Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR135572. The contractual start date was in May 2018. The draft manuscript began editorial review in November 2022 and was accepted for publication in June 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Richards et al. This work was produced by Richards et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Richards et al.
Chapter 1 Introduction
Background
The UK Renal Registry data highlight that the incidence of people commencing renal replacement therapy in the UK increased from 109 per million population in 2013 to 115 per million population in 2014 and, most recently, to 151 per million population in 2019. 1 This equates to some 7500 patients each year, of whom approximately 70% receive haemodialysis as the first option, with as many as two-thirds of these initially reliant on a central venous catheter (CVC). Around 70,000 adults currently receive kidney replacement therapy, and notwithstanding numbers remaining static through 2020 (most likely a consequence of the COVID pandemic), it is anticipated that this number will again increase by 2–2.5% annually, as has occurred over the last decade. 1
Arteriovenous fistulas (AVFs) are considered the best modality for providing haemodialysis care in patients with end-stage renal disease (ESRD). Compared to dialysis via an AVF, haemodialysis via a CVC is associated with an increased risk of catheter-related bloodstream infection, with an estimated incidence of 1.2–2.5 per 1000 patient-days. 2–6 This results in increased hospitalisation and additional costs. 7,8 Patient mortality for patients dialysing via a CVC is ~40% higher than for patients dialysing via an AVF. 9 There is therefore a clear incentive to form AVFs in patients requiring haemodialysis, and current UK tariffs for haemodialysis have been deliberately set to incentivise dialysis via an AVF.
However, only 20% of UK dialysis centres currently achieve the 80% Renal Association target for dialysis of their prevalent population via definitive access, and many fall well short. 10,11 The reasons why such a small proportion of the prevalent dialysis population achieve dialysis via an AVF are multifactorial, but once formed, AVFs must ‘mature’ over several weeks before they can be used for dialysis, and the relatively poor maturation rate, with approximately 30% of fistulas failing to do so,12–17 undoubtedly contributes. Early identification of AVFs that are unlikely to mature may allow timely, and more effective, surgical or radiological salvage. The resulting improvements in assisted primary patency would likely increase AVF usage and, by minimising morbidity and mortality related to CVC dialysis, such interventions are likely to be cost-effective. Furthermore, early salvage of AVFs that are still patent but deemed at risk of non-maturation may preserve precious venous ‘capital’ by avoiding subsequent loss of the draining venous segment of the AVF through late thrombosis.
Successful AVF maturation is marked by a massive increase in blood flow through the fistula, and accompanying augmentation in fistula vein diameter. Early ultrasound (US) surveillance of nascent AVFs may therefore identify patterns of fistula blood flow and vein size that are associated with non-maturation. For example, the fistula vein can develop progressive intimal hyperplasia at the site of mobilisation onto the artery, which may compromise fistula maturation and eventually lead to thrombosis. Such juxta-anastomotic (or ‘swing-segment’) stenosis18 may be detectable by early US. However, the literature relating to the use of US surveillance of AVFs, even once mature and being used for dialysis, is conflicting, and a consensus strategy has not been reached. This may relate to differences in the mode of surveillance, the site of the fistula and the scanning method adopted. US can confirm fistula maturation,19,20 although a consensus definition of what constitutes maturity on US has not been agreed. 21 The use of an US scan as a surrogate for fistula maturity enables pre-dialysis patients to be included in dialysis trials.
Only a few studies have used US immediately after fistula creation to assess early maturation, but these report that maturation is characterised by rapid increases in fistula blood flow, as early as the first day after formation. 22–24 The diameter of the fistula vein also increases rapidly. 23 This raises the possibility that US assessment at early time points may predict subsequent fistula maturation. Itoga et al. reported early duplex US on 153 patients with newly formed fistulas (4–8 weeks after creation). 25 US detected a flow-limiting stenosis in 40% of patients, with radiological salvage intervention subsequently performed in 81% of them. Assisted primary patency of the fistulas in this group was 83% and 64% at 6 months and 1 year, respectively, compared to 96% and 89% in the cohort who had no detectable US abnormality. This study did not include a control cohort who did not receive routine surveillance, but the fistula patency rates achieved for the entire study population were better than generally reported in vascular access studies. Routine early US surveillance has been examined in one randomised study to date of 150 patients, with US performed at 2, 4 and 8 weeks after fistula creation. 15 The reported fistula failure/non-maturation rate in the surveillance group was 13.6%, compared to 25.4% in the control group in whom US was performed on the basis of a perceived clinical indication. This difference did not reach statistical significance, but notably, the study was powered for a relatively large (20%) difference in maturation.
Rationale for study
Although US surveillance programmes of mature fistulas are increasingly incorporated into standard practice, controversy persists regarding their effectiveness, with limited high-quality evidence available. 26–28 Design of prospective controlled trials is hampered by the low rates of thrombosis observed once AVFs have matured, particularly for standard radiocephalic fistulas:13,29 audit of the Cambridge prevalent dialysis population (~450 patients) has revealed a thrombosis rate for mature fistulas of 0.18 per patient/year, which matches existing literature. 14,30,31 The relatively poor reported 1-year unassisted (primary) fistula patency rates of around 55% are instead largely due to early failures occurring within the first 3 months after creation, before the fistula has matured. 13,14 Later failures in the first year may also reflect suboptimal maturation. 32,33 Thus, the trial team felt that a surveillance programme that focuses upon identifying potentially rectifiable lesions in the first 3 months after fistula formation has greater potential to deliver an effective and economic approach to improving 1-year AVF patency.
Hypothesis and objectives
The paucity of evidence supporting a role for surveillance in increasing fistula patency rates, coupled with the significant costs involved and the resources required, leads to significant heterogeneity in practice across the UK and internationally. This study aims to fill this gap in knowledge by determining whether US surveillance of newly formed AVFs can improve fistula patency rates and minimise CVC usage. Our overall aim of this 5-year project is to test the hypothesis that:
Doppler US surveillance of AVFs immediately after creation improves longer-term AVF patency, by directing early and effective surgical or radiological salvage of those AVFs at risk of failing or not maturing.
For a trial to demonstrate that US surveillance improves patency rates for newly created fistulas, several conditions must be met:
-
that US can effectively distinguish those newly formed fistulas that are unlikely to mature
-
that maturity failure occurs commonly enough that clinically meaningful improvements in fistula outcomes by early identification of at-risk fistulas are plausible
-
that salvage interventions performed on those ‘at-risk’ fistulas are effective and improve fistula patency.
As discussed above, evidence supporting these conditions is lacking, and given that failure to meet them would be expected to generate a negative trial result, they were addressed separately and sequentially, according to the following objectives:
-
Run an observational cohort study in which consenting participants undergo serial US assessment of their AVF in the first 3 months after its formation (phase 1).
-
Model whether features on early US can reliably identify those fistulas that will not mature or will fail early.
-
Assess from the observational cohort study whether a randomised controlled trial (RCT) evaluating early US-guided salvage intervention is feasible.
-
Run a multicentre RCT in which 1-year fistula patency in a treatment group receiving early US surveillance of their developing fistulas is compared against standard care: monitoring of fistula maturation by clinical assessment only (phase 2).
Progression to the phase 2 study is therefore contingent upon accomplishing the first three of these objectives, and in particular, demonstrating that US surveillance can accurately predict fistula non-maturation. This manuscript will thus focus on the phase 1 study set-up and outcomes.
Chapter 2 Methods
Study design
A prospective observational cohort study of adult patients undergoing formation of AVF for haemodialysis was performed to test the hypothesis:
Doppler US surveillance early after AVF creation can reliably identify those AVFs that will not mature or will fail early.
Consenting participants underwent serial US scanning at weeks 2, 4, 6 and 10 after fistula formation in addition to standard care (such as regular clinical assessment) as per local centre policy.
Study setting
The study was performed at 17 UK dialysis centres (Table 1).
| Site name | Assessed for eligibility | Approached | % Approached from assessed | Consented and enrolled | % Consented and enrolled from approached |
|---|---|---|---|---|---|
| N | N | N | |||
| Cambridge University Hospital | 76 | 76 | 100.0 | 73 | 96.1 |
| Edinburgh Royal Infirmary | 13 | 13 | 100.0 | 4 | 30.8 |
| Epsom and St Helier University Hospitals NHS Trust | 51 | 51 | 100.0 | 23 | 45.1 |
| Frimley Park Hospital | 18 | 18 | 100.0 | 18 | 100.0 |
| Guy’s Hospital, London | 77 | 27 | 35.1 | 20 | 74.1 |
| Hammersmith Hospital, London | 6 | 6 | 100.0 | 2 | 33.3 |
| Hull Royal Infirmary | 58 | 51 | 87.9 | 17 | 33.3 |
| James Cook Hospital | 7 | 7 | 100.0 | 7 | 100.0 |
| John Radcliffe Hospital and Churchill Hospitals, Oxford | 57 | 57 | 100.0 | 19 | 33.3 |
| Leicester General Hospital | 20 | 20 | 100.0 | 20 | 100.0 |
| Manchester Royal Infirmary | 49 | 47 | 95.9 | 24 | 51.1 |
| Nottingham University Hospitals NHS Trust | 22 | 22 | 100.0 | 22 | 100.0 |
| Royal Free Hospital, London | 43 | 43 | 100.0 | 35 | 81.4 |
| Royal Sussex County Hospital, Brighton | 15 | 15 | 100.0 | 10 | 66.7 |
| Southmead Hospital, Bristol | 62 | 19 | 30.6 | 14 | 73.7 |
| The Royal London Hospital | 23 | 23 | 100.0 | 22 | 95.7 |
| University Hospitals Coventry and Warwick | 85 | 38 | 44.7 | 17 | 44.7 |
| Total | 682 | 533 | 78.2 | 347 | 65.1 |
Selection of participants
Participants were considered eligible for enrolment in this study if they fulfilled all inclusion criteria and none of the exclusion criteria detailed below.
Participant inclusion criteria
-
Adult, aged 16 years or older.
-
The participant had ESRD and was either already established on haemodialysis or likely to start imminently.
-
The participant was due creation of an arm AVF (either wrist or elbow) including the following types of fistulas: radiocephalic, ulnobasilic, brachiocephalic and brachiobasilic (one- or two-stage) fistula, with a minimal acceptable threshold of 2 mm venous diameter at whatever site chosen.
-
Full informed consent to participate was provided.
Participant exclusion criteria
-
Attempted formation of proximal neoanastomosis at the forearm cephalic and basilic venous systems following failure of a standard radiocephalic or ulnobasilic fistula.
-
Participants with known central venous stenosis undergoing simultaneous central venous angioplasty/stenting and AVF creation.
-
Participants in whom it was anticipated that it would not be possible to perform serial US scanning.
Screening procedures
Potentially eligible participants scheduled to have a new fistula created were identified by the local clinical team and screened against the inclusion and exclusion criteria, with a screening log recording all potentially eligible patients. Patients received the patient information sheet and were given time to consider involvement and raise any questions. Participants whose fistula failed could re-enrol and undergo US surveillance of the next AVF created. Participation was confirmed by providing written consent in the approved study consent form. Participation in other studies did not exclude participation in the SONAR study. There were no mandatory pre-enrolment investigations. Patients could withdraw from the study at any point, but data collected until that point were retained and included in the analysis.
Study outcomes
Primary outcome measure(s)
Primary fistula maturation by week 10 according to accepted surrogate US parameters:23
-
wrist fistula: representative venous diameter ≥ 4 mm, with flow > 400 ml/minute
-
elbow fistula: representative venous fistula diameter ≥ 5 mm, with flow > 500 ml/minute.
Secondary outcome measures
-
For those patients established on dialysis, successful use of the fistula for dialysis on three successive occasions.
-
Clinical suitability for dialysis 10 weeks after fistula creation based on examination alone according to local practice.
-
Formation of a new fistula (including fashioning of proximal neoanastomosis) or radiological salvage procedure.
-
Fistula thrombosis.
-
Secondary fistula patency.
-
Patient acceptability, based on the proportion of patients that complete their expected scans.
Assessments and follow-up
The schematic for study participation is shown in Figure 1.
FIGURE 1.
Study schema. (a) The clinical team and the participants will be blinded to the results of the ultrasound scans. Routine clinical assessments may be performed during the trial period, as per standard practice.
