
International HTA database (https://database.inahta.org/)
Text search: brachial, ABPI – no relevant results
Health economics: per-protocol search: automated measurement of ABPI for PAD in patients with leg ulcers
Ovid MEDLINE and Epub Ahead of Print, In-process, In-data-review and Other Non-indexed Citations, Daily and Versions <1946–3 June 2022>
-
Peripheral Arterial Disease“
-
((“Peripheral Arter*” adj3 Disease?) or PAD).tw,kw.
-
Intermittent Claudication/
-
(Intermittent adj3 Claudication).tw.
-
(“lower extremity arter* disease” or “lower limb arter* disease”).tw,kw.
-
or/1-5
-
Ankle Brachial Index/
-
((brachial or ankle or arm) adj4 (index or pressure)).tw,kw.
-
(ABPI or ABI or AAI).tw,kw.
-
7 or 8 or 9
-
Oscillometry/
-
plethysmography/ or photoplethysmography/ or plethysmography, impedance/
-
(Oscillometr* or plethysmogra* or photoplethysmograph *).tw,kw.
-
Ultrasonography, Doppler/
-
doppler.tw,kw.
-
automat*.tw,kw.
-
or/11-16
-
Leg Ulcer/
-
((leg or lower) adj3 ulcer*).tw,kw.
-
18 or 19
-
(BlueDop or MESI or WatchBP or Microlife or Dopplex or Huntleigh or Bosch or boso).af.
-
*economics/
-
economics, hospital/
-
exp economics,medical/
-
economics,pharmaceutical/
-
exp models, economic/
-
exp decision theory/
-
monte carlo method/
-
markov chains/
-
exp technology assessment, biomedical/
-
(cost$ adj2 (effectinnuiti utilit$ or benefit$ or minimis$)).ab.
-
economics model$.tw.
-
(economic$ or pharmacoeconomic$).tw.
-
(price or prices or pricing).tw.
-
budget$.tw.
-
(value adj1 money).tw.
-
(expenditure$ not energy).tw.
-
markov$.tw.
-
monte carlo.tw.
-
(decision$ adj2 (tree? or analy$ or model$)).tw.
-
ec.fs.
-
or/22-42
-
6 and 10 and 17 and 20 and 21 and 42
Health economics: cost-effectiveness of any methods for diagnosis of PAD
Ovid MEDLINE and Epub Ahead of Print, In-process, In-data-review and Other Non-indexed Citations, Daily and Versions <1946–6 May 2022>
-
exp *Peripheral Arterial Disease/di, dg 180
-
exp “costs and cost analysis”/ 257933
-
*economics/ 10795
-
exp economics,medical/ 14336
-
economics,pharmaceutical/ 3067
-
exp models, economic/ 16113
-
exp decision theory/ 12894
-
monte carlo method/ 31240
-
markov chains/ 15705
-
exp technology assessment, biomedical/ 11867
-
(cost$ adj2 (effectiv* or utilit$ or benefit$ or minimis$)).ab. 170989
-
economics model$.tw. 64
-
(price or prices or pricing).tw. 46022
-
(value adj1 money).tw. 35
-
(expenditure$ not energy).tw. 34109
-
markov$.tw. 28588
-
monte carlo.tw. 54448
-
(decision$ adj2 (Tree? or analy$ or model$)).tw. 30691
-
or/2-18 565318
-
(metabolic adj cost).tw. 1601
-
((energy or oxygen) adj (cost or expenditure)).tw. 31349
-
(letter or editorial or note or comment).pt. 2059716
-
19 not (20 or 21 or 22) 535716
-
1 and 23 37
EMBASE <1974–2022 Week 18>
-
*peripheral occlusive artery disease/di [Diagnosis] 3176
-
exp economic evaluation/ 333018
-
exp *economics/ 28954
-
health economics/ 34224
-
exp health care cost/ 317800
-
pharmacoeconomics/ 8814
-
exp decision theory/ 1807
-
Monte Carlo method/ 46075
-
Markov chain/ 8390
-
exp biomedical technology assessment/ 15703
-
(cost$ adj2 (effective* or utilit$ or benefit$ or minimis$)).ab. 232656
-
economics model$.tw. 126
-
(price or prices or pricing).tw. 63780
-
(value adj2 money).tw. 2731
-
(expenditure$ not energy).tw. 46072
-
markov$.tw. 35552
-
monte carlo.tw. 55410
-
(decision$ adj2 (Tree? or analy$ or model$)).tw. 42137
-
or/2-18 890296
-
1 and 19 90
EconPapers
+peripheral +arter* +diagnos* 11
Value in Health
peripheral AND arter* AND diagno* [Research articles, Review articles] 109
EconLit (ProQuest) 1886–Current
-
peripheral 2371
-
arter* 308
-
s1 and s2 6
Health economics: cost-effectiveness of diagnosis or treatment of leg ulcers
Ovid MEDLINE and Epub Ahead of Print, In-process, In-data-review and Other Non-indexed Citations, Daily and Versions <1946–10 May 2022>
-
*Leg Ulcer“ 6895
-
exp ‘costs and cost analysis”/ 258071
-
*economics/ 10797
-
economics, hospital/ 11265
-
exp economics,medical/ 14336
-
economics,pharmaceutical/ 3067
-
exp models, economic/ 16113
-
exp decision theory/ 12896
-
monte carlo method/ 31253
-
markov chains/ 15709
-
exp technology assessment, biomedical/ 11871
-
(cost$ adj2 (effective* or utilit$ or benefit$ or minimis$)).ab. 171177
-
economics model$.tw. 64
-
(economic$ or pharmacoeconomic$).tw. 326492
-
(price or prices or pricing).tw. 46073
-
budget$.tw. 33079
-
(value adj1 money).tw. 35
-
(expenditure$ not energy).tw. 34156
-
markov$.tw. 28624
-
monte carlo.tw. 54488
-
(decision$ adj2 (tree? or analy$ or model$)).tw. 30751
-
ec.fs. 442129
-
or/2-22 1054082
-
1 and 23 283
EMBASE <1974–2022 Week 18>
-
*leg ulcer/ 8294
-
exp economic evaluation/ 333018
-
exp *economics/ 28954
-
health economics/ 34224
-
exp health care cost/ 317800
-
pharmacoeconomics/ 8814
-
exp decision theory/ 1807
-
Monte Carlo method/ 46075
-
Markov chain/ 8390
-
exp biomedical technology assessment/ 15703
-
(cost$ adj2 (effectiv* or utilit$ or benefit$ or minimis$)).ab. 232656
-
economics model$.tw. 126
-
(price or prices or pricing).tw. 63780
-
(value adj2 money).tw. 2731
-
(expenditure$ not energy).tw. 46072
-
markov$.tw. 35552
-
monte carlo.tw. 55410
-
(decision$ adj2 (tree? or analy$ or model$)).tw. 42137
-
or/2-18 890296
-
1 and 19 469
-
conference abstract.pt. 4389743
-
20 not 21 430
EconPapers
+leg + ulcers 31
Value in Health
Leg AND ulcers [Research articles, Review articles] 20
EconLit (ProQuest) 1886–current
NOFT Leg N/3 ulcers 4
Appendix 2 Characteristics of automated devices
| Study ID | Test (order administered) | Rest period | Patient position | No. of operators | Cost | How was the test done |
|---|---|---|---|---|---|---|
| Welsh 201644 | Dopplex Ability (random order) | 15 minutes | N/R | 1 (community vascular specialist nurse or leg ulcer clinic co-ordinator) | £5700 per unit | N/R (but probably according to the manufacturer’s instructions; description of the technical features of the device and its outputs are reported) |
| Manual Doppler (random order) | N/R | 2 (community vascular specialist nurse and leg ulcer clinic co-ordinator) | £470 per unit | N/R (but probably according to the manufacturer’s instructions; description of the technical features of the device and its outputs are reported) | ||
| Green 202045 | MESI ABPI MD (order N/R) |
N/R | N/R | 1 (general practice nurses, healthcare assistants or nursing students) | N/R | N/R |
| Manual Doppler (order N/R) |
N/R | N/R | N/R | N/R | N/R | |
| NCT05073510 202246 | BlueDop Vascular Expert (order N/R) | N/R | N/R | Confidential information has been removed | N/R | According to the Instructions for Use (IFU) |
| Duplex ultrasound (order N/R) | N/R | N/R | N/R | N/R | N/R | |
| Kordzadeh 201857 | BlueDop Vascular Expert (order N/R) |
N/R | N/R | 2 (physician and vascular specialist) | N/R | N/R (but probably according to the manufacturer’s instructions; description of the technical features of the device and its outputs are reported) |
| Duplex sonography (order N/R) |
N/R | N/R | 1 (senior vascular scientist) | N/R | N/R | |
| Homza 201958 | Boso ABI-system 100 (1st) | 10 minutes | Supine | N/R | N/R | In accordance with the device manual using appropriately sized sphygmomanometric cuffs |
| Manual Doppler (order N/R) |
