Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 13/15/02. The contractual start date was in September 2014. The draft report began editorial review in January 2021 and was accepted for publication in March 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this manuscript.
Permissions
Copyright statement
Copyright © 2024 Hutchinson et al. This work was produced by Hutchinson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Hutchinson et al.
Chapter 1 Introduction
Parts of this chapter have been reproduced from Kolias et al. 1
Parts of this chapter have been reproduced with permission from Kolias et al. 2 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Parts of this chapter have been reproduced from Hutchinson et al. 3
Background
Chronic subdural haematoma (CSDH) is an ‘old’ collection of blood and blood breakdown products in the subdural space. It is radiologically defined as a predominantly hypodense or isodense collection in the subdural space along the cerebral convexity on computerised tomography (CT). 1 It is especially common in older patients and in the UK: 5000 people aged > 65 years are diagnosed with a CSDH each year. It can happen following even a minor injury to the head or in the absence of a known trauma.
The incidence of CSDH is increasing4 and is projected to rise further, matching the ageing global population. 5 Therefore, surgical evacuation of CSDH is projected to become the most common cranial neurosurgical operation in the USA by 2030. 6 Although many patients remain asymptomatic, some patients experience symptoms such as headache, gait disturbance, falls, cognitive decline, focal neurological deficit, speech disturbance, decreased consciousness and seizures.
Current practice
Surgery remains the mainstay of managing symptomatic CSDH, typically through evacuation by burr holes or mini-craniotomy. 1 Additional measures, such as correction of coagulopathy or thrombopathy and subdural drains postoperatively, are established in treating CSDH. 1,7
However, other aspects of CSDH management remain controversial owing to the lack of level 1 evidence to inform their role,8 such as adjuvant medications (antiepileptic drugs or steroids) and postoperative care protocols. 1 Determining the clinical effectiveness of adjuncts to surgical evacuation is essential, particularly in the light of the considerable morbidity and mortality associated with cranial surgery in an ageing population.
Rationale
It is postulated that following a traumatic injury to the head an inflammatory reaction drives the growth of abnormal blood vessels and fluid accumulation over the surface of the brain. Several studies have demonstrated locally elevated cytokine levels in the subdural fluid of patients with CSDH,9–13 suggesting a role of inflammation in CSDH pathophysiology.
Therefore, anti-inflammatory agents, such as steroids, may counter this inflammatory response, with evidence suggesting potential to reduce CSDH recurrence and even the rate of primary surgical intervention. 10–12 This, in turn, might be expected to reduce mortality and morbidity, and improve long-term functional outcomes in patients with CSDH. Non-randomised studies have pursued this hypothesis, with promising observational data supporting the use of dexamethasone in treating symptomatic CSDH. 14–16 A single Phase II randomised controlled trial (RCT), published in 2015, 9 years after it was completed, suggested a benefit of combining steroids with surgical evacuation, with a trend towards a lower recurrence rate. 17 However, there was a high risk of bias in the pilot study. Higher-quality evidence through a larger, definitive RCT was, therefore, necessary to determine the clinical effectiveness of dexamethasone in CSDH. 18
Dexamethasone is one of the most potent synthetic analogues of the naturally occurring glucocorticoid hydrocortisone and has practically no water- and salt-retaining properties, so it is suitable for use in patients with cardiac failure or hypertension. 19 The earliest application of steroids in neurosurgery was for patients with brain tumours and surrounding oedema, for whom 4 mg four times per day was established as the dose with maximum effect. 20 This dosing, with subsequent gradual weaning, continues to be used in neuro-oncology, and a 2-week course of dexamethasone was considered likely to provide the best balance in terms of clinical efficacy and risks in this study. 21 Median time to recurrence after surgical evacuation of CSDH has been shown to be 12–15 days,7,8 and longer courses of corticosteroids have greater risks of side effects. 22 The dose and duration are also reflective of other studies in the field. 23
Risks and benefits
The potential impact of this trial is significant because the results will determine whether or not steroids should be prescribed routinely for patients with symptomatic CSDH. If steroids are found to be effective, an impact on the speed of recovery and functional outcome of patients is expected. In addition, this could reduce the rate of surgical interventions required, reduce length of hospital stay, influence discharge destination and reduce adverse events (AEs). In addition to the impact on clinical outcome, there are health economic considerations that will be addressed by the trial.
Steroids are commonly used to treat neurosurgical conditions and are generally well tolerated. However, side effects have been observed in patients with CSDH, including hyperglycaemia, infections, mental disturbance and mortality. 18 Therefore, the clinical effectiveness of steroids must be elucidated to determine their role in CSDH management.
The Dex-CSDH (DEXamethasone in Chronic SubDural Haematoma) trial is a multicentre, pragmatic, clinical phase III, randomised, double-blind, placebo-controlled trial of dexamethasone for up to 2 weeks in patients diagnosed with CSDH.
Objectives
Primary objective
The primary objective was to determine the clinical effectiveness of a 2-week tapering course of dexamethasone for adult patients with a symptomatic CSDH by detecting an 8% absolute difference in the rate of favourable outcome at 6 months between the two arms.
Secondary objectives
The secondary objectives were to compare the following outcomes between the treatment arm and the control arm of the trial:
-
number of CSDH-related surgical interventions undertaken during the index admission
-
number of CSDH-related surgical interventions undertaken during subsequent admissions in the follow-up period
-
Glasgow Coma Scale (GCS)24 at discharge from the neurosurgical unit (NSU) and at 6 months
-
Modified Rankin Scale (mRS)25 score at discharge from the NSU and at 3 months
-
Barthel Index (BI)26 score at discharge from the NSU and at 3 and 6 months
-
mortality (30 days and 6 months)
-
EuroQol-5 Dimensions, five-level version (EQ-5D-5L),27 utility index at discharge from the NSU and at 3 and 6 months
-
length of stay in the NSU
-
discharge destination from the NSU
-
length of stay in secondary care
-
rates of AEs.
Postoperative recurrence is a tertiary outcome measure and is defined as a symptomatic recurrence requiring reoperation of a previously evacuated ipsilateral CSDH.
To estimate the cost-effectiveness of dexamethasone, compared with placebo, an economic evaluation was also undertaken (see Chapter 4).
Chapter 2 Trial design and methods
Parts of this chapter have been reproduced from Kolias et al. 1
Parts of this chapter have been reproduced with permission from Kolias et al. 2 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Parts of this chapter have been reproduced from Hutchinson et al. 3
Trial design
This was a pragmatic, multicentre, parallel-group, double-blind, Phase III, randomised (1 : 1 randomisation stratified by site), superiority, placebo-controlled trial.
Ethics approval and research governance
Ethics approval in the UK was obtained from the North-West Haydock Research and Ethics Committee (reference 15/NW/0171) in 2015.
The trial protocol was designed collaboratively with input from neurosurgeons, neurologists, stroke physicians and care of the elderly physicians from multiple hospitals and universities in the UK. The Cambridge Clinical Trials Unit led the methodological design.
The protocol has been published previously. 2 Appendix 2 outlines the protocol amendments.
Participants
Patients were eligible if they were aged ≥ 18 years, had a symptomatic CSDH confirmed on cranial imaging [e.g. CT/magnetic resonance imaging (MRI) – predominantly hypodense or isodense crescentic collection along the cerebral convexity on CT] and were willing (or had a willing legal representative) and able to provide informed consent. In the absence of a legal representative, an independent healthcare professional (HCP) provided authorisation for patient enrolment.
Exclusion criteria were:
-
patients with conditions for which steroids were clearly contraindicated
-
patients who were on (or within 1 month of) regular oral or intravenous glucocorticosteroids (patients on topical or inhaled steroids were allowed to be recruited into the trial, as were patients who had one intraoperative dose of dexamethasone for anti-emesis)
-
previous enrolment in the trial for a prior episode
-
the time interval from the time of admission to NSU to first dose of trial medication exceeded 72 hours
-
CSDH in the presence of a cerebrospinal fluid shunt
-
severe lactose intolerance or any known hypersensitivity to dexamethasone or any of the investigational medicinal product (IMP) excipients
-
patients with a previous history of psychotic disorders
-
unwillingness to take products containing gelatine
-
concurrent enrolment in any other trial of an IMP.
Patients were reviewed for eligibility on admission to the NSU by the admitting team. The trial was run in parallel with standard clinical care and, therefore, the need for surgical intervention for the CSDH was determined by the clinical team and did not affect eligibility for trial involvement.
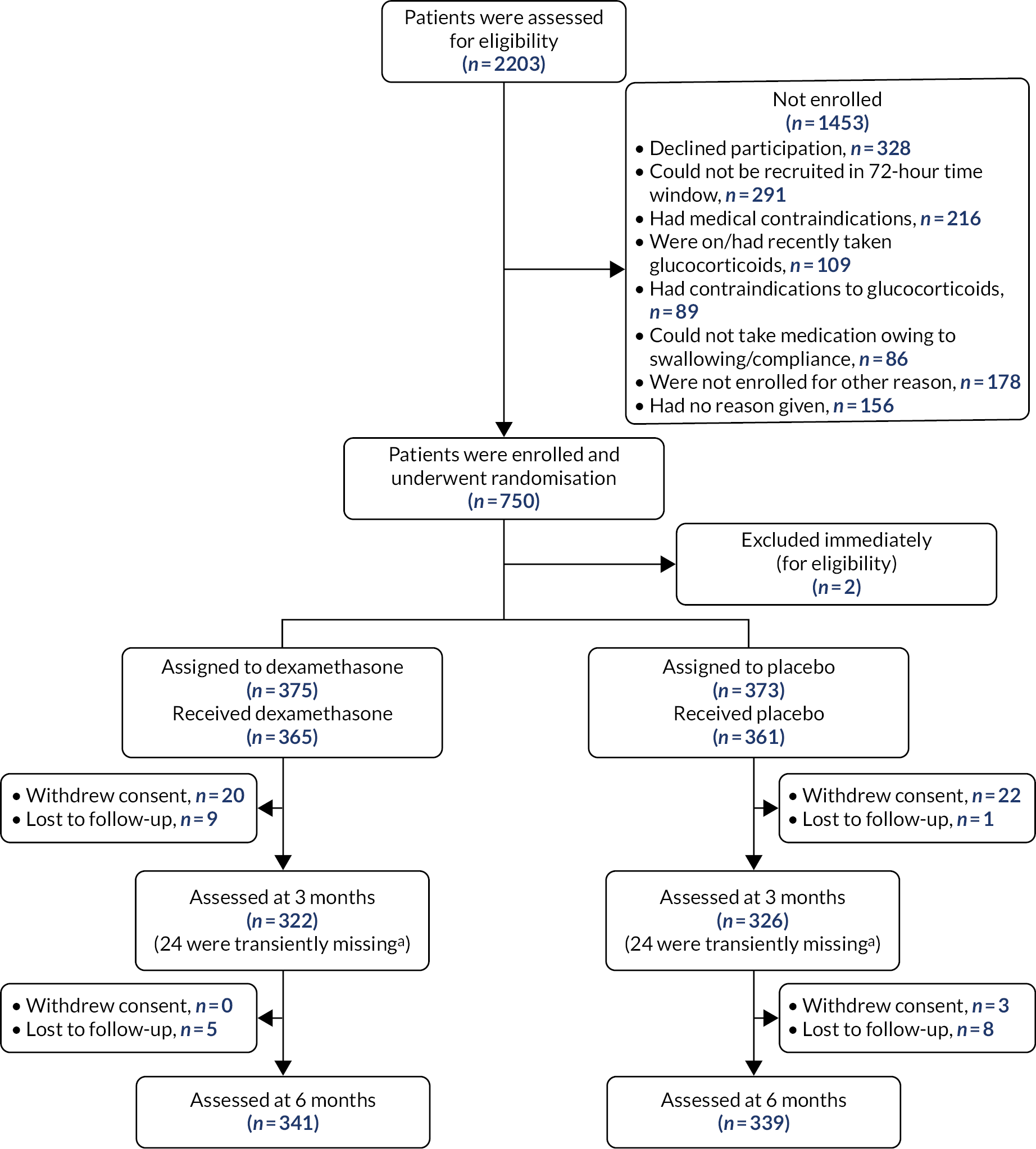
A Consolidated Standards of Reporting Trials (CONSORT) diagram was produced to show patient disposition (Figure 1).
FIGURE 1.
The CONSORT flow diagram. a, Transiently missing refers to patients whose follow-up data were missing at 3 months but available at 6 months.
Study setting
This multicentre study took place from August 2015 to November 2018 in 23 NSUs providing 24-hour acute care in the UK NHS from.
Interventions
Participants received the allocated treatment after randomisation as part of the routine drug rounds by ward nurses once admitted to the NSU. The allocated treatment was a 2-week tapering course of either dexamethasone (overencapsulated 2-mg tablets) or matched placebo (visually indistinguishable from the active treatment and containing inactive excipients only). The excipients used for backfilling the dexamethasone capsules were the same as those used to fill the placebo capsules and were standard tableting excipients.
Dosing schedule
The dosing schedule was as follows:
-
four capsules in the morning and four at lunchtime for days 1, 2 and 3
-
three capsules in the morning and three at lunchtime for days 4, 5 and 6
-
two capsules in the morning and two at lunchtime for days 7, 8 and 9
-
one capsule in the morning and one at lunchtime for days 10, 11 and 12
-
one capsule once daily for days 13 and 14
-
end of allocated treatment.
The maximum duration of treatment was 14 days. This regime was felt to provide the best balance in terms of clinical effectiveness and risks. 21
A missed medication could be taken later, provided that it was taken on the same day and the patient was not nil by mouth for surgery. In the event of missing a dose of medication, doses could be taken when remembered, but only up to the time of the next planned dose on the same day.
Administration and maximum dosage allowed
The drug was administered orally or via nasogastric tube, as required. The maximum dose allowed in a single day was 16 mg (8 mg twice daily for days 1, 2 and 3). The preferred time of administration of once-daily doses (days 13 and 14) was in the morning.
For nasogastric administration, blinded capsules were opened at the point of administration by ward nursing staff. The contents were then dispersed in water to allow administration. This method was also used orally in patients with swallowing difficulties.
All patients completed the 14-day course of trial medication. If discharged or transferred to another hospital, letters were provided to the pharmacy and medical teams at the local hospital alongside any remaining medications. However, if a patient receiving the trial medication via the nasogastric route was transferred or discharged, the medication was stopped at transfer/discharge. Further details on the interventions can be found in the published protocol. 2
Outcomes
Primary outcome measure
The prespecified primary outcome measure was the mRS score at 6 months after randomisation, which was dichotomised into favourable (score of 0–3) versus unfavourable (score of 4–6). 25 This has previously been employed as an outcome measure in CSDH studies. 28 Questionnaires were distributed to patients via post and were collected by the central trial co-ordination team. If after 2 weeks the questionnaire was not returned, patients were followed up by telephone, an equally reliable method of data collection. 25 The mRS scores were calculated by a blinded, clinically trained investigator using a standard algorithm.
Secondary outcome measures
Secondary outcomes were (as detailed in protocol2):
-
number of CSDH-related surgical interventions undertaken during the index admission
-
number of CSDH-related surgical interventions undertaken during subsequent admissions in the follow-up period
-
GCS score at discharge from NSU and at 6 months
-
mRS score at discharge from NSU and at 3 months
-
BI score at discharge from a NSU and at 3 and 6 months
-
mortality at 30 days and at 6 months
-
EQ-5D-5L29 at discharge from NSU and at 3 and 6 months
-
length of stay in NSU
-
discharge destination from NSU
-
length of stay in secondary care
-
AEs.
Recurrence, defined as a symptomatic recurrence requiring reoperation of a previously evacuated ipsilateral CSDH during the study period, was a tertiary outcome measure applying to surgically treated patients only.
Data collection
Patient questionnaires (mRS, BI and EQ-5D-5L) were collected by the local site research team at discharge and the central trial co-ordination team at 3 and 6 months. GCS score was collected by the local site research team. The length of stay in a NSU was a derived variable calculated as:
where the summation was taken over all admissions. Length of stay in secondary care was also a derived variable calculated as the length of stay in NSU plus the self-reported length of stay in hospital or healthcare facility based on the 6-month health service questionnaire.
The EQ-5D-5L questionnaire is a self-report measure consisting of five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). 27 The responses were converted into a utility score (where 0 is death and 1 is full health) using the cross-walk algorithm,30 in accordance with the National Institute for Health and Care Excellence (NICE) position statement. 31 Participants who died during the study were given a score of zero.
Recurrence was defined as a symptomatic recurrence requiring reoperation of a previously evacuated ipsilateral CSDH during the study period. 32
Trial assessments
Table 1 presents a full list and timeline of trial assessments. Participants were followed up for 6 months after randomisation.
| Trial assessment | Prior to randomisation (< 72 hours from admission) | Randomisation (preferable, but not essential for this to occur before surgery) | Day 1 of trial drug (< 72 hours from admission) | Days 2–14 of trial drug | Day 15 (± 1 week) | Day 30 (± 1 week) | Discharge from NSU or at death (± 1 week) | 3-month follow-up (± 4–8 weeks) | 6-month follow-up (± 4–8 weeks) |
|---|---|---|---|---|---|---|---|---|---|
| Eligibility assessment | ✗ | ||||||||
| Informed consent | ✗ | ✗ | If attends OPA | ||||||
| Randomisation | ✗ | ||||||||
| Part 1 of CRF sent to co-ordinating centre | ✗ | ||||||||
| IMP administration | ✗ | ✗ | |||||||
| Review of AEs | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Review of concomitant medication | ✗ | ✗ | |||||||
| Telephone call to assess medication diary | ✗ | ||||||||
| Completed CRF faxed to trial co-ordinating centre | ✗ | ||||||||
| Review of routine lab results | ✗ | ✗ | ✗ | ✗ | |||||
| GCS | ✗ | If attends OPA | |||||||
| mRS | ✗ | ✗ | ✗ | ||||||
| Mortality | ✗ | ✗ | |||||||
| EQ-5D-5L | ✗ | ✗ | ✗ | ||||||
| BI | ✗ | ✗ | ✗ | ||||||
| Health service questionnaire | ✗ |
Sample size
Using a two-sided test at the 5% significance level and assuming a favourable outcome rate of 80–85% in the control arm,14 a sample size of 750 patients (allowing for a 15% loss to follow-up) would have a power of 80–92% to detect an 8% absolute difference in the rate of favourable outcome. An 8% increase in the rate of favourable outcome at 6 months (mRS score of 0–3) was determined to be a plausible and clinically important treatment effect based on the opinion and experiences of the clinicians from multiple specialties involved in the design of the study.
Interim analysis
A pre-planned blinded interim analysis of pooled outcome data was performed after 450 patients had completed 6 months of follow-up to decide if the sample size needed to be adjusted. The possible alternatives after the interim analysis were to increase the sample size (with a maximum of 1000 patients) or to stop the trial for futility if the revised sample size was more than 1000 patients. Because the trial could be stopped only for futility, we did not adjust the confidence interval (CI) and p-value at the end of the trial to account for the interim analysis. The independent Data Monitoring and Ethics Committee (DMEC) recommended that recruitment should continue to the original target sample size of 750 patients.
Trial stopping criteria and end point
No specific criteria were defined for premature discontinuation of the trial. However, both patient safety and efficacy data were under review by the independent DMEC and Trial Steering Committee (TSC) to make recommendations on discontinuation at regular intervals throughout the study. The trial end date was defined as the date of the last expected 6-month follow-up questionnaire completed for the final patient recruited into the trial.
Randomisation
All patients admitted to the NSU with a confirmed CSDH were screened for eligibility, which was assessed by a member of the admitting neurosurgical team. Randomisation took place either before or after initial index surgery.
Patients were randomly assigned to either the dexamethasone or the placebo arm in a 1 : 1 allocation, as per a computer-generated randomisation schedule stratified by NSU using permuted blocks of random sizes (two or four). An interactive web-based response system was used to allocate treatment packs to individual patients once the inclusion criteria being met had been confirmed.
Blinding
Capsules and packaging for the dexamethasone arm and the placebo arm were identical in appearance at the point of issue to patients.
It was estimated that < 10% of eligible patients would have (or develop during the trial) swallowing difficulties making oral IMP administration difficult or impossible/unsafe. To ensure that the trial could proceed in as representative a population as possible, a pragmatic and cost-effective approach to dosing IMP was proposed. The strategy for managing IMP administration in patients with swallowing difficulties was developed after discussion with and advice from the Medicines and Healthcare products Regulatory Agency. The blinded capsules were, with investigator and local pharmacy approval, opened at the point of administration via either the oral route or the nasogastric tube if one had been inserted during the routine course of care. If this scenario occurred, the administering nurse, NHS site pharmacy and, potentially, the trial patient would no longer be blinded because the active dexamethasone was in overencapsulated tablet form, and may have required crushing before dispersal in 15–20 ml of water for nasogastric administration, while the placebo was in powder form. To maintain blinding of the neurosurgeons, the presence of tablets inside the opened capsule was not documented in the medical notes but referred to in generic terms. Although every effort was made to maintain blinding when nasogastric administration was used, if the patient discovered their treatment they were asked not to disclose the information to any of the other medical personnel they interacted with. The research staff and outcome assessors remained blinded.
There were also clinical aspects that could potentially have unblinded trial team members to the treatments allocated. Patients receiving dexamethasone were more likely to have higher blood glucose levels than those receiving placebo. This may have provided an indication, although not proof, that a patient was in the dexamethasone arm. The concealment of glucose measurements was difficult because it may have required clinical action. However, any decision about surgery was made based on the severity of symptoms and/or progression of symptoms, so this was highly unlikely to influence treatment decisions.
The trial statistician performing the analysis was blinded to treatment allocation until version 2.0 of the statistical analysis plan (SAP) had been approved and the database hard locked. Unblinding of the interim DMEC reports, including the interim analysis, was performed by a statistician independent of the trial.
Statistical analysis
Full details of the statistical analyses can be found in the published SAP. 32
Analysis populations
The assignment of participants to analysis populations was undertaken prior to breaking the blinding. The following analysis populations were defined.
Full analysis population
The full analysis population included all participants apart from those who withdrew consent for participation in the trial and those lost to follow-up. Participants were analysed as randomised using a modified intention-to-treat analysis.
Per-protocol population
Separate per-protocol populations were defined for each assessment time point (discharge, 3 months and 6 months). Participants were included if they satisfied the following conditions:
-
were eligible to take part in the study
-
took at least 80% of their medication (50 tablets) based on the daily medication compliance table – if missing, percentage of medication taken was based on remaining pill count
-
completed their assessments within the prespecified time windows (± 1 week for discharge, –4/+ 8 weeks for 3 and 6 months).
Participants in the placebo arm were excluded if they received > 8 mg of dexamethasone during the IMP course. This was based on information on the concomitant medications form and the non-compliance log, and was determined on a per-patient basis by members of the Trial Management Group.
The third criterion (completing assessments within a given time window) applied only to questionnaire outcomes (mRS, GCS, BI and EQ-5D-5L); therefore, a fourth per-protocol population excluding this criterion from the definition was used to analyse non-questionnaire outcomes (surgery outcomes, mortality and discharge information).
Safety population
All randomised participants.
Missing data
The sample sizes of non-missing values were reported for summary tables, with the percentage of missing outcome data shown for the primary and secondary outcomes. The prespecified SAP assumed that any missing data were missing at random and, therefore, missing data were not imputed. Sensitivity analysis (SA) was planned in the event that > 15% of data were missing for the primary outcome. Given that < 15% of data were missing, the primary analysis was based on complete cases.
Patients who died during the study were given a score of ‘6 – Dead’ for all mRS assessments occurring after the date of death (based on the upper time window limit for the assessment) and a score of zero for the EQ-5D-5L utility index. This is in accordance with established practices and user guides for both scores. Management of missing data is explained in Appendix 4.
Summary of study data
Summary statistics were produced for demographics and baseline variables; concurrent illnesses and medical conditions; prior and concurrent medications; treatment compliance; and the primary and secondary outcomes. Continuous variables were summarised using the following descriptive statistics: n (non-missing sample size), mean, standard deviation (SD), median, maximum and minimum. For categorical measures, frequency and percentages (based on the non-missing sample size) of observed levels were reported.
Primary analysis
The primary end point was the proportion of favourable outcomes (mRS score of 0–3 at 6 months) in the two treatment arms. The primary analysis estimated the absolute difference in the proportions achieving a favourable outcome between the two treatment arms. A simple normal approximation (z-test) was used to produce a 95% CI and two-sided p-value testing the null hypothesis that there was no difference in the primary outcome between the two treatment arms. As there was only one primary outcome, no adjustment for multiple testing was required. The analysis was repeated for both the full analysis and the per-protocol populations.
Secondary analyses
All secondary analyses (with the exception of the listing and bar chart of deaths) were performed on both the full analysis and the per-protocol populations.
Surgery
Poisson regression was used to model the effect of treatment (dexamethasone vs. placebo) on the following surgery outcomes:
-
number of CSDH-related surgical interventions undertaken during the index admission
-
number of CSDH-related surgical interventions undertaken during subsequent admissions in the follow-up period.
The former was defined in two ways:
-
including pre-randomisation surgical procedures (which occurred within 72 hours prior to randomisation)
-
excluding pre-randomisation surgical procedures.
The latter was also modelled in two ways:
-
including only patients who had a subsequent admission
-
including all patients – those without a subsequent admission were given a value of zero for number of surgeries.
The treatment effect, 95% CI and p-value were produced for each fitted model.
Questionnaires
The mRS outcomes (original score at discharge from NSU and 3 months) were analysed using proportional odds logistic regression. The following statistics were reported: cumulative probabilities for the placebo arm at each cut-off point of the mRS; global odds ratio (OR) together with the 95% CI and p-value; frequency and percentage of patients with a mRS score less than or equal to each cut-off point for both the dexamethasone arm and the placebo arm; and the marginal OR (95% CI) at each cut-off point.
A stacked bar chart showing the mRS outcomes by treatment arm and assessment time points (pre morbid, admission to NSU, discharge from NSU, 3 months and 6 months) was produced both including and excluding missing data.
Although a proportional odds logistic regression was originally planned, no model fitting was performed on the GCS outcomes (measured at discharge and 6 months). At discharge, this was because the majority of participants received a score of 15, and at 6 months this was due to the lack of data.
Linear regression was used to model the effect of treatment (dexamethasone vs. placebo) on the BI total score and the EQ-5D-5L utility index (both measured at discharge from NSU and at 3 and 6 months). The treatment effect, standard error, 95% CI and p-value were produced for each fitted model. As a secondary analysis, non-parametric Mann–Whitney U-tests (p-value reported) were also performed on the BI outcomes owing to the skewed nature of the data.
No adjustments for baseline covariates were made.
Mortality
The effect of treatment (dexamethasone vs. placebo) on the binary outcome death (yes/no) at 30 days and at 6 months was modelled using logistic regression, and the OR, 95% CI and p-value were produced.
A listing of deaths, including information on site, treatment arm, gender, age and time in the trial, and a bar chart showing the number of deaths by key time points (≤ 14 days, 15–30 days, 31–90 days and ≥ 90 days) were also produced using the full analysis population.
Discharge information
Negative binomial regression was used to model the effect of treatment (dexamethasone vs. placebo) on the length of stay in NSU and length of stay in secondary care. The rate ratio, 95% CI and p-value were reported.
Logistic regression was used to model the effect of treatment on discharge destination from NSU, with the OR, 95% CI and p-value reported. Two separate regression models were fitted for the following outcome categories: home versus other and local hospital versus other (excluding home). This was due to the spread of data in the different discharge destination categories.
Ancillary analyses
A number of additional analyses were performed on the primary outcome.
Model fitting
A logistic regression model adjusted for the baseline covariates, age and GCS on admission, was fitted to the primary outcome, and the OR for the treatment effect (dexamethasone vs. placebo), 95% CI and p-value were reported.
A proportional odds logistic regression model adjusted for age and GCS on admission was fitted to the ordinal mRS at 6 months and the following output: cumulative probabilities for the placebo arm at each cut-off point of the mRS; global OR together with the 95% CI and p-value; frequency and percentage of patients with a mRS score less than or equal to each cut-off point for both the dexamethasone arm and the placebo arm; and the marginal OR (95% CI) at each cut-off point.
Both models were fitted using the full analysis and per-protocol populations.
Mediation
A mediation analysis to investigate the direct effect of treatment on the primary outcome and the indirect effect of treatment via the mediator variable recurrent CSDH was performed by estimating the causal parameters using parametric regression models for the mediator and outcome. The assumption of no unmeasured confounders was made. A plot showing the direct, indirect and total effects (given as an increase in the probability of having a favourable outcome) was produced. The analysis was performed using both the full analysis and the per-protocol populations.
Compliance
The effect of treatment compliance on the primary outcome was explored in three ways. First, a logistic regression model was fitted to test for the interaction between treatment and the percentage of medication taken. The ORs, 95% CIs and p-values were produced for both the main and the interaction effects.
Second, a complier-average causal effect (CACE) analysis was performed. 33 The CACE was calculated for different cut-off points of compliance (> 50%, > 60%, > 70%, > 80%, > 90% and 100% of medication taken). A plot showing the CACE and 95% CI at each cut-off point was produced.
Finally, an instrumental variable analysis was performed to estimate the effect of compliance measured on a continuous percentage scale using randomisation as the instrumental variable and a two-stage residual inclusion method. 34 The OR for the percentage of medication taken and bootstrapped 95% CI were produced.
The above analyses were performed using only the full analysis population, as treatment compliance is a condition of the per-protocol population.
Subgroups
Exploratory subgroup analyses looked for a treatment interaction effect (using logistic regression) with the following subgroups measured at baseline:
-
Cambridge versus other sites
-
age (< 70 years vs. ≥ 70 years)
-
head trauma (no head trauma, occurred ≤ 4 weeks ago, occurred > 4 weeks ago and unknown timing)
-
use of anticoagulants or platelets versus none
-
GCS score on admission to NSU
-
unilateral versus bilateral CSDH – as defined in imaging findings.
The ORs, 95% CIs and p-values were reported for both the main and the interaction effects. The subgroup-specific treatment effect estimates were calculated only if the interaction effect was judged to be statistically and clinically significant.
Summary statistics (frequency and percentage of patients achieving a favourable mRS outcome at 6 months) were produced by treatment arm for the following post-baseline subgroups:
-
recurrent CSDH (one or more reoperation vs. no reoperations)
-
surgical intervention during primary surgery (burr hole, mini-craniotomy)
-
drain during primary surgery versus no drain during primary surgery
-
conservative management versus non-conservative management (no surgery on any admission vs. one or more operation)
-
trial conservative management (surgery within 7 days of randomisation vs. surgery > 7 days after randomisation vs. no surgery at any time point).
All subgroup analyses were performed using both the full analysis and the per-protocol populations.
Adverse event analyses
These analyses were performed on the safety population. Listings of safety events were produced and included:
-
participant ID
-
site
-
treatment arm
-
onset and resolution dates
-
Medical Dictionary for Regulatory Activities (MedDRA) preferred term (PT) and system organ class (SOC)
-
causality
-
outcome.
For serious adverse events (SAEs), the severity, seriousness and SAE reference number were also reported.
The frequency and percentage of MedDRA SOC codes were calculated, with each participant counted only once and the total population size used as the denominator. Figures showing the incidence of safety events and the relative risk (with 95% CI) by treatment arm based on the MedDRA SOC codes were produced.
Safety events were categorised as adverse events of special interest (AESIs), SAEs or AEs. A listing was produced only for AEs.
Adverse events of special interest
Adverse events of special interest were defined as:
-
hyperglycaemia necessitating stopping of trial medication
-
new-onset diabetes necessitating ongoing medical treatment at day 30 follow-up
-
hyperosmolar hyperglycaemic state
-
new-onset psychosis
-
upper gastrointestinal side effects (e.g. heartburn and vomiting)
-
peptic ulceration and gastrointestinal bleeding.
Separate listings and summary statistics were produced for non-serious AESIs and serious AESIs. The incidence and relative risk plot were produced only for non-serious AESIs owing to the small number of serious AESIs.
The following AESI was also summarised by past medical history of diabetes (yes/no) and treatment group: hyperglycaemia necessitating stopping of trial medication.
Serious adverse events
Serious adverse events were defined as any untoward medical occurrence or effect that:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing inpatients’ hospitalisation
-
resulted in persistent or significant disability or incapacity
-
was a congenital anomaly or birth defect
-
was another important medical event.
Initial index surgery was not reported as a SAE unless any of the above criteria were met.
Serious adverse events were categorised into non-reportable SAEs, reportable SAEs occurring within 30 days of starting the IMP and reportable SAEs post day 30. Listings and summary statistics were produced as previously described for each category. Summary statistics were also produced for the outcome of reportable SAEs (split by timing). A post hoc analysis for the difference in proportion of reportable SAEs occurring within 30 days of starting treatment was also performed.
Patient and public involvement
Our trial team sought key stakeholder perspectives in the design of the trial. These included a charity (Age UK, London, UK) and a patient representative from the Public Involvement in Research Group for Cambridgeshire & Bedfordshire. Their views guided the development of the proposed protocol and selection of appropriate outcome measures to ensure acceptability among patients and their families. In addition, we undertook two community consultations to shape participant consenting and enrolment.
Four questions were asked with regard to dissemination of the Dex-CSDH trial to the Cambridge University Hospital patient and public involvement (PPI) panel in July 2019:
-
As trial participants were recruited from all over the UK and many are elderly, and may be in care homes, a post-trial meeting to share findings directly with participants will not be feasible. Would putting a summary of the trial results, in a patient-friendly style of reporting, on the trial website (www.dexcsdh.org/) and the Headway website (URL: www.headway.org.uk) be helpful?
-
From your experience, can you suggest other ways of sharing the results/findings with the general and target population?
-
Will the panel be willing to help design/review dissemination materials from a patient viewpoint?
-
We are aware of the potential difficulties with dissemination of results through social media platforms. Please may we ask your opinions regarding the use of social media for this purpose?
Summary of responses:
-
Responses should primarily be by post owing to the likely demographic of the participants, but an online version would be welcome as well. A separate leaflet for relatives and friends would be welcome.
-
Posters in general practices and care home visits may support the dissemination of results.
-
Widespread interest in helping with this.
-
Very cautious of social media dissemination for the public – often not trusted.
The questionnaire completed by the Cambridge University Hospital PPI panel guided the dissemination plan. It was clear that social media were not the most appropriate platform for this, although it was felt that they should still be utilised cautiously. Owing to unforeseen circumstances, there was a change of PPI lead during the study, which inevitably resulted in a loss of focus for a period of time. However, good engagement with local advisory groups and the Cambridge University Hospital panel helped us to develop a robust dissemination plan. Engagement with a PPI group, rather than an individual lead, from the start would have helped with integration of PPI throughout; however, the advisory groups that we did approach were very helpful with the areas that were discussed.
Chapter 3 Trial results
Recruitment
Patients were recruited between August 2015 and November 2018. The trial was stopped when the required sample size of 750 patients had been reached.
Patient disposition
A CONSORT flow diagram is shown in Figure 1.
A total of 2203 patients were screened, with 750 randomised. Two patients were excluded immediately after randomisation owing to ineligibility; neither patient had received their assigned intervention and no data were collected from these patients. Of the 748 eligible patients, 375 were randomised to receive dexamethasone (with 365 receiving at least one dose of the intervention) and 373 were randomised to receive placebo (with 361 receiving at least one dose of the intervention). Therefore, the full analysis and safety population comprised 748 participants.
In total, 45 patients withdrew consent to participate in the trial (25 in the placebo arm, 20 in the dexamethasone arm) and 23 patients were lost to follow-up (9 in the placebo arm and 14 in the dexamethasone arm). Therefore, 680 patients were followed up to the primary outcome measure at 6 months (339 in the placebo arm and 341 in the dexamethasone arm) and analysed using the modified intention-to-treat analysis.
