Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number HTA 16/31/98. The contractual start date was in March 2018. The draft report began editorial review in June 2022 and was accepted for publication in November 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Blair et al. This work was produced by Blair et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Blair et al.
Chapter 1 Introduction
Structure of this report
In Chapter 1, the background to the research and rationale for reducing antibiotic prescriptions through use of a complex intervention is presented. Chapter 2 describes the methods employed including the details of the intervention, recruitment, outcome measures, how the data were collected, the internal pilot and the study design. Chapter 3 presents the clinical results of the study, Chapter 4 the qualitative findings and Chapter 5 the economic evaluation. Chapter 6 discusses the efficient design of the study including the facilitators and barriers to such a design. Chapter 7 presents the discussion and conclusions.
Background
Antibiotic prescribing
Respiratory tract infections (RTIs) in children present a major resource implication for primary health-care services internationally for five reasons. First, they are extremely common and costly to service providers, families and employers. 1,2 Second, there is clinical uncertainty in primary care regarding the diagnosis and best management of RTIs, as reflected by the variation in the use of antibiotics in primary care for RTIs between nations,3 general practitioner (GP) practices4 and clinicians. 5 Third, antibiotic prescribing by primary care clinicians in the United Kingdom (UK) remains significantly higher than in some of our European neighbours. 6 Fourth, the overuse and misuse of existing antibiotics, combined with the slowing in the development of new antibiotics, are associated with the development and proliferation of antimicrobial resistance between7 and within8 nations as well as individuals. 6,9,10 Finally, the use of antibiotics leads to the subsequent ‘medicalisation of illness’ in which patients believe they should consult for similar symptoms in the future. 11 A number of key publications have highlighted the need for more research to define the appropriate use of antibiotics and healthcare resources for RTIs if the public health disaster of ineffective antibiotics for serious infections is to be averted. 12–14
Findings from the TARGET programme
The design of the CHIldren with Cough (CHICO) trial’s intervention was borne out of evidence from the TARGET programme’s earlier work streams. This has involved data synthesis of qualitative and quantitative evidence,15–19 a qualitative investigation to understand both parents’ information needs and influences on clinical decisions surrounding antibiotic prescribing, quantitative evidence in terms of a large multicentre, prospective cohort study (over 8300 children) to derive and internally validate a clinical rule to predict hospitalisation of children with RTIs,20,21 and the development of an intervention informed by a critical synthesis of all findings. 22 This culminated in a feasibility cluster randomised controlled trial (RCT) study to measure the acceptability of using symptoms, signs and demographic characteristics to predict hospital admittance in children presenting to primary care with RTI. 23,24 Findings across the TARGET programme were synthesised using Greene and Kreuter’s Precede-Proceed model which integrates across several behavioural theories into a unified model. 25
Qualitative work from the National Institute for Health and Care Research (NIHR) TARGET programme for Applied Research in 2016 identified clinician uncertainty as a major driver of antibiotic prescribing. Primary care clinicians acknowledge that they prescribe antibiotics for a range of medical and non-medical reasons, particularly in children, who are seen as vulnerable26 and whose clinical state can change rapidly. Many clinicians report that they prescribe antibiotics just in case to mitigate perceived risk of future hospital admission and complications. 19,26 Our earlier programme work indicated that improved identification of children at very low risk of future hospitalisation could increase confidence to withhold antibiotics in low-risk groups. 15,16 Clinical prediction rules are designed to reduce clinical uncertainty in an outcome (such as a child’s risk of hospitalisation) by assessing the strength of association between the risk of it occurring and baseline characteristics (e.g. sociodemographic characteristics or symptoms and signs of illness).
Clinical prediction rule for hospitalisation
The TARGET cohort study aimed to identify symptoms, signs and demographic characteristics that may predict hospitalisation and poor prognosis of a child. In particular, such an algorithm could potentially identify a large group of children at very low risk of hospitalisation and therefore are potentially unlikely to require antibiotics.
In line with expectations, just under 1% of the children were admitted to hospital up to 30 days after their consultation with the primary care clinician. We found seven characteristics independently associated with increased risk of hospitalisation for their acute cough or RTI during the subsequent 30 days. These are described by the STARWAVe mnemonic: Short illness duration (parent reported ≤ 3 days), Temperature (parent reported severe in previous 24 hours or ≥ 37.8° on examination), Age of patient (< 2 years), Recession (intercostal or subcostal on examination), Wheeze (on listing to chest with stethoscope), Asthma (currently diagnosed) and Vomiting (parent reported moderate or severe in the 24 hours prior to consultation). The area under the receiver operating characteristic (ROC) curve for the coefficient-based algorithm was 0.82 [95% confidence interval (CI) 0.77 to 0.87].
The prediction rule identifies children at very low, medium and high risk of future hospitalisation with advice given on how the clinician can use this information in conjunction with their own clinical judgement to decide the best course of action for each child. Assigning one point per predictive characteristic, a points-based algorithm was used to quantify absolute probabilities to three groups: (1) ‘very low’ (0.3%, 95% CI 0.2% to 0.4%) scoring 0 or 1 point; (2) ‘normal’ (1.5%, 1.0% to 1.9%) scoring 2 or 3 points and (3) ‘high’ (11.8%, 7.3% to 16.2%) scoring ≥ 4 points. The rule can potentially be effective in both reducing the overall prescription of antibiotics by increasing clinician confidence that they are not needed ‘just in case’ in the very low-risk group (just under 70% of children are in this group), as well as better identify those children in need of close monitoring (2% of children are in the high-risk group). Children in the medium-risk group (29%) have a similar risk of future hospitalisation as all children combined, so management should follow current National Institute for Health and Care Excellence (NICE) guidelines,27 which state that clinicians should decide on the use of immediate, delayed or no antibiotics based on their assessment of the child’s illness severity.
The interaction within the consultation
Our finding from our qualitative reviews was that clinicians could misinterpret parent’s communication about their concerns or ideas regarding their child’s illness as pressure for antibiotics and in some cases this led to unnecessary or unwanted antibiotics being prescribed. 26 Clinician communication was focused on differentiating minor and more serious illnesses, with the message (both implicit and explicit) that viral illnesses were minor while those that were ‘serious’ were treated with antibiotics. Clinicians and parents were often talking at cross purposes about the seriousness of the illness; parents emphasising the severity of the symptoms to demonstrate the impact on child health and to justify the consultation; clinicians seeking to justify a no-antibiotic treatment decision by minimising the problem.
The findings of our qualitative study suggested that parents want better information on the symptoms and signs of serious RTIs and when to consult,28 along with more useful advice on home management of symptoms. 29 Parents did not want to know the absolute risk of hospitalisation for their child but they did want advice and information specific to their child. 29 When parents did consult, clinician explanations of diagnosis and treatment recommendations were not well understood by parents, and they remained unclear about how to manage a RTI and when to consult. 27–29 Clinicians reported that most often they gave a simple viral diagnosis, communicating that the illness was self-limiting and did not need antibiotic treatment. 29 However, if the child’s illness appeared severe to the parent, or the parent was concerned about particular symptoms or impacts which were not addressed by the clinician, the parent reported viewing a simple viral diagnosis as inadequate. Parents’ concerns encompassed things that fell outside a simple biomedical model for RTIs, including both child health and psychosocial impacts, but reported that clinicians rarely addressed these.
The feasibility study and an ‘efficient’ design
In the feasibility trial, the intervention group antibiotic prescribing rate at consultation was 25% (compared to 37% in our earlier cohort study); however, among the control group, the overall prescribing rate was even lower at 16%. The paradoxical effects found in the feasibility study were largely explained by a post-randomisation recruitment differential, with possible Hawthorne effects. 24 In the intervention arm, there was a significantly higher recruitment rate, difference in clinician type (proportionally more practice nurses recruiting), the children were significantly younger and importantly the intervention children were more unwell at baseline (significantly higher respiratory rate, significantly higher wheeze prevalence and significantly higher parent and clinician global illness severity scores). Learning from these lessons, we proposed a more ‘efficient’ study design that would not only mitigate recruitment differential but would also be resource efficient, using an intervention designed to be replicable for the National Health Service (NHS). Rather than recruit patients and collect consent during consultation we conducted the CHICO RCT at the practice level (as a cluster RCT) using Clinical Commission Groups (CCGs) and the national NIHR Clinical Research Network (CRN) to recruit practices. Rather than trawl through the practice notes to collect primary outcome data we utilised routinely collected data (dispensing data collected by NHS Business Services Authority (NHSBSA) ePACT230 and hospitalisation rates for RTI collected by CCGs). We also took a ‘lighter touch’ to data collection using short baseline and follow-up questionnaires filled in by the practice champion (GP, nurse, practice manager or pharmacist) and embedded the intervention within the practice system rather than as a stand-alone tool. This both reduced the time it takes to open and close the tool and had the advantage of utilising data already within the system and incorporating data entered as part of the trial into the practice system, thus, not duplicating clinical work. The barriers and facilitators to this efficient design approach are reported in Chapter 6.
Rationale
Antimicrobial resistance is recognised by the UK government, governments around the world and the World Health Organization (WHO) as one of the most pressing public health threats of our time. Around 80% of antibiotics prescribed for human consumption are prescribed in primary care,31 and it has been estimated that around 50% of antibiotic prescribing in this setting is unnecessary. 32 Approaches to modify antibiotic prescribing in primary care have been developed and evaluated, and prescribing rates in England have declined slightly using figures from 2014 to 2015,31 although antibiotic prescribing rates in the UK continue to be substantially higher than many other European countries. 6 The UK Five Year Anti-Microbial Resistance (AMR) Strategy 2013–2018 aims to conserve the effectiveness of existing antibiotics through effective antimicrobial stewardship, including reducing the inappropriate use of antibiotics. There is, therefore, an urgent need for an efficient intervention that can be rolled out at scale that safely addresses many of the key drivers of antibiotic prescribing in children. Recent papers suggest cluster randomised trials aimed at reducing antibiotics may be implemented efficiently in large samples from routine care settings by using primary care electronic health records in the UK. 33–35 We are aware of two ongoing studies: the first investigating the effects of an integrated package of interventions (including delayed prescribing; patient decision aids; communication training; patient information leaflets; and near-patient testing with C-reactive protein)36 and the second (an ‘efficient design’ study) investigating the effects of a multifaceted intervention consisting of practice antibiotic prescribing feedback, delivery of educational and decision support tools and a webinar to explain and promote the intervention. 37 Both studies focus on the general rather than the paediatric population and have different components compared to our complex intervention. Primary care clinicians38 and the research community39 have called for the development of a sound evidence base, currently unavailable, to help them identify children at low and high risk of complications, especially serious complications such as pneumonia, that require hospitalisation. At a minimum, it is essential to demonstrate that any change in practice does not increase the number of children with serious complications. A change in practice should improve health outcomes for children, while distinguishing children for whom antibiotics are certainly not needed and providing precise information regarding the symptoms denoting poor prognosis for which parents should be vigilant.
Aims and objectives
Aim
The aim of the CHICO RCT is to reduce antibiotic prescribing among children presenting with cough or RTI without increasing hospital admission for this condition. Objectives pertain to the practice level, as no data was collected on individual participants.
Primary objectives
To determine:
-
Whether the CHICO intervention decreases the number of paediatric formulation antibiotic suspension items dispensed for RTIs to children presenting with acute cough and RTI to primary care (superiority comparison).
-
Whether the CHICO intervention results in no change in hospital admissions for children with a hospital diagnosis of RTI (non-inferiority comparison).
Secondary objectives
To determine:
-
Whether the CHICO intervention results in no change in the accident and emergency (A&E) attendance rates of children with a hospital diagnosis of RTI (superiority comparison).
-
The costs to the NHS for using the CHICO intervention.
-
Whether any intervention effect is modified by the proportion of locums used.
-
Whether any intervention effect is modified by the practices’ prior antibiotic prescribing rate.
-
Whether any intervention effect differs between GP and nurse prescribers.
-
Whether any intervention effect differs between practices with 1 versus 2+ sites.
-
Whether any intervention effect differs within child age groups.
-
Whether any intervention effect differs between practices and over time.
-
Whether the embedded CHICO intervention is acceptable to, and used by, primary care clinicians (GPs and nurses) in consultations with carers and their children and how does this vary between practices.
Chapter 2 Methods
Study design
The CHICO RCT is an efficient, pragmatic, open-label, parallel, two-arm (intervention vs. control) cluster randomised trial with an embedded qualitative study and health economics. The trial aimed at reducing antibiotic prescribing among children presenting with acute cough and RTI, with randomisation at the practice level, using routine antibiotic dispensing to assess effectiveness and hospitalisation data for RTI to assess non-inferiority. Randomisation of practices, to the two arms, was 1 : 1 with no involvement, or consent, from participants within them. The CHICO trial protocol has been published. 40
Ethics approval and registration
The study received North of Scotland Research Ethics Committee (REC) approval on 29 October 2018 and by Health Research Authority at London-Camden and Kings Cross REC (ref: 18/LO/0345) on 14 November 2018. Trial Registration Number: ISRCTN11405239. This contains all items required to comply with the WHO Trial Registration Data Set. Any amendments to the protocol were reported accordingly to the regulatory bodies.
Practice systems
Egton Medical Information Systems (EMIS®) or EMIS® Health or EMISWeb (formerly known as Egton Medical Information Systems) supplies electronic patient record systems and software used in primary care in England. It is used in more than half the practices (56%) in England. 41 The intervention was embedded in the EMIS® system including pop-ups to identify children that may be eligible for the trial. The facilitators and barriers to this approach are reported in the results section.
As a separate scoping exercise to inform future dissemination, we contacted users or experts in SystmOne (covers 34% of practices)41 and reported in the results section the potential of embedding the intervention in this system.
Recruitment sites and site training
Given we needed access to CCGs to obtain primary outcome data, we endeavoured to recruit CCGs to the study first and then the practices within these CCGs. In 2018, there were over 200 CCGs in England and between 10 and 80 practices per CCG. For recruitment of CCGs, we focused on those having 15 or more EMIS® practices. One of our co-investigators (EB) is a national project lead for healthcare-acquired infections and antimicrobial resistance at NHS England and co-ordinated expressions of interest from CCGs prior to the start of the trial.
The intervention clinicians were provided with print and online evidence-based information to describe how the intervention works. This included an overview of the intervention (see Figure 1), a flowchart of how the intervention works in EMIS®, a description of how the algorithm is scored and how to use the parent leaflet (see Appendix 1).
FIGURE 1.
Children with cough clinician guide (overview of the intervention).

