Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/192/97. The contractual start date was in October 2016. The draft report began editorial review in November 2021 and was accepted for publication in August 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Bisson et al. This work was produced by Bisson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Bisson et al.
Chapter 1 Introduction
Background
Post-traumatic stress disorder (PTSD) is a common mental health condition that may develop following exposure to traumatic events that involve threatened or actual death, serious injury or sexual violence. The two main current classification systems differ slightly in their symptom criteria for a diagnosis of PTSD. 1,2 The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) requires the presence of symptoms in four different clusters that are associated with the traumatic event(s). At least one intrusion symptom (e.g. recurrent distressing memories and nightmares), persistent avoidance of stimuli, at least two negative alterations in cognitions and mood (e.g. exaggerated negative beliefs and feelings of detachment or estrangement from others), and at least two symptoms indicating increased arousal or reactivity (e.g. irritable behaviour and hypervigilance) are required. The eleventh edition of the International Classification of Diseases requires one intrusion symptom (flashbacks or nightmares), one avoidance symptom and one symptom indicating a current sense of threat (hypervigilance or increased startle).
According to the adult psychiatric morbidity survey, about 3% of the United Kingdom (UK) adult population have PTSD3 and average symptom duration is normally prolonged if untreated. 4 Various studies have demonstrated strong associations between PTSD and physical and mental health comorbidity. 5,6 PTSD has also been found to have a large economic burden. 7 Systematic reviews and meta-analyses have repeatedly found that individual, face-to-face trauma-focused psychological treatments (TFPT), in the form of cognitive behavioural therapies with a trauma focus (CBT-TF) and eye movement desensitisation and reprocessing (EMDR), are the best-evidenced treatments for PTSD. TFPTs are recommended as first-line treatments by guidelines across the world, including those of the UK’s National Institute for Health and Care Excellence (NICE), the International Society for Traumatic Stress Studies (ISTSS), Australia and the American Psychological Association. 8–11
There are a limited number of suitably trained therapists available to deliver TFPT in the National Health Service (NHS). Unfortunately, this often prevents timely access to treatment, with NHS waits of a year or more in some areas of the UK. TFPT is usually delivered weekly, in a face-to-face setting over several months, making it very difficult to access for some recipients (e.g. because of stigma, work commitments, travel and childcare). 12–14 Guided self-help (GSH) is an alternative approach to the delivery of treatment. GSH combines the use of self-help materials with regular guidance from a trained professional and requires less therapist time than recommended face-to-face TFPT.
GSH has been developed and evaluated for the treatment of a number of other mental disorders and there is good evidence of the efficacy of GSH for conditions such as anxiety and depression. 15,16 If effective for PTSD, GSH would offer a time-efficient and accessible treatment option (not least in the face of a pandemic), with the potential to reduce waiting times and intervention costs. These impacts, along with the ability to move more treatment delivery from high to low intensity, would herald a step change in the care pathway for people with PTSD. By treating PTSD in a timelier and more efficient manner, the burden of disease would be reduced, preventing avoidable morbidity and improving quality of life.
Development of Spring
Through careful Phase I work,17 Spring was developed systematically following Medical Research Council (MRC) guidance for the development of a complex intervention. 18 The work followed an iterative process incorporating qualitative work to model the intervention, followed by two pilot studies to refine it based on quantitative and qualitative outcomes. Collaboration with a web development agency (Healthcare Learning Smile-on), as part of a Knowledge Transfer Partnership, produced an interactive web-based version of the intervention. Based on principles of CBT-TF, Spring includes eight steps designed for delivery over 8 weeks, which cover psychoeducation, grounding, relaxation, behavioural activation, real-life and imaginal exposure, cognitive therapy and relapse prevention.
Phase II19 work demonstrated Spring to be a potentially highly effective GSH intervention for PTSD. Forty-two adults with DSM-5 PTSD of mild to moderate severity were randomly allocated to receive GSH using Spring or delayed treatment. Immediately after treatment, the GSH group had significantly lower Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) scores than the delayed treatment control group (between-group effect size Cohen’s d = 2.60). The difference was maintained 14 weeks after randomisation and the difference dissipated once the delayed treatment group had received treatment. Similar patterns of difference between the two groups were found for self-reported PTSD, depression, anxiety, health-related quality of life and functional impairment.
Existing evidence
A recently published Cochrane Review20 of internet-based CBT for PTSD in adults identified 13 relevant randomised controlled trials (RCTs) with 808 participants, 10 of which, including our Phase II RCT,19 included therapist guidance. Compared with wait-list, internet-based CBT was associated with a clinically important reduction in PTSD. There was evidence that interventions delivered with guidance were more effective at reducing the severity of PTSD symptoms than those without, in addition to evidence that trauma-focused interventions were more effective than those without a trauma focus. However, the certainty of the evidence was very low due to a small number of eligible trials. The authors concluded that further work was required to establish non-inferiority (NI) to current first-line interventions, explore cost-effectiveness, and measure adverse events.
The available research led to the inclusion of GSH as a possible treatment for people with mild to moderate PTSD in the latest NICE9 and ISTSS8 treatment guidelines. Both NICE and ISTSS recommended GSH less strongly than face-to-face TFPT due to weaker evidence; NICE stated, ‘supported computerised trauma-focused CBT should be considered as an option for adults with PTSD who prefer this to face-to-face trauma-focused CBT or EMDR’. ISTSS gave guided internet-based CBT-TF a ‘standard recommendation’, indicating that there was at least reasonable quality of evidence but with lower certainty of effect than required for a strong recommendation. The guarded recommendations of NICE and ISTSS signal the need for GSH interventions that are non-inferior to CBT-TF to provide greater choice, allow people with PTSD more control over treatment, enhance access and establish a wider range of evidence-based treatment options.
Aims and objectives
The main aim of the RAPID trial was to determine the likely clinical and cost-effectiveness of GSH using Spring, an internet-based programme based on CBT-TF for mild to moderate PTSD in the NHS in the UK. RAPID also aimed to describe the experience of receiving the GSH from the recipient’s perspective, and the delivery of GSH using Spring from the therapist’s perspective.
The objectives were to answer the following research questions:
-
For people with mild to moderate PTSD, is GSH using Spring at least equivalent in effectiveness and cost-effective relative to individual CBT-TF as judged by reduced symptoms of PTSD and improved quality of life? (main research question)
-
For people with PTSD, what is the impact of GSH using Spring on functioning, symptoms of depression, symptoms of anxiety, alcohol use and perceived social support? (secondary outcomes)
-
What factors may impact effectiveness and successful roll-out of GSH for PTSD in the NHS if the GSH programme is shown to be effective? (process evaluation)
Chapter 2 Trial design and methods
Trial design
This was a multicentre pragmatic randomised controlled NI trial with assessors masked to treatment allocation. Individual randomisation was used. A NI design aimed to determine if a new approach, with distinct advantages over existing strongly recommended treatments, was no worse than a current gold-standard treatment for PTSD. 22 We did not expect GSH to be more effective than face-to-face CBT-TF, and therefore, a superiority design was not appropriate. The trial followed a published protocol,21 was supported by a public advisory group and overseen by a trial steering committee and independent data monitoring committee. Nested process evaluation was included to assess fidelity, adherence and factors that influenced outcome. Quantitative and qualitative research methods were used. The trial was conducted between August 2017 and January 2021. It adhered to CONSORT guidelines23 and was granted a favourable ethical opinion by the South East Wales Research Ethics Committee.
Eligibility criteria
Wide eligibility criteria were used to ensure good external validity. Participants were aged 18 or over, had DSM-5 PTSD as their primary diagnosis, as evaluated by the CAPS-5,24 with mild to moderate symptom severity as indicated by a score of < 50 on the CAPS-5 at baseline assessment, had regular access to the internet to complete the steps and homework required by the GSH programme and were willing and able to give informed consent to take part. Exclusion criteria were inability to read and write fluently in English, previous completion of a course of TFPT for PTSD, current PTSD symptoms to more than one traumatic event, current engagement in psychological therapy, diagnosis of psychosis or substance dependence, active suicide risk and change in psychotropic medication in the past 4 weeks.
Recruitment and consent
Participants were recruited from NHS Improving Access to Psychological Therapy (IAPT) services based in primary care in England (Coventry, Warwickshire, Greater Manchester, London and Southwest Yorkshire), and NHS psychological treatment settings based in primary and secondary care in Scotland (Lothian) and South Wales (Cardiff, Gwent, Mid Glamorgan and the Vale of Glamorgan). Potential participants were identified and approached by a clinician involved in their care, screened, and then fully assessed by one of a team of researchers after providing informed consent. If individuals met the eligibility criteria, they were randomised to receive GSH for PTSD using the Spring programme, or to receive face-to-face CBT-TF.
Semistructured interviews for qualitative analysis were conducted with 19 participants and 10 therapists, to gather perspectives of receiving and delivering the interventions as part of the process evaluation. Trial participants were sampled according to intervention allocation, research site, gender, age, ethnicity, education level and nature of trauma. Therapists were sampled by gender and research site.
Randomisation
Individual randomisation was performed by Cardiff University’s Centre for Trials Research (CTR) and conducted using a pre-programmed online minimisation algorithm developed by the database designer in accordance with CTR Standard Operating Procedures (SOPs). The allocation ratio was 1 : 1. This was implemented to ensure balance between trial arms on gender but retained an 80% random element. Randomisation was stratified by research centre. Randomisation was undertaken by the data manager once eligibility was confirmed. Allocations were e-mailed to the trial manager who informed the local Principal Investigators (PIs)/therapists. A randomisation protocol was written and signed off before recruitment began in line with CTR policy. Outcome assessors were blinded to treatment allocation as far as possible. Participants were asked not to reveal the intervention they received to assessors at follow-up interviews. Only when written informed consent was obtained from the participant, and they were randomised/enrolled into the trial, were they considered a trial participant.
Blinding
It was not possible to blind the therapists or the participants, given the complex interventions under investigation. However, the assessors were blind to treatment allocation and the therapists and participants were asked not to discuss their allocation with the assessors. Participants were reminded of the importance of this at each outcome assessment.
Interventions
Face-to-face CBT-TF
CBT-TF is one of the primary treatments for PTSD adopted by IAPT in England and psychological therapy services in Scotland and Wales. Cognitive therapy for PTSD (CT-PTSD),25 one of the CBT-TF implemented by IAPT, was adopted for RAPID. Participants received up to 12 face-to-face, individual sessions, each lasting 60–90 minutes. In-session treatment was augmented by assignments which participants were required to complete between sessions.
CT-PTSD involves identifying the relevant appraisals, memory characteristics, triggers and behavioural and cognitive strategies that maintain PTSD symptoms. These are addressed by: (1) modifying excessively negative appraisals of the trauma and/or its sequelae; (2) reducing re-experiencing by elaboration of the trauma memories through imaginal exposure or narrative-based memory updating with less threatening meanings and discrimination of triggers; (3) dropping dysfunctional behaviours and cognitive strategies, particularly those related to avoidance of triggers for intrusive symptoms; and (4) when possible, visiting the site of the trauma with the therapist to update the trauma memory.
GSH using Spring
Spring is an eight-step online GSH programme based on CBT-TF (see Table 1 for description of steps); it uses the same principles as CBT-TF but aims to reduce contact time with the therapist by providing some of the therapy content and activities in an online format. The therapist initially meets with the participant for an hour to develop a rapport, learn about the participant’s trauma, provide log-in details and describe and demonstrate the programme, which the participant then completes online in their own time. There are four subsequent fortnightly meetings of 30 minutes, normally undertaken face-to-face, but also deliverable via the internet or telephone, according to participant preference. The participant also receives four brief telephone calls or e-mail contacts between sessions to discuss progress, identify any problems that have arisen and agree new goals. The programme was designed to be accessible through a variety of devices including PC, laptop, tablet and smartphone (via a Spring App).
| Step 1: Learning About My PTSD | Psychoeducation about PTSD illustrated by four actors describing their experience of PTSD to different types of traumatic events. |
| Step 2: Grounding Myself | Explanation of grounding and its uses along with descriptions and demonstrations of grounding exercises. |
| Step 3: Managing My Anxiety | Education about relaxation techniques with learning through videos of a controlled breathing technique, applied muscular relaxation and relaxation through imagery. |
| Step 4: Reclaiming My Life | Behavioural reactivation to help individuals return to previously undertaken/new activities. |
| Step 5: Coming to Terms with My Trauma | Provides rationale for imaginal exposure, narratives of the four video characters. The therapist helps the participant to begin writing a narrative, which they complete remotely and read every day. |
| Step 6: Changing My Thoughts | Cognitive techniques to address PTSD symptoms. |
| Step 7: Overcoming My Avoidance | Graded real-life exposure work. |
| Step 8: Keeping Myself Well | This session reinforces what has been learnt during the programme, provides relapse prevention measures and guidance on what to do if symptoms return. |
The eight Spring steps are accompanied by between-session work. At each session, the therapist reviews progress by logging into a clinician dashboard and guides the participant through the programme. The aim of the guidance is to offer continued support, monitoring, motivation and problem-solving. The eight online steps are usually completed in turn with some later steps relying on mastery of techniques taught in earlier steps. Each step provides psychoeducation and the rationale for specific components of treatment, and they also activate a tool that becomes live in the Toolkit area of the website and aims to reduce traumatic stress symptoms. Specific activities become visible (with the participant’s knowledge) to the therapist via the dashboard to facilitate discussions during guidance. The programme can be accessed online via a web browser or through an app.
Therapists
Both trial interventions were delivered by the same, experienced psychological therapists working in high-intensity IAPT services or psychological services at the trial sites. All therapists had previous experience of delivering CBT-TF for PTSD. Study therapists received one and a half days additional training in CT-PTSD, and a half day training in GSH using Spring. Training was delivered by clinicians involved in the development of CT-PTSD and Spring. Trial therapists completed at least one training case using each intervention and were assessed as being competent by a trial clinical supervisor if they were considered to have delivered the interventions appropriately. Therapists followed treatment manuals for both interventions and received trial-specific group clinical supervision once per month throughout the trial via video or telephone conference call by NK or NR. The COVID-19 pandemic resulted in some of the last participants receiving their final therapy sessions via video conferencing, as opposed to in person.
Fidelity
To ensure the interventions were delivered as intended and according to the manuals, each therapist aimed to audio record at least one session with every participant, using a digital voice recorder. The audio recordings were rated using a general and an intervention-specific fidelity checklist by one of two independent, experienced clinicians.
Adherence
The trial focused on the ‘implementation’ of attending therapy sessions as the adherence element of interest. Implementation was defined as the extent to which the participant attended therapy session as intended. Given that the two arms are different in their therapy session structures, this was expressed as a binary (adhered or not adhered) based on the number of sessions attended:
-
in the GSH arm, attending ≥ 3 sessions defined adhered
-
in the CBT-TF arm, attending ≥ 8 defined adhered.
In both arms, if the therapist determined that the participant had attended a sufficient number of sessions such that that number was less than the number stated above, this also defined adhered. In all other cases, the participant was considered to have not adhered.
Outcomes
All outcome measures were completed at baseline, 16 and 52 weeks after randomisation. The primary outcome was the severity of symptoms of PTSD over the previous week as measured by the CAPS-525 at 16 weeks post-randomisation. Sixteen weeks was chosen as a post-intervention measurement. Severity of PTSD symptoms at 52 weeks post-randomisation, measured using the CAPS-5, was a secondary outcome along with self-reported secondary outcomes, measured using validated measures, at both 16 weeks (to determine the effect of the interventions) and 52 weeks post-randomisation (to determine sustained effects). The Impact of Event Scale-Revised (IES-R) was also collected at each therapy contact to provide clinical feedback and to facilitate imputation for missing data, if required. Information on possible adverse events was also collected.
Primary outcome
CAPS-5 (PTSD symptoms): The CAPS-525 is a 29-item structured interview for assessing PTSD diagnostic status and symptom severity. The CAPS-5 is the gold standard in PTSD assessment and can be used to make a current (past month) or lifetime diagnosis of PTSD or to assess symptoms over the past week. Items correspond to the DSM-5 criteria for PTSD. The CAPS-5 has excellent reliability and convergent and discriminant validity, diagnostic utility and sensitivity to clinical change. 25 Twenty of the twenty-nine items are used to create the score.
Secondary outcomes
IES-R (PTSD symptoms): The IES-R26 is a brief PTSD self-report measure and has been used in many international studies. The IES-R is the outcome measure of choice for evaluating improvement in PTSD symptoms in IAPT services in England.
EuroQol-5 Dimensions, five-level version (EQ-5D-5L) (quality of life): The EQ-5D-5L27 is a widely used instrument in health economic (HE) analysis and recognised by NICE as an appropriate measure for health-related quality of life. The questionnaire provides a simple descriptive profile, which translates to a single utility score for health status. The first part of the instrument identifies the extent of perceived problems – across five levels – in each of five life dimensions: mobility; self-care; usual activities; pain and discomfort; and anxiety and depression. The responses to each of the five questions are used to generate a value set score for self-rated health status between −0.594 and 1, where −0.594 represents the worst possible health state and 1 the best possible health state. Given the NICE Position Statement,28 this value set is a crosswalk of EQ-5D-5L to EQ-5D-3L. 29 The second part is a visual analogue scale, which allows the responder to indicate their current health status on a 0–100 scale.
Work and Social Adjustment Scale (WSAS) (functional impairment): The WSAS30 is a self-report measure, which assesses the impact of a person’s mental health difficulties on their ability to function in terms of work, home management, social leisure, private leisure and personal or family relationships. The WSAS is the outcome measure of choice for evaluating improvement in functioning in IAPT services. The WSAS has been demonstrated to show good reliability and validity and is sensitive to change.
Patient Health Questionnaire (PHQ-9) (depression symptoms): The PHQ-931 is a widely used reliable and well-validated brief self-report measure of depression. It is the outcome measure of choice for evaluating improvement in depressive symptoms in IAPT services.
Generalized Anxiety Disorder-7 (GAD-7) (anxiety symptoms): The GAD-732 is a widely used reliable and well-validated brief self-report measure of anxiety. It is the outcome measure of choice for evaluating improvement in anxiety symptoms in IAPT services.
Alcohol Use Disorders Test (AUDIT-O) (alcohol symptoms): The AUDIT-O33 contains 10 multiple-choice questions on quantity and frequency of alcohol consumption, drinking behaviour and alcohol-related problems or reactions over the preceding 3 months.
Multidimensional Scale for Perceived Social Support (MSPSS) (social support): The MSPSS34 is a widely used 12-item Likert scale measuring the subjective assessment of adequacy of social support from family, friends and partners. 35 The reliability, validity and factor structure of the MSPSS have been demonstrated with a number of populations. 34–37
Insomnia Severity Index (ISI) (insomnia): The ISI38 is a widely used seven-item self-report questionnaire assessing the nature, severity and impact of insomnia. It has been shown to be reliable and valid in terms of detecting insomnia and in measuring treatment response in clinical patients.
Post-Traumatic Cognitions Inventory (PTCI) (post-traumatic cognitions): The PTCI39 was developed as a 33-item scale, which is rated on a Likert scale ranging from 1 (totally disagree) to 7 (totally agree); a shortened, 22-item form has also been developed. Scale scores are formed for three subscales: negative cognitions about self, negative cognitions about the world and self-blame. The PTCI shows good internal consistency, high test-retest reliability and good convergent validity with other measures of trauma-related cognitions. The PTCI also shows promise in being able to differentiate individuals with and without PTSD.
General Self-Efficacy Scale (GSES) (self-efficacy): The GSES is a ten-item, 4-point Likert scale that is used to measure self-efficacy. It has been used in more than 1000 studies and is reliable and well-validated. 40,41
Client Satisfaction Questionnaire-8 (CSQ-8) (treatment satisfaction): The CSQ-842 is a widely used eight-item, Likert Scale which was developed through literature review and expert ranking, pretested on 248 mental health clients in five settings. It is a self-report statement of satisfaction with a high degree of internal consistency, good concurrent validity and reliability and is brief and easy to complete. 43
Agnew Relationship Measure-5 (ARM-5) (therapeutic alliance): The ARM-544 is a validated short 5-item version of the 28-item ARM-5, comprising client and therapist versions containing parallel items. There are versions for both the participant (shown first) and the therapist (shown second).
Sample size
As the study aimed to demonstrate NI of GSH using Spring for PTSD compared to face-to-face CBT-TF, the power calculation considered the NI margin as opposed to the effect size. The NI margin (determined a priori by clinical consensus of clinicians involved in the trial design and the research management group) was 5 points on the 80-point CAPS-5 scale. A meta-analysis45 indicated that the standardised mean difference between CBT-TF and wait-list/usual care for the treatment of PTSD is −1.62. This corresponds to 16.6 points on the CAPS-5 with a common standard deviation (SD) of 10.3. This means that if NI was demonstrated to within 5 points of the gold standard, this would also demonstrate superiority over wait-list/usual care in line with International Conference on Harmonisation Harmonised Tripartite Guideline (Statistical Principles for Clinical Trials) E9 (ICH E9) guidance for NI studies. 46,47
Pilot work indicated an intraclass correlation coefficient of 5.6% at the therapist level at 10 weeks. At 22 weeks, however, there was no observable clustering of CAPS-5 scores among therapists. Given our primary outcome (CAPS-5) was measured at 16 weeks, we allowed for 1% clustering and recalculated the sample size. We allowed for 20% attrition. With the anticipated average therapist cluster size anticipated as four, the design effect was 1.03, requiring a 3% inflation of the sample size. This resulted in a final target sample size of 192 (inflated from 186), which provided 90% power [nQuery v7.0 (Statistical Solutions, Saugus, MA, USA)48].
For the qualitative elements of the study, the sample size was guided by preliminary analysis and constant comparison (with themes from other interviews), during each data collection phase, until the research team was satisfied that there was data saturation and no new themes which were important to the research.
Statistical analysis methods
All statistical analyses were described in a statistical analysis plan (SAP) prior to data analysis being performed.
Analysis of the primary outcome
The primary analysis was performed using analysis of covariance (ANCOVA), modelling 16-week follow-up CAPS-5 score, controlling for baseline CAPS-5 score, research centre and the following patient characteristics: gender, comorbid depression (baseline PHQ-9) and time since trauma. Reflecting the sample size calculation, analyses were undertaken with two-level hierarchical models with patients clustered within therapists.
The primary analysis utilised multiple imputation with interim collected IES-R scores as auxiliary variables to the imputation. The number of imputations datasets created from which the analysis was averaged over was 50, which was greater than the percentage of incomplete cases (defined as a case missing the primary outcome) out of all those randomised. Given that IES-R was collected four to five times for GSH arm patients and eight to twelve times for CBT-TF arm patients, there was potential bias created by undertaking any multiple imputation model. For this analysis, we then applied a different imputation model to each arm: both containing the relevant number of auxiliary variables [along with baseline CAPS-5 score, research centre, gender, comorbid depression (baseline PHQ-9) and time since trauma]. Imputed datasets were then combined for the final analyses. Full details of the imputation procedure are given below. 49
The results were summarised using point estimates, and one-sided 95% confidence intervals (CIs) and NI p-values (in line with the sample size calculation). Since this is a NI design, we checked whether the CI for the difference between arms lay entirely within the 5-point NI margin for the primary outcome. Where the treatment effect and one-sided 95% CI were entirely > 0, then superiority was assessed with a two-sided 90% CI and relevant p-value.
The secondary outcome CAPS-5 at 52-week follow-up was analysed in the same manner as the primary analysis. For the other secondary outcomes the notional NI margin was set as 0.5 times the pooled SD of the baseline values in each of the CBT-TF and GSH groups of the outcome.
Covariate adjustment
All analyses contained the following covariates:
-
treatment arm (categorical; GSH or CBT-TF)
-
gender (categorical; male or female; minimisation variable)
-
research centre (categorical; Cardiff and South Wales, Pennine, London, NHS Lothian; Coventry, South-West Yorkshire; stratification variable)
-
comorbid depression (baseline PHQ-9)
-
time since trauma in months.
In all cases, except those of CSQ-8 and ARM-5, the covariate of the relevant baseline version of the same measure was included in the model such that an ANCOVA model was formed. For PHQ-9, this covariate was only included in the model once.
Sensitivity analyses
For the primary outcome, the complete case intention-to-treat (ITT) analysis and per-protocol analysis were conducted and reported under a NI framework. Results are presented using point estimates, and one-sided 95% CIs and p-values (in line with the sample size calculation). Since this is a NI design, we checked whether the CI for the difference between arms lay entirely within the 5-point NI margin.
A further sensitivity analysis (SA) of the primary outcome under a NI framework implemented a different multiple imputation model: IES-R scores taken from five clinic visits for the CBT-TF arm participants that aligned similarly in time to those of the GSH arm participants were used as auxiliary variables in an imputation model (this one with both arms combined) along with baseline CAPS-5 score, research centre, gender, comorbid depression (baseline PHQ-9) and time since trauma. The results were summarised using point estimates, and one-sided 95% CIs and NI p-values (in line with the sample size calculation). Since this was a NI design, we checked whether the CI for the difference between arms lay entirely within the 5-point NI margin.
To explore the impact of departures from randomised treatment on our primary analysis, we estimated the complier average causal effect (CACE), with the following definition of ‘complier’ considered:
-
Participants who attend the necessary number of therapy sessions to be described as having adhered (as defined above).
In this case, ‘compliers’ form a principal stratification strategy as defined in the ICH E9 Draft Addendum. 50 That is, by defining compliers as the stratum in the trial population that do not experience the post-randomisation intercurrent event of non-compliance.
We used instrumental variable methods to conduct these analyses, using randomisation as an instrument. 51–53 The models were fit using two-stage least squares instrumental variables regression, including those covariates used in the primary analysis model. The results were summarised using point estimates, and one-sided 95% CIs and NI p-values (in line with the sample size calculation). Since this is a NI design, we checked whether the CI for the difference between arms lay entirely within the 5-point NI margin.
In addition, for the 52-week outcome measures, we explored the impact of the COVID-19 pandemic by examining the impacts on the primary analysis by further stratifying by two time periods, namely, before the initial national lockdown date of 23 March 2020, and after that date. Since the trial was not powered to explore the interaction effect of the two time periods and the intervention, these analyses were primarily exploratory in nature. Further analyses were also performed examining the effect of changes in the mode of data collection from face-to-face to remote collection via video and telephone calls. This analysis was conducted on the primary outcome at 16 weeks as by the 52-week outcome the choice of method of data collection was confounded by the switch to telephone/video call due to the COVID-19 pandemic.
Analysis of secondary outcomes
Secondary outcomes IES-R, EQ-5D-5L (Value Set and Visual Analogue Scale), WSAS, PHQ-9, GAD-7, AUDIT-O, MSPSS, ISI, PTCI, GSES, CSQ-8 and ARM-5 were also analysed using multiple imputation to account for missing data under a NI framework. The number of imputations datasets created from which the analysis was averaged over was 50, greater than the percentage of incomplete cases (defined as a case missing that secondary outcome) out of all those randomised. The imputation model used the variables defined above. The results are presented as point estimates, one-sided 95% CIs and NI p-values. To allow comparison to be made across all outcomes the results are standardised to Cohen’s d effects size and the relevant point estimates, one-sided 95% CIs and non-inferiority p-values are expressed likewise.
The Cohen’s d effect size was calculated as the estimated mean difference from the regression model divided by the pooled SD calculated by pooling the SD measured in each trial arm at baseline for the relevant secondary outcome. The NI margin for secondary outcomes was set as 0.5 times the pooled SD. The value of 0.5 was chosen as this approximates the effect size used in the sample size calculation for the primary outcome of CAPS-5, that is, a 5-point margin divided by an assumed SD of 10.3. This gives an effect size 0.48 which was rounded to 0.5.
Exploratory analyses
A pre-specified subgroup analysis considered the differences in treatment effects by gender, including an interaction term between treatment arm and gender. Estimates from the statistical models (stratum-specific mean differences) are presented alongside two-sided 95% CIs and p-values.
Missing data
Individual questionnaires were inspected for missing values. If specific items were missing, they were imputed using the guidance for that questionnaire or if that information was not available then by mean imputation. This was a minor issue as most questionnaires were complete if the participant had been assessed.
Dealing with missing data for the primary outcome is listed above. For the multilevel multiple imputation, a joint modelling approach was chosen as implemented in the JOMO R package. 54 This uses a Bayesian approach utilising Monte Carlo Markov Chain to fit a joint two-level model. For the level 1 model this was fit as a random intercept and slope mixed regression model of the IES-R total scores with time. In addition, the following covariates were added to the model: site, baseline CAPS-5 score, gender, baseline depression score (PHQ-9) and time since traumatic event. The level 2 model was the primary analysis model consisting of a regression of the 16-week CAPS-5 score on the baseline CAPS-5 score, gender, baseline depression score (PHQ-9) and time since traumatic event. As described above, for the primary analysis each treatment group was imputed separately due to the varying number of therapist contacts.
For the SA, the same imputation model framework was used except the IES-R scores and other model variables were combined into a single imputation model. For the 52-week outcomes it was not possible to also include the site variable in the imputation model for each group due to data sparseness in the smallest sites. However, when compared to the sensitivity and complete case analyses the results were consistent.
For each multiple imputation run we used a burn-in period of 10,000 iterations, 500 iterations ‘in-between’ to account for serial correlation; and for all outcome variables 50 multiply imputed datasets were generated. The robustness of the imputation models was checked using a range of diagnostic statistics and convergence plots available in the JOMO package and described there. Based on these diagnostic measures there was no evidence of any issues with the imputation framework.
Additional analyses
IES-R scores over time were analysed using a hierarchical modelling fitting a random slope and intercept model, also allowing for clustering by therapist as in the primary analysis. We modelled the time dimension as a linear spline with a knot at 26 weeks. Both elements of the spline were included as random effects in the form of random slopes. We fitted IES-R trajectories over time (since randomisation) interacted with intervention arm, while also controlling for the same covariates as the primary analysis. Note that these were likely collected four to five times for GSH arm patients and 8 to 12 times for CBT-TF arm patients.
An analysis explored the impact of the COVID-19 pandemic by examining the treatment effect stratified by the period before and after lockdown, defined as any contact that took place after 23 March 2020. For participants with missing outcome data, we calculated the notional 16- or 52-week date of the expected contact with their therapist. This notional date was then used to define the before or after lockdown period.
Public participation
A public advisory group, comprising five people with lived experience of PTSD, was formed, and met every 2–3 months to inform study design, conduct, data analysis and dissemination strategy and activity. This included participation in the interpretation of findings and identifying implications. The group was chaired by coauthor SC, a co-applicant with lived experience of PTSD and a participant in a previous study of GSH using Spring. The public advisory group reviewed and approved all participant-facing material. The trial steering committee included two members of the public, who were separate from the public advisory group.
Chapter 3 Quantitative trial results
Introduction
This chapter describes the results of the quantitative analysis of the RAPID trial.
Recruitment
Screening and randomisation
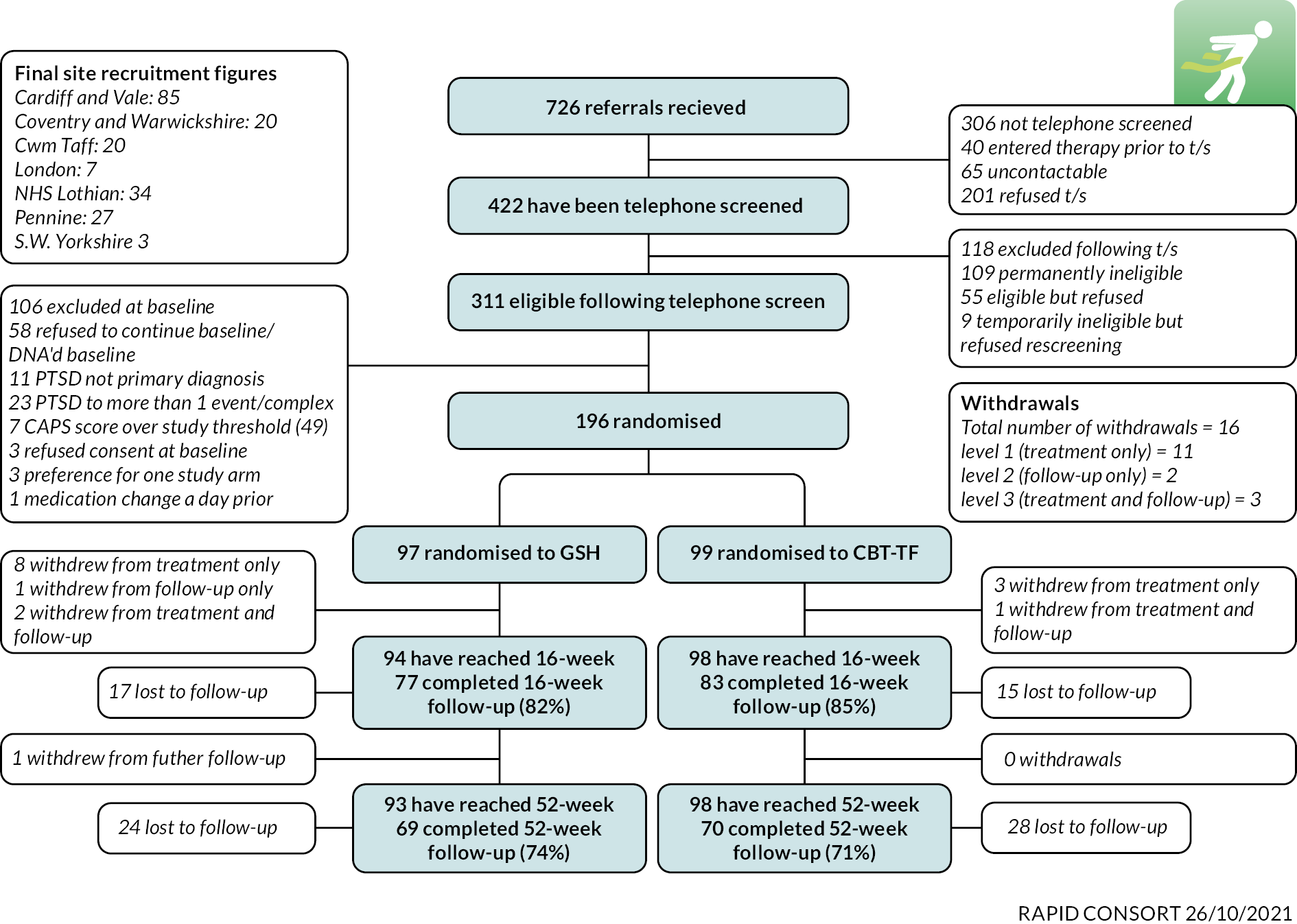
In all, 196 participants were recruited into the trial, 99 in the CBT-TF arm and 97 in the GSH arm. This exceeded the planned sample size of 192 participants. Figure 1 shows the CONSORT patient flow diagram. A more detailed breakdown of the reasons for inclusion and exclusion is shown in Table 2.
FIGURE 1.
Participant flow and CONSORT diagram for the RAPID trial.
| Cardiff and South Wales | Site | ||||||
|---|---|---|---|---|---|---|---|
| Coventry and Warwickshire | East London Foundation Trust | NHS Lothian | Pennine | S.W. Yorks | Total | ||
| Number screened | 250 | 38 | 13 | 73 | 44 | 4 | 422 |
| Number eligible | 178 (71.2) | 28 (73.7) | 9 (69.2) | 53 (72.6) | 39 (88.6) | 4 (100.0) | 311 (73.7) |
| Attended baseline | 178 (100.0) | 24 (85.7) | 9 (100.0) | 49 (92.5) | 39 (100.0) | 4 (100.0) | 303 (97.4) |
| Randomised | 105 (59.0) | 20 (83.3) | 7 (77.8) | 34 (69.4) | 27 (69.2) | 3 (75.0) | 196 (64.7) |
| Inclusion criteria | |||||||
| Are you aged 18 or over? | 250 (100.0) | 38 (100.0) | 12 (92.3) | 73 (100.0) | 43 (97.7) | 4 (100.0) | 420 (99.5) |
| Has the patient experienced a trauma that meets the DSM-5 criteria for PTSD? | 249 (99.6) | 37 (97.4) | 10 (76.9) | 73 (100.0) | 44 (100.0) | 4 (100.0) | 417 (98.8) |
| Have any other traumatic events contributed to your symptoms? | 51 (20.4) | 13 (34.2) | 2 (15.4) | 17 (23.3) | 11 (25.0) | 0 (0.0) | 94 (22.3) |
| Does the patient have PTSD following a single traumatic event? | 229 (91.6) | 33 (86.8) | 10 (76.9) | 62 (84.9) | 39 (88.6) | 4 (100.0) | 377 (89.3) |
| Does the patient answer yes to six or more questions on the Traumatic Screening Questionnaire? | 204 (81.6) | 33 (86.8) | 9 (69.2) | 66 (90.4) | 42 (95.5) | 4 (100.0) | 358 (84.8) |
| Do you have regular access to the internet in order to complete the GSH? | 210 (84.0) | 36 (94.7) | 10 (76.9) | 63 (86.3) | 42 (95.5) | 4 (100.0) | 365 (86.5) |
| Exclusion criteria | |||||||
| Inability to read and write fluently in English | 7 (2.8) | 2 (5.3) | 0 (0.0) | 2 (2.7) | 1 (2.3) | 0 (0.0) | 12 (2.8) |
| Have you previously completed a course of CBT-TF for PTSD? | 3 (1.2) | 2 (5.3) | 1 (7.7) | 3 (4.1) | 1 (2.3) | 0 (0.0) | 10 (2.4) |
| Are you currently receiving any kind of psychological therapy? | 1 (0.4) | 2 (5.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.7) |
| Are you taking any medication for a mental health condition? | 21 (8.4) | 4 (10.5) | 0 (0.0) | 4 (5.5) | 0 (0.0) | 0 (0.0) | 29 (6.9) |
| Are you suffering from psychosis, for example, hearing voices or seeing things? | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 0 (0.0) | 2 (0.5) |
| Are you currently dependent on alcohol or drugs? | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 2 (0.5) |
| Have you been having thoughts of ending your life? | 60 (24.0) | 1 (2.6) | 0 (0.0) | 15 (20.5) | 7 (15.9) | 1 (25.0) | 84 (19.9) |
| Do you feel suicidal? | 2 (0.8) | 0 (0.0) | 0 (0.0) | 4 (5.5) | 1 (2.3) | 0 (0.0) | 7 (1.7) |
Overall, 726 referrals were made to the RAPID team and 422 were telephone screened (58.1%). Of the 422 participants telephone screened, 311/422 (73.7%) were eligible for enrolment and 303/311 (97.4%) attended a baseline assessment. Of these, 196/303 (64.7%) were randomised to the trial. Of those not randomised, 58/303 (19.1%) participants chose not to continue the baseline assessment or did not attend the assessment. A further 48/303 (15.8%) patients did not meet the eligibility criteria for entry to the trial with the reasons being PTSD was attributed to more than one event or was a complex PTSD diagnosis (23/303, 7.6%); not having PTSD as a primary diagnosis (11/303, 3.6%); and 7/303 (2.3%) having a CAPS-5 score above the eligibility threshold of 49. In addition, three patients refused consent at baseline, three participants showed a preference for one trial arm and one patient had a medication change close to the baseline assessment.
Withdrawals during the trial
During the trial, 18/196 (9.2%) participants withdrew from the trial. Of the 18 patients, 12/18 (66.7%) were in the GSH group and 6/18 (33.3%) in the CBT-TF group. 2/18 (11.1%) participants were withdrawn at the request of the therapist and 16/18 (88.9%) withdrew at the request of the participant. Of the 18 participants who withdrew, 14/18 (77.8%) withdrew from the trial intervention but allowed themselves to be followed up, 2/18 (11.1%) withdrew from further data collection and 2/18 (11.1%) withdrew from both the intervention and further data collection.
In terms of timing of withdrawals, 6/18 (33.3%) withdrew prior to randomisation, 11/18 (61.1%) withdrew after randomisation but before the 16-week assessment and only 1/18 (5.6%) withdrew between the 16-week and 52-week assessments.
There was a difference in the number of withdrawals between the CBT-TF group and GSH group. There was one participant withdrawal at the request of the therapist in each group. The participant in the CBT-TF group had an ill family member, and no reason was recorded for the GSH participant. The remaining 16 participants (11 in the GSH group and 5 in the CBT-TF group) who withdrew at their own request gave a variety of reasons for withdrawing. Examining the free text comments revealed that three participants (one in the CBT-TF group and two in the GSH group) had no reason recorded. In the four participants who gave reasons in the CBT-TF group, one patient felt the therapy was not helping and the other three participants had difficulty attending the sessions for work-related or family illness reasons. For the GSH group, where a reason was recorded, three participants indicated a desire for more intensive therapy, one participant felt the intervention was too difficult to use, one participant preferred to do individual research, one participant was taking medication to treat their PTSD symptoms, one participant did not want to revisit their PTSD event, one participant could not commit the time and finally one participant had a cancer diagnosis and did not wish to juggle two health issues. Participant withdrawal information is presented in Table 3.
| CBT-TF | GSH | Total | |
|---|---|---|---|
| N = 6 | N = 12 | N = 18 | |
| Nature of withdrawal | |||
| Participant withdrew consent | 5 (83.3) | 11 (91.7) | 16 (88.9) |
| Participant withdrawn by therapist/trial team | 1 (16.7) | 1 (8.3) | 2 (11.1) |
| Missing (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Withdrawal level | |||
| Withdrawal from the trial intervention | 5 (83.3) | 9 (75.0) | 14 (77.8) |
| Withdrawal from follow-up interviews/questionnaires | 0 (0.0) | 2 (16.7) | 2 (11.1) |
| Withdrawal from both the trial intervention and follow-up interviews/questionnaires | 1 (16.7) | 1 (8.3) | 2 (11.1) |
| Missing (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Lost to follow-up
There were 36/196 (18.4%) patients lost to follow-up at the 16-week assessment, 20/97 (20.6%) in the GSH group and 16/99 (16.2%) in the CBT-TF group. This includes those patients who withdrew permission for future data collection, one in the CBT-TF group and three in the GSH group. As expected, loss to follow-up was higher at the 52-week assessment with 57/196 (29.0%) not reporting data collection overall with 29/99 (29.3%) in the CBT-TF group and 28/97 (28.9%) in the GSH group. There was no strong evidence of a differential loss to follow-up between the CBT-TF or GSH groups.
Description of the trial population
This section describes the trial population in terms of the data collected at the baseline assessments.
Demographics
The average age of participants was 36.5 years (SD 13.4). Female participants represented 63.8% (125/196) of the total trial population. The overwhelming self-reported ethnicity was white (all groups) which comprised 91.8% of the trial population (180/196). In terms of education and qualification, 64/196 (32.7%) participants reported having a higher education qualification and 46/196 (23.5%) reported having two or more A levels. Only 8/196 (4.1%) reported having no qualifications or education achievements.
