Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/129/261. The contractual start date was in October 2012. The draft report began editorial review in January 2020 and was accepted for publication in June 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Christian et al. This work was produced by Christian et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Christian et al.
Chapter 1 Introduction
Material throughout the report has been adapted from the trial protocol by Webb et al. 1 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Trial rationale/introduction
Minimal change nephrotic syndrome is the most common glomerular disease of childhood. 2 The presenting episode is treated with high-dose oral prednisolone and it is expected that > 90% of children will have a complete response, with responders receiving the diagnostic label of steroid-sensitive nephrotic syndrome (SSNS). 2 The optimum duration of prednisone/prednisolone therapy at presentation was recommended by the International Study of Kidney Disease in Children as 60 mg/m2 daily for 4 weeks, followed by 40 mg/m2 on alternate days for 4 weeks. 3 Subsequent trials showed benefits with longer durations of corticosteroid;4 however, in the last decade, four well-designed randomised controlled trials have demonstrated no clinical benefit to an extended course of prednisolone beyond the accepted 8- to 12-week course. 5–8 The most recent of these was the PREDNOS (PREDnisolone in NephrOtic Syndrome) trial, which was undertaken by this trial group. Although the PREDNOS trial demonstrated no clinical benefit to an extended corticosteroid course, it did show evidence of cost-effectiveness. 9
Following successful initial treatment, at least 80% of children develop disease relapses necessitating further courses of high-dose prednisolone. The PREDNOS trial found that 80% of children relapse within 12 months of initial presentation8 and around 50% develop frequently relapsing disease. 10 Long-term low-dose maintenance prednisolone therapy is the most commonly prescribed therapy to reduce relapse frequency, although a number of children will require additional immunosuppressive agents, including levamisole, cyclophosphamide, ciclosporin, tacrolimus, mycophenolate mofetil (MMF) and rituximab.
Nephrotic syndrome relapses are associated with a risk of significant complications, including sepsis, thrombosis, dyslipidaemia and malnutrition. 11 The treatment of relapses with high-dose prednisolone is associated with major short-12 and long-term13 adverse effects, including hip avascular necrosis, hypertension, diabetes and behavioural problems. This places financial pressure on the health-care system and leads to reduced quality of life. Furthermore, children are kept off school during relapses, resulting in impaired education performance and parental absence from work.
It is well recognised that the majority of relapses are precipitated by viral upper respiratory tract infection (URTI). Alwadhi et al. 14 followed 68 Indian children with 76 initial presentations or relapses of nephrotic syndrome over 12 months. Of 68 episodes of nephrosis that occurred while not taking corticosteroid, there was an infectious trigger in 57 (84%). The range of infections included URTI (28%), lower respiratory tract infection (a further 19%) and other causes, including urinary tract infection (23%), peritonitis (16%) and diarrhoeal illness (11%). Arun et al. 15 carried out a study designed to evaluate the effectiveness of supplemental zinc in reducing relapse rate in Indian children with nephrotic syndrome. They reported subgroup data for children with frequently relapsing disease. Within that subgroup, 52 out of 86 relapses (60%) were preceded by infections. MacDonald et al. 16 followed 32 Canadian children with nephrotic syndrome over two successive winters. There were 61 URTIs over that period and 41 exacerbations (29 full relapses) of nephrotic syndrome. Seventy-one per cent of exacerbations (69% of full relapses) were associated with URTIs within the prior 10 days.
Furthermore, in children with frequently relapsing steroid-sensitive nephrotic syndrome (FRSSNS), the development of an URTI frequently precipitates a relapse. Moorani et al. 17 documented infections in 62 Pakistani children with nephrotic syndrome over 12 months. A total of 74 infections were observed. Acute respiratory infections were the most common infection (29%). Nephrotic syndrome relapse or initial presentation occurred with 78% of infections or with 80% of acute respiratory infections. In their Canadian cohort, MacDonald et al. 16 demonstrated 47.5% of URTIs associated with disease exacerbation or 32.8% associated with overt relapse.
Given these strong links between viral URTI and relapse, and the morbidity and cost associated with relapse and its treatment, it is logical that attempts are made to ameliorate the URTI-driven disease modification.
Summary of previous studies investigating the use of daily prednisolone therapy at the time of upper respiratory tract infection
Current practice in the majority of UK centres has been for no change to be made to immunosuppressive therapy at the time of development of an URTI. Between 2000 and 2011, three studies18–20 assessed whether or not the use of daily prednisolone at the time of URTI reduced the subsequent risk of relapse of nephrotic syndrome.
Mattoo and Mahmoud18 published the first of these trials in 2000. Mattoo and Mahmoud18 studied 36 Saudi children with relapsing SSNS who were receiving a long-term maintenance dose of alternate-day prednisone of approximately 0.5 mg/kg per day. Starting on the day of onset of URTI (defined by onset of cough and/or cold with or without fever), children were alternately assigned in an unblinded manner to receive either 5 days of daily prednisone at the same dose or to remain on alternate-day prednisone. Patients who did not relapse and patients who did not achieve remission with cyclophosphamide were excluded. The number of disease relapses [defined as Albustix (Siemens Healthcare Diagnostics Ltd, Frimley, UK) 3+ positivity on morning urinalysis for 3 days] was documented in each group. Patients were followed for 2 years and the main reported outcome was the mean number of relapses per patient over that period for each arm.
The arms were well matched for age, sex, use of prior cyclophosphamide and histology (where performed). In the 18 children assigned to daily prednisone at the time of URTI, the rate of relapse was lower than in the 18 children who continued on alternate-day prednisone [mean ± standard deviation (SD) relapse rate 2.2 ± 0.87 vs. 5.5 ± 1.33; p = 0.04]. No data on the individual risk of relapse following URTI were reported. The intervention arm received a median of seven courses of daily prednisone over the 2-year follow-up period. No difference was noted in the frequency of hospitalisations or the length of treatment for relapses between the two treatment arms.
Abeyagunawardena and Trompeter19 recruited 48 Sri Lankan children to a randomised double-blind crossover study. All were receiving long-term low-dose alternate-day prednisolone (mean 0.36 mg/kg, range 0.1–0.6 mg/kg). Children were studied over two consecutive URTIs (defined as cough, runny nose, sore throat, lethargy, body aches and fever). Throat swabs were taken and children with bacterial infection were excluded. Children were randomised, using sealed envelopes, either to receive prednisolone at their usual maintenance dose for 7 days given daily instead of on alternate days or to continue on alternate-day prednisolone. This was achieved by dispensing investigational medicinal product (IMP) in one of two containers, one of which contained prednisolone and the other placebo. Parents were asked to administer the study drug on the child’s usual non-treatment day and to continue so that a total of 7 days of daily treatment were given. In this way, children received three or four doses of study drug.
In the crossover design, those who received daily prednisolone for the first URTI received alternate-day prednisolone plus placebo for the second URTI, and vice versa. Recruitment continued until 40 children had experienced two URTIs. Children were reviewed on days 3 and 7 to assess for evidence of disease relapse (defined as Albustix 3+ proteinuria for 3 consecutive days). Those who developed prednisolone-related adverse events (AEs), those who required steroid-sparing therapy for frequent relapses, those in whom prednisolone was discontinued because of sustained remission and those who did not have two viral infections were all excluded from the study (8/48 of the recruited number). The main reported outcome was the difference in infection-associated relapse (IAR).
Overall, there were seven (18%) IARs following 40 URTIs treated with daily prednisolone and 19 (48%) IARs following 40 URTIs where alternate-day prednisolone and placebo were administered (p = 0.014; two-sided Fisher’s exact test). Response to the initial URTI was a relapse in 4 of 18 (22%) children in the intervention arm and 10 of 22 (45%) children in the placebo arm (no significance reported). No significant adverse effects were encountered.
The third and largest of these three initial studies was performed in 100 Indian children recently diagnosed with FRSSNS who were on long-term alternate-day prednisolone with or without levamisole. 20 Children were recruited in stable remission, having received alternate-day 1.5 mg/kg of prednisolone for 4 weeks and then tapered by 0.25 mg/kg every 2 weeks until a dose of 0.5–0.75 mg/kg on alternate days was reached. If a dose of prednisolone > 1 mg/kg on alternate days was required, then levamisole was added. Children were randomised, stratified according to whether they received levamisole (n = 32) or not (n = 68), to either receive daily prednisolone for 7 days or remain on alternate-day prednisolone. Prednisolone was given at the same dose for non-treatment-days in an unblinded manner at the time of development of intercurrent infection [defined as fever (i.e. axillary-measured temperature of > 38 °C on two occasions and more than 1 hour apart), rhinorrhoea or cough for more than 1 day or diarrhoea (i.e. three or more semiformed stools per day for more than 2 days)]. Children were reviewed every 2 months for a total of 12 months.
The primary end point was the incidence of IAR, with secondary end points of overall relapse rate, infection frequency and type and cumulative dose of prednisolone. Patients exited the study if there were two or more relapses in any 6-month period. Daily prednisolone therapy at the time of an URTI resulted in a reduction in the incidence of IAR [0.7 ± 0.3 vs. 1.4 ± 0.5; rate difference 0.7, 95% confidence interval (CI) 0.3 to 1.1; p < 0.01] and the overall relapse rate (0.9 ± 0.4 vs. 1.8 ± 0.5; rate difference 0.9, 95% CI 0.4 to 1.4; p < 0.0001). Although not powered to do so, a subanalysis showed that this difference was lost in those receiving levamisole. Nineteen children in the daily prednisolone group, compared with seven in the alternate-day group, remained relapse free over the entire 12-month study period (p = 0.03). There was no difference in cumulative prednisolone dose between the two treatment arms. More infections occurred in the daily prednisolone group (226 vs. 161; p = 0.04), although no difference was detected in height SD score, cushingoid features, cataract or serious infection. Six children (two in the daily steroid group) exited the study because of treatment failure, necessitating treatment with cyclophosphamide or calcineurin inhibitors.
Critique of previous studies investigating the use of daily prednisolone therapy at the time of upper respiratory tract infection and summary of findings
Methodological aspects
Sample size
All of the studies that predate PREDnisolone in NephrOtic Syndrome 2 (PREDNOS2) were small (involving 36–100 children). 18–20 Mattoo and Mahmoud18 did not include a formal sample size calculation. Abeyagunawardena and Trompeter’s19 sample size was based on an observation that nearly 50% of URTIs are followed by a relapse and an assumption that the increase in the maintenance dose of prednisolone would reduce the relapse rate by 50%; however, no details of the power calculation were reported. Only Gulati et al.,20 the largest study, included details of their power calculation, which was based on a relapse rate of 4.6 ± 1.4 relapses per year in patients with frequent relapses (a figure that had been reported in a similar population). 15 Gulati et al. 20 assumed that 70% of relapses follow infections and calculated their sample size on a 50% reduction in frequency of IARs at a power of 80%, an alpha error of 5% and a dropout rate of 10%.
Target population
All three studies18–20 included children with relapsing SSNS receiving long-term alternate-day maintenance corticosteroid treatment. In addition, in Gulati et al. ’s20 study, some children were on alternate-day maintenance treatment with both prednisolone and levamisole. Mattoo and Mahmoud’s18 population was a heterogeneous group that included those with relapsing disease (not formally defined as FRSSNS) or those who had previously received cyclophosphamide for steroid-resistant or frequently relapsing disease and who then, according to local protocol, continued maintenance alternate-day corticosteroid for 2 years. Abeyagunawardena and Trompeter’s19 population had steroid-dependent nephrotic syndrome, implicitly defined because all were taking maintenance corticosteroid. Gulati et al. ’s20 subjects had carefully defined FRSSNS (i.e. more than two relapses in the previous 6 months or more than three relapses in the previous 12 months).
Some studies excluded patients prior to randomisation, which limits the generalisability of results. In Gulati et al. ’s20 population, children with evidence of corticosteroid toxicity, use of non-steroid immunosuppression in the 6 months prior to recruitment and children requiring maintenance prednisolone of > 1 mg/kg on alternate days were excluded.
Study design
In all three studies,18–20 patients were asked to modify maintenance treatment at the time of an infection or URTI for a period of 5–7 days. In the blinded study of Abeyagunawardena and Trompeter,19 this was to commence the trial drug on non-corticosteroid days. In the unblinded studies,18,20 this was to take their maintenance corticosteroid every day instead of every other day.
The definitions of infection/URTI used for the three studies are shown in Table 1.
| Symptom | Study | ||
|---|---|---|---|
| Mattoo and Mahmoud18 | Abeyagunawardena and Trompeter19 | Gulati et al.20 | |
| Duration | Not defined | Not defined | For at least 24 hours |
| Definition | Any one of: | Any three of: | Any one of: |
| Cough | ✓ | ✓ | ✓ |
| Cold, rhinorrhoea, runny nose | ✓ | ✓ | ✓ |
| Fever | ✓ | ✓ | |
| Sore throat or food refusal in younger child | ✓ | ||
| Body aches | ✓ | ||
| Diarrhoea | ✓ | ||
| Other | Bacterial infection excluded | ||
In Abeyagunawardena and Trompeter’s19 study only was there any blinding of patients or researchers. In their crossover design, the recruited patients’ families were provided with two pots of tablets. Pot A contained prednisolone tablets and pot B contained a placebo. Patients were randomised at the start of their first URTI, using sealed envelopes, to use tablets from either pot A or pot B on non-prednisolone days for 7 days. The families were asked to use tablets from the other pot for the second URTI. Although technically double blinded, it is highly possible that researchers could find out which tablet pot was being used. The other two studies were not blinded and patients in the intervention arms took their same maintenance prednisolone dose every day for 5 days (in Mattoo and Mahmoud18) or 7 days (in Gulati et al. 20). There was no placebo controlling for the non-intervention arm for these two studies.
Abeyagunawardena and Trompeter’s19 use of a crossover design, although attempting to use each patient as their own control, may have resulted in the first treatment course influencing the second. Abeyagunawardena and Trompeter19 also required each patient to have two URTIs (which was not the case for three patients).
Randomisation and allocation
Mattoo and Mahmoud18 allocated treatment groups on an alternate basis, which could have introduced selection bias due to the lack of allocation concealment. In Abeyagunawardena and Trompeter’s19 study, patients were randomised at the start of their first URTI using sealed envelopes. Gulati et al. ’s20 patients were randomised by stratified randomisation (with or without levamisole) using opaque sealed envelopes.
Post-randomisation exclusions
Potential bias was introduced by the post-randomisation exclusion of patients from the analysis populations. Eight (16.7%) of Abeyagunawardena and Trompeter’s19 initial patients were excluded from the final analysis. Reasons cited included a need for treatment escalation (n = 4), disease stability leading to discontinuation of maintenance prednisolone (n = 1) and no second URTI (n = 3). Mattoo and Mahmoud18 also excluded children who did not relapse over the 2-year study period, implying a post-randomisation exclusion. Gulati et al. 20 considered those patients who relapsed twice or more in a 6-month period and required escalation of background treatment as treatment failures, but these patients were included in the analysis.
Outcome measures
Primary outcomes were not uniform among the three studies. Mattoo and Mahmoud18 studied the rate of relapses between the two arms. Abeyagunawardena and Trompeter19 assessed IAR following a first infection; however, patients were studied over the course of only two relapses that received different treatments because of the crossover design. In addition, there were no longer-term outcomes. Gulati et al. 20 assessed IAR expressed as episodes per patient per year.
Adverse events
No AE data were formally reported in the studies by Mattoo and Mahmoud18 and Abeyagunawardena and Trompeter. 19 However, in the latter, evidence of corticosteroid toxicity was a reason for maintenance treatment escalation and withdrawal from the study. The presence of cushingoid features, cataracts and the requirement for hospital admission were reported for each arm in Gulati et al. ’s20 study. Despite the high level of importance ascribed to the behavioural effects of corticosteroid,12,21 no study assessed this.
Generalisability of results
The three studies18–20 published prior to PREDNOS2 that reported a possible benefit of increasing prednisolone dose and reducing the risk of relapse were in children taking long-term alternate-day prednisolone. Therefore, there was no evidence to support a role for a short course of daily low-dose prednisolone at the time of an URTI for children not on long-term alternate-day prednisolone or, indeed, for children taking non-steroid second-line agents.
All three studies took place in specific georacial population groups and their definitions of URTI varied. Moreover, for one study,20 this was more broadly defined as ‘infection’ and included children with diarrhoeal illness. It is not appropriate to assume that the pattern of intercurrent illness in these populations can be compared with those in more temperate locations and that each type of infection carries the same risk of precipitating a relapse of nephrotic syndrome.
There was also no systemic evaluation of AEs for each arm to assess the risk/benefit of the intervention, nor was there any evaluation undertaken of cost-effectiveness.
Subsequent research
Since commencement of the PREDNOS2 trial, a second trial from Abeyagunawardena et al. 22 has been published. In contrast to the group’s previous study19 and the other two studies18,20 prior to PREDNOS2, the investigators studied children with SSNS on no maintenance therapy. In a double-blind placebo-controlled crossover study, 48 children were randomised to receive 5 days of daily prednisolone (0.5 mg/kg) or placebo at the start of an URTI. Children on second-line treatments were excluded and children also had to have discontinued corticosteroid treatment at least 3 months prior to recruitment. A sealed envelope method was used to randomise patients in the same way as the group’s previous study. 19 Both investigators and patients/parents were blinded to the contents of the pot until the end of the study. Viral infections were defined more loosely than for the previous study, with the presence of two or more of the following criteria: fever > 38 °C; runny nose; cough; body aches, lethargy or loss of appetite; or sore throat. Study treatment was not given or was subsequently stopped if microbiological evidence of a bacterial infection was found. Patients were followed for 12 months, with any subsequent URTIs treated with the same study drug. Thereafter, a crossover took place, with group 1 taking placebo and group 2 taking prednisolone at the time of an URTI for the next 12 months.
A sample size matching their previous study appears to have been chosen, but no power calculation was provided. Investigators recruited 27 children who were randomised to group 1 and 21 children randomised to group 2. Of the 48 children recruited, only 33 (69%) completed the study (19 children in group 1 and 14 children in group 2). Of those exclusions, 12 children were because of non-compliance and three children needed treatment escalation in the form of maintenance immunosuppressive therapy.
There were 11 relapses following 115 episodes of URTI (9.5%) in the treatment group and 25 relapses following 101 episodes of URTI (24.8%) in the control group. Despite no significant differences in the numbers of URTIs between groups, children in the treatment group had significantly fewer relapses than children in the control group (p = 0.014). Within the treatment group, 22 of 33 children did not relapse compared with 14 of 33 children within the placebo group (p = 0.049).
The IAR in this study, at 25%, was half of that reported in earlier studies. 19,20 The authors explain that this is likely because the study was conducted in children with more stable disease, who do not require long-term prophylactic treatments. Interestingly, the only relapses reported within the study period were those following URTIs.
In common with their previous study,19 the sample size is small and the study was vulnerable to inadvertent unblinding. The high dropout rate weakens the findings and the decision to exclude children who commenced maintenance treatment introduces a potential bias. In addition, along with the studies prior to PREDNOS2, generalisability of the findings to a different georacial group is problematic.
Summary
In the 2015 update of the Cochrane review of corticosteroid treatment for nephrotic syndrome in children,4 the authors noted that the combined weight of the three studies described above18–20 increased the power of the analysis so that low-dose daily prednisolone may be considered at the time of viral infections in children on maintenance alternate-day prednisolone. Abeyagunawardena et al. ’s22 subsequent study, which was available in abstract form only at the time of the Cochrane review, has shown that the same strategy may benefit children on no maintenance treatment. In the 2020 Cochrane update,4 the authors concluded that the limited data provided by a single study of 48 children meant that it remains unclear whether or not children not already on alternate day corticosteroid should restart daily corticosteroid for around a week at the onset of viral infections.
The review authors also stated that there were some concerns about the possibility of bias4 (Table 2). Mattoo and Mahmoud’s study18 was rated as having a high risk of selection bias through inadequate random sequence generation and allocation concealment. Only the two studies by Abeyagunawardena et al. 19,22 were blinded studies. The study by Mattoo and Mahmoud18 reported that fewer than 10% of participants were lost to follow-up or excluded from the analysis. Three of the studies18,19,22 were rated as having a high risk of reporting bias due to a combination of outcome data not including one or more outcomes of FRSSNS, relapse rate and AEs, providing data in a format that could not be entered into the meta-analyses or from inability to separate first and second parts in crossover studies.
| Study | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other bias |
|---|---|---|---|---|---|---|---|
| Mattoo and Mahmoud18 | – | – | – | – | + | – | ? |
| Abeyagunawardena and Trompeter19 | + | + | + | + | – | – | + |
| Gulati et al.20 | + | + | – | – | – | + | + |
| Abeyagunawardena et al.22 | + | + | + | + | – | – | ? |
Therefore, although providing proof of concept that increased corticosteroid dosing may reduce the risk of relapses in children with SSNS, the methodological issues and other limitations of all these studies have not been able to address the following questions:
-
Do children from developed temperate countries where the pattern of childhood URTI is significantly different benefit from the same intervention?
-
Does this effect occur in children receiving long-term maintenance therapy with other immunosuppressive therapies (e.g. levamisole, ciclosporin, tacrolimus and MMF) in conjunction with prednisolone or without prednisolone or, indeed, does this effect occur in children on no long-term immunosuppressive therapy?
-
Is there an effect on the cumulative dose of prednisolone or in the incidence of corticosteroid-related adverse effects, including behavioural issues associated with the use of this intervention?
-
What are the quality-of-life implications of this strategy?
-
What would be the cost-effectiveness of such an approach?
Research question
A meeting was convened in January 2012 to discuss this trial proposal, to which the members of the National Institute for Health Research (NIHR) Medicines for Children Research Network Nephrology Clinical Studies Group, which represents each of the 13 tertiary paediatric nephrology centres in the UK, were invited to attend. A number of consumer representatives, including the chairperson of the UK Nephrotic Syndrome Trust (NeST) (Somerset, UK), and other parents of children with nephrotic syndrome also attended.
It was clear from this meeting that interest lay not only in further generalisability of the use of daily low-dose prednisolone at the time of an URTI in children on alternate-day prednisolone and children on no maintenance treatment, but also in those receiving other immunosuppressant therapies (with or without prednisolone) for their nephrotic syndrome. Therefore, a single pragmatic trial was proposed, which would include all patients with relapsing SSNS, regardless of background therapy, to answer the overarching question of whether or not treatment with a short course of daily prednisolone at the time of URTI in children with relapsing SSNS reduced the subsequent development of nephrotic syndrome relapse.
A reduction in the nephrotic syndrome relapse rate would reduce relapse and treatment-associated morbidity, hospitalisation rates and parental time absent from work.
The research question agreed was ‘Does a 6-day course of daily prednisolone given early in the course of URTI in children with relapsing SSNS effectively and safely reduce the incidence of subsequent URTI-related relapse?’.
A 6-day course was chosen as an even number of days of administration of trial drug would be required to avoid the bias from children already taking alternate-day prednisolone who might receive different dosing of trial drug depending on whether or not it was commenced on a day on which they usually took prednisolone.
Chapter 2 Methods
Trial-related information, including the protocol, trial information sheets, consent and assent forms and the case report forms, are available at the PREDNOS2 website [URL: www.birmingham.ac.uk/research/bctu/trials/renal/prednos2/index.aspx (accessed 17 December 2020)].
Aim
The aim was to evaluate the effectiveness of a 6-day course of daily prednisolone therapy at the time of URTI in reducing the development of subsequent nephrotic syndrome relapse in children with relapsing SSNS.
Objectives
The specific trial objectives were to determine whether or not a 6-day course of oral prednisolone given at the time of an URTI:
-
reduces the incidence of first upper respiratory tract infection-related relapse (URR) in children with relapsing SSNS
-
reduces the overall rate of URR in children with relapsing SSNS
-
reduces the overall rate of relapse in children with relapsing SSNS
-
reduces the cumulative dose of prednisolone over the 12-month study period
-
reduces the incidence and prevalence of adverse effects of prednisolone, including behavioural abnormalities
-
reduces the number of children undergoing escalation of background immunosuppressive therapy
-
increases the number of children undergoing reduction of background immunosuppressive therapy
-
is more cost-effective than standard therapy
-
improves quality of life as measured using the Child Health Utility 9D (CHU-9D), EuroQol-5 Dimensions (EQ-5D) and the Pediatric Quality of Life Inventory (PedsQL).
Trial design
The PREDNOS2 trial was a Phase III randomised parallel-arm placebo-controlled double-blind trial that compared a 6-day course of daily prednisolone with no change in therapy using a matching placebo. Children with relapsing nephrotic syndrome who met eligibility criteria were randomised in a 1 : 1 ratio (with minimisation according to background treatment for nephrotic syndrome) to receive either 6 days of prednisolone or 6 days of placebo at the time of an URTI. The participant, clinician and trial teams were masked to treatment allocation.
The trial protocol for PREDNOS2 was published in open access format in 2014. 1
The trial schema is shown in Figure 1.
FIGURE 1.
Trial schema.
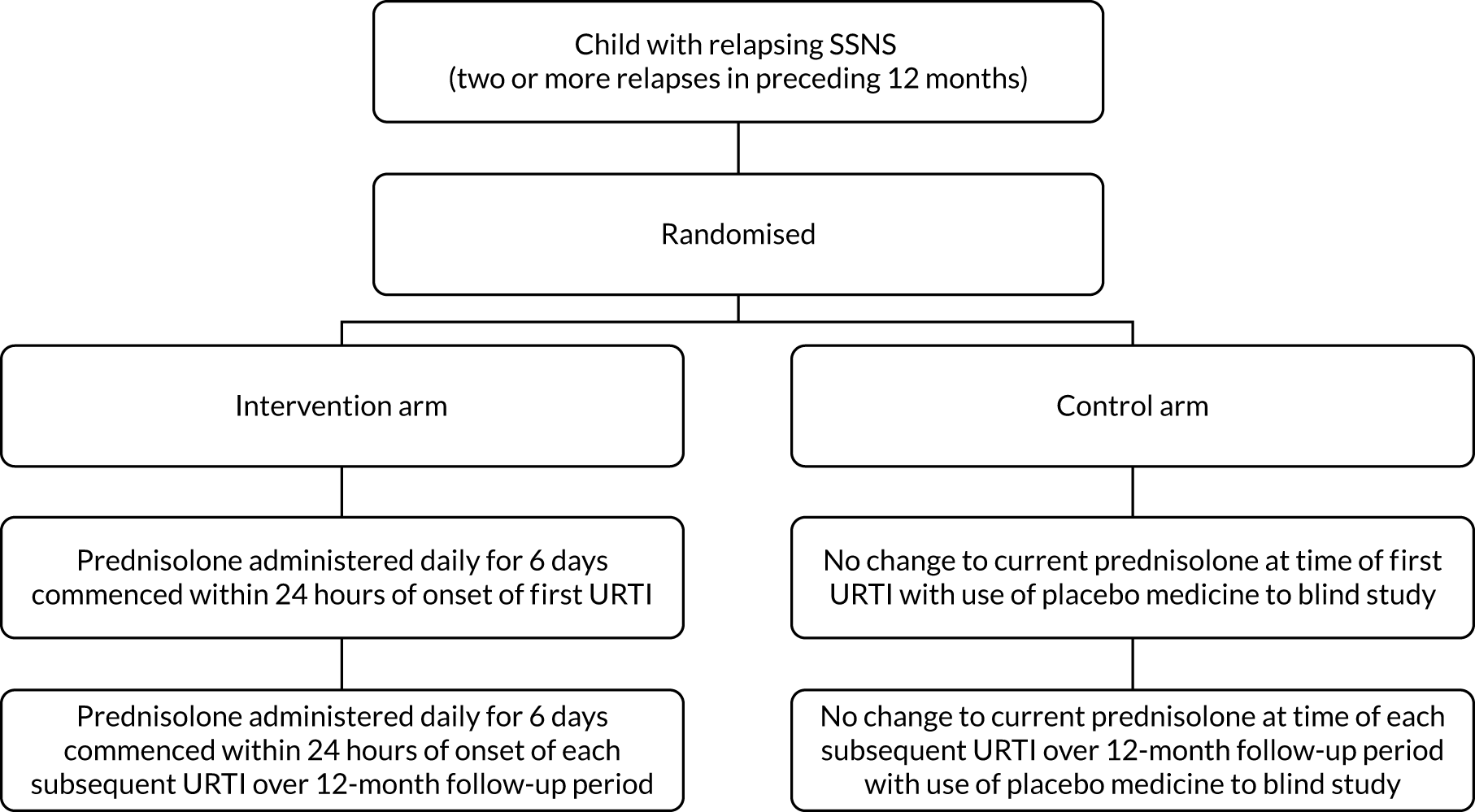
Participants
Inclusion criteria
Children aged > 1 year and < 19 years were eligible for inclusion if they had relapsing SSNS, defined as having experienced two or more relapses in the preceding 12 months. This included the following groups:
-
Children on no long-term immunosuppressive therapy.
-
Children receiving long-term maintenance prednisolone therapy at a dose of up to and including 15 mg/m2 on alternate days. Note that this was the maximum dose at the time of recruitment. If children subsequently received a higher dose, for example after relapse, then they could remain in the trial.
-
Children receiving long-term maintenance prednisolone therapy at a dose of up to and including 15 mg/m2 on alternate days in conjunction with other immunosuppressive therapies, including levamisole, ciclosporin, tacrolimus, MMF, mycophenolate sodium and azathioprine.
-
Children receiving long-term immunosuppressive therapies, including levamisole, ciclosporin, tacrolimus, MMF, mycophenolate sodium and azathioprine, without long-term maintenance prednisolone therapy.
-
Children who had previously received a course of oral or intravenous cyclophosphamide –
-
Children must have experienced two relapses in the 12 months prior to randomisation (in keeping with all other children).
-
Children must have experienced at least one of these relapses following completion of cyclophosphamide therapy.
-
Children must have been at least 3 months post completion of oral or intravenous cyclophosphamide therapy.
-
-
Children who had previously received a single dose or course of intravenous rituximab –
-
Children must have experienced two relapses in the 12 months prior to randomisation (in keeping with all other children).
-
Children must have experienced at least one of these relapses following completion of rituximab therapy.
-
Children must have been at least 3 months post completion of intravenous rituximab therapy.
-
-
Parents and (where age appropriate) child understood the definition of URTI and the need to commence trial drug once this definition was met.
-
Written informed consent obtained from the child’s parents/guardians and written assent obtained from the child (where age appropriate). Young people aged ≥ 16 years provided their own written informed consent.