A practice champion was appointed at each practice to distribute training materials within the practice, co-ordinate training of practice prescribing staff, encourage all clinicians to use the intervention appropriately and report from the EMIS® system how many times the intervention was used. In the training package for clinicians, it was emphasised that the primary purpose of the intervention was to support the care of the larger proportion of children (69%) who have a very low risk of hospitalisation and this intervention was but one tool in their armoury for clinical decision making. The clinicians in practices randomised to the comparator arm were asked to treat children presenting with acute cough and RTI as they would normally.
Participants and recruitment
Recruitment of practices was via CCGs and the 15 CRNs across England using established channels of communication. NIHR CRNs support health and care organisations to be research active42 and can help recruit practices at a national level, including those serving diverse socioeconomic populations, improving the generalisability of findings. 43 CCGs are generally less involved in research but as they communicate regularly with practices can be used to help with recruitment.
A roll-out to three CCGs was performed initially to address any teething issues with the intervention, the internal pilot phase lasted 3 months and included a further four CCGs to help establish best practice for recruiting and communicating with practices before widening to the remaining CCGs.
After the pilot phase, CCGs in other regions of England were approached prioritising those with > 15 EMIS® practices which could potentially be recruited to the study.
Eligibility
Inclusion criteria
General practitioner practices in England using the EMIS® electronic patient record system to house the intervention, where the local CCG has agreed to provide primary outcome data and the practice consented to take part. Children aged 0–9 years presenting with a cough or RTI to the participating practice were flagged for eligibility in CHICO via a pop-up on the EMIS® consultation screen (for practices in the intervention arm of the study). No identifiable information was collected at participant level for the study. The number of times the intervention was used at practice level, over a 12-month period, was collected via an EMIS® search.
Exclusion criteria
Practices were excluded if they were participating in any antimicrobial stewardship activities during the study period involving concurrent intervention studies, where there was a potential to confound or modify the effects of our intervention. Practices involved in the CHICO feasibility study or were merging or planning to merge with another practice were also excluded.
Children aged 10–14 were considered for inclusion but at this age an increasing number are given antibiotics in tablet form and given the much lower consultation rate in older children the research team decided to exclude this group from the study population.
Study population
The study population was children aged 0–9 years presenting with acute cough and RTI. The primary outcomes were antibiotic dispensing rates (amoxicillin and macrolide items) and hospitalisation rates for RTI over a 12-month period at each practice divided by the number of 0–9-year-olds registered at that practice.
Intervention
Overview
The intervention consists of (1) eliciting explicit carer concerns during consultation, (2) a clinician-focused algorithm to predict risk of hospitalisation for children with acute cough and RTI in the following 30 days and (3) a carer-focused personalised printout recording decisions made at the consultation and safety-netting information. 22 The algorithm was to be used as one tool among many the clinicians already have, to decide whether the child needs antibiotics. In the training package for clinicians it was emphasised that the primary purpose of the algorithm was not so much to identify the small proportion of children (2%) at higher risk of hospitalisation but rather the much larger proportion of children (69%), who have a very low risk of hospitalisation.
Installation
Practices randomised to the intervention were sent instructions including screenshots on how to install the intervention application on the EMIS® system. E-mail support was offered to the practice champion to help implement this and encourage appropriate use of the tool. Requests to access a practice EMIS® system remotely to assist installation, subject to appropriate data security clearance, were also used if the installation ran into any problems.
Detail of intervention workflow during a consultation
-
Step 1: Soft Pop-up. Small CHICO pops up when child aged 0–9 years presents; clinician may click to open CHICO input screen.
-
Step 2: CHICO Trigger. Specific EMIS® codes automatically trigger CHICO input screen.
-
Step 3: CHICO Input screen: closed questions (clinical prediction rule) and elicitation of parent concerns.
-
Step 4: CHICO Output. Step 4a – Additional hover text.
-
Step 5: CHICO Letter + printout. Populate EMIS® record, generate Microsoft Word document (via mail merge) for printing.
Once imported, the intervention was embedded within the primary care information system. The steps above outline how the intervention worked in practice. When a child was in the age range of 0–9 years, the healthcare professional received a soft pop-up on their screen asking if the child was presenting with RTI. The pop-up gave the option of opening the CHICO Intervention (see Appendix 2, Figures 23 and 24). The Intervention screen would also open if the healthcare professional input a RTI-specific EMIS® code during the consultation. An embedded form was presented to record the presence or absence of particular symptoms and signs. Two of the seven predictors (age of patient and history of asthma) were already available for automatic entry. The other five predictors (short illness duration, temperature, intercostal or subcostal recession on examination, wheeze and any vomiting) were recorded during the consultation. Clinical details that are required to complete the STARWAVe algorithm were documented in a standardised EMIS® proforma and the electronic medical record, then identified whether the consulting patient was at elevated, average or very low risk of hospitalisation using a 7-point scoring system. The health professional was also prompted to elicit explicit concerns of the carer. The output page appeared as a small pop-up on the screen (see Table 1), requiring no action from the healthcare professional but informed them of the child’s risk of hospitalisation.
| CHICO result | Pop-up text |
|---|---|
| Low-risk group | Very reassuring CHICO score: 0 or 1 CHICO predictors: > 99.6% of children will recover from this illness with home care. Consider a no- or delayed-antibiotic prescribing strategy. CHICO leaflet and letter covers common concerns and safety netting advice. |
| Average-risk group | Reassuring CHICO score: 2 or 3 CHICO predictors: > 98% of children will recover from this illness with home care. Consider no- or delayed-antibiotic prescribing strategy. CHICO leaflet and letter covers common concerns and safety-netting advice. |
| Elevated-risk group | Safety-netting needed: 4+ CHICO predictors: This is more than average, but > 87% of children will still recover from this illness with home care. Highlight SAFETY NETTING advice in CHICO leaflet. |
When the Intervention input page had been saved the health professional had the option to print a short, personalised letter for the carer. This letter captured the following information:
-
patient’s name and age;
-
pre-specified brief description of the function of the consultation;
-
parent/carer’s concerns; manually typed in by the healthcare professional;
-
healthcare professional’s advice regarding raised parent’s concerns;
-
name of healthcare professional.
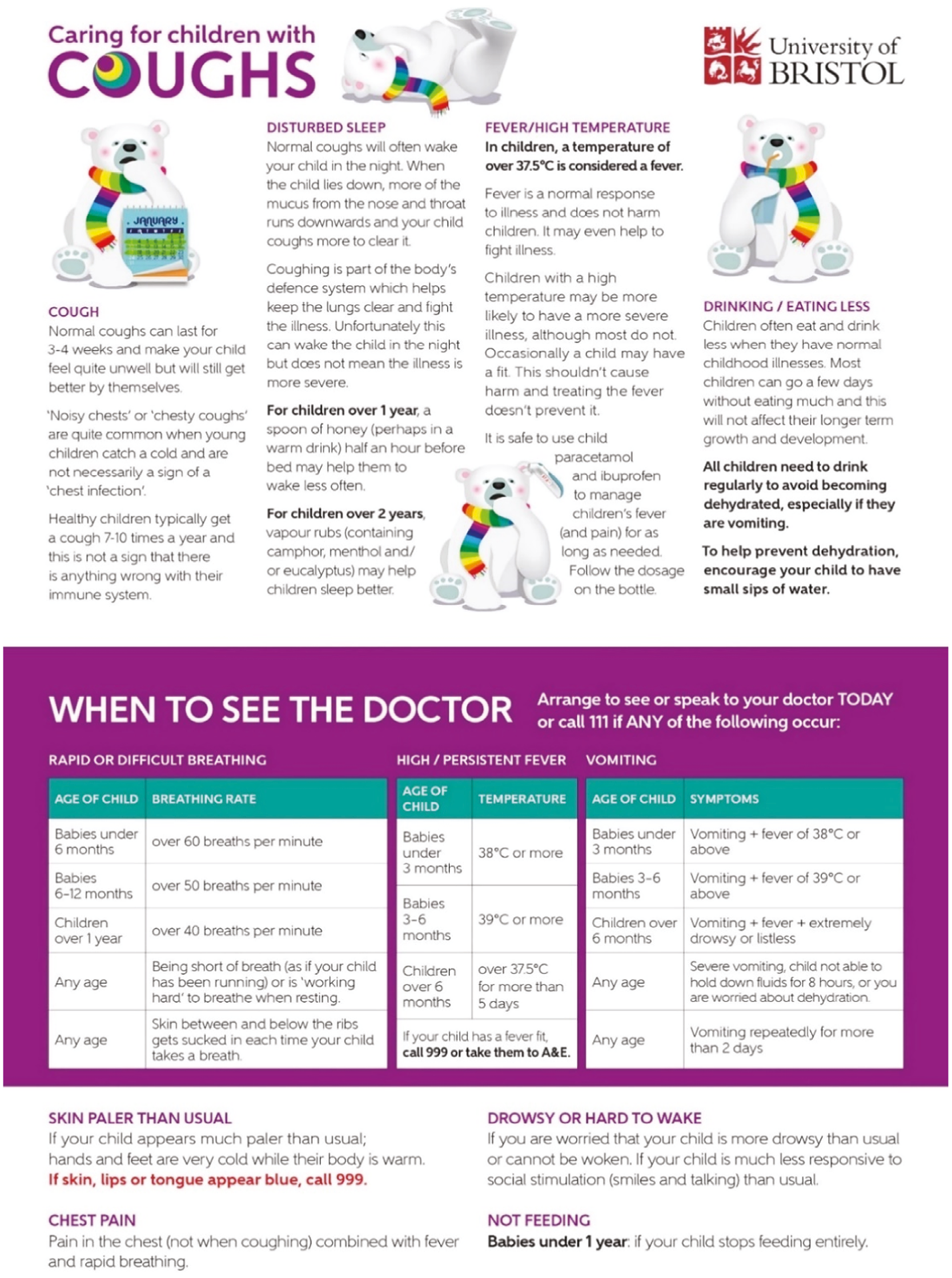
This personalised letter (project website upload) was provided to the carer alongside the ‘Caring for CHICO’ safety-netting leaflet (see Figure 2) providing further information regarding common carer concerns. The leaflet was based on one which was co-designed with parents and drew on our earlier work on the natural course of a typical RTI illness, typical duration of cough, caring for a child with a cough and safety-netting advice about when to re-consult. A slightly amended version was used in this intervention and made available in print or electronic format. 44
FIGURE 2.
Children with cough safety-netting leaflet.
Monitoring intervention use
An internal dashboard system was used for reporting frequency of use of the intervention by clinicians to practices during the 12-month participation period; where a ‘use’ of the intervention was defined as entering five or more symptom codes into the template as part of the consultation. Practices were provided with searches (EMIS® queries) which practice champions were requested to run monthly and feed back to the study team.
Encouraging intervention use
To accomplish this, the monthly searches returned by the practice champions were checked by the study team and relayed back to the practice if the intervention had not been used. In addition, practice champions were to review the search results and remind clinicians to use the intervention. CCGs were asked to support the study at both CCG levels and to endorse the use of the intervention within practices using local engagement processes and Anti-Microbial Stewardship (AMS) activities. Working with the AMS teams already established among practices and CCG pharmacists we selected CCG AMS leads to promote the use of the intervention at practice level.
Fidelity of the intervention
The consistency and reliability (fidelity) measures focused on intervention exposure and the quality of the intervention delivered (using the process interviews as part of the qualitative investigation). Using our light-touch approach to data collection meant it was challenging to measure fidelity.
Capturing intervention usage
Monthly searches collected data on intervention usage, defined as 5+ symptom codes being entered into the CHICO template (see Appendix 2). There may have been consultations that were missed (false negatives) and some that were included with fewer than five symptoms (false positives) but these were difficult to quantify. In addition, in the follow-up questionnaire sent after 12 months participation in CHICO, there were questions about the use of the intervention for practices in the intervention arm of the study. Additional questions were added, concerning change in use during the pandemic, from April 2020 (project web upload).
Usual care (control practices)
The control arm for CHICO trial was usual care for this condition. The clinicians from practices randomised to the control arm were just asked to treat children presenting with cough or RTI as they normally would. Baseline and follow-up questionnaire data on control practices were collected but no data directly from the clinicians aside from routinely requesting information on serious adverse events (SAEs).
Outcome measures
Primary outcome measures
There were two primary outcomes:
-
The rate of amoxicillin and macrolide items dispensed, calculated using the number of items dispensed to 0–9-year-olds and the number of children aged 0–9 registered at each practice over a 12-month period (testing for superiority). The number of items dispensed, for all indications, was used as a proxy measure due to the limitations of routine data.
-
The rate of hospitalisations for RTI, calculated using the number of hospitalisations for RTI among children aged 0–9 years and the number of children aged 0–9 registered at each practice over a 12-month period (testing for non-inferiority).
Secondary outcome measures
-
A&E attendance rates for RTI, calculated using the number of A&E attendances for RTI among children aged 0–9 years and the number of children aged 0–9 registered at each practice over a 12-month period.
-
An exploration into the usage of the intervention, in terms of both usage over a 12-month period and seasonality, and the effects it has on primary outcome P1.
-
A between-arm comparison of mean NHS costs in a cost-consequence analysis (health economics).
-
Acceptability of the intervention and variation in use determined by qualitative interviews with the clinicians.
Subgroup analyses (potential effect modifiers for P1)
-
Comparison of primary outcome P1 stratified by categorisation of proportion of locums (median) used over the 12 months the practice is in the study.
-
Comparison of primary outcome P1 stratified by categorisation of proportion of nurses (median) used over the 12 months the practice is in the study. This was originally planned as a dichotomisation of practices into those with GP prescribers only and those with nurse or other prescribers as well. However, given that the majority of practices had nurse prescribers, it seemed sensible to look at nurses as a proportion of staff.
-
Comparison of primary outcome P1 stratified by categorisation of practice dispensing rates taken from the 12 months prior to the data collection from each practice.
-
Comparison of primary outcome P1 dichotomised by those practices with one site only and those with multiple sites.
-
Comparison of primary outcome P1 dichotomised by those practices with follow-up periods before the COVID-19 pandemic and those with follow-up months on or after March 2020.
-
Comparison of primary outcome P1 dichotomised by those practices with a high level of deprivation versus those with a low level of deprivation.
Data collection
In this efficient design the data were mainly being collected from the practices and CCGs rather than patients and clinicians.
Expression of interest
As part of the recruitment of CCGs to the study, a form was completed by interested CCGs denoting their willingness to participate. One of our co-investigators (EB) is a national project lead for healthcare-acquired infections and antimicrobial resistance at NHS England and co-ordinated expressions of interest from CCGs prior to the start of the trial. Eligible practices were contacted via the CCGs and CRNs to take part in the trial. When GP practices were invited to participate, they were advised on the general principles of the trial, namely that the research will investigate methods to optimise the management of childhood RTI as well to agree to provide a practice champion as our primary contact and willingness of a member of staff to take part in qualitative interviews. If they were randomised to the intervention arm, the practices would need to agree to install the CHICO intervention on EMIS® and for the practice champion to monitor its use and send monthly updates. If practices wished to take part in the study, they completed an expression of interest form confirming agreement.
Randomisation data
When CCGs agreed to take part in the CHICO trial, the latest 12 months of data available, for amoxicillin and macrolide prescribing, was collected for all practices in their area. The latest month of list size data was used as the denominator to calculate the rate of dispensing over the 12-month period. This was then used to divide the data into those with a HIGH or LOW dispensing rate for stratification purposes in the randomisation process. In a similar way, practices within the CCG were split into those with a HIGH or LOW list size, relative to other practices within their CCG. These HIGH/LOW categorisations were not shared with the trial team until a practice agreed to take part and were ready to be randomised.
Baseline questionnaire
A brief questionnaire was sent to each practice to collect data on the characteristics of all practices prior to randomisation. Data were collected on the numbers of staff at the practice (by job type), questions regarding triaging of RTI conditions and postcode (for calculating the Index of Multiple Deprivation).
Routine data
For each practice, baseline data and follow-up data after 12 months were collected along with data for the two primary outcomes (dispensing and hospitalisations) and the secondary outcome (A&E attendances). The items of amoxicillin and macrolides dispensed, each month, were collected for the 12 months prior to randomisation and the 12 months after the implementation period. A short ‘implementation period’ (usually around 1 month) was allowed prior to the 12-month data collection period, giving time for the practices to install the intervention and encourage staff to use it. Any data collected during this period were not used in the analysis. For example, if a practice was randomised on the 10th of January their typical month 1 of follow-up would be February, as routine data were available for each calendar month. The data were obtained from NHSBSA ePACT2 system. 30
For the same 24-month period, data were collected on hospitalisations and A&E attendances, via a secure NHS e-mail inbox. These data were collected directly from each CCG. Data analysts from each CCG were asked to provide the number of hospitalisations for RTI, the number of hospitalisations with a ‘missing’ diagnosis and the total number of hospitalisations. Similar data were collected on A&E attendances. List size data, per month and 5-year epoch, were obtained from the NHS digital website. 45
Intervention usage
Practices were provided with an EMIS® search, which looked at the number of times the intervention template was opened. This was collected for each of the 12 months the intervention practices were in the study. Qualitative interviews with clinicians (GPs and practice nurses) and other practice staff (managers, pharmacists) and CCG staff (medicines managers) explored the use of the intervention, how it was embedded into practice and whether it was used appropriately.
Follow-up questionnaire
Like the baseline questionnaire, practices were asked to complete a follow-up questionnaire 12 months later. This included additional data on items such as the number of locums used during the 12 months of follow-up as well as questions around COVID-19 and how it had affected their triaging. Intervention practices were also asked what they thought of the intervention and if they would want to use it again.
Safety data (serious adverse events)
Intervention and control practices were asked to report SAEs immediately on the practice becoming aware of the event. The CHICO team sent a reminder e-mail, quarterly during follow-up, to remind practices to report SAEs. An additional reminder was sent 3 months after the practice had completed their time in the study. As the trial was collecting data on A&E attendances and hospitalisations routinely, only the following events required reporting.
Intervention and control practices
Fatal SAEs: During the trial the practice should report any deaths that are related to a RTI or its complications that occur in patients under 10 years of age to the CHICO research team. This is only applicable to patients who had attended a consultation at the practice in the 90 days prior to the date of death for a RTI-related medical complaint.
Intervention practices only
Related SAEs: During the trial any medical event that meets the standard definition of an SAE (e.g. is life-threatening), and the attending clinician at the practice suspects may be related to the use of the CHICO intervention should be reported to the CHICO research team. An event may be related if it is possibly, probably or definitely due to an adverse impact on the care of the child following using the information provided in the CHICO intervention.
Fidelity data
The fidelity measures focused on intervention exposure and the quality of the intervention delivered (using the process interviews as part of the qualitative investigation).
Sample size
Both sample size calculations assumed 90% power and a conservative two-sided alpha of 0.025 to take account of the two co-primary outcomes. Both sample sizes also assumed an intracluster correlation coefficient of 0.03 (as suggested in discussion with Professor Sandra Eldridge, an expert in cluster randomised trials and complex designs), an estimated coefficient of variation of 0.65 (to take account of differences in cluster size) and an assumption of 750 children aged 0–9 years registered per practice (based on Bristol and Bath CCG data from 2016). Expected differences assumed: (1) a reduction in dispensing rate from 33 prescriptions per 100 registered children aged 0–9 years to 29 (or fewer) prescriptions (i.e. ≥ 10% overall reduction); and (2) a hospitalisation rate that was no more than 2% in the intervention arm, compared with the control arm which is estimated to be 1%. This was based on a non-inferiority margin of 1%; however, the investigators wanted to err on the side of caution and used a two-sided alpha for the sample size calculation. This gave an overall sample size requirement of 310 practices; 155 intervention and 155 control practices. Although not pre-specified, estimates of the intraclass correlation coefficient (ICC) were carried out for the primary outcomes. Mixed-effects linear regression models were used, instead of Poisson models, to allow for the calculation of an ICC using the ‘estat icc’ command in Stata. Only control practices were used to estimate the ICC and CCGs with less than five practices were not included in the calculation as these were unlikely to provide a true estimate of within-cluster variability.
Random allocation
General practitioner practices were randomised on a 1 : 1 basis by the independent Bristol Randomised Trials Collaboration (BRTC) unit using a web-based system, to ensure allocation concealment. All children registered at a GP practice randomised to the intervention arm followed current standard management along with the additional intervention tool. All children registered at a GP practice randomised to the control arm received current standard management. Randomisation was at the practice level. It was stratified by CCG and then further minimised for by practice list size of children (HIGH/LOW) and the baseline dispensing rates of amoxicillin and macrolides antibiotics given to 0–9-year-olds over the previous 12 months (HIGH/LOW), relative to other practices within the CCG. The trial manager was responsible for inputting these variables into the randomisation system.
Blinding
Due to the nature of the intervention delivery, it was not possible to blind the practices to their allocation of either control or intervention group. Administrative staff had access to individual data items, for entry into the database. The trial statistician and trial manager had access to data, by arm, to be able to monitor hospitalisations and report to the Data Monitoring Committee (DMC). No other member of the research team had access to these data until the findings were revealed.
Statistical methods
A full CHICO statistical analysis plan was developed and agreed by the Trial Steering Committee (TSC) and DMC. 46 All primary and secondary analyses were conducted on an intention-to-treat (ITT) basis. A full CHICO statistical analysis plan was developed and agreed by the TSC and DMC chairs 4 months prior to the end of follow-up and 7 months prior to the final analysis commencement. The analysis plan also included details on the proposed health economic and qualitative outcomes.
Summary of baseline data and flow of participants
A Consolidated Standards of Reporting Trials (CONSORT) diagram was used to present the reasons why CCGs and practices were not recruited (due to ineligibility or declining). It was also used to present the number of withdrawals and the number used in the analysis, by arm.
Descriptive statistics were used to summarise characteristics of practices and compare them between groups in a baseline table. Categorical variables were summarised using frequencies and proportions while continuous variables were summarised using medians and interquartile ranges (IQRs; given their skewed nature). Had any differences, between the groups, been in excess of 0.5 standard deviations (SDs)/IQRs or 10% or more they would have been controlled for in sensitivity analyses. However, this was not the case.
Primary outcome analysis
The co-primary outcomes were the rate of amoxicillin and macrolide items (antibiotics) dispensed, for all indications, and the rate of hospitalisations for RTI. The denominator for each of these outcomes was the mean number of children aged 0–9 years registered at the practice over the 12-month follow-up period. In the analysis plan, the team pre-specified that the median list size would be used; however, given the number of practices that merged it seemed more accurate to calculate the mean. All analyses were carried out in Stata 17.0 and the results were described in terms of ‘strength of evidence’ rather than significance. No formal methods of imputation were carried out as the primary outcome was obtainable for over 99% of practices.
Mixed models were used to account for the within and between CCG level variation, incorporating the latter as a random effect. A random-effects Poisson regression model was used to analyse both of these co-primary outcomes. This has the advantage of incorporating person/years follow-up (number of children at a practice) and examining clustering by practice. The dispensing record of the practices in the 12 months prior to randomisation was used as a minimisation variable and thus balance the dispensing records at baseline. This was adjusted for, in the primary analysis of dispensing rates, to resolve any residual difference. While the dispensing outcome will be testing for superiority, the hospitalisation outcome will be testing for non-inferiority where a difference of no more than 1% higher in the intervention arm is reasonable to suggest non-inferiority. Therefore, emphasis was placed on the CI when assessing this outcome.
Secondary outcome analysis
The rate of A&E attendances for RTI was calculated in the same way as the rate of hospitalisations, although it was a test for superiority rather than non-inferiority.
Intervention usage was explored across the seasons and across the 12 months of follow-up. Its impact on dispensing was explored and the correlation was presented using scatter plots and Pearson’s correlation coefficient.
Sensitivity analyses
Dispensing outcome
-
A per-protocol analysis was utilised to exclude non-compliers in the intervention arm. The level of compliance was calculated as the number of times the intervention was used over a 12-month period divided by the list size of 0–9-year-olds at the practice. Practices were considered compliers if this figure was greater than or equal to 0.05. Practices who did not provide intervention usage data were also excluded from this analysis along with any practices that had merged with another CHICO practice during the follow-up period of the trial.
-
When calculating the level of compliance, it became clear that COVID-19 had been the cause of the majority of ‘non-compliers’. Therefore, a post hoc sensitivity analysis was included that looked at the complier average causal effect (CACE). This analysis utilised data from those who did not comply with the intervention and attempted to find a comparable group in the control arm. This was carried out using the instrumental variable analysis approach, using the ‘ivpoisson gmm’ command in Stata.
-
When the influences of COVID-19 were known, after analysis, a post hoc analysis was included to account for COVID-19. In this analysis, follow-up months prior to March 2020 were included and follow-up months on, or after, March 2020 were excluded. The exposure time variable (list size) was scaled up/down to account for the number of months included; for example, for a practice that began follow-up in January 2020 and completed it in December 2020, the first 2 months of data were utilised as: the number of items dispensed during January and February, across the exposure: list size*(2/12).
-
Small elements to the CHICO intervention were adapted during the pilot phase of the study, such as the generation of a frequently asked questions (FAQ) document; therefore, the dispensing primary outcome was repeated, without the pilot data.
-
At the time of writing the analysis plan, the COVID-19 pandemic had been present in the UK for over a year. The team added a sensitivity analysis that would add a COVID-19 time variable, ranging from 1 to 12, to account for the months of follow-up affected by COVID-19 (on or after March 2020); for example, for practices commencing follow-up between January 2020 and December 2020 the COVID-19 variable would be 10. This variable was added as a covariate in a sensitivity analysis.
-
The amoxicillin and macrolide item data are separated by 5-year epochs and the CHICO trial was primarily interested in the 0–4 and 5–9 year age groups. However, the team also collected data on dispensed items with a ‘missing’ age group. Therefore, in a sensitivity analysis, these additional items were included in the 0–9 age group.
-
A post hoc analysis was included to look at amoxicillin prescribing rates only (excluding macrolides).
-
The primary analysis was repeated for 0–4-year-olds only, utilising the 0–4 age epoch data on dispensing and the list size of 0–4-year-olds at the practice.
-
The primary analysis was repeated for 5–9-year-olds only, utilising the 5–9 age epoch data on dispensing and the list size of 5–9-year-olds at the practice.
-
After follow-up had ended, but before final analysis commenced, a post hoc analysis was added to replace the random effects for CCG with a random effect for Primary Care Network (PCN). PCNs are groups of practices working together to focus on local patient care so, in a similar way to a CCG, this sensitivity analysis accounted for the within and between variation across PCNs. PCNs are often made up of practices across different CCGs and do not lie within CCGs; therefore, the analysis was unable to account for both at the same time.
-
During analysis, an additional post hoc sensitivity analysis was included to adjust for the potential confounding effects of a delay between the baseline 12-month collection period and the 12-month follow-up period.
Hospitalisation/accident and emergency outcome
-
For hospitalisation and A&E data, diagnosis codes are sometimes missing. Therefore, data were collected on the number of ‘diagnosis missing’ hospital/A&Es as well as the total number of hospital/A&Es. Using the proportion of lower respiratory tract infection (LRTI) attendances out of those with a diagnosis we could then deduce the proportion of ‘diagnosis missing’ attendances that were likely to be attributable to RTI and include these in a sensitivity analysis.
-
The previous sensitivity analysis was repeated and included all ‘diagnosis missing’ attendances, rather than just a proportion.
-
As with the dispensing outcome, the team added a sensitivity analysis that would add a COVID-19 time variable, ranging from 1 to 12, to account for the months of follow-up affected by COVID-19 (on or after March 2020); for example, for practices commencing follow up between January 2020 and December 2020 the COVID-19 variable would be 10. This variable was added as a covariate in a sensitivity analysis.
-
Complete data were provided for 46 out of 47 CCGs who provided practices for the study. As monthly data were suppressed to avoid identification of patients (where n < 5), CCGs were asked to also provide annual data to avoid inaccurate summations of n < 5. Unfortunately, West Hampshire CCG did not provide these data. Therefore, their annual data may be a misrepresentation of the true figure and their data were removed in a sensitivity analysis.
Subgroup analyses
Subgroup analyses were carried out for dispensing rates (P1). Formal tests of interaction between the potential effect modifiers and treatment pathway were carried out to test whether the treatment effect differed between the different subgroups. These effect modifiers are listed in Subgroup analyses (potential effect modifiers for P1). These variables were dichotomised to aid presentation and interpretation, taking the median value where the variable was continuous. Models with and without an interaction term were compared using a likelihood ratio test and this p-value was presented.
Internal pilot study
An internal pilot phase lasting 3 months and using 4 CCGs to recruit 60 practices was planned and conducted to help establish the best methods for recruiting and communicating with practices before widening to the remaining CCGs.
Stop/go assessment
There were four stop/go criteria (see Table 2).
| Criteria (all must be met, failure of one or more triggers action) | Proposed action |
|---|---|
| ≥ 80% or 48+ practices recruited | Continue as planned |
| ≥ 80% or 48+ practices naming a champion | |
| ≥ 80% of GPs/nurses using the intervention | |
| ≥ 90% or 54+ practices we can obtain antibiotic dispensing data | |
| 70–79% or 42–47 practices recruited | TSC and HTA discuss problems with the TMG and implement remedies |
| 70–79% or 42–47 practices naming a champion | |
| 70–79% of GPs/nurses using the intervention | |
| 80–89% or 48–53 practices we can obtain antibiotic dispensing data | |
| < 70% or < 42 practices recruiting | Discuss plans with TSC and NIHR HTA. Consider further pilot or stopping trial |
| < 70% or < 42 practices naming a champion | |
| < 70% of GPs/nurses using the intervention | |
| < 80% or < 48 practices we can obtain antibiotic dispensing data |
Our progression criteria at the pilot stage were based on:
-
the percentage of practices recruited against the initial practice target of 60;
-
the percentage of GP practices with a named practice champion;
-
the percentage of intervention GPs/nurses using the intervention at least once;
-
the percentage of antibiotic dispensing data we can obtain for each practice.
Internal pilot areas of assessment
In addition to stop/go criteria, the internal pilot was used to assess areas where we could improve recruitment, communication with practice champions and use of the intervention. This included:
-
the number of eligible practices within the 20 CCGs already approached and whether at this stage we needed to approach further CCGs to increase the number of practices in the study;
-
the recruitment rate of eligible practices within the four CCGs in the pilot phase, again as an indicator of whether we need to approach further CCGs;
-
the recruitment of practice champions, a focus on their role and how we maximise strategies to encourage use of the intervention;
-
the efficiency of embedding the intervention in practice systems and resolution of any barriers or delays;
-
the number of times the intervention is used between practices and over time;
-
the timeliness of the primary outcome data and consistency of format between CCGs;
-
the timeliness of the secondary outcome data and consistency of format between CCGs.
Trial oversight
The University of Bristol was the sponsor for this trial and was responsible for overall oversight of the trial. The TSC provided independent supervision of the trial and monitored trial progress. The TSC had an independent Chair, GP and clinical academic Hazel Everitt (Associate Professor at the University of Southampton) and four other independent members. These are an independent statistician (Dr Beth Stuart from the University of Southampton), a second clinician (Professor Gail Hayward from the University of Oxford) and two patient and public involvement (PPI) representatives. Meetings were held at least annually. The DMC monitored patient safety and trial data efficacy and consisted of an independent chair (Jill Mollison from the University of Oxford), two other independent members (Oliver van Hecke and Sena Jawad) and the trial statistician. The three independent members were nominated in 2018 prior to any data collection activity according to NIHR research governance guidance. The DMC received and reviewed reports on the data accruing to this trial and made recommendations on the conduct of the trial to the TSC. In the final months of follow-up, the chair stepped down from their position and another independent member (Oliver van Hecke) took up the position. Given that the CHICO trial was nearing completion, a new independent member was not sought, this approach was agreed by the funder.
All SAEs were recorded and notified as appropriate to the relevant authorities.
Safety
The trial is a low-risk study (risks to participants are no higher than that of standard medical care); thus, SAEs were only reported if fatal or serious AND potentially related to trial participation.
As one of the outcomes for the trial is hospitalisation, it was expected some participants would be admitted to hospital (e.g. due to a deterioration of their underlying illness). Hospitalisation due to RTI is an expected SAE and would not be subject to expedited reporting.
For SAE reporting, safety forms were completed for each event by the practice healthcare practitioner and subsequently reviewed by the study clinicians. All SAEs were reported by arm to the DMC prior to scheduled meetings. If there were any safety concerns, the DMC would report to the study Chief Investigator and TSC for further action.
Monitoring for SAEs took place for the duration of the 12-month data collection phase and a further 90 days after this period to allow for the collection of information about any fatalities up to 90 days after consultation.
All practices were regularly prompted by the CHICO study team during their participation in the trial to remind them to report any SAEs and to confirm if zero SAEs arose during the reporting period.
Hospitalisation and A&E attendance data were reported each month for participating practices by their local CCG organisation.
Patient and public involvement
Patient and public involvement was represented by a Parent Advisory Group (PAG). The CHICO intervention was developed in collaboration with the PAG during the final year of the CHICO feasibility trial (Workstream 4 of the Applied Health TARGET programme).
We met with the parent group several times during the development of the intervention and design of the study. They felt that it was important to conduct a national study and to use a whole practice intervention. They also thought it would be acceptable to conduct the future study without consenting individual patients, as this took time within GP consultations, and that using a prediction tool on the computer during consultation would be fine and would provide reassurance. They strongly endorsed and encouraged us to use the well-designed parent leaflet as it provided very useful information.
Patient and public involvement was maintained throughout the trial through a group of three parents, two of whom (in rotation) attended and contributed to all the TSC meetings including the final results reveal meeting.
Clinical and Practitioner Advisory Group
As the intervention focus was on GP practices and not on recruiting individual patients, we made extensive contact with the Clinical and Practitioner Advisory Group (CPAG) during the trial period, which was important for the study. The CPAG was made up of clinical staff from seven Bristol practices, including GPs, nurse practitioners with paediatric expertise, practice pharmacists and a practice paramedic. We held several meetings with the group in May 2018 and subsequently communicated by e-mail. Advice was sought on the use of hard or soft pop-ups and trigger codes in a consultation, format of the template and the personalised letter. We discussed potential barriers and how to overcome them and any staff training needs to use CHICO. We also ran some ‘think aloud’ sessions with several GPs to see the intervention in action in EMIS® and gather their thoughts about any problems or changes needed. Input on the draft design of follow-up questionnaires was also requested from the CPAG, including how easy it would be for practices to answer the questions.
Chapter 3 Clinical results
Internal pilot review
A TSC took place on 25 February 2019 to assess the stop/go criteria for the RCT pilot, based on the practices recruited between October and December 2018 (see Table 3).
| Criteria (all must be met, failure of one or more triggers action) | Met | Detail |
|---|---|---|
| 1. ≥ 80% or 48+ practices recruited from 7 CCGs | ☑ | By 31 December, 48 practices had been randomised. |
| 2. ≥ 80% or 48+ practices naming a champion | ☑ | All 48 practices recruited had a named champion. |
| 3. ≥ 80% of GPs/nurses using the interventiona | ☑ | The team received responses from 18/23 intervention practices. Of these, 16 met the criteria. |
| 4. ≥ 90% or 54+ practices we can obtain antibiotic dispensing data | ☑ | 100% of baseline dispensing data were available when the criteria were assessed. |
In addition to the stop/go criteria above, other assessment areas listed in Internal Pilot areas of assessment were used to improve recruitment and resolve any barriers implementing the intervention. Criteria (1) suggested more than 4 CCGs would be required to recruit at least 48 practices; thus, a further 3 CCGs were recruited to the pilot.
The TSC was supportive of the progress that had been made at that time and the achievement of the stop/go criteria. The CHICO trial continued to recruit practices from the pilot CCGs and went on to recruit additional CCGs and practices after the pilot ended.
Recruitment
The CHICO trial recruited CCGs, and randomised practices within them, between October 2018 and October 2020. There was a 3-month pause in recruitment between March 2020 and June 2020, at the onset of the COVID-19 pandemic, while the study team, TSC and funders re-assessed the feasibility and safety of the intervention during the pandemic. By mid-June 2020, there seemed to be an appetite for restarting recruitment again with perceived capacity in practices and new expressions of interest from some regions such as the South-West. A budgeting exercise suggested an un-costed 4-month extension could support the study to reach the target recruitment figure by the end of September 2020. Recruitment restarted again on 16th June, following a non-substantial amendment (following the COVID-related halt) being approved by the sponsor. In total, 52 CCGs agreed to take part in the study but only 47 of the CCGs (see Figure 3) provided the 294 practices (see Figure 4). Practices were randomised between October 2018 and October 2020, with the 12-month follow-up period continuing until September 2021.
FIGURE 3.
Children with cough recruitment of CCGs.
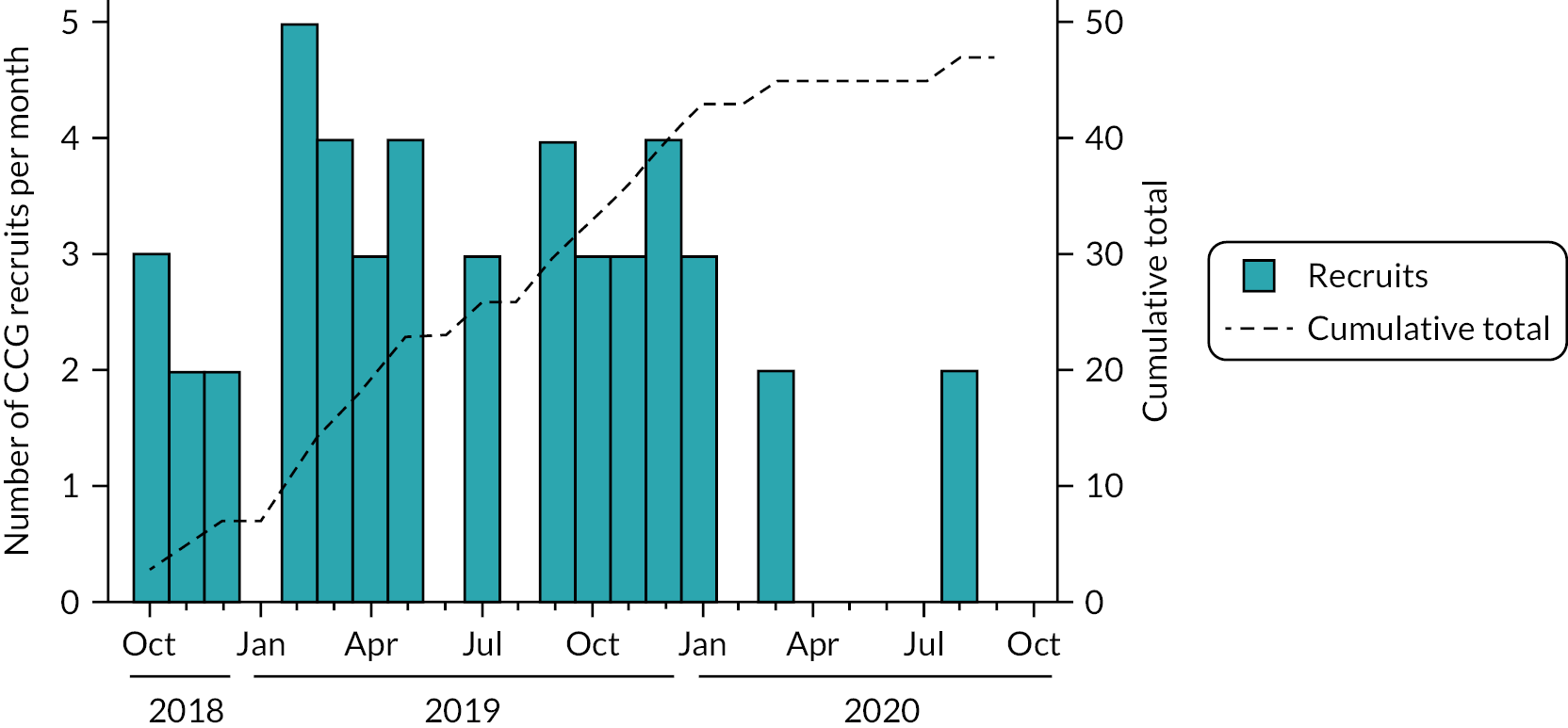
FIGURE 4.
Children with cough recruitment of practices.

