Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the Evidence Synthesis Programme on behalf of NICE as award number NIHR135672. The protocol was agreed in October 2022. The draft manuscript began editorial review in December 2022 and was accepted for publication in September 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Westwood et al. This work was produced by Westwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Westwood et al.
Chapter 1 Objective
The overall aim of this project is to provide a comprehensive summary of all available evidence that may be relevant to the evaluation of CaRi-Heart® [a cloud-based CE-marked medical device (Caristo Diagnostics Ltd, Oxford, UK) that analyses images from computed tomography coronary angiography (CTCA) scans to provide information about inflammation in the coronary arteries], as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain/suspected coronary artery disease (CAD), who are undergoing CTCA. This assessment does not include the development of an executable cost-effectiveness model but does include conceptual modelling which explores the structure and evidence about parameters required for model development (see Chapter 5).
Current National Institute for Health and Care Excellence (NICE) guidelines do not include recommendations about the use of formal risk assessment tools, or intervention(s) based on specific risk thresholds, in this patient group. This Early Value Assessment (EVA), therefore, includes exploration of the potential clinical consequences of the availability of additional risk information from CaRi-Heart.
Given the anticipated limitations of the evidence base, the NICE scope for this assessment1 is broad and includes some evidence about secondary outcomes (Table 1). These outcomes may be used to inform consideration of the potential benefits of implementing CaRi-Heart, as specified in the scope, and to guide further research to enable full assessment of clinical efficacy and safety.
| Question | What is the prognostic performance of CaRi-Heart? | What is the prevalence of ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk? | What are the clinical effects of using CaRi-Heart to assess cardiac risk? | What are the costs, from a UK NHS and PSS perspective, using CaRi-Heart for assessment of cardiac risk? |
|---|---|---|---|---|
| Participants | People undergoing CTCA for the investigation of stable chest pain/suspected CAD Subgroups of interest: people with no evidence of CAD, people with evidence of non-obstructive CAD and people with evidence of obstructive CAD, based on currently available CTCA imaging |
|||
| Setting | Secondary or tertiary care | |||
| Intervention | CaRi-Heart | |||
| Comparators | Current standard of care, for cardiac risk assessment | N/A | Current standard of care, which is CTCA without the addition of CaRi-Heart, alongside clinical risk assessment and patient-appropriate risk factor management | |
| Outcomes | Any reported measure of model performance, e.g. HR or OR for prediction of cardiac death or MACE Secondary outcomes:a
|
Number (%) of patients undergoing CTCA who are classified as ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk and, if reported, the number of cases (cardiac events) in each risk categorya | Cardiac mortality, MACE, HRQoL Secondary outcomes:a
|
Secondary outcomes:a
|
| Study design | Prediction model development and validation studies | RCTs, CCTs and comparative or non-comparative observational studies | RCTs, CCTs or observational before-and-after (implementation) studies | RCTs, CCTs and comparative or non-comparative observational studies and cost-effectiveness analyses |
Based on the NICE scope,1 we have defined a series of research questions that could inform both a full assessment of the clinical and cost effectiveness of using CaRi-Heart, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain/suspected CAD, who are undergoing CTCA and consideration of the potential of this technology to be cost-effective:
-
What is the prognostic performance of CaRi-Heart, in people with stable chest pain, who are undergoing CTCA, where:
-
the dependent variable is cardiac death?
-
the dependent variable is other major adverse cardiovascular events (MACE)?
-
-
What is the prevalence of ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk in people with no evidence of CAD, people with evidence of non-obstructive CAD and people with evidence of obstructive CAD, based on currently available CTCA imaging?
-
What are the clinical effects of using CaRi-Heart to assess cardiac risk?
-
How does CaRi-Heart Risk affect treatment decisions and patient adherence in people with no evidence of CAD, people with evidence of non-obstructive CAD and people with evidence of obstructive CAD, based on currently available CTCA imaging?
-
What are the clinical effects of any changes to treatment, based on CaRi-Heart Risk, in people with no evidence of CAD, people with evidence of non-obstructive CAD and people with evidence of obstructive CAD, based on currently available CTCA imaging?
-
-
What are the costs, from a UK NHS and Personal Social Services (PSS) perspective, using CaRi-Heart, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain, who are undergoing CTCA?
-
How might a conceptual model be specified in terms of structure and evidence required for parameterisation in order to estimate the cost effectiveness of CaRi-Heart in people with stable chest pain, who are undergoing CTCA?
The above questions were defined in line with the NICE scope1 and have been used to inform the inclusion criteria for the rapid review component of this assessment (see Table 1). In addition to the rapid review, evidence that may be required to inform parameterisation of a future cost-effectiveness model has been explored, as part of the conceptual modelling process (see What are the costs, from a UK NHS and Personal Social Services perspective, of using CaRi-Heart, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain, who are undergoing computed tomography coronary angiography? and Chapter 5), using a pragmatic, iterative searching approach; model parameterisation questions, other than costs, were not included in the rapid review.
The available evidence is summarised, with consideration of its relevance to the above research questions, and a detailed description of evidence gaps where further research is needed is provided (see Strengths and limitations).
Chapter 2 Background and definition of the decision problem(s)
The primary indication for this EVA is the assessment of cardiac risk, specifically, the risk of cardiac death.
Coronary artery disease and acute myocardial infarction (AMI) are a significant health burden in the UK, with Office of National Statistics (ONS) mortality data for 2021 showing 20,061 deaths from AMI (3.42% of all deaths recorded in 2021) and ischaemic heart disease being the leading cause of death in males (37,095 deaths, 12.4% of all male deaths). 2,3
Computed tomography coronary angiography is recommended, for the investigation of CAD in people with stable chest pain, in NICE guideline CG95,4 and in European Society of Cardiology (ESC) guidelines. 5 CTCA provides a visualisation of the coronary arteries, which is used to identify plaques (fatty deposits that can form in the artery wall), to quantify the extent of any stenosis (narrowing) of the coronary arteries and the length and location of the affected area, and to quantify the extent of coronary artery calcification [e.g. using the coronary calcium score (CCS)]. Information provided by CTCA is structural rather than functional. However, it is well established that acute coronary events can arise from unstable, but anatomically non-significant, atherosclerotic plaques. 6–8 The vascular inflammatory response is a modulator of atherogenesis and can be a factor in plaque rupture, leading to acute coronary events. 9 A recent prognostic modelling study [Cardiovascular RISk Prediction using Computed Tomography (CRISP-CT)], which included 3912 patients (1872 in the derivation cohort and 2040 in the validation cohort) who were undergoing clinically indicated CTCA, assessed mapping of the fat attenuation index (FAI), a marker of vascular inflammation, as a potential predictor of adverse cardiac events. 10 This study found that high perivascular FAI values (optimal cut-off ≥ −70.1 Hounsfield units) improved prediction of cardiac mortality, over and above clinical risk factors and CTCA parameters (such as extent of atherosclerosis and CCS). 10
The early and accurate identification and characterisation (e.g. plaque burden, atheroma, CCS) of CAD are important to inform treatment decisions and reduce adverse cardiac outcomes. In addition, improvements in the assessment of individual cardiac risk in people being investigated for suspected CAD have the potential to further optimise prevention and treatment strategies.
Population
The population of interest is people with stable, recent onset chest pain, of suspected cardiac origin, who are undergoing CTCA, in line with NICE guideline CG95. 4 The use of CaRi-Heart in this population would represent opportunistic additional risk assessment, as an adjunct to current standard of care. The company have indicated that CaRi-Heart is used to guide preventative interventions NOT to guide or change revascularisation decisions. However, the population specified for this assessment includes all patients undergoing CTCA for the investigation of recent-onset stable chest pain; this is because it is not clear whether a risk assessment based on CaRi-Heart could be used to guide additional interventions in patients requiring revascularisation. Subgroups of interest are patients with no evidence of CAD on CTCA, patients with non-obstructive CAD and patients with obstructive CAD (requiring revascularisation).
Intervention technology
CaRi-Heart is a cloud-based CE-marked medical device (Caristo Diagnostics Ltd) that analyses images from CTCA scans to provide information about inflammation in the coronary arteries. 11,12 This analysis utilises the imaging biomarker perivascular FAI. 10 The main outputs of the CaRi-Heart medical device are:11
-
the FAI for the proximal segments of each major coronary artery [right coronary artery (RCA), left anterior descending artery (LAD) and left circumflex artery (LCX)]
-
the FAI score [FAI weighted for scan parameters, unspecified anatomical parameters related to fat distribution around the arteries age ‘basic demographics (age, sex)’] for each major coronary artery. The FAI score is accompanied by vessel-specific nomograms to allow localised interpretation of the degree of inflammation
-
CaRi-Heart Risk (calculated, individual patient risk of a fatal cardiac event in the next 8 years). CaRi-Heart Risk calculation uses a prognostic model, which includes FAI score, information about atherosclerotic plaque burden as indicated by the modified Duke index13 and clinical risk factors (including diabetes mellitus, smoking, hyperlipidaemia and hypertension). CaRi-Heart Risk scores can be classified as low (< 5%), medium (≥ 5% and < 10%) and high (≥ 10%), with respect to 8-year risk of cardiac death. 11
CaRi-Heart analysis is undertaken centrally, by the company (Caristo Diagnostics Ltd). 1 CTCA scans can be transferred directly to the company from the hospital picture archiving and communication system (PACS) using a gateway appliance installed in the healthcare provider’s network and reports can be electronically transferred back to the originating PACS or sent by e-mail. 11 Segmentation of the epicardial adipose tissue and perivascular space is done by a deep learning network and the device includes a quality control step by a trained analyst. 11 The analysis is performed on a standard CTCA images; the minimum requirements, specified by the company, are:1
-
Patients for CaRi-Heart should be between 30 and 80 years old.
-
Images are acquired using a CTCA protocol on a 64-slice scanner or above.
-
Image scans should include the pulmonary artery bifurcation cranially and fully include the apex of the heart caudally.
The company have stated that CaRi-Heart Risk uses similar information to widely used clinical risk scores such as QRISK®3 and that, therefore, minimal training (30-minute training session) is required to interpret the report because clinicians (who are the intended users of the report) are familiar with using risk calculators. 1
The company have also stated that the technical failure rate of CaRi-Heart analysis is low (< 3%). 1
Potential alternative technologies
No commercially available alternative technologies were identified for this topic. Clinical experts highlighted that FAI can be estimated using other methods but that these methods are not standardised and are used in research only.
Comparator(s)
The comparator, for this EVA, is the current standard of care, which is CTCA without the addition of CaRi-Heart, alongside clinical risk assessment and patient-appropriate risk factor management (see Care pathway).
Care pathway
Diagnostic assessment of people with stable chest pain of suspected cardiac origin
The NICE guideline on assessment and diagnosis of chest pain of recent onset (CG95, updated 2016)4 recommends diagnostic testing for people with stable chest pain, for whom initial clinical assessment (history taking and physical examination) cannot rule out typical or atypical angina.
The CG954 recommends offering 64-slice (or above) CTCA, as the first-line diagnostic investigation, if:
-
clinical assessment indicates typical or atypical angina, or
-
clinical assessment indicates non-anginal chest pain but 12-lead resting electrocardiogram (ECG) has been done and indicates ST-T changes or Q waves.
Additional, non-invasive, functional imaging for myocardial ischaemia is recommended if 64-slice (or above) CTCA has shown CAD of uncertain functional significance or is non-diagnostic. 4 Non-invasive functional testing is also recommended for people with a history of CAD, when there is uncertainty about whether chest pain is being caused by myocardial ischemia. 4
Recommended options for non-invasive functional imaging for myocardial ischemia are:4
-
myocardial perfusion scintigraphy (MPS) with single photon emission computed tomography (SPECT) or
-
stress echocardiography or
-
first-pass contrast-enhanced magnetic resonance (MR) perfusion or
-
magnetic resonance imaging for stress-induced wall motion abnormalities.
Guidelines state that the choice of non-invasive functional imaging technique should consider locally available technologies and expertise, the person and their preferences and any contraindications (e.g. disabilities, frailty, limited ability to exercise). 4
The CG95 recommends offering invasive coronary angiography (ICA) as a third-line investigation when the results of non-invasive functional imaging are inconclusive. 4
Significant CAD or ‘obstructive CAD’, on CTCA or ICA, is defined as ≥ 70% stenosis of at least one major epithelial artery segment or ≥ 50% stenosis of the left main coronary artery (LMCA). 4
A diagnosis of stable angina should be made when:4
-
there is evidence of significant CAD on CTCA or ICA or
-
reversible myocardial ischaemia is found during non-invasive functional imaging.
Management
Options for the management of CAD include:5,14
-
risk-modifying lifestyle advice (e.g. exercise, dietary, smoking cessation and limiting alcohol consumption)
-
risk-modifying pharmacological interventions (e.g. aspirin, statins, antihypertensives, antianginal drugs)
-
revascularisation [percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG)].
The choice of appropriate intervention(s) is multifactorial and is likely to include consideration of: the burden of disease (extent, location and length of stenosis, CCS and atheroma), in patients with CAD detected on CTCA or ICA; history of coronary events; presence of modifiable risk factors; adequacy of symptom control. 14
Risk-modifying interventions may also be offered, for primary prevention, to patients in whom CTCA or ICA shows no evidence of CAD, but where significant risk factors are present. 15
Guidelines for the management of CAD5,14 do not currently include any recommendations for the use of formal risk assessment tools and specific risk thresholds, either for risk of cardiac death or risk of MACE, to guide intervention decisions.
Proposed position of CaRi-Heart in pathway
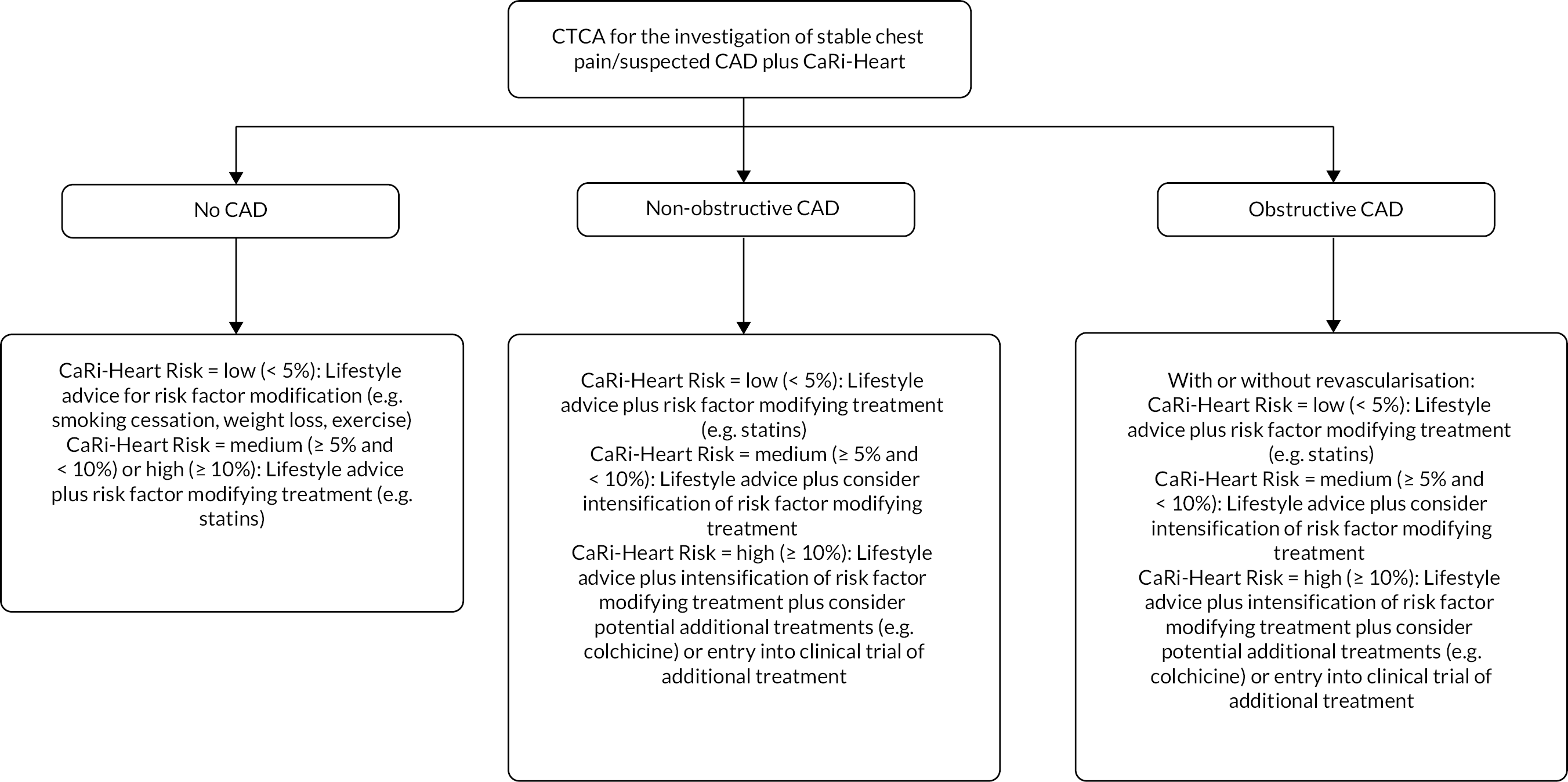
The company have indicated that CaRi-Heart could be used as an adjunctive investigation for all people with stable chest pain/suspected CAD who have been referred for CTCA. 1 The flowchart in Figure 1 provides an illustration of current practice and Figure 2 illustrates the potential position of CaRi-Heart in the care pathway (including possible changes to management based on CaRi-Heart Risk) and is based on discussions with clinicians during the NICE scoping workshop (14 September 2022).
FIGURE 1.
Current care pathway for people with stable chest pain/suspected CAD who have been referred for CTCA.
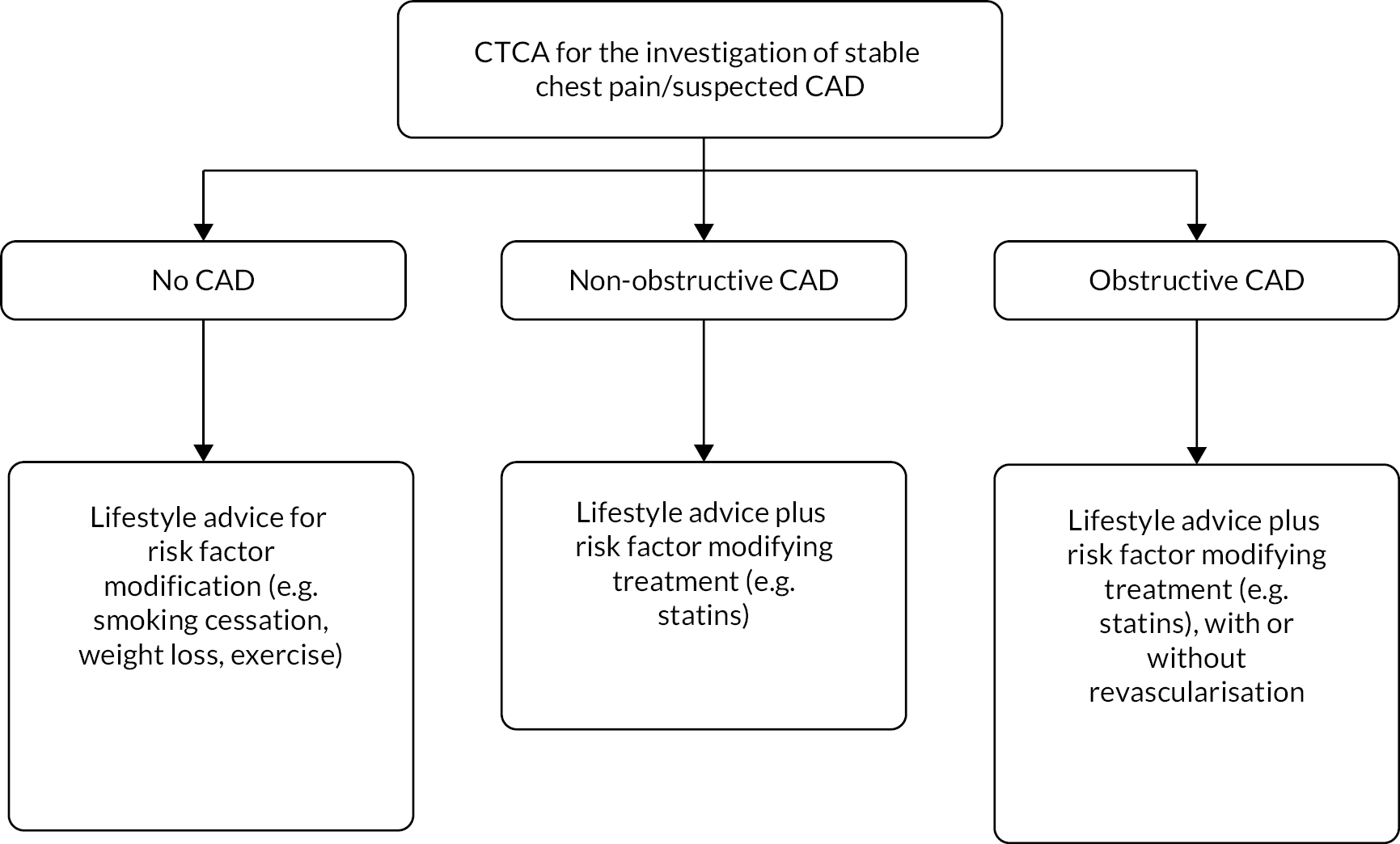
FIGURE 2.
Potential position of CaRi-Heart in the care pathway for people with stable chest pain/suspected CAD who have been referred for CTCA.
Chapter 3 Rapid review methods
Rapid review methods followed the principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care,16 the NICE guide to methods of technology appraisal17 and the Cochrane Rapid Reviews group’s interim methods guidance. 18
Search strategy
Searches were undertaken to identify studies evaluating CaRi-Heart (as described in Table 1), as recommended in the CRD guidance for undertaking reviews in health care. 16
Candidate search terms were identified from target references, browsing database thesauri (e.g. MEDLINE MeSH and EMBASE EMTREE), and existing reviews identified during the initial scoping searches. Strategy development involved an iterative approach, testing candidate text and indexing terms across a sample of bibliographic databases, aiming to reach a satisfactory balance of sensitivity and specificity. Search strategies were developed specifically for each database and the keywords and thesaurus terms were adapted according to the configuration of each database.
The following databases were searched for relevant studies from inception to October 2022:
-
MEDLINE (Ovid): 1946–4 October 2022
-
MEDLINE In-Process Citations (Ovid): 1946–4 October 2022
-
MEDLINE Daily Update (Ovid): 1946–4 October 2022
-
MEDLINE Epub Ahead of Print (Ovid): 1946–4 October 2022
-
EMBASE (Ovid): 1974–4 October 2022
-
Cochrane Database of Systematic Reviews (CDSR) (Wiley): up to October 2022/Iss10
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley): up to October /Iss10
-
Database of Abstracts of Reviews of Effects (DARE) (www.crd.york.ac.uk/CRDWeb/): up to March 2015
-
Health Technology Assessment database (HTA) (www.crd.york.ac.uk/CRDWeb/): up to March 2018
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO): up to 6 October 2022
-
Kleijnen Systematic Reviews Ltd (KSR) Evidence (https://ksrevidence.com/): up to 5 October 2022
-
Epistemonikos (www.epistemonikos.org/): up to 6 October 2022
-
International HTA database (INAHTA) Publication (www.inahta.org/hta-database/): up to 6 October 2022
-
National Institute for Health and Care Research (NIHR) HTA Programme (www.nihr.ac.uk/): up to 6 October 2022
-
International Prospective Register of Systematic Reviews (PROSPERO) (www.crd.york.ac.uk/prospero/): up to 5 October 2022
-
International Platform of Registered Systematic Review and Meta-analysis Protocols (https://inplasy.com/): up to 6 October 2022
-
Latin American and Caribbean Health Sciences Literature (LILACS) (http://regional.bvsalud.org/php/index.php?lang=en): up to 6 October 2022
-
Directory of Open Access Journals (DOAJ) (https://doaj.org/): up to 6 October 2022.
Completed and ongoing trials were identified by searching the following resources:
-
National Institutes of Health (NIH) ClinicalTrials.gov (www.clinicaltrials.gov/): up to 6 October 2022
-
European Union (EU) Clinical Trials Register (www.clinicaltrialsregister.eu/ctr-search/search): up to 6 October 2022
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/): up to 6 October 2022
-
ScanMedicine (https://scanmedicine.com/): up to 6 October 2022.
To identify conference proceedings, searches in EMBASE were not restricted to exclude conference abstracts. In addition, a search was undertaken of the following conference proceedings resource:
-
Northern Light Life Sciences Conference Abstracts (Ovid): 2010–22/wk38.
An additional search of the medRxiv PrePrint server was undertaken. All results retrieved from this resource were treated with due caution, as these are preliminary reports of work that have not been certified by peer review.
-
MedRxiv (www.medrxiv.org): up to 6 October 2022.
No restrictions on language, publication status or date were applied. Searches included generic and other product names for the device where appropriate.
The main EMBASE strategy for each search was independently peer reviewed by a second Information Specialist based on the Canadian Agency for Drugs and Technologies in Health (CADTH) Peer Review Checklist. 19
Inclusion and exclusion criteria
Separate inclusion criteria were developed for each of the research questions listed in Chapter 1. These are summarised in Table 1.
Inclusion screening and data extraction
One reviewer (MW) screened titles and abstracts of all reports identified by the searches, and a minimum of 20% were independently screened by a second reviewer (MP). 18 Full copies of all studies deemed potentially relevant, by either reviewer, were obtained and both reviewers independently assessed these for inclusion; any disagreements were resolved by consensus or discussion with a third reviewer (NA).
Studies cited in materials submitted by the manufacturer and other stakeholders were first checked against the project reference database, in EndNote X20; any studies not already identified by our searches were screened for inclusion following the process described above.
Where available, data were extracted on the following: study design/details, participant characteristics [e.g. demographic characteristics, clinical history, cardiac risk factors, subgroup (no CAD, non-obstructive CAD or obstructive CAD on CTCA)], details of the implementation of CaRi-Heart (protocol for use, definition of risk categories, method of reporting output, experience and training of healthcare professionals using the CaRi-Heart report), measures of prognostic performance [e.g. hazard ratio (HR) for cardiac death or MACE] and test technical performance outcome measures (e.g. failure rate and reasons for failure, time to result), changes to treatment decision, patient adherence to treatment, cardiac outcomes (MACE and cardiac death), health-related quality of life (HRQoL), costs. Data were extracted by one reviewer (MW), using a piloted, standard data extraction form. A second reviewer (MP) checked data extraction and any disagreements were resolved by consensus or discussion with a third reviewer (NA).
Quality assessment
The methodological quality of the included prediction model studies was assessed using Prediction model Risk Of Bias ASsessment Tool (PROBAST). 20 No studies, of any other design, were identified which met the inclusion criteria for the rapid review, as specified in Table 1. A PROBAST assessment was undertaken by an expert statistician (Professor Sue Mallett, Professor in Diagnostic and Prognostic Medical Statistics, University Centre London (UCL) Centre for Medical Imaging, Division of Medicine, Faculty of Medical Sciences, University of London, UK), who is a member of the PROBAST steering group. The supporting information used for the PROBAST assessment was checked by the lead reviewer (MW), who is also a member of the PROBAST steering group.
The results of the quality assessment are presented in the section Study quality.
Methods of analysis/synthesis
The findings of our rapid review are presented as a narrative synthesis, structured by research question. A detailed commentary on the major methodological problems or biases that affected the single included study is also provided, together with a description of how this may have affected the study results and the relevance of the study to the decision problem specified. The evidence gaps identified by the rapid review and additional exploratory searches have been used to inform recommendations for future research.
Chapter 4 Rapid review results
The literature searches conducted for this EVA rapid review used a broad approach, with respect to the intervention, and included terms for both CaRi-Heart and FAI. These searches identified a total of 3230 unique references. After initial screening of titles and abstracts, 50 references10,11,21–68 were considered to be potentially relevant and ordered for full-paper screening; of these, two publications,11,52 one full paper11 and one conference abstract,52 which reported results the same study, were included in the review. All potentially relevant publications provided by the company were identified by our searches. Figure 3 shows the flow of studies through the review process. Appendix 2 provides details, with reasons for exclusion, of all publications excluded at the full-paper screening stage.
FIGURE 3.
Flow of studies through the review process.
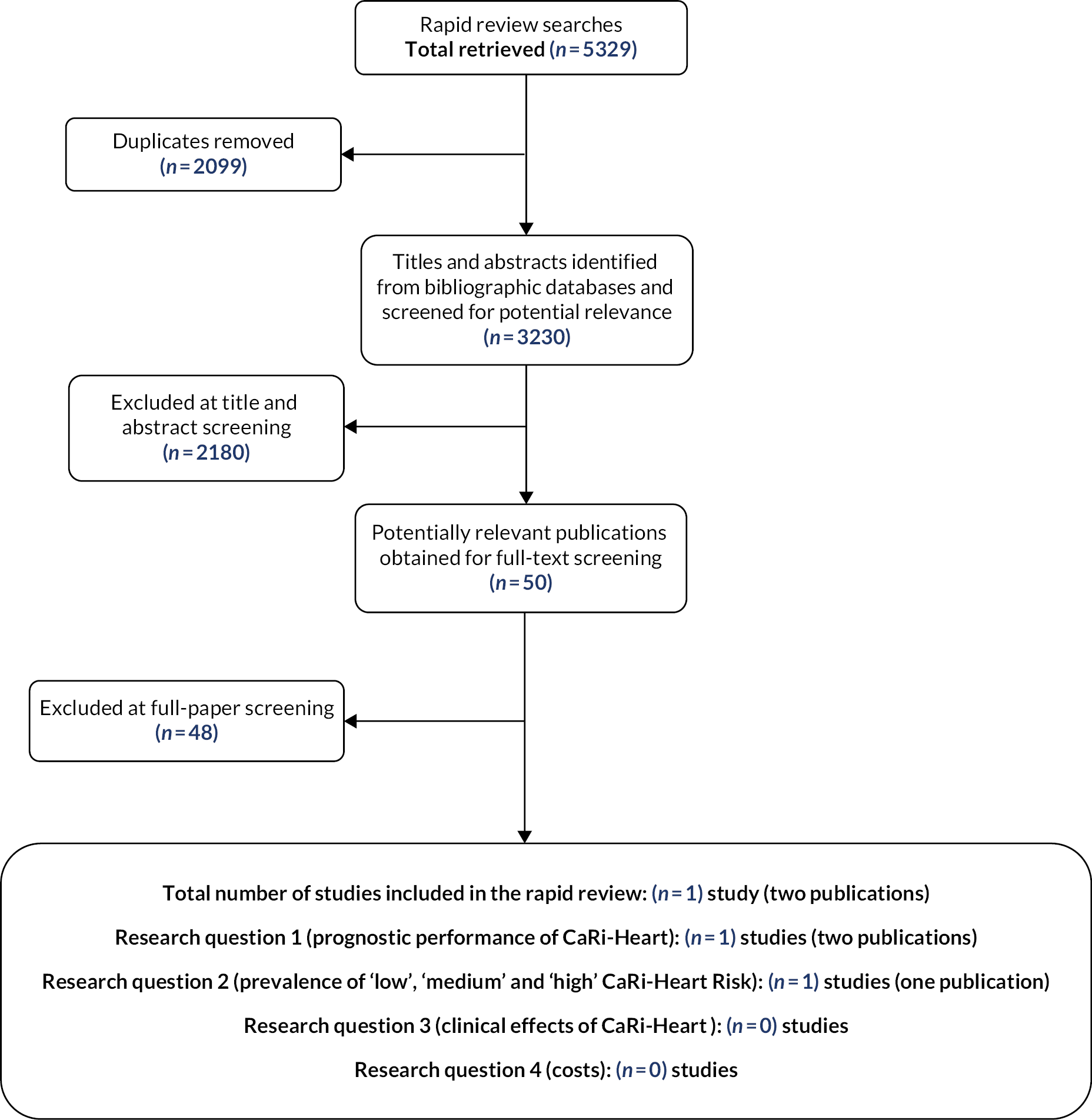
In addition to the studies included in this report, our searches of trials’ registries and information provided by the company identified one relevant ongoing study,69 the details of which are provided in Appendix 2.