Most participants reported having salary or wages as their main source of income (123/128, 96.1%). However, just under 35% of participants declined to report their main source of income (68/196). Participants were more inclined to report their income category (only eight declined) and 110/188 (58.5%) participants reported income below £20,000 per annum. This is lower than the UK median income as reported by the Office for National Statistics (ONS) for 2019 which was £29,900. Of participants’ current occupations the three most common responses were customer service occupations (24/96, 12.2%), teaching and education profession (17/196, 8.7%) and healthcare professionals (16/196, 8.2%). While customer service occupations remained the most common response in terms of lifetime vocation (62/196, 31.6%) the next two categories were sales occupations (31/196, 15.8%) and administrative roles (30/196, 15.3%). Very few participants reported managerial or scientific roles currently or over their lifetime.
There were no major imbalances reported among the two groups indicating the randomisation process had been successful. There were a few smaller imbalances noted. More CBT-TF participants reported having achieved a higher education qualification (37/99, 37.4%) than GSH participants (27/97, 27.8%). Slightly more CBT-TF participants reported being employed than the GSH group, 63/99 (63.6%) versus 56/97 (57.7%). This was reflected in the proportion of participants not receiving benefits which was 69/99 (69.7%) for the CBT-TF group and 57/97 (58.8%) for the GSH group. There were a larger number of participants not able to work in the GSH group (12/97, 12.4%) compared to the CBT-TF group (6/99, 6.1%). Full details of the demographic profile of the trial participants are shown in Table 4.
| Treatment comparison | ||||||
|---|---|---|---|---|---|---|
| CBT-TF | GSH | Total | ||||
| N = 99 | N = 97 | N = 196 | ||||
| Age (years) | ||||||
| Mean (SD) | 37.6 | (13.4) | 35.4 | (13.4) | 36.5 | (13.4) |
| Median (IQR) | 37.0 | (25.7–48.3) | 31.4 | (24.7–43.8) | 32.3 | (25.2–47.1) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Age group (years) | ||||||
| 18–24 | 23 | (23.2) | 26 | (26.8) | 49 | (25.0) |
| 25–34 | 23 | (23.2) | 33 | (34.0) | 56 | (28.6) |
| 35–44 | 20 | (20.2) | 15 | (15.5) | 35 | (17.9) |
| 45–64 | 31 | (31.3) | 20 | (20.6) | 51 | (26.0) |
| 65+ | 2 | (2.0) | 3 | (3.1) | 5 | (2.6) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Gender | ||||||
| Male | 36 | (36.4) | 35 | (36.1) | 71 | (36.2) |
| Female | 63 | (63.6) | 62 | (63.9) | 125 | (63.8) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Site name | ||||||
| Cardiff and South Wales | 52 | (52.5) | 53 | (54.6) | 105 | (53.6) |
| Coventry and Warwickshire | 11 | (11.1) | 9 | (9.3) | 20 | (10.2) |
| East London Foundation Trust | 4 | (4.0) | 3 | (3.1) | 7 | (3.6) |
| NHS Lothian | 17 | (17.2) | 17 | (17.5) | 34 | (17.3) |
| Pennine | 14 | (14.1) | 13 | (13.4) | 27 | (13.8) |
| S.W. Yorks | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Ethnicity | ||||||
| White: Welsh/English/Scottish/Northern Irish/British | 86 | (86.9) | 86 | (88.7) | 172 | (87.8) |
| White: Irish | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| White: Any other white background | 3 | (3.0) | 3 | (3.1) | 6 | (3.1) |
| Mixed/multiple ethnic groups: White and Black Caribbean | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Mixed/multiple ethnic groups: White and Black African | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Mixed/multiple ethnic groups: any other mixed/multiple ethnic background | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Asian/Asian British: Indian | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Asian/Asian British: Pakistani | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Asian/Asian British: Bangladeshi | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Asian/Asian British: Chinese | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Black/African/Caribbean/Black British: African | 2 | (2.0) | 1 | (1.0) | 3 | (1.5) |
| Black/African/Caribbean/Black British: Caribbean | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Black/African/Caribbean/Black British: any other black/African/Caribbean background | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Any other ethnic group | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Highest level of qualification | ||||||
| None | 1 | (1.0) | 7 | (7.2) | 8 | (4.1) |
| 1–4 GCSE/O levels | 12 | (12.1) | 12 | (12.4) | 24 | (12.2) |
| 5 + GCSE/O levels | 19 | (19.2) | 17 | (17.5) | 36 | (18.4) |
| Apprenticeship | 3 | (3.0) | 1 | (1.0) | 4 | (2.0) |
| 2 + A levels | 22 | (22.2) | 24 | (24.7) | 46 | (23.5) |
| Higher education | 37 | (37.4) | 27 | (27.8) | 64 | (32.7) |
| Other | 5 | (5.1) | 9 | (9.3) | 14 | (7.1) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Main income source | ||||||
| Salary/wage | 64 | (94.1) | 59 | (98.3) | 123 | (96.1) |
| State benefits | 3 | (4.4) | 0 | (0.0) | 3 | (2.3) |
| Other | 1 | (1.5) | 1 | (1.7) | 2 | (1.6) |
| Missing (%) | 31 | (31.3) | 37 | (38.1) | 68 | (34.7) |
| Gross income (individual, without benefits) | ||||||
| Up to £10,000 | 36 | (37.1) | 36 | (39.6) | 72 | (38.3) |
| £10,000–20,000 | 19 | (19.6) | 19 | (20.9) | 38 | (20.2) |
| £20,000–30,000 | 23 | (23.7) | 16 | (17.6) | 39 | (20.7) |
| £30,000–40,000 | 14 | (14.4) | 14 | (15.4) | 28 | (14.9) |
| £40,000–50,000 | 3 | (3.1) | 3 | (3.3) | 6 | (3.2) |
| £50,000–60,000 | 0 | (0.0) | 2 | (2.2) | 2 | (1.1) |
| £60,000 + | 2 | (2.1) | 1 | (1.1) | 3 | (1.6) |
| Missing (%) | 2 | (2.0) | 6 | (6.2) | 8 | (4.1) |
| Current employment | ||||||
| Employed (including being on temporary leave from work for any reason) | 63 | (63.6) | 56 | (57.7) | 119 | (60.7) |
| Self-employed or freelance | 5 | (5.1) | 4 | (4.1) | 9 | (4.6) |
| Homemaker | 6 | (6.1) | 3 | (3.1) | 9 | (4.6) |
| Student | 12 | (12.1) | 15 | (15.5) | 27 | (13.8) |
| Retired | 4 | (4.0) | 3 | (3.1) | 7 | (3.6) |
| Volunteering | 3 | (3.0) | 0 | (0.0) | 3 | (1.5) |
| Unable to work (including those receiving DLA) | 6 | (6.1) | 12 | (12.4) | 18 | (9.2) |
| Out of work and looking for work | 4 | (4.0) | 2 | (2.1) | 6 | (3.1) |
| Out of work but not currently looking for work | 3 | (3.0) | 6 | (6.2) | 9 | (4.6) |
| Current vocation | ||||||
| Corporate managers and directors | 4 | (4.0) | 0 | (0.0) | 4 | (2.0) |
| Science, research, engineering and technology professionals | 3 | (3.0) | 0 | (0.0) | 3 | (1.5) |
| Health professionals | 7 | (7.1) | 9 | (9.3) | 16 | (8.2) |
| Teaching and educational professionals | 10 | (10.1) | 7 | (7.2) | 17 | (8.7) |
| Business, media and public service professionals | 4 | (4.0) | 1 | (1.0) | 5 | (2.6) |
| Other managers and proprietors | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Science, engineering and technology associate professionals | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Health and social care associate professionals | 4 | (4.0) | 5 | (5.2) | 9 | (4.6) |
| Protective service occupations | 3 | (3.0) | 1 | (1.0) | 4 | (2.0) |
| Culture, media and sports occupations | 0 | (0.0) | 3 | (3.1) | 3 | (1.5) |
| Business and public service associate professionals | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Skilled agricultural and related trades | 3 | (3.0) | 0 | (0.0) | 3 | (1.5) |
| Skilled metal, electrical and electronic trades | 0 | (0.0) | 3 | (3.1) | 3 | (1.5) |
| Skilled construction and building trades | 4 | (4.0) | 2 | (2.1) | 6 | (3.1) |
| Textiles, printing and other skilled trades | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Administrative occupations | 8 | (8.1) | 3 | (3.1) | 11 | (5.6) |
| Secretarial and related occupations | 4 | (4.0) | 1 | (1.0) | 5 | (2.6) |
| Caring personal service occupations | 6 | (6.1) | 2 | (2.1) | 8 | (4.1) |
| Leisure, travel and related personal service occupations | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Sales occupations | 2 | (2.0) | 2 | (2.1) | 4 | (2.0) |
| Customer service occupations | 10 | (10.1) | 14 | (14.4) | 24 | (12.2) |
| Process, plant and machine operatives | 4 | (4.0) | 2 | (2.1) | 6 | (3.1) |
| Transport and mobile machine drivers and operatives | 1 | (1.0) | 3 | (3.1) | 4 | (2.0) |
| Elementary trades and related occupations | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Elementary administration and service occupations | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Lifetime vocation | ||||||
| Corporate managers and directors | 5 | (5.1) | 3 | (3.1) | 8 | (4.1) |
| Science, research, engineering and technology professionals | 4 | (4.0) | 3 | (3.1) | 7 | (3.6) |
| Health professionals | 5 | (5.1) | 12 | (12.4) | 17 | (8.7) |
| Teaching and educational professionals | 18 | (18.2) | 10 | (10.3) | 28 | (14.3) |
| Business, media and public service professionals | 5 | (5.1) | 4 | (4.1) | 9 | (4.6) |
| Other managers and proprietors | 4 | (4.0) | 7 | (7.2) | 11 | (5.6) |
| Science, engineering and technology associate professionals | 3 | (3.0) | 1 | (1.0) | 4 | (2.0) |
| Health and social care associate professionals | 11 | (11.1) | 9 | (9.3) | 20 | (10.2) |
| Protective service occupations | 3 | (3.0) | 3 | (3.1) | 6 | (3.1) |
| Culture, media and sports occupations | 5 | (5.1) | 4 | (4.1) | 9 | (4.6) |
| Business and public service associate professionals | 2 | (2.0) | 2 | (2.1) | 4 | (2.0) |
| Skilled agricultural and related trades | 3 | (3.0) | 0 | (0.0) | 3 | (1.5) |
| Skilled metal, electrical and electronic trades | 5 | (5.1) | 3 | (3.1) | 8 | (4.1) |
| Skilled construction and building trades | 10 | (10.1) | 9 | (9.3) | 19 | (9.7) |
| Textiles, printing and other skilled trades | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Administrative occupations | 18 | (18.2) | 12 | (12.4) | 30 | (15.3) |
| Secretarial and related occupations | 6 | (6.1) | 5 | (5.2) | 11 | (5.6) |
| Caring personal service occupations | 8 | (8.1) | 7 | (7.2) | 15 | (7.7) |
| Leisure, travel and related personal service occupations | 9 | (9.1) | 12 | (12.4) | 21 | (10.7) |
| Sales occupations | 14 | (14.1) | 17 | (17.5) | 31 | (15.8) |
| Customer service occupations | 30 | (30.3) | 32 | (33.0) | 62 | (31.6) |
| Process, plant and machine operatives | 5 | (5.1) | 3 | (3.1) | 8 | (4.1) |
| Transport and mobile machine drivers and operatives | 3 | (3.0) | 9 | (9.3) | 12 | (6.1) |
| Elementary trades and related occupations | 3 | (3.0) | 1 | (1.0) | 4 | (2.0) |
| Elementary administration and service occupations | 3 | (3.0) | 0 | (0.0) | 3 | (1.5) |
| Homemaker | 7 | (7.1) | 5 | (5.2) | 12 | (6.1) |
| Never worked (including those receiving DLA) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Benefits | ||||||
| No benefits received | 69 | (69.7) | 57 | (58.8) | 126 | (64.3) |
| Income support | 5 | (5.1) | 7 | (7.2) | 12 | (6.1) |
| Jobseeker’s allowance | 4 | (4.0) | 2 | (2.1) | 6 | (3.1) |
| DLA | 10 | (10.1) | 11 | (11.3) | 21 | (10.7) |
| Statutory sick pay | 4 | (4.0) | 5 | (5.2) | 9 | (4.6) |
| Housing benefit | 5 | (5.1) | 6 | (6.2) | 11 | (5.6) |
| State pension | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Child benefit | 12 | (12.1) | 17 | (17.5) | 29 | (14.8) |
Experience of trauma
Participants reported previous experience of trauma using the Life Events Checklist (LEC) tool. 56 Figure 2 shows the prevalence of the worst experience reported and Table 5 reports the full details of the LEC questionnaire. Participants reported on average being 32.7 (SD 13.9, range 5–69) years old at the time of their worst trauma experience and that the event had occurred a median of 16 [interquartile range (IQR) 6–32, range 2–720] months ago. Most participants responded that the event lasted a median of 1 hour (IQR 0.2–4); however, some participants reported much longer durations, range 0–720 hours.
FIGURE 2.
Prevalence of most traumatic event reported.

| CBT-TF | GSH | Total | ||||
|---|---|---|---|---|---|---|
| N = 99 | N = 97 | N = 196 | ||||
| Natural disaster (e.g. flood, hurricane, tornado, earthquake) | ||||||
| Happened to me | 6 | (6.1) | 7 | (7.2) | 13 | (6.6) |
| Witnessed it | 3 | (3.0) | 1 | (1.0) | 4 | (2.0) |
| Learned about it | 9 | (9.1) | 11 | (11.3) | 20 | (10.2) |
| Part of my job | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Not sure | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Does not apply | 81 | (81.8) | 80 | (82.5) | 161 | (82.1) |
| Fire or explosion | ||||||
| Happened to me | 6 | (6.1) | 6 | (6.2) | 12 | (6.1) |
| Witnessed it | 7 | (7.1) | 11 | (11.3) | 18 | (9.2) |
| Learned about it | 10 | (10.1) | 7 | (7.2) | 17 | (8.7) |
| Part of my job | 6 | (6.1) | 4 | (4.1) | 10 | (5.1) |
| Not sure | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Does not apply | 75 | (75.8) | 72 | (74.2) | 147 | (75.0) |
| Transportation accident (e.g. car accident, train wreck, plane crash) | ||||||
| Happened to me | 47 | (47.5) | 46 | (47.4) | 93 | (47.4) |
| Witnessed it | 10 | (10.1) | 9 | (9.3) | 19 | (9.7) |
| Learned about it | 11 | (11.1) | 12 | (12.4) | 23 | (11.7) |
| Part of my job | 3 | (3.0) | 5 | (5.2) | 8 | (4.1) |
| Not sure | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Does not apply | 36 | (36.4) | 36 | (37.1) | 72 | (36.7) |
| Serious accident at work, home or during recreational activity | ||||||
| Happened to me | 25 | (25.3) | 27 | (27.8) | 52 | (26.5) |
| Witnessed it | 9 | (9.1) | 11 | (11.3) | 20 | (10.2) |
| Learned about it | 4 | (4.0) | 5 | (5.2) | 9 | (4.6) |
| Part of my job | 3 | (3.0) | 4 | (4.1) | 7 | (3.6) |
| Not sure | 4 | (4.0) | 1 | (1.0) | 5 | (2.6) |
| Does not apply | 63 | (63.6) | 53 | (54.6) | 116 | (59.2) |
| Exposure to toxic substance (e.g. dangerous chemicals, radiation) | ||||||
| Happened to me | 3 | (3.0) | 0 | (0.0) | 3 | (1.5) |
| Witnessed it | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Learned about it | 5 | (5.1) | 1 | (1.0) | 6 | (3.1) |
| Part of my job | 7 | (7.1) | 1 | (1.0) | 8 | (4.1) |
| Not sure | 1 | (1.0) | 5 | (5.2) | 6 | (3.1) |
| Does not apply | 87 | (87.9) | 89 | (91.8) | 176 | (89.8) |
| Physical assault (e.g. being attacked, hit, slapped, kicked, beaten up) | ||||||
| Happened to me | 43 | (43.4) | 37 | (38.1) | 80 | (40.8) |
| Witnessed it | 7 | (7.1) | 11 | (11.3) | 18 | (9.2) |
| Learned about it | 4 | (4.0) | 12 | (12.4) | 16 | (8.2) |
| Part of my job | 3 | (3.0) | 6 | (6.2) | 9 | (4.6) |
| Not sure | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Does not apply | 45 | (45.5) | 43 | (44.3) | 88 | (44.9) |
| Assault with a weapon (e.g. being shot, stabbed, threatened with a knife, bomb) | ||||||
| Happened to me | 13 | (13.1) | 13 | (13.4) | 26 | (13.3) |
| Witnessed it | 4 | (4.0) | 4 | (4.1) | 8 | (4.1) |
| Learned about it | 5 | (5.1) | 8 | (8.2) | 13 | (6.6) |
| Part of my job | 2 | (2.0) | 6 | (6.2) | 8 | (4.1) |
| Not sure | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Does not apply | 74 | (74.7) | 67 | (69.1) | 141 | (71.9) |
| Sexual assault (rape, attempted rape, forced sexual acts) | ||||||
| Happened to me | 15 | (15.2) | 14 | (14.4) | 29 | (14.8) |
| Witnessed it | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Learned about it | 7 | (7.1) | 9 | (9.3) | 16 | (8.2) |
| Part of my job | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Not sure | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Does not apply | 77 | (77.8) | 73 | (75.3) | 150 | (76.5) |
| Other unwanted or uncomfortable sexual experience | ||||||
| Happened to me | 13 | (13.1) | 15 | (15.5) | 28 | (14.3) |
| Witnessed it | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Learned about it | 1 | (1.0) | 6 | (6.2) | 7 | (3.6) |
| Part of my job | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Not sure | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Does not apply | 84 | (84.8) | 76 | (78.4) | 160 | (81.6) |
| Combat or exposure to a warzone (in the military or as a civilian) | ||||||
| Happened to me | 2 | (2.0) | 1 | (1.0) | 3 | (1.5) |
| Witnessed it | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Learned about it | 7 | (7.1) | 7 | (7.2) | 14 | (7.1) |
| Part of my job | 2 | (2.0) | 2 | (2.1) | 4 | (2.0) |
| Not sure | 2 | (2.0) | 0 | (0.0) | 2 | (1.0) |
| Does not apply | 87 | (87.9) | 88 | (90.7) | 175 | (89.3) |
| Captivity (e.g. being kidnapped, abducted, held hostage, prisoner of war) | ||||||
| Happened to me | 3 | (3.0) | 2 | (2.1) | 5 | (2.6) |
| Witnessed it | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Learned about it | 2 | (2.0) | 6 | (6.2) | 8 | (4.1) |
| Part of my job | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Not sure | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Does not apply | 93 | (93.9) | 89 | (91.8) | 182 | (92.9) |
| Life-threatening illness or injury | ||||||
| Happened to me | 14 | (14.1) | 24 | (24.7) | 38 | (19.4) |
| Witnessed it | 15 | (15.2) | 20 | (20.6) | 35 | (17.9) |
| Learned about it | 7 | (7.1) | 8 | (8.2) | 15 | (7.7) |
| Part of my job | 3 | (3.0) | 4 | (4.1) | 7 | (3.6) |
| Not sure | 2 | (2.0) | 0 | (0.0) | 2 | (1.0) |
| Does not apply | 64 | (64.6) | 50 | (51.5) | 114 | (58.2) |
| Severe human suffering | ||||||
| Happened to me | 4 | (4.0) | 5 | (5.2) | 9 | (4.6) |
| Witnessed it | 6 | (6.1) | 14 | (14.4) | 20 | (10.2) |
| Learned about it | 7 | (7.1) | 4 | (4.1) | 11 | (5.6) |
| Part of my job | 2 | (2.0) | 2 | (2.1) | 4 | (2.0) |
| Not sure | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Does not apply | 83 | (83.8) | 75 | (77.3) | 158 | (80.6) |
| Sudden, violent death (e.g. homicide, suicide) | ||||||
| Happened to me | 6 | (6.1) | 5 | (5.2) | 11 | (5.6) |
| Witnessed it | 9 | (9.1) | 10 | (10.3) | 19 | (9.7) |
| Learned about it | 18 | (18.2) | 12 | (12.4) | 30 | (15.3) |
| Part of my job | 3 | (3.0) | 7 | (7.2) | 10 | (5.1) |
| Not sure | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Does not apply | 63 | (63.6) | 67 | (69.1) | 130 | (66.3) |
| Sudden, unexpected death of someone close to you | ||||||
| Happened to me | 37 | (37.4) | 34 | (35.1) | 71 | (36.2) |
| Witnessed it | 11 | (11.1) | 11 | (11.3) | 22 | (11.2) |
| Learned about it | 12 | (12.1) | 15 | (15.5) | 27 | (13.8) |
| Part of my job | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Not sure | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Does not apply | 42 | (42.4) | 41 | (42.3) | 83 | (42.3) |
| Serious injury, harm or death you caused to someone | ||||||
| Happened to me | 3 | (3.0) | 4 | (4.1) | 7 | (3.6) |
| Witnessed it | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Learned about it | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Part of my job | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Not sure | 0 | (0.0) | 2 | (2.1) | 2 | (1.0) |
| Does not apply | 95 | (96.0) | 88 | (90.7) | 183 | (93.4) |
| Any other stressful event or experience | ||||||
| Happened to me | 28 | (28.3) | 31 | (32.0) | 59 | (30.1) |
| Witnessed it | 5 | (5.1) | 5 | (5.2) | 10 | (5.1) |
| Learned about it | 5 | (5.1) | 2 | (2.1) | 7 | (3.6) |
| Part of my job | 4 | (4.0) | 2 | (2.1) | 6 | (3.1) |
| Not sure | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Does not apply | 60 | (60.6) | 58 | (59.8) | 118 | (60.2) |
| Childhood physical abuse | ||||||
| Happened to me | 10 | (10.1) | 8 | (8.2) | 18 | (9.2) |
| Witnessed it | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Learned about it | 3 | (3.0) | 6 | (6.2) | 9 | (4.6) |
| Part of my job | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Not sure | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Does not apply | 86 | (86.9) | 84 | (86.6) | 170 | (86.7) |
| Childhood sexual abuse or molestation | ||||||
| Happened to me | 5 | (5.1) | 2 | (2.1) | 7 | (3.6) |
| Witnessed it | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Learned about it | 4 | (4.0) | 5 | (5.2) | 9 | (4.6) |
| Part of my job | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Not sure | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Does not apply | 90 | (90.9) | 89 | (91.8) | 179 | (91.3) |
| Which one of these was the worst event that has happened to you? | ||||||
| Natural disaster | 1 | (1.0) | 4 | (4.1) | 5 | (2.6) |
| Fire or explosion | 2 | (2.0) | 2 | (2.1) | 4 | (2.0) |
| Transportation accident | 16 | (16.2) | 17 | (17.5) | 33 | (16.8) |
| Serious accident not transportation | 12 | (12.1) | 11 | (11.3) | 23 | (11.7) |
| Physical assault | 15 | (15.2) | 6 | (6.2) | 21 | (10.7) |
| Assault with a weapon | 8 | (8.1) | 5 | (5.2) | 13 | (6.6) |
| Sexual assault | 9 | (9.1) | 9 | (9.3) | 18 | (9.2) |
| Other unwanted sexual experience | 0 | (0.0) | 2 | (2.1) | 2 | (1.0) |
| Captivity | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Life-threatening illness or injury | 5 | (5.1) | 12 | (12.4) | 17 | (8.7) |
| Severe human suffering | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Sudden, violent death | 9 | (9.1) | 7 | (7.2) | 16 | (8.2) |
| Sudden, unexpected death of someone close to you | 12 | (12.1) | 10 | (10.3) | 22 | (11.2) |
| Serious injury, harm or death you caused to someone | 0 | (0.0) | 2 | (2.1) | 2 | (1.0) |
| Any other stressful event or experience | 4 | (4.0) | 10 | (10.3) | 14 | (7.1) |
| Childhood physical abuse | 2 | (2.0) | 0 | (0.0) | 2 | (1.0) |
| Childhood sexual abuse or molestation | 2 | (2.0) | 0 | (0.0) | 2 | (1.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| How old were you when the event started/happened? (age in years) | ||||||
| Mean (SD) | 33.5 | (14.4) | 31.8 | (13.5) | 32.7 | (13.9) |
| Median (IQR) | 33.0 | (20.0–45.0) | 27.0 | (21.0–42.0) | 30.0 | (21.0–43.0) |
| Minimum, maximum | (5.0, 69.0) | (8.0, 68.0) | (5.0, 69.0) | |||
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| How long did the event last? (time in hours) | ||||||
| Mean (SD) | 36.2 | (128.7) | 25.3 | (80.4) | 30.8 | (107.5) |
| Median (IQR) | 0.5 | (0.2–3.0) | 1.0 | (0.2–6.0) | 1.0 | (0.2–4.0) |
| Minimum, maximum | (0.0, 720.0) | (0.0, 600.0) | (0.0, 720.0) | |||
| Missing (%) | 5 | (5.1) | 6 | (6.2) | 11 | (5.6) |
| How long ago did the event end? (time in months) | ||||||
| Mean (SD) | 38.5 | (73.6) | 36.3 | (80.9) | 37.4 | (77.2) |
| Median (IQR) | 16.0 | (6.0–33.0) | 17.0 | (6.5–28.0) | 16.0 | (6.0–32.0) |
| Minimum, maximum | (2.0, 600.0) | (2.0, 720.0) | (2.0, 720.0) | |||
| Missing (%) | 2 | (2.0) | 1 | (1.0) | 3 | (1.5) |
The most prevalent event reported as the worst experience was a transportation accident (33/196, 16.8%). The next most common category was serious accident (not transportation) (23/196, 11.7%) and then the sudden, unexpected death of someone close to the participant (22/196, 11.2%).
There were some small imbalances observed, for example, physical assault where 15/99 (15.2%) reported this in the CBT-TF arm compared to 6/97 (6.2%) in the GSH arm. Similarly, in the life-threatening illness or injury category only 5/99 (5.1%) participants in the CBT-TF arm reported this as their worst event experience versus 12/97 (12.4%) in the GSH arm. These are relatively small numbers and so some imbalances are to be expected by chance alone even under perfect execution of the randomisation method. There was also a small difference in the ‘Any other stressful event or experience’ but the version of the LEC questions used did not ask the participant to specify the exact event or experience.
Mental health
During the baseline assessment, participants were asked to report if they had ever been told by a health professional that they had a particular mental health diagnosis. Figure 3 shows the prevalence of the reported mental health conditions. The most prevalent condition was PTSD reported by 147/195 (75.4%) participants. Generalised anxiety was the next most prevalent, reported by 129/196 (65.8%) participants, and depressive disorder the third most prevalent with 112/196 (57.1%) reporting having been told they had this condition. These three conditions were the most prevalent by a long margin. The next most prevalent condition was panic disorder which was reported by 21/196 (10.7%) participants with the remaining conditions reported by 0.0–7.7% of participants. Many conditions which were asked about had no participants reporting as being diagnosed.
FIGURE 3.
Prevalence of mental health diagnoses reported at baseline assessment.
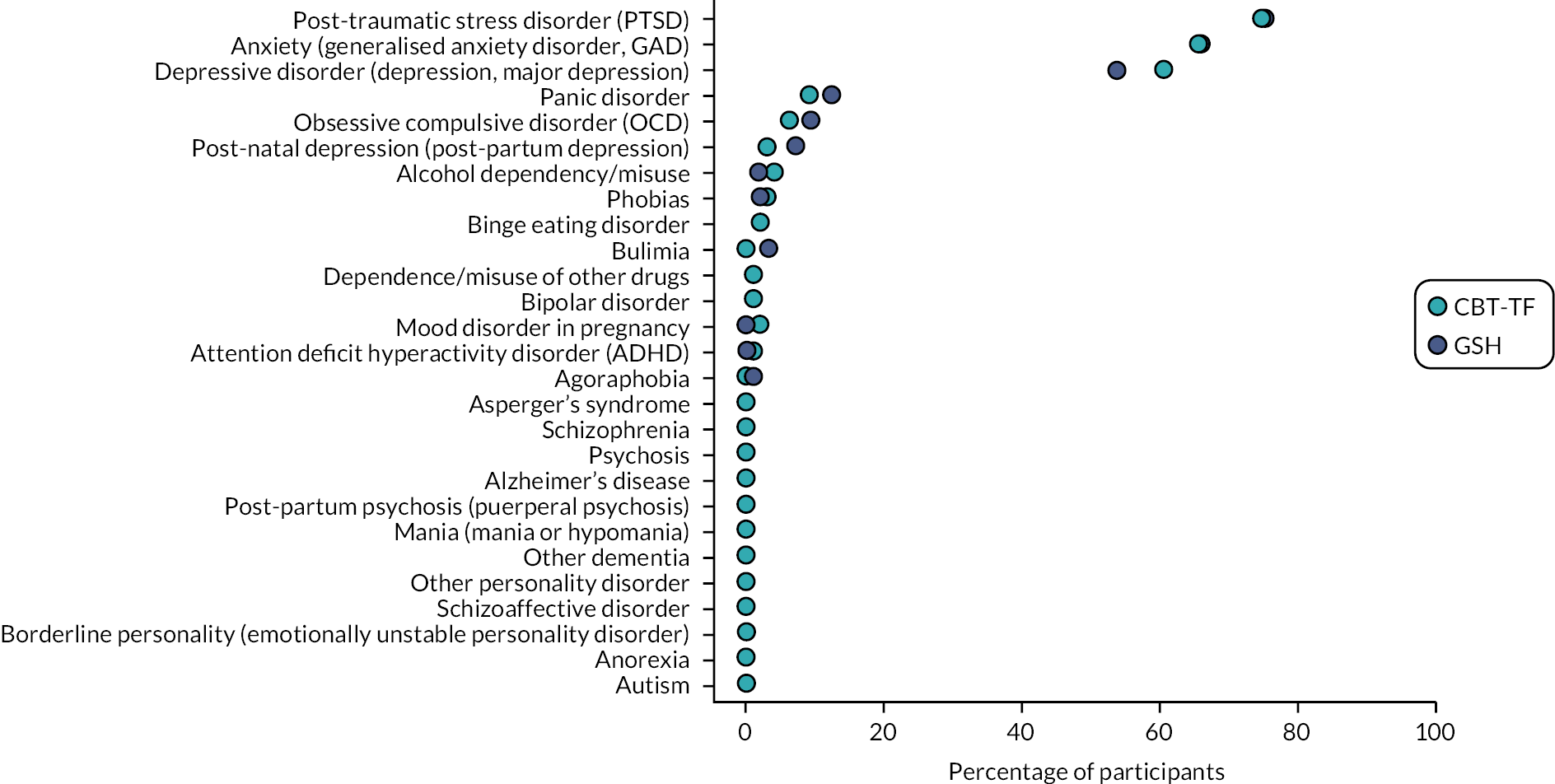
There were no serious imbalances observed between the two intervention arms although a small imbalance was observed in participants reporting depressive disorder with 60/99 (60.6%) in the CBT-TF group compared to 52/97 (53.6%) in the GSH arm. Table 6 shows the complete details of the participant responses.
| CBT-TF | GSH | Total | ||||
|---|---|---|---|---|---|---|
| N = 99 | N = 97 | N = 196 | ||||
| Have you ever been told by a health professional that you have? | ||||||
| PTSD | ||||||
| Yes | 74 | (75.5) | 73 | (75.3) | 147 | (75.4) |
| No | 24 | (24.5) | 24 | (24.7) | 48 | (24.6) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Anxiety (GAD) | ||||||
| Yes | 65 | (65.7) | 64 | (66.0) | 129 | (65.8) |
| No | 34 | (34.3) | 33 | (34.0) | 67 | (34.2) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Agoraphobia | ||||||
| Yes | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| No | 99 | (100.0) | 96 | (99.0) | 195 | (99.5) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Panic disorder | ||||||
| Yes | 9 | (9.1) | 12 | (12.4) | 21 | (10.7) |
| No | 90 | (90.9) | 85 | (87.6) | 175 | (89.3) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Phobias | ||||||
| Yes | 3 | (3.0) | 2 | (2.1) | 5 | (2.6) |
| No | 96 | (97.0) | 95 | (97.9) | 191 | (97.4) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| OCD | ||||||
| Yes | 6 | (6.1) | 9 | (9.3) | 15 | (7.7) |
| No | 93 | (93.9) | 88 | (90.7) | 181 | (92.3) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Depressive disorder (depression, major depression) | ||||||
| Yes | 60 | (60.6) | 52 | (53.6) | 112 | (57.1) |
| No | 39 | (39.4) | 45 | (46.4) | 84 | (42.9) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Bipolar disorder | ||||||
| Yes | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| No | 98 | (99.0) | 96 | (99.0) | 194 | (99.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Mania (mania or hypomania) | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Borderline personality (emotionally unstable personality disorder) | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Other personality disorder | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Schizophrenia | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Schizoaffective disorder | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Psychosis | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Autism | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Asperger’s syndrome | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Attention deficit hyperactivity disorder | ||||||
| Yes | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| No | 98 | (99.0) | 97 | (100.0) | 195 | (99.5) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Anorexia | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Bulimia | ||||||
| Yes | 0 | (0.0) | 3 | (3.1) | 3 | (1.5) |
| No | 99 | (100.0) | 94 | (96.9) | 193 | (98.5) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Binge eating disorder | ||||||
| Yes | 2 | (2.0) | 2 | (2.1) | 4 | (2.0) |
| No | 97 | (98.0) | 95 | (97.9) | 192 | (98.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Mood disorder in pregnancy | ||||||
| Yes | 2 | (2.0) | 0 | (0.0) | 2 | (1.0) |
| No | 97 | (98.0) | 97 | (100.0) | 194 | (99.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Post-natal depression (post-partum depression) | ||||||
| Yes | 3 | (3.0) | 7 | (7.2) | 10 | (5.1) |
| No | 96 | (97.0) | 90 | (92.8) | 186 | (94.9) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Post-partum psychosis (puerperal psychosis) | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Alcohol dependency/misuse | ||||||
| Yes | 4 | (4.0) | 2 | (2.1) | 6 | (3.1) |
| No | 95 | (96.0) | 95 | (97.9) | 190 | (96.9) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Dependence/misuse of other drugs | ||||||
| Yes | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| No | 98 | (99.0) | 96 | (99.0) | 194 | (99.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Alzheimer’s disease | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Other dementia | ||||||
| Yes | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| No | 99 | (100.0) | 97 | (100.0) | 196 | (100.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
Family history of mental health
Participants were asked to give details on their family history of mental health conditions based on whether a relative was a first-degree relative, defined as mother, father, son, daughter, brother or sister; or a second-degree relative which was defined as grandparents, grandchildren, half-brother or sister, aunts or uncles. Specifically, the relative must have been told by a health professional that they had one of the listed conditions.
Figure 4 shows the prevalence of each mental health condition separately for first- and second-degree relatives. In both cases the predominant condition among relatives was depressive disorder with 91/195 (46.7%) among first-degree relatives and 46/194 (23.7%) second-degree relatives. In the case of first-degree relatives, 26/195 (13.3%) reported having two or more relatives with depressive disorder (detailed results shown in Table 7). The second most prevalent mental health condition reported was anxiety, reported for 68/195 (34.9%) first-degree relatives and 33/193 (17.1%) second-degree relatives. In the case of first-degree relatives, participants reported 22/193 (11.4%) having two or more relatives with anxiety-related disorders.
FIGURE 4.
Prevalence of mental health conditions among first- and second-degree relatives of participants.

| CBT-TF | GSH | Total | ||||
|---|---|---|---|---|---|---|
| N = 99 | N = 97 | N = 196 | ||||
| How many first-degree relatives have had? | ||||||
| PTSD | ||||||
| 0 | 89 | (90.8) | 91 | (93.8) | 180 | (92.3) |
| 1 | 7 | (7.1) | 5 | (5.2) | 12 | (6.2) |
| 2 | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| 3 | 2 | (2.0) | 0 | (0.0) | 2 | (1.0) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Anxiety (GAD) | ||||||
| 0 | 66 | (67.3) | 61 | (62.9) | 127 | (65.1) |
| 1 | 19 | (19.4) | 27 | (27.8) | 46 | (23.6) |
| 2 | 8 | (8.2) | 5 | (5.2) | 13 | (6.7) |
| 3 | 3 | (3.1) | 2 | (2.1) | 5 | (2.6) |
| 4 | 2 | (2.0) | 2 | (2.1) | 4 | (2.1) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Agoraphobia | ||||||
| 0 | 95 | (96.9) | 94 | (96.9) | 189 | (96.9) |
| 1 | 3 | (3.1) | 3 | (3.1) | 6 | (3.1) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Panic disorder | ||||||
| 0 | 86 | (88.7) | 90 | (92.8) | 176 | (90.7) |
| 1 | 10 | (10.3) | 7 | (7.2) | 17 | (8.8) |
| 2 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 2 | (2.0) | 0 | (0.0) | 2 | (1.0) |
| Phobias | ||||||
| 0 | 94 | (95.9) | 93 | (95.9) | 187 | (95.9) |
| 1 | 3 | (3.1) | 2 | (2.1) | 5 | (2.6) |
| 2 | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| OCD | ||||||
| 0 | 89 | (90.8) | 93 | (95.9) | 182 | (93.3) |
| 1 | 7 | (7.1) | 3 | (3.1) | 10 | (5.1) |
| 2 | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| 3 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Depressive disorder (including major depression) | ||||||
| 0 | 50 | (51.0) | 54 | (55.7) | 104 | (53.3) |
| 1 | 33 | (33.7) | 32 | (33.0) | 65 | (33.3) |
| 2 | 11 | (11.2) | 6 | (6.2) | 17 | (8.7) |
| 3 | 2 | (2.0) | 3 | (3.1) | 5 | (2.6) |
| 4 | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| 5 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Bipolar disorder | ||||||
| 0 | 94 | (95.9) | 92 | (95.8) | 186 | (95.9) |
| 1 | 4 | (4.1) | 3 | (3.1) | 7 | (3.6) |
| 2 | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Mania (mania or hypomania) | ||||||
| 0 | 98 | (100.0) | 96 | (99.0) | 194 | (99.5) |
| 1 | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Borderline personality disorder | ||||||
| 0 | 93 | (94.9) | 96 | (99.0) | 189 | (96.9) |
| 1 | 5 | (5.1) | 1 | (1.0) | 6 | (3.1) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Other personality disorder | ||||||
| 0 | 97 | (99.0) | 97 | (100.0) | 194 | (99.5) |
| 1 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Schizophrenia | ||||||
| 0 | 97 | (99.0) | 97 | (100.0) | 194 | (99.5) |
| 1 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Schizoaffective disorder | ||||||
| 0 | 98 | (100.0) | 97 | (100.0) | 195 | (100.0) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Psychosis | ||||||
| 0 | 96 | (98.0) | 96 | (99.0) | 192 | (98.5) |
| 1 | 2 | (2.0) | 1 | (1.0) | 3 | (1.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Autism | ||||||
| 0 | 96 | (97.0) | 90 | (92.8) | 186 | (94.9) |
| 1 | 3 | (3.0) | 6 | (6.2) | 9 | (4.6) |
| 2 | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Asperger’s syndrome | ||||||
| 0 | 97 | (98.0) | 93 | (95.9) | 190 | (96.9) |
| 1 | 2 | (2.0) | 4 | (4.1) | 6 | (3.1) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Attention deficit hyperactivity disorder | ||||||
| 0 | 94 | (94.9) | 92 | (94.8) | 186 | (94.9) |
| 1 | 4 | (4.0) | 5 | (5.2) | 9 | (4.6) |
| 2 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Anorexia | ||||||
| 0 | 95 | (96.9) | 95 | (97.9) | 190 | (97.4) |
| 1 | 3 | (3.1) | 2 | (2.1) | 5 | (2.6) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Bulimia | ||||||
| 0 | 98 | (100.0) | 94 | (96.9) | 192 | (98.5) |
| 1 | 0 | (0.0) | 3 | (3.1) | 3 | (1.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Binge eating disorder | ||||||
| 0 | 96 | (98.0) | 97 | (100.0) | 193 | (99.0) |
| 1 | 2 | (2.0) | 0 | (0.0) | 2 | (1.0) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Mood disorder in pregnancy | ||||||
| 0 | 96 | (98.0) | 95 | (97.9) | 191 | (97.9) |
| 1 | 2 | (2.0) | 2 | (2.1) | 4 | (2.1) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Post-natal depression (post-partum depression) | ||||||
| 0 | 86 | (87.8) | 85 | (87.6) | 171 | (87.7) |
| 1 | 12 | (12.2) | 11 | (11.3) | 23 | (11.8) |
| 2 | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Post-partum psychosis (puerperal psychosis) | ||||||
| 0 | 98 | (100.0) | 97 | (100.0) | 195 | (100.0) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Alcohol dependence/misuse | ||||||
| 0 | 84 | (85.7) | 86 | (88.7) | 170 | (87.2) |
| 1 | 13 | (13.3) | 10 | (10.3) | 23 | (11.8) |
| 2 | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Dependence/misuse of drugs | ||||||
| 0 | 89 | (90.8) | 89 | (91.8) | 178 | (91.3) |
| 1 | 7 | (7.1) | 7 | (7.2) | 14 | (7.2) |
| 2 | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| 4 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Alzheimer’s disease | ||||||
| 0 | 95 | (96.0) | 95 | (97.9) | 190 | (96.9) |
| 1 | 3 | (3.0) | 2 | (2.1) | 5 | (2.6) |
| 3 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Other dementia | ||||||
| 0 | 91 | (91.9) | 96 | (99.0) | 187 | (95.4) |
| 1 | 8 | (8.1) | 0 | (0.0) | 8 | (4.1) |
| 2 | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| How many second-degree relatives have had? | ||||||
| PTSD | ||||||
| 0 | 95 | (96.9) | 92 | (95.8) | 187 | (96.4) |
| 1 | 2 | (2.0) | 4 | (4.2) | 6 | (3.1) |
| 2 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Anxiety (GAD) | ||||||
| 0 | 81 | (83.5) | 79 | (82.3) | 160 | (82.9) |
| 1 | 10 | (10.3) | 11 | (11.5) | 21 | (10.9) |
| 2 | 5 | (5.2) | 3 | (3.1) | 8 | (4.1) |
| 3 | 1 | (1.0) | 3 | (3.1) | 4 | (2.1) |
| Missing (%) | 2 | (2.0) | 1 | (1.0) | 3 | (1.5) |
| Agoraphobia | ||||||
| 0 | 97 | (99.0) | 95 | (97.9) | 192 | (98.5) |
| 1 | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Panic disorder | ||||||
| 0 | 94 | (96.9) | 94 | (96.9) | 188 | (96.9) |
| 1 | 3 | (3.1) | 3 | (3.1) | 6 | (3.1) |
| Missing (%) | 2 | (2.0) | 0 | (0.0) | 2 | (1.0) |
| Phobias | ||||||
| 0 | 98 | (100.0) | 94 | (96.9) | 192 | (98.5) |
| 1 | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| 2 | 0 | (0.0) | 2 | (2.1) | 2 | (1.0) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| OCD | ||||||
| 0 | 93 | (94.9) | 95 | (97.9) | 188 | (96.4) |
| 1 | 5 | (5.1) | 2 | (2.1) | 7 | (3.6) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Depressive disorder (including major depression) | ||||||
| 0 | 79 | (80.6) | 69 | (71.9) | 148 | (76.3) |
| 1 | 13 | (13.3) | 21 | (21.9) | 34 | (17.5) |
| 2 | 5 | (5.1) | 5 | (5.2) | 10 | (5.2) |
| 3 | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Missing (%) | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Bipolar disorder | ||||||
| 0 | 97 | (99.0) | 94 | (97.9) | 191 | (98.5) |
| 1 | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| 2 | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Mania (mania or hypomania) | ||||||
| 0 | 98 | (100.0) | 97 | (100.0) | 195 | (100.0) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Borderline personality disorder | ||||||
| 0 | 97 | (99.0) | 94 | (97.9) | 191 | (98.5) |
| 1 | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Missing (%) | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Other personality disorder | ||||||
| 0 | 98 | (100.0) | 96 | (100.0) | 194 | (100.0) |
| Missing (%) | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Schizophrenia | ||||||
| 0 | 94 | (95.9) | 92 | (95.8) | 186 | (95.9) |
| 1 | 4 | (4.1) | 3 | (3.1) | 7 | (3.6) |
| 2 | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Schizoaffective disorder | ||||||
| 0 | 97 | (99.0) | 96 | (100.0) | 193 | (99.5) |
| 1 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Psychosis | ||||||
| 0 | 96 | (98.0) | 96 | (100.0) | 192 | (99.0) |
| 1 | 2 | (2.0) | 0 | (0.0) | 2 | (1.0) |
| Missing (%) | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Autism | ||||||
| 0 | 95 | (96.0) | 89 | (91.8) | 184 | (93.9) |
| 1 | 3 | (3.0) | 7 | (7.2) | 10 | (5.1) |
| 2 | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Asperger’s syndrome | ||||||
| 0 | 96 | (97.0) | 96 | (99.0) | 192 | (98.0) |
| 1 | 3 | (3.0) | 1 | (1.0) | 4 | (2.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Attention deficit hyperactivity disorder | ||||||
| 0 | 96 | (97.0) | 90 | (93.8) | 186 | (95.4) |
| 1 | 3 | (3.0) | 6 | (6.3) | 9 | (4.6) |
| Missing (%) | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Anorexia | ||||||
| 0 | 95 | (96.9) | 96 | (99.0) | 191 | (97.9) |
| 1 | 3 | (3.1) | 1 | (1.0) | 4 | (2.1) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Bulimia | ||||||
| 0 | 96 | (98.0) | 95 | (97.9) | 191 | (97.9) |
| 1 | 2 | (2.0) | 2 | (2.1) | 4 | (2.1) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Binge eating disorder | ||||||
| 0 | 96 | (98.0) | 95 | (97.9) | 191 | (97.9) |
| 1 | 2 | (2.0) | 2 | (2.1) | 4 | (2.1) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Mood disorder in pregnancy | ||||||
| 0 | 98 | (100.0) | 95 | (97.9) | 193 | (99.0) |
| 1 | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| 2 | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Post-natal depression (post-partum depression) | ||||||
| 0 | 95 | (96.9) | 95 | (97.9) | 190 | (97.4) |
| 1 | 3 | (3.1) | 2 | (2.1) | 5 | (2.6) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Post-partum psychosis (puerperal psychosis) | ||||||
| 0 | 98 | (100.0) | 97 | (100.0) | 195 | (100.0) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Alcohol dependence/misuse | ||||||
| 0 | 89 | (90.8) | 86 | (88.7) | 175 | (89.7) |
| 1 | 6 | (6.1) | 5 | (5.2) | 11 | (5.6) |
| 2 | 3 | (3.1) | 3 | (3.1) | 6 | (3.1) |
| 3 | 0 | (0.0) | 3 | (3.1) | 3 | (1.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Dependence/misuse of drugs | ||||||
| 0 | 92 | (93.9) | 87 | (89.7) | 179 | (91.8) |
| 1 | 3 | (3.1) | 7 | (7.2) | 10 | (5.1) |
| 2 | 2 | (2.0) | 3 | (3.1) | 5 | (2.6) |
| 9 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Alzheimer’s disease | ||||||
| 0 | 82 | (82.8) | 87 | (89.7) | 169 | (86.2) |
| 1 | 13 | (13.1) | 10 | (10.3) | 23 | (11.7) |
| 2 | 3 | (3.0) | 0 | (0.0) | 3 | (1.5) |
| 3 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Other dementia | ||||||
| 0 | 86 | (86.9) | 85 | (87.6) | 171 | (87.2) |
| 1 | 11 | (11.1) | 10 | (10.3) | 21 | (10.7) |
| 2 | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| 3 | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
In the case of first-degree relatives, the next two most prevalent conditions were alcohol and drug dependence (25/195, 12.8%) and post-natal depression (24/195, 12.3%). For second-degree relatives the third and fourth most prevalent conditions were Alzheimer’s disease (27/196, 13.8%) and other types of dementia (25/196, 12.8%). This reflects the older age profile of the second-degree relatives which included grandparents and uncles and aunts.