Trial flow
In total, 125 CCGs were assessed for eligibility (see Figure 5). For the CHICO intervention to work, practices had to be using EMIS®.
FIGURE 5.
The CHICO trial CONSORT flowchart. EMIS®, Egton Medical Information Systems® (electronic health records); qnr, questionnaire; a One of the practices randomised is made up of two practices who work closely and were due to merge; b Reasons include lack of staff, lack of resources or lack of consultations for CHICO; c Two practices within the control arm merged, therefore their outcome data was compiled into one practice.
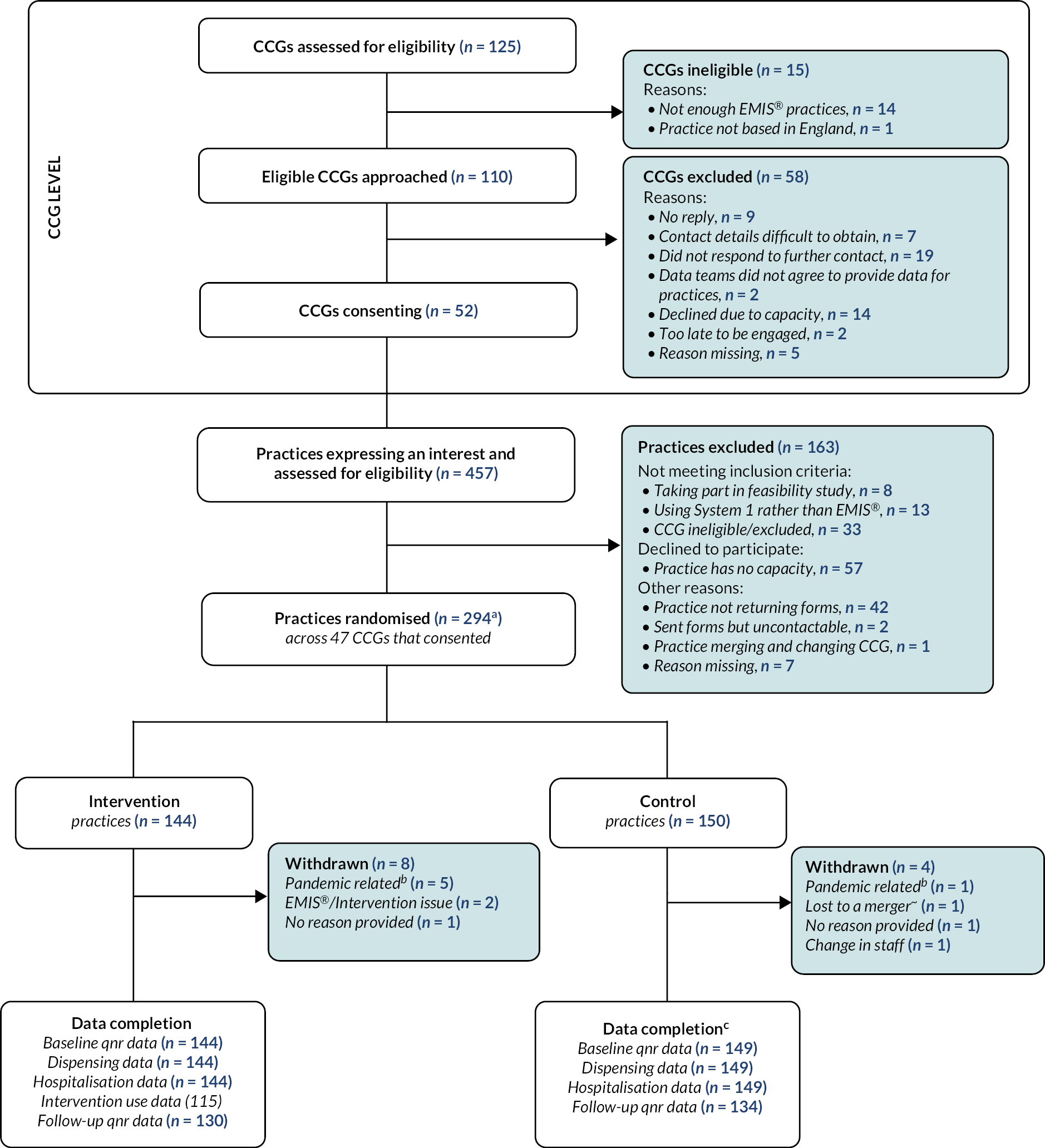
The majority of CCGs found to be ineligible (n = 14) did not have enough practices using EMIS®. Of the 110 eligible CCGs that were then approached, 52 (47%) consented to take part. Practices from within those CCGs were then approached and asked for expressions of interest. An additional 33 practices expressed interest but were not recruited, as their CCG had not consented to take part. The majority of practices were excluded due to having no capacity to take part (n = 57) or because they stopped responding to communications (n = 42). By the end of recruitment, the CHICO study had randomised 294 practices, 144 to the intervention arm and 150 to the control arm.
Over the course of the trial, there were 12 practice withdrawals, 8 in the intervention arm of the trial and 4 in the control arm (see Table 4). The majority of withdrawals in the intervention arm were listed as ‘pandemic related’, which included lack of time/resources to take part in the trial but also a lack of children consulting with a cough. Other reasons include issues with installing the intervention, a complete change in staff at the practice and a merger (two practices in the control arm merged together to form one larger practice).
| Intervention, N (%) | Control, N (%) | p-valuea | |
|---|---|---|---|
| CCG withdrawals | 0 (0) | 0 (0) | – |
| Practice withdrawals | 8 (6) | 4 (3) | 0.211 |
| Pandemic related | 5 (3) | 1 (1) | 0.089 |
| Intervention/EMIS ® issues | 2 (1) | 0 (0) | 0.148 |
| No reason provided | 1 (1) | 1 (1) | 0.977 |
| Complete change of staff | 0 (0) | 1 (1) | 0.326 |
| Merged with another practice | 0 (0) | 1 (1) | 0.326 |
| Loss to follow-upb | 14 (10) | 16 (11) | 0.789 |
Of the 144 practices randomised to the intervention arm, 100% provided baseline data and routine data on dispensing rates and hospitalisations could be obtained for 100% of practices. Intervention usage data were complete (12 months of data) for 115 (80%) practices and partially complete for 16 (11%) of practices. The other 13 (9%) of practices provided no intervention usage data. The follow-up questionnaire was then completed by 130 (90%). Similar proportions of follow-up data were available for the control arm (89%), with one missing due to the Gloucestershire practice merger.
Randomisation
Randomisation of practices was stratified by CCG and minimised for list size and previous dispensing rate. Table 5 shows that the stratification across CCGs allowed for a relatively equal share of intervention and control practices across each CCG.
| Total | Intervention | Control | |
|---|---|---|---|
| N | n (%) | n (%) | |
| Bristol, North Somerset and South Gloucestershire | 39 | 20 (51) | 19 (49) |
| Birmingham and Solihull | 9 | 5 (56) | 4 (44) |
| Brenta | 17 | 7 (41) | 10 (59) |
| Buckinghamshire | 2 | 0 (0) | 2 (100) |
| Cambridge and Peterborough | 2 | 1 (50) | 1 (50) |
| Cannock Chase | 1 | 0 (0) | 1 (100) |
| Canterbury and Coastalb | 2 | 1 (50) | 1 (50) |
| Devon | 3 | 1 (33) | 2 (67) |
| Dudleyc | 13 | 5 (38) | 8 (62) |
| East Berkshired | 5 | 2 (40) | 3 (60) |
| East Lancashire | 7 | 4 (57) | 3 (43) |
| East Staffordshire | 4 | 3 (75) | 1 (25) |
| Eastbournee | 1 | 1 (100) | 0 (0) |
| Gloucestershire | 5 | 3 (60) | 2 (40) |
| Guildford and Waverlyf | 1 | 0 (0) | 1 (100) |
| Hastings and Rothere | 5 | 2 (40) | 3 (60) |
| Herts Valley | 7 | 5 (71) | 2 (29) |
| Kernow | 4 | 2 (50) | 2 (50) |
| Leeds | 1 | 0 (0) | 1 (100) |
| Liverpool | 15 | 7 (47) | 8 (53) |
| Manchester | 12 | 7 (58) | 5 (42) |
| Medwayb | 6 | 4 (67) | 2 (33) |
| Morecambe Bay | 14 | 6 (43) | 8 (57) |
| Newcastle and Gateshead | 1 | 1 (100) | 0 (0) |
| North East Essex | 3 | 2 (67) | 1 (33) |
| North Hampshireg | 1 | 1 (100) | 0 (0) |
| North Staffordshire | 2 | 1 (50) | 1 (50) |
| North West Surreyf | 4 | 1 (25) | 3 (75) |
| Nottinghamh | 3 | 1 (33) | 2 (67) |
| Oxfordshire | 15 | 8 (53) | 7 (47) |
| Salford | 1 | 0 (0) | 1 (100) |
| Sandwell and West Birminghamc | 2 | 2 (100) | 0 (0) |
| Shropshirei | 4 | 3 (75) | 1 (25) |
| Somerset | 27 | 14 (52) | 13 (48) |
| South East Hampshireg | 4 | 2 (50) | 2 (50) |
| South East Staffordshire and Seisdon | 2 | 1 (50) | 1 (50) |
| South Cheshirej | 3 | 1 (33) | 2 (67) |
| Southwarkk | 6 | 3 (50) | 3 (50) |
| Staffordshire and Surrounds | 3 | 2 (67) | 1 (33) |
| Stoke on Trent | 1 | 1 (100) | 0 (0) |
| Sunderland | 8 | 4 (50) | 4 (50) |
| Thanetb | 1 | 1 (100) | 0 (0) |
| Vale of York | 2 | 0 (0) | 2 (100) |
| Walsallc | 9 | 5 (56) | 4 (44) |
| Wandsworthl | 2 | 1 (50) | 1 (50) |
| West Hampshireg | 9 | 5 (56) | 4 (44) |
| West Kentb | 6 | 4 (67) | 2 (33) |
However, some CCGs were only able to recruit one or two practices, which hindered this process. During the 2-year recruitment period, almost half of the CCGs underwent a merger, either with other CCGs in the study or with CCGs outside of the study. Details of these mergers can be found in the footnote of Table 5. There were five errors when randomising practices that will have affected the minimisation process, which are summarised in Table 6.
| Error | Practices affected | Potential issue |
|---|---|---|
| Randomised three times (due to false error message). | 1 | The randomisation system will have counted the same practice three times, therefore this will have affected the minimisation. |
| Typo when entering the CCG number. | 3 | The stratification/minimisation process was not successful in these practices as they were entered as a new CCG. |
| Practice entered as having a ‘High’ list size, when in fact it had a ‘Low’ list size. | 1 | This will have affected the minimisation for this CCG. |
Baseline data
Baseline comparisons for the CHICO study are given in Table 7. The team pre-specified in the analysis plan46 that any baseline characteristics that differed by more than 10% (categorical variables), half a SD or half an IQR (continuous variables) would be investigated. None of the baseline characteristics met these criteria so no investigations were carried out. The two arms were well balanced with respect to baseline characteristics, suggesting that stratification by CCG and minimisation had been successful. Of the practices randomised, 62% had a high list size relative to other practices within their CCG suggesting that smaller practices were less likely to be recruited. In a similar way, 42% of the practices randomised had a high baseline dispensing rate, relative to other practices within their CCG suggesting that high prescribers were less likely to be recruited.
The median number of GPs was 6 per arm and this was well recorded (290/294). However, there were varying completion rates for the numbers of each type of staff, at each practice, therefore these figures may not be a true representation of practice staff. The Index of Multiple Deprivation was used to assess whether the CHICO study was recruiting practices from high or low levels of deprivation is based on data available from 2019. 47 In general, there was a larger proportion of practices in quintiles 1 and 2 (higher levels of deprivation) than quintiles 4 and 5 (lower levels of deprivation) reflecting an excess of practices located in urban areas and a similar distribution to the geographical location of all GP practices in England.
The Income Deprivation Affecting Children Index (IDACI)48 measures the proportion of all children aged 0–15 living in income-deprived families and this was 15% in both arms. There were 43 practices that were randomised on, or after, March 2020. The other 251 were randomised before March 2020. The proportion of RTIs consulted over the phone changed immensely; going from ~25% mostly/always consulting over the phone pre-COVID-19 to ~65% during the pandemic.
Serious adverse events
There were four events that were reported during the CHICO study (see Table 8). There was one hospitalisation in the intervention arm. There were three deaths reported, two of which were in the intervention arm; all unrelated to the intervention.
| SAE | Arm of the study | SAE description | Outcome |
|---|---|---|---|
| SAE-001 | Intervention | Patient admitted to hospital for RTI | Based on information available the event was not considered related to the intervention |
| SAE-002 | Control | Fatality – no details of cause but patient history suggests unrelated to RTI | Not considered related to the intervention |
| SAE-003 | Intervention | Fatality (unrelated to RTI, intervention not used) | Not considered related to the intervention |
| SAE-004 | Intervention | Fatality (unknown whether a RTI consultation took place within 90 days of the fatality) | Not considered related to the intervention |
Intervention usage
Complete 12-month intervention usage data were available for 115/144 (80%) practices. Data from these practices allowed us to look at intervention usage over time, both in terms of calendar year (January–December) and follow-up time point (months 1–12). Twelve practices (8%) provided between 6 and 11 months of intervention usage data and four (2%) practices provided < 6 months of data. The remaining 13 (9%) intervention practices did not provide any intervention usage data. Across the 121 practices that provided at least 1 month of intervention usage data, we recorded a total of 11,944 uses of the intervention. The median usage across the practices with 12 months of data (n = 115) was 70 uses (IQR 9–142). Twenty practices (17%) recorded zero usage over the 12-month period. All of these 20 practices started follow-up after May 2019 and 13 (65%) began follow-up from March 2020.
Users of the intervention
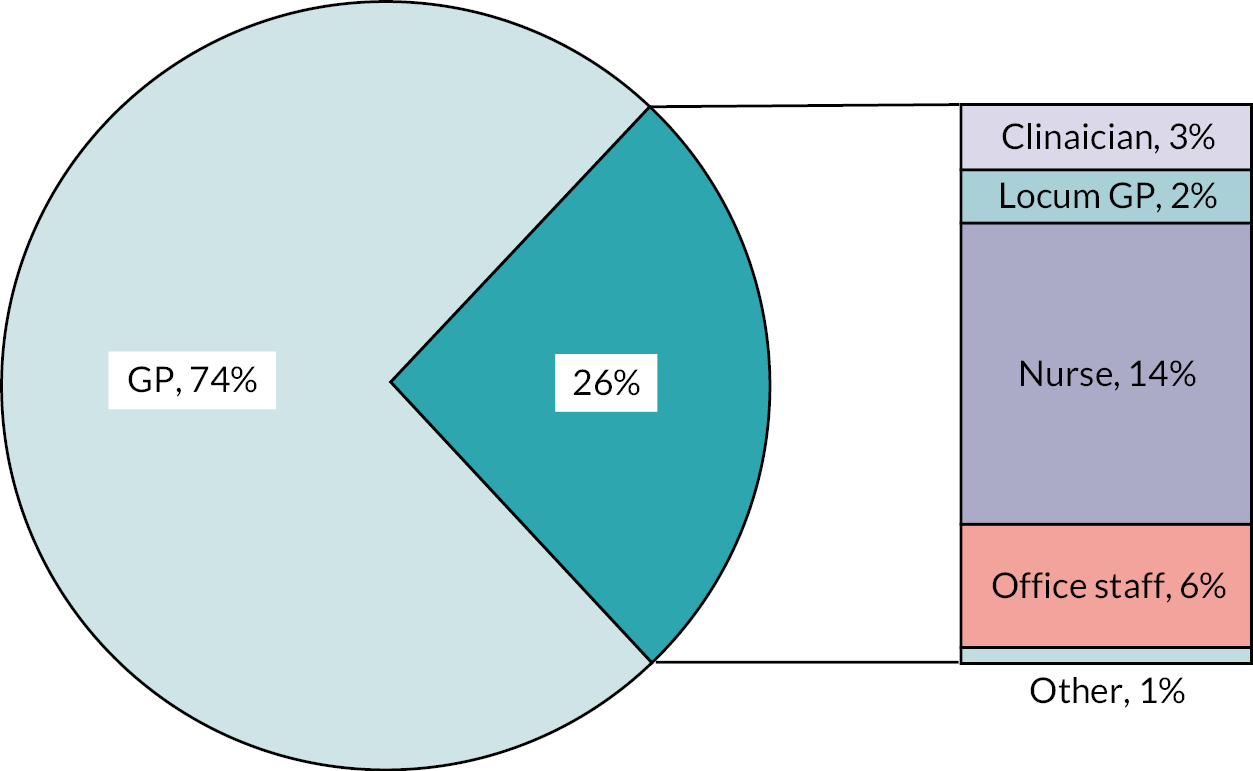
Using the EMIS® user mnemonic (a unique username for each healthcare practitioner using system), the CHICO template recorded 1399 individual users of the intervention. The median number of users per practice (n = 131) was 9 (IQR 3–16). This figure remained the same when looking at practices providing 12 months of data (n = 115); median 9 (IQR 3–17). Figure 6 shows the job roles of the intervention users. Of the 1399 users identified, the job role type was available for 1341 of them. Of these, 994 (74%) were GPs, 187 (14%) were nurses, 77 (6%) were office staff, 40 (3%) were clinicians, 34 (3%) were locum GPs and 7 (1%) were pharmacists. There was also one consultant and one phlebotomist.
FIGURE 6.
Job roles of those using the intervention.
There were four individual users who used the intervention more than 100 times; two GPs, one nurse and one clinician. Overall, the GPs and nurses used the intervention the most, with a median of five uses per GP/nurse over the 12-month period (see Figure 7). Office staff (e.g. analysts and medical secretaries) used it the least, averaging one use per member of staff.
FIGURE 7.
Uses of the intervention, per job role.

Usage of the intervention over the 12 months of participation in the study
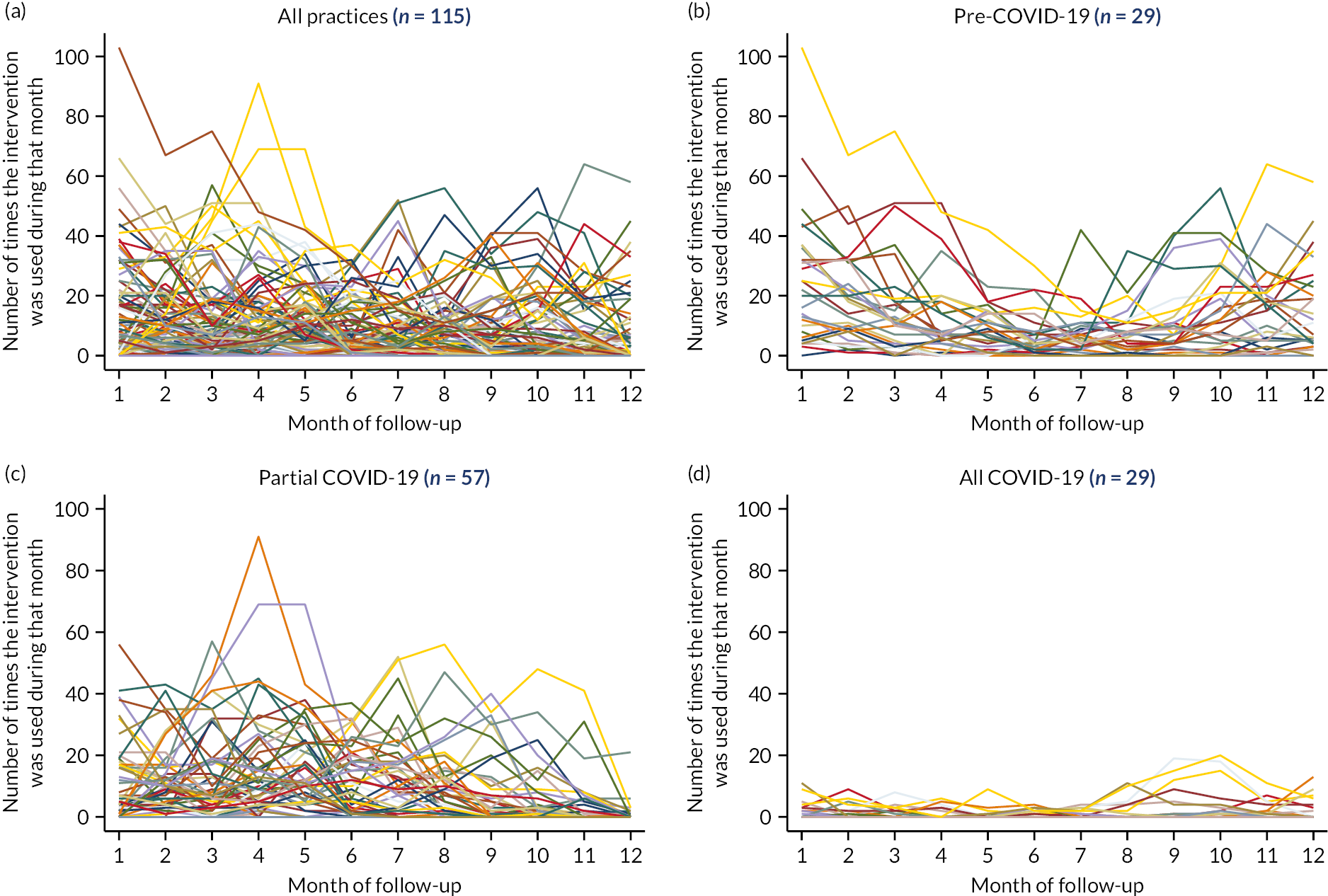
Practices with 12 months of intervention usage data available allowed for a detailed inspection of trends over months 1–12 of follow-up. Panel A of Figure 8 shows usage over the 12-month period for all 115 practices. Due to COVID-19, it is difficult to determine what a typical pattern of intervention usage may look like. Table 9 provides the median use per month based on the individual practices presented in Figure 8. Panel B of Figure 8 and row 2 of Table 9 show the data for the 29 practices that completed follow-up prior to March 2020 and were, therefore, unaffected by COVID-19 lockdowns. Generally, the majority of use was at the start of follow-up, in months 1 (median = 22) and 2 (median = 18). However, for these 29 practices who completed follow-up prior to the pandemic, month 1 had to be between November 2018 and March 2019. Therefore, the increased usage in months 1 and 2 may coincide with the seasons, rather than indicate an increased usage at the start of follow-up. Panel C shows the practices that had 1–11 months of follow-up data on, or after, March 2020 (n = 57). Given the variety of months affected by COVID-19, it is difficult to spot trends or patterns in the intervention usage data.
FIGURE 8.
Intervention usage, by follow-up month for (a) all practices, (b) pre-COVID-19 (completed before March 2020), (c) partial COVID-19 (≥ 1 month from March 2020), (d) all COVID-19 (complete follow-up from March 2020).
| Month of follow-up | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 | M12 | |
| All practices (n = 115) | 4 | 6 | 4 | 5 | 5 | 2 | 2 | 2 | 0 | 1 | 1 | 0 |
| Pre-COVID-19a (n = 29) | 22 | 18 | 11 | 7 | 8 | 4 | 6 | 6 | 7 | 11 | 10 | 7 |
| Partial COVID-19b (n = 57) | 4 | 9 | 7 | 9 | 10 | 6 | 5 | 2 | 0 | 0 | 0 | 0 |
| All COVID-19c (n = 29) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
For practices with complete follow-up on or after March 2020 (n = 29), the median usage was 0 for all months and panel D of Figure 8 shows how the maximum usage recorded over that period was 20.
Usage of the intervention across the seasons
If we combine all practices and look at the data across calendar months, we can see the seasonal effects on intervention usage. In Figure 9, we can see the seasonal variation between October 2018 and February 2020, with increased usage during the winter months and fewer uses during the summer months. This suggests that usage did correlate with the number of children presenting with RTIs. However, from March 2020 the effects of COVID-19 lockdowns are clear, with very little usage of the intervention. There is a small increase in May, June and July 2021, when restrictions were relaxed and RTIs were more prevalent in the population.
FIGURE 9.
Intervention usage over time, with each practice (n = 115) contributing 12 months of data.

Looking at usage per calendar month, with all years combined, the seasonal variation is clear (see Figure 10 and Table 10). Looking specifically at practices that completed follow-up before COVID-19 (n = 29), median usage was highest in November (19), December (14) and January (15). This was also evident in practices with at least 1 month on, or after, March 2020 (n = 57). For practices with all 12 months of follow-up data on, or after March 2020 (n = 29), median usage bucked the usual seasonal trends, with summer months providing the highest number of uses.
FIGURE 10.
Intervention usage, by calendar month for (a) all practices, (b) pre-COVID-19 (completed before March 2020), (c) partial COVID-19 (≥ 1 month from March 2020), (d) all COVID-19 (complete follow-up from March 2020).

| Month of follow-up | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January | Febuary | March | April | May | June | July | August | September | October | November | December | |
| All practices (n = 115) | 5 | 3 | 2 | 0 | 0 | 0 | 1 | 1 | 4 | 5 | 10 | 10 |
| Pre-COVID-19a (n = 29) | 15 | 11 | 12 | 9 | 7 | 4 | 5 | 3 | 7 | 12 | 19 | 14 |
| Partial COVID-19b (n = 57) | 7 | 6 | 3 | 0 | 0 | 0 | 0 | 1 | 5 | 8 | 18 | 19 |
| All COVID-19c (n = 29) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Compliance with the intervention
The definition of compliance was pre-defined in the statistical analysis plan as the number of uses of the intervention divided by the list size of 0–9-year-olds. Figure 11 shows the relationship between these two variables. For the per-protocol analysis, the CHICO trial team pre-specified that a practice would be compliant if the intervention was used in at least 5% of children (0.05*list size). However, this definition may be impacted by COVID-19. The green line indicates the boundary line for practices considered compliant (dots on or above the line).
FIGURE 11.
Assessing compliance with the intervention.
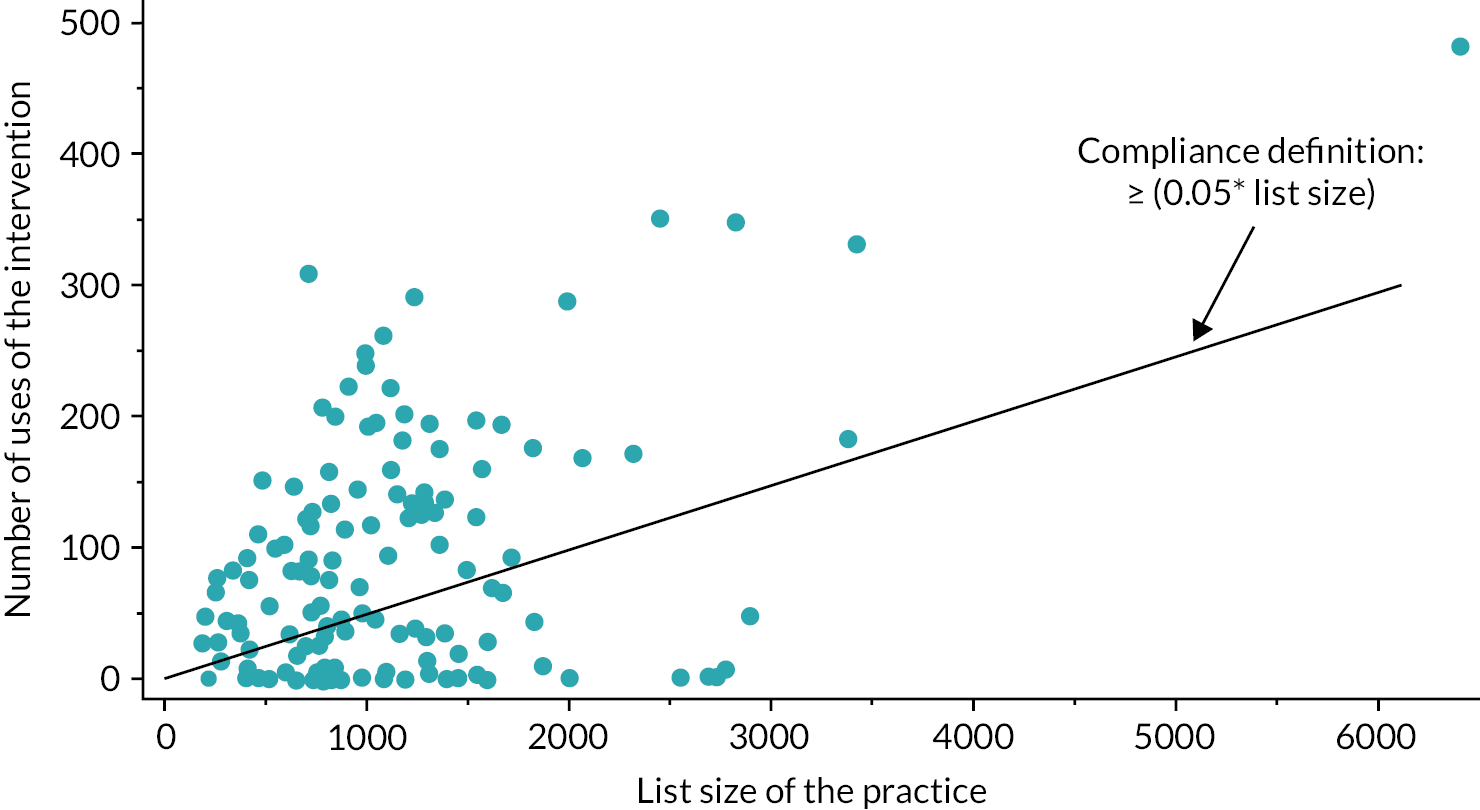
We had planned to look at the proportion of prescribing staff that were using the intervention at least once. As described previously (see Baseline data), the reporting of the number of staff at each practice was quite poor.
Plotting the number of staff (x-axis) versus the number of individual users of the intervention (y-axis), it is clear that, in many cases, the number of users exceeds the number of prescribing staff at the practice (see Figure 12). Given the underreporting, the ‘number of staff’ figures were not utilised when looking at compliance with the intervention.
FIGURE 12.
Number of individual users compared to the number of prescribing staff.