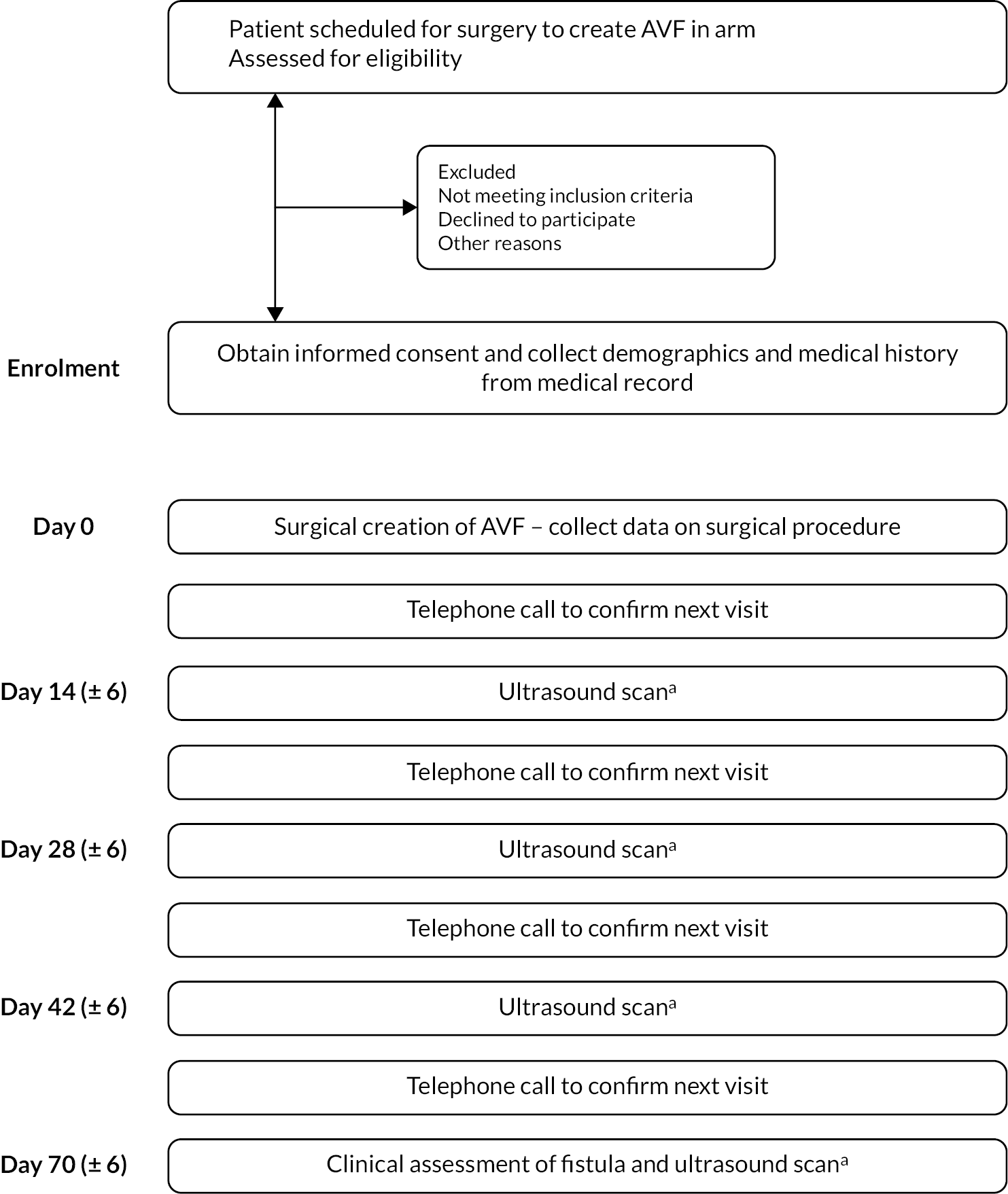
Study assessment schedule
The study assessment schedule is shown in Table 2.
| Time pointa | Screening | Enrolment | AVF surgery study day 0 |
Week 2 study day 14 (± 6 days) |
Week 4 study day 28 (± 6 days) |
Week 6 study day 42 (± 6 days) |
Week 10 study day 70 (± 6 days) |
|---|---|---|---|---|---|---|---|
| Eligibility screen | X | ||||||
| Informed consent | X | ||||||
| Collect demographics and medical history | X | ||||||
| Collect operative data | X | ||||||
| Assessments | |||||||
| Doppler US scan of AVFb | X | X | X | X | |||
| Clinical assessment of suitability of AVF for dialysis | X | ||||||
| Routine assessment of AVF | (as per participating centre standard of care) | ||||||
Procedures at enrolment
Background data (participant characteristics) collected at enrolment included: participant age; gender; heart rate and blood pressure; medical history of ischaemic heart disease, hypertension or diabetes; and current dialysis status (no dialysis, peritoneal or haemodialysis). Anticoagulants such as aspirin, clopidogrel, dipyridamole, warfarin or non-vitamin K anticoagulants were also recorded.
Recorded vascular access history included:
-
previous CVC insertions and attempts at AVF creation
-
whether formal pre-operative US mapping was performed prior to SONAR study enrolment
-
date of surgery and type of fistula (radiocephalic, ulnabasilic, brachiocephalic or brachiobasilic) created for SONAR study.
Procedures during week 2
-
US assessment; all duplex US scans were performed according to the protocol detailed in Appendix 2.
-
Flow characteristics as defined by scanning protocol.
-
Routine clinical examination (if applicable as per local policy).
-
Recording of first detection of ‘at-risk’ fistula – by either clinical examination or US.
-
Recording of time point at which a fistula was no longer patent on US.
-
Recording of the formation of a new AVF.
-
Recording of reported fistula thrombosis.
Procedures at week 4
-
US assessment.
-
Flow characteristics as defined by scanning protocol.
-
Routine clinical examination (if applicable as per local policy).
-
Recording of reported fistula thrombosis.
Procedures at week 6
-
US assessment.
-
Flow characteristics as defined by scanning protocol.
-
Routine clinical examination (if applicable as per local policy).
-
Recording of reported fistula thrombosis.
Procedures at week 10
-
Clinical assessment of fistula function.
-
Number of successful/unsuccessful dialysis visits within 10-week period.
-
Secondary fistula patency at week 10.
-
Recording of first detection of ‘at-risk’ fistula – by either clinical examination or US.
-
Recording of time point at which a fistula was no longer patent on US.
-
The number of fistula operations and radiological salvage interventions (number, nature and timing) reported up to week 10.
-
Flow characteristics as defined by scanning protocol.
-
Recording of the formation of a new AVF.
-
Recording of reported fistula thrombosis.
-
Recording number of days dialysed via a CVC, and CVC-related septic complications.
Blinding of assessments
The US findings were blinded; that is, not relayed to the participant or to the participant’s clinical team, unless either:
-
The participating centre’s local standard of care already included a routine scan, or concern relating to the fistula development would justify an US scan on clinical grounds; in which case, to avoid unnecessary additional scans being performed, the centre was given access to study scan data for that time point (but not the other study scans).
-
A trial scan confirmed that the AVF had thrombosed. This information was shared with the clinical care team to enable appropriate care to continue, and no further study scans were performed. Clinical outcome data were still collected at week 10.
Follow-up 12-month study
Upon analysis of the 10-week outcome data for the SONAR study, the Trial Management Group (TMG) and the Trial Steering Committee (TSC) recommended that the study be extended to examine whether early US surveillance could identify fistulas that were likely to have failed by 6 and 12 months. Thus, approvals were gained for a second observational cohort study, the SONAR-12M study.
Inclusion and exclusion criteria to 12-month follow-up
Previous participants in the SONAR study, who provided informed consent for further follow-up, could participate. Patients who had either died since SONAR participation or withdrawn from the SONAR study were excluded.
Study outcomes
Primary outcome measure
The primary outcome measure for the longer-term study was primary fistula patency at 6 months post creation.
Primary fistula patency at 6 months post AVF creation was calculated from a combination of reported palpable thrill data at 6 months, thrombosis, intervention and abandonment data (with clinical reviews, if appropriate). In cases where the primary outcome could not be determined from reported data, it was imputed as follows:
-
If the SONAR fistula had been used for haemodialysis at 6 months after fistula creation, without interventions, then the fistula status was imputed as patent at 6 months.
-
In other cases, primary fistula patency was imputed based on the end-point review assessment. For instance, if the last known follow-up of the SONAR fistula was at 3 months post creation and a patent status was reported (e.g. through use of the fistula for haemodialysis or palpable thrill in comment boxes), then the fistula status was imputed as patent at 6 months, subject to detailed clinical scrutiny of the case. The fistula patency status of participants who underwent a transplant was imputed, if appropriate, based on end-point review assessments.
Secondary outcome measures
-
Formation of a new fistula.
-
Proximal revision/neoanastomosis or radiological salvage procedure.
-
Fistula thrombosis.
-
Secondary fistula patency at 6 and 12 months post creation.
-
Assisted primary fistula patency at 6 and 12 months post creation.
-
Primary fistula patency at 12 months post creation.
-
Functional patency and, if functionally patent, time to event.
-
Haemodialysis discontinued: improvement in renal function or withdrawn from dialysis or switched to peritoneal dialysis or kidney transplant received.
Missing secondary outcome data were not imputed.
Definitions of fistula patency
For the follow-up study, the longer follow-up raised the potential for radiological or surgical salvage procedures to be performed on the AVFs after the original 10-week scanning window. We adopted the Sidawy definitions of fistula patency for this study. 34 These have become accepted standard and, critically, enable fistula interventions to be captured on fistula survival analysis. Because we thought it likely that those fistulas identified as at risk on early US surveillance were the more likely to undergo a salvage procedure, the relevant definitions are copied below:
Primary patency: The interval between access creation and the earliest fistula thrombosis, abandonment (except abandonment because of steal or pseudoaneurysm), intervention on the fistula (to either maintain or re-establish patency) or the time of measurement of patency.
Assisted primary patency: The interval from access creation until access thrombosis or the time of measurement of patency, including surgical or endovascular interventions designed to maintain the patency of the fistula.
Secondary patency: The interval from access creation to fistula abandonment or the time of measurement of patency. Secondary fistula patency measures the total lifespan of the fistula and includes the time during which the fistula remains patent after an intervention procedure (including radiological and surgical interventions, or the formation of a proximal neoanastomosis in order to re-establish fistula patency).
Clinical maturation: Suitability to cannulate based on clinical examination.
Functional patency: For those patients established on dialysis at the time of AVF creation, successful use of the fistula for dialysis on three successive occasions.
Study details
Primary and secondary outcome data for each participant were obtained from the local Principal Investigator at each site, with up-to-date in-person or remote review of the participant as required.
Statistical analysis plan
All statistical analyses were carried out using Base SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Sample size calculation
We estimated that 20% of AVFs fail within the first 2 weeks post surgery and that US surveillance in the first 6 weeks after AVF creation has a positive predictive value (PPV) (number of true non-mature/number of predicted non-mature) of 72% for predicting non-maturation. To estimate this with ± 10% precision [i.e. the 95% confidence interval (CI) is from 62% to 82%], 78 predicted failures are required. We estimated that US would predict failure in 25% of fistulas, and thus US surveillance of 312 AVFs was required. Allowing for 10% dropout, 347 fistulas was set as the study total.
We anticipated that two models to predict primary fistula non-maturation by week 10 would be required and that we would therefore have two PPVs – one for wrist fistulas and one for elbow AVFs – as well as an overall PPV (based on combining the results of the two models). Assuming a ratio of 50 : 50 for wrist to elbow fistulas, the precision CI for the separate elbow and wrist models would be from 55.3% to 85.2%.
Primary outcome analysis
The primary outcome analysis was whether the study AVFs had ‘matured’ by week 10 after creation, based on an intention-to-treat approach, and included all 333 AVFs created. Three distinct outcomes defined fistula non-maturation:
-
fistula occlusion/thrombosis within the study period (76 days post AVF creation)
-
fistula abandonment within the study period due to failure to mature or due to thrombosis/occlusion
-
failure to achieve (either reported at the week 10 scan or imputed) maturation, according to preset US parameters (wrist: representative venous diameter ≥ 4 mm and average volume flow > 400 ml/minute; elbow: representative venous diameter ≥ 5 mm and average volume flow > 500 ml/minute).
The primary fistula maturity rate at week 10 was calculated alongside a 95% CI based on all participants enrolled and with an AVF created. Supplementary rates were also calculated, based on participants whose fistulas did not fail before week 2, before week 4 and before week 6; and for all enrolled participants with an AVF created by fistula location (elbow or wrist). Maturity was assessed using the results from the week 10 US scan, with the fistula volume flow (ml/minute) based on the average of up to three measurements taken at the scan and the venous diameter (mm) based on the representative measurement taken at the same scan.
Handling of missing data
In anticipation that some participants would be unable to attend all scans and that some scan data would therefore be missing, the following assumptions were adopted:
If the primary outcome data were missing (i.e. the fistula volume flow and/or representative venous diameter on the week 10 scan was unavailable), then:
-
If at least one scan had occurred at week 2, 4 or 6 and fistula maturity was achieved by US criteria at the latest available time point, it was assumed that the fistula was mature at week 10.
-
If fistula non-maturity was consistently reported at weeks 2, 4 and/or 6, or at least one scan at week 2 or 4 confirmed fistula maturation, but a later scan reported non-maturation, then fistula non-maturation was imputed at week 10 unless, for dialysis patients, the fistula had been used successfully at least once or, for pre-dialysis patients, was deemed suitable for dialysis cannulation on clinical examination; in these instances, fistula maturity at week 10 was imputed.
-
If the fistula was abandoned because of development of steal syndrome or pseudoaneurysm within the study period (reported in the end-of-study form) and with at least one scan form available, then 10-week fistula status was imputed as per (1) or (2).
-
If the participant died within the study period (reported in the end-of-study form) and with at least one scan form available, then 10-week fistula status was imputed as per (1) or (2).
For fistula flow and venous diameter at the 2-, 4- and 6-week scans, as well as for exposure factors and confounding variables considered in modelling of primary fistula maturity, missing data were imputed using multiple imputation techniques if the level of missing data was > 10% but < 30%. Any factors with > 30% missing data were excluded from the analysis. Any data for factors with < 10% missing data were not imputed and were used directly in the analysis. For the optimum model(s) selected for primary fistula maturation, a sensitivity analysis was additionally performed, excluding any imputed outcome. Missing secondary outcome data were not imputed.
Predictive modelling for fistula non-maturation
A binary-data population-average model with exchangeable correlation structure was used to model the probability of fistula non-maturity by week 10. This approach enabled modelling of the correlation between subjects from the same hospital. Cases with scan data missing from all time points were excluded. Cases with fistula failure prior to the latest scan time point(s) being considered as explanatory variables in the model were also excluded. Failure to achieve primary fistula maturity is as defined in Primary outcome analysis; otherwise, a fistula was deemed mature.
The following candidate variables were considered for model inclusion: pre-operative vein diameter; quality of artery at the time of surgery; quality of vein at the time of surgery; clinical prediction of fistula maturity; average resistance index at scan time point(s); representative venous diameter at scan time point(s); average flow at scan time point(s); patient sex; patient age; and diabetes. A purposeful variable selection approach was followed to identify the final model for a given scan time point (week 2, week 4 or week 6) and for a given fistula location (elbow, wrist or all-fistula). The significance levels used in order to determine whether a given explanatory variable was included in the model, or not, at each step in the model building process were as recommended by Hosmer et al as described by Hosmer. 35
Because of the different criteria for defining fistula maturity at wrist and elbow fistulas, models were built for elbows and wrists, and for different combinations of 2-, 4- and 6-week scan time points. In addition, an all-fistula predictive model was built, with maturity criteria by week 10 defined according to surrogate US parameters as: representative venous diameter ≥ 4 mm and average volume flow > 400 ml/minute. Fistula location (elbow or wrist) was considered as an additional candidate variable in all-fistula models. Evidence of non-linearity in continuous variables was visually explored using univariable LOWESS smoothing and statistically assessed using quadratic and logarithmic univariable fractional polynomials.
