Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/143/01. The contractual start date was in April 2018. The draft report began editorial review in April 2022 and was accepted for publication in September 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Knight et al. This work was produced by Knight et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Knight et al.
Chapter 1 Introduction
The World Health Organisation recommends exclusive breastfeeding for at least 6 months because of its many health benefits. Breastmilk-fed infants are less likely to have a range of childhood infections, and protection from infection is greater with longer duration of breastfeeding. 1 Increasing breastfeeding rates is likely to have significant economic benefits based on protection from these infections alone. 2 Similarly, breastfeeding is associated with lower rates of childhood overweight and obesity, and hence a lower likelihood of diabetes as an adult. 3 Breastfeeding and breastfeeding duration is associated with better educational attainment. 4 Mothers who have breastfed have lower rates of both breast and ovarian cancer. 3 Significant sociodemographic inequalities in breastfeeding rates persist,5 and the latest figures suggest that only around half of babies are still receiving breastmilk at 6 months of age. 6 Interventions to support breastfeeding and breastfeeding duration are therefore important. Breastfeeding support interventions have been shown to be associated with continuation of breastfeeding beyond 10 days. 7
Breastfeeding difficulties have been associated with many factors, from a societal to an individual level. 8 Tongue-tie can be diagnosed in 3–11% of babies,9 with the variation in reported prevalence thought to relate to the use of different diagnostic or severity criteria. 10 Up to half of babies with tongue-tie are reported to have breastfeeding difficulties, but the reported proportion is highly variable. Some studies report almost universal difficulties, and others report very few feeding difficulties that relate to the tongue-tie itself, instead noting that incorrect positioning and attachment are the primary reasons behind the observed breastfeeding difficulties and not the tongue-tie itself. 9 In a UK survey,10 it was noted that management of tongue-tie in infants with breastfeeding difficulties was therefore highly variable across the country. This is coupled with highly variable provision of breastfeeding support,11 which can range from minimal to expert and intensive, and using a variety of different models including peer supporter, midwife and health visitor.
A Cochrane review12 identified five prior randomised controlled trials (RCTs) of frenotomy including a total of only 302 infants. The trials are small and underpowered and/or include only very short-term or subjective outcomes, suggesting further robust evidence is needed. Hence there is considerable controversy regarding, not only the diagnosis and clinical significance, but also the management of tongue-tie. Current National Institute for Health and Care Excellence (NICE) guidance13 allows for the procedure, based on lack of safety concerns, but notes very limited evidence of efficacy. In preparation for this study, we searched the literature to identify previous economic evaluations assessing the cost-effectiveness of frenotomy in a UK setting but no relevant studies were identified. There is therefore a clear need for an assessment of the clinical and cost-effectiveness of frenotomy for babies diagnosed with tongue-tie in the form of an adequately powered, pragmatic RCT, taking into account the diagnostic controversy and variation in practice.
Objective
The objective of this research was to investigate whether frenotomy is clinically and cost-effective to promote continuation of breastfeeding at 3 months in infants with breastfeeding difficulties diagnosed with tongue-tie.
Chapter 2 Methods
Design
The FROSTTIE trial was a multicentre, randomised, controlled parallel group trial conducted in England.
The trial was registered with the International Standard Randomised Controlled Trial Register (ISRCTN 10268851).
Patient and public involvement
The research question was initially prioritised by the NIHR Health Technology Assessment programme, including patient and public involvement (PPI). In order to obtain the perspective of a wide group of women with recent breastfeeding experience and/or experience of tongue-tie, we included a PPI co-applicant from the Breastfeeding Network, who consulted with other Network members, and also established a Public Advisory Group. These two groups helped design the study processes and materials, and advised throughout the trial.
Participants
Inclusion criteria
-
Any infant aged <10 weeks referred (by parent or other breastfeeding support service) to an infant feeding service with breastfeeding difficulties and judged to have tongue-tie, whose parent had given informed consent for participation.
Exclusion criteria
Infants were not eligible to enter the study if ANY of the following applied:
-
Infant was older than 10 weeks.
-
Infant had breastfeeding difficulties but was not judged to have tongue-tie.
-
Infant was born at <34 weeks’ gestation.
-
Infant had a congenital anomaly known to interfere with breastfeeding, for example cleft palate, Down syndrome.
-
Infant had a known bleeding diathesis.
-
Infant had a frenotomy prior to recruitment.
Setting
The trial was conducted in 12 infant feeding services in England (see Appendix 1).
Informed consent and recruitment
Information about the trial was made widely available throughout the infant feeding units in the form of posters and leaflets (with QR codes to the trial website). Written information about the trial was available to all women at participating centres when they attended for breastfeeding support. In some sites, materials were also available on the postnatal ward.
Potential participants were identified by the infant feeding service staff from the population of infants with breastfeeding difficulties referred to NHS infant feeding services through volunteer breastfeeding supporters, other breastfeeding counsellors, midwives or through self-referral by parents.
As standard, following referral to the infant feeding service, infant feeding was observed (either in person or via video conferencing) and tongue assessment conducted, and mothers received advice on positioning and attachment. Initial discussions may have taken place in person or virtually via telephone or video conferencing if this was what was being offered as part of routine care. The tongue-tie diagnosis was made according to usual hospital practice, which may have included using any suitable tool. However, all babies whose parents consented for their participation in the trial had an assessment of their tongue-tie made using the Bristol Tongue Assessment Tool (BTAT). 14
Following diagnosis of a tongue-tie associated with breastfeeding difficulties, a verbal explanation and written information (the Parent Information Leaflet) was provided to the parent(s) either as a hardcopy in person or via post, electronically via email or by being directed to the study website. The parent(s) were allowed as much time as they needed to consider the information, and the opportunity to question staff before deciding whether they consented for their baby to participate in the study. Written or remote verbal informed consent was obtained.
Written or verbal informed consent also included optional consent for linkage of their baby’s data to routine data sources to allow the potential for further follow-up beyond the funded trial.
Intervention
Infants were randomised via a web randomisation portal to either:
-
frenotomy with standard breastfeeding support (intervention arm), or
-
no frenotomy with standard breastfeeding support (comparator arm).
Breastfeeding support included as a minimum: an initial assessment of breastfeeding, for example using the LATCH (Latch, Audible swallowing, Type of nipple, Comfort, Hold) tool15 or Baby Friendly Initiative assessment tool, and advice on positioning and attachment and at least one follow-up visit, together with drop-in clinic advice as required, but available on more than 1 day a week. Assessments and breastfeeding support were provided face-to-face in person or virtually using video conferencing or telephone dependent on what was being offered as part of routine care.
Intervention arm: infants who were randomised to frenotomy with breastfeeding support underwent frenotomy according to usual hospital practice. Frenotomy was carried out by the usual trained practitioner for participating hospitals using their normal technique. The babies had an immediate postfrenotomy observed feed. Parents received further advice on positioning and attachment together with standard postfrenotomy advice concerning bleeding and other postfrenotomy adverse events. Parents were provided with details about how to access rapid breastfeeding support in the event of ongoing feeding difficulties and an appointment for at least one follow-up visit.
Comparator arm: infants randomised to breastfeeding support only did not undergo frenotomy, but received further advice on positioning and attachment. Parents were provided with details about how to access rapid breastfeeding support in the event of ongoing feeding difficulties and an appointment for at least one follow-up visit.
Randomisation and blinding
The infants entered into the trial were randomised 1 : 1 to either intervention or comparator arm. Multiples (twins or higher-order multiples) were randomised to the same arm. Stratified block randomisation (using variable block sizes) was performed via a secure 24-hour web-based randomisation system [hosted by the National Perinatal Epidemiology Unit (NPEU) Clinical Trials Unit (CTU), University of Oxford] stratified by infant’s age (<2 and ≥2 weeks) at randomisation and mother’s parity (primiparous or multiparous) within the centre. A telephone back-up system was available 24 hours a day (365 days per year).
A statistician independent of the trial generated the stratified block randomisation (using variable block sizes) schedule and the Senior Trials Programmer wrote the web-based randomisation program; both were independently validated. The implementation of the randomisation procedure was monitored by the Senior Trials Programmer and independent statistician throughout the trial and reports provided to the Data Monitoring Committee (DMC).
Parents were blinded to the allocation for the first 20 participants, following which the trial was conducted unblinded. Blinding consisted of parents being asked not to directly observe the procedure (tongue-tie examination with or without frenotomy) immediately before a postprocedure breastfeed. The request for a change to an unblinded design was made by the funder as it was felt to be a barrier to recruitment.
Internal pilot
We conducted an internal pilot during the first 6 months of the trial, when 266 recruits were predicted, to test recruitment and retention assumptions. The pre-defined stop–go criteria were as follows:
-
recruitment 75% or more (N ≥ 199) – continue directly with the main trial
-
recruitment 50–75% (133 ≤ N < 199) – recruit more centres and review in 6 months
-
recruitment < 50% (N < 133) – undertake an urgent detailed review of options with Trial Steering Committee (TSC) to subsequently recommend to the funder.
The pilot was restarted in September 2019, following removal of blinding, but was never completed due to a pause in recruitment between March and May 2020 because of the COVID-19 pandemic, and subsequent closure of the trial by the funder.
Outcomes
Primary outcome
Any breastmilk feeding in the 24 hours prior to the infant reaching 3 months of age. 16,17 A positive response was considered indicative of continuation of breastfeeding.
Secondary outcomes
The secondary outcomes were assessed at 3 months of age and some additionally at first follow-up visit (indicated by *).
-
Mother’s breastfeeding self-efficacy*: measured using the Breastfeeding Self-Efficacy Scale – Short Form
-
Mother’s pain while feeding during the previous 24 hours: measured using visual analogue scale of the Short Form McGill Pain Questionnaire (SF-MPQ), modified into a Likert-type scale
-
Amount of breastfeeding support used: measured by total number of contacts (whether face-to-face or virtual) with any breastfeeding supporter since the FROSTTIE procedure
-
Infant weight gain: measured as difference in weight for age z-scores between birth and 3 months of age
-
Infant postrandomisation weight gain: measured as difference in weight for age z-scores between baseline and 3 months of age
-
Exclusive breastmilk feeding*: exclusive breastmilk feeding in the previous 24 hours
-
Exclusive direct breastfeeding*: exclusive breastfeeding directly from the breast with no bottle feeds of expressed milk in the previous 24 hours
-
Age of child when s/he last received breastmilk: age when child last received breastmilk, to determine when and whether switch to exclusive formula feeding has occurred
-
Time spent breastfeeding in previous 24 hours: time in minutes/hours spent breastfeeding in previous 24 hours
-
Frenotomy in comparator group*/repeat frenotomy*/bleeding following frenotomy or frenulum tear*/post-procedure adverse events (tongue cut*, salivary duct damage*)/maternal and infant NHS health-care resource use): measured by specific questions
-
Maternal anxiety and depression: dimension of EuroQol-5 Dimensions, five-level version*
-
Maternal health-related quality of life: as elicited by the EQ-5D-5L*
-
Any breastmilk feeding at 6 months: according to maternal self-report: defined as any breastmilk feeding in the 24 hours prior to the infant reaching 6 months of age.
Process outcomes
The process outcomes for all infants included BTAT score by adherence status, reasons for non-adherence, type of breastfeeding support. For infants who had undergone frenotomy additional process outcomes were assessed – role of person performing the procedure, whether anaesthetic was used, whether frenotomy was performed using bipolar diathermy, scissors, or other, frenotomy technique, and whether the infant was able to breastfeed after the procedure.
Sample size
It was assumed that a 10% absolute increase in the rate of breastfeeding represented the minimal clinically important difference that should be detectable by the trial; and breastfeeding rates will remain high in this motivated population. Thus, assuming a breastfeeding rate of 70% in the control group and 80% in the intervention group, at 90% power with a 5% level of significance, and allowing for 5% loss to follow-up, with a further 5% increase to account for between-group contamination required a sample size of 870. Given the final sample size achieved with complete primary outcome data (n = 163), the study had 31% power to detect this difference, assuming the same control group rate.
Statistical analyses
Statistical analyses were carried out according to a pre-specified Statistical Analysis Plan finalised prior to unblinding. For the primary analysis for all primary and secondary outcomes infants, we compared the outcomes of all infants allocated to frenotomy with breastfeeding support with all those allocated to breastfeeding support, regardless of deviation from the protocol or treatment received (referred to as the ITT population). Demographic and clinical data were summarised with counts and percentages for categorical variables, means [standard deviations (SDs)] and medians [with interquartile range (IQR) or simple range] for continuous variables. For binary outcomes, risk ratios (RRs) and confidence intervals (CIs) were calculated using log binomial regression or Poisson regression with a robust variance estimator. Continuous outcomes were analysed using linear or median (quantile) regression for normally distributed and skewed variables, respectively. Analyses were adjusted for stratification factors at randomisation where possible (centre, infant’s age at randomisation and mother’s parity). 18 Both unadjusted and adjusted risk ratios (aRRs) are presented, with the primary inference based on the adjusted estimates. Two-sided statistical testing was performed throughout. A 5% level of statistical significance was used, and 95% CIs are presented.
Secondary analyses
Four planned secondary analyses were carried out:
-
A comparison of the characteristics and primary outcome by adherence status in the breastfeeding support arm.
-
An assessment of the impact of non-adherence to the randomised allocation using complier-average causal effect (CACE) analysis. The CACE analysis assumes that the proportion of would-be non-compliers in the frenotomy with breastfeeding support arm (i.e. women in this group who would not have complied had they been randomised to breastfeeding support alone) is the same as the proportion of non-compliers in the breastfeeding support arm. It also assumes that the event rate among the non-compliers in the breastfeeding support arm is the same as the event rate among the would-be non-compliers in the frenotomy with breastfeeding support arm. Applying these two assumptions, the event rate for the primary outcome was calculated for the would-be compliers and would-be non-compliers in the frenotomy with breastfeeding support arm. The unadjusted CACE RR and 95% CI for the primary outcome was calculated using the event rates for compliant groups only (i.e. the observed compliers in the breastfeeding support arm and the would-be compliers in the frenotomy arm). The CI for the CACE estimated RR was estimated using the bootstrapping method.
-
Exploratory analysis: a restricted per protocol analysis, excluding participants in the frenotomy with breastfeeding support arm who had no frenotomy performed and participants in the breastfeeding support group who had a frenotomy performed.
-
Exploratory analysis: an as-treated analysis, grouping participants according to the allocation they received (participants in the breastfeeding only group who had a frenotomy performed and received breastfeeding support included in the frenotomy with breastfeeding support arm and participants in the frenotomy with breastfeeding support arm who had no frenotomy performed but received breastfeeding support included in the breastfeeding support group).
Pre-specified subgroup analyses
Four planned subgroup analyses were carried out, examining the primary outcome in the following groups:
-
infants aged <2 weeks versus ≥2 weeks at randomisation
-
infants with BTAT score 4 or less versus 5–6 versus 7 or more at randomisation
-
prior belief concerning frenotomy: likely to be beneficial versus uncertain versus unlikely
-
recruited pre- or posttrial pause during the COVID-19 pandemic.
The primary outcome is presented within these subgroups using descriptive statistics only due to the small achieved sample size.
Data collection
Baseline information was collected on sociodemographic and other characteristics at trial entry, including the following:
-
infant birthweight
-
infant current weight
-
estimated date of delivery
-
current feeding practices (e.g. expressed breastfeeding, use of infant formula)
-
assessment of the degree of tongue-tie using the BTAT
-
mother’s prior beliefs about frenotomy: using a 3-point Likert scale, the opinions of mothers of infants recruited to the trial were measured at the time of recruitment on their prior belief of the potential benefit of frenotomy
-
EQ-5D-5L
-
mother’s pain while feeding during the previous 24 hours: measured using visual analogue scale of the SF-MPQ, modified into a Likert-type scale (scores ranging from 0 to 10)16
-
exclusive breastmilk feeding: exclusive breastmilk feeding in the previous 24 hours
-
exclusive direct breastfeeding: exclusive breastfeeding directly from the breast with no bottle feeds of expressed milk in the previous 24 hours
-
pre-trial entry breastfeeding support received.
The following data were collected from the clinician performing the frenotomy on the day of the procedure:
-
in-person BTAT assessment if the baseline assessment was done virtually that is not face-to-face
-
intervention undertaken according to randomisation schedule and technique used
-
bleeding following frenotomy or frenulum tear
-
postprocedure adverse events (tongue cut, salivary duct damage).
The following data were collected from the mother at the routine follow-up visit (approximately 1 to 2 weeks posttrial entry):
-
mother’s pain while feeding during the previous 24 hours: measured using visual analogue scale of the SF-MPQ, modified into a Likert-type scale (scores ranging from 0 to 10)16
-
exclusive breastmilk feeding in the previous 24 hours
-
exclusive breastfeeding directly from the breast with no bottle feeds of expressed milk in the previous 24 hours
-
type of breastfeeding support received (in person or virtual)
-
frenotomy/repeat frenotomy (defined as any further procedure on tongue-tie)
-
bleeding following frenotomy or frenulum tear
-
postprocedure adverse events (tongue cut, salivary duct damage): measured by specific questions
-
maternal anxiety or depression as indicated by the anxiety and depression dimension of EQ-5D-5L
-
maternal health-related quality of life (HRQoL): as elicited by the EQ-5D-5L.
Data on the primary outcome (any breastmilk feeding in the previous 24 hours at age 3 months) were collected from mothers by automated text message. The following data were collected using maternal self-report via a follow-up link (by smart phone, tablet, computer, postal questionnaire or telephone) when the infant was 3 months of age:
-
mother’s breastfeeding self-efficacy: measured using the Breastfeeding Self-Efficacy Scale – Short Form19
-
mother’s pain while feeding during the previous 24 hours: measured using visual analogue scale of the SF-MPQ, modified into a Likert-type scale (scores ranging from 0 to 10)16
-
total number of contacts with any breastfeeding supporter since first referral and specific means of support used (in person or virtual)
-
infant weight
-
exclusive breastmilk feeding in the previous 24 hours
-
exclusive breastfeeding directly from the breast with no bottle feeds of expressed milk in the previous 24 hours
-
age when child last received breastmilk, to determine when and whether switch to exclusive formula feeding had occurred20
-
time spent breastfeeding in previous 24 hours: time in minutes/hours
-
frenotomy/repeat frenotomy
-
bleeding following frenotomy or frenulum tear
-
postprocedure adverse events (tongue cut, salivary duct damage): measured by specific questions
-
mother or infant previously diagnosed with COVID-19
-
maternal anxiety or depression as indicated by the anxiety and depression dimension of EQ-5D-5L
-
maternal HRQoL: as elicited by the EQ-5D-5L
-
maternal and infant NHS health-care resource use: collected on general practice visits and hospital admissions.
Data on any breastmilk feeding at 6 months were collected from mothers by automated text message.
Adverse event reporting
Non-serious adverse events were not routinely recorded as the procedure is part of standard clinical practice. However adverse events that were part of the study outcomes [bleeding following frenotomy (unless excessive) or frenulum tear and postprocedure adverse events (tongue cut, salivary duct damage)] were collected as part of standard follow-up.
All serious adverse events (SAEs) were reported immediately, at least within 24 hours; except the following SAEs, which were foreseeable SAEs and were subject to SAE reporting procedures:
-
admission or extension of hospital stay due to the following:
-
breastfeeding difficulties
-
poor milk supply in the mother
-
weight loss or poor weight gain in the baby
-
jaundice.
-
Economic evaluation
A within-trial cost-consequences analysis (CCA) with a time horizon of a 3-month follow-up was conducted from a NHS perspective as the primary analysis for the economic evaluation. In this case, results were presented as benefits and health-care costs in disaggregated format for both mothers and their infants in each treatment arm. 21 Costs included frenotomy and breastfeeding support-related costs, primary care, community care, secondary care and non-NHS related costs. The benefits considered in the CCA were the primary outcome of any breastmilk feeding at 3 months according to maternal self-report, maternal anxiety and depression measured using the relevant EQ-5D-5L domain22 and HRQoL measured using the EQ-5D-5L at different time points.