N/R | N/R | N/R | N/R | In accordance with AHA guidelines for ABI measurement. A digital vascular Doppler HUNTLEIGH Dopplex DMX (Huntleigh Healthcare, UK) with an 8 MHz probe was used to measure the individual systolic pressures. An appropriately sized pneumatic cuff was applied to the right upper arm, inflated to suprasystolic pressure and deflated slowly until a Doppler flow signal was detected. The process was repeated for right leg and values for both dorsal pedal and anterior tibial arteries were measured, followed by left leg and left arm. | |
| Duplex ultrasound (order N/R) |
N/R | Supine | N/R | N/R | Performed using Vivid S6 Ultrasound System (GE Healthcare, USA) equipped with 8L-RS (a 5–13 MHz linear transducer) and 4C-RS (1, 8–6 MHz curvilinear transducer). Each limb was examined in the proximal to distal direction. | |
| Jarai 201859 | Boso ABI-system 100 (order N/R) |
N/R | N/R | 1 (trained nurse) | N/R | N/R |
| Manual Doppler (order N/R) |
N/R | N/R | 1 (trained nurse) | N/R | Performed with the validated ELITE 200 Doppler 5 MHz device | |
| Wohlfahrt 201166 | Boso ABI-system 100 (order N/R) |
5 minutes | Recumbent | N/R | N/R | N/R (but probably according to the manufacturer’s instructions; description of the technical features of the device and its outputs are reported) |
| Manual Doppler (order N/R) |
5 minutes | Supine | 2 (experienced physicians) | N/R | Appropriately sized cuffs of a mercury sphygmomanometer were placed proximal to the malleolus and on the right arm. After a 5-minute resting period in the supine position, systolic blood pressure was measured in the right brachial artery, right dorsal pedal and posterior tibial arteries, left dorsal pedal and tibial arteries (in this order) with a pocket Doppler device with an 8 MHz probe (Dopplex multi, Huntleigh, Cardiff, UK). Next, systolic blood pressure measurement was repeated on the right brachial artery for a second time. If the difference between the first and the second brachial systolic pressure measurements was higher than 10 mmHg, all measurements were repeated. | |
| Diehm 200947 | Boso ABI-system 100 (2nd) | N/R | N/R | 1 (examiner with experience of over 30 years of ABI measurements) | N/R | N/R (but probably according to the manufacturer’s instructions; description of the technical features of the device and its outputs are reported) |
| Manual Doppler (1st) | 10 minutes | Supine | 1 (examiner with experience of over 30 years of ABI measurements) | N/R | Doppler-assisted ABI measurements were performed according to the method described by Lovelace and Moneta using a sphygmomanometer (Erka GmbH, Bad Toelz, Germany) with a cuff width ranging between 29 and 40 cm and a Doppler device with an 8.2 MHz continuous wave probe (Ultrasonic Flow Detector model 811–B, Parks Medical Electronic Inc., Aloha, OR, USA). | |
| In brief, the cuff was inflated to suprasystolic pressure (i.e. >30 mmHg above expected systolic pressure) and deflated slowly until a flow signal was detected by Doppler over the dorsalis pedis artery and posterior tibial artery, respectively, thereby possibly indicating two different systolic pressures at the ankle level. These were recorded as ‘high’ and ‘low’ ankle systolic pressures. Brachial artery systolic pressure was determined similarly on both upper extremities, the higher systolic brachial pressure being used for ABI calculations. Hence, for each limb a ‘high’ and a ‘low’ Doppler-assisted ABI was registered. | ||||||
| Babaei 202054 | Dopplex Ability (1st) | None | Supine | 1 (trained nurse with extensive experience in vascular assessment of lower limbs) | N/R | In accordance with the manufacturer’s guidelines and undertaken first as there is no need for resting before testing |
| Doppler ultrasound (2nd) | 5 minutes | Supine | 1 (trained nurse with extensive experience in vascular assessment of lower limbs) | N/R | After 5 minutes, measured in accordance with the American Heart Association’s scientific statement for ABI measurement | |
| Ultrasound Duplex scan (UDS) (3rd) | N/R | Supine | N/R | N/R | From the iliac and common femoral arteries then distally assessing superficial femoral artery, popliteal and tibial arteries in the longitudinal plane. The extent and severity of any arterial disease were assessed using triplex mode by measuring the PSV from the Doppler waveform just proximal to and through the stenosis. | |
| Millen 201860 | Dopplex Ability (random order) | N/R | N/R | N/R (vascular specialists) | N/R | According to manufacturer’s guidelines. Three repeat readings were performed on each participant and results printed out from the device. The Dopplex did not always obtain a complete set of data at the first attempt (i.e. 2 arm blood pressures, 2 ankle blood pressures and 2 ABIs) and therefore the first successful set of Dopplex ABI data was used. |
| Doppler/air plethysmography-based Parks Flo-Lab system (random order) | N/R | N/R | N/R (vascular specialists) | N/R | ABIs were obtained using the standard Doppler method, using the supplied Parks 8 MHz Doppler probe, obtaining the brachial, posterior tibial and dorsalis pedis arteries. | |
| Davies 201648 | Dopplex Ability (1st) | None | N/R | 1 (registered nurse with significant experience in vascular assessment of lower limb) | N/R | In accordance with manufacturer’s guidelines. All four limbs measured simultaneously before automatic calculation of ABI for each leg. In the event of a failed measurement, the procedure was repeated if acceptable to the participant and the clinician’s time schedule permitted. |
| Doppler ultrasound (2nd) | N/R | N/R | 1 (registered nurse with significant experience in vascular assessment of lower limb) | N/R | In accordance with the American Heart Association’s scientific statement for ABI measurement | |
| Lewis 201649 | Dopplex Ability (1st) | N/R | Supine | 1 (podiatrist or vascular nurse practitioner) | N/R | In accordance with the manufacturer’s guidelines |
| Duplex ultrasound (2nd) | N/R | Supine | 1 (highly experienced medical physicist) | Equipment utilised: Toshiba Aplio 500 with linear PLT-704SBT and curvi-linear PVT-375BT probes. The participant lay supine on the scanning couch with the lower limbs exposed. The distal CFA was imaged and the DW was assessed visually for any loss of triphasic flow due to significant iliac disease. If the DW showed indications of this, then the iliac | ||
| arteries were assessed for the presence of atherosclerotic disease. The scan continued distally from the CFA assessing the superficial femoral artery and popliteal arteries in the longitudinal plane. The extent and severity of any arterial disease were assessed using triplex mode by measuring the peak systolic velocity from the DW just proximal to and through the stenosis. | ||||||
| Lewis 201051 | Dopplex Ability (random order) | 10 minutes (sequence A) 5 minutes (sequence B) |
N/R | 1 (podiatrist) | N/R | N/R (but probably according to the manufacturer’s instructions; description of the technical features of the device and its outputs are reported) |
| Manual Doppler (order random) | 10 minutes (sequence B) 5 minutes (sequence A) |
N/R | N/R | N/R | N/R | |
| Zebari 202250 | MESI ABPI MD (2nd) | N/R | N/R | N/R | N/R | N/R (but probably according to the manufacturer’s instructions; description of the technical features of the device and its outputs are reported) |
