Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 13/04/107. The contractual start date was in December 2014. The draft manuscript began editorial review in May 2022 and was accepted for publication in July 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Hodgetts Morton et al. This work was produced by Hodgetts Morton et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Hodgetts Morton et al.
Chapter 1 Introduction
Preterm birth
Preterm birth (before 37 weeks) is a significant global problem, contributing to worldwide excess neonatal mortality and morbidity. 1 Office for National Statistics data demonstrate the contribution of preterm birth to excess neonatal mortality and morbidity, with over 51,000 preterm births per year in England and Wales. 2 Second trimester miscarriage is the delivery of a baby between 14 and 24 weeks without signs of life, a devastating event for women and their families. The number of women experiencing a second trimester miscarriage is harder to quantify as these losses are not registered through national reporting systems. The aetiology of second trimester miscarriage and preterm birth are complex and multifactorial and the conditions are often unrecognised as a continuum. 3 Romero et al. described the complex underlying mechanism of preterm birth as a pathological activation of the labour pathway, with a range of aetiologies. 4 The pathological processes potentially involved in preterm labour are infection/inflammation, cervical insufficiency, vascular disorders, decidual failure, uterine over distension (twins/polyhydramnios), decline in progesterone action, stress and breakdown of maternal–fetal tolerance. 4
Risk factors for second trimester miscarriage and preterm birth
There are several risk factors that can that predispose women to an increased risk of second trimester miscarriage and preterm birth. Previous history is the strongest predictor of recurrence; a systematic review and meta-analysis of 32 studies including 55,109 women identified a recurrence risk of 30%. 5 Additionally, previous preterm prelabour rupture of membranes (PPROM) is also associated with a risk of recurrence of approximately 7%, with previous early PPROM conferring a higher risk. 5 These risk factors when identified in a subsequent pregnancy enable increased surveillance, screening and interventions to help reduce the risk of recurrence. Previous early birth and PPROM have mixed aetiologies and can result from multiple underlying pathological processes. One of these pathological processes is cervical insufficiency, which is a condition of unknown aetiology but involves cervical shortening [which can be diagnosed by transvaginal ultrasound scanning (TVUS) measuring cervical length] and loss of cervical integrity. Cervical cerclage (CC) is one of several interventions proposed to treat cervical insufficiency. 6 Previous cervical surgery, often for premalignant cervical changes also predisposes to cervical insufficiency and early birth, with the risk of early delivery being directly proportionate to the depth of cervical excision. 7
Cervical cerclage
Vaginal CC is one treatment option for the prevention of second trimester miscarriage and preterm birth. A vaginal CC was first described in the literature over 70 years ago. The surgical procedure involves the placement of a purse string stitch around the cervix to close the cervix, provide mechanical support and potentially prevent ascending infection. There remains uncertainty regarding the surgical technique for performing the cerclage, the magnitude of effect and the population in which to offer CC. One of the largest trials evaluating the efficacy of CC was a joint collaboration between the Medical Research Council (MRC) and the Royal College of Obstetricians and Gynaecologists (RCOG), randomising women to CC or standard care (no intervention/standard care). Eligible women were defined as those women where clinicians felt there was uncertainty regarding the benefit of cerclage, and the trial demonstrated no significant benefit of CC. 8 The trial did not potentially recruit women at the highest risk of second trimester miscarriage and preterm birth, additionally it was conducted before the use of routine transvaginal cervical length scanning. Thus, the trial has significant limitations related to the population risk. However, it did offer important insights into the potential risks of cerclage, including maternal infection. 8 Prior to commencing this trial, a Cochrane review also did not conclusively demonstrate a benefit of CC. 9 At the same time as C-STICH (cerclage suture type for an insufficient cervix and its effects on health outcomes) opened for recruitment, the National Institute for Health and Care Excellence (NICE) 2015 preterm birth guideline identified two groups of women who should be offered a vaginal CC:10 women with a previous preterm birth and a short cervix on transvaginal scanning and women with previous PPROM and short cervical length. 10 A subsequent updated Cochrane review identified a benefit in reducing preterm birth in women at high risk based on their history (second trimester miscarriage and preterm birth) and a short cervical length, supporting the recommendations of the NICE guideline. 11 Vaginal CC is an appropriate treatment option for some, but not all, women, and care must be taken to ensure that background risk is carefully assessed to determine the potential benefit of a CC.
Cerclage technique
There are two main surgical techniques for performing a vaginal CC. A Shirodkar cerclage, which involves a bladder dissection and is placed at the levels of cardinal ligaments, or a McDonald cerclage, which is placed lower in the cervix without a bladder dissection (see Figure 1).
FIGURE 1.
Illustration of cerclage technique.
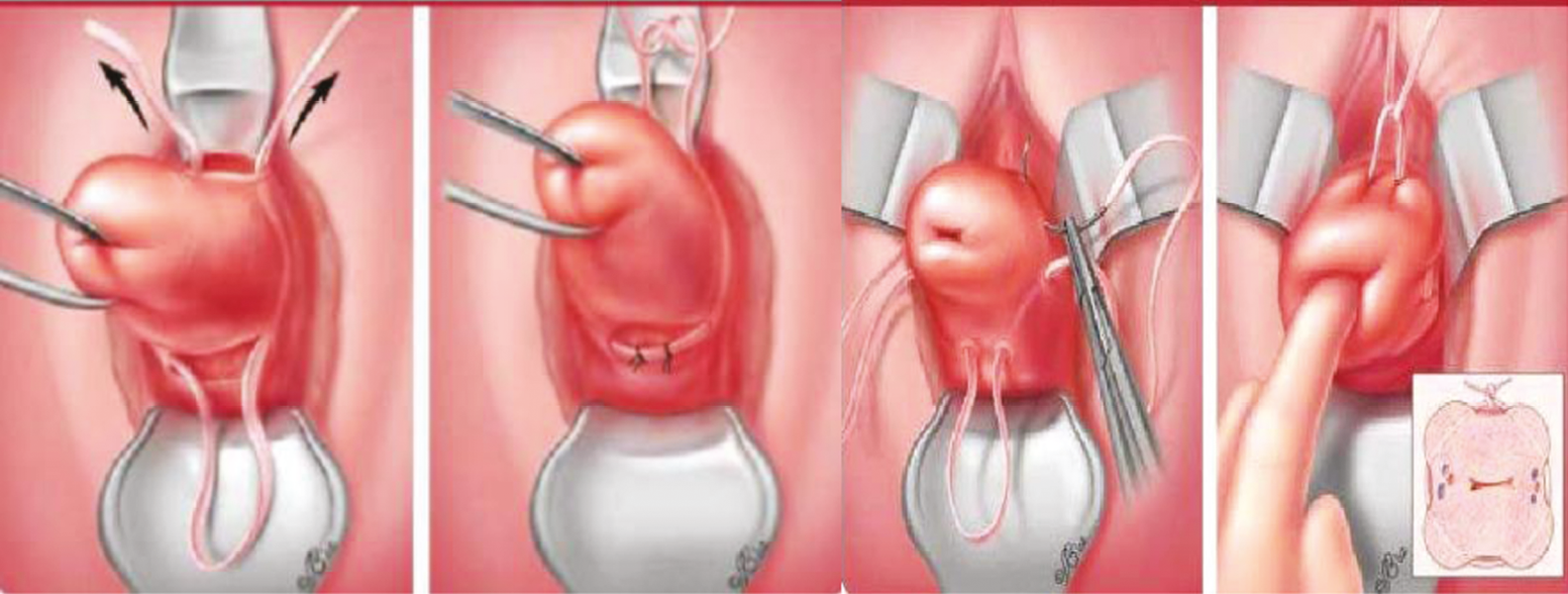
There are no randomised controlled trials (RCTs) comparing these two surgical techniques; a systematic review of the Shirodkar versus the McDonald technique suggests that there is no clear benefit of a bladder-dissected cerclage, and the authors suggest that if using a McDonald technique it should be placed as high as possible. 12
Current practice in the UK to prevent preterm birth
Preterm birth and second trimester prevention is an integral part of the Saving Babies Lives Care Bundle version 2. This bundle details a national approach to reducing stillbirth and neonatal death. Women are screened at booking for risk factors for preterm term birth and identified as either high risk or intermediate risk as per Table 1.
| High risk | Intermediate risk |
|---|---|
| Previous preterm birth or mid-trimester loss (16 to 34 weeks gestation) | Previous delivery by caesarean section at full dilatation |
| Previous PPROM | History of significant cervical excisional event, such as LLETZ where > 10 mm depth removed or > 1 LLETZ procedure carried out or cone biopsy (knife or laser, typically carried out under general anaesthetic) |
| Previous use of CC | |
| Known uterine variant (i.e. unicornuate, bicornuate uterus or uterine septum) | |
| Intrauterine adhesions (Asherman syndrome) | |
| History of trachelectomy (for cervical cancer) |
Women who are at high risk should be counselled regarding the risks and benefits of screening and treatment options, including CC, progesterone and Arabin pessaries. The majority of women will follow a screening pathway and will have a TVUS assessment of their cervix to measure the cervical length. In high-risk women, a cervical length measurement is recommended at least every 4 weeks from 16 weeks until 24 weeks. Women at intermediate risk of preterm birth should be offered at least one cervical assessment between 18 and 22 weeks.
Properties of suture threads
There are different types of suture thread used in surgical practice related to their properties: synthetic versus natural, monofilament versus braided and absorbable versus non-absorbable. Sutures are selected for different procedures based on the required characteristics, for example strength, diameter of suture, type of needle size, handling, ability to remove suture (anatomical location), absorption time. Surgeon preference also plays a role.
In the context of vaginal CC there are two main suture types: a thin, monofilament synthetic, non-absorbable suture and a thicker, multifilament, synthetic, non-absorbable braided tape. Braided suture materials, due to the multifilament threads woven together, have the potential to harbour bacteria. 13 In the context of a vaginal CC, this has the potential to cause vaginal dysbiosis which can predispose the area to infection and cerclage failure. 14 Microbiome studies have identified stability in the lactobacillus vaginal microbiome with a monofilament cerclage and a loss of microbiome stability (vaginal dysbiosis) with the use of a braided thread. 14 Yet, single-stranded monofilament sutures have been shown not to confer the same risk of vaginal dysbiosis. 14
Cerclage outcome by the type of suture material
Prior to C-STICH a feasibility study, cerclage outcome by the type of suture material (COTS), was performed. This study consisted of a literature review, a survey of optimal practice and the development of a protocol and trial documentation which then underwent feasibility-for-recruitment testing with women. 15–17
The survey of practice revealed that 87% of clinicians had a preference for a braided suture thread and 13% had a preference for a monofilament thread. 17 When exploring the decision-making process, the majority of clinicians did not consider suture-thread properties when making a choice, but instead utilised the thread that they were taught to perform the procedure with. Some clinicians, particularly those clinicians who had experience of mesh/woven type sutures within urogynaecology, considered that bacteria could penetrate the thread and potentially cause vaginal dysbiosis and predispose the area to infection. 17 Others felt that a braided tape could offer additional strength and support to a weak cervix. 17
As part of the COTS feasibility study, a literature review was performed which identified that there were limited data to inform suture-thread choice when performing a vaginal CC, with no RCTs. Additionally, most cerclage trials did not report the suture thread utilised during the procedure. Therefore, a retrospective cohort study in the authors’ units was performed and a non-randomised meta-analysis identified a potential difference in pregnancy loss between the different suture materials used for CC. 15–17
The meta-analysis is graphically represented in Figure 2 and identifies a reduction in pregnancy loss of up to 7% in the monofilament group compared with 18% in the braided group. The limitations of these data include the retrospective nature and the potential bias from the operator choice of suture thread, with a suggestion from the survey that those with high levels of experience preferentially choose monofilament suture threads. In view of the potential benefit of a monofilament suture thread and the lack of robust evidence, there was a need for a trial. Therefore, the concluding part of the COTS study included the development of a proposed protocol for a randomised trial and patient information leaflet that was evaluated by women undergoing a CC and demonstrated the willingness of women to be recruited and randomised. 16
FIGURE 2.