There were no obvious serious imbalances observed between the GSH and CBT-TF groups as can be seen from Table 7.
Medical history
Participants were asked about their physical health, specifically if they had been diagnosed with particular conditions. The prevalence of these conditions is shown in Figure 5. The most prevalent condition reported was migraine headaches in 65/196 (33.2%) participants. A small imbalance was present between the CBT-TF arm (29/99, 29.3%) compared to the GSH arm (36/97, 37.1%). The next most common category was ‘Any other physical health problem’ reported by 52/196 (26.5%). This was collected as free text and an examination of these comments tabulated by intervention group revealed no patterns. Again, a small imbalance was observed between the groups with 22/99 (22.2%) in the CBT-TF arm compared to 30/97 (30.9%) in the GSH arm. However, a difference of seven or eight participants could arise from chance even under randomisation. Two conditions had prevalences larger than 20%, namely asthma (52/196, 26.5%) and head injury (40/196, 20.4%). Finally, three conditions were reported in more than 10% of patients: chronic pain 29/196 (14.8%); hypertension and high blood pressure 25/196 (12.8%); and inflammatory bowel disease 20/196 (10.2%). Table 8 lists the detailed prevalence results.
FIGURE 5.
Prevalence of physical health conditions reported at baseline.
| Treatment comparison | Total | |||||
|---|---|---|---|---|---|---|
| CBT-TF | GSH | |||||
| N = 99 | N = 97 | N = 196 | ||||
| Have you ever been told by a health professional that you have? | ||||||
| Asthma | 27 | (27.3) | 25 | (25.8) | 52 | (26.5) |
| Breast cancer | 0 | (0.0) | 2 | (2.1) | 2 | (1.0) |
| Cancer (other) | 2 | (2.0) | 4 | (4.1) | 6 | (3.1) |
| Chronic obstructive pulmonary disease | 3 | (3.0) | 0 | (0.0) | 3 | (1.5) |
| Chronic pain | 12 | (12.1) | 17 | (17.5) | 29 | (14.8) |
| Diabetes – Type 1 | 1 | (1.0) | 0 | (0.0) | 1 | (0.5) |
| Diabetes – Type 2 | 0 | (0.0) | 6 | (6.2) | 6 | (3.1) |
| Elevated lipids/cholesterol | 8 | (8.1) | 7 | (7.2) | 15 | (7.7) |
| Epilepsy/seizure disorder | 3 | (3.0) | 4 | (4.1) | 7 | (3.6) |
| Gastric or duodenal ulcers | 2 | (2.0) | 3 | (3.1) | 5 | (2.6) |
| Head injury | 23 | (23.2) | 17 | (17.5) | 40 | (20.4) |
| Heart disease | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Hypertension/high blood pressure | 12 | (12.1) | 13 | (13.4) | 25 | (12.8) |
| Human immunodeficiency virus | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Kidney disease | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Liver disease | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Memory loss (dementia) | 1 | (1.0) | 2 | (2.1) | 3 | (1.5) |
| Migraine headaches | 29 | (29.3) | 36 | (37.1) | 65 | (33.2) |
| Meningitis | 5 | (5.1) | 1 | (1.0) | 6 | (3.1) |
| Multiple sclerosis | 0 | (0.0) | 2 | (2.1) | 2 | (1.0) |
| Osteoarthritis | 4 | (4.0) | 6 | (6.2) | 10 | (5.1) |
| Osteoporosis | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Parkinson’s disease | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Rheumatoid arthritis | 3 | (3.0) | 1 | (1.0) | 4 | (2.0) |
| Stroke/haemorrhage | 2 | (2.0) | 1 | (1.0) | 3 | (1.5) |
| Overactive thyroid/hyperthyroidism | 3 | (3.0) | 3 | (3.1) | 6 | (3.1) |
| Underactive thyroid/hypothyroidism | 3 | (3.0) | 6 | (6.2) | 9 | (4.6) |
| Inflammatory bowel disease | 12 | (12.1) | 8 | (8.2) | 20 | (10.2) |
| Other autoimmune disease | 4 | (4.0) | 2 | (2.1) | 6 | (3.1) |
| Any other physical health problem | 22 | (22.2) | 30 | (30.9) | 52 | (26.5) |
Contact with the criminal justice system
Five participants reported contact with the criminal justice system in the past 3 months, three in the CBT-TF group and two in the GSH group. These contacts consisted of police interviews or short stays at the police station. There were no overnight stays in a police cell or court appearances. No psychiatric assessments were conducted during these contacts with the criminal justice system.
Quality of life (EQ-5D)
At baseline participants reported their quality of life using the EQ-5D instrument. The participants reported on their quality of life in five domains: mobility, self-care, ability to perform daily tasks, pain and anxiety. Participants are also asked to rate their overall quality of life on a scale of 0–100. For mobility, 140/195 (71.8%) participants reported no problems walking about. 45/195 (23.1%) reported slight or moderate difficulty walking about. In terms of taking care of themselves, 157/195 (80.5%) reported no problems, with 18.5% of participants reporting slight or moderate problems taking care of themselves. When asked about performing their usual activities, more participants reported issues, with only 67/196 (34.2%) reporting no problems with doing their usual activities. Having slight or moderate difficulties with usual activities were reported by just half of the participants (94/196, 48%). A further 14/196 (7.1%) indicated they could not perform their usual activities. A similar pattern was seen on the pain dimension with 88/196 (44.9%) reporting no pain issues and 75/196 (38.3%) reporting moderate to extreme problems with pain. Finally, practically all participants reported issues with anxiety or depression, with only 9/196 (4.6%) reporting not being anxious or depressed. Of the other participants, 113/196 (57.7%) reported being slightly or moderately anxious or depressed and 74/196 (37.8%) indicated a severe or extreme feeling of anxiety or depression.
When asked about ‘Your health today’ on a scale from 0 to 100, participants reported a mean score of 58.1 (SD 20.0). Scores were reported in the range 5–98 with a median of 60 (IQR 50–70). There were no notable imbalances observed between the CBT-TF and GSH groups in terms of quality of life. Table 9 gives a complete breakdown of the baseline EQ-5D.
| CBT-TF | GSH | Total | ||||
|---|---|---|---|---|---|---|
| N = 99 | N = 97 | N = 196 | ||||
| Mobility | ||||||
| I have no problems in walking about | 75 | (75.8) | 65 | (67.7) | 140 | (71.8) |
| I have slight problems in walking about | 13 | (13.1) | 9 | (9.4) | 22 | (11.3) |
| I have moderate problems in walking about | 9 | (9.1) | 14 | (14.6) | 23 | (11.8) |
| I have severe problems in walking about | 2 | (2.0) | 7 | (7.3) | 9 | (4.6) |
| I am unable to walk about | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Missing (%) | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Self-care | ||||||
| I have no problems washing or dressing myself | 81 | (81.8) | 76 | (79.2) | 157 | (80.5) |
| I have slight problems washing or dressing myself | 11 | (11.1) | 12 | (12.5) | 23 | (11.8) |
| I have moderate problems washing or dressing myself | 6 | (6.1) | 7 | (7.3) | 13 | (6.7) |
| I have severe problems washing or dressing myself | 1 | (1.0) | 1 | (1.0) | 2 | (1.0) |
| Missing (%) | 0 | (0.0) | 1 | (1.0) | 1 | (0.5) |
| Usual activities | ||||||
| I have no problems doing my usual activities | 35 | (35.4) | 32 | (33.0) | 67 | (34.2) |
| I have slight problems doing my usual activities | 19 | (19.2) | 25 | (25.8) | 44 | (22.4) |
| I have moderate problems doing my usual activities | 28 | (28.3) | 22 | (22.7) | 50 | (25.5) |
| I have severe problems doing my usual activities | 11 | (11.1) | 10 | (10.3) | 21 | (10.7) |
| I am unable to do my usual activities | 6 | (6.1) | 8 | (8.2) | 14 | (7.1) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Pain/discomfort | ||||||
| I have no pain or discomfort | 47 | (47.5) | 41 | (42.3) | 88 | (44.9) |
| I have slight pain or discomfort | 22 | (22.2) | 11 | (11.3) | 33 | (16.8) |
| I have moderate pain or discomfort | 16 | (16.2) | 30 | (30.9) | 46 | (23.5) |
| I have severe pain or discomfort | 12 | (12.1) | 11 | (11.3) | 23 | (11.7) |
| I have extreme pain or discomfort | 2 | (2.0) | 4 | (4.1) | 6 | (3.1) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Anxiety/depression | ||||||
| I am not anxious or depressed | 4 | (4.0) | 5 | (5.2) | 9 | (4.6) |
| I am slightly anxious or depressed | 17 | (17.2) | 20 | (20.6) | 37 | (18.9) |
| I am moderately anxious or depressed | 46 | (46.5) | 30 | (30.9) | 76 | (38.8) |
| I am severely anxious or depressed | 19 | (19.2) | 25 | (25.8) | 44 | (22.4) |
| I am extremely anxious or depressed | 13 | (13.1) | 17 | (17.5) | 30 | (15.3) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Your health today (VAS) | ||||||
| Mean (SD) | 56.7 | (18.3) | 59.4 | (21.5) | 58.1 | (20.0) |
| Median (IQR) | 60.0 | (50.0–70.0) | 65.0 | (50.0–75.0) | 60.0 | (50.0–70.0) |
| Minimum, maximum | (6.0, 95.0) | (5.0, 98.0) | (5.0, 98.0) | |||
| Missing (%) | 5 | (5.1) | 1 | (1.0) | 6 | (3.1) |
Adherence
Table 10 shows the detailed breakdown of adherence split by intervention group. In the CBT-TF group participants attended an average of 8.6 (SD 3.4) face-to-face therapy sessions with a median of 9 (IQR 6–12) and a range of 0–16. For the GSH group participants attended a mean of 3.9 (SD 1.7) face-to-face sessions with a median of 5 (IQR 3–5) and a range of 0–8. Looking at the detailed distribution of face-to-face sessions attended, as shown in Table 10, in the CBT-TF group 30/97 (30.3%) attended 12 sessions and the most frequent number of sessions in the GSH group was 5, with 47/97 (48.5%) participants.
| CBT-TF | GSH | |||
|---|---|---|---|---|
| N = 99 | N = 97 | |||
| Face-to-face sessions attended | ||||
| Mean (SD) | 8.6 | (3.4) | 3.9 | (1.7) |
| Median (IQR) | 9.0 | (6.0–12.0) | 5.0 | (3.0–5.0) |
| Minimum, maximum | (0.0, 16.0) | (0.0, 8.0) | ||
| Missing (%) | 1 | (1.0) | 2 | (2.1) |
| Face-to-face sessions attended | ||||
| 0 | 3 | (3.1) | 5 | (5.3) |
| 1 | 1 | (1.0) | 9 | (9.5) |
| 2 | 2 | (2.0) | 4 | (4.2) |
| 3 | 3 | (3.1) | 14 | (14.7) |
| 4 | 4 | (4.1) | 13 | (13.7) |
| 5 | 3 | (3.1) | 47 | (49.5) |
| 6 | 10 | (10.2) | 2 | (2.1) |
| 7 | 11 | (11.2) | 0 | (0.0) |
| 8 | 5 | (5.1) | 1 | (1.1) |
| 9 | 10 | (10.2) | 0 | (0.0) |
| 10 | 7 | (7.1) | 0 | (0.0) |
| 11 | 8 | (8.2) | 0 | (0.0) |
| 12 | 30 | (30.6) | 0 | (0.0) |
| 16 | 1 | (1.0) | 0 | (0.0) |
| Missing (%) | 1 | (1.0) | 2 | (2.1) |
| Telephone/video calls attended | ||||
| Mean (SD) | 3.5 | (0.7) | 3.2 | (1.0) |
| Median (IQR) | 3.5 | (3.0, 4.0) | 3.0 | (2.0, 4.0) |
| Minimum, maximum | (3.0, 4.0) | (1.0, 5.0) | ||
| Missing (%) | 97 | (98.0) | 63 | (64.9) |
| Telephone/video calls attended | ||||
| 1 | 0 | (0.0) | 1 | (2.9) |
| 2 | 0 | (0.0) | 8 | (23.5) |
| 3 | 1 | (50.0) | 10 | (29.4) |
| 4 | 1 | (50.0) | 13 | (38.2) |
| 5 | 0 | (0.0) | 2 | (5.9) |
| Missing (%) | 97 | (98.0) | 63 | (64.9) |
| Adherence status | ||||
| Non-complier | 38 | (38.4) | 20 | (20.6) |
| Complier | 61 | (61.6) | 77 | (79.4) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) |
| Per-protocol status | ||||
| Non-protocol | 38 | (38.4) | 34 | (35.1) |
| Per protocol | 61 | (61.6) | 63 | (64.9) |
| Missing (%) | 0 | (0.0) | 0 | (0.0) |
In terms of the agreed definition of adherence, 71/97 (73.2%) of the participants in the GSH group met the criteria for adherence compared to 60/99 (60.6%) of the CBT-TF group participants. When the criteria are tightened to examine those participants who received the intervention as specified in the protocol (the ‘per protocol’ population) then 63/99 (64.9%) of the GSH group achieved this compared to 60/99 (60.6%) in the CBT-TF group.
Distributions and summary statistics of outcome measures
Figure 6 and Table 11 show the distributions and summary statistics for the primary and secondary outcome measures.
FIGURE 6.
Distributions of outcome measures.

| Study outcomes | CBT-TF | GSH | Regression analysis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 16 weeks | 52 weeks | Baseline | 16 weeks | 52 weeks | 16 weeks | 52 weeks | |||||||||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | Effect size | One-sided 95% CI | Non-inferiority, p | Effect Size | One-sided 95% CI | Non-inferiority, p | |
| CAPS-5 | 99 | 35.6 (6.7) | 83 | 13.0 (11.1) | 70 | 10.9 (11.1) | 97 | 34.6 (6.8) | 77 | 13.1 (11.7) | 69 | 12.9 (11.6) | 0.150 | (−∞, 0.585) | 0.012 | 0.476 | (−∞, 0.892) | 0.145 |
| IES-R | 99 | 55.7 (12.2) | 78 | 20.3 (19.1) | 57 | 13.2 (14.8) | 97 | 53.5 (14.5) | 68 | 19.9 (18.0) | 54 | 21.7 (22.1) | 0.102 | (−∞, 0.478) | 0.041 | 0.876 | (−∞, 1.274) | 0.060 |
| WSAS | 99 | 20.9 (9.8) | 75 | 10.4 (10.8) | 55 | 6.5 (8.1) | 97 | 21.1 (10.2) | 68 | 8.9 (9.8) | 53 | 8.0 (10.5) | −0.136 | (−∞, 0.133) | <0.001 | 0.244 | (−∞, 0.526) | 0.068 |
| PHQ-9 | 99 | 15.1 (5.7) | 75 | 7.1 (6.5) | 55 | 4.8 (5.0) | 97 | 15.1 (6.7) | 69 | 7.1 (6.8) | 52 | 6.5 (7.0) | 0.006 | (−∞, 0.253) | <0.001 | 0.315 | (−∞, 0.577) | 0.123 |
| GAD-7 | 99 | 13.4 (4.6) | 75 | 5.3 (5.3) | 55 | 3.8 (4.1) | 97 | 13.9 (4.9) | 67 | 5.6 (5.4) | 52 | 5.3 (5.6) | 0.100 | (−∞, 0.410) | 0.017 | 0.470 | (−∞, 0.781) | 0.436 |
| AUDIT-O | 99 | 5.8 (5.2) | 75 | 4.9 (5.0) | 55 | 4.9 (4.4) | 97 | 5.7 (5.5) | 68 | 5.7 (5.7) | 52 | 5.9 (6.5) | 0.151 | (−∞, 0.322) | <0.001 | 0.129 | (−∞, 0.346) | 0.003 |
| MSPSS | 99 | 5.2 (1.0) | 75 | 5.8 (1.0) | 55 | 5.8 (0.9) | 97 | 5.6 (0.9) | 67 | 6.0 (0.8) | 52 | 6.1 (0.8) | −0.059 | (−∞, 0.195) | <0.001 | −0.174 | (−∞, 0.103) | <0.001 |
| EQ-5D-5L (quality of life) | 94 | 56.7 (18.3) | 75 | 71.3 (17.3) | 55 | 76.6 (16.0) | 96 | 59.4 (21.5) | 67 | 70.1 (20.8) | 52 | 73.3 (20.0) | 0.093 | (−∞, 0.325) | 0.002 | 0.219 | (−∞, 0.497) | 0.048 |
| EQ-5D-5L (utilities) | 99 | 0.6 (0.2) | 75 | 0.8 (0.2) | 55 | 0.8 (0.2) | 97 | 0.5 (0.3) | 67 | 0.7 (0.3) | 52 | 0.7 (0.3) | 0.122 | (−∞, 0.367) | 0.006 | 0.279 | (−∞, 0.566) | 0.102 |
| ISI | 99 | 17.4 (5.4) | 75 | 9.1 (7.6) | 55 | 7.1 (7.1) | 97 | 16.5 (7.5) | 67 | 8.6 (7.7) | 52 | 7.7 (7.8) | 0.057 | (−∞, 0.378) | 0.012 | 0.275 | (−∞, 0.595) | 0.123 |
| PTCI | 99 | 79.2 (20.5) | 77 | 51.0 (26.6) | 56 | 43.3 (23.1) | 97 | 80.6 (23.7) | 68 | 46.3 (23.7) | 54 | 48.3 (25.7) | −0.201 | (−∞, 0.080) | <0.001 | 0.286 | (−∞, 0.619) | 0.144 |
| GSES | 99 | 24.8 (6.3) | 75 | 30.1 (6.8) | 55 | 31.3 (6.8) | 97 | 24.8 (6.8) | 67 | 29.4 (7.0) | 52 | 30.5 (6.6) | 0.258 | (−∞, 0.506) | 0.054 | 0.210 | (−∞, 0.493) | 0.046 |
| CSQ-8 | N/A | - (-) | 75 | 29.8 (3.3) | N/A | - (-) | N/A | - (-) | 70 | 26.9 (6.3) | N/A | - (-) | 0.600 | (−∞, 0.869) | 0.270 | - | (-, -) | - |
Analysis of primary outcome
Primary analysis
The primary outcome was the difference in CAPS-5 score at 16 weeks between the GSH group and CBT-TF group. At 16 weeks this included data on 160/196 (81.6%) participants. Note this exceeded the assumption of 80% retention used in the sample size calculation. Full data were available on all baseline variables used in the analysis models. The results of the primary analysis and SAs are shown in Figure 7.
FIGURE 7.
Results of the primary analysis of CAPS-5 score at 16 weeks.
Analysis adjusted for the following variables at baseline: CAPS-5 score, gender, research centre, comorbid depression (baseline PHQ-9) and time since trauma (months).
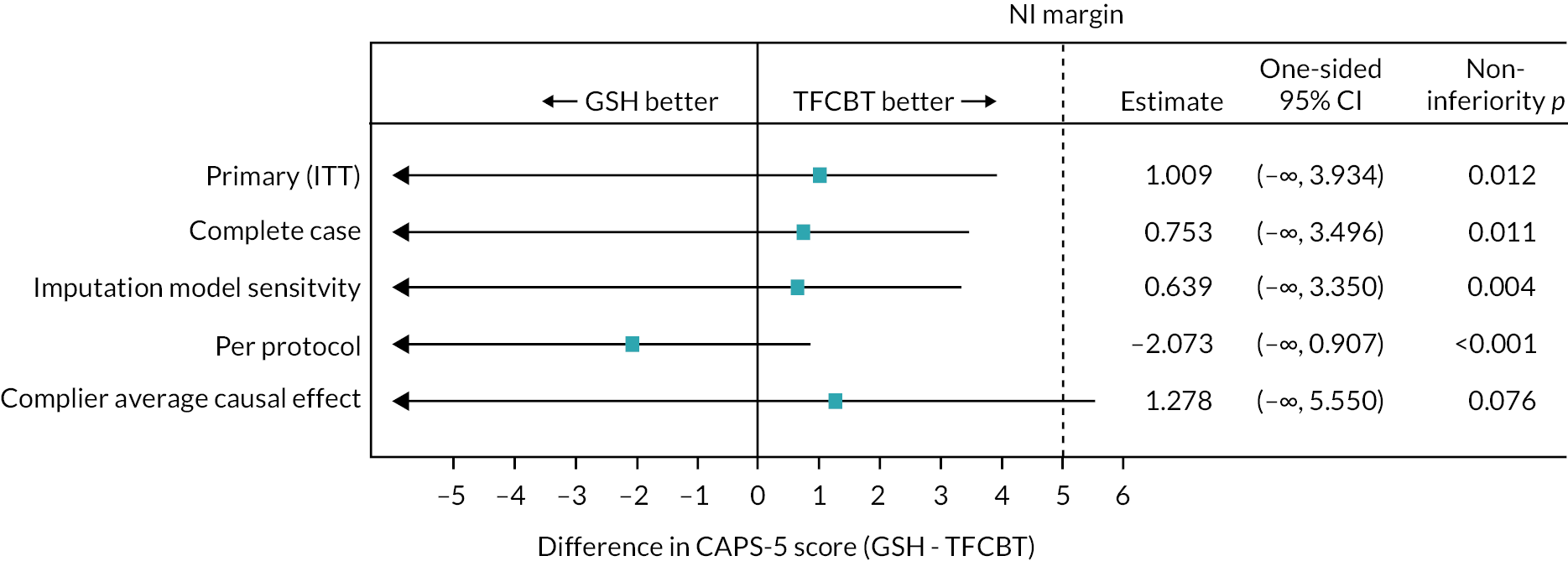
The primary ITT analysis resulted in an estimated mean difference in CAPS-5 score at 16 weeks between the CBT-TF group and GSH group of 1.0 points with a one-sided 95% CI of (−∞, 3.9). Since the mean difference represented the adjusted CAPS-5 mean difference in the GSH group minus the CBT-TF group, the observed difference of 1.0 points can be interpreted as the GSH patients had on average, after adjusting for the pre-specified baseline covariates, a 1.0 point higher CAPS-5 than the CBT-TF group. Since higher CAPS-5 scores are considered a worse outcome, this means that GSH had a very small overall lower impact than CBT-TF. However, the one-sided 95% CI contained 0 and it was consistent with the null hypothesis of no difference in mean CAPS-5 scores at 16 weeks. The primary outcome was assessed on a NI basis with NI attained if the upper limit of the one-sided 95% CI was < 5, indicating a mean CAPS-5 outcome at most 5 points higher in the GSH group. Since the upper limit of the one-sided 95% CI here is 3.9, it excluded five, and so non-inferiority was attained, NI p = 0.012.
Sensitivity analyses
A series of SAs were performed to check the robustness of the primary analysis using only complete cases where the estimated mean difference was 0.8 points with one-sided 95% CI (−∞, 3.5). The complete-case analysis was also consistent with the achievement of NI. A per-protocol analysis also showed NI was achieved with an estimated mean difference of −2.1 and one-sided 95% CI (−∞, 0.9). Finally, the CACE analysis did not quite achieve NI with an estimated mean difference of 1.3 and a one-sided 9% CI (−∞, 5.6). However, the CACE analysis here was highly variable compared to the other analyses although having a consistent estimated mean difference. The two-stage estimation process appears to have inflated the underlying variability for this analysis. Finally, a SA that used a different imputation model also showed evidence of NI with an estimated mean difference of 0.6 and a one-sided 95% CI (−∞, 3.5).
Prevalence of PTSD
At each assessment, participants were assessed, based on the CAPS-5 score, if they met the criteria for a DSM-5 PTSD diagnosis. At baseline all participants had this diagnosis as a function of the inclusion criteria. Overall, assessed PTSD diagnoses declined over the course of the trial with 26/160 (16.3%) participants having a PTSD diagnosis at 16 weeks and 16/139 (11.5%) participants were assessed as having PTSD at 52 weeks. At both time points over 80% of participants were not assessed as having a CAPS-5-based assessment of PTSD.
At the 16-week assessment 12/83 (14.5%) of the CBT-TF group and 14/77 (18.2%) of the GSH group met the criteria for the PTSD diagnosis. However, there was little evidence that the proportions in each group differed (p = 0.5). Similarly, at 52 weeks there was little to suggest a difference between the CBT-TF and GSH groups (p = 0.3), with 6/70 (8.6%) in the CBT-TF group and 10/69 (14.5%) in the GSH group being assessed as having PTSD.
Interpretation
Considering the primary analysis and the additional SAs there is clear evidence that GSH is non-inferior to CBT-TF at 16 weeks and in all analyses the estimated mean difference was around 1 point on the CAPS-5 score. Considering the CAPS-5 has a range from 0 to 80 a 1-point difference is for all intents and purposes a trivial difference and this was shown in all analyses where the data were consistent with the null hypothesis of no difference between the two groups. In terms of prevalence of PTSD assessed by the CAPS-5 instrument there were substantial declines in both groups with little evidence to suggest GSH had significantly higher prevalence than CBT-TF.
Secondary outcomes
Primary outcome at 52 weeks
The analyses of the primary outcome were repeated at 52 weeks and the results are shown in Figure 8. The ITT analysis showed a larger reduction in CAPS-5 score for the CBT-TF group with an estimated mean difference between the GSH group and CBT-TF group of 3.2 with one-sided 95% CI (−∞, 6.0). So, at the 52-week assessment the GSH group narrowly failed to show NI. The complete case analysis was similar with an estimated mean difference of 3.5 and one-sided 95% CI (−∞, 6.3). The multiple imputation SA narrowly showed NI with an estimated mean difference of 2.3 and one-sided 95% CI (−∞, 4.9). Similar results were also observed for the per-protocol and CACE analyses.
FIGURE 8.
Results of the primary analysis of CAPS-5 score at 52 weeks.
Analysis adjusted for the following variables at baseline: CAPS-5 score, gender, research centre, comorbid depression (baseline PHQ-9) and time since trauma (months).
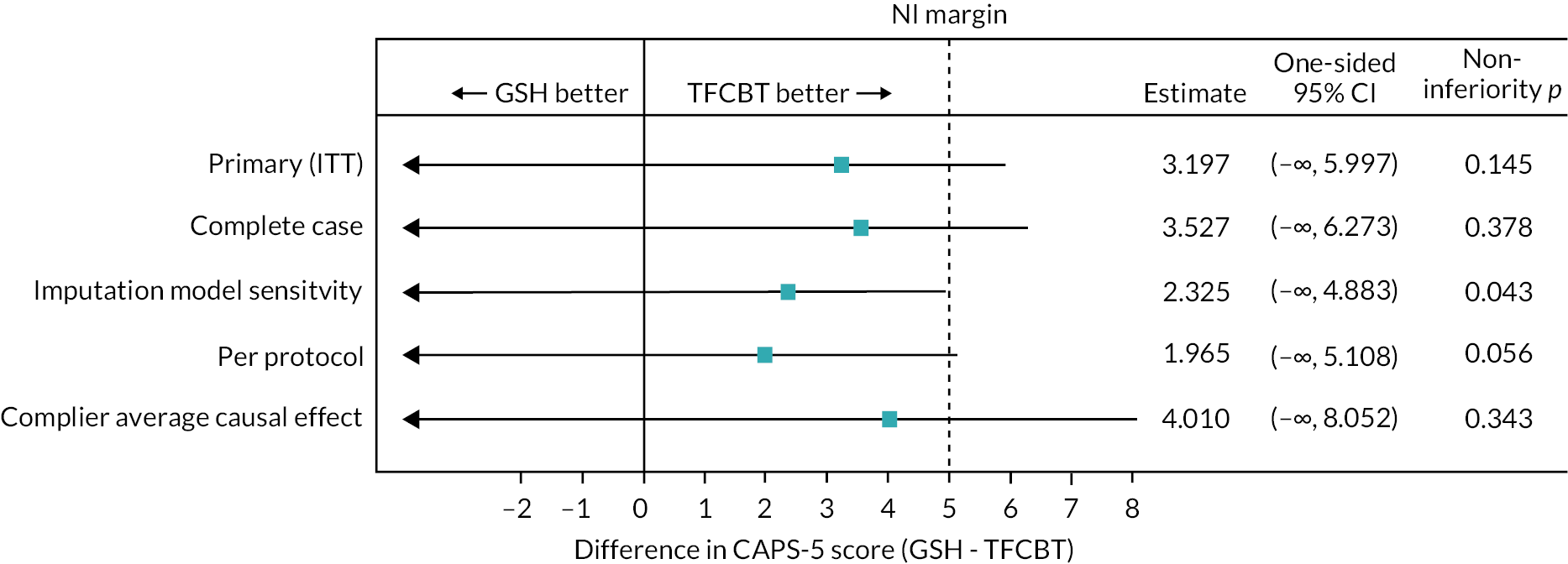
The interpretation at the 52-week assessment was that the CBT-TF scores were roughly 3 points lower on average than the GSH scores and that NI was not achieved although only very narrowly with the ITT analysis showing a one-sided 95% CI with an upper limit of 6 a single point above the NI margin of 5. An examination of the mean scores for each group (see Figure 11) showed that the GSH group sustained their improved CAPS-5 scores while the CBT-TF group continued to improve very slightly between the 16- and 52-week assessments.
Secondary outcomes at 16 weeks
Secondary outcomes at 16 weeks were analysed using the same statistical modelling approach as the primary ITT analysis. To ensure comparability the adjusted mean differences between the GSH and CBT-TF groups were converted to Cohen’s d effect size by dividing the adjusted mean difference estimates by the regression models by the pooled SD of the baseline outcome measures. These results are shown in Figure 9 with full details in Tables 12 and 13.
FIGURE 9.
Analysis results of secondary outcomes at 16 weeks.
Analysis adjusted for the following variables at baseline: baseline score, gender, research centre, comorbid depression (baseline PHQ-9) and time since trauma (months).
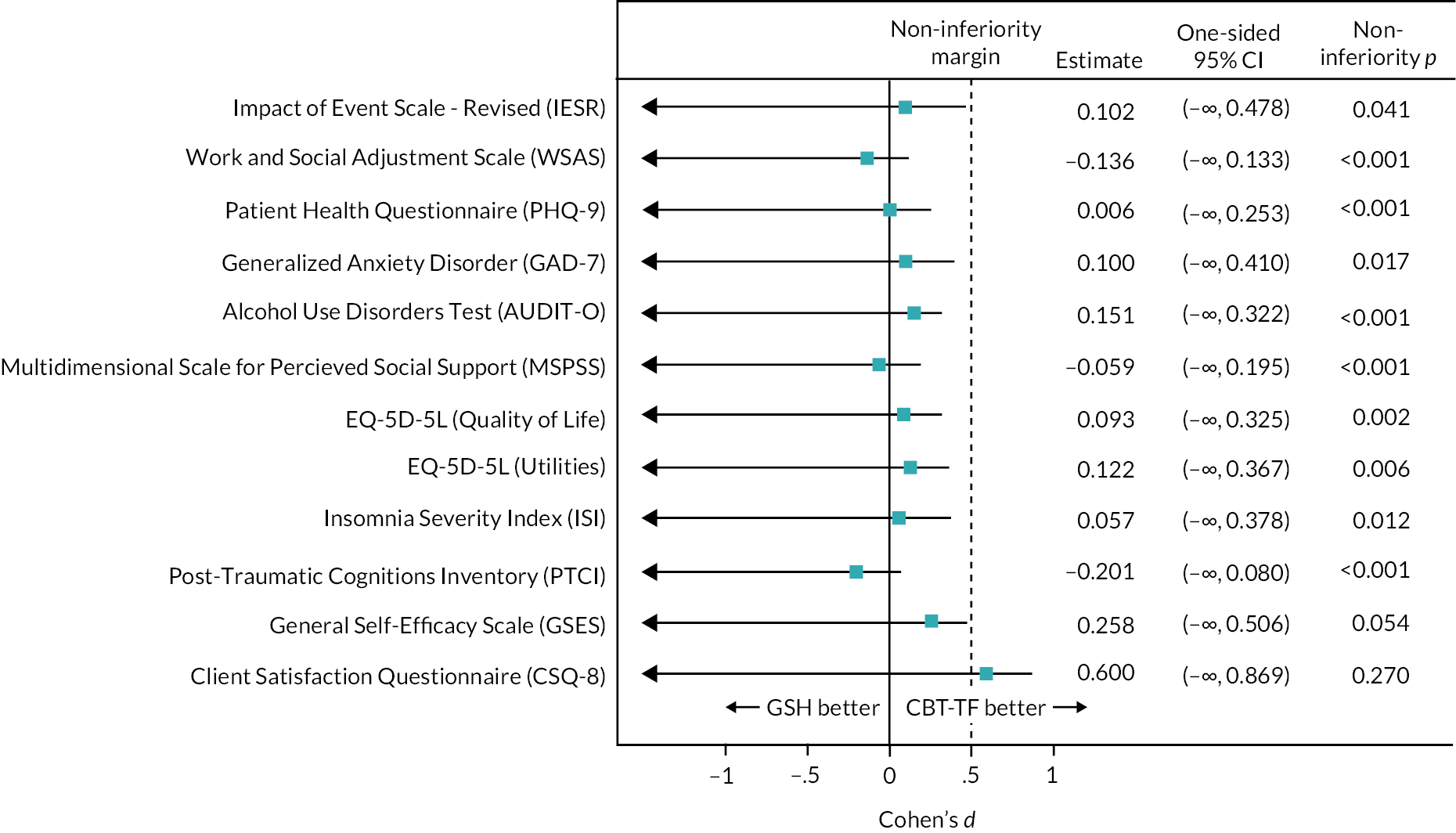
| Study outcomes | Regression analysis | |||||
|---|---|---|---|---|---|---|
| 16 weeks | 52 weeks | |||||
| Effect size | One-sided 95% CI | Non-inferiority, p | Effect size | One-sided 95% CI | Non-inferiority, p | |
| CAPS-5 | 0.150 | (−∞, 0.585) | 0.012 | 0.476 | (−∞, 0.892) | 0.145 |
| IES-R | 0.102 | (−∞, 0.478) | 0.041 | 0.876 | (−∞, 1.274) | 0.060 |
| WSAS | −0.136 | (−∞, 0.133) | < 0.001 | 0.244 | (−∞, 0.526) | 0.068 |
| PHQ-9 | 0.006 | (−∞, 0.253) | < 0.001 | 0.315 | (−∞, 0.577) | 0.123 |
| GAD-7 | 0.100 | (−∞, 0.410) | 0.017 | 0.470 | (−∞, 0.781) | 0.436 |
| AUDIT-O | 0.151 | (−∞, 0.322) | < 0.001 | 0.129 | (−∞, 0.346) | 0.003 |
| MSPSS | −0.059 | (−∞, 0.195) | < 0.001 | −0.174 | (−∞, 0.103) | < 0.001 |
| ISI | 0.057 | (−∞, 0.378) | 0.012 | 0.275 | (−∞, 0.595) | 0.123 |
| PTCI | −0.201 | (−∞, 0.080) | < 0.001 | 0.286 | (−∞, 0.619) | 0.144 |
| GSES | 0.258 | (−∞, 0.506) | 0.054 | 0.210 | (−∞, 0.493) | 0.046 |
| CSQ-8 | 0.600 | (−∞, 0.869) | 0.270 | - | (-, -) | - |
| Study outcomes | Non-inferiority | Regression analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| 16 weeks | 52 weeks | |||||||
| Pooled SD | Margin | Effect size | One-sided 95% CI | Non-inferiority, p | Effect size | One-sided 95% CI | Non-inferiority, p | |
| CAPS-5 | 6.7 | 5.0 | 1.009 | (−∞, 3.934) | 0.012 | 3.197 | (−∞, 5.997) | 0.145 |
| IES-R | 13.4 | 6.7 | 1.370 | (−∞, 6.402) | 0.041 | 11.734 | (−∞, 17.061) | 0.060 |
| WSAS | 10.0 | 5.0 | −1.353 | (−∞, 1.323) | < 0.001 | 2.438 | (−∞, 5.253) | 0.068 |
| PHQ-9 | 6.2 | 3.1 | 0.040 | (−∞, 1.565) | < 0.001 | 1.947 | (−∞, 3.574) | 0.123 |
| GAD-7 | 4.7 | 2.4 | 0.471 | (−∞, 1.932) | 0.017 | 2.213 | (−∞, 3.677) | 0.436 |
| AUDIT-O | 5.3 | 2.7 | 0.805 | (−∞, 1.721) | < 0.001 | 0.690 | (−∞, 1.849) | 0.003 |
| MSPSS | 1.0 | 0.5 | −0.057 | (−∞, 0.189) | < 0.001 | −0.169 | (−∞, 0.100) | < 0.001 |
| ISI | 6.6 | 3.3 | 0.372 | (−∞, 2.484) | 0.012 | 1.808 | (−∞, 3.902) | 0.123 |
| PTCI | 22.1 | 11.1 | −4.444 | (−∞, 1.778) | < 0.001 | 6.322 | (−∞, 13.686) | 0.144 |
| GSES | 6.5 | 3.3 | 1.691 | (−∞, 3.311) | 0.054 | 1.378 | (−∞, 3.227) | 0.046 |
| CSQ-8 | 5.1 | 2.6 | 0.600 | (−∞, 0.869) | 0.270 | - | (-, -) | - |
As can be seen from Figure 9 most of the secondary outcomes showed a modest effect size with most outcomes exhibiting observed NI. The GSES was borderline over the NI margin of 0.5 SD [one-sided 95% CI (−∞, 0.51)]. Only the client satisfaction questionnaire (CSQ-8) showed clear lack of NI at 16 weeks with an effect size of 0.6, one-sided 95% CI (−∞, 0.87), NI p = 0.27 with the upper limit exceeding the NI margin.