Exclusion criteria
-
Children with steroid-resistant nephrotic syndrome.
-
Children receiving or within 3 months of having completed a course of oral or intravenous cyclophosphamide.
-
Children receiving or within 3 months of having received a course of rituximab.
-
Children on daily prednisolone therapy at time of recruitment.
-
Children on a prednisolone dose of > 15 mg/m2 on alternate days at time of recruitment.
-
Children with a documented history of significant non-adherence to medical therapy.
-
Young people who would be transferred from paediatric to adult services during the 12-month trial period.
-
Children unable to take prednisolone tablets, even in crushed form.
-
Known allergy to prednisolone.
Rationale for choice of inclusion and exclusion criteria
Children with relapsing nephrotic syndrome experience relapses whether they are on maintenance immunosuppression with steroids, with other agents, with both or if they are taking no maintenance immunosuppression. In this trial, we aimed to reflect that pragmatism with a broad eligibility criterion of all children with frequently relapsing disease. At the outset of the trial, a precise definition of frequently relapsing disease (i.e. two or more relapses in the 6 months prior to recruitment) was used, but this was later relaxed to more than two relapses within the previous 12 months to optimise recruitment.
The reason for the 3-month window following treatment with cyclophosphamide or rituximab is that patients who respond to this agent (approximately 50–60%) are likely to see a significant reduction in the frequency of disease relapse, particularly when receiving and shortly after completing this therapy. The inclusion of at least one relapse following cyclophosphamide or rituximab treatment confirms the persistence of relapsing disease.
Children became eligible for recruitment if a second relapse occurred within 12 months of a previous one. Once they enter remission, normal practice is to continue prednisolone at a dose of 40 mg/m2 on alternate days for 4 weeks, sometimes followed by a slow weaning process. The cumulative dose of prednisolone required to treat children for 6 days if they developed URTI symptoms while weaning prednisolone following a relapse and taking a dose of > 15 mg/m2 on alternate days was considered unacceptably high. The families of these children were informed about the trial, but recruitment did not take place until the child’s prednisolone dose had been reduced.
Outcome measures
Primary outcome
The primary outcome was the incidence of first URR of nephrotic syndrome following any URTI during the 12-month follow-up period.
Relapse was defined as Albustix-positive proteinuria (i.e. +++ or greater) for 3 consecutive days or the presence of generalised oedema plus 3+ proteinuria. URR was defined as a relapse occurring within 14 days of the development of an URTI. See below for more details.
This was chosen as the primary outcome as it was hypothesised that giving daily prednisolone at the time of URTI would reduce the subsequent development of disease relapse. If this hypothesis was correct, then those children randomised to placebo would experience more URRs than those children randomised to the active drug.
Secondary outcomes
-
Rate of URR of nephrotic syndrome (relapses per year).
-
Rate of relapse (i.e. URTI related and non-URTI related) of nephrotic syndrome (relapses per year).
-
Cumulative dose of prednisolone (mg/kg and mg/m2) received over the 12-month trial period.
-
Incidence of serious adverse events (SAEs).
-
Incidence of adverse effects of prednisolone, including assessment of behaviour using the Achenbach Child Behaviour Checklist (ACBC).
-
Incidence of escalation of background immunosuppressive therapy (e.g. addition of ciclosporin, tacrolimus, cyclophosphamide).
-
Incidence of reduction of background immunosuppressive therapy (i.e. cessation of long-term maintenance prednisolone therapy).
-
Quality of life using the PedsQL, CHU-9D and EQ-5D (the latter two were used for the economic analysis).
-
Cost per quality-adjusted life-year (QALY) gained.
Change to the primary outcome
The original primary outcome was the incidence of URR following the first URTI.
An interim analysis for the Data Monitoring Committee (DMC) in late 2015 showed that the URR rate following the first URTI was 22%. This was much lower than the 50% event rate used in the sample size calculation (see below). It was also noted that a larger number of children than expected were completing the 12-month trial period and not experiencing an URTI (see Sample size).
At the request of the Health Technology Assessment (HTA) programme and the DMC, a futility analysis was undertaken and presented to the DMC in January 2016. Following the results of the futility analysis, the DMC advised the Trial Steering Committee (TSC) that, based on the planned sample size and current event rate, it was unlikely that the trial would show a difference in primary outcome between the two treatment arms, should there be one. They did, however, acknowledge that there were still important secondary end-point data that the trial could address.
Following this, the Trial Management Group (TMG), the TSC and the HTA programme discussed the best way to proceed. It was agreed for the primary outcome to be changed to incidence of first URR following any URTI during the 12-month follow-up period, rather than URR following the first URTI. This had the advantage of being correlated with the original primary outcome and had a (combined) event rate of 42%, which was closer to the sample size assumptions.
Sample size
In children with FRSSNS, the development of an URTI results in relapse in around 50% of instances. 19 In the Abeyagunawardena and Trompeter19 study, 40 URTIs treated with placebo were followed by 19 relapses (48%), compared with seven relapses (18%) in the prednisolone-treated group. This corresponds to an absolute difference of 30% (a 62.5% proportional reduction). In the first treatment period, there were 10 relapses (45%) in 22 placebo-treated children, compared with four relapses (22%) in 18 prednisolone-treated children (i.e. a 23% absolute difference and 51% proportional reduction). 19 This was a large treatment effect based on a small study of children on long-term maintenance alternate-day prednisolone therapy in a developing country. Therefore, to detect a more conservative difference of 17.5% (i.e. 35% proportional reduction) in URR rate (i.e. from 50% to 32.5%), with 80% power, a two-sided test and an alpha of 0.05, required 250 children in total (comparison of two proportions23). An allowance was made for between 10% and 20% dropout (e.g. subject withdrawal, lost to follow-up or subject not having an URTI during the 12-month follow-up period), which required recruitment of between 280 and 320 children. Therefore, it was proposed to recruit 300 children, 150 to each arm. However, with a treatment effect more in line with the 50% reduction observed in the first treatment period of the Abeyagunawardena and Trompeter19 study, the PREDNOS2 trial would have sufficient power (> 95%) to detect this difference [i.e. to detect a 50% proportional reduction (i.e. from 50% to 25%) with 90% power and an alpha of 0.05, required 160 children, increasing to 200 with allowance for 20% dropout].
During the trial, it became apparent that a larger number of children than expected were reaching the end of the 12-month follow-up period without experiencing an URTI and, therefore, were unable to contribute to the analysis. The original sample size was increased by between 10% and 20% to account for this, but, in late 2015, the actual number of children who had completed 12 months’ follow-up without an URTI was 28%. Therefore, the TSC recommended revising the sample size using a 30% attrition rate, which increased the sample size to 360 patients.
Recruitment
Children with relapsing SSNS under the care of a paediatric nephrologist and/or a general paediatrician were recruited from throughout the UK. Potentially eligible children were identified from clinic lists and departmental databases. Information sheets outlining the trial were mailed to the parents or guardians of potentially eligible children (and the child, where age appropriate) 1–2 weeks prior to their next clinical appointment. Following confirmation of eligibility with regard to the inclusion and exclusion criteria, and a further full discussion of the trial, informed consent was sought from the parents (or guardians) and children (informed consent or assent, according to age) at the time of this appointment.
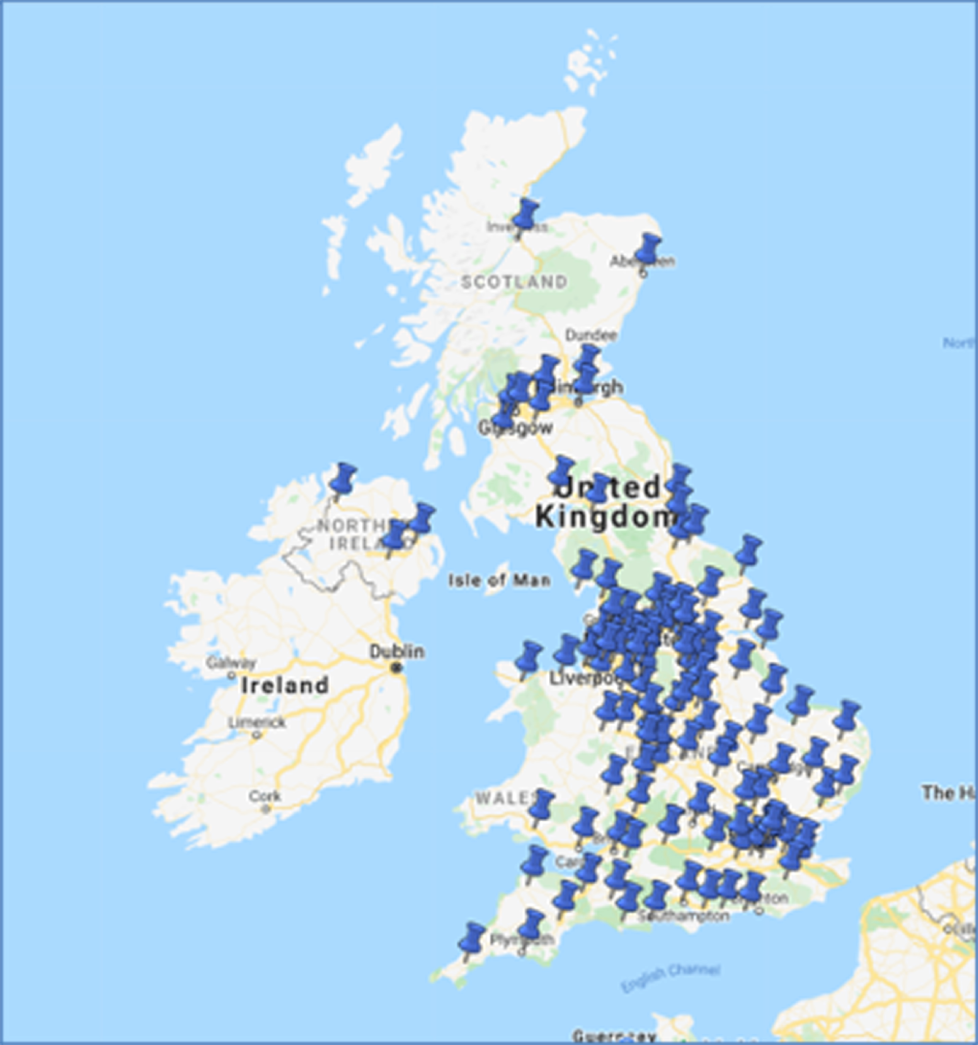
Trial sites
A total of 153 sites were set up throughout the UK and 122 of these proceeded to full approval. Figure 2 shows the location of these centres on a map. These sites included all 13 tertiary paediatric nephrology units, as well as general paediatric units within university and district general hospitals.
FIGURE 2.
Map of recruiting centres for the PREDNOS2 trial. Map data © 2021 GeoBasis-DE/BKG (© 2009) Google, Inst. Geogr. National.
Informed consent
The conduct of the trial was in accordance with the principles of Good Clinical Practice (GCP) and applicable regulatory requirements.
For children aged < 16 years, the parent’s written informed consent for their child to participate in the trial and the child’s assent, as appropriate, given the child’s competence, were obtained before randomisation and after a full explanation had been given of the treatment options and the manner of treatment allocation. Young people aged 16–18 years gave their own consent to participation in the trial. Examples of patient information can be seen in the supporting documentation [see NIHR Journals Library, URL: www.journalslibrary.nihr.ac.uk/programmes/hta/11129261/#/documentation (accessed 21 July 2021)].
Electronic copies of the parent/patient information sheet and informed consent/assent forms were printed or photocopied onto the headed paper of the local institution.
It was the responsibility of the investigator or designate (e.g. research nurse if local practice allowed and this responsibility had been delegated by the principal investigator, as captured on the site signature and delegation log) to obtain written informed consent for each parent/child prior to performing any trial-related procedures. Investigators ensured that they had adequately explained the aim, trial treatment, anticipated benefits and potential hazards of taking part in the trial to the parent/child. The investigator also stressed that the child was completely free to refuse to take part or to withdraw from the trial at any time. The parent/child were given ample time (e.g. up to 1 week) to read the parent/patient information sheet and to discuss their participation with others outside the site research team. The parent/child was given an opportunity to ask questions to be answered to their satisfaction. The right of the parent/child to refuse to participate in the trial without giving a reason was respected.
If the parent expressed an interest in their child participating in the trial, then they were asked to sign and date the current ethics-approved version of the informed consent form. The young person signed their own consent form if aged 16–18 years (inclusive). If the young person was aged < 16 years, then they could sign an assent form, if age appropriate. The investigator or designate then signed and dated the form. A copy of the informed consent/assent form was given to the parent/child, a copy was filed in the hospital notes and the original was placed in the investigator site file (ISF).
Once the child was entered into the trial, their trial number was entered on the informed consent/assent form maintained in the ISF. In addition, a copy of the signed informed consent/assent form was sent by fax to the Birmingham Children’s Hospital Pharmacy Department (Birmingham, UK). Details of the informed consent discussions were recorded in the child’s medical notes, including the date of the initial discussion, information regarding the initial discussion and the outcome, the date consent was given, the name of the trial, the version number of the parent/patient information sheet, the version number of the informed consent form and confirmation that the person signing the consent form on behalf of the child had been determined to have the parental responsibility to do so.
Throughout the trial, the parent/child had the opportunity to ask questions about the trial and any new information that may be relevant to the child’s continued participation was shared with them in a timely manner.
Details of all children approached about the trial were recorded on the subject screening/enrolment log and, with the parent’s/child’s prior consent, their general practitioner (GP) was informed that they were taking part in the trial. A GP letter was provided electronically for this purpose.
Randomisation
Following the informed consent process and completion of the baseline assessments, eligible children were randomised at the level of the individual in a 1 : 1 ratio to either the intervention arm or the control arm. To ensure concealment of treatment allocation, randomisation was provided using a central secure 24-hour internet-based randomisation service, or by a telephone call to the University of Birmingham Clinical Trials Unit (BCTU) (Birmingham, UK). The randomisation process used minimisation by the background treatment regimen that the child was receiving at randomisation (i.e. no treatment, long-term maintenance with prednisolone therapy only, long-term maintenance prednisolone therapy with other immunosuppressant therapy, and other immunosuppressant therapy only).
Randomisation took place at the time of the initial trial visit, prior to the development of the first URTI, to ensure that the trial drug reached the parents/child by the time the first URTI developed. The investigator completed the randomisation notepad to prepare for randomisation. The randomisation notepad was signed by the investigator to indicate that all of the eligibility criteria had been checked. It was also noted in the medical records that the investigator had checked all of the eligibility criteria and that the child met all of the inclusion criteria and none of the exclusion criteria. The signed randomisation notepad was kept in the ISF and a copy sent to the trials office.
After randomisation, each child was assigned a unique trial identification number to be used on all trial-related material for the child. Confirmation of the randomisation and trial identification number was forwarded to the trial manager, the local investigator and the Birmingham Children’s Hospital Pharmacy Department by e-mail immediately on randomisation. Once a PREDNOS2 trial number had been obtained, the investigator faxed or e-mailed a signed copy of the clinical trial prescription form and the consent/assent form(s) to the pharmacy at the Birmingham Children’s Hospital to order the PREDNOS2 trial drug, which was then sent directly to the parents/child (see Investigational medicinal product). Only delegated staff at the Birmingham Children’s Hospital NHS Trust Pharmacy were able to view the treatment allocation to dispatch the pots of trial drug. This was carried out using a secure login link to the randomisation program once the child had been randomised. This ensured that investigators and the trial office remained blind to the treatment allocation.
Blinding
The PREDNOS2 trial was a double-blind placebo-controlled trial. The secure internet-based central randomisation service provided by BCTU ensured concealment of treatment allocation. Essential Nutrition Ltd (Brough, UK), a Medicines and Healthcare products Regulatory Agency (MHRA)-licensed good manufacturing practice manufacturer of solid oral dosage form products, manufactured both the active and placebo tablets for the PREDNOS2 trial. To ensure that the tablets were identical in size, shape, taste and smell, Essential Nutrition Ltd conducted batch testing throughout the trial. To maintain blinding, children randomised to the control arm received the same number of study tablets as they would have received in the intervention arm through supplemental placebo tablets. Therefore, outcome assessments by families and local investigators were carried out masked to treatment allocation. Only staff at the Birmingham Children’s Hospital Pharmacy Department were aware of the individual allocations of children. The trial statistician was unblinded to the intervention code for all of the interim analyses and presented unblinded analyses to the DMC.
Planned interventions
Current practice in the UK has been for no change to be made to immunosuppressive therapy at the time of development of an URTI. The intervention being assessed within the PREDNOS2 trial was a 6-day course of daily prednisolone therapy at the time of onset of URTI.
Those randomised to the intervention arm commenced a 6-day course of daily prednisolone each time they developed an URTI during the 12-month follow-up period (see Definition of upper respiratory tract infection for strict definition). Those randomised to the control arm received an identical number of placebo tablets, therefore allowing double blinding of the trial. If the child was receiving background long-term immunosuppressive therapy (e.g. levamisole or ciclosporin), then this continued unchanged.
Definition of upper respiratory tract infection
An URTI was defined as the presence of at least two of the following for at least 24 hours:
-
sore throat
-
ear pain/discharge
-
runny nose
-
cough (dry/barking)
-
hoarse voice
-
fever of > 37 °C (measured using tympanometric electronic thermometer).
Parents were provided with clear written and (if requested) downloadable electronic information informing them of the trial definition of an URTI, as outlined above. They were also provided with abbreviated versions of this printed onto laminated cards, which could be kept in multiple locations within the family home, including in fridge magnet format.
An electronic tympanometric thermometer (Braun Thermoscan®; Braun, Kronberg, Germany) was provided to the parents of all children to allow them to measure their child’s temperature. A diary was also provided for parents to record the results of the daily morning urinalysis (standard care in children with relapsing SSNS), development of URTI, commencement of trial drug, other ongoing treatment, acute illnesses and other issues.
Investigational medicinal product
The trial drug (5-mg prednisolone tablets and matching placebo tablets) was manufactured, packed and labelled by Essential Nutrition Ltd. Following randomisation, children were provided with a supply of 100 tablets (two containers of 50 tablets) of the trial drug (5 mg of prednisolone) for those randomised to the intervention arm or a matching placebo for those randomised to the control arm. This was sent directly to the family home from the central pharmacy at the Birmingham Children’s Hospital by Royal Mail (London, UK)-registered post on a day convenient for the family. The trial drug containers and their contents appeared identical in every way, therefore maintaining the double blind. Trial drug labelling complied with the applicable regulatory requirements and clinical trial-specific labels were attached to all treatments. The pharmacy at the Birmingham Children’s Hospital maintained drug accountability logs for dispensed and returned trial drug doses according to its local policy. The central pharmacy at the Birmingham Children’s Hospital documented when they were contacted by the parent/patient to confirm receipt of the IMP.
For children unable to swallow the tablets whole, the prednisolone and placebo tablets could be crushed and a tablet crusher was supplied with the trial drug at the prescriber’s request.
Every child participating in the trial was issued with a standard pack containing the following:
-
A diary in which the results of the morning urinalysis, treatment administration and any consultations with health-care professionals [e.g. GP, nurse, hospital accident and emergency (A&E) department], development of URTI, commencement of trial drug and details of medicines prescribed or purchased over the counter could be recorded.
-
Instructions on contacting the central pharmacy at Birmingham Children’s Hospital on receipt of IMP at the family home. This included date of receipt and number of bottles received.
-
An electronic tympanometric thermometer to measure temperature at the time of suspected URTI.
-
Written and (if requested) electronically downloadable information regarding URTI definition.
-
Written and (if requested) electronically downloadable information regarding the dose of the trial drug to be commenced in the event of URTI developing.
-
Other ‘aides-memoires’ regarding definition of URTI, etc., displayed on fridge magnets and laminated sheets.
Compliance with trial drug was assessed at each trial visit. This was carried out by a manual pill count by an appropriate staff member at the local site, using a standard counting triangle.
Commencement of trial drug
Once the child met the definition for URTI (i.e. two or more criteria as listed above for at least 24 hours), the parents or guardians commenced the child on the trial drug (prednisolone tablets for those in the intervention arm or placebo for those in the control arm) (see Dosing of trial drug for dosing schedules). It was anticipated that parents or guardians would have no difficulties in identifying that their child had met the URTI criteria and would commence the trial drug unassisted, having been provided with comprehensive advice on this at the time of their recruitment. However, a back-up service was also provided. If they were in any doubt, parents or guardians were instructed to contact their local trial site or, if this was not possible, to call a PREDNOS2 trial telephone number, which was manned by the chief investigator or his nominated deputy during periods of annual leave. This meant that parents or guardians were able to seek advice regarding whether their child met the URTI criteria, the dose of trial drug required and any other issues or concerns they may have had relating to the trial. A log of any telephone calls was maintained and the chief investigator reported their content to the local principal investigator by e-mail. This back-up service was used on fewer than five occasions during the trial.
To ensure patient safety, the information provided to parents and guardians also contained information about the signs of a more serious infection (e.g. non-blanching rash, leg pain, cool extremities, rapid breathing, blue lips, fitting, unconsciousness, any other major concern). If any of these features were present, parents and guardians were instructed not to start the trial drug and to seek urgent medical attention for their child from their GP or local A&E department.
Parents or guardians were asked to contact their local trial site within 24 hours of commencing the trial drug to inform the principal investigator or nominated deputy and to allow them the opportunity to discuss any of their child’s symptoms that may be of concern to them.
The above intervention was repeated every time the child developed a URTI over the 12-month follow-up period. The only exception to this was if the child was receiving daily prednisolone therapy (e.g. in the early stages of treatment for a previous relapse). In these instances, the trial drug was not commenced.
Dosing of trial drug
The precise dose of the trial drug at time of the development of URTI depended on the child’s current treatment regimen, in particular whether or not they were receiving long-term maintenance prednisolone therapy and, if so, at what dose.
Children not receiving long-term maintenance prednisolone
Those randomised to the intervention arm received prednisolone (15 mg/m2) daily (maximum dose of 40 mg) for a total of 6 days. The dose was rounded up or down to the nearest 5 mg and given as a single morning dose. Children randomised to the control arm received an identical number of placebo tablets.
Children receiving a long-term maintenance prednisolone dose of ≤ 15 mg/m2 on alternate days
Those randomised to the intervention arm received prednisolone (15 mg/m2) daily (maximum dose of 40 mg) for a total of 6 days. The dose was rounded up or down to the nearest 5 mg and given as a single morning dose. Children randomised to the control arm received an identical number of placebo tablets. Children receiving long-term maintenance prednisolone therapy received a different number of tablets of the trial drug on regular treatment-days (i.e. those days when they usually take prednisolone) from non-treatment-days. For example, a child of 1.0 m2 receiving a long-term maintenance dose of 5 mg of prednisolone on alternate days took an additional two 5-mg prednisolone tablets (or matching placebo) on treatment days and three 5-mg prednisolone tablets (or matching placebo) on non-treatment days. Therefore, this particular child received either prednisolone 15 mg (15 mg/m2) daily for 6 days if in the intervention arm or continued unchanged on prednisolone 5 mg on alternate days if in the control arm.
Children receiving a prednisolone dose of > 15 mg/m2 on alternate days
A small number of children may have been receiving a prednisolone dose of > 15 mg/m2 at the time of URTI development. These were likely to be children who had already relapsed during the 12-month trial period and had their prednisolone dose increased accordingly, as the inclusion/exclusion criteria excluded those on a dose of prednisolone > 15 mg/m2 at trial entry. These children converted to the same dose on a daily basis for a total of 6 days. The dose was rounded up or down to the nearest 5 mg and given as a single morning dose (maximum dose 60 mg). Children randomised to the control arm received an identical number of placebo tablets. For example, a child of 1.0 m2 receiving a long-term maintenance dose of 20 mg of prednisolone on alternate days took their regular dose on treatment days and four 5-mg prednisolone tablets (or matching placebo) on non-treatment days. Therefore, this particular child received either 20 mg (20 mg/m2) daily for 6 days if in the intervention arm or continued unchanged on 20 mg on alternate days if in the control arm.
To allow for changes in dosing of trial drug with the child’s growth throughout the trial, the precise regimen to be administered was discussed with parents and guardians, along with a written treatment plan at the time of recruitment, and re-discussed at each trial visit. Information about the precise number of trial drug tablets to administer was provided to the family in written form, using a standard form, which was completed by the local principal investigator.
The trial drug was always given as a single dose in the morning. If the child was receiving any additional immunomodulatory therapy (e.g. ciclosporin, levamisole, etc.), then this continued unchanged throughout the 6-day course of the trial drug. Other drug treatment also continued unchanged. At the end of the 6-day course of the trial drug, the child continued on their previous dose of long-term maintenance prednisolone therapy (or no prednisolone treatment if they were not previously receiving this). If an URTI occurred when a child was receiving daily prednisolone (e.g. in the early stages of treatment for a disease relapse), then the trial drug was not commenced. These children continued to participate in the trial and the subsequent URTIs were treated with the trial drug, provided that they were receiving alternate-day prednisolone at that point.
Trial procedures
Trial visits and data collection
All children underwent a comprehensive assessment at the randomisation visit and were then followed up for 12 months from randomisation, with visits at 3, 6, 9 and 12 months for trial follow-up assessments. This follow-up schedule is entirely in keeping with the normal frequency of follow-up of this population in routine clinical practice. The information captured at the randomisation and subsequent visits are shown in Table 3.
| Information | Visit | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Month | 0 | 3 | 6 | 9 | 12 |
| Window | ± 2 weeks | ± 2 weeks | ± 2 weeks | ± 2 weeks | |
| Inclusion/exclusion criteria | ✓ | ||||
| Informed consent | ✓ | ||||
| Randomisation | ✓ | ||||
| Allocation of trial number | ✓ | ||||
| Documentation of URTI | ✓ | ✓ | ✓ | ✓ | |
| Documentation of commencement of trial drug | ✓ | ✓ | ✓ | ✓ | |
| Documentation of recent relapse | ✓ | ✓ | ✓ | ✓ | ✓ |
| Recent medical/drug history | ✓ | ✓ | ✓ | ✓ | ✓ |
| AE documentation | ✓ | ✓ | ✓ | ✓ | |
| Compliance check (tablet count using counting triangle)a | ✓ | ✓ | ✓ | ✓ | |
| Physical examination | ✓ | ✓ | ✓ | ✓ | ✓ |
| Assessment of steroid toxicity | ✓ | ✓ | ✓ | ✓ | ✓ |
| Height and weight | ✓ | ✓ | ✓ | ✓ | ✓ |
| Blood pressure | ✓ | ✓ | ✓ | ✓ | ✓ |
| Calculation of trial drug dose to be administered in the event of URTI and explanation/documentation | ✓ | ✓ | ✓ | ✓ | ✓ |
| If three or more courses of the trial drug have been administered since the previous visit, confirm parental understanding of URTI definition | ✓ | ✓ | ✓ | ✓ | |
| Blood sample for DNA/RNAb | ✓ | ✓ | ✓ | ✓ | ✓ |
| ACBC | ✓ | ✓ | ✓ | ✓ | ✓ |
| PedsQL | ✓ | ✓ | ✓ | ✓ | ✓ |
| CHU-9D and EQ-5D | ✓ | ✓ | ✓ | ✓ | ✓ |
| Trial drug returned to Birmingham Children’s Hospital Pharmacy Department for accountability | ✓ | ||||
At each visit, there was a full clinical review of the child and a number of different questionnaires were administered. The clinical review included assessment of whether or not the child had experienced any URTI or non-URRs since the last visit and a review of the current treatment regimen.
To evaluate changes in the child’s behaviour associated with the different prednisolone regimens, the ACBC24 was completed by the parents/guardian. The ACBC is a standardised measure made up of 120 items that measure internalising behaviour problems (i.e. withdrawn, somatic complaints, anxiety/depression, thought problems) and externalising behaviour problems (i.e. social problems, attention problems, delinquent and aggressive). It was used as part of the behavioural effects of prednisolone for the PREDNOS trial that preceded this trial. 9 A total behavioural problem score is calculated from these problem scales and forms the basis of comparison with age- and sex-matched normative data.
Information relating to quality of life was also collected using the CHU-9D, EQ-5D (dependent on child age)25 and PedsQL26,27 questionnaires. The information was collected from parents/guardians or the child/young person themselves, depending on their age. The CHU-9D is a newly developed utility measure designed for children aged 5–11 years. The EQ-5D is a validated utility measure routinely used in adult and adolescent populations. Both the CHU-9D and the EQ-5D were used to generate data for the health economic analyses. The PedsQL questionnaire is a well-validated approach to measuring health-related quality of life (HRQoL) in healthy children and adolescents and those with acute and chronic health conditions.
Adverse effects due to prednisolone were assessed by studying growth (i.e. height, weight and body mass index), cushingoid features, hypertrichosis, striae, appetite (all Likert scales), behaviour (ACBC), blood pressure and urine dipstick analysis for glycosuria. The development of significant bacterial, viral or fungal infections and the use of post-varicella exposure prophylaxis (zoster immune globulin or antiviral therapy) were also recorded. Information was also collected regarding all episodes of consultation with GPs or hospital medical teams, including data on treatments prescribed. This was incorporated into the costs measured as part of the health economic analysis (see Chapter 4).
Information recorded by parents and guardians
Parents or guardians performed dipstick testing for proteinuria (using an Albustix, Siemens Healthcare Diagnostics Ltd, Frimley, UK) of the child’s first morning urine on a daily basis in accordance with routine clinical care. They were provided with a record book to enter the results (to allow the early detection of nephrotic syndrome relapses) and the medication administered on a daily basis. This was maintained for the 12 months of the trial. Parents and guardians also used this diary to record any intercurrent illnesses and consultations with health-care professionals (e.g. GP, nurse, hospital A&E department), development of URTI and commencement of trial medicines, along with details of medicines prescribed or purchased over the counter.
Diagnosis and treatment of relapse
Relapse was defined as Albustix 3+ or more for 3 consecutive days or the presence of generalised oedema and Albustix 3+ on urine testing. An URR was defined as a relapse occurring within 14 days of the onset of an URTI. Where disease relapse occurred, parents or guardians contacted their trial centre in accordance with routine clinical care and treatment for disease relapse was commenced. A relapse was treated in accordance with the International Study of Kidney Disease in Children relapse regimen:28 prednisolone was commenced at a dose of 60 mg/m2 daily (maximum dose of 80 mg) until the urine tests were negative or trace for 3 consecutive days, then reduced to 40 mg/m2 (maximum dose of 60 mg) on alternate days for 4 weeks (14 doses). A subsequent tapering dose was used at the individual physician’s discretion. When relapse therapy was commenced, long-term maintenance prednisolone therapy (e.g. 10 mg on alternate days) was discontinued.