In a secondary analysis, a within-trial cost-utility analysis (CUA) from the mother’s perspective with a time horizon up to 3 months was also conducted. Quality-adjusted life-years (QALYs), were used to measure benefits in the CUA with mean difference in costs and QALYs were synthesised using the incremental cost-effectiveness ratio (ICER) expressed as incremental costs per QALYs gained over the trial period. 23 The ICER was compared to the standard cost-effectiveness threshold as recommended by NICE to determine value for money. 24
NHS health-care resource use
Detailed information on health-care resource use was collected for women and their infants and included data on resource utilisation up to 3 months after birth. Frenotomy-related resource use was collected directly from the health-care professional undertaking the procedure. Infant and maternal health-care resource use was collected using questionnaires at the routine follow-up visit (approximately 1 to 2 weeks posttrial entry) and when the infant was three months of age. The latter one could be completed by smart phone, tablet, computer, postal questionnaire or telephone.
Health-care utilisation related to frenotomy intervention
Frenotomy-related resource use data were collected from the health-care professional performing the procedure on the day of the operation and included who performed the procedure and whether any complications occurred. The setting where the frenotomy was conducted (NHS setting or private provided) was facilitated by mothers at her routine follow-up visit or using the 3-month follow-up questionnaire.
Health-care utilisation related to breastfeeding support
Resource use data on breastfeeding support were collected using the maternal questionnaire when the infant was 3 months of age. Resource use data in this category included type of breastfeeding support service, whether it was delivered face-to-face or remotely and how many times the service was used. We also collected information on any out of pocket expenses incurred due to visits to any breastfeeding support service consultations.
Primary, community and secondary health-care utilisation
Primary and community health-care utilisation for both mothers and their infants were collected using the maternal questionnaire when the infant was 3 months of age. Primary care visits included general practitioner and practice nurse visits, medication prescribed to treat anxiety or depression, and antibiotic use (reason and number of courses received). Community care contacts included visits to community nurse or midwife contacts, infant health visitor contacts and community paediatrician visits. Secondary (hospital-based) care contacts included accident and emergency department visits, hospital outpatient clinic appointments and hospital overnight admissions. We also collected any other NHS contact to a health-care professional not captured by the previous categories and visits to non-NHS health-care professionals up to 3 months follow-up. The different items of resource use collected for each category are presented in Appendix 2, Table 22.
Unit cost data collection
Sources and associated estimates of unit costs for the different resource use categories are presented in Appendix 2, Table 22. Unit costs were extracted from national sources, including NHS Reference Costs 2019/2020 Version 2,25 Unit costs of Health and Social Care 202026 and the Electronic Drug Tariff March 2020. 27 Given the reason for the antibiotics prescribed, the generic name of the antibiotic for the medicine costs analysis was assumed based on the national guidelines. 28,29 Prescription cost analysis 2020/21 data were then used to determine the antibiotic’s most prescribed form and dosage. 30 Hospital admissions were costed using the weighted average of a non-elective short stay across relevant Health-care Resource Group codes for the reason for admission from the NHS Reference Costs. We assumed that breastfeeding services received outside a NHS setting did not incur any costs unless reported specifically by the mother as out of pocket expenses. The only other non-NHS health-care professional visits reported was a contact with an osteopath, which was costed individually from the best source available to the research team. 31 All costs were expressed in 2019/20 pounds sterling.
Health outcome measures
In the CCA, health outcome measures included the primary clinical effectiveness of any breastmilk feeding at 3 months according to maternal self-report as described above, maternal anxiety and depression as measured by the relevant EQ-5D-5L dimension, and HRQoL at different follow-up periods as measured by EQ-5D-5L index values.
The EQ-5D-5L is a multiattribute generic instrument for measuring HRQoL. EQ-5D-5L consists of a descriptive system to describe health state and a visual analogue scale to evaluate overall level of health. 22 In this study, only results from the descriptive system are reported. The instrument covers five dimensions: mobility, self-care, usual activities, pain/discomfort, anxiety/depression, and each dimension has associated five-levels ranging from no problems to unable/extreme problems. The EQ-5D-5L describes 3125 health state profiles that need to be converted into a single preference-based score (HRQoL) using a value set obtained from a representative sample of the general population to be used in economic evaluations. 22
At the time of conducting this economic evaluation, the recommended approach to estimate EQ-5D-5L preference-based scores (HRQoL) was to convert EQ-5D-5L responses onto EQ-5D-3L preference-based scores using a mapping algorithm. 32 This mapping was based on the recent exercise conducted by Hernández-Alava and colleagues to derive EQ-5D-3L utility values from the existing EQ-5D-5L data. 33 The EQ-5D-5L questionnaire was completed by mother at the trial entry, at the routine follow-up visit (approximately 1 to 2 weeks posttrial entry) and when the infant was 3 months of age.
In the CUA, the main measure of benefits was maternal QALYs [also expressed as quality-adjusted life-days (QALDs) to facilitate interpretation given short time horizon]. Maternal QALDs and QALYs were derived as the area under the curve for the health profile created connecting EQ-5D-5L values at trial entry, at the routine follow-up visit, and 3 months after birth. A straight line relationship was assumed between the maternal utility values at the different time points.
Statistical analysis
We summarised the information about frenotomy procedures in each group using frequencies and associated proportions. Mean and SD were used to present the different categories of NHS health-care resource use and costs in each trial arm. Resource use is presented across all participants in the study and only for those who consumed a particular health-care resource use category. A mean difference between trial arms adjusted for the centre, infant’s age at randomisation and parity with associated 95% CI was computed using a generalised linear model. We present resource use and costs separately for mothers and their infants, but main categories of costs [primary care, medicines, community care, secondary (hospital-based) care, other NHS health-care professionals’ contacts, other non-NHS health care] were also presented as a single value combining information from both. Mean (SD) for each group with associated adjusted mean difference using the same approach as for costs was calculated to present EQ-5D-5L scores at baseline (trial entry), routine follow-up (approximately 1 to 2 weeks posttrial entry), 3 months after birth and overall QALYs or QALDs. The distribution of responses across the EQ-5D-5L dimensions was presented as frequencies and proportions at baseline (trial entry), routine follow-up (approximately 1 to 2 weeks posttrial entry) and 3 months after birth. Pearson’s chi-squared test was used to examine differences in the distribution of EQ-5D-5L responses between the two groups.
For all categories of NHS resource use, costs, HRQoL and QALYs we report the number of participants with missing data. The summary of the cost-consequence analysis therefore used different sample sizes for each of the components presented. However, the within-trial CUA was presented using a complete case analysis where mothers have complete information on total costs and QALYs over the trial period. The ICER was expressed as the ratio of the mean difference in costs divided by the mean difference in QALYs between the two groups. The breastfeeding support only arm was used as the comparator in the ICER calculation. Uncertainty around the ICER was evaluated using 95% CIs from a non-parametric bootstrap approach using 1000 replicates. Bootstrap replicates of mean difference in costs and effects were presented in the cost-effectiveness plane (CEP). Current thresholds of willingness to pay for QALY gained of £20,000 was used to determine value for money. Cost-effectiveness acceptability curves were derived to evaluate whether frenotomy when compared with breastfeeding support was cost-effective at different thresholds of willingness to pay.
The statistical analysis was conducted in Stata/MP version 17.0 and Microsoft Excel.
Governance and monitoring
A monitoring plan for the trial, including responsibilities, was developed prior to the start of recruitment. In person monitoring of all sites was carried out to identify barriers and facilitators to recruitment and the findings of the visits summarised to guide ongoing actions to enhance recruitment.
The trial was supervised on a day to day basis by a Project Management Group. A TSC was convened including an independent chair, four other independent members, a PPI representative(s), the NPEU CTU Director and the Chief Investigator. A DMC independent of the applicants and of the TSC reviewed the progress of the trial annually and provided advice on the conduct of the trial to the TSC.
Summary of changes to the study protocol
Masking of parents was removed from the trial at the funder request after a short pilot period as it was felt to be a barrier to recruitment. Following the restart after the first wave of the COVID-19 pandemic, changes were made to allow virtual assessments and breastfeeding support, and virtual BTAT assessment. Verbal consent was permitted if written consent was not possible. The COVID-19 status of mother and baby was added to the data collection.
A summary of the other changes made to the original protocol is presented in Appendix 3.
Chapter 3 Results
Recruitment and retention
Between March 2019 and November 2020, 169 infants were randomised; 80 to the frenotomy with breastfeeding support arm and 89 to the breastfeeding support arm. There were no substantial differences in the response rates between the intervention arms during the follow-up period (see Figure 1). The trial was stopped in November 2020 in the context of the COVID-19 pandemic due to withdrawal of breastfeeding support services, slow recruitment and crossover between arms. In the frenotomy with breastfeeding support arm 74/80 infants (93%) received their allocated intervention, compared to 23/89 (26%) in the breastfeeding support arm.
FIGURE 1.
Flow of participants.

The number of participants recruited at each site varied from 1 to 85 (see Table 1).
| Total (n = 169) | Frenotomy w/ breastfeeding support (n = 80) | Breastfeeding support (n = 89) | |
|---|---|---|---|
| Gestational age at birth | |||
| 34+0 to 35+6 weeks, n (%) | 7 (4.1) | 3 (3.8) | 4 (4.5) |
| 36+0 to 37+6 weeks, n (%) | 15 (8.9) | 6 (7.5) | 9 (10.1) |
| 38+0 to 39+6 weeks, n (%) | 71 (42.0) | 35 (43.8) | 36 (40.5) |
| 40+0 to 42+6 weeks, n (%) | 76 (45.0) | 36 (45.0) | 40 (44.9) |
| Age at randomisation a | |||
| <2 weeks, n (%) | 64 (37.9) | 30 (37.5) | 34 (38.2) |
| ≥2 and <4 weeks, n (%) | 48 (28.4) | 24 (30.0) | 24 (27.0) |
| ≥4 and <10 weeks, n (%) | 57 (33.7) | 26 (32.5) | 31 (34.8) |
| ≥10 weeks, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Birthweight (g), mean (SD) | 3439.3 (561.3) | 3409.0 (563.6) | 3466.5 (561.1) |
| Birthweight z-score (adjusted for gestational age and sex at birth),b median (IQR) | −0.4 (−1.0 to 0.2) | −0.5 (−1.2 to 0.3) | −0.3 (−0.8 to 0.2) |
| Current weight (g, within last 7 days), mean (SD) | 3832.2 (857.7) | 3767.8 (841.0) | 3890.7 (873.4) |
| Missing, n | 3 | 1 | 2 |
| Mode of birth | |||
| Unassisted vaginal, n (%) | 87 (51.5) | 42 (52.5) | 45 (50.6) |
| Assisted vaginal, n (%) | 26 (15.4) | 14 (17.5) | 12 (13.5) |
| Vaginal breech, n (%) | 1 (0.6) | 1 (1.3) | 0 (0.0) |
| C-section before labour onset, n (%) | 22 (13.0) | 7 (8.8) | 15 (16.9) |
| C-section after labour onset, n (%) | 33 (19.5) | 16 (20.0) | 17 (19.1) |
| Sex | |||
| Male, n (%) | 100 (59.2) | 51 (63.8) | 49 (55.1) |
| Female, n (%) | 69 (40.8) | 29 (36.2) | 40 (44.9) |
| Missing, n (%) | 0 | 0 | 0 |
| Degree of tongue-tie (BTAT) | |||
| 0–4, n (%) | 55 (32.7) | 29 (36.3) | 26 (29.6) |
| 5–6, n (%) | 53 (31.6) | 21 (26.3) | 32 (36.4) |
| 7–8, n (%) | 60 (35.7) | 30 (37.5) | 30 (34.1) |
| Missing, n | 1 | 0 | 1 |
| Exclusive breastmilk feeding in the previous 24 hours c | |||
| Yes, n (%) | 111 (65.7) | 51 (63.8) | 60 (67.4) |
| No, n (%) | 58 (34.3) | 29 (36.2) | 29 (32.6) |
| Exclusive direct breastfeeding in the past 24 hours d | |||
| Yes, n (%) | 67 (39.6) | 30 (37.5) | 37 (41.6) |
| No, n (%) | 102 (60.4) | 50 (62.5) | 52 (58.4) |
| Use of infant formula | |||
| Yes, n (%) | 57 (33.7) | 28 (35.0) | 29 (32.6) |
| No, n (%) | 112 (66.3) | 52 (65.0) | 60 (67.4) |
| Phototherapy for jaundice | |||
| Yes, n (%) | 23 (13.6) | 10 (12.5) | 13 (14.6) |
| No, n (%) | 146 (86.4) | 70 (87.5) | 76 (85.4) |
| NICU admission | 19 (11.2) | 9 (11.3) | 10 (11.2) |
| 1–2 nights, n (%) | 8 (44.4) | 4 (50.0) | 4 (40.0) |
| 3–4 nights, n (%) | 3 (16.7) | 2 (25.0) | 1 (10.0) |
| >4 nights, n (%) | 7 (38.9) | 2 (25.0) | 5 (50.0) |
| Missing, n | 1 | 1 | 0 |
| Baby is one of a multiple pregnancy | |||
| Yes, n (%) | 1 (0.6) | 1 (1.2) | 0 (0.0) |
| No, n (%) | 168 (99.4) | 79 (98.8) | 89 (100.0) |
| Sibling enrolled in the study (in multiple pregnancies) | |||
| Yes, n (%) | 0 (0.0) | 0 (0.0) | Not applicable |
| No, n (%) | 1 (100.0) | 1 (100.0) | |
| Recruiting centre a | |||
| Cumberland Infirmary, n (%) | 1 (0.6) | 1 (1.2) | 0 (0.0) |
| George Eliot Hospital, n (%) | 2 (1.2) | 2 (2.5) | 0 (0.0) |
| Norfolk and Norwich University Hospital, n (%) | 85 (50.3) | 39 (48.8) | 46 (51.7) |
| Queen Alexandra Hospital, n (%) | 1 (0.6) | 0 (0.0) | 1 (1.1) |
| Royal Albert Edward Infirmary, n (%) | 5 (3.0) | 2 (2.5) | 3 (3.4) |
| Royal Berkshire Hospital, n (%) | 24 (14.2) | 12 (15.0) | 12 (13.5) |
| Royal Blackburn Hospital, n (%) | 13 (7.7) | 7 (8.8) | 6 (6.7) |
| Royal Cornwall Hospital, n (%) | 5 (3.0) | 2 (2.5) | 3 (3.4) |
| Royal United Hospital, Bath, n (%) | 1 (0.6) | 0 (0.0) | 1 (1.1) |
| Royal Victoria Infirmary, n (%) | 26 (15.4) | 12 (15.0) | 14 (15.7) |
| Stoke Mandeville Hospital, Aylesbury, n (%) | 1 (0.6) | 1 (1.3) | 0 (0.0) |
| Sunderland Royal Hospital, n (%) | 5 (3.0) | 2 (2.5) | 3 (3.4) |
Characteristics of participants
Characteristics of participants were similar between the two trial arms. Infants had a mean age of 3 weeks, a mean birthweight of 3439 g and 87% were born at ≥38 weeks’ gestation. Overall 33% of infants had a BTAT score of 4 or less, 66% had exclusive breastmilk feeding in the previous 24 hours, and 40% had exclusive direct breastmilk feeding. Thirty-four per cent of infants had also received formula milk in the previous 24 hours (see Table 1). Mothers were a mean of 32 years old, 94% were of white ethnicity and 48% had a previous live birth. Only 8% were resident in the most deprived quintile of areas. Mothers reported a mean pain score of 4 out of 10 while feeding during the previous 24 hours and 42% had some anxiety or depression. More than half of women recruited to the trial believed a frenotomy would help their baby (see Table 2). Only one infant, in the breastfeeding support group, was reported to have had COVID-19.
| Total n = 169) | Frenotomy w/ breastfeeding support (n = 80) | Breastfeeding support (n = 89) | |
|---|---|---|---|
| Mother’s age (years), mean (SD) | 32.3 (5.0) | 32.7 (4.8) | 31.9 (5.1) |
| Missing, n | 6 | 3 | 3 |
| Mother’s ethnic group | |||
| White, n (%) | 156 (94.0) | 72 (92.3) | 84 (95.5) |
| Asian, n (%) | 7 (4.2) | 5 (6.4) | 2 (2.3) |
| Black, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mixed, n (%) | 3 (1.8) | 1 (1.3) | 2 (2.3) |
| Other, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing, n | 3 | 2 | 1 |
| Index of multiple deprivation of area of residence | |||
| 1 (most deprived), n (%) | 13 (7.7) | 3 (3.8) | 10 (11.2) |
| 2, n (%) | 40 (23.7) | 22 (27.5) | 18 (20.2) |
| 3, n (%) | 38 (22.5) | 21 (26.3) | 17 (19.1) |
| 4, n (%) | 40 (23.7) | 15 (18.8) | 25 (28.1) |
| 5 (least deprived), n (%) | 38 (22.5) | 19 (23.8) | 19 (21.4) |
| Previous live birth(s) a | |||
| Yes, n (%) | 81 (47.9) | 39 (48.7) | 42 (47.2) |
| No, n (%) | 88 (52.1) | 41 (51.3) | 47 (52.8) |
| Breastfed before | |||
| Yes, n (%) | 73 (92.4) | 33 (89.2) | 40 (95.2) |
| No, n (%) | 6 (7.6) | 4 (10.8) | 2 (4.8) |
| Not applicable – no previous live birth, n | 88 | 41 | 47 |
| Missing, n | 2 | 2 | 0 |
| Pre-trial breastfeeding support received | |||
| Yes, n (%) | 140 (84.3) | 66 (84.6) | 74 (84.1) |
| No, n (%) | 26 (15.7) | 12 (15.4) | 14 (15.9) |
| Missing, n | 3 | 2 | 1 |
| Mother’s pain while feeding during previous 24 hours,b median (IQR) | 4 (2–7) | 4 (1–7) | 4 (2–7) |
| Missing, n | 3 | 2 | 1 |
| Mother’s prior beliefs about frenotomy | |||
| Think it will help my baby, n (%) | 86 (51.8) | 41 (52.6) | 45 (51.1) |
| Do not know if it will help my baby, n (%) | 79 (47.6) | 37 (47.4) | 42 (47.7) |
| Think it is unlikely to help my baby, n (%) | 1 (0.6) | 0 (0.0) | 1 (1.1) |
| Missing, n | 3 | 2 | 1 |
| Maternal anxiety or depression c | |||
| Yes, n (%) | 69 (41.8) | 29 (37.2) | 40 (46.0) |
| No, n (%) | 96 (58.2) | 49 (62.8) | 47 (54.0) |
| Missing, n | 4 | 2 | 2 |
| Maternal HRQoL,d mean (SD) | 0.77 (0.17) | 0.77 (0.18) | 0.77 (0.16) |
| Missing, n | 8 | 4 | 4 |
| Recruited pre- or posttrial pausee during the COVID-19 pandemic | |||
| Pre-pause, n (%) | 107 (63.3) | 52 (65.0) | 55 (61.8) |
| Postpause, n (%) | 62 (36.7) | 28 (35.0) | 34 (38.2) |
Outcomes
Primary outcomes
Primary outcome data were available for 163/169 infants (96%). There was no evidence of a difference between the arms in the rate of breastmilk feeding at 3 months, which was high in both groups (67/76, 88% vs. 75/87, 86%; aRR 1.02, 95% CI 0.90 to 1.16) (see Table 3).
