Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR129713. The contractual start date was in September 2020. The draft manuscript began editorial review in August 2022 and was accepted for publication in March 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Snowsil et al. This work was produced by Snowsil et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Snowsil et al.
Chapter 1 Background
Overview
Lynch syndrome (LS), previously referred to as hereditary non-polyposis colorectal cancer (HNPCC), is a hereditary cancer predisposition syndrome caused by pathogenic variants in deoxyribonucleic acid (DNA) mismatch repair (MMR) genes. Although mostly undiagnosed, around 1 in 300 people are born with variants causing LS. 1 LS confers a higher lifetime risk and earlier onset age of developing colorectal (CRC), endometrial (EC) and ovarian (OC) cancers. 2 These cancers are also associated with morbidity and mortality. 3
The risks in those with confirmed MMR pathogenic variants are managed through risk-reducing measures including surveillance, risk-reducing surgery and aspirin chemoprophylaxis. 4
Although colonoscopic surveillance is recommended to manage the risk of CRC and is generally accepted to reduce the risk of mortality from CRC,5 surveillance for gynaecological cancer is more contentious. 6 Instead, risk-reducing gynaecological surgery is recommended to virtually eliminate the risk of endometrial and OC. 7
Risk-reducing surgery can have some negative consequences. Removal of ovaries prior to the age of natural menopause leads to surgical menopause. Hormone replacement therapy (HRT) is generally safe and effective for women with no prior cancer. Removal of female reproductive organs also forecloses natural fertility. Therefore, many women with LS seek to delay risk-reducing surgery until they have completed their families and are closer to the age of natural menopause.
Until they undergo risk-reducing surgery, women with LS may be anxious that their risk of gynaecological cancer is unmanaged. Many will know relatives who have had gynaecological cancer and this can contribute to their anxiety.
Many women with LS are keen to undergo surveillance to manage their risk of gynaecological cancer, and some will resort to private healthcare spending if their local NHS does not offer this surveillance.
The aim of this study was to find out whether surveillance for gynaecological cancer in LS is effective and whether it would be cost-effective in the NHS.
Description of health condition
Aetiology, pathology and prognosis
Lynch syndrome is an inherited autosomal dominant disorder,8–10 meaning that a child with one parent with LS has a 50% chance of inheriting the disease. It is caused by pathogenic mutations in one of four DNA MMR genes (MLH1, MSH2, MSH6 or PMS2). 11,12 MMR proteins recognise and repair errors in DNA replication. As a person with LS only inherits one functional allele, there is a high risk that MMR function is lost because of somatic mutation. Loss of DNA MMR activity in cell division induces inability to repair mutations, eventually leading to cancer. 9,10,13
Lynch syndrome is associated with early-onset cancer as tumours develop at a young age. Depending on which MMR gene is affected, the cumulative cancer risks by 70 years can exceed 50% and 20% for EC and OC,2,8,14 compared with cumulative risks of 1.3% and 1.0% in the general population. 15
Diagnostic criteria/measurement of disease
Historically, families with LS were identified through family history criteria, such as the Amsterdam criteria, followed by MMR gene mutation testing in constitutional DNA. 16 Recently, universal testing for LS among those with new colorectal or endometrial cancer has been recommended. 17,18
Impact of the health problem
Significance for patients
An individual with LS may develop several clinical and pathological features, including cancers such as EC and OC, early onset of cancer, multiple independent cancers. 19 The value of a surveillance regimen for women at risk of EC or OC has yet to be established. 20 The Manchester International Consensus Group concluded that ‘further research is required to establish the value of gynaecological cancer surveillance in LS’. 6
The risks of endometrial and ovarian cancer affect people with female reproductive organs, and this may include transgender men as well as cisgender women. We are not aware of any research beyond isolated case reports in LS involving transgender men, and it is possible that evidence that is collected (believed to relate entirely to cisgender women) may be less reliable when applied to transgender men who have used exogenous sex hormones, GnRH analogues or anti-oestrogens. In this monograph, we may at times refer to women – in this context we mean all people with female reproductive organs, which could include transgender men. Our preference is to avoid the noun ‘females’ for people of female sex, as this term can be seen as dehumanising.
Significance for the NHS
The majority of individuals with LS caused by path_MLH1 or path_MSH2 will go on to develop at least one cancer in their lifetime, and LS accounts for around 3% of CRC and ECs. 21,22 In 2017, there were nearly 35,000 CRC diagnoses in England and over 7000 cases of EC. 23 This suggests that over 1000 cancers each year are being diagnosed in people with LS, which may be preventable through risk management strategies.
Current service provision to manage gynaecological cancer risk in Lynch syndrome
Clinical guidelines and why this research is needed now
As a result of publication of recent National Institute for Health and Care Excellence (NICE) guidance on identifying LS,17,18 more women will have a diagnosis of LS and need to manage their gynaecological cancer risk. Evidence is needed to determine which interventions are effective and cost-effective to reduce the morbidity and mortality from gynaecological cancer and to contribute to the NHS Long Term Plan goal of improving early diagnosis of cancer. 24 Surveillance is a preferred option for patients managing cancer risk, but good-quality estimates of its effectiveness and cost-effectiveness have not been produced.
Care pathways
Two main interventions are available to manage the gynaecological cancer risk in LS: risk-reducing surgery (removal of the uterus and ovaries) and surveillance. Some patients may have surveillance initially and then have risk-reducing surgery. In addition, chemoprevention with aspirin4 and hormone therapy25 may be considered, as well as lifestyle changes.
Gynaecological surveillance can identify precancerous lesions in the uterus, for which there are fertility-sparing treatments. Surveillance may also be able to identify OC in the early stages, where management options could maintain fertility or allow egg harvesting.
Gynaecological surveillance has previously been estimated to cost the NHS £473 per year for a woman with LS. 9 Later-stage gynaecological cancers can be costly to treat; for example, stage 3 OC costs twice as much to treat as stage 1 cancer. 26
Surgery is widely offered, since its effectiveness is well documented,7 typically at 40–45 years when most women have completed their families. 27 This prevents women from becoming pregnant and artificially brings on menopause, which can detrimentally affect health-related quality of life (HRQoL) and long-term health unless managed with HRT. In some cases, women do not have risk-reducing surgery because of technical difficulties due to past surgery for CRC, high anaesthetic risk due to medical comorbidities or patient preferences. 28
Some NHS providers offer surveillance for gynaecological cancer in women with LS not undergoing risk-reducing surgery; others do not because of a lack of evidence-based guidelines and resource constraints. Some women opt to pay privately for surveillance, but not all women can afford this service.
Chapter 2 Decision problem
The key research questions for this study were:
-
Is gynaecological cancer surveillance in LS clinically effective?
-
Is gynaecological cancer surveillance in LS cost-effective in the NHS?
Furthermore, we were interested to determine whether certain groups would benefit more or less from surveillance, experience more or fewer harms from surveillance or whether surveillance would be more or less cost-effective for certain groups.
To answer the first key research question, our objective was to conduct a systematic review of existing studies on the effectiveness of gynaecological cancer surveillance in LS. This systematic review is reported in Chapter 3.
To answer the second key research question, we had the following objectives:
-
To conduct a systematic review of existing studies on the cost-effectiveness of gynaecological cancer surveillance in LS (reported in Chapter 4).
-
To conduct a systematic review of health state utility values which may be relevant for the cost-effectiveness of gynaecological cancer surveillance in LS (reported in Chapter 5).
-
To conduct a model-based economic evaluation of gynaecological cancer surveillance in LS.
We decided that there would be value in developing a whole-disease model for LS rather than a model only capable of addressing the focused research question in this study. The development of the whole-disease model is reported in Chapter 6.
The model-based economic evaluation is reported in Chapter 7.
Population
People with LS (i.e. with confirmed pathogenic variants in MLH1, MSH2, MSH6 or PMS2) or suspected LS at risk of EC and/or OC.
Intervention
Gynaecological cancer surveillance
Strategies for gynaecological cancer surveillance in women with LS may include a number of different modalities. Surveillance is typically conducted at 1- or 2-year intervals and initiated between the ages of 25 and 35 years. 6,8
Hysteroscopy and directed biopsy
An endoscopic technique for inspecting the uterine cavity (in this case to identify endometrial neoplasia) by inserting a hysteroscope via the cervical os. Hysteroscopy can allow for directed biopsy (targeted extraction of tissue for pathological examination). It can often be performed in outpatient or office settings with no anaesthesia or analgesia, although in some cases local or general anaesthesia (GA) may be used.
Undirected biopsy
Techniques for sampling the endometrium without visualising the interior of the uterus. Numerous samples are taken from different parts of the endometrium. Aspirate biopsy (e.g. Pipelle) is typically conducted in an outpatient or office setting while dilatation and curettage is conducted under sedation or GA in an inpatient or daycase setting.
Transvaginal ultrasound
An ultrasound probe is inserted into the vagina to visualise organs in the pelvic cavity, which can identify signs of endometrial and ovarian malignancy (e.g. increased endometrial thickness).
Transabdominal ultrasound
An ultrasound probe is pressed against the abdomen to visualise the uterus and ovaries to identify malignancies.
Cancer antigen-125
A blood serum biomarker which is raised in around 90% of women with advanced ovarian cancer. NICE recommends that serum cancer antigen 125 (CA-125) of 35 iu/ml or greater is an indication for further investigation in women with symptoms of OC. 29
Comparators
The relevant comparators are risk-reducing surgery or no risk reduction.
Hysterectomy with bilateral salpingo-oophorectomy (BSO) is commonly used in clinical practice to all but eliminate the risk of endometrial and ovarian cancers. 7 However, it is an operation under GA, which can carry surgical risk (particularly with women who have had prior surgery, e.g. for CRC) and artificially brings on menopause in premenopausal women.
There is evidence from a retrospective cohort study that prolonged use of hormonal contraceptives lowers the rate of endometrial cancer in women with LS. 25 This comparator falls outside the scope of this research, although it may be a concomitant treatment for some individuals in the included studies as a significant proportion of women engage in prolonged use of hormonal contraceptives.
Symptom awareness, with optional annual clinical review, has been recommended by a consensus group,6 together with rapid access to investigation for suspicious signs and symptoms.
Outcomes
Outcomes are specified in detail in the subsequent chapters.
Chapter 3 Systematic review of clinical effectiveness evidence
Review aims
This systematic review aimed to evaluate the clinical effectiveness of different gynaecological cancer surveillance strategies in LS, the ability of those strategies to detect gynaecological cancers, and the harms associated with those strategies.
Under these three broad aims the review sought to evaluate ten specific research questions which are listed below.
Cancer detection:
-
What are the cancer detection rates/incidence rates (malignancies and premalignancies) for gynaecological surveillance strategies in people with LS?
-
What are the cancer detection rates/incidence rates for gynaecological surveillance among asymptomatic women with LS?
-
What is the incidence of interval cancers among people with LS taking part in gynaecological surveillance programmes?
-
What are the incidental detection rates of other medical findings (e.g. ovarian cysts) among people with LS undergoing gynaecological surveillance?
-
What are the diagnostic test accuracies of different gynaecological surveillance strategies for people with LS?
-
What are the test failure rates for gynaecological surveillance procedures in LS?
Clinical effectiveness:
-
Do gynaecological surveillance strategies improve mortality, survival, cancer prognosis, treatment response and fertility in people with LS?
-
Do gynaecological surveillance strategies improve early diagnosis (i.e. stage at diagnosis) in people with LS?
Harms:
-
What are the rates (and severity) of adverse events (including pain) observed in different gynaecological surveillance strategies among people with LS?
-
What risk factors impact the occurrence (and severity) of adverse events among people with LS undergoing gynaecological surveillance?
Methods
The methods of the review were conducted following a protocol, which was registered on PROSPERO (CRD42020171098). Deviations from the protocol and protocol clarifications are described in the relevant sections that follow or in Protocol amendments. Changes and additions made due to patient and public involvement (PPI) activities (see Patient and public involvement) were not considered to be protocol deviations but are highlighted where applicable.
Study identification
A bibliographical database search was developed using MEDLINE by an information specialist (SB) in consultation with the review team. The search strategy combined two components: (1) search terms for LS, MMR and HNPCC (i.e. the population/problem of interest); and (2) a relevant selection of gynaecological screening methods (i.e. the interventions of interest). A combination of free-text terminology and indexing terms (e.g. MeSH) was used. No date or language limits were applied. The final search was translated for use in an appropriate selection of bibliographic databases including: Cochrane Central Register of Controlled Trials (via the Cochrane Library); Cumulative Index to Nursing and Allied Health Literature (via EBSCO); MEDLINE (via Ovid); EMBASE (via Ovid); Web of Science Science Citation Index (SCI) and Conference Proceedings Citation Index – Science (CPCI – S; via Clarivate Analytics). The search strategies are reported in full in Appendix 1.
Bibliographical database searches were carried out on 21 September 2020, with update searches carried out on 3 August 2021. The results were exported to Endnote X8 (Clarivate Analytics, Philadelphia, PA, USA) and deduplicated using the automated deduplication feature and manual checking. The resulting library was then exported to Covidence (Veritas Health Innovation Ltd, Australia, www.covidence.org) in preparation for the study selection process.
To identify further studies, forward and backward citation searches of all included studies were conducted using Web of Science (Clarivate Analytics). We used Google Scholar to carry out forward citation searching on included studies that were not indexed in Web of Science. Any relevant systematic reviews identified in the process of screening the results of our searches were also manually checked for relevant primary studies. The results of forward and backward citation searches were exported to Endnote X8 for deduplication and then exported to Covidence for the study selection process.
Relevant conference proceedings were scrutinised. Finally, the clinical trials registries ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials on 9 August 2021. The clinical trials registry search strategies are reported in Appendix 1.
Study selection
A two-stage screening process was used to select studies: two reviewers (HC and SB) independently screened titles and abstracts against the Eligibility criteria (see below). The full texts of studies that appeared to meet the eligibility criteria were retrieved and independently screened by the same two reviewers. At both stages of screening, disagreements were resolved by discussion, with involvement of a third reviewer (TS) where necessary.
Screening was carried out in Covidence for both title and abstract screening and full-text screening. This was a deviation from the protocol where the intention had been to conduct screening in EndNote. This change in software was to enable ease of remote working due to the COVID-19 pandemic.
Eligibility criteria
The following prespecified eligibility criteria were used to select studies:
Population
Studies must have been based on an adult population (age ≥ 18 years) at risk of EC and/or OC (individuals born with and retaining a uterus and/or ovaries, including trans men, and intersex individuals at risk of EC and/or OC) and with confirmed or suspected LS.
Prior to screening being conducted, it was clarified that studies based on a wider population should be included if subgroup data were provided for the eligible population. In these cases, only the relevant subgroup data were included. This clarification was an omission from the published protocol.
Intervention
Studies that evaluated any gynaecological surveillance strategy either alone or in combination with a comparator were included. The target conditions of surveillance had to be EC and/or OC. Surveillance strategies included (but were not limited to): hysteroscopy and directed biopsy, undirected biopsy, ultrasound (transvaginal or transabdominal) and cancer antigen-125 testing. Studies were not excluded based on the surveillance schedule.
Comparators
For controlled study designs, eligible comparators included no surveillance, alternative surveillance programmes, surgical or hormonal prevention. In diagnostic test accuracy (DTA) studies, any eligible surveillance test result could be used as a reference standard for another test but, primarily, cancer diagnosis using histology was the preferred reference standard.
Outcomes
To be eligible for inclusion, studies needed to evaluate at least one of the following outcomes: all-cause mortality, cancer-specific mortality, cancer survival, cancer treatment response, fertility, cancer stage, cancer detection rates (in symptomatic and asymptomatic individuals), interval cancer rates, incidental medically important findings, diagnostic accuracy, test failure rates, adverse events (including pain/discomfort), risk factors impacting adverse events or HRQoL.
Study design
Eligible study designs included randomised and non-randomised controlled trials (RCTs; for all review questions), prospective and retrospective comparative and non-comparative observational designs (review questions related to cancer detection and harms), DTA studies, including any study from which 2 × 2 data could be ascertained (for the diagnostics test accuracy question only), surveys, interviews and studies with visual analogue scale (VAS) or Likert-type scales (for the questions related to harms). Case reports, opinion pieces, editorials and studies that only published in abstract form were excluded.
Few comparative studies were found to address the clinical effectiveness questions, so a protocol amendment was made to also present relevant clinical effectiveness data (mortality, survival and stage of cancers) from non-comparative cohort studies.
Data extraction
A bespoke data extraction form was used to extract publication details, study characteristics, participant characteristics, methods and results for each outcome. The form was refined in consultation with PPI representatives, to include data about HRT use (at baseline and during the study) and the need for GA during surveillance procedures. The PPI processes also clarified and expanded the data that should be extracted that are relevant to fertility (parity at baseline, pregnancies and births during the study, comparison between pre-and postmenopausal women).
Data extraction was performed by one reviewer (HC or SB) and checked by a second (HC, NM or SB). Any disagreement was resolved by discussion, with involvement of a third reviewer (one of HC, SB, NM or TS) where necessary.
Risk of bias assessment
Risk of bias (ROB) was assessed at study level using appropriate tools for the study design: the Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I)30 for non-randomised comparative studies and the Critical Appraisal Skills Programme (CASP) cohort study checklist for non-comparative cohort studies. 31 Studies were deemed to be comparative if more than one group of participants was compared for at least one outcome and these groups were either entirely separate or overlapping (e.g. registry data from different time periods). Studies that provided separate data for different tests or procedures from the same sample were deemed to be non-comparative cohort studies. QUADAS-2 (a revised tool for quality assessment of diagnostic accuracy studies) was completed in addition to the ROBINS-I/CASP if DTA data were extracted. 32
Over the course of the data extraction process, and to ensure a consistent approach across studies, a decision was made to complete the CASP checklist, in addition to the ROBINS-I, for comparative studies. This additional assessment was a deviation from the review protocol and was introduced because these studies provided limited comparative data (usually on a single outcome), so the ROBINS-I judgements were not relevant for the other study outcomes where only single-arm data were provided.
Risk of bias assessments were performed by one reviewer (HC or SB) and checked by a second (HC, NM or SB). Any disagreement was resolved by discussion, with involvement of a third reviewer (one of HC, SB, NM or TS) where necessary.
Synthesis of evidence
Study methods and results were described in a narrative synthesis supported by cross-tabulation. There were insufficient numbers of clinically and methodologically homogenous studies to enable meta-analysis to be conducted for any of the review outcomes.
For test accuracy data, where full 2 × 2 data were available, STATA 17 (Stata Press, College Station, TX, USA) was used to generate sensitivity, specificity, positive (PPV) and negative predictive values (NPV), likelihood ratios and prevalence (using the diagti command). These values were reported if they were not already provided by the study report (see Diagnostic test accuracy data).
Where possible, subgroups were considered in narrative and quantitative syntheses according to participant age (and/or pre- or postmenopause status), participant ethnicity, frequency and age of commencement of surveillance, surveillance prior to the study start, diagnostic status, MMR gene affected, previous gynaecological or colorectal surgery, women for whom risk-reducing surgery is not considered appropriate (particularly those with previous CRC), other previous cancer, family history of gynaecological cancer, parity (nulliparous or parous), method of previous deliveries (vaginal or caesarean section).
Protocol amendments
In addition to the clarifications and amendments described above, the protocol stated that quality of life (QoL) data would be extracted, and these data are treated as clinical effectiveness data. Only one study provided QoL data (see Quality of life and mental health outcomes). This study also provided data on anxiety and depression as clinical effectiveness outcomes. It was decided that, for completeness, these data would also be reported.
Results
Studies identified by the review
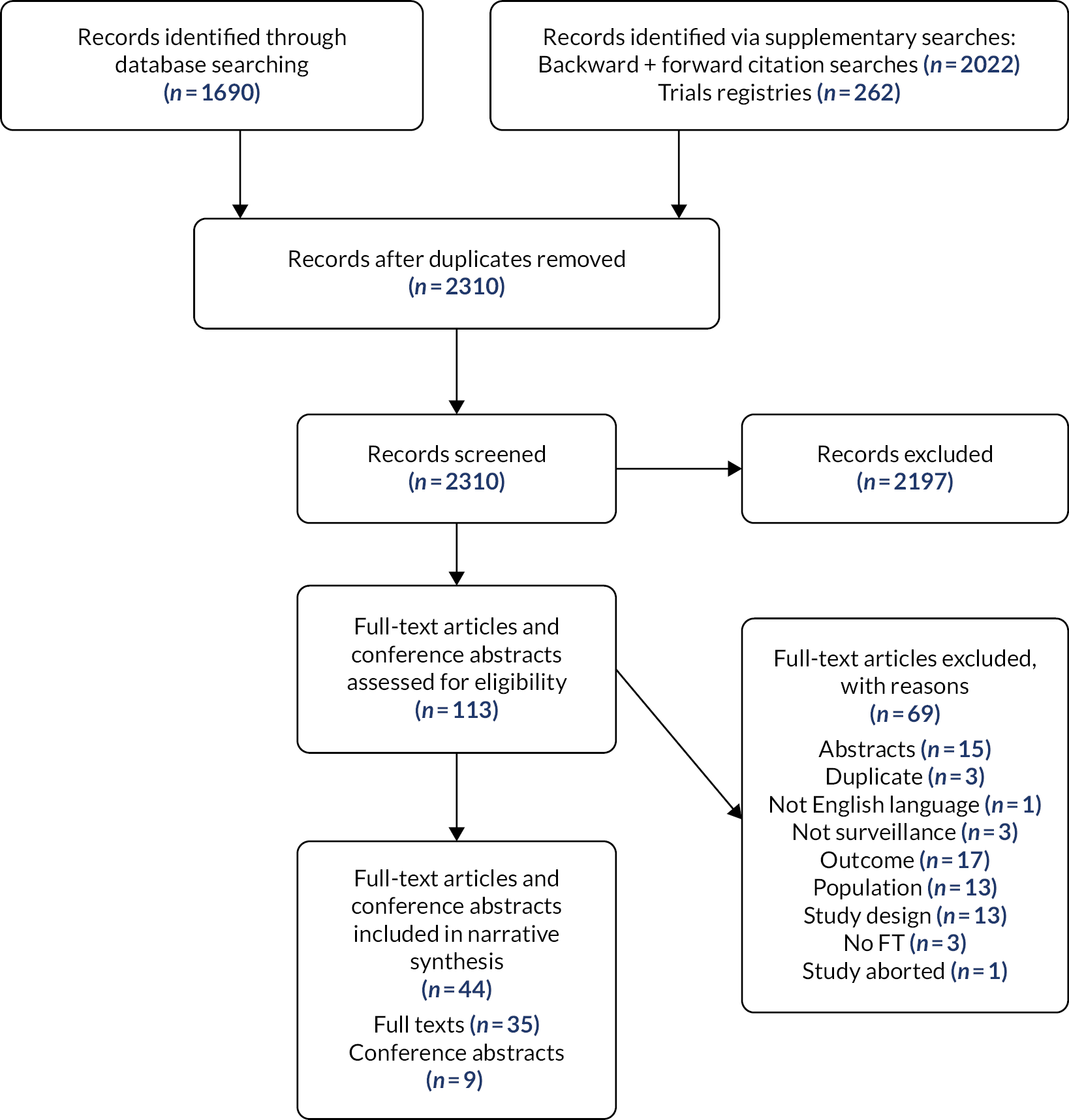
A total of 2310 titles and abstracts were screened (Figure 1). After exclusions based on title and abstract, 113 articles were sought in full, with 110 successfully obtained and assessed for eligibility. Of these, 66 were excluded (reasons for exclusion are provided in Table 25, Appendix 2). The remaining 44 articles (including 34 full-length articles, 9 conference abstracts and 1 correction) covering 30 studies were included in the review.
FIGURE 1.
Study flowchart for the systematic review of clinical effectiveness. FT, full text.
The included studies each contributed to different review questions, with some studies providing data for more than one question (Table 1). Most studies provided data concerning the detection of gynaecological cancers by surveillance, with fewer studies contributing to other review questions. A data map is provided in Table 26, Appendix 3.
| Broad aim | Specific research question | Included studies contributing data |
|---|---|---|
| Detection of gynaecological cancers by surveillance strategies in indivsiduals with LS | What are the cancer detection rates/incidence rates (malignancies and premalignancies)? | 27 studies reported across 41 publications8,33–72 |
| What are the asymptomatic cancer detection rates/incidence rates? | 10 studies reported across 16 publications34,35,40,43–50,52,67,69–71 | |
| What is the incidence of interval cancers? | 18 studies reported across 29 publications37,40,42–52,54–58,60–67,69–71 | |
| What are the incidental detection rates of other medical findings (e.g. ovarian cysts)? | 11 studies reported across 18 publications40,41,43,44,47–52,54–58,60–62 | |
| What are the diagnostic test accuracies of the surveillance strategies/tests? | 5 studies reported across 7 publications34,35,50–52,63,64 | |
| What are the test failure rates? | 5 studies reported across 6 publications34,35,41,50,62,72 | |
| Clinical effectiveness of gynaecological surveillance strategies in individuals with LS | Does surveillance improve mortality, survival, cancer prognosis, treatment response and fertility and QoL?a | Mortality or survival: 11 studies reported across 17 publications8,37–39,42–44,53,59–61,65,66,69–71,73 Cancer prognosis: none Treatment response: noneb Fertility: nonec QoL and mental health: Wood 200868 |
| Does surveillance improve early diagnosis (i.e. stage at diagnosis)? | 8 studies reported across 12 publications38–40,42,47,48,52,65,67,69–71 | |
| Harms associated with gynaecological surveillance strategies in individuals with LS | What are the rates (and severity) of adverse events (including pain)? | 7 studies reported across 13 publications41,43–46,54–58,72,74,75 |
| What risk factors impact the occurrence (and severity) of adverse events? | 4 studies reported across 9 publications41,45,46,54–58,72 |
Characteristics of included studies
Summary of included studies
Of the 30 included studies, 10 were comparative cohort studies (Table 2) and 20 were single-arm cohort studies (Table 3). The comparative studies were those that included data from more than one cohort (either completely separate or overlapping due to the use of two periods) who received different surveillance programmes, or where one group received surveillance and at least one other was an eligible comparator. Cohort studies providing some separate data for different tests within a surveillance programme, but for the same individuals, were not considered to be comparative studies. In these cases, data were extracted separately for the different tests.
| Study | Country | Study design | Centres (N) | Total (N) | Groups; interval | Patients in group (N) | Total visits; visits per person (n) | Programme duration |
|---|---|---|---|---|---|---|---|---|
| de Jong et al. 200673 | Netherlands | Registry data/prospective hospital data/period comparison | UC | 1375a | Surveillance 1990–2004: TVUS, CA-125; 1 year | NRc | NR | NR |
| 1965–75b | NR | NR | NR | |||||
| 1975–90b | NR | NR | NR | |||||
| Dueñas et al. 202038,39 | Spain | Retrospective hospital data | 1 | 531 | Surveillance: clinical examinationd, TVUS; 1 year | 465 | NR | 10.4 years (median), range 0–45 years |
| Surgery (preventative); previous surveillancee | 66 | NR | 8.7 years (median), range 0–43 yearsf | |||||
| Eikenboom et al. 202140 | Netherlands | Retrospective hospital data | 1 | 164g | Surveillance: TVUS, routine biopsy, CA-125; 1–2 years | 111 | 570; 3.48 (mean)h | 5.6 years (median), IQR 3–9 yearsh |
| Surgery (preventative); previous surveillance | 53 | |||||||
| Gerritzen et al. 200942 | Netherlands | Prospective hospital data/period comparison | 1 | 100i | Surveillance post-2006: clinical examination, TVUS, routine biopsy, CA-125; 1 year | NR | 64; NR | NR |
| Surveillance pre-2006: clinical examination, TVUS, non-routine biopsy, CA-125; 1 year | NR | 221j;NR | NR | |||||
| Helder-Woolderink et al. 201343,44 | Netherlands | Retrospective hospital data/period comparison | 1 | 75k | Surveillance 2008–12: TVUS, routine biopsy, CA-125; 1 year | 63 | 149; 2 (median), range 1–3 | 28 months (median), range 2–51 months |
| Surveillance 2003–07: TVUS, non-routine biopsy, CA-125; 1 year | 44 | 117; 3 (median), range 1–6 | 36 months (median), range 1–60 months | |||||
| Kalamo et al. 202074 | Finland | Survey data | 1 | 76l | Surveillance: UCm | 24n | NR | 11 years (median), range 6–29 years |
| Surgery (preventative); some previous surveillance | 42o | n/a | n/ap | |||||
| Renkonen-Sinisalo et al. 200760,61 | Finland | Retrospective hospital data/registry data | UCq | 385r | Surveillance: varieds; 2–3 yearst | 175 | 503; 2.87 (mean) | 3.7 years (median), (range 0–13 years) |
| Surgery (preventative/treatment); not eligible for surveillance | 138 | n/a | n/a | |||||
| Stuckless et al. 201366 | Canada | Retrospective hospital data | UC | 204u | Surveillance: TVUS, endometrial biopsy, CA-125; 1–2 years | 54 | NR | 8.5 years (median) |
| No surveillancev | 120 | n/a | n/a | |||||
| Age-matched controls (no surveillance)w | 54 | n/a | n/a | |||||
| Tzortzatos et al. 201567 | Sweden | Retrospective hospital data | 5 | 86x | Surveillance: TVUS, endometrial biopsy, CA-125; 1–2 years | 45 | NR; 2.8 (mean), range 1–20h | NR |
| Surgery (preventative); previous surveillancey | 41 | NR | ||||||
| Woolderink et al. 201869–71 | Netherlands | Prospective hospital data/registry data | UC | 878 | Surveillance: TVUS, endometrial biopsy, CA-125; 1 year | NR | NR | NR |
| No surveillance | NR | NR | NR |
| Study | Country | Centres (N) | Surveillance interval and strategies | Patients in surveillance (N) | Total visits; visits per person (n) | Programme duration at data cut-off point | Person years at risk |
|---|---|---|---|---|---|---|---|
| Single visit, single-arm, cohort studies (based on hospital data) | |||||||
| Bats et al. 201434,35 | France | 1 | Single visit: uterine cavity washings (MSI analysis) | 9 | 9; 1 (median) | n/a – single visit | n/a |
| Elmasry et al. 200941 | UK | 1 | Single visit: TVUS, hysteroscopy (plus saline hysterosonography)a, endometrial biopsy | 25 | 25; 1 (median) | n/a – single visit | NR |
| Wood et al. 200868 | UK | 1 | Single visitb: TVUS, hysteroscopy, endometrial biopsy, CA-125 | 15 | NR | n/a – single visit | NR |
| Woolderink et al. 202072 | Netherlands | 2 | Single visit: TVUS, endometrial biopsy, endometrial sampling with tampon | 25 | 25; 1 (median) | n/a – single visit | n/a |
| Single-arm cohort studies (based on retrospective registry data) | |||||||
| Ketabi et al. 201447,48 | Denmark | UC | 2 years: varied techniques, likely based on guidelinesc | 871d | 1945; 2.2 (mean), range: 1–11 | 7.9 years (mean), range 0.1–21.7 years | NR |
| Pylvänäinen 2012 et al.59 | Finland | UC | 2 years: varied techniques, likely based on guidelines | 548e | NR | NR | NR |
| Single-arm cohort studies (prospective hospital data) | |||||||
| Bucksch et al. 202036 | Germany | 6f | 1 year: unclear, included clinical examination | 865 | NR | NRg | NR |
| Dove-Edwin 2002 et al.37 | UK, Netherlands | UC | 1–2 years: TVUS/pelvic USh | 292 | 522; 1.79 (mean) | 3.62 years (mean) | 825.7 |
| Helder-Woolderink 201745,46 | Netherlands | 2 | 1 year: TVUS, endometrial biopsy, CA-125 | 52 | 97; 1.87 (mean) | NR | NR |
| Lécuru et al. 200749 | France | 1 | 1 year: TVUS, hysteroscopy, CA-125 | 57i | 91 | NR | NR |
| Lécuru et al. 200850 | France | 1 | 1 year: clinical examination, pelvic US, hysteroscopy, endometrial biopsy, CA-125 | 62 | 125; 2.02 (mean) | NR | 125 |
| Manchanda et al. 201252 | UK | 1 | 1 year: TVUS, hysteroscopy with endometrial biopsyj | 41 | 69; 2 (median), range 1–2 | 12 months (median), range 6–23.5 months | 49.2 |
| Rosenthal et al. 201363,64 | UK | 37 | 1 year: TVUS, CA-125 | 99 | NR | NR | NR |
| Ryan et al. 201765 | UK | 1 | 1 year: TVUS, hysteroscopy, ovarian US, CA-125k | 87l | NR | 50 months (mean) | NR |
| Single-arm cohort studies (retrospective hospital data) | |||||||
| Barrow et al. 200933 | UK | 1 | Interval UC: clinical examination, TVUS, hysteroscopy, endometrial biopsy | Unclearm | NR | NR | NR |
| Lécuru et al. 201051 | France | 1 | 1 year: pelvic US, hysteroscopy, endometrial biopsy | 58 | 96; 1.66 (mean) | 51.4 months (median), range 1–106 months | 246 |
| Nebgen et al. 2014,54–58,86 | USA | 1 | 1–2 years: clinical examination, TVUS, endometrial biopsy, pap smear, CA-125 (combined with colonoscopy at some visits) | 55 | 111; 2 (median), range 1–6 | NR | NR |
| Rijcken 2003 et al.62 | Netherlands | 1 | 1 year: clinical examination, TVUS, endometrial biopsyn, CA-125 | 41 | 179; 4.37 (mean) | 5 years (median), range 5 months – 11 years | 197 years |
| Single-arm cohort study (survey data) | |||||||
| Ryan 2021 et al.75 | UK | 21 | Interval varied: Varied techniqueso | 59p | 204; 3.46 (mean) | n/a | n/a |
| Pooled data from various studies | |||||||
| Møller 2017 et al.8,53 | Multiq | UC | Interval varied; varied techniquesr | 1057s | NR | NR | 7264 |
Comparative cohort studies
The 10 comparative cohort studies varied in size, ranging from 75 participants eligible for surveillance in Helder-Woolderink et al. (2013)43,44 to 1375 participants in de Jong et al. (2006),73 although in the latter study it was unclear how many of these individuals were eligible for or received surveillance. The duration of surveillance was not reported in many of these studies. Where these data were reported, duration varied greatly, ranging from 28 months (median; range 2–51 months) for the most recent period in Helder-Woolderink et al. (2013)43,44 to 11 years (median; range 6–29 years) in the survey by Kalamo et al. (2020). 74
The studies by de Jong et al. (2006),73 Renkonen-Sinisalo et al. (2007)60,61 and Woolderink et al. (2018)69–71 were based primarily on registry data. Renkonen-Sinisalo et al. (2007)60,61 followed 313 individuals relevant to this review (175 under gynaecological surveillance and 138 in a surgery comparator group), but in both Woolderink et al. (2018)69–71 and de Jong et al. (2006)73 it was unclear how many participants were undergoing gynaecological surveillance. The other comparative studies were either based on hospital data38–40,42–44,66,67 or used survey methodology. 74
Three of the comparative studies investigated the differences between different surveillance time periods. 42–44,73 Two of these were single-centre studies with different processes for initiating endometrial biopsy in the different time periods (biopsy was only offered when clinically indicated during the earlier period but added as a routine test in the later period). 42–44 The remaining study was larger,73 and based on registry data, but it was unclear what the differences were between the time periods (although it is probable that gynaecological surveillance was only available during the latest period).
Five studies compared surveillance with surgery. 38–40,60,61,67,74 In Renkonen-Sinisalo et al. (2007),60,61 the comparator group comprised individuals who had surgery prior to the start of the surveillance programme and who were, therefore, not eligible for surveillance. In Kalamo et al. (2020),74 some of the comparator group had previously received surveillance (68/76 survey respondents across both groups), but were not undergoing surveillance at the time of the survey. In the other three surgical comparator studies, all the comparator group started on surveillance but then opted for preventative surgery. 38–40,67
The remaining two comparative studies used ‘no surveillance’ comparator groups. 66,69–71 Stuckless et al. (2013)66 included a comparator group who did not have any surveillance for a variety of reasons including ineligibility (due to gynaecological cancer, hysterectomy or age < 30 years). To mitigate survivor bias, a second comparator group was also used. This second comparator group was a subsample of the first comparator group and comprised individuals who did not receive surveillance, who were matched with the surveillance group on age and who were alive and disease-free when the matched case started surveillance. 66 The study by Woolderink et al. (2018)71 provided limited data for those who had surveillance and those who did not, but it was unclear how many individuals were under surveillance or why individuals were not under surveillance (e.g. whether preventative surgery had taken place). 69–71
Single-arm cohort studies
The 20 single-arm cohort studies included in the review are presented in Table 3. These studies comprise a range of designs, including four small studies that were limited to evaluating a single surveillance visit,34,35,41,68–71 two retrospective registry-based studies,47,48,59 four retrospective studies of hospital data,33,51,54–58,62 eight hospital-based prospective cohort studies,36,37,45,46,49,50,52,63–65 one of which comprised data entered on to a prospective registry,36 one survey75 and one study that pooled individual data from 10 countries. 8,53 Some of the data that were pooled in that study are also included in this review, but the actual samples may have differed from the samples in the individual published studies,8,53 although a high degree of overlap is likely. Further information on the potential overlap between studies is provided in the section Potential overlap between included studies.
The single-arm cohort studies were variable in size, ranging from 9 eligible participants in a pilot study by Bats et al. (2014)34,35 to 871 eligible participants in the study by Ketabi et al. (2014),47,48 not including the pooled data from Møller et al. (2017). 8,53 A wide range of surveillance strategies were used, including pilot or lesser used techniques such as endometrial sampling using tampons72 and microsatellite instability (MSI) analysis of uterine cavity washings,34,35 as well as more standard surveillance techniques such as transvaginal ultrasound (TVUS), pelvic/abdominal ultrasound, hysteroscopy, endometrial biopsy and CA-125 testing. One study included surveillance visits where gynaecological surveillance was combined with colonoscopy. 54–58
Across the 20 single-arm cohort studies, the median or mean length of time that individuals were undergoing surveillance (as part of the study) was not frequently reported. When these data were reported, they also varied greatly, ranging from single visit studies34,35,41,68–71 to registry data which had a mean surveillance duration of 7.9 years (range 0.1–21.7 years). 47,48 For studies with more than one surveillance visit, the interval between visits was generally 1 or 2 years (see Table 3).
Potential overlap between included studies
Two reviewers (HC and SB) initially evaluated 59 articles and abstracts to group together those that clearly represented the same study. Conference abstracts that did not appear to report data connected to any of the included full articles were subsequently excluded (n = 15; see Figure 1 and Table 25, Appendix 2).
The design of the included studies meant that the likelihood of participant overlap between the included studies was high. Two reviewers (HC and NM) evaluated the studies for potential overlap, paying particular attention to studies conducted in the same country. This evaluation concluded that none of the 30 studies clearly overlapped sufficiently to be deemed the same study, but there were several studies based in the UK and the Netherlands, where overlap between participants was probable or possible. Furthermore, the study by Møller et al. (2017) pooled data from several countries and would, therefore, include participants from other included studies. 8,53
Meta-analyses were not conducted for this review, but potential double-counting of participants could impact upon narrative syntheses and was considered.
Overlap between studies based in the UK
Seven of the included studies were based in the UK,33,41,52,63–65,68,75 with one further study based in both the UK and the Netherlands. 37 Scrutiny of the study locations, centres, sample sizes, participant characteristics, recruitment dates and methods could not rule in or out any participant overlap. Some overlap is likely where participants were recruited from the same region, or between regional studies and UK-wide studies, but this was not clear from the study publications. For example, participant overlap is plausible between the Manchester-based samples in Barrow et al. (2009)33 and Ryan et al. (2017),65 and the Ryan et al. (2021) UK-wide survey where almost half of participants were recruited from the north-west of England. 75 Participant overlap between Rosenthal et al. (2013)63,64 and Manchanda et al. (2012)52 is also possible because both studies are associated with the UK Familial OC Screening Study (UKFOCSS). 76
Overlap between studies based in the Netherlands
Eight of the included studies were based in the Netherlands,40,42–46,62,69–73 with one study additionally based in the Netherlands as well as the UK. 37 In most cases, it was not possible to establish clear participant overlap between studies. An exception to this was Helder-Woolderink et al. (2017),45,46 which reported overlap (exact number not reported) with Helder-Woolderink et al. (2013). 43,44 Both studies were based in Groningen with a 1.5-year overlap in data collection. 43–46 Furthermore, recruitment periods for Helder-Woolderink et al. (2013)43,44 and (2017)45,46 fall within the data collection timeframe for Woolderink et al. (2018), which also recruited some participants from Groningen, so participant overlap is possible. 69–71 Two other studies recruited participants from Groningen62,73 and may partially overlap with Helder-Woolderink et al. (2013),43,44 Helder-Woolderink et al. (2017)45,46 and Woolderink et al. (2018)69–71 and also with each other. Finally, the study by Gerritzen (2009) used HNPCC registry data from across the Netherlands and, therefore, overlap with the other studies from the Netherlands is plausible. 42
Overlap between Møller et al. (2017) and other included studies
One included study8,53 pooled data across ten countries and overlaps with three of the other studies included in this review,41,47,48,60,61 although the extent of overlap is unclear; the pooled data may have been collected from different periods to the data published for each of the individual studies. However, a high degree of overlap was deemed likely.
Two of the datasets included in Møller et al. (2017; from Cardiff, UK, and from Spain) were reported to be unpublished. 8,53 It is not clear whether the data collected in Spain were related to the more recently published study by Dueñas et al. (2020). 38,39 The remaining datasets included in Møller et al. (2017) do not appear to be connected to studies already included in this review. 8,53 The references for these studies provided in Møller et al. (2017) report data for colorectal rather than gynaecological surveillance, so although it is not clearly stated, the remaining gynaecological data in Møller et al. (2017) appear to be previously unpublished. 8,53
Risk of bias in included studies
Risk of bias assessments are provided for all studies (see Critical Appraisal Skills Programme checklist for cohort studies) using the CASP31 checklist for cohort studies, and additionally using the ROBINS-I30 for the 10 comparative studies (see ROBINS-I). These assessments were made across all outcomes, with particular attention paid to ROB with regards to the primary outcomes in each study that were relevant to this review. The ROB assessment made for the pooled data study was based upon the information provided in the two Møller et al. (2017) publications rather than cited publications for all of the individual datasets. 8,53
For studies reporting DTA data, QUADAS-232 assessments are provided (see Risk of bias in test accuracy data).
Critical Appraisal Skills Programme checklist for cohort studies
Risk of bias ratings for all 30 included studies, according to the CASP checklist for cohort studies,31 are given in Table 4.
| Study | Section A | Section B | Section C | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clear focus | Acceptable recruitment methods | Exposure accurately measured | Outcome accurately measured | Confounders identified | Confounders accounted for | Follow-up complete enough | Follow-up long enough | Bottom line results | Precision of results | Believe results | Applicable to the UK | Fit with other evidence | Implications for practice | |
| Bats et al. 201434,35 | Y | CT | Y | Y | N | N | Y | Y | R | R | Y | CT | CT | CTa |
| Barrow et al. 200933 | Y | CT | Y | Y | N | N | Y | CT | R | R | Y | Y | CT | CTb,c |
| Bucksch et al. 20204 | Y | Y | CT | Y | N | N | Y | Y | R | R | Y | CT | CT | CTb |
| de Jong et al. 200673 | Nd | Y | CT | Y | N | N | Y | CT | R | R | Y | CT | CT | CTb,c |
| Dove-Edwin et al. 200237 | Y | CT | Y | Ye | N | N | Nf | CT | R | R | Y | CTg | CT | Y |
| Dueñas et al. 202038,39 | Y | CT | Y | Y | N | N | Y | CT | R | R | Y | CT | CT | Y |
| Eikenboom et al. 202140 | Y | Y | Y | Y | N | N | Y | Y | R | R | Y | CT | CT | Y |
| Elmasry et al. 200941 | Y | CT | Y | Y | Y | Yh | Y | Y | R | R | Y | Y | CT | CTa |
| Gerritzen et al. 200942 | Yi | Y | Y | Y | Y | N | Y | N | R | R | Y | CT | CT | CTc |
| Helder-Woolderink et al. 201343,44 | Y | Y | Y | Y | N | N | Y | CT | R | R | Y | CT | CT | Y |
| Helder-Woolderink et al. 201745,46 | Y | Y | Y | Y | Y | Yj | Y | Y | R | R | Y | CT | CT | Y |
| Kalamo et al. 202074 | Y | CT | CT | CT | Yk | N | Y | Y | R | R | Y | CT | CT | Y |
| Ketabi et al. 201447,48 | Y | Y | CT | Y | N | N | Y | Y | R | R | Y | CT | CT | Y |
| Lécuru et al. 200749 | Y | Y | Y | Y | N | N | Y | Y | R | R | Y | CT | CT | Y |
| Lécuru et al. 200850 | Y | Y | Y | Y | Y | N | Y | Y | R | R | Y | CT | CT | Y |
| Lécuru et al. 201051 | Y | CT | Y | Y | N | N | Y | Y | R | R | Y | CT | CT | Y |
| Manchanda et al. 201252 | Y | CT | Y | Y | Y | N | Y | Y | R | R | Y | Y | CT | Y |
| Møller et al. 20178,53 | Y | CT | CT | Y | N | N | CT | CT | R | R | Y | CT | CT | Y |
| Nebgen et al. 201454–58 | Y | CT | Y | Y | Y | Yl | Y | Y | R | R | Y | CT | CT | Y |
| Pylvänäinen et al. 201259 | Y | Y | CT | Y | N | N | Y | CT | R | R | Y | CT | CT | CTb |
| Renkonen-Sinisalo et al. 200760,61 | Y | Y | CT | Y | N | N | Y | N | R | R | Y | CT | CT | Y |
| Rijcken et al. 200362 | Y | Y | Y | Y | Y | N | Y | N | R | R | Y | CT | CT | Y |
| Rosenthal et al. 201363,64 | Y | CT | Y | Y | N | N | Y | Y | R | R | Y | Y | CT | CTb |
| Ryan et al. 201765 | Y | CT | Y | Y | N | N | Y | N | R | R | Y | Y | CT | Y |
| Ryan et al. 202175 | Y | CT | CT | CT | Y | N | CTm | Y | R | R | Y | Y | CT | Y |
| Stuckless et al. 201366 | Y | Y | Y | Y | Y | Y | Y | N | R | R | Y | CT | CT | Y |
| Tzortzatos et al. 201567 | Y | Y | Y | Y | N | N | Y | Y | R | R | Y | CT | CT | Y |
| Wood et al. 200868 | Y | CT | Y | Y | N | N | Y | Y | R | R | Y | Y | CT | CTa |
| Woolderink et al. 201869–71 | Nn | Y | Y | Y | N | N | Y | CT | R | R | Y | CT | CT | CTb,c |
| Woolderink et al. 202072 | Y | Y | Y | Y | Y | Yo | Y | Y | R | R | Y | CT | CT | CTa |
It is important to consider whether the included studies had sufficiently long follow-up periods to assess the primary outcomes of interest. For cancer detection rates and data on adverse events, follow-up periods were likely to be sufficient. However, 12 studies reported data on mortality and/or survival (see Mortality and survival) and none of these reported a follow-up period of 10 years or more: three had a mean/median follow-up period of < 5 years,42,60,61 two had a follow-up period between 5 and 10 years,62,66 and it was also likely that the study by Møller et al. (2017) had a mean follow-up period between 5 and 10 years, but this is assumed (mean observation years were given for each mutation and ranged from 8.0 years for MLH1 to 4.3 years for PMS2). 8,53 The remaining six studies providing mortality and/or survival data did not clearly report the follow-up period. 37–39,43,44,59,69–71,73 However, with the exception of Woolderink et al. (2018),69–71 these studies provided other information indicating that follow-up was unlikely to be long enough to assess mortality/survival.
Risk Of bias In Non-randomized Studies – of Interventions
For the 10 studies providing comparative data, additional ROB ratings using the ROBINS-I tool were made (Table 5). It is important to remember that ratings refer to the level of ROB and not the amount of bias. For each study, ROBINS-I ratings were made in consideration of all outcomes that were provided for both surveillance and an eligible comparator; where ROB differed according to outcome, this was noted and the highest ROB rating was recorded.
| Study | Cause of bias | |||||||
|---|---|---|---|---|---|---|---|---|
| Confounding | Selection of participants into the study | Classification of interventions | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of the reported result | Overallc | |
| de Jong 200673 | S | M | S | NI | NI | L | L | S |
| Dueñas 202038,39 | Ca | M | L | NI | NI | NI | L | C |
| Eikenboom 202140 | Ca,b | M | L | NI | L | NI | L | C |
| Gerritzen 200942 | S | M | S | NI | L | M | L | S |
| Helder-Woolderink 201343,44 | S | M | L | NI | L | M | L | S |
| Kalamo 202074 | S | M | L | NI | L | M | L | C |
| Renkonen-Sinisalo 200760,61 | Ca,b | M | L | NI | L | NI | L | C |
| Stuckless 201366 | S | M | L | NI | NI | NI | L | S |
| Tzortzatos 201567 | Ca | L | L | NI | NI | NI | L | C |
| Woolderink 201869–71 | Cd | M | S | NI | L | NI | L | C |
For all included comparative studies, selection to groups was based on participant preference (likely influenced by confounding factors) or was dictated by comparison of different periods. 42–44,73 Four of the five studies comparing surveillance with RRS were at critical ROB due to confounding, as baseline characteristics of the participants in each group were not considered or accounted for, including factors which could impact upon key outcomes (e.g. previous cancers, family history of gynaecological cancer). 38–40,60,61,67 Additionally, the meaningfulness of comparisons between surveillance and RRS is inherently limited, particularly with regard to cancer detection rates: except for cancers detected during surgery, it is expected that few post-surgical endometrial or ovarian cancers would occur (depending on the exact nature of the surgical procedure) and, conversely, a negative impact on fertility would be expected due to surgery. These critical ROB ratings were, therefore, applicable to data on detection rates (fertility data were not reported).
Participant characteristics at baseline
Baseline participant characteristics data for the 30 included studies are presented in Table 6. In general, few participant characteristics were reported, with several studies reporting very limited data. In particular, Barrow et al. 200933 and de Jong et al. 200673 provided no relevant data on participant characteristics because of a lack of disaggregated data for participants at risk of gynaecological cancer. This paucity of participant baseline characteristics data limits both the conclusions that can be drawn regarding the generalisability of the studies’ results and the assessment of ROB.
| Study | Confirmed LS, n (%) | Suspected LS, n (%) | Age (years) | Menopausal status, n (%) | LS mutations (all female participants in sample unless indicated), n/N (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| MLH1 | MSH2 | MSH6 | PMS2 | EPCAM | |||||
| Barrow et al. 200933 | NR | NR | NR | NR | NR | NR | NR | NR | 0 (0.0) |
| Bats et al. 201434,35 | 8 (88.9) | 1 (11.1) | NR | NR | 3/9 (33.3)a | 2/9 (22.2)a | 3/9 (33.3) | 0 (0.0) | 0 (0.0) |
| Bucksch et al. 202036 | 550 (59.5)b | 315 (34.1)b | 38.0 (median), IQR 31.0–45.0c | NR | 222/865 (25.7) | 265/865 (30.6) | 63/865 (7.3) | 0 (0.0) | 0 (0.0) |
| de Jong et al. 2006d73 | NR | NR | NR | NR | NR | NR | NR | 0 (0.0) | 0 (0.0) |
| Dove-Edwin et al. 200237 | NR | 269 (100.0) | Netherlands: 42.0 (median), range 23.0–68.0; UK AC-positive group: 40.0 (median), (range 24.0–64.0); UK HNPCC-like group: 45.0 (median), range 20.0–71.0 | NR | NR | NR | NR | NR | NR |
| Dueñas et al. 202038,39 | Surveillance: 465 (100.0) | 0 | NR | NR | Surveillance: 226/465 (48.6) | Surveillance: 127/465 (27.3) | Surveillance: 75/465 (16.1) | Surveillance: 22/465 (4.7) | Surveillance: 15/465 (3.2) |
| Surgery: 66 (100.0) |
Surgery: 33/66 (50.0) |
Surgery: 19/66 (28.8) |
Surgery: 10/66 (15.2) |
Surgery: 3/66 (4.5) |
Surgery: 1/66 (1.5) |
||||
| Eikenboom et al. 202140 | Surveillance: 111 (100) | 0 | 46.0 (median), range 21.5–75.0 | NR | Whole sample: 38/164 (23.2)e | Whole sample: 25/164 (15.2)e | Whole sample: 82/164 (50.0)e | Whole sample: 19/164 (11.6)e | Whole sample: 0 (0.0)e |
| Surgery: 53 (100) |
Surgery: 10/53 (18.9) |
Surgery: 3/53 (5.7) |
Surgery: 34/53 (64.1) |
Surgery: 6/53 (11.3) |
Surgery: 0 (0.0) |
||||
| Elmasry et al. 200941 | 1 (4.0) | 24 (96.0) | 43.6 (median), range 30.0–62.0 | Pre: 19 (76.0); post: 6 (24.0) | NR | NR | NR | NR | NR |
| Gerritzen et al. 2009f42 | 67 (67.0)g | 16 (16.0)g | 46.0 (median), range 23.0–72.0 | Pre: 72 (72.0); post: 22 (22.0); unknown: 6 (6.0) | 22/100 (22.0) | 22/100 (22.0) | 23/100 (23.0) | 0 (0.0) | 0 (0.0) |
| Helder-Woolderink et al. 201343,44 | 2008–12: 42 (66.7)h |
2008–12: 21 (33.3)h |
41 (median), range 23–67 | Pre: 61 (81.0); post: 14 (19.0)i | 2008–12: 13/63 (20.6) |
2008–12: 10/63 (15.9) |
2008–12: 10/63 (15.9) |
2008–12: 6/63 (9.5) |
2008–12: 3/63 (4.8) |
| 2003–7: 25 (56.8)h |
2003–7: 19 (43.2)h |
38 (median), range 26–61 | 2003–7: 9/44 (20.5) |
2003–7: 9/44 (20.5) |
2003–7: 6/44 (13.6) |
2003–7: 1/44 (2.3) |
2003–7: 0/44 (0.0) |
||
| Helder-Woolderink et al. 201745,46 | NR | NR | 45.1 (mean), range 33.0–69.0j,k | Pre: 40 (76.9); post: 12 (23.1) | NR | NR | NR | NR | NR |
| Kalamo et al. 202074 | Surveillance: 24 (100.0) |
0 (0.0) | At time of survey, 48.8 (median), range 30.0–76.0; at time of LS diagnosis, 35.2 (median), range 22.0–65.0m,n | NR | 47/76 (61.8)n | 22/76 (28.9)n | 7/76 (9.2)n | 0 (0.0) | 0 (0.0) |
| Surgery: 42 (100.0)l |
|||||||||
| Ketabi et al. 201447,48 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Lécuru et al. 200749 | 11 (16.4) | 46 (68.7) | 42.0 (mean), SD 11 | NR | 2/57 (3.5) | 9/57 (15.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lécuru et al. 200850 | 13 (21.0) | 49 (79.0) | 42.0 (mean), SD 11.3 | Pre: 47 (75.8); post 15 (24.2) | 4/62 (6.5) | 9/62 (14.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lécuru et al. 201051 | 14 (24.1) | 44 (76.0) | 42.5 (mean), SD 11.6 | NR | 4/58 (6.9) | 10/58 (17.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Manchanda et al. 201252 | 16 (39) | 25 (60.9) | 42.9 (median), IQR 39.4–49.7 | Post: 9 (22.0) | 10/41 (24.4) | 6/41 (14.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Møller et al. 20178,53 | 1057 (100.0)o | 0 (0.0) | NRp | NR | 514/1057 (48.6)q | 325/1057 (30.8)q | 170/1057 (16.1)q | 48/1057 (4.5)q | 0 (0.0)r |
| Nebgen et al. 201454–58 | 32 (58.0)s | 23 (42.0)t | 39.5 (mean), range 25.8–73.8u | Pre: 47 (85.5); peri: 2 (3.6); post: 6 (10.9) | 8/55 (14.55)v | 17/55 (30.91)w | 4/55 (13.0)x | 3/55 (9.0)y | 0z |
| Pylvänäinen et al. 201259 | 548 (47.0) | 618 (53.0) | NR | NR | 427/1166 (36.62) | 86/1166 (7.38) | 35/1166 (3.0) | 0 (0.0) | 0 (0.0) |
| Renkonen-Sinisalo et al. 200760,61 | Surveillance: 175 (100.0) | 0 (0.0) | NR | NR | 333/385 (86.5)bb | 32/385 (8.3)bb | 20/385 (5.2)bb | 0 (0.0) | 0 (0.0) |
| Surgery: 138 (100.0) |
|||||||||
| Rijcken et al. 200362 | 11 (26.8) | 30 (73.2) | 37.0 (median), range 27.0–60.0 | Pre: 35 (85.4); post: 6 (14.6) | 8/41 (19.5) | 2/41 (4.9) | 1/41 (2.4) | 0 (0.0) | 0 (0.0) |
| Rosenthal et al. 201363,64 | 65 (65.7) | 34 (34.3) | NRcc | NR | 28/99 (28.3) | 33/99 (33.3) | 4/99 (4.0) | 0 (0.0) | 0 (0.0) |
| Ryan et al. 201765 | 437 (100.0) | 0 (0.0) | NR | NR | 148/437 (33.9) | 210/437 (48.1) | 48/437 (11.0) | 22/437 (5.0) | NR |
| Ryan et al. 202175 | 298 (100.0)eeff | 0 (0.0) | 51 (mean), SD 14.1 | NR | 71/298 (23.8) | 108/298 (36.2) | 52/298 (17.4) | 19/298 (6.4) | 7/298 (2.3) |
| Stuckless et al. 201366 | Surveillance: 53 (98.1) | Surveillance: 1 (1.9)gg | NR | NR | 0 (0.0) | Surveillance: 54/54 (100.0)hh | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No surveillance: 98 (81.7) | No surveillance: 22 (18.3)gg | No surveillance: 120/120 (100.0)hh | |||||||
| Matched controls: 46 (85.2) | Matched controls: 8 (14.8)gg | Matched controls: 54/54 (100.0)hh | |||||||
| Tzortzatos et al. 201567 | Surveillance: 45 (100.0) | 0 (0.0) | NR | NR | 40/86 (46.5)ii | 26/86 (30.3)ii | 17/86 (19.8)ii | 3/86 (3.5)ii | 0 (0.0) |
| Surgery: 41 (100.0) | |||||||||
| Wood et al. 200868 | NR | NR | Survey responders, 41.3 (mean), range 37.2–45.3; survey non-responders, 43.6 (mean), range 38.8–48.5 | NR | NR | NR | NR | NR | NR |
| Woolderink et al. 201869–71 | 871 (99.2) | 7 (0.8) | 56.6 (median), range 23–98jjkk | NR | 268/878 (30.5) | 294/878 (33.5) | 255/878 (29.0) | 54/878 (6.2) | 0 (0.0) |
| Woolderink et al. 2020nn72 | 20 (80.0) | 5 (20.0) | 47.0 (median), (range 37.0–71.0)oo | Pre: 15 (60.0); post: 10 (40.0) | 3/25 (12.0) | 5/25 (20.0) | 6/25 (24.0) | 5/25 (20.0) | NR |
Participants all had female reproductive organs and were, therefore, at risk of gynaecological cancer. None of the studies reported participants’ gender identity, so it is not known how many transgender men and people with genders other than male or female were included in the study samples.
Baseline data on ethnicity, parity, HRT use, previous CRC and previous gynaecological cancer were sparsely reported and are not provided in Table 6. Only two studies reported data on ethnicity. 54–58,75
Only five of the included studies reported baseline data on parity, despite the fact that our PPI workshop highlighted parity data as being of particular importance to patients with LS. 41,45,46,72,74,75 Kalamo et al. (2020) reported that 21 (87.5%) survey respondents who had not undergone gynaecological surgery and 37 (88.1%) of those who had undergone surgery had one or more previous delivery. 74 Similarly, Elmasry et al. (2009) reported that of the 25 women who consented to the study (and underwent a single surveillance visit), 21 (84.0%) had 1 or more previous delivery. 41 Helder-Woolderink et al. (2017) reported that 36 of 52 women (69%) had one or more previous delivery at the time of the first surveillance visit, and 5 (9.6%) had unknown parity status. 45,46 Ryan et al. (2021) reported that 261 (87.6%) of the study sample had been pregnant at least once and 173 (58.1%) had previously had at least 1 live birth. The mean number of pregnancies was 6.4 [standard deviation (SD) 7.2]; the mean number of live births was 1.57 (SD 1.2) and of the participants who had been pregnant, 18 had used assisted reproduction technology (2 as a result of complications related to LS). 75 Woolderink et al. (2020) reported that 16 of 25 (64.0%) women who underwent surveillance had 1 or more previous delivery and 5 (25.0%) had unknown parity status. 72 One study reported HRT use at baseline, reporting that 1 of 25 participants (4.0%) was undergoing treatment. 72 This study also reported that three participants (12.0%) were using oral contraceptives as hormone treatment, five participants (20.0%) were using the Mirena® (Bayer, Leverkusen, Germany) intrauterine system and one participant (4.0%) was using progestogen. 72
Two studies reported numbers of participants with CRC prior to commencing surveillance. 53–58 Nebgen et al. (2014) reported that 11 (20.0%) of participants had CRC at baseline. 54–58 In a subsample of participants from Møller et al. (2017),53 it was reported in a table that 530/718 participants (73.8%) had CRC prior to study inclusion (but a lower percentage, 49%, is reported in the text of the study report). However, the data reported in Møller et al. (2017)53 were specifically subsample data on subsequent cancers (i.e. all participants in the subsample would have had a previous cancer) and therefore rates of CRC would be expected to be high. Two studies reported numbers of participants with gynaecological cancers prior to commencing surveillance. 8,53,66 In the Møller et al. (2017) subsample,53 it was reported that 377 of 718 (52.51%) participants had a previous diagnosis of gynaecological cancer (including 296 ECs, 61 OCs and 20 cervical cancers). 53 Again, all participants in the subsample would have had a previous cancer, so rates of previous gynaecological cancer would be expected to be high. Stuckless et al. (2013) reported 15 of 174 (8.6%) participants had a previous gynaecological cancer, all of which were in the comparator arm (first comparator arm, the second comparator arm used age-matched participants who were alive and disease-free). 66
Detection of gynaecological cancers (and other medical findings)
Detection of malignancies and premalignancies
Data are summarised in Table 7.
| Single-arm studies | |||||||
|---|---|---|---|---|---|---|---|
| Study | Surveillance (frequency) | Detection timea | Participants with cancer detected n/N participants (%) | FIGO stage of cancers | Symptomatic n/N when cancers detected n (%) | Participants with premalignancies n/N participants (%) | Participants with incidental findings n/N participants (%) |
| Barrow et al. 200933 | CE, TVUS, OPH, Bx (frequency UC) | Over study | EC: 86b | NR | NR | NR | NR |
| OC: 24b | |||||||
| Bats et al. 201434,35 | UCW (single visit) | At visit | EC: 2/9(22.2) | NR | 2/2 (100.0) | 2/9 (22.2) | NR |
| OC: NR | |||||||
| Bucksch et al. 202036 | UCc (1 year) | Over study | EC: 28/865 (3.2) | NR | NR | NR | NR |
| OC: 5/865 (0.6) | |||||||
| Dove-Edwin et al. 200237 | TVUS/PUS (1–2 years) | At visit | EC: 0/292 (0.0) | NA | NA | NR | NR |
| OC: NR | |||||||
| Interval | EC: 2/292 (0.7)d | EC: 2× I | 2/2 (100.0) | NR | NA | ||
| OC: NR | |||||||
| Elmasry et al. 200941 | TVUS, OPHe, Bx (single visit) | At visit | EC: 0/25(0.0) | NA | NA | CAH: 1/25 (4.0) | Polyps: 2/25 (8.0) |
| OC: 0/25 (0.0) | |||||||
| Helder-Woolderink et al. 201745,46 | TVUS, Bx, CA-125 (1 year) | At visit | EC: NR | OC: 1× IA | 0/1 (0.0) | NR | NR |
| OC: 1/52 (1.9) | |||||||
| Interval | EC: NR | NA | NA | NR | NA | ||
| OC: 0/52 (0.0) | |||||||
| Ketabi et al. 201447,48 | UCf (2 years) | At visit | EC: 7/871 (0.8) | EC: 1× NR, 1× IA, 2× IB, 2 × IC, 1× IV | 4/7 (57.1) | CAH: 2/871 (0.2) | FTC: 1/871 (0.1); MBOC: 1/871 (0.1) |
| OC: 1/871 (0.1) | OC: 1× IIB | 0/1 (0.0) | CH: 1/871 (0.1) | ||||
| Interval | EC: 6/871 (0.7) | EC: 3× IB, 1× II, 1× IIC, 1× IIIC | 6/6 (100.0) | CAH: 2/871 (0.2) | |||
| OC: 3/871 (0.3) | OC: 2× IC, 1× IIIC | 3/3 (100.0) | |||||
| Lécuru et al. 200749 | TVUS, OPH, CA-125 (1 year) | At visit | EC: 2/57 (3.5) | EC: 1× IB, 1× IC | 2/2 (100) | NR | Endometrial polyps: 12g Fibroids/adenomyosis: 7g |
| OC: NR | |||||||
| Interval | EC: 0/57 (0.0) | NA | NA | NR | NA | ||
| OC: NR | |||||||
| Lécuru et al. 200850 | CE, PUS, OPH, Bx, CA-125 (1 year) | At visit | EC: 3/62 (4.8) | EC: 2× IB, 1× IC | 3/3 (100.0) | CAH: 0/62 (0.0) | Atrophy: 22;h myoma/adenomyosis: 8;h endometrial polyps: 26;h simple hyperplasia: 9h |
| OC: NR | |||||||
| Interval | EC: 0/62 (0.0) | NA | NA | NR | NA | ||
| OC: NR | |||||||
| Lécuru et al. 201051 | PUS, OPH, Bx (1 year) | At visit | EC: 2/58 (3.4) | EC: NR | NR | CAH: 0/58 (0.0) | Atrophy: 21;g endometrial polyps: 7;g simple hyperplasia:2g |
| OC: NR | |||||||
| Interval | EC: 0/58 (0.0) | NA | NA | NR | NA | ||
| OC: NR | |||||||
| Manchanda et al. 201252 | TVUS, OPH, Bx (1 year) | At visit | EC: 3/41 (7.3) | EC: 3× I | 0/3 (0.0) | CAH: 1/41 (2.4) | Simple hyperplasia: 2/41 (4.9) Endometrial polyps: 6/41 (14.6); endocervical polyps: 2/41 (4.9) |
| OC: NR | |||||||
| Interval | EC: 1/41 (2.4)i | EC: 1× IA | 0/1 (0.0) | NR | NA | ||
| OC: NR | |||||||
| Møller et al. 20178,53 | Variedj | Over study | EC: 72/1057 (6.7)k | EC: NR OC: NR |
NR | NR | NR |
| OC: 19/1057 (1.8)k | |||||||
| Nebgen et al. 201454–58 | CE, TVUS, Bx, pap smear, CA-125l (1–2 years) | At visit | EC: 1/55 (1.8) | EC: 1× IA | NR | CAH: 1/55 (1.8) | Simple hyperplasia: 1/55 (1.8);m CH no atypia: 2/55 (3.6)m |
| OC: 0/55 (0.0) | |||||||
| Interval | EC: 0/55 (0.0) | NA | NA | NR | NA | ||
| OC: 0/55 (0.0) | |||||||
| Pylvänäinen et al. 201259 | Varied (2 years) | Over study | EC: 139/548 (25.4) | NR | NR | NR | NR |
| OC: NR | |||||||
| Rijcken et al. 200362 | CE, TVUS, Bxn, CA-125 (1 year) | At visit | EC: 0/41 (0/0) | NA | NA | CAH: 3/41 (7.3) | Myoma: 1/41 (2.4); polyps: 2/41 (4.9); DPE: 2/41 (4.9) |
| OC: 0/41 (0.0) | |||||||
| Interval | EC: 1/41 (2.4) | EC: 1× IB | 1/1 (100) | NR | NA | ||
| OC: 0/41 (0.0) | |||||||
| Rosenthal et al. 201363,64 | TVUS, CA-125 (1 year) | At visit | EC: 0/99 (0.0) | OC: 1× IA, 2× IC | NR | NR | NR |
| OC: 3/99 (3.0)o | |||||||
| Interval | EC: 0/99 (0.0) | NA | NA | NR | NA | ||
| OC: 0/99 (0.0) | |||||||
| Ryan et al. 201765 | TVUS, OPH, OUS, CA-125 (1 year) | At visit | EC: 2/87 (2.3) | EC: 2× IA | NR | AEH: 1/87 (2.3) | NR |
| OC: 2/87 (2.3) | OC: 2× IC | ||||||
| Interval | EC: NR | OC: 1× IA, 1× IB, 1× II | NR | NR | NA | ||
| OC: 3/87p,q(3.4) | |||||||
| Ryan et al. 202175 | Variedr | Survey | NR | NR | NR | NR | NR |
| Wood et al. 200868 | TVUS, OPH, Bx, CA-125 (single visit) | At visit | EC: 0/15 (0.0) | NA | NA | NR | NR |
| OC: 0/15 (0.0) | |||||||
| Woolderink et al. 202072 | TVUS, Bx, ETS (single visit) | At visit | EC: 0/25 (0.0) | NA | NA | 0/25 (0.0) | NR |
| OC: NR | |||||||
| Comparative studies | |||||||
| de Jong et al. 200673 | 1990–2004: TVUS, CA-125 (1 year) | NA | NR | NR | NR | NR | NR |
| 1965–75s | NA | NR | NR | NR | NR | NR | |
| 1975–90s | NA | NR | NR | NR | NR | NR | |
| Dueñas et al. 202038,39 | CE, TVUS; 1 year | Over study | EC: 123/465 (26.5)t | EC: 57× I, 8× II, 8× III, 4× IV, 39× NK, 7× NR | NR | NR | NR |
| OC: 36/465 (7.7)t | OC: 16× I, 2× II, 7× III, 11× NK | ||||||
| RRSu | At surgery | EC: 6/66 (9.1)v | EC: 3× pTis, 1× I, 1× II, 1× III | 0/6 (0.0) | NR | NR | |
| OC: 0/66 (0.0) | |||||||
| Eikenboom et al. 202140 | TVUS, Bx, CA-125 (1–2 years) | At visit | EC: 6/111 (5.4)w | EC: 1× I, 4× IA, 1× IB | 4/6 (66.7) | NRx | EH: 8/111 (7.2)x |
| OC: 1/111 (0.9) | OC: 1× IV | 1/1 (100.0) | |||||
| Interval | EC: 0/111 (0.0) OC: 0/111 (0.0) |
NA | NA | NR | NR | ||
| RRSu | At surgery | EC: 1/53 (1.9) | EC: 1 × 1A | 0/1 (0.0) | 0/53 (0.0) | NR | |
| OC: 0/53 (0.0) | |||||||
| Gerritzen et al. 200942 | Post-2006: CE, TVUS, Bx, CA-125 (1 year) | At visit | EC: 1/64 (1.6)y | EC: 1× IB | NR | CAH: 3/64 (4.7) | NCRaa |
| OC: NCRz | OC: NCRz | ||||||
| Interval | EC: 0/64 (0.0) | NA | NA | NR | NR | ||
| OC: 0/64 (0.0) | |||||||
| Pre-2006: CE, TVUS, non-routine Bx, CA-125 (1 year) | At visit | EC: 2/221 (0.9)y OC: NCRz |
EC: 1× IC, 1× IIIC OC: NCRz |
NR | CAH: 1/221 (0.5) | NCRaa | |
| Interval | EC: 0/221 (0.0) | NA | NA | NR | NR | ||
| OC: 0/221 (0.0) | |||||||
| Helder-Woolderink et al. 201343,44 | 2008–12: TVUS, Bx, CA-125 (1 year) | At visit | EC: 0/63 (0.0) | NA | NA | CAH: 1/63 (1.6) | Simple hyperplasia: 1/63 (1.6); ovarian cysts: 5/63 (7.9)bb |
| OC: 0/63 (0.0) | |||||||
| Interval | EC: 0/63 (0.0) | NA | NA | 0/63 (0.0) | NA | ||
| OC: 0/63 (0.0) | |||||||
| 2003–7: TVUS, non-routine Bx, CA-125 (1 year) | At visit | EC: 1/44 (2.3) | EC: 1× IB | 1/1 (100) | CAH: 1/44 (9.1) | Simple hyperplasia: 3/44 (6.8); ovarian cysts: 2/44 (4.5) | |
| OC: 0/44 (0.0) | |||||||
| Interval | EC: 0/44 (0.0) | NA | NA | 0/44 (0.0) | NA | ||
| OC: 0/44 (0.0) | |||||||
| Kalamo et al. 202074 | PUS, Bxcc | Survey | NR | NR | NR | NR | NR |
| RRSdd | NR | NR | NR | NR | NR | ||
| Renkonen-Sinisalo et al. 200760,61 | Varied (2–3 years)ee | At visit | EC: 11/175 (6.3) | EC: 5× IA, 4× IB, 1× IIB, 1× IIIA | 0/11 (0.0) | CAH: 4/175 (2.3); SAH: 1/175 (0.6) | CH no atypia: 3/175 (1.7); simple hyperplasia: 1/175 (0.6) |
| OC: 0/175 (0.0) | |||||||
| Interval | EC: 3/175 (1.7) | EC: 2× IA, 2 × 1Bff | 2/3 (66.7) | ||||
| OC: 4/175 (2.3) | OC: NR | 2/4 (50.0) | |||||
| RRS/STgg | At visit | EC: 83/138 (60.1) | EC: 27× IA, 32× IB, 8 × IC, 1× IIA, 1× IIB, 4× IIIA, 7× IIIC, 2× IVB, 1× NK | NR | NR | NR | |
| OC: NR | |||||||
| Stuckless et al. 201366 | TVUS, Bx, CA-125 (1–2 years) | At visit | EC: 5/54 (9.3) | EC: 4× I, 1× III | NR | NR | NR |
| OC: 1/54 (1.9) | OC: 1× II | ||||||
| Interval | EC: 4/54 (7.4) | EC: 3× I, 1× NK | NR | NR | NR | ||
| OC: 5/54 (9.3) | OC: 1× I, 2× II, 2× NK | ||||||
| No surveillancehh | Over study | EC: 44/120 (36.7) | NCR | NR | NR | NR | |
| OC: 16/120 (13.3) | |||||||
| Age-matched no surveillanceii | Over study | EC: 20/54 (37.0) | NCR | NR | NR | NR | |
| OC: 6/54 (11.1) | |||||||
| Tzortzatos et al. 201567 | TVUS, Bx, CA-125 (1–2 years) | At visit | EC: 3/45 (6.7) | EC: 1× IA, 2× II | 0/3 (0.0) | CAH: 2/45 (4.4) | NR |
| OC: 2/45 (4.4) | OC: 2× I | 0/2 (0.0) | |||||
| Interval | EC: 4/45 (8.9) | EC: 1× IA, 2× IB, 1× II | 4/4 (100) | 0/45 (0.0) | NR | ||
| OC: 0/45 (0.0) | |||||||
| RRSu | At surgery | EC: 3/41 (7.3) | EC: 3× IA | 0/3 (0.0) | CAH: 2/41 (4.9) | NR | |
| OC: 0/41 (0.0) | |||||||
| Woolderink et al. 201869–71 | TVUS, Bx, CA-125 (1 year) | At visit | EC: NCRjj | OC: 5× IA, 1× IB, 3× IC, 1× IIA, 1× IIB | 8/11 (72.7) | NCRjj | NR |
| OC:11kk | |||||||
| Interval | EC: NCRjj | OC: 1× IA | 1/1 (100.0) | NCRjj | NR | ||
| OC: 1kk | |||||||
| No surveillance | Over study | EC: NCRjj | OC: 28× I, 6× II, 7× III | 41/41 (100.0) | NCRjj | NR | |
| OC: 41kk | |||||||
Detection during surveillance
Fifteen of the single-arm studies and seven of the comparative studies provided data on gynaecological cancers detected during surveillance visits (Table 7). These data are provided separately for EC and OC. However, one of the studies reported analyses of data for EC and OC combined, suggesting that cumulative lifetime incidence of these cancers was similar before and after surveillance being introduced [31.6%, 95% confidence interval (CI) 28.2 to 35.0] and 32.5% (95% CI 29.1 to 35.9), respectively] as were annual incidence rates (0.6 and 0.7%, respectively). 33 This study was treated as a single-arm study and not a comparative study because no data were provided for the separate periods and the study was presented as a single-arm cohort study.
