Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 14/140/63. The contractual start date was in October 2016. The draft manuscript began editorial review in May 2022 and was accepted for publication in November 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Beard et al. This work was produced by Beard et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Beard et al.
Chapter 1 Introduction
Parts of this chapter have been reproduced with permission from Davies et al. 1 The text below includes minor additions and formatting changes to the original text.
Background
Anterior cruciate ligament (ACL) rupture is a common injury, mainly affecting young, active individuals with estimated 200,000 injuries annually in the USA. 2,3 ACL injury can have a profound effect on knee kinematics (knee movement and forces), with recurrent knee instability (giving way) as the main problem. 4 Furthermore, the injury can lead to poor quality of life (QoL), decreased activity5 and increased risk of secondary osteoarthritis (OA) of the knee. 6 Some patients, once recovered from the initial injury, are able to function well without their ACL (copers), usually after undergoing some formal rehabilitation. 7 Other patients continue with episodes of knee instability, and surgery [ACL reconstruction (ACLR) using a graft] is thought necessary to stabilise the knee.
Current treatment/management options
Current management of ACL deficiency (ACLD) includes both surgical and non-surgical treatments (rehabilitation). In the UK, however, a surgical management strategy has become the preferred treatment for individuals with ACL injuries. A survey conducted as part of the feasibility work at the outset of this study showed that the ratio of surgical intervention to non-surgical conservative intervention is 4:1 (unpublished data). Our data suggested that 80% of non-acute patients were directly listed for surgery in the NHS. In England, an estimated 15,000 primary ACLR surgeries are performed each year. 8,9 However, this is a modest estimate based on NHS Hospital Episode Statistics (HES) data, and the real figure for a UK population of 63 million may be closer to 50,000 pa (based on Swedish ACL registry data – incidence 71/100,000 pa). 10 Work related to this project showed that the age-standardised rate of ACLR in the UK increased 12-fold from 1997 to 2017. 9 The rate reached 24.2/100,000 population in 2017 with the largest increase of 48% in 2009. Based on the conservative estimate (n = 15,000), the costs of ACLR to the NHS in 2015 (at inception of this project) was approximately £63 million. Today the cost is more likely to be closer to £85 million.
Rationale for the ACL SNNAP trial
At the outset of this study, it was highlighted that despite ACLR being common, management for ACL injury was based on limited evidence. 7,11–13 At the timing of application for funding, a Cochrane systematic review examined whether surgery or non-surgical (conservative) management was superior for ACL injury14 and concluded no high-quality evidence exists on which to base practice. Surgical stabilisation of the knee joint appears a beneficial intervention, but whether the surgery is more beneficial than non-surgical intervention is unclear, particularly in the later-presenting non-acute patient. Although complications from ACLR have been shown to be infrequent,15 they can still exist and could therefore impact on the outcome for surgery and appropriately influence decision-making.
The unsupported preference for surgical management of the ACL-deficient knee was questioned by evidence obtained in a Scandinavian trial (KANON trial). 16 The benefit of surgery, for all injured patients, was shown to be uncertain, with an operation being unnecessary in many cases. Frobell et al. 16 showed that a period of prior rehabilitation before considering operation can reduce ACL surgery by up to 50%. The clinical implication was that a period of rehabilitation should always be offered prior to surgical reconstruction, and this had become accepted practice, particularly with isolated ACL tears without comorbidity [although more recent randomised controlled trial (RCT) evidence from the COMPARE trial17 has subsequently questioned this reasoning – see Evidence update since study submission]. While this original clinical decision-making evidence was valid for acutely injured individuals, it was not considered applicable to those more typically seen in the NHS, where patients are often non-acute, having sustained injury sometime earlier. By the time NHS patients are diagnosed and begin dedicated ACL injury management, up to 12 months can have passed since initial injury. 18
The mixed acknowledgement and uptake of this evidence and the uncertainty over the applicability to a less acute UK population have resulted in a highly varied approach to managing ACL injury in the NHS. 19–21
The limited evidence, particularly for the non-acute population, means that surgical management may potentially be overused for later-presenting patients. Conversely, an argument can be made in favour of bypassing further formal rehabilitation for the longer-standing ACL-deficient knee and the optimum treatment being immediate reconstructive surgery. Which strategy is the most clinically effective and cost-effective for this subpopulation remains unknown. Because surgery is expensive and may also have greater complications,8,22 provision of strong evidence for automatic default ACLR is even more important. 23
Likewise, the routine prescription of formal rehabilitation, if not beneficial, is considered wasteful and may disadvantage individuals with ACL injuries by delaying optimum treatment or fully stabilising the knee. There was a clear need to identify the most appropriate treatment strategy.
In summary, at the outset of this study there was no evidence-based management of non-acute ACLD occurring, particularly in the NHS. Moreover, little consensus existed on the management of these patients. The aim of the ACL Surgery Necessity in Non-Acute Patients (ACL SNNAP) trial was to address the gap in the evidence base regarding the clinical and cost effectiveness of these approaches and inform standards of care for ACL injury management in longer-standing non-acute patients.
Evidence update since study submission
An update of the initial literature search and review of the clinical trials registries was conducted to inform this report and set the results in context (see Table 1).
| Study ID | Study design | Blinding | Sample size | Participants | Interventions | Primary outcome | Results |
|---|---|---|---|---|---|---|---|
| Completed studies | |||||||
| Frobell et al., 201016,25 ISRCTN84752559 Sweden |
Multicentre RCT, 2 sites | Blinding of participants and care providers not feasible | 121 | 18–35 years Acute (within 4 weeks) |
Structured rehabilitation plus early ACLR and structured rehabilitation with the option of later ACLR if needed | KOOS4 at 24 months | No significant difference between groups Of the 59 patients in the optional delayed-reconstruction group, 23 (39%) underwent ACL surgery (by the 24-month time point) |
| Reijman et al., 202117,26 Netherlands |
Multicentre RCT, 6 sites | Blinding of participants and care providers not feasible | 167 | Acute (within 2 months after the initial trauma) complete primary ACL rupture | Early reconstruction of the ACL or rehabilitation followed by optional delayed reconstruction of the ACL | IKDC at 24 months | At 24 months, early ACLR group had a significantly better (p = 0.026) but not clinically relevant IKDC score [84.7 vs. 79.4 (difference between groups 5.3, 95% CI 0.6 to 9.9)] |
| Of the 82 patients in rehabilitation and optional delayed ACLR group, 41 (50%) underwent ACL surgery (by the 24-month time point) | |||||||
| Ongoing studies | |||||||
| Smeets et al., 202227 Belgium |
Pilot study | Blinding of participants and care providers not feasible | No formal power calculation | Acute (within 4 weeks after the injury) | Conservative treatment (consisting of rehabilitation + optional delayed surgery) and surgical treatment (immediate reconstruction) | Recruitment rate | N/A |
| de Vos et al., 202228 Netherlands |
Multicentre cluster RCT | No blinding | 230 patients | 18 years or older, with a complete primary ACL rupture and maximum of 6 weeks of non-operative treatment | An adjusted treatment decision strategy using a treatment algorithm compared to current used treatment strategy | IKDC at 24 months | N/A |
During the course of this study, the ongoing study by Reijman et al. (COMPARE trial)17,24 examining the clinical and cost effectiveness of two treatment strategies for ACL rupture has also been completed. The COMPARE trial was carried out in the Netherlands and has a sample size of 188 participants. At 2-year follow-up, the results demonstrated slightly better self-reported outcomes (knee symptoms, self-reported knee function and perception of the ability to participate in sports) in the immediate surgery group compared with the conservative group, but none of these findings were considered large enough to be clinically important. 17 As this study also evaluates the newly injured (acute) patients and replicates the Scandinavian study setting, again, it cannot be directly applied to the typical NHS pathway. However, the study does increase the contention on how best to manage the acutely injured population with the former recommendation of undergoing rehabilitation first being brought into some question. This contention, albeit in a different population, provided even greater justification for ACL SNNAP.
Research objectives
The primary objective of the ACL SNNAP study was to determine in patients with non-acute ACLD whether a strategy of non-surgical management (rehabilitation) (with option for later ACLR only if required) was more clinically effective and cost-effective than a strategy of surgical management (reconstruction) without prior rehabilitation with all patients followed up at 18 months.
Secondary objectives were to compare the two management strategies regarding the return to activity/level of sports, generic QoL, knee-specific patient-reported outcomes, intervention-related complications, health economics–cost effectiveness, ability to work (e.g. sickness absences/return to work number of days off work and subjective working ability), resource use and costs, expectations of return to activity and confidence in relation to the knee.
Chapter 2 Pre-trial feasibility
Randomised controlled trials that compare a surgical and non-surgical intervention can be challenging to conduct and many struggle with recruitment. 29,30 Strong treatment preferences of both clinicians and patients can pose a challenge to recruitment, adherence to treatment allocation and ultimately the success of a trial. Stark differences in the level of invasiveness, potential risks, disparity between treatments pathways and views on perceived mechanism of effect, between interventions can influence both clinicians and patients’ treatment preferences, making recruitment challenging. 31
This chapter describes qualitative research [(1) clinician and (2) patient interviews] conducted prior to the main RCT to explore likely challenges to recruitment and to develop optimum procedures for the main trial. 32 Emerging findings were disseminated through focused reports/presentations to the study management group, participating sites and steering committee as research progressed.
Clinician interviews
Semistructured, face-to-face interviews were conducted with purposely sampled 12 clinicians (6 orthopaedic knee surgeons and 6 specialist lower limb physiotherapists) from NHS hospital trusts. All clinicians were experienced in the management of ACL injuries and had expressed an interest in participating in a proposed trial of ACLD management (NIHR HTA 14/140/63).
Recordings were transcribed and analysed using thematic analysis. 33
Findings
Three themes were identified highlighting factors that potentially would impact on clinicians’ ability to recruit to the proposed ACL SNNAP trial: (1) clinician equipoise for specific patients, (2) self-acknowledged clinician bias and (3) lack of patient equipoise.
Clinician equipoise for specific patients
This theme described how being in a position of equipoise for certain patient groups with specific characteristics (age, activity level and time since injury) may be difficult in this proposed trial.
All clinicians indicated that they would be happy to randomise patients to the proposed trial. However, when talking more specifically about certain patients or patient subgroups who were eligible for the study, some were more uncomfortable about the possibility of not offering a particular treatment or about recruiting certain patients to the trial. This discomfort was underpinned by concerns about age and activity levels, and it was particularly evident when considering young active patients hoping to return to sports such as football. Clinicians discussed being uncomfortable with not offering surgical treatment to this patient group.
I think that it is an important question to answer but I think it is going to be difficult. I think most ACL surgeons have reached a point in their decision making that if you have a young active patient wanting to go back to sport it is very difficult to discuss non operative treatment. I think that most people now have got the view that ACL reconstruction is the best way forward for most of those patients.
Surgeon
In contrast, some clinicians described being in a position of equipoise as easier for certain patients.
They are the ones that would tend to be more polarised [young active/older not so active] … and I would agree with them feeling polarised … it is that group in the middle … in their 30s not playing that regularly, it is not clear cut, they would be the group that would be more easy to feel in equipoise and persuade them to enter a trial, I think.
Surgeon
There were mixed feelings regarding equipoise related to a person’s age; some clinicians thought that it was not necessarily age but functional level that should be considered.
I think there are set ideas at the moment that if you are kind of forty plus you are much less likely to have this (operation) done, if you are under forty you are much more likely to have it done … but this sort of pseudo too old too young needs to be shifted to one side and I don’t think it is the primary or should be the primary reason for it, it should be function.
Physiotherapist
Clinicians’ perceptions of patient priorities related to age and ‘stage in life’ were also expressed by many of the clinicians as a reason for recommending surgery with certain patients.
Classically someone who is in their first or second year at university, they have got two more years left at university and want to play for their university football team because that is where their social life is. Whereas people in their 30s who are in the workhouse of their career, they have many other priorities that they are looking at doing and the thought of not having an operation if they have got family and got other things going on, they are happier to wait 6 months.
Surgeon
Some clinicians were aware that this would potentially influence their decision to recruit patients to the trial and therefore the generalisability of trial findings.
Some clinicians indicated that equipoise would be difficult if patients had sustained the injury more than 4 months previously. At this stage, the presenting symptoms would predominately be instability rather than a combination of pain, swelling and instability at the more acute stage and therefore would be more likely to recommend surgery in these cases.
I would struggle with equipoise with certain patient groups actually, perhaps even more so with these chronic ones … Because they have only sought referral because they have got instability and if a lot of those people who play football or rugby have tried to get back not realising what their injury was and then of course we are all worried about secondary injury to the knee.
Surgeon
The reason that some clinicians expressed difficulty in adopting a position of equipoise for certain patients was because they considered these patients as potentially at a greater risk of secondary damage and subsequent development of OA to the knee. Many of the clinicians discussed this with specific reference to the current evidence.
In terms of the Australian and the US evidence base if you leave people with ACL [deficiency] they will irreversibly trash their meniscal and chondral surfaces and get arthritis quicker and you need to fix all these young people, it is contra to what the Scandinavian stuff [Frobell study] that tells us it is not necessarily the case.
Surgeon
Self-acknowledged clinician bias
Clinicians were aware that the way they frame or present information about treatment may influence patient choice and thoughts about treatment. In some cases, the clinicians anticipated that this might be done inadvertently. However, there was also a sense that in some cases they steered certain patients towards a particular treatment.
We all know that if you try, you can talk through the risks and benefits of non-operative treatment and surgical treatment in lots of different ways depending on whether you personally as the surgeon want, think the operation is a good idea or not so yeah you can emphasise the terrible risks of surgery or you can gloss over them almost and completely change the emphasis of your discussion and sometimes you probably do that unwittingly.
Surgeon
Despite indicating that they would be happy to discuss the trial and randomise participants to it, in some cases, clinicians did not seem to be aware of the possible impact phrasing would have on patients’ thoughts about treatment.
I’d be happy to randomise the young ones as long as they are happy with that, the patients, so I say look if you push me to make decision I think you are heading towards surgery, but if you are willing it is perfectly reasonable not to have surgery and if you are not sure about it we are running trial that I am happy to put you in to. So even if I felt that they were more of a surgical candidate, as long as they feel unsure about it and they are perfectly okay to then go into the trial.
Surgeon
Others questioned whether or not it should be the clinicians (surgeons) recruiting patients to the trial. They were aware however of the potential challenge with this because of the requirement for a clinician to make a diagnosis.
It is probably best to approach these patients before they have a detailed discussion with the surgeon. Recruitment done by someone completely separate with no particular bias towards one particular treatment or the other.
Surgeon
When the clinicians discussed the treatments, in general they were not expressed in equivalent terms or considered to provide equivalent outcome. Surgery was considered as a means to getting back to sport or providing the ‘fix’ and preventing your knee from giving way.
At the other end you have got the 45 year old occasional sports person or female skier that does it that doesn’t play any twisting or turning sport who I would say look we could fix it you have ruptured it but actually there is no rush to make that decision and we will see how you go first go down the physio route.
Surgeon
Lack of patient equipoise
Clinicians felt that patients would come to a clinic appointment with certain preconceived ideas about an ACL injury and treatments available. There was a sense that, although some might prefer a conservative approach, others would have stronger preference towards surgical treatment.
Most of them [patients] actually make a decision fairly quickly that actually they really not keen to have surgery they definitely want to push with physiotherapy first or you know, I think I need an operation because I am really keen to play football at a high level and I just know that is what footballers have done.
Surgeon
Some clinicians felt that many patients did not see physiotherapy as a definitive treatment, with patients’ decisions around treatment hinging on whether or not to have surgery, rather than an active choice for physiotherapy. Clinicians considered that patients viewed surgery as a means of fixing a problem, whereas with a physiotherapy approach there would always be the uncertainty of ‘will it be good enough’.
The patients that I have seen that are deficient, most of their treatment has been a conversation around should they or shouldn’t they [have an operation] and they are still thinking that they may well, as opposed to this [physiotherapy] is definitive treatment, I would say.
Physiotherapist
Clinicians felt that as this is a fairly common sporting injury with high media profile, this preference was highly influenced by various sources including patients’ social environment, and experiences of family, friends. In addition, they recognised the impact of information available through social media and the internet.
A patient will say ‘my mate had it done’, ‘I must have a patella tendon graft or a hamstring graft’ and I’m going to need surgery because I’ll never get back to it [sport] because the club has a therapist and has said to them, you will need surgery.
Surgeon
Clinicians also described how previous treatment received and advice from healthcare providers, especially if the patient had been referred from elsewhere on the treatment pathway, for example referral clinics, could highly influence patient’s choices and make it difficult to discuss and recruit to the trial.
The only problem here would be patient expectation. So if someone has been told they need to see me because they need an operation, then it is then difficult to have the conversation because even though I am the specialist, if someone has been told they should have an operation it is very hard to then turn that round into we are not sure about this operation.
Surgeon
Patient interviews
Semistructured interviews were conducted with a convenience sample of 15 patients with ACL injury referred to the Outpatient Clinic at the Nuffield Orthopaedic Centre, Oxford University Hospitals NHS Foundation Trust. The age range of patients was 21–57 years. The patients were an active population with a Tegner activity level score ranging from 3 to 10 (a score of 5 indicates participation in recreational sports to a score of 9 indicating participation in competitive sport on a non-professional level). Ten patients were interviewed face to face and five interviewed over the phone.
Recordings were transcribed and analysed using thematic analysis. 33
Findings
Four themes were identified that underpinned patients’ treatment preference: (1) Implications of the diagnosis, (2) Surgery considered by ‘everyone’ as the best means of returning to being active, (3) Surgery intuitively understood as providing a ‘fix’ to the problem and (4) I’ll try the least invasive first, but surgery is always an option.
Implications of the diagnosis
This theme describes how a diagnosis of an ACL tear influenced patients’ views on prognosis and their decision about treatment. It could also have a strong emotional impact. For some the implications of the diagnosis were positive, while others were negative. Several patients considered the diagnosis of an ACL injury to imply the need for surgical treatment, regardless of their current symptom presentation or their previous level of activity/sport.
Once I was told it was going to be an ACL I was still trying to get my head round it, I knew I had to have it fixed it is just my way, my sporting way. I have to be put back right again.
KS039
Patients also indicated that being given a diagnosis was important because it helped them acknowledge their injury and enabled them to move forward and start to deal with the implications, consider options and make decisions.
Just having a diagnosis really helped because I was okay, right, I know what the problem is with my knee I can sort of go away and understand what this means …
KS152
Hearing the diagnosis, however, could create feelings of shock as it was a ‘bad sign’, and it could escalate patients’ views on the severity of the problem. Patients who were aware of this type of injury expressed that they had ‘feared’ or ‘hoped’ that it was not an ACL injury. Some felt that an alternative diagnosis, such as meniscal tear, would have more positive implications for recovery, not require a surgical intervention, or imply a more immediate return to activities than with an ACL injury.
I had really got my hopes up that it was a dislocated kneecap or something slightly less extreme, because I know that you can have meniscal tears and they can heal on their own. I was hoping it was a strain or a partial tear …
KS137
The diagnosis evoked strong emotions in some patients who had not previously heard of the injury. Hearing that you had ‘completely ruptured your ACL’ resulted in feeling of ‘shock’ and uncertainty as to what the implications of the injury were.
On hearing the diagnosis, mentally I went into meltdown thinking, what does this mean … does it mean that I will never be able to walk, you know all sorts of stupid things.
KS015
Awareness of their diagnosis instantly influenced patients’ thoughts on prognosis, which in the majority of cases was seen to have a negative impact related to their sporting career; the ‘end of my football career’; a ‘career-limiting injury’. It also created thoughts of uncertainty about how it would affect the future, in terms of sporting lifestyle, potential for further injuries and the development of OA.
Because you know if you rupture it you will never play at that level again and you know it is a career limiting injury and then you start thinking … oh god I’m going to have a meniscal injury with it, I am going to increase my chances of medial OA, am I going to have a knee replacement, that sort of thing.
KS060
Awareness of the particular diagnosis also inferred a trajectory of a long recovery, regardless of the treatment decision.
Of course I knew straight away 12 months, a minimum 12 months and obviously the higher level you play the more you put yourself under strain and the longer the physio takes … I was aware of what it entailed which is why I guess it hits you so hard when someone tells you it is that, it all goes through your mind very quickly.
KS039
Surgery considered by ‘everyone’ as the best means of returning to being active
Patients described the strong influence of friends, family, healthcare professionals and the media towards recommending surgical treatment as the best means of returning to activities that were important to them. Patients indicated how ‘everyone’ they spoke to had views on the injury and opinions on treatment. Strong recommendations towards the perceived benefits of surgery influenced patients’ views and decisions about treatment, often before they attended their clinic appointment.
… it was a tough one, sort of everyone I had spoken to, my rugby coach, she had ACL surgery and she plays rugby for England, so she was like have surgery … by the time I had got to the appointment I was thinking I am having surgery. So in my mind it was surgery, there wasn’t really an option not too.
KS122
Positive experiences of friends who had an ‘amazing recovery’ following surgery and were back to sporting activities influenced patients’ preferences towards surgery.
I saw it as a long path, but I did see it as a way to get back to Rugby and I know people who have had an ACL and they have had the surgery and they have gone back to like normal sort of sporting stuff. So I don’t so maybe in ideal world it was sort of other people were doing it so …
KS122
Patients’ views on surgical treatment were reinforced and justified by their awareness of professional athletes often seen to be having surgery following this particular injury.
… it just felt like it would be the thing that worked and again if you see professional athletes having it done and it clearly shows that is the best route to go down if you are wanting to be more active.
KS137
In addition, clinicians were described as indicating surgery as the only means of being able to return to particular activities. Some patients felt that physiotherapy was encouraged for people who were not going to be as active or were prepared to become less active.
It just seemed that the physio route was more encouraged for people who weren’t going to be as active or weren’t necessarily in my stage of life, that was my impression at the very least, it might have been my own biases coming through!, yes that is what I thought.
KS137
Following treatment discussions with clinicians, other patients felt that surgery was inevitable and the only solution to ‘fix’ the problem of their injury.
You can either have the surgery now and recover once or have surgery later and recover twice that was the impression I kind of got … surgery is the failsafe almost, so you know exercise will work well but the surgery is the best thing to do to fix the issue straight away was the impression I got.
KS177
Some patients reflected on the negative experiences of people they knew who did not undergo the surgery, and ‘didn’t play rugby again’, or ‘never felt strong enough’, to support their views towards undergoing surgical management.
Her sister who hasn’t [had the operation] still has a bit of meniscal damage and her knee often locks even during matches … and its constantly a problem during high competitive matches when she is a bit tired and lands funny or not quite control or balance.
KS001
Information sourced on the internet about the injury was also described by patients, regardless of their own preferences for treatment, to imply that surgery was ‘what happened’ following an ACL injury or was expressed positively as the means to return to an active lifestyle.
It just seemed the more and more I read about it [on the internet], the more it seemed that if I was going to lead an active lifestyle in the way that I wanted to, surgery was going to be the way to do that. Despite the setback for about a year or six months I was going to be able to go back to trust both of my knees and physio didn’t seem to be an option that was going to work for me in that kind of thing.
KS137
In contrast, some patients indicated that it was difficult to find information on the outcomes of conservative treatment as an alternative way to manage this injury. Examples of where people had returned to activities without undergoing surgery were described as difficult to find, influencing patients’ thoughts towards treatments.
… Wikipedia, Knee Guru and I also looked at [internet] forums that were specific to the activities I undertake, to see whether [other people] were able to carry on doing things irrespective of route or whatever route they took. It is very difficult to find stuff about the conservative route.
KS005
Information available that described non-surgical treatment implied considerable restrictions on the type of activities patients would be able to do, such as straight-line activities, for example swimming and cycling. This was expressed as having negative implications for patients in terms of the ability to return to activities that were important to them, not just competitively but in terms of social contact and identity.
… the conservative route I say would place considerable restrictions on what I would be able to do … I would need to be holding back for the rest of my life and would have to constantly think about whether I could or could not do something and not be able to join in with friends that sort of thing … That was quite significant because as a single person fairly dependant on the friends for social contact that sort of thing … these activities are very important to me.
KS005
Surgery intuitively understood as providing a ‘fix’ to the problem
Surgical and non-surgical treatments were not viewed by the majority of patients as potentially being able to provide an equivalent outcome. Patients viewed surgery as a means of providing a ‘fix’ to the problem that the injury had caused. The operation was viewed as providing mechanical stability in the knee and therefore reassurance.
The surgery that they offer is the reassurance that a mechanical knee which has been tested on the table. It is never going to be the same as it was but you know there is something there holding it together.
KS060
Surgery was seen to restore strength and stability in the knee by means of repairing something that had been broken. The majority of patients considered that by having surgery the tear would be repaired, a mechanical action providing a definitive treatment. A number of patients viewed surgery as the means to the knee being normal again, while others were aware the knee was never going to be the same again, highlighting patients’ varying expectations of treatment.
I didn’t see surgery as a magic cure, as in have the surgery and everything will be wonderful. I was aware that things perhaps were never going to be quite the same again but I did think that it would give me a lot better chance of getting back to those sorts of things [activities].
KS005
Although patients described how physiotherapy could ‘strengthen’ the knee, concern was expressed as to whether adequate stability would be achieved. Several patients described how it did not seem intuitive that you could return to activities without the ligament in place, particularly in activities which required pivoting, change of direction movements.
I didn’t really think much about not having the surgery, … I knew strengthening it was an option if you didn’t have it reconstructed, but just to get back to playing netball where it is stop start and so much forward pressure of the thigh over the shin without an ACL, it didn’t seem very intuitive that you could get back to playing without having something there.
KS001
In addition to this physical stability, the surgery was described as increasing a patient’s confidence and trust in the knee. Patients considered that if the ACL had been ‘fixed’ it was less likely to ‘give way’ and become unstable and they felt less vulnerable to further injury.
I can’t imagine doing a year of physio, obviously it does things, but I think I would always be worrying about my knee constantly regardless of how much physio I was doing …. I think that you can feel something has happened after the surgery. So I wonder in a way it is like a placebo effect making you feel like it is better to a certain extent, I don’t know.
KS137
I’ll try the least invasive first, but surgery is always an option
For some patients it was preferable to do everything possible to avoid surgery or at least to try the least invasive option first. Surgery, however, was always considered to be an option if patients were unable to return to sport or activities that were important to them.
If I do reach a point where I’m unable to do the things that I would like to do, purely because is such a huge part of my life, … that is alright we can have surgery at some point then, but I am in no rush and I want to give this a try. If I don’t have to have surgery then that is great, no one loves or has casual surgery.
KS141
Some patients viewed an initial non-surgical approach as a potential way to enable a quicker return to work or sport. Others described the potential implications of ‘lost time’ if the non-surgical treatment was taken first and was not effective. Some felt that physiotherapy would be a waste of time and that it would delay return to sport or create concern over the risk of secondary damage.
Cost of time it would take if you go down the conservative route, there is risk of further damage down the line and sort of feeling that there is potential for me to end up settling for a less satisfactory end result.
KS005
Knowing that you could undergo surgery if there was no improvement following non-surgical treatment, however, was described as reassuring.
So I just wanted to give it [physio] a try, because [the clinician] said, you know even if you decide in 2 years we’ll do it then and I thought that is fine, I’ll make that decision if it hurts and if it doesn’t work as well as I want it to, if I start falling over loads at netball I will think about it again that was good to know that I could come back it is not like you have to know now that was really useful.
KS014
Summary
Comprehensive work was carried out under the heading of the ACL SNNAP to address issues of equipoise and decision-making.
Exploring clinician views on recruiting patients to the proposed ACL SNNAP trial helped the trial team highlight and understand the potential difficulties clinicians may have in adopting a position of equipoise and recruiting specific patients to the trial.
Exploring patients’ views on surgical and non-surgical treatments for ACLD helped the trial team understand factors that may influence the development of patients’ treatment preference and the potential implications for recruitment to a trial evaluating these interventions. Overall, patients viewed surgery more positively than a non-surgical, physiotherapy approach. A number of patients, however, did express a preference for an initial non-surgical approach, knowing that the option of surgery could be considered if there was no improvement.
Several strategies were employed on the basis of this work to facilitate recruitment in such an equipoise-challenged environment. These strategies and guidelines were disseminated at site visits and more widely at methodology conferences. The recruitment strategies allowed for strong preference of both patients and surgeons (patients being not eligible for the study, surgeons not being eligible to recruit), but ensured those without a strong preference could be accommodated. Both patients and surgeons were provided with contemporary information to enable an informed decision choice to enter the trial as either clinicians or participants.
