Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/115/76. The contractual start date was in December 2015. The draft report began editorial review in July 2021 and was accepted for publication in September 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Banerjee et al. This work was produced by Banerjee et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Banerjee et al.
Chapter 1 Introduction
Scientific background
Dementia is one of the most common and serious public health issues of our time. 1 Over 46 million people have dementia worldwide, a figure set to double in the next 20 years. 2 The commonest cause of dementia is Alzheimer’s disease (AD), which causes irreversible and progressive decline in memory, reasoning, communication skills and the ability to carry out daily activities. Alongside this cognitive and functional decline, individuals may develop neuropsychiatric symptoms (NPS) such as agitation, sleep disturbance, depression and psychosis. 3 These are common, occurring in up to 90% of people with dementia, with agitation as one of the most persistent symptoms. 4 Agitation is defined as inappropriate verbal, vocal or motor activity that is not thought to be caused by unmet need; it encompasses physical and verbal aggression and is particularly problematic. 5 It affects nearly half of people with AD over a month6 and 80% of those with clinically significant symptoms will have them 6 months later. 7 Agitation is associated with deteriorating relationships with family and professional carers, care home admission, increased costs of care, carer burden and burnout and decreased quality of life. 5,7,8
Agitation in dementia has substantial economic consequences, accounting for between 12% and 44% of dementia care costs annually9,10 and imposing significant costs on unpaid carers. 11 Costs rise as the severity of agitation increases. 10,12 The annual excess health and social care cost of agitation in AD in the UK has been estimated at £2B. 9
Agitation in dementia is therefore a legitimate target for therapeutic intervention, but it is a symptom with a number of possible causes, including pain, physical or psychological distress, misperception of threat (e.g. when receiving personal care), and response to hallucinations or delusions. Using non-pharmacological interventions that investigate aetiology and provide a tailored response as a first-line treatment for agitation in dementia, such as the DICE approach (Describe the problem, Investigate the cause, Create a plan, Evaluate its effectiveness), is recommended as best practice. 1,13 However, given the clinical significance of agitation, there is a need for second-line treatments when no underlying causes are found or when correction of these has not resulted in improvement. The mainstay of drug treatment is the use of antipsychotic medication. These drugs, however, have low efficacy, with the American Psychiatric Association guideline group reporting that they ‘demonstrate minimal or no efficacy with strong placebo effects’14 and have been shown to cause particular harms in those with dementia, including excess dementia-specific mortality. In 2009, around 180,000 people with dementia were prescribed antipsychotic medication across the UK per year and this equated to an additional 1800 deaths and an additional 1620 cerebrovascular adverse events (AEs) attributable to the use of antipsychotics in dementia. 15 While their rate of prescription to people with dementia has decreased,16 they are still commonly used and such treatment is largely unlicensed. In most countries, few or no treatments have been given regulatory approval for such use. In the UK, the only drugs with a relevant license are risperidone and haloperidol and these are highly restrictive. Risperidone is indicated for the ‘short-term treatment (up to 6 weeks) of persistent aggression in patients with moderate to severe Alzheimer’s dementia unresponsive to non-pharmacological approaches and when there is a risk of harm to self or others’ and haloperidol for ‘persistent aggression and psychotic symptoms in moderate to severe Alzheimer’s dementia and vascular dementia [when non-pharmacological treatment is ineffective and there is a risk of harm to self or others]’.
Other drug treatments have been suggested for agitation in dementia but trials of antidementia medication, the acetylcholinesterase donepezil17 and the N-Methyl-D-Aspartate (NMDA) inhibitor memantine,18 have been tested in randomised controlled trials (RCTs) and not demonstrated efficacy. In a large multicentre trial, the anticonvulsant sodium valproate did not delay or prevent NPS in dementia. 19 Benzodiazepines are used short-term clinically but there are no trials and adverse effects such as falls are common and of concern. 20 Antidepressants have also been investigated as an alternative to antipsychotics. The CitAD trial of citalopram for agitated behaviours provided evidence that a target dose of citalopram 30 mg per day had a small positive effect on agitation in dementia21 in those who were less agitated and less cognitively impaired. 22 Adverse cardiac and cognitive effects identified in the trial limit its use in clinical practice. 21 Antidepressants are not mentioned as a potential treatment for agitation in the English National Institute for Health and Care Excellence (NICE) guideline on dementia assessment and management23 but they are increasingly used as a treatment of agitation in dementia. This substitution strategy to seek to avoid the prescription of antipsychotics has been reported in a large study of US nursing homes where the prescription rates of mood stabilisers such as sodium valproate, carbamazepine and particularly gabapentin increased as those for antipsychotics decreased. 24,25 Such prescribing of antidepressants is part of the common polypharmacy seen among people with dementia in the community. 26
Mirtazapine for agitated behaviours in dementia
Mirtazapine, a noradrenergic and specific serotonergic antidepressant (NASSA), is widely used in older people; from 2009 to 2014, in a study of 4.8 million antidepressant initiations in Europe, it was the antidepressant that was most commonly prescribed for older people and also to those with dementia. 27 A centrally active presynaptic α2-antagonist, it stimulates both noradrenergic and serotonergic systems mediated via 5-HT1 receptors, with 5-HT2 and 5-HT3 receptors blocked by mirtazapine. Histamine H1-antagonistic activity is thought to cause its sedative properties. It has little anticholinergic activity, unlike citalopram, and, at therapeutic doses, has few cardiovascular effects. Mirtazapine is a relatively potent antagonist/inverse agonist at key receptors likely to be pivotal in target symptoms including antagonism of α2-adrenergic, 5-HT1A and histamine H1 receptors. The overall effects are to increase noradrenergic and serotonergic neurotransmission which may explain its use in depression while the H1 antagonism is associated with useful acute sedative benefits. The pharmacological profile of mirtazapine is such that at higher dosages, the sedative effect decreases due to noradrenaline stimulation. It is a well-established treatment for depression and is well tolerated by older people and so a popular choice by psychiatrists which will encourage recruitment. It is available generically at low cost in the NHS. Cost implications, were it found to be effective, would be therefore minimal.
In pre-specified secondary analyses of the HTA-SADD data set, reported in the HTA-SADD final report,28 we have found a positive effect of mirtazapine on decreasing Behavioural and Psychological Symptoms in Dementia (BPSD) [as measured by Neuropsychiatric Inventory (NPI) score] at 13 weeks. Taking the top 50% of raw NPI scores (i.e. those with appreciable BPSD), there was a 7.1-point difference in NPI score [95% confidence interval (CI) −0.50 to 14.68; p = 0.067] between mirtazapine and placebo and a 13.2-point difference between mirtazapine and sertraline (95% CI 4.47 to 21.95; p = 0.003). An additional surprising but encouraging positive finding was from the cost-effectiveness analyses. Over the course of the trial, the time spent by unpaid carers caring for participants in the mirtazapine group was almost half that for patients in the placebo group (6.74 vs. 12.27 hours per week) and sertraline group (6.74 vs. 12.32 hours per week). Informal care costs were £1510 (95% CI −3088 to −136) and £1522 (95% CI −3398 to −72) less for the mirtazapine-treated group when compared with placebo and sertraline respectively. In the secondary outcome evaluation, looking at quality-of-life gains and costs, treatment with mirtazapine had a high likelihood of cost-effectiveness compared to placebo or sertraline. 28,29 The improvements in quality of life for mirtazapine relative to the other treatments contributed to the cost-effectiveness result, and there is a plausibility that comes from the putative ability of mirtazapine to ameliorate sleep disturbances and anxiety. 30,31 Improvements in sleep could potentially improve life quality and therefore patient-reported EuroQol-5 Dimension (EQ-5D) scores; they could also release carer time directly and also ameliorate an important source of carer distress. 32 In this way, mirtazapine might have a general effect, beneficial for both the patient and the carer, even without exerting an antidepressant effect. Two small-scale open-label pilot studies give supportive evidence for the potential of a trial in this area [Cakir and Kulaksizoglu33 (those on mirtazapine did better); Reichman et al. 34 (NPI decreased by 5.8 points)]. This would be the first placebo-controlled RCT of mirtazapine for agitation in dementia. Given the paucity of alternatives and the priority of finding safe and effective treatments for BPSD, these data suggest that a placebo-controlled trial of mirtazapine would be of value.
Carbamazepine for agitated behaviours in dementia
Carbamazepine stabilises the inactivated state of voltage-gated sodium channels and potentiates GABA receptors. It is recommended in the BNF for epilepsy, prophylaxis of bipolar disorder and trigeminal neuralgia. It is generally safe within the proposed dose ranges; there are few data on people with AD, but it seems that there is no increase in mortality as in antipsychotics for AD. 35 Carbamazepine has been widely used in psychiatric disorders and AD, off licence, to treat symptoms including agitation, aggression, irritability and impulsivity. Open-label studies and case reports have indicated promise in agitation in AD. 36 Two small 6-week parallel-group RCTs of carbamazepine for BPSD have been published. 37,38 The first in 55 patients (modal dose 300 mg) showed significant symptom decrease. It was well tolerated with no decrease in cognition, function or increased side effects relative to placebo. The second (400 mg in 21 patients not responding to antipsychotics) showed a trend but not a significant advantage over placebo. Meta-analysis indicated significant benefit compared with placebo treatment on the Brief Psychiatric Rating Scale (mean difference −5.5 points, 95% CI −8.5 to −2.5 points) and on the Clinical Global Impression Scale [odds ratio (OR) 10.2, 95% CI 3.1 to 33.1]. 39 A third small trial of the similar compound oxcarbazepine (n = 103) indicated a trend towards benefit (p = 0.07) with active drug performing better than placebo in all analyses. 40
Why is the study needed?
Agitated behaviours drive poor quality of life in dementia and poor outcomes including hospitalisation and care home placement and high cost. They have profound negative effects on people with dementia themselves, their family carers, services and society. They are a major issue in care homes, in general hospitals and in people’s own homes. The non-drug treatments we have are not always successful and the antipsychotic drugs that we use are associated with unacceptable increases in mortality and morbidity and low clinical effectiveness. There is a pressing need nationally and internationally for safe alternative pharmacological treatments. Research into better treatments for agitated behaviours in dementia was identified as a top 10 research priority by the Alzheimer’s Society and the James Lind Alliance. 41 This involved extensive engagement with people with dementia, carers, health and social care practitioners and organisations that represent these groups. Over 4000 questions on prevention, diagnosis, treatment and care of dementia were considered and the top 10 identified, including: ‘What non-pharmacological and/or pharmacological (drug) interventions are most effective for managing challenging behaviour in people with dementia?’ The need for better research into pharmacological treatments for agitation and aggression is also articulated in the National Dementia Strategy,42 the outputs of the 2010 Ministerial Dementia Research Summit and 2011 NIHR Dementia Research Workshop summarised in the Ministerial Advisory Group for Dementia Research (MAGDR) final report ‘Priority Topics in Dementia Research’ published by the MRC in February 2011. 43 MAGDR concluded ‘further research into behavioural and psychological symptoms in order to provide more effective management of challenging behaviour and improved quality of life’ was one of the top six headline priorities for research.
In this study, we therefore aimed to establish the clinical and cost-effectiveness and safety profile of carbamazepine (discontinued when 40 people had been randomised into this arm due to slower than projected recruitment) or mirtazapine in reducing agitation in AD relative to placebo.
Chapter 2 Methods
Study design
This is a pragmatic, multicentre, double-blind, placebo-controlled superiority RCT of safety, clinical and cost-effectiveness of mirtazapine (with usual care) at 6 and 12 weeks on agitated behaviours in dementia. We included a long-term follow-up period to allow limited assessment of longer-term outcomes at 26 and 52 weeks. An internal pilot phase assessed trial recruitment, with progression to a full trial dependent on the number of patients recruited within the pilot recruitment period.
Important changes to methods
SYMBAD was initially designed as a three-arm trial, comparing both carbamazepine and placebo and mirtazapine and placebo for a difference in change in Cohen-Mansfield Agitation Inventory (CMAI) score at 12 weeks as the primary objective. Challenges in recruitment in this population resulted in the funder requesting that the available data be reviewed to July 2018, with the aim of dropping one arm of the trial.
The SYMBAD independent Data Monitoring Committee (DMC) reviewed available data comparing the two active arms with placebo. They were asked to consider efficacy data (the primary end point, CMAI at 12 weeks), safety data [frequency of AEs and serious adverse events (SAEs) on an individual basis] and compliance with treatment (dropouts and adherence with the prescribed amount of treatment medication). This was done subgroup blind: the DMC knew which was the placebo group but not the identity of the two active groups. Based on each set of data, the DMC were asked which arm they would recommend stopping or if they felt unable to make a recommendation. Taking all three sets together, the DMC were again asked whether they would recommend stopping one arm or were unable to make a recommendation.
The DMC could provide no recommendation on the basis of treatment compliance but recommended on the basis of efficacy and safety data the discontinuation of the carbamazepine arm. This recommendation did not provide, in any way, any indication that sufficient evidence had accrued to deem either drug to be effective or non-effective.
In August 2018, the protocol was submitted for a substantial amendment to change to a two-arm trial design, comparing mirtazapine with placebo as the primary objective. Protocol version 2.0 shows the new trial design. Up to the date of approval of this substantial amendment, 40 patients had been randomised to receive carbamazepine. These data have been analysed in the same way as the mirtazapine data. Chapters 2–4 reflect the amended protocol and refer only to the mirtazapine/placebo comparisons.
See Appendix 1 for summary of protocol changes.
Aim
The overall trial aim was to assess the safety, clinical and cost-effectiveness of mirtazapine in the treatment of agitation in dementia.
The null hypothesis is that there is no difference in CMAI scores between patients treated with placebo and mirtazapine at 12 weeks.
The primary objective is to determine if mirtazapine is more clinically effective in reducing agitated behaviours in dementia than placebo, measured by CMAI score 12 weeks post randomisation.
The secondary objectives are:
-
to determine if mirtazapine is more cost-effective than placebo at 12 weeks post randomisation
-
to determine if mirtazapine is more clinically and cost-effective than placebo in reducing CMAI score at 6 weeks post randomisation
-
to determine differences in effectiveness between mirtazapine and placebo on carers
-
to determine whether there are differences between the groups in AEs and adherence
-
to determine long-term differences between those randomised to placebo and mirtazapine in a head-to-head comparison of agitation (measured by CMAI score), institutionalisation, death and clinical management at 26 and 52 weeks post randomisation.
Participants
Inclusion criteria
-
Patients with a clinical diagnosis of probable or possible AD using National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria. 44
-
A diagnosis of co-existing agitated behaviours.
-
Evidence that the agitated behaviours have not responded to management according to the AS/DH algorithm. 45
-
An assessment of CMAI (Long Form46) score of 45 or greater.
-
Written informed consent to enter and be randomised into the trial.
-
Availability of a suitable informant (consenting identifiable family carer or paid carer) to provide information on carer-completed outcome measures and who consents to take part in the trial.
Exclusion criteria
-
Current treatment with antidepressants (including MAOIs) or antipsychotics. Normal clinical practice should be followed, with an appropriate wash-out period before trial drug administration. For MAOIs, this should be at least 2 weeks.
-
Contraindications to the administration of mirtazapine or carbamazepine as per the current Summary of Product Characteristics (SmPC).
-
Patients with second-degree atrioventricular block [patients with third-degree heart block, with a pacemaker fitted, may be included at principal investigator (PI) discretion].
-
Cases too critical for randomisation (i.e. where there is a suicide risk or where the patient presents a risk of harm to others).
-
Female subjects under the age of 55 years of childbearing potential, defined as follows: postmenopausal females who have not had at least 12 months of spontaneous amenorrhea or 6 months of spontaneous amenorrhoea with serum FSH > 40 mIU/ml or females who have not had a hysterectomy or bilateral oophorectomy at least 6 weeks prior to enrolment.
Setting
Participants were drawn from existing patients and new patient referrals to old age psychiatric services, memory clinics, specific Participant Identification Centres (PICs), primary care centres and those in care homes in 26 UK sites.
Interventions
Initially there were three groups: (1) mirtazapine, (2) carbamazepine and (3) placebo. As noted above, the carbamazepine arm was dropped during the course of the study in August 2018. The target dose was 45 mg of mirtazapine or 300 mg of carbamazepine per day. Drugs and their placebos were identically presented with participants aiming to take three capsules orally once a day.
Randomisation
Once a patient’s screening CMAI score has been assessed as being ≥ 45, the research worker discussed the case with the site PI who was permitted to prescribe Investigational Medicinal Product (IMP). The PI confirmed or not the patient’s eligibility to join the study, and on confirmation, the research worker used an online randomisation system to randomise the patient for the trial. This system required confirmation of eligibility criteria. Details of the randomisation were confirmed by e-mail to the research worker, site PI, Chief investigator and co-ordinating team at Norwich Clinical Trials Unit (NCTU). A semi-blinded randomisation e-mail detailing IMP allocation was sent to site pharmacy contact/s only. The PI provided a signed prescription for the patient’s trial medication. The research worker then collected this prescription from the central pharmacy and delivered it to the patient at a scheduled ‘Week 0’ IMP delivery visit. Local policies for treating patients outside of their registered NHS Trust were followed as appropriate. Random allocation was block stratified by centre and type of residence (care home vs. own household) with random block lengths of three or six before the discontinuation of the carbamazepine arm and thereafter of two or four. The trial was double-blind, with drug and placebo identically encapsulated. Referring clinicians, participants, the trial management team and the research workers who did baseline and follow-up assessments were masked to group allocation.
Primary outcome
Cohen-Mansfield Agitation Inventory score (Long Form) at 12 weeks.
Secondary outcomes
-
Costs derived from Client Service Receipt Inventory (CSRI), and quality-adjusted life-years (QALYs) from cost data alongside supplemented information from Dementia-Specific Quality of Life (DEMQOL) and EuroQol-5 Dimensions, five-level version (EQ-5D-5L) interviews 12 weeks post randomisation.
-
CMAI score and cost at 6 weeks post randomisation.
-
Patient and carer quality of life, and carer outcomes at 6 and 12 weeks post randomisation.
-
AEs from week 0 to week 16 and adherence at 6 and 12 weeks post randomisation.
-
CMAI score, AEs and adherence at 6 and 12 weeks, conditional on evidence of effectiveness of IMP over placebo.
-
Longer-term follow-up: CMAI score, institutionalisation, death and clinical management at 26 and 52 weeks post randomisation.
Instruments used in the study – range and scoring
-
CMAI (agitation): score ranges from 29 (no agitated behaviour) to 203 (very agitated behaviour).
-
Mini Mental State Examination (MMSE) (cognition): score ranges from 0 (severe impairment) to 30 (normal).
-
General Health Questionnaire (GHQ-12) (mental health): score ranges from 0 (least severe problems, good) to 36 (most severe).
-
Zarit Carer Burden: range from 0 (no burden) to 88 (most severe).
-
DEMQOL (quality of life): DEMQOL range from 28 (poor) to 112 (good); DEMQOL-Proxy: range from 31 (poor) to 124 (good).
-
NPI (neuropsychiatric symptoms) total: total score range from 0 (none) to 144 (severe); NPI agitation/aggression: range from 0 (none) to 12 (severe); NPI depression/anxiety/irritation: range from 0 (none) to 36 (severe); and NPI carer distress: range 0 (none) to 60 (severe).
Change in outcomes over the time of the trial
There were no changes to the primary outcomes of the trial following the registration of the trial.
Sample size
An initial calculated sample size of 400 (randomised 1 : 1 : 1) provided 90% power using two-sided 5% significance tests to detect a drug versus placebo mean difference in CMAI score at 12 weeks of 6 points. This equated to an effect size of d = 0.4 [assuming a common standard deviation (SD) of 15] or a clinically significant 30% decrease in CMAI from placebo to active drug. With a realistic 15% attrition, a sample of 471 (157 per arm) was aimed for. Due to slower than projected recruitment, an arm of the study (carbamazepine) was dropped and sample size was amended in consultation with the funder, DMC and TMC (see Important changes to methods). Based on the same parameters, an amended sample size target of 222 was calculated (randomised 1 : 1) allowing for a 10% attrition (111 per arm).
The primary outcome measure in this trial was the CMAI. Active drug treatment, compared with placebo, may be associated with changes in the CMAI that are much > 6 points, but SYMBAD was powered to detect the smallest difference in the CMAI that could be considered clinically meaningful. This estimation was based on the changes and SD of change score seen in the CALM trial which included a similar patient population treated with donepezil where 6 CMAI points was 35% of the SD.
Blinding and unblinding
Blinding
All non-statistical members of the trial team, their clinicians, participants and their carers were blinded to trial arm allocation. To maintain the blind, active medication and the placebo were identically encapsulated.
Unblinding
Final unblinding of all trial participants occurred following the creation of a locked analysis data set. The decision to unblind a single case was made when knowledge of an individual’s allocated treatment was required:
-
to enable treatment of severe AE/s, or
-
in the event of an overdose.
Where possible, requests for emergency or unplanned unblinding of individuals were made via the trial manager, and in agreement of the Chief Investigator. However, in circumstances where there is insufficient time to make this request or for agreement to be sought, the treating clinician was able to make the decision to unblind immediately. This was done via the study database (local PIs and the CI have special logins which allowed for unblinding and was closely audited within the database management system) or by contacting the CI who authorised unblinding by the Data Management Team. All instances of unblinding were recorded and reported to NCTU by the local PI, including the identity of all recipients of the unblinding information.
Data management
Confidentiality
Any paper copies of personal trial data were kept at the participating site in a secure location with restricted access. Only non-identifiable data were kept at the NCTU office with authorised NCTU staff members having access. Only staff working on the trial had password access to this information.
Confidentiality of patient’s personal data was ensured by not collecting patient names on Case Report Forms (CRFs) that were be sent to NCTU and storing the data in a pseudo-anonymised fashion at NCTU. At trial enrolment, the patient was issued a Participant Identification Number (PIN) and this acted as the primary identifier for the patient, with secondary identifiers of initials (and date of birth as required).
The patient and carer’s consent forms carried their name and signature. These were kept at the trial site, and a copy was sent to NCTU for monitoring purposes. Consent forms were kept separate from patient data.
Data collection tools and source document identification
Research workers completed paper CRFs during their visits to participants and their carers. They entered data onto a central database via an online system once they had internet access. Research workers received training on data collection and use of the online system. Identification logs, screening logs and enrolment logs were kept locally, either in paper or electronic form.
Source data worksheets were drafted by the trial manager with the CI, trial statistician, data management team and PIs. The database specification was prepared by the NCTU data manager and approved by the CI and trial statistician prior to the database being built. The database was prepared by the CTU data programmer and tested by the trial statistician, trial manager and study site staff for user acceptability prior to the final system being launched.
Data collection, data entry and queries raised by members of the HTA-SYMBAD trial team were conducted in line with NCTU and trial-specific Data Management Standard Operating Procedures.
Clinical trial team members received trial protocol training. All data was handled in accordance with the Data Protection Act 1998 and as updated in the 2018 Act, and the General Data Protection Regulation (GDPR) [European Union (EU)] 2016/679.
Data handling
Within each trial site, patients were allocated a unique trial PIN. Data were entered under this PIN onto the central database stored on the servers based at University of East Anglia (UEA). The database was password protected and only accessible to members of the SYMBAD trial team at NCTU, the participating sites and external regulators (upon request). The server is in a secure room, which is protected by CCTV, where access is restricted to members of the UEA Information Systems team by security door access. The study database was built using Microsoft SQL Server tools and direct access was restricted to NCTU data management staff. Data entry was via web pages created using Microsoft.NET technology. All internet traffic was encrypted using the standard SSL (Secure Sockets Layer) methodology. The data entry system validated data on entry to ensure they were of the expected type (e.g. integers, dates, etc.) and range of values. Periodically and at database lock the data were further validated for errors and inconsistencies. The database was linked to an audit tool where all data additions, modifications and deletions are recorded with date/time and the user ID of the person making the change. The database was designed to comply with the International Conference on Harmonisation (ICH) Guideline for Good Clinical Practice (GCP), within the Standard Operating Procedures for Data Management in NCTU and also where appropriate with UEA Information Technology (IT) procedures.
The database and coding values were developed by the NCTU data manager in conjunction with the Chief Investigator, study statistician and other NCTU members and the trial team. The database software provides a number of features to help maintain data quality, including maintaining an audit trail, allowing custom validations on all data, allowing users to raise data query requests and search facilities to identify validation failure/missing data. Further details can be found in the SYMBAD Trial Data Management Plan. The database will be retained on the servers of UEA for 10 years following the end of the trial.
The identification, screening and enrolment logs, linking participant identifiable data to the PIN, were held locally by the research sites and at NCTU. This was either in written form in a locked filing cabinet or electronically in password-protected form on hospital computers. After completion of the trial, the identification, screening and enrolment logs will be stored securely by the sites for a minimum of 10 years.
Monitoring and site visits
For each site, a site initiation visit (SIV) was arranged by the trial manager with the core study team. A remote visit (teleconference or video conference call) was considered if they met predefined criteria of experience.
The minimum attendance for the SIV (in person or remotely) was the PI, lead research nurse, lead data manager (if applicable), pharmacy lead and research worker, any sub-Investigators were also encouraged to attend. All sites received a standardised copy of site initiation slides to aid the training of new staff working on the trial.
Each site was provided with a paper site file containing all the documents required to be held at site, generated at NCTU to comply with ICH GCP guidelines. A confirmation receipt was sent with the file for completion at site to confirm all documents had been received. Prior to a site being activated, the receipt was required to be received and returned to the TM or delegate at NCTU.
Before COVID-19, all sites recruiting at least five participants received one routine monitoring visit during the course of the trial. After lockdown, three sites (South West London, Central and North West London and Sheffield) that recruited more than five and were not visited, checks for these were made online. Site monitoring included the following checks:
-
ensuing that key eligibility variables match source data
-
blood test results and electrocardiogram (ECG) printouts
-
SAE [and serious adverse reaction (SAR)/suspected unexpected serious adverse reaction (SUSAR) where applicable] reports were verified against clinical notes where possible
-
clinic notes checked for unreported notable or serious events, where possible
-
data from participants experiencing study drug discontinuations/dose lowering
-
50% Source Data Verification from patient packs transcribed to Electronic Case Report Forms (eCRFs) will be checked for at least 20% of patients recruited at the site at the time of the visit; if time allows, more patient packs will be checked. The CMAI questionnaire/score will always be checked for the randomly selected 20% of patients; a selection of the other questionnaires up to a minimum of 50% of the data will be checked
-
sites will not be warned in advance which patient packs will be checked; the monitor will take a list with them and select the listed patient numbers from all of the available packs
-
any major or critical findings should prompt the monitoring team to increase the level of monitoring to cover as many participant files as time allows
-
completeness of trial drug dispensing, accountability and drug supply inventories
-
documentation and procedures will be checked for protocol deviations and serious breaches
-
consent forms
-
delegation logs
-
confirmation that safety checks had been completed and reviewed by the PI prior to randomisation
-
accuracy of site file (and pharmacy file checks where relevant).
After the visit, the PI and site team were provided with a report summarising the documents that had been reviewed and the corrective actions that were required by the site team. A response was required to be provided to the TT. The Trial Manager reviewed responses and compiled them alongside the on-site monitoring findings. The final report was signed off by the TT member performing the visit and the CI (reviewer). Additional monitoring visits were conducted on a ‘for cause’ approach. Monitoring of data quality, recruitment rates, pharmacy, Investigator Site File documents, consent and safety also occurred centrally.
Assessment by time point
For an overview of assessments over time, please see Table 1.
| Assessment | Baselinea | Rx ‘week 0’ visit (0–7 days after last baseline or randomisation visit) | Week 2 (12–16 days after week 0 visit date)b | Week 4 (21–35 days after week 0 visit)b | Week 6 (35–49 days after week 0 visit)a | Week 12 (77–91 days after week 0 visit)a | Week 16h (105–119 days after week 0 visit)b | Week 26c, (week 25–27) | Week 52c,d (week 51–53) |
|---|---|---|---|---|---|---|---|---|---|
| Consent | X | ||||||||
| CMAI (Long Form) | X | X | X | ||||||
| Safety bloodsd (FBC, U&Es, LFT) |
Xe | Xf | |||||||
| ECGd | Xe | Xf | |||||||
| CSRIg | X | X | X | ||||||
| Disease-specific Quality of Life DEMQOL | X | X | X | ||||||
| Carer assessed disease-specific Quality of Life DEMQOL-Proxy | X | X | X | ||||||
| Generic Quality of Life EQ-5D-5L (Proxy) | X | X | X | ||||||
| Cognitive impairment sMMSE | X | X | X | ||||||
| BPSD | X | X | X | ||||||
| C-SSRS (Cognitively impaired version) | X | X | X | ||||||
| Randomisation | X | ||||||||
| Dispensing | X | X | |||||||
| Adherence | X | X | X | X | |||||
| AEs | X | X | X | X | X | ||||
| Medication assessment (for dose changes) | X | X | X | ||||||
| Use of rescue medications | X | X | |||||||
| Concomitant medications | X | X | X | X | X | X | X | X | X |
| Withdrawal of treatment | X | X | X | X | |||||
| Carer mental health GHQ-12h | X | X | X | ||||||
| Carer quality of life EQ-5D-5Lh | X | X | X | ||||||
| Carer burden Zarit CBIh | X | X | X | ||||||
| Carer proxy report of CMAI scoreb | X | X | |||||||
| Carer proxy report of treatmentb | X | X | |||||||
| Institutionalisationb | X | X | |||||||
| Deathb | X | X |
Safety assessments
Definitions of harm of the EU Directive 2001/20/EC Article 2 based on the principles of ICH GCP applied to this trial: any unfavourable and intended sign, symptom or illness that developed or worsened during the period of the study was classified as an AE, whether or not it was considered to be related to the study treatment. AEs included unwanted side effects, sensitivity reactions, abnormal laboratory results, injury or intercurrent illnesses, and may be expected or unexpected. These were recorded on the CRF.