The main per-protocol population (not taking into account time windows) comprised 597 patients (307 in the placebo arm and 290 in the dexamethasone arm). The number of patients in the per-protocol population at discharge was 491 (252 in the placebo arm and 239 in the dexamethasone arm), at 3 months was 539 (280 in the placebo arm and 259 in the dexamethasone arm) and at 6 months was 553 (283 in the placebo arm and 270 in the dexamethasone arm).
Baseline information
Demographic details and health status prior to CSDH are provided in Table 2 and show no apparent differences between the treatment arms. Injury background and imaging details are provided in Table 3. For details of medical conditions and prior and concurrent medications based on the full analysis population, see Appendix 3, Tables 26–28. Appendix 3 also shows summary statistics for the blood and clotting products given on admission, which were similar between treatment arms.
| Demographic | Treatment arm | Total | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Age (years) | |||
| n | 373 | 375 | 748 |
| Mean (SD) | 74.3 (11.0) | 74.5 (11.8) | 74.4 (11.4) |
| Median | 76 | 76 | 76 |
| Minimum, maximum | 21, 95 | 23, 97 | 21, 97 |
| Gender, n/N (%) | |||
| Male | 286/373 (76.7) | 268/375 (71.5) | 554/748 (74.1) |
| Female | 87/373 (23.3) | 107/375 (28.5) | 194/748 (25.9) |
| Ethnicity, n/N (%) | |||
| Caucasian/white | 353/372 (94.9) | 360/373 (96.5) | 713/745 (95.7) |
| Black | 7/372 (1.9) | 4/373 (1.1) | 11/745 (1.5) |
| Asian | 11/372 (3) | 7/373 (1.9) | 18/745 (2.4) |
| Hispanic | 1/372 (0.3) | 0/373 (0) | 1/745 (0.1) |
| Other | 0/372 (0) | 2/373 (0.5) | 2/745 (0.3) |
| Residence prior to CSDH, n/N (%) | |||
| Independent | 328/372 (88.2) | 327/374 (87.4) | 655/746 (87.8) |
| Carers at home | 30/372 (8.1) | 24/374 (6.4) | 54/746 (7.2) |
| Residential home | 1/372 (0.3) | 3/374 (0.8) | 4/746 (0.5) |
| Nursing home | 4/372 (1.1) | 6/374 (1.6) | 10/746 (1.3) |
| Other | 9/372 (2.4) | 14/374 (3.7) | 23/746 (3.1) |
| Mobility prior to CSDH, n/N (%) | |||
| Independent | 307/372 (82.5) | 294/375 (78.4) | 601/747 (80.5) |
| Stick | 40/372 (10.8) | 43/375 (11.5) | 83/747 (11.1) |
| Walking frame | 20/372 (5.4) | 17/375 (4.5) | 37/747 (5) |
| Wheelchair | 1/372 (0.3) | 3/375 (0.8) | 4/747 (0.5) |
| Bedbound | 0/372 (0) | 5/375 (1.3) | 5/747 (0.7) |
| Other | 4/372 (1.1) | 13/375 (3.5) | 17/747 (2.3) |
| Premorbid mRS score, n/N (%) | |||
| 0: No symptoms | 182/373 (48.8) | 178/373 (47.7) | 360/746 (48.3) |
| 1: No significant disability | 53/373 (14.2) | 55/373 (14.7) | 108/746 (14.5) |
| 2: Slight disability | 40/373 (10.7) | 36/373 (9.7) | 76/746 (10.2) |
| 3: Moderate disability | 29/373 (7.8) | 30/373 (8) | 59/746 (7.9) |
| 4: Moderately severe disability | 14/373 (3.8) | 20/373 (5.4) | 34/746 (4.6) |
| 5: Severe disability | 0/373 (0) | 3/373 (0.8) | 3/746 (0.4) |
| Not available | 55/373 (14.7) | 51/373 (13.7) | 106/746 (14.2) |
| Baseline data | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Timing of onset of symptoms related to CSDH | |||
| < 7 days | 133/373 (35.7) | 140/373 (37.5) | 273/746 (36.6) |
| 7–14 days | 116/373 (31.1) | 100/373 (26.8) | 216/746 (29) |
| 15–28 days | 75/373 (20.1) | 64/373 (17.2) | 139/746 (18.6) |
| 29–42 days | 22/373 (5.9) | 26/373 (7) | 48/746 (6.4) |
| > 42 days | 19/373 (5.1) | 35/373 (9.4) | 54/746 (7.2) |
| Not known | 8/373 (2.1) | 8/373 (2.1) | 16/746 (2.1) |
| Known head trauma | |||
| Yes | 267/373 (71.6) | 253/373 (67.8) | 520/746 (69.7) |
| If known head trauma, how long ago did it occur? | |||
| < 2 weeks ago | 56/267 (20.9) | 59/253 (23.4) | 115/518 (22.2) |
| 2–4 weeks ago | 77/267 (28.9) | 72/253 (28.5) | 149/518 (28.8) |
| 1–3 months ago | 110/267 (41.2) | 94/253 (37.1) | 204/518 (39.4) |
| 4–6 months ago | 10/267 (3.7) | 17/253 (6.7) | 27/518 (5.2) |
| > 6 months ago | 6/267 (2.2) | 1/253 (0.4) | 7/518 (1.4) |
| Not known | 7/267 (2.6) | 9/253 (3.6) | 16/518 (3.1) |
| Density of CSDH | |||
| Hypodense | 89/355 (25.1) | 111/361 (30.7) | 200/716 (27.9) |
| Isodense | 96/355 (27.0) | 73/361 (20.2) | 169/716 (23.6) |
| Mixed density | 170/355 (47.9) | 177/361 (49.0) | 347/716 (48.5) |
| Midline shift | |||
| 0–5 mm | 74/318 (23.3) | 68/314 (21.7) | 142/632 (22.5) |
| 6–10 mm | 115/318 (36.2) | 126/314 (40.1) | 241/632 (38.1) |
| > 10 mm | 129/318 (40.6) | 120/314 (38.2) | 249/632 (39.4) |
Treatment compliance
Treatment compliance was similar between treatment arms, with the mean percentage of tablets taken being 89% in the placebo arm and 87% in the dexamethasone arm based on the daily medication records, and 94% compared with 94% for the placebo arm and the dexamethasone arm, respectively, based on remaining pill count for those who completed treatment in the hospital.
Outcomes
Primary analysis
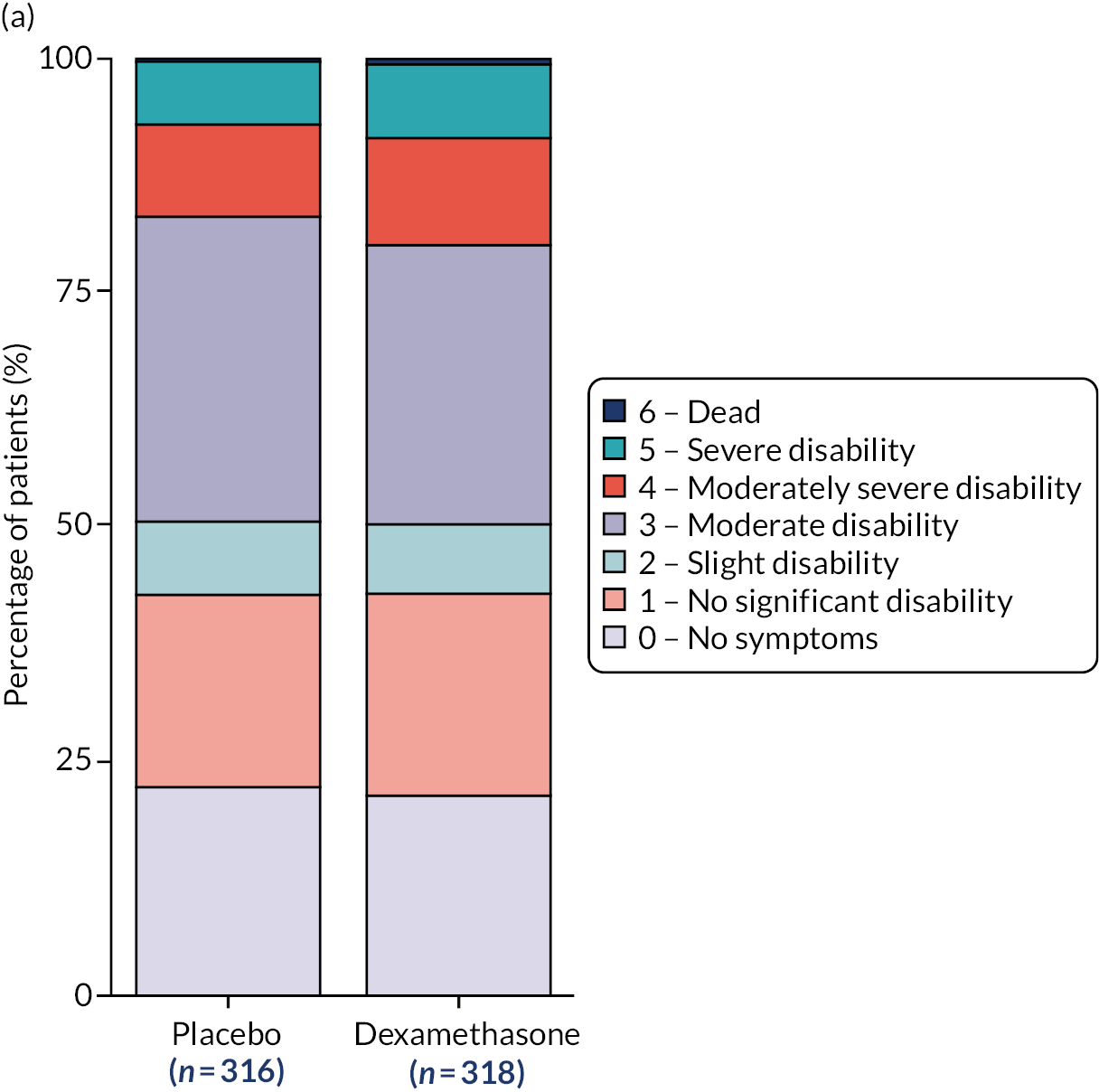
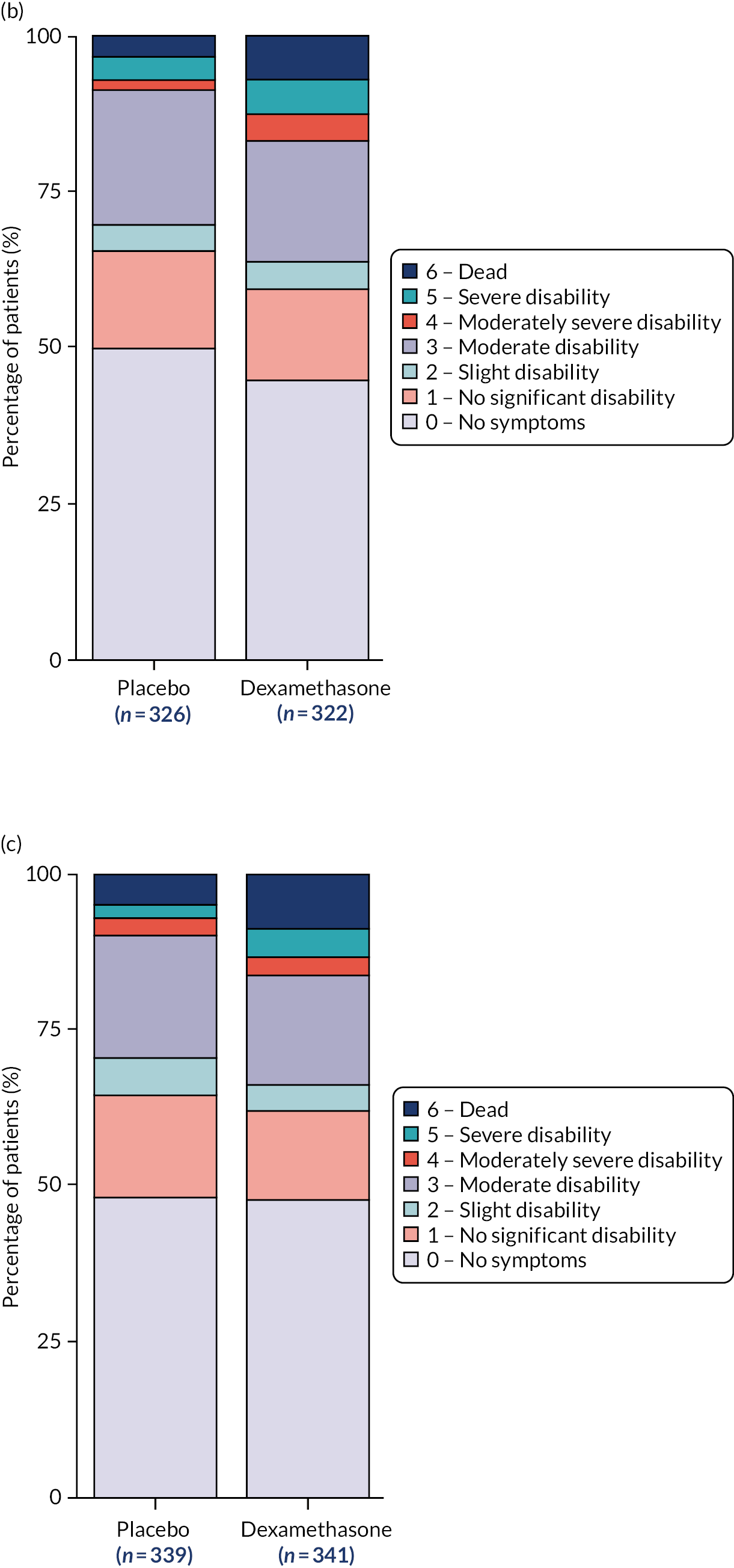
Summary statistics for the primary outcome are presented in Table 4 for the full analysis population, with dichotomous scores at discharge, 3 months and 6 months presented in Figure 2. The per-protocol results can be found in Appendix 3, Table 29. The absolute difference in the proportion achieving a favourable outcome (mRS score of 0–3) at 6 months was –6.4% in the dexamethasone arm compared with the placebo arm (95% CI –11.4% to –1.4%; p = 0.01), based on the full analysis population. The per-protocol analysis gave similar results, with an absolute difference in proportions of –6.4% (95% CI –12% to –1%; p = 0.02).
| mRS | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Score | |||
| 0: No symptoms | 164/339 (48.4) | 163/341 (47.8) | 327/680 (48.1) |
| 1: No significant disability | 55/339 (16.2) | 49/341 (14.4) | 104/680 (15.3) |
| 2: Slight disability | 21/339 (6.2) | 14/341 (4.1) | 35/680 (5.1) |
| 3: Moderate disability | 66/339 (19.5) | 60/341 (17.6) | 126/680 (18.5) |
| 4: Moderately severe disability | 9/339 (2.7) | 10/341 (2.9) | 19/680 (2.8) |
| 5: Severe disability | 7/339 (2.1) | 15/341 (4.4) | 22/680 (3.2) |
| 6: Dead | 17/339 (5.0) | 30/341 (8.8) | 47/680 (6.9) |
| Dichotomised | |||
| Favourable | 306/339 (90.3) | 286/341 (83.9) | 592/680 (87.1) |
| Unfavourable | 33/339 (9.7) | 55/341 (16.1) | 88/680 (12.9) |
FIGURE 2.
Modified Rankin Scale by treatment arm and time point. (a) Discharge mRS score; (b) 3-month mRS score; and (c) 6-month mRS score.
Secondary analyses
Surgery
Summary statistics for the number of surgical interventions undertaken during index and subsequent admissions based on the full analysis population are presented in Table 5, along with the recurrence rate in each treatment arm. For summary statistics based on the per-protocol population, see Appendix 3, Table 30.
| Number of surgeries | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Index admission | |||
| 0 | 29/370 (7.8) | 30/372 (8.1) | 59/742 (8.0) |
| 1 | 330/370 (89.2) | 341/372 (91.7) | 671/742 (90.4) |
| 2 | 10/370 (3.0) | 1/372 (0.2) | 11/742 (1.5) |
| 3 | 1/370 (0.23) | 0/372 (0) | 1/742 (0.1) |
| Subsequent admissions | |||
| 1 | 25/370 (6.7) | 16/372 (4.3) | 41/742 (5.5) |
| 2 | 2/370 (0.5) | 3/372 (0.8) | 5/742 (0.7) |
| 3 | 1/370 (0.3) | 0/375 (0) | 1/742 (0.1) |
| Repeat surgery for recurrence of CSDHa | 25/350 (7.1) | 6/349 (1.7) | 31/699 (4.4) |
The effect of dexamethasone compared with placebo on the number of surgeries undertaken during the index and during subsequent admissions was not significant for both the full analysis (Table 6) and the per-protocol populations (see Appendix 3, Table 31).
| Outcome | Covariate | Estimate | 95% CI | p-value |
|---|---|---|---|---|
| Number of surgeries: index admission (including pre randomisation) | (Intercept) | 0.954 | 0.858 to 1.06 | |
| Dexamethasone vs. placebo | 0.966 | 0.833 to 1.12 | 0.653 | |
| Number of surgeries: index admission (excluding pre randomisation) | (Intercept) | 0.489 | 0.421 to 0.564 | |
| Dexamethasone vs. placebo | 0.896 | 0.724 to 1.11 | 0.308 | |
| Number of surgeries: subsequent admissions (re-admissions only) | (Intercept) | 0.489 | 0.421 to 0.564 | |
| Dexamethasone vs. placebo | 0.896 | 0.724 to 1.11 | 0.308 | |
| Number of surgeries: subsequent admissions (all patients) | (Intercept) | 0.0858 | 0.0594 to 0.119 | |
| Dexamethasone vs. placebo | 0.684 | 0.392 to 1.17 | 0.17 |
For full details of the type of surgical procedures undertaken during primary surgery, see Appendix 3, Table 32. The number and type of surgical procedures performed during primary surgery were similar between treatment arms. Similar details for recurrent surgery are also found in Appendix 3, Table 33.
Modified Rankin Scale
There was no significant difference in mRS scores between the dexamethasone arm and the placebo arm at discharge. At 3 months, the effect of dexamethasone compared with placebo was to significantly decrease the odds of achieving a favourable outcome. This can be clearly seen from both the global ORs and the marginal ORs in Table 7. Results were similar for both the full analysis and the per-protocol populations.
| Cut-off point | Ordinal logistic regression | Sequential ORs | ||||
|---|---|---|---|---|---|---|
| Probability mRS ≤ cut-off point (placebo arm) | Global OR (95% CI)a | p-value | Placebo (n = 316, discharge) (n = 326, 3 months), n (%) | Dexamethasone (n = 318, discharge) (n = 322, 3 months), n (%) | Marginal OR (95% CI) | |
| mRS at discharge | ||||||
| 0 | 0.226 | 0.937 (0.71 to 1.24) | 0.644 | 71 (22) | 69 (22) | 0.956 (0.657 to 1.392) |
| 1 | 0.439 | 136 (43) | 137 (43) | 1.002 (0.732 to 1.372) | ||
| 2 | 0.514 | 161 (51) | 160 (50) | 0.975 (0.714 to 1.331) | ||
| 3 | 0.822 | 263 (83) | 255 (80) | 0.816 (0.545 to 1.222) | ||
| 4 | 0.924 | 293 (93) | 291 (92) | 0.846 (0.474 to 1.51) | ||
| 5 | 0.995 | 315 (100) | 316 (99) | 0.502 (0.045 to 5.56) | ||
| 6 | N/A | N/A | N/A | N/A | ||
| mRS at 3 months | ||||||
| 0 | 0.509 | 0.747 (0.561 to 0.993) | 0.044 | 163 (50) | 144 (45) | 0.809 (0.594 to 1.102) |
| 1 | 0.658 | 213 (65) | 192 (60) | 0.784 (0.57 to 1.078) | ||
| 2 | 0.698 | 227 (70) | 205 (64) | 0.764 (0.551 to 1.06) | ||
| 3 | 0.889 | 298 (91) | 268 (83) | 0.466 (0.287 to 0.758) | ||
| 4 | 0.915 | 303 (93) | 282 (88) | 0.535 (0.313 to 0.916) | ||
| 5 | 0.956 | 315 (97) | 300 (93) | 0.476 (0.227 to 0.999) | ||
| 6 | N/A | N/A | N/A | N/A | ||
Figure 1 shows the percentage of patients in each mRS category at each assessment time point by treatment arm, including missing data. This shows that pre-morbid mRS score was similar between arms, on admission to NSU and at discharge, whereas, at 3 and 6 months, the outcomes in the placebo arm were more favourable than those in the dexamethasone arm. The number of missing data was similar between arms. Summary statistics for the mRS score on admission to the NSU, at discharge and at 3 months are presented in Appendix 3, Tables 34 and 35, for the full analysis population and Tables 36 and 37 for the per-protocol population. Table 7 contains the model-fitting results at discharge and 3 months using the full analysis population. Model-fitting results for the per-protocol population can be found in Appendix 3, Table 38.
Glasgow Coma Scale
On admission to the NSU, 350 out of 371 (94%) patients in both the dexamethasone arm and the placebo arm had a GCS total score of 13–15, based on the full analysis population. These figures were similar for the per-protocol population, with 272 out of 289 (94%) in the dexamethasone arm and 286 out of 306 (94%) in the placebo arm receiving a total score of 13–15. Almost 100% of patients in both arms had a GCS total score of 13–15 on discharge from the NSU, using both the full analysis (dexamethasone: 351/354, 99.2%; placebo: 355/356, 99.7%) and the per-protocol populations (dexamethasone: 236/237, 99.6%; placebo: 250/251, 99.6%). Only 27 patients had 6-month GCS data, with 24 out of 27 (89%) patients having a total score of 13–15.
Barthel Index
The summary statistics for BI score at discharge, 3 months and 6 months using the full analysis and per-protocol populations can be found in Appendix 3, Tables 39–44. There was no significant effect of dexamethasone compared with placebo on BI total score at any time point for either the full analysis (Table 8) or the per-protocol populations (see Appendix 3, Table 45).
| Outcome | Linear regression | Mann–Whitney U-test: p-value | |||
|---|---|---|---|---|---|
| Covariate | Estimate (SE) | 95% CI | p-value | ||
| BI at discharge | (Intercept) | 80.5 (1.54) | 77.4 to 83.5 | 0.414 | |
| Dexamethasone vs. placebo | 0.505 (2.18) | −3.78 to 4.79 | 0.817 | ||
| BI at 3 months | (Intercept) | 89.4 (1.24) | 87 to 91.8 | 0.305 | |
| Dexamethasone vs. placebo | −2.68 (1.77) | −6.16 to 0.8 | 0.131 | ||
| BI at 6 months | (Intercept) | 90.3 (1.17) | 88 to 92.7 | 0.32 | |
| Dexamethasone vs. placebo | −2.29 (1.67) | −5.57 to 0.995 | 0.172 | ||
EuroQol-5 Dimensions, five-level version
The summary statistics for EQ-5D-5L at discharge, 3 months and 6 months using the full analysis and per-protocol populations can be found in Appendix 3, Tables 46–51. The effect of dexamethasone compared with placebo on the EQ-5D-5L utility index at discharge and 6 months using the full analysis population was not significant (Table 9); however, the results were significant at 3 months, with dexamethasone being associated with a worse outcome than placebo (a decrease in utility index of −0.07). Similar results were found using the per-protocol population (see Appendix 3, Table 52).
| Outcome | Covariate | Estimate (SE) | 95% CI | p-value |
|---|---|---|---|---|
| EQ-5D-5L utility index at discharge | (Intercept) | 0.727 (0.016) | 0.695 to 0.758 | |
| Dexamethasone vs. placebo | −0.03 (0.0226) | −0.0743 to 0.0142 | 0.183 | |
| EQ-5D-5L utility index at 3 months | (Intercept) | 0.773 (0.0177) | 0.739 to 0.808 | |
| Dexamethasone vs. placebo | −0.0666 (0.0251) | −0.116 to −0.0174 | 0.008 | |
| EQ-5D-5L utility index at 6 months | (Intercept) | 0.766 (0.0188) | 0.73 to 0.803 | |
| Dexamethasone vs. placebo | −0.0334 (0.0267) | −0.0858 to 0.019 | 0.211 |
Mortality
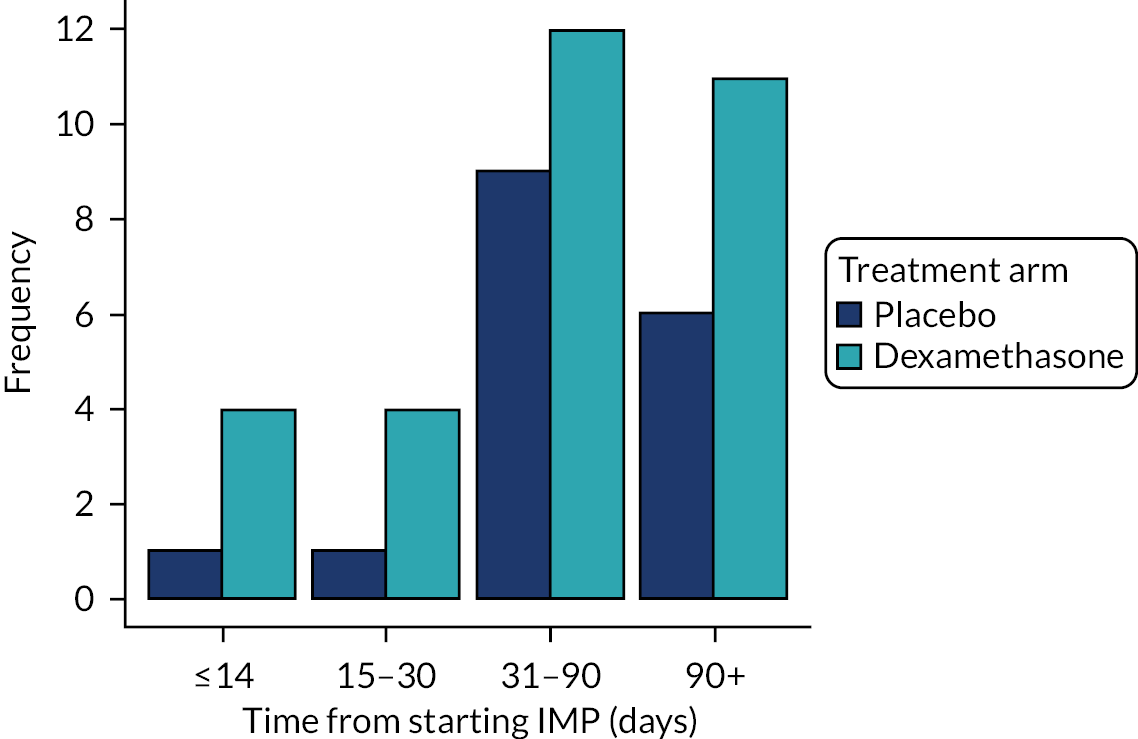
There were 17 deaths (5%) in the placebo arm, compared with 31 (8%) deaths in the dexamethasone arm. Figure 3 shows a bar chart of the number of deaths by key time points. Although there were more deaths in the dexamethasone arm than in the placebo arm, the difference was not significant at either day 30 or 6 months (Table 10).
FIGURE 3.
Number of deaths by time point and treatment arm.
| Outcome | ORa | 95% CI | p-value |
|---|---|---|---|
| Full analysis population | |||
| Mortality at 30 days | 4.08 | 1.01 to 27.2 | 0.077 |
| Mortality at 6 months | 1.83 | 0.99 to 3.45 | 0.062 |
| Per-protocol population | |||
| Mortality at 30 days | 2.13 | 0.413 to 15.5 | 0.384 |
| Mortality at 6 months | 1.97 | 0.908 to 4.5 | 0.094 |
Discharge information
The summary statistics on the discharge destination after index admission and the length of stay in NSU, secondary care and intensive care unit (ICU)/high-dependency unit (HDU) are provided in Table 11 for the full analysis population (see Appendix 3, Table 53). Discharge destination was similar for both treatment arms, with the majority of patients being discharged to either their home or a local hospital. Length of stay in NSU, secondary care and ICU/HDU, as well as the number of patients who stayed in ICU, were also similar between treatment arms. Model-fitting results showed that there were no statistically significant differences between treatment arms for either the full analysis population (Table 12) or the per-protocol population (see Appendix 3, Table 54).
| Outcome | Treatment arm | Total | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Discharge destination after index admission, n/N (%) | |||
| Home | 253/362 (69.9) | 239/361 (66.2) | 492/723 (68) |
| Carers at home | 13/362 (3.6) | 6/361 (1.7) | 19/723 (2.6) |
| Local hospital | 66/362 (18.2) | 84/361 (23.3) | 150/723 (20.7) |
| Rehabilitation centre | 8/362 (2.2) | 8/361 (2.2) | 16/723 (2.2) |
| Residential home | 1/362 (0.3) | 1/361 (0.3) | 2/723 (0.3) |
| Nursing home | 2/362 (0.6) | 5/361 (1.4) | 7/723 (1) |
| Other | 19/362 (5.2) | 18/361 (5) | 37/723 (5.1) |
| Length of stay in NSU (days) | |||
| n | 359 | 362 | 721 |
| Mean (SD) | 9.03 (8) | 9.3 (8.4) | 9.18 (8.18) |
| Median | 6 | 7 | 7 |
| Minimum, maximum | 1, 63 | 2, 70 | 1, 70 |
| Length of stay in secondary care (days)a | |||
| n | 359 | 362 | 721 |
| Mean (SD) | 13.7 (23) | 13.0 (17) | 13.4 (20.0) |
| Median | 7 | 8 | 7 |
| Minimum, maximum | 1, 219 | 2, 198 | 1, 219 |
| Stayed in ICU/HDU, n/N (%) | |||
| Yes | 39/373 (10.5) | 36/375 (9.6) | 75/748 (10) |
| Length of stay in ICU/HDU (days) | |||
| n | 39 | 36 | 75 |
| Mean (SD) | 3.05 (3.19) | 3.08 (2.41) | 3.07 (2.82) |
| Median | 2 | 2 | 2 |
| Minimum, maximum | 1, 17 | 1, 10 | 1, 17 |
| Outcome | Estimatea | 95% CI | p-value |
|---|---|---|---|
| Negative binomial regression model | |||
| Length of stay in NSU (days) | 1.03 | 0.934 to 1.14 | 0.535 |
| Length of stay in secondary care (days) | 0.952 | 0.835 to 1.09 | 0.467 |
| Logistic regression model | |||
| Discharge destination after index admissionb | 1.18 | 0.867 to 1.62 | 0.288 |
| Discharge destination after index admissionc | 0.694 | 0.402 to 1.19 | 0.188 |
Ancillary analyses
Model fitting
Model-fitting results for the primary outcome, adjusting for age and GCS on admission, are presented in Table 13 for the full analysis population. The results show that, even after adjusting for baseline variables (both significant), the odds of achieving a favourable outcome are still significantly lower for dexamethasone than placebo. Per-protocol analyses gave similar results (see Appendix 3, Table 55).
| Covariate | Odds ratio (95% CI) | p-value |
|---|---|---|
| Dexamethasone vs. placebo | 0.553 (0.33 to 0.914) | 0.022 |
| Age (years) | 0.902 (0.873 to 0.93) | < 0.001 |
| GCS at baseline | 1.46 (1.27 to 1.69) | < 0.001 |
Model-fitting results for the ordinal mRS score at 6 months, adjusted for age and GCS on admission, are presented in Table 14 for the full analysis population. Although the global OR for dexamethasone compared with placebo was not statistically significant, it can be seen from the marginal ORs that at a cut-off point of 3, that is, the odds of achieving a favourable outcome, are significantly worse in the dexamethasone arm than the placebo arm. The per-protocol analysis gave similar results (see Appendix 3, Table 56).
| Ordinal logistic regression | Sequential ORs | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | Global OR (95% CI)a | p-value | Cut-off point | Probability mRS ≤ cut-off point (placebo arm) | Placebo (n = 339), n (%) | Dexamethasone (n = 341), n (%) | Marginal OR (95% CI) |
| Dexamethasone vs. placebo | 0.866 (0.651 to 1.15) | 0.324 | 0 | 0.483 | 164 (48) | 163 (48) | 0.977 (0.723 to 1.32) |
| Age (years) | 0.945 (0.931 to 0.958) | < 0.001 | 1 | 0.656 | 219 (65) | 212 (62) | 0.9 (0.659 to 1.23) |
| GCS at baseline | 1.41 (1.26 to 1.57) | < 0.001 | 2 | 0.715 | 240 (71) | 226 (66) | 0.811 (0.586 to 1.121) |
| 3 | 0.904 | 306 (90) | 286 (84) | 0.561 (0.354 to 0.889) | |||
| 4 | 0.929 | 315 (93) | 296 (87) | 0.501 (0.298 to 0.843) | |||
| 5 | 0.952 | 322 (95) | 311 (91) | 0.547 (0.296 to 1.012) | |||
| 6 | . | . | . | . | |||
Mediation
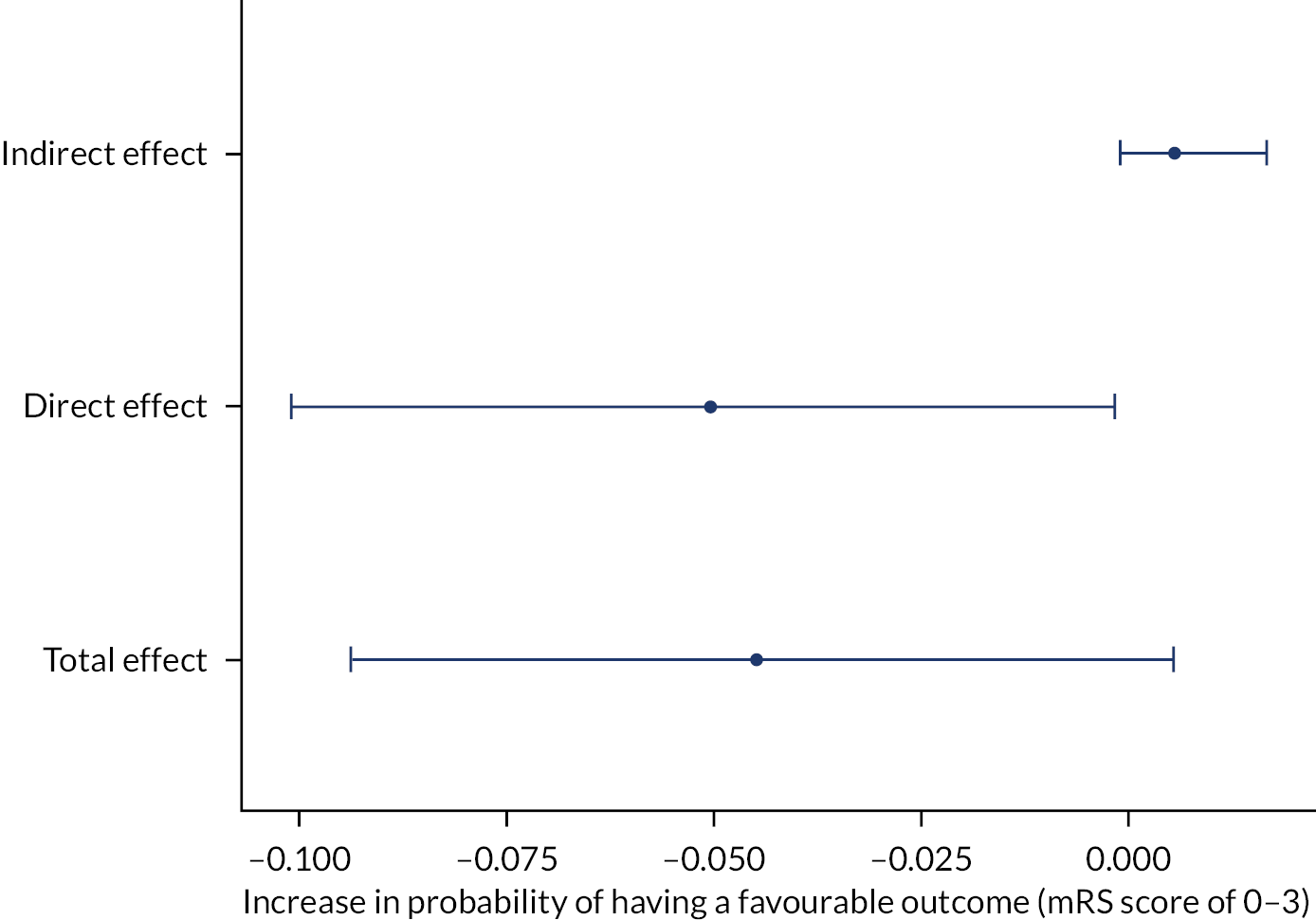
Figure 4 shows the results of the mediation analysis. The indirect effect of treatment via the mediator recurrent CSDH was not significant.
