Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 16/91/04. The contractual start date was in March 2018. The draft manuscript began editorial review in April 2023 and was accepted for publication in November 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Snooks et al. This work was produced by Snooks et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Snooks et al.
Chapter 1 Introduction
Parts of this chapter have been reproduced from Jones et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
In England, over half of drug deaths involve opioids, and death by drug overdose has increased since 2012. 2 In the UK, accidental overdose related to the misuse of opioid drugs (such as heroin, methadone, fentanyl and morphine) is an increasingly prevalent public health problem. 3–5 The number of deaths involving heroin and/or morphine doubled between 2012 and 2015 to the highest on record. 6 The rise in heroin-related deaths has not only caught the attention of the research community but has also received coverage from the popular press – in the UK7,8 and abroad. 9
People who misuse either illicit or prescription opioids are at an increased risk of non-fatal overdose, subsequent hospital or emergency service (ES) utilisation, and death. 10–12 Non-fatal opioid overdose is associated with long-term morbidity and increased demand on health services. 13–15 ES contact for drug-related morbidity has been found to be a predictor of future episodes of poisoning or overdose. 16,17
Naloxone is an effective fast-acting opioid antagonist used to treat opioid overdose. 18 Naloxone blocks opioid receptors to counteract the effects of opioid drugs. It reverses the life-threatening effects of an overdose such as depressed breathing, and has no psychoactive properties or intoxicating effects. 19
Naloxone can be supplied to people at risk of opioid overdose by paramedics or by laypeople in the form of take-home naloxone (THN). 20 However, the safety of naloxone in community settings is unclear. Typically, a THN kit comprises one or more doses of naloxone, an intramuscular (IM) needle and syringe for injecting the dose, and written or pictorial instructions to explain how to prepare and administer the dose and perform basic life support, and the importance of calling the ES. These materials may also describe the duration of effect, and hence why it is important that paramedics attend the patient as soon as possible; the safety of naloxone in terms of adverse events and overdose; and the legality of bystander administration of naloxone.
Non-experimental studies suggest that THN programmes which involve the training of laypersons to administer a naloxone dose in cases of overdose emergency are safe and effective. 21–23 THN kits can be used by people without formal medical training in the event of an opioid overdose. Increased access to THN kits via specialist drug services in the UK and internationally has been motivated by recommendations from influential bodies, including the World Health Organization (WHO) and the British Advisory Council on the Misuse of Drugs (ACMD). 24,25
Numerous THN distribution programmes aiming to reduce death from opioid overdose have been implemented by drug service providers in the UK and internationally since the 1990s. 26,27 However, a significant proportion of people at risk of opioid overdose do not engage with these services. 28 Additionally, high-quality empirical evidence to demonstrate the safety and effectiveness of THN is sparse. Observational data suggest that non-serious adverse reactions to naloxone administration are common while serious adverse reactions are rare. 29,30 However, the risks of inadequate response or return to a state of overdose following the administration of naloxone by laypeople remain poorly quantified. 31,32 Moreover, the uptake of THN kits in at-risk populations remains low33,34 and appropriate THN intervention by peers and witnesses may not be optimal. 35
Members of the research team (CM, HS) have previously conducted a randomised feasibility study of THN distributed through the emergency ambulance service (AS) in a single urban geographic area. 36 Their experiences, consistent with those of other researchers,37,38 have demonstrated that using traditional methods (e.g. telephone or postal methods) for capturing follow-up outcomes of participants in receipt of a THN kit (and of those not in receipt of a THN kit despite eligibility) is not feasible.
Rationale
The theory underpinning THN provision as an intervention is that by distributing a readily administrable dose of naloxone to people likely to witness opioid overdose, naloxone would be administered to victims of overdose earlier post onset of symptoms than standard care (administration of naloxone by health professional on ambulance or in ED), thus improving survival. Currently, THN provision initiatives, primarily aimed at reducing incidence of fatal heroin overdose, usually take place in non-clinical environments such as third-sector drug services or prison, rather than in emergency care settings. This means that a proportion of those at risk of opioid overdose who do not attend drug services or who are not completing a sentence of imprisonment have limited access to THN kits. Based on figures from England, Wales and Scotland, it appears that this is a sizeable population. In 2017, it was estimated that there were 340,000 high-risk opioid users in England and Wales, and 149,420 people receiving opioid substitution treatment. 39 Between 2017 and 2018 in Wales, 25,190 individuals accessed a needle exchange service, of which opioids was the primary use for 48%. 40 In comparison, only 2896 THN kits were supplied for the same year, of which 1372 were supplied to individuals for the first time and 533 were reportedly used. 41
In Scotland in 2017–8, a total of 6924 THN kits were issued in the community, of which 2458 were thought to have been issued to individuals for the first time. 42 To put this into context, figures for 2015–6 in Scotland show an estimated 55,800 to 58,900 users of opioids and/or benzodiazepines. 43 These data tell us that saturation of THN kits among opioid users in the general population remains low. However, the distribution and receipt of THN kits in communities around the country is increasing – for example, in 2019–20, Scotland had a 44.3% increase, England’s THN distribution increased by 13.8% whereas Wales’s distribution stayed the same at an average of 389 kits a month. 44 This increase in uptake underlines the urgency of establishing the safety and effectiveness of THN provision. Despite the growth in THN provision initiatives and implementation programs, questions have been raised regarding potential harms associated with the administration of naloxone to lay people in non-clinical settings. Concerns include adverse events such as acute opioid withdrawal,31,32 and an increase in high-risk drug-taking behaviour (due to the availability of an apparent ‘safety net’) or other unforeseen methods of indirectly abusing naloxone. 45 Furthermore, there is a possibility of naloxone dose being insufficient, or wearing off too quickly, resulting in patients requiring cardiopulmonary resuscitation (CPR) or further treatment and not receiving this. 31,46 Tse et al. ’s47 systematic review of 7 studies with 2578 participants found no evidence that THN provision increased opioid use. Of the included studies which reported overdose frequency, three of the studies saw no change in overdose rate and one found a decrease. Nonetheless, a previous study found no difference in the number of ambulance call outs before or after THN implementation, suggesting that community-based THN programmes do not result in a reduction in ambulance calls for overdose, that overdose rates do not increase, and that users do not become less likely to call 999. 48 However, as the study used anonymised linked ambulance dispatch data, it was not possible to assess whether an increased number of overdoses took place where no 999 call was made.
There is currently limited published evidence regarding THN provision in emergency settings and, with little evidence from RCTs, the safety, wider effects on drug-taking behaviour, and cost-effectiveness of THN provision initiatives in the community are unknown. However, evidence suggests that THN provision programs in emergency settings are acceptable to our target population, and we can therefore expect favourable recruitment among paramedics. 38 Members of our study team completed the recruitment phase of a feasibility study of THN provision to patients following attendance and resuscitation by emergency ambulance paramedics for opioid overdose. 36 We used a cluster stepped wedge design and randomly selected paramedics in one urban centre in south Wales. We randomised paramedics to the week in which they would receive training and THN kits over the first 4 months of the 12-month recruitment period, so that all paramedics had the opportunity to deliver the intervention during the study. Eligible patients attended by participating paramedics during the period before they were trained and allocated THN kits were allocated to the control group, while eligible patients attended by paramedics who had received training and THN kits were allocated to the intervention group. We published preliminary results related to recruitment, follow-up and outcomes. 49 Eighty-five of 102 eligible paramedics took part. The number of opioid-related emergency ambulance contacts (215) exceeded those predicted (100–120) in 12 months. Of 182 cases attended by study paramedics, 148 were attended by paramedics who had been trained and issued with THN kits (intervention group). Thirty-five of 55 patients eligible for the intervention (sufficiently recovered and able to consent) were offered it. Twenty-five accepted, received training from the paramedic, and were given a THN kit; a 71% acceptance rate. Follow-up of participants for self-reported outcomes proved challenging. Of the 215 events for which we have data, 58 were repeat episodes involving 25 individual users. The number of repeat encounters experienced by these individuals ranged from two to five. Six deaths were recorded during the study period.
In addition to this work, Kestler et al. 50 carried out an anonymous survey study in the ED in which patients completed a questionnaire regarding opioid use and were offered THN kit plus training after completion. Two-thirds accepted THN, a similar proportion to that seen in our team members’ feasibility study, and multivariate analysis identified injecting drug use as a factor associated with acceptance of THN. In comparison, researchers at Boston University in the USA carried out a feasibility study of the distribution of intranasal (IN) THN via a drug outreach service based in an ED. 37 They did not use linked data but took contact details for 415 participants seen by the service. Of these 415, 12.3% (n = 51) completed follow-up by telephone and 9% (n = 37) accepted the THN kit, using IM naloxone – a much lower acceptance rate than previously reported. Of those who had received a THN kit and completed follow-up, six reported administering the naloxone.
In summary, though the efficacy of naloxone in reversing opioid overdose is established, the effectiveness of THN provision as an intervention – in either community or ES settings – is unknown. This state of equipoise warrants investigation using experimental methods. We tested the feasibility of carrying out a definitive randomised trial to evaluate safety, clinical effectiveness and cost-effectiveness of THN provision to opioid users at risk of overdose in emergency settings. In this feasibility trial, at randomly selected sites, THN was distributed to patients identified by emergency ambulance paramedics or ED clinical staff as actively taking opioids. Using this approach, we aimed to reach the very highest-risk group, who may not be registered with drug services or general practitioners (GPs). Of drug users in the UK, opioid users have the lowest rates of GP referral to drug services and have been found to be most likely to report insecure housing arrangements,51 representing a barrier to GP access. 52 The ES may be the only healthcare system that this vulnerable and underserved patient group ever access. Testing whether THN can be distributed through such settings to help minimise the risk of preventable death is an important step in establishing an evidence base for distribution of THN for peer administration of naloxone to people in an overdose.
Research aims and objectives
Feasibility study aim
To determine the feasibility of carrying out a fully powered RCT of THN in emergency settings using anonymised linked data to capture outcomes.
Feasibility study objectives
To establish:
-
the best form of THN kit, training and delivery, based on previous experience, evidence, specialist (addiction, emergency care) and service user advice
-
using agreed progression criteria, whether a trial clustered by ED catchment area and associated AS is deliverable.
In order to satisfy our study objectives, we answered the following questions:
-
Can we recruit paramedics and ED staff to trial participation?
-
Is the THN intervention acceptable to service users and practitioners?
-
Can we identify and retrieve (linked routine data) outcomes for two population groups:
-
those eligible for THN kit provision by paramedics or ED staff?
-
a further group who may receive (peer-administered) THN following an overdose?
-
-
What outcomes should we include in a full trial; how should its primary outcome be defined and in what form should it be presented for analysis?
-
What difference in primary outcome would be clinically important and justify the costs and burden on emergency care staff needed to facilitate widespread implementation of THN kit provision?
-
What sample size (and number of clusters) would we need to achieve in a full trial to be confident of detecting a specified THN intervention effect – should it exist – in this primary outcome?
-
Are there any important safety effects of distributing THN kits that we should consider (e.g. increase in risk-taking behaviour or non-fatal overdoses), and can we retrieve data on these?
-
What are the barriers and facilitators to implementation of this THN intervention in emergency settings?
-
What are the patient and peer experiences at intervention sites?
Progression criteria
We assessed whether or not to proceed to a fully powered RCT using the following progression criteria, informed by the previous Cardiff-based feasibility study (CM, HS),35,48 and the Trial Steering Committee (TSC). We used a ‘traffic-light’ system to judge progress against each criteria.
Green: indicates that we have either met a criterion (in which case no modifications to the relevant aspect of the study protocol may be needed), or we are within 10% of our stated progression targets (in which case we reviewed the reasons for this and considered appropriate modifications to study methods).
Amber: indicates that we are within 20% of our stated progression target, in which case we critically reviewed reasons for this and assess whether major changes to study methods are likely to realise significant improvements.
Red: indicates that we are more than 20% from our target, in which case we would not, in the absence of clear extenuating circumstances, consider progression to a full trial.
All percentage changes were measured as relative to target.
Intervention feasibility criteria
-
Sign-up of four sites, including ≥ 50% of eligible staff to complete training in delivering the intervention at each intervention site.
-
Identification of ≥ 75% of people who have presented to ED or associated AS with opioid overdose or an opioid use-related problem over a 12-month period.
-
THN kits issued to ≥ 50% of eligible patients over a 12-month period at intervention sites.
-
Serious adverse event rate [to be defined in agreement with the Data Monitoring and Ethics Committee (DMEC)] of no more than 10% difference in intervention sites to control sites at the conclusion of recruitment.
Trial methods feasibility criteria
-
Identification and inclusion for follow-up of ≥ 75% of people who died of opioid poisoning in the following year in the study areas according to ONS mortality data (previous ONS data suggest between 140 and 180 such deaths across the 4 participating sites during the study period).
-
Matching and data linkage in ≥ 90% of cases not dissented at the conclusion of quantitative data collection.
-
Retrieval of primary and secondary outcomes from NHS Digital and Digital Health and Care Wales (DHCW) within 6 months of projected timeline.
Chapter 2 Methods
Parts of this chapter have been reproduced from Jones et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Study design
We carried out a randomised feasibility trial in the emergency care environment, involving study sites defined geographically as an ED and its catchment area within the local emergency AS.
We were unlikely to know if the naloxone dose included in any individual THN kit was administered to a peer of the recipient of the kit or to the recipient him/herself. Effects of the THN intervention could extend beyond recipients seen in the ED or by ambulance crews. In order to measure treatment effect in those likely to benefit from THN, we needed to define a wider population – those at high risk of death from opioid overdose in the general population at intervention and control study sites. We undertook work, therefore, to develop a discriminant function to identify cohorts to include in outcome comparisons.
Alongside this work, we carried out a RCT clustered by study site. We also collected qualitative data to gain an understanding of the processes of implementation of the intervention and experiences of service users and providers and assessed the feasibility of costing the intervention and its effects.
Study setting
We conducted the feasibility study in the emergency departments (EDs) and their catchment areas within the local emergency AS. The following sites were randomly allocated to intervention or control in ‘pairs’: Bristol Royal Infirmary and South Western Ambulance Service NHS Foundation Trust; Hull Royal Infirmary and Yorkshire Ambulance Service NHS Trust; Northern General Hospital Sheffield and Yorkshire Ambulance Service NHS Trust; Wrexham Maelor Hospital and Welsh Ambulance Service NHS Trust.
Development of the discriminant function
We intended to identify for inclusion in outcome follow-up people at high risk of fatal opioid overdose who may benefit from naloxone from a THN kit. We attempted to define a discriminant function, similar to a risk index, incorporating known and routinely recorded predictors of opioid-related deaths. We used existing linked data about opioid deaths in Wales, including ED and inpatient data, to select predictors closely associated with those who died from opioid poisoning. We then intended to use these predictors in our discriminant function to identify participants in the study site areas to be included in the ‘high-risk population’ for outcome analyses. We previously carried out scoping of NHS Wales ED and hospital routine datasets and linked ONS mortality records with these datasets. We found that we were able to describe circumstances of death for opioid overdose decedents who had visited EDs prior to their death, as well as describe service usage over a prolonged observation period. Mortality data were of high quality, as were data items on times and dates of attendances, outcomes of attendances and demographic characteristics of attendees. Diagnostic and treatment data were of lower quality.
Scope of routine data
This retrospective study was conducted using anonymised routine data for the period from 1 January 2015 until 30 November 2021, including both dates. De-identified individual-level data were provisioned from the secure anonymised information linkage (SAIL) Databank, and made available for analysis within the SAIL Gateway, a Trusted Research Environment. 53
Data sources
The starting point was the Welsh Demographic Service Dataset (WDSD), a centralised database which comprises records for all GP registrations in Wales. WDSD is used to define the study population.
The annual district death extract (ADDE) dataset, based on UK Office of National Statistics (ONS) mortality data, is a register of all deaths relating to Welsh residents, including those that die outside Wales. The ADDE dataset includes details on the cause of death using WHO International Classification of Diseases, tenth revision (ICD-10) codes; these codes are used to categorise deaths as related to an opioid overdose or not.
The Emergency Department Data Set (EDDS) captures attendances at accident and emergency departments and minor injury units (MIUs) in Welsh hospitals. Attendances by Welsh residents at EDs in other UK nations are not included.
The Patient Episode Database for Wales (PEDW) records all episodes of inpatient and day case activity in NHS Wales hospitals, includes planned and emergency admissions, minor and major operations, and hospital stays for giving birth. Hospital activity for Welsh residents treated in other UK nations (primarily England) was also included. PEDW includes clinical information [diagnoses, Operative Procedures ICD-10 and Office of Population Censuses and Surveys 4 (OPCS-4)], hospital admissions, spell and episode level data for all in-patients and day cases undertaken by NHS Wales. 54
The Critical Care Data Set (CCDS) captures all intensive and high-dependency care activity regardless of the patient’s area of residence.
The Substance Misuse Data Set (SMDS) captures data relating to individuals (both young persons and adults), presenting for substance misuse treatment in Wales. A summary of data sources and data sets used is available in Table 1.
| Source | Data items | Coding framework |
|---|---|---|
| WDSD | Age Gender Periods of residence in Wales WIMD Date of death (secondary) |
None |
| ADDE | Date of death (primary) Cause/s of death |
ICD-10 |
| EDDS | Dates of EDDS attendances Reason/s for attendance |
NHS Wales Data Dictionary |
| PEDW | Dates related to hospital admissions Reason/s for admission |
ICD-10 |
| CCDS | Dates related to critical care admissions Reason/s for admission |
NHS Wales Data Dictionary |
| SMDS | Dates related to presentations for substance misuse treatment Reason/s for presentation |
NHS Wales Data Dictionary |
Defining the discriminant function
We used WDSD files to define the study cohort. The WDSD comprises information on periods of residency in Wales, anonymised information on period and place of residency, its lower super output area (LSOA) and characteristics of that LSOA [including its Welsh Index of Multiple Deprivation (WIMD) quintile], and GP registrations. WDSD files also include a date of death, and anonymised linkage fields.
We then checked and reconciled WDSD and ADDE dates of death within the study window, taking ADDE data as primary. Deaths were then classified as related to opioid overdose (or not) using the coding framework outlined in Fuller et al. 55 We calculated an individual-level study end date, based on residency and mortality data and the study window end, forming demographic profiles using this study end date.
We next considered EDDS, PEDW, CCDS and SMDS attendances for the 36 months up to study end dates, also recording whether or not that attendance related to opioid overdose, based on an appropriate coding framework (where available; ICD-10 or NHS Wales Data Dictionary; see Table 2), and further recording whether or not the attendance occurred within 1 month or within 12 months of the study end date.
| ADDE status | Total | |||
|---|---|---|---|---|
| Dead | Alive | |||
| WDSD status | Dead | 233,495 | 9997 | 243,492 |
| Alive | 101 | 2,983,803 | 2,983,904 | |
| Total | 233,596 | 2,993,800 | 3,227,396 | |
We also derived a composite measure of health resource use, primarily to ascertain proportions of cohorts with no healthcare utilisation recorded in routine data within 1 month, 12 months and 36 months of their study end date.
We then used logistic regression to model the relation between the primary binary outcome (death from opioid overdose or not) and potential risk factors and covariates available from routine data sources, specifically age, gender, WIMD quintile and attendances (all, and opioid overdose-related) as recorded in and derived from EDDS, PEDW, CCDS and SMDS. Our primary analysis included data on all variables, based on attendances within 12 months of the study end date.
We undertook further analyses to assess the sensitivity of fitted models with respect to (1) the inclusion or otherwise of factors with high numbers of missing values, and (2) different versions of some explanatory variables. We finally assessed the feasibility of using fitted logistic regression models in identifying a high-risk population to include in outcome comparisons.
Testing the discriminant function
Due to delays with NHS Digital and low THN kit administration we did not apply the discriminant function, as we had intended, in a second data set from study sites in England.