| Total (n = 169) | Frenotomy w/ breastfeeding Support (n = 80) | Breastfeeding support (n = 89) | Unadjusted risk ratio (95% CI) | Adjusteda risk ratio (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Any breastmilk feeding b | ||||||
| Yes, n (%) | 142 (87.1) | 67 (88.2) | 75 (86.2) | 1.02 (0.91 to 1.15) | 1.02 (0.90 to 1.16) | 0.73 |
| No, n (%) | 21 (12.9) | 9 (11.8) | 12 (13.8) | |||
| Missing, n | 6 | 4 | 2 | |||
Secondary outcomes
There was no evidence of differences between the intervention arms for any secondary outcomes (see Tables 4 and 5). Sixty-three infants in the breastfeeding support only arm had undergone frenotomy by their first follow-up visit. An additional two infants underwent frenotomy by the third month of follow-up. None of the infants had a repeat frenotomy. Adverse events were reported for three infants postsurgery (one infant had bleeding, one infant had salivary duct damage, and the third infant had accidental cut to the tongue and salivary duct damage) (see Tables 4–6). No other causally related SAEs were reported.
| Total (n = 169) | Frenotomy w/ breastfeeding support (n = 80) | Breastfeeding support (n = 89) | Unadjusted effect estimate (95% CI) | Adjusteda effect estimate (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Exclusive breastmilk feeding in the previous 24 hours b | ||||||
| Yes, n (%) | 117(70.9) | 51 (65.4) | 66 (75.9) | 0.86 (0.60 to 1.24) | 0.86 (0.59 to 1.24) | 0.42 |
| No, n (%) | 48 (29.1) | 27 (34.6) | 21 (24.1) | |||
| Missing, n | 4 | 2 | 2 | |||
| Exclusive direct breastfeeding in the past 24 hours c | ||||||
| Yes, n (%) | 78 (47.3) | 35 (44.9) | 43 (49.4) | 0.91 (0.58 to 1.42) | 0.92 (0.59 to 1.45) | 0.73 |
| No, n (%) | 87 (52.7) | 43 (55.1) | 44 (50.6) | |||
| Missing, n | 4 | 2 | 2 | |||
| Mother’s pain while feeding during previous 24 hours,d median (IQR) | 2 (0–4) | 2 (0–4) | 2 (0–4) | 0.0 (−0.9 to 0.9) | 0.0 (−0.9 to 0.9) | 0.99 |
| Not currently breastfeeding, n | 3 | 2 | 1 | |||
| Missing, n | 4 | 2 | 2 | |||
| Maternal anxiety or depression e | ||||||
| Yes, n (%) | 52 (31.7) | 23 (29.9) | 29 (33.3) | 0.90 (0.52 to 1.55) | 0.92 (0.53 to 1.63) | 0.79 |
| No, n (%) | 112 (68.3) | 54 (70.1) | 58 (66.7) | |||
| Missing, n | 5 | 3 | 2 | |||
| Frenotomy performed | - | - | - | |||
| Yes, n (%) | 138 (81.7) | 75 (93.8) | 63 (70.8) | - | - | - |
| No, n (%) | 31 (18.3) | 5 (6.2) | 26 (29.2) | - | - | - |
| Missing, n | 0 | 0 | 0 | - | - | - |
| Total (n = 169) | Frenotomy w/ breastfeeding support (n = 80) | Breastfeeding support (n = 89) | Unadjusted effect estimate (95% CI) | Adjusteda effect estimate (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Mother’s breastfeeding self-efficacy,b median (IQR) | 58 (47–65) | 60 (47–65) | 56.5 (47–65) | 4 (−1.8 to 9.8) | 0.3 (−5.2 to 5.8) | 0.92 |
| (Min to max) | (14, 70) | (14, 70) | (14, 70) | |||
| Missing, n | 26 | 11 | 15 | |||
| Exclusive breastmilk feeding in the previous 24 hours c | ||||||
| Yes, n (%) | 95 (65.1) | 45 (63.4) | 50 (66.7) | 0.95 (0.64 to1.42) | 0.92 (0.61 to 1.39) | 0.69 |
| No, n (%) | 51 (34.9) | 26 (36.6) | 25 (33.3) | |||
| Missing, n | 23 | 9 | 14 | |||
| Exclusive direct breastfeeding in the past 24 hours d | ||||||
| Yes, n (%) | 77 (53.1) | 38 (53.5) | 39 (52.7) | 1.02 (0.65 to 1.59) | 1.03 (0.65 to 1.62) | 0.90 |
| No, n (%) | 68 (46.9) | 33 (46.5) | 35 (47.3) | |||
| Missing, n | 24 | 9 | 15 | |||
| Mother’s pain while feeding during previous 24 hours,e median (IQR) | 0 (0–1.5) | 0 (0–1) | 0 (0–2) | 0 (−0.5 to 0.5) | −0.2 (−0.6 to 0.3) | 0.45 |
| Missing, n | 24 | 10 | 14 | |||
| Not currently breastfeeding, n | 21 | 9 | 12 | |||
| Amount of breastfeeding support used (number of contacts),f median (IQR) | 3 (1–4) | 3 (2–5) | 2 (1–4) | 1 (0.1 to 1.9) | −0.3 (−1.5 to 1.0) | 0.68 |
| Missing, n | 22 | 8 | 14 | |||
| Not applicable, ng | 36 | 22 | 14 | |||
| Maternal anxiety or depression h | ||||||
| Yes, n (%) | 55 (37.2) | 29 (39.7) | 26 (34.7) | 1.15 (0.67 to 1.95) | 1.12 (0.65 to 1.93) | 0.69 |
| No, n (%) | 93 (62.8) | 44 (60.3) | 49 (65.3) | |||
| Missing, n | 21 | 7 | 14 | |||
| Maternal HRQoL,i median (IQR) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 0.0 (−0.1 to 0.1) | 0.0 (−0.1 to 0.1) | 0.94 |
| Missing, n | 23 | 8 | 15 | |||
| Infant weight gain from birth (z-score),j mean (SD) | −1.2 (1.2) | −1.1 (1.3) | −1.2 (1.1) | 0.10 (−0.62 to 0.82) | 0.17 (−0.60 to 0.95) | 0.65 |
| Infant weight gain from randomisation (z-score),j mean (SD) | −1.1 (1.4) | −1.0 (1.6) | −1.1 (1.3) | 0.04 (−0.82 to 0.90) | 0.10 (−0.83 to 1.03) | 0.83 |
| Frenotomy performed | ||||||
| Yes, n (%) | 140 (82.8) | 75 (93.8) | 65 (73.0) | - | - | - |
| No, n (%) | 29 (17.2) | 5 (6.2) | 24 (27.0) | |||
| Missing, n | 0 | 0 | 0 | - | - | - |
| Infants who continued to breastfeed | n = 142 | n = 67 | n = 75 | |||
| Time spent breastfeeding in previous 24 hours (hours), median (IQR) | 3 (2–5) | 3 (2–5) | 3 (2–4) | 0 (−1.1 to 1.1) | 0.1 (−1.1 to 1.2) | 0.90 |
| Total (n = 140) | Frenotomy w/ breastfeeding support (n = 75) | Breastfeeding support (n = 65) | |
|---|---|---|---|
| Age of infant at first frenotomy (days), median (IQR) | 24 (13–38) | 23 (11–35) | 26.5 (16–42.5) |
| Missing, n | 1 | 0 | 1 |
| Time from randomisation to frenotomy (days) | |||
| <1 day, n (%) | 42 (30.2) | 30 (40.0) | 12 (18.8) |
| 1–2 days, n (%) | 34 (24.5) | 20 (26.7) | 14 (21.9) |
| 3–6 days, n (%) | 22 (15.8) | 12 (16.0) | 10 (15.6) |
| 7–13 days, n (%) | 26 (18.7) | 10 (13.3) | 16 (25.0) |
| 14–<28 days, n (%) | 10 (7.2) | 3 (4.0) | 7 (10.9) |
| ≥28 days, n (%) | 5 (3.6) | 0 (0.0) | 5 (7.8) |
| Missing, n | 1 | 0 | 1 |
Data on age at cessation of breastfeeding was only available for 6/21 infants, five in the breastfeeding support arm, who stopped breastfeeding at a median of 5 weeks (IQR 5–7).
Mothers had high breastfeeding self-efficacy at 3 months’ follow-up and exclusive breastfeeding rates were high during follow-up (first follow-up: 71%, third month: 65%). Compared to first follow-up, pain during breastfeeding was lower at 3 months (median score out of 10: 0 vs. 2).
Process outcomes
In the frenotomy with breastfeeding support arm, 74/80 (93%) received the allocated intervention whereas in the breastfeeding support arm this was 23/89 (26%). More than four-fifths of the infants in the breastfeeding support arm who adhered to their allocation (19/23, 83%) had a BTAT score >4. Infants in the breastfeeding support arm who had a frenotomy had the operation a median of 5.5 days after randomisation (IQR 2–9 days). Frenotomies were mostly performed by midwives, the technique most commonly used was division of an anterior membrane plus posterior fleshy attachment, and 85% of infants were able to breastfeed immediately after the procedure (see Table 7).
| Total (n = 169) | Frenotomy w/ breastfeeding support (n = 80) | Breastfeeding support (n = 89) | |
|---|---|---|---|
| Allocation adhered to a | |||
| Yes, n (%) | 97 (57.4) | 74 (92.5) | 23 (25.8) |
| BTAT score 0–4 at randomisation, n (%) | 31 (32.0) | 27 (36.5) | 4 (17.4) |
| BTAT score 5–6 at randomisation, n (%) | 28 (28.9) | 20 (27.0) | 8 (34.8) |
| BTAT score 7–8 at randomisation, n (%) | 38 (39.2) | 27 (36.5) | 11 (47.8) |
| No, n (%) | 72 (42.6) | 6 (7.5) | 66 (74.2) |
| BTAT score 0–4 at randomisation, n (%) | 24 (33.8) | 2 (33.3) | 22 (33.9) |
| BTAT score 5–6 at randomisation, n (%) | 25 (35.2) | 1 (16.7) | 24 (36.9) |
| BTAT score 7–8 at randomisation, n (%) | 22 (31.0) | 3 (50.0) | 19 (29.2) |
| Missing, n | 1 | 0 | 1 |
| Received no intervention, n (%) | 2 (1.2) | 1 (1.3) | 1 (1.1) |
| Reason for non-adherence | |||
| Parental wish, n (%) | 5 (23.8) | 4 (80.0) | 1 (6.3) |
| Clinician decision, n (%) | 2 (9.5) | 1 (20.0) | 1 (6.3) |
| Other,b n (%) | 14 (66.7) | 0 (0.0) | 14 (87.5) |
| Missing, n | 51 | 1 | 50 |
| Type of breastfeeding support received | |||
| In person, n (%) | 29 (26.4) | 15 (30.0) | 14 (23.3) |
| Virtual, n (%) | 45 (40.9) | 18 (36.0) | 27 (45.0) |
| In person and virtual, n (%) | 36 (32.7) | 17 (34.0) | 19 (31.7) |
| Missing, n | 56 | 28 | 28 |
| Not applicable,c n | 3 | 2 | 1 |
| Infants who underwent frenotomy | ( n = 140) | ( n = 75) | ( n = 65) |
| Person performing procedure | |||
| Midwife, n (%) | 117 (85.4) | 61 (81.3) | 56 (90.3) |
| Nurse, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Doctor, n (%) | 19 (13.9) | 13 (17.3) | 6 (9.7) |
| Other, n (%) | 1 (0.7) | 1 (1.3) | 0 (0.0) |
| Missing, n | 3 | 0 | 3 |
| Frenotomy performed with | |||
| Bipolar diathermy, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Scissors, n (%) | 129 (100.0) | 75 (100.0) | 54 (100.0) |
| Missing, n | 11 | 0 | 11 |
| Technique | |||
| Division of an anterior membrane only, n (%) | 12 (9.2) | 11 (14.7) | 1 (1.8) |
| Division of an anterior membrane plus posterior fleshy attachment, n (%) | 102 (78.5) | 54 (72.0) | 48 (87.3) |
| Division of posterior fleshy attachment only, n (%) | 14 (10.8) | 8 (10.7) | 6 (10.9) |
| Other, n (%) | 2 (1.5) | 2 (2.7) | 0 (0.0) |
| Missing, n | 10 | 0 | 10 |
| Baby able to breastfeed after procedure | |||
| Straight away, n (%) | 116 (84.7) | 65 (86.7) | 51 (82.3) |
| Within 15 minutes, n (%) | 11 (8.0) | 6 (8.0) | 5 (8.1) |
| No, n (%) | 10 (7.3) | 4 (5.3) | 6 (9.7) |
| Missing, n | 3 | 0 | 3 |
Exploratory analyses
There was no significant difference (aRR 1.27, 95% CI 0.99 to 1.64) in the rate of breastmilk feeding at 3 months between the arms per protocol analysis where the 70 infants who did not receive their allocated intervention were excluded (infants in the frenotomy with breastfeeding support who had no frenotomy performed and infants in the breastfeeding only group who had a frenotomy performed) (see Table 8).
| Total (n = 99) | Frenotomy w/ breastfeeding support (n = 75) | Breastfeeding support (n = 24) | Unadjusted risk ratio (95% CI) | Adjusteda risk ratio (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Any breastmilk feeding b | ||||||
| Yes, n (%) | 81 (86.2) | 65 (90.3) | 16 (72.7) | 1.24 (0.95 to 1.62) | 1.27 (0.99 to 1.64) | 0.06 |
| No, n (%) | 13 (13.8) | 7 (9.7) | 6 (27.3) | |||
| Missing, n | 5 | 3 | 2 | |||
There was a significant difference in the rate of breastmilk feeding at 3 months in the as-treated analysis where infants were analysed according to the intervention received (RR 1.35, 95% CI 1.05 to 1.74), noting that for this analysis, the groups compared were not the groups originally randomised and hence this difference may be due to confounders not accounted for in the analysis (see Table 9).
| Total (n = 166) | Received frenotomy w/ breastfeeding support (n = 139) | Received breastfeeding support only (n = 27) | Unadjusted risk ratio (95% CI) | Adjusteda risk ratio (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Any breastmilk feeding b | ||||||
| Yes, n (%) | 141 (87.0) | 123 (90.4) | 18 (69.2) | 1.31 (1.00 to 1.70) | 1.35 (1.05 to 1.74) | 0.02 |
| No, n (%) | 21 (13.0) | 13 (9.6) | 8 (30.8) | |||
| Missing, n | 4 | 3 | 1 | |||
Pre-specified subgroup analyses
There were no notable differences between both the arms for any of the selected subgroups except that the rate of breastmilk feeding at 3 months appeared higher in the frenotomy with breastfeeding support arm compared to the breastfeeding support arm (92% vs. 83%) before the trial paused due to the coronavirus pandemic (see Table 10). After the trial restarted, the rate was higher in the breastfeeding support arm compared to the frenotomy with breastfeeding support arm (91% vs. 81%).
| Primary outcome | Total (n = 169) | Frenotomy w/ breastfeeding support (n = 80) | Breastfeeding support (n = 89) | |
|---|---|---|---|---|
| Infant’s age at randomisation | ||||
| ≥2 weeks | ||||
| Yes, n (%) | 93 (92.1) | 43 (91.5) | 50 (92.6) | |
| No, n (%) | 8 (7.9) | 4 (8.5) | 4 (7.4) | |
| Missing, n | 4 | 3 | 1 | |
| <2 weeks | ||||
| Yes, n (%) | 49 (79.0) | 24 (82.8) | 25 (75.8) | |
| No, n (%) | 13 (21.0) | 5 (17.2) | 8 (24.2) | |
| Missing, n | 2 | 1 | 1 | |
| Degree of tongue-tie (BTAT score) at randomisation | ||||
| ≤4 | ||||
| Yes, n (%) | 46 (83.6) | 23 (79.3) | 23 (88.5) | |
| No, n (%) | 9 (16.4) | 6 (20.7) | 3 (11.5) | |
| Missing, n | 0 | 0 | 0 | |
| 5–8 | ||||
| Yes, n (%) | 95 (88.8) | 44 (93.6) | 51 (85.0) | |
| No, n (%) | 12 (11.2) | 3 (6.4) | 9 (15.0) | |
| Missing, n | 6 | 4 | 2 | |
| Missing, n | 1 | 0 | 1 | |
| Mother’s prior beliefs about frenotomy | ||||
| Think it will help my baby | ||||
| Yes, n (%) | 73 (88.0) | 34 (87.2) | 39 (88.6) | |
| No, n (%) | 10 (12.0) | 5 (12.8) | 5 (11.4) | |
| Missing, n | 3 | 2 | 1 | |
| Do not know if it will help my baby | ||||
| Yes, n (%) | 66 (85.7) | 32 (88.9) | 34 (82.9) | |
| No, n (%) | 11 (14.3) | 4 (11.1) | 7 (17.1) | |
| Missing, n | 2 | 1 | 1 | |
| Think it is unlikely to help my baby | ||||
| Yes, n (%) | 1 (100.0) | 0 (0.0) | 1 (100.0) | |
| No, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Missing, n | 0 | 0 | 0 | |
| Missing, n | 2 | 1 | 1 | |
| Recruited pre- or posttrial pause a during the COVID-19 pandemic | ||||
| Pre-pause | ||||
| Yes, n (%) | 89 (87.3) | 45 (91.8) | 44 (83.0) | |
| No, n (%) | 13 (12.7) | 4 (8.2) | 9 (17.0) | |
| Missing, n | 5 | 3 | 2 | |
| Postpause | ||||
| Yes, n (%) | 53 (86.9) | 22 (81.5) | 31 (91.2) | |
| No, n (%) | 8 (13.1) | 5 (18.5) | 3 (8.8) | |
| Missing, n | 1 | 1 | 0 | |
Secondary analyses
There were minimal differences between infants of mothers who complied with the intervention compared to non-compliers in the breastfeeding support arm for most baseline characteristics (see Table 11). However, a higher proportion of mothers who did not comply believed that frenotomy was helpful for their baby compared to mothers who complied (39/65, 61% vs. 6/24, 25%). A higher proportion of infants in the complier group had a BTAT score >4 compared to non-compliers (21/24, 88% vs. 41/64, 64%). The rate of breastmilk feeding at 3 months was higher among non-compliers compared to compliers (59/65, 91% vs. 16/24, 73%) (see Table 12), but the results from the CACE analysis showed no evidence for a difference in rates of breastmilk feeding at 3 months between the arms (RR 1.09, 95% CI 0.53 to 1.64) (see Table 13).