Relationship between compliance and change in dispensing
Figure 13 looks at the relationship between the level of compliance (annual intervention usage/list size) and the change in dispensing (follow-up rate – baseline rate). Panel A shows the relationship for all intervention practices with compliance data (n = 129).
FIGURE 13.
Correlation between compliance and change in dispensing in (a) all intervention practices with compliance data and (b) restricted to those with follow-up prior to COVID-19.
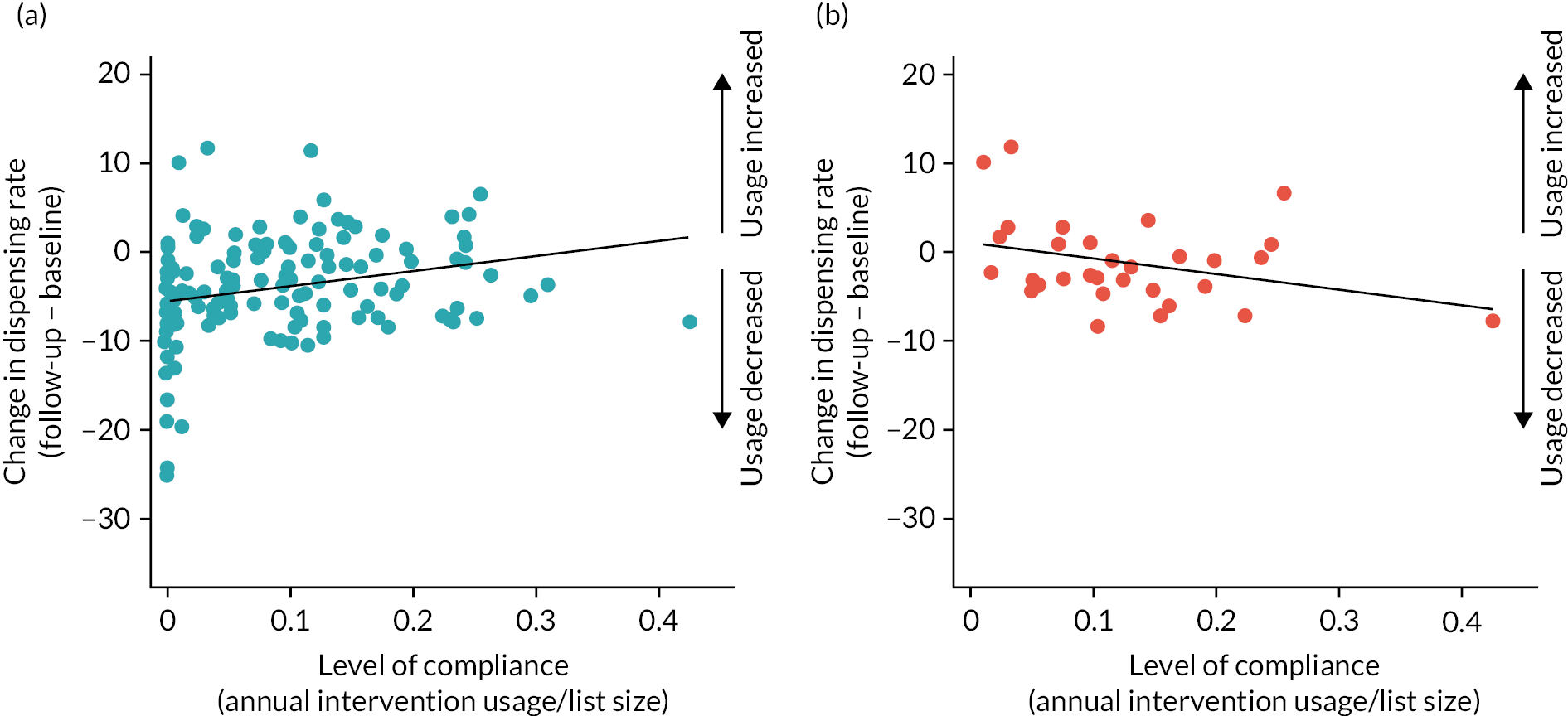
The line of best fit suggested that increased compliance led to increased antibiotic usage; Pearson’s correlation coefficient 0.241, p = 0.006. However, as described in Usage of the intervention across the seasons, practices that had follow-up during COVID-19 often reported 0 compliance, yet had a decrease in dispensing due to lockdowns. Therefore, panel B is perhaps a better representation of the relationship between intervention usage and change in dispensing rate. In this panel, the focus is on those practices that completed follow-up prior to COVID-19, n = 31. The relationship here is in the opposite direction, with increased intervention usage leading to a decrease in dispensing; Pearson’s correlation coefficient −0.326, p = 0.074. After removing the outlier (compliance = 0.42), the correlation observed was weakened to −0.222, p = 0.238.
Dispensing: primary outcome, sensitivity and subgroup analyses
Impact of COVID-19 on dispensing
Figure 14 combines all baseline and follow-up data for all practices, in both arms, and shows the seasonal effects on dispensing. Between October 2017 and February 2020, the dispensing rates reached highs of over 0.35 in the winter and approximately 0.10 in the summer. The effects of COVID-19, and its associated lockdowns, on dispensing are clear from March–July 2020 when it dropped to 0.05. When restrictions were relaxed in late spring/summer of 2021, there was an unseasonably high dispensing rate. Focusing specifically on the control practices (reflecting usual care) comparing the 12 months (March 2019–February 2020) prior to the pandemic to the first 12 months (March 2020–February 2021) during the pandemic (taking account of seasonal variability), the dispensing rates halved from 20.55 items per 100 children (95% CI 19.64 to 21.46) to 9.84 items per 100 children (95% CI 9.18 to 10.50). A similar reduction was observed in the intervention arm.
FIGURE 14.
Mean dispensing rate (95% CI) during the CHICO baseline and follow-up periods. Each practice contributed 24 months of follow-up data.
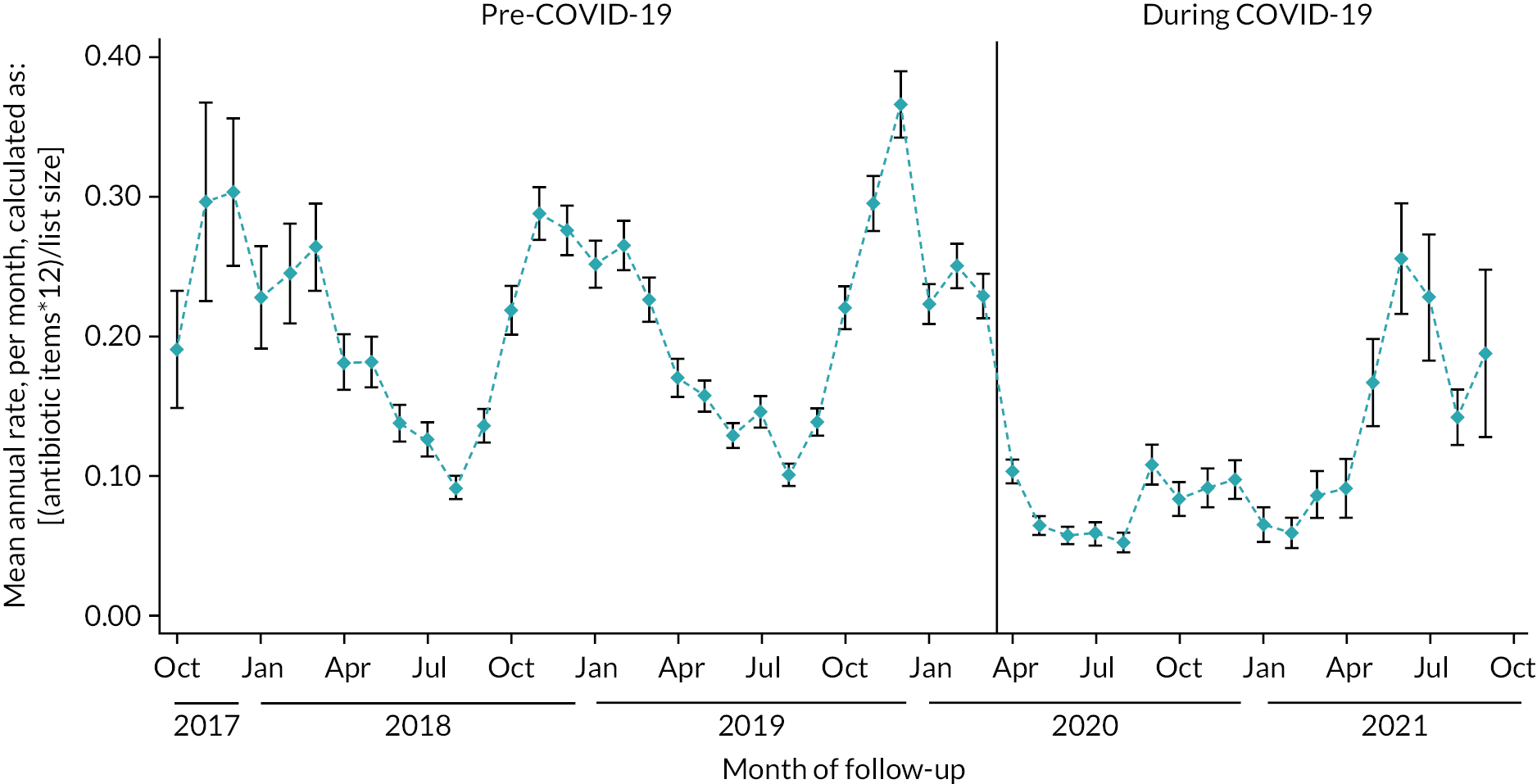
In the sample size calculation, for the dispensing rate outcome, the team had hypothesised a 4% absolute rate reduction, from 0.33 to 0.29 (equivalent to 4 prescriptions per 100 patients, per year). Given the low dispensing rates during COVID-19, it would have been difficult to distinguish any differences between the arms of the trial between March 2020 and April 2021.
Dispensing rate comparison
Figure 15 displays the follow-up data separated by arm. There were fewer practices at either end of the follow-up that could contribute data for these time points; therefore, the CIs are larger at either end. However, even with this uncertainty, there did not appear to be a difference over time between the arms.
FIGURE 15.
Mean dispensing rate (95% CI) during the CHICO follow-up period (October 2018–October 2021), by arm. Each practice contributed 12 months of follow-up data.
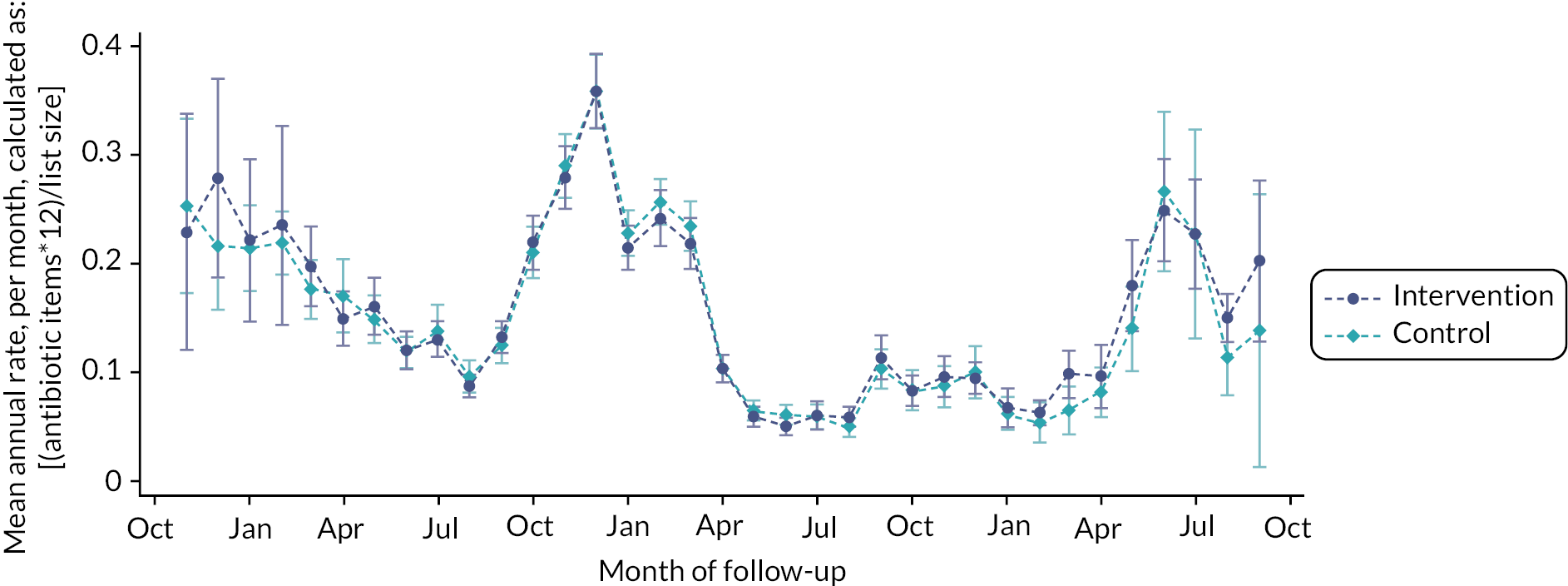
There were 144 practices in the intervention arm with data on dispensing and 149 in the control arm (1 lost due to a merger). Using the list size of 0–9-year-olds as the denominator and adding random effects at the CCG level, the incidence rate, of amoxicillin/macrolide dispensing, was 0.155 and 0.154 in the intervention and control arms, respectively (see Table 11). After adjustment for the baseline dispensing rate, this gave an incidence rate ratio (IRR) of 1.011 (0.992, 1.029). There was no evidence of a difference between the arms for the dispensing rate outcome.
| Intervention rate (95% CI) | Control rate (95% CI) | IRR (95% CI)a | p-valuesa | |
|---|---|---|---|---|
| Dispensing outcome | 0.155 (0.135 to 0.179) | 0.154 (0.130 to 0.182) | 1.011 (0.992 to 1.029) | 0.253 |
Sensitivity analyses for the dispensing primary outcome
There were six pre-specified sensitivity analyses for the dispensing rate primary outcome (see Table 12) and five post hoc analyses that were added during follow-up data collection or after presenting the results to the team. Firstly, a pre-specified per-protocol analysis was carried out that excluded ‘non-compliant’ practices from the intervention arm. Practices were considered non-compliant if the number of uses of the intervention divided by the list size of 0–9-year-olds was less than 5% (n = 50). Intervention practices were also excluded where the intervention usage was unknown (n = 15) or where an intervention practice merged with another practice that had previously taken part in the control arm (n = 1). In total, there were 66 exclusions from this analysis leaving 78 intervention practices to compare with the 149 in the control arm.
| N (I : C) | Intervention rate (95% CI) | Control rate (95% CI) | IRR (95% CI)a | p-valuesa | |
|---|---|---|---|---|---|
| Primary analysis | 144 : 149 | 0.155 (0.135 to 0.179) | 0.154 (0.130 to 0.182) | 1.011 (0.992 to 1.029) | 0.258 |
| Per protocol b | 78 : 149 | 0.166 (0.141 to 0.196) | 0.154 (0.130 to 0.182) | 1.052 (1.029 to 1.076) | < 0.001 |
| Post hoc 1: CACE analysis c | 144 : 149 | 1.056 (0.890 to 1.254) | 0.530 | ||
| COVID-19 month indicator d | 144 : 149 | 1.002 (0.984 to 1.021) | 0.790 | ||
| Post hoc 2: Pre-COVID months e | 105 : 121 | 0.192 (0.163 to 0.227) | 0.204 (0.171 to 0.244) | 0.967 (0.946 to 0.989) | 0.003 |
| Excluding pilot practices f | 117 : 128 | 0.155 (0.134 to 0.179) | 0.154 (0.129 to 0.183) | 1.026 (1.006 to 1.048) | 0.012 |
| Including ‘age unknown’ g | 144 : 149 | 0.187 (0.164 to 0.213) | 0.187 (0.160 to 0.219) | 1.002 (0.986 to 1.018) | 0.835 |
| Post hoc 2: Amoxicillin h | 144 : 149 | 0.120 (0.105 to 0.138) | 0.121 (0.103 to 0.143) | 0.994 (0.974 to 1.014) | 0.555 |
| 0–4-year-olds only | 144 : 149 | 0.224 (0.195 to 0.258) | 0.222 (0.187 to 0.264) | 1.037 (1.014 to 1.060) | 0.001 |
| 5–9-year-olds only | 144 : 149 | 0.093 (0.080 to 0.107) | 0.093 (0.079 to 0.110) | 0.965 (0.935 to 0.997) | 0.030 |
| Post hoc 4: PCN level i | 144 : 149 | 0.155 (0.143 to 0.168) | 0.154 (0.140 to 0.169) | 0.942 (0.916 to 0.969) | < 0.001 |
| Post hoc 5: Delay j | 144 : 149 | 1.043 (1.023 to 1.063) | < 0.001 |
The per-protocol analysis produced strong evidence against the null hypothesis of equal dispensing in both arms of the study, with a higher rate of dispensing in the intervention arm (restricted to compliers). However, when investigated further, a lot of the practices that were non-compliant had joined in the latter half of the study, when COVID-19 had lowered all dispensing rates Figure 16. Therefore, this sensitivity analysis was not considered to be suitable as it falsely inflates the intervention arm dispensing rate as equivalent practices in the control arm were still included.
FIGURE 16.
Baseline and follow-up dispensing rates across the intervention arm compliers and non-compliers. Marker labels indicate the number of months on or after March 2020 (COVID-19).

Therefore, a post hoc CACE analysis was included to allow for compliance to be incorporated but avoid bias caused by only removing practices from one arm of the study. This analysis provided a similar point estimate as the per protocol but with large CIs and therefore no evidence of a difference.
A pre-specified sensitivity analysis had attempted to account for COVID-19 effects, by including a variable in the model which provided the number of years of follow-up that were on, or after, March 2020 (1–12). This analysis produced very similar results as the primary outcome. A post hoc sensitivity analysis was carried out which only utilised follow-up month data prior to COVID-19 and excluded follow-up months after February 2020. The exposure (list size) was then scaled down to the number of months of available data. This provided evidence of a difference, in favour of the intervention [0.192 vs. 0.204, RR = 0.967 (95% CI 0.946 to 0.989). p = 0.003]; assuming 1000 children (0–9-year-olds) per practice this equates to 192 items (intervention) compared to 204 items (controls) per practice or a 5.9% reduction. However, interpretation of this finding is limited given the lack of power and the post hoc nature of this observation.
As previously described in Internal pilot review, there were 48 practices that were recruited as part of a CHICO internal pilot. These practices were removed in a sensitivity analysis as the intervention was adapted for the main trial. Excluding these practices provided an IRR of 1.026 (95% CI 1.005 to 1.048), which provided weak evidence to suggest that the intervention may have led to higher dispensing rates.
Another sensitivity analysis incorporated dispensed amoxicillin/macrolide items with ‘missing’ age. This produced equally higher rates of dispensing in both arms, providing the same conclusion as the primary analysis. We felt that a post hoc analysis may have helped to distinguish whether there was any difference in amoxicillin dispensing only. This analysis did provide a slightly lower dispensing rate in the intervention arm but there was no statistical evidence of a difference.
A difference was seen between the arms when splitting the primary outcome into two separate epochs; 0–4 and 5–9. In the 0–4 group, there was evidence to suggest an increase in dispensing in the intervention arm. Conversely, in the 5–9-year-old group, there was evidence to suggest a decrease in dispensing rate in the intervention arm. This may be because clinical staff, using the intervention, had more confidence when using the intervention in the older age group but this is conjecture.
Another sensitivity analysis replaced the CCG random effects with PCN random effects. The 293 practices were part of 174 unique PCNs, with between 1 and 6 practices in each. Adding PCN as a random effect gave an IRR of 0.942 (95% CI 0.916 to 0.969), providing evidence of lower dispensing in the intervention practices, compared with controls.
The final sensitivity analysis included a covariate to indicate the number of months between baseline and follow-up (implementation period). This was 1 month for 253/293 (86%) of practices, 2 months for 14 (5%) of practices (10 intervention: 4 control) and between 3 and 21 months for the remaining 26 (9%) practices (all intervention). This adjusted analysis provided evidence of a difference, in favour of the control arm.
Subgroup analyses for the dispensing primary outcome
There were six pre-specified subgroup analyses for the dispensing primary outcome,46 which utilised practice-level data to split the data into two subgroups and look for an interaction between the subgroup and trial arm effects, on dispensing rates (see Table 13).
| Variable | N (I : C) | Intervention mean (SD) | Control mean (SD) | Subgroup-specific IRR (95% CI)a |
Interaction IRR (95% CI)b |
p c |
|---|---|---|---|---|---|---|
| Proportion of locums | ||||||
| < 25% | 54 : 41 | 0.15 (0.12, 0.18) | 0.15 (0.12, 0.18) | 0.94 (0.90 to 0.97) | 0.84 (0.74 to 0.95) | 0.005 |
| ≥ 25% | 48 : 58 | 0.17 (0.14, 0.20) | 0.18 (0.15, 0.21) | 0.96 (0.93 to 0.99) | ||
| Proportion of nurses | ||||||
| < 17% | 81 : 72 | 0.15 (0.13, 0.17) | 0.17 (0.15, 0.19) | 0.92 (0.90 to 0.95) | 1.40 (1.21 to 1.62) | < 0.001 |
| ≥ 17% | 62 : 74 | 0.16 (0.13, 0.19) | 0.14 (0.11, 0.18) | 1.05 (1.02 to 1.08) | ||
| Past dispensing rate | ||||||
| < 18% | 68 : 80 | 0.12 (0.11, 0.13) | 0.12 (0.10, 0.13) | 1.03 (1.00 to 1.06) | 1.00 (1.00 to 1.00) | 0.557 |
| ≥ 18% | 76 : 69 | 0.19 (0.17, 0.21) | 0.21 (0.18, 0.23) | 0.99 (0.97 to 1.02) | ||
| Practices site d | ||||||
| 1 site | 95 : 90 | 0.15 (0.13, 0.17) | 0.16 (0.13, 0.20) | 0.93 (0.90 to 0.95) | 1.15 (1.10 to 1.20) | < 0.001 |
| ≥ 2 sites | 34 : 37 | 0.17 (0.14, 0.20) | 0.15 (0.12, 0.18) | 1.03 (0.99 to 1.07) | ||
| Follow-up completed before the COVID-19 pandemic | ||||||
| Yes | 31 : 26 | 0.16 (0.13, 0.20) | 0.16 (0.14, 0.18) | 0.99 (0.96 to 1.04) | 1.02 (0.97 to 1.07) | 0.391 |
| No | 113 : 123 | 0.15 (0.13, 0.18) | 0.15 (0.13, 0.18) | 1.01 (0.99 to 1.03) | ||
| Level of deprivation | ||||||
| Low (rank ≥ 14387) | 72 : 74 | 0.15 (0.12, 0.18) | 0.15 (0.13, 0.18) | 0.96 (0.93 to 0.98) | 1.00 (1.00 to 1.00) | 0.004 |
| High (rank < 14387) | 72 : 75 | 0.16 (0.14, 0.19) | 0.15 (0.12, 0.19) | 1.06 (1.03 to 1.09) | ||
In Table 13, when assessing the impact of the proportion of locums at the practice, there was some evidence to suggest that practices with more locums had a treatment effect in favour of the intervention. There was strong evidence of an interaction between trial arm and proportion of nurses. For those practices with a low proportion of nurses (< 17% of staff), there was a 0.15 dispensing rate in the intervention arm and a 0.17 dispensing rate in the control arm. In practices with a higher proportion of nurses (≥ 17%) the opposite was true; 0.16 and 0.14, respectively. This suggests that the intervention was successful at decreasing dispensing rates in practices with a lower proportion of nurses. Equally, it suggested that the intervention increased the dispensing rate in practices with a high proportion of nurses. A similar interaction was found when looking at the number of sites a practice has. In those with more than one site, the intervention had a negative impact on dispensing, suggesting that the intervention training may be difficult to implement across multiple sites.
There was also some evidence to suggest that the intervention was more successful in practices with lower levels of deprivation and had a negative impact on those that had high levels of deprivation.
There was no evidence of an interaction when looking at past dispensing habits or separating practices into those affected/not affected by COVID-19.
Hospitalisations: primary outcome and sensitivity analyses
Impact of COVID-19 on hospitalisations for respiratory tract infection
In a similar way to dispensing rates, there was seasonal variation in hospitalisation for RTI across all CHICO practices (see Figure 17). Between October 2017 and March 2020, the hospitalisation rates, for RTI, reached highs of nearly 0.06 in the winter and approximately 0.02 in the summer. The effects of COVID-19, and its associated lockdowns, on hospitalisations are clear from April 2020 onwards when it dropped to < 0.01. When restrictions were relaxed in late spring/summer of 2021, hospitalisations climbed back towards normal levels; apart from July 2021 where they were unseasonably high.
FIGURE 17.
Mean hospitalisation rate (95% CI) during the CHICO baseline and follow-up periods. Each practice contributed 24 months of data.

Hospitalisation rate comparison
Hospitalisation rates appeared to be very similar between the arms, over the 3-year follow-up period (see Figure 18).
FIGURE 18.
Mean hospitalisation rate (95% CI) during the CHICO follow-up period (January 2019–August 2021), by arm. October 2018–December 2018 and September 2021 have been excluded due to low numbers. Each practice contributed up to 12 months of follow-up data.
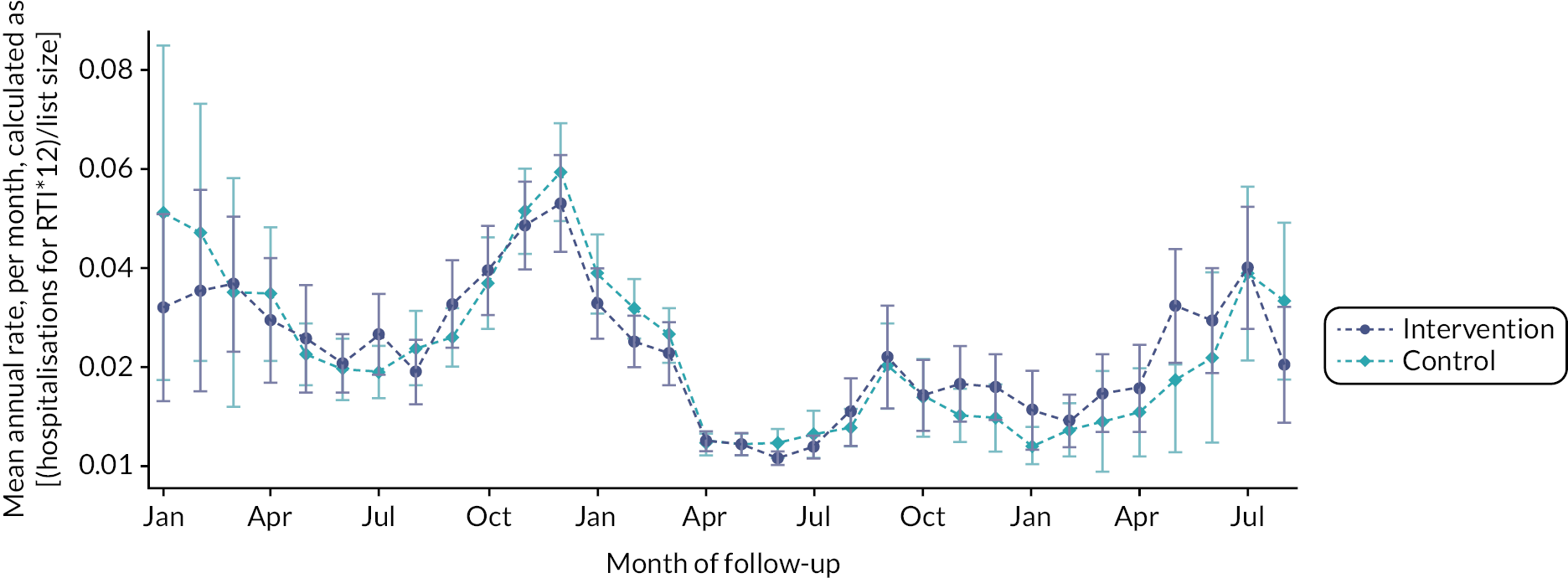
We pre-specified a non-inferiority margin of 0.01 for this outcome. Therefore, when comparing intervention with the control the IRR upper CI needed to be less than 1.01 to conclude non-inferiority. The rate of hospitalisations were 0.019 and 0.021 for the intervention and control arms, respectively (see Table 14). The IRR was 0.952 (95% CI 0.905, 1.003). As 1.003 lies below the 1.01 non-inferiority margin the intervention was considered non-inferior to the control.
| Intervention rate (95% CI) | Control rate (95% CI) | IRR (95% CI)a | |
|---|---|---|---|
| Primary analysis | 0.019 (0.014 to 0.026) | 0.021 (0.014 to 0.029) | 0.952 (0.905 to 1.003) |
| Including % missing diagnoses b | 0.020 (0.014 to 0.026) | 0.021 (0.015 to 0.032) | 0.950 (0.903 to 1.000) |
| Including all missing diagnoses c | 0.021 (0.015 to 0.028) | 0.023 (0.015 to 0.034) | 0.967 (0.920 to 1.016) |
| COVID-19 month indicator d | 0.936 (0.889 to 0.986) | ||
| Excluding West Hampshire e | 0.018 (0.013 to 0.024) | 0.019 (0.013 to 0.026) | 0.951 (0.900 to 1.004) |
Three pre-specified sensitivity analyses were carried out to test the robustness of the non-inferiority result. As West Hampshire did not provide accurate annual figures, their data were removed in a post hoc sensitivity analysis. First, a proportion of the hospitalisations with a missing diagnosis were included as RTI diagnoses. This provided very similar results to the primary outcome; IRR 0.950 (95% CI 0.903 to 1.000). Second, all hospitalisations with a missing diagnosis were included as RTI diagnoses, akin to a worst-case scenario. While there were still more hospitalisations in the control arm, the CI for this sensitivity analysis was (0.920, 1.016), slightly above the non-inferiority margin. Including a variable from 1 to 12 months, as with the dispensing outcome, to account for the number of months affected by COVID-19 provided results that agreed with the non-inferiority conclusion. We observed the same effect following the removal of West Hampshire practices.
Accident and emergency attendances: secondary outcome and sensitivity analyses
As A&E attendances were collected in the same way as hospitalisations, they were also analysed in the same way.
Impact of COVID-19 on accident and emergency attendances for respiratory tract infection
In a similar way to dispensing and hospitalisations, there was obvious seasonal variation in A&E attendances for RTI across all CHICO practices (see Figure 19). Between October 2017 and March 2020, the A&E attendance rates, for RTI, reached highs of nearly 13% in the winter and approximately 4% in the summer. The effects of COVID-19, and its associated lockdowns, on A&E attendances is clear from April 2020 onwards when it dropped to 2%. When restrictions were relaxed in late spring/summer of 2021, they became unseasonably high.
FIGURE 19.
Mean A&E attendance rate (95% CI) during the CHICO baseline and follow-up periods. Each practice contributed 24 months of data.