Secondary outcomes at 52 weeks
Figure 10 shows results for the analysis of secondary outcomes at the 52-week assessment. In general, the results show a more positive outcome for the CBT-TF group compared to the GSH group.
FIGURE 10.
Analysis results of secondary outcomes at 52 weeks.
Analysis adjusted for the following variables at baseline: baseline score, gender, research centre, comorbid depression (baseline PHQ-9) and time since trauma (months).

Two outcomes, the AUDIT-O scale and the MSPSS, were within the NI margin with effect sizes of 0.1, one-sided 95% CI (−∞, 0.3), NI p = 0.003; and −0.2, one-sided 95% CI (−∞, 0.1), non-inferiority p < 0.001, respectively. Two additional outcomes, EQ-5D-5L (quality of life) and the GSES, achieved borderline NI with an effect size of 0.2 m one-sided 95% CI (−∞, 0.497), non-inferiority p = 0.048; and 0.2, one-sided 95% CI (−∞, 0.49), NI p = 0.046, respectively. There was slightly less evidence for borderline NI for the WSAS, effect size of 0.2, one-sided 95% CI (−∞, 0.53), NI p = 0.07.
While none of the other secondary outcomes showed evidence of NI, most were inconclusive in terms of superiority, that is, consistent with the null hypothesis of no difference, between the CBT-TF and GSH groups at 52 weeks. Only two scales, the IES-R, p < 0.001, and GAD-7, p = 0.013, showed evidence of a difference in favour of CBT-TF over GSH at 52 weeks.
Change in mean CAPS-5 scores
Figure 11 shows the change in mean CAPS-5 score over time. In the CBT-TF group the mean CAPS-5 score reduced from 35.6 at baseline to 13.2, 95% CI (10.7, 15.7) at 16 weeks and 10.2, 95% CI (7.9, 12.6) at 52 weeks. For participants in the GSH group their CAPS-5 score reduced from 34.6 at baseline to 14.2, 95% CI (11.4, 17.0) at 16 weeks and a slight further reduction to 13.8, 95% CI (11.4, 16.1) at 52 weeks. This analysis needs to be interpreted slightly carefully as being a within-participant comparison; it may be subject to bias from regression to the mean. However, given that proviso it suggests that both the CBT-TF and GSH groups saw a substantial reduction in their CAPS-5 scores between baseline and the 16-week assessment. Furthermore, the CBT-TF group participants continued to have slightly improved CAPS-5 scores from the week 16 assessment to the week 52 assessment while the GSH group sustained their improvement in CAPS-5 score over the same period.
FIGURE 11.
Change in CAPS-5 mean scores over time.
Means at 16 and 52 weeks adjusted for the following variables at baseline: CAPS-5 score, gender, research centre, comorbid depression (baseline PHQ-9) and time since trauma (months).
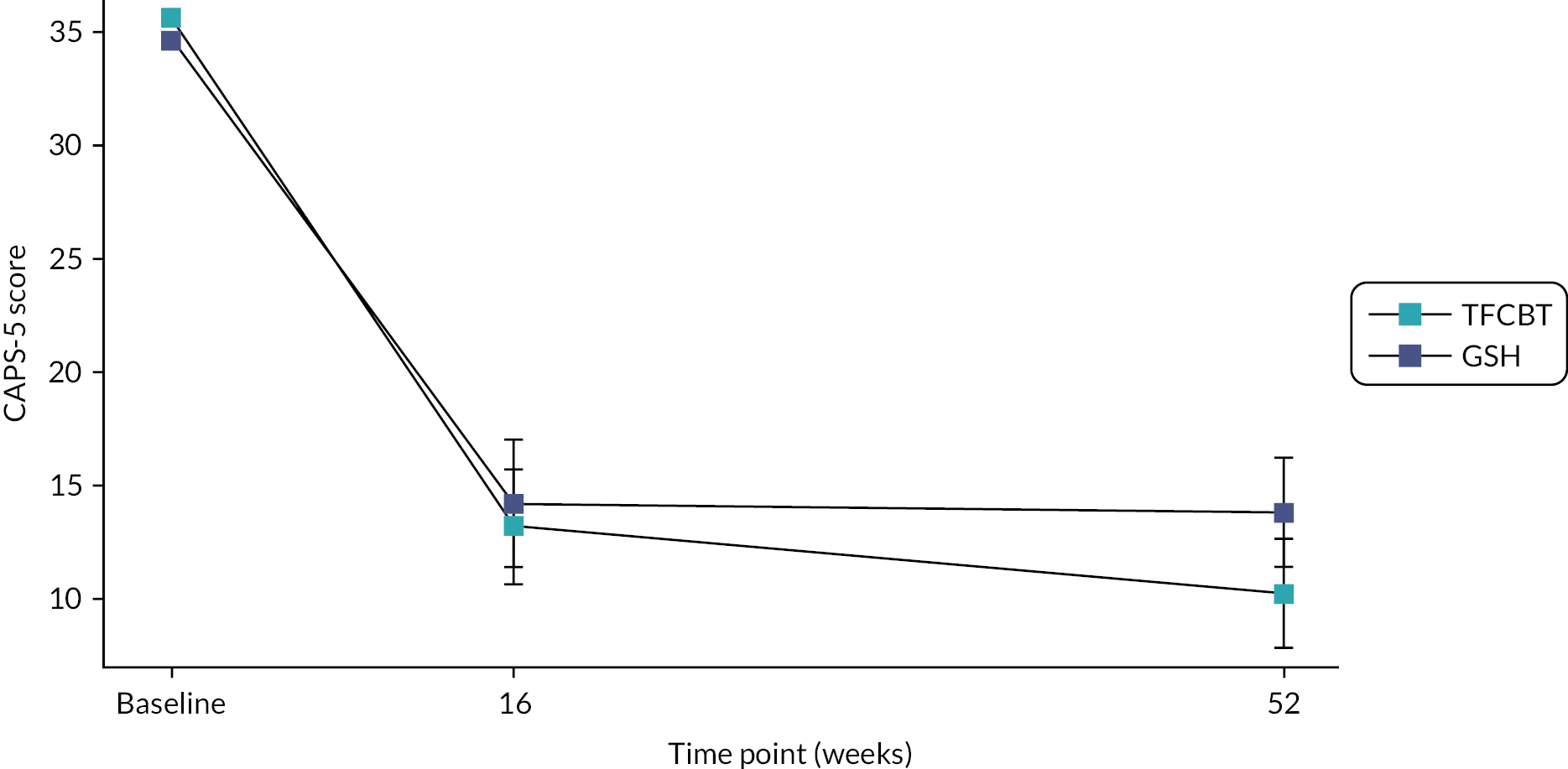
Subgroup analyses
There was only one pre-specified subgroup analysis which was to use the primary analysis to compare the difference in the CBT-TF and GSH groups between females and males. An interaction term between the gender and intervention variables was fit to the primary analysis model and the effect of CBT-TF versus GSH was determined separately for males and females. The results are shown in Figure 12. There was no evidence of a difference in the intervention effect for males versus females [difference 0.5, 95% CI (−6.7, 7.6), p interaction = 0.9] with the difference in CAPS-5 score at 16 weeks 0.7, 95% CI (−5.1, 6.5), for females and 1.2, 95% CI (−3.1, 5.5) for males. It was a similar result looking at the 52-week CAPS-5 score with difference of −1.2, 95% CI (−8.1, 5.6), p interaction = 0.7 and females having a difference in CAPS-5 score of 4.0, 95% CI (−1.5, 9.4), and males having a difference of 2.8, 95% CI (−1.5, 7.0). Although this subgroup analysis was pre-specified the trial was not powered to detect any interaction effects. The subgroup results are shown in Figure 12.
FIGURE 12.
Results of planned and exploratory subgroups.
Analysis adjusted for the following variables at baseline: CAPS-5 score, gender, research centre, comorbid depression (baseline PHQ-9) and time since trauma (months).

Exploratory analyses
There were several exploratory analyses specified in the SAP. The results of these are described in this section. It should be noted that these analyses were not powered as part of the trial sample size calculations and so should be interpreted cautiously.
Analysis of mode of data collection
The trial data were collected from participants either in a face-to-face session or via a telephone or video call. There was interest in exploring if there were differences in the primary outcome when stratified by mode of data collection. It is important to note that only the 16-week assessment was analysed since at 52 weeks the model of data collection had moved to telephone or online due to the COVID-19 pandemic. In addition, there was insufficient information to reliably ‘impute’ the missing mode of data collection and so the analysis was confined to those participants with a 16-week CAPS-5 assessment. The results of this analysis are shown in Figure 12. There was little evidence that the outcome differed based on data collection mode, p interaction = 0.3. The GSH group had a slightly lower (more favourable) CAPS-5 score with a mean difference of −1.5, 95% CI (−7.0, 3.9). It was the reverse for the telephone/video call with the CBT-TF group having lower CAPS-5 scores with a mean difference of 1.8, 95% CI (−2.5, 6.0). However, for both modes of data collection the results are also consistent with there being no difference between the CBT-TF and GSH groups.
Impact of COVID-19 lockdown
Since the trial was partially conducted during the COVID-19 pandemic an exploratory analysis was conducted to examine the potential impact on the primary outcome of having an assessment performed after the start of the lockdown period in the first wave of COVID-19 infections. The lockdown date was 23 March 2020 and assessments conducted after that time were considered made in the ‘lockdown’ period and so potentially impacted by COVID-19. The analysis was conducted at the 52-week assessment as only 16 participants had 16-week assessments after 23 March 2020. The full imputed dataset was used for the analysis and participants with missing 52-week assessments were allocated to the ‘before’ or ‘after’ periods based on their estimated assessment date using their baseline assessment CAPS-5 assessment as the starting point.
Of the 99 CBT-TF participants, 46 (46.5%) either had or were scheduled to have a 52-week assessment after 23 March 2020. For the GSH group, 40/97 (41.2%) had or were scheduled to have a 52-week assessment after 23 March 2020. The results of the analysis are shown in Figure 12. There was little evidence of a difference in the effect of the intervention depending on the period it was assessed, p interaction = 0.2. The estimated mean difference between the 52-week CAPS-5 score between the GSH and CBT-TF group was 4.7, 95% CI (0.4, 9.0) higher in the GSH group compared to the CBT-TF group in the pre-lockdown period and 0.9, 95% CI (−4.0, 5.9) higher in the post-lockdown period. As previously mentioned, this was a post hoc analysis and so was not powered to detect any interaction effects.
Analysis of IES-R trajectories
During the trial, participants were asked to complete the IES-R at each therapist contact. These interim IES-R scores have been used to inform the multiple imputation model as described in Introduction. In addition, it was possible to use this longitudinal data to examine the change over time in the IES-R scores. A multilevel mode was fit using piecewise linear splines (see Introduction) to quantify these changes over time. Figure 13 shows the trajectories of the IES-R scores for the GSH and CBT-TF groups.
FIGURE 13.
Longitudinal IES-R scores.
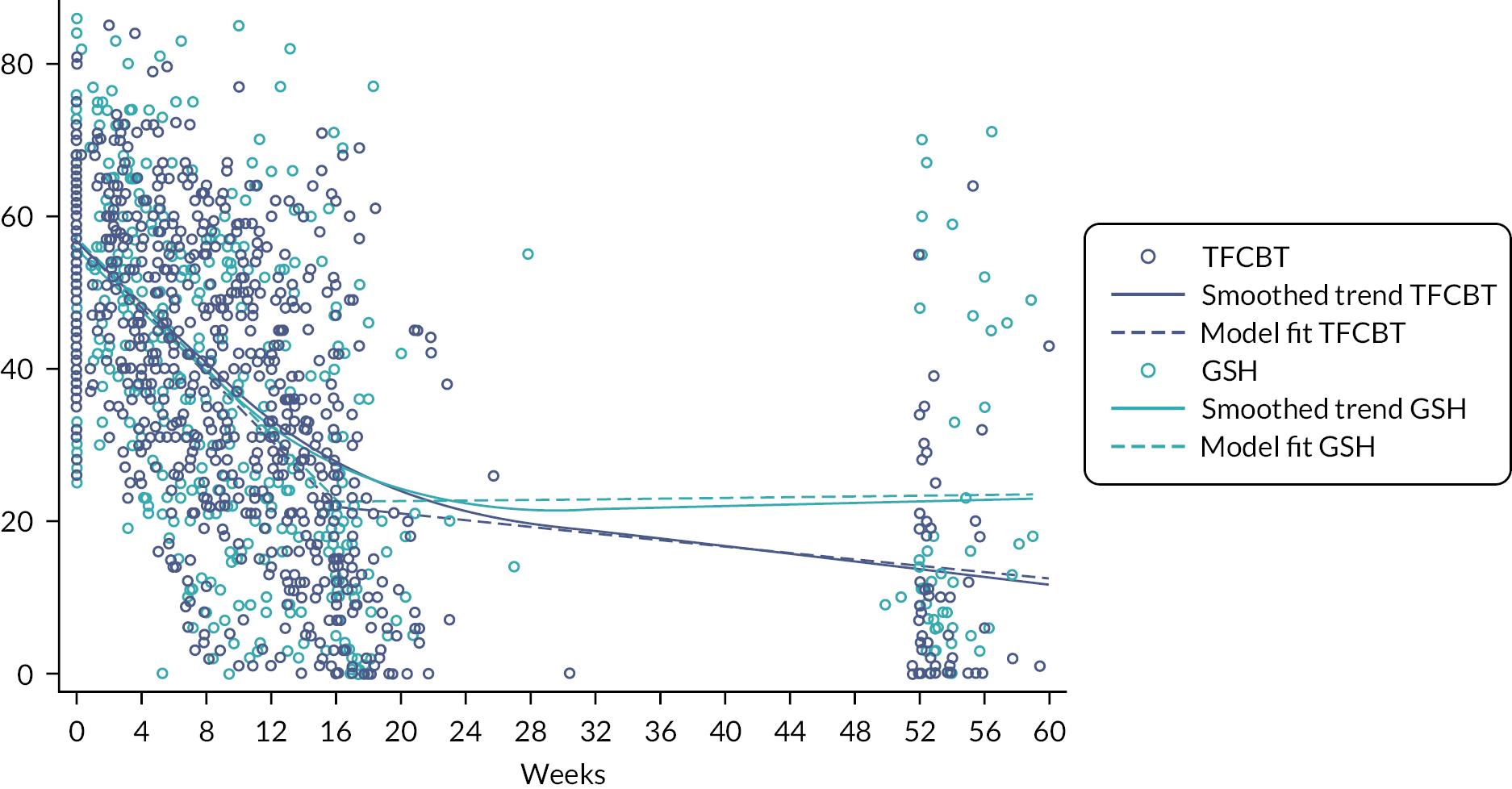
As can be seen from Figure 13 there was a rapid decline over the first 16 to 20 weeks coinciding with participants’ involvement in the GSH or CBT-TF interventions. The multilevel model fits two lines; one over the period of 0 to 16 weeks and a second one from 16 weeks onwards. Figure 13 shows this gives a good fit but slightly overestimates the scores around weeks 12–16. More complex models did not resolve this completely and it was decided to stay with the two piecewise linear lines for interpretability. In the period 0 to 16 weeks on average participants in the CBT-TF group showed an estimated mean reduction (improvement) in IES-R scores of 2.2 points, 95% CI (2.0, 2.4), p < 0.001 per week. The GSH group had a slightly lower rate of improvement of 2.0 points, 95% CI (1.8, 2.3), p < 0.001 per week. However, there was little evidence of a difference between the GSH and CBT-TF groups during this period (p = 0.4). For the period after 16 weeks the CBT-TF group’s reduction slowed to 0.2 points, 95% CI (0.1, 0.3), p < 0.001 per week. For the GSH group the reduction flattened with a small uptick of 0.02 points, 95% CI (−0.1, 0.1), p = 0.7 per week. However, this was consistent with no increase in IES-R score and so for the GSH group they maintained rather than improved their IES-R score reduction. For the CBT-TF group they continued to improve their IES-R by a slower reduction after 16 weeks. There was evidence of a difference after 16 weeks between the CBT-TF and GSH groups’ rate of reduction of 0.2 points, 95% CI (0.1, 0.4), p = 0.002 in favour of the CBT-TF group. This finding is consistent with what was observed for the CAPS-5 score as shown in Figure 11.
Adverse events
The risk assessment framework was triggered 105 times. One risk assessment was triggered due to a report of self-harming, the rest were for reported suicidal ideation. The risk assessment was triggered 70 times during telephone screening; 28 times during the baseline assessment; once during a pre-screening conversation between a RAPID research assistant and a referrer, and once during a qualitative interview. During follow-ups, it was triggered twice at 16-week and three times at 52-week follow-ups. After following the risk assessment framework, none of the referrals were considered to be actively suicidal.
There were six adverse events that were classified as serious adverse events, none of which were found to be related to involvement in the RAPID trial. Two further events were reported as potentially serious but were determined to be non-serious when reviewed. Table 14 provides details of all these events.
| Description | Causality/expectedness | Action taken |
|---|---|---|
| Participant attended for their first treatment session and informed therapist of plan to end life, attempt was prevented by visit from son. Risk protocol enacted; GP informed. Risk management plan developed. | Unrelated/expected | Intervention delayed |
| Learned at 52-week follow-up appt they had relapsed into alcohol addiction not long after 16-week follow-up in April 2018. This resulted in the patient attending a 2-week alcohol detoxification as an inpatient. The participant states they had been abstinent since, and engaged with community services. | Unrelated/expected | N/A |
| Chronic wound problem | Unrelated | Intervention not changed |
| Pneumonia | Unrelated | Intervention delayed |
| Torn tendons in shoulder | Unrelated | Intervention not changed |
| Hospitalised due to influenza | Unrelated | Intervention delayed |
| Possible cancerous lesion found and biopsies taken | N/A | N/A |
| Disclosed repeated self-harm by superficially cutting arms since the age of 13 years | N/A | N/A |
Fidelity
Audio recordings of 74 therapy sessions involving different participants were assessed. All but one session viewed was rated at least satisfactory. For GSH, 1 (3%) was rated ‘mediocre’, 12 (39%) satisfactory, 13 (42%) good and 5 (16%) very good. For CBT-TF, 10 (23%) were rated satisfactory, 20 (42%) good, 10 (23%) very good and 3 (7%) excellent.
Chapter 4 Economic evaluation
Introduction
This chapter presents the methods and results of the economic evaluation conducted alongside the RAPID trial. The aims of the economic evaluation were to:
-
determine the costs associated with Spring GSH compared to individual face-to-face CBT-TF
-
assess the cost-effectiveness of GSH compared to CBT-TF.
Method
A within-trial HE analysis was undertaken of GSH compared to CBT-TF from a health service (UK NHS), and personal social services perspective, reflecting the trial follow-up period of 16 and 52 weeks post-randomisation. Given the time horizon of 52 weeks no discounting was applied. The analysis consisted of:
-
analysis of the costs of GSH compared to CBT-TF
-
cost-effectiveness analysis (CEA) to assess the incremental cost of achieving a percentage reduction:
-
in symptoms of PTSD measured by the CAPS-5 score
-
in distress caused by the traumatic event measured by the IES-R score
-
in functioning measured by the WSAS score.
-
-
a cost–utility analysis (CUA) to assess the incremental cost per quality-adjusted life-year (QALY) gained.
Prior to commencement of the analysis, a HE analysis plan was developed and agreed with the trial team. The HE team followed this analysis plan during the conduct of the economic evaluation. The analysis was developed in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) and Stata. 55 Deviation from the analysis plan was that current medication was only collected at baseline and so the costs were not included in the final analysis. Long-term modelling was not conducted for the evaluation as stated in the protocol. A literature review was conducted prior to developing the analyses which identified the recent evaluation of psychological treatments for PTSD in the UK. 57 This identified that the post-treatment follow-up period reporting changes in PTSD symptoms showed considerable uncertainty, also the annual risk of relapse was assumed to be equal across all treatment arms due to a lack of published evidence. Therefore, it was decided that the inevitable uncertainty surrounding extrapolation from 52 weeks onwards would not produce useful results for decision-making.
The health outcome measures and resource use were collected in the case report forms (CRFs) administered by a researcher for the 3 months preceding baseline, 16-week and 52-week follow-ups. Individual level utility scores were obtained at each assessment point using the EQ-5D-5L questionnaire, and this was mapped back to the UK 3L valuation set as currently recommended28 and summated for the GSH and CBT-TF arms.
The resource use items were collected using an adapted Client Services Receipt Inventory (CSRI) included in the CRFs. 58,59 The adapted CSRI was based on a generic mental health CSRI that was developed to allow economic evaluations to be compared across interventions for similar mental health problems. 60 All healthcare resource use was collected regardless of reason for contact. The QALY is considered to capture productivity,61 therefore, productivity losses in monetary terms have not been included in an analysis.
Costs included in the health economic analysis
The health economic HE analysis considered the following:
-
costs to the NHS of GSH implementation (including staff time for training and supervision)
-
costs to the NHS of CBT-TF (including staff time for training and supervision)
-
cost of additional therapy (psychiatrist, psychologist and counsellor appointments)
-
cost of primary and community health and social care
-
costs of secondary care (inpatient admissions and outpatient appointments, A&E visits).
Costs were reported for all available cases (for the most complete overview) with ITT population (using multiple imputation) for the CEA and CUA. All costs were expressed as 2020 Great British pounds (£), inflated and converted appropriately where required. 62 Costs were calculated at a participant level, multiplying the unit cost by the resource used at each time point. The cumulative implementation and health and social care costs at baseline, 16 weeks and 52 weeks were calculated for all available cases for both the GSH and CBT-TF arms (including 95% CIs, median and IQR). The intervention and health and social care costs were summated and mean difference per patient in costs (including 95% CIs) was calculated at 16 and 52 weeks.
Intervention costs
The time spent by therapists on face-to-face therapy (in person and online), phone calls, note taking and other administration were collected in the trial. Staff costs were estimated using published unit costs (see Appendix 1, Table 32).
The therapy reports were compared to final numbers of therapy sessions reported in the CRFs, with the greater number of sessions reported from either source assumed to be correct. If therapy reports were missing, then the mean time for face-to-face contacts from those reported for that therapy arm was applied. The number of calls included was again based on the therapy reports of the CRFs and the final numbers reported. If calls were recorded in either source it was assumed these were accurate and no additional calls were added. If no calls were reported for GSH therapy, but face-to-face sessions were reported, then one fewer phone call per face-to-face session was estimated to have occurred. Where no therapy reports were submitted, and final number of therapy sessions had not been reported in the CRFs, it was assumed no therapy took place.
GSH therapy was anticipated to take 8 to 10 weeks, self-help material was provided through the intervention website or app, with face-to-face appointments with a therapist, a 1-hour initial session and four further 30-minute sessions, with catch-up calls between these sessions. CBT-TF was delivered over 10–12 weeks as face-to-face therapy sessions lasting 60–90 minutes. In addition, some therapy sessions involve a trauma site visit.
CBT-TF was delivered by mental health professionals (nurses, occupational therapists, psychologists and social workers). Therapy staff were generally NHS Agenda for Change band 7, with some band 6 and band 8a giving CBT-TF therapy. For the base case analysis, it was assumed that all therapy staff were band 7. However, it is anticipated that GSH therapy will be provided in routine practice in primary care with lower-intensity therapy training at band 4/5 and this was tested in a SA.
Training and supervision costs
Resource use resulting from GSH and CBT-TF training (including materials, consumables and staff time for trainers and attendees) was estimated through interviews and direct communications with the clinical staff involved in the trial.
Website costs for Spring
The Spring GSH website and app were developed prior to the RAPID trial; therefore, development costs have not been included in the analysis. The website and app require regular maintenance, and administration support to add users; an estimate of the costs provided by the trial team was included in the analysis. No equipment was provided as participation in the trial required access to a computer, tablet or smartphone.
Cost of health and social care resource use
Healthcare resource use (including primary care consultations, out-of-hours care, outpatient appointments, day-cases, inpatient stays and A&E attendances) and social care (social worker appointments and home help visits) were collected using data from the CSRI to assess the differences in profile of health and social care use as a result of the intervention compared to control.
If one or more items in the CSRI were completed (values of ‘0’ or greater), the CSRI was assumed to have been fully completed and any missing items were imputed with zeroes. If items were missing, for example, if the number of appointments was completed but not the time, then the median time for those appointments recorded in either treatment arm was applied. If the CRF was marked as ‘not done’ or no data were recorded, data were considered missing and multiple imputation was used to account for missing data in the analyses.
The Personal Social Services Research Unit (PSSRU) unit costs for health and social care were applied to therapist time, primary and community care including social care by number of appointments and staff member visited (see Appendix 1, Tables 32–36). NHS reference costs were applied to outpatient appointments, day cases, diagnostic tests and imaging. Outpatient visits and day cases were costed individually according to the number of appointments, reasons for healthcare contact and speciality/department recorded in the trial CRFs (see Appendix 1, Tables 37 and 40). The unit costs for imaging and blood tests were applied, and where a diagnostic test was recorded but no details given, the average cost of pathology tests in the 2018/19 NHS reference costs was applied (uplifted to 2020 prices) (see Appendix 1, Table 39). The most up-to-date NHS reference costs published at the time of analysis were for 2018/19 and so were uplifted to 2020 using the PSSRU inflation index. 63 Inpatient attendances were costed according to number of admissions and length of stay on an acute medical ward, general ward, acute psychiatric ward, long-stay rehabilitation ward or a psychiatric rehabilitation ward, and additionally if a patient was admitted via A&E (see Appendix 1, Tables 38 and 41). A cost per bed day was applied to the length of stay for the general ward and acute medical ward; as bed days were not reported in the 2018/19 NHS reference costs, those from 2017/18 were used and uplifted. The cost per day for a long-term care home for mental health support for adults was identified for the long-stay rehabilitation. The cost per bed day for a secure mental health ward was identified for the acute psychiatric ward in unit costs for 2019 which were uplifted to 2020.
Cost of medication
Current medications were recorded at baseline. Unit costs were taken from the NHS Electronic Drug Tariff64 and British National Formulary65 (see Appendix 1, Table 31). Prescriptions were costed individually based on dose, treatment duration and frequency of use. As medications were only collected at baseline these costs have not been included in the analysis and are reported separately.
Missing data
The problems concerning missing data are particularly relevant to HE health economic analysis as the main outcomes are cumulative measures collected over the trial period. Missing items relating to health-care service use may underestimate the total costs, while missing outcome data may be correlated to effects as those individuals without information may be systematically different from those for whom all information is observed. 66 As such, using complete-case assessments and available case analysis only could result in meaningful data being excluded. We therefore adopted a multiple imputation approach within the incremental economic analysis as the appropriate technique to provide a comprehensive investigation of the impact of missing data on the estimations of cost-effectiveness. 67 MI was performed using chained equations and predictive mean matching (PMM). A total of 46 imputations were used based on the maximum percentage of missingness across imputed variables (see Appendix 1, Table 42).
Impact of COVID-19 on the health economic analysis
The trial follow-up was completed in December 2020. The first UK national lockdown due to COVID-19 started on 23 March 2020 with restrictions eased in June. The majority of trial therapy was completed before the national lockdown, however, for some participants the follow-up period from 16 weeks to 52 weeks would have included full lockdown, and also any subsequent delays to treatment due to the disruption caused by lockdown. Given the randomisation of participants, the impact on face-to-face treatment was assumed to be balanced across arms and would not affect the overall results.
Cost-effectiveness analysis and cost–utility analysis
The CEA expressed the incremental cost of achieving a percentage reduction in symptoms of PTSD at 16 and 52 weeks post-randomisation measured by CAPS-5, with additional analyses expressing the cost of achieving a percentage reduction in distress caused by the traumatic event measured by the IES-R, and achieving a percentage improvement in functioning measured by WSAS score. Total costs at 16 and 52 weeks (including baseline) for the ITT population were considered in the incremental analysis.
A CUA was undertaken to assess the incremental costs per QALY gained as a result of the use of GSH compared to CBT-TF. QALYs for each patient were calculated based on the utility scores at baseline, 16 weeks and 52 weeks using the area-under-the-curve approach and linear interpolation. Total costs at 16 and 52 weeks (including baseline) for the EQ-5D ITT population were used to calculate the incremental cost.
Adjusted mean costs and outcomes, including QALYs, and the differences in adjusted mean costs and outcomes (and associated 95% CIs) at 52 weeks were estimated using seemingly unrelated regressions which accounts for the correlation between costs and outcomes. Costs and outcomes were adjusted for study site, age and time to event. Costs were also adjusted for baseline costs and outcomes for baseline utility.
The incremental cost-effectiveness ratio (ICER) resulting from the CUA was compared to the willingness to pay threshold of £20,000 and £30,000 per QALY gained as standardised by NICE. 68
No conditions for NI were applied in the analysis. Results were reported as net monetary benefit (NMB) presenting the incremental value of the intervention in monetary terms by applying the willingness to pay threshold to the change in QALYs:69
Sensitivity analyses
Deterministic SAs were undertaken to test the robustness of the results of the CUA considering the uncertainty in input parameters such as costs of therapy, and in different scenarios more representative of real-world application of GSH. The health and social care resource use delivered in private and non-NHS institutions was included in an additional analysis to consider wider costs. It is anticipated that GSH will be provided by band 4/5 healthcare professionals rather than band 7 therapists in routine clinical practice, thus this was tested in the SA. Analysis was carried out for the 16-week treatment end point. Subgroup analysis was carried out to determine if cost–utility was improved for specific groups based on demographic and clinical features. The following subgroups were tested: age groups (35 years and under, and over 35 years); level of education of the participant (people with a degree or higher qualification, and all other levels of education); months since the traumatic event (> 18 ≤ 18 months); comorbidity was taken as level 4/5 in the EQ-5D pain or anxiety score at baseline.
Probabilistic sensitivity analysis (PSA) used non-parametric bootstrapping to address joint parameter uncertainty and assess the impact on the ICER during 1000 simulations which were undertaken using random sampling of the distributions of costs and outcomes with results presented as cost-effectiveness acceptability curves (CEAC). A CEAC describes the probability of the intervention being cost-effective at different willingness-to-pay thresholds based on the PSA.
Budget impact analysis
A trial-based budget impact analysis (BIA) was undertaken for the UK population to estimate the likely impact of the use of GSH on NHS budgets through implementation costs and changes in healthcare usage. The BIA was informed by trial data supplemented by the best available published evidence where required and conducted according to recommended good practice. 70
A simple budget impact model was constructed in Microsoft Excel 365. The number of eligible patients was calculated using prevalence and incidence figures. Prevalence and incidence data for PTSD in the UK were obtained from published sources. 50 Age-specific general population mortality was not applied as a 1-year time horizon was used. Approximately 31.4% of adults in England reported experiencing at least one traumatic event according to the Adult Psychiatric Morbidity Survey in 2014. Of the participants of the survey, 4.4% screened positive for PTSD in the previous month, and half were receiving mental health treatment, although the majority were taking medication (43.6%), and only 24.0% were having psychological therapy, which is currently recommended by NICE. 50
Results
One hundred and ninety-six participants were included in the ITT analyses, out of 440 potential participants screened for inclusion.
Training and supervision costs
Training was delivered face-to-face over 2.5 days, 1 day was for GSH training, and 1.5 days for CBT-TF training, by two band 8a–c staff running the sessions for 20 participants (see Table 15). Each trainer required 2 hours of preparation time (e.g. preparation of materials) for the training sessions. There was an additional 10 hours for developing the course materials for GSH training (£823.33). No cost was applied for room rental as training can be given within NHS facilities.
| GSH trainers | GSH trainees | CBT-TF trainers | CBT-TF trainees | |
|---|---|---|---|---|
| One day (8 hours) GSH training | £1317.33 | £464.00 | ||
| One and a half days CBT-TF | £1976 | £696.00 | ||
| Preparation time – 2 hours | £164.67 | £164.67 | ||
| One case + 13% requiring an additional GSH case | £460.35 | £1384.75 | ||
| Supervision of one case + 13% requiring additional case for GSH | £1489.01 | £209.79 | £2627.66 | £370.21 |
| Critique of recorded CBT-TF session | £2058.33 | |||
| Three 1-hour monthly supervision sessions | £617.50 | £87.00 | £617.50 | £87.00 |
| Total | £3588.51 | £1221 | £7444.16 | £2537.96 |
| Cost per trainee attending course plus supervision for 3 months | £1433.50 | £2910.17 |
In addition, for each therapy, one case was supervised as part of the training programme. For 4 out of 30 (13%) therapists, competency was not achieved in GSH from the first supervised case and they required an additional supervised case. For CBT-TF, one session was recorded and sent to the trainers for critique. After completion of training, monthly supervision was given via Skype in groups of three or four therapists.
Supervision for band 4/5 staff will be fortnightly for 4 months after training, with hourly group sessions (with six participants), followed by monthly supervision.
Website costs
It was estimated by the trial team that it will cost £10,000 per year to maintain the website and app, and provide administrative support for 1000 users.
Cost of therapy
The mean number of face-to-face appointments in the GSH arm was 3.93 (95% CIs 3.60, 4.25; median 5, IQR 3–5) and 8.63 (95% CI 7.94, 9.32; median 9, IQR 6–12) in the CBT-TF. The amount of face-to-face therapy time was 208.4 minutes (SD 69.3) for GSH and 767.0 minutes (SD 278.2) for CBT-TF.
In the GSH arm there were check-in phone calls between the face-to-face sessions; the median number of calls was 3 (IQR 2–4). This was based on data from 193 people, two people in the GSH arm and one person in the CBT-TF arm had no data reported for the therapy sessions.
The cost of therapy was calculated as time in face-to-face sessions, phone calls and non-contact time for note-taking. The therapist cost per working hour was applied as time on direct and indirect tasks was collected (see Appendix 1, Table 32). It was not possible to determine if any CBT-TF appointments were site visits which may incur extra costs due to travel time.
The cost of therapy was lower for the GSH arm as there were fewer face-to-face appointments, mean cost £277.31 (95% CI £253.27, £301.34; median £286.15, IQR £210.88–351.53) compared to £729.49 (95% CI £670.76, £788.22; median £753.71, IQR £554.37–950.60) in the CBT-TF arm.
It is anticipated that the GSH therapy will be given by band 4/5 low-intensity therapists if it is used more widely in the NHS. Applying a lower unit cost for staff in the GSH arm led to the mean cost of therapy being £160.09 (95% CI £146.22, £173.97; median £165.20, IQR £121.74–202.94).
Cost of medication at baseline
In the 3 months before baseline, the mean number of prescriptions per person was 1.40 (95% CI 1.08, 1.72) in GSH arm, and 1.35 (95% CI 1.06, 1.65) in the CBT-TF arm (see Table 16).
| Medication | GSH therapy group | CBT-TF therapy group |
|---|---|---|
| Mean number of medications per participant (95% CIs) | 1.40 (1.08, 1.72) | 1.35 (1.06, 1.65) |
| Mean prescription cost per day at baseline (£), per patient (95% CIs) | £1.62 (£0.27, £2.97) | £0.31 (£0.19, £0.44) |
| Median prescription cost per day at baseline per patient (IQR) | £0.09 (£0–0.35) | £0.09 (£0–0.32) |
Cost of healthcare resource use
Additional therapy costs
In the available case population, in the 3 months prior to baseline, 19.6% (19/97) of people in the GSH arm and 34.3% (34/99) of people in the CBT-TF arm had seen an NHS psychologist or counsellor, and five patients in each arm had an appointment with an NHS psychiatrist. In the GSH arm the mean cost of this therapy prior to baseline was £114.05 (95% CI £26.00, £202.11), and £187.25 (95% CI £69.64, £304.86) in the CBT-TF arm (see Table 17).
| Baseline | GSH (N = 97) | CBT-TF (N = 99) |
|---|---|---|
| N of psychiatrist appointments, mean (95% CIs) | 0.062 (0.005, 0.119) | 0.091 (−0.016, 0.198) |
| N of psychologist appointments, mean (95% CIs) | 0.371 (−0.050, 0.792) | 0.626 (0.100, 1.152) |
| N of counsellor appointments, mean (95% CIs) | 0.381 (0.143, 0.620) | 0.545 (0.220, 0.871) |
| 16 weeks | GSH (N = 72) | CBT-TF (N = 80) |
| N of psychiatrist appointments, mean (95% CIs) | 0.042 (−0.006, 0.089) | 0.125 (−0.076, 0.326) |
| N of psychologist appointments, mean (95% CIs) | 0.139 (−0.005, 0.283) | 0.963 (0.322, 1.603) |
| N of counsellor appointments, mean (95% CIs) | 0.208 (−0.100, 0.516) | 0.15 (−0.077, 0.377) |
| 52 weeks | GSH (N = 60) | CBT-TF (N = 59) |
| N of psychiatrist appointments, mean (95% CIs) | 0.083 (−0.003, 0.170) | 0.034 (−0.014, 0.081) |
| N of psychologist appointments, mean (95% CIs) | 0.583 (0.105, 1.06) | 0.220 (−0.066, 0.507) |
| N of counsellor appointments, mean (95% CIs) | 0.333 (−0.005, 0.672) | 0.034 (−0.034, 0.102) |
Of all the participants, 72 were on a waiting list for therapy in the 3 months prior to baseline (38 in the GSH arm and 34 in the CBT-TF arm). Of those on the waiting list for therapy, 14 (6 in the GSH arm and 8 in the CBT-TF arm) saw an NHS psychologist or counsellor in the 3 months prior to baseline.
At 16 weeks after baseline, in addition to the trial therapy, 16 people saw a counsellor or psychologist (or both in one case), 8.33% (6/72) people in the GSH arm and 12.5% (10/80) in the CBT-TF arm. Only one person in the GSH arm and two in the CBT-TF arm had a psychiatrist appointment during the trial therapy period. The mean cost for this additional therapy was £197.30 (95% CI £46.78, £347.83) in the CBT-TF, compared to £48.06 (95% CI £8.58, £87.54) in the GSH arm (see Table 17).
At the 52-week follow-up, six people in the GSH arm visited an NHS psychologist or counsellor in the preceding 3 months, and only two people in the CBT-TF arm. Three people in the GSH arm and one person in the CBT-TF arm had an appointment with a psychiatrist. The mean cost of these additional visits was £95.92 (95% CI £2.31, £189.53) in the GSH arm compared to £29.59 (95% CI −£23.07, £82.25) in the CBT-TF arm (see Table 17).
There were also more people on the waiting list for therapy at the 52-week follow-up in the GSH arm, 21.67% (13/60) compared to 8.47% (5/59) in the CBT-TF arm, with three people in the GSH arm still on the therapy waiting list at the 52-week follow-up if they had been on the waiting list at baseline (see Table 18).
| Therapy waiting list | GSH | CBT-TF |
|---|---|---|
| Baseline | 38 (39.18%) | 34 (34.34%) |
| 16-week follow-up | 11 (15.28%) | 8 (10.0%) |
| 52-week follow-up | 13 (21.67%) | 5 (8.47%) |
Other primary and community care costs
Primary and community care costs in the 3 months prior to baseline were similar in both arms, the mean cost in the GSH arm was £226.95 (95% CIs £162.78, £291.16) and £225.47 (95% CI £145.01, £305.93) in the CBT-TF arm (see Table 19). This included GP appointments, calls and out-of-hour appointments; practice, district and community psychiatric nurse appointments; social worker and home help visits, NHS111 calls, occupational therapist and other community care appointments.
| Baseline | GSH (N = 97) | CBT-TF (N = 99) |
|---|---|---|
| N of GP appointments, mean (95% CIs) | 2.213 (1.684, 2.749) | 2.909 (2.168, 3.650) |
| N calls to GP, mean (95% CIs) | 0.495 (0.258, 0.731) | 0.576 (0.318, 0.834) |
| N of GP practice or district nurse appointments, mean (95% CIs) | 0.588 (0.077, 1.098) | 0.414 (0.232, 0.597) |
| N of community psychiatric nurse appointments, mean (95% CIs) | 0.155 (0.171, 0.292) | 0.162 (0.009, 0.314) |
| N of social worker appointments, mean (95% CIs) | 0.052 (−0.002, 0.105) | 0.051 (−0.016, 0.117) |
| N of occupational therapist appointments, mean (95% CIs) | 0.237 (−0.026, 0.500) | 0.263 (−0.063, 0.588) |
| N of home help visits, mean (95% CIs) | 0.144 (−0.142, 0.431) | 0 |
| N of GP out-of-hours appointments, mean (95% CIs) | 0.155 (−0.003, 0.312) | 0.111 (0.031, 0.191) |
| N of NHS 111 calls, mean (95% CIs) | 0.227 (0.089, 0.365) | 0.040 (0.001, 0.080) |
| N of other community appointments, mean (95% CIs) | 1.062 (0.526, 1.597) | 0.697 (0.120, 1.274) |
| 16 weeks | GSH (N = 72) | CBT-TF (N = 80) |
| N of GP appointments, mean (95% CIs) | 0.806 (0.533, 1.078) | 1.688 (1.252, 2.123) |
| N calls to GP mean (95% CIs) | 0.264 (0.088, 0.440) | 0.313 (0.133, 0.492) |
| N of GP practice or district nurse appointments, mean (95% CIs) | 1.25 (−0.110, 2.610) | 0.263 (0.131, 0.394) |
| N of community psychiatric nurse appointments, mean (95% CIs) | 0.306 (−0.152, 0.763) | 0.025 (−0.025, 0.075) |
| N of occupational therapist appointments, mean (95% CIs) | 0.194 (−0.089, 0.478) | 0.038 (−0.005, 0.080) |
| N of GP out-of-hours appointments, mean (95% CIs) | 0.028 (−0.011, 0.067) | 0.15 (−0.005, 0.305) |
| N of NHS 111 calls, mean (95% CIs) | 0.056 (−0.022, 0.133) | 0.063 (−0.019, 0.144) |
| N of other community appointments, mean (95% CIs) | 0.347 (0.024, 0.670) | 1.175 (−0.105, 2.455) |
| 52 weeks | GSH (N = 60) | CBT-TF (N = 59) |
| N of GP appointments, mean (95% CIs) | 0.75 (0.470, 1.030) | 1.050 (0.665, 1.434) |
| N of calls to GP, mean (95% CIs) | 0.6 (0.190, 1.010) | 0.441 (0.744, 0.807) |
| N of GP practice or district nurse appointments, mean (95% CIs) | 0.6 (0.129, 1.071) | 0.271 (0.119, 0.423) |
| N of community psychiatric appointments, mean (95% CIs) | 0.033 (-0.013, 0.080) | 0.102 (−0.102, 0.305) |
| N of social worker appointments, mean (95% CIs) | 0.083 (−0.083, 0.250) | 0 |
| N of counsellor appointments, mean (95% CIs) | 0.333 (−0.005, 0.672) | 0.034 (−0.034, 0.102) |
| N of occupational therapist appointments, mean (95% CIs) | 0.05 (−0.024, 0.124) | 0.034 (−0.014, 0.081) |
| N of home help visits, mean (95% CIs) | 0.017 (−0.017, 0.050) | 0 |
| N of GP out-of-hours appointments, mean (95% CIs) | 0.033 (−0.013, 0.080) | 0.068 (−0.039, 0.175) |
| N of NHS 111 calls, mean (95% CIs) | 0.067 (0.002, 0.132) | 0.034 (−0.014, 0.81) |
| N of other community appointments, mean (95% CIs) | 0.45 (0.126, 0.774) | 0.525 (0.060, 0.990) |
In the 3 months prior to the 16-week follow-up, the primary and community care mean costs were £105.83 (95% CI £52.89, £158.77) for the GSH arm and £163.92 (95% CI £78.51, £249.33) in the CBT-TF arm. In the 3 months prior to the 52-week follow-up, the mean cost in the GSH arm was £92.97 (95% CI £52.09, £133.84) and £108.04 (95% CI £59.27, £156.80) in the CBT-TF arm (see Table 19).