Endometrial cancers detected during surveillance
Of the 15 single-arm studies that provided data on cancer detection during surveillance visits, 14 reported the proportion of participants who had an endometrial cancer detected. In 6 of these 14 studies, no ECs were detected with surveillance. 37,41,62–64,68,72 Among the remaining eight single-arm studies, the absolute numbers of ECs detected was low; in these studies, detection rates ranged from 0.8%47,48 to 22.2%,34,35 but the study by Bats et al. (2014) was unusual in that it was a pilot study where only a single visit was used, the sample was extremely small (nine participants) and thus likely to produce less accurate detection rates, and the surveillance technique used was not typical of clinical practice or similar to other studies (uterine cavity washings). 34,35 The next highest rate of EC detected during surveillance visits was 7.3%, where participants underwent surveillance with TVUS, outpatient hysteroscopy and endometrial biopsy over a median of two annual surveillance visits. 52
Among the seven comparative studies that provided data on detection of gynaecological cancers during surveillance visits, six provided data on ECs (the remaining study71 only reported ECs among participants with OCs). 69–71 One of these studies found no ECs at surveillance visits where biopsies were routinely conducted, and only one at surveillance visits during the period prior to this (non-routine biopsies). 43,44 The other study providing data on the detection of ECs during surveillance at different periods found low rates during both periods (1.6% and 0.9%, respectively). 42
Stuckless et al. (2013) compared surveillance with two no-surveillance groups (those who did not receive surveillance and age-matched controls). Endometrial cancer was detected in 9.3% of participants during surveillance visits, in 36.7% across the study period in the no-surveillance group and in 37.0% across the study period in the age-matched controls who were alive and disease-free at the point at which the surveillance group participants entered surveillance. 66 Even if interval cancers were added to the surveillance group data, fewer ECs were detected among those receiving surveillance.
The remaining three comparative studies compared surveillance with surgery. 40,60,61,67 In these three studies, detection of ECs at surveillance visits was similar to that reported in the single-arm studies (5.4%, 6.7%, 6.3%, respectively). 40,60,61,67 Two of these studies provided data on ECs detected during RRS in participants who had previously received surveillance (where no cancer was found). 40,67 These could be missed/interval cancers and are discussed in that section of the report [see Interval (missed) cancers]. In the other study, the surgery group included participants undergoing surgical treatment as well as RRS – this would increase the number of cancers found and would not be a meaningful comparator group; indeed, ECs were detected in 60.1% of participants in this group. 60,61
Seven of the single-arm studies and six of the comparative studies reporting data on EC detected during surveillance visits provided some data on the stage of the cancers. There was no clear indication from these data that EC detected at surveillance was detected at an earlier stage to interval ECs or those detected during surgery. This may be, in part, because the low numbers of cancers detected do not allow for any clear patterns to be seen.
Following PPI input, it was agreed that detection rate data would be presented according to LS mutation. Fourteen studies provided some data on ECs detected according to LS mutations; these data are given in Table 8. Data were too sparse for any patterns to be ascertained, although in one study it appears that ECs were more commonly detected among participants with MSH2 and MSH6 mutations. 39
| Study | Surveillance (frequency) | Detection timea | Participants with cancers detected n/N (%) | |||||
|---|---|---|---|---|---|---|---|---|
| All mutations | MLH1 | MSH2 | MSH6 | PMS2 | EPCAM | |||
| Barrow et al. 200933 | CE, TVUS, OPH, Bx (frequency UC) | Over study | EC: 86a | NRb | NRb | NRb | NRb | NA |
| OC: 24a | ||||||||
| Bats et al. 201434,35 | UCW (single visit) | At visit | EC: 2/8(22.2) | EC: 1/3 (33.3) | EC: 1/2 (50.0) | EC: 0/3 (0.0) | NA | NA |
| OC: NR | ||||||||
| Bucksch et al. 202036 | UCc (1 year) | Over study | EC: 23/550 (4.2) | EC: 9/222 (4.1) | EC: 13/265 (4.9) | EC: 1/63 (1.6) | NA | NA |
| OC: 5/550 (0.9) | OC: 0/222 (0.0) | OC: 5/265 (1.9) | OC: 0/63 (0.0) | |||||
| Dove-Edwin et al. 200237 | TVUS/PUS (1–2 years) | At visit | EC: 0/292 (0.0)d | NA | NA | NA | NA | NA |
| OC: NR | ||||||||
| Interval | EC: 2/292 (0.7)d,e | EC: 2f | NA | NA | NA | NA | ||
| Dueñas et al. 202038,39 | CE, TVUS; 1 year | Over study | EC: 123/465 (26.5)h | EC: 39/226 (17.3) | EC: 46/127 (36.2) | EC: 27/75 (36.0) | EC: 4/22 (18.2) | EC: 1/15 (6.7) |
| OC: 36/465 (7.7)h | OC: 12/226 (5.3) | OC: 13/127 (10.2) | OC: 8/75 (10.7) | OC: 2/22 (9.1) | OC: 1/15 (6.7) | |||
| RRSg | At surgery | EC: 6/66 (9.1)i | EC: 4/33 (12.1) | EC: 2/19 (10.5) | EC: 0/10 (0.0) | EC: 0/3 (0.0) | EC: 0/1 (0.0) | |
| OC: 0/66 (0.0) | OC: NA | OC: NA | OC: NA | OC: NA | OC: NA | |||
| Eikenboom et al. 202140 | TVUS, Bx, CA-125 (1–2 years) | At visit | EC: 6/111 (5.4) | EC: 2f | EC: 2f | EC: 2f | EC: 0f | EC: 0f |
| OC: 1/111 (0.9) | OC: 0f | OC: 1f | OC: 0f | OC: 0f | OC: 0f | |||
| Interval | EC: 0/111 (0.0) | NA | NA | NA | NA | NA | ||
| OC: 0/111 (0.0) | ||||||||
| RRSg | At surgery | EC: 1/53 (1.9) | EC: 0/10 (0.0) | EC: 0/3 (0.0) | EC: 1/34 (2.9) | EC: 0/6 (0.0) | NA | |
| OC: 0/53 (0.0) | OC: NA | OC: NA | OC: NA | OC: NA | ||||
| Gerritzen et al. 200942 | Post-2006: CE, TVUS, Bx, CA-125 (1 year) | At visit | EC: 1/64 (1.6)j | EC: 0l | EC: 0l | EC: 1l | EC: 0l | EC: 0l |
| OC: NCRk | OC: NA | OC: NA | OC: NA | OC: NA | OC: NA | |||
| Interval | EC: 0/64 (0.0) | NA | NA | NA | NA | NA | ||
| OC: 0/64 (0.0) | ||||||||
| Pre-2006: CE, TVUS, non-routine Bx, CA-125 (1 year) | At visit | EC: 2/221 (0.9)j | EC: 1l | EC: 1l | EC: 0l | EC: 0l | EC: 0l | |
| OC: NCRk | OC: NA | OC: NA | OC: NA | OC: NA | OC: NA | |||
| Interval | EC: 0/221 (0.0) | NA | NA | NA | NA | NA | ||
| OC: 0/221 (0.0) | ||||||||
| Helder-Woolderink et al. 201343,44 | 2008–12: TVUS, Bx, CA-125 (1 year) | At visit | EC: 0/63 (0.0) | NA | NA | NA | NA | NA |
| OC: 0/63 (0.0) | ||||||||
| Interval | EC: 0/63 (0.0) | NA | NA | NA | NA | NA | ||
| OC: 0/63 (0.0) | ||||||||
| 2003–7: TVUS, non-routine Bx, CA-125 (1 year) |
At visit | EC: 1/44 (2.3) | EC: 0/9 (0.0) | EC: 0/9 (0.0) | EC: 1/6 (16.7) | EC: 0/1 (0.0) | EC: NA | |
| OC: 0/44 (0.0) | OC: NA | OC: NA | OC: NA | OC: NA | OC: NA | |||
| Interval | EC: 0/44 (0.0) | NA | NA | NA | NA | NA | ||
| OC: 0/44 (0.0) | ||||||||
| Ketabi et al. 201447,48 | UCf (2 years) | At visit | EC: 7/871 (0.8) | EC: 4f | EC: 1f | EC: 2f | EC: 0f | EC: 0f |
| OC: 1/871 (0.1) | OC: 0f | OC: 1f | OC: 1f | OC: 1f | OC: 1f | |||
| Interval | EC: 6/871 (0.7) | EC: 2f | EC: 3f | EC: 1f | EC: 0f | EC: 0f | ||
| OC: 3/871 (0.3) | OC: 1f | OC: 2f | OC: 0f | OC: 0f | OC: 0f | |||
| Interval | EC: 0/58 (0.0) | NA | NA | NA | NA | NA | ||
| OC: NR | ||||||||
| Manchanda et al. 201252 | TVUS, OPH, Bx (1 year) | At visit | EC: 3/41 (7.3) | EC: 2/10 (20)n | EC: 0/6 (0.0) | EC: NA | EC: NA | EC: NA |
| OC: NR | OC: NR | OC: NR | OC: NR | OC: NR | OC: NR | |||
| Interval | EC: 1/41 (2.4)m | EC: 0/10 (0.0) | EC: 1/6 (16.7) | EC: NA | EC: NA | EC: NA | ||
| OC: NR | OC: NR | OC: NR | OC: NR | OC: NR | OC: NR | |||
| Møller et al. 20178,53 | Varied | Over study | EC: 72/1057 (6.7)o,p | EC: 35/514 (6.8) | EC: 22/325 (6.8) | EC: 11/170 (6.5) | EC: 2/48 (4.2) | EC: NA |
| OC: 19/1057 (1.8)o | OC: 10/514 (2.0) | OC: 9/325 (2.8) | OC: 0/170 (0.0) | OC: 0/48 (0.0) | OC: NA | |||
| Nebgen et al. 201454–58 | CE, TVUS, Bx, pap smear, CA-125 (1–2 years) | At visit | EC: 1/55 (1.8) | EC:1/8 (12.5) | EC: 0/17 (0.0) | EC: 0/4 (0.0) | EC: 0/3 (0.0) | EC: NA |
| OC: 0/55 (0.0) | OC: NA | OC: NA | OC: NA | OC: NA | OC: NA | |||
| Interval | EC: 0/55 (0.0) | NA | NA | NA | NA | NA | ||
| OC: 0/55 (0.0) | ||||||||
| Renkonen-Sinisalo et al. 200760,61 | Varied (2–3 years) | At visit | EC: 11/175 (6.3) | EC: 8l | EC: 2l | EC: 1l | EC: NA | EC: NA |
| OC: NR | OC: NA | OC: NA | OC: NA | OC: NA | OC: NA | |||
| Interval | EC: 3/175 (1.7) | EC: 3l | EC: 0l | EC: 0l | EC: NA | EC: NA | ||
| OC: 4/175 (2.3) | OC: NR | OC: NR | OC: NR | OC: NR | OC: NR | |||
| RRS/STq | At visit | EC: 83/138 (60.1) | NR | NR | NR | NR | NR | |
| OC: NR | ||||||||
| Rosenthal et al. 201363,64 | TVUS, CA-125 (1 year) | At visit | EC: 0/99 (0.0) | EC: NA | EC: NA | EC: NA | EC: NA | EC: NA |
| OC: 3/99 (3.0) | OC: 1/28 (3.6) | OC: 2/33 (6.1) | OC: 0/4 (0.0) | OC: NA | OC: NA | |||
| Interval | EC: 0/99 (0.0) | NA | NA | NA | NA | NA | ||
| OC: 0/99 (0.0) | ||||||||
| Ryan et al. 201765 | TVUS, OPH, OUS, CA-125 (1 year) | At visit | EC: 2/87 (2.3) | EC: 0/148 (0.0) | EC: 2/210 (1.0) | EC: 0/48 (0.0) | EC: 0/22 (0.0) | EC: NA |
| OC: 2/87 (2.3) | OC: 0/148 (0.0) | OC: 2/210 (1.0) | OC: 0/48 (0.0) | OC: 0/22 (0.0) | OC: NA | |||
| Interval | EC: NR | EC: NA | EC: NA | EC: NA | EC: NA | EC: NA | ||
| OC: 3/87 (3.4) | OC: 1/148 (0.7) | OC: 2/210 (1.0) | OC: 0/48 (0.0) | OC: 0/22 (0.0) | OC: NA | |||
| Tzortzatos et al. 201567 | TVUS, Bx, CA-125 (1–2 years) | At visit | EC: 3/45 (6.7) | EC: 2 | EC: 0 | EC: 1 | EC: 0 | EC: NA |
| OC: 2/45 (4.4) | OC: 0 | OC: 2 | OC: 0 | OC: 0 | OC: NA | |||
| Interval | EC: 4/45 (8.9) | EC: 1 | EC: 2 | EC: 1 | EC: 0 | EC: NA | ||
| OC: 0/45 (0.0) | OC: NA | OC: NA | OC: NA | OC: NA | OC: NA | |||
| RRSg | At surgery | EC: 3/41 (7.3) | EC: 1 | EC: 2 | EC: 0 | EC: 0 | EC: NA | |
| OC: 0/41 (0.0) | OC: NA | OC: NA | OC: NA | OC: NA | OC: NA | |||
Ovarian cancers detected during surveillance
Eight of the single-arm studies reported the proportion of participants who had an OC detected during surveillance and in four of these studies no OCs were detected with surveillance. 41,54–58,62 Among the remaining four single-arm studies, detection rates for OC were generally lower than those for EC, ranging from 0.1% (surveillance strategy unclear but likely to have followed Danish guidance)47,48 to 3.0% (surveillance comprising annual CA-125 and TVUS). 63,64
All seven comparative studies that provided data on detection of gynaecological cancers during surveillance visits, provided data on OCs. However, although Gerritzen et al. (2009)42 reported one OC, it was unclear within which study period this was detected, and in Woolderink et al. (2018)69–71 the number of participants undergoing surveillance was unclear, so detection rates could not be calculated. In two of the remaining five studies, no OCs were detected during surveillance visits. 43,44,60,61 Rates of OCs detected at surveillance visits were low in the other three studies, ranging from 0.9% in Eikenboom et al. (2021)40 to 4.4% in Tzortzatos et al. (2015). 67 Additionally, in the study comparing two surveillance periods, no OCs were detected with surveillance in the comparator (earlier) period either. 43,44 Three of the other comparative studies provided OC detection rates in control groups,40,66,67 two of which reported no OCs detected during RRS. 40,67 The remaining study found higher rates of OCs in the no-surveillance control groups over the study period (13.3% in the no-surveillance group and 11.1% in the age-matched controls) than was detected during surveillance (1.9%). 66 When interval cancers were added to the surveillance group data (to more closely match the across study data in the control groups), the number of OCs detected among those receiving surveillance was identical to the number detected in age-matched controls (see Table 7).
Four of the single-arm studies and four of the comparative studies reporting data on OC detected during surveillance visits provided some data on the stage of the cancers. These data are reported in Table 7. There was no clear indication from these data that OC detected at surveillance was detected at an earlier stage to interval OCs or those detected during surgery. As with the data for EC, this may be, in part, because the low numbers of cancers detected do not allow for any clear patterns to be seen.
Seven studies provided some data on OCs detected according to LS mutations; these data are given in Table 8. Data were too sparse for any patterns to be ascertained.
Pre-malignancies detected during surveillance
Ten of the single-arm studies and four of the comparative studies provided data on hyperplasia with atypia [reported as complex atypical hyperplasia (CAH)/atypical endometrial hyperplasia (AEH) or simple atypical hyperplasia (SAH)] detected during surveillance visits (see Table 7). Across the ten single-arm studies, low rates of premalignancies were generally reported, with three of these studies stating that there were no premalignancies detected. 50,51,72 One study reported a higher rate of premalignancies (22.2%), but this study was unusual, based only on nine participants and an atypical surveillance technique (uterine cavity washings). 34,35 Among the remaining six single-arm studies where premalignancies were detected during surveillance visits, absolute numbers of premalignancies were low (see Table 7) with rates ranging from 0.3% in Ketabi et al. (2014)47,48 to 7.3% in Rijcken et al. (2003). 62 The study by Ketabi et al. (2014) also reported that, in 0.2% of participants, CAH was detected during the interval between visits. 47,48
Four of the comparative studies reported hyperplasia with atypia during surveillance visits (see Table 7). A fifth comparative study reported eight cases of endometrial hyperplasia detected during surveillance (and none in the RRS group) but did not specify whether atypia was present. 40 Two of the four comparative studies reporting premalignancies during surveillance visits compared different surveillance periods. 42–44 Rates of premalignancies were low across all groups, but in Gerritzen et al. (2009) premalignancies appeared to be more common after routine biopsy was introduced (4.7%) than in the period where biopsies were not routinely used (0.5%). 42 However, the opposite was reported in Helder-Woolderink et al. (2013) where the rate of premalignancies was 1.6% after routine biopsy was introduced but 9.1% when biopsies were not routine. 43,44 These differences are likely to be due to the low absolute number of cases and relatively small sample sizes (see Table 7).
The other two comparative studies that reported premalignancies detected during surveillance visits had a surgical comparator. 60,61,67 The study by Renkonen-Sinisalo et al. (2007) only provided these data for the surveillance group and is therefore akin to the single-arm studies (rates of premalignancies were also similar to those reported across the single-arm studies, 2.9%). 67 Premalignancies were also infrequently detected in the Tzortzatos et al. (2015) surveillance group and the rate of premalignancy in this group (4.4%) also fell within the range of rates provided in the single-arm studies. 67 Although this study reported that no interval premalignancies were found in the surveillance group, 4.9% of participants who previously underwent surveillance before opting for RRS had a premalignancy detected during surgery. 67
Asymptomatic cancer detection rates
It is important to consider whether the cancers detected during surveillance were symptomatic or asymptomatic. It could be argued that symptomatic cancers are those which would be more likely to have presented even if surveillance had not taken place, whereas asymptomatic cancers would be unlikely to be detected without surveillance.
Asymptomatic endometrial cancers
Six of the included single-arm studies and four of the comparative studies reported whether ECs detected during surveillance were symptomatic or asymptomatic. In three of the single-arm studies reporting these data,34,35,49,50 all ECs detected with surveillance were symptomatic and in two of these studies all ECs detected with surveillance were asymptomatic. 45,46,52 In the remaining study, just over half (57.1%) of ECs detected with surveillance were symptomatic. 47,48
Among the four comparative studies providing these data, the one EC detected in Helder-Woolderink et al. (2013) during surveillance (in the period where biopsies were not routine) was symptomatic. 43,44 In two of these studies all ECs detected during surveillance were asymptomatic. 60,61,67 Tzortzatos et al. (2015)67 also reported that the ECs detected during RRS were all asymptomatic, whereas Renkonen-Sinisalo et al. (2007) did not report these data for the surgery group. 60,61 In the remaining comparative study, two-thirds of ECs detected during surveillance were symptomatic, whereas the single EC detected during RRS was asymptomatic. 40
These data are clearly mixed, but across the studies reporting these data, over half of ECs detected during surveillance were asymptomatic and would have been unlikely to have been detected without surveillance (at least at the point at which they were detected). However, the number of ECs detected with surveillance was still low across studies (see Table 7) and the two studies actually comparing surveillance with no surveillance did not provide data on whether ECs were symptomatic or asymptomatic.
As expected the majority of ECs detected during the interval between surveillance visits were symptomatic (see Table 7).
Asymptomatic ovarian cancers
Only one of the included single-arm studies and three of the comparative studies reported whether OCs detected during surveillance were symptomatic or asymptomatic. In the single-arm study reporting these data, the single OC detected with surveillance was asymptomatic. 47,48
Among the three comparative studies providing these data, Eikenboom et al. (2021)40 reported one OC detected during surveillance and the case was symptomatic, whereas in Tzortzatos et al. (2015)67 the two OCs detected with surveillance were asymptomatic (in both studies there were no OCs detected during RRS). 67 In Woolderink et al. (2018),69–71 72.7% of OCs detected during surveillance were symptomatic, whereas all of the OCs detected without surveillance were symptomatic.
Again, these data are clearly mixed, but the studies by Woolderink et al. (2018),69–71 Ketabi et al. (2014)47,48 and Tzortzatos et al. (2015)67 provide some limited data to suggest that surveillance can pick up at least some asymptomatic cases of OC. However, the comparative data from Woolderink et al. (2018)69–71 were not clear and it was not possible to establish whether the OC detection rates were different between those who received surveillance and those who did not.
Only three studies reported whether OCs detected during the interval between surveillance visits were symptomatic and in two of these studies all interval OCs were symptomatic. 47,48,69–71 However, in Renkonen-Sinisalo et al. (2007),60,61 two of the four of the interval OCs were asymptomatic (see Table 7).
Interval (missed) cancers
Data on interval cancers can elucidate how many cancers were missed by the surveillance programmes. Interval cancers are generally expected to be symptomatic, although sometimes asymptomatic cancers are found during the interval between surveillance visits during tests for other cancers or for other conditions. It is important to note that the detected interval cancers may not represent all of the cancers missed by surveillance; asymptomatic cancers that are missed by the surveillance programmes may not be detected during the interval between visits, may only present at a later point (e.g. once they become symptomatic, which could be outside of the study timeframe) or may be picked up at a subsequent surveillance visit. Cancers detected during RRS in participants who had previously been undergoing surveillance can also be considered missed cancers and are included here.
Data on interval cancers were reported in 11 single-arm studies and 7 comparative studies (see Table 7).
Interval endometrial cancers
Nine of the single-arm studies and six of the comparative studies provided data on interval EC (see Table 7). Two of these comparative studies also provided data on ECs detected during RRS in participants who had previously received surveillance (where no cancer was found). 40,67 Additionally, Dueñas et al. (2020) did not provide separate data on ECs detected at surveillance and in the interval between surveillance visits, but did provide data on ECs detected during RRS in participants who had previously received surveillance. 38,39
Of the nine single-arm studies providing these data, five reported no interval ECs. 49–51,54–58,63,64 In the other four studies, absolute numbers and rates of interval ECs were low: 0.7% in both Dove-Edwin et al. (2002)37 and Ketabi et al. (2014),47,48 and 2.4% in both Manchanda et al. (2012)52 and Rijcken et al. (2003). 62 Of the six comparative studies reporting these data, three reported no interval ECs,40,42–44 including across both periods in Gerritzen et al. (2009)42 and Helder-Woolderink et al. (2013). 43,44 Of the remaining three comparative studies, Renkonen-Sinisalo et al. (2007)60,61 reported interval EC in the surveillance group at a similarly low rate to the single-arm studies (1.7%) but Stuckless et al. (2013)66 and Tzortzatos et al. (2015)67 reported higher rates of interval EC in their surveillance groups (7.4% and 8.9%). The reasons for this are unclear.
As previously mentioned, Eikenboom et al. (2021)40 and Tzortzatos et al. (2015)67 also provided data on EC detected during RRS in participants who had previously received surveillance (where no cancer was found). Tzortzatos et al. (2015)67 detected 3 ECs among the 41 participants who underwent RRS, and Eikenboom et al. (2021)40 detected 1 EC among the 53 participants who underwent RRS. 40 Additionally, Dueñas et al. (2020) reported 6 ECs among the 66 participants who underwent RRS. 38,39
Although it is clear that in several studies there were no missed ECs detected, it was also clear that surveillance did not detect all ECs. As expected, and has previously been mentioned, the majority of the missed ECs detected during the interval between surveillance visits were symptomatic (see Table 7).
Interval ovarian cancers
Six of the single-arm studies and seven of the comparative studies provided data on interval OCs (see Table 7). As with the data for EC, two of these comparative studies also provided data on OCs detected during RRS in participants who had previously received surveillance (where no cancer was found). 40,67 Again, Dueñas et al. (2020) also provided data on OCs detected during risk-reducing surgery, despite not providing data on OCs detected in the interval between surveillance visits. 39
Of the six single-arm studies providing these data, four reported no interval OCs. 45,46,54–58,62–64 The other two single-arm studies reported low absolute numbers and rates of interval OC: 0.3% in Ketabi et al. (2014) and 3.4% in Ryan et al. (2017). 47,48,65 Of the seven comparative studies reporting these data, four reported no interval OCs,40,42–44 including across both periods in Gerritzen et al. (2009)42 and Helder-Woolderink et al. (2013). 43,44 Of the remaining three comparative studies, Woolderink et al. (2018) reported one interval OC, but the number of participants undergoing surveillance was not clear so the rate of interval OC is not known. 69–71 As with the data for EC, Renkonen-Sinisalo et al. (2007)60,61 reported interval OC in the surveillance group at a similarly low rate to the single-arm studies (2.3%) but Stuckless et al. (2013)66 reported a higher rate of interval OC in their surveillance group (9.3%). Again, the reason for this difference is not clear.
Eikenboom et al. (2021) and Tzortzatos et al. (2015) also provided data on OC detected during RRS in participants who had previously received surveillance (where no cancer was found). 40,67 Neither study found any OC among the participants who underwent RRS. Similarly, Dueñas et al. (2020) reported no OC among participants who underwent RRS. 38,39
As with the data for EC, it is clear that in several studies there were no missed OCs detected, but surveillance did not detect all OCs across the studies. As previously mentioned, two studies reported that all interval OCs were symptomatic. 47,48,69–71 However, in Renkonen-Sinisalo et al. (2007), two of the four interval OCs were asymptomatic. 60,61
Other medical findings
Eleven studies reported other (incidental) medical findings during surveillance (see Table 7). 40,41,43,44,47–52,54–58,60–62 Of the three comparative studies providing these data,40,43,44,60,61 only Helder-Woolderink et al. (2013) reported these data for more than one study group (i.e. the different surveillance time periods). 43,44 In this study, simple hyperplasia was detected more frequently in the earlier period (before biopsy became routine) and ovarian cysts were detected more frequently in the later period. 43,44 However, numbers were small and no conclusions should be drawn from this. Eikenboom et al. (2021) and Renkonen-Sinisalo et al. (2007) only reported data on other medical findings during surveillance and not for surgical comparators, and so effectively provided single-arm data. 40,60,61
During surveillance, the detection of hyperplasia (either simple or complex) without atypia was reported in eight studies. Two studies reported detecting complex hyperplasia without atypia [with an incidence of 3.6% in Nebgen et al. (2014)54–58 and 1.7% in Renkonen-Sinisalo et al. (2007)]. 60,61 Seven studies reported detecting simple hyperplasia, with incidence rates (where calculable) ranging from 0.6% in Renkonen-Sinisalo et al. (2007)60,61 to 6.8% in Helder-Woolderink et al. (2013). 43,44 Eikenboom et al. (2021) reported an incidence of endometrial hyperplasia of 7.2% but it was not clear whether this referred to simple or complex hyperplasia, or indeed whether or not atypia was present in any of the cases (see Detection of malignancies and premalignancies). 40
Six studies reported finding endometrial and/or endocervical polyps during surveillance and three reported the number of participants with polyps (rather than the number of polyps): Elmasry et al. (2009)41 and Rijcken et al. (2003)62 reported incidence rates of polyps (not clear if endometrial or endocervical) of 8.0% and 4.9%, respectively, and Manchanda et al. (2012) reported incidence rates of endometrial polyps as 14.6% and endocervical polyps as 4.9%. 52
In Ketabi et al. (2014), an additional gynaecological cancer which was outside of the scope of this review was found (fallopian tube carcinoma), and additionally a mucinous borderline OC was reported (see Detection of malignancies and premalignancies). 47,48 It was unclear whether these findings were detected with surveillance or during the interval between surveillance visits. Other incidental findings reported across the studies were fibroids/myoma/adenomyosis (reported in three studies), ovarian cysts (two studies), atrophy (two studies) and disturbed proliferative endometrium (one study; see Table 7).
Diagnostic test accuracy
Five studies provided DTA data (either sufficient data to complete a 2 × 2 table or reported both sensitivity and specificity values) for specific tests used in gynaecological surveillance programmes. 34,35,50–52,63,64 In four of these studies, the target condition was EC,34,35,50–52 and in one study the target condition was OC. 63,64
The QUADAS-2 ROB ratings for these studies are reported in the section Risk of bias in test accuracy data. 32 Only one of the studies provided test accuracy data for more than one test. 52 Little between-test comparison of DTA was made in the study report. 52 It was therefore decided not to deviate from protocol by assessing this study using the QUADAS-C for head-to-head test accuracy studies published in 2021. 77 Instead, any differences in the ROB for each test are discussed in the section Risk of bias in test accuracy data.
Diagnostic test accuracy data from these studies are provided in Diagnostic test accuracy data, and should be considered alongside the ROB assessments in Risk of bias in test accuracy data.
Risk of bias in test accuracy data
The results from the QUADAS-2 assessments are divided into ratings regarding ROB and those regarding applicability (Table 9).
| Study | ROB | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Bats et al. 201434,35 | ? | ? | ? | ☺ | ☺ | ? | ☺ |
| Lécuru et al. 201051 | ? | ? | ☺ | ☺ | ☺ | ☺ | ☺ |
| Lécuru et al. 200850 | ☺ | ? | ? | ☺ | ☺ | ☺ | ☺ |
| Manchanda et al. 201252 | ? | ? | ? | ? | ☺ | ☺ | ? |
| Rosenthal et al. 201363,64 | ? | ☺ | ? | ☹ | ☺ | ☺ | ? |
In Manchanda et al. (2012) it was not clear whether all women received the same reference standard; the reference standard was described as ‘histology’, and when the test under assessment was TVUS it was likely that tissue for the reference standard was obtained via endometrial sampling, but it was unclear what tissue was used for the reference standard when endometrial sampling was the index test (some participants, but not all, underwent surgery). 52
In Rosenthal et al. (2013) it is likely that the flow and timing of the study would have resulted in high ROB. 63,64 This is primarily because the reference standard was any cancer diagnosed within 365 days of the index test, and this was considered too long an interval (within 3 months would have been more appropriate). Using a 365-day timeframe rather than a 3-month timeframe risks overestimating true positive and false negative results and underestimating true negative and false positive results. It was also unclear in Rosenthal et al. (2013) what the reference standard was: for participants with a negative index test result who did not opt for risk-reducing surgery and who did not present with a symptomatic interval cancer, it appeared that an absence of cancer was assumed. 63,64
When assessing DTA data, the appropriateness and applicability of the reference standard is dependent upon the treatment pathway of the study participants. For studies where all participants were undergoing surgery following surveillance, the reference standard should be histologically confirmed cancer using surgical tissue samples, and this was the case in Bats et al. (2014). 34,35 For studies based on participants who were not undergoing surgery, the best available reference standard is histologically confirmed cancer based on biopsy tissue samples, and this was the case in Lécuru et al. (2008)50 and (2010). 51 However, in the remaining two studies the appropriateness of the reference standard was unclear (see Risk of bias in test accuracy data). 52,63,64
Diagnostic test accuracy data
The DTA data are given in Table 10. Of these studies, only Manchanda et al. (2012) clearly and fully reported EC prevalence (6.35%, 95% CI 1.76 to 15.47) alongside DTA data and none of the studies clearly and fully reported OC prevalence (see Table 10). 52 For Bats et al. (2014), EC prevalence was calculated as 22.2% (95% CI 2.8 to 60.0) for this review, using the 2 × 2 data provided in the study report. 34,35 Similarly, for Rosenthal et al. (2013), OC prevalence was calculated for this review as 4.0% (95% CI 1.1 to 10.0) using the 2 × 2 data provided. 63,64 The other two studies did not provide sufficient 2 × 2 data for prevalence to be calculated. 50,51 It is important to note that DTA data (in particular PPV and NPV) are impacted by disease prevalence and, therefore, it is important to be able to consider prevalence alongside the DTA data.
| First author (year) | Test | Reference standard | TP n/N (%) | TN n/N (%) | FP n/N (%) | FN n/N (%) | Prevalence n/N (%) (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR− (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hysteroscopy | |||||||||||||
| Lécuru 200850 | Hysteroscopy | EC diagnosis; endometrial biopsya | NR | NR | NR | NR | NR | 50 | 100 | 100 | 40 | NC | 0.5 |
| Endometrial sampling | |||||||||||||
| Manchanda 201252 | OHES | EC diagnosis: histologyb | 4/63 (6.3) | 53/63 (84.1) | 6/63 (9.5) | 0/63 (0.0) | 4/63 (6.3) (1.8 to 15.5) | 100 (39.8 to 100.0) | 90 (79.2 to 96.2) | 40 (12.2 to 73.8) | 100 (93.3 to 100.0) | 9.8 (4.6 to 21.0) | 0.0 |
| US | |||||||||||||
| Lécuru 201051 | Pelvic ultrasound | EC diagnosis: endometrial biopsya | NR | NR | NR | NR | NR | 100 | 55 (45.0 to 65.0) | 6 (1.00 to 10.00) | 100 | 2.2 | 0.0 |
| Manchanda 201252 | TVUS | EC diagnosis: histologyb | 2/63 (3.2) | 50/63 (79.4) | 9/63 (14.3) | 2/63 (3.2) | 4/63(6.3) (1.8 to 15.5) | 50 (6.8 to 93.2) | 85 (73.0 to 92.8) | 18 (2.3 to 51.8) | 96 (86.8 to 99.5) | 3.3 (1.0 to 10.4) | 0.6 (0.2, 1.6) |
| Rosenthal 201363,64 | TVUS + CA-125 | OC diagnosis: test NRc | 4/99 (4.0) | 95/99 (96.0) | 0/99 (0.0) | 0/99 (0.0) | 4/99 (4.0) (1.1 to 10.0)d | 100.0 (39.8 to 100.0)e | 100 (96.1 to 100.0) | 100.0 (9.8 to 100.0)e | 100 (96.1 to 100.0) | NCf | 0.0f |
| Other | |||||||||||||
| Bats 201434,35 | UCW with MSI analysis | EC diagnosis: histologyg | 2/9 (22.2) | 7/9 (77.8) | 0/9 (0.0) | 0/9 (0.0) | 2/9 (22.2) (2.8 to 60.0)h | 100 | 100 | 100 | 100 | NCf | 0.0f |
Ultrasound
Rosenthal et al. (2013) was the only study that provided DTA data (for TVUS plus CA-125) in relation to OC. 63,64 This study reported no false positive or false negative results with TVUS plus CA-125, so sensitivity, specificity, PPV and NPV were all reported as 100% (see Table 10). This should be interpreted with caution because of the high ROB in these data; the reference standard is unclear, but it appears that not all individuals received the same reference standard and that a negative OC diagnosis was assumed for individuals with negative surveillance results who did not have surgery or later present with symptoms. Furthermore, any OC detected within 365 days of the index tests (due to surgery or symptomatic presentation) was interpreted as a positive reference standard result where a 3-month timeframe would be considered more appropriate (see Risk of bias in test accuracy data). This would mean that false positives and true negatives under a 3-month interval may not have been detected under a 365-day interval.
Two studies reported data on the DTA of ultrasound techniques for detecting EC. 51,52 However, the two studies assessed different tests: pelvic US in Lécuru et al. (2010) and TVUS in Manchanda et al. (2012). 51,52 Lécuru et al. (2010) assessed the test accuracy of pelvic ultrasound against endometrial biopsy (Pipelle device) results from the same surveillance time point, and reported sensitivity of 100%, specificity of 55% (95% CI 45.00 to 65.00), a PPV of 6% (95% CI 1.00 to 10.00) and a NPV of 100%, with likelihood ratio of a positive task (LR+) reported as 2.2 (95% CI not provided) and likelihood ratio of a negative task (LR−) as 0. 51 Manchanda et al. (2012) assessed TVUS against histology (possibly based on biopsy tissue but this is unclear, and with the interval between TVUS and the reference standard also unclear) and reported sensitivity of 50% (95% CI 6.8 to 93.2), specificity of 85% (73.0 to 92.8), a PPV of 18% (2.3 to 51.8) and a NPV of 96% (86.8 to 99.5), with LR+ reported as 3.3 (1.0 to 10.4) and LR– as 0.6 (0.2 to 1.6). 52 Owing to the lack of clarity about the reference standard (both the techniques used and the timeframe of the index test and reference standard) in Manchanda et al. (2012), it is important not to use the data from these two studies to make comparisons between TVUS and pelvic ultrasound. 52
Hysteroscopy (with or without endometrial sampling)
Two studies reported DTA data on hysteroscopy for detecting EC but, again, these tests (and the reference standards) were dissimilar (see Table 10). 50,52 Lécuru et al. (2008) used a 3-mm hysteroscope and reported DTA against a reference standard of endometrial biopsy using a Pipelle device at the same surveillance time point. 50 Sensitivity was reported as 50%, specificity as 100%, PPV as 100% and NPV as 40% and the study also reported LR+ to be non-calculable and LR− to be 0.5. CIs were not provided for the DTA data in Lécuru et al. (2008) and these values could not be recalculated because 2 × 2 data were not provided. 50 The study by Manchanda et al. (2012) assessed test accuracy of hysteroscopy with endometrial sampling. 52 It is not clear what the reference standard was or whether all participants received this (see Risk of bias in test accuracy data), which seriously limits any interpretation of these DTA data: sensitivity was reported as 100% (95% CI 39.8 to 100.0), specificity as 90% (95% CI 79.2 to 96.2), PPV 40% (12.2 to 73.8) and NPV 100% (93.3 to 100.0), with LR+ reported as 9.8 (4.6 to 21.0) and LR− as 0.
Other tests
Bats (2014) reported on the DTA of uterine cavity washing (with MSI analysis; mononucleotide repeat markers BAT25, BAT26, NR21, NR24 and NR27) for detecting EC. 34,35 The reference standard was histological EC diagnosis using tissue obtained from total hysterectomy (all participants received a hysterectomy after the uterine cavity washing procedure). No false positive or false negative index test results were reported; sensitivity, specificity, PPV and NPV were all reported as 100%. It should be noted that both cases of EC were known at recruitment, although it was unlikely that the laboratory staff carrying out MSI analysis for the index test would be aware of this. This study was designed as a proof-of-concept study, and due to the very small sample size (n = 9) and small number of participants with cancer detected (n = 2), these data should be considered as extremely preliminary. The study was published in 2014, but no further studies investigating MSI analysis of uterine cavity washings were identified in the relevant population. 34,35
Test failure rates
Five of the included studies reported test failures for at least one test used as part of a surveillance programme. 34,35,41,50,62,72 These are given in Table 11.
| First author (year) | Test | Test failures n/N (%) |
|---|---|---|
| Hysteroscopy | ||
| Elmasry et al. 200941 | 4-mm hysteroscope, saline 0.9% | 6/23 (26.1) |
| Lécuru et al. 200850 | 3-mm hysteroscope, saline | 11/125 (8.8) |
| Endometrial biopsy | ||
| Elmasry et al. 200942 | Pipelle | 6/25 (24.0) |
| Lécuru et al. 200850 | Pipelle | 12/116 (10.3) |
| Rijcken et al. 200362 | Pipelle | 2/17 (11.8) |
| Woolderink et al. 202072 | Pipelle | 5/25 (20.0) |
| US | ||
| Elmasry et al. 200941 | TVUS | 1/25 (4.0) |
| Rijcken et al. 200362 | TVUS | 1/179 (0.6) |
| Other | ||
| Woolderink et al. 202072 | Tampon sampling | 25/25 (100.0)a |
| Bats et al. 201434,35 | UCW with MSI analysis | 0/9 (0.0) |
Test failures for hysteroscopy were reported as 26.1% and 8.8% with a 4-mm hysteroscope41 and a 3-mm hysteroscope,50 respectively. However, because of small sample sizes and other differences between studies (e.g. different samples of patients, different individuals and teams performing the procedures), no inferences or generalisations should be made regarding test failures and the devices/tests themselves. Test failure rates were reported for endometrial biopsy in four studies (see Table 11) and ranged from 10.3% in Lécuru et al. (2008)50 to 24.0% in Elmasry et al. (2009). 41 All of these studies used a Pipelle device; differences between-test failure rates may be due to the small sample sizes in these studies.
Failure of TVUS was reported in two studies [4.0% in Elmasry et al. (2009) and 0.6% in Rijcken et al. (2003)]. 41,62 The remaining two studies reported test failures for tampon sampling72 and uterine cavity washing (with MSI analysis). 34,35 In the Bats et al. (2014) study, it was reported that all procedures were successfully carried out. 34,35 Conversely, in the Woolderink et al. (2020) study it was reported that although tampons were successfully collected, in all cases there was a failure to collect endometrial cells. 72 The authors suggested that this may be due to the nature of their study sample; endometrial cells may not have been shed because all participants were healthy at the time of sampling and no cancers or any other abnormalities were found.
When looking within studies at the test failure data for different tests, Elmasry et al. 2009 found lower rates of test failure with TVUS (4.0%) than with either hysteroscopy (26.1%) or endometrial biopsy (24.0%). 41 This is consistent with the data from Rijcken et al. (2003), where lower rates of test failures were reported with TVUS (0.6%) than with endometrial biopsy (11.8%),62 and Lécuru et al. (2008),50 where similar rates of test failures were reported for hysteroscopy (8.8%) and endometrial biopsy (10.3%).
Clearly, in the feasibility study by Woolderink et al. (2020), test failure rates were much higher for tampon sampling (100.0% failure to collect endometrial cells) than with endometrial biopsy (20.0% test failures). 72 However, this might be interpreted differently if it were to be established that endometrial cells are consistently collected in individuals with endometrial abnormalities (in which case the absence of such cells may not be considered a test failure but a negative test result). Further research, in a larger LS sample, would be needed to clarify this.
Clinical effectiveness of gynaecological surveillance strategies
Mortality and survival
Data on mortality and survival were sought from comparative end-to-end studies (i.e. studies that compared surveillance with a control and thus provided comparative data on the clinical effectiveness outcomes). Ideally comparative RCTs were sought, but these were not found.
Seven comparative cohort studies provided data on mortality and/or survival. 38,39,42–44,60,61,66,69–71,73 However, these data were sparse [see Mortality and survival (time-to-event data)], so we amended the inclusion criteria and additionally present mortality or survival data from the single-arm studies.
All data relating to mortality or survival reported in the included studies are presented in Table 12.
| Comparative studies | |||||
|---|---|---|---|---|---|
| Study | Surveillance (frequency) | All-cause mortalitya n/N (%) |
Cancer-specific mortality n/N (%) | Overall survival | Other survival |
| de Jong et al. 200673 | Whole sample | NR | AC: 205/1375 (14.9)b | NR | NR |
| 1990–2004: TVUS, CA-125 (1 year) | NR | EC: 6c | NR | NR | |
| 1965–75d | NR | EC: 10c | NR | NR | |
| 1975–90d | NR | EC: 14c | NR | NR | |
| Dueñas et al. 202038,39 | Whole sample | 98/531 (18.5) | EC: 11/531 (2.1) | NR | NR |
| OC: 6/531 (1.1) | |||||
| CE, TVUS; 1 year | 98/465 (21.1) | EC: 11/465 (2.4) | NR | NR | |
| OC: 6/465 (1.3) | |||||
| RRS | 0/66 (0.0) | EC: 0/66 (0.0) | NR | NR | |
| OC: 0/66 (0.0) | |||||
| Gerritzen et al. 200942 | Whole sample | 3/100 (3.0) | EC: 0/100 (0.0) | NR | NR |
| OC: 1/100 (1.0) | |||||
| CRC: 2/100 (2.0) | |||||
| Post-2006: CE, TVUS, Bx, CA-125 (1 year) | NR | NR | NR | NR | |
| Pre-2006: CE, TVUS, non-routine Bx, CA-125 (1 year) | NR | NR | NR | NR | |
| Helder-Woolderink et al. 201343,44 | Whole sample | NR | EC: 0/75 (0.0) | NR | NR |
| 2008–12: TVUS, Bx, CA-125 (1 year) | NR | EC: 0/63 (0.0) | NR | NR | |
| 2003–7: TVUS, non-routine Bx, CA-125 (1 year) | NR | EC: 0/44 (0.0) | NR | NR | |
| Renkonen-Sinisalo et al. 200760,61 | Whole sample | NR | EC: 6/313 (1.9) | NR | NR |
| Surveillance, varied (2–3 years)e | NR | EC: 0/175 (0.0) | EC: 100% | NR | |
| RRS/STf | NR | EC: 6/138 (4.3) | EC: 92% | NR | |
| Stuckless et al. 201366 | Whole sample | 57/174 (32.8) | EC: 11/174 (6.3) | NR | NR |
| OC: 7/174 (4.0) | |||||
| TVUS, Bx, CA-125 (1–2 years) | 3/54 (5.6) | EC: 0/54 (0.0) | 79 yearsg | GC: 54 yearsh | |
| OC: 2/54 (3.7) | EC: 54 yearsh | ||||
| No surveillancei | 54/120 (45.0) | EC: 11/120 (9.2) | 66 yearsg | GC: 60 yearsh | |
| OC: 5/120 (4.2) | |||||
| Age-matched no surveillancej | 29/54 (53.7) | EC: 3/54 (5.6) | 69 yearsg | EC: 57 yearsh | |
| OC: 3/54 (5.6) | GC: 56 years | ||||
| Woolderink et al. 201869–71 | Whole sample | NR | OC: 8/878 (0.9) | NR | NR |
| TVUS, Bx, CA-125 (1 year) | NR | OC: 0c | NR | NR | |
| No surveillance | NR | OC: 8c | NR | NR | |
| Single-arm studies | |||||
| Dove-Edwin et al. 200237 | TVUS/PUS (1–2 years) | 8/184 (4.3)k | EC: 0/184 (0.0) | NR | NR |
| Møller et al. 20178,53 | Surveillance, variedl | NR | EC: 71/1057 (6.7) | EC: 98% (95% CI 88.0 to 99.8) at 5 years and 10 years | NR |
| OC: 19/1057 (1.8) | OC: 88% (95% CI 60.0 to 97.0) at 5 years; 89% (95% CI 60.0 to 97.0) at 10ym | ||||
| Pylvänäinen et al. 201259 | Surveillance, varied (2 years) | 65/548 (11.9)n | 42/548 (7.7)o | NR | NR |
| Ryan et al. 201765 | TVUS, OPH, OUS, CA-125 (1 year) | 11/36 (30.6) | OC: 6/36 (16.7)p | 80% at 2 years | NR |
Mortality
The single-arm studies,37,59,65 and the study by Gerritzen et al. (2009)42 reported a wide range of rates of all-cause mortality, from 3.0% in Gerritzen et al. (2009)42 to 30.6% in Ryan et al. (2017). 65 The higher rate in Ryan et al. (2017) was probably due to the fact that the sample was based on participants with complete data who were offered surveillance rather than those who were actually undergoing surveillance and also on the long timeframe of the dataset. 65
In Dueñas et al. (2020), all-cause mortality was higher for people who had surveillance than for those who started on surveillance before opting for RRS, which mostly involved hysterectomy with BSO (21.1% vs. 0.0%, median follow-up 10.4 years for surveillance and 8.7 years for RRS). 38,39 However, it is unclear whether the participants who opted for RRS differed from those who continued on surveillance in a way that may have influenced this outcome. Nevertheless, participants with factors likely to predict worse outcomes (e.g. strong family gynaecological cancer history) might be more inclined to opt for RRS and this would likely bias the data in the opposite direction to the findings.
In Stuckless et al. (2013), all-cause mortality was higher for those who did not undergo surveillance than for those who did (no surveillance 45.0%, no surveillance matched controls 53.7%, surveillance 5.6%, difference between surveillance and matched controls p = 0.000). 66 The data from the matched controls provides the best comparator across all studies (by matching cases with alive, disease-free controls at the age cases entered surveillance). However, the study still did not consider a wide range of potential confounding factors which might have influenced results and was also unclear about the period over which all-cause mortality was assessed.
Two studies provided data on mortality related to any cancer [de Jong et al. (2006), 14.9% over the 44-year study period; Pylvänäinen et al. (2012), 11.6% among LS mutation carriers, timeframe unclear]. 59,73 Despite being a comparative study, de Jong et al. (2006) did not provide these data separately for the three study groups and was, therefore, effectively a single-arm study with regards to these data. 73
Two single-arm studies and seven comparative studies (see Table 12), provided data on mortality due to EC. For one of the comparative studies [Gerritzen et al. (2009)],42 data were only provided for the whole sample and not by group and could therefore only be considered single-arm data; mortality due to EC for the two single-arm studies plus Gerritzen et al. (2009) was low: 0.0% in Gerritzen et al. (2009)42 over the 11 years of the study and Dove-Edwin et al. (2002)37 over a total of 825.7 patient-years at risk and 6.7% in Møller et al. (2017);8,53 the timeframe was unclear.
For two of the comparative studies, data on mortality due to EC were given by the study group but it was not clear how many participants there were in each group (de Jong et al. 2006, Woolderink et al. 2018). 69–71,73 Data from Woolderink et al. (2018) were, therefore, not interpretable;69–71 de Jong et al. (2006), however, did report that standardised mortality ratios for EC were not statistically significantly different between study periods. 73 This study also provided EC mortality by mutation (for all periods combined); the standardised mean mortality ratio for EC was 23.8 for MLH1 and MSH2 families combined and 20.7 for MSH6 families, and this was reported as not statistically significant. 73
Among the remaining four comparative studies providing data on mortality due to EC, two compared surveillance with RRS,38,39,60,61 one compared different surveillance periods,43,44 and one compared surveillance with two no-surveillance groups. 66 Helder-Woolderink et al. (2013) reported no mortality due to EC in either surveillance group over the 4-year period for each group. 43,44 In Stuckless et al. (2013), a higher mortality due to EC was reported for matched controls who did not receive surveillance than for those who did receive surveillance (5.6% vs. 0.0%, over a median surveillance period of 8.5 years). 66
Dueñas et al. (2020) reported higher EC-related mortality with surveillance than with RRS (2.4% vs. 0.0%, median follow-up 10.4 years for surveillance and 8.7 years for RRS). 38,39 However, in Renkonen-Sinisalo et al. (2007), a higher rate of EC-related mortality was reported with RRS than with surveillance: 4.3%, follow-up time-period median 13.7(0–42) years (range (0–42 years) for RRS compared with 0.0%, follow-up period mean 5 years (range 1–9 years) for surveillance. 60,61 This difference between studies may be due to the inclusion of participants who were undergoing surgical treatment as well as those undergoing RRS in the surgery group in Renkonen-Sinisalo et al. (2007). 60,61
Four comparative studies and two single-arm studies provided data on mortality due to OC (see Table 12). For one of the comparative studies, data were only provided for the whole sample and not by group and could therefore only be considered single-arm data. 42 As with the data for EC, mortality due to OC for the single-arm studies plus Gerritzen et al. (2009)42 was generally low: 1.0% in Gerritzen et al. (2009)42 over the 11-year study period and 1.8% in Møller et al. (2017);8,53 the timeframe was unclear. In Ryan et al. (2017) mortality due to OC was reported as 16.7% but all participants with OC who were undergoing surveillance were alive and well at the time that data were reported (i.e. mortality due to OC was 0.0% in those undergoing surveillance). 65
In a comparative study by Woolderink et al. (2018), data on mortality due to OC were given by group but it was not clear how many participants there were in each group; the data were therefore not interpretable. 69–71 The comparative study by Dueñas et al. (2020) reported higher OC-related mortality with surveillance than with RRS but this was low in both groups (1.3% vs. 0.0%, median follow-up 10.4 years for surveillance and 8.7 years for RRS). 38,39 In Stuckless et al. (2013),66 a higher mortality due to OC was reported for matched controls who did not receive surveillance than for those who did receive surveillance (5.6% vs. 3.7% over a median follow-up of 8.5 surveillance years). However, Stuckless et al. (2013)66 reported that deaths due to gynaecological cancer (EC and OC combined) were not statistically significantly different for the surveillance group and the matched controls (p = 0.15).
Møller et al. (2017)8 reported on the crude 5- and 10-year survival of EC (n = 71) and OC (n = 19) patients. Survival at 10 years was equivalent to survival at 5 years (i.e. no deaths were observed in cancer patients between 5 and 10 years post diagnosis). Survival was 98% (95% CI 88 to 99.8) for EC and 88% (95% CI 60 to 97) for OC.
In Renkonen-Sinisalo et al. (2007), 10-year EC survival did not significantly differ (p = 0.4) between those undergoing surveillance (100%, n = 14) and those whose EC was detected at surgery (92%, n = 83). 60,61
In Ryan et al. (2017), OC-specific survival was ‘around 80%’ at 2 years. 65 This was based on a sample including OCs diagnosed prior to LS diagnosis and OCs diagnosed in women with diagnosed LS who had not been offered surveillance, rather than those who were actually undergoing surveillance. Ryan et al. (2017) reported that none of the five women with OCs diagnosed under surveillance had died.
Other studies reported survival outcomes for a very limited number of cancer cases. Gerritzen et al. (2009)42 reported that the three participants with EC detected (two prior to the routine use of endometrial biopsy and one after biopsy was routinely added to the surveillance programme) were alive without recurrence at data collection, which was 8, 11 and 21 months post diagnosis. Rijcken et al. (2003) reported that the participant who had EC detected (interval cancer, surgical treatment received) was alive without EC recurrence at 5 years. 62
One comparative study aimed to provide relevant time-to-event data. 66 This study reported life expectancy and median age to cancer diagnosis (censored at date of gynaecological cancer, surgery, death or last follow-up). Life expectancy was better for those receiving surveillance than for matched controls who did not receive surveillance (mean 79 vs. 69 years, SDs not provided), but this difference was not statistically significant (p = 0.11). 66
This study also reported that the median age to gynaecological cancer diagnosis was similar for all groups [54, 60 and 56 years for the surveillance group, non-surveillance group and non-surveillance matched controls respectively, interquartile ranges (IQRs) not provided]; the comparison between the surveillance group and the matched controls was not statistically significant (p = 0.50). 66 The difference in median age to EC was provided for the surveillance group and the matched controls, and this was similar in the two groups (54 years and 57 years, respectively, IQRs not provided, p = 0.77). The study also reported that the median age to OC was no different in these two groups (data only provided graphically in the study report). 66
Cancer prognosis
None of the included studies provided data on cancer prognosis (e.g. clinically estimated prognosis in months) for women with LS who were undergoing gynaecological surveillance.
Stage of cancer at diagnosis could, however, be used as a proxy for prognosis. Indeed, one included study did report that FIGO stage 3 and 4 disease was associated with poorer prognosis (defined as percent survival over time in months) than FIGO stage 1 and 2 disease, although numbers with advanced disease were small and did not reach statistical significance. 65 Data on stage of cancer at diagnosis are described in the section Stage at diagnosis.
Treatment response
While many of the included studies provided some information on the number of women who had surgical treatment following detection of gynaecological cancer (or premalignancies), only three single-arm studies provided data for more than one treatment and reported the response to the treatments. 37,52,54–58 However, even data in these three studies was extremely limited. None of the studies provided end-to-end data; no comparative studies reported treatment response data, so there was no comparison of the eventual treatment responses of individuals who had surveillance with individuals in control groups.
In Dove-Edwin et al. (2002) it was merely stated that one participant received a hysterectomy with BSO for interval EC and OC, while another received a hysterectomy for interval EC, and that both of these individuals were well at the time the study was reported (i.e. no other data were reported about this). 37 In another study, the treatment of five participants with surveillance-detected endometrial abnormalities was described. 54–58 Three individuals with CAH (one of whom was thought to have EC at the time of biopsy and another of whom was thought to not have atypia at the time of biopsy) were successfully surgically treated with hysterectomy. The other two individuals had complex hyperplasia without atypia and were treated with contraceptives [one with oral contraceptives and the other with a levonorgestrel-releasing intrauterine system (LNG-IUS)]. After 6 months, an endometrial biopsy in the participant treated with oral contraceptives displayed no signs of hyperplasia. Similarly, for the participant treated with the LNG-IUS, there was no sign of hyperplasia at surveillance visits 10.3 months and 14.5 months later. 54–58
Similarly, Manchanda et al. (2012) reported successful treatment in a small subsample of individuals with EC or AEH detected during surveillance. 52 Three participants received a hysterectomy because of EC (one who also had AEH) but response to treatment is not explicitly reported (although surgery is likely to be successful, spread to other organs detected at a later date cannot be completely ruled out). One further participant with AEH was reported to have received treatment with a LNG-IUS and outpatient hysteroscopy and endometrial sampling did not find EC or atypia 4 months later.
These limited data suggest that individuals with premalignancies detected during surveillance can be successfully treated with contraceptives but, in the absence of direct within-study comparator groups (e.g. comparison with no surveillance), we cannot elucidate whether the surveillance had any impact upon the treatment response. The two individuals with interval gynaecological cancers reported in Dove-Edwin et al. (2002)37 were successfully treated with surgery and three individuals with surveillance-detected EC reported in Manchanda et al. (2012)52 also received surgical treatment (success not reported). All premalignancies were detected during surveillance and successfully treated either surgically or with contraceptives.
Fertility
Five included studies provided baseline parity data (see Participant characteristics at baseline). 41,45,46,72,74,75 One study provided parity data at different time points but did not clearly indicate whether new pregnancies occurred during the study period. 45,46 Another study reported that 87.5% of participants undergoing surveillance and 88.1% of participants who had opted for RRS (some who had previously been on surveillance) had one or more previous delivery. 74 However, this study was a cross-sectional survey, and it was not clear whether any of the deliveries occurred during surveillance. 74 Two studies investigated parity (and/or menopause status) as a subgroup for adverse events data. 45,46,54–58 These data do not elucidate the impact of surveillance on fertility.
One of the studies reported survey data (in 261 individuals with LS at risk of gynaecological cancer) on the number of births, number of pregnancies, use of assisted reproduction technology, decisions about starting families earlier due to LS, and reasons why participants could not have children (or their ideal number of children). 75 However, these data were not provided separately for individuals who had undergone surveillance so cannot contribute meaningfully to this review.
The single-arm study by Rijcken et al. (2003) reported that, over 10 years of surveillance, there were six births (four participants delivered one child and one delivered two children). 62 However, without a comparator, the impact of surveillance on fertility cannot be assessed. None of the studies, therefore, provided clear data that would enable the impact of surveillance on fertility to be assessed (e.g. by directly comparing different surveillance programmes or by comparing individuals undergoing surveillance with those who did not attend surveillance – noting that these data would not be expected for surgical comparators where fertility is necessarily impacted).
It should be noted, however, that one of the included studies reported that three individuals did not start surveillance (and did not enter the study) because they were trying to conceive and were concerned about the impact of hysteroscopy on fertility. 41 Of the 25 individuals who did enter the study and were undergoing surveillance, 2 nulliparous participants did not consent to hysteroscopy (but did consent to TVUS and to endometrial biopsy) because they were concerned about risk of infection and potential impact upon fertility. This highlights the need to consider the safety and acceptability of surveillance techniques for individuals who are actively trying to conceive (with some techniques being more invasive than others) but does not provide data to clarify whether certain tests have a greater impact upon fertility in the LS population.
Quality of life and mental health outcomes
One single-arm study provided data on QoL. 68 This study provided data at baseline, 3 and 6 months [using the Short Form 36 Health Survey (SF-36)] in individuals with LS undergoing a surveillance programme that could include outpatient hysteroscopy, endometrial biopsy, TVUS and/or CA-125.
The study also provided data on anxiety and depression [using the Hospital Anxiety and Depression Scale (HADS)]. It was decided that, for completeness, these data would also be presented here. The data regarding anxiety, depression and QoL (n = 15) are, therefore, presented together in Table 13. 68 For all HADS and SF-36 subscales there was a trend towards a reduction in scores from baseline over 6 months of surveillance, indicating a reduction in depression and anxiety, and an improvement in QoL for individuals with LS once gynaecological surveillance is started. However, these trends did not reach statistical significance. It is possible that this is due to the follow-up period only being 6 months (improvements in these types of outcomes may take longer to emerge).
| Type of data | Data point (months) | Mean score (SD) | 95% CI |
|---|---|---|---|
| HADS total | Baseline | 11.3 (9.3) | 6.1 to 16.4 |
| 3 | 11.2 (10.2) | 5.6 to 16.8 | |
| 6 | 10.0 (8.2) | 5.5 to 14.5 | |
| HADS anxiety | Baseline | 7.7 (4.7) | 5.1 to 10.3 |
| 3 | 7.6 (5.1) | 4.8 to 10.4 | |
| 6 | 7.3 (5.5) | 4.3 to 10.4 | |
| HADS depression | Baseline | 3.6 (5.1) | 0.8 to 6.4 |
| 3 | 3.6 (5.4) | 0.6 to 6.6 | |
| 6 | 2.7 (3.2) | 0.9 to 4.4 | |
| SF-36 physical | Baseline | 50.8 (9.3) | 45.6 to 56.0 |
| 3 | 46.6 (13.8) | 39.0 to 54.3 | |
| 6 | 47.3 (15.0) | 39.0 to 55.6 | |
| SF-36 mental | Baseline | 52.9 (9.3) | 47.7 to 58.1 |
| 3 | 53.1 (11.3) | 46.8 to 59.3 | |
| 6 | 50.8 (10.2) | 45.1 to 56.4 |
Additionally, the single-arm design of the study precludes any conclusion that surveillance itself may be driving any reductions in depression and anxiety or improvements in QoL.
Stage at diagnosis
The stage at which malignancies are diagnosed can impact upon prognosis, treatment and end-point clinical outcomes (mortality and survival); detection at an earlier stage is expected to lead to better outcomes. Cancer stage at diagnosis can, therefore, be considered a proxy for prognostic data.
Data on cancer stage at diagnosis were extracted from both single-arm and comparative studies and are provided in Table 7. The study by Stuckless et al. (2013) included a surveillance group and two control groups who did not receive surveillance, but did not provide data on stage of cancers for the groups who did not have surveillance. 66 Woolderink et al. (2018) reported the stage at which OCs were detected for those undergoing surveillance and a no-surveillance control. 69–71 The OCs detected with surveillance appear to be generally lower stage than those detected without surveillance, but as this is based on one study with only 11 patients with OC in the surveillance group, this should be interpreted with caution. 69–71 Similar data were not available for EC.
For single-arm data, an evaluation of the stage of cancers detected at surveillance versus those detected in the interval between surveillance visits can illustrate whether surveillance is likely to miss earlier stage cancers. Three single-arm studies provided data on the stage of cancers detected during surveillance as well as the stage of interval cancers. 47,48,52,65 Across these three studies, data were very sparse and there was no obvious indication that there was a difference in cancer stage between surveillance detected and interval detected OC or EC (see Table 7). Additionally, although one of the comparative studies provided EC staging data for two different surveillance periods, the data were too sparse (low number of cases) to be meaningful. 42
Studies comparing surveillance with RRS (following surveillance) are more akin to single-arm studies; the cancers detected during RRS among those previously undergoing surveillance are similar to interval cancers. Dueñas et al. (2020),38,39 Eikenboom et al. (2021)40 and Tzortzatos et al. (2015)67 reported the stage of cancers detected with RRS and with surveillance. Again, data were sparse, but the cancers detected during surgery were generally as low or lower stage than those detected with surveillance. There are insufficient data to draw any conclusions from this. One further study provided cancer staging data for surveillance and a surgical comparator that included those undergoing surgical treatment (i.e. with cancer previously detected). 60,61 This study lacks meaning in this context because surgery group participants would not have been eligible for surveillance.
Harms associated with gynaecological surveillance procedures and strategies
Rates and severity of adverse events
Seven of the included studies directly provided either (1) rates of adverse events or (2) continuous data using rating scales, with regards to gynaecological surveillance for individuals with LS. 41,45,46,52,54–58,72,74,75 All seven studies were either single-arm cohort designs41,45,46,52,54–58,72 or provided survey data for either a single sample75 or for two subsamples. 74 With the exception of Manchanda et al. (2012),52 all these studies provided data on pain; two on the need for pain relief,45,46,75 one on infections,75 one on vasovagal reactions,41 and one on perforations. 52
An additional study provided data relevant to pain but did not report the proportion of participants experiencing pain, or continuous data using a pain rating scale 43,44 Instead, this study reported some limited data on anxiety about pain. 43,44 Four of the studies that did provide direct data on pain also provided limited data on concerns and worries related to surveillance and the impact of these concerns on surveillance discontinuation or on opting for prophylactic surgery. 41,45,46,72,74
None of the included studies reported data on discomfort or discomfort severity (rather than pain) or on serious infections. Wood et al. (2008) reported data on QoL and on anxiety and depression, but these data were provided as clinical effectiveness outcomes, rather than as data pertaining to harms due to surveillance procedures and are therefore presented in the section Quality of life and mental health outcomes. 68 No other adverse events were reported in the included studies, but this does not mean that other harms did not occur.