The period for SAE reporting was from the time of randomisation until 4 weeks post final trial medication administration. The participants were followed up by a telephone interview 4 weeks after the last dose of trial medication (the week 16 call). All events were followed until resolution, including if that meant beyond 4 weeks’ post final trial medication implementation.
Definitions
Definitions of AEs are presented in Table 2.
| Adverse event (AE) | Any untoward medical occurrence in a patient or clinical trial participant administered a medicinal product and which does not necessarily have a causal relationship with this product |
| Adverse reaction (AR) | Any untoward and unintended response to an IMP related to any dose administered |
| Unexpected adverse reaction | An adverse reaction, the nature or severity of which is not consistent with the applicable product information (e.g. Investigator’s Brochure for an unauthorised product or summary of product characteristics (SmPC) for an authorised product |
| Serious adverse event or serious adverse reaction | Any AE or AR that at any dose: |
Recording and reporting adverse events
NCTU were notified of all SAEs within 24 hours of the investigator becoming aware of the event. Investigators notified NCTU of any SAEs that occurred from the time of randomisation until 4 weeks after the last protocol treatment administration. SARs and SUSARs were notified to NCTU until trial closure. Any subsequent events that could be attributed to treatment were reported to the Medicines and Healthcare Products Regulatory Agency (MHRA) using the yellow card system (https://yellowcard.mhra.gov.uk/the-yellow-card-scheme/).
The SAE form was completed by the investigator (the consultant named on the delegation of responsibilities list who is responsible for the participant’s care in the trial) with attention paid to the grading, causality and expectedness of the event. In the absence of the responsible investigator, the SAE form was completed and signed by a member of the site trial team and e-mailed as appropriate within the timeline. The responsible investigator checked the SAE form at the earliest opportunity, made any changes necessary, signed and then e-mailed it to NCTU. Detailed written reports were completed as appropriate. Systems were in place at each site to enable the investigator to check the form for clinical accuracy.
The minimum criteria required for reporting an SAE were the patient trial number and date of birth, name of reporting investigator and sufficient information on the event to confirm seriousness. Any further information regarding the event that was unavailable at the time of the first report was sent as soon as it became available. The SAE form was scanned and sent by e-mail to the trial team at NCTU.
Participants were followed up until clinical recovery was complete and laboratory results had returned to normal or baseline values, or until the event had stabilised. Follow-up visits continued after completion of protocol treatment and/or trial follow-up if necessary. Follow-up SAE forms were completed and e-mailed to NCTU as further information became available. Additional information and/or copies of test results (etc.) could be provided separately. The participant was identified by trial number, date of birth and initials only. The participant’s name was not used on any correspondence and was blacked out and replaced with trial identifiers on any test results.
Assessment of adverse events
The severity of all AEs and/or ARs (serious and non-serious) in this trial was based on the Research Worker and site PI’s clinical judgement. For general (e.g. non-haematological) AEs/ARs, they were graded using the following definitions:
-
mild: an event that is easily tolerated by the participant, causing minimal discomfort and not interfering with everyday activities
-
moderate: an event that is sufficiently discomforting to interfere with normal everyday activities
-
severe: an event that prevents normal everyday activities.
For haematological (e.g. from blood test results) AEs/ARs, they were graded using the Common Terminology Criteria for Adverse Events v4.03 (CTCAE) 14 June 2010 criteria:
-
Grade 1: mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated
-
Grade 2: moderate; minimal, local or non-invasive intervention indicated; limiting age-appropriate instrumental ADL (preparing meals, shopping for groceries or clothes, using the telephone, managing money, etc.)
-
Grade 3: severe or medically significant but not immediately life-threatening; hospitalisation or prolongation of hospitalisation indicated; disabling; limiting self-care ADL (bathing, dressing and undressing, feeding self, using the toilet, taking medications and not bed-ridden, etc.)
-
Grade 4: life-threatening consequences; urgent intervention indicated
-
Grade 5: death related to AE.
In addition to severity, the investigators assessed the causality of serious events or reactions in relation to the trial therapy using the definitions in Table 3. SAEs that were considered related to the trial treatment were reviewed against the list of expected events in the approved version of the mirtazapine SmPC. Events that did not appear on the list or happened more frequently than listed were considered unexpected and reported as SUSARs.
| Relationship | Description | Event type |
|---|---|---|
| Unrelated | There is no evidence of any causal relationship | Unrelated SAE |
| Unlikely to be related | There is little evidence to suggest that there is a causal relationship (e.g. the event did not occur within a reasonable time after administration of the trial medication). There is another reasonable explanation for the event (e.g. the participant’s clinical condition or other concomitant treatment) | Unrelated SAE |
| Possibly related | There is some evidence to suggest a causal relationship (e.g. because the event occurs within a reasonable time after administration of the trial medication). However, the influence of other factors may have contributed to the event (e.g. the participant’s clinical condition or other concomitant treatment) | SAR |
| Probably related | There is evidence to suggest a causal relationship and the influence of other factors is unlikely | SAR |
| Definitely related | There is clear evidence to suggest a causal relationship and other possible contributing factors can be ruled out | SAR |
Statistical methods
Primary outcome measures
Analyses were based on intention-to-treat (all participants were analysed according to the group to which they were randomised, irrespective of the treatment or dose received). The primary outcome (CMAI at 12 weeks) was analysed using a general linear regression model including baseline CMAI score as a covariate, place of residence as a fixed effect and recruitment centre as a random effect. Treatment group was added as a fixed effect, with two levels (placebo vs. mirtazapine). Model assumptions were checked by use of diagnostic plots. The primary analysis used complete cases (excluding those with missing values). Imputation was done under the MAR assumption. A sensitivity analysis imputed missing values using multiple imputation with chained equations approach [the mi impute chained command in Stata® (StataCorp LP, College Station, TX, USA)].
Secondary outcome measures
Analysis of secondary outcomes, including long-term outcomes at 26 and 52 weeks followed an analogous approach using general linear regression models including baseline outcome, stratification variables and treatment group. We completed a post hoc analysis comparing death rates in the groups using Fisher’s exact test.
Health economics
Economic evaluation
The primary outcome for the economic evaluation was the incremental cost per 6-point difference in CMAI score at 12 weeks, from a health and social care system perspective. A 6-point difference represents a clinically significant minimum difference, or 30% decrease on the measure from placebo to mirtazapine. In addition, we conducted a secondary cost-effectiveness analysis on this outcome measure from the societal perspective. We conducted secondary cost-utility analyses of participants’ and unpaid carers’ QALYs at 12 weeks, from both the health and social care and societal perspectives (encompassing health and social care, unpaid care and out-of-pocket costs of purchasing adaptive equipment). Three measures of health-related quality of life were used to derive participant utilities: informant-rated EQ-5D-5L,47,48 informant-rated DEMQOL-Proxy-U and participant-rated DEMQOL-U. 49,50 Unpaid carers’ utilities were derived from carer self-rated EQ-5D-5L. QALYs were calculated using the area under the curve method, assuming linear change between assessment points. 51 Six-week costs and outcome measures were reported but a full cost-effectiveness analysis of the CMAI outcome at 6 weeks was not undertaken, given the very short time horizon for observing changes in service utilisation.
In addition, a model-based cost-effectiveness analysis was planned, examining lifetime costs and QALY gain beyond the intervention period. However, on the basis of the clinical and cost-effectiveness findings, this was not progressed and no results have been reported.
Resource use
Comprehensive costs of care for participants with dementia were calculated (including the costs of formal/paid care such as that provided by health and social services and also the costs associated with unpaid care) using data gathered using the CSRI52 at baseline, 6 and 12 weeks.
Unit costs
The base year for prices was 2016–17. Unit costs were taken from nationally representative published sources. 53–55 The price of generic mirtazapine was taken from the NHS Prescription costs analysis. 53 Unpaid carer time was valued at opportunity cost in the main analyses (following the lost productivity approach described elsewhere). 56,57 The costs of unpaid care were estimated as either the cost of time spent in caring or of time taken off from work to care, whichever cost was the greater. In estimating the cost of unpaid carer time in caring, those in work were considered to have given up work time (lost production), valued at the national average wage;58 those not working were considered to have given up leisure time, valued at 35% of national average wage. The CSRI, which was used to estimate carers’ caring hours, covered time spent over the previous week in all caring tasks (including supervision and also care home visiting). Unpaid carers chose a time band for the hours of care provided per week (ranging from no hours to 100 + hours per week). A continuous variable for total hours of care was calculated by taking the mid-point of each band. The maximum of the topmost band was first adjusted to account for nightly sleep time (assumed to be 8 hours if carers reported no lost sleep in caring or the hours remaining once hours of lost sleep were deducted). All time spent in caring tasks received the same valuation (rather than attributing a lower value to supervision than hands-on care tasks).
Cost estimation
Items of resource use were grouped into categories for the purposes of costing: hospital services, primary and community health, mental health, accommodation (domestic/communal), overnight respite care (in communal settings), community social care, day services, equipment and adaptations (including memory aids), medications and unpaid care provided to participants. Unpaid care included lost working time (work cut down/given up) and hours of help and support provided by the main carer and family/friends.
Health economic statistical analysis
The cost per unit of effect of the intervention is known as the incremental cost-effectiveness ratio (ICER). It is calculated as the mean difference in costs in mirtazapine and placebo groups (ΔC) divided by the mean difference in outcome (ΔE) between groups.
Mirtazapine would be considered cost-effective if it was significantly more effective and less expensive than placebo. The treatment would also be cost-effective if it was significantly more effective and more expensive than placebo, but the decision-maker was willing to pay the additional cost (up to a threshold, λ) to achieve the additional effect; or, put another way, if the ICER was below some threshold of willingness to pay for a unit of additional effectiveness, λ. 59 The cost-effectiveness decision rule in this case can be expressed as:
Mirtazapine might also be considered cost-effective if it was significantly less effective and less expensive and the decision-maker considered the sacrifice of some effectiveness worth making to achieve the savings. Mirtazapine would be considered unambiguously to be not cost-effective if it is both significantly less effective and more expensive.
The incremental net monetary benefit (NMB)59,60 is the monetary value of gains in effects associated with the treatment at a given value of λ, once the additional cost of the treatment has been deducted. Rearranging the decision rule in (1), NMB is expressed as:
Multilevel bivariate regressions were estimated for costs and outcomes with fixed effects for baseline cost/outcome and living arrangement at randomisation (stratifying variable) and a random effect for centre. Multilevel models (MLM) were estimated by restricted maximum likelihood. Where the sample providing data consisted of 50 or fewer observations, models applied small sample inference for fixed effects and residual denominator degrees of freedom in tests of fixed effects. 61 NMB over a range of willingness-to-pay values was derived from model estimates and their 95% CIs were calculated following Fieller’s theorem. 62,63
There is no societal consensus on what should be paid for a minimum clinically significant difference in the CMAI. A NMB plot and a cost-effectiveness acceptability curve (CEAC) were produced to show the extent to which the primary outcome could be judged cost-effective. The plot of the NMB and its confidence limits over a range of willingness-to-pay thresholds illustrates not only the size of any positive values of NMB but also whether the ICER has confidence limits. The point ICER is found where the NMB line intersects with the x-axis (the net benefit for a unit of effect is zero), that is the point where the decision-maker is prepared to pay just the cost of achieving a benefit. 59 The confidence limits of the ICER are found where the confidence limits of the NMB line intersect with the x-axis. 60 An unbounded ICER (when the NMB confidence limit lines never intersect with the x-axis) indicates that neither the intervention nor the control strategy can be considered more cost-effective. 63 The CEAC depicts the probability that the NMB at a given level of willingness to pay (λ) is > 0. 64 This approach is useful for demonstrating the level of uncertainty associated with deciding that mirtazapine is cost-effective at different levels of willingness-to-pay values.
For secondary analyses of QALY and health and social care costs outcomes, the ICER and the NMB at £20,000 (the lower limit of the NICE threshold for a QALY gain)65 were calculated and presented alongside descriptive and cost-effectiveness analysis results. Probability of cost-effectiveness over a range of willingness-to-pay thresholds was calculated for narrative commentary in the text. MLM analyses were conducted in Stata 16. 66
Sensitivity analyses
Sensitivity analyses explored the impact on results of varying key assumptions made in the base case for primary and secondary analyses: including accommodation of participants in domestic as well as residential care in total health and social care costs; examining total (EQ-5D-5L) QALY and costs for the dyad (person with dementia and unpaid carer); and using an alternative valuation and definition of unpaid carer time. Accommodation costs of domestic residence were sourced from UK Household expenditure statistics (Office for National Statistics 2019) and ‘sheltered’ domestic housing. 54 Unpaid carer time was valued at replacement cost, using the hourly cost of a home care worker. This valuation was also used to calculate unpaid care time defined as the hours of the day that the person with dementia could not be left alone by the carer.
In addition, we explored the impact on results of varying the modelling approach in the primary cost-effectiveness analyses. First, we included a covariate for gender in the MLM to adjust for a baseline imbalance between groups. Second, as an alternative approach to the MLM and to address skewness typical of cost data, we applied seemingly unrelated regressions67 (where cost and outcome equations were the same as in the MLM) to 4000 replicates generated by a two-stage bootstrapping procedure suitable for clustered data. 68 This analysis was conducted in R (The R Foundation for Statistical Computing, Vienna, Austria). 69
Ethics and regulatory approvals
The study was approved by the Hampshire A South Central Research Ethics Committee (15/SC/0606), and the MHRA. It received local NHS Trust approvals and consent or assent (with legal representative consent) was obtained from all participants (see trial protocol for more details). This protocol was submitted to the UK national competent authority (MHRA).
This is a clinical trial of an IMP as defined by the EU Directive 2001/20/EC. The progress of the trial, safety issues and reports, including expedited reporting of SUSARs, was reported to the competent authority (MHRA).
Patient and public involvement
Ensuring the involvement of people living with dementia and their carers has been integral to the SYMBAD trial. This section provides further information on their important role in the trial.
Application for funding and trial design
SN is a co-applicant and has been leading on public/carer involvement in the trial throughout, from support and active involvement in the initial application of funding and trial design to the dissemination of results. Trial design also received input from a Lived Experience Advisory Panel (LEAP) group hosted by Sussex Partnership Foundation Trust (SPFT) and the Dementias and Neurodegenerative Diseases Research Network (DeNDRoN) group. The need for this trial received tremendous support from patients, public representatives and service users, keen to express the great need for a specific, effective and safe medicinal treatment for those with dementia and agitation. The protocol design was influenced through patient and public involvement (PPI) feedback, with increased attention to monitoring of AEs and review of participant burden, particularly with regard to data completion. It was felt that community-based data collection would be appropriate for this population and provide carers with additional support to make trial-based decisions in this vulnerable population.
Trial set up
Our PPI lead co-applicant (SN), along with SPFT LEAP co-ordinators (JF and JS), was involved in frequent communication to develop the trial documents and review the participant/carer-facing information. Among other points, this resulted in the ‘patient summary sheet’, aimed specifically at informing people living with dementia themselves about what being a participant in the trial would entail. Although the ethics committee felt this document alone was not sufficiently detailed to allow informed consent to be taken, it did mean that a concise summary could be provided to participants with dementia and enable them to be fully included in the decision-making process. PPI members also reviewed and advised on initial recruitment strategies, posters and information leaflets. SN, a former carer for her husband, is a member of the Alzheimer’s Society Research Network of volunteers working to raise awareness of the trial. The lead Trust (SPFT) was also supportive in raising awareness through the Clinical Research Network and leading on hosting the ‘Join Dementia Research’ website recruitment strategy [Join dementia research – register your interest in dementia research: Home (nihr.ac.uk)].
Delivery and support of the trial
SN and JF have been members of the Trial Management Group (TMG) throughout. The group had a standing agenda item to discuss trial management and delivery from the patient, carer and public perspective. This included support of the trial when recruitment became challenging and looking at ways to engage further with clinicians in order to raise awareness of the trial for potential participants.
The trial team has been grateful for the input of the two PPI members on the Trial Steering Committee (TSC), who have balanced their support for the trial to continue alongside closely reviewing recruitment levels and strategies, thus ensuring the trial achieved its objectives.
The trial team has also been grateful for the input from the dedicated LEAP, co-ordinated initially by JS, then by JF. Over the course of the trial, nine people were members of the LEAP. All of the members had experience of living with dementia, whether diagnosed themselves or as a family carer. They have asked challenging questions of the trial team and provided excellent and thoughtful guidance on the writing and phrasing of patient facing information, including advising on the content of a participant newsletter where and how to raise awareness of the trial and suggested ways to disseminate the findings accordingly. The LEAP members were keen to balance the need for this trial to answer the important question it posed while stressing the need to reduce participant burden as far as possible.
A video was produced with support from patients and their carers in Norfolk and Suffolk Foundation Trust and CRN Eastern Patient and Public Involvement Manager, as one of the strategies to overcome potential clinician ‘gatekeeper’ behaviours and increase recruitment. Previous participants in the trial volunteered to share their experiences and support of the trial.
Dissemination
SN, JF and our LEAP panel have been involved with the dissemination activities and helped with appropriate wording to convey the, perhaps less hopeful, findings of the trial from a patient and public perspective, while stressing the value of the trial findings. This has been invaluable, since although the results are extremely important in understanding what should (or should not) be prescribed in this population, it is not a step forward regarding finding a treatment that helps. The PPI team have helped in reading the main academic outputs, as well as the preparation of the plain language summary of this report and the end-of-study information sheet for trial participants. They will continue to be involved in the most effective ways to communicate the important outcomes of the trial through their respective networks.
Chapter 3 Mirtazapine versus placebo results
Patient flow
We recruited participants between January 2017 and February 2020 and completed week 12 follow-up interviews by May 2020 (See Figure 1). See Appendix 2 for recruitment by site and month.
FIGURE 1.
CONSORT flow diagram of recruitment and testing for mirtazapine and placebo groups.
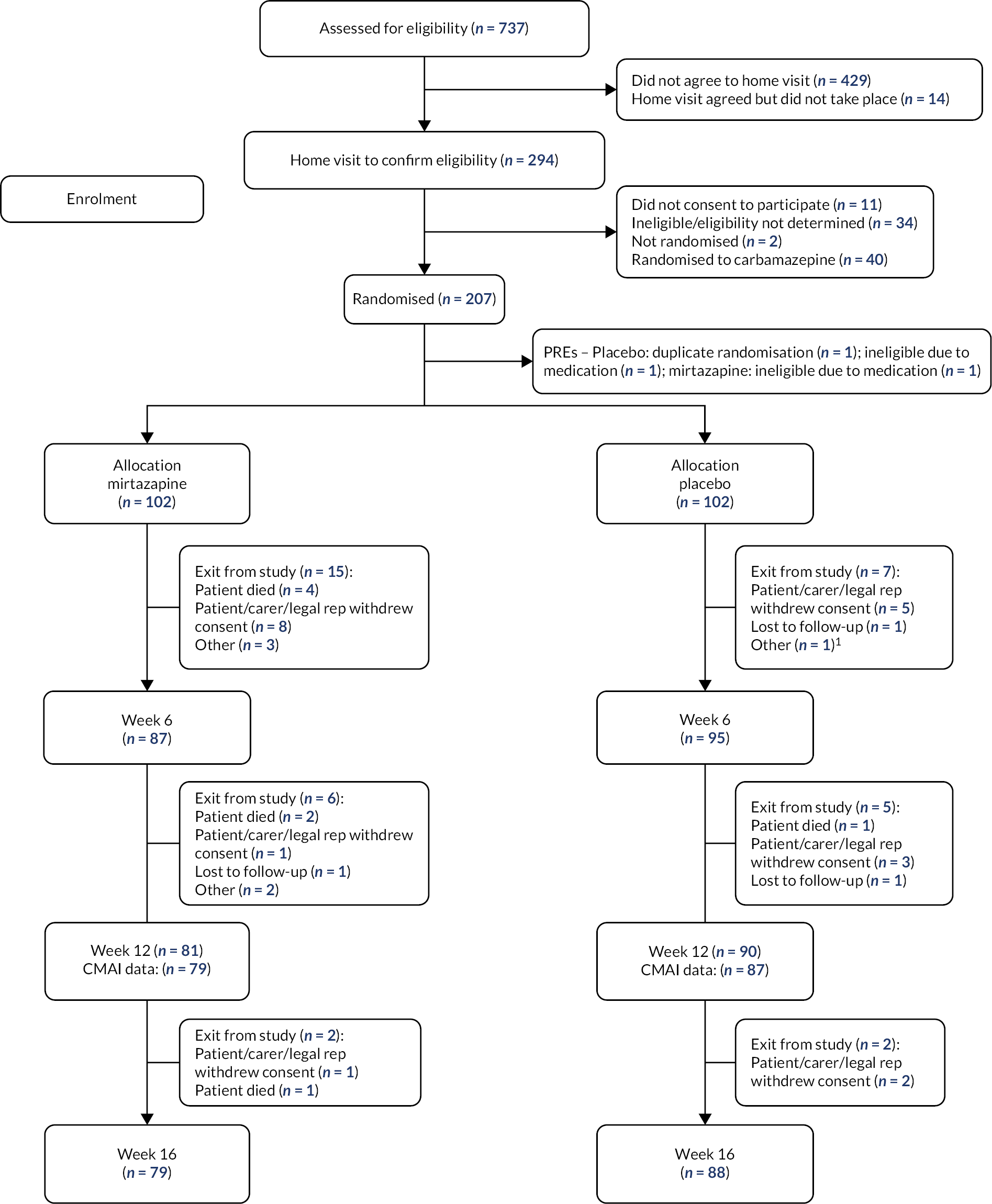
Baseline characteristics
Table 4 shows baseline demographic and clinical characteristics of participants and carers. Groups were similar at baseline except for sex with more females randomised to mirtazapine (n = 77, 75%) than placebo (n = 59, 58%). In light of this difference, sex was included in an additional model as a sensitivity analysis. By week 12, similar numbers remained in the mirtazapine (80/102, 78%) and the placebo group (89/102, 87%).
| Mirtazapine (n = 102) | Placebo (n = 102) |
|
|---|---|---|
| Participants | ||
| Age (years) (SD) | 82.2 (7.8) | 82.8 (7.7) |
| Sex | n = 102 | n = 102 |
| Female | 76 (75%) | 59 (58%) |
| Residence | n = 102 | n = 102 |
| Own household | 55 (54%) | 57 (56%) |
| Care home | 47 (46%) | 45 (44%) |
| Agitation | n = 102 | n = 102 |
| CMAI (29–203) | 71.1 (16.4) | 69.8 (17.1) |
| Cognition | n = 52 | n = 50 |
| Standardised MMSE (0–30) | 13.4 (8.1) | 16.1 (6.7) |
| Condition-specific quality of life | n = 41 | n = 37 |
| DEMQOL (28–122) | 92.4 (10.8) | 95.8 (10.2) |
| DEMQOL-Proxy (31–124) | n = 100 | n = 99 |
| 92.3 (15.0) | 90.9 (14.4) | |
| Generic quality of life | n = 100 | n = 101 |
| EQ-5D (proxy report by carer) (0–1) | 0.46 (0.34) | 0.50 (0.32) |
| Neuropsychiatric symptoms | n = 98 | n = 102 |
| NPI total score (0–144) | 32.7 (16.7) | 34.9 (18.2) |
| NPI agitation/aggression subscore (0–12) | n = 99 | n = 102 |
| 5.6 (3.2) | 5.6 (3.4) | |
| NPI depression/anxiety/irritability subscore (0–36) | n = 99 | n = 102 |
| 9.9 (6.2) | 10.5 (7.0) | |
| Suicidality | ||
| CSSRS | n = 102 | n = 102 |
| Suicidal ideation (lifetime) | 18 (18%) | 13 (13%) |
| Suicidal ideation (past month) | 11 (11%) | 11 (11%) |
| Suicidal behaviour (lifetime) | 4 (4%) | 0 |
| Suicidal behaviour (past 3 months) | 2 (2%) | 0 |
| Carers | ||
| Carer | ||
| Paid | 39 (38%) | 31 (30%) |
| Family | 63 (62%) | 71 (70%) |
| Family carer relationship | ||
| Partner or spouse | 34 (54%) | 35 (49%) |
| Son or daughter | 21 (33%) | 31 (44%) |
| Sibling | 1 (2%) | 0 |
| Other relative | 5 (8%) | 3 (4%) |
| Friend | 1 (2%) | 2 (3%) |
| Other | 1 (2%) | 0 |
| Family carer occupation (pre-retirement) | ||
| Professional | 13 (21%) | 13 (18%) |
| Managerial and technical | 23 (37%) | 22 (31%) |
| Skilled non-manual | 9 (14%) | 11 (15%) |
| Skilled manual | 11 (17%) | 8 (11%) |
| Partly skilled | 2 (3%) | 8 (11%) |
| Unskilled | 3 (5%) | 0 |
| Unemployed or unwaged | 2 (3%) | 5 (7%) |
| Unanswered | 0 | 4 (6%) |
| Carer mental health (family carers only) | n = 61 | n = 66 |
| GHQ-12 | 15.0 (5.8) | 14.5 (4.9) |
| Carer burden (family carers only) | n = 58 | n = 66 |
| Zarit Carer Burden Inventory (CBI) | 33.8 (15.7) | 34.1 (13.9) |
| Carer generic quality of life (family carers only) | n = 61 | n = 66 |
| EQ-5D | 0.79 (0.21) | 0.81 (0.22) |
| NPI carer distress subscore (0–60) | n = 94 | n = 99 |
| 14.1 (8.6) | 15.5 (9.0) | |
Primary outcome measures
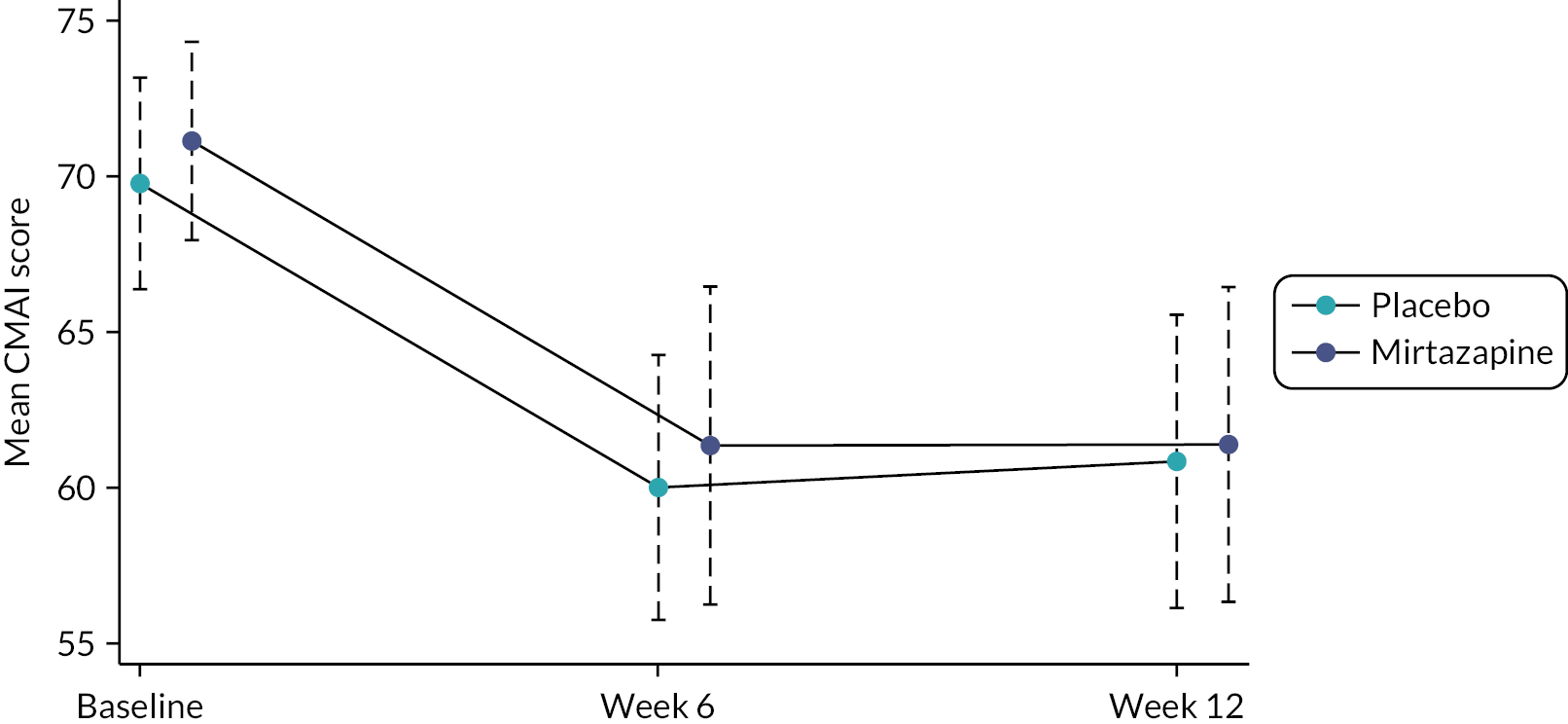
Severity of agitation decreased in both groups at 6 weeks by around 10 points and continued to be lower than baseline scores at 12 weeks (see Figure 2); this change between baseline and 6- and 12-week outcomes is illustrated by the separation in 95% confidence limits. At no point was the unadjusted or adjusted CMAI difference between the groups statistically significant (see Table 5). Table 5 presents the results from the general linear mixed modelling for the primary outcome. There was no evidence that mirtazapine improved agitation relative to placebo. The estimated adjusted effect on the CMAI was −1.74 (95% CI −7.17 to 3.69; p = 0.530). This changed little with the addition of sex into the model.