| Total (n = 89) | Non-complier (frenotomy performed) (n = 65) | Complier (frenotomy not performed) (n = 24) | |
|---|---|---|---|
| Mother’s age (years), mean (SD) | 31.9 (5.1) | 32.1 (4.8) | 31.6 (5.8) |
| Missing, n | 3 | 3 | 0 |
| Mother’s ethnic group | |||
| White, n (%) | 84 (95.5) | 61 (95.3) | 23 (95.8) |
| Asian, n (%) | 2 (2.3) | 1 (1.6) | 1 (4.2) |
| Black, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mixed, n (%) | 2 (2.3) | 2 (3.1) | 0 (0.0) |
| Other, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing, n | 1 | 1 | 0 |
| Deprivation index | |||
| 1 (most deprived), n (%) | 10 (11.2) | 8 (12.3) | 2 (8.3) |
| 2, n (%) | 18 (20.2) | 14 (21.5) | 4 (16.7) |
| 3, n (%) | 17 (19.1) | 11 (16.9) | 6 (25.0) |
| 4, n (%) | 25 (28.1) | 17 (26.2) | 8 (33.3) |
| 5 (least deprived), n (%) | 19 (21.4) | 15 (23.1) | 4 (16.7) |
| Previous live birth(s) a | |||
| Yes, n (%) | 42 (47.2) | 32 (49.2) | 10 (41.6) |
| No, n (%) | 47 (52.8) | 33 (50.8) | 14 (58.3) |
| Breastfed before | |||
| Yes, n (%) | 40 (95.2) | 30 (93.8) | 10 (100.0) |
| No, n (%) | 2 (4.8) | 2 (6.2) | 0 (0.0) |
| Not applicable – no previous live birth, n | 47 | 33 | 14 |
| Pre-trial breastfeeding support received | |||
| Yes, n (%) | 74 (84.1) | 53 (82.8) | 21 (87.5) |
| No, n (%) | 14 (15.9) | 11 (17.2) | 3 (12.5) |
| Missing, n | 1 | 1 | 0 |
| Mother’s pain while feeding during previous 24 hours,b median (IQR) | 4 (2–7) | 5 (2–7) | 3.5 (0–6) |
| Missing, n | 1 | 1 | 0 |
| Mother’s prior beliefs about frenotomy | |||
| Think it will help my baby, n (%) | 45 (51.1) | 39 (60.9) | 6 (25.0) |
| Do not know if it will help my baby, n (%) | 42 (47.7) | 25 (39.1) | 17 (70.8) |
| Think it is unlikely to help my baby, n (%) | 1 (1.1) | 0 (0.0) | 1 (4.2) |
| Missing, n | 1 | 1 | 0 |
| Maternal anxiety or depression c | |||
| Yes, n (%) | 40 (46.0) | 30 (47.6) | 10 (41.7) |
| No, n (%) | 47 (54.0) | 33 (52.4) | 14 (58.3) |
| Missing, n | 2 | 2 | 0 |
| Maternal HRQoL,d mean (SD) | 0.77 (0.16) | 0.75 (0.14) | 0.80 (0.20) |
| Missing, n | 4 | 4 | 0 |
| Recruited pre- or posttrial pausee during the COVID-19 pandemic | |||
| Pre-pause, n (%) | 55 (61.8) | 35 (53.8) | 20 (83.3) |
| Postpause, n (%) | 34 (38.2) | 30 (46.2) | 4 (16.7) |
| Degree of tongue-tie (BTAT) | |||
| 0–4, n (%) | 26 (30.0) | 23 (35.9) | 3 (12.5) |
| 5–6, n (%) | 32 (36.4) | 24 (37.5) | 8 (33.3) |
| 7–8, n (%) | 30 (34.1) | 17 (26.6) | 13 (54.2) |
| Missing, n | 1 | 1 | 0 |
| Total (n = 89) | Non-complier (frenotomy performed) (n = 65) | Complier (frenotomy not performed) (n = 24) | |
|---|---|---|---|
| Any breastmilk feeding a | |||
| Yes, n (%) | 75 (86.2) | 59 (90.8) | 16 (72.7) |
| No, n (%) | 12 (13.8) | 6 (9.2) | 6 (27.3) |
| Missing, n | 2 | 0 | 2 |
| Frenotomy w/ breastfeeding support (n = 76) | Breastfeeding support (n = 87) | CACE risk ratio (95% CI)a | ||||
|---|---|---|---|---|---|---|
| Primary outcome | Event rate (%) | Compliance | Primary outcomeb | Event rate (%) | ||
| Compliers | 15/19 | 78.9 | 22 (25.3) | 16/22 | 72.7 | |
| Non-compliers | 52/57 | 91.2 | 65 (74.7) | 59/65 | 90.8 | 1.09 (0.53 to 1.64) |
| Total | 67/76 | 88.2 | 75/87 | 86.2 | ||
Economic evaluation
NHS health-care resource use
Tables 14 and 15 present health-care resource use associated with the intervention-related health-care resource use. Table 14 presents the number of frenotomies performed from trial entry up to 3 months after birth across all participants. All frenotomies were performed by NHS providers, with 61 (76.25%) and 56 (62.9%) of procedures conducted by midwives in the frenotomy and no frenotomy groups, respectively. In the frenotomy with breastfeeding support group, 75 participants (93.75%) had the procedure whereas 65 participants (73.0%) had the procedure in the breastfeeding support group. Total numbers of frenotomies conducted across all participants in the trial are presented in Appendix 2, Table 23. Table 15 reports the different types of breastfeeding support received up to 3 months of age across all participants. Different types of support services were accessed including the NHS infant feeding service and/or other breastfeeding support services. The latter included access to a breastfeeding café, the Breastfeeding Network, La Leche League, the NCT (formerly National Childbirth Trust) or contacts with any other trained breastfeeding supporter. Table 24 reports the same information as Table 15 but only for those women who received breastfeeding support. There were no statistically significant differences in the breastfeeding support received between trial arms.
| Frenotomy with breastfeeding support (n = 80), n (%) | Breastfeeding support only (n = 89), n (%) | |
|---|---|---|
| Frenotomies performed by a private provider | 0 | 0 |
| Frenotomies performed by the NHS provider | 75 (93.75%) | 65 (73.0%) |
| Doctor | 13 (16.25%) | 6 (6.7%) |
| Midwife | 61 (76.25%) | 56 (62.9%) |
| Other | 1 (1.25%) | 0 |
| Missing | 0 | 3 (3.4%) |
| Frenotomies with complications | 1 (1.25%) | 2 (2.2%) |
| Frenotomies not performed | 5 (6.25%) | 24 (27.0%) |
| Frenotomy with breastfeeding support (n = 80) | Breastfeeding support only (n = 89) | Mean difference in number of contacts (95% CI)a |
|||
|---|---|---|---|---|---|
| Number of patients (%) | Mean number of contacts (SD) | Number of patients (%) | Mean number of contacts (SD) | ||
| Total breastfeeding support received | 50 (62.5) | 2.53 (2.87) | 60 (67.4) | 2.69 (2.65) | −0.26 (−1.12 to 0.61) |
| NHS contacts | 43 (53.75) | 1.62 (1.85) | 49 (55.0) | 2.07 (2.46) | −0.4 (−1.1 to 0.29) |
| Phone | 26 (60.5) | 0.72 (1.13) | 32 (65.3) | 0.73 (1.23) | |
| In person | 29 (67.4) | 0.9 (1.51) | 37 (75.5) | 1.33 (2.09) | |
| Missing | 8 (10.0) | --- | 14 (15.73) | --- | |
| Other contacts | 18 (22.5) | 0.9 (2.06) | 15 (16.85) | 0.59 (1.49) | 0.18 (−0.39 to 0.75) |
| Phone | 9 (50.0) | 0.39 (1.23) | 12 (75.0) | 0.38 (1.18) | |
| In person | 14 (77.8) | 0.51 (1.31) | 10 (62.5) | 0.22 (0.73) | |
| Missing | 8 (10.0) | --- | 15 (16.58) | --- | |
| Not received | 22 (27.5) | --- | 14 (15.73) | --- | |
Maternal health-care resources consumed at 3 months of age is presented in Table 16 across all participants. Similar information is presented in Table 25 but the mean number of resources consumed is calculated only for those consuming the health-care resource use category. No significant differences for any resource use categories between groups were detected in any of the resource use categories: primary care, community care, secondary care and any other health-care professionals.
| Resource use category and item | Frenotomy with breastfeeding support (n = 80) | Breastfeeding support only (n = 89) | Mean difference in number of contacts (95% CI)b | ||
|---|---|---|---|---|---|
| Number of participants (%) | Mean number of contactsa (SD) | Number of participants (%) | Mean number of contactsa (SD) | ||
| Primary care | |||||
| General practitioner visits | 73 (91.25) | 0.92 (1.05) | 73 (82.0) | 0.64 (0.95) | 0.26 (−0.07 to 0.58) |
| Yes | 73 (91.25) | 73 (82.0) | |||
| No | 0 | 0 | |||
| Missing | 7 (8.75) | --- | 16 (18.0) | --- | |
| Practice nurse visits | 73 (91.25) | 0.16 (0.94) | 74 (83.15) | 0.11 (0.48) | 0.07 (−0.19 to 0.33) |
| Yes | 73 (91.25) | 74 (83.15) | |||
| No | 0 | 0 | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Medicines | 73 (91.25) | 0.29 (0.72) | 74 (83.15) | 0.15 (0.51) | 0.17 (−0.03 to 0.37) |
| Yes | 14 (17.5) | 7 (7.9) | |||
| No | 59 (73.75) | 67 (75.25) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Of which antibiotics | 73 (91.25) | 0.25 (0.6) | 74 (83.15) | 0.12 (0.47) | |
| Yes | 13 (16.25) | 6 (6.7) | |||
| No | 60 (75.0) | 68 (76.45) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Of which for anxiety/depression | 73 (91.25) | 0.04 (0.26) | 74 (83.15) | 0.03 (0.16) | |
| Yes | 2 (2.5) | 2 (2.2) | |||
| No | 71 (88.75) | 72 (80.95) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Community care | |||||
| Community nurse/midwife contacts | 73 (91.25) | 0.07 (0.35) | 74 (83.15) | 0.04 (0.26) | −0.01 (−0.1 to 0.08) |
| Yes | 3 (3.75) | 2 (2.2) | |||
| No | 70 (87.5) | 72 (80.95) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Of which virtual | 73 (91.25) | 0 (0) | 74 (83.15) | 0.013 (0.116) | |
| Yes | 0 | 1 (1.15) | |||
| No | 73 (91.25) | 73 (82.0) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Of which in person | 73 (91.25) | 0.07 (0.35) | 74 (83.15) | 0.03 (0.16) | |
| Yes | 3 (3.75) | 2 (2.2) | |||
| No | 70 (87.5) | 72 (80.95) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Secondary (hospital-based) care | |||||
| Accident & emergency department visits | 73 (91.25) | 0.027 (0.234) | 74 (83.15) | 0.013 (0.116) | 0.004 (−0.057 to 0.065) |
| Yes | 1 (1.25) | 1 (1.15) | |||
| No | 72 (90.0) | 73 (82.0) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Hospital outpatient clinic appointments | 73 (91.25) | 0.14 (0.51) | 74 (83.15) | 0.05 (0.37) | 0.07 (−0.08 to 0.22) |
| Yes | 6 (7.5) | 2 (2.2) | |||
| No | 67 (83.75) | 72 (80.95) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Of which virtual | 73 (91.25) | 0.03 (0.23) | 74 (83.15) | 0 (0) | |
| Yes | 1 (1.25) | 0 | |||
| No | 72 (90.0) | 74 (83.15) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Of which in person | 73 (91.25) | 0.11 (0.39) | 74 (83.15) | 0.05 (0.37) | |
| Yes | 6 (7.5) | 2 (2.2) | |||
| No | 67 (83.75) | 72 (80.95) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Hospital admissions | 72 (90.0) | 0.014 (0.118) | 75 (84.25) | 0.013 (0.115) | 0.015 (−0.013 to 0.044) |
| Yes | 1 (1.25) | 1 (1.1) | |||
| No | 71 (88.75) | 74 (83.15) | |||
| Missing | 8 (10.0) | --- | 14 (15.75) | --- | |
| Length of stay (days) | 0.014 (0.118) | 0.027 (0.231) | 0.015 (−0.013 to 0.044) | ||
| Missing | 8 (10.0) | --- | 14 (15.7) | --- | |
| Any other NHS health-care professionals contacts | |||||
| Other NHS health-care professionals contacts | 73 (91.25) | 0.19 (0.64) | 74 (83.15) | 0.16 (0.66) | 0.03 (−0.19 to 0.25) |
| Yes | 7 (8.75) | 5 (5.5) | |||
| No | 66 (82.5) | 69 (77.5) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Of which virtual | 73 (91.25) | 0.05 (0.33) | 74 (83.15) | 0.09 (0.58) | |
| Yes | 2 (2.5) | 2 (2.2) | |||
| No | 71 (88.75) | 72 (80.95) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Of which in person | 73 (91.25) | 0.14 (0.45) | 74 (83.15) | 0.07 (0.34) | |
| Yes | 7 (8.75) | 3 (3.35) | |||
| No | 66 (82.5) | 71 (79.8) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Any other non-NHS health-care professionals contacts | |||||
| Osteopath visits | 73 (91.25) | 0 (0) | 74 (83.15) | 0.07 (0.42) | −0.07 (−0.17 to 0.03) |
| Yes | 0 | 2 (2.2) | |||
| No | 73 (91.25) | 72 (80.95) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
Table 17 presents health-care resource utilization for infants from trial entry up to 3 months across all participants in the study. Table 26 presents health-care utilization but only for those infants consuming the health-care resource use category. Similar to maternal health-care utilization, we did not observe any mean differences in visits for any of the categories: primary care, community care, secondary care and any other health-care professionals.
| Resource use category and item | Frenotomy with breastfeeding support (n = 80) | Breastfeeding support only (n = 89) | Mean difference in number of contacts (95% CI)b | ||
|---|---|---|---|---|---|
| Number of patients (%) | Mean number of contactsa (SD) | Number of patients (%) | Mean number of contactsb (SD) | ||
| Primary care | |||||
| General practitioner contacts | 73 (91.25) | 0.86 (0.92) | 72 (80.9) | 0.86 (1.12) | 0.03 (−0.30 to 0.37) |
| Yes | 42 (52.5) | 37 (41.6) | |||
| No | 31 (38.75) | 35 (39.3) | |||
| Missing | 7 (8.75) | --- | 17 (19.10) | --- | |
| Of which virtual | 73 (91.25) | 0 (0) | 72 (80.9) | 0.014 (0.083) | |
| Yes | 0 | 1 (1.1) | |||
| No | 73 (91.25) | 71 (79.8) | |||
| Missing | 7 (8.75) | --- | 17 (19.10) | --- | |
| Of which in person | 73 (91.25) | 0.86 (0.92) | 72 (80.9) | 0.85 (1.1) | |
| Yes | 42 (52.5) | 37 (41.6) | |||
| No | 31 (38.75) | 35 (39.3) | |||
| Missing | 7 (8.75) | --- | 17 (19.10) | --- | |
| Practice nurse visits | 73 (91.25) | 0.12 (0.47) | 74 (83.15) | 0.13 (0.48) | −0.02 (−0.19 to 0.14) |
| Yes | 5 (6.25) | 6 (6.75) | |||
| No | 68 (85.0) | 68 (76.4) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Medicines (antibiotics) | 73 (91.25) | 0.05 (0.23) | 74 (83.15) | 0.08 (0.27) | −0.03 (−0.11 to 0.06) |
| Yes | 4 (5.0) | 6 (6.75) | |||
| No | 69 (86.25) | 68 (76.4) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Community care | |||||
| Community nurse/midwife visits | 73 (91.25) | 0.11 (0.94) | 74 (83.15) | 0.03 (0.16) | 0.08 (−0.15 to 0.30) |
| Yes | 1 (1.25) | 2 (2.2) | |||
| No | 72 (90.0) | 72 (80.95) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Infant health visitor contacts | 72 (90.0) | 0.25 (1.0) | 74 (83.15) | 0.19 (0.65) | 0.07 (−0.22 to 0.36) |
| Yes | 5 (6.25) | 7 (7.9) | |||
| No | 67 (83.75) | 67 (75.25) | |||
| Missing | 8 (10.0) | --- | 15 (16.85) | --- | |
| Of which virtual | 72 (90.0) | 0.08 (0.71) | 74 (83.15) | 0.01 (0.12) | |
| Yes | 1 (1.25) | 1 (1.1) | |||
| No | 71 (88.75) | 73 (82.05) | |||
| Missing | 8 (10.0) | --- | 15 (16.85) | --- | |
| Of which in-person | 72 (90.0) | 0.17 (0.73) | 74 (83.15) | 0.18 (0.63) | |
| Yes | 4 (5.0) | 7 (7.9) | |||
| No | 68 (85.0) | 67 (75.25) | |||
| Missing | 8 (10.0) | --- | 15 (16.85) | --- | |
| Community paediatrician visits | 73 (91.25) | 0.01 (0.12) | 74 (83.15) | 0.03 (0.16) | −0.02 (−0.07 to 0.03) |
| Yes | 1 (1.25) | 2 (2.2) | |||
| No | 72 (90.0) | 72 (80.95) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Secondary (hospital-based) care | |||||
| Accident & emergency department visits | 73 (91.25) | 0.07 (0.3) | 74 (83.15) | 0.04 (0.2) | 0.03 (−0.06 to 0.12) |
| Yes | 4 (5.0) | 3 (3.35) | |||
| No | 69 (86.25) | 71 (79.8) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Hospital outpatient clinic appointments | 73 (91.25) | 0.16 (0.96) | 74 (83.15) | 0.04 (0.26) | 0.12 (−0.12 to 0.36) |
| Yes | 5 (6.25) | 2 (2.2) | |||
| No | 68 (85.0) | 72 (80.95) | |||
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Hospital admissions | 72 (90.0) | 0.1 (0.51) | 75 (84.25) | 0.13 (0.38) | −0.03 (−0.18 to 0.11) |
| Yes | 4 (5.0) | 9 (10.1) | |||
| No | 68 (85.0) | 66 (74.15) | |||
| Missing | 8 (10.0) | --- | 14 (15.75) | --- | |
| Length of stay (days) | 0.33 (2.16) | 0.24 (0.75) | 0.14 (-0.38 to 0.67) | ||
| Missing | 8 (10.0) | --- | 14 (15.75) | --- | |
| Any other NHS health-care professionals visits | |||||
| Other NHS health-care professionals visits | 72 (90.0) | 0.05 (0.37) | 72 (80.95) | 0.04 (0.26) | 0.01 (−0.09 to 0.12) |
| Yes | 2 (2.5) | 2 (2.2) | |||
| No | 70 (87.5) | 70 (78.75) | |||
| Missing | 8 (10.0) | --- | 17 (19.05) | --- | |
| Any other non-NHS health-care professionals visits | |||||
| Osteopath visits | 72 (90.0) | 0.04 (0.26) | 72 (80.95) | 0.11 (0.49) | −0.07 (−0.21 to 0.07) |
| Yes | 2 (2.5) | 4 (4.55) | |||
| No | 70 (87.5) | 68 (76.4) | |||
| Missing | 8 (10.0) | --- | 17 (19.05) | --- | |
NHS health-care costs
Table 18 reports the cost analysis results for mothers and their babies over the study period. A borderline statistically significant mean cost difference (95% CI) of £27 (£0.3–54) between groups favouring the frenotomy was observed in the intervention-related costs. There were no significant differences in any cost categories incurred by mothers or their babies between the frenotomy with breastfeeding support and breastfeeding support groups. The mean (SD) total cost per woman/infant pair was estimated to be £497 (£854) and £483 (£529) in the frenotomy and no frenotomy groups, respectively, and a non-significant mean cost difference (95% CI) of £21 (−£221 to £263) was detected. Maternal and infant cost analysis across all participants in the study is presented in Table 27.