Overview of the included CaRi-Heart Risk prediction model study
Based on the searches and inclusion screening described above, two publications11,52 relating to a single study were included in this rapid review; the Rapid review results section of this report cites the primary publication only. 11 This publication is a full report of the development and validation of the CaRi-Heart Risk prediction model and provides information relevant to research question 1, ‘What is the prognostic performance of CaRi-Heart®, in people with stable chest pain, who are undergoing CTCA where: a) the dependent variable is cardiac death? b) the dependent variable is MACE?’ and research question 2 ‘What is the prevalence of “low”, “medium” and “high” CaRi-Heart® Risk in people with no evidence of CAD, people with evidence of non-obstructive CAD and people with evidence of obstructive CAD, based on currently available CTCA imaging?’ Table 2 provides a brief overview of the key features of the CaRi-Heart prediction model study and Table 3 provides a summary of the baseline characteristics of the training/development and validation cohorts included in this study. 11
| Study ID | Study details | Objective | Study design and outcomes extracted |
|---|---|---|---|
| Oikonomou 202111 | Population: The study included a total of 3912 patients who were undergoing clinically indicated CTCA for the evaluation of stable coronary disease, comprised of two independent cohorts:
Country:
Funding:
|
To evaluate the performance of a new medical device, CaRi-Heart (Caristo Diagnostics Ltd), in a multinational cohort of patients undergoing CTCA | Study design:
Outcomes extracted:
|
| Variable | US training/development cohort (n = 2040) | Germany validation cohort (n = 1872) | p-valuea |
|---|---|---|---|
| Demographic characteristics | |||
| Age in years, median (IQR) | 53 (43–62) | 62 (52–68) | < 0.001 |
| Male, n (%) | 1126 (55.2) | 1178 (62.9) | < 0.001 |
| Clinical risk factorsb | |||
| Hypertension, n (%) | 949 (46.5) | 1068 (62.0) | < 0.001 |
| Hypercholesterolaemia, n (%) | 1126 (55.2) | 930 (54.7) | 0.78 |
| Diabetes mellitus, n (%) | 219 (10.7) | 215 (12.4) | 0.11 |
| Smoking, n (%) | 465 (22.8) | 221 (12.8) | < 0.001 |
| Modified Duke CAD index | |||
| < 50% stenosis, n (%) | 1690 (82.8) | 1044 (55.8) | < 0.001 |
| ≥ 2 mild stenoses with proximal CAD in one artery or one moderate stenosis, n (%) | 212 (10.4) | 518 (27.7) | N/R |
| 2 moderate stenoses or 1 severe stenosis, n (%) | 100 (4.9) | 66 (3.5) | N/R |
| 3 moderate stenoses or 2 severe stenoses or severe stenosis in the proximal LAD, n (%) | 9 (0.4) | 152 (8.1) | N/R |
| 3 severe stenoses or 2 severe stenoses in the proximal LAD, n (%) | 14 (0.7) | 18 (1.0) | N/R |
| ≥ 50% stenosis in the LMCA, n (%) | 15 (0.7) | 74 (3.9) | N/R |
| CAD maximum stenosis | |||
| None to mild (< 30%), n (%) | 1033 (50.6) | 673 (36.0) | N/R |
| Mild (30–50%), n (%) | 721 (35.4) | 732 (39.0) | N/R |
| Moderate (50–70%), n (%) | 196 (9.6) | 226 (12.1) | N/R |
| Severe (≥ 70%), n (%) | 90 (4.4) | 241 (12.9) | N/R |
| Total CCSc | |||
| 0, n (%) | – | 526 (28.1) | N/R |
| 1–99, n (%) | – | 444 (23.7) | N/R |
| 100–299, n (%) | – | 183 (9.8) | N/R |
| ≥ 300, n (%) | – | 262 (14.0) | N/R |
| Not performed | 2040 (100) | 457 (24.4) | N/R |
| High-risk plaque featuresd | |||
| Any, n (%) | 458 (22.5) | 465 (24.8) | N/R |
| Spotty calcification, n (%) | 407 (20.0) | 417 (22.3) | N/R |
| Low-attenuation plaque, n (%) | 64 (3.1) | 84 (4.5) | N/R |
| Positive remodelling, n (%) | 126 (6.2) | 72 (3.9) | N/R |
| Napkin-ring sign, n (%) | 55 (2.7) | 51 (2.7) | N/R |
| Reason for referral | |||
| Assessment of CAD, n (%) | 1761 (86.4) | 1790 (95.6) | < 0.001 |
| Other non-coronary indications, n (%) | 279 (13.6) | 82 (4.4) | N/R |
| Presenting symptoms | |||
| Chest pain, n (%) | 1184 (58.0) | 764 (43.4) | N/R |
| Dyspnoea, n (%) | 452 (22.2) | 193 (10.8) | N/R |
| Palpitations, n (%) | 225 (11.0) | 240 (13.5) | N/R |
| Baseline medicationse | |||
| Antiplatelets (aspirin/clopidogrel/ticagrelor), n (%) | 987 (48.4) | 606 (37.6) | < 0.001 |
| Statins, n (%) | 813 (39.9) | 557 (34.6) | 0.001 |
| ACEi or ARBs, n (%) | 599 (29.4) | 696 (43.1) | < 0.001 |
| Beta-blockers, n (%) | 303 (14.9) | 721 (44.8) | < 0.001 |
A further publication,10 which reports an assessment of the ability of the perivascular FAI to predict clinical outcomes in patients undergoing CTCA, is also cited in this section. This article did not meet the inclusion criteria for our rapid review because it reports an evaluation of the prognostic performance of FAI and not of CaRi-Heart Risk. The article is cited, where it has provided a source of additional information about the training/development and validation cohorts used in the included study,11 including definitions dependent and independent variables in the CaRi-Heart Risk model.
We did not identify any studies which addressed research question 3, ‘What are the clinical effects of using CaRi-Heart® to assess cardiac risk?’ or research question 4, ‘What are the costs, from a UK NHS and Personal Social Services perspective, using CaRi-Heart®, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain, who are undergoing CTCA?’
Study quality
This section describes the results of PROBAST assessment for Oikonomou study. 11 PROBAST assesses both the risk of bias and concerns regarding applicability of a study that evaluates (develops, validates or updates) a multivariable diagnostic or prognostic prediction model. It is designed to assess primary studies included in a systematic review. 20 PROBAST assessment includes four steps: specification of the systematic review question(s) (Table 4), once per systematic review; classification of the type of prediction model evaluation (Table 5), once for each model in each publication assessed and for each relevant outcome; assessment of risk of bias and applicability (Table 6), once for each development and validation of a distinct prediction model in a publication; overall judgement (Table 7), once for each development and validation of a distinct prediction model in a publication. 20
| Criteria | Specification of systematic review questiona |
|---|---|
| Intended use of model: | To assess cardiac risk in people undergoing CTCA for the investigation of stable chest pain/suspected CAD |
| Participants including selection criteria and setting: | People undergoing CTCA for the investigation of stable chest pain/suspected CAD in secondary or tertiary care settings |
| Predictors (used in prediction modelling), including types of predictors (e.g. history, clinical examination, biochemical markers, imaging tests), time of measurement, specific measurement issues (e.g. any requirements/prohibitions for specialised equipment): | Any reported predictors: components of current UK standard care (e.g. clinical risk factors, history and parameters reported on standard CTCA) are of particular interest |
| Outcome to be predicted: | Cardiac death or MACE |
| Classification of the type of prediction model evaluation | |||
|---|---|---|---|
| Type of prediction study | PROBAST boxes to complete | Definition for type of prediction model study | |
| Development only | Development | ✓ | Prediction model development without external validation. These studies may include internal validation methods, such as bootstrapping and cross-validation techniques. |
| Development and validation | Development and validation | An external validation is reported, but used the same patients who comprised the development cohort in the initial modelling study, which assessed the prognostic value of FAI.10 The methods described in Oikonomou 2021,11 therefore, do not correspond to the definition of external validation in PROBAST. The PROBAST assessment, described in Tables 6 and 7, considers both cohorts (Germany and USA) as development/internal validation cohorts. | Prediction model development combined with external validation in other participants in the same article. |
| Validation only | Validation | External validation of existing (previously developed) model in other participants. | |
| Development (USA/Germany) | Validation | ||
|---|---|---|---|
| Development/internal validation | Validation | ||
| Development/internal validation | Validation | ||
| Development/internal validation | Validation | ||
| DOMAIN 1: Participants | |||
| A. Risk of bias | |||
| Describe the sources of data and criteria for participant selection: The source of data was the CRISP-CT study,10 which comprised two prospective, independent cohorts (Germany and USA) of consecutive patients, undergoing clinically indicated CTCA All consecutive patients (aged 16 years or older) were eligible for inclusion, unless they were referred for evaluation of congenital heart disease |
|||
1993 patients were assessed for inclusion in the Germany cohort and 121 were excluded:
|
|||
| KVp other than 100 or 120 (n = 14) Anatomical/coronary anomalies (n = 2) |
|||
2246 patients were assessed for inclusion in the USA cohort and 206 were excluded:
|
|||
| KVp other than 100 or 120 (n = 62) Anatomical/coronary anomalies (n = 9) |
|||
| 1.1 Were appropriate data sources used, e.g. cohort, RCT or nested case–control study data? | Yes/yes | N/A | |
| 1.2 Were all inclusions and exclusions of participants appropriate? | Unclear/unclear | N/A | |
| Risk of bias introduced by selection of participants |
RISK:
(low/high/unclear) |
Unclear/unclear | N/A |
| Rationale of bias rating: Exclusion of patients with poor image quality CTCA or anatomical/coronary anomalies may result in over estimation of the prognostic performance of CaRi-Heart Risk. If FAI scores cannot be calculated in these patient groups, they should be included and reported as ‘failure rate’ for the CaRi-Heart Risk tool |
|||
| B. Applicability | |||
| Describe included participants, setting and dates: Full details of the baseline characteristics of the study population are provided in Table 3, above. The development (USA) cohort was recruited at the Cleveland Clinic, between 2008 and 2016. This data set had previously been used as the external validation data set for Oikonomou 2018,10 where the model had been developed on the data set from Germany (recruited at Erlangen University Hospital, between 2005 and 2009) The German data set was reported as the external validation data set for this article (Oikonomou 2021);11 however, it was the same German data set used to develop the model in Oikonomou 2018,10 which is cited as the model used in the methods of this article (Oikonomou 2021)11 The impact of risk factors such as BMI and statin or other treatments have not been reported for their impact on model predictions reported, so the generalisability to current patients is unclear |
|||
| Concern that the included participants and setting do not match the review question |
CONCERN:
(low/high/unclear) |
Low/low | N/A |
| Rationale of applicability rating: The included study participants appear to be broadly representative of the population specified in the scope for this EVA1 |
|||
| DOMAIN 2: Predictors | |||
| A. Risk of bias | |||
| List and describe predictors included in the final model, e.g. definition and timing of assessment: The predictors included in the CaRi-Heart Risk were not explicitly reported. The included study describes CaRi-Heart Risk as incorporating FAI score, information about atherosclerotic plaque burden (as described by the modified Duke CAD index) and clinical risk factors (diabetes, smoking, hyperlipidaemia and hypertension)11 Hypertension was defined10 as the presence of a documented diagnosis or treatment with an antihypertensive according to clinical guidelines.71 ‘Similar criteria’ were applied for the definitions of hypercholesterolaemia and diabetes mellitus72,73 Clinical data and demographics were recorded prospectively in the electronic medical records at the time of the initial clinical encounter10 |
|||
| 2.1 Were predictors defined and assessed in a similar way for all participants? | Yes/yes | N/A | |
| 2.2 Were predictor assessments made without knowledge of outcome data? | Unclear/unclear | N/A | |
| 2.3 Are all predictors available at the time the model is intended to be used? | Yes/yes | N/A | |
| Risk of bias introduced by predictors or their assessment | RISK: (low/high/unclear) | Unclear/unclear | N/A |
| Rationale of bias rating: Clinical predictors appear to have been appropriately defined, were recorded prospectively at the initial point of contact and are likely to be representative of clinical risk factors which would be routinely considered/available for this patient group. Imaging parameters would be available or estimable form initial CTCA |
|||
| B. Applicability | |||
| Concern that the definition, assessment, or timing of predictors in the model does not match the review question |
CONCERN:
(low/high/unclear) |
High/high | N/A |
| Rationale of applicability rating: The CaRi-Heart Risk model11 does not appear to have included all imaging parameters that might be reported as part of standard care (current CTCA), for example, maximum stenosis, presence of high-risk plaques or CCS; these parameters were recorded and included in the earlier modelling study, which assessed the prognostic value of FAI,10 but do not appear to have been included in the CaRi-Heart Risk model.11 In addition, some clinical risk factors (e.g. BMI, family history of premature CAD) and prior treatment with risk-modifying agents (e.g. statins) are not reported as having been included |
|||
| DOMAIN 3: Outcome | |||
| A. Risk of bias | |||
| Describe the outcome, how it was defined and determined and the time interval between predictor assessment and outcome determination: In both cohorts, outcome data were assembled through search of medical records, and querying of local/national databases by local investigators not involved in subsequent image/data analysis. It is not explicitly stated whether the investigators who collected outcome data were aware of other predictor information. Since outcome data were taken from medical records, it is likely that these investigators would have been aware of information about other clinical predictors; however, knowledge of other predictors is of limited relevance given the nature of the outcome if this was used as reported in the medical record (cardiac death) Cardiac mortality was defined as any death due to proximate cardiac causes (e.g. MI, low-output heart failure, fatal arrhythmia). Investigators determining outcome followed the guidelines of the ACC/AHA70 and the Academic Research Consortium for definition of the cause of death.74 Deaths fulfilling the criteria of sudden cardiac death were also included. Deaths from other non-cardiac vascular causes such as stroke were not included. Deaths where information on the exact cause could not be collected with certainty were classified as ‘unknown cause’ at the discretion of the local site investigators The time interval, between predictors and outcome, appears to have been data driven (determined by available –follow-up) but is likely to have been adequate for the outcome to occur |
|||
| 3.1 Was the outcome determined appropriately? | Yes/yes | N/A | |
| 3.2 Was a prespecified or standard outcome definition used? | Yes/yes | N/A | |
| 3.3 Were predictors excluded from the outcome definition? | Yes/yes | N/A | |
| 3.4 Was the outcome defined and determined in a similar way for all participants? | Yes/yes | N/A | |
| 3.5 Was the outcome determined without knowledge of predictor information? | Unclear/unclear | N/A | |
| 3.6 Was the time interval between predictor assessment and outcome determination appropriate? | Unclear/unclear | N/A | |
| Risk of bias introduced by the outcome or its determination | RISK: (low/high/unclear) | N/A | N/A |
| Rationale of bias rating: The outcome was objective and was predefined, using standard criteria. The time interval, between predictors and outcome, appears to have been data driven (determined by available follow-up) but is likely to have been adequate for the outcome to occur. |
|||
| B. Applicability | |||
| At what time point was the outcome determined: The outcome was fatal cardiac event at 8 years, with the choice of 8-year time point unclear (median follow-up in cohorts of 4.5 and 6 years). If a composite outcome was used, describe the relative frequency/distribution of each contributing outcome: Not applicable. |
|||
| The study11 only assessed the ability of CaRi-Heart Risk to predict cardiac death at 8 years; no other, potentially relevant, adverse cardiac outcomes (e.g. MACE, MI, stroke, cardiac hospitalisation) were considered. The report of the earlier modelling study, which assessed the prognostic value of FAI,10 indicates that data on MI during follow-up were collected for the US cohort. | |||
| Concern that the outcome, its definition, timing or determination does not match the review question |
CONCERN:
(low/high/unclear) |
High/high | N/A |
| Rationale of applicability rating: The choice of the 8-year time point appears to have been data driven, rather than being determined by clinical considerations and the evaluation of the CaRi-Heart Risk model considers only its ability to predict cardiac death |
|||
| DOMAIN 4. Analysis | |||
| Risk of bias | |||
| Describe numbers of participants, number of candidate predictors, outcome events and events per candidate predictor: The development (USA) and (internal as the same data set was used to derive methods and model in Oikonomou 201810) validation (Germany) cohorts included 2040 and 1872 participants, respectively. The number of candidate predictors was not explicitly stated but appears to have been 8 (assuming that FAI scores were included separately for each coronary artery assessed). During the follow-up period, there were 48 cardiac deaths in the development (USA) cohort and 26 in the validation (Germany) cohort, i.e. 6 outcome events per candidate variable in the development (USA) cohort and 3.25 outcome events per candidate variable in the validation (Germany) cohort. This is considered insufficient to produce a stable model or reliable model calibration estimates based on current methods75 |
|||
| Describe how the model was developed [e.g. in regard to modelling technique (e.g. survival or logistic modelling), predictor selection, and risk group definition]: Participant demographics were described as numbers (percentages) for categorical variables and median and IQR or range for continuous variables. Between-group comparisons were performed using Pearson’s chi-squared for categorical variables and Mann–Whitney’s test or unpaired Student’s t-test (as appropriate) for continuous variables. Correlations between continuous predictors were assessed using Spearman’s rho coefficient. The prognostic value of FAI score of each coronary artery against fatal cardiac events was presented using both univariate analysis and a multivariable Cox regression model, after inclusion of the patient risk factors into the model. It was not clear how predictors (other than FAI score) were selected for inclusion in the CaRi-Heart Risk model |
|||
| Describe whether and how the model was validated, either internally (e.g. bootstrapping, cross-validation, random split sample) or externally (e.g. temporal validation, geographical validation, different setting, different type of participants): The model was developed and internally validated in separate cohorts, from different geographic locations (USA and Germany). The model validation data set is more correctly described as an internal validation, as the German data set used for validation in Oikonomou 202111 was the same patient data set used to develop methods and the FAI scores in Oikonomou 201810 |
|||
| Describe the performance measures of the model, e.g. (re)calibration, discrimination, (re)classification, net benefit and whether they were adjusted for optimism: For internal validation of the USA data set model performance metrics included: Nagelkerke’s R2; discrimination index D; unreliability index U; overall quality index Q (= D − U); c-index (concordance); Somer’s Dxy [= 2 x (C − 0.5)]; calibration slope. All metrics with optimism-adjustment and 95% CI calculated using bootstrapping with 200 replications CaRi-Heart Risk was also compared to a baseline cardiac risk prediction tool consisting of age, sex, hypertension, hypercholesterolaemia, diabetes mellitus and smoking (with and without inclusion of modified Duke CAD index) |
|||
| Improvement in discrimination was assessed by comparing the time-dependent c-statistic of the two models across different follow-up times, as well as by calculating the NRI, IDI, and median improvement at 8 years (95% CI calculated using bootstrapping with 200 replications). Finally, the net benefit of using CaRi-Heart Risk over a baseline clinical risk model was assessed using a decision curve analysis. In this analysis, the y-axis reflects the net benefit, while the x-axis reflects varying probability thresholds (for the outcome of interest, i.e. cardiac mortality over 8 years of follow-up). The probability threshold describes the minimum probability of disease at which further intervention would be warranted. This threshold tends to be lower for interventions with high efficacy and low cost, though higher for minimally effective treatments or those associated with significant morbidity. Conversely, the net benefit reflects the difference between the expected benefit (number of patients truly at risk who will receive an intervention using the proposed strategy) and harm [number of patients without the disease who would be treated unnecessarily (false positives)], weighted by the odds of the risk threshold. This graphical method enables the comparison of the net clinical benefit of different approaches across different levels of estimated risk. Statistical analysis was performed in the R environment (R 4.0.2, The R Foundation for Statistical Computing, www.R-project.org) using R studio (version 4.0.2) and the following packages: rms, survival, riskRegression, survIDINRI, timeROC, survivalROC, caret. Hmisc, Design, rmda11 Text within this box has been reproduced under the Creative Commons licence |
|||
| Describe any participants who were excluded from the analysis: No exclusions were reported. However, the earlier modelling study, which assessed the prognostic value of FAI,10 describes the exclusion of patients from the cohorts used in the CaRi-Heart Risk study. See Domain 1 |
|||
| Describe missing data on predictors and outcomes as well as methods used for missing data: Maximum missingness of 9.2% was reported for the Germany cohort, in relation to clinical variables (hypertension, hypercholesterolaemia, diabetes and smoking); no further details were provided Missing data were imputed using the multiple imputation by chained equations method (package mice in R) with a bootstrapped logistic regression model for categorical (binary) variables and mean imputation for continuous variables |
|||
| 4.1 Were there a reasonable number of participants with the outcome? | No/no | N/A | |
| 4.2 Were continuous and categorical predictors handled appropriately? | No/no | N/A | |
| 4.3 Were all enrolled participants included in the analysis? | No/no | N/A | |
| 4.4 Were participants with missing data handled appropriately? | Yes/yes | N/A | |
| 4.5 Was selection of predictors based on univariable analysis avoided? | Unclear | ||
| 4.6 Were complexities in the data (e.g. censoring, competing risks, sampling of controls) accounted for appropriately? | Unclear/unclear | N/A | |
| 4.7 Were relevant model performance measures evaluated appropriately? | No/no | N/A | |
| 4.8 Were model overfitting and optimism in model performance accounted for? | Yes/no | ||
| 4.9 Do predictors and their assigned weights in the final model correspond to the results from multivariable analysis? | Unclear/unclear | ||
| Risk of bias introduced by the analysis |
RISK:
(low/high/unclear) |
High/high | N/A |
| Rationale of bias rating: There was no external validation of model performance data on an independent set of patients. The German data set was claimed as the external validation data set for this article (Oikonomou 2021);11 however, it was the same German data set used to develop the model in Oikonomou 2018,10 which is cited as the model used in the methods of this article (Oikonomou 2021)11 The stability of model predictions for individual patients is not reported. The number of cardiac events used for model development (or internal validation) was insufficient to enable stable model performance given the number of predictors included in the model, and no data have been provided to substantiate the stability of the model performance for patients Model methods for selection variables and the final model equation (with 95% CI for coefficients) are not reported for either CaRi-Heart or the clinical baseline model, so the model lacks transparency of model performance claims |
|||
| The FAI score nomograms across different age groups are based on both data sets, and methods are not clearly reported, nor IPD points. In addition, IPD points are not shown indicating data from different centres or using different imaging machines, making assessment of any generalisability and bias not possible The calibration plot and metrics of the German data set (second internal validation data set) were not shown, and the calibration plot of the USA data set (3B) did not show the distribution of real data points The reclassification reported in Oikonomou 202111 does not provide justification of the thresholds chosen for clinical risk model risk groups (< 1%, 1–4.99%, 5–10%, > 10%, across 8 years) or whether these were prespecified or data driven. How the risk groups or reclassification between these risk groups would impact on clinical decision-making is not justified The choice of number and thresholds for risk categories will impact on the IDI performance The decision curve analysis in figure 7 does not include 95% CIs and so it is not possible to understand whether benefits were significant between curves The clinical model used for comparison of CaRi-Heart performance of current methods did not include CTCA variables, so comparisons to this are not valid |
|||
| Overall judgement about risk of bias and applicability of the prediction model evaluation | ||
|---|---|---|
| Overall judgement of risk of bias | RISK:(low/high/unclear) | High |
| Summary of sources of potential bias: | ||
|
||
| Overall judgement of applicability |
CONCERN:
(low/high/unclear) |
High |
Summary of applicability concerns:
|
||
What is the prognostic performance of CaRi-Heart, in people with stable chest pain, who are undergoing computed tomography coronary angiography?
Where the dependent variable is cardiac death
The Oikonomou11 study included a total of 3912 patients who were undergoing clinically indicated CTCA for the evaluation of stable coronary disease. The training/development (USA) cohort comprised 2040 patients, with a median (range) follow-up duration of 53.8 (4–105) months; a total of 85 deaths were reported during follow-up, of which 48 were cardiac. 11 The validation (Germany) cohort comprised 1872 patients, with a median (range) follow-up duration of 72 (51–109) months; there were a total of 114 deaths during follow-up, of which 26 were confirmed cardiac deaths and 16 were deaths of unknown cause. 11 Numbers of non-fatal adverse coronary events were not reported.
The unadjusted HR, for 8-year cardiac death, per unit increase in CaRi-Heart Risk was 1.10 [95% confidence interval (CI) 1.07 to 1.12] in the training/development cohort and 1.06 (95% CI 1.04 to 1.08) in the validation cohort. 11 The HRs adjusted for ‘traditional risk factors’ (smoking, hypercholesterolaemia, hypertension, diabetes mellitus, Duke index, presence of high-risk plaque features and epicardial adipose tissue volume) were 1.05 (95% CI 1.03 to 1.06) in the training/development cohort and 1.04 (95% CI 1.03 to 1.06) in the validation cohort. 11
With respect to the subgroups of interest, specified in the scope for this EVA,1 the predictive value of the CaRi-Heart Risk model was consistent across patients with and without obstructive CAD. 11 The unadjusted HRs were similar in patients without obstructive CAD, 1.08 (95% CI 1.05 to 1.10) n = 1754 in both the training/development cohort and 1.07 (95% CI 1.04 to 1.07) n = 1405 in the validation cohort, to those in patients with obstructive CAD, 1.04 (95% CI 1.02 to 1.06) n = 286 in the training development cohort and 1.03 (95% CI 1.01 to 1.05) n = 467 in the validation cohort. 11 The subgroup of patients without obstructive CAD included those with no to mild CAD (maximum stenosis < 30%), n = 1033 in the training/development and n = 673 in the validation cohort and those with mild CAD (maximum stenosis 30–50%), n = 721 in the training development cohort and n = 732 in the validation cohort. 11 No subgroup analysis was presented for patients with no evidence of CAD.
Unadjusted HRs were reported for other clinically relevant subgroups (age, sex, presence or absence of ‘high-risk plaque features’ and CCS and for different race and ethnicity subgroups [white, black and other (Asian, multiethnic)]. 11 The unadjusted HRs, for 8-year cardiac death, per unit increase in CaRi-Heart Risk, for the whole study population and for all reported subgroups are provided in Table 8.
| Subgroup | CaRi-Heart Risk, HR (95% CI) per unit increase, n | |
|---|---|---|
| Training/development (USA) cohort |
Validation (Germany) cohort |
|
| All | 1.10 (1.07 to 1.12), 2040 | 1.06 (1.04 to 1.08), 1872 |
| Age | ||
| < 60 years | 1.08 (1.04 to 1.12), 1467 | 1.07 (1.04 to 1.11), 887 |
| ≥ 60 years | 1.05 (1.03 to 1.06), 573 | 1.04 (1.02 to 1.05), 985 |
| Sex | ||
| Female | 1.05 (1.03 to 1.08), 914 | 1.05 (1.03 to 1.07), 694 |
| Male | 1.06 (1.04 to 1.08), 1126 | 1.04 (1.03 to 1.06), 1178 |
| Obstructive CAD | ||
| No | 1.08 (1.05 to 1.10), 1754 | 1.07 (1.04 to 1.09), 1405 |
| Yes | 1.04 (1.02 to 1.06), 286 | 1.03 (1.01 to 1.05), 467 |
| High-risk plaque featuresa | ||
| No | 1.06 (1.04 to 1.08), 1582 | 1.05 (1.03 to 1.06), 1407 |
| Yes | 1.05 (1.032 to 1.08), 458 | 1.05 (1.02 to 1.08), 465 |
| CCS | ||
| < 300 | N/A | 1.06 (1.03 to 1.09), 1153 |
| ≥ 300 | N/A | 1.04 (1.02 to 1.07), 262 |
| N/A | N/A | 1.03 (1.02 to 1.05), 457 |
| Race/ethnicity | ||
| White | 1.09 (1.06 to 1.13), N/R | N/R |
| Black | 1.13 (1.06 to 1.20), N/R | N/R |
| Other (Asian, multiethnic) | 1.22 (0.99 to 1.51), N/R | N/R |
The HRs associated with FAI score component of the CaRi-Heart Risk model are provided in Table 9. HRs, per unit increase in FAI score, are given for each of the three major coronary arteries (RCA, LAD and LCX), where FAI score was used as a continuous variable in multivariable Cox regression analysis (adjusted for smoking, hypercholesterolaemia, hypertension, diabetes mellitus, Duke index, presence of high-risk plaque features and epicardial adipose tissue volume).
| Variable | HR (95% CI) per unit increase | |
|---|---|---|
| Training/development cohort (USA), n = 2040 | Validation cohort (Germany), n = 1872 | |
| FAI score RCA | 1.07 (1.05 to 1.09) | 1.05 (1.03 to 1.06) |
| FAI score LAD | 1.06 (1.04 to 1.09) | 1.07 (1.05 to 1.09) |
| FAI score LCX | 1.09 (1.05 to 1.12) | 1.04 (1.03 to 1.06) |
When compared to a baseline clinical risk model, which included age, sex, hypertension, hypercholesterolaemia, diabetes mellitus and smoking, the CaRi-Heart Risk model showed improved risk discrimination (Δ c-statistic 0.085, p = 0.01, in the training/development cohort and 0.149, p < 0.001, in the validation cohort). 11 This improved discrimination appeared to be retained when the extent of coronary atherosclerosis (indicated by the modified Duke CAD index) was added to the baseline clinical risk model; however, data were only presented for the training/development and validation cohorts combined; the c-statistic for CaRi-Heart Risk was 0.863 [standard error (SE) 0.029], the c-statistic for the clinical risk model plus modified Duke CAD index was 0.733 (SE 0.057) and the Δ c-statistic was 0.130 (p < 0.001). 11
Where the dependent variable is major adverse cardiovascular events
The Oikonomou11 study evaluated the predictive performance of CaRi-Heart Risk, with cardiac mortality with 8 years as the dependent variable. The study did not assess the ability of CaRi-Heart Risk to predict other outcomes of clinical interest [e.g. MACE or any of the individual components of MACE, such as stroke, myocardial infarction (MI), heart failure or cardiac hospitalisation]. 11
We did not identify any other studies that assessed the prognostic performance of CaRi-Heart Risk for any dependent variable.