FIGURE 2.
Unadjusted mean CMAI scores (95% CI) by treatment group.
| Mirtazapine (n = 102) |
Placebo (n = 102) |
Difference | (95% CI) | Adjusted differencea | (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|
| 12-week primary outcome | |||||||
| Agitation (CMAI) | n = 79 | n = 87 | |||||
| 61.4 (SD 22.6) | 60.8 (SD 21.8) | 0.59 | (−6.22 to 7.40) | −1.74 | (−7.17 to 3.69) | 0.530 | |
| −0.93b | −6.42 to 4.56 | 0.739 | |||||
| 6-week secondary outcomes | |||||||
| Agitation (CMAI) | n = 84 | n = 88 | |||||
| 61.4 (23.5) | 60.0 (19.9) | 1.39 | (−5.15 to 7.93) | −0.55 | (−6.18 to 5.08) | 0.848 | |
| Cognition (sMMSE) | n = 33 | n = 31 | |||||
| 15.5 (7.1) | 16.2 (7.2) | −0.68 | (−4.25 to 2.89) | 0.14 | (−1.17 to 1.45) | 0.836 | |
| Quality of life (DEMQOL) | n = 32 | n = 32 | |||||
| 95.1 (10.2) | 96.8 (8.4) | −1.69 | (−6.38 to 3.00) | 1.12 | (−2.74 to 4.97) | 0.570 | |
| Quality of life (DEMQOL-Proxy) | n = 79 | n = 86 | |||||
| 96.6 (14.7) | 94.6 (16.2) | 2.03 | (−2.74 to 6.79) | 0.80 | (−3.18 to 4.77) | 0.694 | |
| Quality of life EQ-5D (proxy report by carer) | n = 82 | n = 87 | |||||
| 0.48 (0.33) | 0.56 (0.30) | −0.08 | (−0.17 to 0.02) | −0.07 | (−0.13 to 0.00) | 0.061 | |
| Neuropsychiatric symptoms | |||||||
| NPI total score | n = 84 | n = 88 | |||||
| 27.1 (20.0) | 24.8 (20.0) | 2.29 | (−3.73 to 8.31) | 2.03 | (−2.89 to 6.95) | 0.419 | |
| NPI agitation/aggression subscore | n = 84 | n = 88 | |||||
| 4.0 (3.6) | 4.2 (3.5) | −0.20 | (−1.28 to 0.87) | −0.34 | (−1.30 to 0.62) | 0.490 | |
| NPI depression/anxiety/irritability subscore | n = 84 | n = 88 | |||||
| 7.9 (7.7) | 7.2 (8.2) | 0.68 | (−1.72 to 3.07) | 0.70 | (−1.24 to 2.63) | 0.482 | |
| 12-week secondary outcomes | |||||||
| Cognition (sMMSE) | n = 23 | n = 27 | |||||
| 18.0 (6.0) | 15.6 (7.5) | 2.44 | (−1.48 to 6.37) | 1.45 | (−0.20 to 3.10) | 0.084 | |
| Quality of life (DEMQOL) | n = 24 | n = 24 | |||||
| 94.3 (7.1) | 97.1 (8.4) | −2.83 | (−7.35 to 1.68) | −1.36 | (−5.82 to 3.10) | 0.549 | |
| Quality of life (DEMQOL-Proxy) | n = 71 | n = 82 | |||||
| 98.4 (14.5) | 97.5 (12.4) | 0.93 | (−3.37 to 5.23) | 0.44 | (−3.09 to 3.96) | 0.809 | |
| Quality of life EQ-5D | n = 77 | n = 84 | |||||
| (proxy report by carer) | 0.46 (0.35) | 0.50 (0.33) | −0.04 | (−0.14 to 0.07) | −0.01 | (−0.08 to 0.07) | 0.822 |
| Neuropsychiatric symptoms | n = 75 | n = 84 | |||||
| NPI total score | 23.9 (17.8) | 25.7 (19.6) | −1.80 | (−7.69 to 4.09) | −2.02 | (−6.67 to 2.62) | 0.393 |
| NPI agitation/aggression subscore | n = 76 | n = 84 | |||||
| 4.1 (3.4) | 4.5 (3.6) | −0.40 | (−1.49 to 0.70) | −0.52 | (−1.52 to 0.47) | 0.305 | |
| NPI depression/anxiety/irritability subscore | n = 75 | n = 84 | |||||
| 6.9 (6.7) | 7.3 (8.0) | −0.44 | (−2.77 to 1.88) | −0.58 | (−2.43 to 1.27) | 0.541 | |
Secondary outcome measures
Table 5 shows the effect of mirtazapine compared with placebo on secondary outcomes in participants and Table 6 in carers. Again, there was no evidence of difference between the groups, apart from: a single statistically significant difference in the Zarit Carer Burden Inventory at 12 weeks which indicated higher carer burden in the mirtazapine group (adjusted difference 5.01 points, 95% CI 0.80 to 9.23; p = 0.020); weaker evidence at 6 weeks (3.76, −0.03 to 7.83); p = 0.069) in the same variable; and a weak association between higher proxy-rated ED-5D quality of life in the placebo group at 6 weeks (−0.07, −0.13 to 0.00, p = 0.061) that was not maintained at 12 weeks (−0.01, −0.08 to 0.07, p = 0.822).
| Mirtazapine (n = 102) |
Placebo (n = 102) |
Difference | (95% CI) | Adjusted differencea | (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|
| 6-week outcomes | |||||||
| Carer GHQ-12b | n = 50 | n = 54 | |||||
| 12.8 (6.2) | 12.1 (4.9) | 0.69 | (−1.47 to 2.85) | 0.61 | (−1.21 to 2.42) | 0.512 | |
| Carer EQ-5Db | n = 50 | n = 55 | |||||
| 0.83 (0.16) | 0.83 (0.15) | 0.00 | (−0.06 to 0.06) | 0.01 | (−0.04 to 0.05) | 0.821 | |
| Zarit CBIb | n = 46 | n = 49 | |||||
| 34.7 (16.3) | 29.4 (13.9) | 5.35 | (0.82 to 11.53) | 3.76 | (−0.30 to 7.83) | 0.069 | |
| NPI carer distress subscore | n = 78 | n = 84 | |||||
| 11.5 (1.1) | 10.2 (8.8) | 1.37 | (−1.45 to 4.19) | 1.48 | (−0.78 to 3.73) | 0.199 | |
| 12-week outcomes | |||||||
| Carer GHQ-12b | n = 44 | n = 52 | |||||
| 13.1 (6.0) | 12.2 (5.4) | 0.88 | (−1.43 to 3.19) | 0.36 | (−1.58 to 2.31) | 0.714 | |
| Carer EQ-5Db | n = 46 | n = 49 | |||||
| 0.80 (0.16) | 0.82 (0.19) | −0.02 | (−0.09 to 0.06) | 0.02 | (−0.04 to 0.07) | 0.561 | |
| Zarit CBIb | n = 42 | n = 48 | |||||
| 35.5 (17.2) | 29.0 (15.8) | 6.48 | (−0.43 to 13.39) | 5.01 | (0.80 to 9.23) | 0.020 | |
| NPI carer distress subscore | n = 72 | n = 81 | |||||
| 10.0 (8.6) | 10.5 (8.3) | −0.52 | (−3.22 to 2.17) | −0.27 | (−2.34 to 1.80) | 0.798 | |
Adverse events and severe AEs were ascertained to 16 or 4 weeks after last dose of IMP; deaths were recorded up to 16 weeks after randomisation. Examining AEs by week 16, there were 192 in 102 participants in the placebo group, of whom 65 (64%) individuals had at least one AE, compared with 225 events in 102 participants in the mirtazapine group of whom 67 (66%) had at least one. There were 35 SAEs in 18 individuals in the placebo group, compared with 13 in 8 individuals in the mirtazapine group. Mortality differed between groups with a potentially higher rate in the mirtazapine group (seven deaths in the mirtazapine and one in the placebo group by 16-week safety follow-up). Post hoc statistical analysis suggested weak evidence of a mortality difference between groups (Fisher’s exact test p = 0.065). Causes of death coded with MedDRA (Medical Dictionary for Regulatory Activities) terms showed no consistent pattern with the one death in the placebo group attributed to dementia, and the seven in the mirtazapine group to: (i) dementia; (ii) pneumonia, aspiration; (iii) emphysema, dementia, pneumonia, aspiration; (iv) dementia Alzheimer’s type; (v) cardiac failure; (vi) pelvic fracture, osteoporosis, vascular dementia; and (vii) chronic kidney disease, dementia, congestive cardiac failure. See Table 7. See Appendix 3 for a summary of AEs and severe AEs by randomisation group.
| Mirtazapine (n = 102) |
Placebo (n = 102) |
|
|---|---|---|
| AE | ||
| Number of events | 225 | 192 |
| Number of individuals | 67 (66%) | 65 (64%) |
| SAE | ||
| Number of events | 13 | 35 |
| Number of individuals | 8 (8%) | 18 (18%) |
| Deaths | 7 (7%) | 1 (1%) |
| MedDRA codes for deaths |
|
|
Long-term outcomes at 26 and 52 weeks
CMAI outcomes at 26 and 52 weeks
CMAI outcomes at 26 and 52 weeks are presented in Table 8. There were no statistically significant differences between mirtazapine and placebo at either time point. This applied to both the raw and adjusted differences.
| Mirtazapine (n = 102) |
Placebo (n = 102) |
Difference | (95% CI) | Adjusted differencea | (95% CI) | p-value |
|---|---|---|---|---|---|---|
| 26 weeks | ||||||
| n = 67 | n = 62 | |||||
| 61.9 (SD = 21.0) |
56.8 (SD = 19.7) |
5.1 | (−2.02 to 12.20) | 1.36 | (−4.32 to 7.05) | 0.638 |
| 52 weeks | ||||||
| n = 53 | n = 56 | |||||
| 56.8 (SD = 16.2) | 58.5 (SD = 20.8) | −1.6 | (−8.76 to 5.46) | −3.26 | (−9.91 to 3.39) | 0.337 |
| Mirtazapine (n = 102) |
Placebo (n = 102) |
|
|---|---|---|
| Hospitalisations by 26 weeks | ||
| Yes | 4 (5.6%) | 10 (14.3%) |
| No | 68 (94.4%) | 60 (85.7%) |
| No information | 30 | 32 |
| Days in hospital by 26 weeks | ||
| N | 4 | 10 |
| Mean (SD) | 8.3 (4.9) | 16.8 (30.6) |
| Median (IQR) | 10 (5.5–11) | 5.5 (1–14) |
| N missing | 0 | 0 |
| Hospitalisations between 26 and 52 weeks | ||
| Yes | 6 (10.2%) | 9 (15.0%) |
| No | 53 (89.8%) | 51 (85.0%) |
| No information | 43 | 42 |
| Days in hospital between 26 and 52 weeks | ||
| N | 6 | 8 |
| Mean (SD) | 16.3 (23.0) | 20.1 (30.3) |
| Median (IQR) | 2 (1–44) | 9 (3–22) |
| N missing | 0 | 1 |
Hospitalisation at 26 and 52 weeks
Hospitalisation by 26 weeks and between 26 and 52 weeks are presented in Table 9. There were no statistically significant differences between mirtazapine and placebo for either time period.
Deaths at 26 and 52 weeks
The cumulative number of deaths at 26 and 52 weeks are presented in Table 10. The marginal differences observed at 12 weeks were not maintained at 26 and 52 weeks and there were no statistically significant differences between mirtazapine and placebo at either time point.
| Mirtazapine (n = 102) |
Placebo (n = 102) |
|
|---|---|---|
| By 26 weeks | ||
| Died | 7 (9.0%) | 6 (8.1%) |
| Alive | 71 (91.0%) | 68 (91.9%) |
| No information | 24 | 28 |
| By 52 weeks | ||
| Died | 15 (19.7%) | 13 (18.6%) |
| Alive | 61 (80.3%) | 57 (81.4%) |
| No information | 26 | 32 |
Economic evaluation
Data were reasonably complete for most service-use items (see Table 11) (ranging from 96% to 100% at baseline, 94% to 100% at 6 weeks, 94% to 100% at 12 weeks). Data on carers’ care time and service use were similarly complete at baseline (94–99%) but slightly less so at 6 weeks (87–90%) and 12 weeks (91–94%). A filter question in the database classified informants as paid or unpaid carers to determine which carer measures should be completed. A few cases that were reported to be family/friend carers in the demographics question were classified as paid carers on this question, resulting in the loss of unpaid carer resource-use data from placebo participants (three cases at baseline; four at 6 and 12 weeks).
| Service/item | Units | Valid N | Mirtazapine No. users (%) |
Mirtazapine Mean (SE) |
Valid N | Placebo No. users (%) |
Placebo Mean (SE) |
|---|---|---|---|---|---|---|---|
| Baseline (prior 12 weeks) | Expected = 103 | Expected = 103 | |||||
| Community health | |||||||
| GP | Visits | 99 | 65 (66) | 1.61 (0.28) | 101 | 71 (70) | 1.84 (0.30) |
| Practice nurse | Visits | 98 | 25 (26) | 0.52 (0.20) | 101 | 19 (19) | 0.25 (0.06) |
| Community nurse | Visits | 98 | 23 (23) | 0.69 (0.20) | 101 | 19 (19) | 0.75 (0.28) |
| Physiotherapist | Visits | 98 | 6 (6) | 0.11 (0.06) | 101 | … | … |
| OT | Visits | 99 | … | … | 102 | … | … |
| Geriatrician | Visits | 99 | … | … | 102 | … | … |
| Neurologist | Visits | 99 | … | … | 102 | … | … |
| Specialist nurse | Visits | 98 | 8 (8) | 0.12 (0.05) | 101 | 14 (14) | 0.25 (0.09) |
| Mental health | |||||||
| Mental health nurse | Visits | 99 | 37 (37) | 0.75 (0.18) | 101 | 43 (43) | 0.74 (0.11) |
| Psychiatrist | Visits | 98 | 33 (34) | 0.39 (0.06) | 101 | 31 (31) | 0.38 (0.06) |
| Psychologist | Visits | 99 | … | … | 102 | … | … |
| Mental health team | Visits | 98 | 11 (11) | 0.27 (0.12) | 101 | 6 (6) | 0.15 (0.07) |
| Community care | |||||||
| Home care | Visits | 101 | 15 (15) | 9.73 (3.07) | 102 | 14 (14) | 13.28 (4.31) |
| Home care | Hours | 101 | 15 (15) | 30.54 (20.19) | 102 | 14 (14) | 46.13 (27.81) |
| Social worker | Visits | 98 | 16 (16) | 0.19 (0.05) | 101 | 17 (17) | 0.36 (0.13) |
| Cleaner | Visits | 101 | 10 (10) | 2.56 (1.68) | 102 | 8 (8) | 1.07 (0.41) |
| Meals on Wheels | Visits | 102 | … | … | 103 | … | … |
| Sitting service | Visits | 102 | … | … | 103 | … | … |
| Carer support worker | Visits | 102 | … | … | 103 | … | … |
| Day services | |||||||
| Day centre | Attendances | 99 | 15 (15) | 3.84 (1.77) | 101 | 10 (10) | 1.52 (0.56) |
| Lunch club | Attendances | 99 | … | … | 102 | … | … |
| Hospital care | |||||||
| ED | Attendances | 99 | 18 (18) | 0.24 (0.06) | 101 | 15 (15) | 0.20 (0.05) |
| Inpatients services | Days | 99 | 12 (12) | 1.86 (0.79) | 101 | 9 (9) | 0.95 (0.43) |
| Day hospital services | Days | 99 | … | … | 102 | … | … |
| Outpatients services | Visits | 99 | 21 (21) | 0.29 (0.07) | 101 | 19 (19) | 0.32 (0.09) |
| Care home resident | |||||||
| Residential home | Days | 100 | 14 (14) | 16.17 (3.30) | 102 | 12 (12) | 13.73 (3.03) |
| Nursing home | Days | 100 | 32 (32) | 30.01 (4.02) | 102 | 32 (31) | 29.17 (3.95) |
| Residential respite | |||||||
| Residential home | Days | 100 | … | … | 102 | … | … |
| Nursing home | Days | 100 | … | … | 102 | … | … |
| Medications | |||||||
| Number of medications | Units | 102 | 92 (90) | 5.00 (0.34) | 102 | 95 (93) | 5.25 (0.33) |
| Equip. & adaptations | |||||||
| Equip. (HSC) | Items | 102 | 11 (11) | 0.25 (0.08) | 103 | 10 (10) | 0.24 (0.08) |
| Unpaid care;a out-of-pocket | |||||||
| Equipment (private) | Items | 101 | 11 (11) | 0.15 (0.05) | 102 | … | … |
| Unpaid care – carerb | Hours | 60 | 60 (100) | 857.90 (68.60) | 67 | 67 (100) | 716.78 (66.00) |
| Carer cut down work | Hours | 61 | … | … | 68 | … | … |
| Carer stopped work | Weeks | 60 | … | … | 68 | … | … |
| Unpaid care oth. carers | Hours | 60 | 37 (62) | 106.27 (22.33) | 67 | 44 (66) | 178.02 (46.29) |
| Time off work oth. carers | Days | 60 | … | … | 67 | 8 (12) | 1.47 (1.19) |
| Week 6 (prior 6 weeks) | Expected = 87 | Expected = 95 | |||||
| Community health | |||||||
| GP | Visits | 83 | 36 (43) | 0.81 (0.14) | 90 | 31 (34) | 0.89 (0.20) |
| Practice nurse | Visits | 84 | 11 (13) | 0.29 (0.15) | 90 | 7 (8) | 0.10 (0.05) |
| Community nurse | Visits | 84 | 13 (15) | 0.18 (0.05) | 90 | 13 (14) | 0.69 (0.25) |
| Physiotherapist | Visits | 84 | … | … | 90 | … | … |
| OT | Visits | 84 | … | … | 90 | … | … |
| Geriatrician | Visits | 84 | … | … | 90 | … | … |
| Neurologist | Visits | 84 | … | … | 90 | … | … |
| Specialist nurse | Visits | 84 | 6 (7) | 0.08 (0.03) | 90 | 6 (7) | 0.14 (0.06) |
| Mental health | |||||||
| Mental health nurse | Visits | 84 | 8 (10) | 0.20 (0.08) | 90 | 16 (18) | 0.26 (0.07) |
| Psychiatrist | Visits | 84 | 9 (11) | 0.12 (0.04) | 90 | 9 (10) | 0.14 (0.06) |
| Psychologist | Visits | 84 | … | … | 90 | … | … |
| Mental health team | Visits | 84 | … | … | 90 | 7 (8) | 0.10 (0.04) |
| Community care | |||||||
| Home care | Visits | 86 | 12 (14) | 3.48 (1.34) | 95 | 12 (13) | 7.21 (3.06) |
| Home care | Hours | 86 | 12 (14) | 5.58 (1.95) | 95 | 12 (13) | 14.23 (10.65) |
| Social worker | Visits | 84 | … | … | 90 | 12 (13) | 0.17 (0.05) |
| Cleaner | Visits | 86 | 8 (9) | 0.77 (0.39) | 95 | 7 (7) | 0.60 (0.24) |
| Meals on Wheels | Visits | 86 | … | … | 95 | … | … |
| Sitting service | Visits | 86 | … | … | 95 | 7 (7) | 0.51 (0.24) |
| Carer support worker | Visits | 86 | … | … | 95 | … | … |
| Day services | |||||||
| Day centre | Attendances | 84 | 10 (12) | 1.39 (0.59) | 91 | 8 (9) | 0.63 (0.25) |
| Lunch club | Attendances | 84 | … | … | 91 | … | … |
| Hospital care | |||||||
| ED | Attendances | 84 | … | … | 89 | 9 (10) | 0.12 (0.04) |
| Inpatients services | Days | 84 | … | … | 90 | … | … |
| Day hospital services | Days | 84 | … | … | 89 | … | … |
| Outpatients services | Visits | 84 | 8 (10) | 0.12 (0.05) | 89 | 7 (8) | 0.09 (0.03) |
| Care home resident | |||||||
| Residential home | Days | 85 | 16 (19) | 10.05 (1.92) | 91 | 14 (15) | 8.48 (1.75) |
| Nursing home | Days | 85 | 23 (27) | 14.00 (2.16) | 91 | 29 (32) | 15.82 (2.15) |
| Residential respite | |||||||
| Residential home | Days | 84 | … | … | 91 | … | … |
| Nursing home | Days | 84 | … | … | 91 | … | … |
| Medications | |||||||
| Number of medications | Units | 87 | 80 (92) | 5.54 (0.40) | 95 | 91 (96) | 5.96 (0.35) |
| Equip. & adaptations | |||||||
| Equip. (HSC) | Items | 86 | 7 (8) | 0.15 (0.06) | 95 | … | … |
| Unpaid care a ; out-of-pocket | |||||||
| Equipment (private) | Items | 86 | 8 (9) | 0.13 (0.05) | 95 | 8 (8) | 0.18 (0.07) |
| Unpaid care – carerb | Hours | 49 | 49 (100) | 471.49 (35.34) | 55 | 54 (98) | 353.62 (35.07) |
| Carer cut down work | Hours | 51 | … | … | 59 | … | … |
| Carer stopped work | Weeks | 51 | … | … | 59 | … | … |
| Unpaid care oth. carers | Hours | 50 | 33 (66) | 65.37 (16.14) | 56 | 33 (59) | 61.17 (14.94) |
| Time off work oth. carers | Days | 50 | … | … | 56 | … | … |
| Week 12 (prior 6 weeks) | Expected = 81 | Expected = 90 | |||||
| Community health | |||||||
| GP | Visits | 78 | 35 (45) | 0.73 (0.13) | 86 | 38 (44) | 0.94 (0.20) |
| Practice nurse | Visits | 78 | 9 (12) | 0.14 (0.05) | 86 | 9 (10) | 0.12 (0.04) |
| Community nurse | Visits | 78 | 10 (13) | 0.27 (0.12) | 86 | 14 (16) | 0.51 (0.18) |
| Physiotherapist | Visits | 78 | … | … | 86 | … | … |
| OT | Visits | 78 | … | … | 86 | … | … |
| Geriatrician | Visits | 78 | … | … | 86 | … | … |
| Neurologist | Visits | 78 | … | … | 86 | … | … |
| Specialist nurse | Visits | 78 | … | … | 86 | … | … |
| Mental health | |||||||
| Mental health nurse | Visits | 78 | 9 (12) | 0.15 (0.05) | 86 | 10 (12) | 0.16 (0.05) |
| Psychiatrist | Visits | 78 | … | … | 86 | 6 (7) | 0.06 (0.03) |
| Psychologist | Visits | 78 | … | … | 86 | … | … |
| Mental health team | Visits | 78 | … | … | 86 | … | … |
| Community care | |||||||
| Home care | Visits | 81 | 9 (11) | 3.27 (1.41) | 89 | 9 (10) | 6.10 (2.35) |
| Home care | Hours | 81 | 9 (11) | 5.18 (2.15) | 89 | 9 (10) | 17.53 (11.69) |
| Social worker | Visits | 78 | … | … | 86 | … | … |
| Cleaner | Visits | 81 | 6 (7) | 0.30 (0.13) | 89 | 6 (7) | 0.34 (0.15) |
| Meals on Wheels | Visits | 81 | … | … | 89 | … | … |
| Sitting service | Visits | 81 | … | … | 89 | 6 (7) | 0.46 (0.21) |
| Carer support worker | Visits | 81 | … | … | 89 | … | … |
| Day services | |||||||
| Day centre | Attendances | 78 | 8 (10) | 1.53 (0.65) | 86 | … | … |
| Lunch club | Attendances | 78 | … | … | 86 | … | … |
| Hospital care | |||||||
| ED | Attendances | 78 | … | … | 86 | 6 (7) | 0.07 (0.03) |
| Inpatients services | Days | 78 | … | … | 87 | … | … |
| Day hospital services | Days | 78 | … | … | 86 | … | … |
| Outpatients services | Visits | 78 | 8 (10) | 0.12 (0.04) | 86 | 7 (8) | 0.13 (0.05) |
| Care home resident | |||||||
| Residential home | Days | 78 | 15 (19) | 11.05 (2.11) | 87 | 13 (15) | 9.14 (1.86) |
| Nursing home | Days | 78 | 23 (29) | 14.65 (2.25) | 87 | 28 (32) | 15.64 (2.18) |
| Residential respite | |||||||
| Residential home | Days | 77 | … | … | 85 | … | … |
| Nursing home | Days | 77 | … | … | 85 | … | … |
| Medications | |||||||
| Number of medications | Units | 81 | 76 (94) | 5.99 (0.44) | 90 | 83 (92) | 5.98 (0.40) |
| Equip. & adaptations | |||||||
| Equip. (HSC) | Items | 80 | 8 (10) | 0.19 (0.07) | 90 | … | … |
| Unpaid care;a out-of-pocket | |||||||
| Equipment (private) | Items | 80 | … | … | 90 | 8 (9) | 0.17 (0.07) |
| Unpaid care – carerb | Hours | 46 | 46 (100) | 481.37 (35.50) | 52 | 52 (100) | 336.46 (36.89) |
| Carer cut down work | Hours | 46 | … | … | 52 | … | … |
| Carer stopped work | Weeks | 45 | … | … | 52 | … | … |
| Unpaid care oth. carers | Hours | 46 | 30 (65) | 46.13 (11.93) | 52 | 30 (58) | 71.66 (18.35) |
| Time off work oth. carers | Days | 46 | … | … | 52 | … | … |
Table 11 sets out paid and unpaid care services used by participants at baseline and follow-ups. Less than half of participants had used a mental health service in the 12 weeks prior to baseline. Participant use of community and mental health services between the 6-week and 12-week follow-up was similar to use between baseline and 6-week assessment. Relatively few participants (15% mirtazapine; 14% placebo) had home care in the pre-baseline period, for means of 2.5 and 3.8 hours per week of home care in the mirtazapine and placebo groups. In the sample participating at 12 weeks, proportions using home care were similar (11% mirtazapine; 10% placebo), although mirtazapine participants had used less than an hour a week (0.86) while the placebo participants had used almost 3 hours (2.92) in the prior 6 weeks. At baseline, hours provided by unpaid carers greatly exceeded paid home care hours (71 and 60 hours per week in mirtazapine and placebo, respectively). At 12-week follow-up, mirtazapine participants received approximately 80 hours per week while placebo participants received 56 hours per week of unpaid care.
