Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 12/35/45. The contractual start date was in February 2014. The draft manuscript began editorial review in June 2023 and was accepted for publication in February 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Moakes et al. This work was produced by Moakes et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Moakes et al.
Chapter 1 Introduction
This chapter contains material that has been reproduced with permission from Bradbury et al. 1 This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
Chronic limb-threatening ischaemia (CLTI), previously known as critical limb ischaemia (CLI) and severe limb ischaemia of the leg, is the most severe form of peripheral arterial disease (PAD) due to atherosclerosis and presents with ischaemic rest pain, tissue loss (ulceration, gangrene or both) or both, usually affecting the foot. 2 Mainly because of tobacco smoking and the growing prevalence of type 2 diabetes mellitus (DM), CLTI represents a growing burden on health and social care services across the world. 3,4 Unless the blood supply to the affected limb is restored, patients with CLTI are at high risk of amputation or death. Although it is universally agreed that – in addition to best medical therapy (BMT) – virtually all patients with CLTI should at least be considered for revascularisation, there is a continuing debate, as to whether such revascularisation is best achieved by inserting a bypass graft – preferably using a vein taken from the patient’s own leg [vein bypass (VB)] – or through best endovascular treatment (BET), which in most cases will be balloon angioplasty with or without the use of stents. 5 The scarcity of high-quality evidence, especially regarding infra-popliteal (IP) revascularisation,6 is readily apparent in the published literature, and is also reflected in the low strength of recommendations found within various international guidelines. 7–10 Even after initially successful revascularisation, patients with CLTI often require multiple procedures to maintain limb perfusion; as well as frequent hospital re-admissions for limb-related problems and other comorbidities, most commonly ischaemic heart and respiratory disease, which usually coexist in this patient population. As a result, CLTI is associated with high resource use and poor health-related quality of life (HRQoL). 11,12
The UK Bypass versus Angioplasty in Severe Ischaemia of the Leg Trial (BASIL-1) trial, which included 452 participants, suggested that patients with CLTI anticipated to have a life expectancy of 2 years or more and who had a suitable vein for bypass, should be offered VB-first in preference to balloon angioplasty. 13–16 The BEst Surgical Therapy in patients with Chronic Limb threatening Ischaemia (BEST-CLI) trial, which included 1830 participants mainly from the USA, reported that, in the 1434 (78%) patients who had an optimal (single segment great saphenous) vein for bypass (cohort 1), the incidence of a composite end point comprising major adverse limb events (MALE) or death from any cause was significantly lower in the VB-first group than in the BET-first group. Of the 396 (22%) participants who did not have optimal single segment great saphenous vein (GSV) for bypass (cohort 2), outcomes were similar between treatment groups. 17
The severity and anatomical distribution of atherosclerosis affect treatment options and outcomes in patients with CLTI. 18,19 About three-quarters of participants in the BASIL-1 trial had a vein or prosthetic bypass, or a balloon angioplasty, for disease in the femoropopliteal (FP) segment, that is, the arteries that carry blood from the hip to the knee. A subsequent subgroup analysis of participants in the BASIL-1 trial who underwent IP revascularisation also suggested that outcomes might be better with VB than balloon angioplasty, but this finding was associated with a high level of uncertainty. 20
Mainly because of the growing importance of type 2 DM as a major worldwide risk factor for CLTI, but also due to the increased numbers of patients with end-stage renal disease (ESRD), a growing proportion of patients with CLTI have, often heavily calcified, IP disease requiring treatment. Establishing an evidence base for different revascularisation strategies in this specific patient group is increasingly important. In 2012, the UK National Institute for Clinical and Health Excellence (NICE) recommended that a randomised trial be done to compare a VB-first with a BET-first revascularisation strategy specifically in patients with CLTI who require an IP revascularisation with or without an additional more proximal infra-inguinal revascularisation procedure. 21
The aim of BASIL-2 was to specifically compare the effectiveness of a VB-first with a BET-first revascularisation strategy in terms of preventing major (above the ankle) amputation and death from any cause [amputation-free survival (AFS)] in patients with CLTI who required an IP, with or without an additional more proximal infra-inguinal revascularisation procedure, to restore limb perfusion. BASIL-2 also included a health economic analysis.
Chapter 2 Methods
This chapter contains material that has been reproduced with permission from Bradbury et al. 1 This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
Trial design
The BASIL-2 trial was a pragmatic, open-label, parallel, multicentre, randomised, superiority trial. The trial had a favourable ethical opinion from National Research Ethics Service Committee 14/WM/0057 obtained in March 2014. The final protocol version was v7.0 dated 22 September 2022. The protocol for the trial is published22 (based on an earlier version of the protocol), and the full, most recent protocol is available at the BASIL-2 website (www.birmingham.ac.uk/BASIL2).
Participants
The participants in the BASIL-2 trial were recruited from vascular surgery units across the UK as well as in Stockholm, Sweden and Kolding, Denmark. Eligible participants were patients who presented to hospital-based vascular surgery units with CLTI due to atherosclerotic PAD and who required an IP revascularisation with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion. Participants had to have an anticipated life expectancy of more than 6 months, judged by a minimum of two consultants (at least one of whom was performing IP VB and one of whom was performing IP BET in their clinical practice) to require and be suitable for both IP VB and IP BET. Eligible patients were not permitted to have had previous vascular intervention to the target IP artery within the previous 12 months, but did need to have adequate aortoiliac inflow to support both revascularisation strategies. Patients had to be able and willing to complete HRQoL and health economic questionnaires (with help if required) and be able to understand sufficient English, Swedish, or Danish (depending on country of recruitment) to ensure informed consent.
Randomisation
Once eligibility was confirmed and consent obtained, participants were randomised to the BASIL-2 trial by the research staff at participating centres using a secure online, central randomisation service provided by the Birmingham Clinical Trials Unit. A central telephone back-up service was also available. Participants were randomised on a 1 : 1 basis to either a VB-first or a BET-first revascularisation strategy. A minimisation procedure, incorporating a random element, using a computer-based algorithm, was used to reduce the chance of imbalances in important prognostic variables. Strata used in the minimisation were:
-
Age (≤ 60, 61–70, 71–80, > 80 years).
-
Gender (male, female).
-
DM and chronic kidney disease (CKD) {DM, CKD [CKD defined as stage 3 or worse based on estimated glomerular filtration rate (eGFR) of < 60 (ml/minute/1.73 m2)]23, DM and CKD or neither}.
-
Severity of clinical disease (ischaemic rest/night pain only, tissue loss only, or both).
-
Previous (permissible) intervention to the trial leg (yes, no).
-
Intention for hybrid procedure (yes, no).
Procedures
The pragmatic trial design encouraged vascular and endovascular surgeons and interventional radiologists to perform VB and BET using their preferred equipment, devices, and surgical and anaesthetic techniques. For VB, any vein deemed suitable by the responsible vascular surgeons could be used. If at operation it was discovered that the vein could not be used, then composite or prosthetic grafts could be inserted at the surgeon’s discretion in the patient’s best interests. Regarding BET, any device being used as part of standard care in that country was permissible. Drug-coated balloons (DCBs), bare metal stents (BMSs) and drug-eluting stents (DESs) could be used at the operator’s discretion. Atherectomy devices were permitted but not used. In this pragmatic trial, all additional management strategies, including additional procedures, were at the responsible clinicians’ discretion in the patient’s best interests.
Blinding
BASIL-2 was an open-label study; participants, study staff, and investigators were not masked to treatment allocation.
Adherence monitoring
Adherence to the allocated intervention was confirmed on the intervention form where the first revascularisation procedure details were collected. The researchers defined adherence as those participants in the VB-first group who received a VB as their first revascularisation intervention and those participants in the BET-first group who received a BET as their first revascularisation intervention. Further revascularisations, which have been captured as secondary outcomes, were permitted as deemed clinically indicated by the responsible clinicians and were not considered part of the adherence definition.
Participant withdrawal
Participants could voluntarily withdraw from the trial (or any component of it) at any time. Reasons for withdrawal were captured where possible. If a participant explicitly withdrew consent to have any further data recorded (including NHS data), their decision was respected and recorded on the electronic data capture system. All communication surrounding the withdrawal was noted in the patient’s medical notes, and no further data were collected for that participant. In rare instances where a participant was randomised and was later found to have violated one or more inclusion criteria they remained in the trial.
Outcomes and assessments
Primary outcome measure
The primary outcome was AFS, defined as time to major (above the ankle) amputation of the trial leg or death, from any cause (whichever occurred first).
Secondary outcome measures
Secondary outcomes were as follows:
-
Time to death from any cause [overall survival (OS)].
-
Time to major amputation of the trial leg.
-
MALE (defined as major amputation of the trial leg, or any further major revascularisation intervention to the trial leg, following the first revascularisation intervention).
-
Major adverse cardiac event [MACE, defined as CLTI and/or major amputation affecting the non-trial leg, myocardial infarction (MI), transient ischaemic attack or stroke].
-
Thirty-day morbidity and mortality.
-
Relief of ischaemic pain as determined by visual analogue scale (VAS) and opiate usage.
-
HRQoL using generic [EuroQol-5 Dimensions, five-level version (EQ-5D-5L), Short Form questionnaire-12 items (SF-12), ICEpop CAPability measure for Older people (ICECAP-O)] and disease specific [the Vascular Quality of Life Questionnaire (VascuQoL)] tools.
-
Further major revascularisation intervention to the trial leg (following the first revascularisation intervention) including re-intervention and crossover intervention (where re-intervention is defined as the same, and a crossover procedure is defined as an alternative, revascularisation procedure to the first revascularisation procedure post randomisation).
-
Healing of tissue loss (ulcers, gangrene, or both) at or below the ankle presumed to be caused by atherosclerotic PAD as assessed by the perfusion, extent, depth, infection and sensation (PEDIS) score,24 the Wound Ischaemia and foot Infection (WIfI) tool. 25
-
Haemodynamic measurements [these being absolute ankle and toe pressures, ankle–brachial pressure index (ABPI), and toe brachial pressure index (TBPI)].
Outcome assessment details
The schedule for outcome assessment is given in Table 1. Details of how outcomes were generated are given in Table 2. Patients were followed up locally at 1 month after the first revascularisation procedure; 6, 12 and 24 months after randomisation; and then annually until the last recruited participant had been followed up for a minimum of 24 months. HRQoL and clinical data including amputation and death were collected at each visit where possible. However, from March 2020 onwards, components of this data collection that required a face-to-face assessment, such as haemodynamic measurements, were substantially affected by the COVID-19 pandemic. In England and Wales, the primary outcome data were also obtained until the end of follow-up from NHS Digital [NHS Digital (now NHS England) is the statutory custodian for health and social care data for England and Wales]. In the Swedish centre, the Regional Electronic Health Data system was also used to check for amputations, hospitalisations or deaths. Electronic health data systems were not used in the Danish centre where all data were collected locally only.
| Randomisation | Intervention (within 2 weeks of randomisation where possible) | Follow-up month (1, 6, 12 and annually thereafter until the end of the trial) | |
|---|---|---|---|
| Consent | ✓ | ||
| Randomisation | ✓ | ||
| Imaging | ✓ | ✓ | |
| Wound assessment | ✓ | ✓ | |
| VAS | ✓ | ✓ | |
| WIfI | ✓ | ✓ | |
| PEDIS | ✓ | ✓ | |
| EQ-5D-5L | ✓ | ✓ | |
| ICECAP-O | ✓ | ✓ | |
| VascuQoL | ✓ | ✓ | |
| Haemodynamic indices | ✓ | ✓ | |
| Amputation assessment | ✓ | ✓ | |
| Revascularisation intervention review | ✓ | ✓ | |
| Resource usage | ✓ | ||
| Pain relief medication review | ✓ | ✓ | |
| SAE review | ✓ | ✓ |
| Outcome assessed | Time point | Method | Reported by |
|---|---|---|---|
| Death | Up to end of follow-up | Clinical assessment of participant at follow-up visits and medical records | Research nurse or doctor |
| Major amputation | |||
| Further revascularisation intervention | |||
| MALE | |||
| MACE | |||
| Thirty-day morbidity and mortality | Thirty days post first revascularisation intervention | ||
| Opiate usage | 1, 6, 12 months and annually thereafter until end of follow-up | ||
| VAS | Paper case report form | Patient reported | |
| EQ-5D-5L | |||
| SF-12 | |||
| ICECAP-O | |||
| VascuQoL | |||
| PEDIS | Clinical assessment of participant at follow-up visits and medical records | Research nurse or doctor | |
| WIfI | |||
| ABPI | |||
| TBPI |
Serious adverse events
Serious adverse events (SAEs) were recorded on a purpose-designed SAE form and notified by local investigators to the clinical trials unit within 24 hours of the local investigators becoming aware of these events. Local investigators were responsible for additionally reporting SAEs to their host institutions, according to local regulations. SAEs that were categorised by the Chief Investigator as both suspected to be related to the trial intervention and unexpected were classified as related unexpected serious adverse events (RUSAEs) and were subject to expedited reporting. SAEs were collected up to 30 days following the first revascularisation.
Statistical considerations
Sample size
The original sample size was based on a time-to-event analysis to be undertaken 2 years after completion of recruitment. It was anticipated that recruitment would take place over 3 years: 20% of patients recruited in year 1, 40% in year 2 and 40% in year 3. Based on the BASIL-1 trial,13 AFS rates were assumed to be 0.72 in year 1, 0.62 in year 2, 0.53 in year 3, 0.47 in year 4 and 0.35 in year 5. Allowing for a 10% attrition rate and based on the survival estimates calculated using the BASIL-1 data, a population of 600 participants (247 primary outcome events) would have 90% power to detect a reduction in AFS of one-third [hazard ratio (HR) 0.66] at the 5% significance level using the artsurv (version 1.0.7) programme in Stata® (StataCorp LP, College Station, TX, USA).
The initial assumptions made in this trial concerning recruitment rates were not achieved and subsequently recruitment continued beyond year 3. As a result, the median length of follow-up was longer than originally planned such that the number of randomised participants required to observe 247 events (as per the original sample size target) was reduced due to the increased exposure time. With support from the funder and independent oversight from the Data Monitoring Committee (DMC), recruitment rates, length of follow-up, and pooled event rates over time were modelled to predict the number of participants needed to reach 247 events, with 24 months minimum follow-up in each participant. The modelling was updated approximately every 6 months on the basis of emerging data. Due to ongoing challenges with recruitment, largely related to the COVID-19 pandemic, the BASIL-2 trial closed to recruitment on 30 November 2020 with 345 participants randomly assigned.
Statistical analysis
A comprehensive statistical analysis plan was specified before analysis. The primary, secondary and safety outcomes were analysed in the intention-to-treat (ITT) population (which included all randomly assigned participants irrespective of adherence with the treatment protocol). All estimates of differences between groups were presented with two-sided 95% confidence intervals (CIs), adjusted for the minimisation variables as fixed effects (when convergence was possible) and recruiting centre as a random effect (or as a shared frailty variable in time-to-event analyses). EQ-5D-5L, SF-12, ICECAP-O, VascuQoL, ABPI, TBPI, PEDIS and VAS were also adjusted for baseline value.
The primary outcome was analysed using a Cox proportional hazards model to generate a HR adjusted for the minimisation factors and recruitment site. Statistical significance of the treatment group parameter was determined through examination of the associated chi-squared statistic. Kaplan–Meier survival curves were constructed for visual presentation of time-to-event comparisons.
Time-to-event secondary outcomes (OS, major amputation, MALE, MACE) were analysed as per the primary outcome but were not subjected to hypothesis testing. For these outcomes (excluding OS), post hoc sensitivity analyses were considered in a competing risk framework to account for patients who had died before having an event. 26 A cumulative incidence function was used to estimate the probability of occurrence over time. A Fine–Gray model was then used to estimate a subdistribution adjusted HR directly from the cumulative incidence function. In addition, a further Cox proportional hazards model was fitted and applied to the cause-specific hazard function and used to generate an adjusted HR. 27
For continuous secondary outcome measures (EQ-5D-5L, SF-12, ICECAP-O, VascuQoL, ABPI, TBPI, PEDIS, VAS), adjusted mean differences (MDs) were estimated at the primary time points (1, 12 and 24 months) using mixed-effect repeated measures models. 28 Binary secondary outcomes measured at a single time point (MALE, MACE) were analysed using a mixed-effects log-binomial model to generate an adjusted risk ratio (RR) and risk difference (RD) (the latter using an identity link function). Binary secondary outcomes measured at multiple assessment times (opiate usage, WIfI) were analysed using a mixed-effects repeated measures logistic regression model to generate adjusted odds ratios (OR) at the primary time points (1, 12 and 24 months).
Sensitivity and supportive analyses of the primary outcome included a per-protocol analysis, including only participants regarded as adherent and an as-treated analysis, where participants were analysed as per what they received for their first revascularisation intervention. Further analysis of the primary outcome included assessment of the proportional hazards assumption, assessed graphically and by fitting time-dependent effects.
Pre-planned subgroup analyses (limited to the primary outcome only) were completed for the minimisation variables in addition to baseline ABPI (< 0.8, 0.8–1.2, > 1.2 or non-compressible) and baseline TBPI (< 0.6, ≥ 0.6 or non-compressible). The effects of these subgroups were examined by adding the subgroup by treatment group interaction parameters to the regression model. P-values from the tests for statistical heterogeneity were presented alongside the effect estimate and estimates of uncertainty within each subgroup. In addition to this, ratios were provided to quantify the difference between the treatment effects estimated within each subgroup. All analyses were performed in SAS (version 9.4) (SAS Institute Inc., Cary, NC, USA) or Stata® (version 17.0) (StataCorp, Stata Statistical Software: Release 17, College Station, TX, StataCorp LLC) and reported adhering to Consolidation Standards of Reporting Trials guidelines.
Interim analyses of effectiveness and safety end points were performed on behalf of the DMC on an approximately annual basis during the period of recruitment; no reason to recommend halting the trial was identified. These analyses were performed with the use of the Haybittle–Peto principle and hence no adjustment was made in the final p-values to determine significance. 29
Trial oversight
Study oversight was provided by a Trial Steering Committee (TSC) (Chaired by Professor Jonathan Michaels, University of Sheffield) and a DMC (Chaired by Professor Charles McCollum, University of Manchester).
The TSC provided independent supervision for the trial, providing advice to the Chief and Co- Investigators and the Sponsor on all aspects of the trial throughout the study. The DMC adopted the DAMOCLES charter to define its terms of reference and operation in relation to oversight of the BASIL-2 trial. 30
Data sharing
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Chapter 3 Results
This chapter contains material that has been reproduced with permission from Bradbury et al. 1 This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
This chapter reports the results of the BASIL-2 trial.
