Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 12/186/01. The contractual start date was in January 2015. The draft manuscript began editorial review in January 2023 and was accepted for publication in June 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Wittmann et al. This work was produced by Wittmann et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Wittmann et al.
Chapter 1 Introduction/background
Hand eczema (HE) is one of the most common skin disorders and an important cause for morbidity and occupational disability. Estimated prevalence values summarised in a meta-analysis reported by Quaade et al. are 14.5% for the lifetime prevalence, 9.1% for the 1-year prevalence and 4% for the point prevalence in the general population. 1 Women are more frequently affected than men (1.5–2 times more often). This meta-analysis also showed that around one-third of patients had a history of atopic dermatitis (AD). The proportion of patients who develop severe chronic hand eczema (CHE) is estimated to be 5–7% of all patients with HE. 2,3 HE has a strong tendency to become a chronic condition, and epidemiology data have clearly shown that the longer it exists, the more difficult it becomes to treat. 4 Of note, a delay in medical attention to HE has been highlighted. 5
The impact on daily life of sufferers is considerable. 6–8 Due to the hands being constantly in use and being so important for our functioning in daily life, impairment of their use due to pain, losing of grip, loss of tactile properties or avoidance of showing the hands due to embarrassing symptoms has a significant impact – much more so than eczema symptoms on other parts of the body. The psychosocial burden of HE has been highlighted recently, whereby patients with HE have a greater level of anxiety and depression compared with a control group of hospital employees. 9 Ninety-six per cent of people with HE reported impairment of their social life due to HE. 10 Moreover, patients present with intensely itchy lesions on their hands, which can be red, scaly or vesicular, swollen, and often show cracks and splits, which are painful. 2,11 Around 27% of HE patients also experience symptoms on the feet, which can make walking painful. 12
The disease burden for the patient can be considerable, impairing functioning in daily life and at work. As a consequence the economic burden has been estimated to include the following for CHE (thus also including less severe forms) across several countries: on average, total annual costs of illness ranged from EUR 1311 to EUR 9792, and more severe HE and occupational HE resulted in higher costs. 13,14 Moreover, 57% of patients take sick leave and up to 25% reported job loss/job change due to CHE. 15
Hand eczema is a heterogeneous disease that presents with various subtypes. 14,16,17 A variety of underlying genetic and lifestyle-related causes can contribute to this complex disease. 16,18 The multiple underlying trigger factors (mechanical, psychological stress, hormonal influences, irritant exposure, etc.), different morphological presentations and co-morbidities cause difficulties in classifying disease subentities. As a consequence, there is no generally accepted disease classification. Clinical experts distinguish between different causes of HE, including atopic diathesis, delayed-type hypersensitivity reactions (contact allergy) and irritant dermatitis. With regard to morphology, HE is referred to as dyshidrotic, pompholyx, hyperkeratotic, rhagadiform, pulpitis sicca/fingertip dermatitis, tylotic, psoriasiform or combinations of these morphologies. However, morphology alone has not proven sufficient to choose the best-suited therapeutic approach. 19 Of note, while nail involvement has been extensively described, classified and assessed in psoriasis,20 a clear and comprehensive assessment tool for the extent and morphology of eczema-associated nail changes is lacking. There is also a shortcoming in the recognition and description of eczema lesions in skin of colour. It has become apparent that due to difficulties in assessing erythema in darker skin types, eczema severity may be under-recognised in this patient group. In line with this, potential different therapeutic responses across different ethnicities have not been investigated. As a range of assessment tools for HE severity and photographs have been performed for participants with skin of colour, the ALPHA trial provides an opportunity to further explore HE assessment challenges in this subgroup of patients.
Risk and trigger factors
A number of factors have been described that influence the onset or flare-up of HE. As mentioned, HE in the general population affects women about twice as often as men. 21–23 AD and/or childhood eczema, which is almost always associated with dry skin and skin barrier impairment, seems to be one of the most important risk factors. 24,25 Exposure to wet work, frequent hand washing and irritants are recognised relevant trigger factors. A high HE incidence rate is thus associated with contact allergy, AD, wet work and also female sex. 23
Important genetic factors are those linked to AD, and the most recognised ones are loss of function (LOF) mutations in the filaggrin gene. Filaggrin is an important constituent of the skin barrier. 26 Filaggrin significantly contributes to water retention in the skin, while LOF mutations result in increased transepidermal water loss. There are four common filaggrin mutations prevalent among Europeans and more frequently found in patients with AD. 27–29 Deletions in the late cornified envelope (LCE) genes LCE3B and LCE3C have been described to be associated with CHE with allergic contact dermatitis. 30 The LCE complex is also a key component of the skin barrier.
To date, it is not fully elucidated whether the association of HE with AD is primarily due to filaggrin and skin barrier changes or due to atopy resulting in a type 2 shift in the immune response. The ALPHA trial collected information on LOF filaggrin mutation, LCE variants and atopy status.
Lifestyle factors discussed to a great extent include tobacco smoking. However, despite a wealth of available observational, retrospective studies, the data are still not clear, and overall the association seems weak; it may be stronger for foot eczema. 31–37 No study has found an association between alcohol consumption and HE. 35 Predictive factors for HE persistence include (1) age of onset before 20 years, (2) positive history of childhood eczema (3) and moderate to severe extension of HE – thus, HE severity. 23
As there is no clear subclassification of HE, there is also a lack of clear treatment guidelines. 38 Mostly, CHE therapy is delivered in escalating steps. When patient education, allergen/irritant avoidance and topical treatment are not sufficient to control the disease, ultraviolet (UV) therapy or systemic immune-modifying drugs are used. 17,39 Alitretinoin (AL) is licensed for severe CHE unresponsive to treatment with potent topical corticosteroids. However, currently available clinical evidence for the treatment of CHE is not compelling enough to guide clinical practice. 39,40 The lack of clear evidence-based data has been outlined by different national and international expert groups as well as by a recent Cochrane review. 38,41 Given the high socioeconomic impact of the disease, there is a pressing need for comparative studies on available first-line treatments and on the long-term outcome of currently used therapies. The National Institute for Health and Care Research (NIHR) has recognised this need and launched a commissioned call aiming to compare AL with other treatment options. Our choice of comparator for AL was based on published clinical trials and on feedback from UK dermatologists and the United Kingdom Dermatology Clinical Trials Network (UKDCTN). 41 As research and audit data on treatment choices for CHE were unavailable, we performed a survey among 194 UK dermatologists; the most frequent first-choice approaches for CHE were 8-methoxypsoralen combined with ultraviolet A (PUVA), oral steroids and AL. 42 When asked which strategy was thought to be most efficient for long-term outcome, 43% of clinicians reported AL, 30% PUVA and a further 20% of clinicians indicated that they did not know.
The following immunosuppressive treatments have been described as being used for the treatment of CHE: azathioprine, mycophenolate mofetil, methotrexate, ciclosporin A and systemic corticosteroids. Recently described drugs include the interleukin (IL)-4/IL-13R blocker dupilumab and Jak inhibitors including baricitinib and upadicitinib, all of which are licensed for the treatment of AD. 43,44 Clinical trials of topical Jak inhibitors are being conducted, and phase IIb results for delgocitinib have recently been reported. 45
The current literature suggests that dyshidrotic/vesicular subtypes with atopic diathesis respond preferentially to immunosuppressants and dupilumab, whereas hyperkeratotic rhagadiform subtypes may show a better response pattern to AL. However, there is insufficient clarity on which subtypes respond to which therapy approaches.
A generally unfavourable therapeutic response is believed to be linked to smoking, long disease duration and barrier defects such as LOF mutations affecting filaggrin. 5,11,39 There are two important shortcomings affecting clinical trials in CHE. Firstly, there is no consensus regarding the best outcome measures of extent and severity of the disease, disease impact on quality of life (QoL), or assessment of comorbidities, and there is a lack of measures to highlight impairment in functional use of the hands. Numerous disease severity assessments have been proposed, including photoguide, Physician’s Global Assessment (PGA), Hand Eczema Severity Index (HECSI), Hand Eczema Extent Score (HEES) and modified Total Lesion Symptom Score (mTLSS). 46,47 Although none has been fully recognised as ‘gold standard’ yet, the HECSI is predominantly used in clinical studies and has advantages regarding accurate assessment of morphology features and extent. 48,49 ALPHA has used the HECSI as the primary outcome but included other outcome measures frequently used in previous trials. The second shortcoming relates to the short duration of clinical trials, most of which have been limited to 12 weeks. This indeed seems inadequate for a disease characterised by its tendency for chronicity and which often has a long disease duration.
Summary
There is a lack of controlled clinical trials that directly compare treatments or demonstrate effectiveness under daily practice conditions. Moreover, the lack of clear evidence-based data has been outlined by a number of national and international expert groups. 38,41 Given the high socioeconomic impact of the disease, there was a pressing need for comparative studies on available first-line treatments and on long-term outcomes of currently used therapies. Therefore, we conducted a randomised controlled trial (RCT) comparing AL with Immersion PUVA for patients with severe CHE, who were followed up for a maximum period of 52 weeks to understand the longer-term impact of these treatments as first-line therapies.
Chapter 2 Methods
Aims and objectives
The primary aim was to determine the clinical effectiveness and cost-effectiveness of AL compared with 8-methoxypsoralen combined with UV-A (Immersion PUVA) in conjunction with concomitant topical corticosteroids, emollients and patient education as the first-line treatment in patients with severe CHE.
Primary trial objective
The primary objective is to compare AL and Immersion PUVA in conjunction with concomitant topical corticosteroids, emollients and patient education as first-line therapy in terms of disease activity at 12 weeks post planned start of treatment.
Secondary objectives
-
To compare AL and Immersion PUVA in terms of disease activity over time with a focus on disease activity at 24 and 52 weeks post planned start of treatment.
-
To compare AL and Immersion PUVA in terms of time to relapse.
-
To compare AL and Immersion PUVA in terms of QoL and patient benefit over the 52 weeks’ duration post planned start of treatment.
-
To determine the cost-effectiveness of AL compared with Immersion PUVA at week 12, 52 (short term) and over 10 years (long term).
-
To determine the educational need for individual patients.
-
To compare AL and Immersion PUVA in terms of safety.
Exploratory objectives
-
To compare scoring systems HECSI, mTLSS, Dermatology Life Quality Index (DLQI) and PGA used to monitor response to treatment in patients with severe CHE.
-
To evaluate whether response to first-line treatment is affected by the following parameters:
-
duration of disease
-
clinical phenotype
-
disease severity
-
presence of atopy
-
filaggrin LOF mutation and other potential emerging mutations affecting skin barrier or response to Immersion PUVA/AL
-
smoking history
-
body mass index (BMI)
-
foot involvement.
-
-
To collect pilot data on clinical effectiveness of second-line therapies, using HECSI and PGA.
-
To explore treatment responses in HE subgroups defined by molecular inflammatory mediators determined in tape strips or washing solution.
-
To compare AL and Immersion PUVA in terms of time in remission using different definitions of end of remission, including varying the extent of corticosteroid use.
-
To compare AL and Immersion PUVA in terms of assessment of the nails.
-
To explore the use of the photography guide for patients of non-Caucasian ethnicity.
Note that objectives 6 and 7 were added after the trial was funded and are not reported within this monograph, but will be reported in separate publications.
Overview of methods
This chapter outlines the main methods for the trial, including all data collection for the trial and substudy work and the analysis methods for the primary and secondary clinical results, which are detailed in Chapter 3. Analysis methods and results for the main trial health economics are detailed in Chapter 4. The discussion chapter draws the work together in Chapter 5.
Trial design
The trial was a multicentre, Phase III, open, prospective, adaptive, two-arm parallel group RCT, with one planned interim analysis.
Participants were randomised on a 1 : 1 basis to receive either AL at a dose of 30 mg/day (with the option to reduce to 10 mg if participants suffered with headaches and restored to 30 mg dose once headaches ceased) or Immersion PUVA (3 mg/l Meladinine® with UV A, twice weekly) for a 12-week interventional phase. Randomised treatment was given in conjunction with concomitant topical corticosteroids, emollients and patient education. Partial responders in both arms continued their randomised treatment for up to a further 12 weeks. Treatment could be discontinued between 12 and 24 weeks if participants achieved a clear/almost clear assessment (responder) or had a severe assessment (non-responder). All participants stopped treatment by 24 weeks. Participants who relapsed and non-responders continued with ‘standard clinical practice’, as determined by the attending clinical team.
The study protocol for this trial has already been published. 50 Summary details of the methods are given below.
Ethical approval
Ethical approval for the study was given by Leeds West Research Ethics Committee [REC reference: 14/YH/1259, Integrated Research Application System (IRAS) project ID: 163195]. All trial activity took place according to the ethically approved protocol, principles of Good Clinical Practice (GCP) and Declaration of Helsinki 1996.
Eligibility and informed consent
Patients were screened in secondary care dermatology outpatient, community hospital and General Practice settings. Formal eligibility assessment and recruitment was undertaken in secondary care dermatology outpatient clinics. Patients were eligible if they fulfilled the following criteria:
-
suffering from uncontrolled, severe CHE, defined as the presence of both of the following criteria:
-
PGA score of severe
-
resistance to treatment with potent topical corticosteroids for ≥ 4 weeks prior to the point of eligibility screening
-
-
aged ≥ 18 years
-
able to provide written informed consent
-
expected to comply with treatment and protocol schedule.
Patients were excluded if they fulfilled any of the following criteria:
Skin-related:50
-
had a clinically suspected infection (fungal, bacterial or viral) as cause for dermatitis of the hands
-
known clinically relevant allergic contact dermatitis of the hands, unless they had made a reasonable effort to avoid the contact allergen
-
atopic eczema covering more than 10% of body surface (excluding hands)
-
had skin conditions worsened by the sun, that is, do not tolerate UV-light (for example lupus erythematosus, porphyria).
Treatment-related:
-
received systemic vitamin A derivatives or systemic immunosuppressants, for example, methotrexate or biologics treatment for HE, or received phototherapy/photochemotherapy, in the 3 months prior to randomisation
-
received ciclosporin A or systemic glucocorticoid steroid treatment for HE or topical calcineurin antagonist treatment within 1 week prior to randomisation
-
receiving concomitant treatment with tetracyclines, medication with potential for drug–drug interaction with AL (e.g. CYP3A4 inhibitor ketoconazole), or concomitant treatment with relevant photosensitisers, that cannot be suspended or switched to an acceptable alternative
-
history of melanoma skin cancer, or patients with a history of non-melanoma skin cancer depending on history, location and ‘severity’ of the non-melanoma skin cancer based on experience from routine practice
-
received prior treatment with arsenic agents or ionising radiation in the treatment area (e.g. hands).
General:50
-
if female:
-
lactating
-
of child bearing potential [Woman of Child Bearing Potential (WCBP)] and:
-
- with positive pregnancy test (absence of pregnancy confirmed with a negative pregnancy test before randomisation)
-
- unwilling to follow pregnancy prevention programme measures (rigorous contraception for women of childbearing potential, unless exempt according to standard of care practice, was required 1 month before treatment, during the treatment period and 1 month after cessation of treatment as per usual standard practice) while receiving treatment and after the last dose of protocol treatment as indicated in the relevant summary of product characteristics (SmPC)
-
-
-
hepatic insufficiency (alanine aminotransferase and/or aspartate aminotransferase > 2.5 times the upper limit of normal), known severe renal insufficiency, uncontrolled hyperlipidaemia [for all of the following: triglycerides, cholesterol and/or low-density lipoprotein (LDL) cholesterol] or uncontrolled hypothyroidism in the 12-week period prior to randomisation
-
known hypersensitivity to peanut, soya or vitamin A derivatives or with rare hereditary fructose intolerance as determined by patient history
-
suffering from hypervitaminosis A as directed by clinical symptoms or patient history
-
previous participation in the ALPHA trial.
Blood samples were required to confirm eligibility and atopy status prior to randomisation. Research sites could choose to use either a one-stage process to obtain full informed consent or a two-stage consent process involving obtaining consent for blood sampling to confirm eligibility and atopy status, and then full informed consent for trial participation. If a period of > 12 weeks elapsed prior to the baseline visit, the eligibility blood test was redone.
If available and within the 12 weeks prior to baseline visit, existing blood results (taken for other reasons) in participants’ medical notes could have been used to confirm eligibility. Similarly, if existing atopy results {bon presence/absence of specific immune globulin E [IgE] to inhalant or other relevant allergens as appropriate [e.g. via prick test, Radioallergosorbent Test (RAST) blood test]}, were available in participants’ hospital notes, a blood sample to confirm atopy status was not required.
A log was completed of all patients screened but not recruited, either because they were ineligible or because they were eligible but declined participation. The following anonymised information was included:
-
age
-
gender
-
ethnicity
-
date screened
-
how the patient first heard about the trial
-
the reason why they were not eligible for study participation, OR
-
the reason for declining participation.
Interventions
Participants were randomised to either AL or Immersion PUVA for a minimum of 12 weeks and a maximum of 24 weeks. In both arms of the study, it was expected that patients would follow good self-care practices in the use of emollients, irritant avoidance and – if applicable – diligent continuation of contact allergen avoidance. Education on HE in using emollients, avoiding irritants and relevant contact allergens was delivered face to face in a standardised way based on study-specific educational information by the research nurse involved in the trial. The same education material was handed to participants. The information material for participants was based on sources used in clinical practice [British Association of Dermatology (BAD), National Eczema Society, Eczema Society patient information leaflets].
According to standard clinical practice and National Institute for Health and Care Excellence (NICE) guidelines, AL was administered at a starting dose of 30 mg, to be taken once daily with the main meal. Participants self-administered the treatment at home. Dose adjustment of AL down to 10 mg or temporary cessation (i.e. dose interruption) was permitted according to standard practice in participants who suffered from AL-related headaches.
For participants allocated to Immersion PUVA, hands were immersed for 15 minutes in a Meladinine® 0.75% solution diluted to 3 mg/l for 15 minutes followed by up to a 30-minute delay before exposure to UV-A radiation according to standard practice at the participating site; all sites operated based on ‘British Photodermatology Group’ guidelines. The exact dose of UV-A radiation that participants received was individually tailored to the participant depending on phenotype (as per BAD guidelines) and the erythematous response of the skin following treatment.
The treatment was performed in outpatient phototherapy departments within secondary care units and was administered and supervised by the specialised nurses/dermatologists. Treatments were carried out twice weekly.
As directed by NICE guidelines for AL, the PGA score directed treatment pathway decisions as determined by the treating clinician.
Criteria of response
Criteria are shown in the protocol schedule (Figure 1).
FIGURE 1.
Protocol schedule.
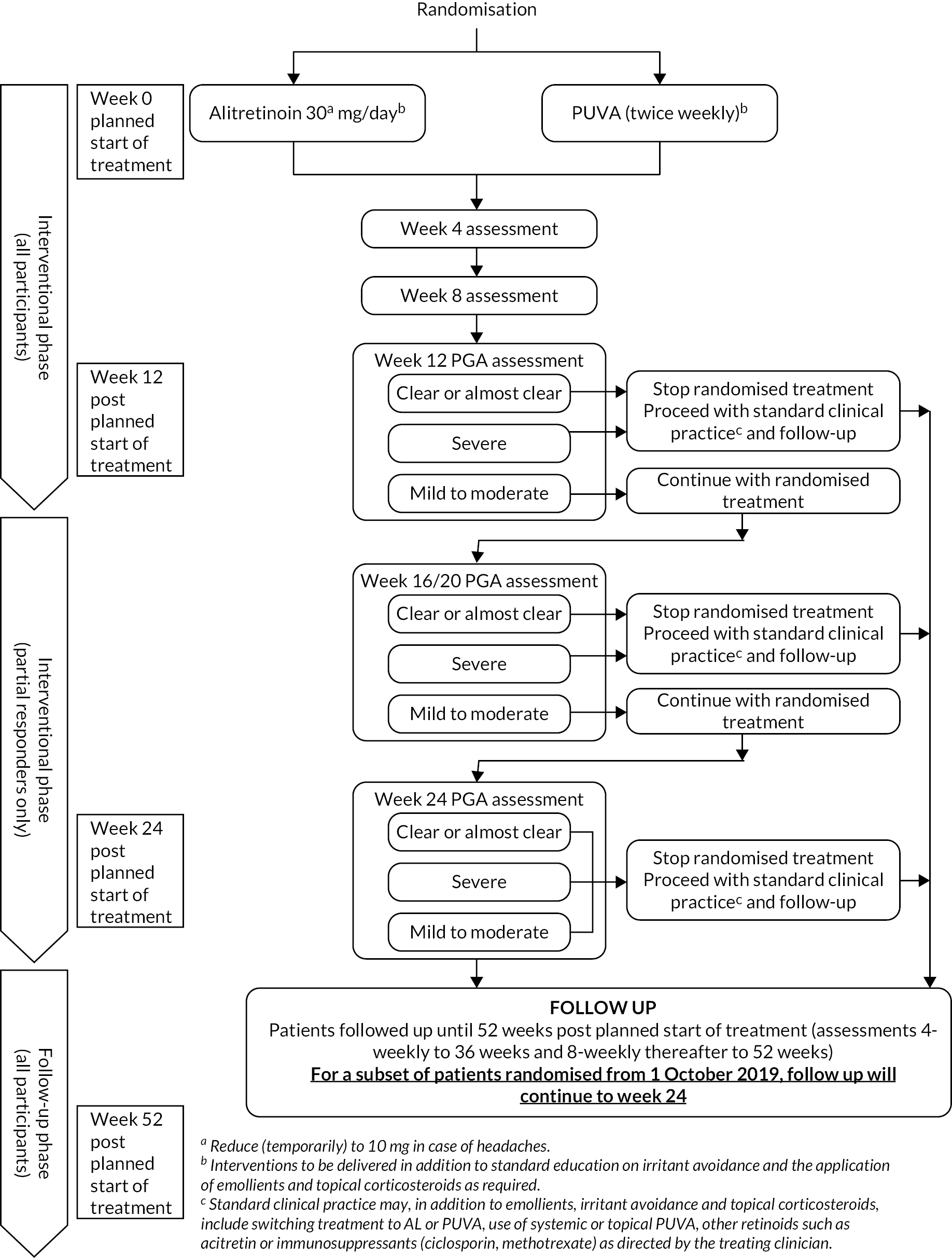
Responders: defined as a PGA score of clear/almost clear at 12 weeks post planned start of treatment, discontinued randomised treatment and continued to receive ‘standard clinical practice’ and follow-up monitoring.
Partial responders: defined as a PGA score of mild/moderate, continued with randomised treatment for up to 24 weeks post planned start of treatment. During this 12- to 24-week treatment period, the patients were monitored at 4-weekly intervals; randomised treatment could be stopped at any time if the patient responded (PGA score of clear/almost clear) or if the symptoms worsened (PGA score of severe) and in the opinion of the attending clinical team there was no clinical benefit to continuation. All randomised treatment was discontinued at the maximum 24 weeks’ treatment period, and patients continued to receive ‘standard clinical practice’ and follow-up monitoring.
Non-responders: defined as a PGA score of severe at 12 weeks post planned start of treatment, discontinued randomised treatment and continued to receive ‘standard clinical practice’ and follow-up monitoring.
At the end of trial treatment and during follow up, treatment was at the discretion of the attending clinical team as per ‘standard clinical practice’. Follow-up monitoring continued until 52 weeks post planned start of treatment, with the exception of participants randomised from 1 October 2019, who completed follow-up assessments for 24 weeks post planned start of treatment.
Randomisation
Recruitment of participants to the ALPHA trial required a blood sample taken within 12 weeks of the baseline visit to confirm eligibility and atopy status, and at least 1 month’s duration of the contraception prevention programme prior to randomisation (if applicable) to confirm eligibility. Patients were registered by an authorised member of the attending clinical or research team and were issued a unique trial number. Registration was performed using either the 24-hour automated registration telephone system or via a web address based at the Clinical Trials Research Unit (CTRU).
Participants were randomised once eligibility was confirmed and the baseline assessments and questionnaires were completed. Participants were randomised in a 1 : 1 allocation ratio to receive either AL or Immersion PUVA in conjunction with concomitant topical corticosteroids, emollients and patient education. A computer-generated minimisation programme that incorporated a random element equal to 0.8 was used to ensure intervention groups were well balanced for the following factors:
-
randomising site
-
disease duration (< 6 months/6–24 months/> 24 months)
-
clinical phenotype (predominantly hyperkeratotic/predominantly vesicular/fingertip dermatitis)
-
atopy status as determined by presence of specific IgE to inhalant allergens (prick test or RAST blood test to detect specific IgE to inhalant or other suspected allergens as appropriate)
-
DLQI (< 15, ≥ 15)
-
skin type (white/fair/dark).
A total of 100 participants who consented to take part in a tape stripping (non-invasive epidermal sampling) substudy were selected at their baseline visit. A quota sampling approach was taken so that the first 25 participants allocated to Immersion PUVA with predominantly hyperkeratotic CHE, the first 25 participants allocated to Immersion PUVA with predominantly vesicular CHE, the first 25 participants allocated to AL with predominantly hyperkeratotic CHE, and the first 25 participants allocated to AL with predominantly vesicular CHE were selected. Participants presenting exclusively with fingertip dermatitis were excluded from this substudy. Epidermal samples in the form of tape strip samples for randomised participants were obtained from both lesional skin and non-lesional, healthy-looking skin of the forearm. Full details of the sample technique are provided in Appendix 1.
Blinding
The trial was openlabel, as participants and investigators could not be blinded to treatment allocation due to the nature of the Immersion PUVA intervention. However, the assessment of the HE severity scores (HECSI, mTLSS and PGA) was undertaken by an assessor who was blinded to the randomised treatment. Participants were reminded not to reveal which treatment they had received to the blinded assessors in order to preserve blinding.
In addition, photographs were taken, for 20% randomly identified, consenting participants of white ethnicity and all consenting participants from minority ethnic groups from each centre, at baseline and 12 weeks post planned start of treatment. A blinded central review of the photographs was conducted to assess intercentre differences in severity scoring.
Outcome measures
Clinical symptoms
The HECSI is a validated scoring system that resembles the clinically well-established Psoriasis Area Severity Index (PASI) score and takes into account disease extent, which is an important prognostic factor for HE. 19,51 The hands are divided into five areas [fingertips, fingers (excluding tips), palm of hands, back of hands, wrists], and each of these five areas is given a score from 0 to 3 for the intensity of pre-specified clinical symptoms (erythema, infiltration/papulation, vesicles, fissures, scaling, oedema) and a score of 0 to 4 for the extent of disease on each area. For each area, the extent of the disease is multiplied (to obtain the product) by the sum of the intensity scores assigned to each clinical symptom. All these products are then added together to give an overall HECSI score, which ranges from 0 to 360, with a higher score indicating greater severity. The PGA is a five-level score (clear, almost clear, mild, moderate, severe) and was used in line with NICE guidelines to determine the HE severity and eligibility of patients for the study. 52 The PGA has been used in all HE studies involving AL. For the treating clinician, this was assessed as a global score for the participant, but for the blinded assessor, the PGA was recorded for each side of each hand, and the most severe score was taken as the overall PGA.
The mTLSS has been used widely in the past. 52 Similarly to the HECSI, the mTLSS considers seven eczema-related symptoms, erythema, scaling, lichenification/hyperkeratosis, vesiculation, oedema, fissures and pruritus/pain, but it does not take extent of HE into account. In line with the PGA assessment, the mTLSS was recorded for each side of each hand. Each symptom was given a score between 0 and 3, such that a score of 1 corresponded to mild, 2 corresponded to moderate and 3 corresponded to severe. The total mTLSS score, on a patient basis, was derived by adding up the scores for each side of each hand (as assessed by the blinded assessor), and the overall mTLSS was the maximum score, so that it corresponded to the most affected side of the most affected hand. The total score ranges from 0 to 21, where a higher score indicates greater severity.
The Person-Centred Dermatology Self-Care Index (PeDeSI) is a validated 10-item questionnaire that measures the education and support needs of people with long-term skin conditions. 53 It is completed in collaboration between patient and practitioner/unblinded nurse and is quick and simple to use in practice. Each item has a score range of 0–3, and total scores indicate as follows: 0–10, needs intensive education and support to develop knowledge, ability and confidence; 11–20, needs some education and support to develop knowledge, ability and confidence; 21–29, needs limited education and support to develop knowledge, ability and confidence; 30, has sufficient knowledge, ability and confidence to manage on their own.
Participant-completed questionnaires
The DLQI is a validated patient-reported outcome measure of the effect of skin disease on a patient’s daily activities and is widely used. 54,55 The DLQI has a simple method of score interpretation: no impact (0–1), small impact (2–5), moderate impact (6–10), very severe impact (11–20) and extremely severe impact (21–30). 56 The DLQI has been used in most large HE studies and is used in the definition of severe CHE in the NICE guidelines (NICE TA177).
The Patient Benefit Index for chronic hand eczema (PBI-HE) is a disease-specific patient-reported tool for HE and is an extension of the PBI, which was developed based on the finding that the physician’s perspective only partly corresponds with the patient’s perspective when it comes to benefit measurements. 57–59 The score is calculated from the importance of needs before therapy and the achievement of these needs. The PBI ranges from 0 (no benefit) to 4 (maximal benefit).
The EuroQol-5 Dimensions, three-level version (EQ-5D-3L), questionnaire consists of five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression, each having three levels of response. 60 The EQ-5D is a generic instrument (www.euroqol.org) and forms part of the NICE reference case for cost per quality-adjusted life-years (QALY) analysis.
The Health Resource Utilisation and Private Costs questionnaire was used to measure participant-reported healthcare use, days off work and private costs due to CHE using a bespoke short self-reported form developed at the University of Leeds. Healthcare use included the number of contacts with clinical staff (occupational health, primary care staff, dermatologists, etc.) and medications because of CHE.
Safety monitoring
This was a RCT using established medicinal products with well-known safety profiles. In recognition of this, events fulfilling the definition of an adverse event (AE) or serious adverse event (SAE) were not reported unless they were: (1) expected and related to trial treatment, (2) related to HE and classified as a- SAE or (3) related to trial treatment and classified as a serious adverse reaction (SAR) or suspected unexpected serious adverse reaction (SUSAR). The following expected AEs/reactions were known to be related to the trial treatments and were not reported unless assessed as serious: headaches and dry skin on regions of the body other than the hands for participants allocated to AL, and erythema (mild to moderate) or itching of skin in Immersion PUVA-treated skin locations for participants allocated to Immersion PUVA. Safety monitoring was conducted throughout each participant’s follow-up period: that is, 52 weeks post planned start of treatment for participants randomised before 1 October 2019 and 24 weeks post planned start of treatment for participants randomised from 1 October 2019.
Data collection schedule
Clinical data and patient-reported data were collected at baseline, every 4 weeks to week 36 and 8-weekly thereafter to week 52. For participants randomised after 1 October 2019, data collection was stopped at week 24. Trial activity was paused due to the COVID-19 pandemic from March 2020 in all centres. Some centres restarted research activity from July 2020. When trial activity restarted, visits were permitted to be conducted in person or virtually (telephone or video call), and QoL questionnaires were posted to participants where possible. The primary end point was only permitted in person, with visits at 12 weeks and 24 weeks prioritised.
Baseline
The baseline visit was booked within 7 days of the first Immersion PUVA appointment, within 12 weeks of the eligibility blood sample and after at least 1 month’s duration of the pregnancy prevention programme (if applicable).
Data collected included: full written informed consent for the study and substudies (if appropriate), treating clinician, mobile phone number for participants consenting to weekly text reminder service, relevant medical history (including date of HE diagnosis, patient and family history of relevant diseases, other eczema locations, previous treatments for HE and exposure to irritants), urine pregnancy test (for all WCBP), smoking history, height and weight, dominant hand, hand that interferes with their daily life the most, IgE results, visual assessment of HE clinical phenotype of the hands and assessment of potential foot involvement, PGA by treating clinician, PeDeSI by treating clinician/unblinded nurse, gene variant analysis blood sample. Also PGA, HECSI and mTLSS assessments by blinded assessor, nail involvement assessment by a blinded assessor (at participating centres) and patient-completed questionnaires (DLQI, PBI-HE and EQ-5D-3L).
After randomisation, the following data were collected: photographs of each side of each hand and substudy sample acquisition (tape stripping) for randomly identified participants.
Interventional phase and follow-up phase
Data collected at 4, 8, 12, 16, 20, 24, 28, 32, 36, 44, 52 weeks included: HE topical corticosteroid usage (obtained from a review of participant medication diaries in conjunction with clinical records), reportable adverse reactions, PGA by treating clinician; PGA and HECSI by blinded assessor, nail assessment by blinded assessor (at participating centres); and patient-completed questionnaires (DLQI).
Data collected at 4, 8, 12, 16, 20, 24 weeks included: randomised treatment compliance (obtained from a review of participant medication diaries in conjunction with clinical records). Note that treatment compliance data were only collected at 16, 20, 24 weeks if the participant continued on treatment; otherwise, they moved into the follow-up phase.
Data collected at 16, 20, 24, 28, 32, 36, 44, 52 weeks included: treatment received under ‘standard clinical practice’. Note that these data were only collected at 16, 20, 24 weeks if the participant had discontinued their randomised treatment phase.
Data collected at 12, 24, 36, 52 weeks included: mTLSS by blinded assessor; patient-completed questionnaires (PBI-HE, EQ-5D and Health Resource Utilisation).
Data collected at week 12 only: photographs of each side of each hand for randomly identified participants.
Data collected at 12 and 52 weeks included: PeDeSI by treating clinician/unblinded nurse. Note that for participants randomised after 1 October 2019, the PeDeSI was collected at week 24 instead of week 52.
End points
Primary end point
The primary end point was defined as the natural logarithm of the HECSI at 12 weeks post planned start of treatment. In the event of HECSI scores of zero, a pre-planned adjustment to take the natural logarithm of the HECSI + 1 was included in the Statistical Analysis Plan (SAP).
Secondary end points
-
The HECSI, mTLSS and PGA over 52 weeks post planned start of treatment.
-
Time to relapse, defined as the time between achieving clear/almost clear overall on the blinded assessor PGA and scoring 75% of their baseline HECSI, with the sensitivity of this definition assessed by redefining relapse as achieving 50% of their baseline HECSI.
-
The DLQI and PBI-HE over 52 weeks post planned start of treatment.
-
The PeDeSi at 12 and 52 (or 24) weeks post planned start of treatment.
-
Reported AEs and SAEs over 52 weeks post planned start of treatment.
-
Cost-effectiveness of AL compared with Immersion PUVA at week 12, 52 (short term) and over 10 years (long term).
Exploratory end points
-
Time to the end of remission defined as time from entering clear/almost clear according to the blinded assessor during the randomised treatment phase, to no longer being clear/almost clear.
Trial organisational structure
The Trial Sponsor was the University of Leeds; responsibilities were delegated to the (CTRU) as detailed in the trial contract.
Trial oversight and management were conducted by the Trial Management Group (TMG), the Trial Steering Committee (TSC) and the Data Monitoring and Ethics Committee (DMEC). All groups met regularly throughout the trial.
Data quality and monitoring were performed by the CTRU.
Statistical methods50
Sample size
Due to the positively skewed nature of the data, the trial was designed to detect a relative difference in treatment effects. A minimum of 500 and maximum of 780 participants were required to detect a relative difference, or fold change, of 1.3 (clinical opinion) in HECSI score between treatment arms at 12 weeks post planned start of treatment (80% power; two-sided 5% significance level) assuming a coefficient of variation (CV) between 1.175 and 1.7 and allowing for 20% attrition. A sample size review was planned after 364 participants (precision of −0.132 and + 0.168 assuming CV = 1.2) had reached 12 weeks post planned start of treatment, to revise the CV and the final sample size.
Planned recruitment rate
At the start of the trial, we estimated that we would need to screen 3200 patients, of whom 50% were expected to be eligible and 50% of those eligible would consent. It was estimated that each centre would recruit 1–2 patients per month so that the maximum target of 780 participants could be attained within the 24 months’ planned recruitment period.
An internal pilot study was planned in order to assess the feasibility of recruitment. The internal pilot targets were set to recruit 63 participants across 12 centres over the first 6 months of the recruitment period. This target represented 8% of participants recruited across 30% of centres after 25% of the recruitment period had been completed and was based on a recruitment rate of 1–2 participants per month per centre taking into account a staggered opening of centres. The decision to continue the trial remained with the funder in the event that the target was not met.
Revised sample size and expected accrual
The trial recruited participants at a much slower rate than originally anticipated, and the sample size re-estimation was requested by the funder and conducted in August 2017 after 126 participants had an available 12 weeks’ HECSI assessment. The re-estimation was reviewed by the DMEC, who recommended a revised sample size of 514 participants based on an updated CV of 1.2, informed by the interim analysis.
Statistical analysis
All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
A SAP was approved prior to the relevant analysis being conducted.
Patient populations
All participants recruited into the trial were included in the analysis using ‘Intention-To-Treat’ (ITT) and analysed according to the randomised allocation and true values of minimisation factors.
A per-protocol (PP) population was defined such that it would exclude all major protocol violators and participants who did not receive at least 80% of their trial treatment or had a treatment break of more than 1 week.
The safety population was defined as all patients who were registered into the trial and was used to summarise patient disposition during the trial. This population was used to summarise any AEs, SAEs and SUSARs that occurred during the trial period.
The biomarker subgroup population consisted of all consenting participants who were selected for the biomarker substudy. The analysis population for the biomarker substudy included those for whom the relevant sample was received. This population was analysed and summarised according to the treatment they were randomised to receive.
The photography subgroup population consisted of all consenting participants who were selected for photography at randomisation.
The population to be used for the assessment of time in remission and time to relapse consisted of all participants who achieved clear/almost clear on the PGA according to the blinded assessor by the end of their randomised treatment phase. This population was analysed and summarised according to the treatment they were randomised to receive.
Participants were excluded from available case analyses if they had no available outcome data or at least one missing covariate for the relevant analysis.
Missing data
Descriptive summaries were used to compare participants with complete data to those with missing data for various variables including baseline characteristics and randomised treatment group. Under the missing at random (MAR) assumption, data were imputed using a multiple imputation technique in order to comply with the ITT analysis principle, stratified by treatment allocation and using ongoing treatment received as an auxiliary variable. 61–65 Missing data were imputed in ascending order of missingness.
Where more than 5% of data were missing for the HECSI, mTLSS, PGA and DLQI secondary end points multiple imputation in line with the method described above was employed to account for these missing data.
Where multiple imputation was used, sensitivity analyses using the available case population for the relevant end point were conducted to assess the sensitivity of the results to the multiple imputation method.
Primary end-point analysis
A multivariable multilevel repeated measures linear regression model was fitted to the loge(HECSI + 1) at 4, 8 and 12 weeks, adjusting for minimisation factors: duration of disease, clinical phenotype, DLQI, atopy status and ethnicity and the covariates: smoking history, BMI, foot involvement, baseline loge(HECSI), time since planned start of treatment and treatment group. Participant and participant–time interaction were fitted as random effects. Filaggrin LOF mutation was originally planned as a fixed effect, but due to high levels of missing data (27.6%), it was not considered appropriate. Centre was originally planned as a random effect; however, this led to challenges with multiple imputation due to the three-level structure of the data, and therefore, it was excluded from the analysis. However, a sensitivity analysis comparing the primary analysis on the available case population was conducted with and without centre as a random effect to ensure that centre was not influential in the analysis results. The relative difference in the HECSI score (+1) at 12 weeks post planned start of treatment, corresponding 95% confidence intervals (CIs) and p-values were reported.
Secondary end-point analyses
Multivariable multilevel repeated measures linear regression models of loge(HECSI score + 1), mTLSS and DLQI, and a multilevel repeated measures ordinal logistic regression model of the PGA over 52 weeks post planned start of treatment, were fitted, adjusting for the minimisation factors, covariates as for the primary end point analysis, corresponding baseline measurement and treatment group, as fixed effects. Participant and participant–time interaction were fitted as random effects. A multivariable ordinal logistic regression model of the PeDeSI was fitted adjusting for the minimisation factors, covariates as for the primary end point analysis, corresponding baseline measurement and treatment group, as fixed effects. The parameter estimates for each of these analyses, corresponding 95% CIs and p-values were reported; treatment effect estimates at 24 and 52 weeks post planned start of treatment were also reported. The PBI-HE was reported using summary statistics.
The method of Kaplan and Meier was used to report time to relapse.
Adverse events and SAEs classified as related to treatment or HE or resulting from administration of any research procedures were reported descriptively.
Exploratory end points
The correlation between HECSI, mTLSS and DLQI was calculated to assess convergent validity of scoring systems used to monitor response to treatment. Box plots and summary statistics of HECSI, mTLSS and DLQI within each level of the overall blinded PGA were produced.
Subgroup analyses were conducted to compare treatment effects within pre-defined subgroups (duration of disease, clinical phenotype, disease severity, presence of atopy, filaggrin LOF mutation, smoking history, BMI and foot involvement). Multivariable linear regression models were fitted to the response, loge(HECSI + 1) and treatment group, and an interaction term between treatment group and the subgroup/biomarker was included in the models to explore if there were potential differential treatment effects at 12 weeks.
Potential biomarker subgroups were explored through the tape stripping substudy. Subpopulation treatment effect pattern plots (STEPP) were produced and examined for four biomarkers [IL-36, Thymus and Activation-Regulated Chemokine (TARC), CCL20 and IL-18] to identify whether there were any potential differential treatment effects for different levels of each biomarker. 66
Receipt of and types of second-line therapies were reported descriptively.
The end of remission, defined as the time point at which participants were no longer clear/almost clear, was reported and analysed using the method of Kaplan and Meier. Frequency of corticosteroid use was reported descriptively.
Agreement between the blinded assessor and the central review of photographs was assessed using cross-tabulations.
Treatment compliance
Descriptive statistics on the time to receiving allocated treatment, compliance with treatment pathway decisions and reasons for treatment discontinuation, proportion of treatment received during the first 12 weeks compared with expected treatment, and length of treatment breaks were reported.
Summary of main changes to the protocol
Centres opened to protocol v3.0 and patient information sheet and consent form v3.0 on 5 November 2015. The following substantial changes were made during the lifetime of the trial:
-
Addition of a nail assessment substudy at the Chief Investigator’s centre, as there are currently no validated tools available for nail assessment in eczema. Note that the results of this are not included in this report because it was outwith the grant application.
-
Following TSC and DMEC feedback, addition of a new stratification factor informed by the Fitzpatrick score for skin colour because erythema may be underestimated in darker skin.
-
As part of the original Health Technology Assessment (HTA) funding envelope, a recruitment time extension was requested and approved, with a reduction in follow up to 6 months for all patients recruited from October 2019 to maximise accruals within the trial extension period. The trial stopped recruitment in accordance with the timelines agreed with the funder.
Chapter 3 Clinical results
This chapter presents the findings of the analysis for the clinical outcomes.
Participant flow
In total, 1557 participants were assessed for trial eligibility, 582 (37.4%) registrations took place to enable trial-specific tests and pregnancy prevention programmes to be implemented as required, and 441 (75.8% of those registered) randomisations took place between October 2015 and June 2021. Of those randomised, 59 (13.4%) were recruited after 1 October 2019 with a follow-up period of 6 months. Thirty-five NHS Secondary Care hospitals opened, of which 31 registered and randomised participants. The recruiting hospitals were located in England in the Yorkshire and Humber, North West, West Midlands, East Midlands, Eastern, London and South West regions, and in Scotland and Wales. The number of participants registered by each recruiting hospital ranged from 1 to 153 with a median of 10 (see Appendix 2 – Clinical results supplementary tables and figures, Figure 13). The mean number of registrations per centre was 0.30 participants per month. The number of participants randomised by each hospital ranged from 1 to 51, with a median of 4 (Figure 2). The mean number of participants randomised per centre was 0.23 per month. Follow-up was completed by 31 December 2021, after the last participant reached 24 weeks post planned start of treatment.
FIGURE 2.
Randomisations by anonymised centres.