Non-randomised meta-analysis of pregnancy loss with monofilament vs. braided suture thread at CC. 18

Hypothesis
Multifilament braided suture threads are susceptible to colonisation by bacteria due to the nature of the woven threads. This colonisation increases the risk of CC failure and pregnancy loss through an infective mechanism which will be reduced by using a monofilament single-stranded thread when performing a CC.
Specific objectives
Primary objective
The primary objective of the study was to examine the effectiveness of using monofilament suture material as opposed to braided suture material on pregnancy loss in women requiring a vaginal CC.
Secondary objectives
The secondary objectives of the study include exploring the effectiveness of suture material on maternal and neonatal outcomes, exploring the variation of effect with regard to surgical technique (with or without bladder dissection) and exploring the variation of effect between indications for cerclage.
Chapter 2 Methods
Design
The C-STICH trial was a pragmatic, open, parallel, multicentre, randomised, superiority trial of monofilament versus braided suture type at CC to prevent pregnancy loss. The trial had a favourable ethical opinion from the National Research Ethics Service (NRES) Committee Cambridgeshire and Hertfordshire obtained in February 2015. The final protocol version was v9.0 dated 25 March 2020. The protocol for the trial has been published,18 and the full protocol is available at the C-STICH website.
Participants
Some text in this chapter has been reproduced from our study protocol,18 published under the Creative Commons Attribution License (CC BY 4.0), which permits its unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited (https://creativecommons.org/licenses/by/4.0/).
The participants in the C-STICH trial were recruited from secondary and tertiary care NHS hospitals located across the UK. Women were eligible for the trial if they required a vaginal CC as part of their routine care within their current pregnancy and they fulfilled the following eligibility criteria:
Inclusion
-
Singleton pregnancy.
-
Indication for CC for either:
-
a history of three or more previous midterm losses or premature births (≤ 28 weeks), OR
-
insertion of cervical sutures in previous pregnancies, OR
-
a history of mid-trimester loss or premature birth with a shortened (≤ 25 mm) cervix, OR
-
women whom clinicians deemed to be at risk of preterm birth either because of their history or because of the results of an ultrasound scan
-
Exclusion
-
Had taken part in C-STICH previously.
-
Aged < 18 years old at the time of presentation.
-
Those requiring a rescue cerclage [for the purposes of the study, a rescue cerclage was defined as an emergency cerclage where stitches are inserted in women who have had their preterm labours (e.g. uterine contractions, progressive cervical dilatation, bulging membranes) sufficiently halted by tocolysis or other means between 15 and 28 weeks].
-
Unwilling or unable to give informed consent.
-
Those in whom a cerclage was to be placed by any route other than vaginally (e.g. via an abdominal route).
-
Immediate need for insertion of a suture [immediate need for the insertion of a suture should not have been delayed by the trial (thus, if giving information about the trial and waiting for the participant to decide upon whether or not she wants to participate would delay the insertion of an urgently needed suture, then treatment should have gone ahead, and the woman should have been excluded from the trial)].
-
Membranes that had ruptured or were surfacing (Women with membranes that are ruptured or bulging through the external OS should have a rescue cerclage and be excluded from trial participation).
Recruitment
Women were identified in antenatal clinics, in delivery suites or in dedicated preterm birth prevention clinics. Women requiring a vaginal CC as part of their standard care were approached by doctors, research nurses and midwives. The decision about CC had already been made by the woman and clinicians and ideally the woman should have already completed the consent process for cerclage. Following consent for cerclage, women were approached to consider taking part in C-STICH. The trial was explained to women and a patient information leaflet was provided. To promote equity in access to the trial, written information was available in five additional languages. All staff approaching women regarding the trial were trained in ‘Good Clinical Practice’ (GCP) and had received dedicated trial-specific training. Participation in the trial was entirely voluntary, and women were informed that participation or non-participation would not affect their usual care. If an eligible woman agreed to participate, written consent was obtained, and both the clinician and the woman signed the consent form. The original copy was kept in the investigator site file, one copy was given to the woman, one copy was retained in the woman’s hospital records and one copy was sent to the clinical trials unit (CTU). Women declining participation were recorded in screening logs and sites were also asked to record women undergoing CC who were not approached about the trial.
Randomisation
Once final eligibility was confirmed and consent obtained, women were randomised to the C-STICH trial by the research staff at sites using a secure online, central randomisation service provided by the Birmingham Clinical Trials Unit. Additionally, there was a central telephone back-up service available.
Randomisation minimisation
Women were randomised on a 1 : 1 basis to either a monofilament or braided suture for the CC. A minimisation procedure, incorporating a random element, using a computer-based algorithm, was used to avoid chance imbalances in important prognostic variables. Strata used in the minimisation were:
-
planned bladder dissection (yes, no)
-
intention to commence patient on progesterone (yes, no)
-
indication for cerclage [a history of three or more previous mid-term losses or premature births (≤ 28 weeks), insertion of cervical sutures in previous pregnancies, a history of mid-trimester loss or premature birth with a (current) shortened (≤ 25 mm) cervix, women whom clinicians deem to be at risk of preterm birth either because of their history or because of the results of an ultrasound scan] (cerclage categories were developed to reflect the varying background risk of preterm birth)
-
randomising centre.
Interventions
Both monofilament and braided suture threads were standard surgical materials already in use. Different brands of suture material were available for both suture threads. Prior to the C-STICH trial commencing, the brands of suture threads used within potential sites were established. The commonest types were Ethilon for a monofilament suture and Mersilene® for a braided suture. Ethilon is a non-absorbable, sterile surgical suture composed of the long-chain aliphatic polymers of nylon. Mersilene is a non-absorbable, sterile surgical suture composed of poly-ethylene terephthalate. Due to the pragmatic nature of the trial, the brand of suture thread was left to the discretion of the clinician inserting the suture. However, Ethilon® and Mersilene sutures were encouraged. An absorbable suture thread was not permitted.
The vaginal CC technique (i.e. with or without bladder dissection) was at the discretion of the surgeon. However, the surgeon’s surgical intention (with or without bladder dissection) was expressed prior to randomisation and used as a minimisation variable. The trial was pragmatic in nature and surgeons were allowed to use antibiotics, tocolytics and to perform the CC according to their local practices. The surgical procedure could include an occlusion stitch to close the external OS with the stipulation that the same suture type as the randomised allocation be used.
Co-enrolment
Women already participating in another study [observational or a clinical trial of an investigational medicinal product (CTIMP)] prior to their confirmed eligibility for C-STICH were permitted to co-enrol into C-STICH. After a woman had been randomised to the C-STICH trial, she could participate in further CTIMP trials, although trials for the prevention of second trimester miscarriage or preterm birth, or any trials with the same primary outcome as C-STICH were excluded (such as trials of tocolytic agents). Women participating in C-STICH were permitted to be recruited into observational studies.
Blinding
The clinicians performing the vaginal CC could not be blinded to the suture thread and the surgical record documented the type of cerclage inserted, the type of suture used and the placement of any knots to facilitate removal. As far as possible, we requested that women and other research staff remained blinded to the suture-thread allocation, including microbiologists and outcome assessors. The duration of blinding continued until both mother and baby had reached their respective trial end points (i.e. final follow-up).
Adherence monitoring
Adherence to the allocated intervention was monitored on the cerclage placement form, where the suture material used to place the primary cerclage was recorded. We defined adherence as those participants in the monofilament suture group who received a monofilament suture for their primary cerclage and those participants in the braided suture group who received a braided suture for their primary cerclage. Subsequent suture replacements following the initial placement were permitted as necessary. For subsequent sutures, we encouraged clinicians to use the same suture thread as per the initial cerclage; however this was not mandated. The type of suture used for subsequent sutures was not considered as part of the adherence definition.
Withdrawal from the trial
Women could voluntarily withdraw from the trial at any time. The reasons for withdrawal were captured where possible. If a participant explicitly withdrew consent to have any further data recorded, their decision was respected and recorded on the electronic data capture system. All communication surrounding the withdrawal was noted in the patient’s medical notes, and no further data were collected for that participant. In rare instances where a participant was randomised and was later found to violate the inclusion criteria, they remained in the trial unless they had a multiple pregnancy or there was no informed consent.
Outcomes and assessment
The outcomes for the C-STICH trial were developed in collaboration with our patient advisory group and followed on from the non-randomised pilot study, COTS. 16 Patient input into the trial outcomes informed the primary outcome for this trial. Pregnancy loss was viewed as the most important outcome for women and their families, with prevention of prematurity considered as a secondary outcome, rather than the originally planned primary outcome.
Early in the trial, the core outcome set for preterm birth was developed and published. 19 The trial management group (TMG) recognised the importance of collecting all core outcomes within this preterm birth trial. Therefore, the outcome set in C-STICH was expanded in February 2016 to include all outcomes in the core outcome set for preterm birth. 19 No outcomes were removed in incorporating the core outcome set (C-STICH protocol amendment 3.0–4.0). In February 2016, the trial had commenced recruitment but the outcomes from the women recruited had not yet been collected and, therefore, all women in the trial had a complete preterm-birth core outcome set collected. 19
Primary outcome
Pregnancy loss (miscarriage and perinatal mortality, including any stillbirth or neonatal death in the first week of life).
Secondary outcomes
Key secondary outcome
-
Time from conception to pregnancy end (any reason).
Maternal outcomes
-
Miscarriage and previable neonatal death (defined as delivery < 24 weeks).
-
Stillbirth (defined as intrauterine death ≥ 24 weeks).
-
Gestation at delivery (in live births ≥ 24 weeks).
-
Gestational age < 28/< 32/< 37 weeks at delivery (in live births ≥ 24 weeks).
-
Time from conception to onset of spontaneous vaginal delivery (in live births ≥ 24 weeks).
-
Sepsis (at any time in pregnancy and until 7 days postnatal).
-
PPROM.
-
Mode of initiation of labour (spontaneous or induced).
-
Mode of delivery (vaginal, operative vaginal or caesarean).
-
Cerclage placement complications (cervical laceration/bleeding from cervix/ruptured membranes/bladder injury).
-
Cerclage removal complications (cervical tears/need for anaesthetic/difficult to remove).
-
Other maternal complications: vaginal bleeding/steroid use/chorioamnionitis/maternal pyrexia of 38°C (intrapartum/postnatal)/admission to HDU or ITU (pre/postdelivery).
Neonatal outcomes
-
Early neonatal death (defined as a death within 7 days after delivery) (in live births ≥ 24 weeks).
-
Late neonatal death (defined as a death beyond 7 days and before 28 days after delivery) (in live births ≥ 24 weeks).
-
Birth-weight centile adjusted for gestational age and sex (in live births ≥ 24 weeks).
-
Small for gestational age and sex (< 10th centile, in live births ≥ 24 weeks).
-
Resuscitation at birth (in live births ≥ 24 weeks).
-
Additional care required [special care baby unit (SCBU)/neonatal intensive care unit (NICU)/HDU/transitional care] and length of stay in additional care (in live births ≥ 24 weeks).
-
Antibiotics within 72 hours (in live births ≥ 24 weeks).
-
Sepsis (clinically diagnosed/proven) (in live births ≥ 24 weeks).
-
Early neurodevelopmental morbidity (severe abnormality on cranial ultrasound scan) (in live births ≥ 24 weeks).
-
Respiratory support and days on respiratory support (in live births ≥ 24 weeks).
-
Supplementary oxygen requirements at 36 weeks post menstrual age (in live births ≥ 24 weeks).
-
Necrotising enterocolitis (Bell’s stage 2 or 3) (in live births ≥ 24 weeks).
-
Retinopathy of prematurity requiring laser treatment (in live births ≥ 24 weeks)
-
Disabilities (live births ≥ 24 weeks).
-
Congenital anomalies (in live births ≥ 24 weeks).