Recruitment
The trial recruited its first participant on 22 July 2014 and closed to recruitment on 30 November 2020 after 345 had participants had been randomised. The trial completed follow-up on 30 November 2022, after all surviving participants were followed up for a minimum of 2 years from randomisation. The database was locked for statistical analysis on 5 January 2023. Recruitment occurred across 39 vascular surgery units in the UK, and one each in Sweden (Stockholm) and Denmark (Kolding) (Table 3).
| Centre – N (%) | All participants (N = 345) |
|---|---|
| Sodersjukhuset, Stockholm, Sweden | 36 (10) |
| Hull Royal Infirmary | 34 (10) |
| St Thomas Hospital, London | 28 (8) |
| Russells Hall Hospital, Dudley | 23 (7) |
| Birmingham Heartlands Hospital | 20 (6) |
| Leeds General Infirmary | 20 (6) |
| Manchester Royal Infirmary | 20 (6) |
| Kolding Hospital, Denmark | 16 (5) |
| Royal Gwent Hospital | 11 (3) |
| Royal Free Hospital, London | 10 (3) |
| Kent and Canterbury Hospital | 10 (3) |
| Leicester Royal Infirmary | 8 (2) |
| Western Infirmary, Glasgow | 8 (2) |
| Frimley Park Hospital | 7 (2) |
| University Hospital Coventry (Walsgrave) | 7 (2) |
| Southmead Hospital, Bristol | 7 (2) |
| St Mary’s Hospital, London | 7 (2) |
| St George’s Hospital, London | 6 (2) |
| Freeman Hospital, Newcastle | 6 (2) |
| John Radcliffe Hospital, Oxford | 6 (2) |
| Royal Bournemouth and Christchurch NHS Trust | 5 (2) |
| Pilgrim Hospital, Boston | 5 (2) |
| Northern General Hospital, Sheffield | 4 (1) |
| Queen Elizabeth Hospital, Birmingham | 4 (1) |
| City Hospital Birmingham | 4 (1) |
| Colchester General Hospital | 3 (1) |
| Addenbrookes Hospital, Cambridge | 3 (1) |
| Cumberland Infirmary | 3 (1) |
| Doncaster Royal Infirmary | 3 (1) |
| Worcestershire Royal Hospital | 3 (1) |
| The Royal Oldham Hospital | 3 (1) |
| Royal Sussex County Hospital, Brighton | 3 (1) |
| Ninewells Hospital, Dundee | 3 (1) |
| Royal Cornwall Hospital (Treliske) | 2 (1) |
| Bradford Royal Infirmary | 1 (< 1) |
| York Health Services Trust | 1 (< 1) |
| Southampton General Hospital | 1 (< 1) |
| Queen Alexandra Hospital, Portsmouth | 1 (< 1) |
| Northwick Park Hospital | 1 (< 1) |
| Forth Valley Royal Hospital, Larbert | 1 (< 1) |
| Barts and the Royal London, London | 1 (< 1) |
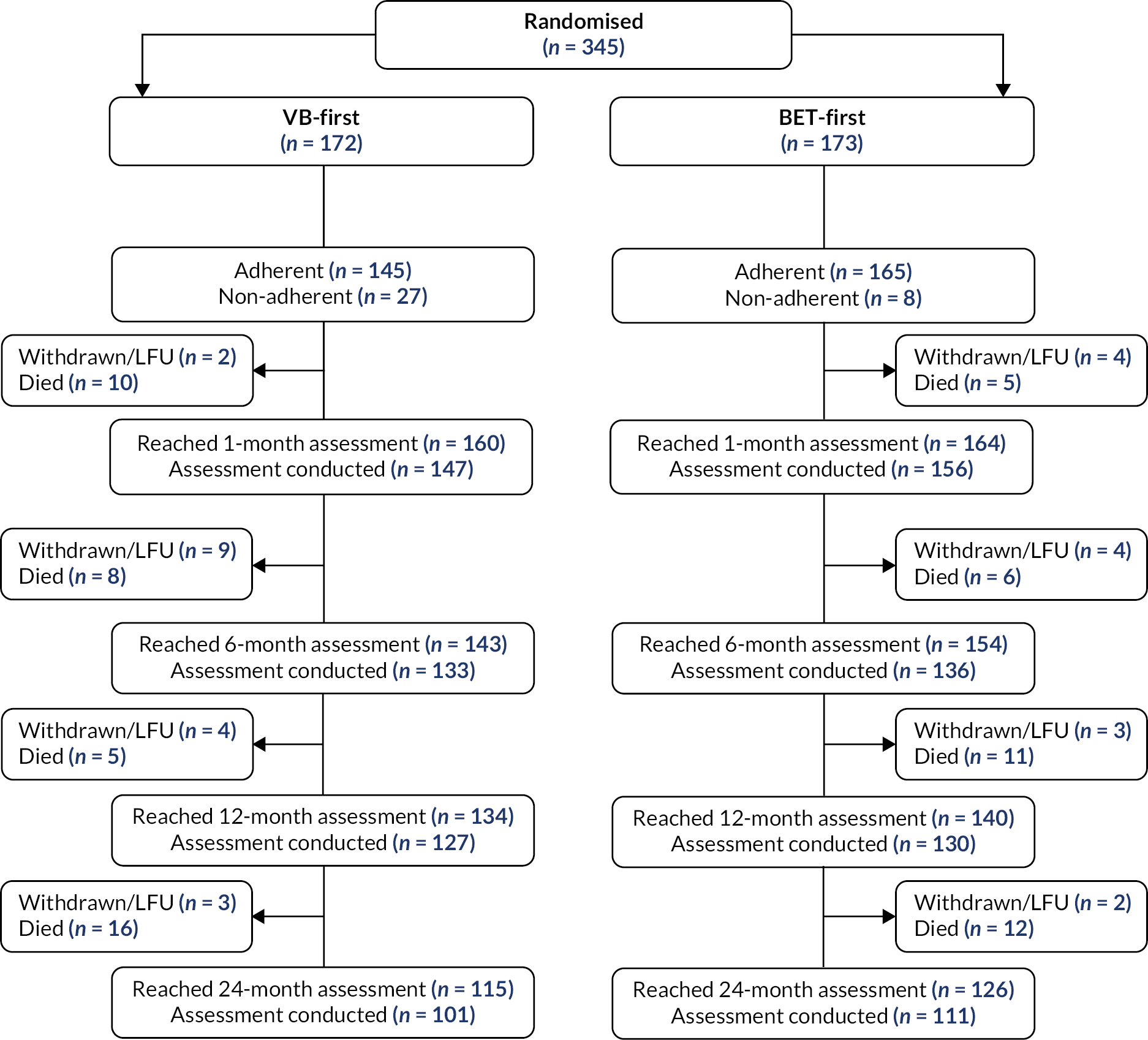
Participant flow (up to 24 months) is illustrated in Figure 1. Three hundred and forty-five participants were randomised to BASIL-2, with 172 participants allocated to a VB-first and 173 participants allocated to a BET-first revascularisation strategy. At the end of follow-up, 74 participants were withdrawn from the study and a further 10 participants were lost to follow-up. Type of withdrawal and median time to withdrawal are presented in Table 4. At the end of follow-up {median 40.0 months [interquartile range (IQR) 20.9–60.6]}, 200 primary outcome events had been observed. All patients were included in the primary ITT analysis of AFS.
FIGURE 1.
Participant flow. LFU, lost to follow-up.
| VB-first (N = 172) | BET-first (N = 173) | |
|---|---|---|
| Withdrawalsa – N (%) | 39 (23) | 35 (20) |
| Type of withdrawal – N | ||
| Full withdrawal – no use of NHS datab | 18 | 14 |
| Time to withdrawal (months) – median (IQR, N) | 12.8 (4.6–30.3, 18) | 18.1 (3.4–54.7, 14) |
| Full withdrawal – use of NHS datac | 9 | 11 |
| Time to withdrawal (months) – median (IQR, N) | 33.5 (2.2–46.1, 9) | 39.1 (3.1–53.8, 11) |
| Withdrawn from HRQoL onlyc | 10 | 10 |
| Time to withdrawal (months) – median (IQR, N) | 14.6 (6.1–24.6, 10) | 13.4 (8.4–23.9, 10) |
| Withdrawn from clinical follow-up onlyc | 2 | 0 |
| Time to withdrawal (months) – median (IQR, N) | 11.2 (3.1–19.4, 2) | – |
| Lost to follow-up – N (%) | 4 (2) | 6 (3) |
Baseline data
The baseline demographic characteristics of participants in the VB-first group and the BET-first group were comparable, with the minimisation algorithm achieving balance across age, gender, DM and CKD, severity of clinical disease, previous (permissible) intervention to the trial leg and intention for a hybrid procedure.
The participants had a mean age of 70.7 years [standard deviation (SD) 11.0 years] and 81% were male. Sixty-nine percentage of participants had predominantly type 2, DM [207/237 (87%)] and a high proportion were insulin-dependent [123/236 (52%)]. In regard to their severity of clinical disease at randomisation, 88% presented with tissue loss (with or without rest pain), which was mainly at or below the ankle. Twenty-one percent of participants declared themselves as current smokers, with an additional 49% declaring to be ex-smokers (Table 5).
| VB-first (N = 172) | BET-first (N = 173) | |
|---|---|---|
| Age (years)a – N (%) | ||
| ≤ 60 | 38 (22) | 36 (21) |
| 61–70 | 42 (24) | 44 (25) |
| 71–80 | 61 (36) | 58 (34) |
| > 80 | 31 (18) | 35 (20) |
| Mean (SD, N) | 70.4 (10.7, 172) | 71.1 (11.4, 173) |
| Gendera-male – N (%) | 139 (81) | 141 (82) |
| DMa – N (%) | 117 (68) | 120 (69) |
| DM typeb – N (%) | ||
| Type 1 | 14/117 (12) | 16/120 (13) |
| Type 2 | 103/117 (88) | 104/120 (87) |
| DM insulin dependentb – N (%) | 62/117 (53) | 61/119 (51) |
| Missing | 0 | 1 |
| CKDa,c – N (%) | 58 (34) | 60 (35) |
| Severity of clinical disease on the trial lega – N (%) | ||
| Rest/night pain only | 22 (13) | 19 (11) |
| Tissue loss only | 39 (23) | 32 (18) |
| Both | 111 (64) | 122 (71) |
| Tissue loss details (on the trial leg)d – N (%) | ||
| At or below ankle only | 139/149 (93) | 132/151 (87) |
| At or below ankle and above ankle | 5/149 (3) | 11/151 (7) |
| Above ankle only | 5/149 (3) | 8/151 (5) |
| Missing | 1 | 3 |
| Previous (permissible) intervention to the trial lega,e – N (%) | 20 (21) | 22 (23) |
| Unknown | 77 | 76 |
| Hybrid procedure planneda,e – N (%) | 4 (4) | 4 (4) |
| Unknown | 77 | 76 |
| Trial leg-right – N (%) | 74 (43) | 95 (55) |
| Body mass index (kg/m²) – mean (SD, N) | 27.1 (4.9, 149) | 26.8 (5.5, 154) |
| Living arrangement – N (%) | ||
| Own home | 135 (94) | 142 (93) |
| Other acute hospital | 1 (1) | 1 (1) |
| Residential home | 0 (–) | 1 (1) |
| Nursing home | 0 (–) | 1 (1) |
| Other | 8 (5) | 6 (4) |
| Missing | 28 | 22 |
| Mobility – N (%) | ||
| Fully ambulant without walking aid | 84 (49) | 69 (40) |
| Ambulant with walking aid | 73 (43) | 93 (53) |
| Wheelchair bound | 13 (7) | 10 (6) |
| Bed-bound | 1 (1) | 1 (1) |
| Missing | 1 | 0 |
| Smoking status – N (%) | ||
| Never | 58 (34) | 48 (28) |
| Ex | 75 (44) | 92 (53) |
| Current | 38 (22) | 33 (19) |
| Missing | 1 | 0 |
| Ethnicity – N (%) | ||
| White | 157 (92) | 158 (92) |
| Black/Black British | 8 (4) | 9 (5) |
| Asian/Asian British | 5 (3) | 5 (3) |
| Otherf | 1 (1) | 0 (–) |
| Declined to provide/missing | 1 | 1 |
| Previous stroke – N (%) | 25 (15) | 34 (20) |
| Missing | 1 | 0 |
| Previous MI – N (%) | 41 (24) | 23 (13) |
| Missing | 1 | 0 |
| Previous angina – N (%) | 22 (13) | 21 (12) |
| Missing | 1 | 1 |
| Previous CABG – N (%) | 22 (13) | 15 (9) |
| Missing | 1 | 0 |
| Previous PCI – N (%) | 23 (13) | 17 (10) |
| Missing | 1 | 2 |
| Previous dialysis – N (%) | 10 (6) | 5 (3) |
| Missing | 1 | 0 |
| Any previous vascular intervention to the trial leg – N (%) | 54 (32) | 67 (39) |
| Missing | 1 | 0 |
| Endovascularg | 37/54 (69) | 40/67 (60) |
| Surgeryg | 13/54 (24) | 14/67 (21) |
| Minor amputationg | 17/54 (31) | 36/67 (54) |
| Trial leg: ABPI – mean (SD, N) | 0.4 (0.3, 73) | 0.5 (0.3, 72) |
| Non-calculableh – N (%) | 23 (13) | 21 (12) |
| Trial leg: TBPI – mean (SD, N) | 0.2 (0.2, 44) | 0.1 (0.2, 41) |
| Any antiplatelet usei – N (%) | 131 (78) | 138 (80) |
| Missing | 3 | 1 |
| Aspirin – N (%) | 100 (59) | 106 (62) |
| Missing | 3 | 1 |
| Clopidogrel – N (%) | 50 (29) | 50 (29) |
| Missing | 2 | 1 |
| Other antiplatelet – N (%) | 5 (3) | 7 (4) |
| Missing | 5 | 2 |
| Treatment for hypercholesterolaemia – N (%) | 129 (76) | 138 (81) |
| Missing | 3 | 2 |
| Treatment for hypertension – N (%) | 128 (76) | 129 (75) |
| Missing | 4 | 1 |
| Any anticoagulant usej – N (%) | 46 (27) | 50 (29) |
| Missing | 3 | 2 |
| Warfarin – N (%) | 12 (7) | 13 (8) |
| Missing | 2 | 2 |
| Other anticoagulant – N (%) | 34 (20) | 39 (23) |
| Missing | 3 | 2 |
| Paracetamol – N (%) | 124 (73) | 122 (71) |
| Missing | 2 | 1 |
| Opiates – N (%) | 89 (53) | 81 (47) |
| Missing | 3 | 1 |
| NSAIDs – N (%) | 18 (11) | 13 (8) |
| Missing | 3 | 1 |
| Gabapentin – N (%) | 26 (15) | 21 (12) |
| Missing | 3 | 1 |
| Amitriptyline – N (%) | 23 (14) | 22 (13) |
| Missing | 3 | 1 |
| Imaging method – N (%) | ||
| Duplex US | 39 (23) | 37 (22) |
| MRA | 34 (20) | 43 (25) |
| CTA | 44 (26) | 45 (27) |
| DSA | 50 (30) | 44 (26) |
| Missing | 5 | 4 |
Adherence data
Adherence to allocated intervention was high: (n = 145, 84%) in the VB-first group and (n = 165, 95%) in the BET-first group. In total, 17 participants received no revascularisation intervention (n = 15 VB-first, n = 2 BET-first), and reported reasons included deterioration or improvement in condition and patient declining. Eighteen participants (n = 12 VB-first, n = 6 BET-first) received alternative revascularisation interventions to the one to which they were allocated at randomisation. Ninety-four percentage of participants who received a first revascularisation intervention did so within 4 weeks of randomisation (Table 6). Further details of the first revascularisation procedures are provided in Tables 7 and 8.
| VB-first (n = 172) | BET-first (n = 173) | |
|---|---|---|
| Adherent – n (%) | ||
| Yes | 145 (84) | 165 (95) |
| No | 27 (16) | 8 (5) |
| First revascularisation intervention received – n (%) | ||
| BET | 10 (6) | 165 (95) |
| Surgical bypass | 145 (84) | 5 (3) |
| Non-bypass surgerya | 2 (1) | 1 (1) |
| No revascularisation intervention received | 15 (9) | 2 (1) |
| Reason no revascularisation intervention received – n | ||
| Deterioration of condition | 5 | 1 |
| Improvement in condition | 2 | 0 |
| Participant declined intervention | 3 | 0 |
| No reason provided | 5 | 1 |
| Time from randomisation to first revascularisation interventionb (weeks) – n (%) | ||
| < 2 | 125/157 (80) | 140/171 (82) |
| 2–4 | 24/157 (15) | 20/171 (12) |
| > 4 | 8/157 (5) | 11/171 (6) |
| Median (IQR, N) | 0.9 (0.3–1.7, 157) | 0.6 (0.3–1.6, 171) |
| VB-first | BET-first | |
|---|---|---|
| Surgical bypass received – n | 145 | 5 |
| Technical success – n (%) | 137 (96) | 4 (80) |
| Missing | 2 | 0 |
| Conduit – n (%) | ||
| Ipsi-GSV reversed | 70 (49) | 1 (20) |
| Ipsi-GSV non-reversed | 48 (34) | 4 (80) |
| Contra-GSV reversed | 7 (5) | 0 (–) |
| Contra-GSV non-reversed | 2 (1) | 0 (–) |
| Ipsi-SSV reversed | 0 (–) | 0 (–) |
| Ipsi-SSV non-reversed | 0 (–) | 0 (–) |
| Contra-SSV reversed | 0 (–) | 0 (–) |
| Contra-SSV non-reversed | 1 (1) | 0 (–) |
| Arm reversed | 5 (4) | 0 (–) |
| Arm non-reversed | 1 (1) | 0 (–) |
| Composite | 5 (4) | 0 (–) |
| Prosthetic | 2 (1) | 0 (–) |
| Missing | 4 | 0 |
| Proximal anastomosis – n (%) | ||
| Common femoral artery | 37 (26) | 3 (60) |
| Superficial femoral artery | 46 (33) | 2 (40) |
| Profunda femoris artery | 0 (–) | 0 (–) |
| Above-knee popliteal artery | 11 (7) | 0 (–) |
| Below-knee popliteal artery | 46 (33) | 0 (–) |
| Previous bypass | 1 (1) | 0 (–) |
| Missing | 4 | 0 |
| Distal anastomosis – n (%) | ||
| Superficial femoral artery | 0 (–) | 0 (–) |
| Above-knee popliteal artery | 0 (–) | 0 (–) |
| Below-knee popliteal artery | 2 (1) | 0 (–) |
| Anterior tibial artery (1) | 13 (9) | 0 (–) |
| Anterior tibial artery (2) | 9 (6) | 0 (–) |
| Anterior tibial artery (3) | 11 (8) | 0 (–) |
| Posterior tibial artery (1) | 6 (4) | 0 (–) |
| Posterior tibial artery (2) | 20 (14) | 0 (–) |
| Posterior tibial artery (3) | 33 (23) | 2 (50) |
| Peroneal artery (1) | 8 (6) | 1 (25) |
| Peroneal artery (2) | 8 (6) | 0 (–) |
| Peroneal artery (3) | 4 (3) | 1 (25) |
| Dorsalis pedis | 24 (17) | 0 (–) |
| Plantar artery | 1 (1) | 0 (–) |
| Missing | 6 | 1 |
| VB-first | BET-first | |
|---|---|---|
| BET received – n | 10 | 165 |
| Technical successa – n (%) | 7 (78) | 130 (87) |
| Missing | 1 | 15 |
| Segments treated – n | ||
| Superficial femoral artery – proximal 1/2 | 3 | 24 |
| Superficial femoral artery – distal 1/2 | 3 | 51 |
| Above-knee popliteal artery | 3 | 57 |
| Below-knee popliteal artery | 5 | 60 |
| Posterior tibial artery – proximal 1/2 | 2 | 42 |
| Posterior tibial artery – middle 1/2 | 0 | 26 |
| Posterior tibial artery – distal 1/3 | 0 | 27 |
| Anterior tibial artery – proximal 1/2 | 4 | 78 |
| Anterior tibial artery – middle 1/2 | 2 | 51 |
| Anterior tibial artery – distal 1/3 | 2 | 47 |
| Peroneal artery – proximal 1/2 | 0 | 44 |
| Peroneal artery – middle 1/2 | 0 | 24 |
| Peroneal artery – distal 1/3 | 1 | 10 |
| Dorsalis pedis | 0 | 17 |
| Other | 2 | 23 |
| Missing | 1 | 6 |
| Devices usedb – n | ||
| Plain balloon angioplasty | 6 | 136 |
| DCB | 0 | 21 |
| BMS | 1 | 28 |
| DES | 0 | 21 |
| Missing | 4 | 21 |
| Number of crural arteries treated – n (%) | ||
| Single crural artery | 5 (83) | 86 (65) |
| Two crural arteries | 1 (17) | 43 (33) |
| Three crural arteries | 0 (–) | 2 (2) |
| Missing | 4 | 33 |
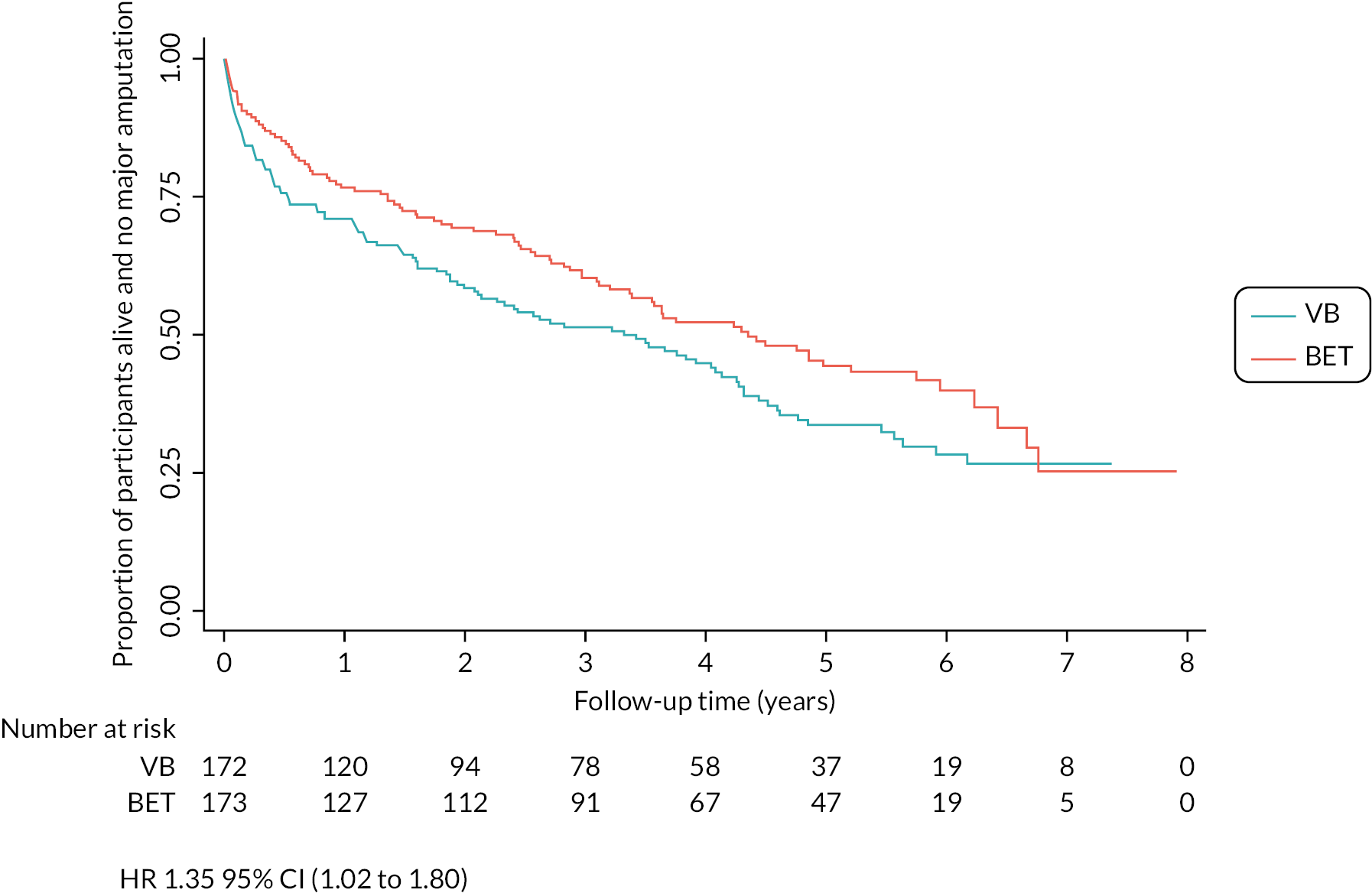
Primary outcome
One hundred and eight (63%) of 172 patients in the VB-first group and 92 (53%) of 173 patients in the BET-first group had a major amputation or died [adjusted HR 1.35 (95% CI 1.02 to 1.80); p = 0.037]. Thirty-five participants (n = 18 VB-first, n = 17 BET-first) had a major amputation and then died (major amputation was considered their first event for the time-to-event analysis) (see Table 9 and Figure 2).
| VB-first (n = 172) | BET-first (n = 173) | HRa (95% CI) | p-value | |
|---|---|---|---|---|
| AFS – n (%) | 64 (37) | 81 (47) | 1.35 (1.02 to 1.80) | 0.037 |
| No AFS – n (%) | 108 (63) | 92 (53) | ||
| Major amputation – n (%) | 35 (20) | 32 (19) | ||
| Death – n (%) | 91 (53) | 77 (45) |
FIGURE 2.
