Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number NIHR129784. The contractual start date was in March 2020. The draft report began editorial review in April 2021 and was accepted for publication in June 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Beyer et al. This work was produced by Beyer et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Beyer et al.
Chapter 1 Background
Description of hepatobiliary cancers
The majority of malignant obstructions of the bile duct are caused by a variety of cancers, including ampullary carcinoma, cholangiocarcinoma, adenocarcinoma of the pancreatic head and carcinoma of the gall bladder, which are inoperable in the majority of scenarios (e.g. < 30% of cholangiocarcinomas and 20% of pancreatic carcinomas are resectable at the time of diagnosis). 1 Furthermore, evidence that the incidence of gall bladder cancer and cholangiocarcinoma is increasing in the Western world and globally. 2,3
Despite years of research, survival in this group of patients continues to be poor, even with chemotherapy and/or radiotherapy and, therefore, palliation of symptoms becomes a key aspect of therapy. 4
Description of current service provision
In patients with inoperable disease, current standard of care involves the insertion of one or more stents during endoscopic retrograde cholangiopancreatography (ERCP), which restores bile flow, alleviating symptoms associated with obstructive jaundice. 5 Around 60,000 ERCPs are performed in the UK per annum, with about 20% being for malignant biliary obstruction. Metal stents are preferred over plastic stents because they remain patent longer. 6 Metal stents remain patent for an average period of about 6–9 months, after which repeat intervention may be necessary. 7 Metal stents necessitate repeated hospital admissions, cause considerable morbidity and expose the patient to further procedure related risks. Efforts have been ongoing to develop adjunctive interventions for improving the patency period of metallic biliary stents. 4 Some interventions that have been studied include photodynamic therapy and intraductal radiotherapy; however, there are many drawbacks to these treatments, and they are usually delivered in multiple sessions. 8
Description of radiofrequency ablation
Delivery of radiofrequency ablation (RFA) in the bile duct has emerged as a promising modality in the last few years. 9 RFA produces coagulative necrosis of tissue and, therefore, reduces tumour volume in the bile duct. RFA has been used both prior to placing biliary stent (i.e. primary RFA) and for management of blocked biliary stents (i.e. secondary RFA) in malignant bile duct obstruction. 9,10 RFA is part of standard care in the treatment of hepatocellular cancer or liver tumours that are unsuitable for resection (including metastatic liver tumours, oesophageal tumours and colorectal cancers). 11 As overall survival in pancreatic and biliary cancers is poor, additional treatments are urgently needed.
Primary RFA delivered at the time of stent insertion is technically straightforward to perform, and feasibility studies have already shown high levels of technical success. 9,10 If primary RFA can improve survival and duration of stent patency, then this has the potential to reduce the rate of repeated admissions and interventions and could conceivably lead to improvements in quality of life in people with unresectable disease.
Secondary RFA is employed in the management of occluded metal stents to treat the cancerous tissue that has grown back into the lumen, causing recurrent obstructive jaundice and often infection (cholangitis). This is often an emergency situation and patients may take several weeks to recover from such an event. In addition, because of the recurrent jaundice, patients may not be able to receive chemotherapy, which may further adversely affect their outcome. 7
There are two commercially available RFA probes that can be used during ERCP, both of which come at additional cost on top of that of standard care. The two RFA probes have slightly different characteristics and, therefore, may not deliver the same outcomes for patients. Furthermore, there have been case reports of adverse events (AEs) occurring in patients undergoing biliary RFA but it is difficult to ascertain whether this is in excess to that expected from standard care at ERCP.
Primary radiofrequency ablation
Initial investigation of RFA delivered at the time of ERCP has shown that this is a technically feasible adjunct with acceptable safety and stent patency rates at 90 days. 9 Two studies9,10 have suggested that RFA prior to stent insertion may confer a doubling in overall survival. The studies,9,10 however, are small single-centre studies that are not randomised and, therefore, are not of sufficient quality to change clinical practice. Many of the data have arisen from retrospective analysis of clinical usage and primarily in patients with cholangiocarcinoma. 12 Review of the previous studies in this area with respect to size, trial design, control group selection and outcomes reveals considerable heterogeneity and a lack of high-quality study design. Only two9,13 of the studies have been of prospective design and only four studies14–17 used a control group. Some studies14–16 used historic controls, and one study17 used the Surveillance, Epidemiology, and End Results database. Given the poor survival of most patients with pancreatic and biliary cancers, more information is urgently required concerning RFA, particularly with reference to any survival benefits, AEs and effects on quality of life. Pilot data from two UK centres (Aberdeen Royal Infirmary and the Hammersmith Hospital) have shown that delivery of RFA during ERCP has a high technical success rate and a low AE rate, and suggests overall improvement in survival. 9,10 The addition of RFA was also acceptable to patients during ERCP. How RFA leads to such effects is not fully understood. It is thought that RFA causes tissue necrosis and increases the diameter of biliary strictures. Drainage and stent insertion may be aided by this. 13,16 Previously noted increased survival times cannot be attributed to this mechanism alone. Rat models of metastatic colorectal cancer have shown that antigen release after RFA can lead to antitumour immunity against hepatocellular carcinoma. 18 The technical feasibility, safety and efficacy of primary RFA have been confirmed; however, very few prospective randomised studies exist.
Secondary radiofrequency ablation
With respect to treatment of tumour ingrowth and subsequent occlusion of biliary metal stents, there are several case series demonstrating technical feasibility and safety of RFA in this setting. 19 Data from Newcastle have shown that RFA significantly increases the stricture diameter, allowing for better flow. 20 However, similar to primary RFA, many of the data have been derived from small single-centre retrospective studies with heterogeneous cohorts and often without suitable control groups. One study19 examined secondary RFA purely in patients with occluded metal stents and matched to control subjects in whom plastic stents were inserted across the occluded metal stent. The study19 found improved stent patency at 90 days and longer overall stent patency, but did not report survival in the two groups. Secondary RFA may improve stent patency and time to further intervention, but overall survival has not been well studied. Indeed, this is likely to be difficult, as, in contrast to patients treated with primary RFA (delivered prior to stent insertion), in patients undergoing secondary RFA (delivered within a previously placed stent), the period since diagnosis of the malignancy will generally be longer, and such patients are therefore likely to have more advanced tumours. There is also the question as to whether or not a further stent (and, therefore, additional time and cost) is required following secondary RFA, as the rates of stent reintervention in current studies appears to vary.
Rationale
Although there appears to be a suggestion from some studies13,44 that primary RFA may improve survival, it is currently unclear if this is cost-effective or associated with an increased AE rate. In addition, true impact on quality of life is not known. For secondary RFA, there is a suggestion of improving stent patency duration, but, again, cost-effectiveness, AE rates and quality of life have not been well studied. This evidence synthesis will evaluate the existing data with respect to these outcomes to determine if there is sufficient evidence for RFA in these circumstances or if further research, and its directions, are required.
Aims and objectives
The aim of this research was to establish the expected value of undertaking additional research to determine the clinical effectiveness, cost-effectiveness and safety of endoscopic bipolar radiofrequency interventions for the treatment of malignant biliary obstruction.
The key objectives were as follows:
-
To undertake a systematic review assessing the clinical effectiveness and potential risks of RFA in patients with malignant biliary obstruction (see Chapter 2).
-
To undertake a second systematic review assessing the cost-effectiveness of RFA in patients with malignant biliary obstruction (see Chapter 3).
-
To develop a decision model to estimate cost-effectiveness based on the data derived from the systematic reviews (see Chapter 6).
-
To assess the value of further research by undertaking a value of information analysis from the data and results generated by the decision model (see Chapter 7).
Chapter 2 Methods of clinical effectiveness review
Arobust systematic review was carried out in accordance with the methods outlined in guidance from the Centre for Reviews and Dissemination (CRD). 21 A protocol was developed and signed off by the project team and Clinical Advisory Board. The review was registered on PROSPERO (reference CRD42020170233) and was reported in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and SwiM (Synthesis Without Meta-analysis) guidance. 1,22 The review aimed to evaluate the impact of RFA, compared with inserting a stent without RFA, on survival, quality of life and AEs. Two patient and public involvement (PPI) colleagues were members of the Clinical Advisory Board (including author LC). The two PPI colleagues contributed to the design of the protocol, in particular helping to identify and prespecify patient-related outcomes that were subsequently reported as an important gap in the literature. In addition the PPI colleagues also contributed to interpretation of results, writing of the Plain English summary and the final report.
Search strategy
An experienced information specialist designed the search in MEDLINE in collaboration with the project team. The search used the following concepts:
-
population: people with cancer that could cause biliary obstruction
-
intervention: endoscopic biliary RFA.
The search was designed using database thesaurus headings and keywords, and the strategy was translated as appropriate to other databases. An example of the full search strategy can be found in Appendix 1.
As the intervention was not available prior to 2008, the search dates were restricted from 2008 to present. No other limits or restrictions were applied to the search. All search results were downloaded to EndNote (Clarivate Analytics, Philadelphia, PA, USA) and de-duplicated.
Update searches were restricted to bibliographic databases and de-duplicated against the primary search results.
Bibliographic databases
-
MEDLINE (OVID), 1946 to May 19 2020 (searched 20 May 2020, updated search 21 January 2021).
-
EMBASE (OVID), 1996 to 2020 week 20 (searched 20 May 2020, updated search 21 January 2021).
-
The Cochrane Library (Wiley) (searched 20 May 2020, updated search 21 January 2021):
-
Cochrane Database of Systematic Reviews
-
Cochrane Central Register of Controlled Trials
-
Cochrane Clinical Answers.
-
-
Scopus (searched 22 May 2020, updated search 21 January 2021).
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost) (searched 20 May 2020, updated search 21 January 2021).