Chapter 3 Methods
The final protocol1 (including the health economics analysis plan) and statistical analysis plan34 for this trial have been published and some of the content has been reproduced in this monograph. The text below includes minor additions and formatting changes to the original text.
A summary of changes to the protocol which occurred during the conduct of the trial are outlined in Appendix 2, Table 30.
Trial design
The ACL SNNAP trial was designed as a pragmatic, multicentre, superiority, RCT with two-arm parallel groups and 1:1 allocation ratio to compare non-surgical management [rehabilitation (with option for later ACLR only if required)] or surgical management (reconstruction) options for patients with a symptomatic non-acute ACL injury. A two-stage internal pilot was included with clear progression criteria regarding recruitment.
Rather than a head-to-head comparison of two interventions, the trial was designed as a ‘management’ assessment in which specific events were expected and permitted. This included the option for later surgical intervention (ACLR) in the non-surgical (rehabilitation) arm, if required.
Interventions
The trial compared two routine and well-established management strategies for patients with symptomatic non-acute ACL-injured knees: (1) non-surgical management (rehabilitation) and (2) surgical management (reconstruction).
Intervention content was based on a minimal set of pre-established criteria in order to ensure the integrity of the comparison while allowing for variation in practice in delivering the interventions between both surgeons and physiotherapists [see Non-surgical management (rehabilitation) and Surgical management (reconstruction)]. This pragmatic approach to the delivery of the intervention allowed the management approach to reflect current practice and resource use within the NHS thus aiding generalisation yet included minimal levels of standardised quality and content for both interventions.
Non-surgical management (rehabilitation)
Patients randomised to rehabilitation were referred to their nearest physiotherapy department to undergo non-surgical management (rehabilitation) delivered (or closely overviewed) by a senior physiotherapist with experience of ACL injury regimens.
Routine ACL rehabilitation protocols used at participating sites were followed. As part of the site selection process, documentary evidence of the use of or willingness to adopt a rehabilitation protocol that reflected the guidelines of the mandatory aims/goals set for the study rehabilitation intervention was required. Part of the requirement was for the site to be in a position to provide a minimum of six rehabilitation sessions delivered over at least a 3-month period.
The rehabilitation protocol was required to include the following components:
-
evidence of interventions aimed at achieving the mandatory aims/goals:
-
control of pain and swelling
-
regaining range of movement
-
improving neuromuscular control
-
regaining muscle strength
-
achieving normal gait pattern
-
returning to function/activity/sport
-
-
clearly identified progression milestones
-
return to sport criteria
-
identification criteria for poor or non-progression.
Rehabilitation protocols commonly used in clinical practice consist of a progressive programme,35 designed to rebuild muscle strength, re-establish joint mobility and neuromuscular control, and enable patients to decrease the risk of reinjury and return to previous levels of activity. 36 As little consensus exists in the literature over the most effective rehabilitation protocol,37 variation in the specific exercises carried out and the use of adjuncts (such as cryotherapy) to reach these aims was permitted.
Flexibility was permitted to adapt treatment to individual needs with no timelines specified for progression. Examples of exercises used to reach the aims were documented in a physiotherapy case report form (PCRF) to facilitate recording of the rehabilitation interventions and to monitor for fidelity to these guidelines.
The progress of patients who had been randomised to non-surgical management (rehabilitation) was monitored by their treating physiotherapist or surgical staff where appropriate. If, after a minimum period of at least 3 months of rehabilitation (or before, if instability or symptoms were more immediate and deemed substantial), the participant continued to experience symptomatic knee instability and/or symptoms related to associated pathology, that is pain or locking, the non-surgical management was considered to have potentially failed. This intermediate outcome was confirmed at a review clinical appointment and the criteria listed below confirmed. Following a policy of shared decision-making, the patient and surgical team then made the decision as to whether to proceed with ACLR surgery to address the instability. These appointments were established within normal practice at each site and could involve surgical staff or extended-scope physiotherapy practitioners allocated with these duties. If appropriate, the participant was listed for surgery, as per usual practice.
All other clinical follow-up occurred as per routine practice at each participating site. The criteria for change in status (from non-surgical to surgical intervention) after a minimum of 3 months of rehabilitation were confirmed at a consensus meeting (surgeon/physiotherapist) held on 20 January 2016. The consensus group agreed that 3 months is considered the minimal time needed for the rehabilitation to provide any effect. The criteria for surgery include one or more of the following:
-
continued feeling of knee instability and/or symptoms, that is pain or locking, related to the associated pathology
-
at least two episodes of physical giving way of the knee
-
unable to return to a Tegner activity level 2 points below pre-injury status.
Outside early conversions (inside 3 months), the above criteria assume all patients will have undergone a comprehensive rehabilitation regime.
Surgical management (reconstruction)
Patients randomised to reconstructive surgery were placed on a surgical waiting list to undergo a standard ACLR procedure. Operations were carried out according to the discretion of the participating surgeon. Two types of commonplace ACLR were acceptable: one using a patella tendon graft and the other using a hamstring graft.
Any physiotherapy advice and any treatment aimed at the acute presentation (i.e. swelling, regaining range of motion etc.) prior to surgery was permitted, but no formal ACL rehabilitation programme or specific ACL remedial exercise prescription beyond basic maintenance exercises. All other care was routine, including immediate postoperative care. Patients were engaged in a postoperative rehabilitation programme as per standard care at the participating hospital. Note the initial content of postoperative physiotherapy was different from that for non-surgical management, in that some aspects of graft protection and caution are necessary following ACLR.
Surgery was performed or supervised in theatre by a specialist consultant knee surgeon with recognised expertise in ACLR (will have performed at least 50 previous ACLRs). See Inclusion criteria for sites and surgeons.
An operation case report form was used to document the operation and monitor compliance with the intervention guidelines. The content of and attendance (adherence) to the postoperative rehabilitation was also recorded for this group.
Participants
Patients with symptomatic knee problems (instability) consistent with an ACL injury (see Inclusion criteria) were eligible for inclusion. ACLD, either partial or complete tear, was confirmed at a patient’s routine outpatient appointment through clinical assessment and magnetic resonance imaging (MRI) scan.
Anterior cruciate ligament tears can occur as isolated injuries but more commonly occur in conjunction with injuries to other structures of the knee, including menisci, articular cartilage and collateral ligaments. Apart from the pathology detailed in the exclusion criteria below, all other patients with an ACL tear combined with associated injuries were considered for participation in the trial.
Inclusion criteria
-
Aged 18 years or above.
-
Symptomatic ACLD of the native ligament (instability episodes of frank giving way or feeling unstable) with ACL injury (either partial or complete rupture/tear) confirmed using clinical assessment and MRI scan (patients who had undergone primary ACL reconstruction on the index knee were not eligible).
Exclusion criteria
-
Acute phase of primary ACL injury; that is the patient had not recovered from acute symptoms relating to their initial ACL injury [pre-existing ACL injury presenting with acute symptoms (from a recent instability episode) allowed a patient to be considered for inclusion. This was assessed by the surgeon at the participating hospital site during routine clinic appointments].
-
Previous knee surgery (other than diagnostic arthroscopy or partial meniscectomy) to the index knee or concomitant severe injury to the contra-lateral knee.
-
Meniscal pathology with characteristics that indicate immediate surgery, that is locked knee or large bucket handle or complex cartilage tear producing mechanical symptoms.
-
Knee joint status of grade 3 or 4 on the Kellgren and Lawrence (KL) scale. 38
-
Grade 3 medial collateral ligament (MCL)/lateral collateral ligament injury/associated posterior cruciate ligament/posterolateral corner injury.
-
Inflammatory arthropathy.
-
Pregnancy.
Setting and locations
Participants were recruited between 1 February 2017 and 12 April 2020 from 29 NHS secondary care hospitals from across the UK. Study sites were selected based on criteria detailed further in the published protocol. 1 This included having an established practice of ACLR and an experienced ACLR knee surgeon and physiotherapy team capable of providing contemporary care.
Whether it was feasible to run the trial at the proposed site was also explored, for example able to offer treatment (ACL surgery or rehabilitation) within the 18-week pathway (in line with current NHS waiting time targets) and provide documentary evidence of the use of a rehabilitation protocol that reflected the guidelines outlined in the protocol. Each site is listed below:
-
Morriston Hospital, Swansea Bay University Health Board, Swansea.
-
Glan Clwyd Hospital, Betsi Cadwaladr University Health Board, North Wales.
-
Wrexham Maelor Hospital, Betsi Cadwaladr University Health Board, Wrexham.
-
Countess of Chester Hospital, Countess of Chester Hospital NHS Foundation Trust, Chester.
-
Frimley Park Hospital, Frimley Health NHS Foundation Trust, Frimley.
-
Wexham Park Hospital, Frimley Health NHS Foundation Trust, Wexham.
-
Cheltenham General Hospital, Gloucestershire Hospitals NHS Foundation Trust, Cheltenham.
-
Great Western Hospital, Great Western Hospitals NHS Foundation Trust, Swindon.
-
King’s College Hospital, Kings College Hospital NHS Foundation Trust, London.
-
Chapel Allerton Orthopaedic Centre, Leeds Teaching Hospitals NHS Trust, Leeds.
-
Manchester Royal Infirmary, Manchester University NHS Foundation Trust, Mnachester.
-
Southmead Hospital, North Bristol NHS Trust, Bristol.
-
Peterborough City Hospital, North West Anglia NHS Foundation Trust, Peterborough.
-
Nuffield Orthopaedic Centre, Oxford University Hospitals NHS Foundation Trust, Oxford.
-
Royal Berkshire Hospital, Royal Berkshire NHS Foundation Trust, Reading.
-
Royal Cornwall Hospital, Royal Cornwall Hospitals NHS Trust, Truro.
-
Royal Surrey County Hospital, Royal Surrey County Hospitals NHS Foundation Trust, Guildford.
-
Salisbury District Hospital, Salisbury NHS Foundation Trust, Salisbury.
-
Northern General Hospital, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield.
-
Kings Mill Hospital, Sherwood Forest Hospitals NHS Foundation Trust, Sutton in Ashfield.
-
Queen Alexandra Hospital, Solent NHS Trust, Portsmouth.
-
Stepping Hill Hospital, Stockport NHS Foundation Trust, Stockport.
-
Musgrove Park Hospital, Taunton and Somerset NHS Foundation Trust, Taunton.
-
Pinderfields Hospital, The Mid Yorkshire Hospitals NHS Trust, Wakefield.
-
University Hospital Coventry, University Hospitals Coventry and Warwickshire NHS Trust, Coventry.
-
Leicester Royal Infirmary, University Hospitals of Leicester NHS Trust, Leicester.
-
Warrington Hospital, Warrington and Halton Hospitals NHS Foundation Trust, Warrington.
-
Wrightington Hospital, Wrightington, Wigan and Leigh NHS Foundation Trust, Wigan.
-
Yeovil Hospital, Yeovil District Hospital NHS Foundation Trust, Yeovil.
Recruitment and consent
The process of patient identification and recruitment varied depending on the local treatment pathways at each participating site. Potential patients were identified in routine orthopaedic outpatient and pre-assessment clinics by the local clinical team.
The participating surgeon or member of the clinical team initially approached potential participants who meet the eligibility criteria and informed them of the study. Patients who expressed a potential interest in participating were then referred to a research nurse/physiotherapist for further details about the study and written information. Patients who wished to participate then completed an informed consent form and baseline questionnaire.
Baseline assessment
The baseline assessment included a patient self-reported questionnaire that examined knee-specific QoL [Knee Injury and Osteoarthritis Outcome Score (KOOS) and ACL-QoL], generic QoL [EuroQol-5 Dimensions, five-level version (EQ-5D-5L)], return to activity/level of sport participation (Tegner/Modified Tegner) and resource use, as detailed in Table 2. Full details of these measures are provided in the Outcomes section. Once the baseline questionnaire was completed by the patient, they were then randomised into the study as detailed below. Details of the baseline level of ACL injury and associated knee pathology from the MRI report were also collected.
| Time point | Visits | Follow-up–postal/e-mail questionnaire | |||||
|---|---|---|---|---|---|---|---|
| Screening | Enrolment and baseline | Intervention | Reassessment | 6 months | 12 months | 18 months | |
| Informed consent | X | ||||||
| Patient demographics | X | ||||||
| Medical history | X | ||||||
| Physical examination | Xa | ||||||
| MRI (as part of routine practice) | X | ||||||
| Eligibility assessment | X | ||||||
| Randomisation | X | ||||||
| AE reporting b | X | x | X | ||||
| Treatment: operation/rehabilitation |
X | ||||||
| Questionnaire: | |||||||
| KOOS | X | Xc | Xc | X | |||
| Return to activity/level of sport participation – modified Tegner | X | X | |||||
| Health economics – EQ-5D | X | X | X | X | |||
| Complications | X | X | X | ||||
| Knee-specific patient-reported outcomes, ACL-QoL | X | Xd | Xd | X | |||
| Patient satisfaction | Xe | Xe | X | ||||
Randomisation
Randomisation was performed by computer allocation (thus ensuring concealment of sequence generation) using a centrally managed web-based automated system provided by Fr3dom limited. Random allocation was to one of two management options: non-surgical management (rehabilitation) or surgical management (reconstruction) on a 1:1 basis. The allocation was generated using permuted block randomisation with varying block sizes stratified by baseline KOOS score (< 30 or ≥ 30) and recruitment site.
Randomisation by local hospital research teams took place following the baseline assessment visit. This occurred either at the time of the patient’s outpatient preoperative assessment visit or at a ‘separate research visit’ around these routine appointments, depending on the local hospital set-up. Following randomisation, the allocation details were displayed on the web-based system for each participant, and an automated e-mail also sent to the designated member of the research team at the site to inform them of the allocation.
A standard letter was used to inform the admissions, care pathway co-ordinators and general practitioner (GP) (with patient consent) of allocation.
Blinding (masking)
Due to the nature of the interventions, there was no blinding of the participants nor healthcare practitioners (surgeons and physiotherapists) to receipt of the intervention.
Outcomes
The primary outcome for the study was the KOOS4 at 18 months post randomisation. This outcome measure is derived from four of five subscales: pain, symptoms, difficulty in sports and recreational activities and knee-related QoL,39 with scores ranging from 0 to 100, and a higher score indicating better health. KOOS is a validated patient-reported outcome used in ACL research (including recent RCT of acute ACL patients16,25 and large-scale databases, that is the National Ligament Registry). 40,41 The KOOS4 is sensitive and specific for detecting functional deficits due to knee instability.
Secondary outcome measures were used to further assess knee-specific QoL, return to activity/level of sport participation, patient health-related QoL, resource use, frequency of complications. The outcomes reflected consensus opinion in a patient and public involvement (PPI) group and the reference standard for evaluating ACL injury/reconstruction. 42 These were as follows:
Return to activity/level of sport participation: Tegner/Modified Tegner
Return to activity/level of sports participation was measured by the modified Tegner43 at baseline and at 18 months post randomisation. The activity level assessed using the Tegner scale is graded from 1 (low activity levels) to 10 (professional level). In addition, on the baseline form the Tegner was modified as follows: three columns with the headings of (1) activity level before your injury, (2) current level of activity (today) and (3) level you expect to return to. At 18 months, the Tegner contains one answer column as follows: current level of activity (today).
Intervention-related complications
Any clinical complications associated with undergoing ACLD treatment which resulted in participants returning to see a healthcare professional or being admitted to hospital associated with undergoing ACLD treatment were recorded.
Generic quality of life: EuroQol-5 Dimensions, five-level version
The EuroQol EQ-5D-5L is a validated, generic, self-reported outcome measure covering five health domains that are used to facilitate the calculation of quality-adjusted life-years (QALYs) in health economic evaluations. The original EQ-5D questionnaire contained three response options within each of five health domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). 44 More recently, the EQ-5D-5L has been developed to overcome problems with ceiling effects and to improve sensitivity. 45 The 5L version consists of the same five domains as the original but with five response options.
Knee-specific patient-reported outcomes
All five subscales of the KOOS39 were included as separate outcomes (the fifth scale being activities of daily living).
In addition, the ACL-QoL,46 a validated 32-item, knee-specific measure for chronic ACLD, was included. This score is divided into five subscales which include symptoms and physical complaints, work-related concerns, physical activity and sports participation, lifestyle issues and social and emotional concerns. The overall score is calculated (0–100), with higher scores indicating better outcome.
Resource usage data
Detailed resource use data on initial treatments received (surgical reconstruction or rehabilitation) and on subsequent healthcare contacts including reoperations (surgery arm), subsequent surgical reconstructions (rehabilitation arm), surgery-related complications, further rehabilitation and primary care and other secondary care contacts out to 18 months post randomisation are secondary outcomes. In addition, data were collected on the ability to work (e.g. sickness absences/return to work number of days off work and subjective working ability – from had no effect on my work, 0, to completely prevented me from working, 1). All data were collected from self-reported questionnaires and assessment of hospital records.
Expectations of return to activity and confidence in relation to the knee: anterior cruciate ligament quality of life score
The ACL-QoL46 was also used to collect data on patient’s expected outcome in relation to their return to activity and on how confident they feel about doing so, considering any limitation related to their injured knee.
Patient satisfaction
A simple Likert scale was used to assess satisfaction with the outcome of treatment.
Data collection and management
Follow-up patient-reported outcome measures
Follow-up outcome data were collected by self-reported questionnaire completed by participants using a web-based data collection system. The option of completing the follow-up questionnaires in a paper hard copy and returning via post was also available.
The 18-month (primary end point) follow-up questionnaire contained the following outcome measures: KOOS, EQ-5D-5L, Modified Tegner, ACL-QoL and patient satisfaction and was sent out at 18 months post randomisation to all participants. A shortened version of the follow-up questionnaire was sent out at the 6- and 12-month time points. The questionnaires also asked participants if they had returned to see a healthcare professional or been admitted to hospital in relation to complications with their study knee. Details of the specific outcomes collected at each of the follow-up time points are outlined in Table 2.
Non-response was minimised through use of e-mail reminders, text message and participants were sent a postal questionnaire if there was no response to completion of the online questionnaire. If there was no response to multiple reminders, phone calls were also made to participants. Questionnaire return rates were monitored throughout the trial and strategies (e.g. shortened version of questionnaire at 6- and 12-month time points and £20 high-street voucher given a small token of appreciation) to improve and maximise return rates were implemented.
Clinical outcome and fidelity data
Clinical outcome and fidelity data were collected throughout the trial by research teams at the local sites as outlined by the schedule in Table 2. A final readmission checklist was completed following review of local hospital records at 18 months post randomisation to ensure that any complication data were collected from all participants. Data from any readmission events identified were recorded. In addition, any complications reported by participants in returned follow-up questionnaires were also queried with the research team at the participant’s local hospitals to obtain any further detail available.
Statistical methods and study analysis
The methods outlined here are primarily for clinical effectiveness. The methods for the cost-effectiveness analysis are included in Chapter 5.
Sample size
The sample size was calculated using the KOOS. The minimal clinically important change (MIC) for the KOOS score has been suggested to be 8–10 points. 47 Estimates of the minimal detectable change (MDC) for the two KOOS subscales most relevant for ACLD vary between 5 and 12 points (Symptoms 5–9, and Sport/Rec 6–12). 47 Conservatively, a mean target difference of eight points in the primary outcome, KOOS4, along with a standard deviation (SD) of 19 (the highest value observed in a trial of acute patients at baseline among the KOOS subscales) was assumed. 48,49
Given these assumptions, 120 participants per group were required (1:1 allocation, 240 in total) to achieve 90% power at two-sided 5% significance level in the absence of any clustering of outcome. However, in order to ensure sufficient power, clustering (clsampsi Stata command50) was allowed for by conservatively assuming an intracluster correlation (ICC) of 0.0651 and cluster size n, mean (SD) of 26, 5 (12) and 43, 3 (5) for the ACLR and rehabilitation groups, respectively. This led to the larger number of 130 participants per group (260), for which just over 80% power is achieved. Given the conservative nature of the assumed values and the anticipated gain in precision from adjusting for the baseline scores and other randomisation factors, actual power was thought likely to be higher even in the presence of clustering. In addition, to allow for just over 15% missing data (response in a similar trial25), 320 participants were needed.
An interim analysis was planned to estimate the magnitude of clustering in order to assess the potential need for an adjustment to the sample size to maintain sufficient statistical power. A single interim analysis was carried out for the 6-month KOOS4 outcome once data were available for 100 participants. The Data Monitoring Committee (DMC) reviewed interim data and a decision was taken not to increase the target sample size.
Statistical analysis
General analysis principles
The trial analysis followed the statistical analysis plan that was agreed in advance by the Trial Steering Committee (TSC). All principal analyses were based on the intention-to-treat (ITT) principle (‘as randomised’), analysing participants in the groups to which they are randomised irrespective of compliance with treatment allocation. Statistical significance was at the two-sided 5% level, with corresponding confidence intervals (CIs) derived, and the analysis was carried out in Stata® (Stata Statistical Software: Release 17. StataCorp LLC, College Station, TX, USA; 2021). Baseline and follow-up data were summarised using the appropriate descriptive statistics. The main analyses were carried out once the 18-month time point had been reached by the last participant.
Two per-protocol (PP) analyses (conservative and pragmatic PP analysis) were also carried out for the primary outcome, excluding patients (in both groups) who did not fulfil minimal protocol criteria. The patients who were excluded from these analyses are described in the Analysis of primary outcomes section.
Loss to follow-up, withdrawals and missing data
Differences in withdrawals between treatment groups were compared. Surgery after 3 months of rehabilitation was not considered a withdrawal from the rehabilitation arm, as this was part of the management strategy described in the protocol.
Item-level missing data for the primary outcome were dealt with according to the KOOS scoring manual47 for the primary outcome analysis. However, participant-level missing data were not imputed in the principal analyses. The impact of missing data at the participant level was explored via sensitivity analyses for the primary outcome.
Compliance
It was anticipated that the ACL SNNAP trial would involve numerous potential treatment pathways due to the complex nature of the interventions. The potential pathway profiles are described below:
(A) Intention-to-treat (ITT) profiles.
-
S: All patients allocated to surgery (surgical reconstruction).
-
R: All patients allocated to rehabilitation (initial non-surgical management).
In addition to the principal ITT analysis-based summaries of the groups, descriptive summaries of patients who completed treatment (within treatment protocol) but with different treatment profiles were carried out. These profiles are as follows:
(B) Complete Pathway Profiles (intervention as intended).
-
SCom: Allocated surgical reconstruction, had surgery, completed postoperative rehabilitation.
-
RCom: Allocated rehabilitation, completed rehabilitation, no reconstruction.
-
RCom S: Allocated rehabilitation, completed rehabilitation but underwent surgery, completed postoperative rehabilitation.
As previously stated, having surgery in the rehabilitation arm (for some patients) was expected and part of the protocol. It was anticipated that some participants in the rehabilitation arm would require surgery; however, those participants who did receive surgery having been allocated to non-surgery in the first instance were analysed as randomised in the principal analysis of the primary outcome.
Some patients did not complete their allocated/intended treatment. For the PP analyses, a further set of patient profiles were categorised according to any deviation from the allocated pathway (listed 6–12 below as incomplete pathway profiles). Note, ‘reconstruction or surgery’ refers to a decision to list for surgical reconstruction and not necessarily the point in time of the surgical procedure.
(C) Incomplete Pathway Profiles – Allocated Surgical Reconstruction (Group S)
-
SX: Did not have surgery (never had ACLR).
-
SX R: Did not have surgery, underwent rehabilitation.
-
SX AS: Did not have surgery, still awaiting surgical reconstruction (at 18 months).
-
SCom IR: Completed surgery but insufficient follow-up time/postoperative rehabilitation (as surgery was delayed).
(D) Incomplete Pathway Profiles – Allocated Rehabilitation (Group R).
-
RX: Did not start rehabilitation (never had any rehab).
-
RI: Started rehabilitation but insufficient rehabilitation or unknown rehab completion.
-
RCom S IR: Completed rehabilitation but underwent surgery, insufficient postoperative rehabilitation.
Primary/secondary outcome analysis
Analysis of primary outcomes
The principal analysis of the primary outcome measure (KOOS4 score) was compared using a linear regression model including treatment arm, with adjustment for the stratification by site and KOOS4 baseline score. The model included the KOOS4 score at baseline as a continuous variable and used the cluster option52 to adjust for stratification by site.
Two PP analyses were also carried out, excluding patients who did not fulfil the minimum protocol criteria.
Conservative PP analysis: Excludes all patients who did not fulfil requirements of the trial for each intervention stated in the protocol [i.e. all the deviations listed above (6–12) in the incomplete pathway profiles].
Pragmatic PP analysis: Replicates the conservative PP analysis above but does not exclude patients who had insufficient physiotherapy or did not complete the physio treatment (as can occur as per normal clinical experience).
A secondary analysis of the primary outcome was also performed on the ITT population using an area under the curve (AUC) approach. The treatment estimates obtained from a mixed model at each time point (baseline, 6 months, 12 months, 18 months) were used to calculate the AUC. The model included repeated measures of the KOOS4 score (level 1), nested within participants (level 2) and adjusted for recruitment site as a random effect (level 3). A treatment by time interaction was also included in the model.
Sensitivity analyses explored the impact of missing data on the main primary outcome ITT analysis. The Stata package rctmiss53 was used to show graphically the difference in treatment effect for each arm if different means are assumed for the missing data. A pattern-mixture model was used to extend the linear regression model for the primary outcome.
A second sensitivity analysis was also conducted to consider the three responder criteria proposed by Roos as an alternative measure of assessing the KOOS score. 54 The three measures MIC (minimal important change – improvement in change of KOOS4 > 9), patient acceptable symptom state (PASS – KOOS4 score ≥ 9) and treatment failure (TF – KOOS4 score ≤ 42) were tabulated by treatment arm, but no formal statistical comparison was performed.
For the secondary outcomes, KOOS subscales, ACL-QoL and EQ-5D-5L were analysed using generalised linear regression models with adjustment for randomisation and baseline variables as described in the analysis of the primary outcome. Modified Tegner Activity Scores were analysed using a Mann–Whitney U-test, with CIs for proportions calculated for patient satisfaction and return to pre-injury activity level. Numbers of complications were summarised by treatment arm.
Planned subgroup analysis
Exploratory subgroup analyses explored the possible treatment effect modification of clinically important baseline factors (age, gender, high vs. moderate or light physical activity as measured by the modified Tegner score, and KOOS4 overall score) by adding treatment by factor interactions to the primary outcome model. The statistical significance level remained at the two-sided 5% level, and results were interpreted cautiously and labelled as ‘exploratory’.
Supplementary/additional analyses and outcomes
The planned supplementary analyses were a CACE analysis, a coronavirus disease discovered in 2019 (COVID-19) exploratory analysis and an alternative time window for the primary outcome analysis.
Complier-average causal effect
The study was designed to test the benefit of a treatment policy, to determine the effectiveness of the pathways of rehabilitation first, or surgery first. However, the study findings may have been criticised due to presence of non-compliance. To strengthen the support of the treatment policy, an estimation of the efficacy was made, with the caveat that this study was designed to estimate effectiveness, not efficacy. The impact of non-compliance was explored via a CACE analysis. Compliance was defined as having had surgery at any time (e.g. profiles 3, 5, 9, 12 defined in the Compliance section).
COVID-19
The COVID-19 pandemic has significantly disrupted all medical research, including the ACL SNNAP trial. To determine the extent of the effect the pandemic had on SNNAP, the number of patients affected by the pandemic (after the first UK nationwide lockdown on 23 March 2020) was explored and reported descriptively.
Primary outcome analysis with 12- to 18-month window
In a supplementary analysis, the primary outcome analysis on the ITT population was repeated using KOOS4 scores collected at 12 months for participants for whom 18-month outcome data were not available and where sufficient time has passed for the participant to recover from treatment.
Patient and public involvement
Patients contributed to the design of the study and supported the development of the funding proposal and conduct of the study. Early in the project, the PPI group helped ensure that patient information sheets and report forms were accessible and user-friendly. A patient representative was an active member of the TSC and, as part of this role, contributed to the monitoring and supervision of the trial progress.
Ethics approval and monitoring
Favourable ethics opinion for the ACL SNNAP trial was given by the National Research Ethics Service, Oxfordshire Research Ethics Committee (REC) in October 2016 (16/SC/0502).
Trial Management Group
The trial was managed through the Surgical Intervention Trial Unit (SITU) and Oxford Clinical Trials Research Unit (OCTRU), University of Oxford, and the research team’s trial management group (TMG). The TMG was responsible for the day-to-day management of the trial and included the chief investigator, lead collaborative investigators and trial staff.