Selection of optimum model(s)
In determining the optimum model from those developed using week 2, 4 or 6 data (or some combination), the number of patients who could potentially benefit in the proposed SONAR phase 2 trial was a key consideration, because this would likely decrease as later scan data were included and fistulas that may have been amenable for surgical or radiological salvage at an earlier time point were confirmed to have thrombosed. Thus, the number of patients for whom fistula failure was correctly predicted by the optimum model is presented, as is the number of patients for whom fistula failure was correctly predicted and where the failure occurred at least 2 weeks after the final scan used in the model (for simplicity, we use ‘patient’ as a proxy for ‘fistula’ but note that the study included patients with more than one SONAR fistula created). This reflects the practicalities in the proposed phase 2 RCT that it would generally take at least 2 weeks to arrange a salvage intervention once a fistula is identified as at risk of failing/not maturing on early surveillance US.
Receiver operating characteristic (ROC) curve analysis of the developed predictive model(s) was used to determine appropriate cut-offs for identifying fistulas at risk of failing or not maturing. Maximising Youden’s J statistic (sensitivity + specificity − 1) was used to guide a choice of cut-off, alongside discussions with clinicians. The PPV for the optimum model(s) was calculated alongside a 95% CI for the chosen risk-score cut-offs. Once the final elbow, wrist and all-fistula models were built for different combinations of 2-, 4- and 6-week scan time points, clinical discussions informed by the models’ performance parameters [PPV, negative predictive value (NPV)] and number of patients who could benefit from predicting a failure and intervention to salvage the fistula in the SONAR phase 2 trial took place to select the optimum model(s). Diagnostic tests for model fit and influential observation analysis were performed on the optimum models.
Progression to proposed randomised controlled trial
As discussed above, this study was performed to assess the practicalities of introducing a multicentre randomised trial in which early surveillance US would be used to identify patients with newly formed fistulas that are at risk of not maturing or failing, and these patients would then be randomised to standard care (continued observation) or to radiological or surgical salvage.
The key condition set for proceeding to the second SONAR phase was that early US surveillance could identify fistula non-maturation with a PPV of at least 70% (i.e. the 95% CI for PPV includes 70%). This target was chosen because, although the impact of early US surveillance on improving fistula patency through informing radiological or surgical salvage is unknown, pragmatically we felt that surveillance programmes were only warranted if relatively sizeable improvements in fistula patency were achieved. In this regard, the study team felt that a minimal improvement of 10% in primary assisted patency rates (from an estimated 55% to 65%) was reasonable, and that the benefits of surveillance would be questionable if the difference were less marked. Power calculations indicated that to detect this difference would require 1224 fistulas at 90% power, 5% significance and allowing for 20% dropout, which, although a large trial, would be attainable within a multicentre UK setting (see Table 9).
Ethical and regulatory considerations
Funding
This study is funded by the NIHR Health Technology Assessment programme, in response to research call, ‘17/27/11 Surveillance of Arteriovenous Fistulae in Haemodialysis’, with Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge as sponsor. Additional funding for patient travel expenses was provided by the Addenbrooke’s Kidney Patient Association.
Chapter 3 Results
Patient characteristics
Between 1 September 2018 and 11 November 2019, 682 assessments for eligibility for inclusion into the SONAR study were carried out at 17 UK haemodialysis sites, with 347 consents (see Table 1). Patient demographics are detailed in Table 3. Patients were generally elderly and male, with ~25% having cardiovascular, cerebrovascular or peripheral vascular disease, and with > 40% diabetic. The study cohort mirrored coincidental UK experience. 36 At enrolment, 191 (55.0%) patients were still pre-dialysis, and a further 8 (2.3%) had a previous renal transplant that was now failing.
| Age (years) | 65 (52–74) |
| Male | 225 (64.8) |
| Cause of renal failure | |
| Glomerulonephritis | 22 (6.3) |
| Polycystic kidney disease | 19 (5.5) |
| Hypertension | 40 (11.5) |
| Diabetes | 108 (31.1) |
| Pyelonephritis | 0 (0.0) |
| Renovascular disease | 13 (3.7) |
| Unknown | 44 (12.7) |
| Other | 100 (28.8) |
| Hypertension | 284 (81.8) |
| Diabetes | |
| No | 197 (56.8) |
| Yes: insulin-dependent | 89 (25.6) |
| Yes: non-insulin-dependent | 61 (17.6) |
| History of IHD/CVA/PVDa | |
| No | 259 (74.6) |
| Yes | 87 (25.1) |
| Dialysis status at enrolment | |
| Pre-dialysis | 191 (55) |
| Haemodialysis | 141 (40.6) |
| Peritoneal | 7 (2.0) |
| Failing transplant | 8 (2.3) |
| Current vascular access for haemodialysis (n = 141) | |
| Fistula | 3 (2.1) |
| Graft | 0 (0.0) |
| Line | 138 (97.9) |
| Number of previous fistulas | |
| 0 | 273 (78.7) |
| 1 | 43 (12.4) |
| 2 | 23 (6.6) |
| > 2 | 8 (2.3) |
| Number of patients re-entering the study | |
| With AVF surgery | 14 |
| Without AVF surgery | 0 (0.0) |
Patient enrolment, fistula creation and adherence to trial protocol
Participant enrolment is shown in Figure 2. Fourteen patients re-enrolled in the study, following failure of their original SONAR AVF. Fourteen patients did not undergo AVF creation, mostly because of ill health. Operative details for the 333 AVFs created are provided in Table 4: the majority (75.1%) of AVFs were created on the left side, reflecting the non-dominant arm, with slightly more (52.3%) AVFs created at the elbow than at the wrist. Twenty-seven participants withdrew from the study; 9 of these withdrew before an AVF was created and 18 afterwards, mainly because of either failing health or death.
FIGURE 2.
Study CONSORT diagram.
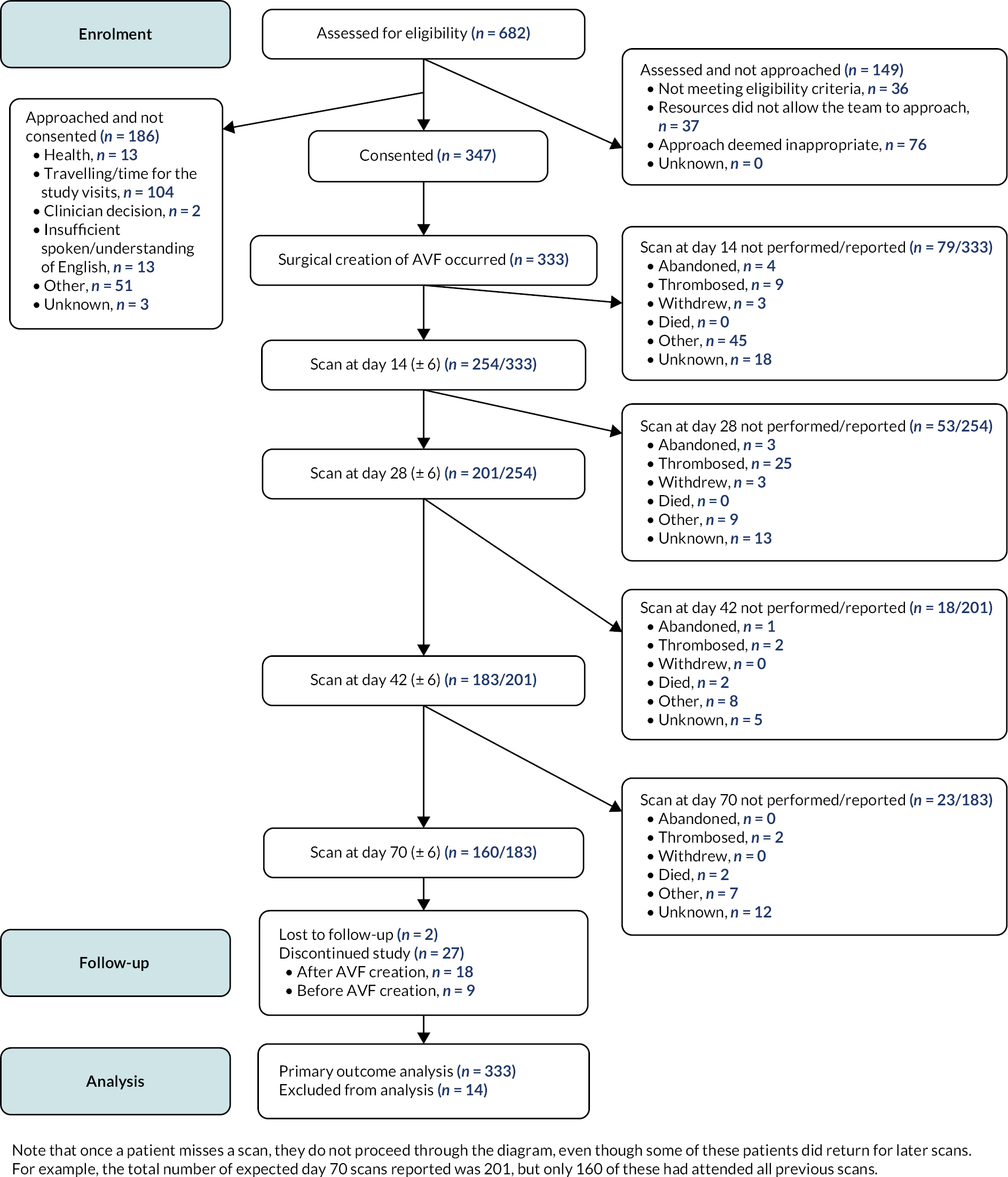
| Pre-operative mapping US scan performed | 240 |
| Side and site of fistula | |
| Left wrist | 116 |
| Left: radiocephalic | 115 |
| Left: ulnobasilic | 1 |
| Left elbow | 134 |
| Left: brachiocephalic | 109 |
| Left: brachiobasilic | 25 |
| Left other | 0 |
| Right wrist | 43 |
| Right: radiocephalic | 43 |
| Right: ulnobasilic | 0 |
| Right elbow | 40 |
| Right: brachiocephalic | 33 |
| Right: brachiobasilic | 7 |
| Right other | 0 |
| Number of enrolled participants who never had an AVF created | 14 |
Participants were scheduled to undergo US scanning of their AVF at weeks 2, 4, 6 and 10 after AVF creation. Despite concerns that this regimen would prove too onerous, particularly for the frailer, older participants, attendance for the scans was good: 126 (37.8% of those with an AVF created) cases missed at least one of their expected scans, but at any particular time point approximately three-quarters of patients attended for the scheduled scan (Table 5; note that further scans were not performed once an AVF was confirmed to have thrombosed).
| No. of fistulas eligible for scanning at time pointa | N (%) where data reported | |
|---|---|---|
| Representative venous diameter and volume flow reportedb | ||
| At day 14 | 292 | 223 (76.4) |
| At day 28 | 284 | 218 (76.8) |
| At day 42 | 272 | 214 (78.7) |
| At day 70 | 260 | 191 (73.5) |
Fistula patency and maturation rates
As detailed in Table 6, by week 10 after AVF creation, four outcomes are possible: the AVF has reached maturation; the AVF is patent but non-mature; the AVF has failed (thrombosed or abandoned); and unknown. By 10 weeks, 219 of 333 (65.8%) of created AVFs had reached maturity according to predefined US criteria (see Primary outcome measures), with 67.2% of elbow and 60.4% of wrist AVFs maturing. Conversely, by week 10, 57 of 333 (17.1%) of AVFs had failed. However, 37 of the fistula failures (64.9%) had occurred by the week 2 scan, and thus too early after fistula creation for salvage procedures to be practical (Table 7). In this regard, it is notable that if these early failures within the first 2 weeks are excluded, fistula maturation of 74.0% at 10 weeks was achieved (see Table 7). A relatively small number of AVFs (n = 29; 8.7%) remained patent, but not mature, on the week 10 scan (see Table 6).
| All fistulasa | Elbows | Wrists | ||||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | n/N | % | |
| Mature | 219/333 | 65.8 | 117/174 | 67.2 | 96/159 | 60.4 |
| Patent but non-mature | 29/333 | 8.7 | 19/174 | 10.9 | 16/159 | 10.1 |
| Failedb | 57/333 | 17.1 | 18/174 | 10.3 | 39/159 | 24.5 |
| Unknown | 28/333 | 8.4 | 20/174 | 11.5 | 8/159 | 5.0 |
| All fistulas | Elbows | Wrists | ||||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | n/N | % | |
| Fistulas with primary outcome data reported | 249/333 | 74.8 | 125/174 | 71.8 | 124/159 | 78.0 |
| Fistulas with primary outcome data imputed | 56/333 | 16.8 | 29/174 | 16.7 | 27/159 | 17.0 |
| Primary fistula maturity by week 10 (95% CI) | 219/333 | 65.8 (60.4 to 70.9) | 117/174 | 67.2 (59.7 to 74.2) | 96/159 | 60.4 (52.3 to 68.0) |
| Fistulas with a failure event before, or at, 2 weeks after AVF creation (‘early failures’) | 37/333 | 11.1 | 13/174 | 7.5 | 24/159 | 15.1 |
| Fistulas patent after 2 weeksa | 296/333 | 88.9 | 161/174 | 92.5 | 135/159 | 84.9 |
| Primary fistula maturity by week 10 for patent fistulas after 2 weeks (95% CI) | 219/296 | 74.0 (68.6 to 78.9) | 117/161 | 72.7 (65.1 to 79.4) | 96/135 | 71.1 (62.7 to 78.6) |
| Fistulas with a failure event before, or at, 4 weeks after AVF creation | 40/333 | 12.0 | 13/174 | 7.5 | 27/159 | 17.0 |
| Fistulas patent after 4 weeksa | 293/333 | 88.0 | 161/174 | 92.5 | 132/159 | 83.0 |
| Primary fistula maturity by week 10 for patent fistulas after 4 weeks (95% CI) | 219/293 | 74.7 (69.4 to 79.6) | 117/161 | 72.7 (65.1 to 79.4) | 96/132 | 72.7 (64.3 to 80.1) |
| Fistulas with a failure event before, or at, 6 weeks after AVF creation | 46/333 | 13.8 | 15/174 | 8.6 | 31/159 | 19.5 |
| Fistulas patent after 6 weeksb | 287/333 | 86.2 | 159/174 | 91.4 | 128/159 | 80.5 |
| Primary fistula maturity by week 10 for patent fistulas after 6 weeks (95% CI) | 219/287 | 76.3 (71.0 to 81.1) | 117/159 | 73.6 (66.0 to 80.3) | 96/128 | 75.0 (66.6 to 82.2) |
Univariate analysis was performed to identify patient and pre-operative anatomical factors associated with fistula non-maturation at week 10. Candidate factors included: pre-operative vein diameter; quality of artery at the time of surgery; quality of vein at the time of surgery; clinical prediction of fistula maturity; patient sex; patient age; and history of diabetes. Pre-operative vein diameter was excluded in the wrist model due to missing values. Out of those factors known at baseline, only sex was univariately significant at the 5% level, and for wrist fistulas only. For elbow fistulas, no baseline factor was significant at the 5% level; pre-operative vein diameter, identified previously as an important predictive factor in AVF maturation,37–39 was not statistically significant.