Trial participants
Randomisation
We approached all UK ASs and received five positive responses from potential sites with matched EDs, who were able to demonstrate the capacity and resources to participate. Of these potential sites, four demonstrated sufficient geographic separation from other study sites to mitigate potential cross-contamination of study populations. From these four sites, we randomly selected two to be intervention sites and two to be control sites. A member of the research team (MJ) picked one set of study site allocations at random from the set of all possible allocations, each contained within separate sealed opaque envelopes.
Blinding
Due to the nature of the intervention, the study did not include blinding of participants or intervention providers.
Randomised controlled trial patient recruitment
Inclusion criteria
We included adult patients (18 +) who were attended by participating (trained) ambulance paramedics following a 999 call or ED clinicians for a problem related to opioid misuse (e.g. opioid overdose or injuries due to opioid use), who were assessed as having the capacity to consent to receipt of the kit and related training.
Exclusion criteria
Patients were excluded if they:
-
were under 18 years of age
-
were known to have previously suffered an adverse reaction to naloxone
-
lacked capacity
-
were aggressive or exhibited other challenging behaviours
-
were seen by untrained staff
-
had already been recruited
-
were in police custody at the time of presentation.
Interventions
Usual care
Treatment as usual (TAU) for suspected opioid overdose involves a clinical assessment during which the healthcare staff who first come into contact with the patient seek to confirm the substance or substances which led to the overdose. Opioid overdose is assumed if the substance which led to overdose is not known and the patient presents with an altered mental state, such as reduced consciousness, bradypnoea and miosis. Treatment includes prolonged and gradual administration of naloxone followed by a period of observation. Ideally, for patients attended by ASs, the treatment begins at the scene of the overdose and then continues at the ED following conveyance. However, patients may refuse to be conveyed should they respond to the naloxone at the scene.
There was no change in usual practice at the two control sites. Patients were not supplied Prenoxad by the ED or AS, although this may have been available from drug support services in the study site areas.
Intervention (experimental) arm: clinical staff training
Paramedics, nurses and doctors at intervention site EDs and ASs, registered with their respective professional bodies, were invited to participate in the study. Volunteer eligible staff members were trained in delivering the intervention in accordance with the study protocol. Patient group directions (PGDs) were established at participating services within intervention sites to allow non-prescribing paramedics and nurses to distribute THN kits. Training, provided in a flexible manner to suit the working practices of individual departments and services, involved face-to-face group-based training, complemented by a ‘cascade’ approach whereby research support paramedics and nurses continue to train their peers on an ad hoc basis. Online resources produced by Martindale Pharma were available as refresher content for staff (www.prenoxadinjection.com). Training per person was estimated to take up to 15 minutes. Staff completed and signed a ‘Record of Completion of Training’ form once they were deemed competent by their trainer.
Intervention arm: administration of THN kits and training
The Take-home naloxone Intervention Multicentre Emergency setting (TIME) intervention is described here according to the guidance for intervention description and replication (TIDieR) checklist. 56 Prenoxad is a multi dose THN kit containing 2 mg naloxone hydrochloride in 1 mg/1 ml solution for IM injection. This kit contains simple textual and pictorial instructions which reiterated the face-to-face training each participant receives as part of the intervention. Participants were not able to receive part of the intervention only — for example the kit but not the training — and therefore needed to consent to the whole intervention or decline the whole intervention.
The kit is manufactured by Martindale Pharma (Woodburn Green, UK) and supported by ‘train-the-trainer’ materials for participating paramedics and ED staff developed by Stephen Malloy, an independent consultant. Each Prenoxad kit retails at £21.00 before value-added tax (VAT). The decision to use the Prenoxad kit, as opposed to an IN alternative, is supported by evidence regarding the bioavailability of naloxone following IM versus IN administration,57,58 and the time taken for improvement in respiratory rate to be observable. 59 We also based our decision on feedback from drug service workers who were approached in the initial setting up of the study.
At intervention sites, participating healthcare professionals based in the specific ED or AS region and caring for patients eligible to receive the intervention offered these patients the THN kit, with an explanation of its purpose. Patients received TAU and then offered the intervention.
In the intervention arm, if the patient consented to receiving the kit, the healthcare professional provided training regarding the preparation and administration of the naloxone dose using the kit materials. The healthcare professional and the patient then completed a training checklist document which was stored as evidence that training was provided as part of the intervention.
Sample size
We expected to identify 200 records for individuals at high risk of overdose and thus eligible for the intervention (THN) in each of the four sites (n = 100 via ED; n = 100 via the corresponding AS n = 800 in total). Allowing for a dissent rate of 5%, we expected to identify prospectively for follow-up n = 760 people. We intended to use routine-linked data to identify a wider population via a discriminative function, which would be fully specified within our study. These individuals, regarded as at high risk of fatal overdose, would represent the peers of those attending during the recruitment phase. We expected the combined follow-up population to provide at least 1520 analysable outcomes. We did not carry out a power calculation for this feasibility study, which was not an attempt to assess the treatment effect, but our ability to do this in a full trial.
Participant consent
We did not attempt to gain consent to participate in the trial prospectively, at the time of attendance for opioid-related emergency, because that setting contradicts the requirements of informed consent. 60 We did not gather consent retrospectively, as the population is likely to be very difficult to reach and low contact rates could invalidate research findings. We did, however, consent patients to receive the intervention. Patients did this by signing a training sheet, giving their name and date of birth as part of this process.
As the wider population for inclusion in follow-up was to be identified through anonymised routine data sources, we would not have identifiable data with which to contact people for consent purposes. We offered the option to dissent from the research at all sites via patient information leaflets supplied with THN kits and made available at ED waiting areas. We also included this information on the Wales Centre for Primary and Emergency (including unscheduled) Care Research (PRIME) website www.primecentre.wales. We gained ethical, research and information governance permissions to allow this study to follow this approach, in which all information about processes and outcomes of care were anonymised to the research team except for clinical members at each site. The clinical researchers then split the identifiable data from clinical and operational study data before sending files separately to NHS Digital in England and DCHW in Wales for linkage to routinely held outcomes in ED, inpatient and mortality datasets held centrally. This split-file approach, in which identifiable and clinical data are separated, preserves patient anonymity. 53
For the qualitative component, we obtained written informed consent from all service users and healthcare professionals who participated in interviews and focus groups. Service user participants were identified by members of the NHS care team and third-sector drug treatment services. Participants were eligible to receive a thank-you gift card voucher with a monetary value of £10 for their time.
Outcomes
We measured outcomes related to the feasibility of the study in terms of the intervention and methodology, as reflected in the progression criteria.
The proposed primary outcome for a future trial was mortality (all deaths and those known to be opioid-related). Secondary outcomes included intensive treatment unit (ITU) admissions, ED-visits, and inpatient admissions (all visits/attendances as well as those known to be opioid-related), further 999 calls as well as THN kits issued and costs. Our feasibility study was not adequately powered to detect statistically significant differences in these proposed outcomes between intervention and control arms.
Qualitative study methods
We used qualitative data to explore the feasibility and acceptability of THN from the perspective of service users, based upon their previous knowledge and experience of overdose. We also explored the feasibility and acceptability of the intervention from the provider perspective by undertaking interviews and focus groups with paramedics, clinical ED-staff, and health service managers at participating sites regarding THN in emergency settings. We explored awareness and experiences of naloxone, perceived benefits and challenges of THN, and views on the feasibility and acceptability of distributing THN via ambulance paramedics and hospital EDs. Interviews were recorded, with participants’ consent, and professionally transcribed prior to analysis. We used normalisation process theory (NPT) to guide analysis of the provider data and to help understand how the intervention can be optimised within the ED and prehospital settings, and to explore whether difficulties in implementation were due to the intervention itself or other factors. 61
Service users
The perspectives of people with lived experiences of opioid use, accessing treatment centres or attending third-sector group counselling sessions were examined qualitatively in order to understand peoples’ knowledge and experience of naloxone, THN and overdoses. A semistructured topic guide was developed to explore issues identified within the literature around experiences of opioid overdose and emergency administration of naloxone by clinical staff and others, as well as experience of, and attitudes to, THN kits for use in overdose situations by peer opioid users or family and friends. Within this context, the study aimed to explore how experiences of opioid use and overdose experiences interact with knowledge, understanding, behaviour and attitudes around access to, and use of, THN kits to reduce risk of death. As well as aiming to develop a more comprehensive understanding of the facilitators and barriers to use of THN kits from an opioid user perspective, we examined specifically how participants felt about receiving THN kits and accompanying training from ED and ambulance or first responder staff.
Recruitment
Interviews were carried out in two drug treatment outpatient clinics (Sheffield and Hull) and one third-sector drug organisation (Bristol) in three major cities in the UK. Participants were referred by clinical staff if they were over eighteen and were either a current or a past drug user with experience of opioid injection use. Carers or partners of opioid users could also participate. All participants were given a patient information sheet and had the opportunity to discuss the research with staff and the researcher before consent was taken. Interviews took place in a private room. Before the start of the interview, consent was confirmed and the patient was informed they could stop the interview at any time and withdraw. At the conclusion of the interview, participants were given the opportunity to ask questions and provide additional information if they wished. All interviews were audio-recorded and transcribed verbatim to maintain data integrity and accuracy. Interviews lasted approximately 15–50 minutes. Interviews loosely followed the topic guide, which focused on the participant’s experiences with overdose, experience of THN kits and attitudes and behaviours surrounding THN kits. The interview guide identified general topic areas for discussion and contained specific prompts designed to elicit more detailed information as needed. Participants received a £10 gift token as an appreciation of their taking part in the study. In total, 28 interviews took place. One participant was a carer but also had past experience of opioid use, and 27 were either current or former opioid users.
Inclusion criteria
Drug service users either currently using opioid drugs or abstinent and in recovery, and their friends and family members (18 years of age or older) who have the ability to consent to involvement were eligible to participate in the qualitative component of TIME.
Service providers
We undertook a focus group and individual interviews with AS and ED staff (paramedics, ED clinical staff and service managers) at intervention sites – Site 1 ED, Site 1 AS, Site 2 AS – to understand barriers and facilitators to THN implementation, and to gain an insight into how the intervention may be assimilated into everyday work practices.
Recruitment
The initial recruitment plan was to conduct up to four focus groups, each involving approximately 6 staff who had recruited patients at each site, to take place during the first 2 months of the recruitment period, with further focus groups planned at 6 months after the start of the recruitment period to understand how the intervention was being delivered. Where rotas did not permit participation in planned focus groups, individual face-to-face interviews were offered as an alternative. In September 2019 we submitted an ethics amendment to HRA to allow us to undertake individual telephone interviews to accommodate shift patterns and to maximise participation among the mobile ambulance workforce. We also requested the provision of staff payments to encourage participation. After low uptake, we expanded the inclusion criteria to include employees who had signed up for the trial but had not recruited patients into the trial, to try to understand reasons for poor recruitment.
At Site 1, we undertook a baseline focus group (n = 8) and supplementary interviews with two ED staff at the 1 ED in August 2019. The planned focus group with Site 1 AS was delayed until February 2020 due to winter pressures, then cancelled due to the coronavirus disease 2019 (COVID-19) pandemic. We were unable to recruit staff for a focus group at Site 2 AS, with only one person responding to initial invitations and recruitment to individual interviews was similarly postponed in February 2020. We recommenced recruitment for individual telephone interviews at both ambulance sites in February 2021 and recruited two further ED staff from Site 1 ED. We were unable to recruit any staff from Site 2 ED, despite several meetings and invitations.
Participant information sheets were provided and informed consent was obtained and documented prior to the interviews. Participants were given a £20 Amazon voucher as a thank-you for participating in the study. All interviews and focus groups were undertaken by either FS or JH, and were audio-recorded and transcribed verbatim. The researcher added reflexive notes immediately following the interview.
Inclusion criteria
Paramedic and ED clinical staff and managerial staff from both branches of the ES (18 years of age or older), who were fully qualified to practice in their chosen discipline, were eligible to participate in the qualitative component of TIME.
Changes to study design
Following difficulty in recruiting staff to qualitative focus groups, it was decided to offer the £20 Amazon voucher to staff as an incentive to take part. Also, due to staff rotas, it was decided to conduct interviews for individual staff rather than focus groups as originally planned.
Due to delays in permissions for routine data access, and low numbers of THN kits distributed during the trial recruitment period, a decision was taken by the Trial Management Group (TMG) to not go ahead with the planned retrieval of datasets from English sites (originally planned to be used for testing of discriminant function and comparison of outcomes between trial arms).
Randomised controlled trial
Data collection
At intervention sites, participating clinical staff completed an intervention flowchart for each eligible participant and recorded if the participant received a THN kit and training, declined the training and/or kit, if the patient was eligible but not offered a kit and if not why. Reasons an individual were considered ineligible were also recorded and this included reduced capacity, in police custody or abusive to staff. We also collected data from electronic records of patients that received naloxone at both control and intervention sites. These data were then uploaded to the SAIL Databank for analysis. 53 THN kit stocks were also audited weekly to ensure all kits were accounted for.
Serious adverse events
We monitored for instances of serious adverse events, including deaths following THN use, by interrogating routine health service data and also requests for data made to services on behalf of coroners across intervention and control sites. We included control sites because we expected THN to be available from specialist drug services in control sites.
Data analysis
The main analysis addressed the progression criteria with regard to the percentage of eligible staff trained, percentage of eligible patients given a THN kit.
Interim analysis and stopping guidelines
No interim analyses were planned or performed.
Health economics
We aimed to assess the feasibility of generating costs relating to staff training, distribution of the THN kits and the use of routine data sources to estimate healthcare costs.
We aimed to calculate training costs using records of completion of training and staff recall of patient training, and then combined with NHS salary data.
Data collection
Separate methods of data collection are required for the four cost components described above and are described in turn.
Naloxone kits
The handing over of naloxone kits to patients was recorded by the Naloxone Case Report Form as described by the intervention flow chart.
Staff time to undergo training
The length of time taken for staff to complete training was measured prospectively using staff-reported estimates which were recorded on the training sign-off form (TSF). This was combined with the number of staff trained and unit costs from the most recent edition of Unit Costs of Health and Social Care,62 to produce a staff training cost for each Trust.
Translating this organisation-level cost into a per-patient cost requires a further mathematical transformation. This requires data on frequency of training within the organisation (which requires information on staff turnover and possible ‘top-up training’) and annual patient numbers. It was not thought necessary to attempt to collect these data and undertake these further calculations for the purposes of a feasibility study.
Staff time to give training to patients
Estimates of these times were to be collected retrospectively using staff recall. The focus groups were used as the vehicle for this. These data were to be combined with unit costs from Unit Costs of Health and Social Care,62 to produce a mean cost per patient.
Associated health service contacts
The assessment of these costs within the feasibility study was opportunistic. Being based on routine data, the feasibility of measuring health service contacts is beyond doubt; however, as a data request was needed for the assessment of effectiveness, it was felt that the opportunity should be taken to refine the analytical approach. In order to undertake this, additional fields relating to healthcare resource groups (HRGs) for all events were to be requested. Alternative ways of incorporating HRGs into the analysis would be tested and estimates of overall costs would be produced. The unit costs for this analysis were to be based on the most recent edition of NHS Reference Costs. 63
Data analysis
The cost data were to be summarised in terms of mean values.
Qualitative data
Data collection instruments
We used NPT64 to guide design of topic and interview guides and analysis. NPT is suitable for use in feasibility studies, can be applied flexibly and can be used to help understand what people do rather than what they say they will do. We developed the topic guides around the four NPT constructs of coherence (what the intervention involves and what its purpose is), cognitive participation (who has a role in delivering the intervention), collective action (how has the intervention been delivered and what has enabled or hindered uptake) and reflexive monitoring (how participants reflect on and appraise effectiveness). Topic guides used open-ended questions to encourage participants to offer their own perceptions and experiences of the THN training, implementation processes and uptake, as well as the potential harms and benefits of THN.
We originally planned to conduct focus groups to draw on the interaction between group members to explore both shared and divergent experiences of the phenomenon under study (in this case, the implementation of THN within the workplace). However, after the first focus group and supplementary interviews (for people who were unable to attend the focus group), we had to change the research plan and undertake individual interviews due to COVID-19 restrictions. To accommodate this change, we created an interview schedule which used the same questions as the intended 0–3-month focus group topic guide, but also incorporated the follow-up questions from the intended 6-month topic guide. This was to enable us to retrospectively examine how work practices were reported to have been adapted over time. In addition, analysis of the initial interviews and focus groups undertaken at Site 1 ED identified that staff found it difficult to respond to some of the questions, and engagement of some members of staff in the initial focus group was limited. We therefore simplified some of the questions and added additional explanatory prompts to obtain more useful data.
Service user data analysis
Data were thematically analysed using QSR International’s NVivo 12 (QSR International, Warrington, UK) qualitative data analysis software program. 65 Audio-recordings were transcribed verbatim and then analysed by JL, JH and FS through an iterative process. 66 Major themes for the initial round of coding were identified in the interview guide as well as from interviewers’ written notes made during and immediately after the interviews. Using a hybrid approach of reading the first few transcripts combined with using codes loosely defined a priori based on the research questions, an initial coding framework was developed. 67 During coding, researchers identified additional thematic categories and subcategories emerging from the analysis. Following this, the research team collaboratively evaluated the initial coding process to assess cross-coder reliability, resolve any coding discrepancies and establish a set of coding categories for the remaining transcripts. Throughout, an iterative process of coding, cross-checking and discussions was carried out to establish consensus around the final set of themes. 66 As data saturation was not felt to be achieved by the initial set of 18 interview transcripts, a further eight interviews were conducted, and the transcripts analysed to reach data saturation. The identified themes centred on experience of overdose either personally or as a bystander, experience of emergency treatment of an overdose; positive and negative attitudes regarding naloxone and THN; experiences, beliefs and behaviours surrounding naloxone-precipitated withdrawal symptoms; and perceptions/experiences regarding risky drug use, opinions about delivery of THN and training by both ambulance staff at scene and staff within the ED.
Service provider data analysis
We analysed data using Framework Analysis, based broadly on the constructs of NPT (using the framework of Huddlestone et al. , 2020)68 but also reported additional themes relating to the trial itself, rather than the intervention. Throughout, we attempted to differentiate between problems relating to the intervention and those relating to the process and conduct of the trial. Specifically, we considered how stakeholders embraced and used THN, any adaptations made to the clinical and research process, and how they reported recruiting patients to the trial itself.
We imported transcripts into NVivo, and read and re-read transcripts to ensure familiarity with the data before coding. Coding was undertaken with reference to the NPT framework while remaining ‘grounded’ in the data. Initial coding was carried out by JL and emerging concepts used to develop themes. Two other researchers (FS and JH) independently coded a subsample of transcripts for comparison and discussion. The coding structure was further refined and analysis undertaken after discussion between JH, FS, JL and PB.
Trial management
A TMG was established to manage the project and report to the independent TSC at appropriate intervals. The Chief Investigator chaired the TMG, which met quarterly. The TMG comprised of all co-applicants, named collaborators, public contributors and researchers.
The independent TSC oversaw the conduct and progress of the trial and adherence to the protocol, patient safety, and the consideration of new information of relevance to the trial. Two public contributors were members of the TSC.
A Data Monitoring Committee (DMC) monitored the study data at interim periods and made recommendations to the TSC on whether there are any ethical or safety reasons why the trial should not continue.
Chapter 3 Epidemiology and discriminant function results
Given difficulties in using conventional methods to follow-up recipients of THN kits and peers who may experience an opioid overdose, we used routine data to test the feasibility of identifying a high-risk cohort, postulating that outcomes associated with the intervention would be most visible within this cohort. This chapter summarises this feasibility work, which was based on routine data for the population of Wales for the period from January 2015 to November 2021. Summaries of analyses, undertaken within the SAIL Databank, are subject to the SAIL Databank’s dissemination policies.