Secondary care costs
Although the same number of people had an inpatient admission in each arm, four people, with three admitted through A&E, the costs were higher for the GSH arm, mean cost £111.41 (95% CI −£13.63, £236.46), than the CBT-TF arm, mean cost £82.12 (95% CI −£14.19, £178.43). More people had outpatient appointments, day case attendances and attended A&E in the GSH arm than in the CBT-TF arm, although the costs were higher in the CBT-TF arm, £95.80 (95% CI £52.50, £139.10) compared to £101.64 (95% CI £1.75, £201.53) (see Table 20).
| Baseline | GSH (N = 97) | CBT-TF (N = 99) |
|---|---|---|
| N of inpatient admissions, mean (95% CIs) | 0.052 (−0.002, 0.104) | 0.051 (−0.002, 0.103) |
| N of outpatient appointments or day case attendances, mean (95% CIs) | 0.289 (0.197, 0.380) | 0.202 (0.122, 0.283) |
| N of A&E visits, mean (95% CIs) | 0.155 (0.034, 0.276) | 0.051 (−0.016, 0.117) |
| 16 weeks | GSH (N = 72) | CBT-TF (N = 80) |
| N of inpatient admissions, mean (95% CIs) | 0.028 (−0.011, 0.067) | 0 |
| N of outpatient appointments or day case attendances, mean (95% CIs) | 0.306 (0.131, 0.480) | 0.563 (0.181, 0.944) |
| N of A&E visits, mean (95% CIs) | 0.042 (−0.020, 0.103) | 0.05 (−0.028, 0.128) |
| 52 weeks | GSH (N = 60) | CBT-TF (N = 59) |
| N of inpatient admissions, mean (95% CIs) | 0.017 (−0.017, 0.050) | 0.017 (−0.017, 0.051) |
| N of outpatient appointments, mean (95% CIs) | 0.267 (0.090, 0.444) | 0.339 (0.119, 0.559) |
| N of A&E visits, mean (95% CIs) | 0.033 (−0.013, 0.080) | 0.085 (−0.003, 0.172) |
In the 3 months prior to the 16-week follow-up only two people had inpatient admissions, both in the GSH arm, mean cost £260.46 (95% CI −£124.45, £645.38). Two people in each arm attended A&E, and 13 people had outpatient appointments in the GSH arm, mean cost £53.62 (95% CI £17.49, £89.75), and 19 people in the CBT-TF arm, mean cost £89.01 (95% CI £29.80, £148.22) (see Table 20). In the 3 months prior to the 52-week follow-up one person in each arm had an inpatient admission, the mean cost for GSH was £29.77 (95% CI −£29.80, £89.33), and for CBT-TF the mean cost was £35.54 (95% CI −£35.60, £106.67). The mean cost of outpatient attendances was £48.54 (95% CI £8.21, £88.86) including two A&E attendances in the GSH arm and £82.08 (95% CI £25.50, £138.67) in the CBT-TF arm (see Table 20).
No inpatient attendances were recorded where patients stayed in a psychiatric ward.
Total costs at baseline, 16 weeks and 52 weeks
The mean total costs from an NHS perspective in the available case population at baseline were £548.21 (95% CI £364.17, £732.25; median £232.51, IQR £86.41–626.48) and £596.48 (95% CI £360.89, £832.07; median £273.32, IQR £112.09−444.07) in the GSH therapy group and CBT-TF groups, respectively (see Figure 14).
FIGURE 14.
Total NHS health and social care costs in the 3 months prior to baseline.
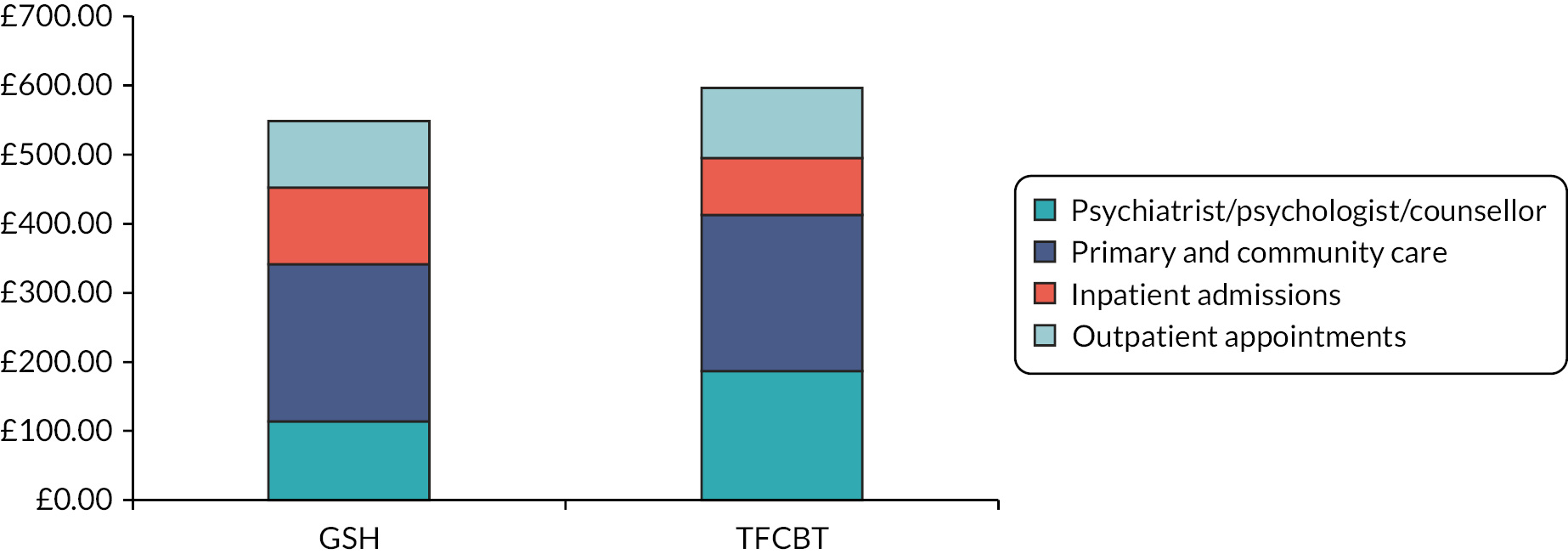
In the 3 months prior to the 16-week follow-up the mean total NHS costs were £467.97 (95% CI £63.66, £872.28; median £62.80, IQR £0–277.34) and £450.24 (95% CI £269.81, £630.67; median £156.00, IQR £14.77–416.64) in the GSH therapy group and CBT-TF groups, respectively (based on the available cases) (see Figure 15).
FIGURE 15.
Total NHS health and social care costs in the 3 months prior to the 16-week follow-up.
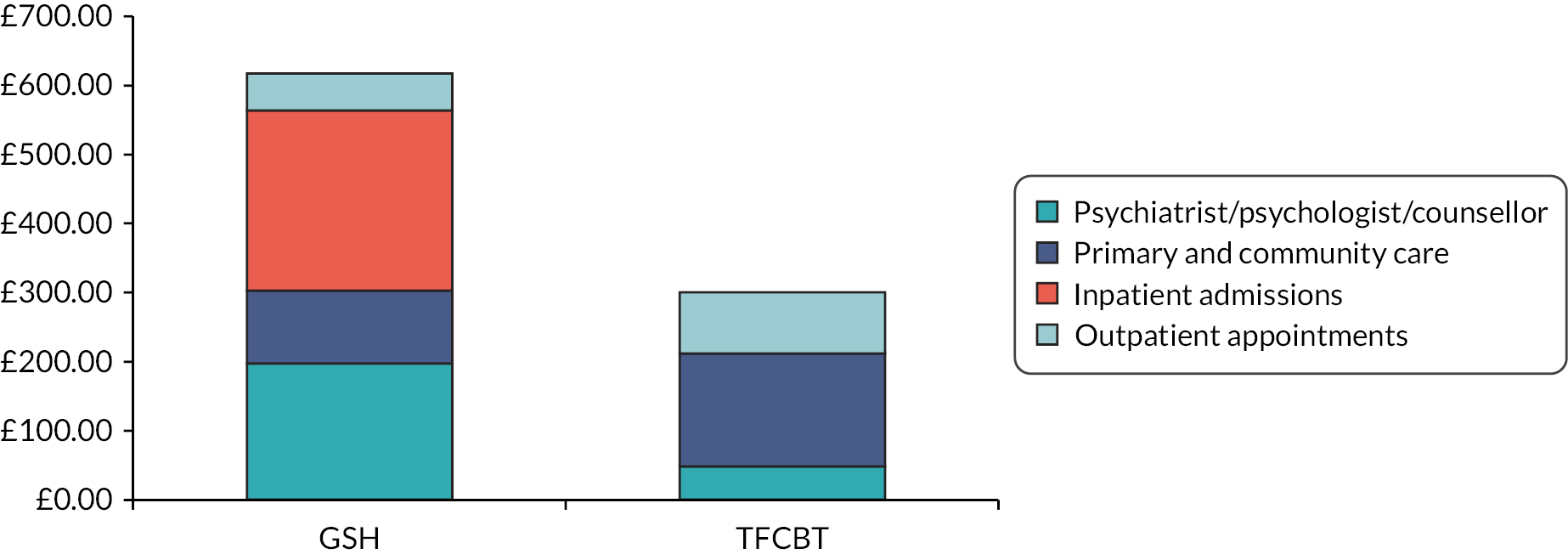
In the 3 months prior to the 52-week follow-up the mean total NHS costs were £267.19 (95% CI £134.57, £399.82; median £89.42, IQR £0–215.72) and £255.25 (95% CI £130.54, £379.96; median £100.17, IQR £0–203.40) in the GSH therapy group and CBT-TF groups, respectively (based on available cases) (see Figure 16).
FIGURE 16.
Total NHS health and social care costs in the 3 months prior to the 52-week follow-up.

Private and other institution-provided care costs
In addition to health and social care provided within the NHS, a number of participants received care privately or through another non-NHS institution. Participants recorded psychiatrist, psychologist, counsellor, GP, occupational therapy and physiotherapy visits provided outside of the NHS. The number of patients receiving additional therapy provided privately or by another institution is presented in Table 21. At baseline, private and institution-provided care in the GSH arm had a mean cost of £11.08 (95% CI −£3.60, £25.75) and £5.71 (95% CI −£0.40, £11.82) in the CBT-TF arm. At the 16-week follow-up in the GSH arm the mean cost £24.57 (95% CI £5.12, £44.01) and in the CBT-TF arm was £41.17 (95% CI −£0.91, £83.25). At the 52-week follow-up additional mean costs were £60.82 (95% CI £10.08, £111.57) in the GSH arm, and £19.92 (95% CI −£7.13, £46.98) in the CBT-TF arm.
| Baseline | GSH (N = 97) | CBT-TF (N = 99) |
|---|---|---|
| Psychiatrist | 0 | 0 |
| Psychologist | 1 (1.0%) | 1 (1.0%) |
| Counsellor | 4 (4.1%) | 4 (4.0%) |
| 16 weeks | GSH (N = 72) | CBT-TF (N = 80) |
| Psychiatrist | 1 (1.4%) | 1 (1.3%) |
| Psychologist | 2 (2.8%) | 2 (2.5%) |
| Counsellor | 0 | 2 (2.5%) |
| 52 weeks | GSH (N = 60) | CBT-TF (N = 59) |
| Psychiatrist | 1 (1.7%) | 1 (1.7%) |
| Psychologist | 5 (8.3%) | 1 (1.7%) |
| Counsellor | 4 (6.7%) | 1 (1.7%) |
Cost-effectiveness analyses
The mean cost (adjusted for site, baseline costs, age and time to event) for the ITT population (n = 196) at the 52-week follow-up point (including baseline) was £1325.36 (95% CIs £941.97, £1708.74) in the GSH group (n = 97) and £1897.91 (95% CIs £1565.24, £2230.58) in the CBT-TF group (n = 99) (see Table 22). This represents an incremental saving of £572.55 (95% CI £1080.14, £64.96) per person in the GSH group.
| N | Adjusted mean costs,a (£) mean (95% CI) | Adjusted mean change in score, mean (95% CI) | Incremental costs (95% CI) | Incremental outcome (95% CI) | Cost per point change | |
|---|---|---|---|---|---|---|
| Change in CAPS b | ||||||
| CBT-TF | 99 | 1897.91 (1565.24, 2230.58) | −24.59 (−26.79, −22.39) | £178 saved per 1-point increase in CAPS | ||
| GSH | 97 | 1325.36 (941.97, 1708.74) | −21.37 (−23.80, −18.94) | −572.55 (−1080.14, −64.96) | 3.22 (−0.20, 6.65) | |
| Change in IES-Rc | ||||||
| CBT-TF | 99 | 1897.91 (1565.24, 2230.58) | −40.12 (−44.57, −35.66) | |||
| GSH | 97 | 1325.36 (941.97, 1708.74) | −29.62 (−35.13, −24.10) | −572.55 (−1080.14, −64.96) | 10.50 (3.01, 17.99) | £55 saved per 1-point increase in IES-R |
| Change in WSASd | ||||||
| CBT-TF | 99 | 1897.91 (1565.24, 2230.58) | −13.19 (−15.67, −10.71) | |||
| GSH | 97 | 1325.36 (941.97, 1708.74) | −10.95 (−13.82, −8.08) | −572.55 (−1080.14, −64.96) | 2.24 (−1.61, 6.09) | £256 saved per 1-point increase in WSAS |
Reduction in symptoms of PTSD is measured by CAPS-5 with high scores representing a greater level of PTSD. There was a greater reduction in CAPS-5 score at the 52-week follow-up point in the CBT-TF arm −24.51 (95% CI −26.67, −22.34), than in the GSH arm −21.51 (95% CI −23.84, −19.18). Reduction in distress is measured by the IES-R score, with higher scores representing greater distress. There was a greater reduction in distress in the CBT-TF arm, −39.82 (95% CI −43.98, −35.67), than in the GSH arm, −29.74 (95% CI −34.79, −24.70). The WSAS score measures functional impairment, with a high score indicating a greater level of impairment. Again, there was a greater reduction in the WSAS score in the CBT-TF arm, −13.29 (95% CI −15.62, 10.95), than in the GSH arm, −11.06 (−13.72, −840). For all cost-effectiveness analyses GSH was less costly but had worse outcomes than CBT-TF (see Table 22).
Cost–utility analysis
The mean QALY gain (adjusted for site, baseline utility, age and time to event) at 52-week follow-up was 0.68 in the GSH (95% CIs 0.64, 0.72) compared to 0.72 in the CBT-TF group (95% CIs 0.69, 0.76). This represents an incremental QALY loss of 0.04 (95% CI −0.10, 0.01) with GSH compared to CBT-TF (see Table 23).
| N | Adjusted mean cost,a (£) mean (95% CI) | Adjusted QALYs,b mean (95% CI) | Incremental costs (95% CI) | Incremental QALYs (95% CI) | Incremental NMB (£) at £20,000/QALY (95% CI) | Incremental NMB (£) at £30,000/QALY (95% CI) | |
|---|---|---|---|---|---|---|---|
| CBT-TF | 99 | 1897.91 (1565.24, 2230.58) | 0.72 (0.69, 0.76) | ||||
| GSH | 97 | 1325.36 (941.97, 1708.74) | 0.68 (0.64, 0.72) | −572.55 (−1080.14, −64.96) | −0.04 (−0.10, 0.01) | −104.56 (−1286.39, 1077.26) | −460.41 (−2143.27, 1222.45) |
The incremental NMB at 52 weeks for GSH was −£104.56 (95% CI −£1286.39, £1077.26) at a WTP threshold of £20,000 per QALY, and −£460.41 (95% CI −£2143.27, £1222.45) at £30,000 per QALY. The probability that GSH represents a cost-effective option compared to CBT-TF at these thresholds was 43.16% and 29.74%, respectively (see Figure 17).
FIGURE 17.
CUA – cost-effectiveness acceptability curve.

Sensitivity analyses
When private and institution-provided care was included in the analysis, the mean cost savings from GSH were reduced from £572 to £431 (see Table 24). The real-world scenario was based on GSH being delivered by band 4/5 staff instead of band 7 staff, with staff seeing more patients, estimated to be 25 patients per year in each arm. Reducing the staff costs from band 7 staff to band 4/5 staff in the GSH arm had little impact on the results and in all the SAs, the direction of the results remained the same (see Table 24). Analysis on the results using the 16-week follow-up as the end point led to increased savings compared to 52 weeks, £597 (95% CI £1064, £130), and reduced QALY loss, 0.01 (95% CI −0.02, 0.01), from GSH compared to CBT-TF with NHS costs included. When private and other institution costs were also included, GSH was cost saving with no loss in health at 16 weeks.
| N | Adjusted mean costs,a (£) mean (95% CI) | Adjusted QALYs,b mean (95% CI) | Incremental costs (95% CI) | Incremental QALYs (95% CI) | |
|---|---|---|---|---|---|
| CUA at 52 weeks – all costs (NHS, private and institution-provided care) | |||||
| CBT-TF | 99 | 1848.25 (1440.81, 2255.70) | 0.75 (0.72, 0.78) | ||
| GSH | 97 | 1416.81 (1012.88, 1820.74) | 0.71 (0.67, 0.75) | −431.45 (−1009.66, −146.77) | −0.04 (−0.09, 0.01) |
| Real-world analysis at 52-weeks – NHS costs (band 4/5 staff delivering GSH) | |||||
| CBT-TF | 99 | 1667.28 (1342.80, 1991.77) | 0.73 (0.69, 0.76) | ||
| GSH | 97 | 1096.31 (699.02, 1493.61) | 0.68 (0.64, 0.72) | −570.97 (−1083.07, −58.86) | −0.04 (−0.10, 0.01) |
| Real-world analysis at 52 weeks – all costs (band 4/5 staff delivering GSH) | |||||
| CBT-TF | 99 | 1629.36 (1222.12, 2036.60) | 0.75 (0.71, 0.78) | ||
| GSH | 97 | 1165.40 (761.68, 1569.12) | 0.71 (0.67, 0.74) | −463.95 (−1041.87, 113.96) | −0.04 (−0.09, 0.01) |
| CUA analysis at 16 weeks – NHS costs | |||||
| CBT-TF | 99 | 1569.68 (1286.22, 1853.14) | 0.20 (0.19, 0.21) | ||
| GSH | 97 | 972.73 (598.21, 1347.26) | 0.19 (0.19, 0.20) | −596.95 (−1064.29, −129.60) | −0.01 (−0.02, 0.01) |
| CUA analysis at 16 weeks – all costs | |||||
| CBT-TF | 99 | 1643.17 (1360.94, 1925.41) | 0.21 (0.20, 0.21) | ||
| GSH | 97 | 1005.47 (707.94, 1303.01) | 0.20 (0.19, 0.21) | −637.70 (−1049.28, −226.12) | 0.00 (−0.01, 0.01) |
| Real-world analysis at 16 weeks – NHS costs | |||||
| CBT-TF | 99 | 1344.42 (1063.03, 1625.81) | 0.20 (0.19, 0.21) | ||
| GSH | 97 | 725.11 (379.33, 1070.90) | 0.20 (0.19, 0.20) | −619.31 (−1064.97, −173.64) | −0.01 (−0.02, 0.01) |
| Real-world analysis at 16 weeks – all costs | |||||
| CBT-TF | 99 | 1416.36 (1133.86, 1698.85) | 0.21 (0.20, 0.21) | ||
| GSH | 97 | 754.63 (456.74, 1052.52) | 0.20 (0.19, 0.21) | −661.73 (−1073.76, −249.71) | 0.00 (−0.01, 0.01) |
The cost savings in the GSH arm increased for people aged 35 years or older, £754 (95% CI −£1497, −£10.28) compared to £388 (95% CI −£1063, −£287) for people younger than 35 years old; however, there was a greater reduction in QALYs, 0.05 (95% CI −0.12, 0.02) compared to 0.04 (95% CI −0.10, 0.03) in the younger group. People who recorded their level of education as less than degree level also had greater cost savings in GSH, £819 (95% CI −£1423, £216) compared to £293 (95% CI −£1053, £447) in those with a degree or above, and also had a lower QALY loss, 0.03 (95% CI −0.11, 0.04) compared to 0.06 (95% CI −0.13, 0.00). People who started therapy more than 18 months after their traumatic event had greater cost savings in the GSH arm, £720 (95% CI £1267, £173) compared to £485 (95% CI £1246, £276), with less QALY loss, 0.03 (95% CI −0.10, 0.05) compared to 0.05 (95% CI −0.11, 0.02). People with severe pain or anxiety and depression had lower cost savings, £433 (95% CI £1157, £292) compared to £507 (95% CI £1143, £129), and with more health loss, 0.06 (95% CI −0.15, 0.04) and 0.02 (95% CI −0.07, 0.04) (see Table 25).
| N | Adjusted mean costs,a (£) mean (95% CI) | Adjusted QALYs,b mean (95% CI) | Incremental costs (95% CI) | Incremental QALYs (95% CI) | |
|---|---|---|---|---|---|
| Subgroup analysis – age < 35 years – NHS costs | |||||
| CBT-TF | 46 | 1716.07 (1243.42, 2188.73) | 0.75 (0.70, 0.79) | ||
| GSH | 59 | 1328.13 (834.27, 1821.99) | 0.71 (0.66, 0.76) | −387.94 (−1062.88, −286.99) | −0.04 (−0.10, 0.03) |
| Subgroup analysis – age ≥ 35 years – NHS costs | |||||
| CBT-TF | 53 | 2049.85 (1632.58, 2467.12) | 0.70 (0.66, 0.75) | ||
| GSH | 38 | 1296.02 (690.24, 1901.80) | 0.65 (0.59, 0.71) | −753.83 (−1497.38, −10.28) | −0.05 (−0.12, 0.02) |
| Subgroup analysis – education less than degree | |||||
| CBT-TF | 56 | 1984.55 (1609.33, 2359.77) | 0.72 (0.67, 0.76) | ||
| GSH | 54 | 1165.45 (657.56, 1673.34) | 0.68 (0.62, 0.74) | −819.10 (−1422.51, −215.69) | −0.03 (−0.11, 0.04) |
| Subgroup analysis – education degree and above | |||||
| CBT-TF | 42 | 1742.08 (1227.51, 2256.64) | 0.75 (0.70, 0.79) | ||
| GSH | 36 | 1448.80 (891.25, 2006.35) | 0.68 (0.63, 0.73) | −293.27 (−1053.27, −446.73) | −0.06 (−0.13, 0.00) |
| Subgroup analysis – time from traumatic event – ≤ 18 months | |||||
| CBT-TF | 55 | 1999.34 (1480.12, 2518.56) | 0.73 (0.68, 0.78) | ||
| GSH | 56 | 1514.32 (930.72, 2097.92) | 0.68 (0.63, 0.73) | −485.02 (−1246.02, −275.97) | −0.05 (−0.11, 0.02) |
| Subgroup analysis – time from traumatic event – > 18 months | |||||
| CBT-TF | 44 | 1787.88 (1497.81, 2077.95) | 0.71 (0.66, 0.76) | ||
| GSH | 41 | 1067.47 (620.33, 1514.62) | 0.68 (0.63, 0.74) | −720.41 (−1267.33, −173.48) | −0.03 (−0.10, 0.05) |
| Subgroup analysis – comorbidities – baseline EQ-5D pain and anxiety and depression < 4 | |||||
| CBT-TF | 57 | 1885.69 (1465.72, 2305.66) | 0.81 (0.77, 0.84) | ||
| GSH | 49 | 1378.52 (908.46, 1848.58) | 0.79 (0.75, 0.83) | −507.17 (−1143.28, −128.94) | −0.02 (−0.07, 0.04) |
| Subgroup analysis – comorbidities – baseline EQ-5D pain and anxiety and depression 4 or more | |||||
| CBT-TF | 42 | 1810.44 (1337.89, 2282.98) | 0.62 (0.56, 0.68) | ||
| GSH | 48 | 1377.68 (802.93, 1952.44) | 0.57 (0.49, 0.64) | −432.76 (−1157.21, −291.70) | −0.06 (−0.15, 0.04) |
Scenario analysis
A scenario analysis was conducted assuming a proportion of patients receiving GSH relapse each year and going on to receive CBT-TF, with no relapses with CBT-TF. At a 9.5% annual probability of relapse over a 5-year period, GSH would no longer be cost saving, when all costs, therapy plus other healthcare costs, were considered. If only therapy costs were included, then GSH was no longer cost saving at an annual probability of relapse of 24.5% (see Appendix 1, Table 43). The secondary outcome of CAPS-5 score at 52 weeks showed a larger reduction for the CBT-TF group than in the GSH group, mean difference 3.2 (95% CI −∞, 6.0). As this narrowly failed to show NI, and the improved CAPS-5 scores were sustained in the GSH group, it would not imply potential for higher rates of relapse in the GSH group.
Budget impact analysis
The UK population in 2020 was 67.1 million [ONS. Source dataset: Population estimates time series dataset. 25 June 2021. ons.gov.uk (accessed 9 September 2021)]. Using the 2018 population projections, 78.85% of the UK population was 18 years and older (52.9 million). 71 The incidence of PTSD was calculated as 4.4% of people who had experienced at least one traumatic event, N = 730,768. 50 Assuming that 10% of people diagnosed with PTSD are considered to be mild or moderate led to an incidence estimate of 73,077. Not all people diagnosed with PTSD will choose to participate in therapy, it has been assumed that 50% of those diagnosed would choose therapy.
The median NHS healthcare costs for available cases collected at baseline, 16 weeks and 52 weeks have been applied to represent other healthcare costs to reflect the positively skewed data. The resource use preceding baseline and the 52-week follow-up have been applied for equal lengths, with the resource use in the 3 months preceding baseline applied for 9 weeks in the GSH arm and 11 weeks in the CBT-TF arm to reflect the average lengths of these therapies. The median cost of therapy for band 7 staff for CBT-TF (£754) and for band 4/5 staff for GSH (£165) is based on the expected delivery in practice. Extrapolated to the UK population, the estimated budget impact over a year is a saving of £12.3 million if GSH is introduced into the current NHS therapy options and 50% of eligible people chose GSH therapy (see Table 26). If only the costs of therapy are included, then the cost savings are reduced to £10.6 million. If the uptake is lower for GSH compared to face-to-face therapy, 25%, then the potential savings will also decrease to approximately £6.2 million. If the proportion of people diagnosed with mild and moderate PTSD is higher, 50%, then cost savings will increase, estimated at £61.6 million.
| 50% uptake GSH | Only therapy costs | 25% uptake GSH | 50% PTSD mild/moderate | |
|---|---|---|---|---|
| Number of eligible people experiencing at least one traumatic event and diagnosed with mild/moderate PTSD | 73,077 | 73,077 | 73,077 | 365,384 |
| Uptake of any therapy estimate (%) | 50 | 50 | 50 | 50 |
| Number of eligible patients taking up therapy (N) | 36,538 | 36,538 | 36,538 | 182,692 |
| Uptake of GSH therapy estimate (%) | 50 | 50 | 25 | 50 |
| Annual cost of GSH therapy | £3,200,765 | £3,200,765 | £1,600,383 | £16,003,825 |
| Annual cost of CBT-TF therapy | £13,769,684 | £13,769,684 | £20,654,526 | £68,848,420 |
| Annual cost of NHS healthcare GSH | £15,892,453 | £0 | £7,946,227 | £79,462,267 |
| Annual cost of NHS healthcare CBT-TF | £17,639,179 | £0 | £26,458,769 | £88,195,896 |
| Net costs (£) | £50,502,081 | £16,970,449 | £56,659,904 | £252,510,408 |
| Only CBT-TF therapy | £62,817,726 | £27,539,368 | £61,914,862 | £314,088,632 |
| Budget impact | £12,315,645 | £10,568,919 | £6,157,822 | £61,578,224 |
Summary
This chapter described in detail the methods and results of the HE evaluation undertaken as part of the RAPID trial. The GSH involved self-help materials through the website or app, with face-to-face sessions from a trained therapist. The median number of face-to-face sessions in the GSH arm was 5 (IQR 3–5), with additional check-in phone calls in between face-to-face sessions. The comparator, CBT-TF, involved face-to-face therapy only, with a median of 9 (IQR 6–12) sessions per participant. The cost of therapy was lower for the GSH arm, mean cost £277.31 (95% CI £253.27, £301.34), compared to £729.49 (95% CI £670.76, £788.22) in the CBT-TF arm. Other healthcare resource use was collected for primary, community and secondary care. All resource use was reported regardless of reason, to capture any increased use which may be indirectly related to PTSD. Medication costs were only collected at baseline, which is a limitation as there may have been changes in prescriptions related to PTSD. The healthcare resource use was similar in both arms at each time point with the main cost driver being the cost of therapy. This was to be expected given the trial population included mild to moderate PTSD with PTSD as their primary diagnosis.
The results suggest that use of GSH is cost saving compared to CBT-TF (−£572.55: 95% CI −£1080.14, −£64.96), but may be associated with poorer outcomes, given the small, non-statistically significant difference (−0.04: 95% CI −0.10, 0.01). Including private and other institution costs led to lower cost savings with GSH, −£431 (95% CI −£1010, −£147). Changing the staff delivering GSH to band 4/5 had little impact on the results, with cost savings of −£571 (95% CI −£1083, −£59). At 16 weeks the NHS cost savings increased, −£597 (95% CI −£1064, −£130), but there was still a small and not statistically significant health loss, 0.01 (95% CI −0.02, 0.01). Subgroup analysis attempted to consider which people may benefit more from each therapy; however, these analyses were based on small, underpowered samples and results should be interpreted with caution. These results reported greater cost savings in the GSH arm for people aged 35 years or older compared to people younger than 35 years, however, with a greater reduction in QALYs. People who recorded their level of education as less than degree level also had greater cost savings in GSH compared to those with a degree or above, and also had a lower QALY loss. People who started therapy more than 18 months after their traumatic event had greater cost savings in the GSH arm with less QALY loss. People with severe pain or anxiety and depression had lower cost savings, compared to people reporting no or mild or moderate pain, anxiety or depression at baseline, and with more health loss.
Self-help with support was found to be more cost-effective than CBT-TF in a recent evaluation of psychological treatments for PTSD, the QALY gain with self-help with support was 1.75 QALYs at a cost of £266, with CBT-TF the QALY gain was 1.74 but the cost of therapy was considerably higher, £1058. The population in this evaluation was adults presenting in primary care with clinically important PTSD symptoms and therefore may include people with more severe symptoms than the RAPID trial. The odds ratio of remission versus no treatment at treatment end point was 14.06 in the CBT-TF group compared to 13.98 in the self-help with support group.
The majority of participants in the RAPID trial were employed (60.7%) at baseline. Participants were not asked if they had to take time off work for face-to-face sessions, but as CBT-TF involves more sessions of longer length, then it is reasonable to assume that CBT-TF will require more time off work than GSH. As part of CBT-TF it was estimated that 75% of participants would have a site visit during a session, this is likely to increase time and costs as increased travel time is required. Therefore, a full societal perspective analysis including the costs of lost work and travel expenses would likely further emphasise the incremental cost savings of GSH compared to CBT-TF.
The reported NMBs from our analysis are presented to assist the decision-making process and are not an absolute statement on whether the intervention can be deemed cost-effective. There are no established willingness-to-pay thresholds for the cost-effectiveness in percentage reduction in CAPS-5, percentage reduction in IES-R or percentage reduction in WSAS score. Based on standard approaches to cost–utility analyses, and using a standard UK threshold, GSH is not a cost-effective option. However, cost-effectiveness analyses should be considered alongside other considerations, for instance budget impact and feasibility. A simple BIA was conducted which explores the potential impact on NHS budgets if the lower-cost GSH therapy is used. The results of the BIA demonstrated the potential cost savings if GSH was introduced could be £12.3 million in 1 year if 50% of eligible people chose GSH therapy. If only the costs of therapy and no other health and social care costs were included in the analysis, the savings would be £10.6 million in 1 year.
As this was a NI trial, it was not powered to detect a difference in the outcomes of two therapies, and this is seen in the CIs around the QALY loss (95% CI −0.10, 0.01) and the wide CIs in the NMB results (see Table 23). However, it is not appropriate to conduct a cost-minimisation analysis as this assumes there is no difference in the effects with no uncertainty. 72 It is anticipated that GSH will be delivered by staff with lower-intensity therapy training, which may allow GSH to be offered more widely, releasing trained therapist time for other interventions. Some participants achieved better health with GSH, this may be due to convenience in accessing the therapy around other time commitments or preference for less face-to-face contact. Additionally, given the impact of COVID-19 on accessing health care, there is increased need for availability of interventions that reduce face-to-face contact. Understanding which participants achieve better health outcomes with GSH will aid provision of an optimal intervention mix for PTSD. 73
Chapter 5 Qualitative study
Introduction
The process evaluation examined acceptability and fidelity of two interventions from the perspective of RAPID participants and therapists. The perspectives of NHS commissioners and managers involved in commissioning and implementing psychological therapies, but not involved in the RAPID trial, nor in the development of the Spring intervention, were also explored. One member of the research team undertook data collection and led on analysis for patient and therapist interviews, and another member of the research team undertook data collection and led on analysis for NHS commissioner and manager interviews. This work was supported by a subgroup of trial members and members from the Cardiff University Traumatic Stress Research Group PTSD Public Advisory Group. Meetings were held to discuss topic guides, data themes and saturation as well as approaches to analysis. A framework approach was applied to the analysis of qualitative data for the process evaluation.
The process evaluation, informed by MRC guidance,74 explored the contextual factors and mechanisms of change that may impact on the effectiveness and successful rollout of the intervention post-trial. In-depth qualitative data were collected from semistructured interviews with therapists and participants at two different time points within the trial, pre- and post-intervention delivery, and with NHS commissioners and managers between January and June 2020. In total 39 participants took part in interviews with 54 interviews completed in total.
The qualitative work undertaken aimed to describe the experience of receiving the GSH intervention from the patients’ perspective, and the delivery from the therapists’ perspective. Qualitative data were obtained from semistructured telephone interviews with a sample of therapists (n = 10) and patients (n = 19) across research sites. Qualitative data were also obtained by telephone (n = 6), in person (n = 3), and via videoconference (n = 1), from NHS commissioners and managers in confidential NHS settings. These data informed a process evaluation undertaken as part of the RAPID trial. Qualitative methods incorporated into trials and process evaluations capture the participant voice and are associated with the how and why of a phenomenon and focus on the experience of participants. 75,76 The telephone interview method has the benefit of wide geographical reach with a lower cost than face-to-face interviews, which is an important cost consideration for trials and funders. A sense of comfort may come from the familiar surroundings for participants taking part in a telephone interview and anonymity with the loss of the visual. Verbal cues, language, patterns, pace and pauses are given attention as a way of managing the loss of visual clues. The data collection, management and approach to analysis are separated into three subsections, beginning with patient participants.
Patient participant sample
A purposeful approach to sampling was applied in the recruitment of patient participants for qualitative interviews. A qualitative researcher received notifications of potential patient participants following randomisation into the main trial. A sampling and recruitment participant workbook was developed in Microsoft Excel and contained preliminary information on (1) arm allocation, (2) age at randomisation, (3) gender, (4) ethnicity, (5) educational level, (6) nature of trauma, (7) trial site and (8) contact attempts and outcome for each participant considered for interview. Further information was gathered on (1) nature of the trauma through notes added to the trial participant database and (2) availability of both researcher and participant to complete a telephone interview before the commencement of intervention delivery. A maximum of three attempts were made to contact a participant to achieve an interview. Fifty-five trial participants included within the participant sample were approached for an interview with nineteen participants agreeing to take part.
Method of data collection
This qualitative component was designed using the principles of the Critical Appraisal Skills Programme (CASP) qualitative checklist, to ensure the quality of qualitative research (CASP 2018). Two interview topic guides were drafted for patients by the lead qualitative researcher and further developed with input from members of the trial management group with public and patient representation. The first interview with patients was undertaken post-randomisation and pre-treatment providing a timeframe of approximately 2 weeks within which to recruit and complete an interview prior to the receipt of any treatment. The first topic guide contained three broad categories exploring (1) trial processes, (2) patient context and (3) treatment. Questions were followed by probes as a means of opening up the topic being discussed. A document for recording field notes and details from the interview was prepared in advance and reflections completed at the end of each data collection.
Patients were approached for post-intervention interviews after their treatment in the trial had concluded and scheduled after a 16-week assessment as part of the broader trial. The topic guide for post-intervention interviews was developed to mirror the content of the first topic guide and followed the development process of the pre-intervention interview schedule. Questions and probes focused on the experience of the trial and treatments. For those patient participants who had completed a pre-intervention interview, field notes and the transcript from their first interview were reviewed for, and preparatory notes were made ahead of, their post-treatment interview. Details relating to (1) arm allocation, (2) allocation preference prior treatment, (3) nature of trauma, (4) pain associated with trauma, (5) medication, if any, (6) previous understanding, views and experience of PTSD and treatments and (7) any points of interest to re-visit from first interview were noted ahead of a second interview with patients.
Data management
Audio recordings of interviews (raw data) were collected on encrypted electronic recording devices. The audio files produced were copied on to the trial shared drive folder in accordance with the trial protocol and labelled with a unique participant identifier (PID). The recordings were deleted from the device following upload. Interviewer’s field notes (reflective notes) were typed up as a Microsoft Word document and stored in the trial shared drive folder. Handwritten copies of interviewer’s field notes were stored in a locked, secured cabinet in CTR.
A password-protected table in a Microsoft Excel document was used to record sampling, dates and outcomes from interviews. All word-processed data were uploaded to NVivo 12 (QSR International, Warrington, UK) qualitative coding software for management and analysis.
An external transcription company transcribed audio recordings which were transferred using an agreed secure procedure. Un-anonymised transcripts produced were returned and saved on the secure server. The anonymisation process involved removing personal identifiers such as name, date of birth, places of birth, etc. and replacing them with an anonymised descriptor. Qualitative data were checked for any errors in transcription or understanding, as part of the anonymisation. A further sweep was then undertaken to identify any errors, typos, missing data and sense making.
Data analysis
The data were analysed using framework analysis. 77 This systematic, five-stage method is increasingly being used in healthcare research81 and allows for a comparison of themes across time point, treatment centre and interviewee category (i.e. patient and therapist). The framework analytic approach was selected as it is a recognised transparent approach to analysis.
A framework approach can accommodate different data sources (interviews at different time points, clinical assessments and usage data for GSH participants), and diverse sampling (patient participants from across trial sites).
This five-stage method involves (1) familiarisation, (2) developing a thematic framework from the trial (protocol) objectives, interview questions and emerging themes, (3) indexing, (4) charting and (5) mapping to search for interpretations. Agreement on anchor codes, subthemes and concepts were sought between members of the research team to ensure reliability. Commonly expressed themes as well as unusual cases were identified. A proportion of the data (~10–20%) was coded by three different team members to check on the reliability of the coding scheme. The qualitative process data collected were regularly reviewed for saturation and discussed within the team. It is recognised that the data collected are context-specific, providing a snapshot into a defined phenomenon or experience. Therefore, in identifying saturation, data were reviewed for density and ability to sufficiently answer the research questions. 79
The analysis framework was tested using data from two pre and two post-intervention interviews and a matrix summarising key themes was completed. This allowed for a review of the framework to see if the pre-defined anchor codes captured the data needed. This was presented to a small group of members of the Trial Management Group for discussion and feedback used to make refinements. A summary of key themes from indexing and charting into the framework was presented and discussed on two occasions with members from the trial PPI group and resulted in suggestions to aid interpretations and proposed variables to consider in further analysis.
Therapist participant sample
Therapists within the trial were asked to provide information to the qualitative researcher relating to their level of experience, current practice and experience of Spring along with demographic information; this was used to guide sampling from across regions. Nineteen trial therapists were approached after they had completed training for the interventions with ten therapists taking part in interviews.
Method of data collection and management
The topic guides for therapist interviews were developed following the same steps as patient interviews. The first topic guide contained four broad categories exploring (1) assessing PTSD, (2) usual care, (3) trial processes and (4) impressions of GSH intervention. Questions were followed by probes as a means of opening up the topic being discussed. A document for recording field notes and details from the interview was prepared in advance and reflections were completed at the end of each data collection. Therapists were approached for the second interview after they had finished delivering treatment in the trial. The data management procedures applied to patient participant interviews were also employed to therapist data.
Data analysis
The therapist data were also analysed using a framework approach and followed a similar process to the analysis of patient participant data. The analytic framework was tested using data from two pre and two post-intervention interviews and a matrix summarising key themes was completed. This allowed for a review of the framework to see if the pre-defined anchor codes captured the data needed. The framework was presented to a subgroup of members of the TMG for discussion and feedback used to make refinements.
NHS commissioners and managers participant sample
Interviewees with specific knowledge and experiences and a range of familiarity with internet-based interventions were purposively sampled, accounting for representation across genders, RAPID recruitment sites and NHS clinical leadership and management roles. Twelve eligible individuals from England, Scotland and Wales were invited and provided written informed consent to participate. Two were unable to progress due to unforeseen changes in their roles due to COVID-19.
Method of data collection
Demographic information was collected at interview, which followed a topic guide, developed with researchers and clinicians of the RAPID Trial Management Group, co-produced with individuals with lived experience of PTSD (Cardiff University’s Traumatic Stress Research PTSD Public Advisory Group), and an independent NHS Consultant Clinical Psychologist. The semistructured interview was informal and included prompts to maintain conversational flow, to encourage individuals to introduce new topics as they saw fit, and to probe for further detail and views. Questions broadly invited discussion of the following topics: the participant’s role, organisation and interventions they were involved with; their reflections on internet-based interventions; and their understanding of the barriers and facilitators to implementing such interventions.
Data management and analysis
Interviews were audio recorded and transcribed to produce orthographic verbal verbatim and field notes were written immediately after each interview to aid the preliminary analysis.
Data collection and analysis occurred in parallel, so that a constant comparison approach to exploring themes could be adopted. This allowed an extra check for sufficient data saturation,78 in addition to our aim for sufficient information power via the recruitment of ten participants. 80 Saturation was monitored through the double-coding process and discussed between members of the research team.