FIGURE 4.
Mediation analysis (full analysis population).
Compliance
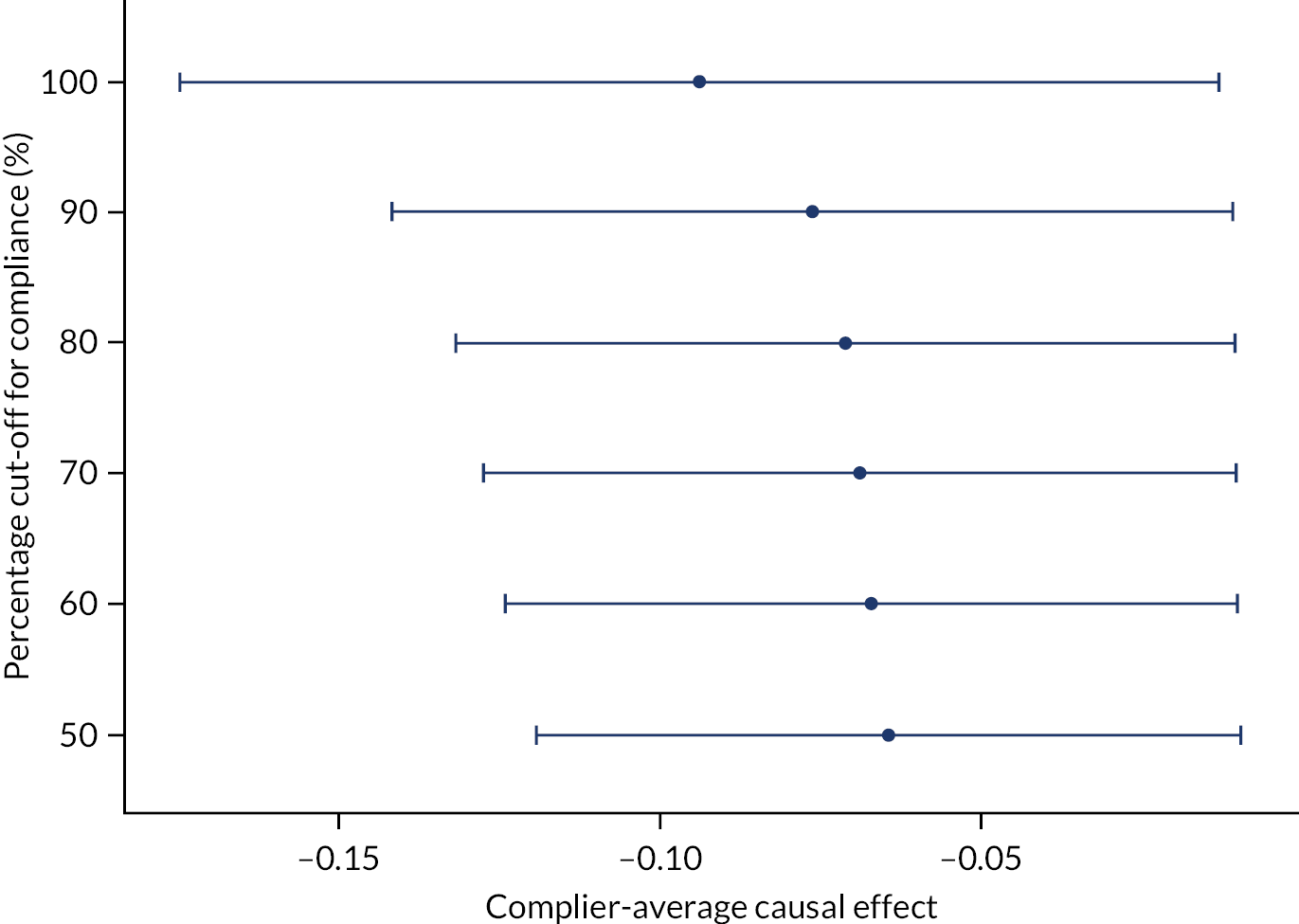
Table 15 shows the results of the logistic regression to investigate the effect of compliance with medication on the primary outcome. The interaction between the treatment arm and the percentage of medication taken was not significant. Figure 5 shows the results of the CACE analysis. This suggests that the more compliant that the patient was with medication in the dexamethasone arm, the worse their mRS outcome was at 6 months. The instrumental variables analysis gave an OR (95% CI) of 0.942 (0.891 to 0.994) of achieving a favourable outcome at 6 months for every 10% increase in medication taken.
| Covariate | OR | 95% CI | p-value |
|---|---|---|---|
| Dexamethasone vs. placebo | 1.06 | 0.195 to 5.43 | 0.941 |
| Percentage of medication taken | 1.02 | 1 to 1.03 | 0.034 |
| Treatment: dexamethasone – percentage of medication taken | 0.993 | 0.975 to 1.01 | 0.447 |
FIGURE 5.
The CACE analysis results.
Subgroups
Table 16 shows the model-fitting results for the baseline subgroup analyses based on the full analysis population. The only subgroup to have a significant interaction effect with treatment was the side of the CSDH (bilateral vs. unilateral), suggesting that the association between the treatment arm and the probability of achieving a favourable mRS outcome at 6 months depends on the side of the CSDH. Further investigation of the subgroup-specific treatment effects showed that the odds of having a favourable outcome in the dexamethasone arm compared with the placebo arm were 0.422 (95% CI 0.244 to 0.711; p = 0.001; n = 530) in patients with a unilateral CSDH, whereas there was no significant difference for patients with a bilateral CSDH (OR 1.55, 95% CI 0.574 to 4.29; p = 0.388; n = 150). No subgroups were significant when analysed using the per-protocol population. Appendix 3, Tables 57 and 58, shows the post-baseline subgroup analyses using the full analysis population. This shows that, although there was a higher proportion of recurrences in the placebo arm (symptomatic recurrence requiring re-operation of a previously evacuated ipsilateral chronic subdural haematoma), 89% of these patients had a favourable mRS outcome at 6 months, compared with 64% of patients with a recurrence in the dexamethasone arm. Results were similar for the per-protocol population (see Appendix 3, Table 59). These comparisons must be interpreted with caution because there may be confounding biases as a result of the subgroups being defined post randomisation.
| Subgroup | Favourable outcome (mRS score 0–3), n/N (%) | |
|---|---|---|
| Placebo | Dexamethasone | |
| Recurrence (one or more reoperation): yes | 25/28 (89.3) | 9/15 (60) |
| Surgical intervention during primary surgery | ||
| Burr hole(s) | 249/278 (89.6) | 229/274 (83.6) |
| Craniotomy | 33/37 (89.2) | 30/35 (85.7) |
| Drain during primary surgery | ||
| Yes | 247/276 (89) | 222/262 (85) |
| No | 43/47 (91) | 46/56 (82) |
| Conservative management (no surgery on any admission) | 16/16 (100) | 18/22 (82) |
| Surgery within 7 days of randomisation | 280/313 (89) | 264/313 (84) |
| Surgery > 7 days after randomisation | 10/10 (100) | 4/6 (67) |
Adverse event analyses
Adverse events of special interest were reported in 41 out of 375 patients (10.9%) in the dexamethasone arm and in 12 out of 373 patients (3.2%) in the placebo arm (OR 3.4, 95% CI 1.81 to 6.85). SAEs occurred in 60 out of 375 (16.0%) and 24 out of 373 (6.4%) patients, respectively (OR 2.49, 95% CI 1.54 to 4.15). The risk of any infection was 6.4% in the dexamethasone arm and 1.1% in the placebo arm.
Adverse events of special interest
A listing of non-serious AESIs is available in Appendix 3, Table 60. Figure 6 shows the incidence and relative risk plot for non-serious AESIs. Patients in the dexamethasone arm had more AESIs, with a significant increase in the relative risk of endocrine and psychiatric disorders. Table 17 provides summary statistics for hyperglycaemia AESIs by past history of diabetes. The majority of patients in the dexamethasone arm with an AESI of hyperglycaemia necessitating treatment had a previous history of diabetes. A listing of serious AESIs is provided in Appendix 3, Table 61.
FIGURE 6.
Incidence and relative risk plot for non-serious AESIs.
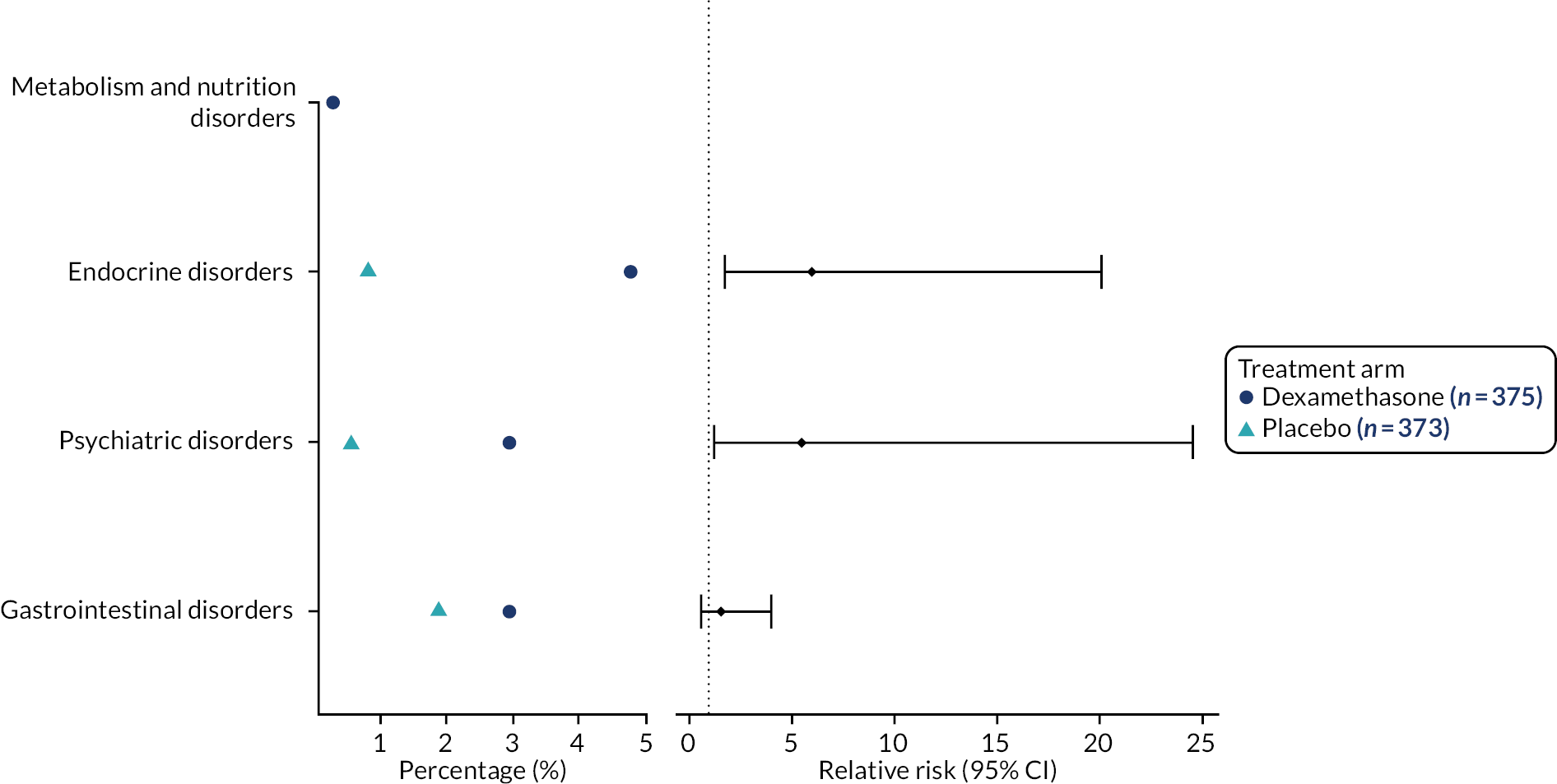
| Variable | History of diabetes | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|---|
| Placebo | Dexamethasone | |||
| Hyperglycaemia necessitating treatment | Yes | 1/373 (0.3) | 13/375 (3.5) | 14/748 (1.9) |
| No | 0/373 (0) | 3/375 (0.8) | 3/748 (0.4) | |
| Hyperglycaemia necessitating stopping of trial medication | Yes | 0/373 (0) | 1/375 (0.3) | 1/748 (0.1) |
Serious adverse events
Non-reportable SAEs are listed in Appendix 3, Table 62. Figure 7 shows the incidence and relative risk plot for non-reportable SAEs. The relative risk of a nervous system disorder was significantly lower in the dexamethasone arm than in the placebo arm.
FIGURE 7.
Incidence and relative risk plot for non-reportable SAEs.

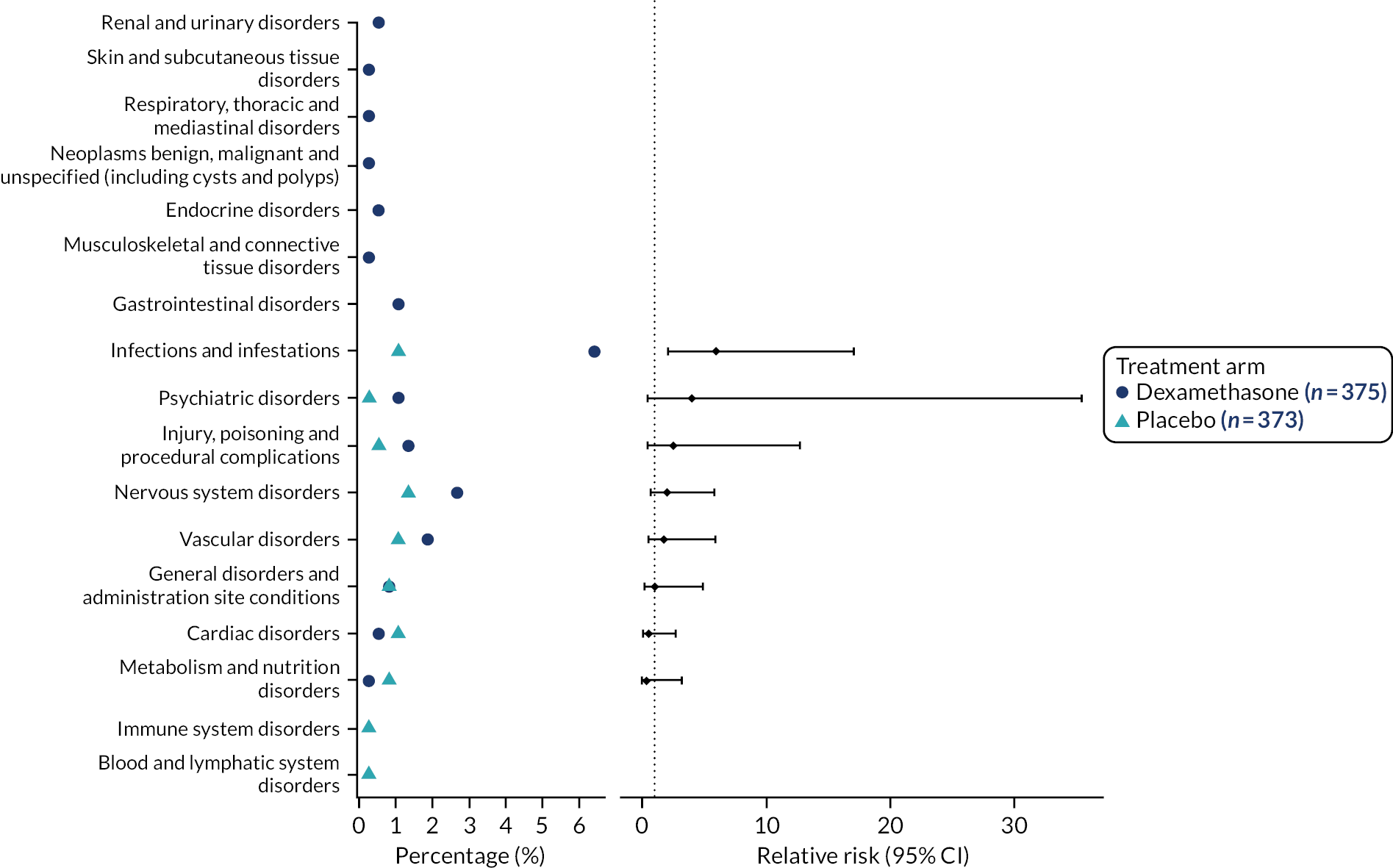
Reportable SAEs (pre-study day 30) are reported in Appendix 3, Table 63. Figure 8 shows the incidence and relative risk plot for reportable SAEs (pre-study day 30). In general, there were more reportable SAEs in the dexamethasone arm than the placebo arm, with the relative risk of infections and infestations significantly higher. Table 18 provides a summary of the pre-study day 30 SAE outcomes by treatment arm. A post hoc analysis showed a significant difference in the number of pre-study day 30 SAEs between treatment arms (19% dexamethasone vs. 8% placebo).
FIGURE 8.
Incidence and relative risk plot for reportable SAEs (pre-study day 30).
| SAE outcome | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Death | 3/373 (0.8) | 13/375 (3.5) | 16/748 (2.1) |
| Ongoing | 5/373 (1.3) | 7/375 (1.9) | 12/748 (1.6) |
| Resolved no residual effects | 14/373 (3.8) | 37/375 (9.9) | 51/748 (6.8) |
| Resolved with residual effects | 2/373 (0.5) | 8/375 (2.1) | 10/748 (1.3) |
Reportable SAEs (post-study day 30) are listed in Appendix 3, Table 64. Figure 9 shows the incidence and relative risk plot for post-study day 30 reportable SAEs. Table 19 provides a summary of the post-study day 30 SAE outcomes by treatment arm.
FIGURE 9.
Incidence and relative risk plot for reportable SAEs (post-study day 30).

| SAE outcome | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Death | 7/373 (1.9) | 2/375 (0.5) | 9/748 (1.2) |
| Ongoing | 1/373 (0.3) | 0/375 (0) | 1/748 (0.1) |
| Resolved no residual effects | 3/373 (0.8) | 8/375 (2.1) | 11/748 (1.5) |
| Resolved with residual effects | 1/373 (0.3) | 1/375 (0.3) | 2/748 (0.3) |
A list of AEs is provided in Appendix 3, Table 65.
Chapter 4 Economic evaluation
Objective
To estimate the cost-effectiveness of dexamethasone, compared with placebo, in patients with CSDH.
Background
Prior to analysis, a health economic analysis plan (HEAP) was developed, demonstrating that all analyses were prespecified (details of the HEAP are provided in this chapter). Here, for ease of reading, we present the methods and results (written in accordance with the Consolidated Health Economic Evaluation Reporting Standards checklist35) in sufficient detail that readers do not need to continually refer to the HEAP.
Methods
Trial design
As described above, the Dex-CSDH trial was a multicentre randomised trial conducted in the UK. The trial compared a tapering 2-week course of dexamethasone with matching placebo in patients with symptomatic CSDH. Patients were eligible for enrolment if they were aged ≥ 18 years and were admitted to a participating NSU with symptomatic CSDH that had been confirmed on cranial imaging.
Intervention
Eligible patients were randomly assigned in a 1: 1 ratio to receive a tapering 2-week course of oral dexamethasone (starting at 8 mg twice daily on day 1 and reducing to 2 mg once per day by day 14) or matching placebo.
Measuring costs
In line with the NICE methods guide,36 in the base-case analysis, costs were estimated from the viewpoint of the NHS and Personal and Social Services (PSS). The subsequently described resource use data were collected, to which unit costs (estimated in Great British pounds for the 2017–18 financial year) were assigned. Where unit costs were taken from previous years, they were inflated using the NHS Cost Inflation Index. 37
There were two main sources for the resource use data: a case report form (CRF) and a patient self-report (6-month follow-up) questionnaire (PSRQ). Both were specifically developed for the study and were informed by the guidance that one should focus on the large cost drivers and those resource items that might differ between study arms. 38 Costs were not assigned to resource items that were undertaken for research purposes.
Data from the case report form
Up to the point of discharge from the NSU, CRF data that were requested (to be completed by site staff) included details of any operation(s) undertaken during the index admission (there could be more than one operation), postoperative imaging, length of stay (in the NSU and any stay in an ICU/a HDU) and dexamethasone medication taken. In addition, sites were asked to record any re-admissions that included a CSDH-related surgical intervention (at any time in the 6-month follow-up period).
Unit costs were assigned to CRF data resource use items (Table 20). In terms of the intervention costs, it was assumed that each participant in the intervention arm was prescribed the aforementioned 14-day course of dexamethasone (62 2-mg tablets) and that tablets could not be reused if they were not taken (i.e. the same cost was incurred regardless of whether or not the regimen was completed). Each 2-mg tablet was costed at £0.24 (net ingredient cost per tablet),39 giving a total dexamethasone regimen estimated cost of £15.01. No extra staff costs were included because the additional time was considered negligible and, therefore, captured by the associated admission costs. No medication costs were applied to those in the control arm (the placebo drug is a research-related cost and would not be provided as part of routine care outside the study).
| Resource use | Unit cost (£) | Assumptions |
|---|---|---|
| Primary admission costs | ||
| 14-day course of dexamethasone (124 mg in total) | 15.0140 | 62 2-mg tablets (£0.24 per tablet), full regimen provided, not reuseable if not taken |
| Surgical procedure (one side) | 1362.8041 | Duration of 1 hour |
| Surgical procedure (on both sides) | 2044.2041 | Duration of 1.5 hours, e.g. left and right burr hole |
| Postoperative imaging | 79.3242 | CT scan |
| Cost per bed-day in critical care ward (ICU/HDU) | 1441.4242 | Neuroscience adult patients, critical care (mean) |
| Cost per bed-day in NSU | 356.3742 | |
| Post discharge from NSU | ||
| Cost per bed-day in neurorehabilitation unit | 492.4143 | |
| Cost per bed-day in NSU | 356.3742 | |
| Cost per bed-day in critical care ward (ICU/HDU) | 1441.4242 | Neuroscience adult patients, critical care (mean) |
| Cost per bed-day (other ward type) | 345.7642 | Weighted average of elective and non-elective excess bed-days |
| Surgical procedure (post discharge) | 1477.3241 | Weighted average of one side two-sides operations, derived from primary admission CRF data |
| Health professional visits | ||
| Hospital doctor | ||
| Community | 31.0044 | As hospital doctors do not work in the community, the unit cost for a community GP visit was applied |
| Hospital | 208.2842 | |
| Home | 55.9144,45 | As hospital doctors do not usually visit homes, the unit cost for a home GP visit was applied |
| Nurse | ||
| Community | 12.1044 | |
| Hospital | 79.1042 | |
| Home | 19.3044,45 | Costed as for community visit, plus 12 minutes of travel time |
| General practitioner | ||
| Community | 31.0044 | |
| Hospital | 208.2842 | As GPs do not work in hospitals, the unit cost for a hospital doctor visit was applied |
| Home | 55.9144,45 | Costed as for community visit, plus 12 minutes of travel time |
| Physiotherapist | ||
| Community | 57.2642 | |
| Hospital | 52.0742 | |
| Home | 64.0242,45 | Costed as for community visit, plus 12 minutes of travel time |
| Occupational therapist | ||
| Community | 81.3142 | |
| Hospital | 65.5842 | |
| Home | 88.0742,45 | Costed as for community visit, plus 12 minutes of travel time |
| Speech therapist | ||
| Community | 28.2344 | |
| Hospital | 97.6242 | |
| Home | 35.0044,45 | Costed as for community visit, plus 12 minutes of travel time |
| Social worker | ||
| Community | 100.3944,46 | |
| Hospital | 100.3944 | |
| Home | 109.1244,45 | Costed as for community visit, plus 12 minutes of travel time |
| Community care assistant | ||
| Community | 19.4447 | |
| Hospital | 19.4447 | |
| Home | 24.1045,47 | Costed as for community visit, plus 12 minutes of travel time |
| Emergency department | ||
| Community | 160.3242 | |
| Hospital | 160.3242 | |
| Home | 160.3242 | |
| Other | ||
| Community | 31.0042 | The unit costs for the most commonly reported visit types from each location were used to cost ‘other’ visits. Community: GP; hospital: hospital doctor; home: occupational therapist |
| Hospital | 208.2842 | |
| Home | 88.0742,45 | |
| Other costs | ||
| MRI scan | 131.1542 | |
| CT scan | 79.3242 | |
| ‘Other’ scan | 133.0342 | Weighted average of CT and MRI scans derived from PSRQ data |
| Care home (cost per week in residence) | 1812.0044 | |
| Carer time | 16.7148 | Gross hourly wage used to value carer time, whether paid or not (opportunity cost method49). Average reported hours assumed to apply to all weeks post discharge |
For each participant, the component costs associated with any operation, imaging, length of stay in ICU/HDU and/or NSU (for the index admission), and dexamethasone could thereby be estimated and were summed to estimate the total index admission costs. Re-admissions that included a CSDH-related surgical intervention were also costed (based on surgery and length of stay details); however, to avoid potential duplication (with the overnight stays detailed in Patient self-reported resource use), these costs were not included in the base-case analysis.
Patient self-reported resource use
At the 6-month follow-up point, participants were sent a questionnaire that requested the following resource use data (if a participant was unable to fill in the questionnaire, proxy responses by a relative/ friend/carer were requested). The questionnaire requested information only in relation to the time since discharge from the NSU, as the resource use associated with the time in the NSU was assumed to be captured by the aforementioned CRF data.
Participants were asked to report any overnight stays in a hospital or other healthcare facility (for any reason), and (if applicable) the number of nights, type of unit and whether or not there was an associated operation on the skull/brain. Participants were also asked to report any HCP visits (for any aspect of their health) and (if applicable) the type of HCP seen and where (most) visits took place. In addition, participants were asked to report any scans of the head/brain and (if applicable) the type and number of investigations. Participants were also asked to report if they had spent any time in a care home and (if applicable) the time in weeks/months. Finally, participants were asked to report if they had received any help from a family member/friend or other carer. If a respondent reported ‘yes’, for each carer they were asked to report the average number of hours of care received (per week in the past 6 months).
Unit costs were assigned to each aforementioned item of resource use data reported by participants (see Table 20). This enabled the component costs associated with any overnight stays (post NSU discharge), HCP visits, scans, stay in a care home and carer costs to be estimated. These were summed to estimate the total post-discharge costs; however, to align with the NHS and PSS cost perspective recommended in the NICE methods guide,36 care home and carer costs (the same hourly cost was assigned to all reported hours of care regardless of whether, for example, a payment was made/the carer lived with the participant) were not included in the base-case analysis.
Measuring outcomes
To estimate the impact that the intervention had on health-related quality of life, and in line with the NICE methods guide,36 the EQ-5D-5L was used, and respondents were asked to report the level of problems (none to extreme/unable) that they had on five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). 29 Participants were asked to complete the EQ-5D-5L at discharge from the NSU, and again at 3 and 6 months post randomisation (when a participant was unable to fill in the questionnaire, proxy responses by a relative/friend/carer were requested). As recommended in the NICE position statement,31 the crosswalk mapping function30 was then used to convert responses into utility scores, where a score of 0 corresponds to death and 1 to full health. 50 Participants who died were assigned a utility score of 0 on their date of death (assuming that this occurred within the 6-month follow-up period). The quality-adjusted life-year (QALY) score that was accrued over the trial period was then estimated for each individual based on the total area-under-the-curve method and the assumption of linear interpolation. 51 In the base-case analysis, the score at the date of discharge was assumed to be the baseline score.
Analyses
Missing data
Missing data are common in randomised trials and can lead to bias and lack of precision. 52 As recommended for within-trial analysis of cost-effectiveness, and in line with previously described methods,52 multiple imputation (MI) was thereby undertaken in the base-case analysis.
Based on the pattern of missing data, costs were aggregated and imputed at the level of ‘total index admission costs’ and ‘total post-discharge costs’. Costs were imputed at this level because the CRF and patient self-report data had different response rates, despite the fact that individual questions within each of these data types had similar levels of completion. Similarly, where missing, overall EQ-5D-5L utility scores were imputed at NSU discharge and at the 3- and 6-month follow-ups, as response rates differed over time, although if EQ-5D-5L data were missing it was generally across all dimensions.
Based on a descriptive analysis demonstrating the association between costs and outcomes and baseline variables, and between missing costs and outcomes and baseline variables, the data were assumed to be missing at random. MI with chained equations under missing at random was, therefore, used and the missing data imputed, by treatment arm, using the ‘mi impute chained’ command (Stata version 16.0; StataCorp LP, College Station, TX) to create 40 data sets (it is recommended that the number of imputed data sets should be similar to the percentage of incomplete cases52), which were then pooled using Rubin’s rules. 53
In addition to the costs (index admission and post discharge) and outcomes (baseline, 3-month and 6-month EQ-5D-5L scores), the MI model included baseline covariates associated (p < 0.05) with missing data, costs, outcomes, age and gender.
The MI model was validated by comparing the distributions of the observed and imputed data to check that the distributions were similar. 52
Cost-effectiveness
The NICE methods guide36 recommends that costs are estimated from the viewpoint of the NHS and PSS. As a consequence, in the base-case analysis, the previously defined total index admission costs and total post-discharge costs (excluding care home and carer costs) were summed to estimate the overall costs.
A 6-month within-trial analysis was undertaken, for which (after excluding any patients randomised in error) an intention-to-treat approach was adopted, in which participants were analysed within the arm to which they were allocated, regardless of whether or not they adhered to the regimen in question. A within-trial analysis was deemed appropriate because we are not aware of any previous economic evaluations that have compared dexamethasone with placebo for patients with symptomatic CSDH. A previous systematic review14–16 also failed to identify any RCTs in this population group. No discounting was undertaken because the analysis period matched that of the trial duration (6 months), which is less than 1 year.
To estimate the mean incremental cost and incremental effect (QALY gain) associated with dexamethasone compared with placebo, regression analysis was undertaken, which is generally robust for skewed data and allows for any correlation between costs and effects. 54 All regressions included those baseline variables found to be predictive of total costs and/or outcomes: age (years) and baseline utility score.
We sought to identify whether or not the use of dexamethasone was cost-effective by calculating the net monetary benefit (NMB). 55 The NMB was estimated at the cost-effectiveness thresholds of £20,000 and £30,000 per QALY; if the NMB is estimated to be > 0, the intervention is estimated to be cost-effective, and if the NMB is estimated to be < 0, the intervention is estimated to be not cost-effective. The NMB was used rather than, for example, the incremental cost-effectiveness ratio (ICER) because a negative ICER is open to misinterpretation: both a negative cost/positive effect and negative effect/positive cost can give the same ICER value, but have different implications for cost-effectiveness. 55
Decision uncertainty
Based on previous work,52 the probability of being cost-effective at different thresholds of cost-effectiveness was estimated to enable the cost-effectiveness acceptability curve (CEAC)56 to be calculated. Here, we report the estimated probability that dexamethasone is cost-effective at the recommended thresholds of £20,000 and £30,000 per QALY. 36
Sensitivity analyses
The following sensitivity analyses were undertaken to assess the robustness of conclusions to changes in key assumptions (unless otherwise stated, all other assumptions remain as in the aforementioned base-case analysis50). To analyse the data from a wider societal perspective, the care home and carer costs (which were excluded from the base-case analysis) were included in the first sensitivity analysis (SA1). A complete-case analysis was also undertaken, in which participants were included only if they had complete CRF, PSRQ and QALY data (SA6). No imputation was undertaken within this SA. Finally, to test for the influence of extreme values, a further SA (SA7, based on winsorising) was undertaken. Here, data values below the 5th percentile were replaced with the 5th percentile value and data values above the 95th percentile were replaced with the 95th percentile value. This was applied to the overall cost and total QALY score data for the base-case analysis. Four further sensitivity analyses (SA2, SA3, SA4 and SA5) were planned but not undertaken for reasons explained in the results.
Results
Participants
Between August 2015 and November 2018, 750 patients were randomly assigned to a treatment arm; however, two patients were excluded shortly after randomisation owing to ineligibility (see Figure 1). Therefore, 748 patients were enrolled in the trial: 375 in the dexamethasone arm and 373 in the placebo arm. These 748 patients, for whom the baseline characteristics have been outlined (see Table 2), constitute the full analysis population used in the base-case and other analyses (unless specified otherwise) in this economic evaluation.
Costs
At NSU discharge, 718 out of 745 (96%) (excluding those that died) participants had complete CRF resource use data.