Accident and emergency attendance rate comparison
As with the dispensing and hospitalisation rates the A&E attendance rates appeared to be very similar between the arms, over the 3-year follow-up period (see Figure 20).
FIGURE 20.
Mean A&E attendance rate (95% CI) during the CHICO follow-up period (January 2019–August 2021), by arm. October 2018–December 2018 and September 2021 have been excluded due to low numbers. Each practice contributed up to 12 months of follow-up data.

The rate of A&E attendances was 0.049 and 0.045 for the intervention and control arms, respectively (see Table 15). The IRR was 1.013 (95% CI 0.980, 1.047; p = 0.437), providing no evidence of a difference between the groups.
| Intervention rate (95% CI) | Control rate (95% CI) | IRR (95% CI)a | p-value | |
|---|---|---|---|---|
| Secondary analysis | 0.049 (0.037 to 0.066) | 0.045 (0.032 to 0.063) | 1.013 (0.980 to 1.047) | 0.437 |
| Including % missing diagnoses b | 0.060 (0.045 to 0.082) | 0.054 (0.038 to 0.078) | 1.006 (0.977 to 1.037) | 0.680 |
| Including all missing diagnoses c | 0.134 (0.093 to 0.193) | 0.120 (0.080 to 0.182) | 0.976 (0.956 to 0.996) | 0.017 |
| COVID-19 month indicator d | 1.012 (0.980 to 1.047) | 0.445 | ||
| Excluding West Hampshire e | 0.050 (0.037 to 0.067) | 0.045 (0.032 to 0.063) | 1.021 (0.987 to 1.056) | 0.226 |
As with the hospitalisation outcome, various sensitivity analyses were carried out to account for the various misgivings in the data. As with hospitalisations, there were A&E attendances with missing diagnoses. Including a proportion of these as RTI-related gave very similar results to the main secondary outcome; however including all of them led to a higher number of A&E attendances in the intervention arm. This was true for both hospitalisations and A&E attendances suggesting that there may have been a higher proportion of ‘missing’ diagnoses in the intervention arm, compared with the control. Including a variable from 1 to 12, to account for the number of months affected by COVID-19, provided results that agreed with the secondary outcomes results. As did the removal of West Hampshire practices.
Exploratory calculations of the intracluster correlation coefficient
Of the 149 control practices, 55 were excluded when estimating the ICC as they were from a CCG with n < 5 practices. Using a mixed-effects linear regression model with follow-up rate as the dependent variable, baseline rate as a covariate and CCG as the random effect the ICC for practices within clusters for the dispensing and hospitalisation outcomes was ~0.05 and ~0.32, respectively. While an estimate has been made, these results should be viewed with caution given the small size of each cluster and the effects of COVID-19 on hospitalisation and dispensing rates.
Acceptability of the intervention
As part of the follow-up questionnaire, practices were asked how they found the intervention in terms of (1) ease of use, (2) helpfulness and (3) willingness to use it again. Practices were asked ‘Did you find the intervention…?’ and they provided the following responses (see Figure 21). The majority (92%) found the intervention ‘OK’ or ‘easy’ to use and over half found it ‘helpful’. For practices that found the intervention unhelpful, reasons provided were conflicts with other templates: ‘Sepsis template’, ‘Didn’t work with the contradictory COVID templates released nationally’, or dislike of the intervention: ‘GPs felt like this was an extra layer of intervention required on top of usual care’, ‘The problems that often the doctor had to close the EMIS® window before printing the leaflets off – if they forgot the patient had often disappeared – it was too late’, ‘It is hard to know the extent to which the information given to parents influence their subsequent behaviour’. When asked if they would use the intervention again, if it became available, 85/117 (73%) said that they would.
FIGURE 21.
Acceptability of the intervention.

COVID-19 impact
From March 2020, the intervention was used very infrequently (see Figure 9). Although various sensitivity and subgroup analyses were carried out in an attempt to account for COVID-19, if practices were not using the intervention then it does call into question the validity of the results. Points to note are:
-
As seen in Impact of COVID-19 on dispensing, Impact of COVID-19 on hospitalisations for respiratory tract infection and Impact of COVID-19 on accident and emergency attendances for respiratory tract infection, COVID-19 had a big impact on dispensing, hospitalisation and A&E rates, causing a drop in rates during lockdowns and an unseasonably high rate during the summer of 2021. Although this should have affected the two arms in a similar way, it would have made any differences between the two arms difficult to distinguish.
-
How patients were triaged was also impacted. From April 2020, in the follow-up questionnaire, practices were asked if the proportion of patients triaged over the telephone had changed due to the pandemic and 141/181 (78%) agreed that it had. Post March 2020, intervention practices were asked if there had been any months where they had been unable to use the intervention due to COVID-19. Of the 80 practices that answered this question, 17 (21%) said that there had been months where they were unable to use the intervention and 24 (30%) said there had been months with limited usage. Prior to March 2020, the median usage per month, per practice, was nine uses. Post March 2020, the median usage was zero.
-
Adverse impact to recruitment of practices during wave 1 of COVID, resulted in requiring an extra 4 months (un-costed) extension to achieve the target number of practices.
-
COVID-19 and subsequent vaccination programs may have contributed to the poor follow-up and communication from several practices, due to staff unavailability and other priorities.
-
Estimated reduction in dispensing antibiotics may not be clearly attributed to use of the intervention, as this could be partly affected by school closures during the pandemic causing a slowdown in RTI infections and children not visiting GP practices as frequently.
-
A reduction in intervention usage occurred during the pandemic when changing patterns of consultations in practices affected the number of children being seen face to face, although an alternative one-page guidance for using the intervention in remote consultations was provided.
-
There may have been an increase in A&E attendances during the pandemic if patients were unable to be seen in GP practices, this could affect the secondary outcome S1.
-
In some intervention practices, COVID-19 pop-ups prevented the CHICO pop-up from being displayed.
Protocol deviations
There was only one protocol deviation noted; during randomisation, four input errors were identified. Corrective and preventative actions were put in place. Both the Chief Investigator and the sponsor concluded the input errors would have minimal impact on the data integrity for the study, and the sample size would ensure there was no impact on the balance of the arms of the study (reviewed and approved on 16 April 2019).
SNOMED/Egton Medical Information Systems upgrade impact
The CHICO intervention resources were created using Template manager (the module for creating templates, protocols and concepts in EMIS®). The EMIS® set-up guide is described in Appendix 3. Certain known errors in EMIS® prevented a handful of practices from being able to implement the CHICO resources at all. The SNOMED upgrade by EMIS® adversely affected intervention practices being able to calculate the CHICO score. Therefore, a SNOMED-compliant version of the CHICO resources had to be written and tested before implementing new practices.
Scoping exercise for SystmOne
SystmOne covers 34% of GP practices In England, and although these practices were excluded from the trial, a scoping exercise was undertaken to investigate the possibility of creating the intervention using this practice system should the results indicate the intervention would be adopted by the NHS. Discussing the intervention with SystmOne users there did not seem to be any barriers to using the intervention in this system. A SystmOne Resources set-up guide was created to describe how the resources (a clinical template, letter templates, protocol and a set of clinical reports) could be distributed and imported into practices (see Appendix 4). The resources were demonstrated to a study clinician; however, there would need to be further testing by clinicians using SystmOne before distributing. In addition, both for the EMIS® and SystmOne versions of the resources, the list of medicines prescribed for asthma (such as drugs for prophylaxis of asthma), would need to be updated to reflect current prescribing behaviour.
Chapter 4 Qualitative study
Introduction
To explore the use of the intervention, how it was embedded into practice and whether it was used appropriately, we conducted interviews with clinicians (GPs and practice nurses). Data collection and analysis were informed by normalisation process theory (NPT),39,49 which was developed to explain the social processes leading to the routine embedding of innovative health technologies or complex interventions in healthcare.
Normalisation process theory proposes that implementation of interventions is dependent on the ability of participants to fulfil four criteria which can be understood using the core constructs of NPT, which are ‘coherence’ (sense making — understanding and opinion of the intervention purpose), ‘cognitive participation’ (buy-in — the work people do to develop new practices), ‘collective action’ (the work to operationalise practices) and ‘reflexive monitoring’ (ways in which people appraise how new practices are working).
Methods
Recruitment and sampling
Clinicians involved in implementing the CHICO intervention were invited to take part in semistructured interviews. To capture maximum variation in views and experience and reflect a range of practices (those with high and low patient lists and antibiotic prescribing rates, serving areas of high and low socioeconomic deprivation) and clinicians (GPs and practice nurses with a range of years’ experience) were purposively sampled. The socioeconomic status of practices was estimated using the Index of Multiple Deprivation decile47 based on the practice post code. The sample size was determined by data saturation, such that no new themes were emerging from the data by the end of data collection. 50 Potential participants were contacted by the qualitative researcher (CLCL) via e-mail and invited to take part in the interview. Clinicians from 56 practices were invited to participate. Participants were asked to provide their audio-recorded verbal consent to take part immediately before the interview.
Interviews
The interviews were all conducted by telephone by the qualitative researcher (CLCL), who is an experienced social scientist. The first set of interviews was conducted during the trial pilot phase and findings were fed back to the Trial Management Group (TMG) to optimise the intervention implementation and guided changes during the remainder of the study. The second phase of interviews was conducted after practices had been using the intervention for 12 months. A flexible topic guide was used to assist questioning during interviews but allow participants to introduce and discuss topics not anticipated by the researchers (see Box 1).
-
Experience and views of CHICO intervention
-
First impressions
-
Preparedness for the intervention
-
Views on credibility and usefulness
-
Frequency of use and changes over time
-
Who used for and why?
-
How is it used in consultation?
-
Parents’ responses
-
-
Impact of CHICO
-
Benefits of CHICO intervention
-
What has not worked well/been less successful
-
Influences on prescribing behaviour
-
Influences on views on hospitalisation
-
Impact on consultation time
-
Any changes made to how CHICO intervention is used
-
Suggested changes to the CHICO intervention
-
-
Views of potential for change in practice
-
Impact on practice
-
Any changes needed for wider implementation?
-
Potential barriers to roll-out
-
Other current influences on antibiotic prescribing
-
-
Any other issues
Topic guides were modified as necessary throughout the course of the study to reflect findings as they emerged. With informed consent from participants, interviews were audio recorded using a digital voice recorder, transcribed using a professional transcription service and anonymised to protect confidentiality. Interviews lasted between 15 and 37 minutes, with an average time of 25 minutes.
Analysis of the interviews
Anonymised transcripts were checked for accuracy and then imported into NVivo Pro (version 10/11)51 data analysis software to aid the management and analysis of the data. Analysis began shortly after data collection started and was ongoing and iterative. Ongoing analysis informed further data collection; for instance, analytic insights from data gathered in earlier interviews helped identify any changes that needed to be made to the topic guide during later interviews, for example, to explore new or underexplored topics raised in earlier interviews. Thematic analysis52 was used to scrutinise the data to identify and analyse patterns and themes of salience for participants across and within the data set. Transcripts were coded inductively to establish an initial analysis framework and three researchers (CLCL, JH and CC) coded a subset of three transcripts; any discrepancies were discussed to ensure a coding consensus and maximise rigour. 53 The remaining transcripts were analysed (by CLCL) using the agreed analysis framework. The four NPT constructs were used to further develop themes across the data sets. To ensure that findings were trustworthy and credible, preliminary findings were discussed with the multidisciplinary trial management team.
Results
Participants
Twenty-six clinicians (20 GPs and 6 practice nurses) were interviewed from across 24 practices and 13 CCGs (see Table 16). Findings are presented for each of the NPT constructs, illustrated with anonymised verbatim quotations.
| Characteristic | Number of participants |
|---|---|
| Years of experience | |
| 0–5 | 2 |
| 6–10 | 7 |
| 11–15 | 6 |
| 16–20 | 1 |
| 21–25 | 8 |
| 31+ | 2 |
| Above CCG median practice patient list size | 14 |
| Below CCG median practice patient list size | 12 |
| Above CCG median antibiotic prescribing rate | 11 |
| Below CCG median antibiotic prescribing rate | 15 |
| Practice deprivation score | |
| High | 7 |
| Medium | 9 |
| Low | 10 |
Coherence (sense making)
Clinicians welcomed the CHICO intervention as it aimed to help with ongoing issues being faced by practices such as the prevalence of children presenting with a cough and perceived parent concerns with not receiving antibiotics.
It was something that we were particularly interested in doing anyway. We do see lots of children, obviously, with coughs and colds and some parents generally are concerned. Some parents do understand that infections can be viral, but some do also expect an antibiotic if it’s had a chesty cough for a certain period of time.
(GP 14)
Just a couple of weeks ago before CHICO was installed, they [nurses] were having numerous complaints from parents saying they felt cheated out of antibiotics.
(GP 5)
The intervention also aligned with existing strategies and efforts to reduce unnecessary antibiotic prescribing. Clinicians were already familiar with similar protocols and templates commonly used in primary care and believed the intervention would fit within their usual practice.
I thought it was good using that kind of direction that we were moving anyway to prescribe less antibiotics. So it was just something that was in time and in tune with what we needed to do.
(GP 21)
It seemed like it would be a simple intervention to use that could be used, you know just within a consultation so we always like that … It’s almost part of what we would normally do.
(GP 11)
Cognitive participation (buy-in)
Clinicians felt they were well prepared for using the intervention and liked the training and materials. They found the training guides useful to refer to throughout the study and liked the ability to practice using a training patient. Although a website that held background information on the study and published literature on the intervention development was available, most clinicians did not feel the need to visit it.
I think [we were] really well prepared. The training results are really good but having a test patient was really good.
(GP 11)
The leaflets that you gave out and the information and the CHICO guides were given, were quite self-explanatory and all seemed quite straightforward.
(Nurse 17)
Collective action (using children with cough in practice)
Most clinicians liked the intervention and thought it was quick and easy to use and used it as a supportive aid within consultations and described it as a safety net. It was a way of reassuring themselves and parents of the appropriateness of some treatment decisions.
I thought it was good, it was easy to use and yes, I thought it was a good idea.
(Nurse 20)
I think if you are not prescribing antibiotics, then it’s a good safety netting tool. So, you can confidently say to the patient that you don’t feel antibiotics are indicated.
(GP 19)
It’s very reassuring for the professional and of course when you’ve printed out the leaflet that this is the scoring we have done; it is very reassuring for parents as well.
(GP 16)
Launching children with cough
During the pilot phase, clinicians were prompted to use the intervention through a ‘pop-up’, which appeared for all children under the age of 10. This limited the use of the intervention for some because it was launched at a point too early in the consultation to be useful and some clinicians did not like it. Based on this finding, the timing of the CHICO template was changed in the main trial phase and clinicians preferred the new form of launching the intervention using a code such as ‘cough’.
It seems the hard pop up was useful, but it comes up too early. So, it comes up before you know what’s actually wrong with the child.
(Nurse 8)
Initially … the flags … came up whenever you had an interactions with a 10-year-old. We had to remove it because it just drove everyone nuts.
(GP 4)
When you type in ‘cough’, a little box would come up with a CHICO template opening up, and then we were quite happy to proceed with completing it.
(GP 14)
However, some practices retained the automatic pop-up to help remind clinicians to use the intervention.
We’ve kept the automatic launch on, even though it is a bit annoying … We thought the best way to try and get people to use it and remember it for the whole year was keep it as automated.
(GP 13)
Prognostic algorithm
Most clinicians like the template where they entered the patient’s symptoms and signs. They found it easy and straightforward to use without adding any more time to consultations.
It was fairly quick to use … it also allowed you to enter free text, and it had all the relevant questions.
(GP 14)
I think in practise I think the templates are not cumbersome. They are not bulky, and it can fit into the time that we have to see patients.
(GP 4)
However, some clinicians felt the template did not capture all the relevant symptoms and they needed to record these separately in the patient’s record. This required ‘moving between two screens’ (GP 11), which could be problematic.
It was quite useful template to record the information. It could have been a little bit more sort of, bit more history of presenting complaint and there was the examination obviously and certain questions to ask but I often found then I went back into the patients notes and wrote a bit more information about the consultation, so, you couldn’t put everything in the template. I found I either use the template and then probably it was a bit of a sketchy history, or, I had to then go into the patients note or save the template and then do a history of presenting complaints and I found it made it a bit more disjointed.
(GP 19)
Clinicians liked that the template helped to elicit parent concerns which were believed to be important but could be forgotten.
I think the parent concerns are good because that’s just making it a bit more patient centred and maybe we forget to ask that sometimes.
(GP 11)
I suppose I wouldn’t necessarily ask about what are you particularly worried about directly.
(Nurse 17)
‘Ask the parent or carer what they were most worried about that day’. I think that’s an invaluable question, really, because you just don’t quite know what the parent is actually worried about … so I think that was really valuable, and again, a good prompt that some doctors may or may not actually ask.
(GP 14)
Clinicians had mixed views about the risk management advice aspect of the intervention. Few of the clinicians remembered this aspect and some did not find it useful. The perceived usefulness of the prognostic algorithm depended on the severity of the child’s symptoms. Clinicians found it most useful with children who were ‘borderline’ cases for hospitalisation or prescribing antibiotics.
It gives the clinician visual cues about the severity of it, which is very good.
(GP 1)
I’ve never seen CHICO give me any kind of management advice.
(GP 12)
I’ve tended not to take that much notice of that. I don’t know if that was just because originally it was quite interesting, but now it’s always the same, because the majority of what we see is mild viral self-limiting respiratory illnesses. Most parents hadn’t even considered that their child might need hospital, or whether that would play any part in how we manage them.
(GP 13)
I personally would probably [have] used it more for the borderline ones.
(GP 14)
Letter/advice leaflet
The advice leaflet was believed to be the most useful intervention component and clinicians liked it for a range of reasons. The leaflet was a good safety-netting tool and a way of facilitating conversations with parents and reinforcing the clinician’s decision not to prescribe antibiotics.
I didn’t find CHICO useful for the score. I found it helpful for the leaflet. I think my whole use was centred around the leaflet.
(GP 11)
It really helped with safety netting if you weren’t giving them antibiotics, so it gave you a lot more confidence that parents knew what the signs were … so I think that was the best bit of the intervention.
(GP 16)
So, if there was a feeling that it was going to be a difficult consultation to try and steer them [parents] away from antibiotics based on the clinical assessment then that would be a really good adjunct tool for that.
(GP 25)
Clinicians felt that parents were more satisfied with receiving the leaflet that explained the clinician’s decisions and having information they could take home with them. The safety-netting advice was a way of educating and empowering parents, which could have longer-term implications for reducing repeat visits to practices.
I think it just makes patients feel more satisfied that they’re not going away empty handed. They’ve been given something, and I feel kind of that what I’ve said to them has been enhanced by going away with a leaflet.
(GP 11)
I think on one of the occasions it was particularly good because I think the mother of my patient did struggle with the idea why antibiotics might be useful down the line but weren’t now and I think that was quite helpful.
(GP 16)
I hope over time it [leaflet] will empower parents a bit to not actually contact us as early as they tend to do in this area with coughs in their children. There’s the first line on the leaflet saying, ‘Normal coughs can last three to four weeks.’ That’s actually what we’ve been trying to educate patients about for ages. The bit about how many times you get a cough a year, and all this is quite normal. I think probably that has been one of the most helpful things as well that healthy children do get a lot of coughs. It doesn’t necessarily mean there’s anything wrong with their child. It’s just normal.
(GP 13)
Nurses found being able to give the parents the leaflet particularly useful as they felt they faced increased scrutiny and push back from parents if they did not prescribe antibiotics.
What we find quite often as an ANP [Advanced Nurse Practitioner] is if we refuse them antibiotics, then they go and make an appointment with the doctor and get antibiotics. So, you know, you’re always aware in the back of your mind that that kind of thing is going to rumble on and then you know, they’ll just go and see and then just keep seeing people until they get what they want … I would say that it gave us that extra back-up to say no.
(Nurse 24)
As with the prognostic algorithm, the advice leaflet was seen to be more useful in children considered to be ‘borderline’.
Like especially when there is a borderline whether to go to the hospital or not and the score is a bit on the lesser side and parents are not keen to go to the hospital and at that time, this has particularly helped. The leaflet you’re giving them the clear-cut advice of when to go and when to seek advice.
(GP 23)
Challenges with children with cough in practice
Clinicians highlighted some challenges with using the CHICO intervention in practice, which led to reduced use or not using all the intervention components. These included the use of the intervention not being aligned to some clinicians’ usual consultation practice. Some clinicians would usually complete the patient record at the end of the consultation or after the patient had left meaning they were not able to use the algorithm to support decision-making or provide the parent with the personalised letter and advice leaflet. Difficulties aligning the intervention with their consultation flow led some clinicians to stop using the intervention. However, in some cases, clinicians did provide parents with pre-printed advice leaflets.
I do my typing up at the end of the consultation so it [intervention] doesn’t alter my thought processes, ‘am I going to prescribe them antibiotics or not’. I have already made that decision from taking the history and doing the examination. It is just back-up at the end of the consultation, because, like I say, I do my typing up at the end. It doesn’t actually give you the scoring until you click, ‘save’ so that pop-up comes right up right at the end. I have already made my decision by then.
(GP 3)
If I then while the child is with me code something like upper respiratory tract infection or chest infection, then the pop up comes again and then I fill it in. But the problem is that I don’t always do that bit until after the kid has gone … I get a pop up saying do I want to print a leaflet about antibiotics or not. But usually, my patient’s long gone by that stage.
(GP 12)
The hardest thing I think is that to load the letters you have to save and actually you save when the patient’s out of the door normally and so it’s just a bit clunky so you’re having to save earlier than you would like to and then go back in and edit in order to launch the leaflet … Most of us find that it gets in the way of our consultation and so therefore we don’t use it, but we like the leaflet, and we give that out.
(GP 11)
Unfortunately the leaflet thing probably got a little bit overlooked because you do the whole template, finish the consultation with the patient and then they would go and then you’d finish writing up your notes which I think most practitioners would do, finish writing up the notes when they’ve gone and then you click on save and then up comes the ‘would you like to print a leaflet’ and it’s ‘oh, I’ve forgotten to do that’.
(Nurse 17)
Some clinicians did not like focusing on the computer and having to input information when the patient was present as they wanted to pay attention to the patient and to maintain a face-to-face contact.
Normally for most consultations, I’ll type it up after they’ve gone so that if we can maintain eye contact and have the conversation with the parent rather than typing.
(GP 13)
Some practices conducted their consultations remotely, which meant clinically assessing the symptoms required for the prognostic algorithm was challenging. This was more of an issue with telephone consultations as some symptoms could still be assessed using video where the child could still be ‘seen’ and ‘heard’.
Can assess using video, can see the child … breathlessness, wheezing.
(GP 18)
I mean we do a lot of video consultations as well ‘cause then you can see whether the child’s running around and what they’re doing and what have you, so yes it could easily be adapted I would have thought.
(Nurse 20)
It was also difficult to provide parents with the printed letters and leaflets in some practices, either because of the remote consultation or because of printing issues. However, some clinicians had found ways around this including using pre-printed versions of the leaflet provided by the study team, saving a PDF version that could then be printed off without using the intervention and e-mailing or texting the leaflets to parents.
Often, I would just give them a nice – you know, they were very attractive leaflets and a bit more probably, yeah more striking than the black and white paper printout.
(GP 19)
We’ve started to e-mail the leaflet to patients … using a text messaging service … So we have used the leaflets via telephone consultation as well, so you can do that, so that bit is good.
(GP 22)
So, a lot of the time we’re texting leaflets now. I don’t know whether that would be something –we use something called [ACCU RX]… that is a technical service, and you can embed leaflets and… that would be quicker… so if we wanted to upload CHICO we could.
(GP 11)
I have to say I really – I do like it but what I do is - we’ve got the CHICO leaflet embedded in EMIS® so sometimes I just give that without using – We’ve also got it, I think there’s a link to it on our website so we don’t so much do the CHICO intervention we just say, ‘Actually, there’s this really nice leaflet … have a look on our website.’ Some of my colleagues do … sometimes I just have some printed leaflets and give it to them without having to use the intervention.
(GP 15)
Frequency of use
How often clinicians believed they used the CHICO intervention was variable with some reporting frequent use early on, but that use reduced over time. This could depend on clinicians remembering to use it, how busy the practice was, and increased familiarity with the algorithm outcomes.
Used on a daily basis.
(GP 15)
She [nurse practitioner] uses it for every single patient.
(GP 5)
In the latter months we sometimes would forget to do the CHICO template … The more you use it, the more you get used to it and you get a feel of what the score might be and what the outcome might be.
(GP 14)
Perceived impact on behaviour/practice
Some clinicians believed the intervention influenced their prescribing behaviour. However, others believed that it supported rather than changed their prescribing decisions and did not change their behaviour.
I’m not sure it massively did [affect prescribing]. Perhaps not directly I would say … We probably went on the history and the physical examination.
(Nurse 17)
The main thing we used it for was safety netting and we do that anyway, so it’s really just enhancing. Not like we’re saying ‘okay we are going to ignore everything in front of us because CHICO is telling us to do this.’ It really just fits in with what we do anyway.
(GP 11)
You know your clinical judgement supersedes any guidelines but they’re always there to support your decision making from that aspect.
(GP 25)
Use during the COVID-19 pandemic (interviews took place during or just after the first wave)
Changes with practice pathways such as increased nurse triaging and the use of COVID-19 protocols led to reduced use of the CHICO intervention during the pandemic. Anyone presenting with a cough was assessed for potential COVID-19 infection and referred for a COVID-19 test.
COVID did definitely affect it. So, I mean just I guess thinking of cough, you’re thinking of COVID so I don’t think we’ve used it for a year I would say. I would say that. But before that we used it quite frequently.
(GP 21)
I didn’t and I’m not sure others did because first of all we were doing remote consultation, especially the first wave and the first wave also I was isolated at home so I wouldn’t have used it then and I would definitely tell anybody with a cough to get a test and refer them to the clinic we have in ((city)).
(GP 25)
The need to conduct consultations in restricted spaces with no computers or materials such as leaflets meant clinicians were unable to use the CHICO prognostic algorithm and they could not print out the personalised letters or use pre-printed leaflets.
The problem it was that was slightly that we were seeing patients with coughs and temperatures in a red room. So, we’d cleared everything from that clinical room to keep things as, so we didn’t have the leaflets readily available and also we weren’t logging onto that computer cause we were trying to keep it that everything we touched we had to clean down, so, I must admit, I don’t think we did use it when patients cause we had to have a red room cause, you know, anyone presenting with a temperature or a cough, which included children with those symptoms, so, yeah, I don’t think we’d probably used it quite as much during COVID.
(GP 19)
Part of the reason that we wouldn’t use it during COVID is that we’re seeing our patients now … we’re seeing outside in the car park, so we don’t have our computer in front of us.
(GP 15)
The increased use of remote consultations during the COVID-19 pandemic further highlighted the challenges discussed. However, having used it during remote consultations during this period, clinicians did perceive some benefits to using the intervention remotely including less need to focus on a face-to-face consultation.
It fit more naturally with remote working because its easier to get whatever you need on the screen, and you’re not worried about eye contact and body language.
(GP 25)
There were also fewer children presenting with respiratory illnesses which reduced the opportunity to use the CHICO intervention during the pandemic.
That’s where it all stopped because majority of our consultations since the COVID started last year, they all went into the telephone consultation and so that’s where we have not used much of CHICO template at all.
(GP 23)
We get a lot of virally coughs and colds and things in children but since lockdown and since COVID, there’s been hardly any and I suppose that’s because people aren’t going out and they’re not going to nurseries are they and they’re not picking it up … So there’s hardly – well we haven’t hardly any children in now.
(Nurse 20)
Both GPs and nurse practitioners found the intervention useful. However, some clinicians believed the intervention would be more useful and have more impact for trainee doctors to support decision-making and help guide patient management. Trainee doctors are likely to be less experienced with sick children and believed to be more likely to prescribe antibiotics and could therefore benefit more from the intervention.
Doctors in training have found this a much more useful than some of the experienced doctors … ‘cause sometimes they haven’t seen lots of children and they really appreciate having a system whereby it helps to guide their management.
(GP 22)
Of course it was useful for us also but sometimes we are seeing registrars might be more inclined to prescribe antibiotics and everything but that was a good guide regarding the intervention.
(GP 23)
Reflexive monitoring (appraisal of CHICO)
When appraising the CHICO intervention and making recommendations for future implementation, participants suggested expanding the template to encompass more information. This could help overcome issues with having to record information in multiple places and switching screens.
Maybe a box, history of presenting complaints, so if there was other information – because I think if you’re filling in a template, especially when we’re busy in the winter, it be good if we could record all the information in that template and then not have to go back into the notes to record things that we think is important to record.
(GP 19)
But if you’re using a template, you want it to capture all the information. So, adding those two, heart rate, respiratory rate and oxygen saturations would help it to be more complete.
(GP 13)
Clinicians also recommended adapting the intervention to be more conducive to remote consultations. This could include informing parents about how to assess symptoms and having parent-reported criteria rather than having the clinician-assessed criteria. However, some clinicians worried about relying on parent-reported symptoms as these could be less accurate and more subjective.
Adapting the template to remote consultation will be a good thing … have parent observed criteria rather than clinician observed.
(GP 23)
I think the tool is little bit reliant on clinical aspect as well which you may not have so it’s difficult to judge on chest signs and symptoms and respiratory distress and that sort of thing, wheeze, based on a conversation with a parent and even temperature. They may not have a temperature probe so you may not be able to get particular aspects of it but then some bits you will be able to get. But if it can be tweaked to amend for things that may not happen on remote working then that may obviously help.
(GP 25)
And asking if they would use the intervention in the future
I would have no problem with starting to use it again now because I feel you know, now we’re gonna start getting back to normal and coughs will just be coughs and colds and it would be really useful to have that back again.
(Nurse 24)
Discussion and conclusions
Summary of the findings and implications
The use of NPT allowed for examination of issues with both the design of the intervention and its implementation. Interview data indicated that while the NPT constructs of coherence and cognitive participation were fulfilled, this was not the case for cognitive participation, with negative consequences for implementation.
The qualitative findings help explain why no difference was found between the control and intervention groups. They confirmed that clinicians used the intervention at the start of the trial, but usage waned over time. Clinicians initially welcomed CHICO in theory but in practice, it proved difficult to align the intervention flow with that of the clinician’s usual consultation practice. Most clinicians liked the algorithm template and found it straightforward to use, without adding any more time to consultations. However, having to close the patient’s record before the end of the consultation to complete the intervention process did not always align with clinicians’ usual processes and was problematic. The COVID-19 pandemic also impacted use of the intervention due to changes to practice pathways, increased use of remote consultations and reduced numbers of children presenting with RTIs.
While some clinicians reported that the intervention influenced prescribing decisions and found it most useful in ‘borderline cases’, others reported that they used it as a supportive aid during consultations rather than a tool to change prescribing behaviour. CHICO helped elicit parent concerns and reassure clinicians and parents of the appropriateness of some treatment decisions. Clinicians particularly liked the safety-netting parental advice leaflet, as it helped explain treatment decisions and home care with parents and this was seen to be the most useful intervention component. To increase use of the intervention, findings suggest it may need to be adapted for use within remote consultations and to fit better with clinicians’ consultation flow.
Strengths and limitations of the qualitative study
Strengths include interviewing both GPs and nurses who used the intervention from a diverse range of practices. The use of NPT to inform data collection and analysis enabled a focus on issues with both the intervention design and the way it was implemented in practice.
Limitations include possible memory bias as most clinicians were interviewed towards the end of the trial, which may have been some time after they had last used the intervention. In addition, we did not interview those clinicians who have never used the tool, and this could have provided useful insights into the barriers of using the tool. Insight into whether and when clinicians would choose to use or ignore the tool is also missing, which limits understanding of its use in different situations. A further limitation that parents of children that CHICO was used with were not interviewed which should be taken into consideration when interpreting the results. However, clinicians did discuss how the intervention was received by parents, which provided some useful insight into parents’ views.
As we were examining the experience of using the intervention, we did not interview people who did not use the intervention. However, as interviews were conducted with those who used the intervention early on but then their use reduced over time, we have provided insight into why clinicians may not engage with the intervention.
Conclusions
Clinicians found the CHICO intervention useful, and it helped discussions with parents about concerns and treatment decisions. Some clinicians believed the intervention influenced their prescribing behaviour and found it most useful in ‘borderline cases’. However, others believed that it supported rather than changed their prescribing decisions and did not change their prescribing behaviour. Clinicians usage of the intervention reduced over time and this was further impacted by the COVID-19 pandemic and subsequent increase in remote consultations and reduction in RTIs.
To increase use of the intervention, findings suggest it may need to be adapted to align more with clinician’s consultation flow and allow use during remote consultations.
Chapter 5 Economic evaluation
Introduction
The economic evaluation of the CHICO trial comprised a between-arm comparison of secondary and primary care costs from an NHS perspective. This analytical approach was informed by the experiences of the related CHICO feasibility trial, as well as the design used for the present trial.
The feasibility trial recruited children aged between 3 months and 11 years with acute cough and RTI. Quality of life was calculated in the feasibility trial using the CHU9D instrument, which is validated for children aged 5–11 years. In the event, only some 25% of children recruited to the feasibility trial were in this age range, and only 9% of participants provided sufficient baseline and follow-up CHU9D data to enable calculated of quality-adjusted life-years (QALYs). This experience indicated the challenges of collecting and calculating data on quality of life and cognate measures such as QALYs in very young populations presenting to primary care with RTIs.
In relation to the present trial, the co-primary outcomes of the trial are rates of amoxicillin and macrolide items dispensed to children aged 0–9 years, and rates of LRTI hospitalisations. Given that both outcomes embody elements of resource consumption (dispensing of medication and hospitalisation), a between-arm comparison of these and other costs was considered the most appropriate way of addressing our secondary aim (S2) of clarifying whether and by how much NHS costs might change in the event of a deployment of the intervention algorithm into routine clinical practice.
The following sets out our approach to identify, quantify and value NHS costs incurred in each arm of the trial, which was undertaken following the publication of the trial’s protocol and was undertaken following the steps described in the statistical analysis plan45 agreed with the trial’s DMC. Our reporting of this economic evaluation follows the updated guidance contained in the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement. 54
Resource use and valuation
The NHS costs included in the between-arm inferential comparison were those of the intervention itself, costs of dispensed amoxicillin and macrolides, A&E attendances and hospital admissions. These costs were calculated from an NHS perspective. As the follow-up period does not extend beyond 1 year, discounting of costs was not applied. Each cost item is considered in turn below. Costs are reported on 2020/21 prices.
Costs of the intervention
The costs of the intervention comprised the non-research related costs involved at the practice level that arose from integrating the intervention into local computers, and training costs borne at the practice level. We consider possible other intervention-related costs that may arise in the event of a large-scale (e.g. national) deployment in Chapter 7 Discussion, but for the between-arm cost analysis we restricted our focus to those costs incurred in the trial itself.
To reflect experiences at some but not all practices in the trial, we made the conservative assumption that time will be required to manage and troubleshoot the integration of the algorithm at the practice level. We assumed that this would be undertaken by a practice manager working for 1 hour in total. This amounts to a cost of £54 per practice in 2020/21 prices, corresponding to a salary of Band 6 on the Agenda for Change (AFC) pay scale. 55
For training time involved in using the intervention, we assumed that it would take some time for prescribing clinicians to understand the rationale for the intervention and to become familiar with its use. This is also a conservative assumption since the ‘implementation’ period of the trial allowed time for practices to install the intervention and to encourage its use by staff. We assumed that the time involved would be equivalent to that of a single consultation. The median number of GP prescribers of amoxicillin and macrolides per practice in the trial was 4. Given a cost per consultation of £39 in 2020/21 prices, we therefore calculated practice-level training costs as 4*£39 = £156.
The costs of the intervention were therefore estimated as £210 per practice. In sensitivity analysis, to test our conservative assumptions on GP training and manager integration of the intervention, we re-ran our main results attributing no costs to the intervention itself.
Costs of amoxicillin and macrolides
Data on both the volume and cost of prescriptions at each practice were taken from the BNFePACT2 (Electronic Prescribing Analysis and Cost) system. The volume data were specific to each practice, collected routinely every month and reflected dispensed amoxicillin and macrolide prescriptions given to children aged 0–9 years, for each practice, and obtained from ePACT2.
Data on the net ingredient cost of amoxicillin and macrolides (see Appendix 6, Table 20) were also obtained from the ePACT system. The cost data were not specific to each practice. Instead, they represented cost of all amoxicillin and macrolides dispensed to this age group in England during the 2020/21 financial year. Using these cost data, therefore, assumes that the costs and composition of prescriptions – reflecting the relative mix of specific medications as well as their formulation, dose and presentation – of all GP practices in England are similar to those of practices included in the trial.
The use of net ingredient cost reflects a basic cost of the drug concerned. Given the unavailability of details on formulation, presentation, dose and other information, it does not account for container costs, dispensing costs and net fees or net prescription charges income.
Costs of accident and emergency attendances
As noted in Chapter 3, the collection of data on A&E attendances is routinely undertaken each month by each CCG. We used these data to identify the volume of attendances. These were costed using NHS Reference Costs 2019/2056 and inflated to 2020/21 prices. The inflation index is used as the average of the last 5 years (2016/17–2020/21 financial years) of the NHS cost inflation index. 55
We did not have information on whether an attendance led to a subsequent admission, the type of investigation conducted or the nature of treatment received while attending. Reference costs also does not distinguish between attendances related to paediatric or adult populations. We, therefore, created an average unit cost, weighted by the number of attendances reported for all admissions. However, we excluded dental admissions, and those where the patient was reported as dead on arrival. The unit cost in 2020/21 prices was £186.
Costs of hospital admissions
Volumes of per-practice hospital admissions related to RTI were also obtained from data routinely collected by CCGs. These were also costed using NHS Reference Costs 2019/20, inflated to 2020/21 prices as for A&E attendance using NHS cost inflation index as described above. 54
The data available to the trial indicated that an admission had taken place, but did not otherwise identify the specific diagnosis associated with an admission nor whether the admission was associated with non-elective long or short stay, or day care. We therefore costed admissions as follows. Within each of non-elective long-stay, non-elective short-stay and day care episodes, we calculated an average of all unit costs that relate to acute respiratory symptoms. We weighted each unit cost by the respective volume of finished consultant episodes. Finished consultant episodes are completed episodes of treatment received by a patient under the care of a single consultant, and are a fundamental way of tracking activity in the NHS. These unit costs were then weighted by the total finished consultant episodes of each of non-elective long-stay care, non-elective short-stay care and day care to give a total average unit cost for LRTI-related hospital admissions.
Clinical Commissioning Groups were asked to identify hospital admissions for children for a broad list of conditions that may have been associated with RTIs. We developed estimates of unit costs that specifically focused on admissions pertaining to these types of infection.
A currency, in NHS reference costs, is a unit of healthcare activity such as a finished consultant episode. The currency codes used and their associated descriptions in, for example, the costing of non-elective long-stay care are summarised (see Appendix 6, Table 21). For the primary economic analysis, we estimated the cost of a hospital admission as £1209. We also conducted a sensitivity analysis in which we repeated this calculation of unit costs, but only including those service descriptions that specifically mention RTIs. In this case, the unit cost was estimated as £1165.
Analysis and sensitivity analysis
The comparison of between-arm costs used a two-way mixed-effect linear regression that accounted for the nesting of practices in CCG clusters. The analysis used this model for mean between-arm cost differences given the sample size of the trial and the desirable coverage properties for these kinds of parametric estimators even when within-arm cost data happen to be skewed. 57,58 The primary analysis model regressed total costs on arm and covariates for list size and dispensing rate, both of which were used for minimisation at randomisation. We conducted sensitivity analyses that repeated this regression but (a) from a per-protocol perspective, following the same analysis steps described in Chapter 2 Methods but applied to the between-arm comparison of costs (b) using unit costs for hospital admissions where the currency description of care episodes specifically mentions ‘RTI’ as in Table 18 below (c) excluding intervention costs from the between-arm comparison.
Results
Data completeness and description of cost data
We used data from 293 of 294 randomised practices; the 294th practice merged with another trial practice after randomisation and was analysed as part of the larger, merged entity. Data were complete on all resource items used to undertake the between-arm cost comparison.
The volume of resource use in each arm is shown in Table 17. All cost data reported below are in 2020/21 prices. All practices reported some costs, ranging between £188 (for a practice in the control arm) and a maximum cost of £452,029 (for a practice in the intervention arm).
| Cost category | Per practice mean resource use in control arm | Per practice mean resource use intervention arm |
|---|---|---|
| Amoxicillin | 142.59 (116.83) | 139.54 (101.04) |
| Macrolides | 38.60 (38.67) | 40.18 (38.50) |
| A&E attendance | 53.05 (50.68) | 57.31 (50.97) |
| Hospital admissions | 24.16 (35.20) | 22.02 (31.98) |
Costs were similar between arms. Mean (£39,826) and median (£29,162) unadjusted per-practice NHS costs during the 12 months of trial follow-up were somewhat lower in the intervention arm than in the control arm (mean £38,246, median £28,216). The distribution of per-practice costs was also similar between arms (see Figure 22).
FIGURE 22.
Distribution of total costs in control and intervention arms.