Pain
Of the six studies providing data on pain associated with surveillance procedures, four reported continuous data using either a VAS45,46,54–58,72 or verbal rating scale (VRS)41 and three provided the proportion of participants reporting pain or severe pain (Table 14). 72,74,75 It should be noted that differences between studies in pain reported may be due to a variety of study-level, participant-level and site-specific variables that may not have been measured, including but not limited to differences in study design, participant recall, rating scales used, surveillance procedures, personnel undertaking the surveillance and use of pain relief.
| Study | Type of data | Surveillance technique | Patients | |
|---|---|---|---|---|
| N, mean (SD) and/or median (range) | n/N (%) | |||
| Pain | ||||
| Elmasry et al. 200941 | VRS (range 1–5) measured at visit | TVUS | N = 24, mean 1.1 | n/a |
| Hysterosonogram | N = 4, mean 1.7 | n/a | ||
| Hysteroscopy | N = 21, mean 3.9 | n/a | ||
| Endometrial sampling (Pipelle) | N = 23, mean 3.3 | n/a | ||
| Helder-Woolderink et al. 201745,46 | VAS (range 0–10) measured at 5 visits | Endometrial sampling (Pipelle) | 1st visit, N = 52, median 5.0 (0–10) | n/a |
| 2nd visit, N = 24, median 5.0 (0–10) | n/a | |||
| 3rd visit, N = 13, median 6.0 (1–9) | n/a | |||
| 4th visit, N = 5, median 7.0 (4–7) | n/a | |||
| 5th visit, N = 3, median 7.0 (1–9) | n/a | |||
| Nebgen et al. 201454–58 a | VAS (range 1–10) measured at visit | Endometrial sampling (Pipelle) without sedationb | N = 19, mean 5.8, median 6.0 (1–10) | n/a |
| Endometrial sampling (Pipelle) + colonoscopy with sedationb | N = 19, mean 1.8, median 1.0, (1–7) | n/a | ||
| Woolderink et al. 202072 | VAS (range 0–10)b; proportion experiencing severe pain (VAS ≥ 7) measured at visit | Endometrial sampling (Pipelle) | N = 25, median 5.5 (1–10)c | NR |
| Endometrial sampling (tampon) | N = 25, median 0.0 (0–10)c | 3/25 (12)d | ||
| Kalamo et al. 202074 | Proportion experiencing ‘strong pain’ from survey data | Endometrial sampling (non-operated individuals)e | n/a | 8/24 (33.33) |
| Endometrial sampling (prophylactic hysterectomy)e | n/a | 7/42 (16.67) | ||
| Ryan et al. 202175 | Proportion reporting pain from survey data | Variesf | n/a | 6/59 (10.17) |
| Pain relief required/requested | ||||
| Helder-Woolderink et al. 201745,46 | Proportion using NSAIDsg recorded at 5 visits | Pipelle endometrial biopsy | n/a | 1st visit, 11/52 (21.15) |
| 2nd visit, 9/24 (37.5) | ||||
| 3rd visit, 5/13 (38.46) | ||||
| 4th visit, 4/5 (80) | ||||
| 5th visit, 2/3 (66.67) | ||||
| Ryan et al. 202175 | Proportion requiring GA based on survey data | Variesf | n/a | 8/59 (13.56) |
All four studies reporting continuous pain ratings did so for endometrial sampling using a Pipelle device and reported similar ratings [mean = 3.3 on a 5-point VRS in Elmasry et al. (2009);41 mean = 5.8, median = 6.0 on an 11-point VAS in Nebgen et al. (2014);54–58 median 5.5 on a 10-point VAS in Woolderink et al. (2020);72 and median ranging from 5 at the first visit to 7 at the fifth visit on a 10-point VAS in Helder-Woolderink et al. (2020)72]. It is not possible to conclude from the Helder-Woolderink et al. (2020) data that endometrial sampling using Pipelle gets more painful the more it is performed (scores appear to increase across visits). 72 This is because sample sizes become very small at later visits (see Table 14). Overall, these data indicate that, on average, individuals experience endometrial sampling using Pipelle as moderately painful, although a wide range of pain experience is evident with some individuals experiencing no pain and others experiencing severe pain (see Table 14).
The data provided by Elmasry et al. (2009) on a 5-point VRS indicate that both endometrial sampling (mean = 3.3, SD not reported) and hysteroscopy (mean = 3.7, SD not reported) appear to be more painful than TVUS (mean = 1.1, SD not reported) and hysterosonogram (mean = 1.7, SD not reported). 41 Analyses of these data indicated that TVUS was significantly less painful than Pipelle biopsy or hysteroscopy (p < 0.01), which did not statistically significantly differ from each other. 41 Woolderink et al. (2020) reported lower pain scores on an 11-point VAS for endometrial sampling using tampons than with a Pipelle device (median = 0.0 vs. median = 5.5, p < 0.001). 72 Not all participants found tampon insertion to be pain free, with three of the 25 participants experiencing severe pain (VAS ≤ 7). It was not clear how many participants experienced severe pain during sampling with Pipelle. 72
The other two studies that provided data on the proportion of participants experiencing pain or strong pain were both surveys. 74,75 Survey data are based on recall of events and may therefore be less accurate than data obtained at the time of surveillance. Comparative data from Kalamo et al. (2020)74 suggested that participants who did not go on to have prophylactic surgery were more likely to report strong pain with endometrial biopsy (33.3%) than those who did go on to have surgery (16.7%). However, it is not clear whether the total number or the recency of biopsy procedures differed between these two subgroups, and this may impact upon pain perception. The data from Ryan et al. (2021)75 suggest that 10.17% of participants experience pain with surveillance, but these data were based on varied surveillance techniques (internal examination, TVUS, pelvic ultrasound, biopsy, CA-125 or hysteroscopy). It was, therefore, not possible to determine which specific procedures the participants had found painful.
Following recommendations from the PPI workshop for this research (see Patient and public involvement), this systematic review primarily sought data on the need for GA during gynaecological surveillance.
Two studies provided data on the use of pain relief,45,46,75 but only one directly provided data on GA use (13.6% of individuals who were currently undergoing surveillance reported receiving hysteroscopy with GA, equating to 24.2% of individuals who had received a hysteroscopy). 75 However, the reasons for GA use were not provided, so it was not clear whether these were patient requests due to previous experience of pain, what the criteria would be to fulfil requests for GA or what proportion of requests were fulfilled. 75 As such, the extent of patient need for GA due to pain during hysteroscopy remains unclear.
Helder-Woolderink et al. (2017) did not specifically collect data on the use of GA but the use of any pain relief before endometrial sampling with Pipelle was collected. 45,46 There were no reports of GA being used: the data instead indicated that non-steroidal anti-inflammatory drugs (NSAIDs) were fairly frequently used before endometrial sampling, ranging from 21.2% of individuals before the first visit to 80.0% before the fourth visit. These data should be interpreted with caution because the number of individuals who had received four or five surveillance visits was very low (see Table 14). However, Helder-Woolderink et al. (2017) also reported that a subgroup of participants who started surveillance before the study period were more likely to use NSAIDs prior to their first study biopsy than those who started surveillance during the study period (see Table 15 and Risk factors impacting adverse events, Other). 45,46 This supports the idea that prior experience of endometrial sampling might increase NSAID use at future surveillance visits.
| Study | Surveillance technique | N | Results |
|---|---|---|---|
| Menopause (pre vs. post) | |||
| Elmasry et al. 200941 | Uncleara | Pre n = 19; post n = 6 | No significant difference in reported pain scores between pre- and postmenopausal women |
| Helder-Woolderink et al. 201745,46 | Endometrial sampling (Pipelle) | Pre n = 40; post n = 12 | VAS: median 4.0 (range 0–10) vs. median 6.5 (range 3–10), p = 0.78 |
| NSAID before 1st visit: 8/40 (20%) vs. 3/12 (25%), p = 0.14 | |||
| Preventative surgery: 9/40 (23%) vs. 0/12 (0%), p = 0.07 | |||
| Woolderink et al. 202072 | Endometrial sampling (Pipelle) | Pre n = 14; post n = 9 | VAS: median 5.0 (range 3–9) vs. median 7.0 (range 1–10) |
| Endometrial sampling (tampon) | Pre n = 15; post n = 10 | VAS: median 0.0 (range 0–2) vs. median 4.5 (range 0–10) | |
| Parity (nulliparous vs. parous) | |||
| Helder-Woolderink et al. 201745,46 | Endometrial sampling (Pipelle) | Nulliparous n = 11; parous n = 36; unknown n = 5 | VAS: median 6.0 (range 2–9) vs. median 4.0 (range 1–10) vs. median 8.0 (range 3–10), p = 0.39 |
| NSAID before 1st visit: 4/11 (36%) vs. 5/36 (14%) vs. 2/5 (40%), p = 0.16 | |||
| Preventative surgery: 1/11 (9%) vs. 8/36 (22%) vs. 0/5 (0%), p = 0.34 | |||
| Nebgen et al. 2014b54–58 | Endometrial sampling (Pipelle) without sedationc | Nulliparous n = 6; parous n = 13 | VAS: mean 7.3, median 8.0 (range 4–10) vs. mean 5.2, median 5.0 (range 1–9) |
| Endometrial sampling (Pipelle) + colonoscopy with sedationc | Nulliparous n = 6; parous n = 13 | VAS: mean 2.7, median 1.0, (range 1–7) vs. mean 1.4, median 1.0 (range 1–2) | |
| Labour delivery (vaginal vs. caesarean section) | |||
| Nebgen et al. 2014b54–58 | Endometrial sampling (Pipelle) without sedationc | Vaginal n = 10; caesarean section n = 3 | VAS: median 4.0 vs. median 7.0 |
| Endometrial sampling (Pipelle) + colonoscopy with sedationc | Vaginal n = 10; caesarean section n = 3 | VAS: median 1.0 vs. median 2.0 | |
| Screening start date (pre vs. post 2011) | |||
| Helder-Woolderink et al. 201745,46 | Endometrial sampling (Pipelle) | Pre n = 28; post n = 24 | VAS: median 5.0 (range 0–10) vs. median 5.0 (range 2–10); p = 0.97 |
| NSAID before 1st visit: 10/28 (36%) vs. 1/24 (4%); p = 0.04 | |||
| Preventative surgery: 4/28 (14%) vs. 5/24 (21%); p = 0.62 | |||
An additional included study did not report rates of pain relief use, but found that participants retrospectively reported lower pain scores for a combined endometrial biopsy and colonoscopy procedure that included sedation than for endometrial sampling alone without sedation (mean 1.8, median 1.0 vs. mean 5.8, median 6.0, using a 10-point VAS). 54–58 There were no data reported in this study for endometrial biopsy with sedation but without colonoscopy.
One case of infection due to surveillance was identified in a cross-sectional survey of 59 participants who were undergoing gynaecological surveillance. 75 However, it was not clear which procedure resulted in the infection and no further details were reported. 75 Other studies did not provide data on infections, but this does not preclude their occurrence.
Elmasry et al. (2009)41 reported that 2 of the 21 participants undergoing hysteroscopy experienced a vasovagal reaction. No further details were given, other than that the procedures were still successful.
Manchanda et al. (2012)52 reported that among 41 participants (69 outpatient hysteroscopy with endometrial sampling procedures), there were no uterine perforations.
Several studies provided data on participants’ concerns and worries, including concerns about pain,41,43–46,72,74 or concerns about infection and fertility,41 and the impact of these concerns or worries on decision-making (whether to continue with surveillance or to undergo prophylactic surgery). These data are all related to either endometrial sampling using a Pipelle device or to hysteroscopy.
As previously mentioned (see Individuals’ experience of pain), Kalamo et al. (2020)74 provided survey data indicating that individuals who were previously on a surveillance programme but underwent prophylactic surgery reported lower levels of endometrial biopsy pain than those who did not opt for surgery. The authors concluded that biopsy pain did not predict the decision to opt for surgery. 74 However, as previously stated, it is not clear if the number or recency of biopsy procedures differed between these two subgroups, and these factors may impact upon pain perception.
The conclusion in Kalamo et al. (2020)74 is not supported by data presented in Helder-Woolderink et al. (2017),45,46 where seven of the nine participants who had preventative surgery cited concerns about surveillance pain (as well as cancer fear) as a reason that they opted for surgery. Although implied, it was not entirely clear that these concerns were about biopsy pain rather than pain due to other procedures (pain from other testing was not reported or discussed). In an earlier study, Helder-Woolderink et al. (2013),43,44 reported that 8 of 75 participants decided to have prophylactic surgery because of cancer worry or anxiety about painful endometrial biopsy. However, it is not clear how many of these eight participants cited worry about the endometrial sampling (rather than cancer worry) as the reason for undergoing surgery.
Helder-Woolderink et al. (2017)45,46 also reported that 4 of 52 participants declined endometrial sampling due to a fear of pain and that 1 participant stopped participating in surveillance after 2 painful biopsies. Similarly, Woolderink et al. (2020)72 reported one individual who refused biopsy because of fear of pain (the total number undergoing surveillance is unclear), and Elmasry et al. (2009)41 reported that 1 of 25 participants ‘did not tolerate’ biopsy, which had to be stopped before any suction was applied.
Overall, these data indicate that concerns about pain (or experience of pain) related to endometrial biopsy using Pipelle might influence individuals’ decisions to continue with surveillance or to undergo prophylactic surgery. However, these data are not plentiful or robust and are sometimes contradictory.
With regard to hysteroscopy, Elmasry et al. (2009)41 reported that 3 individuals declined to take part in the study due to concerns about the procedure impacting fertility and 2 of 24 participants declined hysteroscopy because of concerns about infection and the possibility that this may impact upon future fertility. For another two participants hysteroscopy was abandoned due to pain and anxiety (one of these participants also did not complete a Pipelle biopsy). These limited data indicate that concerns about pain, infection and future fertility may lead to individuals declining or stopping hysteroscopy. Indeed, the authors reported that almost all participants stated that, due to procedural pain with hysteroscopy, TVUS would be preferential if all techniques were equally effective.
Risk factors impacting adverse events
Four of the included studies investigated whether particular subgroups were more likely to experience adverse events or to experience them more severely. 41,45,46,54–58,72
Menopause status
Three of the studies investigated the impact of menopause status on pain severity,41,45,46,72 with Helder-Woolderink et al. (2017)45 also reporting data on NSAID use and on opting to have prophylactic surgery. These data should be cautiously interpreted due to the small subgroup sizes in these studies (see Table 15).
Elmasry et al. (2009)41 reported that pain scores (5-point VRS) did not differ between participants who were pre- or postmenopausal but did not clarify which surveillance technique this referred to (TVUS, hysteroscopy plus saline hysterosonography and endometrial biopsy were all included in the surveillance) or provide any supporting data. However, both Helder-Woolderink et al. (2017)45 and Woolderink et al. (2020)72 reported higher VAS pain ratings related to endometrial sampling with Pipelle (using an 11-point VAS and a 10-point VAS, respectively) among postmenopausal participants than premenopausal participants [median 7.0, range 1–10 vs. median 5.0, range 3–9 in Woolderink et al. (2020),72 and median 6.5, range 3–10 vs. median 4.0, range 0–10 in Helder-Woolderink et al. (2017)]. 45,46 However, Helder-Woolderink et al. (2017)45 reported that this difference was not statistically significant. 45,46 Woolderink et al. (2020)72 also found that postmenopausal participants reported higher pain scores than premenopausal participants when samples were collected using tampons (median 4.5, range 0–10 vs. median 0.0, range 0–2).
Helder-Woolderink et al. (2017)45 additionally reported that there was no statistically significant difference between pre- and postmenopausal participants in NSAID use prior to the first biopsy with Pipelle (20% vs. 25%, p = 0.14). 45,46 However, individuals who were premenopausal were more likely to opt out of surveillance and have preventative surgery (despite reporting less pain with surveillance biopsy) than those who were postmenopausal (23% vs. 0%, p = 0.07). The reasons for this are unclear and may not be related to issues with the surveillance itself.
Parity and method of prior deliveries
Two studies investigated whether 10-point VAS pain ratings for endometrial sampling with Pipelle differed according to parity (see Table 15). 45,46,54–58 Nebgen et al. (2014) also provided these data for pain ratings related to combined endometrial sampling and colonoscopy (under sedation). 54–58 Both studies reported higher pain ratings with Pipelle for nulliparous participants compared to parous participants [mean 7.3, median 8.0, range 4–10 vs. mean 5.2, median 5.0, range 1–9 in Nebgen et al. (2014);54–58 median 6.0, range 2–9 vs. median 4.0, range 1–10 in Helder-Woolderink et al. (2017)]. 45,46 Helder-Woolderink et al. (2017) reported that this difference was not statistically significant. 45,46 For the combined endometrial sampling and colonoscopy procedure (under sedation) reported in Nebgen et al. (2014), higher pain scores were again reported for nulliparous individuals than for parous individuals (mean 2.7, median 1.0, range 1–7 vs. mean 1.4, median 1.0, range 1–2). 54–58 These data should be cautiously interpreted due to the small subgroup sample sizes (see Table 15).
Helder-Woolderink et al. (2017) found higher rates of NSAID use before the first biopsy among nulliparous individuals than in parous individuals, and higher rates still in those whose parity was unknown, but this difference was not statistically significant [4/11 (36%), 5/36 (14%) and 2/5 (40%), respectively, p = 0.16]. 45,46 Similarly, nulliparous participants were less likely to opt for preventative surgery than parous participants (and none of the participants with unknown parity opted for surgery) but this difference was also not statistically significant [1/11 (9%), 8/36 (22%) and 0/5 (0%), respectively, p = 0.34]. Again, it is important to note the small subgroup sizes in this study.
Among parous individuals, Nebgen et al. (2014) reported higher VAS pain ratings for those who had a caesarean section than for those who had a vaginal delivery, for both Pipelle sampling without sedation (median 7.0 vs. median 4.0) and for the combined biopsy and colonoscopy procedure (median 2.0 vs. median 1.0). 54–58 Variability in these ratings was not reported.
Other
Helder-Woolderink et al. (2017) reported that individuals who started surveillance prior to 2011 (when the study started) and those who started surveillance after 2011 reported the same median pain ratings related to endometrial sampling. 45,46 These subgroups also did not differ in the likelihood of opting for preventative surgery (see Table 15). However, as mentioned in Pain relief during surveillance procedures, participants who started surveillance during the earlier period were more likely to use NSAIDs prior to their first study biopsy than those who started surveillance during the study period [10/28 (36%) vs. 1/24 (4%), p = 0.04]. 45,46 This might indicate that prior experience of biopsy increases NSAID use at future biopsies, and fits with the other data from the study that indicated that NSAID use increased from the first to fourth (although those data should be interpreted with caution because only a small number of participants had received later visits; see Table 14).
Chapter 4 Systematic review of cost-effectiveness evidence
This review aimed to identify and assess studies evaluating the cost-effectiveness of gynaecological surveillance for women with LS.
Methods
Search strategy
An electronic search strategy consistent with the systematic review of clinical effectiveness was designed by an information specialist (SB) in consultation with the review team. Searches were limited to English language and human populations when possible. Filters for economic evaluations were not used to increase the sensitivity of the search. No publication date was imposed. The search strategies for each database are detailed in Systematic review of cost-effectiveness, Appendix 1.
Study selection
Titles and abstracts obtained from electronic searches were independently screened by two reviewers (TS and NM). Inclusion was based on criteria described in Table 16. Any disagreement was resolved by discussion between the two reviewers.
| PICOS criteria | Inclusion criteria |
|---|---|
| Population | Women with known or suspected LS at risk of gynaecological cancer |
| Intervention | Any strategy to reduce the risk of gynaecological cancer in LS population |
| Comparator | Alternative risk reduction strategy or no risk reduction |
| Outcomes | Costs from healthcare payer or societal perspective. Health outcomes measured in natural units, utility-based units or monetary terms (e.g. number of cancers, number of deaths, life-years, QALYs) |
| Study type | Full economic evaluations based on decision analytic modelling, trials or observational data. (Systematic reviews of economic evaluations to be included at title/abstract screening and used for citation chasing) |
| Other | English language, full text available |
Forward and backward citation chasing was conducted from relevant systematic reviews of economic evaluations and primary studies identified as included at full text. Titles and abstracts were retrieved and independently screened by the same two reviewers (TS and NM).
Full texts of potentially relevant studies were retrieved and further assessed for inclusion by the same reviewers (TS and NM) with disagreement again resolved by discussion.
Data extraction and quality assessment
Bespoke data extraction forms were designed and piloted prior to use. Quality appraisal was conducted on all included studies using the Philips quality assessment checklist78 supplemented by an additional set of review-specific criteria deemed important a priori for this review. Quality appraisal and data extraction were conducted by one reviewer (NM) and checked by a second (TS).
Synthesis of evidence
Narrative synthesis was performed, supported by cross-tabulation of study characteristics and findings. Costs were not converted into a common currency or inflated to a common price year.
Results
Study identification
The literature was searched for evidence on the effectiveness and cost-effectiveness of gynaecological surveillance for women with LS. Figure 2 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for the screening of these search results.
FIGURE 2.
Study flow diagram for systematic review of cost-effectiveness. Notes: Adapted from Moher et al. (2009);82 a, Forward and backward citation chasing was conducted from included studies and from systematic reviews.
The search strategy yielded 1678 records after deduplication. Following title and abstract screening, nine studies were retrieved at full text and assessed for eligibility. Of these, two were deemed eligible for inclusion in this review. 79,80 A final study, unpublished at the time of database searching, was included following its identification by clinical experts. 81
Study characteristics
All studies constructed a decision analytic model comparing multiple gynaecological cancer management strategies. Studies were set in the USA and considered a population of women diagnosed with LS across a time horizon of at least 40 years. Economic evaluations took the form of cost–utility analyses. Health benefits were measured in QALYs in all studies, and costs measured using the US dollar from a societal perspective in Kwon et al. (2008)79 and Yang et al. (2011),80 and from the healthcare system perspective in Wright et al. (2021). 81 Benefits and costs were each discounted at a rate of 3%. Wright included survival, cancer incidence and cancer mortality as additional measures of health benefit. A cost-effectiveness threshold of $50,000 per QALY was used in Kwon et al. (2008) and Yang et al. (2011), while Wright et al. (2021) considered a $100,000 willingness-to-pay threshold. All studies included the stage of cancer.
Kwon et al. (2008)
Kwon et al. 79 modelled effectiveness and cost-effectiveness through Markov microsimulation. Five treatment strategies evaluating combinations of screening and risk-reducing or prophylactic surgery were included. These strategies were no prevention; prophylactic surgery at 30 years; prophylactic surgery at 40 years; combined annual screening from 30 years and prophylactic surgery at 40 years; and annual screening with endometrial biopsy, CA-125 and TVUS from 30 years. A time horizon of 40 years, from 30 to 70 years of age was assumed. Base case data included lifetime risk, incidental diagnosis during surgery, age at diagnosis, probability of symptoms, sensitivity and specificity of screening, cancer stage distribution, lifetime risk of peritoneal carcinoma and perioperative mortality. Cost estimates were based on existing literature and national registers and statistics from official governing or healthcare bodies. Benefits and QALY weights were derived from existing literature. Evidence on effectiveness for each intervention is presented as an incremental cost-effectiveness ratio (ICER).
Yang et al. (2011)
Yang et al. 80 built a decision analytic model in TreeAge (TreeAge Software LLC, Williamstown, MA, USA) to evaluate the effectiveness and cost-effectiveness of gynaecological cancer management. The model included age-specific cancer risks in HNPCC, diagnosis, surgery and survival by cancer stage, surgical mortality, and efficacy, sensitivity and specificity of screening. The decision tree contained three arms, one for each of the following management strategies: annual examination, annual surveillance (including endometrial biopsy, CA-125 and TVUS as in Kwon et al. 79); and prophylactic surgery at 30 years. Health benefits and utility estimates were identified from the existing literature and the Surveillance, Epidemiology and End Results database and applied against discounted life expectancy to generate QALYs. Costs were obtained from University of California San Francisco Patient Financial Services and departmental billing offices, alongside existing literature. Where screening costs were unavailable they were estimated based on a cost-to-charge ratio of 0.48 per hospital charge. Cost-effectiveness was evidenced by the average cost-effectiveness, or cost per QALY, for each strategy, but we have calculated ICERs as only these are appropriate for decision-making. 83 The model was an extension of a prior publication by Chen et al. (2007). 84
Wright et al. (2021)
Wright et al. 81 analysed the cost-effectiveness of genotype-specific surveillance and prevention of gynaecologic cancers through a Markov state transition cohort-level model. Women began in the healthy state at 25 years of age, progressing through health states following an annual cycle until 75 years, intervention or death. Alternate strategies were examined by varying type and timing of risk-reducing surgery and age of surveillance initiation. Overall 12 different strategies were evaluated: hysterectomy and BSO at 35 years, surveillance from 30 years; hysterectomy and BSO at 4 years, surveillance from 30 years; hysterectomy and BSO at 50 years, surveillance from 30 years; surveillance alone from 30 years; hysterectomy and BSO at 40 years, surveillance from 35 years; hysterectomy and BSO at 50 years, surveillance from 35 years; surveillance alone from 35 years; hysterectomy and BSO alone at 35 years; hysterectomy and BSO alone at 40 years; hysterectomy and BSO alone at 50 years; no intervention; two-stage approach – hysterectomy and bilateral salpingectomy at 40 years, oophorectomy at 50 years. Alongside costs and utilities, the model included base parameters for mortality, surveillance sensitivity and specificity, surgical complication, gynaecological cancer risk by gene, and cancer stage distribution for no intervention, surveillance, surgery and survival. Health benefits and utilities were obtained from existing literature and included a half-cycle correction. Costs were derived from Medicare data and published analyses. They were adjusted for inflation using the Medical Consumer Price Index. The effectiveness for each intervention was assessed by calculating an ICER for each gene mutation.
Study findings
All studies found risk-reducing surgery to be less costly, and provide more QALYs, than screening or surveillance alternatives. A summary of study findings is provided in Table 17; detailed findings are presented in subsections below.
| Strategy | Cost ($) | Life-yearsa | QALYs | ICER ($)b |
|---|---|---|---|---|
| Kwon et al . (2008) 79 | ||||
| No prevention | 13,620 | 19.5910 | 18.4582 | – |
| PS at 30 years | 18,523 | 21.5873 | 18.8115 | Extendedly dominated |
| PS at 40 years | 19,184 | 20.6444 | 18.9430 | 11,477 |
| Combined strategy | 25,726 | 20.7087 | 18.9766 | 194,650 |
| Annual screen from 30 years | 30,912 | 20.0405 | 18.6627 | Dominated |
| Yang et al . (2011) 80 | ||||
| Annual examination | 100,484 | – | 24.60 | Dominated |
| Annual surveillance | 68,392 | – | 25.17 | Dominated |
| PS at 30 years | 23,224 | – | 25.71 | – |
| Wright et al. (2021)81,c | ||||
| MLH1: HBSO at 35 years | 8642 | 47.01 | 22.09 | – |
| MLH1: HBSO at 40 years | 9261 | 46.68 | 22.27 | 3520 |
| MLH1: two-stage approach | 15,935 | 45.97 | 22.47 | 33,269 |
| MSH2: HBSO at 35 years | 8879 | 46.96 | 22.07 | – |
| MSH2: HBSO at 40 years | 9692 | 46.58 | 22.23 | 5180 |
| MSH6: HBSO at 35 years | 8950 | 46.95 | 22.06 | – |
| MSH6: HBSO at 40 years | 9823 | 46.55 | 22.22 | 5726 |
| MSH6: two-stage approach | 15,303 | 46.06 | 22.49 | 20,008 |
| PMS2: no intervention | 4677 | 46.76 | 22.82 | Dominated |
| PMS2: HBSO at 50 years | 4470 | 47.36 | 23.04 | – |
Kwon et al. (2008)
Kwon et al. (2008)79 found the reference strategy of no prevention to be the least costly, but it achieved the lowest QALYs. Annual screening (without risk-reducing surgery) was the most expensive strategy and was dominated by the three strategies which included risk-reducing surgery. Risk-reducing surgery alone was more expensive when conducted at 4 years than 30 years, but it also led to more QALYs and risk-reducing surgery at 30 years was extendedly dominated by no prevention and risk-reducing surgery at 40 years. The combined strategy of surveillance from 30 years until risk-reducing surgery at 40 years provided the most QALYs, but this was at a very high cost, meaning that it was not cost-effective.
Threshold analysis revealed that when utility from risk-reducing surgery rises from its base case value of 0.86–0.88 or higher, the QALYs of risk-reducing surgery at years exceed those of risk-reducing surgery at 40 years.
Overall Kwon et al. found risk-reducing surgery alone to be the cost-effective strategy (cost-effectiveness threshold of $50,000 per QALY gained). 79 Varying the age at which surveillance was initiated or risk-reducing surgery was performed could not make the combined strategy approach cost-effectiveness.
Although an analysis was not conducted by the authors in which risk-reducing surgery is not an option, we have calculated that the ICER for annual surveillance (starting at 30 years) compared with no prevention would be around $85,000 per QALY gained, which is not cost-effective.
Yang et al. (2011)
Yang et al. (2011)80 used average cost-effectiveness ratios rather than ICERs, so many of their results are not useful for decision-makers. They also did not include a strategy of no prevention. Nevertheless, they found that risk-reducing surgery dominated annual gynaecological examination and annual gynaecological surveillance.
Wright et al. (2021)
Wright et al. (2021)81 stratified their economic evaluation according to LS genotype. For path_MLH1 and path_MSH6, they identified the economically optimal approach was two-stage RRS, that is hysterectomy and bilateral salpingectomy at 40 years and bilateral oophorectomy at 50 years. For path_MSH2, the economically optimal approach was risk-reducing hysterectomy and BSO (HBSO) at 40 years, while for path_PMS2 the optimal approach was risk-reducing HBSO at 50 years.
For patients of all genotypes, risk-reducing surgery was dominant over no intervention and over strategies including surveillance.
Numerous one-way sensitivity analyses were conducted and reported:
-
When the utility value associated with surgical menopause was varied from its base case value of 0.90 in the range 0.86–0.95, RRS remained the economically optimal approach for all genotypes, although the precise surgery which was optimal (HBSO at 35, 40 or 50 years, or two-stage surgery) did change in a predictable pattern.
-
When the utility value associated with hysterectomy and with postmenopausal HBSO was varied from its base case value of 1 in the range 0.97–1, the economically optimal strategies were unchanged. When it was varied in the range 0.95–0.96, however, HBSO at 40 years became the optimal strategy for path_MLH1 and no intervention became the optimal strategy for path_PMS2.
-
If risk-reducing HBSO can reduce the risk of OC, this does not affect the economically optimal strategy, at least with a risk reduction of up to 25%.
-
If bilateral oophorectomy can increase the rate of general mortality, this does not affect the optimal strategy over a range of hazard ratios to 1.24. For a range of hazard ratios between 1.32 and 1.4 the optimal strategy for path_MSH2 changed to the two-stage approach.
-
In path_MSH6, if the lifetime risk of OC was in the range 23.2–33.9%, the economically optimal strategy became surveillance from 3 years until risk-reducing HBSO at 35 years.
Probabilistic sensitivity analysis was conducted and confirmed the robustness of findings to parameter uncertainty. The two-stage approach remained optimal in 84.2% of iterations for path_MLH1 and in 71.0% of iterations in path_MSH6. For path_MSH2 HBSO at 40 years was optimal in 86.2% of iterations, the two-stage in 12.2% and HBSO at 35 years in 1.6%. Finally for path_PMS2 HBSO at 50 years remained optimal in 91.6% of iterations.
The authors did not consider a population in which RRS is not an option, but they have reported costs and QALYs for all strategies, so the review authors have been able to investigate this question:
-
For path_MLH1 and path_MSH6, surveillance from 35 years would be cost-effective (ICER ~$9000/QALY vs. no intervention) and would dominate surveillance from 30 years.
-
For path_MSH2, surveillance from 30 years would be cost-effective (ICER ~$8000/QALY vs. surveillance from 35 years) and would dominate no intervention.
-
For path_PMS2, no intervention dominated the surveillance strategies.
Quality assessment
Reporting was mixed in all three studies. No study specified the primary decision-maker, described evidence on the model structure, justified parameter and data selection methods or addressed all four principal types of uncertainty (methodological, structural, heterogeneity, parameter). Kwon et al. (2008)79 did not address any of the four principal types of uncertainty. Yang et al. (2011)80 did not include options for no management or screening with delayed surgery. While a distinction was made in Wright et al. (2021),81 both Kwon et al. (2008)79 and Yang et al. (2011)80 treated LS as homogeneous, without distinction between MMR pathogenic variants. No justification was provided for this homogeneity.
Only Wright et al. (2021)81 included cancer risks separately for carriers of pathogenic variants in different MMR genes. Whether included separately by gene or as a general figure, no study included risks clearly free from ascertainment bias or free from confounding due to surveillance. Furthermore, in no study were cancer survival/mortality estimates suitably sourced as they included some non-LS population data. This was also the case for sensitivity and specificity as estimates were taken from predominantly non-LS populations. Estimates of sensitivity and specificity were stated and taken from the same technologies being modelled in the economic evaluation, but it was unclear how parameters from different papers were combined for use in each of our included studies. In each study, assumptions about the performance of combinations of tests were reasonable but not justified. No study stated occult disease states, thus assumptions regarding the preclinical development of disease could not be assessed. Assumptions regarding detection of disease were neither reasonable nor justified in Kwon et al. (2008)79 and Wright et al. (2021). 81 While these assumptions appeared reasonable in Yang et al. (2011),80 they were not clearly stated and again were not justified.
Chapter 5 Systematic review of utility values
This review aims to understand the impact of EC, OC, gynaecological surveillance and risk-reducing gynaecological surgery on preference-based HRQoL.
Methods
Search strategy
An electronic search strategy was designed by an information specialist (SB) with input from a health economist (TS) and clinical experts. Searches were limited to English language and human populations when possible. Search terms for LS were not included as it was anticipated that insufficient studies would exist focused on LS. No publication date was imposed. The search strategies for each database are detailed in Systematic review of utility values, Appendix 1.
The final strategy was implemented on 10 November 2020 in the following databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL; via the Cochrane Library)
-
EconLit (American Economic Association; via EBSCO)
-
EMBASE (via Ovid)
-
MEDLINE (via Ovid)
-
Web of Science Science Citation Index and Conference Proceedings Citation Index – Science (Clarivate Analytics).
This was supplemented on 24 August 2021 to include the following databases:
-
EuroQol-5 Dimensions (EQ-5D) publications (https://euroqol.org/search-for-eq-5d-publications)
-
International HTA Database (International Network of Agencies for Health Technology Assessment)
-
Cost-Effectiveness Analysis Registry (Center for the Evaluation of Value and Risk in Health, Tufts Medical Center, Boston, MA, USA).
Search results were exported to EndNote X9 and deduplicated.
Study selection
Titles and abstracts obtained from electronic searches were independently screened by two reviewers (TS and NM). Inclusion was based on criteria described in Table 18. Any disagreement was resolved by discussion between the two reviewers. Full texts of potentially relevant studies were retrieved and further assessed for inclusion by the same reviewers (TS and NM) with disagreement again resolved by discussion.
| PICOS criteria | Inclusion criteria |
|---|---|
| Population | Women with EC, OC, undergoing gynaecological surveillance or who have had gynaecological surgery as risk reduction for gynaecological cancer or due to benign gynaecological conditions |
| Outcomes | Studies using generic preference-based HRQoL tools and techniques (e.g. EQ-5D, Short Form 6 Dimensions, standard gamble, time trade-off). Studies only using non-preference-based measures (e.g. FACT-G, VAS, McGill Pain Questionnaire) will not be eligible for inclusion |
| Study type | Primary studies (literature reviews were used for backward citation chasing but were not included) |
| Other | English language, full text available |
Backward citation chasing was undertaken for secondary studies included at title and abstract screening to identify primary studies for inclusion at full text. Forward and backward citation chasing was conducted on all studies included at full text. Citations were independently screened, at title and abstract, and then at full text, by the same two reviewers (TS and NM) following the process outlined above.
Data abstraction and quality assessment
Bespoke data abstraction forms were designed. Quality assessment was performed on all included studies using an established set of quality assessment criteria for health state utility values. 85 Quality assessment and data abstraction were conducted by one reviewer (NM) and checked by a second (TS).
Synthesis of evidence
Narrative synthesis was conducted, supported by cross-tabulation of study characteristics and findings. Subgroup evaluation was performed for groups with: (1) EC; (2) OC; (3) undergoing gynaecological surveillance; and (4) those who had gynaecological surgery.
Results
Study characteristics
Included studies were sorted into subcategories for EC (n = 14), OC (n = 32), gynaecological surveillance (n = 4) and gynaecological surgery (n = 23). Where appropriate studies were included in multiple subcategories. Study characteristics are presented in Table 19 and discussed below.
| EC (n = 14) | OC (n = 32) | Surveillance (n = 4) | Surgery (n = 23) |
|
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Sample size | ||||
| ≤ 100 | 8 (57) | 9 (28) | 4 (100) | 7 (30) |
| 101–500 | 5 (36) | 10 (31) | 0 (0) | 13 (57) |
| 501–999 | 0 (0) | 7 (22) | 0 (0) | 2 (9) |
| ≥ 1000 | 1 (7) | 0 (0) | 0 (0) | 1 (4) |
| Not reported | 0 (0) | 6 (19) | 0 (0) | 0 (0) |
| Age (mean/median) | ||||
| 30–40 | 0 (0) | 1 (3) | 2 (50) | 3 (13) |
| 40–50 | 1 (7) | 0 (0) | 0 (0) | 13 (57) |
| 50–60 | 3 (21) | 5 (16) | 1 (25) | 2 (9) |
| 60–70 | 7 (50) | 3 (9) | 0 (0) | 2 (9) |
| Lifespan (multiple categories) | 2 (14) | 10 (31) | 1 (25) | 2 (9) |
| Not reported | 1 (7) | 13 (41) | 0 (0) | 1 (4) |
| Country | ||||
| Europe | 5 (36) | 3 (9) | 1 (25) | 13 (57) |
| North America | 4 (29) | 8 (25) | 3 (75) | 5 (22) |
| Asia | 3 (21) | 2 (6) | 0 (0) | 0 (0) |
| Australasia | 0 (0) | 1 (3) | 0 (0) | 0 (0) |
| Multiple continents | 2 (14) | 3 (9) | 0 (0) | 2 (9) |
| Not reported | 0 (0) | 15 (47) | 0 (0) | 3 (13) |
| Health condition | ||||
| LSa | 1 (7) | 1 (3) | 2 (50) | 2 (9) |
| BRCA1/2 b | 7 (22) | 1 (25) | 4 (17) | |
| Breast cancer | 1 (25) | 1 (4) | ||
| Menorrhagia or uterine bleeding | 4 (17) | |||
| Fibroids | 1 (4) | |||
| Uterine prolapse | 1 (4) | |||
| Benign gynaecological conditions | 9 (39) | |||
| Multiple conditions/unclear | 1 (4) | |||
| Affected organs | ||||
| Ovaries | 4 (17) | |||
| Uterus | 11 (48) | |||
| Uterus and cervix | 5 (22) | |||
| Uterus, ovaries, fallopian tubes | 1 (4) | |||
| Uterus, cervix, ovaries, fallopian tubes | 1 (4) | |||
| Not reported | 1 (4) | |||
| Disease stage | ||||
| Stage I–III (early-stage) | 4 (29) | 0 (0) | ||
| Stage IV (advanced) | 0 (0) | 9 (28) | ||
| Undefined or multiple stages | 3 (21) | 7 (22) | ||
| Recurrent | 0 (0) | 6 (19) | ||
| Not reported/n/a | 7 (50) | 10 (31) | ||
| Gynaecological surveillance strategy | ||||
| TVUS (alone) | 1 (25) | |||
| CA-125/blood test (alone) | 1 (25) | |||
| TVUS, CA-125, endometrial biopsy | 1 (25) | |||
| Not reported | 2 (50) | |||
| Gynaecological treatment | ||||
| Hysterectomy | 2 (14) | 0 (0) | 16 (70) | |
| HBSO | 2 (14) | 0 (0) | 2 (9) | |
| Oophorectomy | 0 (0) | 0 (0) | 4 (17) | |
| Chemotherapy/medication | 0 (0) | 20 (63) | 0 (0) | |
| Other | 1 (7) | 1 (3) | 0 (0) | |
| Not reported/n/a | 9 (64) | 11 (34) | 1 (4) | |
| Utility measurement instrument | ||||
| SG | 2 (14) | 2 (6) | 1 (25) | 2 (9) |
| Time trade-off | 1 (7) | 6 (19) | 3 (75) | 8 (35) |
| EQ-5D | 10 (71) | 16 (50) | 0 (0) | 12 (52) |
| SF-6D | 1 (7) | 2 (6) | 0 (0) | 0 (0) |
| Converted FACT | 0 (0) | 6 (19) | 0 (0) | 0 (0) |
| 15D | 0 (0) | 0 (0) | 0 (0) | 1 (4) |
| Source population | ||||
| Patients (own health) | 11 (79) | 23 (72) | 0 (0) | 17 (74) |
| Patients and public | 2 (14) | 7 (22) | 4 (100) | 5 (22) |
| Public (for patient preferences) | 1 (7) | 1 (3) | 0 (0) | 1 (4) |
| Patients and clinicians | 0 (0) | 1 (3) | 0 (0) | 0 (0) |
| Follow-up period (last time point) | ||||
| Baseline/< 1 month | 3 (21) | 4 (13) | 0 (0) | 4 (17) |
| 1 month | 0 (0) | 0 (0) | 0 (0) | 1 (4) |
| 3 months | 1 (7) | 0 (0) | 0 (0) | 0 (0) |
| 6 months | 1 (7) | 3 (9) | 0 (0) | 2 (9) |
| 1 year | 0 (0) | 0 (0) | 0 (0) | 5 (22) |
| > 1 year | 0 (0) | 3 (9) | 0 (0) | 3 (13) |
| Not applicable/unclear | 9 (64) | 22 (69) | 3 (100) | 8 (35) |
Endometrial cancer
Fourteen studies88,95–107 were identified providing utility values for EC. Most had a sample size below 500 (93%), with over half (57%) including fewer than 100 participants. Participants were predominantly aged between 50 and 70 years (71%) and came from Europe (36%) or North America (29%). Disease stage was largely unreported, with only four studies clear in their evaluation of early-stage EC. No study reported utility in the context of gynaecological surveillance. Five studies evaluated EC utility in the context of gynaecological surgery including hysterectomy (n = 2), HBSO (n = 2) and minimally invasive surgery (n = 1). Utility was most often measured by the EQ-5D (n = 10), followed by standard gamble (n = 2), time trade-off (n = 1) and Short Form 6 Dimensions (SF-6D; n = 1). These health state valuations were largely made by patients themselves in evaluating their own health (79%, n = 11), although alternative populations including patients and public (n = 2), and the public alone (n = 1) were also used. Follow-up period was often unclear in EC studies (64%, n = 9). Where stated, most considered only baseline cancer (21%), though one study extended follow-up to 3 months and the final study up to 6 months.
Ovarian cancer
We identified 32 studies88–94,99–101,105,107–127 reporting OC utilities. No study on OC reported a sample size greater than 1000, with most (59%) using samples of 500 or less. Age was not consistently reported, being not reported in 41% of studies. Again, 50–70 years was the most common (25%), although evaluating OC at multiple ages across the lifespan was also popular (31%). Study location was reported in only half of the included studies (53%), of which half (n = 8) considered participants from North America. Disease stage was often not reported, undefined or considered multiple stages (53%). No study evaluated early-stage OC, nine studies (28%) evaluated stage IV or advanced cancer and six studies (19%) considered recurrent OC. While not solely reporting surveillance or surgical intervention, other than cytoreductive surgery (n = 1), OC studies did consider treatment through chemotherapy and medication (63%). Utilities were most commonly measured through the EQ-5D (50%), followed by time trade-off (19%) and the converted FACT (19%) while the standard gamble and SF-6D were each used in two (6%) studies. Utilities were evaluated by patients (72%), both patients and the public (22%), the public alone (3%) or patients and clinicians (3%). Three studies reported aspects of data from the ICON7 trial. 110,120,123 Again, most studies did not clearly report a follow-up period (69%). Where follow-up was reported, the last time points were baseline (n = 4), 6 months (n = 3) and over 1 year (n = 3).
Gynaecological surveillance
Four studies28,88,115,128 reporting utilities for gynaecological surveillance were identified. Two studies115,128 considered screening or surveillance for OC, so the surveillance modality was aimed towards detecting OC. Havrilesky et al. (2009)115 were identifying utilities for OC screening (not in a high-risk population) and they explicitly defined the test as either a blood test or TVUS, while van Roosmalen et al. (2002)128 focused on ovarian surveillance for BRCA1/BRCA2 mutation carriers but did not specify the surveillance modality. Sun et al. (2019)28 and Kuppermann et al. (2013)88 sought to identify preferences for management of cancer risk in LS, but while Sun et al. (2019)28 were explicit about the form of surveillance, Kuppermann et al. (2013)88 gave no details of surveillance. Health states were described by vignettes in two studies,28,88,115 while in the remaining study128 it was unclear how health states were described. In all four studies participants were valuing hypothetical health states rather than their own health. Health state valuation was by time trade-off in three studies,88,115,128 while in the remaining study,28 the authors describe their approach as a modified standard gamble, in which the downside risk is developing cancer over an individual’s lifetime rather than dying immediately.
Gynaecological surgery
A total of 23 studies published in 24 papers were included that evaluated gynaecological surgery. 28,86–90,104,114,128–143
Two studies28,88 specifically investigated risk-reducing gynaecological surgery for LS, while another four studies89,90,114,128 investigated risk-reducing gynaecological surgery for hereditary breast OC (pathogenic variants in BRCA1/BRCA2). In these studies, participants gave values to hypothetical disease states using the time trade-off method, except for one study28 where a modified standard gamble was used.
The remaining studies86,87,104,129–143 considered various benign gynaecological conditions and obtained utility values directly from patients, generally using the EQ-5D, although three studies135,139,142 used time trade-off, one study104 used standard gamble and one study143 used the 15D measure.
Utility value estimates
Endometrial cancer
Utility values for EC ranged from 0.5698 to 1.00. 99,107 The lowest utility value of 0.56 was observed immediately 1-week post-laparotomy surgery at mean utility 0.56. 98 The greatest utility values related to EC were observed in primary disease at 0.999,99 and in patients participating in a UK breast cancer trial yielding utility 0.913. 104
In general, utilities valued by both the public and patients were lower than those classified only by patients. Utilities are not observed to consistently differ by geographic location in cross-study comparison; however, within-study assessment utilities as greater in Canada and the USA than the UK population. 105 Additionally, utilities are not seen to dramatically alter by medical facility with only minimal variation observed. 88 There is evidence of a relationship between EC utility and body mass index (BMI) with greater utility identified in individuals with BMI below 35 kg/m2. Furthermore, findings suggest utility associated to EC will decrease as stage and so severity of the cancer increases, whether comparing primary with advanced disease,99 or stage I with stage I–III EC. 102,103
More invasive surgery for EC (e.g. total abdominal hysterectomy or laparotomy) is seen to result in a more dramatic short-term reduction in utility than minimally invasive or laparoscopic surgery. 96,98 However, in the longer term at 3 or 6 months post surgery, this difference is no longer observable, given the dramatic improvement in utility for invasive surgery, with Bijen et al. (2011)96 even implying greater long-term results from invasive abdominal hysterectomy than the less invasive laparoscopic hysterectomy. Further details of EC study results are presented in Table 27, Appendix 5.
Ovarian cancer
Ovarian cancer utility estimates ranged from 0.16115 to 0.977,126 the lower bound being for end-stage OC and the upper in a cluster of patients undergoing chemotherapy. Again within-study comparison by geographical location suggests that utilities are valued higher in North America than in the UK. 105 Utilities based on conversion from FACT-O scores show slight variation dependent on transformation algorithm. The Dobrez algorithm consistently yields the greatest utility values, followed by the Cheung algorithm with the lowest value produced by linear, ordinary least squares and/or Tobit transformation. 91,118
As in EC, utility decreases as cancer stage and so severity increases,99,105,113,115 with the lowest utility across all studies observed at 0.16 in end-stage OC. 115 While these findings are not echoed in the median values identified by Hildebrandt et al. (2014),99 inspection of the range provides evidence of lower utilities in advanced disease. Furthermore, utility is lower following progressive disease than before its onset. 94,111 Treatment through chemotherapy and medication are shown to improve utility for individuals with OC. 108–110,118,120,123 However, this was not observed across all included studies. Treatment by chemotherapy medication docetaxel and carboplatin were unable to prevent a reduction in utility from randomisation to end of study in patients with platinum-sensitive recurrent OC. 116 Likewise, this lack of long-term reduction in utility from screening to post-progression was observed in platinum-sensitive recurrent patients with OC undergoing niraparib maintenance therapy or placebo both with and without BRCA mutations. 92 In comparison with patients receiving chemotherapy, patients under surveillance displayed lower utility regardless or adverse events, treatment efficacy and emotional well-being. 119
Women with BRCA1/2 display lower utility values at the older age of 33–50 years, with mean 0.58, than when aged 20–32 years at mean 0.84. 89 The three studies on BRCA1/2 by Grann et al. also reveal a difference in utility dependent on who the health state is valued by. 89,90,114 Patients and the public, mutation carriers and individuals with no known personal risk are consistent in their utility estimates; however, health professionals present a lower estimate than other individuals. Underestimation from physicians is not present across all OC studies. 119 Additionally, one study ascertaining utility values from patients with advanced OC and healthy female volunteers suggested inconsistency between the groups with patients presenting higher utilities than volunteers for all medication strategies regardless of response. 124 Further details of OC studies are presented in Table 28, Appendix 5.
Gynaecological surveillance
Gynaecological surveillance utilities ranged from 0.7028 to 1.00. 128 The highest levels of utility were found for the health state of screening for breast and OC in patients with BRCA1/2 valued via the time trade-off method. 128 The lowest utility (median 0.70) was obtained by Sun et al. (2019)28 for annual gynaecological surveillance with endometrial biopsy, TVUS and CA-125, although this study did not use a method that gives utility values suitable for QALY calculation.
Havrilesky et al. (2009)115 estimated median utility values of 0.97 for all health states relating to OC screening (blood test or TVUS with or without false positive result), but mean utilities varied more substantially. Unexpectedly, the mean utility was higher for TVUS with a false positive result than for TVUS without a false positive result, which may suggest a problem with using the time trade-off method to evaluate temporary health states.
Additional utility detail from studies on gynaecological surveillance can be found in Table 29, Appendix 5.
Gynaecological surgery
Utilities related to gynaecological surgery ranged from weighted health state 0.03 on day 0 of hysterectomy with GA,144 to utility of 0.99 in presymptomatic BRCA1/2 mutation carriers undergoing prophylactic oophorectomy alongside breast cancer screening. 128 When not confounded by anaesthesia, the lowest reported utility related to gynaecological surgery was observed in premenopausal women with LS undergoing prophylactic HBSO at median 0.40. 28 Utility in the context of prophylactic HBSO was greater in postmenopausal than premenopausal women. 28
For benign gynaecological conditions, while utility is seen to drop immediately following gynaecological surgery (hysterectomy, oophorectomy, endometrial ablation, laparotomy), in the longer term QoL recovers and utility reaches a level better than in the preoperative state. This initial drop is observed in the weeks following surgery, with an improvement in utility compared to preoperative state identified around 1–3 months after surgery. 131,140,144 Conversely, in patients with noncancerous pelvic populations one study identifies an immediate improvement in utility from immediately before to immediately after both hysterectomy and uterus-preserving surgery. 139 Clarification of the term ‘immediately’ is however not provided by the authors. Where data are not collected in the first weeks following surgery, no initial drop in utility is observed, rather only an improvement as a result of surgery. 127,132–138,141,143,145 Improved utility is most evident in the first 3 months post surgery with more of a plateau observed after around 3–6 months. 98,132,134,136,140,145 One study found that while remaining above preoperative levels, there is evidence of a slightly longer-term reduction in utility at around 15 months post randomisation,132 while another study identifies this reduction at 7 years. 139 This finding is, however, not consistent across all included studies, with sustained improvements in utility observed to extend beyond 1 year elsewhere. 134,136
Little difference in outcome utility is observed between inpatient and outpatient total laparoscopic hysterectomies,131 or when comparing vaginal and abdominal hysterectomy to their laparoscopic alternatives. 136 Failure or complication in vaginal hysterectomy is seen to reduce utility below that at baseline. 138 Meanwhile, in uterine preservation surgery, failure results in only a negligible reduction in utility, while a complication still results in an overall improvement in utility compared to that at baseline. 138 When asked to value, by time trade-off, the utility of total abdominal HBSO, greater utility was expected from undergoing rather than forgoing the surgical intervention. 88 Additionally the study found higher utility values from assessing the surgical decision from the sibling state (participants imagine having sibling with CRC and positive test for LS) than the proband state (participants imagine themselves having CRC and suspected LS).
In the BRCA1/2 population, as would be expected owing to the additional burden, combined oophorectomy and mastectomy yielded a lower utility value than oophorectomy alone,89,90 or oophorectomy accompanied by breast cancer screening. 128 This was consistent across patients of different age groups, with high risk, and when positive for breast cancer. This higher utility in oophorectomy alone was also reflected in valuations by ‘other individuals’ in the public, however health professionals did not make a distinction between oophorectomy alone and combined oophorectomy and mastectomy in their utility estimates for the BRCA1/2 population. In considering prophylactic oophorectomy alone, BRCA1/2 mutation carriers placed a slightly higher utility value on the procedure than a control group of women with personal or family history but no known personal risk of breast/OC. 114 This was also observed in a second study by the same authors, however while the public underestimated compared with BRCA1/2 carriers aged 20–32 years, they greatly overstated utility related to prophylactic oophorectomy compared to carriers aged 33–50 years. 89
Additional utility detail from studies on gynaecological surgery can be found in Table 30, Appendix 5.
Quality appraisal
In total, 61 studies were included in this synthesis across the 4 subgroups. Where reported (n = 54), utility was assessed by the full population in 23 studies while the remaining 31 ascertained utility from a subpopulation of participants.
Studies predominantly used data from RCTs (n = 28) or from individuals recruited from a hospital, clinic, university medical department, cancer registry, physician referral or special interest group (n = 19). Further recruitment methods included general public convenience sample (n = 5), data from pre-existing datasets (n = 3) or data obtained from the literature (n = 1). The remaining studies did not report a method for respondent selection (n = 5).
Inclusion and/or exclusion criteria were specified in 74% of studies (45/61). Studies were most commonly limited by age (n = 31), comorbidities or contraindications (n = 24) or stage, grade, severity or type of cancer (n = 21). Other common inclusion/exclusion criteria included sufficient computer, language and/or comprehension skills (n = 11), previous or current surgical experience (n = 10), provision of informed consent (n = 7), preoperative chemotherapy (n = 5) and state of childbearing or pregnancy (n = 5). Also of note, three studies restricted their population by including only individuals with a genetic mutation of BRCA1/2114 or LS. 28,88
Questionnaire return rate was reported in 28 studies (28/61; 46%) ranging from a maximum of 100%115 to 31%,106 with 116 included from 374 eligible participants. Response rates to the utility instrument used were reported in 26 studies (26/61; 43%). The most commonly reported return rate was 100%,86,87,115,128 which was often identified at baseline. However, as previously discussed, response to the utility measure was often not completed by the whole sample. Gordon et al. (2010)113 reported the lowest levels of response rate to the utility instrument used, being the SF-6D. 113 The authors reported that 61 women (72%) completed at least 6 surveys and 32 (38%) completed at least 12 surveys, whereas 5 (6%) completed 18 or more surveys and 3 women completed no surveys.
Loss to follow-up was stated in 22 studies (22/61; 36%). Only 1 study reported no loss to follow-up,115 and reasons for loss to follow-up were provided in only 4 of the 22 studies reporting on the matter. The existence of missing data was discussed in 25 studies, of which 3 studies stated only their method for handling missing data rather than reporting the levels of missing data for any variable. 98,112,127 Methods to address missing data included: multiple imputation,120,127,130,132,136,138 patient stratification by last completed patient-reported outcome;112 substitution with mean values;129,144 excluding patients with incomplete data,143 and one study made no statistical adjustments having observed no patterns for missing data. 94
Further problems were identified by study authors in 77% of studies (47/61). The most commonly discussed limitation was from selection bias which was identified by authors in 15 studies, arising from inclusion criteria (n = 6), lack of randomisation (n = 5), study design (n = 3) or the type of cancer reported (n = 1). Other limitations frequently identified by study authors included small sample size (n = 12), limited generalisability due to inclusion/exclusion criteria, dataset, location and/or period (n = 9), follow-up length (n = 7), poor data quality for quantifying costs (n = 6) or available for included parameters (n = 4), use of cross-sectional data (n = 4) and potential recall bias from self-report questionnaires (n = 3). Also of note, one study suggested some health gains may be missed where health outcomes were not measured until 6 weeks after discharge,145 a finding also identified in the gynaecological surgery subsection of this review. With particular relevance to LS, it is claimed possible that some study responses could be influenced by participating women’s knowledge of the procedures. The authors suggest it is possible participants may have been involved in previous LS studies and so be better informed or more experienced in LS risk management, for example regarding surveillance where participants may be aware that CA-125 and TVUS are not proven screening methods in detecting OC. 28
Further details of the results of quality appraisal are given in Table 31, Appendix 5.
Chapter 6 Development of a whole-disease model for Lynch syndrome
Introduction
Typically bespoke decision analytic models are developed for a particular health economic evaluation, although they may be based on or influenced by past models. Historically, most health economic models have not been made publicly available, but as research culture shifts towards open data and open access, there are increasing calls for health economic models to be made open access or open source. 146
Many open source software projects are supported and maintained by community volunteers and it is possible that communities may also form to maintain and improve health economic models, if they are sufficiently useful.
Whole-disease modelling
Whole-disease modelling is an approach to health economic modelling in which a single model is built to cover the entire care pathway for a particular disease, with the capability of evaluating combinations of interventions that do not all sit in the same place in the care pathway. 147
Research aims
Our aim was to create a whole-disease model for LS which could be used to conduct an economic evaluation of strategies to reduce the risk of gynaecological cancer in people with LS (see Chapter 7), as well as being suitable for economic evaluations of other interventions for people with LS. We also aimed for the model to be adaptable and extensible by other researchers as needed and for it to be open source.
In the remainder of this chapter, we describe the model software, then the model structure in detail, followed by a description of model calibration processes for the natural history components, then costs and utility values.
Software details
The model was built in Python (version 3.7; Python Software Foundation, Wilmington, DE, USA). The Python package Simpy148 (version 4.0.1) is used to coordinate the discrete event simulation. Other key Python packages used are:
-
injector149 (version 0.20.1)
-
NumPy150 (version 1.23.1)
-
SciPy151 (version 1.8.1)
-
Pandas (version 1.4.3)
-
pytest152 (version 7.1.1).
Calibration was generally performed with Stan153 (version 2.30; NumFOCUS, Austin, TX, USA) and R154 (version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria) and Stata (version 17.0; StataCorp LP, College Station, TX, USA) were used for many supporting analyses, but the model does not require Stan, R or Stata to run.
Model structure
The model is a discrete event simulation in which multiple individuals (the population) are simultaneously simulated. For one or more (combinations of) interventions, the same population is simulated with each competing option. This means that differences between the outcomes in the populations will not be affected by differences between the populations at baseline.
Simulating the population simultaneously theoretically allows for interaction between individuals (e.g. a family member undergoing predictive testing for LS following the diagnosis of LS in a cancer patient).
For each individual, we model their constitutional genetic status (i.e. were they born with a LS pathogenic variant, and if so which gene is affected). The model can include individuals who have LS but have no knowledge of it, enabling the modelling of care pathways around LS diagnosis.
For each individual, we model the organs most at risk of cancer due to LS [i.e. the bowel, the endometrium (lining of the womb) and the ovaries]. Within each of these organs, the model can simulate the incidence and development of neoplasia. These neoplasias can lead to symptoms and mortality.
The model incorporates various healthcare interventions, such as biennial colonoscopic surveillance, risk-reducing gynaecological surgery and gynaecological surveillance.
The mathematical approach adopted for competing events within the discrete event simulation is latent event times. In this approach, when an entity (e.g. an individual or a neoplasm) becomes at risk for an event, a random time to event is sampled from the relevant probability distribution. In the absence of any competing events the event then happens at time
where tnow is the current time in the simulation and Tevent is the sampled time to event. If there are multiple competing events then the earliest of these will be processed first, and this may prevent other events from happening. In many cases the model incorporates a state transition model to define the relevant risks.
As the model runs, certain events which affect healthcare resource use and/or HRQoL are recorded at the individual level. Once the simulations have completed, separate components calculate the economic quantities of interest (lifetime discounted costs and QALYs).
Certain aspects of the whole disease model (which are irrelevant for the question of gynaecological surveillance) remain incomplete at the time of writing but the software architecture is such that these aspects can be easily incorporated at a later date.
Constitutional genetic status
An individual is born with a constitutional genetic status in relation to LS. Generally, this is determined by inheritance, although some LS pathogenic variants arise de novo.
We consider four different pathogenic variants:
-
path_MLH1, path_MSH6 and path_PMS2 (monoallelic pathogenic variants of MLH1, MSH6 and PMS2 genes)
-
path_MSH2 (monoallelic pathogenic variant of MSH2 gene, including genomic deletions affecting MSH2 and the neighbouring gene EPCAM).
We have not included pathogenic variants of EPCAM gene, which can lead to mosaic inactivation of the MSH2 gene.
The risks of incidence and development of neoplasia within an individual is affected by their constitutional genetic status. It is not possible for the constitutional genetic status to change during an individual’s life.
We do not model constitutional mismatch repair deficiency, which is when an individual is born with a biallelic pathogenic variant in a MMR gene.
Baseline state
When an individual is simulated in the model, the model has the capability to sample their baseline state given that they have not yet been diagnosed with any cancer. This sampling is done during the initialisation of the population and a common random number generator seed is used so that the baseline state is identical across model arms.
Colorectal cancer
Colorectal cancer is the primary manifestation of LS, as it is the most common cancer in men with LS and the most or second most common cancer in women with LS. CRC is an essential component of any whole-disease model of LS.
Emerging evidence suggests that CRC in LS can develop through three major pathways depending on the order of genetic changes. 155 Some LS-associated CRC begin as MMR-proficient adenomas before losing MMR proficiency and finally becoming carcinomas. The evidence for this is that not all adenomas in individuals with LS are MMR deficient. Other cancers begin as MMR-deficient colonic crypt foci, which then become adenomatous polyps before becoming carcinomas. In the third pathway, the cancers begin as MMR-deficient colonic crypt foci, which remain non-polypous up to the point of becoming invasive cancers. These third pathway cancers are expected to be very difficult to detect through colonoscopic surveillance prior to becoming malignant.
Some cancers identified through surveillance may be ‘overdiagnosed’ – they would not have had clinically meaningful impacts on the patient during their life. In some cases, this is because the cancers are indolent or too slow growing, but it is also possible that some LS-associated precancerous lesions and cancers may be eliminated by an individual’s immune system. 156,157 A comparison of German, Dutch and Finnish people with LS found clear evidence that annual surveillance (the German guidelines) resulted in significantly more adenomas detected than less frequent surveillance but did not find any difference in the incidence or stage distribution of CRC. 158 This study does not distinguish between screen-detected CRC and interval CRC, which makes interpretation somewhat challenging.
Recently, Haupt et al. 159 have developed a mathematical description of the three pathways towards cancerous crypts which takes the form of a system of linear ordinary differential equations (with 1250 different states). Despite not being calibrated to medical data, this model is able (with selected parameter values) to accurately reproduce certain phenomena.
Less recently, Dinh et al. (2011)160 constructed an economic model in which CRCs were modelled using the adenoma–carcinoma sequence assumption. Adenoma growth was modelled and sensitivity of colonoscopy for adenomas was related to adenoma size. Malignant transformation of adenoma to carcinoma was assumed to be independent of adenoma size. Adenomas detected by colonoscopy would be removed, thereby reducing the risk of CRC.
We concluded that our model for CRC must include precursor lesions, some of which are detectable by colonoscopy at some point prior to malignant transformation and some of which are never detectable by colonoscopy.
Including the ability for precursor lesions and cancers to be eliminated by an individual’s immune system is challenging from a mathematical perspective as the model can become underdetermined, but it appears important to model that some precursor lesions which are detectable by colonoscopy can be eliminated.
We included in the model two types of precursor lesions: polypoid adenomas, which are detectable by colonoscopy, and non-polypoid (flat) adenomas, which are not detectable by colonoscopy. Polypoid adenomas can demonstrate MSI (a sign of deficient DNA MMR) or microsatellite stability (MSS – a sign of proficient DNA MMR). Polypoid adenomas can be low risk (< 10 mm) or high risk (≥ 10 mm). Flat adenomas all demonstrate MSI. Flat adenomas can become polypoid, at which point they become detectable by colonoscopy. Flat adenomas can also directly develop into CRC.
We assumed that any MSI adenoma would be at risk of being eliminated by the immune system and that the rate of elimination would initially be zero but would increase with age to an asymptote as the immune system becomes active against MSI neoplasia. We assumed that flat MSI adenomas would not arise in individuals with path_PMS2.
We built the bowel cancer component around the bowel cancer model ‘MiMiC-Bowel’161 which has been calibrated to nationally represented sources for the general population. As we do not have access to the statistical dependency between parameters for the MiMiC-Bowel model, we treated the parameters as fixed.
The MiMiC-Bowel model contains low- and high-risk MSS adenomas, so our additions were low- and high-risk MSI adenomas and flat MSI adenomas, and corresponding transition probabilities for these.
We modelled the annual probability for a flat MSI adenoma to arise from normal colonic epithelium as θ=θ(age)=exp(θconstant+θage⋅age). We modelled the annual probability for any MSI adenoma to be eliminated by the immune system as a scaled logistic (sigmoid) function.
Details of the calibration of the CRC component are given in the section Calibrated parameters, Colorectal cancer.
The model does not at present simulate where in the colorectum the adenoma/CRC are based.
Metachronous cancer
Although metachronous CRC does occur in LS (and is reported in approximately 1 in 10 with LS who are diagnosed with CRC), the model does not at present incorporate metachronous CRC.
Survival
As with other LS-associated cancers, survival from CRC is better among individuals with LS than among individuals with no diagnosis of LS. This could be caused by a number of factors, since CRC tends to be diagnosed at an earlier age and stage in LS (and these are presumably somewhat affected by surveillance), and CRCs in LS may be less likely to metastasise due to immune differences.
Xu et al. (2021)162 have conducted an analysis of CRC survival in LS, which attempts to isolate the impact of LS. After diagnosing 47 patients who had CRC with LS via reflex testing, they used propensity score matching to identify two controls (patients with CRC not diagnosed with LS) for each patient with LS. After this, they conducted survival analysis using Kaplan–Meier curves and evaluated the risk ratios for surviving to 5 years. At 5 years, overall survival was 0.976 in LS and 0.826 in sporadic CRC; in a multivariate analysis the risk ratio for death by 5 years was 0.106 (95% CI 0.025 to 0.446) for LS compared with sporadic CRC.
As a hazard ratio is more useful for modelling, we estimated the unadjusted risk ratio and hazard ratios to be 0.138 and 0.127, respectively. We assumed that the effect of adjustment on the log scale would be approximately equal for the risk ratio and hazard ratio, and therefore estimated a hazard ratio for overall survival of 0.098, lnβLS~N(−2.33,0.88), with the uncertainty estimated by inflating the standard error in the adjusted log risk ratio by 20%.
For sporadic CRC, we fitted an exponential survival model with an unshared frailty component and terms related to stage (I, II, III, IV), sex and age group (15–44, 45–54, 55–64, 75+ years). The data source was 1- and 5-year survival for CRCs diagnosed between 2013 and 2017 and followed up to 2018 as reported by the Office for National Statistics. 163 The fitting was performed using maximum likelihood estimation, using survival estimates and their 95% confidence limits to construct a likelihood function using normal distribution probability density functions. The variance–covariance matrix from the maximum likelihood estimation was used to represent the uncertainty and correlation in these parameters.
Endometrial cancer
In the general population, EC typically affects older women, with peak incidence in the 75–79 years age group. 23 It is also strongly associated with obesity, with an estimated 34% of cases attributable to overweight and obesity. 164 LS (caused by path_MLH1, path_MSH2 or path_MSH6) leads to a significantly elevated risk of EC, with peak incidence in the 50–54 years age group. 2 It is harder to accurately characterise the risk of EC in path_PMS2 LS using only prospective data, but a modified segregation analysis correcting for ascertainment bias found that the cumulative risk of EC by 80 years was 13% in path_PMS2 LS compared with 2.4% in the US general population. 165
Endometrial hyperplasia is the primary precursor lesion to EC. Classification of endometrial hyperplasia has historically been according to glandular crowding (simple vs. complex) and nuclear appearance (presence or absence of atypia), although SAH is very rare. 166 The risk of transformation to EC is by far highest among AEH. 167
Modelling approaches to EC in LS have typically been very simple, even when the interventions of interest are forms of gynaecological cancer risk reduction.
Kwon et al. (2008)79 modelled EC by using three different stage distributions for the different ways EC can be diagnosed (standard diagnostic pathway, RRS, surveillance). In each 1-year cycle there is a probability of EC developing and, if the strategy under evaluation includes surveillance, there is a probability that the cancer is detected by surveillance (i.e. test sensitivity). Different survival curves are applied to the four different stages of EC. The model also appears to include a parameter for the probability of abnormal bleeding, though its use in the model logic is not reported.
Yang et al. (2011)80 conducted a model-based economic evaluation of risk-reducing strategies for gynaecological cancer in LS. Their modelling approach is not clearly described, but it appears similar to that of Kwon et al. (2008). 79
Dinh et al. (2011)160 conducted a model-based economic evaluation of primary care-based genetic screening for LS, and this included a model for EC. The EC model was very simple; it did not break down EC according to stage and it assumed zero effectiveness of gynaecological surveillance.
Wright et al. (2021)81 developed a model to assess risk-reducing strategies for gynaecological cancer in LS. This included three stages of cancer (local, regional, distant), as well as having an ‘undetected EC’ state, but it generally seems similar to the approach used by Kwon et al. (2008). 79
We assume that all ECs develop from precursor lesions, which for the sake of simplicity we denote as AEH. Following clinical expert advice, we assume that LS affects the incidence of AEH. In the absence of evidence to suggest that LS accelerates malignant transformation of AEH into ECs we assume that once an AEH appears, its subsequent development will be independent of whether the individual has LS.
For each AEH there is a chance it will spontaneously resolve, and there is a chance that it will undergo malignant transformation to become a stage I EC. Once it has become a stage I EC, we assume that it cannot spontaneously resolve but will then progress through stage II, stage III and finally to stage IV. At any point, the lesion can become symptomatic, and after it has become symptomatic, it is at risk of being diagnosed. A diagram is shown in Figure 4.
FIGURE 4.
Diagram for development of EC.
We modelled the incidence of AEH using an inhomogeneous Poisson process. This means that it is possible for zero, one or more AEH to be incident during an individual’s lifetime, and that the incidence rate for AEH is not constant. The intensity function for this process was specified in terms of age and genotype by the construction of separate B-splines for the log-intensity for each genotype.