| Resource use category and item | Frenotomy with breastfeeding support (n = 80), mean (SD) | Breastfeeding support only (n = 89), mean (SD) | Mean cost differencea (95% CI) | |||
|---|---|---|---|---|---|---|
| Mother | Infant | Mother | Infant | Mother | Infant | |
| Frenotomy [1] | £120 (£74) | £94 (£97) | £27 (£0.3 to £54) | |||
| Missing, n (%) | 0 | 0 | --- | |||
| Breastfeeding support | ||||||
| NHS contacts, phone | £33 (£52) | £34 (£57) | £2 (−£16 to £20) | |||
| NHS contacts, in person | £73 (£122) | £108 (£170) | −£36 (−£80 to £8) | |||
| Total NHS contacts, breastfeeding support [2] | £106 (£131) | £142 (£180) | −£34 (−£83 to £14) | |||
| Missing, n (%) | 8 (10.0) | 14 (16.0) | ||||
| Primary care | ||||||
| General practitioner visits | £36 (£41) | £34 (£36) | £25 (£37) | £33 (£43) | £10 (−£3 to £23) | £2 (−£11 to £15) |
| Missing, n (%) | 7 (8.75) | 7 (8.75) | 16 (18.0) | 17 (19.1) | ||
| Practice nurse visits | £1.8 (£10.4) | £1.4 (£5.2) | £1.2 (£5.3) | £1.5 (£5.2) | £0.7 (−£2.1 to £3.6) | −£0.3 (−£2.0 to £1.5) |
| Missing, n (%) | 7 (8.75) | 7 (8.75) | 15 (16.85) | 15 (16.85) | ||
| Primary care, total | £38 (£43) | £35 (£36) | £27 (£39) | £35 (£43) | £10 (−£3 to £24) | £2 (−£12 to £15) |
| Missing, n (%) | 7 (8.75) | 7 (8.75) | 17 (19.1) | 18 (20.2) | ||
| Primary care, total mother/infant pair [3] | £73 (£65) | £62 (£67) | £11 (-£11 to £34) | |||
| Missing, n (%) | 7 (8.75) | 18 (20.2) | ||||
| Medicines | ||||||
| Antibiotics | £1.6 (£8.0) | £0.2 (£1.0) | £0.5 (£2.2) | £0.3 (£1.2) | £1.2 (−£0.7 to £3.2) | −£0.1 (−£0.5 to £0.3) |
| For anxiety/depression | £0.3 (£2.4) | --- | £0.1 (£0.4) | --- | £0.3 (−£0.3 to £0.9) | |
| Medicines, total | £2.0 (£10.2) | £0.2 (£1.0) | £0.6 (£2.3) | £0.3 (£1.2) | £1.6 (−£1.0 to £4.1) | −£0.1 (−£0.5 to £0.3) |
| Missing, n (%) | 7 (8.75) | 7 (8.75) | 15 (16.85) | 15 (16.85) | ||
| Medicines, total mother/infant pair [4] | £2.2 (£10.2) | £0.9 (£2.7) | £1.4 (−£1.1 to £4.0) | |||
| Missing, n (%) | 7 (8.75) | 15 (16.85) | ||||
| Community care | ||||||
| Community nurse/midwife contacts | £3 (£16) | £9 (£79) | £2 (£10) | £2 (£14) | −£0.2 (−£4 to £4) | £6 (−£13 to £26) |
| Missing, n (%) | 7 (8.75) | 7 (8.75) | 15 (16.85) | 15 (16.85) | ||
| Infant Health visitor contacts | --- | £17 (£67) | --- | £15 (£52) | --- | £3 (−£17 to £23) |
| Missing, n (%) | --- | 8 (10.0) | --- | 15 (16.85) | ||
| Community paediatrician visits | --- | £5 (£41) | --- | £10 (£57) | --- | −£7 (−£24 to £9) |
| Missing, n (%) | --- | 7 (8.75) | --- | 15 (16.85) | ||
| Community care, total | £3 (£16) | £27 (£116) | £2 (£10) | £27 (£79) | −£0.2 (−£4 to £4) | −£1 (−£33 to £33) |
| 7 (8.75) | 8 (10.0) | 15 (16.85) | 15 (16.85) | |||
| Community care, total mother/infant pair [5] | £30 (£116) | £28 (£79) | −£1 (−£34 to £33) | |||
| Missing, n (%) | 8 (10.0) | 15 (16.85) | ||||
| Secondary (hospital-based) care | ||||||
| Accident & emergency department visits | £5 (£43) | £12 (£55) | £2 (£21) | £7 (£36) | £1 (−£10 to £12) | £5 (−£10 to £21) |
| Missing, n (%) | 7 (8.75) | 7 (8.75) | 15 (16.85) | 15 (16.85) | ||
| Hospital outpatient clinic appointments | £20 (£71) | £38 (£221) | £9 (£60) | £9 (£60) | £8 (−£14 to £30) | £27 (−£27 to £82) |
| Missing, n (%) | 7 (8.75) | 7 (8.75) | 15 (16.85) | 15 (16.85) | ||
| Hospital admissions | £21 (£182) | £54 (£285) | £9 (£77) | £79 (£230) | £23 (−£21 to £68) | −£23 (−£106 to £59) |
| Missing, n (%) | 8 (10.0) | 8 (10.0) | 14 (16.0) | 14 (16.0) | ||
| Secondary (hospital-based) care, total | £47 (£202) | £105 (£540) | £20 (£99) | £97 (£252) | £33 (−£19 to £85) | £8 (−£130 to £146) |
| Missing, n (%) | 8 (10.0) | 8 (10.0) | 15 (16.85) | 15 (16.85) | ||
| Secondary (hospital-based) care, total mother/infant pair [6] | £152 (£568) | £117 (£299) | £42 (-£108 to £191) | |||
| Missing, n (%) | 8 (10.0) | 15 (16.85) | ||||
| Any other NHS health-care professionals contacts | ||||||
| Other NHS health-care professionals contacts, total | £17 (£59) | £7 (£44) | £26 (£160) | £4 (£25) | −£10 (−£52 to £32) | £4 (−£8 to £16) |
| Missing, n (%) | 7 (8.75) | 7 (8.75) | 15 (16.85) | 15 (16.85) | ||
| Other NHS health-care professionals contacts, total mother/infant pair [7] | £25 (£83) | £30 (£164) | -£6 (-£51 to £39) | |||
| Missing, n (%) | 7 (8.75) | 15 (16.85) | ||||
| Any other non-NHS health-care costs | ||||||
| Payment for anything to help breastfeeding | £3 (£9) | --- | £11 (£35) | --- | −£8 (−£17 to £1) | --- |
| Missing, n (%) | 13 (16.25) | 18 (20.22) | ||||
| Non-NHS health-care professionals visits, osteopath | 0 (0) | £2 (£12) | £3 (£20) | £5 (£23) | −£3 (−£8 to £2) | −£3 (−£10 to £3) |
| Missing, n (%) | 7 (8.75) | 8 (10.0) | 15 (16.85) | 17 (19.1) | ||
| Other non-NHS health-care costs, total mother/infant pair [8] | £5 (£18) | £20 (£52) | −£16 (−£29 to −£2) | |||
| Missing, n (%) | 13 (16.25) | 21 (23.6) | ||||
| Total maternal/infant costs [1] + [2] + [3] + [4] + [5] + [6] + [7] | £215 (£274) | £285 (£745) | £218 (£312) | £262 (£349) | −£1 (−£99 to £98) | £31 (−£166 to £227) |
| Missing, n (%) | 9 (11.2) | 9 (11.2) | 17 (19.1) | 18 (20.2) | ||
| Total cost mother/infant pair [1] + [2] + [3] + [4] + [5] + [6] + [7] | £497 (£854) | £483 (£529) | £21 (−£221 to £263) | |||
| Missing, n (%) | 10 (12.5) | 18 (20.2) | ||||
Maternal HRQoL using EQ-5D-5L instrument
The distribution of response across all EQ-5D-5L dimensions at different follow-ups are presented in Table 28. A significant difference in the distribution of responses between groups was observed at the routine follow-up visit for the mobility dimension (χ2 = 10.429 with p-value of 0.005). No other significant difference was observed in any of the other dimensions. Table 19 presents the results of the HRQoL analysis between trial arms at different follow-up points. The mean (SD) HRQoL at baseline was 0.773 (0.179) and 0.766 (0.162), at routine follow-up was 0.851 (0.141) and 0.824 (0.157) and at 3 months after birth was 0.850 (0.183) and 0.868 (0.117) in the frenotomy and no frenotomy groups, respectively. No statistically significant mean differences were detected in overall HRQoL at baseline, routine follow-up and 3 months after birth.
| Frenotomy with breastfeeding support (n = 80) | Breastfeeding support only (n = 89) | Mean differencea (95% CI) | |
|---|---|---|---|
| HRQoL at baseline (trial entry) | |||
| Mean (SD) | 0.773 (0.179) | 0.766 (0.162) | 0.001 (−0.051 to 0.054) |
| Missing, n (%) | 4 (5.0) | 4 (4.5) | |
| HRQoL at routine follow-up (approximately 1 to 2 weeks posttrial entry) | |||
| Mean (SD) | 0.851 (0.141) | 0.824 (0.157) | 0.028 (−0.019 to 0.075) |
| Missing, n (%) | 6 (7.5) | 4 (4.5) | |
| HRQoL at 3 months after birth | |||
| Mean (SD) | 0.850 (0.183) | 0.868 (0.117) | −0.018 (−0.072 to 0.032) |
| Missing, n (%) | 8 (10.0) | 15 (16.85) | |
Maternal QALYs
Table 20 shows QALDs and QALYs between trial arms at 3 months of age. The mean (SD) QALYs was estimated to be 0.2117 (0.0345) and 0.2099 (0.0303) in the frenotomy with breastfeeding support and breastfeeding support groups, respectively. There were no significant mean differences in QALDs or QALYs between the frenotomy with breastfeeding support and breastfeeding support groups.
| Frenotomy with breastfeeding support (n = 80), mean (SD) | Breastfeeding support only (n = 89), mean (SD) | Mean differencea (95% CI) | |
|---|---|---|---|
| QALDs | |||
| Mean (SD) | 77.12 (13.58) | 76.47 (11.03) | 0.55 (−3.46 to 4.56) |
| Missing, n (%) | 11 (13.75) | 15 (16.85) | |
| QALYs | |||
| Mean (SD) | 0.2117 (0.0345) | 0.2099 (0.0303) | 0.0015 (−0.0095 to 0.0125) |
| Missing, n (%) | 11 (13.75) | 15 (16.85) | |
Cost-utility analysis
A summary of cost-effectiveness results comparing frenotomy with breastfeeding support with breastfeeding support only is presented in Table 21. This analysis was undertaken using a complete-case analysis with pairs of total costs and QALYs available for each trial participant. The ICER was estimated to be £6113 per QALY gained indicating that such point estimate is below current thresholds of willingness to pay for QALY gained of £20,000. However, Figure 2 displays the uncertainty around the ICER on the CEP with the joint distribution of mean differences in costs and QALYs. The chart clearly shows bootstrap replicates expanding the four quadrants of the plane indicating that CIs for the ICER in this case would be misleading and difficult to interpret. More informative is Figure 3 that suggests that at a threshold of £20,000 per QALY gained, frenotomy only reaches 50% probability of being cost-effective. Increasing this willingness to pay above that value tends asymptotically towards 60% of being cost-effective.
| Trial arm | Total number of complete cases | Total costs (2019/2020 UK £), mean (SE) | Incremental costs, mean (SE) | QALYs, mean (SE) | Incremental QALYs, mean (SE) |
|---|---|---|---|---|---|
| Breastfeeding support only | 66 | 488 (63) | --- | 0.2082 (0.0036) | --- |
| Frenotomy with breastfeeding support | 71 | 502 (106) | 14 (123) | 0.2105 (0.0043) | 0.0023 (0.0056) |
FIGURE 2.
Bootstrap replications of mean difference in costs and effects plotted on the CEP.
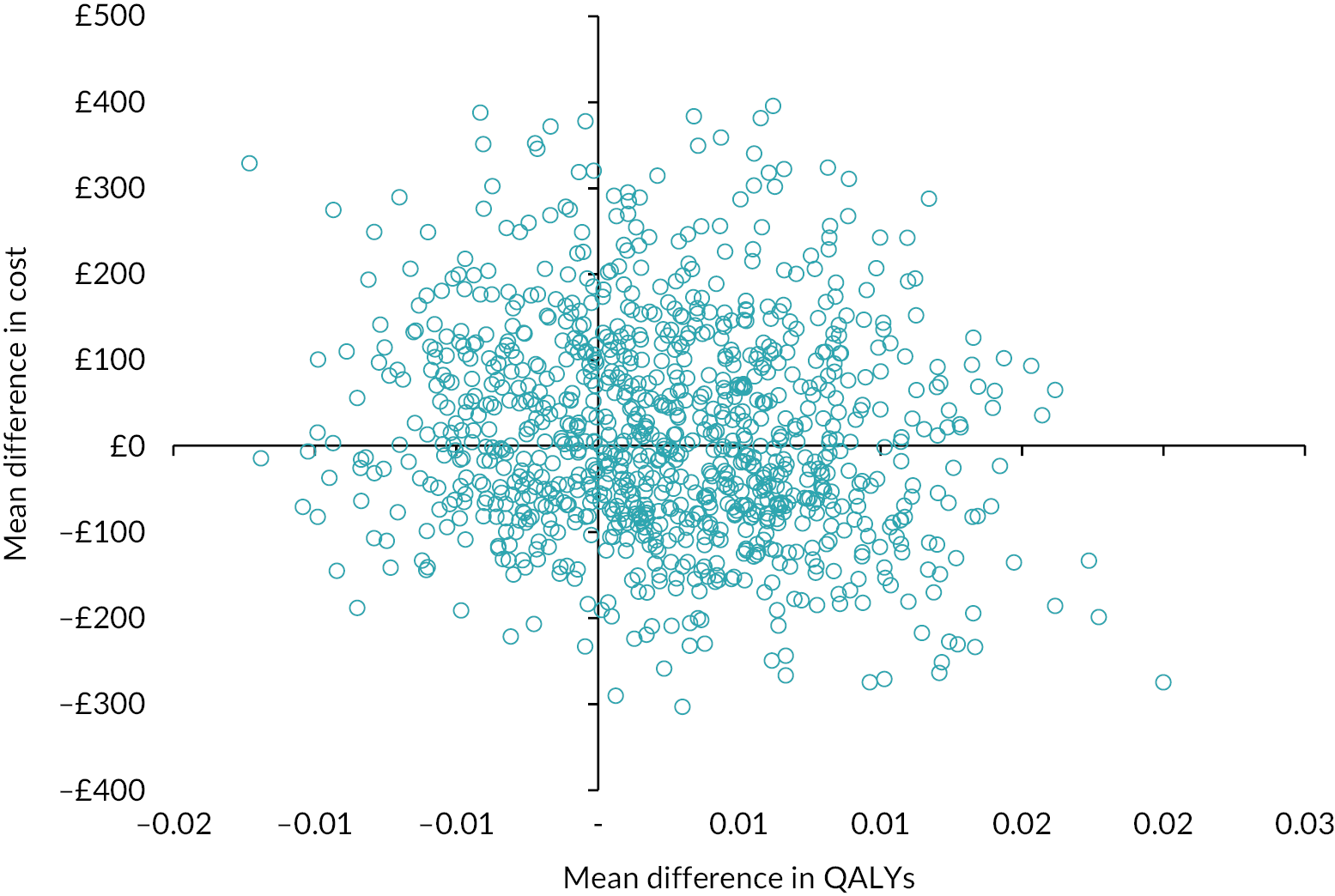
FIGURE 3.
Cost-effectiveness acceptability curves indicating the probability that frenotomy is cost-effective compared to breastfeeding support only.
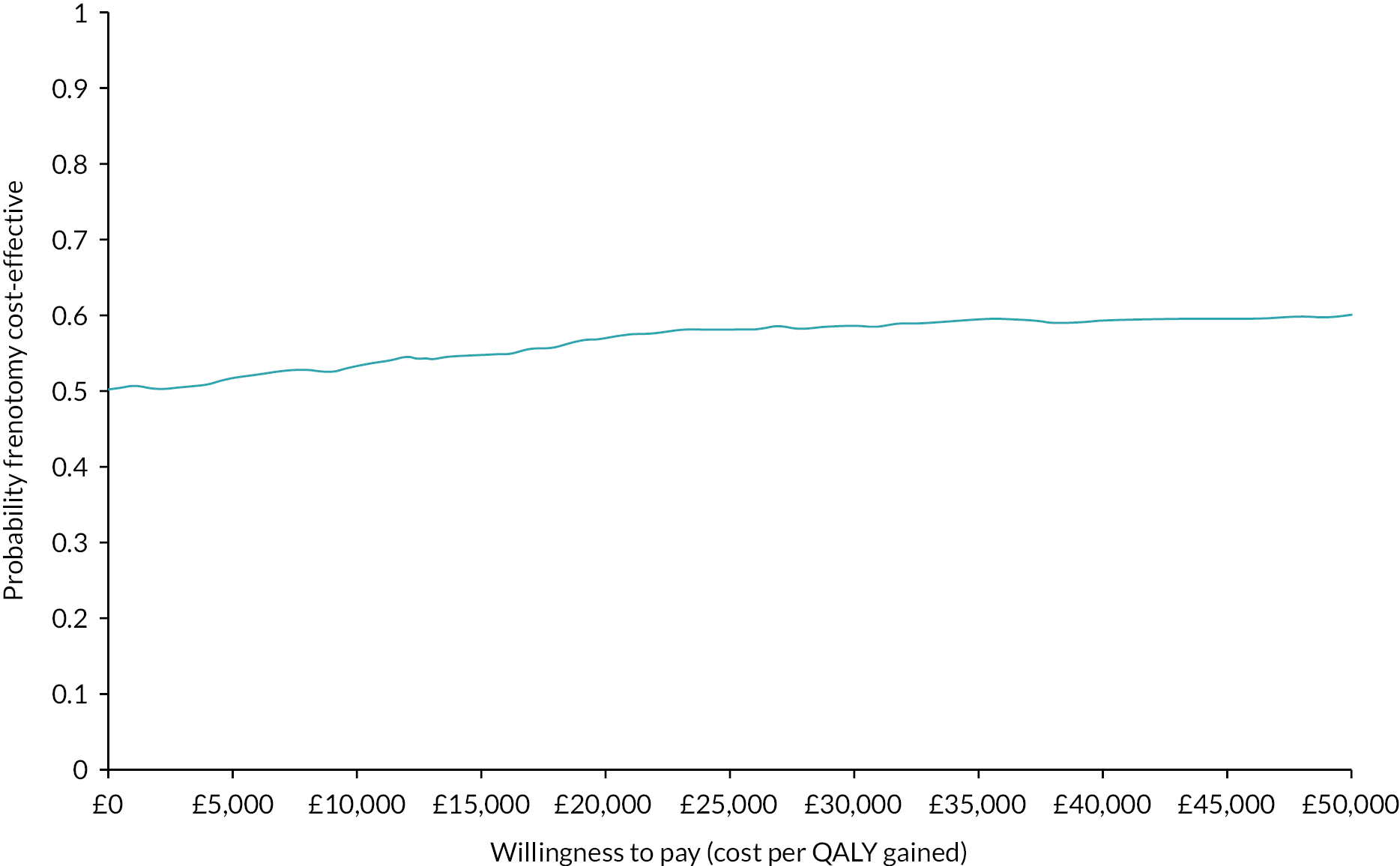
Site monitoring: barriers and facilitators to recruitment
Main factors identified in sites where recruitment was challenging
Several factors were identified, which represented barriers to trial recruitment, mostly related to equipoise among staff and women, but others concerning location and composition of recruiting teams.
Approaching women when they have already received breastfeeding support. In many areas capacity within infant feeding clinics and/or care pathways meant that women attended their first appointment only after they had been having difficulties with breastfeeding for some time. Only a third of babies recruited were <2 weeks old at trial entry (see Table 1). Many women felt that they had therefore already tried breastfeeding support without success, and therefore either were not willing to be randomised, or, if they were randomised to the breastfeeding support arm, they were willing only to try breastfeeding support alone for a few days before requesting frenotomy. Some sites gave women in the breastfeeding support arm ‘just in case’ appointments for frenotomy to address this concern, which had the effect of encouraging crossovers.
In the context of the pandemic, this was exacerbated, since parents were generally less willing to risk having to return for another clinic appointment and/or frenotomy when services were reduced, waiting times were longer and there were concerns over potential COVID-19 exposure by additional visits.
Sites that covered large geographical areas. Women who lived in remote areas wanted their baby to have a frenotomy before they left hospital or within the community so that they did not have to travel. The research team often did not therefore get to see the women before the procedure was carried out or they were discharged. This was a particular issue at one site where up to 10 midwives were qualified to perform a frenotomy. Their numbers of frenotomies performed were high and were often carried out on wards and within homes before there had been time to fully assess the need for a frenotomy and was associated with a lack of equipoise.