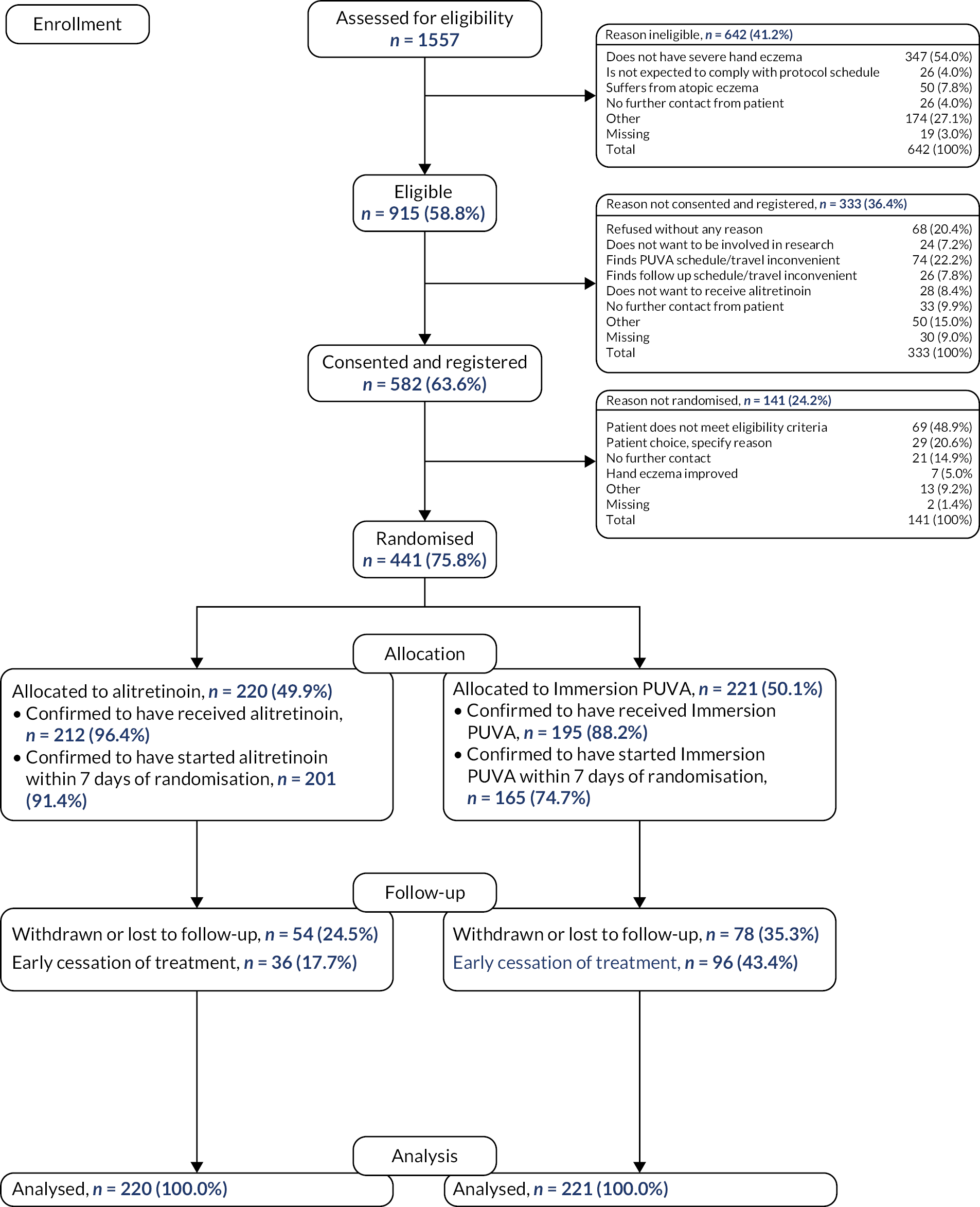
A consolidated standards of reporting trials (CONSORT) flow diagram of trial progress is presented in Figure 3. Of the 1557 patients who were screened, 642 (41.2%) were ineligible, with over half of the patients not having a diagnosis of severe CHE [N = 347 (54.0%)]. Of the 915 eligible patients, 582 (63.6%) were consented and registered. Regarding the reasons for not consenting to the trial, 74 (22.2%) patients thought the Immersion PUVA schedule or travel was inconvenient and 28 (8.4%) did not want to receive AL. A total of 141 (24.2%) of those registered did not progress to randomisation; the main reasons were because they did not meet the eligibility criteria [N = 69 (48.9%)] or due to patient choice [N = 29 (20.6%)]. A total of 441 participants were randomised.
FIGURE 3.
Consolidated standards of reporting trials flow diagram.
The screened population and randomised populations were similar in respect of age, gender and ethnicity (Table 1).
| Screened | Registered | Randomised | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 44.2 (15.8) | 44.7 (15.3) | 45.7 (15.1) |
| Median (range) | 43.8 (10–87) | 43.8 (18–81) | 45.9 (18–81) |
| IQR | 30.1–56.2 | 31.5–57.1 | 32.8–57.9 |
| Missing | 83 | 0 | 0 |
| N | 1474 | 582 | 441 |
| Gender | |||
| Male (%) | 574 (36.9) | 206 (35.4) | 162 (36.7) |
| Female (%) | 968 (62.2) | 365 (62.7) | 273 (61.9) |
| Missing (%) | 15 (1.0) | 11 (1.9) | 6 (1.4) |
| Total (%) | 1557 (100) | 582 (100) | 441 (100) |
| Ethnicity | |||
| White (%) | 1211 (77.8) | 511 (87.8) | 390 (88.4) |
| Mixed – white and Black Caribbean (%) | 12 (0.8) | 5 (0.9) | 3 (0.7) |
| Mixed – white and Black African (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mixed – white and Asian (%) | 18 (1.2) | 1 (0.2) | 0 (0.0) |
| Other mixed background (%) | 6 (0.4) | 3 (0.5) | 2 (0.5) |
| Asian – Indian (%) | 33 (2.1) | 11 (1.9) | 7 (1.6) |
| Asian – Pakistani (%) | 45 (2.9) | 26 (4.5) | 23 (5.2) |
| Asian – Bangladeshi (%) | 6 (0.4) | 4 (0.7) | 3 (0.7) |
| Other Asian background (%) | 19 (1.2) | 5 (0.9) | 3 (0.7) |
| Black – Caribbean (%) | 5 (0.3) | 4 (0.7) | 3 (0.7) |
| Black – African (%) | 4 (0.3) | 2 (0.3) | 2 (0.5) |
| Other black background (%) | 4 (0.3) | 2 (0.3) | 1 (0.2) |
| Chinese (%) | 6 (0.4) | 3 (0.5) | 2 (0.5) |
| Other ethnic group (%) | 11 (0.7) | 3 (0.5) | 2 (0.5) |
| Not stated (%) | 87 (5.6) | 2 (0.3) | 0 (0.0) |
| Missing (%) | 90 (5.8) | 0 (0.0) | 0 (0.0) |
| Total (%) | 1,557 (100) | 582 (100) | 441 (100) |
Of those patients randomised, 220 (49.9%) were allocated to receive AL and 221 (50.1%) to receive Immersion PUVA. Of those allocated to AL, 201 (91.4%) were confirmed to have started treatment within 7 days post randomisation compared with 165 (74.7%) of those allocated to Immersion PUVA. A total of 132 (29.9%) participants were withdrawn or withdrew from the follow-up schedule or were reported as lost to follow-up, with 54 (24.5%) in the AL group and 78 (35.3%) in the Immersion PUVA group. A total of 441 (100.0%) participants were included in the ITT population.
Baseline characteristics
Patient characteristics were balanced across randomised treatment groups and are detailed in Tables 2 and 3. In summary, the study population had a median age of 46 years (range 18–81); 61.9% (n = 273) were female and 88.9% (n = 392) were of white ethnicity. The majority of participants had been suffering with CHE for more than 2 years [n = 311 (70.5%)], and 64.9% (n = 286) participants had predominantly hyperkeratotic CHE. Just over half of the patients were atopic, as verified by the presence of allergen-specific IgE [n = 227 (51.5%)]. The baseline DLQI score was dichotomised based on the NICE TA177 threshold for prescribing AL (≥ 15), and our results showed that the majority of participants recruited with severe CHE actually had a DLQI < 15 (n = 250, 56.7%), with a median [interquartile range (IQR)] of 13 (9–18).
| AL | Immersion PUVA | Total | |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 46.5 (14.9) | 45.1 (15.2) | 45.8 (15.1) |
| Median (range) | 47.7 (20–81) | 44.6 (18–79) | 46.0 (18–81) |
| IQR | 33.5–58.7 | 31.9–56.8 | 32.9–58.0 |
| Missing | 0 | 0 | 0 |
| N | 220 | 221 | 441 |
| Gender | |||
| Male (%) | 85 (38.6) | 77 (34.8) | 162 (36.7) |
| Female (%) | 132 (60.0) | 141 (63.8) | 273 (61.9) |
| Missing (%) | 3 (1.4) | 3 (1.4) | 6 (1.4) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| BMI (kg/m) | |||
| Mean (SD) | 30.1 (6.6) | 28.9 (5.8) | 29.5 (6.2) |
| Median (range) | 29.3 (18–69) | 28.5 (17–50) | 28.7 (17–69) |
| IQR | 25.5–34.0 | 24.9–32.3 | 25.2–33.2 |
| Missing | 9 | 8 | 17 |
| N | 211 | 213 | 424 |
| Participant’s smoking status | |||
| Non-smoker (%) | 89 (40.5) | 85 (38.5) | 174 (39.5) |
| Past smoker (%) | 79 (35.9) | 93 (42.1) | 172 (39.0) |
| Current smoker (%) | 52 (23.6) | 42 (19.0) | 94 (21.3) |
| Missing (%) | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Are the feet involved as well? | |||
| Yes (%) | 57 (25.9) | 60 (27.1) | 117 (26.5) |
| No (%) | 163 (74.1) | 160 (72.4) | 323 (73.2) |
| Missing (%) | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Filaggrin LOF mutation | |||
| No mutation (%) | 141 (64.1) | 126 (57.0) | 267 (60.5) |
| Mutation (%) | 27 (12.3) | 24 (10.9) | 51 (11.6) |
| Samples taken but mutation status could not be determined (%) | 0 (0.0) | 2 (0.9) | 2 (0.5) |
| No sample available for analysis (%) | 52 (23.6) | 69 (31.2) | 121 (27.4) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| AL | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| Skin type | |||
| White (%) | 193 (87.7) | 199 (90.0) | 392 (88.9) |
| Fair (%) | 5 (2.3) | 2 (0.9) | 7 (1.6) |
| Dark (%) | 22 (10.0) | 20 (9.0) | 42 (9.5) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Duration of disease | |||
| < 6 months (%) | 6 (2.7) | 4 (1.8) | 10 (2.3) |
| 6–24 months (%) | 57 (25.9) | 63 (28.5) | 120 (27.2) |
| > 24 months (%) | 157 (71.4) | 154 (69.7) | 311 (70.5) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Clinical phenotype | |||
| Predominantly hyperkeratotic (%) | 143 (65.0) | 143 (64.7) | 286 (64.9) |
| Predominantly vesicular (%) | 62 (28.2) | 62 (28.1) | 124 (28.1) |
| Fingertip dermatitis (%) | 15 (6.8) | 16 (7.2) | 31 (7.0) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Presence of specific IgE | |||
| Yes (%) | 114 (51.8) | 113 (51.1) | 227 (51.5) |
| No (%) | 106 (48.2) | 107 (48.4) | 213 (48.3) |
| Missing (%) | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Baseline DLQI (categorised)a | |||
| < 15 (%) | 121 (55.0) | 129 (58.4) | 250 (56.7) |
| ≥ 15 (%) | 99 (45.0) | 92 (41.6) | 191 (43.3) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Baseline DLQI (continuous) | |||
| Mean (SD) | 13.9 (6.8) | 13.6 (6.0) | 13.8 (6.4) |
| Median (range) | 13.0 (2–30) | 13.0 (2–30) | 13.0 (2–30) |
| IQR | 8.0–20.0 | 9.0–17.0 | 9.0–18.0 |
| Missing | 1 | 2 | 3 |
| N | 219 | 219 | 438 |
Outcomes
Primary end point – HECSI
At baseline, the median (IQR) HECSI was 55 (31.5–91.0). There was a slight imbalance, with the Immersion PUVA group having a lower median and IQR than the AL group (Table 4); however, the baseline score was included as a covariate in the analysis to account for any imbalance. At 12 weeks, both groups had a lower median HECSI score, with an overall median (IQR) of 20 (7.0–44.0). The median (IQR) HECSI score at 12 weeks was equal to 19 (6.0–44.0) in the AL group compared with 25 (8.0–53.0) in the Immersion PUVA group; however, it should be noted that there was a larger proportion of missing data in the Immersion PUVA group, with 33.5% (n = 74) missing compared with 23.2% (n = 51) in the AL group (see Table 4).
| AL | Immersion PUVA | Total | |
|---|---|---|---|
| Baseline | |||
| Mean (SD) | 68.2 (47.5) | 62.2 (42.0) | 65.2 (44.9) |
| CV | 0.7 | 0.7 | 0.7 |
| Median (range) | 57.0 (2–243) | 52.5 (2–206) | 55.0 (2–243) |
| IQR | 34.0–97.0 | 30.0–86.0 | 31.5–91.0 |
| Missing | 6 | 7 | 13 |
| N | 214 | 214 | 428 |
| 12 weeks | |||
| Mean (SD) | 30.4 (33.5) | 35.8 (38.4) | 32.9 (35.9) |
| CV | 1.1 | 1.1 | 1.1 |
| Median (range) | 19.0 (0–196) | 25.0 (0–230) | 20.0 (0–230) |
| IQR | 6.0–44.0 | 8.0–53.0 | 7.0–44.0 |
| Missing | 51 | 74 | 125 |
| N | 169 | 147 | 316 |
| 24 weeks | |||
| Mean (SD) | 29.4 (34.3) | 23.3 (31.3) | 26.6 (33.0) |
| CV | 1.2 | 1.3 | 1.2 |
| Median (range) | 17.5 (0–205) | 9.0 (0–168) | 13.0 (0–205) |
| IQR | 6.0–40.0 | 4.0–30.0 | 5.0–35.0 |
| Missing | 84 | 106 | 190 |
| N | 136 | 115 | 251 |
| 52 weeks | |||
| Mean (SD) | 23.8 (35.9) | 20.1 (26.6) | 22.1 (32.0) |
| CV | 1.5 | 1.3 | 1.4 |
| Median (range) | 12.0 (0–269) | 8.5 (0–120) | 10.0 (0–269) |
| IQR | 4.0–25.0 | 4.0–24.0 | 4.0–25.0 |
| Missing | 111 | 131 | 242 |
| N | 109 | 90 | 199 |
Table 4 demonstrates that the CV for the HECSI score during follow-up ranged from 1.1 to 1.4, which is broadly in line with the original sample size calculation assumption of 1.2.
For those with observed data at 12 weeks, the median (IQR) change in score from baseline was equal to −30 (−61 to −10) for the AL group, compared with −20 (−47 to −2) in the Immersion PUVA group (Table 5). In terms of a relative change, the median (IQR) score at 12 weeks was equal to 30% (10–70%) of that at baseline for the AL group compared with 50% (20–100%) in the Immersion PUVA group (see Table 5). At 24 and 52 weeks, the overall median (IQR) score compared with that at baseline was equal to 30% (10–60%) and 20% (10–50%), respectively (see Table 5).
| Absolute change from baseline | Relative change from baseline | |||||
|---|---|---|---|---|---|---|
| AL | Immersion PUVA | Total | AL | Immersion PUVA | Total | |
| 12 weeks | ||||||
| Mean (SD) | −37.5 (45.0) | −25.8 (46.1) | −32.0 (45.8) | 0.7 (2.4) | 0.8 (2.0) | 0.8 (2.2) |
| Median (range) | −30.0 (−222 to 99) | −20.0 (−150 to 220) | −27.0 (−222 to 220) | 0.3 (0–30) | 0.5 (0–23) | 0.4 (0–30) |
| IQR | −61.0 to −10.0 | −47.0 to −2.0 | −57.0 to −6.0 | 0.1–0.7 | 0.2–1.0 | 0.1–0.8 |
| Missing | 57 | 76 | 133 | 57 | 76 | 133 |
| N | 163 | 145 | 308 | 163 | 145 | 308 |
| 24 weeks | ||||||
| Mean (SD) | −39.3 (46.2) | −42.1 (46.8) | −40.6 (46.4) | 0.5 (0.7) | 0.4 (0.6) | 0.5 (0.7) |
| Median (range) | −37.0 (−191 to 90) | −32.0 (−180 to 104) | −34.0 (−191 to 104) | 0.3 (0–5) | 0.2 (0–4) | 0.3 (0–5) |
| IQR | −65.0 to −12.0 | −70.0 to −13.0 | −66.0 to −12.0 | 0.1–0.6 | 0.1–0.6 | 0.1–0.6 |
| Missing | 89 | 108 | 197 | 89 | 108 | 197 |
| N | 131 | 113 | 244 | 131 | 113 | 244 |
| 52 weeks | ||||||
| Mean (SD) | −46.8 (43.5) | −39.9 (43.3) | −43.6 (43.4) | 0.4 (0.6) | 0.5 (1.1) | 0.4 (0.9) |
| Median (range) | −43.0 (−152 to 112) | −35.0 (−184 to 74) | −38.0 (−184 to 112) | 0.2 (0–4) | 0.2 (0–9) | 0.2 (0–9) |
| IQR | −69.0 to −20.5 | −60.0 to −13.0 | −62.0 to −16.0 | 0.1–0.5 | 0.1–0.6 | 0.1–0.5 |
| Missing | 116 | 132 | 248 | 116 | 132 | 248 |
| N | 104 | 89 | 193 | 104 | 89 | 193 |
The results of fitting a linear mixed model to log(HECSI + 1) show that there was a statistically significant benefit of AL compared with Immersion PUVA at 12 weeks, with an estimate of the fold change of 0.66 (0.52 to 0.82), p = 0.0003 at 12 weeks (Table 6). This is equivalent to a fold change of 1.52 (1.22 to 1.92) for Immersion PUVA compared with AL at 12 weeks. This CI includes the target treatment effect of a fold change equal to 1.3. The estimated parameters of the full model are included in Appendix 2 – Clinical results supplementary tables and figures, Table 39.
| Time since planned start of treatment (weeks) | Randomisation allocation | Adjusted mean log(HECSI + 1) scores (95% CI) | Treatment comparison | Difference in adjusted mean log(HECSI + 1) scores (95% CI) | Adjusted fold change (95% CI) | p-value |
|---|---|---|---|---|---|---|
| 4 | AL | 3.12 (2.81 to 3.42) | Immersion PUVA | −0.29 (−0.46 to −0.11) | 0.75 (0.63 to 0.89) | . |
| 4 | Immersion PUVA | 3.40 (3.09 to 3.72) | . | . | ||
| 8 | AL | 2.83 (2.53 to 3.13) | Immersion PUVA | −0.35 (−0.52 to −0.19) | 0.70 (0.60 to 0.83) | . |
| 8 | Immersion PUVA | 3.18 (2.87 to 3.49) | . | . | ||
| 12 | AL | 2.54 (2.22 to 2.85) | Immersion PUVA | −0.42 (−0.65 to −0.19) | 0.66 (0.52 to 0.82) | 0.0003 |
| 12 | Immersion PUVA | 2.96 (2.63 to 3.29) | . | . |
Secondary objective – hand eczema severity index over 52 weeks
The results of fitting a linear mixed model to log(HECSI + 1) collected over the full 52 weeks show that there is no evidence of a difference between AL and Immersion PUVA at 24 weeks, with the estimate of the fold change (95% CI) equal to 0.92 (0.798 to 1.08) (Figure 4). At 52 weeks, there continues to be no evidence of a difference between AL and Immersion PUVA, with the estimated fold change (95% CI) equal to 1.27 (0.97 to 1.67) (see Figure 4). The estimated parameters of the full model are included in Table 40. The adjusted mean log(HECSI + 1) scores and corresponding differences by treatment arm are included in Table 41.
FIGURE 4.
Estimated fold change in HECSI + 1 scores (AL vs. Immersion PUVA), with ongoing treatment decision up to 24 weeks used as auxiliary variable.