All events once an AEH develops (e.g. spontaneously resolving, becoming symptomatic, progressing) were modelled using exponential distributions (i.e. constant hazard rate). We assumed that the rate of progression through EC states would depend on whether the individual had LS, but that the rates at which asymptomatic lesions became symptomatic, and symptomatic lesions became diagnosed, would not be affected by genotype.
For details of how the EC model was calibrated, see Calibrated parameters, Endometrial cancer.
Determining the baseline state
Even though we consider a population that has not been diagnosed with EC, at the start of the model it is not appropriate to assume the entire population is free from endometrial lesions (unless the population is being modelled from birth). We must model the state of the endometrium conditional on the observation that the individual has not been diagnosed with EC.
While the EC model cannot be described as a finite state cohort model since there is no upper limit on the number of endometrial lesions, it can be approximated by a cohort model in which no more than three endometrial lesions can arise. With this assumption, the size of the state space was reduced to 286. For each genotype, we performed cohort simulation and recorded the probability distribution over the states at each integer value of age.
Management of atypical endometrial hyperplasia
If an AEH is identified, the management may be conservative (hormone therapy via insertion of a LNG-IUS, with or without oral progestin) or may be surgical (hysterectomy with or without BSO). Per clinical expert opinion, this decision is strongly influenced by the age of the patient, since younger patients may wish to subsequently attempt conception and may be concerned about the prospect of surgical menopause (if surgery includes BSO) or premature menopause.
We therefore modelled the probability of surgical management of AEH as:
This model allows for some people to choose surgery regardless of age (θ1), some people to choose conservative management regardless of age (1−θ2−θ1), and for the remainder to be influenced by age. We assumed that the age effect would be centred around θ3~N(40,2) and would have a maximum slope determined by:
We assumed that (θ1,θ2,1−θ1−θ2)~Dir [ (1,8,1) ] , so that 10% would always choose conservative management and 10% would always choose surgical management, but these figures are subject to uncertainty.
We assumed that surgical management would be 100% effective, that is no residual risk of EC from an AEH following hysterectomy. For conservative management we assumed that within 1 year, treatment would result in regression (disappearance of the AEH) with probability. 168–170
Clinical expert opinion is that the probability of successful medical management may be lower for individuals with LS. During conservative management patients would undergo endometrial sampling or hysteroscopy every 3 months to check for disease progression. We assumed that if conservative management was not successful after 1 year, the patient would undergo surgical management.
It is possible that an AEH may be diagnosed when there is fact also an EC. If the AEH is managed surgically we assume that the EC is detected during pathological examination and from that point on it is treated as a diagnosed EC (either due to surveillance or symptoms depending on how the AEH was diagnosed). If the AEH is managed conservatively and the EC is stage I, we assumed there was a probability of the EC regressing within 3 months ofp~Beta(11,5). 171 If the cancer did not regress, we assumed that it would be detected at the first check for disease progression.
Survival
It has been observed that survival for LS-associated EC is better than for sporadic EC. 172 There are multiple possible explanations for this, including different distributions of stage, histology, grade, age at diagnosis and risk factors (e.g. obesity).
As we model age and cancer stage we ideally require an estimate of survival for LS-associated EC according to age and stage, and similar for sporadic EC.
We considered three sources when estimating the survival of LS-associated EC.
First, we considered survival from the Prospective Lynch Syndrome Database (PLSD). 2 Many of the women in this database will have been undergoing gynaecological surveillance, so it is possible that survival in this database is biased upwards. The authors estimated crude overall survival from EC in those diagnosed aged under 65 years to be 0.89 (95% CI 0.82 to 0.94) at 5 years (and also at 10 years). This source has the advantage that all included participants have confirmed LS and it includes data from multiple centres across the world, but it has the disadvantages of possible bias due to surveillance, and it does not present according to stage at diagnosis, nor does it report stage at diagnosis.
Second, we considered a study published by Carr et al. (2020). 173 They reported the results of MMR deficiency testing and MLH1 promoter hypermethylation testing in 1018 ECs treated by hysterectomy at a single centre. Patients were therefore classified as MMR-I (MMR intact), MMR-DM (MMR deficiency explained by MLH1 promoter hypermethylation) and MMR-DU (MMR deficiency without MLH1 promoter hypermethylation). Of the MMR-DU subgroup, approximately half of those undergoing genetic testing for LS had a pathogenic variant of an MMR gene identified (i.e. confirmed LS). The authors presented progression-free and overall survival at 3 years, stratified according to stage at diagnosis: stage I/II, progression-free survival 0.912 (95% CI 0.792 to 1.00), overall survival 1.00 (95% CI 1.00 to 1.00); stage III/IV, progression-free survival 0.656 (95% CI 0.247 to 1.00), overall survival 0.750 (95% CI 0.450 to 1.00).
Finally, we considered the study published by Post et al. (2021),174 which included an evaluation of recurrence-free survival and overall survival in women recruited to the PORTEC-1, -2 and -3 studies who were found to have LS. At 5 years, recurrence-free survival was 0.917 (95% CI 0.831 to 1.00) and overall survival was 0.885 (95% CI 0.785 to 0.998).
We modelled survival from LS-associated EC as follows. For cancers diagnosed at stage III/IV, we assumed an exponential distribution for the time to cancer-specific mortality with rate
on the basis that two of eight participants in the study by Carr et al. (2020) with stage III/IV MMR-DU EC died within 3 years.
For cancers diagnosed at stage I/II, we assumed an exponential distribution for time to recurrence, followed by an exponential distribution for time from recurrence to cancer-specific mortality. We modelled the rate of recurrence as
based on an assumption that three of 34 participants in the study by Carr et al. (2020) with stage I/II MMR-DU EC experienced recurrence within 3 years (and assuming a further three participants were lost to follow-up very early). In the absence of any better data, we modelled the rate of cancer-specific mortality following recurrence as the same as the rate of cancer-specific mortality for those diagnosed in stage III/IV.
For sporadic EC the same approach was adopted as for sporadic OC.
Ovarian cancer
As with EC, OC typically affects older women in the general population, with peak incidence in the 80–84 years age group. 23 Survival of OC is typically poor – less than half survive more than 5 years from diagnosis163 – but survival for LS-associated OC is more favourable, as explored below. The risk of OC in LS is dependent on the MMR gene affected: the cumulative risk to 70 years is over 10% for path_MLH1, path_MSH2 and path_MSH6. 2 The risk for path_PMS2 is harder to characterise, with one analysis suggesting an increased risk compared with the general population but not reaching statistical significance. 165
Existing models have taken the same approach to modelling OC as EC. 79–81,160
In our model, unlike for EC, we neither model whether OC is symptomatic or asymptomatic, nor do we model precursor lesions to OC. There is an age- and genotype-dependent hazard of developing preclinical stage I OC and, once this has occurred, there are then (separate) constant hazard rates for progression (through stages II, III and IV) and clinical presentation. Similarly to AEH, the log-hazard rate for incidence of preclinical stage I OC was modelled using separate B-splines for each genotype.
Unlike with EC, we assumed separate rates of cancer progression for LS and sporadic OCs.
We assumed that if OC was diagnosed, treatment would include HBSO.
Details of the calibration of the OC model are given in the section Calibrated parameters, Ovarian cancer.
Recurrence and survival
Lynch syndrome
If OC is diagnosed in stage I/II, it is treated and is presumed to be cured (no cancer-specific mortality) unless disease recurrence occurs. Two studies informed the estimation of OC recurrence and survival. 71,175 There may be some overlap between the populations of these studies. Woolderink et al. (2018)71 reported that seven of 37 (16%) OCs diagnosed in stage I/II recurred, and reported the mean (56 months), SD (48 months), median (40 months) and range (3–120 months) of the time to recurrence among these seven. Based on these findings, we imputed times for recurrences to be 3, 8.6, 24.5, 40, 63.5, 99.2 and 120 months, and fitted a Gompertz model to this (Figure 5), assuming that the remaining 30 OCs were recurrence-free at 151 months (mean follow-up).
FIGURE 5.
Recurrence-free survival for stage I/II OC.
For survival post-recurrence for OC initially diagnosed in stage I/II, we apply survival as is predicted for OCs when diagnosed in stage III/IV.
For survival for OCs diagnosed in stage III/IV, we refer to the findings of Grindedal et al. (2010)175 that of 25 women with cancers diagnosed in stage III/IV, 59% survived to 5 years and 53% survived to 10 years. Examining the Kaplan–Meier curve, it appears there was minimal censoring prior to 5 years, but there may be considerable censoring after that time. We assume therefore that 10 of 25 died before 5 years, and 1 of 8 died between 5 and 10 years. We fitted exponential, Weibull and Gompertz models on this basis. The exponential distribution appeared to give a poor fit compared with the published Kaplan–Meier curve. We decided to use the Weibull model as it continued to include significant mortality risk beyond 10 years and this was felt to be more clinically realistic (Figure 6).
FIGURE 6.
Cancer-specific survival for stage III/IV OC.
Sporadic ovarian cancer
For sporadic OC, we assumed there was an exponential time-to-event distribution for the time from diagnosis to recurrence/progression, followed by an exponential time-to-event distribution for the time from recurrence/progression to death. We assumed a common distribution for the time from recurrence/progression to death across the stages. We also incorporated heterogeneity by including an individual (unshared) frailty component which was gamma distributed.
We fitted this model to 1- and 5-year stage-specific survival from the Office for National Statistics163 using a Bayesian approach with lognormal(0,1) priors for all rates and for θ.
Gynaecological surveillance
Test performance
Many of the women undergoing gynaecological surveillance due to LS will be premenopausal as uptake of risk-reducing gynaecological surgery increases substantially with age.
Dijkhuizen et al. (2000)176 conducted a systematic review and found that the sensitivity of undirected endometrial sampling (e.g. Pipelle biopsy) to detect endometrial carcinoma was substantially lower in populations including premenopausal women than in populations consisting only of postmenopausal women. TVUS is also less useful for detecting endometrial abnormalities in premenopausal women because of cyclical changes in endometrial thickness.
There is generally a paucity of good-quality data to inform estimates for the performance of gynaecological surveillance tests in LS. This renders any attempt at this time to use economic modelling to choose an optimal set of surveillance tests pointless, and a decision was made to model the performance of gynaecological surveillance as a suite of tests, rather than attempt to model the performance of tests in isolation or in specific combinations.
We modelled the sensitivity of gynaecological surveillance using a multivariate normal distribution and log-odds transformations (Figure 7).
FIGURE 7.
Sampled sensitivity estimates for gynaecological neoplasia.
If uncertainty has been exaggerated in the model, this could lead to an overestimation of the value of perfect information for these test performance parameters, so they should in that case be viewed as upper bounds.
The model allows for multiple endometrial lesions, and the test performance is evaluated independently for each, so that if multiple endometrial lesions are present, the probability of at least one being identified is increased.
Effectiveness
We assumed that if an AEH was identified by surveillance, it would be managed in the same way as a symptom-detected AEH, that is a chance of being managed medically or surgically. We did not incorporate any lead time estimate since AEH itself is not associated with mortality risk.
We assumed that if an EC was identified by surveillance it would be managed surgically, and that there would be a lead time effect on survival as well as a possible effect of identifying in an earlier stage.
As the model allows multiple endometrial lesions, it is possible that an EC is missed on surveillance but surgical management of an AEH (which is detected) leads to detection of the EC during pathological examination.
The model does not include the risk of complications from surveillance.
Schedule
We assumed that gynaecological surveillance would be annual from 25 to 75 years while female reproductive organs are intact. We modelled the interval between surveillance visits using a Pareto distribution with m~N(0.9,0.1) and α~ Γ ,E[α]=4,Var[α]=2.
Lead time
Surveillance has the potential to detect cancer earlier, so it is important to incorporate an estimate of lead time into survival estimates (otherwise surveillance may be associated with earlier death since survival is measured and simulated from time of diagnosis).
We estimate the lead time by the conditional expectation of the time to clinical presentation given the cancer presents in the same stage (I, II, III, IV for sporadic; I/II, III/IV for LS).
For example, a sporadic OC diagnosed in stage I by surveillance would have an expected lead time of:
where ξIsporadic and λIsporadic are the presentation and progression rates in stage I.
Similarly, a LS-associated OC diagnosed in stage I by surveillance would have an expected lead time of:
For EC, development of symptoms is a precursor event to diagnosis in the model, so the lead time takes this into account, and the lead time is shorter if the EC is already symptomatic.
Colorectal surveillance
Test performance
Values for colonoscopy sensitivity for polyps and CRC were taken from MiMiC-Bowel model. 161
Schedule
Colorectal surveillance for LS in the UK is typically done on a biennial basis (every 2 years) from age 25 to age 75 (or when on balance the risks outweigh the benefits). We modelled the interval between surveillance visits using a Pareto distribution with xm~N(1.8,0.1) and α~ Γ ,E[α]=4,Var [ α ] =2.
Lead time
As for gynaecological surveillance, we estimate the lead time as the conditional expectation of the time to clinical presentation given the cancer presents in the stage at which it is detected by surveillance.
As a discrete-time Markov model was used for CRC, we potentially have two relevant probability terms: we let p1 denote the transition probability for clinical presentation and p2 denote the transition probability for disease progression (if applicable).
If the CRC is in stage IV and has no opportunity for further progression then the lead time is estimated as −1/ln(1−p1). If the CRC is in an earlier stage, then the lead time is estimated as −1/ln(1−p1−p2).
Risk-reducing gynaecological surgery
Women with path_MLH1, path_MSH2 or path_MSH6 LS face a significant lifetime risk of endometrial and OC and are therefore often recommended to have risk-reducing gynaecological surgery, particularly if an AEH has been diagnosed. 75
For postmenopausal women it is expected that the surgery will be HBSO. For premenopausal women the choice of surgery may be less clear because the absolute risk of OC remains fairly low (fewer than one in five will go on to develop OC) and bilateral oophorectomy would lead to surgical menopause requiring immediate and potentially long-term HRT. Indeed, Wright et al. (2021)81 explored the cost-effectiveness of different risk-reducing strategies for gynaecological cancer in LS with an explicit strategy of hysterectomy and bilateral salpingectomy at 40 years with delayed oophorectomy at 50 years.
The uptake of risk-reducing surgery is understandably age-related, since the risks that are being reduced are also age-related, and the consequences of risk-reducing surgery (end to natural fertility, surgical menopause) are also age-dependent. Seppälä et al. (2021)177 analysed data from the PLSD and found that HBSO was the most common risk-reducing surgery, but that a significant number of women had hysterectomy alone or BSO alone. Very few women aged under 40 years had undergone risk-reducing surgery. It should be noted that hysterectomy or BSO for benign conditions cannot be excluded, that is not all surgeries can be guaranteed to have been purely for cancer prophylaxis.
We also may expect that the uptake of risk-reducing surgery would be affected by the availability of other interventions to reduce the risk of gynaecological cancer (e.g. gynaecological surveillance). Gynaecological surveillance would have been available for a fair proportion of the women included the PLSD.
We ultimately modelled the uptake of risk-reducing surgery as follows:
-
A certain proportion of women (~8%) will be very keen to undergo risk-reducing surgery as soon as it is offered (at 35 years or when they are diagnosed with LS, whichever is later), and this is not dependent on the specific LS genotype, p~Beta(36,438).
-
A certain proportion of women will not take up risk-reducing surgery at any age (unless they are subsequently diagnosed with an AEH), and this is dependent on the specific LS genotype:
-
40% for path_MLH1, p~Beta(10,15).
-
28% for path_MSH2, p~Beta(7,18).
-
48% for path_MSH6, p~Beta(12,13).
-
60% for path_PMS2, p~Beta(15,10).
-
The remaining population may take up risk-reducing surgery over time; we model the rate of uptake (when surveillance is available) using a log-normal distribution which is specific to the LS genotype.
-
When surveillance is not available, we do not adjust the proportions of women who will always or never take up risk-reducing surgery, but we do incorporate an accelerated failure component, whereby if a woman would undergo risk-reducing surgery in T years if surveillance was available, she would instead undergo risk-reducing surgery in αT years if it was not available. α does not vary between individuals, but is subject to uncertainty, α~ Γ (k=100,θ=0.008),E[α]=0.8.
If an individual has occult endometrial or OC at the time of risk-reducing surgery we assume it is detected, since detailed pathological examination of surgical specimens from LS patients is standard practice.
The model does not explicitly determine whether risk-reducing surgery is inappropriate for some individuals, for example due to prior abdominal surgery for CRC leading to adhesions.
The model also does not account for the possibility that colonoscopy becomes more painful or impossible to complete following risk-reducing gynaecological surgery.
General mortality
General mortality was estimated from the present and projected cohort life tables for the UK from the Office for National Statistics. 178 We extracted life tables for men and women aged 30, 35, …, 65 years in 2022. We fitted a Gompertz model to the extracted life tables by calculating the average hazard of death in a year of life and fitting a linear regression of log hazard against age and year of birth, stratified by sex. The relationship between log hazard and year of birth was assumed to be linear with a changepoint at 1975 following graphical examination.
It is a minor limitation that we have not subtracted the population-level rates of mortality from colorectal, ovarian and EC, but these are very low. For patients with LS, this is compensated for by the fact the model does not include an association between LS and cancers such as small bowel cancer, stomach cancer and urinary tract cancers.
Family structure
An important aspect of LS is that it is hereditary. The offspring of an individual with LS have a 50% probability of inheriting LS. In the absence of other information, this means that a first-degree relative of somebody diagnosed with LS (i.e. a sibling, parent or child) has a 50% probability of also having LS.
We considered three strategies for modelling family structures in the model:
-
simulate full family structures
-
simulate LS and non-LS families separately
-
simulate individuals and only simulate family structures and events when needed.
The first strategy is the most general (most likely to be flexible enough to answer any research question) but will be the most computationally expensive. The third strategy is the least general – certain research questions will not be immediately answerable, and some may not be answerable at all – but it is also the most computationally efficient for some research questions as it avoids simulating unnecessary detail.
We opted to use the third strategy for the economic evaluation of risk-reducing strategies for gynaecological cancer in LS (see Chapter 7), but we have also ensured the model can be adapted or extended to use the other strategies.
Chemoprevention
At present chemoprevention with aspirin is not fully incorporated into the model, although the costs for aspirin chemoprophylaxis have been estimated.
Calibrated parameters
The whole-disease model includes three natural history components that require calibration since they include many parameters which cannot be directly studied, such as the rate at which cancers progress in the absence of treatment.
Endometrial cancer
As previously noted, the time from AEH development to cancer diagnosis can be modelled as a phase-type distribution. We calibrated the EC model in part by modelling the incidence of EC, since the incidence of AEH is rarely observed.
Using the displacement theorem, we used the inhomogeneous Poisson process for AEH incidence and the probability density function for the time from AEH incidence to EC diagnosis to construct an inhomogeneous Poisson process for EC incidence.
We also predicted the stage distribution of endometrial lesions when a population cross-section is taken (assuming perfect sensitivity) and the stage distribution of ECs which are not identified through surveillance.
We fitted to data on EC incidence in England,23 the stage distribution of ECs in England,180 the PLSD,2 and studies by Renkonen-Sinisalo et al. (2007)61 and Eikenboom et al. (2021). 40
Priors for λAEH and ρ informed by Lacey et al. (2010). 181 12.4% risk of EC within 10 years in those diagnosed with AEH, 27.5% risk within 20 years. This is despite the use of hormone therapy, which would in theory increase ρ (and perhaps reduce λAEH).
We use priors of λAEH~lognormal(−2.6,0.22) and ρ~lognormal(−2.0,0.22), which means that 90% of the prior distribution is such that the probability an AEH would become an EC if it was not diagnosed and treated is λAEH/(ρ+λAEH)∈[ 0.256,0.466 ].
Calibration was conducted using Stan153 and three chains were used, with 1000 samples from each chain after 1000 warmup iterations.
The modelled cumulative risk of EC is shown in Figure 8.
FIGURE 8.
Cumulative risk of EC diagnosis (posterior mean and 90% credible interval).
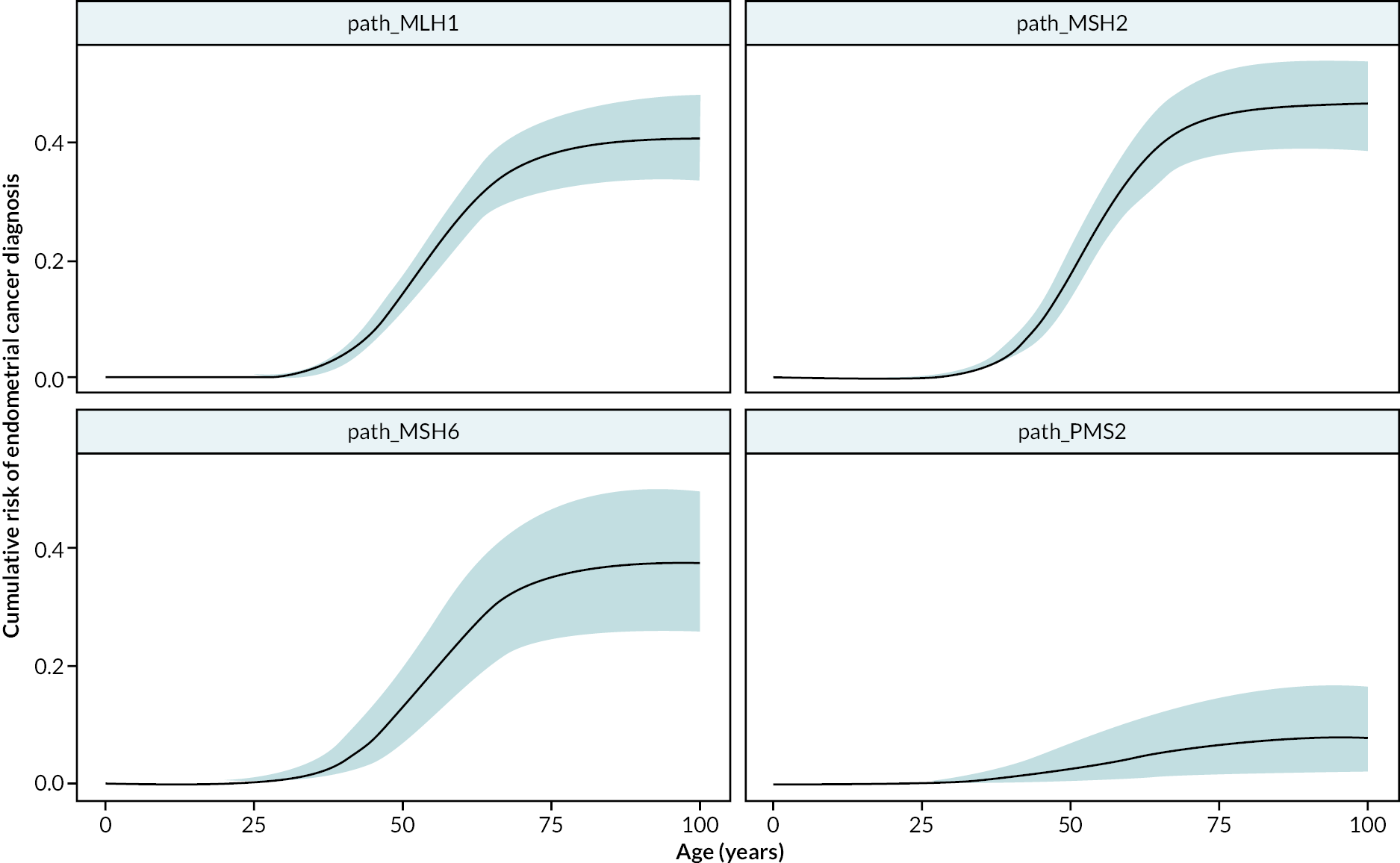
The posterior predictive distributions for the numbers of EC diagnoses in PLSD are shown in Figure 9. These generally show a good fit, although there is some suggestion that the incidence of EC should drop sharply after 50–54 years rather than declining more gradually. This may suggest the need for a less smooth intensity function for AEH incidence, or perhaps for other parameters (particularly rates of progression and of diagnosis of symptomatic lesions) to depend on age with a near discontinuity around the time of menopause. Note that the number of ECs in PLSD is also dependent on the number of patient-years observed at risk (i.e. without risk-reducing hysterectomy) and drops with age.
FIGURE 9.
Posterior predictive distributions for EC diagnoses in PLSD. Note: line ranges show 90% posterior predictive distribution credible interval; circles show posterior predictive distribution means; squares show PLSD data.
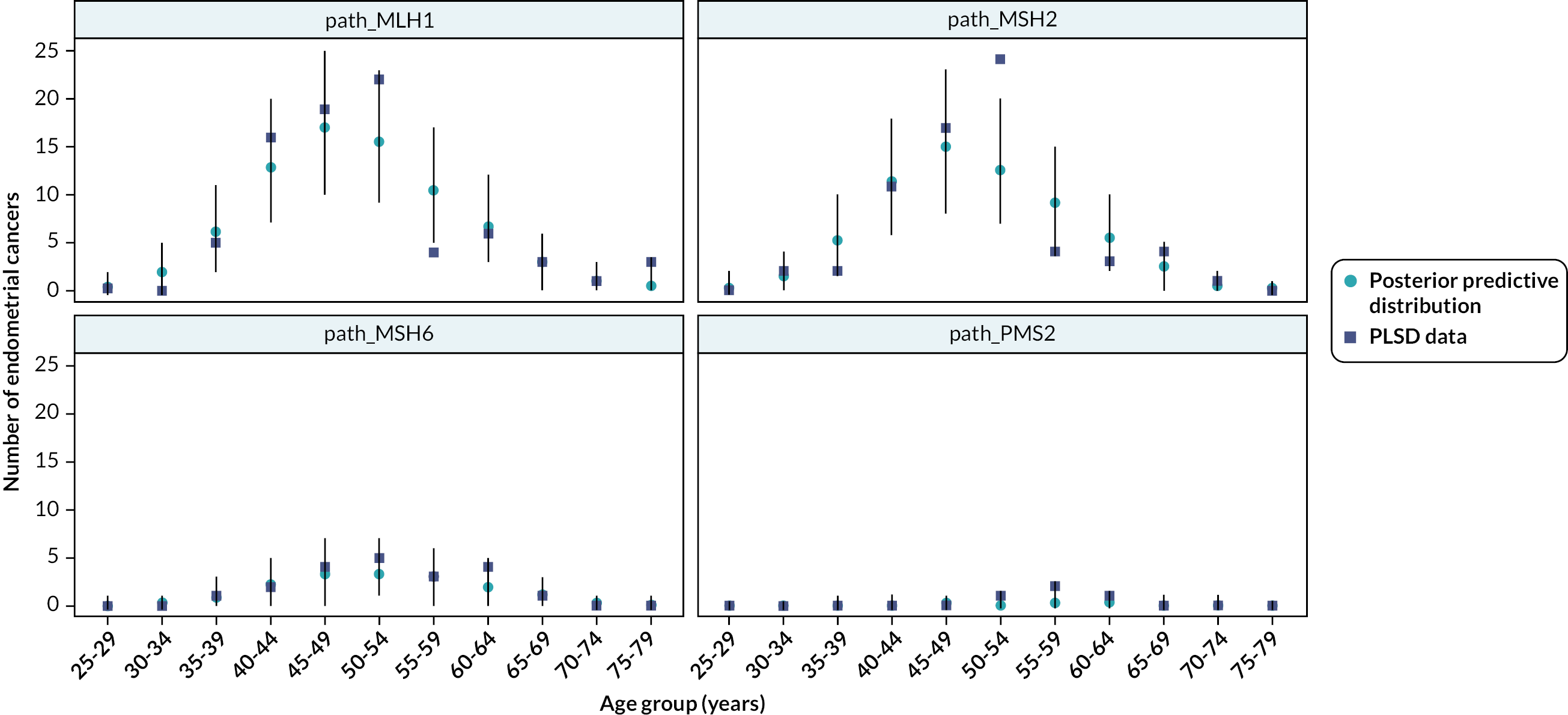
Ovarian cancer
A similar approach was used to calibrate the OC natural history model. We again used the number of cancer diagnoses in the PLSD and in the general population of England across different age groups,2,23 and we used the stages of OC diagnosis in England180 and in two studies reporting stage distributions of LS OCs in the absence of risk-reducing interventions. 39,71
Vague priors were used – the coefficients for the B-spline for log hazard were given priors α~lognormal(−20,22) and the rate parameters (progression, presentation) were given priors λ~lognormal(0,12).
Again, we used Stan153 for calibration, but due to computational limitations, we only ran one chain and obtained 1000 samples after 1000 warmup iterations.
The resulting cancer risk estimates for path_MLH1 and path_MSH2 LS are shown in Figure 10 (the estimates for path_MSH6 and path_PMS2 are near zero). The posterior predictive distributions for the number of OCs in PLSD are shown in Figure 11.
FIGURE 10.
Cumulative OC risk (posterior mean and 90% credible interval).
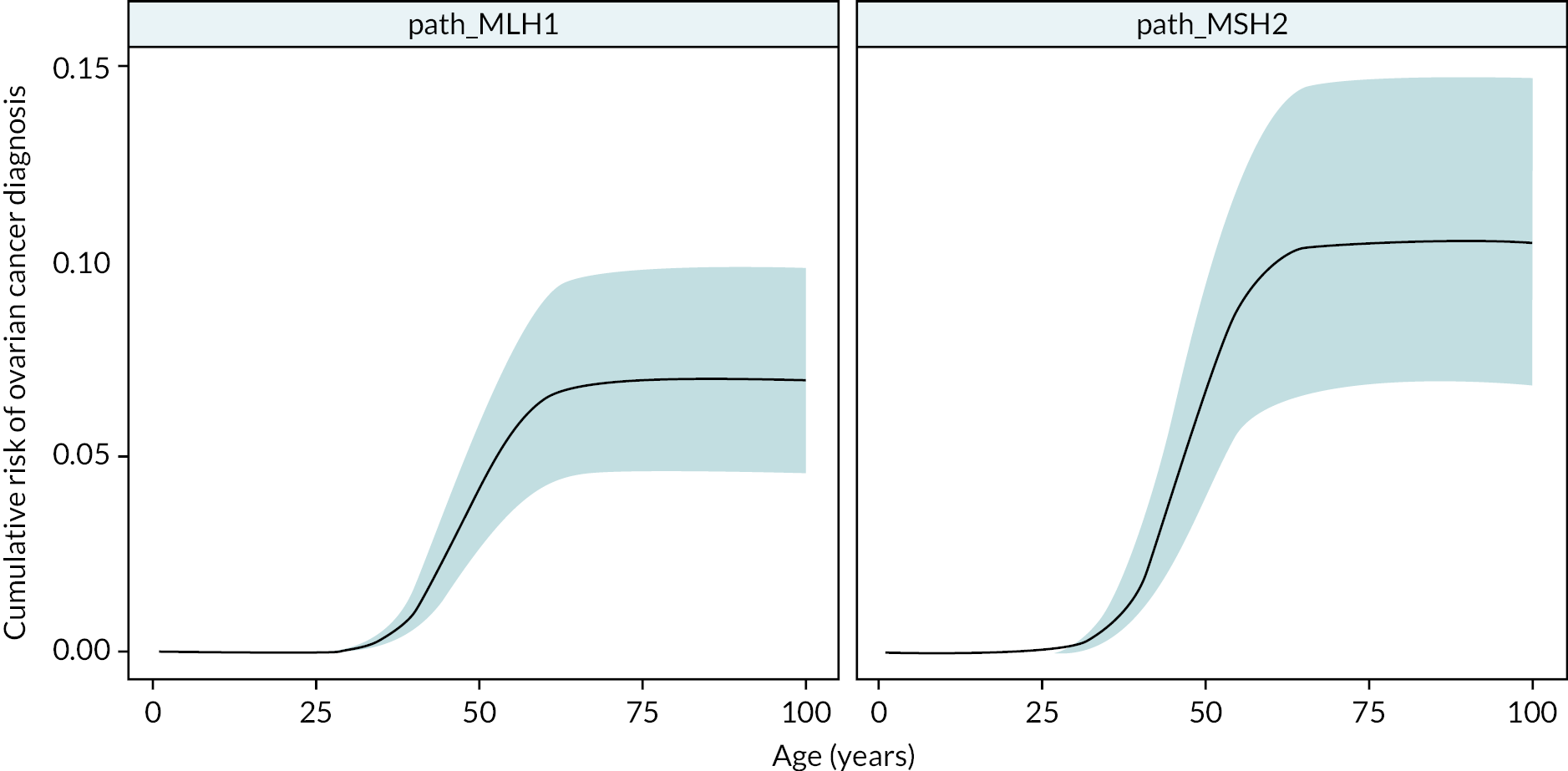
FIGURE 11.
Posterior predictive distributions for OC incidence in PLSD. Note: dark and light line ranges show 50% and 90% posterior predictive distribution credible intervals, respectively; light blue circles show posterior predictive distribution medians; dark blue circles show PLSD data.
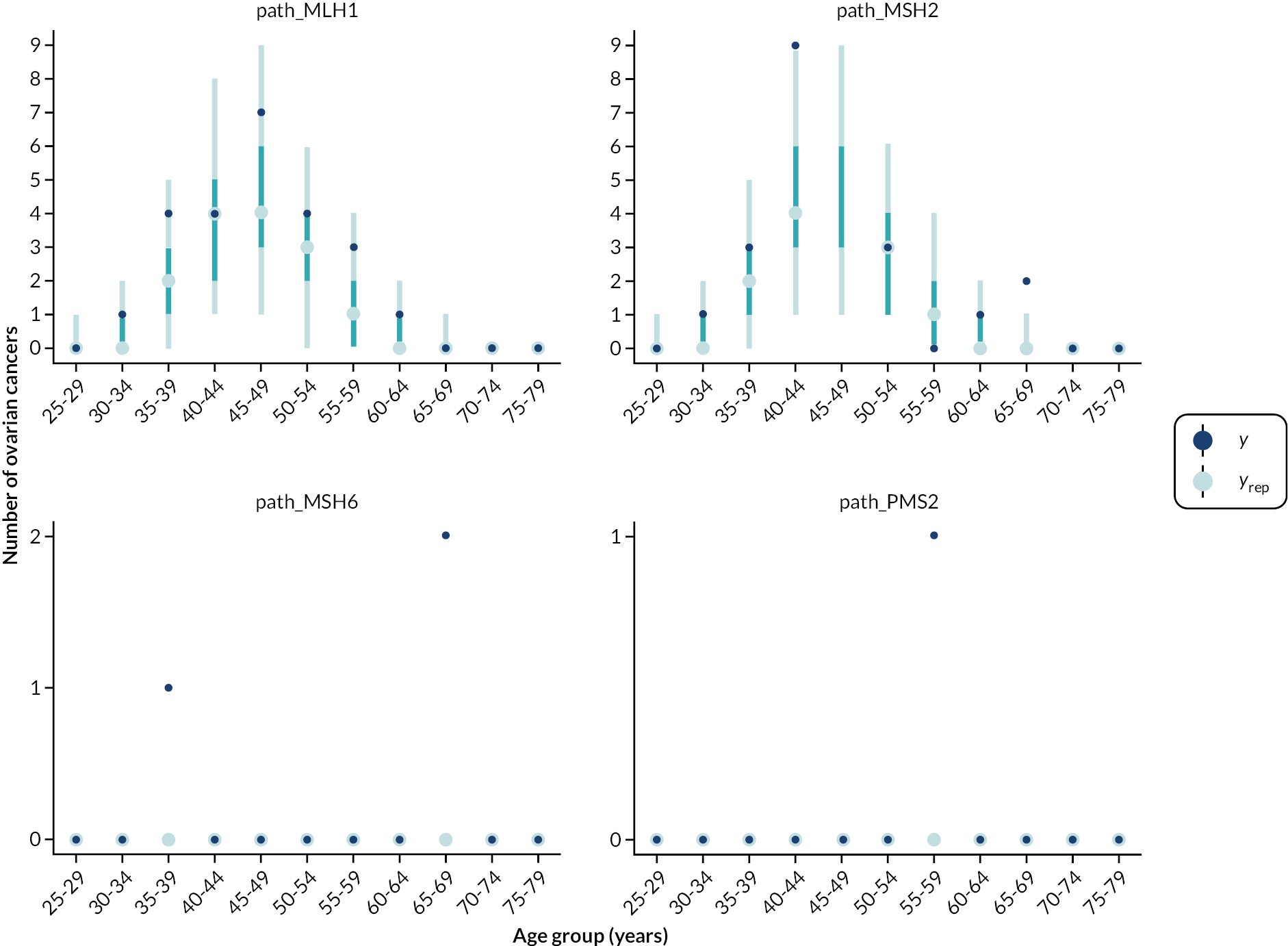
Colorectal cancer
We used Stan to conduct a Bayesian calibration of the CRC model. The key data sources were the PLSD,2 a systematic review of MSI in colorectal adenomas in people with LS by Dabir et al. (2020),182 and a comparison of three different colorectal surveillance intervals in people with LS by Engel et al. (2018). 158
From PLSD, we took the exposure (patient-years) and number of CRCs diagnosed (events) according to age (5-year age groups), genotype and sex, and modelled each of these with a Poisson distribution where the rate parameter was determined by a Markov simulation of the CRC model assuming biennial colonoscopy from 25 years.
From Dabir et al. (2020),182 we modelled the relationships between MSI status and genotype, adenoma size and age, again using a Markov simulation of the CRC model assuming biennial colonoscopy.
From Engel et al. (2018),158 we modelled the findings at the index colonoscopy and the number of follow-up adenomas and CRCs. For this, we used multiple Markov simulations with different assumed colonoscopy intervals.
Prior distributions were generally chosen to avoid a significant amount of prior mass existing where the Markov transition matrix would be invalid (because probabilities would sum to more than one), but were otherwise uninformative. The prior distribution for the parameters for the incidence function for flat MSI adenoma were θ0~N(−4,32) and θage~lognormal(−6,12). The prior distribution for the parameters for the regression transition probabilities were ρ0~Beta(1,20),ρ1~N(40,52),ρ2~lognormal(3,0.252).
The prior distributions for the following (annual) transition probabilities were p~Beta(1,20):
-
MSS polypoid adenoma to MSI polypoid adenoma
-
flat MSI adenoma to high-risk MSI polypoid adenoma
-
flat MSI adenoma to stage I CRC
-
low-risk MSI polypoid adenoma to high-risk MSI polypoid adenoma
-
high-risk MSI polypoid adenoma to stage I CRC.
Four chains were sampled, with 1000 samples from each after a warmup of 1000 iterations.
We simulated from posterior predictive distributions to evaluate the fit of the calibrated model.
Certain calibration targets were not met accurately; for example:
-
The proportion of adenomas with MSI in people with path_MSH2 mutations was overestimated [vs. the findings of Dabir et al. (2020)182].
-
The calibrated model estimated that MSI adenomas would be more associated with younger patients (under 60 years) than older patients, while Dabir et al. (2020)182 found a slight association in the other direction.
-
The calibrated model estimated that low-risk adenomas (< 10 mm) would be significantly less likely to demonstrate MSI, while Dabir et al. (2020),182 found a weaker association in the same direction.
-
The number of adenomas detected by annual surveillance was low compared to the findings by Engel et al. (2018),158 while the number of CRCs was high.
These suggest that further work on specifying or calibrating the CRC model may be necessary, especially where interventions of interest specifically focus on CRC.
Costs
Costs were calculated in Great British pounds in the price year 2021–2 and were valued from the perspective of the NHS and Personal Social Services.
Gynaecological surveillance
We estimated the cost of three combinations of gynaecological surveillance technologies.
First, we estimated the cost of the combination of hysteroscopy with biopsy, TVUS and CA-125 testing, as this was believed to be the most sensitive combination of surveillance technologies for individuals with uterus and ovaries intact.
The Healthcare Resource Group (HRG) MA46Z (diagnostic hysteroscopy with TVUS, with biopsy) was judged to be highly relevant to the cost of hysteroscopy with biopsy and TVUS. The cost for this currency (weighted average across all settings) in the 2019/20 National Schedule of NHS Costs183 was £264.02. The vast majority of activity for this currency was in outpatient procedures (4397/4544, 97%), but there were also daycase procedures (132/4544, 3%), which may have been due to a need for anaesthesia. This was inflated by 3.08% to 2020–1 prices using the NHS Cost Inflation Index184 and then by 2% to 2021–2 prices.
The cost of CA-125 testing was estimated from a tariff for procedures and investigations used to estimate NHS treatment costs for National Institute for Health and Care Research (NIHR) research:185 we assumed 15 minutes of nursing time to collect blood and included the investigation 86304 (pathology: immunoassay for tumour antigen; quantitative, CA-125); after applying multipliers for an average market forces factor (1.080) and overheads (1.7), this resulted in a total cost of £49.60. It should be noted that other economic studies have assumed somewhat lower costs for CA-125 testing [e.g. £10 by Menon et al. (2017),186 US$28.35 by Esselen et al. (2016)187 based on Medicare reimbursement].
We therefore estimated the total cost of the combination of hysteroscopy with biopsy, TVUS and CA-125 testing to be £329.13.
The next combination of tests we considered was undirected endometrial biopsy, TVUS and CA-125 testing, as this was believed to be fairly sensitive and may reduce costs and pain versus the use of hysteroscopy. The relevant HRG was determined to be MA37Z (TVUS with biopsy), but the average cost for this was £261.47. As this was so similar to the cost of hysteroscopy with biopsy and TVUS, we did not estimate a separate cost for this combination.
For women who have undergone hysterectomy but retained their ovaries, we assumed that they would undergo transvaginal or transabdominal ultrasound and CA-125 testing. The HRG MA36Z (TVUS) was judged to be most relevant, with a cost of £162 in 2019/20 prices,183 inflated to £171.26 in 2021–2 prices (as previously described). The total cost was therefore estimated to be £220.86.
For women who have undergone oophorectomy but retained their uterus, we assumed that they would undergo hysteroscopy with biopsy and TVUS. This was estimated to cost £279.53 (see above).
For all these costs, we represented uncertainty using a gamma distribution with shape 100 to obtain a coefficient of variation of 10%.
Colorectal surveillance
We identified the key HRGs to be FE30Z (therapeutic colonoscopy, 19 years and over) and FE32Z (diagnostic colonoscopy, 19 years and over). The average costs for these were £705 and £560 in 2019/20 prices,183 inflated to £749.29 and £593.37 in 2021–2 prices. FE30Z applies when the colonoscopy results in detection of polyps, while FE32Z applies when no findings are made. Again, a gamma distribution with coefficient of variation 10% was used.
Risk-reducing gynaecological surgery
We considered three possible forms of risk-reducing gynaecological surgery: HBSO, hysterectomy alone and BSO alone.
For both hysterectomy alone and HBSO, the appropriate HRG was deemed to be MA02 or MA28Z, depending on whether surgery was open or laparoscopic. We estimated the average cost to be £5932 in 2019/20 prices,183 which was inflated to £6281.03 in 2021–2 prices.
For BSO alone, the appropriate HRG was deemed to be MA07 or MA08, depending on whether surgery was open or laparoscopic. We estimated the average cost to be £4402 in 2019/20 prices,183 which was inflated to £4660.22 in 2021–2 prices.
Again, a gamma distribution with coefficient of variation 10% was used.
We incorporated a cost for HRT for surgical menopause if this took place earlier than 50 years. We assumed a transdermal dose of 100 μg estradiol per day for the first 2 years, then tapered to a dose of 50 μg per day to 50 years or 4 years after RRS (whichever was later). Based on Prescriptions Cost Analysis data,188 we assumed a cost of £0.717064 per 100-μg/24-hour patch and £0.577416 per 50-μg/24-hour patch, corresponding to annual costs of £74.57 for 100-μg/24-hour and £60.05 for 50-μg/24-hour patches. We used a gamma distribution with coefficient of variation 10% for these costs.
Aspirin chemoprophylaxis
For aspirin chemoprophylaxis, we assumed a daily dose of 300 mg and an annual review with a general practitioner. The cost of 100 aspirin tablets (300 mg) is £12.54,189 resulting in an annual drug acquisition cost of £45.78. A general practitioner appointment is estimated to cost £33.19 (excluding qualification costs). 184
Atypical endometrial hyperplasia
For diagnosed AEH managed conservatively by LNG-IUS, we included a cost of coil insertion at the start of management and the cost of coil removal, plus a hysteroscopy with biopsy and TVUS every 3 months for 1 year, plus a cost for oral progestogen.
We deemed HRG MA35Z (implantation of the LNG-IUS) most appropriate for coil insertion, the cost of which was inflated to £317.97 in 2021–2 prices.
For the examination and removal of the IUS after 1 year, we were unable to identify a more appropriate HRG than MA46Z (diagnostic hysteroscopy with TVUS, with biopsy), so we used this after inflating to £279.53.
The cost of oral progestogen (daily 400 mg medroxyprogesterone acetate) is £58.67 per 30 days,190 but some patients may not take oral progestogen and some patients may receive a higher dose than this (up to 600 mg), so there is uncertainty in this cost.
We used gamma distributions with coefficient of variation 10% for these costs.
Endometrial cancer
For the cost of EC, we relied upon a study by Pennington et al. (2016)191 in which relevant costs for 491 women diagnosed with EC within the UKCTOCS trial were estimated over a 5-year time horizon. These costs were presented according to stage at diagnosis in 2012/13 prices. We combined stage IA/IB and stage IC (using the properties of mixture distributions to estimate the resulting SD of cost), inflated to 2021–2 prices, and fitted gamma distributions using the method of moments (with the standard error), as shown in Table 20.
| Stage | Mean cost over 5 years (£) | Uncertainty distribution |
|---|---|---|
| I | 11,642 | Γ (608.3, 19.14) |
| II | 16,237 | Γ (128.5, 126.3) |
| III | 30,323 | Γ (87.08, 348.2) |
| IV | 32,055 | Γ (24.32, 1318) |
We applied this cost at time of diagnosis (it already includes discounting at 3.5% per year). No further costs were applied except in the case of recurrence.
We only applied costs of recurrence for ECs diagnosed in stages I or II. Treatment of recurrence may involve surgery (removing affected lymph nodes, resection of affected organs), radiotherapy and/or chemotherapy. In the study by Pennington et al. (2016),191 the average costs over the first 2 years for ECs diagnosed in stage III and IV were £18,615 and £16,999, respectively. Taking a weighted average of these costs, inflating to 2021–2 prices, and fitting a gamma distribution assuming a coefficient of variance of 20%, results in a cost of c~ Γ (25,858),E[ c ]=£21,449.
Ovarian cancer
Westwood et al. (2018)192 conducted a cost-effectiveness analysis of different risk scores for people with suspected OC, and as part of this they updated cost estimates for OC from the NICE clinical guideline CG12229 in 2015–6 prices. They estimated that the cost of early-stage OC would be £5320 and the cost for advanced OC would be £10,606. They further estimated that follow-up costs would be £398 per year for years 1–3 and £92 per year beyond year 3.
We inflated these costs to 2021–2 prices and applied them according to OC stage at diagnosis (early: stage I and II; advanced: stage III and IV). Upon recurrence from stage I or II OC we applied the cost of advanced OC.
To represent the uncertainty in these costs, we used gamma distributions with shape parameter k=25 and scale parameter c/k, such that the coefficient of variation was 20%.
Colorectal cancer
We utilised costs from Laudicella et al. (2016)193 for CRC. This allowed us to apply a cost for each year that a patient was alive following a diagnosis rather than applying all costs at the time of diagnosis. The authors present costs according to year since diagnosis, stage at diagnosis (I–II vs. III–IV) and age (18–64 years vs. ≥ 65 years; presumed to be age at diagnosis). We subtracted the average annual cost for the period 12–36 months prior to diagnosis (£231.50 for 18–64 years, £453 for age ≥ 65 years) in an attempt to remove unrelated medical costs, and inflated to 2014–5 prices using the hospital and community health service pay and prices index, then to 2020–1 using the NHS cost inflation index, then to 2021–2 at 2% (Table 21). We assumed that the cost beyond the final year estimated by Laudicella et al. would be equal to the cost in the final year.
| Year | Age 18–64 years | Age ≥ 65 years | ||
|---|---|---|---|---|
| Stages 1–2 | Stages 3–4 | Stages 1–2 | Stages 3–4 | |
| 1 | 17,722 | 22,885 | 16,592 | 18,059 |
| 2 | 4134 | 7468 | 3822 | 5662 |
| 3 | 3426 | 5092 | 3116 | 4361 |
| 4 | 2639 | 4151 | 2592 | 3405 |
| 5 | 2371 | 2951 | 2631 | 3182 |
| 6 | 1611 | 2463 | 2658 | 3019 |
| 7 | 1676 | 2878 | 2416 | 1914 |
| 8 | 1534 | 2197 | 2678 | 2499 |
| 9 | 1318 | 1498 | 2236 | 1933 |
Owing to substantial uncertainty in these estimates caused by needing to inflate from 2010 price year and the possibility of substantial changes to costs of clinical practice (e.g. old therapies going off patent, new expensive targeted therapies), we constructed a joint distribution for uncertainty multipliers. For example, if the uncertainty multiplier for year 1, age 18–64 years, stages 1–2 is sampled as 0.9, the resulting cost is £17,722 × 0.9 = £15,950. The uncertainty multipliers were sampled from a multivariate normal distribution with mean 1 and covariance matrix ∑.
For example, ρij for i corresponding to year 1, age 18–64 years, stages 1–2 and j corresponding to year 3, age 18–64 years, stages 3–4 would be:
Failure to include these correlations could result in a substantial underestimation of the uncertainty in total costs.
Death
We assumed that costs of death from CRC, EC and OC would already be included in the costs selected. For deaths from other causes we chose not to include any cost.
Costs not yet estimated
For a whole-disease model, there are other costs of interest that have not yet been estimated as they do not relate to gynaecological cancer surveillance and it is also uncertain what some of these costs will be in years to come, for example due to rapid technological change. These costs are primarily those related to diagnosing LS, that is the costs of tumour-based triage tests, mutation testing and genetic counselling.
The most recent relevant estimation of these costs in the UK (2017–8 prices) can be found in Stinton et al. (2021). 194
Utility values
Utility values, sometimes known as QALY weights, describe the expected preferences between different health states. Perfect health is given a utility value of 1 (1 QALY is accrued for each year lived with perfect health), while other health states may have a lower utility value. When the utility value for a health state is < 1, this suggests people would be willing to give up some life expectancy to get an improvement in QoL.
Baseline utility
It is usually inappropriate to assume that individuals are in perfect health if they do not have the health condition being modelled. A large proportion of the adult population has a longstanding illness (45% of women and 40% of men), and 1 in 13 describe themselves as having bad or very bad general health. 195
We used a statistical model to predict baseline utility (utility when individuals have none of the conditions modelled) based on age and sex, which was published by Ara and Brazier (2010). 196
Combining health state utility values
It is very rare to have estimates for the utility values for joint health states, that is when an individual has multiple diseases.
We estimate the utility value (QALY weight) attached to joint health states using the linear combination method described by Basu et al. (2009)197 We incorporate population norms as an estimate of baseline health, which is dependent on age and sex, U0(age,sex). We can model up to four different conditions affecting an individual’s HRQoL at once, which could include:
-
CRC
-
OC
-
EC
-
surgical menopause caused by risk-reducing surgery including bilateral oophorectomy.
If none of the conditions are present, then the utility is simply the baseline utility. If there is one condition, then we apply the method of Basu et al. (2009)197 by treating the difference between baseline utility and perfect health (1) as a second health condition. If there are two or more conditions, then we apply the method of Basu et al. (2009)197 by taking the two conditions with the biggest effect on utility and subtracting the calculated joint state utility loss from the baseline utility.
Ovarian cancer
Few studies included patients with OC across multiple stages of disease, and these are most useful for modelling interventions such as surveillance.
Gordon et al. (2010)113 reported the results of a nested cohort study of women with primary epithelial OC referred for chemotherapy in Australia. Measurements including QoL (SF-36) were requested at least every 3 months for at least 2 years. SF-6D health states were derived from SF-36 responses and valued using a UK time trade-off tariff.
Naik et al. (2017)105 reported the results of a cross-sectional study of ambulatory cancer survivors in Canada. QoL was measured using the EQ-5D-3L instrument and was valued with preference weights from Canada, the UK and the USA.
Havrilesky et al. (2009)115 developed vignettes relating to OC and obtained valuations for these health states from volunteers using the time trade-off method. Of the 50 participants, 13 had been diagnosed with OC and were only presented with health states relating to chemotherapy toxicity.
The findings of these studies are summarised in Table 22.
| Health state | Participants (N) | Mean (median) (SE) | SD (IQR) (range) |
|---|---|---|---|
| Havrilesky et al . 2009 115 | |||
| Early OC (newly diagnosed) | 16 | 0.81 (0.83) | 0.26 (0.03–0.97) |
| Advanced OC (newly diagnosed) | 14 | 0.55 (0.50) | 0.29 (0.03–0.93) |
| OC – clinical remission | 16 | 0.83 (0.95) | 0.25 (0.03–0.97) |
| Recurrent OC | |||
| Responding to chemotherapy, grade 3–4 toxicity | 14 | 0.61 (0.67) | 0.24 (0.17–0.97) |
| Responding to chemotherapy, grade 1–2 toxicity | 15 | 0.50 (0.50) | 0.34 (0.03–0.93) |
| Progressive, grade 3–4 toxicity | 15 | 0.47 (0.50) | 0.34 (0.03–0.93) |
| Progressive, grade 1–2 toxicity | 16 | 0.40 (0.42) | 0.33 (0.03–0.93) |
| Gordon et al . 2010 113 | |||
| Stage I/II | 13 | 0.74 | 0.11 |
| Stage III | 63 | 0.68 | 0.09 |
| Stage IV | 9 | 0.69 | 0.08 |
| Naik et al . 2017 105 | |||
| Canadian tariff | 85 | 0.79 (0.02) | |
| UK tariff | 85 | 0.76 (0.02) | |
| Stage I/II (Canadian tariff) | 59 | 0.80 (0.02) | |
| Stage III/IV (Canadian tariff) | 25 | 0.78 (0.03) | |
We considered that the estimates from Havrilesky et al. 115 were potentially at ROB due to the use of vignettes. Comparing the results from Gordon et al. (2010)113 and Naik et al. (2017),105 it appears possible that the EQ-5D-3L used by Naik et al. (2017)105 is less sensitive than the SF-6D used by Gordon et al. (2010)113 to detect differences in QoL between early and advanced OC. 105,113 None of the studies included a control group without OC.
We decided that the expected utility for stage I/II OC would be 0.78 (broadly consistent with all three studies) and that expected utility for stage III/IV OC would be 0.72 (i.e. a reduction in utility of 0.06 from stage I/II cancer). We assumed that in the absence of cancer, utility would be 0.80 (roughly the UK population norm for women aged 60–65 years).
These estimates were represented as:
Note that because of the choice of variance, these gamma distributions are in fact exponential distributions.
We assumed that the utility in the event of recurrence would be the same as the utility for advanced OC.
Endometrial cancer
Only one study (Hildebrandt et al. 2014)99 was identified which directly compared the utility for EC according to stage of disease. The authors measured health utility in cancer patients with the EQ-5D-3L and valued health states with a German time trade-off tariff. 198 Of these, nine had ‘primary’ EC and 11 had advanced EC. The median utility was 0.999 for those with primary EC and 0.887 for those with advanced EC. A set of 62 controls (without cancer) were also present and their median utility was 0.9995. Also of note was that on a VAS, there was a potentially meaningful difference in HRQoL between healthy controls and those with primary EC – median VAS (0–100) was 90 for healthy controls and 60 for those with primary EC.
We modelled a utility decrement of 0.02 for stage I/II EC and a utility decrement of 0.11 from stage I/II to stage III/IV. We assumed that the utility in the event of recurrence would be the same as the utility for advanced EC.
Colorectal cancer
We adopted the same mean utility multipliers for CRC as Thomas et al. (2021)161 in the MiMiC-Bowel model, derived from a systematic review and meta-analysis by Djalalov et al. (2014). 199 These utility multipliers are determined by stage at diagnosis (I–III vs. IV) and time since diagnosis (≤ 1 year, > 1 year). In contrast to Thomas et al. (2021),161 we used a beta distribution to represent uncertainty rather than a normal distribution, to avoid the clinically implausible part of the normal distribution in which CRC improves HRQoL.
Cancer surveillance
Although it is acknowledged that cancer surveillance (colonoscopy and gynaecological cancer surveillance) can be painful or uncomfortable, these health states are also very short-lived and only experienced on an annual or biennial basis, so any estimate of utility values to apply for these surveillance procedures would have an infinitesimally small impact on lifetime QALYs.
Further research is needed to determine whether an alternative to QALY calculations is appropriate for transitory health states.
Risk-reducing surgery
No studies directly measured preference-based HRQoL among women with LS undergoing risk-reducing HBSO (see Systematic review of utility values). We therefore consider the best quality evidence among women with LS and the best quality evidence among women with benign gynaecological conditions.
Sun et al. (2019)28 elicited preferences for risk management strategies among women with LS using vignettes and a method derived from the standard gamble. Unfortunately, instead of the anchor points in the standard gamble being ‘perfect health’ and ‘death’ (with QALY weights of 1 and 0), they were instead ‘no cancer in lifetime’ and ‘develop cancer in lifetime’, therefore the preference scores which were elicited can neither be used as nor adapted into QALY weights. Nevertheless, the findings of the study do show that women with LS have a preference towards postmenopausal RRS rather than premenopausal RRS, as the corresponding lifetime risks of cancer at which participants would consider each were 60% and 40% (median across participants).
Kuppermann, Wang et al. (2013)88 elicited utility values for health states related to LS. The population included 35 women recruited from a general medical clinic and eight women recruited from the University of California San Francisco Gastrointestinal Cancer Prevention Program, who had all undergone genetic testing but may or may not have LS. Vignettes were used to describe health states and the time trade-off method was used to value the health states. The authors found that utility values were higher for the health states in which RRS was taken up than the corresponding health states in which RRS was forgone. However, the vignettes do not include any description of physical consequences of RRS, focusing on the effect on cancer risk and alluding to possible psychological consequences.
Kuppermann, Learman et al. (2013)139 directly measured longitudinal preference-based HRQoL in women with non-cancerous pelvic problems. Of the 1491 women enrolled, 205 underwent hysterectomy (two women also had BSO). Preference-based HRQoL was elicited using the time trade-off method. The authors found that the utility value measured after hysterectomy was 0.1 higher than the utility value measured before hysterectomy. Seven years after hysterectomy utility had then decreased by 0.04 but this may be consistent with ageing; the corresponding loss of utility was 0.13 for women undergoing uterus-preserving surgery. The mean utility at the most recent observation was, however, very similar regardless of the management (hysterectomy, uterus-preserving surgery or no surgery).
Garry et al. (2004)136 reported preference-based HRQoL of participants in two RCTs of open compared with laparoscopic-assisted hysterectomy (vaginal and abdominal), which was measured using the EQ-5D-3L and valued using the standard UK time trade-off tariff. The reported distribution of age suggests that the majority of participants would have been premenopausal, although there is no explicit reporting of menopausal status. Of women undergoing abdominal hysterectomy, 45% (376/837) also had bilateral oophorectomy; the corresponding figure for vaginal hysterectomy was 22% (105/476). The mean utility 1 year after abdominal hysterectomy was 0.895, while for vaginal hysterectomy it was 0.919.
If we make the strong assumption that the difference in utility at 1 year between the four arms in the study by Garry et al. (2004)136 are entirely caused by the differences in baseline utility and bilateral oophorectomy rates, a simple linear regression suggests that expected utility without bilateral oophorectomy is 0.9235 while expected utility with bilateral oophorectomy is 0.8700.
We therefore modelled the effect of risk-reducing premenopausal HBSO as a predicted multiplicative effect of (U1/U0)~Beta(94.2,5.8), with U0=0.9235. This means the expected utility was 0.870 but would fall in the range 0.821–0.903 in 95% of simulations. We assumed the effect would last until age 50.
We modelled risk-reducing postmenopausal HBSO, and risk-reducing surgery without removal of both ovaries, as having no effect on utility value.
Validation
Each component of the model was tested as it was written. Some components had specific unit tests written. We tested the implementation of parametric distributions by drawing 500 parameter sets and performing graphical inspection and comparison of moments (mean and variance) to their expected values.
Chapter 7 Economic evaluation of strategies to reduce the risk of gynaecological cancer in Lynch syndrome
We used the whole-disease model developed and described in the previous chapter to conduct an economic evaluation of strategies to reduce the risk of gynaecological cancer in LS.
Evaluation characteristics
Population
We simulated a population of women LS with no known gynaecological cancer. The population was divided equally by genotype, as well as by age (30, 35, 40, 45 years). These combinations led to 16 separate groups; 1000 women were simulated in each group.
Interventions
The following strategies were considered:
-
no interventions to reduce the risk of gynaecological cancer (i.e. no surveillance or RRS)
-
only RRS offered
-
only surveillance offered
-
surveillance and RRS offered.
Outcomes
The primary economic outcomes were lifetime discounted costs and QALYs for each patient and averaged across the population and the relevant subgroups.
We estimated the following secondary outcomes:
-
life expectancy
-
ECs
-
EC deaths
-
OCs
-
OC deaths.
We also timed how long the simulations took.
Handling uncertainty
The simulations were each repeated 500 times, each time sampling a new set of parameter values for all parameters subject to uncertainty in the model.
Setting and country
Hospital and tertiary care, UK.
Currency and price year
Great British pounds, 2021–2.
Perspective on costs
NHS and Personal Social Services.
Time horizon
Each individual was simulated to death or 100 years of age, whichever comes first.
Discounting
Future costs, life-years and QALYs are discounted at 3.5% per year.
Results
The average per-patient costs and QALYs are shown in Table 23, together with incremental net monetary benefit calculations. Incremental net monetary benefit200 is a convenient alternative to the ICER frequently used in cost-effectiveness analyses; the strategy with the highest (incremental) net monetary benefit is the optimal strategy given the cost-effectiveness threshold. Incremental net monetary benefit has been calculated using £20,000 per QALY as the cost-effectiveness threshold.
| Population (years of age) | Costs (£) | QALYs | INMB vs. nothing (at £20k/QALY) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nothing | RRGS | Surv | RRGS and Surv | Nothing | RRGS | Surv | RRGS and Surv | RRGS | Surv | RRGS and Surv | |
| path_MLH1 | |||||||||||
| 30 | 21,046 | 22,128 | 25,343 | 25,382 | 19.471 | 19.591 | 19.901 | 19.873 | 1314 | 4305 | 3711 |
| 35 | 22,272 | 23,578 | 25,915 | 25,990 | 18.284 | 18.449 | 18.783 | 18.746 | 1991 | 6335 | 5513 |
| 40 | 23,055 | 24,387 | 26,205 | 26,355 | 17.086 | 17.275 | 17.592 | 17.571 | 2450 | 6969 | 6397 |
| 45 | 23,452 | 24,880 | 26,212 | 26,512 | 15.840 | 16.051 | 16.328 | 16.333 | 2800 | 6997 | 6806 |
| path_MSH2 | |||||||||||
| 30 | 20,307 | 21,243 | 24,120 | 24,071 | 19.297 | 19.537 | 19.824 | 19.833 | 3875 | 6734 | 6965 |
| 35 | 21,420 | 22,548 | 24,678 | 24,542 | 18.129 | 18.421 | 18.712 | 18.715 | 4717 | 8402 | 8605 |
| 40 | 22,300 | 23,449 | 24,907 | 24,907 | 16.858 | 17.18 | 17.478 | 17.520 | 5308 | 9810 | 10638 |
| 45 | 22,710 | 24,043 | 25,081 | 25,219 | 15.702 | 16.012 | 16.282 | 16.323 | 4872 | 9234 | 9921 |
| path_MSH6 | |||||||||||
| 30 | 16,480 | 17,479 | 20,611 | 20,712 | 19.730 | 19.772 | 20.083 | 20.034 | −155 | 2940 | 1853 |
| 35 | 16,349 | 17,615 | 19,996 | 20,092 | 18.642 | 18.731 | 19.046 | 18.991 | 513 | 4426 | 3232 |
| 40 | 15,889 | 17,096 | 19,029 | 19,178 | 17.480 | 17.594 | 17.893 | 17.859 | 1068 | 5122 | 4293 |
| 45 | 15,182 | 16,465 | 17,904 | 18,233 | 16.261 | 16.408 | 16.662 | 16.655 | 1662 | 5305 | 4822 |
| path_PMS2 | |||||||||||
| 30 | 6671 | 8288 | 12,447 | 12,819 | 20.554 | 20.519 | 20.631 | 20.584 | −2326 | −4234 | −5552 |
| 35 | 6418 | 8326 | 11,812 | 12,244 | 19.493 | 19.449 | 19.572 | 19.513 | −2795 | −3822 | −5432 |
| 40 | 6219 | 8221 | 11,245 | 11,750 | 18.310 | 18.296 | 18.397 | 18.353 | −2277 | −3285 | −4658 |
| 45 | 5759 | 7761 | 10,368 | 10,978 | 17.038 | 17.039 | 17.105 | 17.084 | −1987 | −3278 | −4300 |
The results in this table are averaged over the 500 sampled parameter sets and the 1000 individuals per parameter set, that is each is the average over 500,000 simulated individuals.
We generally found that having no risk reduction was the least costly option, and only offering RRS increased costs a small amount. Higher costs were associated with the strategies including surveillance; generally, the highest costs were associated with the combined strategy but this was only marginally more expensive than the surveillance-only strategy.
For QALYs, the strategies leading to most benefit depended on the genotype. For path_MSH2, the combined strategy led to the most QALYs, while for the other genotypes surveillance alone usually led to the most QALYs. The only exception was for older path_MLH1 carriers (for whom the combined strategy gave marginally greater benefit than surveillance alone). Incremental QALY gains were highest for path_MSH2 carriers and lowest for path_PMS2 carriers.
When taking the effects on costs and QALYs together, we find that the economically optimal strategy is the combined strategy for path_MSH2 carriers, surveillance alone for path_MLH1 and path_MSH6 carriers, and no risk reduction for path_PMS2 carriers (i.e. for LS caused by path_PMS2, none of the intervention strategies were cost-effective). The incremental net monetary benefit was similar for surveillance alone and the combined strategy except for path_PMS2 carriers.
It is important to bear in mind that risk-reducing surgery is expected to be most effective in terms of reducing cancer risk, but that it does have an impact on HRQoL, resulting in some loss of QALYs. An important difference between the risk-reducing surgery-only strategy and the combined strategy is that when surveillance is offered the uptake of risk-reducing surgery is reduced.
Analyses of uncertainty
The results of the economic evaluation are subject to three main sources of uncertainty.
There is stochastic variability (Monte Carlo error), the extent to which results would be different if the experiment were to be conducted again with a different initial state for the pseudorandom number generator. This source of uncertainty can be reduced by increasing the number of simulations conducted, although to reduce this uncertainty by a factor of 10 requires increasing the number of simulations by a factor of 100.
There is also parameter uncertainty, the extent to which the results are uncertain because we do not have perfect information about the parameters of the model. If we could collect more or better data, we may be able to reduce the extent of parameter uncertainty. Parameter uncertainty is typically understood through cost-effectiveness acceptability curves, which calculate the probability any strategy is the economically optimal strategy by calculating the fraction of parameter set samples for which that strategy is economically optimal. These calculations are affected by Monte Carlo error in two ways: first, we may get somewhat different results if we resampled parameter sets; second, the calculation of which strategy is economically optimal is based on a finite simulation subject to Monte Carlo error as described above.
Finally, there is uncertainty about whether the model has been appropriately specified. All models are simplifications and involve assumptions about mechanisms which are frequently mathematically convenient rather than fully justified by biological or other evidence. The consequences of this source of evidence are typically very difficult to quantify.
We used cost-effectiveness acceptability curves to help understand the amount of decision uncertainty due to parameter uncertainty (Figure 12). These tell us that if the combined strategy or surveillance alone is adopted for women with LS caused by path_MLH1, path_MSH2 or path_MSH6, there is a low chance that this is a poor use of limited NHS resources compared to having no gynaecological cancer risk reduction. There is a risk that using risk-reducing surgery only for women with LS caused by path_MSH6 could be a worse use of NHS resources than offering no risk reduction, especially for younger patients. For LS caused by path_PMS2 it is likely that using any form of risk reduction for gynaecological cancer will be a poor use of NHS resources.
FIGURE 12.
Cost-effectiveness acceptability curves.
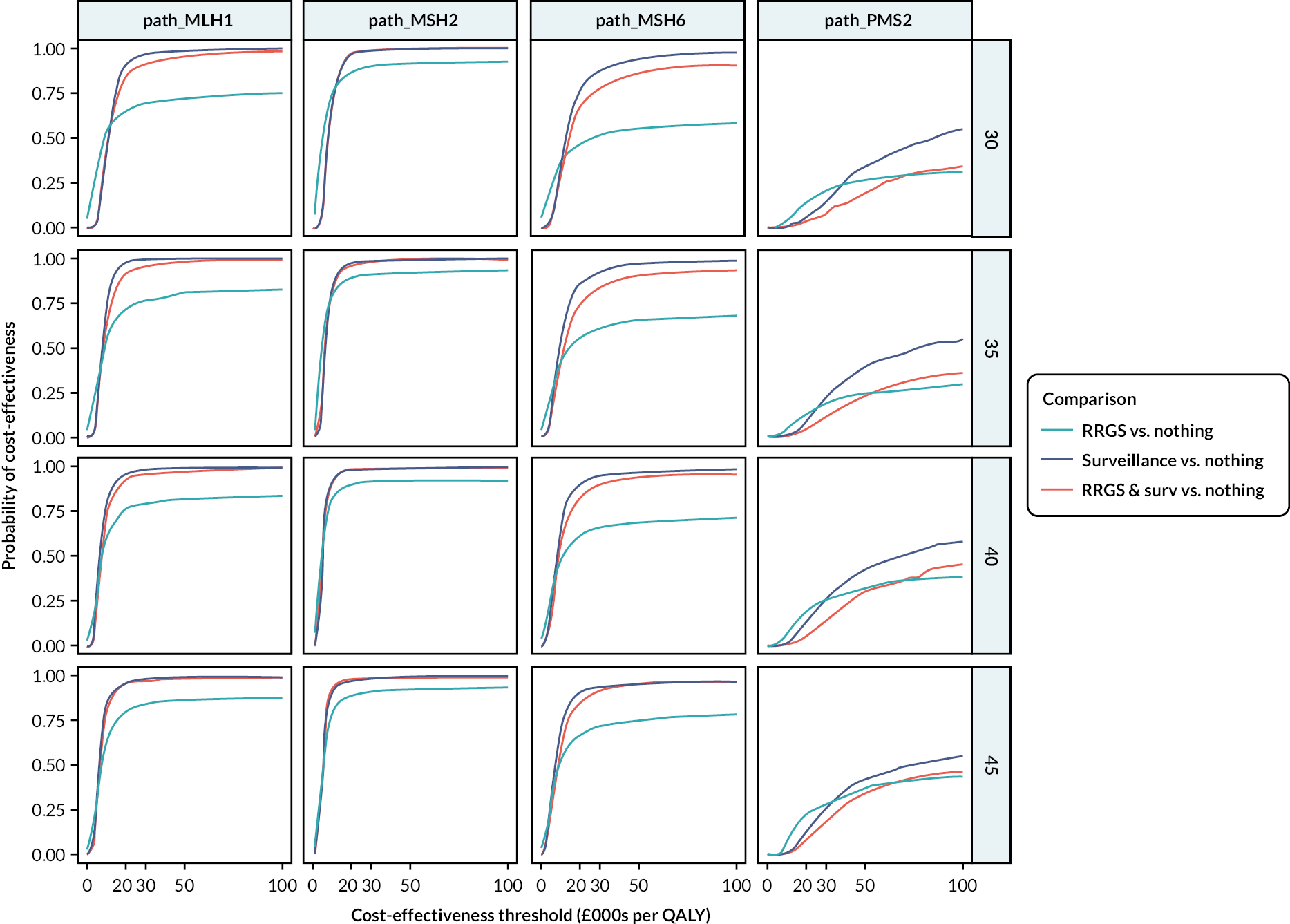
We present a number of analyses relating to uncertainty in Appendix 6 which are summarised below.