Where relapse occurred while the child was receiving the 6-day course of the trial drug, the trial drugs were discontinued and relapse treatment was initiated. Once the relapse regimen was completed, long-term maintenance prednisolone therapy could be recommenced at any dose at the principal investigator’s discretion. Background immunomodulatory therapy other than prednisolone (e.g. ciclosporin, MMF, levamisole, etc.) continued unchanged throughout the relapse treatment period.
Escalation of background immunomodulatory therapy
Children underwent intensification of background immunomodulatory therapy (i.e. the addition of, or change to, a new immunomodulatory agent, e.g. ciclosporin, MMF, levamisole, etc.) only when there were two or more relapses (URTI related or unrelated) in any 6-month period or where there were unacceptable adverse effects of prednisolone or other therapy. These children remained under follow-up, as intensification of immunomodulatory therapy was an important secondary outcome for this trial. Similarly, immunomodulatory therapy was discontinued only when the child remained relapse free for at least 6 months or there were unacceptable adverse effects of therapy.
Changes in certain types of non-corticosteroid immunosuppression were predefined as escalations, for example a move from cyclophosphamide to ciclosporin/tacrolimus, the addition of mycophenolate or the addition of rituximab. This is in line with guidelines, such as those written by the Kidney Disease Improving Global Outcomes (KDIGO) group. 28 Any time cyclophosphamide was given, even if it was following ciclosporin/tacrolimus, it was considered to be an escalation, as the overwhelming rationale for using it would be poorly controlled nephrotic syndrome relapses. As both cyclophosphamide and rituximab are disease-modifying drugs given as short courses that are not repeated at all (usually in the case of cyclophosphamide) or for at least 6 months (in the case of rituximab), absence of either drug in a 3-month time period following one where it had been given was not considered to be a reduction in background therapy.
Safety assessment and reporting
Pharmacovigilance reporting complied with the Medicines for Human Use (Clinical Trials) Regulations 200429 and the Medicines for Human Use (Clinical Trials) Amended Regulations 2006. 30 Annual development safety update reports were submitted to the main Research Ethics Committee (REC) and MHRA.
The investigator assessed the seriousness and causality (relatedness) of all AEs experienced by patients (this was documented in the source data), with reference to the prednisolone tablet (Wockhardt UK Ltd) summary of product characteristics (dated 31 March 2008, section 4.8). 31 This was the reference safety information for the trial. 31
Within the PREDNOS2 trial, the steroid prednisolone and the matching placebo were both defined as IMPs.
Adverse events
An AE is any untoward medical occurrence in a patient or clinical trial subject administered a pharmaceutical product that does not necessarily have a causal relationship with this treatment. An AE could, therefore, be any unfavourable and unintended sign (including an abnormal laboratory finding), symptom or disease temporally associated with the use of a (investigational) medicinal product, whether or not related to the medicinal product.
Expected AEs for the PREDNOS2 trial were those listed in the reference safety information for prednisolone.
The following were not considered AEs for the purposes of this trial:
-
a pre-existing condition (unless it worsened significantly during treatment)
-
diagnostic and therapeutic procedures, such as surgery (although the medical condition for which the procedure was performed was reported if new).
Only data on the AEs listed on the case report forms were routinely collected during the trial.
Serious adverse events
All AEs that met the definition of being SAEs were reported for the duration of the 12-month trial and for 3 months following completion of the trial drug.
A SAE is defined as any AE that results in death, is life-threatening, requires inpatient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, results in a congenital anomaly or a birth defect or is otherwise considered medically significant by the investigator.
Note that life-threatening in this context refers to an event in which the child was at risk of death at the time of the event. It does not refer to an event that, hypothetically, might have caused death if it were more serious. Hospitalisation was an admission that resulted in an overnight stay in hospital. Day admissions did not need to be reported as a SAE. Medical judgement was exercised in deciding whether or not an AE was serious in other situations. Important AEs that were not immediately life-threatening or did not result in death or hospitalisation, but may have jeopardised the child or may have required intervention to prevent one of the other outcomes listed in the definition above, were considered serious.
All AEs were evaluated by a doctor to determine severity and causality between the IMP and/or concomitant therapy and the AE. The causality and severity of all AEs was recorded in the medical notes. Investigators reported all AEs that met the definition of being serious immediately and within 24 hours of being made aware of the event using the trial-specific SAE form, other than the following events.
Hospitalisations for the following expected events did not require expedited reporting:
-
routine treatment or monitoring of the studied indication not associated with any deterioration in condition
-
treatment that was elective or pre-planned for a pre-existing condition that was unrelated to the indication under study, and had not worsened.
These events were reported on the trial-specific SAE form and sent to the trial office at the BCTU within 1 week of the site being made aware of the date of hospitalisation.
Vaccination
Where the child was due for routine vaccination or specialised vaccinations, there were some contraindications that needed to be taken into account, as stated in the Department of Health and Social Care’s Immunisation Against Infectious Disease, otherwise known as ‘The Green Book’. 32
Live vaccines can, in some situations, cause severe or fatal infections in immunosuppressed individuals because of extensive replication of the vaccine strain. For this reason, severely immunosuppressed individuals should not be given live vaccines and vaccination in immunosuppressed individuals should be conducted in consultation with an appropriate specialist only. Inactivated vaccines cannot replicate and so may be administered to immunosuppressed individuals, although they may elicit a lower response than in immunocompetent individuals.
Children who receive prednisolone, orally or rectally, at a daily dose (or its equivalent) of 2 mg/kg per day for at least one week or 1 mg/kg per day for 1 month are classified as a special risk group for being given live vaccines. Administration of live vaccines should be postponed for at least 3 months after immunosuppressive treatment has stopped or 3 months after levels have been reached that are not associated with immunosuppression. Routine live vaccines given in the UK include the MMR (measles, mumps and rubella) vaccine and the BCG (bacillus Calmette–Guérin) vaccine for tuberculosis. For parents who may wish to travel with their children, other live vaccines include Yellow Fever and oral typhoid vaccines. Where these issues arose with a child within the trial, the advice of an immunologist was sought.
Unblinding
Should there have been any medical emergencies where it was necessary for a child’s treatment to be unblinded, a codebreak was available via Birmingham Children’s Hospital Pharmacy Department. Subject to clinical need, where possible, members of the research team were to remain blinded. No unblinding was required during the trial.
Trial withdrawals
Trial participants were followed up for the entire trial provided that consent for their ongoing participation in the trial was not withdrawn. Where consent was withdrawn, parents and children could choose any of the following:
-
The child wished to withdraw from the investigational treatment, but was willing to be followed up in accordance with the trial protocol (i.e. agreed that follow-up data could be collected and used in the final analysis).
-
The child did not want to attend trial-specific follow-up visits, but was willing to be followed up in accordance with standard practice (i.e. agreed that follow-up data could be collected at standard clinic visits and used in the trial final analysis).
-
The child was not willing to be followed up for trial purposes at any further visits (but agreed that any data collected prior to the withdrawal of consent could be used in the trial final analysis).
Blood samples
A genome-wide association study of SSNS and a deoxyribonucleic acid (DNA) mutation substudy are planned as part of the PREDNOS2 trial, and are funded separately. The results do not form part of this report and will be published elsewhere.
There are multiple reasons to suggest that SSNS may be a genetic disorder. As part of the PREDNOS2 trial, we obtained DNA and ribonucleic acid samples to identify changes that may be the cause of or contribute to the disease process of SSNS. The discovery of genetic changes that are unique to SSNS would increase our understanding of the disease and may lead to improved and more specific therapies becoming available.
As part of the consent process, permission was obtained to collect a single 10-ml blood sample for genetic substudies at some point during the trial, preferably at the time of routine venepuncture for clinical purposes. If for any reason the parents and/or child were unwilling to provide a blood sample, this did not preclude them from taking part in the main trial.
The 10-ml blood sample was collected into two 5-ml aliquots containing potassium ethylenediaminetetraacetic acid (EDTA). Samples were labelled with the child’s PREDNOS2 trial number and their date of birth in mmm/yyyy format only (there was no personal information about the child identifiable to the bench researchers).
Parents/guardians and/or children were asked whether or not they would also agree to submit their DNA for future studies to help understand genetic links to related medical conditions, but were equally free to opt out at any point. In this case, they would be able to request for and be assured that their sample had been destroyed after analysis for the PREDNOS2 laboratory subtrial.
Statistical methods
The primary comparison trial arms were those who were randomised to 6 days of daily prednisolone and those randomised to placebo. Analyses were based on the intention-to-treat (ITT) principle. The analysis population did not include all of the randomised population, as analyses were based on a modified ITT population, which included only those participants who had an URTI over the 12-month follow up period. Excluding those participants who did not have an URTI resulted in no bias, as taking the study medication was conditional on the child experiencing an URTI and this should, by chance, have been balanced between the two arms. Participants were analysed in the intervention arm to which they were randomised and participants were included whether or not they received the allocated intervention following an URTI.
Estimates of treatment effects are presented with two-sided 95% CIs. p-values are reported from two-sided tests at the 5% significance level. Analyses were adjusted for the minimisation variable (i.e. background therapy at randomisation) and baseline scores (where appropriate). The placebo arm is the reference group. No corrections for multiple tests were made. All analyses were carried out using SAS®, version 9.4 (SAS Institute Inc., Cary, NC, USA), or Stata®, version 16 (StataCorp LP, College Station, TX, USA).
The primary outcome was the incidence of first URR of nephrotic syndrome following any URTI during the 12-month follow-up period. This outcome is a binary outcome (i.e. yes/no). The number and percentage of children reporting an URR was reported. An adjusted risk difference and relative risk along with the respective 95% CI were estimated using binomial models with the identity and log-links, respectively. The statistical significance of the treatment arm parameter was determined from the p-value generated by the model. The number needed to treat (NNT) was also calculated. Three sensitivity analyses to assess the impact of missing data were undertaken for the primary outcome: (1) no URR (best)/URR (worse), (2) URR (worse)/no URR (best) for the prednisolone and placebo arms, respectively, and (3) an analysis that included the eight participants who had URTIs without relapse, but who did not complete 12 months’ follow-up and so it was not possible to assume that they did not have an URR during the 12-month trial follow-up period. In this third analysis, the data available were used and so they were included as ‘no’ for the primary outcome.
The number of children experiencing any relapse (i.e. both URRs and non-URRs), the number of children having an escalation of background immunosuppressant therapy and the number of children having a reduction in background immunosuppressant therapy were analysed as per the primary outcome measure. The rate of URRs and rate of any relapse were determined in the first instance as numbers of children who had a relapse with the respective percentage and in the second instance as a median and interquartile range (IQR). An adjusted incidence rate ratio with 95% CI was estimated using a Poisson regression model. An offset for the length of time the child was in the trial was included in the model.
The cumulative dose of prednisolone received over the 12-month period was initially planned to be reported as means with SDs, with an adjusted mean difference and 95% CI estimated from a linear regression model. These data were, however, skewed and so the median and IQR were also reported, along with the unadjusted median difference between arms and 95% CI estimated using bootstrapping methods, and the arms were instead compared using a Wilcoxon rank-sum test.
The PedsQL quality-of-life measure and ACBC measures were collected at multiple time points. The scores at each time point were summarised using means and SDs. Data were analysed using mixed-effect repeated measures models, with the minimisation variable and the baseline score included in the model as covariates. Time was included as a continuous variable in the model. In the initial model, a treatment-by-time cross-term was included in the model. If this was not significant, it was assumed that the treatment effect was constant over time and models without the treatment-by-time cross-term were fitted. Adjusted mean differences between arms are presented, alongside the respective 95% CIs.
The prednisolone AE data were collected at each time point (i.e. 3, 6, 9 and 12 months). To assess whether or not each AE occurred over the whole trial period, the 3-, 6-, 9- and 12-month data were also assessed collectively, with statistical significance determined by a chi-squared or Fisher’s exact test (as appropriate). The SAE data were summarised descriptively. The number of children experiencing any SAEs and suspected unexpected serious adverse reactions (SUSARs) was presented by arm. Statistical significance was determined by a chi-squared or Fisher’s exact test (as appropriate).
Subgroup analyses were limited to the primary outcome and the following defined categories based on the child’s background treatment regimen at randomisation:
-
no background immunosuppressive therapy compared with long-term maintenance prednisolone therapy, long-term maintenance prednisolone therapy plus other immunosuppressive therapies and other immunosuppressive therapies alone
-
on prednisolone compared with not on prednisolone
-
on other immunosuppressant compared with not on other immunosuppressant.
The effects of these subgroups were examined by including a treatment arm by subgroup interaction parameter in the final binomial model.
Ethics approval, regulations and trial registration
Ethics approval
Ethics approval for the PREDNOS2 trial was granted by the National Research Ethics Service Committee North West – Greater Manchester Central (reference 12/NW/0766) on 4 December 2012.
Substantial amendments made to the trial protocol are itemised in Table 4.
| Substantial amendment | Date | Details |
|---|---|---|
| 1 | 8 May 2013 | New PIS and consent forms, additional sites and change of PIs |
| 2 | 23 August 2013 | Additional sites and change of PI |
| 3 | 6 September 2013 | Change of PI |
| 4 | 20 February 2014 | Additional sites, change of PIs and protocol V1.2 |
| 5 | 5 February 2014 | Addition of site and change of PI |
| 6 | 1 August 2014 | Additional sites, change of PIs, advertising flyer and text for Renal Patient Support Group and renal/paediatric social media platforms and websites |
| 7 | 9 June 2015 | Additional sites, change of PIs, trust mergers, trust name change and protocol V1.3 |
| 8 | 3 August 2015 | Additional sites and change of PIs |
| 9 | 19 October 2015 | Additional sites, change of PIs, trust merger and parent/participant advice letters V1.2 |
| 10 | 3 December 2015 | Additional site and change of PIs |
| 11 | 28 October 2016 | Change of PI |
| 12 | 24 February 2017 | Changes of PI and change in trust name |
| 13 | 16 June 2017 | Changes of PI, trust merger, protocol V2.0 and other REC-approved documents V2.0, general administrative changes |
| 14 | 26 September 2017 | Additional site, change of PI and trust name change |
| 15 | 5 October 2017 | Letters associated with the re-issue of study medication were due to expire on 30 November 2017 |
| 16 | 7 March 2018 | Change of one of co-sponsor’s trust name (MHRA only) |
| 17 | 31 May 2018 | Changes of PI |
| 18 | 3 October 2018 | Change of CI, protocol V3.0, other REC-approved document changes, general administrative changes |
| 19 | 8 February 2019 | Changes of PI and trust mergers |
| 20 | 5 March 2020 | Changes of PI |
Sponsorship
The PREDNOS2 trial was sponsored by the Central Manchester University Hospitals NHS Foundation Trust (subsequently renamed the Manchester University NHS Foundation Trust) and the University of Birmingham (RG_12-188). The MHRA clinical trial authorisation reference was 21761/0281/001-0001. The EudraCT number was 2012-003476-39.
Regulations
The trial was conducted in compliance with the principles of the Declaration of Helsinki (1996)33 and the principles of GCP and in accordance with all applicable regulatory requirements, including, but not limited to, the Research Governance Framework for Health and Social Care34 and the Medicines for Human Use (Clinical Trials) Regulations 2004,29 as amended in 200630 and any subsequent amendments.
The active trial drug and placebo were manufactured in accordance with current good manufacturing process regulations.
Monitoring and oversight
Monitoring of this trial was carried out to ensure compliance with GCP. A risk-proportionate approach to the initiation, management and monitoring of the trial was adopted (as per Risk-Adapted Approaches to the Management of Clinical Trials of Investigational Medicinal Products35) and outlined in the trial-specific risk assessment.
Study oversight was provided by an independent TSC and DMC. The TSC provided overall supervision for the trial, providing advice on the study and assessing the progress of the trial. The DMC reviewed accumulating data and was responsible for assessing patient safety.
Patient, parent and public involvement
The PREDNOS2 trial design was discussed extensively with a number of patient and public involvement representatives, including representatives from NeST. This provided valuable input regarding the development of essential and patient-facing documentation for the trial and the acceptability of study visit frequency, as well as insight into the issues surrounding self-medication and addressing the complexity of calculating study drug dosage at home.
Patient and public involvement representatives worked with members of the trial team to develop advertising materials for social media to encourage patient recruitment through avenues such as the NeST Facebook page (URL: www.facebook.com, Facebook, Inc., Menlo Park, CA, USA). Representatives also disseminated information about the trial among their nephrotic syndrome contacts, encouraging those families/patients who may have benefited from participation in the PREDNOS2 trial to seek out more information and ask their local clinician about participation. These actions undoubtedly aided the trial’s recruitment.
Patient and public involvement representatives reviewed any changes to essential or patient-facing documentation throughout the trial as amendments were made. Representatives also had input throughout the trial through their membership of the TMG and TSC, and regularly attended meetings for both oversight groups.
Chapter 3 Results
Recruitment
The PREDNOS2 trial opened to recruitment in February 2013 and the first patient was randomised on 19 March 2013. The trial closed to recruitment, after 6 years, on 31 January 2019, with a total of 365 patients randomised from 91 of the 122 approved centres. Five patients more than the 360 patient recruitment target were included, as these patients had all received written information on the trial and verbally indicated their willingness to participate in the trial. The Trial Ethics Committee agreed that these patients should be offered randomisation to the trial.
The average rate of recruitment was 5.1 patients per month. After 2 years where the average recruitment rate had been 6.4 patients per month, the rate slowed to 4.6 patients per month for the remaining 47 months. Quarterly recruitment by randomised treatment is shown in Figure 3. The first period is 4 months (March–June 2013) and the final period is a single month (January 2019).
FIGURE 3.
Quarterly recruitment by randomised treatment.
Patients were recruited from 91 paediatric units of 122 approved units (74.6%) across the whole of the UK. The number of patients recruited per centre varied from 1 to 35. Individual site recruitment data are shown in Appendix 1, Table 31. The 13 UK tertiary paediatric renal units recruited 133 (36%, range 3–35) patients of the total trial population. As was seen with the PREDNOS trial,9 this highlights the important contribution of district general paediatric units, in which individual recruitment ranged from 1 to 17 participants.
Participants had completed 12 months’ follow-up by 31 January 2020.
Participant flow
Of the 365 children randomised into the PREDNOS2 trial, 182 were randomised to the intervention arm and 183 to the placebo arm. Thirty-two children (8.8%) did not complete the 12 months’ follow-up (13 children from the intervention arm and 19 children from the placebo arm). Of these withdrawals, 14 patients were withdrawn before having an URTI (six patients from the intervention arm and eight patients from the placebo arm). Eighteen patients were withdrawn after having an URTI (seven patients from the intervention arm and 11 patients from the placebo arm). Eighty children completed the 12 months’ follow-up without experiencing an URTI (42 children in the prednisolone arm and 38 children in the placebo arm). Therefore, in total, 48 children in the prednisolone arm and 46 children in the placebo arm were excluded from the trial analyses, as they did not have an URTI during the trial. This gave a modified ITT analysis population of 271 patients (i.e. 365 randomised patients – 94 patients with no URTI).
The CONSORT (Consolidated Standards of Reporting Trials) flow diagram36 for all randomised patients is shown in Figure 4. Patient flow for the modified ITT population is shown in Figure 5.
FIGURE 4.
A CONSORT flow diagram for all randomised patients.
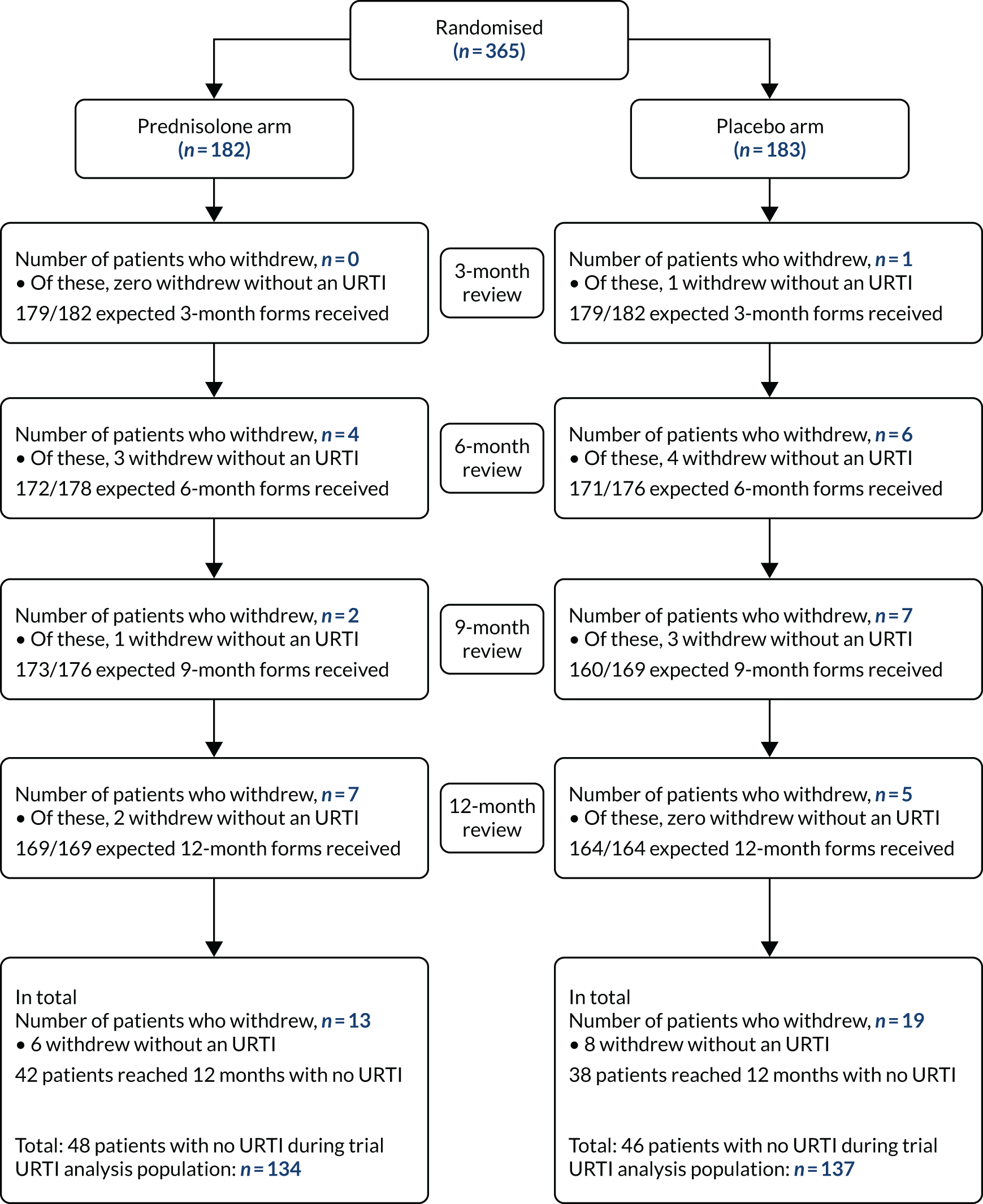
FIGURE 5.
A CONSORT flow diagram for the modified ITT population. Reproduced with permission from Christian et al. 37 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The figure includes minor additions and formatting changes to the original figure.
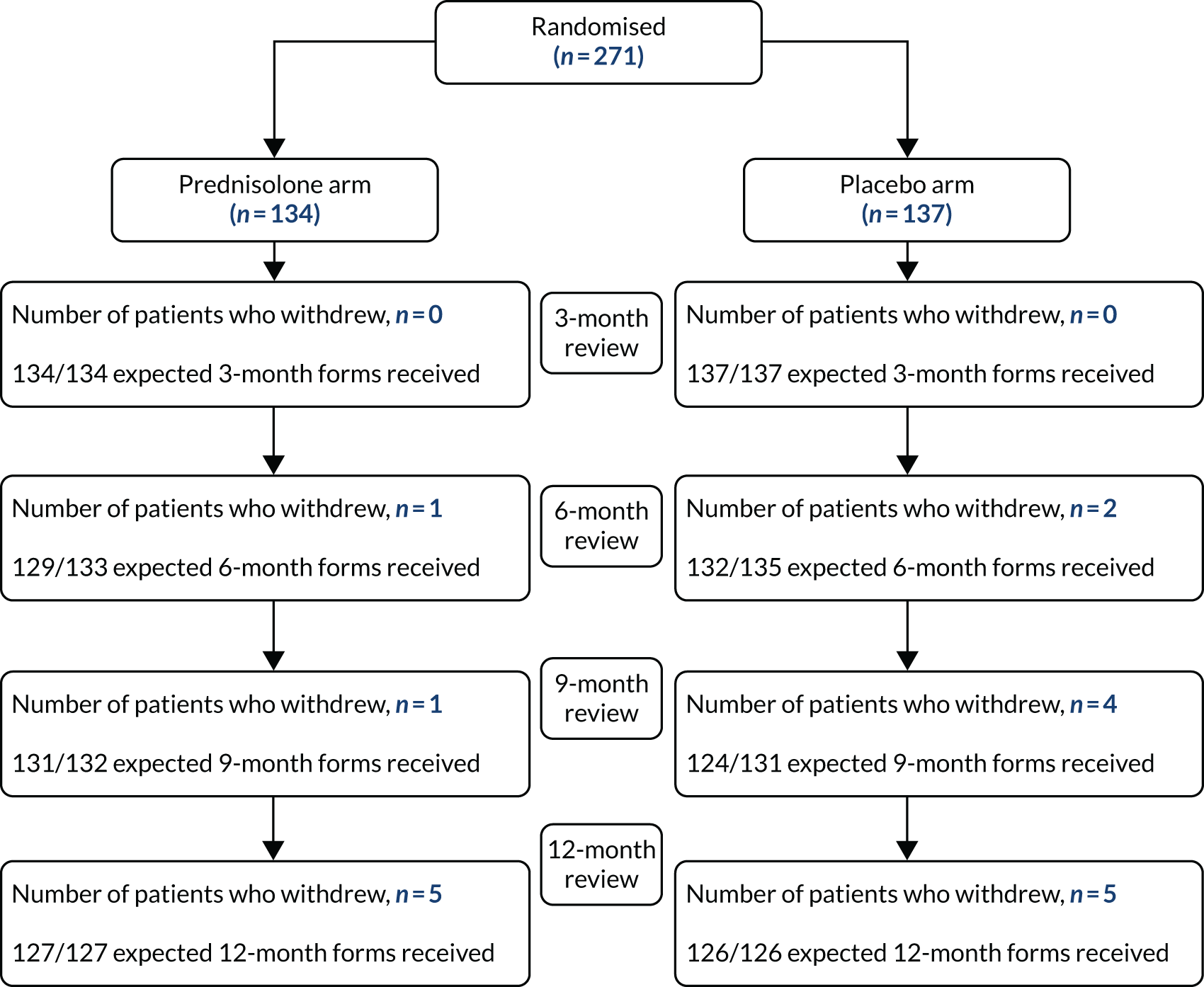
Overall reasons for withdrawal are detailed in Table 5.
| Withdrawals | Treatment arm | Total | |
|---|---|---|---|
| Prednisolone | Placebo | ||
| Number randomised | 182 | 183 | 365 |
| Total withdrawals, n | 13 | 19 | 32 |
| Withdrew consent | 8 | 10 | 18 |
| Moved away | 2 | 5 | 7 |
| Other | 3a | 4b | 7 |
| Number of withdrawals without an URTI | 6 | 8 | 14 |
| Number who reached 12 months without an URTI, n/N (%) | 42/169 (25) | 38/164 (23) | 80/333 (24) |
| Exclusions due to not experiencing an URTI, n/N (%) | 48/182 (26) | 46/183 (25) | 94/365 (26) |
| Total, n/N (%) | 55/182 (30) | 57/183 (31) | 112/365 (31) |
Withdrawals and analysis population
Of the 365 children randomised:
-
32 children were withdrawn or lost to follow-up from the trial prior to completing the 12 months assessment –
-
18 of these children had an URTI prior to withdrawal or being lost to follow-up
-
14 of these children had no URTI prior to withdrawal or being lost to follow-up
-
-
333 children completed the 12-month follow-up assessment –
-
253 of these children had an URTI
-
80 of these children had no URTI at any point in the trial.
-
This gives a modified ITT analysis population of 271 patients (i.e. 253 + 18).
Details of the children who were excluded are shown in Table 5 for the whole randomised population and in Table 6 for the modified ITT population.
| Withdrawals | Treatment arm | Total | |
|---|---|---|---|
| Prednisolone | Placebo | ||
| Modified ITT population, n | 134 | 137 | 271 |
| Total withdrawals, n (%) | 7 (5) | 11 (8) | 18 (7) |
| Withdrew consent, n | 4 | 4 | 8 |
| Moved away, n | 0 | 4 | 4 |
| Other, n | 3a | 3b | 6 |
| Proportion who completed 12 months’ follow-up, n (%) | 127 (95) | 126 (92) | 253 (93) |
Completeness of data
Data completion was very high. For the modified ITT population at all four data collection time points, a total of 1040 forms out of a possible 1055 were received (98.6%; 99.0% from the intervention arm and 98.1% from the placebo arm).