Early surveillance ultrasound
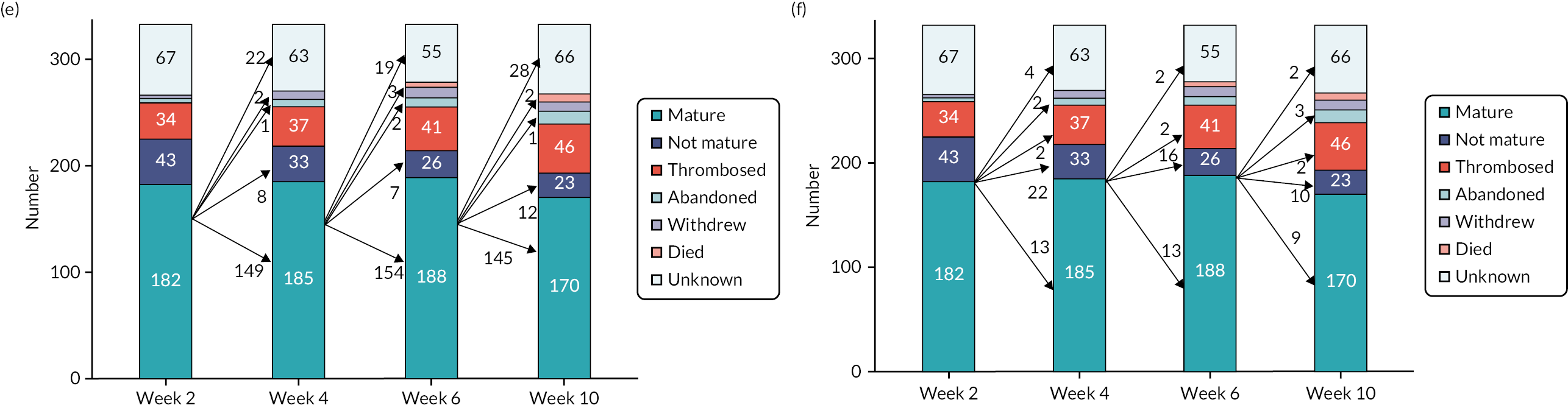
The most immediate and surprising finding from analysis of the US data was how rapidly fistula flow rates and venous diameters increased after AVF creation (Figure 3a and b). Fistula flow rates were calculated by the study vascular scientists by measuring brachial artery flow, on the assumption that blood flow through a brachial artery prior to AVF creation is minimal (< 100 ml/minute) and that increased flow thereafter reflects additional flow through the AVF. Thus, for example, at the first scan at 2 weeks, a median blood flow of 770 ml/minute and a median venous diameter of 5.2 mm were achieved, excluding those AVFs that had already thrombosed. Consequently, at week 2, 50.3% of wrist and 48.9% of elbow AVFs had reached maturation (Figure 3c and d), according to the US-determined surrogates (see Primary outcome measures), with 16.4% of wrist and 20.1% of elbow AVFs still patent but not yet mature. Interestingly, the proportion of AVFs that were classed as mature did not change dramatically in the subsequent 4-, 6- and 10-week scans (Figure 3c and d), largely because once matured, AVFs tended to remain so, although small numbers did either regress to an immature state or had thrombosed on the subsequent scan (Figure 3e). Conversely, the proportion of AVFs that were immature gradually fell with each successive scan (Figure 3f), either because they had matured or because they had thrombosed or had been abandoned.
FIGURE 3.
Ultrasound assessment of fistula maturation. Following AVF creation, participants underwent US assessment of their fistula 2, 4 and 6 weeks later, with fistula maturity assessed at week 10. Box and whisker plot depiction of (a) fistula flow rates and (b) representative fistula venous diameter for all fistulas at each time point, showing minimum value (after excluding outliers), 25th centile, median, 75th centile and maximum value (after excluding outliers) from bottom to top. Stacked 100% bar charts showing the proportion of (c) elbow and (d) wrist fistulas, with the following outcomes at each of weeks 2, 4, 6 and 10: died; withdrawn; abandoned; thrombosed; mature by US parameters (at that scan); not mature by US parameters (at that scan); unknown (did not attend scan, or missing data from the scan prevented determination of maturity). (e and f): as for (c) and (d) but for all fistulas, presented as numbers and including arrows depicting status at next scan of those fistulas (e) mature or (f) immature at previous scan.

Comparison of the early scan data in those AVFs that had matured by the week 10 scan against those AVFs that were still immature revealed marked differences in the recorded fistula vein diameter and in particular, in fistula flow rates, for both wrist and elbow AVFs (Figure 4). For example, the median blood flow at 2 weeks was 1135.5 and 691.0 ml/minute in those elbow and wrist fistulas, respectively, that reached maturation by week 10, whereas flows of 349.0 and 395.5 ml/minute were recorded in those elbow and wrist fistulas, respectively, that did not reach maturation at week 10. Similarly, the median venous diameter at 2 weeks was 6.3 and 5.0 mm in those elbow and wrist fistulas, respectively, that reached maturation by week 10, whereas the corresponding values in those elbow and wrist fistulas that did not mature were 4.6 and 4.3 mm, respectively. Scatterplot diagrams of average fistula flow against fistula vein diameter for the week 2, 4 and 6 scan data (Figure 5) highlight the different patterns of fistula development in those that will mature by week 10 compared to those that remain immature.
FIGURE 4.
Ultrasound scan parameters at weeks 2, 4, 6 and 10, according to maturation status at week 10. Representative (a and b) fistula venous diameter and (c and d) fistula volume flow rate for elbow (a and c) and wrist (b and d) according to maturation status at week 10. Box and whisker plot shows minimum value (after excluding outliers), 25th centile, median, 75th centile and maximum value (after excluding outliers) without imputation of primary outcome. Fistulas that failed before week 10 (thrombosis or abandonment after a failure) were excluded from the analysis.

FIGURE 5.
Scatterplot of representative venous diameter by average volume flow at each scan time point, according to fistula site and maturation status at week 10. Scatterplot of representative venous diameter by average volume flow at (a) 2, (b) 4 and (c) 6 weeks with different symbols for mature/non-mature fistulas at week 10 (as per primary outcome with no imputation).

Predictive modelling for fistula non-maturation
The data presented in the preceding section suggest it may be possible to identify from the early US findings those AVFs that are unlikely to mature. If so, this raises the potential of performing early radiological or surgical salvage of these AVFs in anticipation that this improves fistula assisted patency rates, and would merit progression to the proposed phase 2 RCT. A binary-data population-average model with exchangeable correlation structure was therefore developed to calculate the probability of fistula non-maturation by week 10, as detailed in the Methods section. Fistula maturity was known for 75% of fistulas and imputed (using a prespecified method based on previous scan or clinical data; see Handling of missing data) for 17% of fistulas (see Table 7). The maturity status for 8% of fistulas could not be imputed, and these fistulas were excluded from the modelling.
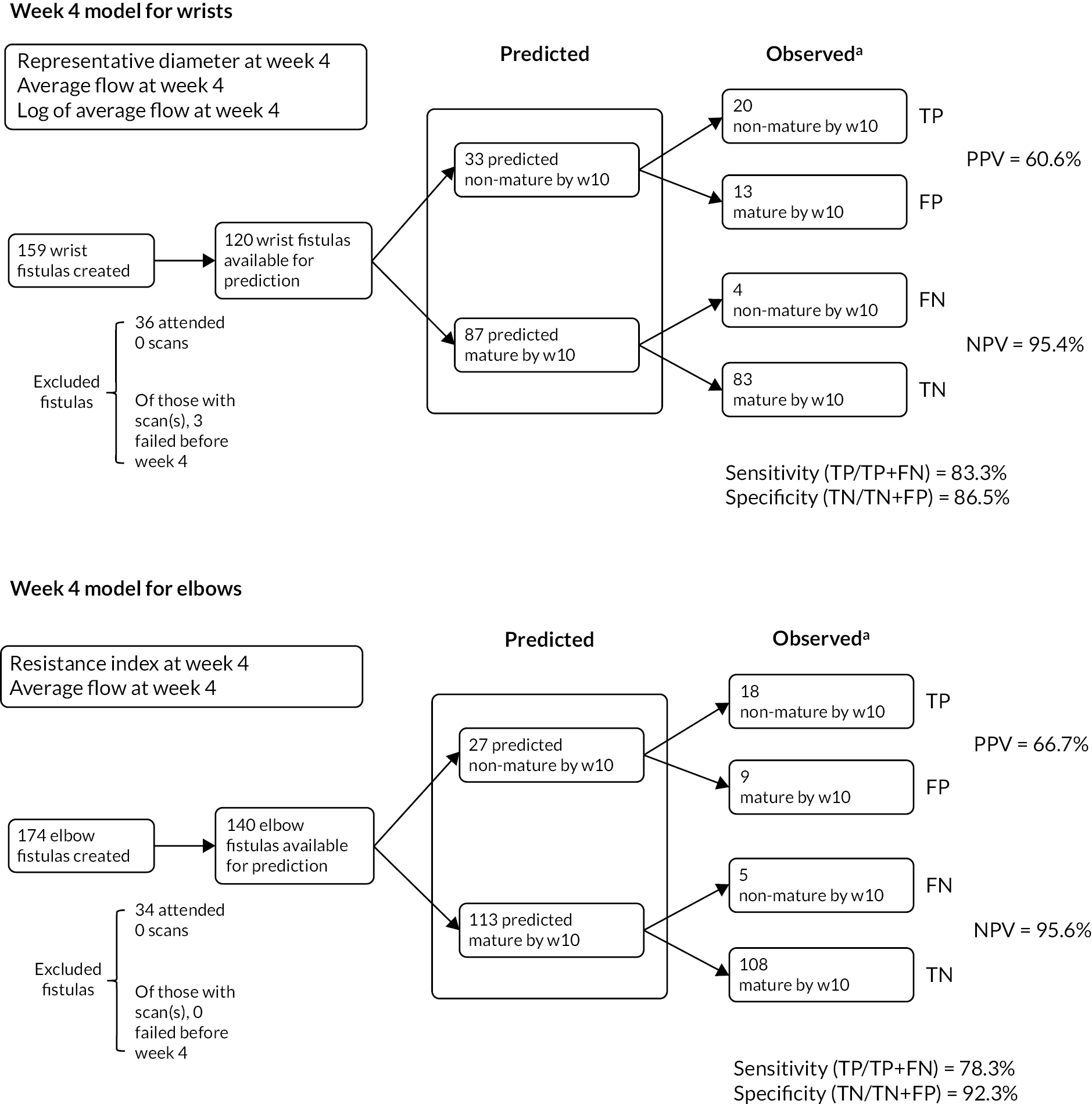
The optimum models identified considered elbow and wrist AVFs separately, and included week 4 scan data only; including data from more than one scan time point did not improve performance (Table 8 and Figure 6). Thus, for elbow AVFs, an algorithm that included the week 4 average resistance index and fistula blood flow predicted fistula non-maturation at week 10 in 27 cases, and correctly so in 18 of these (true positives), giving a PPV of 66.7% (95% CI 48.9% to 84.4%). A similar model could be developed for wrist fistulas, incorporating week 4 fistula venous diameter and fistula blood flow, and predicted fistula non-maturation in 33 cases, with 20 of these true positives (PPV 60.6%, 95% CI 43.9% to 77.3%). Diagnostic tests for model fit and influential observations were run on the optimum models for wrist and elbow AVFs, with both performing well, with area under the curve (AUC) values of at least 0.90 (see Figure 6). Interestingly, although the focus was on identifying on early surveillance US those AVFs that were not going to mature, the NPV – that is, the correct identification of those fistulas that were going to be mature at week 10 – was extremely high for both models (wrist: 95.4%, 95% CI 91.0% to 99.8%; and elbow: 95.6%, 95% CI 91.8% to 99.4%).
| Week 4 factors included in model | Elbow (n = 140) odds ratio (95% CI) |
Wrist (n = 120) odds ratio (95% CI) |
Single model (n = 257) odds ratio (95% CI) |
|---|---|---|---|
| Average resistance index (0.1 unit change from mean) | 5.9 (2.6 to 13.3) p < 0.0001 |
NS | 1.7 (1.1 to 2.7) p = 0.0158 |
| Average volume flow (100 unit change from mean) | 0.8 (0.6 to 1.0) p = 0.0224 |
2.2 (1.2 to 4.0) p = 0.0080 |
1.5 (1.2 to 1.8) p = 0.0004 |
| Representative venous diameter (1 unit change from mean) | NS | 0.5 (0.3 to 0.7) p = 0.0006 |
NS |
| Log of average volume flow (1 unit change from mean) | NS | < 0.001 (< 0.001 to 0.019) p = 0.0005 |
0.005 (< 0.001 to 0.022) p < 0.0001 |
| Clinical prediction of fistula maturity (will mature vs. will not mature) | NS | NS | 0.4 (0.2 to 0.9) p = 0.0270 |
| Model performance | |||
| AUC value | 0.92 | 0.90 | 0.89 |
| Threshold (Youden index) | 0.27 | 0.17 | 0.14 |
| PPV for threshold (95% CI) | 66.7% (48.9 to 84.4) | 60.6% (43.9 to 77.3) | 48.5% (36.7 to 60.4) |
| NPV for threshold (95% CI) | 95.6% (91.8 to 99.4) | 95.4% (91.0 to 99.8) | 95.8% (92.9 to 98.6) |
| Number of predicted failures vs. actual failures | 27 vs. 23 | 33 vs. 24 | 68 vs. 41 |
| Number of correctly predicted failures (true positives that fail at week 6 or later)a | 18 (14) | 20 (16) | 33 (27) |
FIGURE 6.