| Manual Doppler (1st) | 10 minutes | Supine | N/R | N/R | A non-directional vascular pen Doppler (Huntleigh Dopplex D900, Arjo Inc., Addison, IL, USA) and a standard manual blood pressure cuff were used for the measurements, and the highest recorded pressure at ankle level was used in the ABI calculations. | |
| Hageman 202161 | MESI ABPI MD (1st) | 3–5 minutes | Supine | 1 | N/R | N/R (but probably according to the manufacturer’s instructions; description of the technical features of the device and its outputs are reported). Three series of measurements were performed in each patient. For each series, the ABI was measured twice with a 1-minute pause between measurements: first, at the left brachial artery and both ankle arteries; and second, at the right brachial artery and both ankle arteries. ABIs based on the arm with the highest of the two SBPs were used in the analyses, as is standard practice. To assess the validity of the device, the average of the three series was used for calculations. ABI results were blinded for the patients and the other operators. |
| Manual Doppler (2nd) | 15 minutes | N/R | 1 (trained vascular technician) | N/R | In each patient, ABI measurements were repeated with vascular laboratory Doppler equipment (ELCAT vasolab 320; ELCAT Medical Systems, Wolfratshausen, Germany) some 15 minutes after the oscillometric measurements. SBPs of the brachial and ankle arteries (dorsal pedal and posterior tibial) were measured with sphygmomanometer cuffs, which were automatically inflated and deflated. SBP cut-off points of all arteries were defined as the systolic upstroke of the first arterial waveform. At the first characteristic arterial sound and at the simultaneous appearance of the first arterial waveform, the monitor screen was frozen and the SBP cut-off point was defined by precise retrospective positioning of an adjustable marker line. The ABI was calculated in each leg by dividing the highest systolic ankle pressure (either posterior tibial or dorsal pedal) by the highest systolic brachial pressure of both arms. | |
| Boilley 202052 | MESI ABPI MD (1st) | 10 minutes | Supine | 1 (experienced vascular physician) | N/R | For all patients, the ABI was measured using the MESI-ABPI-MD® immediately after lying down (MESI 1) and following a rest period of 10 min (MESI 2), after which ABI–Dop was measured. The measurement was considered a diagnosis of PAD when the value was ≤0.90 or when the device displayed a ‘PAD’ message. Indeed, if the SBP was lower than 70 mmHg or if the ABI was lower than 0.50, the device displayed a ‘PAD’ message since the accuracy of the measurement below 0.50 is low. |
| Manual Doppler (2nd) | N/R | Supine | 1 (experienced vascular physician) | N/R | ABI measurements using the standard manual method (continuous Doppler) were performed with a hand-held Doppler (BASIC, Atys Medical, France) according to the European Society of Cardiology guidelines. The operator rounded up the values to the nearest 5 mmHg. | |
| Catillon 202062 | MESI ABPI MD (1st) | 10 minutes | N/R | 1 (vascular specialist) | N/R | N/R (but probably according to the manufacturer’s instructions; description of the technical features of the device and its outputs are reported). |
| Doppler ultrasound (2nd) | N/R | N/R | 1 (vascular specialist) | N/R | Taken in line with standard procedure (i.e. right brachial artery, right posterior tibial artery, right anterior tibial artery, left posterior tibial artery, left anterior tibial artery, left brachial artery and right brachial artery). The highest brachial and ankle pressure values were used to calculate the ABI. | |
| Varetto 201963 | MESI ABPI MD (order N/R) | N/R | Supine | 1 | N/R | According to manufacturer’s instructions using 3 cuffs. ABI automatically calculated with the same ratio employed for the Doppler method. |
| Manual Doppler (order N/R) | N/R | Supine | 1 | N/R | Performed with a calibrated sphygmomanometer and 8 MHz Doppler probes. ABI was calculated as the ratio of the highest systolic blood pressure (SBP) obtained from both tibial and dorsalis pedis arteries at one ankle to the highest SBP of both brachial arteries. | |
| Span 201653 | MESI ABPI MD (2nd) | N/R | N/R | 1 | N/R | According to the manufacturer’s instructions using three cuffs. |
| Manual Doppler (1st) | ‘a few minutes’ | Supine | 1 | N/R | According to the standard protocol with a calibrated sphygmomanometer and 8 MHz Doppler probes (Dopplex SD2, Huntleigh Healthcare Ltd, Cardiff, UK). ABI was calculated as the ratio of the highest SBP obtained from both tibial and dorsalis pedis arteries at one ankle to the highest SBP of both brachial arteries. | |
| Verma 202255 | WatchBP Office ABI (1st) | 5 minutes | N/R | N/R | N/R | Appropriately sized cuffs were used. BP was measured simultaneously on both arms followed by both ankles. The arm and ankle with the higher SBP were selected for the ABI measurement. |
| Vascular Doppler device (2nd) | N/R | N/R | N/R | N/R | The brachial and posterior tibial systolic pressures were measured using appropriately sized blood pressure cuffs linked to a mercury sphygmomanometer placed successively on the upper arms and just above the ankles. Using a hand‑held continuous wave Doppler probe (8 MHz, HI.dop, BT-200 Vascular Doppler, Bistos Co. Ltd, Korea), the systolic pressure in each artery was measured by inflating the cuffs 30 mmHg above the systolic blood pressure and deflated slowly until a flow signal was detected over the brachial and posterior tibial artery. | |
| Raya 201964 | WatchBP Office ABI (order N/R) | N/R | N/R | N/R (nurses experienced in the technique) | N/R | Three consecutive measurements separated by intervals of 1 minute, obtaining the mean of each arm. A cuff was then placed in the control arm and an anklet on the left leg. ABPI was calculated automatically. The same was done on the right. |
| MESI ABPI MD (order N/R) | N/R | N/R | N/R (nurses experienced in the technique) | N/R | One of the cuffs was placed in the control arm obtained with Doppler and an ankle brace in each of the legs. The ABPI was automatically calculated for each one. | |
| Manual Doppler (order N/R) | N/R | N/R | N/R (nurses experienced in the technique) | N/R | Huntleigh Healthcare Dopplex II model SD2 model and CORYSAN type manual sphygmomanometer. The arm with the highest SBP was determined with the Doppler probe. SBP was then obtained in the legs, first right and then left, in the pedal and tibial arteries. | |
| Rodriguez-Roca 201467 | WatchBP Office ABI (2nd) | N/R | N/R | 1 (nurse specifically trained) | N/R | N/R |
| Manual Doppler (1st) | 5 minutes | Supine decubitus | 1 (nurse specifically trained) | N/R | Measured using a validated and calibrated sphygmomanometer and a two-way Doppler with an 8-MHz probe (BIDOP ES-100 V3). After resting for 5 minutes in the supine decubitus position, systolic BP was measured in both arms and the highest value was selected for calculation of the ABI (denominator). The systolic BP of the posterior tibial artery and the pedal artery was then measured in each leg, and the highest value (whether tibial or pedal) was taken as reference for calculating the individual ABI of each leg (numerator). The ABI of both the left and right legs was recorded and for the definition of PAD, the lowest of the two values was considered. | |