Table 21 presents the reported levels of resource use for the associated index admission to hospital for both the dexamethasone arm and the placebo arm, based on the available CRF data. This includes the mean number of unilateral/bilateral procedures and postoperative imaging procedures and the mean length of stay in the NSU and ICU/HDU; it can be seen that resource levels are broadly comparable across groups. When combined with the associated unit costs (see Table 20), this enabled costs to be estimated for the same resource components and, in turn, the total index admission costs (Table 22), which were, again, similar between the two arms.
| Resource use | Treatment arm | Total | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Number of surgeries: unilateral or bilateral, n/N (%) | |||
| 0 | 29/373 (8) | 30/375 (8) | 59/748 (8) |
| 1 | 330/373 (88) | 341/375 (91) | 671/748 (90) |
| 2 | 10/373 (3) | 1/375 (0.3) | 11/748 (1) |
| 3 | 1/373 (0.2) | 0/375 (0) | 1/748 (0.1) |
| Missing | 3/373 (0.8) | 3/375 (0.8) | 6/748 (0.8) |
| Surgical procedure: unilateral (mean number of operations) | |||
| n | 370 | 372 | 742 |
| Mean (SD) | 0.805 (0.471) | 0.755 (0.437) | 0.780 (0.455) |
| Missing | 3 | 3 | 6 |
| Surgical procedure: bilateral (mean number of operations) | |||
| n | 370 | 372 | 742 |
| Mean (SD) | 0.149 (0.356) | 0.167 (0.373) | 0.158 (0.365) |
| Missing | 3 | 3 | 6 |
| Postoperative imaging, n/N (%) | |||
| 0 | 200/373 (54) | 210/375 (56) | 410/748 (55) |
| 1 | 161/373 (43) | 155/375 (41) | 316/748 (42) |
| 2 | 7/373 (2) | 1/375 (0.3) | 8/748 (1) |
| Missing | 5/373 (1) | 9/375 (2) | 14/748 (2) |
| Postoperative imaging (mean number of procedures) | |||
| n | 368 | 366 | 734 |
| Mean (SD) | 0.476 (0.537) | 0.429 (0.501) | 0.452 (0.520) |
| Missing | 5 | 9 | 14 |
| Stayed in ICU/HDU, n/N (%) | |||
| Yes | 39/373 (10) | 31/375 (8) | 70/748 (9) |
| No | 320/373 (86) | 330/375 (88) | 650/748 (87) |
| Missing | 14/373 (4) | 14/375 (4) | 28/748 (4) |
| Length of stay in ICU/HDU (mean number of days) | |||
| n | 359 | 361 | 720 |
| Mean (SD) | 0.320 (1.320) | 0.241 (0.997) | 0.281 (1.169) |
| Missing | 14 | 14 | 28 |
| Length of stay in NSU (mean number of days) | |||
| n | 359 | 361 | 720 |
| Mean (SD) | 8.203 (6.958) | 8.321 (6.591) | 8.263 (6.772) |
| Missing | 14 | 14 | 28 |
| Cost component | Treatment arm, n; mean (SD) cost (£) | p-value | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Index admission costs | |||
| 14-day course of dexamethasone (124 mg in total) | 373; 0 (0) | 375; 15.01 (0) | – |
| Surgical procedures: unilateral | 370; 1097.60 (642.39) | 372; 1029.43 (595.08) | 0.134 |
| Surgical procedures: bilateral | 370; 303.87 (728.19) | 372; 340.70 (762.85) | 0.501 |
| Postoperative imaging | 368; 37.72 (42.58) | 366; 34.02 (39.75) | 0.225 |
| Length of stay in NSU | 359; 2923.45 (2479.66) | 361; 2965.51 (2348.85) | 0.815 |
| Length of stay in ICU/HDU | 359; 461.74 (1903.25) | 361; 347.38 (1437.41) | 0.363 |
| Total index admission cost per patient | 359;a 4820.38 (3496.69) | 361;a 4726.64 (2873.89) | 0.694 |
| Post-index admission costs | |||
| Overnight stays on rehabilitation unit | 309; 995.98 (6404.51) | 303; 627.30 (3636.01) | 0.383 |
| Overnight stays on NSU | 309; 146.47 (1080.79) | 303; 108.21 (762.37) | 0.614 |
| Overnight stays on ICU/HDU | 309; 65.31 (1147.99) | 303; 66.60 (1159.30) | 0.989 |
| Overnight stays on ‘other’ ward | 309; 1018.24 (5099.23) | 303; 977.93 (3428.18) | 0.909 |
| Number of operations on skull/brain | 313; 80.24b (355.60) | 308; 76.75b (349.37) | 0.902 |
| HCP visits | 272; 237.05 (740.79) | 271; 289.67 (55.47) | 0.461 |
| Head/brain scans | 310; 55.49 (91.66) | 292; 54.08 (109.44) | 0.863 |
| Total post-discharge cost per patient | 258;c 2313.38 (8831.96) | 255;d 1997.36 (6345.51) | 0.642 |
| Overall NHS and PSS cost per patient | 258;c 6869.56 10 to 347.44 | 255;d 6540.55 (7299.00) | 0.641 |
In terms of patient self-reported resource use data, 513 out of 700 (73%) (excluding those who died) participants had complete data at 6 months, all of whom also had complete CRF data. Based on available data, Table 23 presents the associated levels of resource use, including overnight stays, further operations, HCP visits and number of scans. It can be seen that levels were again broadly similar in both arms, although the mean duration of stay on a rehabilitation unit was greater in the placebo arm than in the dexamethasone arm (2.023 vs. 1.274 days). This was in large part due to one participant in the placebo arm, who had an exceptionally long single stay of 200 days on a rehabilitation unit. By combining these resource levels with associated unit costs (see Table 20) post-discharge costs were estimated (see Table 22). Again, costs were broadly similar between arms, and if the aforementioned participant (in the placebo arm) who had an extended stay in a rehabilitation unit is removed from the analysis, then the mean difference is reduced [£1997 (n = 255) dexamethasone arm vs. £1932 (n = 257) placebo arm; p = 0.907]. Results between arms were also similar when the index admission and post-discharge costs (excluding care home and carer costs) were summed to estimate per-patient overall NHS and PSS costs (see Table 22). Again, if the participant who had an extended stay in a rehabilitation unit is removed from the analysis, the mean difference is reduced [£6541 (n = 255) dexamethasone arm vs. £6458 (n = 257) placebo arm; p = 0.903].
| Resource use | Treatment arm | Total | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Overnight stays, n/N (%) | |||
| Yes | 78/373 (21) | 86/375 (23) | 164/748 (22) |
| No | 235/373 (63) | 222/375 (59) | 457/748 (61) |
| Missing | 60/373 (16) | 67/375 (18) | 127/748 (17) |
| Length of stay in rehabilitation unit | |||
| n | 309 | 303 | 612 |
| Mean (SD) | 2.023 (13.006) | 1.274 (7.384) | 1.652 (10.600) |
| Missing | 64 | 72 | 136 |
| Length of stay in NSU | |||
| n | 309 | 303 | 612 |
| Mean (SD) | 0.411 (3.033) | 0.304 (2.139) | 0.358 (2.627) |
| Missing | 64 | 72 | 136 |
| Length of stay in ICU/HDU | |||
| n | 309 | 303 | 612 |
| Mean (SD) | 0.045 (0.796) | 0.046 (0.804) | 0.046 (0.800) |
| Missing | 64 | 72 | 136 |
| Length of stay on other ward | |||
| n | 309 | 303 | 612 |
| Mean (SD) | 2.945 (14.75) | 2.828 (9.915) | 2.887 (12.58) |
| Missing | 64 | 72 | 136 |
| Operations to skull/brain | |||
| n | 313 | 308 | 748 |
| Mean (SD) | 0.054 (0.241) | 0.052 (0.236) | 0.053 (0.238) |
| Missing | 60 | 67 | 127 |
| HCP contact | |||
| Yes | 159/373 (43) | 162/375 (43) | 321/748 (43) |
| No | 155/373 (42) | 144/375 (38) | 299/748 (40) |
| Missing | 59/373 (16) | 69/375 (18) | 128/748 (17) |
| HCP visitsa | |||
| n | 302 | 297 | 599 |
| Mean (SD) | 7.463 (34.922) | 8.199 (42.437) | 7.828 (38.800) |
| Missing | 71 | 78 | 149 |
| Head/brain scans, n/N (%) | |||
| Yes | 126/373 (38) | 110/375 (29) | 236/748 (32) |
| No | 187/373 (50) | 188/375 (50) | 375/748 (50) |
| Missing | 60/373 (16) | 77/375 (21) | 137/748 (18) |
| Head/brain scansb | |||
| n | 310 | 292 | 602 |
| Mean (SD) | 0.597 (0.996) | 0.589 (1.202) | 0.593 (1.100) |
| Missing | 63 | 83 | 146 |
Outcomes
There were ≥ 82% complete EQ-5D-5L data at all time points (NSU discharge and 3 and 6 months post randomisation), and 504 out of 748 (67%) participants had complete EQ-5D-5L data at all time points (those who died were given an EQ-5D-5L score of ‘0’ and would, therefore, not be recorded as ‘missing’) (Table 24).
| Item | Treatment arm, n; mean (SD) | p-value | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Baseline EQ-5D-5L score | 306; 0.727 (0.265) | 307; 0.697 (0.293) | 0.183 |
| 3-month EQ-5D-5L score | 316; 0.773 (0.291) | 316; 0.707 (0.337) | 0.008 |
| 3-month change in EQ-5D-5L score | 275; 0.065 (0.273) | 273; 0.282 (0.287) | 0.126 |
| 6-month EQ-5D-5L score | 315; 0.766 (0.320) | 311; 0.733 (0.348) | 0.211 |
| 6-month change in EQ-5D-5L score | 276; 0.054 (0.294) | 271; 0.036 (0.300) | 0.456 |
| QALY score | 256; 0.395 (0.117) | 248; 0.367 (0.146) | 0.020 |
Table 24 shows the mean EQ-5D-5L utility scores at each time point on an available case basis, along with the change in score at each time point. The EQ-5D-5L scores at each time point were higher (non-significant at baseline and 6 months; p = 0.008 at 3 months) in the placebo arm than the dexamethasone arm. This is in keeping with the primary end point of the study, which reported a favourable outcome in the placebo arm compared with the dexamethasone arm.
Analyses
Cost-effectiveness
Table 25 presents estimates of mean incremental costs and incremental QALYs, which were generated from multiple regression (both costs and QALYs adjusted for EQ-5D-5L at NSU discharge and age), along with NMB and probability of being cost-effective at thresholds (λ) of £20,000 and £30,000 per QALY.
| Analysis (Nd, Np) | Incremental cost (£) (95% CI) | QALY gain (95% CI) | NMB at £20,000 threshold (£) | CEAC at £20,000 threshold (%) | NMB at £30,000 threshold (£) | CEAC at £30,000 threshold (%) |
|---|---|---|---|---|---|---|
| Base case: MI 375, 373 | −143.73 (−1793 to 1505) | −0.012 (−0.027 to 0.003) | −97.19 | 46 | −217.65 | 41 |
| SA0 [analysis without patient N36112 (outlier)]: MI 375, 372 | 381.98 (−1265 to 2029) | −0.013 (−0.028 to 0.002) | −632.39 | 24 | −757.59 | 21 |
| SA1: MI 375, 373 | 2446.70 (−1339 to 6233) | −0.011 (−0.026 to 0.004) | −2664.08 | 9 | −2773.00 | 8 |
| SA6: MI 189, 196 | −64.46 (−1099 to 970) | −0.008 (−0.022 to 0.006) | −93.27 | 44 | −172.14 | 39 |
| SA7: MI 375, 373 | 292.66 (−615 to 1201) | −0.011 (−0.025 to 0.004) | −508.66 | 17 | −616.67 | 14 |
For the base-case analysis (based on intention to treat/MI), the mean difference in cost for the dexamethasone arm compared with the placebo arm was –£143.73 (95% CI –£1793 to £1505), with a mean QALY difference of −0.012 (95% CI −0.027 to 0.003). That is, the mean total cost and QALY scores were both slightly lower in the dexamethasone arm than in the placebo arm, although neither of these differences was significantly different. The resulting NMB for the base-case analysis at a £20,000 threshold was –£97.19, which means that dexamethasone was not estimated to be cost-effective compared with placebo.
Decision uncertainty
In terms of uncertainty, the base-case probability that dexamethasone is cost-effective at a threshold of £20,000 was 46% (see Table 25). This indicates that there was a high degree of uncertainty associated with the decision that dexamethasone was not cost-effective (Figure 10). However, given that it is argued that decisions about provision should be based on the mean estimates of costs and benefits (regardless of the quality of data available57), this does not alter the assertion that dexamethasone was not estimated to be cost-effective compared with placebo.
FIGURE 10.
Cost-effectiveness acceptability curve for dexamethasone compared with placebo.
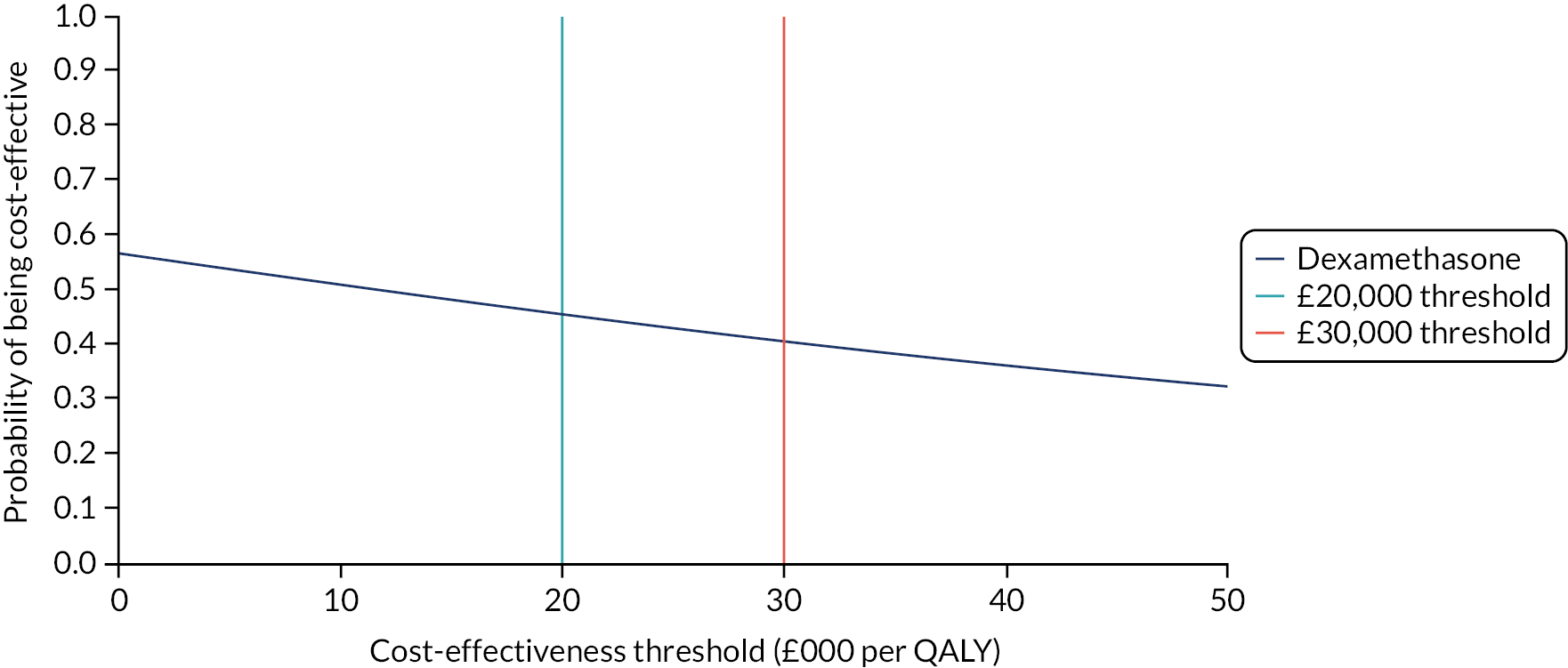
Sensitivity analyses
The results of the sensitivity analyses that were undertaken are shown in Table 25. In all of these analyses (at a willingness-to-pay threshold of both £20,000 and £30,000 per QALY), dexamethasone is not estimated to be cost-effective compared with placebo. SA2 was deemed unnecessary because the mean length of stay in the NSU was estimated to be only 8 days and virtually the same in both arms (see Table 2). Consequently, using linear interpolation to estimate what the EQ-5D-5L scores would have been at the date of admission to the NSU (an average of 8 days prior to their discharge date) was deemed superfluous because it would have had a negligible impact on estimated QALY scores. SA3 was not undertaken for similar reasons: for the primary outcome the results for the full analysis population (see Table 4) were virtually the same as that for the per-protocol analyses (see Table 29). This was also true for the EQ-5D-5L (see Tables 9 and 52). In addition, there would be no impact on intervention costs because it was assumed that each participant in the intervention arm was prescribed the same course of dexamethasone and that the medication could not be reused if it was not taken (see Methods). Similarly, SA4 and SA5 were deemed unnecessary because there was no significant difference between arms in terms of the number of subsequent admissions based on CRF data.
Discussion
Main findings
On the basis of the estimated negative NMB (at willingness-to-pay thresholds of £20,000 and £30,000 per QALY), dexamethasone is not estimated to be cost-effective compared with placebo for the treatment of patients with symptomatic CSDH (see Table 25). This is in keeping with the results for the primary outcome, which also favoured the placebo arm (see Table 25), and the same result was found in each of the sensitivity analyses that were conducted (see Table 25). That said, there is a degree of uncertainty associated with the decision regarding cost-effectiveness, with an estimated 46% probability (at a willingness-to-pay threshold of £20,000 per QALY) of making the wrong decision by not using dexamethasone in these patients. However, given that economists tend to focus on mean values57 (here that is the negative NMB estimates) the implication is not to use dexamethasone in this population of patients.
Comparisons with other studies
We are not aware of any previous economic evaluations that have compared dexamethasone with placebo for patients with symptomatic CSDH. Most patients in this study were treated surgically during their index admission, which limits the ability of the study to draw conclusions regarding the use of dexamethasone as a non-surgical alternative. The DECSA trial,58 which includes an economic evaluation but was yet to report at the time of writing, seeks to compare the effect of primary dexamethasone therapy with primary burr hole craniotomy on functional outcome and cost-effectiveness in a similar population group (symptomatic CSDH).
Study limitations
In line with good practice recommendations for cost-effectiveness analyses,38 we concentrated on the large cost drivers and excluded resources that were not expected to differ between the two treatment arms (e.g. routine monitoring scans or tests). That said, the mean resource use levels could be heavily influenced by outliers owing to the high unit costs associated with resource use, such as length of stay in critical care and rehabilitation units. One such example was the aforementioned participant (in the placebo arm) who had an extended stay in a rehabilitation unit, who, when removed from the analysis, resulted in an increase in the incremental cost for dexamethasone compared with placebo. The conclusions were, however, unchanged when winsorising was undertaken, and the influence of outliers was reduced (see Table 25).
Regarding health-related quality of life, QALY scores (EQ-5D-5L recorded at all time points) were available for approximately 67% of participants only (see Table 24). Some of the missing EQ-5D-5L baseline (NSU discharge) data may be because of patients being discharged at short notice or at the weekend when a research nurse was not available. Such missing data are a limitation, but it should be noted that the same conclusion can be drawn from the results of both the complete-case and the imputed analyses.
A further potential limitation is that, for ethics reasons, baseline quality-of-life (EQ-5D-5L) scores were taken at discharge from the NSU rather than at the point of randomisation. The date of discharge from the NSU is post intervention and there is, therefore, the potential for any benefits associated with the intervention to be underestimated by assuming the score at the date of discharge to be the baseline score. In this study, the mean length of stay was approximately 8–9 days and was similar between arms. For this reason, if there had been an additional quality-of-life measurement point at randomisation, we consider that any change in QALY scores would have been negligible and not have changed the study conclusion. Similarly, given that a per-protocol analysis favoured the placebo arm, there is no evidence that any lack of compliance had an impact on study results.
A further potential limitation is that the conclusions might differ if results were estimated over a longer follow-up period. However, if the treatment effect found in this study was maintained beyond 12 months, the conclusions would be unchanged because extrapolation would result in further QALY gain for the placebo arm compared with the dexamethasone arm, while the costs would remain similar between the two arms.
The main strength of this economic evaluation is that it is based on a large, multicentre, randomised study. Previous evidence, in the form of a systematic review,14–16 was based on five small observational studies and stated that there is a lack of well-designed trials to support or refute the use of corticosteroids for CSDH.
Conclusion
Our economic evaluation has shown that in a UK population of patients with symptomatic CSDH, most of whom underwent surgery during their index admission, dexamethasone was not estimated to be cost-effective compared with placebo. Some uncertainty was estimated to be associated with that decision. In addition, when a number of sensitivity analyses were conducted, the main conclusion, that dexamethasone was not estimated to be cost-effective compared with placebo, was unchanged. Consequently, given that economists tend to focus on the mean estimated values of cost and effect, the health economic analysis supports the aforementioned recommendation (based on the primary outcome) not to use dexamethasone in this population of patients.
Chapter 5 Discussion
Key findings
The results of our literature review indicate that this is the first multicentre RCT of dexamethasone in addition to standard care (including surgery) in patients with CSDH. There was a higher rate of unfavourable outcome (mRS score of 4–6) at 6 months among patients who received a 14-day course of dexamethasone (16.1%), than among those who received placebo (9.70%). Therefore, dexamethasone resulted in an absolute risk increase of 6.4%. This finding does not support routine use of dexamethasone as part of standard care in this patient population because it is associated with harm. In addition, given that there is a cost associated with the medication, which would be saved if it was no longer provided, it is estimated that dexamethasone is not cost-effective and that scarce NHS resources would be better spent on other healthcare interventions.
Consistent with previous studies, dexamethasone did reduce the recurrence rate compared with that in the placebo arm. Previous studies have shown that reductions in recurrence also appear to be related to a reduction in mortality. 7 In this study, patients with recurrent CSDH in the dexamethasone arm also did worse than those with recurrent CSDH in the placebo arm. Overall, despite fewer CSDH recurrences, patients had a worse outcome with dexamethasone and a higher mortality at 6 months (8% vs. 5% for the dexamethasone and placebo arm, respectively).
There is widespread reporting of the potential adverse effects of dexamethasone, which are dose related. A short course of only 14 days was applied in this study; however, the number of SAEs reported was larger in the dexamethasone arm than in the placebo arm. In particular, there were more infections (6.4% vs. 1.1%) in the dexamethasone arm than the placebo arm.
Limitations of this study include the fact that 94% of patients received surgery in addition to the trial treatment; therefore, these results cannot necessarily be generalised to patients who are managed conservatively with medication alone. Exploratory subgroup analysis of patients non-operatively managed were in the same direction of effect as the primary analysis, which could be examined further in appropriately powered research.
The co-ordinating centre recruited approximately one-third of patients, meaning that a large proportion of patients originated from one region of the UK. There was no significant difference in outcome between this site and others, which suggests that this has little impact on the generalisability of the data. The pragmatic study design allowed the recruitment target to be met, and the baseline characteristics of patients reflect those reported in previous CSDH studies; therefore, we think that these results are generalisable to the surgical CSDH population.
In total, 9% of patients were lost to follow-up at 6 months, which reduced the sample size informing the primary outcome measure of the trial. This was, however, within the limits of our sample size calculation, which had allowed for up to 15% loss to follow-up. The percentage was similar in both arms, which suggests that this limitation did not influence the study’s findings.
The characteristic adverse effects, such as steroid-induced hyperglycaemia, may have alerted clinical teams to the trial-arm assignment.
Follow-up imaging to assess the size of CSDHs were not mandated, meaning that the impact of dexamethasone of haematoma size was not assessed. This was unlikely to impact the results of this pragmatic trial given that such follow-up imaging after evacuation is not beneficial in terms of patient outcomes at 6 months. 59
Chapter 6 Conclusions
In conclusion, dexamethasone increases the rate of unfavourable outcome at 6 months in surgically treated CSDH patients compared with placebo. Therefore, this study does not recommend its use in routine clinical practice for this patient group.
Acknowledgements
This paper is dedicated to the memory of Mrs Kate Massey, who was the patient representative involved in study design.
Protocol
The published trial protocol is available online. 2
Contributions of authors
Peter J Hutchinson (https://orcid.org/0000-0002-2796-1835) [PhD, FRCS (SN), FMedSci] (Chief Investigator) made substantial contributions to the conception and design of the study and the acquisition, analysis and interpretation of data.
Ellie Edlmann (https://orcid.org/0000-0002-7253-9115) (MRCS, PhD) (Lead Sub-Investigator, University of Cambridge Research Associate and Royal College of Surgeons Research Fellow) made substantial contributions to the trial management, including site initiations and conception and design of the study; and contributed to the acquisition, analysis and interpretation of data.
John G Hanrahan (https://orcid.org/0000-0003-4584-2298) (BSc, MBBS) (Academic Trainee) made substantial contributions to the interpretation of data and editing of the HTA report.
Diederik Bulters (https://orcid.org/0000-0001-9884-9050) [BSc, FRCS (SN)] (Consultant Neurosurgeon) made substantial contributions to the conception and design of the study and the acquisition, analysis and interpretation of data.
Ardalan Zolnourian (https://orcid.org/0000-0002-0428-179X) (MRCS) (Neurosciences Research Fellow) made substantial contributions to the acquisition, analysis and interpretation of data.
Patrick Holton (https://orcid.org/0000-0003-1548-9649) (MRCS) (Consultant Neurosurgeon) made substantial contributions to the acquisition, analysis and interpretation of data.
Nigel Suttner (https://orcid.org/0000-0002-4784-7271) [FRCS (SN)] (Consultant Neurosurgeon) made substantial contributions to the acquisition, analysis and interpretation of data.
Kevin Agyemang (https://orcid.org/0000-0001-7553-7281) (MRCS) (Neurosurgery Trainee) made substantial contributions to the acquisition, analysis and interpretation of data.
Simon Thomson (https://orcid.org/0000-0003-4827-1961) (FRCS) (Consultant Neurosurgeon) made substantial contributions to the acquisition, analysis and interpretation of data.
Ian A Anderson (https://orcid.org/0000-0003-3965-5963) [FRCS (SN)] (Consultant Neurosurgeon) made substantial contributions to the acquisition, analysis and interpretation of data.
Yahia Al-Tamimi (https://orcid.org/0000-0002-9014-2728) [MD, FRCS (SN)] (Consultant Neurosurgeon) made substantial contributions to the acquisition, analysis and interpretation of data.
Duncan Henderson (https://orcid.org/0000-0002-3916-8455) (MRCS) (Neurosurgery Trainee) made substantial contributions to the acquisition, analysis and interpretation of data.
Peter Whitfield (https://orcid.org/0000-0003-0566-1820) [PhD, FRCS (SN)] (Consultant Neurosurgeon) made substantial contributions to the acquisition, analysis and interpretation of data.
Monica Gherle (https://orcid.org/0000-0003-0614-3291) (MD) (Neurosurgery Trainee) made substantial contributions to the acquisition, analysis and interpretation of data.
Paul M Brennan (https://orcid.org/0000-0002-7347-830X) [PhD, FRCS (SN)] (Consultant Neurosurgeon) made substantial contributions to the conception and design of the study and the acquisition, analysis and interpretation of data.
Annabel Allison (https://orcid.org/0000-0002-9122-6341) (MSc) (Trial Statistician) made substantial contributions to the analysis and interpretation of data, drafted the trial’s statistical analysis plan, and performed the trial’s statistical analysis.
Eric P Thelin (https://orcid.org/0000-0002-2338-4364) (PhD) (Neurosurgery Trainee) made substantial contributions to the acquisition, analysis and interpretation of data.
Silvia Tarantino (https://orcid.org/0000-0003-3283-482X) (BSc) (Clinical Research Nurse) made substantial contributions to the acquisition and interpretation of data.
Beatrice Pantaleo (https://orcid.org/0000-0003-4860-3743) (M Pharm) (Clinical Trials Database Manager) performed data cleaning for the trial, carried out study database creation, completed database updates and made substantial contributions to the acquisition and interpretation of data.
Karen Caldwell (https://orcid.org/0000-0001-5604-8773) (BSc) (Clinical Research Nurse) made substantial contributions to the acquisition and interpretation of data.
Carol Davis-Wilkie (https://orcid.org/0000-0001-7315-4025) (BSc) (Clinical Trial Coordinator) co-ordinated the trial, completed necessary research permission submissions, maintained the trial master file, performed ongoing site management and made substantial contributions to the acquisition and interpretation of data.
Harry Mee (https://orcid.org/0000-0002-1314-3962) (MRCP) (Clinical Research Fellow) made substantial contributions to the acquisition, analysis and interpretation of data.
Elizabeth A Warburton (https://orcid.org/0000-0003-2575-3255) (MA, DM, FRCP) (Consultant Physician of Stroke Medicine) made substantial contributions to the conception and design of the study.
Garry Barton (https://orcid.org/0000-0001-9040-011X) (BA, MSc, PhD) (Health Economist) completed the health economics analysis and made substantial contributions to the conception and design of the study and the analysis and interpretation of data.
Aswin Chari (https://orcid.org/0000-0003-0053-147X) (MRCS) (Trainee Representative) made substantial contributions to the conception and design of the study and the interpretation of data.
Hani J Marcus (https://orcid.org/0000-0001-8000-392X) [PhD, FRCS (SN)] (Trainee Representative) made substantial contributions to the conception and design of the study and the interpretation of data.
Sarah Pyne (https://orcid.org/0000-0003-0093-9125) (MRCP) (Health Economist) completed the health economics analysis and made substantial contributions to the acquisition, analysis and interpretation of data.
Andrew T King (https://orcid.org/0000-0002-6546-7248) [FRCS (SN)] (Consultant Neurosurgeon) made substantial contributions to the conception and design of the study and the interpretation of data.
Antonio Belli (https://orcid.org/0000-0002-3211-9933) [MD, FRCS (SN)] (Consultant Neurosurgeon) made substantial contributions to the conception and design of the study and the interpretation of data.
Phyo K Myint (https://orcid.org/0000-0003-3852-6158) (MD, FRCP) (Professor of Old Age Medicine) made substantial contributions to the conception and design of the study and the interpretation of data.
Ian Wilkinson (https://orcid.org/0000-0001-6598-9399) (DM, FRCP) (Professor of Clinical Pharmacology) made substantial contributions to the conception and design of the study and the interpretation of data.
Thomas Santarius (https://orcid.org/0000-0002-1416-9566) [PhD, FRCS (SN)] (Consultant Neurosurgeon) made substantial contributions to the conception and design of the study and the interpretation of data.
Carole Turner (https://orcid.org/0000-0002-8297-4890) (MSc) (Research Associate) made substantial contributions to the conception and design of the study and the acquisition, analysis and interpretation of data.
Simon Bond (https://orcid.org/0000-0003-2528-1040) (PhD) (Senior Statistician) made substantial contributions to the conception and design of the study and the analysis and interpretation of data.
Angelos G Kolias (https://orcid.org/0000-0003-3992-0587) [PhD, FRCS (SN)] (Co-Chief Investigator) made substantial contributions to the conception and design of the study and the acquisition, analysis and interpretation of data.
All authors drafted and/or critically revised the report for important intellectual content. Please note that job titles may reflect those at the time of the trial rather than current position.
Publications
Kolias A, Edlmann E, Hutchinson PJ. The role of pharmacotherapy in the management of chronic subdural haematoma. Swiss Med Wkly 2017;147:w14479.
Kolias AG, Edlmann E, Thelin EP, Bulters D, Holton P, Suttner N, et al. Dexamethasone for adult patients with a symptomatic chronic subdural haematoma (Dex-CSDH) trial: study protocol for a randomised controlled trial. Trials 2018;19:670.
Allison A, Edlmann E, Kolias AG, Davis-Wilkie C, Mee H, Thelin EP, et al. Statistical analysis plan for the Dex-CSDH trial: a randomised, double-blind, placebo-controlled trial of a 2-week course of dexamethasone for adult patients with a symptomatic chronic subdural haematoma. Trials 2019;20:698.
Edlmann E, Thelin EP, Caldwell K, Turner C, Whitfield P, Bulters D, et al. Dex-CSDH randomised, placebo-controlled trial of dexamethasone for chronic subdural haematoma: report of the internal pilot phase. Sci Rep 2019;9:5885.
Edlmann E, Whitfield PC, Kolias A, Hutchinson PJ. Pathogenesis of chronic subdural hematoma: a cohort evidencing de novo and transformational origins. J Neurotrauma 2021;38:2580–9.
Hutchinson PJ, Edlmann E, Bulters D, Zolnourian A, Holton P, Suttner N, et al. Trial of dexamethasone for chronic subdural hematoma. N Engl J Med 2020;383:2616–27.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data are vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This manuscript presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Kolias AG, Chari A, Santarius T, Hutchinson PJ. Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol 2014;10:570-8. https://doi.org/10.1038/ nrneurol.2014.163.
- Kolias AG, Edlmann E, Thelin EP, Bulters D, Holton P, Suttner N, et al. Correction to: dexamethasone for adult patients with a symptomatic chronic subdural haematoma (Dex-CSDH) trial: study protocol for a randomised controlled trial. Trials 2019;20:1-14. https://doi.org/10.1186/s13063-019-3283-x.
- Hutchinson PJ, Edlmann E, Bulters D, Zolnourian A, Holton P, Suttner N, et al. Trial of dexamethasone for chronic subdural hematoma. N Engl J Med 2020;383:2616-27. https://doi.org/10.1056/NEJMoa2020473.
- Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Andersen KN, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev 2012;35:155-69. https://doi.org/10.1007/s10143-011-0349-y.
- Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet 2016;387:2145-54. https://doi.org/10.1016/S0140-6736(15)00516-4.
- Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg 2015;123:1209-15. https://doi.org/10.3171/2014.9.JNS141550.
- Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet 2009;374:1067-73. https://doi.org/10.1016/S0140-6736(09)61115-6.
- Brennan PM, Kolias AG, Joannides AJ, Shapey J, Marcus HJ, Gregson BA, et al. The management and outcome for patients with chronic subdural hematoma: a prospective, multicenter, observational cohort study in the United Kingdom. J Neurosurg 2017;127:732-9. https://doi.org/10.3171/2016.8.JNS16134.
- Wada T, Kuroda K, Yoshida Y, Ogasawara K, Ogawa A, Endo S. Local elevation of the anti-inflammatory interleukin-10 in the pathogenesis of chronic subdural hematoma. Neurosurg Rev 2006;29:242-5. https://doi.org/10.1007/s10143-006-0019-7.
- Hong HJ, Kim YJ, Yi HJ, Ko Y, Oh SJ, Kim JM. Role of angiogenic growth factors and inflammatory cytokine on recurrence of chronic subdural hematoma. Surg Neurol 2009;71:161-5. https://doi.org/10.1016/j.surneu.2008.01.023.
- Stanisic M, Aasen AO, Pripp AH, Lindegaard KF, Ramm-Pettersen J, Lyngstadaas SP, et al. Local and systemic pro-inflammatory and anti-inflammatory cytokine patterns in patients with chronic subdural hematoma: a prospective study. Inflamm Res 2012;61:845-52. https://doi.org/10.1007/s00011-012-0476-0.
- Frati A, Salvati M, Mainiero F, Ippoliti F, Rocchi G, Raco A, et al. Inflammation markers and risk factors for recurrence in 35 patients with a posttraumatic chronic subdural hematoma: a prospective study. J Neurosurg 2004;100:24-32. https://doi.org/10.3171/jns.2004.100.1.0024.
- Suzuki M, Endo S, Inada K, Kudo A, Kitakami A, Kuroda K, et al. Inflammatory cytokines locally elevated in chronic subdural haematoma. Acta Neurochir 1998;140:51-5. https://doi.org/10.1007/s007010050057.
- Sun TF, Boet R, Poon WS. Non-surgical primary treatment of chronic subdural haematoma: preliminary results of using dexamethasone. Br J Neurosurg 2005;19:327-33. https://doi.org/10.1080/02688690500305332.
- Delgado-López PD, Martín-Velasco V, Castilla-Díez JM, Rodríguez-Salazar A, Galacho-Harriero AM, Fernández-Arconada O. Dexamethasone treatment in chronic subdural haematoma. Neurocirugia 2009;20:346-59. https://doi.org/10.1016/S1130-1473(09)70154-X.
- Berghauser Pont LM, Dirven CM, Dippel DW, Verweij BH, Dammers R. The role of corticosteroids in the management of chronic subdural hematoma: a systematic review. Eur J Neurol 2012;19:1397-403. https://doi.org/10.1111/j.1468-1331.2012.03768.x.
- Chan DYC, Sun TFD, Poon WS. Steroid for chronic subdural hematoma? A prospective phase IIB pilot randomized controlled trial on the use of dexamethasone with surgical drainage for the reduction of recurrence with reoperation. Chinese Neurosurg J 2015;1:30-2. https://doi.org/10.1186/s41016-015-0005-4.
- Holl DC, Volovici V, Dirven CMF, van Kooten F, Miah IP, Jellema K, et al. Corticosteroid treatment compared with surgery in chronic subdural hematoma: a systematic review and meta-analysis. Acta Neurochir 2019;161:1231-42. https://doi.org/10.1007/s00701-019-03881-w.
- Kawai S, Ichikawa Y, Homma M. Differences in metabolic properties among cortisol, prednisolone, and dexamethasone in liver and renal diseases: accelerated metabolism of dexamethasone in renal failure. J Clin Endocrinol Metab 1985;60:848-54. https://doi.org/10.1210/jcem-60-5-848.
- Reulen HJ, Hadjidimos A, Hase U. Brain Edema/Cerebello Pontine Angle Tumors. Advances in Neurosurgery, vol 1. Berlin: Springer; 1973.
- Lipman AG. MARTINDALE: ‘Martindale – the Extra Pharmacopoeia’ (30th ed), edited by J. E. F. Reynolds. Int J Pharm Pract 1993;2. https://doi.org/10.1111/j.2042-7174.1993.tb00740.x.
- Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med 2009;103:975-94. https://doi.org/10.1016/j.rmed.2009.01.003.
- Emich S, Richling B, McCoy MR, Al-Schameri RA, Ling F, Sun L, et al. The efficacy of dexamethasone on reduction in the reoperation rate of chronic subdural hematoma – the DRESH study: straightforward study protocol for a randomized controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-6.
- Teasdale G, Allan D, Brennan P, McElhinney E, Mckinnon L. Forty years on: updating the Glasgow Coma Scale. Nurs Times 2014;110:12-6.