Outcome generation
There were no extra visits beyond the standard care required for women recruited into the C-STICH trial. All outcome data were collected from the medical records. The outcomes were collected either on electronic case report forms (CRFs) and entered directly into the database or on paper CRFs and sent to the trial office. Details of how the outcome measures were generated are given in Table 2.
| Outcome assessed | Time point | Method | Reported by |
|---|---|---|---|
| Pregnancy loss | End of pregnancy | Clinical records | Research midwife or doctor |
| Pregnancy outcomes | End of pregnancy | Clinical records | Research midwife or doctor |
| Maternal outcomes | Up to 28 days post delivery | Clinical records | Research midwife or doctor |
| Neonatal outcomes | Up to 28 days of neonatal life | Neonatal records | Research midwife or doctor |
| Cerclage placement complications | At insertion of primary cerclage | Clinical records | Research midwife or doctor |
| Cerclage removal complications | At removal of primary cerclage | Clinical records | Research midwife or doctor |
Definition of the end of the trial
The end of trial was defined as the time when each participant had reached their defined end point. The maternal end point for the trial was 28 days post delivery or discharge to home (whichever occurred sooner).
For surviving babies, the definition of the end of the trial was different, depending on if the baby was born preterm (< 37 weeks) or at term. For preterm babies, the end of neonatal follow-up was the estimated date of delivery or discharge to home (whichever occurred sooner). For term babies, the end of the trial was 28 days post delivery or discharge to home (whichever occurred sooner). The primary analysis was scheduled to occur after all the corresponding outcome data had been entered into the study database and validated as being ready for analysis.
Serious adverse events
Serious adverse events (SAEs) were recorded on a purpose-designed SAE form and notified by local investigators to the CTU within 24 hours of the local investigators becoming aware of these events. Local investigators were responsible for additionally reporting SAEs to their host institutions, according to local regulations.
Full details of the SAEs reporting within C-STICH can be found in the published protocol. 18 SAEs categorised by the chief investigator as both suspected to be related to the trial intervention and unexpected were classified as suspected unexpected serious adverse reactions (SUSARs) and were subject to expedited reporting.
Sample size
The original sample size for C-STICH was informed by a non-randomised meta-analysis of audit data. 15 The pregnancy loss rate observed was approximately 7% in women who received a monofilament suture and 19% in women who received a braided suture [risk ratio (RR) 0.34, 95% confidence interval (CI) 0.18 to 0.63]. A total sample of 326 women would have been sufficient to detect a difference of this size with 90% power (alpha = 0.05). However, we inflated this to a total sample target of 900 women (which included inflation for an attrition rate of 2.5%) and which enabled us to detect a more plausible relative reduction of 41% (19% with braided sutures to 11.2% with monofilament sutures) with 90% power (alpha = 0.05).
Given that there was uncertainty around the estimates used for the rates of pregnancy loss, it was agreed that the data monitoring and ethics committee (DMEC) would monitor the overall (pooled) event rate throughout the study to assess any deviation from the original sample size assumptions. In July 2017, the DMEC disclosed that the current estimate of the pooled event rate was lower than anticipated and may affect the trial’s ability to detect a difference between groups, should one exist. They advised that to maintain 90% power (alpha = 0.05) to detect the same relative reduction of 41% the sample size should be increased. A final sample size of 2050 women was agreed, which assumed a pooled pregnancy loss rate of 7.4% [9.3% in the braided suture group and 5.5% in the monofilament group (RR 0.59)], with 90% power, an alpha error rate of 5% and allowing for 2.5% attrition. The overall pooled event rate and calculation was not disclosed to the TMG.
Statistical methods
A comprehensive statistical analysis plan (SAP) was drawn up prior to any analysis and provided to the independent DMEC and trial steering committee (TSC) for review. Full details of the statistical analysis can be found in the SAP, which is available on request from Birmingham Clinical Trials Unit.
In the first instance, participants were analysed in the treatment group to which they were randomised [intention to treat (ITT)], irrespective of adherence to the treatment protocol. All estimates of the differences between groups are presented with 95%, two-sided CIs, and were adjusted for the minimisation variables where possible. The time from conception to pregnancy end, gestational age at delivery and time from conception to onset of spontaneous vaginal delivery were further adjusted for gestational age at randomisation. The categorical data were summarised with frequencies and percentages. The normally distributed continuous variables were summarised with means and standard deviations (SDs); otherwise, medians and interquartile ranges (IQRs) were presented.
The primary outcome was analysed using a mixed-effects log-binomial model to generate an adjusted RR and risk difference (RD) (the latter using an identity link function), including the centre as a random effect. The statistical significance of the treatment group parameter was determined (p-value generated) through an examination of the associated chi-squared statistic (obtained from the log-binomial model which produces the RR). A hierarchical approach to testing was planned to control for the overall rate of type I error; if the primary outcome was to meet a conclusion of superiority, then the key secondary outcome (time from conception to pregnancy end) was also to be tested in this manner.
Other binary maternal outcomes and the following neonatal outcomes – early neonatal death, late neonatal death and small for gestational age – were analysed as per the primary outcome but were not subjected to hypothesis testing. For continuous secondary outcome measures (birthweight centile and gestational age at delivery), the adjusted mean differences were estimated using a linear regression model, including the centre as a random effect. Time-to-event outcomes (time from conception to pregnancy end, time from conception to onset of spontaneous vaginal delivery) were summarised using medians and IQRs. A Cox regression model was fitted to generate adjusted hazard ratios; Kaplan–Meier plots were produced to assess the data visually. Cerclage placement complications, cerclage removal complications and all other neonatal outcomes (excluding those previously listed) were analysed descriptively only. Numbers and percentages were provided for binary data and means (or medians) and standard deviations (or IQRs) for continuous normal (or non-normal) data. If the overall event rates exceeded 3%, then formal analysis was undertaken as per the methods outlined above.
Sensitivity and supportive analyses were limited to the primary outcome only. These included a per-protocol analysis (including only those participants who received their randomised allocation), an as-treated analysis and an assessment of missing data by means of a ‘tipping point’ approach, which explored the possibility that missing responses were ‘missing not at random’ (MNAR). Unadjusted models were utilised. Firstly, all women with missing outcome data were considered as having not met the primary outcome (i.e. pregnancy loss was no). Two scenarios were then considered. In the first scenario, in women who originally had missing data in the monofilament suture group, ‘events’ (i.e. pregnancy loss was changed from no to yes) were sequentially added to this group until the number of events added was equal to the number of women with missing outcome data in that group. With the addition of each event, an unadjusted model was run and the RR stored. The tipping point for the monofilament suture group occurred when enough events had been added such that the upper/lower limit of the CI from the corresponding model differed from that of the primary ITT finding (in regard to whether the CI crosses the null value of one), should the tipping point exist. A second scenario was then considered which repeated this process in the braided arm.
Pre-planned subgroup analyses (limited to the primary outcome measure only) were completed for the following: (1) planned bladder dissection (yes/no), (2) intention to commence patient on progesterone (yes/no) and (3) indication for cerclage (a history of three or more previous mid-term losses or premature births (≤ 28 weeks)/insertion of cervical sutures in previous pregnancies/a history of mid-trimester loss or premature birth with a (current) shortened (≤ 25 mm) cervix/women whom clinicians deem to be at risk of preterm birth either because of their history or because of the results of an ultrasound scan). The effects of these subgroups were examined by adding the subgroup-by-treatment-group interaction parameters to the regression model. p-values from the tests for statistical heterogeneity were presented alongside the effect estimate and 95% CI within each subgroup. In addition to this, a ratio(s) [and 95% CI(s)] was provided to quantify the difference between the treatment effects estimated within each subgroup.
Interim analyses of effectiveness and safety end points were performed on behalf of the DMEC on an approximately annual basis during the period of recruitment. These analyses were performed with the use of the Haybittle–Peto principle and, hence, no adjustment was made in the final p-values to determine significance. 20
All analyses were performed in SAS (version 9.4) or Stata (version 17.0).
Trial oversight
Study oversight was provided by a TSC (chaired by Professor Harry Gee, University of Warwick) and a DMEC (chaired by Professor Marian Knight, National Perinatal Epidemiology Unit).
The TSC provided independent supervision for the trial, providing advice to the chief and co-investigators and the sponsor on all aspects of the trial throughout the study. The DMEC adopted the DAMOCLES charter to define its terms of reference and operation in relation to oversight of the C-STICH trial. 21
Chapter 3 Results
This chapter reports the results of the C-STICH trial. Some text in this chapter has been reproduced from our study results paper,22 published under the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited (https://creativecommons.org/licenses/by/4.0/).
Recruitment
The trial opened to recruitment on 30 August 2015 and recruited its first participant on 22 September 2015, completing recruitment to its revised sample size on 28 January 2021. The trial completed follow-up and subsequently the database was locked for statistical analysis on 26 January 2022. There was a pause in recruitment between 26 March 2020 and 5 May 2020 as a result of COVID-19 restrictions and further impacts on recruitment numbers were seen throughout 2020 and 2021 (see Figure 3). However, due to the emergency nature of the situation and the continued support of the non-redeployed clinicians, COVID-19 had minimal impact on the assessments and resulting data. Recruitment occurred across a large preterm-birth-prevention network of 56 trusts relating to 75 individual sites. Figure 4 is a geographical representation of the C-STICH network. Table 3 demonstrates the number of women recruited at each C-STICH trust with the top three sites being Guys and St Thomas, Birmingham Women’s Hospital and Leeds, recruiting 29% of the total sample.
FIGURE 3.
Monthly recruitment into the C-STICH trial.
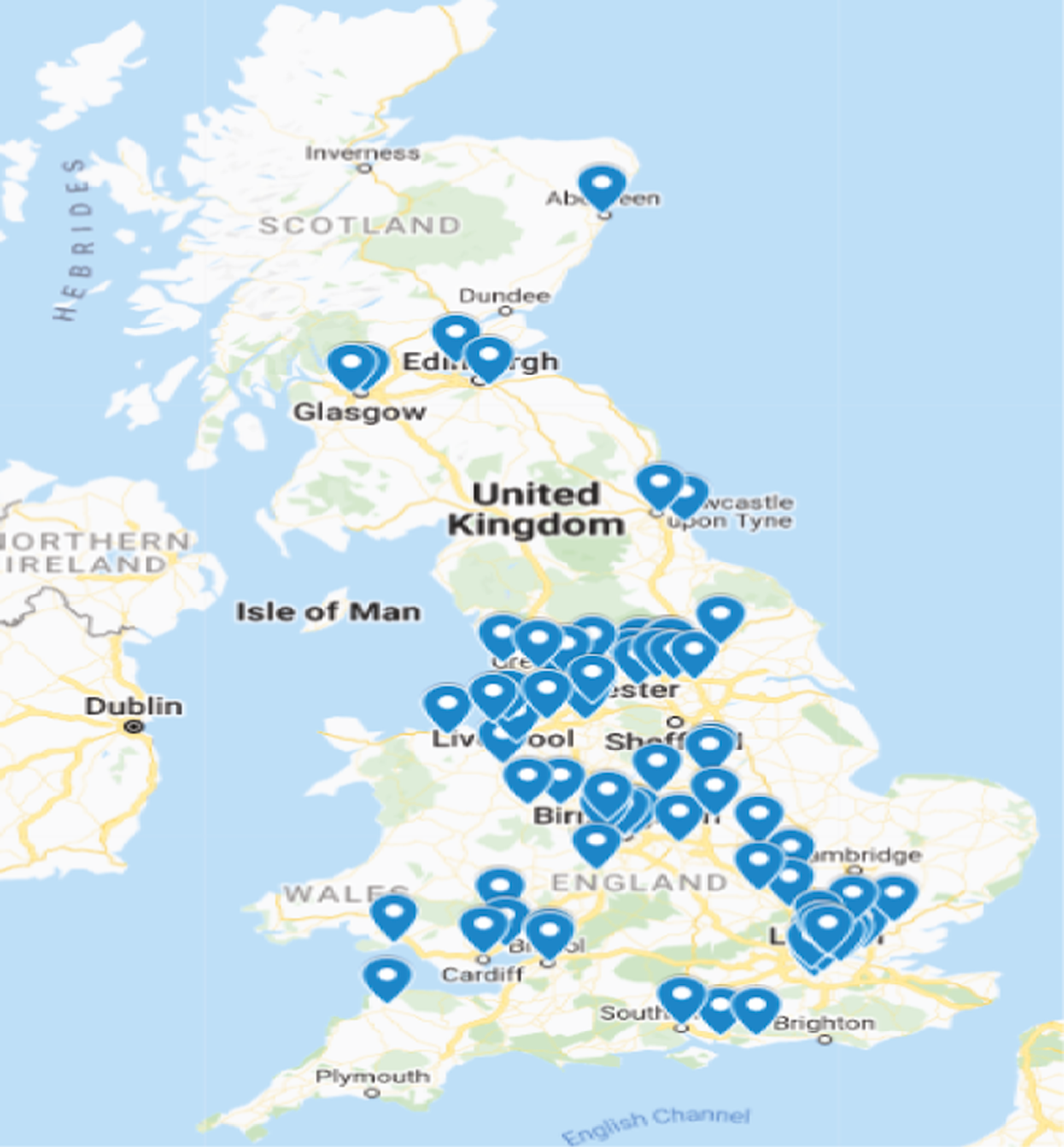
FIGURE 4.