Routine data
The combined WDSD files comprise information on n = 5,640,113 individuals. No ALF_PE (unique patient identifier in SAIL) was recorded for n = 504 cases; a further n = 1,740,317 individuals had no recorded residency in Wales during the study window; and a further n = 151 individuals had a recorded date of death indicating death before the study window – although date of death is recorded in ADDE, only ADDE data on or after the beginning of the study window were available, and so could not determine whether people were alive or not at this timepoint. Excluding these individuals leaves for further consideration n = 3,899,140 individuals deemed to be alive and resident in Wales at the start of the study window.
Of these, we then excluded another n = 664,050 individuals with week of birth recorded as on or after 1 December 2003; these individuals would be < 18 years old at the end of the study window. A further n = 7694 individuals were aged 17 or under at their final date of residency in Wales, or at their date of death. Excluding these individuals leaves n = 3,227,396 individuals in our study population.
For our derived cohort of n = 3,227,396 individuals, we extracted basic demographic data and then assessed mortality and healthcare resource utilisation, as recorded in the available routine data sources. We start with mortality, which defines both our primary outcome (death from opioid overdose) and study end dates.
Baseline characteristics
Mortality and date of death
Deaths within the study window are recorded in both WDSD and ADDE (see Table 2); we note that WDSD records only the date of death, while ADDE also records cause of death. We regard ADDE data as primary, noting generally good agreement between these datasets.
Combination A: there are n = 233,495 cases where both WDSD and ADDE record death on or before 30 November 2021, with good agreement on the date of death – exact agreement in over 99% of cases. Where there are discrepancies, we take the ADDE date as primary unless that date of death is contradicted by records of subsequent health events, defined as a recorded health event extending beyond the day after the ADDE date of death.
Combination B: there are n = 101 cases where ADDE records a date of death, but WDSD does not. We consider three possible scenarios.
-
B1: ADDE date of death is 14 days or more after the last recorded date of residency in Wales.
-
B2: ADDE date of death is within 14 days of the last recorded date of residency in Wales.
-
B3: ADDE date of death is more than 14 days before the last recorded date of residency in Wales.
For cases in B1, we censor at the last recorded date of residency in Wales; no further data processing is necessary. For cases in B2 and B3, we use the ADDE date of death to define the final date in the study for these individuals, except where that date is contradicted by records of subsequent health events.
Combination C: there are n = 9997 cases where WDSD records a date of death but ADDE does not. We consider two possible scenarios.
-
C1: WDSD date of death is 14 days or more after the last recorded date of residency in Wales.
-
C2: WDSD date of death is within 14 days of the last recorded date of residency in Wales.
For cases in C1, we censor at the last recorded date of residency in Wales; no further date processing is required. For cases in C2, we use the WDSD date of death to define the final date in the study for these individuals, except where that date is contradicted by records of subsequent health events. Cases in C2 are deaths still to be confirmed within ADDE, and we have no further information available on the cause of death – specifically, we are unable to classify death as opioid-related or otherwise. We note that including these as non-opioid deaths will dilute further the relatively small number of deaths ascribed to opioid overdose. Further dilution will occur on categorising as opioid-related any health events associated with these cases.
Combination D: there is no date of death recorded for n = 2,983,803 cases. For these individuals, we define the study end to be the earlier of the end of the study window or their final recorded date of residency in Wales.
Mortality due to opioid overdose Coding of ADDE mortality data followed the methods outlined in Fuller et al. ,55 based on the ICD-10 classification system. Specifically, we classified deaths as opioid overdose-related where codes for primary and/or secondary underlying causes of death included (see Table 3):
| Code | Cause of death description |
|---|---|
| F11–F19 | Mental and behavioural disorders due to psychoactive substance use |
| X40-X44 | Unintentional poisoning by and exposure to narcotics and psychodysleptics |
| X60-X69 | Intentional self-poisoning by and exposure to narcotics and psychodysleptics |
| X85 | Assault (homicide) by drugs, medicaments and biological substances |
| Y10-Y19 | Poisoning by and exposure to narcotics and psychodysleptics (undetermined intent) |
| T40 | Opium |
| T40.1 | Heroin |
| T40.2 | Other opioids (morphine, oxycodone, hydrocodone) |
| T40.3 | Methadone |
| T40.4 | Synthetic opioids excluding methadone (fentanyl, propoxyphene, meperidine) |
After linkage with the WDSD cohort, we identified three subcohorts: these comprise n = 1105 cases with deaths related to opioid overdose; a further n = 237,212 cases with deaths from all other causes; and remaining n = 2,989,079 individuals, still alive at their study end date.
Tables 4–6 summarise demographic data for these three study subcohorts.
| Gender | Study subcohort | |||||
|---|---|---|---|---|---|---|
| Alive at study end date (n = 2,989,079) | Death: all other causes (n = 237,212) | Death: opioid overdose-related (n = 1105) | ||||
| Male | 1,486,846 | 49.7% | 118,125 | 49.8% | 785 | 71.0% |
| Female | 1,502,211 | 50.3% | 119,087 | 50.2% | 320 | 29.0% |
| Missing | 22 | < 0.1% | 0 | 0 | ||
| Age band (years) | Study subcohort | |||||
|---|---|---|---|---|---|---|
| Alive at study end date (n = 2,989,079) | Death: all other causes (n = 237,212) | Death: opioid overdose related (n = 1105) | ||||
| 18–25 | 445,029 | 14.9% | 868 | 0.4% | 49 | 4.4% |
| 26–30 | 260,684 | 8.7% | 766 | 0.3% | 93 | 8.4% |
| 31–35 | 251,301 | 8.4% | 1046 | 0.4% | 152 | 13.8% |
| 36–40 | 231,821 | 7.8% | 1490 | 0.6% | 198 | 17.9% |
| 41–45 | 209,045 | 7.0% | 2257 | 1.0% | 142 | 12.9% |
| 46–55 | 459,262 | 15.4% | 9914 | 4.2% | 279 | 25.2% |
| 56–65 | 459,656 | 15.4% | 21,153 | 8.9% | 114 | 10.3% |
| 66–75 | 374,612 | 12.5% | 45,551 | 19.2% | 44 | 4.0% |
| 76 + | 297,669 | 10.0% | 154,167 | 65.0% | 34 | 3.1% |
| WIMD quintile | Study subcohort | |||||
|---|---|---|---|---|---|---|
| Alive at study end date (n = 2,989,079) | Death: all other causes (n = 237,212) | Death: opioid overdose-related (n = 1105) | ||||
| WIMD available | 2,863,661 | 231,794 | 1083 | |||
| 1 (most deprived) | 549,492 | 19.2% | 47,231 | 20.4% | 425 | 39.2% |
| 2 | 563,071 | 19.7% | 48,666 | 21.0% | 297 | 27.4% |
| 3 | 580,844 | 20.3% | 49,131 | 21.2% | 177 | 16.3% |
| 4 | 581,988 | 20.3% | 45,999 | 19.8% | 106 | 9.8% |
| 5 (least deprived) | 588,266 | 20.5% | 40,767 | 17.6% | 78 | 7.2% |
We next categorised records in EDDS, PEDW, CCD and SMDS for the 36 months up to immediately prior to and including an individual’s study end date, censoring at the start of the study window where necessary. For each attendance, admission or presentation, we used further routine data and appropriate coding framework (ICD-10: as per Table 2; codes 10B, 10C, 10D, 10Z and 31B in the NHS Wales Data Dictionary) to determine whether that event was related to opioid overdose, and further recording whether or not the event occurred within 1 month or within 12 months of an individual’s study end date.
Emergency department attendances
After linkage with the WDSD cohort, we identified EDDS records in n = 1,187,033 cases, with breakdown of the number of attendances by subcohort and period summarised in Table 7.
| Time period and attendance category | Number of attendances | Study subcohort | |||||
|---|---|---|---|---|---|---|---|
| Alive at study end date (n = 2,989,079) | Death: all other causes (n = 237,212) | Death: opioid overdose-related (n = 1105) | |||||
| Within 36 months | |||||||
| All attendances | 0 | 1,985,462 | 66.4% | 54,633 | 23.0% | 268 | 24.3% |
| 1–3 | 876,091 | 29.3% | 128,365 | 54.1% | 462 | 41.8% | |
| 4–12 | 121,955 | 4.1% | 51,322 | 21.6% | 306 | 27.7% | |
| 13 + | 5571 | 0.2% | 2892 | 1.2% | 69 | 6.2% | |
| Opioid overdose-related attendances | 0 | 2,975,604 | 99.5% | 235,316 | 99.2% | 887 | 80.3% |
| 1–3 | 13,054 | 0.4% | 1836 | 0.8% | 206 | 18.6% | |
| 4 + | 421 | < 0.1% | 60 | < 0.1% | 12 | 1.1% | |
| Within 12 months | |||||||
| All attendances | 0 | 2,520,674 | 84.3% | 76,000 | 32.0% | 423 | 38.3% |
| 1–3 | 443,898 | 14.9% | 137,261 | 57.9% | 502 | 45.4% | |
| 4–12 | 23,772 | 0.8% | 23,541 | 9.9% | 165 | 14.9% | |
| 13 + | 735 | < 0.1% | 410 | 0.2% | 15 | 1.4% | |
| Opioid overdose-related attendances | 0 | 2,984,543 | 99.8% | 236,191 | 99.6% | 961 | 87.0% |
| 1+ | 4536 | 0.1% | 1021 | 0.4% | 144 | 13.0% | |
| Within 1 month | |||||||
| All attendances | 0 | 2,926,591 | 97.9% | 14,5622 | 61.4% | 815 | 73.8% |
| 1–3 | 61,609 | 2.1% | 91,259 | 38.5% | 279 | 25.2% | |
| 4 + | 879 | < 0.1% | 331 | < 0.1% | 11 | 1.0% | |
| Opioid overdose-related attendances | 0 | 2,988,521 | ~100.0% | 236,974 | 99.9% | 1064 | 96.3% |
| 1 + | 558 | < 0.1% | 238 | 0.1% | 41 | 3.7% | |
Hospital admissions
After linkage with the WDSD cohort, we identified PEDW records in n = 998,563 cases, with breakdown of the number of attendances by subcohort and period summarised in Table 8.
| Time period and admission category | Number of attendances | Study subcohort | |||||
|---|---|---|---|---|---|---|---|
| Alive at study end date (n = 2,989,079) | Death: all other causes (n = 237,212) | Death: opioid overdose-related (n = 1105) | |||||
| Within 36 months | |||||||
| All admissions | 0 | 2,192,076 | 73.3% | 36,309 | 15.3% | 448 | 40.5% |
| 1–3 | 668,456 | 22.4% | 119,399 | 50.3% | 476 | 43.1% | |
| 4–12 | 110,284 | 3.7% | 64,795 | 27.3% | 158 | 14.3% | |
| 13 + | 18,263 | 0.6% | 16,709 | 7.0% | 23 | 2.1% | |
| Opioid overdose-related admissions | 0 | 2,987,393 | 99.9% | 236,842 | 99.8% | 1019 | 92.2% |
| 1–4 + | 1686 | 0.1% | 370 | 0.2% | 86 | 7.7% | |
| Within 12 months | |||||||
| All admissions | 0 | 2,616,002 | 87.5% | 52,332 | 22.1% | 622 | 56.3% |
| 1–3 | 336,773 | 11.3% | 137,906 | 58.1% | 418 | 37.8% | |
| 4 + | 36,304 | 1.2% | 46,974 | 19.8% | 65 | 5.9% | |
| Opioid overdose-related admissions | 0 | 2,988,572 | ~100.0% | 236,992 | 99.9% | 1049 | 94.9% |
| 1 + | 507 | < 0.1% | 220 | <0.1% | 56 | 5.1% | |
| Within 1 month | |||||||
| All admissions | 0 | 2,904,066 | 97.2% | 94,044 | 39.6% | 910 | 82.4% |
| 1+ | 85,013 | 2.8% | 143,168 | 60.4% | 195 | 17.6% | |
| Opioid overdose-related admissions | 0 | 2,989,013 | ~100.0% | 237,146 | ~100.0% | 1081 | 97.8% |
| 1 + | 66 | < 0.1% | 66 | < 0.1% | 24 | 2.2% | |
Critical care admissions
After linkage with the WDSD cohort, we identified CCDS records in n = 35,489 cases, with relatively few cases with multiple admissions within this number; approximately 90% had a single admission over 36 months, and only 2% had more than two such admissions in this period. The breakdown of the number of attendances by subcohort and period summarised in Table 9; no coding was available to categorise admissions as opioid overdose-related or otherwise.
| Time period | Number of attendances | Study subcohort | |||||
|---|---|---|---|---|---|---|---|
| Alive at study end date (n = 2,989,079) | Death: all other causes (n = 237,212) | Death: opioid overdose-related (n = 1105) | |||||
| Within 36 months | |||||||
| All admissions | 0 | 2,974,728 | 99.5% | 216,220 | 91.2% | 959 | 86.8% |
| 1 + | 14,351 | 0.5% | 20,992 | 8.8% | 146 | 13.2% | |
| Within 12 months | |||||||
| All admissions | 0 | 2,984,216 | 99.8% | 219,825 | 92.7% | 987 | 89.3% |
| 1 + | 4863 | 0.2% | 17,387 | 7.3% | 118 | 10.7% | |
| Within 1 month | |||||||
| All attendances | 0 | 2,988,554 | ~100.0% | 224,073 | 94.5% | 1025 | 92.8% |
| 1 + | 525 | < 0.1% | 13,139 | 5.5% | 80 | 7.2% | |
Substance misuse treatment presentations
After linkage with the WDSD cohort, we identified SMDS records in n = 32,774 cases, with relatively few cases with multiple presentations within this number; under 10% had more than three such presentations in this period. The breakdown of the number of attendances by sub-cohort and period summarised in Table 10.
| Time period and presentation category | Number of attendances | Study subcohort | |||||
|---|---|---|---|---|---|---|---|
| Alive at study end date (n = 2,989,079) | Death: all other causes (n = 237,212) | Death: opioid overdose-related (n = 1105) | |||||
| Within 36 months | |||||||
| All presentations | 0 | 2,959,595 | 99.0% | 234,283 | 98.8% | 744 | 67.3% |
| 1 + | 29,484 | 1.0% | 2929 | 1.2% | 361 | 32.7% | |
| Opioid overdose-related presentations | 0 | 2,974,825 | 99.5% | 236,576 | 99.7% | 851 | 77.0% |
| 1 + | 14,254 | 0.5% | 636 | 0.3% | 254 | 23.0% | |
| Within 12 months | |||||||
| All presentations | 0 | 2,976,864 | 99.6% | 235,559 | 99.3% | 862 | 78.0% |
| 1 + | 12,215 | 0.4% | 1653 | 0.7% | 243 | 22.0% | |
| Opioid overdose-related presentations | 0 | 2,983,446 | 99.8% | 236,867 | 99.9% | 940 | 85.1% |
| 1 + | 5633 | 0.2% | 345 | 0.1% | 165 | 14.9% | |
| Within 1 month | |||||||
| All presentations | 0 | 2,987,754 | ~100.0% | 236,979 | 99.9% | 1076 | 97.4% |
| 1 + | 1325 | < 0.1% | 233 | 0.1% | 29 | 2.6% | |
| Opioid overdose-related admissions | 0 | 2,988,468 | ~100.0% | 237,173 | ~100.0% | 1083 | 98.0% |
| 1 + | 611 | < 0.1% | 39 | < 0.1% | 22 | 2.0% | |
Proportions of individuals with no records in routine data in the 12 months prior to study end date
Based on linkages between the WDSD cohort and EDDS, PEDW, CCDS and SMDS records, we assessed the numbers of individuals within cohorts with no records in the 12 months prior to study end date; the breakdown of numbers and proportions by subcohort and period is summarised in Table 11.
| Time period (months prior to study end date) | Study subcohort | |||||
|---|---|---|---|---|---|---|
| Alive at study end date (n = 2,989,079) | Death: all other causes (n = 237,212) | Death: opioid overdose-related (n = 1105) | ||||
| 12 months | 2,316,451 | 77.5% | 39,236 | 16.5% | 307 | 27.8% |
| 1 month | 2,857,958 | 95.6% | 81,882 | 34.3% | 755 | 68.3% |
Discriminant function data analysis
For analysis, we combined the group of those alive at their study end date with the group of deaths from all other causes and contrasted this combined group with the (much) smaller group of decedents from opioid overdose. We examined the extent to which factors and covariates (recorded in routine data) (1) are associated with death from opioid overdose; and (2) enable us to identify these decedents, or a relatively small subset of an overall population that contains most or all of them.
Prediction via logistic regression
We fitted alternative versions of the logistic regression model, using (1) raw rather than banded counts of EDDS attendances and PEDW admissions, (2) all admissions and attendances rather than just those coded as opioid-related and (3) omitting WIMD quintile as a factor, thereby including data on all but 22 individuals in fitting the model.
Sensitivity and specificity
We obtain predicted probabilities by a logistic transformation of the linear predictor, including the constant term.
Logistic regression analysis
The appropriate methodology here is logistic regression; we illustrate its potential effectiveness using age and gender. Table 4 above shows that the decedents from opioid overdose are disproportionately male – 785 males and 320 females; compared with a near-equal split in both other groups in Table 4, and hence in the combined group. This observed difference in proportions is highly statistically significant.
Table 5 shows differences in the age profiles across the three original groups; for the combined group, as described above, the mean (standard deviation) age (in years) is 50.7 (20.6), while the corresponding value for the 1105 deaths from opioid overdose is 44.3 (13.3). Again, this observed difference is highly statistically significant.
For (1), therefore, the raw data indicate that both variables – separately and jointly – appear as statistically significant explanatory variables in logistic regression models for the binary outcome of death from opioid overdose (or not). Table 12 gives details on the fitted model, including age, gender, WIMD quintile; and the number of EDDS, PEDW, CCDS and SMDS attendances within 12 months of study end date.
| Variable | Coefficienta,b | OR | 95% CI | p- value |
|---|---|---|---|---|
| Constant | –8.145 | |||
| Femalec | –0.774 | 0.461 | (0.403 to 0.527) | < 0.001 |
| Age (years) | –0.013 | 0.987 | (0.984 to 0.990) | < 0.001 |
| WIMDd | ||||
| Quintile 1 | 1.439 | 4.218 | (3.302 to 5.388) | < 0.001 |
| Quintile 2 | 1.188 | 3.280 | (2.551 to 4.217) | < 0.001 |
| Quintile 3 | 0.696 | 2.006 | (1.533 to 2.623) | < 0.001 |
| Quintile 4 | 0.244 | 1.276 | (0.951 to 1.712) | 0.104 |
| EDDSe (opioid-related attendances) | 2.726 | 15.273 | (12.024 to 19.401) | < 0.001 |
| PEDWc (opioid-related admissions) | 2.309 | 10.064 | (6.793 to 14.908) | < 0.001 |
| CCDSe (all admissions) | 2.235 | 9.343 | (7.429 to 11.751) | < 0.001 |
| SMDSe (drug-related attendances) | 3.094 | 22.071 | (17.872 to 27.256) | < 0.001 |
We fitted alternative versions of this model, using (1) raw rather than banded counts of EDDS attendances and PEDW admissions, (2) all admissions and attendances rather than just those coded as opioid-related, and (3) omitting WIMD quintile as a factor, thereby including data on all but 22 individuals in fitting the model.
All fitted models had broadly similar characteristics to those summarised in Table 12; factors and covariates were (generally) highly statistically significant, and the predicted probabilities of death from opioid overdose are lower for females, reduce with age, and increase with WIMD quintile, and opioid-related attendances and admissions in EDDS, PEDW, CCDS and SMDS.
For (2), we consider the predictive ability of fitted logistic regression models: we obtain predicted probabilities by a logistic transformation of the linear predictor, including the constant term. For illustration, based on the fitted model above, a female aged 23, living within a LSOA categorised as the most deprived (WIMD Quintile 1), and with an opioid-related attendance at a substance misuse centre but no opioid-related ED attendances or hospital or critical care admissions, would have a predicted probability of 0.00916 of death from opioid overdose.
For the current routine data cohort, these predicted probabilities (of death from opioid overdose) range from –0.00003 to –0.96150, distributed with considerable skewness – 99% of individuals have a predicted probability < 0.001; 99.5% of individuals have a predicted probability < 0.0035.