- Bruno A, Akinwuntan AE, Lin C, Close B, Davis K, Baute V, et al. Simplified modified rankin scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke 2011;42:2276-9. https://doi.org/10.1161/STROKEAHA.111.613273.
- Sulter G, Steen C, De Keyser J. Acute stroke trials. Stroke 1999:1538-41. https://doi.org/10.1161/01.STR.30.8.1538.
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. https://doi.org/10.1007/s11136-012-0322-4.
- Chari A, Hocking KC, Broughton E, Turner C, Santarius T, Hutchinson PJ, et al. Core outcomes and common data elements in chronic subdural hematoma: a systematic review of the literature focusing on reported outcomes. J Neurotrauma 2016;33:1212-9. https://doi.org/10.1089/neu.2015.3983.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019) 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 5 October 2020).
- Allison A, Edlmann E, Kolias AG, Davis-Wilkie C, Mee H, Thelin EP, et al. Statistical analysis plan for the Dex-CSDH trial: a randomised, double-blind, placebo-controlled trial of a 2-week course of dexamethasone for adult patients with a symptomatic chronic subdural haematoma. Trials 2019;20. https://doi.org/10.1186/s13063-019-3866-6.
- Peugh JL, Strotman D, Mcgrady M, Rausch J, Kashikar-Zuck S. Beyond intent to treat (ITT): a complier average causal effect (CACE) estimation primer. J Sch Psychol 2017;60:7-24. https://doi.org/10.1016/j.jsp.2015.12.006.
- Koladjo BF, Escolano S, Tubert-Bitter P. Instrumental variable analysis in the context of dichotomous outcome and exposure with a numerical experiment in pharmacoepidemiology. BMC Med Res Methodol 2018;18. https://doi.org/10.1186/s12874-018-0513-y.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc 2013;11. https://doi.org/10.1186/1478-7547-11-6.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9] 2013. www.nice.org.uk/guidance/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 15 September 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- NHS Digital . Prescription Cost Analysis – England, 2018 [PAS] 2018. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018 (accessed 5 September 2021).
- NHS Digital . Prescription Cost Analysis – England, 2017 2017. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/prescription-cost-analysis-england-2017 (accessed January 2020).
- Public Health Scotland . R142X: Theatre – Direct Cost Per Hour, by Specialty 2018.
- NHS Improvement . NHS Reference Costs 2018.
- Turner-Stokes L, Williams H, Bill A, Bassett P, Sephton K. Cost-efficiency of specialist inpatient rehabilitation for working-aged adults with complex neurological disabilities: a multicentre cohort analysis of a national clinical data set. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010238.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Curtis L. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Office for National Statistics . Earnings and Hours Worked, All Employees: ASHE Table 1 2018.
- Hoefman RJ, van Exel J, Brouwer W. How to include informal care in economic evaluations. PharmacoEconomics 2013;31:1105-19. https://doi.org/10.1007/s40273-013-0104-z.
- Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Manca A, Palmer S. Handling missing data in patient-level cost-effectiveness analysis alongside randomised clinical trials. Appl Health Econ Health Policy 2005;4:65-7. https://doi.org/10.2165/00148365-200504020-00001.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Little R, Rubin D. Statistical Analysis with Missing Data. Hoboken, NJ: Wiley; 2014.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:68-80. https://doi.org/10.1177/0272989X98018002S09.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64. https://doi.org/10.1016/S0167-6296(98)00039-3.
- Miah IP, Holl DC, Peul WC, Walchenbach R, Kruyt N, de Laat K, et al. Dexamethasone therapy versus surgery for chronic subdural haematoma (DECSA trial): study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2945-4.
- Schucht P, Fischer U, Fung C, Bernasconi C, Fichtner J, Vulcu S, et al. Follow-up computed tomography after evacuation of chronic subdural hematoma. N Engl J Med 2019;380:1186-7. https://doi.org/10.1056/NEJMc1812507.
Appendix 1 Trial collaborators
Dex-CSDH trial collaborators (to be indexed on PubMed)
-
Daniela Georgieva, Southampton University Hospital, Southampton, UK.
-
Carol Dalton, Queen Elizabeth University Hospital, Glasgow, UK.
-
Mary Kambafwile, Leeds General Infirmary, Leeds, UK.
-
Charlotte Eglington, Derriford Hospital, Plymouth, UK.
-
Giles Critchley, Royal Sussex County Hospital, Brighton, UK.
-
Laura Ortiz-Ruiz De Gordoa, Royal Sussex County Hospital, Brighton, UK.
-
Manjunath Prasad, James Cook University Hospital, Middlesbrough, UK.
-
Philip Kane, James Cook University Hospital, Middlesbrough, UK.
-
Emanuel Cirstea, James Cook University Hospital, Middlesbrough, UK.
-
Nikolaos Tzerakis; Royal Stoke University Hospital, Stoke-on-Trent, UK.
-
Marios C Papadopoulos, St George’s Hospital, London, UK.
-
Jothy Kandasamy,Western General Hospital, Edinburgh, UK.
-
Masood Hussain, Hull Royal Infirmary, Hull, UK.
-
Dimitrios Paraskevopoulos, Royal London Hospital, London, UK.
-
Chris Uff, Royal London Hospital, London, UK.
-
Peter Bodkin, Aberdeen Royal Infirmary, Aberdeen, UK.
-
Damian Holliman, Royal Victoria Infirmary, Newcastle, UK.
-
Nihal Gurusinghe, Royal Preston Hospital, Preston, UK.
-
Taha Lilo, Royal Preston Hospital, Preston, UK.
-
Terrie Louise Cromie, Royal Preston Hospital, Preston, UK.
-
Jash Patel, John Radcliffe Hospital, Oxford, UK.
-
Nick Haliasos, Queen’s Hospital, Romford, UK.
-
Kismet Hossain-Ibrahim, Ninewells Hospital, Dundee, UK.
-
Dipankar Nandi, Charing Cross Hospital, London, UK.
-
Kevin Tsang, Charing Cross Hospital, London, UK.
-
Ravindra Nannapaneni, University Hospital of Wales, Cardiff, UK.
-
Pietro D’Urso, Salford Royal Hospital, Salford, UK.
-
Hu-Liang Low, Queen’s Hospital, Romford, UK.
BNTRC trial collaborators (to be indexed on PubMed)
-
Taiwo Akhigbe, Southampton University Hospital, Southampton, UK.
-
Michael Canty, Queen Elizabeth University Hospital, Glasgow, UK.
-
Paul Fivey, Queen Elizabeth University Hospital, Glasgow, UK.
-
Isaac Phang, Queen Elizabeth University Hospital, Glasgow, UK.
-
Souyma Mukherjee, Leeds General Infirmary, Leeds, UK.
-
Oliver Richards, Leeds General Infirmary, Leeds, UK.
-
Fozia Saeed, Leeds General Infirmary, Leeds, UK.
-
Chris Akhunbay-Fudge, Leeds General Infirmary, Leeds, UK.
-
Adam Wahba, Royal Hallamshire Hospital, Sheffield, UK.
-
Dan Gatt, Royal Hallamshire Hospital, Sheffield, UK.
-
Arif Zafar, Royal Hallamshire Hospital, Sheffield, UK.
-
Stuart Stokes, Royal Hallamshire Hospital, Sheffield, UK.
-
Boh Sofela, Derriford Hospital, Plymouth, UK.
-
Sam Jeffrey, Derriford Hospital, Plymouth, UK.
-
Kamal M Yakoub, Queen Elizabeth Hospital, Birmingham, UK.
-
Emma Toman, Queen Elizabeth Hospital, Birmingham, UK.
-
Marian Vintu, Royal Sussex County Hospital, Brighton, UK.
-
Mathew Gallagher, St George’s Hospital, London, UK.
-
Florence Hogg, St George’s Hospital, London, UK.
-
Hamza Solieman, Western General Hospital, Edinburgh, UK.
-
Julie Woodfield, Western General Hospital, Edinburgh, UK.
-
Adam Razak, Hull Royal Infirmary, Hull, UK.
-
Shumaila Hasan, Royal London Hospital, London, UK.
-
Anthony Wiggins, Aberdeen Royal Infirmary, Aberdeen, UK.
-
Aimun Jamjoom, Aberdeen Royal Infirmary, Aberdeen, UK.
-
Ian Coulter, Royal Victoria Infirmary, Newcastle, UK.
-
Venetia Giannakaki, Royal Victoria Infirmary, Newcastle, UK.
-
James Manfield, Royal Preston Hospital, Preston, UK.
-
Rory Piper, John Radcliffe Hospital, Oxford, UK.
-
Khaled Badran, Ninewells Hospital, Dundee, UK.
-
Edward Dyson, Charing Cross Hospital, London UK.
-
Malik Zaben, University Hospital of Wales, Cardiff, UK.
Appendix 2 List of protocol amendments
The following changes were introduced in version 2 of the protocol (1 March 2016):
-
Removal of Montreal Cognitive Assessment (MOCA) from secondary outcomes.
-
Eligibility criteria refined with respect to previous history of steroids.
-
Exclusion criterion changed from ‘patients who are on steroids’ to ‘patients who are on (or within 1 month of) regular oral or intravenous steroids’.
-
It was also clarified that ‘patients on topical or inhaled steroids are allowed to be recruited into the trial, as are patients who have had 1 intraoperative dose of dexamethasone for anti-emesis’.
-
Addition of objectives/end points for a mechanistic substudy (blood and CSDH fluid biomarkers and imaging – this study recruited only at Addenbrooke’s and is not part of the accompanying publication ‘Trial of dexamethasone for chronic subdural hematoma’).
The following changes were introduced in version 3 of the protocol (27 April 2017):
-
The interim analysis wording was amended from ‘after 100 patients in the stage 1 phase have observed 6-month follow-up in order to confirm the sample size’ to ‘after an appropriate number of patients have observed 6-month follow-up, in order to confirm the sample size, the TSC, independent DMEC and statistical team will agree jointly on the most appropriate timing of this interim analysis, taking into account the case mix and parameters the independent DMEC wishes to estimate’.
-
‘CT/MRI’ was added in the second inclusion criterion to further clarify what ‘cranial imaging’ stands for.
-
‘Glucocorticoids’ was added in the second exclusion criterion to clarify the type of steroids.
-
The eligibility criteria for the mechanistic substudy were refined (please note that these did not affect the eligibility criteria for the main study reported in the accompanying publication).
-
The windows for the 3-month and 6-month follow-up periods changed from ± 3 weeks to −4 weeks/+ 8 weeks.
-
‘Hyperglycaemia necessitating stopping of trial medication’ was added as an AESI.
Appendix 3 Trial results
| Comorbidity | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Diabetes | 54/373 (14.5) | 55/375 (14.7) | 109/748 (14.6) |
| Ischaemic heart disease | 50/373 (13.4) | 58/375 (15.5) | 108/748 (14.4) |
| Atrial fibrillation | 68/373 (18.2) | 88/375 (23.5) | 156/748 (20.9) |
| Metallic heart valve | 7/373 (1.9) | 9/375 (2.4) | 16/748 (2.1) |
| DVT/PE | 19/373 (5.1) | 24/375 (6.4) | 43/748 (5.7) |
| Stroke | 39/373 (10.5) | 34/375 (9.1) | 73/748 (9.8) |
| Previous CSDH | 5/373 (1.3) | 9/375 (2.4) | 14/748 (1.9) |
| Epilepsy | 11/373 (2.9) | 15/375 (4) | 26/748 (3.5) |
| Dementia | 21/373 (5.6) | 19/375 (5.1) | 40/748 (5.3) |
| COPD | 25/373 (6.7) | 33/375 (8.8) | 58/748 (7.8) |
| Liver disease | 9/373 (2.4) | 9/375 (2.4) | 18/748 (2.4) |
| Current malignancy | 16/373 (4.3) | 13/375 (3.5) | 29/748 (3.9) |
| Other | 284/373 (76.1) | 273/375 (72.8) | 557/748 (74.5) |
| Treatment | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Anticoagulants | |||
| Aspirin only | 57/368 (15.5) | 63/370 (17) | 120/738 (16.3) |
| Clopidogrel only | 18/368 (4.9) | 16/370 (4.3) | 34/738 (4.6) |
| Warfarin only | 52/368 (14.1) | 77/370 (20.8) | 129/738 (17.5) |
| Other anticoagulant only | 21/368 (5.7) | 17/370 (4.6) | 38/738 (5.1) |
| Combination of anticoagulants | 18/368 (4.9) | 5/370 (1.4) | 23/738 (3.1) |
| Other anticoagulant categories | |||
| Apixaban | 8/373 (2.1) | 7/375 (1.9) | 15/748 (2) |
| Dabigatran | 1/373 (0.3) | 1/375 (0.3) | 2/748 (0.3) |
| Dipyridamole | 3/373 (0.8) | 1/375 (0.3) | 4/748 (0.5) |
| Edoxaban | 1/373 (0.3) | 1/375 (0.3) | 2/748 (0.3) |
| LMWH | 6/373 (1.6) | 2/375 (0.5) | 8/748 (1.1) |
| Rivaroxaban | 8/373 (2.1) | 7/375 (1.9) | 15/748 (2) |
| Ticagrelor | 2/373 (0.5) | 1/375 (0.3) | 3/748 (0.4) |
| Other treatments | |||
| Non-steroidal anti-inflammatory drugs | 22/368 (6) | 30/371 (8.1) | 52/739 (7) |
| Diuretics | 52/368 (14.1) | 53/371 (14.3) | 105/739 (14.2) |
| Immunosuppressants | 7/368 (1.9) | 3/371 (0.8) | 10/739 (1.4) |
| ACE inhibitors | 91/368 (24.7) | 75/371 (20.2) | 166/739 (22.5) |
| Antacids/proton pump inhibitors | 102/368 (27.7) | 115/371 (31) | 217/739 (29.4) |
| Treatment arm, n/N (%) | Total, n/N (%) | ||
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Blood or clotting products given | |||
| Yes | 80/373 (21.4) | 96/374 (25.7) | 176/747 (23.6) |
| Product type | |||
| Vitamin K only | 6/373 (1.6) | 13/374 (3.5) | 19/747 (2.5) |
| PCC only | 5/373 (1.3) | 7/374 (1.9) | 12/747 (1.6) |
| Platelets only | 36/373 (9.7) | 28/374 (7.5) | 64/747 (8.6) |
| FFP only | 0/373 (0) | 1/374 (0.3) | 1/747 (0.1) |
| PRBCs only | 0/373 (0) | 1/374 (0.3) | 1/747 (0.1) |
| Other only | 1/373 (0.3) | 3/374 (0.8) | 4/747 (0.5) |
| Combination of treatments | 32/373 (8.6) | 43/374 (11.5) | 75/747 (10) |
| mRS | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Score | |||
| 0: No symptoms | 142/283 (50.2) | 129/270 (47.8) | 271/553 (49) |
| 1: No significant disability | 45/283 (15.9) | 40/270 (14.8) | 85/553 (15.4) |
| 2: Slight disability | 18/283 (6.4) | 12/270 (4.4) | 30/553 (5.4) |
| 3: Moderate disability | 53/283 (18.7) | 48/270 (17.8) | 101/553 (18.3) |
| 4: Moderately severe disability | 8/283 (2.8) | 9/270 (3.3) | 17/553 (3.1) |
| 5: Severe disability | 6/283 (2.1) | 12/270 (4.4) | 18/553 (3.3) |
| 6: Dead | 11/283 (3.9) | 20/270 (7.4) | 31/553 (5.6) |
| Dichotomised | |||
| Favourable | 258/283 (91.2) | 229/270 (84.8) | 487/553 (88.1) |
| Unfavourable | 25/283 (8.8) | 41/270 (15.2) | 66/553 (11.9) |
| Treatment arm, n/N (%) | Total, n/N (%) | ||
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Number of surgeries: all admissions | |||
| 0 | 15/307 (4.9) | 19/290 (6.6) | 34/597 (5.7) |
| 1 | 268/307 (87.3) | 260/290 (89.7) | 528/597 (88.4) |
| 2 | 21/307 (6.8) | 10/290 (3.4) | 31/597 (5.2) |
| 3 | 1/307 (0.3) | 1/290 (0.3) | 2/597 (0.3) |
| 5 | 2/307 (0.7) | 0/290 (0) | 2/597 (0.3) |
| Number of surgeries: all admissions (excluding pre randomisation) | |||
| 0 | 146/307 (47.6) | 151/290 (52.1) | 297/597 (49.7) |
| 1 | 146/307 (47.6) | 132/290 (45.5) | 278/597 (46.6) |
| 2 | 13/307 (4.2) | 7/290 (2.4) | 20/597 (3.4) |
| 4 | 2/307 (0.7) | 0/290 (0) | 2/597 (0.3) |
| Number of surgeries: index admission | |||
| 0 | 23/307 (7.5) | 24/290 (8.3) | 47/597 (7.9) |
| 1 | 275/307 (89.6) | 266/290 (91.7) | 541/597 (90.6) |
| 2 | 8/307 (2.6) | 0/290 (0) | 8/597 (1.3) |
| 3 | 1/307 (0.3) | 0/290 (0) | 1/597 (0.2) |
| Number of surgeries: index admission (excluding pre randomisation) | |||
| 0 | 161/307 (52.4) | 161/290 (55.5) | 322/597 (53.9) |
| 1 | 141/307 (45.9) | 129/290 (44.5) | 270/597 (45.2) |
| 2 | 5/307 (1.6) | 0/290 (0) | 5/597 (0.8) |
| Number of surgeries: subsequent admissions | |||
| 0 | 1/307 (0.3) | 5/290 (1.7) | 6/597 (1) |
| 1 | 22/307 (7.2) | 13/290 (4.5) | 35/597 (5.9) |
| 2 | 2/307 (0.7) | 2/290 (0.7) | 4/597 (0.7) |
| 3 | 1/307 (0.3) | 0/290 (0) | 1/597 (0.2) |
| Recurrence (one or more reoperation)a | |||
| Yes | 24/292 (8.2) | 11/271 (4.1) | 35/563 (6.2) |
| Outcome | Covariate | Estimate | 95% CI | p-value |
|---|---|---|---|---|
| Number of surgeries: index admission (including pre randomisation) | (Intercept) | 0.958 | 0.852 to 1.07 | |
| Dexamethasone vs. placebo | 0.958 | 0.811 to 1.13 | 0.61 | |
| Number of surgeries: index admission (excluding pre randomisation) | (Intercept) | 0.492 | 0.418 to 0.575 | |
| Dexamethasone vs. placebo | 0.904 | 0.714 to 1.14 | 0.402 | |
| Number of surgeries: subsequent admissions (re-admissions only) | (Intercept) | 0.492 | 0.418 to 0.575 | |
| Dexamethasone vs. placebo | 0.904 | 0.714 to 1.14 | 0.402 | |
| Number of surgeries: subsequent admissions (all patients) | (Intercept) | 0.0945 | 0.0641 to 0.133 | |
| Dexamethasone vs. placebo | 0.621 | 0.334 to 1.12 | 0.118 |
| Variable | Treatment arm, n/N (%) | |
|---|---|---|
| Placebo | Dexamethasone | |
| Primary surgery a | ||
| Burr hole(s) evacuation | 304/350 (86.8) | 302/349 (86.5) |
| Mini-craniotomy | 44/350 (12.6) | 40/349 (11.5) |
| Other | 2/350 (0.6) | 7/349 (2) |
| Postoperative drain b | ||
| Subdural | 287/350 (82) | 277/349 (79.4) |
| Subgaleal | 11/350 (3) | 11/349 (3.2) |
| No drain/not recorded | 53/350 (15) | 61/349 (17.4) |
| Anaesthesia used | ||
| General | 293/340 (86.2) | 297/342 (86.8) |
| Local | 23/340 (6.8) | 18/342 (5.3) |
| Sedation | 24/340 (7) | 27/342 (7.9) |
| Primary surgery | ||
| Burr hole(s) (total) | 304/350 (86.8) | 302/349 (86.5) |
| One burr hole | 78/304 | 63/302 |
| Two burr holes | 217/304 | 232/302 |
| Three burr holes | 1/304 | 0/302 |
| Unknown number of burr holes | 1/304 | 0/302 |
| Combination of one/two (in bilateral cases) | 7/304 | 6/302 |
| Mini-craniotomy | 44/305 (12.6) | 40/349 (11.5) |
| Other | 2/350 (0.6) | 7/349 (2) |
| Bilateral surgery with combination of burr hole(s) and mini-craniotomy | 1/2 | 4/7 |
| Reopening of old burr hole(s) or mini-craniotomy from previous surgery | 1/2 | 2/7 |
| Craniectomy | 0/2 | 1/7 |
| Recurrent surgery | Treatment arm, n/N (%) | |
|---|---|---|
| Placebo | Dexamethasone | |
| New burr hole(s) | 3/28 (10.7) | 1/14 (7.1) |
| Mini-craniotomy | 5/28 (17.8) | 2/14 (14.3) |
| Previous burr hole(s) reopened | 21/28 (75) | 9/14 (64.3) |
| Previous burr hole(s) extended to mini-craniotomy | 6/28 (21.4) | 3/14 (21.4) |
| Subdural/subgaleal drain | 27/28 (96.4) | 14/14 (100) |
| mRS | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Score | |||
| 0: No symptoms | 71/316 (22.5) | 69/318 (21.7) | 140/634 (22.1) |
| 1: No significant disability | 65/316 (20.6) | 68/318 (21.4) | 133/634 (21) |
| 2: Slight disability | 25/316 (7.9) | 23/318 (7.2) | 48/634 (7.6) |
| 3: Moderate disability | 102/316 (32.3) | 95/318 (29.9) | 197/634 (31.1) |
| 4: Moderately severe disability | 30/316 (9.5) | 36/318 (11.3) | 66/634 (10.4) |
| 5: Severe disability | 22/316 (7) | 25/318 (7.9) | 47/634 (7.4) |
| 6: Dead | 1/316 (0.3) | 2/318 (0.6) | 3/634 (0.5) |
| Dichotomised | |||
| Favourable | 263/316 (83.2) | 255/318 (80.2) | 518/634 (81.7) |
| Unfavourable | 53/316 (16.8) | 63/318 (19.8) | 116/634 (18.3) |
| mRS | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Score | |||
| 0: No symptoms | 163/326 (50) | 144/322 (44.7) | 307/648 (47.4) |
| 1: No significant disability | 50/326 (15.3) | 48/322 (14.9) | 98/648 (15.1) |
| 2: Slight disability | 14/326 (4.3) | 13/322 (4) | 27/648 (4.2) |
| 3: Moderate disability | 71/326 (21.8) | 63/322 (19.6) | 134/648 (20.7) |
| 4: Moderately severe disability | 5/326 (1.5) | 14/322 (4.3) | 19/648 (2.9) |
| 5: Severe disability | 12/326 (3.7) | 18/322 (5.6) | 30/648 (4.6) |
| 6: Dead | 11/326 (3.4) | 22/322 (6.8) | 33/648 (5.1) |
| Dichotomised | |||
| Favourable | 298/326 (91.4) | 268/322 (83.2) | 566/648 (87.3) |
| Unfavourable | 28/326 (8.6) | 54/322 (16.8) | 82/648 (12.7) |
| mRS | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Score | |||
| 0: No symptoms | 60/251 (23.9) | 55/239 (23) | 115/490 (23.5) |
| 1: No significant disability | 53/251 (21.1) | 48/239 (20.1) | 101/490 (20.6) |
| 2: Slight disability | 21/251 (8.4) | 20/239 (8.4) | 41/490 (8.4) |
| 3: Moderate disability | 82/251 (32.7) | 76/239 (31.8) | 158/490 (32.2) |
| 4: Moderately severe disability | 19/251 (7.6) | 25/239 (10.5) | 44/490 (9) |
| 5: Severe disability | 16/251 (6.4) | 15/239 (6.3) | 31/490 (6.3) |
| Dichotomised | |||
| Favourable | 216/251 (86.1) | 199/239 (83.3) | 415/490 (84.7) |
| Unfavourable | 35/251 (13.9) | 40/239 (16.7) | 75/490 (15.3) |
| mRS | Treatment arm, n/N (%) | Total, n/N (%) | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Score | |||
| 0: No symptoms | 148/280 (52.9) | 118/259 (45.6) | 266/539 (49.4) |
| 1: No significant disability | 38/280 (13.6) | 41/259 (15.8) | 79/539 (14.7) |
| 2: Slight disability | 12/280 (4.3) | 9/259 (3.5) | 21/539 (3.9) |
| 3: Moderate disability | 59/280 (21.1) | 51/259 (19.7) | 110/539 (20.4) |
| 4: Moderately severe disability | 4/280 (1.4) | 10/259 (3.9) | 14/539 (2.6) |
| 5: Severe disability | 11/280 (3.9) | 15/259 (5.8) | 26/539 (4.8) |
| 6: Dead | 8/280 (2.9) | 15/259 (5.8) | 23/539 (4.3) |
| Dichotomised | |||
| Favourable | 257/280 (91.8) | 219/259 (84.6) | 476/539 (88.3) |
| Unfavourable | 23/280 (8.2) | 40/259 (15.4) | 63/539 (11.7) |
| Cut-off point | Ordinal logistic regression | Sequential ORs | ||||
|---|---|---|---|---|---|---|
| Probability mRS ≤ cut-off (placebo arm) | Global OR (95% CI)a | p-value | Placebo (n = 251, discharge) (n = 280, 3 months), n (%) | Dexamethasone (n = 239, discharge) (n = 259, 3 months), n (%) | Marginal OR (95% CI) | |
| mRS score at discharge | ||||||
| 0 | 0.243 | 0.913 (0.665 to 1.25) | 0.572 | 60 (24) | 55 (23) | 0.952 (0.626 to 1.446) |
| 1 | 0.452 | 113 (45) | 103 (43) | 0.925 (0.647 to 1.322) | ||
| 2 | 0.535 | 134 (53) | 123 (51) | 0.926 (0.649 to 1.32) | ||
| 3 | 0.853 | 216 (86) | 199 (83) | 0.806 (0.492 to 1.32) | ||
| 4 | 0.939 | 235 (94) | 224 (94) | 1.017 (0.491 to 2.105) | ||
| 5 | . | . | . | . | ||
| 6 | . | . | . | . | ||
| mRS score at 3 months | ||||||
| 0 | 0.532 | 0.724 (0.528 to 0.992) | 0.045 | 148 (53) | 118 (46) | 0.746 (0.532 to 1.048) |
| 1 | 0.675 | 186 (66) | 159 (61) | 0.804 (0.565 to 1.143) | ||
| 2 | 0.712 | 198 (71) | 168 (65) | 0.765 (0.532 to 1.099) | ||
| 3 | 0.899 | 257 (92) | 219 (85) | 0.49 (0.284 to 0.844) | ||
| 4 | 0.922 | 261 (93) | 229 (88) | 0.556 (0.305 to 1.014) | ||
| 5 | 0.964 | 272 (97) | 244 (94) | 0.478 (0.199 to 1.148) | ||
| 6 | . | . | . | . | ||
| Treatment arm | Total | ||
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Eating my meals, n/N (%) | |||
| I need help for all aspects of eating meals | 18/316 (5.7) | 17/317 (5.4) | 35/633 (5.5) |
| I need some help eating my meal (e.g. cutting/spreading butter) | 38/316 (12) | 33/317 (10.4) | 71/633 (11.2) |
| I can eat without help | 260/316 (82.3) | 267/317 (84.2) | 527/633 (83.3) |
| Bathing/showering, n/N (%) | |||
| I need help with bathing/showering | 108/316 (34.2) | 97/317 (30.6) | 205/633 (32.4) |
| I can bath/shower without help | 208/316 (65.8) | 220/317 (69.4) | 428/633 (67.6) |
| Personal care, n/N (%) | |||
| I need help with personal care | 79/316 (25) | 79/317 (24.9) | 158/633 (25) |
| I can do all my personal care without help | 237/316 (75) | 238/317 (75.1) | 475/633 (75) |
| Getting dressed, n/N (%) | |||
| I need help with all aspects of dressing | 43/315 (13.7) | 43/317 (13.6) | 86/632 (13.6) |
| I need some help with dressing but can do about half without help | 61/315 (19.4) | 48/317 (15.1) | 109/632 (17.2) |
| I can get dressed without help | 211/315 (67) | 226/317 (71.3) | 437/632 (69.1) |
| Controlling bowels, n/N (%) | |||
| I cannot control my bowel function | 11/316 (3.5) | 13/317 (4.1) | 24/633 (3.8) |
| I sometimes have an accident with my bowels | 33/316 (10.4) | 26/317 (8.2) | 59/633 (9.3) |
| I have no problems controlling my bowels | 272/316 (86.1) | 278/317 (87.7) | 550/633 (86.9) |
| Controlling bladder, n/N (%) | |||
| I cannot control my bladder function or I have a catheter | 27/316 (8.5) | 31/317 (9.8) | 58/633 (9.2) |
| I sometimes have an accident with my bladder | 35/316 (11.1) | 37/317 (11.7) | 72/633 (11.4) |
| I have no problems controlling my bladder | 254/316 (80.4) | 249/317 (78.5) | 503/633 (79.5) |
| Toilet use: getting on/off the toilet, wiping and dressing, n/N (%) | |||
| I need help to do all of these things when going to the toilet | 38/316 (12) | 38/317 (12) | 76/633 (12) |
| I need help to do some of these things, but can do some things alone | 38/316 (12) | 35/317 (11) | 73/633 (11.5) |
| I can do all of these things without help | 240/316 (75.9) | 244/317 (77) | 484/633 (76.5) |
| Getting from the bed to the chair and back, n/N (%) | |||
| I am unable to sit in a chair | 7/316 (2.2) | 13/317 (4.1) | 20/633 (3.2) |
| I need one or two people to help me get into the chair and back | 39/316 (12.3) | 36/317 (11.4) | 75/633 (11.8) |
| I need a little bit of help getting into the chair and back | 33/316 (10.4) | 23/317 (7.3) | 56/633 (8.8) |
| I can get into the chair and back without help | 237/316 (75) | 245/317 (77.3) | 482/633 (76.1) |
| Walking on a flat surface, n/N (%) | |||
| I cannot walk more than 50 yards | 54/316 (17.1) | 60/317 (18.9) | 114/633 (18) |
| I use a wheelchair on my own to go more than 50 yards | 4/316 (1.3) | 4/317 (1.3) | 8/633 (1.3) |
| I can walk more than 50 yards with another person helping me | 54/316 (17.1) | 39/317 (12.3) | 93/633 (14.7) |
| I can walk more than 50 yards without help from another person | 204/316 (64.6) | 214/317 (67.5) | 418/633 (66) |
| Walking up the stairs, n/N (%) | |||
| I cannot walk up the stairs on my own | 74/315 (23.5) | 75/316 (23.7) | 149/631 (23.6) |
| I can walk up the stairs with some help | 65/315 (20.6) | 53/316 (16.8) | 118/631 (18.7) |
| I can walk up the stairs without help | 176/315 (55.9) | 188/316 (59.5) | 364/631 (57.7) |
| Total score | |||
| n | 315 | 316 | 631 |
| Mean (SD) | 80.5 (26.7) | 81.0 (28.0) | 80.7 (27.4) |
| Median | 95 | 95 | 95 |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| Treatment arm | Total | ||
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Eating my meals, n/N (%) | |||
| I need help for all aspects of eating meals | 3/314 (1) | 13/299 (4.3) | 16/613 (2.6) |
| I need some help eating my meal (e.g. cutting/spreading butter) | 20/314 (6.4) | 16/299 (5.4) | 36/613 (5.9) |
| I can eat without help | 291/314 (92.7) | 270/299 (90.3) | 561/613 (91.5) |
| Bathing/showering, n/N (%) | |||
| I need help with bathing/showering | 61/313 (19.5) | 59/299 (19.7) | 120/612 (19.6) |
| I can bath/shower without help | 252/313 (80.5) | 240/299 (80.3) | 492/612 (80.4) |
| Personal care, n/N (%) | |||
| I need help with personal care | 36/314 (11.5) | 37/299 (12.4) | 73/613 (11.9) |
| I can do all my personal care without help | 278/314 (88.5) | 262/299 (87.6) | 540/613 (88.1) |
| Getting dressed, n/N (%) | |||
| I need help with all aspects of dressing | 14/313 (4.5) | 25/299 (8.4) | 39/612 (6.4) |
| I need some help with dressing but can do about half without help | 46/313 (14.7) | 37/299 (12.4) | 83/612 (13.6) |
| I can get dressed without help | 253/313 (80.8) | 237/299 (79.3) | 490/612 (80.1) |
| Controlling bowels, n/N (%) | |||
| I cannot control my bowel function | 9/313 (2.9) | 13/300 (4.3) | 22/613 (3.6) |
| I sometimes have an accident with my bowels | 33/313 (10.5) | 33/300 (11) | 66/613 (10.8) |
| I have no problems controlling my bowels | 271/313 (86.6) | 254/300 (84.7) | 525/613 (85.6) |
| Controlling bladder, n/N (%) | |||
| I cannot control my bladder function or I have a catheter | 18/314 (5.7) | 24/300 (8) | 42/614 (6.8) |
| I sometimes have an accident with my bladder | 51/314 (16.2) | 42/300 (14) | 93/614 (15.1) |
| I have no problems controlling my bladder | 245/314 (78) | 234/300 (78) | 479/614 (78) |
| Toilet use: getting on/off the toilet, wiping and dressing, n/N (%) | |||
| I need help to do all of these things when going to the toilet | 15/314 (4.8) | 20/299 (6.7) | 35/613 (5.7) |
| I need help to do some of these things, but can do some things alone | 24/314 (7.6) | 23/299 (7.7) | 47/613 (7.7) |
| I can do all of these things without help | 275/314 (87.6) | 256/299 (85.6) | 531/613 (86.6) |
| Getting from the bed to the chair and back, n/N (%) | |||
| I am unable to sit in a chair | 6/314 (1.9) | 7/299 (2.3) | 13/613 (2.1) |
| I need one or two people to help me get into the chair and back | 7/314 (2.2) | 18/299 (6) | 25/613 (4.1) |
| I need a little bit of help getting into the chair and back | 13/314 (4.1) | 15/299 (5) | 28/613 (4.6) |
| I can get into the chair and back without help | 288/314 (91.7) | 259/299 (86.6) | 547/613 (89.2) |
| Walking on a flat surface, n/N (%) | |||
| I cannot walk more than 50 yards | 31/314 (9.9) | 39/299 (13) | 70/613 (11.4) |
| I use a wheelchair on my own to go more than 50 yards | 5/314 (1.6) | 5/299 (1.7) | 10/613 (1.6) |
| I can walk more than 50 yards with another person helping me | 19/314 (6.1) | 21/299 (7) | 40/613 (6.5) |
| I can walk more than 50 yards without help from another person | 259/314 (82.5) | 234/299 (78.3) | 493/613 (80.4) |
| Walking up the stairs, n/N (%) | |||
| I cannot walk up the stairs on my own | 37/313 (11.8) | 54/299 (18.1) | 91/612 (14.9) |
| I can walk up the stairs with some help | 32/313 (10.2) | 27/299 (9) | 59/612 (9.6) |
| I can walk up the stairs without help | 244/313 (78) | 218/299 (72.9) | 462/612 (75.5) |
| Total score | |||
| n | 312 | 298 | 610 |
| Mean (SD) | 89.4 (19.7) | 86.7 (23.9) | 88.1 (21.9) |
| Median | 100 | 100 | 100 |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| Treatment arm | Total | ||
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Eating my meals, n/N (%) | |||
| I need help for all aspects of eating meals | 3/322 (0.9) | 12/309 (3.9) | 15/631 (2.4) |
| I need some help eating my meal (e.g. cutting/spreading butter) | 23/322 (7.1) | 11/309 (3.6) | 34/631 (5.4) |
| I can eat without help | 296/322 (91.9) | 286/309 (92.6) | 582/631 (92.2) |
| Bathing/showering, n/N (%) | |||
| I need help with bathing/showering | 52/322 (16.1) | 62/310 (20) | 114/632 (18) |
| I can bath/shower without help | 270/322 (83.9) | 248/310 (80) | 518/632 (82) |
| Personal care, n/N (%) | |||
| I need help with personal care | 32/322 (9.9) | 41/310 (13.2) | 73/632 (11.6) |
| I can do all my personal care without help | 290/322 (90.1) | 269/310 (86.8) | 559/632 (88.4) |
| Getting dressed, n/N (%) | |||
| I need help with all aspects of dressing | 16/322 (5) | 21/309 (6.8) | 37/631 (5.9) |
| I need some help with dressing but can do about half without help | 38/322 (11.8) | 35/309 (11.3) | 73/631 (11.6) |
| I can get dressed without help | 268/322 (83.2) | 253/309 (81.9) | 521/631 (82.6) |
| Controlling bowels, n/N (%) | |||
| I cannot control my bowel function | 8/321 (2.5) | 13/309 (4.2) | 21/630 (3.3) |
| I sometimes have an accident with my bowels | 38/321 (11.8) | 37/309 (12) | 75/630 (11.9) |
| I have no problems controlling my bowels | 275/321 (85.7) | 259/309 (83.8) | 534/630 (84.8) |
| Controlling bladder, n/N (%) | |||
| I cannot control my bladder function or I have a catheter | 9/321 (2.8) | 20/309 (6.5) | 29/630 (4.6) |
| I sometimes have an accident with my bladder | 56/321 (17.4) | 50/309 (16.2) | 106/630 (16.8) |
| I have no problems controlling my bladder | 256/321 (79.8) | 239/309 (77.3) | 495/630 (78.6) |
| Toilet use: getting on/off the toilet, wiping and dressing, n/N (%) | |||
| I need help to do all of these things when going to the toilet | 14/321 (4.4) | 21/309 (6.8) | 35/630 (5.6) |
| I need help to do some of these things, but can do some things alone | 19/321 (5.9) | 18/309 (5.8) | 37/630 (5.9) |
| I can do all of these things without help | 288/321 (89.7) | 270/309 (87.4) | 558/630 (88.6) |
| Getting from the bed to the chair and back, n/N (%) | |||
| I am unable to sit in a chair | 4/321 (1.2) | 5/310 (1.6) | 9/631 (1.4) |
| I need one or two people to help me get into the chair and back | 10/321 (3.1) | 15/310 (4.8) | 25/631 (4) |
| I need a little bit of help getting into the chair and back | 10/321 (3.1) | 10/310 (3.2) | 20/631 (3.2) |
| I can get into the chair and back without help | 297/321 (92.5) | 280/310 (90.3) | 577/631 (91.4) |
| Walking on a flat surface, n/N (%) | |||
| I cannot walk more than 50 yards | 28/322 (8.7) | 31/310 (10) | 59/632 (9.3) |
| I use a wheelchair on my own to go more than 50 yards | 9/322 (2.8) | 9/310 (2.9) | 18/632 (2.8) |
| I can walk more than 50 yards with another person helping me | 26/322 (8.1) | 18/310 (5.8) | 44/632 (7) |
| I can walk more than 50 yards without help from another person | 259/322 (80.4) | 252/310 (81.3) | 511/632 (80.9) |
| Walking up the stairs, n/N (%) | |||