Carers’ own use of health and support services is presented in Table 12. Data were fairly complete from carers classified as unpaid (95–99% at baseline, 90–94% at 6 weeks and 91–94% at 12 weeks). More than half had made use of at least one service over the 12 weeks prior to baseline, and approximately half made use of a service over each follow-up. Carers were asked to estimate the proportion of all services related to their caring role, judging this to be 22% and 23% in mirtazapine and placebo groups, respectively at baseline. Estimated proportions were similar at 6 weeks; however, the sample completing 12-week assessments reported divergent estimates (mirtazapine: mean of 50.8% vs. placebo mean of 19.2%). Carers were also asked at each point whether they felt that their care situation had improved since they had used these services and whether their health had been affected as a result of caring (see Table 13). While groups did not differ on the status of their care situation at baseline or 6 weeks, at 12 weeks, more mirtazapine than placebo carers in receipt of at least one service agreed their situation had improved [12/24 (50%) vs. 4/24 (16.7%), respectively]. The proportion of carers reporting that their health was affected by their caring role was at least 50% at each time point and proportions were similar between groups. At baseline, carers of people with dementia living at home reported substantial numbers of hours of sleep lost per week as a result of assisting the person or because of the person’s agitation (approximately 8 and 7 hours weekly in mirtazapine and placebo, respectively) (see Table 14). Hours of lost sleep were similar at the 6- and 12-week follow-ups. At baseline, approximately half of the carers in each group reported that the person could be left alone at home. Participants could be left alone for an average of < 3 hours a day. These estimates were similar at 6- and 12-week follow-ups.
| Service/item | Units | Valid N | Mirtazapine No. users (%) |
Mirtazapine Mean (SE) |
Valid N | Placebo No. users (%) |
Placebo Mean (SE) |
|---|---|---|---|---|---|---|---|
| Baseline (prior 12 weeks) | Expected = 63 | Expected = 68 | |||||
| Community health | |||||||
| GP | Visits | 60 | 21 (35) | 0.83 (0.23) | 67 | 24 (36) | 0.58 (0.12) |
| Practice nurse | Visits | 60 | 15 (25) | 0.27 (0.07) | 67 | 18 (27) | 0.45 (0.14) |
| Community nurse | Visits | 60 | … | … | 67 | … | … |
| Mental health | |||||||
| Admiral Nurse | Visits | 60 | … | … | 67 | … | … |
| Psychiatrist | Visits | 60 | … | … | 67 | … | … |
| Psychologist | Visits | 60 | … | … | 67 | … | … |
| Mental health team | Visits | 60 | … | … | 67 | … | … |
| Counsellor | Visits | 60 | … | … | 67 | … | … |
| Hospital care | |||||||
| Outpatients services | Visits | 60 | 16 (27) | 0.63 (0.24) | 67 | 15 (22) | 0.31 (0.08) |
| Support services | |||||||
| Online support | Visits | 60 | 6 (10) | 0.18 (0.09) | 67 | … | … |
| Support groups | Visits | 60 | … | … | 67 | … | … |
| Education groups | Visits | 60 | … | … | 67 | … | … |
| Expert relative groups | Visits | 60 | 6 (10) | 0.20 (0.11) | 67 | … | … |
| Alternative therapies | Visits | 60 | 6 (10) | 0.18 (0.09) | 67 | … | … |
| Proportion of use related to caring role – carera | Percentage | 60 | 41 (68) | 22.20 (4.68) | 67 | 37 (55) | 23.27 (4.58) |
| Medications | |||||||
| Prescription medications | Any used | 60 | 42 (70) | 67 | 46 (69) | ||
| OTC medications | Any used | 60 | 25 (42) | 67 | 28 (42) | ||
| Week 6 (prior 6 weeks) | Expected = 53 | Expected = 60 | |||||
| Community health | |||||||
| GP | Visits | 50 | 11 (22) | 0.30 (0.09) | 55 | 12 (22) | 0.29 (0.08) |
| Practice nurse | Visits | 50 | … | … | 55 | 7 (13) | 0.13 (0.05) |
| Community nurse | Visits | 50 | … | … | 55 | … | … |
| Mental health | |||||||
| Admiral Nurse | Visits | 50 | … | … | 55 | … | … |
| Psychiatrist | Visits | 50 | … | … | 55 | … | … |
| Psychologist | Visits | 50 | … | … | 55 | … | … |
| Mental health team | Visits | 50 | … | … | 55 | … | … |
| Counsellor | Visits | 50 | … | … | 55 | … | … |
| Hospital care | |||||||
| Outpatients services | Visits | 50 | 8 (16) | 0.36 (0.18) | 55 | 11 (20) | 0.27 (0.08) |
| Support services | |||||||
| Online support | Visits | 50 | … | … | 55 | … | … |
| Support groups | Visits | 50 | … | … | 55 | … | … |
| Education groups | Visits | 50 | … | … | 55 | … | … |
| Expert relative groups | Visits | 50 | … | … | 55 | … | … |
| Alternative therapies | Visits | 50 | … | … | 55 | … | … |
| Proportion of use related to caring role – carera | Percentage | 50 | 24 (48) | 36.04 (6.09) | 55 | 25 (45) | 33.38 (5.87) |
| Medications | |||||||
| Prescription medications | Use – y/n | 50 | 32 (64) | 54 | 35 (65) | ||
| OTC medications | Use – y/n | 50 | 18 (36) | 54 | 19 (35) | ||
| Week 12 (prior 6 weeks) | Expected = 49 | Expected = 56 | |||||
| Community health | |||||||
| GP | Visits | 46 | 12 (26) | 0.43 (0.12) | 52 | 10 (19) | 0.29 (0.09) |
| Practice nurse | Visits | 46 | 7 (15) | 0.41 (0.26) | 52 | 6 (12) | 0.12 (0.04) |
| Community nurse | Visits | 46 | … | … | 52 | … | … |
| Mental health | |||||||
| Admiral Nurse | Visits | 46 | … | … | 52 | … | … |
| Psychiatrist | Visits | 46 | … | … | 52 | … | … |
| Psychologist | Visits | 46 | … | … | 52 | … | … |
| Mental health team | Visits | 46 | … | … | 52 | … | … |
| Counsellor | Visits | 46 | … | … | 52 | … | … |
| Hospital care | |||||||
| Outpatients services | Visits | 46 | 8 (17) | 0.67 (0.46) | 52 | 8 (15) | 0.17 (0.06) |
| Support services | |||||||
| Online support | Visits | 46 | … | … | 52 | … | … |
| Support groups | Visits | 46 | … | … | 52 | … | … |
| Education groups | Visits | 46 | … | … | 52 | … | … |
| Expert relative groups | Visits | 46 | … | … | 52 | … | … |
| Alternative therapies | Visits | 46 | … | … | 52 | … | … |
| Proportion of service use related to caring – carera | Percentage | 46 | 26 (57) | 50.80 (6.52) | 52 | 25 (48) | 19.20 (5.23) |
| Medications | |||||||
| Prescription medications | Use – y/n | 46 | 33 (72) | 51 | 34 (67) | ||
| OTC medications | Use – y/n | 46 | 20 (43) | 52 | 23 (44) | ||
| Care situation improved by servicesa | p (Fisher’s) | Health affected as a result of caringb | p | |||
|---|---|---|---|---|---|---|
| Mirtazapine | Placebo | Mirtazapine | Placebo | |||
| Users/N (%) | Users/N (%) | Users/N (%) | Users/N (%) | |||
| Baseline | 10/37 (27.03) | 8/37 (21.62) | p = 0.787 | 32/60 (53.33) | 39/67 (58.21) | χ2 = 0.305, p = 0.581 |
| 6 weeks | 4/22 (18.18) | 9/24 (37.5) | p = 0.197 | 29/50 (58) | 33/54 (61.11) | χ2 = 0.104, p = 0.747 |
| 12 weeks | 12/24 (50) | 4/24 (16.67) | p = 0.030 | 23/46 (50) | 27/51 (52.94) | χ2 = 0.084, p = 0.772 |
| Service/item | Units | Valid N | Mirtazapine Yes (%) |
Mirtazapine Mean (SE) |
Valid N | Placebo Yes (%) |
Placebo Mean (SE) |
|---|---|---|---|---|---|---|---|
| Baseline | Expected = 52 | Expected = 57 | |||||
| Left alone at homea | Hours/day | 51 | 28 (55) | 2.50 (0.59) | 57 | 32 (56) | 2.76 (0.66) |
| Sleep disrupted | Hours lost/week | 51 | 28 (55) | 7.71 (1.38) | 57 | 33 (58) | 6.89 (1.24) |
| Week 6 | Expected = 46 | Expected = 47 | |||||
| Left alone at homea | Hours/day | 45 | 22 (49) | 1.62 (0.36) | 46 | 27 (59) | 3.02 (0.79) |
| Sleep disrupted | Hours lost/week | 45 | 25 (56) | 6.30 (1.27) | 44 | 21 (48) | 5.91 (1.35) |
| Week 12 | Expected = 40 | Expected = 45 | |||||
| Left alone at homea | Hours/day | 40 | 17 (43) | 1.53 (0.46) | 45 | 26 (58) | 2.89 (0.81) |
| Sleep disrupted | Hours lost/week | 40 | 22 (55) | 6.31 (1.41) | 45 | 17 (38) | 5.24 (1.25) |
Costs
At baseline, of cases with economic data available, there were no differences between groups in any subcategory of cost, in total health and social care or in societal costs of participants with dementia (see Table 15). Appendix 4 presents the unit costs used. Apart from the costs of trial medication, there were no between-group cost differences in the sample participating at 6 weeks. Of those participating in the 12-week follow-up, the costs of unpaid care by the dyadic carer over the prior 6 weeks were significantly higher in the mirtazapine than placebo group [difference: £1120 (95% CI £56 to £2184)]. There were no between-group differences in carers’ health and social care costs (see Table 16).
| Cost categories | Mirtazapine | Placebo | Mirtazapine-placebo difference | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SE | N | Mean | SE | Mean | 95% CI | |
| Baseline (prior 12 weeks) | Expected = 102 | Expected = 102 | ||||||
| Health and social care costs | ||||||||
| Hospital | 97 | 649 | 253 | 101 | 446 | 162 | 203 | −384 to 790 |
| Primary and community health care | 96 | 171 | 29 | 101 | 197 | 37 | −27 | −120 to 67 |
| Community social care | 98 | 553 | 172 | 100 | 511 | 184 | 43 | −455 to 540 |
| Community mental health | 97 | 68 | 13 | 101 | 62 | 7 | 6 | −22 to 34 |
| Day care services | 98 | 170 | 66 | 100 | 82 | 30 | 88 | −54 to 230 |
| Equipment – NHS and social services | 101 | 2 | 1 | 102 | 3 | 1 | −1 | −4 to 2 |
| Respite in residential/nursing home | 100 | 0 | 0 | 101 | 103 | 62 | −103 | −225 to 20 |
| Residential/nursing home residence | 100 | 3850 | 444 | 102 | 3988 | 482 | −138 | −1431 to 1155 |
| Trial medication | 102 | 0 | 0 | 102 | 0 | 0 | 0 | 0 to 0 |
| Concomitant medications | 102 | 95 | 12 | 102 | 109 | 18 | −15 | −58 to 28 |
| Total health and social care a | 93 | 5504 | 501 | 99 | 5475 | 530 | 29 | −1412 to 1471 |
| Unpaid care and out-of-pocket costs | Expected = 63 | Expected = 68 | ||||||
| Opportunity cost to participating carer | 59 | 6653 | 673 | 67 | 5512 | 536 | 1140 | −546 to 2827 |
| Opportunity cost to other carers | 57 | 1808 | 376 | 67 | 3000 | 758 | −1192 | −2959 to 575 |
| Equipment – self or family | 62 | 3 | 2 | 68 | 2 | 1 | 1 | −3 to 6 |
| Total societal b | 52 | 11,488 | 894 | 65 | 11,924 | 1126 | −436 | −3390 to 2519 |
| Week 6 (prior 6 weeks) | Expected = 87 | Expected = 95 | ||||||
| Health and social care costs | ||||||||
| Hospital | 84 | 21 | 6 | 89 | 228 | 157 | −207 | −527 to 112 |
| Primary and community health care | 83 | 78 | 13 | 90 | 109 | 22 | −31 | −81 to 19 |
| Community social care | 84 | 211 | 64 | 89 | 296 | 97 | −85 | −318 to 147 |
| Community mental health | 84 | 19 | 5 | 90 | 23 | 5 | −4 | −19 to 10 |
| Day care services | 84 | 103 | 60 | 90 | 38 | 14 | 66 | −52 to 183 |
| Equipment – NHS and social services | 86 | 0 | 0 | 95 | 0 | 0 | 0 | −1 to 1 |
| Respite in residential/nursing home | 85 | 44 | 32 | 91 | 45 | 37 | 0 | −97 to 97 |
| Residential/nursing home residence | 85 | 1968 | 237 | 91 | 2197 | 262 | −229 | −929 to 472 |
| Trial medication | 87 | 11 | 0 | 95 | 0 | 0 | 11*** | 11 to 11 |
| Concomitant medications | 87 | 63 | 8 | 95 | 61 | 9 | 1 | −23 to 26 |
| Total health and social care a | 83 | 2522 | 229 | 89 | 3043 | 299 | −521 | −1273 to 231 |
| Unpaid care and out-of-pocket costs | Expected = 53 | Expected = 60 | ||||||
| Opportunity cost to participating carer | 49 | 3719 | 404 | 54 | 2897 | 324 | 822 | −197 to 1840 |
| Opportunity cost to other carers | 49 | 1150 | 273 | 56 | 1111 | 256 | 39 | −703 to 781 |
| Equipment – self or family | 52 | 2 | 1 | 60 | 4 | 2 | −1 | −5 to 3 |
| Total societal b | 48 | 6167 | 496 | 54 | 5797 | 501 | 371 | −1035 to 1776 |
| Week 12 (prior 6 weeks) | Expected = 81 | Expected = 90 | ||||||
| Health and social care costs | ||||||||
| Hospital | 78 | 321 | 219 | 86 | 305 | 225 | 16 | −606 to 638 |
| Primary and community health care | 78 | 77 | 14 | 86 | 96 | 19 | −18 | −66 to 29 |
| Community social care | 78 | 203 | 76 | 85 | 280 | 115 | −77 | −354 to 200 |
| Community mental health | 78 | 14 | 4 | 85 | 13 | 3 | 1 | −9 to 11 |
| Day care services | 78 | 104 | 63 | 86 | 33 | 15 | 71 | −51 to 194 |
| Equipment – NHS and social services | 80 | 2 | 1 | 90 | 0 | 0 | 2* | 0 to 4 |
| Respite in residential/nursing home | 78 | 104 | 60 | 86 | 21 | 15 | 83 | −35 to 201 |
| Residential/nursing home residence | 78 | 2131 | 257 | 87 | 2250 | 271 | −119 | −860 to 622 |
| Trial medication | 81 | 17 | 0 | 90 | 0 | 0 | 17*** | 16 to 17 |
| Concomitant medications | 81 | 62 | 8 | 90 | 64 | 10 | −2 | −27 to 24 |
| Total health and social care a | 77 | 3072 | 310 | 85 | 2895 | 281 | 177 | −647 to 1002 |
| Unpaid care and out-of-pocket costs | Expected = 49 | Expected = 56 | ||||||
| Opportunity cost to participating carer | 45 | 3772 | 416 | 51 | 2652 | 344 | 1120* | 56 to 2184 |
| Opportunity cost to other carers | 45 | 816 | 202 | 52 | 1303 | 312 | −487 | −1250 to 276 |
| Equipment – self or family | 48 | 2 | 1 | 56 | 3 | 1 | −1 | −5 to 3 |
| Total societal b | 43 | 6642 | 640 | 50 | 5456 | 470 | 1186 | −364 to 2736 |
| Cost categories | Mirtazapine | Placebo | Mirtazapine-placebo difference | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SE | N | Mean | SE | Mean | 95% CI | |
| Baseline (prior 12 weeks) | Expected = 63 | Expected = 68 | ||||||
| Carer costs | ||||||||
| Primary care | 60 | 31 | 8 | 67 | 27 | 6 | 5 | −15 to 24 |
| Community mental health | 60 | 72 | 25 | 67 | 51 | 12 | 21 | −33 to 75 |
| Hospital Outpatient Department | 60 | 67 | 25 | 67 | 33 | 8 | 34 | −16 to 84 |
| Support services | 60 | 10 | 5 | 67 | 1 | 1 | 9 | −0 to 17 |
| Health and support attributable to rolea | 60 | 25 | 9 | 67 | 20 | 9 | 5 | −19 to 30 |
| Opportunity cost to participating carer over period | 59 | 6653 | 673 | 67 | 5512 | 536 | 1140 | −546 to 2827 |
| Opportunity cost to other carers over period | 57 | 1808 | 376 | 67 | 3000 | 758 | −1192 | −2959 to 575 |
| Costs of equipment – self or family | 62 | 3 | 2 | 68 | 2 | 1 | 1 | −3 to 6 |
| Societal costsb | 56 | 8639 | 833 | 67 | 8532 | 923 | 107 | −2397 to 2610 |
| Dyad costs | ||||||||
| Dyadic total health and social care | 56 | 2763 | 485 | 65 | 3298 | 608 | −536 | −2109 to 1038 |
| Week 6 (prior 6 weeks) | Expected = 53 | Expected = 60 | ||||||
| Carer costs | ||||||||
| Primary care | 50 | 12 | 3 | 55 | 32 | 13 | −20 | −49 to 9 |
| Community mental health | 50 | 45 | 19 | 55 | 38 | 11 | 7 | −35 to 49 |
| Hospital Outpatient Department | 50 | 38 | 19 | 55 | 29 | 9 | 9 | −30 to 49 |
| Support services | 50 | 2 | 1 | 55 | 16 | 14 | −14 | −42 to 14 |
| Health and support attributable to rolea | 50 | 21 | 8 | 54 | 30 | 14 | −9 | −42 to 24 |
| Opportunity cost to participating carer over period | 49 | 3719 | 404 | 54 | 2897 | 324 | 822 | −197 to 1840 |
| Opportunity cost to other carers over period | 49 | 1150 | 273 | 56 | 1111 | 256 | 39 | −703 to 781 |
| Costs of equipment – self or family | 52 | 2 | 1 | 60 | 4 | 2 | −1 | −5 to 3 |
| Societal costsb | 48 | 4969 | 545 | 53 | 4129 | 445 | 841 | −544 to 2226 |
| Dyad costs | ||||||||
| Dyadic total health and social care | 50 | 1208 | 222 | 54 | 1834 | 375 | −626 | −1508 to 256 |
| Week 12 (prior 6 weeks) | Expected = 49 | Expected = 56 | ||||||
| Carer costs | ||||||||
| Primary care | 46 | 20 | 5 | 52 | 38 | 28 | −18 | −78 to 42 |
| Community mental health | 46 | 79 | 49 | 52 | 26 | 9 | 53 | −39 to 146 |
| Hospital Outpatient Department | 46 | 71 | 49 | 52 | 18 | 6 | 53 | −39 to 144 |
| Support services | 46 | 2 | 1 | 52 | 1 | 1 | 0 | −2 to 3 |
| Health and support attributable to rolea | 45 | 86 | 52 | 52 | 7 | 4 | 79 | −17 to 175 |
| Opportunity cost to participating carer over period | 45 | 3772 | 416 | 51 | 2652 | 344 | 1120* | 56 to 2184 |
| Opportunity cost to other carers over period | 45 | 816 | 202 | 52 | 1303 | 312 | −487 | −1250 to 276 |
| Costs of equipment – self or family | 48 | 2 | 1 | 56 | 3 | 1 | −1 | −5 to 3 |
| Societal costsb | 44 | 4668 | 503 | 51 | 3949 | 445 | 719 | −609 to 2047 |
| Dyad costs | ||||||||
| Dyadic total health and social care | 44 | 2031 | 454 | 51 | 1592 | 326 | 439 | −650 to 1529 |
Outcome measures
Raw CMAI scores in both groups summarised from available cases’ data were similar at baseline and both follow-ups (see Table 17). Mean CMAI scores in the sample participating at 6 and 12 weeks (regardless of allocation) were approximately 10 points lower than those in the baseline sample. Raw index scores (utilities) derived from informant-reported quality-of-life measures were similar between groups. EQ-5D-5L-derived utilities were much lower than those derived from the DEMQOL-Proxy-U. Utilities derived from the participant-reported DEMQOL-U (completed by less than half of the people with dementia participating at each time point) were somewhat higher than scores of the proxy-completed version in both groups. At each assessment point, carers’ EQ-5D-5L scores were similar between groups (see Table 18).
| Mirtazapine | Placebo | Mirtazapine-placebo difference | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SE | N | Mean | SE | Mean | 95% CI | |
| Baseline | Expected = 102 | Expected = 102 | ||||||
| CMAI | 102 | 71.108 | 1.622 | 102 | 69.794 | 1.695 | 1.314 | −3.312 to 5.939 |
| EQ-5D-5L-PROXY | 100 | 0.458 | 0.034 | 101 | 0.498 | 0.032 | −0.039 | −0.132 to 0.053 |
| DEMQOL-U | 44 | 0.85 | 0.017 | 38 | 0.874 | 0.018 | −0.024 | −0.074 to 0.025 |
| DEMQOL-PROXY-U | 101 | 0.684 | 0.014 | 101 | 0.68 | 0.013 | 0.003 | −0.034 to 0.041 |
| Week 6 | Expected = 87 | Expected = 95 | ||||||
| CMAI | 84 | 61.357 | 2.567 | 88 | 59.966 | 2.116 | 1.391 | −5.151 to 7.933 |
| EQ-5D-5L-PROXY | 82 | 0.482 | 0.037 | 88 | 0.553 | 0.032 | −0.071 | −0.168 to 0.025 |
| DEMQOL-U | 34 | 0.883 | 0.014 | 33 | 0.893 | 0.014 | −0.011 | −0.050 to 0.029 |
| DEMQOL-PROXY-U | 82 | 0.709 | 0.015 | 87 | 0.7 | 0.015 | 0.009 | −0.032 to 0.050 |
| Week 12 | Expected = 81 | Expected = 90 | ||||||
| CMAI | 79 | 61.392 | 2.539 | 87 | 60.805 | 2.341 | 0.588 | −6.221 to 7.397 |
| EQ-5D-5L-PROXY | 78 | 0.467 | 0.04 | 85 | 0.494 | 0.037 | −0.027 | −0.133 to 0.080 |
| DEMQOL-U | 26 | 0.89 | 0.014 | 24 | 0.882 | 0.022 | 0.009 | −0.043 to 0.061 |
| DEMQOL-PROXY-U | 73 | 0.724 | 0.016 | 84 | 0.711 | 0.015 | 0.012 | −0.031 to 0.055 |
| Mirtazapine | Placebo | Mirtazapine-placebo difference | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SE | N | Mean | SE | Mean | 95% CI | |
| Baseline | Expected = 63 | Expected = 68 | ||||||
| EQ-5D-5L | 61 | 0.789 | 0.027 | 66 | 0.814 | 0.028 | −0.025 | −0.101 to 0.052 |
| Week 6 | Expected = 53 | Expected = 60 | ||||||
| EQ-5D-5L | 50 | 0.826 | 0.023 | 54 | 0.826 | 0.021 | 0 | −0.061 to 0.062 |
| Week 12 | Expected = 49 | Expected = 56 | ||||||
| EQ-5D-5L | 46 | 0.806 | 0.024 | 50 | 0.825 | 0.027 | −0.019 | −0.092 to 0.054 |
Cost-effectiveness analyses
Primary analysis
Mean raw outcome scores and costs of the complete cases samples showed no differences between groups (see Table 19). Adjusting for baseline measure and living arrangement, the estimate for the difference between groups in both CMAI and costs had wide CIs crossing zero. The point estimate for the ICER on CMAI was negative because costs were slightly lower and outcome slightly better in the mirtazapine group compared to the placebo group. The NMB line (see Figure 3) shows that net benefit is positive at all willingness-to-pay thresholds from £0 to £30,000: there is monetary benefit once the cost of the intervention has been deducted. However, the CIs of the line do not cross zero, illustrating that 95% confidence limits of the ICER could not be defined and therefore neither mirtazapine nor placebo can be judged to be the more cost-effective strategy with a high level of confidence. The CEAC (see Figure 4) illustrates that probability of cost-effectiveness was 81% at a willingness to pay of £3000 and 80% at £20,000; also that a 10% CI for the ICER can be defined between willingness to pay of approximately £0 and £3000 per QALY, giving a low degree of certainty that mirtazapine is cost-effective (see Glick et al. 63 2014; Gray et al. 59).
| Outcomes and costs | Mirtazapine | Placebo | Mirtazapine-placebo difference | ICER | ||
|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (95% CI) | Adjusted (95% CI) | p | Cost per 6-point differencea |
|
| Health and social care | ||||||
| Observations | N = 72 | N = 79 | ||||
| CMAI score | 61.847 (2.659) | 60.848 (2.49) | 0.999 (−6.193 to 8.191) | −2.446 (−8.243 to 3.352) | 0.408 | −273/0.408 = −670 |
| Total costs | 5752 (513) | 5877 (591) | −125 (−1686 to 1435) | −273 (−1754 to 1208) | 0.718 | |
FIGURE 3.
Primary outcome: NMB plot.
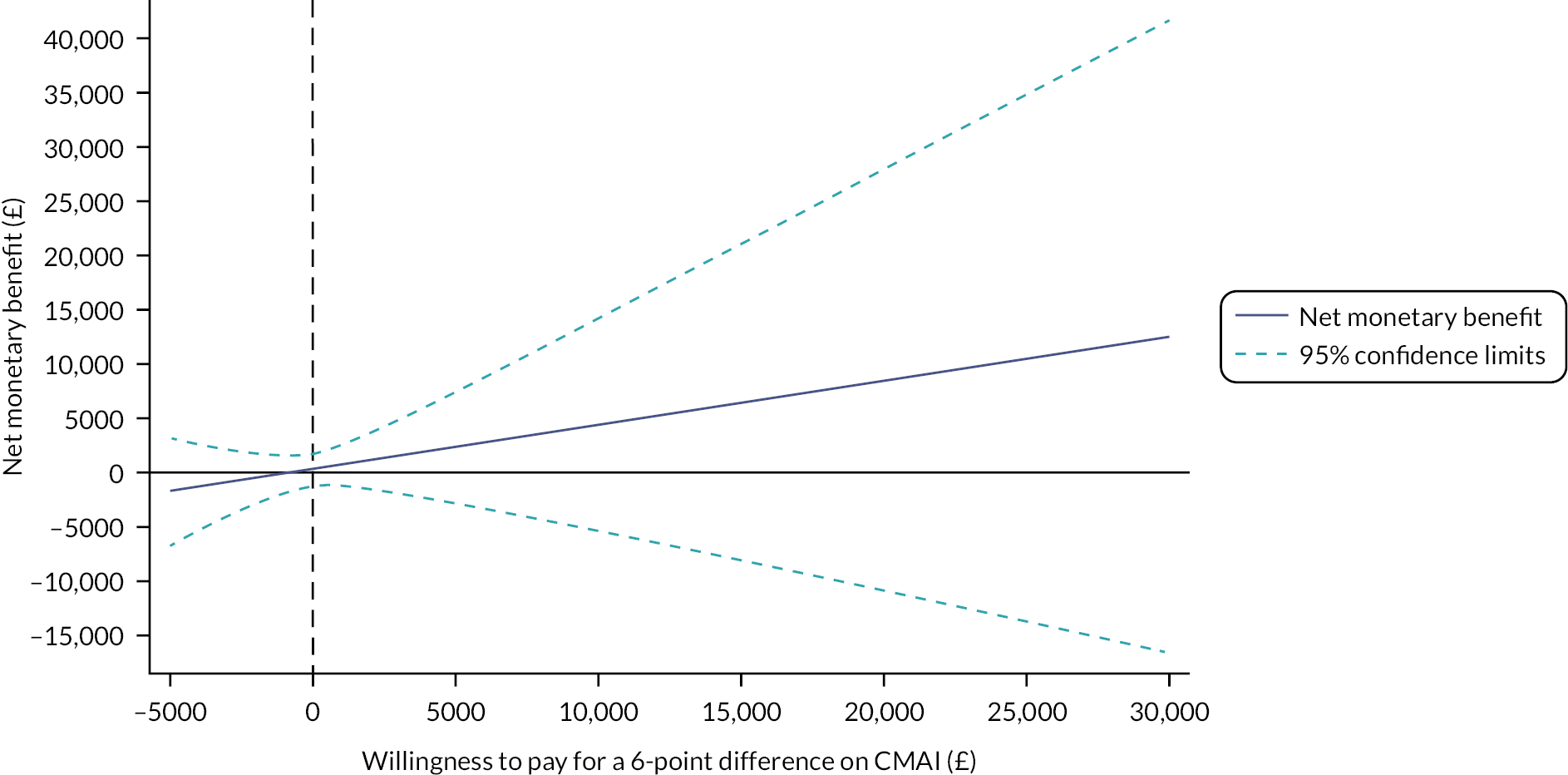
FIGURE 4.