Baseline data
Randomisation was minimised according to baseline treatment. Details of baseline demographics for the whole randomised population and for the modified ITT population are shown in Tables 7 and 8, respectively. For the modified ITT analysis population, the mean age at recruitment was 7.6 (SD 3.6) years, 64% of participants were male, 64% of participants were white and the duration of SSNS at trial entry was 3.2 years.
| Demographic | Treatment arm | |
|---|---|---|
| Prednisolone | Placebo | |
| Total randomised, n | 182 | 183 |
| Background treatment regimen, n (%)a | ||
| No long-term treatment | 44 (24) | 42 (23) |
| Long-term maintenance prednisolone | 48 (26) | 49 (27) |
| On other immunosuppressant therapy plus long-term maintenance prednisolone therapy | 61 (34) | 63 (34) |
| On other immunosuppressant therapy only | 29 (16) | 29 (16) |
| Age (years), mean (SD) | 7.6 (3.5) | 7.6 (3.5) |
| Sex, n (%) | ||
| Male | 117 (64) | 121 (66) |
| Female | 65 (36) | 62 (34) |
| BMI percentile | ||
| Median (IQR) | 85.0 (64.4–97.2) | 84.8 (65.2–96.9) |
| Underweight (< 5th), n (%) | 0 (0) | 2 (1) |
| Healthy (5th–84th), n (%) | 91 (50) | 90 (49) |
| Overweight (85th–94th), n (%) | 34 (19) | 34 (19) |
| Obese (≥ 95th), n (%) | 57 (31) | 57 (31) |
| Prednisolone dose (mg on alternate days), mean (SD) [n] | 9.2 (3.8) [109b] | 8.8 (3.4) [111b] |
| Missing, n | 0 | 1 |
| Ethnicity, n (%) | ||
| British | 118 (65) | 115 (63) |
| Irish | 0 (0) | 4 (2) |
| Other white background | 12 (7) | 5 (3) |
| African | 2 (1) | 4 (2) |
| Other black British background | 2 (1) | 3 (2) |
| Indian | 11 (6) | 10 (5) |
| Pakistani | 12 (7) | 14 (8) |
| Bangladeshi | 6 (3) | 6 (3) |
| Sri Lankan | 3 (2) | 1 (< 1) |
| Other Asian background | 3 (2) | 3 (2) |
| White and black Caribbean | 1 (< 1) | 2 (1) |
| White and black African | 1 (< 1) | 4 (2) |
| White and Asian | 5 (3) | 5 (3) |
| Other mixed background | 2 (1) | 2 (1) |
| Chinese | 0 (0) | 1 (< 1) |
| Other ethnic group | 2 (1) | 3 (2) |
| Not stated | 2 (1) | 1 (< 1) |
| Age (years) at diagnosis of nephrotic syndrome, mean (SD) | 4.4 (2.5) | 4.6 (2.8) |
| Time (days) from last relapse to randomisation | ||
| Median (IQR) | 89 (58–138) | 91 (61–126) |
| Minimum, maximum | 7, 280 | 7, 293 |
| Time (days) from second last relapse to randomisation | ||
| Median (IQR) | 209.5 (148–287) | 189 (146–264) |
| Minimum, maximum | 42, 364 | 36, 365 |
| Demographic | Treatment arm | |
|---|---|---|
| Prednisolone | Placebo | |
| Modified ITT analysis population, n | 134 | 137 |
| Background treatment regimen, n (%)a | ||
| No long-term treatment | 31 (23) | 31 (23) |
| Long-term maintenance prednisolone | 40 (30) | 34 (25) |
| On other immunosuppressant therapy plus long-term maintenance prednisolone therapy | 43 (32) | 48 (35) |
| On other immunosuppressant therapy only | 20 (15) | 24 (17) |
| Age (years), mean (SD) | 7.7 (3.6) | 7.5 (3.5) |
| Sex, n (%) | ||
| Male | 83 (62) | 91 (66) |
| Female | 51 (38) | 46 (34) |
| BMI percentile | ||
| Median (IQR) | 84.1 (63.7–96.9) | 86.4 (68.4–97.0) |
| Underweight (< 5th), n (%) | 0 (0) | 0 (0) |
| Healthy (5th–84th), n (%) | 69 (51) | 64 (47) |
| Overweight (85th–94th), n (%) | 24 (18) | 30 (22) |
| Obese (≥ 95th), n (%) | 41 (31) | 43 (31) |
| Prednisolone dose (mg on alternate days), mean (SD) [n] | 9.2 (3.7) [83b] | 8.4 (3.1) [81b] |
| Missing, n | 0 | 1 |
| Ethnicity, n (%) | ||
| British | 85 (63) | 88 (64) |
| Irish | 0 (0) | 2 (1) |
| Other white background | 11 (8) | 2 (1) |
| African | 1 (1) | 3 (2) |
| Other black British background | 1 (1) | 2 (1) |
| Indian | 9 (7) | 7 (5) |
| Pakistani | 9 (7) | 9 (7) |
| Bangladeshi | 4 (3) | 4 (3) |
| Sri Lankan | 2 (1) | 1 (1) |
| Other Asian background | 3 (2) | 2 (1) |
| White and black Caribbean | 0 (0) | 2 (1) |
| White and black African | 1 (1) | 4 (3) |
| White and Asian | 3 (2) | 5 (4) |
| Other mixed background | 1 (1) | 2 (1) |
| Other ethnic group | 2 (1) | 3 (2) |
| Not stated | 2 (1) | 1 (1) |
| Age (years) at diagnosis of nephrotic syndrome, mean (SD) | 4.4 (2.5) | 4.4 (2.8) |
| Time (days) from last relapse to randomisation | ||
| Median (IQR) | 90 (58–143) | 87 (58–126) |
| Minimum, maximum | 14, 280 | 7, 280 |
| Time (days) from second last relapse to randomisation | ||
| Median (IQR) | 209.5 (153–287) | 189 (146–252) |
| Minimum, maximum | 42, 363 | 36, 365 |
Adherence
Adherence to the trial medication was summarised at each follow-up visit according to the following:
-
Did the parent commence their child on trial medication at the time of the URTI?
-
If yes, what was the number of days to starting medication from the start of the URTI?
-
-
Was the whole 6-day course of trial mediation taken?
-
If no, how many days were missed?
-
-
Was the 6-day course prematurely discontinued?
Overall adherence for the 12-month trial period is shown in Table 9. In total, trial medication was commenced at the time of an URTI for 691 of 791 URTIs (87.3%; 85.4% of URTIs in the intervention arm and 89.2% of URTIs in the placebo arm). There were no differences in rates of adherence for each 3-month period throughout the trial. Reasons for not starting the trial medication at the time of URTI included the parent/guardian forgetting, medication had not arrived in time and child already relapsed and/or on treatment for relapse.
| Adherence | Treatment arm | |
|---|---|---|
| Prednisolone | Placebo | |
| Total number of URTI’s reported | 384 | 407 |
| Trial medication commenced at time of URTI?, n (%) | 384 | 407 |
| No | 52 (13.5) | 42 (10.3) |
| Yes | 328 (85.4) | 363 (89.2) |
| Missing | 1 (0.3) | 0 (0.0) |
| Unknowna | 3 (0.8) | 2 (0.5) |
| If yes: the number of days to start medication from URTI | 326 | 362 |
| Median (IQR) | 0 (0–1) | 0 (0–1) |
| Minimum, maximum | 0, 7 | 0, 6 |
| If yes: was the whole 6-day course of trial medication taken?, n | ||
| No | 55 | 50 |
| Yes | 272 | 313 |
| Missing | 1 | 0 |
| If no: how many days missed? | 55 | 50 |
| Median (IQR) | 3 (2–4) | 2 (1–3) |
| Minimum, maximum | 1, 5 | 1, 5 |
| Was the 6-day course prematurely discontinued?, n | ||
| No | 273 | 319 |
| Yes | 54 | 44 |
| Missing | 1 | 0 |
Primary outcome
All outcome analyses were carried out on the modified ITT population of 271 children and excluded the 80 children who completed 12 months of follow-up without having had an URTI and the 14 children who were withdrawn without having had an URTI.
The median time from randomisation to first URTI was 61 (IQR 21–126) days in the intervention arm and 54 (IQR 23–98) days in the placebo arm. There were 384 URTIs and 82 URRs in the intervention arm and 407 URTIs and 82 URRs in the placebo arm. One patient in the placebo arm had an URTI, but no information was provided on whether or not they had a URR and so their data were classed as missing. Three patients in the intervention arm and five patients in the placebo arm had URTIs but withdrew before the 12-month follow-up and did not report any URR for any time points for which they provided data. These patients were excluded from the primary analysis, but were included in a sensitivity analysis (Table 10).
| Outcome/analysis | Treatment arm | Risk difference (95% CI) | Risk ratio (95% CI) | p-valueb | |||
|---|---|---|---|---|---|---|---|
| Prednisolone | Placebo | Unadjusted | Adjusteda | Unadjusted | Adjusteda | ||
| Number of modified ITT patients | 134 | 137 | |||||
| Total number of URTIs | 384 | 407 | |||||
| Number of URRs | 82 | 82 | |||||
| Primary outcome | |||||||
| Number of patients experiencing an URR, n | 131 | 131 | |||||
| No, n (%) | 75 (57.3) | 73 (55.7) | –0.02 (–0.14 to 0.10) | –0.02 (–0.14 to 0.09) | 0.97 (0.73 to 1.27) | 0.96 (0.74 to 1.26) | 0.70 |
| Yes, n (%) | 56 (42.7) | 58 (44.3) | |||||
| Sensitivity analyses for primary outcome | |||||||
| Best case (no URR) for prednisolone/worse case (URR) for placebo, n | 134 | 137 | |||||
| No, n (%) | 78 (58.2) | 73 (53.3) | –0.05 (–0.17 to 0.07) | –0.05 (–0.17 to 0.06) | 0.89 (0.68 to 1.17) | 0.90 (0.69 to 1.16) | 0.35 |
| Yes, n (%) | 56 (41.8) | 64 (46.7) | |||||
| Worst case (URR) for prednisolone/best case (no URR) for placebo, n | 134 | 137 | |||||
| No, n (%) | 75 (56.0) | 79 (57.7) | 0.02 (–0.10 to 0.13) | 0.01 (–0.10 to 0.13) | 1.04 (0.79 to 1.37) | 1.04 (0.80 to 1.36) | 0.84 |
| Yes, n (%) | 59 (44.0) | 58 (42.3) | |||||
| Sensitivity analysis including the eight participants who did not complete 12 months’ follow-up who, from the available data, did not have an URR, n | 134 | 136 | |||||
| No, n (%) | 78 (58.2) | 78 (57.4) | –0.01 (–0.13 to 0.11) | –0.01 (–0.13 to 0.10) | 0.98 (0.74 to 1.30) | 0.99 (0.75 to 1.30) | 0.81 |
| Yes, n (%) | 56 (41.8) | 58 (42.6) | |||||
There was no difference in the proportion of children experiencing an URR between the two arms of the trial. In the intervention arm, 56 children (42.7%) experienced an URR, compared with 58 children (44.3%) in the placebo arm. The adjusted risk difference was –0.02 (95% CI –0.14 to 0.09; p = 0.70) and the adjusted risk ratio was 0.96 (95% CI 0.74 to 1.26). The NNT with prednisolone to avoid one URR was 42.
There were three predefined subgroup analyses according to background treatment. There was no evidence that the treatment effect differed when the data were analysed by these subgroups (Table 11).
| Subgroup | Treatment arm, n/N (%) | Interaction p-value | Risk ratio (95% CI) | |
|---|---|---|---|---|
| Prednisolone (N = 131) | Placebo (N = 131) | |||
| Background treatment | ||||
| No long-term treatment | 19/31 (61) | 16/29 (55) | 0.85 | 1.11 (0.72 to 1.71) |
| Long-term maintenance prednisolone | 16/39 (41) | 15/31 (48) | 0.85 (0.50 to 1.43) | |
| On other immunosuppressant therapy only | 7/20 (35) | 8/23 (35) | 1.01 (0.44 to 2.28) | |
| On other immunosuppressant therapy plus long-term maintenance prednisolone therapy | 14/41 (34) | 19/48 (40) | 0.86 (0.50 to 1.50) | |
| On background prednisolone | ||||
| No | 26/51 (51) | 24/52 (46) | 0.38 | 1.09 (0.74 to 1.59) |
| Yes | 30/80 (38) | 34/79 (43) | 0.85 (0.58 to 1.25) | |
| On background immunosuppressants | ||||
| No | 35/70 (50) | 31/60 (52) | 0.73 | 1.00 (0.71 to 1.39) |
| Yes | 21/61 (34) | 27/71 (38) | 0.90 (0.57 to 1.43) | |
Secondary outcomes
Rate of upper respiratory tract infection-related relapses
There was no difference in the number of URRs experienced by any one child between the two trial treatment arms (adjusted incidence rate ratio 0.98, 95% CI 0.72 to 1.33) (Table 12).
| URR | Treatment arm | Rate ratio (95% CI) | p-valueb | ||
|---|---|---|---|---|---|
| Prednisolone | Placebo | Unadjusted | Adjusteda | ||
| Number of modified ITT patients | 134 | 137 | |||
| Number of patients by URR rate, n | 134 | 136 | |||
| Zero, n (%) | 78 (58.2) | 78 (57.4) | 0.99 (0.73 to 1.34) | 0.98 (0.72 to 1.33) | 0.88 |
| One, n (%) | 36 (26.9) | 41 (30.1) | |||
| Two, n (%) | 15 (11.2) | 10 (7.4) | |||
| Three, n (%) | 4 (3.0) | 7 (5.1) | |||
| Four, n (%) | 1 (0.8) | 0 (0) | |||
| Number of URRs | |||||
| Median (IQR) | 0 (0–1) | 0 (0–1) | |||
| Range | 0–4 | 0–3 | |||
| Number of URRs when excluding children who did not relapse | |||||
| Median (IQR) | 1 (1–2) | 1 (1–2) | |||
| Range | 1–4 | 1–3 | |||
Rate of upper respiratory tract infection-related and non-upper respiratory tract infection-related relapses
There were 216 URRs and non-URRs in 132 children in the prednisolone arm, compared with 237 URRs in 132 children in the placebo arm. There was no difference in the proportion of children experiencing any relapse between the two arms of the trial. In the intervention arm, 91 children (68.9%) experienced a relapse, compared with 98 children (74.2%) in the placebo arm. The adjusted risk difference was –0.05 (95% CI –0.16 to 0.06; p = 0.33) (Table 13).
| Overall relapses | Treatment arm | Risk difference (95% CI) | Risk ratio (95% CI) | p-valueb | |||
|---|---|---|---|---|---|---|---|
| Prednisolone | Placebo | Unadjusted | Adjusteda | Unadjusted | Adjusteda | ||
| Number of modified ITT patients | 134 | 137 | |||||
| Number of relapses | 216 | 237 | |||||
| Patients experiencing any relapse, n | 132 | 132 | –0.05 (–0.16 to 0.06) | –0.05 (–0.16 to 0.05) | 0.93 (0.80 to 1.08) | 0.93 (0.80 to 1.09) | 0.33 |
| No, n (%) | 41 (31.1) | 34 (25.8) | |||||
| Yes, n (%) | 91 (68.9) | 98 (74.2) | |||||
The overall number of relapses per child varied between zero and nine over the 12-month trial. There was no difference in the overall rate of relapses between the two trial arms (Table 14).
| Overall relapse rate | Treatment arm | Rate ratio (95% CI) | p-valueb | ||
|---|---|---|---|---|---|
| Prednisolone | Placebo | Unadjusted | Adjusteda | ||
| Number of modified ITT patients | 134 | 137 | |||
| Number of patients by relapse rate | 134 | 136 | |||
| Zero, n (%) | 43 (32.1) | 38 (27.9) | 0.90 (0.75 to 1.08) | 0.89 (0.74 to 1.07) | 0.23 |
| One, n (%) | 28 (20.9) | 39 (28.7) | |||
| Two, n (%) | 24 (17.9) | 24 (17.7) | |||
| Three, n (%) | 22 (16.4) | 11 (8.1) | |||
| Four, n (%) | 11 (8.2) | 14 (10.3) | |||
| Five, n (%) | 6 (4.5) | 5 (3.7) | |||
| Six, n (%) | 0 (0) | 2 (1.5) | |||
| Seven, n (%) | 0 (0) | 1 (0.7) | |||
| Eight, n (%) | 0 (0) | 1 (0.7) | |||
| Nine, n (%) | 0 (0) | 1 (0.7) | |||
| Number of relapses | |||||
| Median (IQR) | 1 (0–3) | 1 (0–3) | |||
| Range | 0–5 | 0–9 | |||
| Number of relapses when excluding children who did not relapse | |||||
| Median (IQR) | 2 (1–3) | 2 (1–3) | |||
| Range | 1–5 | 1–9 | |||
Escalation and reduction in background therapy
Background treatment regime was categorised into the following four categories: (1) no long-term alternate-day prednisolone or other long-term immunosuppressive therapies, (2) on long-term alternate-day prednisolone only, (3) on both long-term alternate-day prednisolone and other long-term immunosuppressive therapies and (4) on other long-term immunosuppressive therapies only.
The background treatment regime and second-line immunosuppressant information was used in the following way to calculate escalation/reduction of background immunosuppressant therapy.
Escalation was computed based on the following criteria:
-
If a child was in category 1 of the background treatment regime at the previous visit and then at a following time point was in category 2, 3 or 4 of the background treatment regime, then this was considered an escalation.
-
If a child was in category 2 of the background treatment regime at the previous visit and then at a following time point was in category 3 or 4 of the background treatment regime, then this was considered an escalation.
-
If a child was in category 4 of the background treatment regime at the previous visit and then at a following time point was in category 3 of the background treatment regime, then this was considered an escalation.
-
If a child was not on cyclophosphamide or rituximab at the previous visit but was then given cyclophosphamide or rituximab at the following time point, then this was considered an escalation.
-
If a child had an addition of any immunosuppressant (e.g. addition of ciclosporin) in between follow-up visits, then this was considered an escalation.
-
If a child had a change in medication from levamisole to one of tacrolimus, ciclosporin or mycophenolate, then this was considered an escalation.
Reduction was computed based on the following criteria:
-
If a child was in category 3 of the background treatment regime at the previous visit and then at a following time point was in category 4, 2 or 1 of the background treatment regime, then this was considered a reduction.
-
If a child was in category 4 of the background treatment regime at the previous visit and then at a following time point was in category 2 or 1 of the background treatment regime, then this was considered a reduction.
-
If a child was in category 2 of the background treatment regime at the previous visit and then at a following time point was in category 1 of the background treatment regime, then this was considered a reduction.
-
If a child was on an immunosuppressant (except cyclophosphamide or rituximab) at one time point but was no longer on it at the following time point, then this was considered a reduction.
There were no significant differences in the incidence of escalation or reduction in background therapy between the arms of the trial (Table 15). Background treatment was escalated on at least one occasion in 58 patients (44.6%) in the prednisolone arm compared with 57 patients (44.5%) in the placebo arm (p = 0.96). Fifty-five patients (43.0%) in the prednisolone arm had at least one treatment reduction during the trial, compared with 62 patients (48.1%) in the placebo arm (p = 0.42).
| Incidence of escalation and reduction | Treatment arm | Risk difference (95% CI) | Risk ratio (95% CI) | p-valueb | |||
|---|---|---|---|---|---|---|---|
| Prednisolone | Placebo | Unadjusted | Adjusteda | Unadjusted | Adjusteda | ||
| Number of modified ITT patients | 134 | 137 | |||||
| Escalation of background immunosuppressant therapy | |||||||
| Number of patients having an escalation | 130 | 128 | 0.00 (–0.12 to 0.12) | –0.00 (–0.12 to 0.12) | 1.00 (0.76 to 1.32) | 0.98 (0.75 to 1.29) | 0.96 |
| No, n (%) | 72 (55.4) | 71 (55.5) | |||||
| Yes, n (%) | 58 (44.6) | 57 (44.5) | |||||
| Reduction of background immunosuppressant therapy | |||||||
| Number of patients having a reduction | 128 | 129 | –0.05 (–0.17 to 0.07) | –0.04 (–0.15 to 0.06) | 0.89 (0.68 to 1.17) | 0.91 (0.71 to 1.16) | 0.42 |
| No, n (%) | 73 (57.0) | 67 (51.9) | |||||
| Yes, n (%) | 55 (43.0) | 62 (48.1) | |||||
Cumulative dose of prednisolone over the 12-month trial period
Over the 12-month trial period, the median cumulative dose of prednisolone received was 2060 mg (IQR 1127.5–3355 mg) for children in the prednisolone arm and 1880 mg (IQR 1115–3295 mg) in the placebo arm (Table 16). There was no significant difference in these cumulative doses between the trial arms (unadjusted difference between medians 180 mg, 95% CI –302 to 662 mg; p = 0.72 from a Wilcoxon rank-sum test).
| Cumulative prednisolone dose (mg) | Treatment arm | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| Prednisolone | Placebo | Unadjusted | ||
| Number of modified ITT patients | 134 | 137 | ||
| n | 126 | 125 | ||
| Mean (SD) | 2371.9 (1669.0) | 2481.2 (2190.6) | ||
| Median (IQR) | 2060 (1127.5–3355) | 1880 (1115–3295) | 180 (–301.83 to 661.83) | 0.72a |
| Range | 60–7155 | 0–13185 | ||
There were 13 children in the placebo who received no prednisolone during the trial. None of these children experienced a relapse.
Incidence of serious adverse events
There were 93 SAEs reported in 60 children (60/271, 22%). There were no SUSARs or deaths (Table 17).
| SAE | Treatment arm | p-value | |
|---|---|---|---|
| Prednisolone | Placebo | ||
| Total number of SAEs | 40 | 53 | |
| Number of patients experiencing at least one SAE | 29/134 (21.6%) | 31/137 (22.6%) | 0.85 |
| Number of SUSARs | 0 | 0 | |
| Number of deaths | 0 | 0 | |
The most common reason for a SAE was an admission directly due to a relapse of SSNS. The second most common SAE was an admission for another reason, such as the development of chickenpox.
Incidence of adverse effects of prednisolone
The incidence of specific corticosteroid AEs by treatment arm was as expected (Table 18).
| AE | Treatment arm | p-value | |
|---|---|---|---|
| Prednisolone | Placebo | ||
| n | 134 | 137 | |
| Cushingoid facies, n (%) | 41 (30.6) | 41 (29.9) | 0.90 |
| Striae, n (%) | 9 (6.7) | 8 (5.8) | 0.77 |
| Hypertrichosis, n (%) | 16 (11.9) | 18 (13.1) | 0.77 |
| Acne, n (%) | 12 (9) | 11 (8) | 0.78 |
| Increased appetite, n (%) | 68 (50.7) | 80 (58.4) | 0.21 |
| Poor behaviour, n (%) | 60 (44.8) | 74 (54) | 0.13 |
| Glycosuria, n (%) | 11 (8.2) | 8 (5.8) | 0.45 |
| Abdominal pain, n (%) | 35 (26.1) | 33 (24.1) | 0.70 |
Achenbach Child Behaviour Checklist
There were no differences in behaviour scores for either the T-score or total score between each arm of the trial (Table 19). The ACBC T-scores and total scores were, on average, 1 point and 1.4 points lower in the prednisolone arm than in the placebo arm.
| ACBC scores | Treatment arm | Adjusteda mean difference (95% CI); p-value | Treatment-by-time interaction p-value | |
|---|---|---|---|---|
| Prednisolone | Placebo | |||
| Total problems T-score | ||||
| Baseline | –1.02 (–2.91 to 0.86); 0.29 | 0.11 | ||
| n | 131 | 131 | ||
| Mean (SD) | 50.2 (13.3) | 50.9 (13.6) | ||
| 3 months | ||||
| n | 127 | 133 | ||
| Mean (SD) | 48 (13.7) | 49.7 (14.2) | ||
| 6 months | ||||
| n | 117 | 123 | ||
| Mean (SD) | 47.4 (13.5) | 48.1 (13.9) | ||
| 9 months | ||||
| n | 121 | 115 | ||
| Mean (SD) | 45.8 (13.8) | 46.4 (13.2) | ||
| 12 months | ||||
| n | 118 | 121 | ||
| Mean (SD) | 45 (12.9) | 47.8 (14.4) | ||
| Total problems total score | ||||
| Baseline | –1.43 (–5.00 to 2.14); 0.43 | 0.05 | ||
| n | 131 | 132 | ||
| Mean (SD) | 30.2 (27.5) | 31.5 (26.6) | ||
| 3 months | ||||
| n | 127 | 133 | ||
| Mean (SD) | 26.8 (28) | 29.7 (29.5) | ||
| 6 months | ||||
| n | 119 | 124 | ||
| Mean (SD) | 24.9 (25.7) | 26.5 (26.9) | ||
| 9 months | ||||
| n | 122 | 115 | ||
| Mean (SD) | 23.1 (24.6) | 23.1 (24) | ||
| 12 months | ||||
| n | 119 | 121 | ||
| Mean (SD) | 20.7 (22.6) | 26.1 (29.7) | ||
Quality-of-life measures
Quality of life, as measured using the PedsQL questionnaire, was recorded at each trial visit. Small differences in scores were seen favouring the prednisolone arm. The physical health summary score was, on average, 2.8 points better, psychosocial health summary score was 0.98 points better and total score was 1.5 points better with prednisolone than placebo. These differences are not considered clinically meaningful and they did not reach statistical significance (Table 20).
| PedsQL scores | Treatment arm | Adjusteda mean difference (95% CI); p-value | Treatment-by-time interaction p-value | |
|---|---|---|---|---|
| Prednisolone | Placebo | |||
| Physical health summary score | ||||
| Baseline | 2.77 (–0.27 to 5.80); 0.07 | 0.85 | ||
| n | 133 | 135 | ||
| Mean (SD) | 83.5 (20.2) | 84.3 (16.9) | ||
| 3 months | ||||
| n | 131 | 132 | ||
| Mean (SD) | 84.2 (19) | 79.9 (20.8) | ||
| 6 months | ||||
| n | 124 | 124 | ||
| Mean (SD) | 84.5 (19) | 83.5 (17.7) | ||
| 9 months | ||||
| n | 125 | 117 | ||
| Mean (SD) | 85.5 (18.2) | 83.9 (19.9) | ||
| 12 months | ||||
| n | 124 | 124 | ||
| Mean (SD) | 85.6 (18.6) | 82.2 (20.7) | ||
| Psychosocial health summary score | ||||
| Baseline | 0.98 (–1.40 to 3.36); 0.42 | 0.95 | ||
| n | 133 | 135 | ||
| Mean (SD) | 81 (16.8) | 79.4 (15.5) | ||
| 3 months | ||||
| n | 131 | 132 | ||
| Mean (SD) | 81.8 (15.8) | 78.3 (15.2) | ||
| 6 months | ||||
| n | 124 | 124 | ||
| Mean (SD) | 82.1 (16.4) | 81.5 (14.8) | ||
| 9 months | ||||
| n | 125 | 117 | ||
| Mean (SD) | 83.4 (15.3) | 82.4 (14.6) | ||
| 12 months | ||||
| n | 124 | 124 | ||
| Mean (SD) | 84.8 (16) | 81.7 (16.3) | ||
| Total score | ||||
| Baseline | 1.52 (–0.83 to 3.87); 0.20 | 0.98 | ||
| n | 133 | 135 | ||
| Mean (SD) | 81.9 (16.5) | 81.2 (14.5) | ||
| 3 months | ||||
| n | 131 | 132 | ||
| Mean (SD) | 82.7 (15.4) | 78.9 (15.9) | ||
| 6 months | ||||
| n | 124 | 124 | ||
| Mean (SD) | 83 (16.2) | 82.2 (14) | ||
| 9 months | ||||
| n | 125 | 117 | ||
| Mean (SD) | 84.2 (15) | 82.9 (15.5) | ||
| 12 months | ||||
| n | 124 | 124 | ||
| Mean (SD) | 85.1 (15.3) | 81.9 (16.9) | ||
Chapter 4 Economic analysis and evaluation
Introduction
The economic evaluation was undertaken alongside the PREDNOS2 trial to estimate the cost-effectiveness of a short course of daily prednisolone therapy at the time of URTI compared with usual care (use of placebo) for treating children with SSNS. The primary evaluation was a model-based cost–utility analysis, with the outcome measured in terms of QALYs.
Aim
The aim was to estimate the cost-effectiveness of a 6-day course of daily prednisolone given early in the course of an URTI compared with standard care in treating children with relapsing SSNS.
Methods
A model-based economic evaluation in the form of a cost–utility analysis based on the outcome of cost per QALY was performed alongside the PREDNOS2 trial. The analysis assessed the difference in costs and difference in QALYs from a NHS perspective. An ITT analysis was adopted, as the economic evaluation was conducted alongside the clinical trial. Two different time horizons were considered: (1) the primary analysis assessed the cost-effectiveness at 1 year and (2) the secondary analysis extrapolated the results over 16 years (when the model cohort reached 18 years). Although a societal perspective was considered, this was not feasible within the resource constraints of the trial.
Participants and data collection
The model included 271 children aged from 1 to 17 years randomised within the trial who had experienced at least one URTI during the 12-month follow-up period. Data on both resource use and outcomes were collected within the study using case report forms. To calculate the overall cost of the treatment, resource use data were multiplied by relevant unit costs. Quality of life (i.e. HRQoL) was measured using the CHU-9D,25 PedsQL26 and EQ-5D instruments,38 depending on the age of the participants. HRQoL and cost data were collected at baseline and then at 3, 6, 9 and 12 months.
Economic model
A decision-analytic model was developed to estimate the incremental cost and incremental QALYs from administering a 6-day course of prednisolone therapy at the time of an URTI compared with standard care. The time horizon for the primary analysis was 12 months, based on the follow-up period of the PREDNOS2 trial. According to the trial follow-up data, children frequently changed their background therapy throughout the 12-month period, which had an impact on the costs and HRQoL. A decision-analytic model was, therefore, deemed the most appropriate method to capture these changes in background therapy in a way that a typical trial-based analysis following a regression framework could not. Therefore, it was decided to develop a state transition Markov cohort model using TreeAge Pro (TreeAge Software, Inc., Williamstown, MA, USA). Markov models are especially useful for handling multiple treatment consequences on both costs and health outcomes concomitantly. 39 In addition, they are ideally suited to model chronic conditions with fluctuating severity, while taking into account the repetitive nature of events. 39 All model parameters came from the trial data.
Model structure
The model structure was based on the treatment pathways that patients followed in the trial and was informed by clinical input. At the 1-year time point, the inclusion of death was not considered appropriate, as the intervention did not cause death and the likelihood of death from natural causes was minimal because of the age of the patients. Although SSNS is not considered a fatal disease, all-cause mortality was incorporated into the extrapolated model to account for deaths by other causes, and mortality tables were retrieved from the Office for National Statistics. 40 Figure 6 presents the clinical pathways that patients followed in the PREDNOS2 trial. This was the same for both treatment arms.
FIGURE 6.
Treatment pathways for the daily prednisolone and standard-care arms in the Markov model.