-
Health Technology Assessment database (CRD) (searched 22 May 2020).
-
Database of Abstracts of Reviews of Effects (CRD) (searched 22 May 2020).
Grey literature databases
-
OpenGrey (searched 12 June 2020).
-
Web of Science Conference Proceedings Index (searched 17 June 2020).
Specific websites
-
Royal College of Surgeons.
-
Health Management Information Consortium.
-
Annual conference meetings:
-
digestive disease week (accessed 12 June 2020).
-
united European gastroenterology week (accessed 12 June 2020)
-
International Digestive Endoscopy Network (accessed 12 June 2020)
-
the British Society of Gastroenterology (accessed 12 June 2020).
-
Trial registries
A range of trials registers were searched to ensure that international trials were identified:
-
ClinicalTrials.gov (accessed 17 June 2020)
-
European Union Drug Regulating Authorities Clinical Trials (accessed 17th June 2020)
-
International Standard Randomised Control Trials Number registry (accessed 12 June 2020)
-
International Conference on Harmonization in Good Clinical Practice (accessed 17 June 2020)
-
Korean Clinical Research Information Service (accessed 12 June 2020)
-
National Institute of Public Health Japan Primary Registry Network (accessed 12 June 2020)
-
Thai Clinical Trials Registry (accessed 12 June 2020).
Reference lists/hand-searching
The references of included studies and relevant systematic reviews were checked for eligible studies potentially missed in the search.
As the intervention was not available prior to 2008, the search dates were restricted from 2008 to present. All search results were downloaded to EndNote and de-duplicated.
Inclusion and exclusion criteria
Population
Studies that recruited the following types of patients were included:
-
patients with biliary obstruction caused by any form of unresectable malignancy who were ineligible for surgical resection (malignancies could include cancer of the pancreas, bile duct, gall bladder and duodenum, and also ampullary and metastatic cancers)
-
patients undergoing a first procedure or patients with recurrent obstruction of a previously inserted stent
-
adult patients aged ≥ 18 years
-
patients with either first diagnosis or previous history of cancer, including patients receiving ongoing treatment
-
patients with underlying health issues, such as diabetes or asthma.
Studies that recruited the following types of patients were excluded:
-
Patients with benign biliary obstruction (studies with patients presenting with both benign and malignant strictures were included if the malignant data were reported separately)
-
Patients with hepatocellular cancer or liver tumours, unless there was also biliary obstruction.
Interventions and comparators
Interventions
Endoscopic biliary RFA used to ablate malignant tissue that obstructed the bile or pancreatic ducts, either to fit a stent (metal or plastic) or to clear obstructed stents.
Studies that used RFA that was not endoscopic were excluded.
Comparators
Comparators include insertion of a stent to clear the bile or pancreatic duct or standard care of patients with an occluded stent. ‘Standard care’ was deemed likely to be different between different countries and at different time points (e.g. ‘standard’ types of chemotherapy would be different now from types of chemotherapy 10 years ago, even in the same hospital). Where detail was available about what was provided as ‘standard care’, this was extracted.
Outcomes
Outcomes were defined in consultation with clinician and PPI colleagues during the first Clinical Advisory Group meeting. Studies that reported any of the following primary outcomes were included:
-
survival
-
quality of life
-
procedure-related AEs (e.g. bleeding, perforation, liver infarction, infection, pancreatitis, cholangitis or biliary leakage).
Studies were combined in a meta-analysis only if outcome measures matched; otherwise the studies were included in the narrative synthesis. Secondary outcomes included technical success, relief of biliary obstruction, pain, nausea, resource use, number of further interventions, length of hospital stays and reintervention and re-admission rates.
Study design
Scoping had uncovered a limited and heterogeneous literature, and so we considered all articles except editorials, letters and opinion pieces to make the most use of available data. Studies reported in abstract form were considered for inclusion if sufficient data were available to extract.
Data collection
Selection of studies
Two reviewers (FB, JL, NOC, HOK, GOL or MS) independently screened the title and abstracts of the studies retrieved by the search in Rayyan (Doha, Qatar), a software designed to aid screening of results for systematic reviews. 23 A set of 253 records were pilot screened, and reviewers met to resolve disagreements and to clarify eligibility criteria. For studies deemed eligible, or where it was impossible to decide eligibility from the abstract, the full text was retrieved, and two reviewers independently assessed the full text for inclusion. Any disagreements were resolved through discussion or by reference to a third reviewer or the Clinical Advisory Board.
Data extraction
Data were extracted by one reviewer and checked by a second reviewer, and, when required, discrepancies were resolved by consultation with a third reviewer. Where studies were reported in multiple publications, relevant data were extracted from all publications, but they were considered as one study. Where data were missing or unclear, authors were contacted to request details or clarification.
For the effectiveness review, we extracted the following data from included studies:
-
citation information
-
study design
-
participant characteristics (e.g. diagnosis, source and extent of obstruction, new or existing stent, disease stage, age, other relevant treatments, clinical measurements that are proposed as a mechanism of action of the RFA)
-
intervention characteristics (e.g. type of stent, RFA settings used, duration of ablation, type of probe used, detail of proposed mechanism of action)
-
comparator characteristics (e.g. type of stent, alternative treatment details, details of ‘standard care’ provision)
-
primary outcomes [e.g. survival, relief of biliary obstruction, time to occlusion or reocclusion, AE details (quantitative or qualitative)]
-
secondary outcomes, where reported (e.g. technical success, relief of biliary obstruction, pain, nausea, resource use, number of further interventions, length of hospital stays, reintervention and readmission rates)
-
carer perspectives, where available (e.g. personal costs in terms of personal and physical health, well-being, financial impacts of the disease on patients and carers)
-
details of study methods to facilitate an assessment of risk of bias.
Risk-of-bias assessment of included studies
Risk-of-bias assessment was conducted by two reviewers independently at a study level using the following tools, according to study design:
-
Randomised controlled trials (RCTs) were assessed using the Cochrane Risk of Bias 2.0 tool.24 Domains under consideration included risk of bias arising from the randomisation process, from deviations from intended assignment to interventions, from missing outcome data and the way the outcome was measured, and in selection of the reported result.
-
Non-randomised controlled studies were assessed using criteria based on the ROBINS-I (Risk Of Bias In Non-randomized Studies – of Interventions) tool.25 Domains under consideration included risk of bias arising from confounders, from selection of participants into the study, from classification of and deviation from interventions, from missing outcome data, and in selection of the reported result.
-
Uncontrolled studies were not formally assessed using a specific tool.
-
Studies published only as abstracts were not formally assessed for risk of bias, as there was a risk that brevity of reporting would confound the assessment.
Disagreements were resolved by the two reviewers or in team discussions.
Data analysis
In the first instance, data were presented as study characteristics, results and risk-of-bias assessments in a series of structured tables to give a clear picture of the available evidence.
For the clinical effectiveness synthesis, controlled studies were prioritised. The primary analysis estimated the hazard ratio (HR) of mortality using a random-effects generic inverse variance model, with separate analyses for primary and secondary RFA. All meta-analyses were conducted using RevMan software (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). Chemotherapy was identified as a key confounding factor. The base-case analysis was restricted to full-text papers and studies that were RCTs or were non-randomised controlled trials that adjusted for chemotherapy treatment if the study included some patients receiving chemotherapy treatment, as these were considered better-quality studies. The result of the analysis was included in the economic model for patients not receiving chemotherapy treatment, as the economic model distinguished between patients receiving chemotherapy treatment and patients not receiving chemotherapy treatment. Results from conference abstracts and non-randomised studies that did not adjust for chemotherapy but did include chemotherapy patients were included in a sensitivity analysis.
Adverse events were analysed using an exploratory approach, utilising all reported AEs so as not to bias the results of the review with the author preconceptions. AE data were pooled in a random-effects meta-analysis using Mantel–Haenszel weighting. Heterogeneity between studies was assessed by visual inspection of plots of the data, from the chi-squared test for heterogeneity and the I2-statistic. Possible reasons for heterogeneity were explored where possible, such as differences in the populations studied (e.g. concomitant treatments, cancer type and stage), the interventions (whether patients were receiving primary RFA with a stent being newly inserted or secondary RFA to unblock an existing stent), the detail of ‘standard care’ provided and the way in which the outcomes were assessed.
A sensitivity analysis included studies that were reported in conference abstracts because there were usually insufficient data to fully assess the risk of bias in the studies. Subgroup analyses were also planned according to the type of probe, stent (i.e. metal or plastic) and cancer. However, during the review, it became clear that there were insufficient data to carry out these subgroup analyses.
Where there were insufficient data or it was inappropriate to pool data because of differences between studies in comparisons or reported outcomes, a narrative synthesis of the data was provided, structured by outcome. The effectiveness estimates fed into the economic model.
Meta-analyses were conducted with and without adjustment for bias. On average, the characteristics of participants (e.g. average age, severity of disease and whether or not people receive adjuvant treatment in each group) should be similar in both arms of a RCT because of the randomisation process. Conversely, in a non-randomised study, it is useful to adjust for the potential differences between groups that may occur in the absence of randomisation.
The key confounding factor, raised in the initial Clinical Advisory Board meeting, was whether or not patients received chemotherapy, as this has its own impact on survival. Non-randomised studies were included in the primary meta-analyses only if they had adjusted for the chemotherapy given to patients or if no or similar chemotherapy was received by patients in each group.
Chapter 3 Methods of cost-effectiveness review
A second systematic review was planned, looking at economic evaluations of RFA for malignant biliary obstruction. Searches and screening were carried out as described below, but no studies were found for inclusion.
Search strategy
An experienced information specialist designed the search in MEDLINE in collaboration with the project team. The search used the following concepts:
-
Population: people with cancer that could cause biliary obstruction.