Primary outcome: cost-effectiveness acceptability curve.

Secondary analyses
Raw mean outcomes and costs from the health and social care and societal perspectives are presented alongside their raw and adjusted between-group differences, ICER and NMB at £20,000 in Table 20.
| Outcomes and costs |
Mirtazapine | Placebo | Mirtazapine-placebo difference | Adjusted (95% CI) | p | ICER | NMB at £20,000 |
|---|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (95% CI) | C/E | Mean (95% CI) | |||
| QALY | |||||||
| Health and social care | |||||||
| Observations | N = 70 | N = 77 | |||||
| QALY-EQ-5D-5L | 0.107 (0.008) | 0.128 (0.007) | −0.021 (−0.043 to 0.001) | −0.007 (−0.021 to 0.007) | 0.314 | 60,976 | 289 (−1347 to 1925) |
| Total costs | 5689 (526) | 5844 (596) | −155 (−1740 to 1429) | −430 (−1920 to 1061) | 0.572 | ||
| Observations | N = 67 | N = 76 | |||||
| QALY-DEMQOL-Proxy-U | 0.162 (0.003) | 0.163 (0.003) | −0.002 (−0.010 to 0.007) | 0.002 (−0.004 to 0.008) | 0.540 | −227,589 | 449 (−1102 to 1999) |
| Total costs | 5604 (535) | 5816 (604) | −211 (−1824 to 1402) | −412 (−1942 to 1117) | 0.597 | ||
| N = 18 | N = 20 | ||||||
| QALY-DEMQOL-U | 0.203 (0.004) | 0.205 (0.004) | −0.002 (−0.014 to 0.009) | 0.001 (−0.006 to 0.007) | 0.832 | 692,101 | −406 (−2521 to 1710) |
| Total costs | 2858 (800) | 2512 (735) | 346 (−1853 to 2545) | 458 (−1677 to 2592) | 0.674 | ||
| Societal | |||||||
| Observations | N = 37 | N = 43 | |||||
| QALY-EQ-5D-5L | 0.124 (0.009) | 0.145 (0.007) | −0.021 (−0.044 to 0.003) | −0.003 (−0.017 to 0.011) | 0.708 | −848,348 | −2320 (−5382 to 742) |
| Total costs | 13,235 (1219) | 10,940 (1026) | 2295 (−853 to 5444) | 2266 (−855 to 5388) | 0.153 | ||
| Observations | N = 36 | N = 43 | |||||
| QALY-DEMQOL-Proxy-U | 0.157 (0.005) | 0.158 (0.003) | −0.001 (−0.012 to 0.01) | 0.004 (−0.005 to 0.012) | 0.395 | 638,829 | −2239 (−5405 to 928) |
| Total costs | 13,252 (1254) | 10,940 (1026) | 2313 (−880 to 5506) | 2311 (−869 to 5491) | 0.153 | ||
| Observations | N = 14 | N = 18 | |||||
| QALY-DEMQOL-U | 0.204 (0.004) | 0.206 (0.005) | −0.002 (−0.015 to 0.011) | −0.001 (−0.015 to 0.014) | 0.923 | −775,304 | −550 (−5372 to 4272) |
| Total costs | 10,325 (1705) | 9790 (1963) | 536 (−4955 to 6026) | 536 (−4226 to 5299) | 0.822 | ||
| CMAI and societal | ICER | ||||||
| Observations | N = 38 | N = 45 | Cost per 6-point differencea |
||||
| CMAI score | 61.895 (2.829) | 59.778 (3.139) | 2.117 (−6.425 to 10.659) | −3.028 (−9.702 to 3.646) | 0.374 | 1944/0.505 = 3851 | |
| Total costs | 13,169 (1189) | 11,204 (1001) | 1965 (−1104 to 5034) | 1944 (−1076 to 4964) | 0.207 | ||
Participant outcomes
Health and social care perspective
On raw participant QALY derived from EQ-5D-5L, DEMQOL-U and DEMQOL-U-Proxy and costs from a health and social care perspective, there were no differences between groups. Similarly, on adjusted mean differences between groups from the multilevel analyses, there were no differences between groups. Cost-effectiveness results on the DEMQOL-U are not discussed further because of the small numbers involved.
The ICER of EQ-5D-5L-derived QALYs and costs from the health and social care perspective was positive as the sign of the cost difference was negative and there was a small QALY loss. Results are not discussed further given the latter result.
The ICER from the DEMQOL-U-Proxy was negative as there was a small QALY gain and the sign of the cost difference was negative. The probability of cost-effectiveness ranged from 70% to 72% across a WTP range of £0–50,000. NMB at the lower NICE threshold (£20,000) was positive, but the 95% CIs crossed zero. The ICER was unbounded, indicating that neither mirtazapine nor placebo could be considered a cost-effective strategy at any level of willingness to pay to gain a QALY.
Societal perspective
Groups did not differ on costs or CMAI outcomes from the societal perspective. The societal cost per 6-point difference was £3851, the ICER being unbounded. The net benefit at a willingness to pay of £0 was −£1944 (95% CI −£4964 to £1076) and at a WTP of £20,000 was £8150 (95% CI −£14,195 to £30,494). Probability of cost-effectiveness ranged between 10% and 77% over this range.
There were no between-group differences in QALYs derived from EQ-5D-5L, DEMQOL-U or DEMQOL-U-Proxy and costs from the societal perspective, before or after adjustment. Given the low numbers on the DEMQOL-U, cost-effectiveness results have not been discussed.
The ICER of EQ-5D-5L-derived QALYs and costs from the societal perspective was negative as the sign of the cost difference was positive and there was a small QALY loss. Results are not discussed further for this reason. The ICER from the DEMQOL-U-Proxy was positive and very large; probability of cost-effectiveness ranged from 8% to 10% across WTP values from £0 to £50,000.
Carer outcomes
In cases with complete health and support and QALY data, unadjusted and adjusted between-group differences in QALYs were not significant (see Table 21). The NMB of mirtazapine at £20,000 was negative but the CI crossed zero. The ICER on this measure was unbounded. Results for carers’ societal costs and QALY were similar. Probability of cost-effectiveness from the health and social care perspective did not exceed 45% over WTP-per-QALY thresholds ranging between £0 and £50,000; probability of cost-effectiveness from the societal perspective did not exceed 19% over the same range.
| Outcomes and costs | Mirtazapine | Placebo | Mirtazapine-placebo difference | Adjusted (95% CI) | p | ICER | NMB at £20,000 |
|---|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (95% CI) | C/E | Mean (95% CI) | |||
| Health and social care | |||||||
| Observations | N = 44 | N = 45 | |||||
| QALY (EQ-5D-5L) | 0.186 (0.005) | 0.19 (0.006) | −0.004 (−0.019 to 0.011) | 0.001 (−0.008 to 0.01) | 0.764 | 70,668 | −286 (−69 to 149) |
| Total costs | 111 (55) | 22 (10) | 90 (−20 to 199) | 96 (−12 to 203) | 0.082 | ||
| Societal | |||||||
| Observations | N = 41 | N = 43 | |||||
| QALY (EQ-5D-5L) | 0.187 (0.005) | 0.189 (0.006) | −0.003 (−0.018 to 0.013) | 0.004 (−0.006 to 0.013) | 0.437 | 361,088 | −1283 (−4045 to 1480) |
| Total costs | 9648 (1073) | 8245 (983) | 1403 (−1488 to 4294) | 1358 (−1491 to 4206) | 0.348 | ||
Sensitivity analysis
Mean costs used in sensitivity analyses are reported in Table 22 and results of sensitivity analyses of the primary outcome are displayed in Table 23. Results of analysis of CMAI scores and health and social care costs that included the costs of domestic accommodation were similar to the base case results, with no significant differences between groups. The cost per 6-point difference was slightly lower than in the primary analysis but unbounded as in the base case results. Analyses adjusting for the baseline imbalance between groups in proportion of female participants yielded similar results to the base case, with an unbounded ICER. Results of a SUR model applied to samples from a two-stage bootstrapping routine indicated no differences between groups in costs or CMAI scores; model estimates produced a small positive unbounded ICER of £136 per 6-point difference.
| Cost categories | Mirtazapine | Placebo | Mirtazapine-placebo difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SE | N | Mean | SE | Mean | 95% CI | ||
| Baseline (prior 12 weeks) | |||||||||
| Accommodation costs | |||||||||
| Domestic residence (includes sheltered) | 100 | 1596 | 146 | 101 | 1662 | 144 | −66 | −469 to 338 | |
| Permanent residence | 100 | 5447 | 308 | 101 | 5555 | 345 | −108 | −1020 to 804 | |
| Total accommodation, health and social care costs | 93 | 7136 | 403 | 99 | 7112 | 422 | 24 | −1130 to 1178 | |
| Expected = 63 | Expected = 68 | ||||||||
| Total societal, replacement cost | 53 | 29,461 | 2002 | 65 | 27,514 | 2076 | 1947 | −3847 to 7740 | |
| Total societal, alternative care time | 49 | 50,224 | 1488 | 58 | 51,220 | 1466 | −995 | −5162 to 3171 | |
| Week 6 (prior 6 weeks) | |||||||||
| Accommodation costs | |||||||||
| Domestic residence (includes sheltered) | 85 | 771 | 79 | 91 | 769 | 77 | 2 | −215 to 219 | |
| Permanent residence | 85 | 2739 | 162 | 91 | 2966 | 192 | −227 | −726 to 273 | |
| Total accommodation, health and social care costs | 83 | 3294 | 160 | 89 | 3797 | 242 | −503 | −1084 to 79 | |
| Expected = 53 | Expected = 60 | ||||||||
| Total societal, replacement cost | 48 | 15,774 | 1008 | 55 | 12,899 | 1027 | 2875* | 3 to 5748 | |
| Total societal, alternative care time | 45 | 26,039 | 475 | 50 | 25,364 | 952 | 675 | −1510 to 2860 | |
| Week 12 (prior 6 weeks) | |||||||||
| Accommodation costs | |||||||||
| Domestic residence (includes sheltered) | 78 | 731 | 83 | 86 | 756 | 79 | −25 | −251 to 201 | |
| Permanent residence | 78 | 2862 | 180 | 86 | 3032 | 200 | −170 | −705 to 365 | |
| Total accommodation, health and social care costs | 77 | 3794 | 267 | 85 | 3643 | 220 | 151 | −527 to 830 | |
| Expected = 49 | Expected = 56 | ||||||||
| Total societal, replacement cost | 44 | 16,078 | 1125 | 51 | 12,415 | 1008 | 3663* | 671 to 6654 | |
| Total societal, alternative care time measure | 40 | 26,754 | 742 | 47 | 25,026 | 963 | 1728 | −754 to 4211 | |
| Outcomes and costs | Mirtazapine | Placebo | Mirtazapine-placebo difference | Cost per 6-point differencea | ||
|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (95% CI) | Adjusted (95% CI) | p | C/E | |
| CMAI – participant | ||||||
| Total HSC costs including domestic accommodation | ||||||
| Observations | N = 72 | N = 79 | ||||
| CMAI | 61.847 (2.659) | 60.848 (2.490) | 0.999 (−6.193 to 8.191) | −2.506 (−8.299 to 3.287) | 0.396 | −212/0.418 = −507 |
| Total costs | 7234 (390) | 7380 (462) | −146 (−1353 to 1060) | −212 (−1375 to 951) | 0.721 | |
| Statistical model adding sex as covariate | ||||||
| Observations | N = 72 | N = 79 | ||||
| CMAI | 61.847 (2.659) | 60.848 (2.49) | 0.999 (−6.193 to 8.191) | −1.726 (−7.613 to 4.162) | 0.566 | −278/0.288 = −956 |
| Total HSC costs | 5752 (513) | 5877 (591) | −125 (−1686 to 1435) | −275 (−1756 to 1206) | 0.716 | |
| Alternative statistical modelb | ||||||
| Observations | N = 72 | N = 79 | ||||
| CMAI | 61.847 (2.659) | 60.848 (2.49) | 0.999 (−6.193 to 8.191) | −2.253 (−9.847 to 7.794) | 0.453 | 51/0.376 = 136 |
| Total HSC costs | 5752 (513) | 5877 (591) | −125 (−1686 to 1435) | 51 (−1304 to 1432) | 0.900 | |
In terms of secondary outcomes, analyses explored the impact on results for EQ-5D-5L QALY and societal costs of valuing unpaid carer time at replacement cost, alone and in combination with an alternative method of estimating hours of unpaid carer time (see Table 24). The societal costs were significantly greater in the mirtazapine group if valuing unpaid carer time at replacement cost. Valuation at replacement cost resulted in a negative NMB at £20,000 with negative upper and lower confidence limits (the costs outweighed the benefit of the intervention). Using an alternative calculation of unpaid carer hours increased the cost of both groups (doubling it in the placebo group) but the groups did not differ. Valuation of the alternative estimation of unpaid care time at replacement cost resulted in an unbounded ICER and negative NMB at £20,000 with CIs crossing zero.
| Outcomes and costs | Mirtazapine | Placebo | Mirtazapine-placebo difference | Adjusted (95% CI) | p | ICER | NMB at £20,000 |
|---|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (95% CI) | C/E | Mean (95% CI) | |||
| Societal | |||||||
| Replacement cost of unpaid care | |||||||
| Observations | N = 38 | N = 45 | |||||
| QALY-EQ-5D-5L | 0.125 (0.009) | 0.147 (0.007) | −0.022 (−0.045 to 0.001) | −1.813 (−8.426 to 4.799) | 0.589 | −3,366,204 | −8169 (−14,178 to −2160) |
| Total costs | 33,090 (2246) | 24,966 (2105) | 8125* (1989 to 14,260) | 7263 (1328 to 13,197) | 0.017 | ||
| Societal | |||||||
| Replacement cost of unpaid care + alternative unpaid care estimate | |||||||
| Observations | N = 35 | N = 39 | |||||
| QALY-EQ-5D-5L | 0.129 (0.010) | 0.150 (0.008) | −0.022 (−0.046 to 0.003) | −2.291 (−9.273 to 4.691) | 0.518 | −842,854 | −3730 (−8173 to 712) |
| Total costs | 53,241 (1145) | 49,685 (1887) | 3556 (−966 to 8079) | 3242 (−1114 to 7597) | 0.143 | ||
| QALY – participant and carer | |||||||
| HSC | |||||||
| Observations | N = 40 | N = 44 | |||||
| QALY-EQ-5D-5L | 0.311 (0.010) | 0.336 (0.009) | −0.025 (−0.051 to 0.001) | 0.000 (−0.014 to 0.014) | 0.988 | −4,439,387 | −465 (−2242 to 1312) |
| Total costs | 3291 (632) | 2987 (696) | 303 (−1580 to 2187) | 463 (−1362 to 2288) | 0.617 | ||
Examining combined QALY and health and social care costs of participant and dyadic carer, the groups did not differ. The ICER was unbounded and had a negative sign because the mirtazapine group had slightly lower QALY and slightly higher costs.
Chapter 4 Carbamazepine versus placebo results
Introduction
As discussed above, this trial was designed to include a carbamazepine arm as well as mirtazapine and placebo arms. The carbamazepine arm was discontinued after 40 randomisations due to slower than projected recruitment. In this chapter, we present the rationale for this arm and mirrored analyses of the clinical effectiveness of carbamazepine versus placebo in the treatment of agitation in dementia using the same methodology that was used for mirtazapine versus placebo.
Carbamazepine for agitated behaviours in dementia
Carbamazepine stabilises the inactivated state of voltage-gated sodium channels and potentiates GABA receptors. It is recommended in the BNF for epilepsy, prophylaxis of bipolar disorder and trigeminal neuralgia. It is generally safe within the proposed dose ranges; there are few data on people with AD, but it seems that there is no increase in mortality as in antipsychotics for AD. 35 Carbamazepine has been widely used in psychiatric disorders and AD, off licence, to treat symptoms including agitation, aggression, irritability and impulsivity. Open-label studies and case reports have indicated promise in agitation in AD. 36 Two small 6-week parallel-group RCTs of carbamazepine for BPSD have been published. 37,38 The first in 55 patients (modal dose 300 mg) showed significant symptom decrease. It was well tolerated with no decrease in cognition, function or increased side effects relative to placebo. The second (400 mg in 21 patients not responding to antipsychotics) showed a trend but not a significant advantage over placebo. Meta-analysis indicated significant benefit compared with placebo treatment on the Brief Psychiatric Rating Scale (mean difference −5.5 points, 95% CI −8.5 to −2.5 points) and on the Clinical Global Impression Scale (OR 10.2, 95% CI 3.1 to 33.1). 39 A third small trial of the similar compound oxcarbazepine (n = 103) indicated a trend towards benefit (p = 0.07) with active drug performing better than placebo in all analyses. 40
Aim
To determine if carbamazepine is more clinically effective in reducing agitated behaviours in dementia than placebo measured by change in CMAI score 12 weeks post randomisation.
Methods
Please see Chapter 3.
Results
Patient flow
We recruited participants between January 2017 and August 2018 to the three-arm trial and recruitment continued until February 2020 for mirtazapine and placebo arms. Follow-up interviews were completed accordingly. See Figure 5.
FIGURE 5.
CONSORT flow diagram of recruitment and testing for carbamazepine and placebo groups.

Baseline characteristics
Table 25 shows baseline demographic and clinical characteristics of participants. Groups were similar at baseline except for sex with more females randomised to carbamazepine (n = 32, 80%) than placebo (n = 59, 58%). Table 26 shows baseline demographics for carers.
| Carbamazepine (n = 40) |
Placebo (n = 102) |
|
|---|---|---|
| Age (years) (SD) | 83.2 (8.1) | 82.8 (7.7) |
| Sex | n = 40 | n = 102 |
| Female | 32 (80%) | 59 (58%) |
| Residence | n = 40 | n = 102 |
| Own household | 20 (50%) | 57 (56%) |
| Care home | 20 (50%) | 45 (44%) |
| Agitation | n = 40 | n = 102 |
| CMAI (29–203) | 70.0 (21.0) | 69.8 (17.1) |
| Cognition | n = 23 | n = 50 |
| Standardised MMSE (0–30) | 12.0 (6.0) | 16.1 (6.7) |
| Condition-specific quality of life | n = 17 | n = 37 |
| DEMQOL (28–122) | 93.5 (12.7) | 95.8 (10.2) |
| DEMQOL-Proxy (31–124) | n = 39 | n = 99 |
| 94.7 (14.8) | 90.9 (14.4) | |
| Generic quality of life | n = 40 | n = 101 |
| EQ-5D (proxy report by carer) (0–1) | 0.47 (0.35) | 0.50 (0.32) |
| Neuropsychiatric symptoms | n = 40 | (n = 102) |
| NPI total score (0–144) | 40.5 (26.1) | 34.9 (18.2) |
| NPI agitation/aggression subscore (0–12) | n = 40 | n = 102 |
| 6.5 (4.0) | 5.6 (3.4) | |
| NPI depression/anxiety/irritability subscore (0–36) | n = 40 | n = 102 |
| 11.6 (8.9) | 10.5 (7.0) | |
| Suicidality | n = 40 | n = 102 |
| CSSRS | ||
| Suicidal ideation (lifetime) | 10 (25%) | 13 (13%) |
| Suicidal ideation (past month) | 5 (13%) | 11 (11%) |
| Suicidal behaviour (lifetime) | 1 (3%) | 0 |
| Suicidal behaviour (past 3 months) | 0 | 0 |
| Carbamazepine (n = 40) |
Placebo (n = 102) |
|
|---|---|---|
| Carer | ||
| Paid | 16 (40%) | 31 (30%) |
| Family | 24 (60%) | 71 (70%) |
| Family carer relationship | ||
| Partner or spouse | 13 (54%) | 35 (49%) |
| Son or daughter | 9 (38%) | 31 (44%) |
| Sibling | 0 | 0 |
| Other relative | 2 (8%) | 3 (4%) |
| Friend | 0 | 2 (3%) |
| Other | 0 | 0 |
| Family carer occupation (pre-retirement) | ||
| Professional | 6 (25%) | 13 (18%) |
| Managerial and technical | 5 (21%) | 22 (31%) |
| Skilled non-manual | 5 (21%) | 11 (15%) |
| Skilled manual | 6 (25%) | 8 (11%) |
| Partly skilled | 1 (4%) | 8 (11%) |
| Unskilled | 1 (4%) | 0 |
| Unemployed or unwaged | 0 | 5 (7%) |
| Unanswered | 0 | 4 (6%) |
| Carer mental health (family carers only) | n = 24 | n = 66 |
| GHQ-12 | 13.2 (5.2) | 14.5 (4.9) |
| Carer burden (family carers only) | n = 21 | n = 66 |
| Zarit CBI | 29.4 (13.2) | 34.1 (13.9) |
| Carer generic quality of life (family carers only) | n = 24 | n = 66 |
| EQ-5D | 0.82 (0.15) | 0.81 (0.22) |
| NPI carer distress subscore (0–60) | n = 38 | n = 99 |
| 14.5 (11.5) | 15.5 (9.0) | |
Primary outcome measures
Severity of agitation decreased in the placebo group by 9.6 points on the CMAI and by 4.4 points in the carbamazepine group. At 12 weeks, the placebo group was 60.8 and the carbamazepine group 63.9 (see Figure 6). At no point was the unadjusted or adjusted CMAI difference between the groups statistically significant (see Tables 27 and 28). Table 27 presents the results from the general linear mixed modelling for the primary outcome. There was no evidence that carbamazepine improved agitation relative to placebo. The estimated adjusted effect on the CMAI was 2.46 (95% CI −5.01 to 9.93; p = 0.518). This changed little with the addition of sex into the model.
FIGURE 6.
Unadjusted mean CMAI scores (95% CI) by treatment group, carbamazepine vs. placebo.
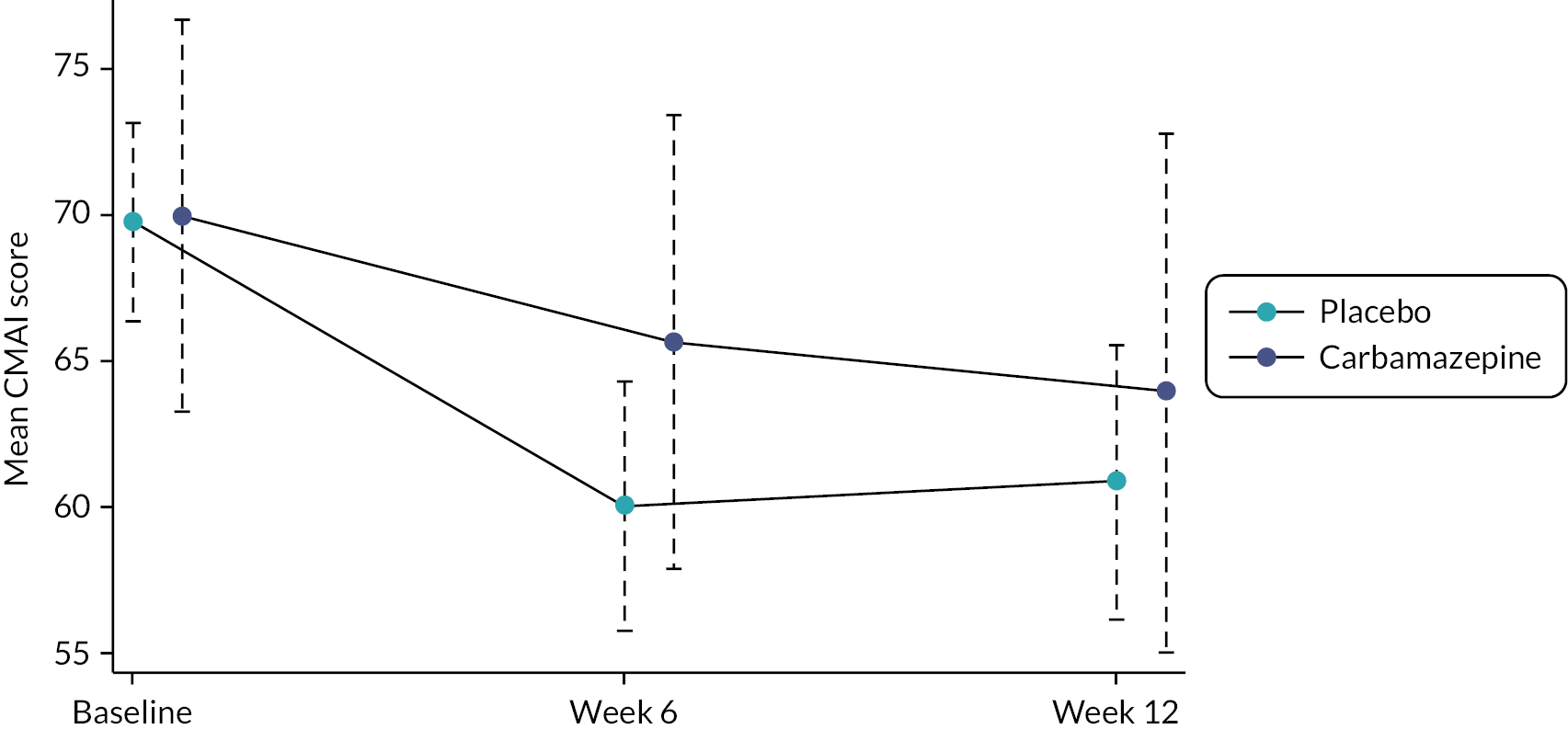
| Carbamazepine (n = 40) | Placebo (n = 102) | Difference | (95% CI) | Adj diffa | (95% CI) | p-value | Adj diffb | (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|---|
| n = 32 | n = 87 | ||||||||
| 63.9 (sd 24.7) | 60.8 (sd 21.8) |
3.1 | (−6.17 to 12.37) | 2.46 | (−5.01 to 9.93) | 0.518 | 2.67 | (−5.05 to 10.40) | 0.498 |
Secondary outcome measures
Table 28 shows the effect of carbamazepine compared with placebo on secondary outcomes in participants and Table 29 in carers. Again, there was no evidence of difference between the groups, apart from: a single statistically significant difference in the NPI carer distress subscore at 6 weeks which indicated higher carer distress in the carbamazepine group (adjusted difference 3.31 points, 95% CI 0.44 to 6.18; p = 0.024) which did not persist at 12 weeks. Table 30 presents data on dose escalation and compliance with carbamazepine and placebo.