| I cannot walk up the stairs on my own | 35/319 (11) | 51/310 (16.5) | 86/629 (13.7) |
| I can walk up the stairs with some help | 28/319 (8.8) | 26/310 (8.4) | 54/629 (8.6) |
| I can walk up the stairs without help | 256/319 (80.3) | 233/310 (75.2) | 489/629 (77.7) |
| Total score | |||
| n | 318 | 309 | 627 |
| Mean (SD) | 90.3 (19.0) | 88.1 (22.8) | 89.2 (20.9) |
| Median | 100 | 100 | 100 |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| Treatment arm | Total | ||
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Eating my meals, n/N (%) | |||
| I need help for all aspects of eating meals | 17/252 (6.7) | 11/239 (4.6) | 28/491 (5.7) |
| I need some help eating my meal (e.g. cutting/spreading butter) | 29/252 (11.5) | 22/239 (9.2) | 51/491 (10.4) |
| I can eat without help | 206/252 (81.7) | 206/239 (86.2) | 412/491 (83.9) |
| Bathing/showering, n/N (%) | |||
| I need help with bathing/showering | 84/252 (33.3) | 71/239 (29.7) | 155/491 (31.6) |
| I can bath/shower without help | 168/252 (66.7) | 168/239 (70.3) | 336/491 (68.4) |
| Personal care, n/N (%) | |||
| I need help with personal care | 60/252 (23.8) | 53/239 (22.2) | 113/491 (23) |
| I can do all my personal care without help | 192/252 (76.2) | 186/239 (77.8) | 378/491 (77) |
| Getting dressed, n/N (%) | |||
| I need help with all aspects of dressing | 31/251 (12.4) | 29/239 (12.1) | 60/490 (12.2) |
| I need some help with dressing but can do about half without help | 47/251 (18.7) | 31/239 (13) | 78/490 (15.9) |
| I can get dressed without help | 173/251 (68.9) | 179/239 (74.9) | 352/490 (71.8) |
| Controlling bowels, n/N (%) | |||
| I cannot control my bowel function | 8/252 (3.2) | 8/239 (3.3) | 16/491 (3.3) |
| I sometimes have an accident with my bowels | 28/252 (11.1) | 18/239 (7.5) | 46/491 (9.4) |
| I have no problems controlling my bowels | 216/252 (85.7) | 213/239 (89.1) | 429/491 (87.4) |
| Controlling bladder, n/N (%) | |||
| I cannot control my bladder function or I have a catheter | 17/252 (6.7) | 17/239 (7.1) | 34/491 (6.9) |
| I sometimes have an accident with my bladder | 30/252 (11.9) | 32/239 (13.4) | 62/491 (12.6) |
| I have no problems controlling my bladder | 205/252 (81.3) | 190/239 (79.5) | 395/491 (80.4) |
| Toilet use: getting on/off the toilet, wiping and dressing, n/N (%) | |||
| I need help to do all of these things when going to the toilet | 28/252 (11.1) | 22/239 (9.2) | 50/491 (10.2) |
| I need help to do some of these things, but can do some things alone | 31/252 (12.3) | 26/239 (10.9) | 57/491 (11.6) |
| I can do all of these things without help | 193/252 (76.6) | 191/239 (79.9) | 384/491 (78.2) |
| Getting from the bed to the chair and back, n/N (%) | |||
| I am unable to sit in a chair | 6/252 (2.4) | 7/239 (2.9) | 13/491 (2.6) |
| I need one or two people to help me get into the chair and back | 27/252 (10.7) | 24/239 (10) | 51/491 (10.4) |
| I need a little bit of help getting into the chair and back | 26/252 (10.3) | 14/239 (5.9) | 40/491 (8.1) |
| I can get into the chair and back without help | 193/252 (76.6) | 194/239 (81.2) | 387/491 (78.8) |
| Walking on a flat surface, n/N (%) | |||
| I cannot walk more than 50 yards | 43/252 (17.1) | 39/239 (16.3) | 82/491 (16.7) |
| I use a wheelchair on my own to go more than 50 yards | 2/252 (0.8) | 3/239 (1.3) | 5/491 (1) |
| I can walk more than 50 yards with another person helping me | 37/252 (14.7) | 28/239 (11.7) | 65/491 (13.2) |
| I can walk more than 50 yards without help from another person | 170/252 (67.5) | 169/239 (70.7) | 339/491 (69) |
| Walking up the stairs, n/N (%) | |||
| I cannot walk up the stairs on my own | 52/251 (20.7) | 52/239 (21.8) | 104/490 (21.2) |
| I can walk up the stairs with some help | 52/251 (20.7) | 39/239 (16.3) | 91/490 (18.6) |
| I can walk up the stairs without help | 147/251 (58.6) | 148/239 (61.9) | 295/490 (60.2) |
| Total score | |||
| n | 251 | 239 | 490 |
| Mean (SD) | 81.5 (26.4) | 83.2 (26.2) | 82.3 (26.3) |
| Median | 95 | 100 | 95 |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| Treatment arm | Total | ||
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Eating my meals, n/N (%) | |||
| I need help for all aspects of eating meals | 3/272 (1.1) | 10/243 (4.1) | 13/515 (2.5) |
| I need some help eating my meal (e.g. cutting/spreading butter) | 18/272 (6.6) | 15/243 (6.2) | 33/515 (6.4) |
| I can eat without help | 251/272 (92.3) | 218/243 (89.7) | 469/515 (91.1) |
| Bathing/showering, n/N (%) | |||
| I need help with bathing/showering | 50/271 (18.5) | 46/243 (18.9) | 96/514 (18.7) |
| I can bath/shower without help | 221/271 (81.5) | 197/243 (81.1) | 418/514 (81.3) |
| Personal care, n/N (%) | |||
| I need help with personal care | 28/272 (10.3) | 28/243 (11.5) | 56/515 (10.9) |
| I can do all my personal care without help | 244/272 (89.7) | 215/243 (88.5) | 459/515 (89.1) |
| Getting dressed, n/N (%) | |||
| I need help with all aspects of dressing | 11/271 (4.1) | 20/243 (8.2) | 31/514 (6) |
| I need some help with dressing but can do about half without help | 42/271 (15.5) | 28/243 (11.5) | 70/514 (13.6) |
| I can get dressed without help | 218/271 (80.4) | 195/243 (80.2) | 413/514 (80.4) |
| Controlling bowels, n/N (%) | |||
| I cannot control my bowel function | 7/271 (2.6) | 11/244 (4.5) | 18/515 (3.5) |
| I sometimes have an accident with my bowels | 28/271 (10.3) | 29/244 (11.9) | 57/515 (11.1) |
| I have no problems controlling my bowels | 236/271 (87.1) | 204/244 (83.6) | 440/515 (85.4) |
| Controlling bladder, n/N (%) | |||
| I cannot control my bladder function or I have a catheter | 14/272 (5.1) | 18/244 (7.4) | 32/516 (6.2) |
| I sometimes have an accident with my bladder | 45/272 (16.5) | 36/244 (14.8) | 81/516 (15.7) |
| I have no problems controlling my bladder | 213/272 (78.3) | 190/244 (77.9) | 403/516 (78.1) |
| Toilet use: getting on/off the toilet, wiping and dressing, n/N (%) | |||
| I need help to do all of these things when going to the toilet | 12/272 (4.4) | 16/244 (6.6) | 28/516 (5.4) |
| I need help to do some of these things, but can do some things alone | 22/272 (8.1) | 17/244 (7) | 39/516 (7.6) |
| I can do all of these things without help | 238/272 (87.5) | 211/244 (86.5) | 449/516 (87) |
| Getting from the bed to the chair and back, n/N (%) | |||
| I am unable to sit in a chair | 5/272 (1.8) | 5/243 (2.1) | 10/515 (1.9) |
| I need one or two people to help me get into the chair and back | 7/272 (2.6) | 13/243 (5.3) | 20/515 (3.9) |
| I need a little bit of help getting into the chair and back | 12/272 (4.4) | 13/243 (5.3) | 25/515 (4.9) |
| I can get into the chair and back without help | 248/272 (91.2) | 212/243 (87.2) | 460/515 (89.3) |
| Walking on a flat surface, n/N (%) | |||
| I cannot walk more than 50 yards | 30/272 (11) | 29/243 (11.9) | 59/515 (11.5) |
| I use a wheelchair on my own to go more than 50 yards | 4/272 (1.5) | 2/243 (0.8) | 6/515 (1.2) |
| I can walk more than 50 yards with another person helping me | 13/272 (4.8) | 19/243 (7.8) | 32/515 (6.2) |
| I can walk more than 50 yards without help from another person | 225/272 (82.7) | 193/243 (79.4) | 418/515 (81.2) |
| Walking up the stairs, n/N (%) | |||
| I cannot walk up the stairs on my own | 33/271 (12.2) | 40/243 (16.5) | 73/514 (14.2) |
| I can walk up the stairs with some help | 26/271 (9.6) | 22/243 (9.1) | 48/514 (9.3) |
| I can walk up the stairs without help | 212/271 (78.2) | 181/243 (74.5) | 393/514 (76.5) |
| Total score | |||
| n | 270 | 243 | 513 |
| Mean (SD) | 89.4 (19.9) | 87.3 (23.4) | 88.4 (21.6) |
| Median | 100 | 100 | 100 |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| Treatment arm | Total | ||
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Eating my meals, n/N (%) | |||
| I need help for all aspects of eating meals | 3/272 (1.1) | 10/248 (4) | 13/520 (2.5) |
| I need some help eating my meal (e.g. cutting/spreading butter) | 18/272 (6.6) | 11/248 (4.4) | 29/520 (5.6) |
| I can eat without help | 251/272 (92.3) | 227/248 (91.5) | 478/520 (91.9) |
| Bathing/showering, n/N (%) | |||
| I need help with bathing/showering | 44/272 (16.2) | 49/249 (19.7) | 93/521 (17.9) |
| I can bath/shower without help | 228/272 (83.8) | 200/249 (80.3) | 428/521 (82.1) |
| Personal care, n/N (%) | |||
| I need help with personal care | 26/272 (9.6) | 36/249 (14.5) | 62/521 (11.9) |
| I can do all my personal care without help | 246/272 (90.4) | 213/249 (85.5) | 459/521 (88.1) |
| Getting dressed, n/N (%) | |||
| I need help with all aspects of dressing | 13/272 (4.8) | 18/248 (7.3) | 31/520 (6) |
| I need some help with dressing but can do about half without help | 34/272 (12.5) | 26/248 (10.5) | 60/520 (11.5) |
| I can get dressed without help | 225/272 (82.7) | 204/248 (82.3) | 429/520 (82.5) |
| Controlling bowels, n/N (%) | |||
| I cannot control my bowel function | 6/271 (2.2) | 11/248 (4.4) | 17/519 (3.3) |
| I sometimes have an accident with my bowels | 32/271 (11.8) | 30/248 (12.1%) | 62/519 (11.9) |
| I have no problems controlling my bowels | 233/271 (86) | 207/248 (83.5) | 440/519 (84.8) |
| Controlling bladder, n/N (%) | |||
| I cannot control my bladder function or I have a catheter | 8/271 (3) | 17/248 (6.9) | 25/519 (4.8) |
| I sometimes have an accident with my bladder | 48/271 (17.7) | 43/248 (17.3) | 91/519 (17.5) |
| I have no problems controlling my bladder | 215/271 (79.3) | 188/248 (75.8) | 403/519 (77.6) |
| Toilet use: getting on/off the toilet, wiping and dressing, n/N (%) | |||
| I need help to do all of these things when going to the toilet | 12/271 (4.4) | 17/248 (6.9) | 29/519 (5.6) |
| I need help to do some of these things, but can do some things alone | 15/271 (5.5) | 16/248 (6.5) | 31/519 (6) |
| I can do all of these things without help | 244/271 (90) | 215/248 (86.7) | 459/519 (88.4) |
| Getting from the bed to the chair and back, n/N (%) | |||
| I am unable to sit in a chair | 4/271 (1.5) | 5/249 (2) | 9/520 (1.7) |
| I need one or two people to help me get into the chair and back | 9/271 (3.3) | 12/249 (4.8) | 21/520 (4) |
| I need a little bit of help getting into the chair and back | 8/271 (3) | 10/249 (4) | 18/520 (3.5) |
| I can get into the chair and back without help | 250/271 (92.3) | 222/249 (89.2) | 472/520 (90.8) |
| Walking on a flat surface, n/N (%) | |||
| I cannot walk more than 50 yards | 22/272 (8.1) | 24/249 (9.6) | 46/521 (8.8) |
| I use a wheelchair on my own to go more than 50 yards | 9/272 (3.3) | 6/249 (2.4) | 15/521 (2.9) |
| I can walk more than 50 yards with another person helping me | 23/272 (8.5) | 15/249 (6) | 38/521 (7.3) |
| I can walk more than 50 yards without help from another person | 218/272 (80.1) | 204/249 (81.9) | 422/521 (81) |
| Walking up the stairs, n/N (%) | |||
| I cannot walk up the stairs on my own | 27/269 (10) | 42/249 (16.9) | 69/518 (13.3) |
| I can walk up the stairs with some help | 24/269 (8.9) | 21/249 (8.4) | 45/518 (8.7) |
| I can walk up the stairs without help | 218/269 (81) | 186/249 (74.7) | 404/518 (78) |
| Total score | |||
| n | 268 | 248 | 516 |
| Mean (SD) | 90.4 (18.9) | 87.7 (23.4) | 89.1 (21.2) |
| Median | 100 | 100 | 100 |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| Outcome | Linear regression | Mann–Whitney U-test: p-value | |||
|---|---|---|---|---|---|
| Covariate | Estimate (SE) | 95% CI | p-value | ||
| BI at discharge | (Intercept) | 81.5 (1.66) | 78.2 to 84.7 | 0.319 | |
| Dexamethasone vs. placebo | 1.73 (2.38) | −2.94 to 6.39 | 0.468 | ||
| BI at 3 months | (Intercept) | 89.4 (1.32) | 86.8 to 92 | 0.432 | |
| Dexamethasone vs. placebo | −2.08 (1.91) | −5.84 to 1.68 | 0.278 | ||
| BI at 6 months | (Intercept) | 90.4 (1.29) | 87.9 to 93 | 0.324 | |
| Dexamethasone vs. placebo | −2.71 (1.86) | −6.36 to 0.953 | 0.147 | ||
| Variable | Treatment arm | Total | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Mobility, n/N (%) | |||
| I have no problems in walking about | 159/308 (51.6) | 149/306 (48.7) | 308/614 (50.2) |
| I have slight problems in walking about | 82/308 (26.6) | 74/306 (24.2) | 156/614 (25.4) |
| I have moderate problems in walking about | 32/308 (10.4) | 44/306 (14.4) | 76/614 (12.4) |
| I have severe problems in walking about | 23/308 (7.5) | 21/306 (6.9) | 44/614 (7.2) |
| I am unable to walk about | 12/308 (3.9) | 18/306 (5.9) | 30/614 (4.9) |
| Self-care, n/N (%) | |||
| I have no problems washing or dressing myself | 196/308 (63.6) | 197/306 (64.4) | 393/614 (64) |
| I have slight problems washing or dressing myself | 53/308 (17.2) | 46/306 (15) | 99/614 (16.1) |
| I have moderate problems washing or dressing myself | 32/308 (10.4) | 30/306 (9.8) | 62/614 (10.1) |
| I have severe problems washing or dressing myself | 16/308 (5.2) | 16/306 (5.2) | 32/614 (5.2) |
| I am unable to wash or dress myself | 11/308 (3.6) | 17/306 (5.6) | 28/614 (4.6) |
| Usual activities, n/N (%) | |||
| I have no problems doing usual activities | 128/306 (41.8) | 122/306 (39.9) | 250/612 (40.8) |
| I have slight problems doing usual activities | 89/306 (29.1) | 74/306 (24.2) | 163/612 (26.6) |
| I have moderate problems doing usual activities | 45/306 (14.7) | 44/306 (14.4) | 89/612 (14.5) |
| I have severe problems doing usual activities | 25/306 (8.2) | 35/306 (11.4) | 60/612 (9.8) |
| I am unable to do usual activities | 19/306 (6.2) | 31/306 (10.1) | 50/612 (8.2) |
| Pain/discomfort, n/N (%) | |||
| I have no pain or discomfort | 153/307 (49.8) | 171/306 (55.9) | 324/613 (52.9) |
| I have slight pain or discomfort | 122/307 (39.7) | 101/306 (33) | 223/613 (36.4) |
| I have moderate pain or discomfort | 26/307 (8.5) | 30/306 (9.8) | 56/613 (9.1) |
| I have severe pain or discomfort | 6/307 (2) | 3/306 (1) | 9/613 (1.5) |
| I have extreme pain or discomfort | 0/307 (0) | 1/306 (0.3) | 1/613 (0.2) |
| Anxiety/depression, n/N (%) | |||
| I am not anxious or depressed | 217/307 (70.7) | 196/305 (64.3) | 413/612 (67.5) |
| I am slightly anxious or depressed | 53/307 (17.3) | 71/305 (23.3) | 124/612 (20.3) |
| I am moderately anxious or depressed | 27/307 (8.8) | 27/305 (8.9) | 54/612 (8.8) |
| I am severely anxious or depressed | 9/307 (2.9) | 7/305 (2.3) | 16/612 (2.6) |
| I am extremely anxious or depressed | 1/307 (0.3) | 4/305 (1.3) | 5/612 (0.8) |
| Visual analogue scale | |||
| n | 285 | 293 | 578 |
| Mean (SD) | 72.3 (18.5) | 74.3 (17.3) | 73.3 (17.9) |
| Median | 75 | 75 | 75 |
| Minimum, maximum | 5, 100 | 0, 100 | 0, 100 |
| Utility index | |||
| n | 306 | 307 | 613 |
| Mean (SD) | 0.727 (0.265) | 0.697 (0.293) | 0.712 (0.279) |
| Median | 0.795 | 0.767 | 0.778 |
| Minimum, maximum | −0.166, 1 | −0.358, 1 | −0.358, 1 |
| Variable | Treatment arm | Total | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Mobility, n/N (%) | |||
| I have no problems in walking about | 192/310 (61.9) | 172/296 (58.1) | 364/606 (60.1) |
| I have slight problems in walking about | 61/310 (19.7) | 42/296 (14.2) | 103/606 (17) |
| I have moderate problems in walking about | 37/310 (11.9) | 51/296 (17.2) | 88/606 (14.5) |
| I have severe problems in walking about | 12/310 (3.9) | 19/296 (6.4) | 31/606 (5.1) |
| I am unable to walk about | 8/310 (2.6) | 12/296 (4.1) | 20/606 (3.3) |
| Self-care, n/N (%) | |||
| I have no problems washing or dressing myself | 237/309 (76.7) | 223/296 (75.3) | 460/605 (76) |
| I have slight problems washing or dressing myself | 38/309 (12.3) | 33/296 (11.1) | 71/605 (11.7) |
| I have moderate problems washing or dressing myself | 16/309 (5.2) | 19/296 (6.4) | 35/605 (5.8) |
| I have severe problems washing or dressing myself | 11/309 (3.6) | 8/296 (2.7) | 19/605 (3.1) |
| I am unable to wash or dress myself | 7/309 (2.3) | 13/296 (4.4) | 20/605 (3.3) |
| Usual activities, n/N (%) | |||
| I have no problems doing usual activities | 178/307 (58) | 154/294 (52.4) | 332/601 (55.2) |
| I have slight problems doing usual activities | 63/307 (20.5) | 59/294 (20.1) | 122/601 (20.3) |
| I have moderate problems doing usual activities | 41/307 (13.4) | 41/294 (13.9) | 82/601 (13.6) |
| I have severe problems doing usual activities | 8/307 (2.6) | 17/294 (5.8) | 25/601 (4.2) |
| I am unable to do usual activities | 17/307 (5.5) | 23/294 (7.8) | 40/601 (6.7) |
| Pain/discomfort, n/N (%) | |||
| I have no pain or discomfort | 195/308 (63.3) | 185/296 (62.5) | 380/604 (62.9) |
| I have slight pain or discomfort | 71/308 (23.1) | 64/296 (21.6) | 135/604 (22.4) |
| I have moderate pain or discomfort | 33/308 (10.7) | 36/296 (12.2) | 69/604 (11.4) |
| I have severe pain or discomfort | 8/308 (2.6) | 10/296 (3.4) | 18/604 (3) |
| I have extreme pain or discomfort | 1/308 (0.3) | 1/296 (0.3) | 2/604 (0.3) |
| Anxiety/depression, n/N (%) | |||
| I am not anxious or depressed | 222/307 (72.3) | 202/296 (68.2) | 424/603 (70.3) |
| I am slightly anxious or depressed | 53/307 (17.3) | 53/296 (17.9) | 106/603 (17.6) |
| I am moderately anxious or depressed | 24/307 (7.8) | 32/296 (10.8) | 56/603 (9.3) |
| I am severely anxious or depressed | 6/307 (2) | 8/296 (2.7) | 14/603 (2.3) |
| I am extremely anxious or depressed | 2/307 (0.7) | 1/296 (0.3) | 3/603 (0.5) |
| Visual analogue scale | |||
| n | 306 | 291 | 597 |
| Mean (SD) | 78.7 (20.4) | 76.4 (22.2) | 77.6 (21.3) |
| Median | 85 | 80 | 85 |
| Minimum, maximum | 10, 100 | 0, 100 | 0, 100 |
| Utility index | |||
| n | 316 | 316 | 632 |
| Mean (SD) | 0.773 (0.291) | 0.707 (0.337) | 0.740 (0.317) |
| Median | 0.877 | 0.836 | 0.837 |
| Minimum, maximum | −0.51, 1 | −0.208, 1 | −0.51, 1 |
| Variable | Treatment arm | Total | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Mobility, n/N (%) | |||
| I have no problems in walking about | 190/300 (63.3) | 186/287 (64.8) | 376/587 (64.1) |
| I have slight problems in walking about | 51/300 (17) | 43/287 (15) | 94/587 (16) |
| I have moderate problems in walking about | 37/300 (12.3) | 31/287 (10.8) | 68/587 (11.6) |
| I have severe problems in walking about | 15/300 (5) | 18/287 (6.3) | 33/587 (5.6) |
| I am unable to walk about | 7/300 (2.3) | 9/287 (3.1) | 16/587 (2.7) |
| Self-care, n/N (%) | |||
| I have no problems washing or dressing myself | 240/299 (80.3) | 225/284 (79.2) | 465/583 (79.8) |
| I have slight problems washing or dressing myself | 29/299 (9.7) | 24/284 (8.5) | 53/583 (9.1) |
| I have moderate problems washing or dressing myself | 15/299 (5) | 20/284 (7) | 35/583 (6) |
| I have severe problems washing or dressing myself | 9/299 (3) | 5/284 (1.8) | 14/583 (2.4) |
| I am unable to wash or dress myself | 6/299 (2) | 10/284 (3.5) | 16/583 (2.7) |
| Usual activities, n/N (%) | |||
| I have no problems doing usual activities | 191/299 (63.9) | 186/285 (65.3) | 377/584 (64.6) |
| I have slight problems doing usual activities | 47/299 (15.7) | 37/285 (13) | 84/584 (14.4) |
| I have moderate problems doing usual activities | 33/299 (11) | 34/285 (11.9) | 67/584 (11.5) |
| I have severe problems doing usual activities | 19/299 (6.4) | 10/285 (3.5) | 29/584 (5) |
| I am unable to do usual activities | 9/299 (3) | 18/285 (6.3) | 27/584 (4.6) |
| Pain/discomfort, n/N (%) | |||
| I have no pain or discomfort | 203/300 (67.7) | 199/285 (69.8) | 402/585 (68.7) |
| I have slight pain or discomfort | 56/300 (18.7) | 48/285 (16.8) | 104/585 (17.8) |
| I have moderate pain or discomfort | 32/300 (10.7) | 32/285 (11.2) | 64/585 (10.9) |
| I have severe pain or discomfort | 4/300 (1.3) | 4/285 (1.4) | 8/585 (1.4) |
| I have extreme pain or discomfort | 5/300 (1.7) | 2/285 (0.7) | 7/585 (1.2) |
| Anxiety/depression, n/N (%) | |||
| I am not anxious or depressed | 222/300 (74) | 210/283 (74.2) | 432/583 (74.1) |
| I am slightly anxious or depressed | 47/300 (15.7) | 40/283 (14.1) | 87/583 (14.9) |
| I am moderately anxious or depressed | 23/300 (7.7) | 27/283 (9.5) | 50/583 (8.6) |
| I am severely anxious or depressed | 3/300 (1) | 5/283 (1.8) | 8/583 (1.4) |
| I am extremely anxious or depressed | 5/300 (1.7) | 1/283 (0.4) | 6/583 (1) |
| Visual analogue scale | |||
| n | 293 | 281 | 574 |
| Mean (SD) | 81.3 (19.7) | 81.5 (18.2) | 81.4 (19.0) |
| Median | 90 | 85 | 85 |
| Minimum, maximum | 5, 100 | 10, 100 | 5, 100 |
| Utility index | |||
| n | 315 | 311 | 626 |
| Mean (SD) | 0.766 (0.320) | 0.733 (0.348) | 0.750 (0.334) |
| Median | 0.877 | 0.877 | 0.877 |
| Minimum, maximum | −0.594, 1 | −0.594, 1 | −0.594, 1 |
| Variable | Treatment arm | Total | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Mobility, n/N (%) | |||
| I have no problems in walking about | 134/248 (54) | 122/232 (52.6) | 256/480 (53.3) |
| I have slight problems in walking about | 62/248 (25) | 59/232 (25.4) | 121/480 (25.2) |
| I have moderate problems in walking about | 27/248 (10.9) | 29/232 (12.5) | 56/480 (11.7) |
| I have severe problems in walking about | 17/248 (6.9) | 13/232 (5.6) | 30/480 (6.2) |
| I am unable to walk about | 8/248 (3.2) | 9/232 (3.9) | 17/480 (3.5) |
| Self-care, n/N (%) | |||
| I have no problems washing or dressing myself | 162/248 (65.3) | 155/232 (66.8) | 317/480 (66) |
| I have slight problems washing or dressing myself | 41/248 (16.5) | 36/232 (15.5) | 77/480 (16) |
| I have moderate problems washing or dressing myself | 27/248 (10.9) | 19/232 (8.2) | 46/480 (9.6) |
| I have severe problems washing or dressing myself | 9/248 (3.6) | 10/232 (4.3) | 19/480 (4) |
| I am unable to wash or dress myself | 9/248 (3.6) | 12/232 (5.2) | 21/480 (4.4) |
| Usual activities, n/N (%) | |||
| I have no problems doing usual activities | 106/246 (43.1) | 100/232 (43.1) | 206/478 (43.1) |
| I have slight problems doing usual activities | 70/246 (28.5) | 59/232 (25.4) | 129/478 (27) |
| I have moderate problems doing usual activities | 36/246 (14.6) | 30/232 (12.9) | 66/478 (13.8) |
| I have severe problems doing usual activities | 20/246 (8.1) | 22/232 (9.5) | 42/478 (8.8) |
| I am unable to do usual activities | 14/246 (5.7) | 21/232 (9.1) | 35/478 (7.3) |
| Pain/discomfort, n/N (%) | |||
| I have no pain or discomfort | 123/247 (49.8) | 136/232 (58.6) | 259/479 (54.1) |
| I have slight pain or discomfort | 98/247 (39.7) | 75/232 (32.3) | 173/479 (36.1) |
| I have moderate pain or discomfort | 23/247 (9.3) | 19/232 (8.2) | 42/479 (8.8) |
| I have severe pain or discomfort | 3/247 (1.2) | 2/232 (0.9) | 5/479 (1) |
| I have extreme pain or discomfort | 0/247 (0) | 0/232 (0) | 0/479 (0) |
| Anxiety/depression, n/N (%) | |||
| I am not anxious or depressed | 182/247 (73.7) | 155/231 (67.1) | 337/478 (70.5) |
| I am slightly anxious or depressed | 40/247 (16.2) | 54/231 (23.4) | 94/478 (19.7) |
| I am moderately anxious or depressed | 17/247 (6.9) | 14/231 (6.1) | 31/478 (6.5) |
| I am severely anxious or depressed | 7/247 (2.8) | 6/231 (2.6) | 13/478 (2.7) |
| I am extremely anxious or depressed | 1/247 (0.4) | 2/231 (0.9) | 3/478 (0.6) |
| Visual analogue scale | |||
| n | 231 | 223 | 454 |
| Mean (SD) | 73.3 (17.6) | 75.6 (16.4) | 74.4 (17.0) |
| Median | 77 | 80 | 80 |
| Minimum, maximum | 20, 100 | 10, 100 | 10, 100 |
| Utility index | |||
| n | 245 | 231 | 476 |
| Mean (SD) | 0.743 (0.251) | 0.730 (0.270) | 0.737 (0.260) |
| Median | 0.796 | 0.778 | 0.795 |
| Minimum, maximum | −0.166, 1 | −0.247, 1 | −0.247, 1 |
| Variable | Treatment arm | Total | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Mobility, n/N (%) | |||
| I have no problems in walking about | 167/269 (62.1) | 135/240 (56.2) | 302/509 (59.3) |
| I have slight problems in walking about | 51/269 (19) | 37/240 (15.4) | 88/509 (17.3) |
| I have moderate problems in walking about | 32/269 (11.9) | 46/240 (19.2) | 78/509 (15.3) |
| I have severe problems in walking about | 12/269 (4.5) | 14/240 (5.8) | 26/509 (5.1) |
| I am unable to walk about | 7/269 (2.6) | 8/240 (3.3) | 15/509 (2.9) |
| Self-care, n/N (%) | |||
| I have no problems washing or dressing myself | 208/268 (77.6) | 184/240 (76.7) | 392/508 (77.2) |
| I have slight problems washing or dressing myself | 32/268 (11.9) | 26/240 (10.8) | 58/508 (11.4) |
| I have moderate problems washing or dressing myself | 13/268 (4.9) | 15/240 (6.2) | 28/508 (5.5) |
| I have severe problems washing or dressing myself | 10/268 (3.7) | 5/240 (2.1) | 15/508 (3) |
| I am unable to wash or dress myself | 5/268 (1.9) | 10/240 (4.2) | 15/508 (3) |
| Usual activities, n/N (%) | |||
| I have no problems doing usual activities | 164/266 (61.7) | 127/238 (53.4) | 291/504 (57.7) |
| I have slight problems doing usual activities | 44/266 (16.5) | 46/238 (19.3) | 90/504 (17.9) |
| I have moderate problems doing usual activities | 36/266 (13.5) | 35/238 (14.7) | 71/504 (14.1) |
| I have severe problems doing usual activities | 8/266 (3) | 12/238 (5) | 20/504 (4) |
| I am unable to do usual activities | 14/266 (5.3) | 18/238 (7.6) | 32/504 (6.3) |
| Pain/discomfort, n/N (%) | |||
| I have no pain or discomfort | 171/267 (64) | 150/240 (62.5) | 321/507 (63.3) |
| I have slight pain or discomfort | 59/267 (22.1) | 53/240 (22.1) | 112/507 (22.1) |
| I have moderate pain or discomfort | 31/267 (11.6) | 28/240 (11.7) | 59/507 (11.6) |
| I have severe pain or discomfort | 6/267 (2.2) | 8/240 (3.3) | 14/507 (2.8) |
| I have extreme pain or discomfort | 0/267 (0) | 1/240 (0.4) | 1/507 (0.2) |
| Anxiety/depression, n/N (%) | |||
| I am not anxious or depressed | 193/266 (72.6) | 162/240 (67.5) | 355/506 (70.2) |
| I am slightly anxious or depressed | 45/266 (16.9) | 44/240 (18.3) | 89/506 (17.6) |
| I am moderately anxious or depressed | 23/266 (8.6) | 27/240 (11.2) | 50/506 (9.9) |
| I am severely anxious or depressed | 4/266 (1.5) | 6/240 (2.5) | 10/506 (2) |
| I am extremely anxious or depressed | 1/266 (0.4) | 1/240 (0.4) | 2/506 (0.4) |
| Visual analogue scale | |||
| n | 265 | 236 | 501 |
| Mean (SD) | 78.6 (20.5) | 77.1 (21.1) | 77.9 (20.8) |
| Median | 85 | 80 | 85 |
| Minimum, maximum | 10, 100 | 0, 100 | 0, 100 |
| Utility index | |||
| n | 272 | 253 | 525 |
| Mean (SD) | 0.785 (0.278) | 0.720 (0.327) | 0.754 (0.304) |
| Median | 0.877 | 0.836 | 0.837 |
| Minimum, maximum | −0.071, 1 | −0.208, 1 | −0.208, 1 |
| Variable | Treatment arm | Total | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Mobility, n/N (%) | |||
| I have no problems in walking about | 160/254 (63) | 147/230 (63.9) | 307/484 (63.4) |
| I have slight problems in walking about | 44/254 (17.3) | 39/230 (17) | 83/484 (17.1) |
| I have moderate problems in walking about | 30/254 (11.8) | 25/230 (10.9) | 55/484 (11.4) |
| I have severe problems in walking about | 14/254 (5.5) | 12/230 (5.2) | 26/484 (5.4) |
| I am unable to walk about | 6/254 (2.4) | 7/230 (3) | 13/484 (2.7) |
| Self-care, n/N (%) | |||
| I have no problems washing or dressing myself | 203/253 (80.2) | 180/227 (79.3) | 383/480 (79.8) |
| I have slight problems washing or dressing myself | 25/253 (9.9) | 21/227 (9.3) | 46/480 (9.6) |
| I have moderate problems washing or dressing myself | 14/253 (5.5) | 14/227 (6.2) | 28/480 (5.8) |
| I have severe problems washing or dressing myself | 8/253 (3.2) | 5/227 (2.2) | 13/480 (2.7) |
| I am unable to wash or dress myself | 3/253 (1.2) | 7/227 (3.1) | 10/480 (2.1) |
| Usual activities, n/N (%) | |||
| I have no problems doing usual activities | 161/253 (63.6) | 148/228 (64.9) | 309/481 (64.2) |
| I have slight problems doing usual activities | 39/253 (15.4) | 31/228 (13.6) | 70/481 (14.6) |
| I have moderate problems doing usual activities | 30/253 (11.9) | 27/228 (11.8) | 57/481 (11.9) |
| I have severe problems doing usual activities | 17/253 (6.7) | 8/228 (3.5) | 25/481 (5.2) |
| I am unable to do usual activities | 6/253 (2.4) | 14/228 (6.1) | 20/481 (4.2) |
| Pain/discomfort, n/N (%) | |||
| I have no pain or discomfort | 168/254 (66.1) | 161/228 (70.6) | 329/482 (68.3) |
| I have slight pain or discomfort | 51/254 (20.1) | 39/228 (17.1) | 90/482 (18.7) |
| I have moderate pain or discomfort | 27/254 (10.6) | 23/228 (10.1) | 50/482 (10.4) |
| I have severe pain or discomfort | 3/254 (1.2) | 3/228 (1.3) | 6/482 (1.2) |
| I have extreme pain or discomfort | 5/254 (2) | 2/228 (0.9) | 7/482 (1.5) |
| Anxiety/depression, n/N (%) | |||
| I am not anxious or depressed | 189/254 (74.4) | 165/226 (73) | 354/480 (73.8) |
| I am slightly anxious or depressed | 41/254 (16.1) | 36/226 (15.9) | 77/480 (16) |
| I am moderately anxious or depressed | 19/254 (7.5) | 19/226 (8.4) | 38/480 (7.9) |
| I am severely anxious or depressed | 2/254 (0.8) | 5/226 (2.2) | 7/480 (1.5) |
| I am extremely anxious or depressed | 3/254 (1.2) | 1/226 (0.4) | 4/480 (0.8) |
| Visual analogue scale | |||
| n | 250 | 225 | 475 |
| Mean (SD) | 81.3 (19.4) | 81.4 (17.2) | 81.4 (18.4) |
| Median | 90 | 85 | 85 |
| Minimum, maximum | 5, 100 | 10, 100 | 5, 100 |
| Utility index | |||
| n | 263 | 244 | 507 |
| Mean (SD) | 0.777 (0.304) | 0.745 (0.336) | 0.762 (0.320) |
| Median | 0.877 | 0.877 | 0.877 |
| Minimum, maximum | −0.594, 1 | −0.594, 1 | −0.594, 1 |
| Outcome | Covariate | Estimate (SE) | 95% CI | p-value |
|---|---|---|---|---|
| EQ-5D-5L utility index at discharge | (Intercept) | 0.743 (0.0166) | 0.71 to 0.776 | |
| Dexamethasone vs. placebo | −0.0129 (0.0239) | −0.0598 to 0.034 | 0.588 | |
| EQ-5D-5L utility index at 3 months | (Intercept) | 0.785 (0.0184) | 0.749 to 0.821 | |
| Dexamethasone vs. placebo | −0.0652 (0.0265) | −0.117 to −0.0132 | 0.014 | |
| EQ-5D-5L utility index at 6 months | (Intercept) | 0.777 (0.0197) | 0.738 to 0.816 | |
| Dexamethasone vs. placebo | −0.0322 (0.0284) | −0.0881 to 0.0237 | 0.258 |
| Variable | Treatment arm | Total | |
|---|---|---|---|
| Placebo | Dexamethasone | ||
| Discharge destination after index admission, n/N (%) | |||
| Home | 217/307 (70.7) | 197/290 (67.9) | 414/597 (69.3) |
| Carers at home | 11/307 (3.6) | 6/290 (2.1) | 17/597 (2.8) |
| Local hospital | 53/307 (17.3) | 61/290 (21) | 114/597 (19.1) |
| Rehabilitation centre | 8/307 (2.6) | 8/290 (2.8) | 16/597 (2.7) |
| Residential home | 1/307 (0.3) | 1/290 (0.3) | 2/597 (0.3) |
| Nursing home | 1/307 (0.3) | 4/290 (1.4) | 5/597 (0.8) |
| Other | 16/307 (5.2) | 13/290 (4.5) | 29/597 (4.9) |
| Length of stay in NSU (days) | |||
| n | 307 | 290 | 597 |
| Mean (SD) | 8.72 (7.25) | 9.08 (8.52) | 8.90 (7.89) |
| Median | 6 | 6.5 | 6 |
| Minimum, maximum | 2, 57 | 2, 70 | 2, 70 |
| Length of stay in secondary care (days)a | |||
| n | 307 | 290 | 597 |
| Mean (SD) | 13.7 (23.0) | 13.1 (18.2) | 13.4 (20.8) |
| Median | 7 | 7 | 7 |
| Minimum, maximum | 2, 219 | 2, 198 | 2, 219 |
| Stayed in ICU/HDU: yes, n/N (%) | 32/307 (10.4) | 29/290 (10) | 61/597 (10.2) |
| Length of stay in ICU/HDU (days) | |||
| n | 32 | 29 | 61 |
| Mean (SD) | 3.03 (2.95) | 3.07 (2.63) | 3.05 (2.78) |
| Median | 2 | 2 | 2 |
| Minimum, maximum | 1, 17 | 1, 10 | 1, 17 |
| Outcome | Estimatea | 95% CI | p-value |
|---|---|---|---|
| Negative binomial regression model | |||
| Length of stay in NSU (days) | 1.04 | 0.934 to 1.16 | 0.468 |
| Length of stay in secondary care (days) | 0.962 | 0.831 to 1.12 | 0.611 |
| Logistic regression model | |||
| Discharge destination after index admissionb | 1.14 | 0.804 to 1.61 | 0.466 |
| Discharge destination after index admissionc | 0.751 | 0.411 to 1.37 | 0.35 |
| Covariate | Odds ratio (95% CI) | p-value |
|---|---|---|
| Dexamethasone vs. placebo | 0.506 (0.278 to 0.904) | 0.023 |
| Age (years) | 0.893 (0.858 to 0.925) | < 0.001 |
| GCS score at baseline | 1.5 (1.29 to 1.76) | < 0.001 |
| Ordinal logistic regression | Sequential OR | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | Global OR (95% CI)a | p-value | Cut-off point | Probability mRS ≤ cut-off point (placebo arm) | Placebo (N = 283), n (%) | Dexamethasone (N = 270), n (%) | Marginal OR (95% CI) |