-
Intervention: endoscopic biliary RFA.
Bibliographic databases
-
MEDLINE (OVID), 1946 to 19 May 2020 (searched 20 May 2020).
-
EMBASE (OVID), 1996 to week 20 2020 (searched 20 May 2020).
-
Scopus (searched 22 May 2020).
-
CINAHL (EBSCOhost) (searched 20 May 2020).
-
NHS Economic Evaluation Database (NHS EED) (CRD) (searched 22 May 2020).
The search was designed using database thesaurus headings and keywords. The strategy was translated, as appropriate, to other databases. An example of the full search strategy can be found in Appendix 2.
Grey literature databases
-
Web of Science Conference Proceedings Index (accessed 17 June 2020).
-
Cost-Effectiveness Analysis Registry (accessed 12 June 2020).
-
DEAS (Research Papers in Economics) database (accessed 12 June 2020).
Reference lists/hand-searching
References were checked from previous relevant systematic reviews.
An economic study filter was applied (NHS EED, MEDLINE using OvidSp) and the search was restricted from 2008 (as the intervention was not available prior to 2008). No other limits or restrictions were applied.
All search results were downloaded to EndNote and de-duplicated.
Inclusion and exclusion criteria
Population
Studies had to include patients with biliary obstruction caused by any form of unresectable malignancy who were ineligible for surgical resection (see Chapter 2, Population, for further details).
Interventions
Studies were included where endoscopic biliary RFA was used to ablate malignant tissue that obstructed the bile or pancreatic ducts, either to fit a stent (metal or plastic) or to clear obstructed stents (see Chapter 2, Interventions and comparators, for further details).
Outcomes
The aim was to include full economic evaluations, including trial- and model-based evaluations. No restrictions were imposed on the type of economic evaluation (i.e. cost-effectiveness analyses, cost–utility analyses, cost–benefit analyses, cost–consequences analyses), as long as the studies fitted the Drummond et al. 26 definition of a full economic evaluation (i.e. a comparative analysis of alternative courses of action in terms of costs and consequences).
Data collection
Selection of studies
Two reviewers (FB and GOL) independently screened the title and abstracts of the studies retrieved by the search in Rayyan. 23 For studies deemed eligible or where it was impossible to decide eligibility from the abstract, the full text was retrieved and two reviewers independently assessed the full text for inclusion.
Data extraction
We planned to extract the following data from included studies using a standardised data extraction form:
-
citation information
-
study design
-
participant characteristics (e.g. diagnosis, source and extent of obstruction, new or existing stent, disease stage, age, other relevant treatments, clinical measurements that are proposed as a mechanism of action of the RFA)
-
intervention characteristics (e.g. type of stent, RFA settings used, duration of ablation, type of probe used, detail of proposed mechanism of action)
-
comparator characteristics (e.g. type of stent, alternative treatment details, details of ‘standard care’ provision)
-
primary outcomes [e.g. survival, relief of biliary obstruction, time to occlusion or reocclusion, AE details (quantitative or qualitative)]
-
method of economic evaluation
-
principal study findings.
Risk-of-bias assessment of included studies
We planned to use the Drummond et al. 26 checklist to assess the risk of bias in the included economic evaluations.
Data synthesis
We planned to assess the transferability of the included evaluations and to carry out a narrative synthesis.
Chapter 4 Results of clinical effectiveness review
The search retrieved 4131 results after de-duplication and update searches retrieved a further 287 de-duplicated results, giving a total of 4418 results. After title and abstract screening, a total of 697 results were deemed potentially eligible for inclusion.
EndNote was used to assist the full-text screening of 697 records. All records were screened in duplicate by independent reviewers, blinded to each other’s decisions. After removal of the blind, conflicting decisions were resolved by discussion or by a third reviewer if an agreement could not be reached (see Appendix 3 for excluded studies).
Characteristics of included studies
Following eligibility assessment, 68 studies were included in this review (Figure 1, and see Appendix 4).
FIGURE 1.
Flow of studies through the effectiveness review. Reproduced with permission from Moher et al. 116 This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The figure above includes minor additions and formatting changes to the original figure.
Less than half (n = 32, 47%) of the studies were reported as peer-reviewed published articles, and the rest (n = 36, 53%) were available only as conference abstracts. In total, there were 18 comparative and 50 non-comparative studies, with a total of 1742 patients (plus one study that did not report numbers). The studies were conducted in Asia (n = 24), European countries (n = 20), the USA (n = 20), South America (n = 2) and Australia (n = 2) (Table 1). Most patients had biliary obstruction arising from cholangiocarcinoma (where reported). The most commonly reported probe used for the ablation procedure was the Habib™ EndoHPB27 (Boston Scientific Corporation, Marlborough, MA, USA) (n = 35), although many studies did not report the detail of the equipment used. Studies reported the insertion of a first stent (i.e. primary RFA; n = 40), the unblocking of an existing stent (i.e. secondary RFA; n = 15) or both (n = 11); this aspect was unclear in two studies.
| Study | Paper/abstract | Prospective? | Study design; number of participants | Country | Diagnosis | Primary/secondary RFA | Type of probe |
|---|---|---|---|---|---|---|---|
| Gao et al.28 | Paper | Yes | RCT; 174 | China | Extrahepatic CCA; AC | Primary | Habib EndoHPB |
| Yang et al.29 | Paper | Yes | RCT; 65 | China | Extrahepatic hilar CCA, except Bismuth III–IV | Primary | Habib EndoHPB |
| Hu et al.30 | Abstract | Yes | RCT; 63 | China | Hilar CCA; mid-CBD tumour; AC | Primary | NR |
| Hucl et al.31 | Abstract | Yes | RCT; 31 | Czech Republic | CCA; PC | Primary | NR |
| Teoh et al.32 | Abstract | Yes | RCT; 47 | China | Malignant distal biliary obstruction at least 2 cm away from the hilum | Primary | NR |
| Yang et al.33 | Abstract | Yes | RCT; 59 | China | Extrahepatic CCA | Primary | NR |
| Bokemeyer et al.12 | Paper | No | Case control; 32 | Germany | Hilar CCC; PC; GBC; other malignancy | Primary | NR |
| Kallis et al.14 | Paper | No | Case control; 69 | UK | PC | Primary | Habib EndoHPB |
| Sharaiha et al.16 | Paper | No | Case control; 66 | USA | CCA; PC; GBC; gastric cancer; liver metastases from colon cancer | Primary | Habib EndoHPB |
| Dutta et al.34 | Paper | No | Cohort; 31 | UK | MBO | Primary/secondary | Habib EndoHPB |
| Kadayifci et al.35 | Paper | No | Cohort; 50 | USA | MBO | Secondary | Habib EndoHPB |
| Andalib et al.36 | Abstract | No | Cohort; 406 | USA | CCA | Primary | NR |
| Buerlein et al.37 | Abstract | No | Cohort; 47 | USA | Perihilar CCA | Primary | NR |
| Kallis et al.38 | Abstract | No | Cohort; 24 | UK | CCA; PC | Primary | NR |
| Nair et al.39 | Abstract | Yes | Cohort; unclear | India | Hilar CCA | Primary | TaeWoong RFA catheter (TaeWoong Medical Co., Ltd, Gyeonggi-do, Republic of Korea) |
| Sampath et al.40 | Abstract | No | Cohort; 26 | USA | Unresectable perihilar CCA | Primary | NR |
| Schwarzer et al.41 | Abstract | No | Cohort; 9 | Austria | CCA | Primary | NR |
| Wu et al.42 | Abstract | No | Cohort; 39 | China | Malignant distal biliary obstruction | Secondary | NR |
| Alis et al.43 | Paper | No | Non-comparative; 10 | Turkey | CCA | Primary | NR |