We can now, in any cohort with the requisite individual-level data, take the high-risk population to include the set of individuals each with a predicted probability greater than some (arbitrary) threshold value. For instance, with a threshold value of 0.0004, applying this model across the entire cohort identifies 708 of the 1083 decedents – a true positive rate (sensitivity) of 65.4%; however, it also identifies 646,750 individuals with no death from an opioid overdose. This gives a total of 647,458 individuals to include in the high-risk population. Table 13 gives the full 2 × 2 classification for this threshold value and shows a true negative rate (specificity) of 2448685/3226269 or 79.1%.
| Predicted status | |||
|---|---|---|---|
| Opioid overdose death | Alive or non-opioid death | ||
| Recorded status | Opioid overdose death | 708 | 375 |
| Alive or non-opioid death | 646,750 | 2,448,685 | |
The sensitivity, specificity and potential contributions to the high-risk population for a range of threshold values are shown in Table 14, along with a summary of known outcomes.
| Threshold value | Opioid overdose decedents identified (sensitivity, %) | Identified for high-risk population | Total deaths in high-risk population | Excluded from high-risk population and without opioid overdose-related mortality (specificity, %) |
|---|---|---|---|---|
| 0.0002 | 933 (86.1) | 1,597,342 | 87,797 | 1,499,026 (48.4) |
| 0.0003 | 809 (74.7) | 989,151 | 60,980 | 2,107,093 (68.1) |
| 0.0004 | 708 (65.4) | 647,458 | 40,751 | 2,448,685 (79.1) |
| 0.0005 | 662 (61.1) | 432,962 | 25,943 | 2,663,135 (86.0) |
| 0.0006 | 566 (52.3) | 312,131 | 20,006 | 2,783,870 (89.9) |
| 0.0007 | 473 (43.7) | 193,751 | 17,597 | 2,902,157 (93.8) |
| 0.0008 | 381 (35.2) | 108,487 | 16,677 | 2,987,329 (96.5) |
| 0.0009 | 350 (32.3) | 57,022 | 15,839 | 3,038,763 (98.2) |
| 0.0010 | 346 (31.9) | 28,345 | 14,827 | 3,067,436 (99.1) |
Table 14, in conjunction with Tables 7 – 13, illustrates the extent of the ability of predictive methods using this set of routine outcomes; the majority of decedents from opioid overdose are essentially indistinguishable both from other decedents and those still alive at their study end date. As the latter two groups are considerably more numerous than the cohort of decedents from opioid overdose, it follows that one must either include in the high-risk population a substantial minority (or even a majority) of the overall population, or restrict the high-risk population to a relatively small proportion of the overall population, with a lower proportion of decedents from opioid overdose.
Accuracy of discriminant function
From the data we had available, we were not able to distinguish between decedents from opioid overdose and other decedents and those still alive using the discriminant function, partly due to the number of decedents from opioid overdose cohort being considerably smaller.
Based on logistic regression models we would need to monitor approximately one-third of the population to capture 75% of the decedents from opioid overdose in 1-year follow-up. Furthermore, as mortality in the 1-year period is estimated to be 6% of the third of the population, it is estimated that only 1–2% of these deaths would be categorised as a result of opioid overdose. As a high proportion of this population have no records of a healthcare event in the 12 months prior to death, it is generally the case that usage by decedents is matched or surpassed by individuals in other cohorts, therefore making the predictive link between death and healthcare events associated with opioid overdose weak.
Summary
We have provided details on healthcare utilisation and proportion of decedents from opioid overdose with no such records up to 12 months prior to death. We found a small number of decedents, which is a limiting factor in the development and accuracy of the discriminant function. Predictive variables produced in this study have shown that there are statistically significant differences in the factors and covariates in opioid overdose decedents and other cohorts, however, at an individual level and not sufficiently different from members of the other cohorts.
In order to identify and include 75% of the high-risk population for 1-year follow-up, we would need to monitor approximately one-third of the population. As only 1–2% of the deaths within the follow-up period would be the result of opioid overdose, it follows that reducing substantially or even eliminating entirely such deaths would have little overall effect on mortality rates in the high-risk population. Given the weak predictive link between deaths from and healthcare events related to opioid overdose, using routine data to identify with sufficient precision those at high risk of death from opioid overdose currently seems infeasible.
Chapter 4 Randomised controlled trial results
Our results are reported in line with the CONSORT extension for reporting feasibility studies. 69
Setting and recruitment
Site 2 AS was the first site to open to recruitment on 13 May 2019; Site 1 AS was the last on 10 October 2019.
Site 1 AS suspended recruitment on 28 November 2019 with concerns that paramedics could not supply THN under the agreed PGD – ‘our Pharmaceutical Advisor has raised the issue with her colleagues in the Specialist Pharmacy Service and been advised that administration under PGD by paramedics is not supported by the current legal framework’. Site 2 AS then paused recruitment as well. The Trial Office wrote to the MHRA to reassure the ASs that paramedics could supply THN under a PGD, and received this response on 4 December 2019: ‘Paramedics can supply Naloxone under a PGD. Naloxone is not usually supplied on a named patient basis. Once the Naloxone has been supplied an exemption in Section 17 of the Human Medicines Regulations 2012 would allow it to be administered by anyone in a medical emergency’. Site 2 AS recommenced recruitment the following day. Site 1 AS recommenced recruitment on 18 December 2019.
The recruitment period for TIME was also disrupted by COVID-19; all recruitment was paused on 17 March 2020 (except for Site 2 ED where study activity paused on 23 March 2020). Table 15 shows when each site originally opened; opened after the pause for COVID-19; and then closed to recruitment. Site 2 ED took the longest time to reopen as their research nurse had been redeployed during COVID-19 and recruitment could not recommence until he was reinstated.
| Site | Original start date | COVID closure | Restart date | Closure |
|---|---|---|---|---|
| Site 2 ED | 4 April 2019 | 23 March 2020 | 3 February 2021 | 2 June 2021 |
| Site 2 AS | 13 May 2019 | 17 March 2020 | 4 September 2020 | 30 October 2020 |
| Site 1 ED | 1 July 2019 | 17 March 2020 | 19 August 2020 | 5 February 2021 |
| Site 1 AS | 10 October 2019 | 17 March 2020 | 6 August 2020 | 1 March 2021 |
Clinical staff trained
In total, 299 staff were trained to supply THN kits to eligible patients (Site 1 ED: 107, Site 2 ED: 25, Site 1 AS: 121, Site 2 AS: 46).
Trial recruitment
In total, 277 patients were identified as eligible to receive a THN kit and training in this trial (see Figure 1). Sixty THN kits were supplied to eligible patients during the recruitment period (Site 1 ED = 36, Site 2 ED = 16, Site 1 AS = 4, Site 2 AS = 4). In 16 cases (all in Site 1 ED), the patient agreed to the THN kit and accompanying training but ultimately did not receive the kit (n = 4) or declined the THN kit because they already had one (n = 12). In 37 cases, the patient declined the THN kit for reasons other than already having one Site 1 ED = 25, Site 2 ED = 9, Site 1 AS = 1, Site 2 AS = 2. Eligible patients were not offered THN kits 164 times (Site 1 ED = 159, Site 1 AS = 2, Site 2 AS = 3). Reasons reported for not offering eligible patients kits were: staff forgot (n = 136); no kit available (n = 2); staff too busy (n = 15); suspected intentional overdose (n = 3); already given by drugs nurse (n = 4); and other (n = 3).
FIGURE 1.
CONSORT flow diagram. a, We are unable to report patient numbers by site due to small numbers of cases at some sites.
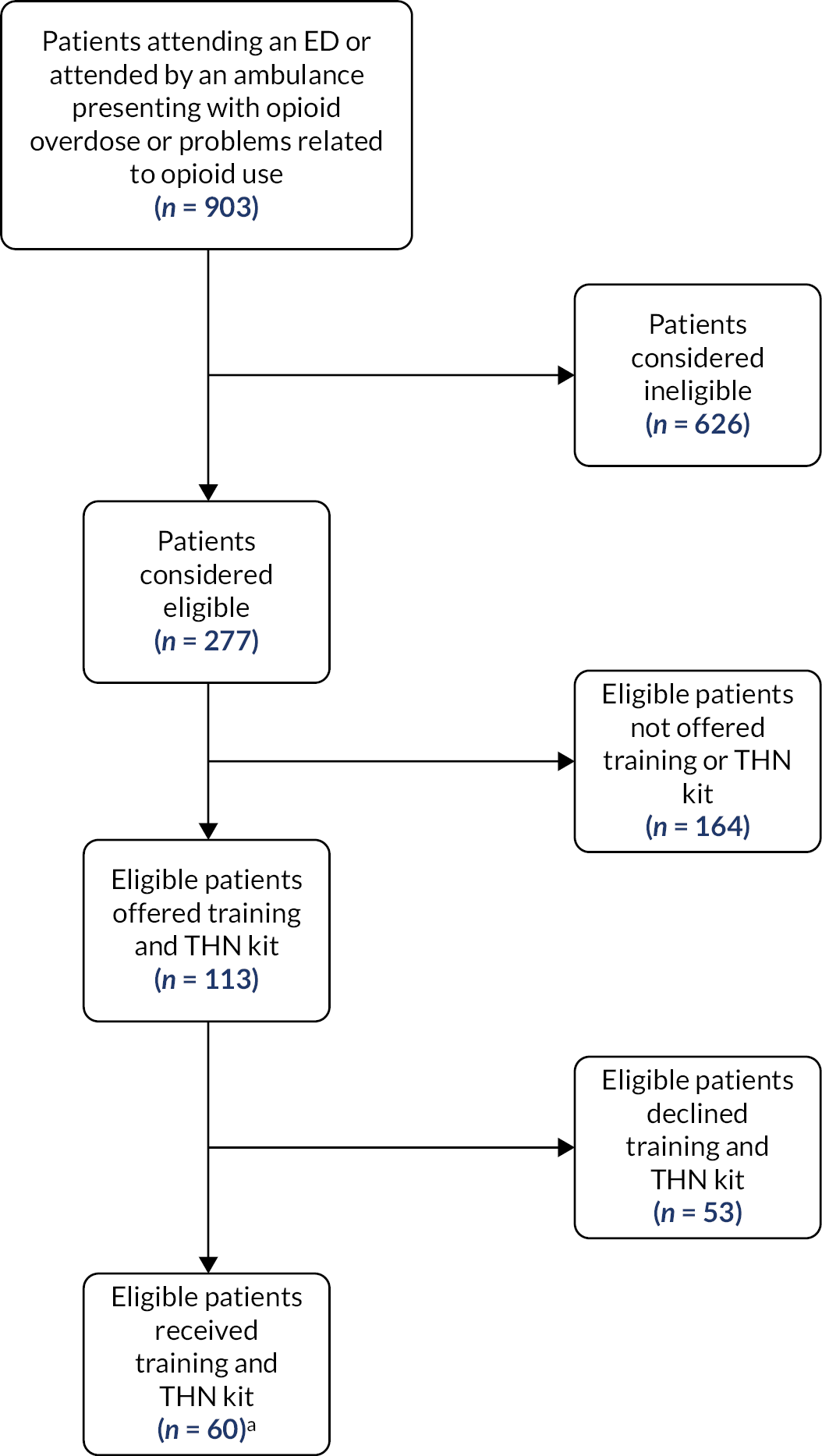
Over 90% of cases in Site 1 ED and Site 2 AS occurred between July 2019 and March 2020 – that is, before the start of the COVID-19 pandemic. In contrast, only a third of cases at Site 2 ED occurred before the pandemic, with two-thirds between February 2021 and July 2021. At Site 1 AS, equal numbers of cases were reported before and after the start of the pandemic, over the period from November 2019 to October 2020. We are unable to provide further details or more precise numbers due to the small numbers of cases at some sites.
We had no direct access to identifiable or other demographic data on patients presenting with opioid overdose or problems related to opioid use. Instead, some sites sent data to NHS Digital with the intention of generating demographic data from linked data. As no data linkage was undertaken, we are unable to report any characteristics of these patients.
Staff recorded 626 people as being considered for inclusion but found not to be eligible (Site 1 ED = 532; Site 2 ED = 4; Site 1 AS = 49; Site 2 AS = 41). Reasons given were: uncooperative including being abusive towards staff (n = 55); lack of capacity (n = 35); reduced consciousness level (n = 41); patient in custody (n = 21); patient admitted to hospital (n = 194, see Protocol deviations); 6); staff not trained (n = 29); locum staff (n = 14); already recruited (n = 64); other (n = 12).
Completeness of data
The data recorded and uploaded by each site were not uniform. Each site was supposed to record information about THN eligibility, offered, patients trained, received, declined and presenting complaint. There was no eligibility information recorded by Site 2 ED but the other three sites identified a total of 278 patients eligible to receive the intervention [Site 1 ED (n = 264), Site 1 AS (n = 8), Site 2 AS (n = 6)]. Site 1 ED documented 504 ineligible patients as these were either admitted (25%), self-discharged (20%), aggressive (3.4%) or were in police custody (1.4%). The majority of patients (n = 49) were identified to be ineligible by Site 1 AS with over 50% having reduced consciousness and few lacking the capacity or being abusive. Everyone recorded as having attended by Site 2 ED was deemed eligible.
Site 1 ED trained 37 patients receiving the kit and offered training to 25 but was declined due to reasons unknown while further 12 declined as they already had kits. Other sites did not record how many were trained.
Sample size and recruitment required for a full trial
To extend this study into a full trial we were required to identify at least 75% of people presented to ED or attended by the AS with an opioid overdose or use-related problem. Across the 4 sites we identified 848 people presented to ED or attended by the AS using the routine data; however, only 453 were reported to us.
Additionally, we were required to issue kits to 50% of the eligible patients, we identified 277 patients as eligible; however, we only issued kits to 60.
Adverse events
No adverse events were reported.
Fulfilment of progression criteria
-
Sign-up of four sites, including ≥ 50% eligible staff to complete training in delivering the intervention at each intervention site.
-
Assessment: RED: Four sites did recruit patients to the TIME trial. At the time of staff training, there were 132 eligible staff in Site 1 ED; 197 in Site 2 ED; 222 in Site 1 AS; and 136 in Site 2 AS. Both Site 1 ED and Site 1 AS trained more than 50% of their eligible staff (82.6% and 54.5%, respectively). Site 2 AS, however, trained only 33.8% (46/136) and Site 2 ED only trained 8.1% (16/197) of eligible staff. This progression criterion was not met.
-
-
Identification of ≥ 75% of people who have presented to ED or AS with opioid overdose or an opioid use-related problem over a 12-month period.
-
We were unable to identify all those who presented to ED or the AS with opioid overdose or other related problem, so were not able to ascertain whether this criterion was met.
-
-
Take-home naloxone kits issued to ≥ 50% eligible patients over a 12-month period at intervention sites.
-
RED: Sixty kits were given to eligible patients, out of n = 277 identified by trial staff as eligible. As this is just 21.7% of eligible patients, we did not meet this progression criterion.
-
-
Serious adverse event rate (to be defined in agreement with DMEC) of no more than 10% difference in intervention sites to control sites at the conclusion of recruitment.
-
GREEN: No serious adverse events were reported; therefore, this criterion was met.
-
Trial method feasibility
-
Identification and inclusion for follow-up of ≥ 75% of people who died of opioid poisoning in the following year in the study areas according to ONS mortality data (previous ONS data suggest between 140 and 180 such deaths across the 4 participating sites during the study period).
-
RED: We were unable to identify this population to include in the 1-year follow-up.
-
-
Matching and data linkage of quantitative data collected.
-
RED: The linkage of quantitative data was no longer pursued due to issues with NHS Digital approvals resulting in significant delays that did not allow for linkage and analysis to be possible within the timeframe of this project, as well as low distribution of THN kits at intervention sites. Therefore, we did not meet this criterion.
-
-
Retrieval of primary and secondary outcomes from NHS Digital and National Welsh Informatics Service within 6 months of projected timeline.
-
RED: We did not retrieve primary and secondary outcomes from NHS Digital and NWIS within 6 months of our projected timeline; therefore, we did not meet this criterion.
-
Protocol deviations
There were 201 protocol deviations reported during the trial. One protocol deviation was reported to the Research Ethics Committee (REC) and Sponsor, both on 23 February 2021. There was miscommunication between the Trial Office and Site 1 ED, with the Trial Office being under the impression that Site 1 ED had stopped recruiting on 2 December 2020 as agreed. Site 1 ED actually continued to recruit until 5 February 2021 (a total of 61 weeks instead of 52). The underlying reasons for this miscommunication were reported as related to the COVID-19 pandemic (with research staff at site having not met in December 2020 or January 2021 as planned), and a change in Trial Manager at Swansea University (a new Trial Manager was appointed in December 2020). No further action was taken regarding this.
Other protocol deviations reported to the Trial Office were:
-
Site 1 ED: 194 protocol deviations were recorded for patients who were going to be admitted and were not offered a THN kit; this was not one of our exclusion criteria.
-
Site 1 AS: TIME Patient Information Leaflet not left with the patient 5 November 2019.
-
Site 2 AS: Reduced frequency of THN kit audit following reopening of recruitment after the pause for COVID-19. Prior to the pandemic, Site 2 AS audited there THN kits every fortnight. Subsequently, this was done once at reopening and once at closure (8 weeks apart). This was due to availability of staff members.
-
Site 2 AS: on three occasions, THN kits were not accounted for during audit. One of these was eventually located, two were not.
-
Site 2 AS: a THN kit was used on a patient on one occasion.
Chapter 5 Health economics results
Naloxone kits
The data from the Naloxone Case Report Forms were complete.
Staff time to undergo training
Training data were collected from all four intervention Trusts.
Site 1 AS and Site 2 AS
Within Site 2 AS, records were available for 121 trainees with each session taking, on average, 24 minutes. Trainees were predominantly paramedics, but there were also other grades of staff including consultant paramedics and specialist practitioner paramedics. Training was undertaken by three separate people – an external contractor, a staff member of Martindale and a research paramedic. The majority of training sessions were provided one-to-one, but group training was also undertaken for groups ranging from two to six.
Within Site 1 AS, records were available for 121 trainees, averaging 22 minutes per session. Trainees were predominantly paramedics or Hazardous Area Response Team (HART) paramedics. Training was principally undertaken through a cascade approach, with early trainees going on to train other staff. A slight majority of training was group sessions with groups ranging from two to eight.
For illustrative purposes, trainee data were combined with a unit cost based on an agenda for change (AfC) Band 6 salary for a paramedic (together with associated AfC on-costs and overheads for hospital staff), which is associated with an hourly cost of £51 (2020–1 price levels). The grade of the trainer outside a research setting is uncertain, and so for the purposes of this study we considered that it would be undertaken by a paramedic (which is broadly in line with both the site 2 AS and Site 1 AS training models). This resulted in a cost per staff member trained of £40 in Site 2 AS and £40 in Site 1 AS. Due to discrepancies in the recording of groups at both sites, with group numbers not always matching the number of paramedics being registered on the same day, costs were calculated assuming all training was undertaken one-to-one.
Site 1 ED and Site 2 ED
At Site 1 ED, records were available for 109 trainees, averaging 14 minutes per session. The majority of trainees had nursing roles (55%) with the remainder being doctors of various grades. Training was principally undertaken by a research nurse, with some being provided by a consultant physician and a staff member of Martindale. The vast majority of training was undertaken in group sessions with groups comprising up to 15 staff members.
For illustrative purposes, nurses undertaking training were costed using an AfC Band 6 salary and doctors using a Registrar salary, both with on-costs and overheads, which are associated with hourly costs of £51 and £52, respectively (2020–1 price levels). For the purposes of this study, we costed trainer time at the rate of a Grade 8B Research Nurse, which is £82 per hour. Group training data included only minor inconsistencies and so these were used to in the calculation of costs. This resulted in a cost per staff member trained of £17.