| Sinski 201365 | WatchBP Office ABI (1st) | 20–30 minutes | Supine | 1 (experienced technician) | N/R | According to the device’s manual using appropriately sized cuffs. Blood pressure was measured simultaneously on both arms and the arm with the higher systolic blood pressure selected for the ABI measurement. One of the brachial cuffs was then replaced with the ankle cuff. The ankle cuff was placed over the posterior tibial artery on the ankle. Both cuffs were inflated simultaneously, and the ABI was calculated automatically. The same measurement was performed on the other ankle. |
| Ultrasound Doppler (2nd) | 5–10 minutes | N/R | 1 (experienced physician) | N/R | Measured within 5–10 minutes of the automated oscillometric measurement using a linear vascular probe with the ultrasound unit (GE Vivid 5, GE Vingmed, Horten, Norway) or Philips IE 33 (Philips Medical Systems, Andover, MA, USA) and a sphygmomanometer (Heine G5, Heine Optotechnik, Herrsching, Germany). The measurements were started by determining systolic blood pressure on the brachial arteries. A cuff was placed over the brachial artery and inflated 20 mm Hg above systolic pressure and then released until the first signal of the Doppler flow was recorded. The higher systolic blood pressure was recorded for the ABI calculation. After the brachial artery measurements, systolic blood pressure was measured in the same way on both ankles. Specifically, a Doppler probe was placed over the posterior tibial artery, which was the site used for the automatic oscillometric measurement. | |
| Kollias 201156 | WatchBP Office ABI (random order) | 10 minutes | Supine | 1 | N/R | N/R (but probably according to the manufacturer’s instructions; description of the technical features of the device and its outputs are reported). |
| Manual Doppler (order random) | Supine | 1 | N/R | Manual Doppler ABI was measured according to the American Heart Association guidelines using a continuous wave Doppler device with an 8 MHz probe. |
Appendix 3 Baseline characteristics of included studies
| Study ID | Age, years, mean (SD) | Male sex (%) | Comorbidities, n (%) |
|---|---|---|---|
| Welsh 201644 | N/R | N/R | N/R |
| Green 202045 | N/R | N/R | N/R |
| Confidential information has been removed46 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Kordzadeh 201857 | Median (IQR) 73 (65–81) | 62.0 | Hypertension, 111 (66.9) Hypercholesterolaemia, 100 (60.2) Active smoking, 71 (42.8) Ischaemic heart disease, 54 (32.5) Cardiac arrhythmias, 37 (22.3) COPD, 29 (17.5) Renal failure, 26 (15.7) |
| Homza 201958 | 67.6 (min, max 41.8, 83.2) | 74.2 | Diabetes, 62 (100) Coronary artery disease, 42 (67.7) Angina pectoris, 19 (30.6) MI, 15 (24.2) Stroke, 13 (21.0) Cardiostimulator, 8 (12.9) Polyneuropathy, 20 (32.3) Nephropathy, 2 (32.2) Current smoker, 13 (21.0) |
| Jarai 201859 | 63.9 (11.5) | 44.6 | Smoking, 96 (24.2) Peripheral vascular disease, 105 (26.4) Diabetes mellitus, 110 (27.7) |
| Wohlfahrt 201166 | 54.3 (13.8) | 46.8 | Coronary heart disease, 47 (5.6) Diabetes mellitus, 78(9.3) Hyperlipoproteinaemia, 383 (45.6) Stroke or TIA, 19 (2.3) Claudications, 6 (< 1%) |
| Diehm 200947 | 65 (6) | 62.0 | Diabetes, 19 (38) Arterial hypertension, 27 (54) Hyperlipidaemia, 30 (60) Renal insufficiency, 6(12) Current smoking, 41 (82) Coronary heart disease, 17 (34) Cerebrovascular disease, 7 (14) Claudication, 68 legs (68) Critical limb ischaemia, 32 legs (32) |
| Babaei 202054 | 60.1 (0.3) | 39.8 | T2DM, 100% (mean duration 13.29 ± 0.34 years) Known CAD, 12.5% Known stroke, 1.6% Known hypertension, 69.7% Retinopathy, 21.1% Neuropathy, 42.8% Claudication, 17.1% Rest pain, 20.1% Foot ulcer, 6.6% Vascular surgery, 17.4% Current smoker, 4.6% (n N/R for above) |
| Millen 201860 | 69.5 (12) | 77.3 | T1DM, 4 (6.1) T2DM, 14 (21.2) Hypertension, 52 (78.8) Hyperlipidaemia, 45 (68.2) IHD, 29 (44.0) CVA/TIA, 11 (16) Current smoker, 10 (15.2) Claudication, 36 (54.5) Rest pain, 4 (6.1) Oedema, 5 (7.6) Renal failure, 1 (1.5) Amputees, 3 (4.5) |
| Davies 201648 | 64 (9) | 57.0 | Hypertension, 550 limbs (76.0) |
| Lewis 201649 | 67 (12) | 65.1 | Hypertensive, 119 (62.9) Hyperlipidaemia, 108 (57.1) Previous CVA, 25 (13.2) Family history of CVA, 45 (23.8) Known CHD, 59 (31.2) Family history of CHD, 95 (50.3) Known PAD, 49 (25.9) Family history of PAD, 28 (14.8) Diabetes, 49 (25.9) DVT history, 15 (7.9) Retinopathy, 9 (4.8) Smoker, 59 (31.2) |
| Lewis 201051 | N/R | N/R | N/R |
| Zebari 202250 | 72 (10) | 63.4 | Hypertension, 110 (71.9) Hyperlipidaemia, 68 (44.4) Diabetes, 35 (22.9) Cardiac disease, 42 (27.5) Pulmonary disease, 22 (14.4) Renal disease, 10 (6.5) PAD, 80 (52.3) Lower limb pain during physical activity, 77 (50.3) Rest pain, 30 (19.6) Ulceration/gangrene, 13 (8.5) Aortic aneurysm, 53 (34.6) Aortic dissection, 3 (2.0) Other vascular disease, 9 (5.9) Current smoker, 26 (17.0) |
| Hageman 202161 | 67 (11) | 55.7 | Current smoker, 89 (44.3) Hypertension, 118 (58.7) Hypercholesterolaemia, 85 (42.3) Obesity, 38 (18.9) Diabetes mellitus, 61 (30.3) Renal insufficiency, 17 (8.4) Atrial fibrillation, 11 (5.5) Coronary artery disease, 73 (36.3) Cerebrovascular disease, 33 (16.4) |
| Boilley 202052 | 63 (11) | 84.3 | Diabetes, 20/102 (19.6) Dyslipidaemia, 78/102 (76.5) Medical history: PAD, 82/102 (80.4) Atrial fibrillation, 14/102 (13.7) Vascular bypass, 15/102 (14.7) Beta-blocker medication, 37/102 (37.3) Antiplatelet medication, 75/102 (73.5) ACE inhibitor medication, 28/102 (27.5) |
| Catillon 202062 | 66 (14.4) | 67.4 | Hypertension, 30 (69.8) Tobacco, 4 (9.3) Diabetes, 11 (25.6) Dyslipidaemia, 18 (41.9) Renal insufficiency, 16 (37.2) Cardiovascular diseases, 20 (46.5) Anticoagulants, 8 (18.6) |
| Varetto 201963 | 72.5 (13.6) | 62.7 | Arteriopathy, 116 (62.7) Diabetes, 46 (24.9) Hypertension, 139 (75.1) Smoker/ex-smoker, 80 (43.2) Coronary artery disease, 42 (22.7) |
| Span 201653 | 64 (7.8) | N/R | Hypertension, 66 (48.5) Dyslipidaemia, 58 (42.6) Diabetes mellitus, 19 (14.0) Current smoker, 22 (16.2) |
| Verma 202255 | 27.5 (4.1) | 100.0 | N/R |
| Raya 201964 | 63 (7) | 44.1 | Hypertension, 114 (56.4) Dyslipidaemia, 117 (57.9) Diabetes mellitus, 57 (28.2) Cardiovascular or cerebrovascular disease, 30 (14.9) |
| Rodriguez-Roca 201467 | 47.7 | 45.7 | Dyslipidaemia, 227 (70.5) Hypertension 86 (26.7) Current smoker, 82 (25.5) Obesity, 80 (24.8) Diabetes mellitus, 27 (8.4) Ischaemic heart disease, 13 (4.0) Heart failure, 5 (1.6) Cerebrovascular disease, 5 (1.6) |
| Sinski 201365 | 70.1 (9.4) | 66.3 | Coronary artery disease, 80 (100.0) MI, 19 (23.8) Percutaneous coronary intervention, 22 (27.5) CABG, 21 (26.3) Current smoking, 27 (33.8) Previously diagnosed PAD, 10 (12.5) Hypertension, 63 (78.8) Hypercholesterolaemia, 60 (75.0) Diabetes mellitus, 26 (32.5) History of atrial fibrillation, 17 (21.3) History of lower leg pain, 34 (42.5) |
| Kollias 201156 | 62.5 (11.1) | 62.4 | Hypertension, 77 (82.8) Diabetes, 42 (45.2) Dyslipidaemia, 6 (68.8) Current smoking, 14 (15.1) Cardiovascular disease, 21 (22.6) Chronic renal disease, 6 (6.5) Treatment with beta-blockers, 20 (21.5) |
Appendix 4 Risk-of-bias assessments
FIGURE 16.
Quality assessment of diagnostic accuracy studies-2 results.
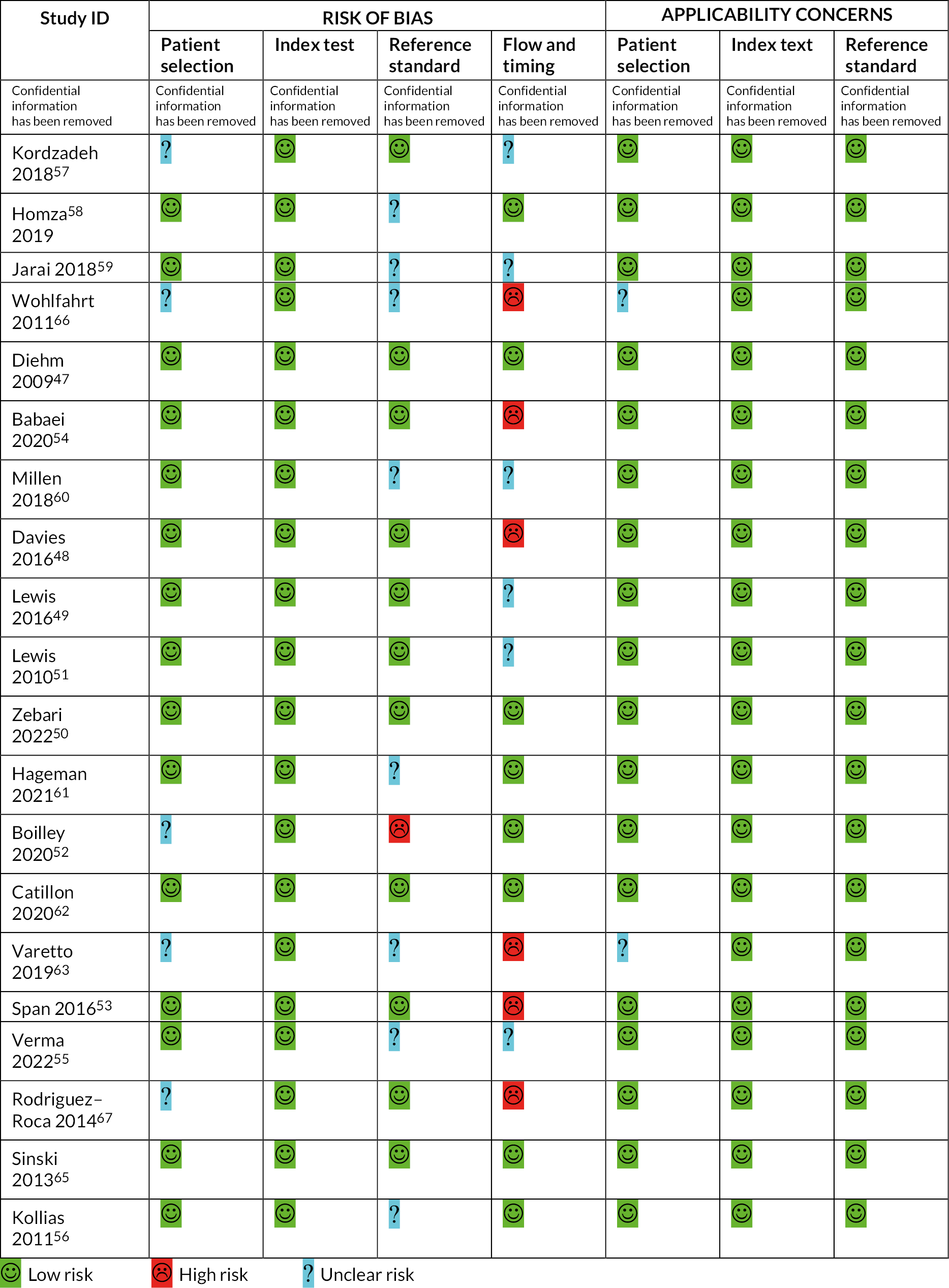
FIGURE 17.
Quality assessment of diagnostic accuracy studies-C results.