What is the prevalence of ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk in people with no evidence of coronary artery disease, people with evidence of non-obstructive coronary artery disease and people with evidence of obstructive coronary artery disease, based on currently available computed tomography coronary angiography imaging?
We did not identify any studies that reported the prevalence of ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk scores for people in the specified subgroups (no evidence of CAD, people with evidence of non-obstructive CAD and people with evidence of obstructive CAD) based on findings on conventional CTCA imaging. However, the Oikonomou study11 reported information about the numbers of patients in various CaRi-Heart Risk categories versus clinical risk categories. These data allowed calculation of the prevalence of ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk scores in the overall study population.
The prevalence of ‘low’ (< 5%) CaRi-Heart Risk score was 3060/3912 (78.2%) for the whole study population, 1415/2040 (69.4%) for the training/development cohort and 1645/1872 (87.9%) for the validation cohort.
The prevalence of ‘medium’ (5–10%) CaRi-Heart Risk score was 423/3912 (10.8%) for the whole study population, 302/2040 (14.8%) for the training/development cohort and 121/1872 (6.5%) for the validation cohort.
The prevalence of ‘high’ (> 10%) CaRi-Heart Risk score was 429/3912 (11.0%) for the whole study population, 323/2040 (15.8%) for the training/development cohort and 106/1872 (5.7%) for the validation cohort.
Table 10 shows the rates of reclassification, upwards and downwards, using CaRi-Heart Risk score, compared to a risk score derived from the baseline clinical risk model (age, sex, hypertension, hypercholesterolaemia, diabetes mellitus and smoking). Data are reported separately for the training/development and validation cohorts.
| Cohort analysed | Clinical risk model | CaRi-Heart Risk model | ||
|---|---|---|---|---|
| < 5% | 5–10% | > 10% | ||
| Training/development (USA) cohort, n = 2040 | < 5% | 1230/2040 (60.3) |
107/2040 (5.2) |
17/2040 (0.8) |
| 5–10% | 167/2040 (8.2) |
138/2040 (6.8) |
96/2040 (4.7) |
|
| > 10% | 18/2040 (0.9) |
57/2040 (2.8) |
210/2040 (10.3) |
|
| Validation (Germany) cohort, n = 1872 | < 5% | 1595/1872 (85.2) |
81/1872 (4.3) |
36/1872 (1.9) |
| 5–10% | 44/1872 (2.4) |
28/1872 (1.5) |
38/1872 (2.0) |
|
| > 10% | 6/1872 (0.3) |
12/1872 (0.6) |
32/1872 (1.7) |
|
Overall, 242 of 2040 (11.9%) patients in the training/development cohort and 62 of 1872 (3.3%) patients in the validation cohort were reclassified to a lower-risk category when cardiac risk was assessed using the CaRi-Heart Risk model, compared to the baseline clinical risk model. Conversely, 220 of 2040 (10.8%) patients in the training/development cohort and 155 of 1872 (8.3%) patients in the validation cohort were reclassified to a higher-risk category when cardiac risk was assessed using the CaRi-Heart Risk model, compared to the baseline clinical risk model. The rate of reclassification from ‘low’ (< 5%) to ‘high’ (> 10%) risk was 17/1354 (1.3%) in the training/development cohort and 36/1712 (2.1%) in the validation cohort.
What are the clinical effects of using CaRi-Heart to assess cardiac risk?
What are the clinical effects of any changes to treatment, based on CaRi-Heart Risk, in people with no evidence of coronary artery disease, people with evidence of non-obstructive coronary artery disease and people with evidence of obstructive coronary artery disease, based on currently available computed tomography coronary angiography imaging?
We did not identify any studies that assessed the clinical effects of any changes to treatment, based on CaRi-Heart Risk, either for the whole population or for any of the subgroups of interest (people with no evidence of CAD, people with evidence of non-obstructive CAD and people with evidence of obstructive CAD, based on currently available CTCA imaging).
How does CaRi-Heart Risk affect treatment decisions and patient medication adherence in people with no evidence of coronary artery disease, people with evidence of non-obstructive coronary artery disease and people with evidence of obstructive coronary artery disease, based on currently available computed tomography coronary angiography imaging?
We did not identify any studies that assessed whether and how the availability of a CaRi-Heart Risk score might affect treatment decisions or people’s willingness to take medication, either for the whole population or for any of the subgroups of interest (people with no evidence of CAD, people with evidence of non-obstructive CAD and people with evidence of obstructive CAD, based on currently available CTCA imaging).
What are the costs, from a UK NHS and PSS perspective, of using CaRi-Heart, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain, who are undergoing computed tomography coronary angiography?
We did not identify any studies that reported information of the costs, from a UK NHS and PSS perspective or any other perspective, of using CaRi-Heart, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain, who are undergoing CTCA.
Caristo Diagnostics Ltd provided the following response, regarding costs, to the NICE request for information:76
The price of CaRi-Heart® technology per CTCA scan of a patient to the NHS is yet to be specified but it will cover the costs of:
-
performing the AI-based analysis;
-
providing the CaRi-Heart® report to the clinicians;
-
training the clinicians to interpret the CaRi-Heart® report [minimal, as the main outputs are relative and absolute CV risk which are outputs familiar to all cardiologists as it is a metric provided by other prognostic risk assessment tools (e.g. QRISK, ESC-SCORE)]. The CTCA scan itself is already being performed as part of clinical practice and NICE guidelines; CaRi-Heart® simply provides additional information to enhance risk stratification in patients.
No further direct costs are expected from the adoption of the CaRi-Heart® technology to the NHS. However, we will test this expectation in the NHS AI award evaluation.
The downstream costs of the clinical action taken as a result of the CaRi-Heart® technology (e.g. further investigations or initiation of clinical management if the CaRi-Heart® risk of a patient is high) will be included in the economic evaluation of CaRi-Heart® and will be compared with the respective costs of care as usual (e.g. downstream costs without CaRi-Heart® analysis).
Caristo Diagnostics Ltd provided the following additional response, regarding costs, to NICE, following submission of our draft report:
CaRi-Heart® analysis is currently available in the private sector at a price of (confidential information has been removed) per case. It is Caristo’s intention to offer the analysis to the NHS at a discounted price. This will be determined by the ongoing health economic work that is currently being conducted by the Department of Epidemiology and Public Health at the University of Oxford. The price is expected to be between (confidential information has been removed) per case. However, this may change on the health economic results are available.
Chapter 5 Exploration of intervention technology-specific parameters
Pragmatic exploration of the literature, to inform parameterisation, is part of the process of developing a full, executable cost-effectiveness model. This process is used to inform those parameters that fall outside the scope of the clinical effectiveness systematic review; it is designed to identify studies that can be used to support the development of a health economic model and to estimate the model input parameters, but not to perform a systematic review or define evidence gaps.
When developing cost-effectiveness models for diagnostic technologies, using a ‘linked evidence’ approach (e.g. combining evidence from one group of studies about properties of the technology, such as accuracy for detecting a particular group of patients, with evidence from other studies about the efficacy of a treatment in that group of patients), the additional parameters required can be broadly classified into two groups:
-
Those which relate to the mapping of the disease state, and which are not specific to the diagnostic technology being assessed (e.g. utilities, effects of current treatments).
-
Those which are specific to the diagnostic technology being assessed (e.g. costs, effects of any new treatments that may be introduced as a result of information provided by the diagnostic technology).
There will usually, though not always, be evidence available to inform group 1 parameters. When assessing a new diagnostic technology, evidence gaps are more likely in respect of group 2 parameters. A summary of the anticipated most relevant input parameters is presented in Input parameters.
Development and parameterisation of a full, executable cost-effectiveness model are outside the scope of an EVA, as currently defined. However, in order to provide as much information as possible about those areas where evidence gaps are most likely, this EVA has included a pragmatic exploration of some group 2 parameters. The following group 2 parameters were specified in our protocol:77
-
exploration of evidence about the link between FAI and adverse cardiac events
-
exploration of evidence about the efficacy of treatments (e.g. colchicine) which target coronary artery inflammation (e.g. as indicated by FAI) and which are not currently part of standard care for the treatment or prevention of CAD
-
exploration of evidence about the effects of changing or introducing treatments which are currently part of standard care for the treatment or prevention of CAD (e.g. statins) based on measures of coronary artery inflammation (e.g. FAI).
As a result of discussions that informed the development of the conceptual cost-effectiveness model, we also sought information about the efficacy of statins for secondary prevention of adverse cardiac outcomes (thus, group 1 parameters), conditional upon baseline risk category, because these were considered to be potentially important for the cost-effectiveness model.
It should be noted that this section of the EVA has been informed by pragmatic searching and cannot be used to make definitive statements about evidence gaps.
Search strategy
Two sets of focused literature searches were performed to inform this section of the EVA. These searches were conducted separately from the main searches used to inform the rapid review described in Chapter 3. The main searches, described in the section Search strategy, included terms for FAI and were used to identify the studies described in the section Exploration of evidence about the link between fat attenuation index and cardiac events. Searches, to identify the studies described in the sections Exploration of evidence about the efficacy of treatments which target coronary artery inflammation (as indicated by fat attenuation index) and which are not currently part of standard care for the treatment or prevention of coronary artery disease and Exploration of evidence about the effects of changing or introducing treatments which are currently part of standard care for the treatment or prevention of coronary artery disease (e.g. statins) based on measures of coronary artery inflammation (e.g. fat attenuation index), were conducted in EMBASE (Ovid) and KSR Evidence (https://ksrevidence.com/), from inception to October 2022; no language restrictions were applied. Full search strategies are provided in Appendix 1.
Exploration of evidence about the link between fat attenuation index and cardiac events
The scope for this EVA did not include any alternative technologies to the CaRi-Heart device. 1 During scoping discussions for this topic, the question was raised as to whether FAI measurement (without the use of the CaRi-Heart device) should be considered as an alternative technology. This question arose because FAI has been presented as the unique feature of the CaRi-Heart device. 12 In addition, analysis of data from the CRISP-CT study,10 which preceded the development and validation study for the CaRi-Heart Risk model11 and which assessed the ability of FAI to predict clinical outcomes, concluded that FAI is independently predictive of cardiac mortality ‘over and above current state-of-the-art assessment in coronary CTA’. 10 The FAI was not considered to be an alternative technology for this EVA because no commercially available method of measurement (other than the CaRi-Heart device) was identified. 1
In order to inform possible future reconsideration of the FAI as a potential alternative technology, our protocol for this EVA77 included exploratory searches to identify evidence about the link between FAI and adverse cardiac events (e.g. MACE, MI, stroke) in addition to the reported evidence about the link with cardiac mortality. 10 The summary provided below focuses on systematic review evidence.
Two potentially relevant systematic reviews were identified: Kato (2022)48 and Antonopoulos (2022). 25 It should be noted that Antonopoulos (2022)25 included studies with a range of different populations (e.g. general population, chronic kidney disease) and was not limited to people undergoing CTCA for suspected CAD, and Kato (2022)48 included four studies conducted in people with suspected CAD and one study that was conducted in people with end-stage renal disease. Both these systematic reviews included the CRISP-CT study. 10
Kato (2022)48 included five studies looking at the ability of the FAI to predict adverse cardiac events. The adverse cardiac events varied by study: cardiac mortality in one study, major adverse cardiac events in three studies and all-cause mortality in the other study. These were combined as a single outcome: adverse cardiac events. The ‘predictive ability’ of FAI was quantified by the HR a : b, where a = the hazard of an adverse cardiac event for people with FAI values above a cut-off value and b = the hazard of an adverse cardiac event for people with FAI values below that cut-off value. Kato (2022)48 did not specify the cut-off value but appears to have included all cut-off values reported by included primary studies. Higher FAI was reported to be predictive of adverse cardiac events, when the FAI was measured in the RCA [HR 2.15 (95% CI 1.67 to 2.77)], and the LAD [HR 2.09 (95% CI 1.63 to 2.68)], with a borderline statistically significant effect for measurement in the LCX [HR 1.30 (95% CI 1.00 to 1.70)]. 48 When using the coronary artery with the highest ‘predictive’ value within each study, the summary estimate of the ‘predictive ability’ of higher FAI was reported as a HR of 2.23 (95% CI 1.80 to 2.77). 48 These results indicate a positive association between FAI and risk of adverse cardiac events.
Antonopoulos (2022)25 included 39 studies that evaluated the association between various biomarkers of vascular inflammation [C-reactive protein (CRP), interleukin-6 (IL-6)/tumour necrosis factor-alpha (TNF-α), arterial positron emission tomography (PET)/CT and CT angiography-derived biomarkers of vascular inflammation, including anatomical high-risk plaque features and perivascular fat imaging] on cardiac events. The results pertaining to CT angiography-derived biomarkers of vascular inflammation (CT-PVAT) are included here, since this is analogous to the measure of FAI. The CT-PVAT, which was used in three large studies (n = 5507), showed a good accuracy for prediction of the composite outcome of MACE and all-cause mortality across different CT-PVAT thresholds, as measured by the median c-index of 0.880 (range 0.838–0.962). Of all the biomarkers used, CT-PVAT had the highest added prognostic value (above coronary atherosclerosis extent) for MACE and all-cause mortality, %Δ c-index 8.2 (95% CI 4.0 to 12.5). The results of regression analysis indicated that predictive effects were independent of potential confounders such as study size, follow-up, population event incidence, performance of the baseline model and statistical adjustment.
The ROBIS evaluations, for these two systematic reviews, are provided in Table 11 and supporting information for these assessments is provided in Appendix 4. The findings from these reviews should be interpreted with caution, given that both were rated as high risk of bias with respect to all48 or most25 of the key components of systematic review methodology assessed by the ROBIS tool.
| Paper | Study eligibility criteria | Identification and selection of studies | Data collection and study appraisal | Synthesis and findings | Overall rating of risk of bias |
|---|---|---|---|---|---|
| Kato (2022)48 | High concerns | High concerns | High concerns | High concerns | High risk of bias |
| Antonopoulos (2022)25 | Low concerns | High concerns | High concerns | High concerns | High risk of bias |
Although systematic reviews were preferentially included in this pragmatic exploration of evidence, relevant primary prognostic studies were also considered if they were not included in any identified systematic review. The only such study was Chatterjee (2022)36 which involved 381 stable patients undergoing ICA. Pericoronary adipose tissue attenuation (PCAT) measurements were made, which are a type of FAI. The PCAT values in the RCA, the LAD and LCX were each reported to have poor ability to predict MACE [HRs of 0.96 (95% CI 0.75 to 1.22), 1.31 (95% CI 0.96 to 1.78) and 0.98 (95% CI 0.78 to 1.22), respectively]. For the prediction of the composite outcome of death, stroke or MI, HRs of 0.68 (95% CI 0.44 to 1.07), 0.85 (95% CI 0.56 to 1.29) and 0.57 (95% CI 0.41 to 0.80) were recorded for PCAT measurements in the RCA, LAD and LCX, respectively. The authors suggested that results in this study were less favourable than previous findings because of more severe disease. This could indicate that FAI measures may be most useful in low- to intermediate-risk patients.
Evaluation of the Chatterjee (2022)36 study with the Qualitative Impact Assessment Protocol (QUIP) tool indicated that this study had low risk of bias for the domains of study participants, study attrition, prognostic factor measurement, outcome measurement, study confounding and statistical analysis and reporting, yielding an overall rating of low risk of bias (see Appendix 5).
In summary, notwithstanding the concerns about the quality of the systematic review evidence, the evidence presented in this section is broadly supportive of a positive relationship between FAI and risk of adverse coronary events and hence of the future inclusion of FAI as an alternative technology (in evaluations of the CaRi-Heart device) should a method of measurement become commercially available in the UK NHS.
Exploration of evidence about the efficacy of treatments which target coronary artery inflammation (as indicated by fat attenuation index) and which are not currently part of standard care for the treatment or prevention of coronary artery disease
Because CaRi-Heart Risk utilises an imaging biomarker of perivascular inflammation (FAI), information about the effects of potential CAD treatments which target coronary artery inflammation (e.g. colchicine), which are not currently part of standard care but might be introduced as a result of assessment using CaRi-Heart Risk, is important to inform full cost-effectiveness modelling. The ideal source of such information would be studies where the efficacy of such treatments is tested in populations selected using CaRi-Heart Risk, or by measurements of coronary inflammation such as FAI. Such studies could provide an indication of the potential for assessment using CaRi-Heart Risk to inform treatment changes that could improve clinical outcomes. Analyses stratified by levels of coronary inflammation could be used to inform considerations of optimal treatment targeting. Unfortunately, exploratory searches did not identify any studies of colchicine efficacy, where participants were selected by any measures of coronary inflammation.
The following text provides a summary of recent systematic reviews, which have assessed the efficacy of colchicine for secondary prevention of adverse cardiac events in unselected patients with CAD and which also reported the intermediate outcomes of inflammatory markers. These studies provide an indication of the general efficacy of colchicine in the population of interest, but do not provide any indication of the efficacy of targeting colchicine treatment using CaRi-Heart Risk or separate measures of coronary inflammation, such as FAI. It should also be noted that colchicine is not currently recommended by NICE, or licensed in the UK, for this indication.
Colchicine is an anti-inflammatory drug that has been repurposed from a gout treatment to a treatment for CAD. Our pragmatic literature searches identified 27 systematic reviews, which evaluated the effects of colchicine in patients with CAD. 78–104 The findings of these systematic reviews indicated a consistent benefit of colchicine for secondary prevention of all outcomes assessed, with the exception of all-cause mortality and cardiac mortality. In general, results also indicated that colchicine treatment was associated with an increase in gastrointestinal symptoms such as diarrhoea; however, these effects tended to be reversible and non-serious. As colchicine is an anti-inflammatory agent, it has been assumed that its beneficial effects on cardiac outcomes are at least partially mediated by its anti-inflammatory effects. However, this assumption may require further objective evaluation. In addition, it is unclear whether any anti-inflammatory effect is specific to the coronary arteries or part of a more systemic effect. Only one of the systematic reviews identified, Bytyci (2022),79 evaluated the effects of colchicine on intermediate outcomes (inflammatory markers), as well as clinical outcomes, and this review is summarised below.
Bytyci (2022)79 included 12 randomised controlled trials (RCTs), comprising 13,073 patients with CAD, with a mean follow-up of 22.5 months. Random-effects meta-analyses indicated that colchicine treatment was associated with reduced risks (compared to control) for recurrent MI [risk ratio (RR) 0.78 (95% CI 0.65 to 0.93)], stroke [RR 0.47 (95% CI 0.29 to 0.76)], hospitalisation [RR 0.32 (95% CI 0.12 to 0.87)] and MACE [RR 0.67 (95% CI 0.55 to 0.83)]. However, the results of meta-analyses indicated that colchicine (compared to control) had no effect on all-cause mortality [RR 1.05 (95% CI 0.71 to 1.53] or cardiovascular mortality [RR 0.75 (95% CI 0.40 to 1.43)]. Colchicine treatment was also associated with an increase in the risk of gastrointestinal symptoms [RR 1.49 (95% CI 1.02 to 2.18)], but other adverse effects were not demonstrably different between colchicine and control arms. These clinical findings were typical of most of the other 26 systematic literature reviews (SLRs) included in this review. In addition, Bytyci (2022)79 evaluated effects on four inflammatory marker outcomes: hs-CRP, IL-6, IL-β1 and IL-18. The FAI was not measured. At a mean follow-up of 19 days, there was a mean difference of −1 (p = 0.001) between colchicine and control groups in change of hs-CRP and a mean difference of −3.84 (p = 0.001) between colchicine and control groups in change of IL-6. However, there were no significant differences observed between colchicine and control for IL-β1 and IL-18. Unfortunately, these inflammatory outcomes were not meta-analysed in a conventional way, as the mean difference in outcome between arms was not used as the measure of effect. Instead, the within-arm ‘before to after’ change was meta-analysed for each arm separately, and the effect in each arm was presented (the mean differences given above were calculated by the authors of the report). 79 A ROBIS evaluation of this systematic review led to a rating of low risk of bias, and it is summarised in Table 12 with details of supporting information provided in Appendix 4.
| Paper | Study eligibility criteria | Identification and selection of studies | Data collection and study appraisal | Synthesis and findings | Overall rating of risk of bias |
|---|---|---|---|---|---|
| Bytyci (2022)79 | Low concerns | Low concerns | Low concerns | Unclear | Low risk of bias |
Taken at face value, these results do support the notion that colchicine may reduce some inflammatory markers. However, this is unsurprising, given that colchicine is an anti-inflammatory drug. Therefore, these results do not, in themselves, provide unequivocal evidence that colchicine exerts its clinical effects on CAD outcomes via an anti-inflammatory mechanism. In addition, this study does not provide any information about whether any anti-inflammatory effects were specific to the coronary arteries or more systemic. Further studies looking at the differing strength of associations between clinical effects and both local and systemic measures of inflammation may be informative.
In summary, the evidence identified supports the efficacy of colchicine for secondary prevention of some adverse cardiac events in unselected patients with CAD. However, there is no evidence about the effects of colchicine on cardiac mortality.
The evidence also does not provide unequivocal evidence about the mechanism by which this effect is mediated. Importantly, for the aims of this EVA, the evidence identified does not provide any indication of the efficacy of targeting colchicine treatment using CaRi-Heart Risk or separate measures of coronary inflammation, such as FAI. Our searches have identified a small (n = 40), ongoing, randomised, placebo-controlled trial (NCT05347316),105 with the potential to inform this question. The study aims to assess the effects of colchicine treatment on FAI (primary outcome), and all-cause mortality, cardiovascular mortality, AMI, stroke and need for revascularisation (secondary outcomes). 105 It is being conducted in adults undergoing CTCA, who have non-calcified or mixed coronary plaques and FAI values > −70.1 HU. 105
Exploration of evidence about the effects of changing or introducing treatments which are currently part of standard care for the treatment or prevention of coronary artery disease (e.g. statins) based on measures of coronary artery inflammation (e.g. fat attenuation index)
Information about the effects of changing or introducing treatments, which are currently part of standard care for the treatment or prevention of CAD (e.g. statins), based on measures of coronary artery inflammation was identified, a priori, as being potentially important to inform modelling. A pragmatic exploration of the evidence was included in our protocol for this EVA. 77 As a result of discussions that informed the development of the conceptual cost-effectiveness model, we also sought information about the efficacy of statins for secondary prevention of adverse cardiac outcomes, conditional upon baseline risk category.
The optimum evidence, to inform this EVA, would include studies assessing the efficacy of statins, where participants are stratified by CaRi-Heart Risk or by baseline levels of coronary inflammation (e.g. measured by FAI). Such studies could inform consideration of how risk assessment, based on or including measures of coronary inflammation, could be used to select those patients most likely to benefit from treatment. A further important consideration for this assessment is the efficacy of any changes to the dose of existing statin treatment, based on CaRi-Heart Risk or, alternatively, on any assessment of coronary artery inflammation. Exploratory searches did not identify any studies about the effects of introducing or changing statin treatment, based on any measure of coronary artery inflammation or any risk assessment that included a measure of coronary artery inflammation.
The following text describes studies which provide information about the efficacy of statins for secondary prevention of adverse cardiac outcomes, conditional upon the baseline risk category of study participants. We did not identify any studies that provided information about the effects of changing statin treatment (e.g. different doses) based on any method of risk assessment.
Two systematic reviews from the Cholesterol Treatment Trialists’ (CTT) Collaboration were identified, Fulcher (2015)106 and Mihaylova (2012),107 which both used individual patient data (IPD) from the same 27 RCTs. The reporting of these studies focused on meta-analyses of efficacy, stratified by gender, but results were also reported for efficacy stratified by baseline cardiovascular risk. The 27 included RCTs investigated the effects of statins on major vascular events (MVEs),106,107 any vascular death107 and non-vascular death. 107 Twenty-two of the included trials evaluated statins versus no statins (n = 134,537) and five trials evaluated higher doses of statins versus lower doses (n = 39,612). However, in the reported meta-analyses, studies were pooled as higher-dose statin or lower-dose statin versus lower-dose statin or control. In the meta-analyses, the benefit of statins was measured by the event rate ratio (denoted as ‘RR’ in both papers). The ratio of the event rates of the chosen cardiovascular outcome was between the statins/more statins arm and the no-statins/less-statins arm. A RR value of < 1 would therefore denote a benefit for statins. The event RR was normalised to the reduction in low-density lipoprotein (LDL) cholesterol effected by treatment and was expressed as the event RR per 1 mmol/l reduction in LDL cholesterol.
Fulcher (2015)106 and Mihaylova (2012)107 reported that the benefit of statins in terms of MVEs was very similar for participants at > 10 to < 20% baseline 5-year MVE risk RR 0.79 (95% CI 0.75 to 0.84), > 20 to < 30% baseline 5-year MVE risk RR 0.81 (95% CI 0.78 to 0.85) and > 30% baseline 5-year MVE risk RR 0.79 (95% CI 0.75 to 0.83). However, for participants at the lowest level of risk (< 10% baseline 5-year MVE risk), the benefit of statins was greater RR 0.68 (95% CI 0.62 to 0.74).
Mihaylova (2012)107 analysed the effects on any vascular death and non-vascular death in the same way. Using a Cox regression model, the rate ratio (RR) for any vascular death was reported to be very similar across all levels of baseline 5-year MVE risk. At < 5% baseline 5-year MVE risk, the RR was 0.87 (95% CI 0.58 to 1.31), and it was similar at > 5 to < 10% baseline 5-year MVE risk RR: 0.92 (95% CI 0.74 to 1.13), > 10 to < 20% baseline 5-year MVE risk RR: 0.88 (95% CI 0.79 to 0.97), > 20 to < 30% MVE RR: 0.88 (95% CI 0.81 to 0.96) and > 30% baseline 5-year MVE risk RR: 0.87 (95% CI 0.80 to 0.95). For the outcome of non-vascular death, Mihaylova (2012)107 reported some differences between baseline risk levels in the benefits of statins. At < 5% 5-year MVE risk, the RR was 1.16 (95% CI 0.80 to 1.68), which was different to effects at > 5% to < 10% baseline 5-year MVE risk RR 0.88 (95% CI 0.71 to 1.09), > 10% to < 20% baseline 5-year MVE risk RR 0.94 (95% CI 0.83 to 1.07), > 20% to < 30% baseline 5-year MVE risk RR 1.00 (95% CI 0.89 to 1.13) and > 30% baseline 5-year MVE risk RR 0.96 (95% CI 0.83 to 1.10). Although there is a weak signal suggesting lower efficacy of statins in preventing non-vascular death in people at the lowest level of baseline risk, the imprecision of the estimates at all levels of risk needs to be considered when interpreting these figures.
Taken at face value, these results suggest that people at all levels of baseline cardiovascular risk may experience a benefit from statins in terms of a reduction in event rate of MVEs and any vascular death. For MVEs, while this benefit does not depend on the level of risk when risk is moderate or high, the benefit may actually increase at the lowest levels of risk. The findings for the outcome of non-vascular death do suggest that there could be a tendency for people at the lowest levels of risk to have a relatively reduced benefit from statins, but there is much uncertainty in these findings. In particular, it is important to note that the level of uncertainty in the non-vascular death analysis is consistent with no benefit at all risk levels. Taken together, these results suggest that knowledge of baseline risk may not be helpful in determining those who will benefit best from statin therapy, because the level of benefit does not appear to have a strong relationship with baseline risk.
The ROBIS evaluation of both systematic reviews led to a rating of high risk of bias (Table 13); full details of supporting information are provided in Appendix 4. Interpretation of the findings from these reviews should consider the results of ROBIS assessment. A key concern, with respect to the meta-analyses presented in both studies, arises from the normalisation of the event RR of the cardiac outcome to a unit reduction in LDL. This methodology is surprising, as it would be expected that the absolute level of LDL reduction would drive the magnitude of clinical effects. Therefore, because statins principally work by reducing LDL levels, normalising to LDL reduction may ‘adjust out’ the clinical effects of interest. It is perhaps surprising that there was any difference in effect noted between statins and no-statins arms when such a normalisation was applied. This anomaly in the methodology calls into question the validity of findings in these studies. In fact, it may explain the tendency for no difference in apparent efficacy at different risk levels. At a low-risk level, there may be lower levels of LDL and so smaller absolute drops in LDL with statins treatment. These smaller drops are likely to lead to a smaller difference in the unadjusted risk of cardiac events between statins and no-statins groups. At a higher-risk level, there may be higher levels of LDL and so there is the potential for larger drops in LDL with statins treatment. These greater drops are likely to lead to a greater difference in the risk of unadjusted cardiac events between statins and no-statins groups. Taken together, these unadjusted observations lead to the conclusion that the higher-risk group benefit more from statins, which is probably the empirical information that clinicians require. However, if the risk of the outcome is normalised to the reductions in LDL, then this effect may be ‘adjusted out’, leaving the impression that statins work equally well across all risk levels.
| Paper | Study eligibility criteria | Identification and selection of studies | Data collection and study appraisal | Synthesis and findings | Overall rating of risk of bias |
|---|---|---|---|---|---|
| Fulcher 2015106 | High concerns | High concerns | High concerns | High concerns | High risk of bias |
| Mihaylova 2012107 | High concerns | High concerns | High concerns | High concerns | High risk of bias |
In summary, there remains some uncertainty about whether and to what extent the efficacy of statins, for the secondary prevention of MACE in people with CAD, may vary with baseline risk assessed using currently available methods. In addition, there is currently no information about the effects introducing statin treatment or changing the dose of existing statin treatment, based on CaRi-Heart Risk or on any assessment of coronary artery inflammation.