| Dolak et al.44 | Paper | No | Non-comparative; 58 | Austria | Klatskin tumour; distal CCA; pancreatic adenocarcinoma; central HCC; mixed HCC/CCA; GBC; metastatic colorectal cancer | Primary/secondary | Habib EndoHPB |
| Figueroa-Barojas et al.13 | Paper | Yes | Non-comparative; 20 | USA | CCA; PC; intraductal papillary mucinous neoplasm with high grade dysplasia; gastric cancer with metastatic tumour in the bile duct | Primary | Habib EndoHPB |
| Han et al.45 | Paper | Yes | Non-comparative; 21 | Republic of Korea | Combined HCC with bile duct invasion | N/A | StarMed (Intersurgical Ltd, Wokingham UK) |
| Lee et al.46 | Paper | Yes | Non-comparative; 30 | Republic of Korea | CCA; PC; GBC | Primary | ELRA™ (TaeWoong Medical Co., Ltd, Gyeonggi-do, Republic of Korea) |
| Ogura et al.47 | Paper | No | Non-comparative; 12 | Japan | PC; CCA | Primary | Habib EndoHPB |
| Sharaiha et al.17 | Paper | No | Non-comparative; 69 | USA | CCA; PC | Primary | Habib EndoHPB |
| Steel et al.9 | Paper | Yes | Non-comparative; 22 | UK | PC; CCA | Primary/secondary | Habib EndoHPB |
| Tal et al.48 | Paper | No | Non-comparative; 12 | Germany | Malignant bile duct obstruction of the hepatic hilus (Klatskin-like tumours); intrahepatic CCA (Bismuth stage IV); CCA; GBC; metastases of gastric small cell carcinoma | Unclear | Habib EndoHPB |
| Battish et al.49 | Abstract | No | Non-comparative; 19 | USA | CCA; PC; HCC; metastatic colon cancer, pancreatobiliary origin; metastatic breast cancer | Primary/secondary | NR |
| De Nucci et al.50 | Abstract | No | Non-comparative; 6 | Italy | Extrahepatic CCA (with or without ongoing chemotherapy) | Primary | Habib EndoHPB |
| Ermerak et al.51 | Abstract | Yes | Non-comparative; 9 | Australia | MBO | Primary | Habib EndoHPB |
| Han et al.52 | Abstract | No | Non-comparative; 9 | Republic of Korea | Perihilar CCA | Primary | StarMed |
| Hashimoto et al.53 | Abstract | Yes | Non-comparative; 12 | Japan | Malignant hilar biliary obstruction | Primary | NR |
| Kahaleh et al.54 | Abstract | No | Non-comparative; 62 | USA | CCA; PC; GBC; gastric cancer; liver metastasis from colon cancer | Primary/secondary | Habib EndoHPB |
| Kallis et al.55 | Abstract | No | Non-comparative; 11 | UK | PC; CCA; hepatic metastases | Secondary | NR |
| Ribeiro56 | Abstract | No | Non-comparative; 16 | USA | CCA; liver metastases of PC; colon cancer metastasis; GBC; HCC | Primary | Habib EndoHPB |
| Samuel et al.57 | Abstract | No | Non-comparative; 8 | USA | CCA; PC; colon cancer; papillary neoplasm of CBD with type I choledochal cyst | Primary | Habib EndoHPB |
| Saraswat et al.58 | Abstract | No | Non-comparative; 10 | India | GBC; CCA | Primary | Habib EndoHPB |
| Ueno et al.59 | Abstract | No | Non-comparative; 16 | Japan | Malignant biliary stricture | Primary/secondary | NR |
| Laquière et al.60 | Paper | No | Non-comparative; 12 | France | Extrahepatic CCA | Secondary | Habib EndoHPB |
| Martí Romero et al.61 | Paper | No | Non-comparative; 3 | Spain | CCA | Primary | SpyGlass® (Boston Scientific Corporation, Marlborough, MA, USA) |
| Mukund et al.62 | Paper | N | Non-comparative; 2 | India | Rising serum bilirubin and signs of cholangitis secondary to occlusion of MBS | Secondary | NR |
| Nayar et al.20 | Paper | N | Non-comparative; 7 | UK | Pancreaticobiliary cancer; blocked biliary stents | Primary/secondary | ELRA |
| Lewis et al.63 | Abstract | No | Non-comparative; 5 | USA | Primary CCA; biliary implant of colon adenocarcinoma to the left hepatic duct | Primary | Habib EndoHPB |
| Morales et al.64 | Abstract | No | Non-comparative; 10 | Mexico | Malignant biliary stenosis | Primary/secondary | Habib EndoHPB |
| Mukund et al.65 | Abstract | No | Non-comparative; 8 | India | Adenocarcinoma; malignant hilar obstruction | Secondary | Habib EndoHPB |
| Watson and Habr66 | Abstract | No | Non-comparative; 3 | USA | Hilar CCA | Primary | Habib EndoHPB |
| Bastos et al.67 | Paper | No | Case report; 1 | Brazil | CCA | Primary/secondary | Habib EndoHPB |
| Gunasingam et al.68 | Paper | No | Case report; 1 | Australia | CCA | Primary | Habib EndoHPB |
| Han et al.69 | Paper | No | Case report; 1 | Republic of Korea | Advanced hilar CCA | Primary/secondary | ELRA |
| Inoue et al.70 | Paper | No | Case report; 1 | Japan | Obstructive jaundice due to malignant hilar biliary obstruction | Primary | Habib EndoHPB |
| Kruger and Krishna71 | Paper | No | Case report; 1 | USA | Extrahepatic CCA and recurrent malignant biliary strictures | Secondary | Habib EndoHPB |
| Lee et al.72 | Paper | No | Case report; 1 | Republic of Korea | Adenocarcinoma | Primary | StarMed |
| Lorenzo et al.73 | Paper | No | Case report; 1 | France | Stenosis of the main pancreatic duct | Secondary | Habib EndoHPB |
| Lui and Li74 | Paper | No | Case report; 1 | China | CCA | Secondary | NR |
| Mansilla-Vivar et al.75 | Paper | No | Case report; 1 | Spain | Cryptogenic liver cirrhosis; spontaneous bacterial peritonitis; hilar CCA | Primary | ELRA |
| Mok et al.76 | Paper | No | Case report; 1 | USA | CCA | Secondary | NR |
| Monga et al.77 | Paper | No | Case report; 1 | India | CCA; adenocarcinoma | Primary | Habib EndoHPB |
| Ogura et al.78 | Paper | No | Case report; 1 | Japan | CCA with liver metastasis | Primary | Habib EndoHPB |
| Linz et al.79 | Abstract | No | Case report; 1 | USA | Metastatic PC involving the head of the pancreas | Secondary | NR |
| Ludvik et al.80 | Abstract | No | Case report; 1 | USA | Pancreatic adenocarcinoma with numerous metastases | Secondary | NR |
| Morais et al.81 | Abstract | No | Case report; 1 | Portugal | Perihilar CCA | Primary | Habib EndoHPB |
| Musquer et al.82 | Abstract | No | Case report; 1 | France | Peritoneal carcinomatosis | Secondary | Habib EndoHPB |
| Saumoy et al.83 | Abstract | No | Case report; 1 | USA | CCA | Primary | NR |
| Schlosser et al.84 | Abstract | No | Case report; 1 | Switzerland | Klatskin tumour | Secondary | ELRA |
| Sonpal et al.85 | Abstract | No | Case report; 1 | USA | Klatskin tumour | Secondary | Erbe (Erbe Medical UK Ltd, Leeds, UK) |
| Tian et al.86 | Abstract | No | Case report; 1 | China | Periampullary carcinoma | Primary | Habib EndoHPB |
| Tyberg et al.87 | Abstract | No | Case report; 1 | USA | MBS | Primary | Habib EndoHPB |
| Yoon and Brugge88 | Abstract | No | Case report; 1 | Republic of Korea | Malignant occlusion in the common hepatic duct | Primary | Habib EndoHPB |
Risk-of-bias assessment of included studies
Two full-text RCTs were assessed for risk of bias using the Cochrane Risk of Bias 2.0 algorithm. 28,29 One study28 was judged to be at a high risk of bias overall and one study29 was judged to be of ‘some concern’. More detailed results for each domain of the Risk of Bias 2.0 tool can be seen in Figure 2.
FIGURE 2.
Risk-of-bias assessments for RCTs. (a) Risk-of-bias domains; and (b) risk-of-bias assessments.

In the case of Yang et al. ,29 concerns were around the intervention being reported differently in the paper compared with its cited trial registry record. 29 It was not possible to establish why this was the case, however, and, as the paper matched our inclusion criteria, it was included, with concerns raised in the risk-of-bias judgement.
The Gao et al. 28 study was deemed at high risk of bias because patients and clinicians delivering the intervention were not blinded to the intervention received. 28
A total of five full-text non-randomised studies12,14,16,34,35 were assessed for risk of bias (Figure 3). Four12,16,34,35 of these studies were judged to be at moderate risk of bias overall, whereas the study by Kallis et al. 14 was judged to be at low risk.
FIGURE 3.
Risk-of-bias assessments for non-randomised comparative studies. (a) Risk-of-bias domains; and (b) risk-of-bias assessments.

Risk of bias was not formally assessed for studies for which only abstracts were available.