Secondary objective – mTLSS over 52 weeks
At baseline, the median (IQR) mTLSS score was equal to 12 (9–15), and the distribution of scores between randomised treatment groups was similar. At 12 weeks, both groups had a lower median mTLSS score, with an overall median (IQR) of 6 (3–10) (Table 7). The overall median (IQR) reduction in mTLSS was equal to 5 (2–8) at 12 weeks and equal to 6 (3–10) at 52 weeks (see Table 7). The proportions of missing data at each time point were similar to that observed for the primary end point.
| Raw score | Absolute difference from baseline | |||||
|---|---|---|---|---|---|---|
| AL | Immersion PUVA | Total | AL | Immersion PUVA | Total | |
| Baseline | ||||||
| Mean (SD) | 11.4 (4.3) | 11.7 (4.2) | 11.5 (4.2) | |||
| Median (range) | 12.0 (0–21) | 12.0 (0–20) | 12.0 (0–21) | |||
| IQR | 8.5–14.0 | 9.0–15.0 | 9.0–15.0 | |||
| Missing | 0 | 4 | 4 | |||
| N | 220 | 217 | 437 | |||
| 12 weeks | ||||||
| Mean (SD) | 6.1 (4.3) | 7.0 (4.7) | 6.5 (4.5) | −5.4 (4.9) | −4.7 (4.9) | −5.1 (4.9) |
| Median (range) | 5.0 (0–18) | 6.5 (0–21) | 6.0 (0–21) | −6.0 (−16 to 8) | −4.0 (−17 to 12) | −5.0 (−17 to 12) |
| IQR | 3.0–9.0 | 3.0–10.0 | 3.0–10.0 | −8.0 to −2.0 | −8.0 to −2.0 | −8.0 to −2.0 |
| Missing | 50 | 75 | 125 | 50 | 77 | 127 |
| N | 170 | 146 | 316 | 170 | 144 | 314 |
| 24 weeks | ||||||
| Mean (SD) | 6.1 (4.2) | 5.0 (3.8) | 5.6 (4.0) | −5.5 (5.2) | −7.1 (4.8) | −6.2 (5.1) |
| Median (range) | 6.0 (0–19) | 4.0 (0–14) | 5.0 (0–19) | −6.0 (−18 to 9) | −7.0 (−19 to 3) | −6.0 (−19 to 9) |
| IQR | 3.0–9.0 | 2.0–8.0 | 3.0–8.0 | −9.0 to −2.0 | −10.0 to −3.5 | −10.0 to −3.0 |
| Missing | 89 | 107 | 196 | 89 | 109 | 198 |
| N | 131 | 114 | 245 | 131 | 112 | 243 |
| 52 weeks | ||||||
| Mean (SD) | 5.1 (4.0) | 4.9 (4.2) | 5.0 (4.1) | −6.3 (4.9) | −6.7 (4.8) | −6.5 (4.9) |
| Median (range) | 4.5 (0–21) | 4.0 (0–15) | 4.0 (0–21) | −6.0 (−17 to 6) | −7.0 (−18 to 8) | −6.0 (−18 to 8) |
| IQR | 2.0–7.0 | 2.0–8.0 | 2.0–7.0 | −10.0 to −3.0 | −10.0 to −3.0 | −10.0 to −3.0 |
| Missing | 77 | 96 | 173 | 77 | 96 | 232 |
| N | 114 | 95 | 209 | 114 | 95 | 209 |
After fitting a linear mixed model to the mTLSS scores collected over 52 weeks, the results show that there was no evidence of a difference between treatment groups over 52 weeks, with each CI for the estimated difference at each time point including 0 (no difference) (Table 8). Note that model checking gave slight concern that the residuals might deviate from the normal distribution due to a potential floor effect; however, a sensitivity analysis on transformed scores (square root) led to consistent conclusions with the untransformed data. The estimated parameters of the full model are included in Table 42.
| Time since planned start of treatment (weeks) | Randomisation allocation | Adjusted mean mTLSS scores (95% CI) | Treatment comparison | Difference in adjusted mean mTLSS scores (95% CI) |
|---|---|---|---|---|
| 12 | AL | 5.50 (4.24 to 6.76) | Immersion PUVA | −0.37 (−1.23 to 0.48) |
| 12 | Immersion PUVA | 5.87 (4.68 to 7.07) | . | |
| 24 | AL | 5.18 (3.98 to 6.37) | Immersion PUVA | −0.14 (−0.84 to 0.56) |
| 24 | Immersion PUVA | 5.31 (4.15 to 6.48) | . | |
| 52 | AL | 4.42 (3.16 to 5.68) | Immersion PUVA | 0.41 (−0.59 to 1.40) |
| 52 | Immersion PUVA | 4.01 (2.62 to 5.40) | . |
Secondary objective – Physician’s Global Assessment over 52 weeks
At 12 weeks, the proportion of participants with available data who had achieved clear or almost clear according to the blinded assessor was equal to 27.6% (47/170) for the AL group and 23.6% (35/148) for the Immersion PUVA group (Table 9). Over the full 52 weeks, 59.4% (123/207) of AL participants with available data achieved at least one clear/almost clear assessment compared with 61.5% (118/192) of those in the Immersion PUVA group with available data (Table 10).
| AL (%) | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| 12 weeks | |||
| Clear | 14 (6.4) | 10 (4.5) | 24 (5.4) |
| Almost clear | 33 (15.0) | 25 (11.3) | 58 (13.2) |
| Mild | 37 (16.8) | 29 (13.1) | 66 (15.0) |
| Moderate | 62 (28.2) | 52 (23.5) | 114 (25.9) |
| Severe | 24 (10.9) | 32 (14.5) | 56 (12.7) |
| Missing | 50 (22.7) | 73 (33.0) | 123 (27.9) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| 24 weeks | |||
| Clear | 9 (4.1) | 10 (4.5) | 19 (4.3) |
| Almost clear | 24 (10.9) | 39 (17.6) | 63 (14.3) |
| Mild | 37 (16.8) | 23 (10.4) | 60 (13.6) |
| Moderate | 58 (26.4) | 29 (13.1) | 87 (19.7) |
| Severe | 9 (4.1) | 15 (6.8) | 24 (5.4) |
| Missing | 83 (37.7) | 105 (47.5) | 188 (42.6) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| 52 weeks | |||
| Clear | 9 (4.1) | 12 (5.4) | 21 (4.8) |
| Almost clear | 33 (15.0) | 27 (12.2) | 60 (13.6) |
| Mild | 31 (14.1) | 25 (11.3) | 56 (12.7) |
| Moderate | 28 (12.7) | 21 (9.5) | 49 (11.1) |
| Severe | 6 (2.7) | 8 (3.6) | 14 (3.2) |
| Missing | 113 (51.4) | 128 (57.9) | 241 (54.6) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| AL (%) | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| PGA | |||
| Clear | 50 (22.7) | 49 (22.2) | 99 (22.4) |
| Almost clear | 73 (33.2) | 69 (31.2) | 142 (32.2) |
| Mild | 45 (20.5) | 37 (16.7) | 82 (18.6) |
| Moderate | 30 (13.6) | 28 (12.7) | 58 (13.2) |
| Severe | 9 (4.1) | 9 (4.1) | 18 (4.1) |
| Missing | 13 (5.9) | 29 (13.1) | 42 (9.5) |
| Total | 220 (100) | 221 (100) | 441 (100) |
After fitting an ordinal logistic mixed model, the results show that there was no evidence of a difference between treatment groups over 52 weeks, with the CI at each time point for the estimated odds ratios (AL vs. Immersion PUVA) for achieving lower PGA scores including 1 (no difference) (Table 11). The estimated parameters of the full model are included in Table 43.
| Randomisation allocation | Treatment comparison | Time since planned start of treatment (weeks) | Estimate | 95% CI |
|---|---|---|---|---|
| AL | Immersion PUVA | 12 weeks | 1.22 | (0.90 to 1.64) |
| AL | Immersion PUVA | 24 weeks | 1.18 | (0.89 to 1.56) |
| AL | Immersion PUVA | 52 weeks | 1.10 | (0.64 to 1.89) |
Secondary objective – DLQI over 52 weeks
At baseline, the median (IQR) DLQI score was 13 (9–18), and the distribution of scores between randomised treatment groups was similar. At 12 weeks, both groups had a lower median DLQI score, with an overall median (IQR) of 4 (1–9) (Table 12). At 12 weeks, the median (IQR) reduction was equal to 7 (3–12), and at 24 and 52 weeks, the median (IQR) reduction was equal to 9 (5–13) and 9 (6–14), respectively (see Table 12). At 12 weeks, the proportion of missing data overall was lower than the blinded assessments (primary end point), with 16.8% (37/220) in the AL group, 27.1% (60/221) in the Immersion PUVA group and 22.0% (97/441) overall (see Table 12).
| Raw score | Absolute difference from baseline | |||||
|---|---|---|---|---|---|---|
| AL | Immersion PUVA | Total | AL | Immersion PUVA | Total | |
| Baseline | ||||||
| Mean (SD) | 13.9 (6.8) | 13.6 (6.0) | 13.8 (6.4) | |||
| Median (range) | 13.0 (2–30) | 13.0 (2–30) | 13.0 (2–30) | |||
| IQR | 8.0–20.0 | 9.0–17.0 | 9.0–18.0 | |||
| Missing | 1 | 2 | 3 | |||
| N | 219 | 219 | 438 | |||
| 12 weeks | ||||||
| Mean (SD) | 5.3 (5.8) | 6.7 (6.2) | 5.9 (6.1) | −8.3 (7.1) | −6.7 (6.5) | −7.6 (6.9) |
| Median (range) | 3.0 (0–30) | 5.0 (0–30) | 4.0 (0–30) | −7.0 (−30 to 9) | −6.0 (−30 to 8) | −7.0 (−30 to 9) |
| IQR | 1.0–8.0 | 2.0–9.0 | 1.0–9.0 | −12.0 to −4.0 | −10.5 to −3.0 | −12.0 to −3.0 |
| Missing | 37 | 60 | 97 | 38 | 61 | 99 |
| N | 183 | 161 | 344 | 182 | 160 | 342 |
| 24 weeks | ||||||
| Mean (SD) | 4.7 (4.8) | 4.0 (4.7) | 4.4 (4.7) | −9.0 (7.1) | −9.6 (5.9) | −9.3 (6.6) |
| Median (range) | 3.0 (0–25) | 2.0 (0–27) | 3.0 (0–27) | −8.0 (−30 to 7) | −9.0 (−30 to 3) | −9.0 (−30 to 7) |
| IQR | 1.0–7.0 | 1.0–6.0 | 1.0–7.0 | −14.0 to −4.0 | −13.0 to −6.0 | −13.0 to −5.0 |
| Missing | 62 | 82 | 144 | 63 | 83 | 146 |
| N | 158 | 139 | 297 | 157 | 138 | 295 |
| 52 weeks | ||||||
| Mean (SD) | 4.2 (4.9) | 4.3 (4.8) | 4.2 (4.9) | −9.3 (6.9) | −9.5 (5.9) | −9.4 (6.5) |
| Median (range) | 2.0 (0–24) | 3.0 (0–29) | 3.0 (0–29) | −9.0 (−29 to 13) | −9.0 (−30 to 6) | −9.0 (−30 to 13) |
| IQR | 1.0–6.0 | 1.0–6.0 | 1.0–6.0 | −15.0 to −4.0 | −12.5 to −6.0 | −14.0 to −6.0 |
| Missing | 92 | 117 | 209 | 93 | 117 | 210 |
| N | 128 | 104 | 232 | 127 | 104 | 231 |
After fitting a linear mixed model to the DLQI scores collected over 52 weeks, the model results are broadly consistent with the analysis of the primary end point, such that there is a statistically significant benefit of AL compared with Immersion PUVA at 12 weeks, with mean scores estimated to be 0.95 (CI 0.09 to 1.82) lower (Table 13). The model suggests that there is no statistically significant treatment effect at 24 weeks [estimated difference in mean scores = −0.18 (−0.92 to 0.56)], but at 52 weeks, there is a statistically significant treatment effect, with an estimated increase in the AL group of 1.62 (0.62 to 2.62) compared with the Immersion PUVA group (see Table 13). Note that as with the analysis of the mTLSS end point, model checking gave slight concern that the residuals might deviate from the normal distribution due to a potential floor effect; however, a sensitivity analysis analysing transformed scores (log transformation) led to consistent conclusions with the untransformed data. The estimated parameters of the full model are included in Table 44.
| Time since planned start of treatment (weeks) | Randomisation allocation | Adjusted mean DLQI scores (95% CI) | Treatment comparison | Difference in adjusted mean DLQI scores (95% CI) |
|---|---|---|---|---|
| 12 | AL | 5.46 (4.10 to 6.81) | Immersion PUVA | −0.95 (−1.82 to −0.09) |
| 12 | Immersion PUVA | 6.41 (4.96 to 7.86) | . | |
| 24 | AL | 5.10 (3.77 to 6.43) | Immersion PUVA | −0.18 (−0.92 to 0.56) |
| 24 | Immersion PUVA | 5.28 (3.88 to 6.68) | . | |
| 52 | AL | 4.25 (2.83 to 5.67) | Immersion PUVA | 1.62 (0.62 to 2.62) |
| 52 | Immersion PUVA | 2.63 (1.17 to 4.09) | . |
Secondary objective – PeDeSI over 52 weeks
At baseline, just 16.1% (n = 71) were assessed as having sufficient knowledge, ability and confidence to manage their condition on their own according to the PeDeSI, with a similar distribution of education needs across the treatment groups (Figures 5 and 6). At 12 weeks, the proportion of participants with available data who had sufficient knowledge and education was equal to 26.2% (117/324), with 28.0% (49/175) in the AL group and 24.2% (36/149) in the Immersion PUVA group (Appendix 2, Table 45). The results observed at 24 weeks should be treated with caution due to the smaller number of participants who provided data at 24 weeks instead of at 52 weeks due to the protocol amendment.
FIGURE 5.
Distribution of PeDeSI categories over time, AL.
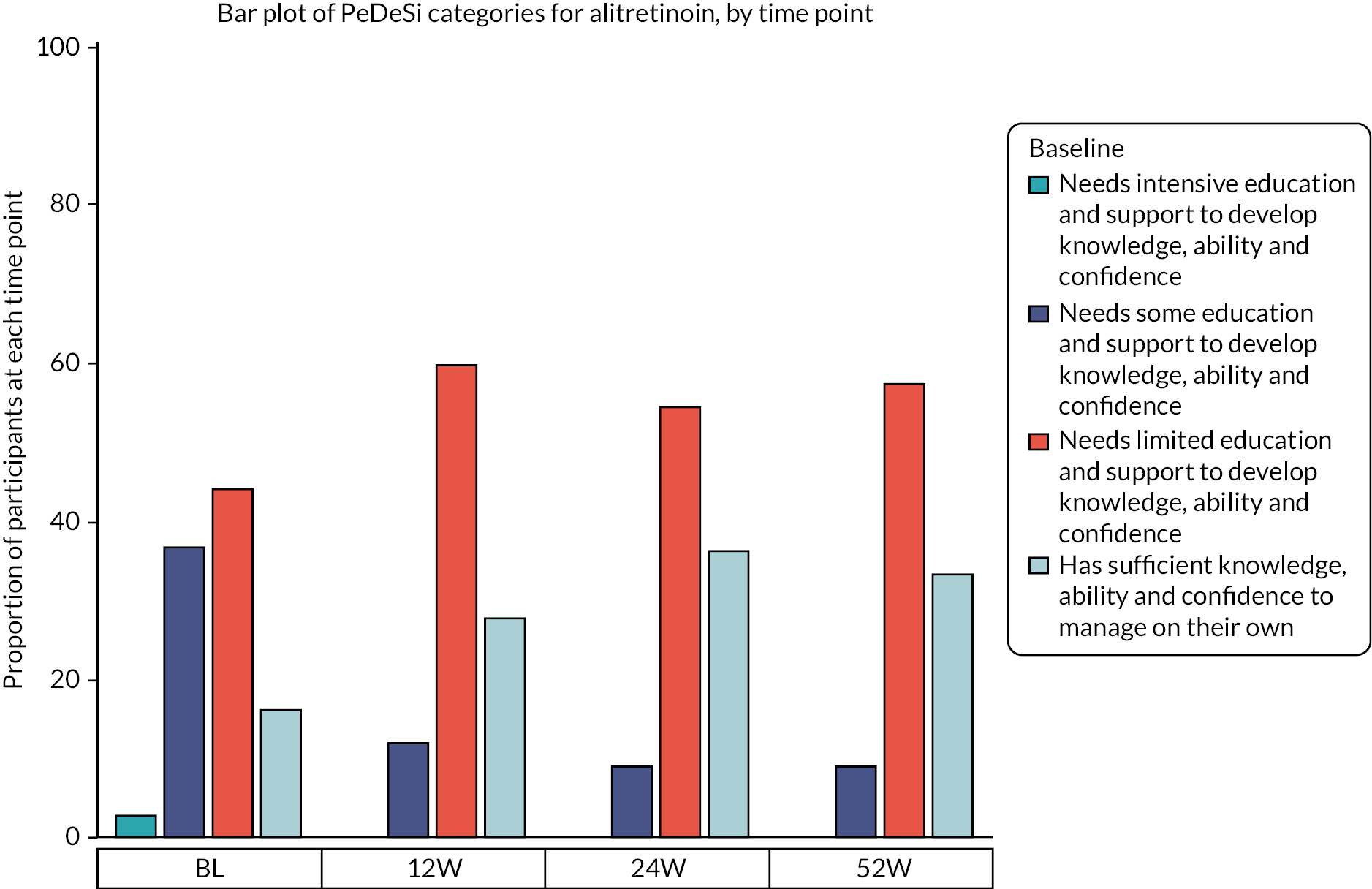
FIGURE 6.
Distribution of PeDeSI categories over time, Immersion PUVA.
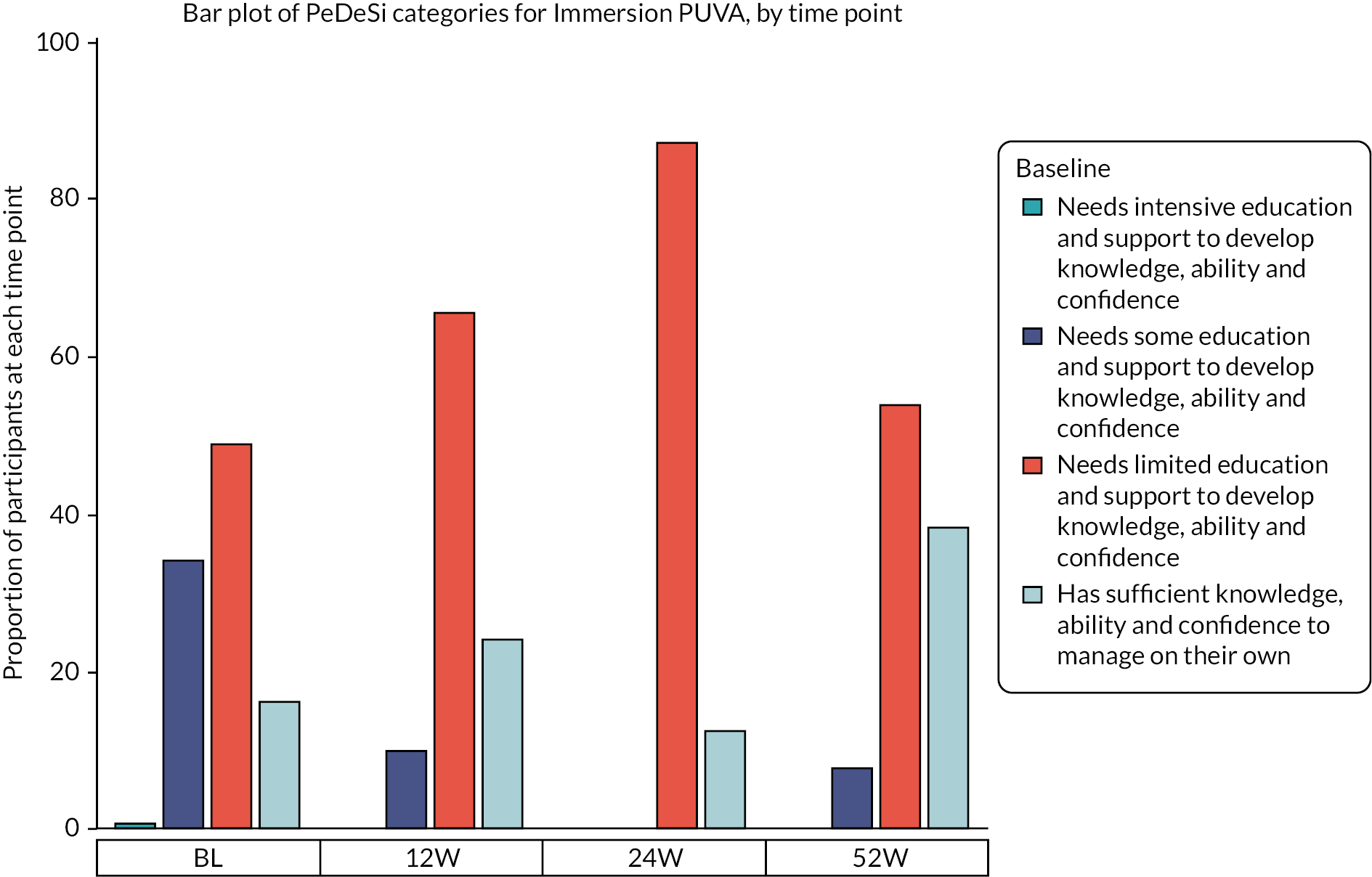
After fitting an ordinal logistic mixed model to the PeDeSI scores at 12 weeks, the results show that there was no evidence of a difference in terms of educational need between treatment groups at 12 weeks, with an estimated odds ratio (AL vs. Immersion PUVA) for lower educational need (i.e. higher PeDeSI score) of 0.65 (CI 0.39 to 1.08) (Appendix 2 – Clinical results supplementary tables and figures, Table 46).
Secondary objective – Patient Benefit Index (hand eczema) over 52 weeks
The PBI-HE was derived in relation to identified needs at baseline. At 12 weeks, the median (IQR) score was equal to 2.3 (1.4–3.2) in the AL group and 1.9 (0.9–2.8) in the Immersion PUVA group (Table 14). A PBI score > 1 is considered to show a treatment benefit. At 24 weeks, the median (IQR) score was unchanged at 2.3 (1.2–3.5) in the AL group but had increased to 2.8 (1.4–3.5) in the Immersion PUVA group (see Table 14). At 52 weeks, the median (IQR) score was equal to 2.6 (1.6–3.3) in the AL group and 3.0 (1.7–3.6) in the Immersion PUVA group (see Table 14). These results are purely descriptive and should be treated with caution, given the high proportion of missing data at each visit.
| AL | Immersion PUVA | Total | |
|---|---|---|---|
| 12 weeks | |||
| Mean (SD) | 2.2 (1.1) | 1.9 (1.2) | 2.1 (1.1) |
| Median (range) | 2.3 (0–4) | 1.9 (0–4) | 2.1 (0–4) |
| IQR | 1.4–3.2 | 0.9–2.8 | 1.1–3.0 |
| Missing | 71 | 87 | 158 |
| N | 149 | 134 | 283 |
| 24 weeks | |||
| Mean (SD) | 2.3 (1.2) | 2.4 (1.2) | 2.4 (1.2) |
| Median (range) | 2.3 (0–4) | 2.8 (0–4) | 2.5 (0–4) |
| IQR | 1.2–3.5 | 1.4–3.5 | 1.3–3.5 |
| Missing | 93 | 108 | 201 |
| N | 127 | 114 | 241 |
| 52 weeks | |||
| Mean (SD) | 2.4 (1.1) | 2.6 (1.2) | 2.5 (1.1) |
| Median (range) | 2.6 (0–4) | 3.0 (0–4) | 2.7 (0–4) |
| IQR | 1.6–3.3 | 1.7–3.6 | 1.7–3.5 |
| Missing | 115 | 133 | 248 |
| N | 105 | 88 | 193 |
Secondary objective – time to relapse
By the end of the treatment phase, 34.1% (N = 75) of participants allocated to AL were confirmed to have achieved a clear or almost clear response, compared with 25.8% (N = 57) in the Immersion PUVA group (Table 15). Two definitions of relapse were considered. When achieving 75% of the baseline HECSI score was used as the relapse definition, 18.2% (n = 24) were confirmed to have relapsed, corresponding to 20% (n = 15) in the AL group and 15.8% (n = 9) in the Immersion PUVA group (see Table 15). Meanwhile, when achieving 50% of the baseline HECSI score was used as the relapse definition, 37.1% (n = 49) were confirmed to have relapsed, corresponding to 37.3% (n = 28) in the AL group and 36.8% (n = 21) in the Immersion PUVA group (see Table 15). Kaplan–Meier curves for the time to relapse under each definition indicate there is no evidence of a difference between treatment groups (Appendix 2, Figures 14 and 15).
| AL (%) | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| Responder | |||
| Yes | 75 (34.1) | 57 (25.8) | 132 (29.9) |
| No | 145 (65.9) | 164 (74.2) | 309 (70.1) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| Relapse defined as achieving 75% of the baseline score | |||
| Yes | 15 (20.0) | 9 (15.8) | 24 (18.2) |
| No | 55 (73.3) | 45 (78.9) | 100 (75.8) |
| Missing | 5 (6.7) | 3 (5.3) | 8 (6.1) |
| Total | 75 (100) | 57 (100) | 132 (100) |
| Relapse defined as achieving 50% of the baseline score | |||
| Yes | 28 (37.3) | 21 (36.8) | 49 (37.1) |
| No | 42 (56.0) | 33 (57.9) | 75 (56.8) |
| Missing | 5 (6.7) | 3 (5.3) | 8 (6.1) |
| Total | 75 (100) | 57 (100) | 132 (100) |
Secondary objective – safety
A total of 135 reportable AEs were observed, corresponding to 79 participants who had at least one reportable AE recorded. Of those participants allocated to AL, 25.0% (N = 55) had at least one reportable AE observed (total of 107 AEs) compared with 10.9% (N = 24) of those allocated to Immersion PUVA (total of 28 AEs) (Table 16). Of the 135 AEs reported, the majority were mild or moderate, with 11.1% (N = 15) assessed as severe, and no AEs were assessed as life-threatening (Table 17). The most common types of AEs reported in the AL arm were gastrointestinal AEs (N = 22, 20.6%) and depressive mood changes (N = 11, 10.3%), while for Immersion PUVA, 50.0% (N = 14) of the reportable AEs were PUVA burn (Appendix 2 – Clinical results supplementary tables and figures, Table 47). There were four AEs that were considered to meet the criteria of serious (two AL, two Immersion PUVA); one was suspected and expected to be related to treatment, and three were related to the underlying CHE. In addition to the AEs reported in Tables 47 and 17, four pregnancies were reported (three AL, one Immersion PUVA). The participant allocated to Immersion PUVA was in follow-up when their pregnancy was reported. Of those who were allocated to AL, there was one participant who stopped their randomised treatment (AL) following a positive pregnancy test, one was in their treatment phase but had not been taking treatment and one was in the follow-up phase. These were discussed by the Data Monitoring and Ethics and TSCs, and a work instruction issued to sites which detailed the current standard care definition of rigorous contraception for patients of child bearing potential taking AL, which was in line with guidance provided on the British Association of Dermatologists website and current AL SmPC. Centres were asked to confirm with patients that they are complying with rigorous contraception at each clinical assessment. Additionally, Leeds CTRU requested confirmation from the Principal Investigator at each site that they followed processes as detailed in the work instruction, to provide details of current local standard care practice for pregnancy prevention for patients receiving AL in their clinic, and that this process had been followed for participants randomised to AL where pregnancy had been reported. No deaths were reported during the trial.
| At least one AE was reported | AL | Immersion PUVA | Total |
|---|---|---|---|
| At least one AE was reported | |||
| Yes (%) | 55 (25.0) | 24 (10.9) | 79 (17.9) |
| No (%) | 161 (73.2) | 179 (81.0) | 340 (77.1) |
| Missing (%) | 4 (1.8) | 18 (8.1) | 22 (5.0) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Number of times AE reported | |||
| 0 (%) | 161 (73.2) | 179 (81.0) | 340 (77.1) |
| 1 (%) | 32 (14.5) | 21 (9.5) | 53 (12.0) |
| 2 (%) | 8 (3.6) | 2 (0.9) | 10 (2.3) |
| 3 (%) | 6 (2.7) | 1 (0.5) | 7 (1.6) |
| 4 (%) | 5 (2.3) | 0 (0.0) | 5 (1.1) |
| 5 (%) | 3 (1.4) | 0 (0.0) | 3 (0.7) |
| 6 (%) | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| Missing (%) | 4 (1.8) | 18 (8.1) | 22 (5.0) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Number of weeks from randomisation to first AE | |||
| Mean (SD) | 9.0 (6.5) | 9.5 (8.2) | 9.2 (7.0) |
| Median (range) | 8.0 (4–44) | 5.4 (3–34) | 7.9 (3–44) |
| IQR | 4.3–11.9 | 4.3–13.0 | 4.3–12.0 |
| Missing | 1 | 0 | 1 |
| N | 54 | 24 | 78 |
| AL (%) | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| Participant received any randomised treatment? | |||
| Yes | 96 (89.7) | 26 (92.9) | 122 (90.4) |
| No | 9 (8.4) | 2 (7.1) | 11 (8.1) |
| Missing | 2 (1.9) | 0 (0.0) | 2 (1.5) |
| Total | 107 (100) | 28 (100) | 135 (100) |
| Intensity grade | |||
| Mild | 67 (62.6) | 11 (39.3) | 78 (57.8) |
| Moderate | 33 (30.8) | 9 (32.1) | 42 (31.1) |
| Severe | 7 (6.5) | 8 (28.6) | 15 (11.1) |
| Life-threatening | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 107 (100) | 28 (100) | 135 (100) |
| Does the event meet the criteria of a SAE? | |||
| Yes | 2 (1.9) | 2 (7.1) | 4 (3.0) |
| No | 105 (98.1) | 26 (92.9) | 131 (97.0) |
| Total | 107 (100) | 28 (100) | 135 (100) |
| Action taken | |||
| None | 67 (62.6) | 10 (35.7) | 77 (57.0) |
| Delayed, modified or stopped | 14 (13.1) | 9 (32.1) | 23 (17.0) |
| Permanently stopped | 26 (24.3) | 9 (32.1) | 35 (25.9) |
| Total | 107 (100) | 28 (100) | 135 (100) |
Exploratory objective – comparison of outcome measures
Figures 7, 8 and 9 show the distribution of the HECSI, mTLSS and DLQI scores, respectively within each PGA category according to the blinded assessor, with further detail provided in Appendix 2 – Clinical results supplementary tables and figures (Table 48). These assessments were taken across all patients and all time points with no adjustment for repeated assessments within participants in the analysis. In general, the PGA categories are reflected well by the HECSI and mTLSS scores, with little overlap of the HECSI and mTLSS IQRs for each PGA. Where the CHE was assessed as severe, the median (IQR) HECSI score was equal to 67 (44–324), and the median (IQR) mtLSS was equal to 13 (10–15). In contrast, the IQRs for the DLQI within each category of the PGA do overlap. Of note, the median (IQR) DLQI when the CHE is assessed as severe is equal to 11 (6–16).
FIGURE 7.
Box plot of HECSI by PGA categories over all time points.

FIGURE 8.
Box plot of mTLSS by PGA categories over all time points.

FIGURE 9.
Box plot of DLQI by PGA categories over all time points.
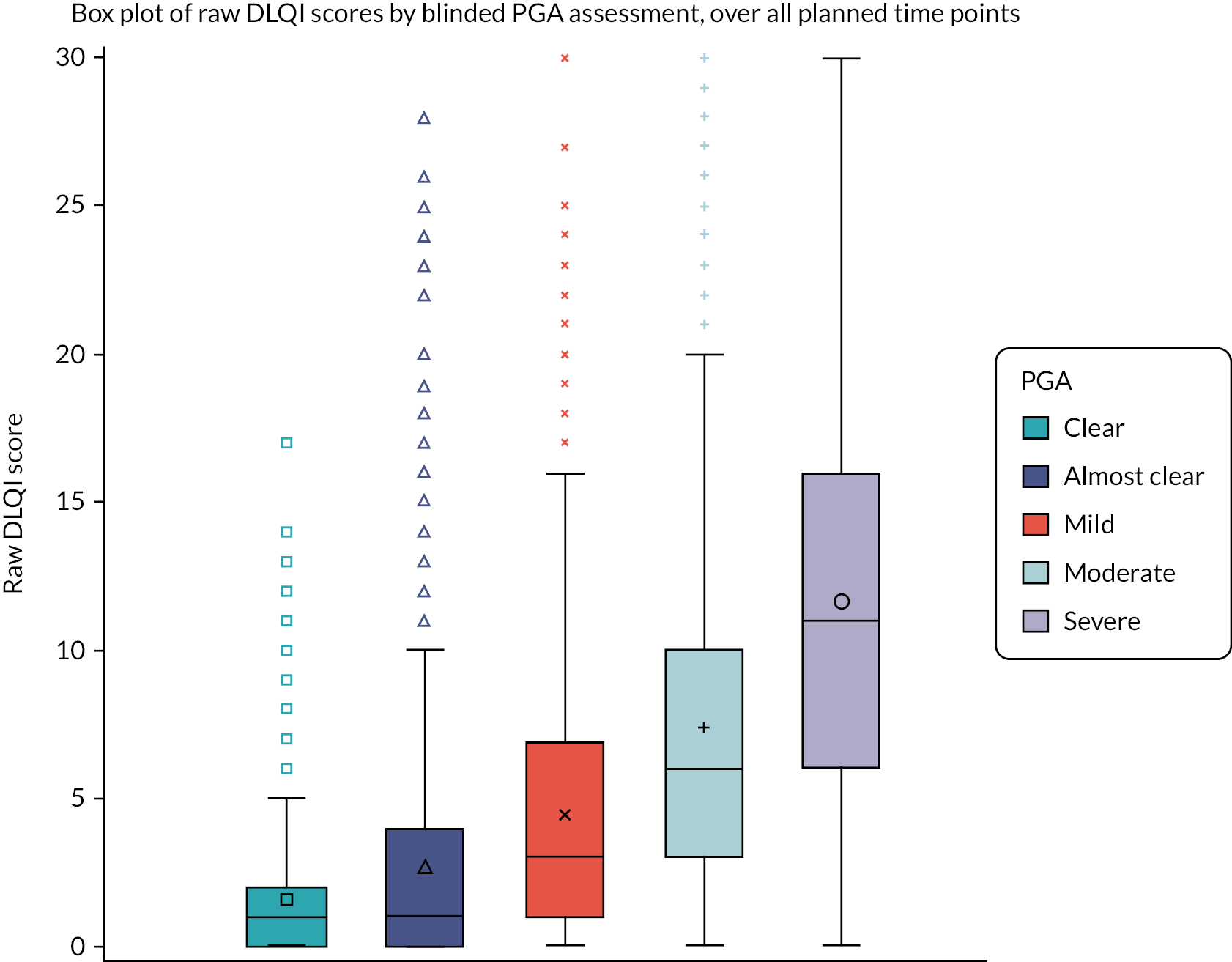
There was high positive correlation of the raw HECSI scores with the raw mTLSS scores over all time points, with an estimated correlation (95% CI) of 0.84 (0.83 to 0.86). Moderate positive correlation was observed for the raw DLQI scores compared with both the raw HECSI scores [estimated correlation (95% CI) = 0.56 (0.53 to 0.58)] and the raw mTLSS scores [estimated correlation (95% CI) = 0.65 (0.62 to 0.68)]. Scatterplots of the scores are in Appendix 2 – Clinical results supplementary tables and figures, Figures 16–18.
Exploratory objective – subgroup analyses
Figure 10 shows the estimated fold change in the HECSI + 1 scores between AL and Immersion PUVA. Estimates and CIs that lie fully to the left of the reference line of 1 indicate a benefit of AL compared with Immersion PUVA, while estimates and CIs that lie fully to the right of the reference line indicate a benefit of Immersion PUVA compared with AL. These analyses are exploratory and should be treated with caution because they are underpowered and conducted on the available case population. For each variable, there is overlap of each subgroup CI, suggesting that there is no evidence of a differential treatment effect for any of the subgroups.
FIGURE 10.
Forest plot of effect sizes within pre-planned subgroups (AL vs. Immersion PUVA).
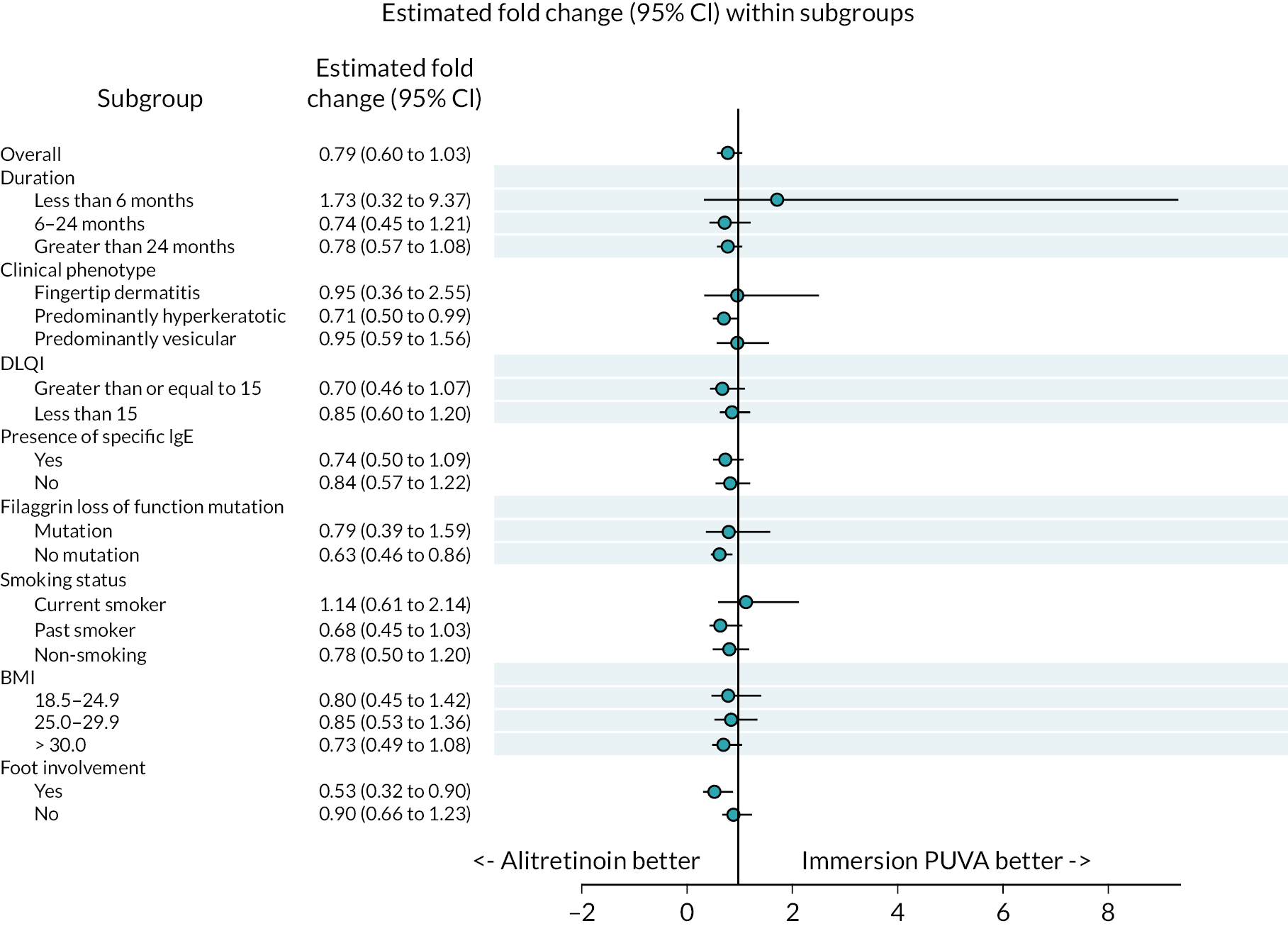
Exploratory objective – biomarker subgroups
Table 18 summarises the detectable levels of biomarkers by treatment allocation and by whether the sample was taken from a lesional sample. IL36 values are lower overall when the sample is non-lesional compared with lesional [median (IQR) of 12.9 (5.3–31.3) compared with 86.9 (41.2–184.8), respectively]. Differences in IL18, TARC and CCL20 between lesional/non-lesional samples are more difficult to ascertain due to small values of the biomarkers. Furthermore, Table 18 is affected by high levels of missing data due to samples being below the limit of detection, but IL18, TARC and CCL20 all have higher proportions of samples below the limit of detection for non-lesional samples compared with lesional samples.
| Lesional samples | Non-lesional samples | |||||
|---|---|---|---|---|---|---|
| AL | Immersion PUVA | Total | AL | Immersion PUVA | Total | |
| IL36 | ||||||
| Mean (SD) | 136.1 (130.9) | 163.3 (255.7) | 150.1 (204.4) | 29.5 (37.8) | 23.1 (30.0) | 26.3 (34.1) |
| Median (range) | 90.3 (2–625) | 73.5 (6–1415) | 86.9 (2–1415) | 16.5 (0–148) | 9.3 (0–123) | 12.9 (0–148) |
| IQR | 64.4–153.0 | 28.2–208.1 | 41.2–184.8 | 7.5–36.7 | 4.2–23.6 | 5.3–31.3 |
| Missing | 0 | 1 | 1 | 1 | 0 | 1 |
| N | 45 | 48 | 93 | 37 | 37 | 74 |
| IL18 | ||||||
| Mean (SD) | 2.9 (7.1) | 1.3 (1.9) | 2.1 (5.3) | 0.3 (0.6) | 0.1 (0.1) | 0.2 (0.4) |
| Median (range) | 1.1 (0–44) | 0.5 (0–9) | 0.7 (0–44) | 0.0 (0–2) | 0.0 (0–0) | 0.0 (0–2) |
| IQR | 0.3–2.4 | 0.2–1.5 | 0.3–2.1 | 0.0–0.2 | 0.0–0.1 | 0.0–0.2 |
| Missinga | 3 | 8 | 11 | 22 | 25 | 47 |
| N | 42 | 41 | 83 | 16 | 12 | 28 |
| TARC | ||||||
| Mean (SD) | 0.3 (0.3) | 0.3 (0.5) | 0.3 (0.4) | 0.2 (0.2) | 0.1 (0.0) | 0.1 (0.1) |
| Median (range) | 0.2 (0–1) | 0.2 (0–2) | 0.2 (0–2) | 0.1 (0–1) | 0.1 (0–0) | 0.1 (0–1) |
| IQR | 0.1–0.4 | 0.1–0.3 | 0.1–0.4 | 0.1–0.2 | 0.1–0.1 | 0.1–0.1 |
| Missinga | 14 | 21 | 35 | 33 | 32 | 65 |
| N | 31 | 28 | 59 | 5 | 5 | 10 |
| CCL20 | ||||||
| Mean (SD) | 0.3 (0.4) | 0.3 (0.7) | 0.3 (0.6) | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) |
| Median (range) | 0.1 (0–1) | 0.1 (0–3) | 0.1 (0–3) | 0.0 (0–0) | 0.0 (0–0) | 0.0 (0–0) |
| IQR | 0.0–0.3 | 0.0–0.3 | 0.0–0.3 | 0.0–0.1 | 0.0–0.1 | 0.0–0.1 |
| Missinga | 22 | 22 | 44 | 31 | 30 | 61 |
| N | 23 | 27 | 50 | 7 | 7 | 14 |
Inspection of the STEPP plots (see Appendix 2, Figures 19–26) indicates that there is no evidence of a differential treatment effect for any of the biomarkers assessed as part of the biomarker substudy.
Exploratory objective – second-line therapies
Overall, 212 participants were confirmed to receive other treatments for their CHE over 52 weeks, with 55.0% (n = 121) in the AL group and 41.2% (n = 91) in the Immersion PUVA group (Table 19). These descriptive summaries are subject to high proportions of missing data, and when excluded from the denominator, we observe that 83.4% (121/145) of AL participants with available data received other treatments for CHE compared with 76.5% (91/119) of Immersion PUVA participants. Of those who were confirmed to receive other treatments, 27.3% (n = 33) of those originally allocated to AL went on to receive phototherapy, and 71.4% (n = 65) of those originally allocated to Immersion PUVA went on to receive AL (see Table 19). Almost half of those originally allocated to receive AL who were confirmed to receive further treatment for CHE received AL again (N = 58, 47.9%), compared with just 4.4% (n = 4) participants originally allocated to receive Immersion PUVA who were confirmed to receive further phototherapy (see Table 19).
| AL | Immersion PUVA | Total | |
|---|---|---|---|
| Other treatments received for CHE up to 24 weeks, all participants | |||
| Yes (%) | 65 (29.5) | 71 (32.1) | 136 (30.8) |
| No (%) | 71 (32.3) | 58 (26.2) | 129 (29.3) |
| Missing (%) | 84 (38.2) | 92 (41.6) | 176 (39.9) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Other treatments received for CHE up to 52 weeks, participants randomised before 1 October 2019 | |||
| Yes (%) | 112 (58.6) | 80 (41.9) | 192 (50.3) |
| No (%) | 16 (8.4) | 21 (11.0) | 37 (9.7) |
| Missing (%) | 63 (33.0) | 90 (47.1) | 153 (40.1) |
| Total (%) | 191 (100) | 191 (100) | 382 (100) |
| Other treatments received for CHE, all participants | |||
| Yes (%) | 121 (55.0) | 91 (41.2) | 212 (48.1) |
| No (%) | 24 (10.9) | 28 (12.7) | 52 (11.8) |
| Missing (%) | 75 (34.1) | 102 (46.2) | 177 (40.1) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Types of other treatments received (denominator is the number of participants who received alternative treatments) | |||
| Any type of phototherapy (%) | 33 (27.3) | 4 (4.4) | 37 (17.5) |
| Ciclosporin A (%) | 14 (11.6) | 11 (12.1) | 25 (11.8) |
| Oral glucocorticosteroids (%) | 21 (17.4) | 14 (15.4) | 35 (16.5) |
| Methotrexate (%) | 16 (13.2) | 12 (13.2) | 28 (13.2) |
| Azathioprine (%) | 4 (3.3) | 0 (0.0) | 4 (1.9) |
| AL (%) | 58 (47.9) | 65 (71.4) | 123 (58.0) |
| Acitretin (%) | 9 (7.4) | 5 (5.5) | 14 (6.6) |
| Other (%) | 24 (19.8) | 19 (20.9) | 43 (20.3) |
| Total number of patients who received alternative therapies | 121 | 91 | 212 |
Exploratory objective – remission and corticosteroid use
The end of remission is defined as no longer having a clear/almost clear PGA, and of the 132 participants who were observed to be clear/almost clear by the end of the treatment phase, 81.8% (n = 108) were confirmed to have a more severe PGA at a subsequent assessment, corresponding to 90.7% (n = 68) of those in the AL group and 70.2% (n = 40) in the Immersion PUVA group (Table 20).
| AL (%) | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| Participants remained in remission | |||
| Yes | 7 (9.3) | 17 (29.8) | 24 (18.2) |
| No | 68 (90.7) | 40 (70.2) | 108 (81.8) |
| Total | 75 (100) | 57 (100) | 132 (100) |
Figure 11 shows Kaplan–Meier curves for the time to the end of remission. Inspection of the curves leads to the conclusion that there is no evidence of a difference between treatment groups.
FIGURE 11.
Kaplan–Meier plot for the time to the end of remission, where end of remission is defined as no longer having clear/almost clear PGA.