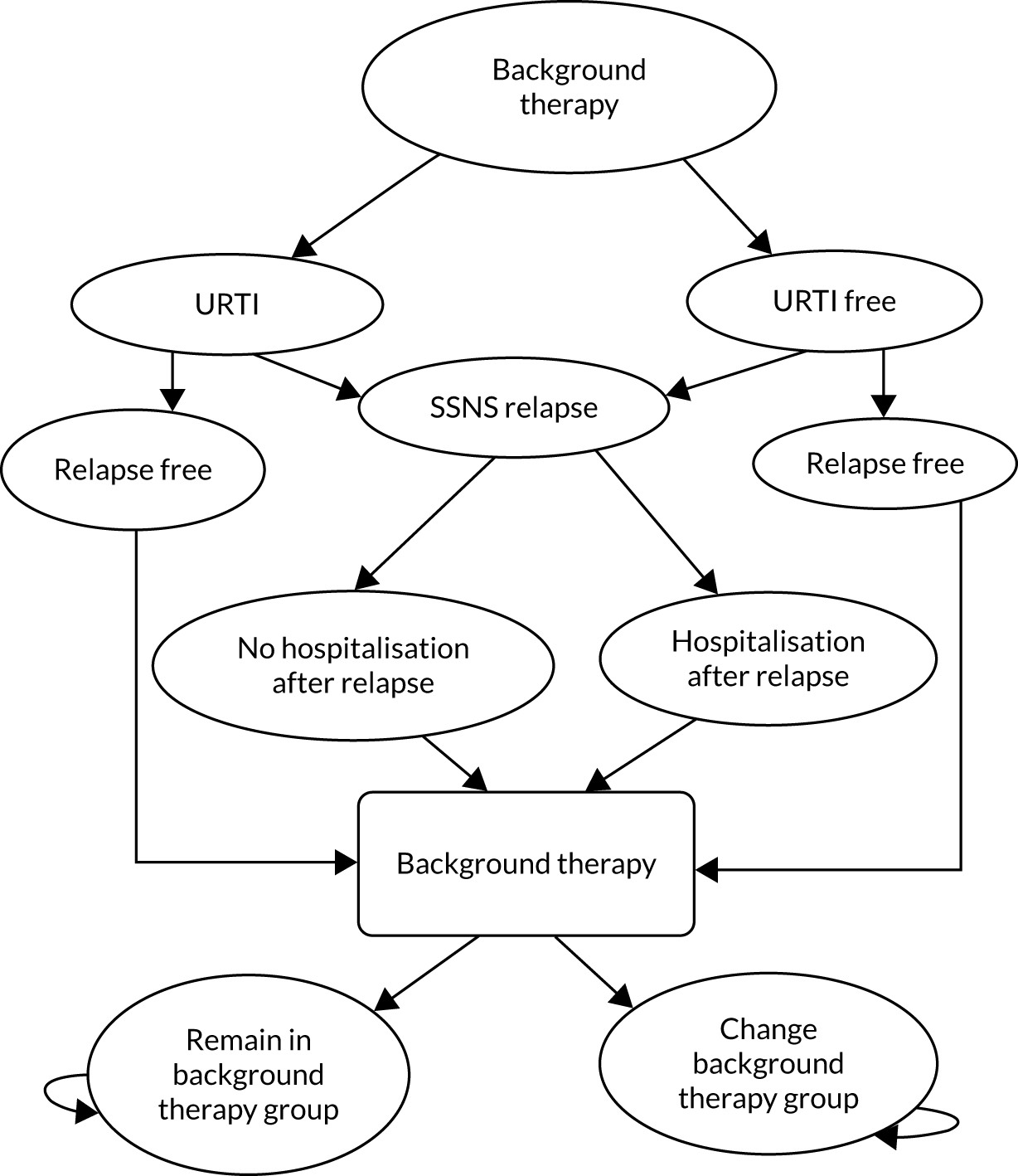
Patients in both the daily prednisolone and standard-care arms were initially allocated into one of the following four health states (groups 1–4), based on their background therapy:
-
patients on no long-term immunosuppressive therapy
-
patients receiving long-term maintenance prednisolone therapy only
-
patients receiving long-term maintenance prednisolone therapy in conjunction with other immunosuppressive therapies
-
patients receiving long-term immunosuppressive therapy only.
The clinical pathway within each background therapy group follows the same structure. After allocation to a background therapy group, the patient will have an URTI and move to ‘SSNS relapse’ (i.e. URR), move to ‘SSNS relapse’ without prior URTI (i.e. non-URR) or remain ‘relapse free’. From the ‘SSNS relapse’ state the patient will either move to ‘hospitalisation after relapse’ or ‘no hospitalisation after relapse’. From either of these three states (i.e. ‘hospitalisation after relapse’, ‘no hospitalisation after relapse’ and ‘relapse free’), patients will either remain on the current background therapy or change to a new background therapy at the end of the model cycle.
The model made a direct comparison between two treatment strategies at the time a patient develops URTI symptoms: (1) administering a course of daily prednisolone for 6 days and (2) no change to ongoing therapy (with the use of placebo tablets to maintain blinding).
Time cycle
The model adopted a 2-week time cycle, as this represents a clinically meaningful transition period between the health states. A 2-week cycle length was chosen based on the definition of an URR, which can occur within 14 days of the URTI diagnosis. Every patient in the PREDNOS2 trial had experienced at least one URTI within 12 months. A half-cycle correction was applied to costs and QALYs.
The mean costs and utility weights, as well as transition probabilities, were derived from the trial data and used to define the model parameters. QALYs were incorporated into the model by multiplying the mean utility value for each health state with the 2-week cycle length, expressed as a fraction of a year. In the primary analysis, treatment costs and effects were estimated for 12 months (i.e. 24 model cycles).
As part of the secondary analysis, the long-standing treatment effects were observed by varying the time horizon of the analysis beyond the trial follow-up period of 12 months and up to age 18 years. For extrapolating the findings, a starting age of 2 years was assumed for the model cohort and then the model was run for 16 years (384 model cycles). The extrapolation period up to age 18 years was deemed appropriate because of the paediatric nature of the disease and the low rates of SSNS relapses among adults. Costs and benefits were discounted at the standard rate (3.5%).
Model assumptions
The following set of model assumptions were applied based on the standard treatment protocol and clinical expert input. The assumptions used for the 1-year time horizon were maintained for the 16-year time horizon. The only additional assumption that was required for the 16-year time horizon was the probability of death from other causes:
-
Patients can experience only one URTI and/or one relapse in any one model cycle (2 weeks).
-
Patients can move between background therapy groups based on clinical decisions according to the number of relapses experienced over the last 3 months. Patients can change to a new background therapy every 3 months (six cycles) only and not more frequently.
-
Patients can move to background therapy group 1 from any other group if they are relapse free.
-
The allocation of patients into background therapy groups on randomisation was assumed to be the same for the two treatment arms.
-
Patients are administered the study drug (daily prednisolone) when they experience an URTI. Daily placebo was used for masking and it was assigned a zero cost in the model.
-
For hospital-related costs, if the hospital visit/admission was related to a relapse, this cost was assigned to the ‘hospitalisation after relapse’ health state and a weighted average applied from a combination of A&E visits, outpatient visits and hospital admissions. If the hospital costs were unrelated to a relapse, then, within the model, this was assumed to have happened because of any other reason and was assigned as a general mean cost applied to each respective background therapy group.
-
For costs associated with primary care visits, data were not collected on the precise reason for the visit (e.g. if relapse related or not) and were, therefore, assumed to have happened for any reason and applied as a general mean cost to each respective background therapy group.
-
Medication costs from background therapy and other prescribed medication were assigned as mean general costs to each therapy group.
-
For costs associated with prednisolone intake (over and above the study drug), these were presented as cumulative doses. Costs associated with taking prednisolone as background therapy were assigned to groups 2 and 3. For groups 1 and 4, any extra prednisolone costs were applied to the ‘SSNS relapse’ health state on the assumption that prednisolone was required for treating a relapse.
-
Each background therapy group was assigned a general mean utility score of patients who have been in the group without having a relapse.
-
A utility decrement was then applied to the SSNS relapse state as the disutility as a consequence of having a relapse.
-
The same disutility and hospitalisation costs were applied in the SSNS relapse state regardless of whether or not an URTI had preceded.
-
The probability of being hospitalised after an SSNS relapse was the same regardless of whether or not an URTI had preceded.
-
In the secondary (16-year) analysis, death from other causes was added to the model based on Office for National Statistics data.
Model parameters
All costs, utility parameters and transition probabilities are summarised in Tables 21–23, respectively.
| Health-care resource use | Unit cost (£) | Source |
|---|---|---|
| Primary care resource use | ||
| GP consultation (9.22 minutes) | 39.20 | Unit Costs of Health and Social Care 2019 41 |
| Practice nurse consultation (band 5 staff) | 9.55 | Unit Costs of Health and Social Care 2019 41 |
| Other staff (band 4 staff) | 7.49 | Unit Costs of Health and Social Care 2019 41 |
| Secondary care resource use | ||
| Renal emergency visit (non-elective) | 553.00 | NHS National Schedule of Reference Costs 2018/19 42 |
| Renal elective admission (flat rate) | 1285.00 | NHS National Schedule of Reference Costs 2018/19 42 |
| Renal elective beyond trim point (per day/regular day tariff) | 331.00 | NHS National Schedule of Reference Costs 2018/19 42 |
| Paediatric outpatient (consultant led) | 232.00 | NHS National Schedule of Reference Costs 2018/19 42 |
| Paediatric outpatient (non-consultant led) | 139.00 | NHS National Schedule of Reference Costs 2018/19 42 |
| Medication costs | ||
| Background therapy | Patient specific | BNF for Children (Online)43 and the PREDNOS2 trial |
| Other prescribed medication | Patient specific | BNF for Children (Online)43 and the PREDNOS2 trial |
| Health state cost | Mean (SE) | Distribution | Source of estimates |
|---|---|---|---|
| Mean cost of health care (£) | |||
| Group 1 | 84.74 (11.13) | Gamma | PREDNOS2 trial |
| Group 2 | 94.48 (19.95) | ||
| Group 3 | 128.21 (17.56) | ||
| Group 4 | 147.32 (23.36) | ||
| Hospitalisation after relapse | |||
| A&E visits | 691.25 (40.70) | ||
| Hospital admissions | 1411.86 (202.05) | ||
| Outpatient visits | 241.68 (8.96) | ||
| Mean cost of medication (£) | |||
| Group 1 | 47.73 (7.04) | Gamma | PREDNOS2 trial |
| Group 2 | 49.39 (8.90) | ||
| Group 3 | 214.03 (18.45) | ||
| Group 4 | 208.23 (24.00) | ||
| URTI (daily prednisolone) | |||
| Group 1 | 1.21 (0.08) | ||
| Group 2 | 1.22 (0.07) | ||
| Group 3 | 1.04 (0.07) | ||
| Group 4 | 1.56 (0.15) | ||
| Relapse | |||
| Group 1 | 20.98 (1.33) | ||
| Group 4 | 21.41 (1.84) | ||
| Utilities | |||
| Group 1 | 0.9468 (0.0052) | Beta | PREDNOS2 trial |
| Group 2 | 0.9419 (0.0050) | ||
| Group 3 | 0.9432 (0.0042) | ||
| Group 4 | 0.9365 (0.0062) | ||
| Disutility relapse | 0.01 (0.018) | ||
| Transition probability parameter | Probability | Distribution (parameters) |
|---|---|---|
| Daily prednisolone | ||
| Group 1 (general state) to URTI | 0.150 | Beta(129,729) |
| URTI to (URTI-related) SSNS relapse in group 1 | 0.279 | Beta(36,93) |
| Group 1 (general state) to (non-URR) SSNS relapse | 0.063 | Beta(46,683) |
| SSNS relapse to hospitalisation in group 1 | 0.462 | Beta(22.97,26.75) |
| Group 2 (general state) to URTI | 0.117 | Beta(86,646) |
| URTI to (URTI-related) SSNS relapse in group 2 | 0.186 | Beta(16,70) |
| Group 2 (general state) to (non-URR) SSNS relapse | 0.053 | Beta(34,612) |
| SSNS relapse to hospitalisation in group 2 | 0.189 | Beta(9.38,40.28) |
| Group 3 (general state) to URTI | 0.110 | Beta(104,844) |
| URTI to (URTI-related) SSNS relapse in group 3 | 0.202 | Beta(21,83) |
| Group 3 (general state) to (non-URR) SSNS relapse | 0.037 | Beta(31,813) |
| SSNS relapse to hospitalisation in group 3 | 0.165 | Beta(17.37,87.93) |
| Group 4 (general state) to URTI | 0.111 | Beta(65,523) |
| URTI to (URTI-related) SSNS relapse in group 4 | 0.138 | Beta(9,56) |
| Group 4 (general state) to (non-URR) SSNS relapse | 0.044 | Beta(23,500) |
| SSNS relapse to hospitalisation in group 4 | 0.102 | Beta(6.72,59.18) |
| Group 1 to group 2 | 0.064 | Dirichlet(121;9;5;6) |
| Group 1 to group 3 | 0.035 | |
| Group 1 to group 4 | 0.043 | |
| Group 2 to group 1 | 0.149 | Dirichlet(89;18;10;4) |
| Group 2 to group 3 | 0.083 | |
| Group 2 to group 4 | 0.033 | |
| Group 3 to group 1 | 0.032 | Dirichlet(130;5;3;18) |
| Group 3 to group 2 | 0.019 | |
| Group 3 to group 4 | 0.115 | |
| Group 4 to group 1 | 0.103 | Dirichlet(76;10;1;10) |
| Group 4 to group 2 | 0.010 | |
| Group 4 to group 3 | 0.103 | |
| Placebo | ||
| Group 1 (general state) to URTI | 0.140 | Beta(106,650) |
| URTI to (URTI-related) SSNS relapse in group 1 | 0.264 | Beta(28,78) |
| Group 1 (general state) to (non-URR) SSNS relapse | 0.060 | Beta(39,611) |
| SSNS relapse to hospitalisation in group 1 | 0.333 | Beta(25.99,52) |
| Group 2 (general state) to URTI | 0.139 | Beta(92,568) |
| URTI to (URTI-related) SSNS relapse in group 2 | 0.141 | Beta(13,79) |
| Group 2 (general state) to (non-URR) SSNS relapse | 0.056 | Beta(32,536) |
| SSNS relapse to hospitalisation in group 2 | 0.227 | Beta(12.03,40.97) |
| Group 3 (general state) to URTI | 0.124 | Beta(120,846) |
| URTI to (URTI-related) SSNS relapse in group 3 | 0.158 | Beta(19,101) |
| Group 3 (general state) to (non-URR) SSNS relapse | 0.059 | Beta(50,796) |
| SSNS relapse to hospitalisation in group 3 | 0.211 | Beta(11.83,44.25) |
| Group 4 (general state) to URTI | 0.122 | Beta(89,643) |
| URTI to (URTI-related) SSNS relapse in group 4 | 0.247 | Beta(22,67) |
| Group 4 (general state) to (non-URR) SSNS relapse | 0.053 | Beta(34,609) |
| SSNS relapse to hospitalisation in group 4 | 0.213 | Beta(11.17,41.28) |
| Group 1 to group 2 | 0.112 | Dirichlet(98;14;6;7) |
| Group 1 to group 3 | 0.048 | |
| Group 1 to group 4 | 0.056 | |
| Group 2 to group 1 | 0.167 | Dirichlet(83;18;5;2) |
| Group 2 to group 3 | 0.046 | |
| Group 2 to group 4 | 0.019 | |
| Group 3 to group 1 | 0.013 | Dirichlet(128;2;3;27) |
| Group 3 to group 2 | 0.019 | |
| Group 3 to group 4 | 0.169 | |
| Group 4 to group 1 | 0.051 | Dirichlet(101;6;1;9) |
| Group 4 to group 2 | 0.009 | |
| Group 4 to group 3 | 0.077 | |
Resource use and costs
The cost analysis adopted a UK health sector (NHS) perspective. Resource use data were collected at 3, 6, 9 and 12 months using case report forms within the trial. The resource use collected include primary care, hospital admissions, outpatient care, emergency visits and any prescribed medication. All costs are reported in 2019 prices in GBP.
Staff costs were calculated using nationally recognised reference costs. 41 For hospital admissions, episodes with ≤ 5 bed-days were within what is known as a ‘trim point’ and were priced at a flat rate. Admission episodes with > 5 bed-days first incurred the trim-point flat rate, and then the excess bed-days were priced per additional bed-day at a ‘regular day rate’. A 5-day admission episode, which is the maximum expected length of stay for nephrotic syndrome in the UK, was assumed for cases in which the admission date was recorded but the discharge date was unavailable. Zero bed-stays, when the patient was discharged within 24 hours, incurred one regular day-rate tariff. For outpatient care, different unit costs were used for consultant- and non-consultant-led visits. The costs of resources used were estimated as the product of the resource use and its unit cost. Only renal-related admissions and visits were considered for the economic analysis. For missing information, the cause of admission was not assumed to be renal aetiology. The unit costs of the hospital-related services were derived from the NHS National Schedule of Reference Costs 2018/19. 42
Serious adverse events required either outpatient or inpatient hospital care. Therefore, to avoid double-counting, SAEs were not costed separately and, instead, were assumed to be captured within the inpatient and outpatient hospital care data.
All drug unit costs were estimated using the BNF for Children (Online). 43 Treatment costs included prednisolone (as the study medicine and as a background therapy, or for treating relapses), immunosuppressants and any other prescribed medication. With regard to the other prescribed medication, these were classified into 36 groups and a mean unit cost was attached to groups with more than one drug. Owing to the large discrepancies, missing and non-specific information within prescription data, the most common dose was assumed for each group. This microcosting approach was considered important because the treatment of SSNS is associated with medications that have high daily costs. Non-prescribed or over-the-counter medications were not included in the economic evaluation, as these costs fall outside the health-care perspective for the analysis. All unit costs used in the analysis are presented in Table 21.
A total mean cost of health-care resource use and medication was applied to each therapy group in the model. This included primary care costs, secondary care costs from episodes that were not reported as related to a relapse and medication costs. For secondary care visits/admissions that were related to a relapse, the average cost was applied as a transition cost to the ‘hospitalisation after relapse’ state. In the therapy groups for which there was no maintenance prednisolone therapy (i.e. groups 1 and 4), any extra prednisolone cost was applied to the relapse state. Table 22 presents the total mean cost for each therapy group and state transition costs. As recommended by the National Institute for Health Care Excellence, a discount rate of 3.5% was applied to both costs and utilities when a model time horizon beyond 1 year was used. 44
Quality of life
The primary economic outcome measure was QALYs, estimated from utility values. For the utility, three different instruments were used: (1) the non-preference-based PedsQL Generic Core Scale, (2) the preference-based CHU-9D questionnaire and (3) the generic EQ-5D instrument [EuroQol-5 Dimensions, three-level version (EQ-5D-3L)]. The PedsQL and the CHU-9D questionnaires (for children aged ≥ 5 years) were proxy completed by parents or guardians at the relevant time points within the trial.
In the base-case analysis, three approaches were used to estimate the utility values depending on the age of the patients at the time of completion. For patients aged between 2 and 4 years, a crosswalk/mapping technique was applied to generate utility values using the responses to the PedsQL questionnaire. 27 Although the PedsQL is a validated measure of HRQoL, it is not preference based and, therefore, not suitable for deriving utility weights. Therefore, a mapping technique was used to estimate CHU-9D scores from the patients’ responses to the PedsQL. 27 For patients aged between 5 and 17 years, utility values were estimated directly using the proxy responses to the CHU-9D instrument and from applying the UK value set. For older participants (aged 18 years at the time of completion), the EQ-5D-3L instrument was used.
Utility values for each therapy group were calculated by averaging the utility values obtained from each participant in the respective therapy group who was relapse free at any given time. A utility decrement was applied to those patients who experienced a SSNS relapse, regardless of whether or not an URTI had preceded the relapse (i.e. the disutility was applied to both URRs and non-URRs). The level of disutility applied was calculated by subtracting the utility value of patients who experienced a relapse from the utility value of those who remained relapse free at any given time. QALYs were then estimated in the model by multiplying the utility values with the time spent in the health state. The state-specific utility values used in the model are presented in Table 22. As part of a sensitivity analysis, additional scenarios were explored to assess how uncertain the results were to assumptions made within the HRQoL estimation. Full details are presented in the following sections.
Transition probabilities
The PREDNOS2 trial data were used to determine the transition probabilities for movement between the health states within the model. All patients recruited to the trial were diagnosed with relapsing SSNS. The proportion of patients in each therapy group was determined by their initial background therapy on randomisation. For each arm of the trial, the movements of all patients between therapy groups for every time cycle were determined to derive the transition probability for each model cycle. The probability of changing therapy group was time dependent, as a decision about whether to change or continue with existing background therapy was made every 3 months in the trial. This decision was based on a clinical assessment of the patient’s overall condition. Therefore, a transition probability to a different therapy group was applied every sixth cycle and this probability was zero for all intermediate cycles. Transitions to therapy group 1 from each of the other groups could happen only if the patient was relapse free.
Within each therapy group, transition probabilities were calculated by dividing the number of transitions to each health state by the number of transitions to the previous health state. The clinical definitions of an URR (i.e. a relapse following an URTI) and a non-URR (i.e. a relapse without prior URTI) were used to differentiate between the two types of SSNS relapses. For example, the transition probability to the ‘SSNS relapse’ state after an URTI (URR) was calculated by dividing the number of URRs by the number of URTIs. To account for the fact that patients had multiple URTIs and relapses while in the therapy group, the time spent in each group was converted to 2-week cycles and this number was used for the calculation of the transition probabilities to the URTI state. Therefore, the transition probability to the URTI state was the total number of URTIs divided by the total number of group cycles for each therapy group. All transition probabilities are presented in Table 23.
Analysis
An incremental cost–utility analysis was conducted for both the primary and secondary analyses, which provides the incremental costs and incremental QALYs comparing daily prednisolone with standard care. The results are reported as cost per QALY gained in terms of incremental cost-effectiveness ratios (ICERs). The treatment is considered to be dominant if it generates lower costs and a greater number of QALYs over time. Analysis was by ITT. The primary analysis had a time horizon of 1 year and the secondary analysis costs and outcomes were extrapolated over 16 years.
Uncertainty in the model was explored by conducting both probabilistic sensitivity analysis (PSA) and deterministic sensitivity analysis (DSA).
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was used to account for the level of parameter uncertainty from the variance in the model inputs. A PSA simultaneously changes all model parameters and was performed in both the primary and secondary analysis. For each model parameter (i.e. transition probabilities, utilities and costs), the distribution of possible values was used for the PSA (see Tables 22 and 23). A Dirichlet distribution was used for the transition probabilities between the different therapy groups, as this distribution allows for possible movement to more than two states. A beta distribution is assigned to all other transition probabilities and to the utility parameters. A gamma distribution is assigned to resource use costs and medication costs.
In the Monte Carlo simulation, values of the model parameters were resampled from their distributions and incremental costs and effects were recalculated. The process of resampling and recalculating the results was repeated 10,000 times to generate a distribution around the estimated ICER. A cost-effectiveness acceptability curve (CEAC) and a cost-effectiveness plane were plotted from the simulated data. The CEAC shows the probability of daily prednisolone being more cost-effective than placebo and the impact of uncertainty in relation to a range of thresholds that decision-makers are willing to pay for an additional QALY.
Deterministic sensitivity analysis
Three DSAs were performed at 1 year to account for the methodological uncertainty around the model parameters and model structure. These were as follows:
-
Methodological uncertainty around the HRQoL was tested by varying the instrument used for each age group within the trial population. The CHU-9D was used to obtain utility values for participants aged 5–11 years (vs. those aged 5–17 years in the primary analysis) and the EQ-5D-3L was used for participants aged 12–18 years (vs. those aged 18 years in the primary analysis). The age cut-off point was based on the initial design of CHU-9D for use in children up to age 11 years,45 as its validity in adolescents up to age 17 years has been demonstrated by recent research only. 46 The mapped PedsQL for the younger ages (2–4 years) remained in the sensitivity analysis, as neither of the other two measures is validated for use with this age range.
-
Uncertainty around the structural assumption that patients change their background therapy every 3 months in the model was tested by allowing patients to move to a different therapy group every month within the model.
-
In the base case, a utility decrement of 0.01 was used as the disutility from having a relapse. A one-way DSA was applied using a lower (–0.04) and upper (0.03) value of disutility derived from the 95% CI. As disutility appears as a negative value in the model, the value of –0.04 represents the extreme scenario that there is a utility gain, rather than a utility decrement, after a relapse.
In the secondary analysis, the base-case analysis was extrapolated over 16 years, but a complete set of deterministic analysis was not deemed necessary if the cost-effectiveness results remained the same as at 1 year.
Subgroup analysis
A subgroup analysis was performed to explore the uncertainty from using a mapping technique to derive utility values from the PedsQL responses for patients aged between 2 and 4 years. The base-case analysis at 1 year was performed again after excluding participants aged between 2 and 4 years to observe possible differences in the results.
Results
The results for the base-case and sensitivity analyses are presented separately for the primary and secondary analyses.
Primary analysis results
Table 24 summarises the base-case analysis and DSA results at 1 year. The base-case results show that daily prednisolone had a mean cost of £252 and cost less than standard care. Moreover, daily prednisolone generated 0.003 more QALYs than placebo, as the mean number of QALYs in the daily prednisolone arm was 0.9427 compared with 0.9424 in the standard-care arm. The ICER for the base-case analysis is negative, indicating that there is a cost saving from administering daily prednisolone at the time of an URTI.
| Analysis | Total mean cost per intervention (£) | Total mean QALYs per intervention | ICER (£) |
|---|---|---|---|
| Base case | |||
| Daily prednisolone | 252 | 0.9427 | |
| Placebo | 254 | 0.9424 | –5878 |
| Mean difference | 2 | –0.0003 | |
| DSA 1a | |||
| Daily prednisolone | 252 | 0.9431 | |
| Placebo | 254 | 0.9427 | –3805 |
| Mean difference | 2 | –0.0004 | |
| DSA 2b | |||
| Daily prednisolone | 242 | 0.9433 | |
| Placebo | 249 | 0.9428 | –12,200 |
| Mean difference | 7 | –0.0005 | |
| DSA 3 (one way): –0.04c | |||
| Daily prednisolone | 252 | 0.9462 | |
| Placebo | 254 | 0.9462 | –72,209 |
| Mean difference | 2 | < –0.0001 | |
| DSA 3 (one way): 0.03c | |||
| Daily prednisolone | 252 | 0.9412 | |
| Placebo | 254 | 0.9408 | –4024 |
| Mean difference | 2 | –0.0004 | |
Background therapy was a key driver of costs and QALYs, as proportionally more patients moved to no therapy (i.e. group 1) in the prednisolone arm than in the standard-care arm after 1 year (43% vs. 39%, respectively). In addition, there was a larger relative proportion of patients in group 3 in the standard arm than in the prednisolone arm (32% vs. 30%, respectively) (see Appendix 2, Table 32). Although patients were ‘allowed’ to change background therapy every 3 months in the model, even these small differences at 1 year will have influenced the incremental cost and QALY differences between treatment arms.
In all three of the DSAs, the findings supported the base-case results. Although the cost and QALY differences between the two treatment arms did change, daily prednisolone remained the dominant option. DSA 1 increased the mean number of QALYs in both treatment arms and daily prednisolone was slightly more effective, with 0.0004 more QALYs compared with 0.0003 in the base case. In DSA 2, there was a greater cost and QALY difference between the two arms compared with the base case, with daily prednisolone being cheaper and more effective. Similarly, the favourable effect of daily prednisolone was sustained in DSA 3 for both the upper and lower values.
The results from the PSA are illustrated in the cost-effectiveness plane and CEAC. The cost-effectiveness plane shows that the majority of the cost and effect distributions from 10,000 Monte Carlo simulations lie in the south-east quadrant (Figure 7). This indicates that daily prednisolone is less costly and more effective than placebo. The uncertainty is then summarised in the CEAC in relation to different willingness-to-pay (WTP) thresholds of decision-makers (Figure 8). As demonstrated in Figure 8, daily prednisolone is more cost-effective than standard care at any given WTP threshold and has a probability of 80% of being cost-effective at a WTP threshold of £20,000.
FIGURE 7.
Cost-effectiveness plane from the PSA results of the primary analysis.
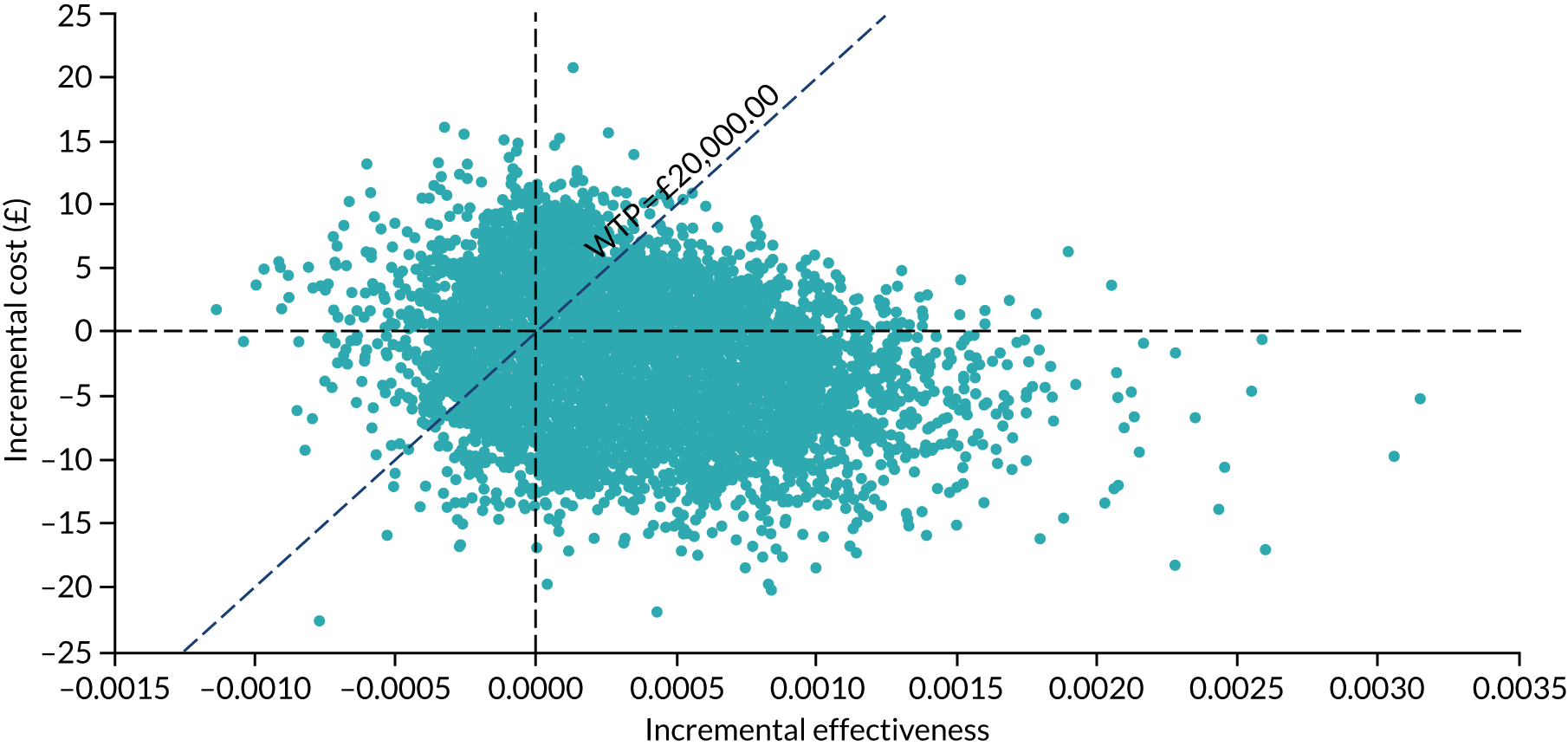
FIGURE 8.