Trial Steering Committee
The TSC was responsible for monitoring and supervising the progress of the ACL SNNAP trial to ensure it was conducted to high standards in accordance with the protocol, the principles of good clinical practice (GCP), relevant regulations and guidelines with regard to participant safety. The committee met eight times between November 2016 and February 2022, at time points agreed by the committee. The TSC consisted of six independent experts including a patient representative, the chief investigator and key members of the TMG. Membership of the TSC is given in the Acknowledgements section.
Data Monitoring Committee
The DMC was independent of the trial and was tasked with monitoring efficacy, safety and compliance data. The committee met nine times between November 2016 and February 2022. The trial statistician provided the data and reports requested by the DMC at each of the meetings. Membership of the DMC is given in the Acknowledgements section.
Chapter 4 Clinical results
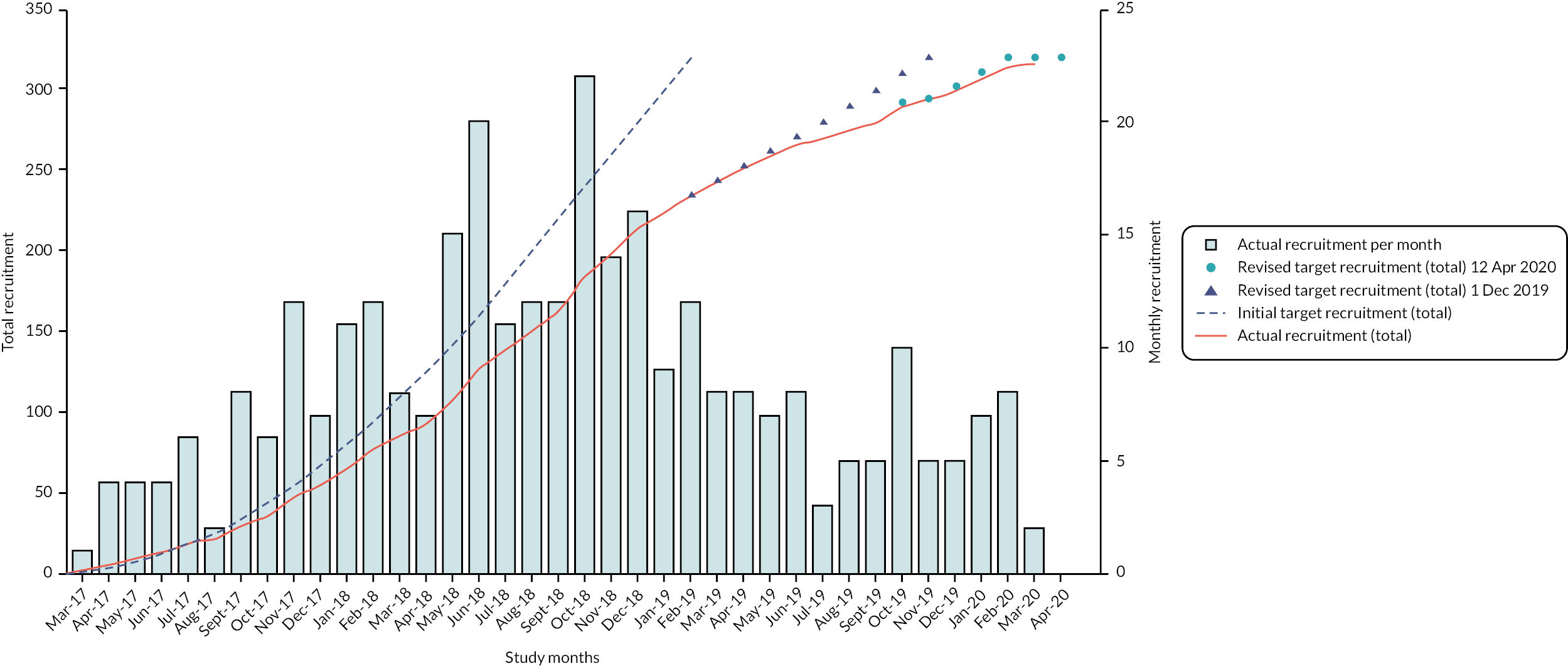
Trial recruitment
Twenty-nine sites across the UK recruited 316 trial participants between 1 February 2017 and 12 April 2020 (see Figure 1). One hundred and fifty-six participants were randomised to the surgical management arm and 160 to the rehabilitation arm.
FIGURE 1.
Participant recruitment throughout duration of trial.
Participant flow through trial
Figure 2 shows the Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the trial, summarising participants’ movement through the trial from screening to randomisation, and on to treatment received. Also detailed are the numbers of participants who declined to be included in the trial, as well as those who were ineligible for the study.
FIGURE 2.
Consolidated Standards of Reporting Trials flow diagram. aReasons patients ineligible and declined to participate are detailed in Table 3.

| Total screened | 1403 |
| Total not eligible (reasons) | 602 |
| Other | 159 |
| Pregnancy | 2 |
| Inflammatory arthropathy | 3 |
| Grade 3 MCL/LCL injury | 55 |
| Grade 3 MCL/LCL injury + other | 1 |
| Grade 3 or 4 on KL scale | 17 |
| Grade 3 or 4 on KL scale + other | 1 |
| Meniscal pathology | 148 |
| Meniscal pathology + other | 3 |
| Meniscal pathology + grade 3 MCL/LCL injury | 3 |
| Previous knee surgery | 91 |
| Previous knee surgery + grade 3 or 4 on KL scale | 2 |
| Previous knee surgery + meniscal pathology | 3 |
| Previous knee surgery + meniscal pathology + grade 3 MCL/LCL injury | 1 |
| Previous knee surgery + meniscal pathology + grade 3 or 4 KL scale | 1 |
| Acute injury | 81 |
| Acute injury + other | 9 |
| Acute injury + grade 3 MCL/LCL injury | 3 |
| Acute injury + meniscal pathology | 15 |
| Acute injury + meniscal pathology + grade 3 MCL/LCL injury | 1 |
| Acute injury + meniscal pathology + grade 3 or 4 KL scale | 1 |
| Acute injury + previous knee surgery | 1 |
| Grade 3 MCL/LCL injury + previous knee surgery | 1 |
| Total eligible to be randomised | 801 |
| Total eligible but not participating (reasons) | 485 |
| Patient preferred surgery | 276 |
| Patient preferred physiotherapy | 115 |
| Other | 67 |
| No reason given | 27 |
| Total randomised | 316 |
Baseline comparability
Participant randomisation was stratified by both KOOS4 score at baseline and recruiting centre. A breakdown by these two stratification factors is shown in Table 4. The randomised groups were similar for these factors as anticipated.
| Surgical reconstruction (N = 156) | Rehabilitation (N = 160) | Total (N = 316) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| KOOS category at randomisation | ||||||
| High (≥ 30) | 116 | 74 | 124 | 78 | 240 | 76 |
| Low (< 30) | 40 | 26 | 36 | 23 | 76 | 24 |
| Centre | ||||||
| Bristol | 2 | 1 | 4 | 3 | 6 | 2 |
| Cheltenham | 2 | 1 | 1 | 1 | 3 | 1 |
| Cornwall | 1 | 1 | 1 | 1 | 2 | 1 |
| Countess of Chester | 1 | 1 | 2 | 1 | 3 | 1 |
| Coventry | 3 | 2 | 3 | 2 | 6 | 2 |
| Frimley | 1 | 1 | 0 | 0 | 1 | < 1 |
| Kings College | 8 | 5 | 9 | 6 | 17 | 5 |
| Leeds | 3 | 2 | 3 | 2 | 6 | 2 |
| Leicester | 9 | 6 | 7 | 4 | 16 | 5 |
| Manchester | 5 | 3 | 5 | 3 | 10 | 3 |
| MidYorks | 3 | 2 | 4 | 3 | 7 | 2 |
| Musgrove | 2 | 1 | 3 | 2 | 5 | 2 |
| North Wales | 0 | 0 | 1 | 1 | 1 | < 1 |
| Oxford | 21 | 13 | 19 | 12 | 40 | 13 |
| Peterborough | 6 | 4 | 6 | 4 | 12 | 4 |
| Royal Berkshire NHS Foundation Trust | 1 | 1 | 1 | 1 | 2 | 1 |
| Royal Surrey County Hospitals NHS Foundation Trust | 10 | 6 | 10 | 6 | 20 | 6 |
| Salisbury | 7 | 4 | 7 | 4 | 14 | 4 |
| Sheffield | 2 | 1 | 1 | 1 | 3 | 1 |
| Solent/Portsmouth | 3 | 2 | 4 | 3 | 7 | 2 |
| Stockport | 1 | 1 | 0 | 0 | 1 | < 1 |
| Sutton in Ashfield | 6 | 4 | 7 | 4 | 13 | 4 |
| Swansea | 19 | 12 | 20 | 13 | 39 | 12 |
| Swindon | 8 | 5 | 9 | 6 | 17 | 5 |
| Warrington | 11 | 7 | 11 | 7 | 22 | 7 |
| Wexham Park Hospital | 14 | 9 | 13 | 8 | 27 | 9 |
| Wrexham | 3 | 2 | 4 | 3 | 7 | 2 |
| Wrightington | 2 | 1 | 2 | 1 | 4 | 1 |
| Yeovil | 2 | 1 | 3 | 2 | 5 | 2 |
Table 5 shows the breakdown of baseline characteristics for each treatment group and overall. Groups were well balanced in general. The mean participant age was 33 years. A slightly higher proportion of patients were male in the surgical group compared to the rehabilitation group (71% vs. 62%). Around a third (34%) of patients had < 4 months between their injury and randomisation. The numbers of associated knee injuries reported on baseline MRI scan (see Appendix 3, Table 31) were well balanced. Baseline patient reported outcome measures (PROMs) were also well balanced between the two treatment groups.
| Surgical reconstruction (N = 156) | Rehabilitation (N = 159) | Total (N = 315) | |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 110 (71) | 98 (62) | 208 (66) |
| Female | 46 (29) | 61 (38) | 107 (34) |
| Age at randomisation, n , mean (SD) | 156, 32.9 (10.0) | 159, 32.9 (9.6) | 315, 32.9 (9.8) |
| Knee side, n (%) | |||
| Right | 84 (54) | 94 (59) | 178 (56) |
| Left | 71 (46) | 65 (41) | 136 (43) |
| Time since injury, months, n (%) | |||
| ≤ 1 | 13 (8) | 12 (8) | 25 (8) |
| > 1–< 4 | 45 (29) | 38 (24) | 83 (26) |
| 4–< 6 | 34 (22) | 37 (23) | 71 (23) |
| 6–< 9 | 23 (15) | 25 (16) | 48 (15) |
| 9–< 12 | 6 (4) | 14 (9) | 20 (6) |
| 12–< 24 | 11 (7) | 16 (10) | 27 (9) |
| 24 + | 24 (15) | 17 (11) | 41 (13) |
| KOOS4 at baseline, n , mean (SD) | 156, 45.7 (19.6) | 159, 43.3 (18.1) | 315, 44.5 (18.9) |
| KOOS pain score at baseline, n, mean (SD) | 156, 62.9 (20.5) | 159, 59.4 (19.6) | 315, 61.1 (20.1) |
| KOOS symptoms score at baseline, n, mean (SD) | 156, 57.1 (21.8) | 159, 54.3 (19.3) | 315, 55.7 (20.5) |
| KOOS ADL score at baseline, n, mean (SD) | 156, 67.8 (22.8) | 159, 67.9 (21.3) | 315, 67.8 (22.0) |
| KOOS sport/rec score at baseline, n, mean (SD) | 156, 34.6 (27.1) | 159, 33.4 (26.5) | 315, 34.0 (26.7) |
| KOOS QoL score at baseline, n, mean (SD) | 156, 28.3 (20.4) | 159, 26.3 (19.1) | 315, 27.3 (19.7) |
| KOOS5 score at baseline, n , mean (SD) | 156, 50.1 (19.8) | 159, 48.3 (18.1) | 315, 49.2 (19.0) |
| Tegner activity level before injury at baseline, n (%) | |||
| Level 0 | 0 (0) | 0 (0) | 0 (0) |
| Level 1 | 1 (1) | 1 (1) | 2 (1) |
| Level 2 | 0 (0) | 2 (1) | 2 (1) |
| Level 3 | 3 (2) | 12 (8) | 15 (5) |
| Level 4 | 14 (9) | 9 (6) | 23 (7) |
| Level 5 | 21 (13) | 17 (11) | 38 (12) |
| Level 6 | 22 (14) | 20 (13) | 42 (13) |
| Level 7 | 44 (28) | 35 (22) | 79 (25) |
| Level 8 | 10 (6) | 9 (6) | 19 (6) |
| Level 9 | 26 (17) | 42 (27) | 68 (22) |
| Level 10 | 15 (10) | 11 (7) | 26 (8) |
| Tegner activity level today at baseline, n (%) | |||
| Level 0 | 11 (7) | 13 (8) | 24 (8) |
| Level 1 | 28 (18) | 40 (25) | 68 (22) |
| Level 2 | 37 (24) | 33 (21) | 70 (22) |
| Level 3 | 43 (28) | 41 (26) | 84 (27) |
| Level 4 | 23 (15) | 20 (13) | 43 (14) |
| Level 5 | 6 (4) | 6 (4) | 12 (4) |
| Level 6 | 3 (2) | 2 (1) | 5 (2) |
| Level 7 | 4 (3) | 2 (1) | 6 (2) |
| Level 8 | 0 (0) | 1 (1) | 1 (< 1) |
| Level 9 | 0 (0) | 1 (1) | 1 (< 1) |
| Level 10 | 1 (1) | 0 (0) | 1 (< 1) |
| Tegner activity level you expect to return to at baseline, n (%) | |||
| Level 0 | 0 (0) | 0 (0) | 0 (0) |
| Level 1 | 0 (0) | 2 (1) | 2 (1) |
| Level 2 | 1 (1) | 2 (1) | 3 (1) |
| Level 3 | 6 (4) | 7 (4) | 13 (4) |
| Level 4 | 11 (7) | 13 (8) | 24 (8) |
| Level 5 | 23 (15) | 20 (13) | 43 (14) |
| Level 6 | 24 (15) | 27 (17) | 51 (16) |
| Level 7 | 42 (27) | 37 (23) | 79 (25) |
| Level 8 | 10 (6) | 11 (7) | 21 (7) |
| Level 9 | 28 (18) | 31 (20) | 59 (19) |
| Level 10 | 11 (7) | 8 (5) | 19 (6) |
| ACL-QoL at baseline, n, mean (SD) | 156, 26.1 (17.4) | 157, 23.2 (14.6) | 313, 24.6 (16.1) |
| ACL-QoL subscale symptoms and physical complaints at baseline, n, mean (SD) | 156, 42.4 (23.2) | 157, 39.9 (20.3) | 313, 41.2 (21.8) |
| ACL-QoL subscale work-related concerns at baseline, n, mean (SD) | 155, 37.5 (26.7) | 156, 34.4 (24.9) | 311, 36.0 (25.8) |
| ACL-QoL subscale recreational activities and sport participation at baseline, n, mean (SD) | 155, 14.8 (15.9) | 156, 12.8 (14.2) | 311, 13.8 (15.1) |
| ACL-QoL subscale lifestyle at baseline, n, mean (SD) | 155, 26.7 (22.8) | 156, 22.0 (19.4) | 311, 24.3 (21.3) |
| ACL-QoL subscale social and emotional at baseline, n, mean (SD) | 155, 26.6 (20.4) | 156, 21.6 (18.0) | 311, 24.1 (19.4) |
| EQ-5D-5L VAS, n, mean (SD) | 154, 64.2 (20.8) | 156, 68.4 (20.6) | 310, 66.3 (20.8) |
| EQ-5D-5L Index, n, mean (SD) | 156, 0.56 (0.25) | 158, 0.57 (0.26) | 314, 0.56 (0.26) |
Loss to follow-up
Data completion was generally good at the 18-month primary outcome time point, although it was poorer at the 6- and 12-month collection time points. Of the 313 forms sent out at 18 months, 248 (79%) were completed and returned. At 6 and 12 months, 64% and 54% of forms were completed and returned, respectively.
Three patients withdrew from ACL SNNAP, with one patient requesting that none of their data collected up to the point of withdrawal be used for purposes of the study.
Table 6 shows a breakdown of the reasons for injury by intervention group. Over one-third (38%) of injuries were sustained playing football. Other sports (including netball, rugby, skiing/snowboarding and non-specific sports) made up a significant proportion of other injuries.
| Surgical reconstruction (N = 156) | Rehabilitation (N = 159) | Total (N = 315) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| American football | 3 | 2 | 0 | 0 | 3 | 1 |
| Athletics/running | 4 | 3 | 0 | 0 | 4 | 1 |
| Basketball | 1 | 1 | 2 | 1 | 3 | 1 |
| Car/cycle/motorcycle RTA | 9 | 6 | 8 | 5 | 17 | 5 |
| Cricket | 1 | 1 | 1 | 1 | 2 | 1 |
| Dancing | 3 | 2 | 3 | 2 | 6 | 2 |
| Football | 56 | 36 | 63 | 40 | 119 | 38 |
| Hockey | 0 | 0 | 1 | 1 | 1 | < 1 |
| Horse riding | 2 | 1 | 1 | 1 | 3 | 1 |
| Jumping | 1 | 1 | 1 | 1 | 2 | 1 |
| Misc trauma | 1 | 1 | 0 | 0 | 1 | < 1 |
| Martial arts/wrestling | 3 | 2 | 4 | 3 | 7 | 2 |
| Netball | 6 | 4 | 11 | 7 | 17 | 5 |
| Rugby | 16 | 10 | 17 | 11 | 33 | 10 |
| Skiing/snowboarding | 15 | 10 | 15 | 9 | 30 | 9 |
| Skydiving | 1 | 1 | 0 | 0 | 1 | < 1 |
| Trampolining | 3 | 2 | 6 | 4 | 9 | 3 |
| Trip/fall/twisting injury (non-specific sport) | 26 | 17 | 22 | 14 | 48 | 15 |
| Ultimate frisbee | 1 | 1 | 0 | 0 | 1 | < 1 |
| Unknown mechanism | 0 | 0 | 1 | 1 | 1 | < 1 |
| Volleyball | 1 | 1 | 0 | 0 | 1 | < 1 |
| Water sports | 1 | 1 | 0 | 0 | 1 | < 1 |
| Weight training | 0 | 0 | 1 | 1 | 1 | < 1 |
| Missing | 2 | 1 | 3 | 2 | 5 | 2 |
Treatment received
Of the 156 participants allocated to surgical management, 110 (71%) received surgery and completed postoperative rehabilitation as per the original treatment pathway. Seventeen (11%) participants in the surgical management arm did not undergo surgery and instead underwent only rehabilitation. In the rehabilitation arm, 125 (78%) of 160 participants received initial rehabilitation treatment within the trial. Of these, 61 (49%) patients completed rehabilitation treatment with no subsequent surgery, 39 (31%) had subsequent reconstruction for continued symptoms and 25 (20%) started rehabilitation but did not complete the treatment (see Figure 2). Thirty-five (22%) of 160 patients in the rehabilitation group did not undergo the allocated rehabilitation treatment, of whom 26 (74%) had subsequent reconstruction. Sixty-five (41%) patients allocated to rehabilitation had subsequent reconstruction for ongoing symptoms in line with the protocol. The numbers of participants in each PP analysis population are shown in Table 7.
| Analysis population | Surgical reconstruction | Rehabilitation |
|---|---|---|
| PPC | 110 | 86 |
| PPP | 113 | 125 |
Details of preoperative rehabilitation for those who received surgery are shown in Table 8. Data were available for 14 participants (9%) in the surgical arm who received some form of preoperative rehabilitation, compared to 121 participants (76%) in the rehabilitation arm. As expected, due to the clinical pathway for each arm, there were far more preoperative rehabilitation sessions in the rehabilitation arm compared to the surgical arm overall, and more on average when they did receive some rehabilitation which was unrelated to an operation. Staff grades were comparable across treatment groups, as was the average session length. There was a higher proportion of one-to-one sessions in the rehabilitation group compared to the surgical arm (58% vs. 48%), and the content of sessions also varied between arms.
| Surgical reconstruction | Rehabilitation | Total | |
|---|---|---|---|
| Rehabilitation data available, n | 14 (9%) | 121 (76%) | 135 (43%) |
| Average number of sessions prior to surgery (if received), n , median (IQR) (range) | 14, 3 (2, 5) (1–20) | 121, 5 (3, 7) (1–34) | 135, 4 (3, 7) (1–34) |
| Session length (minutes), mean (SD) | 14, 42 (12) | 121, 38 (12) | 135, 39 (12) |
| Total session time per person (minutes), mean (SD); median (IQR) | 14, 213 (297) 150 (90, 270) |
121, 259 (346) 120 (80, 180) |
135, 255 (340) 150 (90, 270) |
| Total number of sessions prior to surgery, n | 64 | 751 | 815 |
| Staff grade, n (%) | |||
| 5 | 10 (16) | 81 (11) | 91 (11) |
| 6 | 38 (59) | 376 (50) | 414 (51) |
| 7 | 15 (23) | 166 (22) | 181 (22) |
| 8 | 1 (2) | 61 (8) | 62 (8) |
| Other | 0 (0) | 67 (9) | 67 (8) |
| Type of session, n (%) | |||
| One to one | 31 (48) | 436 (58) | 467 (57) |
| Group based | 33 (52) | 315 (42) | 348 (43) |
| Content of session, n (%) | |||
| Advice and education | 63 (98) | 672 (89) | 735 (90) |
| Supervised exercises (strengthening) | 58 (91) | 663 (88) | 721 (88) |
| Supervised exercises (stretching) | 38 (59) | 377 (50) | 415 (51) |
| Supervised exercises (sport specific) | 8 (13) | 240 (32) | 248 (30) |
| Home exercises (instructions/review) | 60 (94) | 471 (63) | 531 (65) |
| Gait re-education | 10 (16) | 108 (14) | 118 (14) |
| Supervised exercises (proprioception) | 48 (75) | 565 (75) | 613 (75) |
| Hydrotherapy | 0 (0) | 9 (1) | 9 (1) |
Data on ACLR operative data are shown in Table 9. One hundred and thirteen participants (72%) received ACLR surgery in the surgical arm, compared with 65 participants (41%) in the rehabilitation arm. A comparable proportion of surgeries were day cases (87% in surgical arm, 93% in rehabilitation arm). Operation and theatre times were similar in both arms (around 115 minutes). Most of participants (71%) in the surgical arm were classified as American Society of Anaesthesiologists (ASA) grade 1, compared to 80% in the rehabilitation arm. The majority of surgeries were arthroscopic in approach (97% in surgical arm, 100% in rehabilitation arm).
| Surgical reconstruction (N = 113) |
Rehabilitation (N = 65) |
|
|---|---|---|
| Data available on surgical reconstruction, n (%) | 106 (94) | 58 (89) |
| Day case, n (%) | 92 (87) | 54 (93) |
| Operation time (minutes), n , mean (SD) | 100, 79 (21) | 56, 76 (24) |
| Theatre time (minutes), n , mean (SD) | 100, 116 (28) | 56, 114 (38) |
| Anaesthetic used, n (%) | ||
| General | 105 (99) | 56 (97) |
| Periarticular LA infiltration | 53 (50) | 25 (43) |
| Epidural | 0 (0) | 0 (0) |
| Sciatic block | 1 (1) | 2 (3) |
| Femoral block | 7 (7) | 4 (7) |
| Spinal | 1 (1) | 2 (3) |
| Other | 22 (21) | 9 (16) |
| ASA grade, n (%) | ||
| I | 52 (71) | 36 (80) |
| II | 21 (29) | 9 (20) |
| III | 0 (0) | 0 (0) |
| IV | 0 (0) | 0 (0) |
| Approach, n (%) | ||
| Open | 3 (3) | 0 (0) |
| Arthroscopic | 102 (97) | 58 (100) |
| Articular cartilage | ||
| Normal throughout, n (%) | ||
| Yes | 70 (71) | 39 (70) |
| No | 29 (29) | 17 (30) |
| ACLR | ||
| Type of graft used, n (%) | ||
| Hamstring tendon | 94 (90) | 53 (91) |
| Patella tendon | 2 (2) | 0 (0) |
| Other | 8 (8) | 5 (9) |
| Notchplasty performed?, n (%) | ||
| Yes | 10 (10) | 2 (4) |
| No | 87 (90) | 53 (96) |
| Femoral tunnel drilling technique, n (%) | ||
| Outside-in | 2 (2) | 1 (2) |
| Trans-tibial | 4 (4) | 2 (4) |
| AM portal | 64 (67) | 36 (68) |
| All inside | 26 (27) | 14 (26) |
| Tibial tunnel drilling technique, n (%) | ||
| Outside-in | 73 (77) | 40 (74) |
| Inside-out | 3 (3) | 1 (2) |
| All inside | 19 (20) | 13 (24) |
| Additional surgery, n (%) | ||
| Partial medial meniscectomy | 15 (14) | 9 (16) |
| Medial meniscal repair | 14 (13) | 10 (17) |
| Medial replacement (synthetic) | 0 (0) | 0 (0) |
| Partial lateral meniscectomy | 12 (11) | 5 (9) |
| Lateral meniscal repair | 13 (12) | 4 (7) |
| Lateral replacement (synthetic) | 0 (0) | 0 (0) |
| Medial transplant (allograft) | 0 (0) | 0 (0) |
| Saucerisation medial discoid meniscus | 0 (0) | 0 (0) |
| PLC surgery | 0 (0) | 0 (0) |
| Lateral transplant (allograft) | 0 (0) | 0 (0) |
| Saucerisation lateral discoid meniscus | 0 (0) | 0 (0) |
| Collateral ligament surgery | 0 (0) | 0 (0) |
| Extensor mechanism surgery | 0 (0) | 0 (0) |
| Articular cartilage surgery | 3 (3) | 2 (3) |
| PCL surgery | 0 (0) | 0 (0) |
| Other | 7 (7) | 4 (7) |
| Medial meniscal status at end of procedure, n (%) | ||
| Normal | 82 (82) | 42 (76) |
| 2/3 remaining | 12 (12) | 9 (16) |
| 1/3 remaining | 5 (5) | 3 (5) |
| < 10% remaining | 1 (1) | 1 (2) |
| Lateral meniscal status at end of procedure, n (%) | ||
| Normal | 81 (82) | 48 (89) |
| 2/3 remaining | 18 (18) | 5 (9) |
| 1/3 remaining | 0 (0) | 1 (2) |
| < 10% remaining | 0 (0) | 0 (0) |
Table 10 gives details on postoperative rehabilitation sessions. Data of postoperative rehabilitation details were available for just under half of the patients who received surgery in each treatment group (surgical 48%, rehabilitation 40%). Postoperative rehabilitation was broadly similar across the treatment groups, although there were some minor differences in terms of the session content.
| Surgical reconstruction (N = 113) | Rehabilitation (N = 65) | Total (N = 178) | |
|---|---|---|---|
| Data availability on postoperative rehabilitation, n (%) | 54 (48) | 26 (40) | 80 (45) |
| Average number of sessions prior to surgery (if received), n , median (IQR) (range) | 54, 8 (4, 16) (1–27) | 26, 6 (2, 12) (1–28) | 80, 7 (3, 13) (1–28) |
| Session length, n , mean (SD) | 53, 40 (14) | 26, 41 (15) | 79, 40 (14) |
| Total session time per person, n , mean (SD); median (IQR) | 53, 375 (316) 270 (150, 525) |
26, 323 (397) 180 (105, 360) |
79, 358 (343) 225 (120, 470) |
| Total number of sessions post surgery, n | 532 | 200 | 732 |
| Staff grade, n (%) | |||
| 5 | 47 (9) | 16 (8) | 63 (9) |
| 6 | 226 (42) | 131 (66) | 357 (49) |
| 7 | 137 (26) | 37 (19) | 174 (24) |
| 8 | 53 (10) | 10 (5) | 63 (9) |
| Other | 69 (13) | 6 (3) | 75 (10) |
| Type of session, n (%) | |||
| One to one | 367 (69) | 130 (65) | 497 (68) |
| Group based | 165 (31) | 70 (35) | 235 (32) |
| Content of session, n (%) | |||
| Advice and education | 468 (88) | 189 (95) | 657 (90) |
| Supervised exercises (strengthening) | 458 (86) | 152 (76) | 610 (83) |
| Supervised exercises (stretching) | 337 (63) | 104 (52) | 441 (60) |
| Supervised exercises (sport specific) | 120 (23) | 102 (51) | 222 (30) |
| Home exercises (instructions/review) | 394 (74) | 131 (66) | 525 (72) |
| Gait re-education | 141 (27) | 84 (42) | 225 (31) |
| Supervised exercises (proprioception) | 293 (55) | 96 (48) | 389 (53) |
| Hydrotherapy | 7 (1) | 2 (1) | 9 (1) |
Primary outcome – Knee Injury and Osteoarthritis Outcome Score
The mean KOOS4 scores at baseline were 45.7 for participants allocated to surgical management and 43.3 for those allocated to rehabilitation (see Table 5).