In general, there seemed to be a higher proportion of participants allocated to AL who reported no use of corticosteroids compared with participants allocated to Immersion PUVA; however, these data are subject to high levels of missing data (Appendix 2, Figures 27 and 28, Table 49).
Exploratory objective – photography
Overall, 418 (94.8%) participants consented to have photographs taken, of whom 149 (35.6%) were selected for the photography substudy. Of those selected for the photography substudy, 113 (75.8%) were of white ethnicity, and 36 (24.2%) were from ethnic minority groups (Appendix 2, Table 50).
At baseline and 12 weeks, each participant who consented and was selected for the photography substudy was expected to have four photographs taken (one of each side of each hand). Therefore, 596 photos were expected at each time point, or 1192 in total.
At baseline, a total of 425 photographs were received, of which 387 (91.1%) had a successful central review assessment, defined as at least 2 reviewers being in agreement with the severity assessment. There was no agreement among the central reviewers for 35 (8.2%) photos. At 12 weeks, a total of 287 photographs were received, of which 247 (86.1%) had a successful central review assessment, with no agreement among the central reviewers for 32 (11.1%) photos. This means there were a total of 712 photos received, of which 634 (89.0%) had a central review assessment (see Appendix 2, Table 50).
Overall, there was agreement in 274 (43.7%) photos, with 154 (47.2%) in the AL group and 120 (39.9%) in the Immersion PUVA group (Table 21). A total of 251 (40.0%) photographs were assessed as more severe than the corresponding blinded PGA assessment, and 102 (16.3%) were assessed as less severe. Overall, there was agreement for 220 (43.2%) photographs taken of white participants and agreement for 54 (45.8%) photographs taken of ethnic minority participants, with similar proportions of photographs assessed as more or less severe than the corresponding blinded PGA (see Table 21).
| Blinded PGA | Photographic assessment | Total | |||||
|---|---|---|---|---|---|---|---|
| Clear | Almost clear | Moderate | Severe | Very severe | |||
| All photos | Clear | 56 (8.9) | 69 (11.0) | 32 (5.1) | 6 (1.0) | 0 (0.0) | 163 (26.0) |
| Almost clear | 16 (2.6) | 44 (7.0) | 21 (3.3) | 10 (1.6) | 1 (0.2) | 92 (14.7) | |
| Mild | 4 (0.6) | 34 (5.4) | 49 (7.8) | 13 (2.1) | 2 (0.3) | 102 (16.3) | |
| Moderate | 4 (0.6) | 19 (3.0) | 61 (9.7) | 41 (6.5) | 7 (1.1) | 132 (21.1) | |
| Severe | 1 (0.2) | 17 (2.7) | 41 (6.5) | 48 (7.7) | 31 (4.9) | 138 (22.0) | |
| Total | 81 (12.9) | 183 (29.2) | 204 (32.5) | 118 (18.8) | 41 (6.5) | 627 (100.0) | |
| Agreement | 274 (43.7) | Photographs more severe | 251 (40.0) | Photographs less severe | 102 (16.3) | ||
| AL | Clear | 38 (11.7) | 35 (10.7) | 17 (5.2) | 3 (0.9) | 0 (0.0) | 93 (28.5) |
| Almost clear | 6 (1.8) | 23 (7.1) | 10 (3.1) | 2 (0.6) | 0 (0.0) | 41 (12.6) | |
| Mild | 1 (0.3) | 17 (5.2) | 25 (7.7) | 8 (2.5) | 1 (0.3) | 52 (16.0) | |
| Moderate | 2 (0.6) | 11 (3.4) | 28 (8.6) | 24 (7.4) | 1 (0.3) | 66 (20.2) | |
| Severe | 1 (0.3) | 7 (2.1) | 18 (5.5) | 27 (8.3) | 21 (6.4) | 74 (22.7) | |
| Total | 48 (14.7) | 93 (28.5) | 98 (30.1) | 64 (19.6) | 23 (7.1) | 326 (100.0) | |
| Agreement | 154 (47.2) | Photographs more severe | 126 (38.7) | Photographs less severe | 46 (14.4) | ||
| Immersion PUVA | Clear | 18 (6.0) | 34 (11.3) | 15 (5.0) | 3 (1.0) | 0 (0.0) | 70 (23.3) |
| Almost clear | 10 (3.3) | 21 (7.0) | 11 (3.7) | 8 (2.7) | 1 (0.3) | 51 (16.9) | |
| Mild | 3 (1.0) | 17 (5.6) | 24 (8.0) | 5 (1.7) | 1 (0.3) | 50 (16.6) | |
| Moderate | 2 (0.7) | 8 (2.7) | 33 (11.0) | 17 (5.6) | 6 (2.0) | 66 (21.9) | |
| Severe | 0 (0.0) | 10 (3.3) | 23 (7.6) | 21 (7.0) | 10 (3.3) | 64 (21.3) | |
| Total | 33 (11.0) | 90 (29.9) | 106 (35.2) | 54 (17.9) | 18 (6.0) | 301 (100.0) | |
| Agreement | 120 (39.9) | Photographs more severe | 125 (41.5) | Photographs less severe | 56 (18.6) | ||
| White ethnicity | Clear | 44 (8.6) | 57 (11.2) | 23 (4.5) | 6 (1.2) | 0 (0.0) | 130 (25.5) |
| Almost clear | 12 (2.4) | 38 (7.5) | 17 (3.3) | 9 (1.8) | 0 (0.0) | 76 (14.9) | |
| Mild | 4 (0.8) | 28 (5.5) | 42 (8.3) | 10 (2.0) | 1 (0.2) | 85 (16.7) | |
| Moderate | 3 (0.6) | 16 (3.1) | 50 (9.8) | 34 (6.7) | 4 (0.8) | 107 (21.0) | |
| Severe | 0 (0.0) | 15 (2.9) | 36 (7.1) | 36 (7.1) | 24 (4.7) | 111 (21.8) | |
| Total | 63 (12.4) | 154 (30.3) | 168 (33.0) | 95 (18.7) | 29 (5.7) | 509 (100.0) | |
| Agreement | 220 (43.2) | Photographs more severe | 203 (39.9) | Photographs less severe | 86 (16.9) | ||
| Ethnic minorities | Clear | 12 (10.2) | 12 (10.2) | 9 (7.6) | 0 (0.0) | 0 (0.0) | 33 (28.0) |
| Almost clear | 4 (3.4) | 6 (5.1) | 4 (3.4) | 1 (0.8) | 1 (0.8) | 16 (13.6) | |
| Mild | 0 (0.0) | 6 (5.1) | 7 (5.9) | 3 (2.5) | 1 (0.8) | 17 (14.4) | |
| Moderate | 1 (0.8) | 3 (2.5) | 11 (9.3) | 7 (5.9) | 3 (2.5) | 25 (21.2) | |
| Severe | 1 (0.8) | 2 (1.7) | 5 (4.2) | 12 (10.2) | 7 (5.9) | 27 (22.9) | |
| Total | 18 (15.3) | 29 (24.6) | 36 (30.5) | 23 (19.5) | 12 (10.2) | 118 (100.0) | |
| Agreement | 54 (45.8) | Photographs more severe | 48 (40.7) | Photographs less severe | 16 (13.6) | ||
Treatment compliance
Treatment pathway
There was imbalance between treatment groups, with 45% (N = 99) of participants allocated to AL who were confirmed to comply with the protocol treatment pathway, compared with 28.1% (N = 62) in the Immersion PUVA group (Table 22). Further imbalance is noted with 18.2% (N = 40) of participants in the AL group who continued treatment beyond the protocol treatment phase, compared with 10.4% (N = 23) of the Immersion PUVA group. Meanwhile, 17.7% (N = 39) of participants in the AL group stopped treatment early, compared with 43.4% (N = 96) of participants in the Immersion PUVA group. The proportion of participants for whom compliance with the treatment pathway could not be determined was similar across groups, with 19.1% (N = 42) in the AL group and 18.1% (N = 40) in the Immersion PUVA group (see Table 22).
| AL (%) | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| Treatment pathway compliance a | |||
| Complied with protocol pathway | 99 (45.0) | 62 (28.1) | 161 (36.5) |
| Continued treatment beyond protocol pathway | 40 (18.2) | 23 (10.4) | 63 (14.3) |
| Discontinued treatment early | 39 (17.7) | 96 (43.4) | 135 (30.6) |
| Unclear whether protocol pathway was followed | 42 (19.1) | 40 (18.1) | 82 (18.6) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| Reason for early discontinuation | |||
| Does not require treatment as clear/almost clearb | 2 (5.1) | 2 (2.1) | 4 (3.0) |
| End of maximum randomised treatment periodc | 0 (0.0) | 5 (5.2) | 5 (3.7) |
| Insufficient response to randomised treatmentd | 9 (23.1) | 25 (26.0) | 34 (25.2) |
| Participant request | 13 (33.3) | 33 (34.4) | 46 (34.1) |
| Side effects | 10 (25.6) | 10 (10.4) | 20 (14.8) |
| Other reason | 4 (10.3) | 18 (18.8) | 22 (16.3) |
| Missing | 1 (2.6) | 3 (3.1) | 4 (3.0) |
| Total | 39 (100) | 96 (100) | 135 (100) |
Figure 29 in Appendix 2 shows the treatment pathway decisions by time point over the full 24 weeks. At 4 weeks, 91.4% (N = 201) of the participants allocated to AL were known to be compliant with the treating decisions, compared with 81.0% (N = 179) of the Immersion PUVA participants, because 13.1% (N = 29) of the participants allocated to Immersion PUVA had discontinued treatment by 4 weeks compared with 5.0% (N = 11) of the participants allocated to AL. This discrepancy continued. At 8 weeks, 93.5% (N = 188) of those who complied at 4 weeks continued treatment as per the protocol in the AL arm compared with 83.8% (N = 150) of the Immersion PUVA group who were compliant at 4 weeks. By 12 weeks, a total of 144 (65.5%) were known to have complied with the treatment pathway decisions in the AL group compared with 103 (46.6%) in the Immersion PUVA group. These differences were consistent at all time points, with a higher proportion of Immersion PUVA participants discontinuing treatment early compared with the AL patients.
The most common reasons for early discontinuation were participant request and insufficient response to treatment, with similar proportions observed for both treatment groups, for those who discontinued early. There were differences between the groups, with a higher proportion of participants in the AL group who stopped due to side effects, 25.6% of those who stopped treatment early, compared with 10.4% of Immersion PUVA participants who stopped early. Meanwhile, a higher proportion of Immersion PUVA patients who stopped early stopped for other reasons, at 18.8% (N = 18), compared with 10.3% (N = 4) of AL participants who stopped early. There were eight patients (one allocated to AL and seven allocated to Immersion PUVA) who stopped treatment early due to COVID-19, and a further two patients who reported early discontinuation due to the Immersion PUVA schedule (see Table 22).
Treatment received
Over 12 weeks, participants allocated to AL should have received 30 mg per day (with reduction to 10 mg for side effects, if required, in line with standard practice) from the day of randomisation, and participants allocated to Immersion PUVA should have received twice-weekly treatments with no more than 7 days between treatments. This section also summarises the proportion of treatment received compared with that expected over the 12 weeks, together with the number of missed treatments, and length of any treatment breaks. Overall, 92.5% (N = 408) participants were confirmed to receive their randomised treatment, with an imbalance between the groups; 96.4% (N = 212) of those allocated to AL received their treatment compared with 88.7% (N = 196) in the Immersion PUVA group (Table 23). As expected, the number of days from randomisation to receipt of first treatment was smaller in the AL group compared with the Immersion PUVA group, with a median (IQR) of 0 (0–1) days and 5 (4–7) days, respectively (see Table 23). A total of 91.4% (N = 201) of the participants allocated to AL were confirmed to receive their treatment within 7 days compared with 74.7% (N = 165) of the participants allocated to Immersion PUVA (see Table 23).
| AL | Immersion PUVA | Total | |
|---|---|---|---|
| Received any randomised treatment | |||
| Yes (%) | 212 (96.4) | 196 (88.7) | 408 (92.5) |
| No (%) | 1 (0.5) | 10 (4.5) | 11 (2.5) |
| Missing (%) | 7 (3.2) | 15 (6.8) | 22 (5.0) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Number of days from randomisation to starting treatment | |||
| Mean (SD) | 1.8 (5.6) | 7.0 (9.1) | 4.3 (7.9) |
| Median (range) | 0.0 (0–51) | 5.0 (0–84) | 2.0 (0–84) |
| IQR | 0.0–1.0 | 4.0–7.0 | 0.0–5.0 |
| Missing | 9 | 26 | 35 |
| N | 211 | 195 | 406 |
| Started treatment within 7 days of randomisation | |||
| Yes (%) | 201 (91.4) | 165 (74.7) | 366 (83.0) |
| No (%) | 12 (5.5) | 34 (15.4) | 46 (10.4) |
| Missing (%) | 7 (3.2) | 22 (10.0) | 29 (6.6) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
A total of 67.7% (N = 149) participants allocated to AL were confirmed to receive at least 80% of their treatment compared with 39.8% (N = 88) of those allocated to Immersion PUVA (Table 24). There was a similar imbalance in terms of treatment breaks, with 72.3% (N = 159) of participants allocated to AL confirmed as having no treatment break > 7 days, compared with just 26.7% (N = 59) of those allocated to Immersion PUVA (see Table 24). There was therefore a large imbalance in the observed treatment compliance; 65.9% (N = 145) of the participants allocated to AL were confirmed to be within the trial definition of a complier, compared with 24.0% (N = 53) of the participants allocated to Immersion PUVA (Table 25).
| AL | Immersion PUVA | Total | |
|---|---|---|---|
| Compliance | |||
| Mean (SD) | 0.9 (0.2) | 0.7 (0.3) | 0.8 (0.2) |
| Median (range) | 1.0 (0–1) | 0.8 (0–1) | 0.9 (0–1) |
| IQR | 0.9–1.0 | 0.7–0.9 | 0.8–1.0 |
| Missing | 51 | 64 | 115 |
| N | 169 | 157 | 326 |
| Received at least 80% of their treatment | |||
| Yes (%) | 149 (67.7) | 88 (39.8) | 237 (53.7) |
| No (%) | 20 (9.1) | 69 (31.2) | 89 (20.2) |
| Missing (%) | 51 (23.2) | 64 (29.0) | 115 (26.1) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| Did not have a treatment interruption of more than 7 days | |||
| Yes (%) | 159 (72.3) | 59 (26.7) | 218 (49.4) |
| No (%) | 17 (7.7) | 89 (40.3) | 106 (24.0) |
| Missing (%) | 44 (20.0) | 73 (33.0) | 117 (26.5) |
| Total (%) | 220 (100) | 221 (100) | 441 (100) |
| AL (%) | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| Compliance with reasons for non-compliance | |||
| Compliant | 145 (65.9) | 53 (24.0) | 198 (44.9) |
| Proportion of treatment received < 80% | 13 (5.9) | 26 (11.8) | 39 (8.8) |
| Treatment interruption of more than 7 days | 10 (4.5) | 46 (20.8) | 56 (12.7) |
| Both proportion of treatment received < 80% and treatment interruption of more than 7 days | 7 (3.2) | 43 (19.5) | 50 (11.3) |
| Early discontinuation of treatment | 13 (5.9) | 23 (10.4) | 36 (8.2) |
| Missing | 32 (14.5) | 30 (13.6) | 62 (14.1) |
| Total | 220 (100) | 221 (100) | 441 (100) |
In light of this large imbalance of treatment compliance, the DMEC and the TSC agreed that it would be inappropriate to conduct a PP population analysis.
Chapter 4 Health economics
Aims and objectives
Aims of economic evaluation
The primary aim of the health economic analysis was to assess the cost-effectiveness of AL compared with Immersion PUVA in the management of severe chronic HE.
Objectives of economic evaluation
The primary objective of the health economic analysis was to estimate the short- and long-term cost-effectiveness of AL versus Immersion PUVA in the management of severe chronic HE. To achieve the latter, two sets of economic evaluations will be undertaken:
-
a within-trial analysis to estimate the cost-effectiveness at weeks 12 and 52
-
a long-term decision analytic model to estimate the cost-effectiveness of AL versus Immersion PUVA up to 10 years post intervention.
The analysis performed here is based on the previously developed health economics analysis plan.
Jurisdiction and perspective
The trial was conducted in the NHS, UK. Both analyses followed the NHS and personal and social (PSS) perspective. An additional secondary analysis for the within-trial analysis included the societal perspective or that of the patients, which was aimed at accounting for out-of-pocket expenditure. All the analyses adhered to the methods guidance produced by the NICE. 67 Such guidance has been developed to produce unbiased and complete economic evaluations set in the UK.
Within-trial analysis
The within-trial analysis was aimed at estimating the cost-effectiveness of the intervention(s) from the NHS perspective and the societal perspective. The analysis compared costs and consequences for over 12 and 52 weeks post start of treatment. The outcome measures were QALYs estimated from utility values collected during the trial, while costs were based on estimates from resources used by the trial participants (more details below). The analysis was performed using R studio®.
Outcome measures
Quality-adjusted life-years were used as an outcome measure; these are a generic measure of health that take account of both quality and length of life, such that one QALY is equal to 1 year of life lived in a state of full health. 68 For the primary analysis, health-related QoL was estimated using responses from the EQ-5D-3L. 69 A further analysis mapped the responses of the DLQI to the EQ-5D-3L instrument to estimate QALYs (more details below).
Measurement and valuation of outcomes
Responses to both the EQ-5D-3L and the DLQI instrument were collected at baseline and weeks 12, 24, 36 and 52 post planned start of treatment. To estimate QALYs from the EQ-5D-3L instrument, we used standard UK tariff values (based on UK general population preferences) to estimate patients’ utility at each collected time point. QALYs were then calculated using the ‘area under the curve’ approach. 69 These methods use two adjacent time points to estimate QALYs for a specific time period. We used the following formula to estimate QALYs at week 12 (1) and week 52 (2).
Where EQ5DBaseline, EQ5D12, EQ5D24, EQ5D36 and EQ5D52 are the EQ-5D scores at baseline, week 12, week 24, week 36 and week 52, respectively, while ti is the time in weeks between two given measurements over the 12- or 52-week period (t1 = 12/52; t2 = 12/52; t3 = 12/52; t4 = 16/52).
The DLQI responses were mapped to EQ-5D-3L using the method described by Ali et al. 70 This method was used by Blank et al. 71 The mapped responses were then used to estimate the DLQI QALYs using the area under the curve approach described above.
Cost
All healthcare resources used to estimate the costs of both interventions were collected via a health resource use questionnaire. This was aimed to collect information on resources used or costs incurred by the patients during their participation in the trial and follow-up period. The questionnaire was administered at weeks 12, 24, 36 and 52.
The questionnaire captures information to estimate the costs incurred by NHS in the provision of the treatment and other healthcare resource utilisation, including medications over the trial follow-up. It also included a section to capture patients’ out-of-pocket expenditures, including travel expenses, creams, medical aids such as gloves, and the potential cost of a day out of work to estimate the potential overall burden of both treatments on patients’ income.
Valuation of resource use data
Wherever possible, unit costs for resources were obtained from national sources: NHS reference costs were employed to value hospital resource use (e.g. outpatient attendances). Costs of medical personnel, such as general practitioner (GP) and nurse consultations, were obtained from the PSS Research Unit Costs of Health and Social Care. 72 Medication costs were taken from the British National Formulary (BNF) and the Prescription Cost Analysis (PCA) for England. 73,74 Table 26 contains the costs of the resources used during the trial. These unit costs were then used to estimate the total healthcare costs per patient during the intervention and follow-up period.
| Item | Unit cost (£) | Additional notes | Source |
|---|---|---|---|
| Community-based services | |||
| GP visit at a general practice | 40.00 | Cost per surgery consultation (9.22 minutes), including direct care | PSSRU 202172 |
| District nurse visit at a general practice | 21.00 | Cost per visit lasting 20 minutes | |
| Physiotherapist (community services) | 103.14 | Average cost per session (community services) | |
| Occupational therapist (community services) | 51.57 | Average cost 30 minutes session | |
| Hospital-based services | |||
| A&E attendance | 124.15 | A weighted average of category 1–2 treatment non-admitted | National cost collection 2020/2176 |
| Dermatology | 128.40 | Average per visit, | |
| Cardiology | 213.00 | Average per visit | NHS Tariffs 2022/2377 |
| Haematology | 346.50 | Average per visit | |
| Gastroenterology | 224.50 | Average per visit | |
| Radiotherapy | 256.40 | National average, adjusted to 2022 | National cost collection 2020/2176 |
| Monitoring | |||
| Blood test | 4.00 | Phlebotomy. National schedule of NHS costs (2019/20)78 | |
| Pregnancy test | 1.00 | Cost per pregnancy strip test (private pharmacy) | |
| Medication/phototherapy | |||
| Toctino (AL) | 493.72 | Cost per box of 30 capsules | BNF73 |
| Phototherapy or Photo-chemotherapy | 112.00 | Assumed as the cost per Immersion PUVA session and any other phototherapy offered | National schedule of NHS costs (2019/20)78 |
| Other alternative treatments | |||
| Ciclosporin A | Doses and duration based on patient responses | BNF73 | |
| 50 mg (30 capsules) | 35.97 | ||
| 100 mg (30 capsules) | 68.28 | ||
| Oral glucocorticoids | 29.12 | Drug and pharmaceutical electronic market information tool79 | |
| Methotrexate 2.5 mg (100 tablets) | 4.23 | ||
| Azathioprine 50 mg (56 tablets) | 1.57 | ||
| Mycophenolate mofetil | |||
| 250 mg (100 tablets) | 9.81 | ||
| 500 mg (50 tablets) | 6.83 | ||
| Acitretin | 9.06 | ||
Treatment costs were then added to the estimated healthcare costs to estimate the total NHS and PSS costs. According to protocol, patients were to receive their allocated treatment up to week 12 depending on their HE status. However, patients could withdraw from their allocated treatment at any time point during the intervention and follow-up phase due to side effects, lack of effectiveness or other. A proportion of these patients were offered an alternative treatment option, some including AL or Immersion PUVA. Our estimates of treatment costs were then based on patients’ responses on the duration of their original randomised treatment and the offered alternative if applicable. Table 26 contains the resources used by participants with their unit costs. Resources used related to their allocated intervention are described in Table 27. Patients’ responses along with the details described in Tables 26 and 27 were used to estimate the healthcare costs of both interventions at weeks 12 and 52 (excluding out-of-pocket expenditure). These healthcare costs estimates comprise those incurred during the intervention phase, follow-up phase and pharmacological treatment alternatives offered to patients who stopped their initial allocated intervention and were offered an alternative. This information was captured monthly. All costs were updated to 2022 British pound sterling (GBP) prices using the healthcare index. 75
| Time point | AL | Immersion PUVA |
|---|---|---|
| Resources/items costed | Resources/items costed | |
| Baseline to week 12 (all patients) |
|
|
| Weeks 12–16: patients with moderate PGA score or those whose treatment was considered adequate to continue, irrespective of their HE status |
|
|
| Weeks 16–20: patients with moderate PGA score or those whose treatment was considered adequate to continue, irrespective of their HE status |
|
|
| Weeks 20–24: patients with moderate PGA score or those whose treatment was considered adequate to continue, irrespective of their HE status |
|
|
| Weeks 24–36: only for those patients whose treatment was considered adequate to continue despite the intervention concluding at week 24 |
|
|
| Weeks 36–52: only for those patients whose treatment was considered adequate to continue despite the intervention concluding at week 24 |
|
Personal and social or societal costs were collected at the same time as the resource use collection schedule (weeks 12, 24, 36 and 52). Information collected included items such as gloves and creams (including steroids and emollients), travel costs and loss of workdays because of HE.
Total costs for the base case analysis were those incurred by the NHS and PSS. These were calculated by adding all resources used by participants as reported in the questionnaires plus the treatment cost based on their initial treatment allocation and treatment alternatives if treatment was changed or stopped during the treatment and follow-up phase.
Secondary analysis was aimed at exploring the impact of both interventions including the patient perspective. To achieve the latter, we added to the total NHS and PSS costs the total out-of-pocket expenditure as reported by the participants. These costs included those due to loss in productivity, measured by loss of workdays because of HE. To estimate the average cost of a workday lost, we use the average wage or income per day for people of working age depending on their work status (i.e. full-time, part-time, self-employed or government-supported training). Loss of income was accounted for those patients who reported being at work during the follow-up period and indicated that they required time off work due to their skin condition. This information was obtained from the Office of National Statistics UK and the UKRI. 80,81
Population, temporality, discounting rates and cost-effectiveness threshold
The analysis adopted the ITT perspective. Alternative population analysis such as complete data was also performed as part of the sensitivity analysis (details below).
The within-trial analysis was carried out at the end of weeks 12 and 52. At these time points, we estimated the mean QALYs and costs associated with each treatment option and compared them to evaluate which strategy was considered cost-effective. Costs and QALYs were not discounted, as they incurred within a year from randomisation, as recommended by NICE. Cost-effectiveness was determined by comparing the estimated incremental cost-effectiveness ratio (ICER) against the NICE threshold value (λ). The ICER was estimated as follows (3):
The ICER represents the additional cost per QALY gained for each intervention compared with the next best alternative. 82 The more expensive intervention will be considered cost-effective as long as its ICER is below λ = £20,000 or within the £20,000–30,000 per QALY range.
Data cleaning and analysis
Data were analysed using R Studios®. Face validity tests were conducted on data (e.g. to identify mis-spelled text) and checked against the source documents. Mis-spelled text or different connotations for the same item were found in the responses, particularly on the out-of-pocket expenditure data sets. Any corrections or standardised use of terms was done on a copy of the data set to avoid altering the original responses from the questionnaires.
Missing data
We found missing data on the HE status and utility responses. Missingness on these two variables was considered relevant due to its impact on the estimation of the treatment costs and QALYs derived from DLQI. To deal with the latter, we perform multiple imputations. The analysis was conducted on the assumption of MAR. Regression analysis showed that some observed variables predicted missingness, indicating that MAR was a plausible assumption. We recognise, however, that missingness not at random (MNAR) cannot be completely ruled out. The multiple imputation tools include randomness to reflect the uncertainty inherent in missing data. The process uses iterative multivariable regression techniques. 83
Baseline adjustments and correlation between cost and quality-adjusted life-years
Seemingly unrelated regression (SUR) was used to adjust QALYs and costs to estimate outcomes and account for the correlation between observed costs and utilities. The estimated outcomes were costs and QALY differences at weeks 12 and 52. The analysis took into consideration the potential of utility imbalance at randomisation. This is of particular importance because a patient’s utility at baseline is likely to be correlated with their utility over the follow-up period. 84 We account for these potential imbalances by including the baseline utility responses in our SUR analysis. Other variables included to estimate the costs and QALYs difference were treatment, skin type, duration of HE, phenotype, presence of specific IgE to inhalant or other relevant allergens, ethnicity, foot involvement, BMI and smoking status at baseline. Centre was not included as a variable, as it was not found to have an impact on the results or adjustment.
Sampling uncertainty
The level of sampling uncertainty around the ICER was determined by randomly sampling 10,000 iterations based on a normal distribution using the mean and standard error estimates of QALY and cost difference from the SUR analysis using the estimated variance/covariance matrix. The random samples were used to estimate the mean cost and QALY difference to estimate the mean ICER. 85 Net monetary benefit (NMB) was also estimated from these iterations. NMB combines cost-effectiveness and willingness to pay to give an explicit monetary valuation of the health outcome. NMB is estimated as follows (4):
For this analysis, and assuming the NICE recommended threshold value (λ) of £20,000 per QALY gained, the treatment with an average incremental NMB ≥ 0 should be adopted. The incremental net monetary benefit (INMB) was estimated by substituting QALYs to incremental QALYs (difference in QALYs between two interventions) and costs to incremental costs (difference in costs between the two interventions) in the equation above.
The expected INMB was used to estimate the probability of the intervention being cost-effective given a range of threshold values (λ = £1000 to λ = £100,000). These estimates were then plotted to construct the cost-effectiveness acceptability curve (CEAC). 86 The CEAC illustrates the probability that AL is cost-effective compared with Immersion PUVA as a function of the willingness-to-pay threshold (λ). The CEAC is constructed by using the 10,000 samples from the mean estimated differences and its standard error from the SUR adjustment to plot the proportion of times each treatment has a positive INMB for a range of willingness-to-pay thresholds (λ).
Sensitivity analyses
Alternate scenarios were explored in the sensitivity analysis to test the robustness of the main trial analysis results. The effect of not imputing missing data was considered with an analysis that includes only complete cases. We also explored the cost-effectiveness of both interventions using the DLQI responses mapped to QALYs. Further, we explored the impact of out-of-pocket expenditure on the cost-effectiveness results. Table 28 summarises the four sensitivity scenarios included in the analysis.
| Analysis | Perspective | Time horizon | Outcome measure | Resource use measure | Baseline adjustment | Missing data | Cost-effectiveness analysis | |
|---|---|---|---|---|---|---|---|---|
| Primary | ITT | Healthcare and personal social service provider | 12 and 52 weeks | QALYs (EQ-5D) | Resource use questionnaires and intervention costs | EQ-5D | Imputed | Cost per incremental QALY |
| Sensitivity | DLQI mapping | Healthcare and personal social service provider | 12 and 52 weeks | DLQI mapped QALYs | Resource use questionnaires and intervention costs | EQ-5D | Imputed | Cost per incremental QALY |
| Societal perspective | Societal perspective | 12 and 52 weeks | QALYs (EQ-5D) | Resource use questionnaires and intervention costs | EQ-5D | Imputed | Cost per incremental QALY | |
| Complete case | Healthcare and personal social service provider | 12 and 52 weeks | QALYs (EQ-5D) | From the resource use questionnaires and intervention costs | EQ-5D | Excluded | Cost per incremental QALY |
Long-term cost-effectiveness analysis
Decision analytic modelling was undertaken to extrapolate costs and outcomes beyond the duration of the trial, irrespective of statistical significance in trial results. The model had a 10-year duration, as previous work had demonstrated that the ICER was likely to change marginally on a higher time horizon; an analytic 10-year horizon, therefore, appeared long enough to capture all the main costs and benefits of both treatments. 41
To achieve the latter, we developed a Markov decision analytic model to compare both interventions (AL vs. Immersion PUVA) in the management of severe chronic HE. The starting point was at the end of week 24, corresponding to the end of the intervention phase. This allows some data collected during the last 28 weeks of follow-up to be used to populate the model (more details below).
The model structure is shown in Figure 12. The main elements of the model were aimed to capture patients’ response to treatment at week 24 and every 3 months until 10 years. The model included three mutually exclusive health states depending on the status of the patients’ HE: clear/almost clear; mild/moderate; and severe. Death from natural causes at any stage of the above-mentioned states was also considered.
FIGURE 12.
Markov model structure.
Health states and treatment effects beyond the end of the intervention phase
The long-term impact of both interventions was sourced from the trial data. The proportion of patients considered to have a HE that was clear/almost clear, moderate and severe at week 24 was used to determine the initial proportion of patients in the Markov chain. Transitions from weeks 24 to 36 and then to week 52 were those observed in the trial. Transitions from week 52 onwards were assumed to be the same as those observed between weeks 36 and 52. According to the trial protocol, after week 24, all patients will have stopped their initially allocated treatment at randomisation. After this week, patients who required additional treatment followed what was considered standard practice. This includes ciclosporin A, methotrexate, acitretin and azathioprine but also intensified topical treatment with immunomodulatory agents such as glucocorticoids, calcineurin inhibitors or Jak (Janus kinase) inhibitors. The standard practice may also include treatment with Immersion PUVA or AL irrespective of the patient’s initial treatment allocation. Treatment decisions were reviewed every 4 weeks and continued or modified accordingly. After consulting with the clinical team of the trial, it was agreed that the clinical transitions between HE status observed between weeks 36 and 52 could be assumed to be carried on for transitions from week 52 onwards. This assumption implies that self-awareness, recognition of symptoms, treatment experience and standard of care management, if required, would allow patients to maintain or fluctuate between clear/almost clear and moderate, while patients who had severe HE after 52 weeks would either remain there or move towards moderate or clear. Other studies have assumed that the time to relapse for patients under AL was 24 weeks. Based on the data collected from the trial, most patients will likely fluctuate between clear/almost clear and moderate from 24 weeks onwards, while not many patients return to the severe state. The proportion of patients in the clear state for AL was 32%, while this proportion slightly increased to 33% at week 36 and 38% at week 52. Similarly, for Immersion PUVA, the proportion of patients with a clear/almost clear status at week 24 was 41%; at week 36, this proportion reduced to 33%, increasing again to 45% at week 52. The transition probabilities used for the model are shown in Appendix 3, Tables 51–54. The length of the model cycle was 3 months. To fit this with the actual data, and in consultation with the clinical team of the trial, we assumed that patient status at week 52 was equivalent to that observed at week 48.
Death for natural causes was considered from transitions beyond week 52. The proportion of patients dying from natural causes was obtained from the Office for National Statistics (ONS) life tables for all causes of mortality and was considered depending on the age of the patient.
Adverse events of treatment
Two of the main AEs reported by using AL are the potential increase of cholesterol and triglyceride levels and persistent headaches. Both AEs were considered during the trial design and were closely monitored by the treating physician. If the patient showed any of these events in a severe form, treatment dose was reduced, or treatment was suspended. In the case of discontinuation of AL as randomised treatment, alternative treatment according to standard of care was given. Given that these events were mainly observed, and action was taken during the intervention phase and follow-up period (up to 52 weeks), there were no concerns in terms of potential long-term implications or future dropouts after 52 weeks for these reasons.
Quality of life
Utility values collected at baseline, week 12 and week 24 were used to estimate QALYs at week 24. We follow the under-the-curve approach described above for this estimation. As the Markov model’s initial population was divided by HE status, we estimated QALYs at week 24 by this variable (clear/almost clear; moderate; and severe). These estimates were adjusted by baseline unbalances to avoid potential initial bias at the randomisation stage. The utility reported from the trial at week 36 was used for the initial cycle, while the utility reported at week 52 was assumed for the second and future cycles of the model (Appendix 4, Tables 56 and 57).
Costs
Costs up to week 24 were estimated based on those reported in the health economics questionnaires up to that week. To these costs, we added the treatment costs based on the treatment offered at randomisation but using the treatment continuation reported by patients to accurately cost the time under AL or Immersion PUVA.
Table 27 shows the items included in each treatment arm. As described above, if a patient had their treatment discontinued, they were offered alternative treatment according to standard care. These costs were estimated from the resource use questionnaires and may include AL or Immersion PUVA as well as other alternative treatments offered, according to the patient’s treatment plan. A treatment plan for these patients was reviewed and updated every 4 weeks up to week 36 and then at week 52. We adjusted the estimated costs between weeks 36 and 52 to fit with the model cycle duration (every 3 months). After discussion with the clinical team of the trial, the average costs during the last 4 months of the intervention phase (between weeks 36 and 48) were considered representative of those related to a long-term HE standard of care. Up to this point in time (and confirmed by the treatment continuation responses), it was unlikely that patients would be still receiving AL or Immersion PUVA, even as part of their standard care package. This was confirmed by the data analysis, as no patient remained under such treatments at week 52. As such, the adjusted costs between weeks 36 and 52 (adjusted to 48) were assumed as standard care costs and were used from this point onwards in the model.
Discounting
Discounting of costs and outcomes was used after week 52 according to NICE guidelines. 67 The discounting rate was 3.5%.
Model uncertainty
Parameter uncertainty was addressed through probabilistic sensitivity analysis (PSA) using Monte Carlo simulation. Transitions between HE states followed a Dirichlet distribution, while 3-monthly costs and QALYs followed a gamma and beta distribution, respectively (Appendix 3 – Long-term model transition probabilities, cost, QALYs parameters; Tables 51–55). From these iterations, we estimated the mean ICER comparing AL versus Immersion PUVA. We also developed a scatterplot and cost-effectiveness acceptability (CEAC) curves and calculated the INMB of both interventions. Cost-effectiveness was determined by comparing the ICER against the NICE recommended threshold of £20,000 per QALY gained. This was supported by the NMB, in which case the intervention with a positive or above-zero INMB was cost-effective. We performed one-way and scenario deterministic sensitivity analysis. These were: both interventions had equal costs after week 52; both interventions had equal costs and outcomes after week 52; and lastly, both interventions had equal costs, outcomes and transition probabilities between health states after week 52.
Expected value of perfect information
The long-term decision analytic cost-effectiveness model was used to estimate the value of further research on the use of AL or Immersion PUVA [expected value of perfect information (EVPI)]. We estimated a 5-year EVPI, assuming a 1-year HE prevalence of severe HE of 1.2% and a yearly incidence of 0.955 per 1000 people, based on Quaade et al. , and a £20,000 willingness to pay. 1 The 5-year population was discounted at 3.5% yearly rate.
Results
The overall within-trial analysis results indicate that AL is the cost-effective strategy. These results are robust, as the probability of cost-effectiveness is over 96% in favour of AL in all scenarios. The long-term (10 years) analysis indicates a slight advantage for Immersion PUVA; however, the small difference in costs and QALYs indicates that the results are uncertain, as only 50% of the iterations favour Immersion PUVA.
The within-trial estimated costs indicate that Immersion PUVA is more costly than AL. Treatment costs are the main drivers of Immersion PUVA costs (these include outpatient visits, the cost of therapy, medication and follow-up). Out-of-pocket expenditures are also higher for patients originally assigned to Immersion PUVA. Alternative treatments offered were higher for AL at week 52 (Table 29). The resource use breakdown can be found in Appendix 5, Tables 58–61.
| Cost | Week 12 | t-test (p-value) | Week 52 | t-test (p-value) | ||
|---|---|---|---|---|---|---|
| AL | Immersion PUVA | AL | Immersion PUVA | |||
| Health care | £33 | £50 | 0.050 | £163 | £219 | 0.021 |
| Treatment (AL or Immersion PUVA) | £1858 | £3128 | < 0.001a | £2431 | £3571 | < 0.001a |
| Alternative treatments | £15 | £57 | 0.015 | £759 | £599 | 0.135 |
| Out-of-pocket | £310 | £541 | 0.119 | £1209 | £1684 | 0.055 |
| Total without out-of-pocket | £1907 | £3235 | < 0.001 a | £3353 | £4389 | < 0.001 a |
| Total with out-of-pocket | £2216 | £3776 | < 0.001 a | £4562 | £6074 | 0.000 a |
| QALYs | 0.1589 | 0.1651 | 0.239 | 0.7618 | 0.7984 | 0.025 |
The adjusted QALYS for both interventions at weeks 12 and 52 are shown in Table 29. Immersion PUVA’s QALYs are higher at both time points. The difference at week 12 is negligible (< 1 full day of health). Similarly, at week 52, Immersion PUVA participants reported an unadjusted increase in QALYs of 0.036, which is equivalent to around 13 days of full health.
Within-trial analysis
Primary analysis results
The estimated mean adjusted costs of AL at week 12 were £1904 compared with £3236 for Immersion PUVA, while the estimated QALYs were 0.1589 and 0.1651, respectively. At 52 weeks, the estimated costs were £3366 for AL and £4424 for Immersion PUVA, while the QALYs were 0.761 and 0.798, respectively. Despite Immersion PUVA offering more QALYs at both time points, the cost for Immersion PUVA is much greater than AL (over £1000 more expensive) at both time points. AL is the cost-effective strategy, as the estimated ICER for Immersion PUVA is above £20,000, while the INMB for Immersion PUVA is negative. The probability of cost-effectiveness indicates that the results are robust, as AL is cost-effective at 100% at week 12 and 96% at week 52, when the threshold is set at £20,000 per QALY gained (Tables 30 and 31). The scatterplot and CEAC of these analyses can be found in Appendix 6, Figures 30–33.
| Intervention | Costs (£) | QALYs | Incremental adjusted cost (£) | Incremental adjusted QALYs | ICER (£) | INMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| AL | 1907 | 0.159 | 0 | 1.00 | |||
| Immersion PUVA | 3256 | 0.165 | 1333 | 0.002 | 699,682 | −1295 | 0.00 |
| Interventiona | Costs (£) | QALYs | Incremental adjusted cost (£)b | Incremental adjusted QALYsb | ICER (£) | INMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| AL | 3353 | 0.761 | 0 | 0.96 | |||
| Immersion PUVA | 4398 | 0.798 | 1081 | 0.027 | 39,787 | −537 | 0.04 |
Dermatology life quality index
When estimating the QALYs based on the DLQI, the estimated QALYs were 0.158 for AL and 0.154 for Immersion PUVA at 12 weeks, and 0.784 and 0.782, respectively, at week 52. The cost of both interventions remains the same as in the base case scenario (where Immersion PUVA is more expensive), while the estimated QALYs at 12 weeks favour AL slightly (making it dominant, i.e. less expensive, and more effective). The QALY difference is reduced at week 52 when compared with the primary analysis; however, AL maintains cost-effectiveness. In both scenarios, AL is the cost-effective strategy with at least 91% probability of cost-effectiveness (Tables 32 and 33).
| Interventiona | Costs (£) | QALYs | Incremental adjusted costb (£) | Incremental adjusted QALYsb | ICER (£) | INMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| AL | 1907 | 0.158 | 0 | 1.00 | |||
| Immersion PUVA | 3256 | 0.154 | 1333 | −0.004 | −298,286 | −1422 | 0.00 |
| Interventiona | Costs (£) | QALYs | Incremental adjusted costb (£) | Incremental adjusted QALYsb | ICER | INMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| AL | 3353 | 0.7840 | 0 | 0.91 | |||
| Immersion PUVA | 4398 | 0.7820 | 1080 | −0.002 | −£670,338 | −1112 | 0.09 |
Societal perspective
When out-of-pocket expenditure is added, the costs increase by £567 for Immersion PUVA (from £3236 to £3803) and by £280 for AL (from £1904 to £2184) at week 12. At week 52, costs increase by £1788 (from £4424 to £6212) for Immersion PUVA and by £1249 (from £3366 to £4615) for AL. The cost difference between the two interventions is increased from £1333 to £1650 at week 12 and from £1081 to £1841 at week 52. This increases the ICER in favour of AL, with a probability of cost-effectiveness of 100% for both time points (Tables 34 and 35).
| Intervention | Costs (£) | QALYs | Incremental adjusted cost (£) | Incremental adjusted QALYs | ICER (£) | INMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| AL | 2216 | 0.1589 | 0 | 1.00 | |||
| Immersion PUVA | 3776 | 0.1651 | 1650 | 0.002 | 905,461 | −1614 | 0.00 |
| Interventiona | Mean costs (£) | Mean QALYs | Incremental adjusted cost (£)b | Incremental adjusted QALYsb | ICER (£) | INMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| AL | 4562 | 0.761 | 0 | 1.00 | |||
| Immersion PUVA | 6074 | 0.798 | 1844 | 0.027 | 68,442 | −1305 | 0.00 |
Complete case analysis
At weeks 12 and 52, AL is the cost-effective strategy. At week 52, however, the estimated costs and QALYs difference indicate that AL is a dominant strategy, as it is cheaper and more effective, resulting in a negative ICER. The probability of cost-effectiveness indicates that the results are robust, as 100% and 99% of the iterations, respectively, indicate that AL is the cost-effective strategy (Tables 36 and 37).
| Interventiona | Costs (£) | QALYs | Incremental adjusted costb (£) | Incremental adjusted QALYsb | ICER | INMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| AL | 1938 | 0.156 | 0 | 1.00 | |||
| Immersion PUVA | 3233 | 0.159 | 1297 | 0.001 | £1,335,016 | –1277 | 0.00 |
| Interventiona | Costs (£) | QALYs | Incremental adjusted costb (£) | Incremental adjusted QALYsb | ICER (£) | INMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| AL | 3585 | 0.622 | 0 | 0.99 | |||
| Immersion PUVA | 4537 | 0.607 | 986 | −0.021 | −46,942 | −1406 | 0.01 |
Long-term cost-effectiveness model results
The estimated costs at 10 years were £5432 for AL and £5361 for Immersion PUVA, while the estimated QALYs were 6.530 and 6.536, respectively, indicating that Immersion PUVA is dominant over AL and therefore is the cost-effective strategy (Table 38). However, the results are uncertain, as the probability of cost-effectiveness is equal between the two interventions (50%). The latter is due to the negligible difference in QALYs (< 0.01) and a small difference in costs (< £75) (see Table 38).
| Intervention | Costs (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) | NMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| Immersion PUVA | 5361.65 | 6.5357 | 0 | 0.50 | |||
| AL | 5432.89 | 6.5303 | 71.23 | −0.0054 | −13,123 | −180 | 0.50 |
The scatterplot shown in Appendix 6 (see Figures 34 and 35) shows the uncertainty of the results, as the cloud of iterations is equally distributed between the two interventions, while the CEAC at £20,000 indicates that both interventions have an equal probability of being cost-effective.