The CEAC for daily prednisolone and placebo for the primary and secondary analysis.
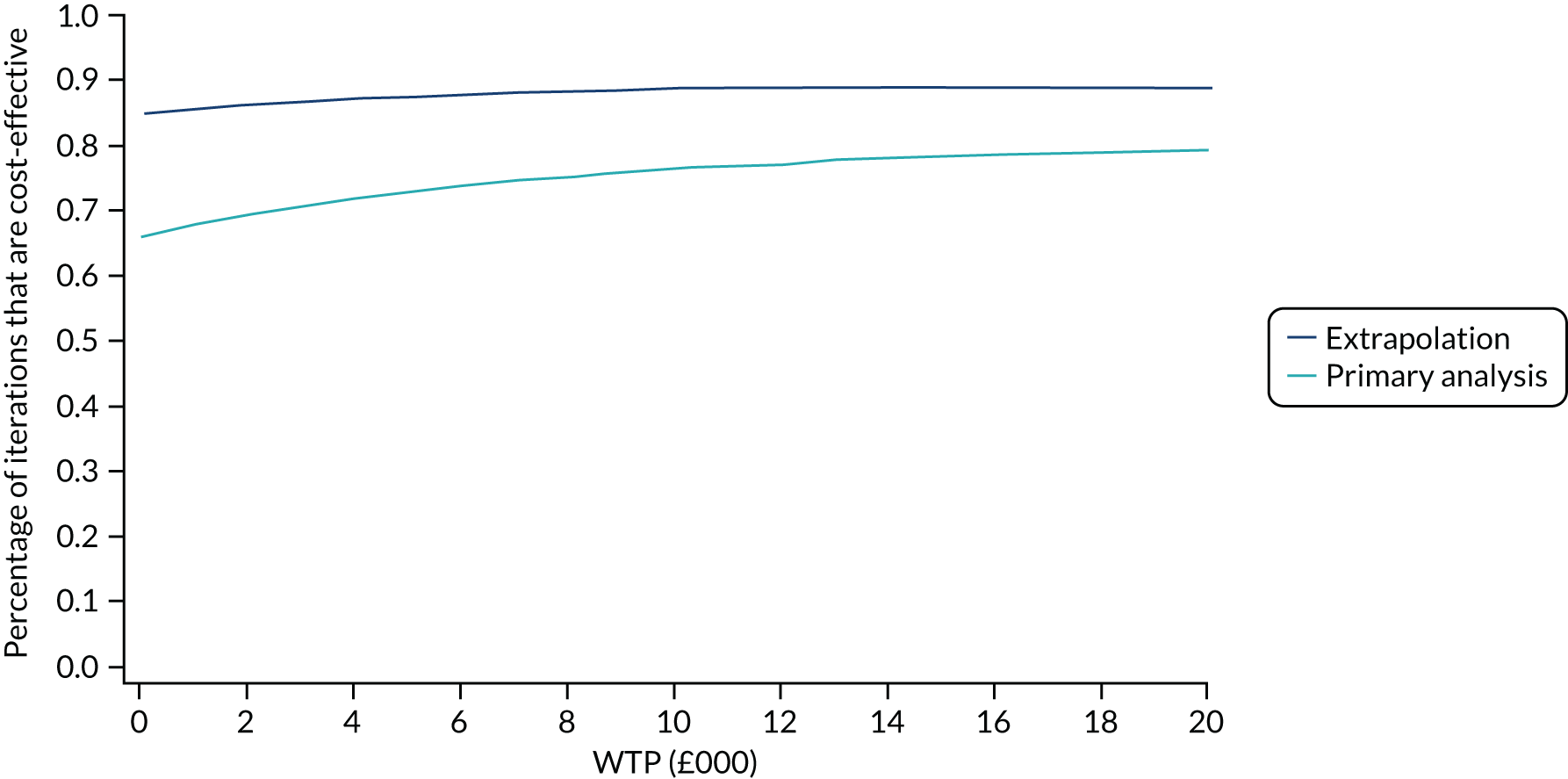
In the subgroup analysis, daily prednisolone was more effective than standard care and generated 0.0004 more QALYs (Table 25). This suggests that using a mapping technique can be considered the second-best option when there is no validated utility-based HRQoL instrument available.
| Subgroup analysis | Total mean cost (£) per intervention | Total mean QALYs per intervention | ICER (£) |
|---|---|---|---|
| Daily prednisolone | 252 | 0.9434 | |
| Placebo | 254 | 0.9430 | –4190 |
| Mean difference | 2 | –0.0004 |
Secondary analysis (extrapolation)
The results for the extrapolated analysis are summarised in Table 26 and show that daily prednisolone costs £176 less and generates 0.01 more QALYs than placebo (11.61 vs. 11.60 QALYs, respectively). Therefore, as with the primary analysis, daily prednisolone dominated the standard-care arm (i.e. less costs and more QALYs).
| Secondary analysis | Total mean cost (£) per intervention | Total mean QALYs per intervention | ICER (£) |
|---|---|---|---|
| Daily prednisolone | 2690 | 11.61 | |
| Placebo | 2866 | 11.60 | –16,278 |
| Mean difference | 176 | –0.01 |
The PSA results for the secondary analysis show that daily prednisolone is less costly and more effective, as most incremental cost and effect points lie in the south-east quadrant of the cost-effectiveness plane (Figure 9). Moreover, daily prednisolone had a high probability (approximately 90%) of being cost-effective at a WTP of £20,000 and is, therefore, considered the most cost-effective option at all WTP thresholds (see Figure 8).
FIGURE 9.
Cost-effectiveness plane from the PSA results of the secondary analysis.
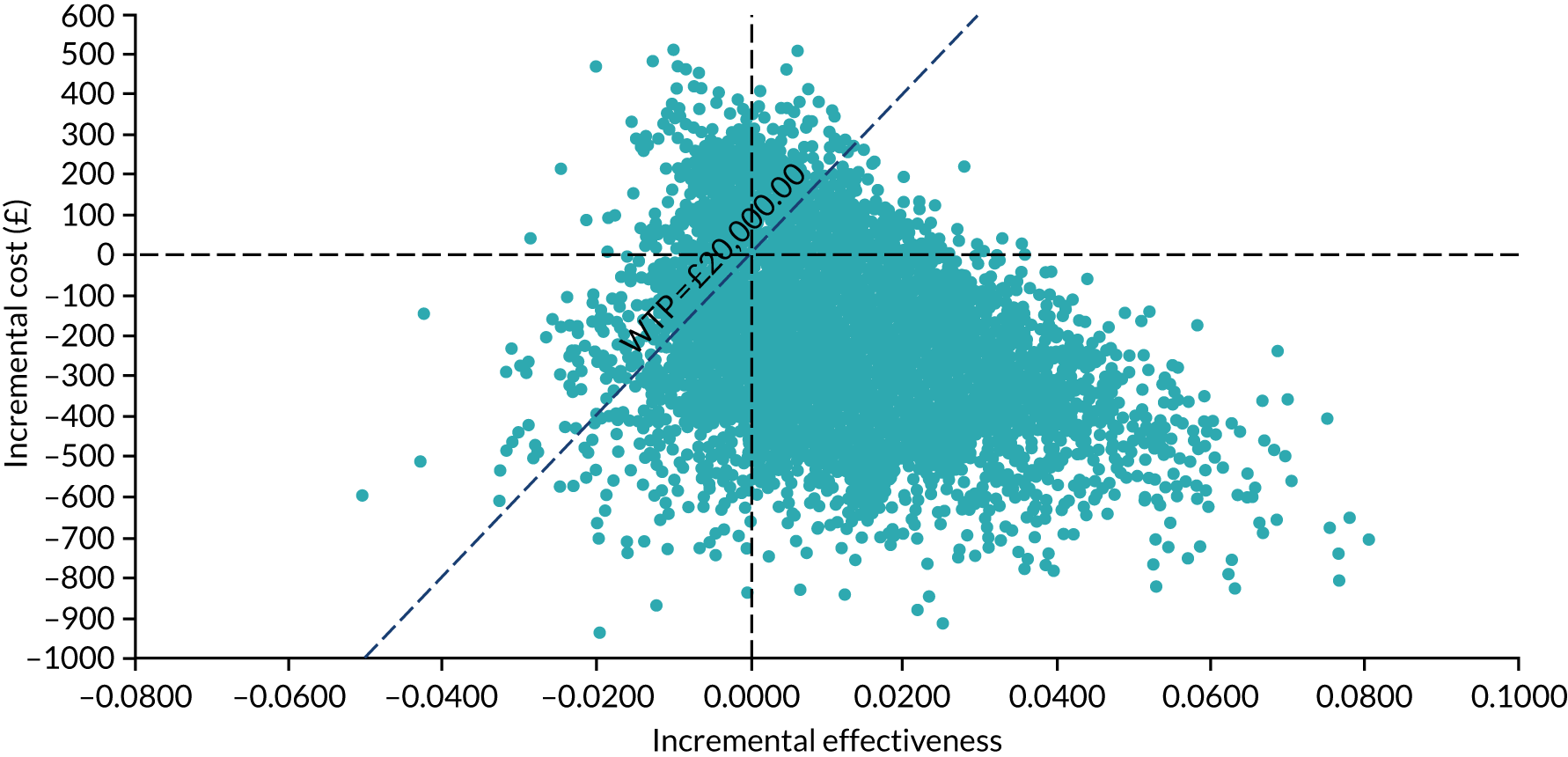
Chapter 5 Discussion
Summary of findings
In, to the best of our knowledge, the largest ever clinical trial of an IMP in children with nephrotic syndrome, the PREDNOS2 trial has shown that giving 6 days of low-dose prednisolone to children with relapsing SSNS at the time of an URTI does not reduce the risk of a subsequent URR. The findings apply across the four subgroups: (1) children on no background treatment, (2) children on long-term maintenance prednisolone only, (3) children on non-corticosteroid maintenance immunosuppression only and (4) children on both long-term maintenance prednisolone and non-corticosteroid maintenance immunosuppression.
There were also no differences in secondary outcomes between the two treatment arms, including the overall incidence of relapses (whether URTI related or not), escalations and reductions in background therapy, cumulative dose of prednisolone, adverse effects of prednisolone, ACBC scores or quality of life (measured using the PedsQL).
However, the economic analysis showed that giving daily prednisolone at the time of an URTI, compared with standard care, led to reduced overall health-care and background therapy costs, increased QALYs and was, therefore, the ‘dominant’ treatment option. This result was maintained over a 16-year time horizon and was robust to all sensitivity analyses.
Comparison of demographics with other studies
The findings do not support the four previously published trials,18–20,22 which all showed that low-dose daily prednisolone for 5–7 days given at the time of an intercurrent infection was effective in preventing a relapse, although, with p-values of 0.04, 0.01, 0.01 and 0.049, the results were not overwhelmingly in favour of daily prednisolone. Rather, the findings suggested that it may work. Furthermore, the small populations and the methodological issues with these studies limited their impact, leading the latest Cochrane review to conclude that ‘clinicians are unlikely to use this regimen without additional data to confirm its efficacy and safety’. 4
Table 27 shows how the PREDNOS2 trial compares with the four previous trials of daily corticosteroid at the time of an intercurrent illness. The PREDNOS2 trial sample size was larger than those of all four previous studies combined. In terms of demographics, the average age at recruitment for the PREDNOS2 trial population (7.6 years) was in the middle of the age ranges for previous trials, with Abeyagunawardena and Trompeter19 having the youngest population (median age 5.3 years) and Abeyagunawardena et al. 22 having the oldest population (mean age 12.3 years). There was a considerable difference in the duration of nephrotic syndrome prior to recruitment in the two trials that reported this. Gulati et al. ’s20 subjects had been diagnosed only 9.8 and 10.5 months prior to recruitment for intervention and control arms, respectively, whereas children in the trial by Abeyagunawardena et al. 22 were older, with a much longer nephrotic syndrome course (diagnosed 90 and 76.8 months prior to recruitment for intervention and control arms, respectively). The length of time that the PREDNOS2 trial ITT population had SSNS was between these values (i.e. 40 months for those taking prednisolone and 37 months for the placebo arm).
| Variable | Study | ||||
|---|---|---|---|---|---|
| PREDNOS2 | Mattoo and Mahmoud18 | Abeyagunawardena and Trompeter19 | Gulati et al.20 | Abeyagunawardena et al.22 | |
| Recruited sample size (n) | 365 (271 in the modified ITT analysis population) | 36 | 48 (crossover) | 100 | 48 (crossover) |
| Number completing trial | 253 | 40 | 89 | 33 | |
| Age (years) at recruitment | 7.7 ± 3.6 for intervention and 7.5 ± 3.5 for placebo | Median 7.2 for intervention arm and median 6.8 for control | Median 5.3 (range 1.5–13.2) | 6.5 ± 2.97 for intervention arm and 6.8 ± 3.23 for control arm | Mean 12.3 for intervention arm and 9.9 for placebo arm |
| Mean time (months) from nephrotic syndrome diagnosis to recruitment | 39.7 for intervention arm and 36.8 for control arm | NR | NR | 9.8 for intervention arm and 10.5 for control arm | 90 for intervention arm and 76.8 for control arm |
| Population definition | SSNS with two or more relapses in last 12 months | SSNS on low-dose maintenance prednisolone because of either FRSSNS (n = 22) or post cyclophosphamide (n = 14) | SSNS on low-dose maintenance prednisolone | SSNS on low-dose maintenance prednisolone | Previous SDNS but off corticosteroids and other immunosuppression for ≥ 3 months |
| Relapse rate prior to recruitment | NR | NR | NR | 4.1 ± 0.2 for intervention arm and 4.2 ± 0.4 for control arm | NR |
| Average background prednisolone dose (mg/kg/48 hours) | 0.3a in those taking maintenance prednisolone | 0.5 | 0.36 (range 0.1–0.6) | 0.6 ± 0.1 in intervention arm and 0.7 ± 0.2 in placebo arm | |
| Other background immunosuppression |
Low-dose prednisolone alone: n = 74 Low-dose prednisolone with other immunosuppression: n = 91 |
No | No | Levamisole in 32 out of 100 | No |
| Number of infections (patient per year) | Mean 2.95 and median 2 | 3.5b | N/A | 3.8c | 3.3d |
| Children excluded because of no URTI | 80 out of 333 who completed 12 months’ follow-up (24%) | 0 | 3 out of 48 (6.3%) | 0 | 0 |
| Total number of relapses in trial population (patient per year) | 1.67 | 1.93 | NR | 1.34 | 0.55 |
| URR frequency in control arm (as per cent of URTIs) | 20.15 | NR | 47.5 | 35 | 24.75 |
No comment about ethnicity is made in any of the previous studies and it is assumed that the populations comprised a single ethnicity that corresponded to the location of the trial. Nephrotic syndrome is much more common in individuals of South Asian ethnicity47 and three of these previous studies19,20,22 were undertaken in ethnic groups that are considered to have a higher incidence of SSNS. In the mixed-ethnic PREDNOS2 modified ITT population, 21.4% of the population reported South Asian ethnicity (i.e. Pakistani, Indian, Bangladeshi, Sri Lankan, other Asian or mixed white Asian). Thirty of 134 (22.3%) patients in the prednisolone arm and 28 of 137 (20.4%) patients in the placebo arm were of South Asian ethnicity. We undertook a post hoc subgroup analysis that assessed the primary outcome in those of South Asian ethnicity (risk ratio 0.66, 95% CI 0.4 to 1.10), compared with those of other ethnicity (risk ratio 1.11, 95% CI 0.81 to 1.54), which suggested that there may be evidence that daily prednisolone is effective in those of South Asian ethnicity (test for interaction p = 0.09). However, as this was not a planned subgroup analysis, the numbers are small and this needs confirmation in a larger study.
In comparison with previous studies, the PREDNOS2 trial included children on a range of background treatments. Three of the previous studies18–20 included children on maintenance prednisolone only (with levamisole in addition for some patients in one study), with the fourth study22 comprising children on no background therapy only. In Gulati et al. ’s study,20 it is noteworthy that the lower relapse rate seen in children taking background levamisole as well as prednisolone was non-significant.
Abeyagunawardena et al. ’s22 second study replicated their previous design in children on no background immunosuppression and is the only previous study examining the effect of daily low-dose prednisolone on preventing the risk of URR. The results mirrored the group’s earlier findings of a benefit to the intervention. However, compared with all other studies, including the PREDNOS2 trial, this population was an outlier in terms of the longer time for which children had nephrotic syndrome and the lower relapse frequency during the trial, suggesting that their nephrotic syndrome was progressing towards long-term remission. Only the PREDNOS2 trial has considered a real-world scenario of children with relapsing SSNS taking a range of background treatments or none.
The PREDNOS2 trial was also the only trial to include patients by the number of relapses experienced prior to recruitment. The three earlier studies18–20 inferred frequently relapsing or steroid-dependent disease through the need for maintenance corticosteroid. Abeyagunawardena et al. ’s second study22 included children with previous steroid-dependent nephrotic syndrome (SDNS) but who had been off maintenance prednisolone for at least 3 months (it is noteworthy that they had the lowest frequency of relapses during the study period for any trial). The only trial that reported rate of relapses prior to recruitment was Gulati et al. ,20 with a mean of 4.1 relapses per year. That metric is noteworthy because Gulati et al. 20 reported a lower frequency of relapse during the trial itself for both arms [mean (± SD) 0.9 (± 0.4) and 1.8 (± 0.5) for intervention and control arms, respectively]. Reasons for this reduction are not discussed. Given that it was a population of children early in their nephrotic syndrome history, it is most likely that maintenance prednisolone was commenced at some point in the year prior to the trial as a response to frequent relapses. Whatever the explanation, the background reduction in frequency mitigates the extent of the treatment effect they report.
During the trial periods, there were differences in the overall rate of relapses, varying between 1.93 relapses per patient per year for Mattoo and Mahmoud’s population18 and 0.55 relapses per patient per year for Abeyagunawardena et al. ’s second trial. 22 The rate of relapse for the PREDNOS2 trial was 1.67 relapses per patient per year. This difference in relapse frequency may reflect the fact that Abeyagunarwardena et al. ’s second trial comprised a population of children who may have been outgrowing the disease, as discussed above. Interestingly, however, in all of the studies, including the PREDNOS2 trial, the average relapse rate of the control groups did not meet the definition of FRSSNS (i.e. four or more relapses per year) over the study period.
The rate of infections, where reported, was similar across the previous trials, varying between 3.3 and 3.8 infections per patient per year. The overall rate of infections in the PREDNOS2 trial was slightly lower, at 2.95 URTIs per patient per year. The PREDNOS2 trial-recruited population included 80 children (24% of those who completed 12 months of follow-up) who did not have any URTI. Analysis of a modified ITT population of those who did have an URTI was part of the PREDNOS2 trial design, but was not considered by previous studies. Three of these studies18,20,22 did not mention children who did not experience an intercurrent infection during the trial period and the inference is that all children had at least one infection. The study by Abeyagunawardena and Trompeter19 was the only other study to have excluded children for this reason, but with a much lower percentage (6.3%) than that of the PREDNOS2 trial. There was a small difference in age at recruitment between the PREDNOS2 trial and the three earlier studies18–20 and, in general, viral URTIs are more common in younger children. 48 When the modified ITT PREDNOS2 population was split by age into < 4 years (pre-school) and ≥ 4 years (school age), the breakdown of URTI confirmed a greater incidence of URTIs in younger children (Table 28).
| Age (years) | Treatment arm (mean) | Total | |
|---|---|---|---|
| Prednisolone | Placebo | ||
| < 4 | 72 URTIs in 21 children (3.4 URTIs per child) | 74 URTIs in 18 children (4.1 URTIs per child) | 146 URTIs in 39 children (3.7 URTIs per child) |
| ≥ 4 | 312 URTIs in 113 children (2.8 URTIs per child) | 333 URTIs in 119 children (2.8 URTIs per child) | 645 URTIs in 232 children (2.8 URTIs per child) |
A lower rate of intercurrent infection but similar rate of relapses means that either there is a higher incidence of non-URRs in the PREDNOS2 trial population or the children have had more URTIs than have been recorded in the trial. Differences in the URTI-to-relapse ratio may be explained partly by the epidemiology of viral URTIs, suggesting that the types of infections encountered in the UK are less likely to trigger infections than those in more tropical countries. One justification for the PREDNOS2 trial was the need to determine if findings were generalisable to a European temperate climate. The difference in definitions of viral infections, as presented in Table 1, shows that fever was an essential component for three of the four previous studies (although not with Mattoo and Mahmoud’s population18), whereas it could be one or two out of six symptoms to define URTI in the PREDNOS2 trial. In two of those trials,20,22 a temperature of > 38 °C was required, implying a more virulent infection than a typical URTI in the UK. The lower URTI-to-relapse ratio in the PREDNOS2 trial may be explained by a milder viral illness that was less likely to trigger a relapse. Overall, there were more relapses in the placebo arm (237 relapses in 137 patients in the placebo arm vs. 216 relapses in 134 patients in the prednisolone arm), but this small increase in number was non-significant (p = 0.33).
The risk of any individual URTI triggering a relapse, what was described as the event rate in planning the sample size for the trial design, is a different measure to the relapse-to-URTI ratio, as it eliminates the possibility of other relapses being caused by undiagnosed infections. It can be extracted from published data for most of the trials where the number of relapses following URTIs is documented. The appropriate comparator between trials of the property of the infections within the trial population is the measurement of the event rate within the control arm. In Abeyagunawardena and Trompeter’s first (and younger) population,19 a viral infection was likely to trigger a relapse 48% of the time, but only 25% of the time in the second (older) population. 22 In the PREDNOS2 trial, the likelihood was even lower, with just 82 of 407 URTIs (20.1%) within the placebo arm resulting in a relapse. Again, this might be a property of the epidemiology of viral URTIs, but interpretation is problematic because of the large differences in event rates seen between the two trials of Abeyagunawardena et al. 19,22 and it suggests differences in how relapses are triggered in individuals with SSNS. In Abeyagunawardena and Trompeter’s first trial,19 the population comprised children early on in their history of nephrotic syndrome who were taking alternate-day maintenance corticosteroid, whereas in the population of Abeyagunawardena et al. ’s second trial22 children had experienced SSNS for a longer duration and had stopped taking background corticosteroid for at least 3 months before recruitment. The natural history of SSNS is for the disease to enter permanent remission in the majority of patients and this mostly occurs in teenage years. 11 It is self-evident then that as children grow older the risk of URTIs triggering relapses must reduce.
It is also possible, however, that children on maintenance alternate-day prednisolone may be more vulnerable to relapses triggered by intercurrent infection. Evidence to support this hypothesis comes from the historical finding that adrenal suppression is common in children with SSNS and may increase the risk of relapse49 and the more recent finding that low-dose maintenance corticosteroid is more effective in preventing relapses if the same total 48-hour dose is divided into daily doses rather than given in a standard alternate-day fashion. 50
Gulati et al. ’s study20 was the only study to calculate NNT. The authors presented mean (± SD) numbers of relapses per infection in the intervention and control groups of 0.13 (± 0.1) and 0.35 (± 0.2), respectively, giving a mean difference of 0.22 (95% CI 0.16 to 0.28). Using adjusted Poisson regression, Gulati et al. 20 calculated a 59% independent reduction in the risk of relapse following an URTI and estimated that the intervention would result in a reduction in the frequency of relapses to fewer than three per year for one out of six patients with frequent relapses. Note that the NNT calculated is one to reduce relapses below the FRSSNS definition, rather than one required to abolish relapses completely. Given the a priori reduction in relapse frequency seen in the control group from the year prior to recruitment to the 12 months of the trial, this NNT may well be an underestimate. The NNT increases as the URTI rate and the event rate reduce. Therefore, the optimistic NNT calculated by Gulati et al. 20 would be even greater if applied to the lower event rate of Abeyagunawardena et al. ’s second trial,22 implying that the intervention effect may have been considerably overestimated prior to the PREDNOS2 trial.
Comparison of trial design with previous research
Many similar demographics and trial design issues allow for careful comparison of results (Table 29). The average background dose of prednisolone/prednisone was similar for all trials that included children on long-term maintenance prednisolone, including the relevant subgroups in the PREDNOS2 trial. The dose of prednisolone used as trial medication was also similar between all trials. In the PREDNOS2 trial, the dose of prednisolone for children not already taking it as part of long-term maintenance treatment was 15 mg/m2, which is equivalent to Abeyagunawardena et al. ’s dose of 0.5 mg/kg. 22,51
| Trial design | Study/trial | ||||
|---|---|---|---|---|---|
| PREDNOS2 | Mattoo and Mahmoud18 | Abeyagunawardena and Trompeter19 | Gulati et al.20 | Abeyagunawardena et al.22 | |
| Recruited sample size (n) | 365 | 36 | 48 (crossover) | 100 | 48 (crossover) |
| Number of participants completing trial | 253 | 36 | 40 | 89 | 33 |
| Blinding | Yes | No | Yes | No | Yes |
| Length of trial medication (days) | 6 | 5 (two or three extra doses) | 7 (three or four extra doses) | 7 | 5 |
| Dose of trial medication | 15 mg/m2 (or topping up to alternate-day prednisolone dose if higher) | Same as alternate-day dosing | Same as alternate-day dosing | Same as alternate-day dosing | 0.5 mg/kg |
| Definition of URR | Within 2 weeks of onset of URTI symptoms | Not relevant | Not defined | 8–14 days following onset of URTI symptoms | Not defined |
| Adherence to trial medication |
Commenced mediation at time of URTI: 85.4% in the prednisolone arm and 89.2% in the placebo arm Completed 6-day course and commenced medication at time of URTI: 70.8% in the prednisolone arm and 76.9% in the placebo arm |
Not reported | Not reported | Not reported | Suspected non-adherents excluded from analysis |
| Length of follow-up (months) | 12 | 24 | 24 | 12 | 24 |
| Primary outcome | Proportion of children in each arm experiencing an URR following any URTI | Number of relapses in each arm over 2-year trial period | Proportion of URTIs followed by relapse in each arm | Average number of URRs in each arm | Proportion of URTIs followed by relapse in each arm |
| Corticosteroid adverse effects | No difference in specific symptoms of ACBC scores between arms | None reported | Not detailed | Cushingoid features, infections and cataracts described | Not reported |
The PREDNOS2 trial’s large sample size, double-blinded randomised controlled design and low dropout rate make its data more reliable than those of previous trials. Lack of blinding, exclusion of children whose background therapy either escalated or reduced and exclusion of children where concerns about adherence became apparent may all have introduced bias in previous trials. One other trial design issue that may also have unwittingly introduced bias into previous studies is the duration of trial medication, which ranged from 5 to 7 days. We chose 6 days in the trial design of the PREDNOS2 trial so that an even number would remove any inadvertent bias caused if there were an imbalance in the actual number of doses of trial medication between arms for those children already taking long-term maintenance prednisolone. As this factor was not addressed in previous studies, results may have been inadvertently biased.
Adherence
To the best of our knowledge, the PREDNOS2 trial is the only study of daily corticosteroid at the time of an URTI in children with SSNS that has systematically reported adherence data. Abeyagunawardena et al. 22 reported that 12 patients who failed to report with viral infections, did not maintain daily urine protein records or failed to comply with trial protocol at the time of acute infections were excluded from the final assessment of the study. Other previous studies made no assessment of adherence.
In the PREDNOS2 trial, adherence was assessed by verbal recall of how trial medication had been given during any URTI prior to the trial visit. Counting trial tablets at trial visits, as was performed with the PREDNOS trial,9 was felt to document the number of trial tablets remaining only and not necessarily to reflect how it had been given. Three questions were asked of parents/carers:
-
Was trial medication started at the time of an URTI? If yes, after what length of time?
-
Was the whole 6-day course of trial mediation taken? If no, how many days were missed?
-
Was the 6-day course prematurely discontinued?
Answers to questions 2 and 3 were very similar, but not identical. Question 2 was felt to be a more robust question, covering the eventuality that mid-course doses were missed without the 6-day course finishing prematurely. The percentage of URTIs where medication was commenced at the time of URTI symptoms was 85.4% in the prednisolone arm and 89.2% in the placebo arm. Of those URTIs where medication was commenced, the 6-day course was completed in 82.9% of URTIs in the prednisolone arm and 86.2% of URTIs in the placebo arm. Overall, medication was commenced and a 6-day course completed for 70.8% of URTIs in the prednisolone arm and 76.9% of URTIs in the placebo arm (see Table 29).