Adjusted and unadjusted analyses were carried out on the ITT, conservative per-protocol (PPC) and pragmatic per-protocol (PPP) populations using linear regression. Unadjusted analyses included only the treatment variable in the analysis models, with adjusted analyses further adjusting for baseline KOOS4 scores and allowing for ICC between recruitment sites.
Analyses on the KOOS4 primary outcome for all analysis populations are shown in Table 11. For the ITT analysis of the KOOS4 at 18 months, 128 participants (82%) in the surgical management arm had scores available for analysis, compared to 120 (75%) participants in the rehabilitation arm. Adjusted mean KOOS4 scores at 18 months post randomisation had increased to 73.0 in the surgical management arm, and to 64.6 in the rehabilitation arm. The adjusted mean difference was 7.9 (95% CI 2.5 to 13.2; p = 0.005) in favour of surgical management. The PPP and PPC analyses supported the ITT results, with all treatment effects favouring surgical management at a level reaching statistical significance. All unadjusted analyses also produced statistically significant effects in favour of surgical management.
| Analysis population | KOOS4 average treatment effect | Surgical reconstruction | Rehabilitation | Surgical management – non-surgical management | |
|---|---|---|---|---|---|
| n, mean (SD) | n, mean (SD) | Mean difference (95% CI) | p-value | ||
| ITT | Adjusted | 128, 73.0 (18.3) | 120, 64.6 (21.6) | 7.9 (2.5 to 13.2) | 0.0053 |
| Unadjusted | 128, 73.0 (18.3) | 120, 64.6 (21.6) | 8.3 (3.3 to 13.3) | 0.0012 | |
| PPC | Adjusted | 94, 75.9 (16.1) | 73, 69.1 (18.7) | 7.3 (0.8 to 13.8) | 0.030 |
| Unadjusted | 94, 75.9 (16.1) | 73, 69.1 (18.7) | 6.8 (1.5 to 12.2) | 0.012 | |
| PPP | Adjusted | 95, 75.7 (16.2) | 100, 64.8 (21.05) | 11.2 (5.7 to 16.8) | 0.0003 |
| Unadjusted | 95, 75.7 (16.2) | 100, 64.8 (21.5) | 10.9 (5.5 to 16.3) | 0.0001 | |
A secondary AUC analysis was performed on the ITT population using the KOOS4 scores at baseline, 6, 12 and 18 months (see Table 12). AUC values were calculated by extracting parameters from a mixed-effects repeated measures model. The difference in AUC values was 4.1 (95% CI 0.4 to 7.7; p = 0.028), which was statistically significant in favour of surgical management. Figure 3 shows the marginal mean KOOS4 scores over the duration of the trial period split by treatment arm. The score in both groups increases over time with mean value being very similar in both groups at 6 months but higher in the surgery arm at 12 and 18 months.
| Surgical reconstruction | Rehabilitation | Surgical management – non-surgical management | |
|---|---|---|---|
| n, mean (SD) | n, mean (SD) | Mean difference (95% CI) (p-value) | |
| KOOS4 AUC | 156, 61.7 (17.2) | 159, 57.6 (17.7) | 4.1 (0.4 to 7.7) (0.028) |
FIGURE 3.
Marginal mean and 95% CIs for KOOS4 scores at each time point (ITT population).
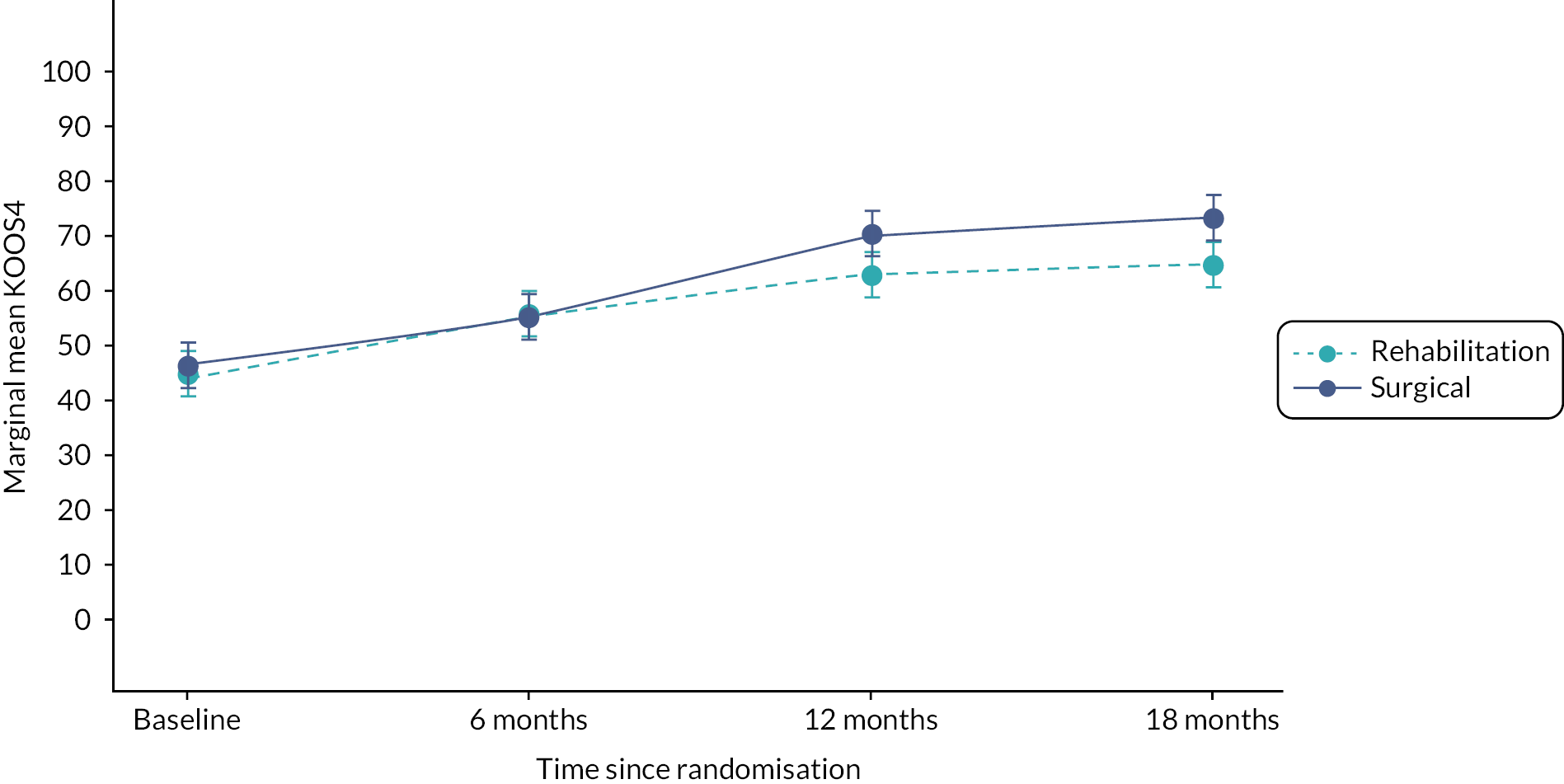
Figure 4 shows the marginal mean KOOS4 scores with the rehabilitation treatment arm split into those who received surgery and those who did not. Although the rehabilitation patients who did not receive surgery initially perform better than the surgical management arm in the first 6 months, this benefit levels off at 12 months and decreases between 12 and 18 months. Patients in the rehabilitation arm who received rehabilitation then required surgery start off with lower KOOS4 scores at baseline than those who did receive surgery without rehabilitation and also those who received only rehabilitation. This difference in baseline level perhaps explains to some degree why these individuals went on to have surgical treatment. Despite these participants starting off lower, the marginal means KOOS4 scores eventually surpass those of the patients in the rehabilitation arm who did not receive surgery at the 18-month time point.
FIGURE 4.
Marginal mean and 95% CIs for KOOS4 scores at each time point, broken down by receipt of surgery in the rehabilitation arm.

Additional analyses on primary outcome
Analyses were carried out on the KOOS4 score to assess sensitivity to missing data, compliance with the allocated treatment and to determine if there were any subgroup effects present.
Sensitivity to missing data was assessed using the rctmiss package in Stata. A pattern-mixture model was used to extend the adjusted linear regression model used for the primary outcome analysis, in order to show graphically the difference in treatment effect for each treatment arm if different mean values are assumed for the missing data. For up to a 5-point difference in KOOS4 scores (in favour and against surgical management group) for the participants with missing outcome data versus those for whom data were available, there remained a significant difference in favour of surgical management. The output from the rctmiss command is shown in Figure 5.
FIGURE 5.
rctmiss sensitivity analysis for KOOS4 primary outcome.

A CACE analysis was carried out on the KOOS4 outcome to assess the impact of non-compliance with allocated treatment, where compliance was defined as having had surgery at any time between randomisation and 18 months.
Of the 316 participants randomised, 248 had primary outcome data available (120 in the rehabilitation arm, 128 in the surgical management arm). Out of the 120 participants randomised to rehabilitation, 49 (40.8%) received surgery (the ‘always-takers’), while 33 out of the 128 participants (25.8%) randomised to surgical management did not receive surgery (the ‘never-takers’). The unadjusted CACE estimate can be calculated by dividing the unadjusted ITT treatment effect by the observed proportion of compliers. Accordingly, the unadjusted CACE estimate was 25.0 (95% CI 9.4 to 40.5; p = 0.002).
For the adjusted CACE analysis, the adjusted proportion of compliers was calculated using a first-stage linear regression on participants with available primary outcome data, with compliance as the outcome variable and randomised treatment as the dependent group variable, with additional adjustments as specified in the main primary outcome model. This gave an adjusted CACE estimate of 22.8 (95% CI 6.9 to 38.8; p = 0.005) (see Table 13).
| Surgical management – non-surgical management | |
|---|---|
| CACE mean difference (95% CI) (p-value) | |
| KOOS4 CACE treatment effect at 18 months, adjusted for baseline KOOS4 and recruitment site | 22.8 (6.9 to 38.8) (0.0051) |
| KOOS4 CACE treatment effect at 18 months, unadjusted | 25.0 (9.4 to 40.5) (0.0017) |
An additional pre-specified analysis which imputed 12-month KOOS4 scores if participants were missing their 18-month KOOS4 scores was carried out (limited form of last observation carried forward). This added an additional 12 scores to the analysis (6 in each treatment arm). The analysis results remained similar to the ITT analysis (see Table 14).
| Surgical reconstruction | Rehabilitation | Surgical management – non-surgical management | |
|---|---|---|---|
| n, mean (SD) | n, mean (SD) | Mean difference (95% CI) (p-value) | |
| KOOS4 average treatment effect at 18 months, adjusted for baseline KOOS4 and site | 128, 73.0 (18.3) | 120, 64.6 (21.6) | 7.5 (2.1 to 12.9) (0.008) |
| KOOS4 average treatment effect at 18 months, unadjusted | 134, 73.1 (18.0) | 126, 65.2 (21.7) | 8.0 (3.1 to 12.8) (0.001) |
Subgroup analyses were carried out on four subgroups: sex (male vs. female), baseline KOOS4 scores (low vs. high), age (< 40 vs. 40 years and over) and baseline Tegner Activity Scale values (moderate/light vs. high activity). No difference in treatment effect was found for any of the subgroups, although there was substantial uncertainty (see Table 15).
| Subgroup | Subgroup Strata | Mean difference (95% CI) | Interaction effect (95% CI) | Interaction p-value |
|---|---|---|---|---|
| Gender | Male (n = 209) | 8.1 (−0.4 to 16.7) | 0.5 (−15.4 to 16.4) | 0.949 |
| Female (n = 107) | 7.6 (−2.9 to 18.2) | |||
| KOOS4 scores | Low (< 30) (n = 76) | 4.8 (−2.5 to 12.0) | −11.8 (−30.7 to 7.0) | 0.210 |
| High (≥ 30) (n = 240) | 16.6 (1.6 to 31.6) | |||
| Age (years) | < 40 (n = 236) | 8.0 (−4.7 to 20.7) | 0.3 (−12.2 to 12.8) | 0.962 |
| 40 and over (n = 80) | 7.7 (3.1 to 12.3) | |||
| Tegner Activity Scale | Moderate/light activity (< 5) (n = 42) | 6.4 (0.8 to 12.0) | −9.1 (−28.7 to 10.5) | 0.350 |
| High activity (≥ 5) (n = 274) | 15.5 (−3.2 to 34.2) |
Secondary outcomes
Knee Injury and Osteoarthritis Outcome Score subscales
The subscales of the KOOS score (pain, symptoms, activities of daily living, sports and recreation, knee-related QoL) were analysed separately as a secondary outcome, with results shown in Table 16. Each subscale was analysed using linear regression, adjusting for baseline KOOS and respective subscale scores and allowing for ICC between recruitment sites. All subscales showed statistically significant differences in favour of surgical management at the 5% level of significance.
| KOOS subscale | Surgical reconstruction | Rehabilitation | Mean difference (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||
| Pain | 128 | 85.3 (15.5) | 120 | 79.3 (19.2) | 5.4 (0.9 to 9.9) | 0.020 |
| Symptoms | 128 | 79.4 (15.7) | 119 | 71.9 (20.8) | 6.8 (2.7 to 10.9) | 0.002 |
| Activities of daily living | 105 | 91.2 (14.5) | 88 | 85.0 (20.3) | 8.1 (3.2 to 13.0) | 0.002 |
| Sports and recreation | 128 | 68.9 (24.9) | 120 | 59.2 (29.8) | 9.3 (0.3 to 18.3) | 0.043 |
| Knee-related QoL | 128 | 58.1 (25.0) | 120 | 48.1 (26.6) | 9.7 (2.9 to 16.4) | 0.006 |
Tegner Activity Score
The median Tegner Activity Scores at 18 months were five in the surgical management arm and four in the rehabilitation arm. There were 95 18-month scores available in the surgical management arm and 86 in the rehabilitation arm. The distribution of Tegner Activity Scores was compared between treatment groups at 18 months post randomisation using a Mann–Whitney U-test. The difference between treatment arms was statistically significant in favour of surgical management (p = 0.006), with a 38% chance that a random Tegner score drawn from the rehabilitation arm being greater than a score drawn from the surgical arm.
Patients were asked to complete a modified version of the Tegner Activity Score at baseline, asking them their pre-injury activity level as well as the level they expected to return to after treatment. At 18 months, 28% of participants in the surgical arm had returned to their pre-injury activity level, compared to 24% in the rehabilitation arm. Sixty-five out of the 95 (68%) of participants with available scores in the surgical arm did not reach the activity level they expected to return to post treatment, compared to 63 of the 86 patients with scores (73%) in the rehabilitation arm. Analyses on the Tegner Activity Score are shown in Table 17.
| Surgical reconstruction | Rehabilitation | p-value | |
|---|---|---|---|
| Tegner Activity Score at 18 months, n, median (IQR) | 95, 5 (3, 6) | 86, 4 (3, 5) | 0.006 |
| Return to pre-injury activity level, n (%) | 27 (28) | 21 (24) | - |
| Did not reach expected return level, n (%) | 65 (68) | 63 (73) | - |
Complications
Table 18 shows complications from surgery which occurred prior to discharge. Out of the 156 participants in the surgical management arm, 113 (72.4%) underwent surgery compared to 65 (40.6%). In the rehabilitation arm, one intraoperative complication was recorded in the surgical management arm, with correspondingly only two in the rehabilitation arm. No postoperative complications were recorded in either treatment arm.
| Surgical reconstruction | Rehabilitation | |
|---|---|---|
| Total first surgeries | 113 | 65 |
| Patients with intraoperative complications | 1 | 2 |
| Patients with postoperative complications | 0 | 0 |
| Time in hospital (days), median (range) | 0 (0–4) | 0 (0–1) |
| Hours in ICUa | – | – |
| Hours in HDUa | – | – |
Details of clinical events where a medical professional was encountered are shown in Table 19. Eleven complications were recorded in the surgical management arm (from 10 patients) compared to 12 in the rehabilitation arm (from 11 patients). Numbers of each complication were generally very low, with the most common being newly acquired meniscal pathology (one in surgical management, three in rehabilitation).
| Surgical reconstruction | Rehabilitation | |
|---|---|---|
| Received surgery, n (%) | 113 (72) | 65 (41) |
| Total number of complications | 11 | 12 |
| Total participants with complications | 10 | 11 |
| Complication, n (%) | ||
| Anterior knee pain | 0 | 0 |
| Back/mobility problems | 0 | 0 |
| Instability | 1 | 0 |
| Meniscal pathology (newly acquired) | 1a | 3 |
| Numbness and weakness | 0 | 0 |
| Suspected DVT | 1 | 0 |
| Swelling/haematoma | 1 | 1 |
| Twisting injury | 0 | 0 |
| Unexplained knee pain | 0 | 1 |
| Unexplained knee pain/haemarthrosis | 0 | 0 |
| DVT | 1 | 0 |
| Graft failure | 2 | 1 |
| Infection | 1b | 1 |
| Meniscal and PLC pathology newly acquired | 1 | 0 |
| Patellofemoral related pain | 1 | 2 |
| Superficial skin infection (graft harvest) | 1 | 0 |
| Suspected ligament damage | 0 | 2 |
| Suspected vascular abnormality | 0 | 1 |
Generic quality of life (EuroQol-5 Dimensions, five-level version)
Table 20 shows the outcome of the analysis on the EQ-5D-5L at the 18-month time point. Formal analysis was carried out on the EQ-5D-5L index using linear regression, adjusting for baseline KOOS4 and EQ-5D-5L scores and allowing for ICC between recruitment sites.
| EQ-5D-5L | Surgical reconstruction | Rehabilitation | Mean difference (95% CI) | p-value |
|---|---|---|---|---|
| Index, n, mean (SD) | 115, 0.77 (0.23) | 116, 0.72 (0.24) | 0.04 (−0.02 to 0.10) | 0.22 |
| VAS, n, mean (SD) | 114, 77.7 (16.3) | 113, 75.9 (16.2) | - | - |
One hundred and fifteen participants had EQ-5D-5L scores available in each treatment arm. The mean index score was 0.77 in the surgical management arm and 0.72 in the rehabilitation arm. The adjusted mean difference was 0.04 (95% CI −0.02 to 0.10; p = 0.219), which was not statistically significant at the 5% significance level.
Anterior cruciate ligament quality of life score
Anterior cruciate ligament quality of life scores at 18 months were analysed using linear regression, adjusting for baseline KOOS and ACL-QoL scores and allowing for ICC between recruitment sites (see Table 21).
| ACL-QoL | Surgical reconstruction | Rehabilitation | Mean difference (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||
| Overall score | 89 | 59.7 (24.5) | 82 | 48.2 (26.3) | 11.6 (4.4 to 18.8) | 0.0028 |
Eighty-nine participants had ACL-QoL scores taken at 18 months available in the surgical management arm, compared to 81 participants in the rehabilitation arm (57% vs. 51%, respectively). The mean ACL-QoL scores were 59.7 and 48.2 in the surgical and rehabilitation arms, respectively. The adjusted mean difference was 11.6 (95% CI 4.4 to 18.8; p = 0.003) and was statistically significant in favour of surgical management at the 5% significance level.
Patient satisfaction
Patient satisfaction was assessed in two ways: asking patients the nature of their problems at the 18-month time point compared to before their treatment and also if they would still choose to have the same treatment if they were able to go back in time.
A CI for the difference in proportions of participants who said their knee was better at 18 months than before their treatment (but after their injury) was calculated using the Newcombe Method 10 (also referred to as the score method).
One hundred and twenty-three participants had available responses for the ‘better than before’ question in the surgical arm, compared to 116 in the rehabilitation arm. One hundred and two participants (83%) in the surgical management arm said their knee was better than before treatment at 18 months, compared to 79 (68%) in the rehabilitation arm. This gave a difference in proportions of 15% (95% CI 4% to 25%) in favour of surgical management.
A higher proportion of participants in the surgical management arm said they would choose the same treatment again compared to those in the rehabilitation arm (80% vs. 61%, respectively). Conversely, a higher proportion said they would not choose the same treatment again in the rehabilitation arm compared to the surgical management arm (18% vs. 5% respectively). Table 22 gives full details of the patient satisfaction outcome analysis.
| Patient satisfaction | Surgical reconstruction | Rehabilitation | Difference in proportions (95% CI) |
|---|---|---|---|
| Better than before, n (%) (95% CI) | 102 (83) (75.1 to 89.1) | 79 (68) (58.8 to 76.4) | 15 (4 to 25) |
| Same treatment again, n (%) | |||
| Yes | 98 (80) | 71 (61) | - |
| No | 6 (5) | 21 (18) | - |
| Unsure | 19 (15) | 24 (21) | - |
Fidelity to rehabilitation
A more detailed analysis of the compliance and fidelity to the management interventions was conducted. 55 Compliance (adherence) in the rehabilitation arm was defined as participants completing sufficient physiotherapy over a period of at least a 90-day time span. Compliance in the surgical arm was defined as participants undergoing surgical reconstruction and postsurgical rehabilitation over at least a 5-month time span. This revealed that adherence (compliance) to treatment was higher in the surgical arm: 110 of 156 participants completed surgical reconstruction and were compliant with postsurgical rehabilitation for a compliance rate of 70% while compliance was 59% in the rehabilitation arm (see Table 23). Compliance to intervention allocation was higher in the rehabilitation arm (78%) than in the surgical arm (72%) (see Table 23).
| Compliance and fidelity | Group | n | Compliance or fidelity rate (%) |
|---|---|---|---|
| Non-surgical arm | Participants randomised to rehabilitation arm | 160 | |
| Participants completed any amount of rehab | 125 | ||
| Compliance Physiotherapy takes place over ≥ 60-day time span |
Participants were randomised to the non-surgical arm (rehab), met compliance criteria. | 95/160 | 59 |
| Partial compliance (compliance with intervention allocation) |
Participants were randomised and started therapy but did not meet compliance criteria (completed any amount of therapy) | 125/160 | 78 |
| High compliance | Participants randomised to and began physiotherapy, met compliance criteria. | 95/125 | 76 |
| Fidelity Physiotherapy included progression of activities towards return to sport or functional activity |
Participants who completed any amount of rehab and rehab programme met fidelity criteria. | 90/125 | 72 |
| High fidelity | Participants had high compliance to rehab and physiotherapy programme met fidelity criteria. | 55/95 | 58 |
| Number of days in rehab | Participants who completed any amount of physiotherapy | 125 | |
| Participants with high compliance (≥ 60-day time span) |
95 | ||
| Surgical arm | Participants randomised to the surgical reconstruction arm | 156 | |
| Compliance (compliance with intervention allocation) |
Participants had surgical reconstruction | 113/156 | 72 |
| High compliance | Participants had surgical reconstruction and completed postsurgical rehab over at least a 5-month time span. | 110/156 | 71 |
| Partial compliance | Surgical reconstruction, no or insufficient rehab | 3/156 | 2 |
Intervention fidelity is the similarity of the interventions delivered in the trial to interventions specified in the protocol. 56 In the rehabilitation arm, this was defined as participants’ physiotherapy programmes including a progression of therapeutic activities towards those targeting return to sports or functional activities. Intervention fidelity was high in the rehabilitation arm, with 72% of participants who were randomised to and began physiotherapy meeting fidelity criteria (see Table 23).
Chapter 5 Cost-effectiveness analysis
Introduction
This chapter reports the methods and results of a within-trial cost-effectiveness analysis of ACL SNNAP. Between February 2017 and April 2020, ACL SNNAP randomly allocated 316 adults with symptomatic non-acute ACL-deficient knees to receive: (1) surgical management (reconstruction n = 156), in which patients were placed on a waiting list to undergo standard ACLR procedure, or (2) non-surgical management (rehabilitation n = 160), in which patients were referred to their nearest physiotherapy department and undergo rehabilitation. Participants have been followed up to 18 months following randomisation. Information on recruitment, including inclusion and exclusion criteria, is presented in more detail in Chapter 3. Participant characteristics at recruitment and clinical results are presented in Chapter 4.
In this chapter, we compared surgery and rehabilitation in terms of QALYs gained and healthcare costs and calculated incremental cost-effectiveness ratios (ICERs), which give the additional spending required in order to generate one additional QALY.
Methods
Health economic data collection
Resource use data were collected for each participant in the trial using questionnaires at baseline, 6, 12 and 18 months. At these time points, participants were asked to report their use of healthcare resources and impact on employment in the previous 6 months. The questionnaires were originally designed to collect data on visits to and from healthcare practitioners (NHS and private), admissions to hospital, medication use, equipment provided or purchased, informal care received, and time away from paid employment and time at work affected by their knee. However, the pilot phase demonstrated that early follow-up data were not as comprehensive as anticipated in terms of retention and completion rates. Hence, an amendment (Amendment number 5, 12 October 2018) was made comprising a series of strategies to improve follow-up such as shorter follow-up questionnaires and the use of vouchers. The health economics data collection therefore underwent the following changes:
-
Items were removed: medication use, equipment provided or purchased, informal care received and time at work affected by their knee.
-
Questions concerning time away from paid employment were removed from follow-up at 6 and 12 months. In the final questionnaire (at 18 months), individuals were asked to recall time off work in the previous 18 months rather than 6 months.
Questions concerning the contact with healthcare services were left unchanged for all time points. As a result, we used in this analysis the healthcare contact data. Patients were asked to complete the EQ-5D-5L questionnaire at randomisation and at 6, 12 and 18 months after randomisation. In the EQ-5D-5L questionnaire, participants are asked to report whether they have no problems, slight problems, moderate problems, severe problems and extreme problems in five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression.
Hospital admissions related to the study knee were identified from data reported in the self-reported questionnaires, clinical events reported and assessment of hospital records for all participants by local research teams. Where potentially relevant admissions were identified, a data extraction sheet was completed providing details of the admission.
Rehabilitation sessions related to the study knee were identified from data reported in the self-reported questionnaires and documented in PCRFs completed by local physiotherapist/research teams. Where rehabilitation sessions were available from PCRFs and self-reported questionnaires for the same period, we used data from the source reporting the highest number of sessions. Where rehabilitation session data were available from PCRFs but were missing from self-reported questionnaires, we used data from PCRFs.
Healthcare costs
To estimate healthcare costs, unit costs were derived from NHS Reference Costs and Unit Costs of Health and Social Care57–59 and are reported in Appendix 3 (see Tables 32 and 33). All unit costs are inflated, where necessary, to 2019–20 prices using the healthcare and community health services inflation index. 57
Hospital admissions concerning ACLR surgery and other relevant knee-related issues were converted into a Healthcare Resource Group (HRG) and valued using NHS Reference Costs. HRGs are groups of International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnoses and Office of Population Censuses and Surveys Classification of Interventions and Procedures (OPCS) procedures which use comparable levels of healthcare resources. Following clinical and hospital coder expert opinion, we identified ICD-10 and OPCS for all hospital admissions (see Appendix 3, Table 33) and used these to derive HRGs. Mean costs for each HRG were obtained from the NHS Reference Cost Schedule 2019–20 (see Appendix 3, Table 32).
Rehabilitation sessions were costed using mean costs from the NHS Reference Cost Schedule 2019–20 (see Appendix 3, Table 32). We used a weighted average of individual and group-based sessions (using number of finished consultant episodes) to cost rehabilitation sessions. Unit costs were also attached to self-reported data on consultations with a GP (surgery, home, or telephone), physiotherapist (NHS or private), chiropractor/osteopath/acupuncturist (NHS or private), outpatient clinic (NHS or private) and contacts with accidents and emergency departments. Private healthcare contacts were also costed using the unit costs reported in Tables 32 and 33 (see Appendix 3).
Quality-adjusted life-years
Responses to EQ-5D-5L questionnaires were converted into utility scores using the cross-walk to the three-level version. 60 QALYs were calculated using the AUC approach, which involves estimating the average EQ-5D utility between each follow-up time, and weighting it by survival time. Partially completed EQ-5D-5L questionnaires were considered missing.