The change in the results from the long-term sensitivity analysis in all the scenarios analysed (equal costs; equal costs and outcomes; and equal costs, outcomes and transition probabilities from week 52) showed a relatively small variation in costs and QALYs with no change in the cost-effectiveness of Immersion PUVA. Similarly, results remain uncertain, as only 51% of the iterations indicate that Immersion PUVA is the preferred option. The latter is given by the relatively bigger proportion of patients in the clear/almost clear and moderate states in the Immersion PUVA arm at the end of week 52 (see transition probabilities). Results of these analyses can be found in Appendix 7, Tables 62–64.
Expected value of perfect information
Considering all adults in the UK between the age of 15 and 75 years (49.3 million people), we estimated that 610,000 people may suffer from severe HE in one given year, with a yearly incidence of 46,950. 87 Using those estimates, the individual EVPI was £5145, while the total EVPI indicates that research in this area may be up to £4 billion in a 5-year time period.
Discussion
The within-trial and long-term cost-effectiveness analysis comparing AL versus Immersion PUVA indicate that AL is the cost-effective strategy at weeks 12 and 52, while the long-term analysis indicates that Immersion PUVA is cost-effective, but with a high degree of uncertainty.
The within-trial analysis results are, however, robust, as the probability of cost-effectiveness indicates that in 96% (or above) of the 10,000 iterations, AL is the cost-effective strategy, while the long-term analysis is uncertain, as the PSA analysis indicates that both interventions have equal probability of being cost-effective.
In both analyses, the cost difference is the driver of the cost-effectiveness results. The within-trial analysis estimates that AL is over £1300 cheaper during the intervention phase (up to week 12) and over £1000 cheaper at week 52 (intervention and follow-up phase). The latter reflects the relevant cost incurred by repeated outpatient visits to receive Immersion PUVA during the intervention phase. The follow-up phase between weeks 12 and 52 indicates that costs incurred by patients initially under Immersion PUVA are lower than for those under AL. As a result, the long-term costs of Immersion PUVA (at 10 years after the intervention) are almost equal to those of AL, as the cost difference between the two at 10 years is almost equal (£71 difference).
Within-trial analysis estimating QALYs based on patients’ DLQI responses offers similar results to those of the base case analysis, where AL is the cost-effective strategy. In contrast, however, the mean estimated QALY difference favours AL instead of Immersion PUVA. These estimates indicate that AL is the dominant strategy (less costly and more effective than Immersion PUVA).
The out-of-pocket analysis performed indicates that patients undertaking Immersion PUVA spend on average £287 more than patients under AL at 12 weeks and £539 over 52 weeks. The latter is likely to be related to the increased travel expenditure and/or potential loss of workdays to attend treatment. The increase in out-of-pocket expenditure reaffirmed the cost-effectiveness of AL during the treatment and follow-up phases.
As a limitation of this study, the results obtained from the 10-year analysis assume that patients who were relatively successful in managing their HE during the treatment and follow-up phase will be more likely to effectively treat their HE if they experience a recurrence. This means that patients are likely to remain or fluctuate between almost clear and moderate HE for the 10 years following the intervention, as they will be able to identify and manage their condition better along with their treatment physician. People with severe HE at the end of the follow-up period will likely remain there or move to moderate eczema. We made this assumption based on clinical expertise due to the lack of long-term data on the effectiveness of AL or Immersion PUVA. Other studies evaluating AL have assumed that patients relapse after 24 weeks and assumed retreatment of eligible patients with AL. 71 However, these assumptions contradict our observations in the trial period, which indicate that patients on average remain in either the clear/almost clear and/or the moderate state after 24 weeks post treatment. Furthermore, phototherapy guidelines generally do not recommend more than one Immersion PUVA course per year, and there is an upper limit of (lifetime) exposure sessions for hand and foot Immersion PUVA of around 200. We evaluated alternative scenarios to evaluate the potential impact of this assumption; on those scenarios, however, the results remain unchanged.
Although expected value of parameter information was not performed, it is clear that further research in the area investigating the long-term impact of these two interventions would help reduce the uncertainty of the long-term results, as indicated by the EVPI, who estimated that further research in the area up to £4 billion is considered good use of public funds. Future research including expected value of perfect partial information (EVPPI) would help determine which other parameters future research should focus on.
Chapter 5 Discussion
The initial trial for AL, a retinoid used in the treatment of HE, was a comparison with placebo. 52 Since this initial trial, which led to European Medicines Agency (EMA)/National Institutes of Health (NIH) approval, many observational, real-world data have been reported, including a wealth of information on the safety profile of AL. However, AL has not been compared with any other active treatment in a clinical trial,38 which prompted the design of the ALPHA trial. Only recently have other studies been initiated; these include a commercial trial, currently recruiting, comparing the topical Jak inhibitor delgocitinib with AL (NCT05259722) and a trial focused on vesicular HE only, which compares AL with ciclosporin A. 88 A trial comparing AL with azathioprine was discontinued due to high drop-out rates. 89
ALPHA is the largest randomised trial to compare AL with another active treatment, specifically Immersion PUVA. The trial demonstrated that both treatments as first-line therapies lead to a reduction in disease severity in patients with severe CHE over a period of 52 weeks. AL was shown to have superior clinical effectiveness in terms of disease severity, as measured by the HECSI, in the short term (3 months) compared with Immersion PUVA in the ITT population. However, there was no evidence of a difference between AL and Immersion PUVA in disease severity (HECSI) at 12 months, indicating that the initial superiority of AL as a first-line therapy was not maintained in the longer term, with similar improved outcomes observed in both groups. The latter conclusion is also consistent with other outcome measures of disease severity, including the PGA and mTLSS, and although it is acknowledged that the trial was underpowered for detecting differences in these two outcomes, the estimates of treatment effect support the conclusion of no difference in the long term.
It should be noted that treatment compliance was much lower in the Immersion PUVA arm compared with AL and hence there is uncertainty as to whether participants randomised to Immersion PUVA, in this group as a whole, received an adequate dose of treatment. However, the situation represents clinical reality in the UK. Moreover, the protocol allowed for treatment according to standard care in case of relapse or failure to respond, resulting in ‘mixed’ treatment delivery in both arms during the follow-up period. Overall, 212 (48.1%) participants were confirmed to receive second-line therapies, which may in part explain the lack of difference observed between treatment groups in the longer term; however, it should be noted that the data reported on second-line therapies received is limited.
Despite there being no evidence of a difference in clinical effectiveness over the longer term, AL was shown to have superior cost-effectiveness compared with Immersion PUVA over a time period of 12 months. These results are robust, as AL has a probability of cost-effectiveness of 96%. The 10-year cost-effectiveness analysis, however, shows an advantage to Immersion PUVA. The estimates indicate that Immersion PUVA is slightly cheaper and slightly more effective. These results are, however, uncertain as the probability of cost-effectiveness is equal for both interventions. The results suggest that AL may be considered as the recommended treatment choice when prescribed as a first-line therapy.
In terms of QoL, as measured by the DLQI, there was a statistically significant difference observed in favour of AL at 12 weeks and then of Immersion PUVA at 52 weeks. However, the treatment effects reported are small, and the upper limit of the CI lies below previously reported Minimally Clinically Important Differences for the DLQI of 3–5. 90 Therefore, the treatment effects reported are considered not to be clinically meaningful; however, both groups were observed to have a reduction in DLQI compared with their baseline assessment. There was no evidence of differences between treatment groups in the Patient Benefit Index (PB-HEI) and the PeDeSI; however, the data for these two outcomes are limited due to high levels of missing data.
Sample size and primary outcome measure
Choosing the best-suited outcome measure is of high importance for a clinical trial. Within ALPHA, we have used several outcome measures. For the assessment of HE severity, the HECSI, mTLSS and PGA were used. The reason for this was that none of the outcome measures were the gold standard (see discussion below) and that we wanted to be in a position to compare our data with other HE trial results when different outcome measures have been used.
The HECSI was chosen as the primary outcome measure because it incorporated more details about the condition compared with PGA, which had been used in previous trials. 52,91–94 Furthermore, the HECSI is continuous rather than discrete or binary, which meant that a smaller sample size could be achieved for a desired level of power. There were few published data on the target treatment effect for the HECSI, but due to the lognormal distribution of the outcome, it was reasonable to base the sample size on a relative difference, or fold change. This was discussed at length with the clinical co-applicants, including demonstrating what different fold change values equated to in terms of absolute differences in the HECSI score. This led to an agreed target fold change of 1.3 (or 0.77). At the trial design stage, there was great uncertainty in the assumptions for the sample size calculation, which was based on the HECSI. The trial therefore included a pre-planned sample size re-estimation. This was conducted earlier than planned due to slower than anticipated recruitment, but the final analysis showed that the estimates of the CV obtained at the sample size re-estimation were consistent with the final trial results. Furthermore, the trial was originally powered at 80%, and the pre-planned sample size re-estimation provided confidence in the underlying sample size assumptions to ensure that the revised target would provide sufficient power.
Participants responding to treatment
Previous trials have reported on the efficacy of AL. Depending on the patient selection criteria, around half of the patients treated with AL 30 mg/day achieved a PGA of clear/almost clear after 12 or 24 weeks, respectively. In an open-label study by Dirschka et al. , the responder rate was 46.6%. 93 This study enrolled HE patients with PGA severe, and 91% of that cohort (n = 249) showed a hyperkeratotic HE morphology. Thaci et al. found in a cohort (n = 631) with moderate to severe HE at baseline (62% PGA severe; 34.1% PGA moderate), of which 61.2% had a hyperkeratotic morphology, that 31.7% and 29.8% of all patients receiving at least one dose of AL had a PGA status of clear/almost clear at week 12 and 24, respectively. 95 The initial AL study by Ruzicka et al. recruited 1032 participants with severe CHE, of whom more than 80% had hyperkeratotic eczema morphology: at the end of the treatment phase (maximum 24 weeks), 47.7% of patients in the 30 mg dose group and 27.5% in the 10 mg dose group achieved clear/almost clear compared with 16.6% in the placebo group. 52 The ALPHA trial was balanced regarding predominant eczema morphology (hyperkeratotic rhagadiform vs. vesicular) as well as atopy status. Participants for the ALPHA trial were eligible if they had a severe PGA according to the treating clinician at the time of randomisation. By the end of the treatment phase in ALPHA, 34.1% of AL participants were confirmed to have achieved a clear or almost clear response, but this lower rate compared with previous trials may be due to the differences in the patient population recruited.
Regarding Immersion PUVA, Brass et al. reported a clear/almost clear response rate to Immersion PUVA of 43% after 12 weeks in 23 participants. 91 We are not aware of other larger trials on Immersion PUVA in severe HE that used the PGA as outcome measure. In the ALPHA trial, the proportion of participants with available data who had achieved clear or almost clear according to the blinded assessor was equal to 23.6% (35/148) for the Immersion PUVA group. This difference in proportion may be due to the small sample size reported in Brass et al. 91
Ethnicity/skin of colour
Information on HE in skin of colour is sparse. Indeed, we failed to identify any publication or trial that specifically mentions skin of colour. However, it has been highlighted in several reports that eczema and other inflammatory skin diseases may be underestimated in severity due to difficulties in scoring erythema intensity in skin of colour. 96,97 The ALPHA trial included 11.6% of participants with different ethnic backgrounds, and the proportion observed in those randomised was similar to the proportion of the participants who were screened for participation. At the start of the trial, the issue of assessment challenges for skin of colour was raised by the PPI representative on the TSC. Following discussion with the clinical members of both the TMG and the TSC, a decision was made to incorporate skin colour as a minimisation factor, and to take photographs at baseline and week 12 for all consenting participants with skin of colour. There was already a pre-planned substudy to take photographs of 30% of consenting participants, and this remained for participants with white skin. Each photograph was assessed by three blinded assessors, and there was no evidence in our trial of a disadvantaged assessment regarding to people with darker skin colour. However, there does not exist a ‘gold standard’ regarding assessment of erythema, which is a morphological feature in most composite scores for inflammatory skin diseases, in this patient population. Furthermore, the photographic guide for HE was used to guide the central review; however, it was developed on photographs of white patients only. 98 A later study was conducted to determine whether the photographic guide could be used for HE self-assessment; however, the study only included participants with white skin due to the limitations of the photographic guide, stating that further research would be required for patients with darkly pigmented skin. 99 Within ALPHA, consent was also received for any photographs taken to be used in further research, which may include extending a photographic guide for patients of ethnic minority background.
Subtypes of hand eczema and therapy response
As mentioned above, a multitude of HE subtypes have been described, some based on morphology (dishydrotic, hyperkeratotic, rhagadiform, tylotic, nummular, psoriasiform) or assumed aetiology (irritant, atopic, contact allergic). In general, there is currently no strong evidence to support a relationship between morphology of HE and response to therapy. However, some important information is well known and accepted.
Firstly, the only viable solution to treat contact dermatitis to a relevant contact allergen is allergen avoidance. None of the treatments available will overcome allergen-triggered contact allergic inflammatory activity in the long term. Of note, allergic contact dermatitis is a common feature of occupational HE. In the ALPHA trial, non-avoidance of relevant contact allergens was an exclusion criterion. However, there have been challenges across UK centres regarding testing for allergic contact dermatitis (patch test), and this will be discussed below.
Secondly, AL has been described as showing higher response rates in hyperkeratotic rhagadiform as compared with dishydrotic or vesicular/pompholyx phenotypes. 52 However, that does not imply that the latter group fails to respond to AL. Studies are under way to investigate specifically the response to AL as compared with ciclosporin A in vesicular types of HE. 88
In ALPHA, the proportions of participants with hyperkeratotic versus vesicular HE types were balanced across both treatment groups. In the exploratory analysis, there was no evidence of a differential treatment effect by morphology on disease severity [see Forest plot (see Figure 10)]. However, an analysis exploring the relationship between morphology and outcome was not conducted because it was outside the trial aims and objectives. There is also the expert opinion that AL may work better on lesions in the palmar than on the dorsal side of the hands. Although dorsal/palmar lesion data were collected, the relationship between hand side and treatment group on treatment response was not a pre-specified analysis and therefore is the subject of future research.
Diagnosis/differential diagnosis
In cases where eczema is restricted to the palmoplantar area and when presenting with hyperkeratotic morphology, it can be difficult to distinguish between eczema and palmoplantar psoriasis. 100,101 Experience in clinical practice and throughout the ALPHA trial reflects that absence of other signs of psoriasis or eczema results in diagnostic uncertainties. In some patients, diagnostic biopsies were necessary to confirm the diagnosis. Other differential diagnoses that came up included palmoplantar keratodermas and Bazex syndrome. Palmoplantar psoriasis, however, causes the largest diagnostic challenge.
Allergic contact dermatitis
It is a generally accepted expert view that every patient with chronic hand and/or foot eczema should have a patch test assessment to exclude contribution of a relevant contact allergen to the clinical symptoms. Thyssen et al. reported full consensus regarding recommendation for a patch test in all patients with HE of more than 3 months duration and who are non-responsive to adequate treatment or if there is clinical suspicion of contact allergy. 14 However, the current situation in UK dermatology is not ideal, and the severe understaffing and waiting lists fail to allow appropriate patch testing for patients in need within a reasonable time frame. There is, therefore, underdiagnosis of contact allergies. It is mentioned above that avoidance therapy is an absolute necessity in the face of relevant contact sensitisations. However, the ALPHA trial aimed to mimic clinical practice and thereby followed a pragmatic approach, resulting in the following pathway: in the case of severe CHE without a strong patient history indicating a contact allergic trigger, treatment was started even without available patch test results. Although far from ideal, it was considered unethical to leave patients untreated who suffer from severe HE, impairing functioning in daily life and causing significant pain and itch. This is in line with routine clinical practice in the UK. This approach implies that a minority of the participants included in the trial may have suffered from a non-identified, relevant contact allergy. In addition, it is important to highlight that even with patch test clinics in place, these may not be supervised by experienced allergologists in many centres. In-depth knowledge in the area of contact allergy is necessary to be aware of potential allergens to screen for and to include relevant chemicals regarding co- and cross-sensitisations. As such, even in patients in whom patch test had been performed, appropriate batteries/panels may not have been tested due to lack of specialists and training in contact allergy in some centres in the UK. Furthermore, in-depth knowledge about cross-reacting chemicals and where relevant, contact allergens that may be expected to be encountered is necessary for optimal medical avoidance strategy advice.
Factors influencing hand eczema severity and response to treatment: lifestyle factors, atopy status and genetic background
Disease duration
Disease duration has been shown in epidemiology studies to be a factor influencing therapy response. 102 In general, it has been shown that long disease duration is associated with a less favourable therapy response. In the ALPHA trial, exploratory subgroup analysis was conducted to explore whether there was a differential treatment effect within different lengths of disease duration, but there was no evidence of a differential treatment effect.
Smoking status
As outlined in the introduction chapter, many previous epidemiology studies have discussed smoking as a trigger factor for HE and/or a factor leading to poorer therapeutic response. One of the known facts regarding smoking is that the blood circulation in acral body parts, including fingers, is impaired. In the ALPHA trial, participants were asked to state their current and past smoking habits; the majority (78.5%) were non-smokers or past smokers at the time of randomisation. The exploratory subgroup analysis showed there was no evidence of a differential treatment effect by smoking status.
Atopy
Atopy, the likelihood to react to innocuous environmental molecules in an allergic way, represents a clear risk factor for developing eczema, including HE. Many atopic individuals also show a LOF mutation in the filaggrin gene (see below). It is international expert consensus that in order to show that an individual is ‘atopic’, sensitisations to specific allergens need to be shown. 103 The following are appropriate tests to prove specific sensitisations: the RAST test performed in serum, showing specific IgE antibodies to allergens, and the prick test, an in vivo test that is positive if a wheal reaction is seen to a specific allergen as compared with the positive (histamine) and negative controls. Unfortunately, allergy training is limited among dermatologists in the UK, as assessment of immediate type hypersensitivities is largely performed by clinical immunology. Therefore, it is not current practice to screen for atopy. If this is conducted, doctors check the total IgE; however, this is not sufficient to show atopy. Information on specific IgE was collected in the ALPHA trial and used as a randomisation factor.
Current literature suggests that atopic HE may be difficult to treat and may be less responsive to AL than non-atopic HE. Conversely, not every HE in an atopic individual (e.g. showing symptoms of allergic rhinitis and/or allergic asthma) represents a variant of AD. The exploratory subgroup analysis showed there was no evidence of a differential treatment effect (comparing AL with Immersion PUVA) for atopy status.
Filaggrin/genetics
As highlighted in the introduction chapter, LOF mutations in the filaggrin gene are quite common, particularly among atopic individuals. Filaggrin is an important barrier protein, and its breakdown products are also referred to as natural moisturising factor. Reduced filaggrin expression is the cause of dry skin, increased transepidermal water loss and increased likelihood of environmental molecules entering deeper layers of the skin – thus a reduced barrier function of the skin. LOF mutations also present with an increased risk of acquiring contact allergies.
In the ALPHA trial, LOF mutations were investigated through exploratory subgroup analyses to explore whether there was a differential treatment effect by mutation status, but there was no evidence of a differential treatment effect.
Treatment compliance and missing data
The majority of trial participants received at least one dose of treatment, with 212 (96.4%) participants randomised to AL and 196 (88.7%) participants randomised to Immersion PUVA. There was differential treatment compliance observed, with 65.9% of the participants allocated to AL confirmed to be within the trial definition of a complier (at least 80% received and no treatment breaks more than 7 days during the first 12 weeks) within the first 12 weeks, compared with 24.0% of those allocated to Immersion PUVA. This is likely due to the differences in treatment modality, with Immersion PUVA requiring twice-weekly hospital visits, compared with AL, which is a tablet to be taken once daily. This is discussed further below. As a result of the imbalance in treatment compliance, an analysis on the pre-defined PP population would have led to biased estimates of the treatment effect; a decision endorsed by both the DMEC and TSC was made not to conduct an analysis on the PP population. Furthermore, there were differential levels of missing data, with higher dropout in the Immersion PUVA group. The DMEC and TSC both recommended that the primary analysis model should be a repeated measures model to utilise data collected at earlier time points as well as the 12-week data. The treatment effects reported are on the ITT population and are comparing first-line therapies, with ongoing treatment receipt used as an auxiliary variable for multiple imputation of missing data. There was a statistically significant difference in the primary end point at 12 weeks, but there is no evidence of a difference at 24 or 52 weeks. This conclusion is likely to be confounded by second-line therapies received, and causal inference-based analyses may be a useful area for secondary analysis of the trial data to further understand the effect of first-line therapies in compliers, and to understand the treatment pathway and disease response in this chronic condition.
Immersion 8-methoxypsoralen combined with ultraviolet A as comparator treatment arm
8-Methoxypsoralen combined with ultraviolet A is a well-recognised treatment for HE. Our survey performed among UK dermatologists supported PUVA being among the first-line therapies for severe chronic HE in the UK. 42 Debate is ongoing about the best modality to deliver PUVA, with the following options being available: systemic PUVA requiring oral administration of a photosensitising agent, Immersion (also referred to as bath or soak) PUVA requiring soaking of hands in 8-methoxypsoralen prior to Ultraviolet A (UVA) exposure, and cream and gel PUVA, where the photosensitising agent is applied prior to UVA exposure. The most widely used approach in UK centres and Europe is topical PUVA, mostly using PUVA soaks. There are no published trials comparing the different PUVA modalities for HE, but only smaller case series. 104 For palmoplantar psoriasis, a case series showed that both bath and systemic PUVA showed a potential benefit at the 4-week time point, with systemic PUVA showing rapid and possible increased efficacy at earlier time points but also more systemic side effects. 105 It is well documented that systemic administration of oral methoxypsoralen (8-MOP), a deoxyribonucleic acid (DNA) intercalating substance, comes with potential side effects and contraindications. A proportion of patients wish to avoid oral medication and are worried about side effects, and it is thus important to also offer patients the choice of Immersion PUVA. Numerous studies have shown the efficacy of topical PUVA in HE conditions. It has been suggested that atopic HE may respond better than hyperkeratotic HE variants. 106,107
Although the decision to use Immersion PUVA as the comparator was supported by the survey results, it was recognised that there may be challenges with implementation in the trial. Firstly, Immersion PUVA delivery schedule is limited in many UK dermatology centres due to staff shortage. While it was common practice in the past to offer slots to patients on Saturdays and evening times, treatment slots outside general working hours are no longer offered by most phototherapy units. This situation had significant consequences for recruitment, as patients with strict work-hour commitments (e.g. teachers) were unable to participate. Indeed, a large proportion of patients approached mentioned inability to attend Immersion PUVA treatment due to work, travel difficulties or other problems as the main reason for not participating in the trial. The Immersion PUVA treatment arm was also more affected by the COVID pandemic than the AL treatment arm.
The difficulty of accessing Immersion PUVA treatment is of concern. The trial shows that Immersion PUVA and AL led to a reduction in disease severity, and in the longer term there was no evidence of a difference in clinical effectiveness compared with AL as a first-line therapy. This means that Immersion PUVA could be considered as a potential first-line treatment choice for longer-term outcomes, particularly in patients where AL is not considered an appropriate treatment. However, compliance with Immersion PUVA was low, and so there is a need to improve accessibility to treatment, such as through home delivery solutions, which have been explored for ultraviolet B (UVB) treatment in a recent NIHR vitiligo trial. 108
Treatment feasibility – pregnancy prevention programme for alitretinoin
Alitretinoin is a retinoid and thus belongs to a group of drugs that are teratogenic. Pregnancy prevention programme (PPP) rules must be followed. The need for two independent contraceptive measures is of concern for some patients. The need for one monthly face-to-face appointment with pregnancy testing can be a challenge in some understaffed dermatology units. The COVID pandemic adopted some remote solutions, where pregnancy tests were sent to patients, who had to report back the results.
Pregnancies and ALPHA
In the context of the ALPHA trial, which prohibited any pregnancy while receiving trial treatments as intervention as well as during follow-up, four pregnancies were reported. Only two pregnancies occurred under retinoid therapy, one under AL and another one under Isotretinoin, which was given as standard-of-care medication during the follow-up period for acneiform lesions unrelated to the HE. Interestingly, none of the pregnancies occurred during the COVID pandemic/lockdown, during which it was difficult to perform in-attendance pregnancy tests.
With regard to PPP, the ALPHA trial followed standard of care. Lessons learned from the occurrences are the following: (1) patients who are assumed to be knowledgeable regarding pregnancy prevention (e.g. healthcare professionals) should be given the same attention and degree of information as all other patients; (2) exceptions to the PPP measures (e.g. BAD leaflet for Isotretinoin with exceptions) should be handled with utmost care. We have sufficient evidence that not being in a current relationship does not preclude ‘pregnancies’.
Assessment tools for hand eczema
Currently, there is no gold standard for HE assessment tools. This has prompted some researchers to use the initial photographic assessment guide, which has been the basis for the development of assessment tools, including PGA. 88,98 However, the photographic guide has significant shortcomings, including the absence of a ‘mild’ category due to lack of expert consensus on this item. HECSI has been used more recently in research, as it most accurately captures extent and morphology items. However, it requires some training and dermatology lesion knowledge. The items oedema and infiltration are difficult to distinguish. HECSI also does not allow separate assessment of each hand; the areas assessed include, for example, palm/dorsum or fingers of both hands. 51 The PGA is much faster to perform but very unclear in its criteria, and it only focuses on the most severely affected side of the most severely affected hand. mTLSS was also included in ALPHA to allow comparison with previous trials, including the Ruzicka et al. study. 52
We identified shortcomings in the QoL assessment, in particular the DLQI. This widely used score is derived as the sum of scores provided for 10 questions. Current NICE guidelines (TA177) require a score of 15 for eligibility for AL treatment. This cut-off is considered to be an unusually high threshold, given that any DLQI above 11 is considered as ‘very large effect on patient’s life’. 57 However, in ALPHA, only 43.3% of our participants with severe CHE had a DLQI score of 15 or above. Moreover, clinicians’ experience throughout the trial was that elderly patients, patients with pre-existing disabilities that restricted mobility, patients not working and patients who were perceived as ‘coping’ very well with their disease were ‘disadvantaged’. Marron et al. reported a median DLQI of 7 in a cohort of HE patients who showed increased anxiety and depression parameters. 9 Agner et al. reported in a cohort of 416 patients a significant correlation between HE severity as assessed by HECSI and DLQI. 109 A median score of 8 was considered to be in agreement with a reported 7.8 in severe occupational HE. 6 This further underlines that a DLQI of 15 or higher seems an unrealistic high threshold for a guideline not supported by clinical reality.
None of the current assessment tools considers fingernail changes. There is no specific tool [such as nail psoriasis severity index (NAPSI) for psoriasis] to score nail involvement in HE. However, HE affecting the nail fold is well recognised to result in visible nail changes, which can impair function and/or be distressing for patients upon social exposure. We here included nail assessment in a single participating centre as a ‘pilot’ assessment: results of this will be published elsewhere.
Photography
The agreement of the photographic assessments with the blinded PGA was consistent between treatment groups and ethnicity groups, but there was generally a low level of agreement (43.7% overall). Further research may be beneficial to further understand the reliability of the photographic guide and how it could be used in trial research. 98,99
Moreover, the extent of misclassification between the photoguide and blinded PGA further supports the use of HECSI, as it measures extent and morphology.
Time in remission and time to relapse
Results on time in remission/time to relapse should be treated with caution and considered only as exploratory. The analysis was based on a small number of participants who had achieved remission, and therefore, the estimated treatment effects are subject to bias.
Lessons learned: recruitment
From our work with patient representatives, we know that many people with severe HE self-manage at home. Based on the experience on the ALPHA trial, reaching patients who were not under the care of a GP or dermatologist posed many challenges on how best to ‘advertise’ the study outside health-care settings. Numerous avenues were explored (online/social media, newspaper, radio, TV, poster campaigns, magazines), and professions associated with HE were approached (hairdressing, building, garden/landscaping, health care/occupational health). We identified that communications/advertising and media required specialist expertise and a significant amount of time to manage, and this represents a specialty/role in its own right distinct from trial management. In future studies with a similar patient population, we would consider employing a member of staff dedicated to this role within the trial.
We know that many patients remain under their GP without referral, and recruitment/patient identification from primary care was incorporated; however, challenges existed as (1) there is no commonly used ‘read code’ on GP systems specifically for HE, using a search term of eczema would produce extensive lists with many not eligible for the study (eczema not on the hands); (2) opportunistic screening during standard care appointments relied upon every GP at a single practice being trained on the study; (3) we could only approach GP practices in areas associated with a secondary care recruiting centre. Many GP practices supported the study by displaying posters with information on the study self-referral website; however, this may have been for a limited duration only rather than the full duration of the recruitment period. Engagement with primary care networks and a structured approach to primary care recruitment, including payment for screening activities, is key, and we note that this has become much more established since ALPHA. Early liaison with clinical research networks and allocation of dedicated resource are required, and this understanding will be taken forward into future studies.
Access to treatment slots for Immersion PUVA treatment was a limiting factor at most participating centres, with a restriction on the number of Immersion PUVA ‘slots’ for trial participants alongside standard care treatment appointments. Where only one or two slots were available for trial participants, once these were in use, the participating centre was unable to randomise another participant until the course of treatment had been completed for the existing participant. This meant that our estimate of one to two per centre per month was not achievable at the majority of centres. Through ALPHA we have developed an excellent understanding of the pathway for patients with severe HE, which can be taken into future studies, and understanding the key questions to ask of participating centres when developing estimated recruitment rates.
Equipoise between the two treatment options proved difficult, as administration of the two treatments required an extremely different level of commitment from participants, and as discussed previously, this prevented a number of people from taking part, as they were unable to commit to the Immersion PUVA schedule. However, we must also acknowledge that a number of participants did not want to take AL (or any other oral medications) due to potential side effects or the requirement to complete a pregnancy prevention programme, and it is important to provide options suitable for all patients. Consideration of alternative ways to deliver Immersion PUVA would be of value in any future trials.
Patient and public involvement
Patients were involved in the design of the trial during development of the grant application, and throughout the trial via membership on the TMG and the TSC.
Feedback from our TMG patient representative and PPI group held in September 2019 detailed that patients often self-manage at home with over-the-counter creams for many months prior to seeking a GP appointment, and this is ‘the last resort’ as they have ‘tried everything else but nothing is working’. There is frustration at not being taken seriously, and despite meeting NICE criteria for referral to a secondary care dermatology centre, many receive repeated prescriptions for steroid creams prior to a referral being made, resulting in delays of months to years from first GP visit to seeing a dermatologist. At this point, patients describe feeling ‘desperate’, with their severe HE having had a significant impact on their daily lives, QoL and well-being for an extended period of time. Importantly, delay in medical attention to HE is recognised to be associated with a poorer prognosis. 102
When discussing the findings of ALPHA, our patient representative felt strongly that ‘getting early relief is really important’ as it had been such a long time managing at home, and the study results showing benefit of AL at 12 weeks would provide this ‘quick fix’, which would be so important.
Easy self-management is a key consideration, and generally a preference, particularly for those who have work or child-care commitments; however, it must be acknowledged that some patients are unable to, or choose not to, take AL, and having alternative treatment options available is important.
Equality, diversity and inclusion
We have previously discussed the considerations and steps taken in ALPHA regarding assessment of different skin types and the photography guide. In addition, ALPHA was able to maximise research engagement of underserved populations (i.e. low socioeconomic, minority ethnic and rural/urban) by opening a large number of centres across the UK representing the devolved nations, large urban city centres and rural communities, including large acute Trusts/Teaching Hospitals and smaller District General Hospitals (DGHs), and incorporating recruitment pathways/strategies that allowed the trial to be accessible to all people, including those not currently under the care of a healthcare professional.
We worked with our patient representatives to develop patient information materials in different accessible formats such as video, website, posters, summary sheets and content for social media platforms (Twitter, Instagram). ALPHA was the first Leeds CTRU trial to use Instagram as a social media platform after it was flagged that this was important to ensure trial information reached people with wider-ranging demographics (such as age). A significant amount of time was dedicated to developing the self-referral pathway via a dedicated website and e-mail account to ensure that it was simple and user friendly, and this has been used as an exemplar moving forward for other large multicentre studies.
Outlook
ALPHA delivers important evidence to feed into standard-of-care situations. It was a pragmatic real-life study with direct relevance to UK dermatology care. The main recommendations for which we provide evidence at this point include the following:
-
Patients with severe chronic HE have a considerable benefit from treatment in specialised secondary care. Referrals should be encouraged.
-
AL works faster and is more effective at week 12 when comparing Immersion PUVA with AL.
-
AL is more cost-effective than Immersion PUVA.
-
Immersion PUVA leads to a reduction in symptoms for chronic severe HE and has the potential to be cost-effective after 10 years post treatment. However, access to Immersion PUVA treatment is limited in the UK. There is a strong clinical need to improve access to this treatment for a larger group of patients.
-
Current guideline for treatment based on a DLQI of 15 or more is too high and may prevent treatment access for patients with severe CHE.
-
Assessment of HE severity requires assessor training.
-
HECSI may be a useful primary outcome measure for HE trials, and the results from the ALPHA trial will be useful to inform future CHE trial designs.
Further analysis of the ALPHA data set
Exploratory analyses would be useful to understand the effect of covariates on disease trajectory, including palmar/dorsal site of hands, foot involvement, smoking history, atopy status, LOF filaggrin mutation, etc. Furthermore, a more detailed analysis of the way DLQI can be answered by different participant subpopulations (working age vs. retired) will be considered. We will also analyse pilot data on nail involvement and its response to therapy. Statistical analysis approaches to understand treatment pathways after first-line therapy will be explored.
Open questions to be addressed in future trials
-
Which treatment works best for which subgroup of HE, and which parameters allow us to distinguish between responders and non-responders?
-
How can assessment of severity be improved?
-
Which educational measures work best for primary and secondary prevention of HE?
-
How can treatment compliance be improved, and explored through statistical analysis?
-
Is there a benefit of combining Immersion PUVA and AL (‘Re-PUVA’) or combining AL with novel therapies such as dupilumab or topical Jak inhibitors (e.g. delgocitinib)?
Additional information
Acknowledgements
Trial Steering Committee members
Chair
Professor Pieter-Jan Coenraads, Professor of Occupation and Environmental Dermatology and Dermato-allergy, Hanzeplein 1, 9713 GZ Groningen, the Netherlands.
External/independent members
Dr Matthew Ridd, Consultant Senior Lecturer in Primary Healthcare, University of Bristol.
Mrs Amanda Roberts, Patient Representative.
Mrs Gill Worthy, Statistician.
2014–2020 Dr Ira Madan, Consultant Occupational Physician, Guy’s and St. Thomas’ NHS Foundation Trust.
Data Monitoring and Ethics Committee members
Chair
Professor Alan Montgomery, Professor of Medical Statistics and Clinical Trials, Nottingham Clinical Trials Unit.
External/independent members
Dr Miriam Santer, Clinical Lecturer and GP, Primary Care and Population Sciences, University of Southampton.
Dr Jonathan Batchelor, Consultant Dermatologist.
Research team members at participating sites
We are grateful to all the clinical research team members from the NHS Trusts who have participated in this research. Without the commitment and support of the Principal Investigators (PIs), Dermatology colleagues, clinical Research Nurses (RNs), Registered Healthcare Professionals (RHCPs) and support staff in gaining local permissions, recruiting patients and collecting data, this trial would not have been possible.
Aneurin Bevan University Health Board
Dr Nabil Ponnambath, Consultant Dermatologist.
Kym Wyness, Research Nurse.
Barts Health NHS Trust
Dr Portia Goldsmith, Consultant Dermatologist.
Cardiff and Vale University Health Board
Dr Mahbub M. U. Chowdhury, Consultant Dermatologist.
Beverly Gambles, Research Nurse.
Chapel Allerton Hospital (Leeds Teaching Hospitals NHS Trust)
Eileen O’Grady, Study Nurse.
Julie Bush, Research Sister.
Karen Hughes, Research Nurse.
Harrogate and District NHS Foundation Trust
Alison Layton, Principal Investigator.
Helen Lyon, Pharmacist.
Samantha Jackson, Pharmacy Technician.
Ipswich Hospital (East Suffolk and North Essex NHS Foundation Trust)
Dr Liberta Labimoti, Consultant Dermatologist.
Sue Hood, Nurse.
James Paget University Hospitals NHS Foundation Trust
Dr Ingrid Asalvary, Consultant Dermatologist.
Mid Yorkshire Hospitals NHS Trust
Dr Rebecca Rose, Consultant Dermatologist.
Julie Ball, Lead Administrator.
Kate Lindley, Research Nurse.
NHS Lothian
Dr Richard Weller, Reader and Honorary Consultant Dermatologist.
Catherine Beveridge, Senior Community Research Nurse.
NHS Fife
Dr Allan Matthews, Consultant Dermatologist.
Anna Gill, Senior Research Nurse.
Linda Lister, Research Nurse.
Maria Simpson, Senior Pharmacy Technician, Clinical Trials.
NHS Tayside
John Forester, Subinvestigator.
Caroline Tee, Senior Clinical Research Nurse.
Shona Murray, Research Nurse.
NHS Greater Glasgow and Clyde
Dr Donna Torley, Consultant Dermatologist.
Stephanie McEnhill, Senior Research Nurse.
Norfolk and Norwich University Hospitals NHS Foundation Trust
Professor Nick Levell, Consultant Dermatologist.
Dr Anne-Marie Skellett, Consultant Dermatologist.