Table 30 shows adherence data for individual URTIs in each arm of the trial according to whether or not an URR resulted. Adherence appeared to be better when a relapse was not triggered compared with when there was an URR. In the prednisolone arm, trial medication was commenced and 6 days’ treatment completed in 253 of 298 (84.9%) URTIs where an URR did not occur, compared with 19 of 82 (23.2%) URTIs where an URR did occur. However, this difference in adherence was observed for both the prednisolone arm and placebo arm and, on careful inspection of the data, the most common reason for ‘non-adherence’ was the need to stop trial medication to begin treatment for a relapse.
| Adherence | URTI that did not result in an URR | URTI that did result in an URR | ||
|---|---|---|---|---|
| Prednisolone arm | Placebo arm | Prednisolone arm | Placebo arm | |
| Trial medication commenced at time of URTI? | ||||
| n | 298 | 323 | 82 | 82 |
| No, n (%) | 30 (10.1) | 22 (6.8) | 22 (26.8) | 20 (24.4) |
| Yes, n (%) | 268 (89.9) | 301 (93.2) | 59 (72.0) | 62 (75.6) |
| Missing, n (%) | 0 (0) | 0 (0) | 1 (1.2) | 0 (0) |
| Whole 6 days of trial medication taken? | ||||
| n | 268 | 301 | 59 | 62 |
| No, n (%) | 15 (5.6) | 15 (5) | 40 (67.8) | 35 (56.5) |
| Yes, n (%) | 253 (94.4) | 286 (95) | 19 (33.2) | 27 (43.5) |
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| If no, how many days missed? | ||||
| n | 15 | 15 | 40 | 35 |
| Median (IQR) | 1 (1–5) | 1 (1–2) | 3 (2–4) | 2 (2–4) |
| Minimum, maximum | 1, 5 | 1, 5 | 1, 5 | 1, 5 |
It is still possible that higher adherence within the prednisolone arm with those URTIs that did not result in URRs may also have meant that beneficial effects of the intervention treatment have been missed because the treatment was not administered as intended, either through medication not being given at all or through an incomplete course of prednisolone. An exploratory per-protocol analysis of those URTIs where medication was started within 2 days of URTI gives an adjusted risk difference of –0.05 (95% CI –0.17 to 0.08).
Secondary outcomes
There was a small difference in the number of relapses between the two trial arms (216 relapses were reported in 91 patients the prednisolone arm vs. 237 relapses in 98 patients in the placebo arm). The difference in the number of patients experiencing a relapse was non-significant (p = 0.33), but the difference in the number of relapses between trial arms was sufficient for the health economic analysis to conclude a benefit in the trial intervention. If a small increase in relapses in the placebo arm was genuine, the NNT calculation that 42 URTIs are required to be treated with 6 days of daily low-dose prednisolone to prevent one URR would be considered unacceptable by most clinicians.
The cumulative dose of prednisolone, which was calculated including the prednisolone used as the trial drug in the intervention arm, was not different between the two arms (median 2060 mg, IQR 1127.5–3355 mg in the prednisolone arm; median 1880 mg, IQR 1115–3295 mg in the placebo arm; p = 0.72 from a Wilcoxon rank-sum test). Therefore, there was no benefit or detriment from occasional use of this additional 6-day course of low-dose prednisolone.
Similar to findings of the PREDNOS study,8 the incidence of most specific known corticosteroid adverse effects was as expected and not different between treatment arms. There was a tendency for increased reporting of poor behaviour in the placebo arm (54% vs. 45%). This was also supported by ACBC scores, which were, on average, lower (better) in the prednisolone arm (although the differences were small). The scores are comparable to those found in the PREDNOS trial;9 however, these ACBC mean total problem T-scores are low compared with those found in a study by Mishra et al. 52 Mishra et al. 52 looked at ACBC individual and total problem T-scores for children with SSNS at different points of the disease course and matched controls. Total problems T-scores for children in both arms of the PREDNOS2 trial fall between the newly diagnosed pre-corticosteroid treatment and the control group in Mishra et al. ’s study (59.8 and 44.7, respectively). In addition, the differences between groups in that study were much greater than the differences seen between treatment arms in the PREDNOS2 trial. Given that the PREDNOS2 trial involved giving low doses of prednisolone and the average relapse rate was relatively low, meaning that relatively few courses of high-dose prednisolone were required, it is perhaps not surprising to see these low ACBC scores.
An expected number of SAEs was seen, with no significant difference between treatment arms. Absence of SUSARs and deaths is consistent with known safety data on prednisolone.
Escalation and reduction of background therapy were considered important secondary outcomes. They were not included in the design of previous trials, in which children who escalated or reduced background therapy were often excluded from the analysis, therefore introducing potential bias. Should the trial intervention be effective, it was hypothesised that patients in the treatment arm should see a reduction in the level of background therapy. Similarly, patients in the placebo arm continuing to experience frequent relapses would be more likely to require escalation in background therapy. There was no difference in escalation of background therapy between treatment arms, with an adjusted risk ratio close to 1 (p = 0.96). In terms of reduction of background therapy, more patients in the placebo than in the prednisolone arm (48.1% vs 43%) moved to a lower level of maintenance treatment, although the difference was non-significant (p = 0.42).
Health economic findings
The economic evaluation combined the costs associated with giving daily low-dose prednisolone at the time of an URTI with the HRQoL benefits and found it to increase QALYs and decrease costs when compared with standard care. This finding was robust to sensitivity analysis and was maintained over the long term, until all patients had reached adulthood (i.e. 18 years).
There are two main reasons for this finding. First, the main driver of the cost difference was the difference in costs of background therapy plus the hospitalisation costs associated with relapse. After 1 year, proportionally more patients in the prednisolone arm had discontinued background therapy and moved to group 1 than in the standard care arm. In addition, there were fewer cases of hospitalisation after relapse (see Table 23). These differences led to an incremental cost saving that favoured the daily low-dose prednisolone arm that was sustained and accumulated over 16 years. Second, when the utility decrement associated with having a relapse was accounted for, this led to low-dose prednisolone gaining more QALYs than standard care.
The model used the trial data over 12 months’ follow-up and projected the findings over 16 years (i.e. until all patients reached 18 years). The robustness of the findings was tested in a PSA by plotting 10,000 paired cost and QALY estimates on the cost-effectiveness plane and CEAC. According to published recommendations, CEACs are considered an effective tool for dealing with uncertainty in economic evaluations and remove reliance from statistics, such as the 95% CIs, which are not always defined for cost-effectiveness ratios. 53 Although the difference in overall relapse rate between the two trial arms did not reach statistical significance in the clinical analysis, the health economic model provided insight into the trade-off between the costs and effects when considered simultaneously. It provided an economic case for administering low-dose prednisolone at the time of an URTI to avoid the high-cost and HRQoL impacts associated with relapse events, and to take account of the costs associated with background therapy, therefore leading to daily prednisolone at the time of an URTI being the recommended option.
These economic findings are particularly interesting as the clinical effectiveness results concluded that using prednisolone at the time of an URTI does not lead to a statistically significant reduction in SSNS relapses and, therefore, should not be routinely recommended as a strategy. Therefore, it appears that the clinical effectiveness and cost-effectiveness results from the PREDNOS2 trial are producing different recommendations. The explanation for this stems from the underlying differences in the theoretical approaches. Unlike the clinical effectiveness analysis, the economic analysis is focused on the ratio of costs and outcomes and assessing value, based on society’s WTP for a unit gain in outcome (QALYs). With this in mind, although the PREDNOS2 trial showed that the difference in SSNS relapse between the treatment arms did not reach statistical significance, the direction of effect favoured the prednisolone arm. As these relapse episodes are frequently associated with high hospital costs and a corresponding reduction in HRQoL, and when this is combined with the relatively cheap costs of the intervention (prednisolone), this small difference in relapse rate, albeit not statistically significant for the clinical effectiveness, combined with the difference in costs associated with background therapy led to the low-dose prednisolone strategy being the ‘dominant’ treatment option. This apparent difference in findings between the clinical effectiveness and cost-effectiveness of this trial emphasises the importance of the morbidity and economic consequences of SSNS relapses and on the underlying costs associated with background therapy.
Strengths of the trial
The strengths of the PREDNOS2 trial are its size and methodology. With 365 patients recruited and 271 patients forming the modified ITT population, it, to the best of our knowledge, constitutes the largest clinical trial of an IMP in childhood nephrotic syndrome ever undertaken. A trial of this scale could be undertaken as a multicentre study only and the PREDNOS2 trial was built on the successful research network developed with its predecessor study (i.e. the PREDNOS trial9). The extremely high case report form return rate (98.6% data completion) (see Figure 5) demonstrates the effectiveness of this research network and adds to the robustness of the data.
The PREDNOS2 trial is unique among other trials evaluating the use of daily corticosteroid to prevent relapses in SSNS in its comprehensive trial design and methodology. In contrast to other trials, the PREDNOS2 trial was double blinded and placebo controlled. Care was taken to avoid bias from excluding groups such as those experiencing corticosteroid adverse effects or escalating background treatment. Rather than excluding patients in whom inadequate adherence was suspected, it sought to capture adherence data systematically and explore reasons for this non-adherence. It was also the only trial, to the best of our knowledge, that attempted to record corticosteroid AEs systematically, include an objective measure of the impact of corticosteroid on behaviour and include a health economic analysis.
Challenges of the trial
The long duration of the trial did present a challenge to try and minimise recruitment fatigue among local research teams. However, from an initial slowing of recruitment, a rate of 4.6 recruits per month was maintained for the last 4 years of the trial, although this might also have reflected a ready prevalent population at the start of the trial, moving to incident patients and slower recruitment once that initial eligible pool had been recruited.
The finding of a lower than anticipated event rate combined with a higher than anticipated number of children not experiencing an URTI within the 12 months of the trial prompted a review of the study that recommended two changes: (1) changing the primary outcome from the incidence of URR following first URTI to incidence of URR following any URTI within the trial duration and (2) increasing the sample size to allow for a 30% attrition rate. In the light of the trial results, the decision taken by the TMG to continue the trial with this change in primary outcome has been vindicated.
Towards the end of the trial, there was a change in chief investigator due to a change in situation for the previous incumbent. The new chief investigator had been a member of the TSC and changeover occurred smoothly.
Possible weaknesses
The trial may be criticised for the large number of excluded children who did not experience an URTI during their 12 months’ follow-up. With hindsight, an alternative strategy might have been to offer re-recruitment for a further 12 months as a more cost-effective way to reach the target population, but those children may not have fulfilled the inclusion criteria of a minimum of two relapses in the 12 months prior to their re-recruitment, therefore bringing into question the application of the trial’s findings.
In contrast to previous trial design, we did not review and examine these children at the time of their URTI and it is possible that some excluded children had experienced URTIs that were not captured within the trial definition. If we had made a type II error by failing to find a significant result that is present, we would have expected to see an increased number of relapses. As discussed above, the small increase in patients experiencing any relapses in the placebo arm was non-significant (p = 0.33).
The economic analysis also had some limitations. First, the cross-walked/mapping technique used to derive utility values for children aged < 5 years in the absence of a validated HRQoL instrument may have introduced additional uncertainty to the economic model. Second, the model ‘allowed’ patients to change their background therapy every 3 months and not more frequently and, although this assumption was based on clinical expert opinion, it may have biased the results. Any uncertainty related to these assumptions was explored in a sensitivity analyses by excluding the under-5s cohort in a subgroup analysis and by relaxing the change in background therapy to every 1 month, respectively, although neither had an impact on the direction of the ICER.
Conclusion
-
This is, to the best of our knowledge, the largest by far and most methodologically robust trial addressing this specific clinical question. We have shown that giving 6 days of daily low-dose prednisolone at the time of an URTI does not reduce the risk of relapse of nephrotic syndrome in children on a range of background treatments for relapsing nephrotic syndrome and in a temperate country.
-
Although the reduction in relapse associated with the intervention was not statistically significant, health economic analysis has shown a benefit to the intervention (with the high cost and reduction of HRQoL from having a relapse alongside background therapy costs and the low cost of prednisolone).
-
Results from previous trials that supported use of daily low-dose corticosteroid at the time of an URTI were small and underpowered. The direction of the much larger, mixed-ethnicity PREDNOS2 trial result was the same, but with non-significant results. This may mean that previous trials produced false-positive results or that there is an ethnicity effect. The size of the PREDNOS2 trial population will outweigh the combined results of all previous studies when future meta-analyses are undertaken.
Recommendations for future research
The following research recommendations, in priority order, follow on from the PREDNOS2 trial conclusions:
-
The PREDNOS2 trial data has highlighted the importance of relapse in terms of cost and quality-of-life impact. More research is needed to understand how this might be explained by variations in treatment and across different patient populations.
-
More research is needed to establish if the findings of the PREDNOS2 trial, compared with previous trials, are purely a result of a more robust trial design or whether or not disease, georacial or viral epidemiological factors also play a role.
-
Large registry studies, such as the RaDaR rare disease registry,54 have the potential to study differences in SSNS disease course and the reasons for them in different racial groups within a diverse population, such as the UK.
-
The PREDNOS2 trial recruited more efficiently than the PREDNOS trial. 9 The PREDNOS trial research network, comprising local research teams in > 100 local hospitals and an experienced central clinical trials team, has now delivered two large multicentre interventional trials. This puts the network in a unique position to deliver further efficient trials towards evidence-based management of SSNS in children.
-
Virology studies of the specific causes of URTIs experienced by children with SSNS would help us to understand whether or not specific organisms are more likely to trigger relapses and, in so doing, provide the potential for greater understanding of the pathophysiology that results in a relapse.
-
The PREDNOS2 trial has shown that low-dose corticosteroid at the time of an URTI before the onset of any proteinuria does not reduce the risk of relapse and, moreover, the risk of an URTI resulting in a relapse is relatively low at 21%. A related research question is whether or not a relapse can be prevented by the earlier-than-usual commencement of a lower-than-usual dose of prednisolone. In the last few years, there has also been renewed interest in whether or not relapses can be treated with lower doses of prednisolone. 55–57 In addition, in the latest Cochrane update published in 2020,4 authors have called for a large trial to verify these findings, which would open the gateway to exploring more creative, personalised ways to manage relapses that limit the cumulative dose of corticosteroid. With its strong research network, the PREDNOS trial group would be well placed to continue to deliver the multicentre trials that are required to provide the evidence basis for this debilitating chronic childhood illness.
-
The role of the adrenal axis in nephrotic syndrome and the potential association of adrenal suppression with an increased risk of relapse is a long-forgotten area of SSNS that has been highlighted anew in the latest Cochrane update of corticosteroid use in SSNS. 4
Acknowledgements
The PREDNOS2 investigators would like to thank the NIHR HTA programme for funding this trial. We thank all of the parents and participants who agreed to enter the trial, the local investigators who contributed to the trial, the local research teams that, so assiduously, completed and returned case report forms and the NIHR Clinical Research Network: Children for its support with trial set-up and recruitment.
We also thank the Service Users Group from NeST and the Renal Patient Support Group, in particular Mrs Wendy Cook, Mrs Samantha Davies-Abbott and Dr Shahid Muhammad who provided valuable input regarding trial design and conduct and reviewed the Plain English summary. The support of the British Association for Paediatric Nephrology (London, UK) and the Royal College of Paediatrics and Child Health (London, UK) is much appreciated.
Trial Management Group
Professor Nicholas Webb (Royal Manchester Children’s Hospital, Manchester University NHS Foundation Trust, chief investigator up to May 2018); Dr Martin Christian (Nottingham Children’s Hospital, chief investigator from June 2018); Miss Natalie Ives (Senior Statistician, Birmingham Clinical Trial Unit); Professor Emma Frew (Senior Lecturer in Health Economics, University of Birmingham); and Mrs Elizabeth Brettell (Renal Trials Manager, BCTU).
PREDNOS2 trial management and day-to-day co-ordination
Birmingham Clinical Trials Unit, University of Birmingham
Mrs Elizabeth A Brettell, Team Leader; Emma Barsoum, Trial Manager; Ms Helen Bodenham-Chilton, Trial Manager; Mr Adam R Khan, Trial Manager; Ms Noreen Akhtar, Senior Data Manager; Mrs Charmaine Hunt, Senior Data Manager; Ms Carla Galinha, Data Manager; Ms Dominique Smith, Data Manager; and Mr Neil Winkles, Senior Systems Developer.
PREDNOS2 trial statistics, Birmingham Clinical Trials Unit, University of Birmingham
Miss Natalie Ives, Statistics Team Leader; Ms Rebecca Woolley, Senior Medical Statistician; Mr Samir Mehta, Senior Medical Statistician; and Ms Catherine Hewitt, Senior Medical Statistician.
PREDNOS2 trial health economics
Professor Emma Frew, Health Economics Lead (Institute of Applied Health Research, University of Birmingham) and Ms Nafsika Afentou, Health Economics Research Associate.
Patient and public representatives
Mrs Wendy Cook (Director, NeST) and Mrs Sandra Cope.
Trial Steering Committee
Dr Megan Thomas (Consultant Community Paediatrician, Blackpool Teaching Hospitals NHS Trust, independent chairperson); Professor Nicholas JA Webb (Consultant Paediatric Nephrologist, Manchester Children’s Hospital, chief investigator, non-independent member); Dr Martin T Christian (Consultant Paediatric Nephrologist, Nottingham Children’s Hospital, chief investigator, non-independent member); Mrs Sandra Cope (consumer representative, independent member); Dr Andrew Duncan (Consultant Paediatrician, Borders General Hospital, Melrose, independent member); Dr Kate Hillman (Consultant Nephrologist, Manchester Royal Infirmary, independent member); Dr Zala Ibrahim (Consultant Paediatrician, Russells Hall Hospital, Dudley, independent member); Dr Ly-Mee Yu (Senior Medical Statistician, Nuffield Department of Primary Care Health Sciences, University of Oxford, independent member); Dr Nigel Coad (Consultant Paediatrician, University Hospitals of Coventry and Warwickshire NHS Trust, independent member); Natalie Ives (co-investigator, Senior Statistician, non-independent member); and Darren Green (Consultant Acute Physician and Nephrologist, Salford Royal NHS Foundation Trust, independent member).
Data Monitoring Committee
Professor Philip Kalra (chairperson, Consultant Nephrologist and Honorary Professor, Salford Royal Hospital NHS Trust); Professor Saul Faust (Reader in Paediatric Immunology and Infectious Diseases, Southampton General Hospital); and Dr Andrea Marshall (Senior Research Fellow in Medical Statistics, Warwick Clinical Trials Unit).
Participating centres and PREDNOS2 trial collaborative group members
Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust: Dr Birgit Ulbrich (principal investigator), Dr Peter Heinz and Sara Foster.
Airedale General Hospital, Airedale NHS Foundation Trust: Dr Pronab Bala (principal investigator).
Altnagelvin Area Hospital, Western Health and Social Care Trust: Dr Damien Armstrong (principal investigator), Julie Brown and team.
Arrowe Park Hospital, Wirral University Teaching Hospital NHS Foundation Trust: Dr Elizabeth Breen (principal investigator), Sharon Hughes and team.
Barnsley Hospital, Barnsley Hospital NHS Foundation Trust: Dr Rajeev Gupta (principal investigator) and Miranda Murray.
Doncaster Royal Infirmary & Bassetlaw Hospital, Doncaster and Bassetlaw Hospitals NHS Foundation Trust: Dr Mathew Kurian (principal investigator), Dr Sunitha Sampath, Dr Sarah Didier, Sarah Shortland, Sharon Allen, Lisa Warren and team.
Birmingham Children’s Hospital, Birmingham Women’s and Children’s NHS Foundation Trust: Dr David Milford (principal investigator), Dr Mordi Muorah, Alison Watson, Fatima Bibi and team.
Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Foundation Trust: Dr Eduardo Moya (principal investigator).
Bristol Royal Hospital for Children, University Hospitals Bristol NHS Foundation Trust: Professor Moin Saleem (principal investigator), Dr Alison Kelly, Michelle Ross, Natalie Fineman and team.
Calderdale Royal Hospital & Huddersfield Royal Infirmary, Calderdale and Huddersfield NHS Foundation Trust: Dr Eilean Crosbie (principal investigator), Rachel Swingler, Susan Kilroy and team.
Chesterfield Royal Hospital, Chesterfield Royal Hospital NHS Foundation Trust: Dr Oyekunle Ayonrinde (principal investigator) and Amanda Smith.
Colchester General Hospital, Colchester Hospital University NHS Foundation Trust: Dr Andrea Turner (principal investigator), Dr Jonathan Campbell and Aine Turner.
Countess of Chester Hospital, Countess of Chester Hospital NHS Foundation Trust: Dr Stephen Brearey (principal investigator), Caroline Burchett and Sarah De-Beger.
Croydon University Hospital, Croydon Health Services NHS Trust: Dr Theo Fenton (principal investigator).
Cumberland Infirmary, North Cumbria University Hospitals NHS Trust: Dr Glyn Jones (principal investigator) and Nicci Kelsall.
Darent Valley Hospital, Dartford and Gravesham NHS Trust: Dr Selwyn D’Costa (principal investigator).
Darlington Memorial Hospital, University Hospital of North Durham, County Durham and Darlington NHS Foundation Trust: Dr Dinakaran Jayachandran (principal investigator), Dr Asha Nair, Catherine Tarn Nozedar and Dawn Egginton.
Derriford Hospital, Plymouth Hospitals NHS Trust: Dr Oliver Cuthell (principal investigator), Dr Catherine Derry, Dr Kathiresan Natesan and Sarah-Jane Sharman.
Dewsbury and District Hospital, Pinderfields Hospital, The Mid Yorkshire Hospitals NHS Trust: Dr Rajeeva Singh (principal investigator), Dr Kathryn Deakin, Gail Castle and team.
Diana, Princess of Wales Hospital (Grimsby), Northern Lincolnshire and Goole Hospitals NHS Foundation Trust: Dr Bukar Wobi (principal investigator), Dr Bemigho Etuwewe, Caroline Burnett and team.
East Surrey Hospital, Surrey and Sussex Healthcare NHS Trust: Dr Kamal Khoobarry (principal investigator).
Eastbourne District General Hospital, Conquest Hospital, East Sussex Healthcare NHS Trust: Dr Graham Whincup (principal investigator) and Anne Cowley.
Evelina London Children’s Hospital, Guy’s and St Thomas’ NHS Foundation Trust: Dr Ania Koziell (principal investigator), Dr Manish Sinha, Dr Christopher Reid, Mohammad Ahmad and team.
Fairfield General Hospital, North Manchester General Hospital, The Royal Oldham Hospital, Rochdale Infirmary, The Pennine Acute Hospitals NHS Trust: Dr Beena Padmakumar (principal investigator), Dr Talaivirichan Magadevan, Grainne O’Connor and Louise Woodhead.
Royal Lancaster Infirmary & Furness General Hospital, University Hospitals of Morecambe Bay NHS Foundation Trust: Dr Mireille Formosa (principal investigator), Dr Nayan Peepah Nardeosingh and Kathryn Allison.
Glan Clwyd Hospital, Betsi Cadwaladr University Health Board: Dr Markus Hesseling (principal investigator), Annette Bolger, Lucie Hobson and team.
Gloucestershire Royal Hospital, Gloucestershire Hospitals NHS Foundation Trust: Dr Adamu Sambo (principal investigator), Dr Lyda Jadresic and Susan Beames.
Great Ormond Street Hospital, Great Ormond Street Hospital for Children NHS Foundation Trust: Professor Detlef Bockenhauer (principal investigator), Dr Daljit Hothi, Elizabeth Vella, Corinne Linton, Shaima Yussuf, Tendai Bazaya, Mahmoud Abou-Rayyah and team.
Great Western Hospital, Great Western Hospitals NHS Foundation Trust: Dr Nick West (principal investigator).
Homerton University Hospital, Homerton University Hospital NHS Foundation Trust: Dr Rajiv Sood (principal investigator), Hilarious De Jesus and team.
Hull Royal Infirmary, Hull and East Yorkshire Hospitals NHS Trust: Dr Vikas Gupta (principal investigator) and Dr Verghese Mathew.
James Paget University Hospital, James Paget University Hospitals NHS Foundation Trust: Dr Esi Bentsi-Enchill (principal investigator) and Allyson Davison.
John Radcliffe Hospital, Oxford University Hospitals NHS Trust: Dr Janet Craze (principal investigator).
Kent and Canterbury Hospital, Queen Elizbaeth the Queen Mother Hospital, William Harvey Hospital, East Kent Hospitals University NHS Foundation Trust: Dr Elhussein Rfidah (principal investigator), Janine Musselwhite, Angela Moon and team.
Kettering General Hospital, Kettering General Hospital NHS Foundation Trust: Dr Harsha Bilolikar (principal investigator), Sonia White and team.
King’s Mill Hospital, Sherwood Forest Hospitals NHS Foundation Trust: Dr Simon Rhodes (principal investigator) and Caroline Moulds.
Leeds General Infirmary, Leeds Teaching Hospitals NHS Trust: Dr Hitesh Prajapati (principal investigator), Dr Eric Finlay, Dr Pallavi Yadav, Dr Amanda Newnham, Dr Kay Tyerman, Majorie Allen, Lucy Wellings and team.
Leicester Royal Infirmary, University Hospitals of Leicester NHS Trust: Dr Angela Hall (principal investigator), Jackie Philps and team.
Leighton Hospital, Mid Cheshire Hospitals NHS Foundation Trust: Dr Subajini Kaviethasan (principal investigator), Sally Smith and team.
Pilgrim Hospital & Lincoln County Hospital, United Lincolnshire Hospitals NHS Trust: Dr David Broodbank (principal investigator), Dr Sourabh Mukhopadhyay, Dr Ruchika Gupta, Amanda Roper, Susie Butler and team.
Luton and Dunstable University Hospital, Luton and Dunstable Hospital NHS Foundation Trust: Dr Tomasz Rajkowski (principal investigator), Dr Michael Eisenhut, Karen Duncan, Karen Samm, Samantha Clough and team.
Macclesfield District General Hospital, East Cheshire NHS Trust: Dr David Wright (principal investigator), Dr Krishnakumar Thattakkat, Dr Ignatius Losa, Natalie Keenan and team.
Maidstone Hospital, Tunbridge Wells Hospital, Maidstone and Tunbridge Wells NHS Trust: Dr Krishnan Balasubramanian (principal investigator).
Manor Hospital, Walsall, Walsall Healthcare NHS Trust: Dr Muhammad Javed (principal investigator), Sharon Kempson, Marie Phipps and team.
Medway Maritime Hospital, Medway NHS Foundation Trust: Dr Janette Cansick (principal investigator) and Maines Msiska.
Milton Keynes Hospital, Milton Keynes University Hospital NHS Foundation Trust: Dr Lazarus Anguvaa (principal investigator), Dr Mya Aye, Sally Conway, Natalie Beer, Francesca Wright and team.
Musgrove Park Hospital, Taunton and Somerset NHS Foundation Trust: Dr Jennifer Langlands (principal investigator), Kirsty O’Brien, Nicola Thorne and team.
New Cross Hospital, The Royal Wolverhampton Hospitals NHS Trust: Dr Karen Davies (principal investigator), Sharon Kempson, Marie Phipps and team.
Newham University Hospital, The Royal London Hospital, Whipps Cross Hospital, Barts Health NHS Trust: Dr Ami Parikh (principal investigator), Dr Nimze Gadong, Dr Bahadur Anjum, Nicolene Plaatjies, Ivone Lancoma-Malcolm, Hilarious De Jesus and team.
Norfolk and Norwich University Hospital, Norfolk and Norwich University Hospitals NHS Foundation Trust: Dr Vipan Datta (principal investigator), Dr Chris Upton, Louisa Fear, Louise Coke and team.
North Devon District Hospital, Northern Devon Healthcare NHS Trust: Dr Dermot Dalton (principal investigator), Becky Holbrook and team.
Northampton General Hospital, Northampton General Hospital NHS Trust: Dr Imogen Norton (principal investigator).
Nottingham Children’s Hospital (Queens Medical Centre), Nottingham University Hospitals NHS Trust: Dr Martin Christian (principal investigator), Dr Andrew Lunn, Olivia Vincent, Helen Navarra, Neelam Khan and team.
Peterborough City Hospital, Peterborough and Stamford Hospitals NHS Foundation Trust: Dr Mona Aslam (principal investigator) and Paula Goodyear.
Poole Hospital, Poole Hospital NHS Foundation Trust: Dr Steve Wadams (principal investigator), Susan Power, Amy Roff and team.
Princess Royal Hospital, Royal Shrewsbury Hospital, The Shrewsbury and Telford Hospital NHS Trust: Dr Manish Gupta (principal investigator), Dr Naeem Ayub, Charlotte Owen and team.
Queen Alexandra Hospital (Portsmouth), Portsmouth Hospitals NHS Trusta: Dr Judith Scanlan (principal investigator), Sharon McCready and Andrew Gribbin.
Queen’s Hospital, Burton, Burton Hospitals NHS Foundation Trust: Dr Mansoor Ahmed (principal investigator), Dr Dominic Muogbo, Dr Heather Carswell, Stephanie Boswell, Claire Backhouse and team.
Queen’s Hospital, Romford, Barking, Havering and Redbridge University Hospitals NHS Trust: Dr Junaid Solebo (principal investigator) and Helen Smith.
Raigmore Hospital, NHS Highland: Dr Alan Webb (principal investigator), Ing-Marie Logie and Sandra Dekker.
Rotherham Hospital, The Rotherham NHS Foundation Trust: Dr Sanjay Suri (principal investigator), Janet Shackleton and team.
Royal Aberdeen Children’s Hospital, NHS Grampian: Dr Craig Oxley (principal investigator), Margaret Connon and team.
Royal Albert Edward Infirmary, Wrightington, Wigan and Leigh NHS Foundation Trust: Dr Vineeta Joshi (principal investigator), Nicola Pemberton and team.
Royal Alexandra Hospital, NHS Greater Glasgow and Clyde: Dr Heather Maxwell (principal investigator), Dr Amita Sharma, Elizabeth Waxman and team.
Royal Belfast Hospital for Sick Children, Belfast Health and Social Care Trust: Dr Karl McKeever (principal investigator), Muriel Millar and team.
Royal Berkshire Hospital, Royal Berkshire NHS Foundation Trus: Dr Ann Gordon (principal investigator), Dr Susan Edees, Susan Hallett and team.
Royal Blackburn Hospital, East Lancashire Hospitals NHS Trust: Dr Javed Iqbal (principal investigator), Dr Beate von Bremen, Heather Collier, Andrew Lancaster and team.
Royal Bolton Hospital, Bolton NHS Foundation Trust: Dr Fiona Watson (principal investigator), Joanne Henry and team.
Royal Derby Hospital, Derby Hospitals NHS Foundation Trust: Dr Richard Bowker (principal investigator) and Coral Smith.
Royal Devon & Exeter Hospital (Wonford), Royal Devon & Exeter NHS Foundation Trust: Dr Hannah Cottis (principal investigator), Dr Rebecca Samuel, Caroline Harrill, Suzanne Wilkins and team.
Royal Hospital for Children (Glasgow), NHS Greater Glasgow and Clyde: Dr Heather Maxwell (principal investigator), Dr Ben Reynolds, Dr David Hughes, Elizabeth Waxman and team.
Royal Hospital for Sick Children (Edinburgh), NHS Lothian: Dr Ben Reynolds (principal investigator), Dr David Hughes, Tracey McGregor, Maxine Ramsay, Julie Baggott, Naomi Matos and team.