Chapter 6 Conceptual modelling
This section describes a conceptual decision-analytic model that could be used to inform an EVA of the cost-effectiveness of CaRi-Heart in addition to CTCA in patients with stable, recent-onset chest pain, of suspected cardiac origin, who are undergoing CTCA, in line with NICE guideline CG95. 4 The comparator technology is the current standard of care, which is CTCA without the addition of CaRi-Heart.
Review of existing economic models
We did not identify any completed economic model exploring the cost effectiveness of using CaRi-Heart, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain, who are undergoing CTCA. However, we did identify that a model was in development by a team based in University of Oxford, led by Apostolos Tsiachristas, Associate Professor of Health Economics (see Appendix 3). To the best of our knowledge, the Oxford model is the only one in development. Although the time frame for this EVA precludes the full construction and reporting of the Oxford model, aspects of it and how the EVA has contributed to its parameterisation are described below. The conceptual model described below is not the Oxford model.
De novo conceptual model
Model structure
A combination of a short-term diagnostic model component (e.g. decision tree) and a long-term model component that evaluated the downstream consequences (e.g. decision tree or cohort state-transition model) is anticipated to capture the diagnosis and the progression of CAD, respectively. The model begins with a patient population with stable, recent-onset chest pain, of suspected cardiac origin, who are referred for CTCA. The alternatives that will be compared for this cohort are (1) CTCA only (the comparator) and (2) CTCA plus CaRi-Heart. If other competing alternatives are identified, those could be added to the model, provided that there is sufficient available evidence. The following is a brief description of a potential model structure and its implications for parameterisation (e.g. baseline risks, treatments effects).
Short-term model (diagnostic decision tree)
The first part of the model is a short-term decision tree that is used to simulate the risk assessment part of the strategies. The time horizon for this part of the model should reflect the duration of the diagnostic phase in clinical practice.
In the comparator strategy (CTCA only), the patient population is diagnosed as either having (1) no CAD, (2) non-obstructive CAD or (3) obstructive CAD, with treatment determined by current practice (including any risk assessment).
For the CaRi-Heart strategy, the patient population is first diagnosed as either (1) no CAD, (2) non-obstructive CAD or (3) obstructive CAD based on the CTCA results. Those diagnostic groups are, in turn, further split by the CaRi-Heart information into groups of low, medium or high CaRi-Heart Risk. There could also be a group where CaRi-Heart was not able to estimate the risk score; for those patients, only the CTCA results are available, and these results would be used to guide treatment. The risk group/health state of the patient determines the type of treatment/intervention that is offered to the patient. The consequences of treatment/intervention decisions will be considered in developing the structure of the long-term model.
Note that it is assumed that CaRi-Heart would have no influence on decisions regarding coronary intervention to decrease or remove any coronary artery obstruction. Instead, it is assumed that only decisions regarding risk-reducing treatments with statins or possibly colchicine can be affected by the results of CaRi-Heart Risk assessment.
Long-term model (alive, dead, with/out cardiac event)
The aim of the long-term model will be to incorporate the effects of the potential treatment strategies (e.g. statins) that could be implemented, based on risk category. It should be noted that, in general, the CTCA procedure, with or without CaRi-Heart, is not expected to be repeated over time. Therefore, it is anticipated that the model will assume that patients do not change CAD status after their initial diagnosis in the model.
If the long-term model structure is implemented using a Markov model, a cycle length relevant to capture CAD events (e.g. 1 year, but to be defined based on the literature and/or clinical experts) should be used to simulate a cohort of patients through the model to observe relevant (CAD) events based on their associated risks. At each model cycle, patients are at risk of experiencing MACE. The economic analyses should be conducted from the perspective of the UK NHS and PSS. The model should have a lifetime time horizon, as CAD is a condition where the relevant outcomes are spread throughout the lifetime. Costs and quality-adjusted life-years (QALYs) should be discounted at 3.5% per annum according to the NICE method guidance. Model assumptions and parameter values should reflect clinical practice as well as possible and must be supported by the literature whenever possible, or otherwise informed by expert opinion.
Main differences between the Early Value Assessment conceptual model and the Oxford model
Currently, the Oxford model consists of a decision tree only with time horizon of 8 years. This was selected as the most appropriate choice given the available data. Also, as explained below, the Oxford model stratifies patients based on CaRi-Heart Risk but not on CAD status.
Input parameters
A summary of the most relevant input parameters for the conceptual model is presented below.
Computed tomography coronary angiography stratification
In the comparator arm, the cohort is split according to the CTCA results in combination with other currently available methods. Thus, patients can be diagnosed as either having (1) no CAD, (2) non-obstructive CAD or (3) obstructive CAD. The NICE guideline prohibits the use of a risk assessment tool for patients with CAD (categories 2 and 3) and clinical experts have indicated that treatment decisions are not made according to any risk assessment tool, but a composite of clinical information.
For the CaRi-Heart strategy, the initial diagnostic groups based on CTCA only would be, in turn, further split by the CaRi-Heart information into groups of low, medium or high CaRi-Heart Risk.
However, as noted in the section What is the prevalence of ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk in people with no evidence of coronary artery disease, people with evidence of non-obstructive coronary artery disease and people with evidence of obstructive coronary artery disease, based on currently available computed tomography coronary angiography imaging?, no studies reporting the prevalence of ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk scores for people in the specified subgroups (no evidence of CAD, people with evidence of non-obstructive CAD and people with evidence of obstructive CAD) based on findings on conventional CTCA imaging were identified. Therefore, a health economic model based on the clinical pathway depicted in Figure 2 could not be informed with the current available evidence.
The Oikonomou 202111 study reported information about the numbers of patients in various CaRi-Heart Risk categories versus clinical risk categories. These data allowed calculation of the prevalence of ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk scores in the overall study population. The Oxford model can make use of the proportion of patients reclassified between low, medium and high-risk levels with CaRi-Heart. The comparator arm in the Oxford model is either real-world practice or patient stratification based on their phenotyping, CTCA and other risk scores such as QRISK®3 or ESC risk.
Treatment change
In the comparator arm, given that current practice does not entail treatment according to any specific risk assessed by a risk assessment tool, the distribution of treatments would have to be according to clinical practice in the CTCA diagnostic categories. These data have been collected as part of the NHS artificial intelligence (AI) award study,69 which will be instrumental in parameterising the Oxford model.
In the intervention arm, treatment could be determined only by CaRi-Heart Risk level. These risks could be stratified by CTCA categories. However, data by CTCA category are currently not available (see Chapter 4). Also, CaRi-Heart does not currently include independent variables for CTCA findings and it seems unlikely that clinicians would use only CaRi-Heart to determine risk-reducing treatments. An alternative would be to mirror the approach for the comparator arm by estimating the treatment distribution, as described in Figure 2. These data have also been collected as part of the NHS AI award study,69 which will be instrumental in parameterising the Oxford model.
It should also be noted that, as explained in the section Exploration of evidence about the effects of changing or introducing treatments which are currently part of standard care for the treatment or prevention of coronary artery disease (e.g. statins) based on measures of coronary artery inflammation (e.g. fat attenuation index), the current evidence available for the long-term modelling suggests that there is uncertainty as to whether and to what extent the efficacy of statins, for the prevention of MACE in people with CAD, may vary with baseline risk assessed using currently available methods. In addition, no information about the effects of introducing statin treatment or changing the dose of existing statin treatment, based on CaRi-Heart Risk or on any assessment of coronary artery inflammation, was identified.
Utilities
Utility values would be derived from literature sources to be incorporated in the economic model for the various health states to calculate QALYs. QALYs are calculated by multiplying the time patients spend in each health states by the associated utility.
Disutilities would be subtracted from the QALY estimation to reflect a temporary reduction of the utility value in case of a clinical event. To assist the parameterisation of the Oxford model, pragmatic literature searches were conducted to identify utility values associated with MACE. These searches were conducted in KSR Evidence (https://ksrevidence.com/), from inception to October 2022. Full search strategies are provided in Appendix 1.
As explained above, the search focused on systematic reviews, which were therefore the preferred source of utilities. When specific evidence gaps were identified (e.g. utilities for a certain event not reported), source papers (not systematic reviews) were pragmatically searched. A pragmatic approach was also taken to extract utilities from the selected papers. Only papers reporting the EuroQol-5 Dimension (EQ-5D) were included; other HRQoL measures were excluded. Studies in low- and/or middle-income countries were excluded. Studies where the underlying condition was not cardiac were excluded too. No time filter was applied. An overview of the utilities for MACE reported by the included studies is presented in Appendix 7, Table 15.
Resource use and costs
Resource use and cost data should be specific to the UK setting. Fortunately, these data were collected for the Oxford model, as explained below.
Resource use data were collected through the Oxford risk factors and non-invasive imaging (ORFAN) 469 study, where individual-level hospital records are available up to 8 years after initial cardiac risk assessment. 108 When healthcare resource group codes were not available in the data, 2020–1 unit costs from the NHS National Cost Collection and the Personal Social Services Research Unit (PSSRU) Costs of Health and Social Care were used to value presentations to accident and emergency (A&E) departments, visits to outpatients cardiology clinics, diagnostic tests, day cases and elective and non-elective admissions to hospital wards. 109,110 For costing non-invasive diagnostic tests, Healthcare Resource Groups (HRGs) that were used in the HTA accompanying the updated NICE guidelines were considered. Following the structure of NHS reference costs, diagnostic imaging performed on the same day of cardiology outpatient visits were costed separately, while they were excluded from the costs of hospital admission to avoid double counting if they were performed during a hospital admission. All costs of diagnostic tests that did not take place during a hospital admission were grouped together. Hospital admissions were grouped into day cases, elective or non-elective short stay, non-elective long stay and costed based on the primary procedure provided using the respective unit costs. The length of hospital stay was used to determine short (i.e. < 2 days) or long (i.e. 2 or more days) as well as excess bed-days. Medication costs (e.g. cost of statin treatment) were derived from the British National Formulary (BNF). 108
Costs, including cardiologists’ time, and the implementation costs per CTCA were added to the price of a CaRi-Heart analysis to estimate the total cost per CTCA of introducing CaRi-Heart into the NHS. Average intervention costs to the NHS of adding CaRi-Heart to CTCA per patient were calculated by multiplying the total minutes spent by the cardiologists in training to interpret CaRi-Heart analyses with their time’s unit cost and dividing it with the number of patients with a CaRi-Heart score. The time for delivering the interventions was derived from the NHS AI prospective study using a costing template. Average intervention costs were added to the NHS costs of all patients in the CaRi-Heart branch of the short-term (decision tree) model.
Model analysis and validation
Standard cost-effectiveness analysis, including scenario analyses on relevant assumptions, deterministic and probabilistic sensitivity analyses should be conducted. Validation should include all relevant aspects [conceptual model, input data, TECHnical VERification (TECH-VER) and validation of model outcomes] and could be guided by using the Assessment of the Validation Status of Health Economic (AdViSHE) and TECH-VER111,112 health economic validation-specific tools.
Chapter 7 Discussion
Statement of principal findings
The rapid review component of this EVA identified a single study which evaluated the CaRi-Heart device. 11 This study reported the training/development and validation of the CaRi-Heart Risk model. 11
The results of the included study indicate that CaRi-Heart Risk is predictive of a patient’s absolute 8-year risk of a fatal cardiac event, when applied in a population undergoing clinically indicated CTCA for the investigation of suspected CAD. 11 The unadjusted HR per unit increase in CaRi-Heart Risk, for 8-year cardiac death, in the model validation cohort, was 1.06 (95% CI 1.04 to 1.08). 11 The corresponding HR, adjusted for ‘traditional risk factors’ (smoking, hypercholesterolaemia, hypertension, diabetes mellitus, Duke index, presence of high-risk plaque features and epicardial adipose tissue volume), was 1.04 (95% CI 1.03 to 1.06). 11
With respect to the subgroups of interest, specified in the scope for this EVA,1 the predictive value of the CaRi-Heart Risk model was consistent across patients with and without obstructive CAD. 11 Patients without obstructive CAD were defined as those with maximum stenosis from none to 50%, and no subgroup analysis was presented for patients with no evidence of CAD. 11
The results of the included study also indicated that the CaRi-Heart Risk model showed improved risk discrimination, when compared to a baseline clinical risk model, which included age, sex, hypertension, hypercholesterolaemia, diabetes mellitus and smoking (Δ c-statistic 0.149, p < 0.001, in the validation cohort). 11 This improved discrimination appeared to be retained when the extent of coronary atherosclerosis (indicated by the modified Duke CAD index) was added to the baseline clinical risk model; however, data for this comparison were only presented for the training/development and validation cohorts combined. 11
The included study also reported data that allowed the calculation of the prevalence of ‘low’ (< 5%), ‘medium’ (5–10%) and ‘high’ (> 10%) CaRi-Heart Risk scores, for the whole study population. The majority of the included participants, 3060/3912 (78.2%), were in the ‘low’ (< 5%) CaRi-Heart Risk category. 11 Prevalence information was not available for the subgroups of clinical interest,1 subgroups (no evidence of CAD, people with evidence of non-obstructive CAD and people with evidence of obstructive CAD) based on findings on conventional CTCA imaging. The source table from which these prevalence estimates were calculated11 presented rates of reclassification (change of risk category) when patients were assessed using CaRi-Heart Risk, compared to a baseline clinical risk model comprising age, sex, hypertension, hypercholesterolaemia, diabetes mellitus and smoking. Considering the whole study population, this comparison (see Table 10) appears to indicate that the use of CaRi-Heart Risk could result in approximately 10% of patients being reclassified to a higher-risk group and approximately 1.4% of patients being reclassified from the low-risk (< 5%) to the high-risk (> 10%) group. However, these data have important limitations (see Strengths and limitations).
Our rapid review did not identify any studies, published or unpublished, that provided information about:
-
the clinical effects of any changes to treatment, based on CaRi-Heart Risk
-
whether and how the availability of a CaRi-Heart Risk score might affect treatment decisions or people’s willingness to take medication
-
the costs, from a UK NHS and PSS perspective or any other perspective, of using CaRi-Heart, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain, who are undergoing CTCA.
Strengths and limitations
Strengths and limitations of the methods used in this Early Value Assessment
This report describes the findings of an assessment, which was conducted as part of a NICE EVA pilot. The EVA process has been introduced to provide an assessment route for new diagnostic technologies, where the evidence base is, as yet, underdeveloped. This process is intended to be applied where topic scoping has indicated that there is not sufficient evidence to inform a full Diagnostic Assessment Report (DAR) and to support the development of a cost-effectiveness model(s). The use of an EVA approach acknowledges that there is currently insufficient evidence to inform decision-making about routine use in UK NHS clinical practice. The stated aim of the EVA process is to: ‘Actively draw in medical devices, diagnostics and digital products that address national unmet needs, and to provide quicker assessments of early value to identify the most promising technologies, conditional on further evidence generation.’113 The methodological approaches and processes of an EVA are being developed, iteratively, during the pilot period.
The potential benefits, as indicated by NICE, of using an EVA process to assess new technologies are:113
-
‘quicker early value signals to the health and care system on promising medical technologies that address national unmet need’
-
‘better evidence to inform clinical and long-term commissioning decisions’
-
‘earlier access for patients’
-
‘clearer pathway to market access for industry’
-
‘collective contributions to system wide productivity and efficiency’.
The EVA process, as implemented in this assessment, comprised a rapid review of the evidence about the prognostic performance, clinical effects and costs of using the CaRi-Heart device, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain/suspected CAD, who are undergoing CTCA. The rapid review followed standard methods, described in Chapter 3 of this report.
The decision problem, for this assessment, was defined using the same process of scoping, expert and public consultation and iterative drafting that would be used for a full Diagnostic Assessment; the decision problem, defined by this process, has informed our recommendations for research needed to inform a full Diagnostic Assessment (see Suggested research priorities).
Our rapid review used the same comprehensive approach to literature searching that would be used for a full Diagnostic Assessment. Extensive literature searches were conducted to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as clinical trial registers and conference abstracts to identify unpublished studies. Search strategies were developed to maximise sensitivity at the expense of reduced specificity. To be as inclusive as possible, we also conducted a search of medRxiv, the preprint server.
The use of a rapid review approach is inherently less robust than full systematic review methods. In the current assessment, the initial screening of retrieved references was not done independently, in duplicate, increasing the potential for error and bias in the initial stage of study selection, by comparison with standard systematic review methods. The rapid review approach was taken, for this topic, because the recency of development of the CaRi-Heart device (the CaRi-Heart Risk model development and validation study was published in 2021)11 meant that it was not anticipated that the device would yet have been widely studied.
In addition to the rapid review, this EVA has included conceptual cost-effectiveness modelling and an exploration of the available evidence about key parameters that are likely to be required to inform future development of a full cost-effectiveness model. This work has been undertaken in synergy with the ongoing cost-effectiveness modelling, which is being undertaken at the Nuffield Department of Population Health, University of Oxford (lead by Apostolos Tsiachristas), with the aim of providing faster and better-quality evidence to the EVA and making information from the EVA rapidly available to the Oxford group.
Strengths and limitations of the evidence available to inform this Early Value Assessment
The following text describes the key limitations of the evidence identified by the rapid review, with respect to informing the decision problem defined by the NICE scope1 and described in the section Inclusion and exclusion criteria of this report.
The only study included in our rapid review reported the development and validation of the CaRi-Heart Risk model but has been rated as having high risk of bias, based on PROBAST (see Study quality). There was no external validation of the model,11 as the reported validation data set was used in a previous study10 to develop methods and thresholds for the main imaging predictors (FAI scores). The reliability data were not fully reported with only intraclass correlation coefficient (ICC) measures averaged over three readers and an unspecified number of patients included in the reliability assessment being reported. The actual absolute risk changes for individual patient changes when the FAI scores were assessed by different technical experts using the CaRi-Heart model were not reported; it was, therefore, not possible to verify the claim of reliable absolute risk score verification by expert technicians using the model. The number and thresholds for risk groups for absolute risk (a major claim of model) were not reported as prespecified or justified clinically, and so performance measures based on reclassification of patients may be data driven. The comparison clinical model was not fully reported, as there was no equation for the model, and the variables claimed to be used were not the same as the variables in the reference used to report the methods for the clinical model. 10 The clinical comparison model did not appear to include any clinical observations from the CTCA scan that is required by clinical guidelines for assessment of patients, and so does not appear to be consistent with current practice information. As such, any claims of CaRi-Heart to be superior to a relevant clinical practice have not been evaluated in this study.
As described in the section Study quality of this report, there are a number of concerns regarding the applicability to the decision problem for UK clinical practice of the current version of the CaRi-Heart Risk model and its evaluation, as reported in the included study;11 this study has been rated as having high applicability concerns, based on PROBAST. The decision problem specified the evaluation of CaRi-Heart, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain/suspected CAD, who are undergoing CTCA. The comparator was specified as current standard of care, which was defined as CTCA without the addition of CaRi-Heart, alongside clinical risk assessment and patient-appropriate risk factor management. The included study11 only evaluated CaRi-Heart Risk for the prediction of 8-year cardiac death; it does not consider prediction of cardiac risk, as specified in the scope for this EVA1 (i.e. including risk of non-fatal adverse cardiovascular events). The comparisons presented in the included study11 are not directly applicable to UK clinical practice in that they primarily focus on the effects of CaRi-Heart Risk, in terms of improved risk discrimination and frequency of risk reclassification, relative to a risk model based on clinical factors alone (age, sex, hypertension, hypercholesterolaemia, diabetes mellitus and smoking). Formal, quantitative risk assessment (e.g. using tools such as QRISK®3) is not part of standard care and is, explicitly, not recommended for this patient group. 15 Nonetheless, it may seem implausible that risk factors [e.g. body mass index (BMI), family history of premature (CAD) would not be considered by clinicians, when assessing this patient group. It may also seem implausible that any informal clinical consideration of an individual patient’s, future risk and appropriate management would exclude information currently provided by CTCA (without the addition of CaRi-Heart)]. However, discussion with clinical experts (cardiologists and radiologists who are specialist committee members for this topic), both at scoping and subsequently, during the development of this report, has indicated that there remains some uncertainty about what should be considered standard of care in this patient group. Questions circulated to clinical specialist committee members, together with responses received are reported in Appendix 6. In summary, it was noted that components of QRISK®3 (e.g. BMI, family history of premature CAD) are more likely to be considered where there is no stenotic disease evident (CTCA normal), but also noted that QRISK®3 is used to estimate the risk of MI or stroke, rather than cardiac death (as with CaRi-Heart Risk) and that clinical risk models generally overestimate risk; suggestions for radiological parameters included the use of CADRAD-2114 to report CT angiograms, heart flow for predicting whether anatomical stenoses are causing symptoms and helping to determine which patients may benefit from revascularisation but not for predicting the overall vascular risk and assessment of high-risk plaques. Hence, while it would seem reasonable that an appropriate comparator for CaRi-Heart Risk should include information currently available CTCA (e.g. presence of high-risk plaques, CCS), in addition to all potentially relevant clinical risk factors, the precise definition of such a comparator remains subject to debate.
The potential effects of the choice of comparator are of particular note when considering the data presented for rates of reclassification (change of risk category) when patients were assessed using CaRi-Heart Risk. These data, as described in the section What is the prevalence of ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk in people with no evidence of coronary artery disease, people with evidence of non-obstructive coronary artery disease and people with evidence of obstructive coronary artery disease, based on currently available computed tomography coronary angiography imaging? and summarised in the section Statement of principal findings, appear to indicate that the use of CaRi-Heart Risk could be associated with potentially clinically important rates of reclassification of patients to higher-risk groups. However, the choice of comparator (risk model based on clinical factors only) combined with the lack of separate data for clinically relevant subgroups based on the findings of standard CTCA (no evidence of CAD, non-obstructive CAD, obstructive CAD), given that some patients had obstructive CAD, means that these results are potentially misleading. It is unsurprising that, when such a population are evaluated based on clinical risk factors alone and subsequently re-evaluated using a tool which includes a component for an imaging-based assessment of atherosclerotic plaque burden (the CaRi-Heart Risk model includes modified Duke score), some patients who are at low clinical risk will be reclassified as high risk; this reclassification may simply be the result of adding imaging results (information about the degree of CAD) which would be available from a standard CTCA examination, rather than being an effect which is specifically attributable to the use of CaRi-Heart Risk.
Of note, the report of the CRISP-CT study,10 which preceded the development and validation study for the CaRi-Heart Risk model11 and which assessed the ability of FAI to predict clinical outcomes, concluded that FAI is independently predictive of cardiac mortality ‘over and above current state-of-the-art assessment in coronary CTA’. 10 This study assessed the prognostic value of FAI (as a dichotomous variable) using Cox regression analysis, where models included age, sex, hypertension, hypercholesterolaemia, diabetes mellitus, smoking, epicardial obesity (measured as total epicardial adipose tissue volume), tube voltage, modified Duke CAD index, number of high-risk plaque features and log-transformed CCS. 10 It is not clear why the current version of the CaRi-Heart Risk model11 appears to include a reduced set of variables (in addition to FAI). However, the following information was provided by the company in response to a request from NICE:76
(confidential information has been removed).
Uncertainties
Evidence to inform the aims of an Early Value Assessment
The evidence about the clinical utility of CaRi-Heart Risk is, as yet, sparse and is subject to some limitations, both in terms of risk of bias and applicability to UK clinical practice (see Strengths and limitations). There is some evidence to indicate that CaRi-Heart Risk is predictive of an individual patient’s risk of cardiac death, for patients undergoing CTCA for suspected CAD. However, whether and to what extent CaRi-Heart represents an improvement relative to the current standard of care remains uncertain, as described in the section Strengths and limitations; this is, in part, because the definition of standard of care and hence the applicability of the comparator used in the CaRi-Heart study11 are uncertain.
In addition, this EVA has not identified any information about:
-
the costs, from a UK NHS and PSS perspective or any other perspective, of using CaRi-Heart, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain, who are undergoing CTCA
-
intermediate measures of clinical effects of CaRi-Heart (secondary outcomes), such as change to treatment/management or patients’ adherence to treatment.
Evidence to inform a full Diagnostic Assessment, including cost-effectiveness modelling
During scoping, clinical experts (cardiologists and radiologists who are specialist committee members for this topic) indicated that the use of any risk assessment tools, including CaRi-Heart Risk, is unlikely to affect treatment decisions in patients who have evidence of obstructive CAD on CTCA. Clinical experts further indicated that the patient group for whom improved risk assessment is most likely to be beneficial are those symptomatic patients in whom CTCA shows no evidence of CAD. The NICE scope for this topic, therefore, defined potential subgroups for consideration:1
-
patients with no CAD
-
patients with non-obstructive CAD
-
patients with obstructive CAD.
To inform a full Diagnostic Assessment, including cost-effectiveness modelling, it is important to be able to assess (for each relevant patient group), whether and how treatment is changed as a result of the availability of CaRi-Heart Risk score (over and above information from standard CT plus clinical information) and what are the effects of any treatment changes on clinical outcome (cardiac mortality, MACE, HRQoL). There is currently a lack of granularity in the information provided by the CaRi-Heart Risk study,11 in that although the predictive value of the CaRi-Heart Risk model was reported to be consistent across patients with and without obstructive CAD, no distinction was made between patients with non-obstructive CAD and those with no evidence of CAD. 11 There is also a lack of information about the prognostic performance of CaRi-Heart Risk for the prediction of non-fatal cardiac events, both for the whole population and for clinically relevant subgroups. In addition, the CaRi-Heart Risk model does not appear to include the prior use of risk-modifying treatments (e.g. statins) and no subgroup analyses have been presented to assess the prognostic performance of CaRi-Heart Risk in treated versus untreated patients; it is therefore not clear whether the prognostic performance of CaRi-Heart Risk may vary between treated versus untreated patients.
A key component of any full Diagnostic Assessment is establishment of a link between test result, change to treatment/management and subsequent clinical outcome. The rapid review, conducted for this EVA, did not identify any evidence about the effects of CaRi-Heart Risk on treatment/management decisions or clinical outcomes.
In addition to the rapid review, this EVA included a pragmatic exploration of the literature to identify information about the possible effects of new treatments or changes to existing treatments that may occur as a result of adding CaRi-Heart Risk to current CTCA (see Chapter 5). As indicated in our published protocol,77 we sought information about:
-
the efficacy of treatments (e.g. colchicine) which target coronary artery inflammation (e.g. as indicated by FAI) and which are not currently part of standard care for the treatment or prevention of CAD
-
the effects of changing or introducing treatments which are currently part of standard care for the treatment or prevention of CAD (e.g. statins) based on measures of coronary artery inflammation (e.g. FAI).
As a result of discussions that informed the development of the conceptual cost-effectiveness model, we also sought information about:
-
The efficacy of statins for secondary prevention of adverse cardiac outcomes, conditional upon baseline risk category.
Our exploratory searches did not identify any studies about the efficacy of colchicine, where participants were selected by any measures of coronary inflammation. Systematic review evidence (summarised in the section Exploration of evidence about the efficacy of treatments which target coronary artery inflammation (as indicated by fat attenuation index) and which are not currently part of standard care for the treatment or prevention of coronary artery disease), about the efficacy of colchicine for secondary prevention of adverse cardiac events, was derived from the general population of patients with CAD and did not include any stratification by measures of coronary inflammation or baseline risk category. There was systematic review evidence that colchicine treatment may reduce the risk of recurrent MI, stroke, MACE and hospitalisation, compared to control. 79 However, it was not clear what treatments were received by the control groups in the included studies and the results of meta-analyses indicated that colchicine treatment had no significant effect on all-cause mortality or cardiac mortality. 79 Importantly, for the aims of this EVA, the evidence identified does not provide any indication of the efficacy of targeting colchicine treatment using CaRi-Heart Risk or separate measures of coronary inflammation, such as FAI. It should also be noted that colchicine is not currently recommended by NICE, or licensed in the UK, for this indication. Our searches have identified a small (n = 40), ongoing randomised, placebo-controlled trial (NCT05347316),105 with the potential to provide some information on this question (limited by every small sample size). The study aims to assess the effects of colchicine treatment on FAI (primary outcome), and all-cause mortality, cardiovascular mortality, AMI, stroke and need for revascularisation (secondary outcomes). 105 It is being conducted in adults undergoing CTCA, who have non-calcified or mixed coronary plaques and FAI values > −70.1 HU. 105
Our exploratory searches did not identify any studies about the effects of changing or introducing treatments which are currently part of standard care for the treatment or prevention of CAD (e.g. statins) based on CaRi-Heart Risk or measures of coronary artery inflammation (e.g. FAI). Systematic review evidence [summarised in Exploration of evidence about the effects of changing or introducing treatments which are currently part of standard care for the treatment or prevention of coronary artery disease (e.g. statins) based on measures of coronary artery inflammation (e.g. fat attenuation index)] suggested that people at all levels of baseline cardiovascular risk may experience a benefit from statins in terms of a reduction in event rate of MVEs and of any vascular death. 106,107 However, for participants at the lowest level of risk (< 10% baseline 5-year risk), there was some evidence to suggest a greater benefit of statins, in reducing MVEs, as expressed by relative risk per mmol LDL cholesterol reduction. 106,107 There remains some uncertainty about whether and to what extent the efficacy of statins, for the secondary prevention of MACE in people with CAD, may vary with baseline risk. Given this uncertainty, it may be considered preferable for cost-effectiveness modelling of CaRi-Heart Risk to assume a differential treatment effect for statins, which is conditional upon baseline cardiovascular risk. Without such an assumption and/or data supporting clinical benefit of targeted introduction of new treatments (e.g. colchicine), it is difficult to see how the cost effectiveness of the CaRi-Heart device could be established. For example, if ‘flat’ treatment effects are assumed (i.e. statins are equally effective across all risk groups), then it would follow that simply treating more patients (irrespective of any risk assessment) would be more effective. Whether this would be cost-effective would depend on the effect on absolute risk if effectiveness is estimated as a relative risk.