Missing data
We followed best practice methods for addressing missing data in cost-effectiveness studies. 61 Missing data on participant characteristics, EQ-5D and costs at baseline were imputed using unconditional mean imputation. Data on allocation to treatment arm and death were assumed to be complete.
We examined patterns of missing data and their similarity between trial arms. In particular, we examined whether there was some evidence that the probability of data missing was conditional on baseline participant characteristics (age, sex, KOOS score) or on lagged outcomes (EQ-5D utility). The association between the probability of data missing was estimated using logistic random-effects regressions. We used multiple imputation by chained equations to impute missing data on EQ-5D-5L utility scores and visual analogue scale (VAS) scores, and cost components, at each follow-up time point. Each missing value was imputed as a function of follow-up period, sex, age, recruitment site, treatment allocation, baseline EQ-5D data (score and VAS), baseline KOOS4 score, physical activity prior to injury (as measured by the modified Tegner score), updated EQ-5D score (utility and VAS) and costs (by category and payer – NHS or private). The imputation model was run separately by randomised treatment. We used predictive mean matching to create 30 imputed datasets (proportion of data missing across all time periods × 100) with 10 nearest neighbours. We imputed costs and EQ-5D-5L utility and VAS scores in each follow-up period.
Within-trial analysis
The perspective of the analysis was of the NHS and personal social services. In sensitivity analyses, we considered a wider perspective and included private healthcare contacts and lost productivity losses. Furthermore, our analysis followed ITT principles wherein healthcare resource use, costs and EQ-5D scores were analysed according to treatment allocation, regardless of the treatment actually received.
We report descriptive statistics (means, SD as a minimum) for resource use, costs and EQ-5D utilities at each follow-up time point using only complete data. Differences between arms were estimated using multilevel mixed-effects linear regression models to allow for multiple follow-ups clustered within participant. The models were adjusted for treatment allocation, an interaction between follow-up time and treatment allocation and, in the case of EQ-5D, baseline utility score. Clustering by site was accounted for by using robust standard errors, using the ‘cluster’ option in Stata.
Following multiple imputation, we estimated total costs and QALYs for all 316 participants in the ACL SNNAP study from the date of study recruitment until the earliest of death, withdrawal from study or the end of follow-up at 18 months. We discounted total costs and QALYs at the recommended 3.5% rate as the time horizon of the analysis was above 12 months. On each imputed dataset, we estimated mean costs (by type) and QALYs using separate linear regression models controlling for treatment allocation, and, for QALYs, baseline EQ-5D utility, and cluster-robust standard errors (by site). Estimates derived from each imputed dataset were combined using Rubin’s rule to estimate the adjusted mean difference and standard error for each outcome.
We estimated the ICER by dividing the mean cost difference between surgical management (reconstruction) and non-surgical management (rehabilitation) by the mean QALY difference. We judged surgical management to be cost-effective if the ICER relative to non-surgical management was below the threshold of £20,000–30,000 per QALY gained. 62,63 This choice of threshold was informed by recent National Institute for Health and Care Excellence (NICE) guidance and revealed thresholds from NICE’s past decisions. 64 We also estimated the ICER within participant subgroups defined by age at recruitment (< 40 or 40 or older), baseline KOOS4 (< 30 or ≥ 30), gender (male or female), physical activity prior to injury (high – Tegner score 5 or above – or moderate/low – Tegner score below 5).
We estimated the joint uncertainty around incremental total costs and QALYs (i.e. the difference between surgical and non-surgical management), and in the cost effectiveness, by bootstrapping 1000 times from each of the n imputed datasets (creating at least 1000 × 30 bootstraps), running the estimation model on each bootstrapped dataset and extracting the estimated treatment effects. From these bootstrapped results, we calculated the probability that surgical management is more cost-effective than non-surgical management for different threshold values per QALY gained. 65 These were calculated by estimating the proportion of bootstrap replicates with a net monetary benefit (NMB) above 0 for each threshold value, where the NMB is given by the product of the mean difference in QALYs and the threshold value minus the mean difference in costs.
Finally, the COVID-19 pandemic has significantly disrupted all medical research, including the ACL SNNAP trial. To determine the extent of the effect the pandemic had on ACL SNNAP, we also estimated the incremental NHS costs and QALYs of those who completed the 18-month follow-up before (n = 159) and after (n = 157) the first UK nationwide lockdown on 23 March 2020.
All analyses were conducted using Stata version 17.0 (StataCorp 2021).
Results
Study follow-up and missing data
Of the 316 study participants, there were no deaths and 3 participants withdrew from the study.
Table 24 reports the percentage of data observations for resource use and EQ-5D utility at each follow-up point by treatment allocation. Overall, the levels of missing data were 30% across all self-reported data items and EQ-5D utility scores. The levels of missing data were similar between trial arms. Age at baseline was associated with the probability of missingness (univariate, p = 0.007 for resource use and p = 0.003 for EQ-5D utility) suggesting that data are not missing completely at random. Previous lagged EQ-5D utility was significantly associated with the probability of missingness (univariate, p < 0.001 for resource sue and EQ-5D utility) suggesting that missing at random (MAR) may be a plausible assumption.
| Follow-up time | Resource use data | EQ-5D data | ||
|---|---|---|---|---|
| Surgical (n = 156) | Rehabilitation (n = 160) | Surgical (n = 156) | Rehabilitation (n = 160) | |
| Baseline | – | – | 156 (100%) | 159 (99%) |
| 6 months | 88 (56%) | 93 (58%) | 85 (54%) | 89 (56%) |
| 12 months | 87 (56%) | 78 (49%) | 84 (54%) | 75 (47%) |
| 18 months | 127 (81%) | 120 (75%) | 115 (74%) | 116 (73%) |
Resource use and costs during follow-up
Table 25 presents mean costs for each cost type and totals by treatment allocation and follow-up period and adjusted mean differences. Appendix 3, Table 34 provides comprehensive data on resource use by treatment allocation and follow-up period. Total NHS and healthcare costs were substantially greater between baseline and 6 months than in subsequent periods. In the first 6 months, total NHS costs were significantly higher in the surgical arm compared to rehabilitation arm (£1612, p < 0.001). In the surgical arm, hospitalisation costs were higher in the first 6 months (£1392, p < 0.001) but lower between 12 and 18 months (−£180, p = 0.016). In the rehabilitation arm, physiotherapy costs were higher in the first 6 months (£128, p < 0.001) but lower between 6 and 12 months (−£81, p = 0.003). Private healthcare costs were substantially smaller than NHS costs in all periods with no significant differences between trial arms.
| Cost category | Baseline to 6 months | 6–12 months | 12–18 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | Rehabilitation | Differencea | Surgery | Rehabilitation | Differencea | Surgery | Rehabilitation | Differencea | |||||||
| n | Mean (SD) | n | Mean (SD) | (Surgical vs. rehabilitation) | n | Mean (SD) | n | Mean (SD) | (Surgical vs. rehabilitation) | n | Mean (SD) | n | Mean (SD) | (Surgical vs. rehabilitation) | |
| Total NHS costs | 88 | 2680 (1805) | 93 | 1068 (1196) | 1612 (1225 to 1999) | 87 | 765 (1256) | 78 | 671 (1144) | 93 (−190 to 377) | 127 | 146 (375) | 120 | 330 (803) | −184 (−39 to −28) |
| Hospital admissions (total) | 155 | 1803 (1637) | 159 | 411 (1028) | 1392 (1092 to 1693) | 155 | 456 (1161) | 159 | 518 (1190) | −62 (−356 to 231) | 155 | 34 (301) | 159 | 214 (768) | −180 (−325 to −34) |
| ACLR | 155 | 1736 (1649) | 159 | 394 (1013) | 1342 (1029 to 1656) | 155 | 402 (1131) | 159 | 518 (1190) | −116 (−395 to 163) | 155 | 34 (301) | 159 | 214 (768) | −180 (−325 to −34) |
| Other admissions | 155 | 67 (373) | 159 | 17 (211) | 50 (−17 to 117) | 155 | 54 (337) | 159 | 0 (0) | 54 (8 to 100) | 155 | 0 (0) | 159 | 0 (0) | - |
| Physiotherapy sessions | 152 | 225 (279) | 159 | 354 (313) | −128 (−200 to −56) | 152 | 217 (332) | 156 | 138 (263) | 81 (28 to 134) | 153 | 64 (150) | 159 | 52 (132) | 13 (−16 to 42) |
| Primary care | 88 | 41 (75) | 93 | 23 (62) | 17 (−4 to 39) | 87 | 15 (34) | 78 | 10 (28) | 5 (−1 to 10) | 127 | 8 (25) | 120 | 13 (40) | −5 (−11 to 0) |
| Outpatient care | 88 | 189 (262) | 93 | 147 (231) | 43 (−49 to 134) | 87 | 52 (135) | 78 | 84 (188) | −36 (−78 to 6) | 127 | 18 (56) | 120 | 49 (117) | −33 (−51 to −14) |
| Other healthcare contacts | 88 | 39 (106) | 93 | 57 (192) | −18 (−74 to 37) | 87 | 2 (20) | 78 | 31 (127) | −29 (−53 to −6) | 127 | 4 (36) | 120 | 26 (95) | −22 (−40 to −5) |
| Total private health care | 88 | 53 (151) | 93 | 43 (142) | 9 (−35 to 52) | 87 | 38 (133) | 78 | 31 (112) | −6 (−50 to 37) | 127 | 76 (356) | 120 | 106 (455) | −30 (−106 to 45) |
| Total health care (NHS and private) | 88 | 2733 (1828) | 93 | 1111 (1220) | 1622 (1233 to 2011) | 87 | 803 (1285) | 78 | 702 (1195) | 100 (−193 to 394) | 127 | 222 (509) | 120 | 436 (913) | −214 (−400 to −28) |
EuroQol-5 dimensions utility during follow-up
Table 26 presents EQ-5D utility scores and differences by treatment allocation at each time point. EQ-5D scores improved between baseline and 18 months. Participants in the surgical arm reported significantly higher EQ-5D scores 12-month follow-up compared to the rehabilitation arm (p = 0.028). The distribution of responses to each EQ-5D domain at each follow-up point is presented by treatment allocation in Table 35 (see Appendix 3).
| Follow-up time | Surgical reconstruction | Rehabilitation | Differencea (surgical vs. rehab) |
||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | ||
| EQ-5D-5L utility | |||||
| Baseline | 156 | 0.558 (0.252) | 159 | 0.568 (0.258) | |
| 6 months | 85 | 0.642 (0.232) | 89 | 0.642 (0.271) | 0.016 (−0.068 to 0.100) |
| 12 months | 84 | 0.781 (0.175) | 75 | 0.730 (0.243) | 0.067 (0.007 to 0.127) |
| 18 months | 115 | 0.766 (0.227) | 116 | 0.724 (0.244) | 0.052 (−0.012 to 0.117) |
| EQ-5D-VAS | |||||
| Baseline | 154 | 64.2 (20.8) | 157 | 68.4 (20.5) | |
| 6 months | 84 | 69.8 (18.0) | 89 | 67.6 (19.2) | 2.9 (−0.2 to 6.0) |
| 12 months | 84 | 75.5 (17.1) | 75 | 75.9 (18.5) | −0.4 (−5.2 to 4.4) |
| 18 months | 114 | 77.7 (16.3) | 113 | 75.9 (16.2) | 3.5 (−1.2 to 8.1) |
Within-trial cost-effectiveness analysis
Table 27 shows the main analysis results at 18 months (see Appendix 3, Table 36 for descriptive statistics on costs and QoL at each follow-up point following multiple imputation). Participants in the surgical arm reported higher QALYs compared to rehabilitation, but the difference was not statistically significant (0.052; p = 0.177). Total NHS costs were larger in the surgical arm (£1017; p < 0.001), as a result of higher hospitalisation costs (£1150; p < 0.001).
| Surgical reconstruction (n = 156) | Rehabilitation (n = 160) | Differencea (surgical vs. rehabilitation) | |
|---|---|---|---|
| N | - | ||
| QALYs | 1.03 (0.02) | 0.98 (0.03) | 0.05 (−0.03 to 0.13) |
| Total NHS costs | £3186 (155) | £2169 (141) | £1017 (557 to 1476) |
| Hospital admissions | £2287 (122) | £1138 (118) | £1150 (773 to 1523) |
| Rehabilitation sessions | £510 (45) | £550 (40) | −£40 (−171 to 90) |
| Total private health care | £197 (44) | £191 (48) | £6 (−77 to 90) |
| Total healthcare costs (NHS and private) | £3383 (156) | £2360 (147) | £1023 (538 to 1508) |
| ICERsb | |||
| NHS costs only | - | - | £19,346 |
| Total healthcare costs (NHS and private) | - | - | £19,473 |
| Probability that surgical management is the most cost-effective option | |||
| At £20,000 per QALY (NHS costs only) | 51% | ||
| At £30,000 per QALY (NHS costs only) | 72% | ||
Adopting an NHS perspective, the ICER for surgical management programme versus rehabilitation was £19,346 per QALY gain, below the standard threshold for cost-effectiveness in the UK (£20,000–30,000 per QALY gain). Similar results were derived when using all healthcare costs (NHS and private), with the ICER for surgical management versus rehabilitation being £19,473.
Figure 6 presents the cost-effectiveness scatter plot giving differences in mean total costs and QALYs for surgical versus rehabilitation management adopting the NHS health and social care perspective. Most bootstrap replicates remained largely in the north-west quadrant of the cost-effectiveness scatter plot, indicating that surgical management resulted in higher QALYs but and also higher costs relative to rehabilitation. Figure 7 presents the cost-effectiveness acceptability curve, which gives the probability that surgical management is cost-effective compared to rehabilitation for different threshold values for a QALY (from £0 to £100,000 per QALY). Adopting an NHS or a healthcare cost perspective, the probability that surgical management is cost-effective is 51% and 72% at a threshold value of £20,000 and £30,000 per QALY, respectively.
FIGURE 6.
Cost-effective scatter plot for the base-case analysis. Scatter plot of estimated joint density of incremental costs and QALYs of surgical management relative to rehabilitation obtained by bootstrap resampling from each of the 30 imputed datasets, running the regression models on each bootstrapped dataset and extracting the estimated incremental costs and QALYs. Dashed lines represent threshold values of £20,000 and £30,000 per QALY gained. Bootstrapped results falling below the lines are deemed cost-effective. From the bootstrapped results, we calculated the probability that surgical management was more cost-effective than rehabilitation for different threshold values per QALY gained.

FIGURE 7.
Cost-effectiveness acceptability curve. Figure plots the probability (y-axis) that surgical management is cost-effective compared to rehabilitation for different willingness-to-pay thresholds per QALY gain (x-axis). Probability captures the joint uncertainty in incremental costs and QALYs of surgical management compared to rehabilitation and was obtained by estimating the proportion of bootstrapped results that were cost-effective for each threshold value. The interpretation is that, given a willingness-to-pay threshold of £30,000 per QALY gained, the probability that surgical management is cost-effective compared to rehabilitation is 0.72.

Subgroup analysis
Table 28 presents cost-effectiveness results at 18 months by subgroups of participants. There is considerable variation in the cost-effectiveness estimates between participant subgroups by age and physical activity prior to injury. Adopting a £30,000 per QALY threshold, surgical management was cost-effective for participants under 40 years of age and for those with high level of physical activity prior to injury. All subgroups by baseline KOOS4 and sex reported ICERs below the £30,000 per QALY threshold.
| N | NHS costs | QALYs | ICER | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Surg | Rehab | Surg | Rehab | Difference | Surg | Rehab | Difference | ||
| All participants | 156 | 160 | £3186 (155) | £2169 (141) | £1017 (557–1476) | 1.03 (0.02) | 0.98 (0.03) | 0.05 (−0.03 to 0.13) | £19,346 |
| Sex | |||||||||
| Female | 46 | 61 | £3776 (279) | £2123 (230) | £1653 (842–2464) | 1.00 (0.04) | 0.97 (0.04) | 0.07 (−0.06 to 0.20) | £23,369 |
| Male | 110 | 99 | £2939 (181) | £2198 (180) | £741 (213–1270) | 1.04 (0.03) | 0.98 (0.03) | 0.05 (−0.04 to 0.13) | £15,501 |
| Age at baseline | |||||||||
| < 40 | 114 | 122 | £3093 (174) | £2154 (163) | £939 (413–1466) | 1.04 (0.02) | 0.97 (0.03) | 0.07 (−0.02 to 0.15) | £13,597 |
| 40 or older | 42 | 38 | £3438 (325) | £2218 (284) | £1219 (144–2295) | 1.01 (0.04) | 0.97 (0.04) | < 0.01 (−0.13 to 0.14) | £313,069 |
| Baseline KOOS4 | |||||||||
| < 30 | 40 | 36 | £3471 (250) | £2363 (266) | £1108 (456–1760) | 0.93 (0.04) | 0.79 (0.05) | 0.11 (−0.07 to 0.29) | £10,047 |
| 30 or higher | 116 | 124 | £3087 (188) | £2113 (165) | £975 (379–1570) | 1.06 (0.02) | 1.03 (0.03) | 0.03 (−0.03 to 0.10) | £27,849 |
| Activity prior injury (modified Tegner score) | |||||||||
| Moderate/low | 17 | 24 | £4120 (548) | £1644 (377) | £2476 (1336–3615) | 0.95 (0.07) | 0.90 (0.06) | 0.06 (−0.15 to 0.27) | £43,416 |
| High | 139 | 136 | £3078 (159) | £2262 (151) | £810 (337–1283) | 1.04 (0.02) | 0.98 (0.03) | 0.05 (−0.02 to 0.13) | £15,262 |
COVID impact
Of the total sample, 157 completed the 18-month follow-up after 23 March 2020 and 159 completed it before this date. Table 29 reports the total NHS costs and QALYs for these two subgroups of participants. Before the lockdown date, the difference in QALYs between trial arms was higher (0.09, p = 0.080) compared to after the lockdown date (0.02, p = 0.668), albeit there were no significant differences. In terms of difference in NHS costs, this was smaller before the lockdown date (£925, p = 0.001) compared to after the lockdown date (£1101, p = 0.003). These differences translated into an ICER of £10,782 using the participants who completed the 18-month follow-up before 23 March 2020 and an ICER of £54,789 using participants who completed it after this date.
| Follow-up time | N | NHS costs | QALYs | ICER | |||||
|---|---|---|---|---|---|---|---|---|---|
| Surg | Rehab | Surg | Rehab | Difference | Surg | Rehab | Difference | ||
| All participants | 156 | 160 | £3186 (155) | £2169 (141) | £1017 (557–1476) | 1.03 (0.02) | 0.98 (0.03) | 0.05 (−0.03 to 0.13) | £19,346 |
| Before 23 March 2020 | 80 | 79 | £3251 (222) | £2326 (210) | £925 (417–1434) | 1.06 (0.03) | 0.97 (0.03) | 0.09 (−0.01 to 0.18) | £10,782 |
| After 23 March 2020 | 76 | 81 | £3117 (214) | £2016 (187) | £1101 (412–1789) | 0.99 (0.03) | 0.99 (0.04) | 0.02 (−0.08 to 0.12) | £54,789 |
Cost-effectiveness discussion
Over 18 months of follow-up in the ACL SNNAP trial, we found that surgical management led to improved health-related QoL compared to rehabilitation management but with higher healthcare costs. Using £20,000–30,000 per QALY thresholds, we report surgical management to be cost-effective in the UK setting. The probability that surgical management is the most cost-effective option was 51% and 72% at £20,000 and £30,000 per QALY gained, respectively. This is the first study estimating the effectiveness and cost effectiveness of two common interventions for non-acute ACL-injured knees.
Recent research comparing surgical management with rehabilitation in individuals with recent ACL injury (< 2 months) concluded that early ACLR was not cost-effective. 26 The study, conducted in the Netherlands, comprised an economic evaluation alongside a clinical trial where participants were followed up for 2 years. Consistent with our findings, the authors reported early reconstruction to be more effective (0.04 QALYs) but more costly than rehabilitation. The difference in costs between the two trial arms was considerably higher than what we found in the ACL SNNAP trial. This is partially explained by the larger proportion of participants undergoing surgery in the Dutch study compared to the UK study (96% vs. 78%) among those allocated to surgical management. Surgery reconstruction costs accounted largely for the difference in costs observed between the trial arms in both trials.
When trial participants were divided into those completing the trial prior to or after the UK national lockdown (23 March 2020), we found differences in the QALY gains associated with the surgical management arm. These resulted in surgical management being cost-effective in the cohort of participants that completed the trial prior to national lockdown but not cost-effective in those who completed it after the national lockdown. The number of reported rehabilitation sessions among those allocated to the surgical arm was significantly lower (four fewer sessions per individual) for those who completed the trial after the lockdown. There was no significant difference in rehabilitation sessions for those allocated to the rehabilitation arm.
Our cost-effectiveness analysis is based on the largest randomised trial comparison of surgical and non-surgical management of non-acute ACL in the world. The analysis has several limitations, including the sizeable amount of missing data on use of healthcare resources and EQ-5D-5L. We accounted for this using multiple imputation. 61 This assumes data are MAR conditional on modelled covariates and we found no strong evidence to contradict this assumption. The trial was not powered to estimate differences in incremental costs and QALYs, which may explain the probability of surgical management being cost-effective of 51% and 72% at £20,000–30,000 per QALY gained thresholds, respectively. However, with an ICER below £20,000 per QALY, surgical management is cost-effective and should be recommended for implementation relative to non-surgical management. In addition, results were calculated over only 18 months and longer follow-up could confirm whether the observed differences in costs and QoL are maintained. Finally, the trial amendment resulted in the exclusion of data concerning medication use, equipment, informal care and productivity losses. Productivity costs are likely to be high in the participants of ACL SNNAP given their age and working status. Further research would be needed to ascertain differences between the two groups.
Chapter 6 Discussion and conclusion
Research question
Anterior cruciate ligament SNNAP was a large, multicentre, parallel-group, superiority RCT designed to assess the clinical and cost effectiveness of surgical management compared to initial non-surgical management (and subsequent surgery if necessary) in patients with non-acute more long-standing ACL injuries. The research question arose due to uncertainty around the best treatment for patients with a damaged ACL, particularly in patients who, in the NHS, presented late with their injury and therefore diagnosis. Previous work16,25 had already ascertained that it was beneficial to prescribe remedial exercises and undergo rehabilitation prior to surgical reconstruction, but these findings could not be applied to the less acute population as often seen in the NHS. While the findings and approach still hold for acute patients and may be appropriate for more long-standing patients, clinical anecdote suggested that patients who had been ACL deficient for longer may have already achieved their maximum level of stability with self-exercise or normal activities. An additional round of rehabilitation would therefore likely offer no further benefit. Accordingly, there were two clear treatment pathways to evaluate: firstly, immediate surgical reconstruction as soon as possible after diagnosis and, secondly, rehabilitation, followed by a surgical reconstruction option if the rehabilitation treatment failed to stabilise the knee sufficiently.
Design of the study
For these reasons, a management-style study design was selected. The two-group multi-intervention design allowed for patients in the rehabilitation arm to legitimately undergo surgical reconstruction if necessary. In a standard two-group head-to-head treatment comparison trial, this would be designated as crossover.
The trial, as all non-surgery versus surgery study designs, could not be blinded in any meaningful way as treatments were entirely disparate and the primary outcome was self-reported.
The end point of final KOOS4 score at 18 months was compared irrespective of how many patients underwent subsequent reconstruction. However, these data were still important and collected. In the event of no significant differences between treatment groups, the percentage of patients ‘failing’ rehabilitation and requiring surgical reconstruction would have become a more important variable.
One other feature of the trial that was accounted for in a comprehensive analysis plan was the fidelity and compliance of the interventions, particularly the non-surgical care. It was apparent from the outset that compliance to the rehabilitation programme had the potential to directly affect interpretation. For this reason, compliance data were collected and sub-categorisations made based on compliance/fidelity levels for insertion into different analyses. As there was a clear result from the trial, these multiple PP and compliance-based analyses were not needed for the main question but still provided a valuable insight into ACL rehabilitation compliance in a pragmatic setting.
Summary of main findings
The trial found that both groups improved over time but patients in the surgical management group had substantially better outcomes at 18 months post randomisation than those in the non-surgical management group. For the ITT population, there was a statistically significant difference between the groups for the KOOS4 primary outcome at the 18-month time point in favour of surgical management. This was supported by an AUC analysis over the duration of the study, as well as the analyses on each of the PP populations and CACE analysis which sought to compensate for non-compliance. The main result was also robust when investigating assumptions around missing data. Exploratory subgroups’ analyses suggested benefit for surgical management by age, gender, KOOS4 baseline score and Tegner Activity Score. There was a possible suggestion that those who had lower KOOS4 score at baseline and lower activity on Tegner might get more benefit.
The secondary outcome measures also generally favoured surgical management over initial non-surgical management. There were statistically significant differences shown for each of the KOOS domains, Tegner Activity Score, ACL-QoL and patient satisfaction. A higher proportion of patients said they would choose the same treatment again if they could go back in time in the surgical management group compared to the rehabilitation management group. No difference was found between the groups for the EQ-5D-5L outcome index with the surgical group having a slightly higher observed value, and there were a relatively low number of clinical events recorded in both treatment groups.
Due to the complex nature of the interventions, there were various treatment pathways participants randomised to each group could have taken. Of those randomised to surgical management, 110 out of 156 (71%) had surgery and completed their postoperative rehabilitation, which was below the level expected. Sixty-one out of 160 (38%) patients randomised to non-surgical management completed their rehabilitation without undergoing surgery. Of 160 patients, 65 (40.6%) in the non-surgical group underwent surgery, although this was allowed for in the study protocol.
A subsequent analysis that split the rehabilitation group into those who completed the rehabilitation management in full and those in that allocation who required subsequent surgery because of continued persistent symptoms revealed interesting findings. Patients in the rehabilitation only group (not requiring subsequent reconstruction) improved quickly (as per KOOS4 score) but then rapidly plateaued ending up some 12 points below the surgical group. In contrast, the patients who underwent subsequent reconstruction surgery after failed rehabilitation were slow to progress initially (presumably because of a continuation of instability) but had a final outcome (at 18 months) very similar to the allocated surgery group. This latter pattern in these patients is somewhat expected, as essentially effective treatment is ‘delayed’.
Recruitment and conduct
In the early part of the study, it was found that recruitment was slow and investigations were performed to explore this feature. The screening data collected were invaluable for this exploration and were a significant learning point for the trial. The detail and comprehensive nature of the screening data greatly assisted with re-establishing appropriate recruitment. Recruitment was inhibited for several reasons:
-
lack of equipoise of patients
-
lack of equipoise of clinicians (surgeons)
-
eligibility and inclusion criteria features, including comorbidity and time since injury
-
patient pathway issues at some sites where patients had already undertaken formal rehabilitation interventions.
Equipoise in patients
Many patients were found to have difficulties with equipoise. The main bias was towards surgical reconstruction with the final recruitment characteristics clearly showing this phenomenon. There was a large percentage of patients not enrolling due to having a preference for surgery despite being eligible (276/485). Conversely, 115 patients from 485 (eligible but declined) preferred the non-operative management.
Although a lot of the bias was generated from media influence, friends and family and those who had undergone either successful or unsuccessful treatment in the past, some education in clinic was found to help with this equipoise problem. Efforts were made early in the trial to explain and clarify the uncertainty that existed around the benefit for both treatment options. Additional educational input was given to sites to this aim. The chief investigator presented on several occasions at knee meetings and trial meetings on this subject.
Equipoise in clinicians
This was largely an issue for the surgical community where the main preference was for surgical intervention. This was expected, as a profession trained to perform surgical procedures will naturally want to employ these skills, efforts were made to minimise this effect. Surgeons were encouraged to recognise their own lack of equipoise and therefore deem themselves not suitable as a recruiting surgeon/site. In those who expressed equipoise, continual reinforcement of this position and the importance of upholding this position for the benefit of the trial were required.
Eligibility and inclusion criteria
Early in the trial, it was found from screening data that comorbidity resulted in many patient exclusions. Specifically meniscal pathology (148 from 602 patients deemed not eligible) and MCL injury pathology (55 from 602 patients deemed not eligible). After consensus meetings, these criteria were relaxed to include patients who had non-serious meniscal injury or MCL not requiring urgent surgery. This added to the pragmatic nature of the study and represented usual practice.