Royal Liverpool Children’s Hospital (Alder Hey), Alder Hey Children’s NHS Foundation Trust: Dr Caroline Jones (principal investigator), Dr Henry Morgan, Dr Richard Holt, Dr Louise Oni, Theresa Moorcroft, Joanne Shakeshaft and team.
Royal Manchester Children’s Hospital, Central Manchester University Hospitals NHS Foundation Trust: Dr Mohan Shenoy (principal investigator), Professor Nicholas Webb, Dr Amrit Kaur, Dr Dean Wallace, Dr Nicholas Plant, Dr Shaila Sukthankar, Angela Branson, Helen Blackburn, Jane Howell, Jess Nichols and team.
Royal Stoke University Hospital, The County Hospital (Stafford Hospital), University Hospitals of North Midlands NHS Trust: Dr Furqan Basharat (principal investigator), Dr Saeeda Raja, Marie Phipps, Helen Parker, Joanne Tomlinson, Eric Roe and team.
Royal United Hospital (Bath), Royal United Hospital Bath NHS Foundation Trust: Dr Lynn Diskin (principal investigator) and Alison Barratt.
Russells Hall Hospital, The Dudley Group NHS Foundation Trust: Dr Subramanian Mahadevan-Bava (principal investigator), Abigail Weston and Daljit Kaur.
Scarborough Hospital, York Teaching Hospital NHS Foundation Trust: Dr Udupa Venkatesh (principal investigator), Emma Temlett, Simon Dyer, Kerry Elliott, Rosie Furness and team.
Sheffield Children’s Hospital, Sheffield Children’s NHS Foundation Trust: Dr Andrew Lunn (principal investigator), Janet Shackleton, Sarah Shortland, Miranda Murray and team.
Southampton Children’s Hospital, University Hospital Southampton NHS Foundation Trust: Dr Rodney Gilbert (principal investigator), Dr Matthew Harmer, Dr Shuman Haq, Lisa Fairhead, Louise Haskell, Victoria Bingham and team.
Southend Hospital, Southend University Hospital NHS Foundation Trust: Dr Anupam Shrivastava (principal investigator), Onie Hove and Bernard Hadebe.
St Mary’s Hospital, Isle of Wight NHS Trust: Dr Christopher Magier (principal investigator), Bettina Harms and Sian Butterworth.
St Peter’s Hospital, Ashford and St Peter’s Hospitals NHS Foundation Trust: Dr Tariq Bhatti (principal investigator), Dr Aisling Parker, Lorna Walding and team.
St Richard’s Hospital, Worthing Hospital, Western Sussex Hospitals NHS Foundation Trust: Dr Nicholas Brennan (principal investigator).
Stepping Hill Hospital, Stockport NHS Foundation Trust: Dr Chris Cooper (principal investigator), Sara Bennett and team.
Tameside Hospital, Tameside Hospital NHS Foundation Trust: Dr Anjali Date (principal investigator), Dr Anjali Petkar, Wendy Hulse and team.
Worcestershire Royal Hospital, The Alexandra Hospital (Redditch), Worcestershire Acute Hospitals NHS Trust: Dr Munir Ahmed (principal investigator), Dr Tom Dawson, Connie Rowlands and Stephanie Chamberlain.
The County Hospital, Hereford, Wye Valley NHS Trust: Dr Simon Meyrick (principal investigator), Dr Iain Darwood and Emma Collins.
The Friarage Hospital, The James Cook University Hospital, South Tees NHS Foundation Trust: Dr Rajesh Lall (principal investigator), Dr Elizabeth Onifade, Joanna Green and team.
The Great North Children’s Hospital, The Newcastle Upon Tyne Hospitals NHS Foundation Trust: Dr Sally Johnson (principal investigator), Dr Heather Lambert, Dr Yincent Tse, Dr Michal Malina, Dr Vijaya Sathyanarayan, Jenny Booth, Kathryn Bell, Stephen Crulley and team.
The Ipswich Hospital, The Ipswich Hospital NHS Trust: Dr Jackie Buck (principal investigator), Deborah Beeby, Louise Hunt and team.
The York Hospital, York Teaching Hospital NHS Foundation Trust: Dr Sundeep Sandhu (principal investigator), Dr Gur Millman, Dr Murray Wheeler, Anna Clayton, David Thompson and team.
University Hospital Crosshouse, NHS Ayrshire and Arran: Dr Bridget Oates (principal investigator), Claire Bell, Joanna Wardrop and team.
University Hospital of North Tees, North Tees and Hartlepool NHS Foundation Trust: Dr Vijay Tandle (principal investigator), Carolyn Campbell, Dawn Egginton and team.
University Hospital of Wales, Cardiff and Vale University Health Board: Dr Shivaram Hegde (principal investigator), Dr Rajesh Krishnan, Zoe Morrison, Jennifer Muller, Louise Yendle and team.
Warrington Hospital, Warrington and Halton Hospitals NHS Foundation Trust: Dr Delyth Webb (principal investigator) and Natalie Rogers.
West Middlesex University Hospital, Chelsea and Westminster Hospital NHS Foundation Trust: Dr Nour Elhadi (principal investigator), Dr Dipali Shah, Amrinder Sayan and team.
West Suffolk Hospital, West Suffolk NHS Foundation Trust: Dr Karine Cesar (principal investigator), Dr Raman Lakshman and Helen Cockerill.
Wexham Park Hospital, Frimley Health NHS Foundation Trust: Dr Zilla Huma (principal investigator).
Wishaw General Hospital, NHS Lanarkshire: Dr Thin Thin Saing (principal investigator), Angela Brown, Karen Leitch and team.
Wythenshawe Hospital, University Hospital of South Manchester NHS Foundation Trust: Dr Gopi Vemuri (principal investigator), Claire Holliday, Jessica Carey, Louise Woodhead and team.
Ysbyty Gwynedd, Betsi Cadwaladr University Health Board: Dr Madalitso Kubwalo (principal investigator), Annette Bolger and team.
Contributions of authors
Martin T Christian (https://orcid.org/0000-0003-1642-1961) (subsequent chief investigator, Consultant Paediatric Nephrologist) took over as chief investigator in 2018, led and developed the trial design, led the TMG and provided causality assessment for all SAEs, was an original co-applicant of the grant application, was a principal investigator for their hospital and was the lead author of the report.
Nicholas JA Webb (https://orcid.org/0000-0001-8572-5446) (original chief investigator, Consultant Paediatric Nephrologist) led and developed the trial design, led the TMG and provided causality assessment for all SAEs until 2018, was an original co-applicant of the grant application and was a principal investigator for their hospital.
Rebecca L Woolley (https://orcid.org/0000-0001-5119-1431) (Statistician) provided statistical support throughout the study and developed and led the planned statistical analyses.
Nafsika Afentou (https://orcid.org/0000-0003-1337-7029) (Fellow in Health Economics) designed, conducted and wrote the health economic evaluation.
Samir Mehta (https://orcid.org/0000-0001-7096-513X) (Statistician) designed and undertook the final statistical analysis.
Emma Frew (https://orcid.org/0000-0002-5462-1158) (Professor in Health Economics) designed, conducted and wrote the health economic evaluation.
Elizabeth A Brettell (https://orcid.org/0000-0002-0669-3085) (Clinical Trials Team Leader) led the trial team at BCTU, overseeing the day-to-day management of the trial and data collection.
Adam R Khan (https://orcid.org/0000-0001-5366-233X) (Trial Manager) led the trial team at BCTU, overseeing the day-to-day management of the trial and data collection.
David V Milford (https://orcid.org/0000-0003-4737-2744) (Consultant/Senior Paediatric Nephrologist) was an original co-applicant of the grant application and was a principal investigator for their hospital.
Detlef Bockenhauer (https://orcid.org/0000-0001-5878-941X) (Consultant Paediatric Nephrologist) was principal investigator for his hospital.
Moin A Saleem (https://orcid.org/0000-0002-9808-4518) (Consultant Paediatric Nephrologist) was an original co-applicant of the grant application and was a principal investigator for their hospital.
Angela S Hall (https://orcid.org/0000-0003-2300-5421) (Associate Specialist Paediatrician with Interest in Nephrology) was an original co-applicant of the grant application and was a principal investigator for their hospital.
Ania Koziell (https://orcid.org/0000-0003-4882-0246) (Consultant Paediatric Nephrologist) was principal investigator for her hospital.
Heather Maxwell (https://orcid.org/0000-0002-5904-1718) (Consultant Paediatric Nephrologist) was an original co-applicant of the grant application and was a principal investigator for their hospital.
Shivaram Hegde (https://orcid.org/0000-0003-3573-9940) (Consultant Paediatric Nephrologist) was an original co-applicant of the grant application and was a principal investigator for their hospital.
Eric R Finlay (Consultant Paediatric Nephrologist) was an original co-applicant of the grant application and was a principal investigator for their hospital.
Rodney D Gilbert (https://orcid.org/0000-0001-7426-0188) (Consultant Paediatric Nephrologist) was an original co-applicant of the grant application and was a principal investigator for their hospital.
Caroline Jones (https://orcid.org/0000-0001-9442-9286) (Consultant Paediatric Nephrologist) was an original co-applicant of the grant application and was a principal investigator for their hospital.
Karl McKeever (https://orcid.org/0000-0002-5010-0398) (Consultant/Senior Paediatric Nephrologist) was an original co-applicant of the grant application and was a principal investigator for their hospital.
Wendy Cook (https://orcid.org/0000-0001-8360-2813) (Director for NeST) provided patient and public involvement at the point of trial concept and design, was a member of the TSC and read the report manuscript.
Natalie Ives (https://orcid.org/0000-0002-1664-7541) (Reader in Clinical Trials, Senior Statistician) provided the statistical support throughout the study, and developed and led the planned statistical analyses.
Publications
Webb NJA, Frew E, Brettell EA, Milford DV, Bockenhauer D, Saleem MA, et al. Short course daily prednisolone therapy during an upper respiratory tract infection in children with relapsing steroid-sensitive nephrotic syndrome (PREDNOS 2): protocol for a randomised controlled trial. Trials 2014;15:1–12.
Christian MT, Webb NJA, Mehta S, Woolley RL, Afentou N, Frew E, et al. Evaluation of daily low-dose prednisolone during upper respiratory tract infection to prevent relapse in children with relapsing steroid-sensitive nephrotic syndrome: the PREDNOS randomized clinical trial [published online ahead of print December 20 2021]. JAMA Pediatr 2021.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Webb NJ, Frew E, Brettell EA, Milford DV, Bockenhauer D, Saleem MA, et al. Short course daily prednisolone therapy during an upper respiratory tract infection in children with relapsing steroid-sensitive nephrotic syndrome (PREDNOS 2): protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-147.
- Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS. Long-term outcome for children with minimal-change nephrotic syndrome. Lancet 1985;1:368-70. https://doi.org/10.1016/S0140-6736(85)91387-X.
- Abramowicz M, Barnett HL, Edelmann CM, Greifer I, Kobayashi O, Arneil GC, et al. Controlled trial of azathioprine in children with nephrotic syndrome. A report for the international study of kidney disease in children. Lancet 1970;1:959-61. https://doi.org/10.1016/s0140-6736(70)91093-7.
- Hahn D, Hodson EM, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children (review). Cochrane Database Syst Rev 2015;3. https://doi.org/10.1002/14651858.CD001533.pub5.
- Teeninga N, Kist-van Holthe JE, Van Rijswijk N, de Mos NI, Hop WCJ, Wetzels JFM, et al. Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. J Am Soc Nephrol 2013;24:149-59. https://doi.org/10.1681/ASN.2012070646.
- Yoshikawa N, Nakanishi K, Sako M, Oba MS, Mori R, Ota E, et al. A multicenter randomized trial indicates initial prednisolone treatment for childhood nephrotic syndrome for two months is not inferior to six-month treatment. Kidney Int 2015;87. https://doi.org/10.1038/ki.2014.260.
- Sinha A, Saha A, Kumar M, Sharma S, Afzal K, Mehta A, et al. Extending initial prednisolone treatment in a randomized control trial from 3 to 6 months did not significantly influence the course of illness in children with steroid-sensitive nephrotic syndrome. Kidney Int 2015;87:217-24. https://doi.org/10.1038/ki.2014.240.
- Webb NJA, Woolley RL, Lambe T, Frew E, Brettell EA, Barsoum EN, et al. Long term tapering versus standard prednisolone treatment for first episode of childhood nephrotic syndrome: phase III randomised controlled trial and economic evaluation. BMJ 2019;365. https://doi.org/10.1136/bmj.l1800.
- Webb NJ, Woolley RL, Lambe T, Frew E, Brettell EA, Barsoum EN, et al. Sixteen-week versus standard eight-week prednisolone therapy for childhood nephrotic syndrome: the PREDNOS RCT. Health Technol Assess 2019;23. https://doi.org/10.3310/hta23260.
- Early identification of frequent relapsers among children with minimal change nephrotic syndrome . A report of the International Study of Kidney Disease in Children. J Pediatr 1982;101:514-18. https://doi.org/10.1016/S0022-3476(82)80692-6.
- Noone DG, Iijima K, Parekh R. Idiopathic nephrotic syndrome in children. Lancet 2018;392:61-74. https://doi.org/10.1016/S0140-6736(18)30536-1.
- Aljebab F, Choonara I, Conroy S. Systematic review of the toxicity of short-course oral corticosteroids in children. Arch Dis Child 2016;101:365-70. https://doi.org/10.1136/archdischild-2015-309522.
- Aljebab F, Choonara I, Conroy S. Long-course oral corticosteroid toxicity in children. Arch Dis Child 2016;101. https://doi.org/10.1136/archdischild-2016-311535.57.
- Alwadhi RK, Mathew JL, Rath B. Clinical profile of children with nephrotic syndrome not on glucorticoid therapy, but presenting with infection. J Paediatr Child Health 2004;40:28-32. https://doi.org/10.1111/j.1440-1754.2004.00285.x.
- Arun S, Bhatnagar S, Menon S, Saini S, Hari P, Bagga A. Efficacy of zinc supplements in reducing relapses in steroid-sensitive nephrotic syndrome. Pediatr Nephrol 2009;24:1583-6. https://doi.org/10.1007/s00467-009-1170-5.
- MacDonald NE, Wolfish N, McLaine P, Phipps P, Rossier E. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr 1986;108:378-82. https://doi.org/10.1016/S0022-3476(86)80876-9.
- Moorani KN, Khan KM, Ramzan A. Infections in children with nephrotic syndrome. J Coll Physicians Surg Pak 2003;13:337-9. https://doi.org/06.2003/JCPSP.337339.
- Mattoo TK, Mahmoud MA. Increased maintenance corticosteroids during upper respiratory infection decrease the risk of relapse in nephrotic syndrome. Nephron 2000;85:343-5. https://doi.org/10.1159/000045684.
- Abeyagunawardena AS, Trompeter RS. Increasing the dose of prednisolone during viral infections reduces the risk of relapse in nephrotic syndrome: a randomised controlled trial. Arch Dis Child 2008;93:226-8. https://doi.org/10.1136/adc.2007.116079.
- Gulati A, Sinha A, Sreenivas V, Math A, Hari P, Bagga A. Daily corticosteroids reduce infection-associated relapses in frequently relapsing nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 2011;6:63-9. https://doi.org/10.2215/CJN.01850310.
- Hall AS, Thorley G, Houtman PN. The effects of corticosteroids on behavior in children with nephrotic syndrome. Pediatr Nephrol 2003;18:1220-3. https://doi.org/10.1007/s00467-003-1295-x.
- Abeyagunawardena AS, Thalgahagoda RS, Dissanayake PV, Abeyagunawardena S, Illangasekera YA, Karunadasa UI, et al. Short courses of daily prednisolone during upper respiratory tract infections reduce relapse frequency in childhood nephrotic syndrome. Pediatr Nephrol 2017;32:1377-82. https://doi.org/10.1007/s00467-017-3640-5.
- Machin D, Campbell MJ, Tan SB, Tan SH. Sample Size Tables for Clinical Studies. Chichester: Wiley-Blackwell; 2009.
- Achenbach T, Edelbrock C. Manual for the Child Behavior Checklist and Revised Behavior Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1983.
- Stevens K. Valuation of the Child Health Utility 9D Index. PharmacoEconomics 2012;30:729-47. https://doi.org/10.2165/11599120-000000000-00000.
- Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003;3:329-41. https://doi.org/10.1367/1539-4409(2003)003<0329:TPAAPP>2.0.CO;2.
- Lambe T, Frew E, Ives NJ, Woolley RL, Cummins C, Bretell WA, et al. Mapping the Paediatric Quality of Life Inventory (PedsQL™) generic core scales onto the Child Health Utility Index – 9 Dimension (CHU-9D) score for economic evaluation in children. PharmacoEconomics 2018;36:451-65. https://doi.org/10.1007/s40273-017-0600-7.
- Kidney Disease Improving Global Outcomes . KDIGO Clinical Practice Guideline for Glomerulonephritis 2012. https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-English.pdf (accessed 20 July 2021).
- Great Britain . Medicines for Human Use (Clinical Trials) Regulations 2004 2004.
- Great Britain . Medicines for Human Use (Clinical Trials) Amendment Regulations 2006 2006.
- Electronic medicines compendium . Prednisolone 5mg Tablets 2021. https://www.medicines.org.uk/emc/product/2427/smpc#gref (accessed October 2021).
- Department of Health and Social Care . Immunisation Against Infectious Disease. 1996. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/147832/Green-Book-updated-140313.pdf (accessed 15 July 2021).
- World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Department of Health and Social Care (DHSC) . Research Governance Framework for Health and Social Care 2005.
- Medical Research Council, Department of Health and Social Care and Medicines and Healthcare products Regulatory Agency . Risk-Adapted Approaches to the Management of Clinical Trials of Investigational Medicinal Products 2011. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/343677/Risk-adapted_approaches_to_the_management_of_clinical_trials_of_investigational_medicinal_products.pdf (accessed 15 July 2021).
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- Christian MT, Webb NJA, Mehta S, Woolley RL, Afentou N, Frew E, et al. Evaluation of daily low-dose prednisolone during upper respiratory tract infection to prevent relapse in children with relapsing steroid-sensitive nephrotic syndrome: the PREDNOS randomized clinical trial. JAMA Pediatr 2021.
- Devlin N, Parkin D, Janssen B. Methods for Analysing and Reporting EQ-5D Data 2020. https://doi.org/10.1007/978-3-030-47622-9.
- Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. PharmacoEconomics 1998;13:397-409. https://doi.org/10.2165/00019053-199813040-00003.
- Office for National Statistics . Avoidable Mortality in the UK: 2019 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/bulletins/avoidablemortalityinenglandandwales/latest (accessed October 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- NHS . NHS National Schedule of Reference Costs 2018 19 n.d. www.england.nhs.uk/wp-content/uploads/2020/08/1_-_NCC_Report_FINAL_002.pdf (accessed 17 December 2020).
- BNF for Children (Online). London: BMJ Group, Pharmaceutical Press and RCPCH Publications; 2020.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Canaway AG, Frew EJ. Measuring preference-based quality of life in children aged 6–7 years: a comparison of the performance of the CHU-9D and EQ-5D-Y – the WAVES pilot study. Qual Life Res 2013;22:173-83. https://doi.org/10.1007/s11136-012-0119-5.
- Stevens K, Ratcliffe J. Measuring and valuing health benefits for economic evaluation in adolescence: an assessment of the practicality and validity of the child health utility 9D in the Australian adolescent population. Value Health 2012;15:1092-9. https://doi.org/10.1016/j.jval.2012.07.011.
- McKinney PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol 2001;16:1040-4. https://doi.org/10.1007/s004670100021.
- Denny FW. The clinical impact of human respiratory virus infections. Am J Respir Crit Care Med 1995;152:S4-12. https://doi.org/10.1164/ajrccm/152.4_pt_2.s4.
- Leisti S, Koskimies O. Risk of relapse in steroid-sensitive nephrotic syndrome: effect of stage of post-prednisone adrenocortical suppression. J Pediatr 1983;103:553-7. https://doi.org/10.1016/S0022-3476(83)80582-4.
- Yadav M, Sinha A, Khandelwal P, Hari P, Bagga A. Efficacy of low-dose daily versus alternate-day prednisolone in frequently relapsing nephrotic syndrome: an open-label randomized controlled trial. Pediatr Nephrol 2019;34:829-35. https://doi.org/10.1007/s00467-018-4071-7.
- Saadeh SA, Baracco R, Jain A, Kapur G, Mattoo TK, Valentini RP. Weight or body surface area dosing of steroids in nephrotic syndrome: is there an outcome difference?. Pediatr Nephrol 2011;26:2167-71. https://doi.org/10.1007/s00467-011-1961-3.
- Mishra OP, Basu B, Upadhyay SK, Prasad R, Schaefer F. Behavioural abnormalities in children with nephrotic syndrome. Nephrol Dial Transplant 2010;25:2537-41. https://doi.org/10.1093/ndt/gfq097.
- O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455-68. https://doi.org/10.1191/0962280202sm304ra.
- Mason AE, Sen ES, Bierzynska A, Colby E, Afzal M, Dorval G, et al. Response to first course of intensified immunosuppression in genetically stratified steroid resistant nephrotic syndrome. Clin J Am Soc Nephrol 2020;15:983-94. https://doi.org/10.2215/CJN.13371019.
- Raja K, Parikh A, Webb H, Hothi D. Use of a low-dose prednisolone regimen to treat a relapse of steroid-sensitive nephrotic syndrome in children. Pediatr Nephrol 2017;32:99-105. https://doi.org/10.1007/s00467-016-3458-6.
- Borovitz Y, Haskin O, Levi S, Kas S, Alfandary H, Davidovits M, et al. Lower prednisolone dosing for nephrotic syndrome relapse: a prospective, randomised study. Pediatr Nephrol 2017;32.
- Sheikh S, Mishra K, Kumar M. Low versus conventional dose prednisolone for nephrotic syndrome relapses: randomised-controlled, non-inferiority trial. Pediatr Nephrol 2019;34.
Appendix 1 Site recruitment
| Site name | Date site opened | Number of patients randomised |
|---|---|---|
| Addenbrooke’s Hospital, Cambridge | 28 March 2013 | 4 |
| Alder Hey Hospital, Liverpool | 14 June 2013 | 10 |
| Altnagelvin Area Hospital | 29 September 2014 | 2 |
| Arrowe Park Hospital, Wirral | 13 May 2013 | 1 |
| Barnsley Hospital | 25 September 2013 | 1 |
| Birmingham Children’s Hospital | 27 March 2013 | 11 |
| Bristol Royal Hospital For Children | 21 March 2013 | 6 |
| Calderdale Royal Hospital | 10 June 2013 | 2 |
| Chesterfield Royal Hospital | 22 May 2013 | 1 |
| Colchester General Hospital | 17 April 2013 | 8 |
| Conquest Hospital | 29 April 2015 | 1 |
| Countess of Chester Hospital | 27 March 2013 | 3 |
| Derriford Hospital, Plymouth | 11 June 2013 | 4 |
| Diana, Princess of Wales Hospital, Grimsby | 8 July 2014 | 2 |
| Doncaster Royal Infirmary | 27 May 2014 | 2 |
| East Surrey Hospital | 19 February 2014 | 1 |
| Evelina London Children’s Hospital | 17 June 2013 | 9 |
| Fairfield General Hospital | 8 May 2013 | 1 |
| Glan Clwyd Hospital | 3 July 2013 | 3 |
| Gloucestershire Royal Hospital | 17 April 2013 | 6 |
| Great Ormond Street Hospital | 3 September 2013 | 10 |
| Great Western Hospital, Swindon | 1 July 2015 | 4 |
| Homerton University Hospital, London | 21 March 2013 | 1 |
| Hull Royal Infirmary | 29 April 2014 | 1 |
| James Paget University Hospital, Great Yarmouth | 24 July 2014 | 1 |
| John Radcliffe Hospital, Oxford | 19 February 2014 | 3 |
| Kettering General Hospital | 6 September 2013 | 4 |
| King’s Mill Hospital, Mansfield | 18 April 2013 | 1 |
| Leeds General Infirmary | 18 March 2013 | 5 |
| Leicester Royal Infirmary | 22 April 2013 | 17 |
| Lincoln County Hospital | 4 December 2013 | 2 |
| Luton and Dunstable University Hospital | 29 April 2013 | 4 |
| Macclesfield District General Hospital | 29 January 2016 | 4 |
| Manor Hospital, Walsall | 11 September 2015 | 5 |
| Medway Maritime Hospital, Gillingham | 7 May 2014 | 1 |
| Milton Keynes Hospital | 4 November 2015 | 3 |
| Musgrove Park Hospital | 1 April 2014 | 3 |
| New Cross Hospital, Wolverhampton | 16 May 2013 | 4 |
| Newham University Hospital, London | 9 May 2013 | 3 |
| Norfolk and Norwich University Hospital | 19 February 2014 | 3 |
| North Devon District Hospital | 19 March 2014 | 1 |
| North Manchester General Hospital | 8 May 2013 | 1 |
| Northampton General Hospital | 10 January 2014 | 1 |
| Nottingham Children’s Hospital (Queen’s Medical Centre) | 9 April 2013 | 13 |
| Peterborough City Hospital | 11 October 2013 | 3 |
| Pilgrim Hospital, Boston | 7 March 2016 | 2 |
| Poole Hospital | 5 October 2015 | 3 |
| Princess Royal Hospital, Telford | 22 April 2013 | 1 |
| Queen Elizabeth The Queen Mother Hospital, Kent | 23 October 2015 | 1 |
| Queen’s Hospital, Burton | 16 May 2013 | 5 |
| Queen’s Hospital, Romford | 16 February 2015 | 6 |
| Raigmore Hospital | 26 April 2013 | 1 |
| Rotherham Hospital | 24 August 2015 | 3 |
| Royal Aberdeen Children’s Hospital | 24 June 2014 | 4 |
| Royal Albert Edward Infirmary, Wigan | 28 June 2013 | 1 |
| Royal Alexandra Hospital, Paisley | 18 July 2013 | 2 |
| Royal Belfast Hospital for Sick Children | 19 August 2014 | 12 |
| Royal Berkshire Hospital | 7 January 2014 | 2 |
| Royal Blackburn Hospital | 18 June 2013 | 7 |
| Royal Bolton Hospital | 7 May 2013 | 6 |
| Royal Derby Hospital | 2 July 2014 | 3 |
| Royal Devon and Exeter Hospital, Wonford | 12 April 2013 | 5 |
| Royal Hospital for Sick Children, Edinburgh | 8 July 2013 | 6 |
| Royal Hospital for Children, Glasgow | 13 May 2014 | 3 |
| Royal Lancaster Infirmary | 18 December 2013 | 1 |
| Royal Manchester Children’s Hospital | 5 March 2013 | 35 |
| Royal Oldham Hospital | 8 May 2013 | 2 |
| Royal Stoke University Hospital | 27 January 2014 | 5 |
| Royal United Hospital, Bath | 28 February 2014 | 5 |
| Sheffield Children’s Hospital | 15 May 2013 | 2 |
| Southampton Children’s Hospital | 1 December 2014 | 7 |
| Southend Hospital | 10 June 2013 | 6 |
| St Peter’s Hospital, Chertsey | 22 May 2013 | 4 |
| Stepping Hill Hospital, Stockport | 25 March 2013 | 5 |
| The Alexandra Hospital, Redditch | 22 February 2016 | 1 |
| The County Hospital, Hereford | 6 December 2013 | 3 |
| The Great North Children’s Hospital, Newcastle | 20 May 2013 | 8 |
| The Ipswich Hospital | 10 May 2013 | 3 |
| The James Cook University Hospital, Middlesbrough | 5 August 2014 | 1 |
| The Royal London Hospital | 12 April 2013 | 2 |
| University Hospital Crosshouse, Kilmarnock | 11 September 2013 | 2 |
| University Hospital of North Durham | 8 July 2014 | 1 |
| University Hospital of North Tees | 16 July 2013 | 1 |
| University Hospital Of Wales, Cardiff | 12 August 2013 | 4 |
| Warrington Hospital | 7 May 2013 | 2 |
| West Middlesex University Hospital | 18 December 2013 | 6 |
| West Suffolk Hospital | 13 December 2013 | 2 |
| Whipps Cross University Hospital, London | 14 June 2013 | 2 |
| William Harvey Hospital, Kent | 23 October 2015 | 1 |
| Wishaw General Hospital | 13 August 2014 | 3 |
| Ysbyty Gwynedd | 8 July 2013 | 2 |
Appendix 2 Initial and final cohort allocation at 1 year
Table 32 illustrates that, after 1 year, proportionally more patients moved to the group 1 background therapy group within the prednisolone arm than within the standard-care arm and, therefore, this provides a further explanation for the difference in cost between the two treatment arms.
| Health state | Allocation (%) | ||
|---|---|---|---|
| Initial | Final (after 1 year) | ||
| Prednisolone arm | Placebo arm | ||
| Group 1 | 0.23 | 0.43 | 0.39 |
| Group 2 | 0.27 | 0.15 | 0.15 |
| Group 3 | 0.34 | 0.30 | 0.32 |
| Group 4 | 0.16 | 0.12 | 0.15 |
List of abbreviations
- A&E
- accident and emergency
- ACBC
- Achenbach Child Behaviour Checklist
- AE
- adverse event
- BCTU
- Birmingham Clinical Trials Unit
- CEAC
- cost-effectiveness acceptability curve
- CHU-9D
- Child Health Utility 9D
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DMC
- Data Monitoring Committee
- DNA
- deoxyribonucleic acid
- DSA
- deterministic sensitivity analysis
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FRSSNS
- frequently relapsing steroid-sensitive nephrotic syndrome
- GCP
- Good Clinical Practice
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IAR
- infection-associated relapse
- ICER
- incremental cost-effectiveness ratio
- IMP
- investigational medicinal product
- IQR
- interquartile range
- ISF
- investigator site file
- ITT
- intention to treat
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MMF
- mycophenolate mofetil
- NeST
- Nephrotic Syndrome Trust
- NIHR
- National Institute for Health Research
- NNT
- number needed to treat
- PedsQL
- Pediatric Quality of Life Inventory
- PREDNOS
- PREDnisolone in NephrOtic Syndrome
- PREDNOS2
- PREDnisolone in NephrOtic Syndrome 2
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SSNS
- steroid-sensitive nephrotic syndrome
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- URR
- upper respiratory tract infection-related relapse
- URTI
- upper respiratory tract infection
- WTP
- willingness to pay