During scoping discussions for this topic, the question was raised as to whether FAI measurement (without the use of the CaRi-Heart device) should be considered as an alternative technology. This question arose because FAI has been presented as the unique feature of the CaRi-Heart device. 12 There is evidence to indicate that FAI is independently predictive of cardiac mortality ‘over and above current state-of-the-art assessment in coronary CTA’. 10 Further explorations of the evidence, conducted for this EVA (see Exploration of evidence about the link between fat attenuation index and cardiac events), support a positive relationship between FAI and risk of adverse coronary events; the ability of CaRi-Heart Risk to predict non-fatal adverse cardiovascular events (e.g. MI and stroke) has not yet been assessed. Studies indicating that FAI is predictive of these clinically important outcomes may be considered indicative of the likelihood that CaRi-Heart Risk will be similarly predictive. Such studies also support the value of the future inclusion of FAI as an alternative technology (in evaluations of the CaRi-Heart device) should a method of measurement become commercially available in the UK NHS. Information about the precise nature of the way in which the CaRi-Heart device combines clinical information, FAI and other CT parameters is not in the public domain and the clinicians’ perceptions about the potential value of having clinical risk and FAI and/or other imaging parameters ‘wrapped up’ in a single tool/report have not been assessed.
A more minor additional point concerns uncertainty around the technical failure rate that may be associated with the CaRi-Heart device. The company’s response to the request for information from NICE stated that: ‘The technical failure rate of CaRi-Heart® analysis (< 3%) is much lower than other imaging technologies, primarily because perivascular adipose tissue (the structure analysed by CaRi-Heart®) is less influenced by CT scan quality’. However, it should be noted that the report of the CRISP-CT study,10 which preceded the development and validation study for the CaRi-Heart Risk model11 and from which the study cohorts were taken, reported that approximately 5.7% of patients who met the study inclusion criteria were subsequently excluded on the basis of poor image quality, technical criteria or the presence of anatomic/coronary anomalies. If these patients were excluded because they could not be assessed by the CaRi-Heart device, then the real-world failure rate may be as high as 10%.
It should be noted that the company’s response to the request for information from NICE76 included a description of ongoing work, which is being undertaken as part of an NHS AI Stage 3 award. In support of the cost-effectiveness modelling, described in Appendix 3 of this report, this ongoing work includes an observational study comparing data from implementation sites with data from a large registry study linking CaRi-Heart with the risk of fatal and non-fatal events, in patients who have had a clinically indicated CTCA. 76 This study will collect data from 800 patients, with analysis to be completed in question 1 of 2023. 76 The company summarised data to be collected as including:76
-
clinical presentation of patients referred for CTCA, to map the potential patient pool for CaRi-Heart analysis in the NHS
-
patient risk reclassification, to inform modelling the cost of changes in medication to the NHS and total effect size of CaRi-Heart analysis on downstream events and costs to the NHS
-
costs to the NHS of adding CaRi-Heart to CTCA, including cardiologists’ time in training to interpret CaRi-Heart analyses, and the implementation costs per CTCA (if any) added to the price of a CaRi-Heart analysis to estimate the total cost per CTCA of introducing CaRi-Heart into the NHS.
The company further stated76 that patients from the study sites will be matched with patients from an existing CTCA registry (https://oxhvf.com/the-orfan-study/) using propensity score matching (PSM), as recommended in the Medical Research Council (MRC) guidelines on performing natural or quasi-experimental studies. 115
This study, along with the ongoing cost-effectiveness modelling described in Appendix 3 of this report, has the potential to inform some of the areas of uncertainty described in this EVA, particularly in relation to costs. Further information about this study (NCT05169333)69 is provided in Appendix 3; however, it is not clear whether the study will collect key information about clinical outcomes (i.e. information about changes to treatment following CaRi-Heart analysis or the information about the long-term clinical effects of any such changes). If information about clinical outcomes is being collected, it is very important that both the presentation of the observation study data (particularly data on reclassification and changes to treatment) and the subsequent modelling consider the clinically relevant subgroups (no evidence of CAD, non-obstructive CAD and obstructive CAD based on standard CTCA) defined during the scoping of this topic. 1
Unsurprisingly, the key areas of outstanding uncertainty concern the clinical effects of any changes to treatment/management that may be made as a result of adding assessment using CaRi-Heart Risk to current standard of care, in patients undergoing CTCA for suspected CAD. The optimum method of resolving this uncertainty would be collection of long-term outcomes data, which could be undertaken as part of ongoing or new company studies (e.g. the ORFAN study, described in Appendix 3). Acknowledging that such data collection will take a number of years, alternative, pragmatic approaches to populating this component of a full cost-effectiveness model may be considered useful. Such approaches could include estimation of the potential effects of treatment changes based on risk-stratified effects of treatment (e.g. statins), where risk stratification has been based on methods other than CaRi-Heart Risk and/or estimation of the potential effects of introducing new treatments (e.g. colchicine) where the ‘target condition’ (coronary inflammation) has been assessed by methods other than CaRi-Heart Risk (e.g. FAI alone; see Chapters 5 and 6). As described above, exploratory searches have indicated that there may also be a lack of data to fully support such approaches. However, it should be noted that, while initial exploratory searches can be used to identify relevant studies for modelling, they cannot be used to conclusively rule out the availability of such studies.
Conceptual cost-effectiveness modelling
A de novo conceptual decision-analytic model that could be used to inform an early assessment of the cost effectiveness of CaRi-Heart has been described in Chapter 6 of this report. A combination of a short-term diagnostic model component and a long-term model component that evaluated the downstream consequences is anticipated to capture the diagnosis and the progression of CAD, respectively. In the EVA conceptual model, it is expected that for the CaRi-Heart strategy, the initial diagnostic groups based on CTCA only would be, in turn, further split by the CaRi-Heart information into groups of low, medium or high CaRi-Heart Risk. However, there are currently no studies reporting the prevalence of ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk scores for people in the specified CAD subgroups. In addition, the current evidence available for the long-term modelling suggests that there is uncertainty as to whether and to what extent the efficacy of statins, for the prevention of MACE in people with CAD, may vary with baseline risk assessed using currently available methods. Also, no information about the effects of introducing statin treatment or changing the dose of existing statin treatment, based on CaRi-Heart Risk or on any assessment of coronary artery inflammation, was identified. Therefore, it is concluded that a health economic model based on the full clinical pathway depicted in Figure 2 could not be informed with the current available evidence.
Patient and public involvement
This study was secondary research with a short (8-week) project duration. These factors limit the opportunity for and potential contribution of patient and public involvement. However, patient representatives were included as members of the NICE specialist committee for the assessment. This means that patients were actively involved both in setting the scope for the assessment and in discussions of the evidence and its implications for decision-making.
Reporting equality, diversity and inclusion
This study was secondary research and followed a scope defined by NICE. The NICE scoping process includes consideration of equality and diversity issues. The following text describes the potential equality and diversity issues identified. 1
Angina and CAD can sometimes have a substantial and long-term adverse effect on a person’s ability to carry out normal day-to-day activities. Therefore, people with these conditions may be covered under the disability provision of the Equality Act (2010).
Coronary artery disease is more common in people who are older, live in deprived areas and men; however, women are often underdiagnosed. People of African and South Asian heritage have higher rates of CAD than people who are white and East Asian. Sex, race and age are protected characteristics. An objective measure of cardiac risk could help address this and promote equality.
Information governance statement
This study was secondary research and did not handle any personal information.
Chapter 8 Conclusions
Implications for service provision
The evidence about the clinical utility of CaRi-Heart Risk is, as yet, sparse and is subject to considerable limitations, both in terms of risk of bias and applicability to UK clinical practice. There is some evidence to indicate that CaRi-Heart Risk may be predictive of an individual patient’s 8-year risk of cardiac death, for patients undergoing CTCA for suspected CAD. However, it should be noted that the only study included in our rapid review11 has been rated as having high risk of bias, based on PROBAST. Importantly, there was no external validation of the model,11 as the reported validation data set was used in a previous study10 to develop methods and thresholds for the main imaging predictors (FAI scores). With respect to applicability, the CaRi-Heart study evaluated CaRi-Heart Risk for the prediction of 8-year cardiac death; it did not consider prediction of cardiac risk, as specified in the scope for this EVA1 (i.e. including risk of non-fatal adverse cardiovascular events). In addition, whether and to what extent CaRi-Heart represents an improvement relative to current standard of care remains unclear. This is, in part, because the definition of standard of care and hence the applicability of the comparator used in the CaRi-Heart study are uncertain. However, the clinical comparison model, reported in the CaRi-Heart study, did not appear to include any clinical observations from the CTCA scan, as required by clinical guidelines,4 and so does not appear to be consistent with current practice. As such, any claims of CaRi-Heart to be superior to a relevant clinical practice have not been adequately evaluated in this study.
The evaluation of the CaRi-Heart device is ongoing and currently available data are insufficient to fully inform cost-effectiveness modelling. Hence, there is currently insufficient evidence to inform decision-making about routine use in UK NHS clinical practice. The Oxford model, which is currently in development, could be used to conduct a preliminary assessment to the cost effectiveness of CaRi-Heart. However, it should be noted that there are key differences between the Oxford model and the EVA conceptual model presented in this report. The Oxford model consists of a decision tree only with time horizon of 8 years. This seems an appropriate choice given the available data. Also, the Oxford model stratifies patients based on CaRi-Heart Risk but not on CAD status and can make use of the proportion of patients reclassified between low-, medium- and high-risk levels with CaRi-Heart. The comparator arm in the Oxford model is either real-world practice or patient stratification based on their phenotyping, CTCA and other risk scores such as QRISK®3 or ESC risk. Therefore, despite the anticipated ability to provide an early assessment of the cost effectiveness of CaRi-Heart®, the Oxford model cannot be used to answer all the cost-effectiveness research questions presented in this EVA.
Suggested research priorities
There are a number of key areas of uncertainty, with respect to the information required to support a full Diagnostic Assessment evaluating the clinical and cost effectiveness of the CaRi-Heart device. Some of these uncertainties will be or could potentially be addressed by the ongoing ORFAN study, NCT05169333:69
-
External validation of the CaRi-Heart Risk model should be considered a high priority. Without external validation, in a population which is independent from that in which the model was developed, claims about the prognostic performance of the CaRi-Heart Risk score cannot be considered reliable. The company could be asked to undertake an external validation study and this process could also be used to address some of the applicability concerns, for example the ability of CaRi-Heart Risk to predict non-fatal adverse cardiovascular events (in addition to cardiac death) could be considered.
-
It remains unclear whether and to what extent CaRi-Heart represents an improvement relative to current standard of care; this is largely because the definition of standard of care and hence the applicability of the comparator used in the CaRi-Heart study are uncertain. If a consensus could be reached, among clinical experts (e.g. during committee discussions), as to what should constitute standard of care, then the company could be asked to provide an analysis of the prognostic performance of CaRi-Heart Risk compared to this standard. Given that clinical experts have indicated that there is variation in practice and uncertainty with respect to current standard care in the UK NHS, a definition could be based on consensus with respect to the preferred comparator or ideal practice.
-
There is currently a lack of information about the costs, from a UK NHS and PSS perspective, using CaRi-Heart, as an adjunctive investigation for assessment of cardiac risk, in people with stable chest pain, who are undergoing CTCA. The company have indicated76 that the ORFAN study will collect data on costs to the NHS of adding CaRi-Heart to CTCA, including cardiologists’ time in training to interpret CaRi-Heart analyses, and the implementation costs per CTCA (if any) added to the price of a CaRi-Heart analysis to estimate the total cost per CTCA of introducing CaRi-Heart into the NHS.
-
There is also a lack of information about the clinical effects of any changes to treatment/management that may be made as a result of adding assessment using CaRi-Heart Risk to current standard of care, in patients undergoing CTCA for suspected CAD. The optimum study design would be a RCT or cluster RCT, where patients or study centres are randomised to receive CTCA with or without the addition of CaRi-Heart Risk assessment, and information about changes to treatment/management and long-term clinical effects is collected. Observational study designs, including ‘before and after’ implementation studies or using matching techniques to provide a control, could provide an alternative approach. The collection of information about changes to treatment/management and long-term outcomes could be undertaken as part of the ongoing ORFAN study (NCT05169333);69 however, it is not clear whether collection of these data is currently planned. Irrespective of the chosen study design, it is very important that data are collected to inform estimates of effect for the clinically relevant subgroups (no evidence of CAD, non-obstructive CAD and obstructive CAD based on standard CTCA) defined during the scoping of this topic.
-
Acknowledging that the collection of data about the long-term clinical effects of using CaRi-Heart Risk will take a number of years, alternative, pragmatic approaches to populating this component of a full cost-effectiveness model may be considered useful. Such approaches could include estimation of the potential effects of treatment changes based on risk-stratified effects of treatment (e.g. statins), where risk stratification has been based on methods other than CaRi-Heart Risk and/or estimation of the potential effects of introducing new treatments (e.g. colchicine) where the ‘target condition’ (coronary inflammation) has been assessed by methods other than CaRi-Heart Risk (e.g. FAI alone). Our exploratory searches have indicated that there may also be a lack of data to fully support such approaches. However, it should be noted that, while initial exploratory searches can be used to identify relevant studies for modelling, they cannot be used to conclusively rule out the availability of such studies. It may therefore be useful to conduct a full systematic review.
Additional information
Contributions of authors
Marie Westwood (https://orcid.org/0000-0002-6257-0653) (Reviews Manager and Diagnostic Assessment Lead) planned and performed the systematic review and interpretation of evidence.
Nigel Armstrong (https://orcid.org/0000-0002-7443-4798) (Senior Health Economist) contributed to the planning and interpretation of the systematic review and conceptual cost-effectiveness modelling.
Eline Krijkamp (https://orcid.org/0000-0003-3970-2205) (Health Economist) conducted the conceptual cost-effectiveness modelling.
Mark Perry (https://orcid.org/0000-0002-5459-6554) (Systematic Reviewer) planned and performed the systematic review and interpretation of evidence.
Caro Noake (https://orcid.org/0000-0003-0329-4772) (Information Specialist) devised and performed the literature searches and provided information support to the project. All parties were involved in drafting and/or commenting on the report.
Apostolos Tsiachristas (https://orcid.org/0000-0002-4662-8915) (Associate Professor of Health Economics) leads the work on conceptual cost-effectiveness modelling which has contributed to the ongoing development of a full cost-effectiveness model by the Nuffield Department of Population Health at the University of Oxford; he is also a co-author on this report and has contributed to discussions of conceptual modelling.
Isaac Corro-Ramos (https://orcid.org/0000-0002-1294-8187) (Senior Health Economist) conducted the conceptual cost-effectiveness modelling.
Acknowledgements
The authors would like to thank Professor Sue Mallett (Professor in Diagnostic and Prognostic Medical Statistics, UCL Centre for Medical Imaging, Division of Medicine, Faculty of Medical Sciences, University of London, UK) for her assistance in completing the PROBAST assessment included in this report and for her provision of specialist statistical advice.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration.
Ethics statement
This report concerns secondary research, for which ethics approval is not required.
Information governance statement
This study did not handle any personal information.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/WYGC4096.
Primary conflicts of interest: None declared.
All authors have completed the unified competing interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare (1) no financial support for the submitted work from anyone other than their employer; (2) no financial relationships with commercial entities that might have an interest in the submitted work; (3) no spouses, partners or children with relationships with commercial entities that might have an interest in the submitted work; and (4) no financial interests that may be relevant to the submitted work.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- National Institute for Health and Care Excellence . Diagnostics Assessment Programme: Early Value Assessment: CaRi-Heart for Predicting Cardiac Risk in Suspected Coronary Artery Disease: Final Scope 2022.
- Office for National Statistics . Heart Attack Deaths in 2019, 2020, 2021 and 2022 2022. www.ons.gov.uk/aboutus/transparencyandgovernance/freedomofinformationfoi/heartattackdeathsin201920202021and2022 (accessed 8 September 2022).
- Office for National Statistics . Deaths Registered in England and Wales: 2021 2022. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregistrationsummarytables/2021 (accessed 8 September 2022).
- National Institute for Health and Care Excellence . Recent-Onset Chest Pain of Suspected Cardiac Origin: Assessment and Diagnosis. 2010. www.nice.org.uk/guidance/cg95 (accessed 8 September 2022).
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. ESC Scientific Document Group . 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407-77.
- Fishbein MC, Siegel RJ. How big are coronary atherosclerotic plaques that rupture?. Circulation 1996;94:2662-6.
- Matter CM, Stuber M, Nahrendorf M. Imaging of the unstable plaque: how far have we got?. Eur Heart J 2009;30:2566-74.
- Fleg JL, Stone GW, Fayad ZA, Granada JF, Hatsukami TS, Kolodgie FD, et al. Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging 2012;5:941-55.
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med 1999;340:115-26.
- Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Centeno EH, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392:929-39.
- Oikonomou EK, Antonopoulos AS, Schottlander D, Marwan M, Mathers C, Tomlins P, et al. Standardized measurement of coronary inflammation using cardiovascular computed tomography: integration in clinical care as a prognostic medical device. Cardiovasc Res 2021;117:2677-90.
- Caristo Diagnostics Ltd . CaRi-Heart Analysis. Uncover Inflammation. Predict Heart Attacks (Doc No. 1413) 2021. https://caristo.com/img/pdfs/Caristo_Flyer_1413.pdf (accessed 15 September 2022).
- Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161-70.
- National Institute for Health and Care Excellence . Stable Angina: Management 2011. www.nice.org.uk/guidance/cg126/resources/stable-angina-management-pdf-35109453262021 (accessed 15 September 2022).
- National Institute for Health and Care Excellence . Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification 2014. www.nice.org.uk/guidance/cg181/resources/cardiovascular-disease-risk-assessment-and-reduction-including-lipid-modification-pdf-35109807660997 (accessed 21 September 22).
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009. www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm (accessed 23 March 2011).
- National Institute for Health and Care Excellence . NICE Health Technology Evaluations: The Manual. Process and Methods [PMG36] 2022. www.nice.org.uk/process/pmg36 (accessed 8 February 2022).
- Garritty C, Gartlehner G, Kamel C, King VJ, Nussbaumer-Streit B, Stevens A, et al. Cochrane Rapid Reviews: Interim Guidance from the Cochrane Rapid Reviews Methods Group 2020. http://methods.cochrane.org/sites/methods.cochrane.org.rapidreviews/files/uploads/cochrane_rr_-_guidance-23mar2020-v1.pdf (accessed 27 September 22).
- Canadian Agency for Drugs and Technologies in Health . PRESS – Peer Review of Electronic Search Strategies: 2015 Guideline Explanation and Elaboration (PRESS E&Amp;E) 2016. https://cadth.ca/press-peer-review-electronic-search-strategies (accessed 16 November 2020).
- Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST Group . PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 2019;170:51-8.
- Abbasi J. Could a new method to detect coronary inflammation prevent heart attacks?. JAMA 2017;318:1527-8.
- Antoniades C, Kotanidis CP, Berman DS. State-of-the-art review article. Atherosclerosis affecting fat: what can we learn by imaging perivascular adipose tissue?. J Cardiovasc Comput Tomogr 2019;13:288-96.
- Antoniades C, Shirodaria C. Detecting coronary inflammation with perivascular fat attenuation imaging: making sense from perivascular attenuation maps. JACC Cardiovasc Imaging 2019;12:2011-4.
- Antoniades C, Antonopoulos AS, Deanfield J. Imaging residual inflammatory cardiovascular risk. Eur Heart J 2020;41:748-58.
- Antonopoulos AS, Angelopoulos A, Papanikolaou P, Simantiris S, Oikonomou EK, Vamvakaris K, et al. Biomarkers of vascular inflammation for cardiovascular risk prognostication: a meta-analysis. JACC Cardiovasc Imaging 2022;15:460-71.
- Antonopoulos AS, Angelopoulos A, Tsioufis K, Antoniades C, Tousoulis D. Cardiovascular risk stratification by coronary computed tomography angiography imaging: current state-of-the-art. Eur J Prev Cardiol 2022;29:608-24.
- Antonopoulos AS, Angelopoulos A, Papanikolaou P, Simantiris S, Oikonomou EK, Vamvakaris K, et al. Biomarkers of vascular inflammation for cardiovascular risk prognostication: a meta-analysis. JACC Cardiovasc Imaging 2022;15:460-71.
- Antonopoulos AS, Antoniades C. Perivascular fat attenuation index by computed tomography as a metric of coronary inflammation. J Am Coll Cardiol 2018;71:2708-9.
- Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 2017;9.
- Bao W, Yang M, Xu Z, Yan F, Yang Q, Li X, et al. Coronary inflammation assessed by perivascular fat attenuation index in patients with psoriasis: a propensity score-matched study. Dermatology 2022;238:562-70.
- Bengs S, Haider A, Warnock GI, Fiechter M, Pargaetzi Y, Rampidis G, et al. Quantification of perivascular inflammation does not provide incremental prognostic value over myocardial perfusion imaging and calcium scoring. Eur J Nucl Med Mol Imaging 2021;48:1806-12.
- Berman DS, Kwiecinski J. Imaging coronary inflammatory risk. JACC Cardiovasc Imaging 2022;15:472-5.
- Bittner DO, Goeller M, Zopf Y, Achenbach S, Marwan M. High level of EPA is associated with lower perivascular coronary attenuation as measured by coronary CTA. Presented at European Society of Cardiology Congress (ESC 2019); 31 Aug–4 Sept 2019; Paris, France. Eur Heart J 2019;40.
- Cabrelle G, Pergola V, Cattarin S, Dellino C, Continisio S, Montonati C, et al. 514 Usefulness and clinical implications of plaque analysis and Pfai for the evaluation of cardiovascular risk. Presented at Society of Cardiovascular Computed Tomography 17th Annual Scientific Meeting; 15–17 July 2022; Las Vegas, US. J Cardiovasc Comput Tomogr 2022;16.
- Cecere A, De Kreutzenberg S, Motta R, Benvenuti F, Iliceto S, Avogaro A, et al. Coronary perivascular inflammation in type 2 diabetes mellitus patients: the missing piece in the puzzle of their increased cardiovascular risk? Presented at European Society of Cardiology Congress (ESC 2021); 27–30 Aug 2021; Virtual. Eur Heart J 2021;42.
- Chatterjee D, Shou BL, Matheson MB, Ostovaneh MR, Rochitte C, Chen MY, et al. Perivascular fat attenuation for predicting adverse cardiac events in stable patients undergoing invasive coronary angiography. J Cardiovasc Comput Tomogr 2022;16:483-90.
- Chatterjee D, Shou B, Ostovaneh M, Matheson M, Ortman J, Cox C, et al. Perivascular fat attenuation index for predicting outcome in patients referred for invasive coronary angiography. Presented at 16th Annual Scientific Meeting of the Society of Cardiovascular Computed Tomography; 16–17 July 2021; Virtual. J Cardiovasc Comput Tomogr 2021;15:S53-4.
- Chen X, Dang Y, Hu H, Ma S, Ma Y, Wang K, et al. Pericoronary adipose tissue attenuation assessed by dual-layer spectral detector computed tomography is a sensitive imaging marker of high-risk plaques. Quant Imaging Med Surg 2021;11:2093-103.
- Dai X, Hou Y, Tang C, Lu Z, Shen C, Zhang L, et al. Long-term prognostic value of the serial changes of CT-derived fractional flow reserve and perivascular fat attenuation index. Quant Imaging Med Surg 2022;12:752-65.
- Dai X, Yu L, Lu Z, Shen C, Tao X, Zhang J. Serial change of perivascular fat attenuation index after statin treatment: insights from a coronary CT angiography follow-up study. Int J Cardiol 2020;319:144-9.
- Dang Y, Chen X, Ma S, Ma Y, Ma Q, Zhou K, et al. Association of pericoronary adipose tissue quality determined by dual-layer spectral detector CT with severity of coronary artery disease: a preliminary study. Front Cardiovasc Med 2021;8.
- Elnabawi YA, Oikonomou EK, Dey AK, Mancio J, Rodante JA, Aksentijevich M, et al. Association of biologic therapy with coronary inflammation in patients with psoriasis as assessed by perivascular fat attenuation index. JAMA Cardiol 2019;4:885-91.
- Gaibazzi N, Tuttolomondo D, Nicolini F, Tafuni A, Sartorio D, Martini C, et al. The histopathological correlate of peri-vascular adipose tissue attenuation on computed tomography in surgical ascending aorta aneurysms: is this a measure of tissue inflammation?. Diagnostics (Basel) 2021;11.
- Hoshino M, Yang S, Sugiyama T, Zhang J, Kanaji Y, Hamaya R, et al. Characteristic findings of microvascular dysfunction on coronary computed tomography angiography in patients with intermediate coronary stenosis. Eur Radiol 2021;31:9198-210.
- Hoshino M, Zhang J, Sugiyama T, Yang S, Kanaji Y, Hamaya R, et al. Prognostic value of pericoronary inflammation and unsupervised machine-learning-defined phenotypic clustering of CT angiographic findings. Int J Cardiol 2021;333:226-32.
- Hoshino M, Yang S, Sugiyama T, Zhang J, Kanaji Y, Yamaguchi M, et al. Prognostic value of peri-coronary adipose tissue attenuation and whole vessel and lesion plaque quantification on Coronary Computed Tomography Angiography. Presented at European Society of Cardiology Congress (ESC 2020); 29 Aug–1 Sep 2020; Virtual. Eur Heart J 2020;41.
- Kanaji Y, Sugiyama T, Hoshino M, Hirano H, Horie T, Kanno Y, et al. The association between global coronary flow reserve and coronary inflammation assessed by fat attenuation index on computed tomography in patients with acute coronary syndrome. Presented at European Society of Cardiology Congress (ESC 2019); 31 Aug–4 Sep 2019; Paris, France. Eur Heart J 2019;40.
- Kato S, Utsunomiya D, Horita N, Hoshino M, Kakuta T. Prognostic significance of the perivascular fat attenuation index derived by coronary computed tomography: a meta-analysis. Hellenic J Cardiol 2022;67:73-5.
- Li JH, Tang CX, Liu TY, Chen YC, Zhou CS, Lu GM, et al. [Association of coronary perivascular fat attenuation index, the parameters of plaque and fractional flow reserve]. Zhonghua Yi Xue Za Zhi 2021;101:3214-20.
- Liu Y, Sun Y, Hu C, Liu J, Gao A, Han H, et al. Perivascular adipose tissue as an indication, contributor to, and therapeutic target for atherosclerosis. Front Physiol 2020;11.
- Montonati C, Pergola V, Dellino C, Continisio S, Mattesi G, Zolin A, et al. Coro-CT plaque analysis in assessment of cardiovascular risk. Presented at 53rd Congress of the Italian Association of Hospital Cardiologists (ANMCO 2022); 19–21 May 2022; Rimini, Italy. Eur Heart J Suppl 2022;24:C23-4.
- Oikonomou EK, Schottlander D, Antonopoulos AS, Marwan M, Kotanidis CP, Kluner LV, et al. Standardised quantification of coronary inflammation using cardiac computed tomography: the Fat Attenuation Index Score (FAI-Score). Presented at ESC Preventive Cardiology 2021; 15–17 April 2021; Online. Eur J Prev Cardiol 2021;28:i440-1.
- Oikonomou EK, Desai MY, Marwan M, Kotanidis CP, Antonopoulos AS, Schottlander D, et al. Perivascular fat attenuation index stratifies cardiac risk associated with high-risk plaques in the CRISP-CT study. J Am Coll Cardiol 2020;76:755-7.
- Oikonomou EK, Marwan M, Mancio J, Kotanidis CK, Thomas KE, Alashi A, et al. Perivascular fat attenuation index stratifies the cardiac risk associated with high-risk plaque features on coronary computed tomography angiography. Presented at European Society of Cardiology Congress (ESC 2019); 31 Aug–4 Sep 2019; Paris, France. Eur Heart J 2019;40.
- Oikonomou EK, Williams MC, Kotanidis CP, Desai MY, Marwan M, Antonopoulos AS, et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur Heart J 2019;40:3529-43.
- Oikonomou EK, Thomas S, Mancio J, Antonopoulos AS, Sabharwal N, Kelion A, et al. Computed tomography-based perivascular fat phenotyping identifies unstable coronary lesions and active vascular calcification. Presented at European Society of Cardiology Congress (ESC 2018); 25–29 Aug 2018; Munich, Germany. Eur Heart J 2018;39.
- Pandey NN, Sharma S, Jagia P, Kumar S. Epicardial fat attenuation, not volume, predicts obstructive coronary artery disease and high risk plaque features in patients with atypical chest pain. Br J Radiol 2020;93.
- Pergola V, Cabrelle G, Mattesi G, Cattarin S, Furlan A, Dellino CM, et al. Added value of CCTA-derived features to predict MACEs in stable patients undergoing coronary computed tomography. Diagnostics (Basel) 2022;12.
- Pergola V, Previtero M, Cecere A, Storer V, Castiello T, Baritussio A, et al. Clinical value and time course of pericoronary fat inflammation in patients with angiographically nonobstructive coronaries: a preliminary report. J Clin Med 2021;10.
- Plackett B. Caristo Diagnostics: taking a fresh look at CT scans. Nature 2020;588:S70-2.
- Sagris MS, Antonopoulos AA, Simantiris SS, Panagiotopoulos GP, Papanikolaou PP, Oikonomou EO, et al. Pericoronary adipose tissue attenuation index (FAI) – a newimaging biomarker and its diagnostic and prognostic utility: a systematic review and meta-analysis. Presented at ESC Congress 2021; 27–30 Aug 2021; Virtual. Eur Heart J 2021;42.
- Sen N, Tanwar S. Correlation of coronary inflammatory markers like high sensitive C reactive protein, tumor necrosis factor, interleukin 6 with perivascular fat attenuation index to stratify the cardiac risk associated with the presence of adverse plaque morphology. Presented at ACC20 – World Congress of Cardiology; 28–30 Mar 2020; Chicago, US. J Am Coll Cardiol 2020;75.
- Shan D, Wang G, Wang X, Ding Y, Chen Y, Chen J. [Value of maximum area stenosis combined with perivascular fat attenuation index in predicting hemodynamically significant coronary artery disease]. Nan Fang Yi Ke Da Xue Xue Bao 2021;41:988-94.
- Simantiris S, Antonopoulos AS, Angelopoulos A, Papanikolaou P, Oikonomou EK, Vamvakaris K, et al. Prognostic value of vascular inflammation biomarkers over clinical risk factors for cardiovascular risk: a meta-analysis. Presented at ESC Preventive Cardiology 2021; 15–17 Apr 2021; Online. Eur J Prev Cardiol 2021;28.