The acuteness of injury was also found to be a reason for non-eligibility of patients with several centres seeing patients earlier in their clinical pathway than anticipated. It was not known if this was a manifestation of running a national trial where heightened awareness can often induce a change in practice before any results are known. To address this, the acuteness boundary was relaxed which improved recruitment. The 81 patients deemed non-eligible for an acute injury were excluded early in the trial. The guidance provided to recruiting centres to address the issue was to exclude patients only if their acute episode had not settled (around 3–4 weeks). The prior strict 4-month boundary for acuteness was removed. This necessary amendment is discussed further in Patient characteristics section and can be considered a limitation and is discussed in the Limitations section.
Patient pathway and prior treatment
It was discovered that several potential patients were attending screening visits having already undertaken a comprehensive rehabilitation programme (and therefore rendering themselves not eligible). Sometimes this non-surgical management had been set up on exposure, advertising and discussion about the study. Verbal discussion was undertaken with such sites to see if the treatment pathways could be restored to pre-trial existence status. The justification for this rewind (to potential sites) was that any such change in treatment approach had not been made with any new evidence (notably the very reason for the ACL SNNAP study). This resetting of pathways was successful in some sites, but not in others where the adoption of early decision-making (without evidence to support) had become more established and mature. This was another trial learning point and showed how evaluation pieces (such as RCTs) can have strong effects and influences even before they are concluded.
Data collection platform
This was one of the first trials (within SITU) to engage in electronic data collection (EDC) methods. It was thought that the young and mobile ACL-injured population would welcome and respond well to mobile and computer-based platforms to respond to questionnaires such as the KOOS4 score. The trial team linked with appropriate IT and PROMS collection expertise and engaged F3dom Ltd (subsequently PRO-Mapp Ltd) to design a dedicated randomisation and data collection platform for ACL SNNAP.
The final platform allowed baseline data to be collected on tablets and on clinic computers. Patients could then be randomised by a computerised randomisation system with appropriate stratification variables. The validity of the randomisation was checked on several occasions and by the quality assurance processes within the host clinical trial unit, OCTRU.
Overall, the system was largely successful. However, the engagement with EDC was not as strong as expected, especially with follow-up. Both paper and telephone follow-up consultations were required to complete the study. Appendix 3, Table 37 shows that at 18 months, the respective % returns from online, paper/post and phone surveys were 32%, 39% and 28%, respectively. Note the trial mostly pre-dated COVID and sudden increase in remote data collection methods. A trial started today may have enjoyed a better EDC rate of return due to the increased comfort of virtual, mobile and electronic data transference.
Patient characteristics
Three hundred and sixteen patients with an ACL injury were recruited over 3 years. It is the largest RCT to compare a surgical management with ACLR of the ACL versus initial rehabilitation (and subsequent surgery when necessary) in a routine clinical setting. Baseline demographics were generally very similar at baseline as anticipated given randomisation and particularly for the stratification factors (site and KOOS4 at baseline). There was a small gender imbalance with more women in the non-surgical management group. The average age of the sample was 33 years.
The environment, cause or mechanism of injury showed that the highest percentage of patients in the sample had footballing injuries (n = 121, 38%). High percentages of patients also damaged their knee twisting or falling (15%), in rugby (11%) and snow sports (10%). In the UK, it is thought that this pattern of injury mechanism is generalisable. 66
The change in protocol to allow a wider acuteness boundary resulted in the recruitment of 108 patients (34%) who had sustained their injury inside 4 months previously. The remaining 66% all sustained injury longer than 4 months previously. Twenty-two per cent of patients had sustained their injury longer than a year before recruitment. While the original intention was to explore a highly ‘chronic’ and long-standing population, the trial conduct constraints precipitated some protocol changes to allow a less long-standing injury and full recruitment within the given time. While the change is listed as a limitation, the patients remained non-acute by definition, and therefore the population under evaluation remained valid and for the desired population for the research question, albeit slightly less ‘chronic’. A key interpretative point is to examine how this shift may have influenced the results and interpretation of the trial. The modified inclusion criteria created a population that would more likely benefit from rehabilitation (according to the Frobell findings and recommendations). The change would therefore have served to potentially dilute or moderate any results in favour of surgical management. However, the highly significant findings in favour of surgical management showed the converse to be true and the influence of any adjustment and amendment can be considered negligible.
The baseline characteristics of the population were unremarkable and expected. The overall KOOS4 score was 44.5/100. The patients were predominantly active with 86% of the sample having a Tegner Activity Score above level 5 prior to injury. This dropped to 8% at recruitment to the study. These results show the remarkable drop-off in activity and consequence of ACL injury.
Distribution of treatment received
The adherence (compliance) to allocation and treatment received was reviewed.
The findings for adherence and fidelity were of interest. There was substantial non-adherence in the surgical group, with only 72% of participants receiving ACLR as per allocation, including patients who ultimately declined surgery for work duties (self-employed) or an improvement in their condition. Twenty-eight per cent of patients did not have surgery for a variety of reasons. Seven per cent of patients in the surgical management group were still awaiting surgery at the final follow-up point, and 10% of patients in this group did not have surgery for unknown reasons. Despite this, the ITT analysis still provided a clear indicator of superiority for surgical management.
A substantial 41% of patients allocated to the rehabilitation arm underwent surgery following failure to stabilise the knee by rehabilitation alone. The conversion to surgery was expected and in line with the protocol, but the magnitude of conversion could not be predicted in this non-acute population. The conversion rate at around 2 years follow-up (41%) seen in SNNAP was slightly higher than expected in a non-acute population (39%)16,25 but slightly lower than the 50% reported by Reijman et al. 17 However, without examining outcome scores, it shows there is much uncertainty for individual patients around two very disparate treatment choices, surgical or non-surgical treatment. The high percentage of failure for rehabilitation management probably reflects the limited capability of remedial exercises to stabilise an unstable joint, especially for high levels of activity. This is not a new phenomenon and the characteristics of what allows some patients, and not others, to ‘cope’ without a ligament has not yet been fully delineated despite many attempts. 67
The data for partial and lower-level adherence in rehabilitation group patients were varied, with 16% of these patients starting rehabilitation, but it was unknown how much of the intervention they completed, and 6% having no record of starting rehabilitation or receiving surgery. This shows the difficulties in collecting and validating these types of data.
Of those patients who went on to have surgery after a course of rehabilitation, 16% did so very early on or had very little rehabilitation treatment and 24% completed the full course of therapy but still had an unstable knee at the end of treatment. These patients with ongoing instability (in consideration of the primary and secondary outcome results) probably reflect the overall results and again, the relative ineffectiveness of non-surgical management to stabilise the unstable knee. Nevertheless, it is important to highlight that many patients in the rehabilitation group did not undergo reconstruction and achieved knee stability without surgery. Hence, rehabilitation cannot be dismissed as a viable option and this point should be emphasised. It should be remembered that a trial is comparison and presentation of averages and does not allow or account for individual benefits. Despite evidence of superiority for one management strategy, both treatment groups did improve over time.
Overall, a similar proportion of participants complied with the randomised allocation (in some way) in the non-surgical management group (78%) and surgical group (72%). Various factors will have contributed to the completion of both groups’ management, including the impact of COVID-19 pandemic on scheduling of surgery and provision of rehabilitation, which included ‘virtual’ physiotherapy sessions in some cases. Thematic analysis of information for non-adherence in the PCRFs found that the most frequently reported reasons for non-adherence were as follows: patients not being satisfied with their progress with rehabilitation (26%), reinjury or flare-up of knee pain or instability (17%), difficulties with scheduling, child care, or getting to physiotherapy (15%), the COVID-19 pandemic (9%), participants having difficulty performing rehabilitation exercises (7%) and patient illness (6%). The cause of poor or non-adherence was unknown in 20% of those cases. In the surgical reconstruction group, the reason for not undergoing surgery was unknown in 32% of patients, the COVID-19 pandemic (14%), work commitments (7%) and participants moving out of the area (7%).
These adherence patterns demonstrated how difficult it can be to enforce rehabilitation management in pragmatic trials. Even in a high-profile trial to address a lifestyle affecting pathology, such as in ACL SNNAP, the uptake is often poor. The pragmatic nature of the trial helped give a true reflection of what could be expected in normal NHS practice. The results cannot be extrapolated to high-level sports or high-intensity rehabilitation that is sometimes offered for elite athletes. The trial assessed adequate-quality rehabilitation provision only.
Compliance and fidelity
A more detailed analysis of the compliance to the management interventions was conducted and showed that compliance was asymmetrical, being more of a problem for the rehabilitation group. Compliance (adherence) in the rehabilitation arm was defined as participants completing sufficient physiotherapy over a period of at least a 90-day time span. Compliance in the surgical arm was defined as participants undergoing surgical reconstruction and postsurgical rehabilitation over at least a 5-month time span. This revealed that adherence (compliance) to treatment was higher in the surgical arm: 110 of 156 participants completed surgical reconstruction and were compliant with postsurgical rehabilitation for a compliance rate of 70%, while compliance was 59% in the rehabilitation arm (see Table 23). Compliance to intervention allocation was higher in the rehabilitation arm (78%) than in the surgical arm (72%) (see Table 23).
Intervention fidelity is the similarity of the interventions delivered in the trial to interventions specified in the protocol. 56 In the rehabilitation arm, this was defined as participants’ physiotherapy programmes including a progression of therapeutic activities towards those targeting return to sports or functional activities.
Despite stipulating senior rehabilitation staff were required, in reality this did not always occur and the breakdown on grades (see Table 8) shows that some staff delivering the rehabilitation intervention were less senior and not especially well aligned to the protocol. It is hoped that the more junior staff had some level of supervision, again as stipulated in the protocol.
Safety
The trial did not investigate a life-threatening condition, but there were some aspects of safety to be addressed. A concern when designing the study was that a significant number of patients in the rehabilitation group, while performing exercise on an unstable knee, would have episodes of instability and sustain secondary damage to their menisci. The outcome for an ACL-injured knee with concomitant meniscal damage is potentially suboptimal. However, this did not occur. Only four patients in total had acquired meniscal damage (1%): three in the rehabilitation arm and one in the surgical arm.
Methodological points
The three pre-specified analyses to account for potential non-compliance showed surprising consistency in terms of indicating a benefit for surgical management over initial rehabilitation management in terms of KOOS4 outcome at 18 months. None of the three analyses led to a different overall conclusion with as large or larger gain in KOOS4 for surgical management estimated. It could be argued that these pre-set analyses were not needed in the light of the primary outcome results analysed as ITT.
The increase in KOOS4 over time supports the interpretation that reconstruction provides a greater benefit even if received some time later with the increase in the surgical groups. However, it is worth noting that the very large effect from the CACE will be overly optimistic, in that only non-compliance to surgery is considered and a subset of these patients will not need surgery. Eleven per cent in the surgical management group ended up undergoing rehabilitation and did not have surgery. This may be representative of real life in which a number of listed patients for ACLR do not always complete the surgery.
We also looked at the potential impact of missing data given the sizeable missing data observed at 18 months. The method we used was a pattern-mixture model-based approach where the magnitude of difference in the outcome between those observed and those missing can be specified. We assessed the impact of a difference in one group or both over a reasonable range of values (5-point difference in either direction). The findings were remarkably consistent and did not indicate sensitivity to missing data in terms of the overall conclusion.
Health economics
Over 18 months of follow-up in the ACL SNNAP trial, we found that surgical management led to improved health-related QoL compared to the rehabilitation management but with higher healthcare costs. Using £20,000–30,000 per QALY thresholds, we report surgical management to be cost-effective in the UK setting. The probability that surgical management is the most cost-effective option was 51% and 72% at £20,000 and £30,000 per QALY gained, respectively. This is the first study estimating the effectiveness and cost effectiveness of two common interventions for non-acute ACL-injured knees.
Recent research comparing surgical management with rehabilitation in individuals with recent ACL injury (< 2 months) concluded that early ACLR was not cost-effective. 26 The study, conducted in the Netherlands, comprised an economic evaluation alongside a clinical trial where participants were followed up for 2 years. Consistent with our findings, the authors reported early reconstruction to be more effective (0.04 QALYs) but more costly than rehabilitation. The difference in costs between the two trial arms was considerably higher than what we found in the ACL SNNAP trial. This is partially explained by the larger proportion of participants undergoing surgery in the Dutch study compared to the UK study (96% vs. 78%) among those allocated to surgical management. Surgery reconstruction costs accounted largely for the difference in costs observed between the trial arms in both trials.
When trial participants were divided into those completing the trial prior to or after the UK national lockdown (23 March 2020), we found differences in the QALY gains associated with the surgical management arm. These resulted in surgical management being cost-effective in the cohort of participants that completed the trial prior to national lockdown but not cost-effective in those who completed it after the national lockdown. The number of reported rehabilitation sessions among those allocated to the surgical arm was significantly lower (four fewer sessions per individual) for those who completed the trial after the lockdown. There was no significant difference in rehabilitation sessions for those allocated to the rehabilitation arm.
Our cost-effectiveness analysis is based on the largest randomised trial comparison of surgical and rehabilitation management of non-acute ACL in the world. The analysis has several limitations, including the sizeable amount of missing data on use of healthcare resources and EQ-5D-5L. We accounted for this using multiple imputation. 61 This assumes data are MAR conditional on modelled covariates and we found no strong evidence to contradict this assumption. The trial was not powered to estimate differences in incremental costs and QALYs, which may explain the probability of surgical management being cost-effective of 51% and 72% at £20,000–30,000 per QALY gained thresholds, respectively. However, with an ICER below £20,000 per QALY, surgical management is cost-effective and should be recommended for implementation relative to non-surgical management. In addition, results were calculated over only 18 months, and longer follow-up could confirm whether the observed differences in costs and QoL are maintained. Finally, the trial amendment resulted in the exclusion of data concerning medication use, equipment, informal care and productivity losses. Productivity costs are likely to be high in the participants of ACL SNNAP given their age and working status. Further research would be needed to ascertain differences between the two groups.
Equality, diversity and inclusion
As the trial was embedded in routine NHS care, it was intended to be inclusive (all patients who meet the selection criteria at participating sites were candidates). Outcomes were collected by questionnaire only to reduce participant burden; there were no additional clinical visits to routine care for participants. We maximised methods of follow-up, that is online, postal and phone calls to be inclusive of patients’ preferences for completion.
In addition to study participants, we included a representative sample of clinicians and trial personnel at recruiting sites. The trial was promoted at national conferences to increase awareness of the project and encourage interest and geographic diversity of trial sites. To help remove any potential barriers perceived by inexperienced teams/sites, we emphasised the support available throughout the study from the central study team to enable them to participate. As this was a management study including both rehabilitation exercises (physiotherapy) and surgery, we encouraged co-principal investigators from each discipline to lead the study locally where possible.
Patient and public involvement
Patients contributed to the design of the study, supported the development of the funding proposal and conduct of the study. The proposed research was presented and discussed at a PPI study development meeting supported by the Research Design Service South Central. Early in the project, the PPI group of four patients helped ensure that patient information sheets and report forms were accessible and user-friendly. In addition, the patients from Swansea and Oxford supported work around the testing of the EDC and method of follow-up. A patient representative was an active member of the TSC and, as part of this role, contributed to the monitoring and supervision of the trial progress.
Limitations
Several potential limitations and influences on the study have already been discussed including modifications to the inclusion criteria, issues of equipoise, adherence (compliance) to allocated treatment and fidelity of treatment content. Most of these have been well described and caveats provided to explain why any threat from these factors might be low. There are, however, other aspects that warrant mention.
A surgical versus non-surgical trial, such as this, suffers from an imbalance in sequencing options. Rehabilitation is nearly always considered as the first option and surgery the second. The surgery option is perceived as an escalation in intervention (and therefore can be perceived as having greater a priori efficacy). This has many manifestations. It can produce a perceived suboptimal treatment option in the non-surgical group and create resentful demoralisation, especially if not progressed as soon as expected. We have no evidence or detailed information to describe the effect or influence of this in SNNAP, but it is likely present.
Another potential limitation is the ability to control the professional profiles of clinicians treating patients in the different arms of the study. Unfortunately, we could not describe or enforce any grade or profile of the staff (mainly physiotherapists) treating non-surgical patients a priori, as was possible for surgeons. This information could only be obtained post study and is presented in Results section (see Table 8). Physiotherapy treatment was administered in line with a pragmatic trial design, which resulted in varied experience and expertise. However, a senior physiotherapist was required to overview the treatment regime and provide guidance. This issue is common to most surgery versus non-surgery trials with a surgical operation being highly specialised with risk for serious, even life-threatening complications. Therefore, ACLR should not be carried out by surgeons without the necessary experience, for which it was thought necessary to stipulate for the trial (> 50 operations). The nature of the two main interventions, one a very clearly defined surgical procedure and the other a less stark and defined intervention (rehabilitation) with over 80 different therapeutic interventions, can create difficulties with interpretation. The quality of compliance data then becomes an important factor. Despite detailed recording and reporting for compliance for the rehabilitation arm, it remains a limitation of the RCT. Fortunately, the signal from the ITT analysis was so great in favour of one group that it counteracted this concern and was sufficient to allow confidence in reporting that patients in the surgical arm had greater benefit.
The follow-up, while adequate for power, was not as great as hoped, particularly with early data points. Many strategies were included to improve this, but, as with all surgical trials, solutions to obtaining high rates of follow-up from self-reported instruments remain elusive.
As previously discussed, the change in the injury acuteness of the population should be highlighted. The study set out to examine just long-standing ACL-injured patients but ended up including some patients much closer in time to their original injury.
Strong opinion on the compliance issues from rehabilitation is negated by the pragmatic design. However, it is not possible to say whether the results may have been different if compliance had been higher for the rehabilitation group. As a question we were interested in the real-world delivery and not proof of principle.
Crossover could be considered an issue, but it must be remembered that the change from rehabilitation to surgery was expected and accounted for in the protocol. Several patients in both groups failed to complete treatment, but these were not considered crossovers. The only true crossover patients are those who were allocated to surgery and chose not to undergo reconstruction for various reasons (n = 17). The ITT analysis accounted for all the non-compliance.
It should also be noted that the trial had a substantial number of patients who had a strong preference and were ineligible. The trial results therefore can only apply to patients without preference (in equipoise) over treatment.
Recommendations for future research
The trial was pragmatic and studies to explore the influence of treatment fidelity and compliance, especially in the rehabilitation arm, will be useful. Other recommendations for future research include evaluation of innovative surgical reconstruction or even ligament repair. The best form and content of postoperative rehabilitation following ACLR should also be explored. Future research could also focus on identifying characteristics of those patients who are more likely to benefit from either surgery or rehabilitation. This can be used to support shared decision-making on management and treatment. Further methodology work exploring the challenges in conducting trials comparing surgical versus non-surgical interventions, including patient preference and clinician equipoise, and compliance would also be beneficial. How to obtain improved compliance for rehabilitation (and reporting), perhaps using wearables or assessing other biomarkers, may be of value.
Conclusion and clinical impact
The ACL SNNAP trial showed that, while benefit can be obtained from initial non-surgical treatment (with reconstruction performed only if required), treatment with more immediate surgical reconstruction of the ACL without further rehabilitation provides superior outcome. The null hypothesis was rejected, and the effect size observed for this surgical trial can be considered unusual for a surgical trial exploring true uncertainty.
The superiority of surgical management was shown in self-reported outcome of function and pain, complication rate and all secondary outcomes, including activity level. Interestingly neither group had good evidence of returning to their pre-injury level (28% surgery and 24% rehabilitation). In terms of patient satisfaction, 83% of patients in the surgery group reported they were better than before treatment compared to only 68% in the rehabilitation group. Eighty per cent of patients allocated to the surgical management would have the treatment and management strategy again compared to 61% in the rehabilitation group. Eighteen per cent of patients undergoing initial non-surgical management would not opt for that treatment again. Immediate ACLR management, despite generating higher costs, was shown to be cost-effective in the NHS.
The clinical implications are clear and important. In a shared decision-making setting with patients who have injured their ACL previously and are no longer acute, it should be communicated that ACLR (as soon as possible to stabilise the joint) is likely to provide superior outcome compared to attempting a rehabilitation strategy with subsequent reconstruction, if and when, needed. Patients who do not want surgery (for any reason) should be reassured that they can still improve with non-operative care and the option for later surgical reconstruction remains open.
Pathways being provided for ACL injury should incorporate this evidence and with practice change where appropriate. Sufficient availability and resourcing of ACLR for the non-acute population are warranted. Non-operative management and access to physiotherapy should still remain available, but it may be that efforts for progressing rehabilitation of ACL injury should be directed to postoperative care and return to sport/activity after surgery.
Additional information
Contributions of authors
David J Beard (https://orcid.org/0000-0001-7884-6389) (Professor of Musculoskeletal and Surgical Science) was chief investigator; conceived and planned the study; acted as study lead; led the writing of the report and all aspects of study design, conduct and analysis.
Loretta Davies (https://orcid.org/0000-0002-4721-356X) (Senior Trial Manager, Clinical Trials Unit) contributed to conception, design and conduct; was project manager; and contributed to the writing, editing and review of the report.
Jonathan A Cook (https://orcid.org/0000-0002-4156-6989) (Senior Statistician, Clinical Trials Unit) contributed to the conception, design and conduct; oversaw the statistical analyses, and contributed to the writing, editing and review of the report.
Jamie Stokes (https://orcid.org/0000-0002-5279-2332) (Trial Statistician, Clinical Trials Unit) was the trial statistician, led the design of the statistical analysis plan, undertook the statistical analyses and contributed to the writing of the report.
Jose Leal (https://orcid.org/0000-0001-7870-6730) (Senior Health Economist) provided expertise in health economics, co-designed the health economics analysis, carried out the analysis of the health economics data and contributed to the writing and review of the report.
Heidi Fletcher (https://orcid.org/0000-0002-6518-6024) (Data Manager, Clinical Trials Unit) contributed to the design and management of the trial, data analysis and review of the report.
Simon Abram (https://orcid.org/0000-0002-4452-6499) (Clinical Lecturer in Orthopaedic Surgery) contributed to the design and management of the trial and review of the report.
Katie Chegwin (https://orcid.org/0000-0002-8671-5976) (Trial Administrator) contributed to the management of the trial and review of the report.
Akiko Greshon (https://orcid.org/0000-0002-8006-2943) (Data Management Assistant) contributed to the management of the trial and review of the report.
William Jackson (https://orcid.org/0000-0002-1851-5921) (Consultant in Trauma and Orthopaedic Surgery, Oxford University Hospitals) was a trial co-investigator and contributed to the study design and review of the report.
Nicholas Bottomley (https://orcid.org/0000-0001-8441-630X) (Clinical Lead Consultant in Trauma and Orthopaedic Surgery, Oxford University Hospitals) was the principal investigator for one of the recruiting sites; and contributed to the review of the report.
Matthew Dodd (https://orcid.org/0000-0002-8229-8175) (Consultant in Orthopaedic Surgery, Swansea University Hospitals) was the principal investigator for one the recruiting sites; and contributed to the review of the report.
Henry Bourke (https://orcid.org/0000-0003-3341-8545) (Consultant in Orthopaedic Surgery) was principal investigator for one of the recruiting sites; and contributed to the review of the report.
Beverly A Shirkey (https://orcid.org/0000-0002-4347-6784) (Trial Statistician) was the trial statistician, contributed to the design and conduct and review of the report.
Arsenio Paez (https://orcid.org/0000-0002-7984-514X) (Doctoral Student) contributed to the analysis and interpretation of the compliance data and the write-up and review of the report.
Sarah E Lamb (https://orcid.org/0000-0003-4349-7195) (Professor of Rehabilitation) was a trial co-investigator and contributed to the study design and review of the report.
Karen L Barker (https://orcid.org/0000-0001-9363-0383) (Professor of Physiotherapy) was a trial co-investigator and contributed to study design and review of the report.
Michael Phillips (https://orcid.org/0000-0002-6381-0727) (Founder, Director of PRO-MAPP Ltd) contributed to the study design, data collection processes and review of the report.
Mark Brown (https://orcid.org/0000-0002-9618-2480) (Programming PRO-MAPP Ltd) contributed to the study design, data collection processes and review of the report.
Vanessa Lythe (https://orcid.org/0000-0002-6372-2743) (Health Economics) provided expertise in health economics, carried out the analysis of the health economics data and contributed to the writing of the report.
Burhan Mirza (https://orcid.org/0000-0003-0369-7707) (Health Economics) provided expertise in health economics, carried out the analysis of the health economics data and contributed to the writing of the report.
Andrew Carr (https://orcid.org/0000-0001-5940-1464) (Nuffield Professor of Orthopaedics) was a trial co-investigator and contributed to the study design and review of the report.
Paul Monk (https://orcid.org/0000-0002-1673-2148) (Clinical Lecturer in Orthopaedics) was a trial co-investigator and contributed to the study design and review of the report.
Carlos Morgado Areia (https://orcid.org/0000-0002-4668-7069) (Trial Manager, Clinical Trials Unit) contributed to the design and management of the trial and review of the report.
Sean O’Leary (https://orcid.org/0000-0002-5650-9768) (Consultant in Orthopaedics) was a trial co-investigator, site lead for Reading and contributed to the study design and review of the report.
Fares Haddad (https://orcid.org/0000-0002-6272-0156) (Consultant in Orthopaedics) was a trial co-investigator, site lead for King’s College, London, and contributed to the study design and review of the report.
Chris Wilson (https://orcid.org/0000-0001-5730-0651) (Consultant in Orthopaedics) was a trial co-investigator and contributed to the study design and review of the report.
Andrew Price (https://orcid.org/0000-0002-4258-5866) (Consultant in Orthopaedics) was a trial co-investigator contributed to conception, design and conduct, advised on clinical aspects of trial. Contributed to the writing, editing and critical review of the report.
All authors contributed to the design and development of the trial protocol. All authors read, gave input and approved the final manuscript.
Acknowledgements
ACL SNNAP study group
Trial Management Group
Chief investigator: David Beard.
Trial co-investigators: Karen Barker (Oxford), Helen Campbell (Oxford) (submission of funding application), Andrew Carr (Oxford), Jonathan Cook (Oxford), Loretta Davies (Oxford), Fares Haddad (London), William Jackson (Oxford), Sallie Lamb (Oxford then Exeter), Jose Leal (Oxford), Paul Monk (Oxford), Sean O’Leary (Reading), Andrew Price (Oxford), Chris Wilson (Cardiff).
Research fellows: Simon Abram, Arsenio Páez.
Trial management: Loretta Davies, Carlos Morgado Areia (until October 2018), Jackie Davies (until June 2016).
Trial administration: Katie Chegwin, Jiyang Li (until February 2018).
Data management: Heidi Fletcher, Akiko Greshon.
Database/programming management: Christina Bagg, Mark Brown, Michael Phillips (Fr3dom Limited).
Trial statisticians: Jamie Stokes, Beverly Shirkey (until April 2020).
Health economists: Jose Leal, Vanessa Lythe, Burhan Mirza.
Qualitative researcher: Francine Toye (Oxford).
The study was managed by the Surgical Intervention Trials Unit (SITU) in collaboration with the Oxford Clinical Trials Research Unit (OCTRU).
Research teams
We are grateful to the participants and research teams at collaborating hospital sites.
Basildon and Thurrock University Hospitals NHS Foundation Trust
Laura Haywood, Anne Nicholson, Joanne Riches, Sean Symons (PI), Mark Vertue.
North Bristol NHS Trust
Louay Al Mouazzen, Rachel Bray, Damian Clark, James Coulthard, Tim Holland, Nick Howells, Andrew Jones, Richard Kapur, Alastair Kiszely, Harry Krishnan, Karen MacDonald-Taylor, Jon Manara, James Murray (PI), Corina Negrut, Vishai Pai, Andrew Porteous, Sven Putnis, James Robinson, Shav Rupasinghe, Veenesh Selvaratnam, James Smith, Nick Smith, Jarrad Stevens, Clare Taylor, Anthony Theodorides, Niraj Vetharajan, Helen Vint, Lucy Young.
Gloucestershire Hospitals NHS Foundation Trust
Susan Bullock, Rebecca Cook, Alexander Dodds (PI), Amanda Freeman-Hicks, Paula Hillout.
Royal Cornwall Hospitals NHS Trust
Thomas Cornell, Abbie Coutts, Suzy Dean, Nicki Devooght-Johnson, Emma Ferrell, Eve Fletcher, Chrissie Hall, Benjamin Kent, Sandra Kessly, Robin Kincaid (PI), Mohamed Lazizi, Ahmed Mostafa, Toby Nisbett, Tim Powell, Peter Riddlestone, Andrew Roberton, Jessica Summers, Lucy Whitbread, Belinda Wroath.
Countess of Chester Hospital NHS Foundation Trust
Emma Fenlon, Andrew Hall, Helen Jeffrey, Raghuram Thonse (PI).