Map of C-STICH recruiting centres.

| Trust, n (%) | All participants (N = 2049) |
|---|---|
| Guy’s and St Thomas’ NHS Foundation Trust | 248 (12) |
| Leeds Teaching Hospitals NHS Trust | 178 (9) |
| Birmingham Women’s and Children’s NHS Foundation Trust | 161 (8) |
| University College London Hospitals NHS Foundation Trust | 99 (5) |
| University Hospitals of Leicester NHS Trust | 92 (5) |
| Chelsea and Westminster Hospital NHS Foundation Trust | 88 (4) |
| Barts Health NHS Trust | 84 (4) |
| Central Manchester University Hospital NHS Trust | 81 (4) |
| Heart of England NHS Foundation Trust | 78 (4) |
| NHS Lothian | 71 (4) |
| East Lancashire Hospitals NHS Trust | 70 (3) |
| University Hospitals Coventry and Warwickshire NHS Trust | 50 (2) |
| Imperial College Healthcare NHS Trust | 48 (2) |
| The Newcastle Upon Tyne NHS Foundation Trust | 46 (2) |
| Kingston Hospital NHS Foundation Trust | 37 (2) |
| Kettering General Hospital NHS Trust | 36 (2) |
| Portsmouth Hospitals NHS Trust | 35 (2) |
| Barking, Havering and Redbridge Hospitals NHS Trust | 32 (2) |
| Lewisham and Greenwich NHS Trust | 32 (2) |
| Aneurin Bevan University Health Board | 31 (2) |
| Sandwell and West Birmingham Hospitals NHS Trust | 28 (1) |
| Nottingham University Hospitals NHS Foundation Trust | 25 (1) |
| Liverpool Women’s NHS Foundation Trust | 24 (2) |
| Milton Keynes University Hospital NHS Foundation Trust | 24 (1) |
| NHS Fife | 19 (1) |
| City Hospitals Sunderland NHS Foundation Trust | 18 (1) |
| Royal free London NHS Foundation Trust | 18 (1) |
| University Hospitals Bristol NHS Foundation Trust | 18 (1) |
| Burton Hospitals NHS Foundation Trust | 17 (1) |
| Betsi Cadwaladr University Health Board | 16 (1) |
| Epsom and St Helier University Hospitals NHS Trust | 16 (1) |
| NHS Grampian | 16 (1) |
| Pennine Acute Hospitals NHS Trust | 16 (1) |
| The Mid Yorkshire Hospitals NHS Trust | 16 (1) |
| Cardiff and Vale | 15 (1) |
| Calderdale and Huddersfield NHS Trust | 14 (1) |
| NHS Greater Glasgow and Clyde | 14 (1) |
| The Royal Wolverhampton NHS Foundation Trust | 14 (1) |
| Bradford Teaching Hospital NHS Foundation Trust | 13 (1) |
| Wirral University Teaching Hospital NHS Foundation Trust | 13 (1) |
| Lancashire Teaching Hospitals NHS Foundation Trust | 12 (1) |
| Blackpool Teaching Hospitals NHS Foundation Trust | 9 (< 1) |
| The Shrewsbury and Telford Hospital NHS Trust | 9 (< 1) |
| Luton and Dunstable University Hospital NHS Foundation Trust | 8 (< 1) |
| Worcestershire Acute Hospitals NHS Trust | 8 (< 1) |
| Bolton NHS Foundation Trust | 6 (< 1) |
| North Bristol NHS Trust | 6 (< 1) |
| University Hospital Southampton NHS Foundation Trust | 6 (< 1) |
| The Dudley Group NHS Foundation Trust | 5 (< 1) |
| Warrington and Halton Hospitals NHS Foundation Trust | 5 (< 1) |
| York Teaching Hospital NHS Foundation Trust | 5 (< 1) |
| Northern Devon Healthcare NHS Trust | 4 (< 1) |
| Mid Essex Hospital Services NHS Trust | 4 (< 1) |
| Western Sussex University Hospitals NHS Trust | 3 (< 1) |
| Countess of Chester NHS Trust | 3 (< 1) |
| Princess Alexandra Hospital NHS Trust | 3 (< 1) |
| Abertawe Bro Morgannwg University Health Board | 1 (< 1) |
| Bedford Hospital NHS Trust | 1 (< 1) |
Participant flow
The participant flow is illustrated in Figure 5. A total of 2937 women were screened for eligibility. Of these, 326 women were not eligible to be randomised and 526 women were not randomised. In total, 2049 women were randomised to C-STICH, with 1025 women allocated to a monofilament suture thread and 1024 women allocated to a braided suture thread. Seven women were withdrawn from the study and a further 40 women were lost to follow-up, meaning that 2002 women (97.7% of those randomised) were available for analysis of the primary outcome. The reasons for withdrawal are provided in Table 4.
FIGURE 5.
CONSORT diagram representing the flow of women through the C-STICH trial.

| Monofilament suture (N = 1025) |
Braided suture (N = 1024) |
|
|---|---|---|
| Withdrawals, n (%) | 4 (< 1) | 3 (< 1) |
| Reason for withdrawal, n | ||
| Withdrawn consent, no reason provided | 1 | 2 |
| Ineligible, multiple pregnancy | 3 | 0 |
| Ineligible, no consent (randomised in error) | 0 | 1 |
Monthly recruitment into the C-STICH trial was consistent across the developed preterm birth network following the start-up phase.
Baseline data
The baseline demographic characteristics of women in the monofilament suture group and the braided suture group were comparable, with the minimisation algorithm ensuring balance across indication for cerclage, intended bladder dissection and concomitant progesterone intended use.
The randomised women had a mean age of 32.9 years (SD 5.0 years). Ethnicity, as documented within the maternity booking records, was similar across both groups: 57% were white, 19% were Asian, 19% were black and 5% were from other ethnic groups demonstrating a good representation of ethnicity. The mean body mass index (BMI) at booking was 27.7 kg/m2.
The majority of women (70%) had a cervical length scan prior to cerclage placement, with a mean shortest cervical length of 23.1 mm (SD 9.4 mm). The majority of women (61%) in whom a cerclage was placed were deemed at-risk on the basis of their history or the results of ultrasound. A total of 439 women (21%) had had a CC placed in a previous pregnancy and then opted for a repeat cerclage; 324 (16%) of women had a history of a mid-trimester loss and/or preterm birth and a short cervix in this pregnancy. Previous cervical surgery as a risk factor for preterm birth was present in 538 women (27%), of which the majority had undergone a single previous large loop excision of the cervical transformation zone (LLETZ) treatment. Further details are provided in Table 5.
| Monofilament suture (N = 1025) |
Braided suture (N = 1023a) |
|
|---|---|---|
| Participant characteristics | ||
| Gestational age at randomisation (weeks), mean (SD, N) | 16.5 (3.7, 1025) | 16.6 (3.8, 1023) |
| Maternal age (years), mean (SD, N) | 32.8 (5.0, 1025) | 33.0 (5.1, 1023) |
| Ethnicity, n (%) | ||
| White | 592 (58) | 566 (56) |
| Asian | 197 (19) | 196 (19) |
| Black | 181 (18) | 205 (20) |
| Mixed | 36 (4) | 37 (4) |
| Other | 13 (1) | 10 (1) |
| Missing | 6 | 9 |
| BMI at bookingb (kg/m2), mean (SD, N) | 27.2 (6.3, 347) | 28.1 (6.5, 337) |
| Pregnancy history | ||
| Gravida, median [IQR, N] | 2.0 [1.0–4.0, 1025] | 2.0 [1.0–4.0, 1023] |
| Parity, n (%) | ||
| Nulliparous | 307 (30) | 309 (30) |
| 1–3 | 661 (64) | 661 (65) |
| > 3 | 57 (6) | 53 (5) |
| Median [IQR, N] | 1.0 [0–2.0, 1025] | 1.0 [0–2.0, 1023] |
| Number of 1st trimester losses, median [IQR, N] | 0 [0–1.0, 1025] | 0 [0–1.0, 1023] |
| Number of mid-trimester losses, median [IQR, N] | 1.0 [0–1.0, 1025] | 1.0 [0–1.0, 1023] |
| Number of termination of pregnancies, median [IQR, N] | 0 [0–0, 1025] | 0 [0–0, 1023] |
| Number of live births ≤ 33 + 6 weeks, median [IQR, N] | 0 [0–1.0, 1025] | 0 [0–1.0, 1023] |
| Number of live births 34 to 36 + 6 weeks, median [IQR, N] | 0 [0–0, 1025] | 0 [0–0, 1023] |
| Number of live births ≥ 37 weeks, median [IQR, N] | 0 [0–1.0, 1025] | 0 [0–1.0, 1023] |
| Clinical characteristics | ||
| Cervical length ultrasound scan performed, n (%) | 709 (69) | 718 (70) |
| Shortest cervical length before cerclage,c mean (SD, N) | 23.2 (9.8, 709) | 23.1 (9.0, 718) |
| Cervical funnelling, n (%) | ||
| Yes | 239 (34) | 248 (34) |
| No | 419 (59) | 435 (61) |
| Do not know | 51 (7) | 35 (5) |
| Primary indication for cerclage,d n (%) | ||
| Deemed at risk of preterm birth through history or ultrasound | 620 (61) | 619 (61) |
| Insertion of cervical sutures in previous pregnancies | 219 (21) | 220 (21) |
| History of mid-trimester loss/premature birth with a shortened cervix | 164 (16) | 160 (16) |
| History of ≥ 3 previous mid-term losses/premature births | 22 (2) | 24 (2) |
| Planned cerclage technique includes bladder dissection,d n (%) | 174 (17) | 175 (17) |
| Intention to commence patient on progesterone,d n (%) | 415 (40) | 414 (40) |
| Previous cervical surgery, n (%) | 258 (26) | 280 (28) |
| Missing | 19 | 28 |
| Type of previous cervical surgery,e n (%) | ||
| 1 × previous LLETZ | 123 (48) | 131 (47) |
| 2 × previous LLETZ | 45 (18) | 53 (19) |
| Knife cone biopsy | 29 (11) | 32 (11) |
| Otherf | 59 (23) | 64 (23) |
| Missing | 2 | 0 |
Adherence
The CC was placed in 98% of women in both the monofilament and braided arms. In the women where the CC was not placed, the reasons included change of decision for cerclage, cervix too short, membranes ruptured and cervical bleeding. Most women (87%) had their cerclage placed within 3 days of randomisation. Adherence to the randomised allocation was 94% in the monofilament arm and 96% in the braided arm, as detailed in Table 6.