Summary of clinical effectiveness results
Primary outcomes
Survival
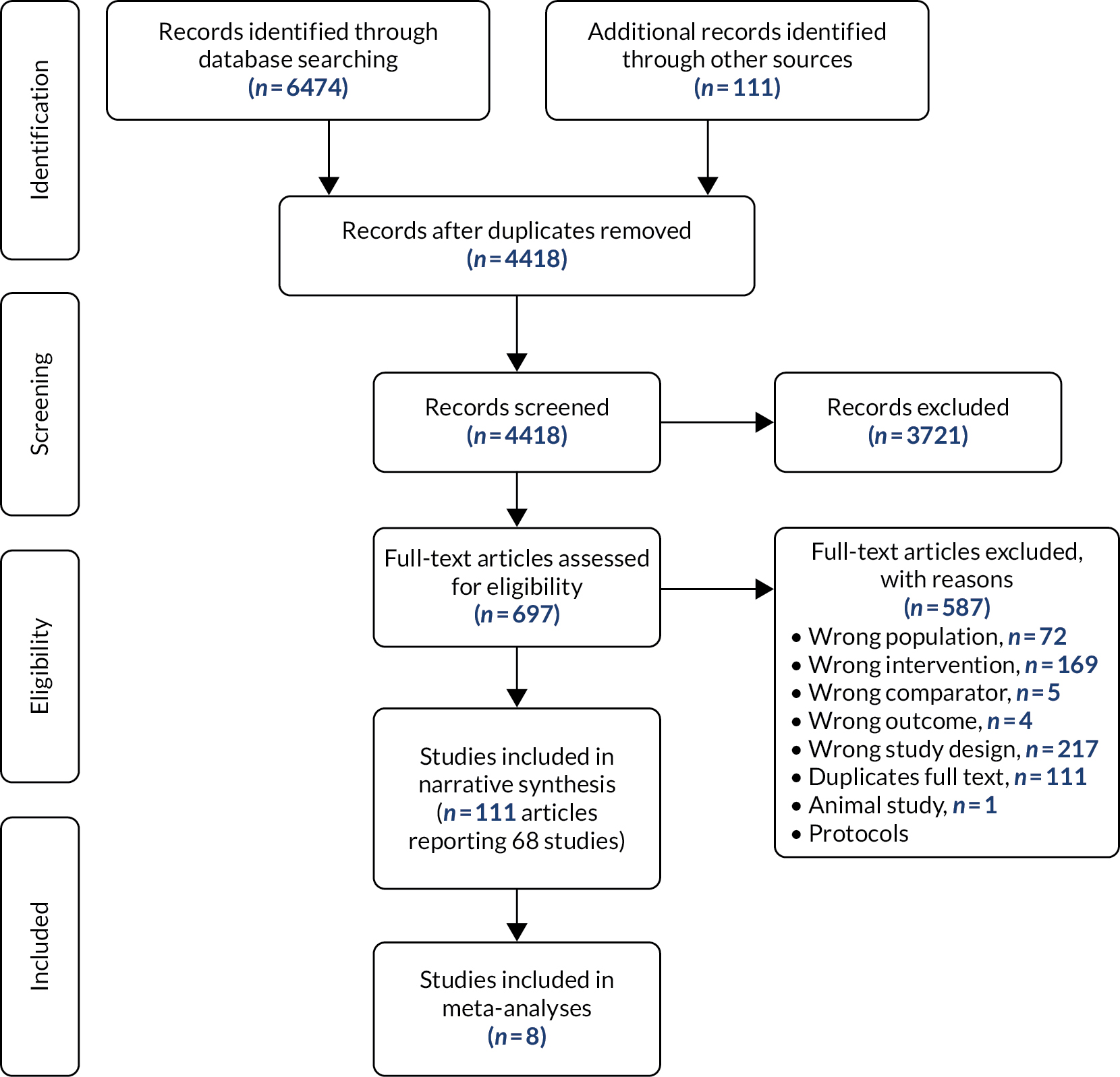
Of the 18 comparative studies, 16 reported a measure of survival. Two RCTs,28,29 one case–control study14 and three cohort studies16,34,37 reported a HR for death for primary RFA compared with stent-only control. Four16,28,29,34 of these (full-text papers and either RCTs or non-randomised controlled studies that adjusted for receiving chemotherapy treatment where necessary) were used for the base-case meta-analysis, two14,37 were used only in a sensitivity analysis (one was an abstract37 and one did not report a Cox proportional regression estimate). 14
The pooled HR in the base-case analysis (336 participants) was 0.34 [95% confidence interval (CI) 0.21 to 0.55], meaning that RFA reduces the hazard of dying by 66%, and this is statistically significant at a 95% level of confidence (Figure 4). There was moderate heterogeneity, indicated by an I2-value of 53%. Heterogeneity was not apparent in the characteristics of the participants where reported (age), but stage of cancer, comorbidities and sex were all poorly reported. The individual study effect sizes were consistently in favour of RFA.
FIGURE 4.
Hazard ratio of mortality, base-case meta-analysis (336 participants). SE, standard error.
The sensitivity analysis that included the studies at higher risk of bias (452 participants) showed a potentially slightly less beneficial effect of RFA, with the pooled HR estimated at 0.39 (95% CI 0.27 to 0.57) and this was statistically significant at a 95% level of confidence (Figure 5). There was moderate heterogeneity indicated by an I2-value of 53%. The effect sizes are consistently in favour of RFA.
FIGURE 5.
Hazard ratio of mortality, subgroup analysis including studies at higher risk of bias (452 participants). SE, standard error.

Of the 12 comparative studies that did not report a HR, most reported mean or median survival times and results were mixed. Of the four prospectively designed studies, two RCTs reported no difference in survival,30,32 whereas one RCT and one prospective cohort study reported significantly prolonged survival in patients who received RFA. 33,39 Seven retrospective comparative studies12,35,36,38,40–42 reported similarly mixed results.
None of the studies that assessed secondary RFA reported HRs, and so this planned meta-analysis was not possible. Two cohort studies35,42 reported survival following secondary RFA, and neither reported a significant difference in survival between groups.
Of the 15 non-comparative studies9,13,17,20,43–48,50,54,60–62 that reported a measure of survival, 11 reported mean or median survival and four reported the proportion of patients who died.
Quality of life
None of the studies reported quality of life using a conventional tool. Two studies28,29 reported Karnofsky Performance Score as a measure of quality of life. Karnofsky Performance Score is designed to measure functional status from a clinician perspective rather than quality of life from a patient perspective. In both studies,28,29 Karnofsky Performance Scores were reported to be significantly higher] (p < 0.001) in the RFA groups up to 9 months after the intervention.
Adverse events
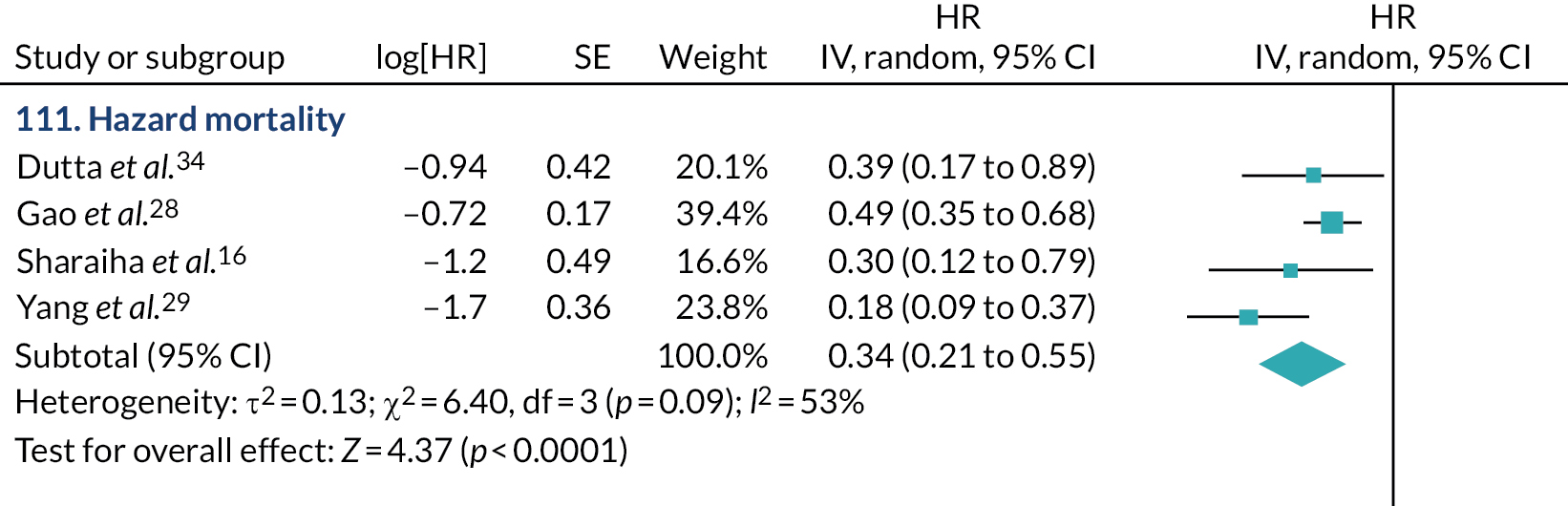
The most commonly reported AEs were cholangitis, pancreatitis and cholecystitis (Figure 6).
FIGURE 6.
Frequency of reported AEs.
Sixteen comparative studies reported AEs. Five studies32,33,36,39,42 reported that there were no statistically significant differences in AEs between groups, but did not specify particular AEs. Three comparative studies reported events in the RFA group only12,38 or events in all participants without specifying whether they were in the control or intervention groups. 16 One study reported that no patients experienced any AEs. 35 Seven studies14,28–31,34,37 specified the number of AEs in both intervention and control arms and were pooled in meta-analyses.
We found 24 non-comparative studies9,13,17,20,43–62 and 14 single case reports68,70–73,76,78–85 that reported AEs.
Cholangitis
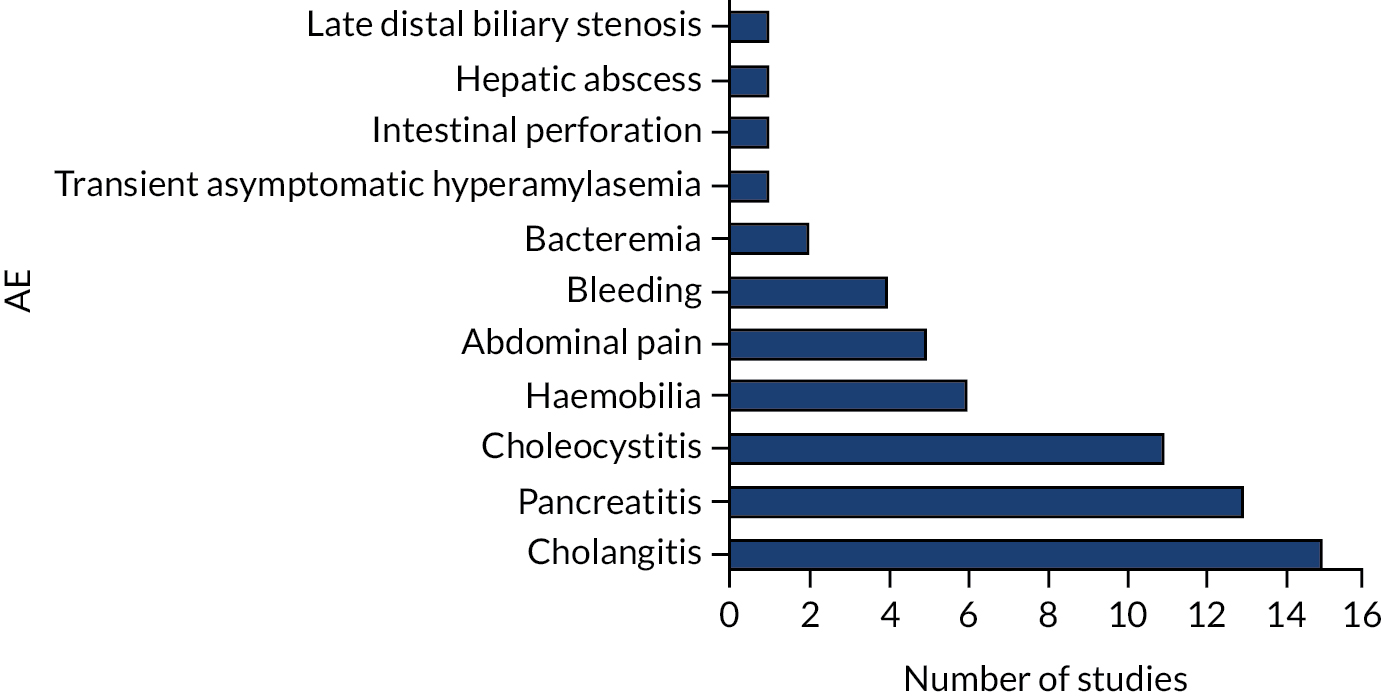
Cholangitis is typically inflammation and fibrosis of the biliary tract, commonly caused by infection. 89 Cholangitis was reported in 15 studies. 12,14,28–30,34,37,44,46–48,52,58–60 Data from five comparative studies14,28–30,34 were pooled in a meta-analysis (Figure 7), which showed no evidence of difference between groups (risk ratio 1.15, 95% CI 0.63 to 2.12). One further study12 reported AEs in the intervention group only.