Ava S.W. Lee, Speciality Doctor in Dermatology.
Azaharry Yaakub, Speciality Doctor in Dermatology.
Catherine Haughton, Dermatology Research Nurse.
David Tomlinson, Research Nurse.
Iman Abulela, Clinical Fellow.
Priya Patel, Dermatology Registrar.
Dr Pura Gurung, Locum Consultant.
Rona Applewaite, Clinical Fellow.
Northampton General Hospital NHS Trust
Lorraine Campey, Research Nurse.
Lucy Dudgeon, Research Nurse.
Nottingham University Hospitals NHS Trust
Dr John English, Consultant Dermatologist.
Royal Cornwall Hospitals NHS Trust
Dr Preshita Divekar, Consultant Dermatologist.
Zoe Berry, Research Nurse.
Royal Devon and Exeter NHS Trust
Rob James, Senior Research Nurse.
Royal Hallamshire Hospital (Sheffield Teaching Hospitals NHS Foundation Trust)
Dr Sarah Cockayne, Consultant Dermatologist.
Royal Liverpool and Broadgreen University Hospitals NHS Foundation Trust
Dr Mili Shah, Consultant Dermatologist.
Dr Richard Azurbia, Consultant Dermatologist.
Sarah Aldwinckle, Research Nurse.
Tessa Garland, Research Nurse.
Salford Royal Hospital (Northern Care Alliance)
Caroline White, Research Nurse.
Gill Aarons, Research Nurse.
Jean Bastrilles, Senior Research Nurse.
Marie Durkin, Research Nurse.
St Luke’s Hospital (Bradford Teaching Hospitals NHS Foundation Trust)
Carol Denniss, Research Sister.
Jenny Ott, Research Nurse.
St Helens and Knowsley Teaching Hospitals NHS Trust
Nicola Collins, Research Nurse.
Surrey and Sussex Healthcare NHS Trust
Dr Irene Man, Consultant Dermatologist.
Louise Nimako, Dermatology Research Nurse.
The Newcastle upon Tyne Hospitals NHS Foundation Trust
Sarah Wilkinson, Senior Research Nurse.
The James Cook University Hospital
Dr Philippa Cousen, Consultant Dermatologist.
Jackie Dodds, Research Nurse.
The Dudley Group NHS Foundation Trust
Dr Evmorfia Ladoyanni, Consultant Dermatologist.
Tracy Henn, Research Nurse.
Wara Mudunge, Lead Research Nurse.
University Hospitals Bristol NHS Foundation Trust
Dr Debbie Shipley, Consultant Dermatologist.
Lucy-Belle Guthrie, Research Coordinator.
Victoria Taylor, Research Nurse.
University Hospitals of Leicester NHS Trust
Dr Graham Johnston, Consultant Dermatologist.
Dr Elizabeth Roberts, Co-Investigator.
Adam Lewszuk, Senior Research Nurse.
Aidan Dunphy, Senior Research Nurse.
Anna Shah, Dermatology Registrar.
Denny Vail, Senior Research Assistant.
Rhiannon Cotter, Clinical Research Assistant.
Sally Batham, Research Space Clinical Manager.
Sophie Devine, Research Nurse.
York and Scarborough Teaching Hospitals NHS Foundation Trust
Dr Calum Lyon, Dermatologist.
Emma Temlett, Research Nurse.
We would also like to sincerely thank Dr Catherine Fernandez (Head of Trial Management 2014–20), Dr Moninder Bhogal (Senior Trial Manager 2014–16), Catherine Reynolds (Senior Data Manager 2014–20), Dr Victoria Goss (Senior Trial Coordinator 2016–18) and Jennifer Langridge (Trial Management Assistant 2014–18).
The study was developed with support from the UK Dermatology Clinical Trials Network (UK DCTN). The UK DCTN is grateful to the British Association of Dermatologists and the University of Nottingham for financial support of the Network.
Contributions of authors
Miriam Wittmann (https://orcid.org/0000-0003-2328-4926) (Associate Professor in Inflammatory Skin Diseases, Chief Investigator) contributed to the design, protocol development, protocol implementation and interpretation of data; was involved in the implementation of protocol and coordination of local approvals at Leeds Teaching Hospitals NHS Trust and Bradford Teaching Hospitals NHS Foundation Trust; contributed to drafting or revising for intellectual content and approval of monograph final version.
Isabelle L Smith (https://orcid.org/0000-0002-8326-1075) (Principal Statistician) contributed to the design, protocol development, protocol implementation, statistical design, statistical analysis of the clinical results and interpretation of data; contributed to drafting or revising for intellectual content and approval of the monograph final version.
Sarah Tess Brown (https://orcid.org/0000-0002-1840-3786) (Principal Statistician) was a co-applicant and contributed to the design, protocol development, protocol implementation, statistical design, and oversight and interpretation of data; contributed to drafting or revising for intellectual content and approval of the monograph final version.
Anna Berekméri (https://orcid.org/0000-0001-5894-8812) (ALPHA Clinical Coordinator) led design of the tape stripping substudy, including protocol development, data analysis and interpretation; developed and delivered the skin assessment scoring tool training package; blinded expert photography review panel; contributed to drafting and revising for intellectual content and approval of the monograph final version.
Armando Vargas-Palacios (https://orcid.org/0000-0002-6503-0134) (Research Fellow in Health Economics) contributed to protocol implementation, health economic analysis plan, data analysis and data interpretation; contributed to drafting or revising for intellectual content and approval of the monograph final version.
Lesley Sunderland (https://orcid.org/0000-0002-5807-530X) (General Practitioner) was a co-applicant and contributed to the design, protocol development, protocol implementation and interpretation of data; contributed to revising for intellectual content and approval of the monograph final version.
Amy Barker (https://orcid.org/0000-0003-1626-0934) (Patient Representative) was a co-applicant and contributed to the design, protocol development, protocol implementation and interpretation of data; contributed to drafting and approval of the monograph final version.
Fiona Cowdell (https://orcid.org/0000-0002-9355-8059) (Professor of Nursing and Health Research) was a co-applicant and contributed to the design, protocol development, protocol implementation and interpretation of data; contributed to revising for intellectual content and approval of the monograph final version.
Steven Ersser (https://orcid.org/0000-0001-6995-6121) (Head and Professor of Nursing and Dermatology Care) was a co-applicant and contributed to the design, protocol development, protocol implementation and interpretation of data; contributed to revising for intellectual content and approval of the monograph final version.
Rachael Gilberts (https://orcid.org/0000-0003-3326-7900) (Head of Trial Management) contributed to protocol implementation and interpretation of data; contributed to revising for intellectual content and approval of the monograph final version.
Cathy Green (https://orcid.org/0000-0003-0920-036X) (Consultant Dermatologist) was a co-applicant and contributed to the design, protocol development, protocol implementation and interpretation of data; carried out protocol implementation and coordination of local approvals and data acquisition for Ninewells Hospital, Dundee; contributed to revising for intellectual content and approval of the monograph final version.
Philip Hampton (https://orcid.org/0000-0001-6797-2697) (Consultant Dermatologist) was a co-applicant and contributed to the design, protocol development, protocol implementation and interpretation of data; carried out protocol implementation and coordination of local approvals and data acquisition for The Newcastle upon Tyne Hospitals NHS Foundation Trust; contributed to revising for intellectual content and approval of the monograph final version.
Catherine Smith (https://orcid.org/0000-0001-9918-1144) (Professor of Dermatology and Therapeutics) was a co-applicant and contributed to the design, protocol development, protocol implementation and interpretation of data; carried out protocol implementation and coordination of local approvals and data acquisition for Guy’s and St Thomas’ NHS Foundation Trust; contributed to revising for intellectual content and approval of the monograph final version.
Jane Nixon (https://orcid.org/0000-0003-1705-7698) (Professor of Tissue Viability and Clinical Trials Research) contributed to the design, protocol development, protocol implementation and interpretation of data; contributed to drafting or revising for intellectual content and approval of the monograph final version.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/TWQC0141.
Primary conflicts of interest: Miriam Wittmann reports grants from Pfizer, UCB, British Skin Foundation, Psoriasis UK Foundation and Sanofi. Miriam Wittmann has received payment for lectures from Sanofi, Leo, Astra Zeneca, UCB, Euforea Masterclass, Novartis and Janssen. Miriam Wittmann reports membership of advisory boards for UCB, Leo, Biogen and Astra Zeneca. Miriam Wittmann reports board or committee membership for Journal of Investigative Dermatology, British Skin Foundation, and British Society of Investigative Dermatology. Sarah Brown reports grants from NIHR (NIHR151863, NIHR131475, NIHR201612). Anna Berekméri reports grants from Leeds Teaching Hospitals NHS Trust. Anna Berekméri reports travel support from Health Professional Scholarship, Health Care Provider Scholarship, and ESDR Travel Grant. Lesley Sunderland reports payment paid to employers for attending TMG meetings. Rachael Gilberts reports grants from NIHR (NIHR151863). Catherine Smith reports grants from the European Commission. Catherine Smith reports payments for speaking at various conferences. Jane Nixon reports membership of the HTA Commissioning Committee 2018–24.
No competing interests are declared by Isabelle L Smith, Sarah Tess Brown, Armando Vargas-Palacios, Amy Barker, Fiona Cowdell, Steven Ersser, Cathy Green and Philip Hampton.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Ethics statement
The trial was conducted in accordance with the World Medical Association Declaration of Helsinki (www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/) and was reviewed and approved by the Yorkshire and Humber Research Ethics Committee (REC reference: 14/YH/1259 on 9 January 2015), the Health Research Authority (HRA) and Medicine and Healthcare products Regulatory Agency (MHRA).
Information governance statement
All personal information was handled in line with the Data Protection Act (2018) and General Data Protection Regulation (EU GDPR) 2016/679. Under Data Protection legislation, the University of Leeds is the Data Processor and Data Controller, and personal data are processed in accordance with their instructions. You can find out more about how personal data are handled here: https://ctru.leeds.ac.uk/ctru-comprehensive-privacy-guide/ and the contact details for the University of Leeds Data Protection Officer below:
-
e-mail: DPO@leeds.ac.uk
-
Telephone number: +44 (0)113 343 7641
-
Postal address for data protection issues: University of Leeds, Room 11.72 EC Stoner Building, Leeds, LS2 9JT
Publications
The following associated publications to this report are listed below:
Smith IL, Brown S, Nixon J, Cowdell FC, Ersser S, Fernandez C, et al. Treatment of severe, chronic hand eczema: results from a UK-wide survey. Clin Exp Dermatol 2016. https://doi.org/10.1111/ced.13015
Berekméri A, Latzko A, Alase A, Macleod T, Ainscough JS, Laws P, et al. Detection of IL-36γ through noninvasive tape stripping reliably discriminates psoriasis from atopic eczema. J Allergy Clin Immunol 142(3):988–991.e4.
Smith IL, Gilberts R, Brown S, Fernandez C, Nixon J, Reynolds C, et al. Comparison of ALitretinoin with PUVA as the first-line treatment in patients with severe chronic HAnd eczema (ALPHA): study protocol for a randomised controlled trial. BMJ Open 2022;0:e060029. https://doi.org/10.1136/bmjopen-2021-060029
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Quaade AS, Simonsen AB, Halling AS, Thyssen JP, Johansen JD. Prevalence, incidence, and severity of hand eczema in the general population – a systematic review and meta-analysis. Contact Derm 2021;84:361-74.
- Diepgen TL, Andersen KE, Chosidow O, Coenraads PJ, Elsner P, English J, et al. Guidelines for diagnosis, prevention and treatment of hand eczema. J Dtsch Dermatol Ges 2015;13:ee1-22.
- Diepgen TL, Agner T, Aberer W, Berth-Jones J, Cambazard F, Elsner P, et al. Management of chronic hand eczema. Contact Derm 2007;57:203-10.
- Malkonen T, Alanko K, Jolanki R, Luukkonen R, Aalto-Korte K, Lauerma A, et al. Long-term follow-up study of occupational hand eczema. Br J Dermatol 2010;163:999-1006.
- Hald M, Agner T, Blands J, Veien NK, Laurberg G, Avnstorp C, et al. Clinical severity and prognosis of hand eczema. Br J Dermatol 2009;160:1229-36.
- Cvetkovski RS, Zachariae R, Jensen H, Olsen J, Johansen JD, Agner T. Quality of life and depression in a population of occupational hand eczema patients. Contact Derm 2006;54:106-11.
- Fowler JF, Ghosh A, Sung J, Emani S, Chang J, Den E, et al. Impact of chronic hand dermatitis on quality of life, work productivity, activity impairment, and medical costs. J Am Acad Dermatol 2006;54:448-57.
- Moberg C, Alderling M, Meding B. Hand eczema and quality of life: a population-based study. Br J Dermatol 2009;161:397-403.
- Marron SE, Tomas-Aragones L, Navarro-Lopez J, Gieler U, Kupfer J, Dalgard FJ, et al. The psychosocial burden of hand eczema: data from a European dermatological multicentre study. Contact Derm 2018;78:406-12.
- Meding B, Wrangsjo K, Jarvholm B. Fifteen-year follow-up of hand eczema: persistence and consequences. Br J Dermatol 2005;152:975-80.
- Agner T, Elsner P. Hand eczema: epidemiology, prognosis and prevention. J Eur Acad Dermatol Venereol 2020;34:4-12.
- Brans R, Hubner A, Gediga G, John SM. Prevalence of foot eczema and associated occupational and non-occupational factors in patients with hand eczema. Contact Derm 2015;73:100-7.
- Politiek K, Oosterhaven JA, Vermeulen KM, Schuttelaar ML. Systematic review of cost-of-illness studies in hand eczema. Contact Derm 2016;75:67-76.
- Thyssen JP, Schuttelaar MLA, Alfonso JH, Andersen KE, Angelova-Fischer I, Arents BWM, et al. Guidelines for diagnosis, prevention, and treatment of hand eczema. Contact Derm 2022;86:357-78.
- Armstrong A, Hahn-Pedersen J, Bartlett C, Glanville J, Thyssen JP. Economic burden of chronic hand eczema: a review. Am J Clin Dermatol 2022;23:287-300.
- Diepgen TL, Andersen KE, Brandao FM, Bruze M, Bruynzeel DP, Frosch P, et al. European Environmental and Contact Dermatitis Research Group . Hand eczema classification: a cross-sectional, multicentre study of the aetiology and morphology of hand eczema. Br J Dermatol 2009;160:353-8.
- English J, Aldridge R, Gawkrodger DJ, Kownacki S, Statham B, White JM, et al. Consensus statement on the management of chronic hand eczema. Clin Exp Dermatol 2009;34:761-9.
- Giwercman C, Lerbaek A, Bisgaard H, Menne T. Classification of atopic hand eczema and the filaggrin mutations. Contact Derm 2008;59:257-60.
- Meding B, Wrangsjo K, Jarvholm B. Hand eczema extent and morphology – association and influence on long-term prognosis. J Invest Dermatol 2007;127:2147-51.
- Rich P, Scher RK. Nail psoriasis severity index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol 2003;49:206-12.
- Meding B. Epidemiology of hand eczema in an industrial city. Acta Derm Venereol Suppl (Stockh) 1990;153:1-43.
- Montnemery P, Nihlen U, Lofdahl CG, Nyberg P, Svensson A. Prevalence of hand eczema in an adult Swedish population and the relationship to risk occupation and smoking. Acta Derm Venereol 2005;85:429-32.
- Thyssen JP, Johansen JD, Linneberg A, Menne T. The epidemiology of hand eczema in the general population – prevalence and main findings. Contact Derm 2010;62:75-87.
- Bryld LE, Hindsberger C, Kyvik KO, Agner T, Menne T. Risk factors influencing the development of hand eczema in a population-based twin sample. Br J Dermatol 2003;149:1214-20.
- Johannisson A, Ponten A, Svensson A. Prevalence, incidence and predictive factors for hand eczema in young adults – a follow-up study. BMC Dermatol 2013;13.
- Kezic S, Novak N, Jakasa I, Jungersted JM, Simon M, Brandner JM, et al. Skin barrier in atopic dermatitis. Front Biosci (Landmark Ed) 2014;19:542-56.
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006;38:441-6.
- Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol 2006;118:214-9.
- Sandilands A, Terron-Kwiatkowski A, Hull PR, O’Regan GM, Clayton TH, Watson RM, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet 2007;39:650-4.
- Molin S, Vollmer S, Weiss EH, Weisenseel P, Ruzicka T, Prinz JC. Deletion of the late cornified envelope genes LCE3B and LCE3C may promote chronic hand eczema with allergic contact dermatitis. J Investig Allergol Clin Immunol 2011;21:472-9.
- Loman L, Politiek K, Schuttelaar MLA. Smoking and obesity are associated with chronic hand eczema and severity of hand eczema: data from the Dutch general population. Contact Derm 2022;87:103-6.
- Molin S, Ruzicka T, Herzinger T. Smoking is associated with combined allergic and irritant hand eczema, contact allergies and hyperhidrosis. J Eur Acad Dermatol Venereol 2015;29:2483-6.
- Lai YC, Yew YW. Smoking and hand dermatitis in the United States adult population. Ann Dermatol 2016;28:164-71.
- Patruno C, Ayala F, Zagaria O, Balato N. Is cigarette smoking dangerous for chronic hand eczema in housewives?. Dermatitis 2014;25:201-4.
- Koskelo M, Sinikumpu SP, Jokelainen J, Huilaja L. Risk factors of hand eczema: a population-based study among 900 subjects. Contact Derm 2022;87:485-91.
- Bo K, Thoresen M, Dalgard F. Smokers report more psoriasis, but not atopic dermatitis or hand eczema: results from a Norwegian population survey among adults. Dermatology 2008;216:40-5.
- Agrawal PV, Kumar A, Sharma YK, Deora M, Ranpariya RH. Comparative analysis of epidemiological data as well as quality of life in patients having hand eczema vis-a-vis foot eczema. Indian Dermatol Online J 2019;10:519-23.
- Christoffers WA, Coenraads PJ, Svensson A, Diepgen TL, Dickinson-Blok JL, Xia J, et al. Interventions for hand eczema. Cochrane Database Syst Rev 2019;4.
- Coenraads PJ. Hand eczema. N Engl J Med 2012;367:1829-37.
- Van Coevorden AM, Coenraads PJ, Svensson A, Bavinck JN, Diepgen TL, Naldi L, et al. European Dermato-Epidemiology Network (Eden) . Overview of studies of treatments for hand eczema: the EDEN hand eczema survey. Br J Dermatol 2004;151:446-51.
- Paulden M, Rodgers M, Griffin S, Slack R, Duffy S, Ingram JR, et al. Alitretinoin for the treatment of severe chronic hand eczema. Health Technol Assess 2010;14:39-46.
- Smith IL, Brown S, Nixon J, Cowdell FC, Ersser S, Fernandez C, et al. Treatment of severe, chronic hand eczema: results from a UK-wide survey. Clin Exp Dermatol 2017;42:185-8.
- Stingeni L, Bianchi L, Antonelli E, Caroppo ES, Ferrucci SM, Ortoncelli M, et al. DADA – Dupilumab for Atopic Dermatitis of the Adolescence Study Group . Moderate-to-severe atopic dermatitis in adolescents treated with dupilumab: a multicentre Italian real-world experience. J Eur Acad Dermatol Venereol 2022;36:1292-9.
- Voorberg AN, Romeijn GLE, de Bruin-Weller MS, Schuttelaar MLA. The long-term effect of dupilumab on chronic hand eczema in patients with moderate to severe atopic dermatitis-52 week results from the Dutch BioDay Registry. Contact Derm 2022;87:185-91.
- Worm M, Thyssen JP, Schliemann S, Bauer A, Shi VY, Ehst B, et al. The pan-JAK inhibitor delgocitinib in a cream formulation demonstrates dose response in chronic hand eczema in a 16-week randomized phase IIb trial. Br J Dermatol 2022;187:42-51.
- Agner T, Jungersted JM, Coenraads PJ, Diepgen T. Comparison of four methods for assessment of severity of hand eczema. Contact Derm 2013;69:107-11.
- Carlsson A, Svensson A, Anderson CD, Baranovskaya I, Hindsen-Stenstrom M, Holt I, et al. Scoring of hand eczema: good reliability of hand eczema extent score (HEES). Acta Derm Venereol 2017;97:193-7.
- Yuksel YT, Norreslet LB, Olesen CM, Agner T. Assessment of hand eczema severity: what’s new?. Contact Derm 2022;87:556-7.
- Oosterhaven JAF, Schuttelaar MLA. Responsiveness and interpretability of the hand eczema severity index. Br J Dermatol 2020;182:932-9.
- Smith IL, Gilberts R, Brown S, Fernandez C, Nixon J, Reynolds C, et al. Comparison of ALitretinoin with PUVA as the first-line treatment in patients with severe chronic HAnd eczema (ALPHA): study protocol for a randomised controlled trial. BMJ Open 2022;12.
- Held E, Skoet R, Johansen JD, Agner T. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. A study of inter- and intraobserver reliability. Br J Dermatol 2005;152:302-7.
- Ruzicka T, Lynde CW, Jemec GB, Diepgen T, Berth-Jones J, Coenraads PJ, et al. Efficacy and safety of oral alitretinoin (9-cis retinoic acid) in patients with severe chronic hand eczema refractory to topical corticosteroids: results of a randomized, double-blind, placebo-controlled, multicentre trial. Br J Dermatol 2008;158:808-17.
- Cowdell F, Ersser SJ, Gradwell C, Thomas PW. The person-centered dermatology self-care index: a tool to measure education and support needs of patients with long-term skin conditions. Arch Dermatol 2012;148:1251-5. https://doi.org/10.1001/archdermatol.2012.1892.
- Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The dermatology life quality index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008;159:997-1035.
- Finlay AY, Basra MKA, Piguet V, Salek MS. Dermatology life quality index (DLQI): a paradigm shift to patient-centered outcomes. J Invest Dermatol 2012;132:2464-5.
- Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean?. J Invest Dermatol 2005;125:659-64.
- Augustin M, Radtke MA, Zschocke I, Blome C, Behechtnejad J, Schafer I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res 2009;301:561-71.
- Blome C, Maares J, Diepgen T, Jeffrustenbach S, Augustin M. Measurement of patient-relevant benefits in the treatment of chronic hand eczema – a novel approach. Contact Derm 2009;61:39-45.
- Zschocke I, Hammelmann U, Augustin M. Therapeutic benefits in dermatological therapy. Evaluation of therapy from the physician’s and patient’s perspective in psoriasis and atopic dermatitis. Der Hautarzt; Zeitschrift Fur Dermatologie, Venerologie, Und Verwandte Gebiete 2005;56:839844-42836.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72.
- EMA . Guideline on Missing Data in Confirmatory Clinical Trials 2010. www.ema.europa.eu/en/documents/scientific-guideline/guideline-missing-data-confirmatory-clinical-trials_en.pdf (accessed 19 December 2022).
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99.
- Carpenter JR, Roger JH, Kenward MG. Analysis of longitudinal trials with protocol deviation: a framework for relevant, accessible assumptions, and inference via multiple imputation. J Biopharm Stat 2013;23:1352-71.
- Guizzaro L, Pétavy F, Ristl R, Gallo C. The use of a variable representing compliance improves accuracy of estimation of the effect of treatment allocation regardless of discontinuation in trials with incomplete follow-up. Stat Biopharm Res 2021;13:119-27.
- Sullivan TR, White IR, Salter AB, Ryan P, Lee KJ. Should multiple imputation be the method of choice for handling missing data in randomized trials?. Stat Meth Med Res 2018;27:2610-26.
- Ballarini NM, Chiu YD, König F, Posch M, Jaki T. A critical review of graphics for subgroup analyses in clinical trials. Pharm Stat 2020;19:541-60.
- NICE . Introduction to Health Technology Evaluation: NICE Health Technology Evaluations: The Manual: Guidance n.d. www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation (accessed 19 December 2022).
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. New York, USA: Oxford University Press; 2014.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108.
- Ali FM, Kay R, Finlay AY, Piguet V, Kupfer J, Dalgard F, et al. Mapping of the DLQI scores to EQ-5D utility values using ordinal logistic regression. Qual Life Res 2017;26:3025-34.
- Blank PR, Blank AA, Szucs TD. Cost-effectiveness of oral alitretinoin in patients with severe chronic hand eczema: a long-term analysis from a Swiss perspective. BMC Dermatol 2010;10.
- Jones KC, Burns A. Unit Costs of Health and Social Care 2021. Canterbury: PSSRU, University of Kent; 2021.
- NICE . British National Formulary (BNF) 2022. https://bnf.nice.org.uk/ (accessed 19 December 2022).
- Prescription Cost Analysis England.
- Office for National Statistics, CPI INDEX 06: HEALTH 2015=100 n.d. www.ons.gov.uk/economy/inflationandpriceindices/timeseries/d7bz/mm23 (accessed 19 December 2022).
- NHS Choices n.d. www.england.nhs.uk/costing-in-the-nhs/national-cost-collection/ (accessed 19 December 2022).
- NHS England . National Tariffs 2022 23.
- NHS England . National Schedule of NHS Costs.
- NHS England . Drugs and Pharmaceutical Electronic Market Information Tool (eMIT) 2022.
- UK Government . Earnings and Hours Worked, Place of Residence by Local Authority: ASHE Table 8 2022. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/placeofresidencebylocalauthorityashetable8 (accessed 19 December 2022).
- UK Research and Innovation . MRC Studentships 2022. www.ukri.org/what-we-offer/developing-people-and-skills/mrc/mrc-studentships/minimum-amounts-for-studentship-stipends-and-allowances/ (accessed 19 December 2022).
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-Effectiveness analysis alongside clinical trials II: an ISPOR good research practices task force report. Value Health 2015;18:161-72.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96.
- O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455-68.
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401.
- Office of National Statistics . Principal Projection: UK Population in Age Groups 2022.
- Oosterhaven JAF, Schuttelaar MLA. Study protocol: efficacy of oral alitretinoin versus oral cyclosporine A in patients with severe recurrent vesicular hand eczema (ALICsA): a randomised prospective open-label trial with blinded outcome assessment. BMJ Open 2018;8.
- Voorberg AN, Kamphuis E, Christoffers WA, Romeijn GLE, Oosterhaven JAF, Schuttelaar MLA. Efficacy and safety of oral alitretinoin versus oral azathioprine in patients with severe chronic hand eczema: results from a prematurely discontinued randomized controlled trial. Contact Derm 2022;87:366-8.
- Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015;230:27-33.
- Brass D, Fouweather T, Stocken DD, Macdonald C, Wilkinson J, Lloyd J, et al. An observer-blinded randomized controlled pilot trial comparing localized immersion psoralen-ultraviolet A with localized narrowband ultraviolet B for the treatment of palmar hand eczema. Br J Dermatol 2018;179:63-71.
- Tan J, Maari C, Nigen S, Bolduc C, Bissonnette R. Open-label exploratory study of acitretin for the treatment of severe chronic hand dermatitis. J Dermatolog Treat 2015;26:373-5.
- Dirschka T, Reich K, Bissonnette R, Maares J, Brown T, Diepgen TL. An open-label study assessing the safety and efficacy of alitretinoin in patients with severe chronic hand eczema unresponsive to topical corticosteroids. Clin Exp Dermatol 2011;36:149-54.
- Schmitt-Hoffmann AH, Roos B, Sauer J, Spickermann J, Stoeckel K, Edwards D, et al. Pharmacokinetics, efficacy and safety of alitretinoin in moderate or severe chronic hand eczema. Clin Exp Dermatol 2011;36:29-34.
- Thaci D, Augustin M, Westermayer B, Kamps A, Hennig M. Effectiveness of alitretinoin in severe chronic hand eczema: PASSION, a real-world observational study. J Dermatolog Treat 2016;27:577-83.
- Wilson BN, Alexis A, Murase JE. Art of prevention: atopic dermatitis in women and families of color – prevalence, recognition, and prevention. Int J Womens Dermatol 2022;8.
- Zhao CY, Hao EY, Oh DD, Daniel BS, Martin LK, Su JC, et al. A comparison study of clinician-rated atopic dermatitis outcome measures for intermediate- to dark-skinned patients. Br J Dermatol 2017;176:985-92.
- Coenraads PJ, Van Der Walle H, Thestrup-Pedersen K, Ruzicka T, Dreno B, De La Loge C, et al. Construction and validation of a photographic guide for assessing severity of chronic hand dermatitis. Br J Dermatol 2005;152:296-301.
- Hald M, Veien NK, Laurberg G, Johansen JD. Severity of hand eczema assessed by patients and dermatologist using a photographic guide. Br J Dermatol 2007;156:77-80.
- Politiek K, Loman L, Pas HH, Diercks GFH, Lemmink HH, Jan SZ, et al. Hyperkeratotic hand eczema: eczema or not?. Contact Derm 2020;83:196-205.
- Garzorz-Stark N, Eyerich K. Molecular diagnostics of hand eczema. Hautarzt 2019;70:760-5.
- Hald M, Agner T, Blands J, Johansen JD. Danish Contact Dermatitis Group . Delay in medical attention to hand eczema: a follow-up study. Br J Dermatol 2009;161:1294-300.
- Bos JD, Van Leent EJ, Sillevis Smitt JH. The millennium criteria for the diagnosis of atopic dermatitis. Exp Dermatol 1998;7:132-8.
- Grundmann-Kollmann M, Behrens S, Peter RU, Kerscher M. Treatment of severe recalcitrant dermatoses of the palms and soles with PUVA-bath versus PUVA-cream therapy. Photodermatol Photoimmunol Photomed 1999;15:87-9.
- Hofer A, Fink-Puches R, Kerl H, Quehenberger F, Wolf P. Paired comparison of bathwater versus oral delivery of 8-methoxypsoralen in psoralen plus ultraviolet: a therapy for chronic palmoplantar psoriasis. Photodermatol Photoimmunol Photomed 2006;22:1-5.
- Behrens S, von Kobyletzki G, Gruss C, Reuther T, Altmeyer P, Kerscher M. PUVA-bath photochemotherapy (PUVA-soak therapy) of recalcitrant dermatoses of the palms and soles. Photodermatol Photoimmunol Photomed 1999;15:47-51.
- Schempp CM, Muller H, Czech W, Schopf E, Simon JC. Treatment of chronic palmoplantar eczema with local bath-PUVA therapy. J Am Acad Dermatol 1997;36:733-7.
- Thomas KS, Batchelor JM, Akram P, Chalmers JR, Haines RH, Meakin GD, et al. UK Dermatology Clinical Trials Network’s HI-Light Vitiligo Trial Team . Randomized controlled trial of topical corticosteroid and home-based narrowband ultraviolet B for active and limited vitiligo: results of the HI-Light Vitiligo Trial. Br J Dermatol 2021;184:828-39.
- Agner T, Andersen KE, Brandao FM, Bruynzeel DP, Bruze M, Frosch P, et al. , European Environmental and Contact Dermatitis Research Group . Hand eczema severity and quality of life: a cross-sectional, multicentre study of hand eczema patients. Contact Derm 2008;59:43-7.
Appendix 1 Tape stripping method
D-squame adhesive discs of 3.8 cm2 (Cuderm Corporation, Dallas, TX, USA) were used to non-invasively sample the skin. The sampled area was marked following the placement of the first tape by applying four marks with a pen around the outside of the tape (at 12, 3, 6 and 9 o’clock). In order to remove any impurities from the surface area, the first tape applied to the skin was discarded. Ten subsequent tapes were collected from the same location. Tapes were applied with gentle pressure for approx. 5 seconds, removed with a sudden movement and placed in a collection tube. Samples for protein extraction were immediately placed on dry ice following sampling for transport and stored at −80 °C until processing. For samples intended for ribonucleic acid (RNA) isolation, RNAlater (Sigma-Aldrich, Gillingham, Dorset, UK) was used in order to avoid RNA degradation.
Selection of sampled area
Lesional tape samples were taken from the most representative lesion of either the palmar or the dorsal hand, depending on the lesion localisation. In addition, samples were preferentially taken from non-treated sites, or at sites with no topical treatment for a minimum of 2 days. Lesions with oozing or broken skin (erosions, vesicles, bleeding/oozing fissures) were avoided. Sampling was stopped if any bleeding or oozing occurred during the procedure. Non-lesional samples were collected from the forearm of patients.
Sample processing and protein extraction
Samples for protein quantification by ELISA-based techniques were transferred tape by tape into an Eppendorf tube with 1.5 ml lysis buffer containing 20 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X100, 5 mM EDTA and 1X protease inhibitor cocktail (Roche, Welwyn Garden City, UK). Following transfer, samples were labelled and incubated on ice for 30 minutes. Subsequently, samples were sonicated three times for 20 seconds, with 20-second intervals of cooling on ice between sonication steps in order to avoid overheating of sample and protein denaturation. Finally, samples were centrifuged at 15,000g (maximum rpm) for 10 minutes, and the supernatant was carefully collected. Samples obtained were stored at −80 °C.
The total soluble protein content of each tape stripping sample was determined using bicinchoninic acid assay (BCA – microplate procedure, Life Technologies Ltd, Paisley, UK).
Protein quantification
IL36γ ELISA
A custom-made sandwich ELISA was used for the quantification of IL36γ in samples following general ELISA protocols. 96-Well immunosorbent ELISA plates (Nunc Life Technologies, Paisley, UK) were coated with 100 μl/well B5A2 monoclonal capture antibody in phosphate-buffered saline (PBS) at a concentration of 2 µg/ml. The IL36γ capture antibody B5A2 was generated and provided by Dr Tom Macleod from the University of Leeds. The plate was incubated at 4 °C overnight. Plates were then washed four times with 0.1% Tween-20/PBS and blocked for 1 hour with 2% bovine serum albumin (BSA) in 0.1% Tween-20/PBS. Following repeated washing, samples were added to the plate and subsequently incubated for 2 hours at room temperature with agitation. At the end of the incubation period, samples were discarded, followed by repeated washing. Biotinylated monoclonal IL36γ detection antibody HCL17 was added to each well at a concentration of 250 ng/ml and incubated for 1 hour. After subsequent washing steps, streptavidin-horseradish peroxidase (HRP) was added to the wells, and the plate was incubated for 20 minutes. Plates were then washed and tetramethylbenzidine (TMB) was used as chromogenic substrate for HRP detection. Plates were incubated in the dark until colour development before the addition of an equal amount of 2 N H2SO4. The absorbance of each well was read at 450 and 550 nm, followed by subtraction of 550-nm values from 450-nm values to correct for optical imperfections in the microplate.
Commercially available ELISAs
Elafin (R&D systems, Abingdon) and CCL27/CTACK (Bio-Techne, Abingdon, UK) proteins from the tape stripping samples were quantified by ELISA according to the manufacturers’ protocols.
Multiplex bead-based quantification assays
Multiplex bead-based quantification immunoassays (LEGENDplex, Biolegend, London, UK) were used to detect and measure cytokines and chemokines in tape stripping samples, following the manufacturer’s recommendations. These assays are based on the principles of sandwich immunoassays, capturing a soluble analyte between two antibodies. Beads are differentiated by size and fluorescence intensities. Each bead set is combined with a specific capture antibody (capture bead), allowing capture of the target analyte from the sample. In this way, multiple proteins of interest can be identified and quantified by a selected panel of capture beads. To the capture bead–analyte complex, biotinylated detection antibodies are added. Finally, by adding streptavidin-phycoerythrin (SA-PE), a fluorescent signal is generated with intensities correlated to the proportion of the amount of bound analyte. The fluorescence signal intensity was acquired by flow cytometry (LSRII, Oxford, UK). The results were analysed using LEGENDplex™ data analysis software (Biolegend), determining the concentration of an analyte based on a given standard curve. Based on the relevant literature and previous preliminary results, a customised kit (Biolegend) containing the following cytokines of interest was used: IL1α, IL1β, IL8, IL18, CCL17 (TARC), CXCL1 (GROα), CXCL10 (IP10), CCL20 (MIP-3α).
Appendix 2 Clinical results supplementary tables and figures
FIGURE 13.
Number of registrations for anonymised centres.
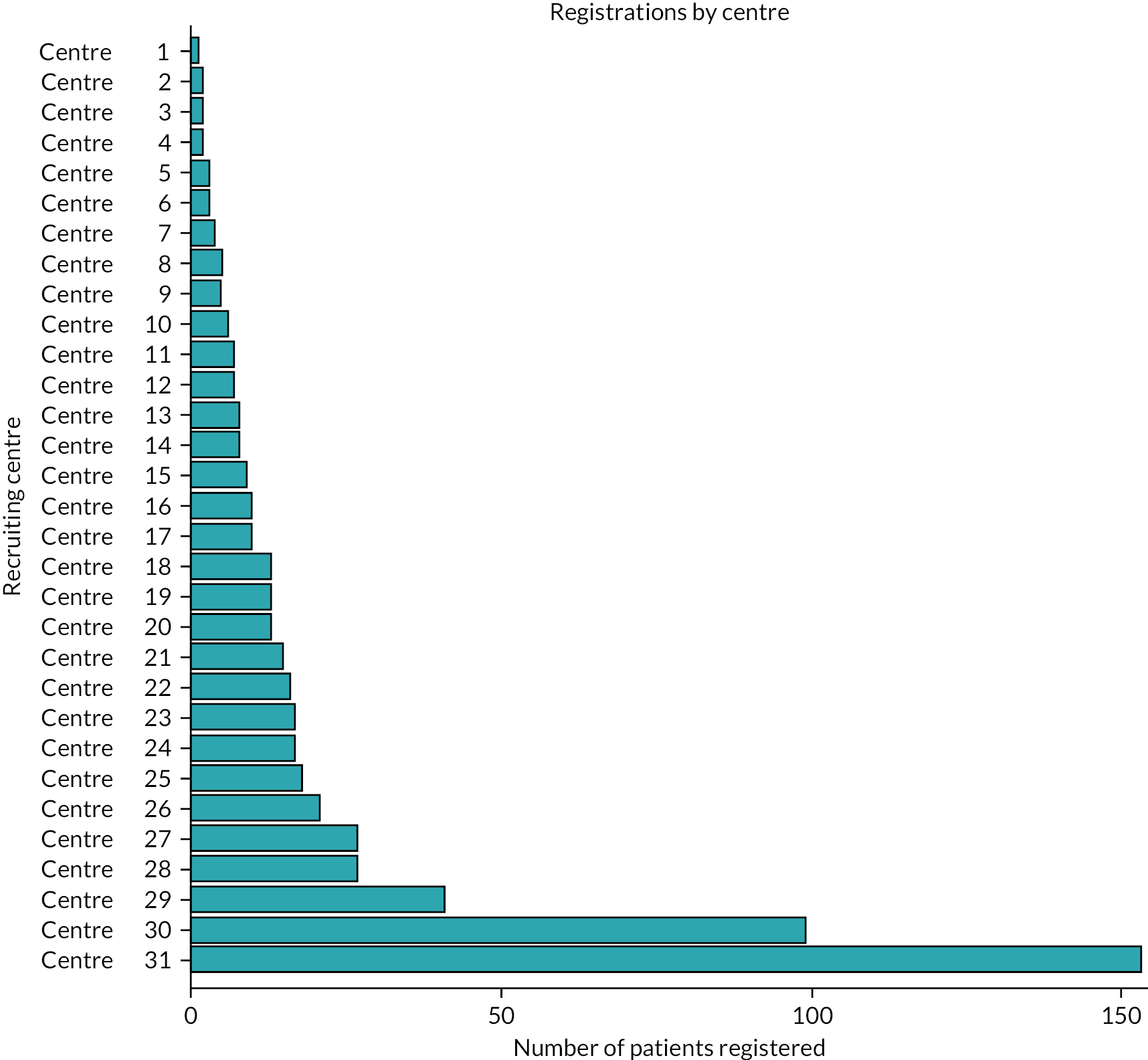
| Effect | Level | Estimate | 95% CI | p-value |
|---|---|---|---|---|
| Intercept | 0.98 | (0.12 to 1.83) | 0.0256 | |
| Log(HECSI + 1) at baseline | 0.56 | (0.45 to 0.67) | < 0.0001 | |
| Duration of disease (ref ≤ 6 months) | 6–24 months | 0.38 | (−0.17 to 0.92) | 0.1740 |
| Duration of disease (ref ≤ 6 months) | > 24 months | 0.46 | (−0.07 to 1.00) | 0.0895 |
| Clinical phenotype (ref = Predominantly vesicular) | Predominantly hyperkeratotic | −0.14 | (−0.32 to 0.05) | 0.1385 |
| Clinical phenotype (ref = Predominantly vesicular) | Fingertip dermatitis | −0.59 | (−0.93 to −0.25) | 0.0008 |
| Skin type (ref = White) | Fair | 0.40 | (−0.30 to 1.09) | 0.2619 |
| Skin type (ref = White) | Dark | −0.06 | (−0.34 to 0.22) | 0.6692 |
| DLQI (ref = < 15) | ≥ 15 | 0.13 | (−0.04 to 0.30) | 0.1302 |
| Presence of atopy (ref = No) | Yes | 0.11 | (−0.07 to 0.28) | 0.2341 |
| Smoking status (ref = Non-smoker) | Past smoker | 0.13 | (−0.05 to 0.31) | 0.1571 |
| Smoking status (ref = Non-smoker) | Current smoker | 0.19 | (−0.02 to 0.40) | 0.0837 |
| BMI | 0.00 | (−0.01 to 0.02) | 0.5772 | |
| Foot involvement (ref = No) | Yes | −0.12 | (−0.31 to 0.08) | 0.2344 |
| Randomised treatment (ref = Immersion PUVA) | AL | −0.22 | (−0.46 to 0.03) | 0.0817 |
| Time from planned start of treatment (weeks) | −0.06 | (−0.08 to −0.03) | < 0.0001 | |
| Time*randomised treatment (ref = Immersion PUVA) | AL | −0.02 | (−0.05 to 0.01) | 0.2525 |
| Effect | Level | Estimate | 95% CI | p-value |
|---|---|---|---|---|
| Intercept | 1.23 | (0.48 to 1.98) | 0.0013 | |
| Log(HECSI + 1) at baseline | 0.41 | (0.31 to 0.52) | 0.0000 | |
| Duration of disease (ref ≤ 6 months) | 6–24 months | 0.40 | (−0.09 to 0.90) | 0.1113 |
| Duration of disease (ref ≤ 6 months) | > 24 months | 0.59 | (0.09 to 1.08) | 0.0195 |
| Clinical phenotype (ref = Predominantly vesicular) | Predominantly hyperkeratotic | −0.18 | (−0.36 to −0.01) | 0.0383 |
| Clinical phenotype (ref = Predominantly vesicular) | Fingertip dermatitis | −0.56 | (−0.88 to −0.24) | 0.0007 |
| Skin type (ref = White) | Fair | 0.10 | (−0.51 to 0.71) | 0.7458 |
| Skin type (ref = White) | Dark | −0.11 | (−0.38 to 0.16) | 0.4199 |
| DLQI (ref = < 15) | ≥ 15 | 0.10 | (−0.05 to 0.25) | 0.2079 |
| Presence of atopy (ref = No) | Yes | 0.07 | (−0.08 to 0.23) | 0.3603 |
| Smoking status (ref = Non-smoker) | Past smoker | 0.13 | (−0.03 to 0.30) | 0.1201 |
| Smoking status (ref = Non-smoker) | Current smoker | 0.23 | (0.03 to 0.43) | 0.0275 |
| BMI | −0.00 | (−0.01 to 0.01) | 0.8991 | |
| Foot involvement (ref = No) | Yes | −0.03 | (−0.21 to 0.14) | 0.7226 |
| Randomised treatment (ref = Immersion PUVA) | AL | −0.36 | (−0.56 to −0.16) | 0.0004 |
| Time from planned start of treatment (weeks) | −0.02 | (−0.03 to −0.02) | < 0.0001 | |
| Time*randomised treatment (ref = Immersion PUVA) | AL | 0.01 | (0.00 to 0.02) | 0.0007 |
| Time since planned start of treatment (weeks) | Randomisation allocation | Adjusted mean log(HECSI + 1) scores (95% CI) | Treatment comparison | Difference in adjusted mean log(HECSI + 1) scores (95% CI) | Adjusted fold change (95% CI) |
|---|---|---|---|---|---|
| 24 | AL | 2.59 (2.32 to 2.86) | Immersion PUVA | −0.08 (−0.24 to 0.08) | 0.92 (0.79 to 1.08) |
| 24 | Immersion PUVA | 2.81 (2.53 to 3.08) | . | ||
| 52 | AL | 2.63 (2.36 to 2.91) | Immersion PUVA | 0.24 (−0.03 to 0.51) | 1.27 (0.97 to 1.67) |
| 52 | Immersion PUVA | 2.90 (2.62 to 3.18) | . |
| Effect | Level | Estimate | 95% CI | p-value |
|---|---|---|---|---|
| Intercept | 3.02 | (0.13 to 5.91) | 0.0407 | |
| mTLSS at baseline | 0.20 | (0.12 to 0.28) | < 0.0001 | |
| Duration of disease (ref ≤ 6 months) | 6–24 months | 1.57 | (−0.42 to 3.55) | 0.1212 |
| Duration of disease (ref ≤ 6 months) | > 24 months | 1.98 | (0.02 to 3.93) | 0.0473 |
| Clinical phenotype (ref = Predominantly vesicular) | Predominantly hyperkeratotic | −0.60 | (−1.34 to 0.15) | 0.1151 |
| Clinical phenotype (ref = Predominantly vesicular) | Fingertip dermatitis | −1.77 | (−3.12 to −0.41) | 0.0106 |
| Skin type (ref = White) | Fair | 0.52 | (−2.08 to 3.12) | 0.6949 |
| Skin type (ref = White) | Dark | −0.35 | (−1.48 to 0.78) | 0.5402 |
| DLQI (ref = < 15) | ≥ 15 | 0.45 | (−0.22 to 1.13) | 0.1874 |
| Presence of atopy (ref = No) | Yes | 0.32 | (−0.34 to 0.99) | 0.3413 |
| Smoking status (ref = Non-smoker) | Past smoker | 0.83 | (0.09 to 1.58) | 0.0283 |
| Smoking status (ref = Non-smoker) | Current smoker | 0.92 | (0.04 to 1.80) | 0.0403 |
| BMI | −0.01 | (−0.07 to 0.05) | 0.8082 | |
| Foot involvement (ref = No) | Yes | −0.20 | (−0.93 to 0.53) | 0.5866 |
| Randomised treatment (ref = Immersion PUVA) | AL | −0.61 | (−1.73 to 0.51) | 0.2853 |
| Time from planned start of treatment (weeks) | −0.05 | (−0.07 to −0.02) | 0.0009 | |
| Time*randomised treatment (ref = Immersion PUVA) | AL | 0.02 | (−0.01 to 0.05) | 0.2134 |
| Parameter | Category | Log odds ratio | 95% CI |
|---|---|---|---|
| Intercept | Clear | −0.69 | (−1.67 to 0.3) |
| Intercept | Almost clear | −1.13 | (−2.12 to −0.13) |
| Intercept | Mild | 0.38 | (−0.6 to 1.36) |
| Intercept | Moderate | 1.97 | (0.99 to 2.96) |
| Disease duration | 6–24 months | −0.56 | (−1.27 to 0.15) |
| Disease duration | > 24 months | −0.67 | (−1.34 to 0.0032) |
| Clinical phenotype | Predominantly hyperkeratotic | 0.21 | (−0.085 to 0.5) |
| Clinical phenotype | Fingertip dermatitis | 0.57 | (0.011 to 1.14) |
| Skin type | Fair | −0.23 | (−1.18 to 0.73) |
| Skin type | Dark | 0.27 | (−0.17 to 0.71) |
| Presence of specific IgE | Yes | −0.031 | (−0.28 to 0.22) |
| DLQI category | ≥ 15 | −0.29 | (−0.55 to −0.038) |
| Smoking status | Past smoker | −0.21 | (−0.48 to 0.069) |
| Smoking status | Current smoker | −0.38 | (−0.72 to −0.035) |
| BMI | −0.0076 | (−0.029 to 0.014) | |
| Foot involvement | Yes | −0.043 | (−0.33 to 0.24) |
| Randomised treatment: AL vs. Immersion PUVA | AL | 0.23 | (−0.18 to 0.63) |
| Time since planned start of treatment (weeks) | 0.023 | (0.012 to 0.035) | |
| Time × allocation (AL vs. Immersion PUVA) | AL | −0.0025 | (−0.017 to 0.012) |
| Effect | Level | Estimate | 95% CI | p-value |
|---|---|---|---|---|
| Intercept | 2.96 | (−0.23 to 6.15) | 0.0689 | |
| DLQI at baseline | 0.25 | (0.20 to 0.31) | < 0.0001 | |
| Duration of disease (ref ≤ 6 months) | 6–24 months | 0.91 | (−1.52 to 3.34) | 0.4648 |
| Duration of disease (ref ≤ 6 months) | > 24 months | 1.79 | (−0.58 to 4.15) | 0.1388 |
| Clinical phenotype (ref = Predominantly vesicular) | Predominantly hyperkeratotic | −0.70 | (−1.53 to 0.13) | 0.0975 |
| Clinical phenotype (ref = Predominantly vesicular) | Fingertip dermatitis | −1.40 | (−2.96 to 0.17) | 0.0798 |
| Skin type (ref = White) | Fair | −0.01 | (−3.03 to 3.02) | 0.9970 |
| Skin type (ref = White) | Dark | 0.71 | (−0.58 to 1.99) | 0.2814 |
| Presence of specific IgE (ref = No) | Yes | 0.30 | (−0.47 to 1.07) | 0.4386 |
| Smoking status (ref = Non-smoker) | Past smoker | 0.34 | (−0.51 to 1.19) | 0.4349 |
| Smoking status (ref = Non-smoker) | Current smoker | 0.53 | (−0.47 to 1.52) | 0.3019 |
| BMI | −0.01 | (−0.07 to 0.06) | 0.8585 | |
| Foot involvement (ref = No) | Yes | 0.77 | (−0.09 to 1.64) | 0.0801 |
| Randomised treatment (ref = Immersion PUVA) | AL | −1.72 | (−2.81 to −0.64) | 0.0020 |
| Time from planned start of treatment (weeks) | −0.09 | (−0.12 to −0.07) | 0.0000 | |
| Time*randomised treatment (ref = Immersion PUVA) | AL | 0.06 | (0.04 to 0.09) | 0.0000 |
| AL (%) | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| Baseline | |||
| Needs intensive education and support to develop knowledge, ability and confidence | 6 (2.7) | 1 (0.5) | 7 (1.6) |
| Needs some education and support to develop knowledge, ability and confidence | 79 (35.9) | 75 (33.9) | 154 (34.9) |
| Needs limited education and support to develop knowledge, ability and confidence | 95 (43.2) | 107 (48.4) | 202 (45.8) |
| Has sufficient knowledge, ability and confidence to manage on their own | 35 (15.9) | 36 (16.3) | 71 (16.1) |
| Missing | 5 (2.3) | 2 (0.9) | 7 (1.6) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| 12 weeks | |||
| Needs intensive education and support to develop knowledge, ability and confidence | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Needs some education and support to develop knowledge, ability and confidence | 21 (9.5) | 15 (6.8) | 36 (8.2) |
| Needs limited education and support to develop knowledge, ability and confidence | 105 (47.7) | 98 (44.3) | 203 (46.0) |
| Has sufficient knowledge, ability and confidence to manage on their own | 49 (22.3) | 36 (16.3) | 85 (19.3) |
| Missing | 45 (20.5) | 72 (32.6) | 117 (26.5) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| 24 weeks (only collected for participants recruited from 1 October 2019) | |||
| Needs intensive education and support to develop knowledge, ability and confidence | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Needs some education and support to develop knowledge, ability and confidence | 1 (3.4) | 0 (0.0) | 1 (1.7) |
| Needs limited education and support to develop knowledge, ability and confidence | 6 (20.7) | 7 (23.3) | 13 (22.0) |
| Has sufficient knowledge, ability and confidence to manage on their own | 4 (13.8) | 1 (3.3) | 5 (8.5) |
| Missing | 18 (62.1) | 22 (73.3) | 40 (67.8) |
| Total | 29 (100) | 30 (100) | 59 (100) |
| 52 weeks (only collected for participants recruited before 1 October 2019) | |||
| Needs intensive education and support to develop knowledge, ability and confidence | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Needs some education and support to develop knowledge, ability and confidence | 11 (5.8) | 8 (4.2) | 19 (5.0) |
| Needs limited education and support to develop knowledge, ability and confidence | 69 (36.1) | 55 (28.8) | 124 (32.5) |
| Has sufficient knowledge, ability and confidence to manage on their own | 40 (20.9) | 39 (20.4) | 79 (20.7) |
| Missing | 71 (37.2) | 89 (46.6) | 160 (41.9) |
| Total | 191 (100) | 191 (100) | 382 (100) |
| Effect | Category | Log odds ratio estimate | Log odds ratio 95% CI |
|---|---|---|---|
| Intercept | Needs some education and support to develop knowledge, ability and confidence | −0.36 | (−3.74 to 3.03) |
| Intercept | Needs limited education and support to develop knowledge, ability and confidence | 3.69 | (0.28 to 7.11) |
| Randomised treatment: AL vs. Immersion PUVA | AL | −0.44 | (−0.95 to 0.078) |
| Baseline PeDeSI category | Has sufficient knowledge, ability and confidence to manage on their own | −5.69 | (−7.70 to −3.69) |
| Baseline PeDeSI category | Needs some education and support to develop knowledge, ability and confidence | −2.52 | (−4.40 to −0.64) |
| Baseline PeDeSI category | Needs limited education and support to develop knowledge, ability and confidence | −3.92 | (−5.83 to −2.02) |
| Disease duration | > 24 months | −0.12 | (−1.75 to 1.51) |
| Disease duration | 6–24 months | 0.17 | (−1.49 to 1.84) |
| Clinical phenotype | Predominantly hyperkeratotic | 0.53 | (−0.38 to 1.45) |
| Clinical phenotype | Predominantly vesicular | 1.12 | (0.13 to 2.12) |
| Skin type | White | 0.42 | (−1.63 to 2.47) |
| Skin type | Dark | 0.2 | (−2.03 to 2.44) |
| Presence of specific IgE | Yes | 0.19 | (−0.33 to 0.72) |
| DLQI category | < 15 | 0.16 | (−0.37 to 0.68) |
| Smoking status | Past smoker | 0.45 | (−0.12 to 1.02) |
| Smoking status | Current smoker | −0.11 | (−0.81 to 0.58) |
| BMI | 0.0026 | (−0.038 to 0.043) | |
| Foot involvement | Yes | 0.38 | (−0.21 to 0.97) |
FIGURE 14.
Kaplan–Meier plots for the time to relapse, relapse defined as achieving 75% of the baseline HECSI score.