- Sugiyama T, Kanaji Y, Hoshino M, Yamaguchi M, Hada M, Misawa T, et al. Prognostic value of fat attenuation index of pericoronary adipose tissue surrounding left anterior descending artery on coronary computed tomography angiography. presented at European Society of Cardiology Congress, ESC 2020; 29 Aug–1 Sep 2020; Virtual. Eur Heart J 2020;41.
- Sutanto SA, James NE, Loy RHY. Using perivascular fat attenuation index to monitor coronary inflammation in patients with psoriasis. JAMA Cardiol 2020;5.
- Yan H, Zhao N, Geng W, Hou Z, Gao Y, Lu B. The perivascular fat attenuation index improves the diagnostic performance for functional coronary stenosis. J Cardiovasc Dev Dis 2022;9.
- Zhu X, Chen X, Ma S, Zhou K, Hou Y. Dual-layer spectral detector CT to study the correlation between pericoronary adipose tissue and coronary artery stenosis. J Cardiothorac Surg 2021;16.
- University of Oxford, Caristo Diagnostics Limited, National Consortium of Intelligent Medical Imaging, Oxford University Hospitals NHS Trust, British Heart Foundation, Innovate UK . The Oxford Risk Factors and Non-Invasive Imaging Study (ORFAN). NCT05169333 2021. https://clinicaltrials.gov/show/NCT05169333 (accessed 23 November 22).
- Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403-69.
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507-20.
- American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37:S81-90.
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889-934.
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344-51.
- Riley RD, Snell KI, Ensor J, Burke DL, Harrell FE, Moons KG, et al. Minimum sample size for developing a multivariable prediction model: PART II – binary and time-to-event outcomes. Stat Med 2019;38:1276-96.
- National Institute for Health and Care Excellence . Diagnostics Assessment Programme: Early Value Assessment: CaRi-Heart® for Predicting CTCA Risk in Suspected Coronary Artery Disease (CAD): Request for Information 2022.
- Westwood M, Armstrong N, Krijkamp E, Perry M, Noake C, Corro-Ramos I. CaRi-Heart® for Predicting Cardiac Risk in Suspected Coronary Heart Disease (CAD): A Rapid Review and Conceptual Economic Model to Inform Early Value Assessment (EVA). Final Protocol. York: Kleijnen Systematic Reviews Ltd; 2022.
- Ma Z, Chen J, Jin K, Chen X. Colchicine and coronary heart disease risks: a meta-analysis of randomized controlled clinical trials. Front Cardiovasc Med 2022;9.
- Bytyci I, Bajraktari G, Penson PE, Henein MY, Banach M. Lipid and Blood Pressure Meta-Analysis Collaboration (LBPMC) Group; International Lipid Expert Panel (ILEP) . Efficacy and safety of colchicine in patients with coronary artery disease: a systematic review and meta-analysis of randomized controlled trials. Br J Clin Pharmacol 2022;88:1520-8.
- Xu H, Mao L, Liu H, Lin Z, Zhang Y, Yang J. Colchicine for secondary prevention of coronary artery disease: a meta-analysis of randomised controlled trials. Heart Lung Circ 2022;31:685-95.
- Niu Y, Bai N, Ma Y, Zhong PY, Shang YS, Wang ZL. Safety and efficacy of anti-inflammatory therapy in patients with coronary artery disease: a systematic review and meta-analysis. BMC Cardiovasc Disord 2022;22.
- Fiolet ATL, Opstal TSJ, Mosterd A, Eikelboom JW, Jolly SS, Keech AC, et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur Heart J 2021;42:2765-75.
- Sen S, Karahan E, Buyukulas C, Polat YO, Uresin AY. Colchicine for cardiovascular therapy: a drug interaction perspective and a safety meta-analysis. Anatol J Cardiol 2021;25:753-61.
- Samuel M, Tardif JC, Bouabdallaoui N, Khairy P, Dube MP, Blondeau L, et al. Colchicine for secondary prevention of cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Can J Cardiol 2021;37:776-85.
- Masson W, Lobo M, Lavalle-Cobo A, Molinero G. Can colchicine prevent acute myocardial infarction? Systematic review and meta-analysis. Rev Argent Cardiol 2021;89:42-9.
- Aimo A, Pascual Figal DA, Bayes-Genis A, Emdin M, Georgiopoulos G. Effect of low-dose colchicine in acute and chronic coronary syndromes: a systematic review and meta-analysis. Eur J Clin Invest 2021;51.
- Ullah W, Haq S, Zahid S, Gowda SN, Ottman P, Saleem S, et al. Safety and efficacy of colchicine in patients with stable CAD and ACS: a systematic review and meta-analysis. Am J Cardiovasc Drugs 2021;21:659-68.
- Xia M, Yang X, Qian C. Meta-analysis evaluating the utility of colchicine in secondary prevention of coronary artery disease. Am J Cardiol 2021;140:33-8.
- Tien YY, Huang HK, Shih MC, Tu YK. Drug repurposing? Cardiovascular effect of colchicine on patients with coronary artery disease: a systematic review and meta-analysis. J Cardiol 2021;77:576-82.
- Xiang Z, Yang J, Yang J, Zhang J, Fan Z, Yang C, et al. Efficacy and safety of colchicine for secondary prevention of coronary heart disease: a systematic review and meta-analysis. Intern Emerg Med 2021;16:487-96.
- Kofler T, Kurmann R, Lehnick D, Cioffi GM, Chandran S, Attinger-Toller A, et al. Colchicine in patients with coronary artery disease: a systematic review and meta-analysis of randomized trials. J Am Heart Assoc 2021;10.
- Roman YM, Hernandez AV, White CM. The role of suppressing inflammation in the treatment of atherosclerotic cardiovascular disease. Ann Pharmacother 2020;54:1021-9.
- Webb CA, Barry AR. Colchicine for secondary cardiovascular prevention: a systematic review. Pharmacotherapy 2020;40:575-83.
- Verma S, Eikelboom JW, Nidorf SM, Al-Omran M, Gupta N, Teoh H, et al. Colchicine in cardiac disease: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2015;15.
- Varotto L, Bonanno C, Caprioglio F. Network meta-analysis evaluating the utility of non-corticosteroid anti-inflammatory therapy in secondary prevention of coronaryartery disease. Eur Heart J Suppl 2021;23:C114-5.
- Iskandarani ME, Shatla I, Khalid M, Paul T. Colchicine in stable coronary artery disease, a systematic review and metanalysis of randomized clinical trials. J Am Coll Cardiol 2021;77.
- Kassab K, Chuy KL, Vij A. Use of colchicine for secondary prevention of cardiovascular events: systematic review and meta-analysis. J Am Coll Cardiol 2021;77.
- Galli M, Princi G, Crea F, D’Amario D. Colchicine significantly increases the risk of non-cardiovascular death in patients with coronary artery disease. Eur Heart J Suppl 2020;22.
- Fong HK, Tan JL, Eniezat M, Yap J, Low KM, Desai R, et al. Outcomes of anti-inflammatory agents in coronary artery disease. Catheter Cardiovasc Interv 2020;95:S204-5.
- Wudexi I, Shokri E, Abo-Aly M, Shindo K, Abdel-Latif A. Comparative effectiveness of anti-inflammatory drug treatments in coronary heart disease patients: a systematic review and network meta-analysis. Mediators Inflamm 2021;2021.
- Abrantes AM, Nogueira-Garcia B, Alves M, Teixeira Passos D, Brito D, Pinto FJ, et al. Low-dose colchicine in coronary artery disease – systematic review and meta-analysis. Circ Rep 2021;3:457-64.
- Chen Y, Zhang H, Chen Y, Li M, Luo W, Liu Y, et al. Colchicine may become a new cornerstone therapy for coronary artery disease: a meta-analysis of randomized controlled trials. Clin Rheumatol 2022;41:1873-87.
- Grajek S, Michalak M, Urbanowicz T, Olasinska-Wisniewska A. A meta-analysis evaluating the colchicine therapy in patients with coronary artery disease. Front Cardiovasc Med 2021;8.
- Al-Atta A, Kuzemczak M, Alkhalil M. Colchicine for the prevention of ischemic stroke: an updated meta-analysis of randomized clinical trials. Brain Circ 2021;7:187-93.
- University of Sao Paulo General Hospital . Colchicine Effect on Perivascular Inflammation Index on Coronary CTA (COPIX). NCT05347316 2023. https://clinicaltrials.gov/show/NCT05347316 (accessed 9 November 2022).
- Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397-405.
- Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581-90.
- British National Formulary (BNF) . British National Formulary 2022. https://bnf.nice.org.uk/ (accessed 23 November 2022).
- Jones K, Burns A. Unit Costs of Health and Social Care 2021. Canterbury: Personal Social Services Research Unit, University of Kent; 2021.
- NHS (England) . 2019/20/National/Cost/Collection/Data/Publication 2021. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/ (accessed 23 November 22).
- Vemer P, Corro Ramos I, van Voorn GAK, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. PharmacoEconomics 2016;34:349-61.
- Büyükkaramikli NC, Rutten-van Mölken MPMH, Severens JL, Al M. TECH-VER: a verification checklist to reduce errors in models and improve their credibility. PharmacoEconomics 2019;37:1391-408.
- National Institute for Health and Care Excellence . Early Value Assessment for Medtech: Actively Drawing in Medical Devices, Diagnostics and Digital Products That Address National Unmet Needs. Providing Quicker Assessments of Early Value to Identify the Most Promising Technologies, Conditional on Further Evidence Generation. [PowerPoint Slides Provided by NICE] 2022.
- Cury RC, Leipsic J, Abbara S, Achenbach S, Berman D, Bittencourt M, et al. CAD-RADS™ 20 – 2022 coronary artery disease – reporting and data system an expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR) and the North America Society of Cardiovascular Imaging (NASCI). Radiol Cardiothorac Imag 2022;4.
- Craig P, Cooper C, Gunnell D, Haw S, Lawson K, Macintyre S, et al. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health 2012;66:1182-6.
- Blieden Betts M, Gandra SR, Cheng LI, Szatkowski A, Toth PP. Differences in utility elicitation methods in cardiovascular disease: a systematic review. J Med Econ 2018;21:74-8.
- Betts MB, Rane P, Bergrath E, Chitnis M, Bhutani MK, Gulea C, et al. Utility value estimates in cardiovascular disease and the effect of changing elicitation methods: a systematic literature review. Health Qual Life Outcomes 2020;18.
- Creber RM, Dimagli A, Spadaccio C, Myers A, Moscarelli M, Demetres M, et al. Effect of coronary artery bypass grafting on quality of life: a meta-analysis of randomized trials. Eur Heart J Qual Care Clin Outcomes 2022;8:259-68.
- De la Puente C, Vallejos C, Bustos L, Zaror C, Velasquez M, Lanas F. Latin American Clinical Epidemiology Network Series – Paper 8: Ticagrelor was cost-effective vs. clopidogrel in acute coronary syndrome in Chile. J Clin Epidemiol 2017;86:117-24.
- Di Tanna GL, Urbich M, Wirtz HS, Potrata B, Heisen M, Bennison C, et al. Health state utilities of patients with heart failure: a systematic literature review. PharmacoEconomics 2021;39:211-29.
- Duarte A, Llewellyn A, Walker R, Schmitt L, Wright K, Walker S, et al. Non-invasive imaging software to assess the functional significance of coronary stenoses: a systematic review and economic evaluation. Health Technol Assess 2021;25:1-230.
- Gao L, Nguyen P, Dunstan D, Moodie M. Are office-based workplace interventions designed to reduce sitting time cost-effective primary prevention measures for cardiovascular disease? A systematic review and modelled economic evaluation. Int J Environ Res Public Health 2019;16.
- Health Quality Ontario . Mechanical thrombectomy in patients with acute ischemic stroke: a health technology assessment. Ont Health Technol Assess Ser 2016;16:1-79.
- Health Quality Ontario . Percutaneous ventricular assist devices: a Health Technology Assessment. Ont Health Technol Assess Ser 2017;17:1-97.
- Ontario Health (Quality) . Transcatheter aortic valve implantation in patients with severe, symptomatic aortic valve stenosis at intermediate surgical risk: a Health Technology Assessment. Ont Health Technol Assess Ser 2020;20:1-121.
- Joundi RA, Adekanye J, Leung AA, Ronksley P, Smith EE, Rebchuk AD, et al. Health state utility values in people with stroke: a systematic review and meta-analysis. J Am Heart Assoc 2022;11.
- Perera R, McFadden E, McLellan J, Lung T, Clarke P, Pérez T, et al. Optimal strategies for monitoring lipid levels in patients at risk or with cardiovascular disease: a systematic review with statistical and cost-effectiveness modelling. Health Technol Assess 2015;19:1-401, vii.
- Shan L, Shan J, Saxena A, Robinson D. Quality of life and functional status after carotid revascularisation: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2015;49:634-45.
- Health Quality Ontario. Left atrial appendage closure device with delivery system: a Health Technology Assessment. Ont Health Technol Assess Ser 2017;17:1-106.
- Sterne JA, Bodalia PN, Bryden PA, Davies PA, López-López JA, Okoli GN, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess 2017;21:1-386.
- Stevanovic J, Pechlivanoglou P, Kampinga MA, Krabbe PFM, Postma MJ. Multivariate meta-analysis of preference-based quality of life values in coronary heart disease. PLOS ONE 2016;11.
- Westwood M, Ramaekers B, Grimm S, Worthy G, Fayter D, Armstrong N, et al. High-sensitivity troponin assays for early rule-out of acute myocardial infarction in people with acute chest pain: a systematic review and economic evaluation. Health Technol Assess 2021;25:1-276.
Appendix 1 Literature search strategies
Rapid review searches
| Database | Date span | Hits retrieved |
|---|---|---|
| MEDLINE + Med in P | 1946 to 4 October 2022 | 1203 |
| EMBASE | 1974 to 4 October 2022 | 1746 |
| PubMed-not-MEDLINE | 1946 to 4 October 2022 | 142 |
| PubMed | up to 5 October 2022 | 415 |
| CDSR + CDSR P | up to October 2022/Iss10 | 21 |
| CENTRAL | up to October 2022/Iss10 | 225 |
| DARE | up to March 2015 | 0 |
| HTA (CRD) | up to March 2018 | 0 |
| CINAHL | up to 6 October 2022 | 510 |
| KSR Evidence | up to 5 October 2022 | 28 |
| Epistemonikos | up to 6 October 2022 | 138 |
| INAHTA | up to 6 October 2022 | 2 |
| NIHR HTA | up to 6 October 2022 | 0 |
| PROSPERO | up to 5 October 2022 | 225 |
| INPLASY | up to 6 October 2022 | 0 |
| LILACS | up to 6 October 2022 | 9 |
| DOAJ | up to 6 October 2022 | 80 |
| ClinicalTrials.gov | up to 6 October 2022 | 277 |
| EUCTR | up to 6 October 2022 | 40 |
| WHO ICTRP | up to 6 October 2022 | 16 |
| ScanMedicine | up to 6 October 2022 | 114 |
| Northern Light | 2010–22/wk38 | 103 |
| MedRxiv | up to 6 October 2022 | 35 |
| Total | 5329 |
MEDLINE(Ovid) and Epub Ahead of Print, In-process, In-data-review and Other Non-indexed Citations and Daily: 1946–4 October 2022
Searched 5 October 2022
-
((CaRi adj3 heart) or CaRi-Heart or CaRiHeart).af. (2)
-
(Caristo or CariCloud).ti,ab,ot. (1)
-
(CRD42020181158 or CRD42021229491 or CRD42021297228 or NCT05169333).af. (2)
-
1 or 2 or 3 (5)
-
((Fat or PVAT or ‘perivascular adipose tissue’) adj2 Attenuation$).ti,ab,ot. (282)
-
(FAI adj3 (Scor$ or Index$ or indic$ or measure$ or map$ or coronary or plaque$ or arter$ or heart$ or athero$)).ti,ab,ot. (974)
-
(FAITM or pFAI).ti,ab,ot. (17)
-
or/5–7 (1229)
-
4 or 8 (1232)
-
exp animals/not (exp animals/and humans/) (5,053,872)
-
9 not 10 (1203)
EMBASE (Ovid): 1974–4 October 2022
Searched 5 October 2022
-
((CaRi adj3 heart) or CaRi-Heart or CaRiHeart).af. (8)
-
(Caristo or CariCloud).ti,ab,ot. (1)
-
(CRD42020181158 or CRD42021229491 or CRD42021297228 or NCT05169333).af. (2)
-
1 or 2 or 3 (11)
-
((Fat or PVAT or ‘perivascular adipose tissue’) adj2 Attenuation$).ti,ab,ot. (443)
-
(FAI adj3 (Scor$ or Index$ or indic$ or measure$ or map$ or coronary or plaque$ or arter$ or heart$ or athero$)).ti,ab,ot. (1381)
-
(FAITM or pFAI).ti,ab,ot. (26)
-
or/5–7 (1783)
-
4 or 8 (1791)
-
animal/ (1,589,563)
-
animal experiment/ (2,876,011)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (7,353,556)
-
or/10–12 (7,353,556)
-
exp human/ (24,163,444)
-
human experiment/ (596,121)
-
or/14–15 (24,165,573)
-
13 not (13 and 16) (5,550,502)
-
9 not 17 (1746)
MEDLINE(Ovid) PubMed-not-MEDLINE: 1946–4 October 2022
Searched 5 October 2022
-
((CaRi adj3 heart) or CaRi-Heart or CaRiHeart).af. (0)
-
(Caristo or CariCloud).ti,ab,ot. (0)
-
(CRD42020181158 or CRD42021229491 or CRD42021297228 or NCT05169333).af. (0)
-
1 or 2 or 3 (0)
-
((Fat or PVAT or ‘perivascular adipose tissue’) adj2 Attenuation$).ti,ab,ot. (56)
-
(FAI adj3 (Scor$ or Index$ or indic$ or measure$ or map$ or coronary or plaque$ or arter$ or heart$ or athero$)).ti,ab,ot. (95)
-
(FAITM or pFAI).ti,ab,ot. (4)
-
or/5–7 (142)
-
4 or 8 (142)
-
exp animals/ not (exp animals/ and humans/) (1)
-
9 not 10 (142)
PubMed (NIH): up to 5 October 2022
Searched 5 October 2022
(PubMed top up)
-
10 #2 or #4 or #5 or #7 or #8 or #9 (415)
-
9 FAITM[Title/Abstract] OR pFAI[Title/Abstract] (18)
-
8 (FAI[Title/Abstract] AND (coronary[Title/Abstract] OR plaque*[Title/Abstract] OR arter*[Title/Abstract] OR heart*[Title/Abstract] OR athero*[Title/Abstract]) (135)
-
7 ‘Fat attenuation’ or ‘PVAT attenuation’ or ‘perivascular adipose tissue attenuation’ (306)
-
5 (Caristo[Title/Abstract] OR CariCloud[Title/Abstract]) (1)
-
4 CRD42020181158 or CRD42021229491 or CRD42021297228 or NCT05169333 (2)
-
2 ‘CaRi heart’ or CaRi-Heart or CaRiHeart (1)
Cochrane Database of Systematic Reviews (Wiley): up to 2022/10/Iss10
Cochrane Database of Systematic Reviews – Protocols (CDSR_P) (Wiley): up to 2022/10/Iss10
Cochrane Central Register of Controlled Trials (Wiley): up to 2022/10/Iss10
Searched 5 October 2022
ID Search Hits
-
((CaRi Near3 heart) or CaRi-Heart or CaRiHeart) 0
-
(Caristo or CariCloud) 5
-
(CRD42020181158 or CRD42021229491 or CRD42021297228 or NCT05169333) 0
-
#1 or ‘#2 or #3 5
-
((Fat or PVAT or ‘perivascular adipose tissue’) Near3 Attenuation*) 34
-
(FAI Near3 (Scor* or Index* or indic* or measure* or map* or coronary or plaque* or arter* or heart* or athero*)) 211
-
(FAITM or pFAI) 0
-
#5 or #6 or #7 242
-
#4 or #8 247
CDSR = 20
CDSR Protocols = 1
CENTRAL = 225
Database of Abstracts of Reviews of Effects (CRD): up to March 2015
Health Technology Assessment Database (CRD): up to March 2018
Searched 5 October 2022
-
(((CaRi Near3 heart) or CaRi-Heart or CaRiHeart)) 0 Delete
-
((Caristo or CariCloud)) 0 Delete
-
((CRD42020181158 or CRD42021229491 or CRD42021297228 or NCT05169333)) 0 Delete
-
(((Fat or PVAT or ‘perivascular adipose tissue’) Near3 Attenuation*)) 0 Delete
-
((FAI Near3 (Scor* or Index* or indic* or measure* or map* or coronary or plaque* or arter* or heart* or athero*))) 0 Delete
-
((FAITM or pFAI)) 0 Delete
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 0 Delete
CINAHL (EBSCOhost Research Databases): up to 6 October 2022
Searched 6 October 2022
-
S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 510
-
S6 TI ((FAI N3 (Scor* or Index* or indic* or measure* or map* or coronary or plaque* or arter* or heart* or athero*))) OR AB ((FAI N3 (Scor* or Index* or indic* or measure* or map* or coronary or plaque* or arter* or heart* or athero*))) 367
-
S5 TI ((Fat or PVAT or ‘perivascular adipose tissue’) N3 Attenuation*)) OR AB ((Fat or PVAT or ‘perivascular adipose tissue’) N3 Attenuation*)) 138
-
S4 (FAITM or pFAI) 4
-
S3 (CRD42020181158 or CRD42021229491 or CRD42021297228 or NCT05169333) 0
-
S2 (Caristo or CariCloud) 11
-
S1 (CaRi N3 heart) or CaRi-Heart or CaRiHeart) 1
KSR Evidence (https://ksrevidence.com/): up to 5 October 2022
Searched 5 October 2022
-
((CaRi Adj/3 heart) or CaRi-Heart or CaRiHeart) in All text 0 results
-
(Caristo or CariCloud) in All text 0 results
-
(CRD42020181158 or CRD42021229491 or CRD42021297228 or NCT05169333) in All text 2 results
-
((Fat or PVAT or ‘perivascular adipose tissue’) adj/3 Attenuation*) in All text 4 results
-
(FAI adj/3 (Scor* or Index* or indic* or measure* or map* or coronary or plaque* or arter* or heart* or athero*)) in All text 24 results
-
(FAITM or pFAI) in All text 0 results
-
#1 or #2 or #3 or #4 or #5 or #6 in All text 28 results
Epistemonikos (https://www.epistemonikos.org/): up to 6 October 2022
Searched 6 October 2022
-
Advanced search Limits: Systematic Review/ (advanced_title_en:(‘CaRi heart’ OR CaRi-Heart OR CaRiHeart) OR advanced_abstract_en:(‘CaRi heart’ OR CaRi-Heart OR CaRiHeart)) OR (advanced_title_en:(Caristo OR CariCloud) OR advanced_abstract_en:(Caristo OR CariCloud)) OR (advanced_title_en:(CRD42020181158 OR CRD42021229491 OR CRD42021297228 OR NCT05169333) OR advanced_abstract_en:(CRD42020181158 OR CRD42021229491 OR CRD42021297228 OR NCT05169333)) OR (advanced_title_en:(‘Fat attenuation’ OR ‘PVAT attenuation’ OR ‘perivascular adipose tissue attenuation’) OR advanced_abstract_en:(‘Fat attenuation’ OR ‘PVAT attenuation’ OR ‘perivascular adipose tissue attenuation’)) OR (advanced_title_en:((FAI AND (Scor* OR Index* OR indic* OR measure* OR map* OR coronary OR plaque* OR arter* OR heart* OR athero*))) OR advanced_abstract_en:((FAI AND (Scor* OR Index* OR indic* OR measure* OR map* OR coronary OR plaque* OR arter* OR heart* OR athero*)))) OR (advanced_title_en:(FAITM OR pFAI) OR advanced_abstract_en:(FAITM OR pFAI)) [Filters: classification = systematic-review, protocol = no]
Search retrieved 138 records
INAHTA (https://www.inahta.org/): up to 6 October 2022
Searched 6 October 2022
Advanced search
-
(‘CaRi heart’ OR CaRi-Heart OR CaRiHeart) OR (Caristo OR CariCloud) OR (CRD42020181158 OR CRD42021229491 OR CRD42021297228 OR NCT05169333) OR (‘Fat attenuation’ OR ‘PVAT attenuation’ OR ‘perivascular adipose tissue attenuation’) OR ((FAI AND (Scor* OR Index* OR indic* OR measure* OR map* OR coronary OR plaque* OR arter* OR heart* OR athero*))) OR (FAITM OR pFAI)
Search retrieved two records
NIHR HTA (Journals: www.journalslibrary.nihr.ac.uk/): up to 6 October 2022
Searched 6 October 2022
Simple search
| Search terms | Journal reports | Research projects |
|---|---|---|
| ‘CaRi-Heart’ | 0 | 0 |
| CaRiHeart | 0 | 0 |
| Caristo | 0 | 0 |
| CariCloud | 0 | 0 |
| ‘fat attenuation’ | 0 | 0 |
| Total | 0 | 0 |
Results = 0
PROSPERO (CRD): up to 5 October 2022
Searched 5 October 2022
-
‘CaRi heart’ or CaRi-Heart or CaRiHeart
-
(Caristo or CariCloud) 0
-
(CRD42020181158 or CRD42021229491 or CRD42021297228 or NCT05169333) 3
-
Fat or PVAT or ‘perivascular adipose tissue’ 3622
-
Attenuation* 196
-
#3 AND #4 37
-
FAI 203
-
Scor* or Index* or indic* or measure* or map* or coronary or plaque* or arter* or heart* or athero* 145,490
-
#6 AND #7 190
-
FAITM or pFAI 0
-
#1 OR #2 OR #5 OR #8 OR #9 OR #10 225
INPLASY (https://inplasy.com/): up to 6 October 2022
Searched 6 October 2022
| Keyword | Hits |
|---|---|
| ‘CaRi-Heart’ OR CaRiHeart OR Caristo OR CariCloud | 0 |
| ‘Fat attenuation’ OR ‘perivascular adipose tissue attenuation’ OR ‘PVAT attenuation’ | 0 |
| FAI OR FAITM OR pFAI | 0 |
| Total | 0 |
LILACS (http://regional.bvsalud.org/php/index.php?lang=en): up to 6 October 2022
Searched: 6 October 2022
(Limit = Not MEDLINE)
| Keywords | Hits |
|---|---|
| (‘CaRi heart’ OR CaRi-Heart OR CaRiHeart OR Caristo OR CariCloud OR CRD42020181158 OR CRD42021229491 OR CRD42021297228 OR NCT05169333) | 0 |
| ((‘Fat attenuation’ OR ‘PVAT attenuation’ OR ‘perivascular adipose tissue attenuation’)) | 1 |
| (FAI) AND ((coronary OR plaque* OR arter* OR heart* OR athero*)) | 8 |
| Results | 9 |
Retrieved 9 hits (LILACs only)
DOAJ (https://doaj.org/): up to 6 October 2022
Searched 6 October 2022
| Keywords | All fields |
|---|---|
| CaRi-Heart OR CaRiHeart OR Caristo OR CariCloud OR CRD42020181158 OR CRD42021229491 OR CRD42021297228 OR NCT05169333 OR ‘Fat attenuation’ OR ‘perivascular adipose tissue attenuation’ OR FAITM OR pFAI | 80 |
| Total | 80 |
ClinicalTrials.gov (https://clinicaltrials.gov/ct2/home): up to 6 October 2022
Searched 6 October 2022
| Expert search | Hits |
|---|---|
| ‘CaRi-Heart’ OR CaRiHeart OR Caristo OR CariCloud | 3 |
| ((Fat OR PVAT OR ‘perivascular adipose tissue’) AND (attenuation)) | 235 |
| NCT05169333 | 1 |
| (FAI AND (coronary OR plaque OR plaques OR artery OR Arteries OR heart OR atherosclerosis)) | 38 |
| Total | 277 |
EUCTR (www.clinicaltrialsregister.eu): up to 6 October 2022
Searched 6 October 2022
| Expert search | Hits |
|---|---|
| ‘CaRi-Heart’ OR CaRiHeart OR Caristo OR CariCloud | 0 |
| ((Fat OR PVAT OR ‘perivascular adipose tissue’) AND (attenuation)) | 12 (exports 25) |
| (FAI AND (coronary OR plaque* OR arter* OR heart* OR athero*)) | 5 (export 15) |
| Total | 17 (exports 40) |