Transcripts were prepared for analysis, assigning pseudonyms to participants and removing the names of others and their roles and institutions, to help preserve anonymity. Cleaned transcripts were imported into QSR NVivo 12 qualitative data analysis software (Q.I.P. Ltd., 2020, Denver, USA).
An inductive approach was chosen for analysis due to the theoretical flexibility, as well as the ‘thick descriptions’ afforded by the method (Braun and Clarke, 2006). The Framework Method was used to support the thematic analysis, which allows for an inductive approach and also provides a systematic model for managing and mapping data. 81 Two researchers generated codes for 100% of the interviews, meeting regularly while coding, initially to develop the analytic framework, for example, as new codes were generated from further interviews. They met with other members of the research team at regular intervals to discuss codes and themes, to ensure clear understanding and interpretation and to be able to reconcile any inter-rater reliability discrepancies, though this was not required. The researchers applied the analytic framework when coding the remainder of the transcripts and finally populated the codes into a framework matrix. The matrix comprised rows based on participants and columns based on codes, with each cell therefore including verbatim quotes for the corresponding participant and code. Final interpretations were made with input from members of Cardiff University’s Traumatic Stress Research Group PTSD Public Advisory Group.
Participant characteristics
The patient sample in the process evaluation reflected the trial population in terms of gender, ethnicity and age. The sample included patient participants from each trial site and represented a range of traumatic experiences as illustrated in Table 27. Six males and 13 females participated; 17 pre-intervention and 10 post-intervention. Seven participants took part in both pre- and post-intervention (paired) interviews.
| PID | Pseudonym | Gender | Age | Ethnicity | Educational level | Trauma type | Arm |
|---|---|---|---|---|---|---|---|
| 18 | Mike | Male | 51 | White Irish | Other vocational/work-related qualification | Serious accident at work, home or during recreational activity | GSH |
| 32 | Rachel | Female | 34 | White | Degree level or above | Witness to death | CBT-TF |
| 37 | Matilda | Female | 32 | White | Other vocational/work-related qualification | Near-death experience | GSH |
| 38 | Katie | Female | 32 | White | 2 + A levels | Witness to death | CBT-TF |
| 39 | Molly | Female | 24 | White | 2 + A levels | Witness to death | CBT-TF |
| 48 | Sheila | Female | 51 | White British | 2 + A levels | Personal injury | CBT-TF |
| 51 | Kay | Female | 69 | White British | Other vocational/work-related qualification | Life-threatening illness or injury | GSH |
| 60 | Terry | Male | 52 | Other ethnic background | Degree level or above | Transportation accident | CBT-TF |
| 73 | Ann | Female | 60 | White | 5 + GCSEs | Other stressful experience | GSH |
| 76 | Ellen | Female | 25 | White | 5 + GCSEs | Witness to death | GSH |
| 98 | Stuart | Male | 53 | White | Other vocational/work-related qualification | Transportation accident | GSH |
| 100 | Becky | Female | 27 | White | 2 + A levels | Transportation accident | GSH |
| 102 | Ross | Male | 40 | White | Degree level or above | Personal injury | GSH |
| 104 | Miriam | Female | 60 | White | Other vocational/work-related qualification | Near-death experience | CBT-TF |
| 108 | Hugo | Male | 58 | White | 1–4 GCSEs | Transportation accident | CBT-TF |
| 112 | Karen | Female | 44 | White | 5 + GCSEs | Any other stressful event or experience | CBT-TF |
| 114 | Clare | Female | 24 | White other | Degree level or above | Sexual assault | GSH |
| 182 | Emma | Female | 34 | White | Degree level or above | Any other stressful experience or event | GSH |
| 183 | Luke | Male | 31 | White | 5 + GCSEs | Transportation accident | GSH |
Table 28 details the therapist participant sample. Ten therapists, sampled from each research site, participated in pre-intervention delivery interviews, six were female and four male. Seven of these therapists went on to complete a post-delivery interview, three male and four female. Most participant therapists had had low exposure and experience of the Spring application prior to the trial.
| PID | Pseudonym | Gender | GSH familiarity | Research site |
|---|---|---|---|---|
| 102 | Christian | Male | Very high | Cardiff and Vale UHB |
| 103 | Laura | Female | Low | Cardiff and Vale UHB |
| 106 | Jenny | Female | Low | Cardiff and Vale UHB |
| 107 | William | Male | Low | Cardiff and Vale UHB |
| 214 | Erica | Female | Low | Coventry and Warwickshire |
| 218 | Annabel | Female | Low | Coventry and Warwickshire |
| 309 | Roy | Male | Low | Newham |
| 423 | Meg | Female | Low | NHS Lothian |
| 424 | Sophie | Female | Low | NHS Lothian |
| 427 | Gavin | Male | Low | NHS Lothian |
The stakeholder sample included five males and five females. The group were mostly White British, with a mean age of 50.7, and with a degree level of education or over. Interview lengths ranged from 27 to 62 minutes, with a mean of 48.9. Six interviews were conducted prior to the COVID-19 UK National Lockdown commencing 23 March 2020, and four were conducted after (see Table 29).
| Pseudonym | Gender | Age | Ethnic origin | Type of NHS role | Length of interview (minutes) | Interview conducted pre-/post 23 March COVID-19 UK lockdown |
|---|---|---|---|---|---|---|
| Phil | Male | 59 | White British | Clinician Clinical service management |
50 | Pre |
| Tim | Male | 44 | White British | Clinical service management | 57 | Pre |
| Sue | Female | 59 | White British | Clinical service(s) strategic lead | 56 | Pre |
| Patrick | Male | 51 | White British | Clinical service management | 57 | Pre |
| Isla | Female | 55 | White British | Clinical service(s) strategic lead | 43 | Pre |
| Geoff | Male | 53 | White British | Clinical service management | 47 | Pre |
| Sarah | Female | 49 | White British | Clinician Clinical service(s) strategic lead |
40 | Post |
| Robert | Male | 34 | White Irish | Clinical service management | 62 | Post |
| Gwendolyn | Female | 52 | White British | Clinical service(s) strategic lead | 27 | Post |
| Rose | Female | 51 | White and Black African | Clinician Clinical service management |
50 | Post |
Results
The results from the qualitative process evaluation add a narrative complementary to the quantitative components of the trial examining intervention effectiveness and fidelity. The qualitative results presented provide an overview of intervention implementation in the RAPID trial. There is a focus on how the intervention was delivered and how this was received, in order to explore the range of effects and functioning of the intervention as well as overall acceptability. Contextual factors external to the intervention are also included to further inform issues of implementation, delivery in real-world setting, future roll-out and sustainability.
Trial design and processes
The following results relate to the trial design and processes in RAPID. Acceptability was examined in interviews with patient and therapist participants and explored through their experience of recruitment, screening, consent and randomisation. Table 30 provides an overview of the key themes identified in relation to each trial process component. Appendix 2 provides additional supporting quotes for the themes identified.
| RAPID participants | RAPID therapists |
|---|---|
| THEME: BARRIERS/CHALLENGES TO ENGAGEMENT WITH ‘SPRING’ GSH | |
| Themes common across participant and therapist interviewees | |
| Difficulties fitting in homework | Participant lack of time and competing demands |
| Treatment too short and slow paced | Sessions felt too short |
| Technical difficulties | Technology challenges |
| Difficulties engaging with some programme components | Perception that participants were finding some programme components to be challenging |
| Limited support in therapy sessions | Limited therapeutic alliance |
| Language limitations | Participant literacy levels |
| Preference for face-to-face | Perception that participants preferred face-to-face |
| Themes unique to participant and therapist interviewees | |
| Concentration difficulties | |
| Therapist unfamiliarity with intervention and modality | |
| Not knowing how long participants were spending on the programme | |
| Therapist concerns about exposure work conducted through GSH | |
| Challenge of participant complexities | |
| Less flexibility in intervention content | |
| THEME: FACILITATORS/OPPORTUNITIES FOR ENGAGEMENT WITH ‘SPRING’ GSH | |
| Themes common across participants and therapist interviewees | |
| Positive and calming programme | |
| Good pace and length | Adequate treatment length |
| Supportive sessions with therapist | Therapeutic alliance could be established |
| Beneficial programme components | Positivity about programme components |
| Themes unique to participant and therapist interviewees | |
| Flexible treatment | |
| Technology worked well | |
| Empowering | |
| A good option for individuals who would rather not talk to someone over weekly sessions | |
| Participant treatment preference and motivation to get better | |
| Liking the intervention helped therapists to encourage participant engagement | |
| Participant digital literacy | |
| Structured and containing | |
| Value to therapist of an alternative to face-to-face | |
| Supervision | |
| THEME: TREATMENT OUTCOMES | |
| Feeling better | |
| Feeling like back to square one | |
| Better understanding of PTSD and its treatment | |
| Seeing more friends | |
| THEME: CONSIDERATIONS FOR THE FUTURE OF GSH for PTSD | |
| Therapist preconceptions of GSH have been challenged | |
| Potential for GSH in widening access to psychological therapies | |
| ‘COVID-proof’ intervention | |
| Intervention applicability | |
| Recommendations for sustainability | |
Consistency and clarity
The majority of patient participants report referral into the trial via a health professional such as a GP or mental health nurse. There was also evidence of referrals from mental health services and charities.
the doctor referred me to [service provision], a lady that I spoke to on the phone asked me if I would take part in it.
Sheila
There were varying recollections about the information received in relation to participant information sheets and content of conversations for recruitment into the trial; however, participants recalled initial engagement with members of the trial team, and the consistency of these reports demonstrate a standardised approach to recruitment that was acceptable to participants.
Oh, I’ve got a really bad memory so let me, erm, so, erm, I had, firstly a lady called me … Erm, and she explained everything about the trial, she said that you have to be assessed to come onto the trial … Erm, and she gave me a phone assessment, erm, she, and then she sent some things through to me to the post.
Rachel
Participants described contact with a number of health professionals or services in their search for treatment and it was also evident that some patients could be engaged with a number of agencies and healthcare professionals as a result of their trauma.
I think it came from [service provision], well initially I went to [OTHER SERVICE PROVISION] at work … And then they put me on to [service provision] and I think that’s where this came from … it was all a blur, I think they gave me um a leaflet.
Stuart
Overall, participants understood the information they received about the trial and further clarification was gained through interactions with trial staff undertaking screening and consent.
Erm, and then after that I can’t remember his name, but erm a man from the study called me and explained a bit about the study and then did an initial assessment I think, over the phone. Err and then after that I received a pamphlet and some information in the mail.
Clare
There was one isolated case where a participant indicated that the information provided at recruitment may have been lacking:
Erm yeah, I mean I don’t mean to say vague in a negative way … but just erm, it was just erm it didn’t fully explain the, the sort of study erm but I didn’t expect it to at that point anyway erm but yeah.!
Molly
Consistency of both approach and messaging in recruitment can help support participants in making an informed decision about their participation in a trial. Early contact between research team members and participants is also important, helping shape initial engagement and creating opportunities to provide further clarification for participants. The varying and sometimes limited recall from participants about the information they received and the process of giving consent could be attributed to difficulties with memory associated with their PTSD, but also as a result of engagement with other services in seeking support. However, motivations for taking part are also a contributing factor potentially influencing how much attention participants paid to the recruitment information and consenting and reported in further detail below.
Volume, intensity and impact
There were a variety of experiences reported about the screening process, with a predominant theme relating to the volume of the screening tools and the time taken to complete baseline assessments.
Sorry, when [rater] was running though all of the questionnaires with me, I was finding it not hard, but it would’ve taken us a lot longer. There was a lot of questions. About me as a person to answer on the, on her laptop. It did seem a lot of things, I think, that people would struggle with.
Mike
Participants were challenged when they felt that responses and scales represented on a screening tool did not fit their situation.
And to actually fill in a box that wasn’t quite me left me feeling a, erm, distraught when I came out.
Kay
Miriam, reflected on how a lack of understanding about the use and purpose of the questions asked as part of the screening could put people off, ‘It could because I felt like saying, what you know, hang on a minute, what, what’s that got to do with my accident?’
There was a recognition among participants that screening and baseline assessments can be challenging for people experiencing PTSD, given their recent trauma and associated symptoms. However, participants indicated that they were supported by recruitment staff.
And you have to and she’s very sensitive to then, her questions afterwards, it’s not just like, oh I’m reading questions off a list … And, erm, really just taking down, she understand that you know, you have something that’s really, you know, hurt your heart. So she was really, she was really good.
Rachel
Of interest was a connection drawn by a few participants between the experience of assessments having an influence on what participants might expect going forward in the trial. They talked about how the experience of how the screening tools and process may negatively influence perceptions of what the interventions might involve, such as answering lots of questionnaires and inputting them into a computer.
that [completing baseline assessments] could flavour how people think that the online training’s going to go. Um, as in that is essentially an online form, and is it going to be just along these lines where I just sit there and click boxes?
Ross
However, some participants acknowledged that the screening tools and measures were a necessary and an important part of the trial design.
Because I think you need to know where you are to begin with to see how far you’ve come, you know in a few month’s time … That would be a bit … you know if anything has worked or not … But I do think it is all relevant because there’s no use putting somebody through a programme if it’s not really going to be as much help.
Ann
In exploring the acceptability of recruitment and screening in the RAPID study, the results from the process evaluation data demonstrate an overall acceptability of the processes, but also highlight the potential burden of screening processes on participants in completing baseline assessments. In RAPID, some participants expressed feeling distressed during assessments and having an emotional impact after. This was not always reported as a negative experience though, and was acknowledged as having some cathartic qualities. In supporting the recruitment of patients experiencing PTSD, participants in RAPID acknowledged the professionalism and sensitivity of recruiters and valued contact with team recruiters and seeking clarification about taking part.
To explore the acceptability of providing consent, patient participants were asked what they remembered and invited to share their thoughts or concerns they may have had at the time. Two focal themes were identified: (1) confidentiality and reservations and (2) motivations for taking part. The process data confirms that, overall, participants found the consent process acceptable, reporting it as clear, understandable and easy.
Erm yeah it was, it was fine they talked me through all of the paperwork and everything and erm I understood everything that I was signing up to.
Molly
Confidentiality and reservations
In exploring concerns about taking part in the trial, participants paid attention to the confidentiality of their information and data.
Well I knew it was going to be kept like, er, not quiet, how would you say it, like, erm, I was, my identity was going to be protected.
Rachel
Participants also voiced reservations about the interventions being offered in the trial. This included thoughts about the ability to engage in GSH, treatment length and effectiveness. These themes are developed and reported further on in relation to intervention implementation and delivery, facilitators and barriers.
Motivations for taking part
Four themes were identified in relation to participant motivations for taking part: (1) the need for help, (2) quicker access to treatment, (3) to help others and (4) help improve services. The severity of symptoms and impact on quality of life was evident within the process data and it is natural to think that the need for help is an instinctive motivation for taking part. As Mike illustrates, ‘Um, I did. I, I really needed to talk to someone … at the end of the day I just, I just want to get better’.
So it’s what motivated me to take part was that I could, I realised that I was just getting worse and worse.
Sheila
Patient participants acknowledged challenges in accessing treatment and long waiting times within the NHS, and that taking part in the trial would result in quicker access to treatment.
Erm, well initially because I was at my worst stages then that, erm, my initial thoughts was anything that would speed up my treatment.
Kay
Participants expressed altruistic views in that by taking part they are able to help others and as Becky expressed, ‘yeah I mean if I can help other people by taking part in it …’. This help for others also extended to the research team and those working to find new treatments.
I just wanted to help somebody else out and obviously like, if, if it’s a big thing that people want to learn more about, so ….
Katie
Alongside the theme of helping others, participants made connections between taking part in the trial and the potential benefit and improvements to services as a result of their contribution.
It, this is trying to help the NHS as well innit you know … Cos of the list, to get on the waiting list, it’s horrendous isn’t it.
Miriam
Hugo expressed a social responsibility to take part in trials and stated, ‘Like I said to you, to me it’s like giving, err organ donation’. This is further illuminated in the quote below.
You know I went with my gut instinct because everything felt really good about it and I … and it’s only like giving blood or leaving your organs if something happens to you. To me it’s doing some good. You know even community or at your establishment, if it’s helping you, it’s not costing me nothing.
Hugo
Understanding participant motivations can help inform recruitment in future trials, and also illuminate factors that may impact on future roll-out and engagement. It could be surmised that patients in need and wanting access to treatment for PTSD could be receptive to engage in a treatment such as GSH in their desire for help in a timely fashion.
Preference and expectations
A key component of the RAPID trial design is randomisation, and this was explored with both patients and therapists. We focused on interviewees’ descriptions and reflections to explore the acceptability of randomisation within the trial. The process data demonstrate that, in principle, randomisation is acceptable to both patients and therapists.
Patient participants overall demonstrated an understanding of randomisation within the trial.
Er, well, he kind of explained to me that one was through conventional means, and I presume by that he was talking about CBT, um, and one was through self help, and well, guided um, online um, approach, um, and you would fall into one of the two camps, er, obviously randomly selected to see which you went into.
Ross
Patient participants also expressed an openness to receiving either of the treatments being offered.
So whichever one I was offered I would commit to.
Kay
In participantsʼ openness to randomisation and potentially receiving a new treatment, there is an implied trust in the research team and their professionalism and that any treatment offered could help.
I just thought I’d leave it to you, to be honest, they’re the experts, they know what they’re doing, I’m happy with whatever they put me, put me forward to, to be honest.
Katie
Even though participants indicated an openness to receive either intervention, overall, there was a preference for CBT-TF.
I mean when you’ve just got the Therapist is, is best for what I would like. But the, the other one, I’m, I’m okay with.
Stuart
The preference for CBT-TF expressed by participants highlighted that face-to-face contact and having human interaction was an important aspect of treatment for them.
Erm I understand the need to, to sort of assess the success of both erm, personally for me I think the, the face to face element of, of erm the therapy would be more beneficial, but I understand the need to sort of get it a bit more automated so that it can be more accessible.
Molly
It is possible that the need for treatment is a factor shaping the acceptability of the treatments available through the trial.
For therapists interviewed, there was an understanding of the trial design and processes and that patients would be randomised to receive one of two interventions which they had been trained to deliver. Although therapists were not involved directly in allocation, they were in a position of informing and confirming to participants which treatment they were going to receive.
So I, I would say to my participants, um, yeah I’ve, I’ve, you’ve been err referred to me as your Therapist on the trial um, you’ve been allocated to whichever arm, and, and then I’d just follow that up with, was you hoping to get this arm? Or, or you know, did you have a preference? Or are you disappointed?
Christian
This process resulted in mixed feelings for therapists and how they described managing patient expectation and preferred treatment option, and as Christian commented that, ‘some people were disappointed’. Therapists also reflected on the suitability and appropriateness of an allocation for some patients – they considered they may have benefited in the alternative arm option if not under trial conditions.
Um, so all my cases were along that, the guided … for guided self help. And half of them were … weren’t suitable either. I felt like they were shoehorning er, people into the … the intervention, and primarily because I felt you know … and I got emails just to say that I was sharing a concern about the … certain things that were getting missed for example was, one off trauma, there’s people that had other traumas, that were clearly going to impact the past on that one off trauma.
Gavin
Recommendations
-
Less intensive screening process; consider reducing the number of measures or the option to spread over more than one sitting; being considerate of the potential impact for patients with PTSD.
-
Actively seek to provide clarification to participants at each contact point during recruitment to provide further clarification; this includes the use and purpose of measures.
-
Provide a summary of the nature of the trauma for patients allocated to therapists ahead of first contact and commencing treatment; preparedness of therapists.
Intervention delivery and acceptability
Acceptability of the interventions was explored in interviewees’ descriptions and experiences of delivery and receipt of treatment. We examined anticipatory notions about treatment and participant expectations. We focused on the barriers and facilitators highlighted by participants and appraised intervention activities and outcomes reported by both patient and therapist participant groups as a way of probing mechanisms.
We explored with patient participants the prospect of receiving either GSH to CBT-TF and a theme emerged around uncertainty and not knowing what to expect. Patient participants expressed their uncertainty about what might be involved in either of the treatments. Mike stated of GSH, ‘I haven’t got a clue’, and Kay responded, ‘I can’t visualise the online therapy’. Similar uncertainty was expressed about what might be involved in the CBT-TF treatment.
I don’t know, I think, I don’t, I honestly have no idea, cos I’ve never been on, done anything like this before.
Miriam
Others who did articulate their thoughts about what the treatments might look like expressed an uncertainty in terms of the role of the therapist and application of the technology.
I am not sure what the therapist’s role is, do you speak to a therapist directly through the computer or is it a, or do they observe?
Ellen
Therapist views of GSH pre-delivery were less favourable than for TF-CBT, with some hesitancy around a new model and mode for treating PTSD. However, through experience of intervention delivery, preconceptions of therapists changed.
Facilitators
Participant narratives revealed numerous facilitators and demonstrated an acceptability of the interventions. They accentuated factors that are important to participants and the engagement in psychological interventions and treatment such as Spring and TF-CBT. The process data presented provide evidence in support of GSH and overall acceptability of the intervention. It is, however, acknowledged that some participants did not find the GSH intervention acceptable, and their views are presented within the barriers theme that follows.
Data were themed around (1) accessibility, (2) therapeutic relationships, (3) structure and format, (4) pace and length, (5) use of technology, (6) effects and outcomes and (7) contextual factors.
Accessibility
GSH was seen as an accessible mode of treatment for some patients. The ability to engage in treatment from home and at a time that suits them fitting around other commitments and accommodating personal circumstances was seen as a positive aspect to the GSH approach. Ellen commented that, ‘When you go to an appointment you usually have to put aside like two/three hours. But it was with a phone call, you can do the phone call and then carry on with your day’.
Erm, it, it sort of, it, it, I mean luckily it fit in around work as well, so I’d gone back to work at the time.
Stuart
The balance of therapeutic input in GSH was also seen as positive in providing participants space and time to engage in Spring and perceived as less intense than weekly appointments.
I realise that you have, you, you, you have to use it every day, it was nice to just do half an hour every day.
Luke
The balance of contact with therapists through check-in sessions were described as reassuring and helpful to participants.
Erm, so that sort of happened I think it was every two weeks and, erm, in between my, er, clinician was very good at keeping in touch by phone so we have phone consultations or check in by email which was also very helpful.
Emma
The flexibility of the treatment to fit around individual circumstances was highly valued and evidenced in the recurring positive narrative from some of the participants. Therapists noted the benefits of a flexible mode of delivery as Meg reflected, ‘the fact that people could do this in their own time, um, and in their own way, I think that probably made a difference’.
There was a lot more flexibility with the guided self-help.
Jenny
The benefits associated with the flexible format of GSH also extend to therapists as Christian remarked further, ‘Yes, yeah it’s helpful for them and me as well’.
Therapeutic relationship
Both patients and therapists acknowledged the importance of the therapeutic relationship in the delivery and receipt of psychological interventions. Those patients reporting positively of GSH valued the support provided by their therapist.
As far as the course went, yeah, it were, and [therapist] who, who was me contact she was very good.
Stuart
There was a positivity expressed about therapists, and patients highlighted the benefits they felt as a result.
He was brilliant, err he made me feel comfortable … he really made me you know, open up and really get into, err the accident you know ….
Luke
Sheila who received TF-CBT commented of her therapist, ‘I had a connection with him yeah because he was just, erm he was just nice the way that he … and if there was something I didn’t understand which was quite a lot of stuff I didn’t understand, then he was explaining it to me until I did understand’.
Therapists also reflected on the relationship with patients within GSH. Their thoughts centred around building rapport and the therapeutic relationship. Of interest is that the face-to-face contact and interaction remains an important aspect for developing these relationships.
Um, and I don’t know how you get rapport … I don’t know the theories you use in such a thing as rapport when you’re just emailing backwards and forwards; I don’t know … Um, I … I think the reason why it worked was because we did have that contact.
Meg
The first face-to-face appointment in GSH not only provides an opportunity to begin building rapport, but also an opportunity to demonstrate how patients can access Spring and make best use of the programme.
Programme structure and format
In exploring the delivery and receipt of GSH, we examined interviewees’ narrative about the Spring programme, its structure and format. We reviewed the steps and hypothesised outcomes identified in the logic model for GSH identified to help gain a better understanding of what worked and what did not. Patient participants provided description about the treatment that they had received. In recalling the various components of Spring that they found helpful they highlighted a value in the grounding activities and exposure to narrative writing.
Erm, what I found helpful with [trial therapist] is that I was … erm the one thing I really got stuck on with the self-help was writing what had happened.
Ellen
It, it’s got some very good grounding stuff on there.
Stuart
Luke was another patient participant who found the grounding tools to be beneficial, as he highlights here, ‘I would just log on um and … did err breathing exercises and stuff like that, so if ever I felt anxious about it I would log on … it was a comfort for me … just really brought me back down to, to a level that was, right, you know … I wouldn’t say I felt happy, but I felt grounded’. He also found the narrative writing exercise to be particularly beneficial for him.
I’d say that the, the most useful was the actual err, reading back of the event … the explanation of what happened that day … My thoughts, what I saw that day err and really brought everything back … Just reading over it every time … I kept adding and adding, and eventually I had quite a long paragraph, well a long story of what had happened that day ….
Luke
Although patient participants acknowledged that aspects of the intervention and programme activities were challenging, they still inferred an acceptability and reported positively about the intervention overall, and as Kay stated, ‘I think the programme was fantastic’.
Erm, well I mean it was, it was really interesting and I think a lot of it was really, really useful … I liked that erm, the whole like self you know learning thing, self-therapy I thought it was really good and you know, quite a lot of the time going through it I thought oh okay, yeah that makes sense, yeah that’s how I feel and okay yeah.
Becky
Therapists also reflected on components of the Spring programme they found to be helpful in the delivery of the GSH intervention. As with patient participants, therapists highlighted a value in the grounding activities and trauma narrative writing components of the programme.
the anxiety and the grounding stuff … they’re really kind of well explained and people sort of tended to, when you rang them, they were like, oh yeah, it all made sense and yeah no problems with it ….
Annabel
Jenny also noted, ‘I think they liked the, erm, grounding techniques’.
In reflecting on the trauma narrative writing exercise, therapists highlighted the importance of this step within the intervention and component within the Spring application.
so I’m very keen … That people do step five, so you know, writing the narrative down … Um in terms of reading it for about half an hour or more a day, every day, until people habituate.
Christian
Gavin also acknowledged the importance of the narrative writing, ‘there were steps, now step four is reclaiming my life, and then step five is the narrative. And I always remember that, obviously it’s an important part of the whole thing …’. Annabel reflected how the narrative writing can be challenging for patients, but also intimated a potential benefit for patients engaging in this component of treatment, ‘being able to, er, sort of break the whole situation down and I think facing it, facing the actual memory in itself, because often people are avoided it and staying away from it … So I think the feedback usually from those sessions is that at, that the time of doing it, it’s extremely difficult, erm, but then they’ve, they take a lot from that part’.
The positive reflections from therapists not only illustrate an appreciation of the various programme components, but they also highlight the value from the behavioural and cognitive tasks and exercises.
Um, or if I felt, as I said, about step six, and the cognitive therapy element, if I didn’t think that was that important for that patient, I would you know, spend less time on that, and, and major on step five probably or, or something else that I thought was important.
Christian
Some therapists reflected on the benefit provided by the psychoeducational aspects of the programme as well as the consistent messaging across the platform, and as Gavin noted, ‘There was, other than the classic oh, I thought that this only happened to soldiers … I can’t remember if it’s four or five, examples of people’s stories, I think it’s four isn’t it? … then understanding … it’s actually PTSD they had … that’s a little … little light bulb, urm, moment for them … I imagine’.
the message was so consistent … I then remember thinking, gosh … work with people will never be as consistent as … I think that probably surprised me … I think having that basic education at the start is really important ….
Meg
Laura also referred to the blueprint exercise at the end of the programme being of help, ‘I think that was really good was the blue print at the end, it was really helpful ‘cause again they had something to take away and it was as really good summary and frame for the therapy and a really helpful way of ending …’.
Pace and length of treatment
Although there were mixed reports about the pace and length of treatment, an acceptability was inferred in some patient narratives.
Um I think it was just, just perfect for me … Um I had, I, I, on that last time that I went to see my Counsellor, he said “This is the last session”, and I … I walked into err, it was just before COVID actually, before, we, we, we went into lockdown and everything you know … It was, I was lucky I got that … That was my last session you know, because I can imagine, it’s all kind of gone, I’ve gone off the road, but yeah it was perfect, it was perfect timing for me, I felt, I felt great.
Luke
Emma remarked on the length of treatment, ‘Erm, er, I think for me it was, it was right …’ Ellen also commented positively in relation to the pace and length of treatment, ‘I think it was just right … I think it was the perfect balance to be honest’.
Similarly, therapists indicated an overall acceptability in relation to the pace and length of the GSH intervention. Laura stated that, ‘It seems okay’. Jenny said, ‘Erm, generally I thought that was fine’.
Meg reflected on her assumption prior to delivery of GSH that the length of treatment might not be sufficient. ‘Well, I thought that was … that would make a difference … because it was different. And I remember thinking that … that felt like that would be quite short. But actually, I think it was completely adequate’. She then went on to remark on the length of treatment, ‘it’s not too long, it’s not too onerous …’.
William also noted that the length of treatment was acceptable, but he also went on to suggest a ‘tweak’ to the intervention in relation to the face-to-face component.
Erm, yeah it was probably adequate for the guided self-help, but, erm, if it could be tweaked in any way I would say a bit more face-to-face contact before jumping into it, perhaps.
Christian reflected on administering GSH and managing time, highlighting the skills needed by therapists to deliver the treatment within the timeframe specified. ‘So you know, you need to be organised, you need to be pretty structured and good at containing patients’. In talking about the pace of delivery in GSH, Christian raises an important point for implementation and roll-out.
Effects and outcomes
Patient participants provided reflections about the treatment they received revealing a mixture of outcomes. For some, they reported on the positive effects experienced and outcomes attributed to their treatment. These reports focused primarily on an increased understanding of PTSD, symptoms and management.
But I’ve got a better understanding why I feel like I do now.
Mike
This new insight for some not only revealed an awareness of how a trauma can affect anyone, but also informed their understanding of why they were feeling this way.
my understanding of it [PTSD] has certainly, you know broadened and deepened because I now understand, you know, what I was experiencing was, was in that group of, of mental health conditions as well … that it was actually, er, erm, a, a medical, clinical, physiological change in, in my brain … That explained the change in how I was feeling and so, yeah, there was a degree of validation, definitely.
Emma
Treatment satisfaction and acceptability were also indicated in patient’s reports of feeling better, improvement in symptoms and impact on relationships with family and friends.
I was sleeping better … I was, I was having less flashbacks so … massively in a better place yeah, massively ….
Luke
I just didn’t wanna be around anyone cos I felt angry all the time for no reason and I didn’t wanna put that on anybody else but it kinda helped me realise that it’s normal and I can stop being angry. I can realise that I’m being angry but other than just sitting there feeling it, I can tell myself you’re being angry, there’s no need to be and you know, and then I can be around people more and I did then start making more of an effort to see my friends again.
Becky
The benefits and outcomes highlighted by patients also included the empowering nature of the GSH intervention and the tools that help manage symptoms and change thoughts and behaviours.
Erm, being able to calm myself from erm … if I’m having a bit of a flashback or something, being able to bring myself down and put myself in positive placement and breathing … That helped me massively.
Ellen
erm, well I realised that I was doing some things that I hadn’t realised I was doing and that some things were affecting me and I hadn’t even realised or I was you know, I think I definitely changed my behaviours towards certain things because I realised that I was making myself worse and that I didn’t need to do certain things and you know using a lot of the techniques and stuff to help me you know, relax and stuff.
Becky
Of interest are the similarities in reporting of patient participants following receipt of the CBT-TF intervention. They also highlighted an increased understanding about their PTSD and valued the in vivo exposure work.
No, I was … when I was talking to [trial therapist], you might know about this, I don’t know, you know vivo exposure, [trial therapist] was telling me about that … You know when you go back to the place where the trauma occurred and erm overcome it.
Sheila
Use of technology
Both patient participants and therapists reflected on the use of technology in the delivery and receipt of the GSH intervention. As acknowledged above, some patient participants identified particular components of the Spring programme as beneficial. They also talked about the use and interaction with the technology used as part of the GSH intervention. Some patient participants highlighted the usefulness of using the app and how they were using it.
Erm, yeah, so I, I, I, I used the app a lot … I still, I still am using it … Just for the relax, er, techniques … It, it’s got some very good grounding stuff on there ….
Stuart
Although some technological issues were reported by some patient interviewees, there was an indication that the use of technology was acceptable, and as Becky highlights, ‘it worked really well … I think it was all quite, quite user friendly … it seemed well structured’. Kay also went on to say about using Spring, ‘I think the programme was fantastic’.
I had my username and password given to me and I could do my on my laptop at home. I had no issues with it at all.
Ellen
Just like the patients, some therapists also reflected on the usefulness of the app.
… I had it on my phone, you had it as an app … Um, it took some negotiating to begin with, to learn how to use it, um, and to learn how to get into it from the … wherever … and then to learn how to do all that. But, once you’d got it, it was fine.
Meg
Jenny commented, ‘… a lot of people were kind of doing it on their phone which, although it’s handy having it on the phone, you know, I think it was more helpful if they had it on the computer and the phone’.
Some therapists also reflected on the ease of use of the programme.
Erm, it got easier with, erm, each participant that I went through it with, erm, but the programme itself was so well designed, erm, that, erm, yeah, you know, it didn’t really require much heavy lifting from me.
William
Meg reflected on how the ease of use for patients might encourage engagement going forward.
I wonder … I wonder if the first … You know, if you can get somebody to get through the first couple of steps … then they … they become … Once they’ve done that, they’re more engaged in the process. And if they get benefit from that, and it feels like an easy process, I wonder if they stay in more? I mean, for me, there is no doubt about the reason why it worked.
Meg
Some therapists interviewed elaborated further on the acceptability of the technology. They reflected on how this mode of delivery may be more accessible to more digitally literate patients and some highlighted how this approach could be a facilitator for younger patients.
I think it’s generational, I think they’re used to properly engaging with IT material, you know, like, you know, some young people can spend all day on computers can’t they and they’re virtual world friendships. They’re used to emotionally engaging with IT material.
Laura
Contextual factors
Thoughts and opinions about both GSH and TF-CBT were sought ahead of intervention delivery and examined for contextual factors external to the trial that may impact on the implementation. The process data reveal anticipatory thoughts and views that are pertinent to the reach and delivery of psychological interventions and important considerations for the roll-out of a new treatment.
The majority of patient participants interviewed expressed an openness towards talking therapies with some reporting previous engagement in interventions or services. Interviewees articulated their views on therapies around three main themes: one-to-one nature of support and how talking helps, therapist qualities and patient characteristics.
Like it helps to just talk to somebody.
Matilda
Patient participants identified with the format of talking to someone face-to-face and highlighted the need for someone impartial to talk with and not wanting to burden family members.
… as much as you have people to talk to you that you know you don’t want to burden them or … bring them down with you, but with someone who is impartial you can go there and have a rant and go home.
Ellen
Patient participant narratives revealed assumptions and expectations associated with therapies. They associate a professionalism with therapists, indicating an expertise and expected standard associated with talking therapies.
Definitely, ‘cause, definitely I do think that, I think as well talking to somebody who knows what they’re on about as well.
Katie
Interviewees identified with a professional who can listen and guide. They also highlighted the need for a professional with expertise and understanding.
Erm, I don’t know. It’s nice to speak to someone that’s not a friend or a relative … my feeling you know you can speak to someone, you can say anything you like to them … erm, and they’re not gonna judge you for it ….
Karen
Interviewees indicate an acceptability towards talking therapies in providing a safe space to talk about feelings in confidence and it is this that is seen as helpful.
Reflecting on the type of people who could benefit from treatment, patient participants suggested the characteristics necessary. Some interviewees talked about a ‘readiness’ for treatment as well as patients needing a level of motivation to engage in therapy.
I’m ready as well, you know ‘cause some people aren’t ready, they’re not ready to say goodbye or, or to change their ways you know.
Katie
I accept that not everything works for everyone, but you’ve got to try it, at least, for it to work, or potentially work.
Ross
Some therapists also reflected on the type of person that might be suitable for the GSH intervention and the motivation needed to engage in treatment. Christian commented, ‘I think it’s true for, for all patients, who receive psychological therapies … those that are keen to access treatment and you know, want to do anything that can, that will hopefully get better and, and do homework, as directed … usually do well …’.
I think, erm, perhaps people who, you know, don’t have complex backgrounds, have had a single event trauma and, erm, you know, haven’t lost a person as a result of that trauma.
Jenny
There was an element of scepticism expressed by some patient participants about talking therapies as well as some uncertainty about the therapeutic process.
Err, maybe I’m a bit cynical about people that just go and see [a counsellor] … especially when you look at American films everybody’s got a therapist haven’t they?
Hugo
Although there was some recognition of a growing awareness of mental health within the broader population, some patient participants highlighted a stigma associated with mental health and PTSD being associated with ‘other people’. These views may also be a factor likely to inform views on talking therapies and acceptability of therapeutic interventions.
Barriers
Interviewees’ narratives revealed some barriers associated with the delivery and receipt of the Spring programme and GSH intervention. Like the facilitators, they draw attention to the factors that are important for the engagement in psychological interventions and treatment such as Spring and TF-CBT. The process data presented revealed that the GSH intervention was not acceptable in some cases and provide contrasting evidence to the facilitators reported and are also themed around (1) accessibility, (2) therapeutic relationships, (3) structure and format, (4) pace and length, (5) effects and outcomes and (6) contextual factors.
Accessibility
In contrast to the accessibility reported as facilitators, the approach of the GSH intervention combining computer-mediated self-direction with therapist contact was also indicated as a barrier. Ellen commented, ‘if you’ve got an appointment with the therapist you’re more inclined to turn up than maybe turn on the computer’.
That ironically in my situation perhaps would have been a lot easier because I could have dedicated, you know, two hours of childcare in order to allow me to do that … Whereas factoring in something that was self-driven myself, at home when I had a new born and when I suffering from trauma was very difficult to do.
Emma
Therapeutic relationship
For both patient participants and therapists, the face-to-face nature of therapy and contact with a therapist was highlighted as an important factor and facilitator; however, the preference of patients for face-to-face treatment might be considered as a barrier for engagement in a GSH intervention and approach.
Erm, but I’m just … I feel like it would be easier to, erm like explore issues more if you were just talking to somebody rather than, erm some online, erm thing.
Clare
Christian also reflected on patients’ preference for face-to-face and stated in relation to the GSH intervention, ‘… people that were disappointed, that wanted face-to-face … you know, I think people vote with their feet …’.
Clare felt that face-to-face contact with a therapist could help facilitate communication, ‘Erm, but I’m just … I feel like it would be easier to, erm like explore issues more if you were just talking to somebody rather than, erm some online, erm thing’.
Although the majority of patients receiving GSH reported a positive relationship with their therapist, this was not expressed by some and as Becky illustrates, ‘… it wasn’t very personal …’ and went on to explain further, ‘… the lady I saw I didn’t have that connection so yeah, you know … the majority of it being at home you know on your own sorta thing but I thought there would be a bit more of an element of support there off somebody’.
As reported earlier in relation to the acceptability of delivering the GSH intervention, overall therapists indicated that therapeutic alliance with patients was achievable. However, some therapists reflected on the impact GSH can have on the relationship and how the GSH approach might be perceived as lesser or inferior.
Yeah, it did, erm, and I noticed that I had, erm, obviously a much stronger therapeutic alliance with the patients I was doing the face-to-face sessions with as compared to the guided self-help.
William
Structure and format
Interviewees highlighted components of GSH structure and format that they found to be helpful, but also acknowledged challenges in engaging with some elements. For patient participants, there was some criticism of the sample trauma accounts that highlighted authenticity, relevance and impact of this part of Spring.
what I found with the case studies … I just found that none of them related to what I went through … And I couldn’t really relate with them or empathise ….
Ellen
Patient participants acknowledged some challenges with other Spring components including the challenging thoughts and pie chart activities.
I think there was a, like a pie chart thing that said how much of the responsibility over the incident erm, do you attribute to yourself … Erm and there wasn’t an option saying I don’t attribute any of it to myself … because you know, I couldn’t have helped what happened to me … So I know, I know it wasn’t my fault and I kind of felt that I had to put that it was partially my fault, I think that, that was something, that was the only thing that I found going through it that I thought I don’t like that ….
Becky
Therapists interviewed also reflected on components of the Spring programme and some highlighted reservations and potential limitations in relation to the structure and content of the GSH programme. Jenny noted in relation to the trauma writing activity, ‘… it probably is the most difficult part of the programme’. Some therapists also drew comparisons between GSH and TF-CBT and acknowledged the challenges of facilitating exposure work within the format of the GSH intervention. William noted the benefits that can be gained from working with patients in vivo and exposure to work ‘out of the office’ but also stated, ‘we couldn’t do anything with that with the guided self-help package’. Jenny also noted, ‘I guess with the guided self-help it’s a little more like, okay you’re going to go off and do this thing on your own’.
I think you’ve got ways to respond in CT [Cognitive Therapy] that would be informed by the formulation, and you can … you can use or not use those … ways of dealing with it. Whereas, in the guided self-help, you just have to go through the steps, regardless.
Meg
Pace and length of treatment
The pace and length of treatment was identified as a barrier for some interviewees in the delivery and receipt of the GSH intervention. Some patients felt that the length of treatment in GSH was too short.
… I would imagine if, if it could have been better a bit longer, I would imagine if you had just had like a plan, sort of last year and you’re still coming round, and I would imagine a bit longer would help. I think the eight weeks is, is just, you know, just touching the nub of the problem.
Stuart
Therapists reflected on the pace of delivering GSH and some indicated that sessions felt too short. In particular they suggested that some additional time at different stages could be helpful.
it could make more sense to be longer, and with more steps within the key steps, rather than steps as I said, at the end, even though it’s equally as important, but er, they um, you know, the steps four and five and six probably.
Gavin
The shortness of the face-to-face sessions was also indicated as a possible barrier for some therapists and reflected in comments from some patients.
the sessions were very short … I would have liked a little more time for the face-to-face meetings ….
Jenny
There was some indication that an eight-week intervention may not be sufficient for more complex cases.
Effects and outcomes
Dissatisfaction with the GSH intervention was evident for some participants, and as Mike illustrates, ‘to be honest I feel like it’s gone back to square one to be honest with you’.
From the framework analysis, stand-out and divergent cases were identified, and further exploratory analysis was undertaken. From this, six participants were identified from the sample whose quantitative outcome measures (CAPS-5/IES-R/WAS) were different to the rest of the participant sample. As a way of trying to understand what might be happening, demographic information and patient characteristics were reviewed within the analysis framework and then interview transcripts re-examined to help provide some explanation for these outcomes.
For Mike, their CAPS score had gone down as was hypothesised but only by three points, and in contrast their self-reported IES-R score had increased. This participant presented a complex background and came into the trial 6 years post-trauma incident. They had received a diagnosis of PTSD following a work-related trauma that resulted in serious injury and there was evidence of engagement with multiple health professionals and services over the years.