FIGURE 15.
Kaplan–Meier plots for the time to relapse, relapse defined as achieving 50% of the baseline HECSI score.

| AL (%) | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| Adverse r eaction/ SAE category | |||
| Other – gastrointestinal | 22 (20.6) | 0 (0.0) | 22 (16.3) |
| PUVA burn | 0 (0.0) | 14 (50.0) | 14 (10.4) |
| Depressive mood changes | 11 (10.3) | 0 (0.0) | 11 (8.1) |
| Pruritus | 7 (6.5) | 4 (14.3) | 11 (8.1) |
| Increased lipids | 10 (9.3) | 0 (0.0) | 10 (7.4) |
| Other | 9 (8.4) | 1 (3.6) | 10 (7.4) |
| Dry eyes | 9 (8.4) | 0 (0.0) | 9 (6.7) |
| Arthralgia/myalgia | 8 (7.5) | 0 (0.0) | 8 (5.9) |
| Exacerbation of eczema outside hands and feet | 5 (4.7) | 0 (0.0) | 5 (3.7) |
| Fatigue | 5 (4.7) | 0 (0.0) | 5 (3.7) |
| Other – skin related | 3 (2.8) | 2 (7.1) | 5 (3.7) |
| Dizziness | 4 (3.7) | 0 (0.0) | 4 (3.0) |
| Increased liver function | 4 (3.7) | 0 (0.0) | 4 (3.0) |
| Exacerbation of eczema (hands and feet) | 2 (1.9) | 1 (3.6) | 3 (2.2) |
| Hair loss | 3 (2.8) | 0 (0.0) | 3 (2.2) |
| PUVA itch | 0 (0.0) | 3 (10.7) | 3 (2.2) |
| PUVA pain | 0 (0.0) | 2 (7.1) | 2 (1.5) |
| Benign intracranial hypertension | 1 (0.9) | 0 (0.0) | 1 (0.7) |
| Dyspepsia | 1 (0.9) | 0 (0.0) | 1 (0.7) |
| Erythema | 0 (0.0) | 1 (3.6) | 1 (0.7) |
| Eye irritation | 1 (0.9) | 0 (0.0) | 1 (0.7) |
| Increased sun sensitivity symptoms | 1 (0.9) | 0 (0.0) | 1 (0.7) |
| Sleep/concentration problems | 1 (0.9) | 0 (0.0) | 1 (0.7) |
| Total | 107 (100) | 28 (100) | 135 (100) |
| PGA | |||||
|---|---|---|---|---|---|
| Clear | Almost clear | Mild | Moderate | Severe | |
| Raw HECSI score | |||||
| Mean (SD) | 2.0 (4.9) | 6.8 (7.8) | 15.1 (12.1) | 38.1 (27.4) | 77.8 (47.6) |
| Median (range) | 0.0 (0–49) | 4.0 (0–63) | 11.0 (0–77) | 31.0 (0–224) | 67.0 (4–324) |
| IQR | 0.0–2.0 | 2.0–8.0 | 6.0–20.0 | 19.0–51.0 | 44.0–102.0 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| N | 203 | 606 | 712 | 1074 | 725 |
| Raw mTLSS score | |||||
| Mean (SD) | 0.4 (0.8) | 2.4 (1.6) | 4.7 (2.2) | 8.5 (3.2) | 12.7 (3.9) |
| Median (range) | 0.0 (0–4) | 2.0 (0–9) | 5.0 (0–13) | 9.0 (0–20) | 13.0 (0–21) |
| IQR | 0.0–0.0 | 1.0–3.0 | 3.0–6.0 | 6.0–11.0 | 10.0–15.0 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| N | 81 | 242 | 273 | 451 | 399 |
| Raw DLQI score | |||||
| Mean (SD) | 1.6 (2.8) | 2.7 (4.0) | 4.5 (4.6) | 7.4 (6.0) | 11.7 (7.0) |
| Median (range) | 1.0 (0–17) | 1.0 (0–28) | 3.0 (0–30) | 6.0 (0–30) | 11.0 (0–30) |
| IQR | 0.0–2.0 | 0.0–4.0 | 1.0–7.0 | 3.0–10.0 | 6.0–16.0 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| N | 201 | 602 | 701 | 1073 | 731 |
FIGURE 16.
Scatterplot of raw HECSI scores by raw mTLSS scores over all time points, estimated correlation (95% CI) = 0.84 (0.83 to 0.86).
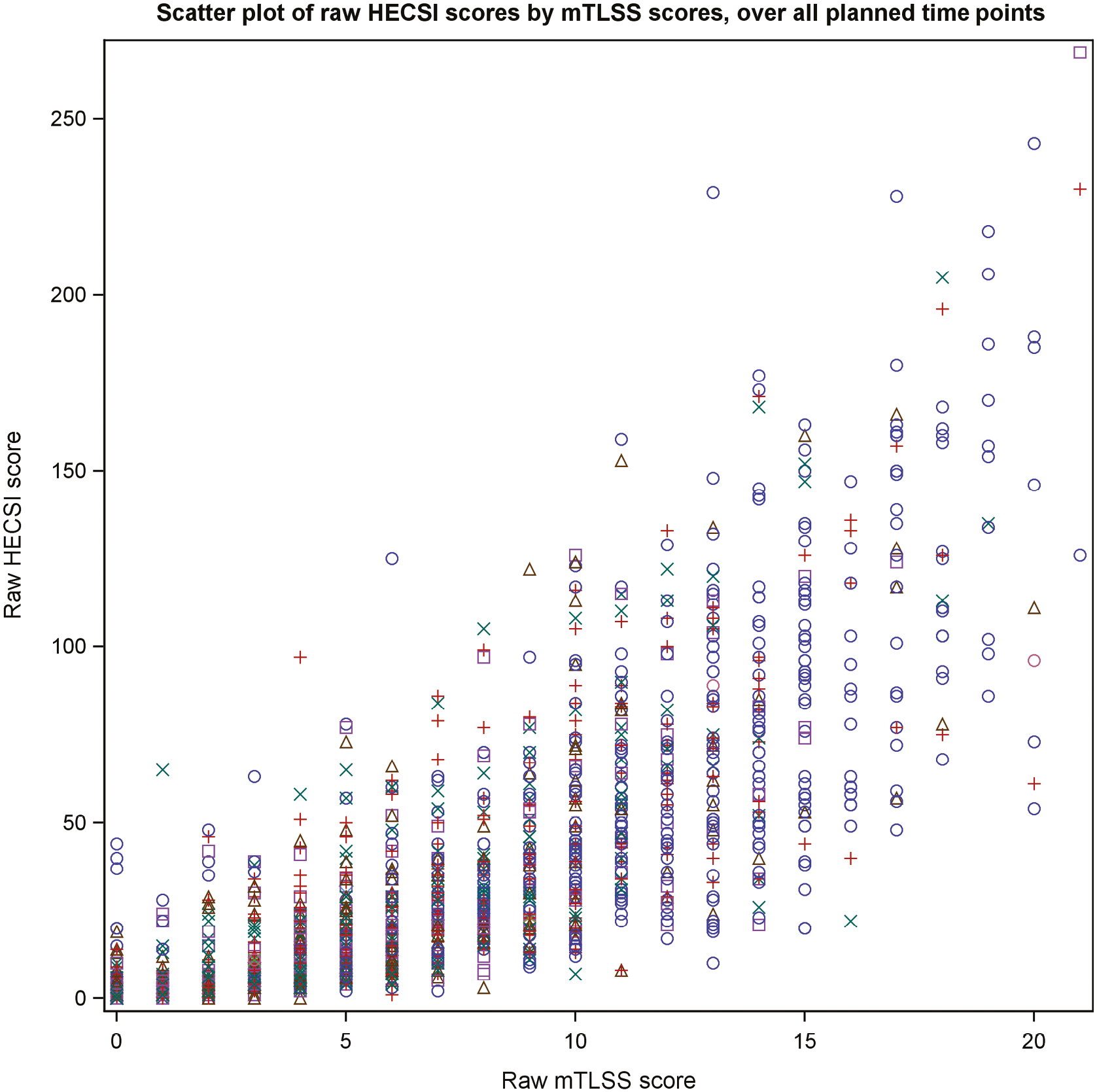
FIGURE 17.
Scatterplot of raw HECSI scores by raw DLQI scores over all time points, estimated correlation (95% CI) = 0.56 (0.53 to 0.58).