| Carbamazepine (n = 40) | Placebo (n = 102) |
Difference | (95% CI) | Adjusted difference | (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|
| 6-week outcomes | |||||||
| Agitation (CMAI) | n = 35 | n = 88 | |||||
| 65.6 (22.7) | 60.0 (19.9) | 5.66 | (−2.52 to 13.85) | 4.51 | (−2.37 to 11.39) | 0.199 | |
| Cognition (sMMSE) | n = 12 | n = 31 | |||||
| 12.5 (6.1) | 16.2 (7.2) | −3.69 | (−8.46 to 1.08) | 0.64 | (−1.35 to 2.62) | 0.531 | |
| Quality of life (DEMQOL) | n = 11 | n = 32 | |||||
| 94.6 (15.8) | 96.8 (8.4) | −2.11 | (−9.68 to 5.45) | −1.79 | (−7.90 to 4.33) | 0.566 | |
| Quality of life (DEMQOL-Proxy) | n = 33 | n = 86 | |||||
| 100.6 (13.8) | 94.6 (16.2) | 6.07 | (−0.26 to 12.39) | 1.94 | (−3.19 to 7.08) | 0.459 | |
| Quality of life EQ-5D (proxy report by carer) | n = 35 | n = 87 | |||||
| 0.49 (0.35) | 0.56 (0.30) | −0.06 | (−0.19 to 0.06) | −0.03 | (−0.12 to 0.05) | 0.427 | |
| Neuropsychiatric symptoms NPI total score |
n = 34 | n = 88 | |||||
| 34.3 (4.3) | 24.8 (20.0) |
9.48 | (0.86 to 18.01) | 4.88 | (−2.03 to 11.78) | 0.166 | |
| NPI agitation/aggression subscore | n = 34 | n = 88 | |||||
| 5.4 (3.7) | 4.2 (3.5) | 1.14 | (−0.30 to 2.58) | 0.40 | (−0.82 to 1.62) | 0.519 | |
| NPI depression/anxiety/irritability subscore | n = 34 | n = 88 | |||||
| 9.1 (8.1) | 7.2 (8.2) | 1.88 | (−1.39 to 5.14) | 0.47 | (−2.05 to 3.00) | 0.712 | |
| 12-week outcomes | |||||||
| Cognition (sMMSE) | n = 14 | n = 27 | |||||
| 12.3 (6.8) | 15.6 (7.5) | −3.27 | (−8.12 to 1.58) | 0.39 | (−1.67 to 2.45) | 0.709 | |
| Quality of life (DEMQOL) | n = 11 | n = 24 | |||||
| 96.8 (9.9) | 97.1 (8.4) | −0.31 | (−6.89 to 6.27) | −1.49 | (−6.74 to 3.77) | 0.579 | |
| Quality of life (DEMQOL-Proxy) | n = 29 | n = 82 | |||||
| 101.7 (11.9) | 97.5 (12.4) | 4.26 | (−1.00 to 9.52) | 0.11 | (−4.04 to 4.26) | 0.958 | |
| Quality of life EQ-5D (proxy report by carer) | n = 31 | n = 84 | |||||
| 0.43 (0.34) | 0.50 (0.33) | −0.07 | (−0.21 to 0.07) | −0.02 | (−0.11 to 0.08) | 0.743 | |
| Neuropsychiatric symptoms | n = 31 | n = 84 | |||||
| NPI total score | 31.1 (25.0) | 25.7 (19.6) | 5.43 | (−3.39 to 14.25) | 0.35 | (−6.10 to 6.80) | 0.916 |
| NPI agitation/aggression subscore | n = 31 | n = 84 | |||||
| 4.6 (4.0) | 4.5 (3.6) | 0.12 | (−1.42 to 1.67) | −0.60 | (−1.92 to 0.72) | 0.374 | |
| NPI depression/anxiety/irritability subscore | n = 31 | n = 84 | |||||
| 8.0 (8.3) | 7.3 (8.0) | 0.67 | (−2.67 to 4.03) | −0.53 | (−3.04 to 1.98) | 0.680 | |
| Carbamazepine (n = 40) |
Placebo (n = 102) |
Difference | 95% CI | Adjusted difference | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| 6-week outcomes | |||||||
| Carer GHQ-12a | n = 18 | n = 54 | |||||
| 13.0 (4.8) | 12.1 (4.9) | 0.85 | (−1.80 to 3.50) | 1.55 | (−0.46 to 3.56) | 0.130 | |
| Carer EQ-5Da | n = 18 | n = 55 | |||||
| 0.81 (0.14) | 0.83 (0.15) | −0.02 | (−0.10 to 0.07) | −0.02 | (−0.08 to 0.03) | 0.428 | |
| Zarit CBIa | n = 16 | n = 49 | |||||
| 29.2 (14.7) | 29.4 (13.9) | −0.20 | (−8.33 to 7.93) | 2.23 | (−3.40 to 7.86) | 0.438 | |
| NPI carer distress subscore | n = 32 | n = 84 | |||||
| 13.1 (11.3) | 10.2 (8.8) | 2.97 | (−0.95 to 6.89) | 3.31 | (0.44 to 6.18) | 0.024 | |
| 12-week outcomes | |||||||
| Carer GHQ-12a | n = 14 | n = 52 | |||||
| 13.1 (5.3) | 12.2 (5.4) | 0.93 | (−2.29 to 4.15) | 0.75 | (−1.58 to 3.08) | 0.527 | |
| Carer EQ-5Da | n = 16 | n = 49 | |||||
| 0.82 (0.13) | 0.82 (0.19) | 0.00 | (−0.10 to 0.11) | 0.01 | (−0.05 to 0.07) | 0.750 | |
| Zarit CBIa | n = 13 | n = 48 | |||||
| 28.6 (14.4) | 29.0 (15.8) | -0.41 | (−10.11 to 9.30) | −0.64 | (−6.34 to 5.07) | 0.827 | |
| NPI carer distress subscore | n = 28 | n = 81 | |||||
| 11.0 (10.3) | 10.5 (8.3) | 0.48 | (−3.36 to 4.32) | 0.64 | (−1.88 to 3.17) | 0.615 | |
| Carbamazepine (n = 40) |
Placebo (n = 102) |
|
|---|---|---|
| End of week 4 | n = 35 | n = 97 |
| 3 study meds/day | 20 (57%) | 59 (61%) |
| 2 study meds/day | 2 (6%) | 16 (16%) |
| 1 study med/day | 3 (9%) | 8 (8%) |
| 0 study meds/daya | 10 (29%) | 14 (14%) |
| Dose information missing/inconsistent | 0 | 0 |
| End of week 6 | n = 33 | n = 95 |
| 3 study meds/day | 15 (45%) | 49 (52%) |
| 2 study meds/day | 4 (12%) | 23 (24%) |
| 1 study med/day | 5 (15%) | 8 (8%) |
| 0 study meds/daya | 5 (15%) | 7 (7%) |
| Dose information missing/inconsistent | 4 (12%) | 8 (8%) |
| In trial at 6 weeks | ||
| % compliance:a mean (SD) | 79 (20) | 84 (16) |
| Compliance missing or inconsistent | n = 13 | n = 49 |
| In trial at 12 weeks | n = 31 | n = 88 |
| Taking trial medication at 12 weeks | n = 24 | n = 76 |
| % compliance:b mean (SD) | 60 (28) | 74 (27) |
| Compliance missing or inconsistent | n = 13 | n = 50 |
Adverse events and SAEs were ascertained to 16 or 4 weeks after last dose of IMP; deaths were recorded up to 16 weeks after randomisation (see Table 31). Examining AEs by week 16, there were 192 in 102 participants in the placebo group, of whom 65 (64%) individuals had at least one AE, compared with 106 events in 40 participants in the mirtazapine group of whom 27 (68%) had at least one. There were 35 SAEs in 18 individuals in the placebo group, compared with 12 in 5 individuals in the carbamazepine group. There was one death (1%) in the placebo group and two (5%) in the carbamazepine group.
| Carbamazepine (n = 40) |
Placebo (n = 102) |
|
|---|---|---|
| Adverse events | ||
| Number of events | 106 | 192 |
| Number of individuals | 27 (68%) | 65 (64%) |
| Serious adverse events | ||
| Number of events | 12 | 35 |
| Number of individuals | 5 (13%) | 18 (18%) |
| Deaths | 2 (5%) | 1 (1%) |
| MedDRA codes for deaths | 1 cause unknown 1 gastric haemorrhage |
1. Dementia |
Long-term outcomes at 26 and 52 weeks
CMAI outcomes at 26 and 52 weeks
CMAI outcomes at 26 and 52 weeks are presented in Table 32. There were no statistically significant differences between carbamazepine and placebo at either time point. This applied to both the raw and adjusted differences.
| Carbamazepine (n = 40) |
Placebo (n = 102) |
Difference | (95% CI) | Adjusted differencea | (95% CI) | p-value |
|---|---|---|---|---|---|---|
| 26 weeks | ||||||
| n = 26 | n = 62 | |||||
| 58.5 (SD = 18.0) | 56.8 (SD = 19.7) | 1.7 | (−7.26 to 10.57) | 0.08 | (−7.16 to 7.33) | 0.982 |
| 52 weeks | ||||||
| n = 17 | n = 56 | |||||
| 56.8 (SD = 21.8) | 58.5 (SD = 20.8) | −1.7 | (13.29 to −9.97) | −6.18 | (−16.02 to 3.66) | 0.218 |
Hospitalisation at 26 and 52 weeks
Hospitalisation by 26 weeks and between 26 and 52 weeks are presented in Table 33. There were no statistically significant differences between carbamazepine and placebo at either time period.
| Carbamazepine (n = 40) |
Placebo (n = 102) |
|
|---|---|---|
| Hospitalisations by 26 weeks | ||
| Yes | 1 (3.8%) | 10 (14.3%) |
| No | 25 (96.2%) | 60 (85.7%) |
| No information | 14 | 32 |
| Days in hospital by 26 weeks | ||
| N | 1 | 10 |
| Mean (SD) | 4 (−) | 16.8 (30.6) |
| Median (IQR) | 4 | 5.5 (1 to 14) |
| N missing | 0 | 0 |
| Hospitalisations between 26 and 52 weeks | ||
| Yes | 1 (5.3%) | 9 (15.0%) |
| No | 18 (94.7%) | 51 (85.0%) |
| No information | 21 | 42 |
| Days in hospital between 26 and 52 weeks | ||
| N | 1 | 8 |
| Mean (SD) | 7 (−) | 20.1 (30.3) |
| Median (IQR) | 7 | 9 (3 to 22) |
| N missing | 0 | 1 |
Deaths at 26 and 52 weeks
The cumulative number of deaths at 26 and 52 weeks are presented in Table 34. There were no statistically significant differences between death rates on carbamazepine and placebo at either time point.
| Carbamazepine (n = 40) |
Placebo (n = 102) |
|
|---|---|---|
| By 26 weeks | ||
| Died | 4 (13.4%) | 6 (8.1%) |
| Alive | 25 (86.6%) | 68 (91.9%) |
| No information | 11 | 28 |
| By 52 weeks | ||
| Died | 5 (17.9%) | 13 (18.6%) |
| Alive | 23 (82.1%) | 57 (81.4%) |
| No information | 12 | 32 |
Chapter 5 Discussion
This is a trial with negative findings, but these have important clinical implications for practice. Our results indicate that mirtazapine, given with normal clinical care, is not clinically effective compared with placebo for the treatment of clinically significant agitation in people with dementia. This finding implies a need to change the present practice of prescription of mirtazapine, and possibly other antidepressants, for agitation in dementia. In this study, there were clear decreases in agitation scores overall, with a clinically and statistically significant 10-point drop in the first 6 weeks of treatment, which was then maintained from 6 to 12 weeks; however, this drop was not attributable to mirtazapine since it was also seen in the placebo group. These clinical effectiveness data taken with those from the cost-effectiveness analyses make clear that there is no evidence to support the use of mirtazapine for agitation in dementia. These data are unequivocal in there is no clinical or economic reason for mirtazapine being used for the treatment of agitation in dementia. The data presented here are novel. While there is economic literature on psychosocial interventions for agitation, to our knowledge, no other formal economic analyses have been published of an RCT of an IMP for agitation in dementia. As such, this study provides data that may be of use in subsequent analyses of the cost-effectiveness of other IMPs for agitation in dementia.
Limitations
Our study has important potential limitations. First, there was a major adjustment to the initial trial protocol. We dropped the proposed carbamazepine arm from the trial in response to slower-than-anticipated recruitment, which means we are unable to test hypotheses concerning the clinical effectiveness of carbamazepine in the treatment of agitation in dementia with any confidence. The data presented on carbamazepine versus placebo must therefore be seen as exploratory only. The incomplete recruitment into the carbamazepine arm also meant that we could not complete an economic evaluation on this group. Stopping recruitment to this arm did not affect our ability to compare the clinical effectiveness of mirtazapine with placebo. However, the data from this trial apply only to mirtazapine and it is possible that other antidepressants from other classes might have a different effect; in the CitAD trial,21 citalopram, a SSRI, was reported to have had a modest positive effect, though with concerning adverse effects.
Second, the difference in mortality observed at 12 weeks may have been by chance. This study was not powered to investigate a mortality difference between the groups. The analysis was post hoc, its statistical significance was marginal and the difference was not observed at 6 or 12 months. In our previous study of depression in dementia, there were no more deaths in 108 randomisations to mirtazapine than in 111 randomised to placebo. 70 We therefore need to be careful in the interpretation of the mortality data in this study with the most likely conclusion being that in the long run, there are no mortality differences between taking mirtazapine or placebo. Third, recruitment beyond February 2020 was constrained by health research restrictions secondary to the COVID-19 pandemic. We only recruited 204 (92%) of our target of 222, but the closeness of the findings in both groups makes it highly unlikely that the results we found would have been different had there been another 18 randomisations as planned. Fourth, there was a relatively high level of missing data in descriptive (e.g. MMSE score) and secondary outcomes. This was most likely a function of participant ability to complete multiple questionnaires given their having clinically significant agitation. This is not likely to have introduced bias since there is no reason falling differentially between the randomisation groups, but it may limit inferences on secondary outcomes due to power, even with imputation.
Generalisability
This study was designed to reflect clinical populations and interventions as closely as possible. We kept exclusion criteria to a minimum and had permissive inclusion criteria, but the findings will not apply to individuals who are too critically ill to risk random allocation (such as those with high risk of harm to themselves or others). Only two potential participants were excluded for this reason, but there will have been others who were not referred to the trial. However, there are potential limits in generalisability that come from our having recruited most participants from old-age psychiatry services and care homes; outcomes might possibly have been different in those living in the community treated by primary care services alone. In the UK, those with significant agitation at home are likely to be referred to psychiatric services and would represent those for whom drug treatment might be indicated. In terms of generalisability, participants were not drawn only from specialist research clinics or tertiary care, but from 26 geographically diverse areas with a correspondingly high number of clinicians who therefore are likely to cover the range of services in general.
Strengths
The three main strengths of our study were high follow-up rates, large sample size and the broad nature of the study group (in terms of severity of agitation as measured by CMAI score and severity and type of dementia with mean sMMSE scores in the moderately severe range [for mirtazapine vs. placebo 25.4% mild (sMMSE 21–30), 46.1% moderate (10–20), 28.4% severe (0–9)]). We were able to follow up 81 (79%) of the mirtazapine group and 90 (87%) of the placebo group at 12 weeks and complete primary outcome assessment. Dropouts might introduce bias if those not followed up had a different response to mirtazapine or placebo compared with those completing the trial. However, our rates of follow-up are relatively high, and the difference between the groups seems attributable to the six additional deaths in the mirtazapine group compared with placebo. We included individuals with probable and possible AD, not just narrowly defined AD; this is important since agitation can affect dementia of all causes and most people with dementia have mixed aetiology. Participants were therefore closer to populations encountered in clinical practice, in which there is often mixed dementia. However, our inclusion criteria mean that we should restrict generalisation of our findings to AD and mixed dementia and be cautious in applying them to other subtypes (e.g. vascular, Lewy body or frontotemporal dementia).
Carbamazepine
We only have data on 40 people randomised to carbamazepine. This number, even when compared to the whole placebo group does not have sufficient statistical power for us to be able to draw definitive conclusions from the analyses presented above; these will therefore not be discussed in detail here. However, the data that we do have provide no signal that carbamazepine might have any positive effect on agitation in dementia above that seen in the placebo group.
Economic evaluation
The mirtazapine group had a marginally lower mean CMAI score than the placebo group at 12-week primary outcome follow-up. This difference was not statistically significant and much smaller than the pre-specified effectiveness criterion of a 6-point difference in favour of the mirtazapine group. The groups had similar costs from both the health and social care and societal perspectives. On the secondary analyses, groups were similar in both costs and health-related quality-of-life outcomes. Sensitivity analyses of the primary outcome yielded similar results to the base case. Results of sensitivity analyses of most secondary outcomes were also similar to base-case findings. Between-group differences in societal costs were sensitive to assumptions about valuing unpaid care time. Valuing carer time at replacement cost resulted in mirtazapine being definitely less cost-effective than placebo at a willingness to pay per QALY threshold of £20,000.
Taken with our findings that carers in the mirtazapine group attributed a higher proportion of their use of health and support services to their caring role, reported more hours of unpaid care at 12 weeks and more improvement in their care situation because of these services than did carers in the placebo group, it is possible that receipt of mirtazapine resulted in increased carer burden and related help-seeking and help-giving.
The substantial costs of caring reported by unpaid carers should be of concern. Carers from both groups lost approximately an hour of sleep nightly to care for the agitated person; the mean number of hours they felt able to leave the person alone at home was < 3 hours. Agitation is a distressing state for people experiencing it and for those around them. Effective strategies for managing agitation and supporting carers are required, tailored to the needs of the person with dementia and their families.
We were unable to locate previous trial-based economic evaluations of pharmacological interventions specifically focused on agitation in dementia. The cost-effectiveness of non-pharmacological interventions to manage agitation in care home residents with dementia has been evaluated in trials71,72 and model-based studies. 73 Non-pharmacological management approaches included person-centred care, communication, care mapping and care planning, and combinations of interventions in multicomponent programmes, in care home settings. 73 A trial of a person-centred care intervention in English care homes was found to be cost-effective in terms of agitation and quality of life, and the intervention was no more costly from the health and social care perspective than usual care. 71 Little is known on the effectiveness of any interventions for people with dementia and agitation living in the community. 73 There is a need for further research to address this evidence gap and future trials should include economic evaluations such as those completed here, given the high costs experienced by carers in terms of hours of care, sleep loss and carer burden identified across the SYMBAD effectiveness and cost-effectiveness studies.
Clinical context
The US National Health and Nutrition Examination Survey showed that the highest rates of antidepressant use between 2015 and 2018 were in those over 60, where 19.0% of people were prescribed such medication. 74 Mirtazapine is commonly prescribed for older adults. In a study of people living in long-term care facilities in Helsinki, there was a marked increase in use of mirtazapine between 2003 and 2017: from 15.7% to 22.7% in nursing homes, and 14.0% to 23.8% in assisted-living facilities, both settings with very high prevalence of residents with dementia. 75 In the MEDALZ cohort of 70,718 community-dwelling people with AD in Europe, mirtazapine was responsible for most new prescriptions (n = 6462, 39.2%). 76 One reason for high rates of prescription of mirtazapine in later life is to avoid the use of antipsychotics. 77 The influential NICE dementia guideline for the management of dementia is clear that antipsychotics should only be used in ‘agitation, aggression, distress and psychosis’ when the person with dementia is at risk of harming themselves or others or where the agitation or psychosis is causing the person with dementia severe distress. 23 The only other medication advice is that valproate should not be offered; there is no mention of antidepressants. This absence of guidance on the use of alternative medications for agitation in all but the most extreme clinical situations means that clinicians will seek to use other medications. Antidepressants that are perceived to have sedative effects such as mirtazapine, with which they are familiar, may appear an attractive and safe alternative to proscribed antipsychotics. However, there are reports that this may not be the case. Analyses of a primary care cohort showed increased all-cause mortality in people aged 20–64 prescribed mirtazapine. 78 The reports of potentially serious adverse effects of citalopram in the CitAD trial,21 of increased falls in trials of dextromethorphan-quinidine,79 and the higher mortality in mirtazapine group in this trial, present a growing evidence base that the assumption that the substitution of antidepressants, or other novel compounds, for antipsychotics for the treatment agitation in dementia is a safe alternative may well not be tenable.
In terms of secondary outcomes, the absence of any positive effects on participant and carer quality of life, on participant cognition and on broader neuropsychiatric symptoms as measured by the NPI is striking. The potential positive effects for people with agitation in dementia and for their family carers observed in secondary analyses of our HTA-SADD28 study of people with depression in dementia were not found in this definitive study of people with agitation in dementia. Our study provides strong evidence that the overall improvement seen over the 12 weeks of the study is not attributable to mirtazapine, but SYMBAD cannot tell us what has caused it. The improvement may be a function of the non-drug treatment-as-usual provided by old-age psychiatric and primary care services, or it could be part of the natural course of agitation in dementia. The latter is perhaps less likely given the observed persistence of agitation. 7,80 It might also be due in part to artefacts such as regression to the mean or the Hawthorne effect, though the magnitude of the effect means that these are unlikely to be the whole reason for the changes observed. In current systems, the data therefore suggest that waiting for a 6-week period (by which the improvement was noted), with reassessment following that might be a reasonable and safe course of action for agitation in dementia. A policy of such ‘active monitoring’ without the prescription of medication is recommended in the NICE guideline for depression as part of its stepped-care model for the treatment of depression in adults. 81 As with our earlier study of the treatment of depression in dementia (the HTA-SADD trial),28 our data suggest that finding agitation in dementia may be an appropriate trigger for referral to specialist services in which detailed assessment can be completed and non-drug treatments and active monitoring deployed, perhaps avoiding the use of medication.
Our findings suggest that there is little reason to recommend the use of mirtazapine for people living with dementia who experience agitation. Effective and cost-effective medicinal management strategies for agitation in dementia are needed, particularly where non-pharmacological approaches have been unsuccessful, and for people with dementia and their carers living in community settings.
Equality, diversity and inclusion
Attention was taken to ensure the inclusion of people with dementia and family carers at each stage of the research from design to reporting via our study specific PPIE group. We collected data on participants and our recruitment was designed to ensure the research sample is representative of the population the study is targeted at. We recruited from 26 geographically diverse areas across England and Northern Ireland, which were selected to be representative of the range of services in general. We designed the study so that it could be completed in any recruiting location with access to normal NIHR CRN support.
Interpretation
The main message from this trial is that the NASSA, mirtazapine, one of the most widely prescribed antidepressants for older people, is no more effective than placebo in the treatment of agitation in dementia. The carbamazepine data, while limited and lacking statistical power provide no support for its use in agitation in dementia either. In terms of economic benefits, there appears no evidence for there being any value in the use of mirtazapine for agitation in dementia. Just as our clinical effectiveness data concluded there was no clinical benefit over placebo, these data are unequivocal in there being no economic reason for mirtazapine being used for the treatment of agitation in dementia. Costs to dyadic unpaid carers were higher in those receiving mirtazapine at 12 weeks, suggesting that the intervention could be associated with higher costs to unpaid carers.
The first line of management for agitation in dementia is a full assessment to identify if there is a modifiable cause for the behaviour. In all but the most urgent of situations, the next line is non-pharmacological treatment since such approaches have been shown to be at least as effective as drug treatment. The data from this study provide support for ‘active monitoring’ of agitation in dementia without the prescription of medication as recommended in guidelines for depression. Antipsychotics and SSRI antidepressants are associated with significant harms when used for the treatment of agitation in dementia. This study suggests that substituting the antidepressant mirtazapine in order to avoid such harms is not a clinically or cost-effective treatment strategy.
Chapter 6 Conclusions
Implications for health care
This study finds no evidence to support the use of mirtazapine as a treatment for people with agitation in dementia as many cases will resolve with usual care and without mirtazapine. An important exclusion to this is the most critical of cases (by reason, e.g. of self-harm or other risk) which were not included in this study. Stepped care, with ‘watchful waiting’, is advocated currently for the general treatment of depression in the community. The first step is provision of ‘low-intensity psychosocial interventions’ with more complex psychosocial interventions as an alternative to antidepressants at the next stage of severity. Those recruited into this trial had received non-drug treatment and also during the study received non-drug ‘treatment as usual’ provided largely by the community mental-health teams to whom they were referred. This will have included a broad range of supportive and problem-solving interventions, commonly delivered by community psychiatric nurses, often in their own household. This will have focused on problems encountered by the person with dementia and the carer, covering aspects of dementia as well as agitation, and ranging in intensity from low to high as needed. Identifying which components of ‘usual care’ may be effective is an important area for future research. Compared with this personalised care, the Hawthorne effect of the study assessments is likely to have had only a minor impact. These data suggest that having agitation in dementia may be an appropriate trigger for referral to specialist services where non-drug treatments can be deployed, perhaps avoiding the use of medication with potential for ARs.
The practical implications of this study are that we should reframe the way we think about the treatment of people with dementia who are agitated, as the evidence does not support the routine prescription of antidepressants for agitation in dementia. It suggests that potential cases might be more appropriately managed by specialist services that are able to offer non-drug interventions for agitation and case management, which may not be available in primary care. Based on the data (a decrease at 12 weeks and this then maintained), except for those for whom medication is indicated by risk or extreme severity, and in the absence of evidence to the contrary, it might be appropriate to refrain from prescribing for 12 weeks and only reconsider prescribing for those who have not ‘responded’ or recovered within that period. There is also no reason to support mirtazapine being the drug to be prescribed at that point in this trial.
Overall, this study adds to the evidence base that shows pharmacological interventions for agitation in dementia are limited in their effectiveness82,83 and associated with significant risk of harm. The implications of this study are just that, with the minor limitations in generalisability noted above, that mirtazapine does not work in terms of clinical or cost-effectiveness. There are also reasons to be positive that ‘treatment as usual’ by current primary and secondary health care services may well enable people with agitation and dementia to recover from that agitation without the use of medication and its potential harms. Antipsychotics and SSRI antidepressants are associated with significant harms when used for the treatment of agitation in dementia. This study suggests that substituting the antidepressant mirtazapine in order to avoid such harms is not a clinically effective strategy.
Recommendations for research:
-
Given the multiple demonstrations of the clinical and cost-ineffectiveness of antidepressants in the treatment of agitation and depression in dementia and their high level of prescription alongside possible harm, a RCT of the effects of withdrawal of antidepressants in people with dementia who have been prescribed these drugs is needed.
-
Research into the overall effectiveness of community mental health services in the treatment of agitation would be useful along with analyses of what elements of care provided are effective in decreasing agitation.
-
Further formulation and testing of stepped care protocols for agitation in dementia are needed to expand the limited advice in current NICE guidance.
-
Epidemiological work is needed on the natural history of symptoms of agitation in dementia in the community including those managed in primary care alone.
-
This trial covered probable and possible AD, further work is needed in dementia with a different aetiology.
-
Further work is needed to examine the heterogeneity of the syndrome of agitation in dementia and the extent to which blanket approaches to treatment such as the prescription of antidepressants and other sedative medication is appropriate for the syndrome as a whole.
This report shares data with the paper published in the Lancet presenting a summary of the findings reported here and reproduces some sections of that paper as well as the study protocol published under licence CC-BY-NC-ND. 84
Acknowledgements
Trial Steering Committee
Sube Banerjee, Ann Marie Swart, Lee Shepstone, Peter Connolly, Andy Barker, Chris Penrose and Julie West.
Observer: Juliet High and sponsor representative.
Data Safety and Monitoring Committee
Bart Sheehan, Siobhan Creanor and Adrian Treloar.
Trial Management Group
Sube Banerjee, Martin Knapp, Gill Livingston, Shirley Nurock, Alan Thomas, Peter Bentham, Alistair Burns, Iracema Leroi, John O’Brien, Naji Tabet, Chris Fox, Robert Howard, Lee Shepstone, Ann Marie Swart, Juliet High, Ramin Nilforooshan, Clive Ballard, Paul Francis, sponsor representative and a PPI member.
Contributing sites
Sussex Partnership NHS Foundation Trust, Norfolk and Suffolk NHS Foundation Trust, Gateshead Health NHS Foundation Trust, Greater Manchester Mental Health NHS Foundation Trust, Camden and Islington NHS Foundation Trust, Birmingham and Solihull Mental Health NHS Foundation Trust, Cambridgeshire and Peterborough NHS Foundation Trust, Surrey and Borders Partnership NHS Foundation Trust, Devon Partnership NHS Trust, Barnet Enfield and Haringey Mental Health NHS Trust, Bradford District Care NHS Foundation Trust, Midlands Partnership NHS Foundation Trust, Dudley and Walsall Mental Health Partnership NHS Foundation Trust, South West London and St George’s Mental Health NHS Trust, 2Gether NHS Foundation Trust, South West Yorkshire Partnership NHS Foundation Trust, Central and North West London NHS Foundation Trust, Northamptonshire Healthcare NHS Foundation Trust, Coventry and Warwickshire Partnership NHS Trust, Sheffield Health and Social Care NHS Foundation Trust, Leicestershire Partnership NHS Trust, Kings College Hospital NHS Foundation Trust, Worcestershire Health and Care NHS Trust, The Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust, Rotherham Doncaster and South Humber NHS Foundation Trust, Belfast Health and Social Care Trust.
Contributions of authors
Sube Banerjee (https://orcid.org/0000-0002-8083-7649) (Professor, Chief Investigator) was the chief investigator for the study and conceived, designed and managed the study with input from the group, led the acquisition, analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Nicolas Farina (https://orcid.org/0000-0002-0635-2547) (Associate Professor, Dementia Studies) drafted the first and subsequent versions of this report under the guidance of Sube Banerjee, contributed to the analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Catherine Henderson (https://orcid.org/0000-0003-4340-4702) (Assistant Professorial Research Fellow, health economics) led and completed the economic evaluation with senior input from Martin Knapp; she drafted the economic sections of the report, contributed to the acquisition, analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Juliet High (https://orcid.org/0000-0003-2555-2349) (Senior Trials Manager, trial management), managed the trial and the sites, contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Susan Stirling (https://orcid.org/0000-0001-6663-7846) (Trial Statistician) completed the statistical analyses, contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Lee Shepstone (https://orcid.org/0000-0001-5524-7818) (Professor, Chief Statistician) designed and led the statistical analyses which were completed by Susan Stirling, contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Julia Fountain (https://orcid.org/0000-0003-2169-493X) (Coordinator for Service User and Carer Involvement in Research) led patient and carer engagement with Sheila Nurock, contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Clive Ballard (https://orcid.org/0000-0003-0022-5632) (Professor, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Peter Bentham (https://orcid.org/0000-0002-6443-3353) (Professor, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Alistair Burns (https://orcid.org/0000-0002-9837-0645) (Professor, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Chris Fox (https://orcid.org/0000-0001-9480-5704) (Professor, neurochemistry) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Paul Francis (https://orcid.org/0000-0001-8159-4469) (Professor, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Robert Howard (https://orcid.org/0000-0003-2349-0287) (Professor, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Martin Knapp (https://orcid.org/0000-0003-1427-0215) (Professor, health economics and policy) supervised health economics, contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Iracema Leroi (https://orcid.org/0000-0003-1822-3643) (Professor, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Gill Livingston (https://orcid.org/0000-0001-6741-5516) (Professor, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Ramin Nilforooshan (https://orcid.org/0000-0001-9801-183X) (Professor, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Shirley Nurock (https://orcid.org/0000-0002-7962-4072) (Former Carer, Alzheimer’s Society Research Network member) led patient and carer engagement with Julia Fountain, contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
John O’Brien (https://orcid.org/0000-0002-0837-5080) (Professor, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Annabel Price (https://orcid.org/0000-0002-5505-5231) (Consultant, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Alan J Thomas (https://orcid.org/0000-0002-6667-9533) (Professor, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Ann Marie Swart (https://orcid.org/0000-0002-9359-6995) (Director, Clinical Trials Unit) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Tanya Telling (https://orcid.org/0000-0003-0220-7083) (Director, Joint Clinical Research Office) managed the study finances, contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Naji Tabet (https://orcid.org/0000-0003-4629-6196) (Professor, old age psychiatrist) contributed to the design of the study and the analysis and interpretation of the data and critically reviewed the manuscript.