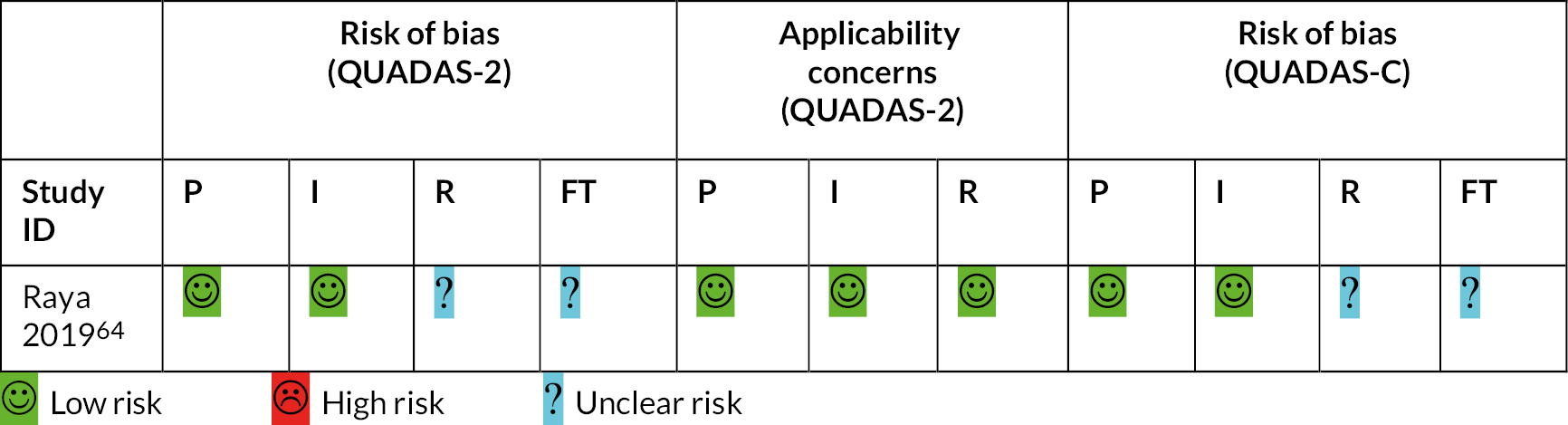
| ReBIP item | Welsh et al.44 | Green et al.45 |
|---|---|---|
| Representative sample | ✓ | ? |
| Inclusion/exclusion criteria clearly defined | ✓ | ✗ |
| Participants at similar point in disease progression | ✓ | ? |
| Prospective data collection | ✓ | ✓ |
| Intervention and comparator clearly defined | ✓ | ✓ |
| Intervention delivered by experienced person | ? | ✓ |
| Intervention delivered in appropriate setting | ✓ | ✓ |
| Important outcomes considered | ✓ | ✓ |
| Objective outcome measures used | ✓ | ✓ |
| Information on dropouts | ✗ | ✗ |
| Dropouts similar to those who completed study | ? | ? |
| Important prognostic factors identified | ? | ? |
Appendix 5 Other outcomes related to the use of automated devices
| Study ID (automated device) | Time required for using the device | Technical failure rate | Acceptability and experience of using the device |
|---|---|---|---|
| Welsh 201644 (Dopplex Ability) |
|
N/R |
|
| Green 202045 (MESI ABPI MD) |
|
N/R |
|
|
|||
| Confidential information has been removed46 Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Kordzadeh 201857 (BlueDop) |
N/R | N/R | N/R |
| Homza 201958 (BOSO ABI-System 100) |
N/R | N/R | N/R |
| Jarai 201859 BOSO ABI-System 100) |
|
|
N/R |
| Wohlfahrt 201166 BOSO ABI-System 100) |
N/R | N/R | N/R |
| Diehm 200947 BOSO ABI-System 100) |
|
N/R | N/R |
| Babaei 202054 (Dopplex Ability) |
N/R | N/R | N/R |
| Millen 201860 (Dopplex Ability) | N/R | ABI was unobtainable by Dopplex over 3 attempts in 3/129 limbs (2.3%) | N/R |
| Davies 201648 (Dopplex Ability) |
|
Failed Dopplex measurements in 28/724 (3.9%) limbs, associated with presence of hypertension (p = 0.015). No failed Doppler measurements | N/R |
| Lewis 201649 (Dopplex Ability) | N/R | N/R | N/R |
| Lewis 201051 (Dopplex Ability) |
|
||
| Zebari 202250 (MESI ABPI MD) | N/R |
|
N/R |
| Hageman 202161 (MESI ABP MD) |
N/R |
|
N/R |
| Boilley 202052 (MESI ABPI MD) | N/R | N/R | N/R |
| Catillon 202062 (MESI ABPI MD) |
|
N/R | N/R |
| Varetto 201963 (MESI ABPI MD) |
|
|
N/R |
| Span 201653 (MESI ABPI MD) |
|
|
N/R |
| Verma 202255 (WatchBP Office ABI) | N/R | N/R | N/R |
| Raya 201964 (WatchBP Office ABI, MESI ABPI MD) |
|
|
N/R |
|
|||
| Rodriguez-Roca 201467 (WatchBP Office ABI) |
N/R | N/R | N/R |
| Sinski 201365 (WatchBP Office ABI) |
N/R |
|
N/R |
| Kollias 201156 (WatchBP Office ABI) |
|
|
N/R |
Appendix 6 Supplementary cost-effectiveness search strategies
Search 1: Cost-effectiveness of PAD diagnostic methods MEDLINE and Epub Ahead of Print, In-process, In-data-review and Other Non-indexed Citations, Daily and Versions <1946–6 May 2022>
-
exp *Peripheral Arterial Disease/di, dg
-
exp “costs and cost analysis”/
-
*economics/
-
exp economics,medical/
-
economics,pharmaceutical/
-
exp models, economic/
-
exp decision theory/
-
monte carlo method/
-
markov chains/
-
exp technology assessment, biomedical/
-
(cost$ adj2 (effectiv* or utilit$ or benefit$ or minimis$)).ab.
-
economics model$.tw.
-
(price or prices or pricing).tw.
-
(value adj1 money).tw.
-
(expenditure$ not energy).tw.
-
markov$.tw.
-
monte carlo.tw.
-
(decision$ Adj2 (tree? or analy$ or model$)).tw.
-
or/2-18
-
(metabolic adj cost).tw.
-
((energy or oxygen) adj (cost or expenditure)).tw.
-
(letter or editorial or note or comment).pt.
-
19 not (20 or 21 or 22)
-
1 and 23
Search 2: Cost-effectiveness of diagnosis or treatment of leg ulcer
Ovid MEDLINE and Epub Ahead of Print, In-process, In-data-review and Other Non-indexed Citations, Daily and Versions <1946–10 May 2022>
-
Leg Ulcer/
-
exp “costs and cost analysis”/
-
*economics/
-
economics, hospital/
-
exp economics,medical/
-
economics,pharmaceutical/
-
exp models, economic/
-
exp decision theory/
-
monte carlo method/
-
markov chains/
-
exp technology assessment, biomedical/
-
(cost$ adj2 (effectiv* or utilit$ or benefit$ or minimis$)).ab.
-
economics model$.tw.
-
(economic$ or pharmacoeconomic$).tw.
-
(price or prices or pricing).tw.
-
budget$.tw.
-
(value adj1 money).tw.
-
(expenditure$ not energy).tw.
-
markov$.tw.
-
monte carlo.tw.
-
(decision$ adj2 (tree? or analy$ or model$)).tw.
-
ec.fs.
-
or/2-22
-
1 and 23
Appendix 7 Cost of amputation
The per-cycle cost of amputation used within the model was based on the methods used in CG147. 127 CG147 used a combination of clinical expert opinion (Guideline Development Group) and the external literature to determine the care needs of patients following the procedure. Within our analysis, we used the same clinical assumptions supplemented with updated literature sources where possible. Furthermore, the unit costs used within CG147 are based on the PSSRU 2010 and the NHS reference costs 2009–10; these have been updated with the 2020–1 cost year applied in our analysis. Details of the clinical assumptions, sourced from CG147, which we used in our calculations, are provided below.
To generate the most representative subsequent care costs, we have supplemented the assumptions made in CG147 with additional screening of the literature. A study conducted in Glasgow in 2014 of 118 patients who underwent lower extremity amputations informed the number of patients who received a prosthetic limb, where 56% of patients were referred for prosthetic rehabilitation. 114 We utilised the same study as CG147 to inform the proportion of formally independent patients who retain their independence following amputation. 115 This study reports a KM analysis of formally independent patients who lose their independence following amputation. We assumed that the number at risk at baseline represents the proportion of the total population who were independent prior to the procedure, 71.9%. This is in line with the Glasgow study, where 73% of patients had no home care pre-amputation. Therefore, we assume that 28.1% of patients were in a care home prior to the amputation. We did not include these costs as they cannot be attributed directly to amputation. At 3 months post amputation, the KM analysis finds that 77% of formally independent patients retained their independence and remained in the community, which represents 55.3% of the total population. We then utilised the assumption from CG147 that 50% of these will require care in the community (27.7%). In line with the costs of the procedure itself, we used the ratio of above-to-below-knee amputations reported in the National Vascular Registry to inform the cost of wound care. 98
| Category | Assumption | First year | Subsequent years |
|---|---|---|---|
| Prosthetics and wheelchairs | 3 prosthetist appointments per patient | ✓ | ✗ |
| Wheelchairs replaced every 5 years | ✓ | ✓ | |
| 50% of wheelchairs are motorised | ✓ | ✓ | |
| Rehabilitation | All patients receive inpatient and outpatient rehabilitation services in the first year following amputation | ✓ | ✗ |
| Inpatient rehabilitation | 1 rehabilitation assessment | ✓ | ✗ |
| 50 days of rehabilitation | ✓ | ✗ | |
| Outpatient rehabilitation | 1 rehabilitation assessment | ✓ | ✗ |
| 8.5 and 13 weeks of rehabilitation for below and above-knee amputations, respectively | ✓ | ✗ | |
| 2 hours of class per week | ✓ | ✗ | |
| 10 patients per class | ✓ | ✗ | |
| 2 physiotherapists and 1 physiotherapy technician per class | ✓ | ✗ | |
| Wound care | 2.5 home nurse visits per week | ✓ | ✗ |
| 90% of wounds are non-complicated with average healing time of 12 weeks | ✓ | ✗ | |
| 10% of wounds are non-complicated with average healing time of 32 weeks | ✓ | ✗ | |
| Care home | 47 weeks per year | ✓ | ✓ |
| Community care | 50% of patients who remain in the community will receive care | ✓ | ✓ |
| Home modifications | All patients who remain in the community will have home modifications | ✓ | ✓ |
| Category | Assumption | Source |
|---|---|---|
| Prosthetics and wheelchairs | 56% receive a prosthetic limb | 56% of lower extremity amputation patients are referred to prosthetic rehabilitation services. Davie-Smith et al.114 |
| 44% receive a wheelchair | ||
| Prosthetics would be replaced every 5 years | Assumed equal to useful lifespan of wheelchairs in CG147. | |
| Care requirements (as a result of amputation) | 27.7% live independently and do not receive care within the community | KM analysis of maintenance of independent living status after major amputation. Where 383/533 (71.9%) at risk at baseline with 77% of these (55.3%) retaining their independent living status at 3 months post amputation and 23% of these (16.5%) would then require full-time care post amputation. Therefore, it is assumed that 150/533 (28.1%) were not living independently prior to amputation and required full-time care. Taylor et al.115 Following the assumption from CG147 reported in Table 32, 50% of those who remain in the community will receive care (50% of 55.3% = 27.65%). |
| 27.7% live independently but receive care within the community | ||
| 16.5% require full-time care as a result of amputation | ||
| 28.1% required full-time care (care home) prior to amputation | ||
| Proportion of above-knee and below-knee amputations | 49.8% above knee | 3203/6429 of major unilateral lower limb amputation procedures were above the knee in 2019 and 2020. National Vascular Registry 2021. |
| 50.1% below knee |
Costs are detailed for each category of resource use: prosthetics, wheelchairs, rehabilitation, wound care, care homes, community care and home modifications. All costs are adjusted for the assumptions detailed above. Finally, we report the total cost by category and year. The cost of care in the first year following amputation is calculated as £35,813.44 and £14,293.65 for all subsequent years. These costs are comparable to those used within CG147 in the first year (£34,464.70 uplifted to 2020–1 prices). 108 The cost used for subsequent years is substantially less in our analysis compared to CG147, £28,651.90 in 2020–1 prices. This is primarily driven by care home costs. CG147 assumed that 36% of formally independent patients require a care home which is higher than our analysis (23%). Furthermore, unlike our analysis, CG147 bases their costing analysis upon the formally independent population only and does not account for patients who were not independent prior to the procedure. Further details of the costs are provided in Tables 34 and 35.