There was no evidence of a between-arm difference when comparing cost categories (see Table 18).
| Cost category | Unadjusted control practices mean cost (SD) | Unadjusted intervention practices mean cost (SD) | Adjusted between-arm cost difference | 95% CI |
|---|---|---|---|---|
| Dispensed amoxicillin and macrolides | £736 (£676) | £740 (£638) | £11 | −£29 to £52 |
| A&E attendance | £9880 (£9439) | £10,673 (£9493) | £629 | −£257 to £1515 |
| Hospital admissions | £29,209 (£42,557) | £26,662 (£38,664) | −£2924 | −£7233 to £1386 |
| Intervention cost | £0 | £210 (–) | £210 | – |
Note that the costs of the intervention itself are modest compared to other cost categories, especially that of hospitalisation and A&E attendance.
Primary economic analysis
The primary analysis model regressed total costs on trial allocation and on covariates for list size and dispensing rate (see Table 19), adjusting for variance in 47 CCG clusters.
| Analysis | Adjusted between-arm cost difference | 95% CI |
|---|---|---|
| Primary economic analysis | −£1999 | −£6627 to £2630 |
| Sensitivity analyses | ||
| Per-protocol analysis | −£1724 | −£7663 to £4216 |
| LRTI-specific hospital unit costs | −£1892 | −£6366 to £2582 |
| Assuming intervention costs of £0 | −£2908 | −£7221 to £1404 |
Although mean NHS costs were lower in the intervention arm, primarily because of the impact of lower hospitalisation costs, all results were consistent with a null effect of the intervention on NHS costs.
This conclusion did not change in any of the sensitivity analyses. Hospitalisation was the largest cost element, and the results of analysis in which a narrower definition of unit costs attributable to admissions was used were similar to the primary analysis. Costs attributable to the intervention itself included training time as well as potential integration and troubleshooting time. Our sensitivity analysis in which we assumed zero costs of the intervention, a possible consequence of long-term use and familiarity of the intervention, was similar to the other between-arm cost comparisons. As noted in Chapter 3, the pre-specified per-protocol analysis is likely to be biased by the effect of non-compliance among practices that joined in the second half of the study, during which period the COVID-19 pandemic had reduced dispensing rates.
Discussion
Given the light-touch design of the trial, the economic evaluation of the RCT was limited to a between-arm comparison of mean NHS costs. NHS costs were calculated from the costs of the intervention itself, prescriptions of amoxicillin and macrolides per the co-primary outcome, A&E attendances and hospital admissions. Data were complete. All models – the primary adjusted analysis and sensitivity analyses – were consistent with no effect of the intervention on NHS costs.
Strengths of the economic evaluation
The design of the trial permitted the efficient analysis of very large volumes of data, encompassing millions of dispensed items, and tens of thousands of intervention usages, A&E attendance and hospital admissions. The reliance on routine data for all elements of the economic evaluation met the protocol’s objective of assessing the costs of the intervention to the NHS, while reducing the overall costs of the trial.
Limitations of the economic evaluation
The CHICO feasibility trial confirmed the difficulty of obtaining comprehensive, useable quality-of-life data in this young patient population. This reflected both the absence of quality-of-life instruments designed for young children (especially those aged 0–4 years), issues in interpreting quality-of-life measures in this population and the completeness of data collected from parents on behalf of participating children. By design, we did not measure the quality of life of children in this trial. This meant that the present analysis was limited to a comparison of costs, rather than a full economic evaluation.
Without patient-specific information, it was necessary to make assumptions about the type and costs of care received for those children attending A&E and those admitted to hospital. We used volume-weighted unit costs to account for uses of this service. This was likely to cause material bias for between-arm cost comparisons only if the composition of care (with respect to complications, comorbidities and any attendant complexity) was markedly different in one arm or another.
Our unit cost data on dispensed amoxicillin and macrolides also involved a compositional assumption. We used unit costs drawn from all dispensed amoxicillin and macrolides in England in the 2020–21 financial year. We, therefore, assumed that the practices included in the trial had the same costs as all other practices in England. We also lacked data on the precise formulation and presentation of dispensed items, but it is not clear that including this information would have had a noticeable impact on between-arm comparisons.
The analysis reported here was – by design – limited to a within-trial evaluation of the impact of the intervention on NHS costs during the 12-month period of trial follow-up. Distal impacts of the intervention on antimicrobial resistance and its associated costs over the long term, a critical motivation for undertaking this work, were not accounted for in the within-trial cost comparison. We also did not intend to evaluate the wider costs to the NHS of a full national roll-out, not least because this would be to presuppose both the efficacy outcome of the trial as well as the interest and capacity of systems providers to integrate the intervention.
Nevertheless, the research team did undertake very preliminary, high-level assessments of how costs might be impacted by a wider deployment of the intervention. Based on discussions with CCG ‘Research and Development’ staff, it appears that costs related to licensing, maintenance and support would be modest, especially when considered on a per-practice basis. The level of these costs would also depend on the specific way in which providers implemented and provided the intervention to practices. Nevertheless, there appear to be few grounds on which a wider use of the intervention would drastically increase NHS costs relative to the experiences observed in the CHICO trial.
Conclusion
There was no evidence of a difference in mean NHS costs in those practice randomised to use the intervention compared to those that did not. This conclusion held under various sensitivity analyses, including a per-protocol analysis.
Chapter 6 Facilitators and barriers to an efficient design
The problems with conducting research in primary care
Primary care is a difficult environment in which to conduct research. Effective recruitment strategies generally require practitioner involvement59 but this is difficult in the time allowed for consultations and can lead to the exclusion of patients for whom recruitment might be more challenging and therefore increase the risk of selection bias. 60 In England, general practices are already divided into those who are willing to conduct research and those who are not, which challenges the external validity of any successful trial when rolling out an intervention. 61 The time pressure can be compounded if the intervention is not fully integrated into the practice computer systems; a stand-alone tool takes time to open and close, may not draw upon information already collected within the system and may be less amenable to modification on a wider scale. In primary care research, there are also difficulties in collecting patient outcomes. This is largely dependent on physical access to patient notes and is both costly and time-consuming.
In this section, we look at some of the facilitators and barriers to an efficiently designed trial including semistructured interviews with three members of a CRN network and two from CCGs involved in the set-up of CHICO using a framework approach. 62
Recruiting practices
Practices were recruited using the national network of all 15 CRNs in England and 47 of the 211 CCGs covering the English regions in 2019. Of the 310 practices required, 294 were recruited over a 24-month period (initially we allowed 12 months for this).
Facilitators to recruitment
The 15 CRNs were helpful with the recruitment of practices; presenting our study at CRN meetings and using the national CRN lead for support helped facilitate a co-ordinated national approach. Some CRNs contact practices that opt-in to conduct research while others contact all the practices in their area. Feedback from 11 of the 15 CRNs suggested around half the practices had joined the Research Sites Initiative scheme (NIHR-funded scheme to enable research delivery) and were often the first to be contacted. Recruitment via CCGs had a wider reach of practices than via CRNs, although CCG participation in recruitment was more variable depending on capacity. Working with these two networks provided a large geographical spread across England (see Appendix 5) and provided us with over a quarter of the practices (26%) in the most deprived socioeconomic quintile.
Using quarterly study newsletters for practices, CRNs and CCGs with league tables monitoring levels of recruitment improved responses.
Barriers to recruitment
Recruitment took longer than expected, partly because we had to recruit CCGs first (who provide hospitalisation data for practices). It was difficult to know which individuals to contact and how to do so (some CCGs did not appear to be public-facing), response times were often slow, their role in research was often misunderstood, staff changes hindered communication and a number of CCGs merged during the study period. Some CCGs were averse to getting involved in research or cited lack of capacity as a reason to be excluded from the research.
I mean in reality they [CCG] don’t play a major part in – and never actually have a major role in research. It’s not a core business of a CCG like it would be in a provider. So we don’t have a research department, which is probably why the CHICO trial ended up at my door because it was about prescribing.
[P4 CCG]
At the start of recruitment, October 2018, there were 211 CCGs across England. By the end of follow-up, September 2021, there were only 106 CCGs. While this did reduce the number of CCG contacts required to obtain the data, it sometimes resulted in change of staff who were not familiar with the trial or its requirements.
The level of engagement was much better with CRNs although access to practices was not straightforward. Limiting the contact to practices that want to opt-in to research via some of the CRNs missed the opportunity of letting research-naïve practices know about light-touch efficient design studies. Although other CRNs contacted all practices.
I would say that about a third of the practices were what I would call research naïve or inexperienced, green as in they hadn’t really had a sort of established relationship with us in the past.
[P2 CRN]
Of the 294 practices we recruited, we are aware of at least 22 practices (7%) who merged during their baseline/follow-up annual data capture. We excluded practices who anticipated a merger with another practice but had no control over this once the practice was randomised, especially in the rare instances when the merging practices were randomised to different arms of the trial. The length of time from expression of interest from practices to randomisation was longer than expected due to the delayed site agreements returned from the practices.
Using routinely collected data for the primary outcomes
The co-primary outcomes in the trial were (1) practice-dispensed prescription data for amoxicillin and macrolide antibiotics for children 0–9 years retrieved from the NHSBSA ePACT230 reporting system and (2) hospital admission rate for RTI among 0–9 years routinely collected by all English CCGs. The ascertainment of these data was 99.7% (293/294) for antibiotic dispensing and hospitalisation rates. Data for one practice were only lost due to a merger.
Facilitators to collecting routine data
Using aggregated data avoids the need for the consulting physician to solicit individual consent, reduces or eliminates the risk of selection bias and removes the task of accessing individual patient notes. Routine data are often collected monthly so data completeness and quality can be monitored throughout the collection period, which was particularly important in this trial to scrutinise and report any sudden changes in hospital admission rates to the DMC. The routinely collected data can be imported to data management software; thus, there is less likelihood of data entry errors and missing data are rare. The cost of research staff and time taken to collect the primary outcome data was much reduced compared to a traditional trial. In total 11/294 practices asked to be withdrawn from the study (3.7%) and 14/294 (4.8%) were lost to follow-up from the study; the main reasons cited being lack of capacity and prioritising the COVID-19 pandemic. A further advantage of collecting routine data is that withdrawal of a practice does not necessarily mean a loss of the primary outcome data for that practice (if the data are already in the public domain).
Barriers to collecting routine data
A potential problem with aggregate data collected from a third party is where data are suppressed, owing to a low number of events, although in this study data were collected over a 12-month period largely avoiding this problem. Some liaison was needed between the trial team and the CCGs to know exactly what data were available and in what format this could be presented needing additional time to import and utilise data from different formats. Dispensed prescription data, practice list size data and hospital admission data are reliable as they feed into financial transactions; however, data reporting A&E attendance were less so due to a limited coding set. Many A&E attendances do not have a diagnosis coded; therefore, the number of attendances attributable to RTI is likely to be inaccurate.
Integrating the intervention within the electronic medical record system
The trial only included practices using the EMIS® system. The practice champion was given written instructions on how to install the intervention within EMIS®. Intervention use was monitored using searches on the EMIS® system, run by the practice champion and shared with the research team.
Facilitators to integrating the intervention
Installation of the intervention was relatively straightforward for EMIS®. Practice systems allow for user-friendly manipulation so interventions can be integrated and interfaced with system data already collected; thus, negating any additional clinical workload. Screen pop-ups can also be used to notify clinicians of eligible patients for the study.
Barriers to integrating the intervention
Familiarity with the EMIS® system varied between practices; thus the level of support required from the research team to help install the intervention and download usage also varied. Provision to help install or use third-party algorithms is not offered by system providers. EMIS® upgrades to the system during the trial meant that rewriting installation instructions, resources and testing had to be carried out and fed back to the practices. For instance, the READ codes used to identify clinical terms within consultations were upgraded to SNOMED codes by EMIS® in the early months of 2020, which meant the algorithm had to be amended so the intervention would function correctly. We found that our funded provision of IT support throughout the study was crucial to the smooth running of the integrated intervention. Although pop-ups can be used, some clinicians found them irritating and switched them off while other practices had so many pop-ups (especially during the COVID-19 pandemic) that the CHICO pop-up was often obscured.
A light-touch approach to data collection
Clinicians were not required to provide any data about individual participants (apart from reporting SAEs), those in the intervention group were asked to familiarise themselves with the tool and use it, while those in the control arm were asked to provide usual care. Data collection from the practices directly was limited to short baseline and follow-up questionnaires. Data on intervention usage were downloaded from EMIS® and the co-primary outcome was downloaded from ePACT2 and the relevant CCGs.
Facilitators to a light-touch approach
The light-touch approach reduced the time needed during consultation to record information, the trawling of patient notes and provided a more objective data resource downloaded from the system rather than from individual input. Practice champions, familiar with practice systems, played an important role in obtaining the required data and training clinicians in the use of the intervention. Baseline questionnaires were received from 294/294 practices (100%), follow-up questionnaires were received from 265/294 practices (90%) while intervention usage was collected from 116/144 of the intervention practices (81%), indicating the data burden was not too onerous. Training consisted of a brief conversation between the practice champion and clinicians, signposting to further information available if needed and a 1-month run-in period before data collection where clinicians could familiarise themselves with the intervention. Feedback suggested the clinicians were happy in terms of training to use the intervention tool.
Barriers to a light-touch approach
Conversely, this approach reduced the level of interpretation that can be gleaned from the data. Removing patient-level recruitment results in a loss of denominator data, in our case not knowing how many individual children consulted for RTI and cough. As a proxy, we used the number of children registered at each practice. Given the diversity of the practices included in the trial, we are assuming that those children taking part in the trial were no different from children in the general population, but we have no way of testing this assumption. We also lost detail that may be taken from the consultation of whether antibiotics were prescribed immediately or delayed relying instead on the number of antibiotics being dispensed. The lack of contact with control practices (apart from reminders to provide SAE reports) also ran the risk of practices wanting to withdraw from the study, especially with changes of staff. Several strategies were needed to obtain follow-up paperwork back from practices, including help from the CRN network and the managed recovery team, newsletters and time and resources spent trying to contact the practices at varying times and days. Consideration also needs to be given to the ethical implications of not seeking consent from individual patients. We asked sites to display posters informing patients the research was taking place and no concerns were raised but we did not proactively seek a response on this issue.
Chapter 7 Discussion
Summary and interpretation of the main findings
There was no evidence overall that the antibiotic dispensing rate, for amoxicillin or macrolides, in the intervention practices differed from practices in the control arm. There were no differences seen in the rate of hospitalisations for RTI between study arms. Subgroup analyses did show decreased dispensing rates in the intervention arm for older children and increased rates in the younger children. This analysis also showed decreased dispensing rates in the intervention arm for practices restricted to one site, in less deprived areas and proportionally fewer nurse practitioners. Conversely, the intervention arm increased practice dispensing rates in those practices with more than one site, situated in the more deprived areas and where there was a higher proportion of nurse practitioners. Intervention use was low in relation to the likely number of children in whom it could have been used. The impact of the COVID-19 pandemic on the trial was noticeable in terms of the primary outcomes and made interpretation of the findings more difficult. Impact of the COVID-19 pandemic on dispensing rates and intervention usage
Follow-up data were collected from practices over a 35-month period from November 2018 to September 2021 which included a 19-month period from March 2020, when the pandemic spread across England. Taking account of seasonal fluctuation, by focusing on the 12-month periods before and after 1 March 2020, the average dispensing rate in the control practices halved from 20.55 items per 100 children (95% CI 19.64 to 21.46) to 9.84 items per 100 children (95% CI 9.18 to 10.50). A similar reduction was also evident among the intervention practices but would make any differences between the two arms more difficult to distinguish. The pandemic also had an impact on intervention usage. Prior to March 2020, the median usage per month, per intervention practice, was nine uses. Post March 2020, the median usage per month, per practice, was zero. Half of the practices questioned responded that there had been months where they were unable to use the intervention or they had limited usage. The qualitative interviews with the clinicians also suggested the changing patterns of consultations in practices affected the number of children being seen face to face, while school closures caused a slowdown in RTI infections and thus reduced consultations. A post hoc sensitivity analysis focused on the months of data collected prior to the pandemic and showed reduced dispensing in the intervention arm compared to the control arm, that did provide statistical evidence of a difference. However, the absolute difference was so small the clinical benefit would be negligible. There was also a correlation between increased intervention usage and decreased dispensing rates in the pre-pandemic data collected but again the number of practices was too low to draw any conclusions. Although the impact of the pandemic does not invalidate our main findings, it does question just how negative these findings may have been in the absence of the pandemic.
Impact of the COVID-19 pandemic on hospitalisation rates
The effects of COVID-19, and its associated lockdowns on hospitalisation admission were clear. From April 2020, the rates dropped and only started to climb back towards normal levels in the summer of 2021 when the rates were unseasonably high. A similar pattern was observed for A&E attendance. Despite these uncommon fluctuations, the rates for attendance and admission were similar for both arms of the trial.
How the intervention was received
Intervention usage over time
The intervention was used nearly 12,000 times in the trial with a median average of 70 uses per practice. It was difficult to gauge usage over the 12-month follow-up period, given the seasonal influence and impact of the pandemic, although the data suggest usage fell after the first few months and slightly picked up towards the end of follow-up. The qualitative interviews confirmed that clinicians used the intervention at the start of the trial, but usage waned over time. We did not collect data on how many RTI consultations were conducted in the trial. A rough estimate can be made from the dispensing data and intervention practice characteristics if we assume the dispensed items were all for RTI consultations and 50% of the children consulting were given these items. The dispensing rate in the intervention arm was 15.5 items per 100 children, given the median number of 0–9-year-olds was 975 children in 144 intervention practices, this yields 21,762 dispensed items and 43,524 consultations for RTI. This is purely speculative but would suggest the 11,944 uses of the intervention was around 1 in every 4 consultations. The qualitative interviews suggested some clinicians found the intervention most useful in ‘borderline cases’, which suggests clinicians may have been selective in terms of when they used the tool and partly explains the low usage.
Acceptance of the intervention
Generally the intervention was easy to use and few thought it unhelpful; nearly three-quarters suggested they would use the intervention again, if it became available. The qualitative interviews suggested the clinicians liked the algorithm template and found it straightforward to use, without adding any more time to consultations. Clinicians particularly liked the safety-netting parental advice leaflet, as it helped explain treatment decisions and home care with parents and this was seen to be the most useful intervention component. While some clinicians reported that the intervention influenced prescribing decisions, others reported that they used it as a supportive aid during consultations rather than a tool to change prescribing behaviour. Some clinicians reported the intervention interrupted the consultation flow, having to close the patient’s record before the end of the consultation to complete the intervention process, which did not always align with clinicians’ usual processes.
Strengths and limitations
The CHICO study demonstrated that an efficiently designed practice-level large trial in primary care using routinely collected data was feasible and potentially good value for money. The average cost of an NIHR RCT was £1.25 million in 2019–20,63 whereas the cost of the CHICO RCT, which included over 300,000 children (5% of the entire national 0–9-year-old population) was below £1 million. The study was geographically widespread recruiting 4% of all GP practices in England. Using different recruitment strategies64 and existing networks such as the CRNs helped recruit some research-naïve practices and gave us a sample that reflected the national breakdown of practices located in more socioeconomically deprived areas. Conducting the trial at the practice level removed the need for patient recruitment and potential for recruitment differential between arms while focusing the clinician’s time on using the intervention, reflecting real-life practice. Utilising routinely collected data as the primary outcome reduced problems with missing data while removing the burden of searching through patient notes. Integrating the intervention within the practice system both exploited the data already available and added to the patient’s record avoiding duplicating of effort and saved time. Primary care practices are often very busy and the average length of face-to-face consultation in the UK is around 10 minutes; less than half the time given to patients in, for example, Sweden and the USA. 65 Thus, embedding the intervention and avoiding patient recruitment protected the limited time available within the consultation. The NIHR is keen to see more efficient, innovative studies which provide robust evidence to inform clinical practice and policy,66 and this trial is a good example of that.
Efficient design studies are not suitable if individual patient consent is required and face their own challenges in the changing landscape of primary care in England. Around 2.5% of practices close or merge each year while some new practices open and recognition of the current research portfolio within practices, especially when signing up to a trial, needs to be part of this process. 67 We were also surprised by the wide variability in practice list size, 8% of practices in the study having three sites or more. The expansion of multisite practices has implications for future trials in terms of factoring in variable list sizes for sample size calculations and checking that multiple sites use the same electronic record systems. For these larger multiple-site practices further provision may be needed; in terms of training, monitoring and data collection a practice champion might be required for each of the different sites.
Growing demands on primary care services have also led to policymakers promoting telephone and video consultations, even before the COVID-19 pandemic, and these sometimes do not lend themselves to enlisting patients in research. 68 Interventions like ours, that require face-to-face consultations, cannot be used if the child cannot be assessed and future studies need to take into account this changing landscape of consultations. Embedding the intervention within the record system makes it easier for clinicians to use and to monitor use but also means that IT support is needed, especially if the system manufacturers make changes that might impact on intervention use. Utilising CCGs to collect one of the primary outcomes also added an extra layer of recruitment in that we needed to get CCGs on board before we could recruit practices in their area. The number of CCGs in England almost halved during the study period due to organisational restructuring which made it difficult to administer the trial. There was also a lack of uniformity when approaching these commissioning groups and their role in research needed clarifying. 69 Another limitation of the design was the allowance for a delayed start date. While this was rare in the control arm (3%), a quarter of intervention practices delayed their start date resulting in a ‘gap’ between their baseline and follow-up period. In the majority of cases, this was to allow the practice more time to implement the intervention due to staffing/resource issues caused by COVID-19.
One of the main limitations of a light-touch approach to data collection is the loss of detail available for analysis. Losing denominator data such as patients consulting for different conditions will depend on the hypothesis being tested and needs to strike a balance between the accuracy of what you are trying to measure and whether a proxy marker will deliver the population impact of the intervention. Using the number of 0–9-year-olds registered at the practice as a denominator accounted for variation in practice size but meant we could not measure the number of children consulting for RTI, which is both a more precise denominator and a metric for measuring intervention usage. The routinely collected dispensing data served as a robust proxy marker for prescribing data but cannot distinguish between immediate and delayed prescriptions, nor antibiotics prescribed for non-RTI indications. Fidelity in this study was also difficult to measure; we monitored intervention usage from symptoms recorded but not how the different components of this complex intervention were used or how long this took. Our light-touch approach meant we did not record the number of RTI consultations or have any means to investigate the nature of these consultations and how this was associated with intervention usage which would have been particularly useful given the impact of the COVID-19 pandemic. We estimate that our intervention was used in approximately one in four consultations. The qualitative interviews suggest clinicians often used the intervention for borderline cases which reflects our advice that the intervention should just be one tool used in the toolbox to deal with these consultations and partly explains why the average use of the intervention was just 70 per practice over 12 months. Finally, an unavoidable limitation is that we have not included the effects of antimicrobial resistance. Antimicrobial resistance is a long-term problem where the overuse of antibiotics may lead to greater resistance for many common diseases, which in turn will have long-term and extensive adverse impacts on health. This RCT is part of a preventative strategy to reduce this potential by testing the efficacy of a tool that may reduce the use of antibiotics. To test any effects on whether we are reducing the problem of antimicrobial resistance would need a different study design over many more years.