| Dexamethasone vs. placebo | 0.818 (0.595 to 1.12) | 0.215 | 0 | 0.498 | 142 (50) | 129 (48) | 0.908 (0.651 to 1.268) |
| Age (years) | 0.944 (0.929 to 0.959) | < 0.001 | 1 | 0.671 | 187 (66) | 169 (63) | 0.859 (0.606 to 1.217) |
| GCS at baseline | 1.4 (1.24 to 1.58) | < 0.001 | 2 | 0.732 | 205 (72) | 181 (67) | 0.774 (0.538 to 1.113) |
| 3 | 0.914 | 258 (91) | 229 (85) | 0.541 (0.319 to 0.918) | |||
| 4 | 0.94 | 266 (94) | 238 (88) | 0.475 (0.257 to 0.878) | |||
| 5 | 0.963 | 272 (96) | 250 (93) | 0.506 (0.237 to 1.076) | |||
| Subgroup | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Site | |||
| Dexamethasone vs. placebo | 0.59 | 0.337 to 1.02 | 0.06 |
| Cambridge vs. other sites | 1.48 | 0.684 to 3.46 | 0.341 |
| Treatment: dexamethasone: site – Cambridge | 0.84 | 0.297 to 2.29 | 0.736 |
| Age | |||
| Dexamethasone vs. placebo | 0.554 | 0.338 to 0.895 | 0.017 |
| < 70 years vs. ≥ 70 years | 6.77 | 1.99 to 42.3 | 0.01 |
| Treatment: dexamethasone: age – < 70 years | 1.14 | 0.14 to 7.46 | 0.892 |
| Timing of head trauma | |||
| Dexamethasone vs. placebo | 0.445 | 0.153 to 1.15 | 0.109 |
| ≤ 4 weeks ago (reference: no head trauma) | 0.404 | 0.141 to 1.02 | 0.068 |
| > 4 weeks ago | 0.816 | 0.265 to 2.35 | 0.71 |
| Not known | 0.404 | 0.0549 to 8.27 | 0.435 |
| Treatment: dexamethasone: trauma – ≤ 4 weeks ago | 1.07 | 0.335 to 3.71 | 0.907 |
| Treatment: dexamethasone: trauma – > 4 weeks ago | 1.94 | 0.496 to 8.06 | 0.348 |
| Treatment: dexamethasone: trauma – not known | 2.62 | 0.0804 to 86.2 | 0.547 |
| Anticoagulants/platelets | |||
| Dexamethasone vs. placebo | 0.58 | 0.293 to 1.12 | 0.108 |
| Anticoagulants/platelets vs. none | 0.731 | 0.353 to 1.51 | 0.393 |
| Treatment: dexamethasone: anticoagulants/platelets – yes | 0.948 | 0.375 to 2.4 | 0.909 |
| GCS at baseline | |||
| Dexamethasone vs. placebo | 0.019 | 0.00028 to 0.941 | 0.054 |
| GCS at baseline | 1.35 | 1.12 to 1.63 | < 0.001 |
| Treatment: dexamethasone: GCS score at baseline | 1.27 | 0.96 to 1.71 | 0.105 |
| Side of CSDH | |||
| Dexamethasone vs. placebo | 0.422 | 0.244 to 0.711 | 0.001 |
| Bilateral vs. unilateral | 0.549 | 0.254 to 1.26 | 0.14 |
| Treatment: dexamethasone: side – bilateral | 3.66 | 1.2 to 11.6 | 0.024 |
| Subgroup | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Site | |||
| Dexamethasone vs. placebo | 0.568 | 0.293 to 1.08 | 0.086 |
| Cambridge vs. other sites | 1.22 | 0.521 to 3.08 | 0.66 |
| Treatment: dexamethasone: site – Cambridge | 0.867 | 0.275 to 2.64 | 0.803 |
| Age | |||
| Dexamethasone vs. placebo | 0.527 | 0.298 to 0.914 | 0.024 |
| < 70 years vs. ≥ 70 years | 5.08 | 1.45 to 32.1 | 0.03 |
| Treatment: dexamethasone: age – < 70 years | 1.66 | 0.183 to 15 | 0.631 |
| Timing of head trauma | |||
| Dexamethasone vs. placebo | 0.455 | 0.138 to 1.31 | 0.161 |
| ≤ 4 weeks ago (reference: no head trauma) | 0.422 | 0.129 to 1.2 | 0.121 |
| > 4 weeks ago | 0.787 | 0.23 to 2.46 | 0.685 |
| Not known | 374,000 | [3.81e-05 to 1.83e+ 96] | 0.983 |
| Treatment: dexamethasone: trauma – ≤ 4 weeks ago | 1.04 | 0.278 to 4.24 | 0.949 |
| Treatment: dexamethasone: trauma – > 4 weeks ago | 2.3 | 0.498 to 11.6 | 0.295 |
| Treatment: dexamethasone: trauma – not known | 1.91e-06 | [3.89e-97 to 18700] | 0.982 |
| Anticoagulants/platelets | |||
| Dexamethasone vs. placebo | 0.551 | 0.242 to 1.21 | 0.142 |
| Anticoagulants/platelets vs. none | 0.635 | 0.272 to 1.45 | 0.282 |
| Treatment: dexamethasone: anticoagulants/platelets – yes | 0.994 | 0.341 to 2.92 | 0.992 |
| GCS at baseline | |||
| Dexamethasone vs. placebo | 0.046 | 0.000587 to 2.76 | 0.149 |
| GCS at baseline | 1.41 | 1.15 to 1.72 | < 0.001 |
| Treatment: dexamethasone: GCS at baseline | 1.18 | 0.883 to 1.62 | 0.269 |
| Side of CSDH | |||
| Dexamethasone vs. placebo | 0.46 | 0.247 to 0.833 | 0.012 |
| Bilateral vs. unilateral | 0.634 | 0.261 to 1.7 | 0.334 |
| Treatment: dexamethasone: side – bilateral | 2.09 | 0.579 to 7.58 | 0.257 |
| Subgroup | Favourable outcome (mRS score 0–3), n/N (%) | |
|---|---|---|
| Placebo | Dexamethasone | |
| Recurrence (one or more reoperation) | ||
| Yes | 20/21 (95) | 6/10 (60) |
| No | 224/248 (90) | 208/242 (86) |
| Surgical intervention during primary surgery | ||
| Burr hole(s) | 211/232 (91) | 185/218 (85) |
| Craniotomy | 26/30 (87) | 22/26 (85) |
| Drain during primary surgery | ||
| Yes | 212/234 (91) | 181/212 (85) |
| No | 32/35 (91) | 33/40 (82) |
| Conservative management (no surgery on any admission) | ||
| Yes | 14/14 (100) | 15/18 (83) |
| No | 244/269 (91) | 214/252 (85) |
| Trial conservative management | ||
| No surgery | 14/14 (100) | 15/18 (83) |
| Surgery within 7 days of randomisation | 235/260 (90) | 211/249 (85) |
| Surgery > 7 days after randomisation | 9/9 (100) | 3/3 (100) |
| Participant ID | Site | Treatment arm | Event | Onset date | Resolution date | MedDRA PT | MedDRA SOC | Causality | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| N17–104 | Plymouth | Placebo | Upper gastrointestinal side effects | 10 December 2015 | 10 December 2015 | Dyspepsia | Gastrointestinal disorders | Unlikely | Resolved: no residual effects |
| N25–108 | Glasgow | Placebo | New-onset psychosis | 29 August 2016 | 29 August 2016 | Acute psychosis | Psychiatric disorders | Possibly | Resolved: no residual effects |
| N01–218 | Cambridge | Placebo | New-onset diabetes necessitating ongoing medical treatment at day 30 follow-up | 16 November 2016 | 6 February 2017 | Hyperglycaemia | Endocrine disorders | Possibly | Resolved: no residual effects |
| N35–129 | Southampton | Placebo | New-onset psychosis | 24 November 2016 | Hallucination | Psychiatric disorders | Unlikely | Ongoing | |
| N01–231 | Cambridge | Placebo | Upper gastrointestinal side effects | 12 January 2017 | 13 January 2017 | Dyspepsia | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N01–246 | Cambridge | Placebo | Hyperglycaemia necessitating treatment | 17 March 2017 | 19 March 2017 | Hyperglycaemia | Endocrine disorders | Possibly | Resolved: no residual effects |
| N34–123 | Sheffield | Placebo | Upper gastrointestinal side effects | 28 July 2017 | 11 August 2017 | Vomiting | Gastrointestinal disorders | Unlikely | Resolved: no residual effects |
| N23–104 | Dundee | Placebo | Hyperglycaemia necessitating stopping of trial medication | 21 October 2017 | 22 October 2017 | Hyperglycaemia | Endocrine disorders | Definitely | Resolved: no residual effects |
| N24–105 | Edinburgh | Placebo | Upper gastrointestinal side effects | 16 December 2017 | 19 December 2017 | Nausea | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N24–105 | Edinburgh | Placebo | Upper gastrointestinal side effects | 16 December 2017 | 19 December 2017 | Vomiting | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N35–159 | Southampton | Placebo | Upper gastrointestinal side effects | 6 March 2018 | 4 October 2018 | Vomiting | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N26–111 | Hull | Placebo | Upper gastrointestinal side effects | 23 April 2018 | 24 April 2018 | Vomiting | Gastrointestinal disorders | Unlikely | Resolved: no residual effects |
| N35–183 | Southampton | Placebo | Upper gastrointestinal side effects | 1 September 2018 | 2 September 2018 | Vomiting | Gastrointestinal disorders | Unrelated | Resolved: no residual effects |
| N01–105 | Cambridge | Dexamethasone | Upper gastrointestinal side effects | 26 August 2015 | 28 August 2015 | Dyspepsia | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N01–126 | Cambridge | Dexamethasone | Hyperglycaemia necessitating treatment | 27 October 2015 | 23 December 2015 | Hyperglycaemia | Endocrine disorders | Probably | Resolved: no residual effects |
| N17–103 | Plymouth | Dexamethasone | New-onset psychosis | 5 November 2015 | 6 November 2015 | Hallucination | Psychiatric disorders | Unlikely | Resolved: no residual effects |
| N01–130 | Cambridge | Dexamethasone | Hyperglycaemia necessitating treatment | 1 December 2015 | 4 December 2015 | Hyperglycaemia | Endocrine disorders | Possibly | Resolved: no residual effects |
| N01–151 | Cambridge | Dexamethasone | Upper gastrointestinal side effects | 4 March 2016 | 4 April 2016 | Dyspepsia | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N01–159 | Cambridge | Dexamethasone | Hyperglycaemia necessitating treatment | 9 March 2016 | 11 March 2016 | Hyperglycaemia | Endocrine disorders | Probably | Resolved: no residual effects |
| N01–158 | Cambridge | Dexamethasone | Hyperglycaemia necessitating treatment | 12 March 2016 | 21 March 2016 | Hyperglycaemia | Endocrine disorders | Probably | Resolved: no residual effects |
| N35–105 | Southampton | Dexamethasone | Hyperglycaemia necessitating stopping of trial medication | 12 March 2016 | 17 March 2016 | Hyperglycaemia | Endocrine disorders | Definitely | Resolved: no residual effects |
| N35–105 | Southampton | Dexamethasone | Hyperglycaemia necessitating treatment | 29 March 2016 | Hyperglycaemia | Endocrine disorders | Probably | Ongoing | |
| N12–101 | Imperial | Dexamethasone | Hyperglycaemia necessitating treatment | 4 April 2016 | Hyperglycaemia | Endocrine disorders | Unrelated | Ongoing | |
| N01–160 | Cambridge | Dexamethasone | New-onset diabetes necessitating treatment | 20 April 2016 | Type 2 diabetes | Endocrine disorders | Possibly | Ongoing | |
| N25–101 | Glasgow | Dexamethasone | Hyperglycaemia necessitating treatment | 25 May 2016 | 31 May 2016 | Hyperglycaemia | Endocrine disorders | Definitely | Resolved: no residual effects |
| N07–104 | Birmingham | Dexamethasone | New-onset diabetes necessitating treatment | 25 June 2016 | Type 2 diabetes mellitus | Metabolism and nutrition disorders | Unlikely | Ongoing | |
| N01–195 | Cambridge | Dexamethasone | Hyperglycaemia necessitating treatment | 1 August 2016 | 15 August 2016 | Hyperglycaemia | Endocrine disorders | Possibly | Resolved: no residual effects |
| N35–120 | Southampton | Dexamethasone | New-onset psychosis | 19 August 2016 | 21 August 2016 | Hallucination | Psychiatric disorders | Probably | Resolved: no residual effects |
| N01–212 | Cambridge | Dexamethasone | Upper gastrointestinal side effects | 18 October 2016 | 18 October 2016 | Gastrointestinal tract irritation | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N35–127 | Southampton | Dexamethasone | Upper gastrointestinal side effects | 18 November 2016 | 3 December 2016 | Dyspepsia | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N25–117 | Glasgow | Dexamethasone | Hyperglycaemia necessitating treatment | 22 November 2016 | 24 November 2016 | Hyperglycaemia | Endocrine disorders | Probably | Resolved: no residual effects |
| N01–221 | Cambridge | Dexamethasone | New-onset psychosis | 26 November 2016 | 29 November 2016 | Acute psychosis | Psychiatric disorders | Possibly | Resolved: no residual effects |
| N12–103 | Imperial | Dexamethasone | Upper gastrointestinal side effects | 8 December 2016 | 8 December 2016 | Dyspepsia | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N01–230 | Cambridge | Dexamethasone | Hyperglycaemia necessitating treatment | 7 January 2017 | 19 January 2017 | Hyperglycaemia | Endocrine disorders | Probably | Resolved: no residual effects |
| N34–113 | Sheffield | Dexamethasone | New-onset psychosis | 3 March 2017 | Psychotic disorder | Psychiatric disorders | Definitely | Ongoing | |
| N01–244 | Cambridge | Dexamethasone | Upper gastrointestinal side effects | 7 March 2017 | 10 March 2017 | Vomiting | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N25–119 | Glasgow | Dexamethasone | New-onset psychosis | 26 May 2017 | 27 May 2017 | Euphoric mood | Psychiatric disorders | Unlikely | Resolved: no residual effects |
| N01–262 | Cambridge | Dexamethasone | Hyperglycaemia necessitating treatment | 9 June 2017 | 14 June 2017 | Hyperglycaemia | Endocrine disorders | Possibly | Resolved: no residual effects |
| N25–125 | Glasgow | Dexamethasone | Hyperglycaemia necessitating treatment | 5 July 2017 | Hyperglycaemia | Endocrine disorders | Possibly | Ongoing | |
| N25–126 | Glasgow | Dexamethasone | Upper gastrointestinal side effects | 9 July 2017 | 9 July 2017 | Abdominal pain | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N35–150 | Southampton | Dexamethasone | New-onset psychosis | 3 November 2017 | 29 November 2017 | Delirium | Psychiatric disorders | Probably | Resolved with residual effects |
| N24–104 | Edinburgh | Dexamethasone | New-onset psychosis | 23 November 2017 | 29 November 2017 | Delirium | Psychiatric disorders | Probably | Resolved: no residual effects |
| N34–127 | Sheffield | Dexamethasone | Upper gastrointestinal side effects | 6 December 2017 | 7 December 2017 | Vomiting | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N35–156 | Southampton | Dexamethasone | New-onset psychosis | 3 January 2018 | 6 January 2018 | Hallucination | Psychiatric disorders | Possibly | Resolved: no residual effects |
| N34–130 | Sheffield | Dexamethasone | Hyperglycaemia necessitating treatment | 16 January 2018 | 8 February 2018 | Hyperglycaemia | Endocrine disorders | Definitely | Resolved: no residual effects |
| N34–131 | Sheffield | Dexamethasone | Hyperglycaemia necessitating treatment | 24 January 2018 | 5 February 2018 | Hyperglycaemia | Endocrine disorders | Definitely | Resolved: no residual effects |
| N25–138 | Glasgow | Dexamethasone | New-onset psychosis | 7 February 2018 | 13 February 2018 | Agitation | Psychiatric disorders | Probably | Resolved: no residual effects |
| N25–141 | Glasgow | Dexamethasone | New-onset psychosis | 11 March 2018 | 12 March 2018 | Delirium | Psychiatric disorders | Probably | Resolved: no residual effects |
| N34–133 | Sheffield | Dexamethasone | Hyperglycaemia necessitating treatment | 17 March 2018 | Hyperglycaemia | Endocrine disorders | Possibly | Ongoing | |
| N24–113 | Edinburgh | Dexamethasone | Hyperglycaemia necessitating treatment | 8 August 2018 | Hyperglycaemia | Endocrine disorders | Unlikely | Unknown | |
| N24–113 | Edinburgh | Dexamethasone | Hyperglycaemia necessitating stopping of trial medication | 16 August 2018 | Hyperglycaemia | Endocrine disorders | Probably | Unknown | |
| N48–149 | Leeds | Dexamethasone | New-onset psychosis | 25 September 2018 | Delirium | Psychiatric disorders | Probably | Ongoing | |
| N48–146 | Leeds | Dexamethasone | Upper gastrointestinal side effects | 2 October 2018 | 2 October 2018 | Dyspepsia | Gastrointestinal disorders | Probably | Resolved: no residual effects |
| N36–113 | St George’s | Dexamethasone | Hyperglycaemia necessitating treatment | 9 October 2018 | 11 October 2018 | Hyperglycaemia | Endocrine disorders | Probably | Resolved with residual effects |
| N31–108 | Newcastle | Dexamethasone | Upper gastrointestinal side effects | 13 October 2018 | 13 October 2018 | Dyspepsia | Gastrointestinal disorders | Probably | Resolved: no residual effects |
| N36–115 | St George’s | Dexamethasone | Gastrointestinal bleeding | 9 November 2018 | 9 November 2018 | Melaena | Gastrointestinal disorders | Unlikely | Resolved: no residual effects |
| Participant ID | Site | Treatment arm | SAE reference number | Event | Onset date | Resolution date | MedDRA PT | MedDRA SOC | Causality | Severity | Seriousness | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N34–123 | Sheffield | Placebo | N34–123–02 | Upper gastrointestinal side effects | 28 July 2017 | 11 August 2017 | Intestinal obstruction | Gastrointestinal disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N48–104 | Leeds | Dexamethasone | N48–104–01 | New-onset psychosis | 9 June 2016 | 10 June 2016 | Acute psychosis | Psychiatric disorders | Possibly | Mild | Hospitalisation | Resolved: no residual effects |
| N01–193 | Cambridge | Dexamethasone | N01–193–01 | Hyperglycaemia necessitating treatment | 4 August 2016 | 6 August 2016 | Hyperglycaemia | Endocrine disorders | Probably | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–345 | Cambridge | Dexamethasone | N01–345–01 | Upper gastrointestinal side effects | 20 August 2018 | 27 August 2018 | Dyspepsia | Gastrointestinal disorders | Probably | Moderate | Hospitalisation | Resolved: no residual effects |
| Participant ID | Site | Treatment arm | Event | Onset date | Resolution date | MedDRA PT | MedDRA SOC | Causality | Severity | Seriousness | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N01–117 | Cambridge | Placebo | Seizure | 1 October 2015 | 2 October 2015 | Seizure | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–124 | Cambridge | Placebo | Subdural empyema | 15 November 2015 | 20 November 2015 | Brain empyema | Infections and infestations | Unlikely | Severe | Hospitalisation | Resolved: no residual effects |
| N01–128 | Cambridge | Placebo | Recurrent CSDH | 17 November 2015 | 20 November 2015 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N35–103 | Southampton | Placebo | Recollection of CSDH | 18 February 2016 | 18 February 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N35–106 | Southampton | Placebo | Partial seizures | 13 March 2016 | 13 March 2016 | Seizure | Nervous system disorders | Unlikely | Mild | Hospitalisation | Resolved: no residual effects |
| N35–106 | Southampton | Placebo | Seizures | 13 March 2016 | 14 March 2016 | Seizure | Nervous system disorders | Unlikely | Mild | Hospitalisation | Resolved: no residual effects |
| N01–164 | Cambridge | Placebo | Reoperation owing to recollection of CSDH (same admission) | 3 April 2016 | 3 April 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N12–102 | Imperial | Placebo | Worsening of CSDH | 15 April 2016 | 19 April 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–167 | Cambridge | Placebo | Residual CSDH | 29 April 2016 | 5 May 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N35–112 | Southampton | Placebo | Recollection of CSDH | 1 May 2016 | 5 May 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N48–101 | Leeds | Placebo | Recollection of CSDH | 23 May 2016 | 23 May 2016 | Subdural haematoma | Nervous system disorders | Possibly | Moderate | Hospitalisation | Resolved: no residual effects |
| N34–103 | Sheffield | Placebo | Rebleed into CSDH (conservatively managed) and subarachnoid haemorrage | 17 June 2016 | 20 June 2016 | Subdural haematoma | Nervous system disorders | Possibly | Moderate | Hospitalisation | Resolved with residual effects |
| N01–188 | Cambridge | Placebo | Residual CSDH (operated) | 13 July 2016 | 15 July 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–190 | Cambridge | Placebo | Recurrent CSDH | 3 August 2016 | 5 August 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–198 | Cambridge | Placebo | Residual CSDH | 22 August 2016 | 23 August 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N48–105 | Leeds | Placebo | Recollection | 12 September 2016 | 19 September 2016 | Subdural haematoma | Nervous system disorders | Unlikely | Severe | Hospitalisation | Resolved: no residual effects |
| N01–210 | Cambridge | Placebo | Recurrent CSDH | 13 October 2016 | 15 October 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N35–124 | Southampton | Placebo | Recollection of CSDH | 31 October 2016 | 7 November 2016 | Subdural haematoma | Nervous system disorders | Unlikely | Severe | Hospitalisation | Resolved: no residual effects |
| N35–124 | Southampton | Placebo | Seizure | 9 November 2016 | 9 November 2016 | Seizure | Nervous system disorders | Unlikely | Mild | Hospitalisation | Resolved: no residual effects |
| N01–218 | Cambridge | Placebo | Reoperation owing to recollection of CSDH (same admission) | 14 November 2016 | 14 November 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–218 | Cambridge | Placebo | Seizure | 4 December 2016 | 5 December 2016 | Seizure | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–222 | Cambridge | Placebo | Residual CSDH | 8 December 2016 | 13 December 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–228 | Cambridge | Placebo | Recurrent CSDH | 2 January 2017 | 3 January 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–248 | Cambridge | Placebo | Re-admission for recollection of CSDH | 8 April 2017 | 8 April 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–248 | Cambridge | Placebo | Surgical site infection | 8 April 2017 | 16 April 2017 | Postoperative wound infection | Infections and infestations | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N34–112 | Sheffield | Placebo | Recollection of CSDH | 20 April 2017 | 23 April 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N17–110 | Plymouth | Placebo | Small rebleed | 14 May 2017 | 16 May 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved with residual effects |
| N31–105 | Newcastle | Placebo | Evacuation of acute on chronic subdural haematoma | 21 May 2017 | Depressed level of consciousnesses | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Ongoing | |
| N31–105 | Newcastle | Placebo | Patient deterioration. Second recurrent haemorrhage | 4 June 2017 | 22 June 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved with residual effects |
| N07–113 | Birmingham | Placebo | Late recollection of CSDH. No surgery required | 9 June 2017 | 12 June 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N08–110 | Brighton | Placebo | Recurrence of CSDH | 20 June 2017 | 21 June 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N31–105 | Newcastle | Placebo | Re-admission: R Pupil 4, L Pupil 3, slurred speech, facial droop. Reopening of cranial wound, removal of infected bone flap and washout of empyema on 8 July 2017 and wound washout on 11 July 2017. R-sided facial weakness, dysphasia, evidence of collection and brain oedema on CT on 7 July 2017 | 4 July 2017 | Brain empyema | Infections and infestations | Unrelated | Severe | Hospitalisation | Ongoing | |
| N34–123 | Sheffield | Placebo | Pneumocephalus | 23 July 2017 | 25 July 2017 | Pneumocephalus | Injury, poisoning and procedural complications | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N26–106 | Hull | Placebo | Expansion CSDH | 17 August 2017 | 18 August 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N17–122 | Plymouth | Placebo | Recollection of CSDH | 24 August 2017 | 24 August 2017 | Subdural haematoma | Nervous system disorders | Definitely | Severe | Hospitalisation | Resolved: no residual effects |
| N25–128 | Glasgow | Placebo | Return to theatre for recollection of CSDH | 29 August 2017 | 29 August 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N07–117 | Birmingham | Placebo | Seizure | 5 October 2017 | 6 October 2017 | Seizure | Nervous system disorders | Unlikely | Mild | Hospitalisation | Resolved with residual effects |
| N12–104 | Imperial | Placebo | Repeat burr hole surgery | 12 October 2017 | 12 October 2017 | Subdural haematoma | Nervous system disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N33–107 | Preston (Lancashire) | Placebo | Recollection of CSDH | 20 October 2017 | 21 June 2018 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved with residual effects |
| N26–108 | Hull | Placebo | Left-sided weakness and residual bleeding | 4 November 2017 | 18 November 2017 | Subdural haematoma | Nervous system disorders | Unlikely | Mild | Considered medically significant by the investigator | Resolved: no residual effects |
| N23–104 | Dundee | Placebo | Recurrent bilateral subdural haematoma (midline shift to right) | 6 December 2017 | 14 December 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved with residual effects |
| N01–328 | Cambridge | Placebo | Recollection of CSDH with consequent reoperation | 31 January 2018 | 31 January 2018 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N46–118 | Stoke/North Staffs | Placebo | Recurrent L CSDH | 17 February 2018 | 10 March 2018 | Subdural haematoma | Nervous system disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N35–158 | Southampton | Placebo | Frontal empyema (subdural) | 23 February 2018 | 28 February 2018 | Brain empyema | Infections and infestations | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N34–134 | Sheffield | Placebo | Subclinical seizures | 24 March 2018 | Seizure | Nervous system disorders | Unlikely | Severe | Hospitalisation | Ongoing | |
| N35–161 | Southampton | Placebo | Recollection of CSDH: right side | 1 April 2018 | 3 April 2018 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N35–161 | Southampton | Placebo | Recollection of CSDH: right side | 9 April 2018 | 9 April 2018 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N18–108 | Aberdeen | Placebo | Recollection of CSDH | 14 April 2018 | 23 April 2018 | Subdural haematoma | Nervous system disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N26–111 | Hull | Placebo | Post-surgery seizures/epilepsy | 22 April 2018 | 1 May 2018 | Seizure | Nervous system disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–338 | Cambridge | Placebo | Seizure post operation | 16 May 2018 | 16 May 2018 | Seizure | Nervous system disorders | Unrelated | Mild | Considered medically significant by the investigator | Resolved: no residual effects |
| N26–111 | Hull | Placebo | Rebleed causing subdural haematoma | 22 May 2018 | 30 May 2018 | Subdural haematoma | Nervous system disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N35–175 | Southampton | Placebo | Recollection of left CSDH | 15 August 2018 | 18 August 2018 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N48–148 | Leeds | Placebo | Recollection of CSDH | 18 September 2018 | 18 September 2018 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved with residual effects |
| N36–109 | St George’s | Placebo | Acute on chronic subdural haematoma (recurrence) | 3 October 2018 | 10 October 2018 | Subdural haematoma | Nervous system disorders | Unlikely | Severe | Hospitalisation | Worsening |
| N36–112 | St George’s | Placebo | Recollection of right CSDH | 20 October 2018 | 25 October 2018 | Subdural haematoma | Nervous system disorders | Unlikely | Moderate | Hospitalisation | Resolved with residual effects |
| N36–112 | St George’s | Placebo | Recollection of right CSDH | 29 October 2018 | 12 November 2018 | Subdural haematoma | Nervous system disorders | Unlikely | Severe | Hospitalisation | Resolved with residual effects |
| N36–112 | St George’s | Placebo | (Recurrent) acute plus chronic subdural haematoma | 31 October 2018 | 12 November 2018 | Subdural haematoma | Nervous system disorders | Unlikely | Severe | Hospitalisation | Resolved with residual effects |
| N36–112 | St George’s | Placebo | Residual collection of CSDH | 9 November 2018 | 12 November 2018 | Subdural haematoma | Nervous system disorders | Unlikely | Moderate | Hospitalisation | Resolved with residual effects |
| N31–109 | Newcastle | Placebo | Recollection of CSDH | 3 December 2018 | 14 December 2018 | Subdural haematoma | Nervous system disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N14–105 | Middlesbrough | Placebo | Tension pneumocephalus | 15 July 2016 | Pneumocephalus | Injury, poisoning and procedural complications | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects | |
| N25–108 | Glasgow | Placebo | Seizure | 29 August 2016 | Seizure | Nervous system disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects | |
| N17–101 | Plymouth | Dexamethasone | Seizure (post D/C) | 7 November 2015 | 7 November 2015 | Seizure | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–148 | Cambridge | Dexamethasone | Recurrent CSDH | 23 February 2016 | 1 March 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–181 | Cambridge | Dexamethasone | Re-admission reoperation for recollection | 29 June 2016 | 1 July 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–186 | Cambridge | Dexamethasone | Expansion of contralateral CSDH | 8 September 2016 | 11 September 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–200 | Cambridge | Dexamethasone | Seizures | 9 September 2016 | 11 September 2016 | Seizure | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–200 | Cambridge | Dexamethasone | Recurrent CSDH | 9 September 2016 | 11 September 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N34–106 | Sheffield | Dexamethasone | Residual CSDH | 10 September 2016 | 11 September 2016 | Subdural haematoma | Nervous system disorders | Unlikely | Mild | Hospitalisation | Resolved: no residual effects |
| N01–221 | Cambridge | Dexamethasone | Residual CSDH | 27 November 2016 | 1 December 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N35–131 | Southampton | Dexamethasone | Non-reportable recollection of CSDH | 14 December 2016 | 6 January 2017 | Subdural haematoma | Nervous system disorders | Probably | Moderate | Is life-threatening | Resolved with residual effects |
| N18–101 | Aberdeen | Dexamethasone | Recollection of CSDH | 7 January 2017 | 9 January 2017 | Subdural haematoma | Nervous system disorders | Definitely | Moderate | Is life-threatening | Resolved with residual effects |
| N18–101 | Aberdeen | Dexamethasone | Subdural empyema | 7 January 2017 | 20 February 2017 | Brain empyema | Infections and infestations | Possibly | Severe | Is life-threatening | Resolved: no residual effects |
| N18–101 | Aberdeen | Dexamethasone | Seizures | 7 January 2017 | 8 January 2017 | Seizure | Nervous system disorders | Possibly | Moderate | Hospitalisation | Resolved: no residual effects |
| N48–115 | Leeds | Dexamethasone | Reaccumulation of haematoma (regarded as recollection of CSDH) | 20 January 2017 | 31 January 2017 | Subdural haematoma | Nervous system disorders | Unlikely | Severe | Hospitalisation | Resolved: no residual effects |
| N17–107 | Plymouth | Dexamethasone | Subdural empyema | 2 March 2017 | Brain empyema | Infections and infestations | Unlikely | Severe | Hospitalisation | Ongoing | |
| N01–244 | Cambridge | Dexamethasone | Re-admission recurrence reoperation | 16 April 2017 | 18 April 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–244 | Cambridge | Dexamethasone | Re-admission recurrence reoperation | 25 April 2017 | 27 April 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N34–117 | Sheffield | Dexamethasone | Pneumocephalus | 5 May 2017 | Pneumocephalus | Injury, poisoning and procedural complications | Unrelated | Moderate | Hospitalisation | Ongoing | |
| N01–260 | Cambridge | Dexamethasone | Re-admission operation (recollection of CSDH) | 1 June 2017 | 1 June 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N16–103 | Romford | Dexamethasone | Subdural empyema | 6 July 2017 | Brain empyema | Infections and infestations | Possibly | Severe | Hospitalisation | Ongoing | |
| N01–268 | Cambridge | Dexamethasone | Re-admission recurrence CSDH reoperation | 7 July 2017 | 8 July 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–279 | Cambridge | Dexamethasone | Empyema | 19 August 2017 | 20 August 2017 | Brain empyema | Infections and infestations | Unrelated | Severe | Hospitalisation | Resolved with residual effects |
| N31–106 | Newcastle | Dexamethasone | Surgical site infection | 16 September 2017 | 17 October 2017 | Postoperative wound infection | Infections and infestations | Probably | Severe | Is life-threatening | Resolved: no residual effects |
| N31–106 | Newcastle | Dexamethasone | Seizures | 16 September 2017 | 16 September 2017 | Seizure | Nervous system disorders | Probably | Severe | Hospitalisation | Resolved: no residual effects |
| N31–106 | Newcastle | Dexamethasone | Surgical site infection | 26 October 2017 | 21 November 2017 | Postoperative wound infection | Infections and infestations | Probably | Moderate | Hospitalisation | Resolved: no residual effects |