FIGURE 7.
Risk of cholangitis.
Eight non-comparative studies44,46–48,52,58–60 reported that between 6% and 33% of patients experienced cholangitis.
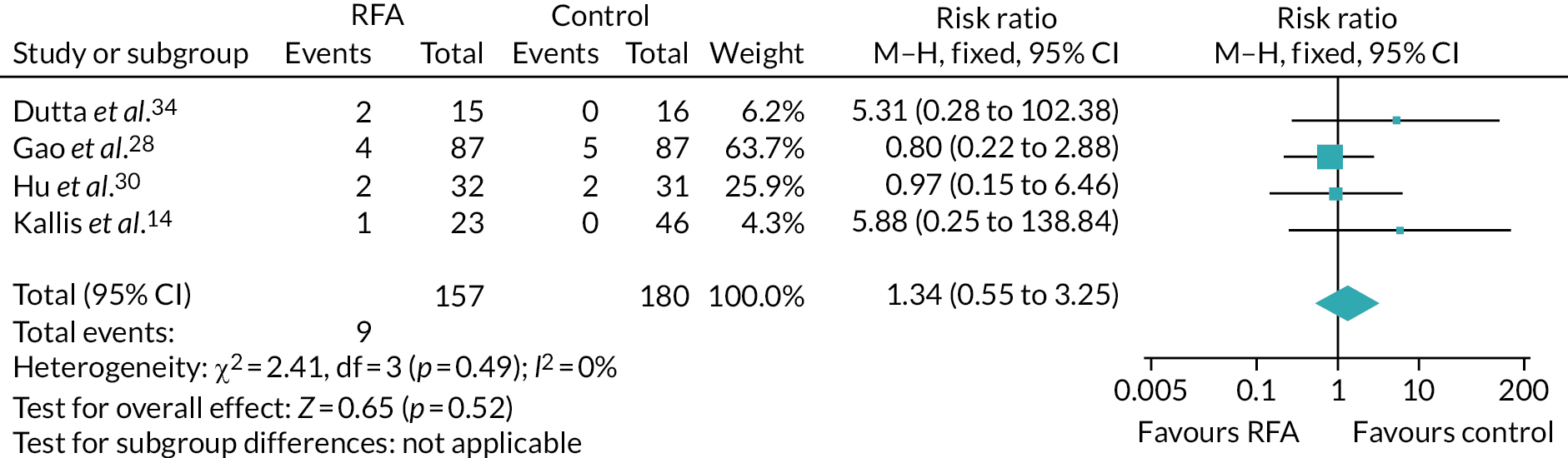
Pancreatitis
Pancreatitis is an inflammation of the pancreas with many aetiologies. 90 Pancreatitis (mild or severe) was reported in 13 studies. 9,12–14,16,17,28,30,34,38,43,46,71 Four comparative studies14,28,30,34 contributed data to a meta-analysis (Figure 8), which showed no evidence of a significant difference between groups (risk ratio 1.34, 95% CI 0.55 to 3.25). Three studies reported AEs in either the intervention group only or across all participants without distinction between the groups. 12,16,38 One study38 reported that the incidence of pancreatitis was similar between groups.
FIGURE 8.
Risk of pancreatitis.
Six observational studies9,13,17,43,46,71 reported that between 4% and 7% of patients experienced pancreatitis.
Cholecystitis
Cholecystitis is an inflammation of the gallbladder, commonly due to a blockage. 91 Eleven studies12,13,16, 17,28,30,31,38,50,52,54 reported incidence of cholecystitis. Three comparative studies28,30,31 contributed data to a meta-analysis. The estimate was very imprecise and none of the studies reported any cholecystitis in control group patients, but it seems likely that RFA carries a higher risk of cholecystitis than stent placement alone (risk ratio 11.47, 95% CI 2.28 to 57.66) (Figure 9). 28,30,31 A further three studies12,16,38 reported cholecystitis in either the intervention group only or in participants from both groups. One study38 reported that the incidence of cholecystitis was similar between groups.
FIGURE 9.
Risk of cholecystitis.

Five non-comparative studies13,17,50,52,54 reported that between 2% and 17% of patients experienced cholecystitis.
Abdominal pain
Two studies17,75 reported that a small number of patients experienced mild, self-limiting abdominal pain after the RFA procedure. One study16 reported a small number of instances of abdominal pain across the intervention and comparator groups, one study58 reported that most (9/10) patients experienced abdominal pain and abdominal pain was also reported in a case study patient. 71
Secondary outcomes
Technical success
Even though the majority of the included studies did not report the ‘technical success’ outcome explicitly, the inference about it was made if study authors reported the RFA procedure as ‘being successful’ or as having ‘no complications’ or ‘no technical problems’, or described it in other similar phrases implying technical success. The vast majority of studies reported 100% technical success. Two studies43,52 explicitly reported a different rate of technical success. One study43 reported that 10 out of 17 RFA procedures were successful. In the remaining cases, RFA was either not attempted or not successful. A second study52 reported that eight out of nine RFA procedures were successful, but ‘functional success was not achieved in one patient’.
Time to occlusion or reocclusion
Occlusion occurs when a stent becomes blocked and reocclusion is when a stent becomes blocked again having been cleared. Two RCTs28,29 reported mean stent patency and were included in a meta-analysis that showed no evidence of improvement in stent patency with primary RFA (Figure 10). In the two studies,28,29 the direction of effect was different and statistical heterogeneity was high, with an I2-value of 79%. The uncertainty in the estimate is consequently very high, and very little can be concluded from this analysis. Two further RCTs30,33 and one comparative cohort study14 also reported no benefit of primary RFA in terms of stent patency. None studies reported mean duration and so this could not be included in the meta-analysis.
FIGURE 10.
Time to occlusion (months).

One cohort study42 and one case–control study35 reported a benefit of secondary RFA compared with stent only in stent patency (median 152 vs. 83 days, p = 0.024; and mean 119.5 vs. 65.3 days, p = 0.01, respectively).
Five non-comparative studies and one case report43,45,47,52,55,86 described different measures of time to occlusion for primary RFA, ranging from 22 months to 23 days.
One cohort study and two case reports60,65,85 described time to reocclusion for secondary RFA, ranging from 2 to 10 months.
Two cohort studies9,59 reported time to occlusion or reocclusion for a population that had received either primary or secondary RFA.
Other secondary outcomes
Very few data were reported about nausea, resource use, number of further interventions, length of hospital stays, and reintervention and re-admission rates.
Chapter 5 Results of cost-effectiveness review
A search retrieved 73 results after de-duplication (see Appendix 5). Rayyan was used to assist title and abstract screening of 73 records. 23 All records were screened in duplicate by independent reviewers, blinded to each other’s decisions. After removal of the blind, conflicting decisions were resolved by discussion or by a third reviewer where an agreement could not be met. A total of 13 results were deemed potentially eligible for inclusion at this stage.
EndNote was used to assist full-text screening of 13 records. All records were screened in duplicate by independent reviewers, blinded to each other’s decisions. After removal of the blind, conflicting decisions were resolved by discussion or by a third reviewer where an agreement could not be met. No records were deemed eligible for inclusion in the review (see Appendices 5 and 6).
Chapter 6 Development of a cost-effectiveness model
The objectives of the economic analysis were to (1) evaluate the cost-effectiveness of endoscopic RFA for the treatment of malignant biliary obstruction and (2) estimate the value of information that may be obtained from conducting future research. RFA alongside stent placement is compared with stent placement alone. Analyses were planned for four populations:
-
patients with pancreatic cancer and receiving primary stent placement
-
patients with pancreatic cancer and receiving secondary stent placement
-
patients with cholangiocarcinoma causing a blockage along the biliary tree and receiving primary stent placement
-
patients with cholangiocarcinoma causing a blockage along the biliary tree and receiving secondary stent placement.
Owing to a lack of effectiveness evidence for secondary stent placement, analyses could be run for primary stent placement only.
As the cost-effectiveness analysis used the effectiveness and AE evidence obtained from the systematic review to inform the consequences of endoscopic RFA compared with no RFA, an economic model was required to estimate the survival for each technology and to estimate the quality of life and cost outcomes over time. The systematic review of economic evaluations found no published model-based economic evaluations of endoscopic RFA interventions. Therefore, a de novo decision-analytic model was developed. The model was developed in TreeAge Pro (TreeAge Software, Inc., Williamstown, MA, USA). The model was probabilistic, meaning that most of the input parameters were entered into the model as probability distributions to reflect parameter uncertainty (i.e. uncertainty in the mean estimates).