| Monofilament suture (N = 1025) |
Braided suture (N = 1023a) |
|
|---|---|---|
| Cerclage placed, n (%) | 999 (98) | 999 (98) |
| Cerclage not placed, n (%) | 23 (2) | 22 (2) |
| Missing | 3 | 2 |
| Reason cerclage not placed,b n | ||
| Participant refused/did not attend | 6 | 3 |
| Cervix too short | 7 | 14 |
| Cervix too dilated | 0 | 1 |
| Membranes ruptured | 5 | 1 |
| Cervical bleeding | 3 | 4 |
| Infection | 0 | 0 |
| Otherc | 10 | 6 |
| Time to cerclage placement (days),d,e n (%) | ||
| ≤ 3 | 866 (87) | 868 (87) |
| > 3 | 133 (13) | 131 (13) |
| Median [IQR, N] | 0 [0–1.0, 999] | 0 [0–1.0, 999] |
| Minimum–maximum | 0–48.0 | 0–51.0 |
| Suture material placed,d n (%) | ||
| Monofilament suture | 963 (96) | 23 (2) |
| Braided suture | 36 (4) | 976 (98) |
| Second suture placed following primary cerclage,d n (%) | 20 (2) | 9 (1) |
| Missing | 48 | 62 |
| Adherent, n (%) | 963 (94) | 976 (96) |
| Missing | 3 | 2 |
Primary outcome: pregnancy loss
Overall, 155 out of 1996 women (8%) had a pregnancy loss. The pregnancy loss rate in the monofilament suture group was 8% (80/1003) and the rate in the braided suture group was 8% (75/993) (RR 1.05, 95% CI 0.79 to 1.40). Full details of the components of pregnancy loss are detailed in Table 7.
| Monofilament suture (N = 1025) |
Braided suture (N = 1023a) |
RRb (95% CI) |
RDc (95% CI) |
p-value | |
|---|---|---|---|---|---|
| Pregnancy loss, n (%) | |||||
| Yes | 80 (8) | 75 (8) | 1.05 (0.79 to 1.40) | 0.002 (−0.02 to 0.03) | 0.73 |
| No | 923 (92) | 918 (92) | |||
| Missing | 22 | 30 | |||
| Type of pregnancy loss, n (%) | |||||
| Miscarriage | 44/80 (55) | 36/75 (48) | – | – | – |
| Spontaneous | 37 | 34 | |||
| Missed | 5 | 1 | |||
| Septic | 2 | 1 | |||
| Termination | 9/80 (11) | 14/75 (19) | |||
| Fetal anomaly | 4 | 3 | |||
| Maternal medical condition | 1 | 1 | |||
| Maternal sepsis | 2 | 6 | |||
| Otherd | 2 | 4 | |||
| Stillbirth | 8/80 (10) | 11/75 (15) | |||
| Othere | 0/80 (–) | 1/75 (1) | |||
| Neonatal death < 7 days | 19/80 (24) | 13/75 (17) | |||
Sensitivity and supportive analyses
In the per-protocol analysis of the primary outcome comparison, including only the 1939 women defined as adherent to their allocated suture thread, the rates of pregnancy loss remained similar across the two arms. Similarly, when women were analysed as per what suture thread they primarily received, there were no significant differences in pregnancy loss rates between the suture threads, as detailed in Table 8.
| Monofilament suture | Braided suture | RRa (95% CI) |
RDb (95% CI) |
|
|---|---|---|---|---|
| Pregnancy loss, n/N (%) | ||||
| Per-protocol analysisc | 67/946 (7) | 68/949 (7) | 0.99 (0.71 to 1.37) | −0.01 (−0.03 to 0.02) |
| As-treated analysisd | 67/968 (7) | 73/984 (7) | 0.93 (0.68 to 1.28) | −0.01 (−0.03 to 0.01) |
The sensitivity analyses to assess the impact of missing data using a ‘tipping point’ approach are summarised in Figures 6 and 7 and support the conclusion that our primary outcome analysis was robust to the small amount of missing data. In the first scenario, Figure 6 demonstrates that if we assume no cases of pregnancy loss (i.e. pregnancy loss = no) in all women with missing data in the braided arm, we must assume > 95% of the women with missing data in the monofilament arm had a pregnancy loss in order to change the conclusion of the outcome (in favour of a braided suture). In the second scenario, Figure 7 shows that even if we assume no cases of pregnancy loss (i.e. pregnancy loss = no) in all women with missing data in the monofilament arm and assume all women with missing data in the braided arm had a pregnancy loss, we do not change the conclusion of the outcome (i.e. under the most extreme assumptions the conclusion will not change in favour of a monofilament suture).
FIGURE 6.
Tipping point analysis in the monofilament suture group. Risk ratios < 1 favour monofilament suture. a, In the base case, the estimate is derived from the model where we assume the event rate in the missing data is equal to the event rate in the non-missing data in the monofilament arm. All missing data in the braided arm are assumed to be non-events.
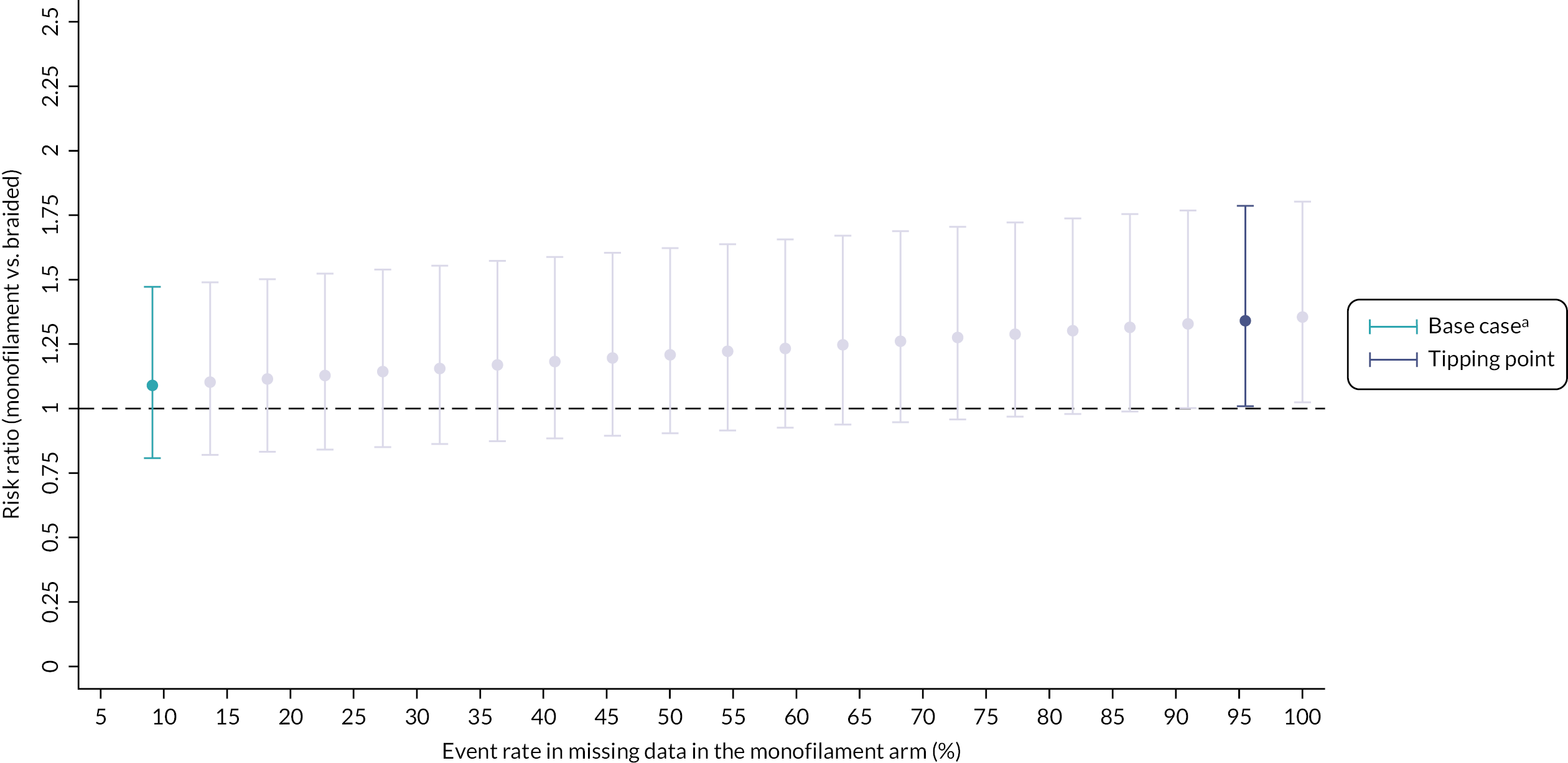
FIGURE 7.
Tipping point analysis in the braided suture group. Risk ratios <1 favour monofilament suture. a, In the base case, the estimate is derived from the model where we assume the event rate in the missing data is equal to the event rate in the non-missing data in the braided arm. All missing data in the monofilament arm are assumed to be non-events.

Subgroup analyses
The output from the subgroup analyses for the primary outcome can be seen in Table 9. For indication for cerclage and current or intended progesterone use, there was no evidence of a differential treatment effect within these subgroups. For the key subgroup of bladder dissection, there was evidence of a potential differential treatment effect (p = 0.05 test for interaction). In women where it was planned to dissect their bladder during the CC, the rate of pregnancy loss was lower in the monofilament suture group than in the braided suture group [6/169 (4%) vs. 14/172 (8%)]; however, the numbers were small and the effect within this stratum was not statistically significant.
| Monofilament suture | Braided suture | Interaction p-value |
RRa (95% CI) |
Ratiob (95% CI) |
|
|---|---|---|---|---|---|
| Key subgroup | |||||
| Planned bladder dissection, n/N (%) | |||||
| Yes | 6/169 (4) | 14/172 (8) | 0.05 | 0.44 (0.17 to 1.12) |
0.37 (0.01 to 0.74)c |
| No | 74/834 (9) | 61/821 (7) | 1.19 (0.86 to 1.64) |
||
| Exploratory subgroups | |||||
| Indication for cerclage, n/N (%) | |||||
| A history of three or more previous mid-term losses or premature births (≤ 28 weeks) | 1/22 (5) | 2/24 (8) | 0.56 | 0.55 (0.05 to 5.65) |
0.46 (0.00 to 1.56)d |
| Insertion of cervical sutures in previous pregnancies | 14/213 (7) | 19/212 (9) | 0.72 (0.37 to 1.39) |
0.60 (0.14 to 1.07)e |
|
| A history of mid-trimester loss or premature birth with a (current) shortened (≤ 25 mm) cervix | 16/163 (10) | 13/160 (8) | 1.20 (0.60 to 2.41) |
1.01 (0.20 to 1.82)f |
|
| Women whom clinicians deem to be at risk of preterm birth either because of their history or because of the results of an ultrasound scan | 49/605 (8) | 41/597 (7) | 1.19 (0.80 to 1.76) |
REF | |
| Progesterone treatment (either current or intention to commence), n/N (%) | |||||
| Yes | 31/402 (8) | 27/403 (7) | 0.66 | 1.15 (0.70 to 1.89) |
1.15 (0.43 to 1.87)g |
| No | 49/601 (8) | 48/590 (8) | 1.00 (0.68 to 1.46) |
||
Secondary outcome results
The secondary maternal outcomes are reported in Table 10. There was no evidence of any difference between the groups in any of the outcomes, apart from maternal sepsis and chorioamnionitis, which was lower in the monofilament group than in the braided group (RR 0.58, 95% CI 0.40 to 0.82 and RR 0.45, 95% CI 0.29 to 0.71, respectively).