Publication
Banerjee S, High J, Stirling S, Shepstone L, Swart AM, Telling T, et al. Study of mirtazapine for agitated behaviours in dementia (SYMBAD): a randomised, double-blind, placebo-controlled trial. Lancet 2021;398:1487–97.
Ethics and regulatory approval
The study was approved by the Hampshire A South Central Research Ethics Committee (15/SC/0606), and the Medicines and Healthcare Products Regulatory Agency (Clinical Trial Authorisation Number: 58810/0001/001-0001).
Data-sharing statement
Requests for access to trial data will be considered, and approved in writing where appropriate, after formal application to the TMG and TSC. Considerations for approving access are documented in the TMG and TSC Terms of Reference. The Chief Investigator and trial statistician at NCTU will have access to the full trial data set. All data requests should be submitted to the corresponding author for consideration.
Disclaimers
None.
References
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673-734. https://doi.org/10.1016/S0140-6736(17)31363-6.
- Prince M, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M. World Alzheimer Report 2015: The Global Impact of Dementia. Alzheimer’s Disease International; 2015.
- Savva GM, Zaccai J, Matthews FE, Davidson JE, McKeith I, Brayne C. Prevalence, correlates and course of behavioural and psychological symptoms of dementia in the population. Br J Psychiatry J Ment Sci 2009;194:212-9. https://doi.org/10.1192/bjp.bp.108.049619.
- van der Linde RM, Dening T, Stephan BCM, Prina AM, Evans E, Brayne C. Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry J Ment Sci 2016;209:366-77. https://doi.org/10.1192/bjp.bp.114.148403.
- Cohen-Mansfield J, Billig N. Agitated behaviors in the elderly. I. A conceptual review. J Am Geriatr Soc 1986;34:711-21.
- Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the aging, demographics, and memory study. J Am Geriatr Soc 2010;58:330-7. https://doi.org/10.1111/j.1532-5415.2009.02680.x.
- Ryu S-H, Katona C, Rive B, Livingston G. Persistence of and changes in neuropsychiatric symptoms in Alzheimer disease over 6 months: the LASER-AD study. Am J Geriatr Psychiatry 2005;13:976-83. https://doi.org/10.1176/appi.ajgp.13.11.976.
- Wetzels RB, Zuidema SU, de Jonghe JFM, Verhey FRJ, Koopmans RTCM. Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2-year period. Am J Geriatr Psychiatry 2010;18:1054-65.
- Morris S, Patel N, Baio G, Kelly L, Lewis-Holmes E, Omar RZ, et al. Monetary costs of agitation in older adults with Alzheimer’s disease in the UK: prospective cohort study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007382.
- Panca M, Livingston G, Barber J, Cooper C, La Frenais F, Marston L, et al. Healthcare resource utilisation and costs of agitation in people with dementia living in care homes in England – the Managing Agitation and Raising QUality of LifE in Dementia (MARQUE) study. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0211953.
- Buylova Gola A, Morris S, Candy B, Davis S, King M, Kupeli N, et al. Healthcare utilization and monetary costs associated with agitation in UK care home residents with advanced dementia: a prospective cohort study. Int Psychogeriatr 2020;32:359-70. https://doi.org/10.1017/S1041610219002059.
- Burley CV, Livingston G, Knapp MRJ, Wimo A, Norman R, Brodaty H. Time to invest in prevention and better care of behaviors and psychological symptoms associated with dementia. Int Psychogeriatr 2020;32:567-72. https://doi.org/10.1017/S104161022000037X.
- Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ 2015;350. https://doi.org/10.1136/bmj.h369.
- Rabins PV, Rovner BW, Rummans T, Schneider LS, Tariot PN. Guideline Watch (October 2014): Practice Guideline for the Treatment of Patients With Alzheimer’s Disease and Other Dementias. Focus Am Psychiatr Publ 2017;15:110-28. https://doi.org/10.1176/appi.focus.15106.
- Banerjee S. The Use of Antipsychotic Medication for People With Dementia: Time for Action. A Report for the Minister of State for Care Services 2009.
- Donegan K, Fox N, Black N, Livingston G, Banerjee S, Burns A. Trends in diagnosis and treatment for people with dementia in the UK from 2005 to 2015: a longitudinal retrospective cohort study. Lancet Public Health 2017;2:e149-56. https://doi.org/10.1016/S2468-2667(17)30031-2.
- Howard RJ, Juszczak E, Ballard CG, Bentham P, Brown RG, Bullock R, et al. Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med 2007;357:1382-92. https://doi.org/10.1056/NEJMoa066583.
- Fox C, Crugel M, Maidment I, Auestad BH, Coulton S, Treloar A, et al. Efficacy of Memantine for agitation in Alzheimer’s dementia: a randomised double-blind placebo controlled trial. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0035185.
- Tariot PN, Schneider LS, Cummings J, Thomas RG, Raman R, Jakimovich LJ, et al. Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch Gen Psychiatry 2011;68:853-61. https://doi.org/10.1001/archgenpsychiatry.2011.72.
- Defrancesco M, Marksteiner J, Fleischhacker WW, Blasko I. Use of benzodiazepines in Alzheimer’s disease: a systematic review of literature. Int J Neuropsychopharmacol 2015;18. https://doi.org/10.1093/ijnp/pyv055.
- Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014;311:682-91.
- Schneider LS, Frangakis C, Drye LT, Devanand DP, Frangakis C, Ismail Z, et al. Heterogeneity of treatment response to Citalopram for patients with Alzheimer’s disease with aggression or agitation: The CitAD Randomized Clinical Trial. Am J Psychiatry 2016;173:465-72. https://doi.org/10.1176/appi.ajp.2015.15050648.
- National Institute for Health and Care Excellence (UK) . Dementia: Assessment, Management and Support for People Living With Dementia and Their Carers 2018. www.ncbi.nlm.nih.gov/books/NBK513207/ (accessed 12 October 2020).
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ national partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018;178:640-7. https://doi.org/10.1001/jamainternmed.2018.0379.
- Gerlach LB, Fashaw S, Strominger J, Ogarek J, Zullo AR, Daiello LA, et al. Trends in antipsychotic prescribing among long-term care residents receiving hospice care. J Am Geriatr Soc 2021;69:2152-62. https://doi.org/10.1111/jgs.17172.
- Maust DT, Strominger J, Kim HM, Langa KM, Bynum JPW, Chang C-H, et al. Prevalence of central nervous system-active polypharmacy among older adults with dementia in the US. JAMA 2021;325:952-61. https://doi.org/10.1001/jama.2021.1195.
- Forns J, Pottegård A, Reinders T, Poblador-Plou B, Morros R, Brandt L, et al. Antidepressant use in Denmark, Germany, Spain, and Sweden between 2009 and 2014: incidence and comorbidities of antidepressant initiators. J Affect Disord 2019;249:242-52. https://doi.org/10.1016/j.jad.2019.02.010.
- Banerjee S, Hellier J, Romeo R, Knapp M, Ballard C, Baldwin R, et al. Study of the use of antidepressants for depression in dementia: the HTA-SADD trial – a multicentre, randomised, double-blind, placebo-controlled trial of the clinical effectiveness and cost-effectiveness of sertraline and mirtazapine. Health Technol Assess 2013;17:1-166. https://doi.org/10.3310/hta17070.
- Romeo R, Knapp M, Hellier J, Dewey M, Ballard C, Baldwin R, et al. Cost-effectiveness analyses for mirtazapine and sertraline in dementia: randomised controlled trial. Br J Psychiatry 2013;202:121-8. https://doi.org/10.1192/bjp.bp.112.115212.
- Schittecatte M, Dumont F, Machowski R, Cornil C, Lavergne F, Wilmotte J. Effects of mirtazapine on sleep polygraphic variables in major depression. Neuropsychobiology 2023;46:197-201. https://doi.org/10.1159/000067812.
- Mühlbacher M, Konstantinidis A, Kasper S, Eichberger G, Hinterhuber H, Hofmann P, et al. Intravenous mirtazapine is safe and effective in the treatment of depressed inpatients. Neuropsychobiology 2006;53:83-7. https://doi.org/10.1159/000091724.
- Naglie G, Tomlinson G, Tansey C, Irvine J, Ritvo P, Black SE, et al. Utility-based quality of life measures in Alzheimer’s disease. Qual Life Res 2006;15:631-43. https://doi.org/10.1007/s11136-005-4364-8.
- Cakir S, Kulaksizoglu IB. The efficacy of mirtazapine in agitated patients with Alzheimer’s disease: a 12-week open-label pilot study. Neuropsychiatr Dis Treat 2008;4:963-6.
- Reichman WE, Coleman J, Aupperle P, Sohnle S. An Open Label Pilot Study of Mirtazapine for the Treatment of Dementia-Associated Behavioral Problems. Chicago, IL, USA: International Psychogeriatric Association Eleventh International Congress; 2003.
- Hollis J, Grayson D, Forrester L, Brodaty H, Touyz S, Cumming R. Antipsychotic medication dispensing and risk of death in veterans and war widows 65 years and older. Am J Geriatr Psychiatry 2007;15:932-41. https://doi.org/10.1097/JGP.0b013e31813547ca.
- Tariot PN, Erb R, Leibovici A, Podgorski CA, Cox C, Asnis J, et al. Carbamazepine treatment of agitation in nursing home patients with dementia: a preliminary study. J Am Geriatr Soc 1994;42:1160-6.
- Tariot PN, Erb R, Podgorski CA, Cox C, Patel S, Jakimovich L, et al. Efficacy and tolerability of carbamazepine for agitation and aggression in dementia. Am J Psychiatry 1998;155:54-61.
- Olin JT, Fox LS, Pawluczyk S, Taggart NA, Schneider LS. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry 2001;9:400-5.
- Ballard CG, Gauthier S, Cummings JL, Brodaty H, Grossberg GT, Robert P, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol 2009;5:245-55. https://doi.org/10.1038/nrneurol.2009.39.
- Sommer OH, Aga O, Cvancarova M, Olsen IC, Selbaek G, Engedal K. Effect of oxcarbazepine in the treatment of agitation and aggression in severe dementia. Dement Geriatr Cogn Disord 2009;27:155-63. https://doi.org/10.1159/000199236.
- James Lind Alliance, Alzheimer’s Society . Outcomes of the James Lind Alliance Dementia Priority Setting Partnership 2013.
- Department of Health . Living Well With Dementia: A National Dementia Strategy 2009.
- Department of Health . The Ministerial Advisory Group on Dementia Research: Headline Report 2011.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939-44.
- Alzheimer’s Society . Optimising Treatment and Care for People With Behavioural and Psychological Symptoms of Dementia. A Best Practice Guide for Health and Social Care Professionals 2011.
- Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol 1989;44:M77-84.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: University of York, Centre for Health Economics; 1995.
- Van Hout B, Janssen MF, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15.
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005;9:1-93.
- Rowen D, Mulhern B, Banerjee S, van Hout B, Young TA, Knapp M, et al. Estimating preference-based single index measures for dementia using DEMQOL and DEMQOL-Proxy. Value Health 2012;15:346-56.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96.
- Beecham J, Knapp M. Costing psychiatric interventions. Meas Ment Health Needs 2001;2:200-24.
- NHS Digital . Prescription Cost Analysis – England, 2017 2018.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- NHS Improvement . Reference Cost Collection: National Schedule of Reference Costs, 2016–17 – NHS Trusts and NHS Foundation Trusts 2017.
- Wimo A, Reed CC, Dodel R, Belger M, Jones RW, Happich M, et al. The GERAS study: a prospective observational study of costs and resource use in community dwellers with Alzheimer’s disease in three European countries – study design and baseline findings. J Alzheimers Dis 2013;36:385-99.
- Gustavsson A, Brinck P, Bergvall N, Kolasa K, Wimo A, Winblad B, et al. Predictors of costs of care in Alzheimer’s disease: a multinational sample of 1222 patients. Alzheimers Dement 2011;7:318-27. https://doi.org/10.1016/j.jalz.2010.09.001.
- Office for National Statistics . Annual Survey of Hours and Earnings: 2017 Provisional and 2016 Revised Results 2017.
- Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied Methods of Cost-Effectiveness Analysis in Healthcare. Oxford: Oxford University Press; 2011.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- StataCorp LP . Stata Multilevel Mixed-Effects Reference Manual: Release 15 2017.
- Willan AR, O’Brien BJ. Confidence intervals for cost-effectiveness ratios: an application of Fieller’s theorem. Health Econ 1996;5:297-305.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Löthgren M, Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ 2000;9:623-30.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- StataCorp LP . Stata: Release 16 2019.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Gomes M, Grieve R, Nixon R, Ng ES-W, Carpenter J, Thompson SG. Methods for covariate adjustment in cost-effectiveness analysis that use cluster randomised trials. Health Econ 2012;21:1101-18. https://doi.org/10.1002/hec.2812.
- R. Core Team . R: A Language and Environment for Statistical Computing 2020.
- Banerjee S, Hellier J, Dewey M, Romeo R, Ballard C, Baldwin R, et al. Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet 2011;378:403-11. https://doi.org/10.1016/S0140-6736(11)60830-1.
- Romeo R, Zala D, Knapp M, Orrell M, Fossey J, Ballard C. Improving the quality of life of care home residents with dementia: cost-effectiveness of an optimized intervention for residents with clinically significant agitation in dementia. Alzheimers Dement 2019;15:282-91. https://doi.org/10.1016/j.jalz.2018.08.010.
- Livingston G, Barber J, Marston L, Stringer A, Panca M, Hunter R, et al. Clinical and cost-effectiveness of the Managing Agitation and Raising Quality of Life (MARQUE) intervention for agitation in people with dementia in care homes: a single-blind, cluster-randomised controlled trial. Lancet Psychiatry 2019;6:293-304. https://doi.org/10.1016/S2215-0366(19)30045-8.
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess 2014;18:1-226. https://doi.org/10.3310/hta18390.
- Brody DJ, Gu Q. Antidepressant use among adults: United States, 2015–2018. NCHS Data Brief 2020. NCHS Data Brief, No 377. Hyattsville, Maryland: National Center for Health Statistics 2020:1-8.
- Aalto UL, Roitto H-M, Finne-Soveri H, Kautiainen H, Pitkälä KH. Temporal trends in the use of anticholinergic drugs among older people living in long-term care facilities in Helsinki. Drugs Aging 2020;37:27-34. https://doi.org/10.1007/s40266-019-00720-6.
- Kettunen R, Taipale H, Tolppanen A-M, Tanskanen A, Tiihonen J, Hartikainen S, et al. Duration of new antidepressant use and factors associated with discontinuation among community-dwelling persons with Alzheimer’s disease. Eur J Clin Pharmacol 2019;75:417-25. https://doi.org/10.1007/s00228-018-2591-5.
- Marcinkowska M, Śniecikowska J, Fajkis N, Paśko P, Franczyk W, Kołaczkowski M. Management of dementia-related psychosis, agitation and aggression: a review of the pharmacology and clinical effects of potential drug candidates. CNS Drugs 2020;34:243-68. https://doi.org/10.1007/s40263-020-00707-7.
- Coupland C, Hill T, Morriss R, Moore M, Arthur A, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in people aged 20–64 years: cohort study using a primary care database. BMC Med 2018;16. https://doi.org/10.1186/s12916-018-1022-x.
- Cummings JL, Lyketsos CG, Peskind ER, Porsteinsson AP, Mintzer JE, Scharreet DW, et al. Effect of dextromethorphan-Quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015;314:1242-54. https://doi.org/10.1001/jama.2015.10214.
- Marston L, Livingston G, Laybourne A, Cooper C. Becoming or remaining agitated: the course of agitation in people with dementia living in care homes. The English Longitudinal Managing Agitation and Raising Quality of Life (MARQUE) Study. J Alzheimers Dis 2020;76:467-73. https://doi.org/10.3233/JAD-191195.
- National Collaborating Centre for Mental Health (UK) . Depression: The Treatment and Management of Depression in Adults (Updated Edition) 2010.
- Kongpakwattana K, Sawangjit R, Tawankanjanachot I, Bell JS, Hilmer SN, Chaiyakunapruk N. Pharmacological treatments for alleviating agitation in dementia: a systematic review and network meta-analysis. Br J Clin Pharmacol 2018;84:1445-56. https://doi.org/10.1111/bcp.13604.
- Watt JA, Goodarzi Z, Veroniki AA, Nincic V, Khan PA, Ghassemi M, et al. Comparative efficacy of interventions for aggressive and agitated behaviors in dementia: a systematic review and network meta-analysis. Ann Intern Med 2019;171:633-42. https://doi.org/10.7326/M19-0993.
- Banerjee S, High J, Stirling S, Shepstone L, Swart AM, Telling T, et al. Study of mirtazapine for agitated behaviours in dementia (SYMBAD): a randomised, double-blind, placebo-controlled trial. Lancet 2021;398:1487-97.
- Curtis LA. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Banks L, Barnes M. Evaluation of the East Sussex Carers’ Breaks Demonstrator Site. Brighton: University of Brighton; 2011.
- Romeo R, Knapp M, Banerjee S, Morris J, Baldwin R, Tarrier N, et al. Treatment and prevention of depression after surgery for hip fracture in older people: cost-effectiveness analysis. J Affect Disord 2011;128:211-9. https://doi.org/10.1016/j.jad.2010.07.026.
- Hartfiel N, Clarke G, Havenhand J, Phillips C, Edwards RT. Cost-effectiveness of yoga for managing musculoskeletal conditions in the workplace. Occup Med Oxf Engl 2017;67:687-95. https://doi.org/10.1093/occmed/kqx161.
- General Osteopathic Council . General Osteopathic Council: What to Expect 2021. www.osteopathy.org.uk/visiting-an-osteopath/what-to-expect/ (accessed 12 October 2020).
- Office for National Statistics . Consumer Prices Index Including Owner Occupiers’ Housing Costs (CPIH) 2019.
- Quinn C, Toms G, Jones C, Brand A, Edwards RT, Sanders F, et al. A pilot randomized controlled trial of a self-management group intervention for people with early-stage dementia (The SMART study). Int Psychogeriatr 2016;28:787-800.
- Dementia Partnerships . Peer Support Projects 2014. https://dementiapartnerships.com/ (accessed 14 April 2014).
Appendix 1 Summary of changes to the SYMBAD protocol
Amendments made to protocol v1.1
Version 1.0 was considered a draft version and did not meet full trial requirements, the version submitted to ethics for initial approvals was v1.1. Therefore, changes made to v1.0 are numerous and not included here.
Amendments made to protocol v1.2
-
Version and date details updated.
-
Exclusions criteria amended in line with MHRA comments; pages 3, 18–19.
-
New abbreviations added in line with amended text.
-
New section 4.1.7 added ‘Risks and benefits’ in line with MHRA discussions.
-
Safety blood and ECG testing and Columbia Suicide Rating Scale (C-SSRS) added as requested by MHRA; pages 20–21, 23–25, 29.
-
Clarification of week 16 phone call added in line with MHRA comments; pages 21, 25.
-
Expanded list of con-meds to be more specific, as requested by MHRA, new sections 5.4.8.1 and 5.4.8.2; pages 29, 30–31.
-
Amended wording for notification of SAEs to Clinical Trials Unit (CTU) from ‘one working day’ to ‘within 24 hours’; pages 41, 43.
-
Approval of protocol amendments wording changed to clarify that competent authority and European Commission (EC) approval must be received before being implemented, where relevant. Page 46.
Amendments made to protocol v1.3
-
Version and date details updated.
-
Minor typographical errors and amendments for consistency and clarity added throughout.
-
New abbreviations added in line with amended text.
-
TMG lists updated, there haven’t been any changes to the groups themselves, but not all names were listed on the protocol when it was first produced.
-
CMAI questionnaire should be the Long Form and this has been updated throughout the protocol for clarity and Appendix 3 has been amended to show the correct version; pages 11–12, 17, 19, 31.
-
The word ‘tablet’ has been changed to ‘capsule’ throughout the protocol, procedures haven’t changed but as the product will be a capsule the wording has been made consistent for clarity; pages 10, 23, 26–29, 36.
-
In some places, the word ‘bottle’ had been used to describe packaging, as with point 6, this has been amended for clarity and consistency to packs/boxes as relevant; page 34.
-
Wording has been added to the participant timeline table (5.3.1) to clarify windows of acceptability for visits/tests and confirm that face-to-face visits may take place over more than one visit if required.
-
Window for acceptability of blood tests has been amended from 4 weeks to 28 days, to be consistent throughout all documents. Postdosing blood test window has been changed from 7 days to 28 days as requested by TMG, to aid compliance.
-
Stratifying has been changed from ‘by centre’ to ‘by independent living’ and text has been updated; pages 11, 33–34.
-
Safety e-mail address has been updated with the new contact details, the procedure remains the same, it’s just the e-mail address that has been updated – recruitment hasn’t started so this doesn’t need to be immediately notified to sites.
-
This section (8) has been updated as there was no previous record of amendments.
Amendments made to protocol v1.4
-
Version and date details updated.
-
The trial is now registered in the publicly accessible ISRCTN database, number added as the primary registry reference, page 10.
-
Inclusion criteria wording changed to make clear that dose of cholinesterase inhibitors and memantine must be stable, only if the patient is already on these medications. (Also updated on page 25.)
-
Exclusion criteria amended following cardiologists review; previously worded that atrioventricular block is always excluded, this has been clarified to exclude as follows: Patients with second-degree atrioventricular block (patients with third-degree heart block, with a pacemaker fitted, may be included at PI discretion). This exclusion has been given its own bullet point, for clarity. (Also updated on pages 21 and 25.)
-
Secondary outcome point 4 has been amended to reflect that AE data are collected from week 0 to week 16 and will be analysed as such (also updated on page 39).
-
Blood AEs should be graded according to the CTCAE criteria, to further operationalise the MHRA requirement for blood safety tests and reporting. The previous system for classifying AEs would not always be relevant to blood AEs, so text has been inserted to explain this new requirement. Text has been added in section 5.10.2.3 to clarify that a blood AE grade 3 or higher is a notifiable event and should be reported using a SAE form. CTCAE full reference has been added to the references in section 9.
-
Minor amendments for consistency and clarity have been added throughout, these include:
-
– Page 13, Martin Knapp’s affiliation changed to LSE.
-
– Page 22, secondary objective 3 amended, removing ‘patients’ form this sentence, to show that the emphasis is on carers in this objective.
-
– Page 23, clarity added on pilot phase recruitment period, in line with delayed start.
-
– Page 24, section 5.1.2.1 PI agreement amended to reflect process for this trial.
-
– Pages 27–29, minor clarity updates to information in table.
-
– Page 31, clarity about the meaning of ‘absence of symptoms’.
-
– Page 32, section 5.3.5.8 defined long-term follow-up period.
-
– Pages 41–45, minor clarity changes in statistical analysis section, including making clear that the statistics team are not blinded.
-
– Page 46, SAE reporting previously defined as up to ‘30 days’ after last IMP dose, changed to ‘4 weeks’ to be consistent with other areas of the protocol.
-
– Page 52, defined main trial closure and made clear that this is separate to long-term follow-up.
-
Amendments made to protocol v1.5
-
Version and date details updated.
-
Removal of inclusion criteria; participants taking memantine or other cholinesterase inhibitors do not need to be on a stable dose of 3 months or more when trial drug is initiated.
-
Exclusion criteria amended following review, to align prohibited medications washout periods with normal clinical practice. For MAOIs, this should still be 2 weeks. Antidepressants (and MAOIs), anticonvulsants and antipsychotics are still prohibited medications during trial drug administration.
-
Absolute requirement for blood and ECG tests prior to trial medication being prescribed has been replaced with a recommendation for these tests to be carried out and a reasonable attempt should be made to collect them in all cases. If this is not possible, PI judgement should be used. Text referring to this has been amended throughout the protocol, including table in section 5.3.4.
-
Wording added regarding approval for recruitment from Participant Identification Centres, which may also include GP practices.
-
Wording added to give clarity to what a treatment interruption is and appropriate circumstances for a participant to continue in the trial after a break in treatment.
-
Minor amendments for consistency and clarity, these include:
-
– Clearer wording on who receives randomisation e-mails and who receives semi-blinded treatment allocation e-mails.
-
– Section 5.3.4, participant timeline, clarifications added on interpretation of baseline visit window, for greater consistency across sites.
-
Amendments made to protocol v2.0
This version of the protocol is for a re-designed two-arm study, continuing with the existing study set-up and patients recruited to date, but with no further patients being recruited to the carbamazepine arm and a statistical re-design to reflect the requirements of the change to two-arm.
-
Version and date details updated.
-
Removal of references to carbamazepine throughout, including change of title.
-
Exclusion criteria ‘patients with a history of bone marrow depression or history of hepatic porphyrias’ removed as this risk related only to carbamazepine.
-
Exclusion criteria related to prohibited concomitant medications updated to remove anticonvulsants as these were only prohibited with carbamazepine.
-
Outcomes and objectives have small amendments to reflect new main analyses.
-
Update to introduction and background, to remove carbamazepine-specific information and references.
-
Trial diagram updated to reflect two-arm design.
-
Study will now open to recruitment in more locations, text added to reflect this throughout.
-
Participant timeline (page 27) updated for clarity and to reflect that all future visits are calculated from the week 0 dispensing visit (to match practice and the rest of the protocol).
-
Clarity added that week 16 phone call is not required where patients stopped trial medication prior to week 8, but that a call is required within 4 weeks of stopping trial medication (pages 29, 32).
-
Pharmacy review of medicines no longer requiring additional monitoring at removal of carbamazepine and addition of a few others for mirtazapine, to reflect current standard practice (list is not exhaustive and these changes should have been considered by clinicians prior to this update).
-
Sample size and statistical considerations updated to reflect requirements of two-arm trial and change to 80% power with 10% attrition rate.
-
Reference to GDPR included, to reflect change in law.
-
Archive period clarified as 10 years throughout (sponsor decision as current trial documents have a variety of dates, all will be made consistent with this).
-
Health economics analysis more clearly demarcated from statistical section but no change of procedures.
-
Minor changes for consistency and clarity in the safety reporting section, no change of procedures.
Appendix 2 Recruitment by site and by month
Number of participants reported refers to patient-carer dyads in this trial. Names of sites were accurate at the time of recruitment.