| N34–127 | Sheffield | Dexamethasone | Recurrence of CSDH requiring operation | 14 December 2017 | 19 December 2017 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N25–136 | Glasgow | Dexamethasone | Drainage of left subdural empyema | 27 January 2018 | 8 March 2018 | Brain empyema | Infections and infestations | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N32–101 | Oxford | Dexamethasone | Reaccumulation of CSDH | 24 June 2018 | 26 June 2018 | Subdural haematoma | Nervous system disorders | Unlikely | Mild | Hospitalisation | Resolved: no residual effects |
| N35–180 | Southampton | Dexamethasone | Right CSDH | 19 July 2018 | 7 September 2018 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N08–121 | Brighton | Dexamethasone | CSDH residual (originally treated conservatively) | 24 July 2018 | 26 July 2018 | Subdural haematoma | Nervous system disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–347 | Cambridge | Dexamethasone | Recollection of CSDH | 29 August 2018 | 29 August 2018 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–347 | Cambridge | Dexamethasone | Site infection surgical | 29 August 2018 | 29 August 2018 | Postoperative wound infection | Infections and infestations | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N48–143 | Leeds | Dexamethasone | Subdural empyema | 20 September 2018 | 10 October 2018 | Brain empyema | Infections and infestations | Possibly | Severe | Hospitalisation | Resolved: no residual effects |
| N48–151 | Leeds | Dexamethasone | Symptomatic left CSDH | 15 October 2018 | 22 October 2018 | Subdural haematoma | Nervous system disorders | Unlikely | Severe | Hospitalisation | Resolved: no residual effects |
| Participant ID | Site | Treatment arm | SAE reference number | Event | Onset date | Resolution date | MedDRA PT | MedDRA SOC | Causality | Severity | Seriousness | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N25–105 | Glasgow | Placebo | N25–105–01 | Stroke | 11 July 2016 | 21 July 2016 | Cerebrovascular accident | Nervous system disorders | Unrelated | Severe | Death | Death |
| N14–108 | Middlesbrough | Placebo | N14–108–01 | Worsening of left acute subdural haematoma | 28 July 2016 | Subdural haematoma | Nervous system disorders | Unrelated | Severe | Is life-threatening | Ongoing | |
| N01–190 | Cambridge | Placebo | N01–190–01 | Anaphylaxis related to flucloxacillin | 2 August 2016 | 5 August 2016 | Anaphylactic reaction | Immune system disorders | Unlikely | Severe | Is life-threatening | Resolved: no residual effects |
| N01–202 | Cambridge | Placebo | N01–202–01 | Laceration | 23 September 2016 | 25 September 2016 | Laceration | Injury, poisoning and procedural complications | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N14–114 | Middlesbrough | Placebo | N14–114–01 | Lethargy and feeling unwell | 23 October 2016 | Malaise | General disorders and administration site conditions | Unrelated | Moderate | Hospitalisation | Ongoing | |
| N35–132 | Southampton | Placebo | N35–132–01 | Pyrexia from unknown origin | 22 January 2017 | 25 January 2017 | Pyrexia | General disorders and administration site conditions | Unrelated | Mild | Hospitalisation | Resolved with residual effects |
| N01–242 | Cambridge | Placebo | N01–242–01 | Fall | 13 March 2017 | 14 March 2017 | Fall | Injury, poisoning and procedural complications | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N25–120 | Glasgow | Placebo | N25–120–01 | Cardiac instability | 7 June 2017 | 7 June 2017 | Cardiac failure | Cardiac disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N01–263 | Cambridge | Placebo | N01–263–01 | Deep-vein thrombosis | 17 June 2017 | 24 June 2017 | Deep-vein thrombosis | Vascular disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N46–108 | Stoke/North Staffs | Placebo | N46–108–01 | Hyponatremia | 8 July 2017 | Hyponatraemia | Metabolism and nutrition disorders | Unrelated | Mild | Hospitalisation | Ongoing | |
| N34–123 | Sheffield | Placebo | N34–123–01 | Right anterior cerebral artery infarction | 17 July 2017 | 19 July 2017 | Cerebrovascular accident | Nervous system disorders | Unlikely | Severe | Hospitalisation | Resolved with residual effects |
| N01–291 | Cambridge | Placebo | N01–291–01 | Postoperative swelling | 29 August 2017 | 13 September 2017 | Brain oedema | Nervous system disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N34–129 | Sheffield | Placebo | N34–129–01 | Aspiration pneumonia | 6 January 2018 | 14 January 2018 | Pneumonia | Infections and infestations | Unlikely | Severe | Hospitalisation | Resolved: no residual effects |
| N48–127 | Leeds | Placebo | N48–127–01 | Reduced appetite and fluid intake | 23 January 2018 | 26 January 2018 | Hypophagia | Metabolism and nutrition disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N34–132 | Sheffield | Placebo | N34–132–01 | Low platelets | 21 March 2018 | Thrombocytopenia | Blood and lymphatic system disorders | Unlikely | Mild | Hospitalisation | Ongoing | |
| N26–111 | Hull | Placebo | N26–111–01 | Pneumonia | 25 April 2018 | 30 April 2018 | Pneumonia | Infections and infestations | Possibly | Moderate | Hospitalisation | Resolved: no residual effects |
| N26–111 | Hull | Placebo | N26–111–02 | Pulmonary embolism | 10 May 2018 | 19 May 2018 | Pulmonary embolism | Vascular disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N08–117 | Brighton | Placebo | N08–117–01 | Death discovered in retrospect. Cause of death reported by coroner as acute cardiac failure | 11 May 2018 | 11 May 2018 | Cardiac failure acute | Cardiac disorders | Unlikely | Severe | Death | Death |
| N46–120 | Stoke/North Staffs | Placebo | N46–120–01 | Attended A&E with neck/arm pain and tingling in tips of left hand | 20 May 2018 | 20 May 2018 | Cervical radiculopathy | Nervous system disorders | Unlikely | Mild | Considered medically significant by the investigator | Resolved: no residual effects |
| N34–143 | Sheffield | Placebo | N26–143–01 | Pneumonia | 7 June 2018 | Pneumonia | Infections and infestations | Possibly | Severe | Hospitalisation | Ongoing | |
| N32–102 | Oxford | Placebo | N01–102–01 | Pyrexia of unknown origin | 3 July 2018 | 4 July 2018 | Pyrexia | General disorders and administration site conditions | Possibly | Moderate | Hospitalisation | Resolved: no residual effects |
| N26–113 | Hull | Placebo | N48–113–01 | Chest infection | 4 August 2018 | 13 August 2018 | Pneumonia | Infections and infestations | Unlikely | Mild | Considered medically significant by the investigator | Resolved: no residual effects |
| N01–346 | Cambridge | Placebo | N25–346–01 | Electrolyte imbalance | 20 August 2018 | 22 August 2018 | Electrolyte imbalance | Metabolism and nutrition disorders | Unlikely | Mild | Hospitalisation | Resolved: no residual effects |
| N48–148 | Leeds | Placebo | N34–148–01 | Worsened heart failure | 29 September 2018 | 23 October 2018 | Cardiac failure chronic | Cardiac disorders | Unrelated | Severe | Death | Death |
| N25–127 | Glasgow | Placebo | N34–127–01 | Collapse | a | a | Syncope | Vascular disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N34–143 | Sheffield | Placebo | N34–143–02 | Slow ventricular response | a | Cardiac disorder | Cardiac disorders | Unlikely | Severe | Hospitalisation | Ongoing | |
| N34–143 | Sheffield | Placebo | N34–143–03 | Gangrene in toes | a | Gangrene | Vascular disorders | Unlikely | Severe | Hospitalisation | Ongoing | |
| N34–143 | Sheffield | Placebo | N34–143–04 | Delirium | a | Delirium | Psychiatric disorders | Possibly | Severe | Hospitalisation | Ongoing | |
| N01–123 | Cambridge | Dexamethasone | N01–123–01 | General physical health deterioration | 14 October 2015 | 24 October 2015 | General physical health deterioration | General disorders and administration site conditions | Unrelated | Severe | Death | Death |
| N01–130 | Cambridge | Dexamethasone | N01–130–01 | Aspiration bronchopneumonia | 12 December 2015 | 1 January 2016 | Pneumonia | Infections and infestations | Unrelated | Severe | Death | Death |
| N01–134 | Cambridge | Dexamethasone | N01–134–01 | Urinary tract infection | 18 December 2015 | 20 December 2015 | Urinary tract infection | Infections and infestations | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N12–101 | Imperial | Dexamethasone | N12–101–01 | Bowel perforation secondary to diverticulitis | 15 March 2016 | 22 March 2016 | Large intestinal perforation | Gastrointestinal disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–154 | Cambridge | Dexamethasone | N01–154–01 | Hyperparathyroidism | 19 March 2016 | 13 April 2016 | Hyperparathyroidism | Endocrine disorders | Unlikely | Moderate | Hospitalisation | Resolved with residual effects |
| N35–110 | Southampton | Dexamethasone | N35–110–01 | Stroke | 31 March 2016 | 13 April 2016 | Cerebrovascular accident | Nervous system disorders | Unrelated | Severe | Hospitalisation | Resolved with residual effects |
| N12–101 | Imperial | Dexamethasone | N12–101–02 | Traumatic subdural (acute) | 4 April 2016 | 22 April 2016 | Subdural haematoma | Nervous system disorders | Unlikely | Severe | Death | Death |
| N01–175 | Cambridge | Dexamethasone | N01–175–01 | Meningitis | 13 May 2016 | 7 June 2016 | Meningitis | Infections and infestations | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N34–101 | Sheffield | Dexamethasone | N34–101–01 | Fall | 13 May 2016 | 17 May 2016 | Fall | Injury, poisoning and procedural complications | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N07–102 | Birmingham | Dexamethasone | N07–102–01 | Infective endocarditis | 12 June 2016 | 6 July 2016 | Endocarditis | Infections and infestations | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N25–101 | Glasgow | Dexamethasone | N25–101–01 | Chest pain | 15 June 2016 | 22 June 2016 | Non-cardiac chest pain | General disorders and administration site conditions | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N48–104 | Leeds | Dexamethasone | N48–104–02 | Delirium (multifactorial delirium with advanced dementia) | 23 June 2016 | 2 July 2016 | Senile dementia | Psychiatric disorders | Possibly | Mild | Hospitalisation | Resolved: no residual effects |
| N14–104 | Middlesbrough | Dexamethasone | N14–104–01 | Clostridium difficile infection | 13 July 2016 | 1 August 2016 | Clostridium difficile colitis | Infections and infestations | Unrelated | Severe | Hospitalisation | Resolved with residual effects |
| N25–106 | Glasgow | Dexamethasone | N25–106–01 | Pneumothorax | 29 July 2016 | 8 August 2016 | Pneumothorax spontaneous | Respiratory, thoracic and mediastinal disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–193 | Cambridge | Dexamethasone | N01–193–02 | Stroke | 9 August 2016 | 14 August 2016 | Cerebrovascular accident | Nervous system disorders | Unrelated | Severe | Death | Death |
| N25–106 | Glasgow | Dexamethasone | N25–106–02 | Deep-vein thrombosis | 11 August 2016 | 12 August 2016 | Deep-vein thrombosis | Vascular disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N01–200 | Cambridge | Dexamethasone | N01–200–01 | Chest infection | 21 August 2016 | 28 August 2016 | Pneumonia | Infections and infestations | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–200 | Cambridge | Dexamethasone | N01–200–02 | Alcohol withdrawal | 21 August 2016 | 27 August 2016 | Alcohol withdrawal syndrome | Psychiatric disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N48–106 | Leeds | Dexamethasone | N48–106–02 | Shingles | 21 August 2016 | 22 August 2016 | Herpes zoster | Infections and infestations | Probably | Moderate | Hospitalisation | Resolved: no residual effects |
| N48–106 | Leeds | Dexamethasone | N48–106–01 | Pulmonary embolism | 30 August 2016 | 2 September 2016 | Pulmonary embolism | Vascular disorders | Unlikely | Severe | Hospitalisation | Resolved with residual effects |
| N14–111 | Middlesbrough | Dexamethasone | N14–111–01 | Acute kidney injury | 2 September 2016 | 12 September 2016 | Acute kidney injury | Renal and urinary disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N07–108 | Birmingham | Dexamethasone | N07–108–01 | Urosepsis with acute kidney injury | 6 September 2016 | 11 September 2016 | Urinary tract infection | Infections and infestations | Unlikely | Mild | Considered medically significant by the investigator | Resolved: no residual effects |
| N01–212 | Cambridge | Dexamethasone | N01–212–01 | Vomiting | 2 November 2016 | 3 November 2016 | Vomiting | Gastrointestinal disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–217 | Cambridge | Dexamethasone | N01–217–01 | Adrenal insufficiency | 15 November 2016 | Adrenal insufficiency | Endocrine disorders | Definitely | Moderate | Hospitalisation | Ongoing | |
| N01–220 | Cambridge | Dexamethasone | N01–220–01 | Confusional state | 21 November 2016 | Confusional state | Psychiatric disorders | Unrelated | Severe | Hospitalisation | Ongoing | |
| N25–117 | Glasgow | Dexamethasone | N25–117–01 | Aspiration pneumonia | 24 November 2016 | 1 December 2016 | Pneumonia | Infections and infestations | Unrelated | Severe | Death | Death |
| N18–101 | Aberdeen | Dexamethasone | N18–101–01 | Influenza A | 8 December 2016 | 14 December 2016 | Influenza | Infections and infestations | Unlikely | Mild | Hospitalisation | Resolved: no residual effects |
| N35–131 | Southampton | Dexamethasone | N35–131–01 | Urinary sepsis | 14 December 2016 | 16 January 2017 | Urinary tract infection | Infections and infestations | Possibly | Moderate | Hospitalisation | Resolved: no residual effects |
| N35–131 | Southampton | Dexamethasone | N35–131–02 | Hospital-acquired pneumonia | 23 December 2016 | 2 January 2017 | Pneumonia | Infections and infestations | Unlikely | Mild | Hospitalisation | Resolved with residual effects |
| N34–111 | Sheffield | Dexamethasone | N34–111–01 | Fall | 22 February 2017 | 3 March 2017 | Fall | Injury, poisoning and procedural complications | Unlikely | Mild | Hospitalisation | Resolved with residual effects |
| N08–105 | Brighton | Dexamethasone | N08–105–01 | Pseudo-obstruction | 21 March 2017 | 30 March 2017 | Intestinal pseudo-obstruction | Gastrointestinal disorders | Unlikely | Severe | Hospitalisation | Resolved: no residual effects |
| N18–103 | Aberdeen | Dexamethasone | N18–103–01 | Speech disturbance | 26 March 2017 | 26 March 2017 | Speech disorder | Nervous system disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N17–111 | Plymouth | Dexamethasone | N17–111–01 | General decline | 20 May 2017 | 15 June 2017 | General physical health deterioration | General disorders and administration site conditions | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N48–121 | Leeds | Dexamethasone | N48–121–01 | Fall | 25 May 2017 | 8 June 2017 | Fall | Injury, poisoning and procedural complications | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N34–117 | Sheffield | Dexamethasone | N34–117–02 | Death: cause – aspiration pneumonia, Alzheimer’s. Prostate carcinoma present at death but not cause of death | 30 May 2017 | 30 May 2017 | Pneumonia | Infections and infestations | Unlikely | Severe | Death | Death |
| N25–125 | Glasgow | Dexamethasone | N25–125–01 | Mesenteric infarction | 18 July 2017 | 19 July 2017 | Intestinal infarction | Vascular disorders | Unrelated | Severe | Death | Death |
| N01–270 | Cambridge | Dexamethasone | N01–270–01 | Stroke | 1 August 2017 | 1 August 2017 | Cerebrovascular accident | Nervous system disorders | Unrelated | Severe | Death | Death |
| N01–276 | Cambridge | Dexamethasone | N01–276–01 | Deep-vein thrombosis | 21 August 2017 | 25 August 2017 | Deep-vein thrombosis | Vascular disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N01–284 | Cambridge | Dexamethasone | N01–284–01 | Left facial swelling | 30 August 2017 | 1 September 2017 | Swelling face | Skin and subcutaneous tissue disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N18–107 | Aberdeen | Dexamethasone | N18–107–01 | Stroke | 19 September 2017 | 17 October 2017 | Cerebrovascular accident | Nervous system disorders | Unlikely | Moderate | Hospitalisation | Ongoing |
| N18–107 | Aberdeen | Dexamethasone | N18–107–02 | Aspiration pneumonia | 28 September 2017 | 17 October 2017 | Pneumonia | Infections and infestations | Unlikely | Moderate | Death | Death |
| N35–146 | Southampton | Dexamethasone | N35–146–01 | Loss of consciousness | 11 October 2017 | 12 October 2017 | Syncope | Vascular disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–300 | Cambridge | Dexamethasone | N01–300–01 | Confusion | 24 October 2017 | 26 October 2017 | Confusional state | Psychiatric disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N01–302 | Cambridge | Dexamethasone | N01–302–01 | Lower respiratory tract infection | 28 October 2017 | 31 October 2017 | Pneumonia | Infections and infestations | Possibly | Moderate | Hospitalisation | Resolved: no residual effects |
| N35–150 | Southampton | Dexamethasone | N35–150–01 | Cholangiocarcinoma | 14 November 2017 | 29 November 2017 | Cholangiocarcinoma | Neoplasms benign, malignant and unspecified (including cysts and polyps) | Unrelated | Severe | Death | Death |
| N34–127 | Sheffield | Dexamethasone | N34–127–01 | Bilateral axillary abscess | 28 November 2017 | 14 December 2017 | Subcutaneous abscess | Infections and infestations | Possibly | Moderate | Hospitalisation | Resolved: no residual effects |
| N34–126 | Sheffield | Dexamethasone | N34–126–01 | Chest infection | 11 December 2017 | Pneumonia | Infections and infestations | Possibly | Severe | Considered medically significant by the investigator | Ongoing | |
| N17–125 | Plymouth | Dexamethasone | N17–125–01 | New acute subdural haemorrhage | 21 December 2017 | 19 January 2018 | Subdural haematoma | Nervous system disorders | Unrelated | Moderate | Considered medically significant by the investigator | Resolved with residual effects |
| N34–130 | Sheffield | Dexamethasone | N34–130–01 | Pneumonia | 21 January 2018 | 8 February 2018 | Pneumonia | Infections and infestations | Unlikely | Severe | Death | Death |
| N25–139 | Glasgow | Dexamethasone | N25–139–02 | (Suspected CSDH recurrence) Headache | 21 February 2018 | 24 February 2018 | Headache | Nervous system disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N35–157 | Southampton | Dexamethasone | N35–157–01 | Pulmonary embolism | 23 February 2018 | 1 March 2018 | Pulmonary embolism | Vascular disorders | Unrelated | Severe | Hospitalisation | Resolved with residual effects |
| N25–139 | Glasgow | Dexamethasone | N25–139–01 | Intermittent back pain | 11 March 2018 | 15 March 2018 | Back pain | Musculoskeletal and connective tissue disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N35–160 | Southampton | Dexamethasone | N35–160–02 | Head injury | 25 March 2018 | 26 March 2018 | Head injury | Injury, poisoning and procedural complications | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N35–160 | Southampton | Dexamethasone | N35–160–01 | Hospital-acquired pneumonia | 25 March 2018 | 2 April 2018 | Pneumonia | Infections and infestations | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N48–131 | Leeds | Dexamethasone | N48–131–01 | Dizziness and unsteadiness | 25 March 2018 | 26 March 2018 | Dizziness | Vascular disorders | Unlikely | Mild | Hospitalisation | Resolved: no residual effects |
| N34–136 | Sheffield | Dexamethasone | N34–136–01 | Chest infection | 28 March 2018 | 17 April 2018 | Infections and infestations | Infections and infestations | Possibly | a | Hospitalisation | Resolved: no residual effects |
| N48–131 | Leeds | Dexamethasone | N48–131–02 | Dizziness and unsteadiness | 3 April 2018 | 4 April 2018 | Dizziness | Vascular disorders | Unlikely | Mild | Hospitalisation | Resolved: no residual effects |
| N17–130 | Plymouth | Dexamethasone | N17–130–01 | Non-ST elevation myocardial infarction | 10 May 2018 | 14 May 2018 | Acute myocardial infarction | Cardiac disorders | Unrelated | Moderate | Is life- threatening | Resolved: no residual effects |
| N07–123 | Birmingham | Dexamethasone | N07–123–01 | Acute bronchitis | 10 July 2018 | 10 July 2018 | Pneumonia | Infections and infestations | Unrelated | Severe | Death | Death |
| N35–171 | Southampton | Dexamethasone | N35–171–01 | Hyponatremia | 12 July 2018 | 30 July 2018 | Hyponatraemia | Metabolism and nutrition disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N25–151 | Glasgow | Dexamethasone | N25–151–01 | Frontal headache | 15 July 2018 | 16 July 2018 | Headache | Nervous system disorders | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N17–132 | Plymouth | Dexamethasone | N17–132–01 | Haematoma over right eye | 20 July 2018 | Periorbital haematoma | Injury, poisoning and procedural complications | Unlikely | Mild | Considered medically significant by the investigator | Ongoing | |
| N23–105 | Dundee | Dexamethasone | N23–105–01 | Pneumonia | 31 August 2018 | Pneumonia | Infections andinfestations | Possibly | Severe | Death | Death | |
| N36–110 | St George’s | Dexamethasone | N36–110–01 | Acute kidney injury | 26 September 2018 | 4 October 2018 | Acute kidney injury | Renal and urinary disorders | Unlikely | Mild | Hospitalisation | Resolved: no residual effects |
| N34–153 | Sheffield | Dexamethasone | N34–153–01 | Stroke | 10 October 2018 | Cerebrovascular accident | Nervous system disorders | Unlikely | Severe | Hospitalisation | Ongoing | |
| N25–157 | Glasgow | Dexamethasone | N25–157–01 | Delirium, treated for UTI | 12 October 2018 | 18 October 2018 | Urinary tract infection | Infections and infestations | Unrelated | Mild | Hospitalisation | Resolved: no residual effects |
| N25–160 | Glasgow | Dexamethasone | N25–160–01 | Inferior myocardial infarction | 15 October 2018 | 24 October 2018 | Acute myocardial infarction | Cardiac disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N48–154 | Leeds | Dexamethasone | N48–154–01 | Sepsis | 11 November 2018 | 3 December 2018 | Sepsis | Infections and infestations | Probably | Severe | Hospitalisation | Resolved: no residual effects |
| N07–129 | Birmingham | Dexamethasone | N07–129–01 | Chest infection – hospital-acquired pneumonia | 4 December 2018 | 29 January 2019 | Pneumonia | Infections and infestations | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N34–117 | Sheffield | Dexamethasone | N34–117–01 | Swallowing difficulties | a | Dysphagia | Gastrointestinal disorders | Unrelated | a | Hospitalisation | Ongoing |
| Participant ID | Site | Treatment arm | SAE reference number | Event | Onset date | Resolution date | MedDRA PT | MedDRA SOC | Causality | Severity | Seriousness | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N17–105 | Plymouth | Placebo | N17–105–01 | Scalp laceration | 28 April 2016 | 3 May 2016 | Laceration | Injury, poisoning and procedural complications | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–183 | Cambridge | Placebo | N01–183–01 | Cardiac failure | 15 July 2016 | 15 July 2016 | Cardiac failure | Cardiac disorders | Unlikely | Severe | Death | Death |
| N34–103 | Sheffield | Placebo | N34–103–01 | Death: cause unknown | 23 July 2016 | 23 July 2016 | Death | General disorders and administration site conditions | Unlikely | Severe | Death | Death |
| N01–188 | Cambridge | Placebo | N01–188–01 | Intracerebral bleed | 26 August 2016 | 16 September 2016 | Cerebral haemorrhage | Nervous system disorders | Unrelated | Severe | Death | Death |
| N48–105 | Leeds | Placebo | N48–105–01 | Fall | 15 September 2016 | 20 September 2016 | Fall | Injury, poisoning and procedural complications | Unrelated | Severe | Hospitalisation | Resolved with residual effects |
| N07–101 | Birmingham | Placebo | N07–101–01 | Death (cause not known) | 16 October 2016 | 16 October 2016 | Death | General disorders and administration site conditions | Unlikely | Severe | Death | Death |
| N14–114 | Middlesbrough | Placebo | N14–114–02 | Death (cause unknown) | 2 November 2016 | 2 November 2016 | Death | General disorders and administration site conditions | Unrelated | Moderate | Death | Death |
| N25–104 | Glasgow | Placebo | N25–104–01 | Pneumonia | 18 November 2016 | 22 November 2016 | Pneumonia | Infections and infestations | Unrelated | Severe | Death | Death |
| N17–108 | Plymouth | Placebo | N17–108–01 | Hospitalisation | 2 June 2017 | 8 June 2017 | Cardiac failure | Cardiac disorders | Unlikely | Moderate | Hospitalisation | Resolved: no residual effects |
| N26–111 | Hull | Placebo | N26–111–03 | Heart failure | 14 May 2018 | Cardiac failure | Cardiac disorders | Unlikely | Moderate | Hospitalisation | Ongoing | |
| N36–109 | St George’s | Placebo | N36–109–01 | Bronchopneumonia | 8 October 2018 | 10 October 2018 | Pneumonia | Infections and infestations | Unlikely | Severe | Death | Death |
| N25–159 | Glasgow | Placebo | N25–159–01 | Acute kidney injury | 27 October 2018 | 7 November 2018 | Acute kidney injury | Renal and urinary disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–125 | Cambridge | Dexamethasone | N01–125–01 | Deep-vein thrombosis | 20 November 2015 | 27 November 2015 | Deep-vein thrombosis | Vascular disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–111 | Cambridge | Dexamethasone | N01–111–01 | Acute subdural haematoma | 23 November 2015 | 28 November 2015 | Subdural haematoma | Injury, poisoning and procedural complications | Unrelated | Severe | Death | Death |
| N01–108 | Cambridge | Dexamethasone | N01–108–01 | Scalp laceration | 11 March 2016 | 12 March 2016 | Laceration | Injury, poisoning and procedural complications | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–142 | Cambridge | Dexamethasone | N01–142-01 | Fracture: left hip | 3 May 2016 | 13 May 2016 | Femur fracture | Injury, poisoning and procedural complications | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–135 | Cambridge | Dexamethasone | N01–135-01 | Incarcerated right groin hernia | 6 June 2016 | 8 June 2016 | Femoral hernia incarcerated | Gastrointestinal disorders | Unrelated | Moderate | Hospitalisation | Resolved: no residual effects |
| N01–174 | Cambridge | Dexamethasone | N01–174-01 | Pulmonary embolism | 7 June 2016 | 26 July 2016 | Pulmonary embolism | Vascular disorders | Unlikely | Moderate | Is life-threatening | Resolved: no residual effects |
| N35–111 | Southampton | Dexamethasone | N35–111-01 | Pulmonary embolism | 7 July 2016 | 11 July 2016 | Pulmonary embolism | Vascular disorders | Unrelated | Severe | Hospitalisation | Resolved with residual effects |
| N14–104 | Middlesbrough | Dexamethasone | N14–104-02 | Colitis (diffuse) secondary to C. difficile infection | 1 September 2016 | 6 September 2016 | Clostridial sepsis | Infections and infestations | Unrelated | Severe | Death | Death |
| N01–200 | Cambridge | Dexamethasone | N01–200-03 | Clostridium difficile diarrhoea | 17 September 2016 | 10 November 2016 | Clostridium difficile colitis | Infections and infestations | Unlikely | Severe | Hospitalisation | Resolved: no residual effects |
| N01–227 | Cambridge | Dexamethasone | N01–227-01 | Pulmonary embolism | 17 January 2017 | 19 January 2017 | Pulmonary embolism | Vascular disorders | Unrelated | Severe | Hospitalisation | Resolved: no residual effects |
| N01–320 | Cambridge | Dexamethasone | N01–320-01 | Transient ischaemic attack | 24 January 2018 | 24 January 2018 | Transient ischaemic attack | Nervous system disorders | Unrelated | Moderate | Considered medically significant by the investigator | Resolved: no residual effects |
| Participant ID | Site | Treatment arm | Event | Onset date | Resolution date | MedDRA PT | MedDRA SOC | Causality | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| N25–110 | Glasgow | Placebo | Haematemesis | 23 September 2016 | 23 September 2016 | Haematemesis | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N31–103 | Newcastle | Placebo | Nausea | 16 March 2017 | 16 March 2017 | Nausea | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N31–103 | Newcastle | Placebo | Diarrhoea | 19 March 2017 | 19 March 2017 | Diarrhoea | Gastrointestinal disorders | Possibly | Resolved: no residual effects |
| N14–106 | Middlesbrough | Dexamethasone | Agitation and aggressiveness | 16 July 2016 | 16 July 2016 | Agitation | Psychiatric disorders | Possibly | Resolved: no residual effects |
| N35–133 | Southampton | Dexamethasone | Constipation | 29 January 2017 | 31 January 2017 | Constipation | Gastrointestinal disorders | Unrelated | Resolved: no residual effects |
| N35–123 | Southampton | Dexamethasone | Appetite gain | a | a | Increased appetite | Metabolism and nutrition disorders | Possibly | a |
Appendix 4 Missing data
In the case of the primary end point, 9% of values were missing, with an identical number of missing values in each arm. A similar proportion of values were missing in the case of the other secondary end points and visits, with the one exception being the GCS score at 6 months.
Thus, the incidence rate of missing values for the primary end point is below the threshold stated in the SAP at which further techniques beyond complete-case analysis are required, which assumes missing at random. The CONSORT diagram provides the most detailed view of reasons for missing values (see Figure 1).
A SA has been added below (Figure 11), in which missing not at random assumptions were quantified by two parameters (delta_pla and delta_dex) that assume that the missing values have a predicted response rate within each arm that differs from the observed values by the value of the parameter, on an absolute risk difference scale. The response rate (favourable outcome rate) in the control arm was near 90%, hence values for these two parameters are considered between –10% and +10% as being the limits of plausibility. MI techniques are applied and the results are as shown in Figure 11, leading to the original conclusion that the extent of missing data are too small to affect the conclusions.
FIGURE 11.
Sensitivity analysis. (a) delta_dex: –0.1; (b) delta_dex: –0.08; (c) delta_dex: –0.06; (d) delta_dex: –0.04; (e) delta_dex: –0.02; (f) delta_dex: 0; (g) delta_dex: 0.02; (h) delta_dex: 0.04; (i) delta_dex: 0.06; (j) delta_dex: 0.08; and (k) delta_dex: 0.1.

List of abbreviations
- AE
- adverse event
- AESI
- adverse event of special interest
- BI
- Barthel Index
- CACE
- complier-average causal effect
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standard of Reporting Trials
- CRF
- case report form
- CSDH
- chronic subdural haematoma
- CT
- computerised tomography
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GCS
- Glasgow Coma Scale
- HCP
- healthcare professional
- HDU
- high-dependency unit
- HEAP
- health economic analysis plan
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IMP
- investigational medicinal product
- MedDRA
- Medical Dictionary for Regulatory Activities
- MI
- multiple imputation
- MRI
- magnetic resonance imaging
- mRS
- Modified Rankin Scale
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NMB
- net monetary benefit
- NSU
- neurosurgical unit
- OR
- odds ratio
- PPI
- patient and public involvement
- PSRQ
- patient self-report questionnaire
- PSS
- Personal Social Services
- PT
- preferred term
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SA
- sensitivity analysis
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SOC
- system organ class
- TSC
- Trial Steering Committee