| Category | Assumption | Timeframe | Unit cost | Source |
|---|---|---|---|---|
| Prosthetics | Annuitised cost of 3 prosthetist appointments per patient | Annual | £248.08 | Based on £373.36 unit cost (total cost of £1120.08) discounted by 3.5% per annum over 5 years. Prosthetics Non-admitted F2F attendance, follow-up (WF01A). NHS reference costs 2020–1. |
| Annuitised cost of prosthetic | Annual | £232.21 | Based on £1048.43 discounted by 3.5% per annum over 5 years. Bespoke orthopaedic prosthesis (DEV03). NHS reference costs 2020–1 | |
| 56% of patients | £268.96 | 56% of £480.28 | ||
| Wheelchairs | Annuitised cost of non-motorised wheelchair (50% of wheelchairs) | Annual | £35.50 | Based on £71 annual cost of self- or attendant-propelled chair discounted by 3.5% per annum over 5 years. PSSRU 2021–2 |
| Annuitised cost of motorised wheelchair (50% of wheelchairs) | Annual | £177.50 | Based on £355 annual cost of powered chair discounted by 3.5% per annum over 5 years. PSSRU 2021–2 | |
| 44% of patients | £93.72 | 44% of £213 | ||
| Inpatient rehabilitation | 1 rehabilitation assessment | – | £793.09 | Complex specialised rehabilitation services level 1. Assessment for rehabilitation, unidisciplinary (VC01Z) |
| 50 days of rehabilitation | – | £20,500 | Based on £410 per occupied ed day of local specialist rehabilitation services. PSSRU 2020–1 | |
| 100% of patients | £21,293.09 | |||
| Outpatient rehabilitation | 2 physiotherapists and 1 physiotherapy technician | Per hour | £139 | 2× Band 6 (£52). 1× Band 4 (£35). Cost per working hour of hospital-based scientific and professional staff. PSSRU 2020–1 |
| 2 hours of class per week | Per class | £278 | 2 multiplied by £139. | |
| 10 patients per class | Per patient | £27.80 | £278 divided by 10 | |
| 8.5 weeks of classes (below knee) | Per course | £118.57 | Multiplied by number of weeks and weighted to 50.1%. National Vascular Registry 2021 | |
| 13 weeks of classes (above knee) | Per course | £180.05 | Multiplied by number of weeks and weighted to 49.8%. National Vascular Registry 2021 | |
| 100% of patients | £1091.72 | Based on weighted cost of classes (£298.63) plus 1 rehabilitation assessment (£793.09) | ||
| Wound care | 2.5 home nurse visits | Per week | £72.50 | £29 per visit. Based on data of N = 644 patients, aged over 70, who were discharged from acute medical units within 72 hours of admission. PSSRU 2020–1 |
| 12 weeks of home nurse visits | - | £783.00 | Multiplied by number of weeks and weighted to 90%. National Vascular Registry 2021 | |
| 32 weeks of home nurse visits | - | £232 | Multiplied by number of weeks and weighted to 10%. National Vascular Registry 2021 | |
| 100% of patients | £1015 | Weighted cost of classes by severity of wound | ||
| Care home | First year (40 weeks) | Annual | £54,360.00 | Based on £1359 establishment cost per permanent resident week in a local authority own-provision residential care home (PSSRU 2020–1) for 47 weeks per year minus any inpatient rehabilitation in year 1 |
| 16.5% require full-time care | £8969.40 | |||
| Subsequent years (47 weeks) | Annual | £63,873.00 | ||
| 16.5% of patients require full-time care | £10,539.05 | |||
| Community care | First year (45 weeks) | Annual | £7425.00 | Based on £165 per client week within local authority own-provision day care for older people (PSSRU 2020–1) for 52 weeks per year minus any inpatient rehabilitation in year 1 |
| 27.7% of patients require ongoing community care | £2053.01 | |||
| Subsequent year (52 weeks) | Annual | £8580.00 | ||
| 27.7% of patients require ongoing community care | £2372.37 | |||
| Home modifications | Fit handrail – external | Annual | £5.70 | Mean cost annuitised over 10 years by 3.5%. PSSRU 2019/20 |
| Fit handrail – internal | Annual | £4.00 | ||
| Fit handrail to bath | Annual | £2.50 | ||
| Relocation of toilet | Annual | £1383 | ||
| Ramp to front/back door | Annual | £44.00 | ||
| Widen doorway for wheelchair access | Annual | £75.00 | ||
| Stair lift | Annual | £263.00 | ||
| Raise electrical sockets/lower light switches | Annual | £11.40 | ||
| 55.3% of patients remain in the community | £1019.56 | £1788.60 uplifted to 2020–1 prices (£1843.69). PSSRU 2020–1 | ||
| Item | First year | Subsequent years |
|---|---|---|
| Prosthetics | £268.96 | £268.96 |
| Wheelchairs | £93.72 | £93.72 |
| Inpatient rehabilitation | £21,293.09 | – |
| Outpatient rehabilitation | £1091.72 | – |
| Wound care | £1015.00 | – |
| Care home | £8969.40 | £10,539.05 |
| Community care | £2053.01 | £2372.37 |
| Home modifications | £1019.56 | £1019.56 |
| Total | £35,804.46 | £14,293.65 |
Appendix 8 Additional cost-effectiveness analysis results (addendum to the DAR)
Reason for addendum submission
This addendum was prepared by the EAG in response to a request from the NICE technical team to provide additional scenario analyses prior to the first committee meeting. Additional scenarios in the addendum further explore the impact on cost-effectiveness of several different possible alternative time gains for automated tests (i.e. reductions in ulcer healing time) for venous ulcers with a TN test result. Version 2.0 of the addendum provides further scenarios which replicate the time gain scenario analyses from version 1.0, but with removal of the costs of manual Doppler testing. The EAG’s view is that any modelled time gains may only be achievable in settings where there is limited access to manual Doppler testing.
Additional cost-effectiveness results
Three additional scenario analyses are conducted on the ‘moderate’ base case (see Table 36) reporting the impact on results of TN time gains of 6, 8, 12 and 16 weeks. Tables 37 and 38 provide threshold analyses (using NMB) illustrating the reductions in ulcer healing times for TN results that would be required before the tests would be considered cost-effective at threshold values of £20,000 and £30,000 per QALY, respectively.
| Test | Total cost (£) | Incremental cost (£) | Total QALY | Incremental QALY | ICER (ranked) | ICER (vs. manual) |
|---|---|---|---|---|---|---|
| Base case (moderate) – deterministic | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,099 | 138 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,392 | 431 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,424 | 463 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,427 | 466 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,501 | 540 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario A1 – Time gains for TN tests of 6 weeks (42 days) | ||||||
| BlueDop Vascular Expert | 11,782 | 0 | 8.038 | 0.000 | Dominant | |
| WatchBP Office ABI | 11,929 | 147 | 8.038 | 0.000 | Dominated | Dominant |
| WatchBP Office Vascular | 11,930 | 147 | 8.038 | 0.000 | Dominated | Dominant |
| Manual Doppler | 11,961 | 179 | 8.032 | −0.006 | Dominated | |
| boso ABI-system 100 | 12,053 | 271 | 8.037 | −0.001 | Dominated | £20,045 |
| MESI ABPI MD | 12,074 | 292 | 8.037 | −0.001 | Dominated | £23,510 |
| MESI mTABLET ABI | 12,077 | 295 | 8.037 | −0.001 | Dominated | £24,042 |
| Dopplex Ability | 12,153 | 371 | 8.036 | −0.002 | Dominated | £45,662 |
| Scenario A2 – Time gains for TN tests of 8 weeks (56 days) | ||||||
| BlueDop Vascular Expert | 11,677 | 0 | 8.041 | 0.000 | Dominant | |
| WatchBP Office ABI | 11,814 | 138 | 8.041 | +0.000 | £358,512 | Dominant |
| WatchBP Office Vascular | 11,814 | 0 | 8.041 | −0.000 | Dominated | Dominant |
| boso ABI-system 100 | 11,941 | 127 | 8.040 | −0.001 | Dominated | Dominant |
| MESI ABPI MD | 11,958 | 144 | 8.040 | −0.001 | Dominated | Dominant |
| MESI mTABLET ABI | 11,961 | 146 | 8.040 | −0.001 | Dominated | Dominant |
| Manual Doppler | 11,961 | 147 | 8.032 | −0.009 | Dominated | |
| Dopplex Ability | 12,037 | 223 | 8.040 | −0.001 | Dominated | £10,253 |
| Scenario A3 – Time gains for TN tests of 12 weeks (84 days) | ||||||
| BlueDop Vascular Expert | 11,466 | 0 | 8.046 | 0.000 | Dominant | |