Kaplan–Meier plot of AFS (ITT analysis).
Sensitivity analyses which included a per-protocol and an as-treated analysis supported a trend towards an increased risk of major amputation or death in the VB-first group (Table 10). No evidence of non-proportional hazards was observed.
| No AFS – n/n (%) | VB-first | BET-first | HRa (95% CI) |
|---|---|---|---|
| ITT analysis | 108/172 (63) | 92/173 (53) | 1.35 (1.02 to 1.80) |
| Per-protocol analysisb | 88/145 (61) | 90/165 (55) | 1.30 (0.94 to 1.80) |
| As-treated analysisc | 89/150 (59) | 98/175 (56) | 1.16 (0.87 to 1.56) |
Subgroup analysis was performed for the pre-specified variables used in the minimisation algorithm and baseline ABPI and TBPI. There was no evidence for varying treatment effects in each subgroup analysis performed. The proportion of participants who had a major amputation or death in each subgroup is shown in Figure 3.
FIGURE 3.
Forest plot for subgroup analyses for AFS (ITT analysis).

Secondary outcomes
Secondary outcomes are reported in Tables 11 and 12.
| Secondary outcomes | VB-first (n = 172) | BET-first (n = 173) | Estimate (95% CI) | |
|---|---|---|---|---|
| Death from any cause (OS) – n (%) | 91 (53) | 77 (45) | HR 1.37a (1.00 to 1.87) | |
| Major amputation – n (%) | 35 (20) | 32 (19) | HR 1.23a (0.75 to 2.01) | |
| Thirty-day morbidity – n (%) | 79 (46) | 73 (42) | RR 1.11b (0.89 to 1.39) RD 0.06c (−0.04 to 0.16) |
|
| Thirty-day mortality – n (%) | 10 (6) | 5 (3) | RR 2.45b (0.84 to 7.20) RD 0.03d (−0.01 to 0.07) |
|
| MALE – n (%) | 71 (41) | 77 (45) | HR 0.93a (0.67 to 1.29) RR 0.94b (0.73 to 1.20) RD −0.04c (−0.15 to 0.06) |
|
| MACE – n (%) | 68 (40) | 73 (42) | HR 1.09a (0.78 to 1.53) RR 0.95b (0.79 to 1.15) RD −0.03d (−0.13 to 0.08) |
|
| Opiate use – n/n (%) | 1 month | 58/146 (40) | 58/151 (38) | OR 1.10e (0.51 to 2.41) |
| 12 months | 33/124 (27) | 31/128 (24) | OR 1.39e (0.57 to 3.42) | |
| 24 months | 21/99 (21) | 32/111 (29) | OR 0.53e (0.20 to 1.43) | |
| Further intervention – n (%) | 50 (29) | 56 (32) | RR 0.94b (0.68 to 1.28) RD −0.03d (−0.13 to 0.06) |
|
| Re-intervention – n (%) | 9 (5) | 33 (19) | RR 0.27b (0.13 to 0.55) RD −0.14d (−0.21 to −0.07) |
|
| Crossover – n (%) | 46 (27) | 33 (19) | RR 1.43b (0.94 to 2.18) RD 0.08d (−0.01 to 0.17) |
|
| PEDISf-Mean (SD, n) | 1 month | 6.1 (1.8, 66) | 7.1 (2.0, 90) | MD −0.66g (−1.27 to −0.06) |
| 12 months | 5.7 (2.5, 19) | 5.8 (2.1, 23) | MD −0.05g (−1.21 to 1.11) | |
| 24 months | 6.5 (0.7, 2) | 5.4 (1.3, 17) | MD 0.03g (−2.57 to 2.62) | |
| WIfIh – n/n (%) | 1 month | 17/51 (33) | 30/66 (45) | OR 0.49e (0.18 to 1.31) |
| 12 months | 5/12 (42) | 2/11 (18) | OR 4.18e (0.45 to 39.02) | |
| 24 months | 1/3 (33) | 4/10 (40) | OR 1.64e (0.06 to 47.28) | |
| ABPI-Mean (SD, n) | 1 month | 1.0 (0.3, 60) | 0.9 (0.3, 67) | MD 1.28i (−0.01 to 0.26) |
| 12 months | 0.9 (0.4, 38) | 0.8 (0.3, 36) | MD 0.08i (−0.09 to 0.25) | |
| 24 months | 1.0 (0.3, 23) | 0.8 (0.3, 26) | MD 0.08i (−0.13 to 0.28) | |
| TBPI-Mean (SD, n) | 1 month | 0.4 (0.4, 22) | 0.3 (0.3, 25) | MD 0.08i (−1.00 to 0.26) |
| 12 months | 0.5 (0.4, 12) | 0.4 (0.5, 9) | MD −0.01i (−0.28 to 0.25) | |
| 24 months | 0.7 (0.4, 7) | 0.3 (0.3, 10) | MD 0.22i (−0.05 to 0.49) | |
| Mean (SD, n) | VB-first (n = 172) | BET-first (n = 173) | Mean difference (95% CI) |
|---|---|---|---|
| VAS | |||
| 1 month | 3.9 (3.0, 122) | 4.0 (3.0, 129) | −0.22a (−0.98 to 0.49) |
| 12 months | 3.1 (3.1, 98) | 3.7 (3.0, 98) | −0.15a (−0.94 to 0.63) |
| 24 months | 2.9 (2.8, 70) | 3.2 (2.8, 83) | −0.13a (−0.99 to 0.73) |
| VascuQoL composite total score | |||
| 1 month | 4.1 (1.6, 116) | 4.1 (1.4, 116) | −0.02b (−0.39 to 0.35) |
| 12 months | 4.7 (1.6, 91) | 4.5 (1.5, 95) | 0.00b (−0.40 to 0.40) |
| 24 months | 4.8 (1.4, 64) | 4.6 (1.4, 72) | 0.11b (−0.34 to 0.56) |
| EQ-5D-5L health state score | |||
| 1 month | 60.5 (22.1, 130) | 64.5 (19.7, 137) | −1.66b (−6.72 to 3.40) |
| 12 months | 62.4 (23.4, 106) | 64.2 (22.9, 100) | −1.63b (−7.26 to 4.00) |
| 24 months | 58.5 (22.7, 76) | 63.2 (21.6, 85) | −2.98b (−9.19 to 3.22) |
| EQ-5D-5L index score (UK participants) | |||
| 1 month | 0.5 (0.3, 106) | 0.5 (0.3, 110) | 0.02b (−0.06 to 0.10) |
| 12 months | 0.6 (0.3, 86) | 0.5 (0.3, 82) | 0.02b (−0.07 to 0.11) |
| 24 months | 0.5 (0.3, 63) | 0.6 (0.3, 65) | 0.02b (−0.07 to 0.12) |
| EQ-5D-5L index score (Danish/Swedish participants) | |||
| 1 month | 0.5 (0.4, 24) | 0.6 (0.2, 25) | −0.09b (−0.23 to 0.05) |
| 12 months | 0.5 (0.3, 19) | 0.7 (0.1, 18) | −0.14b (−0.29 to 0.01) |
| 24 months | 0.7 (0.2, 13) | 0.7 (0.2, 17) | −0.04b (−0.20 to 0.12) |
| ICECAP-O | |||
| 1 month | 0.7 (0.2, 118) | 0.7 (0.2, 132) | 0.01b (−0.04 to 0.05) |
| 12 months | 0.7 (0.2, 100) | 0.7 (0.2, 97) | 0.01b (−0.04 to 0.07) |
| 24 months | 0.8 (0.2, 75) | 0.7 (0.2, 80) | 0.04b (−0.02 to 0.10) |
| SF12v2 physical component score | |||
| 1 month | 33.1 (12.3, 110) | 34.9 (10.9, 114) | −0.47b (−3.36 to 2.42) |
| 12 months | 37.6 (11.5, 92) | 36.0 (11.2, 95) | 0.87b (−2.16 to 3.91) |
| 24 months | 37.9 (10.7, 70) | 36.7 (10.8, 74) | 0.52b (−2.84 to 3.88) |
| SF12v2 mental component score | |||
| 1 month | 44.8 (8.0, 110) | 44.6 (8.8, 114) | −0.27b (−2.43 to 1.90) |
| 12 months | 45.9 (8.7, 92) | 45.8 (7.1, 95) | −0.08b (−2.39 to 2.23) |
| 24 months | 46.3 (7.8, 70) | 45.6 (7.5, 74) | −0.28b (−2.92 to 2.35) |
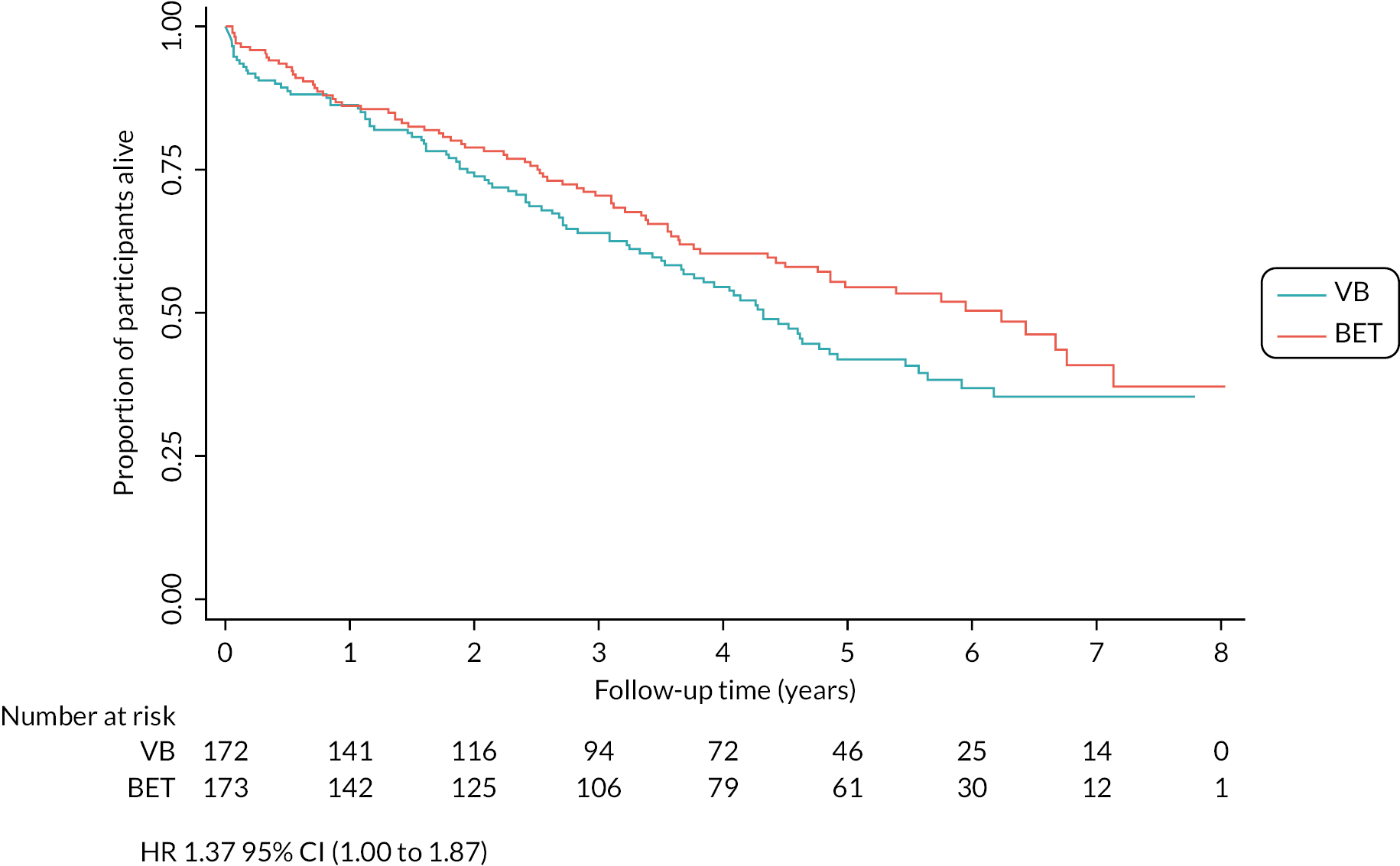
Ninety-one (53%) of 172 patients in the VB-first group and 77 (45%) of 173 in the BET-first group died from any cause [adjusted HR 1.37 (95% CI 1.00 to 1.87); Figure 4]. Cardiovascular and respiratory diseases were the most common causes of death in both groups as expected. There were no specific causes of death identified in either group which would explain the differences in number of deaths observed between the two groups (Tables 13 and 14).
| IHD, HF, upper GI bleed |
| Acute MI, HAP, PAD, AV disease |
| IHD, PAD, pneumonia, ruptured CFA patch repair |
| Pneumonia |
| Malignant neoplasm of prostate |
| IHD, HF, HT |
| Acute MI, IHD, PAD, HF |
| IHD, AKI, CKD, HF, PAD, DM |
| Influenza |
| IHD, HF |
| DM, PAD, CLTI non-trial leg |
| Acute MI, HF |
| Pneumonia, PAD |
| Cellulitis of other parts of limb, sepsis |
| Acute MI |
| Acute exacerbation of COPD |
| PAD, CLTI, RA, leukaemia |
| Malignant neoplasm of orbital sinus |
| Acute exacerbation COPD |
| Acute exacerbation COPD, CKD, DM |
| Acute myeloid leukaemia |
| Pneumonia, HF, PAD |
| COVID-19 |
| Sepsis, CKD Stage 5, AKA requiring revision, opiate toxicity, CLTI non-trial leg |
| Ischaemic stroke, PAD, AKA |
| Acute MI |
| PAD, CLTI trial leg, AKI |
| PAD, colitis, MOF, PAD, end-stage dementia, frailty |
| IHD |
| IHD, CCF, HT, DM |
| Cardiac arrest, CAP, DM, CLTI trial leg |
| COVID-19 |
| Malignancy |
| IHD, HF |
| PAD, CLTI non-trial leg |
| MOF, DM |
| Malignancy, ARF, pneumonia |
| Malignancy |
| Acute MI |
| PAD |
| Stroke unspecified |
| Acute MI |
| Malignancy |
| Acute MI |
| IHD, VF cardiac arrest |
| Acute MI, unspecified GI bleed, stroke |
| IHD, HF, DM, CKD |
| DKA, pneumonia |
| IHD, RF, sepsis |
| Acute MI |
| Ischaemic stroke |
| Respiratory failure, pneumonia, PE, stroke |
| Acute MI, DM, PAD |
| IHD, HT |
| HF, PAD, bilateral CLTI |
| DM, aspiration pneumonia, AF, HT, chronic pancreatitis |
| DM with multiple complications |
| AF, stroke, PAD, DM |
| IHD, cardiac arrest |
| Malignancy |
| Pneumonia, PAD, BKA |
| HF, AV replacement, CABG, DM |
| Senility |
| Malignancy |
| Intracerebral haemorrhage, HT, DM, PAD |
| DM, CLTI trial leg |
| Perforated bowel, aspiration pneumonia and adrenal insufficiency |
| Acute MI, IHD, DM |
| HF |
| Natural causes |
| Intracerebral haemorrhage |
| Cardiac arrest, IHD |
| HAP, IHD, HF, PAD, AKA |
| Cardiac arrest, HF |
| PAD |
| COVID-19 pneumonia, ESRD on dialysis, DM, AKA |
| Acute MI, HF, IHD |
| Acute MI |
| CLTI non-trial leg, malignancy |
| Bowel obstruction, withdrawal of renal dialysis |
| Stroke unspecified, COPD |
| Malignancy |
| HAP, IHD, AF, HF, PVD |
| Acute MI, IHD, CLTI trial leg, DM |
| Acute MI, HF, IHD |
| COVID-19, MOF, pneumonitis. CKD stage 3; DM |
| CKD |
| Acute MI, IHD, malignancy |
| Pneumonia, sepsis, AKI |
| DM, HF, ESRD, HT, PAD |
| Acute exacerbation of COPD, HAP |
| Acute exacerbation of COPD with pneumonia, DM, AF, HTN |
| IHD, DM |
| IHD, MOF, DM |
| RA, unspecified sepsis, AKI, peripheral oedema, poor oral nutrition |
| IHD, DM, MOF, bilateral CLTI |
| Intracerebral haemorrhage, COPD |
| Stroke unspecified, aspiration pneumonia, frailty, Clostridium difficile, AF, DM, CLTI non-trial leg |
| Malignant neoplasm of lung, COPD, pneumonia |
| Acute MI, IHD |
| Stricture of artery |
| Acute MI, IHD. HF, DM, PAD |
| Cholecystitis, sepsis from perforated bowel, CLTI trial-leg |
| IHD, HF |
| Acute exacerbation COPD, HF |
| Old age, DM, CLTI trial leg, BKA |
| Old age |
| Acute MI, IHD, HT, cirrhosis, urinary sepsis, CKD |
| PAD, sepsis, CLTI in trial leg |
| IHD, ESRD |
| IHD, pneumonia, DM, PAD, CKD |
| DM with multiple complications, general deterioration, confusion, infection |
| COVID-19, pneumonia suspected, dementia, IHD, DM |
| CLI trial leg, osteomyelitis |
| PAD |
| COPD, HF, respiratory failure, PAD |
| Acute MI |
| Perforation of gastric ulcer |
| GI haemorrhage |
| Malignancy unspecified |
| IHD, MOF, DM, CLTI |
| COVID-19 pneumonia |
| Senility |
| Malignancy, metastatic disease, unknown primary |
| DM, CLTI trial leg |
| Oesophagitis, GI bleed, pneumonia |
| Sepsis unknown aetiology |
| Malignant neoplasm of pancreas |
| Cardiac arrest, PAD |
| Bronchopneumonia |
| Pneumonia, respiratory failure |
| Malignant neoplasm of lung |
| PE, AKI, DM, IHD |
| PAD, CLTI non-trial leg |
| Malignancy, unknown primary |
| Vascular dementia, HAP |
| Cardiac arrest, hypoxia, aspiration, paralytic ileus |
| IHD |
| IHD |
| Fall, intra-abdominal haemorrhage, IHD |
| AV stenosis |
| Malignant neoplasm of bladder, ESRD |
| Malignant neoplasm of lung |
| Acute exacerbation of COPD, respiratory failure, DM |
| PAD, DKA |
| COVID-19, IHD, HF |
| Interstitial pulmonary disease |
| COVID-19 |
| Recurrent sigmoid volvulus, bowel obstruction, paralytic ileus, PAD, DM, Clostridium difficile |
| DM, PAD, CLTI, sepsis |
| Ruptured aneurysm |
| Unknown, found dead, no PM |
| Stroke unspecified, CAP, PAD |
| Suspected cardiac death |
| COVID-19, DM, PAD |
| HF |
| DM with renal complications, CLTI trial leg |
| IHD, ESRD, DM |
| CLTI trial leg |
| Acute MI, IHD, HF, PVD, AF, DM |
| Sepsis, chest and infection |
| Found dead, previous PE and HF |
| CAP |
| DM with multiple complications |
| Acute MI, HF, jaundice, GI bleeding, pancreatitis |
| Acute lower respiratory infection, DM, PAD |
| Pneumonia |
| DM with multiple complications, frailty, CKD, HF, PAD |
FIGURE 4.
Kaplan–Meier plot for OS (ITT analysis).