We visualised the Monte Carlo error in the overall study results and the variability of costs and QALYs across parameter sets in Figure 13, Appendix 6. These figures show that Monte Carlo variability is low, that is there is no suggestion that more simulations are needed in total, but that parameter uncertainty is leading to substantial uncertainty in costs and QALYs. There is a significant risk that relying on risk-reducing surgery alone could lead to a loss of QALYs, and for women with LS caused by path_PMS2 there is a substantial risk any risk reduction measures could lead to QALY loss.
We quantified the probability that the determination of which strategy is economically optimal is correct for a particular parameter set sample using bootstrapping (see Table 32, Appendix 6). These results suggest that for the comparisons of surveillance versus nothing and the combined strategy versus nothing the results should be robust, while for risk-reducing surgery alone versus nothing the conclusions around cost-effectiveness are not necessarily that robust. This may suggest that simulating more individuals per parameter set would improve cost-effectiveness acceptability curve calculation for risk-reducing surgery.
Secondary outcomes
In Appendix 6 we provide detailed summaries of the secondary outcomes measured in the model, which we summarise here.
The number of ECs diagnosed was lower with the strategies for gynaecological cancer risk reduction (see Figure 14, Appendix 6), although the difference was small for LS caused by path_PMS2 because the rates of EC were already comparatively low. The majority of the difference in EC incidence was in stage I cancer, which is not surprising since most ECs are diagnosed in stage I.
For OC the picture was somewhat more complex (see Figure 15, Appendix 6). As the rate of OC was indistinguishable from zero for path_MSH6 and path_PMS2 there was no effect from any risk reduction strategies. Strategies involving risk-reducing surgery lowered the incidence of OC in LS caused by path_MLH1 and path_MSH2, but surveillance alone had a less clear effect – there was an increase in stage I OCs but a decrease in later-stage OC, with a modest reduction in total OCs. Based on the model of OC this is not unexpected; there is no precursor lesion that can be detected and removed, so the best hope of surveillance is to identify OC in an earlier stage (which it is predicted to do). The small overall reduction in OC is probably caused by surveillance detecting endometrial lesions leading to gynaecological surgery including BSO.
The number of CRCs increased a certain amount with gynaecological cancer risk reduction (see Figure 16, Appendix 6). This can be explained by women living longer due to risk reduction and therefore being at risk for CRC for a longer time. This effect was most pronounced for path_MLH1 and path_MSH2 carriers.
Cancer deaths from colorectal and OC were mostly unaffected by the strategies (see Figure 17, Appendix 6), but gynaecological cancer risk reduction did lead to a reduction in EC mortality. The effect was very limited for LS caused by path_PMS2 but was substantial for the other genotypes. The combined strategy led to the greatest reduction in mortality.
The probability of remaining free from gynaecological cancer was affected by the interventions (see Figure 18, Appendix 6). The strategies with surveillance led to an immediate drop in cancer-free survival because some occult cancers are discovered upon the first surveillance visit. Across all genotypes and age groups, the cancer-free survival curve for no intervention was eventually lower than for the risk-reducing interventions, suggesting that some cancers are being prevented and not merely delayed. Cancer-free survival was highest for the combined strategy, and surveillance alone outperformed risk-reducing surgery alone. For path_PMS2 LS the overall risks of gynaecological cancer are relatively low so the effects of risk reduction are less significant.
The effect of interventions on life expectancy are shown in Figures 19 and 20, Appendix 6. These figures show that for LS caused by path_MLH1, path_MSH2 or path_MSH6 there are considerable survival benefits. The benefits are substantially lower for path_PMS2 carriers.
Value of information analyses
Value of information analyses can help to prioritise further research or development of an economic evaluation. The expected value of perfect information is an estimate of the upper bound on the value of conducting further research – it is an estimate of the value that can be obtained by reducing uncertainty in all parameters to zero. The expected value of perfect information was calculated for each population group and is shown in Table 24. These calculations suggest that there is considerable potential value in conducting further research, particularly for LS caused by path_MSH2 and when the patients are aged 45 years.
| Age (years) | Genotype | |||
|---|---|---|---|---|
| path_MLH1 | path_MSH2 | path_MSH6 | path_PMS2 | |
| 30 | 771 | 1037 | 649 | 365 |
| 35 | 794 | 1147 | 666 | 672 |
| 40 | 855 | 891 | 823 | 745 |
| 45 | 1091 | 857 | 986 | 818 |
Further analyses can be conducted by identifying parameter groups and using regression analyses to determine for which groups of parameters it would be most valuable to obtain perfect information. 201 We could use these methods to investigate the value of better understanding the disease natural history, the performance characteristics of surveillance or utility values.
Running time
The running of the model was separated into two stages: simulation and analysis. The simulation step focuses solely on simulating a population and the events that affect the individuals in that population. The analysis step takes the event listings and uses them to construct costs, QALYs and other outcomes. The stages were split into 80 subtasks; each subtask handled 100 parameter sets for a population subgroup. The simulation subtasks took a median 244 minutes (80% of tasks took between 193 and 335 minutes). The analysis subtasks took a median 224 minutes (80% took between 191 and 261 minutes). By running on a high-performance cluster allowing up to 50 subtasks to run simultaneously, all analyses were completed in 18 hours.
Chapter 8 Discussion
Principal findings
Systematic review of clinical effectiveness evidence
Evidence regarding the impact of gynaecological surveillance on clinical effectiveness outcomes for people with LS was sparse and methodologically limited. Limited data suggested that all-cause and cancer-specific mortality might be lower with RRS than with surveillance (Dueñas et al. 2020),38,39 and that all-cause mortality and OS might be better with surveillance than no surveillance (Stuckless et al. 2013). 66 These findings should be interpreted cautiously due to the paucity of data, small sample sizes and the likelihood of at least some bias due to confounding [which, for example, may have increased the rates of cancer, and therefore mortality, in the no surveillance group in Stuckless et al. (2013)]. 66
Extremely limited data from Woolderink et al. (2018) suggested that OCs detected with surveillance might be lower stage than those detected without surveillance. 69–71 Similar data were not available for EC. Across the included studies, there was no clear indication that cancer stage differed between EC/OC detected with surveillance and interval detected cancers. Although cancers detected during surgery were generally as low or lower stage than those detected with surveillance, the absolute number of cases was too low to draw conclusions.
One study found a trend, from baseline to 6 months of surveillance, towards a reduction in HADS depression and anxiety scores and an improvement in SF-36 QoL (Wood et al. 2008). 68 The single-arm design of the study precludes any conclusion that surveillance itself may be driving these findings. Similarly, there was no comparative data to elucidate whether surveillance had any impact upon treatment response or fertility. Although surveillance is used as a fertility-sparing option, one study indicated that some patients may prefer to avoid hysteroscopy due to fertility concerns (Elmasry et al. 2009). 41
The DTA of the surveillance techniques was rarely, and poorly, reported. There were insufficient data to draw any firm conclusions on the accuracy of any of the tests. EC and OC detection rates were generally low across all studies. A key study by Stuckless et al. (2013)66 reported lower rates of EC and OC with surveillance than with no surveillance (even when interval cancers were added to the surveillance group data, fewer ECs were detected among those receiving surveillance). The reasons for lower rates of EC detection with surveillance are unclear and may be due to confounding variables that were not considered (e.g. family cancer history).
Across the included studies, some asymptomatic ECs and OCs were detected with surveillance, albeit in low numbers. RRS can also detect (and treat) asymptomatic ECs and OCs. It should not be assumed that asymptomatic cancers would not be detected in a timely manner at a later point without surveillance (incidentally or once they become symptomatic). Prospective end-to-end studies following participants on surveillance and those in control groups through cancer detection, treatment and in the longer term are needed. It was also clear that rates of missed cancers (and premalignancies) were generally low, but that they do exist. Additionally, the detected interval cancers and premalignancies may not represent all of the cases missed by surveillance.
Most of the available data on harms was about patients’ experience of pain. The data suggested that Pipelle biopsy is experienced as moderately painful, although a wide range of pain experience was evident. 41,45,46,54–58,72 Based on limited data, it appears that premenopausal people may experience greater pain with biopsy than postmenopausal people,45,46,72 and that those who had previously experienced childbirth may experience less biopsy pain than those who had not. 45,46,54–58 One study reported that Pipelle biopsy and hysteroscopy were significantly more painful than TVUS (p < 0.01) but similarly painful to each other. 41
With regards to pain relief used during surveillance procedures, NSAID use before endometrial sampling appears to be common, and might increase due to previous pain experience. 45,46 Extremely little information was found regarding GA use – a single survey reported that 13.6% of individuals who had undergone a hysteroscopy had needed a GA, but it was unclear how this related to patient requests for GA. 75
There was some indication that patient concerns about pain related to Pipelle biopsy might influence individuals’ decisions to undergo/stop the procedure,41,45,46,72 or opt for risk-reducing surgery. 43–46 In Elmasry et al. (2009),41 there were seven participants who either declined to take part in the study, or in hysteroscopy, due to concerns about the impact of hysteroscopy on infections and fertility or due to pain and anxiety. In this study, the majority of participants stated that, due to procedural pain with hysteroscopy, TVUS would be preferential if all techniques were equally effective. 41 Unfortunately, the data identified in this review were too sparse (few cases of cancer were detected) to evaluate whether certain techniques were more likely to detect cancers than others.
Systematic review of cost-effectiveness evidence
All studies concluded that RRS was the economically optimal strategy to manage the risk of gynaecological cancer in LS. No studies found that gynaecological surveillance was cost-effective when risk-reducing strategies were included as options or investigated the cost-effectiveness of surveillance in people unable to undergo RRS. We were able to estimate the cost-effectiveness of surveillance compared with no risk reduction from figures published by Kwon et al. (2008)79 and Wright et al. (2021):81 surveillance would not be found to be cost-effective by Kwon et al. (2008)79 but would be cost-effective for Wright et al. (2021)81 except for patients with path_PMS2.
All studies made some assumptions which are unreasonable – for example, Wright patients (2021)81 estimated survival for gynaecological cancer from the general population rather than from patients with LS, which could lead to a substantial overestimate of the value of risk reduction strategies.
No studies have been conducted in the UK setting; all three studies were conducted in the US setting.
Overall, this review supports the need for an economic evaluation, conducted in the UK setting and building and improving upon the methods used in existing publications.
Systematic review of utility values
There is considerable published literature on utility values for endometrial and OC, as well as for surgery for benign gynaecological conditions. Utility values have been elicited for risk reduction for hereditary breast OC and for LS, but these studies tend to be small and suffer from poor reporting or suboptimal methodology.
Studies generally did not use control groups, which would have allowed a better estimation of the effects of the conditions and interventions on utility. Few studies included cancer patients across a broad range of stages and reported utility separately, but where they did, a trend was generally observed of worsening utility with advancing stage.
The majority of studies obtained utility estimates from patients reporting their own health, either via the use of a generic HRQoL instrument with an established value set (such as EQ-5D), a condition-specific QoL instrument with known mappings to utility values (such as FACT-O), or directly eliciting health utility using standard gamble or time trade-off. Some studies instead asked participants to value hypothetical health states, typically through the use of vignettes; patients typically were included in these studies as participants but also members of the public, and sometimes people with clinical expertise. All utility estimates directly related to LS were for hypothetical health states.
A number of studies utilised the converted FACT-O to ascertain utilities, and the maping method was seen to impact the resulting utility score with the Dobrez algorithm yielding the greatest utility values. 91,118
Considering gynaecological surveillance, individuals with experience of LS preferred a biennial schedule of gynaecological surveillance (endometrial biopsy, TVUS and CA-125) to an annual schedule. 28 One study found, unexpectedly, that utility values could be higher from a false positive screening result than from a screening test with a negative result,115 which may suggest that the methodology employed to elicit utilities is flawed: participants were asked to imagine living in a health state for 30 years which generally lasts 2 weeks – in the ‘negative screen’ health state patients are waiting for their result and anxious, while in the ‘false positive screen’ state patients undergo removal of an ovary, which may relieve their anxiety. Challenges in obtaining valuations for temporary health states have been noted in the literature, with no gold standard method yet identified,202,203 and it is possible that the value attached to avoiding undesirable health in an acute setting may be disproportionate to the chronic setting204 in a violation of the assumption of proportionality in QALY calculations. 205 We have not specifically sought studies investigating the willingness to pay for surveillance modalities, and this may be an alternative avenue of research to estimating QALY weights.
Although not included in the review of utilities, as the methodology was deemed too distant from health utility valuation, Sakala et al. (2018)206 used the wait-time trade-off method to investigate preferences between TVUS and MRI for investigation of pelvic symptoms and found TVUS was slightly preferred to MRI, although this has limited relevance as MRI has not been proposed as a method for gynaecological surveillance in LS.
Regarding risk-reducing gynaecological surgery, only two studies attempted to elicit utilities in LS,28,88 and both of these had significant methodological deficiencies. Sun et al. 28 failed to specify the anchors for their modified standard gamble in a way that allows calculation of QALY weights, while the vignettes used by Kuppermann et al. (2013)88 do not describe any physical consequences of risk-reducing surgery and focus significant attention on the potential for surgery to relieve anxiety.
As a research team (including clinical experts and patient representatives), we have discussed what may be the most appropriate proxies for utility following risk-reducing surgery for LS. It was argued that in studies of hysterectomy for benign gynaecological conditions one would expect HRQoL to improve, as the purpose of the surgery is to resolve a physical health condition which is worsening QoL. This improvement would not be seen with RRS as no physical condition is being resolved, although it is possible that RRS would affect mental well-being. It was also noted that not all benign gynaecological conditions will be comparable; continued symptoms following hysterectomy are not uncommon if performed for endometriosis, or pain (e.g. pelvic pain, dyspareunia), while symptoms are generally improved or resolved for bleeding, prolapse, fibroids and myomas, and failed ablation. Most surgery for benign gynaecological conditions does not involve removal of both ovaries (which is generally a constituent of RRS for LS) unless women are near or beyond menopause. In premenopausal women, in particular, the removal of ovaries can have a significant impact on HRQoL.
The long-term utility impact of RRS is an important research topic deserving of more attention than it has so far received.
In the short term, utility is observed to drop immediately in the days and weeks following gynaecological surgery as patients undergo GA and enter a recovery phase. In studies of benign gynaecological conditions, QoL recovers and utility surpasses that of the pre-operative state around 1–3 months post surgery, but for people undergoing risk-reducing surgery it is not expected that their QoL will surpass their preoperative state, indeed it may be worse. The hope is that the RRS will prevent future cancer and its effects on QoL and life expectancy.
Economic evaluation of strategies to reduce the risk of gynaecological cancer in Lynch syndrome
We have described the first whole disease model for LS. While certain aspects of the model are incomplete (e.g. relating to case finding of LS), the model is built in such a way that implementing these does not require substantial alterations to existing code. The model can compare competing options which include different sets of interventions at different parts of the disease pathway. The model is open source, which means it is free for anybody to study, use and adapt. There is also a guideline for contributions so that these uses and adaptations, as well as general improvements, can all be in one place.
We have used this whole disease model for LS to investigate the cost-effectiveness of strategies to reduce the risk of gynaecological cancer in LS. We found that gynaecological risk reduction strategies are effective and cost-effective except for LS caused by path_PMS2. While offering surveillance (alone or in combination with RRS being offered) is expected to be more costly than RRS alone, it is expected to lead to greater health benefits and be cost-effective. Surveillance alone and the combination strategy are generally expected to lead to similar costs and QALYs. There is a risk, if RRS alone is offered, that the loss of HRQoL due to surgical menopause will outweigh the benefits in terms of risk reduction.
It is important to note that, unlike other economic evaluations, we have modelled offering RRS, rather than assuming full uptake and compliance. In fact, only a small minority of patients are assumed to immediately take up RRS, and some patients are assumed never to take it up. We assume that uptake and compliance with surveillance is 100%, which may be an overestimate. The results may have been substantially different if we instead assumed all individuals immediately underwent RRS at a particular age.
There is significant potential value in reducing the uncertainty around parameters in the model and these suggest many avenues for future research.
While formal analysis using the partial expected value of perfect information will tell us which parameters it may be most fruitful to research further, it is already clear that there is substantial uncertainty around the potential gains in quality-adjusted life-years from risk-reducing interventions, and parameters relating to this (including disease natural history and utility parameters) are very likely to have high partial expected value of perfect information.
Our findings suggest that women with path_PMS2 LS stand to benefit little from gynaecological cancer risk reduction interventions because gynaecological cancer incidence is comparatively low, and gynaecological cancer survival is good in LS (assuming there is no reason why survival should be affected by the specific LS genotype).
Relation to existing work
At the time the systematic review of clinical effectiveness was conducted, the authors were aware of two existing related systematic reviews. 207,208 Auranen et al. (2011)207 published a systematic review of gynaecological surveillance in LS over 10 years ago, while Helder-Woolderink et al. (2016)208 published a systematic review of OC in LS, including the effectiveness of surveillance on OC outcomes.
After our review was conducted, but prior to monograph submission, Lim et al. (2022) 209 published a systematic review of surveillance and risk-reducing surgery for gynaecological cancer in LS. Our review has included more studies, has considered a much broader range of outcomes and has rigorously and systematically appraised the ROB of included studies, but on the common outcomes we have reached broadly similar conclusions.
Existing cost-effectiveness analyses79–81 have concluded that risk-reducing surgery is clearly cost-effective, generally leading to a substantial gain in QALYs while lowering or only slightly increasing costs, and that surveillance will not be cost-effective if RRS is an option. Those analyses did not consider the possibility of RRS not being an option, but if it were removed, those studies would have produced a mixed picture for the cost-effectiveness of surveillance alone.
Although existing model-based economic evaluations have included many of the sequelae of LS (i.e. CRC, EC and OC),9,160,210 they were created to answer a single decision problem at a single part of the disease pathway.
Ours is not the first model in LS to be open source/open access,81,172 but we hope that the broad scope of this model will mean it is taken up and used to answer further research questions.
Strengths and limitations
Systematic review of clinical effectiveness evidence
The review was conducted in line with a prespecified and preregistered protocol, and any deviations from the protocol have been documented. It has benefited from PPI to ensure that, where possible, it answers questions which are important to patients as well as to clinicians. The search strategy was comprehensive and designed by an information specialist. The review included a thorough assessment of the ROB of included studies.
The primary limitation of the review was the inability to draw firm conclusions due to the lack of high-quality evidence identified.
Systematic reviews of cost-effectiveness evidence and utility values
These reviews were conducted in a rigorous and systematic manner, including comprehensive searching of multiple appropriate bibliographic databases. All studies were quality appraised.
Economic evaluation of strategies to reduce the risk of gynaecological cancer in Lynch syndrome
The analysis has a number of strengths. It has been developed by a researcher with a decade of experience of decision analytic modelling in LS. The underlying model for cancer development is more realistic than those in existing models. The model is open source, which means it is fully transparent and can also be used and adapted freely.
There are also some notable limitations. Certain phenomena have been omitted, such as complications arising from surveillance and RRS. We have also assumed that RRS has no impact on general mortality.
We have not assumed full uptake of risk-reducing gynaecological surgery. Although our assumptions in this area are in line with clinical experience and will be generally appropriate for inclusion in the whole disease model, it may have been more instructive in this economic evaluation to assume full uptake so that any differences in costs and QALYs can be clearly attributed.
Patient and public involvement
Patient and public involvement was key to the development of this project from the earliest stages. The importance of the research question (particularly regarding clinical effectiveness) was made clear during discussions between researchers, patients and their families at a LS UK conference.
Our research team included a PPI co-applicant, Mrs Smith, who is a trustee of the patient charity LS UK and a coauthor of this report. Mrs Smith reviewed and commented on the research proposal at the outline and full application stages, and consulted the LS UK Facebook group (which she moderated and had > 1900 members at the time) about their views on the proposed research. This consultation provoked over 200 responses, with many respondents saying they thought it was important women with LS received gynaecological surveillance as well as colorectal surveillance, and that their experience with the NHS had included limited awareness of the gynaecological risks in LS. Many had paid for surveillance privately as a result.
We ensured PPI throughout the project by the inclusion of three patient representatives on the project advisory group, one of whom was Mrs Smith and the other two were invited to join at the first PPI workshop for the project (Shelley Rossiter and Laurie Meister). We offered to provide mentorship and support to PPI contributors before and after meetings, addressing queries, language and documentation. Throughout the project, we adhered to the INVOLVE guidelines and reimbursed PPI members according to INVOLVE recommendations.
During the project, we held two PPI workshops with participants recruited via LSUK. The workshops were designed and delivered by study authors (KB, HC, TS, TMS). The first workshop took place on 3 November 2020 and was attended by eight women with direct experience of LS and/or gynaecological surveillance. The purposes of the workshop were for participants to share their personal experiences, to learn about the planned research, to comment on the outcomes that were being sought in the clinical effectiveness systematic review (how would they prioritise these? were any missing?) and to discuss whether any particular groups of people with LS might experience surveillance differently or derive different benefits from surveillance.
The key messages from the first workshop were that the participants were most interested to know about fertility outcomes, how accurate the tests were and what effect surveillance might have on cancer. Participants also wanted to know how frequently surveillance was carried out under GA; this was not an outcome that the researchers had considered prior to the workshop and it was incorporated into the outcomes sought in the systematic review. There was also a lot of discussion that women with LS may not get enough information about the consequences of risk-reducing surgery, for example the impact on general HRQoL and on outcomes relating to having sex. Participants thought that people with prior abdominal surgery (e.g. due to CRC) may affect the level of pain during gynaecological surveillance; at least one participant reported personal experience of this. Participants also thought that the experience of surveillance could be different depending on whether recipients had given birth by vaginal delivery.
A second workshop was held on 8 February 2022 and was attended by many of the participants of the first workshop. The purpose of the workshop was to share the findings of the systematic review of clinical effectiveness and to discuss the best way to communicate the complex findings to an audience of patients and their families, particularly with a view to presenting the findings at the LS UK annual conference subsequently held in April 2022. We also asked for suggestions of other ways in which the findings of the research could be disseminated to make sure they reached patients.
Equality, diversity and inclusion
Transgender
As previously noted (Significance for patients), gynaecological cancer can affect transgender men, who may feel excluded from research and clinical guidance directed at women. Also, transgender women will not be affected by gynaecological cancer. Our aim as a group was to ensure that our research was undertaken with appropriate awareness of these factors and that our use of language when reporting our research was not exclusionary.
Our data abstraction forms for the systematic review of clinical effectiveness evidence included columns for gender identity and sex (assigned at birth). Ultimately, gender identity was not described in any of the studies included in our review.
Following discussion with the project advisory group, we concluded that our preferred term in reporting would be ‘women’ rather than ‘females’ or ‘individuals with female reproductive organs’, but that we would note this as a deliberate choice and be clear that the research had relevance for transgender men.
Outstanding areas of uncertainty
Clinical effectiveness
It remains unclear how effective risk-reducing strategies for gynaecological cancer in LS are, versus each other and versus no risk reduction. It is unclear whether technologies used for surveillance are performant in this population and setting.
Utility values
It is unclear what the HRQoL actually is of people with LS receiving surveillance or undergoing RRS. Thus far studies have only elicited utility values for hypothetical states relating to surveillance and RRS.
Few studies of gynaecological surgery for benign conditions were good proxies for RRS, since bilateral oophorectomy was frequently not performed (probably to avoid surgical menopause).
On a philosophical level, it is uncertain how value should be attached to temporary health states, such as those relating to surveillance.
Chapter 9 Conclusions
Clinical effectiveness evidence for gynaecological cancer surveillance in LS is sparse and methodologically limited. There is some evidence that surveillance can prevent some deaths compared with no intervention, but there is also evidence that RRS prevents more deaths. Some asymptomatic cancers are detected by surveillance, but some cancers are also missed. Recipients of surveillance have a wide range of pain experiences.
In the absence of compelling clinical effectiveness evidence, modelling can be helpful. Our work includes models of endometrial and OC and how these can be affected by surveillance and RRS. We predict that these interventions can reduce the risk of cancer and cancer mortality, but this must be weighed against the potential negative consequences: increased costs and decreased HRQoL if RRS occurs before natural menopause.
For women with LS caused by path_PMS2, we predict that strategies currently proposed to reduce the risk of gynaecological cancer are unlikely to be cost-effective and could lead to net harm. For women with LS of other genotypes, we predict that offering surveillance is cost-effective compared with no intervention, and cost-effective compared with offering RRS (which many women will not take up immediately or in the short term).
Implications for health care
People with LS should be informed that gynaecological cancer surveillance is not expected to reduce the risk of gynaecological cancer and cancer death to the same extent as RRS. There is some evidence that surveillance could be beneficial versus no risk reduction (e.g. some asymptomatic cancers detected), but there is also evidence that some cancers are missed and that some individuals find surveillance severely painful. The prognosis from endometrial and OC appears to be better for people with LS than for unselected patients.
Gynaecological cancer surveillance is estimated to cost the NHS over £300 per year per patient, while RRS is estimated to cost over £6000. Only offering women RRS may not be the best use of NHS resources and RRS may adversely affect HRQoL.
Recommendations for research
There have been 30 studies of gynaecological surveillance in LS published, yet they have not been able to definitively answer whether surveillance is effective because of methodological weaknesses. More research of the same quality will not advance knowledge any further.
We recommend that any future research should be designed and conducted with the involvement of a biostatistician or epidemiologist or other methodological expert to minimise the ROB.
It may be possible for researchers to collaborate and pool existing research data in order to answer questions surrounding gynaecological surveillance; this has already helped to increase understanding of the effectiveness of colonoscopy on CRC in LS. 158 Again, this should be accompanied with suitable methodological expertise.
The current evidence base does not preclude an argument of equipoise, and though a RCT would need to be large and run for a considerable period (we have previously estimated an RCT would need to recruit over 1300 participants and follow them up for 10 years), it would offer high-quality evidence which is currently lacking. It would need to be carefully designed to avoid bias, for example because many participants would choose to undergo risk-reducing surgery during follow-up.
More in-depth value of information analyses should be conducted to identify which parameters or groups of parameters are most critical to research further.
Health utilities should be directly elicited from individuals with LS to identify the potential effects of surveillance and RRS on HRQoL and QALYs; relatedly, it may be beneficial to consider whether willingness to pay is a better indication of the value of undergoing or avoiding surveillance.
Additional information
Contributions of authors
Tristan M Snowsill (https://orcid.org/0000-0001-7406-2819) (Senior Lecturer, Health Economics) was the chief investigator and lead for health economics; he conceptualised, designed and implemented the whole-disease model and conducted the economic evaluation; he contributed to the writing of the report.
Helen Coelho (https://orcid.org/0000-0002-4799-4300) (Postdoctoral Research Fellow, Systematic Review) was the lead for the systematic review of effectiveness; she contributed to the writing of the report.
Nia G Morrish (https://orcid.org/0000-0002-7206-4957) (Postdoctoral Research Associate, Health Economics) contributed to the systematic review of effectiveness, the systematic review of cost-effectiveness studies and the systematic review of utility values; she contributed to the writing of the report.
Simon Briscoe (https://orcid.org/0000-0002-6982-4521) (Research Fellow, Information Specialist/Systematic Review) was the information specialist and contributed to the systematic review of effectiveness; he designed and executed literature searches; he contributed to the writing of the report.
Kate Boddy (https://orcid.org/0000-0001-9135-5488) (Research Fellow, Patient and Public Involvement) was the lead for patient and public involvement; she developed, organised and led the patient and public involvement workshops.
Tracy Smith (Patient Representative) was the principal patient representative.
Emma J Crosbie (https://orcid.org/0000-0003-0284-8630) (Consultant, Gynaecological Oncology) was an expert clinical advisor.
Neil AJ Ryan (https://orcid.org/0000-0003-3117-3257) (Registrar, Gynaecological Oncology) was an expert clinical advisor.
Fiona Lalloo (https://orcid.org/0000-0002-0612-8377) (Consultant, Clinical Genetics) was an expert clinical advisor and member of the Project Advisory Group.
Claire T Hulme (https://orcid.org/0000-0003-2077-0419) (Professor, Health Economics) was an expert methodological advisor and chair of the project advisory group.
Acknowledgements
We acknowledge the invaluable administrative assistance of Leala Watson (Health Economics Group, University of Exeter), including collating this report and reference management.
The authors would like to acknowledge the use of the University of Exeter high-performance computing facility in carrying out this work.
We acknowledge the assistance of Georgia Jenkins and Tanya Hynd in supporting the patient and public involvement in this project.
Project advisory group
Chair: Prof Claire Hulme (University of Exeter).
Members: Prof Emma Crosbie, Dr Fiona Lalloo, Tracy Smith, Laurie Meister, Shelley Rossiter.
Patient and public involvement workshops
We are grateful to the attendees of two patient and public involvement workshops which greatly informed the conduct and reporting of the research.
Data-sharing statement
Systematic reviews
Requests for access to systematic review data should be addressed to the corresponding author.
Whole disease model and economic evaluation
The source code for the whole-disease model and economic evaluation is publicly available (open source) at GitHub: https://github.com/tristansnowsill/lynchsyndrome.
The economic evaluation simulation results are publicly available on Figshare at https://doi.org/10.6084/m9.figshare.20496654.
Ethics statement
This study was granted exemption from ethical approval by the Chair of the University of Exeter College of Medicine and Health Research Ethics Committee (CA381, 27 February 2020).
Information governance statement
No personal data were handled during this project.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/VBXX6307.
Primary conflicts of interest: Tracy Smith is a trustee of the patient charity Lynch Syndrome UK. Neil AJ Ryan is a National Institute for Health and Care Excellence Clinical Fellow currently working on the familial ovarian cancer guideline. He is a trainee representative on the Royal College of Obstetricians and Gynaecologists Genomics Task Force; he is the treasurer of the Blair Bell Society and has been awarded an Academy of Medical Sciences clinical lecturer grant. Claire T Hulme declares membership of the NIHR HTA Commissioning Committee (January 2012–February 2017). All other authors report no potential conflicts of interest.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Win AK, Jenkins MA, Dowty JG, Antoniou AC, Lee A, Giles GG, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev 2017;26:404-12. https://doi.org/10.1158/1055-9965.Epi-16-0693.
- Dominguez-Valentin M, Sampson JR, Seppälä TT, Ten Broeke SW, Plazzer JP, Nakken S, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med 2020;22:15-2. https://doi.org/10.1038/s41436-019-0596-9.
- Watson P, Bützow R, Lynch HT, Mecklin JP, Järvinen HJ, Vasen HF, et al. International Collaborative Group on HNPCC . The clinical features of ovarian cancer in hereditary nonpolyposis colorectal cancer. Gynecol Oncol 2001;82:223-8. https://doi.org/10.1006/gyno.2001.6279.
- Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, et al. CAPP2 Investigators . Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011;378:2081-7. https://doi.org/10.1016/s0140-6736(11)61049-0.
- Järvinen HJ, Aarnio M, Mustonen H, Aktan–Collan K, Aaltonen LA, Peltomäki P, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000;118:829-34. https://doi.org/10.1016/s0016-5085(00)70168-5.
- Crosbie EJ, Ryan NAJ, Arends MJ, Bosse T, Burn J, Cornes JM, et al. Manchester International Consensus Group . The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet Med 2019;21:2390-400. https://doi.org/10.1038/s41436-019-0489-y.
- Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med 2006;354:261-9. https://doi.org/10.1056/NEJMoa052627.
- Møller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Mallorca Group . Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut 2017;66:464-72. https://doi.org/10.1136/gutjnl-2015-309675.
- Snowsill T, Coelho H, Huxley N, Jones-Hughes T, Briscoe S, Frayling IM, et al. Molecular testing for Lynch syndrome in people with colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess 2017;21:1-238. https://doi.org/10.3310/hta21510.
- Snowsill T, Huxley N, Hoyle M, Jones-Hughes T, Coelho H, Cooper C, et al. A systematic review and economic evaluation of diagnostic strategies for Lynch syndrome. Health Technol Assess 2014;18:1-406. https://doi.org/10.3310/hta18580.
- Bonis PA, Trikalinos TA, Chung M, Chew P, Ip S, DeVine DA, et al. Hereditary nonpolyposis colorectal cancer: diagnostic strategies and their implications. Evid Rep Technol Assess (Full Rep) 2007;1.
- Shia J, Klimstra DS, Nafa K, Offit K, Guillem JG, Markowitz AJ, et al. Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J Surg Pathol 2005;29:96-104. https://doi.org/10.1097/01.pas.0000146009.85309.3b.
- Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261-8. https://doi.org/10.1093/jnci/djh034.
- Bonadona V, Bonaïti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. French Cancer Genetics Network . Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011;305:2304-10. https://doi.org/10.1001/jama.2011.743.
- Office for National Statistics . Cancer Registration Statistics, England: 2016 2018. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/final2016 (accessed 16 April 2019).
- Vasen HF, Möslein G, Alonso A, Bernstein I, Bertario L, Blanco I, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer). J Med Genet 2007;44:353-62. https://doi.org/10.1136/jmg.2007.048991.
- National Institute for Health and Care Excellence (NICE) . Molecular Testing Strategies for Lynch Syndrome in People With Colorectal Cancer (DG27) 2017. www.nice.org.uk/guidance/dg27 (accessed 11 September 2019).
- National Institute for Health and Care Excellence (NICE) . Testing Strategies for Lynch Syndrome in People With Endometrial Cancer (DG42) 2020. www.nice.org.uk/guidance/dg42/ (accessed 11 September 2019).
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999;116:1453-6. https://doi.org/10.1016/s0016-5085(99)70510-x.
- Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, et al. Mallorca Group . Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut 2013;62:812-23. https://doi.org/10.1136/gutjnl-2012-304356.
- Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol 2008;26:5783-8. https://doi.org/10.1200/jco.2008.17.5950.
- Ryan NAJ, McMahon R, Tobi S, Snowsill T, Esquibel S, Wallace AJ, et al. The proportion of endometrial tumours associated with Lynch syndrome (PETALS): a prospective cross-sectional study. PLOS Med 2020;17. https://doi.org/10.1371/journal.pmed.1003263.
- Office for National Statistics . Cancer Registration Statistics, England, 2017 2019. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/2017 (accessed 30 November 2021).
- National Health Service England . The NHS Long Term Plan 2019. www.longtermplan.nhs.uk (accessed 2 May 2019).
- Dashti SG, Chau R, Ouakrim DA, Buchanan DD, Clendenning M, Young JP, et al. Female hormonal factors and the risk of endometrial cancer in Lynch syndrome. JAMA 2015;314:61-7. https://doi.org/10.1001/jama.2015.6789.
- Incisive Health, Cancer Research UK . Saving Lives, Averting Costs – An Analysis of the Financial Implications of Achieving Earlier Diagnosis of Colorectal, Lung and Ovarian Cancer 2014.
- Office for National Statistics . Conceptions in England and Wales: 2017 2019. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/conceptionandfertilityrates/bulletins/conceptionstatistics/2017 (accessed 2 May 2019).
- Sun CC, Meyer LA, Daniels MS, Bodurka DC, Nebgen DR, Burton-Chase AM, et al. Women’s preferences for cancer risk management strategies in Lynch syndrome. Gynecol Oncol 2019;152:514-21. https://doi.org/10.1016/j.ygyno.2018.11.027.
- National Institute for Health and Care Excellence (NICE) . Ovarian Cancer: The Recognition and Initial Management of Ovarian Cancer (CG122) 2011. www.nice.org.uk/guidance/cg122 (accessed 14 July 2022).
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. https://doi.org/10.1136/bmj.i4919.
- Critical Appraisal Skills Programme (CASP) . CASP Cohort Study Checklist [Online] 2018. https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Cohort-Study-Checklist-2018_fillable_form.pdf (accessed 11 January 2022).
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Barrow E, Robinson L, Alduaij W, Shenton A, Clancy T, Lalloo F, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet 2009;75:141-9. https://doi.org/10.1111/j.1399-0004.2008.01125.x.
- Bats A, Blons H, Narjoz C, Le Frere-Belda M, Laurent-Puig P, Lecuru F. Diagnostic value of microsatellite instability analysis in uterine cavity washings to detect endometrial cancer in Lynch syndrome. Gynecol Oncol 2013;130.
- Bats AS, Blons H, Narjoz C, Le Frere-Belda MA, Laurent-Puig P, Lecuru F. Microsatellite instability analysis in uterine cavity washings to detect endometrial cancer in Lynch syndrome. Anticancer Res 2014;34:3211-5.
- Bucksch K, Zachariae S, Aretz S, Büttner R, Holinski-Feder E, Holzapfel S, et al. German Consortium for Familial Intestinal Cancer . Cancer risks in Lynch syndrome, Lynch-like syndrome, and familial colorectal cancer type X: a prospective cohort study. BMC Cancer 2020;20. https://doi.org/10.1186/s12885-020-06926-x.
- Dove-Edwin I, Boks D, Goff S, Kenter GG, Carpenter R, Vasen HF, et al. The outcome of endometrial carcinoma surveillance by ultrasound scan in women at risk of hereditary nonpolyposis colorectal carcinoma and familial colorectal carcinoma. Cancer 2002;94:1708-12. https://doi.org/10.1002/cncr.10380.
- Dueñas N, Navarro M, Teulé A, Solanes A, Salinas M, Iglesias S, et al. Correction: Dueñas et al. Assessing effectiveness of colonic and gynecological risk reducing surgery in Lynch syndrome individuals. Cancers (Basel) 2021;12. https://doi.org/10.3390/cancers13133104.
- Dueñas N, Navarro M, Teulé A, Solanes A, Salinas M, Iglesias S, et al. Assessing effectiveness of colonic and gynecological risk reducing surgery in Lynch syndrome individuals. Cancers (Basel) 2020;12. https://doi.org/10.3390/cancers12113419.
- Eikenboom EL, van Doorn HC, Dinjens WNM, Dubbink HJ, Geurts-Giele WRR, Spaander MCW, et al. Gynecological surveillance and surgery outcomes in Dutch Lynch syndrome carriers. Cancers (Basel) 2021;13. https://doi.org/10.3390/cancers13030459.
- Elmasry K, Davies AJ, Evans DG, Seif MN, Reynolds K. Strategies for endometrial screening in the Lynch syndrome population: a patient acceptability study. Fam Cancer 2009;8:431-9. https://doi.org/10.1007/s10689-009-9259-3.
- Gerritzen LH, Hoogerbrugge N, Oei AL, Nagengast FM, van Ham MA, Massuger LF, et al. Improvement of endometrial biopsy over transvaginal ultrasound alone for endometrial surveillance in women with Lynch syndrome. Fam Cancer 2009;8:391-7. https://doi.org/10.1007/s10689-009-9252-x.
- Helder-Woolderink JM, De Bock GH, Hollema H, Hofstra RMW, Sijmons RH, Mourits MJE. Annual gynaecological surveillance in women with Lynch syndrome: what’s the additional value of microcurettage?. Int J Gynecol Cancer 2011;21.
- Helder-Woolderink JM, De Bock GH, Sijmons RH, Hollema H, Mourits MJ. The additional value of endometrial sampling in the early detection of endometrial cancer in women with Lynch syndrome. Gynecol Oncol 2013;131:304-8. https://doi.org/10.1016/j.ygyno.2013.05.032.
- Helder-Woolderink J, de Bock G, Hollema H, van Oven M, Mourits M. Pain evaluation during gynaecological surveillance in women with Lynch syndrome. Fam Cancer 2017;16:205-10. https://doi.org/10.1007/s10689-016-9937-x.
- Helder-Woolderink JM, De Bock GH, Mourits MJE. Perception of pain at gynaecological surveillance in women with Lynch syndrome. Int J Gynecol Cancer 2015;25.
- Ketabi Z, Gerdes AM, Mosgaard B, Ladelund S, Bernstein I. The results of gynecologic surveillance in families with hereditary nonpolyposis colorectal cancer. Gynecol Oncol 2014;133:526-30. https://doi.org/10.1016/j.ygyno.2014.03.012.
- Boilesen AE, Bisgaard ML, Bernstein I. Risk of gynecologic cancers in Danish hereditary non-polyposis colorectal cancer families. Acta Obstet Gynecol Scand 2008;87:1129-35. https://doi.org/10.1080/00016340802443806.
- Lécuru F, Metzger U, Scarabin C, Le Frère Belda MA, Olschwang S, Laurent Puig P. Hysteroscopic findings in women at risk of HNPCC. Results of a prospective observational study. Fam Cancer 2007;6:295-9. https://doi.org/10.1007/s10689-007-9123-2.
- Lécuru F, Le Frère Belda MA, Bats AS, Tulpin L, Metzger U, Olschwang S, et al. Performance of office hysteroscopy and endometrial biopsy for detecting endometrial disease in women at risk of human non-polyposis colon cancer: a prospective study. Int J Gynecol Cancer 2008;18:1326-31. https://doi.org/10.1111/j.1525-1438.2007.01183.x.
- Lécuru F, Huchon C, Metzger U, Bats AS, Le Frère Belda MA, Olschwang S, et al. Contribution of ultrasonography to endometrial cancer screening in patients with hereditary nonpolyposis colorectal cancer/Lynch syndrome. Int J Gynecol Cancer 2010;20:583-7. https://doi.org/10.1111/IGC.0b013e3181d7283a.
- Manchanda R, Saridogan E, Abdelraheim A, Johnson M, Rosenthal AN, Benjamin E, et al. Annual outpatient hysteroscopy and endometrial sampling (OHES) in HNPCC/Lynch syndrome (LS). Arch Gynecol Obstet 2012;286:1555-62. https://doi.org/10.1007/s00404-012-2492-2.
- Møller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Mallorca Group . Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: a report from the prospective Lynch syndrome database. Gut 2017;66:1657-64. https://doi.org/10.1136/gutjnl-2016-311403.
- Huang M, Sun C, Boyd-Rogers S, Burzawa J, Milbourne A, Keeler E, et al. Prospective study of combined colon and endometrial cancer screening in women with Lynch syndrome: a patient-centered approach. J Oncol Pract 2011;7:43-7. https://doi.org/10.1200/jop.2010.000038.
- Huang M, Sun CC, Boyd-Rogers S, Burzawa JK, Milbourne A, Keeler E, et al. A prospective study of combined colon and endometrial cancer screening in women with Lynch syndrome: a novel, patient-centered approach. J Clin Oncol 2010;28:1563-.
- Nebgen DR, Lu K, Chisholm GB, Sun CC, Earles T, Soletsky BR, et al. International society for gastrointestinal hereditary tumours-InSiGHT. Fam Cancer 2019;18:1-88. https://doi.org/10.1007/s10689-019-00124-w.
- Nebgen DR, Lu KH, Rimes S, Keeler E, Broaddus R, Munsell MF, et al. Combined colonoscopy and endometrial biopsy cancer screening results in women with Lynch syndrome. Gynecol Oncol 2014;135:85-9. https://doi.org/10.1016/j.ygyno.2014.08.017.
- Ring K, Boyd-Rogers S, Amin Y, Daniels M, Batte B, Schmeler K, et al. Endometrial cancer screening in Lynch syndrome: do patients report symptoms prior to diagnosis?. Gynecol Oncol 2013;130:e100-1.
- Pylvänäinen K, Lehtinen T, Kellokumpu I, Järvinen H, Mecklin JP. Causes of death of mutation carriers in Finnish Lynch syndrome families. Fam Cancer 2012;11:467-71. https://doi.org/10.1007/s10689-012-9537-3.
- Järvinen HJ, Renkonen-Sinisalo L, Aktán-Collán K, Peltomäki P, Aaltonen LA, Mecklin JP. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol 2009;27:4793-7. https://doi.org/10.1200/jco.2009.23.7784.
- Renkonen-Sinisalo L, Bützow R, Leminen A, Lehtovirta P, Mecklin JP, Järvinen HJ. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer 2007;120:821-4. https://doi.org/10.1002/ijc.22446.
- Rijcken FE, Mourits MJ, Kleibeuker JH, Hollema H, van der Zee AG. Gynecologic screening in hereditary nonpolyposis colorectal cancer. Gynecol Oncol 2003;91:74-80. https://doi.org/10.1016/s0090-8258(03)00371-8.
- Rosenthal A, Philpott S, Rizzuto I, Fraser L, Manchanda R, Badman P, et al. Incidental diagnosis of endometrial cancer during the UK familial ovarian cancer screening study (UKFOCSS). Int J Gynecol Cancer 2012;22.
- Rosenthal AN, Fraser L, Manchanda R, Badman P, Philpott S, Mozersky J, et al. Results of annual screening in phase I of the United Kingdom familial ovarian cancer screening study highlight the need for strict adherence to screening schedule. J Clin Oncol 2013;31:49-57. https://doi.org/10.1200/jco.2011.39.7638.
- Ryan NAJ, Evans DG, Green K, Crosbie EJ. Pathological features and clinical behavior of Lynch syndrome-associated ovarian cancer. Gynecol Oncol 2017;144:491-5. https://doi.org/10.1016/j.ygyno.2017.01.005.
- Stuckless S, Green J, Dawson L, Barrett B, Woods MO, Dicks E, et al. Impact of gynecological screening in Lynch syndrome carriers with an MSH2 mutation. Clin Genet 2013;83:359-64. https://doi.org/10.1111/j.1399-0004.2012.01929.x.
- Tzortzatos G, Andersson E, Soller M, Askmalm MS, Zagoras T, Georgii-Hemming P, et al. The gynecological surveillance of women with Lynch syndrome in Sweden. Gynecol Oncol 2015;138:717-22. https://doi.org/10.1016/j.ygyno.2015.07.016.
- Wood NJ, Munot S, Sheridan E, Duffy SR. Does a ‘one-stop’ gynecology screening clinic for women in hereditary nonpolyposis colorectal cancer families have an impact on their psychological morbidity and perception of health?. Int J Gynecol Cancer 2008;18:279-84. https://doi.org/10.1111/j.1525-1438.2007.01009.x.
- Helder-Woolderink J, De Bock G, De Hullu J, Hollema H, Zweemer R, Slangen R, et al. Characteristics of ovarian cancer in women with Lynch syndrome. Int J Gynecol Cancer 2017;27.
- Helder-Woolderink J, De Bock G, Vasen H, Hollema H, Mourits M. Characteristics of ovarian cancer in women with Lynch syndrome. Int J Gynecol Cancer 2016;26.
- Woolderink JM, De Bock GH, de Hullu JA, Hollema H, Zweemer RP, Slangen BFM, et al. Characteristics of Lynch syndrome associated ovarian cancer. Gynecol Oncol 2018;150:324-30. https://doi.org/10.1016/j.ygyno.2018.03.060.
- Woolderink JM, De Bock GH, van Hemel BM, Geuken E, Hollema H, Werner N, et al. Feasibility of endometrial sampling by vaginal tampons in women with Lynch syndrome. BMC Womens Health 2020;20. https://doi.org/10.1186/s12905-020-00920-y.
- de Jong AE, Hendriks YM, Kleibeuker JH, de Boer SY, Cats A, Griffioen G, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006;130:665-71. https://doi.org/10.1053/j.gastro.2005.11.032.
- Kalamo MH, Mäenpää JU, Seppälä TT, Mecklin JP, Huhtala H, Sorvettula K, et al. Factors associated with decision-making on prophylactic hysterectomy and attitudes towards gynecological surveillance among women with Lynch syndrome (LS): a descriptive study. Fam Cancer 2020;19:177-82. https://doi.org/10.1007/s10689-020-00158-5.
- Ryan N, Nobes M, Sedgewick D, Teoh SN, Evans DG, Crosbie EJ. A mismatch in care: results of a United Kingdom-wide patient and clinician survey of gynaecological services for women with Lynch syndrome. BJOG 2021;128:728-36. https://doi.org/10.1111/1471-0528.16432.
- ISRCTN Registry . United Kingdom Familial Ovarian Cancer Screening Study 2010. www.isrctn.com/ISRCTN32794457 (accessed 12 August 2022).
- Yang B, Mallett S, Takwoingi Y, Davenport CF, Hyde CJ, Whiting PF, et al. QUADAS-C Group . QUADAS-C: a tool for assessing risk of bias in comparative diagnostic accuracy studies. Ann Intern Med 2021;174:1592-9. https://doi.org/10.7326/m21-2234.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. PharmacoEcon 2006;24:355-71. https://doi.org/10.2165/00019053-200624040-00006.
- Kwon JS, Sun CC, Peterson SK, White KG, Daniels MS, Boyd-Rogers SG, et al. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer 2008;113:326-35. https://doi.org/10.1002/cncr.23554.
- Yang KY, Caughey AB, Little SE, Cheung MK, Chen LM. A cost-effectiveness analysis of prophylactic surgery versus gynecologic surveillance for women from hereditary non-polyposis colorectal cancer (HNPCC) families. Fam Cancer 2011;10:535-43. https://doi.org/10.1007/s10689-011-9444-z.
- Wright JD, Silver ER, Tan SX, Hur C, Kastrinos F. Cost-effectiveness analysis of genotype-specific surveillance and preventive strategies for gynecologic cancers among women with Lynch syndrome. JAMA Netw Open 2021;4. https://doi.org/10.1001/jamanetworkopen.2021.23616.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Briggs A, Fenn P. Trying to do better than average: a commentary on ‘statistical inference for cost-effectiveness ratios’. Health Econ 1997;6:491-5. https://doi.org/10.1002/(sici)1099-1050(199709)6:5<491::Aid-hec293>3.0.Co;2-r.
- Chen L-m, Yang KY, Little SE, Cheung MK, Caughey AB. Gynecologic cancer prevention in Lynch syndrome/hereditary nonpolyposis colorectal cancer families. Obstet Gynecol 2007;110:18-25. https://doi.org/10.1097/01.Aog.0000267500.27329.85.
- Papaioannou D, Brazier J, Paisley S. Systematic searching and selection of health state utility values from the literature. Value Health 2013;16:686-95. https://doi.org/10.1016/j.jval.2013.02.017.
- Hurskainen R, Teperi J, Rissanen P, Aalto AM, Grenman S, Kivelä A, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet 2001;357:273-7. https://doi.org/10.1016/s0140-6736(00)03615-1.
- Hurskainen R, Teperi J, Rissanen P, Aalto AM, Grenman S, Kivelä A, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA 2004;291:1456-63. https://doi.org/10.1001/jama.291.12.1456.
- Kuppermann M, Wang G, Wong S, Blanco A, Conrad P, Nakagawa S, et al. Preferences for outcomes associated with decisions to undergo or forgo genetic testing for Lynch syndrome. Cancer 2013;119:215-25. https://doi.org/10.1002/cncr.27634.
- Grann VR, Jacobson JS, Sundararajan V, Albert SM, Troxel AB, Neugut AI. The quality of life associated with prophylactic treatments for women with BRCA1/2 mutations. Cancer J Sci Am 1999;5:283-92.
- Grann VR, Panageas KS, Whang W, Antman KH, Neugut AI. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J Clin Oncol 1998;16:979-85. https://doi.org/10.1200/jco.1998.16.3.979.
- Hettle R, Borrill J, Suri G, Wulff J. Estimating health-state utility values for patients with recurrent ovarian cancer using functional assessment of cancer therapy – general mapping algorithms. Clinicoecon Outcomes Res 2015;7:615-27. https://doi.org/10.2147/ceor.S92078.
- Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. ENGOT-OV16/NOVA Investigators . Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016;375:2154-64. https://doi.org/10.1056/NEJMoa1611310.
- Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N, et al. Patient-centered outcomes in ARIEL3, a Phase III, randomized, placebo-controlled trial of rucaparib maintenance treatment in patients with recurrent ovarian carcinoma. J Clin Oncol 2020;38:3494-505. https://doi.org/10.1200/jco.19.03107.
- Oza AM, Matulonis UA, Malander S, Hudgens S, Sehouli J, Del Campo JM, et al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): results from a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2018;19:1117-25. https://doi.org/10.1016/s1470-2045(18)30333-4.
- Armfield NR, Janda M, Obermair A. Obesity in total laparoscopic hysterectomy for early stage endometrial cancer: health gain and inpatient resource use. Int J Qual Health Care 2019;31:283-8. https://doi.org/10.1093/intqhc/mzy162.
- Bijen CB, Vermeulen KM, Mourits MJ, Arts HJ, Ter Brugge HG, van der Sijde R, et al. Cost effectiveness of laparoscopy versus laparotomy in early stage endometrial cancer: a randomised trial. Gynecol Oncol 2011;121:76-82. https://doi.org/10.1016/j.ygyno.2010.11.043.
- Cykert S, Phifer N, Hansen C. Tamoxifen for breast cancer prevention: a framework for clinical decisions. Obstet Gynecol 2004;104:433-42.
- Ferguson SE, Panzarella T, Lau S, Gien LT, Samouëlian V, Giede C, et al. Prospective cohort study comparing quality of life and sexual health outcomes between women undergoing robotic, laparoscopic and open surgery for endometrial cancer. Gynecol Oncol 2018;149:476-83. https://doi.org/10.1016/j.ygyno.2018.04.558.
- Hildebrandt T, Thiel FC, Fasching PA, Graf C, Bani MR, Loehberg CR, et al. Health utilities in gynecological oncology and mastology in Germany. Anticancer Res 2014;34:829-35.
- Kent EE, Ambs A, Mitchell SA, Clauser SB, Smith AW, Hays RD. Health-related quality of life in older adult survivors of selected cancers: data from the SEER-MHOS linkage. Cancer 2015;121:758-65. https://doi.org/10.1002/cncr.29119.
- Kimman M, Jan S, Monaghan H, Woodward M. The relationship between economic characteristics and health-related quality of life in newly diagnosed cancer patients in Southeast Asia: results from an observational study. Qual Life Res 2015;24:937-49.
- Lundin ES, Carlsson P, Wodlin NB, Nilsson L, Kjölhede P. Cost-effectiveness of robotic hysterectomy versus abdominal hysterectomy in early endometrial cancer. Int J Gynecol Cancer 2020;30:1719-25. https://doi.org/10.1136/ijgc-2020-001611.
- Lundin ES, Wodlin NB, Nilsson L, Kjölhede P. A prospective randomized assessment of quality of life between open and robotic hysterectomy in early endometrial cancer. Int J Gynecol Cancer 2019;29:721-7. https://doi.org/10.1136/ijgc-2019-000285.
- Mansel R, Locker G, Fallowfield L, Benedict A, Jones D. Cost-effectiveness analysis of anastrozole vs tamoxifen in adjuvant therapy for early stage breast cancer in the United Kingdom: the 5-year completed treatment analysis of the ATAC (‘Arimidex’, Tamoxifen alone or in combination) trial. Br J Cancer 2007;97:152-61. https://doi.org/10.1038/sj.bjc.6603804.
- Naik H, Howell D, Su S, Qiu X, Brown MC, Vennettilli A, et al. EQ-5D health utility scores: data from a comprehensive Canadian cancer centre. Patient 2017;10:105-15. https://doi.org/10.1007/s40271-016-0190-z.
- Setiawan D, Dusafitri A, Galistiani GF, van Asselt ADI, Postma MJ. Health-related quality of life of patients with HPV-related cancers in Indonesia. Value Health Reg Issues 2018;15:63-9. https://doi.org/10.1016/j.vhri.2017.07.010.
- Ueno T, Watanabe K, Ikemoto T, Matsushita H, Kawanami K, Arai YC, et al. Patient-reported outcomes after surgery among patients with gynecological diseases in Japan. J Psychosom Obstet Gynaecol 2021;42:22-8. https://doi.org/10.1080/0167482x.2019.1708321.
- Cohn DE, Barnett JC, Wenzel L, Monk BJ, Burger RA, Straughn JM, et al. A cost-utility analysis of NRG Oncology/Gynecologic Oncology Group Protocol 218: incorporating prospectively collected quality-of-life scores in an economic model of treatment of ovarian cancer. Gynecol Oncol 2015;136:293-9. https://doi.org/10.1016/j.ygyno.2014.10.020.
- Cole AL, Barber EL, Gogate A, Tran AQ, Wheeler SB. Economic analysis of neoadjuvant chemotherapy versus primary debulking surgery for advanced epithelial ovarian cancer using an aggressive surgical paradigm. Int J Gynecol Cancer 2018;28:1077-84. https://doi.org/10.1097/igc.0000000000001271.
- Duong M, Wright E, Yin L, Martin-Nunez I, Ghatage P, Fung-Kee-Fung M. The cost-effectiveness of bevacizumab for the treatment of advanced ovarian cancer in Canada. Curr Oncol 2016;23:e461-7. https://doi.org/10.3747/co.23.3139.
- Friedlander M, Rau J, Lee CK, Meier W, Lesoin A, Kim JW, et al. Quality of life in patients with advanced epithelial ovarian cancer (EOC) randomized to maintenance pazopanib or placebo after first-line chemotherapy in the AGO-OVAR 16 trial. Measuring what matters-patient-centered end points in trials of maintenance therapy. Ann Oncol 2018;29:737-43. https://doi.org/10.1093/annonc/mdx796.
- Fujiwara K, Monk BJ, Lhommé C, Coleman RL, Brize A, Oaknin A, et al. Health-related quality of life in women with recurrent ovarian cancer receiving paclitaxel plus trebananib or placebo (TRINOVA-1). Ann Oncol 2016;27:1006-13. https://doi.org/10.1093/annonc/mdw147.
- Gordon LG, Scuffham PA, Beesley VL, Green AC, DeFazio A, Wyld DK, et al. Australian Ovarian Cancer Study Group . Medical costs and outcomes for Australian women with ovarian cancer: a patient-level analysis over 2.5 years. Int J Gynecol Cancer 2010;20:757-65. https://doi.org/10.1111/igc.0b013e3181dbd13f.
- Grann VR, Patel P, Bharthuar A, Jacobson JS, Warner E, Anderson K, et al. Breast cancer-related preferences among women with and without BRCA mutations. Breast Cancer Res Treat 2010;119:177-84. https://doi.org/10.1007/s10549-009-0373-6.
- Havrilesky LJ, Broadwater G, Davis DM, Nolte KC, Barnett JC, Myers ER, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol 2009;113:216-20. https://doi.org/10.1016/j.ygyno.2008.12.026.
- Havrilesky LJ, Pokrzywinski R, Revicki D, Higgins RV, Nycum LR, Kohler MF, et al. Cost-effectiveness of combination versus sequential docetaxel and carboplatin for the treatment of platinum-sensitive, recurrent ovarian cancer. Cancer 2012;118:386-91. https://doi.org/10.1002/cncr.26199.
- Havrilesky LJ, Yang JC, Lee PS, Secord AA, Ehrisman JA, Davidson B, et al. Patient preferences for attributes of primary surgical debulking versus neoadjuvant chemotherapy for treatment of newly diagnosed ovarian cancer. Cancer 2019;125:4399-406.
- Hess LM, Brady WE, Havrilesky LJ, Cohn DE, Monk BJ, Wenzel L, et al. Comparison of methods to estimate health state utilities for ovarian cancer using quality of life data: a Gynecologic Oncology Group study. Gynecol Oncol 2013;128:175-80. https://doi.org/10.1016/j.ygyno.2012.10.024.
- Hess LM, Malone DC, Reed PG, Skrepnek G, Weihs K. Preferences of patients and oncologists for advanced ovarian cancer treatment-related health states. Health Outcomes Res MEd 2010;1.
- Hinde S, Epstein D, Cook A, Embleton A, Perren T, Sculpher M. The cost-effectiveness of bevacizumab in advanced ovarian cancer using evidence from the ICON7 trial. Value Health 2016;19:431-9. https://doi.org/10.1016/j.jval.2016.01.013.
- Krasner CN, Poveda A, Herzog TJ, Vermorken JB, Kaye SB, Nieto A, et al. Patient-reported outcomes in relapsed ovarian cancer: results from a randomized Phase III study of trabectedin with pegylated liposomal doxorubicin (PLD) versus PLD alone. Gynecol Oncol 2012;127:161-7. https://doi.org/10.1016/j.ygyno.2012.06.034.
- Luealon P, Khempech N, Vasuratna A, Hanvoravongchai P, Havanond P. Cost effectiveness analysis of different management strategies between best supportive care and second-line chemotherapy for platinum-resistant or refractory ovarian cancer. Asian Pac J Cancer Prev 2016;17:799-805.
- National Institute for Health and Care Excellence (NICE) . Bevacizumab in Combination With Paclitaxel and Carboplatin for First-Line Treatment of Advanced Ovarian Cancer: Technology Appraisal Guidance (TA284) 2013.
- Ortega A, Dranitsaris G, Sturgeon J, Sutherland H, Oza A. Cost-utility analysis of paclitaxel in combination with cisplatin for patients with advanced ovarian cancer. Gynecol Oncol 1997;66:454-63. https://doi.org/10.1006/gyno.1997.4786.
- Rowland MR, Lesnock JL, Farris C, Kelley JL, Krivak TC. Cost-utility comparison of neoadjuvant chemotherapy versus primary debulking surgery for treatment of advanced-stage ovarian cancer in patients 65 years old or older. Am J Obstet Gynecol 2015;212:763.e1-8.
- Stein K, Sugar C, Velikova G, Stark D. Putting the ‘Q’ in quality adjusted life years (QALYs) for advanced ovarian cancer: an approach using data clustering methods and the internet. Eur J Cancer 2007;43:104-13. https://doi.org/10.1016/j.ejca.2006.09.007.
- van de Vrie R, van Meurs HS, Rutten MJ, Naaktgeboren CA, Opmeer BC, Gaarenstroom KN, et al. Cost-effectiveness of laparoscopy as diagnostic tool before primary cytoreductive surgery in ovarian cancer. Gynecol Oncol 2017;146:449-56. https://doi.org/10.1016/j.ygyno.2017.06.019.
- van Roosmalen MS, Verhoef LC, Stalmeier PF, Hoogerbrugge N, van Daal WA. Decision analysis of prophylactic surgery or screening for BRCA1 mutation carriers: a more prominent role for oophorectomy. J Clin Oncol 2002;20:2092-100. https://doi.org/10.1200/jco.2002.08.035.
- Borendal Wodlin N, Nilsson L, Kjølhede P. Health-related quality of life and postoperative recovery in fast-track hysterectomy. Acta Obstet Gynecol Scand 2011;90:362-8. https://doi.org/10.1111/j.1600-0412.2010.01058.x.
- Bouwsma EVA, Bosmans JE, van Dongen JM, Brölmann HAM, Anema JR, Huirne JAF. Cost-effectiveness of an internet-based perioperative care programme to enhance postoperative recovery in gynaecological patients: economic evaluation alongside a stepped-wedge cluster-randomised trial. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-017782.
- Christiansen UJ, Kruse AR, Olesen PG, Lauszus FF, Kesmodel US, Forman A. Outpatient vs inpatient total laparoscopic hysterectomy: a randomized controlled trial. Acta Obstet Gynecol Scand 2019;98:1420-8. https://doi.org/10.1111/aogs.13670.
- Cooper K, Breeman S, Scott NW, Scotland G, Hernández R, Clark TJ, et al. Laparoscopic supracervical hysterectomy compared with second-generation endometrial ablation for heavy menstrual bleeding: the HEALTH RCT. Health Technol Assess 2019;23:1-108. https://doi.org/10.3310/hta23530.
- Davies JE, Doyle PM. Quality of life studies in unselected gynaecological outpatients and inpatients before and after hysterectomy. J Obstet Gynaecol 2002;22:523-6. https://doi.org/10.1080/0144361021000003681.
- Dickersin K, Munro MG, Clark M, Langenberg P, Scherer R, Frick K, et al. Surgical Treatments Outcomes Project for Dysfunctional Uterine Bleeding (STOP-DUB) Research Group . Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized controlled trial. Obstet Gynecol 2007;110:1279-89. https://doi.org/10.1097/01.Aog.0000292083.97478.38.
- Fennessy FM, Kong CY, Tempany CM, Swan JS. Quality-of-life assessment of fibroid treatment options and outcomes. Radiology 2011;259:785-92. https://doi.org/10.1148/radiol.11100704.
- Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al. EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy. Health Technol Assess 2004;8:1-154. https://doi.org/10.3310/hta8260.
- Gorlero F, Lijoi D, Biamonti M, Lorenzi P, Pullè A, Dellacasa I, et al. Hysterectomy and women satisfaction: total versus subtotal technique. Arch Gynecol Obstet 2008;278:405-10. https://doi.org/10.1007/s00404-008-0615-6.
- Hemming C, Constable L, Goulao B, Kilonzo M, Boyers D, Elders A, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess 2020;24:1-220. https://doi.org/10.3310/hta24130.
- Kuppermann M, Learman LA, Schembri M, Gregorich SE, Jackson RA, Jacoby A, et al. Contributions of hysterectomy and uterus-preserving surgery to health-related quality of life. Obstet Gynecol 2013;122:15-2. https://doi.org/10.1097/aog.0b013e318292aea4.
- Lashen H, Jones GL, Duru C, Pitsillides C, Radley S, Jacques RM, et al. Bowel dysfunction after total abdominal hysterectomy for benign conditions: a prospective longitudinal study. Eur J Gastroenterol Hepatol 2013;25:1217-22. https://doi.org/10.1097/MEG.0b013e328362dc5e.
- Radosa JC, Meyberg-Solomayer G, Kastl C, Radosa CG, Mavrova R, Gräber S, et al. Influences of different hysterectomy techniques on patients’ postoperative sexual function and quality of life. J Sex Med 2014;11:2342-50. https://doi.org/10.1111/jsm.12623.
- Sculpher M. A cost-utility analysis of abdominal hysterectomy versus transcervical endometrial resection for the surgical treatment of menorrhagia. Int J Technol Assess Health Care 1998;14:302-19. https://doi.org/10.1017/s0266462300012277.
- Taipale K, Leminen A, Räsänen P, Heikkilä A, Tapper AM, Sintonen H, et al. Costs and health-related quality of life effects of hysterectomy in patients with benign uterine disorders. Acta Obstet Gynecol Scand 2009;88:1402-10. https://doi.org/10.3109/00016340903317990.
- Borendal Wodlin N. Fast Track Abdominal Hysterectomy: On the Mode of Anesthesia, Postoperative Recovery and Health Economics. Linköping: Linköping University Electronic Press; 2011.
- Sculpher M, Manca A, Abbott J, Fountain J, Mason S, Garry R. Cost effectiveness analysis of laparoscopic hysterectomy compared with standard hysterectomy: results from a randomised trial. BMJ 2004;328. https://doi.org/10.1136/bmj.37942.601331.EE.
- Dunlop WCN, Mason N, Kenworthy J, Akehurst RL. Benefits, challenges and potential strategies of open source health economic models. PharmacoEcon 2017;35:125-8. https://doi.org/10.1007/s40273-016-0479-8.
- Tappenden P, Chilcott J, Brennan A, Squires H, Stevenson M. Whole disease modeling to inform resource allocation decisions in cancer: a methodological framework. Value Health 2012;15:1127-36. https://doi.org/10.1016/j.jval.2012.07.008.
- Team SimPy . SimPy 2022. https://simpy.readthedocs.io/ (accessed 8 August 2022).
- Thomas A. Welcome to Injector’s Documentation! 2022. https://injector.readthedocs.io/ (accessed 8 August 2022).
- Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, et al. Array programming with NumPy. Nature 2020;585:357-62. https://doi.org/10.1038/s41586-020-2649-2.
- Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0 Contributors . SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 2020;17:261-72. https://doi.org/10.1038/s41592-019-0686-2.
- Krekel H, Oliveira B, Pfannschmidt R, Bruynooghe F, Laugher B, Bruhin F. Pytest 7.1 2004. https://github.com/pytest-dev/pytest (accessed 23 July 2022).
- Stan Development Team . Stan Modeling Language Users Guide and Reference Manual, 2.30 2022.
- R Core Team . A Language and Environment for Statistical Computing. R Foundation for Statistical Computing 2021.
- Ahadova A, Gallon R, Gebert J, Ballhausen A, Endris V, Kirchner M, et al. Three molecular pathways model colorectal carcinogenesis in Lynch syndrome. Int J Cancer 2018;143:139-50. https://doi.org/10.1002/ijc.31300.
- Ahadova A, Seppälä TT, Engel C, Gallon R, Burn J, Holinski-Feder E, et al. The ‘unnatural’ history of colorectal cancer in Lynch syndrome: lessons from colonoscopy surveillance. Int J Cancer 2021;148:800-11. https://doi.org/10.1002/ijc.33224.
- Seppälä TT, Ahadova A, Dominguez-Valentin M, Macrae F, Evans DG, Therkildsen C, et al. Lack of association between screening interval and cancer stage in Lynch syndrome may be accounted for by over-diagnosis; a prospective Lynch syndrome database report. Hered Cancer Clin Pract 2019;17. https://doi.org/10.1186/s13053-019-0106-8.
- Engel C, Vasen HF, Seppälä T, Aretz S, Bigirwamungu-Bargeman M, de Boer SY, et al. German HNPCC Consortium, the Dutch Lynch Syndrome Collaborative Group, and the Finnish Lynch Syndrome Registry . No difference in colorectal cancer incidence or stage at detection by colonoscopy among 3 countries with different Lynch syndrome surveillance policies. Gastroenterology 2018;155:1400-1409.e2. https://doi.org/10.1053/j.gastro.2018.07.030.
- Haupt S, Zeilmann A, Ahadova A, Bläker H, von Knebel Doeberitz M, Kloor M, et al. Mathematical modeling of multiple pathways in colorectal carcinogenesis using dynamical systems with Kronecker structure. PLOS Comput Biol 2021;17. https://doi.org/10.1371/journal.pcbi.1008970.
- Dinh TA, Rosner BI, Atwood JC, Boland CR, Syngal S, Vasen HF, et al. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev Res (Phila) 2011;4:9-22. https://doi.org/10.1158/1940-6207.Capr-10-0262.
- Thomas C, Mandrik O, Saunders CL, Thompson D, Whyte S, Griffin S, et al. The costs and benefits of risk stratification for colorectal cancer screening based on phenotypic and genetic risk: a health economic analysis. Cancer Prev Res (Phila) 2021;14:811-22. https://doi.org/10.1158/1940-6207.Capr-20-0620.