WHO ICTRP (www.who.int/ictrp/search/en/): up to 6 October 2022
Searched 6 October 2022
| Simple search | Hits |
|---|---|
| ‘CaRi-Heart’ OR CaRiHeart OR Caristo OR CariCloud | 1 |
| ((Fat OR PVAT OR ‘perivascular adipose tissue’) AND (attenuation)) | 12 |
| (FAI AND (coronary OR plaque OR plaques OR artery OR Arteries OR heart OR atherosclerosis)) | 3 |
| Total | 16 |
ScanMedicine (https://scanmedicine.com/): up to 6 October 2022
Searched: 6 October 2022
| Keywords | Hits |
|---|---|
| ‘CaRi-Heart’ | CaRiHeart | Caristo | CariCloud | 2 |
| ‘Fat attenuation’ | ‘perivascular adipose tissue attenuation’ | ‘PVAT attenuation’ | 7 |
| FAI | FAITM | pFAI | 105 |
| Total | 114 |
Northern Light Life Sciences Conference Abstracts (Ovid): 2010–22/wk38
Searched 5 October 2022
-
((CaRi adj3 heart) or CaRi-Heart or CaRiHeart).af. (1)
-
(Caristo or CariCloud).af. (16)
-
(CRD42020181158 or CRD42021229491 or CRD42021297228 or NCT05169333).af. (0)
-
1 or 2 or 3 (17)
-
((Fat or PVAT or ‘perivascular adipose tissue’) adj2 Attenuation$).af. (42)
-
(FAI adj3 (Scor$ or Index$ or indic$ or measure$ or map$ or coronary or plaque$ or arter$ or heart$ or athero$)).af. (44)
-
(FAITM or pFAI).af. (2)
-
or/5-7 (86)
-
4 or 8 (103)
MedRxiv (medRxiv.org): up to 6 October 2022
Searched 6 October 2022
Advanced search
| Keywords | Hits |
|---|---|
| full text or abstract or title ‘CaRi-Heart CaRiHeart Caristo CariCloud CRD42020181158 CRD42021229491 CRD42021297228 NCT05169333’ (match whole any) | 3 |
| abstract or title ‘Fat attenuation’ (match all words) | 8 |
| abstract or title ‘perivascular adipose tissue attenuation’ (match all words) | 0 |
| ‘FAI FAITM pFAI’ (match any words) | 24 |
| abstract or title ‘PVAT attenuation’ (match all words) | 0 |
| Total | 35 |
Additional focused searches
Search (1) Statins and risk of CAD
| Database | Date span | Hits retrieved |
|---|---|---|
| EMBASE | 1974–24 October 2022 | 746 |
| KSR Evidence | up to 26 October 2022 | 45 |
| Total | 791 |
EMBASE < 1974–24 October 2022
Searched 26 October 2022
Statins + Risk of CAD + Symptomatic + RCTS No A
-
exp coronary artery disease/ or CAD.ti,ab,ot. (403,106)
-
(coronary artery adj3 (disease$ or syndrome$ or anomal$ or aneurysm or atherosclerosis or calcification or constriction or dissection or obstruction or occlusion or perforation or thrombosis)).ti,ab,ot. (174,891)
-
or/1-2 (438,155)
-
risk$.ti,ab,ot. (3,926,246)
-
3 and 4 (151,732)
-
exp hydroxymethylglutaryl coenzyme A reductase inhibitor/ or (statin$ or vastatin$).ti,ab,ot. (199,326)
-
((HMG or hydroxymethylglutaryl) adj2 (CoA or coenzyme A) adj2 (‘reductase inhibitor’ or ‘reductase inhibitors’)).ti,ab,ot. (6241)
-
(Atorvastatin or astator or ator or atorab or atoris or atorlip or atorvadivid or atorvastine or atostin or atovans or atovarol or cardyl or ci 981 or ci981 or glustar or lipibec or Lipitor or liprimar or liptonorm or lowlipen or obradon or orbeos or prevencor or sortis or statorva or storvas or tahor or torvast or totalip or xarator or ym 548 or ym548 or zarator or 110862-48-1 or 134523-00-5 or 134523-03-8).ti,ab,ot,tn,rn. (44,225)
-
(Simvastatin or avastinee or belmalip or cholestat or clinfar or colastatina or coledis or colemin or colestricon or corolin or covastin or denan or epistatin or esvat or ethical or eucor or flolipid or ifistatin or jabastatina or kavelor or klonastin or kolestevan or ‘l 644128’ or l644128 or labistatin or lipcut or lipecor or lipex or lipinorm or liponorm or lipovas or lodales or medipo or mersivas or ‘mk 0733’ or mk 733 or mk0733 or mk733 or nivelipol or nor-vastina or normofat or orovas or pantok or Rechol or rendapid or simbado or simcard or simchol or simovil or simtin or simva or simvac or Simvahex or simvalord or simvastar or simvastatina or simvastatine or simvata or simvatin or simvor or simvotin or sinvacor or simvastatin or sinvinolin or sivastin or starzoco or synvinolin or torio or valemia or vasilip or vasotenal or vazim or velostatin or vidastat or zimmex or zocor or zocord or zorced or zosta or zovast or 79902-63-9).ti,ab,ot,tn,rn. (171571)
-
(Rosuvastatin or coupet or crestor or epri or ezallor or ‘hgp 0816’ or hgp0816 or mertenil or provisacor or rostat or rosudia or rosumop or rosuvador or rosuvas or rosuvastatina or rosuvastatine or roswera or roxera or rozuva-teva or ‘s 4522’ or s4522 or simestat or sorvasta or visacor or xeter or zahron or zaranta or zd 4522 or zd4522 or 147098-18-8 or 147098-20-2 or 287714-41-4).ti, ab,ot,tn,rn. (18,972)
-
or/6–10 (330,511)
-
symptomatic.ti,ab,ot,hw. (321,504)
-
stable angina pectoris/ or (stable adj2 angina$).ti,ab,ot,hw. (18,774)
-
secondary prevention/ or (‘secondary prevention’ or ‘secondary preventions’).ti,ab,ot,hw. (46,952)
-
computed tomographic angiography/ or ((computed tomographic or CT) adj2 angiograph$).ti,ab,ot. (87,721)
-
thorax pain/ or ((chest or thorax or thoracic) adj3 (discomfort or pain$ or ache$ or ‘abnormal feeling’)).ti,ab,ot,hw. (131,468)
-
or/12–16 (584,720)
-
5 and 11 and 17 (4165)
-
crossover-procedure/or double-blind procedure/or randomized controlled trial/or single-blind procedure/ (812,505)
-
(random$ or factorial$ or crossover$ or cross over$ or cross-over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).ti,ab,ot. (2,641,780)
-
19 or 20 (2,750,001)
-
animal/or animal experiment/ (4,459,556)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (7,369,171)
-
22 or 23 (7,369,171)
-
exp human/or human experiment/ (24,240,337)
-
24 not (24 and 25) (5,560,969)
-
21 not 26 (2,472,774)
-
18 and 27 (746)
KSR Evidence: up to 26 October 2022
Searched 26 October 2022
-
CAD in Title or Abstract 743 results
-
(coronary artery adj3 (disease* or syndrome* or anomal* or aneurysm or atherosclerosis or calcification or constriction or dissection or obstruction or occlusion or perforation or thrombosis)) in All text 1974 results
-
#1 or #2 in All text 2138 results
-
risk* in Title or Abstract 90,582 results
-
#3 and #4 in All text 1300 results
-
(statin* or vastatin*) in All text 1384 results
-
((HMG or hydroxymethylglutaryl) adj2 (CoA or coenzyme A) adj2 (‘reductase inhibitor’ or ‘reductase inhibitors’)) in All text 237 results
-
(Atorvastatin or astator or ator or atorab or atoris or atorlip or atorvadivid or atorvastine or atostin or atovans or atovarol or cardyl or ci 981 or ci981 or glustar or lipibec or Lipitor or liprimar or liptonorm or lowlipen or obradon or orbeos or prevencor or sortis or statorva or storvas or tahor or torvast or totalip or xarator or ym 548 or ym548 or zarator) in All text 301 results
-
9 (Simvastatin or avastinee or belmalip or cholestat or clinfar or colastatina or coledis or colemin or colestricon or corolin or covastin or denan or epistatin or esvat or ethical or eucor or flolipid or ifistatin or jabastatina or kavelor or klonastin or kolestevan or ‘l 644128’ or l644128 or labistatin or lipcut or lipecor or lipex or lipinorm or liponorm or lipovas or lodales or medipo or mersivas or ‘mk 0733’ or mk 733 or mk0733 or mk733 or nivelipol or nor-vastina or normofat or orovas or pantok or Rechol or rendapid or simbado or simcard or simchol or simovil or simtin or simva or simvac or Simvahex or simvalord or simvastar or simvastatina or simvastatine or simvata or simvatin or simvor or simvotin or sinvacor or simvastatin or sinvinolin or sivastin or starzoco or synvinolin or torio or valemia or vasilip or vasotenal or vazim or velostatin or vidastat or zimmex or zocor or zocord or zorced or zosta or zovast) in All text 21,697 results
-
10 (Rosuvastatin or coupet or crestor or epri or ezallor or ‘hgp 0816’ or hgp0816 or mertenil or provisacor or rostat or rosudia or rosumop or rosuvador or rosuvas or rosuvastatina or rosuvastatine or roswera or roxera or rozuva-teva or ‘s 4522’ or s4522 or simestat or sorvasta or visacor or xeter or zahron or zaranta or zd 4522 or zd4522) in All text 35,033 results
-
#6 or #7 or #8 or #9 or #10 in All text 52,705 results
-
symptomatic in All text 3969 results
-
(stable adj2 angina*) in All text 98 results
-
(‘secondary prevention’ or ‘secondary preventions’) in All text 828 results
-
((computed tomographic or CT) adj2 angiograph*) in All text 118 results
-
((chest or thorax or thoracic) adj3 (discomfort or pain* or ache* or ‘abnormal feeling’)) in All text 519 results
-
in All text 0 results
-
#12 or #13 or #14 or #15 or #16 in All text 5451 results
-
#5 and #11 and #18 in All text 45 results
Search (2) Major adverse cardiac events and utilities
| Database | Date span | Hits retrieved |
|---|---|---|
| KSR Evidence | up to 26 October 2022 | 282 |
| Total | 282 |
KSR Evidence: up to 24 October 2022
Searched 24 October 2022
(MACE/4named + Focused HRQoL filter)
-
MACE or ‘major adverse cardiac event’ or ‘major adverse cardiac events’ in All text 1184 results
-
stroke or strokes or apoplexia or apoplexy or ‘insultus cerebralis’ in All text 9081 results
-
((brain or cerebral or cerebrum) Adj/3 (accident* or attack* or insult* or insufficiency)) in All text 140 results
-
(cerebrovascular Adj/3 (arrest* or failure* or injury* or insult*)) in All text 100 results
-
CVA in Title or Abstract 92 results
-
(ischaemic or ischemic) Adj/2 seizure in All text 4 results
-
((cardiac or heart or cardial or myocardial or myocardium or subendocardial) Adj/3 (infarct* or attack)) in All text 4301 results
-
angina or anginal or stenocardia in All text 608 results
-
revascularisation or revascularization in All text 1760 results
-
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 in All text 12,451 results
-
sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D in All text 110 results
-
Quality adjusted life or Quality-adjusted-life in All text 1216 results
-
euroqol or euro qol or eq5d* or eq 5d in All text 465 results
-
QALY* or DALY* or HALY* or YHL or HYES or YPLL or YHLL or qald* or qale* or qtime* or AQoL* in All text 703 results
-
timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble* or ‘willingness to pay’ in All text 315 results
-
HSUV* or health state* value* or health state* preference* or HSPV* in All text 2511 results
-
(utilit* Adj/3 (‘quality of life’ or valu* or scor* or measur* or health or life or estimat* or elicit* or disease*)) in All text 555 results
-
#11 or #12 or #13 or #14 or #15 or #16 or #17 in All text 4668 results
-
#10 and #18 in All text 282 results
Search (3) Colchicine and CAD
| Database | Date span | Hits retrieved |
|---|---|---|
| EMBASE | 1974–24 October 2022 | 918 |
| KSR Evidence | up to 26 October 2022 | 45 |
| Total | 791 |
EMBASE (Ovid): 1974–24 October 2022
Searched 25 October 2022
-
Colchicine/or (aqua colchin or colchichine or colchicine or colchicine or colchicum-dispert or colchily or colchimedio or colchiquim or colchisol or colchysat or colcine or colcrys or colctab or colgout or colrefuz or gloperba or goutichine or goutnil or kolkicin or kolkisin or mitigare or ‘mpc 004’ or mpc004 or myinfla or nsc 757 or tolchicine or 64-86-8).ti,ab,ot,hw,tn,rn. 38,959
-
exp coronary artery disease/or CAD.ti,ab,ot. 403,106
-
(coronary artery adj3 (disease$ or syndrome$ or anomal$ or aneurysm or atherosclerosis or calcification or constriction or dissection or obstruction or occlusion or perforation or thrombosis)).ti,ab,ot. 174,891
-
or/2-3 438,155
-
1 and 4 918
KSR Evidence (https://ksrevidence.com/): up to 25 October 2022
Searched: 25 October 2022
-
‘aqua colchin’ or colchichine or colchicine or colchicine or ‘colchicum-dispert’ or colchily or colchimedio or colchiquim or colchisol or colchysat or colcine or colcrys or colctab or colgout or colrefuz or gloperba or goutichine or goutnil or kolkicin or kolkisin or mitigare or ‘mpc 004’ or mpc004 or myinfla or ‘nsc 757’ or tolchicine in All text 192 results
-
CAD in All text 822 results
-
‘coronary artery’ adj3 (disease* or syndrome* or anomal* or aneurysm or atherosclerosis or calcification or constriction or dissection or obstruction or occlusion or perforation or thrombosis) in All text 1906 results
-
#2 or #3 in All text 2136 results
-
#1 and #4 in All text 37 results
Search (4) Heart failure and utilities
| Database | Date span | Hits retrieved |
|---|---|---|
| KSR Evidence | up to 15 November 2022 | 77 |
| Total | 77 |
KSR Evidence: up to 15 November 2022
Searched 15 November 2022
(Heart failure + Focused HRQoL filter)
# Query Results
-
((Heart or cardiac or cardial or myocardial or cordis or cardis) adj3 (failure* or decompensat* or incompetence or insufficien* or ‘stand still’)) in All text 4002 results
-
(HF or CHF) in Title or Abstract 1267 results
-
#1 or #2 in All text 4175 results
-
sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D in All text 112 results
-
Quality adjusted life or Quality-adjusted-life in All text 1223 results
-
euroqol or euro qol or eq5d* or eq 5d in All text 467 results
-
QALY* or DALY* or HALY* or YHL or HYES or YPLL or YHLL or qald* or qale* or qtime* or AQoL* in All text 711 results
-
timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble* or ‘willingness to pay’ in All text 319 results
-
HSUV* or health state* value* or health state* preference* or HSPV* in All text 2531 results
-
(utilit* Adj/3 (‘quality of life’ or valu* or scor* or measur* or health or life or estimat* or elicit* or disease*)) in All text 558 results
-
#4 or #5 or #6 or #7 or #8 or #9 or #10 in All text 4707 results
-
#3 and #11 in All text 111 results
Appendix 2 Details of excluded studies with rationale
To be included in the review, studies had to fulfil the following criteria:
| Research Question 1: | ‘What is the prognostic performance of CaRi-Heart®, in people with stable chest pain, who are undergoing CTCA, where:
|
| Population: | People undergoing CTCA for the investigation of stable chest pain/suspected CAD |
| Setting: | Secondary or tertiary care |
| Intervention: | CaRi-Heart |
| Comparator: | Current standard of care, for cardiac risk assessment |
| Outcomes: | Primary outcomes: |
|
|
| Study design: | Prediction model development and validation studies |
| Research Question 2: | ‘What is the prevalence of “low”, “medium” and “high” CaRi-Heart® Risk?’ |
| Population: | People undergoing CTCA for the investigation of stable chest pain/suspected CAD |
| Setting: | Secondary or tertiary care |
| Intervention: | CaRi-Heart |
| Comparator: | Not applicable |
| Outcomes: | Number (%) of patients undergoing CTCA who are classified as ‘low’, ‘medium’ and ‘high’ CaRi-Heart Risk and, if reported, number of cases (cardiac events) in each risk category |
| Study design: | RCTs, CCTs and comparative or non-comparative observational studies |
| Research Question 3: | ‘What are the clinical effects of using CaRi-Heart® to assess cardiac risk?’ |
| Population: | People undergoing CTCA for the investigation of stable chest pain/suspected CAD |
| Setting: | Secondary or tertiary care |
| Intervention: | CaRi-Heart |
| Comparator: | Current standard of care, which is CTCA without the addition of CaRi-Heart, alongside clinical risk assessment and patient-appropriate risk factor management |
| Outcomes: | Primary outcomes: |
| Cardiac mortality, MACE, HRQoL | |
| Secondary outcomes: | |
|
|
| Study design: | RCTs, CCTs or observational before and after (implementation) studies |
| Research Question 4: | ‘What are the costs, from a UK NHS and Personal Social Services perspective, using CaRi-Heart® for assessment of cardiac risk?’ |
| Population: | People undergoing CTCA for the investigation of stable chest pain/suspected CAD |
| Setting: | Secondary or tertiary care |
| Intervention: | CaRi-Heart |
| Comparator: | Current standard of care, which is CTCA without the addition of CaRi-Heart, alongside clinical risk assessment and patient-appropriate risk factor management |
| Outcomes: | Costs of CaRi-Heart testing (including test cost, time to interpret results, and staff training/implementation costs) |
| Study design: | RCTs, CCTs, comparative or non-comparative observational studies and cost-effectiveness analyses |
Table 14 summarises studies which were screened for inclusion based on full-text publication, but which failed to fulfil all inclusion criteria, for any research question.
| Author | Principal exclusion reason(s) |
|---|---|
| Abbasi 201721 | Review article |
| Antoniades 201922 | Review article |
| Antoniades 201923 | Editorial |
| Antoniades 202024 | Review article |
| Antonopoulos 202225 | Systematic review: intervention was not CaRi-Heart (FAI only) |
| Antonopoulos 202226 | Review article |
| Antonopoulos 202027 | Systematic review: intervention was not CaRi-Heart (FAI only) |
| Antonopoulos 201928 | Letter |
| Antonopoulos 201729 | Intervention was not CaRi-Heart (FAI only) |
| Bao 202230 | Intervention was not CaRi-Heart; population was patients with psoriasis |
| Bengs 202131 | Intervention was not CaRi-Heart (FAI only) |
| Berman 202232 | Editorial |
| Bittner 201933 | Intervention was not CaRi-Heart (FAI only) |
| Cabrelle 202234 | Intervention was not CaRi-Heart (FAI only) |
| Cecere 202135 | Intervention was not CaRi-Heart (FAI only) |
| Chatterjee 202236 | Intervention was not CaRi-Heart (FAI only) |
| Chatterjee 202137 | Intervention was not CaRi-Heart (FAI only) |
| Chen 202138 | Intervention was not CaRi-Heart (FAI only) |
| Dai 202239 | Intervention was not CaRi-Heart (FAI only) |
| Dai 202040 | Intervention was not CaRi-Heart (FAI only) |
| Dang 202141 | Intervention was not CaRi-Heart (FAI only) |
| Elnabawi 201942 | Intervention was not CaRi-Heart; population was patients with psoriasis |
| Gaibazzi 202143 | Intervention was not CaRi-Heart; population was patients with aortic aneurysm |
| Hoshino 202144 | Intervention was not CaRi-Heart |
| Hoshino 202145 | Intervention was not CaRi-Heart (FAI only) |
| Hoshino 202046 | Intervention was not CaRi-Heart (FAI only) |
| Kanaji 201947 | Intervention was not CaRi-Heart (FAI only) |
| Kato 202248 | Systematic review: intervention was not CaRi-Heart |
| Li 202149 | Intervention was not CaRi-Heart (FAI only) |
| Liu 202050 | Review |
| Montonati 202251 | Intervention was not CaRi-Heart (FAI only) |
| Oikonomou 202053 | Letter |
| Oikonomou 201954 | Intervention was not CaRi-Heart (FAI only) |
| Oikonomou 201955 | Intervention was not CaRi-Heart (FAI only) |
| Oikonomou 201856 | Intervention was not CaRi-Heart (FAI only) |
| Oikonomou 201810 | Intervention was not CaRi-Heart (FAI only) |
| Pandey 202057 | Intervention was not CaRi-Heart |
| Pergola 202258 | Intervention was not CaRi-Heart (FAI only) |
| Pergola 202159 | Intervention was not CaRi-Heart (FAI only) |
| Placket 202060 | News article/comment |
| Sagris 202161 | Intervention was not CaRi-Heart (FAI only) |
| Sen 202062 | Intervention was not CaRi-Heart (FAI only) |
| Shan 202163 | Intervention was not CaRi-Heart (FAI only) |
| Simantiris 202164 | Intervention was not CaRi-Heart (FAI only) |
| Sugiyama 202065 | Intervention was not CaRi-Heart (FAI only) |
| Sutanto 202066 | Intervention was not CaRi-Heart; population was patients with aortic aneurysm |
| Yan 202267 | Intervention was not CaRi-Heart (FAI only) |
| Zhu 202168 | Intervention was not CaRi-Heart |
Appendix 3 Relevant ongoing studies
Our rapid review searches (described in the section Search strategy) and scoping searches, undertaken by NICE, identified one ongoing trial, NCT05169333,69 the ORFAN study. This is a UK prospective, multicentre, multiethnic cohort observational study collecting CT scans, biological material and outcomes data. The study will combine imaging data with patient demographics and clinical information to aid the development and/or validation of new or existing image analysis algorithms and software tools to improve diagnosis, clinical risk discrimination and prediction. The study will recruit 15,500 participants who have been asked to undergo a CTCA by their clinical team or who have had a CTCA in the previous 6 months. The study will also retrospectively collect a data set of 250,000 cardiac, abdomen and pelvis CT scans. Prospectively recruited participants will be followed up for 15 years and the anticipated completion date is February 2030. 1
The NICE request, to the company, for information76 included the following question and response:
‘Can you please provide a list of any ongoing studies relevant to CaRi-Heart® including details such as study descriptions, study populations, outcomes, expected completion dates, etc.?
As mentioned, ongoing health economic work is currently being undertaken by the Department of Epidemiology and Public Health at the University of Oxford to evaluate the cost-effectiveness and impact of CaRi-Heart on healthcare pathways and outcomes. Part of this work is a component of Caristo’s NHS AI Stage 3 Award. A protocol for the health economic work will also be shared with NICE shortly. The results are expected in Q1 2023.
A component of this work is being undertaken as part of Caristo’s NHS AI Stage 3 award, including a model-based early economic evaluation alongside the implementation to provide the potential cost-effectiveness of adding CaRi-Heart® to conventional CTCA analysis. We will compare data from the implementation sites with data from a large registry study linking CaRi-Heart® with the risk of fatal and non-fatal CTCA events, in patients who have had a clinically indicated CTCA. A protocol for this study will be shared with NICE shortly. In brief, we intend to collect data from 800 patients, and the analysis is to be finished in Q1 2023.
Data collected from each site will include:
-
Clinical presentation of patients referred for CTCA, enabling mapping of the referral patient pool for CaRi-Heart® analysis in the NHS;
-
Patient risk reclassification, to model the cost of the change in the patient’s medication to the NHS and to model the total effect size of CaRi-Heart® analysis on downstream events and costs to the NHS;
-
Costs to the NHS of adding CaRi-Heart® to CTCA, including cardiologists’ time in training to interpret CaRi-Heart® analyses, and the implementation costs per CTCA (if any) added to the price of a CaRi-Heart® analysis to estimate the total cost per CTCA of introducing CaRi-Heart® into the NHS.
Patients from the sites will be matched with patients from an existing CTCA registry (https://oxhvf.com/the-orfan-study/) using PSM, as recommended in the MRC guidelines on performing natural or quasi-experimental studies. A range of PSM techniques will be compared based on Rubin’s rules, and the one that achieves the best covariate balance will be chosen. Incremental cost-effectiveness ratios will be expressed as cost per life-year gained. Bootstrapping with replacement will be used to construct cost-effectiveness planes in order to display uncertainty around the ICERs. The probability of CaRi-Heart® to be cost-effective at different willingness-to-pay values of a life year will be displayed on cost-effectiveness acceptability curves. Heterogeneity will be explored in subgroup analysis based on the different pathways where CaRi-Heart® will be implemented (e.g. stable chest pain vs. acute setting). This early health economic evaluation of CaRi-Heart® will be used to inform the design of larger studies. We will follow NICE guidance and estimate the expected value of perfect information (EVPI). This value represents the monetary value of eliminating the uncertainty in the cost–utility results. In other words, it provides decision-makers with the value of acquiring further information on costs and outcomes for a number of people who may benefit from the additional research. EVPI can potentially be used to set research priorities’.
The cost-effectiveness modelling, described above, is being undertaken by the Nuffield Department of Population Health at the University of Oxford; this work is led by Apostolos Tsiachristas, who is also a co-author on this report and has contributed to discussions of conceptual modelling. The anticipated completion date for this work is March 2023.