Comparison with other literature
Antimicrobial stewardship interventions can seek to either change the type of antibiotic prescribed, and/or reduce the quantity of antibiotics prescribed. Most existing literature focuses on the latter (quantity) with a recent systematic review synthesising the evidence for interventions targeted at reducing antibiotic prescribing for respiratory infections. 70 This identified four types of intervention with moderate-strength evidence of reduced antibiotic prescribing compared with usual care. These were: (1) clinic-based parent education (21% overall prescribing reduction; similar return visits); (2) public patient education campaigns combined with clinician education (7% reduction in overall prescribing); (3) procalcitonin point-of-care testing in adults (12–72% overall prescribing reduction); and (4) electronic decision support systems (5–9% reduction in overall prescribing).
We previously showed that interventions were most likely to be effective in children with respiratory infections if they (1) target both parents and clinicians during consultation; (2) provide automatic prescribing prompts; and (3) include clinician leadership in the intervention design. 16
The present study used an electronic decision support system intended to reduce antibiotic prescribing (and therefore dispensing) in children at very low risk of hospitalisation in the 30 days following consultation, finding no evidence of overall reductions in antibiotic dispensing.
We are aware of one other UK study resembling CHICO. The REDUCE cluster randomised trial also randomised GP practices to receive an electronic medical record embedded decision support tool aiming to reduce antibiotic prescribing for respiratory infections. It found evidence that the intervention was effective in adults (15–84 years) but, as with our study, did not find evidence of reduced prescribing in children (0–14 years, nor adults aged > 84 years). This study also monitored safety by reporting hospital admissions for serious bacterial infections, finding no evidence these were increased in the intervention group. 71
The reduction in consultation and antibiotic dispensing rates we observed between pre-pandemic and pandemic periods is consistent with that reported in a number of other studies, both in the UK and globally. 72–74
Implications for policy, clinical practice and research
While showing promise in some subgroups, we did not find evidence to support the widespread adoption of the CHICO intervention in routine clinical practice. We suspect the main reasons for ineffectiveness were: (1) relatively low frequency of intervention use in relation to the number of children presenting in whom it could have been used; (2) the purpose of the algorithm element of the intervention not being entirely clear to intervention clinicians (to identify children at very low risk of future hospitalisation in whom antibiotics could be safely withheld); and (3) problems with delivery timing of the intervention (often not being presented to the clinician until after the consultation end).
Electronic medical record providers could improve intervention effectiveness if platforms could improve timing of intervention delivery (e.g. decision aids appearing before consultations are closed). Although the intervention does not appear to change prescribing behaviour, elements of the approach may be used in the design of future interventions. Further research is also needed to develop and evaluate effective electronic record-based antimicrobial stewardship interventions for children, who appear to be a ‘hard to reach’ group with regard to this methodology.
Conclusion
Embedding a multifaceted intervention into general practice for children presenting with acute cough and RTI did not reduce antibiotic dispensing or impact on hospital attendance for RTI. There was weak evidence of reduced dispensing levels in the intervention arm prior to the COVID-19 pandemic although the numbers were small. Inference of the findings was made difficult as the pandemic affected intervention usage, dispensing levels and hospital attendance, and it could be argued that the pandemic critically impacted the ability of the trial to demonstrate its intended outcome. The clinicians liked the intervention and used it as a supportive aid during consultations rather than a tool to change behaviour. The use of an efficient design was successful in this trial suggesting using routinely collected data for primary outcomes at the practice level is viable in England. An infrastructure for recruiting practices is in place and with more investment should be promoted for primary care research where appropriate.
Acknowledgements
This study was designed and delivered in collaboration with the Bristol Randomised Trials Collaboration (BRTC), part of the Bristol Trials Centre, is in receipt of National Institute for Health and Care Research CTU support funding. Study data were collected and managed using REDCap75 hosted at the University of Bristol. The University of Bristol is acting as the sponsor for this trial and the trial is hosted by the NHS Bristol, North Somerset and South Gloucestershire CCG. The study received North of Scotland Research Ethics Committee (REC) approval on 29 October 2018 and by Health Research Authority at London-Camden and Kings Cross REC (ref:18/LO/0345) on 14 November 2018. Trial Registration Number: ISRCTN11405239. The authors would like to thank all General practices, CCGs and CRNs for their involvement in CHICO. The authors would also like to thank the members of the TSC and DMC.
Contributions of authors
Peter S Blair (https://orcid.org/0000-0002-7832-8087) (Professor of Epidemiology and Statistics) was chief investigator for the trial, and as such participated in its design, was responsible for oversight of the trial and wrote this final report.
Grace J Young (https://orcid.org/0000-0002-5210-1183) (Medical Statistician) was the trial statistician, involved in writing the statistical analysis plan, co-ordinating data collection and conducting the statistical analysis.
Clare Clement (https://orcid.org/0000-0002-5555-433X) (Qualitative Researcher) was the qualitative researcher for the trial, involved in managing and conducting the qualitative analysis.
Padraig Dixon (https://orcid.org/0000-0001-5285-409X) (Health Economist) was a co-applicant, and health economist for the trial, involved in designing, managing and conducting the health economic evaluation.
Penny Seume (https://orcid.org/0000-0001-9407-5828) (Trial Manager) was responsible for managing the trial, acquisition of data and contributing to aspects of trial design.
Jenny Ingram (https://orcid.org/0000-0003-2366-008X) (Qualitative Researcher) was a co-applicant, and one of the lead qualitative researchers for the trial, involved in the design of the trial and qualitative analysis.
Jodi Taylor (https://orcid.org/0000-0001-7171-8923) (Head of Trial Operations) contributed to aspects of the trial design, and was involved in the day-to-day management of the trial.
Jeremy Horwood (https://orcid.org/0000-0001-7092-4960) (Qualitative Researcher) was a co-applicant, and one of the lead qualitative researchers for the trial, involved in the design of the trial and qualitative analysis.
Patricia J Lucas (https://orcid.org/0000-0002-0469-8085) (Behavioural Scientist) was a co-applicant and involved in the design of the trial, behavioural modelling and development of the intervention.
Christie Cabral (https://orcid.org/0000-0002-9884-0555) (Qualitative Researcher) was a co-applicant and involved in the design of the trial, development of the intervention and qualitative analysis.
Nick A Francis (https://orcid.org/0000-0001-8939-7312) (Professor of Primary Care Research) was a co-applicant and involved in the design of the trial and provided expertise in practice-based RTI interventions.
Elizabeth Beech (https://orcid.org/0000-0001-7288-1167) (NHS Antimicrobial Stewardship Lead) was a co-applicant, involved in the design of the trial, acquisition of data and provided expertise in the collection of routine data.
Martin Gulliford (https://orcid.org/0000-0003-1898-9075) (Professor of Public Health) was a co-applicant, involved in the design of the trial and provided expertise on routine data analysis.
Sam Creavin (https://orcid.org/0000-0002-6772-7111) (GP and Clinical Research Fellow) was a co-applicant, involved in the design of the trial and provided expertise in EMIS® systems and development of the intervention.
Janet A Lane (https://orcid.org/0000-0002-7578-4925) (Reader in Trials Research, codirector of the BRTC) was a co-applicant, contributed to the trial design and was involved in management of the study.
Scott Bevan (https://orcid.org/0000-0002-4567-5111) (Trial Manager) was responsible for managing the trial during the first 18 months and contributed to aspects of trial design.
Alastair D Hay (https://orcid.org/0000-0003-3012-375X) (GP and Professor of Primary Care Research) was a co-applicant and clinical lead, who initiated the idea and led the development with a NIHR Programme grant. He was involved in the design of the trial, further development of the intervention and co-wrote parts of the final report.
All authors read, approved and/or commented on the final report.
Funding
This research is funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme (funder ref: 16/31/98). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The project start date was 1 March 2018, and the planned study end date was 30 November 2020. Due to delays with practice recruitment, a 12-month costed extension was granted by the HTA in July 2019 to enable the target figure to be reached, resulting in the study end date being 30 November 2021. Due to delays with COVID-19 (including pausing recruitment during the first wave of the pandemic), a further 4-month un-costed extension was granted in June 2020, resulting in the study’s final end date being 31 March 2022.
Dissemination
We are planning to write to all the GP practices, CCGs and CRNs that have been involved in the study with a brief summary of the main findings the day before either the final report or the peer-reviewed paper is published. We have also submitted two abstracts of our main findings (quantitative and qualitative) for oral presentation to the Society of Academic Primary Care (SAPC) national conference held in Lancaster in July 2022:
Blair PS, Young G, Seume P, Ingram J, Clement C, Taylor J, Cabral C, Lucas PJ, Beech E, Horwood J, Dixon P, Gulliford M, Francis N, Creavin S, Lane AJ, Bevan S, Hay AD. (2022), Evaluation of a complex intervention to improve antibiotic prescribing for CHIldren presenting to primary care with acute COugh and respiratory tract infection: the CHICO randomised controlled trial. Submitted to the Society for Academic Primary Care (SAPC ASM) 50th Annual Scientific Meeting, University of Central Lancashire, 4–6 July.
Clement C, Ingram J, Cabral C, Blair PS, Lucas P, Hay AD, Seume P, Horwood J. (2022), What are clinician’s views and experiences of implementing a complex intervention to inform antibiotic prescribing in children with respiratory tract infections in primary care? Submitted to the Society for Academic Primary Care (SAPC ASM) 50th Annual Scientific Meeting, University of Central Lancashire, 4–6 July.
We have presented one abstract of preliminary findings at the South-West SAPC Conference 2020:
Clement C, Ingram J, Cabral C, Blair PS, Lucas P, Horwood J. (2020), Clinicians views and experiences of implementing a complex intervention to reduce antibiotic prescribing in children with Respiratory Tract Infections in primary care. Submitted to the Society for Academic Primary Care South-West (SAPC SW) Annual Research Meeting, Bristol, UK, 6 March.
Two abstracts of preliminary findings at the South-West SAPC Conference 2021:
Seume P, Young G, Ingram J, Lucas P, Taylor J, Hay AD, Dixon P, Creavin S, Horwood J, Lane AJ, Beech E, Blair PS. (2021), An efficiently designed RCT in primary care clustered at the practice level using routinely collected CCG data as the primary outcome: barriers and facilitators from the CHIldren’s COugh (CHICO) study. Submitted to the Society for Academic Primary Care South-West (SAPC SW) Annual Research Meeting, University of Warwick Onlin,e 18 March.
Clement C, Ingram J, Cabral C, Blair PS, Lucas P, Hay AD, Seume P, Horwood J. (2021), Clinicians’ views and experiences of implementing a complex intervention to inform antibiotic prescribing in children with respiratory tract infections in primary care. Submitted to the Society for Academic Primary Care South-West (SAPC SW) Annual Research Meeting, University of Warwick Online, 18 March.
One abstract of preliminary findings at the South-West SAPC Conference 2022:
Seume P, Blair PS, Ingram J, Clement C, Young G, Taylor J, Cabral C, Lucas PJ, Beech E, Horwood J, Dixon P, Gulliford M, Francis N, Creavin S, Lane AJ, Bevan S, Hay AD. (2022), A more efficient approach to randomised controlled trials in primary care using routinely collected practice-level data. Submitted to the Society for Academic Primary Care South-West (SAPC SW) Annual Research Meeting, University of Exeter (Online), 10–11 March.
We have one paper describing the protocol published:
Seume P, Bevan S, Young G, Ingram J, Clement C, Cabral C, Lucas PJ, Beech E, Taylor J, Horwood J, Dixon P, Gulliford MC, Francis N, Creavin ST, Lane AJ, Hay AD, Blair PS. Protocol for an ‘efficient design’ cluster randomised controlled trial to evaluate a complex intervention to improve antibiotic prescribing for CHIldren presenting to primary care with acute COugh and respiratory tract infection: the CHICO study. BMJ Open. 2020 April 11(3). http://bmjopen.bmj.com/cgi/content/full/bmjopen-2020-041769.
One paper describing the efficient design has now published:
Blair PS, Ingram J, Clement C, Young G, Seume P, Taylor J, Cabral C, Lucas P, Beech E, Horwood J, Dixon P, Gulliford M, Francis N, Creavin S, Lane A, Bevan S, Hay AD. A more efficient approach to randomised controlled trials in primary care using routinely collected practice-level data. BMJ Open 2022;12(7):e061574.
And we have submitted our main findings to the BMJ.
Blair PS, Young G, Clement C, Dixon P, Seume P, Ingram J, Taylor J, Cabral C, Lucas PJ, Beech E, Horwood J, Gulliford M, Francis NA, Creavin S, Lane JA, Bevan S & Hay AD. A multifaceted intervention to improve management of antibiotics for CHIldren presenting to primary care with acute COugh and respiratory tract infection (CHICO): an efficient cluster randomised controlled trial. Submitted to the BMJ, August 2022.
We intend to submit a separate qualitative paper.
Data-sharing statement
This study used secondary data which can be obtained from the NHS digital website and NHSBSA ePACT2 website (https://www.nhsbsa.nhs.uk/access-our-data-products/epact2) (accessed 5 September 2022) for practice-level list sizes and dispensing data by practice, respectively. The hospitalisation data are not available for redistribution as it was not agreed as part of the contracts with the CCGs. For any further information, please contact the corresponding author.
Equality, diversity and inclusion
The CHICO research team was a multidisciplinary team made up of clinical academics, child health researchers, clinical trial methodologists, statisticians, health economists, qualitative researchers and a NHS antimicrobial stewardship lead. Practices were recruited from every CRN in England, in an effort to gain a representative sample of general practices and also involve less ‘research active’ practices. Involvement at the CRN/CCG level, insured that all practices were provided with an opportunity to take part. Any EMIS® practice was eligible but those using alternative electronic health systems could not be included. The patient leaflet was translated into other languages (Punjabi and Urdu) so that non-English-speaking families could take part.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Hay AD, Heron J, Ness A. The ALSPAC Study Team . The prevalence of symptoms and consultations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): a prospective cohort study. Fam Pract 2005;22:367-74.
- Hollinghurst S, Gorst C, Fahey T, Hay A. Measuring the financial burden of acute cough in pre-school children: a cost of illness study. BMC Fam Pract 2008;9.
- Ferech M, Coenen S, Malhotra-Kumar S, Dvorakova K, Hendrickx E, Suetens C, et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe. J Antimicrob Chemother 2006;58:401-7.
- Ashworth M, Charlton J, Ballard K, Latinovic R, Gulliford M. Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK practices 1995–2000. Br J Gen Pract 2005;55:603-8.
- Howie JG. Clinical judgement and antibiotic use in general practice. BMJ 1976;2:1061-4.
- Goossens H, Ferech M, van der Stichele R, Elseviers M. ESAC Project Group . Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005;365:579-87.
- Sharland M, Kendall H, Yeates D, Randall A, Hughes G, Glasziou P, et al. Antibiotic prescribing in general practice and hospital admissions for peritonsillar abscess, mastoiditis, and rheumatic fever in children: time trend analysis. BMJ 2005;331:328-9.
- Little P, Watson L, Morgan S, Williamson I. Antibiotic prescribing and admissions with major suppurative complications of respiratory tract infections: a data linkage study. Br J Gen Pract 2002;52:187-93.
- Priest P, Yudkin P, McNulty C, Mant D, Wise R. Antibacterial prescribing and antibacterial resistance in English general practice: cross sectional study commentary: antibiotic resistance is a dynamic process. BMJ 2001;323:1037-41.
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340.
- Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ 1997;315:350-52.
- Standing Medical Advisory Committee . The Path of Least Resistance 1998.
- NICE . Respiratory Tract Infections: Prescribing of Antibiotics for Self-Limiting Respiratory Tract Infections in Adults and Children in Primary Care 2008.
- Why Children Die: A Pilot Study (2006). London: CEMACH; 2008.
- Andrews T, Thompson M, Buckley DI, Heneghan C, Deyol R, Redmond N, et al. Interventions to influence consulting and antibiotic use for acute respiratory tract infections in children: a systematic review and meta-analysis. PLOS ONE 2012;7.
- Vodicka TA, Thompson M, Lucas P, Heneghan C, Blair PS, Buckley DI, et al. Reducing antibiotic prescribing for children with respiratory tract infections in primary care: a systematic review. Br J Gen Pract 2013;63:445-54.
- Thompson M, Vodicka TA, Blair PS, Buckley DI, Heneghan C, Hay AD. Duration of symptoms of respiratory tract infections in children: a systematic review. BMJ 2013;347.
- Cabral C, Horwood J, Hay AD, Lucas PJ. How communication affects prescription decisions in consultations for acute illness in children: a systematic review and meta-ethnography. BMC Fam Pract 2014;15.
- Lucas PJ, Cabral C, Hay AD, Horwood J. A systematic review of parent and clinician views and perceptions that influence prescribing decisions in relation to acute childhood infections in primary care. Scand J Prim Health Care 2015;33:11-20.
- Redmond NM, Davies R, Christensen H, Blair PS, . The TARGET cohort study protocol: a prospective primary care cohort study to derive and validate a clinical prediction rule to improve the targeting of antibiotics in children with respiratory tract illnesses. BMC Health Serv Res 2013;13.
- Hay AD, Redmond NM, Turnbull S, Christensen H, Thornton H, Little P, et al. Development and internal validation of a clinical rule to improve antibiotic use in children presenting to primary care with acute respiratory tract infection and cough: a prognostic cohort study. Lancet Respir Med 2016;4:902-10.
- Lucas P, Ingram J, Redmond NM, Cabral C, Turnbull S, Hay A. Development of an intervention to reduce antibiotic use for childhood coughs in UK primary care using critical synthesis of multi-method research. BMC Med Res Methodol 2017;17:1-10.
- Turnbull SL, Redmond NM, Lucas P, Cabral C, Ingram J, Hollinghurst S, et al. The CHICO (Children’s Cough) Trial protocol: a feasibility randomised controlled trial investigating the clinical and cost-effectiveness of a complex intervention to improve the management of children presenting to primary care with acute respiratory tract infection. BMJ Open 2015;5.
- Blair PS, Turnbull S, Ingram J, Redmond N, Lucas PJ, Cabral C, et al. Feasibility cluster randomised controlled trial of a within-consultation intervention to reduce antibiotic prescribing for children presenting to primary care with acute respiratory tract infection and cough. BMJ Open 2017;7.
- Green LW, Kreuter MW. Health promotion as a public health strategy for the 1990s. Ann Rev Public Health 1990;11:319-34.
- Cabral C, Lucas PJ, Ingram J, Hay AD, Horwood J. ‘It’s safer to …’ parent consulting and clinician antibiotic prescribing decisions for children with respiratory tract infections: an analysis across four qualitative studies. Soc Sci Med 2015;136–137:156-64.
- National Institute for Clinical Excellence (NICE): Department of Health . Respiratory Tract Infections: Prescribing of Antibiotics for Self-Limiting Respiratory Tract Infections in Adults and Children in Primary Care 2008.
- Ingram J, Cabral C, Hay AD, Lucas PJ, Horwood J. TARGET Team . Parents’ information needs, self-efficacy and influences on consulting for childhood respiratory tract infections: a qualitative study. BMC Fam Pract 2013;14.
- Horwood J, Cabral C, Hay AD, Ingram J. Primary care clinician antibiotic prescribing decisions in consultations for children with RTIs: a qualitative interview study. Br J Gen Pract 2016;66:e207-13.
- The EPACT Website n.d. www.nhsbsa.nhs.uk/epact2 (accessed 5 September 2022).
- Public Health England . English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) 2016.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, . Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016;315:1864-73.
- Gulliford MC, Moore MV, Little P, Hay AD, Fox R, Prevost AT, et al. Safety of reduced antibiotic prescribing for self limiting respiratory tract infections in primary care: cohort study using electronic health records. BMJ 2016;354.
- Gulliford MC, van Staa T, Dregan A, McDermott L, McCann G, Ashworth M, et al. Electronic health records for intervention research: a cluster randomized trial to reduce antibiotic prescribing in primary care (eCRT study). Ann Fam Med 2014;12:344-51.
- Gulliford MC, van Staa TP, McDermott L, McCann G, Charlton J, Dregan A. eCRT Research Team . Cluster randomized trials utilizing primary care electronic health records: methodological issues in design, conduct, and analysis (eCRT study). Trials 2014;15.
- Avent ML, Hansen MP, Gilks C, Del Mar C, Halton K, Sidjabat H, et al. General Practitioner Antimicrobial Stewardship Programme Study (GAPS): protocol for a cluster randomised controlled trial. BMC Fam Pract 2016;17.
- Juszczyk D, Charlton J, McDermott L, Soames J, Sultana K, Ashworth M, et al. Electronically delivered, multicomponent intervention to reduce unnecessary antibiotic prescribing for respiratory infections in primary care: a cluster randomised trial using electronic health records – REDUCE Trial study original protocol. BMJ Open 2016;6.
- Horwood J., Cabral C., Hay A., Ingram J. Primary healthcare practitioners’ diagnostic and antibiotic prescribing decisions in children’s consultations for respiratory tract infections. Br J Gen Pract 2016;66:e207-13.
- May CR, Mair F, Finch T, . Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4.
- Seume P, Bevan S, Young G, Ingram J, Clement C, Cabral C, et al. Protocol for an ‘efficient design’ cluster randomised controlled trial to evaluate a complex intervention to improve antibiotic prescribing for CHIldren presenting to primary care with acute COugh and respiratory tract infection: the CHICO study. BMJ Open 2021;11.
- Kontopantelis E, Stevens RJ, Helms PJ, Edwards D, Doran T, Ashcroft DM. Spatial distribution of clinical computer systems in primary care in England in 2016 and implications for primary care electronic medical record databases: a cross-sectional population study. BMJ Open 2018;8.
- Clinical Research Network n.d. www.nihr.ac.uk/explore-nihr/support/clinical-research-network.htm (accessed 5 September 2022).
- Bower P, Grigoroglou C, Anselmi L, Kontopantelis E, Sutton M, Ashworth M, et al. Is health research undertaken where the burden of disease is greatest? Observational study of geographical inequalities in recruitment to research in England 2013–2018. BMC Med 2020;18.
- Cabral C, Ingram J, Redmond N, Horwood J, Blair P, Hollinghurst S, et al. Caring for Children with Coughs: Information for Parents. Bristol: Centre for Academic Primary Care, University of Bristol; 2016.
- Practice List Size via the NHS Digital Website n.d. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/general-practice-data-hub/patients-registered-at-a-gp-practice (accessed 5 September 2022).
- CHICO Statistical Analysis Plan n.d. https://research-information.bris.ac.uk/ws/portalfiles/portal/278667871/CHICO_SAP_draft_v1.0_26.05.21_SIGNED.pdf.pdf (accessed 5 September 2022).
- IMD Postcodes n.d. https://imd-by-postcode.opendatacommunities.org/imd/2019 (accessed 5 September 2022).
- IDACI Measures n.d. https://opendatacommunities.org/def/concept/general-concepts/imd/idaci#:~:text=The%20Income%20Deprivation%20Affecting%20Children,deprivation%20relating%20to%20low%20income (accessed 5 September 2022).
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8.
- Sandelowski M. Sample size in qualitative research. Res Nurs Health 1995;18:179-83.
- QSR International . What Is NVivo? | NVivo 2019. www.qsrinternational.com/nvivo/what-is-nvivo (accessed 5 September 2022).
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101.
- Tracy SJ. Qualitative quality: eight ‘big-tent’ criteria for excellent qualitative research. Qual Inq 2010;16:837-51.
- Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs A H, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ 2022;376.
- NHS Employers, Annual Pay Scales 2020 21 n.d. www.nhsemployers.org/articles/annual-pay-scales-202021 (accessed 1 February 2022).
- NHS . 2019/20/National/Cost/Collection/Data/Publication 2021. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/ (accessed 15 January 2022).
- Nixon RM, Wonderling D, Grieve RD. Non-parametric methods for cost-effectiveness analysis: the central limit theorem and the bootstrap compared. Health Econ 2010;19:316-33. https://onlinelibrary.wiley.com/doi/abs/10.1002/hec.1477?casa_token=bWPOoMTRWOgAAAAA:BZi2CJKW76Ewgy9jvcbSGiq8dDe7_zTHGO26sINKlu1Duq9t-73TmErbOQ1WTt-CR3H3rhpCTAwt (accessed 14 September 2022).
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II: an ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. www.sciencedirect.com/science/article/pii/S1098301515000169 (accessed 14 September 2022).
- Ngune I, Jiwa M, Dadich A, Lotriet J, Sriram D. Effective recruitment strategies in primary care research: a systematic review. Qual Prim Care 2012;20:115-23.
- Foster JM, Sawyer SM, Smith L, Reddel HK, Usherwood T. Barriers and facilitators to patient recruitment to a cluster randomized controlled trial in primary care: lessons for future trials. BMC Med Res Methodol 2015;15.
- Allcott H. Site selection bias in program evaluation. Q J Econ 2015;130:1117-65.
- Ritchie J, Spencer L. Analyzing Qualitative Data. London: Routledge; 1994.
- NIHR Annual Report 2019 20 n.d. www.nihr.ac.uk/documents/annual-report-20192020/26565 (accessed 5 September 2022).
- Ellis SD, Bertoni AG, Bonds DE, Randall Clinch C, Balasubramanyam A, Blackwell C, et al. Value of recruitment strategies used in a primary care practice-based trial. Contemp Clin Trials 2007;28:258-67.
- Irving G, Neves AL, Dambha-Miller H, Oishi A, Tagashira H, Verho A, et al. International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open 2017;7.
- NIHR . Annual Efficient Studies Funding Calls for CTU Projects n.d. www.nihr.ac.uk/documents/ad-hoc-funding-calls-for-ctu-projects/20141 (accessed 5 September 2022).
- Practices Closing or Merging n.d. https://digital.nhs.uk/data-and-information/find-data-and-publications/supplementary-information/2018-supplementary-information-files/number-of-gp-practices-closed-or-merged-in-england-between-1-july-2016-and-31-december-2017-by-nhs-geographies (accessed 5 September 2022).
- Hammersley V, Donaghy E, Parker R, McNeilly H, Atherton H, Bikker A, et al. Comparing the content and quality of video, telephone, and face-to-face consultations: a non-randomised, quasi-experimental, exploratory study in UK primary care. Br J Gen Pract 2019;69:e595-604.
- Checkland K, McDermott I, Coleman A, Perkins N. Complexity in the new NHS: longitudinal case studies of CCGs in England. BMJ Open 2016;6.
- McDonagh M, Peterson K, Winthrop K, . Improving Antibiotic Prescribing for Uncomplicated Acute Respiratory Tract Infections. Rockville, MD: Agency for Healthcare Research and Quality (US); 2016.
- Gulliford MC, Prevost AT, Charlton J, . Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ 2019;364.
- Andrews A, Bou-Antoun S, Guy R, Brown CS, Hopkins S, Gerver S. Respiratory antibacterial prescribing in primary care and the COVID-19 pandemic in England, winter season 2020-21. J Antimicrob Chemother 2022;77:799-802.
- Khouja T, Mitsantisuk K, Tadrous M, Suda KJ. Global consumption of antimicrobials: impact of the WHO Global Action Plan on Antimicrobial Resistance and 2019 coronavirus pandemic (COVID-19). J Antimicrob Chemother 2022;77:1491-9.
- Rezel-Potts E, L’Esperance V, Gulliford MC. Antimicrobial stewardship in the UK during the COVID-19 pandemic: a population-based cohort study and interrupted time-series analysis. Br J Gen Pract 2021;71:e331-8.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
Appendix 1 Clinician’s guide