Sussex Partnership NHS Foundation Trust (34 patients, opened 15 December 2016)
Naji Tabet (PI), Andrew Risbridger, Gosia Raczek, Richard Hoile, Andrea Meredith, Angela Ozduran, Elise Armsby, Keren Teichmann, Kim McCabe, Marcela Carvajal, Natalie Portwine, Rachel Russell, Sam Holden, Sharne Berwald, Tamsin Eperson, Yvonne Feeney. Pharmacy: James Atkinson, Jed Hewitt, Nana Tomova, Sinead Clarke-O’Neill
Norfolk and Suffolk NHS Foundation Trust (16 patients, opened 15 December 2016)
Chris Fox (PI), Heather Cooke, Nigel Gill, Caroline Sheldon, Claire Rischmiller, Kim Clipsham, Zoe Inman. Pharmacy: Dennis Liew
Gateshead Health NHS Foundation Trust (36 patients, opened 15 December 2016)
Alan Thomas (PI), Karen Franks, Bryony Storey, Elaine Siddle. Pharmacy: David Sproates
Greater Manchester Mental Health NHS Foundation Trust (13 patients, opened 19 December 2016)
Ross Dunne (PI), Iracema Leroi (previous PI), Alistair Burns, Clare Smith, Preeti Tekur, Anita Davies, Dee Leonard, Emma Oughton, Lewis Harpin, Phillip Tinkler, Rebecca Davies, Robert Bedford, Selina Sonola. Pharmacy: Maxine Syme
Camden and Islington NHS Foundation Trust (28 patients, opened 13 January 2017)
Gill Livingston (PI), Rob Howard, Alessandro Borca, Beena Bauluck, Liam Pikett, Narin Aker. Pharmacy: Jonathan Flor, Silvia Ceci
Birmingham and Solihull Mental Health NHS Foundation Trust (29 patients, opened 16 December 2016)
Peter Bentham (PI), Abdul Patel, Analisa Smythe, Di Baines, Jan Wright, Jane Dyer. Pharmacy: Akram Ali, Nigel Barnes
Cambridgeshire and Peterborough NHS Foundation Trust (nine patients, opened 16 December 2016)
Annabel Price (PI), Catherine Hatfield, Catherine Inkley, Julie Philps, Naomi Thomas. Pharmacy: Christine Rowe
Surrey and Borders Partnership NHS Foundation Trust (17 patients, opened 15 December 2016)
Ramin Nilforooshan (PI), Brian Parsons, Gareth O’Leary, George Shaya, Jessica True, Mariana Gavrilla, Sally Gosling. Pharmacy: Sam Francis
Devon Partnership NHS Trust (24 patients, opened 12 July 2017)
Clive Ballard, Carol Bannister (previous PI), Joseph Butchart, Simona Brown, Amanda Henderson, Anna Grice, Olga Borejko, Sarah Broom, Stacey Horne, Sue Dyson
Barnet Enfield and Haringey Mental Health NHS Trust (four patients, opened 28 November 2017)
Elizabeth Sampson (PI), Ayesha Dar, Luiza Grycuk, Serafeim Papakostas, Tom Freeth. Pharmacy: Neil Spencer, Helen Tsegay-Seyoum
Bradford District Care NHS Foundation Trust (two patients, opened 15 January 2019)
Sushanth Kamath (PI), Gregor Russell, Nasir Khan, Jason Cook, Sarah Kirkland, Zarina Mirza. Pharmacy: Jaspreet Sohal, Edward Sykes
Midlands Partnership NHS Foundation Trust (two patients, opened 18 March 2019)
Rashi Negi (PI), Caroline Wnkle, Sajeev Kshemendran, Lucy Hamilton, Paula Coventry, Susan Lavendar. Pharmacy: Rachel Walsh
Dudley and Walsall Mental Health Partnership NHS Foundation Trust (now Black Country Healthcare NHS Foundation Trust, three patients, opened 13 February 2019)
Udaya Balakrishna (PI), Dee Gayan, Sharada Abilash, Aurora Balalia. Pharmacy: Lisa Stanton
South West London and St George’s Mental Health NHS Trust (six patients, opened 30 January 2019)
Robert Lawrence (PI), Heloise Mongue-Din, Na’ilah Firdaws. Pharmacy: Seema Shah
2Gether NHS Foundation Trust (now Gloucestershire Health and Care NHS Foundation Trust, one patient, opened 24 January 2019)
Emma Abbey (PI), Marelle Harvey, Sarah Little. Pharmacy: Bethan Cartwright
South West Yorkshire Partnership NHS Foundation Trust (no patients recruited, opened 4 April 2019)
Suba Thiyagesh (PI), Amber Hemingway, Lisa Horner, Mark Harper, Wajid Khan. Pharmacy: Mark Payne, Peter Bermingham
Central and North West London NHS Foundation Trust (seven patients, opened 8 March 2019)
Erum Nomani (PI), Desiree Fyle, Narin Aker. Pharmacy: Nawal Arif, Shradha Patel
Northamptonshire Healthcare NHS Foundation Trust (two patients, opened 27 August 2019)
Paul Koranteng (PI), Chetan Lakhani, Sharon Aujla. Pharmacy: Alpa Patel
Coventry and Warwickshire Partnership NHS Trust (one patient, opened 4 April 2019)
Demi Onalaja (PI), Emily Benson, Nyaradzo Nyamayaro. Pharmacy: Wendy Roughan
Sheffield Health and Social Care NHS Foundation Trust (five patients, opened 9 May 2019)
Aparna Mordekar (PI), Janet Hutchinson, Katherine Mewton, Poovanna Pemmaiah, Hannah Gower. Pharmacy: Shrewti Moerman
Leicestershire Partnership NHS Trust (four patients, opened 11 March 2019)
Matthew Noble (PI), Matthew Critchfield (previous PI), Iain Termie, Sarah Ballion. Pharmacy: Robyn McAskill
Kings College Hospital NHS Foundation Trust (two patients, opened 15 October 2019)
Adenike Dare (PI), Fabio Speranza, Shaula Candido. Pharmacy: Kam Sahota
Worcestershire Health and Care NHS Trust (no patients recruited, opened 10 June 2019)
Dhanjeev Marrie (PI), Sinha Tandrila, Angela Hoadley, Harriet Davies, Remi George. Pharmacy: Amanda Critchley
The Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust (now University Hospitals Dorset NHS Foundation Trust, no patients recruited, opened 10 July 2019)
Divya Tiwari (PI), Emma Gunter. Pharmacy: Cathy Howe
Rotherham Doncaster and South Humber NHS Foundation Trust (one patient, opened 8 August 2019)
Oluwafemi Adio (PI), Kevin Williamson, Helen Oldknow, Ken Hindle-May. Pharmacy: Steve Davies
Belfast Health and Social Care Trust (no patients recruited, opened 18 September 2019)
Bernadette McGuinness (PI), Alison Clinton, Debbie Rainey, Nicola Milligan. Pharmacy: Peter Gray
PIC sites assisting with recruitment included the following:
South East England: Care Homes: Autumn Lodge (Care home), Weald Hall (Care home), Windlesham Manor (Care home), Havelock House (Care home), Garland House (Care home), Birchwood House (Care home), Beaconsfield Medical Practice (GP), Preston Park Surgery (GP), Stanford Medical Centre (GP), Warmdene Surgery (GP), Poundhill Medical Group (GP), Parklands Surgery (GP), Furnace Green Surgery (GP), Charter Medical Centre (GP), Brighton Health and Wellbeing Centre (GP), Trinity Medical Centre (GP), Benfield Valley Healthcare Hub (GP), Park Surgery (GP), St John’s Practice (GP), Maidstone and Tunbridge Wells NHS Trust (MTW), Brighton and Sussex University Hospital (BSUH), Kent and Medway NHS Social Care Partnership Trust (KMPT).
East England: Hoveton and Wroxham Medical Centre (GP), Cambridge University Hospitals Trust
North East England: Tees, Esk and Wear Valley NHS Trust (TEWV), Newcastle Tyne and Wear NHS Trust (NTW) and Northumbria Healthcare NHS Trust (NHCT)
Northwest England: Pennine Care NHS Foundation Trust
South West England: Dorset Healthcare NHS Trust (linked with King’s College London).
Appendix 3 SYMBAD adverse events and severe adverse events by randomisation group
| System organ class | Adverse event | Serious |
|---|---|---|
| Cardiac disorders (2) | Atrioventricular block (1) | |
| Angina pectoris (1) | ||
| Gastrointestinal disorders (23) | Abdominal pain (2) | |
| Anal incontinence (1) | ||
| Constipation (4) | ||
| Diarrhoea (1) | ||
| Dry mouth (1) | ||
| Dyspepsia (1) | ||
| Faecaloma (1) | ||
| Gastric dilatation (1) | ||
| Haematochezia (1) | ||
| Mouth ulceration (1) | ||
| Nausea (1) | ||
| Salivary hypersecretion (2) | ||
| Toothache (1) | ||
| Vomiting (5) | ||
| General disorders and administration site conditions (14) | Crepitations (1) | |
| Death (3) | (2) | |
| Fatigue (2) | ||
| Gait disturbance (2) | ||
| Oedema (2) | ||
| Peripheral swelling (1) | ||
| Pyrexia (1) | ||
| Swelling face (1) | ||
| Swelling; contusion (1) | ||
| Infections and infestations (25) | Infection (1) | (1) |
| Influenza (1) | ||
| Low respiratory tract infection (5) | ||
| Nasopharyngitis (6) | ||
| Pneumonia (1) | (1) | |
| Sepsis (1) | ||
| Urinary tract infection (9) | (1) | |
| Vulvovaginal candidiasis (1) | ||
| Injury, poisoning and procedural complications (28) | Accidental overdose (2) | |
| Fall (24) | (3) | |
| Femoral neck fracture (1) | ||
| Forearm fracture (1) | ||
| Investigations (12) | Blood alkaline phosphatase increased (1) | |
| Blood creatinine increased (1) | ||
| Blood urea abnormal (1) | ||
| Electrocardiogram qt prolonged (1) | ||
| Gamma GT increased (1) | ||
| Glomerular filtration rate decreased (1) | ||
| Mean cell volume abnormal (1) | ||
| Oxygen saturation decreased (1) | ||
| Platelet count increased (1) | ||
| Protein total decreased (1) | ||
| Weight decreased (1) | ||
| Weight increased (1) | ||
| Metabolism and nutrition disorders (9) | Decreased appetite (3) | |
| Dehydration (1) | (1) | |
| Fluid intake reduced (1) | ||
| Hyperglycaemia (1) | ||
| Increased appetite (3) | ||
| Musculoskeletal and connective tissue disorders (9) | Arthralgia (1) | |
| Back pain (2) | ||
| Joint swelling (1) | ||
| Muscular weakness (1) | ||
| Musculoskeletal pain (1) | ||
| Neck pain (1) | ||
| Posture abnormal (2) | ||
| Nervous system disorders (29) | Depressed level of consciousness (1) | |
| Dizziness (1) | ||
| Drooling (1) | ||
| Facial paralysis (1) | ||
| Headache (1) | ||
| Lethargy (8) | ||
| Sedation (1) | ||
| Somnolence (14) | ||
| Syncope (1) | ||
| Psychiatric disorders (45) | Abnormal behaviour (1) | |
| Abnormal dreams; sleep talking (2) | ||
| Aggression (5) | ||
| Agitation (12) | ||
| Anxiety (3) | ||
| Confusional state (3) | ||
| Delusion (1) | ||
| Depression (2) | ||
| Depressed mood (1) | ||
| Hallucination (2) | ||
| Insomnia (2) | ||
| Irritability (1) | ||
| Mood altered (2) | ||
| Panic attack (1) | ||
| Restlessness (5) | ||
| Sleep disorder (2) | ||
| Renal and urinary disorders (8) | Pollakiuria (2) | |
| Renal impairment (2) | (1) | |
| Urinary incontinence (4) | ||
| Reproductive system and breast disorders (1) | Genital prolapse (1) | |
| Respiratory, thoracic and mediastinal disorders (11) | Choking (1) | (1) |
| Chronic obstructive pulmonary disease (1) | ||
| Cough (3) | ||
| Dyspnoea (3) | ||
| Pleural effusion (1) | ||
| Pleuritic pain (1) | ||
| Pneumonia aspiration (1) | (1) | |
| Skin and subcutaneous tissue disorders (4) | Decubitus ulcer (1) | |
| Eczema (1) | ||
| Skin disorder (1) | ||
| Skin lesion (1) | ||
| Social circumstances (1) | Bedridden (1) | |
| Surgical and medical procedures (1) | Hospitalisation (1) | (1) |
| Vascular disorders (2) | Aortic aneurysm (1) | |
| Hypertension (2) |
| System organ class | Adverse event | Serious |
|---|---|---|
| Blood and lymphatic system disorders (1) | Anaemia (1) | |
| Cardiac disorders (1) | Bradycardia (1) | |
| Ear and labyrinth disorders (1) | Excessive cerumen production (1) | |
| Gastrointestinal disorders (18) | Abdominal pain (1) | |
| Constipation (7) | (2) | |
| Diarrhoea (4) | ||
| Duodenogastric reflux (3) | ||
| Nausea (1) | ||
| Vomiting (2) | ||
| General disorders and administration site conditions (10) | Abasia (1) | (1) |
| Chest pain (2) | (2) | |
| Death (1) | (1) | |
| Fatigue (1) | ||
| Gait disturbance (1) | ||
| Malaise (2) | (1) | |
| Peripheral swelling (1) | ||
| Swelling; contusion (1) | (1) | |
| Infections and infestations (30) | Bacterial infection (1) | |
| Cellulitis (1) | ||
| COVID-19 (1) | ||
| Fungal infection (1) | ||
| Low respiratory tract infection (7) | (1) | |
| Omphalitis (1) | ||
| Oral candidiasis (1) | ||
| Pneumonia (2) | (1) | |
| Urinary tract infection (15) | (3) | |
| Injury, poisoning and procedural complications (33) | Fall (24) | (4) |
| Femoral neck fracture (1) | ||
| Hip fracture (4) | (4) | |
| Humerus fracture (1) | (1) | |
| Joint injury (1) | ||
| Laceration (1) | ||
| Skin injury (1) | ||
| Investigations (12) | Alanine aminotransferase increased (1) | |
| Blood alkaline phosphatase increased (1) | ||
| Blood creatinine decreased (1) | ||
| C-reactive protein increased (1) | (1) | |
| Gamma GT increased (2) | ||
| Haemoglobin decreased (1) | ||
| Neutrophil count increased (1) | (1) | |
| Protein urine present (1) | ||
| Red blood cell count decreased (2) | ||
| White blood cell count increased (1) | (1) | |
| Metabolism and nutrition disorders (5) | Decreased appetite (4) | |
| Increased appetite (1) | ||
| Musculoskeletal and connective tissue disorders (6) | Arthralgia (1) | |
| Mobility decreased (2) | (1) | |
| Musculoskeletal stiffness (1) | ||
| Pain in extremity (2) | ||
| Nervous system disorders (29) | Altered state of consciousness (1) | |
| Dementia (1) | (1) | |
| Depressed level of consciousness (1) | (1) | |
| Dizziness (5) | (1) | |
| Hypersomnia (1) | ||
| Lethargy (4) | ||
| Somnolence (16) | ||
| Psychiatric disorders (33) | Abnormal dreams; sleep talking; nightmares (1) | |
| Aggression (4) | (1) | |
| Agitation (11) | (1) | |
| Anxiety (1) | ||
| Confusional state (5) | (1) | |
| Insomnia (2) | ||
| Irritability (1) | ||
| Restlessness (4) | ||
| Sleep disorder (1) | ||
| Suicidal behaviour (1) | (1) | |
| Tearfulness (2) | ||
| Respiratory, thoracic and mediastinal disorders (5) | Cough (2) | |
| Dyspnoea (2) | (1) | |
| Epistaxis (1) | ||
| Blister (1) | (1) | |
| Skin and subcutaneous tissue disorders (6) | Pruritus (2) | |
| Rash (3) | ||
| Haematoma (1) | ||
| Vascular disorders (2) | Pallor (1) |
| System organ class | Adverse event | Serious |
|---|---|---|
| Eye disorders (1) | Exophthalmos (1) | |
| Gastrointestinal disorders (16) | Abdominal pain (2) | |
| Constipation (2) | ||
| Diarrhoea (4) | ||
| Gastric haemorrhage (1) | ||
| Nausea (3) | ||
| Oral pain (1) | ||
| Salivary hypersecretion (1) | ||
| Vomiting (2) | ||
| General disorders and administration site conditions (5) | Fatigue (1) | |
| Gait disturbance (1) | ||
| General physical health deterioration (1) | ||
| Malaise (1) | ||
| Oedema (1) | ||
| Infections and infestations (15) | Cellulitis (2) | |
| Fungal infection (1) | ||
| Herpes zoster (1) | ||
| Low respiratory tract infection (2) | ||
| Nasopharyngitis (2) | ||
| Urinary tract infection (7) | (1) | |
| Injury, poisoning and procedural complications (11) | Fall (9) | |
| Hip fracture (1) | (1) | |
| Laceration (1) | ||
| Investigations (28) | Blood albumin decreased (1) | (1) |
| Blood alkaline phosphatase increased (2) | ||
| Blood creatinine increased (2) | ||
| Blood urea increased (2) | ||
| Blood test abnormal (5) | (2) | |
| Electrocardiogram qt prolonged (1) | ||
| Gamma GT increased (1) | (1) | |
| Glomerular filtration rate decreased (2) | ||
| Haemoglobin decreased (1) | (1) | |
| Neutrophil count increased (1) | ||
| Red blood cell count decreased (1) | (1) | |
| White blood cell count increased (1) | ||
| Weight decreased (8) | ||
| Metabolism and nutrition disorders (1) | Diabetes mellitus inadequate control (1) | |
| Nervous system disorders (11) | Dysarthria (1) | |
| Headache (1) | ||
| Lethargy (2) | ||
| Somnolence (7) | ||
| Psychiatric disorders (8) | Aggression (1) | |
| Agitation (6) | ||
| Restlessness (1) | ||
| Renal and urinary disorders (2) | Incontinence (1) | |
| Renal impairment (1) | (1) | |
| Respiratory, thoracic and mediastinal disorders (5) | Chronic obstructive pulmonary disease (1) | (1) |
| Dyspnoea (3) | ||
| Rhinorrhoea (1) | ||
| Skin and subcutaneous tissue disorders (1) | Skin ulcer (1) | |
| Surgical and medical procedures (2) | Hospitalisation (2) | (1) |
Appendix 4 Unit costs
| Item | Unit cost (£, 2016–17) | Unit | Source | Notes/assumptions |
|---|---|---|---|---|
| Living and accommodation expenses | ||||
| Private sector residential care for older people | 94 | Day | Unit Costs of Health and Social Care 2017, table 1.254 | Includes personal living expenses |
| LA residential care for older people | 162 | Day | Unit Costs of Health and Social Care 2017, table 1.354 | Includes personal living expenses |
| Private sector nursing home for older people | 119 | Day | Unit Costs of Health and Social Care 2017, table 1.154 | Includes personal living expenses |
| Domestic accommodation | 239.7 | Week | ONS household expenditure: table A17 | Living Costs and Food Survey data. All retired households. Includes: food and non-alcoholic drinks, alcoholic drinks, tobacco and narcotics, clothing and footwear, housing (net), fuel and power, household goods and services, health, transport, communication, recreation and culture, education, restaurants and hotels, miscellaneous goods and services. Housing spend is net of mortgage interest payments and council tax |
| Sheltered accommodation | 302.0 | Week | Unit Costs of Health and Social Care 2017, table 1.6 | Cost of extra care housing. Includes housing management and support, accommodation and living expenses |
| Community health and social care services | ||||
| GP time, home visit | 88 | Visit | Unit Costs of Health and Social Care 2017, table 10.3b; Unit Costs of Health and Social Care 2013, table 10.3b54 | Ratio of clinic to home cost minute and average duration of visit in 2013 UC table 10.3b. Assumes average home visit duration of 23.4 minutes |
| GP time, surgery | 28 | Visit | Unit Costs of Health and Social Care 2017, table 10.3b54 | No direct care staff and no qualification costs, surgery consultation of 9.22 minutes |
| Practice nurse, face-to-face time | 9.30 | Consultation | Unit Costs of Health and Social Care 2017, table 10.254 | 15.5-minute consultation. Excludes qualification costs |
| Community nursing time | 0.73 | Minute | Unit Costs of Health and Social Care 2017, table 10.154 | Assumes AfC band 6 |
| Community nursing time | 37 | Contact | NHS Reference Costs 2016/1755 | CHS tab |
| Specialist nursing | Range: 65–89 |
Contact | NHS Reference Costs 2016/1755 | CHS tab |
| Nurse (mental health) time | 44 | Contact | Unit Costs of Health and Social Care 2017, table 12.1 | Average visit of 60 minutes in community mental health teams for older people |
| Consultant: Psychiatrist time | 1.8 | Minute | Unit Costs of Health and Social Care 2017, table 15 | Excludes qualification costs |
| Consultant: Neurologist time | 1.8 | Minute | Unit Costs of Health and Social Care 2017, table 15 | Cost of medical consultant. Excludes qualification costs |
| Consultant: Geriatrician time | 1.8 | Minute | Unit Costs of Health and Social Care 2017, table 15 | Cost of medical consultant. Excludes qualification costs |
| Social worker, face-to-face time | 59 | Visit | Unit Costs of Health and Social Care 2017, table 11.254 | Excludes qualification costs. One hour of client-related work |
| Social worker, face-to-face time | 0.98 | Minute | Unit Costs of Health and Social Care 2017, table 11.254 | Excludes qualification costs. One hour of client-related work |
| Physiotherapist | 53 | Contact | NHS Reference Costs 2016/1755 | CHS tab |
| NHS occupational therapist | 76.73 | Contact | NHS Reference Costs 2016/1755 | CHS tab |
| NHS community mental health team (CMHT) worker for older people (OP) with mental health problems, team member | 44 | Contact | Unit Costs of Health and Social Care 2017, table 12.1 | Average visit of 60 minutes in community mental health teams for older people |
| Counselling services in primary care | 0.87 | Minute | Unit Costs of Health and Social Care 2014, table 2.7 | |
| Counselling services in primary care | 47.70 | Consultation | Unit Costs of Health and Social Care 2014, table 2.7 | 55-minute visit |
| Home care – average of independent and social services | 0.44 | Minute | Unit Costs of Health and Social Care 2017, table 11.654 | Face-to-face time: average cost of private and Social Services costs; weighted average of weekday and weekend costs |
| Home care – average of independent and social services | 13.21 | Contact | Unit Costs of Health and Social Care 2017, table 11.654 | Face-to-face time: average cost of private and Social Services costs; weighted average of weekday and weekend costs. Assumes 30-minute visit |
| Cleaner | £20 | Visit | Commercial websites | Internet search. Assumes 2-hour visit |
| Meals on Wheels | 6 | Meal | Unit Costs of Health and Social Care 2014, table 8.1.185 | Uprated with HCHS Pay & Prices Index54 |
| Sitting service i.e. Crossroads Carer support worker |
45 | Visit | Banks and Barnes86 | Short break for carers, 2.5 hours. Uprated with HCHS Pay & Prices Index |
| Day care for older people | 63 | Session | Unit Costs of Health and Social Care 2017, table 1.454 | |
| Day care in NHS facilities | 132.23 | Attendance | NHS Reference Costs 2016/1755 | CHS tab |
| Day care for people with mental health problems | 34 | Session | Unit Costs of Health and Social Care 2017, table 2.454 | |
| Lunch club | 8 | Session | Romeo et al.87 | Uprated with HCHS Pay & Prices Index |
| Alternative therapies: Osteopath, Yoga, Tai Chi, Naturopath |
Range: 8.2–62.3 |
Session | Hartfiel, Clarke et al.,88 General Osteopathic Council,89 commercial websites | Internet search. Items that were sourced from internet searches were deflated using the Consumer Price Index90 |
| Education group | 9.0 | Session | Dementia Self-Management Programme91 | |
| Support/expert relative groups | 3.1 | Session | Community memory café run by voluntary sector92 | Uprated with HCHS Pay & Prices Index |
| Equipment and adaptations | ||||
Various:
|
Range: 1.10–614.00 |
Item, per annum | Unit Costs of Health and Social Care 2017, table 7.254 PSSRU unit costs 2013, table 7.3.1; commercial websites |
Unit Costs Compendium and internet search Annuitised over 5 years (electronic items) or 10 years (non-electronic items) Cost of the item was calculated for the relevant retrospective period (12 weeks at baseline, 6 weeks at 6 and 12-week follow-up); items that were sourced from internet searches were deflated using the Consumer Price Index90 |
| Medications | ||||
| Various | Range: 0.01–225.72 |
Standard quantity units | Prescription cost analysis, England53 | |
| Unpaid carer costs | ||||
| National average wage – value of lost work time | 16.20 | Hour | Annual survey of hours and earnings tables58 | Gross mean wage for all employee jobs, 2017 |
| National average wage – value of lost leisure time | 5.67 | Hour | Annual survey of hours and earnings tables58 | 35% of gross mean wage for all employee jobs, 2017 |
| Hospital services | ||||
| A&E attendances, weighted average of admitted and non-admitted attendances | 148.36 | Attendance | NHS Reference Costs 2016/1755 | EM tab |
| Inpatients | ||||
| Subchapter AA: Nervous System Procedures and Disorders | 477.75 295.42 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter CB: Ear, Nose, Mouth, Throat and Neck Disorders | 521.46 295.1 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter DZ: Respiratory System Procedures and Disorders | 402.23 271.11 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter EB: Cardiac Disorders | 452.02 291.01 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter EY: Interventional Cardiology for Acquired Conditions | 820.12 383.32 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter FD: Digestive System Disorders | 452.79 294.18 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter HE: | 436.19 276.32 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter HN: Orthopaedic Non-Trauma Procedures | 731.69 325.66 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter JD: | 433.25 280.84 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter LA: Renal Procedures and Disorders | 415.24 272.35 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter LB: Urological and Male Reproductive System Procedures and Disorders | 505.19 304.76 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter SA: Haematological Procedures and Disorders | 549.53 349.89 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter VC: Rehabilitation | 362.06 | Day | NHS Reference Costs 2016/1755 | NEL tab |
| Subchapter WD: Treatment of Mental Health Patients by Non-Mental Health Service Providers | 356.25 264.04 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter WH: Poisoning, Toxic Effects, Special Examinations, Screening and Other Healthcare Contacts | 440.99 274.22 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Subchapter WJ: | 439.00 287.38 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Inpatients, weighted average across specialities | 645 299 |
Day Excess day |
NHS Reference Costs 2016/1755 | NEL tab NEL_XS tab |
| Mental health inpatient stay | 451.73 | Day | NHS Reference Costs 2016/1755 | MHCC tab, weighted average for clusters 18–21, cognitive impairment |
| Day cases | ||||
| Subchapter BZ: Eyes and Periorbita Procedures and Disorders | 825.04 | Day | NHS Reference Costs 2016/1755 | DC tab |
| Subchapter DZ: Respiratory System Procedures and Disorders | 606.65 | Day | NHS Reference Costs 2016/1755 | DC tab |
| Subchapter FD: | 313.72 | Day | NHS Reference Costs 2016/1755 | DC tab |
| Subchapter HE: | 553.28 | Day | NHS Reference Costs 2016/1755 | DC tab |
| Outpatients | ||||
| Service code 101: Urology | 102.88 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 103: Breast Surgery |
130.51 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 107: Vascular Surgery | 141.35 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 110: Trauma and Orthopaedics | 109.78 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 120: ENT | 87.94 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 130: Ophthalmology | 82.93 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 141: Restorative Dentistry |
123.26 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 301: Gastroenterology | 138.29 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 304: Clinical Physiology | 72.41 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 307: Diabetic Medicine | 141.00 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 320: Cardiology | 117.34 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 324: Anticoagulant Service | 30.04 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 330: Dermatology | 98.36 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 340: Respiratory Medicine | 144.26 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 361: Nephrology | 148.53 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 370: Medical Oncology | 163.93 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 400: Neurology | 149.30 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 430: Geriatric Medicine | 194.56 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 653: Podiatry | 41.87 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 715: Old Age Psychiatry | 179.66 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 800: Clinical Oncology (Previously Radiotherapy) | 126.39 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 812: Diagnostic Imaging | 80.65 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 840: Audiology | 87.04 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Memory clinic | 406.45 | Follow-up att. | Unit Costs of Health and Social Care 2014, table 1.1085 | Uprated using HCHS Pay & Prices Index54 |
| Weighted average of follow-up attendances across service codes | 105.52 | Follow-up att. | NHS Reference Costs 2016/1755 | Consultant and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
List of abbreviations
- AD
- Alzheimer’s disease
- AE
- adverse event
- BPSD
- Behavioural and Psychological Symptoms in Dementia
- CI
- confidence interval
- CMAI
- Cohen-Mansfield Agitation Inventory
- CRF
- Case Report Form
- CSRI
- Client Service Receipt Inventory
- CTCAE
- Common Terminology Criteria for Adverse Events
- DEMQOL
- Dementia-Specific Quality of Life
- DMC
- Data Monitoring Committee
- ECG
- electrocardiogram
- eCRF
- Electronic Case Report Form
- EU
- European Union
- GCP
- Good Clinical Practice
- GDPR
- General Data Protection Regulation
- GHQ-12
- General Health Questionnaire
- ICH
- International Conference on Harmonisation
- IMP
- Investigational Medicinal Product
- LEAP
- Lived Experience Advisory Panel
- MHRA
- Medicines and Healthcare Products Regulatory Agency
- MLM
- Multilevel models
- NASSA
- noradrenergic and specific serotonergic antidepressant
- NCTU
- Norwich Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- NPI
- Neuropsychiatric Inventory
- PI
- principal investigator
- PIC
- Participant Identification Centre
- PIN
- Participant Identification Number
- PPI
- patient and public involvement
- QALYs
- quality-adjusted life-years
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SAR
- serious adverse reaction
- SD
- standard deviation
- SDV
- Source Data Verification
- SmPC
- Summary of Product Characteristics
- SPFT
- Sussex Partnership Foundation Trust
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UEA
- University of East Anglia