Appendix 4 ROBIS evaluations
Kato (2022) 48
DOMAIN 1: STUDY ELIGIBILITY CRITERIA
| Signalling question | Rating |
|---|---|
| 1.1 Did the review adhere to predefined objectives and eligibility criteria? | N |
| 1.2 Were the eligibility criteria appropriate for the review question? | N |
| 1.3 Were eligibility criteria unambiguous? | N |
| 1.4 Were all restrictions in eligibility criteria based on study characteristics appropriate? | N |
| 1.5 Were any restrictions in eligibility criteria based on sources of information appropriate? | N |
| Concerns regarding specification of study eligibility criteria | HIGH |
| Rationale for concern | No pre hoc protocol presented or described |
DOMAIN 2: IDENTIFICATION AND SELECTION OF STUDIES
| Signalling question | Rating |
|---|---|
| 2.1 Did the search include an appropriate range of databases/electronic sources for published and unpublished reports? | Y |
| 2.2 Were methods additional to database searching used to identify relevant reports? | NI |
| 2.3 Were the terms and structure of the search strategy likely to retrieve as many eligible studies as possible? | PY |
| 2.4 Were restrictions based on date, publication format or language appropriate? | NI |
| 2.5 Were efforts made to minimise errors in selection of studies? | NI |
| Concerns regarding identification and selection of studies | HIGH |
| Rationale for concern | No additional searching methods used, such as perusal of reference lists. No reference to any restrictions on dates, etc. |
DOMAIN 3: DATA COLLECTION AND STUDY APPRAISAL
| Signalling question | Rating |
|---|---|
| 3.1 Were efforts made to minimise error in data collection? | NI |
| 3.2 Were sufficient study characteristics available for both review authors and readers to be able to interpret the results? | Y |
| 3.3 Were all relevant study results collected for use in the synthesis? | Y |
| 3.4 Was risk of bias (or methodological quality) formally assessed using appropriate criteria? | NI |
| 3.5 Were efforts made to minimise error in risk-of-bias assessment? | NI |
| Concerns regarding data collection and study appraisal | HIGH |
| Rationale for concern | No information was provided on methodology of extracting or analysing data |
DOMAIN 4: SYNTHESIS AND FINDINGS
| Signalling question | Rating |
|---|---|
| 4.1 Did the synthesis include all studies that it should? | PN |
| 4.2 Were all predefined analyses followed or departures explained? | NI |
| 4.3 Was the synthesis appropriate given the nature and similarity in the research questions, study designs and outcomes across included studies? | NI |
| 4.4 Was between-studies variation (heterogeneity) minimal or addressed in the synthesis? | NI |
| 4.5 Were the findings robust, for example as demonstrated through funnel plot or sensitivity analyses? | NI |
| 4.6 Were biases in primary studies minimal or addressed in the synthesis? | NI |
| Concerns regarding data collection and study appraisal | HIGH |
| Rationale for concern | In the absence of a clear pre hoc protocol, it is difficult to be sure that decisions on inclusion and exclusion were made prior to knowledge of the data revealed in the sourced papers |
OVERALL RATING – HIGH RISK OF BIAS
| Signalling question | Rating |
|---|---|
| A. Did the interpretation of findings address all of the concerns identified in the Phase 2 assessment? | No – high risk of bias |
| B. Was the relevance of identified studies to the review’s research question appropriately considered? | Y |
| C. Did the reviewers avoid emphasising results on the basis of their statistical significance? | Y |
Antonopoulos (2022) 25
DOMAIN 1: STUDY ELIGIBILITY CRITERIA
| Signalling question | Rating |
|---|---|
| 1.1 Did the review adhere to predefined objectives and eligibility criteria? | PY |
| 1.2 Were the eligibility criteria appropriate for the review question? | PY |
| 1.3 Were eligibility criteria unambiguous? | Y |
| 1.4 Were all restrictions in eligibility criteria based on study characteristics appropriate? | PY |
| 1.5 Were any restrictions in eligibility criteria based on sources of information appropriate? | PY |
| Concerns regarding specification of study eligibility criteria | LOW |
| Rationale for concern | No major concerns |
DOMAIN 2: IDENTIFICATION AND SELECTION OF STUDIES
| Signalling question | Rating |
|---|---|
| 2.1 Did the search include an appropriate range of databases/electronic sources for published and unpublished reports? | N – MEDLNE only |
| 2.2 Were methods additional to database searching used to identify relevant reports? | NI |
| 2.3 Were the terms and structure of the search strategy likely to retrieve as many eligible studies as possible? | PY |
| 2.4 Were restrictions based on date, publication format or language appropriate? | PY |
| 2.5 Were efforts made to minimise errors in selection of studies? | NI |
| Concerns regarding identification and selection of studies | HIGH |
| Rationale for concern | MEDLINE only searched (via PubMed) |
DOMAIN 3: DATA COLLECTION AND STUDY APPRAISAL
| Signalling question | Rating |
|---|---|
| 3.1 Were efforts made to minimise error in data collection? | PY – two reviewers made final selection, but no information on how this was done |
| 3.2 Were sufficient study characteristics available for both review authors and readers to be able to interpret the results? | N |
| 3.3 Were all relevant study results collected for use in the synthesis? | NI |
| 3.4 Was risk of bias (or methodological quality) formally assessed using appropriate criteria? | Y – QUality In Prognostic Studies (QUIPS) tool |
| 3.5 Were efforts made to minimise error in risk-of-bias assessment? | NI |
| Concerns regarding data collection and study appraisal | HIGH |
| Rationale for concern | No information was provided on patient characteristics |
DOMAIN 4: SYNTHESIS AND FINDINGS
| Signalling question | Rating |
|---|---|
| 4.1 Did the synthesis include all studies that it should? | PN |
| 4.2 Were all predefined analyses followed or departures explained? | PY |
| 4.3 Was the synthesis appropriate given the nature and similarity in the research questions, study designs and outcomes across included studies? | PY |
| 4.4 Was between-studies variation (heterogeneity) minimal or addressed in the synthesis? | Y – RE model used |
| 4.5 Were the findings robust, for example as demonstrated through funnel plot or sensitivity analyses? | NI |
| 4.6 Were biases in primary studies minimal or addressed in the synthesis? | PY |
| Concerns regarding data collection and study appraisal | HIGH |
| Rationale for concern | In the absence of a clear pre hoc protocol, it is difficult to be sure that decisions on inclusion and exclusion were made prior to knowledge of the data revealed in the sourced papers |
OVERALL RATING – HIGH RISK OF BIAS
| Signalling question | Rating |
|---|---|
| A. Did the interpretation of findings address all of the concerns identified in the Phase 2 assessment? | No – high risk of bias |
| B. Was the relevance of identified studies to the review’s research question appropriately considered? | Y |
| C. Did the reviewers avoid emphasising results on the basis of their statistical significance? | Y |
Bytyci (2022) 79
DOMAIN 1: STUDY ELIGIBILITY CRITERIA
| Signalling question | Rating guidance |
|---|---|
| 1.1 Did the review adhere to predefined objectives and eligibility criteria? | PY |
| 1.2 Were the eligibility criteria appropriate for the review question? | Y |
| 1.3 Were eligibility criteria unambiguous? | PN |
| 1.4 Were all restrictions in eligibility criteria based on study characteristics appropriate? | PY |
| 1.5 Were any restrictions in eligibility criteria based on sources of information appropriate? | NI |
| Concerns regarding specification of study eligibility criteria | LOW |
| Rationale for concern | No major concerns |
DOMAIN 2: IDENTIFICATION AND SELECTION OF STUDIES
| Signalling question | Rating guidance |
|---|---|
| 2.1 Did the search include an appropriate range of databases/electronic sources for published and unpublished reports? | PY |
| 2.2 Were methods additional to database searching used to identify relevant reports? | Y |
| 2.3 Were the terms and structure of the search strategy likely to retrieve as many eligible studies as possible? | PY |
| 2.4 Were restrictions based on date, publication format or language appropriate? | PY |
| 2.5 Were efforts made to minimise errors in selection of studies? | PY |
| Concerns regarding identification and selection of studies | LOW |
| Rationale for concern | No major concerns |
DOMAIN 3: DATA COLLECTION AND STUDY APPRAISAL
| Signalling question | Rating |
|---|---|
| 3.1 Were efforts made to minimise error in data collection? | Y |
| 3.2 Were sufficient study characteristics available for both review authors and readers to be able to interpret the results? | Y |
| 3.3 Were all relevant study results collected for use in the synthesis? | PY |
| 3.4 Was risk of bias (or methodological quality) formally assessed using appropriate criteria? | Y |
| 3.5 Were efforts made to minimise error in risk-of-bias assessment? | Y |
| Concerns regarding data collection and study appraisal | LOW |
| Rationale for concern | No major concerns |
DOMAIN 4: SYNTHESIS AND FINDINGS
| Signalling question | Rating |
|---|---|
| 4.1 Did the synthesis include all studies that it should? | PY |
| 4.2 Were all predefined analyses followed or departures explained? | PY |
| 4.3 Was the synthesis appropriate given the nature and similarity in the research questions, study designs and outcomes across included studies? | PY – but not for inflammatory markers |
| 4.4 Was between-studies variation (heterogeneity) minimal or addressed in the synthesis? | Y – RE model used |
| 4.5 Were the findings robust, e.g. as demonstrated through funnel plot or sensitivity analyses? | N |
| 4.6 Were biases in primary studies minimal or addressed in the synthesis? | NI |
| Concerns regarding data collection and study appraisal | UNCLEAR |
| Rationale for concern | No comment on ROB in included studies |
OVERALL RATING – HIGH RISK OF BIAS
| Signalling question | Rating guidance |
|---|---|
| A. Did the interpretation of findings address all of the concerns identified in the Phase 2 assessment? | PY |
| B. Was the relevance of identified studies to the review’s research question appropriately considered? | Y |
| C. Did the reviewers avoid emphasising results on the basis of their statistical significance? | Y |
Fulcher (2015) 106
DOMAIN 1: STUDY ELIGIBILITY CRITERIA
| Signalling question | Rating |
|---|---|
| 1.1 Did the review adhere to predefined objectives and eligibility criteria? | PN – very vague inclusion criteria only given |
| 1.2 Were the eligibility criteria appropriate for the review question? | N |
| 1.3 Were eligibility criteria unambiguous? | N |
| 1.4 Were all restrictions in eligibility criteria based on study characteristics appropriate? | NI |
| 1.5 Were any restrictions in eligibility criteria based on sources of information appropriate? | NI |
| Concerns regarding specification of study eligibility criteria | HIGH |
| Rationale for concern | Very vague protocol |
DOMAIN 2: IDENTIFICATION AND SELECTION OF STUDIES
| Signalling question | Rating |
|---|---|
| 2.1 Did the search include an appropriate range of databases/electronic sources for published and unpublished reports? | PN |
| 2.2 Were methods additional to database searching used to identify relevant reports? | NI |
| 2.3 Were the terms and structure of the search strategy likely to retrieve as many eligible studies as possible? | PN |
| 2.4 Were restrictions based on date, publication format or language appropriate? | NI |
| 2.5 Were efforts made to minimise errors in selection of studies? | PN |
| Concerns regarding identification and selection of studies | HIGH |
| Rationale for concern | No information on databases or methodology |
DOMAIN 3: DATA COLLECTION AND STUDY APPRAISAL
| Signalling question | Rating |
|---|---|
| 3.1 Were efforts made to minimise error in data collection? | NI |
| 3.2 Were sufficient study characteristics available for both review authors and readers to be able to interpret the results? | PY |
| 3.3 Were all relevant study results collected for use in the synthesis? | PN |
| 3.4 Was risk of bias (or methodological quality) formally assessed using appropriate criteria? | NI |
| 3.5 Were efforts made to minimise error in risk-of-bias assessment? | NI |
| Concerns regarding data collection and study appraisal | HIGH |
| Rationale for concern | No information provided on methodology of extracting or analysing data |
DOMAIN 4: SYNTHESIS AND FINDINGS
| Signalling question | Rating |
|---|---|
| 4.1 Did the synthesis include all studies that it should? | PN |
| 4.2 Were all predefined analyses followed or departures explained? | NI |
| 4.3 Was the synthesis appropriate given the nature and similarity in the research questions, study designs and outcomes across included studies? | NI |
| 4.4 Was between-studies variation (heterogeneity) minimal or addressed in the synthesis? | NI |
| 4.5 Were the findings robust, for example as demonstrated through funnel plot or sensitivity analyses? | NI |
| 4.6 Were biases in primary studies minimal or addressed in the synthesis? | NI |
| Concerns regarding data collection and study appraisal | HIGH |
| Rationale for concern | In the absence of a clear pre hoc protocol, it is difficult to be sure that decisions on inclusion and exclusion were made prior to knowledge of the data revealed in the sourced papers |
OVERALL RATING – HIGH RISK OF BIAS
| Signalling question | Rating |
|---|---|
| A. Did the interpretation of findings address all of the concerns identified in the Phase 2 assessment? | No – high risk of bias |
| B. Was the relevance of identified studies to the review’s research question appropriately considered? | N/A – no research question. High risk of bias |
| C. Did the reviewers avoid emphasising results on the basis of their statistical significance? | Y |
Mihaylova (2012) 107
DOMAIN 1: STUDY ELIGIBILITY CRITERIA
| Signalling question | Rating |
|---|---|
| 1.1 Did the review adhere to predefined objectives and eligibility criteria? | PN – very vague inclusion criteria only given |
| 1.2 Were the eligibility criteria appropriate for the review question? | N |
| 1.3 Were eligibility criteria unambiguous? | N |
| 1.4 Were all restrictions in eligibility criteria based on study characteristics appropriate? | NI |
| 1.5 Were any restrictions in eligibility criteria based on sources of information appropriate? | NI |
| Concerns regarding specification of study eligibility criteria | HIGH |
| Rationale for concern | Very vague protocol |
DOMAIN 2: IDENTIFICATION AND SELECTION OF STUDIES
| Signalling question | Rating |
|---|---|
| 2.1 Did the search include an appropriate range of databases/electronic sources for published and unpublished reports? | PN |
| 2.2 Were methods additional to database searching used to identify relevant reports? | NI |
| 2.3 Were the terms and structure of the search strategy likely to retrieve as many eligible studies as possible? | PN |
| 2.4 Were restrictions based on date, publication format or language appropriate? | NI |
| 2.5 Were efforts made to minimise errors in selection of studies? | PN |
| Concerns regarding identification and selection of studies | HIGH |
| Rationale for concern | No information on databases or methodology |
DOMAIN 3: DATA COLLECTION AND STUDY APPRAISAL
| Signalling question | Rating |
|---|---|
| 3.1 Were efforts made to minimise error in data collection? | NI |
| 3.2 Were sufficient study characteristics available for both review authors and readers to be able to interpret the results? | PY |
| 3.3 Were all relevant study results collected for use in the synthesis? | PN |
| 3.4 Was risk of bias (or methodological quality) formally assessed using appropriate criteria? | NI |
| 3.5 Were efforts made to minimise error in risk-of-bias assessment? | NI |
| Concerns regarding data collection and study appraisal | HIGH |
| Rationale for concern | No information provided on methodology of extracting or analysing data |
DOMAIN 4: SYNTHESIS AND FINDINGS
| Signalling question | Rating |
|---|---|
| 4.1 Did the synthesis include all studies that it should? | PN |
| 4.2 Were all predefined analyses followed or departures explained? | NI |
| 4.3 Was the synthesis appropriate given the nature and similarity in the research questions, study designs and outcomes across included studies? | NI |
| 4.4 Was between-studies variation (heterogeneity) minimal or addressed in the synthesis? | NI |
| 4.5 Were the findings robust, for example as demonstrated through funnel plot or sensitivity analyses? | NI |
| 4.6 Were biases in primary studies minimal or addressed in the synthesis? | NI |
| Concerns regarding data collection and study appraisal | HIGH |
| Rationale for concern | In the absence of a clear pre hoc protocol, it is difficult to be sure that decisions on inclusion and exclusion were made prior to knowledge of the data revealed in the sourced papers |
OVERALL RATING – HIGH RISK OF BIAS
| Signalling question | Rating |
|---|---|
| A. Did the interpretation of findings address all of the concerns identified in the Phase 2 assessment? | No – high risk of bias |
| B. Was the relevance of identified studies to the review’s research question appropriately considered? | N/A – no research question. High risk of bias |
| C. Did the reviewers avoid emphasising results on the basis of their statistical significance? | Y |
Appendix 5 QUality In Prognostic Studies evaluations
QUPIS tool evaluation for Chatterjee (2022) 36
| Domain | Description | Rating |
|---|---|---|
| Study participation | The relationship between the PF and outcome is unlikely to be different for participants and eligible non-participants | Low risk of bias |
| Study attrition | The relationship between the PF and outcome is unlikely to be different for completing and non-completing participants | Low risk of bias |
| Prognostic factor measurement | The measurement of the PF is unlikely to be different for different levels of the outcome of interest | Low risk of bias |
| Outcome measurement | The measurement of the outcome is unlikely to be different related to the baseline level of the PF | Low risk of bias |
| Study confounding | The observed effect of the PF on outcome is unlikely to be distorted by another factor related to PF and outcome | Low risk of bias |
| Statistical analysis and reporting | The reported results are unlikely to be spurious or biased related to analysis or reporting | Low risk of bias |
Appendix 6 Questions to CLINICAL specialist committee members and responses received
For this EVA, we have identified only one study evaluating CaRi-Heart Risk: Oikonomou EK, Antonopoulos AS, Schottlander D, Marwan M, Mathers C, Tomlins P, et al. Standardized measurement of coronary inflammation using cardiovascular computed tomography: integration in clinical care as a prognostic medical device. Cardiovasc Res 2021;117(13):2677–90.
The focus of the evidence review is, therefore, consideration of the extent to which this study addresses the clinical question defined at scope. As part of this process, we would like to request your input with respect to the ‘appropriateness’ of the variables (additional to FAI score) included in the CaRi-Heart Risk model, and the ‘standard care’ method of risk assessment to which it is compared, specifically:
The above publication describes the CaRi-Heart Risk model as incorporating (in addition to FAI score), atherosclerotic plaque burden (as described by the modified Duke CAD index), diabetes, smoking, hyperlipidaemia and hypertension.
When considering cardiac risk, in patients who are undergoing CTCA for the investigation of suspected CAD:
-
Are there any additional clinical risk factors (other than diabetes, smoking, hyperlipidaemia and hypertension) that you would routinely consider? Please list any additional clinical risk factors that you consider form part of standard care for risk assessment.
-
What imaging parameters (available from current standard CTCA) would routinely be reported/considered as part of standard care for risk assessment?
‘Easy answer to the first question. There are a number of other risk factors which are on the QRISK®3 calculation. They include heart attack in first degree relative < 60, chronic kidney disease, BMI, severe mental illness, use of antipsychotic drugs, atrial fibrillation and steroid use. Of these a very strong family history of premature coronary disease is a particularly potent risk factor (genetics)’.
The imaging question you post is relevant. In the UK cardiac imaging is generally performed when patients present with chest pains etc. rather than as a risk assessment tool. I suspect it may have been different in the US cohort in the Cardiovac Research paper (2021). It would be a big leap for general practitioners (GPs) to go from QRISK®3 scoring to sending hundreds of thousands of patients for CT scans that they wouldn’t normally be considered for.
In the Cardiovasc Res (2021) paper, the cardiac death rates were very low at 1.4% over 6 years – this comes to 0.23% per year in the European cohort (i.e. 1 in 450 chance of dying per year). I can’t see any details of the mode of death and in particular whether this was AMI.
For me there are a couple of missing pieces in the jigsaw. (1) Do the people with markers of inflammation in the coronary arteries also have inflammation in their abdominal fat (i.e. is this a systemic effect which is a marker of bad health – potentially related to kidney disease, obesity, mental illness etc. … all of which have mortality implications). (2) They must have looked for people with an inflamed RCA being admitted with a heart attack due to a blocked RCA. I can’t see any data on FAI predicting a heart attack in a specific artery.
Would be interested in others’ views’.
‘The patients who have a CTCA are largely going to be referred for investigation of chest pain. I agree with the comments about Q Risk 3, but these clinical risk models in general overestimate risk. The proposed CaRi-Heart score incorporates both the most important clinical risk factors and CT imaging markers, including information about the atherosclerotic plaque burden, and the fat attenuation index. As was described at our last meeting this is a ‘black box’ and we don’t know the contribution of each of these components. However, this score does outperform the clinical model and the outcome is death. Q-risk is MI or stroke risk rather than death’.
In answer to the specific questions:
-
In patients undergoing CTCA I suspect that if there is no disease evident and the CTCA is ‘normal’ then treatment would be guided by standard guidelines including Q risk assessment by the GP. If plaque disease is present, then most recommend aspirin and statin, with attention to other cardiac risk factors. I don’t think a risk score would change this recommendation in the presence of anatomical plaque. One key question is whether the CaRi-Heart score can improve this stratification perhaps most importantly in that large cohort with ‘normal’ CTCA because those with plaque are going to be treated anyway. That population with ‘normal’ CTCA may benefit from refined risk assessment.
-
Atherosclerotic plaque burden is reported – usually in a subjective way. Although there is probably variation in practice, it would be interesting to hear views on how this is used by colleagues. It doesn’t directly alter my approach to recommending treatment in the presence of anatomical disease, recognising that those with more plaque are likely to do worse, and if we could better stratify that risk it might be helpful – although I don’t know what we would do differently at this stage other than aspirin, high-dose statins and addressing the other modifiable risk factors.
‘I am less concerned than Gerald about the vessel specific prediction of event by FAI, and I think that one of the key drivers for the improved risk assessment maybe of the CaRi-Heart score is that the anatomical extent of disease is incorporated into the black box model, and we may not be able to separate those components’.
‘Replying from the perspective of a radiologist reporting CTCA:
(1) What imaging parameters (available from current standard CTCA), would routinely be reported/considered as part of standard care for risk assessment?
In addition to severity of stenosis and length of stenosis, I report the position of the lesion (e.g. left main stem and proximal LAD are particularly important) and whether it is fully calcified/mixed density/soft tissue density (i.e. can be induced to calcify with statins). The overall plaque burden is subjectively reported (i.e. overall amount, what proportion of vessels, age is taken into account in the emphasis – the younger with disease being more worrisome). Whether the lesion has features of vulnerability (aka napkin ring sign) or an obvious dissection is apparent, or whether a lesion appears more as vessel wall irregularity or thickening with perivascular fat stranding – i.e. implying there is localised vasculitis. Whether there is an anatomical variation putting the patient at more severe risk from a particular plaque is commented up if present.
We use HeartFlow for all lesions subjectively assessed as moderate or greater in severity of stenosis. Utilising computational fluid dynamics modelling to compute an estimate of lesions fractional flow reserve (CT-FFR), this in effect assesses whether the stenoses are tight and long enough to cause ‘flow limiting disease’ that is likely to be symptomatic. We also see a falloff in CT-FFR in distal vessels which is currently thought of equivocal significance but may possibly represent otherwise unmeasurable microscopic diffuse CAD. (One thought is that since HeartFlow also estimates myocardial volume for each vascular territory, what it might be reflecting is whether a vessel is large enough to supply that amount of myocardium.)
Note that the widely (but not universally) used CADRADs system of reporting CTCA plaque severity now includes in it’s recent ‘2.0’ update scoring of overall plaque burden.
https://pubs.rsna.org/doi/10.1148/ryct.220183
(2) Are there any additional clinical risk factors (other than diabetes, smoking, hyperlipidaemia and hypertension) that you would routinely consider? Please list any additional clinical risk factors that you consider form part of standard care for risk assessment.
Gerald’s list of risk factors as implied by the QRISK®3 calculator is quite reasonable and I see these listed in CTCA referrals to me. I would comment anecdotally that we have large numbers of patients with high BMI on our lists, who turn out to have no obvious CAD, whereas all too many thin patients who have lots of disease. I suspect BMI as a risk factor is more about downstream demand upon the heart rather than not of CAD itself. Furthermore, genetics is not the only reason for the familial risk factor – lifestyle habits and exposure (both food and pathogens) frequently have commonality in families.
I find the comments about RCA FAI and inflammation elsewhere (e.g. abdominal vasculature) intriguing. One increasingly important set of risk factors perhaps not yet fully recognised to be put on the CTCA request history by the referrers (i.e. cardiologists at my hospital) are the systemic inflammatory diseases. I see plenty of referrals with ‘COVID’, but are yet to see any with psoriasis, rheumatoid arthritis and gout which are growing in interest as risk factors for CAD due to their possible vascular aetiologies. (Many systemic diseases like these may however be reflected in the ‘use of steroids’ risk factor.)
www.ncbi.nlm.nih.gov/pmc/articles/PMC7462628/
There is a chicken and egg question here. Do the perivascular fat changes represent a response to CAD, or does it represent a measurable feature that is a precursor to the actual development of plaque? Perhaps both. If the latter is true – CaRi-Heart therefore could potentially be useful in identifying patients where a treatment could be aimed at settling the inflammation before it develops into (initially soft tissue) coronary arterial plaques. Statins are thought to convert soft-tissue plaques more quickly into calcified plaques, which is not a cure but a mitigation hoping to reduce the risk of future plaque rupture. For those of the panel who were at the BSCI conference in Bath: – anti-inflammatory medications such as colchicine (used to treat gout flare-ups) were mentioned as potential anti-inflammatory treatment.
Lastly CaRi-Heart does not have to be just applied to CTCA scans. Having worked with a GE revolution scanner which was being used principally for A&E traumas, the newer generation of scanners is producing remarkably good images of the heart (although not intentionally) when visualising the thorax for other reasons. Just as the BSCI has previously advised a review of the degree of calcification of coronary arteries when the thorax is imaged (under the premise that young with calcified disease are at increased risk of future coronary events, whereas the old with none are conversely at much lower risk), CaRi-Heart may potentially be a useful way of screening patients for risk of developing plaques in future from pre-existing scans’.
www.birpublications.org/doi/10.1259/bjr.20200894
‘I’m answering from a radiologist’s perspective so will try not to stray too far into territory outside my expertise! And note that some good points have been made already.
When considering cardiac risk, in patients who are undergoing CTCA for the investigation of suspected CAD:
-
Are there any additional clinical risk factors (other than diabetes, smoking, hyperlipidaemia and hypertension) that you would routinely consider? Please list any additional clinical risk factors that you consider form part of standard care for risk assessment. As per comments below there are various additional risk factors and scores which are relevant although still relatively crude. Going forward, I think more accurate risk prediction is going to play a greater role in clinical medicine. Moving towards personalised medicine in an increasingly multimorbid population with ever more treatments and interventions available could provide benefits to individuals and society but requires such prediction models to be better tailored to the individual. An interesting paradigm in medicine is the need to have better diagnostic tools to assess the impact of novel treatments and interventions; therefore you often can’t have one without the other and these develop in parallel. Having said that, not all diagnostic investigations will find a role beyond a research setting if they don’t have a use on a patient-by-patient basis.
-
What imaging parameters (available from current standard CTCA) would routinely be reported/considered as part of standard care for risk assessment? As Rob said, practice is heterogeneous and will vary by centre and individual expertise. Coronary calcium score has been best validated as an additional risk factor in an asymptomatic population and not routinely performed in patients referred for chest pain assessment. CADRAD-2 is probably the best ‘template’ for a comprehensive CT coronary angiogram report but recently described and therefore use won’t be widespread. Even where it is in use, much of the risk is subjective and we know that interobserver variability for many imaging findings is generally poor and interpretation subject to bias. Standardisation is therefore limited in this context. HeartFlow is useful for predicting whether anatomical stenoses are causing symptoms and can help determine which patients may benefit from revascularisation but doesn’t predict the overall vascular risk. I suspect in some centres the CT report will focus mainly on functionally significant stenosis and role for interventions rather than a more holistic view of overall burden of atheroma. Again, not standard of care and I’m not sure what is commercially available, but software to assess high-risk plaques may also have a role for risk prediction.
Finally, as we discussed at the meeting, there may be scope for the use of CaRi-Heart outside the population and parameters studied, for example asymptomatic patients with some risk factors, acute chest pain presentations, non-cardiac gated studies, but obviously evidence is not currently available in these patients/settings’.
When considering cardiac risk, in patients who are undergoing CTCA for the investigation of suspected CAD:
-
Are there any additional clinical risk factors (other than diabetes, smoking, hyperlipidaemia and hypertension) that you would routinely consider? Please list any additional clinical risk factors that you consider form part of standard care for risk assessment.
-
‘age
-
gender
-
post code.
-
The advantage of Qrisk over Euroscore (predicts mortality) etc. is the inclusion of post code which includes IMD status. In Scotland they use ASSIGN.
I believe the initial CRISP study publication compared to standard risk model and found a small incremental benefit in AUC for using the FAI model.
(Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP-CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392:929–39.)
However, this improved AUC was lost if you gave the patient a statin.
So the real Q is how Cari-Heart helps in the 30% that you are not going to advise a statin (normal coronaries). The metanalysis Mani sent around touches on this’.
What imaging parameters (available from current standard CTCA) would routinely be reported/considered as part of standard care for risk assessment?
‘The risk parameters include:
-
coronary calcium scoring (if performed);
-
presence absence of plaque;
-
severity of plaque – CAD RADS 2 includes metrics standardly used which incorporates features of increased risk:
-
degree stenosis;
-
number of vessels and LMS involvement;
-
amount of plaque (not quantified – visual: P1–4; you can use CACS as well for this);
-
high-risk plaque features (yes/no) and number of HRP features;
-
ischaemia testing (yes/no) from CT FFR.
-
This is ‘best standard of care’ – and should be reported on every CCTA scan report. However, I suspect the majority of report in the UK don’t include this level of detail or nuance. Nor do the people receiving the report understand the nuances. Finally, there is no final % risk given in the report. We can’t do this at the moment. You can say the relative risk is 32× if you have 3 HRP features – but what does that mean?!
I am aware that Caristo have ORFAN running and a NHSE award in 4 trusts so that they may be able to answer many of these Q in the future. They also now are incorporating plaque quantification. However, none of these data are available’.
Appendix 7 Overview of utilities for major adverse cardiovascular events
| Source (year) | AMI | MI | Stroke | Post stroke | Stable angina | Unstable/unspecified angina | PAD | Revascularisation | Heart failure | CABG | Country |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blieden Betts 2018116 – Barton 2008 | N/R | N/R | N/R | 0.612 (SE: 0.318) |
N/R | N/R | N/R | N/R | N/R | N/R | N/R |
| Blieden Betts 2018116 – Darlington 2007 – 1 year | 0.72 (SE: 0.22) |
N/R | |||||||||
| Blieden Betts 2018116 – Darlington 2007 – 2 months | 0.69 (SE: 0.25) |
N/R | |||||||||
| Blieden Betts 2018116 – De smedt 2014 | 0.78 (95% CI 0.66 to 1) | N/R | N/R | ||||||||
| N/R | 0.69 (95% CI 0.52 to 0.85) |
N/R | |||||||||
| Blieden Betts 2018116 – Fenny 2012 – 6 months | N/R | 0.76 (SE: 0.18) |
N/R | ||||||||
| Blieden Betts 2018116 – Fenny 2012 – baseline | 0.77 (SE: 0.18) |
N/R | |||||||||
| Blieden Betts 2018116 – Han 2012 | 0.75 (SE: 0.19) (95% CI 0.73 to 0.77) |
N/R | N/R | ||||||||
| Blieden Betts 2018116 – Hebert 2008 – HF patient in nurse-led program – 1 year | N/R | 0.708 | N/R | ||||||||
| Blieden Betts 2018116 – Hebert 2008 – HF patient receiving usual care – 1 year | 0.6651 | N/R | |||||||||
| Blieden Betts 2018116 – Kaplan 2011 – from baseline to 1 month | 0.005 (SE: 0.14) |
N/R | |||||||||
| Blieden Betts 2018116 – Kaplan 2011 – from baseline to 6 months | 0.000 (SE: 0.16) |
N/R | |||||||||
| Blieden Betts 2018116 – Sanchez 2010 | 0.4305 | N/R | |||||||||
| Blieden Betts 2018116 – Sanchez 2010 – HF treated with peritoneal dialysis – 6 months | 0.6727 | N/R | |||||||||
| Blieden Betts 2018116 – Stevanociv 2014 | 0.6385 | UK | |||||||||
| Blieden Betts 2018116 – Kraai 2013 | 0.68 (SE: 0.26) |
N/R | |||||||||
| Blieden Betts 2018116 – Wannasiri 2011 | 0.55 | N/R | N/R | ||||||||
| Blieden Betts 2020 117 | 0.79 (95% CI 0.73 to 0.85) |
0.65 (95% CI 0.44 to 0.78) |
0.75 (95% CI 0.67 to 0.78) |
0.71 (95% CI 0.63 to 0.86) |
0.72 (95% CI 0.64 to 0.78) |
0.80 (95% CI 0.73 to 0.84) |
N/R | ||||
| Creber 2022118 – baseline | N/R | N/R | N/R | N/R | N/R | N/R | 0.76 (95% CI 0.74 to 0.78) |
Multicountry | |||
| Creber 2022118 – follow-up | 0.86 (95% CI 0.85 to 0.97) |
Multicountry | |||||||||
| De la Puente 2017119 – Clopidogrel | 0.7770 | 0.677 | N/R | Germany | |||||||
| De la Puente 2017119 – Ticagrelor | 0.7940 | 0.7360 | Germany | ||||||||
| Di Tanna 2021120 – HF | N/R | N/R | 0.64 to 0.72 | N/R | |||||||
| Duarte 2021 121 | −0.0626 (0.0132) | −0.0368 (SE: 0.0257) |
−0.0092 | N/R | −0.033 (SE: 0.001) | Focus on UK | |||||
| Gao 2019 122 | N/R | N/R | 0.76 | N/R | N/R | Australia | |||||
| Health Quality Ontario 2016123 – Berkhemer intervention | 0.69 (95% CI 0.33 to 0.85) | Canada | |||||||||
| Health Quality Ontario 2016123 – Berkhemer – Control | 0.66 (95% CI 0.30 to 0.81) | Canada | |||||||||
| Health Quality Ontario 2017 124 | 0.59 (SE: 0.001) | 0.68 (SE: 0.0018) | N//R | UK | |||||||
| Health Quality Ontario 2020 125 | N/R | N/R | N/R | ||||||||
| Joundi et al. 2022 126 | 0.66 (0.63 to 0.67) | Multicountry | |||||||||
| Perera 2015127 – Greving 2011 | 0.88 (95% CI 0.80 to 0.96) | 0.50 (0.00 to 0.75) | Multicountry | ||||||||
| Shan 2015 128 | N/R | N/R | 0.61 | N//R | |||||||
| Stahl 2017 129 | 0.870 (SE: 0.2) | 0.74 (0.25) | N/R | 0.630 (0.02) | UK (= stroke) | ||||||
| Sterne 2017 130 | 0.683 (SE: 0.233) | 0.718 (SE: 0.243) | –0.59 Uniform (–0.885 to –0.295) |
0.69 (0.18) | N/R | UK | |||||
| Stevanovic 2016 131 | N/R | 0.7638 (SE: 0.0246) | N/R | N/R | 0.7792 (0.025) | N/R | |||||
| Westwood 2021 132 | Age-specific post MI – linear regression model | N/R | UK |
List of abbreviations
- A&E
- accident and emergency
- AI
- artificial intelligence
- AMI
- acute myocardial infarction
- BHF
- British Heart Foundation
- BMI
- body mass index
- BNF
- British National Formulary
- CABG
- coronary artery bypass graft
- CAD
- coronary artery disease
- CCS
- coronary calcium score
- CCT
- controlled clinical trial
- CTTC
- Cholesterol Treatment Trialists Collaboration
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CRD
- Centre for Reviews and Dissemination
- CRISP-CT
- Cardiovascular RISk Prediction using Computed Tomography
- CRP
- C-reactive protein
- CT
- computed tomography
- CTCA
- computed tomography coronary angiography
- DARE
- Database of Abstracts of Reviews of Effects
- DOAJ
- Directory of Open Access Journals
- ECG
- electrocardiogram
- ESC
- European Society of Cardiology
- EU
- European Union
- EVA
- Early Value Assessment
- FAI
- fat attenuation index
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICA
- invasive coronary angiography
- ICC
- intraclass correlation coefficient
- ICTRP
- International Clinical Trials Registry Platform
- IDI
- integrated discrimination improvement
- IL-6
- interleukin-6
- INAHTA
- International Network of Agencies for Health Technology Assessment
- IPD
- individual patient data
- IQR
- interquartile range
- KSR
- Kleijnen Systematic Reviews Ltd
- LAD
- left anterior descending artery
- LCX
- left circumflex artery
- LDL
- low-density lipoprotein
- LILACS
- Latin American and Caribbean Health Sciences Literature
- LMCA
- left main coronary artery
- MACE
- major adverse cardiovascular events
- MI
- myocardial infarction
- MPS
- myocardial perfusion scintigraphy
- MR
- magnetic resonance
- MRC
- Medical Research Council
- MVE
- major vascular event
- N/A
- not applicable
- NICE
- National Institute for Health and Care Excellence
- NIH
- National Institutes of Health
- NIHR
- National Institute for Health and Care Research
- N/R
- not reported
- OR
- odds ratio
- ORFAN
- Oxford risk factors and non-invasive imaging
- PACS
- picture archiving and communication system
- PCAT
- pericoronary adipose tissue attenuation
- PROSPERO
- International Prospective Register of Systematic Reviews
- PSM
- propensity score matching
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-years
- QUIP
- Qualitative Impact Assessment Protocol
- RCA
- right coronary artery
- RCT(s)
- randomised controlled trial(s)
- RR
- risk ratio/rate ratio
- TECH-VER
- TECHnical VERification
- WHO
- World Health Organization
Notes
Note
This monograph is based on the Diagnostic Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Diagnostic Advisory Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