| Maternal outcome | Monofilament suture | Braided suture | Estimate (95% CI) |
RD (95% CI) |
|---|---|---|---|---|
| Time from conception to pregnancy end (weeks), median [IQR, N] | 37.9 [35.6–39.1, 1008] |
38.0 [35.4–39.1, 998] |
1.04a (0.95 to 1.14) |
– |
| Miscarriage and previable neonatal death, n/N (%) | 60/1003 (6) | 49/993 (5) | 1.21b (0.84 to 1.74) |
0.01c (−0.01 to 0.03) |
| Still birth, n/N (%) | 8/1003 (1) | 11/993 (1) | 0.72b (0.29 to 1.77) |
0.00d (−0.01 to 0.01) |
| Gestational age at deliverye (weeks). mean (SD, N) | 37.2 (3.3, 926) | 37.2 (3.4, 919) | 0.02f (−0.29 to 0.32) |
– |
| Gestational age at delivery < 28 weeks,e n/N (%) | 34/926 (4) | 35/919 (4) | 0.96g (0.61 to 1.53) |
0.00d (−0.02 to 0.02) |
| Gestational age at delivery < 32 weeks,e n/N (%) | 77/926 (8) | 86/919 (9) | 0.89b,h (0.66 to 1.19) |
−0.01c,h (−0.03 to 0.02) |
| Gestational age at delivery < 37 weeks,e n/N (%) | 258/926 (28) | 265/919 (29) | 0.96b,h (0.83 to 1.11) |
−0.01c,h (−0.05 to 0.03) |
| Time from conception to onset of spontaneous vaginal deliveryi (weeks), median [IQR, N] | 37.9 [35.6–39.4, 342] |
38.0 [35.7–39.6, 420] |
1.07a (0.91 to 1.26) |
- |
| Maternal sepsis, n/N (%) | 39/1000 (4) | 67/988 (7) | 0.58j (0.40 to 0.82) |
−0.03c (−0.05 to −0.01) |
| PPROM, n/N (%) | 199/1006 (20) | 201/997 (20) | 0.98b (0.82 to 1.16) |
−0.01c (−0.04 to 0.03) |
| Mode of initiation of birth, n/N (%) | ||||
| Spontaneous | 446/957 (47) | 437/951 (46) | 0.99b (0.91 to 1.07) |
−0.01c (−0.05 to 0.04) |
| Induced | 327/957 (34) | 338/951 (36) | ||
| Caesarean section | 184/957 (19) | 176/951 (18) | ||
| Mode of delivery, n/N (%) | ||||
| Vaginal | 490/953 (51) | 513/943 (54) | 0.94k (0.86 to 1.02) |
−0.03l (−0.08 to 0.01) |
| Operative vaginal | 112/953 (12) | 81/943 (9) | ||
| Caesarean | 351/953 (37) | 349/943 (37) | ||
| Vaginal bleeding, n/N (%) | 142/995 (14) | 154/988 (16) | 0.91j (0.74 to 1.13) |
−0.01c (−0.04 to 0.02) |
| Steroid use, n/N (%) | 294/1000 (29) | 303/993 (31) | 0.97b (0.85 to 1.10) |
−0.01c (−0.05 to 0.03) |
| Chorioamnionitis, n/N (%) | 26/956 (3) | 57/957 (6) | 0.45j (0.29 to 0.71) |
−0.03d (−0.05 to −0.01) |
| Maternal pyrexia (intrapartum), n/N (%) | 22/1003 (2) | 35/992 (4) | 0.98j (0.65 to 1.48) |
0.00c (−0.02 to 0.02) |
| Maternal pyrexia (postnatal), n/N (%) | 44/1001 (4) | 44/989 (4) | 0.80j (0.43 to 1.51) |
−0.01c (−0.02 to 0.01) |
| Admission to HDU (pre delivery), n/N (%) | 17/1003 (2) | 21/996 (2) | 0.80j (0.43 to 1.51) | −0.01c (−0.02 to 0.01) |
| Admission to ITU (pre delivery), n/N (%) | 0/1002 (–) | 1/995 (< 1) | – | – |
| Admission to HDU (post delivery), n/N (%) | 52/999 (5) | 50/986 (5) | 1.02j (0.70 to 1.49) |
0.00c (−0.02 to 0.02) |
| Admission to ITU (post delivery), n/N (%) | 1/998 (< 1) | 5/986 (1) | 0.20b (0.02 to 1.69) |
0.00d (−0.01 to 0.00) |
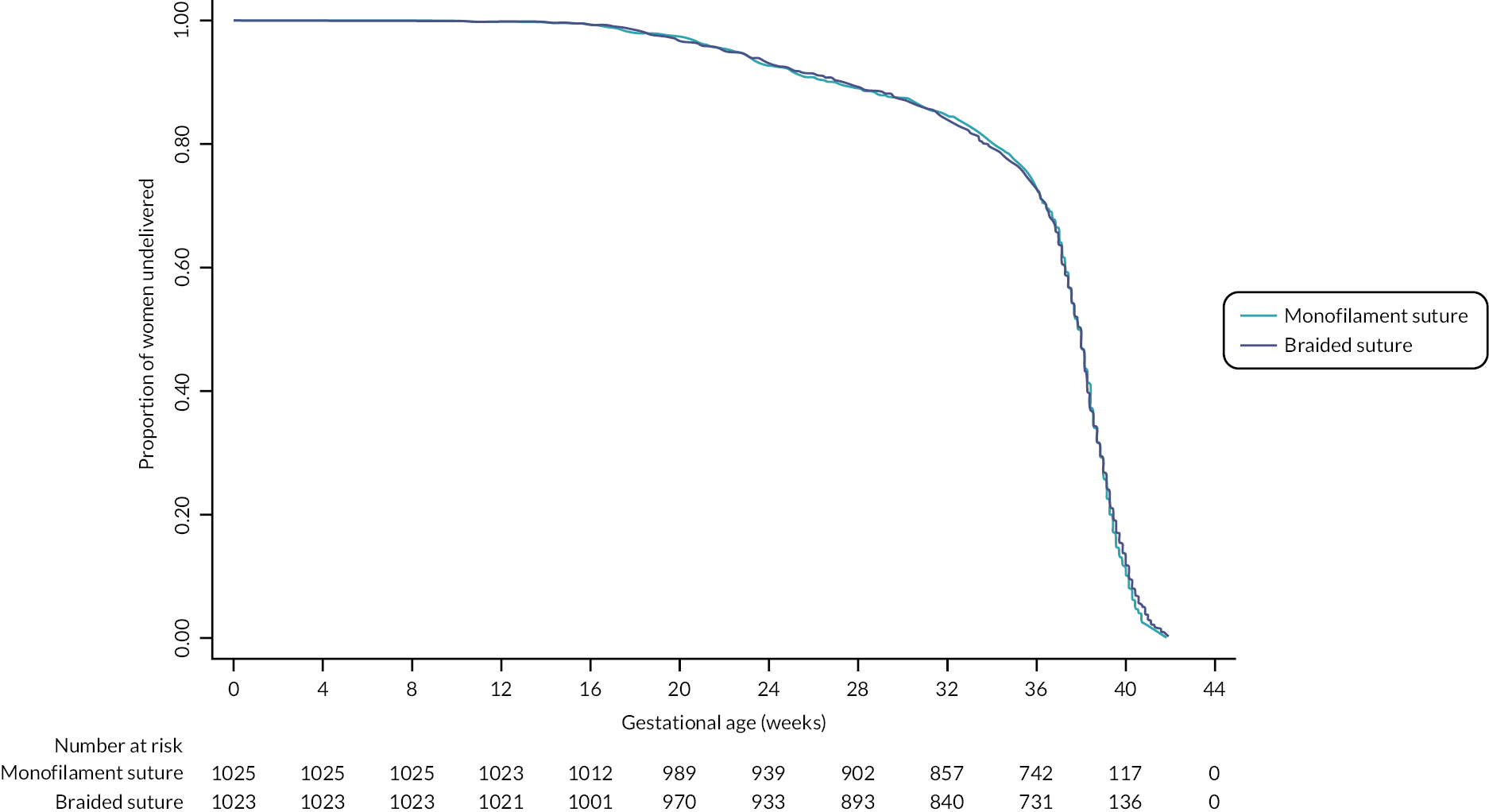
For conception to pregnancy end, no difference was demonstrated between a monofilament suture thread and a braided suture thread (see Figure 8).
FIGURE 8.
Kaplan–Meier plot for time from conception to pregnancy end.
The neonatal outcomes are presented in Table 11. There was no evidence of a difference between any of the outcomes collected.
| Neonatal outcomea | Monofilament suture | Braided suture | Estimate (95% CI) |
RD (95% CI) |
|---|---|---|---|---|
| Early neonatal death (< 7 days), n/N (%) | 5/920 (1) | 3/913 (< 1) | 1.63b (0.39 to 6.80) |
0.00c (0.00 to 0.01) |
| Late neonatal death (≥ 7 and < 28 days), n/N (%) | 1/920 (< 1) | 0/913 (–) | – | – |
| Birth-weight (centile), mean (SD, N) | 41.0 (29.2, 923) | 42.4 (28.8, 912) | −1.44d (−4.09 to 1.21) |
– |
| Small for gestational age, n/N (%) | 147/923 (16) | 132/912 (14) | 1.10e (0.84 to 1.45) |
0.02f (−0.02 to 0.05) |
| Resuscitation at birth, n/N (%) | 61/916 (7) | 62/911 (7) | 0.98b (0.70 to 1.38) |
0.00f (−0.02 to 0.02) |
| Additional care, n/N (%) | 265/920 (29) | 268/912 (29) | 0.98b (0.85 to 1.13) |
0.00f (−0.05 to 0.04) |
| Length of stay in additional care, median [IQR, N] | ||||
| SCBU | 4.0 [0–15.0, 243] | 4.0 [0–16.0, 258] | – | – |
| NICU | 0 [0–7.0, 231] | 0 [0–6.0, 247] | – | – |
| HDU | 0 [0–4.0, 233] | 0 [0–4.0, 244] | – | – |
| Transitional care | 0 [0–1.5, 220] | 0 [0–1.0, 241] | – | – |
| Antibiotics within 72 hours after birth, n/N (%) | 236/915 (26) | 249/906 (27) | 0.94e (0.81 to 1.09) |
−0.01f (−0.06 to 0.03) |
| Sepsis (clinically diagnosed), n/N (%) | 102/909 (11) | 113/904 (13) | 0.90e (0.71 to 1.14) |
−0.01f (−0.04 to 0.02) |
| Sepsis (confirmed), n/N (%) | 15/908 (2) | 19/904 (2) | – | – |
| Early neurodevelopmental morbidity, n/N (%) | 13/912 (1) | 19/903 (2) | – | – |
| Respiratory support, n/N (%) | 130/915 (14) | 144/910 (16) | 0.90e (0.72 to 1.11) |
−0.01f (−0.05 to 0.02) |
| Days on respiratory support, median [IQR, N] | 5.0 [1.0–28.0, 125] |
4.0 [1.0–27.0, 142] |
– | – |
| Supplementary oxygen requirements, n/N (%) | 27/910 (3) | 30/914 (3) | 0.91b (0.54 to 1.52) |
0.00f (−0.02 to 0.01) |
| Necrotising enterocolitis (Bell’s stage 2 or 3), n/N (%) | 7/908 (1) | 11/909 (1) | – | – |
| Retinopathy of prematurity requiring laser treatment, n/N (%) | 4/906 (< 1) | 5/908 (1) | – | – |
| Disabilities, n/N (%) | 1/910 (< 1) | 5/904 (1) | – | – |
| Details of disabilities, n | ||||
| Brachial plexus injury | 0 | 2 | – | – |
| Hypoxic-ischemic encephalopathy (grade one) | 0 | 1 | ||
| Feeding difficulty | 0 | 1 | ||
| Retinopathy of prematurity | 1 | 1 | ||
| Hearing disability | 0 | 1 | ||
| Congenital anomalies, n/N (%) | 18/914 (2) | 18/909 (2) | – | – |
| Details of congenital anomalies, n | ||||
| Chromosomal abnormalities | 2 | 4 | – | – |
| Cleft lip and cleft palate | 3 | 3 | ||
| Congenital malformations and deformations of the musculoskeletal system | 5 | 4 | ||
| Congenital malformations of genital organs | 2 | 0 | ||
| Congenital malformations of the circulatory system | 5 | 5 | ||
| Congenital malformations of the digestive system | 2 | 0 | ||
| Congenital malformations of the nervous system | 0 | 1 | ||
| Congenital malformations of the urinary system | 1 | 2 | ||
Cervical cerclage is known to be associated with both insertion and removal risks (see Table 12). Within C-STICH, 4% of women in the monofilament group and 3% in the braided group experienced insertion complications. There were two cases of ruptured membranes during CC and these both occurred within the monofilament group. The most common insertion complication was bleeding from the cervix, which occurred in 4% of women in the monofilament group and 3% of women in the monofilament group. With regard to cerclage removal complications, there was a higher proportion of women who experienced a removal complication in the monofilament group compared with the braided group RR 1.25 (95% CI 1.15 to 1.36). Removal complications comprised clinician-judged difficulty in suture removal and an increased need for anaesthetic, with 41% of women requiring an anaesthetic for removal in the monofilament group compared with 32% in the braided group. There were no statistically significant differences in the number of women who experienced a SAE between the two treatment groups. There was a small increase in the number of neonates who experienced a SAE in the braided group but overall, the numbers were small. Twelve maternal SAEs and one neonatal SAE were considered to be related to the trial allocation and classified as unexpected.