Thirty-five (20%) of 172 in the VB-first group and 32 (18%) of 173 patients in the BET-first group had a major amputation [adjusted HR 1·23 (95% CI 0.75 to 2.01); Figure 5]. There was no difference in the number of participants who had at least one further revascularisation procedure in the trial leg between the VB-first group [50 (29%) of 172 patients] and the BET-first group [56 (32%) of 173; adjusted RR 0.94 (95% CI 0.68 to 1.28)]. However, the number of patients who had a re-intervention was higher in the BET-first group [33 (19%) patients] than in the VB-first group [9 (5%) patients; RR 0.27 (95% CI 0.13 to 0.55)]. Conversely, cross-over interventions were more common in the VB-first group [46 (27%) patients] than in the BET-first group [33 (19%) patients; RR 1.43 (95% CI 0.94 to 2.18)]. There were no differences between the two treatment groups in 30-day morbidity and death, MALE, MACE, relief of ischaemic pain, or HRQoL (see Tables 11 and 12). The results from the post hoc sensitivity analyses for time-to-event secondary outcomes are reported in Table 15.
| Outcome | Cause specific HRa (95% CI) | Subdistribution HRb (95% CI) |
|---|---|---|
| Major amputation | 1.23 (0.76 to 2.01) | 1.14 (0.70 to 1.84) |
| MALE | 0.93 (0.67 to 1.29) | 0.88 (0.63 to 1.21) |
| MACE | 1.06 (0.76 to 1.49) | 0.95 (0.68 to 1.32) |
FIGURE 5.
Kaplan–Meier plot for major amputation (ITT analysis).

Serious adverse events
Twenty-nine (17%) patients in the VB-first group and 23 (13%) patients in the BET-first group reported 33 and 26 SAEs, respectively. Only one SAE (biliary sepsis due to gallstones complicated by pancreatitis and organ failure) in the BET-first group was considered related to the trial intervention and a RUSAE.
Chapter 4 Health economics analysis
Introduction
This chapter contains material that has been reproduced with permission from Bradbury et al. 1 This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
This chapter describes the health economic analysis conducted for the BASIL-2 trial. The objective of the analysis was to assess the cost-effectiveness of a VB-first versus a BET-first revascularisation strategy in CLTI patients who required an IP, with or without a more proximal infra-inguinal revascularisation procedure, to restore limb perfusion. A comprehensive health economic analysis plan was specified before analysis.
Methods
Overview
A within-trial cost-effectiveness analysis (CEA), based on the primary outcome of the trial, was conducted with results presented in terms of cost per year of AFS. An additional cost–utility analysis (CUA) was carried out to calculate the cost per additional quality-adjusted life-year (QALY) gained. The base-case analysis was based on the ITT population and conducted from the perspective of UK NHS and Personal Social Services (PSS). Costs were presented in Great British pounds (2022 price year). The NICE recommendations31 were used to guide the methods of the health economic analysis. The study findings are reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards guidelines. 32
Resource use and costs
Resource use data over the trial period were collected and included: (1) hospital-based services including procedure-related resource use for the primary interventions and any other secondary procedures, inpatient admissions and day case admissions. Other hospital-based services including outpatient appointments and diagnostic imaging were also collected; (2) primary and community healthcare contacts (general practitioner, practice nurse, district nurse, physiotherapists and occupational therapists, vascular and diabetic nurse, and other secondary healthcare contacts); (3) out-of-pocket expenses incurred by patients; and (4) time off work.
Unit costs were obtained from a set of standard sources. Unit costs for hospital-based services were obtained primarily from NHS Reference costs. 33 Primary and community healthcare services costs were derived from the PSS Research Unit (PSSRU) Unit Costs of Health and Social Care 2022 compendium. 34
Overall total healthcare costs for the duration of the study period were estimated using the micro-costing technique by multiplying the resource items used by the respective unit cost and summing over all items. 35 Unit costs were inflated to a 2022 price year using the NHS Cost Inflation Index (NHSCII),34 where values were obtained from sources prior to 2022. Details on unit costs can be found in Appendix 1, Table 25.
Secondary (hospital-based) healthcare services
Direct personnel costs associated with the interventions (VB-first and BET-first) and additional procedures (major amputation) were obtained from a previous micro-costing study. 11 The cost of revascularisation procedures was generated for individual patients by multiplying the number of reported interventions received (first, repeat and additional interventions) by the cost of procedure. The number and type of endovascular devices (BMS, DCB and DES) were reported for patients who received an endovascular intervention as either a first or further intervention. The total number of devices per patient was generated and multiplied by the unit cost. Additionally, procedural costs of non-bypass procedures (thrombectomy, thrombolysis, wound debridement, fasciotomy, and endarterectomy) were also included in the total hospital costs.
For inpatient costs, the duration of hospital stay for the index admission and subsequent re-admissions in general ward settings, intensive treatment unit (ITU) and high-dependency unit (HDU) were recorded for the index and subsequent admissions for each patient. The cost of hospital stay in a general ward, ITU and ITU for the index admission was calculated from the reported number of days. The total number of hospital days was generated during the revascularisation procedure, reintervention, and further admission and then multiplied by the unit cost of general wards, ITUs and HDUs. Hospital-related resource use and costs were measured from the date the patient was randomised to one of the revascularisation interventions.
Several assumptions were required in order to generate the costs related to hospital-based resources within the trial and these are described below:
-
Given that the primary analysis is based on the ITT population, the cost of the intervention (as randomised) was considered even if the VB and BET forms were not completed. Any other revascularisation intervention received, different to the randomised intervention, was treated as an additional procedure. For example, a patient could be randomised to BET-first and they had VB-first as their first intervention. In this case, the cost of BET-first procedure will be added, and the cost associated with VB-first will be considered as cost of additional procedure.
-
If it was indicated that a revascularisation procedure was received but the inpatient form was not completed, it was assumed that the number of hospital stays was missing, and the cost associated with a hospital stay will be replaced.
-
For patients who had BET-first, if the type and number of endovascular devices in the BET-first form were not provided, it was assumed that a plain balloon angioplasty (PBA) was used which is not associated with additional cost.
-
Where patients reported that they were admitted to the hospital and discharged on the same day, it was assumed that the intervention was obtained as a day case.
-
Other non-bypass revascularisation procedures (thrombolysis, thrombectomy and endarterectomy) and non-bypass non-revascularisation procedures (wound debridement and fasciotomy) were valued as day case procedures.
Data on other hospital healthcare services were also collected including the number of outpatient appointments and the use of diagnostic imaging and other radiological investigations (X-ray). However, these were not considered in the base-case analysis and later included in the sensitivity analysis.
Primary and community-based healthcare services
A resource usage questionnaire, completed by patients with the research nurse, captured data on primary healthcare services use during the trial period. Primary healthcare resource use included visits to the general practitioner, practice nurse, district nurse, physiotherapist and occupational therapist or other healthcare professional, for example podiatrist/chiropodist. The total number of visits was attached to the corresponding unit costs obtained from Unit Costs of Health and Social Care 2022. 34 In cases where a participant responded that services were used without providing details on the number of visits, the median number of visits replaced the missing numbers.
Patients and productivity costs
Patients’ incurred costs and productivity losses were also included in the analysis. Patients were asked to report out-of-pocket expenses incurred (e.g. transport costs) when attending treatment, employment status and time lost from work. In order to estimate productivity costs, self-reported days off work were multiplied by the average gender-specific wage rate obtained from the Annual Survey of Hours and Earnings (ASHE). 36 The analysis of productivity costs was based on the human capital approach. 37
Outcomes
The primary outcome measure for the CEA was AFS based on the principal outcome of the BASIL-2 trial.
For the CUA, HRQoL was measured using EQ-5D-5L where patients completed the EQ-5D-5L questionnaire at baseline, 1, 6 and 12 months and then annually until the last follow-up point up to 7 years. This measurement consists of five domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each domain has five levels ranging from no problems to extreme problems or unable to perform. 38 In line with NICE recommendations,39 the UK crosswalk mapping algorithm was applied40 to convert patients’ 5L responses recruited in the UK onto 3L preference-based summary scores. EQ-5D-5L crosswalk score values range from −0.594 (the worst health state) to 1.000 (full health), with 0 equivalent to death. The Danish value sets41 were applied to patients recruited in Denmark and Sweden. A utility score of zero was assigned to all patients with missing values after death. 42
QALYs were generated from the utility scores at different time points over 24 and 36 months for individual participants using the area under the curve method. 35 The specific time horizons were selected as a result of incomplete EQ-5D-5L data beyond 36 months.
The ICECAP-O43 measure was also used to evaluate changes in patient’s capability, allowing a broader assessment of benefit in patients with CLTI. The ICECAP-O tool is a preference-based measure for older people over the age of 65, and consists of five attributes (attachment, security, role, enjoyment and control) measured across four levels. 44 Responses to ICECAP-O questionnaire were used to generate capability scores derived from a set of tariff values for UK population. 43 The score ranges from 1 (full capability) to 0 (no capability). 43 These UK values were applied to patients recruited from Denmark and Sweden because there are no specific ICECAP-O value sets in these countries.
EQ-5D-5L and ICECAP-O overall scores were considered as missing if the patient failed to respond to all questionnaire items.
Statistical analysis
Due to cost data being positively skewed, 95% CIs around the mean difference in costs were estimated using the bias-corrected and accelerated bootstrap method calculated from 1000 resamples. 45 The same method was also applied to estimate the mean difference in outcomes. Differences between groups are deemed significant if the CIs of the difference in mean costs or outcomes between groups did not cross zero. These differences were adjusted for age group (≤ 60, 61–70, 71–80, > 80 years), gender, DM and CKD, severity of clinical disease, previous (permissible) intervention to the trial leg. EQ-5D-5L and ICECAP-O baseline scores were also considered in the adjustment for health and capability outcome measures, respectively.
Missing values for costs and health outcomes were replaced using the multiple imputation approach by chained equations technique, assuming that values were missing at random (MAR). 46 The minimisation variables of DM, CKD, severity of clinical disease, previous (permissible) intervention to the trial leg were included in the imputation models to replace missing costs and outcomes values. This approach is widely used in economic evaluation where missing observations for participants with available baseline data are replaced with values drawn from the posterior predictive distribution. Fifty imputed data sets were generated based on the percentage of incomplete observation and estimates obtained from the imputation were combined using Rubin’s rules. 47 Additionally, the recruitment centre was also considered in the model for imputing EQ-5D-5L missing values. All analyses were performed using Stata® (version 17.0).
Economic evaluation analysis
The primary analysis was conducted in the form of CEA expressed as cost per year of AFS out to 7 years. A CUA was also carried out with the outcome reported as cost per QALYs gained out to 2 years and 3 years. NICE’s willingness-to-pay (WTP) thresholds of 20,000 and 30,000 per QALY gained were applied to assess the cost-effectiveness of the revascularisation strategies. 31 Both costs and outcomes were discounted at 3.5% rate following the recommendation by HM Treasury. 31,48 Findings from previous studies have found that the main incremental costs were those incurred by inpatient hospital stay. 49,50 Therefore, the base-case analysis considered costs associated with the use of hospital services including revascularisation and additional procedures, and related hospital stays, to the end of follow-up.
Sensitivity analysis
Bootstrapping was used to account for the overall uncertainty that occurs because of variations in sampling, by jointly bootstrapping mean cost and outcome differences. 51 Five thousand paired values of incremental costs and outcomes were generated and displayed on a cost-effectiveness plane as a scatterplot to facilitate interpretation. 51,52 A cost-effectiveness acceptability curve (CEAC) was then constructed to reflect the probability that an intervention is cost-effective at different WTP values per AFLY and QALY gained at 2 and 3 years. 53 A scenario analysis was undertaken to assess the robustness of the results from the main analysis to alternative perspectives, assumptions and to explore the broader issue of the generalisability of the results. All analyses were expressed as the cost associated with additional QALYs gained out to 2 years. The following scenarios were considered to explore the cost-effectiveness of the interventions:
-
Including the imputed cost of primary and other hospital healthcare services in addition to the cost of hospital care services.
-
Adopting a broader costing perspective (Societal) where expenditures borne by patients and costs associated with productivity loss were included.
-
Patient adherence with the revascularisation strategy defined as patients receiving their allocated intervention for their first revascularisation strategy and including the cost of hospital care.
-
A complete case analysis considering patients with complete data on hospital costs and EQ-5D-5L to assess the impact of imputation technique on the difference in costs and QALYs.
Subgroup analysis was also conducted to investigate how costs and effects of revascularisation strategies may change due to different patients’ characteristics. The analysis focused on prespecified subgroups of interest including age groups, sex, CKD, DM, previous intervention to the trial leg, and severity of clinical disease.
Results
Response rate and data completeness
A total of 345 participants were recruited in the trial (172 in the VB-first group and 173 in the BET-first group) and all were included in the base-case analysis. Questionnaires capturing information on the interventions were completed by 147 participants who were randomised to VB-first and 168 randomised to BET-first. The inpatient/day-case form was completed by 100 participants in the VB-first group, who reported that they had revascularisation when they were admitted to hospital, and 114 in the BET-first group. Complete data on primary and other hospital healthcare services use at all time-points over 2 years following randomisation were reported by 54 patients in both groups. Complete EQ-5D-5L data that can be used to generate QALYs, 2 years after randomisation, were available for 80 patients in the VB-first group and 79 in the BET-first group.
Resource use and cost data
Secondary (hospital-based) healthcare services
Hospital length of stay
Details of hospitalisation related to the trial leg are reported in Table 16. When considering initial hospital stay only, patients were mainly admitted to general ward settings in both groups to receive the revascularisation strategy. Few patients required specialised care delivered in ITU and HDU. On average, patients in the VB-first group spent 5 more days after randomisation to receive the revascularisation strategy in a general ward than those in the BET-first group (19.97 days for VB-first vs. 14.46 days for BET-first). The median initial duration of hospital stay was 12.50 days in the VB-first group and 9.00 days in the BET-first group. Similarly, patients in the VB-first group stayed longer in specialised care units when compared to those in the BET-first group. Following the initial admission, patients randomised to BET-first required slightly more hospital care than patients randomised to VB-first.
| VB-first revascularisation strategy (n = 100) | BET-first revascularisation strategy (n = 114) | |||
|---|---|---|---|---|
| Mean unit (SD) | Median (IQR) | Mean unit (SD) | Median (IQR) | |
| Initial hospitalisation | ||||
| LOS (days) | ||||
| Ward | 19.97 (23.51) | 12.50 (7.00–23.00) | 14.46 (16.94) | 9.00 (3.00–19.00) |
| HDU | 0.26 (1.12) | – | 0.11 (0.69) | – |
| ITU | 0.36 (1.32) | – | 0.06 (0.47) | – |
| Total hospitalisation | ||||
| Number of admissions | 2.21 (1.46) | 2.00 (1.00–3.00) | 2.15 (1.38) | 2.00 (1.00–3.00) |
| LOS (days) | ||||
| Ward | 30.89 (35.65) | 18.50 (10.00–41.00) | 29.17 (40.33) | 18.50 (7.00–36.00) |
| HDU | 0.32 (1.16) | – | 0.26 (1.07) | – |
| ITU | 0.50 (2.18) | – | 0.10 (0.51) | – |
| Day case | 0.04 (0.20) | – | 0.04 (0.24) | – |
Over the trial period and prior to any imputation, patients in VB-first and BET-first had a similar number of admissions. The total mean length of stay (LOS) in the general ward was similar in both groups with an average of 30.89 (median, 18.50) days for patients in the VB-first group compared with 29.17 (median, 18.50) days for patients in the BET-first group. Patients in the VB-first group spent almost half a day longer in ITU than those in the BT-first group while patients in both groups spent the same duration of time in HDU (see Table 16). Very few patients were admitted to the hospital and discharged on the same day and treated as a day case in both the BET-first and VB-first group.
Procedural related costs
The average number of different types of revascularisation devices for patients in the BET-first and VB-first groups is reported in Table 17. Overall, patients in the BET-first group utilised more devices than those in the VB-first group. For those in the BET-first group, the average number of different types of endovascular devices consumed was fairly similar (0.30 unit of BMS, 0.29 unit of DES and 0.31 unit of DCB). For the few patients in the VB-first group who crossed over to BET-first, BMS (0.35 units) was mainly used followed by DCB (0.15 units) and none required DES.
| VB-first (n = 52) | BET-first (n = 168) | |
|---|---|---|
| Mean unit (SD) | Mean unit (SD) | |
| BMS | 0.35 (0.84) | 0.30 (0.69) |
| DES | – | 0.29 (0.88) |
| DCB | 0.15 (0.50) | 0.31 (0.81) |
Costs related to the revascularisation strategy were calculated for individual patients based on the number of procedures performed over the trial period. The estimation included the human resource costs associated with each revascularisation strategy alongside the cost of endovascular devices. Total cost of revascularisation (VB-first and BET-first) strategy and other procedures (non-bypass and major amputation) are presented later in the chapter.
Other hospital-based services
Information about resource use and costs associated with outpatient appointments and diagnostic imaging, using complete data, is shown in Table 18. Patients randomised to BET-first had more outpatient appointments (5.17) compared to those randomised to VB-first (4.13). Patients in the BET-first group in general required slightly more diagnostic imaging services compared to their counterpart in the VB-first group. However, data from ultrasound (US) imaging showed usage were more in the VB-first group compared to the BET-first group (mean, 1.81 and 0.94 respectively) and the adjusted cost difference in US usage (mean −£43.65, 95% CI −91.81 to −4.05) was statistically significant.
| Resource use | VB-first (n = 54) | BET-first (n = 54) | Mean adjusteda bootstrapped difference in £ costs (95% CI) | ||
|---|---|---|---|---|---|
| Mean unit (SD) | Mean cost £ (SD) | Mean unit (SD) | Mean cost £ (SD) | ||
| Primary healthcare services | |||||
| GP at practice | 5.09 (8.40) | 213.89 (352.84) | 3.46 (4.29) | 145.44 (180.18) | −62.79 (−206 to 42.73) |
| Nurse at practice | 7.2 (16.18) | 96.75 (217.36) | 10.06 (23.06) | 135.05 (309.75) | 46.65 (−39.87 to 161.31) |
| District nurse | 16.04 (24.84) | 465.07 (720.31) | 39.98 (75.49) | 1159.46 (2189.21) | 732.33 (173.31 to 1333.36)b |
| Physiotherapist | 1.89 (8.42) | 52.89 (235.75) | 0.39 (1.17) | 10.89 (32.82) | −35.20 (−111.34 to 0.37) |
| Occupational therapist | 0.19 (0.65) | 4.63 (16.16) | 0.09 (0.35) | 2.31 (8.78) | −1.52 (−6.69 to 3.03) |
| Other (chiropodist, podiatrist, diabetic and vascular nurse, carer) | 9.28 (16.47) | 73.11 (366.35) | 14.13 (25.96) | 183.93 (681.07) | 149.06 (−39.08 to 367.18) |
| Other secondary (hospital-based) healthcare services | |||||
| Outpatient visit | 4.13 (4.81) | 578.15 (672.82) | 5.17 (7.31) | 723.33 (1023.29) | 216.32 (−59.03 to 686.22) |
| Diagnostic imaging | |||||
| US | 1.81 (3.12) | 116.15 (199.72) | 0.94 (1.16) | 60.44 (73.99) | −43.65 (−91.81 to −4.05)b |
| CT scan | 0.09 (0.35) | 9.17 (34.77) | 0.11 (0.32) | 11.00 (31.40) | 1.67 (−13.38 to 15.06) |
| Magnetic resonance angiogram | 0.13 (0.48) | 23.59 (86.93) | 0.17 (0.57) | 30.33 (104.58) | 9.36 (−33.46 to 56.10) |
| DSA | 0.04 (0.19) | 4.33 (22.30) | 0.17 (0.75) | 19.50 (87.29) | 9.61 (−6.60 to 33.47) |
| Other (X-ray) | 0.31 (0.75) | 9.44 (22.44) | 0.56 (1.31) | 16.67 (39.38) | 7.19 (−4.67 to 23.08) |
Primary healthcare services
The average use and costs per patient of different primary healthcare services are presented by intervention group in Table 18 for those with complete resource use data, 2 years after randomisation. Overall, the level of resource utilisation was relatively similar across both groups. However, those in the BET-first group had more visits to district nurses (40 visits) than those in the VB-first group (16 visits). The difference in mean costs of district nurse visits was statistically significant.