At Site 2 ED, records of training were kept, but the data necessary for the calculation of costs (e.g. times, grades of staff involved and numbers present) were not collected. Consequently, costs have not been calculated.
Staff time to give training to patients
With the change in the format of qualitative data collection following the pandemic, and low recruitment, it was decided to drop the questions relating to patient training. Consequently, no data relating to this are available.
Associated health service contacts
Due to changes in the project, the request for routine data was abandoned, and so these costs could not be calculated.
Summary
The study has shown that the methods for collecting costs relating to the naloxone kits and the training of NHS staff are feasible and can take into account differences in the form of training (e.g. one-to-ones group, nominated trainers vs. cascade trainers). The unit costs used in our calculations are approximate, and further calculations are also needed to produce per-patient costs, but the feasibility of these changes is beyond doubt.
It should be noted, however, that there were some problems with the staff training data. One centre produced high-quality data, two centres generated data with some discrepancies and one centre failed to use the data collection tool at all. All of these problems are considered resolvable in any future trial. 70
We have been unable to assess the feasibility of collecting patient training times via staff recall. It is unlikely that this finding represents a serious barrier to the conduct of any future evaluation of THN. However, the precise method of collection of these data is still open to question; we planned to use practitioner recall, while other methods could include prospective collection as part of the Naloxone Case Report Form. It should be noted that a UK-based evaluation of THN demonstrated that cost-effectiveness is unlikely to be sensitive to uncertainties related to training costs,71 and so this should be recognised when designing future data collection; expensive or disruptive data collection is unlikely to be worthwhile.
We were also unable to test the costing of other health service contacts. However, as stated previous, this work wasn’t strictly an issue of feasibility; the approach is feasible, although the precise way in which the data would be used needs finalising.
Chapter 6 Qualitative results
Service user perspectives
Our findings suggested that service users had significant experience of overdose (their own or others) and were keen to maximise provision of THN which they perceived as an acceptable and easy-to-use intervention. They valued provision of THN via ambulance (ES) or ED staff in addition to community provision. Although they expressed concerns about risks of wanting to leave the ED or ambulance quickly after reversal, due to potential side effects of rapid withdrawal associated with naloxone, they valued its life-saving benefits. Service users spoke about THN training and provision giving them a sense of self-agency and empowerment and users perceived this as an opportunity to save lives. Users also felt there were opportunities for friends and family to also undergo training and receive the THN kits.
Theme 1: service users had wide experience of overdose and high level of knowledge of how to manage overdose, with or without THN
Almost a third of the interviewees in the study had experienced an overdose themselves and nearly all had observed others overdosing at some point. In discussing their own overdoses, participants described a range of different experiences, with or without naloxone or THN. One participant described being found by her mum:
I was at my mum’s and luckily my mum knew what was happening ‘cause my mum knew about what I was doing at the time and I was sort of not responsive…I was sort of quite ashamed of myself ‘cause it was – at that time it was an accidental overdose.
Site 2 ED 06
Another participant was revived in the street by someone carrying THN:
I was lucky, yeah. It wasn’t actually on these premises [treatment centre] but it wasn’t far away. And there was a member of staff who had naloxone in her bag and she brought me round.
Site 1 ED 04
Participants demonstrated a high level of knowledge of how to respond to others in an overdose situation and described either observing or trying to help others who had overdosed. Participants discussed calling the ambulance, using CPR, getting the patient conscious and moving, or putting them in the recovery position:
So I’ve gone up to him with another lad that I know and we picked him up and basically forced him to walk around the park. Which I – we thought was right.
Site 2 ED 08
Called the ambulance. Put them into recovery. At that time though, there wasn’t Naloxone pens about. So, yeah, so I just called an ambulance.
Site 1 ED 01
Some participants also described administering THN if someone had a kit on them:
I was on the stairwell … and I had my Naloxone with me and my friend went over and I phoned the ambulance. They asked me if I had one [THN kit] with me. I said I did and they told me to use it and I did.
Site 1 ED 02
But basically they say one dose is one, but what we did to a friend was we give him two and then when that weren’t really doing owt we give him the rest.
Site 2 ED 01
Generally there was a high level of knowledge overall about the existence of an overdose reversal drug, with only one participant stating they were unaware of a reversal drug being available. All were aware the drug worked instantly. Participants described having received naloxone from paramedics, but some had also been given THN by a peer drug user:
I’ve been using drugs since I was fourteen. I’ve gone over twice in that time … my friend gave me it the first time and brought me round. And I was quite ill after, and angry. But it brought me around. And the second time it was from the ambulance people.
Site 1 ED 01
Some participants believed that ambulance crews used different, stronger drugs than THN, commenting ‘the Naloxone that paramedics carry is different to the Naloxone that you give us’ (Site 1 ED 02) with one participant stating that he had been given ‘Ketamine’ (Site 2 ED 10) and another ‘Adrenaline’ (Site 1 ED 04).
Users valued naloxone and THN as a life-saving drug and acknowledged the paradox of the ambivalence of how it felt to be treated at the time, recognising that the immediate behaviour did not demonstrate gratitude. However, users appeared to strongly value the opportunity to have access to the life-saving drug, and valued their own lives and that of other drug users:
You know, you’re quite selfish, they’ve saved your life and then you throw it in their faces.
Site 2 ED 04
Theme 2: THN was perceived to be an easy-to-use, simple and effective intervention that users would be willing to carry and use and increased their sense of self-agency
Interviewees valued the opportunity to have access to a THN kit, with participants who had experienced THN being used by other peer users being particularly positive about the benefits of THN and feeling encouraged to carry a kit themselves:
If I knew somebody’s life were at risk I would 100 percent use that without any shadow of a doubt.
Site 3 ED 04
So my experience of Take-Home Naloxone, it’s dead handy, especially for if someone goes over, and I’ve actually witnessed it and used it, so yeah, it’s good stuff.
Site 1 ED 04
An associate come round into the flat … overdosed and everybody’s head fell off, but this was given to him. Now when this was given to him he was coming onto his hands and knees as the ambulance was coming through the door, so I seen it actually work you see, so then that’s what made me ask for this (THN kit).
Site 2 ED 01
Several interviewees felt that being trained and given resources to help themselves and others and given them a sense of agency and empowerment:
I felt a bit big-headed actually … Cos I had the kit and I knew what to do with it … I felt quite privileged for them to say that this is not just for you, this is for – you know what I mean? ‘Cos it gives me a bit more responsibility … it makes me want to be around people, like if I need to inject or what not, instead of just going home and being on my own with the dog, I’ll make sure I’m with somebody now. Now I’ve been able to educate people myself.
-Site 2 ED 07
Some participants had received THN training in the past, with some carrying kits themselves or had a family member who was trained to use a kit. Those who had received the short training expressed confidence in being able to administer THN if necessary:
If I had to find a vein or owt’ like that I wouldn’t be confident enough, but the fact that it goes straight in a muscle and it can go through clothes, it’s just, it’s really simple.
Site 2 ED 01
Interviewees were positive about the size of the THN kit with many commenting on the clear, easy-to follow instructions as well as the small size which meant it could be carried in a pocket, handbag or rucksack easily:
I wouldn’t mind having it in my backpack and keeping it in there.
Site 2 ED 06
No, it’s a perfect size, it’s perfect.
Site 3 ED 07
Many participants supported access for family, friends and partners as well as peers who may be users themselves. When discussing the training and how to use the kit, some felt that it might be difficult for family and friends to administer the THN but overall most were positive about this option:
I mean most people have got a family member who’s worried about them, might have a mum who’s worried about them, a sister or something like that. So obviously they might want to get some training and have this little pack just in case, you know.
Site 2 ED 04
I think it’s a good thing. I think people should be encouraged to have one, certainly if they’re in a house where other people come to use there.
Site 2 ED 05
Theme 3: participants expressed mixed views around the risk of using naloxone, in terms of a recipient being aggressive on revival, and around calling the emergency services
Participants expressed some concerns about calling for help for overdoses due to the additional involvement of the police, with fears around arrest for possession and supply of drugs. Longer-term drug users recounted more negative police experiences and fear of arrest etc., but this was usually when they recollected overdose experiences from some time ago:
The thing is there’s a fear round drug users that if you start ringing 999, the police are going to come, but a life’s a life, isn’t it?
Site 2 ED 02
I didn’t dare hang around and do it. Because, like I say, I had loads of drugs in my pocket.
Site 2 ED 06
Overall, there was general agreement that police were now concerned with safety rather than any issues around taking an illegal drug:
Well, we’d heard that in the past, that people were abandoning people and we’d heard that they’d changed the way they dealt with the people helping somebody because of that very reason, because they were getting abandoned. So we kind of were led to believe that we wouldn’t be held to any accountability for helping him.
Site 2 ED 05
I would still, you know, phone them up and say, look, you know, ambulance, he’s overdosed, he’s had whatever, you know, explain everything onto the phone. Whether the police come or not, I know I’m not getting arrested.
Site 2 ED 08
Due to the potential of the naloxone drug wearing off faster than the opioid effects, and the risk of a person who has overdosed going back into respiratory depression, it is important to phone ES and stay with the person, to administer another dose of Naloxone if necessary. However, some interviewees did recount experiences when resuscitation using THN was carried out without calling ES:
Cos, you know, if you’ve got Naloxone and someone’s gone over and you put Naloxone in them and they come back round why do you need to waste time phoning an ambulance?
Site 1 ED 05
I kind of felt – with the Naloxone there, I kind of felt in control. If she’d – if I’d given her the Naloxone and it hadn’t brought her round, the first thing I’d have done was ring an ambulance.
Site 2 ED 05
A predominant theme among those interviewed who had experienced an overdose and then been given naloxone, was feelings of anger and confusion at the first instance. Almost all respondents recounted the physical side of very quickly losing the ‘buzz’ of the opioids’ effect, resulting in going into rapid ‘withdrawal’. This was described as frequently causing headaches, paranoia and confusion and also provoked feelings of anger:
Yeah, I’m just like where has my buzz gone, what the **** have you done like, do you know what I mean, where’s my drugs? I thought he’d robbed me, I woke up and I had no like no recollection of what was going on, I was just like what’s going on, where’s my drugs, why ain’t I buzzing, do you know what I mean?
Site 1 ED 04
And I was furious, I wouldn’t go in the ambulance. I was like, no, they’re going to take my drug, you know, they’re going to give me Naltrexone or something, no.
Site 1 ED 08
This was also viewed by some, in terms of caution in administering the naloxone to others, particularly if it was felt that the person might become combative or angry:
My friend was a bit pissed off and that’s because he had no opiates in his system and straight away he wanted to use drugs.
Site 2 ED 01
However, even though there was acknowledgement of how someone might behave after they have been given the naloxone, almost all of the interviewees felt they would still give THN:
I’d be more hesitant but at the end of the day like I’d still, you know, I’d value the person’s life more than getting a bit of grief over them coming out of their high.
Site 3 ED 08
I think I’d still – knowing that, still use it because I think it’s still more important to have someone confront you than potentially lose a life, so yeah, I’d still administer it knowing that.
Site 2 ED 09
There is some evidence that taking another dose after receiving naloxone to reverse an overdose carries a higher risk of overdosing again and this has been outlined as a concern around THN in the literature. Some participants felt this would be the case:
I didn’t realise there was a twenty minute period where you’ll start – you withdraw for twenty minutes and then you don’t, so I was just really pissed off with that, I thought I’d lost my hit. Then ran round trying to get more money [for more drugs].
Site 1 ED 05
Yeah but I mean, the first thing they want to do is go and get some more drugs. That’s the problem really, rattling is not comfortable at all.
Site 3 ED 01
Linked to this, participants’ opinions were very mixed concerning whether or not having access to THN would lead to opioid users taking more risks, knowing that a safety net was available. A few participants believed that others might be encouraged to take higher amounts of opioids:
If I’m with someone taking drugs and I knew that person had Naloxone would I take more to overdose? Probably, yeah.
Site 1 ED 04
If there’s a safety net there, I think someone will be greedy as well. So, that could make it worser.
Site 3 ED 05
Even though some interviewees acknowledged that this may be a potential risk, overall, the consensus was that quantities of drugs taken were more influenced by factors such as amount of money available or type of drugs available, with some commenting that they would take as much as they could afford and were not in a position to increase this at will:
You can only take what you can afford at the end of the day.
Site 3 ED 08
No, they don’t, they take what the money can buy, it’s not about well I’ve got this much, do you know what I mean?
Site 2 ED 04
Importantly, it was commonly stated that the risk of overdose was something to be avoided at all costs, and therefore it was unlikely that people would take more drugs and knowing it could increase overdose risk:
...if it was me personally I don’t think I would, … cause I wouldn’t want to ever be in that position of overdosing again anyway.
Site 2 ED 01
Nobody wants to go over, nobody wants to go over because it’s big rigmarole. You don’t know what’s going to happen, you don’t know whether you’re going to be here or not. And it’s scary, it’s a scary thing.
Site 3 ED 04
Theme 4: opioid users supported much wider provision of THN
There was a common consensus that THN should be widely available, with almost all of the participants citing chemists, needle exchanges and places like drug treatment services as places where THN kits could be given out. Participants felt that chemists in particular, where some obtained methadone and therefore visited on a daily basis, would be a good place to get the THN kits and training:
Oh, doctors, pharmacies that give out pills. Like, places that do needle exchange should be offering Naloxone. If they’re giving out bags of pins, they should give out Naloxone, I think.
Site 1 ED 01
I think the chemist ‘cos where are they going to get their pins from?
Site 3 ED 05
All participants in the study were asked specifically about their opinion on THN kits and training being given out by both paramedics on scene and also in hospital ED settings. Although overall, interviewees were positive about widening access to THN training, there was less clarity when considering the hospital ED and ES THN provision specifically. A common experience was being treated on scene for an overdose but not going in the ambulance to hospital. Many explained this was due to the instant withdrawal caused by reversing the opioids:
If they’ve got it on the mind to get drugs. Drugs is all important to them and if it’s the case that they don’t hang about, it’s the rattling.
Site 3 ED 01
Almost all the participants commented on either not wanting to go into hospital themselves or observing others refusing to be admitted to hospital by the ambulance staff. This was generally felt to be due to the experience of rapid withdrawal outlined. One participant, however, did feel coping with withdrawal symptoms was a reason for being in the ED, while at the same time expressing concerns about this leading to being more at risk of an overdose by taking more opioids straight away:
If you’ve got it IVd it will clear all the opiates out of you instantly and you’ll be clucking and then you want more. And the risk is because you’ve got Naloxone you’re not going to feel it, so you’re going to go over again. So, it’s best to take it with the hospital if they have a decent place for Naloxone, because they’re going to try and use to get rid of the withdrawal symptoms. And they won’t feel it and then they’ll use more, you know. It’s a catch-22 situation. It’s quite a bad one. You can’t get rid of that feeling. You’ve just got to ride it. Yeah.
Site 1 ED 03
Although interviewees were ambivalent about provision in the ED, possibly due to less experience of being treated for overdose in the ED setting, there was more support expressed for THN provision by ES staff attending overdose calls. Participants felt there would be an opportunity for ambulance staff to give out kits and training at this point:
Both the people that overdosed in my eyes didn’t go in the ambulance. Because they probably wanted to go get more drugs they didn’t go in the ambulance. People just don’t like to go in the ambulance … it maybe would be ideal to give the five, ten minute training or whatever it is, show them, leave them one.
Site 2 ED 01
They come round and they want to go home. They normally will see an ambulance staff … even if they don’t get in the ambulance.
Site 3 ED 05
Treatment on-scene and being given THN training at that point was also viewed as more effective by some participants:
Anything that will cut down on A&E waiting times is a good idea in my book.
Site 3 ED 08
Interviewees felt that there were opportunities for ambulance staff to give out THN kits to peers and family/friends or bystanders while attending overdoses. Not as much support specifically for THN training in the ED setting was expressed, but many included ED provision more generally in widening access to THN:
The more they get out and the more they get it to circulate, the sooner the better, as far as I’m concerned.
Site 2 ED U08
Summary
Few studies have examined THN provision in the ED and ES, particularly from the perspectives of those who have direct knowledge and experience of opioid overdose and treatment. Participants in the qualitative interviews, attending an outpatient treatment clinic or drug support organisation, had a high level of THN knowledge and were highly supportive of widening of THN kit provision. Current and previous access to kits varied across those interviewed depending on different community, third-sector, and NHS support service provision. Many participants felt that THN should be given out in a variety of settings including chemists and needle exchanges as well as in the ED and ES settings. Participants acknowledged that there was resistance to attending hospital in an overdose situation and this therefore may have increased ambivalence about THN provision in this specific setting. Training from paramedics was generally considered a good idea, but some acknowledged the drawbacks, the main one being people going into withdrawal and wanting to immediately either go home or re-use.
It is important to note that universally in our study, overdosing from opioid use was viewed as an accidental event to be avoided and ameliorated as much as possible. Participants were highly motivated to both help others in that situation and to avoid it for themselves. THN provision increased self-agency and empowerment in service users. Inclusion of friends and family in being trained to use a THN kit was important to many in the study. This was viewed in the wider context of support for increasing access to THN.
Service provider perspectives
In total we conducted 14 interviews (8 paramedics, 4 ED staff) plus one focus group with 8 ED staff (see Table 16 for details of characteristics of participants).
| ID | Length of interview (m) | Gender | Role | Organisation | |
|---|---|---|---|---|---|
| FG1- FG 8 | August 2019 | 32 | Mixed | 8 ED staff | Site 1 ED |
| SP02 | August 2019 | 47 | M | ED medic | Site 1 ED |
| SP03 | August 2019 | 61 | F | ED nurse | Site 1 ED |
| SP04 | February 2021 | 34 | F | Paramedic | Site 2 AS |
| SP02 | February 2021 | 57 | M | Paramedic | Site 2 AS |
| SP08 | February 2021 | 47 | F | ED nurse | Site 1 ED |
| SP06 | February 2021 | 67 | M | Paramedic | Site 1 AS |
| SP09 | February 2021 | 38 | F | Paramedic | Site 1 ED |
| SP14 | February 2021 | 50 | M | ED nurse | Site 2 AS |
| SP11 | March 2021 | 40 | M | Paramedic | Site 2 AS |
| SP18 | March 2021 | 25 | M | Paramedic | Site 1 AS |
| SP5 | March 2021 | 40 | M | Paramedic | Site 2 AS |
| SP7 | March 2021 | 27 | F | Paramedic | Site 1 AS |
Findings
We present the findings broadly in relation to the NPT constructs, then discuss issues relating to the trial and recruitment to the study. The first two constructs describe how staff perceived the purpose of the intervention, specifically how it works (coherence) and who has a role in implementing it (cognitive participation). Issues relating to the implementation of the trial itself (rather than the intervention) are discussed mainly within the latter two constructs (collective action and reflexive monitoring). Within collective action we anticipated exploring the question ‘how does the work get done’, focussing on the compatibility of the intervention with current work, training and resources required to enable the provision of THN. However, due to low uptake of the trial, we focus instead on recruitment and implementation of the trial itself. Within reflexive monitoring, we considered whether the intervention was perceived as advantageous, whether the effects of the intervention were clear and whether it could be adapted or improved. 64
Normalisation process theory 1 – Coherence (what does the intervention involve and what is its purpose?)
-
Participants demonstrated high levels of coherence in terms of understanding the aim and purpose of the intervention.
-
Some scepticism around benefits of TNH when given in emergency situations due to patients not wishing to engage.
-
Recognised benefits of THN as distinct from naloxone itself in terms of providing opportunities for education and empowering patients and their families.
Overall, both ED staff and paramedics recognised the value of the intervention and had a clear understanding of the aims of the trial and how it related to their work practice. Participants demonstrated a high level of coherence in describing the purpose and potential benefits of the intervention: reduced mortality and morbidity, as well as wider benefits to the health service in terms of reduced attendance at ED and/or ambulance journeys for future overdose. Participants recognised naloxone as key to reducing fatalities and morbidity for opioid users, with many having witnessed opioid reversal themselves:
I think from an intervention, I mean, I hope the benefit is that it reduces mortality and morbidity for those patients. They’re a very high-risk group of patients. The local addiction service here are pretty good and have been giving out Take-Home naloxone for probably quite a long time, and I’ve been to numerous patients who had Take-Home naloxone administered to them. I mean, you don’t know what would have happened to them but in my eyes, when you get there they’re alert and they’re a good colour, and there’s been some clinical benefit for that for me.