- Xu Y, Li C, Zheng CZ, Zhang YQ, Guo TA, Liu FQ, et al. Comparison of long-term outcomes between Lynch sydrome and sporadic colorectal cancer: a propensity score matching analysis. BMC Cancer 2021;21. https://doi.org/10.1186/s12885-020-07771-8.
- Office for National Statistics . Cancer Survival in England: Adults Diagnosed Between 2013 and 2017 and Followed up to 2018 2019. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed (accessed 21 July 2022).
- Brown KF, Rumgay H, Dunlop C, Ryan M, Quartly F, Cox A, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer 2018;118:1130-41. https://doi.org/10.1038/s41416-018-0029-6.
- ten Broeke SW, van der Klift HM, Tops CMJ, Aretz S, Bernstein I, Buchanan DD, et al. Cancer risks for PMS2-associated Lynch syndrome. J Clin Oncol 2018;36:2961-8. https://doi.org/10.1200/jco.2018.78.4777.
- Lacey JV, Chia VM. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas 2009;63:39-44. https://doi.org/10.1016/j.maturitas.2009.02.005.
- Lacey JV, Ioffe OB, Ronnett BM, Rush BB, Richesson DA, Chatterjee N, et al. Endometrial carcinoma risk among women diagnosed with endometrial hyperplasia: the 34-year experience in a large health plan. Br J Cancer 2007;98:45-53. https://doi.org/10.1038/sj.bjc.6604102.
- Contreras NA, Sabadell J, Verdaguer P, Julià C, Fernández-Montolí ME. Fertility-sparing approaches in atypical endometrial hyperplasia and endometrial cancer patients: current evidence and future directions. Int J Mol Sci 2022;23. https://doi.org/10.3390/ijms23052531.
- Westin SN, Fellman B, Sun CC, Broaddus RR, Woodall ML, Pal N, et al. Prospective phase II trial of levonorgestrel intrauterine device: nonsurgical approach for complex atypical hyperplasia and early-stage endometrial cancer. Am J Obstet Gynecol 2021;224:191.e1-191.e15. https://doi.org/10.1016/j.ajog.2020.08.032.
- Barr CE, Ryan NAJ, Derbyshire AE, Wan YL, MacKintosh ML, McVey RJ, et al. Weight loss during intrauterine progestin treatment for obesity-associated atypical hyperplasia and early-stage cancer of the endometrium. Cancer Prev Res 2021;14:1041-50. https://doi.org/10.1158/1940-6207.Capr-21-0229.
- Pal N, Broaddus RR, Urbauer DL, Balakrishnan N, Milbourne A, Schmeler KM, et al. Treatment of low-risk endometrial cancer and complex atypical hyperplasia with the levonorgestrel-releasing intrauterine device. Obstet Gynecol 2018;131:109-16. https://doi.org/10.1097/aog.0000000000002390.
- Snowsill TM, Ryan NAJ, Crosbie EJ, Frayling IM, Evans DG, Hyde CJ. Cost-effectiveness analysis of reflex testing for Lynch syndrome in women with endometrial cancer in the UK setting. PLOS ONE 2019;14. http://hdl.handle.net/10871/38303.
- Carr C, Son J, Yao M, Priyadarshini A, Marquard J, Vargas R, et al. Clinicopathologic characteristics and outcomes of endometrial cancer patients with mismatch repair deficiency in the era of universal Lynch syndrome screening. Gynecol Oncol 2020;159:712-20. https://doi.org/10.1016/j.ygyno.2020.09.039.
- Post CCB, Stelloo E, Smit V, Ruano D, Tops CM, Vermij L, et al. Prevalence and prognosis of Lynch syndrome and sporadic mismatch repair deficiency in endometrial cancer. J Natl Cancer Inst 2021;113:1212-20. https://doi.org/10.1093/jnci/djab029.
- Grindedal EM, Renkonen-Sinisalo L, Vasen H, Evans G, Sala P, Blanco I, et al. Survival in women with MMR mutations and ovarian cancer: a multicentre study in Lynch syndrome kindreds. J Med Genet 2010;47:99-102. https://doi.org/10.1136/jmg.2009.068130.
- Dijkhuizen FP, Mol BW, Brölmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer 2000;89:1765-72.
- Seppälä TT, Dominguez-Valentin M, Crosbie EJ, Engel C, Aretz S, Macrae F, et al. Uptake of hysterectomy and bilateral salpingo-oophorectomy in carriers of pathogenic mismatch repair variants: a prospective Lynch syndrome database report. Eur J Cancer 2021;148:124-33. https://doi.org/10.1016/j.ejca.2021.02.022.
- Office for National Statistics . Past and Projected Period and Cohort Life Tables: 2020-Based, UK, 1981 to 2070 2021. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/pastandprojecteddatafromtheperiodandcohortlifetables/latest (accessed 8 August 2022).
- Snowsill TM, Ryan NAJ, Crosbie EJ. Cost-effectiveness of the Manchester approach to identifying Lynch syndrome in women with endometrial cancer. J Clin Med 2020;9. https://doi.org/10.3390/jcm9061664.
- National Disease Registration Service (NDRS) . CancerData: Staging Data in England 2021. www.cancerdata.nhs.uk/stage_at_diagnosis (accessed 14 April 2022).
- Lacey JV, Sherman ME, Rush BB, Ronnett BM, Ioffe OB, Duggan MA, et al. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J Clin Oncol 2010;28:788-92. https://doi.org/10.1200/jco.2009.24.1315.
- Dabir PD, Bruggeling CE, van der Post RS, Dutilh BE, Hoogerbrugge N, Ligtenberg MJL, et al. Microsatellite instability screening in colorectal adenomas to detect Lynch syndrome patients? A systematic review and meta-analysis. Eur J Hum Genet 2020;28:277-86. https://doi.org/10.1038/s41431-019-0538-7.
- National Health Service England . National Cost Collection for the NHS 2022. www.england.nhs.uk/costing-in-the-nhs/national-cost-collection (accessed 13 July 2022).
- Jones K, Burns A. Unit Costs of Health and Social Care 2021. Canterbury: Personal Social Services Research Unit, University of Kent; 2021.
- National Institute for Health and Care Research (NIHR) . Interactive Costing Tool (iCT): Getting Started 2019. www.nihr.ac.uk/documents/interactive-costing-tool-ict-getting-started/12170 (accessed 13 July 2022).
- Menon U, McGuire AJ, Raikou M, Ryan A, Davies SK, Burnell M, et al. The cost-effectiveness of screening for ovarian cancer: results from the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Br J Cancer 2017;117:619-27. https://doi.org/10.1038/bjc.2017.222.
- Esselen KM, Cronin AM, Bixel K, Bookman MA, Burger RA, Cohn DE, et al. Use of CA-125 tests and computed tomographic scans for surveillance in ovarian cancer. JAMA Oncol 2016;2:1427-33. https://doi.org/10.1001/jamaoncol.2016.1842.
- National Health Service Business Services Authority . Prescription Cost Analysis (PCA) – Financial Year 2021 22 2022. https://opendata.nhsbsa.net/dataset/prescription-cost-analysis-pca-annual-statistics/resource/c0861d07-b0c7-440b-9657-e25b2a9fb769 (accessed 8 August 2022).
- National Health Service Business Services Authority, NHS Prescription Services . NHS Electronic Drug Tariff (July 2022) 2022. www.drugtariff.nhsbsa.nhs.uk/#/00818824-DC/DC00818821/Home (accessed 13 July 2022).
- Joint Formulary Committee . British National Formulary 2022.
- Pennington M, Gentry-Maharaj A, Karpinskyj C, Miners A, Taylor J, Manchanda R, et al. Long-term secondary care costs of endometrial cancer: a prospective cohort study nested within the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0165539.
- Westwood M, Ramaekers B, Lang S, Grimm S, Deshpande S, de Kock S, et al. Risk scores to guide referral decisions for people with suspected ovarian cancer in secondary care: a systematic review and cost-effectiveness analysis. Health Technol Assess 2018;22:1-264. https://doi.org/10.3310/hta22440.
- Laudicella M, Walsh B, Burns E, Smith PC. Cost of care for cancer patients in England: evidence from population-based patient-level data. Br J Cancer 2016;114:1286-92. https://doi.org/10.1038/bjc.2016.77.
- Stinton C, Jordan M, Fraser H, Auguste P, Court R, Al-Khudairy L, et al. Testing strategies for Lynch syndrome in people with endometrial cancer: systematic reviews and economic evaluation. Health Technol Assess 2021;25:1-216. https://doi.org/10.3310/hta25420.
- National Health Service Digital . Health Survey for England 2019: Adults’ Health 2020.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Basu A, Dale W, Elstein A, Meltzer D. A linear index for predicting joint health-states utilities from single health-states utilities. Health Econ 2009;18:403-19. https://doi.org/10.1002/hec.1373.
- Greiner W, Claes C, Busschbach JJ, von der Schulenburg JM. Validating the EQ-5D with time trade off for the German population. Eur J Health Econ 2005;6:124-30. https://doi.org/10.1007/s10198-004-0264-z.
- Djalalov S, Rabeneck L, Tomlinson G, Bremner KE, Hilsden R, Hoch JS. A review and meta-analysis of colorectal cancer utilities. Med Decis Making 2014;34:809-18. https://doi.org/10.1177/0272989x14536779.
- Stinnett AA, Mullahy J. Net health benefits. Med Decis Making 2016;18:S68-80. https://doi.org/10.1177/0272989x98018002s09.
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample. Med Decis Making 2013;34:311-26. https://doi.org/10.1177/0272989x13505910.
- Ogwulu CB, Jackson LJ, Kinghorn P, Roberts TE. A systematic review of the techniques used to value temporary health states. Value Health 2017;20:1180-97. https://doi.org/10.1016/j.jval.2017.03.009.
- Wright DR, Wittenberg E, Swan JS, Miksad RA, Prosser LA. Methods for measuring temporary health states for cost-utility analyses. PharmacoEcon 2009;27:713-23. https://doi.org/10.2165/11317060-000000000-00000.
- Franic DM, Pathak DS, Gafni A. Quality-adjusted life years was a poor predictor of women’s willingness to pay in acute and chronic conditions: results of a survey. J Clin Epidemiol 2005;58:291-303. https://doi.org/10.1016/j.jclinepi.2004.10.005.
- Craig BM, Rand K, Bailey H, Stalmeier PFM. Quality-adjusted life-years without constant proportionality. Value Health 2018;21:1124-31. https://doi.org/10.1016/j.jval.2018.02.004.
- Sakala MD, Carlos RC, Mendiratta-Lala M, Quint EH, Maturen KE. Understanding patient preference in female pelvic imaging. Acad Radiol 2018;25:439-44. https://doi.org/10.1016/j.acra.2017.10.011.
- Auranen A, Joutsiniemi T. A systematic review of gynecological cancer surveillance in women belonging to hereditary nonpolyposis colorectal cancer (Lynch syndrome) families. Acta Obstet Gynecol Scand 2011;90:437-44. https://doi.org/10.1111/j.1600-0412.2011.01091.x.
- Helder-Woolderink JM, Blok EA, Vasen HFA, Hollema H, Mourits MJ, De Bock GH. Ovarian cancer in Lynch syndrome; a systematic review. Eur J Cancer 2016;55:65-73. https://doi.org/10.1016/j.ejca.2015.12.005.
- Lim N, Hickey M, Young GP, Macrae FA, Kelly C. Screening and risk reducing surgery for endometrial or ovarian cancers in Lynch syndrome: a systematic review. Int J Gynecol Cancer 2022;32:646-55. https://doi.org/10.1136/ijgc-2021-003132.
- Ladabaum U, Wang G, Terdiman J, Blanco A, Kuppermann M, Boland CR, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer. Ann Intern Med 2011;155. https://doi.org/10.7326/0003-4819-155-2-201107190-00002.
Appendix 1 Search strategies for systematic reviews
Systematic review of clinical effectiveness
Bibliographic databases
CENTRAL
| Database | CENTRAL |
|---|---|
| Host | Cochrane Library |
| Issue | Issue 9 of 12, September 2020 |
| Date searched | 21 September 2020 |
| Searcher | SB |
| Hits | 9 |
Strategy
| #1 | (‘lynch syndrome’):ti,ab,kw |
| #2 | (‘Muir Torre syndrome’):ti,ab,kw |
| #3 | (‘mismatch repair’ or MMR):ti,ab,kw |
| #4 | ((HNPCC or ‘hereditary nonpolyposis colorectal cancer’ or ‘hereditary non polyposis colorectal cancer’)):ti,ab,kw |
| #5 | MeSH descriptor: [Colorectal Neoplasms, Hereditary Nonpolyposis] this term only |
| #6 | ((Amsterdam NEAR/2 criter*)):ti,ab,kw |
| #7 | PREMM*:ti,ab,kw |
| #8 | (bethesda NEAR/2 guideline*):ti,ab,kw |
| #9 | ((MLH1 or ‘path_MLH1’ or MSH2 or ‘path_MSH2’ or MSH6 or ‘path_MSH6’ or hMSH2 or hMLH1 or hPMS2 or hMSH6 or PMS2 or ‘path_PMS2’) NEAR/3 carrier*):ti,ab,kw |
| #10 | {or #1-#9} |
| #11 | ((endometr* or uter* or pipelle) NEAR/3 (aspirat* or biops* or cytology or sampl*)):ti,ab,kw |
| #12 | ((dilation or dilatation or vacuum) NEAR/2 curettage):ti,ab,kw |
| #13 | MeSH descriptor: [Dilatation and Curettage] explode all trees |
| #14 | ((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) NEAR/3 (echograph* or sonogra* or ultraso* or ultra NEXT so*)):ti,ab,kw |
| #15 | (hysteroscop*):ti,ab,kw |
| #16 | (‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4):ti,ab,kw |
| #17 | (((gynaecolog* or gynecolog*) NEAR/3 (exam* or screen* or surveill*))):ti,ab,kw |
| #18 | (((endometr* or uter*) NEAR/3 (exam* or screen* or surveill*))):ti,ab,kw |
| #19 | ((ovar* NEAR/3 (exam* or screen* or surveill*))):ti,ab,kw |
| #20 | ((uter* or intrauter* or ‘intra uter*’) NEAR/3 (clean* or wash*)):ti,ab,kw |
| #21 | {or #11-#20} |
| #22 | #10 and #21 in Trials |
CENTRAL (supplementary search without MMR or surveillance terms)
| Database | CENTRAL |
|---|---|
| Host | Cochrane Library |
| Issue | Issue 9 of 12, September 2020 |
| Date searched | 21 September 2020 |
| Searcher | SB |
| Hits | 173 |
Strategy
| #1 | (‘lynch syndrome’):ti,ab,kw |
| #2 | (‘Muir Torre syndrome’):ti,ab,kw |
| #3 | ((HNPCC or ‘hereditary nonpolyposis colorectal cancer’ or ‘hereditary non polyposis colorectal cancer’)):ti,ab,kw |
| #4 | MeSH descriptor: [Colorectal Neoplasms, Hereditary Nonpolyposis] this term only |
| #5 | ((Amsterdam NEAR/2 criter*)):ti,ab,kw |
| #6 | PREMM*:ti,ab,kw |
| #7 | (bethesda NEAR/2 guideline*):ti,ab,kw |
| #8 | ((MLH1 or ‘path_MLH1’ or MSH2 or ‘path_MSH2’ or MSH6 or ‘path_MSH6’ or hMSH2 or hMLH1 or hPMS2 or hMSH6 or PMS2 or ‘path_PMS2’) NEAR/3 carrier*):ti,ab,kw |
| #9 | {or #1-#8} in Trials |
CINAHL
| Database | CINAHL |
|---|---|
| Host | EBSCO |
| Issue | n/a |
| Date searched | 21 September 2020 |
| Searcher | SB |
| Hits | 57 |
Strategy
| S1 | TI ‘lynch syndrome’ OR AB ‘lynch syndrome’ |
| S2 | TI ‘Muir Torre syndrome’ OR AB ‘Muir Torre syndrome’ |
| S3 | TI (‘mismatch repair’ or MMR) OR AB (‘mismatch repair’ or MMR) |
| S4 | TI (HNPCC or ‘hereditary nonpolyposis colorectal cancer’ or ‘hereditary non polyposis colorectal cancer’) OR AB (HNPCC or ‘hereditary nonpolyposis colorectal cancer’ or ‘hereditary non polyposis colorectal cancer’) |
| S5 | (MH ‘Colorectal Neoplasms, Hereditary Nonpolyposis+’) |
| S6 | TI (Amsterdam N1 criter*) OR AB (Amsterdam N1 criter*) |
| S7 | TI PREMM* OR AB PREMM* |
| S8 | TI (bethesda N1 guideline*) OR AB (bethesda N1 guideline*) |
| S9 | TI ((MLH1 or ‘path_MLH1’ or MSH2 or ‘path_MSH2’ or MSH6 or ‘path_MSH6’ or hMSH2 or hMLH1 or hPMS2 or hMSH6 or PMS2 or ‘path_PMS2’) N2 carrier*) OR AB ((MLH1 or ‘path_MLH1’ or MSH2 or ‘path_MSH2’ or MSH6 or ‘path_MSH6’ or hMSH2 or hMLH1 or hPMS2 or hMSH6 or PMS2 or ‘path_PMS2’) N2 carrier*) |
| S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 |
| S11 | TI ((endometr* or uter* or pipelle) N2 (aspirat* or biops* or cytology or sampl*)) OR AB ((endometr* or uter* or pipelle) N2 (aspirat* or biops* or cytology or sampl*)) |
| S12 | TI (((dilation or dilatation or vacuum) N1 curettage)) OR AB (((dilation or dilatation or vacuum) N1 curettage)) |
| S13 | (MH ‘Dilatation and Curettage+’) |
| S14 | TI ((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) N2 (echograph* or sonogra* or ultraso* or ‘ultra so*’)) OR AB ((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) N2 (echograph* or sonogra* or ultraso* or ‘ultra so*’)) |
| S15 | TI hysteroscop* OR AB hysteroscop* |
| S16 | (MH ‘Hysteroscopy’) |
| S17 | TI ((‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4)) OR AB ((‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4)) |
| S18 | TI ((gynaecolog* or gynecolog*) N2 (exam* or screen* or surveill*)) OR AB ((gynaecolog* or gynecolog*) N2 (exam* or screen* or surveill*)) |
| S19 | TI (((endometr* or uter*) N2 (exam* or screen* or surveill*))) OR AB (((endometr* or uter*) N2 (exam* or screen* or surveill*))) |
| S20 | TI ((ovar* N2 (exam* or screen* or surveill*))) OR AB ((ovar* N2 (exam* or screen* or surveill*))) |
| S21 | (MH ‘Gynecologic Examination’) |
| S22 | TI (((uter* or intrauter* or ‘intra uter*’) N2 (clean* or wash*))) OR AB (((uter* or intrauter* or ‘intra uter*’) N2 (clean* or wash*))) |
| S23 | S11 OR S12 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 |
| S24 | S12 AND S25 |
EMBASE
| Database | EMBASE |
|---|---|
| Host | Ovid |
| Issue | 1974–18 September 2020 |
| Date searched | 21 September 2020 |
| Searcher | SB |
| Hits | 663 |
Strategy
-
‘lynch syndrome’.tw.
-
‘Muir Torre syndrome’.tw.
-
(‘mismatch repair’ or MMR).tw.
-
mismatch repair/
-
(HNPCC or ‘hereditary nonpolyposis colorectal cancer’ or ‘hereditary non polyposis colorectal cancer’).tw.
-
exp hereditary nonpolyposis colorectal cancer/
-
(Amsterdam adj2 criter*).tw.
-
PREMM*.tw.
-
(bethesda adj2 guideline*).tw.
-
((MLH1 or ‘path_MLH1’ or MSH2 or ‘path_MSH2’ or MSH6 or ‘path_MSH6’ or hMSH2 or hMLH1 or hPMS2 or hMSH6 or PMS2 or ‘path_PMS2’) adj3 carrier*).tw.
-
MutL protein homolog 1/
-
DNA mismatch repair protein MSH2/
-
protein MSH6/
-
mismatch repair protein PMS2/
-
or/1-14
-
((endometr* or uter* or pipelle) adj3 (aspirat* or biops* or cytology or sampl*)).tw.
-
endometrium biopsy/
-
((dilation or dilatation or vacuum) adj2 curettage).tw.
-
‘dilatation and curettage’/
-
((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) adj3 (echograph* or sonogra* or ultraso* or ‘ultra so*’)).tw.
-
exp transvaginal echography/
-
hysteroscop*.tw.
-
hysteroscopy/
-
(‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4).tw.
-
CA-125 antigen/
-
(gyn?ecolog* adj3 (exam* or screen* or surveill*)).tw.
-
gynecological examination/
-
((endometr* or uter*) adj3 (exam* or screen* or surveill*)).tw.
-
(ovar* adj3 (exam* or screen* or surveill*)).tw.
-
((uter* or intrauter* or ‘intra uter*’) adj3 (clean* or wash*)).tw.
-
or/16-30
-
15 and 31
MEDLINE
| Database | MEDLINE (ALL) |
|---|---|
| Host | Ovid |
| Issue | 1946–18 September 2020 |
| Date searched | 21 September 2020 |
| Searcher | SB |
| Hits | 253 |
Strategy
-
‘lynch syndrome’.tw.
-
‘Muir Torre syndrome’.tw.
-
(‘mismatch repair’ or MMR).tw.
-
(HNPCC or ‘hereditary nonpolyposis colorectal cancer’ or ‘hereditary non polyposis colorectal cancer’).tw.
-
Colorectal Neoplasms, Hereditary Nonpolyposis/
-
(Amsterdam adj2 criter*).tw.
-
PREMM*.tw.
-
(bethesda adj2 guideline*).tw.
-
((MLH1 or ‘path_MLH1’ or MSH2 or ‘path_MSH2’ or MSH6 or ‘path_MSH6’ or hMSH2 or hMLH1 or hPMS2 or hMSH6 or PMS2 or ‘path_PMS2’) adj3 carrier*).tw.
-
or/1-9
-
((endometr* or uter* or pipelle) adj3 (aspirat* or biops* or cytology or sampl*)).tw.
-
((dilation or dilatation or vacuum) adj2 curettage).tw.
-
exp ‘Dilatation and Curettage’/
-
((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) adj3 (echograph* or sonogra* or ultraso* or ‘ultra so*’)).tw.
-
hysteroscop*.tw.
-
(‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4).tw.
-
(gyn?ecolog* adj3 (exam* or screen* or surveill*)).tw.
-
((endometr* or uter*) adj3 (exam* or screen* or surveill*)).tw.
-
(ovar* adj3 (exam* or screen* or surveill*)).tw.
-
((uter* or intrauter* or ‘intra uter*’) adj3 (clean* or wash*)).tw.
-
or/11-20
Web of Science
| Database | SCI; CPCI – S |
|---|---|
| Host | Web of Science, Clarivate Analytics |
| Issue | n/a |
| Date searched | 21 September 2020 |
| Searcher | SB |
| Hits | 276 |
Strategy
-
TOPIC: (‘lynch syndrome’)
-
TOPIC: (‘Muir Torre syndrome’)
-
TOPIC: ((‘mismatch repair’ or MMR))
-
TOPIC: (HNPCC or ‘hereditary nonpolyposis colorectal cancer’ or ‘hereditary non polyposis colorectal cancer’)
-
TOPIC: (Amsterdam near/1 criter*)
-
TOPIC: (PREMM*)
-
TOPIC: (bethesda near/1 guideline*)
-
TOPIC: ((MLH1 or ‘path_MLH1’ or MSH2 or ‘path_MSH2’ or MSH6 or ‘path_MSH6’ or hMSH2 or hMLH1 or hPMS2 or hMSH6 or PMS2 or ‘path_PMS2’) near/2 carrier*)
-
#8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
-
TOPIC: ((endometr* or uter* or pipelle) near/2 (aspirat* or biops* or cytology or sampl*))
-
TOPIC: ((dilation or dilatation or vacuum) near/1 curettage)
-
TOPIC: ((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) near/2 (echograph* or sonogra* or ultraso* or ‘ultra so*’))
-
TOPIC: (hysteroscop*)
-
TOPIC: (‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4)
-
TOPIC: ((gynecolog* or gynaecolog*) near/2 (exam* or screen* or surveill*))
-
TOPIC: ((endometr* or uter*) near/2 (exam* or screen* or surveill*))
-
TOPIC: (ovar* near/2 (exam* or screen* or surveill*))
-
TOPIC: ((uter* or intrauter* or ‘intra uter*’) near/2 (clean* or wash*))
-
#18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10
-
#19 AND #9
Summary of search results
| Database | Hits |
|---|---|
| CENTRAL | 9 |
| CENTRAL (supplementary search) | 173 |
| CINAHL | 57 |
| EMBASE | 663 |
| MEDLINE | 253 |
| SCI and CPCI – S | 276 |
| Total records | 1431 |
| Duplicate records | 562 |
| Unique records | 869 |
Update searches
On 3 August 2021, SB conducted update searches using the search strategies described above and date limited from 2020 to the date of the search.
| Database, Host | Issue | Hits |
|---|---|---|
| CENTRAL, Cochrane Library | Issue 8 of 12, August 2021 | 3 |
| CENTRAL (supplementary search), Cochrane Library | Issue 8 of 12, August 2021 | 32 |
| CINAHL, EBSCO | N/A | 14 |
| EMBASE, Ovid | 1974–30 July 2021 August 3 |
126 |
| MEDLINE (ALL), Ovid | 1946–2 August 2021 | 42 |
| SCI and CPCI – S, Web of Science | N/A | 42 |
| Total records | 259 | |
| Duplicate records (internal to search results) | 97 | |
| Duplicate records (with original search) | 42 | |
| Duplicate records (identified by Covidence) | 10 | |
| Unique records | 110 |
Clinical trials registries
ClinicalTrials.gov
| Registry | ClinicalTrials.gov |
|---|---|
| URL | https://clinicaltrials.gov/ |
| Date searched | 9 August 2021 |
| Searcher | SB |
| Hits | 105 |
| Strategy | lynch OR HNPCC |
WHO ICTRP
| Registry | WHO ICTRP |
|---|---|
| URL | https://trialsearch.who.int/Default.aspx |
| Date searched | 9 August 2021 |
| Searcher | SB |
| Hits | 157 |
| Strategy | lynch OR HNPCC |
Systematic review of cost-effectiveness
Bibliographic databases
This systematic review used the same set of bibliographic database search results as the systematic review of clinical effectiveness (see above) and in addition searched the following databases.
Cost-effectiveness analysis registry
Search terms below were searched using ‘ratios’ setting in the basic search interface. Results were screened ‘on screen’ by the review team.
| Database | Cost-effectiveness analysis registry |
|---|---|
| Host | CEVR Tufts Medical Center |
| Issue | n/a |
| Date searched | 5 November 2020 |
| Searcher | NM |
| Hits | 12 |
Strategy
| Lynch syndrome | 9 |
|---|---|
| Muir–Torre syndrome | 0 |
| hereditary nonpolyposis colorectal cancer | 2 |
| hereditary non polyposis colorectal cancer | 0 |
| HNPCC | 1 |
EconLit
| Database | EconLit |
|---|---|
| Host | EBSCO |
| Issue | n/a |
| Date searched | 28 September 2020 |
| Searcher | SB |
| Hits | 30 |
Strategy
| S1 | TI ‘lynch syndrome’ OR AB ‘lynch syndrome’ |
| S2 | TI ‘Muir Torre syndrome’ OR AB ‘Muir Torre syndrome’ |
| S3 | TI (‘mismatch repair’ or MMR) OR AB (‘mismatch repair’ or MMR) |
| S4 | TI (HNPCC or ‘hereditary nonpolyposis colorectal cancer’ or ‘hereditary non polyposis colorectal cancer’) OR AB (HNPCC or ‘hereditary nonpolyposis colorectal cancer’ or ‘hereditary non polyposis colorectal cancer’) |
| S5 | TI (Amsterdam N1 criter*) OR AB)Amsterdam N1 criter*) |
| S6 | TI PREMM* OR AB PREMM* |
| S7 | TI (bethesda N1 guideline*) OR AB (bethesda N1 guideline*) |
| S8 | TI ((MLH1 or ‘path_MLH1’ or MSH2 or ‘path_MSH2’ or MSH6 or ‘path_MSH6’ or hMSH2 or hMLH1 or hPMS2 or hMSH6 or PMS2 or ‘path_PMS2’) N2 carrier*) OR AB ((MLH1 or ‘path_MLH1’ or MSH2 or ‘path_MSH2’ or MSH6 or ‘path_MSH6’ or hMSH2 or hMLH1 or hPMS2 or hMSH6 or PMS2 or ‘path_PMS2’) N2 carrier*) |
| S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 |
International Health Technology Assessment database
| Database | International HTA database |
|---|---|
| Host | INAHTA |
| Issue | n/a |
| Date searched | 28 September 2020 |
| Searcher | SB |
| Hits | 12 |
| Strategy | Title: ‘lynch syndrome’ OR ‘Muir Torre syndrome’ OR HNPCC OR ‘hereditary nonpolyposis colorectal cancer’ OR ‘hereditary non polyposis colorectal cancer’ OR Abstract: ‘lynch syndrome’ OR ‘Muir Torre syndrome’ OR HNPCC OR ‘hereditary nonpolyposis colorectal cancer’ OR ‘hereditary non polyposis colorectal cancer’ OR MeSH:‘Colorectal Neoplasms, Hereditary Nonpolyposis’ |
Summary of search results
| Database | Hits |
|---|---|
| Cost-Effectiveness Analysis Registry | 12 |
| CENTRAL | 9 |
| CENTRAL (supplementary search) | 173 |
| CINAHL | 57 |
| EconLit | 30 |
| EMBASE | 663 |
| International HTA Database | 12 |
| MEDLINE | 253 |
| SCI and CPCI – S | 276 |
| Total records | 1485 |
| Duplicate records | 558 |
| Unique records | 927 |
Systematic review of utility values
Bibliographic databases
Cost-Effectiveness Analysis Registry
| Database | Cost-Effectiveness Analysis Registry |
|---|---|
| Host | CEVR Tufts Medical Center |
| Issue | n/a |
| Date searched | 24 August 2021 |
| Searcher | NM |
| Hits | 217 |
Strategy
Search terms below were searched using ‘utility weights’ setting in the basic search interface. Results were screened ‘on screen’ by the review team.
-
endometrial (n = 45);
-
uterine (n = 38);
-
ovarian (n = 77);
-
hysterectomy (n = 52);
-
hysteroscopy (n = 0);
-
transvaginal (n = 5).
CENTRAL
| Database | CENTRAL |
|---|---|
| Host | Cochrane Library |
| Issue | 11 of 12 November 2020 |
| Date searched | 10 November 2020 |
| Searcher | SB |
| Hits | 378 |
Strategy
| #1 | (((endometr* or uter* or pipelle) NEAR/3 (aspirat* or biops* or cytology or sampl*))):ti,ab,kw |
| #2 | ((dilation or dilatation or vacuum) NEAR/2 curettage):ti,ab,kw |
| #3 | MeSH descriptor: [Dilatation and Curettage] explode all trees |
| #4 | (((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) NEAR/3 (echograph* or sonogra* or ultraso* or ultra NEXT so*))):ti,ab,kw |
| #5 | (hysteroscop*):ti,ab,kw |
| #6 | (‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4):ti,ab,kw |
| #7 | (((gynaecolog* or gynecolog*) NEAR/3 (exam* or screen* or surveill*))):ti,ab,kw |
| #8 | (((endometr* or uter*) NEAR/3 (exam* or screen* or surveill*))):ti,ab,kw |
| #9 | ((ovar* NEAR/3 (exam* or screen* or surveill*))):ti,ab,kw |
| #10 | ((uter* or intrauter* or ‘intra uter*’) NEAR/3 (clean* or wash*)):ti,ab,kw |
| #11 | {or #1-#10} |
| #12 | (hysterectom*.):ti,ab,kw |
| #13 | ((‘salpingo oophorectom*’ or ‘salpingo ovariectom*’ or salpingooophorectom*)):ti,ab,kw |
| #14 | (salpingectom* near/7 (oophorectom* or ovariectom*)):ti,ab,kw |
| #15 | MeSH descriptor: [Hysterectomy] explode all trees |
| #16 | MeSH descriptor: [Salpingectomy] this term only |
| #17 | MeSH descriptor: [Ovariectomy] this term only |
| #18 | (‘H BSO’ or ‘TAH BSO’ or ‘TLH BSO’ or ‘LAVH BSO’):ti,ab,kw |
| #19 | 17-#18 |
| #20 | ((endom* or uter*) near/3 (cancer* or neoplas* or carcinom* or adenocarcinom* or malignan* or tumor* or tumour*)):ti,ab,kw |
| #21 | MeSH descriptor: [Endometrial Neoplasms] explode all trees |
| #22 | #20 or #21 |
| #23 | (ovar* near/3 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)):ti,ab,kw |
| #24 | MeSH descriptor: [Ovarian Neoplasms] explode all trees |
| #25 | #23 or #24 |
| #26 | (quality near/2 (life or wellbeing or ‘well being’)):ti,ab,kw |
| #27 | (hql or hqol or ‘h qol’ or hrqol or ‘hr qol’):ti,ab,kw |
| #28 | MeSH descriptor: [Quality of Life] this term only |
| #29 | (preference*):ti,ab,kw |
| #30 | {or #26-#28} |
| #31 | #29 and #30 |
| #32 | (qaly* or qald* or qale* or qtime*):ti,ab,kw |
| #33 | MeSH descriptor: [Quality-Adjusted Life Years] this term only |
| #34 | (‘disability adjusted life’ or daly*):ti,ab,kw |
| #35 | (sf36 or ‘sf 36’ or ‘short form 36’ or ‘shortform 36’ or ‘sf thirtysix’ or ‘sf thirty six’ or ‘shortform thirtysix’ or ‘shortform thirty six’ or ‘short form thirtysix’ or ‘short form thirty six’):ti,ab,kw |
| #36 | (sf6 or ‘sf 6’ or ‘short form 6’ or ‘shortform 6’ or ‘sf six’ or sfsix or ‘shortform six’ or ‘short form six’):ti,ab,kw |
| #37 | (sf12 or ‘sf 12’ or ‘short form 12’ or ‘shortform 12’ or ‘sf twelve’ or sftwelve or ‘shortform twelve’ or ‘short form twelve’):ti,ab,kw |
| #38 | (sf6D or ‘sf 6D’ or ‘short form 6D’ or ‘shortform 6D’ or ‘sf six D’ or sfsixD or ‘shortform six D’ or ‘short form six D’):ti,ab,kw |
| #39 | (sf20 or ‘sf 20’ or ‘short form 20’ or ‘shortform 20’ or ‘sf twenty’ or sftwenty or ‘shortform twenty’ or ‘short form twenty’):ti,ab,kw |
| #40 | (sf20 or ‘sf 20’ or ‘short form 20’ or ‘shortform 20’ or ‘sf twenty’ or sftwenty or ‘shortform twenty’ or ‘short form twenty’):ti,ab,kw |
| #41 | (euroqol or ‘euro qol’ or eq5d or ‘eq 5d’ or ‘eq 5d 3l’ or ‘eq 5d 5l’):ti,ab,kw |
| #42 | (AQoL):ti,ab,kw |
| #43 | (‘health* year* equivalent*’ or hye or hyes):ti,ab,kw |
| #44 | (utilit* near/3 (analys* or assess* or estimat* or scor* or valu*)):ti,ab,kw |
| #45 | (‘health utility index’ or hui or hui1 or hui2 or hui3):ti,ab,kw |
| #46 | (disutili*):ti,ab,kw |
| #47 | (‘standard gamble*’):ti,ab,kw |
| #48 | (‘time trade off’ or ‘time tradeoff’ or tto):ti,ab,kw |
| #49 | {or #31-#48} |
| #50 | #11 or #19 or #22 or #25 |
| #51 | #49 and #50 |
EconLit
| Database | EconLit |
|---|---|
| Host | EBSCO |
| Issue | n/a |
| Date searched | 10 November 2020 |
| Searcher | SB |
| Hits | 49 |
Strategy
-
TI ((endometr* or uter* or pipelle) N2 (aspirat* or biops* or cytology or sampl*)) OR AB ((endometr* or uter* or pipelle) N2 (aspirat* or biops* or cytology or sampl*))
-
TI (((dilation or dilatation or vacuum) N1 curettage)) OR AB (((dilation or dilatation or vacuum) N1 curettage))
-
TI ((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) N2 (echograph* or sonogra* or ultraso* or ‘ultra so*’)) OR AB ((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) N2 (echograph* or sonogra* or ultraso* or ‘ultra so*’))
-
TI hysteroscop* OR AB hysteroscop*
-
TI ((‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4)) OR AB ((‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4))
-
TI ((gynaecolog* or gynecolog*) N2 (exam* or screen* or surveill*)) OR AB ((gynaecolog* or gynecolog*) N2 (exam* or screen* or surveill*))
-
TI (((endometr* or uter*) N2 (exam* or screen* or surveill*))) OR AB (((endometr* or uter*) N2 (exam* or screen* or surveill*)))
-
TI ((ovar* N2 (exam* or screen* or surveill*))) OR AB ((ovar* N2 (exam* or screen* or surveill*)))
-
TI (((uter* or intrauter* or ‘intra uter*’) N2 (clean* or wash*))) OR AB (((uter* or intrauter* or ‘intra uter*’) N2 (clean* or wash*)))
-
TI hysterectom* OR AB hysterectom*
-
TI ((‘salpingo oophorectom*’ or ‘salpingo ovariectom*’ or salpingooophorectom*)) OR AB ((‘salpingo oophorectom*’ or ‘salpingo ovariectom*’ or salpingooophorectom*))
-
TI ((salpingectom* N6 (oophorectom* or ovariectom*))) OR AB ((salpingectom* N6 (oophorectom* or ovariectom*)))
-
TI (‘H BSO’ or ‘TAH BSO’ or ‘TLH BSO’ or ‘LAVH BSO’) OR AB (‘H BSO’ or ‘TAH BSO’ or ‘TLH BSO’ or ‘LAVH BSO’)
-
TI (((endom* or uter*) N2 (cancer* or neoplas* or carcinom* or adenocarcinom* or malignan* or tumor* or tumour*))) OR AB (((endom* or uter*) N2 (cancer* or neoplas* or carcinom* or adenocarcinom* or malignan* or tumor* or tumour*)))
-
TI ((ovar* N2 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*))) OR AB ((ovar* N2 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)))
-
S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15
EMBASE
| Database | EMBASE |
|---|---|
| Host | Ovid |
| Issue | 1974–9 November 2020 |
| Date searched | 10 November 2020 |
| Searcher | SB |
| Hits | 2465 |
Strategy
-
((endometr* or uter* or pipelle) adj3 (aspirat* or biops* or cytology or sampl*)).tw.
-
endometrium biopsy/
-
((dilation or dilatation or vacuum) adj2 curettage).tw.
-
‘dilatation and curettage’/
-
((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) adj3 (echograph* or sonogra* or ultraso* or ‘ultra so*’)).tw.
-
exp transvaginal echography/
-
exp transvaginal echography/
-
hysteroscop*.tw.
-
hysteroscopy/
-
(‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4).tw.
-
CA-125 antigen/
-
(gyn?ecolog* adj3 (exam* or screen* or surveill*)).tw.
-
gynecological examination/
-
((endometr* or uter*) adj3 (exam* or screen* or surveill*)).tw.
-
(ovar* adj3 (exam* or screen* or surveill*)).tw.
-
((uter* or intrauter* or ‘intra uter*’) adj3 (clean* or wash*)).tw.
-
or/1-16
-
hysterectom*.tw.
-
(‘salpingo oophorectom*’ or ‘salpingo ovariectom*’ or salpingooophorectom*).tw.
-
(salpingectom* adj7 (oophorectom* or ovariectom*)).tw.
-
exp hysterectomy/
-
salpingooophorectomy/
-
(‘H BSO’ or ‘TAH BSO’ or ‘TLH BSO’ or ‘LAVH BSO’).tw.
-
or/18-23
-
((endom* or uter*) adj3 (cancer* or neoplas* or carcinom* or adenocarcinom* or malignan* or tumor* or tumour*)).tw.
-
exp endometrium tumor/
-
exp uterus tumor/
-
or/25-27
-
(ovar* adj3 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)).tw.
-
exp ovary tumor/
-
29 or 30
-
(quality adj2 (life or wellbeing or ‘well being’)).tw.
-
(hql or hqol or ‘h qol’ or hrqol or ‘hr qol’).tw.
-
quality of life/
-
preference*.tw.
-
(or/32-34) and 35
-
(qaly* or qald* or qale* or qtime*).tw.
-
quality-adjusted life years/
-
(‘disability adjusted life’ or daly*).tw.
-
(sf36 or ‘sf 36’ or ‘short form 36’ or ‘shortform 36’ or ‘sf thirtysix’ or ‘sf thirty six’ or ‘shortform thirtysix’ or ‘shortform thirty six’ or ‘short form thirtysix’ or ‘short form thirty six’).tw.
-
(sf6 or ‘sf 6’ or ‘short form 6’ or ‘shortform 6’ or ‘sf six’ or sfsix or ‘shortform six’ or ‘short form six’).tw.
-
(sf12 or ‘sf 12’ or ‘short form 12’ or ‘shortform 12’ or ‘sf twelve’ or sftwelve or ‘shortform twelve’ or ‘short form twelve’).tw.
-
(sf6D or ‘sf 6D’ or ‘short form 6D’ or ‘shortform 6D’ or ‘sf six D’ or sfsixD or ‘shortform six D’ or ‘short form six D’).tw.
-
(sf20 or ‘sf 20’ or ‘short form 20’ or ‘shortform 20’ or ‘sf twenty’ or sftwenty or ‘shortform twenty’ or ‘short form twenty’).tw.
-
(euroqol or ‘euro qol’ or eq5d or ‘eq 5d’ or ‘eq 5d 3l’ or ‘eq 5d 5l’).tw.
-
exp ‘european quality of life 5 dimensions questionnaire’/
-
AQoL.tw.
-
(‘health* year* equivalent*’ or hye or hyes).tw.
-
(utilit* adj3 (analys* or assess* or estimat* or scor* or valu*)).tw.
-
(‘health utility index’ or hui or hui1 or hui2 or hui3).tw.
-
disutili*.tw.
-
‘standard gamble*’.tw.
-
(‘time trade off’ or ‘time tradeoff’ or tto).tw.
-
or/36-52
-
17 or 24 or 28 or 31
-
54 and 55
EuroQol group
We attempted to search (24 August 2021) for EQ-5D publications using the interface at https://euroqol.org/search-for-eq-5d-publications but the search interface was not operational so we could not complete this part of the search strategy.
International Health Technology Assessment database
| Database | International HTA database |
|---|---|
| Host | INAHTA |
| Issue | n/a |
| Date searched | 24 August 2021 |
| Searcher | NM |
| Hits | 383 |
| Strategy | Title: endom* OR uter* OR ovar* OR Abstract: endom* OR uter* OR ovar* |
National Institute for Health and Care Excellence guidance
| Database | NICE guidance |
|---|---|
| Host | NICE website (https://nice.org.uk/) |
| Issue | n/a |
| Date searched | 24 August 2021 |
| Searcher | NM |
| Hits | 27 |
| Strategy | Searched the NICE website for published guidance on ‘ovarian cancer’; ‘endometrial cancer’; ‘hysterectomy’; ‘hysteroscopy’; and ‘transvaginal’ |
MEDLINE
| Database | MEDLINE (ALL) |
|---|---|
| Host | Ovid |
| Issue | 1946–9 November 2020 |
| Date searched | 10 November 2020 |
| Searcher | SB |
| Hits | 754 |
Strategy
-
((endometr* or uter* or pipelle) adj3 (aspirat* or biops* or cytology or sampl*)).tw.
-
((dilation or dilatation or vacuum) adj2 curettage).tw.
-
exp ‘Dilatation and Curettage’/
-
((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) adj3 (echograph* or sonogra* or ultraso* or ‘ultra so*’)).tw.
-
hysteroscop*.tw.
-
(‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4).tw.
-
(gyn?ecolog* adj3 (exam* or screen* or surveill*)).tw.
-
((endometr* or uter*) adj3 (exam* or screen* or surveill*)).tw.
-
(ovar* adj3 (exam* or screen* or surveill*)).tw.
-
((uter* or intrauter* or ‘intra uter*’) adj3 (clean* or wash*)).tw.
-
or/1-10
-
hysterectom*.tw.
-
(‘salpingo oophorectom*’ or ‘salpingo ovariectom*’ or salpingooophorectom*).tw.
-
(salpingectom* adj7 (oophorectom* or ovariectom*)).tw.
-
exp Hysterectomy/
-
Salpingectomy/
-
Ovariectomy/
-
(‘H BSO’ or ‘TAH BSO’ or ‘TLH BSO’ or ‘LAVH BSO’).tw.
-
or/12-18
-
((endom* or uter*) adj3 (cancer* or neoplas* or carcinom* or adenocarcinom* or malignan* or tumor* or tumour*)).tw.
-
exp Endometrial Neoplasms/
-
20 or 21
-
(ovar* adj3 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)).tw.
-
exp Ovarian Neoplasms/
-
23 or 24
-
(quality adj2 (life or wellbeing or ‘well being’)).tw.
-
(hql or hqol or ‘h qol’ or hrqol or ‘hr qol’).tw.
-
‘Quality of Life’/
-
preference*.tw.
-
(or/26-28) and 29
-
(qaly* or qald* or qale* or qtime*).tw.
-
quality-adjusted life years/
-
(‘disability adjusted life’ or daly*).tw.
-
(sf36 or ‘sf 36’ or ‘short form 36’ or ‘shortform 36’ or ‘sf thirtysix’ or ‘sf thirty six’ or ‘shortform thirtysix’ or ‘shortform thirty six’ or ‘short form thirtysix’ or ‘short form thirty six’).tw.
-
(sf6 or ‘sf 6’ or ‘short form 6’ or ‘shortform 6’ or ‘sf six’ or sfsix or ‘shortform six’ or ‘short form six’).tw.
-
(sf12 or ‘sf 12’ or ‘short form 12’ or ‘shortform 12’ or ‘sf twelve’ or sftwelve or ‘shortform twelve’ or ‘short form twelve’).tw.
-
(sf6D or ‘sf 6D’ or ‘short form 6D’ or ‘shortform 6D’ or ‘sf six D’ or sfsixD or ‘shortform six D’ or ‘short form six D’).tw.
-
(sf20 or ‘sf 20’ or ‘short form 20’ or ‘shortform 20’ or ‘sf twenty’ or sftwenty or ‘shortform twenty’ or ‘short form twenty’).tw.
-
(euroqol or ‘euro qol’ or eq5d or ‘eq 5d’ or ‘eq 5d 3l’ or ‘eq 5d 5l’).tw.
-
AQoL.tw.
-
(‘health* year* equivalent*’ or hye or hyes).tw.
-
(utilit* adj3 (analys* or assess* or estimat* or scor* or valu*)).tw.
-
(‘health utility index’ or hui or hui1 or hui2 or hui3).tw.
-
disutili*.tw.
-
‘standard gamble*’.tw.
-
(‘time trade off’ or ‘time tradeoff’ or tto).tw.
-
or/30-46
-
11 or 19 or 22 or 25
-
47 and 48
Web of Science
| Database | SCI; CPCI – S |
|---|---|
| Host | Web of Science, Clarivate Analytics |
| Issue | n/a |
| Date searched | 10 November 2020 |
| Searcher | SB |
| Hits | 820 |
Strategy
-
TOPIC: ((endometr* or uter* or pipelle) near/2 (aspirat* or biops* or cytology or sampl*))
-
TOPIC: ((dilation or dilatation or vacuum) near/1 curettage)
-
TOPIC: ((pelvi* or transabdominal or transvaginal or ‘trans vaginal’) near/2 (echograph* or sonogra* or ultraso* or ‘ultra so*’))
-
TOPIC: (hysteroscop*)
-
TOPIC: (‘cancer antigen 125’ or ‘carbohydrate antigen 125’ or ‘CA-125’ or CA-125 or ‘human epididymis protein 4’ or HE4)
-
TOPIC: ((gynecolog* or gynaecolog*) near/2 (exam* or screen* or surveill*))
-
TOPIC: ((endometr* or uter*) near/2 (exam* or screen* or surveill*))
-
TOPIC: (ovar* near/2 (exam* or screen* or surveill*))
-
TOPIC: ((uter* or intrauter* or ‘intra uter*’) near/2 (clean* or wash*))
-
#9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
-
TOPIC: (hysterectom*)
-
TOPIC: (‘salpingo oophorectom*’ or ‘salpingo ovariectom*’ or salpingooophorectom*)
-
TOPIC: (salpingectom* near/6 (oophorectom* or ovariectom*))
-
TOPIC: (‘H BSO’ or ‘TAH BSO’ or ‘TLH BSO’ or ‘LAVH BSO’)
-
#14 OR #13 OR #12 OR #11
-
TOPIC: ((endom* or uter*) near/2 (cancer* or neoplas* or carcinom* or adenocarcinom* or malignan* or tumor* or tumour*))
-
TOPIC: (ovar* near/2 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*))
-
#17 OR #16
-
TOPIC: (quality near/1 (life or wellbeing or ‘well being’))
-
TOPIC: (hql or hqol or ‘h qol’ or hrqol or ‘hr qol’)
-
TOPIC: (preference*)
-
#20 OR #19
-
#22 AND #21
-
TOPIC: (qaly* or qald* or qale* or qtime*)
-
TOPIC: (‘disability adjusted life’ or daly*)
-
TOPIC: (sf36 or ‘sf 36’ or ‘short form 36’ or ‘shortform 36’ or ‘sf thirtysix’ or ‘sf thirty six’ or ‘shortform thirtysix’ or ‘shortform thirty six’ or ‘short form thirtysix’ or ‘short form thirty six’)
-
TOPIC: (sf6 or ‘sf 6’ or ‘short form 6’ or ‘shortform 6’ or ‘sf six’ or sfsix or ‘shortform six’ or ‘short form six’)
-
TOPIC: (sf12 or ‘sf 12’ or ‘short form 12’ or ‘shortform 12’ or ‘sf twelve’ or sftwelve or ‘shortform twelve’ or ‘short form twelve’)
-
TOPIC: (sf6D or ‘sf 6D’ or ‘short form 6D’ or ‘shortform 6D’ or ‘sf six D’ or sfsixD or ‘shortform six D’ or ‘short form six D’)
-
TS = (sf20 or ‘sf 20’ or ‘short form 20’ or ‘shortform 20’ or ‘sf twenty’ or sftwenty or ‘shortform twenty’ or ‘short form twenty’)
-
TOPIC: (euroqol or ‘euro qol’ or eq5d or ‘eq 5d’ or ‘eq 5d 3l’ or ‘eq 5d 5l’)
-
TOPIC: (AQoL)
-
TOPIC: (‘health* year* equivalent*’ or hye or hyes)
-
TOPIC: (utilit* near/2 (analys* or assess* or estimat* or scor* or valu*))
-
TOPIC: (‘health utility index’ or hui or hui1 or hui2 or hui3)
-
TOPIC: (disutili*)
-
TOPIC: (‘standard gamble*’)
-
TOPIC: (‘time trade off’ or ‘time tradeoff’ or tto)
-
#38 OR #37 OR #36 OR #35 OR #34 OR #33 OR #32 OR #31 OR #30 OR #29 OR #28 OR #27 OR #26 OR #25 OR #24 OR #23
-
#18 OR #15 OR #10
-
#40 and #39
Summary of search results
| Database | Hits |
|---|---|
| Cost-Effectiveness Analysis Registry | 217 |
| CENTRAL | 378 |
| EconLit | 49 |
| EMBASE | 2465 |
| EQ-5D publications | n/a |
| International HTA Database | 383 |
| NICE published guidance | 27 |
| MEDLINE | 754 |
| SCI and CPCI – S | 820 |
| Total records | 5093 |
Appendix 2 Studies excluded at full-text screening from the systematic review of clinical effectiveness
| Journal article | Reason for exclusion |
|---|---|
| Aiyer K, Bartosch C, Cohen D, Dreef E, Nielsen M, Oliva E, et al. Mismatch repair deficient gland foci in histologically normal endometrium of Lynch syndrome patients. Virchows Archiv 2017;471:S81. https://doi.org/10.1007/s00428-017-2205-0 | Not surveillance |
| Arai M, Koyama M, Honjyo K, Tanakaya K, Akagi K, Yamaguchi T, et al. Clinical significance of FDG-PET examinations for surveillance in patients with Lynch syndrome. Fam Cancer 2011;10:S11–2. http://dx.doi.org/10.1007/s10689-011-9424-3 | Abstract |
| Arts-De Jong M, Van Ham MA, Massuger LF, Hoogerbrugge N, De Hullu JA. Efficacy of gynecological surveillance on ovarian cancer in women with Lynch syndrome. Int J Gynecol Cancer 2012;22:E264. https//doi.org/10.1097/01.IGC.0000422085.58592.d3 | Abstract |
| Auranen A, Joutsiniemi T. A systematic review of gynecological cancer surveillance in women belonging to hereditary nonpolyposis colorectal cancer (Lynch syndrome) families. Acta Obstet Gynecol Scand 2011;90:437–44. https://dx.doi.org/10.1111/j.1600-0412.2011.01091.x | Study design |
| Barrett J, Jenkins V, Farewell V, Menon U, Jacobs I, Kilkerr J, et al. Psychological morbidity associated with ovarian cancer screening: results from more than 23,000 women in the randomised trial of ovarian cancer screening (UKCTOCS). BJOG 2014;121:1071–9. https://doi.org/10.1111/1471-0528.12870 | Population |
| Bats A, Le Frere-Belda M, Metzger U, Laurent-Puig P, Lecuru F. Endometrial cancer screening in patients with Lynch syndrome. J Clin Oncol 2011;29. | Abstract |
| Berchuck A, Havrilesky LJ, Kauff ND. Is there a role for ovarian cancer screening in high-risk women? J Clin Oncol 2017;35:1384–6. https://doi.org/10.1200/JCO.2016.72.0045 | Study design |
| Bouquier J, Blons H, Narjoz C, Lecuru F, Laurent-Puig P, Bats AS. Microsatellite instability analysis in uterine cavity washings as a screening tool for endometrial cancer in Lynch syndrome. Fam Cancer 2011;10:655–7. https://doi.org/10.1007/s10689-011-9470-x | Study design |
| Brown GJ, St John DJ, Macrae FA, Aittomaki K. Cancer risk in young women at risk of hereditary nonpolyposis colorectal cancer: implications for gynecologic surveillance. Gynecol Oncol 2001;80:346–9. | Not surveillance |
| Burton AM, Sun CC, Daniels MS, Boyd-Rogers S, Lynch PM, Lu KH, et al. ‘The Proceedings of the Collaborative Group of the Americas on Inherited Colorectal Cancer’ Delta Centre-Ville Hotel, Montreal, Quebec, Canada, October 10–11, 2011. Abstracts. Fam Cancer 2011;10:713–43. https://doi.org/10.1007/s10689-011-9492-4 | Outcomes |
| Burton AM, Sun CC, Daniels MS, Boyd-Rogers S, Lynch PM, Lu KH, et al. Screening and communication with physicians for women with Lynch syndrome: findings from a qualitative study. Cancer Prev Res 2011;4. https://doi.org/http://dx.doi.org/10.1158/1940-6207.PREV-11-B29 | Outcomes |
| Burton-Chase AM, Hovick SR, Sun CC, Boyd-Rogers S, Lynch PM, Lu KH, et al. Gynecologic cancer screening and communication with health care providers in women with Lynch syndrome. Clin Genet 2014;86:185–9. https://doi.org/10.1111/cge.12246 | Outcomes |
| Chen LM, Yang KY, Little SE, Cheung MK, Caughey AB. Gynecologic cancer prevention in Lynch syndrome/hereditary nonpolyposis colorectal cancer families. Obstet Gynecol 2007;110:18–25. https://doi.org/10.1097/01.AOG.0000267500.27329.85 | Study design |
| ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2003 Jan 27. Identifier: NCT00033488; Screening Women at High Generic Risk for Ovarian Cancer; 2013 Dec 18. URL: https://clinicaltrials.gov/ct2/show/NCT00033488 (accessed 7 September 2021) | Duplicate (published study screened at FT) |
| ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2007 Aug 2. Identifier: NCT00510796; Combined Colon and Endometrial Cancer Screening in Women With HNPCC; 2016 Feb 17. URL: https://clinicaltrials.gov/ct2/show/NCT00033488 (accessed 7 September 2021) | Duplicate (published study screened at FT) |
| ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2007 July 30. Identifier: NCT00508846; Screening for Gynecologic Cancers in Hereditary Nonpolyposis Colorectal Cancer (HNPCC) Patients; 2016 Mar 11. URL: https://clinicaltrials.gov/ct2/show/NCT00508846 (accessed 7 September 2021) | Unable to retrieve FT |
| Collins V, Meiser B, Gaff C, St John DJ, Halliday J. Screening and preventive behaviors one year after predictive genetic testing for hereditary nonpolyposis colorectal carcinoma. Cancer 2005;104:273–81. https://doi.org/10.1002/cncr.21183 | Outcomes |
| Cornou C, Bats AS, Vannieuwenhuyse G, Capmas P, Bensaid C, Ngo C, et al. Impact of gynecologic screening in Lynch syndrome. Gynecol Oncol 2016;141:27. https://doi.org/10.1016/j.ygyno.2016.04.094 | Abstract |
| Crawford R, Newcombe B, Bolton H, Ngu SF, Freeman S, Addley H, et al. The ten year experience of a regional specialist gynaecology cancer genetics clinic with Lynch syndrome. Int J Gynecol Cancer 2017;27:95. https://doi.org/10.1097/01.IGC.0000527296.86225.87 | Abstract |
| Chu MMY, Ngu SF, Tse KY, Chan KKL, Ngan HYS. Patients’ acceptability of different screening tests for gynaecological malignancy in Lynch syndrome carriers. Int J Gynecol Cancer 2017;27:1315–6. https://doi.org/10.1097/01.IGC.0000527296.86225.87 | Abstract |
| Debniak T, Gromowski T, Scott RJ, Gronwald J, Huzarski T, Byrski T, et al. Management of ovarian and endometrial cancers in women belonging to HNPCC carrier families: review of the literature and results of cancer risk assessment in Polish HNPCC families. Hered Cancer Clin Practice 2015;13:3. https://doi.org/10.1186/s13053-015-0025-2 | Study design |
| Del Pup L, Fornasarig M, Giorda G, Sopracordevole F, Zanin G, Lucia E, et al. Prevention and early diagnosis of gynaecological malignancies in hereditary non-polyposis cancer (or Lynch) syndrome. G Ital Ostet Ginecol 2012;34:45–8. | Study design |
| Douay-Hauser N, Bats AS, Huchon C, Bensaid C, Seror J, Cellier C, et al. Diagnostic value of diagnostic hysteroscopy for gynaecologic screening in Lynch syndrome: office vs general anaesthesia. Int J Gynecol Cancer 2013;23:1027. | Abstract |
| Douay-Hauser NDH, Bats ASB, Bensaid CB, Seror JS, Ngo CN, Huchon CH, et al. Which strategy for the gynaecological screening in Lynch syndrome? a retrospective comparison of clinical examination, transvaginal ultrasound, and diagnostic hysteroscopy. Int J Gynecol Cancer 2014;24:1520. https://doi.org/10.1097/01.IGC.0000457075.08973.89 | Abstract |
| Dutton PJ, Green K, Whyte L, Lalloo F, Evans G, Seif MW. Experience of screening for endometrial cancer in high-risk population. BJOG 2017;124:40. https://doi.org/10.1111/1471-0528.10_14571 | Abstract |
| Escobar PF. Office hysteroscopy in women at risk of human nonpolyposis colon cancer. Int J Gynecol Cancer 2009;19:1152. https://doi.org/10.1111/IGC.0b013e3181a7f6eb | Study design |
| EU Clinical Trials Register [Internet]. European Medicines Agency. 2006 May 05. Identifier: EUCTR2006-001815-30-GB 2006; Prevention of Endometrial Tumours (POET). URL: www.clinicaltrialsregister.eu/ctr-search/trial/2006-001815-30/GB (accessed 7 September 2021) | Study aborted |
| Evans DG, Gaarenstroom KN, Stirling D, Shenton A, Maehle L, Dorum A, et al. Screening for familial ovarian cancer: poor survival of BRCA1/2 related cancers. J Med Genet 2009;46:593–7. https://doi.org/10.1136/jmg.2008.058248 | Population |
| Fambrini M, Sorbi F, Guaschino S. Risk factors for developing endometrial cancer after benign endometrial sampling. Obstet Gynecol 2013;121:381–2. https://doi.org/10.1097/AOG.0b013e318280a16a | Unable to retrieve full text |
| Fambrini M, Sorbi F, Guaschino S, Fambrini M, Sorbi F, Guaschino S. Risk factors for developing endometrial cancer after benign endometrial sampling … Obstet Gynecol 2012 Nov;120(5):998–1004; Obstet Gynecol 2012 Nov;120(5):989–91. Obstet Gynecol 2013;121:381–2. https://doi.org/10.1097/aog.0b013e318280a16a | Duplicate |
| Fornasarig M, Tabuso M, Orzes E, Talamini R, Canzonieri V, Cannizzaro R, et al. Getting old with Lynch syndrome in northeastern. Gastroenterology 2013;144:S395–6. | Abstract |
| Fornasarig M, Magris R, Maiero S, Viel A, Canzonieri V, Cannizzaro R. Precancerous condition as risk factor for gastric cancer in Lynch syndrome. Gastroenterology 2018;154:S-794. https://doi.org/10.1016/S0016-5085%2818%2932728-8 | Abstract |
| Frey MK, Pauk SJ, Caputo TA, Moss HA, Sapra KJ, Gerber DA, et al. Availability and scope of integrated screening for patients with Lynch syndrome. Obstet Gynecol Surv 2016;71:26–7. https://doi.org/10.1097/01.ogx.0000475862.62078.8e | Study design |
| Gaarenstroom KN, van der Hiel B, Tollenaar RA, Vink GR, Jansen FW, van Asperen CJ, et al. Efficacy of screening women at high risk of hereditary ovarian cancer: results of an 11-year cohort study. Int J Gynecol Cancer 2006;16(Suppl. 1):54–9. https://doi.org/10.1111/j.1525-1438.2006.00480.x | Population |
| Gosset M, Rossi L, Cornou C, Ngo C, Delomenie M, Nos C, et al. Impact of gynecologic screening in Lynch syndrome. Int J Gynecol Cancer 2017;27:221. https://doi.org/10.1097/01.IGC.0000527296.86225.87 | Abstract |
| Guillen-Ponce C, Martinez-Sevila C, Perea R, Arenas M, Molina-Garrido MJ, Goicoechea M, et al. Gynecologic cancer screening in women at high risk of Lynch syndrome. J Clin Oncol 2011;29. | Abstract |
| Helder-Woolderink JM, Blok EAW, Vasen HFA, Hollema H, Mourits MJE, Bock De GH. Ovarian cancer in Lynch syndrome: a systematic review. Int J Gynecol Cancer 2015;25:449. https://doi.org/10.1097/01.IGC.0000473498.85773.6e | Study design |
| Helder-Woolderink JM, Blok EA, Vasen HFA, Hollema H, Mourits MJ, De Bock GH. Ovarian cancer in Lynch syndrome; a systematic review. Eur J Cancer (Oxford, England: 1990) 2016;55:65–73. https://doi.org/10.1016/j.ejca.2015.12.005 | Study design |
| Helder-Woolderink J, De Bock G, Van Hemel B, Hollema H, Werner N, Mourits M. Analysis of endometrial cells obtained by vaginal tampons during annual gynaecological surveillance in women with Lynch syndrome. Int J Gynecol Cancer 2017;27:1102. https://doi.org/10.1097/01.IGC.0000527296.86225.87 | Outcomes |
| Jia S, Wu X, Zhang Y, Zhang M. Chinese Lynch syndrome-associated colorectal cancer patients’ self-concept and adherence to surveillance. Eur J Cancer Care (Engl) 2021;30(2):e13379. https://doi.org/10.1111/ecc.13379 | Outcomes |
| Karlan BY, Thorpe J, Watabayashi K, Drescher CW, Palomares M, Daly MB, et al. Use of CA-125 and HE4 serum markers to predict ovarian cancer in elevated-risk women. Cancer Epidemiol Biomarkers Prev 2014;23:1383–93. https://doi.org/10.1158/1055-9965.EPI-13-1361 | Population |
| Katz LH, Burton-Chase AM, Advani S, Fellman B, Polivka KM, Yuan Y, et al. Screening adherence and cancer risk perceptions in colorectal cancer survivors with Lynch-like syndrome. Clin Genet 2016;89:392–8. https://doi.org/10.1111/cge.12653 | Outcomes |
| Ketabi Z, Mosgaard BJ, Bernstein IT. Knowledge of gynecologic cancer risk and screening in women from hereditary non-polyposis colorectal cancer families. Fam Cancer 2011;10:S8. https://doi.org/10.1007/s10689-011-9424-3 | Outcomes |
| Ketabi Z, Mosgaard BJ, Gerdes AM, Ladelund S, Bernstein IT. Awareness of endometrial cancer risk and compliance with screening in hereditary nonpolyposis colorectal cancer. Obstet Gynecol 2012;120:1005–12. https://doi.org/10.1097/aog.0b013e31826ba2aa | Outcomes |
| Lai T, Kessel B, Ahn HJ, Terada KY. Ovarian cancer screening in menopausal females with a family history of breast or ovarian cancer. J Gynecol Oncol 2016;27:e41. https://doi.org/10.3802/jgo.2016.27.e41 | Population |
| Lindor NM, Petersen GM, Hadley DW, Kinney AY, Miesfeldt S, Lu KH, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006;296:1507–17. | Study design |
| Lynch HT, Casey MJ. Prophylactic surgery prevents endometrial and ovarian cancer in Lynch syndrome. Nat Clin Pract Oncol 2007;4:672–3. https://doi.org/10.1038/ncponc1002 | Study design |