Receiver operating characteristic curve analysis of optimum models for predicting fistula non-maturation at week 10. Receiver operating characteristic curves for optimum models for predicting week 10 fistula non-maturation from week 4 US findings for: (a) elbow, (b) wrist and (c) all fistulas.

A composite, all-fistula model was then built using a similar approach to that followed for the elbow and wrist-specific models, incorporating a covariate indicating fistula location (elbow or wrist; see Table 8 and Figure 6c). This performed less well than the separate wrist and elbow models, in that it predicted fistula non-maturation in 68 cases, with 33 of these true positives (PPV 48.5%, 95% CI 36.7% to 60.4%). Unlike the individual models for wrist and elbow non-maturation, which relied entirely on parameters of the week 4 US scan, the surgeon’s prediction at creation as to whether the fistula would mature was an independent, albeit weak, factor for fistula non-maturation in the composite model (see Table 8).
Finally, the above modelling was repeated but including only reported outcome data; these models performed very similarly to those incorporating imputed outcome data (not shown).
Twelve-month fistula outcomes
As discussed above, the phase 1 SONAR study was designed as a feasibility study to determine, firstly, whether early surveillance could detect those fistulas that would fail or not mature, but also, critically, whether it would be practical to then perform a multicentre RCT examining whether early surveillance-directed salvage intervention improves fistula outcomes. In this regard, Figure 7 details the potential numbers of wrist and elbow AVFs that could be rescued by the week 4 surveillance US; it is notable (see Table 7) that the 10-week maturation rates for 293 of the original 333 (88.0%) study fistulas that were still patent at 4 weeks were much higher (74.7%; 95% CI 69.4% to 79.6%) than anticipated in the original trial design. Thus week 4 US surveillance could alter maturation in a maximum of 22.0% of AVFs, and factoring in an overall sensitivity of 80.5% (data not shown) for detecting those fistulas that will not mature, this figure is further reduced to 17.8%. Although this is acknowledged to be an estimate, the initial power calculations for the phase 2 RCT (see Table 9) had proposed a sample size of 1224 fistulas, based on detecting a 10% increase in 1-year assisted primary patency. However, from the SONAR phase I study, it seems unlikely that this difference would be achieved, because the intervention either could not be arranged in time or was ineffective. Instead, if one assumes that 50% of the at-risk fistulas identified on the week 4 scan are successfully salvaged, this would provide an effect of 8.9% in the intervention arm.
FIGURE 7.
Summary of week 4 US modelling on identifying 10-week fistula status. FN, false negative; FP, false positive; TN, true negative; TP, true positive. (a) Observed or imputed as per determination of fistula maturity/non-maturity by week 10 criteria.
| Event rate in control arm (%) | Event rate in intervention arm (%) | Sample size | Sample size + 20% loss to follow-up | |
|---|---|---|---|---|
| Original phase 2 | 55 | 65 | 980 | 1224 |
| 9% treatment difference | 55 | 64 | 1214 | 1518 |
| 8% treatment difference | 55 | 63 | 1544 | 1930 |
| 7% treatment difference | 55 | 62 | 2026 | 2532 |
| 9% difference with control rate changed to 60% | 60 | 69 | 1160 | 1450 |
| 8% difference with control rate changed to 60% | 60 | 68 | 1478 | 1848 |
| 9% difference with control rate changed to 65% | 65 | 74 | 1074 | 1344 |
| 8% difference with control rate changed to 65% | 65 | 73 | 1376 | 1720 |
The power calculations for the phase 2 RCT based on the phase 1 study findings are detailed in Table 9 and confirm that to show an intervention effect of 8% with early US surveillance, very large numbers of fistulas would be required, raising doubts as to whether this would be achievable. The recommendation from the TMG, endorsed by the TSC, was therefore to assess longer-term outcomes (6- and 12-month patency) in the original study group, on the basis that longer-term patency is more relevant clinically than early maturation and thus more pertinent to the design of the proposed RCT. Additionally, longer follow-up would provide greater insight into the eventual outcomes of those fistulas that remained patent, but immature, at week 10 (8.7% of the original study group; see Table 6); an important distinction in the latter group is whether the fistulas eventually matured spontaneously, or whether they required further intervention or were abandoned.
The longer follow-up study thus addresses the hypothesis that early Doppler US of AVFs predicts 6-month fistula patency failure. As detailed in Follow-up 12-month study, primary fistula patency was selected as the primary outcome analysis, which differs subtly from the primary analysis performed for the original SONAR study: fistula maturation at 10 weeks. We reasoned that if, by 6 months after creation, fistulas had not matured to a functional state, this would have prompted further salvage interventions or creation of a new fistula; hence patency at 6 months would indicate successful maturation. Thus, further US scanning at 6 and 12 months was not proposed.
Twelve-month follow-up
Patient enrolment for the 12-month follow-up study is shown in Figure 8. Of the original 333 SONAR AVFs, 192 (57.7%) were eligible and provided consent for follow-up. Notably, 56 (16.8%) of the original SONAR cohort had died in the first year after fistula creation, and hence the SONAR 12-month cohort (SONAR-12M) does have a survivorship bias, although the participant demographics still closely match the original SONAR cohort (Table 10).
FIGURE 8.
CONSORT diagram for 12-month follow-up study.

| Age (years) | 64.5 (50–73) |
| Male | 124 (64.6) |
| Cause of renal failure | |
| Glomerulonephritis | 11 (5.7) |
| Polycystic kidney disease | 9 (4.7) |
| Hypertension | 24 (12.5) |
| Diabetes | 56 (29.2) |
| Pyelonephritis | 0 (0.0) |
| Renovascular disease | 9 (4.7) |
| Unknown | 30 (15.6) |
| Other | 53 (27.6) |
| Hypertension | 160 (83.3) |
| Diabetes | |
| No | 113 (58.9) |
| Yes: insulin-dependent | 48 (25.0) |
| Yes: non-insulin-dependent | 31 (16.1) |
| IHD/CVA/PVD | |
| No | 150 (78.1) |
| Yes | 41 (21.4) |
Primary fistula patency at 6 months for all fistulas was 76.6% (95% CI: 69.9% to 82.4%) and was higher at 6 months for elbow fistula than for wrist fistula (elbow: 83.0%, 95% CI 73.8% to 89.9%; wrist: 70.4%, 95% CI 60.3% to 79.2%). This partly reflects the greater rates of early failure observed for wrist fistulas in the original SONAR study, but Kaplan–Meier analysis shows that fistula failure also occurred beyond the original 10-week SONAR study window (Figure 9). Primary fistula patency at 12 months was 68.3% (95% CI 61.2% to 74.5%). Primary patency rates for the SONAR-12M cohort at 6 months are therefore surprisingly greater than the 10-week maturation rates reported for the original SONAR cohort. This at least partly reflects inherent bias associated with the SONAR-12M cohort. For example, at 6 weeks, 46 of the 333 (13.8%) fistulas of the original SONAR cohort had failed, whereas the corresponding figure for the SONAR-12M cohort was 8.3% (16 failures of 192). Additionally, Figure 10 details that small numbers of fistulas that were immature at 10 weeks can still mature to a functional state by 6 months (conversely, some mature fistulas at 10 weeks fail by 6 months).
FIGURE 9.
Kaplan–Meier curve for AVF primary patency of SONAR-12M cohort. Primary patency to 12 months after AVF creation for (a) elbows (76%, 95% CI 66% to 84%) and (b) wrists (61%, 95% CI 50% to 70%). Assisted primary patency to 12 months after AVF creation for (c) elbows (85%, 95% CI 74% to 90%) and (d) wrists (65%, 95% CI 54% to 73%). Secondary patency to 12 months after AVF creation for (e) elbows (90%, 95% CI 82% to 95%) and (f) wrists (69%, 95% CI 59% to 77%).

FIGURE 10.
Fistula status at key study time points.
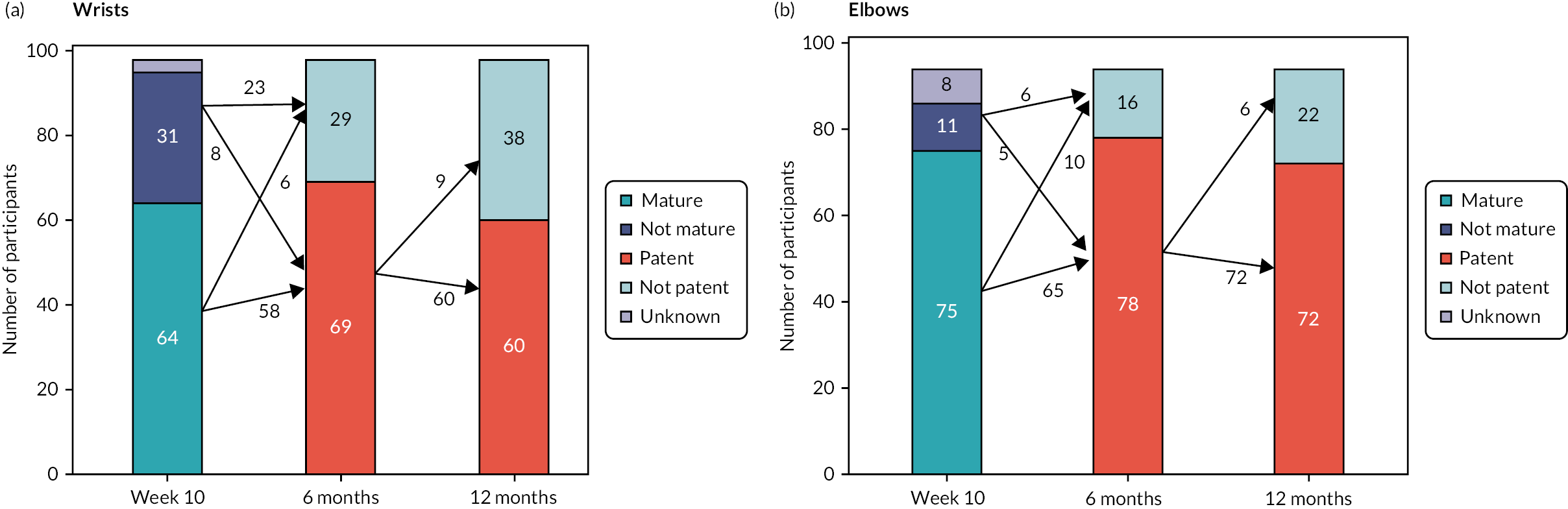
Surgical or radiological ‘salvage’ procedures were attempted on 43 occasions in the first year after AVF creation to either maintain or restore fistula patency and were reported as part of the 12-month follow-up. Radiological salvage generally consisted of angioplasty of perianastomotic stenotic venous segments, whereas of the surgical interventions, the majority (14/23; 60.9%) involved formation of a proximal neoanastomosis. These interventions were successful in 79.1% of the procedures. Assisted primary and secondary patency rates at 6 months (80.7% and 83.3%) and 12 months (74.1% and 79.5%) were therefore substantially higher than the primary patency rates (see Figure 9). For those patients established on dialysis, functional patency (rates of fistula usage on three successive occasions) was broadly similar (79.2%) to the calculated assisted primary patency rates, and substantially greater than recorded at 10 weeks for the original SONAR cohort (26.6%). Given the favourable maturation rates in the original SONAR cohort, the low rate of functional patency within 10 weeks presumably reflects either concerns regarding needling a fistula too early after creation or that fistulas continue to enlarge and mature beyond week 10.
Predictive model for fistula non-patency at 6 months
In similar fashion to predicting fistula non-maturation at 10 weeks, a binary-data population-average model with exchangeable correlation structure was built to assess the probability of fistula non-patency at 6 months. As with the SONAR modelling, optimum models could be developed using US data from a single week, but separate models for predicting non-patency at 6 months for wrist and elbow fistulas performed superiorly, and relied on a different week’s scan data (Table 11 and Figure 11). Thus, for elbow AVFs, an algorithm that included pre-operative vein diameter, week 4 average resistance index and fistula blood flow predicted 6-month non-patency in seven cases, and correctly so (true positives) in four of these, giving a PPV of 57.1% (95% CI 20.5% to 93.8%). A similar model could be developed for wrist fistulas, based on the week 6 scan data and incorporating fistula blood flow and average resistance index, with additional main effects and an interaction between sex of the participant and fistula venous diameter (see Table 11).
| Factors included in modela | Elbow (n = 83) odds ratio (95% CI) |
Wrist (n = 80) odds ratio (95% CI) |
|---|---|---|
| Pre-operative vein diameter (1 unit change from mean) | 1.57 (0.91 to 2.72) p = 0.1030 |
N/A |
| Average resistance indexb (0.1 unit change from mean) | 1.65 (0.61 to 4.46) p = 0.3146 |
2.59 (1.46 to 4.58) p = 0.0015 |
| Average volume flowb (100 unit change from mean) | 0.93 (0.83 to 1.05) p = 0.2471 |
1.13 (1.06 to 1.20) p = 0.0003 |
| Sexc | NS | p = 0.0067 |
| Representative venous diameterb,c | NS | p < 0.0001 |
| Interaction between sex and representative venous diameterb | N/A | p = 0.0003 |
| 1 unit change of representative diameter from mean for males | 0.71 (0.53 to 0.95) | |
| 1 unit change of representative diameter from mean for females | 0.09 (0.03 to 0.26) | |
| Model performance | ||
| AUC value | 0.71 | 0.81 |
| Threshold (Youden index) | 0.37 | 0.32 |
| PPV for threshold (95% CI) | 57.1% (20.5% to 93.8%) | 72.7% (46.4% to 99.0%) |
| NPV for threshold (95% CI) | 88.2% (80.9% to 95.4%) | 91.3% (84.7% to 98.0%) |
| Number of predicted failures vs. actual failures | 7 vs. 13 | 11 vs. 14 |
| Number of correctly predicted failuresd [true positives that fail at week 6 (elbow)/week 8 (wrist) or later]e | 4 (3) | 8 (8) |
FIGURE 11.
Receiver operating characteristic curve analysis of optimum models for predicting wrist and elbow fistula non-patency at 6 months. Receiver operating characteristic curves for optimum models for predicting 6-month fistula non-patency from (a) week 6 US findings for wrist and (b) week 4 US findings for elbow fistulas.