| Monofilament suture | Braided suture | RR (95% CI) |
RD (95% CI) |
|
|---|---|---|---|---|
| Cerclage complications | ||||
| Cerclage placement complication,a n/N (%) | 43/999 (4) | 30/999 (3) | 1.44b (0.91 to 2.27) |
0.01c (0.00 to 0.03) |
| Details of cerclage placement complications,a n/N (%) | ||||
| Cervical laceration | 5/999 (1) | 2/999 (< 1) | – | |
| Bleeding from cervix | 39/999 (4) | 29/999 (3) | – | |
| Ruptured membranes | 2/999 (< 1) | 0/999 (–) | – | |
| Bladder injury | 0/999 (–) | 0/999 (–) | – | |
| Cerclage removal complication,d n/N (%) | 506/896 (56) | 373/883 (42) | 1.25c (1.15 to 1.36) |
0.14e (0.10 to 0.18) |
| Details of cerclage removal complications,d n/N (%) | ||||
| Cervical tears | 20/863 (2) | 8/865 (1) | – | |
| Difficulty in removal | 276/885 (31) | 128/875 (15) | – | |
| Need for anaesthetic | 382/934 (41) | 293/922 (32) | – | |
| Adverse events | ||||
| Total number of women experiencing a SAE, n/N (%) | 108/1025 (11) | 99/1023f (10) | – | |
| Number of maternal SAEs reported, n | 126 | 115 | – | |
| Total number of neonates experiencing a SAE, n/N (%) | 8/1025 (1) | 19/1023f (2) | – | |
| Number of neonate SAEs reported, n | 15 | 25 | – | |
| Total number of women experiencing a SUSAR, n/N (%) | 6/1025 (1) | 3/1023f (< 1) | – | |
| Number of maternal SUSAEs reported, n | 9 | 3 | – | |
| Total number of neonates experiencing a SUSAR, n/N (%) | 1/1025 (< 1) | 0/1023f (–) | – | |
| Number of neonate SUSARs reported, n | 1 | 0 | – | |
Chapter 4 Discussion
Some text in this chapter has been reproduced from our study results paper,22 published under the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited (https://creativecommons.org/licenses/by/4.0/).
C-STICH was a large multicentre, randomised trial designed to assess any difference in the pregnancy outcome comparing monofilament and braided suture material at vaginal CC. Its primary end point showed no difference in pregnancy loss between the materials when performing a vaginal CC. Following conclusion of the C-STICH RCT we can be relatively confident that the use of a monofilament suture is unlikely to have a substantial impact on pregnancy loss compared to the use of a braided suture. The uncertainty around our comparative estimate for the pregnancy loss outcome is, at most, 2% in favour of monofilament in absolute terms. The prevention of pregnancy loss means that a small difference in outcomes would still be important clinically, and while this margin may not completely rule out missing a clinically important difference, we consider this scenario to be unlikely.
This was a well-conducted trial which has sought to establish the optimal suture thread with scientific rigour. One of the strengths of this trial is the appropriate increase in the sample size, implemented following the independent DMEC advising the TMG of a lower-than-anticipated pooled event rate during the trial and, thus, recommending the sample size was increased from 900 to 2050 women to ensure a conclusive trial was delivered. In the original retrospective study that was used to inform the sample size, pregnancy loss rates were between 12% and 9%. 15 Within C-STICH, the pregnancy loss rate was 8%. There are several possible reasons why the observed event rate was lower than anticipated. One reason is that women with complicated pregnancy histories who were perceived to be at higher risk of pregnancy loss were not approached about the trial, although from our screening logs and monitoring with sites we identified high levels of approach to women undergoing a CC. Alternatively, this lower rate could represent improvements in outcomes secondary to changes in preterm prevention in the UK with the Saving Babies Lives Care Bundle version 2 advocating care within specialist clinics and the expanded use of cervical length screening. It may also represent an increased use of CC in women who have a low background risk of pregnancy loss. The rate within the trial is consistent with the 2017 Cochrane review of CC where the pregnancy loss rate in the cerclage group was 10.7%, with this review being published during the trial. 11 There were no differences between the suture threads in terms of time from conception to pregnancy end and the prevention of prematurity, with 9% of deliveries occurring before 32 weeks and 28% of deliveries before 37 weeks.
We identified a decreased risk of maternal sepsis in the monofilament group (4%) compared with the braided group (7%) and a decreased risk of chorioamnionitis, of 3%, in the monofilament group compared with 6% in the braided group. The decreased risk of infection in the monofilament group is in keeping with the hypothesis that braided sutures are reservoirs for bacteria that predispose to infection. 14 This potential reduction in infectious morbidity could have important health benefits for mothers. A systematic review evaluating the effect of chorioamnionitis on maternal morbidity identified increased risks of haemorrhage, operative interventions and HDU admission. 23 This potential benefit in reduced infectious morbidity must be considered relative to the increase in the rate of difficulty in removing the suture (usually close to term) and the need for an anaesthetic for removal.
There were no detectable differences in neonatal outcomes between the two suture threads. The neonatal outcomes collected in this trial are rare neonatal outcomes related to prematurity, and the trial was not powered to detect differences between the two groups. Maternal chorioamnionitis is associated with an increased risk of both early and late neonatal sepsis,23 yet this trial did not detect a difference in clinically diagnosed neonatal sepsis between the monofilament group (11%) and the braided group (13%) [RR 0.90 (95% CI 0.72 to 1.11)]. There was also no significant difference in the proportion of neonates with confirmed neonatal sepsis. There were high levels of neonatal antibiotic use within both groups at 26–27%. The longer-term neonatal consequences of chorioamnionitis are unclear in the medical literature, with some studies reporting an increased risk of adverse neonatal complications and others demonstrating no difference, with most studies only finding a link between histologically confirmed chorioamnionitis and worsening infant neurodevelopmental outcomes. 22 Infection in combination with prematurity confers an additional risk of cerebral palsy and a worsening of neurodevelopmental outcomes. There were no long-term neurodevelopmental outcomes collected within C-STICH and, therefore, limited conclusions can be drawn from this finding at present. There is an opportunity within C-STICH to seek funding to evaluate longer-term paediatric outcomes. The maternal infections are outcomes that could be subjected to bias, as the outcome collectors were unable to be masked to the suture thread used and may have been biased in diagnosing infection based upon the hypothesis of the trial.
Cerclage insertion complications included a low rate of ruptured membranes during insertion, occurring in two cases; this is in keeping with the anticipated rate. 8 These data can support the counselling of women, with the risk of rupture of membranes during cerclage insertion at approximately 1 : 1000 where the suture is placed electively or on the basis of a short cervix seen on ultrasound. Removal complications for the monofilament group include an increase in clinician-reported difficulty in removing the suture, an increased requirement for anaesthetic and an increase in cervical tears during removal (2% vs. 1%). There were two cases of retained thread in the monofilament group and one case of retained thread in the braided group; these required removal postnatally and this was an unexpected complication of a CC identified through the SAE process. These insertion and removal complications are already-known risks of CC, particularly where a Shirodkar technique is employed, which by definition buries the knot of the suture, but there are limited data published to accurately inform counselling. Therefore, this trial will improve the counselling of women undergoing a CC procedure due to the generalisability and reliability of the trial, having recruited a large proportion of all CCs being placed within units across the UK.
The surgical technique for CC placement was included as a minimisation criterion within C-STICH to ensure balance between the treatment groups in whom bladder dissection was planned. It was hypothesised that a bladder-dissected cerclage would result in a lower pregnancy loss rate, since the placement of a cerclage is closer to the cervical internal OS. In the planned key subgroup analysis of planned bladder dissections, there was evidence of a potential differential treatment effect. In women where it was planned to dissect their bladder during the CC, the rate of pregnancy loss was lower in the monofilament suture group than in the braided group; however, the effect within this stratum was not statistically significant. The result must be interpreted with caution in view of the small number of women within this analysis and its exploratory nature. This is an important area of future research and any reduction in pregnancy loss in this high-risk group is clinically important. Given the low event rate, a further RCT to evaluate this would not be feasible and, therefore, we would recommend utilising a large observational cohort or registry approach.
There was no evidence of a differential treatment effect for concomitant progesterone treatment in our pre-defined subgroup analysis. Whether or not concomitant progesterone is a predictor of pregnancy loss is a prioritised research recommendation within the NICE preterm birth guideline,10 and this trial did not evaluate this. Therefore, we would recommend further research within this cohort utilising the data collected within the C-STICH trial to inform predictors of pregnancy loss and preterm birth.
Study strengths
This study is the largest RCT evaluating suture materials during a vaginal CC. A total of 2049 women, from 56 trusts and 75 individual sites within the UK, were randomised to have their cerclage performed with either monofilament or braided suture threads. The centres varied in their size (related to the number of births and, thus, the number of sutures performed), geographical location and the characteristics of their populations. Standard care was conducted according to national guidance and, thus, the results are generalisable to the UK population.
This trial was robustly conducted with central randomisation and included important minimisation criteria that has allowed a valid comparison to be made between the groups. The primary outcome and the majority of the secondary outcomes collected during the C-STICH trial were objective (rather than subjective descriptions) which allowed a true comparison between the groups with minimal bias. We had a low attrition rate. Primary outcome data were available for 98% of those randomised, and an exploration of the impact of this minimal data loss were undertaken to support the ITT analysis.
Limitations and critique
In our opinion, the trial was designed and conducted in a methodologically robust manner. Nevertheless, there are some limitations of the study that should be considered. There were no long-term infant and paediatric outcomes collected and we did not collect data on the outcomes for women who did not take part in the C-STICH trial. The trial could have been strengthened by further considering the effect of suture thread colonisation on outcomes other than pregnancy loss and preterm birth.
Patient and public involvement
In the C-STICH trial, patient and public involvement (PPI) was utilised in the study design, development and monitoring. We powered the trial to evaluate the difference between suture threads in terms of evaluating pregnancy loss, which is the outcome that was considered the most important following our PPI work. We have continued to receive support from our PPI representatives and their voice was essential in supporting the increase in the sample size to ensure a powered trial and in reopening the trial and extending recruitment timelines to allow us to reach sample size. The trial was supported by patient advocacy groups such as the Miscarriage Association and Tommy’s. We aim to develop infographics explaining the results of the trial to support dissemination.
Generalisability
The sites participating in C-STICH were geographically spread across the UK and included large preterm birth centres performing significant numbers of cerclages and small sites performing less frequent cerclages. This improved the generalisability of the results for women. Women in the trial also had a broad range of indications for CC, which reflected the population of women who have a CC to reduce pregnancy loss. The exclusion criteria were kept to a minimum and the heterogeneity of the population was reflected in the characteristics of the trial participants.
Chapter 5 Conclusions
This study set out to establish whether there was any difference in pregnancy loss rates in women undergoing CC comparing a monofilament and braided suture material. The hypothesis of the study was that braided sutures would harbour bacteria, predisposing to infection, pregnancy loss and premature birth. The trial showed no evidence of differences in pregnancy outcomes between the suture groups. Therefore, clinicians can be justified in using either suture thread when performing a vaginal CC, based on clinical judgement and the individual scenario. We observed an increased risk of maternal sepsis and chorioamnionitis within the braided group. We did not see this translate into differences in neonatal outcomes with regard to clinically diagnosed sepsis or histologically confirmed sepsis. Maternal chorioamnionitis is associated with increased adverse outcomes in mothers and this may influence clinicians’ decision-making when determining whether to perform a CC with a monofilament or braided suture thread. 23 Additionally, there was an increase in removal complications in the monofilament group, mainly the need for anaesthetic for removal.
Implications for health care
We would advocate that clinicians consider these findings when deciding which suture thread to use during a vaginal CC.