University Hospitals Coventry and Warwickshire NHS Trust
Debra Dunne, Andy Metcalfe (PI), Kerri McGowan, Simon Middleton, Feisal Shah, Tim Spalding, Charlie-Marie Suddens, Tamar Sweed, Joanna Teuke, Peter Thompson, David Wright.
Frimley Health NHS Foundation Trust
Justine Amero, Emma Brown, Hugh Chissell, Andrea Croucher, Gareth Dickinson, Catherine Hawkes-Blackburn, Alice Peacocke, Graham Smith (PI), Carol Snipe.
Hull and East Yorkshire Hospitals NHS Trust
Kim Dearnley, Reza Mayahi (PI).
Kings College Hospital NHS Foundation Trust
Barry Andrews (PI), Massimo Barcelona (PI), Hazel Giles, Abdulkerim Gokturk, Paul Harnett, Katie Jeeves, Joyce Kadunyi, Sheena Mendoza, Ines Reichert, Marta Santamaria, Harshinder Virdee.
Leeds Teaching Hospitals NHS Trust
Sanjeev Anand (PI), Nayef Aslam-Pervez, Stephen Draycott, Faye Howarth, Irfan Jina, Niall Maher, Denise Ross (PI), Lindsey Worstenholme.
University Hospitals of Leicester
Abdul Baig, Arun Bhaskaran, Daniel Banks, Tracy Brear, Carla Christie, Laura Cowen, Jack Davis, Ross Dixey, Colin Esler (PI), Amirah Essop-Adam, Christina Haines, Linzy Houchen-Wolloff, Husein Varachia, Richard Wood.
Manchester University NHS Foundation Trust
Glaxy Gray, Jessica Nichols, Alice Panes, Susan Partridge, Lawrie Rogerson, Pankaj Sharma (PI), David Triggs, Ian Venables, Danielle Wilcock.
The Mid Yorkshire Hospitals NHS Trust
Sarah Buckley, Thelma Darian, Elizabeth Denis, Jo Duncan, Charlotte Hirst, James Newman, Fern Richardson, Jon Smith (PI).
Taunton and Somerset NHS Foundation Trust, Musgrove
Megan Adcode, Megan Cottingham, Eliza Foster, Andrew Kelly (PI), Niamh McKay, Jane Rewbury, Alison Whitcher, James Williams, Esther Zebracki.
Betsi Cadwaladr University Health Board, North Wales
Llinos Davies, Jayadeep Jayachandran (PI), Alison Tardivel, Victoria Whitehead.
Oxford University Hospitals NHS Foundation Trust
Simon Abram, Carlos Morgado Areia, Martha Batting, Amy Bond, Nick Bottomley (PI), Loretta Davies, Marc Deakin, Christopher Dodd, Alison Hudak, Samantha Hynes, Will Jackson, Luke Jones, Gail Lang, David McKenna, Susan Morris, Andrew Price, Clare Scott-Dempster, Adam Sykes, Iason Vichos, Simon Wood.
North West Anglia NHS Foundation Trust, Peterborough
Rupert Clifton (PI), Stephanie Diaz, Craig Hendy, Nishil Modi, Brendan O’Mahony, Susan O’Sullivan, Nicola Parker, Mira Pecheva, Rowan Rumonovic.
Solent NHS Trust, Portsmouth
Emma McLoughlin, Jeremy Rushbrook (PI), Anna Thornhill.
Royal Berkshire NHS Foundation Trust
Emily Bannister, Chloe Brown, Debbie Burden, Terence Campbell, Emma Craig, Rashmi Easow, Julie Foxton, Alexandra Hazlerigg, Chethan Jayabev, Rosie Murdoch, Sean O’Leary (PI), Georgie Parsons.
Royal Surrey County Hospitals NHS Foundation Trust
Harry Brown, Paula Carvelli, Rugaia Montaser, Ali Pepper, Sinduja Sivarajan, Oliver Templeton-Ward (PI), Eva Wilson.
Salisbury NHS Foundation Trust
Julie Cronin, Sarah Diment, Victoria King, Katharine Shean, Leonidas Vachtsevanos (PI), Katharine Wilcocks, Ben Wilson.
Sheffield Teaching Hospitals NHS Foundation Trust
Paul McNestry, Joanna Ollerenshaw (PI), James Stoddard, Paul Sutton.
Stockport NHS Foundation Trust
Sanjay Anand, Judith Bell, Albert Chikate, Diane Daniel, Timothy Davies, Tom Finnigan, Antonio Frasquet-Garcia, Susan Hopkins, Sharon Kerrison, Angela McGowan, David Sands Johnson (PI), Lara Smith, Philip Turner, Helen Wilkinson.
Sherwood Forest Hospitals NHS Foundation Trust, Sutton in Ashfield
Lynne Allsop, Deborah Anthony, Rebecca Boulton, Sarah Brown, Vikram Desai (PI), Mandy Gill, Cheryl Heeley, Sushrut Kulkarni, Wayne Lovegrove, Dominic Nash, Terri-Ann Sewell, Sarah Shelton, Katie Slack.
Swansea Bay University Health Board, Swansea
David Beard, James Cartwright, Lynda Connor, Andrew Davies, Caroline Davies, Matthew Dodd (PI), Glyn Gainard, Dave Graham-Woollard, Carl Murphy, Leanne Quinn, Caradog Thomas, Jenny Travers, Marie Williams.
Great Western Hospitals NHS Foundation Trust, Swindon
Amanda Bell, Sunny Deo, Katharine Francis, Tracy Jackson, Laura McCafferty, Basalingappa Navadgi, Karan Plank, Venkat Satish (PI), Claire Thelwall.
University College London Hospitals NHS Foundation Trust
Fares Haddad (PI), Rachel Knight, Rahul Patel, Bruce Paton.
Warrington and Halton Hospitals NHS Foundation Trust
Ashutosh Acharya, Utuman Aland, Miltiades Areirobulos, Pascal de Feyter, Lisa Ditchfield, Hafiz Iqbaz, Daniel Massey, Gareth Stables (PI).
Frimley Health NHS Foundation Trust, Wexham
Sarah Appleby, Henry Bourke (PI), Michael Brown, Sarah Cable, Alexander Damen, Joana Da Rocha, Louise Foster, Elizabeth Hamilton, Catriona Hatton, Cassie Honeywell, Kunal Kulkarni, Lucy Markham, Haadiya Mohammed, John O’Grady.
Betsi Cadwaladr University Health Board, Wrexham
Yogesh Joshi (PI), Heather Mclintock, Tania Morgan, Jane Stockport, Victoria Whitehead.
Wrightington, Wigan and Leigh NHS Foundation Trust
Pranshu Agrawal, Jo Armstrong, Shannon Briggs, Ben Coupe (PI), Anne Evans, Rob Gilbert, Sandra Latham, Aslam Mohammed, Valerie Parkinson, Valerie Parkinson, Rafael Sales, Katja Van De Snepscheut-Jones, David Wilcock, Daniel Wright.
Yeovil District Hospital NHS Foundation Trust
Joanna Allison, Simon Baker, Kate Beesley, Gill Ferrari, Benedict Lankester (PI), Alison Lewis, Joanne Lyons, Jamie O’Callaghan, Sarah Sutcliffe, Dianne Wood.
We would like to thank the external members of the Trial Steering Committee and Data Monitoring Committee for their advice and support for the project.
Trial Steering Committee
Marion Campbell (Chair), Tessa Howell (Patient Representative), Hilary Johnson, Stephen McDonnell, Thomas Pinkney, Mark Williams.
Data Monitoring Committee
Richard Emsley (Chair), George Peat, Martyn Snow.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
Favourable ethics opinion for the ACL SNNAP trial was given by the National Research Ethics Service, Oxfordshire Research Ethics Committee (REC) in October 2016 (16/SC/0502).
Information governance statement
The University of Oxford is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, The University of Oxford is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://compliance.web.ox.ac.uk/individual-rights
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/VDKB6009.
Primary conflicts of interest: David Beard holds a NIHR Senior Investigator award. Andrew Price was President of the British Association for Surgery of the Knee (BASK) during this period. William Jackson reports FH Ortho consulting fees and stock: Smith and Nephew. Fares Haddad reports grants, royalties/licenses and consulting fees from: Corin, MatOrtho Limited, Smith and Nephew, Inc., Stryker. Leadership roles: director ISEH; divisional director UCLH and EIC at Bone and Joint Journal.
Publications
Monk AP, Davies LJ, Hopewell S, Harris K, Beard DJ, Price AJ. Surgical versus conservative interventions for treating anterior cruciate ligament injuries. Cochrane Database Syst Rev 2016;4:CD011166.
Abram SGF, Judge A, Beard DJ, Price AJ. Rates of adverse outcomes and revision surgery after anterior cruciate ligament reconstruction: a study of 104,255 procedures using the National Hospital Episode Statistics Database for England, UK. Am J Sports Med 2019;47(11):2533–42
Davies LJ. Design Issues and Challenges in Clinical Trials with a Surgical and Non-surgical Comparison. Doctoral dissertation. Oxford: University of Oxford; 2019.
Davies L, Cook J, Leal J, Areia CM, Shirkey B, Jackson W, et al. Comparison of the clinical and cost effectiveness of two management strategies (rehabilitation versus surgical reconstruction) for non-acute anterior cruciate ligament (ACL) injury: study protocol for the ACL SNNAP randomised controlled trial. Trials 2020;21(1):405.
Stokes JR, Beard DJ, Davies L, Shirkey BA, Price A, Cook JA. ACL Surgery Necessity in Non-Acute Patients (ACL SNNAP): a statistical analysis plan for a randomised controlled trial. Trials 2022;23(1):389.
Beard DJ, Davies L, Cook JA, Stokes J, Leal J, Fletcher H, et al. ; ACL SNNAP Study Group. Rehabilitation versus surgical reconstruction for non-acute anterior cruciate ligament injury (ACL SNNAP): a pragmatic randomised controlled trial. Lancet 2022 Aug 20;400(10352):605–15. https://doi.org/10.1016/S0140-6736(22)01424-6. PMID: 35988569.
Paez A. Faithful but Flexible: Intervention Fidelity in Clinical Trials of Complex Interventions in Healthcare. Doctoral dissertation. Oxford: University of Oxford]; 2023.
Leal J, Mirza B, Davies L, Fletcher H, Stokes J, Cook JA, et al. Cost-effectiveness analysis of a pragmatic randomized trial evaluating surgical reconstruction versus rehabilitation in patients with long-standing anterior cruciate ligament injury. Bone Joint J 2024;106-B (1), 38–45. https://doi.org/10.1302/0301-620X.106B1.BJJ-2023-0175.R1
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Davies L, Cook J, Leal J, Areia CM, Shirkey B, Jackson W, et al. Comparison of the clinical and cost effectiveness of two management strategies (rehabilitation versus surgical reconstruction) for non-acute anterior cruciate ligament (ACL) injury: study protocol for the ACL SNNAP randomised controlled trial. Trials 2020;21.
- Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. New Engl J Med 2008;359:2135-42.
- Kaeding CC, Léger-St-Jean B, Magnussen RA. Epidemiology and diagnosis of anterior cruciate ligament injuries. Clin Sports Med 2017;36:1-8.
- Hernandez LM, Micheo WF, Amy E. Rehabilitation update for the anterior cruciate ligament injured patient: current concepts. Bol Asoc Med P R 2006;98:62-7.
- Thorstensson CA, Lohmander LS, Frobell RB, Roos EM, Gooberman-Hill R. Choosing surgery: patients’ preferences within a trial of treatments for anterior cruciate ligament injury. A qualitative study. BMC Musculoskelet Disord 2009;10.
- Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med 2009;37:1434-43.
- Grindem H, Eitzen I, Engebretsen L, Snyder-Mackler L, Risberg MA. Nonsurgical or surgical treatment of ACL injuries: knee function, sports participation, and knee reinjury: the Delaware-Oslo ACL cohort study. J Bone Jt Surg Am 2014;96:1233-41.
- Jameson SS, Dowen D, James P, Serrano-Pedraza I, Reed MR, Deehan D. Complications following anterior cruciate ligament reconstruction in the English NHS. Knee 2012;19:14-9.
- Abram SGF, Price AJ, Judge A, Beard DJ. Anterior cruciate ligament (ACL) reconstruction and meniscal repair rates have both increased in the past 20 years in England: hospital statistics from 1997 to 2017. Br J Sports Med 2020;54:286-91.
- Peat G, Bergknut C, Frobell R, Joud A, Englund M. Population-wide incidence estimates for soft tissue knee injuries presenting to healthcare in southern Sweden: data from the Skane Healthcare Register. Arthritis Res Ther 2014;16.
- Grindem H, Eitzen I, Moksnes H, Snyder-Mackler L, Risberg MA. A pair-matched comparison of return to pivoting sports at 1 year in anterior cruciate ligament-injured patients after a nonoperative versus an operative treatment course. Am J Sports Med 2012;40:2509-16.
- Meuffels DE, Favejee MM, Vissers MM, Heijboer MP, Reijman M, Verhaar JA. Ten year follow-up study comparing conservative versus operative treatment of anterior cruciate ligament ruptures. A matched-pair analysis of high level athletes. Br J Sports Med 2009;43:347-51.
- Dawson AG, Hutchison JD, Sutherland AG. Is anterior cruciate reconstruction superior to conservative treatment?. J Knee Surg 2016;29:74-9.
- Monk AP, Davies LJ, Hopewell S, Harris K, Beard DJ, Price AJ. Surgical versus conservative interventions for treating anterior cruciate ligament injuries. Cochrane Database Syst Rev 2016;4.
- Abram SGF, Judge A, Beard DJ, Price AJ. Rates of adverse outcomes and revision surgery after anterior cruciate ligament reconstruction: a study of 104,255 procedures using the National Hospital Episode Statistics Database for England, UK. Am J Sports Med 2019;47:2533-42.
- Frobell RB, Roos HP, Roos EM, Roemer FW, Ranstam J, Lohmander LS. Treatment for acute anterior cruciate ligament tear: five year outcome of randomised trial. BMJ 2013;346.
- Reijman M, Eggerding V, van Es E, van Arkel E, van den Brand I, van Linge J, et al. Early surgical reconstruction versus rehabilitation with elective delayed reconstruction for patients with anterior cruciate ligament rupture: COMPARE randomised controlled trial. BMJ 2021;372.
- Bollen SR, Scott BW. Rupture of the anterior cruciate ligament – a quiet epidemic?. Injury 1996;27:407-9.
- Francis A, Thomas RD, McGregor A. Anterior cruciate ligament rupture: reconstruction surgery and rehabilitation. A nation-wide survey of current practice. Knee 2001;8:13-8.
- Kapoor B, Clement DJ, Kirkley A, Maffulli N. Current practice in the management of anterior cruciate ligament injuries in the United Kingdom. Br J Sports Med 2004;38:542-4.
- Evans S, Shaginaw J, Bartolozzi A. ACL reconstruction – it’s all about timing. Int J Sports Phys Ther 2014;9:268-73.
- Cvetanovich GL, Chalmers PN, Verma NN, Cole BJ, Bach BR. Risk factors for short-term complications of anterior cruciate ligament reconstruction in the United States. Am J Sports Med 2016;44:618-24.
- Lohmander LS, Roos EM. The evidence base for orthopaedics and sports medicine. BMJ 2015;350.
- Identifier NL33702.078.10 . Cost-effectiveness of two treatment strategies of an anterior cruciate ligament rupture. A randomized clinical study 2011. www.onderzoekmetmensen.nl/en/trial/26654 (accessed 3 November 2023).
- Frobell RB, Roos EM, Roos HP, Ranstam J, Lohmander LS. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med 2010;363:331-42.
- Eggerding V, Reijman M, Meuffels DE, van Es E, van Arkel E, van den Brand I, et al. ACL reconstruction for all is not cost-effective after acute ACL rupture. Br J Sports Med 2022;56:24-8.
- Smeets A, Ghafelzadeh Ahwaz F, Bogaerts S, De Groef A, Berger P, Kaux JF, et al. Pilot study to investigate the feasibility of conducting a randomised controlled trial that compares Immediate versus Optional Delayed surgical repair for treatment of acute Anterior cruciate ligament injury: IODA pilot trial. BMJ Open 2022;12.
- de Vos FH, Meuffels DE, de Mul M, Askari M, Ista E, Polinder S, et al. ROTATE Study Group . Study protocol ROTATE-trial: anterior cruciate ligament rupture, the influence of a treatment algorithm and shared decision making on clinical outcome – a cluster randomized controlled trial. BMC Musculoskelet Disord 2022;23.
- Paramasivan S, Huddart R, Hall E, Lewis R, Birtle A, Donovan JL. Key issues in recruitment to randomised controlled trials with very different interventions: a qualitative investigation of recruitment to the SPARE trial (CRUK/07/011). Trials 2011;12.
- Hamilton DW, de Salis I, Donovan JL, Birchall M. The recruitment of patients to trials in head and neck cancer: a qualitative study of the EaStER trial of treatments for early laryngeal cancer. Eur Arch Otorhinolaryngol 2013;270:2333-7.
- Davies L, Beard D, Cook JA, Price A, Osbeck I, Toye F. The challenge of equipoise in trials with a surgical and non-surgical comparison: a qualitative synthesis using meta-ethnography. Trials 2021;22.
- Davies LJ. Design Issues and Challenges in Clinical Trials with a Surgical and Non-surgical Comparison. Oxford: University of Oxford; 2019.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101.
- Stokes JR, Beard DJ, Davies L, Shirkey B, Price A, Cook J. ACL Surgery Necessity in Non-Acute Patients (ACL SNNAP): a statistical analysis plan for a randomised controlled trial. Trials 2022;23:1-9.
- Micheo W, Hernandez L, Seda C. Evaluation, management, rehabilitation, and prevention of anterior cruciate ligament injury: current concepts. PM&Amp;R J Injury Funct Rehabil 2010;2:935-44.
- Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med 2004;34:269-80.
- Negus J, Fransen M, Chen JS, Parker DA, March L. Exercise-based interventions for conservatively or surgically treated anterior cruciate ligament injuries in adults. Cochrane Database Syst Rev 2012;10:1465-858.
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494-502.
- Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS) – development of a self-administered outcome measure. J Orthop Sports Phys Ther 1998;28:88-96.
- Granan LP, Bahr R, Lie SA, Engebretsen L. Timing of anterior cruciate ligament reconstructive surgery and risk of cartilage lesions and meniscal tears: a cohort study based on the Norwegian National Knee Ligament Registry. Am J Sports Med 2009;37:955-61.
- Dunn WR, Spindler KP, Amendola A, Andrish JT, Bergfeld JA, Flanigan DC, et al. Predictors of activity level two years after ACL reconstruction: MOON ACLR cohort study. Am J Sports Med 2010;38:2040-50.
- Lynch AD, Logerstedt DS, Grindem H, Eitzen I, Hicks G, Axe MJ, et al. Consensus criteria for defining ‘successful outcome’ after ACL injury and reconstruction: a Delaware-Oslo ACL cohort investigation. Br J Sports Med 2015;49:335-42.
- Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 1985;198:43-9.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36.
- Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. Am J Sports Med 1998;26:350-9.
- Roos EM. KOOS User Guide 2012. www.koos.nu/ (accessed 3 November 2023).
- Cook JA, Julious SA, Sones W, Hampson LV, Hewitt C, Berlin JA, et al. DELTA(2) guidance on choosing the target difference and undertaking and reporting the sample size calculation for a randomised controlled trial. BMJ 2018;363.
- Cook JA, Julious SA, Sones W, Hampson LV, Hewitt C, Berlin JA, et al. Practical help for specifying the target difference in sample size calculations for RCTs: the DELTA(2) five-stage study, including a workshop. Health Technol Assess 2019;23:1-88.
- Batistatou E, Roberts C, Roberts S. Sample size and power calculations for trials and quasi-experimental studies with clustering. Stata J 2014;14:159-75.
- Cook JA, Bruckner T, MacLennan GS, Seiler CM. Clustering in surgical trials – database of intracluster correlations. Trials 2012;13.
- Over M, Jolliffe D, Foster A. Huber correction for two-stage least squares estimates. Stata Tech Bull 1996;5.
- White IR, Carpenter J, Horton NJ. A mean score method for sensitivity analysis to departures from the missing at random assumption in randomised trials. Stat Sin 2018;28:1985-2003.
- Roos EM, Boyle E, Frobell RB, Lohmander LS, Ingelsrud LH. It is good to feel better, but better to feel good: whether a patient finds treatment ‘successful’ or not depends on the questions researchers ask. Br J Sports Med 2019;53:1474-8.
- Paez A. Faithful but Flexible: Intervention Fidelity in Clinical Trials of Complex Interventions in Healthcare. Doctoral Dissertation 2023.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Treatment Fidelity Workgroup of the NIH Behavior Change Consortium . Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018 2018. https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2018/ (accessed 3 November 2023).
- Department of Health . National Schedule of NHS Costs 2019 20 2021.
- Hobbs FDR, Bankhead C, Mukhtar T, Stevens S, Perera-Salazar R, Holt T, et al. National Institute for Health Research School for Primary Care Research . Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007–14. Lancet 2016;387:2323-30.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEcon 2014;32:1157-70.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9] 2013. www.nice.org.uk/process/pmg9/chapter/the-reference-case (accessed 3 November 2023).
- National Institute for Health and Care Excellence . NICE Health Technology Evaluations: The Manual. Process and Methods [PMG36] 2022. www.nice.org.uk/process/pmg36/chapter/economic-evaluation (accessed 3 November 2023).
- Dakin H, Devlin N, Feng Y, Rice N, O’Neill P, Parkin D. The influence of cost-effectiveness and other factors on nice decisions. Health Econ 2015;24:1256-71.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15.
- Gabr A, De Medici A, Haddad F. The National Ligament Registry 2020. www.uknlr.co.uk/pdf/uknlr-2020-annual-report.pdf (accessed 3 November 2023).
- Beard DJ, Dodd CA, Simpson HA. Sensorimotor changes after anterior cruciate ligament reconstruction. Clin Orthop Relat Res 2000;372:205-16.
Appendix 1 Members of ACL SNNAP study group
Independent members of the Data Monitoring Committee
Richard Emsley (Chair) (King’s College London).
George Peat (Keele University).
Martyn Snow (Royal Orthopaedic Hospital, Birmingham).
Independent members of the Trial Steering Committee
Marion Campbell (Chair) (University of Aberdeen).
Tessa Howell (Patient Representative).
Hilary Johnson (Gloucestershire Hospitals NHS Foundation Trust).
Stephen McDonnell (Cambridge University Hospitals NHS Foundation Trust).
Thomas Pinkney (University of Birmingham).
Mark Williams (Oxford Brookes University).
Trial co-investigators
Helen Campbell, Andrew Carr, Jonathan Cook, Loretta Davies, Jose Leal, Paul Monk and Andrew Price (University of Oxford).
Sarah E Lamb (College of Medicine and Health, University of Exeter).
Fares Haddad (University College London Hospitals NHS Foundation Trust).
Karen Barker and William Jackson (Oxford University Hospitals NHS Foundation Trust).
Sean O’Leary (Royal Berkshire NHS Foundation Trust).
Chris Wilson (Cardiff and Vale University Health Board).
Research fellows: Simon Abram and Arsenio Páez (University of Oxford).
Trial management: Loretta Davies, Carlos Morgado Areia and Jackie Davies (University of Oxford).
Trial administration: Katie Chegwin and Jiyang Li (University of Oxford).
Data management: Heidi Fletcher and Akiko Greshon (University of Oxford).
Database/programming management: Christina Bagg, Mark Brown and Michael Phillips (Fr3dom Limited).
Trial statisticians: Jamie Stokes and Beverly Shirkey (until Apr 2020); Health economists: Jose Leal, Vanessa Lythe and Burhan Mirza (University of Oxford).
Qualitative researcher: Francine Toye (Oxford).
Principal investigators (PIs) and research teams:
Laura Haywood, Anne Nicholson, Joanne Riches, Sean Symons (PI) and Mark Vertue (Basildon and Thurrock University Hospitals NHS Foundation Trust).
Louay Al Mouazzen, Rachel Bray, Damian Clark, James Coulthard, Tim Holland, Nick Howells, Andrew Jones, Richard Kapur, Alastair Kiszely, Harry Krishnan, Karen MacDonald-Taylor, Jon Manara, James Murray (PI), Corina Negrut, Vishai Pai, Andrew Porteous, Sven Putnis, James Robinson, Shav Rupasinghe, Veenesh Selvaratnam, James Smith, Nick Smith, Jarrad Stevens, Clare Taylor, Anthony Theodorides, Niraj Vetharajan, Helen Vint and Lucy Young (North Bristol NHS Trust).
Susan Bullock, Rebecca Cook, Alexander Dodds (PI), Amanda Freeman-Hicks and Paula Hillout (Gloucestershire Hospitals NHS Foundation Trust).
Thomas Cornell, Abbie Coutts, Suzy Dean, Nicki Devooght-Johnson, Emma Ferrell, Eve Fletcher, Chrissie Hall, Benjamin Kent, Sandra Kessly, Robin Kincaid (PI), Mohamed Lazizi, Ahmed Mostafa, Toby Nisbett, Tim Powell, Peter Riddlestone, Andrew Roberton, Jessica Summers, Lucy Whitbread and Belinda Wroath (Royal Cornwall Hospitals NHS Trust).
Emma Fenlon, Andrew Hall, Helen Jeffrey and Raghuram Thonse (PI) (Countess of Chester Hospital NHS Foundation Trust).
Debra Dunne, Andy Metcalfe (PI), Kerri McGowan, Simon Middleton, Feisal Shah, Tim Spalding, Charlie-Marie Suddens, Tamar Sweed, Joanna Teuke, Peter Thompson and David Wright (University Hospitals Coventry and Warwickshire NHS Trust).
Justine Amero, Emma Brown, Hugh Chissell, Andrea Croucher, Gareth Dickinson, Catherine Hawkes-Blackburn, Alice Peacocke, Graham Smith (PI) and Carol Snipe (Frimley Health NHS Foundation Trust).
Kim Dearnley and Reza Mayahi (PI) (Hull and East Yorkshire Hospitals NHS Trust).
Barry Andrews (PI), Massimo Barcelona (PI), Hazel Giles, Abdulkerim Gokturk, Paul Harnett, Katie Jeeves, Joyce Kadunyi, Sheena Mendoza, Ines Reichert, Marta Santamaria and Harshinder Virdee (Kings College Hospital NHS Foundation Trust).
Sanjeev Anand (PI), Nayef Aslam-Pervez, Stephen Draycott, Faye Howarth, Irfan Jina, Niall Maher, Denise Ross (PI) and Lindsey Worstenholme (Leeds Teaching Hospitals NHS Trust).
Abdul Baig, Arun Bhaskaran, Daniel Banks, Tracy Brear, Carla Christie, Laura Cowen, Jack Davis, Ross Dixey, Colin Esler (PI), Amirah Essop-Adam, Christina Haines, Linzy Houchen-Wolloff, Husein Varachia and Richard Wood (University Hospitals of Leicester).
Glaxy Gray, Jessica Nichols, Alice Panes, Susan Partridge, Lawrie Rogerson, Pankaj Sharma (PI), David Triggs, Ian Venables and Danielle Wilcock (Manchester University NHS Foundation Trust).
Sarah Buckley, Thelma Darian, Elizabeth Denis, Jo Duncan, Charlotte Hirst, James Newman, Fern Richardson and Jon Smith (PI) (The Mid Yorkshire Hospitals NHS Trust).
Megan Adcode, Megan Cottingham, Eliza Foster, Andrew Kelly (PI), Niamh McKay, Jane Rewbury, Alison Whitcher, James Williams and Esther Zebracki (Taunton and Somerset NHS Foundation Trust, Musgrove).
Llinos Davies, Jayadeep Jayachandran (PI), Alison Tardivel and Victoria Whitehead (Betsi Cadwaladr University Health Board, North Wales).
Simon Abram, Carlos Morgado Areia, Martha Batting, Amy Bond, Nick Bottomley (PI), Loretta Davies, Marc Deakin, Christopher Dodd, Alison Hudak, Samantha Hynes, Will Jackson, Luke Jones, Gail Lang, David McKenna, Susan Morris, Andrew Price, Clare Scott-Dempster, Adam Sykes, Iason Vichos and Simon Wood (Oxford University Hospitals NHS Foundation Trust).
Rupert Clifton (PI), Stephanie Diaz, Craig Hendy, Nishil Modi, Brendan O’Mahony, Susan O’Sullivan, Nicola Parker, Mira Pecheva and Rowan Rumonovic (North West Anglia NHS Foundation Trust, Peterborough).