| WatchBP Office ABI | 11,585 | 119 | 8.047 | 0.001 | 93,736 | Dominant |
| WatchBP Office Vascular | 11,585 | 0 | 8.047 | 0.000 | Dominated | Dominant |
| boso ABI-system 100 | 11,718 | 133 | 8.046 | −0.001 | Dominated | Dominant |
| MESI ABPI MD | 11,727 | 142 | 8.047 | 0.000 | Dominated | Dominant |
| MESI mTABLET ABI | 11,730 | 145 | 8.047 | 0.000 | Dominated | Dominant |
| Dopplex Ability | 11,808 | 223 | 8.046 | −0.001 | Dominated | Dominant |
| Manual Doppler | 11,961 | 376 | 8.032 | −0.015 | Dominated | |
| Scenario A4 – Time gains for TN tests of 16 weeks (112 days) | ||||||
| BlueDop Vascular Expert | 11,255 | 8.051 | Dominant | |||
| WatchBP Office ABI | 11,357 | 102 | 8.053 | 0.002 | £46,934 | Dominant |
| WatchBP Office Vascular | 11,357 | 0 | 8.053 | 0.000 | Dominated | Dominant |
| boso ABI-system 100 | 11,495 | 139 | 8.052 | −0.001 | Dominated | Dominant |
| MESI ABPI MD | 11,498 | 141 | 8.053 | 0.000 | Dominated | Dominant |
| MESI mTABLET ABI | 11,500 | 144 | 8.053 | 0.000 | Dominated | Dominant |
| Dopplex Ability | 11,580 | 224 | 8.053 | 0.000 | Dominated | Dominant |
| Manual Doppler | 11,961 | 605 | 8.032 | −0.021 | Dominated | |
| Net monetary benefits | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time gain (automated, days) | BlueDop Vascular Expert (£) | Dopplex Ability (£) | MESI ABPI MD (£) | MESI mTABLET ABI (£) | Manual Doppler (£) | WatchBP office ABI (£) | WatchBP office vascular (£) | boso ABI-system 100 (£) |
| 0 | 148,509 | 148,033 | 148,125 | 148,123 | 148,684 | 148,305 | 148,304 | 148,162 |
| 7 | 148,588 | 148,124 | 148,216 | 148,213 | 148,684 | 148,392 | 148,392 | 148,249 |
| 14 | 148,666 | 148,214 | 148,306 | 148,303 | 148,684 | 148,480 | 148,480 | 148,336 |
| 21 | 148,745 | 148,305 | 148,396 | 148,394 | 148,684 | 148,568 | 148,568 | 148,423 |
| 28 | 148,823 | 148,395 | 148,487 | 148,484 | 148,684 | 148,656 | 148,656 | 148,510 |
| 35 | 148,901 | 148,486 | 148,577 | 148,574 | 148,684 | 148,744 | 148,744 | 148,597 |
| 42 | 148,980 | 148,576 | 148,667 | 148,664 | 148,684 | 148,831 | 148,831 | 148,684 |
| 49 | 149,058 | 148,666 | 148,757 | 148,754 | 148,684 | 148,919 | 148,919 | 148,770 |
| 56 | 149,137 | 148,756 | 148,847 | 148,844 | 148,684 | 149,007 | 149,006 | 148,857 |
| 63 | 149,215 | 148,846 | 148,937 | 148,934 | 148,684 | 149,094 | 149,094 | 148,943 |
| 70 | 149,293 | 148,936 | 149,026 | 149,024 | 148,684 | 149,181 | 149,181 | 149,030 |
| 77 | 149,372 | 149,026 | 149,116 | 149,114 | 148,684 | 149,269 | 149,269 | 149,116 |
| 84 | 149,450 | 149,116 | 149,206 | 149,203 | 148,684 | 149,356 | 149,356 | 149,202 |
| 91 | 149,528 | 149,206 | 149,295 | 149,293 | 148,684 | 149,444 | 149,444 | 149,289 |
| 98 | 149,607 | 149,295 | 149,385 | 149,382 | 148,684 | 149,531 | 149,531 | 149,375 |
| 105 | 149,685 | 149,385 | 149,474 | 149,472 | 148,684 | 149,618 | 149,618 | 149,461 |
| 112 | 149,763 | 149,474 | 149,563 | 149,561 | 148,684 | 149,705 | 149,705 | 149,547 |
| Net monetary benefits | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time gain (automated, days) | BlueDop Vascular Expert (£) | Dopplex Ability (£) | MESI ABPI MD (£) | MESI mTABLET ABI (£) | Manual Doppler (£) | WatchBP office ABI (£) | WatchBP office vascular (£) | boso ABI-system 100 (£) |
| 0 | 228,813 | 228,299 | 228,400 | 228,397 | 229,006 | 228,595 | 228,595 | 228,439 |
| 7 | 228,904 | 228,407 | 228,506 | 228,504 | 229,006 | 228,698 | 228,698 | 228,541 |
| 14 | 228,996 | 228,514 | 228,613 | 228,610 | 229,006 | 228,801 | 228,801 | 228,644 |
| 21 | 229,087 | 228,620 | 228,719 | 228,716 | 229,006 | 228,903 | 228,903 | 228,746 |
| 28 | 229,178 | 228,727 | 228,825 | 228,823 | 229,006 | 229,006 | 229,006 | 228,848 |
| 35 | 229,270 | 228,834 | 228,931 | 228,929 | 229,006 | 229,109 | 229,109 | 228,950 |
| 42 | 229,361 | 228,940 | 229,037 | 229,035 | 229,006 | 229,212 | 229,212 | 229,052 |
| 49 | 229,452 | 229,047 | 229,143 | 229,141 | 229,006 | 229,314 | 229,314 | 229,154 |
| 56 | 229,543 | 229,153 | 229,249 | 229,247 | 229,006 | 229,417 | 229,417 | 229,256 |
| 63 | 229,635 | 229,260 | 229,355 | 229,353 | 229,006 | 229,520 | 229,520 | 229,358 |
| 70 | 229,726 | 229,366 | 229,461 | 229,458 | 229,006 | 229,622 | 229,622 | 229,459 |
| 77 | 229,817 | 229,472 | 229,567 | 229,564 | 229,006 | 229,725 | 229,724 | 229,561 |
| 84 | 229,908 | 229,578 | 229,672 | 229,670 | 229,006 | 229,827 | 229,827 | 229,662 |
| 91 | 229,999 | 229,684 | 229,778 | 229,775 | 229,006 | 229,929 | 229,929 | 229,764 |
| 98 | 230,090 | 229,790 | 229,883 | 229,881 | 229,006 | 230,032 | 230,032 | 229,865 |
| 105 | 230,181 | 229,896 | 229,989 | 229,986 | 229,006 | 230,134 | 230,134 | 229,967 |
| 112 | 230,273 | 230,001 | 230,094 | 230,091 | 229,006 | 230,236 | 230,236 | 230,068 |
Tables 39–41 replicate the scenarios, removing the costs of manual Doppler testing.
| Test | Total cost (£) | Incremental cost (£) | Total QALY | Incremental QALY | ICER (ranked) | ICER (vs. manual) |
|---|---|---|---|---|---|---|
| Base case (moderate) – deterministic | ||||||
| Manual Doppler | 11,961 | 8.032 | ||||
| BlueDop Vascular Expert | 12,099 | 138 | 8.030 | −0.002 | Dominated | Dominated |
| WatchBP Office ABI | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| WatchBP Office Vascular | 12,276 | 315 | 8.029 | −0.003 | Dominated | Dominated |
| boso ABI-system 100 | 12,392 | 431 | 8.028 | −0.005 | Dominated | Dominated |
| MESI ABPI MD | 12,424 | 463 | 8.027 | −0.005 | Dominated | Dominated |
| MESI mTABLET ABI | 12,427 | 466 | 8.027 | −0.005 | Dominated | Dominated |
| Dopplex Ability | 12,501 | 540 | 8.027 | −0.006 | Dominated | Dominated |
| Scenario A1 – Time gains for TN tests of 6 weeks (42 days) | ||||||
| BlueDop Vascular Expert | 11,782 | 8.038 | 0.000 | Dominant | ||
| WatchBP Office ABI | 11,929 | 147 | 8.038 | 0.000 | Dominated | Dominant |
| WatchBP Office Vascular | 11,930 | 147 | 8.038 | 0.000 | Dominated | Dominant |
| Manual Doppler | 11,941 | 158 | 8.032 | −0.006 | Dominated | |
| boso ABI-system 100 | 12,053 | 271 | 8.037 | −0.001 | Dominated | £24,492 |
| MESI ABPI MD | 12,074 | 292 | 8.037 | −0.001 | Dominated | £27,764 |
| MESI mTABLET ABI | 12,077 | 295 | 8.037 | −0.001 | Dominated | £28,296 |
| Dopplex Ability | 12,153 | 371 | 8.036 | −0.002 | Dominated | £50,541 |
| Scenario A2 – Time gains for TN tests of 8 weeks (56 days) | ||||||
| BlueDop Vascular Expert | 11,677 | 0 | 8.041 | 0.000 | Dominant | |
| WatchBP Office ABI | 11,814 | 138 | 8.041 | 0.000 | £358,512 | Dominant |
| WatchBP Office Vascular | 11,814 | 0 | 8.041 | 0.000 | Dominated | Dominant |
| Manual Doppler | 11,941 | 126 | 8.032 | −0.009 | Dominated | |
| boso ABI-system 100 | 11,941 | 127 | 8.040 | −0.001 | Dominated | £82 |
| MESI ABPI MD | 11,958 | 144 | 8.040 | −0.001 | Dominated | £2205 |
| MESI mTABLET ABI | 11,961 | 146 | 8.040 | −0.001 | Dominated | £2525 |
| Dopplex Ability | 12,037 | 223 | 8.040 | −0.001 | Dominated | 13,001 |
| Scenario A3 – Time gains for TN tests of 12 weeks (84 days) | ||||||
| BlueDop Vascular Expert | 11,466 | 0 | 8.046 | 0.000 | Dominant | |
| WatchBP Office ABI | 11,585 | 119 | 8.047 | 0.001 | £93,736 | Dominant |
| WatchBP Office Vascular | 11,585 | 0 | 8.047 | 0.000 | Dominated | Dominant |
| boso ABI-system 100 | 11,718 | 133 | 8.046 | −0.001 | Dominated | Dominant |
| MESI ABPI MD | 11,727 | 142 | 8.047 | −0.000 | Dominated | Dominant |
| MESI mTABLET ABI | 11,730 | 145 | 8.047 | −0.000 | Dominated | Dominant |
| Dopplex Ability | 11,808 | 223 | 8.046 | −0.001 | Dominated | Dominant |
| Manual Doppler | 11,941 | 356 | 8.032 | −0.015 | Dominated | |
| Scenario A4 – Time gains for TN tests of 16 weeks (112 days) | ||||||
| BlueDop Vascular Expert | 11,255 | 8.051 | Dominant | |||
| WatchBP Office ABI | 11,357 | 102 | 8.053 | 0.002 | £46,934 | Dominant |
| WatchBP Office Vascular | 11,357 | 0 | 8.053 | 0.000 | Dominated | Dominant |
| boso ABI-system 100 | 11,495 | 139 | 8.052 | −0.001 | Dominated | Dominant |
| MESI ABPI MD | 11,498 | 141 | 8.053 | 0.000 | Dominated | Dominant |