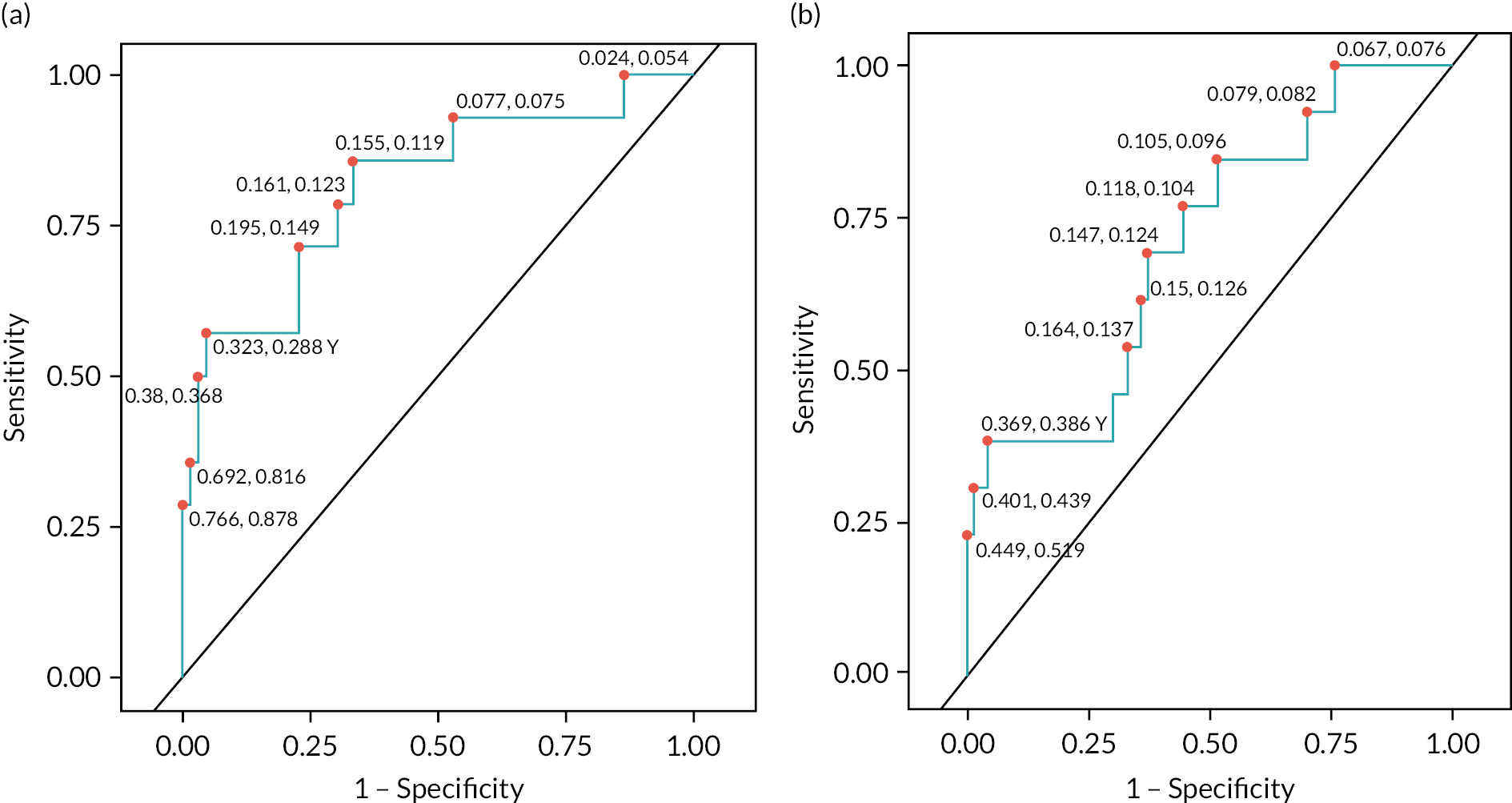
This predicted non-patency in 11 cases, with 8 true positives, giving a PPV of 72.7% (95% CI 46.4% to 99.0%). Diagnostic tests for model fit show that the models perform moderately well, with AUC values on ROC analysis of 0.71 for elbow and 0.81 for wrist (see Figure 11). These values are less than calculated for predicting non-maturation at 10 weeks. As with the modelling for maturation status at 10 weeks, the models for 6-month patency were remarkably effective at identifying those fistulas that would be patent, with NPV of 88.2% (95% CI 80.9% to 95.4%) and 91.3% (95% CI 84.7% to 98.0%) for elbow and wrist fistulas, respectively. The negative and positive predictive powers for modelling 6-month fistula patency are depicted in Figure 12.
FIGURE 12.
Summary of week 4 and week 6 US modelling on identifying 6-month fistula status. FN, false negative; FP, false positive; TN, true negative; TP, true positive. (a) Observed or imputed as per determination of fistula patency/non-patency by 6M criteria.

Wider application of selected models
One would intuit that the US factors identified as predicting fistula maturation status at week 10 would be similar to those predicting 6-month patency, and that consequently, the models established for both would perform reasonably when interchanged. Table 12 details, however, that this is not the case; when the model for 10-week fistula maturation is applied to the SONAR-12M data set to predict 6-month primary fistula failure, the PPV falls to 31.8% and 22.2% for wrist and elbow fistulas, respectively. Similarly, neither the SONAR nor SONAR-12M models could reliably predict assisted primary fistula failure at 6 months; that is, those fistulas that fail despite potentially being subject to additional salvage procedures (see Table 12). This is a particular concern, because assisted primary patency was the primary end point chosen for the proposed RCT.
| SONAR models | SONAR-12M models | |
|---|---|---|
| Against primary fistula failure at 6 months | ||
| PPV for optimum wrist model (95% CI) | 31.8% (18.1% to 45.6%) | 72.7% (46.4% to 99.0%) |
| NPV for optimum wrist model (95% CI) | 94.7% (87.6% to 100.0%) | 91.3% (84.7% to 98.0%) |
| PPV for optimum elbow model (95% CI) | 22.2% (6.5% to 37.9%) | 57.1% (20.5% to 93.8%) |
| NPV for optimum elbow model (95% CI) | 87.5% (78.8% to 96.2%) | 88.2% (80.9% to 95.4%) |
| Against assisted primary fistula failure at 6 months | ||
| PPV for optimum wrist model (95% CI) | 31.6% (16.8% to 46.4%) | 29.3% (15.3% to 43.2%) |
| NPV for optimum wrist model (95% CI) | 95.5% (89.3% to 100.0%) | 100.0% (100.0% to 100.0%) |
| PPV for optimum elbow model (95% CI) | 14.3% (1.3% to 27.3%) | 17.9% (3.7% to 32.0%) |
| NPV for optimum elbow model (95% CI) | 94.5% (88.5% to 100.0%) | 96.4% (91.4% to 100.0%) |
One possible explanation for the poor performance of the SONAR-12M model in predicting 6-month assisted primary failure is that those fistulas identified by the model as at risk of primary failure have their patency successfully maintained by a salvage procedure. In support, Table 13 demonstrates that those elbow or wrist fistulas identified from modelling of the 4- or 6-week US data as at risk of primary failure at 6 months were far more likely to undergo a salvage procedure. For example, of the 80 wrist fistulas considered in the week 6 predictive model, 8 salvage interventions were performed on the 11 (72.7%) that were predicted as a primary patency failure at 6 months, whereas 6 interventions were performed on the 69 fistulas (8.7%) predicted as patent at 6 months (Fisher’s exact test, p < 0.0001). Similarly, of the 83 elbow fistulas considered in the week 4 predictive model, 3 salvage interventions were performed on the 7 (42.8%) that were predicted as a primary patency failure at 6 months, whereas 11 interventions were performed on the 76 fistulas (14.5%) predicted as patent at 6 months (Fisher’s exact test, p = 0.0896). As detailed above, interventions were generally successful at maintaining or restoring fistula patency, and thus fistulas that suffer assisted primary failure at 6 months are perhaps more likely to have had favourable early US scans than those that suffered primary failure at 6 months.
| Predicted primary non-patency at 6 months | Predicted primary patency at 6 months | ||
|---|---|---|---|
| Elbow (n = 83) | Intervention | 3 | 11 |
| No intervention | 4 | 65 | |
| Wrist (n = 80) | Intervention | 8 | 6 |
| No intervention | 3 | 63 |
Chapter 4 Discussion
Chronic kidney disease (CKD) presents a substantial health and economic burden to the NHS, with approximately 6% of adults in the UK suffering from stage 3 to 5 CKD, and with CKD absorbing about 1–2% of the annual NHS budget. 41 There are consequently approximately 23,000 fistula operations annually in the UK, making it one of the more common surgical procedures performed; as reference, there are about 80,000 hip replacement operations annually in the UK, considered to be the most frequently performed operation. The relatively low risk of complications – and particularly of infection – allied to its longevity means that an AVF represents the best haemodialysis option for most patients. The main drawback with fistula provision is the relatively high rate of early failure, with publications generally detailing that between 30% and 40% of fistulas fail to mature or suffer early thrombosis. Thus, improvements in outcomes for fistula surgery and early fistula patency rates would potentially benefit many thousands of individuals, as well as generate substantial cost savings.
The SONAR consortium was established to examine whether US surveillance of AVFs in the first few weeks after formation could identify those fistulas unlikely to either mature or achieve long-lasting patency, and if so, whether this could inform timely salvage intervention to improve outcomes for fistula surgery. Thus, the study differs from previous reports of early US surveillance23,24,38 in that its principal objective was not to assess whether early Doppler US predicted fistula maturation and patency, but rather to address the feasibility of performing a prospective RCT that compares fistula patency in the intervention arm (early US-guided salvage intervention) against standard care. Thus, despite confirming a relationship between fistula flow parameters on early US and subsequent fistula outcomes, we conclude from the study that formal trial evaluation of early US fistula surveillance would require very large numbers of participants, and even then, would be unlikely to show a statistically significant difference. In any event, a large proportion of those fistulas identified on early US screening as at risk of early failure underwent successful salvage at late stages, and presumably without reference to the early US findings. This raises doubts on the premise that, by avoiding thrombosis and loss of the draining fistula vein for further fistula creation, early identification and salvage of at-risk fistulas maximises fistula patency. Rather, the study results highlight that an observant approach, with interventions guided by the later clinical findings, achieves very respectable patency rates.
It is perhaps surprising, given that early US parameters correlate so strongly with both fistula maturation at 10 weeks and fistula patency at 6 months (the week 4 US identified, e.g., 10-week fistula maturation with > 95% predictive power) that the study concluded that running a RCT of early US-guided intervention would be so exacting. However, a critical aspect of progressing to the RCT was the ability of early US to identify those fistulas that were still patent on that particular scan but which would subsequently have either failed or not matured by 10 weeks, and the week 4 US could identify such fistulas with a PPV of 66.7% for elbow and 60.6% for wrist. The much better ability of early US to identify those fistulas that will mature than those that will fail or remain immature partly reflects that failure to mature is a rather uncommon event (thereby strengthening the predictive power for maturation). However, an unexpected finding of the study was how quickly in general fistulas reached maturation according to the predefined US parameters. Consequently, of those attending for a week 4 scan, 54.0% of elbow and 51.6% of wrist fistulas had already reached maturation. One would anticipate that maturation is a relatively static and unchanging state: that, once matured, the most likely outcome is continued gradual increases in fistula flow and venous diameter. Hence, the ability of the week 4 scan to predict 10-week maturation may reflect that it is largely identifying those fistulas that have already matured. In contrast, it is likely that the 10-week outcome of an immature state at week 4 is more uncertain, with some fistulas still maturing successfully and others either remaining immature or failing. In support, Figure 13 depicts fistula flow rates and venous diameter at week 4 US, labelled according to predicted 10-week maturation status, and highlights that those fistulas correctly identified as not going to mature (true positives) were largely still immature at week 4, whereas those fistulas correctly predicted to mature (true negatives) had high flows and large diameters at week 4. Those fistulas incorrectly predicted to not mature (false positives) or incorrectly predicted to mature (false negative) tended to have flow rates and venous diameters around the cut-off for defining maturity.
FIGURE 13.
Week 4 fistula flow rates and venous diameters, labelled according to predicted 10-week maturation status. Week 4 US findings for (a) wrist and (b) elbow fistulas and indicating 10-week maturation status according to predictive modelling. Group 1 (●): True positives – fistulas correctly predicted to not mature. Group 2 (∆): False negatives – fistulas incorrectly predicted to mature. Group 3 (○): False positives – fistulas incorrectly predicted to not mature. Group 4 (▲): True negatives – fistulas correctly predicted to mature. The cut-off for defining maturation is shown as dashed lines.

Although not the main focus of the study, this ability to use early US to identify, with a high degree of certainty, those fistulas that will reach maturity and be patent at 6 months, is not without clinical relevance. Vascular access surgery is generally a tertiary specialty, and an early US scan that provides strong reassurance of short- and medium-term fistula patency would potentially allow the patient to be discharged back to their referring centre at a much earlier stage, thereby avoiding duplications in patient care while minimising costs and travel times. In this regard, participants have also been asked for their consent to 5-year follow-up; this will permit assessment of the longer-term relationship between early US scan data and fistula patency.
In evaluating the proposed RCT, on the basis that compared to standard care (clinical observation only), early US surveillance improved fistula patency from an estimated 55% to 65%, our preliminary power calculations suggested that ~1200 fistulas would be required, allowing for a 20% dropout. However, as detailed in Twelve-month fistula outcomes, when considering the proportion of ‘at-risk’ fistulas that could be identified and then salvaged by the week 4 US, our initial study highlighted that there was the potential, at best, to improve fistula maturation rates by ~18%. Given this, the TMG felt that an improvement of 10% was overly ambitious, in that some fistulas would undoubtedly fail before an intervention could be organised, and in others, salvage intervention would not be possible or would be unsuccessful. However, a more conservative intervention effect of 8% relative to an event rate in the control arm of 65% raised the number of fistulas required for the RCT substantially: to 1720 (allowing for dropout), with 1 : 1 randomisation to standard care or to the treatment arm (salvage intervention of the fistula based on results of a week 4 US scan). Of the 860 fistulas randomised to the treatment group, we estimated that surveillance would result in 175 salvage interventions, with 90 of these unnecessary (the fistula would have matured successfully if managed conservatively). Of the 85 true positives and assuming an intervention success rate of 79%, 67 interventions would be successful, thus improving maturation rates from 65% to 73%. Surveillance would miss a further 20 fistulas that would fail to mature.