Emma McLoughlin, Jeremy Rushbrook (PI) and Anna Thornhill (Solent NHS Trust, Portsmouth).
Emily Bannister, Chloe Brown, Debbie Burden, Terence Campbell, Emma Craig, Rashmi Easow, Julie Foxton, Alexandra Hazlerigg, Chethan Jayabev, Rosie Murdoch, Sean O’Leary (PI) and Georgie Parsons (Royal Berkshire NHS Foundation Trust).
Harry Brown, Paula Carvelli, Rugaia Montaser, Ali Pepper, Sinduja Sivarajan, Oliver Templeton-Ward (PI) and Eva Wilson (Royal Surrey County Hospitals NHS Foundation Trust).
Julie Cronin, Sarah Diment, Victoria King, Katharine Shean, Leonidas Vachtsevanos (PI), Katharine Wilcocks and Ben Wilson (Salisbury NHS Foundation Trust).
Paul McNestry, Joanna Ollerenshaw (PI), James Stoddard and Paul Sutton (Sheffield Teaching Hospitals NHS Foundation Trust).
Sanjay Anand, Judith Bell, Albert Chikate, Diane Daniel, Timothy Davies, Tom Finnigan, Antonio Frasquet-Garcia, Susan Hopkins, Sharon Kerrison, Angela McGowan, David Sands Johnson (PI), Lara Smith, Philip Turner and Helen Wilkinson (Stockport NHS Foundation Trust).
Lynne Allsop, Deborah Anthony, Rebecca Boulton, Sarah Brown, Vikram Desai (PI), Mandy Gill, Cheryl Heeley, Sushrut Kulkarni, Wayne Lovegrove, Dominic Nash, Terri-Ann Sewell, Sarah Shelton and Katie Slack (Sherwood Forest Hospitals NHS Foundation Trust, Sutton in Ashfield).
David Beard, James Cartwright, Lynda Connor, Andrew Davies, Caroline Davies, Matthew Dodd (PI), Glyn Gainard, Dave Graham-Woollard, Carl Murphy, Leanne Quinn, Caradog Thomas, Jenny Travers and Marie Williams (Swansea Bay University Health Board, Swansea).
Amanda Bell, Sunny Deo, Katharine Francis, Tracy Jackson, Laura McCafferty, Basalingappa Navadgi, Karan Plank, Venkat Satish (PI) and Claire Thelwall (Great Western Hospitals NHS Foundation Trust, Swindon).
Fares Haddad (PI), Rachel Knight, Rahul Patel and Bruce Paton (University College London Hospitals NHS Foundation Trust).
Ashutosh Acharya, Utuman Aland, Miltiades Areirobulos, Pascal de Feyter, Lisa Ditchfield, Hafiz Iqbaz, Daniel Massey and Gareth Stables (PI) (Warrington and Halton Hospitals NHS Foundation Trust).
Sarah Appleby, Henry Bourke (PI), Michael Brown, Sarah Cable, Alexander Damen, Joana Da Rocha, Louise Foster, Elizabeth Hamilton, Catriona Hatton, Cassie Honeywell, Kunal Kulkarni, Lucy Markham, Haadiya Mohammed and John O’Grady (Frimley Health NHS Foundation Trust, Wexham).
Yogesh Joshi (PI), Heather Mclintock, Tania Morgan, Jane Stockport and Victoria Whitehead (Betsi Cadwaladr University Health Board, Wrexham).
Pranshu Agrawal, Jo Armstrong, Shannon Briggs, Ben Coupe (PI), Anne Evans, Rob Gilbert, Sandra Latham, Aslam Mohammed, Valerie Parkinson, Valerie Parkinson, Rafael Sales, Katja Van De Snepscheut-Jones, David Wilcock and Daniel Wright (Wrightington, Wigan and Leigh NHS Foundation Trust).
Joanna Allison, Simon Baker, Kate Beesley, Gill Ferrari, Benedict Lankester (PI), Alison Lewis, Joanne Lyons, Jamie O’Callaghan, Sarah Sutcliffe and Dianne Wood (Yeovil District Hospital NHS Foundation Trust).
Appendix 2 Changes to protocol
| Protocol version number | Date issued | Details of changes made |
|---|---|---|
| Version 2 | 7 March 2017 | Randomisation method changed. The new form involved stratification rather than minimisation. A small number of other changes were made in order to provide greater clarity; collection of MRI details at baseline; ‘failure of intervention’ collected and detailed as ‘intervention-related complications’. |
| Version 3 | 18 September 2017 | Exclusion criteria changed from a specific time point since injury (4 months) to instead exclude patients in the acute phase of primary ACL injury. Further detail was added to the meniscal pathology and previous knee surgery exclusion criteria to provide greater clarity. Further clarification on participant identification and recruitment. Guidance on level of preoperative physiotherapy in the surgical management group included. |
| Version 4 | 27 July 2018 | Updated details to reflect the questionnaire changes at 6-, 12- and 18-month follow-up to reduce patient burden with the aim to improve retention rates. |
Appendix 3 Clinical and health economics supplementary data
| Surgical (n = 156) | Non-surgical (n = 159) | |
|---|---|---|
| Completed MRI forms | 151/156 (97%) | 148/159 (93%) |
| ACL Tear | ||
| Complete | 143/151 (95%) | 137/148 (93%) |
| Partial | 8/151 (5%) | 11/148 (7%) |
| Associated injuries | ||
| Medial meniscus | ||
| Tear/damage | 67/151 (44%) | 73/148 (49%) |
| Degenerative changes | 3/151 (2%) | 2/148 (2%) |
| No damage reported | 81/151 (54%) | 73/148 (49%) |
| Lateral meniscus | ||
| Tear/damage | 38/151 (25%) | 34/148 (23%) |
| Degenerative changes | 1/151 (1%) | 2/148 (1%) |
| No damage reported | 112/151 (74%) | 112/148 (76%) |
| PCL | ||
| Any injury (combined ACL/PCL) | 4/151 (3%) | 7/148 (5%) |
| Tear of PCL | 1/151 (< 1%) | 1/148 (< 1%) |
| No damage reported | 146/151 (97%) | 140/148 (95%) |
| MCL | ||
| Grade 1 | 28/151 (19%) | 32/148 (22%) |
| Grade 2 | 24/151 (16%) | 9/148 (6%) |
| Grade 3 | 3/151 (2%) | 3/148 (2%) |
| No damage reported | 96/151 (63%) | 104/148 (70%) |
| Resource use type | Unit cost (£) | Source/details |
|---|---|---|
| GP – surgerya | 39 | Cost GP patient contact consultation: PSSRU 2019–20 (chapter 10, page 120). Average consultation length of 9.22 minutes59 |
| Theatre time (per minute) | 16.49 | Table R142X from Scottish cost tables 2019 (https://beta.isdscotland.org/find-publications-and-data/healthcare-resources/finance/scottish-health-service-costs) |
| GP – homea | 90 | Cost GP patient contact consultation (including qualification costs and direct care staff costs): PSSRU 2019–20 (chapter 10, page 120). Average consultation length of 9.22 minutes;59 assume average 12 minutes travel time for home visits: PSSRU 2009 |
| GP – telephonea | 21 | Cost GP patient contact consultation: PSSRU 2019–20 (chapter 10, page 124). Average consultation length of 5 minutes59 |
| Physiotherapist, one to one | 66.82 | NHS Reference Cost schedule 2019–20, tab CHS, service code A08A1 |
| Physiotherapist, group-based | 65.34 | NHS Reference Cost schedule 2019–20, tab CHS, service code A08AG |
| A&E | 182.28 | NHS Reference Cost schedule 2019–20, tab AE, weighted average of all attendances to a type 1 A&E unit with the exception of dental care and dead on arrival attendances |
| Outpatient visit | 122 | NHS Reference Cost schedule 2019–20, tab total outpatient attendances, service code 110 (Trauma and orthopaedics) |
| Acupuncture/chiropractor/osteopath | 99.18 | Estimated as average of costs for acupuncture, chiropractor and osteopath sessions. Acupuncture: NHS Reference Cost schedule 2019–20, tab OPROC, service code 191 (pain management), currency code AB23Z (£163.90) Chiropractor: assumed to cost the same as physiotherapy session (£66.82) Osteopath: assumed to cost the same as physiotherapy session (£66.82) |
| Reason for admission | ICD-10 | OPCS | HRG | Daycase cost | Inpatient cost (elective) |
|---|---|---|---|---|---|
| ACLR (open) | M23.8 + M23.5 + T93.3 | W74.2 + Z84.6 + Z94.2 (or Z94.3) + Y65.8 + Z94.2 (or Z94.3) | HN23C Major knee procedures for non-trauma | £2656 | £4772 |
| ACLR (arthroscopic) | M23.8 + M23.5 + T93.3 | W74.2 + Y76.7 + Z84.6 + Z94.2 (or Z94.3) + Y65.8 + Z94.2 (or Z94.3) | HN23C Major knee procedures for non-trauma | £2656 | £4772 |
| ACLR with medial/lateral meniscal repair and meniscectomy | M23.8 + M23.5 + T93.3 | ACLR + W82.3 + Z94.2 (or Z94.3) + W82.2 + Z94.2 (or Z94.3) | HN22E Very major knee procedures for non-trauma | £3778 | £6414 |
| ACL operation but no reconstruction (ACL intact) | M23.8 + M23.5 + T93.3 | W87.9 + Z94.2 (or Z94.3) | HN24C Intermediate knee procedures for non-trauma | £1901 | £3523 |
| Arthroscopic arthrolysis | M23.8 + M23.5 | W85.8 | HN25A Minor knee procedures for non-trauma | £1650 | £2262 |
| PCL injury with medial/lateral meniscal repair | S83.5 | W82.3 + Z94.2 (or Z94.3) | HT24C Intermediate knee procedures for trauma | £2261 | £3909 |
| Infection (cellulitis) | L03.1 + R22.4 + T81.4 | - | JD07K Skin disorders without intervention | £344a | £1658a |
| Surgical group (N = 156) | Rehab group (N = 160) | Difference (surgical vs. rehabilitation) | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Median | Range | n | Mean | Median | Range | |||
| Hospital admission (NHS) | ||||||||||
| 6 months | 156 | 0.6 (0.5) | 1 (0, 1) | 0–2 | 160 | 0.1 (0.4) | 0 (0, 0) | 0–1 | 0.5 (0.4 to 0.5) | <0.001 |
| 12 months | 156 | 0.1 (0.4) | 0 (0, 0) | 0–1 | 160 | 0.2 (0.4) | 0 (0, 0) | 0–1 | 0.0 (−0.1 to 0.1) | 0.751 |
| 18 months | 156 | 0.0 (0.1) | 0 (0, 0) | 0–1 | 160 | 0.1 (0.3) | 0 (0, 0) | 0–1 | −0.1 (−0.1 to 0.0) | 0.017 |
| ACLR (NHS) | ||||||||||
| 6 months | 156 | 0.6 (0.5) | 1 (0, 1) | 0–1 | 160 | 0.1 (0.4) | 0 (0, 0) | 0–1 | 0.4 (0.3 to 0.5) | <0.001 |
| 12 months | 156 | 0.1 (0.3) | 0 (0, 0) | 0–1 | 160 | 0.2 (0.4) | 0 (0, 0) | 0–1 | 0.0 (−0.1 to 0.0) | 0.293 |
| 18 months | 156 | 0.0 (0.2) | 0 (0, 0) | 0–1 | 160 | 0.1 (0.3) | 0 (0, 0) | 0–1 | −0.1 (−0.1 to 0.0) | 0.019 |
| Physiotherapy session (NHS) | ||||||||||
| 6 months | 152 | 3.4 (4.2) | 2 (0, 6) | 0–20 | 159 | 5.4 (4.7) | 4 (2, 7) | 0–22 | −1.9 (−3.0 to −0.9) | <0.001 |
| 12 months | 152 | 3.3 (5.0) | 0 (0, 6) | 0–20 | 156 | 2.1 (4.0) | 0 (0, 3) | 0–19 | 1.2 (0.4 to 2.0) | 0.003 |
| 18 months | 153 | 1.0 (2.3) | 0 (0, 0) | 0–11 | 159 | 0.8 (2.0) | 0 (0, 0) | 0–10 | 0.2 (−0.2 to 0.6) | 0.396 |
| GP surgery | ||||||||||
| 6 months | 88 | 0.9 (1.5) | 0 (0, 1) | 0–8 | 93 | 0.5 (1.5) | 0 (0, 0) | 0–8 | 0.3 (−0.1 to 0.7) | 0.132 |
| 12 months | 87 | 0.4 (0.9) | 0 (0, 0) | 0–5 | 78 | 0.2 (0.6) | 0 (0, 0) | 0–3 | 0.1 (0.0 to 0.3) | 0.100 |
| 18 months | 127 | 0.1 (0.5) | 0 (0, 0) | 0–3 | 120 | 0.2 (0.8) | 0 (0, 0) | 0–6 | −0.1 (−0.2 to 0.0) | 0.075 |
| GP telephone | ||||||||||
| 6 months | 88 | 0.4 (1.1) | 0 (0, 0) | 0–6 | 93 | 0.1 (0.5) | 0 (0, 0) | 0–3 | 0.2 (−0.1 to 0.5) | 0.127 |
| 12 months | 87 | 0.1 (0.4) | 0 (0, 0) | 0–3 | 78 | 0.1 (0.3) | 0 (0, 0) | 0–2 | 0.0 (−0.1 to 0.1) | 0.882 |
| 18 months | 127 | 0.1 (0.5) | 0 (0, 0) | 0–4 | 120 | 0.2 (0.7) | 0 (0, 0) | 0–4 | −0.1 (−0.2 to 0.1) | 0.256 |
| Chiropractor (NHS) | ||||||||||
| 6 months | 88 | 0.1 (0.4) | 0 (0, 0) | 0–3 | 93 | 0.1 (1.0) | 0 (0, 0) | 0–10 | 0.0 (−0.3 to 0.2) | 0.709 |
| 12 months | 87 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 78 | 0.1 (0.7) | 0 (0, 0) | 0–5 | −0.2 (−0.4 to 0.0) | 0.069 |
| 18 months | 127 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 120 | 0.0 (0.1) | 0 (0, 0) | 0–1 | −0.1 (−0.1 to 0.0) | 0.154 |
| Outpatient visit (NHS) | ||||||||||
| 6 months | 88 | 1.5 (2.1) | 1 (0, 3) | 0–10 | 93 | 1.2 (1.9) | 1 (0, 2) | 0–10 | 0.3 (−0.4 to 1.1) | 0.363 |
| 12 months | 87 | 0.4 (1.1) | 0 (0, 0) | 0–8 | 78 | 0.7 (1.5) | 0 (0, 1) | 0–10 | −0.3 (−0.6 to 0.0) | 0.093 |
| 18 months | 127 | 0.1 (0.5) | 0 (0, 0) | 0–2 | 120 | 0.4 (1.0) | 0 (0, 0) | 0–5 | −0.3 (−0.4 to −0.1) | 0.001 |
| A&E visit (NHS) | ||||||||||
| 6 months | 88 | 0.2 (0.5) | 0 (0, 0) | 0–3 | 93 | 0.2 (0.7) | 0 (0, 0) | 0–4 | −0.1 (−0.3 to 0.1) | 0.504 |
| 12 months | 87 | 0.0 (0.1) | 0 (0, 0) | 0–1 | 78 | 0.1 (0.5) | 0 (0, 0) | 0–4 | −0.1 (−0.2 to 0.0) | 0.080 |
| 18 months | 127 | 0.0 (0.2) | 0 (0, 0) | 0–2 | 120 | 0.1 (0.5) | 0 (0, 0) | 0–3 | −0.1 (−0.2 to 0.0) | 0.014 |
| Hospital admission (private) | ||||||||||
| 6 months | 156 | 0.0 (0.1) | 0 (0, 0) | 0–1 | 160 | 0.0 (0.1) | 0 (0, 0) | 0–1 | 0.0 (0.0 to 0.0) | 0.550 |
| 12 months | 156 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 160 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 0.0 (0.0 to 0.0) | 1.000 |
| 18 months | 156 | 0.0 (0.1) | 0 (0, 0) | 0–1 | 160 | 0.0 (0.1) | 0 (0, 0) | 0–1 | 0.0 (0.0 to 0.0) | 0.597 |
| Physiotherapy session (private) | ||||||||||
| 6 months | 88 | 0.6 (1.9) | 0 (0, 0) | 0–10 | 93 | 0.6 (2.1) | 0 (0, 0) | 0–15 | 0.0 (−0.5 to 0.4) | 0.847 |
| 12 months | 87 | 0.5 (1.8) | 0 (0, 0) | 0–12 | 78 | 0.3 (1.3) | 0 (0, 0) | 0–10 | 0.1 (−0.3 to 0.6) | 0.520 |
| 18 months | 127 | 0.4 (1.9) | 0 (0, 0) | 0–16 | 120 | 0.4 (1.5) | 0 (0, 0) | 0–10 | 0.0 (−0.4 to 0.5) | 0.911 |
| Chiropractor (private) | ||||||||||
| 6 months | 88 | 0.1 (0.6) | 0 (0, 0) | 0–5 | 93 | 0.0 (0.2) | 0 (0, 0) | 0–1 | 0.1 (0.0 to 0.2) | 0.260 |
| 12 months | 87 | 0.0 (0.3) | 0 (0, 0) | 0–2 | 78 | 0.0 (0.1) | 0 (0, 0) | 0–1 | 0.0 (0.0 to 0.1) | 0.428 |
| 18 months | 127 | 0.1 (0.5) | 0 (0, 0) | 0–5 | 120 | 0.1 (1.2) | 0 (0, 0) | 0–12 | −0.1 (−0.3 to 0.1) | 0.462 |
| Outpatient visit (private) | ||||||||||
| 6 months | 88 | 0.0 (0.2) | 0 (0, 0) | 0–1 | 93 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 0.0 (0.0 to 0.1) | 0.116 |
| 12 months | 87 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 78 | 0.0 (0.2) | 0 (0, 0) | 0–2 | 0.0 (−0.1 to 0.0) | 0.312 |
| 18 months | 127 | 0.0 (0.1) | 0 (0, 0) | 0–1 | 120 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 0.0 (0.0 to 0.0) | 0.327 |
| A&E visit (private) | ||||||||||
| 6 months | 88 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 93 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 0.0 | |
| 12 months | 87 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 78 | 0.0 (0.2) | 0 (0, 0) | 0–2 | 0.0 | |
| 18 months | 127 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 120 | 0.0 (0.0) | 0 (0, 0) | 0–0 | 0.0 | |
| Surgical group (N = 156) | Rehabilitation group (N = 160) | Total (N = 316) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SC | UA | PD | AD | M | SC | UA | PD | AD | M | SC | UA | PD | AD | |
| Baseline | |||||||||||||||
| Available data | 156 (100%) | 156 (100%) | 156 (100%) | 156 (100%) | 156 (100%) | 159 (99%) | 159 (99%) | 159 (99%) | 159 (99%) | 159 (99%) | 315 (100%) | 315 (100%) | 315 (100%) | 315 (100%) | 315 (100%) |
| No problems | 46 (29%) | 101 (65%) | 17 (11%) | 14 (9%) | 60 (38%) | 40 (25%) | 106 (67%) | 21 (13%) | 12 (8%) | 68 (43%) | 86 (27%) | 207 (66%) | 38 (12%) | 26 (8%) | 128 (41%) |
| Slight problems | 53 (34%) | 29 (19%) | 40 (26%) | 53 (34%) | 43 (28%) | 51 (32%) | 36 (23%) | 49 (31%) | 51 (32%) | 48 (30%) | 104 (33%) | 65 (21%) | 89 (28%) | 104 (33%) | 91 (29%) |
| Some problems | 44 (28%) | 22 (14%) | 61 (39%) | 60 (38%) | 36 (23%) | 49 (31%) | 17 (11%) | 54 (34%) | 71 (45%) | 24 (15%) | 93 (30%) | 39 (12%) | 115 (37%) | 131 (42%) | 60 (19%) |
| Severe problems | 12 (8%) | 4 (3%) | 24 (15%) | 26 (17%) | 11 (7%) | 18 (11%) | 0 (0%) | 19 (12%) | 22 (14%) | 11 (7%) | 30 (10%) | 4 (1%) | 43 (14%) | 48 (15%) | 22 (7%) |
| Extreme problems | 1 (1%) | 0 (0%) | 14 (9%) | 3 (2%) | 6 (4%) | 1 (1%) | 0 (0%) | 16 (10%) | 3 (2%) | 8 (5%) | 2 (1%) | 0 (0%) | 30 (10%) | 6 (2%) | 14 (4%) |
| 6 months | |||||||||||||||
| Available data | 85 (54%) | 85 (54%) | 85 (54%) | 85 (54%) | 85 (54%) | 90 (56%) | 90 (56%) | 89 (56%) | 90 (56%) | 90 (56%) | 175 (55%) | 175 (55%) | 174 (55%) | 175 (55%) | 175 (55%) |
| No problems | 39 (46%) | 65 (76%) | 19 (22%) | 10 (12%) | 36 (42%) | 42 (47%) | 74 (82%) | 23 (26%) | 18 (20%) | 37 (41%) | 81 (46%) | 139 (79%) | 42 (24%) | 28 (16%) | 73 (42%) |
| Slight problems | 32 (38%) | 11 (13%) | 35 (41%) | 45 (53%) | 32 (38%) | 30 (33%) | 10 (11%) | 24 (27%) | 34 (38%) | 31 (34%) | 62 (35%) | 21 (12%) | 59 (34%) | 79 (45%) | 63 (36%) |
| Some problems | 10 (12%) | 7 (8%) | 20 (24%) | 23 (27%) | 8 (9%) | 14 (16%) | 3 (3%) | 26 (29%) | 30 (33%) | 10 (11%) | 24 (14%) | 10 (6%) | 46 (26%) | 53 (30%) | 18 (10%) |
| Severe problems | 4 (5%) | 2 (2%) | 7 (8%) | 5 (6%) | 6 (7%) | 4 (4%) | 3 (3%) | 9 (10%) | 6 (7%) | 8 (9%) | 8 (5%) | 5 (3%) | 16 (9%) | 11 (6%) | 14 (8%) |
| Extreme problems | 0 (0%) | 0 (0%) | 4 (5%) | 2 (2%) | 3 (4%) | 0 (0%) | 0 (0%) | 7 (8%) | 2 (2%) | 4 (4%) | 0 (0%) | 0 (0%) | 11 (6%) | 4 (2%) | 7 (4%) |
| 12 months | |||||||||||||||
| Available data | 84 (54%) | 85 (54%) | 85 (54%) | 85 (54%) | 85 (54%) | 76 (48%) | 76 (48%) | 76 (48%) | 76 (48%) | 75 (47%) | 160 (51%) | 161 (51%) | 161 (51%) | 161 (51%) | 160 (51%) |
| No problems | 59 (70%) | 79 (93%) | 45 (53%) | 25 (29%) | 53 (62%) | 56 (74%) | 70 (92%) | 39 (51%) | 21 (28%) | 38 (51%) | 115 (72%) | 149 (93%) | 84 (52%) | 46 (29%) | 91 (57%) |
| Slight problems | 21 (25%) | 6 (7%) | 30 (35%) | 47 (55%) | 19 (22%) | 9 (12%) | 4 (5%) | 16 (21%) | 40 (53%) | 21 (28%) | 30 (19%) | 10 (6%) | 46 (29%) | 87 (54%) | 40 (25%) |
| Some problems | 4 (5%) | 0 (0%) | 8 (9%) | 11 (13%) | 10 (12%) | 6 (8%) | 2 (3%) | 10 (13%) | 8 (11%) | 12 (16%) | 10 (6%) | 2 (1%) | 18 (11%) | 19 (12%) | 22 (14%) |
| Severe problems | 0 (0%) | 0 (0%) | 1 (1%) | 2 (2%) | 0 (0%) | 4 (5%) | 0 (0%) | 4 (5%) | 5 (7%) | 4 (5%) | 4 (3%) | 0 (0%) | 5 (3%) | 7 (4%) | 4 (3%) |
| Extreme problems | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 3 (4%) | 1 (1%) | 0 (0%) | 7 (9%) | 2 (3%) | 0 (0%) | 1 (1%) | 0 (0%) | 8 (5%) | 2 (1%) | 3 (2%) |
| 18 months | |||||||||||||||
| Available data | 116 (74%) | 116 (74%) | 115 (74%) | 116 (74%) | 116 (74%) | 116 (73%) | 116 (73%) | 116 (73%) | 116 (73%) | 116 (73%) | 232 (73%) | 232 (73%) | 231 (73%) | 232 (73%) | 232 (73%) |
| No problems | 89 (77%) | 105 (91%) | 65 (57%) | 37 (32%) | 72 (62%) | 77 (66%) | 99 (85%) | 54 (47%) | 28 (24%) | 67 (58%) | 166 (72%) | 204 (88%) | 119 (52%) | 65 (28%) | 139 (60%) |
| Slight problems | 17 (15%) | 7 (6%) | 28 (24%) | 51 (44%) | 21 (18%) | 21 (18%) | 10 (9%) | 21 (18%) | 56 (48%) | 30 (26%) | 38 (16%) | 17 (7%) | 49 (21%) | 107 (46%) | 51 (22%) |
| Some problems | 8 (7%) | 4 (3%) | 14 (12%) | 24 (21%) | 13 (11%) | 14 (12%) | 6 (5%) | 27 (23%) | 24 (21%) | 14 (12%) | 22 (9%) | 10 (4%) | 41 (18%) | 48 (21%) | 27 (12%) |
| Severe problems | 2 (2%) | 0 (0%) | 5 (4%) | 3 (3%) | 7 (6%) | 3 (3%) | 1 (1%) | 11 (9%) | 6 (5%) | 0 (0%) | 5 (2%) | 1 (0%) | 16 (7%) | 9 (4%) | 7 (3%) |
| Extreme problems | 0 (0%) | 0 (0%) | 3 (3%) | 1 (1%) | 3 (3%) | 1 (1%) | 0 (0%) | 3 (3%) | 2 (2%) | 5 (4%) | 1 (0%) | 0 (0%) | 6 (3%) | 3 (1%) | 8 (3%) |
| EQ-5D utility | NHS costs | Total healthcare costs (NHS + private) | ||||
|---|---|---|---|---|---|---|
| Follow-up time | Surgical | Rehabilitation | Surgical | Rehabilitation | Surgical | Rehabilitation |
| 6 months | 0.634 (0.028) | 0.630 (0.028) | £2306 (147) | £1011 (92) | £2397 (148) | £1084 (97) |
| 12 months | 0.780 (0.018) | 0.705 (0.025) | £755 (103) | £805 (105) | £796 (105) | £838 (108) |
| 18 months | 0.770 (0.020) | 0.724 (0.021) | £129 (28) | £365 (71) | £196 (38) | £452 (77) |
| Time point | Number of questionnaires sent out | Received n (%) | Method of completion | |||
|---|---|---|---|---|---|---|
| Online n (%) | Post n (%) | Phone n (%) | Clinic n (%) | |||
| 6 months | 313 | 201 (64) | 94 (47) | 93 (46) | 14 (7) | – |
| 12 months | 314 | 168 (54) | 64 (38) | 90 (53) | 15 (9) | – |
| 18 months | 313 | 248 (79) | 79 (32) | 97(39) | 69 (28) | 3 (1) |
List of abbreviations
- ACL
- anterior cruciate ligament
- ACLD
- anterior cruciate ligament deficiency
- ACL-QoL
- anterior cruciate ligament quality of life score
- ACLR
- anterior cruciate ligament reconstruction
- ACL SNNAP
- Anterior Cruciate LigamentSurgery Necessity in Non-AcutePatients
- AUC
- area under the curve
- CACE
- complier-average causal effect
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COVID-19
- coronavirus disease discovered in 2019
- DMC
- Data Monitoring Committee
- EDC
- electronic data collection
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HRG
- Healthcare Resource Group
- ICC
- intracluster correlation
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- KOOS
- Knee Injury and Osteoarthritis Outcome Score
- MAR
- missing at random
- MCL
- medial collateral ligament
- MIC
- minimal important change
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NMB
- net monetary benefit
- OA
- osteoarthritis
- OCTRU
- Oxford Clinical Trials Research Unit
- PCRF
- physiotherapy case report form
- PP
- per-protocol
- PPC
- conservative per-protocol
- PPI
- patient and public involvement
- PPP
- pragmatic per-protocol
- QALYs
- quality-adjusted life-years
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SD
- standard deviation
- SITU
- surgical intervention trials unit
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