| MESI mTABLET ABI | 11,500 | 144 | 8.053 | 0.000 | Dominated | Dominant |
| Dopplex Ability | 11,580 | 224 | 8.053 | 0.000 | Dominated | Dominant |
| Manual Doppler | 11,941 | 584 | 8.032 | −0.021 | Dominated | |
| Net monetary benefits | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time gain (automated, days) | BlueDop Vascular Expert (£) | Dopplex Ability (£) | MESI ABPI MD (£) | MESI mTABLET ABI (£) | Manual Doppler (£) | WatchBP office ABI (£) | WatchBP office Vascular (£) | boso ABI-system 100 (£) |
| 0 | 148,509 | 148,033 | 148,125 | 148,123 | 148,704 | 148,305 | 148,304 | 148,162 |
| 7 | 148,588 | 148,124 | 148,216 | 148,213 | 148,704 | 148,392 | 148,392 | 148,249 |
| 14 | 148,666 | 148,214 | 148,306 | 148,303 | 148,704 | 148,480 | 148,480 | 148,336 |
| 21 | 148,745 | 148,305 | 148,396 | 148,394 | 148,704 | 148,568 | 148,568 | 148,423 |
| 28 | 148,823 | 148,395 | 148,487 | 148,484 | 148,704 | 148,656 | 148,656 | 148,510 |
| 35 | 148,901 | 148,486 | 148,577 | 148,574 | 148,704 | 148,744 | 148,744 | 148,597 |
| 42 | 148,980 | 148,576 | 148,667 | 148,664 | 148,704 | 148,831 | 148,831 | 148,684 |
| 49 | 149,058 | 148,666 | 148,757 | 148,754 | 148,704 | 148,919 | 148,919 | 148,770 |
| 56 | 149,137 | 148,756 | 148,847 | 148,844 | 148,704 | 149,007 | 149,006 | 148,857 |
| 63 | 149,215 | 148,846 | 148,937 | 148,934 | 148,704 | 149,094 | 149,094 | 148,943 |
| 70 | 149,293 | 148,936 | 149,026 | 149,024 | 148,704 | 149,181 | 149,181 | 149,030 |
| 77 | 149,372 | 149,026 | 149,116 | 149,114 | 148,704 | 149,269 | 149,269 | 149,116 |
| 84 | 149,450 | 149,116 | 149,206 | 149,203 | 148,704 | 149,356 | 149,356 | 149,202 |
| 91 | 149,528 | 149,206 | 149,295 | 149,293 | 148,704 | 149,444 | 149,444 | 149,289 |
| 98 | 149,607 | 149,295 | 149,385 | 149,382 | 148,704 | 149,531 | 149,531 | 149,375 |
| 105 | 149,685 | 149,385 | 149,474 | 149,472 | 148,704 | 149,618 | 149,618 | 149,461 |
| 112 | 149,763 | 149,474 | 149,563 | 149,561 | 148,704 | 149,705 | 149,705 | 149,547 |
| Net monetary benefits | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time gain (automated, days) | BlueDop Vascular Expert (£) | Dopplex Ability (£) | MESI ABPI MD (£) | MESI mTABLET ABI (£) | Manual Doppler (£) | WatchBP office ABI (£) | WatchBP office vascular (£) | boso ABI-system 100 (£) |
| 0 | 228,813 | 228,299 | 228,400 | 228,397 | 229,027 | 228,595 | 228,595 | 228,439 |
| 7 | 228,904 | 228,407 | 228,506 | 228,504 | 229,027 | 228,698 | 228,698 | 228,541 |
| 14 | 228,996 | 228,514 | 228,613 | 228,610 | 229,027 | 228,801 | 228,801 | 228,644 |
| 21 | 229,087 | 228,620 | 228,719 | 228,716 | 229,027 | 228,903 | 228,903 | 228,746 |
| 28 | 229,178 | 228,727 | 228,825 | 228,823 | 229,027 | 229,006 | 229,006 | 228,848 |
| 35 | 229,270 | 228,834 | 228,931 | 228,929 | 229,027 | 229,109 | 229,109 | 228,950 |
| 42 | 229,361 | 228,940 | 229,037 | 229,035 | 229,027 | 229,212 | 229,212 | 229,052 |
| 49 | 229,452 | 229,047 | 229,143 | 229,141 | 229,027 | 229,314 | 229,314 | 229,154 |
| 56 | 229,543 | 229,153 | 229,249 | 229,247 | 229,027 | 229,417 | 229,417 | 229,256 |
| 63 | 229,635 | 229,260 | 229,355 | 229,353 | 229,027 | 229,520 | 229,520 | 229,358 |
| 70 | 229,726 | 229,366 | 229,461 | 229,458 | 229,027 | 229,622 | 229,622 | 229,459 |
| 77 | 229,817 | 229,472 | 229,567 | 229,564 | 229,027 | 229,725 | 229,724 | 229,561 |
| 84 | 229,908 | 229,578 | 229,672 | 229,670 | 229,027 | 229,827 | 229,827 | 229,662 |
| 91 | 229,999 | 229,684 | 229,778 | 229,775 | 229,027 | 229,929 | 229,929 | 229,764 |
| 98 | 230,090 | 229,790 | 229,883 | 229,881 | 229,027 | 230,032 | 230,032 | 229,865 |
| 105 | 230,181 | 229,896 | 229,989 | 229,986 | 229,027 | 230,134 | 230,134 | 229,967 |
| 112 | 230,273 | 230,001 | 230,094 | 230,091 | 229,027 | 230,236 | 230,236 | 230,068 |
Interpretation
The additional analyses in this addendum illustrate that there may be potential for automated tests to be a cost-effective use of resource if it is possible to achieve reductions in ulcer healing time for venous ulcers in the presence of a TN test result. Such reductions in ulcer healing time could only plausibly be achieved in settings where there is limited access to, or required skills among healthcare professionals to complete, manual Doppler testing. The threshold value of reduction in venous ulcer healing time that would need to be achieved before an automated test might be considered cost-effective ranges from 3 to 7 weeks across the different automated test strategies. Tests with higher sensitivity (i.e. fewer FN test results) would likely require a lower reduction in venous ulcer healing time to offset the additional risks of FN test results. Removing the costs of manual testing from these scenarios has minimal impact on results; it only slightly increases the threshold value of time gains for WATCH BP and only at the £30,000 threshold (see Table 7 compared to Table 3).
Despite the potential for cost-effectiveness in a limited setting demonstrated in the threshold analyses presented here, these results should be interpreted cautiously and considered together with the other uncertainties described in the DAR. In particular, the threshold (minimum) reduction in venous ulcer healing time that would be required before a test could be considered cost-effective will depend on the true diagnostic accuracy of the test, the underlying prevalence of arterial disease and assumptions about whether or not inaccurate test results (FN and FP) are acted on in clinical practice. For example, the moderate base-case analysis on which these scenarios are based assumes that a FN test result in a patient with purely arterial disease would not be acted upon (i.e. it is assumed that a holistic patient assessment would mean that strong compression would not be applied to the arterial ulcer). However, in more pessimistic scenarios, where all FN results are acted upon (both arterial and mixed ulcers), the threshold value of reduction in venous ulcer healing time would need to increase further before the automated tests could be considered cost-effective.
The EAG is of the view that these additional assumptions, together with uncertainty surrounding the underlying diagnostic accuracy in a population of leg ulcer patients, mean that the threshold value of reductions in venous ulcer healing time that an automated test would need to achieve before being cost-effective is highly uncertain.
List of abbreviations
- ABPI
- ankle–brachial pressure index
- AUC
- area under the curve
- CEAC
- cost-effectiveness acceptability curve
- CKS
- clinical knowledge summary
- CLI
- critical limb ischaemia
- CRD
- Centre for Reviews and Dissemination
- CTA
- computerised tomography angiography
- CUA
- cost–utility analysis
- DAP
- Diagnostics Programme
- EAG
- external assessment group
- EVRA
- early venous reflux ablation
- F (2,3,4)
- Fontaine stages of arterial disease (stages 2, 3 and 4)
- FN
- false negative
- FP
- false positive
- GP
- general practitioner
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICH
- intracranial haemorrhage
- IQR
- interquartile range
- KM
- Kaplan–Meier
- LN
- log normal
- MI
- myocardial infarction
- MRA
- magnetic resonance angiography
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- NR
- not reported
- NWCSP
- National Wound Care Strategy Programme
- PAD
- peripheral artery disease
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALW
- quality-adjusted life week
- QALY
- quality-adjusted life-year
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies – version 2
- ROC
- receiving operating characteristics
- RR
- relative risk
- SCM
- Specialist Committee Members
- SD
- standard deviation
- SIGN
- Scottish Intercollegiate Guidelines Network
- THIN
- The Health Improvement Network
- TN
- true negative
- TP
- true positive
- VLU
- venous leg ulcer
Notes
Note
This monograph is based on the Diagnostic Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Diagnostic Advisory Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