Total aggregated costs
National Health Service costs
Details regarding the aggregated imputed cost categories are presented in Table 19. In total, patients in the VB-first group consumed more health resources compared to those in the BET-first group and the mean (SD) imputed total NHS cost per patient was £17,996.31 (£16,491.92) and £15,986.28 (£16,217.52) in the VB-first and BET-first group, respectively. This resulted in a £2069.33 (95% CI £−5524.43 to £1417.67) additional cost for those in the VB-first group compared to their counterpart in the BET-first group.
| Cost category | VB-first (n = 172) | BET-first (n = 173) | Mean adjusteda bootstrapped £ difference (95% CI) |
|---|---|---|---|
| Mean cost £ (SD) | Mean cost £ (SD) | ||
| NHS costs | |||
| Secondary (hospital-based) healthcare costs | |||
| Revascularisation strategy (first intervention and re-intervention) | 3011.75 (883.43) | 1834.00 (2019.06) | −1183.11 (−1489.29 to −848.73)b |
| Non-bypass procedures | 56.86 (156.59) | 54.38 (148.36) | −2.76 (−41.39 to 28.17) |
| Major amputation | 258.63 (513.19) | 249.79 (542.35) | −17.32 (−129.90 to 83.47) |
| Hospital stay (inpatient and day case) | 12,467.33 (16,020.37) | 11,185.31 (15,304) | −1319.72 (−4567.79 to 2188.80) |
| Primary and other secondary healthcare costs | |||
| Primary care services | 1148.06 (1044.59) | 1526.75 (1617.58) | 389.89 (105.06 to 674.42)b |
| Outpatient visits | 880.73 (1319.00) | 1020.14 (1270.17) | 119.00 (−151.02 to 367.79) |
| Diagnostic imaging | 172.96 (192.85) | 115.69 (127.17) | −55.76 (−94.53 to −23.66)b |
| Total NHS costs | 17,996.31 (16,491.92) | 15,986.28 (16,217.52) | −2069.33 (−5524.43 to 1417.67) |
| Patients incurred and productivity costs | |||
| Productivity cost | 372.75 (1144.56) | 97.19 (452.87) | −245.77 (−425.62 to −91.24)b |
| Out-of-pocket expenditure (travel and medication) | 128.48 (771.72) | 27.37 (158.01) | −99.30 (−248.23 to −8.82)b |
| Total societal costs (NHS, patients incurred and productivity costs) | 18,497.54 (16,553.23) | 16,110.63 (15,958.52) | −2414.40 (−5867.91 to 1270.63) |
Resource use cost from hospitalisations contributed to almost 70% of all NHS costs in both groups followed by the cost of revascularisation strategy (representing 17% and 11% of total NHS costs in both the VB-first and BET-first groups, respectively). Costs related to the non-bypass procedure and major amputation were relatively similar with slight additional costs in the VB-first group than the BET-first group. Overall, hospital costs were higher in the VB-first group compared to the BET-first group while primary healthcare-related service use and other hospital-based services (outpatient appointment and imaging) were slightly higher in the BET-first group when compared to the VB-first group. The mean cost differences of revascularisation strategies, primary care services and diagnostic imaging were statistically significant (see Table 19).
Wider societal cost
The total mean (SD) cost per patient in the VB-first group increased to £18,497.54 (£16,5538.23) and £16,110.63 (£15,958.52) in the BET-first group when a broader societal perspective was considered, including out-of-pocket expenditure and productivity loss. Cost of productivity loss was relatively higher in patients in the VB-first group compared to those in the BET-first group (see Table 19). However, the number of patients who reported that they were employed was very small in both groups.
Outcome
Health and capability measures
Patients’ responses to EQ-5D-5L reduced over time while the response rate was relatively similar in both the VB- and BET-first groups. A summary of HRQoL measures (adjusted for death) at different follow-up points over a 36-month period using complete EQ-5D-5L data is presented in Table 20. The summary showed that patients in the VB-first group had slightly lower EQ-5D-5L scores at baseline compared to those in the BET-first group. There was an improvement in utility scores within the first 6 months after randomisation in both groups. Afterwards, a reduction in EQ-5D-5L scores was observed in both intervention groups after year 1 until 3 years after randomisation. At 3 years, the BET-first group had improved HRQoL compared to those in the VB-first group (additional 0.132 score) and the adjusted bootstrapped difference was statistically significant (95% CI 0.034 to 0.225).
| Follow-up time point | VB-first | BET-first | Mean adjusteda bootstrapped difference (95% CI) |
|---|---|---|---|
| Mean score (SD) | Mean score (SD) | ||
| Baseline | |||
| n | 159 | 164 | 0.066b (0.001 to 0.134)c |
| Mean (SD) | 0.377 (0.327) | 0.433 (0.297) | |
| 1 month | |||
| n | 141 | 141 | −0.004 (−0.075 to 0.064) |
| Mean (SD) | 0.436 (0.332) | 0.478 (0.312) | |
| 6 months | |||
| n | 128 | 122 | 0.007 (−0.073 to 0.080) |
| Mean (SD) | 0.459 (0.319) | 0.497 (0.336) | |
| 12 months | |||
| n | 128 | 123 | −0.003 (−0.085 to 0.083) |
| Mean (SD) | 0.458 (0.353) | 0.470 (0.351) | |
| 24 months | |||
| n | 119 | 117 | 0.064 (−0.032 to 0.146) |
| Mean (SD) | 0.357(0.355) | 0.418 (0.344) | |
| 36 months | |||
| n | 109 | 111 | 0.132 (0.034 to 0.225)c |
| Mean (SD) | 0.219 (0.338) | 0.348 (0.361) | |
For the capability measure (ICECAP-O), patients in the VB-first group also had slightly lower scores (mean score of 0.693) at baseline than those in the BET-first group (mean score of 0.737). Over time, there was a slight reduction in the mean score in both groups in general; the mean adjusted differences suggest that patients randomised to BET-first had slightly better ICECAP-O scores than those randomised to VB-first (Table 21).
| Follow-up time point | VB-first | BET-first | Mean adjusteda bootstrapped difference (95% CI) |
|---|---|---|---|
| Mean score (SD) | Mean score (SD) | ||
| Baseline | |||
| n | 150 | 159 | 0.048b (0.005 to 0.095)c |
| Mean (SD) | 0.693 (0.221) | 0.737 (0.207) | |
| 1 month | |||
| n | 125 | 137 | 0.005 (−0.050 to 0.068) |
| Mean (SD) | 0.664 (0.275) | 0.707 (0.242) | |
| 6 months | |||
| n | 120 | 120 | 0.002 (−0.080 to 0.074) |
| Mean (SD) | 0.649 (0.316) | 0.672 (0.293) | |
| 12 months | |||
| n | 119 | 119 | −0.030 (−0.112 to 0.056) |
| Mean (SD) | 0.620 (0.340) | 0.596 (0.345) | |
| 24 months | |||
| n | 114 | 113 | 0.027 (−0.065 to 0.132) |
| Mean (SD) | 0.501 (0.392) | 0.528 (0.370) | |
| 36 months | |||
| n | 104 | 109 | 0.088 (−0.033 to 0.201) |
| Mean (SD) | 0.348 (0.398) | 0.437 (0.410) | |
To assess the impact of replacing utility scores of deaths with zero, the mean utility scores for complete data without replacement are presented in the supplementary (see Appendix 1, Tables 26 and 27).
Final health outcomes
The primary and secondary outcomes (discounted and undiscounted) can be found in Table 22. For the primary outcome expressed as a year free of amputation at the end of the trial period, BET-first was more effective than VB-first (2.937 vs. 2.575 respectively). This translates to a difference of approximately 0.429 additional AFLYs in favour of BET-first.
| VB-first | BET-first | Mean adjusteda bootstrapped difference (95% CI) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Amputation-free life year over 7 years | |||
| n = 172 | n = 173 | – | |
| Undiscounted | 2.760 (2.252) | 3.130 (2.224) | 0.443 (−0.001 to 0.841) |
| Discounted | 2.575 (2.070) | 2.937 (2.031) | 0.429 (0.030 to 0.877)b |
| QALYs – imputed data | |||
| 2 years | n = 172 | n = 173 | |
| Undiscounted | 0.848 (0.544) | 0.897 (0.546) | 0.015 (−0.088 to 0.117) |
| Discounted | 0.832 (0.515) | 0.884 (0.515) | 0.016 (−0.083 to 0.116) |
| 3 years | n = 172 | n = 173 | |
| Undiscounted | 1.162 (0.798) | 1.293 (0.831) | 0.089 (−0.079 to 0.242) |
| Discounted | 1.125 (0.749) | 1.253 (0.776) | 0.085 (−0.067 to 0.238) |
| QALYs – complete data | |||
| 2 years | n = 80 | n = 79 | |
| Undiscounted | 0.807 (0.544) | 1.000 (0.552) | 0.122 (−0.049 to 0.286) |
| Discounted | 0.794 (0.534) | 0.984 (0.542) | 0.119 (−0.050 to 0.267) |
| 3 years | n = 66 | n = 66 | |
| Undiscounted | 1.050 (0.815) | 1.392 (0.875) | 0.258 (−0.022 to 0.544) |
| Discounted | 1.018 (0.786) | 1.346 (0.843) | 0.246 (−0.013 to 0.521) |
In order to generate QALYs, imputation was required for the base-case analysis to replace missing EQ-5D-5L. Patients in the BET-first group had slightly improved QALYs compared to those in the VB-first group with an average discounted difference of 0.016 (95% CI −0.083 to 0.116) and 0.085 (95% CI −0.067 to 0.238) at 2 and 3 years follow-up respectively. To explore the impact of imputation on QALY estimates, the generated QALYs using complete EQ-5D-5L data are also presented separately in Table 22. QALYs obtained from complete EQ-5D-5L data resulted in slightly lower quality of life compared to those obtained from imputed data in the VB-first group and not the BET-group. Therefore, the discounted difference in QALYs increased to 0.119 (95% CI −0.050 to 0.267) and 0.246 (95% CI −0.013 to 0.521) in favour of BET-first after 2 years and 3 years follow-up, respectively.
Economic evaluation analyses (base case)
Results of the base-case analyses (discounted and undiscounted) are presented in Table 23.
| Analysis | Mean (SD) total £cost | Incremental £cost (95% CI)a | Mean (SD) outcome | Incremental outcome (95% CI)b | ICER (£cost per outcome) |
|---|---|---|---|---|---|
| CEA – Years alive without amputation, 7 years post randomisation | |||||
| Undiscounted | |||||
| VB-first | 17,059.75 (19,214.25) | 2.760 (2.252) | |||
| BET-first | 15,485.22 (17,696.17) | −1597.12 (−5740 to 2219.33) | 3.130 (2.224) | 0.443 (−0.001 to 0.841) | Dominantc |
| Discounted | |||||
| VB-first | 16,874.27 (18,672.31) | 2.575 (2.070) | |||
| BET-first | 15,213.06 (17,163.85) | −1690.34 (−5689.09 to 1975.93) | 2.937 (2.031) | 0.429 (0.030 to 0.877)d | Dominant |
| CUA – QALY, 2 years post randomisation | |||||
| Undiscounted | |||||
| VB-first | 15,794.57 (16,302.16) | 0.848 (0.544) | |||
| BET-first | 13,323.49 (15,616.87) | −2522.91 (−5598.37 to 1062.41) | 0.897 (0.546) | 0.015 (−0.113 to 0.109) | Dominant |
| Discounted | |||||
| VB-first | 15,742.59 (16,182.60) | 0.832 (0.515) | |||
| BET-first | 13,273.66 (15,446.92) | −2524.23 (−5844.93 to 1131.52) | 0.884 (0.515) | 0.016 (−0.083 to 0.116) | Dominant |
| CUA – QALY, 3 years post randomisation | |||||
| Undiscounted | |||||
| VB-first | 16,255.22 (16,945.33) | 1.162 (0.798) | |||
| BET-first | 14,088.07 (15,954.24) | −2211.20 (−5832.51 to 1335.83) | 1.293 (0.831) | 0.089 (−0.074 to 0.246) | Dominant |
| Discounted | |||||
| VB-first | 16,172.73 (16,774.44) | 1.125 (0.749) | |||
| BET-first | 13,987.41 (15,742.31) | −2233.25 (−5830.15 to 1396.49) | 1.253 (0.776) | 0.085 (−0.086 to 0.218) | Dominant |
Cost-effectiveness analysis
The results of the base-case analysis suggest that BET-first is dominant over VB-first, meaning that it is a cost saving and more effective intervention compared with VB-first (see Table 23). BET-first was associated with lower hospital-related costs of £1690.34 (95% CI £ −5689.09 to £1975.93) and a mean additional 0.429 (95% CI 0.030 to 0.877) AFLY compared to VB-first.
Cost–utility analysis
At the end of 2 years follow-up, the results show that BET-first was associated with slightly more QALYs (mean 0.016, 95% CI −0.083 to 0.116) compared to VB-first at a lower hospital cost of −£2524.23 (95% CI, £ −£5844.93 to £1131.52). When a longer time horizon of 3 years was considered, the results were similar, and BET-first was still less costly and more effective than VB-first. The difference in QALYs was increased to 0.085 (−0.086 to 0.218) and the cost difference was reduced to −£2233.25 (95% CI −£5830.15 to £1396.49). The results are presented in Table 23.
Sensitivity analysis
The joint distributions of incremental costs and effects (AFLY), derived from the non-parametric adjusted bootstrap, provide an estimate of the distribution of the population costs and effects presented on the cost-effectiveness plane in Figure 6. Most of the point estimates support the base-case finding and were located in the southeast and northeast quadrants where BET-first is more effective and either less costly or more costly than VB-first. In the absence of an official WTP value for the primary outcome, the probability of BET-first being cost saving was 80% and the probability of the intervention being cost-effective can increase to 98% if an arbitrary WTP value per AFLY of £15,000 is used (Figure 7).
FIGURE 6.
Cost-effectiveness plane (BET-first vs. VB-first): years alive without amputation including hospital costs only, 7 years after randomisation (base-case analysis – discounted).
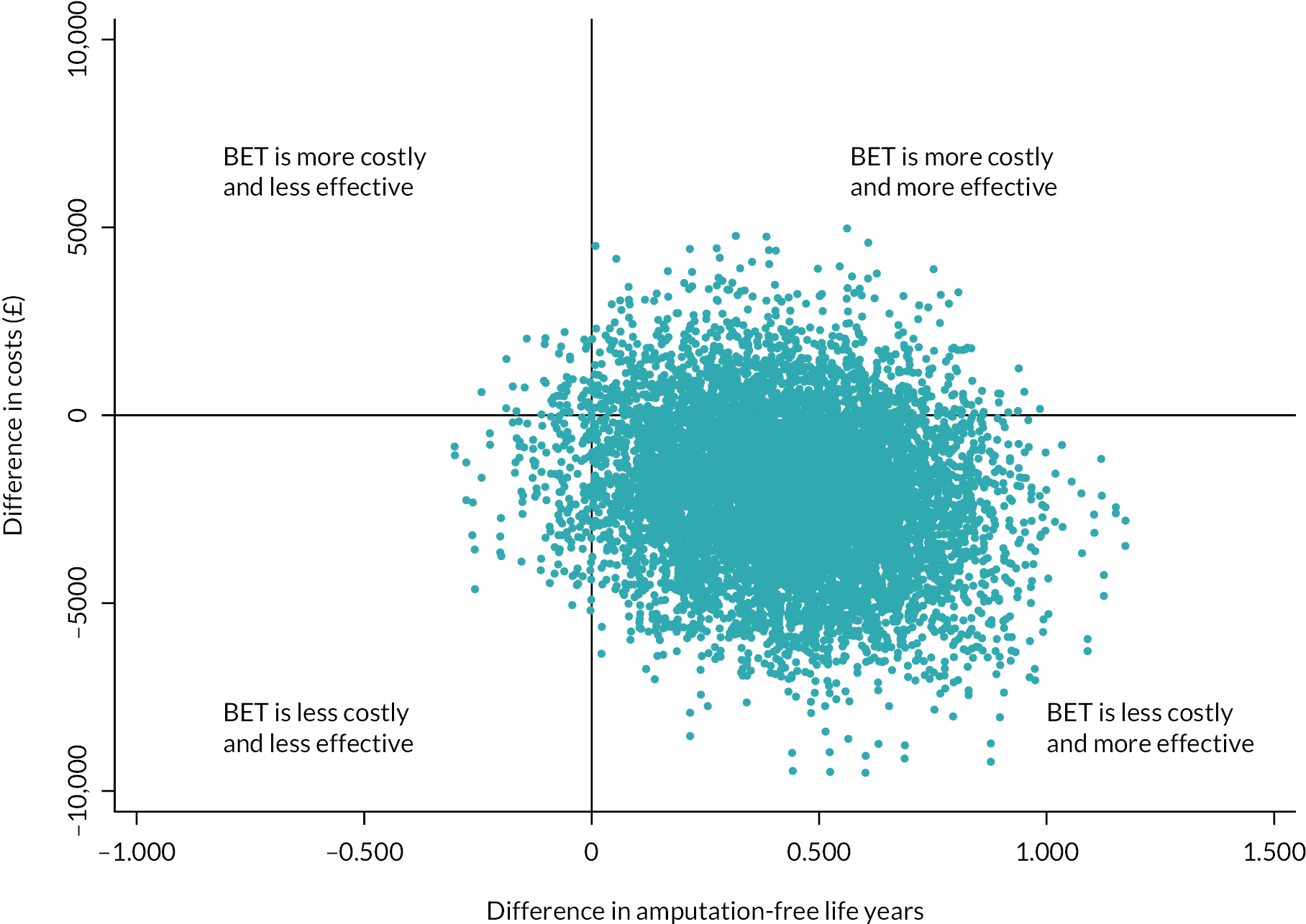
FIGURE 7.
Cost-effectiveness acceptability curve: years alive without amputation including hospital costs only, 7 years after randomisation (base-case analysis – discounted).
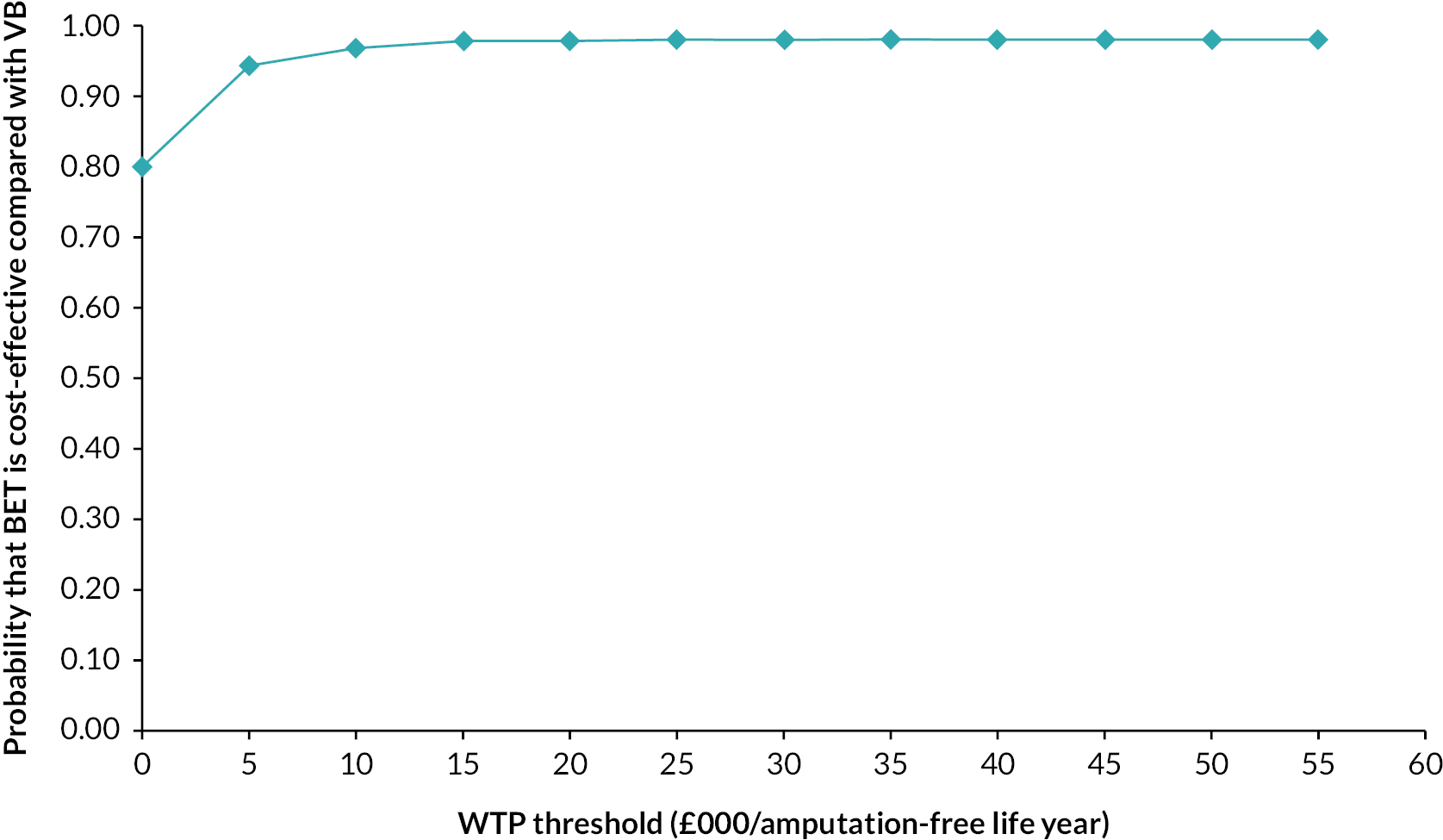
Considering the cost per additional QALY gained, the CE planes of the CUA for 2 and 3 years post randomisation are displayed in Figures 8 and 9, respectively. The figures revealed that BET-first is likely to be less costly and less or more effective when compared with VB-first.
FIGURE 8.
Cost-effectiveness plane (BET-first vs. VB-first): QALY including hospital costs only, 2 years after randomisation (base-case analysis – discounted).

FIGURE 9.
Cost-effectiveness plane (BET-first vs. VB-first): QALY including hospital costs only, 3 years after randomisation (base-case analysis – discounted).