After discussion within the TMG and the independent TSC, we further assessed whether early surveillance US could predict longer-term (6- and 12-month) fistula patency with sufficient accuracy to justify a RCT of early US-guided salvage, but with assisted primary patency as primary end point. This decision was reached on the basis that longer-term fistula outcomes are more important than 10-week patency, but also because longer-term follow-up may clarify, firstly, whether those fistulas that were immature on week 10 US scanning (8.7%) eventually mature spontaneously, or whether they fail or require salvage intervention; and secondly, whether those fistulas that reach maturity at 10 weeks despite unfavourable early US characteristics are nevertheless still prone to early failure. By reinforcing the relationship between early US findings and eventual fistula outcome, either of these outcomes may strengthen the case for proceeding to the RCT. However, if anything, the ability of the early US to model 6-month primary fistula failure was poorer than for predicting 10-week maturation status, with only the wrist model worthy of consideration. Even then, power calculations for a RCT of early surveillance of wrist fistulas (see Table 9) confirm that over 1500 fistulas would be required for an 8% improvement in the primary end point of assisted primary patency, which, as detailed in Figure 12, could only be achieved if virtually all the at-risk fistulas identified on the week 6 US were successfully salvaged.
As the primary outcome analysis for the longer-term follow-up study, we chose primary fistula failure – the point at which either the fistula failed or an intervention was performed to either maintain or restore patency. It was surprising, therefore, that the optimum model for predicting 6-month primary fistula failure performed very poorly at identifying 6-month primary assisted fistula failure; essentially, despite predicting those fistulas that would fail or require an intervention, the model could not predict those that failed despite that intervention. As discussed, this most likely reflects that a similar cohort of fistulas to those identified as at risk on the early surveillance US are subject to late salvage intervention. Given that the responsible clinical teams were generally blinded to the results of the early US scans, these interventions were presumably initiated principally because of clinical concerns relating to fistula maturation and patency. These later salvage interventions were able to restore or maintain fistula patency in approximately 80% of cases, which is certainly higher than the trial group had initially envisaged, and this success raises questions as to the merit of an aggressive approach to early identification of at-risk fistulas.
An additional or alternative explanation is that the modelling was predicated upon identifying primary fistula failure, and its ability to predict assisted primary failure is therefore dependent upon a biological or physiological link between the two outcomes. One would expect this to be the case, but it is perhaps possible that salvage procedures were performed on fistulas that were largely going to remain patent without any intervention, whereas another cohort of fistulas were destined to fail, irrespective of attempts at salvage. This dissociation between the two fistula states would further undermine the proposed RCT, which, because it assesses the role of US to guide salvage intervention, would have used primary assisted fistula patency as the primary end point. Modelling whether the early US parameters could predict assisted fistula patency was not performed, as it would be illogical from the trial perspective.
On consideration of all of the above points, the TMG recommendation, endorsed by the TSC, was that SONAR should not progress to the phase 2 RCT, and the project was closed.
Patient and public engagement
An initial survey of 29 Cambridge haemodialysis patients informed development of our proposal. All surveyed patients thought it reasonable to have an US scan to assess development of their fistula, with 96.6% indicating that they ‘agree’ or ‘strongly agree’ with the statement that ‘it was acceptable that the scans would be for research purposes and would not affect their care directly’ (one patient was neutral on this point); 58.6% of patients responded that they would be willing to be scanned every fortnight for the first 6 weeks (a further 20.7% were neutral). The panel were, however, unanimous that participants in the first phase should have the opportunity to review the results of the trial, so that they could be reassured that their participation was worthwhile.
The proposed application was further discussed with a patient and public involvement (PPI) panel, consisting of lay members of the public and retired doctors, and provided by the Cambridge University Hospitals PPI team. This panel recommended that US scans in phase 1 should be performed during dialysis sessions to minimise inconvenience, and agreed that blinding during the first phase of the study was important for the study design (one member thought it ‘morally preferable’). The PPI group unanimously supported the two-stage design, as focusing first on the ability of early US to identify AVFs that were unlikely to mature would, they felt, provide ‘better information’ and ‘cleaner data’, and crucially, would provide critical information for justifying progression, or otherwise, to a much larger and costly RCT, thus rationalising the numbers of participants required.
One member of the PPI panel, Andrew Norton, joined the TSC to provide lived experience input as the trial progressed. Andrew shared his experiences as a dialysis, fistula and then transplant recipient with the wider trial group, providing great insight and suggestions to support the success of the research. Andrew also promoted the trial with interviews and articles in local and national press and successfully sought funding from the Addenbrooke’s Kidney Patient Association, for patient travel expenses related to the trial US visits.
The SONAR trial was featured in the National Kidney Federation magazine, Kidney Life in the Spring 2020 issue. This article raised awareness of the research within the kidney patient population, and shared feedback from a trial participant. A further article was published in the Spring 2023 edition, sharing the results of the research.
Additional information
Acknowledgements
The research team would like to thank the study participants for their support and contributions. We would like to acknowledge the TSC: Professor Patrick Mark (chair); Dr Kate Steiner; Professor Julie Brittenden; Mr Andrew Norton; Mr Laszlo Szabo, and the Independent Data Monitoring Committee: Dr Sian Griffin (Chair); Ms Kerri Barber; and Ms Helen Dixon. In addition, we would like to acknowledge the Addenbrooke’s Kidney Patient Association for their financial support towards patients’ travel expenses related to the study.
Equality, diversity and inclusion
The SONAR and SONAR-12M inclusion and exclusion criteria were kept as simple and succinct as possible, to facilitate wide participation of the adult ESRD population. CKD stage 3–5 prevalence is higher in women than in men, but men are more likely to reach kidney failure sooner. SONAR recruitment was not limited by sex, and 64.5% of our participants were men. People of black or South Asian origin are more likely to develop CKD. We did not collect ethnicity data from participants, but our centres provide care for diverse populations. We therefore cannot assess the ethnic diversity of our participants, and this is something we will collect prospectively for future AVF research projects, along with measures to support the inclusion of any under-served ethnic minority groups.
The trial participant information sheet was provided in English and participating centres made use of NHS Trust-provided translation and communication services where appropriate, to enable informed conversations about the trial with patients and family members, when verbal explanations or written information in English might not be adequately understood. As the participant information sheet was not translated into any languages other than English, this will inevitably have limited inclusion of patients on occasion when translation services were not available or acceptable. In future studies, budgeting for the translation of written information into several suitable languages could improve inclusion. It was also a requirement of the inclusion criteria that patients could provide informed consent, and therefore we did not include patients lacking capacity.
The SONAR Trial Group identified early on in the study design stage that attending for additional US scans would incur travel costs for some patients. In order to reduce the likelihood of this adversely impacting on the ability of any patient to afford to take part, funding for travel costs was identified as a priority. The Addenbrooke’s Kidney Patient Association generously funded patient travel costs for all participants.
Articles and documents for patients and the public were written with minimal technical or medical terminology. Quotes from patients were used to share real-life experiences.
The SONAR research team includes individuals with a range of experience and expertise, and more junior members of the team were actively encouraged to be involved to gain experience and develop their skills. Vascular scientists and sonographers were provided with training sessions to broaden knowledge and skills for fistula scanning. Research fellows and clinical trainees were encouraged to be co-investigators at the participating centres. An apprentice within the Clinical Trials Unit joined the trial team to develop clinical trial administrator experience. All members of the team are acknowledged in publications, to reflect that this trial relied on the skills of a wide range of clinical trial staff, nurses, vascular scientists, doctors and surgeons. This research involved 16 centres in England and 1 in Scotland. The centres provided good geographical coverage, with a mix of large teaching hospitals in cities (e.g. Manchester, London, Coventry, Edinburgh) and medium-sized hospitals.
Contributions of authors
James Richards (https://orcid.org/0000-0001-9049-5745) (Locum Consultant in NORS, HPB and Transplant Surgery) was involved in the conception and design of the study, patient recruitment and data acquisition and drafting of the report.
Dominic Summers (https://orcid.org/0000-0003-3360-0726) (Consultant Transplant and Vascular Access Surgeon) was involved in the conception and design of the study, data acquisition and drafting of the report.
Anna Sidders (https://orcid.org/0000-0003-2687-4710) (Trial Manager) was involved in the conception and design of the study and drafting of the report.
Elisa Allen (https://orcid.org/0000-0001-5220-9015) (Trial Statistician) was involved in the conception and design of the study, analysis and interpretation of the data and drafting of the report.
Mohammed Ayaz Hossain (https://orcid.org/0000-0002-1408-647X) (Consultant Transplant Surgeon) was involved in the conception and design of the study, data acquisition and drafting of the report.
Subhankar Paul (https://orcid.org/0000-0001-7553-0733) (Senior Clinical Fellow in Transplant Surgery and Organ Retrieval) was involved in the conception and design of the study, data acquisition and drafting of the report.
Matthew Slater (https://orcid.org/0000-0002-5773-3657) (Vascular Scientist) was involved in the conception and design of the study, ultrasound data acquisition and drafting of the report.
Matthew Bartlett (https://orcid.org/0000-0003-4898-7468) (Vascular Scientist) was involved in the conception and design of the study, ultrasound data acquisition and drafting of the report.
Regin Lagaac (https://orcid.org/0000-0003-4964-5101) (Renal and Vascular Access Nurse) was involved in the conception and design of the study, data acquisition and drafting of the report.
Emma Laing (https://orcid.org/0000-0002-8309-0990) (Clinical Operations Manager) was involved in the conception and design of the study and drafting of the report.
Valerie Hopkins (https://orcid.org/0000-0002-2167-1650) (Clinical Trial Coordinator) was involved in the conception and design of the study and drafting of the report.
Chloe Fitzpatrick-Creamer (https://orcid.org/0009-0000-8064-6108) (Clinical Trial Administrator) was involved in the conception and design of the study and drafting of the report.
Cara Hudson (https://orcid.org/0000-0002-5831-5959) (Trial Statistician) was involved in the conception and design of the study, analysis and interpretation of the data and drafting of the report.
Joseph Parsons (https://orcid.org/0000-0001-5542-2460) (Trial Statistician) performed statistical analysis and interpretation of the data and drafting of the report.
Samuel Turner (https://orcid.org/0000-0002-4744-0826) (Consultant Renal Transplant and Vascular Access Surgeon) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Andrew Tambyraja (https://orcid.org/0000-0002-9393-6291) (Consultant Vascular Surgeon) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Subash Somalanka (https://orcid.org/0000-0002-1547-5127) (Consultant Nephrologist) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
James Hunter (https://orcid.org/0000-0002-4972-6879) (Consultant Renal Transplant and Vascular Access Surgeon) is a Grant Co-applicant and Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Sam Dutta (https://orcid.org/0009-0003-0993-8142) (Consultant Transplant and General Surgeon) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Neil Hoye (https://orcid.org/0000-0002-5379-0523) (Consultant Nephrologist) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Sarah Lawman (https://orcid.org/0009-0000-9865-8799) (Consultant Nephrologist) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Tracey Salter (https://orcid.org/0000-0003-3259-917X) (Consultant Nephrologist) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Mohammed Farid Aslam (https://orcid.org/0000-0002-1930-0498) (Clinical Vascular Scientist) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Atul Bagul (https://orcid.org/0000-0002-8146-1946) (Consultant Transplant and Endocrine Surgeon) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Rajesh Sivaprakasam (https://orcid.org/0000-0002-6145-4642) (Consultant Transplant Surgeon) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
George E Smith (https://orcid.org/0000-0002-8085-0886) (Honorary Consultant Vascular Surgeon) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Helen L Thomas (https://orcid.org/0000-0002-7017-7739) (Head of Clinical Trial Statistics) was involved in the conception and design of the study, analysis and interpretation of the data and drafting of the report.
Zia Moinuddin (https://orcid.org/0000-0002-9125-8116) (Consultant Transplant Surgeon) is a Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Simon R Knight (https://orcid.org/0000-0003-4837-9446) (Honorary Consultant Transplant and Vascular Access Surgeon) is a Grant Co-applicant and Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Nicholas Barnett (https://orcid.org/0000-0002-7997-7492) (Consultant Transplant and Vascular Access Surgeon) is a Grant Co-applicant and Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Reza Motallebzadeh (https://orcid.org/0000-0002-5399-9546) (Consultant Renal Transplant Surgeon) is a Grant Co-applicant and Principal Investigator involved in the conception and design of the study, data acquisition and drafting of the report.
Gavin J Pettigrew (https://orcid.org/0000-0003-3724-9945) (Professor of Clinical and Experimental Transplantation) is the Grant Lead Applicant and Chief Investigator responsible for the conception and design of the study, data acquisition, the analysis and interpretation of the data and writing the report.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/YTBT4172.
Primary conflicts of interest: Mr Simon R Knight was a non-paid member of the Health Technology Assessment (HTA) Interventional Procedures (IP) Panel from 1 May 2017 to 31 May 2018. The authors have no other interests to declare.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Ethics statement
The SONAR study received a favourable opinion from the Cambridgeshire and Hertfordshire Research Ethics Committee on 26 July 2018, REC Ref: 18/EE/0234. The SONAR-12M study received a favourable opinion from the West Midlands – Edgbaston Research Ethics Committee on 13 January 2021, REC Ref: 20/WM/0331.
Information governance statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data for the trial have been stored anonymised and within secure NHS servers.
Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge are committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679.
Under the Data Protection legislation, Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge are the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here https://www.cuh.nhs.uk/patient-privacy/.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the HTA programme or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- UK Renal Registry . UK Renal Registry 24th Annual Report – Data to 31 12 2020 2022. https://ukkidney.org/audit-research/annual-report (accessed 14 February 2023).
- Taylor G, Gravel D, Johnston L, Embil J, Holton D, Paton S. Canadian Nosocomial Infection Surveillance Program . Incidence of bloodstream infection in multicenter inception cohorts of hemodialysis patients. Am J Infect Control 2004;32:155-60.
- D’Agata EM, Mount DB, Thayer V, Schaffner W. Hospital-acquired infections among chronic hemodialysis patients. Am J Kidney Dis 2000;35:1083-8.
- Marr KA, Kong L, Fowler VG, Gopal A, Sexton DJ, Conlon PJ, et al. Incidence and outcome of Staphylococcus aureus bacteremia in hemodialysis patients. Kidney Int 1998;54:1684-9.