The perspective of the economic analyses was the NHS and Personal Social Services perspective. The measure of benefit was quality-adjusted life-years (QALYs) and the time horizon was lifetime. Costs and benefits were discounted at an annual rate of 3.5%.
The effectiveness and adverse effects associated with endoscopic RFA with stent placement compared with stent placement alone were obtained from the systematic review. Value of information analyses estimate the value of reducing or eliminating decision uncertainty.
Key uncertainty in an economic analysis is the uncertainty in the effectiveness estimate. If a study is at risk of bias, there is uncertainty about whether or not the effectiveness estimate accurately estimates the true effect in the study population. The uncertainty associated with bias is not captured in the standard errors (SEs) or CIs of an effectiveness estimate. As the existing clinical studies of endoscopic RFA were anticipated to be at high risk of bias for the study populations, the effectiveness estimates from the clinical studies were adjusted for bias, as described in Chapter 2.
Model structure
A cohort Markov model with time-varying mortality and occlusion probabilities was developed to estimate the cost-effectiveness of endoscopic stent insertion and RFA (primary RFA) in a cohort of patients with unresectable cholangiocarcinoma receiving or not receiving chemotherapy. A cohort Markov model was selected, rather than a discrete event simulation, because (1) the only heterogeneity factor for mortality included was use of chemotherapy, and no evidence for this factor influencing the effectiveness of RFA was available; (2) no time-dependent heterogeneity factors were identified by the clinical advisors; (3) the only time-to-occlusion statistic available was mean time to occlusion for patients who experienced an occlusion, which meant that the simplest method to model this was using a Markov model and checking that the model predictions were reasonably accurate (competing risk could not be explicitly modelled); and (4) the quality of the evidence available meant that the impact of model uncertainties on the results needed to be addressed through scenario analyses, and it is far quicker to run a cohort Markov model than to run a discrete event simulation, especially when conducting expected value of partial perfect information (EVPPI) analyses. A microsimulation state-transition model would be more time-consuming to run than a discrete event simulation.
All patients receive a stent at the start of the model. The difference between the cohorts is whether or not patients also receive RFA. The structure of the Markov model is shown in Figure 11. The ellipses in Figure 11 represent the six states in the Markov model. All patients start in the ‘post initial stent’ state, meaning that patients have had a stent inserted and the occlusion has been cleared. Patients may transition between states at every cycle of the model. A 1-month cycle length was chosen because of the short life expectancy of the patients and the short time to occlusion and reintervention. Considering the short cycle length, a half-cycle correction was not expected to make a significant difference in the results and, hence, was not applied. The arrows in Figure 11 indicate the state to which patients may transition during a cycle. Patients start the next cycle period in the new state. If an arrow points back to the same state, then the patient remains in that state. There is a monthly probability of making a transition to another state or remaining in the same state. As no-one can leave the dead state, the proportion of the population cohort that is in the dead state increases over time.
FIGURE 11.
Economic model diagram.
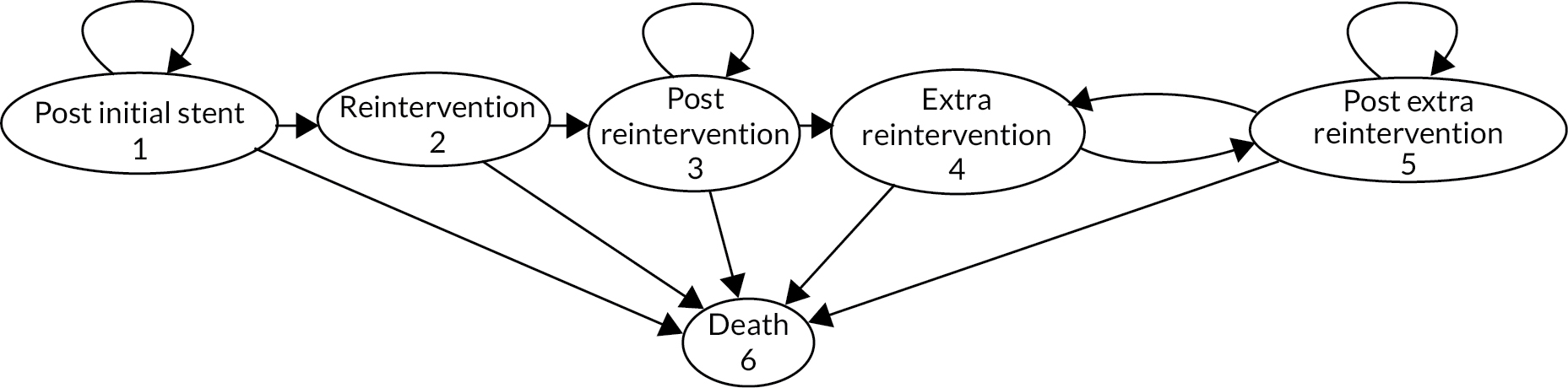
The key outcomes for RFA were survival and time to occlusion (i.e. blockage). The Markov states, therefore, represent whether patients have an occlusion and require a reintervention or they do not. Patients may die at any time. The utility of a patient who does not experience an occlusion is assumed to remain constant and no occlusion or stent or RFA costs are incurred. When a patient has an occlusion and has a reintervention (i.e. a new stent with or without RFA), they enter a reintervention state for 1 month. The utility for this month is a weighted average of the utilities accounting for the procedure and the risk of AEs. The cost incurred during this 1-month period is a weighted average of the cost of the procedure and the cost of treating AEs.
It is possible that a patient may experience more than one occlusion, requiring more than one reintervention. Effectiveness evidence was available for time to the first occlusion. Consequently, the model included a state for reintervention following the first occlusion (see Figure 11, state 2), and a state for subsequent reinterventions following subsequent occlusions (see Figure 11, state 4). This provides the option of making the risk of a second or third occlusion different from the risk of the first occlusion. Following a reintervention, patients enter a post-intervention state until another occlusion occurs, or they die. Following the first reintervention, patients enter the ‘post-reintervention’ state. Following an extra reintervention, patients enter the ‘post extra reintervention’ state. No patient experiences a reintervention within a month of the previous reintervention because of the method of modelling the risk of an extra reintervention (see Time to occlusion).
The population cohort is divided into patients who receive palliative chemotherapy and patients who do not (either because they are not fit to receive chemotherapy or because they choose not to receive chemotherapy). Patients who are fit enough to receive chemotherapy are expected, on average, to survive longer than patients who are not fit enough to receive chemotherapy (see Chemotherapy). For the month when a patient is in a reintervention state, it is assumed that the chemotherapy regimen is halted and is resumed the following month. No cost of chemotherapy is incurred during the month. It is also assumed that the risk of dying changes to the risk for someone who is not fit to receive chemotherapy.
Survival
Time to death
Effectiveness
The HR of mortality was estimated by conducting a meta-analysis of the studies reported in the systematic review (see Figure 4). The effectiveness evidence was expected to be poor quality and so bias-adjusted meta-analysis was also planned (see Reviewer risk-of-bias assessment). Only one of the included studies in the meta-analysis was conducted in the UK, and it is possible that there are treatment practices that differ from those in the UK, which may affect the outcome. The type of stent is one possible confounding factor, although this is more likely to factor in secondary RFA.
The HR was modelled on the log-scale using a normal distribution. A HR of mortality was estimated for only bile duct cancer.
No patient-level data were available to test different survival models. In the base-case analysis, proportional hazards were assumed. Visual observation of the Kaplan–Meier plots suggested that this was a reasonable assumption. Of the four16,28–29,34 studies included in the HR meta-analysis, three reported Kaplan–Meier curves. Of these, two graphs showed increasing divergence of survival curves consistent with proportional hazards until the survival curves converged with 100% dead in both groups within a short period of each other. The other Kaplan–Meier graph showed parallel survival curves to begin with, indicating a falling HR, before diverging until the end of follow-up.
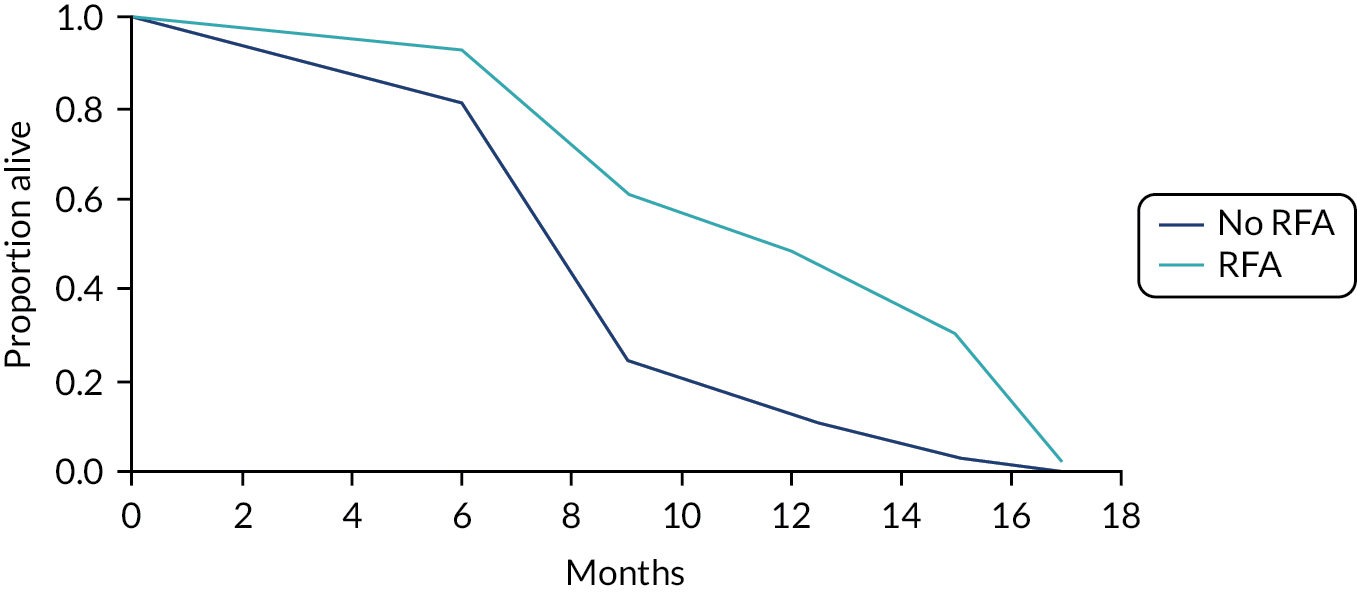
The baseline hazard rate was very high from 15 months (> 4) and so, even with a HR of 0.34, the survival curves for RFA and no RFA followed a pattern seen in the reported Kaplan–Meier curves. The survival curves with and without RFA are reported in Figure 12.