| Makris GM, Siristatidis C, Margari N, Chrelias C, Papanota AM, Sergentanis TN, et al. Office endometrial cytological sampling: examining predictors of strenuousness. In Vivo 2016;30:309–14. | Population |
| McGarragle KM, Aronson M, Semotiuk K, Holter S, Hare CJ, Ferguson SE, et al. Patient-physician relationships, health self-efficacy, and gynecologic cancer screening among women with Lynch syndrome. Hered Cancer Clin Pract 2019;17:24. https://doi.org/10.1186/s13053-019-0123-7 | Outcomes |
| Mints M, Tzortzatos G, Andersson E, Soller M, Askmalm MS, Zagoras T, et al. The gynecological surveillance of women with Lynch syndrome in Sweden. Int J Gynecol Cancer 2015;25:1111. | Unable to retrieve FT |
| Oei AL, Massuger LF, Bulten J, Ligtenberg MJ, Hoogerbrugge N, de Hullu JA. Surveillance of women at high risk for hereditary ovarian cancer is inefficient. Br J Cancer 2006;94:814–9. https://doi.org/10.1038/sj.bjc.6603015 | Population |
| Persson E, Lindholm E, Berndtsson I, Lundstam U, Hulten L, Carlsson E. Experiences of living with increased risk of developing colorectal and gynaecological cancer in individuals with no identified gene mutation. Scand J Caring Sci 2012;26:20–7. https://doi.org/10.1111/j.1471-6712.2011.00898.x | Outcomes |
| Peterson SK, Watts BG, Daniels M, Dinh M, Lynch PM, Lu KH. Screening for gynecologic cancers among women in HNPCC families. J Clin Oncol 2004;22 :102S–S, abstract number 1023. https://ascopubs.org/doi/10.1200/jco.2004.22.90140.1023 | Outcomes |
| Rosenthal AN, Fraser LSM, Philpott S, Manchanda R, Burnell M, Badman P, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom familial ovarian cancer screening study. J Clin Oncol 2017;35:1411. https://doi.org/10.1200/jco.2016.69.9330 | Population |
| Ruvalcaba-Limon E, Cantu-de-Leon D, Leon-Rodriguez E, Cortes-Esteban P, Serrano-Olvera A, Morales-Vasquez F, et al. [The first Mexican consensus of endometrial cancer. Grupo de Investigacion en Cancer de Ovario y Tumores Ginecologicos de Mexico]. Rev Invest Clin 2010;62:583, 5-605. | Not English language |
| Sakala MD, Stein E, Wasnik AP, Curci NE, Masch WR, Mendiratta-Lala M, et al. Scientific Paper Abstracts Presented at the Society of Abdominal Radiology 2019 Annual Scientific Meeting and Educational Course (17–22 March 2019, Orlando, Florida). Abdom Radiol (NY) 2019;44:3210–34. | Population |
| Stadler ZK, Stern R, Devlin V, Glogowski E, Kauff N, Offit K, et al. Adherence to extracolonic cancer screening in Lynch syndrome kindreds. J Clin Oncol 2009;27:1513. | Outcomes |
| Stigliano V, Sanchez Mete L, Caterino M, Baldelli R, Lapenta R, Assisi D, et al. Extracolonic screening strategies in hereditary colorectal cancer syndromes: preliminary data. United European Gastroenterol J 2013;1:A292. http://dx.doi.org/10.1177/2050640613502900 | Abstract |
| Stirling D, Evans DG, Pichert G, Shenton A, Kirk EN, Rimmer S, et al. Screening for familial ovarian cancer: failure of current protocols to detect ovarian cancer at an early stage according to the International Federation of Gynecology and Obstetrics system. J Clin Oncol 2005;23:5588–96. https://doi.org/10.1200/JCO.2005.05.097 | Population |
| Sun CC, Meyer LA, Daniels MS, Bodurka DC, Nebgen DR, Burton-Chase AM, et al. Women’s preferences for cancer risk management strategies in Lynch syndrome. Gynecol Oncol 2019;152:514–21. https://doi.org/10.1016/j.ygyno.2018.11.027 | Outcomes |
| Tabuso M, Orzes E, Talamini R, Viel A, Canzonieri V, Cannizzaro R, et al. Natural history of Lynch syndrome in northeastern Italy. Dig Liver Dis 2014;46:S20. | Abstract |
| Tzur T, Kessous R, Weintraub AY. Current strategies in the diagnosis of endometrial cancer. Arch Gynecol Obstet 2017;296:5–14. https://doi.org/10.1007/s00404-017-4391-z | Population |
| Vrieling A, Visser A, Hoedjes M, Hurks M, Gomez Garcia E, Hoogerbrugge N, et al. Increasing awareness and knowledge of lifestyle recommendations for cancer prevention in Lynch syndrome carriers: randomized controlled trial. Clin Genet 2018;93:67–77. https://doi.org/10.1111/cge.13076 | Outcomes |
| Wagner A, van Kessel I, Kriege MG, Tops CM, Wijnen JT, Vasen HF, et al. Long term follow-up of HNPCC gene mutation carriers: compliance with screening and satisfaction with counseling and screening procedures. Fam Cancer 2005;4:295–300. https://doi.org/10.1007/s10689-005-0658-9 | Outcomes |
| Wentzensen NA, Clarke M, Shridhar V, Lemens M, Hopkins M, Ahlberg L, et al. A prospective study of endometrial cancer detection in women presenting for evaluation of abnormal peri- and postmenopausal bleeding. Cancer Res 2018;78. https://doi.org/10.1158/1538-7445.AM2018-2203 | Population |
| Wong S, Hui P, Buza N. Frequent loss of mutation-specific mismatch repair protein expression in nonneoplastic endometrium of Lynch syndrome patients. Mod Pathol 2020;33:1172–81. https://doi.org/10.1038/s41379-020-0455-x | Not surveillance |
| Wood NJ, Duffy SR, Sheridan E. The outcome of endometrial carcinoma surveillance by ultrasound scan in women at risk of hereditary nonpolyposis colorectal carcinoma and familial colorectal carcinoma. Cancer 2003;98:1772–4. | Study design |
| Yang K, Allen B, Conrad P, Powell CB, Terdiman J, Chen LM. Awareness of gynecologic surveillance in women from hereditary non-polyposis colorectal cancer families. Fam Cancer 2006;5:405–9. https://doi.org/10.1007/s10689-006-0012-x | Outcomes |
| Zhang Y, Biscotti C, Zhu H, Abdul-Karim F. Significance of atypical endometrial cells in women aged younger than 40 years. Lab Invest 2015;95:114A. https://doi.org/10.1038/labinvest.2015.7 | Population |
Appendix 3 Data map of evidence for the systematic review of clinical effectiveness
| Study | Clinical effectiveness | Detection of cancers | Harms | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Does surveillance improve mortality, survival, cancer prognosis, treatment response and fertility and QoL? | Does surveillance improve early diagnosis (i.e. stage at diagnosis)? | What are the cancer detection rates/incidence rates (malignancies and premalignancies)? | What are the asymptomatic cancer detection rates/incidence rates? | What is the incidence of interval cancers? | What are the incidental detection rates of other medical findings (e.g. ovarian cysts)? | What are the diagnostic test accuracies of the surveillance strategies/tests? | What are the test failure rates? | What are the rates (and severity) of adverse events (including pain)? | What risk factors impact the occurrence (and severity) of adverse events? | |
| Single-arm studies | ||||||||||
| Barrow et al. 200933 | ● | |||||||||
| Bats et al. 201434,35 | ● | ● | ● | ● | ||||||
| Bucksch et al. 202036 | ● | |||||||||
| Dove-Edwin et al. 200237 | ● | ● | ● | |||||||
| Elmasry et al. 200941 | ● | ● | ● | ● | ● | ● | ||||
| Helder-Woolderink et al. 201745,46 | ● | ● | ● | ● | ● | ● | ||||
| Ketabi et al. 201447,48 | ● | ● | ● | ● | ● | |||||
| Lécuru et al. 200749 | ● | ● | ● | ● | ||||||
| Lécuru et al. 200850 | ● | ● | ● | ● | ● | ● | ||||
| Lécuru et al. 201051 | ● | ● | ● | ● | ||||||
| Manchanda et al. 201252 | ● | ● | ● | ● | ● | ● | ||||
| Møller et al. 20178,53 | ● | ● | ||||||||
| Nebgen et al. 201454–58 | ● | ● | ● | ● | ● | |||||
| Pylvänäinen et al. 201259 | ● | ● | ||||||||
| Rijcken et al. 200362 | ● | ● | ● | ● | ● | |||||
| Rosenthal et al. 201363,64 | ● | ● | ● | |||||||
| Ryan et al. 201765 | ● | ● | ● | ● | ||||||
| Ryan et al. 202175 | ● | ● | ||||||||
| Wood et al. 200868 | ● | ● | ||||||||
| Woolderink et al. 202072 | ● | ● | ● | ● | ● | |||||
| Comparative studies | ||||||||||
| de Jong et al. 200673 | ● | |||||||||
| Dueñas et al. 202038,39 | ● | ● | ● | |||||||
| Eikenboom et al. 202140 | ● | ● | ● | ● | ● | |||||
| Gerritzen et al. 200942 | ● | ● | ● | ● | ||||||
| Helder-Woolderink et al. 201343,44 | ● | ● | ● | ● | ● | ● | ||||
| Kalamo et al. 202074 | ● | ● | ||||||||
| Renkonen-Sinisalo et al. 200760,61 | ● | ● | ● | ● | ||||||
| Stuckless et al. 201366 | ● | ● | ● | |||||||
| Tzortzatos et al. 201567 | ● | ● | ● | ● | ||||||
| Woolderink et al. 201869–71 | ● | ● | ● | ● | ● | |||||
Appendix 4 Detailed footnotes from the systematic review of clinical effectiveness evidence
| Table | Footnote | Detail |
|---|---|---|
| Table 1: Review questions and identified studies | a | Data were also extracted on depression and anxiety (protocol addition) |
| b | None of the included studies provided end-to-end comparative data for treatment response, although some limited surveillance group data were provided by Dove-Edwin et al. (2002), 37 Manchanda et al. (2012) 52 and Nebgen et al. (2014) 54 - 58 | |
| c | None of the included studies provided data that would clarify the relationship between surveillance programmes or technique and fertility, although limited data were provided by Elmasry et al. (2009), 41 Helder-Woolderink et al. (2017), 45,46 Kalamo et al. (2020), 74 Rijcken et al. 2003, 62 Ryan et al. (2021) 75 and Woolderin et al. (2020) 72 | |
| Table 2: Comparative studies included in the review | a | There were 1375 women registered but it is unclear how many were undergoing surveillance |
| b | Likely no surveillance, but it is unclear when gynaecological surveillance started (periods were largely chosen due to the introduction of CRC screening around 1990) | |
| c | Unclear how many participants were undergoing surveillance | |
| d | Physical examination and history taking | |
| e | These participants started surveillance but then opted to have surgery. Data are provided separately for the two groups in this study and also for the groups combined (i.e. for all those who started on surveillance). Of these 66 participants, 57 underwent HBSO, 1 hysterectomy and salpingectomy and 8 hysterectomy alone | |
| f | Follow-up time for mortality data | |
| g | 505 participants were in the database, but 164 had available data, were eligible for surveillance and provided consent | |
| h | Surveillance visits, data for both groups combined only | |
| i | Five participants were excluded after testing negative for MMR gene mutations, although it is not clear at what point in the study this occurred | |
| j | 32 visits included a non-routine biopsy (i.e. when clinically indicated) | |
| k | 98 participants were in the database, but 75 had sufficient data, were eligible for surveillance and started surveillance | |
| l | Survey sent to 112 participant, 76 (68%) responded | |
| m | Exact surveillance strategy unclear, but did include pelvic ultrasound and endometrial biopsy; surveillance interval also unclear | |
| n | 68 participants across the two groups had ever had surveillance, but 24 were undergoing surveillance and had not had surgery | |
| o | A further 10 participants had surgery as treatment rather than prophylactically but are not included in this sample | |
| p | The programme duration is n/a for surgery; however, for the surgery group, the length of time between LS diagnosis and prophylactic surgery was 6 years (median; range 0–14 years) and then the median time between surgery and the questionnaire was 9 years (1–38 years) | |
| q | There were 28 hospital centres and data were taken from the Finnish Cancer Registry, but it is unclear whether all participants were from the 28 centres or whether there were additional participants with registry data only | |
| r | 385 recruited but 72 withdrew, leaving 313 across the two groups | |
| s | Surveillance strategies varied but included clinical examination and could include any of TVUs, biopsy, Pap smear, CA-125, serum tumour-associated trypsin inhibitor (s-TATI) | |
| t | Every 2 years until age 30–35 years, and then every 3 years | |
| u | Number of eligible participants, 174 entered the study | |
| v | Did not have any screening (47 of the 120 (39.17%) would not have been eligible for screening in any case: 15 already had gynaecological cancer, 21 had a hysterectomy, 6 were < 30 years of age and 5 for reasons unknown) | |
| w | A subsample of the 120 participants from the no-screening group were analysed. These were age-matched controls who were not screened and who were alive and disease-free at the time the matched index case entered screening | |
| x | Although 170 were in the initial records, 10 were clinically ineligible, 26 ineligible due to age < 30 years, 5 did not attend any screening and 43 had surgery prior to knowing LS diagnosis | |
| y | These participants started surveillance but then opted to have surgery. Data are provided separately for the two groups in this study and also for the groups combined (i.e. for all those who started on surveillance). Of these 41 participants, 32 underwent HBSO, 7 hysterectomy alone and 2 BSO alone | |
| Table 3: Single-arm cohort studies included in the review | a | For individuals with abnormal TVUS results |
| b | Although participants were recruited into a yearly surveillance programme, data were collected after a single visit via questionnaires | |
| c | This was unclear, and likely varied over time and between centres (national guidelines include clinical examination and TVUS; study reports that endometrial sampling was conducted at 3.5% of visits) | |
| d | 2959 participants on the database were offered surveillance, but data available for 871 who had surveillance visits. An earlier publication (Boilesen et al. 2008) 48 provided data for the whole database at that earlier time point but not for those who had received surveillance | |
| e | Overall number of female participants is 1166, but data are primarily reported for 548 female mutation carriers | |
| f | Data from the six centres were entered into a registry, so this was a prospective registry study, the study included 924 participants of which 550 has LS and 315 Lynch-like syndrome | |
| g | Not reported for gynaecological surveillance/cancer, but median follow-up time for any cancer for women reported as 6.9 years | |
| h | For UK participants, transabdominal pelvic scans were conducted when TVUS was not available | |
| i | 67 recruited into the study, but 57 were willing to undergo follow-up and had not previously had a hysterectomy | |
| j | Endometrial sampling methods were mixed, comprising guided biopsies/polypectomy where indicated and/or Pipelle sampling | |
| k | Ovarian ultrasound and CA-125 was conducted when indicated by family history | |
| l | 577 women in the study but 87 enrolled on surveillance | |
| m | Unclear how many women were in the study | |
| n | Endometrial biopsy was only conducted where there were clinical symptoms, when the double-layer endometrium thickness was > 12 mm during the second week of the menstruation cycle (premenopausal) or > 5 mm (postmenopausal), or if the endometrium was irregular or not well assessable by TVUS | |
| o | Survey-based study, surveillance varied, but median interval was yearly or less (range < 1 year -to > 5 years) and could include clinical examination, TVUS, abdominal ultrasound, hysteroscopy, endometrial biopsy or CA-125 | |
| p | 298 respondents to survey, of which 59 were undergoing surveillance | |
| q | 10 countries: Australia, Denmark, Finland, Germany, Italy, Norway, the Netherlands, UK, Spain, Sweden | |
| r | Surveillance intervals and techniques varied across countries, but the interval was reported as generally being 1–2 years | |
| s | 1057 participants in Møller et al. (2017), 8 where only first cancers are reported, and 718 in Møller et al. (2017) 53 where subsequent cancers are reported | |
| Table 4: Summary of ROB ratings based on the CASP checklist for cohort studies | a | Small study with only a single surveillance visit |
| b | Unclear how many surveillance visits were conducted, and how long the surveillance programme was | |
| c | Unclear how many participants were in each group | |
| d | Although aims were clearly stated, they were not clearly addressed because neither the number of participants receiving surveillance or the number of surveillance visits was reported | |
| e | Mortality data may be less accurate (obtained by contacting general practitioners and relatives of non-responders to a follow-up questionnaire) | |
| f | Limited all-cause mortality data were reported (none due to EC) but these data were only obtained from participants at one of the study sites. It is also unclear in this study why some participants did not receive TVUS | |
| g | Cannot tell for whole sample (study based in both the UK and the Netherlands) | |
| h | Pre- or postmenopause status was included in some analyses. Parity was considered but there were insufficient nulliparous participants to enable inclusion in analyses | |
| i | Aims were clearly stated and although the number of participants who received surveillance was unclear the number of surveillance visits was reported | |
| j | Pre- or postmenopause status, parity and time period were included in some analyses | |
| k | Parity considered but not accounted for in analyses | |
| l | Parity was included in some analyses | |
| m | Response rates were not reported for this survey, so it is unclear how much data might be missing | |
| n | Aims were unclear, and despite reporting that assessing the impact of surveillance was an aim, the numbers receiving surveillance were not provided | |
| o | Some separate data available for pre- and postmenopausal participants. Sample was small with very little missing data | |
| Table 5: Summary of ROBINS-I ratings for comparative studies | a | Critical ROB when comparing RRS with surveillance for cancer detection rates |
| b | For other outcomes the ROB would be serious rather than critical | |
| c | This relates to the highest ROB rating across all domains and all outcomes | |
| d | The participants who were not under routine surveillance had all received a diagnosis of OC prior to their LS diagnosis | |
| Table 6: Baseline participant characteristics | a | Inclusive of 1 participant with Muir–Torre syndrome for both MLH1 and MHS2 mutations |
| b | Unclear how many participants are index patients and how many are relatives. Study also included participants with familial CRC type X (FCCX) (n = 59) | |
| c | Age at baseline per LS mutation: MLH1, 37.0 (median) (IQR, 28.0–41.0); MSH2, 36.0 (median) (IQR, 30.0–43.0); MSH6, 40.0 (median) (IQR, 38.0–46.0); Lynch-like syndrome, 39.0 (median) (IQR, 29.0–48.0); FCCX, 41.0 (IQR, 36.0–55.0) | |
| d | Comparative study, participant characteristics data not reported separately for surveillance and comparator or male and female participants | |
| e | Data were available for the whole sample (n = 164) and comparator group (n = 53) but not the surveillance-only group (n = 111) | |
| f | Comparative study, participant characteristics data not reported separately for surveillance and comparator | |
| g | Of 100 patients in whole sample (all female), a further 5 were awaiting results at time of write up, 7 declined testing and 5 tested negative despite having a mutation-positive relative and did not receive further surveillance | |
| h | Data from 2 screening periods with overlapping participants (total participants, n = 75) | |
| i | Also reports menopausal status of patients screened in each screening period: 2003–7, 38/44 (86.37%) were premenopausal and 6/44 (13.63%) were postmenopausal; 2008–12, 49/63 (77.7%) were premenopausal and 14/63 (22.2%) were postmenopausal | |
| j | Based on 52 participants in whole sample | |
| k | Age at second visit, 46.4 years (mean; range 34.0–70.0; n = 33 participants); age at third visit, 46.2 years (mean; range 35.0–71.0; n = 17 participants); age at fourth visit, 50.4 years (mean; range 40.0–66.0; n = 5 participants); age at fifth visit, 53.2 years (mean; range 41.0–67.0; n = 3 participants) | |
| l | A further 10 participants had surgery as treatment rather than prophylactically but are not included in this sample | |
| m | At time of survey (including non-respondents), 49.0 (median; range 30.0–89.0); at time of LS diagnosis (including non-respondents), 38.0 (median; range 20.0–72.0) | |
| n | Data not reported separately for those who had surgery and those who only had surveillance | |
| o | Female participants confirmed or obligate, 1057/1057 (100%) | |
| p | Age per mutation (combined and obligate combined): MLH1, 36.1 (mean; range 18–84); MSH2, 37.9 (mean; range 17–73); MSH6, 43.1 (mean; range 19–79); PMS2, 45.9 (mean; range 24–78) | |
| q | Includes both confirmed and obligate carriers | |
| r | EPCAM mutation data reported within MSH2 mutation data | |
| s | In subgroup in Huang et al. (2011) 54 65, 27 (64.29%) | |
| t | In subgroup in Huang et al. (2011) 54 65, NR | |
| u | In subgroup in Huang et al. (2011) 54 65, 37 (median; range 25–73) | |
| v | In subgroup in Huang et al. (2011) 54 65, 11 (40.74%); not clear why there are more MLH1 mutations in Huang et al. (2011) 54 65, than Nebgen et al. (2014) 56 68 | |
| w | In subgroup in Huang et al. (2011) 54 65, 15 (55.56%) | |
| x | In subgroup in Huang et al. (2011) 54 65, 1 (3.7%) | |
| y | In subgroup in Huang et al. (2011) 54 65, 0 | |
| z | In subgroup in Huang et al. (2011) 54 65, 0 | |
| aa | Age at death for female participants, 65.6 (mean) (range 31.0–102.0); age at death specifically due to EC, 54.8 (mean) (range 47.0–62.0) | |
| bb | Data on LS mutations only available for all 385 females potentially eligible for inclusion prior to withdrawals (including: living abroad, n-12; age < 35 years, n = 48; refused surveillance, n = 6; not yet in surveillance, n = 6) | |
| cc | Whole sample, 44.6 (median) (range 35.0–81.0) | |
| dd | One additional participant is likely to be in the possible LS group (deemed likely by referring centre and clinical geneticist) but this is unclear. If including this participant then mean age is 47.25 years (range 35.0–60.0 years) | |
| ee | All respondents described as women with LS, however 257 reported having a confirmed mutation, the other 41 were ‘don’t know’. Unclear if this means they had a confirmed mutation but did not know which, or were unsure if they had a confirmed mutation | |
| ff | 59 (19.8%) women reported currently undergoing surveillance | |
| gg | Surveillance: obligate, 0/54 included with confirmed LS; presumed, 1/54; no surveillance: obligate, 22/120 included with confirmed LS; presumed, 22/120; matched controls: obligate, 10/54 included with confirmed LS; presumed, 8/54 | |
| hh | Including confirmed, obligate and presumed LS mutation carriers (all from families with MSH2) | |
| ii | Only reports LS mutation data for whole sample | |
| jj | Median age at start of analysis of results not baseline and does not include data for 15 women who died during study | |
| kk | Also reports mean age at start of analysis of results, 57.2 (SD 13.9) | |
| ll | Among women with OC | |
| mm | Also reported categorically: age 20–29 years, n = 3 (6%); 30–39 years, n = 10 (19%); 40–49 years, n = 20 (38%); 50–59 years, n = 16 (30%); 60–59 years, n = 3 (6%); 70–79 years, n = 1 (2%) | |
| nn | Comparative study, participant characteristics data not reported separately for surveillance and comparator | |
| oo | Age 48.8 years (mean) (SD 9.9) | |
| pp | 0 cancers detected | |
| Table 7: Detection of malignancies, pre-malignancies and other medical findings | a | At surveillance visit/during surgery, over study period, interval (missed cancers), where data were available for cancers detected at a study visit and over the whole study period, the data collected at visits are preferentially reported |
| b | The number of at risk of gynaecological cancer is unclear, proportions could not be calculated, study reported that for mutation carriers, cumulative lifetime incidence of endometrial and OC combined was 32.5% (95% CI 29.1 to 35.9) and annual incidence rate was 0.7%. When data were censored at family ascertainment (i.e. prior to entering surveillance), cumulative lifetime risk was 31.6% (95% CI 28.2 to 35.0) and annual incidence rate was 0.6% | |
| c | Surveillance programme was not clearly described (except that CE was included) | |
| d | Or 2/222 (0.9%) of participants who had at least one scan | |
| e | Plus saline hysterosonography for those with abnormal TVUS results | |
| f | Unclear but likely based on Danish guidelines | |
| g | Number of incidental findings rather than number of participants with an incidental finding | |
| h | Number of incidental findings with OPH rather than number of participants with an incidental finding, an additional 13 cases of atrophy, 3 polyps and 3 cases of simple hyperplasia were found with Bx that were not detected with OPH | |
| i | This interval cancer was detected as a CAH with surveillance and diagnosed as EC after surgery | |
| j | Pooled data, surveillance strategies and intervals varied between studies, but interval was generally 1–2 years | |
| k | Only first cancers (and not subsequent cancers) were reported for the whole sample, in the Møller et al. (2017) 53 subsample there were 30/718 (4.18%) subsequent ECs and 7/718 (0.97%) subsequent OCs | |
| l | Gynaecological surveillance was combined with colonoscopy at some visits | |
| m | At final pathology the simple hyperplasia was found to be an additional CAH, and the two CH without atypia were categorised as ‘no hyperplasia’ | |
| n | Endometrial biopsy was only conducted where there were clinical symptoms, when the double-layer endometrium thickness was > 12 mm during the second week of the menstruation cycle (premenopausal) or > 5 mm (postmenopausal) or if the endometrium was irregular or not well assessable by TVUS | |
| o | An additional stage IIIC OC was found in a participant who had not undergone mutation testing for LS, but it appeared unlikely that this participant could be considered to have ‘suspected LS’ from the information provided | |
| p | 3 people undergoing surveillance had incidental OCs detected during surgery, 2 due to screen-detected EC, and 1 due to screen-detected AEH | |
| q | The stage II OC was not found with ovarian ultrasound but CA-125 levels were elevated (and identified incidentally during surgery) | |
| r | Survey-based study, surveillance techniques and interval likely to be varied | |
| s | Likely no surveillance, but it is unclear when gynaecological surveillance started (periods were largely chosen due to the introduction of CRC screening around 1990) | |
| t | Including 9 participants with synchronous EC and OC | |
| u | These participants were previously undergoing surveillance | |
| v | All 6 participants had incidental OC detected at surgery (had previously received negative screening results) | |
| w | First and subsequent visit data combined (1 EC was identified at the first surveillance visit and 5 at subsequent visits) | |
| x | It was not clear if the 8 EH had atypia | |
| y | This is based on the number of participants with cancer/number of visits (rather than the number of participants) – the number of participants included in the pre-2006 and post-2006 data sets was not reported | |
| z | There was 1 stage IIIC OC reported in this study and 1 ‘borderline malignancy’ but it was unclear whether these were in the pre- or post-2006 sample | |
| aa | There were 2 ovarian cysts, and 1 teratoma (presumably benign) detected with surveillance (unclear of pre- or post-2006) | |
| bb | Six cysts in five participants | |
| cc | Surveillance methods unclear but included pelvic ultrasound and Bx | |
| dd | Some participants had previously had surveillance but this is not clearly described | |
| ee | Surveillance was every 2 years until 30–35 years, and then every 3 years, strategies varied but included clinical examination and could include any of TVUS, Bx, Pap smear, CA-125, serum tumour-associated trypsin inhibitor (s-TATI) | |
| ff | One participant had 2 interval ECs (stage IA and IB) | |
| gg | These participants were not eligible for surveillance | |
| hh | 47/120 (39.17%) would not have been eligible for screening due to gynaecological cancer (n = 15), hysterectomy (n = 21), < 30 years (n = 6) or reasons unknown (n = 5) | |
| ii | Age-matched controls were a subsample of the no-screening group who were alive and disease-free at the time the matched index case entered screening | |
| jj | Only reported among participants with OC | |
| kk | Not clear how many participants were in each group (percentages could not be calculated) | |
| Table 8: Gynaecological cancers detected by participant LS mutation | a | The number of participants with confirmed mutations is unclear, proportions could not be calculated |
| b | Absolute numbers NR, but study reported a cumulative incidence of EC of 29.2% for MLH1, 24.4% for MSH2 and 48.8% for MSH6 | |
| c | Surveillance programme was not clearly described (except that CE was included) | |
| d | 292 is the total number of participants, number with confirmed mutation was not known | |
| e | Or 2/222 (0.9%) of participants who had at least one scan | |
| f | Number of participants undergoing surveillance with each mutation was not clear | |
| g | These participants were previously undergoing surveillance | |
| h | Including nine participants with synchronous EC and OC | |
| i | All six participants had incidental OC detected at surgery (had previously received negative screening results) | |
| j | This is based on the number of participants with cancer/number of visits (rather than the number of participants) – the number of participants included in the pre-2006 and post-2006 data sets was not reported | |
| k | There was one stage IIIC OC reported in this study and one ‘borderline malignancy’ but it was unclear whether these were in the pre- or post-2006 sample, the stage IIIC OC was in a participant with an MSH2 mutation | |
| l | Number of participants within group with each mutation was not clear | |
| m | This interval cancer was detected as a CAH with surveillance and diagnosed as EC after surgery | |
| n | Two of the 3 ECs were in participants with MLH1 mutations, 1 was in a participant with suspected rather than confirmed LS | |
| o | Only first cancers (and not subsequent cancers) were reported for the whole sample | |
| p | There were 72 ECs reported over the study period. The breakdown by mutation type totals 70 and not 72 ECs. The reason for this is unclear | |
| q | These participants were not eligible for surveillance | |
| Table 10: Diagnostic test accuracy data | a | Using a Pipelle device |
| b | The reference standard is reported as histology but it is unclear what tissue samples were used for the reference standard (this could be from biopsy when the index test is TVUS, but not when the index test is OHES) | |
| c | NR, stated as OC diagnosis, most likely from surgery which was not provided for all participants | |
| d | NR in the study report but calculated for this review (TP + FN/total) | |
| e | Calculated for this review, PPV and sensitivity both given in the study report as 100% (29.2 to 100.0) for prevalent cancers and 100% (2.5 to 100.0) for incident cancers | |
| f | Likelihood ratios were not mentioned in the study report, these data were from the outputs produced for this review | |
| g | Based on tissue obtained from surgery (all participants underwent a total hysterectomy) | |
| h | NR but calculated for this review (TP + FN/total) | |
| Table 12: Mortality and survival | a | Inclusive of cancer-specific mortality |
| b | Additional mortality data provided for whole sample: non-CRC/EC 74/1375 (5.4%), CRC 89/1375 (6.5%), EC and CRC 119/1375 (8.7%), EC 30/1375 (2.2%), OC 9/1375 (0.7%) | |
| c | Unclear how many participants were in each group | |
| d | Likely no surveillance, but it is unclear when gynaecological surveillance started (periods were largely chosen due to the introduction of CRC screening around 1990) | |
| e | Surveillance was every 2 years until 30–35 years, and then every 3 years, strategies varied but included clinical examination and could include any of TVUS, Bx, Pap smear, CA-125, serum tumour-associated trypsin inhibitor (s-TATI) | |
| f | These participants were not eligible for surveillance | |
| g | Life expectancy (i.e. mean overall survival with birth as time zero) | |
| h | Median age to cancer, IQR not reported | |
| i | 47/120 (39.17%) would not have been eligible for screening due to gynaecological cancer (n = 15), hysterectomy (n = 21), < 30 years old (n = 6) or reasons unknown (n = 5) | |
| j | Age-matched controls were a subsample of the no-screening group who were alive and disease-free at the time the matched index case entered screening | |
| k | Only reported for the UK subsample, and not the Netherlands subsample | |
| l | Pooled data, surveillance strategies and intervals varied between studies, but interval was generally 1–2 years | |
| m | Crude survival estimated via the Kaplan–Meier method with date of first cancer diagnosis set to time zero, note it is likely the original report contains a typographical error as increasing survival is not possible using the Kaplan–Meier method | |
| n | Among mutation carriers, all-cause mortality among those without a LS mutation (suspected LS) was 20/618 (3.2%) | |
| o | Among mutation carriers, cancer-specific mortality among those without a LS mutation (suspected LS) was 6/618 (1.0%) | |
| p | This was based on participants with full datasets who were offered surveillance, the study reports that all participants with OC who were undergoing surveillance were alive and well at the time data were reported | |
| Table 14: Reported pain and pain relief required during surveillance | a | Data provided in Huang et al. (2011) 54 |
| b | Participants first received endometrial biopsy in an office setting without sedation and later received endometrial biopsy with colonoscopy with sedation | |
| c | Difference between techniques reported as statistically significant (p < 0.001) | |
| d | Severe pain (VAS ≥ 7) on insertion of tampon | |
| e | Two subgroups (individuals who had gone on to receive prophylactic surgery by the time of the survey and those who had not) | |
| f | Data relate to a surveillance programme which could include internal examination, TVUS, pelvic ultrasound, biopsy, CA-125 or hysteroscopy | |
| g | 1–2 hours before visit | |
| Table 15: Adverse events data for important subgroups | a | Surveillance included TVUS, hysteroscopy and endometrial sampling (Pipelle) but it is not clear which technique these results are related to |
| b | Data provided in Huang et al. (2011) 54 | |
| c | Participants first received endometrial biopsy in an office setting without sedation and later received endometrial biopsy with colonoscopy with sedation |
Appendix 5 Detailed results from the systematic review of utility values
Endometrial cancer
| Study | How valued, presented as | Who valued, location | Health state description | Utility value |
|---|---|---|---|---|
| Armfield et al. (2019)a95 | EQ-5D-3L, mean (SD) | Early-stage EC patients, in Australia, New Zealand, Hong Kong | TLH at baseline | |
| BMI < 35 kg/m² | 0.84 (0.16) | |||
| BMI > 35 kg/m² | 0.78 (0.18) | |||
| Bijen et al. (2011)96 | EQ-5D, median (range) | Early-stage EC patients, in the Netherlands | TAH and BSO (n = 94) | |
| 6 weeks | 0.80 (0.3–1.0) | |||
| 3 months | 0.85 (0.6–1.0) | |||
| TLH and BSO (n = 185) | ||||
| 6 weeks | 0.81 (−0.2 to 1.0) | |||
| 3 months | 0.81 (−0.4 to 1.0) | |||
| Cykert et al. (2004)97 | Standard gamble Point estimate (range) |
Public (scenarios designed to measure patient preferences), in the USA | Curable EC, Tamoxifen | 0.83 (0.0–1.0) |
| Ferguson et al. (2018)98 | EQ-5D, mean (SD) | Patients, in Canada | Minimally invasive surgery ( n = 346) | |
| Pre-surgery | 0.83 (0.02) | |||
| 1 week post surgery | 0.71 (0.02) | |||
| 3 weeks post surgery | 0.79 (0.02) | |||
| 3 months post surgery | 0.88 (0.02) | |||
| 6 months post surgery | 0.89 (0.02) | |||
| Laparotomy ( n = 83) | ||||
| Pre surgery | 0.82 (0.02) | |||
| 1 week post surgery | 0.56 (0.03) | |||
| 3 weeks post surgery | 0.70 (0.02) | |||
| 3 months post surgery | 0.84 (0.02) | |||
| 6 months post surgery | 0.88 (0.02) | |||
| Hildebrandt et al. (2014)99 | EQ-5D-3L, median (range) | Primary and advanced cancer patients, in Germany | Overall (n = 20) | 0.8870 (0.676–0.999) |
| Primary disease (n = 9) | 0.9990 (0.701–1.000) | |||
| Advanced disease (n = 11) | 0.8870 (0.676–1.000) | |||
| Kent et al. (2015)100 | SF-6D, mean (95% CI) | Patients and public, in the USA | EC (n = 1565) | 0.70 (95% CI 0.69 to 0.71) |
| Kimman et al. (2015)101 | EQ-5D, mean (SD) | Patients, in Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines, Thailand, Vietnam | EC (n = 177) | 0.67 (0.18) |
| Kupperman et al. (2013)88 | TTO, mean (SD), median (IQR) | Patients and public with LS, in the USA | Whole sample (n = 70) | 0.728 (0.179) 0.760 (0.640–0.870) |
| General medicine clinic (n = 49) | 0.726 (0.184) 0.758 (0.633–0.877) |
|||
| CRC prevention programme (n = 21) | 0.744 (0.160) 0.760 (0.737–0.804) |
|||
| Lundin et al. (2019)103 | EQ-5D, median (range) | Stage I–III EC patients, in Sweden | Pre-operative state | |
| Robotic hysterectomy (n = 25) | 0.81 (0.12–1.00) | |||
| Abdominal hysterectomy (n = 25) | 0.82 (0.12–1.00) | |||
| Lundin et al. (2020)102 | EQ-5D-3L, ‘average QoL weight’ | Stage I EC patients, in Sweden | Robotic hysterectomy (n = 25) | 0.87 |
| Abdominal hysterectomy (n = 25) | 0.72 | |||
| Mansel et al. (2007)104 | Standard gamble, mean (SD) | Patients in ATAC breast cancer trial, in the UK | EC (n = 23) | 0.913 (0.101) |
| Naik et al. (2017)105 | EQ-5D-3L, mean (SD; SEM) | Patients, in Canada | EC (n = 46) | |
| Canadian tariff | 0.78 (0.13; 0.02) | |||
| US tariff | 0.80 (0.14; 0.02) | |||
| UK tariff | 0.74 (0.21; 0.03) | |||
| Setiawan et al. (2018)106 | EQ-5D-5L, mean (SD) | Patients, in Indonesia | EC (n = 11) | 0.84 (0.29) |
| Ueno et al. (2020)107 | EQ-5D, mean; median (IQR) | Patients undergoing gynaecological surgery, in Japan | EC diagnosis (n = 14) | 0.88; 1.00 (0.77–1.00) |
Ovarian cancer
| Study | How valued, presented as | Who valued, location | Health state description | Utility value |
|---|---|---|---|---|
| Cohn et al. (2015)108 | FACT-O converted to utilities using the Dobrez method, mean (SD) | Patients with advanced OC | Paclitaxel/carboplatin | |
| Baseline | 0.79 (0.118) | |||
| Cycle 4 | 0.82 (0.115) | |||
| Cycle 7 | 0.83 (0.057) | |||
| Cycle 13 | 0.86 (0.108) | |||
| Cycle 21 | 0.85 (0.152) | |||
| 6 months after treatment completion | 0.84 (0.095) | |||
| Paclitaxel/carboplatin/bevacizumab | ||||
| Baseline | 0.79 (0.116) | |||
| Cycle 4 | 0.80 (0.115) | |||
| Cycle 7 | 0.81 (0.111) | |||
| Cycle 13 | 0.85 (0.106) | |||
| Cycle 21 | 0.86 (0.098) | |||
| 6 months after treatment completion | 0.85 (0.094) | |||
| Paclitaxel/carboplatin/bevacizumab + maintenance bevacizumab | ||||
| Baseline | 0.79 (0.119) | |||
| Cycle 4 | 0.79 (0.058) | |||
| Cycle 7 | 0.81 (0.114) | |||
| Cycle 13 | 0.85 (0.109) | |||
| Cycle 21 | 0.85 (0.052) | |||
| 6 months after treatment completion | 0.85 (0.147) | |||
| Cole et al. (2018)109 | QLQ-C30 converted to EQ-5D utility weights | Patients with bulky advanced epithelial OC | NACT | |
| NACT | 0.58 | |||
| Post-surgery + 3 rounds ACT | 0.66 | |||
| Primary debulking surgery | ||||
| Post-surgery + 3 rounds ACT | 0.48 | |||
| 4–6 rounds ACT | 0.57 | |||
| Duong et al. (2016)110 | EQ-5D, mean | Patients with advanced OC, in Canada | Bevacizumab | |
| Cycle 1 (n = 340) | 0.7252 | |||
| Cycle 2 (n = 383) | 0.767 | |||
| Cycle 3 (n = 380) | 0.7798 | |||
| Cycle 4 (n = 365) | 0.7971 | |||
| Cycle 5 (n = 367) | 0.7968 | |||
| Cycle 6 (n = 360) | 0.7835 | |||
| Cycle 8 (n = 308) | 0.7969 | |||
| Cycle 10 (n = 299) | 0.8059 | |||
| Cycle 12 (n = 287) | 0.804 | |||
| Cycle 14 (n = 226) | 0.8136 | |||
| Cycle 16 (n = 206) | 0.7985 | |||
| Cycle 18 (n = 181) | 0.815 | |||
| Follow-up (n = 395) | 0.8438 | |||
| Friedlander et al. (2018)111 | EQ-5D-3L, mean (SD) | Patients with progressive disease OC | Pazopanib ( n = 442) | |
| Baseline | 0.80 (0.20) | |||
| Before progressive disease | 0.79 (0.15) | |||
| After progressive disease | 0.69 (0.28) | |||
| Placebo ( n = 441) | ||||
| Baseline | 0.80 (0.20) | |||
| Before progressive disease | 0.81 (0.16) | |||
| After progressive disease | 0.77 (0.21) | |||
| Fujiwara et al. (2016)112 | EQ-5D-3L, mean (SD) | Patients with recurrent OC, in Western Europe, North America, Australia, rest of world | Baseline | |
| Trebananib (n = 408) | 0.75 (0.20) | |||
| Placebo (n = 426) | 0.74 (0.24) | |||
| Gordon et al. (2010)113 | SF-6D, mean (SD) | Patients with stages 1–4 OC (referred for primary OC), in Australia | Referred for chemotherapy at diagnosis | |
| Stage 1 or 2 (n = 13) | 0.74 (0.11) | |||
| Stage 3 (n = 63) | 0.68 (0.09) | |||
| Stage 4 (n = 9) | 0.69 (0.08) | |||
| Total (n = 85) | 0.69 (0.10) | |||
| Grann et al. (1998)90 | TTO, mean (IQR) | Public (described 5 BRCA1/2 health state scenarios) | OC (n = 54) | 0.82 (0.75–1.00) |
| Grann et al. (1999)89 | TTO, mean (SD) | BRCA1/2 patients, public and health professionals | Age 20–32 years (n = 93) | 0.84 (0.22) |
| Age 33–50 years (n = 42) | 0.58 (0.36) | |||
| Age 33–50 years high risk (n = 22) | 0.71 (0.30) | |||
| Age 33–50 years breast cancer (n = 20) | 0.81 (0.21) | |||
| Health professionals (n = 20) | 0.68 (0.38) | |||
| Other individuals (n = 105) | 0.79 (0.27) | |||
| Grann et al. (2010)114 | TTO, mean (SD) | Women with personal or family history but no known personal risk of breast/OC, and women with known BRCA1/2, in the USA | Known mutation carriers (n = 83) | 0.84 (0.23) |
| No personal/family history and not high risk (n = 160) | 0.83 (0.17) | |||
| Havrilesky et al. (2009)115 | TTO, mean (SD); median (range) | Patients and public (health valuation based on described health state), in the USA | Clinical remission (n = 16) | 0.83 (0.25); 0.95 (0.03–0.97) |
| Early, newly diagnosed (n = 16) | 0.81 (0.26); 0.93 (0.03–0.97) |
|||
| Advanced, newly diagnosed (n = 14) | 0.55 (0.29); 0.50 (0.03–0.93) |
|||
| End stage (n = 15) | 0.16 (0.25); 0.03 (0.03–0.83) |
|||
| Grade 1–2 toxicity | ||||
| Newly diagnosed, chemotherapy (n = 16) | 0.60 (0.31); 0.67 (0.03–0.97) |
|||
| Recurrent, responding to chemo (n = 15) | 0.50 (0.34); 0.50 (0.03–0.93) |
|||
| Recurrent, progressive (n = 16) | 0.40 (0.33); 0.42 (0.03–0.93) |
|||
| Grade 3–4 toxicity | ||||
| Newly diagnosed, chemotherapy (n = 15) | 0.49 (0.36); 0.50 (0.03–0.97) |
|||
| Recurrent, responding to chemo (n = 14) | 0.61 (0.24); 0.67 (0.17–0.97) |
|||
| Recurrent, progressive (n = 15) | 0.47 (0.34); 0.50 (0.03–0.93) |
|||
| Havrilesky et al. (2012)116 | FACT-G subscale scores converted to utilities, estimate (range) | Platinum-sensitive recurrent OC patients | Combination docetaxel and carboplatin | |
| Randomisation | 0.87 (0.84–0.89) | |||
| Cycle 4 | 0.79 (0.75–0.83) | |||
| Cycle 6 | 0.82 (0.79–0.84) | |||
| End of study | 0.83 (0.00–0.86) | |||
| Planned sequential docetaxel followed by carboplatin | ||||
| Randomisation | 0.87 (0.85–0.89) | |||
| Cycle 4 | 0.85 (0.82–0.87) | |||
| Cycle 6 | 0.87 (0.84–0.90) | |||
| End of study | 0.84 (0.81–0.87) | |||
| Havrilesky et al. (2019)117 | EQ-5D-5L, mean (SD); median | Newly diagnosed OC patients | Time of survey (n = 101) | 0.956 (0.095); 0.981 |
| Hess et al. (2010)119 | Standard gamble | Physicians, and advanced OC patients receiving chemotherapy or surveillance only, in the USA | Physicians | |
| Low AE, low TE, poor EWB | 0.39 | |||
| Low-moderate AE, low TE, moderate EWB | 0.43 | |||
| Moderate-high AE, moderate TE, poor EWB | 0.5 | |||
| High AE, moderate TE, positive EWB | 0.51 | |||
| Extremely high AE, high TE, positive EWB | 0.69 | |||
| Extremely high AE, high TE, poor EWB | 0.63 | |||
| Patients receiving chemotherapy | ||||
| Low AE, low TE, poor EWB | 0.57 | |||
| Low-moderate AE, low TE, moderate EWB | 0.52 | |||
| Moderate-high AE, moderate TE, poor EWB | 0.52 | |||
| High AE, moderate TE, positive EWB | 0.57 | |||
| Extremely high AE, high TE, positive EWB | 0.61 | |||
| Extremely high AE, high TE, poor EWB | 0.57 | |||
| Patients under surveillance | ||||
| Low AE, low TE, poor EWB | 0.32 | |||
| Low-moderate AE, low TE, moderate EWB | 0.33 | |||
| Moderate-high AE, moderate TE, poor EWB | 0.3 | |||
| High AE, moderate TE, positive EWB | 0.37 | |||
| Extremely high AE, high TE, positive EWB | 0.37 | |||
| Extremely high AE, high TE, poor EWB | 0.3 | |||
| Hess et al. (2013)118 | FACT-O scores converted to utilities using different methods, mean (range) | GOG-0152: advanced OC patients after initial surgery and combination chemotherapy | Cheung algorithm | |
| Time point 1 (baseline) (n = 373) | 0.79 (0.33–1.00) | |||
| Time point 2 (n = 345) | 0.78 (0.34–1.00) | |||
| Time point 3 (n = 342) | 0.84 (0.44–1.00) | |||
| Time point 4 (n = 302) | 0.84 (0.42–1.00) | |||
| All (n = 1362) | 0.81 (0.33–1.00) | |||
| Dobrez algorithm | ||||
| Time point 1 (baseline) (n = 363) | 0.82 (0.46–1.00) | |||
| Time point 2 (n = 344) | 0.81 (0.46–1.00) | |||
| Time point 3 (n = 334) | 0.86 (0.61–1.00) | |||
| Time point 4 (n = 301) | 0.87 (0.46–1.00) | |||
| All (n = 1342) | 0.84 (0.46–1.00) | |||
| FACT linear transformation | ||||
| Time point 1 (baseline) (n = 371) | 0.75 (0.34–0.96) | |||
| Time point 2 (n = 342) | 0.74 (0.38–0.99) | |||
| Time point 3 (n = 340) | 0.81 (0.35–1.00) | |||
| Time point 4 (n = 301) | 0.81 (0.39–0.99) | |||
| All (n = 1354) | 0.77 (0.34–1.00) | |||
| GOG-0172: stage 3 OC patients | Cheung algorithm | |||
| Time point 1 (baseline) (n = 397) | 0.73 (0.31–1.00) | |||
| Time point 2 (n = 320) | 0.72 (0.38–0.99) | |||
| Time point 3 (n = 330) | 0.76 (0.34–1.00) | |||
| Time point 4 (n = 276) | 0.84 (0.38–1.00) | |||
| All (n = 1323) | 0.76 (0.31–1.00) | |||
| Dobrez algorithm | ||||
| Time point 1 (baseline) (n = 385) | 0.79 (0.46–1.00) | |||
| Time point 2 (n = 313) | 0.77 (0.46–1.00) | |||
| Time point 3 (n = 323) | 0.79 (0.46–1.00) | |||
| Time point 4 (n = 273) | 0.87 (0.46–1.00) | |||
| All (n = 1294) | 0.80 (0.46–1.00) | |||
| FACT linear transformation | ||||
| Time point 1 (baseline) (n = 396) | 0.70 (0.22–0.99) | |||
| Time point 2 (n = 320) | 0.70 (0.38–0.96) | |||
| Time point 3 (n = 330) | 0.74 (0.34–0.99) | |||
| Time point 4 (n = 276) | 0.81 (0.40–1.00) | |||
| All (n = 1322) | 0.73 (0.22–1.00) | |||
| Hettle et al. (2015)91 | FACT-O scores converted to utilities using different methods, mean (SE); median (IQR) | Recurrent OC patients undergoing olaparib maintenance therapy | Screening (intention-to-treat group) | |
| OLS | 0.802 (0.009); 0.821 (0.719–0.912) |
|||
| Tobit | 0.799 (0.009); 0.815 (0.704–0.914) |
|||
| Cheung | 0.828 (0.007); 0.842 (0.762–0.912) |
|||
| Dobrez | 0.860 (0.006); 0.852 (0.822–0.922) |
|||
| Scheduled visits (intention-to-treat group) | ||||
| OLS | 0.786 (0.008); 0.799 (0.699–0.885) |
|||
| Tobit | 0.786 (0.008); 0.787 (0.696–0.885) |
|||
| Cheung | 0.811 (0.007); 0.820 (0.733–0.907) |
|||
| Dobrez | 0.845 (0.006); 0.849 (0.788–0.909) |
|||
| Unscheduled visits (intention-to-treat group) | ||||
| OLS | 0.720 (0.015); 0.729 (0.632–0.820) |
|||
| Tobit | 0.751 (0.013); 0.736 (0.676–0.858) |
|||
| Cheung | 0.769 (0.012); 0.775 (0.697–0.868) |
|||
| Dobrez | 0.816 (0.011); 0.852 (0.743–0.878) |
|||
| Screening (BRCAm subgroup) | ||||
| OLS | 0.787 (0.013); 0.820 (0.694–0.888) |
|||
| Tobit | 0.784 (0.013); 0.813 (0.677–0.875) |
|||
| Cheung | 0.812 (0.010); 0.815 (0.731–0.895) |
|||
| Dobrez | 0.853 (0.009); 0.852 (0.815–0.886) |
|||
| BRCAm subgroup scheduled visits | ||||
| OLS | 0.768 (0.013); 0.784 (0.681–0.871) |
|||
| Tobit | 0.774 (0.011); 0.774 (0.694–0.874) |
|||
| Cheung | 0.799 (0.010); 0.810 (0.706–0.893) |
|||
| Dobrez | 0.837 (0.008); 0.842 (0.789–0.894) |
|||
| BRCAm subgroup unscheduled visits | ||||
| OLS | 0.708 (0.024); 0.707 (0.594–0.811) |
|||
| Tobit | 0.737 (0.020); 0.720 (0.676–0.806) |
|||
| Cheung | 0.763 (0.017); 0.765 (0.706–0.856) |
|||
| Dobrez | 0.831 (0.015); 0.852 (0.809–0.852) |
|||
| Hildebrandt et al. (2014)99 | EQ-5D-3L, median (range) | Primary and advanced OC patients, in Germany | Primary disease (n = 13) | 0.8870 (0.465–1.000) |
| Advanced disease (n = 21) | 0.8870 (0.313–1.000) | |||
| Total (n = 37) | 0.8870 (0.313–1.000) | |||
| Hinde et al. (2016)120 | EQ-5D, mean (range) (SE) | Advanced OC patients | Pre progression (time since randomisation) (weeks) | |
| 0 | 0.65 (0.58–0.70) | |||
| 20 | 0.73 (0.64–0.79) | |||
| 40 | 0.78 (0.71–0.83) | |||
| 60 | 0.81 (0.75–0.87) | |||
| 80 | 0.82 (0.77–0.88) | |||
| 100 | 0.82 (0.77–0.88) | |||
| 120 | 0.82 (0.76–0.88) | |||
| 140 | 0.83 (0.77–0.89) | |||
| Post progression | ||||
| Chemotherapy alone | 0.75 (0.016) | |||
| Bevacizumab | 0.71 (0.020) | |||
| Total | 0.74 (0.013) | |||
| Kent et al. (2015)100 | SF-6D, mean (95% CI) | Public and OC patients, in the USA | OC | 0.68 (0.67 to 0.69) |
| Kimman et al. (2015)101 | EQ-5D, mean (SD) | OC patients, in Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines, Thailand, Vietnam | OC | 0.63 (0.19) |
| Krasner et al. (2012)121 | EQ-5D-3L, mean (SD) | OC patients | Baseline | |
| Pegylated liposomal doxorubicin (PLD) (n = 318) | 0.78 (0.163) | |||
| Trabectedin + PLD (n = 323) | 0.78 (0.171) | |||
| Kupperman et al. (2013)88 | TTO, mean (SD); median (IQR) | Public and patients (including individuals with LS and/or with history of colorectal and other cancers), in the USA | Whole sample | 0.593 (0.272); 0.713 (0.257 to 0.828) |
| General medicine clinic | 0.588 (0.276); 0.727 (0.240 to 0.823) |
|||
| CRC prevention programme | 0.624 (0.261); 0.627 (0.490 to 0.833) |
|||
| Luealon et al. (2016)122 | FACT-G scores converted to EQ-5D utility; estimate (lower – upper value) | Platinum resistant or refractory OC patients | Salvage chemotherapy | 0.766 (0.464 to 0.984) |
| Best supportive care | 0.766 (0.464 to 0.984) | |||
| Mirza et al. (2016)92 | EQ-5D-5L, adjusted mean | Platinum-sensitive, recurrent OC, in the USA, Canada, Hungary | Niraparib maintenance therapy with gBRCAmut ( n = 136) | |
| Screening | 0.851 | |||
| Cycle 2 | 0.843 | |||
| Cycle 4 | 0.839 | |||
| Cycle 6 | 0.849 | |||
| Cycle 8 | 0.849 | |||
| Cycle 10 | 0.838 | |||
| Cycle 12 | 0.841 | |||
| Cycle 14 | 0.84 | |||
| Post progression | 0.816 | |||
| Niraparib maintenance therapy no gBRCAmut ( n = 231) | ||||
| Screening | 0.839 | |||
| Cycle 2 | 0.834 | |||
| Cycle 4 | 0.839 | |||
| Cycle 6 | 0.848 | |||
| Cycle 8 | 0.844 | |||
| Cycle 10 | 0.838 | |||
| Cycle 12 | 0.837 | |||
| Cycle 14 | 0.837 | |||
| Post progression | 0.8 | |||
| Placebo with gBRCAmut ( n = 65) | ||||
| Screening | 0.849 | |||
| Cycle 2 | 0.841 | |||
| Cycle 4 | 0.822 | |||
| Cycle 6 | 0.844 | |||
| Cycle 8 | 0.825 | |||
| Cycle 10 | 0.836 | |||
| Cycle 12 | 0.827 | |||
| Cycle 14 | 0.834 | |||
| Post progression | 0.832 | |||
| Placebo no gBRCAmut ( n = 114) | ||||
| Screening | 0.836 | |||
| Cycle 2 | 0.824 | |||
| Cycle 4 | 0.819 | |||
| Cycle 6 | 0.821 | |||
| Cycle 8 | 0.819 | |||
| Cycle 10 | 0.835 | |||
| Cycle 12 | 0.804 | |||
| Cycle 14 | 0.827 | |||
| Post progression | 0.78 | |||
| Naik et al. (2017)105 | EQ-5D-3L, mean (SD; SEM) | OC patients (ambulatory cancer survivors), in Canada | Time since diagnosis or treatment | |
| < 1 year since diagnosis (n = 19) | 0.77 (NR; 0.04) | |||
| ≥ 1 year since diagnosis (n = 64) | 0.81 (NR; 0.02) | |||
| Recent treatment (≤ 3 months) (n = 54) | 0.78 (NR; 0.02) | |||
| Not treated or treatment > 3 months prior (n = 31) | 0.82 (NR; 0.03) | |||
| Tariff | ||||
| Canadian | 0.79 (0.15; 0.02) | |||
| USA | 0.81 (0.14; 0.02) | |||
| UK | 0.76 (0.21; 0.02) | |||
| Disease stage | ||||
| Local/regional (n = 59) | 0.80 (NR; 0.02) | |||
| Distant/metastatic (n = 25) | 0.78 (NR; 0.03) | |||
| NICE TA284 (2013)123 | EQ-5D, mean (SE) | Advanced OC patients | Bevacizumab, carboplatin, paclitaxel (progression-free survival) | |
| Weeks 0–2 (n = 335) | 0.6571 (0.0133) | |||
| Weeks 3–5 (n = 378) | 0.7153 (0.0118) | |||
| Weeks 6–8 (n = 375) | 0.7443 (0.0110) | |||
| Weeks 9–11 (n = 361) | 0.7683 (0.0100) | |||
| Weeks 12–14 (n = 363) | 0.7643 (0.0112) | |||
| Weeks 15–20 (n = 353) | 0.7444 (0.0121) | |||
| Weeks 21–26 (n = 303) | 0.7638 (0.0131) | |||
| Weeks 27–32 (n = 295) | 0.7718 (0.0129) | |||
| Weeks 33–38 (n = 282) | 0.7638 (0.0136) | |||
| Weeks 39–44 (n = 220) | 0.7785 (0.0155) | |||
| Weeks 45–50 (n = 202) | 0.7533 (0.0165) | |||
| Weeks 51–53 (n = 178) | 0.7760 (0.0170) | |||
| Weeks 54 + (n = 338) | 0.8129 (0.0113) | |||
| Ortega et al. (1997)124 | TTO, mean | Advanced OC patients and healthy female volunteers, in Canada | Ifosfamide | |
| Patients with response | 0.671 | |||
| Patients without response | 0.533 | |||
| Volunteers with response | 0.5 | |||
| Volunteers without response | 0.367 | |||
| Tamoxifen | ||||
| Patients with response | 0.754 | |||
| Patients without response | 0.667 | |||
| Volunteers with response | 0.646 | |||
| Volunteers without response | 0.517 | |||