Recommendations for research
We would recommend the long-term follow-up of neonates born within the C-STICH trial to determine any difference in long-term neurodevelopmental outcomes.
In a post hoc exploration of the data, there was a suggestion that a planned bladder-dissected cerclage with a monofilament suture thread had a lower pregnancy loss rate of 4% than the overall pregnancy loss rate of 8%. This could be a potentially important reduction in pregnancy loss, and it is important to investigate this further to determine the optimal cerclage technique for use within clinical practice.
There are several co-interventions utilised during the cerclage procedure, including antibiotics, tocolytics and progesterone, that need to be further evaluated to determine the optimal management of women undergoing CC.
Additional information
Contributions of authors
Victoria Hodgetts Morton (https://orcid.org/0000-0001-9817-4313) was the research fellow on the trial and was involved with the design, implementation and recruitment, and was responsible for authoring this manuscript.
Catherine A Moakes (https://orcid.org/0000-0002-0473-0532) was the trial statistician and was responsible for the analysis of the trial data and for co-authoring this manuscript.
Jane Daniels (https://orcid.org/0000-0003-3324-6771) was involved with the design of the trial and provided trial oversight and a critical review of the manuscript.
Lee Middleton (https://orcid.org/0000-0003-4621-1922) was the senior statistician for the trial and was responsible for the analysis of the trial data and for co-authoring this manuscript.
Andrew Shennan (https://orcid.org/0000-0001-5273-3132) was involved with recruitment to the trial and provided expert advice on preterm birth prevention and co-authored the manuscript.
Peter Brocklehurst (https://orcid.org/0000-0002-9950-6751) provided senior input to the design of the trial and co-authored the manuscript.
Fidan Israfil-Bayli (https://orcid.org/0000-0002-4751-8857) designed the trial protocol and secured funding for the trial.
Andrew K Ewer (https://orcid.org/0000-0002-3825-4781) was a co-applicant for the trial and was responsible for the design advising on neonatal outcomes and contributed to the critical review of the manuscript.
James Gray (https://orcid.org/0000-0003-4169-3141) provided microbiological advice to the trial and co-authored the manuscript.
Nigel AB Simpson (https://orcid.org/0000-0002-0758-7583) was involved with recruitment to the trial and provided expert advice on preterm birth prevention and co-authored the manuscript.
Jane E Norman (https://orcid.org/0000-0001-6031-6953) was involved with recruitment to the trial and provided expert advice on preterm birth prevention and co-authored the manuscript.
Christoph Lees (https://orcid.org/0000-0002-2104-5561) was involved with recruitment to the trial and provided expert advice on preterm birth prevention and co-authored the manuscript.
Konstatinos Tryposkiadis (https://orcid.org/0000-0002-2516-1180) was the original statistician.
Clive Stubbs (https://orcid.org/0000-0003-2690-6583) provided governance oversight and supported the trial to continue to reopen for recruitment post pandemic.
Max Hughes (https://orcid.org/0000-0001-7058-0109) was the trial co-ordinator for the trial.
R Katie Morris (https://orcid.org/0000-0003-1247-429X) provided senior input into the trial design and delivery and has oversight of the analysis as director of the clinical trials unit.
Philip Toozs-Hobson (https://orcid.org/0000-0002-1859-9934) was the chief investigator for the trial; he conceived the project, supervised the COTS study and was involved with the design, implementation and oversight of the trial, and provided oversight of the manuscript.
Acknowledgements
The final protocol version was v9.0 dated 25 March 2020. The full trial protocol is available online at the NIHR Journals Library and at the C-STICH website.
We would like to thank all the women who agreed to take part in this study, without whom this would not have been possible.
C-STICH has been supported by a trial steering committee and data monitoring committee and we would like to express our sincere thanks for all their guidance, expertise and advice. The TSC members include Professor Harry Gee (chair), Professor Christine Kettle, Professor Jon Dorling, Mr Andrew Elders, Dr Ruth Curry, Ms Sarah Machin and Ms Joanne Deery. The DMC members include Professor Marian Knight (chair), Professor Doug Tincello (withdrew when a potential conflict of interest arose due to working at the recruiting trust), Professor John Norrie, Mr Richard Smith and Professor Graeme Maclennan.
C-STICH has been supported by a large network of sites, principal investigators, research midwives, research nurses, sonographers, clinical data collectors, consultants, specialty doctors, research fellows and lecturers; although too extensive to mention individually, we are extremely grateful for their support.
We would like to personally thank our PPI representatives, Ms Sarah Machin and Ms Joanne Deery.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
The trial had a favourable ethical opinion from the National Research Ethics Service (NRES) Committee Cambridgeshire and Hertfordshire obtained in February 2015.
Information governance statement
Birmingham Women’s Hospital (sponsor) and the University of Birmingham is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, University of Birmingham is the Data Processor; Birmingham Women’s Hospital is the Data Controller and we process personal data in accordance with their instructions. You can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for University of Birmingham’s Data Protection Officer here: https://www.birmingham.ac.uk/documents/university/legal/university-of-birmingham-data-protection-policy.pdf
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/YKTW8402.
Primary conflicts of interest: Victoria Hodgetts Morton is a previous NIHR clinical lecturer, NHS England regional preterm birth lead (Midlands), has received an honorarium from Hologic and is a co-investigator on NIHR grants C-STICH2 (16/141/01) and CHAPTER. Jane Daniels declares membership of the NIHR Clinical Trials Unit Standing Advisory Committee (1/5/2016 to 30/09/2023). Jane E Norman declares membership of the HTA MNCH panel, currently receives funding from the NIHR EME programme, is chair of the Global Health Research Funding committee and Wellcome Trust Science panel and participates in NIHR and Wellcome trust data safety and trial steering committees; she also participates in a data monitoring and ethics committee for GlaxoSmithKline and is a paid consultant for DILAFOR; Jane E Norman is also non-executive director at University Hospitals Bristol and Weston and receives funding from grants for NIHR HTA (17/22/02) and STOPPIT-3. R Katie Morris currently receives funding from NIHR HTA (CSTICH2 16/151/01) and programme grants for applied health research schemes; she is a steering committee member of the Saving Babies Lives Care Bundle and a paid clinical advisory board member for Surepulse (a company designing neonatal monitors). R Katie Morris is also a member of the Tommy’s, RCOG and WOW scientific advisory committees (chair of the Preterm Birth clinical study group) and a member of the RCOG research and academic committees. Andrew K Ewer is President of the Neonatal Society and receives honoraria from Medtronic for educational events. James Gray is a co-investigator on NIHR call 17/86 ‘The clinical and cost effectiveness of screening for Group B Streptococcus (GBS) in pregnancy’ (NIHR HTA reference number: NIHR134534) and a co-investigator with the Healthcare Infection Society [Walker K, et al. (2020) ‘Maternity services response to the COVID-19 pandemic: how PHE guidance was implemented and what we can learn for the future’]. Nigel AB Simpson is a member of the research advisory board for Tommy’s. Andrew Shennan is a previous member of the NIHR commissioning committee. Peter Brocklehurst has been employed by clinical trials units receiving funding from the NIHR and is a past member of several NIHR boards (HTA Efficient Study Designs, HTA MNCH Methods Group, HTA MNCH Panel, HTA Post-funding Committee and HTA Commissioning Committee).
Publication
Hodgetts Morton V, Toozs-Hobson P, Moakes CA, Middleton L, Daniels J, Simpson NAB, et al. Monofilament suture versus braided suture thread to improve pregnancy outcomes after vaginal cervical cerclage (C-STICH): a pragmatic randomised, controlled, phase 3, superiority trial. Lancet 2022;400(10361):1426–36.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Preterm birth: what can be done?. Lancet 2008;371.
- Office for National Statistics . Birth Characteristics in England and Wales: 2014 2015.
- Berghella V. Every 30 seconds a baby dies of preterm birth. What are you doing about it?. Am J Obstet Gynecol 2010;203:416-7.
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760-5.
- Phillips C, Velji Z, Hanly C, Metcalfe A. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open 2017;7.
- Owen J, Hankins G, Iams JD, Berghella V, Sheffield JS, Perez-Delboy A, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol 2009;201:375.e1-8.
- Kyrgiou M, Athanasiou A, Kalliala IEJ, Paraskevaidi M, Mitra A, Martin-Hirsch PP, et al. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst Rev 2017;11.
- Final report of the Medical Research Council/Royal College of Obstetricians and Gynaecologists multicentre randomised trial of cervical cerclage. MRC/RCOG Working Party on Cervical Cerclage. Br J Obstet Gynaecol 1993;100:516-23.
- Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev 2012;18.
- National Institute for Health and Care Excellence (NICE) . Prevention of Preterm Birth 2015.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev 2017;6.
- Berghella V, Ludmir J, Simonazzi G, Owen J. Transvaginal cervical cerclage: evidence for perioperative management strategies. Am J Obstet Gynecol 2013;209:181-92.
- Durdey P, Bucknall TE. Assessment of sutures for use in colonic surgery: an experimental study. J R Soc Med 1984;77:472-7.
- Kindinger LM, MacIntyre DA, Lee YS, Marchesi JR, Smith A, McDonald JA, et al. Relationship between vaginal microbial dysbiosis, inflammation, and pregnancy outcomes in cervical cerclage. Sci Transl Med 2016;8.
- Israfil-Bayli F, Toozs-Hobson P, Lees C, Slack M, Ismail KMK. Pregnancy outcome after elective cervical cerclage in relation to type of suture material used. Med Hypotheses 2013;81:119-21.
- Israfil-Bayli F, Toozs-Hobson P, Lees C, Slack M, Ismail K. Cerclage outcome by the type of suture material (COTS): study protocol for a pilot and feasibility randomised controlled trial. Trials 2014;15.
- Israfil-Bayli F, Toozs-Hobson P, Lees C, Slack M, Daniels J, Vince A, et al. Cervical cerclage and type of suture material: a survey of UK consultants’ practice. J Matern Fetal Neonatal Med 2014;27:1584-8.
- Israfil-Bayli F, Morton VH, Hewitt CA, Ewer AK, Gray J, Norman J, et al. C-STICH: cerclage suture type for an insufficient cervix and its effect on health outcomes – a multicentre randomised controlled trial. Trials 2021;22.
- van ‘t Hooft J, Duffy JM, Daly M, Williamson PR, Meher S, Thom E, et al. A core outcome set for evaluation of interventions to prevent preterm birth. Obstet Gynecol 2016;127:49-58.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer 1977;35:1-39.
- DAMOCLES Study Group, NHS Health Technology Assessment Programme . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22.
- Hodgetts Morton V, Toozs-Hobson P, Moakes CA, Middleton L, Daniels J, Simpson NAB, et al. Monofilament suture versus braided suture thread to improve pregnancy outcomes after vaginal cervical cerclage (C-STICH): a pragmatic randomised, controlled, phase 3, superiority trial. Lancet 2022;400:1426-36.
- Beck C, Gallagher K, Taylor LA, Goldstein JA, Mithal LB, Gernand AD. Chorioamnionitis and risk for maternal and neonatal sepsis: a systematic review and meta-analysis. Obstet Gynecol 2021;137:1007-22.
List of abbreviations
- BMI
- body mass index
- CC
- cervical cerclage
- CI
- confidence interval
- COTS
- cerclage outcome by the type of suture material
- CRF
- case report form
- C-STICH
- cerclage suture type for an insufficient cervix and its effects on health outcomes
- CTIMP
- clinical trial of an investigational medicinal product
- CTU
- clinical trials unit
- DMEC
- data monitoring and ethics committee
- HDU
- high dependency unit
- IQR
- interquartile range
- ITT
- intention to treat
- LLETZ
- large loop excision of the cervical transformation zone
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NICU
- neonatal intensive care unit
- PPROM
- preterm prelabour rupture of membranes
- PPI
- patient and public involvement
- RCT
- randomised controlled trial
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RR
- risk ratio
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SCBU
- special care baby unit
- SD
- standard deviation
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- trial management group
- TSC
- trial steering committee
- TVUS
- transvaginal ultrasound scanning
- WOW
- Wellbeing of Women