Online information regarding tongue tie. Once a diagnosis of tongue-tie has been suggested there is a substantial amount of pro-frenotomy information available online as well as chat groups suggesting that it is the magic fix to breastfeeding issues.
Lack of cohesion among the various professional teams. Often there were difficulties between ward-based research teams and community midwifery teams. Because women are now discharged from hospitals very early following delivery the community midwives are most often the ones to offer breastfeeding support within women’s homes, unless they have frequent feeding support clinics provided by the Trust. In an ideal scenario they would be the ones best placed to recruit women to the trial. However, their caseloads are such that they do not have the time to include this within their practice and most are not research active and have not completed GCP training. Some breastfeeding support midwives who run clinics were willing to undergo training and did recruit to the trial. Some community teams were willing to hand out FROSTTIE leaflets, some were not.
Gatekeeping. One site that withdrew from the trial had a single member of staff who controlled the flow of women coming to the frenotomy clinic appointments and who refused to refer women to the research team. Some clinics were also set in remote sites away from the research team, which made recruitment challenging.
The naming of clinics. If women were invited to attend a tongue-tie clinic then they were expecting to receive a frenotomy for their babies. Feeding support clinics seemed to recruit better.
Lack of feeding support clinics. Some sites had very few clinics, for example just one 4-hour clinic per week. There was a major lack of funding for breastfeeding support services at all sites.
Frenotomies being performed within a different department to the feeding support clinics. Sometimes there was only one member of staff trained to perform frenotomies. This was particularly difficult in one hospital when the service ceased due to long-term sickness. Recruitment was also challenging if the frenotomy teams had no breastfeeding support experience, for example if they were maxillofacial surgery teams.
Feeding support clinics held in a different location to the research teams. This meant that a member of the research team either had to wait throughout the clinic for a potentially eligible recruit, which prevented them from working on other trials, or they had to drop everything and travel to a satellite site at short notice. Many women were not willing to wait for a member of the research team to arrive in this situation. Related to this there was felt, in some sites, to be a lack of funding for research midwife hours.
Paediatric research nurses or research practitioners appointed to cover FROSTTIE as opposed to research midwives. In sites where there was a lack of cohesion between the groups (midwives and nurses identifying as very different professional groups), this caused some issues with cooperation between community staff and research staff.
Research staff time limitations. Research staff did not generally cover weekends within the hospitals, only worked office hours and in some cases only provided cover 3 or 4 days per week. This obviously impacted on recruitment and even more so during annual leave and sickness.
Main factors identified at successful recruiting sites
These were often, not always, the converse of those observed at sites where recruitment was challenging, and showed that recruitment within hospital settings was possible.
Clinics named ‘feeding’ or ‘breastfeeding support’ clinics.
Frequent clinics, some offering drop-in clinics up to 5 days per week.
Trial ‘fit’ with their current practice. For example, sites where women and their babies were assessed and if found to have a tongue tie and feeding difficulties they would not be automatically referred to a frenotomy clinic. They would be sent home to try changes in positioning prior to being re-assessed and possibly then referred for frenotomy.
Recruited early. Babies were recruited at the point of first contact within the clinics prior to breastfeeding support.
Cohesive teams. Clinics for support and frenotomy were held within the same department. Research teams had a good relationship with the feeding support midwives and those who perform the frenotomy.
Pandemic changes to services
In several areas the onset of the COVID-19 pandemic saw the withdrawal of breastfeeding support services, either in person or at all. In areas where all support was withdrawn, as Trusts did not consider breastfeeding support to be an essential service, the trial had to stop. Similarly in some areas frenotomy lists ceased. Other areas moved to providing a remote breastfeeding support service with subsequent frenotomy if necessary, and it was suggested that the trial could move to a centralised model providing fully remote breastfeeding support with referral for frenotomy for those randomised. This would have had the added benefit of enabling breastfeeding support for women in areas where support had been withdrawn and could have enhanced recruitment, but at the time was not considered an appropriate change by the funder.
Chapter 4 Discussion and conclusions
Summary of main findings
The trial did not reach its pre-specified sample size and there was no evidence of differences between trial arms in any outcomes. Rates of continued breastmilk feeding were high at 3 months in both the frenotomy with breastfeeding support and breastfeeding support groups. Compared to first follow-up, pain during breastfeeding was lower at 3 months in both groups. Complications of the procedure were not uncommon, occurring in around 1 in 50 infants. There was a high rate of crossover between arms with 73% of babies in the breastfeeding support arm undergoing frenotomy. Almost two-thirds of women in the breastfeeding support without frenotomy arm whose babies went on to have a frenotomy believed at recruitment that a frenotomy would benefit their child, indicating clear evidence of lack of equipoise. A higher proportion of infants in the non-complier group had a BTAT score of 4 or below, indicating a more restricted tongue compared to compliers. Substantial uncertainty still remains about whether frenotomy with breastfeeding support is a cost-effective use of NHS resources compared with breastfeeding support only.
Limitations
The statistical power of the analysis was extremely limited due to not achieving the target sample size because of the early cessation of the trial and the high proportion of infants in the breastfeeding support arm who underwent frenotomy. Mothers recruited to the trial were more likely to be older, less likely to be of minority ethnicity and more likely to live in more affluent areas than the general population of women giving birth in the UK,6 which may further limit generalisability of the findings.
Comparison with the existing literature
Most infants in the control groups of the five previous trials identified in the Cochrane review12 also underwent frenotomy. Two studies offered frenotomy to all participants, and the other three trials, most of which compared frenotomy to standard care, reported frenotomy rates between 77% and 97%. On this basis all five trials were considered of low quality and at high risk of bias. The 73% frenotomy rate in the breastfeeding support arm that we observed in FROSTTIE is therefore very comparable, but on this basis it must also be regarded as at high risk of bias. The Cochrane review authors concluded that, in the settings where these trials were carried out, equipoise concerning frenotomy was lacking, and FROSTTIE echoes this. Equipoise was a barrier to recruitment due to both staff attitudes and parents’ expectations. Staff broadly fell into two groups. The first group felt strongly that frenotomy was an important intervention, which might aid breastfeeding, and as it had minimal risk of harm should not be denied to women whose babies were diagnosed with tongue-tie and who had breastfeeding difficulties. These staff were reluctant to randomise infants unless the infants were very young, that is before breastfeeding had really been established. It is important to note that although the Cochrane review did not identify any complications of the procedure among the 302 included infants,12 three infants in the FROSTTIE trial had significant complications (bleeding or salivary duct damage), around 1 in every 50 infants. Others have noted concerns over the potential harms of the procedure when its benefits have not been established in high-quality trials. 34
The second group of staff were very clear that expert breastfeeding support was the most important part of the intervention to assist with breastfeeding difficulties and recognised that frenotomy was not without potential harm. These staff were not willing to randomise infants to possible frenotomy until they had a substantial period of breastfeeding support, at which point women themselves were less willing to be randomised as they were desperate for any intervention which might help. Almost all staff fell into one of these two groups with few staff opinions in between.
As evidenced from Tables 2 and 11, which show that more than half of women recruited to the trial believed that frenotomy would help their baby and that fewer than half were truly in equipoise, one of the commonest reported challenges by sites was low numbers of women willing to be randomised. One site reported that around 10 women had to be approached for everyone who expressed willingness to participate. While most women approached who did not wish to be randomised felt that frenotomy would benefit their baby and therefore were not willing to be randomised to a breastfeeding support only arm, there were a few women who strongly felt the opposite, that frenotomy was an unnecessary intervention and did not want their babies to have it. Other studies have reported that many parents feel that frenotomy is a beneficial intervention for breastfeeding difficulties and will go to considerable lengths to access the procedure;35 however, analyses of online forum posts showed more variable experiences of its outcome. 36
In many areas, capacity within infant feeding clinics and/or care pathways meant that women attended their first appointment only after they had been having difficulties with breastfeeding for some time. Only a third of babies recruited were <2 weeks old at trial entry (see Table 1). Many women felt that they had therefore already tried breastfeeding support without success, and therefore either were not willing to be randomised, or, if they were randomised to the breastfeeding support arm, they were willing only to try breastfeeding support alone for a few days before requesting frenotomy. Some sites gave women in the breastfeeding support arm ‘just in case’ appointments for frenotomy to address this concern, which had the effect of encouraging crossovers.
In the context of the COVID-19 pandemic, this was exacerbated, since parents were less willing to risk having to return for another clinic appointment and/or frenotomy when services were reduced, waiting times were longer and there were concerns over potential COVID-19 exposure by additional visits. Breastfeeding support services were known to be highly variable prior to the pandemic,11 and this was exacerbated during the pandemic, with many services ceasing completely, or moving entirely online. Almost half of women completing the 2020 national maternity survey reported that they would have liked more support with breastfeeding compared to around 30% in the 2014 and 2018 surveys. 6
Several sites had clinics that were named ‘the frenotomy clinic’ as opposed to the ‘infant feeding clinic’ or ‘breastfeeding support service’. This naming meant that parents anticipated prior to arrival that their child would undergo frenotomy at the clinic, or shortly afterwards, which again contributed to a lack of equipoise. Some sites renamed their clinics to mitigate this barrier, which was felt to be making a difference to recruitment immediately prior to trial closure.
In some sites, assessment of tongue-tie and breastfeeding support advice was undertaken very early, on the postnatal ward prior to discharge. These sites felt that women were more in equipoise concerning whether or not frenotomy might be beneficial at this time, before they had been struggling with breastfeeding for days or weeks, and therefore that recruitment in the postnatal ward setting might be more effective.
Women in both arms had high rates of breastfeeding continuation, reflecting that they were a highly motivated population. Women recruited were more likely to live in more affluent areas and were older than the general population of women giving birth,6 and almost all of those who had older children had breastfed before. All these factors are known to be associated with higher rates of breastfeeding3 and may account for the high breastfeeding rates seen rather than any impact of the intervention.
It is of concern that women reported high levels of anxiety and depression both at recruitment, and, to a lesser extent, at 3 months, indicating an important need for mental health support.
Equality diversity and inclusion
The majority of women recruited to the trial were from White ethnic groups, despite the trial being conducted at sites with substantial ethnic minority populations. Six per cent of women recruited were from Asian or mixed ethnic backgrounds, and no participants recruited were from Black ethnic minority groups. It is unclear whether this reflects better community support for breastfeeding for women from Black and other ethnic minority groups, a lack of access to hospital breastfeeding support services or differential equipoise concerning frenotomy. Mothers from Black, mixed or other ethnic minority groups are more likely to breastfeed at 6 weeks. 5 Cultural traditions have been shown to be important in women’s decisions about continuing breastfeeding37 and there is a need for tailored support,38 both of which may have influenced decisions to seek support from hospital infant feeding clinics and around the use of frenotomy.
Public and patient involvement
We included a PPI co-applicant from the Breastfeeding Network, who consulted with other Network members, and also established a Public Advisory Group. These two groups helped design the study processes and materials, and advised throughout the trial. We were also guided by a PPI member on the TSC. Patient and public involvement was challenged by the COVID-19 pandemic, and would have benefitted from greater diversity. At the time of trial closure we were planning further PPI work to determine whether there were areas of redesign that might help to address equipoise issues, but this was not carried out.
Implications for practice
The results of this trial provide no evidence of benefit of frenotomy with breastfeeding support over breastfeeding support given the small sample size. The high crossover rate indicates that the trial is at high risk of bias and cannot be used to guide practice. There is nevertheless some useful information to guide counselling of women with babies with tongue-tie. Babies in the breastfeeding support group with more restricted tongue movement, as measured using the BTAT, and whose mothers were in more pain, were more likely to undergo frenotomy. Mothers in both groups had high rates of breastfeeding continuation and reported less pain when feeding at 3 months, emphasising the importance of breastfeeding support to provide advice on latch and correct positioning. Complications of frenotomy were observed in about 1 in 50 babies.
Implications for research
The FROSTTIE trial has indicated that sufficient equipoise does not exist to conduct a randomised trial of frenotomy in NHS hospital infant feeding clinic settings. Such a trial may be possible if infants are recruited early, when initially consulting for breastfeeding support, ideally within community settings. However, it is likely that other study designs will need to be considered in the UK setting, for example observational studies alongside trials of other breastfeeding interventions such as the ABA-Feed study. 39
Conclusions
This trial does not provide sufficient information to assess whether frenotomy in addition to breastfeeding support improves breastfeeding rates in infants diagnosed with tongue-tie. However, there were high breastfeeding rates in both arms. Complications of frenotomy were observed in about 1 in 50 infants. There is a clear lack of equipoise in the UK concerning the use of frenotomy, however, the effectiveness and cost-effectiveness of the procedure still need to be established. Other study designs will need to be considered to address this objective.
Acknowledgements
The authors would like to acknowledge all the women and infants who participated in the trial and the site staff, without whom this research would not have been possible (see Appendix 1). We would also like to thank NPEU CTU and design staff Ann Kennedy, Alan Downs, Alison Stockford, Sarah Chamberlain and Andy Kirk.
Funding and sponsorship
FROSTTIE was funded by the National Institute for Health and Care Research HTA Programme (project number 16/143/01) and sponsored by the University of Oxford. The funder had no role in study design or data collection, analysis and interpretation. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Independent TSC
The trial was overseen by the Trial Steering Committee (TSC) who had ultimate responsibility for considering and, as appropriate, acting on the recommendations of the DMC. The TSC included an independent chair, four independent members, a PPI representative, a co-applicant and the Chief Investigator. Membership consisted of:
Professor Pat Hoddinott, Chair, Chair in Primary Care, NMAHP Research Unit, University of Stirling
Mr Mike Bradburn, Independent member, Senior Medical Statistician, University of Sheffield
Professor Debra Bick, Independent member, Professor of Clinical Trials in Maternal Health, Warwick Clinical Trials Unit, University of Warwick
Dr Mark Johnson, Independent member, Consultant Neonatologist, Department of Neonatal Medicine, University Hospital Southampton NHS Foundation Trust
Dr Sarah McMullen, Independent member (PPI member), Head of Knowledge, National Childbirth Trust
Dr Jenny Ingram, Independent member, Senior Research Fellow, Centre for Academic Child Health, University of Bristol
Professor Marian Knight, Non-independent member, FROSTTIE Chief Investigator, Professor of Maternal and Child Population Health, National Perinatal Epidemiology Unit, University of Oxford
Professor Ed Juszczak, Non-independent member, Professor of Clinical Trials and Statistics in Medicine, Nottingham Clinical Trials Unit, University of Nottingham. During Trial conduct: Director, National Perinatal Epidemiology Unit Clinical Trials Unit (NPEU CTU), University of Oxford.
Independent DMC
The Data Monitoring Committee (DMC), independent of the applicants and the TSC, reviewed the progress of the trial and interim analysis at least annually. They provided advice on the conduct of the trial to the TSC and (via the TSC) to the NIHR HTA programme.
Membership included the following:
Professor Ben Stenson, Chair, Independent member, Consultant Neonatologist, Simpson Centre for Reproductive Health, Royal Infirmary of Edinburgh
Professor Graeme MacLennan, Independent member, Director, Centre for Health-care and Randomised Trials, University of Aberdeen
Professor Mary Renfrew, Independent member, Professor in Mother and Infant Health, School of Nursing and Health Sciences, University of Dundee.
Contributions of authors
Marian Knight (https://orcid.org/0000-0002-1984-4575) (Professor, Maternal and Child Population Health) wrote the first draft of the manuscript, contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Rema Ramakrishnan (https://orcid.org/0000-0002-6784-8319) (Senior Research Fellow, Statistics) conducted the statistical analysis, contributed to the first draft of the manuscript, contributed to the interpretation of the results, and edited and approved the final version of the manuscript.
Svetlana Ratushnyak (https://orcid.org/0000-0001-7967-5112) (Research Fellow, Health Economics) conducted the economic evaluation, contributed to the first draft of the manuscript, contributed to the interpretation of the results, and edited and approved the final version of the manuscript.
Oliver Rivero-Arias (https://orcid.org/0000-0003-2233-6544) (Associate Professor, Health Economics) conducted the economic evaluation, contributed to the first draft of the manuscript, contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Jennifer Bell (https://orcid.org/0000-0001-9571-0715) (Research Fellow, Statistics) developed the statistical analysis plan, contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Ursula Bowler (https://orcid.org/0000-0002-0100-0155) (Senior Trials Manager, Clinical Trials) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Phyll Buchanan (https://orcid.org/0000-0002-1436-4396) (Public Contributor) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Claire Carter (https://orcid.org/0000-0002-0656-1146) (Infant Feeding Specialist) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Christina Cole (https://orcid.org/0000-0002-8798-2136) (Senior Trials Manager, Clinical Trials) contributed to the first draft of the manuscript, contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Oliver Hewer (https://orcid.org/0000-0002-6251-7174) (Trial Manager, Clinical Trials) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Madeleine Hurd (https://orcid.org/0000-0002-2797-2358) (Data Coordinator, Clinical Trials) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Andy King (https://orcid.org/0000-0001-7175-2718) (Head of Programming, Clinical Trials) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Ed Juszczak (https://orcid.org/0000-0001-5500-2247) (Professor, Statistics) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Louise Linsell (https://orcid.org/0000-0003-3205-6511) (Associate Professor, Statistics) developed the statistical analysis plan, contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Anna-May Long (https://orcid.org/0000-0001-6142-2117) (Consultant, Paediatric Surgery) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Linda Mottram (https://orcid.org/0000-0002-5758-7629) (Midwife, Clinical Trials) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
David Murray (https://orcid.org/0000-0001-9010-2905) (Senior Programmer, Clinical Trials) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Sam Oddie (https://orcid.org/0000-0001-8701-4912) (Consultant, Neonatology) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Maria Quigley (https://orcid.org/0000-0002-8058-6181) (Professor, Statistical Epidemiology) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Victoria Stalker (https://orcid.org/0000-0001-5157-7043) (Trial Manager, Clinical Trials) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Kayleigh Stanbury (https://orcid.org/0000-0002-8726-2411) (Head of Operations, Clinical Trials) contributed to the first draft of the manuscript, contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Richard Welsh (https://orcid.org/0000-0001-9892-7581) (Senior Software Developer, Clinical Trials) contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Pollyanna Hardy (https://orcid.org/0000-0003-2937-8368) (Director, Clinical Trials Unit) conducted the statistical analysis, contributed to the first draft of the manuscript, contributed to the development and conduct of the trial and interpretation of the results, and edited and approved the final version of the manuscript.
Ethics statement
The FROSTTIE trial protocol was approved by South Central – Oxford B Research Ethics Committee (ref. 18/SC/0580) on 10/12/2018 and the Health Research Authority (HRA) and may be found at https://www.npeu.ox.ac.uk/assets/downloads/frosttie/protocol/FROSTTIE_IRAS235355_clinical_research_protocol_v5_29Jul2020_clean_signed.pdf.
Local approval and site-specific assessments were obtained from each NHS hospital site.
Data-sharing statement
Data will be shared in accordance with the National Perinatal Epidemiology Unit Data Sharing policy. Requests for access to the data will be considered by the National Perinatal Epidemiology Unit Data Sharing committee. Access to anonymised data can be requested from general@npeu.ox.ac.uk or by contacting the corresponding author.
FROSTTIE Trial Collaborative Group
Professor Marian Knight, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford. marian.knight@npeu.ox.ac.uk
Professor Ed Juszczak, Nottingham Clinical Trials Unit, University of Nottingham. Ed.Juszczak@nottingham.ac.uk
Ms Phyll Buchanan, PPI representative, The Breastfeeding Network. phyll.buchanan@breastfeedingnetwork.org.uk
Associate Professor Oliver Rivero-Arias, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford. oliver.rivero@npeu.ox.ac.uk
Professor Sam Oddie, Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Trust. sam.oddie@bthft.nhs.uk
Professor Maria Quigley, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford. maria.quigley@npeu.ox.ac.uk
Mrs Claire Carter, Registered Midwife and Infant Feeding Lead, Royal Berkshire NHS Foundation Trust (now retired). claire_carter3@hotmail.co.uk
Dr Anna-May Long, Addenbrooke’s, Cambridge University Hospitals NHS Foundation Trust. anna-may.long@addenbrookes.nhs.uk
Dr Louise Linsell, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford.