| Hexamethylmelamine | ||||
| Patients with response | 0.638 | |||
| Patients without response | 0.557 | |||
| Volunteers with response | 0.552 | |||
| Volunteers without response | 0.429 | |||
| Paclitaxel | ||||
| Patients with response | 0.645 | |||
| Patients without response | 0.586 | |||
| Volunteers with response | 0.427 | |||
| Volunteers without response | 0.371 | |||
| Paclitaxel + cisplatin | ||||
| Patients | 0.839 | |||
| Volunteers | 0.711 | |||
| Cyclophosphamide + cisplatin | ||||
| Patients | 0.823 | |||
| Volunteers | 0.654 | |||
| Oza et al. (2018)94 | EQ-5D-5L, mean (SE) | Patients with recurrent OC, with and without BRCA1/2, in the USA, Canada, Europe, Israel | Niraparib with BRCA | |
| Baseline (n = 134) | 0.850 (0.0105) | |||
| Pre progression (n = 129) | 0.838 (0.0097) | |||
| Post progression (n = 60) | 0.801 (0.0210) | |||
| Niraparib no BRCA | ||||
| Baseline (n = 227) | 0.837 (0.0078) | |||
| Pre progression (n = 208) | 0.833 (0.0077) | |||
| Post progression (n = 139) | 0.810 (0.0119) | |||
| Placebo with BRCA | ||||
| Baseline (n = 64) | 0.847 (0.0163) | |||
| Pre progression (n = 59) | 0.834 (0.0173) | |||
| Post progression (n = 46) | 0.794 (0.0178) | |||
| Placebo no BRCA | ||||
| Baseline (n = 112) | 0.824 (0.0128) | |||
| Pre progression (n = 97) | 0.815 (0.0122) | |||
| Post progression (n = 94) | 0.783 (0.0138) | |||
| Oza et al. (2020)93 | EQ-5D-3L, ‘average’ | Patients with platinum- sensitive recurrent OC, with and without BRCA1/2 | Rucaparib maintenance treatment | |
| Intent-to-treat | 0.89 | |||
| BRCA mutant | 0.9 | |||
| Homologous recombination deficient | 0.9 | |||
| BRCA wild type/loss of heterozygosity high | 0.91 | |||
| BRCA wild type/loss of heterozygosity low | 0.85 | |||
| BRCA wild type/loss of heterozygosity indeterminate | 0.97 | |||
| Rowland et al. (2015)125 | FACT-O scores converted to utility index, utility index (range) | Advanced stage OC patients age 65 years or older | Neoadjuvant chemotherapy | |
| Active treatment | 0.791 (0.40–0.84) | |||
| Immediate recovery | 0.779 (0.38–0.84) | |||
| Ongoing recovery (> 6 months) | 0.840 (0.42–0.84) | |||
| Primary debulking surgery | ||||
| Active treatment | 0.791 (0.40–0.84) | |||
| Immediate recovery | 0.779 (0.38–0.84) | |||
| Ongoing recovery (> 6 months) | 0.840 (0.42–0.84) | |||
| Stein et al. (2007)126 | Standard gamble, mean (SE); median (range) | Panel of patients and public regarding advanced OC on chemotherapy, in the UK | Chemotherapy | |
| Cluster 1 (n = 40) | 0.977 (0.044); 0.995 (0.775–1.000) |
|||
| Cluster 2 (n = 34) | 0.930 (0.071); 0.960 (0.675–0.990) |
|||
| Cluster 3 (n = 34) | 0.886 (0.139); 0.955 (0.325–0.990) |
|||
| Cluster 4 (n = 20) | 0.817 (0.175); 0.875 (0.225–0.985) |
|||
| Cluster 5 (n = 18) | 0.788 (0.189); 0.875 (0.125–0.980) |
|||
| Cluster 6 (n = 17) | 0.694 (0.221); 0.775 (0.125–0.970) |
|||
| Ueno et al. (2020)107 | EQ-5D, mean; median (IQR) | Patients who underwent gynaecological surgery, in Japan | OC tumour (n = 25) | 0.78; 0.77(0.65–1.00) |
| van de Vrie et al. (2017)127 | EQ-5D, mean (SD) | OC patients, in the Netherlands | Laparoscopy before primary cytoreductive surgery | |
| Baseline (n = 76) | 0.69 (0.24) | |||
| During treatment – 3 months after start of treatment (n = 54) | 0.72 (0.27) | |||
| End of treatment – 6 months after initial completion (n = 57) | 0.71 (0.29) | |||
| Primary cytoreductive surgery | ||||
| Baseline (n = 69) | 0.63 (0.26) | |||
| During treatment – 3 months after start of treatment (n = 59) | 0.69 (0.21) | |||
| End of treatment – 6 months after initial completion (n = 61) | 0.69 (0.26) | |||
Gynaecological surveillance
| Study | How valued, presented as | Who valued, location | Health state description | Utility value |
|---|---|---|---|---|
| Kuppermann et al. (2013)88 | TTO, mean (SD); median (IQR) | Patients from a general medicine clinic (n = 49), in the USA | Test positive for LS, decline RRGS, told should have surveillance for colorectal, endometrial and OCa | 0.667 (0.232); 0.727 (0.500–0.889) |
| Patients at high risk of LS (n = 21), in the USA | 0.679 (0.239); 0.760 (0.500–0.878) |
|||
| Havrilesky et al. (2009)115 | TTO, mean (SD); median (range) | Patients with OC and public (health valuation from described health state), in the USA | Blood test (n = 15) | 0.90 (0.18); 0.97 (0.33–0.97) |
| TVUS (n = 15) | 0.83 (0.27); 0.97 (0.03–0.97) |
|||
| Blood test with false positive (n = 16) | 0.88 (0.26); 0.97 (0.03–0.97) |
|||
| TVUS with false positive (n = 15) | 0.90 (0.14); 0.97 (0.50–0.97) |
|||
| Sun et al. (2019)28 | Standard gamble, median (SD) (range) | Patients with LS (health states describing potential LS cancer risk management strategies), in the USA | Annual endometrial biopsy, TVUS, CA-125 | 0.70 (0.20) (0.30–1.00) |
| Biannual endometrial biopsy, TVUS, CA-125 | 0.80 (0.17) (0.40–1.00) | |||
| Annual combined screeningb | 0.75 (0.23) (0.20–1.00) | |||
| Biannual combined screeningb | 0.80 (0.18) (0.30–1.00) | |||
| van Roosmalen et al. (2002)128 | TTO, utility (range) | Patients with BRCA1/2 | Screening for breast and OC | 1.00 (0.50–1.00) |
| Study | How valued, presented as | Who valued, location | Health state description | Utility value |
|---|---|---|---|---|
| Borendal Wodlin et al. (2011)129 | EQ-5D-3L, weighted health state index | Patients with benign gynaecological conditions, in Sweden | Hysterectomy with GA (n = 80) | |
| Preoperative | 0.75 | |||
| Day 0 | 0.03 | |||
| Day 1 | 0.24 | |||
| Day 2 | 0.39 | |||
| Day 3 | 0.44 | |||
| Day 4 | 0.46 | |||
| Day 5 | 0.48 | |||
| Day 6 | 0.49 | |||
| Day 7 | 0.51 | |||
| Day 14 | 0.64 | |||
| Day 21 | 0.73 | |||
| Day 28 | 0.85 | |||
| Day 35 | 0.90 | |||
| 6 months | 0.95 | |||
| Hysterectomy with spinal anaesthesia ( n = 82) | ||||
| Preoperative | 0.78 | |||
| Day 0 | 0.12 | |||
| Day 1 | 0.36 | |||
| Day 2 | 0.48 | |||
| Day 3 | 0.51 | |||
| Day 4 | 0.54 | |||
| Day 5 | 0.57 | |||
| Day 6 | 0.56 | |||
| Day 7 | 0.60 | |||
| Day 14 | 0.69 | |||
| Day 21 | 0.79 | |||
| Day 28 | 0.86 | |||
| Day 35 | 0.89 | |||
| 6 months | 0.96 | |||
| EQ-5D-3L, mean (SD) | Hysterectomy with complications | |||
| Week 5 | 0.82 (0.18) | |||
| 6 months | 0.94 (0.09) | |||
| Hysterectomy without complications | ||||
| Week 5 | 0.94 (0.11) | |||
| 6 months | 0.96 (0.11) | |||
| Bouwsma et al. (2018)130 | EQ-5D-3L, mean (SEM) QALYs gained over 12 months | Patients with benign gynaecological conditions receiving hysterectomy or laparoscopic adnexal surgery, in the Netherlands | Care programme (n = 227) | 0.96 (0.008) |
| Usual care (n = 206) | 0.96 (0.007) | |||
| Christiansen et al. (2019)131 | EQ-5D-5L, mean (SD) | Patients with abnormal uterine bleeding, fibroma, dysmenorrhea, dysplasia, endometriosis, dyspareunia, predisposition to gynaecological cancer or breast cancer, in Denmark | Outpatient TLH ( n = 75) | |
| Preoperative | 0.86 (0.12) | |||
| Day 1 | 0.61 (0.14) | |||
| Day 7 | 0.75 (0.11) | |||
| Day 14 | 0.79 (0.13) | |||
| Day 21 | 0.86 (0.13) | |||
| Day 28 | 0.91 (0.12) | |||
| Inpatient TLH ( n = 83) | ||||
| Preoperative | 0.85 (0.15) | |||
| Day 1 | 0.62 (0.17) | |||
| Day 7 | 0.75 (0.13) | |||
| Day 14 | 0.83 (0.12) | |||
| Day 21 | 0.88 (0.12) | |||
| Day 28 | 0.91 (0.1) | |||
| Cooper et al. (2019)132 | EQ-5D-3L, mean (SD) | Patients with heavy menstrual bleeding (HEALTH trial), in the UK | Laparoscopic supracervical hysterectomy | |
| Baseline | 0.7065 (0.30) | |||
| 6 weeks post surgery | 0.8279 (0.22) | |||
| 6 months post surgery | 0.8315 (0.27) | |||
| 15 months post randomisation | 0.8357 (0.24) | |||
| Endometrial ablation | ||||
| Baseline | 0.6983 (0.31) | |||
| 6 weeks post surgery | 0.8282 (0.28) | |||
| 6 months post surgery | 0.8269 (0.25) | |||
| 15 months post randomisation | 0.8005 (0.28) | |||
| SF-6D (from SF-12), mean (SD) | Laparoscopic supracervical hysterectomy | |||
| Baseline | 0.6174 (0.12) | |||
| 6 weeks post surgery | 0.6762 (0.14) | |||
| 6 months post surgery | 0.8036 (0.14) | |||
| 15 months post randomisation | 0.8094 (0.14) | |||
| Endometrial ablation | ||||
| Baseline | 0.6249 (0.14) | |||
| 6 weeks post surgery | 0.7506 (0.16) | |||
| 6 months post surgery | 0.7757 (0.15) | |||
| 15 months post randomisation | 0.7818 (0.14) | |||
| Davies et al. (2002)133 | EQ-5D-3L, mean | Unselected gynaecological outpatients and inpatients, in the UK | Hysterectomy ( n = 131) | |
| Preoperative | 0.72 | |||
| 3 months | 0.84 | |||
| 6 months | 0.89 | |||
| Dickersin et al. (2007)134 | EQ-5D, mean (SD) | Patients with dysfunctional uterine bleeding, in the UK and USA | Hysterectomy (UK) | |
| Baseline | 0.549 (0.328) | |||
| 6 months | 0.784 (0.245) | |||
| 12 months | 0.734 (0.273) | |||
| 18 months | 0.747 (0.282) | |||
| 24 months | 0.760 (0.280) | |||
| Hysterectomy (USA) | ||||
| Baseline | 0.678 (0.215) | |||
| 6 months | 0.836 (0.168) | |||
| 12 months | 0.803 (0.181) | |||
| 18 months | 0.813 (0.191) | |||
| 24 months | 0.818 (0.193) | |||
| Endometrial ablation (UK) | ||||
| Baseline | 0.546 (0.316) | |||
| 6 months | 0.685 (0.306) | |||
| 12 months | 0.735 (0.286) | |||
| 18 months | 0.727 (0.291) | |||
| 24 months | 0.740 (0.275) | |||
| Endometrial ablation (USA) | ||||
| Baseline | 0.672 (0.207) | |||
| 6 months | 0.770 (0.202) | |||
| 12 months | 0.805 (0.193) | |||
| 18 months | 0.799 (0.196) | |||
| 24 months | 0.806 (0.189) | |||
| Fennessy et al. (2011)135 | TTO, mean (SD) | Patients with uterine fibroids, in the USA | Abdominal hysterectomy ( n = 61) | |
| Before treatment | 0.766 (0.351) | |||
| After treatment | 0.986 (0.067) | |||
| Uterine artery embolisation ( n = 72) | ||||
| Before treatment | 0.819 (0.262) | |||
| After treatment | 0.920 (0.204) | |||
| MRI-guided focused US surgery ( n = 59) | ||||
| Before treatment | 0.860 (0.239) | |||
| After treatment | 0.925 (0.128) | |||
| Garry et al. (2004)136 | EQ-5D, mean; median (IQR) | Patients with gynaecological symptoms that, in the opinion of a gynaecologist and patient, justified hysterectomy. This includes: DUB, fibroids, endometriosis, failed ablation, pelvic pain (dysmenorrhoea, dyspareunia, menorrhagia, premenstrual syndrome), other, in the UK and South Africa | Vaginal laparoscopic hysterectomy ( n = 324) | |
| Baseline | 0.746; 0.760 (0.725–1.00) |
|||
| 6 weeks | 0.875; 0.907 (0.812–1.00) |
|||
| 4 months | 0.911; 0.971 (0.848–1.000) |
|||
| 1 year | 0.920; 1.000 (0.881–1.000) |
|||
| Vaginal hysterectomy ( n = 163) | ||||
| Baseline | 0.758; 0.796 (0.691–1.000) |
|||
| 6 weeks | 0.852; 0.863 (0.760–1.000) |
|||
| 4 months | 0.918; 0.959 (0.848–1.000) |
|||
| 1 year | 0.917; 1.000 (0.861–1.000) |
|||
| Abdominal laparoscopic hysterectomy ( n = 573) | ||||
| Baseline | 0.716; 0.760 (0.691–0.848) |
|||
| 6 weeks | 0.832; 0.869 (0.760–1.000) |
|||
| 4 months | 0.886; 0.959 (0.812–1.000) |
|||
| 1 year | 0.897; 0.929 (0.848–1.000) |
|||
| Abdominal hysterectomy ( n = 286) | ||||
| Baseline | 0.690; 0.725 (0.689–0.812) |
|||
| 6 weeks | 0.833; 0.883 (0.760–1.000) |
|||
| 4 months | 0.866; 0.888 (0.796–1.000) |
|||
| 1 year | 0.892; 0.959 (0.822–1.000) |
|||
| Gorlero et al. (2008)137 | EQ-5D, mean | Patients with benign indication (symptomatic myomas, menorrhagia, pelvic pain or dysmenorrhoea, ovarian disease), in Italy | Total hysterectomy ( n = 54) | |
| Baseline (2 weeks before surgery) | 0.690 | |||
| 1 year (after surgery) | 0.780 | |||
| Subtotal hysterectomy ( n = 51) | ||||
| Baseline (2 weeks before surgery) | 0.716 | |||
| 1 year (after surgery) | 0.930 | |||
| Grann et al. (1998)90 | TTO, mean (IQR) | Public convenience sample to develop community-based preferences on BRCA1/2. Scenario described for the 5 health states | Oophorectomy | 0.91 (0.875–1.00) |
| Oophorectomy and mastectomy | 0.86 (0.750–1.00) | |||
| Grann et al. (1999)89 | TTO, mean (SD) | Patients with BRCA1/2, public and health professionals | Prophylactic oophorectomy | |
| Age 20–32 years (n = 93) | 0.89 (0.18) | |||
| Age 33–50 years (n = 42) | 0.68 (0.36) | |||
| Age 33–50 years high risk (n = 22) | 0.82 (0.27) | |||
| Age 33–50 years breast cancer (n = 20) | 0.86 (0.17) | |||
| Health professionals (n = 20) | 0.81 (0.25) | |||
| Other individuals (n = 105) | 0.84 (0.26) | |||
| Prophylactic oophorectomy and mastectomy | ||||
| Age 20–32 years (n = 93) | 0.84 (0.22) | |||
| Age 33–50 years (n = 42) | 0.67 (0.33) | |||
| Age 33–50 years high risk (n = 22) | 0.73 (0.25) | |||
| Age 33–50 years breast cancer (n = 20) | 0.80 (0.23) | |||
| Health professionals (n = 20) | 0.81 (0.22) | |||
| Other individuals (n = 105) | 0.79 (0.27) | |||
| Grann et al. (2010)114 | TTO Mean (SD) |
Women with personal or family history but no known personal risk of breast/OC, and women with known BRCA1/2, in the USA | Prophylactic oophorectomy | |
| BRCA1/2 mutation carriers | 0.95 (0.10) | |||
| Control | 0.90 (0.14) | |||
| Hemming et al. (2020)138 | EQ-5D-3L Mean (SD) |
Patients in uterine trial with uterine prolapse, and utility parameters used in economic model, in the UK | Uterine preservation – uterine trial | |
| Baseline (n = 264) | 0.738 (0.221) | |||
| 6 months after surgery (n = 225) | 0.855 (0.211) | |||
| 12 months after randomisation (n = 225) | 0.871 (0.187) | |||
| Total QALYs (n = 198) | 0.845 (0.158) | |||
| Vaginal hysterectomy – uterine trial | ||||
| Baseline (n = 263) | 0.781 (0.178) | |||
| 6 months after surgery (n = 243) | 0.880 (0.188) | |||
| 12 months after randomisation (n = 235) | 0.886 (0.187) | |||
| Total QALYs (n = 209) | 0.866 (0.140) | |||
| EQ-5D-3L Mean (SE) |
Vaginal hysterectomy – used in economic model | |||
| Baseline | 0.781 (0.011) | |||
| Well | 0.904 (0.011) | |||
| Failure | 0.675 (0.063) | |||
| Complication | 0.674 (0.064) | |||
| Uterine preservation – used in economic model | ||||
| Baseline | 0.739 (0.014) | |||
| Well | 0.873 (0.013) | |||
| Failure | 0.728 (0.044) | |||
| Complication | 0.820 (0.036) | |||
| Hurskainen et al. (2001, 2004)86,87 | Difference in EQ-5D-3L, mean (95% CI) | Patients with menorrhagia treated by hysterectomy, in Finland | Difference from baseline (mean 0.78, 95% CI 0.70 to 0.80) | |
| 12 months post surgery (n = 117) | 0.10 (0.06 to 0.14) | |||
| 5 years post surgery (n = 115) | 0.10 (0.05 to 0.15) | |||
| Kupperman et al. (2013)139 | TTO, mean | Patients with non-cancerous pelvic problems including abnormal uterine bleeding with or without leiomyomas, chronic pelvic pain, or pressure resulting from leiomyomas, in the USA | Hysterectomy ( n = 205) | |
| 4 years before | 0.805 | |||
| Immediately before | 0.743 | |||
| Immediately after | 0.843 | |||
| 7 years after | 0.803 | |||
| Uterus-preserving surgery ( n = 134) | ||||
| 4 years before | 0.789 | |||
| Immediately before | 0.77 | |||
| Immediately after | 0.834 | |||
| 7 years after | 0.701 | |||
| Kuppermann et al. (2013)88 | TTO, mean (SD); median (IQR) | Two patient groups, one who were not particularly knowledgeable about or at high risk for LS (general medicine clinic), the other patients who were knowledgeable about and at high risk for LS (colorectal prevention program), in the USA | Sibling state a , undergo TAH/BSO | |
| Whole sample | 0.697 (0.245); 0.750 (0.500–0.907) |
|||
| General medicine clinic | 0.691 (0.253); 0.750 (0.548–0.907) |
|||
| Colorectal prevention programme | 0.724 (0.220); 0.786 (0.495–0.910) |
|||
| Sibling state a , forgo TAH/BSO | ||||
| Whole sample | 0.669 (0.231); 0.750 (0.500–0.889) |
|||
| General medicine clinic | 0.667 (0.232); 0.727 (0.500–0.889) |
|||
| Colorectal prevention programme | 0.679 (0.239); 0.760 (0.500–0.878) |
|||
| Proband state b , undergo TAH/BSO | ||||
| Whole sample | 0.666 (0.241); 0.750 (0.485–0.870) |
|||
| General medicine clinic | 0.655 (0.242); 0.750 (0.484–0.865) |
|||
| Colorectal prevention programme | 0.710 (0.247); 0.783 (0.556–0.902) |
|||
| Proband state b , forgo TAH/BSO | ||||
| Whole sample | 0.605 (0.252); 0.661 (0.451–0.833) |
|||
| General medicine clinic | 0.616 (0.258); 0.692 (0.470–0.833) |
|||
| Colorectal prevention programme | 0.559 (0.235); 0.462 (0.364–0.758) |
|||
| Lashen et al. (2013)140 | EQ-5D, mean (SD); median (95% CI) | Patients receiving TAH for benign conditions, in the UK | Preoperative (n = 84) | 0.87 (0.13); 0.84 (0.84 to 0.90) |
| 6 weeks (n = 72) | 0.80 (0.14); 0.82 (0.76 to 0.83) |
|||
| 12 weeks (n = 75) | 0.88 (0.12); 0.84 (0.85 to 0.90) |
|||
| 24 weeks (n = 65) | 0.89 (0.14); 1.00 (0.86 to 0.93) |
|||
| 52 weeks (n = 65) | 0.92 (0.13); 1.00 (0.88 to 0.95) |
|||
| Mansel et al. (2007)104 | Standard gamble, mean (SD) | Patients in ATAC breast cancer trial, in the UK | Hysterectomy (n = 23) | 0.899 (0.101) |
| Radosa et al. (2014)141 | EQ-5D-3L, mean (SD) | Patients with benign uterine disorders, in Germany | Supracervical laparoscopic hysterectomy ( n = 72) | |
| Preoperative | 0.89 (0.19) | |||
| Postoperative | 0.96 (0.13) | |||
| Total laparoscopic hysterectomy ( n = 98) | ||||
| Preoperative | 0.84 (0.22) | |||
| Postoperative | 0.95 (0.11) | |||
| Vaginal hysterectomy ( n = 67) | ||||
| Preoperative | 0.68 (0.23) | |||
| Postoperative | 0.95 (0.13) | |||
| Sculpher et al. (1998)142 | TTO, mean (SE); median (range) | Patients with menorrhagia, in the UK | Abdominal hysterectomy | |
| Chronic state, premenopausal following surgery recovery | 0.86 (0.03); 0.95 (0.05–1.00) |
|||
| Temporary health state, convalescence following surgery | 0.74 (0.05); 0.95 (0.00–1.00) |
|||
| Sun et al. (2019)28 | Standard gamble, median (SD) (range) | LS patients (health states describing potential LS cancer risk management strategies), in the USA | Prophylactic hysterectomy and BSO | |
| Premenopausal | 0.40 (0.28) (0.00–1.00) | |||
| Postmenopausal | 0.60 (0.26) (0.00–1.00) | |||
| Taipale et al. (2009)143 | 15D, mean (SD) | Patients with benign disease including: benign uterine or ovarian cause, endometriosis, uterovaginal prolapse, menorrhagia, in Finland | Hysterectomy for benign uterine or ovarian cause | |
| Baseline | 0.908 (0.070) | |||
| 6 months | 0.926 (0.083) | |||
| Hysterectomy for endometriosis | ||||
| Baseline | 0.878 (0.090) | |||
| 6 months | 0.926 (0.068) | |||
| Hysterectomy for prolapse | ||||
| Baseline | 0.910 (0.066) | |||
| 6 months | 0.927 (0.060) | |||
| Hysterectomy for menorrhagia | ||||
| Baseline | 0.914 (0.073) | |||
| 6 months | 0.937 (0.070) | |||
| All women | ||||
| Baseline | 0.907 (0.071) | |||
| 6 months | 0.928 (0.077) | |||
| Baseline by age | ||||
| Age-standardised general female population | 0.911 (0.087) | |||
| Age < 50 years (n = 127) | 0.909 (0.071) | |||
| Age 50–60 years (n = 140) | 0.913 (0.065) | |||
| Age > 60 years (n = 70) | 0.895 (0.083) | |||
| van Roosmalen et al. (2002)128 | TTO, utility (range) | Presymptomatic BRCA1/2 mutation carriers | Screening for breast cancer and prophylactic oophorectomy | 0.99 (0.5–1.0) |
Quality appraisal
| Study | Sample size | Respondent selection | Inclusion/exclusion criteria | Response rates to instrument | Questionnaire return rate | Loss to follow-up | Missing data |
|---|---|---|---|---|---|---|---|
| Armfield (2019)96 | 404 | Secondary analysis of data collected in a RCT | Included: early-stage EC receiving TLH | NR | NR | 3 (2 prior to surgery; 1 1 week after surgery) | 16/404 for adverse events |
| Bijen (2011)96 | 279 (283) | Patients and gynaecologists in multicentre RCT | Included: women with histologically proven endometrioid adenocarcinoma, grade 1 or 2, or CAH, clinically confined to the uterine corpus. Exclusion criteria: any non-endometrioid adenocarcinoma histological types, uterine size larger than that expected at 12 weeks pregnancy and cardiopulmonary contraindications for laparoscopy or laparotomy | EQ-5D = 83% | 90% (88%–93%) | EQ-5D: 4 (within 6 weeks after surgery). Additional detail of loss to follow-up presented in Figure 1 however unclear how this relates to final sample of 279 | 4 (2 patients in each arm were randomised, although it was known before randomisation that they did not fulfil the inclusion criteria) |
| Borendal Wodlin (2011)129 | 162 | Multicentre trial | Included: women age 18–60 scheduled for total or subtotal abdominal hysterectomy, benign indications, expect at least one ovary to be preserved, fluent in Swedish language and understanding, informed consent. | NR | 39% randomised (180/464 eligible). Of the randomised 90% participated (162/180) | NR | 1.68% on EQ-5D. In cases of missing data the health state index score was substituted by mean value of the group |
| Exclusion criteria: contraindication against general or spinal anaesthesia, ASA ≥ class 3, allergic to study medications e.g. morphine, present or historical gynaecological malignancy, previous bilateral oophorectomy, operation expected to include more than hysterectomy/salpingectomy/appendectomy, postmenopausal without hormone therapy, physical disability affecting postoperative mobilisation, severe psychiatric disease or mental disability | |||||||
| Bouwsma (2018)130 | 433 | Stepped-wedge cluster-RCT. Patients recruited from hysterectomy and laparoscopic adnexal surgery waiting lists | Include: women aged 18–65 years who were employed for at least 8 hours a week. Exclude: severe benign comorbidity, had a malignancy, were pregnant, were computer or internet illiterate, were involved in a lawsuit against their employer, were on disability sick leave before surgery or had insufficient command of Dutch | NR | NR | Complete follow-up data from 92.6% of participants on return to work, 71.8% on secondary outcomes (including QoL), 70.0% on healthcare utilisation | Multiple imputation by chained equations. Only 70.4% full cost data. Other missing data NR |
| Christiansen (2019)131 | 158 (203) | Regional hospital RCT. Participants recruited from outpatient clinic | Inclusion: ability to read and write Danish, < 56 years of age, premenopausal, an estimated uterine weight below 500 g by TVUS performed by a senior consultant, and a benign indication for hysterectomy | 158 completed EQ-5D | 162 complete data | 7 (preoperative-day 28: 2 outpatient loss, 5 inpatient loss) | 45 |
| Cohn (2015)109 | NR | RCT data | NR | NR | NR | NR | NR |
| Cole (2018)109 | NR | NR (states event probabilities based on trial data) | NR | NR | NR | NR | NR |
| Cooper (2019)132 | 641 (660) | Multicentre RCT | Include: Women aged < 50 years with HMB who wanted surgical treatment, and had no plans to conceive. Exclusion criteria: endometrial atypia; abnormal cytology; uterine cavity size > 11 cm; any fibroids > 3 cm; contraindications to laparoscopic surgery; previous EA; and inability to give informed consent or complete trial paperwork | EQ-5D: 641 at baseline, 497 at 6 weeks post-surgery, 488 at 6 months post-surgery, 562 at 15 months post randomisation. Also reported for resource use and costs | 558/660 = 84.5% | At 15 months post randomisation 3 lost from LASH [reasons included: unwillingness to have surgery (n = 1), private treatment (n = 1), no reason given (n = 1)]; and 8 lost from EA [reasons included: unwillingness to have surgery (n = 2), requested a different operation (n = 2), family illness (n = 1), moved abroad (n = 1), did not want to complete questionnaires (n = 2)]. Also reported for resource use and costs | Collected data allowed the estimation of total cost and total QALYs for 57% (53% for EA, 60% for LASH) and 63% (65% for EA, 69% for LASH) of the study sample. EQ-5D only: baseline LASH = 11 (3.33%), EA = 8 (2.42%); 6-week post-surgery LASH = 78 (25.08%), EA = 82(26.37%); 6-month post-surgery LASH = 75 (24.12%), EA = 90 (28.94%); 15-month post-randomisation LASH = 49 (14.85%), EA = 49 (14.85%); |
| QALYs LASH = 103 (31.21%), EA = 117(35.45%) (% over 330 possible observations post randomisation or over 311 post-surgery as 19 participants did not have surgery). Missing data dealt with by multiple imputation using chained equations with predicted mean matching (kth-nearest neighbour = 5). Also reported for resource use and costs | |||||||
| Cykert (2004)97 | 106 | Recruited consecutive individuals at libraries, churches, medical clinics and health fairs. Sites chosen to obtain a minimum of one-third African-American representation and wide distribution of income and education in both racial groups | Included: women aged 50 years or older, from urban areas of central North Carolina and south Florida | NR | NR | NR | NR |
| Davies (2002)133 | 131 (348) | Outpatient clinic attendees and hysterectomy patients | Included: women attending outpatient clinic as new patients. QoL for hysterectomy patients | 131/348 measured QoL. Baseline n = 131; 3 months n = 117/131 (89% return rate); 6 months n = 109/131 (83% return rate) | 117/131 (89%) at 3 months; 109/131 (83%) at 6 months | 14 at 3 months, 22 at 6 months. Reasons not given | 7.20% |
| Dickersin (2007)134 | 237 | Multicentre RCT | Include: at least 18 years of age, premenopausal, with dysfunctional uterine bleeding for at least 6 months, and refractory to medical therapy for at least 3 months. Exclude: postmenopausal or had bilateral oophorectomy, was pregnant, wished to retain her fertility, or refused to consider surgery | EQ-5D: baseline EA = 123, Hyst = 114; 6 months EA = 111, Hyst = 109; 12 months EA = 107, Hyst = 103; 18 months EA = 105, Hyst = 104; 24 months EA = 106, Hyst = 107 | Overall women were followed from enrolment to end of follow-up such that those assigned later had shorter follow-up. Those completing follow-up were: 36 of 47 (76.6%) women enrolled for 5 years, 135 of 141 (95.7%) enrolled for 4 years, 191 of 202 (94.6%) enrolled for 3 years, 225 of 237 (94.9%) enrolled for 2 years and 225 of 237 (94.9%) enrolled for 1 year completed follow-up | NR | NR |
| Duong (2016)110 | 340 (502) | RCT subpopulation at high risk of progression | Include: At high risk of progression in ICON7 trial — defined as having suboptimally debulked (> 1 cm) stage iii disease or unresectable stage iii or iv disease |
Health state utilities: cycle1 = 340, cycle2 = 383, cycle3 = 380, cycle4 = 365, cycle5 = 367, cycle6 = 360, cycle8 = 308, cycle10 = 299, cycle12 = 287, cycle14 = 226, cycle16 = 206, cycle18 = 181, follow-up = 395 | NR | NR | NR |
| Fennessy (2011)135 | 192 (197) | Retrospective study – recruited patients with symptomatic uterine fibroids treated within author’s institution 2004–6. All patients sent a recruitment letter | Included: patients with symptomatic uterine fibroids treated with hysterectomy, UAE or MRI-guided focused US surgery at our institution between January 2004 and December 2006 | TTO: 61/62 hysterectomy; 72/74 UAE; 59/61 MRI | Completed questionnaires from patients with whom contact was made: 62/82 (76%) hysterectomy; 74/100 (74%) UAE; 61/85 (72%) MRI- guided US surgery | NR | NR |
| Ferguson (2018)98 | 429 (520) | Multicentre prospective cohort study. Recruited from 1 of 8 gynaecologic centres 2012–4 | Include: age 18+, undergoing primary surgery for a histologically confirmed EC, clinically confined to the uterus, ECOG performance status < 2, English or French speaking, could complete questionnaire independently. Exclude: had preoperative radiation or chemotherapy, evidence of disease beyond the uterus on preoperative imaging or clinical exam, or were medically unfit to undergo surgery |
Total completing some portion of FACT-G = 434/520, pre-surgery = 429 (98.9%), 1 week post surgery = 403 (92.9%), 3 weeks post = 403 (92.9%), 3 months post = 372 (85.7%), 6 months post = 351 (80.9%) | 468/520 = 90% | 52 (10%) did not complete any questionnaire | Missing data represented as a separate category and assumed missing at random using maximum likelihood method (MAR) with sensitivity analysis for MCAR and MNAR |
| Friedlander (2018)111 | 883 (940) | RCT data | Include: patients with advanced EOC whose disease had not progressed at the completion of first-line platinum-based chemotherapy | 894/940 randomised patients had baseline HRQoL scores. For EQ-5D: pazopanib: baseline = 442; week13 = 293; month7 = 226; m10 = 181; m13 = 138; m16 = 87; m25 = 56. Placebo: baseline = 441; w13 = 376; m7 = 303; m10 = 228; m13 = 185; m16 = 129; m25 = 88 | NR | Discontinued placebo n = 333 (disease progression = 238; adverse event = 26; protocol deviation = 4; investigator decision = 39; patient decision = 24; lost to follow-up = 2). Discontinued pazopanib n = 372 (disease progression = 113; adverse event = 159; protocol deviation = 0; investigator decision = 29; patient decision = 71; lost to follow-up = 0) | 46/940 did not have baseline HRQoL scores |
| Fujiwara (2016)112 | 834 (919) | RCT data | Include: ≥ 18 years, PFI ≤ 12 months and had evaluable disease per RECIST version 1.1 with modifications. Exclude: received > 3 previous lines of chemotherapy; platinum refractory disease; or borderline, mucinous, or clear-cell histology | 834/919 91% PRO-evaluable responses. For EQ-5D: baseline = 783, week5 = 733, week9 = 647, week13 = 537, week17 = 476, week25 = 309 | NR | NR | Stratifies patients by last completed PRO to account for data missing not-at-random |
| Garry (2004)136 | 1346 (1380) | Multicentre RCT | Include: women with gynaecological symptoms justifying hysterectomy, giving informed consent, having previous failed medical or conservative treatments. Exclude: confirmed or suspected malignant disease in genital tract, second- or third- degree uterine prolapse, uterine mass bigger than 12-week pregnancy size, associated medical illness precluding laparoscopic surgery, bladder or pelvic support surgery required, patients deemed unsuitable by consultant, refused consent for trial | 801 forms analysed at 6-weeks (363 lost to follow-up, 1017 QoL forms returned); 783 forms analysed at 4-months (443 lost to follow-up, 89 missing, 848 QoL returned); 866 forms analysed at 1-year (443 lost to follow-up, 937 QoL returned) | 1380 recruited from sample size 1800 | 34/1380 dropped out prior to surgery; 83 lost to follow-up at 6-week visit | Total ‘missing’ NR – missing data reported for each measure/question e.g. 89 missing from QoL at 4-months follow-up and ‘One hundred patients had one of the two EQ-5D assessments missing between baseline and the 6-week follow-up visit (between 5.6% and 9.9% in the trial groups)’. Missing data for resource use and EQ-5D were imputed using a multivariate multiple imputation procedure |
| Gordon (2010)113 | 85 | Participants recruited through their treating oncologist or staff clinic from 7 participating hospitals | Included: age 18–79, referred for chemotherapy for primary epithelial ovarian or peritoneal cancer, able to complete the study surveys in English, women with both newly diagnosed disease and those presenting with recurrent disease between April 2003 and January 2005, provided consent for the investigators to collect clinical resource data from their hospital medical charts |
SF-6D: 61 women (72%) completed at least 6 surveys and 32 (38%) completed at least 12 surveys, whereas 5 (6%) completed 18 or more surveys, 3 women completed no surveys | NR | 9 withdrew due to incapacity. 49 (58%) died during study period | 20 (2.2%) surveys had incomplete SF-36 items |
| Gorlero (2008)137 | 105 (117) | Patients referred to authors department 2003–5 for benign indication needing abdominal hysterectomy | Include: patients who needed an abdominal hysterectomy for a benign indication were eligible. Exclusion criteria: second- or third- degree uterine prolapse, age over 75 years, malignancy, BMI > 29, previous pelvic surgery, endometriosis or history of chronic pelvic pain, abnormal cervical smears and psychiatric disorders | NR | 105/117 (89.7%) | 12 not completed at 1 year | NR |
| Grann (1998)90 | 54 | Convenience sample | NR | NR | NR | NR | NR |
| Grann (1999)89 | 177 (184) patients; 125 public | Advertisement, fliers, word of mouth to recruit | Include: women age 20–50 years without cancer or known high risk, with known breast cancer risk factors and breast cancer patients without known metastatic disease | TTO: 96% (177/184) patients. NR public and health professionals | 70% of patients initially approached participated. NR public and health professionals | NR | Participants completed 97.5% of the items on the questionnaires. NR public and health professionals |
| Grann (2010)114 | 243 | Advertisement, fliers, word of mouth to recruit women without cancer or known high risk; NR for BRCA patients | Included: women aged 20–65 who had tested positive for BRCA1/2 mutations, had no personal history of cancer, and were participating in a study of MRI and other screening methods for high-risk women | NR | NR | NR | NR |
| Havrilesky (2009)115 | 50 | Recruited via blackboard fliers at the University Medical Centre | Inclusion criteria: no personal history of OC | TTO: 100% | 100% | 0 | NR |
| Havrilesky (2012)116 | NR | RCT data | NR | NR | NR | NR | NR |
| Havrilesky (2019)117 | 101 | Recruited from University Gynaecologic Oncology outpatient clinics; OC Registry; www.researchmatch.org; social media and online interest groups | Included: English-speaking women ≥ 18 years old with a personal diagnosis of ovarian, peritoneal or tubal cancer (hereafter referred to as OC). Exclusion criteria: age < 18 years, non-English speakers, individuals accessing the survey from outside the USA, and those not providing informed consent | NR | NR | NR | NR |
| Hemming (2020)138 | 527 (565) | RCT in patients from UK hospitals with surgeons of relevant experience (2013–7) | Included: women age 18+ requiring surgery for vault or uterine prolapse based on symptoms and/or anatomical findings. Exclude: unable to consent or unable to complete questionnaires | EQ-5D: baseline = 527/563; 6 months = 468; 12 months = 460. Complete data were available across domains and time points, enabling QALY calculation for 198 out of 279 (71%) in uterine preservation group and 209 out of 283 (74%) in hysterectomy | 563/565 | 469/563 received their randomisation allocation thus would be eligible for follow-up. 468 provided primary outcome at 12-months follow-up while 33 declined further follow-up and 62 did not respond | NR for QoL. Some missing data presented from more primary/clinical outcomes. Multiple imputation of missing data by predictive mean matching used to calculate ICER (190/562 had incomplete cost and QALY profiles) |
| Hess (2010)119 | 75 (86) | Contacted via telephone or email | Included gynaecologic or medical oncologists who prescribed treatment for women with OC and patients at least 18 years old with histologically or pathologically confirmed diagnosis of stage III–IV epithelial ovarian carcinoma or primary peritoneal carcinoma | NR | 75/86 eligible of those enrolled and completed the study | NR | NR |
| Hess (2013)118 | 746 (965) | RCT data | NR | NR | NR | NR | NR |
| Hettle (2015)91 | 247 (264) | RCT data | NR | NR | NR | NR | NR |
| Hildebrandt (2014)99 | 57 (580) | Questionnaires distributed to surgical and oncology wards and University departments | NR | NR | NR | NR | NR |
| Hinde (2016)120 | NR (502) | RCT on patients recently undergone debulking surgery | Include: ICON7 trial women recently undergone debulking surgery for previously untreated advanced OC, or early-stage disease deemed to be at high risk of progression | NR | NR | NR | Total = 15.3% (HRQoL = 24.0% missing; resource use = 7.4% missing). Multiple imputation with chained equations assuming data missing at random |
| Hurskainen (2001)86 | 236 | RCT – women referred by GPs or gynaecologists from 1 of 5 university hospitals | Included: women 35–49 years old, were menstruating, had completed their family and were eligible for hysterectomy. Exclude: sub mucous fibroids, endometrial polyps, ovarian tumours or cysts (diameter > 5 cm), cervical disease, urinary and bowel symptoms or pain due to large fibroids, lack of indication for hysterectomy, history of cancer, menopause, severe depression, menorrhagia as a main complaint, previous treatment failure with levonorgestrel-releasing IUS, severe acne and uterine malformation | EQ-5D: baseline = 100% 236/236, change at 12 months = 228/236 | 236/598 women eligible and willing to take part | 8 (lost when calculating change at 12 months) | NR |
| Hurskainen (2004)87 | 236 | RCT – women referred by GPs or gynaecologists from 1 of 5 university hospitals | Included: women 35–49 years old, were menstruating, had completed their family and were eligible for hysterectomy. Exclude: submucous fibroids, endometrial polyps, ovarian tumours or cysts (diameter > 5 cm), cervical disease, urinary and bowel symptoms or pain due to large fibroids, lack of indication for hysterectomy, history of cancer, menopause, severe depression, menrorhagia as a main complaint, previous treatment failure with levonorgestrel- releasing IUS, severe acne and uterine malformation | EQ-5D: baseline = 100% 236/236, change over 5 years = 232/236 | 236/598 women eligible and willing to take part | 4 (lost when calculating change at 5 years) | NR |
| Kent (2015)100 | 1909 (124,0644) | Data from the pre-existing SEER-MHOS dataset | NR | NR | Ranged 63–72% across study years | NR | NR |
| Kimman (2015)101 | 419 (9513) | Data from the pre-existing ACTION study | Include: age 18+, with a first-time cancer diagnosis received in hospital in the last 12 weeks | NR | NR | NR | NR (reported for each demographic characteristic) |
| Krasner (2012)121 | 641 (672) | RCT data | Include: women age 18+ with histologically proven epithelial ovarian, epithelial fallopian tube or primary peritoneal cancer, previously treated with one platinum-based chemotherapy regimen. Exclude: refractory disease, isolated rise in CA-125 without documented radiological evidence of disease progression | NR | NR | Retention rates: PLD (trabectedin + PLD) cycle 3 = 71% (82%); c5 = 53% (62%); c7 = 24% (37%); c9 = 14% (22%); c11 = 9% (15%); c13 = 5% (8%); c15 = 3% (4%); c17 = 2% (2%); c19 = 1% (1%); c21 = 1%(0%) |
15% in PRO questionnaire |
| Kuppermann, Learman (2013)139 | 339 (1491) | Recruited women who had sought care within the last year for noncancerous pelvic problems at affiliated clinics and practices | Include: premenopausal women age 31–54 who had sought care in previous year for non-cancerous pelvic problems | NR | 1491/1503 with complete information | NR | NR |
| Kuppermann, Wang (2013)88 | 70 | Recruited from university clinics, general clinic and cancer prevention programme. General medical patients recruited via primary care physicians who opted into an appointment schedule review | Include: individuals who had undergone genetic risk assessment and counselling for LS | NR | NR | NR | NR |
| Lashen (2013)140 | 84 (85) | Recruited from university- based treatment hospital | Include: women age 18+ scheduled for hysterectomy. Exclude: malignant or radical hysterectomy | EQ-5D at pre-op n = 84; 6 weeks n = 72; 12 weeks n = 75; 24 weeks n = 65; 52 weeks n = 65 | NR | 15% (15/100 excluded by end of study) | For EQ-5D: at pre-op n = 1; 6 weeks n = 3; 12 weeks n = 0; 24 weeks n = 3; 52 weeks n = 0 |
| Luealon (2016)122 | NR | NR | NR | NR | NR | NR | NR |
| Lundin (2019)103 | 49 | RCT of patients in a university hospital | Include: women age 18+ speaking fluent Swedish with WHO-performance status ≤ 2 admitted for surgical treatment of stage 1 EC and scheduled for hysterectomy, BSO with peritoneal washings. Exclude: laparoscopic, planned midline incision, more extensive surgery planned, any condition excluding woman from having intrathecal morphine analgesia; immunosuppressive medication; physically disabled; severe psychiatric or mental disorder | NR | NR | 2% (1/50) | NR |
| Lundin (2020)103 | 49 | RCT of patients in a university hospital | Include: women age 18+ speaking fluent Swedish with WHO-performance status ≤ 2 admitted for surgical treatment of stage 1 EC and scheduled for hysterectomy, BSO with peritoneal washings. Exclude: laparoscopic, planned midline incision, more extensive surgery planned, any condition excluding woman from having intrathecal morphine analgesia; immunosuppressive medication; physically disabled; severe psychiatric or mental disorder | 98.54% completed EQ-5D forms | NR | 2% (1/50) | NR |
| Mansel (2007)104 | 26 (23) | NR | NR | 88% (23/26) | NR | NR | NR |
| Mirza (2016)92 | 546 | RCT | Include: women age 18+ with histologically diagnosed ovarian, fallopian tube or predominantly high-grade serous primary peritoneal cancer. Cancer shown to be sensitive to platinum-based treatment and at least two platinum-based regimens received, with sensitivity demonstrated in the penultimate platinum-based chemotherapy prior to enrolment | NR | 99% (546/553) received treatment | 80% (437/546 lost to follow-up – 53 had an adverse event, 339 had disease progression, 4 had treatment- associated risk, 2 had noncompliance, 28 requested to stop treatment, 11 had other reason) | NR |
| Naik (2017)105 | 131 (1759) | Convenience sampling across multiple outpatient clinics | Include: age 18+ ambulatory cancer survivors able to communicate in English with no significant cognitive impairment | EQ-5D: 91% (1759/1929) | 64% (1929/3019) | NR | NR |
| NICE (2013)123 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Ortega (1997)124 | 85 | Data obtained from the literature (randomised comparative trials) where possible | NR | NR | NR | NR | NR |
| Oza (2018)94 | 537 (553) | RCT data | Include: age 18+ with histologically diagnosed epithelial ovarian, fallopian tube or primary peritoneal cancer. Patients must have achieved a partial or complete response to the last platinum-based chemotherapy. Patients must have ECOG performance status of 0 or 1 and adequate organ function as assessed appropriate by a laboratory. Excluded: immunocompromised patients, those with active hepatic disease, or symptomatic, uncontrolled brain or leptomeningeal metastases | EQ-5D: baseline = 537; pre-progression = 493; post-progression = 339 | NR | NR overall though could be interpreted from response rates for EQ-5D and for individual FOSI measures from Figure 2 | < 8.5% for reasons other than progression. Observed no patterns for missing data so made no statistical adjustments |
| Oza (2020)93 | 564 | Post hoc exploratory analysis of RCT data | Include: age 18+ had platinum-sensitive, high-grade serous or endometrioid ovarian, primary peritoneal or fallopian tube carcinoma, had received ≤ 2 previous platinum-based chemotherapy regimens, and achieved any of: a complete, partial or a serologic response based on GCIG cancer antigen 125 criteria to their last platinum-based regimen | NR | NR | NR | NR |
| Radosa (2014)141 | 237 (402) | University hospital gynaecology and obstetrics | Include: premenopausal at time of surgery, hysterectomy for benign condition and performed without concurrent unilateral or bilateral adnexectomy, American Society of Anesthesiologists physical status classification of I or II | EQ-5D TTO: 93% (237/255) | 63% (255/402) | NR | 7% (18/255) |
| Exclude: refusal to participate, severe (Clavien–Dindo grade IV or V) perioperative complication with need for intraoperative conversion to laparotomy or abandonment of the intended surgical procedure, second- to fourth-degree uterine descensus requiring prolapse repair, intraoperatively diagnosed adnexal pathology requiring subsequent unilateral or bilateral oophorectomy | |||||||
| Rowland (2015)125 | NR | NR | NR | NR | NR | NR | NR |
| Sculpher (1998)142 | 60 | Women with uncomplicated menorrhagia recently referred to hospital recruited through referral letters | Include: women with apparently uncomplicated menorrhagia | NR | 115 completed forms of whom 89 agreed to be interviewed. Of 29/69 exclusions were made | NR | NR |
| Setiawan (2018)106 | 11 (116) | Hospital registered patients 2010–5 | Include: patients age 18+ diagnosed with any HPV-related cancer who had received any care in the hospital. Exclude: patients with chronic disease comorbidity | NR | 116/374 – 116 included in study from 374 eligible participants | NR | NR |
| Stein (2007)126 | 39 (66) | Outpatient clinic attendees | Include: women with advanced OC on chemotherapy who participated in a RCT of routine QoL measurement | NR | 39 panel members (35%) | NR | NR (0.8% missing ‘response at 6 months’; 3.7% missing ‘marital status’) |
| Sun (2019)28 | 61 | Recruited from a LS registry, physician referral and self-referral | Include: women age 25+ without prior gynaecologic cancer diagnosis, diagnosed with LS through genetic counselling and testing or meeting revised Amsterdam II criteria and so following LS screening recommendations | NR | NR | NR | NR |
| Taipale (2009)143 | 337 | Invited women with scheduled operative treatment in gynaecology | NR | 15D: 337/341 | 360/812 responded with additional 23 excluded (of which 4 due to missing HRQoL data) | NR | NR [4 patients excluded because of incomplete HRQoL data (more than 3 missing answers to 15 dimensions)] |
| Ueno (2020)107 | 39 (100) | Recruited women who underwent gynaecological surgery in authors’ hospital | Include: female age 18+ at least 3 months since surgery. Exclude: ongoing malignancy identified, rheumatic disease or a general inflammatory condition, acute traumatic condition such as fracture neurological disease, dementia or the inability to answer the questionnaires |
NR | 100/120 | NR | 17% (20/120) |
| van de Vrie (2017)127 | 145 (201) | RCT data | NR | EQ-5D TTO: 72% (145/201) baseline; 56% (113/201) during treatment; 59% (118/201) end of treatment | Response rates to questionnaire: 73% at baseline, 57% during treatment and 53% at the end of treatment |
NR (though see change in response rate over time Q4) | Multiple imputation used for missing data |
| van Roosmalen (2002)128 | 23 | Secondary analysis of data e.g OC registry | NR | Utility: 100% | NR | NR | NR |
Appendix 6 Detailed results from the economic evaluation
Detailed analyses of uncertainty
For each parameter set (n = 500) in each population group (n = 16), we used bootstrapping (one replicate) to estimate the probability that different cost-effectiveness conclusions would be reached if a fresh population of 4000 individuals were simulated. These are averaged across the 500 parameter sets. We then present the average percentage for each population group in Table 32.
| Population group | RRGS vs. nothing | Surveillance vs. nothing | RRGS and surveillance vs. nothing | Optimal |
|---|---|---|---|---|
| path_MLH1 | ||||
| Age 30 | 0.262 | 0.150 | 0.132 | 0.358 |
| Age 35 | 0.196 | 0.088 | 0.124 | 0.310 |
| Age 40 | 0.234 | 0.066 | 0.078 | 0.334 |
| Age 45 | 0.200 | 0.070 | 0.088 | 0.348 |
| path_MSH2 | ||||
| Age 30 | 0.168 | 0.056 | 0.076 | 0.314 |
| Age 35 | 0.154 | 0.056 | 0.068 | 0.318 |
| Age 40 | 0.122 | 0.042 | 0.034 | 0.298 |
| Age 45 | 0.200 | 0.070 | 0.088 | 0.348 |
| path_MSH6 | ||||
| Age 30 | 0.262 | 0.156 | 0.194 | 0.374 |
| Age 35 | 0.234 | 0.138 | 0.204 | 0.328 |
| Age 40 | 0.238 | 0.110 | 0.142 | 0.302 |
| Age 45 | 0.212 | 0.142 | 0.164 | 0.346 |
| path_PMS2 | ||||
| Age 30 | 0.170 | 0.078 | 0.028 | 0.204 |
| Age 35 | 0.148 | 0.084 | 0.040 | 0.200 |
| Age 40 | 0.158 | 0.136 | 0.066 | 0.272 |
| Age 45 | 0.208 | 0.136 | 0.098 | 0.294 |
For each population group, we estimated 95% prediction ellipses for the costs and QALYs for each strategy, that is ellipses constructed such that 1 in 20 new parameter set samples would be expected to have costs and QALYs outside the ellipse. These ellipses primarily quantify the uncertainty in costs and QALYs caused by parameter uncertainty, but they are also affected by Monte Carlo error within each parameter set. These prediction ellipses are shown in Figure 13 as dashed ellipses.
FIGURE 13.
Prediction and confidence ellipses for strategy costs and QALYs. RRGS, risk-reducing gynaecological surgery; Surv, surveillance.
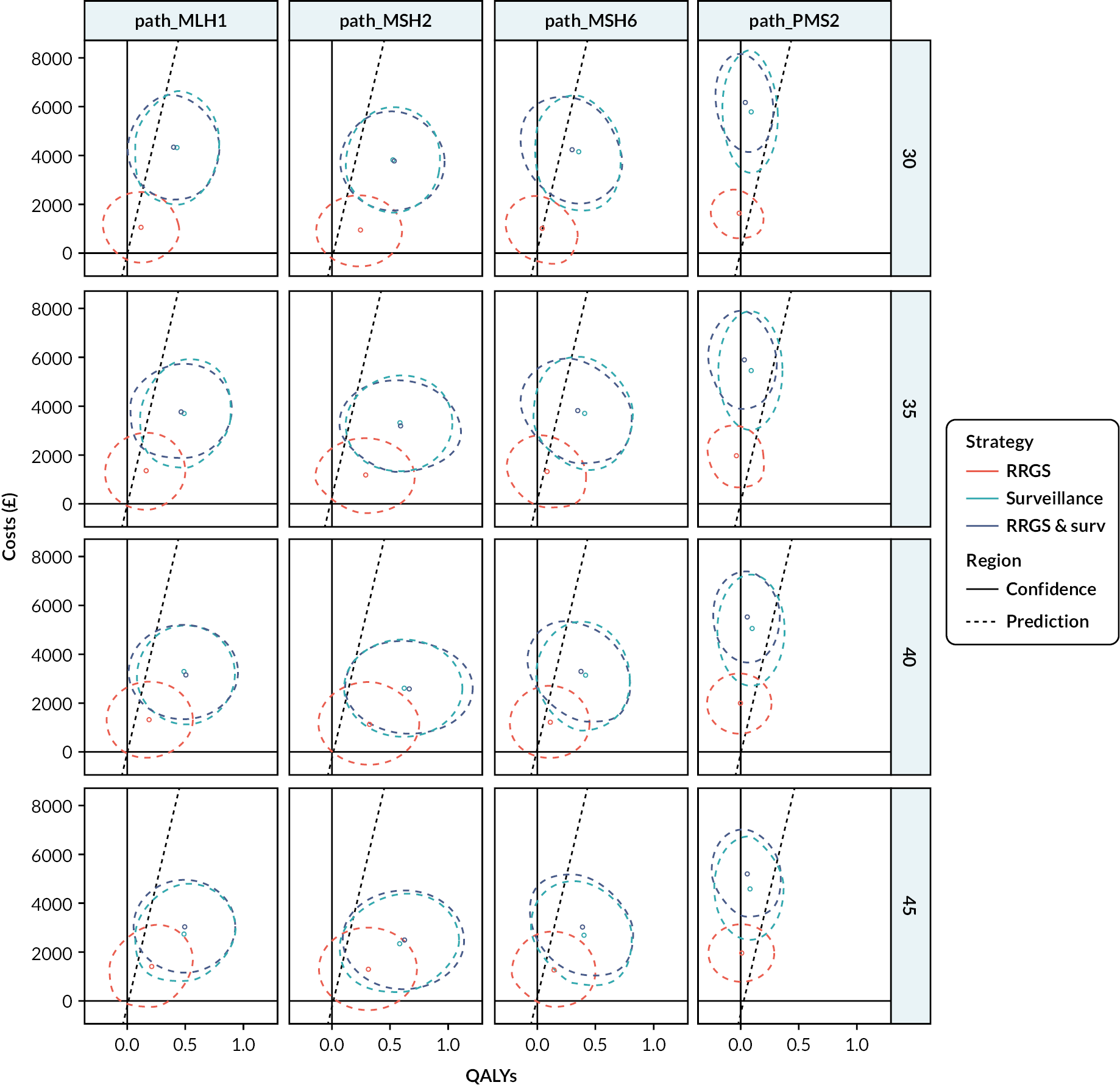
For each population group, we estimated 95% confidence ellipses for the mean costs and QALYs for each strategy. These confidence ellipses tell us the Monte Carlo error in the estimates in Table 23, which could be reduced by increasing the number of simulations conducted. These confidence ellipses are shown in Figure 13 as solid ellipses.
Cancer outcomes
We counted the number and stage of incident endometrial, ovarian and CRCs in the simulations. For each population group and for each parameter set, we calculated the difference in the number of cancers of each stage between the three intervention arms and the no intervention arms. These differences were visualised in box plots as shown in Figures 14–16.
FIGURE 14.
Effects of interventions on incidence and stage of ECs. RRGS, risk-reducing gynaecological surgery; Surv, surveillance.
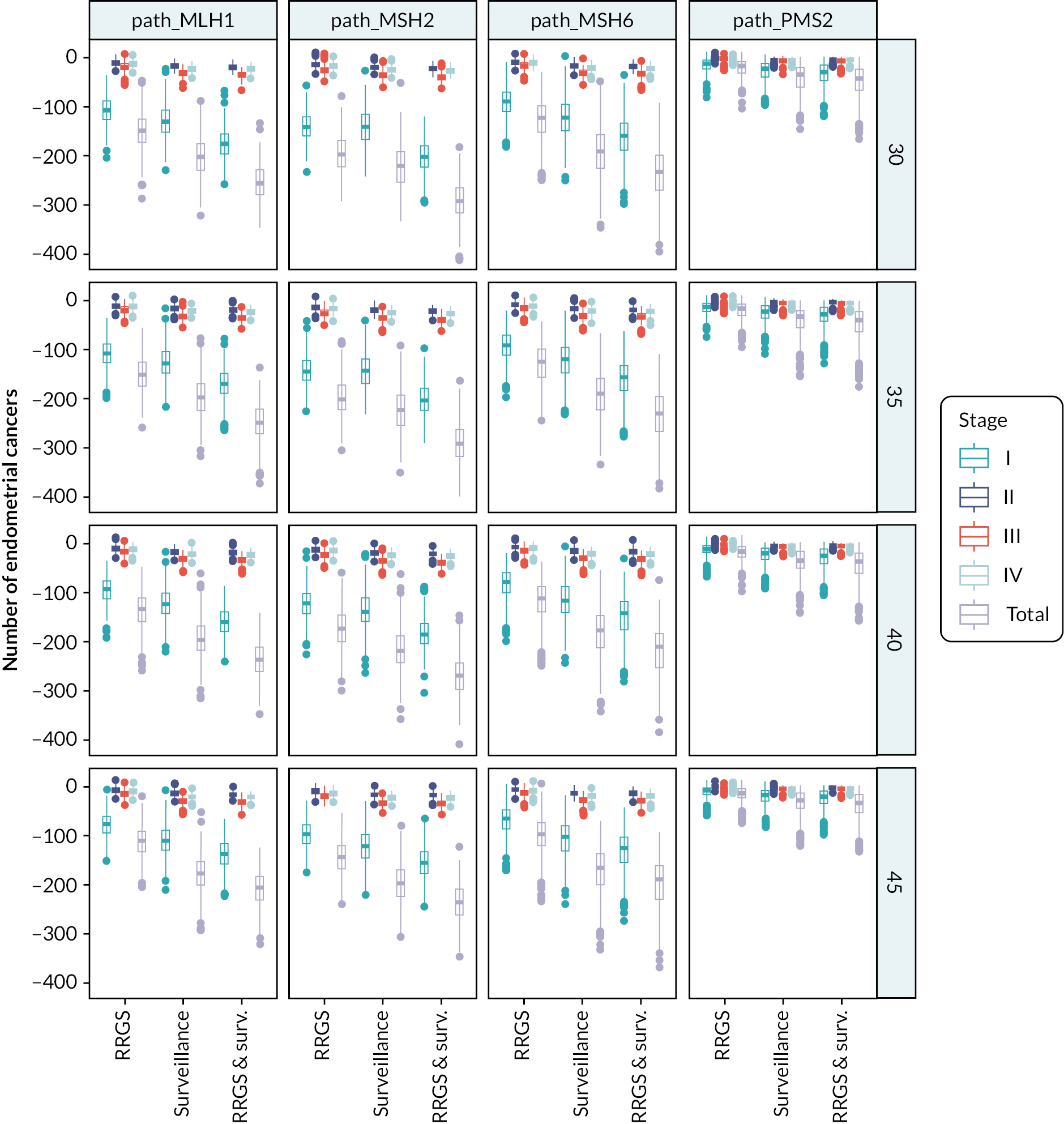
FIGURE 15.
Effects of interventions on incidence and stage of OCs. RRGS, risk-reducing gynaecological surgery; Surv, surveillance.
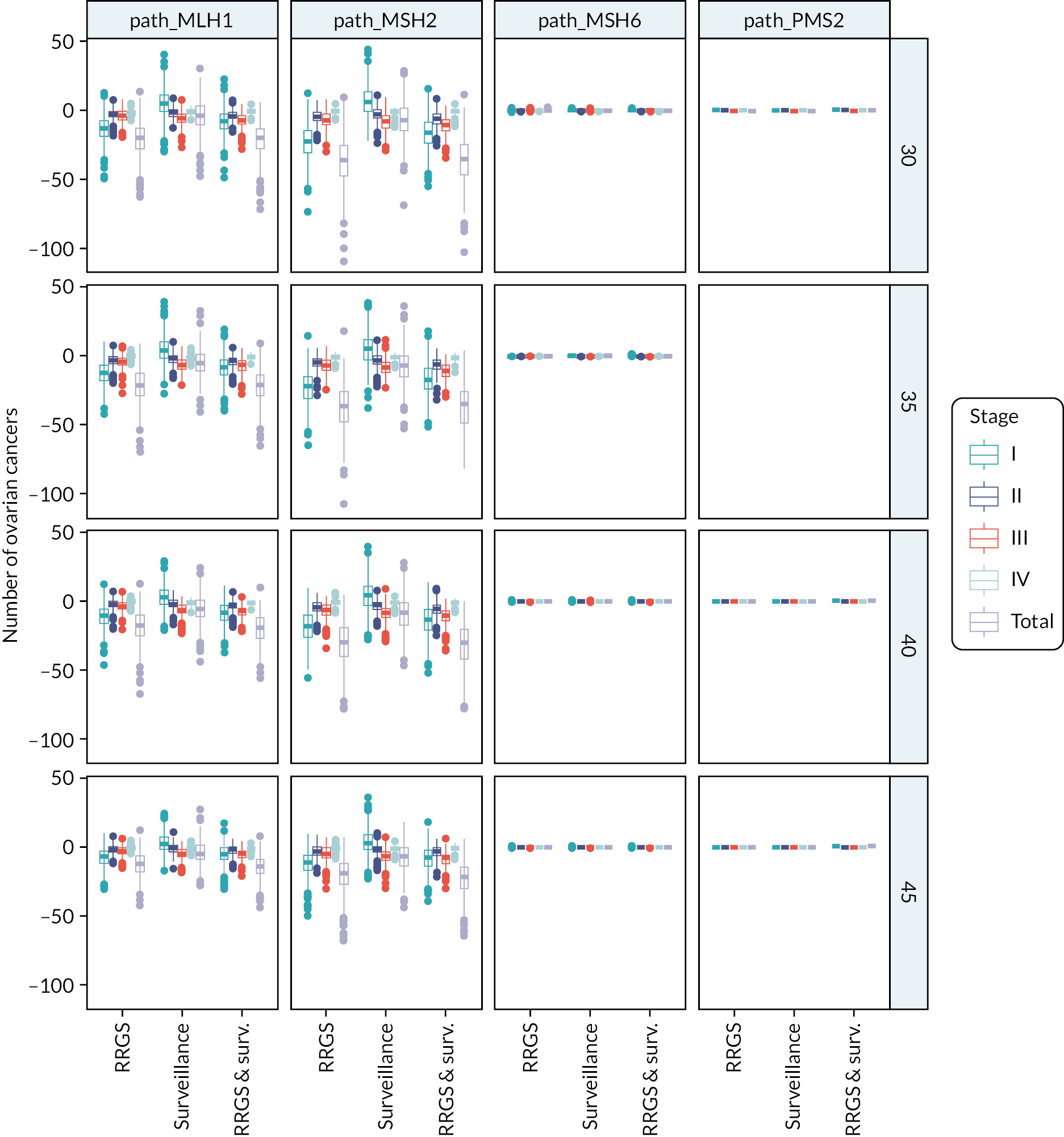
FIGURE 16.
Effects of interventions on incidence and stage of CRCs. RRGS, risk-reducing gynaecological surgery; Surv, surveillance.
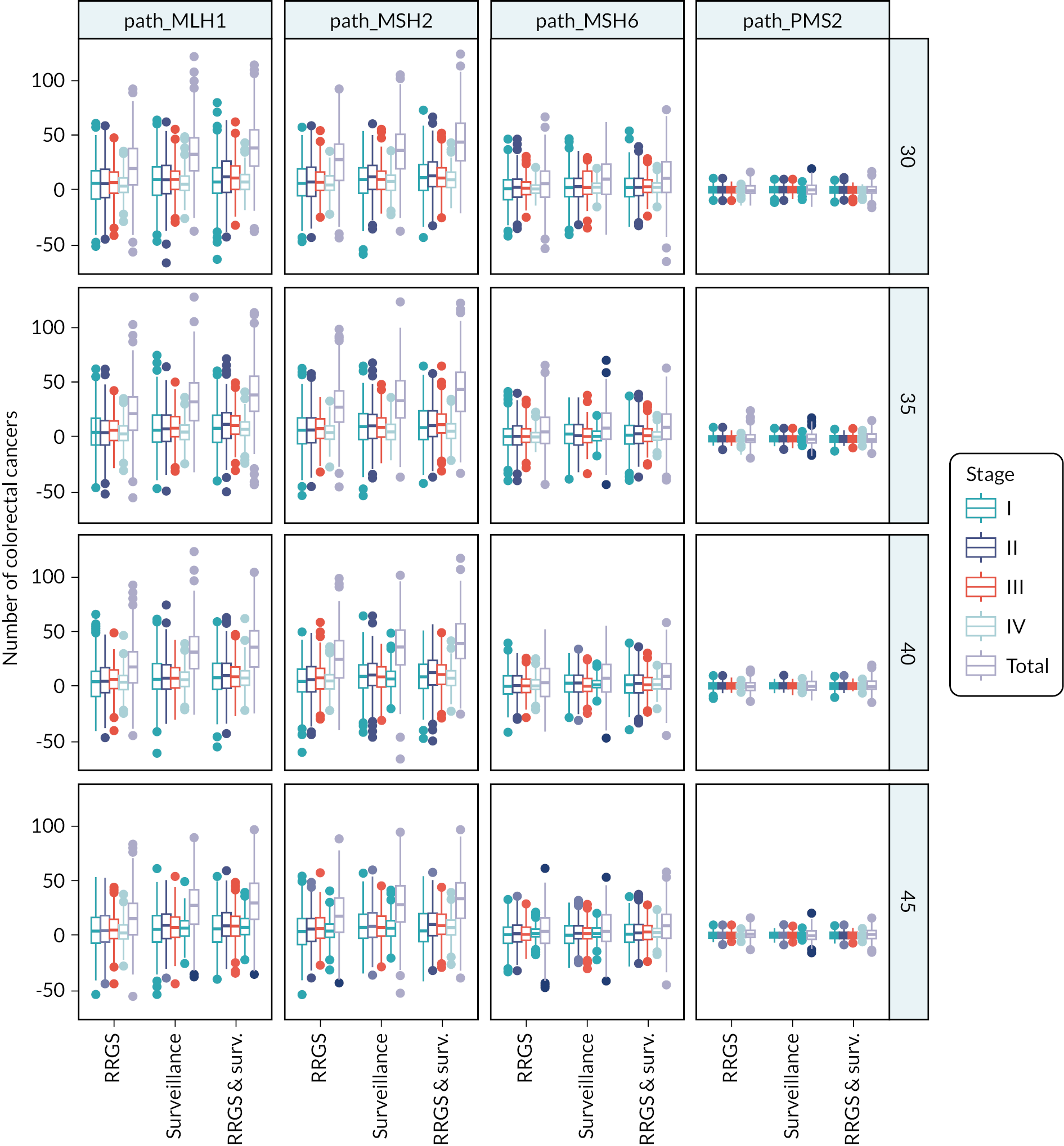
Similar calculations were performed for deaths from endometrial, ovarian and CRC, as shown in Figure 17.
FIGURE 17.
Effects of interventions on mortality from endometrial, ovarian and CRC. RRGS, risk-reducing gynaecological surgery; Surv, surveillance.
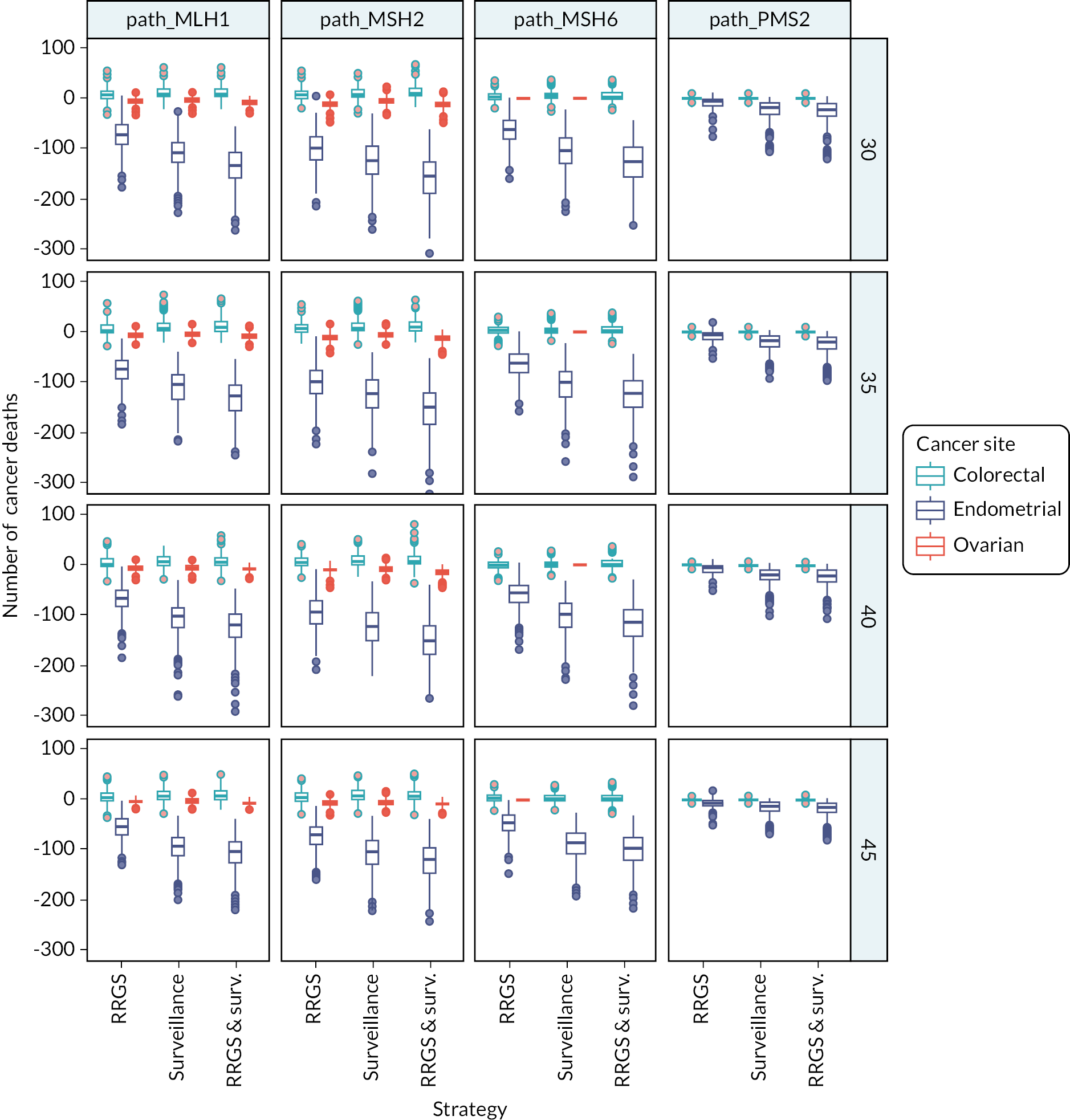
We estimated the probability of cancer-free survival as a function of time since entering the model using the Kaplan–Meier method and visualised these according to the intervention, as shown in Figure 18.
FIGURE 18.
Cancer-free survival. RRGS, risk-reducing gynaecological surgery.
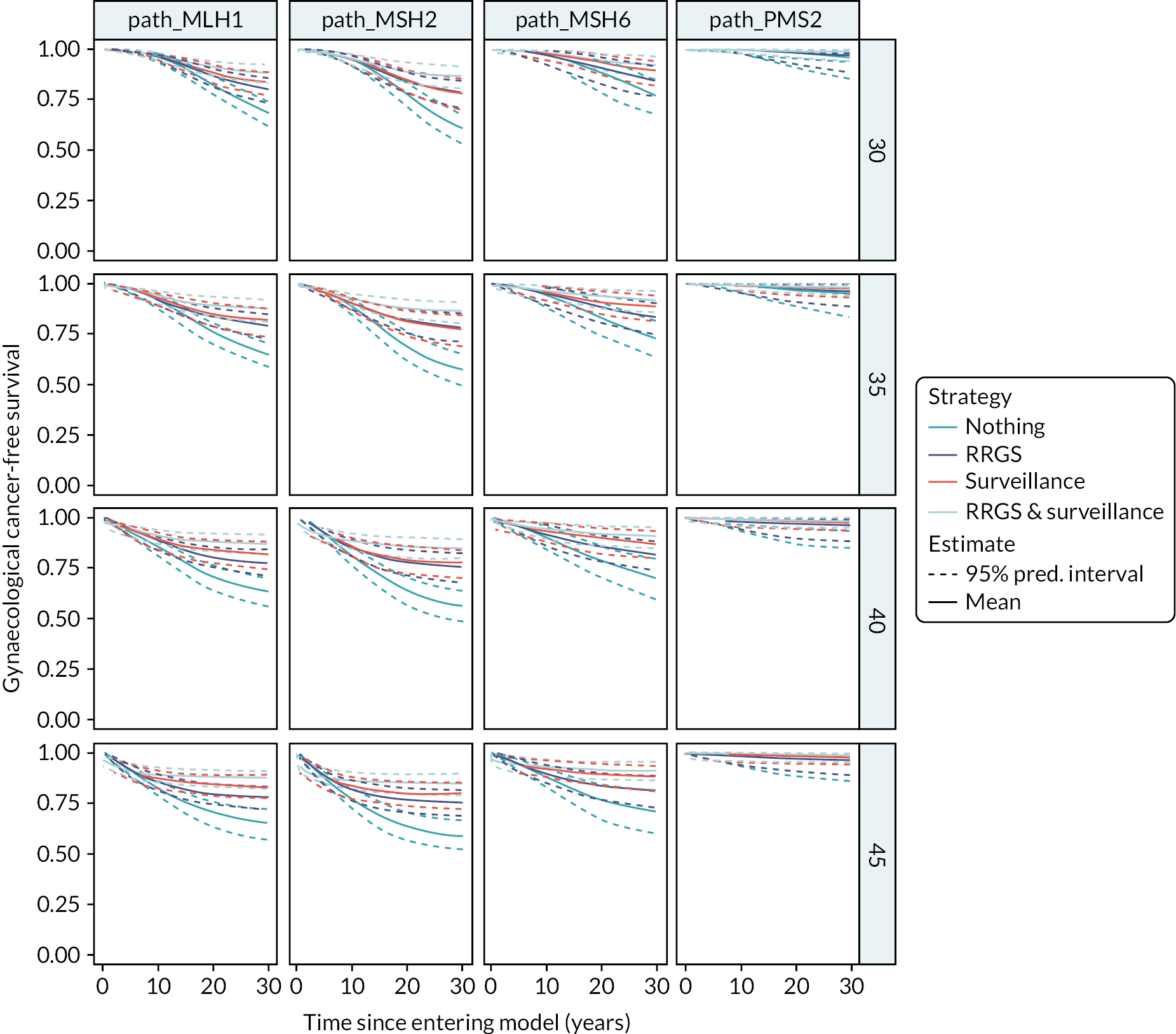
Life expectancy
We calculated the age at death for each simulated individual. The mean age at death across the population groups and interventions are shown in Table 33. Kaplan–Meier curves for overall survival according to age and genotype are shown in Figure 19.
| Population | Life expectancy | |||
|---|---|---|---|---|
| Nothing | RRGS | Surveillance | RRGS and surveillance | |
| path_MLH1 | ||||
| 30 | 80.3 | +1.53 | +2.46 | +2.97 |
| 35 | 80.4 | +1.60 | +2.44 | +2.91 |
| 40 | 80.9 | +1.33 | +2.15 | +2.50 |
| 45 | 81.5 | +1.02 | +1.79 | +2.02 |
| path_MSH2 | ||||
| 30 | 79.4 | +2.28 | +2.97 | +3.74 |
| 35 | 79.7 | +2.27 | +2.82 | +3.52 |
| 40 | 80.0 | +1.90 | +2.63 | +3.22 |
| 45 | 81.0 | +1.40 | +2.13 | +2.48 |
| path_MSH6 | ||||
| 30 | 81.5 | +1.12 | +2.22 | +2.56 |
| 35 | 81.7 | +1.24 | +2.13 | +2.48 |
| 40 | 82.1 | +1.02 | +1.92 | +2.16 |
| 45 | 82.7 | +0.81 | +1.62 | +1.77 |
| path_PMS2 | ||||
| 30 | 85.3 | +0.17 | +0.50 | +0.52 |
| 35 | 85.3 | +0.14 | +0.42 | +0.43 |
| 40 | 85.3 | +0.17 | +0.41 | +0.42 |
| 45 | 85.4 | +0.10 | +0.28 | +0.29 |
FIGURE 19.
Overall survival. RRGS, risk-reducing gynaecological surgery.
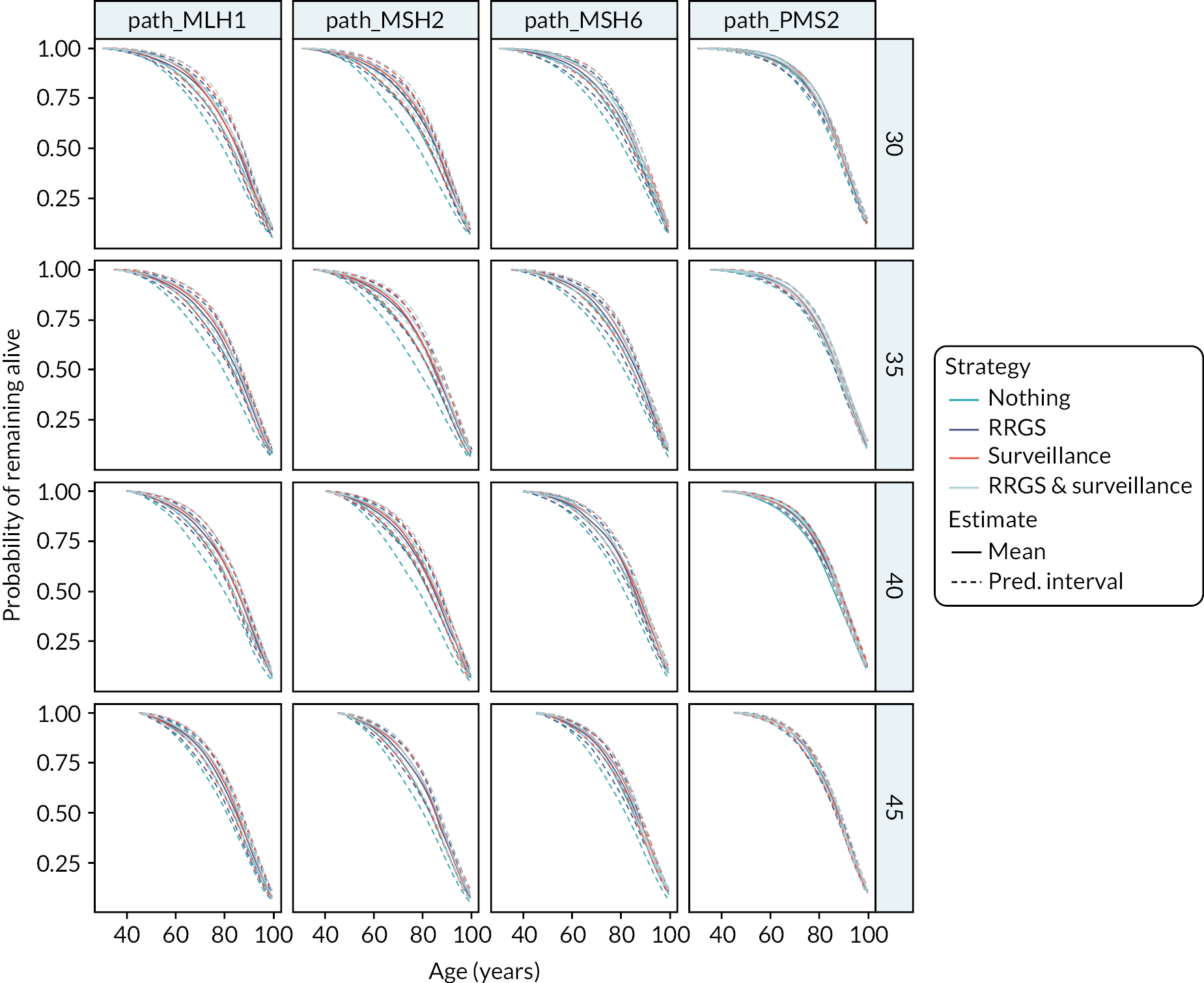
As it is difficult to see differences in the figure above, we also plotted the difference in the mean estimates between the intervention strategies and the no intervention strategy, as shown in Figure 20.
FIGURE 20.
Overall survival differences. RRGS, risk-reducing gynaecological surgery; Surv, surveillance.
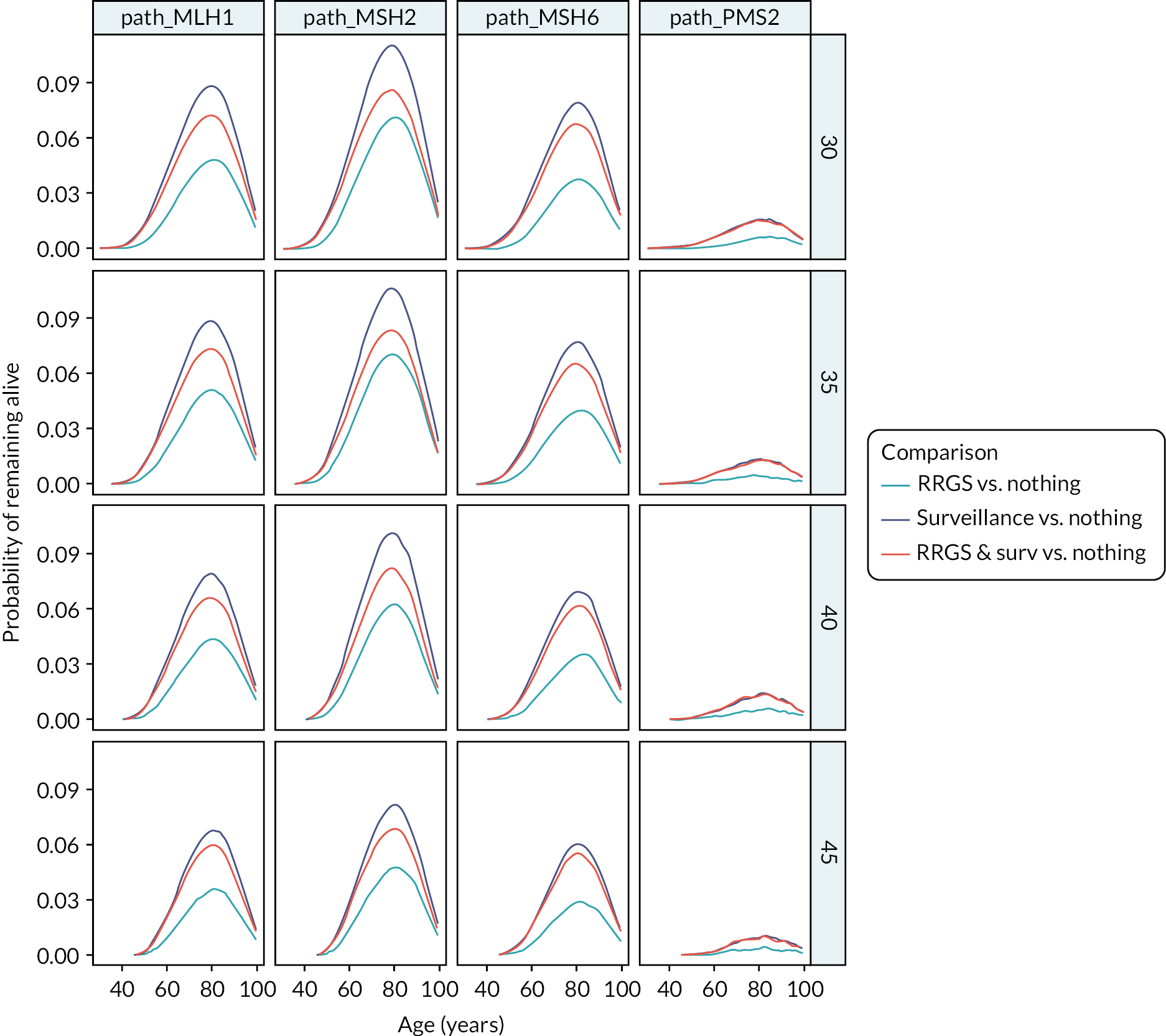
List of abbreviations
- AEH
- atypical endometrial hyperplasia
- BMI
- body mass index
- BSO
- bilateral salpingo-oophorectomy
- CA-125
- cancer antigen 125
- CAH
- complex atypical hyperplasia
- CASP
- Critical Appraisal Skills Programme
- CPCI – S
- Conference Proceedings Citation Index – Science
- CRC
- colorectal cancer
- DTA
- diagnostic test accuracy
- EC
- endometrial cancer
- EQ-5D (-3L, -5L)
- EuroQol-5 Dimensions (Three Levels, Five Levels)
- FACT (-G, -O)
- Functional Assessment of Cancer Therapy (-General, -Ovarian)
- GA
- general anaesthetic/anaesthesia
- HADS
- Hospital Anxiety and Depression Scale
- HBSO
- hysterectomy and bilateral salpingo-oophorectomy
- HNPCC
- hereditary non-polyposis colorectal cancer
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HRT
- hormone replacement therapy
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- IUD
- intrauterine device
- LNG-IUS
- levonorgestrel-releasing intrauterine system
- LR+, LR−
- likelihood ratio of a positive/negative test
- LS
- Lynch syndrome
- MMR
- mismatch repair
- MSI
- microsatellite instability
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NPV
- negative predictive value
- NSAID
- non-steroidal anti-inflammatory drug
- OC
- ovarian cancer
- PLSD
- prospective Lynch syndrome database
- PPI
- patient and public involvement
- PPV
- positive predictive value
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- ROB
- risk of bias
- ROBINS-I
- Risk Of Bias In Non-Randomized Studies – of Interventions
- RRS
- risk-reducing surgery
- QUADAS
- Quality Appraisal of Diagnostic Accuracy Studies
- SAH
- simple atypical hyperplasia
- SCI
- Science Citation Index
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- TVUS
- transvaginal ultrasound
- VAS
- visual analogue scale
- VRS
- verbal rating scale