SP11
The reduction of fatal overdoses, to us, makes it worthwhile straight away.
FG3
Overall it would benefit all round because it would prevent admissions to A&E so I think it would be cost effective, and it’s a relatively simple thing to take on board and to cascade down to patients.
SP5
In addition to the benefits of naloxone, participants also recognised potential additional benefits from the interactions associated with providing THN and the opportunity to encourage opioid users to engage in further treatment. This interaction was viewed as a mechanism for enabling ED and paramedic staff to spend time taking part in education and health promotion conversations and engage with a hard-to-reach population who may otherwise not have much contact with health services:
I think it’s obviously to reduce avoidable death through opiate abuse. I think it enables practitioners to have health promotion conversations with people in that lifestyle. And really to some extent it’s a kind of ‘make every conversation – make every contact count’ message but the patient goes away with something useful at the end of it.
SP2
Like I said, I mean, we all know that people shouldn’t be taking opiates and things like that, but they will. And if we can get it so we’re – we can stop some preventable deaths, a lot of people die from taking drugs. If we can help support them to stop them dying, and even if it gives them an education enough to get off the drugs in the end, you know, we can make it a little bit safer for them and give them that opportunity to actually get through it.
SP04
Delivering the intervention via ES was seen as an opportunity to widen access to THN for a specific, high-risk population, and participants felt that they were in a unique position to be able to connect with opioid users who were not accessing services elsewhere. Some staff saw also implementation of the intervention as a way to relieve the pressure on other drug support services and increase levels of collaboration generally across support services:
think we catch a lot of things. Like I was saying, we are privileged to be accepted into these places that people wouldn’t normally see. So we can catch those people who maybe – they wouldn’t go to the GP for help. They might not engage with services, they might not even know they exist. Whereas we’re called at the crisis point, people panic, they call us. So we’ve got that chance to actually engage with them.
SP4
I guess just to maybe catch a population that aren’t accessing – these people live very chaotic lifestyles so they’re not all accessing addiction services and GPs and things like that. I guess just to potentially access a group that may not be accessing conventional services.
SP11
Participants suggested that ES provision of THN with education and training had potential to increase self-efficacy and empower both opioid users and their peer and support groups. It also gave providers a sense of being able to help a population for whom they felt they could offer little support:
So, that’s what I saw as the key bonus to this scheme, was that it wasn’t just about supporting and educating people and saying, you know, “Recognise this risk and call help”, but also being able to say, “This is something you can do”, and kind of like empowering them at the point of care.
SP06
I guess, friends and carers and relatives, as in, they might feel more powerful to help in that situation or have something to do.
SP09
It is a nice thing to leave people with, it sort of shows that we care. It’s another thing we can say in good faith, we saw this person, we discharged them after a heroin abuse episode that went a bit awry and we have left them with a life saving drug. And I think that’s a nice thing to do to people.
SP02
Normalisation process theory 2 – Cognitive participation – who has a role in delivering the intervention?
-
Participants within this study saw provision of THN as compatible with their role and that of their organisation.
-
Low engagement with the trial suggests that buy-in may be lower from other colleagues. Differing views may then feed into reduction in collective action.
-
Evidence of work being undertaken to increase engagement.
Interviewees saw the provision of THN as broadly compatible with their roles and the role of their organisation. ED staff were used to dealing with opioid users in their day-to-day work and the intervention was not felt to represent a significant change from their normal work practices. Nursing staff were involved in identifying patients for recruitment, with the prescribing of THN and training undertaken either by medical staff, or nursing staff with PGDs. The training and additional conversations required with opioid users were integrated into existing discharge procedures and acknowledged to take some additional time, particularly when the patient was less able to engage following overdose reversal:
So the only difference is just before the patient was discharged you would just ask them if they wanted a Take-Home Naloxone, or if they’d heard of it, and if they were interested in having one. So that would be the only change. It’s just before discharge we would ask them if they wanted a Take-Home Naloxone kit.
SP8
From a point of caring for the patient, I don’t think there is a lot of difference really. It’s more than just offering them the chance to take the kit home. I guess that involves a bit more time and education, because we wouldn’t necessarily routinely go through all the recovery position and talking through effects of overdose and things before.
SP9
Paramedics within this sample described the THN intervention as compatible with their job roles and perceived the provision of THN as an opportunity to be proactive and engaged in public health and preventive medicine. They welcomed expanding their scope of practice and recognised this as an opportunity to increase the value of their interaction with opioid users, compatible with the ‘make every contact count’ approach being advocated by the ASs:
As a paramedic I’d say yeah it’s very compatible ‘cos the frontline ambulance paramedics are going to those patients on a more regular basis than we [specialist role] are. I know from previous experience, certain hotspots within the [name] area that when an address pops up on the screen on the computer you know instantly what the job’s going to be, nine times out of ten. So it would be good to know with the regular callers and the regular addresses, that it’s a high likelihood it will be an opiate related incident and to have the kit there ready to take in with you.
SP18
I think it enables practitioners to have health promotion conversations with people in that lifestyle. And really to some extent it’s a kind of make every conversation – make every contact count message but the patient goes away with something useful at the end of it.
SP2
Participants described frequent interactions with opioid users within their day-to-day work and saw THN as an opportunity to provide an additional service not only to opioid users but also the friends and family, particularly when attending the home setting. They felt that having the ‘captive audience’ in the ambulance put them at a potential advantage over the ED, where staff may struggle more to engage opioid users after their overdose:
But in terms of us paramedics, I think we’re ideally suited for it being a pre-hospital medicine. It’s quite different to in-hospital medicine and I think we get, you know, a different take on what kind of these people – what happens to these people, you know, in the initial stages of an overdose anyway. And certainly, in the pre-hospital interventions that we do, often they don’t go to hospital because like I say, often they just walk off or tell us to leave. So, I think we’re ideally placed really, to try and – at least try our best to try and get people to engage with this.
SP14
Yeah, I think it’s an extra tool I can use. It has its place, and I feel like it’s that kind of thing where because historically we were kind of reactive, using this we could be more proactive as well. So personally myself I think it’s a win/win situation.
SP5
I would say that a lot of times we would go to overdoses, that would then refuse to come in because you’d give them the NARCAN and they’d wake up, and have capacity to refuse. So it would, potentially, be good because you’re also in the environment of the house that they live in, with other people there in their actual environment. So I feel like I probably would have had more opportunities.
FG1
There was evidence of commitment from staff in helping patients to access the THN and managing the anxiety of patients who were coming out of overdose. Although interviewees primarily identified potential trial participants as opioid users who were experiencing overdose, THN had also been given pre-emptively for known opioid users who were attending for other reasons, at a point when they would potentially be more amenable to receiving the kit:
So I think I issued one for someone who had a groin abscess due to injecting, I knew was a heroin addict and just sort of said, “What do you think about this?” He was like, “Yeah, that’s probably a good idea, isn’t it?” So we signed him up.
SP11
Or sometimes we’d recruit them if they were active drug users, but they’d come in for another problem. A lot of the time we see people who come in with health problems relating to their drug use, but they haven’t actually overdosed, but they are at risk of overdose. So those people, ‘cos they’re here for a different reason, we would offer it to them.
SP08
Even people who’ve been quite difficult in A&E when I distributed it to someone, he was quite an aggressive patient. Yeah, post NARCAN treatment, they can be quite aggressive from it. But, once we sat down and talked him through it, he was actually sort of very switched on, compliant. I’ve given it the once, so my – I mean, my experience was good from it.
SPO2
Although recognising their role in widening access to THN, staff spoke of the inherent limitations to providing it through the ESs. They expressed reservations about the willingness and ability of this group of patients to engage with the THN training during their episode of emergency care due to reduced capacity to retain information, concerns about obtaining further drugs and desire to return home as soon as possible:
From my point of view, I think … it’s not been very successful, because of the reasons that I said about the engagement and the fact that we often struggle. Well, I admit that we often struggle to convince people to take it, to take the training or to even take the – what we’re offering them, in terms of the take-home naloxone. Often because they just don’t want to engage … and the other reason that just we’ve sort of spoilt their hit, which is awful, when you say it like that, it’s awful, I’m sort of in two minds about that, but yeah, I think that’s the number one thing I’ve found anyway.
SP14
So, I think, obviously, the people that generally present to ED having overdosed, will be drowsy, you know, take a while to recover, which obviously means that they’re not going to be able to take information in well, or engage. And, as I’ve said before, at the point that they then become more awake, then they don’t always want to stay to engage. So, that would be a barrier, I think.
SP09
While participants within this study expressed support for THN provision, their ability and willingness to drive it forward may be hindered by limited engagement from other staff. Sign-up for the trial had been limited and participants indicated that the beliefs and behaviours required to enable widespread acceptance of the intervention may not held by wider colleagues, with some evidence of wider scepticism about the provision of THN by ESs. Concerns arose from apprehension about the ability to make a significant difference at a population level and about the ‘safety net’ effect, whereby opioid users take a higher dose because they know the drug reversal drug is on hand if they do overdose:
I don’t personally agree with them, but other paramedics on station I spoke to were like, ‘Why on earth are we doing this? This isn’t something we should be doing. We’re just giving them kits and we’re essentially encouraging them to overdose again.’ Again, I disagree with that strongly, but I would say it’s split opinion a bit.
SP11
When we discussed it within our team, so over a cup of tea, I think there was a variation in enthusiasm for it. Some people wondering how effective it would be, feeling that it was difficult to target the people who would be most vulnerable. […] Some people felt it was, you know, a thimble full effect in a bucket full of problem.
SP6
Some patients are their own worst enemy, so they might have some controversial view of them. Just thinking “well, people who take drugs are a waste of space” and all this kind of stuff. They are getting fewer and fewer, but there is still this – because of the patient exposure that we get and the demand that we get, the amount of jobs that we’re going to on an hourly or daily basis, it is phenomenal[…], sometimes I think people’s empathy buckets have run out.
SP5
Normalisation process theory 3 – Collective action (how has it been delivered and what has enabled or hindered uptake?)
-
The trial was felt to be well resourced but high staff turnover impacted on numbers of staff recruiting patients in some sites.
-
Recruitment of patients to the trial was lower than expected due to lower-than-expected numbers of eligible patients (with many in the prehospital setting having persistent low consciousness levels, and others having already received a kit), low engagement from patients and changes in protocol.
-
Changes to protocol midway through the trial led to confusion about who was eligible, and when the trial was recruiting.
-
Opioid users were not always receptive and there were high numbers of refusals in the ED setting.
-
Recruitment of clinicians was low at some sites, particularly EDs, due to other competing trials, COVID-19 exhaustion and lack of conviction.
Staff generally felt that the trial intervention was well resourced, and was a positive, experience in particular with regards to the delivery and content of the training, but they struggled with recruitment of both other clinicians to train and opioid users to enrol in the trial. There was evidence of work undertaken by research leads to encourage and facilitate recruitment to the trial, particularly at the ED where there was a specific research manager who ‘championed’ the project:
She’s done very well, you know. The forms are very easy to find, you know, the – where the medication’s kept, it’s really easy to find. You know, there’s signs up.
SPO3
It all got cascaded down quite well for us from [Research Lead Name]. She was very helpful, and she was always on the end of an email. If we had any query or question, she would reply within a few hours, so that part was really good.
SP18
However, high staff turnover in both the AS and ED resulted in difficulties in recruiting and training sufficient staff for the trial to be adequately cascaded and maintained. Competing trials taking place at the same time was also felt to be an issue for recruitment and retention, as was the administrative burden of trial procedures (logging patients, Site 2 ASF paramedics signing the THN kit in and out for every shift) for paramedics in particular. This was particularly problematic in the context of the COVID-19 pandemic due to increased movement of staff to different locations and roles, and increased time and energy expended on dealing with the pandemic:
The difficulty was the trial had been running for quite a long time and the department has a really high turnover of staff. So I would train people and then they would leave, or there will be people that haven’t been trained in it that because they’re just coming through constantly, I couldn’t keep up. Also, even if they did have the training the trial’s been running over a period of two years so they might forget everything I’ve said or some of the things I’ve said.
SP8
We also had quite a lot of other trials going on at the same time. So, I think you need to – if you’ve got lots of people involved in trials, sometimes they just tend to concentrate on one. Whereas we had quite a lot of trials going on at the same time as this one.
SP7
Um, I think it was a bit – I understand this is study design, I do understand, but having the additional administrative burden of having to sign it out and take it everyday, and then remember to put it back at the end of the shift, to sign it in and out was probably a bit, what’s the word? A bit, er, a bit of a detractor from people’s passion for doing it.
SP2
Recruitment of patients to the trial was reported to be affected by limited numbers of eligible patients, high numbers of patient refusal and confusion around the trial design. Recruitment pauses due to protocol variations and the COVID-19 pandemic meant that staff were not always aware when recruitment was open and may have missed recruiting suitable patients when recruitment had re-opened without their knowledge. Participants reported a reduction in numbers of people using opioids on the streets and lower numbers of overdoses during the COVID-19 pandemic, with paramedics particularly noting a reduction in the number of potentially eligible patients that they saw:
Also, I think another barrier is having something like a global pandemic because everyone’s focus is on that and so we’re probably not treating other stuff as well as we normally would when we’ve got that focus. Although, to be honest, we are still functioning as a normal department, so we treat all manner of problems. It’s just having that focus makes it a bit difficult because it’s a distraction, and it changes a lot of our practices, and causes a lot of upheaval.
SP8
I think so. I think it can be difficult from a medical staff point of view, because in A&E we have a fairly high turnover. So, a lot of SHOs are only in the department for four months. And I think there was a big push with the trial and, you know, putting up the posters and really trying to make people aware. But with COVID and obviously other things that have gone on, I don’t know that the message has been as clear or consistent throughout the whole time.
SP9
Because we struggled initially, before coronavirus, and obviously when coronavirus came along with the first wave we stepped back because there was no way anybody was going to sort of have time or energy or anything to do. And I think the trial itself was paused as well.
SP2
In addition, management protocols for opioid overdose limited the number of potential candidates to the trial, as they recommend that ambulance crews tried to keep patients from fully coming out of overdose so they were safe but not fully alert. This precluded recruitment to the trial as patients with a low Glasgow Coma Score could not consent to participation in the trial:
So, I know when I went through my paramedic training, there was a big emphasis on if the, you know, respiratory rate is good and the observations are good, just keep them in that groggy state until we can get to somewhere that’s more – that’s safer. Be that a hospital or wherever it might be. But yeah, it’s – I think a majority of colleagues that I’ve worked with have found the same problem.
SP14 ES
Similarly, the increase in THN kits from elsewhere impacted on trial recruitment as patients with a THN kit either did not wish to be enrolled in the trial, or were ineligible to be recruited. THN kits had been obtained from drug services, peers or from the trial on a previous occasion. One paramedic stated that drug services had ‘already saturated the market’ (SP2 ES), and the community drugs team were identified to be handing out THN within one ED which temporarily prevented staff from recruiting ED patients to the trial:
They’ve already got sort of naloxone given to them by drug and alcohol services and they’re quite happy to just be on their way. Yeah, so I’d say maybe one colleague, I think I’ve heard of who has managed to be successful so far. Which isn’t a lot.
SP14 ES
I never actually managed to give any out. So, either they’ve already had it, because obviously, the [City] Drugs Project also give it out. The [ED] were also giving it out as well.
SP7
Staff reported finding many patients difficult to engage, either due to side effects of naloxone treatment or because they were unreceptive to the idea of THN, and not understanding their own risks. Additional time and commitment from staff were necessary due to the difficulties in recruiting and training patients when coming out of overdose, particularly when ensuring that instructions for use of THN were understood. This could be particularly challenging if patients were drowsy, confused or combative, as was the case when they were recruited post overdose and suffering with the side effects of naloxone treatment:
And often, if we give them such you know, a rapid reversal of the opiate, some of them can become quite aggressive, because they come around, they don’t know what’s happened. They’re confused, they’re hypoxic, you know, they can become quite aggressive, and there’s not much room to work in the back of an ambulance sometimes.
SP14ES
Well, it is an extra thing because you’ve got to go through the whole, what you do when you find an unconscious patient and CPR and all that kind of thing. Which can be quite time consuming, to get the person to understand and demonstrate that they understand.
FS4
Well, most of the time you have to talk them into it I would say. Most of the time they’re like, ‘No, I’ve got one already, I don’t want one. I’ve had one’. Or they’re just not interested at all, or they’re in denial because they’ll say, ‘Well no, ‘cos I don’t..’., even if they’ve attended the department with a life-threatening opiate overdose as a result of their use, they’ll say, ‘Well no, ‘cos I don’t use very much and I’m not at risk’.
SP8
Normalisation process theory 4 – Reflexive monitoring (how do participants reflect on or appraise the intervention?)
-
Staff struggled to assess the benefits of the intervention within emergency settings, partly due to contamination (THN kits from other sources).
-
Participants supported the use of the intervention but wished to see it adopted as standard practice in emergency settings, not as part of a wider trial.
-
Participants supported enabling staff in other roles to train and distribute THN, and also make the kits available to friends and family.
We asked participants how they perceived the success of the intervention. Overall, there appeared to have been little evaluation of the intervention in terms of formalised evaluation or audit, but support for continuation of the intervention outside of a trial.
Participants expressed concerns about how they could evaluate the benefits of the TIME trial itself (rather than just whether the trial was feasible) due to the proliferation of THN kits provided by other settings within the intervention areas (e.g. community providers or even drug services elsewhere within the Trusts). This not only influenced recruitment to the trial but made it difficult to understand whether changes in outcomes were due to the TIME trial or other initiatives. Due to the difficulties in engaging patients in the ambulance or ED when coming out of overdose, there was some scepticism about provision in the emergency settings being as valuable as other settings where staff were better able to engage patients:
So my experience in [City] is I’d say – I understand the – I think there’s saturation – not saturation but I think that drug services have got a longer term relationship with people, have done very well. I don’t know, and having said I don’t know I’m not saying this in a cynical way, I don’t know how much of an impact us carrying give away Naloxone has been for patients, if you see what I’m saying.
SP2
So I think the main barrier is the patients [laughs], because with the drugs project I think they find it easy to give out these kits because the patients are choosing to engage with them already. Whereas in the department, they have attended for another reason or they’ve attended because they’ve taken an overdose, but they’re here accidentally if you see what I mean. So a lot of the time they’re actually not interested in taking it because they’re not here for that.
SP8
However, participants understood the benefits of THN from seeing naloxone being used successfully outside of the TIME trial, and did not appear to be awaiting the results of the trial to understand the benefits of the intervention. Overall, both ED and paramedic staff perceived ES THN provision to be a low-cost, low-risk intervention that could and should be incorporated into standard work practices. Participants strongly supported the incorporation of the intervention into normal practice and rolling out to other EDs and ASs, outside the confines of a trial, which would address problems associated with consenting patients into the trial. Participants suggested ways of incorporating the intervention into usual practice, including incorporating distribution of THN into the induction and training process and ensuring THN was part of the usual ambulance kit:
I suppose it’s just mindsets, basically. Because a lot of the things that come in, say, thromboprophylaxis for people who can’t have a weight-bearing cast. That was brought in, this is now the policy, this is the checklist. And then it’s just gradually instilled in people. So just part of the process. So I suppose it’s just around that, isn’t it, really, just getting it into people’s mindsets that if you see somebody who’s had an opiate overdose, that is just part of their patient journey, it’s supposed to be part of their assessment and treatment, two parts.
FG4
Yeah, I mean, I would like it to be rolled out as a standard operating procedure, as a care pathway that is available to all staff in [the ambulance service]. And you know, every ambulance carries a drug box with naloxone. And I would like us to be able and empowered to hand that out in an appropriate way, as part of a standard operating procedure.