- Rodriguez-Aranda A, Alcazar JM, Sanz F, Garcia-Martin F, Otero JR, Aguado JM, et al. Endoluminal colonization as a risk factor for coagulase-negative staphylococcal catheter-related bloodstream infections in haemodialysis patients. Nephrol Dial Transplant 2011;26:948-55.
- Rosenbaum D, MacRae JM, Djurdjev O, Levin A, Werb R, Kiaii M. Surveillance cultures of tunneled cuffed catheter exit sites in chronic hemodialysis patients are of no benefit. Hemodial Int 2006;10:365-70.
- McCann M, Moore ZE. Interventions for preventing infectious complications in haemodialysis patients with central venous catheters. Cochrane Database Syst Rev 2010;2010.
- Engemann JJ, Friedman JY, Reed SD, Griffiths RI, Szczech LA, Kaye KS, et al. Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect Control Hosp Epidemiol 2005;26:534-9.
- Astor BC, Eustace JA, Powe NR, Klag MJ, Fink NE, Coresh J. CHOICE Study . Type of vascular access and survival among incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol 2005;16:1449-55.
- The UK Kidney Association . Vascular Access Guidelines 2015. https://ukkidney.org/sites/renal.org/files/vascular-access.pdf (accessed 14 February 2023).
- Caskey F, Cullen R. UK Renal Registry 18th annual report: introduction. Nephron 2016;132:1-8.
- Schinstock CA, Albright RC, Williams AW, Dillon JJ, Bergstralh EJ, Jenson BM, et al. Outcomes of arteriovenous fistula creation after the fistula first initiative. Clin J Am Soc Nephrol 2011;6:1996-2002.
- Wells AC, Fernando B, Butler A, Huguet E, Bradley JA, Pettigrew GJ. Selective use of ultrasonographic vascular mapping in the assessment of patients before haemodialysis access surgery. Br J Surg 2005;92:1439-43.
- Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Moll FL, Blankestijn PJ. CIMINO Study Group . Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol 2008;3:714-9.
- Han A, Min SK, Kim MS, Joo KW, Kim J, Ha J, et al. A prospective, randomized trial of routine duplex ultrasound surveillance on arteriovenous fistula maturation. Clin J Am Soc Nephrol 2016;11:1817-24.
- Voorzaat BM, Janmaat CJ, van der Bogt KEA, Dekker FW, Rotmans JI. Patency outcomes of arteriovenous fistulas and grafts for hemodialysis access: a trade-off between nonmaturation and long-term complications. Kidney360 2020;1:916-24.
- Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 2014;63:464-78.
- Badero OJ, Salifu MO, Wasse H, Work J. Frequency of swing-segment stenosis in referred dialysis patients with angiographically documented lesions. Am J Kidney Dis 2008;51:93-8.
- Ives CL, Akoh JA, George J, Vaughan-Huxley E, Lawson H. Pre-operative vessel mapping and early post-operative surveillance duplex scanning of arteriovenous fistulae. J Vasc Access 2009;10:37-42.
- Hindle P, Ritchie J, Griffiths G, Howd A. Use of early scanning in fistula formation for vascular access. J Vasc Access 2011;12:127-9.
- Beathard GA, Lok CE, Glickman MH, Al-Jaishi AA, Bednarski D, Cull DL, et al. Definitions and end points for interventional studies for arteriovenous dialysis access. Clin J Am Soc Nephrol 2018;13:501-12.
- Saucy F, Haesler E, Haller C, Deglise S, Teta D, Corpataux JM. Is intra-operative blood flow predictive for early failure of radiocephalic arteriovenous fistula?. Nephrol Dial Transplant 2010;25:862-7.
- Robbin ML, Greene T, Cheung AK, Allon M, Berceli SA, Kaufman JS, et al. Hemodialysis Fistula Maturation Study Group . Arteriovenous fistula development in the first 6 weeks after creation. Radiology 2016;279:620-9.
- Robbin ML, Greene T, Allon M, Dember LM, Imrey PB, Cheung AK, et al. Hemodialysis Fistula Maturation Study Group . Prediction of arteriovenous fistula clinical maturation from postoperative ultrasound measurements: findings from the hemodialysis fistula maturation study. J Am Soc Nephrol 2018;29:2735-44.
- Itoga NK, Ullery BW, Tran K, Lee GK, Aalami OO, Bech FR, et al. Use of a proactive duplex ultrasound protocol for hemodialysis access. J Vasc Surg 2016;64:1042.e-1049.e1.
- Moist L, Lok CE. Con: vascular access surveillance in mature fistulas: is it worthwhile?. Nephrol Dial Transplant 2019;34:1106-11.
- Tessitore N, Poli A. Pro: vascular access surveillance in mature fistulas: is it worthwhile?. Nephrol Dial Transplant 2019;34:1102-6.
- Anvari E, Vachharajani TJ. The hemodialysis access surveillance controversy continues. Kidney Int Rep 2020;5:1848-50.
- Goh MA, Ali JM, Iype S, Pettigrew GJ. Outcomes of primary arteriovenous fistulas in patients older than 70 years. J Vasc Surg 2016;63:1333-40.
- Mousa AY, Dearing DD, Aburahma AF. Radiocephalic fistula: review and update. Ann Vasc Surg 2013;27:370-8.
- Kazemzadeh GH, Modaghegh MHS, Ravari H, Daliri M, Hoseini L, Nateghi M. Primary patency rate of native AV fistula: long term follow up. Int J Clin Exp Med 2012;5:173-8.
- Robbin ML, Chamberlain NE, Lockhart ME, Gallichio MH, Young CJ, Deierhoi MH, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 2002;225:59-64.
- Singh P, Robbin ML, Lockhart ME, Allon M. Clinically immature arteriovenous hemodialysis fistulas: effect of US on salvage. Radiology 2008;246:299-305.
- Sidawy AN, Gray R, Besarab A, Henry M, Ascher E, Silva M, et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg 2002;35:603-10.
- Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. John Wiley & Sons, Inc.; 2013.
- UK Renal Registry . UK Renal Registry 23rd Annual Report – Data to 31 12 2019 2021. https://ukkidney.org/sites/renal.org/files/23rd_UKRR_ANNUAL_REPORT.pdf (accessed 14 February 2023).
- Feldman HI, Joffe M, Rosas SE, Burns JE, Knauss J, Brayman K. Predictors of successful arteriovenous fistula maturation. Am J Kidney Dis 2003;42:1000-12.
- Farrington CA, Robbin ML, Lee T, Barker-Finkel J, Allon M. Early predictors of arteriovenous fistula maturation: a novel perspective on an enduring problem. J Am Soc Nephrol 2020;31:1617-27.
- Mendes RR, Farber MA, Marston WA, Dinwiddie LC, Keagy BA, Burnham SJ. Prediction of wrist arteriovenous fistula maturation with preoperative vein mapping with ultrasonography. J Vasc Surg 2002;36:460-3.
- Collett D. Modelling Survival Data in Medical Research. London: Chapman & Hall; 2015.
- Kerr M, Bray B, Medcalf J, O’Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012;27:iii73-80.
Notes
Appendix 1 SONAR trial group
-
Anna Sidders, Cara Hudson, Claire Foley, Valerie Hopkins, Emma Laing, Chloe Fitzpatrick-Creamer, Renate Hodge, David Cope, Lisha Gracias, Elisa Allen, Joseph Parsons, Helen L Thomas, Alison Deary (NHS Blood and Transplant Clinical Trials Unit).
-
Gavin J Pettigrew, James Richards, Mohammed Ayaz Hossain, Dominic Summers, Matthew Slater, Laura Scott, Regin Lagaac, Veena Surendrakumar, Tobi Ayorinde, Igor Chipurovski, Manikandan Kathirvel, Manoj Thialli, Subhankar Paul, Andrew Norton (Cambridge University Hospital).
-
Simon R Knight, Klaus Bond, Elizabeth Hardy, Joanne Widdup, Rachael Potter, Elisabeth Pugh, Karen Parsons, Kathryn Lafferty, Madita Gavrila, Sheera Sutherland, Ria Rabara (Oxford University Hospitals NHS Foundation Trust).
-
Rajesh Sivaprakasam, Kate Crawford, Amy Bolsworth, Naavalah Ngwa-Ndifor, Laura Clementoni (Bart’s Health NHS Trust).
-
Reza Motallebzadeh, Mohammad Ayaz Hossain, Matthew Bartlett, Rani Badhan, Fernando Yuenchang, Phil Gardiner, Natasha Irani (Royal Free London NHS Foundation Trust).
-
Zia Moinuddin, Helena Edlin, Anna Jerram, Jessica Lai, Joyce Banda, Janet Bendle (Manchester University NHS Foundation Trust).
-
Samuel Turner, Maria Morgan, William Owen, Sue Dawson, Simon Daniel, Karen Allsop (North Bristol NHS Trust).
-
Andrew Tambyraja, Sarah-Jane Carmichael, Tom Eadie, Rona Lochiel, Midel Lena, Karen Gallagher (Royal Infirmary of Edinburgh, NHS Lothian).
-
Nicholas Barnett, Soundrie Padayachee, Philip Eldridge, May Rabuya, Naomi Hare (Guy’s and St Thomas’ NHS Foundation Trust).
-
Subash Somalanka, Jashree Patel, Abbas Ghazanfar, Judy van Selm, Caroline Bodneck, Martia Augustin, Kwame Ansu, Nalin Khosla, Kashif Burney, Karen Dear, Duminda Basnayake, Laijee Benny (Epsom and St Helier University Hospitals NHS Trust).
-
James Hunter, Carl Tiivas, Samantha Hyndman, Maria Truslove, Gail Evans, Kerry Read (University Hospital Coventry and Warwickshire NHS Trust).
-
Sam Dutta, Andrew Beech, Sarah Brand, Tara MacCormick-Swanson (Nottingham University Hospitals NHS Trust).
-
Sarah Lawman, Darren Cheal, Mel Smith, Kate Trivedi, Valentina Toska, Lorraine Shah-Goodwin (Brighton and Sussex University Hospitals NHS Trust).
-
Tracey Salter, Adnan Bajwa, John Kerr, Ana Fleet, Lianne Chapman, Sarah Gee, Thanuja Weerasinghe, Lisa Kavanagh, Louise Rowe-Leete (Frimley Health NHS Foundation Trust).
-
George E Smith, Paris Cai, Judith Long, Tracey Rowe (Hull University Teaching Hospitals NHS Trust).
-
Mohammed Farid Aslam, Jeremy Crane, Wael Faqihi (Imperial College Healthcare NHS Trust).
-
Atul Bagul, Mary Quashie-Akponeware, Kate Waters, Alexandra Howson, Caroline Gardiner-Hill (University Hospitals of Leicester NHS Trust).
-
Neil Hoye, Alycon Walker (South Tees Hospitals NHS Foundation Trust)
Appendix 2 Arteriovenous fistula duplex examination protocol
Each patient will be given a trial number, and this will enable images to be anonymised.
Within the Patient ID field and surname field please type SONAR followed by the trial number.
-
Scan from the proximal artery up to and including the outflow deep vein.
-
Within the brachial artery measure volume flow (over three cardiac cycles) in roughly the same place three times and record.
-
Within the brachial artery measure the resistance index (over one cardiac cycle) three times and record.
-
Measure the outflow AVF at its smallest point, largest point and give a representative size of the majority of the AVF.
-
Measure the depth of the outflow AVF (within the area that would be used for dialysis) at its shallowest point, deepest point and give a representative depth of the majority of the outflow vein.
-
Measure the diameter and the PSV at the anastomosis.
-
Assess for stenosis or any other pathology.
-
Grade type of stenosis in the outflow vein using the criteria below:
| Type 1 – Vessel diameter | Type 4 – Thrombus |
| Type 2 – Vessel diameter and intimal hyperplasia | Type 5 – Valve |
| Type 3 – Intimal hyperplasia | Type 6 – Other or not able to identify |
Ignore any branches/perforators unless in the opinion of the sonographer they are draining substantial flow away from the main outflow vein. If there is a substantial branch, note this in the written report and detail on the diagram.
The following images should be recorded as a minimum:
-
Volume flow ×3 within the brachial artery
-
Resistance index (RI) ×3 within the brachial artery
-
Outflow vein diameters (maximum, minimum and representative)
-
AVF depth (shallowest, deepest and representative)
-
PSV and diameter at the anastomosis
-
PSV at any stenosis, and prior to stenosis
-
Any other pathology.
The minimum number of images for each scan will depend on whether the machine will allow volume flow and RI to be calculated on the same image.
If the machine allows RI and volume flow on the same image, the minimum images will be 11.
If the machine does not allow RI and volume flow on the same image, the minimum images will be 14.
Image labelling
Acceptable abbreviations are in brackets.
Axillary artery (AX A, Axillary A) – Only if needed in the event of a very high brachial bifurcation
Brachial artery (BA, Brachial A)
Radial artery (RA, Radial A)
Proximal (Prox, P)
Distal (Dist, D)
It is acceptable to use proximal and distal within the context of arteries as normal (e.g. BA prox) but please do not use these in the context of the AVF.
Anastomosis (Anas)
Cephalic vein lower arm (CV LA)
Cephalic vein upper arm (CV UA)
Basilic vein upper arm (BV UA)
If the name of the vein is unknown, then it is acceptable to use the label AVF. Any branches may be labelled AVF Branch or Cephalic vein branch etc.
Any other pathology can be named by its description (e.g. Pseudoaneurysm, Seroma etc.) and its location
For example: Anterior to Radial artery.
After the scan
Complete a blank SONAR AVF ultrasound proforma.
Log in to the electronic Case Report Form (MACRO) and enter the data. Keep completed proforma in the SONAR Ultrasound file provided by the study team.
Ensure images are stored in case of audit, but ensure this is offline and that the clinical team do not have access to the images to ensure they remain blinded.
DO NOT inform the patients of the results (to ensure they do not bias clinicians by passing on the findings).
However, if the AVF is occluded please inform the clinical team at your centre ASAP.
List of abbreviations
- AUC
- area under the curve
- AVF
- arteriovenous fistula
- CI
- confidence interval
- CKD
- chronic kidney disease
- CVC
- central venous catheter
- ESRD
- end-stage renal disease
- NPV
- negative predictive value
- NS
- non-significant
- PPI
- patient and public involvement
- PPV
- positive predictive value
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- US
- ultrasound