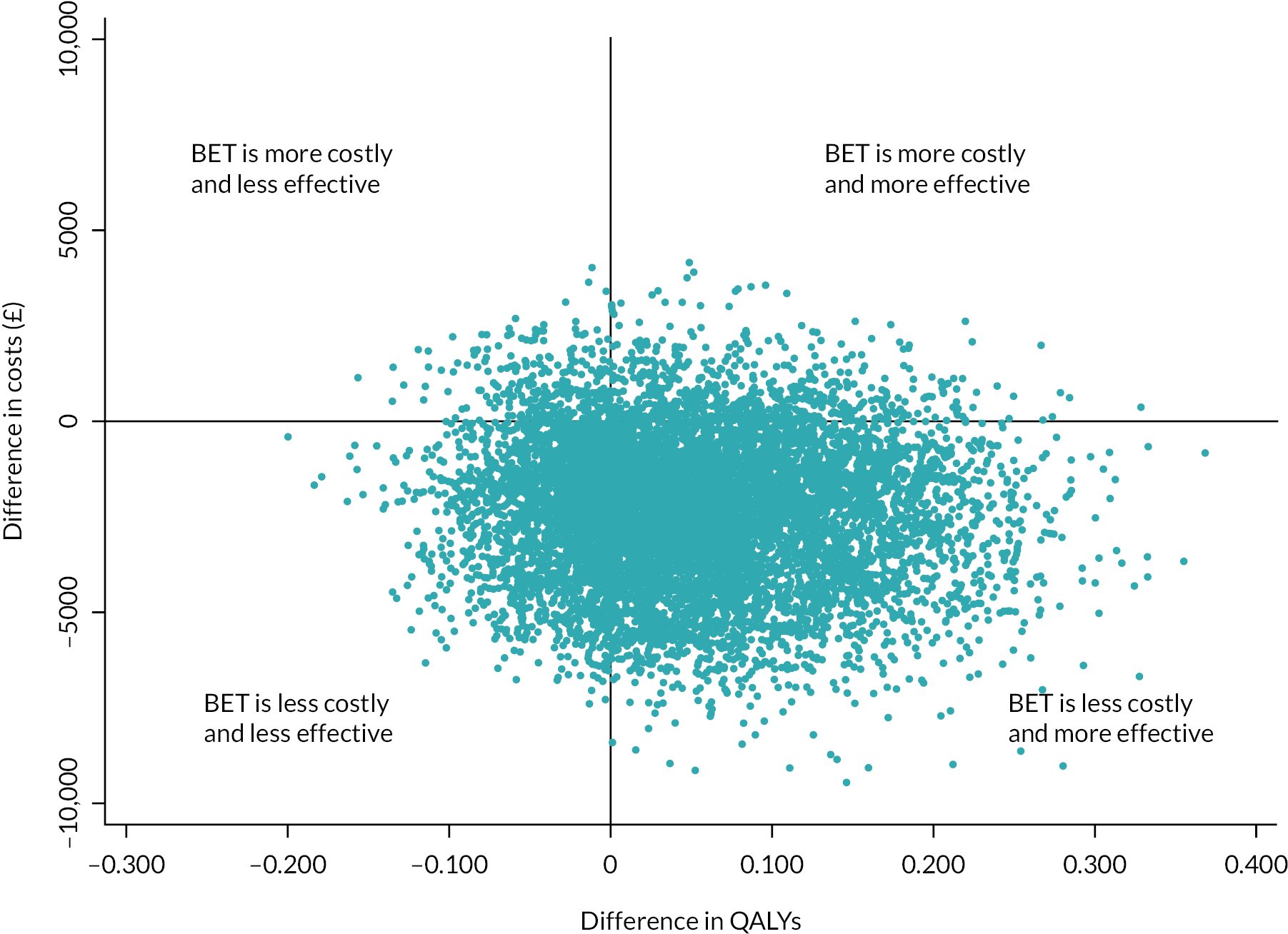
The probability that BET-first is cost-effective at different WTP thresholds over 2 and 3 years is plotted on the CEAC in Figures 10 and 11, respectively. Compared to VB-first, BET-first is likely to be 93% and 89% cost saving at 2 years and 3 years, respectively. When NICE’s WTP threshold of 20,000 is applied, BET-first is 91% cost-effective at 2 years and 94% cost-effective at 3 years.
FIGURE 10.
Cost-effectiveness acceptability curve: QALY including hospital costs only, 2 years post randomisation (base-case analysis – discounted).
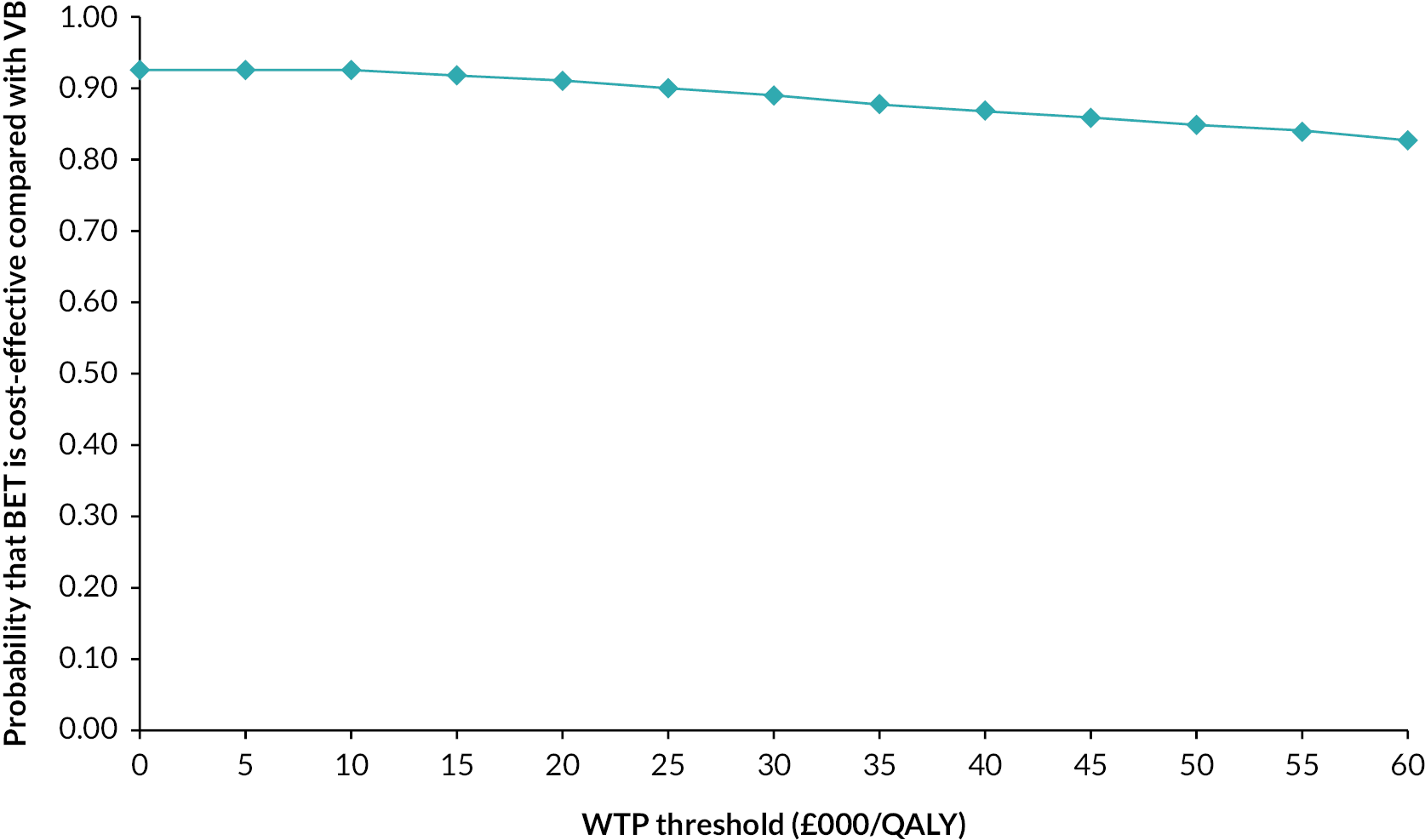
FIGURE 11.
Cost-effectiveness acceptability curve: QALY including hospital costs only, 3 years after randomisation (base-case analysis – discounted).
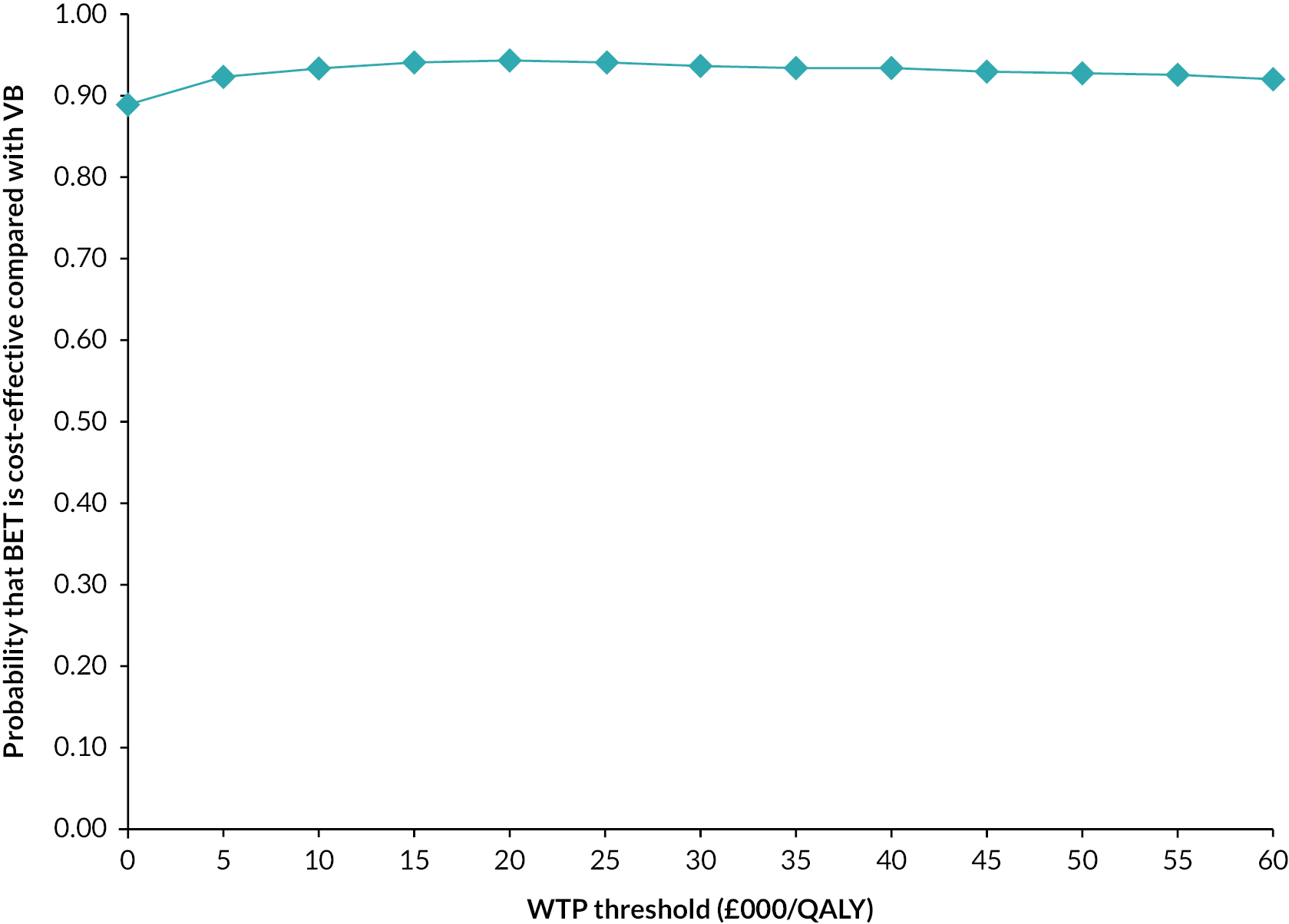
Scenario analyses
Results of different scenario analyses are presented in Table 24. The inclusion of primary health care and other hospital-based services costs, taking the wider societal perspective and considering those who were adherent to the trial intervention, did not change the direction of the findings from the base-case analyses. Overall, BET-first remained a cost-saving and more effective strategy compared with VB-first. When including only patients with complete data, BET-first was still cost saving by £4366 less hospital costs and resulted in additional 0.211 QALY gains. The complete case analysis suggests that the differences in cost and effect (QALY) between BET-first compared to VB-first were statistically significant. The CE plane in Figure 12 shows that a high proportion of the generated point estimates of incremental costs and QALYs using complete data of hospital costs and QALYs imply that BET-first is less costly and more effective than VB-first.
| Mean total (SD) £cost | Incremental £cost (95% CI)a | Mean QALY (SD) | Incremental QALY (95% CI)b | ICER (£cost per QALY) | |
|---|---|---|---|---|---|
| UK NHS perspective [secondary (hospital based), other secondary and primary NHS healthcare costs] | |||||
| VB n = 172 | 17,927.84 (16,366.52) | 0.832 (0.515) | |||
| BET n = 173 | 15,912.35 (15,748.35) | −2078.36 (−5409.00 to 1858.61) | 0.884 (0.515) | 0.016 (−0.083 to 0.116) | Dominantc |
| Societal perspective (all NHS, patient incurred and productivity costs) | |||||
| VB n = 172 | 18,425.47 (16,410.69) | 0.832 (0.515) | |||
| BET n = 173 | 16,036.25 (15,781.83) | −2420.54 (−5791.18 to 1290.36) | 0.884 (0.515) | 0.016 (−0.083 to 0.116) | Dominant |
| Per protocol (adherent) | |||||
| VB n = 145 | 16,338.23 (17,208.12) | 0.863 (0.497) | |||
| BET n = 165 | 13,372.85 (15,712.11) | −3283.94 (−7181.42 to 239.27) | 0.887 (0.523) | 0.038 (−0.082 to 0.146) | Dominant |
| Complete case | |||||
| VB n = 66 | 14,975.07 (13,060.71) | 0.743 (0.523) | |||
| BET n = 66 | 10,412.35 (9986.97) | −4366.45 (−9117.07 to −600.90)d | 0.933 (0.516) | 0.211 (0.043 to 0.382)d | Dominant |
FIGURE 12.
Cost-effectiveness plane: QALY including hospital costs only, 2 years after randomisation (complete case analysis – discounted).

Subgroup analysis
The cost-effectiveness results, associated with the prespecified subgroups, are illustrated in Figure 13. Overall, BET-first is dominant (less costly and more effective) over VB-first in most of the subgroups. However, the results showed that differences in costs and QALYs varied from positive to negative, and this highlights the level of uncertainty over the direction of cost and QALY changes. The differences were also associated with relatively wide CIs, for selected groups,indicating the uncertainty in the size of the estimated QALY gain or loss associated with BET-first relative to VB-first strategy.. In some subgroups (female, those who had unknown intervention to the trial leg, patients with tissue loss only and patients below the age of 60 years), BET-first was dominated by VB-first where it resulted in higher costs and lower QALYs. However, the number of patients in these subgroups was relatively small. In other subgroups, BET-first was more costly and more effective than VB-first and the estimated ICER was above NICE threshold of 20,000 per QALY gained; therefore BET-first was not deemed to be cost-effective. These findings should be interpreted with caution given the level of uncertainty around QALY changes.
FIGURE 13.
Forest plot of BET-first vs. VB-first estimated difference in hospital costs and difference in QALY, 2 years after randomisation for specific subgroups. ICER, incremental cost-effectiveness ratio; dominant, intervention (BET-first) less costly and more effective; dominated, intervention (BET-first) more costly and less effective.
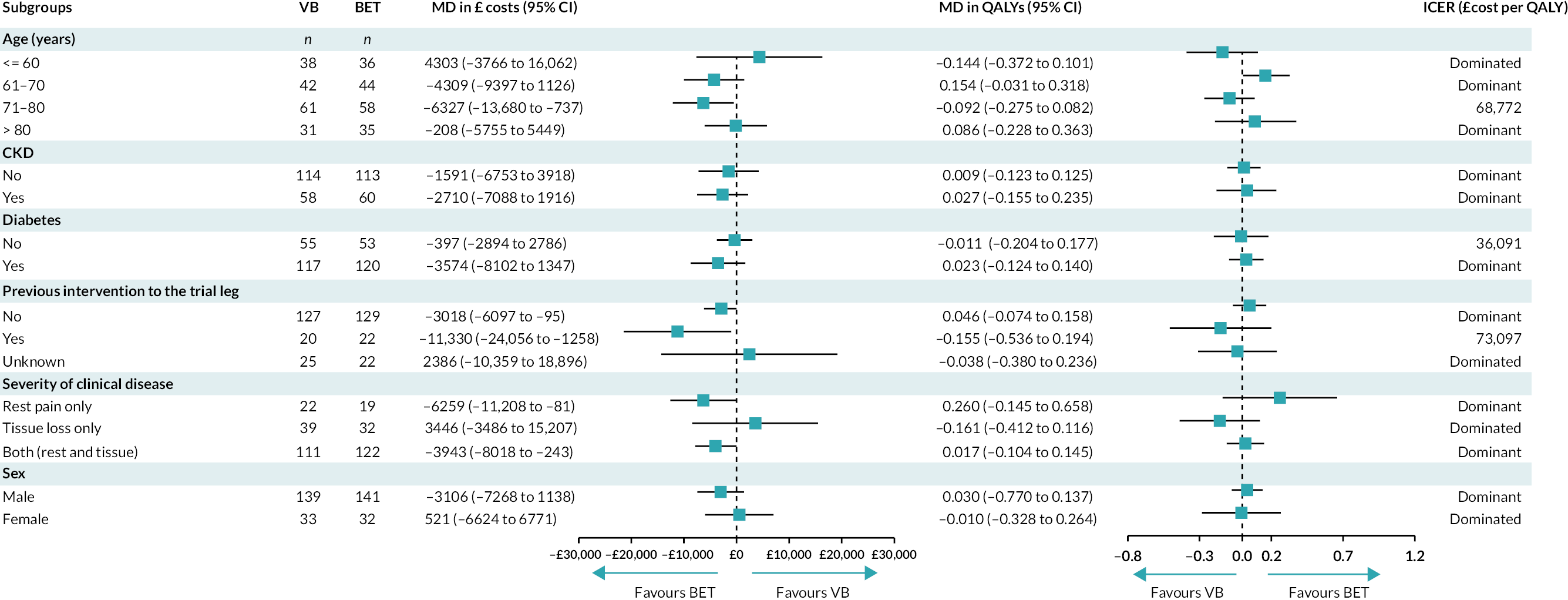
Chapter 5 Discussion
Clinical findings
The BASIL-2 trial shows that, in patients with CLTI who require an IP, with or without an additional more proximal infra-inguinal revascularisation procedure, to restore limb perfusion, a VB-first revascularisation strategy was associated with an approximately one-third increased risk of major amputation or death from any cause compared with a BET-first revascularisation strategy. The difference in AFS was mainly driven by fewer deaths in the BET-first group as limb-based outcomes were similar between the groups. The reported 30-day post-procedural morbidity and mortality rates were not significantly different between the two groups, and the causes of death in the two groups were not unexpected. Further analyses of the BASIL-2 data set and other similar cohorts of patients with CLTI will be required to understand the reasons for the differences observed. However, differences in time to first revascularisation, the timing and nature of additional procedures, and in BMT might be important. 13,54,55 BASIL-2 recruitment was stopped early due to the COVID-19 pandemic. COVID-19 also had a major adverse effect on follow-up, particularly for end points that required face-to-face assessment. However, the study showed no evidence that the observed difference in outcomes between the groups is COVID-19 related.
In 2012, when the BASIL-2 trial was being developed, an IP VB-first revascularisation strategy was hypothesised to be superior to an IP BET-first strategy. This was mainly based on the results of the BASIL-1 trial. 13 However, only around 25% of participants in the BASIL-1 trial had an IP revascularisation. This reflected the fact that at the time (1999–2004) most patients with CLTI were having only more proximal (mainly FP) infra-inguinal revascularisation procedures.
Despite advances in vascular and endovascular techniques and devices, in patients with often heavily calcified IP disease both IP VB and BET are technically challenging. As a result, controversy remains as to whether patients with CLTI, requiring an IP revascularisation and who are suitable for both procedures, should be offered VB- or BET-first. 56 For all these reasons, the results from the BASIL-2 trial have been keenly awaited.
At first glance, this study’s results appear to conflict with the BEST-CLI trial17 which showed that VB in patients with an optimal, single segment GSV (cohort 1), VB was associated with a better outcome, in terms of a composite primary outcome of any MALE or death, than BET. Of note, there were no differences in outcome in participants who did not have an optimal vein for bypass (cohort 2). 17 However, there are many differences between the two trials, importantly including the primary end point. The clinical experience of the BASIL-2 investigators suggests that relatively few patients with CLTI are deemed suitable and have an optimal vein for bypass. Further work is required to determine if BASIL-2 participants are more like patients randomised in cohort 2 of the BEST-CLI trial. Although BASIL-2 and the BEST-CLI trial19 were developed, run and analysed entirely independently, the researchers of this study have collaborated closely with the BEST-CLI trial investigators, and entered into a data-sharing agreement with the BEST-CLI trial investigators before either trial was analysed. A data-sharing agreement will allow an in-depth comparison of the two trials that will hopefully explain why some of the outcomes observed appear to be different. One of the outputs will be an individual-patient-level data meta-analysis. Until this is completed, this study can only speculate as to why the two trials appear to have reached different conclusions in certain respects.