SP6ES
Suggestions about how to improve distribution included use of champions to explain the study, and broadening out the provision of THN to other people (friends and family) and by other roles (e.g. ambulance technicians). Staff felt that the intervention may be more effective if staff in different clinical roles were eligible to take part in the training and the distribution of the kit and if the intervention was made available to friends and family:
It’s being restricted to registered healthcare professionals, whereas naloxone can be administered by a much wider range of people. You don’t need to be a registered healthcare professional to administer it. But I understand why registered healthcare professionals have been recruited to the trial as the practitioners, but don’t necessarily see that as being a barrier to who could be involved in kind of take-home naloxone kind of distribution.
SP6
It’s difficult, it’s a fact, yeah, at the moment, because it needs to be signed out as a controlled drug, it’s mainly for the patient. Whereas, actually, sometimes it would be better if it wasn’t just the patient, if we could have the ability to give other people who are with them the kits as well.
FG3
Reflections on recruitment of staff to the qualitative interviews
Within our later interviews we asked why we had struggled to recruit stakeholders to undertake interviews. Paramedics described the impact of the COVID-19 pandemic where staff were prioritising urgent COVID-19 related work and had less time and energy available for non-urgent work. Participants related reasons mainly around poor communications, which were principally done via e-mail, with people being unaware of the thank-you payment offered or that we wanted to talk to anyone who had undertaken the training and not just those who had handed out kits:
We’re talking about staff groups who are traumatised and exhausted after a year of a lot going on. Um, and perhaps it’s just not been the optimal time to try and recruit people.
SP02
Summary
Overall, participants were positive about the intervention and perceived the intervention to be compatible with their roles. There was surprisingly limited discussion of potential risks associated with the intervention, with participants focussing principally on potential benefits, albeit with an acknowledgement that benefits may be limited to a subset of the intended population. Although there was some additional work associated with delivering the intervention, there was evidence of work being undertaken to improve provision of THN and to try to recruit patients. Recruitment into the trial was problematic for a number of reasons, including the COVID-19 pandemic, and staff supporting the idea of incorporating THN into everyday work without continuing the trial.
Incorporating THN into everyday practice may potentially remove some of the barriers associated with the trial (e.g. difficulty in consenting and training high-risk opioid users, choosing the best method to provide staff with kits, cascading the intervention in a setting with high staff turnover) and may encourage more widespread use when considered normal practice rather than research. Provision of training to wider staff groups may also help to mitigate negative attitudes towards opioid users that may hinder provision of the intervention. Similarly, provision of THN kits to friends and family may be more beneficial and reduce the problems associated with recruiting patients during overdose.
Chapter 7 Public and patient involvement and equality, diversity and inclusion
Public and patient involvement
We involved public contributors throughout this study in order to ensure research relevance, quality and accountability strengthen research rigour. 72
Our aim was to include people with experience of opioid addiction, as drug users or with a close connection to someone who used drugs. We identified individuals with experience of opioid addiction through family relationships, voluntary networks or individual circumstances. We involved them in designing the study proposal and in delivering the commissioned research. When designing this study, we sought the views of different groups linked to opioid use. When developing and designing the study we met with third-sector workers and individuals using a drug service, to discuss attitudes and experiences relating to overdose, THN and the proposed research. We also worked closely with individuals with experience within their family of opioid overdose who drew on personal experience to highlight the relevance of the research questions and comment on data collection methods and selection of outcomes. We wanted to be sure the research would be feasible so explored whether it seemed relevant and coherent to them and how likely people would be to take part. We also gained insight into personal experiences of overdose and THN, including how they perceived the services which respond to emergency overdose calls. These contacts enabled us to gain people’s views on the research question and the proposed methods. We also discussed how we could involve individuals in delivering the research and identified individuals and support groups interested in maintaining a relationship with the study team. The people we were in touch with gave their active support to the research aim to reduce death from opioid overdose. One was named a study co-applicant.
Throughout the process of delivering the TIME trial, we followed our intention to work closely with people with personal experience of opioid addiction and overdose. We sought their input to all stages of research implementation, oversight and dissemination.
Our public contributor co-applicant was a TMG member and included in all meeting discussions and communication. Her experience in relation to opioid overdose, which contributed to her commitment to the research, also interrupted her ability to routinely attend research meetings. We recognised her personal commitments. When domestic responsibilities limited her ability to attend meetings, the public and patient involvement (PPI) lead met with her at another convenient time to discuss project progress and obtain her feedback. We discussed other ways she could contribute to the study and agreed she would give comments and feedback by e-mail between meetings.
We wanted to supplement involvement in delivering the study by creating a second, more accessible route for linking with people with relevant experience. We recognised and had been advised by people we talked to when planning the study, that it would be challenging for people to join a formal research team meeting. We identified the Sheffield Addiction Research Recovery Panel (ShARRP), which is a group of people with experience of dependent/problematic drug and alcohol use, either personally or as carer, partner or relative of someone. Selected team members met periodically with ShARRP. The team members reported study progress and sought comments, suggestions and relevant experience from Panel members to inform research implementation. Through this route they also identified individuals to attend TMG meetings, to speak from the insight provided by membership of ShARRP. 73 These individuals joined some meetings but were not able to consistently attend, so the ShARRP meetings provided a useful supplement to accessing individuals and gaining feedback.
Personal commitments and circumstances made it difficult for any individual to sustain their involvement for the full study period. While the research team made regular efforts to involve people with relevant experience and enable their contributions, we did not have the opportunity to build long-term relationships. 74,75 However, we minimised the lack of continuity by meeting public contributors in their settings and groups. Hearing their stories reinforced for us the challenging circumstances of experiencing, or associating with, opioid addiction. 76
We also recruited two public contributors to join the TSC to provide experience-based expertise alongside input from members with academic, clinical and methodological skills. They were involved in all the TSC meetings. We recruited these individuals through the Public Involvement Community in Wales supported by Health and Care Research Wales and through networks known to the study team. They had relevant experience and also skills which enabled them to be active members of the TSC.
We supported all public contributors in line with the UK Standards for Public Involvement. 72 We offered honoraria and reimbursement of all expenses. We sought flexible routes to seek public contributions and communicate with individuals with relevant experience. We named a co-applicant as PPI lead, who was supported by other team members able to use their skills and geographic location for this aspect of our collaboration. We have reported our experiences in line with best practice. 77
Equality, diversity and inclusion
The methods of participant recruitment for this study did not proactively target specific groups, but maintained an equitable approach. Due to the nature of this project, participants are often marginalised and from deprived socioeconomic backgrounds. We offered an honorarium to all people we invited to be involved as public contributors and provided access to a free Citizens Advice Bureau-supported helpline for advice on claiming this.
Chapter 8 Discussion, conclusion and recommendations
Key findings
Discriminant function
The small proportion of opioid-related deaths limited our ability to identify a small high-risk subsection of the overall population. While there were differences between decedents from opioid overdose, decedents from all other causes, and those still alive, at individual level, people that died from opioid overdose were insufficiently different from members of the other two (more numerous) cohorts. In addition, a relatively high proportion of those that died from opioid poisoning had no healthcare event recorded in the year prior to death. This meant that we would need to monitor roughly one-third of the population in order to include approximately 75% of decedents (from opioid overdose) within that year. With mortality over 1 year of approximately 6% in that third of the population, and approximately 1–2% of these categorised as deaths from opioid overdose, it follows that reducing substantially or even eliminating entirely such deaths would have little effect on mortality rate in the identified ‘high-risk’ subsection.
Given the weak predictive link between deaths from and healthcare events related to opioid overdose, using routine data to identify with sufficient precision those at high risk of death from opioid overdose currently seems infeasible.
Feasibility randomised trial
Four sites participated in the trial and 299 clinical staff were trained. Sixty THN kits were supplied to patients during the 1-year recruitment. Eligible patients were not offered THN kits 164 times: ‘forgot’ (n = 136); ‘too busy’ (n = 15); suspected intentional overdose (n = 3).
In this trial, recruitment of clinical staff and distribution of THN kits were low, with considerable variation between participating sites. Distribution of kits was particularly low in the prehospital setting.
Qualitative
Service users supported the provision of THN kits and training in the emergency setting, as did the service providers. However, both raised concerns with regards to reluctance of the target group attending hospital and their ability to give consent. Both also suggested that a greater focus on family and friends of opioid users may be more appropriate and could improve uptake of THN kits.
Health economics
This study has shown that the methods for collecting costs relating to the naloxone kits and the training of NHS staff are feasible and can take into account differences in the form of training. However, we have been unable to assess the feasibility of collecting patient training times via staff recall. It is unlikely that this finding represents a serious barrier to the conduct of any future evaluation of THN. Cost-effectiveness is unlikely to be sensitive to uncertainties related to training costs,71 in a UK-based evaluation of THN provision in an emergency setting, so this should be recognised when designing future data collection; expensive or disruptive data collection is unlikely to be worthwhile.
Study limitations
-
Quality of coding: currently, many healthcare events have missing inadequate codes. There is a linked lack of granularity – currently events recorded in EDDS and PEDW have been categorised as opioid overdose-related or not. It is unclear that if there would be improvement in an extended classification of events.
-
We have noted above the major limitation that a considerable number of decedents from opioid overdose have no recorded events in routine data in the 12 months (or even in the 36 months) prior to the date of death. Our approach extends in principle to include further data sources, but we note the regulatory and logistical hurdles to doing so in practice. Further data sources include: the Welsh Longitudinal GP data set, potentially including data on smoking/alcohol usage; drug prescriptions; AS records (including records on reasons for emergency call, ambulance attendance and disposition; police and probation/justice data; and data from third-sector organisations working with opioid and other drug and substance users, and their peers.
-
The COVID-19 pandemic resulted in pauses in recruitment and an increase in strain on the ES which may have impacted recruitment of both staff and patients.
-
Unforeseen difficulties with Confidentiality Advisory Group (CAG) and NHS Digital approval to retrieve data for application of the discriminant function to a second population in England and to then retrieve outcomes for the population identified contributed to delays and the decision to discontinue this stage of the research. We were caught between CAG and NHS Digital for many months without clarity as to how to move forward. NHS Digital required detail of the discriminant function prior to approval; however, we were unable to produce the function without a means to test it on the English data.
-
We found some problems with variations in the quality of data. Regarding the staff training data, one centre produced high-quality data; two centres generated data with some discrepancies; and one centre failed to use the data collection tool at all. The number of patients identified as eligible for the trial was also vastly different across sites. While this may have been the case, it is more likely that some sites were more invested in the trial and proactively looking for eligible patients compared to others. In Site 2 ED, where the research nurse was redeployed following COVID-19 for several months, this seemed to be a particular issue. All of these problems are considered resolvable in any future trial.
-
Not all sites were able to provide staff for focus groups, which may have resulted in missing some perspectives of the service providers. It may have been that those who felt more positively towards the intervention were willing to take part in the qualitative aspect of the study. Within the study timetable and resources, and considering COVID-19 delays, we could not pursue this further.
Public and patient involvement
We involved public contributors throughout this study in order to ensure research relevance, quality and accountability and strengthen research rigour. We recruited two public contributors to join the TSC to provide experience-based expertise alongside input from members with academic, clinical and methodological skills. They were involved in all the TSC meetings. However, personal commitments and circumstances made it difficult for any individual to sustain their involvement for the full study period. To minimise the impact of the lack of continuity, we met public contributors in their settings and groups, allowing us to hear their stories, which reinforced for us the challenging circumstances of experiencing, or associating with, opioid addiction.
Interpretation
We found that THN kit administration was overall low in this study, with considerable variation between sites. Reasons given for this were related to the emergency care setting – staff were busy and under pressure; patients were undergoing emergency treatment, mainly for overdoses, and were often not fit or willing to consent to receipt of the kit or the training. There may be ways to overcome these barriers – the protocol for administration of kits could, for instance, be more flexible. A greater focus on relatives and friends may increase the chance of success of TNH provision in the emergency setting. Research conducted in the USA reported that nearly half of the kits distributed by ESs were given to family members with the patient themselves being the second largest group to receive the kit. 78 A study assessing the acceptance of nasal naloxone in the ED reported low uptake, with barriers such as difficulties identifying the ‘right’ patient, access to the kits and lack of clarity as to when to offer the kit due to the patient typically not waiting for formal discharge so they may not get a chance to be offered a kit. 79 The barriers mentioned are similar to those mentioned by service users and providers in this study and indicate that although there is a general consensus that the ED is a suitable setting to provide THN kits due to the contact with the patient group which may not receive contact with the health service in other means, there are issues with regard to the feasibility of the intervention. These findings are in line with other research which found the ED to be a suitable point for THN kit distribution and training but reported ED staff didn’t have enough time for training and patient identification workflow which could hinder the implementation of this intervention in the ED. 38,80 A more recent study assessing methods of increasing THN prescribing in the ED found that although barriers remain, with improved, targeted staff training, the use of works aid such as best-practice advisory tools can increase the prescription of THN kits in the ED. 81 Interestingly, we found that both the service users and service providers believed in focusing on the relatives and friends of opioid users; however, a 2022 European study assessing the attitudes and likelihood of using THN kits reported that opioid users were significantly more likely to witness an overdose and use a THN kit compared to the family. 44 It may also be possible to identify patients for administration of a THN kit at the time of follow-up, rather than during the emergency episode.
There is overall support from both ED staff and opioid users to provide THN kits and training in the emergency setting. Further findings in this study highlighted issues with regards to recruiting eligible staff in some sites; we were unable to reach the target of training 50% or more of eligible staff in two of the four intervention sites, and therefore did not meet he progression criteria. It is however worth noting that Site 2 ED, which only recruited 8% of staff, and Site 1 ED, which recruited 55% of staff, are similar-sized EDs with similar throughputs and have used an identical approach to the recruitment of staff and contact with the academic research team, but have had very different outcomes in training staff, recording training data and were also unable to provide staff to contribute to focus groups. This indicates that the issues with regards to staff recruitment may lie within the EDs themselves, potentially including pressures of the department, staff support for the project and other factors which could not be accounted for in this study but may be work investigating for future research. It could be beneficial for future research to tailor recruitment and training to each ED and provide different support to each ED depending on their requirements. Furthermore, barriers in recruiting ED staff identified from this research have been documented and include general pressures of the ED including large numbers of patients with challenging circumstances of admittance and unpredictable working environment. Other barriers were identified to be specific to research including a lack of face-to-face communication between the academic research team and research nurses recruiting, as well as a lack of support for research nurses during recruitment which can cause a sense of unease and concern about their confidence in identifying eligible staff and patients. 70
Methodologically, this is a challenging area for research and evaluation. There are four main interrelated issues, some of which relate to the specific target population for the intervention, and some of which apply more generally to the conduct of trials in emergency care:
-
Identification of trial population: The THN kits are intended for peer administration. Therefore, the person who benefits from the naloxone may not be the recipient of the kit. Standard RCT recruitment strategies will not work, an alternative has to be found to capture outcomes. We tried to do this through attempting to identify a high-risk population to include in a trial; however, we found that sensitivity and specificity of a discriminant function were both low, and it would not be feasible to use this method in a trial.
-
Consenting patients to any trial in the emergency setting is challenging. In practice, with ethical approval, we often consent them to treatment at the point of recruitment, and to follow up at a later point. In this study we knew from our previous feasibility study49 that follow-up rates would be extremely low and we would not be able to count on consenting patients to follow up after the emergency episode itself.
-
Further to this point, it is very challenging to include self-reported outcomes in a trial in the population. For this reason, we proposed using anonymised linked routine data outcomes, but were unable to define the high-risk population to include in outcome comparisons.
-
With opioid-related death being a relatively rare event, and low THN administration levels, data linkage becomes problematic, as individuals may be at risk of becoming identifiable.
Recommendations for research
The evidence base for distribution of THN in terms of safety, costs and effects is very thin – in any setting, but particularly so in the emergency care environment. It remains the case that the safety and effectiveness of this intervention needs to be evaluated, and that those who suffer an opioid-related emergency may be at highest risk. However, in the light of our findings related to feasibility of the intervention as designed in this study, and our proposed trial methods, in order of priority we recommend the following research be undertaken:
-
What modifications could be made to the intervention to increase its uptake in the emergency setting?
-
Could the intervention be distributed to family or friends of the opioid user?
-
Could the intervention be distributed at follow up, rather than during the initial emergency episode?
-
How can the rate of missed recruitments be reduced? – how could we make sure healthcare staff are more invested in trials? – particularly in this patient group.
-
-
Is there another setting in which THN could be distributed and evaluated? For example, could the intervention be feasible in the primary care setting?
-
What are the self-reported outcomes of patients who are given THN kits?
-
How can a randomised trial be designed for this population in this setting? How can those who may benefit from the naloxone in the THN kits be identified and followed up? Is there an alternative rigorous study design that could be used?
Conclusion
Rigorous research evidence about safety, costs and effectiveness of THN is still needed to inform policy and practice. We recognise the emergency setting may be a good starting point for identification of people at high risk of harm from opioids and, potentially, for THN kit provision. However, in this feasibility study we did not meet our preset progression criteria related to staff and patient recruitment and to the identification of high-risk patients for outcome comparisons. Therefore, we conclude that a full RCT based on the intervention and methods that we tested is not feasible.
Additional information
Contributions of authors
Helen Snooks (https://orcid.org/0000-0003-0173-8843) (Professor of Health Services Research at Swansea University), chief investigator, led the development of the research question and study design, was responsible for trial delivery and conduct, led the drafting and editing of the final report, contributed to the writing of the report and approved the final version.
Jonathan Benger (https://orcid.org/0000-0001-6131-0916) (Professor of Emergency Care in the School of Health and Social Wellbeing at the University of the West of England, Bristol), co-applicant, contributed to the study design, protocol development and delivery of the trial at the Bristol Royal Infirmary, contributed to the writing of the report and approved the final version.
Fiona Bell (https://orcid.org/0000-0003-4503-1903) (Head of Research at Yorkshire Ambulance Service), principal investigator for Yorkshire Ambulance Service, contributed to the writing of the report and approved the final version.
Sarah Black (https://orcid.org/0000-0001-6678-7502) (Head of Research, Audit and Quality Improvement at South Western Ambulance Service NHS Foundation Trust) co-applicant, principal investigator for South Western Ambulance Service NHS Foundation Trust, contributed to the study design, supported the delivery of the trial at this site, contributed to the writing of the report and approved the final version.
Simon Dixon (https://orcid.org/0000-0001-7394-7009) (Health Economist based at the University of Sheffield) wrote the analysis plan for the health economic evaluation, analysed health economics data and led the drafting of the health economics chapter, contributed to the writing of the report and approved the final version.
Helena Emery (https://orcid.org/0000-0003-1890-4254) (Research Officer, Swansea University), project manager; co-ordinated the drafting and editing of final report, contributed to the writing of the report and approved the final version.
Bridie Angela Evans (https://orcid.org/0000-0003-0293-0888) (Research Officer, Swansea University), co-applicant, public and patient involvement lead, led the writing of the public involvement chapter, contributed to the writing of the report and approved the final version.
Gordon Fuller (https://orcid.org/0000-0001-8532-3500) (Consultant in emergency medicine and a NIHR fellow in health data science), co-applicant, contributed to the writing of the report and approved the final version.
Rebecca Hoskins (https://orcid.org/0000-0001-7883-437X) (Consultant Nurse and Senior Lecturer in the School of Health and Social Wellbeing at the University of the West of England, Bristol), co-applicant, principal investigator at the Bristol Royal Infirmary, contributed to the writing of the report and approved the final version.
Jane Hughes (https://orcid.org/0000-0003-1389-3402) (Site Researcher based at Sheffield University), service user involvement lead and qualitative support. Led writing of the service user chapter, contributed to the writing of the report and approved the final version.
Jenna Jones (https://orcid.org/0000-0002-1280-4941) (Research Officer and GP Registrar in Swansea Bay University Health Board), previous research manager, co-led the writing of the final report, contributed to the writing of the report and approved the final version.