Dr Jennifer Bell, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford.
Dr Rema Ramakrishnan, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford.
Dr Pollyanna Hardy, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
Appendix 1 Participating units, principal investigators and site midwives
| Recruiting sites for FROSTTIE | Principal Investigator(s) | Research Staff |
|---|---|---|
| Bradford Royal Infirmary | Sam Oddie | Jenny Eadle, Jennifer Syson |
| Cumberland Infirmary, Carlisle | John Elliott | Rachel Hardy |
| Darlington Memorial Hospital | Mehdi Garbash | Dawn Egginton, Jacqueline Jennings |
| George Eliot Hospital, Nuneaton | Olumuyiwa Olufemi Oso | Michelle Baxter, Alex Dunderdale, Jessica Gunn, Karen Shorthose, Tracy Truslove |
| Great Western Hospital, Swindon | Sarah Bates | KerryAnn Hanks, Kath Townsend |
| Homerton Hospital | Philippa Cox | Lisa Canclini, Angela Chiapparino, Rachel Frowd, Lisa Giacometti, Abigail Laurie |
| Leeds General Infirmary | Kathryn Johnson | Lindsay Uryn, Jackie Mullaney |
| Norfolk and Norwich University Hospital | Ashish Minocha | Louise Coke, Luisa Lyons |
| Queen Alexandra Hospital, Portsmouth | Zoe Garner | Andrew Gribbin, Hayley Nelson, Michelle Pople, Deirdre Rodgers, Amanda Tiller |
| Royal Albert and Edward Infirmary, Wigan | Steve Izzat | Kathryn Ashton, Claire Williams, Michelle Cooper, Jane Davies |
| Royal Berkshire Hospital, Reading | Fidelma Lee | Claire Carter |
| Royal Blackburn Hospital | Bev Hammond, Catharina Schram | Heather Collier, Gary Cousin, Laura Hindle, Louise Hoole, Jennifer McCallum, Matthew Milner, Maire Morton, Frances Pickering, Raheela Rafiq, Jeethendra Rao, Sam White |
| Royal Cornwall Hospital, Truro | Ruth Bowen | Barbara Bromage, Hannah Osborn |
| Royal United Hospital, Bath | Melody Rich | Catherine Bressington, Sara Burnard, Emma James, Annette Moreton, Jennifer Pullen, Sally Tedstone |
| Royal Victoria Infirmary, Newcastle | Paul Ayuk | Andrea Fenn, Lynne McDonald |
| Russells Hall Hospital, Dudley | Subramanian Mahadevan-Bava | Katy Penn, Lisa Williams |
| Stoke Mandeville Hospital, Aylesbury | Eliza Jones | Lisa Frankland, Julie Tebbutt, Danielle Thornton |
| Sunderland Royal Hospital | Lesley Hewitt | Judith Ormonde, Lucy Rowland |
| Worcestershire Royal Hospital | Catherine Townsend | Jessie Brain, Rebecca Davenport, Caroline Payne, Caroline Thunder, Hannah Wood |
Appendix 2 Sources of unit costs (UK British pounds 2017/18) used in the cost-analysis
| Health-care resource use item | Unit cost | Source |
|---|---|---|
| Intervention-specific: Frenotomy | ||
| Frenotomy with complications | £610 | Frenotomy or Frenectomy Day Case (Code CA65Z). NHS Reference Costs 2019/2020 Version 2. |
| Frenotomy performed by doctor | £208 | Weighted average of Frenotomy or Frenectomy Outpatient Procedures across service codes 217 (Paediatric Maxillo-Facial Surgery), 171 (Paediatric Surgery) and 420 (Paediatrics). NHS Reference Costs 2019/2020 Version 2. |
| Frenotomy performed by midwife and when the role of the person performing the procedure is missing | £103 | Frenotomy or Frenectomy Outpatient Procedures, Midwifery Service (Service code 560). NHS Reference Costs 2019/2020 Version 2. |
| Frenotomy performed by other | £86 | Weighted average of Frenotomy or Frenectomy Outpatient Procedures across service codes with the numbers of the procedures <100. NHS Reference Costs 2019/2020 Version 2. |
| Intervention-specific: breastfeeding support | ||
| NHS, phone | £46 | Health Visitor, Other Statutory Contact, Non-Face-to-Face [Code N03J, Community Health Services Health Visiting and Midwifery (HVM)]. NHS Reference Costs 2019/2020 Version 2. |
| NHS, in person | £81 | Health Visitor, Other Statutory Contact, Face-to-Face [Code N03G, Community Health Services Health Visiting and Midwifery (HVM)]. NHS Reference Costs 2019/2020 Version 2. |
| Other, phone and in person | --- | Assumed to be no expenses |
| Primary care | ||
| General practitioner consultation | £39 | Per typical 9.22 min consultation. Section 10.3b, Unit costs of Health and Social Care 2020. |
| General practitioner virtual consultation | £11 | Average of GP face-to-face appointments and GP telephone calls. Section 10.4, Unit costs of Health and Social Care 2020. |
| Practice nurse consultation | £11 | Per typical 15.5 minutes consultation (duration of consultation extracted from Unit Costs of Health and Social Care 2015). Section 10.2, Unit costs of Health and Social Care 2020. |
| Medications | ||
| Course of promethazine hydrochloride assigned to mother`s prescription | £4.9 | Cost of the pack is based on NHS drug tariff price for 25 mg tablets, size 56, one pack for the course. Electronic Drug Tariff March 2020. |
| Course of lamotrigine assigned to mother`s prescription | £17.3 | Cost of the pack is based on NHS drug tariff price for 100 mg tablets (the most prescribed dosage based on Prescription Cost Analysis – England 2020/21), size 56, four packs for the course. Electronic Drug Tariff March 2020. |
| Course of citalopram assigned to mother’s prescription | £2.7 | Cost of the pack is based on NHS drug tariff price for 20 mg tablets (the most prescribed dosage based on Prescription Cost Analysis – England 2020/21), size 28, three packs for the course. Electronic Drug Tariff March 2020. |
| Course of fluoxetine assigned to mother’s prescription | £2.0 | Cost of the pack is based on NHS drug tariff price for 20 mg capsules (the most prescribed dosage based on Prescription Cost Analysis – England 2020/21), size 30, two packs for the course. Electronic Drug Tariff March 2020. |
| Course of sertraline assigned to mother’s prescription | £2.5 | Cost of the pack is based on NHS drug tariff price for 50 mg tablets (the most prescribed dosage based on Prescription Cost Analysis – England 2020/21), size 28, two packs for the course. Electronic Drug Tariff March 2020. |
| Course of flucloxacillin assigned to mother’s prescriptions for ductal infection and mastitis in lactating women | £5.4 | Cost of the pack is based on NHS drug tariff price for 500 mg capsules (the most prescribed dosage based on Prescription Cost Analysis – England 2020/21), size 28, two packs for the course. Electronic Drug Tariff March 2020. |
| Course of co-amoxiclav assigned to mother’s prescriptions for C-section wound infection | £2.5 | Cost of the pack is based on NHS drug tariff price for 500 mg/125 mg tablets (the most suitable dosage based on single dose), size 21, one pack for the course. Electronic Drug Tariff March 2020. |
| Course of amoxicillin assigned to mother’s prescriptions for pericoronitis and acute bronchitis | £1.1 | Cost of the pack is based on NHS drug tariff price for 500 mg capsules (the most prescribed dosage based on Prescription Cost Analysis – England 2020/21), size 15, one pack for the course. Electronic Drug Tariff March 2020. |
| Course of trimethoprim assigned to mother’s prescriptions for low urinary tract infection | £0.4 | Cost of the pack is based on NHS drug tariff price for 200 mg tablets (the most prescribed dosage based on Prescription Cost Analysis – England 2020/21), size 6, one pack for the course. Electronic Drug Tariff March 2020. |
| Course of cefalexin assigned to mother’s prescriptions for sepsis | £2.2 | Cost of the pack is based on NHS drug tariff price for 500 mg capsules (the most prescribed dosage based on Prescription Cost Analysis – England 2020/21), size 21, one pack for the course. Electronic Drug Tariff March 2020. |
| Course of fluconazole assigned to mother’s prescriptions for candida – female genital | £0.9 | Cost of the pack is based on NHS drug tariff price for 150 mg capsules (the most prescribed dosage based on Prescription Cost Analysis – England 2020/21), size 1, one pack for the course. Electronic Drug Tariff March 2020. |
| Course of gentamicin with hydrocortisone assigned to mother’s prescriptions for acute otitis externa | £33.3 | Cost of the pack is based on NHS drug tariff price for Gentamicin 0.3%/Hydrocortisone acetate 1% ear drops, 10 ml, one pack for the course. Electronic Drug Tariff March 2020. |
| Course of amoxicillin assigned to infant’s prescriptions for suspected throat infection and stomach infection | £3.2 | Cost of the bottle is based on NHS drug tariff price for 125 mg/5 ml oral suspension, 100 ml, one bottle for the course. Electronic Drug Tariff March 2020. |
| Course of nystatin assigned to infant’s prescriptions for oral candidiasis | £2.7 | Cost of the pack is based on NHS drug tariff price for 100,000 units/ml oral suspension, 30 ml, one pack for the course. Electronic Drug Tariff March 2020. |
| Course of chloramphenicol assigned to infant’s prescriptions for conjunctivitis | £4.0 | Cost of the pack is based on NHS drug tariff price for 0.5% drops, 10 ml, one pack for the course. Electronic Drug Tariff March 2020. |
| Course of flucloxacillin assigned to infant’s prescriptions for infected eczema and fungal skin infection | £5.7 | Cost of the bottle is based on NHS drug tariff price for 125 mg/5 ml oral suspension, 100 ml, two bottles for the course. Electronic Drug Tariff March 2020. |
| Course of trimethoprim assigned to infant’s prescriptions for vesicoureteral Reflux (VUR) | £5.0 | Cost of the bottle is based on NHS drug tariff price for 50 mg/5 ml oral suspension sugar free, 100 ml, one bottle for the course. Electronic Drug Tariff March 2020. |
| Course of amoxicillin assigned to infant’s prescriptions for bacterial infection | £6.4 | Cost of the bottle is based on NHS drug tariff price for 125 mg/5 ml oral suspension, 100 ml, two bottles for the course. Electronic Drug Tariff March 2020. |
| Course of cefalexin assigned to infant’s prescriptions for urinary infection | £2.0 | Cost of the bottle is based on NHS drug tariff price for 125 mg/5 ml oral suspension, 100 ml, one bottle for the course. Electronic Drug Tariff March 2020. |
| Community care | ||
| Community nurse/midwife contact, virtual | ||
| Mother | £27 | Weighted average of Community Health Services Non-Face-to-Face Nursing (NURS) N02AN and N29AN codes. NHS Reference Costs 2019/2020 Version 2. |
| Community nurse/midwife contact, in person | ||
| Mother | £47 | Weighted average of Community Health Services Health Visiting and Midwifery (HVM) N01P code and Face-to-Face Nursing (NURS) N09AF and N29AF codes. NHS Reference Costs 2019/2020 Version 2. |
| Infant | £84 | Weighted average of Community Health Services Health Visiting and Midwifery (HVM) N01P code and Face-to-Face Nursing (NURS) N29CF code. NHS Reference Costs 2019/2020 Version 2. |
| Infant Health visitor contact, virtual | £46 | Health Visitor, Other Statutory Contact, Non Face-to-Face [Code N03J, Community Health Services Health Visiting and Midwifery (HVM)]. NHS Reference Costs 2019/2020 Version 2. |
| Infant Health visitor contact, in person | £81 | Health Visitor, Other Statutory Contact, Face-to-Face [Code N03G, Community Health Services Health Visiting and Midwifery (HVM)]. NHS Reference Costs 2019/2020 Version 2. |
| Community paediatrician visit | £350 | Weighted average of consultant and non-consultant Face-to-Face Community Paediatrics Outpatient Attendance (Service Code 290) WF01A and WF01B codes. NHS Reference Costs 2019/2020 Version 2. |
| Secondary (hospital-based) care | ||
| Accident & Emergency department visit, mother or infant | £182 | Weighted average of all Emergency Medicine contact codes excluding cases dead on arrival. NHS Reference Costs 2019/2020 Version 2. |
| Hospital outpatient clinic appointment, virtual | ||
| Mother | £72 | Weighted average of Non-Face-to-Face General Medicine and General Surgery Outpatient Attendance (Service Codes 300 and 100) WF01C, WF01D, WF02C, WF02D codes. NHS Reference Costs 2019/2020 Version 2. |
| Hospital outpatient clinic appointment, in person | ||
| Mother | £165 | Weighted average of Face-to-Face General Medicine and General Surgery Outpatient Attendance (Service Codes 300 and 100) WF01A, WF01B, WF02A, WF02B codes. NHS Reference Costs 2019/2020 Version 2. |
| Infant | £231 | Weighted average of Face-to-Face Paediatrics, Paediatric Surgery and Neonatology Outpatient Attendance (Service Codes 420, 171 and 422) WF01A, WF01B, WF02A, WF02B codes. NHS Reference Costs 2019/2020 Version 2. |
| Hospital admission due to mother’s sepsis | £668 | Weighted average of Non-elective Short Stay hospital admissions across codes WJ06A, WJ06B, WJ06C, WJ06D, WJ06E, WJ06F, WJ06G, WJ06H, WJ06J (Sepsis). NHS Reference Costs 2019/2020 Version 2. |
| Hospital admission due to mother’s infusion of rituximab | £1548 | Sum of Follow-up Examination for Other Conditions, with Interventions, Day case admissions, code WH53A and national average unit cost of rituximab, high cost drugs, code PHCD00089. NHS Reference Costs 2019/2020 Version 2. |
| Hospital admissions due to infant’s stomach infection and clostridium difficile | £540 | Weighted average of Non-elective Short Stay hospital admissions across codes PF21A and PF21B (Paediatric, Infectious or Non-Infectious Gastroenteritis). NHS Reference Costs 2019/2020 Version 2. |
| Hospital admissions due to infant’s prolonged jaundice | £517 | Weighted average of Non-elective Short Stay hospital admissions across codes GC18A and GC18B (Non-Obstructive Jaundice). NHS Reference Costs 2019/2020 Version 2. |
| Hospital admissions due to infant’s bronchiolitis | £612 | Weighted average of Non-elective Short Stay hospital admissions across codes PD15A, PD15B, PD15C, PD15D (Paediatric Acute Bronchiolitis). NHS Reference Costs 2019/2020 Version 2. |
| Hospital admissions due to infant’s poor weight gain or weight loss | £558 | Weighted average of Non-elective Short Stay hospital admissions across codes PX57A, PX57B, PX57C (Paediatric, Examination, Follow-up, Special Screening or Other Admissions). NHS Reference Costs 2019/2020 Version 2. |
| Hospital admissions due to infant’s high temperature | £543 | Weighted average of Non-elective Short Stay hospital admissions across codes PW20A, PW20B, PW20C (Paediatric Fever of Unknown Origin). NHS Reference Costs 2019/2020 Version 2. |
| Hospital admissions due to infant’s breathing issues | £651 | Weighted average of Non-elective Short Stay hospital admissions across codes PD12A, PD12B, PD12C (Paediatric, Asthma or Wheezing). NHS Reference Costs 2019/2020 Version 2. |
| Hospital admissions due to infant’s accidental injury | £622 | Weighted average of Non-elective Short Stay hospital admissions across codes PV08A, PV08B, PV31A, PV31B, PV32A, PV32B, PV32C (Paediatric Minor Injury without Intracranial Injury, Paediatric Intermediate Injury without Intracranial Injury, Paediatric Major Injury without Intracranial Injury). NHS Reference Costs 2019/2020 Version 2. |
| Hospital admissions due to infant’s suspected sepsis | £574 | Sepsis without Interventions, with CC Score 0-4, Non-elective Short Stay hospital admissions, code WJ06J. NHS Reference Costs 2019/2020 Version 2. |
| Hospital admissions due to infant’s urinary tract infections | £593 | Weighted average of Non-elective Short Stay hospital admissions across codes PW01A, PW01B, PW01C, PW16A, PW16B, PW16C, PW16D, PW16E, PW17D, PW17E, PW17F, PW17G (Paediatric Minor Infections, Paediatric Intermediate Infections, Paediatric Major Infections). NHS Reference Costs 2019/2020 Version 2. |
| Other health-care professional contacts | ||
| Health visitor visits, mother | £81 | Health Visitor, Other Statutory Contact, Face-to-Face [Code N03G, Community Health Services Health Visiting and Midwifery (HVM)]. NHS Reference Costs 2019/2020 Version 2. |
| Neurologist visits, mother | £220 | Weighted average of consultant and non-consultant led Face-to-Face Neurology Outpatient Attendance (Service Code 400) across codes WF01A and WF01B. NHS Reference Costs 2019/2020 Version 2. |
| Physiotherapist contacts, mother | £42 | Weighted average of consultant and non-consultant led Non-Face-to-Face Physiotherapy Outpatient Attendance (Service Code 650) across codes WF01C and WF01D. NHS Reference Costs 2019/2020 Version 2. |
| Physiotherapist visits, mother | £64 | Weighted average of consultant and non-consultant led Face-to-Face Physiotherapy Outpatient Attendance (Service Code 650) across codes WF01A, WF01B and Physiotherapist, Adult, One to One code A08A1 (Community Health Services, service code AHP). NHS Reference Costs 2019/2020 Version 2. |
| Physiotherapist visits, infant | £68 | Weighted average of consultant and non-consultant led Face-to-Face Physiotherapy Outpatient Attendance (Service Code 650) across codes WF01A, WF01B and Physiotherapist, Child, One to One code A08C1 (Community Health Services, service code AHP). NHS Reference Costs 2019/2020 Version 2. |
| Therapist contacts, mother | £337 | Weighted average of consultant and non-consultant led Non-Face-to-Face Adult Mental Illness (Service Code 710) across codes WF01C, WF01D. NHS Reference Costs 2019/2020 Version 2. |