Appendix 2 View of template on Egton Medical Information Systems screen during consultation
FIGURE 23.
Soft pop-up reminder.

FIGURE 24.
Algorithm template.

Appendix 3 Egton Medical Information Systems resources set-up guide



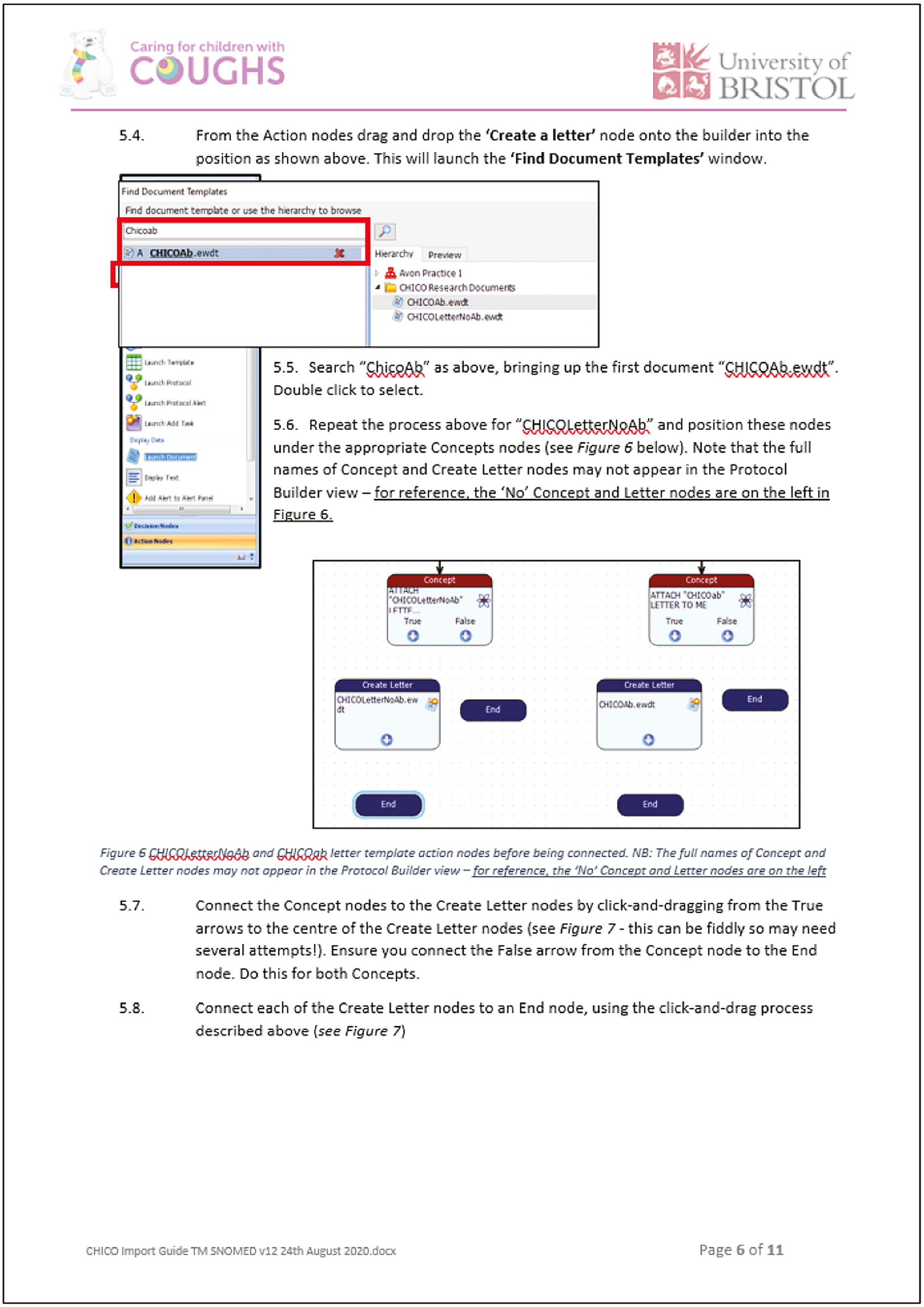




Appendix 4 SystmOne resources set-up guide
This document describes how to set up the CHICO intervention resources in SystmOne. The resources are a clinical Template, two Letter Templates, a Protocol and a set of Clinical Reports.
Preparing for the import
Note: Practices already using the CHICO intervention but needing to upgrade the resources should delete or archive the previous version in SystmOne before importing the new version.
The resources are distributed as a set of files:
-
a letter templates file called ‘CHICO WordLetterTemplates 151021.xml’ (or similar);
-
a clinical template called ‘SystmOneTemplates CHICO child cough RCT 151021.xml’ (or similar);
-
a reporting file called ‘CHICO Exported Reports 151021.rpt’ (or similar);
-
a protocol file called ‘SystmOneProtocol CHICO patient alert protocol 151021.xml’ (or similar);
-
a protocol file called ‘SystmOneProtocol CHICO template launch + outcomes protocol 151021.xml’ (or similar).
Save these files onto your computer or a shared drive.
If these resources have been provided in a single compressed (zip) file, right click the file in File Explorer and select ‘Extract all’ from the pop-up menu. This will create a new folder containing the five individual files.
Setting up the letter templates
There are two CHICO Letter templates – for when antibiotics are prescribed and not prescribed.
To import the letter templates into SystmOne:
-
Go to Setup → Referrals and Letters → Word Letter Templates.
-
Select Import Templates.

-
Select the file called ‘CHICO WordLetterTemplates 151021.xml’ (or similar).

Two Word Letter Templates called ‘CHICOLetterAntibiotics’ and ‘CHICOLetterNoAntibiotics’ will be created in a category called ‘CHICO’.

Setting up the clinical template
There are two steps in setting up the clinical template:
-
Importing the Template.
-
Publishing the Template.
Importing the template
-
Go to Setup → Data Entry → New Template Maintenance.
-
Select Import Templates.

-
Select the file called ‘SystmOneTemplates CHICO child cough RCT 151021.xml’ (or similar).
-
The New Template window is displayed. Select the category called ‘CHICO’ if it already exists. Otherwise create and select a ‘New Category’ called ‘CHICO’. Select Ok to complete the template import.

Publishing the template
-
Select ‘Unpublished Templates’ on the left-hand side of the New Template Maintenance screen. Right click the ‘CHICO child cough RCT’ template and select ‘Publish Template’ from the pop-up menu.

-
Select ‘Publish Locally’ from the available options. Select Ok.
Setting up the clinical reports
-
Go to Reporting → Clinical Reporting.
-
Select Import.

-
Select the file called ‘CHICO Exported Reports 151021.rpt’ (or similar).
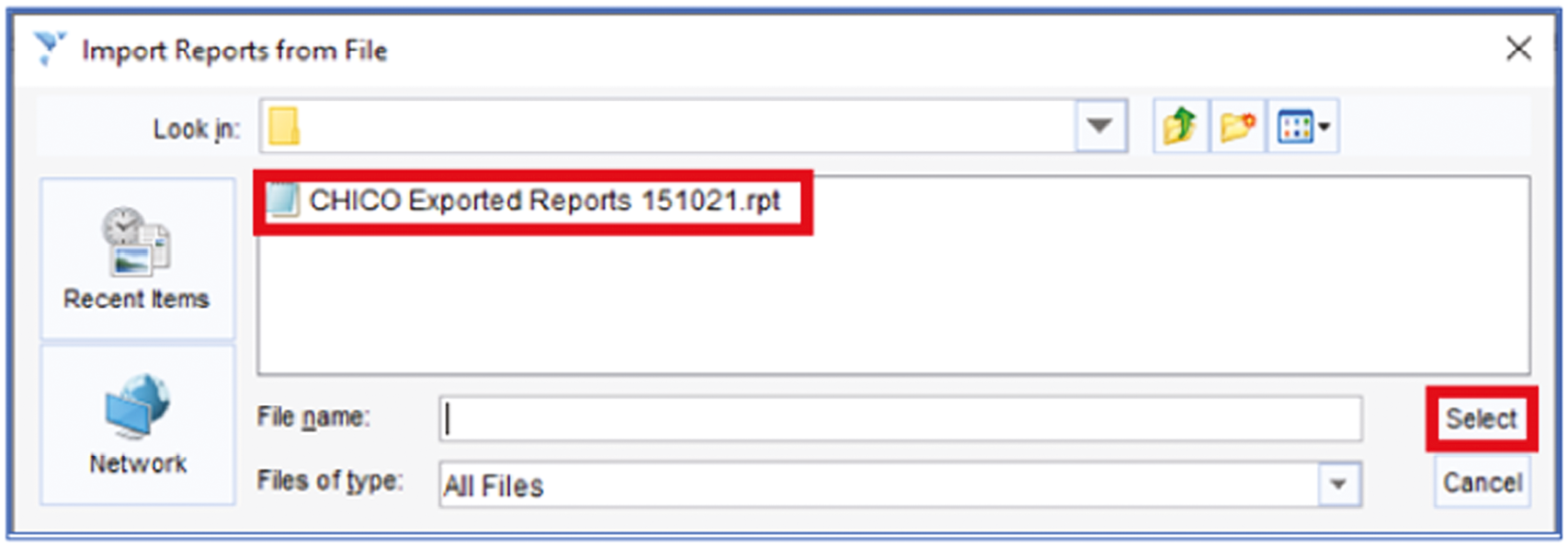
-
An import confirmation message is displayed. Select Ok to clear the message.

-
Select the category called ‘CHICO’ if it already exists. Otherwise create and select a ‘New Category’ called CHICO. Select Ok.

-
A confirmation message is displayed. Select Yes to confirm that you want to move the reports.
A set of clinical reports will be created in a category called ‘CHICO’.
Setting up the protocols
There are three steps in setting up the Protocols:
-
Importing the Protocols.
-
Editing the ‘launch + outcomes’ Protocol.
-
Publishing the Protocols.
Importing the protocols
-
Go to Setup → Workflow Support → Protocols.
-
Select Import Protocol.

-
Select the file called ‘SystmOneProtocol CHICO patient alert protocol 151021.xml’ (or similar).
-
Select the category called ‘CHICO’ if it already exists or create and select a ‘New Category’ called ‘CHICO’. Select Ok.

-
A confirmation creation message is displayed. Select Ok.

-
You may see the following information message in which case select Ok.
-
Now import the protocol file called ‘SystmOneProtocol CHICO template launch + outcomes protocol 151021.xml’ (or similar) in the same way as described above.
Editing the ‘launch ± outcomes’ Protocol
When the ‘launch + outcomes’ Protocol is imported, links from it to the clinical template and letter templates are lost. The Protocol therefore must be amended to reinstate the links.
-
Select ‘Unpublished’ on the left-hand side of the Protocols screen. Right click the Protocol called ‘CHICO template launch + outcomes protocol’ and select ‘Amend Protocol’ from the pop-up menu.

-
Select the tab called Design.
Three steps in the design flowchart need to be amended:
-
The Action step that identifies the clinical template.
-
The two steps that identify the letter templates.
Linking the clinical template
-
Double left-click the Action step near the top of the design.

-
In the Select Quick Action window, search for ‘template’ and select the ‘CHICO child cough RCT’ template. Select Ok.
-
Select Pause Protocol.

-
Check that this part of the design now looks like this.

Linking the two Letter Templates
-
Towards the bottom of the Design, there are two steps with unknown letter templates. Double left-click the one on the left.

-
In the Select Quick Action window, search for ‘letter’ and select New Letter → Specific Word Letter Template. Select Ok.

-
Search for ‘chico’ and select ‘CHICOLetterAntibiotics’. Select Ok.

-
Select Pause Protocol.

-
Repeat the above process for the other unknown template but this time selecting the ‘CHICOLetterNoAntibiotics’ template.

-
Check that this part of the design now looks like this making sure that the CHICOLetterAntibiotics is on the left and CHICOLetterNoAntibiotics is on the right.

-
Select Ok in the top-left corner of the Amend Protocol window to save the amended protocol design.
Publishing the protocols
-
Select ‘Unpublished’ on the left-hand side of the Protocols screen.
-
Right click the Protocol called ‘CHICO patient alert protocol’ and select ‘Publish Protocol’ from the pop-up menu.
-
Select ‘Publish locally’. Select Ok.

-
A confirmation message is displayed. Select Yes.
-
Repeat the above publication sequence for the Protocol called ‘CHICO template launch + outcomes’.
Using SystmOne organisation groups
The instructions above describe publishing the clinical Template and Protocols locally.
If the CHICO resources are to be made available to other organisations, you can publish to the appropriate SystmOne organisation instead of locally. In addition to publishing the clinical Template and Protocols, the Letter Templates and Clinical Reports will also need to be published to the organisation group.
To publish a Letter Template:
-
Go to Setup → Referrals and Letters → Word Letter Templates.
-
Right-click the letter template and select Publish in the pop-up menu.
-
Select the Organisation Group.
-
Select Ok.
To publish Clinical Reports:
-
Go to Reporting → Clinical Reporting.
-
Select the report(s).
-
Select the Publish icon .
-
Select the Organisation Group.
-
Select Ok.
Appendix 5 The Clinical Commissioning Groups taking part in the children with cough study (bold), as defined in 2019

Clinical Commissioning Groups are shaded in red/pink according to the number of items of amoxicillin and macrolides, per 1000 list size at the mid-point of recruitment, October 2019. Copyright Mapbox, Open Street Map. For up to date data please refer to: https://openprescribing.net/analyse/#org= CCG&orgIds=15E,15C,D2P2L,14Y,06H,04Y,27D,15N, 01A,05D,97R,D4U1Y,11M,D9Y0V,06N,91Q,11N, 15F,99A,14L,01K,13T,06T,05G,W2U3Z,52R,10Q, 01G,05Q,11X,M2L0M,05W,72Q,36L,05V,00P,92A, 03Q&numIds=0501013B0,5.1.5&denom= total_list_size&selectedTab=map.
Appendix 6 Health economics: unit codes and currency costs
| Dispensed item | Unit cost – net ingredient cost |
|---|---|
| Children aged 0–4 years | |
| Amoxicillin | £1.83 |
| Macrolides | £6.05 |
| Children aged 5–9 years | |
| Amoxicillin | £3.06 |
| Macrolides | £12.28 |
| Currency code | Currency description | Finished consultant episodes |
|---|---|---|
| PD11A | Paediatric, Acute Upper RTI or Common Cold, with CC Score 4+ | 723 |
| PD11B | Paediatric, Acute Upper RTI or Common Cold, with CC Score 1–3 | 1916 |
| PD11C | Paediatric, Acute Upper RTI or Common Cold, with CC Score 0 | 1608 |
| PD12A | Paediatric, Asthma or Wheezing, with CC Score 4+ | 460 |
| PD12B | Paediatric, Asthma or Wheezing, with CC Score 1–3 | 2850 |
| PD12C | Paediatric, Asthma or Wheezing, with CC Score 0 | 2024 |
| PD13A | Paediatric Cystic Fibrosis, with CC Score 5+ | 188 |
| PD13B | Paediatric Cystic Fibrosis, with CC Score 2–4 | 124 |
| PD13C | Paediatric Cystic Fibrosis, with CC Score 1 | 41 |
| PD13D | Paediatric Cystic Fibrosis, with CC Score 0 | 26 |
| PD14A | Paediatric Lower Respiratory Tract Disorders without Acute Bronchiolitis, with CC Score 11+ | 1198 |
| PD14B | Paediatric Lower Respiratory Tract Disorders without Acute Bronchiolitis, with CC Score 8–10 | 1331 |
| PD14C | Paediatric Lower Respiratory Tract Disorders without Acute Bronchiolitis, with CC Score 4–7 | 3466 |
| PD14D | Paediatric Lower Respiratory Tract Disorders without Acute Bronchiolitis, with CC Score 2–3 | 4048 |
| PD14E | Paediatric Lower Respiratory Tract Disorders without Acute Bronchiolitis, with CC Score 1 | 3839 |
| PD14F | Paediatric Lower Respiratory Tract Disorders without Acute Bronchiolitis, with CC Score 0 | 6192 |
| PD15A | Paediatric Acute Bronchiolitis with CC Score 5+ | 1252 |
| PD15B | Paediatric Acute Bronchiolitis with CC Score 2–4 | 3007 |
| PD15C | Paediatric Acute Bronchiolitis with CC Score 1 | 3179 |
| PD15D | Paediatric Acute Bronchiolitis with CC Score 0 | 7174 |
| PD65A | Paediatric Upper Respiratory Tract Disorders with CC Score 5+ | 215 |
| PD65B | Paediatric Upper Respiratory Tract Disorders with CC Score 2–4 | 390 |
| PD65C | Paediatric Upper Respiratory Tract Disorders with CC Score 1 | 272 |
| PD65D | Paediatric Upper Respiratory Tract Disorders with CC Score 0 | 535 |
List of abbreviations
- AMR
- antimicrobial resistance
- AMS
- antimicrobial stewardship
- BRTC
- Bristol Randomised Trials Collaboration
- CCG
- Clinical Commissioning Group
- CHICO RCT
- CHIldren with COugh Randomised Controlled Study
- CI
- confidence interval
- CONSoRT
- Consolidated Standards of Reporting Trials (Guidelines)
- CPAG
- Clinician and Pharmacist Advisory Group
- CRN
- Clinical Research Network
- DMC
- Data Monitoring Committee
- EMIS®
- Egton Medical Information Systems
- FTE
- full-time equivalent
- GP
- general practitioner
- HTA
- Health Technology Assessment
- IMD
- Index of Multiple Deprivation
- ISRCTN
- International Standard Randomised Controlled Trials Number
- ITT
- intention to treat
- NHS
- National Health Service
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute of Health and Care Research
- NPT
- normalisation process theory
- PAG
- Parent Advisory Group
- PPI
- patient and public involvement
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RTI
- respiratory tract infection
- SAE
- serious adverse event
- SD
- standard deviation
- TARGET
- name given to the 5-year National Institute of Health and Care Research funded programme of research underpinning the children with cough trial to develop an intervention to improve the targeting of antibiotics for children with respiratory tract infections (Ref No: RP-PG-0608-10018)
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