Matthew Jones (https://orcid.org/0000-0002-6974-8725) (Research Officer at Swansea University and Psychotherapist), co-applicant, previous research manager, led the writing of the study protocol, contributed to the writing of the report and approved the final version.
Sasha Johnston (https://orcid.org/0000-0002-9904-8834) (Health Education England Clinical Fellow (Mental Health) and Research Paramedic with South Western Ambulance Service NHS Foundation Trust), PRSO for principal investigator for South Western Ambulance Service NHS Foundation Trust, contributed to the writing of the report and approved the final version.
Jaqui Long (https://orcid.org/0000-0002-6889-6195) (Researcher, Sheffield University) undertook some of the analysis for both of the qualitative chapters, contributed to writing the qualitative chapters, contributed to the writing of the report and approved the final version.
Chris Moore (https://orcid.org/0000-0002-2192-3002) (Head of Medicines Management and Principal Investigator for Welsh Ambulance Services NHS Trust), co-applicant, contributed to the writing of the report and approved the final version.
Rakshita Parab (https://orcid.org/0000-0003-1822-657X) (Research Officer at Swansea University) led the RCT recruitment data analysis, drafted chapter related to epidemiology and discriminant function, contributed to the writing of the report and approved the final version.
Richard Pilbery (https://orcid.org/0000-0002-5797-9788) (Research data analyst at Yorkshire Ambulance Service NHS Trust), PRSO for Yorkshire Ambulance Service NHS Trust, contributed to the writing of the report and approved the final version.
Fiona C Sampson (https://orcid.org/0000-0003-2321-0302) (Site Researcher, Sheffield University), service provider involvement lead and qualitative support, led writing of the service provider data chapter, contributed to the writing of the report and approved the final version.
Alan Watkins (https://orcid.org/0000-0003-3804-1943) (Professor of statistics at Swansea University), senior statistician, developed analysis plan, discriminant function and analysed data. Led draft of epidemiology and discriminant function chapter, contributed to the writing of the report and approved the final version.
Acknowledgements
This study was only possible thanks to the expertise and commitment of a wide range of people and organisations. Although we specifically acknowledge the following parties, we would like to extend our thanks to all of those who supported this work.
-
All the participating hospitals, ASs and their staff.
-
All patients and stakeholders who participated in the trial.
-
NIHR HTA for funding and then supporting this research throughout.
-
Anne Surman, Daniel Holohan, Neil Jenkinson and Hayley Dash for administrative support.
-
Members of our DMC: Ranjit Lall (Chair), Andy Rosser, Dalya Marks and Matthew Reed.
-
Members of our TSC: Kathy Rowan (Chair), Derin Omole, Ben Carter, Elwyn Thomas, Ian Mursell, Andrew McAuley and John Ryan.
-
Our patient and public representatives: Jackie McCarthy and Rosita Wilkins.
-
The SAIL Databank team at Swansea University.
-
A special thanks to Jake Alhiliali, Penny Buykx, Timothy Driscoll, Adrian Edwards, Christopher Evans, Steve Goodacre, Kelly Hird, Ann John, Barbara Lawrence, Rosanna Oretti, Emma Parry, Robin Roop and Paul Williams for their support throughout the project.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
TIME data within SAIL is securely stored within the Databank. Applications to access data held within the SAIL Databank require approval following independent review by their Information Governance Review Panel (IGRP), and are subject to SAIL’s terms, conditions and data disclosure and dissemination policies and procedures. TIME data within the SAIL Databank are held in compliance with its data retention, archiving and data destruction policies. All other queries should be addressed to the Corresponding author.
Ethics statement
The TIME study was approved by the Health Research Authority on 8 January 2019 with a favourable opinion received from the Research Ethics Committee (reference number: 18/WA/0337) on 16 October 2018.
Information governance statement
Swansea University is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679.
Under the Data Protection legislation, Swansea University is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://www.swansea.ac.uk/aboutus/compliance/data-protection/.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/YNRC8249.
Primary conflicts of interest: Alan Watkins declares his role as a member of the NIHR HS&DR Funding Committee, 2018–present. Helen Snooks declares her role as a member of the NIHR HTA & EME Editorial Board, 2015–present. Swansea University is the study sponsor. The protocol is available at https://doi.org/10.1186/s40814-020-00626-w.
Publication
Snooks HA, Jones JK, Bell FB, Benger JR, Black SL, Dixon S, et al. Take-home naloxone administered in emergency settings: feasibility of intervention implementation in a cluster randomized trial. BMC Emerg Med 2024;24(1):155. https://doi.org/10.1186/s12873-024-01061-3
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Jones M, Bell F, Benger J, Black S, Buykx P, Dixon S, et al. Protocol for Take-home naloxone In Multicentre Emergency (TIME) settings: feasibility study. Pilot Feasib Stud 2020;6:1-10.
- Lewer D, Brothers TD, Van Hest N, Hickman M, Holland A, Padmanathan P, et al. Causes of death among people who used illicit opioids in England, 2001–18: a matched cohort study. Lancet Publ Health 2022;7:e126-35.
- Snowdon J. Drug overdose death rates in different countries: who should be alarmed?. Austral Psychiat 2022;30:26-30.
- van Amsterdam J, van den Brink W, Pierce M. Explaining the differences in opioid overdose deaths between Scotland and England/Wales: implications for European opioid policies. Eur Addict Res 2021;27:399-412.
- Pierce M, van Amsterdam J, Kalkman GA, Schellekens A, van den Brink W. Is Europe facing an opioid crisis like the United States? An analysis of opioid use and related adverse effects in 19 European countries between 2010 and 2018. Eur Psychiat 2021;64.
- Black C. Review of Drugs: Phase One Report. London: Gov. UK; 2020.
- Shearing H. Drug deaths in England and Wales highest since 1993. BBC 2021.
- Ely J. Britain’s cocaine epidemic laid bare: Drug deaths hit record high with coke fatalities up 7-FOLD in decade as experts blame COVID lockdowns for fuelling addiction crisis and middle class’s love for ‘gear’ (which is delivered quicker than pizza!). Daily Mail 2022.
- McKenzie S. CNN 2021. https://edition.cnn.com/2021/08/07/europe/trainspotting-scotland-drug-related-deaths-cmd-intl/index.html (accessed 28 September 2023).
- Lewer D, Padmanathan P, ul Arfeen Q, Denaxas S, Forbes H, Gonzalez-Izquierdo A, et al. Healthcare use by people who use illicit opioids (HUPIO): development of a cohort based on electronic primary care records in England. Wellcome Open Res 2020;5.
- O’Mara B. The effectiveness of changes to drug policy, regulation and legislation for reducing harms associated with opioids and supporting their medicinal use in Australia, Canada and the UK: a systematic review. Evid Base: J Evid Rev Key Policy Areas 2020;2020:79-110.
- Alho H, Dematteis M, Lembo D, Maremmani I, Roncero C, Somaini L. Opioid-related deaths in Europe: strategies for a comprehensive approach to address a major public health concern. Int J Drug Policy 2020;76.
- Zibbell J, Howard J, Clarke SD, Ferrell A, Karon S. Non-Fatal Opioid Overdose and Associated Health Outcomes: Final Summary Report. US Department of Health and Human Services; 2019.
- Jiang R, Lee I, Lee TA, Pickard AS. The societal cost of heroin use disorder in the United States. PLOS ONE 2017;12.
- Warner‐Smith M, Darke S, Day C. Morbidity associated with non‐fatal heroin overdose. Addiction 2002;97:963-7.
- Stoové MA, Dietze PM, Jolley D. Overdose deaths following previous non‐fatal heroin overdose: record linkage of ambulance attendance and death registry data. Drug Alcohol Rev 2009;28:347-52.
- Ryan JM, Spronken I. Drug related deaths in the community: a preventive role for accident and emergency departments?. J Accid Emer Med 2000;17:272-3.
- Baca CT, Grant KJ. Take‐home naloxone to reduce heroin death. Addiction 2005;100:1823-31.
- Take-Home Naloxone for Opioid Overdose in People Who Use Drugs. London: Public Health England; 2017.
- Yealy DM, Paris PM, Kaplan RM, Heller MB, Marini SE. The safety of prehospital naloxone administration by paramedics. Ann Emerg Med 1990;19:902-5.
- McDonald R, Strang J. Are take‐home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 2016;111:1177-87.
- Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, Sorensen-Alawad A, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ 2013;346.
- Kerensky T, Walley AY. Opioid overdose prevention and naloxone rescue kits: what we know and what we don’t know. Addict Sci Clin Pract 2017;12.
- WHO-UNODC ‘Stop Overdose Safely (SOS)’ Initiative. Geneva: World Health Organization; 2020.
- Advisory Council on the Misuse of Drugs . Consideration of Naloxone 2012.
- European Monitoring Centre for Drug and Drug Addiction . Take-Home Naloxone 2020.
- McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids: conception and maturation. Drug Alcohol Depend 2017;178:176-87.
- Substance Misuse: Harm Reduction Database Wales (HRD). Wales: Public Health Wales; 2018.
- Skulberg AK, Tylleskär I, Valberg M, Braarud A-C, Dale J, Heyerdahl F, et al. Comparison of intranasal and intramuscular naloxone in opioid overdoses managed by ambulance staff: a double-dummy, randomised, controlled trial. Addiction 2022;117:1658-67.
- Rege SV, Ngo DA, Ait-Daoud N, Sharma S, Verplancken E, Holstege CP. Trends and characteristics of naloxone therapy reported to US poison centers. Addiction 2018;113:2309-15.
- Rzasa Lynn R, Galinkin J. Naloxone dosage for opioid reversal: current evidence and clinical implications. Therap Adv Drug Safe 2018;9:63-88.
- Moustaqim-Barrette A, Dhillon D, Ng J, Sundvick K, Ali F, Elton-Marshall T, et al. Take-home naloxone programs for suspected opioid overdose in community settings: a scoping umbrella review. BMC Publ Health 2021;21.
- Harm Reduction Database Wales: Take-Home Naloxone. Wales: Public Health Wales; 2016.
- National Naloxone Programme Scotland: Annual Monitoring Report 2019/20 and 2020/21. Glasgow: Public Health Scotland; 2022.
- Stam NC, Gerostamoulos D, Smith K, Pilgrim JL, Drummer OH. Challenges with take-home naloxone in reducing heroin mortality: a review of fatal heroin overdose cases in Victoria, Australia. Clin Toxicol 2019;57:325-30.
- Moore C, Lloyd G, Oretti R, Russell I, Snooks H. Paramedic-supplied ‘Take Home’ Naloxone: protocol for cluster randomised feasibility study. BMJ Open 2014;4.
- Dwyer K, Walley AY, Langlois BK, Mitchell PM, Nelson KP, Cromwell J, et al. Opioid education and nasal naloxone rescue kits in the emergency department. Western J Emerg Med 2015;16:381-4.
- Gunn AH, Smothers ZP, Schramm-Sapyta N, Freiermuth CE, MacEachern M, Muzyk AJ. The emergency department as an opportunity for naloxone distribution. Western J Emerg Med 2018;19:1036-42.
- European Monitoring Centre for Drug and Drug Addiction . United Kingdom Country Drug Report 2019.
- Working Together To Reduce Harm: Substance Misuse Annual Report and Forward Look 2018. Welsh: Welsh Government; 2018.
- Turner D, Morgan G, Smith J. Harm Reduction Database Wales; Take Home Naloxone. Wales: Public Health Wales; 2018.
- NHS Scotland . National Naloxone Programme Scotland Monitoring Report 2017 2018 2018.
- NHS Scotland . Prevalence of Problem Drug Use in Scotland 2015 16 Estimates 2019.
- McDonald R, Eide D, Abel-Ollo K, Barnsdale L, Carter B, Clausen T, et al. A rapid assessment of take-home naloxone provision during COVID-19 in Europe. Int J Drug Policy 2022;107.
- Tas B, Humphreys K, McDonald RS, Strang JS. Should we worry that take-home naloxone availability may increase opioid use?. Addiction 2019;114:1723-5.
- Abdelal R, Banerjee AR, Carlberg-Racich S, Darwaza N, Ito D, Epstein J. The need for multiple naloxone administrations for opioid overdose reversals: a review of the literature. Subst Abus 2022;43:774-84.
- Tse WC, Djordjevic F, Borja V, Picco L, Lam T, Olsen A, et al. Does naloxone provision lead to increased substance use? A systematic review to assess if there is evidence of a ‘moral hazard’ associated with naloxone supply. Int J Drug Policy 2022;100.
- McAuley A, Bouttell J, Barnsdale L, Mackay D, Lewsey J, Hunter C, et al. Evaluating the impact of a national naloxone programme on ambulance attendance at overdose incidents: a controlled time–series analysis. Addiction 2017;112:301-8.
- Moore C, Lloyd G, Oretti R, Russell IT, Snooks H. Paramedic Supplied ‘Take Home’ Naloxone: A Randomised Feasibility Study. BMJ Publishing Group Ltd and the British Association for Accident; 2016.
- Kestler A, Buxton J, Meckling G, Giesler A, Lee M, Fuller K, et al. Factors associated with participation in an emergency department–based take-home naloxone program for at-risk opioid users. Ann Emerg Med 2017;69:340-6.
- Adult Substance Misuse Treatment Statistics 2019–2020: Report. London: Public Health England; 2020.
- Bradbury M, Lewer D. Role of community drug and alcohol services in physical healthcare for people who use illicit opioids: a qualitative study of clinical staff in the UK. BMJ Open 2021;11.
- Lyons RA, Jones KH, John G, Brooks CJ, Verplancke J-P, Ford DV, et al. The SAIL databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak 2009;9:1-8.
- Digital Health and Care Wales . PEDW Data Online n.d. https://dhcw.nhs.wales/information-services/health-intelligence/pedw-data-online/ (accessed 28 September 2023).
- Fuller GW, Jones M, Bradshaw CA, Jones J, John A, Snooks H, et al. The socio-demographics and health service use of opioid overdose decedents in Wales: a cross-sectional data linkage study. Eur Addict Res 2022;28:226-30.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348.
- Dowling J, Isbister GK, Kirkpatrick CM, Naidoo D, Graudins A. Population pharmacokinetics of intravenous, intramuscular, and intranasal naloxone in human volunteers. Ther Drug Monit 2008;30:490-6.
- Kerr D, Kelly AM, Dietze P, Jolley D, Barger B. Randomized controlled trial comparing the effectiveness and safety of intranasal and intramuscular naloxone for the treatment of suspected heroin overdose. Addiction 2009;104:2067-74.
- Kelly AM, Kerr D, Koutsogiannis Z, Dietze P, Patrick I, Walker T. Randomised trial of intranasal versus intramuscular naloxone in prehospital treatment for suspected opioid overdose. Med J Aust 2005;182:24-7.
- Furyk JS, Lawton LD, Ting JY, McD Taylor D. Group ACfEMCT . Informed consent in emergency care research: an oxymoron?. Emerg Med Austral 2017;29:110-2.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54.
- Jones KC, Burns A. Unit Costs of Health and Social Care 2021. n.d.
- NHS England . NHS England National Cost Collection 2020 21 Index 2022.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8:1-11.
- Corbin J, Strauss A. Basics of Qualitative Research. Thousand Oaks, CA: SAGE Publications Ltd; 2015.
- Silverman D. Doing Qualitative Research: A Practical Handbook. Thousand Oaks, CA: SAGE Publications Ltd; 2013.
- Berends L, Johnston J. Using multiple coders to enhance qualitative analysis: The case of interviews with consumers of drug treatment. Addict Res Theor 2005;13:373-81.
- Huddlestone L, Turner J, Eborall H, Hudson N, Davies M, Martin G. Application of normalisation process theory in understanding implementation processes in primary care settings in the UK: a systematic review. BMC Fam Pract 2020;21:1-16.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. PAFS Consensus Group . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355.
- Wait S. Engaging nurses in patient recruitment to research trials in the emergency department. Emerg Nurse 2022;31:27-31.
- Langham S, Wright A, Kenworthy J, Grieve R, Dunlop WC. Cost-effectiveness of take-home naloxone for the prevention of overdose fatalities among heroin users in the United Kingdom. Value Health 2018;21:407-15.
- UK Standards for Public Involvement n.d. https://sites.google.com/nihr.ac.uk/pi-standards/standards (accessed 15 January 2024).
- Evans BA, Porter A, Snooks H, Burholt V. A co-produced method to involve service users in research: the SUCCESS model. BMC Med Res Methodol 2019;19:1-10.
- Jinks C, Carter P, Rhodes C, Taylor R, Beech R, Dziedzic K, et al. Patient and public involvement in primary care research: an example of ensuring its sustainability. Res Invol Engag 2016;2.
- Evans BA, Carson-Stevens A, Cooper A, Davies F, Edwards M, Harrington B, et al. Implementing public involvement throughout the research process: experience and learning from the GPs in EDs study. Health Expect 2022;25:2471-84.
- Staley K. Changing what researchers’ think and do’: is this how involvement impacts on research?. Research for All 2017;1:158-67.
- Staniszewska S, Brett J, Mockford C, Barber R. The GRIPP checklist: strengthening the quality of patient and public involvement reporting in research. Int J Technol Assess Health Care 2011;27:391-9.
- Scharf BM, Sabat DJ, Brothers JM, Margolis AM, Levy MJ. Best practices for a novel EMS: based naloxone leave behind program. Prehosp Emerg Care 2021;25:418-26.
- Drainoni M-L, Koppelman EA, Feldman JA, Walley AY, Mitchell PM, Ellison J, et al. Why is it so hard to implement change? A qualitative examination of barriers and facilitators to distribution of naloxone for overdose prevention in a safety net environment. BMC Res Notes 2016;9.
- Lacroix L, Thurgur L, Orkin AM, Perry JJ, Stiell IG. Emergency physicians’ attitudes and perceived barriers to the implementation of take-home naloxone programs in Canadian emergency departments. CJEM 2018;20:46-52.
- Funke M, Kaplan MC, Glover H, Schramm-Sapyta N, Muzyk A, Mando-Vandrick J, et al. Increasing naloxone prescribing in the emergency department through education and electronic medical record work-aids. Joint Commiss J Qual Patient Safe 2021;47:364-75.
List of abbreviations
- ACMD
- British Advisory Council on the Misuse of Drugs
- ADDE
- annual district death extract
- AfC
- agenda for change
- AS
- ambulance service
- BLS
- basic life support
- CAG
- Confidentiality Advisory Group
- CCDS
- Critical Care Data Set
- COVID
- coronavirus disease
- DHCW
- Digital Health and Care Wales
- DMC
- Data Monitoring Committee
- DMEC
- Data Monitoring and Ethics Committee
- ED
- emergency department
- EDDS
- Emergency Department Data Set
- ES
- emergency service
- GCS
- Glasgow Coma Scale
- GP
- general practitioner
- HRGs
- Healthcare Resource Groups
- HTA
- Health Technology Assessment
- ICD
- International Classification of Diseases
- IM
- intramuscular
- IN
- intranasal
- ITU
- intensive treatment unit
- LSOA
- lower super output areas
- MIUs
- minor injury units
- NIHR
- National Institute for Health and Care Research
- NPT
- normalisation process theory
- ONS
- Office of National Statistics
- OPCS
- Office of Population Censuses and Surveys
- PEDW
- Patient Episode Database for Wales
- PGD
- patient group direction
- PPI
- public and patient involvement
- PRIME
- Primary and Emergency (including unscheduled) Care Research
- RCT
- randomised controlled trial
- SAIL
- secure anonymised information linkage
- ShARRP
- Sheffield Addiction Research Recovery Panel
- SMDS
- Substance Misuse Data Set
- TAU
- treatment as usual
- THN
- take-home naloxone
- TIME
- Take-home naloxone Intervention Multicentre Emergency setting
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TSF
- training sign-off form
- WDSD
- Welsh Demographic Service Dataset
- WHO
- World Health Organization
- WIMD
- Welsh Index of Multiple Deprivation