I’d been diagnosed because I, I’ve seen, er, numerous psychiatrists all over the country, obviously, through the accident.
Mike also expressed disappointment from previous treatment provided through the NHS and lack of support provided by healthcare professionals. The trauma for Mike resulted in both physical and psychological injury impacting on relationships, their ability to work and social activities. Possible variation might be explained by the self-report nature of some of the measures employed. It might also be anticipated that variation between IES-R and CAPS score changes can be explained by the impact of the pandemic; however, this participant completed their treatment pre-COVID.
The WSAS scores went up for the following participants: Sheila, Stuart and Becky; Sheila had experienced a work-related trauma in 2016 resulting in physical and psychological injury and she had continued to work for some time before recognising the impact and trigger of being in her work environment and then seeking treatment. One possible explanation for the WAS score could lie in a change in perception and how they view their ability to work, however, responses to individual items would need to be examined further.
I’ve been speaking to my doctor only on today and obviously she’s saying that I … she’s going to do a report for work. She feels like it’s not an environment I should be in because it’s not healthy for my mind to go back.
Sheila
Stuart had been in a ‘near fatal’ road traffic accident in 2009 and at the time had felt they had dealt with their trauma until subsequently experiencing psychological sequela 2 years later. It was evident that the trauma and associated health adversities experienced after the trauma event impacted on their ability to work and type of employment, resulting in many work-related changes. Stuart spoke about being off work but then also mentioned using the app and tools while at work.
I’d gone back to work, they found me another job on the loading bay … I used it [App] at work when I found myself getting a bit wound up ….
Although intervention treatment had ended, Stuart was still in receipt of psychological treatment but now for depression and was receiving this from their trial therapist as part of another service provision outside of the trial. The depression which perhaps was not previously realised may be a factor shaping views and perceptions about their capability and capacity to work.
Er, well I’m still working with her, er, erm, it’s been, well what did and didn’t work for me, I’ve obviously got different issues from what we thought we had in the first place … whilst I have underlying PTSD, but the depression that I have … hasn’t left me.
Stuart
For Becky both WAS and EQ-5D scores went up but only by three and five points, respectively. Unusually there was a large decrease in CAPS and IES-R measures. Becky had been involved in a road traffic accident and sustained some physical injuries and was pursuing a compensation claim as a result. She did not feel the need for psychological treatment after the accident and only sought treatment following a trigger event which took place in her work’s car park. There was no cohesive evidence to help explain these outcome scores and work did not feature in her post-intervention interview. In fact, Becky expressed positive outcomes from treatment and referred to seeing more friends.
Participants Mike, Matilda, Becky and Clare reported lower satisfaction compared to the rest of the patient participants interviewed. For Mike, treatment in the trial had not been effective, reporting they were ‘back to square one’. Spring usage data indicated that Matilda had not engaged in any of the Spring steps which might explain their low score. Further investigation revealed that gender, ethnicity, nor education level appeared to be significant here, and no indication that research site delivery centre was a factor either.
Use of technology
Although the use of technology and mode of delivery of GSH was identified as a facilitator, some interviewees revealed contrasting views about the acceptability of this aspect. Technical difficulties were reported by some patient participants. These related to accessing the website, interaction with tools and activities and use of the app.
It was okay, I, I, I gained a lot from it and possibly lost a little from it because of the teething problems of the, er the web-site … It’s just that elements that weren’t quite there.
Kay
Luke elaborated further on what it was like when the technology failed. ‘Um I had a few occasions where it was down … I couldn’t log on … Err and I couldn’t log onto it, and … It was, you know, some, some days it was on a, err, I could have really been having a bad day and I, and I really tried to get on … I was almost, almost using the, the App as a bit of a safe haven you know, that was, that was the way I was dealing with it’.
Therapist interviews also revealed some potential barriers relating to the use of technology, and indicated some hesitancy about using the technology as a factor impacting acceptability for them.
there are a lot of therapists who are a bit, can’t undertake it themselves, who don’t like using computers and they’re not very confident with computers and websites …
Christian
Gavin reported some issues with technology, ‘some things were logging on, some were just connective issues … or out something in, and hadn’t saved’.
Contextual factors
Motivation to engage in the programme and complete steps in between sessions with the therapist was presented as a facilitator in the engagement of GSH. However, interviews revealed that symptom presentation and severity may impact on people’s capacity to engage in treatment.
Mike went on to express concern about his capacity to engage because of difficulties with concentrating and said, ‘the iPad work, well it was alright I done it all no problem at all but it’s hard for people that can’t concentrate, I think … I was struggling with my concentration’.
Interviews with both patients and therapist also highlighted that GSH requires commitment and dedicated time to complete the steps in Spring that could be challenging for some.
I didn’t realise that it would be so intensive … when it got to the point of writing down … your erm trauma and then having to go over it for an extra forty minutes or whatever it was a day … that was an hour and ten minutes a day … I didn’t have an hour and ten minutes.
Becky
Gavin also alluded to the effort needed from participants and that some are unprepared for this.
there are certain people that … they must have been blinded by desperation of … they wanted something rather than waiting the two years or whatever, when the reality sunk in … they had to go and … do something themselves … when it comes down to putting the effort into it … it’s not always there ….
Jenny highlighted how personal circumstances such as time and space could also be a barrier for some patients.
I think it was people, erm, having that sort of space at home to do it … if they had children or, you know … if, if people were working, you know, when they kind of said they could come home sometimes and they didn’t feel like then going onto the computer and doing that ….
Many of the patient participants interviewed talked about their experiences of seeking help and obtaining a diagnosis, highlighting how previous negative encounters may influence acceptability of the GSH intervention. The influence of family, friends and work was also themed both in terms of contextual facilitators as well as barriers.
Recommendations
Interviewees proposed a selection of recommendations in relation to the delivery of the GSH intervention when talking about possible barriers for the delivery and receipt of treatment. These included minor changes and modifications to Spring content as well as suggestions for improving the GSH intervention overall.
“I think understanding the circumstances of the participant and being able to modify that programme slightly would potentially increase the adherence to the programme.”
Emma
So I think there were a few erm, things that probably could’ve been improved, like I think there was a, like a pie chart thing that said how much of the responsibility over the incident erm, do you attribute to yourself.
Becky
Therapists inferred that a flexibility in delivering the programme is important as is the ability to make adaptions to respond to patients’ needs. Adaptions to the balance of therapeutic input and extending some of the face-to-face sessions were also proposed.
Roll-out and sustainability
Issues of roll-out and sustainability were explored within the framework analysis and themes emerged around patient and organisational factors. These data were then integrated with results from interviews with NHS commissioners and managers.
Patient factors
NHS commissioners and managers expressed a range of attitudes towards internet-based therapies, including their own views and perceived views of patients. These interviewees highlighted some reservations and perceived that patients might expect face-to-face therapy. Patrick suggested, ‘often patients don’t want group offer or e-therapy, they want to see somebody’.
Concerns were raised over patients’ use of internet-based interventions in the proximity of others, for example, those with whom they live. Rose was interviewed post-COVID-19 UK lockdown and talked about this: ‘So one of the things we’ve learnt with, with this … pandemic is there’s a challenge around people doing therapy in their own home you know … particularly in trauma when you may have you know, perpetrator or something like that in the next room … about safety and boundaries’.
Both patient and therapist interviews revealed a preference for face-to-face approaches. The presumption of some therapists about patients’ preference for face-to-face treatment was later contested by some. Laura reflected on this stating, ‘For the guided self-help, I think it’s slightly surprising because people weren’t as shocked as you think about only offering them that’.
NHS commissioners and managers perceived patients expecting face-to-face approaches, interviewees also perceived that some patients may prefer therapy that is more remote, and that internet-based interventions may facilitate openness and was echoed by some patients and therapists. Isla suggested, ‘I think some people would want to see somebody face-to-face initially and actually might be more comfortable doing something through the internet or through, a bit more remote …’. Robert, who was interviewed post-COVID-19 UK lockdown, reflected on his experience of patients entering information into a website ‘more openly than they would face-to-face’.
NHS commissioners and managers preferred guided internet-based therapies over self-directed therapies, with guidance viewed as important for treatment uptake, engagement and enrichment. Phil said ‘it would probably be a good idea for somebody using this method-based therapy to actually come into some centre and … sit down with a person who’s very familiar with the material … that person would meet them again and ask how things are going … it might be some little areas that aren’t quite covered perhaps they’re a bit tangential and the individual therapist then might be able to just enrich the process further by adding some … localised idiosyncratic examples or ways of expressing certain concepts’.
Interviewees suggested internet-based interventions were empowering treatment approaches for people with mild to moderate severity conditions. Gwendolyn said, ‘if, for instance, somebody has milder levels of, erm, psychological morbidity or mental illness and they are able to engage in those kind of [internet-based] interventions then they are going to find it empowering’. The empowering qualities of GSH were also evident in narrative of both patient participants and therapists interviewed in this trial. Sue expressed this further, with respect to general healthcare movements encouraging people to take responsibility for their health, stating, ‘unless we find a way of helping people be more open and take responsibility for their own health, and access stuff that’s really good for them on the internet and things like that, we will never manage to reach them all’.
Facilitators and barriers identified by some patients highlighted the severity of symptoms as a factor relevant to engagement in therapy and internet-mediated interventions. Therapists also reflected on the potential of GSH to help people with complex and severe conditions. NHS commissioners and managers also remarked upon the advantages of internet-based interventions as first-stage interventions for people with complex or severe conditions. Sarah said, ‘I think we have to have a digital, a digital first mentality … the least intensive intervention first, see how somebody responds to that … if somebody does need a kind of one to one situation, that’s gonna cost a lot of money, that we haven’t got a lot of people delivering, at least it’s reserved for the people who really, really need it …’.
A movement towards digital proficiency and acceptance of internet-based therapies was described, for example, when reflecting on a digital intervention for depression, Sarah remarked ‘It wasn’t very, wasn’t successful, um, the uptake of licences was very low, but I think people’s digital … capability was lower back then’. A shift in attitude and growing acceptance of this mode of delivery was also reported by patient participants and therapists.
Organisational factors
A number of organisational factors were identified and further analysis uncovered themes around capacity and capability, readiness and buy-in. The flexibility reported in the delivery and receipt of the GSH will be beneficial going forward, with opportunities to provide a more tailored experience and increase access to treatments. Both patients and therapists identified increased capacity and cost-effectiveness with the shorter delivery time and therapist input. Therapists also highlighted the potential to train other healthcare professionals to deliver the intervention and increase the workforce.
NHS commissioners and managers were invited to talk about interventions they were involved with and their understanding of the barriers and facilitators to accessing mental health treatment in general. Interviewees described capacity issues, stretched services and the impact of this on patient access to treatment, evidenced by unmet governmental targets. The reliability of waiting times as a measure of treatment access was debated, although long waiting times were identified as being of concern.
Tim described difficulties meeting targets for face-to-face therapy: ‘anyone that is referred into, er, psychological therapies should be seen within 18 weeks, erm, but I think it’s interesting that some of my understanding is that there’s no board in [country] that’s currently meeting that target … for face to face therapy’.
Rose talked about limited resources: ‘people come and they want to be treated straight away don’t they and to keep them waiting is, is a challenge when you know, actually a lot of that is about resources when you’ve just got one therapist and one team … What can you do?’.
Interviewees suggested staffing and deployment solutions alone would not be sufficient in increasing patient access to therapies.
Tim stated ‘… even in those areas where they do have a full, er complement of staff that you tend to find that there’s high demand of services…. as investment has been put in, you know, increasing the workforce but the demand is still going up … and digital technologies are becoming much more prevalent … Because they now recognise that the traditional models of service are not really going to meet that demand if the rates continue’.
NHS commissioners and managers also talked positively about quick access to GSH. Patrick suggested, ‘there is some evidence I think that err people who wait longer have poorer outcomes, so the quicker you can start treatment the better, for me that’s a plus, it helps the patient err and it also helps towards our waiting times, achieving our waiting time targets, so it’s a win-win’.
I guess we could see more patients which is … at the end of the day is … is a good thing.
Meg
NHS commissioners and managers offered many considerations for the successful implementation of internet-based interventions.
NICE and other country-specific guidelines and practice-based evidence were considered an important but interestingly, not a sufficient factor for intervention implementation and acceptance among staff. Sue noted NICE guidelines, ‘should be part of the conversation and evidence is really important, but it’s not you know, sometimes we don’t have the evidence and we just have to try things’.
Rose expressed her interest in practice-based evidence, ‘randomised control trials are great but what they miss is most people that come to our door are not, you know, a neat little box or they’re not going to fit into a neat little box … so I suppose it’s, I’m very much in favour of practise-based evidence’.
NHS inflexibility was considered a barrier. Sarah stated, ‘we have been a bit slow on the uptake, it, it’s really about the way I think the NHS bureaucracy works, a lot of the time, it doesn’t allow itself to have the agility to implement …’.
Tim expressed problematic implementation delays due to information governance and procurement processes: ‘within digital what you’re trying to do is streamline the processes as quickly as possible because the technologies always evolving and changing and if it takes you two years to get past information governance and procurement then actually you’re already two years behind where the technology is’.
Interviewees highlighted NHS funding barriers. Phil explained, ‘there isn’t one overarching form of budgetary control … So you could argue there isn’t a great deal of central coordination because of that’.
Tim reflected on an experience of potentially prohibitive intervention set-up costs: ‘one of the biggest barriers, er, when we initially tried to bring CCBT [computerised CBT] into [country] was the cost of the product … the actual ability for them [smaller health boards] to, erm, purchase the product in addition to then the service infrastructure means that many, many areas, particularly smaller board are prohibitive to the set up’.
Rose reflected on her experience regarding timely training and supervision as a facilitator: ‘So a therapist came to me saying, look there’s this training and at the end of it I get a, erm a treatment manual that’s tailored to our service and I’ll be up and running and ready to run this group immediately after I’ve finished this course … that’s quite a big selling point … something that is erm, accessible and useful straight away so that after a training in it, people could, could run with it very quickly … maybe after training thinking about some supervision … to enable implementation and to pick upon any problems’.
Interviewees reflected on implementation facilitated through opportunistic ventures. Isla talked about externally directed funding: ‘a lot of investment for new service tends to come from directed investments … [country] Government may decide they want to invest in that area …’.
Sarah, interviewed prior to the COVID-19 UK lockdown, reflected, ‘I think COVID’s helped … We’ve just managed to get Silver Cloud [internet-based intervention for stress, anxiety and depression] in, um I’ve been struggling for two years … and suddenly we’ve got it within three weeks …’.
Readiness and buy-in
Therapists considered organisational preparedness and technological capacity. They referred to a professional readiness and culture of acceptance in relation to the future roll-out of the GSH intervention. Christian illustrated the importance of early adopters and ‘champions’ to promote the acceptability of a new intervention to delivery teams and support roll-out and implementation.
Yeah again, I think you need people that champion it and want to use it versus being mandated and they have to do it, I think that would be a more helpful way, initially anyway um … Um champions that just roll with it and build some expertise and sell it to other people.
Christian
Sarah suggested internet-based interventions would start to happen with a ‘change in culture from commissioners and that comes from the top … If it was expected that you know, um, seventy-five per cent of your workforce were bums on seats and twenty-five per cent was digital … cos it would hold that accountability in the system’.
Tim reflected on a positive experience of a coordinated national approach and commissioning services at scale: ‘We have one implementation approach which we did across [country] but … we built into the implementation programme ability to then allow people to go different speeds … with a national deployment … you’re able to then look at the costs and identify what the big costs are, and then extract them … within [country] we fund the national CCBT licence for the whole of the country … for every single person’.
Knowledge of set-up and ongoing requirements was recommended. Isla said, ‘setting up a service you would have sort of initial costs … And then the ongoing costs … So it could be that every year they [staff] go on a refresher training or, so you … just build that in really so you haven’t got any surprises really’.
NHS commissioners and managers expressed perceived views of NHS colleagues towards internet-based therapies and their implementation.
Reservations
Interviewees perceived limited staff knowledge of internet-based interventions and who they are aimed at helping. Tim remarked, ‘there’s still quite a lot of, er, misconceptions about what computerised therapies are or internet interventions are’.
Interviewees perceived staff resistance to change. Sue suggested, ‘people often don’t like changing what they’re already doing … sometimes, um, you almost have to get to the point where people understand they can’t carry on delivering things a certain way, before you all realise other opportunities’.
Reservations also included perceptions of internet-based interventions being a threat in terms of how staff interact with and work with people, for example, Tim talked about ‘pushback’ due to clinicians’ ‘ … strong belief on the kind of therapeutic relationships that occurs between the clinician and patient’.
A change in attitude
Rose reflected on resistance to telephone-based assessments prior to the COVID-19 pandemic and how ‘the staff didn’t want it to succeed and it didn’t succeed. Now, we’re talking about you know, telephone assessments are fantastic, we’ve been able to keep the service going, we must do more of these’.
NHS commissioners and managers preferred internet-based therapies that were guided compared to stand-alone interventions. Geoff weighed up the costs and benefits of clinician guidance in GSH: ‘adding a lot of layer and more money because you’ve got a one-to-one session with a clinician, but if it gets them in and using it then that’s probably going to be quite useful’.
Interviewees expressed the opinion that guidance need not necessarily be provided by a clinician, but it would depend on skills required. Rose suggested, ‘so is it something that could be done by somebody with level one skills or do you need to have somebody who’s got a therapy training, who erm, who knows [pause] erm, who knows more than that that is provided in the actual treatment.’
Interviewees perceived patients would value the convenience of accessing treatments at their own pace, in their own time. Gwendolyn, interviewed post-COVID-19 UK lockdown, suggested internet-based interventions were ‘a really important part of the suite of offers that we have for patients … there are also real benefits in terms of being able to provide that kind of input for people at a time and place that most suits them, as opposed to needing to make appointments with an individual during the day which may not be convenient for the patients.’
The potential for continued access to the internet-based intervention after the treatment period had ended was also considered a positive, for example, Isla said, ‘it may be something you would then want to go back to the beginning and do again.’
Geoff acknowledged that different ways of connecting with patients are required: ‘I think what’s come through in our staff group here is that we’ve got to think of different ways of connecting with our patients’.
Patients experiencing PTSD are in urgent need of access to timely treatment and may be inclined to accept an internet-based therapy. There is a need to consider the accessibility to the technological hardware required to make the most of what the app has to offer.
Fit with service
NHS commissioners and managers talked about the importance of an intervention linking outcome data with NHS patient record systems and key performance indicators. Sarah said: ‘otherwise we’ve got an administrator going into the programme, getting the data off, taking that data to another programme … it creates the potential for an Information Governance risk’.
Interviewees were positive about offering internet-based interventions within primary mental health services, for example, Geoff said, ‘we are very keen to be offering interventions for that [primary care] cohort rather than referring on … If we can be offering interventions at the right level … we want to be doing that’.
Chapter 6 Discussion and conclusions
Main findings
The main findings of the RAPID trial were:
-
The RAPID trial slightly exceeded its planned recruitment by recruiting 196 participants, 97 in the GSH group and 99 in the CBT-TF group versus a planned total of 192. There were no serious balances observed in the baseline data between the two groups.
-
The GSH intervention was found to be non-inferior to CBT-TF on the primary end point of CAPS-5 measured at the 16-week assessment using the ITT principle. SAs of the primary analysis were consistent with the primary ITT analysis of NI at 16 weeks.
-
Non-inferiority was shown for all secondary outcomes at 16 weeks, except for client satisfaction which was inconclusive but in favour of CBT-TF. At 52-weeks post-randomisation, NI was shown for MSPSS, AUDIT-O and GSES but not for the other outcomes. The results for the other outcomes, which were inconclusive, were in favour of CBT-TF.
-
Further examination of the IES-R longitudinal measurements indicated that while the GSH group maintained their reduction (improvement) in IES-R scores between the 16- and 52-week assessments the CBT-TF group continued to improve at a slow rate over the same period.
-
There were no subgroup effects that showed any evidence of difference between the interventions including gender (pre-specified), mode of data collection or assessments conducted after the introduction of the COVID-19 lockdown. The last two subgroup analyses were post hoc and exploratory. It is important to note that the study was not powered to detect subgroup effects.
-
GSH using Spring was not shown to be more cost-effective than face-to-face CBT-TF using standard HE health economic methodology but was significantly cheaper to deliver and appeared to be well-tolerated. The results support GSH using Spring being recognised as a clinically effective treatment that provides the first evidence-based, low-intensity treatment option for people with mild to moderate PTSD.
-
The process data provided evidence of acceptability of the overall trial methodology, although key points were identified for consideration in future RCT design, especially concerning burden and impact of outcome measures on participants, and how they are delivered and explained.
-
Intervention acceptability was indicated for both GSH and CBT-TF interventions, although there was a preference for face-to-face treatment. Therapeutic relationship was an important factor highlighted in the acceptability of the interventions.
-
Flexibility identified with GSH was seen as positive and some activities within Spring were described as more helpful than others.
-
Outcomes reported by both patient participants and therapists reflected outcomes identified in the logic model of GSH. Additionally, interviews with NHS commissioners and managers provide detailed information on factors pertinent to both the sustainability and future roll-out.
Results in the context of other research
The results of RAPID add to existing research that has demonstrated the efficacy of GSH using Spring17,19 and other GSH approaches based on CBT-TF for the treatment of PTSD. 20 The trial also confirmed the effectiveness of face-to-face CBT-TF for people with PTSD. The mean levels of improvement found for both face-to-face CBT-TF and GSH using Spring were greater than have been found for CBT-TF in previous meta-analyses. 82 It is, however, possible that participants in other studies may have had more severe PTSD, given the focus on mild to moderate PTSD to a single event in RAPID. That said, the mean (SD) participant score of 35.1 (6.7) on the CAPS-5 at baseline suggests that many participants had more severe forms of PTSD, given the fact that the minimum CAPS-5 score required for a diagnosis of PTSD is 12. The results suggest that GSH using Spring is likely to be an effective treatment for some people with more severe forms of PTSD to a single traumatic event and could be of some benefit to people with PTSD to multiple events. Although not explicitly investigated, and, therefore, not known, the mechanism of action of Spring is probably similar to other CBT-TFs, with processing of the trauma through imaginal and in vivo exposure, coupled with effective challenges to patterns of thinking, ameliorating the symptoms of PTSD. 25,83 The results for the cognitive (PTCI) process measure are in line with this conclusion.
The RAPID trial has demonstrated that GSH using Spring is one of an increasing number of web-assisted GSH interventions that can be delivered as clinically effective as face-to-face interventions, but with reduced therapist time and lower cost, for various common mental disorders. 15,16 Other forms of GSH for PTSD have not been shown to be as effective as GSH using Spring in this study or an earlier efficacy trial. 19,20 Key differences between Spring and apparently less effective GSH approaches are the degree of guidance, its careful coproduction with people with lived experience of PTSD and its adherence to a CBT-TF approach. To the best of our knowledge, this is the largest study of web-assisted GSH to date and the only study of GSH against a manualised gold standard treatment for PTSD. Previous studies have primarily compared GSH for PTSD to wait-list controls, with recent meta-analyses determining effect sizes of around 0.6,20,84 significantly lower than the −1.62 found in a recent meta-analysis for CBT-TF compared to wait-list/usual care. 82 Significant concerns have also been raised about the heterogeneous GSH approaches used, overall methodological quality, absence of follow-up and higher dropout rates than found in this trial. 20,84
The results of RAPID suggest that NICE’s recommendation that GSH for PTSD should meet minimum standards is justified. 9 GSH using Spring is compliant with all the standards set by NICE9 as it is ‘based on a validated programme; provided over 8 steps; involves elaboration and processing of the trauma memories; processes trauma-related emotions; restructures trauma-related meanings for the individual; helps to overcome avoidance; re-establishes adaptive functioning in work, social relationships and other domains; and includes guidance and support from a trained practitioner’. The fact GSH using Spring is compliant with the NICE standards, the magnitude of improvement in a real-world setting and NI to manualised CBT-TF demonstrated in RAPID, makes the authors believe that GSH using Spring may have been recommended as a first-line treatment for PTSD of mild to moderate severity by NICE and ISTSS if the results had been available at the time of their latest treatment guideline updates. 8,9
The largely inconclusive findings with respect to NI at 52 weeks appear to be secondary to some ongoing improvements in the CBT-TF group that were not found in the GSH group. It is difficult to determine why but may indicate a higher ‘dose’ of treatment facilitating ongoing improvement. Other possible explanations or contributory factors are the slightly lower levels of education and greater physical comorbidity in the GSH group; both have previously been found to influence treatment outcome. 16,53 Further improvement after face-to-face CBT-TF has been found before85 and has been hypothesised to be due to ongoing trauma processing and practise of techniques learnt in treatment. Ongoing practise of techniques learnt is likely required for maintenance of symptom improvements too and the absence of evidence of loss of treatment gains in the GSH group is encouraging. It was not our expectation that GSH would outperform CBT-TF, hence the NI design of the study, and the additional benefits with respect to time, cost and convenience, and having another evidence-based treatment option could be argued as outweighing what appear to be minor differences at 52 weeks.
The qualitative analysis of in-depth interviews revealed GSH to be acceptable and this is further supported by a relatively low dropout rate of 10% from treatment, albeit greater than from CBT-TF (4%). This may suggest that GSH was less acceptable than CBT-TF although greater full adherence to GSH (79%) than CBT-TF (56%) does not support this position. It is noteworthy that issues with equipoise were encountered in the study. Some participants and therapists felt CBT-TF would result in better outcomes and this antipathy towards GSH has been found in previous research. 86,87 It may appear counter-intuitive to suggest that receiving less therapist contact is as desirable as receiving more and it is likely that the use of empirical data, such as the results of this trial, that demonstrate NI of a treatment option that may potentially have less face validity, will be needed to fully address this bias. The qualitative finding that therapists reported challenging their views with respect to equipoise after delivering GSH using Spring is encouraging and suggests that this potential barrier to effective dissemination and implementation could be overcome.
As with many treatments, it is likely that GSH using Spring is more suitable for some people with PTSD than others. Further planned quantitative analyses to identify predictors of treatment outcome and the qualitative analyses undertaken will advance our knowledge with respect to factors that can identify which people with PTSD are most likely to benefit from GSH using Spring. The qualitative analysis identified a desire to receive and deliver GSH in a flexible manner, adapting GSH to suit an individual’s needs and preferences and supporting calls to develop a more personalised approach to the delivery of care to people with PTSD. 88 GSH is unlikely to be a ‘one size fits all’ solution, and its suitability must be considered on a person-by-person basis; there is great scope for personalising Spring and other GSH approaches.
The HE analysis confirms that GSH using Spring is a cheaper alternative to face-to-face CBT-TF, both in terms of treatment costs and total NHS costs at 16- and 52-week assessment points. This is consistent with clinically effective GSH interventions for other conditions. 15,16 The lack of evidence that GSH is more cost-effective than face-to-face treatment using standard methodology is perhaps not surprising when it is considered that the standard, NICE-adopted methodology, was designed to determine this in the context of superiority versus NI. 20 Using a £20,000 willingness-to-pay threshold, the additional cost of CBT-TF could be considered worthwhile for the additional health benefit which equates to 14 days in full health per year compared to GSH. However, cost-effectiveness analyses should be considered alongside other considerations, including those discussed above and budget impact and feasibility. 73
As this is a NI evaluation, it is important to consider possible benefits that may offset the lower relative cost-effectiveness, such as greater availability, and the overall budget impact of the lower cost of therapy. GSH using Spring allows reconfiguration of current care pathways with the introduction of a low-intensity treatment alternative for people with mild to moderate PTSD and one that is less time intensive and more flexible for participants, and can potentially be delivered by low-intensity therapists. For many, it will justifiably be seen as a first step in a treatment pathway with other more demanding and resource-intensive treatments prioritised for people with PTSD who have more complex difficulties or who do not respond to or engage with GSH.
Strengths and limitations
This was a well-designed RCT that adhered to current methodological recommendations. 23 A risk of bias assessment for the trial against the Cochrane Risk of Bias checklist89 confirmed a low risk of bias that compares very favourably with and is superior to most RCTs of treatments for PTSD. Blinding of participants and personnel (performance bias) was rated as a high risk, as is true for almost all psychological treatment trials; the participants and therapists could not be blinded due to the fact that GSH or face-to-face therapy was being delivered. An additional risk was the fact the originators of Spring played key roles in the trial although this is mitigated, at least in part, by robust methodology and involvement of an independent trial manager, statistician and qualitative and HE researchers. The outcome raters demonstrated good inter-rater reliability based on training videos. It is also noteworthy that a small number of participants received the final sessions of their treatment during the COVID-19 pandemic although post hoc analyses have not detected a ‘pandemic effect’ on the results.
The sample size of 196 is a major strength and makes it one of the largest-ever RCTs of psychological treatments for PTSD and, to the best of our knowledge, the largest-ever RCT of GSH for PTSD. The fact it recruited from urban and rural sites in England, Scotland and Wales means the results are likely to be generalisable across NHS settings in the UK and beyond. There was, however, a lack of ethnic diversity and more females than males than would be fully representative of the UK population.
A major strength of the trial was the careful training and supervision of the therapists. Fidelity checks demonstrated reasonably good adherence to the treatment manuals although the ratings were not as high as have been found in efficacy studies of CT-PTSD,90 which likely reflects the real-world challenges of replicating quality of delivery achieved by the originators of this model. The fidelity ratings were slightly better for CBT-TF than for GSH which may reflect greater familiarity with working face-to-face among the trial therapists. A limitation is that not all participants had a session of their treatment fidelity rated. Several therapists reported gaining confidence as they treated more participants, and it may be that earlier participants could have done better if treated when the therapists had more confidence and experience with the trial interventions. Equipoise may also have increased over time as therapists became more experienced in delivering GSH using Spring.
A further strength of the trial was the utilisation of both quantitative and qualitative approaches. This allowed cross-referencing of results from different sources to corroborate or challenge outcomes. The quantitative and qualitative results were consistent, further supporting the belief that the results are likely to provide a true reflection of the effectiveness of GSH using Spring.
It is always difficult to identify a perfect control condition; it was felt that a gold-standard CBT-TF comparator would make it easier to interpret the results than one of usual care, and facilitate very robust evaluation of GSH using Spring. Unfortunately, usual care is not standard for PTSD across the UK and treatment variation would have made results very difficult to interpret. The fact that all therapists received formal CT-PTSD training and treated a case under supervision in the control condition before seeing trial participants, means that the results for the control condition would likely have been better than if usual care was the comparator.
The research team agreed that it would be best to have the same therapists deliver both treatments. This meant that ‘high-intensity therapists’ provided all the treatments. The ultimate goal is for GSH using Spring to be effectively delivered by ‘low-intensity therapists’ but further work is required to determine if the results of this trial would translate to effective delivery by less qualified therapists, or clinicians who might be less knowledgeable about PTSD and other trauma-related disorders. It is premature to draw any conclusions but early dissemination work with low-intensity therapists and counsellors in NHS Wales is producing good results and it has been argued that GSH is less reliant on the skills and experience of the therapist. 91
Clinical implications
The results of the RAPID trial could herald a step change in the approach of services to the provision of evidence-based treatment to people with mild to moderate PTSD. The authors suggest that making GSH using Spring available as a low-intensity treatment option for people with PTSD could save time and money and allow more people to receive effective treatment.
The COVID-19 pandemic has seen a major increase in interest in digitally facilitated health care with more people becoming aware and keen to receive treatment in this way. 92 Successful dissemination and implementation of GSH using Spring in clinical practice could allow thousands more people with PTSD to recover. The simplicity of the treatment and the fact it can be provided purely remotely underlines its potential as a more affordable, scalable intervention for the future than current gold-standard treatments for PTSD. Clinicians may also wish to consider recommendations that have been offered for the sustainable roll-out of GSH using Spring across the NHS, based on findings from interviews with NHS commissioners and managers. 93
Research implications
How best to effectively disseminate and implement GSH using Spring at scale, to maximise its impact, is a key research question. Identification of the specific skill set and competencies required by a guiding clinician to foster effective alliance and engagement, and the optimal level of training and supervision required for the provision of GSH using Spring, would help determine if it can be effectively delivered by less qualified therapists. Effective dissemination and implementation would also be facilitated by work to address digital poverty. Further research, including dismantling studies, would help establish the actual mechanism of GSH using Spring. Replication by researchers not involved in the development of GSH using Spring and studies including under-represented populations and would strengthen the evidence for its effectiveness.
The optimal amount of guidance is unclear. The quantitative and qualitative results strongly suggest that the current number of facilitation sessions is right for most people but that some people could probably benefit with more. This points to the need for increased flexibility in delivery and more personalised adaptations seem desirable. ‘For example, it might be important for the clinician and person with PTSD to consider together whether the intervention, for example the pace, should be adapted to suit the person with PTSD’s needs and preferences, perhaps allocating additional time to certain components and less to others’.
Research is required to understand the extent to which individuals may or may not be excluded from internet-based treatments due to language and literacy issues, and online access issues, and how best to address these. Spring programme features that foster engagement may be important for some people. Further work is required to enhance the power of digital assistance, by harnessing innovative advances in information technology. For example, the development of interactive programmes that allow ecological momentary sampling, whereby people are prompted to do things and provide information on how they are feeling and what they are doing could increase effectiveness and reduce the amount of therapist guidance required. Future versions of the programme could allow bespoke versions to be created, for example, to allow choices concerning the gender of the programme voice-over and types of traumatic events included. There is also a need to investigate the use of bespoke GSH-based approaches for people with complex PTSD and other more complex presentations following traumatic events.
Digitally assisted GSH has clear potential to become more effective in the future and the best way to realise this will be through a ‘learning health system’,94 where care and research occur side by side, and increasingly innovative and effective personalised interventions are coproduced and evaluated. ‘Routine data collection should be used to create practice-based evidence around the impact of adaptations to programmes and approaches on their effectiveness, creating a continuously improving system’.
Conclusions
The RAPID trial showed GSH using Spring to be a clinically effective, cheaper, well-tolerated and non-inferior treatment to face-to-face CBT-TF for people with mild to moderate PTSD to a single traumatic event. The results should provide more choices and facilitate improvements to current care pathways for people with PTSD that result in improved health and wellbeing.
Acknowledgements
The research team would like to thank NIHR for funding the research costs of the study and the Welsh Government, through Health and Care Research Wales, for funding the NHS costs of the study. We thank the therapists, Nigel Short and Louise Waddington for undertaking fidelity assessments, Nigel Kirby as data manager, Sam Clarkstone and Francisco Fernandez as database designers, Kali Barawi for supporting the development of documents, Megan Phillips-Laird, Linda John and Jade Williams as CTR trial administrators, and staff at Healthcare Learning for their technical support. Most of all, we thank the participants of the study for taking part.
Contributions of authors
Jonathan I Bisson (https://orcid.org/0000-0001-5170-1243) (Professor in Psychiatry) conceived the study, designed the trial, obtained grant funding, led the management of the trial, and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Cono Ariti (https://orcid.org/0000-0001-7615-0935) (Study Statistician) did the statistical analysis and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Katherine Cullen (https://orcid.org/0000-0002-3704-4598) (Research Officer) did the health economics analysis and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Neil Kitchiner (https://orcid.org/0000-0003-0499-9520) (Director and Consultant Clinical Lead) conceived the study, designed the trial, obtained grant funding, oversaw management of the trial, and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Catrin Lewis (https://orcid.org/0000-0002-3818-9377) (Research Associate) conceived the study, designed the trial, obtained grant funding, oversaw management of the trial, and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Neil P Roberts (https://orcid.org/0000-0002-6277-0102) (Consultant Clinical Psychologist and Honorary Senior Research Fellow) conceived the study, designed the trial, obtained grant funding, oversaw management of the trial, and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Natalie Simon (https://orcid.org/0000-0001-5712-9460) (Research Assistant) acquired data and did the qualitative analysis and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Kim Smallman (https://orcid.org/0000-0002-9283-8120) (Research Associate) acquired data and did the qualitative analysis and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Katy Addison (https://orcid.org/0000-0002-5388-6437) (Research Associate) managed the trial and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Vicky Bell (https://orcid.org/0000-0002-2405-0673) (Research Associate) acquired data and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Lucy Brookes-Howell (https://orcid.org/0000-0002-8263-7130) (Senior Research Fellow) conceived the study, designed the trial, obtained grant funding, oversaw management of the trial and did the qualitative analysis, and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Sarah Cosgrove (https://orcid.org/0000-0001-8816-5878) (Public Advisory Group Chair) conceived the study, designed the trial, obtained grant funding, oversaw management of the trial, oversaw public involvement, and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Anke Ehlers (https://orcid.org/0000-0002-8742-0192) (Professor of Experimental Psychopathology and Wellcome Trust Principal Research Fellow) conceived the study, designed the trial, obtained grant funding, oversaw management of the trial, and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Deborah Fitzsimmons (https://orcid.org/0000-0002-7286-8410) (Professor of Health Economics and Outcomes Research) did the health economics analysis and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Paula Foscarini-Craggs (https://orcid.org/0000-0001-9511-696X) (Research Associate) managed the trial and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Shaun R S Harris (https://orcid.org/0000-0001-7724-6621) (Research Officer) did the health economics analysis and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Mark Kelson (https://orcid.org/0000-0001-7744-3780) (Associate Professor) conceived the study, designed the trial, obtained grant funding, oversaw management of the trial and did the statistical analysis, and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Karina Lovell (https://orcid.org/0000-0001-8821-895X) (Professor of Mental Health) conceived the study, designed the trial, obtained grant funding, oversaw management of the trial, and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Maureen McKenna (https://orcid.org/0000-0002-3477-1626) (Lead Consultant Psychological Therapist) managed the trial and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Rachel McNamara (https://orcid.org/0000-0002-7280-1611) (Principal Research Fellow) conceived the study, designed the trial, obtained grant funding, oversaw management of the trial, and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Claire Nollett (https://orcid.org/0000-0001-6676-4933) (Research Associate) managed the trial and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Tim Pickles (https://orcid.org/0000-0001-7743-0234) (Health and Care Research Wales NIHR Doctoral Fellow) did the statistical analysis and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
Rhys Williams-Thomas (https://orcid.org/0000-0002-1779-3460) (Research Associate) managed the trial and was responsible for the interpretation of the data and for drafting and approving the final submitted manuscript.
All available data can be obtained from the corresponding author.
Department of Health disclaimer
This report presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, CCF, NETSCC, the HTA programme or the Department of Health.
Ethical approval
The trial was granted a favourable ethical opinion by the South East Wales Research Ethics Committee (17/WA/0008).
Data sharing
The dataset is available from the corresponding author at bissonji@cardiff.ac.uk.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders 2013.
- World Health Organization . International Classification of Diseases for Mortality and Morbidity Statistics (11th Revision) 2018. https://icd.who.int/browse11/l-m/en (accessed 22 June 2022).
- McManus S, Bebbington PE, Jenkins R, Brugha T. Mental Health and Wellbeing in England: The Adult Psychiatric Morbidity Survey 2014. Leeds, UK: NHS Digital; 2016.
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995;52:1048-60.
- Brady KT, Killeen TK, Brewerton T, Lucerini S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry 2000;61:22-3.
- Ryder AL, Azcarate PM, Cohen BE. PTSD and physical health. Curr Psychiatry Rep 2018;20:1-8.
- Ferry FR, Brady SE, Bunting BP, Murphy SD, Bolton D, O’Neill SM. The economic burden of PTSD in Northern Ireland. J Trauma Stress 2015;28:191-7.
- ISTSS . Posttraumatic Stress Disorder Prevention and Treatment Guidelines: Methodology and Recommendations 2018. http://www.istss.org/getattachment/Treating-Trauma/New-ISTSS-Prevention-and-Treatment-Guidelines/ISTSS_PreventionTreatmentGuidelines_FNL.pdf.aspx (accessed 22 June 2022).
- NICE . Post-Traumatic Stress Disorder (NICE Guideline NG116) 2018. https://www.nice.org.uk/guidance/ng116 (accessed 22 June 2022).
- Phoenix Australia . The Australian Guidelines for the Prevention and Treatment of Acute Stress Disorder (ASD), Posttraumatic Stress Disorder (PTSD) and Complex PTSD 2020. www.phoenixaustralia.org/australian-guidelines-for-ptsd/ (accessed 22 June 2022).
- American Psychological Association . Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults 2017.
- Davis RG, Ressler KJ, Schwartz AC, Stephens KJ, Bradley RG. Treatment barriers for low‐income, urban African Americans with undiagnosed posttraumatic stress disorder. J Trauma Stress 2008;21:218-22.
- Kantor V, Knefel M, Lueger-Schuster B. Perceived barriers and facilitators of mental health service utilization in adult trauma survivors: A systematic review. Clin Psychol Rev 2017;52:52-68.
- Lovell K, Richards D. Multiple access points and levels of entry (MAPLE): ensuring choice, accessibility and equity for CBT services. Behav Cogn Psychother 2000;28:379-91.
- Lewis C, Pearce J, Bisson JI. Efficacy, cost-effectiveness and acceptability of self-help interventions for anxiety disorders: systematic review. Br J Psychiatry 2012;200:15-21.
- Karyotaki E, Efthimiou O, Miguel C, Bermpohl FM, Furukawa TA, Cuijpers P, et al. Internet-based cognitive behavioral therapy for depression: a systematic review and individual patient data network meta-analysis. JAMA Psychiatry 2021;78:361-71. https://doi.org./10.1001/jamapsychiatry.2020.4364.
- Lewis C, Roberts NP, Vick TL, Bisson JI. Development of a guided self-help (GSH) program for the treatment of mild-to-moderate posttraumatic stress disorder (PTSD). Depress Anxiety 2013;30:1121-8.
- Medical Research Council . A Framework for the Development and Evaluation of Randomised Controlled Trials for Complex Interventions to Improve Health 2000.
- Lewis CE, Farewell D, Groves V, Kitchiner NJ, Roberts NP, Vick T, et al. Internet-based guided self-help for posttraumatic stress disorder (PTSD): randomized controlled trial. Depress Anxiety 2017;34:555-65.
- Simon N, Robertson L, Lewis C, Roberts NP, Bethell A, Dawson S, et al. Internet-based cognitive and behavioural therapies for post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev 2021;5. https://doi.org./10.1002/14651858.CD011710.pub3.
- Nollett C, Lewis C, Kitchiner N, Roberts N, Addison K, Brookes-Howell L, et al. Pragmatic randomised controlled trial of a trauma-focused guided self-help programme versus individual trauma-focused cognitive behavioural therapy for post-traumatic stress disorder (RAPID): trial protocol. BMC Psychiatry 2018;18. https://doi.org/10.1186/s12888-018-1665-3.
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594-604.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11:1-8.
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) 2013. www.ptsd.va.gov (accessed 22 June 2022).
- Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behav Res Ther 2000;38:319-45.
- Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale – Revised. Behav Res Ther 2003;41:1489-96.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy (Amsterdam) 1990;16:199-208.
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Value Set for England 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed October 2021).
- Van Hout B, Janssen MF, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15.
- Mundt JC, Marks IM, Shear MK, Greist JM. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461-4.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13.
- Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7.
- Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. Addiction 1993;88:791-804.
- Dahlem NW, Zimet GD, Walker RR. The multidimensional scale of perceived social support: a confirmation study. J Clin Psychol 1991;47:756-61.
- Zimet GD, Dahlem NW, Zimet SG, Farley GK. The multidimensional scale of perceived social support. J Pers Assess 1988;52:30-41.
- Cecil H, Stanley MA, Carrion PG, Swann A. Psychometric properties of the MSPSS and NOS in psychiatric outpatients. J Clin Psychol 1995;51:593-602.
- Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. J Pers Assess 1990;55:610-7.
- Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601-8.
- Foa EB, Ehlers A, Clark DM, Tolin DF, Orsillo SM. The posttraumatic cognitions inventory (PTCI): Development and validation. Psychol Assess 1999;11:303-14.
- Luszczynska A, Gutiérrez‐Doña B, Schwarzer R. General self‐efficacy in various domains of human functioning: evidence from five countries. Int J Psychol 2005;40:80-9.
- Scholz U, Doña BG, Sud S, Schwarzer R. Is general self-efficacy a universal construct? Psychometric findings from 25 countries. Eur J Psychol Assess 2002;18.
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979;2:197-20.
- Nguyen TD, Attkisson CC, Stegner BL. Assessment of patient satisfaction: development and refinement of a service evaluation questionnaire. Eval Program Plann 1983;6:299-313.
- Cahill J, Stiles WB, Barkham M, Hardy GE, Stone G, Agnew-Davies R, et al. Two short forms of the Agnew Relationship Measure: The ARM-5 and ARM-12. Psychother Res 2012;22:241-55.
- Bisson JI, Roberts NP, Andrew M, Cooper R, Lewis C. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev 2013.
- International Conference on Harmonisation . ICH Harmonised Tripartite Guideline for Statistical Principles for Clinical Trials 1998. https://database.ich.org/sites/default/files/E9_Guideline.pdf (accessed October 2021).
- Chow SC, Shao J. On non‐inferiority margin and statistical tests in active control trials. Stat Med 2006;25:1101-13.
- nQuery v 7.0 . Sample Size and Power Calculation 2017.
- Audigier V, White IR, Jolani S, Debray TP, Quartagno M, Carpenter J, et al. Multiple imputation for multilevel data with continuous and binary variables. Stat Sci 2018;33:160-83.
- European Medicines Agency . ICH E9(R1) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials n.d.;44:1-23. www.ema.europa.eu/en/documents/scientific-guideline/draft-ich-e9-r1-addendum-estimands-sensitivity-analysis-clinical-trials-guideline-statistical_en.pdf (accessed October 2021).
- Gillespie D, Farewell D, Barrett-Lee P, Casbard A, Hawthorne AB, Hurt C, et al. The use of randomisation-based efficacy estimators in non-inferiority trials. Trials 2017;18:1-11.
- Gillespie D, Hood K, Farewell D, Butler CC, Verheij T, Goossens H, et al. Adherence-adjusted estimates of benefits and harms from treatment with amoxicillin for LRTI: secondary analysis of a 12-country randomised placebo-controlled trial using randomisation-based efficacy estimators. BMJ Open 2015;5.
- Wiles N, Fischer K, Cowen P, Nutt D, Peters T, Lewis G, et al. Allowing for non-adherence to treatment in a randomized controlled trial of two antidepressants (citalopram versus reboxetine): an example from the GENPOD trial. Psychol Med 2014;44:2855-66.
- Quartagno M, Grund S, Carpenter JJ. A flexible package for two-level joint modelling multiple imputation. R Journal 2019;9:205-28.
- Stata Statistical Software: Release 16.1 [program]. College Station, TX: StataCorp LLC; 2019.
- Weiss DS. The Impact of Event Scale: Revised. Cross-cultural Assessment of Psychological Trauma and PTSD. Springer; 2007.
- Mavranezouli I, Megnin-Viggars O, Grey N, Bhutani G, Leach J, Daly C, et al. Cost-effectiveness of psychological treatments for post-traumatic stress disorder in adults. PLoS One 2020;15.
- Chisholm D, Knapp MRJ, Knudsen HC, Amaddeo F, Gaite L, Van Wijngaarden B, et al. Client socio-demographic and service receipt inventory – European version: development of an instrument for international research: EPSILON Study 5. Br J Psychiatry 2000;177:s28-s33.
- Beecham J, Knapp M. Costing Psychiatric Interventions. Measuring Mental Health Needs. London: Gaskell; 2001.
- Personal Social Services Research Unit (PSSRU) . Generic Mental Health CSRI n.d. www.pssru.ac.uk/csri/featured-examples-of-the-csri/generic-mental-health-csri/ (accessed October 2021).
- Pritchard C, Sculpher M. Productivity costs: principles and practice in economic evaluation. Monographs 2000;00464.
- Curtis L, Burns A. Unit Costs of Health and Social Care. PSSRU; 2013.
- NHS . NHS Reference Cost Collection. https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed 8 October 2021).
- NHS Business Services Authority . NHS Drug Tariff Online January 2021 2021. www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff (accessed October 2021).
- British National Formulary 2017. www.medicinescomplete.com/about/publications.htm?pub=bnf (accessed January 2018).
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEcon 2014;32:1157-70.
- Francis NA, Gillespie D, White P, Bates J, Lowe R, Sewell B, et al. C-reactive protein point-of-care testing for safely reducing antibiotics for acute exacerbations of chronic obstructive pulmonary disease: the PACE RCT. Health Technol Assess (Winchester, England) 2020;24.
- NICE . The Principles That Guide the Development of NICE Guidance and Standards 2021 n.d. www.nice.org.uk/about/who-we-are/our-principles (accessed 15 September 2021).
- Net Monetary Benefit . York: York Health Economics Consortium 2016. https://yhec.co.uk/glossary/net-monetary-benefit/ (accessed 3 September 2021).
- Sullivan SD, Mauskopf JA, Augustovski F, Caro JJ, Lee KM, Minchin M, et al. Budget impact analysis – principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014;17:5-14.
- ONS . ONS National Population Projections: 2018-Based 2018.
- Dakin H, Wordsworth S. Cost‐minimisation analysis versus cost‐effectiveness analysis, revisited. Health Econ 2013;22:22-34.
- Chisholm D, Evans DB. Economic evaluation in health: saving money or improving care?. J Med Econ 2007;10:325-37.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350.
- Chen H, Chen C, Bai S, Gao Y, Metcalfe G, Cheng W, et al. Multiplexed detection of cancer biomarkers using a microfluidic platform integrating single bead trapping and acoustic mixing techniques. Nanoscale 2018;10:20196-206.
- Palinkas LA. Qualitative and mixed methods in mental health services and implementation research. J Clin Child Adolesc Psychol 2014;43:851-61.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Qualitative data analysis for applied policy research. Analyz Qual Data 1994;173.
- Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52:1893-907.
- Braun V, Clarke V. Reflecting on reflexive thematic analysis. Qualitative Research in Sport, Exercise and Health 2019;11:589-97.
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753-60.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13:1-8.
- Lewis C, Roberts NP, Andrew M, Starling E, Bisson JI. Psychological therapies for post-traumatic stress disorder in adults: systematic review and meta-analysis. Eur J Psychotraumatol 2020;11.
- Schnyder U, Ehlers A, Elbert T, Foa EB, Gersons BPR, Resick PA, et al. Psychotherapies for PTSD: what do they have in common?. Eur J Psychotraumatol 2015;6.
- Lewis C, Roberts N, Simon N, Bethell A, Bisson J. Internet‐delivered cognitive behavioural therapy for post‐traumatic stress disorder: systematic review and meta‐analysis. Acta Psychiatr Scand 2019;140:508-21.
- Macedo T, Barbosa M, Rodrigues H, Coutinho ESF, Figueira I, Ventura P. Does CBT have lasting effects in the treatment of PTSD after one year of follow-up? A systematic review of randomized controlled trials. Trends Psychiatry Psychother 2018;40:352-9.
- Schuster R, Topooco N, Keller A, Radvogin E, Laireiter A-R. Advantages and disadvantages of online and blended therapy: replication and extension of findings on psychotherapists’ appraisals. Internet Interv 2020;21.
- Lovell K, Bower P, Gellatly J, Byford S, Bee P, McMillan D, et al. Clinical effectiveness, cost-effectiveness and acceptability of low-intensity interventions in the management of obsessive–compulsive disorder: the Obsessive–Compulsive Treatment Efficacy randomised controlled Trial (OCTET). Health Technol Assess (Winchester, England) 2017;21:1-132.
- Deisenhofer AK, Delgadillo J, Rubel JA, Boehnke JR, Zimmermann D, Schwartz B, et al. Individual treatment selection for patients with posttraumatic stress disorder. Depress Anxiety 2018;35:541-50.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, LI T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ, Chichester: Wiley-Blackwell; 2019.
- Duffy M, Gillespie K, Clark DM. Post-traumatic stress disorder in the context of terrorism and other civil conflict in Northern Ireland: randomised controlled trial. BMJ 2007;334.
- Andersson G, Titov N. Advantages and limitations of Internet‐based interventions for common mental disorders. World Psychiatry 2014;13:4-11.
- Wind TR, Rijkeboer M, Andersson G, Riper H. The COVID-19 pandemic: The ‘black swan’ for mental health care and a turning point for e-health. Internet Interv 2020;20.
- Simon N, Ploszajski M, Lewis C, Smallman K, Roberts NP, Kitchiner NJ, et al. Internet-based psychological therapies: a qualitative study of National Health Service commissioners and managers views. Psychol Psychother 2021;94:994-1014. https://doi.org./10.1111/papt.12341.
- Knudsen SV, Laursen HVB, Johnsen SP, Bartels PD, Ehlers LH, Mainz J, et al. Can quality improvement improve the quality of care? A systematic review of reported effects and methodological rigor in plan-do-study-act projects. BMC Health Serv Res 2019;19.
Appendix 1 Supplementary health economic data
NHS Electronic Drug Tariff64 and British National Formulary65
| Drug name | Dose | Pack size | Price per pack |
|---|---|---|---|
| Adalimumab | 20 mg/0.2 ml | 2 | £352.14 |
| 40 mg/0.4 ml | 2 | £704.28 | |
| Amitriptyline | 10 mg | 28 | £1.08 |
| 25 mg | 28 | £1.03 | |
| 50 mg | 28 | £1.80 | |
| Amlodipine | 10 mg | 28 | £1.03 |
| Ampicillin | 250 mg | 28 | £24.38 |
| 500 mg | 28 | £47.50 | |
| Apixaban | 5 mg | 56 | £53.30 |
| Atorvastatin chewable tablets | 10 mg | 30 | £13.80 |
| 20 mg | 30 | £26.40 | |
| Azathioprine | 25 mg | 28 | £2.14 |
| 50 mg | 56 | £3.10 | |
| Bendroflumethiazide | 2.5 mg | 28 | £0.84 |
| Bisoprolol | 5 mg | 28 | £0.95 |
| Budesonide with formoterol | 100 µg/6 µg | 120 | £28.00 |
| Buprenorphine transdermal patches | 35 µg/hour | 4 | £15.80 |
| 15 µg/hour | 4 | £49.15 | |
| 52.5 µg/hour | 4 | £23.71 | |
| Calcium carbonate chewable tablets | 500 mg | 48 | £2.72 |
| Candesartan | 8 mg | 28 | £1.53 |
| 16 mg | 28 | £1.57 | |
| Cinnarizine | 15 mg | 84 | £4.70 |
| Citalopram | 20 mg | 28 | £1.23 |
| 40 mg | 28 | £1.42 | |
| Co-codamol tablets | 15 mg/500 mg | 100 | £3.94 |
| 30 mg/500 mg | 30 | £1.85 | |
| Codeine | 30 mg | 28 | £1.32 |
| 100 | £4.71 | ||
| 60 mg | 28 | £2.19 | |
| Diazepam | 2 mg | 28 | £0.89 |
| 5 mg | 28 | £0.93 | |
| 10 mg | 28 | £1.05 | |
| Diltiazem hydrochloride | 120 mg | 28 | £9.14 |
| 180 g | 28 | £10.37 | |
| Digoxin | 250 µg | 28 | £1.63 |
| Disulfiram | 200 mg | 50 | £106.05 |
| Diclofenac | 200 ml | £12.95 | |
| Docusate sodium | 100 mg | 30 | £2.09 |
| Duloxetine | 30 mg | 28 | £1.74 |
| 60 mg | 28 | £2.36 | |
| Ethinylestradiol/drospirenone (Eloine) | 20 µg/3 mg | 84 | £14.70 |
| Ethinylestradiol/levonorgestrel (Rigevidon) | 30 µg/150 µg | 63 | £2.82 |
| Fluoxetine | 30 mg | 30 | £1.80 |
| 60 mg | 30 | £6.76 | |
| Fluticasone with salmeterol | 500 µg/50 µg | 60 dose | £32.74 |
| Fluticasone with vilanterol | 184 µg/22 µg | 30 | £29.50 |
| 92 µg/22 µg | 30 | £22.00 | |
| Folic acid | 5 mg | 28 | £1.15 |
| Fostair | 100 µg/6 mg or 200 µg/6 mg | 120 | £29.32 |
| Gabapentin | 300 mg | 100 | £3.45 |
| 400 mg | 100 | £4.47 | |
| 600 mg | 100 | £9.17 | |
| Gliclazide | 40 mg | 28 | £1.57 |
| 80 mg | 28 | £1.19 | |
| Ibuprofen | 200 mg | 24 | £1.48 |
| 200 mg | 84 | £5.18 | |
| 400 mg | 24 | £2.19 | |
| 400 mg | 84 | £7.67 | |
| Iron supplements | 14 mg | 180 | £6.50 |
| Labetalol | 50 mg | 56 | £3.79 |
| 100 mg | 56 | £6.08 | |
| 200 mg | 56 | £8.80 | |
| Lansoprazole | 15 mg | 28 | £1.12 |
| 30 mg | 28 | £1.43 | |
| Latanoprost | 2.5 ml | 1 | £3.01 |
| Losartan | 12.5 mg | 28 | £5.70 |
| 25 mg | 28 | £1.42 | |
| 50 mg | 28 | £1.61 | |
| 100 mg | 28 | £1.97 | |
| Levothyroxine | 12.5 µg | 28 | £12.49 |
| 25 µg | 28 | £1.54 | |
| 50 µg | 28 | £1.26 | |
| 75 µg | 28 | £3.07 | |
| 100 µg | 28 | £1.26 | |
| Medroxyprogesterone acetate | 104 mg/0.65 ml | 1 | £6.90 |
| 150 ml/1 ml | 1 | £6.01 | |
| Mefenamic acid | 250 mg | 100 | £23.90 |
| Mesalazine | 4 g | 30 | £73.78 |
| Metformin | 500 mg modified release | 56 | £4.00 |
| 1 g modified release | 56 | £6.40 | |
| 500 mg tablets | 28 | £1.53 | |
| Mesalazine | 1.2g | 60 | £42.95 |
| Millinette (oral contraceptive) ethinylestradiol/gestodene | 20 µg/75 µg | 63 | £8.85 |
| 30 µg/75 µg | 63 | £6.73 | |
| Mirtazapine | 15 mg | 30 | £2.44 |
| 30 mg | 30 | £2.71 | |
| 45 mg | 30 | £2.96 | |
| Montelukast | 10 mg | 28 | £2.54 |
| MST | 15 mg | 60 | £9.10 |
| Naproxen | 500 mg | 28 | £2.35 |
| Omeprazole | 20 mg | 28 | £1.18 |
| 40 mg | 7 | £0.88 | |
| Oramorph (Morphine) | 20 mg/ml | 120 ml | £19.50 |
| Oxycontin (longtec) | 5 mg | 28 | £12.52 |
| Paracetamol | 500 mg | 100 | £3.06 |
| 1 g | 100 | £3.50 | |
| Promethazine hydrochloride | 25 mg | 56 | £4.61 |
| Paroxetine | 20 mg | 30 | £1.74 |
| 30 mg | 30 | £2.22 | |
| Prazosin | 1 mg | 60 | £3.46 |
| Prednisolone | 5 mg | 28 | £1.28 |
| Pregabalin | 75 mg | 56 | £2.33 |
| 100 mg | 84 | £3.58 | |
| 200 mg | 84 | £4.98 | |
| 300 mg | 56 | £4.32 | |
| Prochlorperazine | 5 mg | 28 | £1.26 |
| Propranolol | 10 mg | 28 | £1.57 |
| 40 mg | 28 | £1.55 | |
| 80 mg | 56 | £3.36 | |
| 160 mg | 56 | £5.88 | |
| Quetiapine | 25 mg | 60 | £2.08 |
| Ramipril | 10 mg | 28 | £1.22 |
| Salbutamol | 100 µg | 200 dose | £1.50 |
| Sertraline | 50 mg | 28 | £4.39 |
| 100 mg | 28 | £8.88 | |
| Simvastatin | 40 mg | 28 | £1.22 |
| Sumatriptan | 6 mg/0.5 ml | 2 | £45.00 |
| Tablets 50 mg | 6 | £1.4 | |
| Testosterone | 250 mg/1 ml | 1 | £2.45 |
| Tacrolimus | 1 mg | 50 | £80.28 |
| Temazepam | 10 mg | 28 | £1.71 |
| Thiamine | 100 mg | 100 | £7.11 |
| Tiotropium | 2.5 µg | 60 | £23.00 |
| Tramadol | 50 mg | 60 | £7.24 |
| 100 g/ml oral drops | 10 ml | £25.00 | |
| Tranexamic acid | 500mg | 60 | £9.05 |
| Trazadone | 50 mg | 84 | £3.90 |
| 100 mg | 56 | £3.77 | |
| Triptorelin | 3 mg | 1 | £69.00 |
| 11.25 mg | 1 | £207.00 | |
| Tysabri (Natalizumab) | 22.5 mg | 1 | £414.00 |
| Venlafaxine | 75 mg | 30 | £2.60 |
| 150 mg | 30 | £3.90 | |
| 225 mg | 30 | £33.60 | |
| Vitamin B12 | 10 µg | 180 | £6.00 |
| Vitamin D | 10 µg | 90 | £2.30 |
| Zopiclone | 3.75 mg | 28 | £1.27 |
| 7.5 mg | 28 | £1.26 |
| Band | Cost per working hour | Cost per patient contact | Source | |
|---|---|---|---|---|
| Therapist | 4 | £31 | £59.21 | PSSRU 2020 1 : 0.91 ratio of direct to indirect time for clinical psychologist (band7) |
| Therapist | 5 | £36 | £68.76 | |
| Therapist | 7 | £58 | £110.78 | |
| Therapist | 8a | £69 | £131.79 | |
| Therapist | 8b | £82 | £156.62 | |
| Therapist | 8c | £96 | £183.36 | |
| General practice nurse | 5 | £42.00 | £76.29 | PSSRU 2020 Ratio for district nurse used to calculate cost per patient contact |
| District nurse | 6 | £49.00 | £89.00 | PSSRU 2020 |
| Community psychiatric nurse/case manager | 6 | £49.00 | £89.00 | PSSRU 2020 |
| Mental health nurse | 5 | £39.00 | £63.00 | PSSRU 2020 |
| Social worker (adult services) | £51.00 | £82.38 | Ratio for mental health nurse used to calculate cost per patient contact | |
| Counsellor | 6 | £48.00 | £87.18 | Ratio for district nurse used to calculate cost per patient contact |
| Community occupational therapist | £49.00 | £89.00 | Ratio for district nurse used to calculate cost per patient contact |
| Unit cost | Adjusted cost | Source | |
|---|---|---|---|
| GP appointment 9.22 minutes (including direct care staff) | £39.00 | N/A | PSSRU 2020 |
| GP in-person cost per working hour | £255.00 | N/A | PSSRU 2020 |
| GP telephone appointment | £8.41 | N/A | PSSRU 2020 |
| GP out-of-hours appointment | £68.30 | £73.09 | National Audit Office 2014 (1) uplifted to 2020 |
| Unit cost | Uplifted cost | NHS Reference Costs 2018/19 | |
|---|---|---|---|
| OT adult one-to-one | £83.00 | £84.83 | A06A1 |
| OT adult group | £104.00 | £106.30 | A06AG |
| PT adult, one-to-one | £63.00 | £64.39 | A08A1 |
| Other therapist, adult, one-to-one | £83.00 | £84.83 | A01A1 |
| Other therapist, adult, group | £50.00 | £51.11 | A01AG |
| Community midwife, antenatal visit | £58.00 | £59.28 | N01A |
| District nurse, adult face-to-face | £40.00 | £40.88 | N02AF |
| Specialist nursing, active case management adult, face-to-face | £80.00 | £81.77 | N06AF |
| Specialist nursing, Stoma Care Services, Adult face-to-face | £46.00 | £47.02 | N24AF |
| Health visitor | £67.94 | £69.44 | N03A-G, J, N |
| Alcohol services – community care contact | £93.00 | Drug and alcohol services (adults) PSSRU 2020 | |
| Drugs services – outpatient | £121.00 | Drug and alcohol services (adults) PSSRU 2020 |
| Home care worker | Mean unit cost (weekday/weekend/night-time) | Source |
|---|---|---|
| Private purchaser face-to-face | £29.75 | PSSRU 2020 |
| Social services face-to-face | £30.75 | PSSRU 2020 |
| Unit cost 2018/19 | Unit cost 2020 | Source | |
|---|---|---|---|
| Band 1 | N/A | £22.70 | PSSRU 2020 |
| Band 2 | N/A | £62.10 | PSSRU 2020 |
| Band 3 | N/A | £269.30 | PSSRU 2020 |
| Emergency dental service, attendance | £99.00 | £101.19 | M01C NHS reference cost 2018/19 |
| Unit cost 2018/19 | Unit cost 2020 | NHS Reference Costs 2018/19 | |
|---|---|---|---|
| Psychiatric | £315 | £321.96 | 710 adult mental illness |
| Medical | £167 | £170.69 | 300 general medicine |
| Orthopaedic | £120 | £122.65 | 110 trauma and orthopaedics |
| Gastroenterology | £141 | £144.12 | 301 gastroenterology |
| Urology | £108 | £110.39 | 101 urology |
| Audiology | £108 | £110.39 | 840 audiology |
| Maxillofacial | £124 | £126.74 | 144 maxillo-facial surgery |
| Antenatal | £99 | £101.19 | 560 midwifery service |
| Pain management | £157 | £160.47 | 191 pain |
| Complex specialised rehabilitation service | £94 | £96.08 | 344 |
| Physiotherapy | £58 | £59.28 | 650 physiotherapy |
| Occupational therapy | £71.00 | £72.57 | 651 occupational therapy |
| Orthoptics | £74 | £75.64 | 655 |
| Clinical psychology | £199.00 | £203.04 | 656 |
| Orthotics | £124.00 | £126.74 | 658 |
| Genitourinary medicine | £116.00 | £118.56 | 360 |
| Trauma and orthopaedics | £120.00 | £122.65 | 110 |
| Family planning clinic | £90.00 | £91.99 | FPC |
| Neurosurgery | £183.00 | £187.04 | 150 |
| Ophthalmology | £98.00 | £100.17 | 130 |
| Gynaecology | £141.00 | £144.12 | 502 |
| Neurology | £177.00 | £180.91 | 400 |
| General surgery | £134.00 | £136.96 | 100 |
| Nephrology | £164.00 | £167.62 | 361 |
| Cardiology | £139.00 | £142.07 | 320 |
| Rheumatology | £147.00 | £150.25 | 410 |
| Respiratory medicine | £157.00 | £160.47 | 340 |
| Dermatology | £113.00 | £115.50 | 330 |
| Colorectal surgery | £121.00 | £123.67 | 104 |
| Obstetrics | £135.00 | £137.98 | 501 |
| Endocrinology | £161.00 | £164.56 | 302 |
| Respiratory nurse or AHP, education or support | £160.90 | £164.46 | DZ49Z |
| Respiratory sleep study | £183.35 | £187.40 | DZ50Z |
| Injection of RH immune globulin or other blood transfusion 19+ | £153.00 | £156.38 | SA45A |
| Colposcopy | £233.57 | £238.73 | MA38/39/40Z |
| Electrocardiogram | £136.66 | £139.68 | EC22Z/EY51Z |
| IAPT per contact | £25.56 | £261.2 | Cluster 01/02 |
| Unit cost 2018/19 | Unit cost 2020 | Source | |
|---|---|---|---|
| NHS 111 | N/A | £13.24 | Pope et al. BMJ Open 2017 (1) uplifted from 2016 to 2020 |
| Minor injury unit | £72.54 | £74.14 | Type 3 and 4 non-admitted A&E |
| A&E admitted | £268.27 | £274.20 | All emergency medicine investigations admitted |
| A&E non-admitted | £159.48 | £163.00 | All emergency medicine investigations non-admitted |
| Unit cost 2018/19 | Unit cost 2020 | Source | |
|---|---|---|---|
| Diagnostic test | £2.00 | £2.04 | Average of pathology reference costs |
| Blood test | £1.00 | £1.02 | DAPS04 clinical biochemistry, N = 279,917,477 |
| Imaging MRI/CT one area | £119.42 | £122.06 | Imaging – outpatient, MRI, CT, aged 19 and over, one area, no contrast, post-contrast only, pre- and post-contrast |
| Imaging MRI one area | £147.78 | £151.05 | Imaging – outpatient, MRI aged 19 and over, one area, no contrast, post-contrast only, pre- and post-contrast |
| Imaging U/S | £55.21 | £56.43 | Imaging – Outpatient, U/S, less than or more than 20 minutes, with and without contrast |
| Endoscopy/endometriosis | £313.76 | £320.69 | Gynaecology – 502, MA09B and MA10Z; laparoscopic or endoscopic upper genital tract procedures |
| X-ray | £22.00 | £22.49 | IMAGOP Plain film |
| Unit cost 2018/19 | Unit cost 2020 | Source | |
|---|---|---|---|
| Asthma | £282.14 | £288.38 | DZ15M, N, P, Q, R asthma with and without interventions |
| Infections or other complications of procedures | £411.02 | £420.10 | WHO7A-6 |
| Procedure | £914.11 | £934.31 | Mean of all-day case procedures for adults |
| Unit cost 2017/18 | Unit cost 2019/20 | Source | |
|---|---|---|---|
| Acute medical ward | £853.99 | £893.02 | Weighted average of all elective and non-elective inpatient adult stays bed days, NHS reference costs 2017/18 (1) |
| General ward | £334 | £349.45 | Weighted average of all XS bed days for elective and non-elective inpatient stays, NHS reference costs 2017/18 |
| Acute psychiatric ward | £510.03 | Average of low-level and medium-level secure mental health services bed days PSSRU 2019 | |
| Long stay/residential | £124 | Per day for care home – adults requiring long-term mental health support (18–64) PSSRU 2020 | |
| Psychiatric rehabilitation | £429 | £438.48 | VC28Z rehabilitation for other psychiatric disorder, NHS reference costs 2017/18 |
| Variable | Time point | CBT-TF (n = 99) (% missing) | GSH (n = 96) (% missing) |
|---|---|---|---|
| EQ-5D VAS | Baseline | 5 (5.05) | 1 (1.03) |
| 16 weeks | 24 (24.24) | 30 (30.93) | |
| 52 weeks | 44 (44.44) | 45 (46.39) | |
| EQ-5D utility | Baseline | 0 (0.00) | 1 (1.03) |
| 16 weeks | 24 (24.24) | 30 (30.93) | |
| 52 weeks | 44 (44.44) | 45 (46.39) | |
| NHS costs | Baseline | 0 (0.00) | 0 (0.00) |
| 16 weeks | 19 (19.19) | 25 (25.77) | |
| 52 weeks | 40 (40.40) | 37 (38.14) | |
| All cost | Baseline | 0 (0.00) | 0 (0.00) |
| 16 weeks | 19 (19.19) | 25 (25.77) | |
| 52 weeks | 40 (40.40) | 37 (38.14) | |
| CAPS total score | Baseline | 0 (0.00) | 0 (0.00) |
| 16 weeks | 16 (16.16) | 20 (20.62) | |
| 52 weeks | 29 (29.29) | 28 (28.87) | |
| IES-R total score | Baseline | 0 (0.00) | 0 (0.00) |
| 16 weeks | 21 (21.21) | 29 (29.90) | |
| 52 weeks | 42 (42.42) | 43 (44.33) | |
| WSAS total score | Baseline | 0 (0.00) | 0 (0.00) |
| 16 weeks | 24 (24.24) | 29 (29.90) | |
| 52 weeks | 44 (44.44) | 44 (45.36) | |
| Total therapy | 1 (1.01) | 2 (2.06) | |
| Real-world therapy | 1 year | 0 (0.00) | 0 (0.00) |
Appendix 2 Supporting quotes for process evaluation analysis
Trial acceptability, design and processes
Consistency and clarity
Um I think my doctor um put me forward for it, and she, she um, she gave me, she basically put me forward for this while I was waiting to see a Counsellor as well.
Matilda
I think it was like a little black and white leaflet that they gave me. But I, like I say it was just all you know, a blur.
Stuart
Um, I’m not, they did explain, [therapist] did explain stuff to me, but I kind of, I hate to say it goes in one ear and out the other.
Matilda
But erm a man from the study called me and explained a bit about the study and then did an initial assessment I think, over the phone. Err and then after that I received a pamphlet and some information in the mail.
Clare
Volume, intensity and impact
In terms of erm, I dunno there’s quite, there’s quite a lot of questions and I made this clear to [site rater] and [site staff member], quite a lot of questions that ask erm about the link between a certain incident and the way you’ve been feeling erm which I found difficult to answer cos I wasn’t sure erm, in fact I’m still not sure if there is a link between the situation and the way I’ve been feeling because it’s so difficult to explain.
Molly
And I found the diary that I had to fill in was, erm very strangely worded. So like it was hard to describe my symptoms based on the scales that were in it.
Clare
Um so fair play to [rater], [rater] sat beside me and we went through it together. But she was using the, the laptop … But I could see everything she was doing. She didn’t fill in any answers for me.
Mike
So I’m just wondering if it’s pages and pages and pages of questions and … And [stutter] that … I don’t know if that’s for me. That’s my only reservation of this study.
Mike
Um everything would be quite anonymised, you just use the statistics, and stuff like that. So everything was quite really well, you know, really well explained.
Matilda
Consent, confidentiality and reservations
Oh yeah no I was happy to sign for sure. Erm, I think I was … I asked a couple of questions about, erm when the study would be done just because I’m interested in what the results of it will be.
Clare
No it was all really, really clear, straightforward.
Becky
My concerns was sharing the personal information, but then when, erm, [trial site rater] said it’s, it’s anonymous.
Sheila
Motivations
Um and I didn’t really mind how I got it, I just wanted help.
Matilda
I thought it would be a good thing for me to do so that I wasn’t waiting for treatment as long ‘cause she said that it could be about eight months on the … waiting list and, erm, I just thought it was a good idea to go through with it …
Ellen
That people are looking into how to help people, so like say and I, I’m, as a person normally, I’m a very helpful, kind person anyway, I like to help people … So I just thought that’s like two things in one then that I could do.
Sheila
so if there’s a way and if, you know if I can help as well … in that respect to getting help for people quickly I would happily do that.
Karen
Well it was a way to study what would be helpful to people … You know what … and people, err probably suffer different symptoms so the fact that this study would maybe help to tailor treatment in the future for people.
Ann
Preference and expectations
Err and how you … when you was put in the system it was a randomisation, err to compare results and I fully understand, you know that … how that … why that’s done and how that’s done and the whole idea that it works random and why. Err the results are compared at the end.
Hugo
I’m quite happy to receive either one.
Ellen
I’d probably choose, I don’t know, you know, I don’t know. I was going to say I’ll probably choose face to face, but it depends on what the computer side would be, what, what would that be.
Sheila
You see I’ve only had the face-to-face, erm that’s been my only experience. Erm, so I would probably go for that because it’s all that I’ve experienced before. But obviously I would be open to the other treatments.
Ann
Intervention acceptability
Accessibility
-
– Facilitators
Erm, because I’ve got a little one, erm I … it was nice to be able to just sit down and make new things and do it instead of having to go to treatments all the time which with work and life commitments is … can be quite stressful.
Ellen
That was good because like it gave you a break off and even though you had basically two weeks to do your homework in.
Mike
Um but I quite like the flexibility that it gives the participant, because um you know, if, if they weren’t able to make it for any reason, we’d just um resort to email, so I’d say you know, rather than me phoning you then, um if you don’t want me to phone you after a week, for, for a catch up, I can email you, if you prefer that.
Christian
-
– Barriers
It wouldn’t have been ideal for me anyway … I haven’t got a computer, I’ve only got my phone so that wouldn’t have been ideal for me.
Miriam
I mean not, it’s not so much the younger generation, but older people might struggle with internet and, cos I mean I know my mum doesn’t have internet and … my mum doesn’t know how to use a computer.
Karen
Therapeutic relationship and alliance
-
– Facilitators
Even birds in the garden frightened the living daylights out of me. And bit by bit and then suddenly, I’d sit, sit out in the garden, there’s birds fluttering around me, I live on a farm and, and I didn’t care. I, I was happy and, and I put that down actually down to her.
Kay
you’re still able to develop a therapeutic relationship quite, quite quickly. I think the, maybe the boundaries would be a bit tighter. I don’t think they would be restricting completely …
Jenny
-
– Barriers
I don’t think that, I don’t think that would have worked for me, I think I would have walked away I think.
Miriam
there is a very strong collective belief that, actually, that [therapeutic relationship] is what matters, above all; you know … Guided self-help will always be second best to the real gold standard treatment.
Meg
I think there’s a bit of societal expectation in certain thoughts of trauma … something about being attended to by a human being … in a compassionate way.
Laura
Structure and format
-
– Facilitators
Erm, well I liked all the kind of grounding techniques and I appreciated although it was difficult, to do the erm writing down and reading like exposure therapy … I think that really helped because after that, because before when I was talking about it I would get myself really like anxious and talk really quite quick and get really tense and then afterwards, it became just like I would just tell it like it hadn’t happened to me because … it was just, it was just a story that I’d read to myself over and over and over again.
Becky
But I found the app very immersive and actually I found the process in its entirety quite immersive so I was with my clinician at appointments I felt very immersed in my situation and I felt that, erm, he was with me in that experience … Which was very important to me.
Emma
I think the most important one was the, err, narrative writing. Erm, yeah so, you know, they needed to have done that and really, erm, you know emotionally engaged with their story to have the best kind of outcome. It was all useful but I think that’s really the most important part.
William
-
– Barriers
my feeling was that the four different accounts were real and truthful accounts but read by actors … And, erm, or at least as I say read by someone other than that … person who, who gave the account … And the effect that it had on me, and the reason I’m flagging it is because it was quite, erm, quite a strong response that listening to those accounts took me out of that immersive experience … Erm, because I felt, oh hang on I know you’re talking about someone who’s really experienced this but I don’t think that’s you … but I felt as difficult as this might be from a triallist’s perspective my, my urge or plea would be really trying to think is there a way that we could find people, erm, who would be willing to talk about their own account.
Emma
the activity where you had to challenge … your thoughts … like both were generalisations or catastrophizing … And it felt a little bit like, it apply to [my trauma experience] …
Clare
Pace and length of treatment
-
– Facilitators
I think the pace was, erm okay … It works for me and it worked out perfect on you know the timescale of it and the week on, week off sort of thing.
Mike
-
– Barriers
Yeah, it’s just not long enough for me and I understand that some people it could be long enough for them, but like I said I can’t … all my case, all my things erm when they do like a scoring at the end, the end of each, err thing, I wasn’t really getting any better.
Mike
I think being warned you know, being warned that it was so intensive that it has to be that way you know, if it was stretched over you know a few extra weeks or something or …
Becky
… if it could be tweaked in any way I would say a bit more face-to-face contact before jumping into it, perhaps.
William
I think it maybe needed to be a bit, maybe a little bit more one to one as well, but I think the time that I should’ve had, an hour every other week and a five minute phone call every week or whatever it was, would’ve been sufficient you know …
Becky
Effects and outcomes
-
– Facilitators
I think you know now my opinion is it’s you know, lots of different people can have it in varying stages and people can have it severely from something really minor and people can you know go through horrible things and only have a small bit of it.
Becky
You tend to, to think of PTSD with serving erm soldiers … and of course it, it affects every one of us er in life that goes through a trauma. So yes it did make me sit up and, and think about that and recognising it in people … But when traumas do come along in all shapes and sizes then erm the way it affects the brain is, is actually quite a shock and having experienced it … it was quite a shock to, to realise just how, what trauma does do to the brain.
Kay
it was basically my mind, erm reacting to what happened and not being able to process it essentially …
Ellen
now I’m out of the other side of it my quality of life is definitely better … Erm, a lot less symptomatic.
Ellen
So I think that really, really helped me feel less guilty about it … [Pause] erm, well I realised that I was doing some things that I hadn’t realised I was doing and that some things were affecting me and I hadn’t even realised or I was you know, I think I definitely changed my behaviours towards certain things because I realised that I was making myself worse and that I didn’t need to do certain things and you know using a lot of the techniques and stuff to help me you know, relax and stuff.
Becky
There were points that was good for me but I would say that biggest plus for me was the understanding of it … and [therapist] sort of convinced me to go and face my fears and go and see the boys and.. erm and I managed to do that so that was a big plus.
Mike
Erm, well I mean it was, it was really interesting and I think a lot of it was really, really useful.
Becky
can I also put-on record, I mean I don’t know how, erm, whether this would be, be sort of taken or how, how this may be used but, erm, I feel so passionate about the benefit that I received and you know, I’m aware that I picked up and helpfully critiqued some aspects of, of the programme, erm, but I, I’d really be happy to be an advocate for this.
Emma
Use of technology
-
– Facilitators
I would do breathing exercises on the, from the App first.
Luke
Erm, no, but technologically wise I felt that the app was, was very good.
Emma
the cognitive therapy part, when the, they used the um, responsibility pie chart, I thought that worked very well on the mobile that way.
Christian
The programme itself was easy, because you just clicked on and then you could see what they were doing. Things like, there were times where you actually read their narratives … I remember doing that. And it was … As long as they … And they don’t even have to be that tech savvy, you know? And once you get … Once … No, that was easy … I’m not going to say any more; it was easy.
Meg
if they were kind of … accessing their computer more regularly anyway … perhaps that made if a little bit easier than, erm, perhaps for people who don’t often use the computer in their lives …
Jenny
I had one person actually, the guided self help, which worked … He was actually a student, because they were … they got … they were young as well, and they … they were used to using electronics, it was you know, they just said it was just a part of their life, so it was just what they would expect, having a phone in their hand and their way. But that’s just an observation.
Gavin
-
– Barriers
Yeah um so a couple of times um, when I would like load the programme, the tool err box at the bottom or the toolbelt, um at the bottom wouldn’t load um … So it’d take a couple of times with me, just like the exiting out of my internet browser and then loading it again.
Clare
Eventually, yes, it took some, it took some getting on the phone, it didn’t want to, it didn’t want to load on my phone but the boffins at your end sorted that out.
Stuart
at the beginning it felt a bit challenging because … . It felt a very different way of working.
Jenny
Contextual factors
-
– Facilitators
Yeah, yeah. Someone to talk to. To me. That, that, that’s what … that’s what helps me.
Mike
My wife is my best friend but I just … certain things you can’t talk about you know?
Mike
Um, just, just you know, to get that off my chest for people, like for someone that’s impartial, someone’s that’s not going to give me an ultimatum.
Matilda
That’s not what it’s about, what you need is somebody that is an ear for your problems … and can guide you in the right direction but not compete with you and I think that that’s a necessary part of getting over PTSD.
Kay
I want someone to listen to me rambling on … Pick the bones out of it and tell me what I should be looking out for’.
Stuart
‘um well, a bit dubious you know.
Miriam
I just thought I was quite sceptical of it and thought no one’s going to be able to help me because I can’t help myself.
Sheila
-
– Barriers
is there a way that we could find people, erm, who would be willing to talk about their own account.
Emma
I think if there was a bit more er, tweaks here and there, regarding with the add ins, cater for the … maybe expanding on er, reclaiming life and the narrative bit in the step six, then um, yeah. And I can’t see why not.
Gavin
| Annual probability of relapse after GSH | Total cost of GSH and health care use | Total cost of GSH therapy only |
|---|---|---|
| 3% | £1526 | £354 |
| 5% | £1649 | £402 |
| 7% | £1766 | £447 |
| 9% | £1875 | £489 |
| 10% | £1928 | £509 |
| 15% | £2164 | £600 |
| 20% | £2364 | £677 |
| 25% | £2531 | £741 |
List of abbreviations
- ANCOVA
- analysis of covariance
- ARM-5
- Agnew Relationship Measure
- AUDIT-O
- Alcohol Use Disorders Test
- BIA
- budget impact analysis
- CACE
- complier average causal effect
- CAPS-5
- Clinician-Administered PTSD Scale for DSM-5
- CASP
- Critical Appraisal Skills Programme
- CBT-TF
- cognitive behavioural therapies with a trauma focus
- CCBT
- computerised cognitive behavioural therapy
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curves
- CI
- confidence interval
- CRF
- case report forms
- CSQ-8
- Client Satisfaction Questionnaire
- CSRI
- Client Services Receipt Inventory
- CT-PTSD
- cognitive therapy for PTSD
- CTR
- Centre for Trials Research
- CUA
- cost–utility analysis
- DSM-5
- Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- EMDR
- eye movement desensitisation and reprocessing
- GAD-7
- Generalized Anxiety Disorder-7
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GSES
- General Self-Efficacy Scale
- GSH
- guided self-help
- HE
- health economic
- IAPT
- Improving Access to Psychological Therapies
- ICER
- incremental cost-effectiveness ratio
- ICH E9
- International Conference on Harmonisation Harmonised Tripartite Guideline
- IES-R
- Impact of Event Scale-Revised
- ISI
- Insomnia Severity Index
- IQR
- interquartile range
- ISTSS
- International Society for Traumatic Stress Studies
- ITT
- intention to treat
- LEC
- Life Events Checklist
- MRC
- Medical Research Council
- MSPSS
- Multidimensional Scale for Perceived Social Support
- NHS
- National Health Service
- NI
- non-inferiority
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- ONS
- Office for National Statistics
- PHQ-9
- Patient Health Questionnaire
- PID
- participant identifier
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- PTCI
- Post-Traumatic Cognitions Inventory
- PTSD
- post-traumatic stress disorder
- QALY
- quality-adjusted life-year
- RCT
- randomised control trial
- SA
- sensitivity analysis
- SAP
- statistical analysis plan
- SD
- standard deviation
- TFPT
- trauma-focused psychological treatments
- WSAS
- Work and Social Adjustment Scale