FIGURE 18.
Scatterplot of raw mTLSS scores by raw DLQI scores over all time points, estimated correlation (95% CI) = 0.65 (0.62 to 0.68).

Figures 19–26 STEPP plots to assess differential treatment effects at 12 weeks for CCL20, non-lesional samples are STEPP plots of overlapping subgroups defined by biomarker levels. Each subgroup has a sample size of around 20 and is controlled to have about 15 subjects overlapping with the neighbouring subgroups. If the overall estimated treatment effect (represented by the green line) does not lie in the region formed by simultaneous CIs, an interaction may exist. 66
The legend of each subgroup is defined by: Subgroup treatment effect (red); Overall treatment effect (green); Boundaries for 95% CI (blue); Boundaries for 95% SCI (yellow).
FIGURE 19.
Subpopulation treatment effect pattern plot to assess differential treatment effects at 12 weeks for IL36, lesional samples.
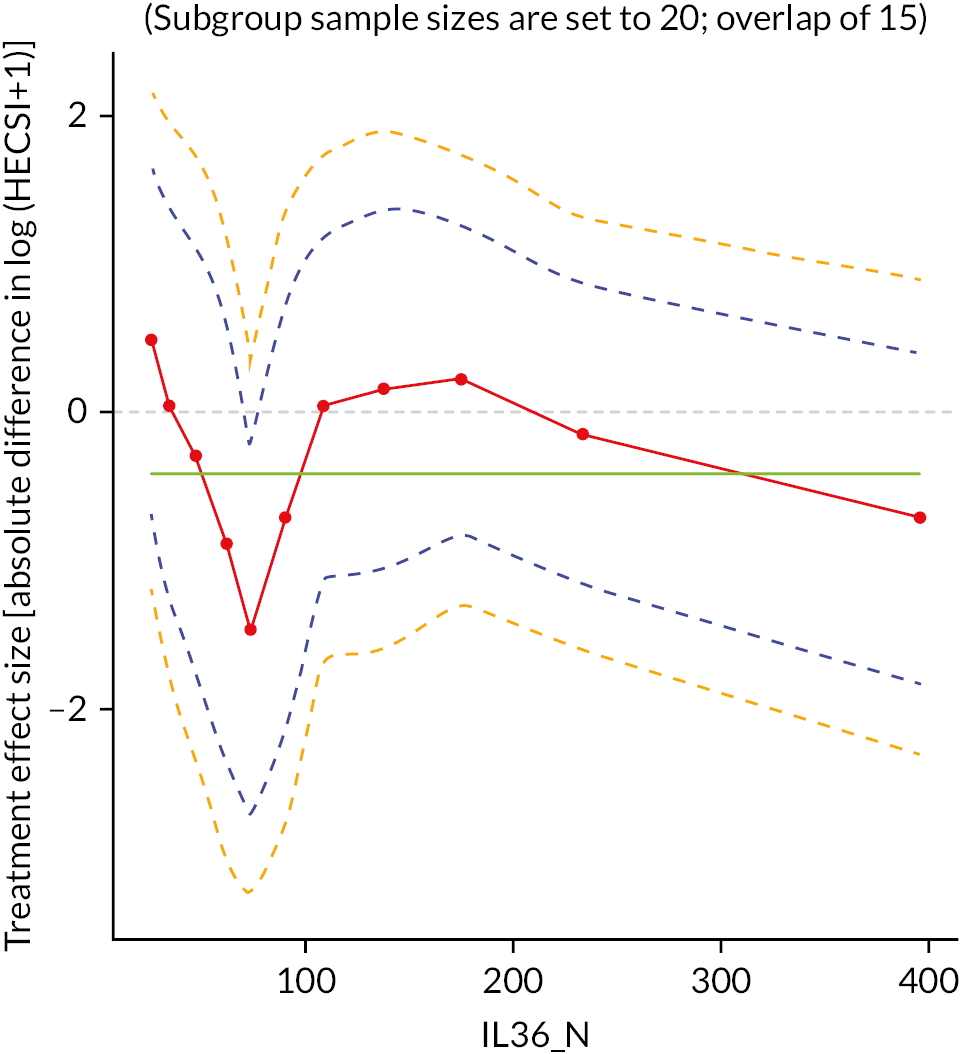
FIGURE 20.
Subpopulation treatment effect pattern plot to assess differential treatment effects at 12 weeks for IL36, non-lesional samples.

FIGURE 21.
Subpopulation treatment effect pattern plot to assess differential treatment effects at 12 weeks for IL18, lesional samples.

FIGURE 22.
Subpopulation treatment effect pattern plot to assess differential treatment effects at 12 weeks for IL18, non-lesional samples.

FIGURE 23.
Subpopulation treatment effect pattern plot to assess differential treatment effects at 12 weeks for TARC, lesional samples.
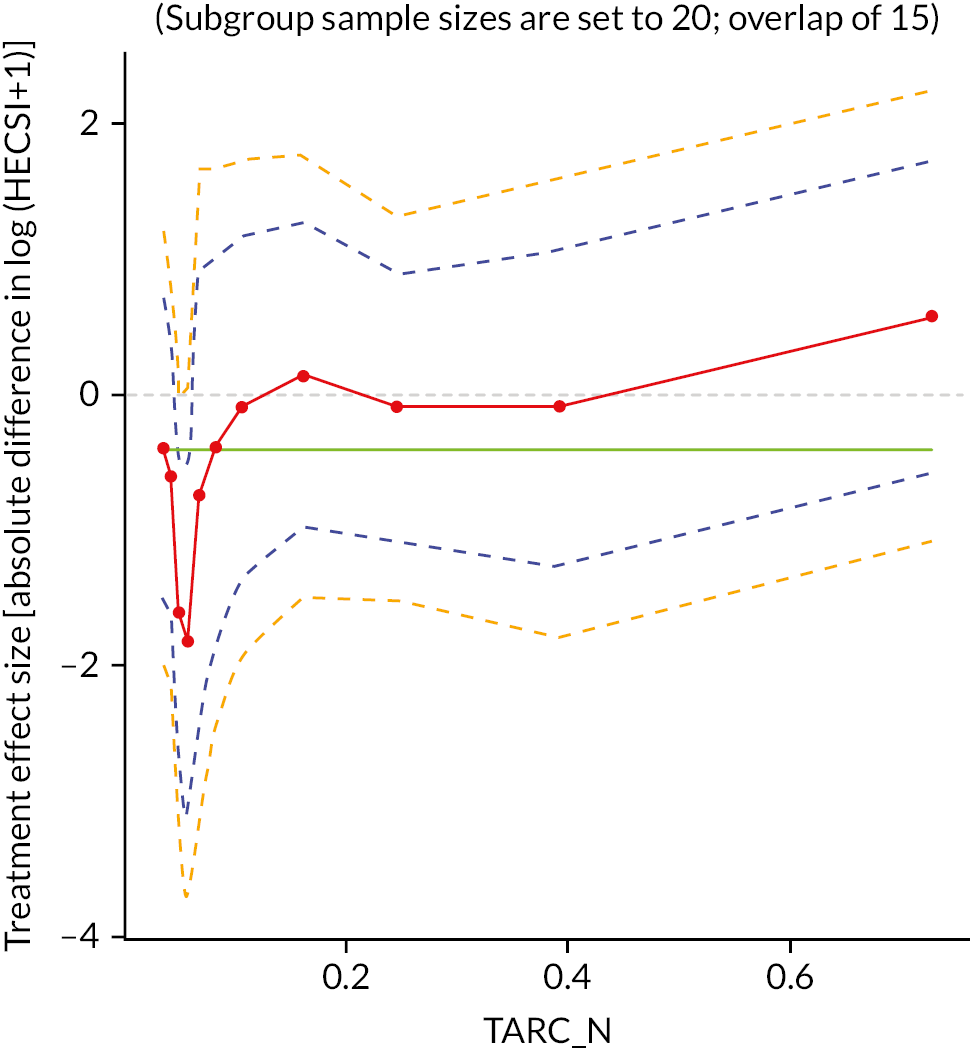
FIGURE 24.
Subpopulation treatment effect pattern plot to assess differential treatment effects at 12 weeks for TARC, non-lesional samples.

FIGURE 25.
Subpopulation treatment effect pattern plot to assess differential treatment effects at 12 weeks for CCL20, lesional samples.

FIGURE 26.
Subpopulation treatment effect pattern plot to assess differential treatment effects at 12 weeks for CCL20, non-lesional samples.

FIGURE 27.
Maximum reported weekly corticosteroid use at each assessment, AL, available data only.

FIGURE 28.
Maximum reported weekly corticosteroid use at each assessment, Immersion PUVA, available data only.
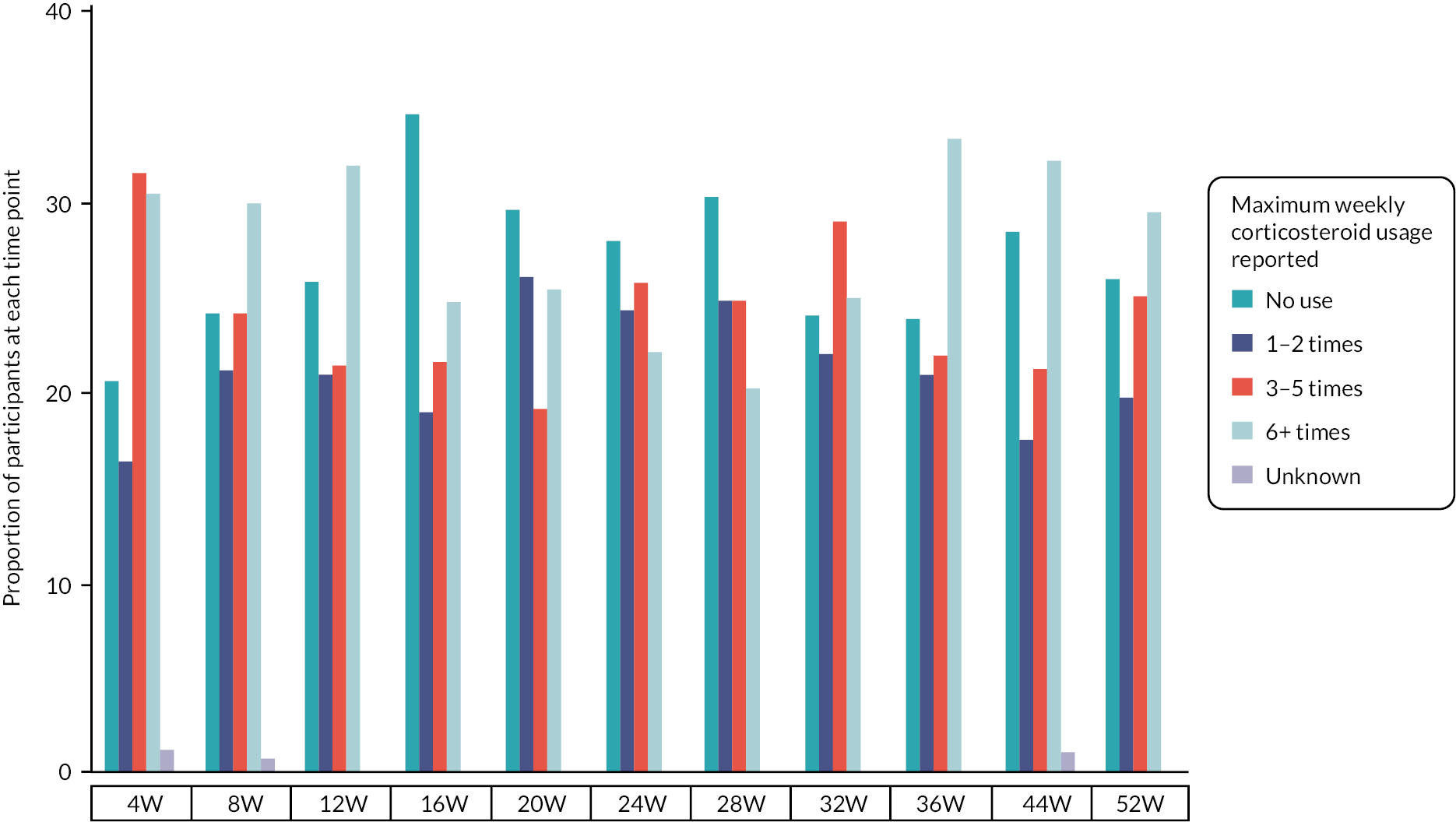
| AL (%) | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| Maximum weekly corticosteroid usage reported at 4 weeks | |||
| No use | 56 (25.5) | 38 (17.2) | 94 (21.3) |
| 1–2 times | 35 (15.9) | 30 (13.6) | 65 (14.7) |
| 3–5 times | 50 (22.7) | 58 (26.2) | 108 (24.5) |
| 6+ times | 61 (27.7) | 56 (25.3) | 117 (26.5) |
| Unknown | 0 (0.0) | 2 (0.9) | 2 (0.5) |
| Missing | 18 (8.2) | 37 (16.7) | 55 (12.5) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| Maximum weekly corticosteroid usage reported at 8 weeks | |||
| No use | 69 (31.4) | 41 (18.6) | 110 (24.9) |
| 1–2 times | 30 (13.6) | 36 (16.3) | 66 (15.0) |
| 3–5 times | 44 (20.0) | 41 (18.6) | 85 (19.3) |
| 6+ times | 46 (20.9) | 51 (23.1) | 97 (22.0) |
| Unknown | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Missing | 31 (14.1) | 51 (23.1) | 82 (18.6) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| Maximum weekly corticosteroid usage reported at 12 weeks | |||
| No use | 72 (32.7) | 42 (19.0) | 114 (25.9) |
| 1–2 times | 28 (12.7) | 34 (15.4) | 62 (14.1) |
| 3–5 times | 44 (20.0) | 35 (15.8) | 79 (17.9) |
| 6+ times | 40 (18.2) | 52 (23.5) | 92 (20.9) |
| Missing | 36 (16.4) | 58 (26.2) | 94 (21.3) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| Maximum weekly corticosteroid usage reported at 16 weeks | |||
| No use | 57 (25.9) | 53 (24.0) | 110 (24.9) |
| 1–2 times | 26 (11.8) | 29 (13.1) | 55 (12.5) |
| 3–5 times | 38 (17.3) | 33 (14.9) | 71 (16.1) |
| 6+ times | 49 (22.3) | 38 (17.2) | 87 (19.7) |
| Missing | 50 (22.7) | 68 (30.8) | 118 (26.8) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| Maximum weekly corticosteroid usage reported at 20 weeks | |||
| No use | 55 (25.0) | 42 (19.0) | 97 (22.0) |
| 1–2 times | 28 (12.7) | 37 (16.7) | 65 (14.7) |
| 3–5 times | 38 (17.3) | 27 (12.2) | 65 (14.7) |
| 6+ times | 38 (17.3) | 36 (16.3) | 74 (16.8) |
| Missing | 61 (27.7) | 79 (35.7) | 140 (31.7) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| Maximum weekly corticosteroid usage reported at 24 weeks | |||
| No use | 52 (23.6) | 38 (17.2) | 90 (20.4) |
| 1–2 times | 30 (13.6) | 33 (14.9) | 63 (14.3) |
| 3–5 times | 30 (13.6) | 35 (15.8) | 65 (14.7) |
| 6+ times | 40 (18.2) | 30 (13.6) | 70 (15.9) |
| Unknown | 2 (0.9) | 0 (0.0) | 2 (0.5) |
| Missing | 66 (30.0) | 85 (38.5) | 151 (34.2) |
| Total | 220 (100) | 221 (100) | 441 (100) |
| Maximum weekly corticosteroid usage reported at 28 weeks | |||
| No use | 52 (27.2) | 31 (16.2) | 83 (21.7) |
| 1–2 times | 19 (9.9) | 27 (14.1) | 46 (12.0) |
| 3–5 times | 24 (12.6) | 26 (13.6) | 50 (13.1) |
| 6+ times | 39 (20.4) | 21 (11.0) | 60 (15.7) |
| Missing | 57 (29.8) | 86 (45.0) | 143 (37.4) |
| Total | 191 (100) | 191 (100) | 382 (100) |
| Maximum weekly corticosteroid usage reported at 32 weeks | |||
| No use | 47 (24.6) | 24 (12.6) | 71 (18.6) |
| 1–2 times | 25 (13.1) | 22 (11.5) | 47 (12.3) |
| 3–5 times | 26 (13.6) | 29 (15.2) | 55 (14.4) |
| 6+ times | 36 (18.8) | 25 (13.1) | 61 (16.0) |
| Missing | 57 (29.8) | 91 (47.6) | 148 (38.7) |
| Total | 191 (100) | 191 (100) | 382 (100) |
| Maximum weekly corticosteroid usage reported at 36 weeks | |||
| No use | 43 (22.5) | 25 (13.1) | 68 (17.8) |
| 1–2 times | 24 (12.6) | 22 (11.5) | 46 (12.0) |
| 3–5 times | 40 (20.9) | 22 (11.5) | 62 (16.2) |
| 6+ times | 30 (15.7) | 35 (18.3) | 65 (17.0) |
| Missing | 54 (28.3) | 87 (45.5) | 141 (36.9) |
| Total | 191 (100) | 191 (100) | 382 (100) |
| Maximum weekly corticosteroid usage reported at 44 weeks | |||
| No use | 43 (22.5) | 30 (15.7) | 73 (19.1) |
| 1–2 times | 22 (11.5) | 19 (9.9) | 41 (10.7) |
| 3–5 times | 40 (20.9) | 23 (12.0) | 63 (16.5) |
| 6+ times | 27 (14.1) | 34 (17.8) | 61 (16.0) |
| Unknown | 0 (0.0) | 1 (0.5) | 1 (0.3) |
| Missing | 59 (30.9) | 84 (44.0) | 143 (37.4) |
| Total | 191 (100) | 191 (100) | 382 (100) |
| Maximum weekly corticosteroid usage reported at 52 weeks | |||
| No use | 44 (23.0) | 28 (14.7) | 72 (18.8) |
| 1–2 times | 26 (13.6) | 21 (11.0) | 47 (12.3) |
| 3–5 times | 18 (9.4) | 28 (14.7) | 46 (12.0) |
| 6+ times | 39 (20.4) | 33 (17.3) | 72 (18.8) |
| Missing | 64 (33.5) | 81 (42.4) | 145 (38.0) |
| Total | 191 (100) | 191 (100) | 382 (100) |
| AL (%) | Immersion PUVA (%) | Total (%) | |
|---|---|---|---|
| Baseline | |||
| Clear | 16 (7.4) | 15 (7.2) | 31 (7.3) |
| Almost clear | 52 (24.1) | 47 (22.5) | 99 (23.3) |
| Moderate | 71 (32.9) | 69 (33.0) | 140 (32.9) |
| Severe | 40 (18.5) | 42 (20.1) | 82 (19.3) |
| Very severe | 19 (8.8) | 16 (7.7) | 35 (8.2) |
| No agreement between assessors | 17 (7.9) | 18 (8.6) | 35 (8.2) |
| Unable to assess | 1 (0.5) | 2 (1.0) | 3 (0.7) |
| Total | 216 (100) | 209 (100) | 425 (100) |
| 12 weeks | |||
| Clear | 32 (21.1) | 18 (13.3) | 50 (17.4) |
| Almost clear | 41 (27.0) | 46 (34.1) | 87 (30.3) |
| Moderate | 27 (17.8) | 40 (29.6) | 67 (23.3) |
| Severe | 24 (15.8) | 13 (9.6) | 37 (12.9) |
| Very severe | 4 (2.6) | 2 (1.5) | 6 (2.1) |
| No agreement between assessors | 16 (10.5) | 16 (11.9) | 32 (11.1) |
| Unable to assess | 8 (5.3) | 0 (0.0) | 8 (2.8) |
| Total | 152 (100) | 135 (100) | 287 (100) |
FIGURE 29.
Treatment pathway compliance by assessment time.
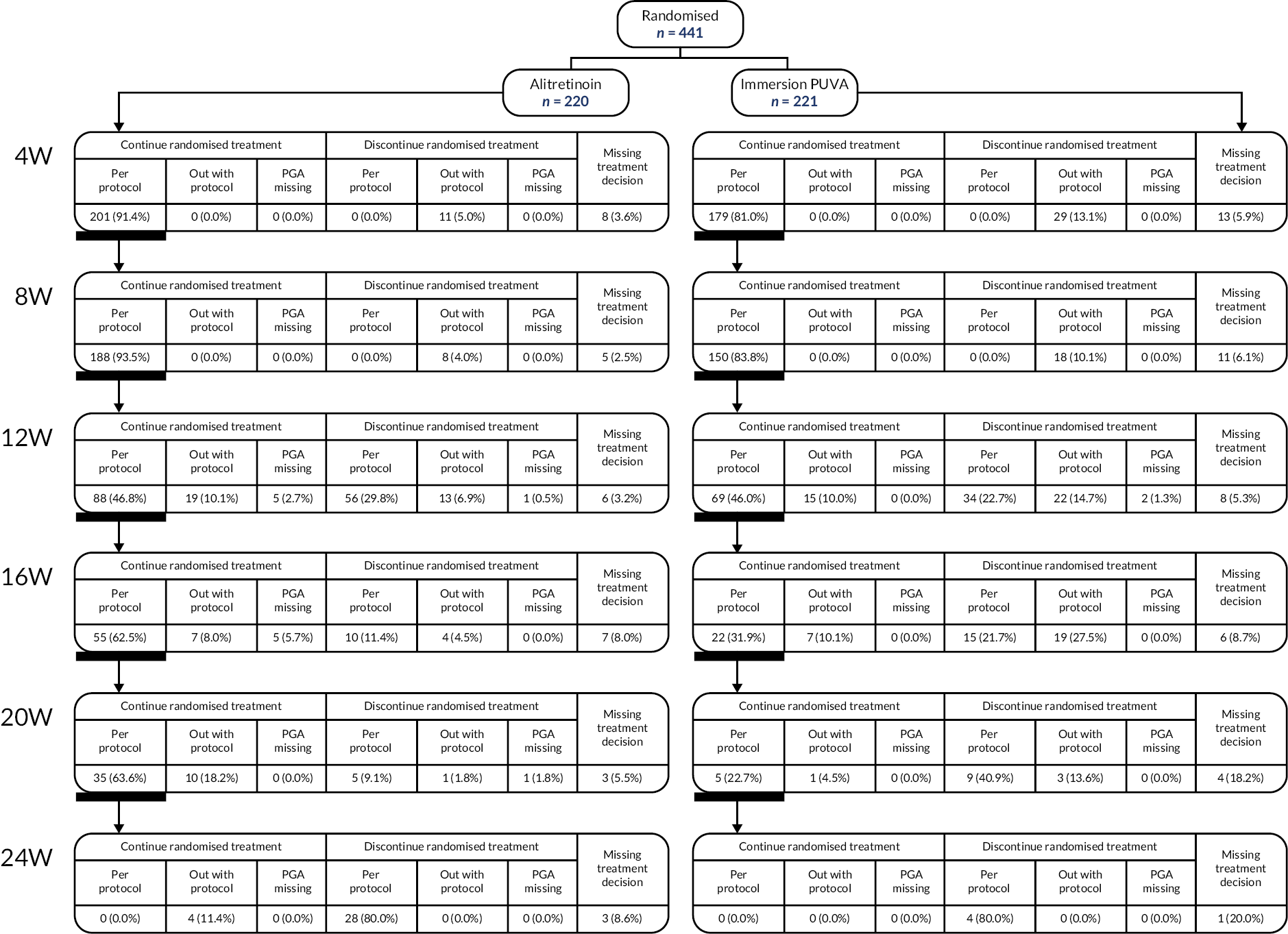
Appendix 3 Long-term model transition probabilities, cost, quality-adjusted life-years parameters
| Proportion of patients at week 24 (initial state) (%) | Proportion of patients at the end of week 36 | |||
|---|---|---|---|---|
| Clear/almost clear (%) | Moderate (%) | Severe (%) | ||
| Clear/almost clear | 32 | 38 | 54 | 8 |
| Moderate | 53 | 30 | 58 | 12 |
| Severe | 15 | 30 | 45 | 24 |
| Proportion of patients at week 36 (%) | Proportion of patients at the end of the 3-month cycle | |||
|---|---|---|---|---|
| Clear/almost clear (%) | Moderate (%) | Severe (%) | ||
| Clear/almost clear | 33 | 44 | 51 | 4 |
| Moderate | 55 | 36 | 53 | 11 |
| Severe | 13 | 32 | 54 | 14 |
| Proportion of patients at week 24 (initial state) (%) | Proportion of patients at the end of week 36 | |||
|---|---|---|---|---|
| Clear/almost clear (%) | Moderate (%) | Severe (%) | ||
| Clear/almost clear | 41 | 38 | 55 | 7 |
| Moderate | 47 | 24 | 65 | 11 |
| Severe | 12 | 50 | 38 | 12 |
| Proportion of patients at week 36 (%) | Proportion of patients at the end of the 3-month cycle | |||
|---|---|---|---|---|
| Clear/almost clear (%) | Moderate (%) | Severe (%) | ||
| Clear/almost clear | 33 | 51 | 42 | 7 |
| Moderate | 58 | 43 | 50 | 7 |
| Severe | 9 | 35 | 45 | 20 |
A proportion of patients in any health state transit/remain to/in a particular state every 3 months until the time frame of the model elapses; for example, of the 32% of patients starting at clear/almost clear status for AL at week 24, 38% will remain as clear, 54% will transit to moderate and 8% will transit to severe. Similarly, from the 53% of patients starting at the moderate state, 30% will move to clear/almost clear, 58% will remain in moderate, while 12% will move to severe.
| Costs | Mean (standard error) | Distribution | |||||
|---|---|---|---|---|---|---|---|
| Clear/almost clear | Moderate | Severe | |||||
| AL | Immersion PUVA | AL | Immersion PUVA | AL | Immersion PUVA | ||
| Randomisation to week 24 | £561 (81.48) | £566 (105.3) | £1340 (115.9) | £1888 (156.1) | £1015 (67.1) | £1738 (102.9) | Gamma |
| Between weeks 24 and 36 | £188 (55.1) | £121 (52.3) | £253 (68.64) | £201 (61.9) | £269 (166.7) | £113 (67.7) | Gamma |
| 12 weeks cost from week 36 | £154 (66.9) | £104 (45.4) | £112 (49.3) | £84 (40.4) | £104 (66.0) | £107 (76.4) | Gamma |
| QALYs/utility | Mean (standard error) | Distribution | |||||
| Clear/almost clear | Moderate | Severe | |||||
| AL | Immersion PUVA | AL | Immersion PUVA | AL | Immersion PUVA | ||
| QALYs from baseline to week 24 | 0.3295 (0.006) | 0.3649 (0.003) | 0.3384 (0.004) | 0.3429 (0.004) | 0.3344 (0.003) | 0.3468 (0.003) | Beta |
| QALYs from weeks 24 to 36 | 0.1915 (0.026) | 0.1976 (0.020) | 0.1809 (0.018) | 0.1894 (0.020) | 0.1642 (0.050) | 0.1756 (0.081) | Beta |
| QALYs from weeks 36 to 52 and every 3 months | 0.2022 (0.019) | 0.2077 (0.014) | 0.1805 (0.024) | 0.1881 (0.022) | 0.1553 (0.049) | 0.1578 (0.063) | Beta |
Appendix 4 Unadjusted utility values
| State | Baseline | 12 | 24 | 36 | 52 |
|---|---|---|---|---|---|
| Mild | 0.721 | 0.876 | 0.900 | 0.830 | 0.876 |
| Moderate | 0.601 | 0.792 | 0.786 | 0.784 | 0.782 |
| Severe | 0.618 | 0.589 | 0.708 | 0.711 | 0.673 |
| State | Baseline | 12 | 24 | 36 | 52 |
|---|---|---|---|---|---|
| Mild | 0.607 | 0.869 | 0.914 | 0.856 | 0.899 |
| Moderate | 0.780 | 0.767 | 0.707 | 0.820 | 0.815 |
| Severe | 0.535 | 0.653 | 0.719 | 0.761 | 0.683 |
Appendix 5 Resource use breakdown
| AL | Immersion PUVA | Comments | |
|---|---|---|---|
| A&E | 0.04 | 0.06 | Eight and 14 visits, respectively, reported to the A&E for the entire 52 weeks |
| Dermatology | 0.17 | 0.13 | Thirty-eight and 29 visits, respectively, reported to the dermatology department for the entire 52 weeks |
| Radiology | 0.04 | 0.05 | Nine and 11 visits, respectively, reported to the radiology for the entire 52 weeks |
| Other (cardiology, gastroenterology, physiotherapy) | 0.04 | 0.01 | Nine and two visits, respectively, reported to other departments for the entire 52 weeks |
| AL | Immersion PUVA | Comments | |
|---|---|---|---|
| GP | 0.23 | 0.43 | Fifty-one and 94 visits, respectively, reported to the GP for the entire 52 weeks |
| Practice or district nurse | 0.05 | 0.10 | Ten and 21 consultations, respectively, with a district or practice nurse for the entire 52 weeks |
| Occupational health specialist | 0.01 | 0.01 | Three and three visits, respectively, reported to the radiology for the entire 52 weeks |
| Physiotherapy | 0.01 | 0.00 | Three and zero visits, respectively, reported to physiotherapist for the entire 52 weeks |
| Other | 0.09 | 0.01 | Nineteen and three visits, respectively, reported to other departments for the entire 52 weeks |
| Average per-patient reported use of aids, appliances and adaptations over 52 weeks | |||
| Cotton, rubber, latex gloves | 6.00 | 7.59 | Reported items were mainly cotton, latex or rubber gloves, either disposable (boxes) or reusable. For costing purposes, we assume the average cost of all items reported (£19.65). |
| Parameters | 12 weeks | 52 weeks | ||
|---|---|---|---|---|
| AL | Immersion PUVA | AL | Immersion PUVA | |
| Hospital admissions | £15.10 | £28.00 | £74.90 | £87.20 |
| Community care | £5.40 | £6.40 | £25.70 | £30.60 |
| Aids | £10.00 | £12.10 | £37.80 | £41.90 |
| Medications | £2.60 | £3.60 | £24.50 | £59.50 |
| Parameters | Week 12 | Week 52 | ||
|---|---|---|---|---|
| AL (£) | Immersion PUVA (£) | AL (£) | Immersion PUVA (£) | |
| Items purchased (gloves, painkillers, washing liquid, soap, etc.) | 64.00 | 70.10 | 263.85 | 306.00 |
| Travel costs | 15.50 | 15.90 | 56.23 | 54.00 |
| Time off work | 179.00 | 411.00 | 700.20 | 1073.70 |
| Other people time off work and paid assistance | 0.27 | 6.10 | 12.25 | 44.50 |
| Other people time off work and unpaid assistance | 51.80 | 38.35 | 177.21 | 206.40 |
Appendix 6 Cost-effectiveness acceptability curve and scatterplot within-trial and long-term cost-effectiveness analysis
FIGURE 30.
Within-trial analysis CEAC at week 12. x-axis: Threshold value; y-axis: probability of cost-effectiveness.

FIGURE 31.
Within-trial analysis scatterplot Immersion PUVA vs. AL at week 12. y-axis: Incremental costs; x-axis: incremental QALYs. Black line: threshold line at £20,000 per QALY gained. All PSA iterations are at the left of the threshold line, which indicates that AL has 100% probability of being cost-effective at the £20,000 cost-per-QALY threshold.

FIGURE 32.
Within-trial analysis CEAC at week 52. x-axis: threshold value; y-axis: probability of cost-effectiveness.

FIGURE 33.
Within-trial analysis scatterplot Immersion PUVA vs. AL at week 52. y-axis: incremental costs; x-axis: incremental QALYs. Black line: threshold line at £20,000 per QALY gained. Ninety-six per cent of the PSA iterations are at the left of the threshold line, which indicates that AL has a 96% probability of being cost-effective at the £20,000 cost-per-QALY threshold.

FIGURE 34.
Long-term cost-effectiveness CEAC. x-axis: Threshold value; y-axis: probability of cost-effectiveness.

FIGURE 35.
Long-term cost-effectiveness scatterplot. y-axis: Incremental costs; x-axis: incremental QALYs. Black line: threshold line at £20,000 per QALY gained. Cloud of iteration is equally distributed to the left and right of the threshold line (black line), indicating a 50% probability of cost-effectiveness for Immersion PUVA.

Appendix 7 Results of the long-term sensitivity analysis
| Intervention | Costs (£) | QALYs | IncCOst (£) | IncQALYs | ICER (£) | NMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| Immersion PUVA | 4806 | 6.535 | 0 | 0.50 | |||
| AL | 4871 | 6.530 | 64.37 | −0.0054 | −11,859 | –173 | 0.50 |
| Intervention | Costs (£) | QALYs | IncCOst (£) | IncQALYs | ICER | NMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| Immersion PUVA | 4816 | 6.663 | 0 | 0.51 | |||
| AL | 4876 | 6.655 | 60.24 | −0.0083 | −£7292 | −225 | 0.49 |
| Intervention | Costs (£) | QALYs | IncCOst (£) | IncQALYs | ICER (£) | NMB (£) | Probability of cost-effectiveness |
|---|---|---|---|---|---|---|---|
| Immersion PUVA | 4805 | 6.666 | 0 | 0.51 | |||
| AL | 4869 | 6.652 | £63.72 | −0.0133 | −4794 | −330 | 0.49 |
List of abbreviations
- AD
- atopic dermatitis
- AE
- adverse event
- AL
- alitretinoin
- BAD
- British Association of Dermatology
- BMI
- body mass index
- CEAC
- cost-effectiveness acceptability curve
- CHE
- chronic hand eczema
- CI
- confidence interval
- CONSORT
- CONsolidated Standards Of Reporting Trials
- CTRU
- Clinical Trials Research Unit
- CV
- coefficient of variation
- DLQI
- Dermatology Life Quality Index
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- HE
- hand eczema
- HECSI
- Hand Eczema Severity Index
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ID
- identifier
- IQR
- interquartile range
- ITT
- intention-to-treat
- LCE
- late cornified envelope
- LOF
- loss of function
- MAR
- missing at random
- mTLSS
- modified total lesion symptom score
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- PBI
- Patient Benefit Index
- PGA
- Physician’s Global Assessment
- PH
- proportional hazards
- PP
- per protocol
- PSS
- personal and social
- PUVA
- 8-methoxypsoralen combined with ultraviolet A
- QALY
- quality-adjusted life-years
- RAST
- radioallergosorbent test
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SAR
- serious adverse reaction
- SmPC
- summary of product characteristics
- STEPP
- subpopulation treatment effect pattern plots
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TOR
- terms of reference
- TSC
- Trial Steering Committee
- UV
- ultraviolet
- WCBP
- Woman of Child Bearing Potential