Outcomes for the patients in the BASIL-2 trial were poor [median AFS of 3.8 years (IQR 3.1–4.4)] and not materially different from those reported in the BASIL-1 trial. 57 In both trials, around half of the patients had died by 5 years post randomisation. Although limb salvage rates of around 80% at 5 years seem encouraging, many patients had died and were no longer at risk of limb loss. 58
At randomisation, most BASIL-2 participants were on what has been termed BMT. The severe multilevel atherosclerotic disease that causes CLTI develops over many years. The researchers do not have information on the quality of previous BMT and lifestyle (e.g. stop smoking) interventions. Randomisation, as researchers have described, helps ensure that variations in the quality of previous BMT and lifestyle interventions are equally represented in both groups. Around 20% of patients admitted that they were still smoking and around 70% of patients had (mostly type 2) diabetes of whom around 50% required insulin. At presentation around 90% of the participants had, often quite extensive tissue loss. These baseline data suggest that there may still be missed opportunities in public health and primary care to prevent CLTI through medical therapy and lifestyle interventions, as well as missed opportunities to refer patients to secondary care earlier once CLTI begins to develop. 1,59 Better prevention and timely referral are important as the BASIL-2 trial shows once again that by the time patients present to vascular and endovascular surgeons and interventional radiologists with established CLTI, their prognosis is often poor in terms of major amputation and death regardless of what form of revascularisation they are offered. 60
The BASIL-2 trial has some statistical and clinical limitations. The total number of participants aimed to enrol was not met due to challenges in recruitment, including COVID-19. Since the planned sample size was based on the number of events required, the reduction in participants recruited was mitigated by the increased duration of follow-up. However, as the study did not reach its planned target of event numbers, it is important that the uncertainty in the findings be considered. The uncertainty in the estimate is best judged by the point estimate and the CI of the primary end point [AFS; HR 1.35 (95% CI 1.02 to 1.80)]. This shows that an increase in risk of 35% (HR 1.35) is the most likely value, with increases in risk of 2% (HR 1.02) and 80% (HR 1.80) the least probable points in this range. Although most of this range covers point estimates likely to be considered clinically important differences, it also includes smaller differences. However, it is very unlikely (around a 2% chance) that a VB-first could be more clinical effective than BET-first revascularisation strategy in this patient cohort.
Clinical colleagues will need to consider some important issues when deciding to what extent this study’s findings can be applied to their patients, practice, and healthcare system. Vascular specialists in some countries might be unable to offer BET due to cost. 61 Patients with CLTI, presenting in other countries, might be different in several ways, for example, inter alia age, sex, prevalence of risk factors, and racial background. 3,17 It is also important to note that many patients with CLTI are offered primary amputation or conservative (end of life) care rather than revascularisation; many patients do not have a suitable vein for VB, so they are deemed only suitable for BET; and even patients who are deemed suitable for VB and have a good vein might choose the less invasive endovascular option. 3
Collecting reliable screening data, other than in Birmingham, proved impossible due to changes in the funding model and the need to increase in the number of centres. However, in Birmingham, the researchers established the BASIL prospective cohort study, which includes all (nearly 500) patients with CLTI admitted to Heartlands Hospital during the recruitment period. These data will be the subject of a further report. The researchers hope the prospective cohort study will allow the BASIL-2 findings to be viewed in the context of the CLTI patient population as a whole, as well as help establish the generalisability of the BASIL-2 trial to similar patients who were not recruited to the trial.
Recruiting patients to the BASIL-2 trial was much more difficult than anticipated. The BEST-CLI trial17 also faced similar difficulties despite much greater funding and a larger potential patient population.
As well as many patients being deemed unsuitable for both procedures (especially for VB) for a variety of reasons, an absence of equipoise on the part of clinicians and patients was an important issue. Colleagues explained it was often easier to offer early BET than it was to offer early VB, and easier to obtain imaging confirming suitability for BET. These logistical issues may cause delay to revascularisation, and this can be associated with a worse outcome. 62 In BASIL-2, most patients received their allocated revascularisation procedures in a timely manner that was clinically appropriate for that individual. Nevertheless, the BASIL-2 trial is a pragmatic randomised trial that compares two different revascularisation strategies, rather than two different sets of procedures, in a real-world context of what can be realistically achieved within the national, publicly funded healthcare systems of the UK, Sweden and Denmark. It is important that the BASIL-2 primary comparative analysis be performed, and the results interpreted, on an ITT basis as results in alternative analysis populations may provide biased estimates (since randomisation can no longer preserve balance for known risk factors).
In conclusion, BASIL-2 has shown that, in patients with CLTI who require an IP revascularisation, with or without an additional more proximal infra-inguinal revascularisation procedure, and who are deemed suitable for both VB and BET, randomisation to a VB-first revascularisation strategy led to a 35% increased risk of major amputation or death when compared to a BET-first revascularisation strategy. This difference was mainly driven by fewer deaths in the BET-first revascularisation group as limb-based outcomes were similar between the two groups.
Health economics findings
Summary of findings
In patients with CLTI who required an IP, with or without an additional more proximal infra-inguinal revascularisation procedure, to restore limb perfusion, a BET-first revascularisation strategy was associated with reduced hospital costs (of £1690.34) and improved AFS (0.429 AFLY), out to 7 years following randomisation. BET-first therefore dominated VB-first in the CEA. Similarly, in the CUA, BET-first was cost-saving with improved QALYs (£2524 and £2233 less hospital costs and 0.016 and 0.085 more QALYs at 2 and 3 years, respectively) and so dominated VB-first. This economic analysis therefore shows that BET-first is a cost-effective option from an NHS perspective. The sensitivity analysis supported the base-case analysis and BET-first was found to be cost-effective at different WTP thresholds. Similar findings were found in all other scenario analyses when considering costs of primary and other hospital healthcare services, taking a broader societal perspective, which includes out-of-pocket expenditure and the costs associated with productivity loss and patient’s adherence to study protocol. However, these findings should be interpreted cautiously given the large number of imputed cost values and the substantial probability of a very small QALY difference. The impact of imputation by focusing on participants with complete hospital cost and EQ-5D-5L data only was evaluated and a BET-first revascularisation strategy was also found to be a cost-saving option with improved QALYs.
Hospital costs of BET-first were lower primarily because of a relatively shorter initial hospital stay and cost of the procedure compared with the VB-first group. The main cost driver in both groups was the hospital stay and, overall, procedural and initial hospital stay costs were higher in the VB-first group. This was expected given that VB-first is a more invasive strategy that is associated with more hospital resource use. Other procedural costs (non-bypass and major amputation) were similar in both intervention groups with slightly more cost in the VB-first group. Both types of revascularisation strategies showed an increase in mean EQ-5D scores in the first 6 months after randomisation. Thereafter, there was a substantial decline in EQ-5D-5L scores up until 3 years. This could be linked to the increased number of deaths in both groups. When the capability measurement of ICECAP-O is considered, a slight reduction in the mean score in both groups was observed over the trial period up until 3 years.
The findings from the productivity cost estimation should be treated with caution. Data were only available from a small number of participants, and information on participants’ baseline employment status were not collected; therefore it was not possible to determine if the intervention had a direct impact on time off work or work continuity. BET-first was also a cost-saving intervention in most patient groups, but the subgroup analysis identified differences in relative cost-effectiveness in some patient subgroups. The mean hospital costs were higher and slightly lower QALYs in female patients, those who are below the age of 60 years and had an unknown previous intervention to trial leg and those with tissue loss. However, the number of patients in these specific groups included in the analysis was relatively small. Therefore, these analyses are uncertain and further analysis is required to focus on the relative cost-effectiveness of both interventions in these specific groups.
Strengths and limitations
Few economic evaluation studies have estimated the cost-effectiveness of interventions due to the complexity of these interventions as well as the difficulty in following up the patient treatment journey due to repeated re-admissions and the need for further procedures. Therefore, this economic analysis is the first since BASIL-1 to estimate the cost-effectiveness of revascularisation strategies considering different types of healthcare services and wider costs. Another key strength of this economic analysis lies in the randomised controlled design of the trial, which provided an opportunity to collect comprehensive data on effectiveness and both NHS and societal costs over a 7-year time horizon of the trial. The analysis was also performed using recommended statistical approaches for analysing cost-effectiveness data. For example, the multiple imputation technique was performed on a cost component level for each year after randomisation. This technique distinguishes the uncertainty associated with the missing data and uses characteristics of patients to predict missing values in the imputation model. Moreover, the ICECAP-O questionnaire was used to explore the impact of the intervention on the broader capability. Extensive sensitivity analysis was performed considering different time horizons, perspectives, adherence and subgroup analysis and therefore presents a robust analysis.
However, there were some limitations. Resources were obtained from self-report data over longer periods of time. A limitation with this approach is that respondents could potentially under-report resource utilisation, over a longer period of time. Additionally, the health economics analysis was performed from a UK NHS perspective and therefore used a costing approach with unit costs only from the UK for all participants. However, the main limitation of this approach is that the theoretical relationship between the relative costs and resource use data might be affected. The level of missing data in the cost and outcome data in this patient group is also a limitation. Imputation of up to 60% data was performed at some follow-up time points. In order to evaluate the impact of missing data on the sensitivity of the overall findings, a complete-case analysis was conducted, and findings showed similar results to the imputation led analysis. Inevitably a number of assumptions were made as part of the analysis. However, it is expected that these will impact both groups. A decision model was initially planned to estimate the long-term cost-effectiveness of both strategies. However, due to the relatively long length of follow-up and lack of uncertainty regarding the cost-effectiveness results here within, a decision model was not deemed necessary in this analysis. Models are commonly used with 6 or 12 months follow-up to extrapolate to a longer time horizon. 35
Comparison with other studies
A recent systematic review found that CUA of revascularisation strategies in CLTI to estimate the cost of additional QALY is very limited and evidence to support recommendations for the best strategy is insufficient. 63
A previous related study by Bradbury et al. 13 found similar findings that showed that a first balloon angioplasty strategy was saving costs but with slightly lower QALYs and shorter survival without imputation when compared with a bypass-first revascularisation strategy.
Recommendations for future research
Although the economic analysis conducted provides useful evidence into the costs and effects of revascularisation strategies in the trial period of 2–7 years, further research is required to stipulate evidence on the cost-effectiveness for VB-first and BET-first. For example, alternative methods to estimate the cost of hospital inpatient care without including the cost hospital procedures associated with peripheral vascular disorders could be explored. Additionally, the use of social services could be included in future analysis to assess the impact of different types of revascularisation procedure and prevented amputation on social service utilisation such as the use of institutional care and day care services for adults requiring physical support and home adaptation to facilitate patients’ daily activities.
The collected out-of-pocket expenditure data were mainly on travel costs; however, it is anticipated some patients might use privately funded healthcare services related to rehabilitation and prosthesis following a major amputation. Therefore, future health economic analyses could include other wider cost implications not only on the patients but also on their caregivers. Different revascularisation strategies may also have positive or negative spillover effects on caregivers. It is recommended that future economic evaluations contain caregiver- related costs and quality64 of life using HRQoL outcome measures or carer specific measures, such as the CarerQol. 65
Patient and public involvement
The researchers have been supported throughout the trial by two patient and public involvement (PPI) members who comprised part of their TSC. The researchers engaged with the PPI members throughout, improving their understanding of the needs of patients with CLTI. PPI members commented on all patient-facing materials to ensure that they were clear and comprehensive. The researchers organised collaboration days during the course of the trial and the PPI representatives attended these meetings. The researchers will engage with their PPI members regarding the dissemination of their results. Any future research groups, taking forward the research recommendations from this project, would benefit from engaging with PPI representatives.
Equality, diversity and inclusion
As this trial had restrictive inclusion criteria, the researchers were unable to target specific under-represented groups. However, they were open to recruitment across 41 centres in Scotland, England, Wales, Sweden and Denmark, some rural and some inner city, and the ethnic diversity of the principal investigators (PIs) and research staff at these sites was broad. Of the 345 participants recruited to this trial, 280 were male (81%), 315 were white (92%); 10 were Asian (3%); 17 were black (4%) and 3 (1%) were from other ethnic groups or declined to provide information. Data from the BASIL prospective cohort study, which includes all (nearly 500) patients with CLTI admitted to Heartlands Hospital during the BASIL-2 recruited period, showed a similar demographic composition of ethnicity and gender which suggests that the BASIL-2 population was generalisable to the CLTI population as a whole (BASIL prospective cohort study awaiting publication).
Additional information
CRediT contribution statement
Catherine A Moakes (https://orcid.org/0000-0002-0473-0532): Data curation (equal), Formal analysis (lead), Methodology (supporting), Writing – original draft (lead), Writing – editing and reviewing (lead), Visualisation (equal), Writing – original draft (supporting).
Andrew W Bradbury (https://orcid.org/0000-0003-0530-9667): Conceptualisation (lead), Funding acquisition (lead); Methodology (lead), Writing – editing and reviewing (supporting), Visualisation (equal), Writing – original draft (supporting).
Zainab Abdali (https://orcid.org/0000-0002-2736-5427): Formal analysis (lead), Writing – editing and reviewing (supporting), Visualisation (equal), Writing – original draft (supporting).
Gareth R Bate (https://orcid.org/0009-0005-3084-070X): Conceptualisation (supporting), Data curation (equal), Funding acquisition (supporting), Methodology (supporting), Visualisation (equal), Writing – original draft (supporting).
Jack Hall (https://orcid.org/0000-0002-3016-7624): Data curation (equal), Validation (lead), Visualisation (equal), Writing – original draft (supporting).
Hugh Jarrett (https://orcid.org/0000-0001-7374-3700): Data curation (equal), Project administration (equal), Resources (equal), Supervision (equal), Visualisation (equal), Writing – original draft (supporting).
Lisa Kelly (https://orcid.org/0009-0000-0105-1967): Data curation (equal), Visualisation (equal), Writing – original draft (supporting).
Jesse Kigozi (https://orcid.org/0000-0001-7608-4923): Validation (lead), Visualisation (equal), Writing – original draft (supporting).
Suzanne Lockyer (https://orcid.org/0009-0000-9188-2261): Data curation (equal), Project administration (equal), Resources (equal), Visualisation (equal), Writing – original draft (supporting).
Lewis Meecham (https://orcid.org/0000-0003-4698-7333): Methodology (supporting), Visualisation (equal), Writing – original draft (supporting).
Smitaa Patel (https://orcid.org/0000-0002-4997-3829): Conceptualisation (supporting), Data curation (equal), Funding acquisition (supporting), Methodology (supporting), Visualisation (equal), Writing – original draft (supporting).
Matthew Popplewell (https://orcid.org/0000-0002-6239-4039): Methodology (supporting), Visualisation (equal), Writing – original draft (supporting).
Gemma Slinn (https://orcid.org/0000-0003-1096-4148): Project administration (equal), Resources (equal), Supervision (equal), Visualisation (equal), Writing – original draft (supporting).
Jonathan J Deeks (https://orcid.org/0000-0002-8850-1971): Conceptualisation (supporting), Funding acquisition (supporting), Methodology (supporting), Visualisation (equal), Writing – original draft (supporting).
Acknowledgements
The researchers would like to thank all the participants who agreed to take part in this study, without whom this would not have been possible.
Additional Birmingham Clinical Trials Unit staff
Samir Mehta, University of Birmingham
Karim Jafri, University of Birmingham
Tinatin Griffin, University of Birmingham
Lucy Casley, University of Birmingham
Joe Humphries, University of Birmingham
Hetal Bhatt, University of Birmingham
Rebecca Record, University of Birmingham
Manmeet Sehejpal, University of Birmingham
Adrian Wilcockson, University of Birmingham
Nicholas Hilken, University of Birmingham
Recruiting site PIs and support staff
BASIL-2 has been supported by a large network of sites, PIs and research nurses, the researchers of this study are extremely grateful for their support.
Recruiting site principal investigators and support staff
| Trust | PI | Site research team |
|---|---|---|
| Sodersjukhuset, Sweden | Dr Jonas Malmstedt | Caroline Larsson |
| Hull and East Yorkshire NHS Trust | Professor Ian Chetter | Academic Vascular team |
| Guy’s and St Thomas’ NHS FT | Mr Hany Zayed | Eleanor Davis |
| Black Country Vascular Unit (Russell’s Hall Hospital) | Mr Simon Hobbs | |
| University Hospitals Birmingham NHS FT (Heartlands Hospital) | Professor Andrew Bradbury | Gareth Bate Lisa Kelly |
| Leeds Teaching Hospitals NHS Trust | Professor Julian Scott | Joanne Fletcher |
| Manchester University NHS FT | Mr Tawqeer Rashid Dr Ray Ashleigh Dr Stephen Butterfield |
Siva Saranya Schvearn Allen |
| Kolding Hospital of University of South Denmark | Dr Kim Houlind | Anita Schrader |
| Aneurin Bevan University Health Board | Dr Rebecca Wallace Mr Christopher Twine |
Laura Smith Nancy Hawkings |
| Royal Free London NHS FT | Mr Toby Richards Mr Lim Chung |
Yvonne Gleeson |
| East Kent Hospitals NHS FT | Mr Thomas Rix | David Loader |
| University Hospitals of Leicester NHS Trust | Mr Robert Davies | Sally Barton |
| NHS Greater Glasgow and Clyde | Mr Wesley Stuart | Lesley Gilmour |
| Frimley Health NHS FT | Mr Patrick Chong | Andrea Croucher |
| University Hospitals Coventry and Warwickshire | Professor Chris Imray | Alex Sergiou |
| North Bristol NHS Trust | Mr William Neary | Jane Ashby-Styles |
| Imperial College Healthcare NHS Trust | Professor Alun Davies | Caitlin Carr |
| St George’s Healthcare NHS Trust | Mr Robert Hinchliffe Mr Peter Holt |
Ayomide Salau and Adam Barton |
| Newcastle upon Tyne Hospitals NHS FT | Professor Gerard Stansby | Martin Catterson and Noala Parr |
| Oxford University Hospitals NHS FT | Dr Raman Uberoi | Juliana Umunna |
| Royal Bournemouth And Christchurch NHS Trust | Mr Lasantha Wijesinghe | Lindsay Rogers |
| United Lincolnshire Hospitals NHS Trust | Mr Nityanand Arya | Kimberley Netherton |
| Sheffield Teaching Hospitals NHS Foundation Trust | Dr Stephen Goode | Helen Newell |
| University Hospitals Birmingham NHS FT (Queen Elizabeth Hospital) |
Mr Rajiv Vohra Mr Radu Rogoveanu |
Gareth Bate Lisa Kelly |
| Sandwell and West Birmingham NHS Trust | Mrs Rachel Sam | |
| East Suffolk and North Essex NHS FT | Mr Sohail Choksy | Alison O’Kelly |
| Cambridge University Hospitals NHS FT | Mr Majit Gohel | Debbie Read |
| North Cumbria University Hospitals NHS Trust | Mr Thomas Joseph Mr Ron Eifell |
Toni Wilson |
| Doncaster and Bassetlaw Hospitals NHS FT | Mr Woolagasen Pillay | Rebecca Pugh |
| Worcestershire Acute Hospitals NHS Trust | Mr Isaac Nyamekye | |
| Northern Care Alliance NHS FT | Mr Damian Kelleher Mr Georgios Antoniou |
Sarah Warran |
| University Hospitals Sussex NHS FT | Dr Mario Caruana | Laura Ortiz-Ruiz de Gordoa |
| NHS Tayside | Mr Murray Flett | Jennifer Taylor |
| Royal Cornwall Hospitals NHS Trust | Dr Harvey Chant Miss Rachel Barnes |
Suzanne Dean |
| Bradford Teaching Hospitals NHS FT | Mr Kevin Mercer | Jackie Todd |
| York Teaching Hospitals NHS FT | Mr Marco Baroni | Victoria Jones |
| University Hospital Southampton NHS FT | Miss Nandita Pal | Tomas Hannam-Penfold |
| Portsmouth Hospitals NHS Trust | Mr Mark Pemberton | Elizabeth Hawes |
| London North West University Healthcare NHS Trust | Mr Tahir Hussain | Sean Connarty |
| NHS Forth Valley | Mr Mike Yapanis | Stephanie Brogan |
| Bart’s Health NHS Trust | Mr Sandip Sarkar | Vish Ramatour |
Data Monitoring Committee, Trial Steering Committee and patient and public involvement representatives
BASIL-2 has been supported by a TSC and DMC and the researchers would like to express their sincere thanks for all guidance, expertise and advice. TSC members included: Professor Jonathan Michaels (Chair, Honorary Professor of Clinical Decision Science, ScHARR, University of Sheffield); Dr Nick Latimer (Senior Research Fellow, Health Economics, ScHARR, University of Sheffield); Mr Andrew Beech (Chief Vascular Scientist, University Hospitals of Nottingham); Mr James Griffin (Research Associate, University of Warwick); Mr Martin Fox (Vascular Specialist Podiatrist); Professor Jon Moss (Professor of Interventional Radiology, University of Glasgow); Professor Frances Game (Consultant Diabetologist, Derby Teaching Hospitals) and Lisa Smith (Clinical Nurse Specialist, Northern Care Alliance). The DMC members included: Professor Charles McCollum (chair, Professor of Surgery, University of Manchester); Dr Richard Jackson (Senior Statistician, Liverpool Cancer Trials Unit); Dr Samin Chakraverty (retired Interventional Radiologist); Professor Doug Altman (former chair, Director of Centre for Statistics in Medicine and Cancer Research, University of Oxford); Professor Peter Gaines (former member, Professor of Vascular Interventional Radiology, Sheffield Vascular Institute) and Dr Michael Flynn (former member, Consultant Diabetologist, Kent and Canterbury Hospital). The researchers would like to personally thank our PPI representatives of our TSC: Mr Barry Attwood and Mr Peter Maufe.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted after review.