| Perinatal psychiatrist visits, mother | £223 | Weighted average of Community Contacts (code SPHMSMBUCC) and Outpatient Attendances (code SPHMSMBUOP) Specialist Perinatal Mental Health Services, Mental Health, service code SPMHS. NHS Reference Costs 2019/2020 Version 2. |
| Dietitian contacts, mother | £92 | Dietitian (code A03), Allied Health Professionals (service code AHP), Community Health Services. NHS Reference Costs 2019/2020 Version 2. |
| Ophthalmologists visits, mother | £108 | Weighted average of consultant and non-consultant led Face-to-Face Outpatient Attendances (Ophthalmology, Service Code 130) across codes WF01A, WF01B. NHS Reference Costs 2019/2020 Version 2. |
| Gynaecologist visits, mother | £157 | Weighted average of consultant and non-consultant led Face-to-Face Outpatient Attendances (Gynaecology, Service Code 502) across codes WF01A, WF01B. NHS Reference Costs 2019/2020 Version 2. |
| Allergy paediatrician visits | £255 | Weighted average of consultant and non-consultant led Face-to-Face Outpatient Attendances (Paediatric Clinical Immunology and Allergy Service, Service Code 255) across codes WF01A, WF01B. NHS Reference Costs 2019/2020 Version 2. |
| Consultant orthopaedic nurse visits | £144 | Weighted average of consultant led Face-to-Face Outpatient Attendances (Paediatric Trauma and Orthopaedics, Service Code 214) across codes WF01A, WF01B. NHS Reference Costs 2019/2020 Version 2. |
| Paediatric surgeon visits | £172 | Weighted average of consultant and non-consultant led Face-to-Face Outpatient Attendances (Paediatric Surgery, Service Code 171) across codes WF01A, WF01B. NHS Reference Costs 2019/2020 Version 2. |
| Sonographer visits | £54 | Weighted average of Consultant Led, Non Consultant Led and Outpatient Procedures Ultrasound (non-obstetric) (code DIM007). NHS Reference Costs 2019/2020 Version 2. |
| Radiographer visits | £58 | Weighted average of Consultant Led, Non Consultant Led and Outpatient Procedures Ultrasound (non-obstetric) (code DIM007). NHS Reference Costs 2019/2020 Version 2. |
| Ostheopath visits | £47 | Assumed to be out of pocket expenses. The average cost of £47 as a unit cost for the osteopath session is based on £40 to £55 for a 30- to 40-minute session. https://www.nhs.uk/conditions/osteopathy/ |
| Total (n = 169), n (%) | |
|---|---|
| Frenotomies performed by a private provider | 0 |
| Frenotomies performed by the NHS provider | 140 (82.8) |
| Doctor | 19 (11.2) |
| Midwife | 117 (69.2) |
| Other | 1 (0.6) |
| Missing | 3 (1.8) |
| Frenotomies with complications | 3 (1.8) |
| Frenotomies not performed | 29 (17.2) |
| Frenotomy with breastfeeding support (n = 80) | Breastfeeding support only (n = 89) | Mean difference in number of contacts (95% CI)* | |||
|---|---|---|---|---|---|
| Number of patients (%) | Mean number of contacts (SD) | Number of patients (%) | Mean number of contacts (SD) | ||
| Total breastfeeding support received | 50 (62.5) | 3.64 (2.8) | 60 (67.4) | 3.32 (2.57) | 0.2 (−0.81 to 1.21) |
| NHS contacts | 43 (53.75) | 2.72 (1.65) | 49 (55.0) | 3.16 (2.4) | −0.31 (−1.2 to 0.57) |
| Phone | 26 (60.5) | 2 (0.98) | 32 (65.3) | 1.72 (1.37) | |
| In person | 29 (67.4) | 2.24 (1.64) | 37 (75.5) | 2.7 (2.28) | |
| Missing | 8 (10.0) | --- | 14 (15.73) | --- | |
| Other contacts | 18 (22.5) | 3.61 (2.7) | 15 (16.85) | 2.93 (2.05) | 0.93 (−1.21 to 3.08) |
| Phone | 9 (50.0) | 3.11 (1.96) | 12 (75.0) | 2.33 (1.92) | |
| In person | 14 (77.8) | 2.64 (1.82) | 10 (62.5) | 1.6 (1.35) | |
| Missing | 8 (10.0) | --- | 15 (16.58) | --- | |
| Not received | 22 (27.5) | --- | 14 (15.73) | --- | |
| Resource use category and item | Frenotomy with breastfeeding support (n = 80) | Breastfeeding support only (n = 89) | Mean difference in number of contacts (95% CI)b | ||
|---|---|---|---|---|---|
| Number of patients (%) | Mean number of contacts* (SD) | Number of patients (%) | Mean number of contacts (SD)a | ||
| Primary care | |||||
| General practitioner visits | 42 (52.5) | 1.59 (0.91) | 32 (36.0) | 1.47 (0.91) | 0.07 (−0.39 to 0.53) |
| Missing | 7 (8.75) | --- | 16 (17.98) | --- | |
| Practice nurse visits | 3 (3.75) | 4 (3) | 4 (4.5) | 2 (0.82) | 2.81 (−37.36 to 42.98) |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Medicines, of which | 14 (17.5) | 1.5 (0.94) | 7 (7.9) | 1.57 (0.79) | 0.16 (−0.86 to 1.19) |
| Antibiotics | 13 (16.25) | 1.38 (0.65) | 6 (6.7) | 1.5 (0.84) | |
| For anxiety/depression | 2 (2.5) | 1.5 (0.7) | 2 (2.2) | 1 (0) | |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Community care | |||||
| Community nurse/midwife contacts, of which | 3 (3.75) | 1.67 (0.58) | 2 (2.25) | 1.5 (0.71) | Not possible to calculate |
| Virtual | 0 | - | 1 (1.1) | 1(-) | |
| In person | 3 (3.75) | 1.67 (0.58) | 2 (2.25) | 1 (0) | |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Secondary (hospital-based) care | |||||
| Accident & emergency department visits | 1 (1.25) | 2 (-) | 1 (1.1) | 1 (-) | Not possible to calculate |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Hospital outpatient clinic appointments, of which | 6 (7.5) | 1.67 (0.82) | 2 (2.25) | 2 (1.41) | −0.25 (−1.89 to 1.4) |
| Virtual | 1 (1.25) | 2 (-) | 0 | - | |
| In person | 6 (7.5) | 1.33 (0.52) | 2 (2.25) | 2 (1.41) | |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Hospital admissions | 1 (1.25) | 1 (-) | 1 (1.1) | 1 (-) | Not possible to calculate |
| Length of stay (days) | 1 (-) | 2 (-) | Not possible to calculate | ||
| Missing | 8 (10.0) | --- | 14 (15.7) | --- | |
| Any other NHS health-care professionals contacts | |||||
| Other NHS health-care professional contacts, of which | 7 (8.75) | 2 (0.82) | 5 (5.6) | 2.4 (1.14) | −0.84 (−2.51 to 0.83) |
| Virtual | 2 (2.5) | 2 (0) | 2 (2.25) | 3.5 (0.71) | |
| In person | 7 (8.75) | 1.43 (0.53) | 3 (3.3) | 1.67 (0.58) | |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Any other non-NHS health-care professional contacts | |||||
| Osteopath visits | 0 | - | 2 (2.2) | 2.5 (0.71) | Not possible to calculate |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Resource use category and item | Frenotomy with breastfeeding support (n = 80) | Breastfeeding support only (n = 89) | Mean difference in number of contacts (95% CI)b | ||
|---|---|---|---|---|---|
| Number of patients (%) | Mean number of contacts (SD)a | Number of patients (%) | Mean number of contacts (SD)a | ||
| Primary care | |||||
| General practitioner contacts, of which | 42 (52.5) | 1.5 (0.71) | 37 (41.57) | 1.68 (1.03) | −0.18 (−0.59 to 0.23) |
| Virtual | 0 | - | 1 (1.1) | 1 (-) | |
| In person | 42 (52.5) | 1.5 (0.71) | 37 (41.6) | 1.65 (1.01) | |
| Missing | 7 (8.75) | --- | 17 (19.10) | --- | |
| Practice nurse visits | 5 (6.25) | 1.8 (0.45) | 6 (6.75) | 1.67 (0.52) | 0.11 (−1.24 to 1.45) |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Medicines (antibiotics) | 4 (5.0) | 1 (0) | 6 (6.75) | 1 (0) | |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Community care | |||||
| Community nurse/midwife visits | 1 (1.25) | 8 (-) | 2 (2.2) | 1 (0) | Not possible to calculate |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Infant health visitor contacts, of which | 5 (6.25) | 3.6 (1.67) | 7 (7.9) | 2.0 (1.0) | 2.4 (−0.82 to 5.56) |
| Virtual | 1 (1.25) | 6 (-) | 1 (1.1) | 1 (-) | |
| In person | 4 (5.0) | 3 (1.15) | 7 (7.9) | 1.86 (1.07) | |
| Missing | 8 (10.0) | --- | 15 (16.85) | --- | |
| Community paediatrician visits | 1 (1.25) | 1 (-) | 2 (2.2) | 1 (0) | Not possible to calculate |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Secondary (hospital-based) care | |||||
| Accident & emergency department visits | 4 (5.0) | 1.25 (0.5) | 3 (3.4) | 1 (0) | Not possible to calculate |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Hospital outpatient clinic appointments | 5 (6.25) | 2.4 (3.13) | 2 (2.2) | 1.5 (0.71) | 5.3 (−45.62 to 56.24) |
| Missing | 7 (8.75) | --- | 15 (16.85) | --- | |
| Hospital admissions | 4 (5.0) | 1.75 (1.5) | 9 (10.1) | 1.11 (0.33) | 0.5 (−1.74 to 2.74) |
| Length of stay (days) | 6 (8) | 2 (1) | 4 (−7 to 15) | ||
| Missing | 8 (10.0) | --- | 14 (15.7) | --- | |
| Any other NHS health-care professional visits | |||||
| Other NHS health-care professional visits | 2 (2.5) | 2 (1.41) | 2 (2.2) | 1.5 (0.71) | Not possible to calculate |
| Missing | 8 (10.0) | --- | 17 (19.1) | --- | |
| Any other non-NHS health-care professional visits | |||||
| Osteopath visits | 2 (2.5) | 1.5 (0.71) | 4 (4.5) | 2.0 (0.82) | −1.03 (−7.45 to 5.4) |
| Missing | 8 (10.0) | --- | 17 (19.1) | --- | |
| Resource use category and item | Total (n = 169), mean cost (SD) | |
|---|---|---|
| Mother | Infant | |
| Frenotomy [1] | £106 (£87) | |
| Missing, n (%) | --- | |
| Breastfeeding support | ||
| NHS contacts, phone | £33 (£54) | |
| NHS contacts, in person | £91 (£149) | |
| Total NHS contacts breastfeeding support [2] | £124 (£158) | |
| Missing, n (%) | 22 (13.0) | |
| Primary care | ||
| General practitioner visits | £30 (£39) | £33 (£39) |
| Missing, n (%) | 23 (13.6) | 24 (14.2) |
| Practice nurse visits | £1.5 (£8.2) | £1.4 (£5.2) |
| Missing, n (%) | 22 (13.0) | 22 (13.0) |
| Primary care, total | £32 (£41) | £35 (£40) |
| Missing, n (%) | 24 (14.2) | 25 (14.8) |
| Primary care, total mother/infant pair [3] | £67 (£66) | |
| Missing, n (%) | 25 (14.8) | |
| Medicines | ||
| Antibiotics | £1.1 (£5.8) | £0.3 (£1.1) |
| For anxiety/depression | £0.2 (£1.7) | --- |
| Medicines, total | £1.3 (£7.4) | £0.3 (£1.1) |
| Missing, n (%) | 22 (13.0) | 22 (13.0) |
| Medicines, total mother/infant pair [4] | £1.6 (£7.5) | |
| Missing, n (%) | 22 (13.0) | |
| Community care | ||
| Community nurse/midwife contacts | £2 (£13) | £6 (£56) |
| Missing, n (%) | 22 (13.0) | 22 (13.0) |
| Infant Health Visitor contacts | --- | £16 (£59) |
| Missing, n (%) | --- | 23 (13.6) |
| Community Paediatrician visits | --- | £7 (£50) |
| Missing, n (%) | --- | 22 (13.0) |
| Community care, total | £2 (£13) | £27 (£99) |
| Missing, n (%) | 22 (13.0) | 23 (13.6) |
| Community care, total mother/infant pair [5] | £29 (£99) | |
| Missing, n (%) | 23 (13.6) | |
| Secondary (hospital-based) care | ||
| Accident & emergency department visits | £4 (£33) | £10 (£47) |
| Missing, n (%) | 22 (13.0) | 22 (13.0) |
| Hospital outpatient clinic appointments | £14 (£66) | £24 (£162) |
| Missing, n (%) | 22 (13.0) | 22 (13.0) |
| Hospital admissions | £15 (£139) | £67 (£258) |
| Missing, n (%) | 22 (13.0) | 22 (13.0) |
| Secondary (hospital-based) care, total | £33 (£158) | £101 (£418) |
| Missing, n (%) | 23 (13.6) | 23 (13.6) |
| Secondary (hospital-based) care, total mother/infant pair [6] | £135 (£451) | |
| Missing, n (%) | 23 (13.6) | |
| Any other NHS health-care professional contacts | ||
| Other NHS health-care professional contacts, total | £22 (£121) | £5 (£36) |
| Missing, n (%) | 22 (13.0) | 22 (13.0) |
| Other NHS health-care professional contacts, total mother/infant pair [7] | £27 (£130) | |
| Missing, n (%) | 22 (13.0) | |
| Any other non-NHS health-care costs | ||
| Payment for anything to help breastfeeding | £7 (£26) | --- |
| Missing, n (%) | 31 (18.0) | |
| Non-NHS health-care professional visits, osteopath | £2 (£14) | £4 (£18) |
| Missing, n (%) | 22 (13.0) | 25 (15.0) |
| Other non-NHS health-care costs, total mother/infant pair [8] | £7 (£26) | |
| Missing, n (%) | 34 (20.0) | |
| Total maternal/infant costs [1] + [2] + [3] + [4] + [5] + [6] + [7] | £217 (£293) | £274 (£580) |
| Missing, n (%) | 26 (15.4) | 27 (16.0) |
| Total cost mother/infant pair [1] + [2] + [3] + [4] + [5] + [6] + [7] | £490 (£707) | |
| Missing, n (%) | 28 (16.6) | |
| Mobility | Self-care | Usual activities | Pain/discomfort | Anxiety/depression | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up point | Frenotomy w/ breastfeeding support (n = 80) | Breastfeeding support (n = 89) | Frenotomy w/ breastfeeding support (n = 80) | Breastfeeding support (n = 89) | Frenotomy w/ breastfeeding support (n = 80) | Breastfeeding support (n = 89) | Frenotomy w/ breastfeeding support (n = 80) | Breastfeeding support (n = 89) | Frenotomy w/ breastfeeding support (n = 80) | Breastfeeding support (n = 89) |
| Baseline (trial entry) | ||||||||||
| No problems | 57 (74) | 66 (76) | 65 (83) | 77 (88.5) | 50 (64.1) | 50 (58.1) | 23 (29.5) | 24 (28) | 49 (62.8) | 47 (54) |
| Slight problems | 15 (19.5) | 17 (19.5) | 12 (15.5) | 8 (9.2) | 18 (23.1) | 21 (24.4) | 36 (46) | 38 (44) | 16 (20.5) | 27 (31) |
| Moderate problems | 5 (6.5) | 4 (4.5) | 1 (1.5) | 2 (2.3) | 9 (11.5) | 12 (14) | 17 (22) | 18 (21) | 10 (12.8) | 11 (12.7) |
| Severe problems | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (2.3) | 2 (2.5) | 6 (7) | 3 (3.9) | 2 (2.3) |
| Unable to | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.3) | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 77 (100) | 87 (100) | 78 (100) | 87 (100) | 78 (100) | 86 (100) | 78 (100) | 86 (100) | 78 (100) | 87 (100) |
| χ2 (p-value) | 0.2860 | 0.867 | 1.6615 | 0.436 | 2.2745 | 0.685 | 1.7177 | 0.633 | 2.6201 | 0.454 |
| Missing | 3 (3.7) | 2 (2.3) | 2 (2.5) | 2 (2.3) | 2 (2.5) | 3 (3.4) | 2 (2.5) | 3 (3.4) | 2 (2.5) | 2 (2.3) |
| Routine follow-up (approximately one to 2 weeks post-trial entry) | ||||||||||
| No problems | 66 (85.7) | 66 (76) | 70 (90.9) | 75 (86.2) | 54 (70) | 61 (70) | 42 (55.3) | 41 (47) | 360 (50) | 58 (66.7) |
| Slight problems | 7 (9.1) | 21 (24) | 5 (6.5) | 11 (12.6) | 16 (20.8) | 19 (21.8) | 28 (36.8) | 39 (45) | 313 (44) | 25 (28.7) |
| Moderate problems | 4 (5.2) | 0 (0) | 2 (2.6) | 1 (1.2) | 6 (7.8) | 6 (7) | 5 (6.5) | 7 (8) | 44 (6) | 2 (2.3) |
| Severe problems | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.4) | 1 (1.2) | 1 (1.3) | 0 (0) | 0 (0) | 2 (2.3) |
| Unable to | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 77 (100) | 87 (100) | 77 (100) | 87 (100) | 77 (100) | 87 (100) | 76 (100) | 87 (100) | 77 (100) | 87 (100) |
| χ2 (p-value) | 10.429 | 0.005 | 2.154 | 0.341 | 0.0737 | 0.995 | 2.42 | 0.49 | 5.0758 | 0.166 |
| Missing | 3 (3.7) | 2 (2.3) | 3 (3.7) | 2 (2.3) | 3 (3.7) | 2 (2.3) | 4 (5) | 2 (2.3) | 3 (3.7) | 2 (2.3) |
| 3 months after birth | ||||||||||
| No problems | 63 (86.3) | 68 (90.3) | 66 (90.5) | 71 (95) | 59 (80.8) | 62 (82.7) | 46 (63) | 48 (64) | 44 (60.3) | 49 (65) |
| Slight problems | 6 (8.3) | 6 (8) | 5 (6.8) | 4 (5) | 10 (13.7) | 8 (10.7) | 20 (27.4) | 22 (29) | 22 (30) | 18 (24) |
| Moderate problems | 2 (2.7) | 1 (1.3) | 2 (2.7) | 0 (0) | 3 (4.1) | 5 (6.7) | 4 (5.5) | 3 (4) | 5 (7) | 8 (11) |
| Severe problems | 2 (2.7) | 0 (0) | 0 (0) | 0 (0) | 1 (1.4) | 0 (0) | 3 (4.1) | 2 (3) | 2 (2.7) | 0 (0) |
| Unable to walk | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 73 (100) | 75 (100) | 73 (100) | 75 (100) | 73 (100) | 75 (100) | 73 (100) | 75 (100) | 73 (100) | 75 (100) |
| χ2 (p-value) | 2.4976 | 0.476 | 2.267 | 0.322 | 1.7699 | 0.622 | 0.4537 | 0.929 | 3.3347 | 0.343 |
| Missing | 7 (8.7) | 14 (15.7) | 7 (8.7) | 14 (15.7) | 7 (8.7) | 14 (15.7) | 7 (8.7) | 14 (15.7) | 7 (8.7) | 14 (15.7) |
Appendix 3 Summary of changes to the study protocol
| Amendment no. | Protocol version no. | Date issued | Author(s) of changes | Details of changes made |
|---|---|---|---|---|
| N/A | 1 | 27/09/18 | Initial version submitted to REC. | |
| N/A | 2 | 06/12/18 | Changes made on behalf of PMG | Amendments made in accordance with NIHR (funder) and REC recommendations. Additional minor corrections to ensure consistency throughout. |
| N/A | 3 | 25/03/19 | Changes made on behalf of PMG | Amendments made to exclusion criteria and SAE reporting; and minor corrections to wording in sections 3, 7 and 10. |
| N/A | 4 | 28/06/19 | Changes made on behalf of PMG | Masking removed from study design as per NIHR (funder) request. Adverse event data collection updated. Minor clarification to number of recruiting centres needed. Authorship criteria clarified. Minor changes to grammar for clarification. |
| N/A | 5 | 28/07/2020 | Changes made on behalf of PMG | Virtual assessments and breastfeeding support, and virtual BTAT assessment permitted within trial. Verbal consent permitted if written consent is not possible. COVID-19 status of mother and baby collected. Minor corrections to references. |
List of abbreviations
- aRR
- adjusted risk ratio
- BTAT
- Bristol Tongue Assessment Tool
- CACE
- complier-average causal effect
- CI
- confidence interval
- CTU
- Clinical Trials Unit
- CUA
- cost-utility analysis
- DMC
- Data Monitoring Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- NICE
- National Institute for Health and Care Excellence
- NPEU
- National Perinatal Epidemiology Unit
- PPI
- patient and public involvement
- QALDs
- quality-adjusted life-days
- QALYs
- quality-adjusted life-years
- RCT
- randomised controlled trial
- RR
- risk ratio
- SAE
- serious adverse event
- SD
- standard deviation
- SF-MPQ
- Short Form McGill Pain Questionnaire
- TSC
- Trial Steering Committee