Ethics statement
The BASIL-2 trial received ethical approval from the West Midlands Research Ethics Committee (reference 14/WM/0057) on 4 March 2014. Study registration: ISRCTN 27728689.
Information governance statement
The University of Birmingham is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, the University of Birmingham is the Data Controller, and you can find out more about how the researchers handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://www.birmingham.ac.uk/privacy.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/YTFV4524.
Primary conflicts of interest: Andrew W Bradbury has the following memberships: HTA Efficient Study Designs – 2, HTA Efficient Study Designs Board, HTA IP Methods Group, HTA IP Panel, HTA Surgery Themed Call Board, HTA Prioritisation Committee B (In hospital), HTA Remit and Competitiveness Group, HTA Prioritisation Committee B Methods Group, HTA Post-Funding Committee teleconference (POC members to attend) and HTA Programme Oversight Committee.
Jonathan J Deeks has the following memberships: DTSP Methods Group Teleconference, HTA Additional Capacity Funding Board, HTA Efficient Study Designs – 2, HTA End of Life Care and Add-on Studies, HTA Medical Tests Methods Group, HTA Primary Care Themed Call board, HTA Primary Care Themed Call Remit meeting and PGfAR EOIs – HTA projects Remit meeting.
Publication
Bradbury AW, Moakes CA, Popplewell M, Meecham L, Bate GR, Kelly L, et al. A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (BASIL-2): an open-label, randomised, multicentre, phase 3 trial. Lancet 2023;401:1798–809.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Bradbury AW, Moakes CA, Popplewell M, Meecham L, Bate GR, Kelly L, et al. A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (BASIL-2): an open-label, randomised, multicentre, phase 3 trial. The Lancet 2023;401:1798-809.
- Barraclough K, Bradbury A. Chronic limb threatening ischaemia. BMJ 2018;360.
- Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. GVG Writing Group for the Joint Guidelines of the Society for Vascular Surgery (SVS), European Society for Vascular Surgery (ESVS), and World Federation of Vascular Societies (WFVS) . Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019;58:S1-109.e33.
- Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health 2019;7:e1020-30.
- Bradbury AW, Popplewell M. Rutherford’s Vascular Surgery and Endovascular Therapy. Philadelphia, PA: Elsevier; 2019.
- Enzmann FK, Nierlich P, Aspalter M, Hitzl W, Dabernig W, Hölzenbein T, et al. Nitinol stent versus bypass in long femoropopliteal lesions: 2-year results of a randomised controlled trial. JACC Cardiovasc Interv 2019;12:2541-9.
- Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. ESC Scientific Document Group . 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke Organization (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763-816.
- Lawrence PF, Gloviczki P. Global Vascular Guidelines for patients with critical limb-threatening ischemia. J Vasc Surg 2019;69:1653-4.
- Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, et al. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg 2016;63:3S-21S.
- Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011;124:2020-45.
- Popplewell MA, Andronis L, Davies HOB, Meecham L, Kelly L, Bate G, et al. Procedural and 12-month in-hospital costs of primary infra-popliteal bypass surgery, infra-popliteal best endovascular treatment, and major lower limb amputation for chronic limb threatening ischemia. J Vasc Surg 2022;75:195-204.
- Shan LL, Yang LS, Tew M, Westcott MJ, Spelman TD, Choong PF, et al. Quality of life in chronic limb threatening ischaemia: systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2022;64:666-83.
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial. Health Technol Assess 2010;14:1-210, iii.
- Simons JP, Schanzer A, Flahive JM, Osborne NH, Mills JL, Bradbury AW, et al. Survival prediction in patients with chronic limb-threatening ischemia who undergo infrainguinal revascularization. Eur J Vasc Endovasc Surg 2019;58:S120-34.e3.
- Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. BASIL trial participants . Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366:1925-34.
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, et al. BASIL trial participants . Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomised to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg 2010;51:5S-17S.
- Farber A, Menard MT, Conte MS, Kaufman JA, Powell RJ, Choudhry NK, et al. BEST-CLI Investigators . Surgery or endovascular therapy for chronic limb-threatening ischemia. N Engl J Med 2022;387:2305-16.
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, et al. BASIL trial participants . Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial: a description of the severity and extent of disease using the Bollinger angiogram scoring method and the TransAtlantic Inter-Society Consensus II classification. J Vasc Surg 2010;51:32S-42S.
- Kodama A, Meecham L, Popplewell M, Bate G, Conte MS, Bradbury AW. Relationship between the global anatomic staging system (GLASS) and clinical outcomes after bypass surgery and endovascular therapy for chronic limb threatening ischaemia in the bypass versus angioplasty in severe ischaemia of the leg (BASIL)-1 trial. Eur J Vasc Endovasc Surg 2020;60:687-95.
- Popplewell MA, Davies HOB, Narayanswami J, Renton M, Sharp A, Bate G, et al. A comparison of outcomes in patients with infrapopliteal disease randomised to vein bypass or plain balloon angioplasty in the bypass vs. angioplasty in severe ischaemia of the leg (BASIL) Trial. Eur J Vasc Endovasc Surg 2017;54:195-201.
- National Institute of Health and Care Excellence . Peripheral Arterial Disease: Diagnosis and Management 2012. www.nice.org.uk/guidance/cg147 (accessed 24 January 2014).
- Popplewell MA, Davies H, Jarrett H, Bate G, Grant M, Patel S, et al. BASIL-2 Trial Investigators . Bypass versus angio plasty in severe ischaemia of the leg – 2 (BASIL-2) trial: study protocol for a randomised controlled trial. Trials 2016;17.
- NICE . Quality Statement 1: Identification and Monitoring n.d. www.nice.org.uk/guidance/qs5/chapter/Quality-statement-1-Identification-and-monitoring (accessed 24 January 2014).
- Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev 2004;20:S90-5.
- van Reijen NS, Ponchant K, Ubbink DT, Koelemay MJW. Editor’s choice – the prognostic value of the wifi classification in patients with chronic limb threatening ischaemia: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2019;58:362-71.
- Austin PC, Fine JP. Accounting for competing risks in randomized controlled trials: a review and recommendations for improvement. Stat Med 2017;36:1203-9.
- Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol 2013;66:648-53.
- Brown H, Prescott R. Applied mixed models in medicine. John Wiley & Sons Inc; 2006.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 1976;34:585-612.
- DAMOCLES Study Group, NHS Health Technology Assessment Programme . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9] n.d. www.nice.org.uk/article/pmg9/chapter/foreword (accessed 28 February 2023).
- Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value Health 2022;25:10-31. https://doi.org/10.1016/j.jval.2021.10.008.
- NHS National Schedule of Reference Costs 2019 20 n.d. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/ (accessed 1 March 2023).
- Jones KC, Weatherly H, Birch S, Castelli A, Chalkley M, Dargan A, et al. Unit Costs of Health and Social Care 2022 Manual 2022. https://doi.org/10.22024/UniKent/01.02.100519 (accessed 2 March 2023).
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Annual Survey of Hours and Earnings 2022 n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours (accessed 10 April 2023).
- Krol M, Brouwer W. How to estimate productivity costs in economic evaluations. PharmacoEconomics 2014;32:335-44.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Value Set for England 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 2 March 2023).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15.
- Jensen CE, Sørensen SS, Gudex C, Jensen MB, Pedersen KM, Ehlers LH. The Danish EQ-5D-5L value set: a hybrid model using cTTO and DCE data. Appl Health Econ Health Policy 2021;19:579-91.
- Billingham LJ, Abrams KR. Simultaneous analysis of quality of life and survival data. Stat Methods Med Res 2002;11:25-48.
- Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JJ, et al. Valuing the ICECAP capability index for older people. Soc Sci Med 2008;67:874-82. https://doi.org/10.1016/j.socscimed.2008.05.015.
- Grewal I, Lewis J, Flynn T, Brown J, Bond J, Coast J. Developing attributes for a generic quality of life measure for older people: preferences or capabilities?. Soc Sci Med 2006;62:1891-901. https://doi.org/10.1016/j.socscimed.2005.08.023.
- Barber JA, Thompson SG. Analysis and interpretation of cost data in randomised controlled trials: review of published studies. BMJ 1998;317:1195-200. https://doi.org/10.1136/bmj.317.7167.1195.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991;10:585-98. https://doi.org/10.1002/sim.4780100410.
- Her Majesty’s Treasury . The Green Book n.d. www.gov.uk/government/publications/the-green-book-appraisal-and-evaluation-in-central-governent/the-green-book-2020#a6-discounting (accessed 15 April 2023).
- Forbes JF, Adam DJ, Bell J, Fowkes FGR, Gillespie I, Raab GM, et al. BASIL trial participants . Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: health-related quality of life outcomes, resource utilization, and cost-effectiveness analysis. J Vasc Surg 2010;51:43S-51S.
- Margolis J, Barron JJ, Grochulski D. Health care resources and costs for treating peripheral artery disease in a managed care population: results from analysis of administrative claims data. J Manag Care Pharm 2005;11:727-34.
- Glick HA, Briggs AH, Polsky D. Quantifying stochastic uncertainty and presenting results of cost-effectiveness analyses. Expert Rev Pharmacoecon Outcomes Res 2001;1:25-36. https://doi.org/10.1586/14737167.1.1.25.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-4. https://doi.org/10.1177/0272989X9001000308.
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. https://doi.org/10.1002/hec.4730030505.
- Birmpili P, Johal A, Li Q, Waton S, Chetter I, Boyle JR, et al. Factors associated with delays in revascularization in patients with chronic limb-threatening ischaemia: population-based cohort study. Br J Surg 2021;108:951-9.
- Meecham L, Patel S, Bate GR, Bradbury AW. Editor’s choice – a comparison of clinical outcomes between primary bypass and secondary bypass after failed plain balloon angioplasty in the bypass versus angioplasty for severe ischaemia of the limb (BASIL) trial. Eur J Vasc Endovasc Surg 2018;55:666-71.
- Patel SD, Biasi L, Paraskevopoulos I, Silickas J, Lea T, Diamantopoulos A, et al. Comparison of angioplasty and bypass surgery for critical limb ischaemia in patients with infrapopliteal peripheral artery disease. Br J Surg 2016;103:1815-22.
- Popplewell MA, Davies HOB, Renton M, Bate G, Patel S, Deeks JJ, et al. Comparison of outcomes following infrapopliteal plain balloon angioplasty in the BASIL trial (1999–2004) and in a contemporary series (2009–2013). Vasc Endovascular Surg 2020;54:141-6.
- Miyata T, Kumamaru H, Mii S, Kinukawa N, Miyata H, Shigematsu K, et al. Prediction models for two year overall survival and amputation free survival after revascularisation for chronic limb threatening ischaemia. Eur J Vasc Endovasc Surg 2022;64:367-76.
- Nickinson ATO, Coles B, Zaccardi F, Gray LJ, Payne T, Bown MJ, et al. Missed opportunities for timely recognition of chronic limb threatening ischaemia in patients undergoing a major amputation: a population based cohort study using the UK’s clinical practice research datalink. Eur J Vasc Endovasc Surg 2020;60:703-10.
- Popplewell MA, Davies HOB, Meecham L, Bate G, Bradbury AW. Comparison of clinical outcomes in patients selected for infra-popliteal bypass or plain balloon angioplasty for chronic limb threatening ischemia between 2009 and 2013. Vasc Endovascular Surg 2020;55.
- Wijeyaratne M, Cassim R, Bradbury A, Hyrin A, Jayawickrema B, Weerasekera A, et al. Clinical outcomes following lower extremity vein bypass for chronic limb-threatening ischaemia (CLTI) at the University of Colombo, Sri Lanka. Eur J Vasc Endovasc Surg 2020;60:560-6.
- Li Q, Birmpili P, Johal AS, Waton S, Pherwani AD, Boyle JR, et al. Delays to revascularization for patients with chronic limb-threatening ischaemia. Br J Surg 2022;109:717-26.
- Shan LL, Wang J, Westcott MJ, Tew M, Davies AH, Choong PF. A systematic review of cost–utility analyses in chronic limb-threatening ischemia. Ann Vasc Surg 2022;85:9-21.
- Al-Janabi H, van Exel J, Brouwer W, Coast J. A framework for including family health spillovers in economic evaluation. Med Decis Making 2016;36:176-86.
- Brouwer WB, van Exel NJ, van Gorp B, Redekop WK. The CarerQol instrument: a new instrument to measure care-related quality of life of informal caregivers for use in economic evaluations. Qual Life Res 2006;15:1005-21.
- National Institute for Clinical Excellence . Drug-Eluting Stents for the Treatment of Coronary Artery Disease. Part Review of NICE Technology Appraisal Guidance, 71 2008. www.nice.org.uk/Guidance/TA152 (accessed 4 April 2023).
Appendix 1
| Unit cost | Description | Source | |
|---|---|---|---|
| Primary healthcare (community-based) services | |||
| GP at practice | £42 | 9.22 minutes contact including direct care with qualification | PSSRU 202233 |
| Nurse at practice | £13.43 | 15.5 minutes contact with qualification | PSSRU 202233 |
| District nurse | £29 | Specialised nurse (band 6), 30-minute appointment | PSSRU 202233 |
| Vascular/diabetic nurse | £27 | Hospital-based specialised nurse (band 6), 30-minute appointment | PSSRU 202233 |
| Physiotherapist | £28 | Community-based scientific and professional staff average of band 6, 30-minute appointment | PSSRU 202233 |
| Occupational therapist | £25 | Community occupational therapist (local authority), 30-minute appointment | PSSRU 202233 |
| Chiropodist/podiatrist | £28 | Community-based scientific and professional staff average of band 6, 30-minute appointment | PSSRU 202233 |
| Carer | £101 | Local authority own-provision day care for adults requiring physical support (age 18–64) | PSSRU 202233 |
| Secondary healthcare (hospital-based) services a | |||
| Hospital stay (per day) | |||
| General ward | £428 | Weighted average of peripheral vascular disorders, YQ50A to YQ50F – non-elective long inpatient stay | NHS reference cost 2019–2032 |
| HDU/ITU | £1372 | Non-specific, general adult critical care patients predominate, XC06Z (adult critical care, 1 organ supported) | NHS reference cost 2019–2032 |
| Day case | £545 | Weighted average of peripheral vascular disorders, YQ50A to YQ50F – non-elective long inpatient stay | NHS reference cost 2019–2032 |
| Surgical procedure (human resource) | |||
| Vascular bypass | £2681 | Average cost | Popplewell et al. 202210 |
| Best endovascular/non-bypass revascularisation procedures (thrombectomy, thrombolysis and endarterectomy) | £384 | Average cost | Popplewell et al. 202210 |
| Major amputation | £1271 | Average cost | Popplewell et al. 202210 |
| Non-revascularisation procedure (wound debridement and fasciotomy) | £396 | Weighted average of infection or inflammatory reaction, due to, internal orthopaedic prosthetic devices, implants or grafts, HE81A to HE81C – day case | NHS reference cost 2019–2032 |
| Outpatient visit | |||
| General outpatient | £140 | Weighted average of all outpatient attendances (consultant and non-consultant led attendances) | NHS reference cost 2019–2032 |
| Diagnostic imaging | |||
| US | £64 | Vascular US | NHS reference cost 2019–2032 |
| CT scan | £99 | General CT, DIM001 – outpatient | NHS reference cost 2019–2032 |
| MRA | £182 | Cost of MRI was used, general magnetic resonance imaging, DIM004 – outpatient | NHS reference cost 2019–2032 |
| DSA | £117 | Fluoroscopy, DIM003 – outpatient | NHS reference cost 2019–2032 |
| X-ray | £30 | NHS reference cost 2019–2032 | |
| Endovascular devices | |||
| BMS | £749 | Assumption – cost of BMS is £300 less than DES | NICE 200866 |
| DCB | £1200 | Drug-eluting peripheral angioplasty balloon, DEV11 | NHS reference cost 2019–2032 |
| DES | £1049 | Peripheral vascular stents (includes peripheral vascular DESs), DEV23 | NHS reference cost 2019–2032 |
| Productivity | |||
| Male – full time employee | £809.6 | Average weekly pay | ASHE 202235 |
| Female – full time employee | £697.10 | Average weekly pay | ASHE 202235 |
| Male – part time employee | £257.60 | Average weekly pay | ASHE 202235 |
| Female – part time employee | £270.00 | Average weekly pay | ASHE 202235 |
| Follow-up time point | VB-first | BET-first | Mean adjusteda bootstrapped difference (95% CI) |
|---|---|---|---|
| Mean score (SD) | Mean score (SD) | ||
| Baseline | |||
| n | 159 | 164 | 0.066b (0.001 to 0.134)c |
| Mean (SD) | 0.377 (0.327) | 0.433 (0.297) | |
| 1 month | |||
| n | 130 | 135 | −0.015 (−0.083 to 0.056) |
| Mean (SD) | 0.473 (0.319) | 0.500 (0.302) | |
| 6 months | |||
| n | 109 | 109 | −0.010 (−0.084 to 0.068) |
| Mean (SD) | 0.539 (0.276) | 0.557 (0.305) | |
| 12 months | |||
| n | 105 | 100 | 0.003 (−0.079 to 0.087) |
| Mean (SD) | 0.558 (0.309) | 0.578 (0.298) | |
| 24 months | |||
| n | 76 | 82 | 0.026 (−0.059 to 0.112) |
| Mean (SD) | 0.559 (0.290) | 0.596 (0.250) | |
| 36 months | |||
| n | 51 | 63 | 0.118 (0.004 to 0.0238)c |
| Mean (SD) | 0.467 (0.358) | 0.614 (0.258) | |
| Follow-up time point | VB-first | BET-first | Mean adjusteda bootstrapped difference (95% CI) |
|---|---|---|---|
| Mean score (SD) | Mean score (SD) | ||
| Baseline | |||
| n | 150 | 159 | 0.048b (0.005 to 0.095)c |
| Mean (SD) | 0.693 (0.221) | 0.737 (0.207) | |
| 1 month | |||
| n | 118 | 132 | −0.005 (−0.058 to 0.046) |
| Mean (SD) | 0.704 (0.229) | 0.734 (0.202) | |
| 6 months | |||
| n | 105 | 108 | −0.031 (−0.083 to 0.018) |
| Mean (SD) | 0.741 (0.212) | 0.747 (0.198) | |
| 12 months | |||
| n | 100 | 80 | −0.032 (−0.096 to 0.032) |
| Mean (SD) | 0.738 (0.224) | 0.745 (0.172) | |
| 24 months | |||
| n | 75 | 80 | −0.025 (−0.077 to 0.027) |
| Mean (SD) | 0.762 (0.183) | 0.745 (0.172) | |
| 36 months | |||
| n | 50 | 61 | 0.048 (−0.027 to 0.138) |
| Mean (SD) | 0.724 (0.236) | 0.781 (0.172) | |
List of abbreviations
- ABPI
- ankle–brachial pressure index
- AFS
- amputation-free survival
- ASHE
- Annual Survey of Hours and Earnings
- BEST-CLI
- BEst Surgical Therapy in patients with Chronic Limb threatening Ischaemia
- BET
- best endovascular treatment
- BMS
- bare metal stent
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CKD
- chronic kidney disease
- CLI
- critical limb ischaemia
- CLTI
- chronic limb-threatening ischaemia
- CUA
- cost–utility analysis
- DCB
- drug-coated balloon
- DES
- drug-eluting stent
- DM
- diabetes mellitus
- DMC
- Data Monitoring Committee
- eGFR
- estimated glomerular filtration rate
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ESRD
- end-stage renal disease
- FP
- femoropopliteal
- GSV
- great saphenous vein
- HDU
- high-dependency unit
- HRQoL
- health-related quality of life
- ICECAP-O
- ICEpop CAPability measure for Older people
- IP
- infra-popliteal
- ITT
- intention to treat
- ITU
- intensive treatment unit
- LOS
- length of stay
- MACE
- major adverse cardiac event
- MALE
- major adverse limb event
- MI
- myocardial infarction
- NHSCII
- NHS Cost Inflation Index
- NICE
- National Institute for Health and Care Excellence
- OS
- overall survival
- PAD
- peripheral arterial disease
- PBA
- plain balloon angioplasty
- PEDIS
- perfusion, extent, depth, infection and sensation
- PI
- principal investigator
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RD
- risk difference
- RUSAE
- related unexpected serious adverse event
- SAE
- serious adverse event
- SF-12
- Short Form questionnaire-12 items
- TBPI
- toe brachial pressure index
- TSC
- Trial Steering Committee
- US
- ultrasound
- VAS
- visual analogue scale
- VascuQoL
- Vascular Quality of Life Questionnaire
- VB
- vein bypass
- WIfI
- Wound Ischaemia and foot Infection
- WTP
- willingness to pay