FIGURE 12.
Survival curve without RFA and with RFA.
Chemotherapy
The clinical experts on the Project Advisory Group were consulted on the proportion of patients in the study populations who might receive chemotherapy or radiotherapy. In the base case, we assumed that 20% of patients would receive chemotherapy. Alternative values of 10% and 40% were used in sensitivity analyses.
PubMed was searched for systematic reviews of studies evaluating the difference in mortality outcomes in patients who receive chemotherapy (i.e. patients who are fit to receive chemotherapy and who choose to receive chemotherapy) and in patients who do not receive chemotherapy (i.e. patients who are either not fit to receive chemotherapy or choose not to receive chemotherapy) in patients with unresectable bile duct cancer or unresectable pancreatic cancer. No systematic reviews were found.
A simple search of Google (Google Inc., Mountain View, CA, USA) was conducted to identify studies that evaluated the HR of mortality for patients receiving chemotherapy in advanced, unresectable cancer with biliary obstruction. Two studies92,93 were identified. Two studies16,94 were also identified from the clinical effectiveness review in this study. A random-effects meta-analysis of the HR of mortality on the log-scale was conducted using the generic inverse variance method in R statistical software (The R Foundation for Statistical Computing, Vienna, Austria). The HR of mortality estimates extracted from the four studies16,92–94 were estimated in those studies using multivariable regression methods that adjusted for confounding factors. The study populations and type of chemotherapy of the four studies16,92–94 are reported in Table 2. The HRs and the 95% CIs for each study are reported in the forest plot (see Figure 12), along with the results of the meta-analysis. The pooled estimate from the random-effects meta-analysis was 0.6123 (95% CI 0.47 to 0.8), indicating that patients who receive chemotherapy are likely to survive for longer than patients who do not receive chemotherapy. There was a moderate degree of heterogeneity indicated by an I2-value of 44.3%.
| Study | Population | Chemotherapy regimen |
|---|---|---|
| Sharaiha et al.16 | Patients with biliary obstruction from advanced stage pancreatic cancer or cholangiocarcinoma. Median survival 5.9 months | Not stated |
| Afshar et al.93 | Patients who underwent biliary stenting for obstructive jaundice related to advanced malignant disease. Curative surgery patients excluded | Not stated |
| Yonemoto et al.92 | Unresectable, locally advanced or metastatic adenocarcinoma arising from intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, gall bladder cancer or papilla of Vater cancer | Gemcitabine used in analysis because of greatest sample size. HR reported for gemcitabine 0.53 (95% CI 0.34 to 0.82) and CDDP 0.49 (95% CI 0.36 to 0.99) |
| Liang et al.94 | Patients with confirmed extrahepatic biliary adenocarcinoma but who are ineligible for curative surgery because of locally advanced or metastatic disease or because they are unfit for or not willing to undergo a major operation | 22 gemcitabine/cisplatin combinations; 18 other gemcitabine based; and 13 fluoropyrimidine based |
The time to death in the stent-only arm among patients who did not receive chemotherapy was modelled using the survival curve for the stent-only group in Yang et al. ,29 as this was the only study included in the meta-analysis that excluded patients on chemotherapy, excluded secondary RFA patients and provided sufficient data to model time to death. The exclusion of patients on chemotherapy was necessary, as both patients receiving and not receiving chemotherapy were modelled. A HR of mortality for chemotherapy compared with no chemotherapy was included in the model. The probability of death was calculated for the time periods of 1–6 months, 7–9 months, 10–12 months and 13–15 months from the Kaplan–Meier curve, as the survival probabilities were reported after 6, 9, 12 and 15 months. The rate per cycle was derived for each period using the formula:
where t is the number of months in the period to which the probability applies. For example, the probability of dying over the first 6 months was 0.182 and t = 6. The hazard rate for the period following 15 months was assumed to the same as the 13- to 15-month period. Regardless of the number reinterventions (the model state), the probability of dying was related to the time since the start of the model.
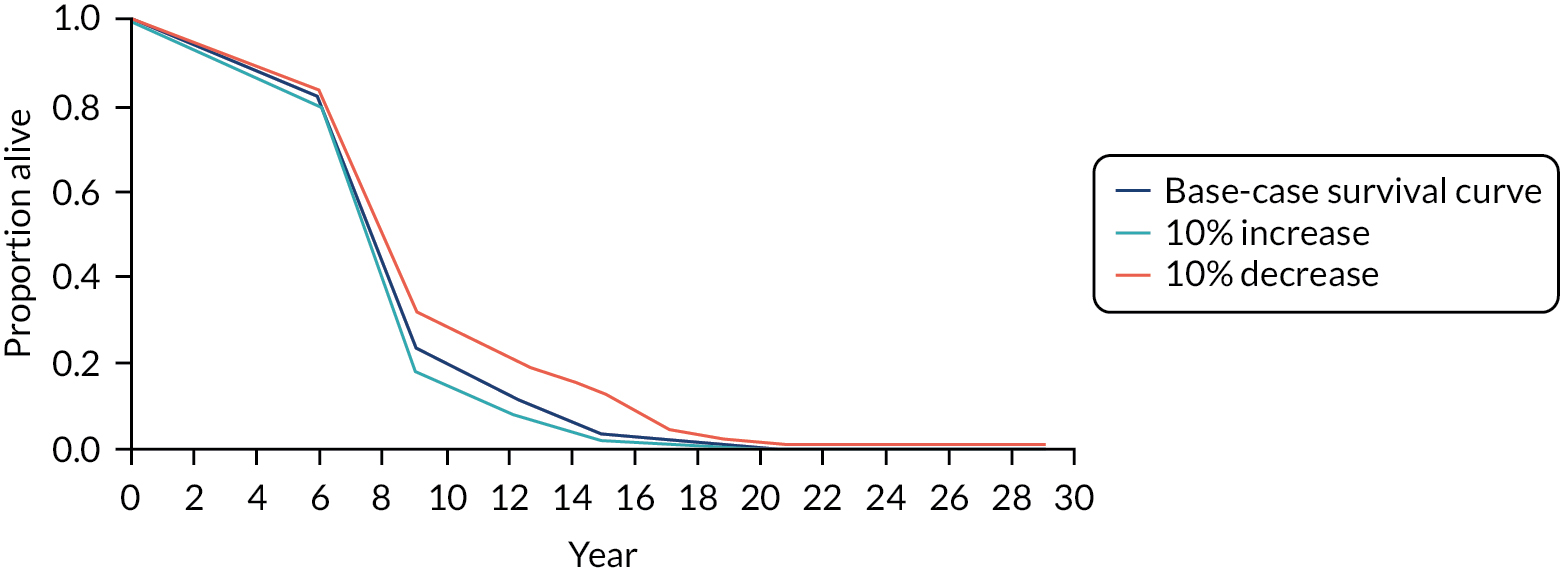
Sensitivity analyses were conducted in which the probability of death was increased and decreased by 10% over each time period. For example, the probability of dying during the first 6 months was increased from 0.182 to 0.2 in one analysis and decreased to 0.164 in another. The survival curves for the stent-only group for the base case and scenario analyses are presented in Figures 13 and 14. The survival curves show a significant increase in the hazard rate of mortality from 6 to 9 months. All patients have died by month 17.
FIGURE 13.
Random-effects meta-analysis of mortality HRs for chemotherapy vs. no chemotherapy.

FIGURE 14.
Survival curves for stent only: base case and sensitivity analyses (% changes in mortality).
Occlusion
Effectiveness
Occlusion and death are competing risks. If a study has sufficient sample size, competing risk survival analysis can be conducted to estimate the HR of occlusion for RFA plus stent compared with stent only. The comparative studies included in the systematic review were small and did not conduct competing risk time-to-occlusion analysis, nor did the studies conduct standard time-to-occlusion analysis. Two studies28,29 included in the systematic review reported average time to occlusion. A random-effects meta-analysis was conducted in the systematic review (see Chapter 4, Survival) and the forest plot is shown in Figure 10. The pooled estimate of the difference in time to occlusion was 1.16 (95% CI –2.5 to 4.83) months, and this was modelled using a normal distribution, with parameters of a mean of 1.16 and a SE of 1.87.
In the base-case analysis, this estimate of difference in time to occlusion was applied to additional RFA procedures, as well as to the initial RFA procedure. Owing to a lack of evidence on the effectiveness of RFA for additional procedures, this estimate of effectiveness was halved for additional procedures in a scenario analysis.
Time to occlusion
The mean time to presentation with a blocked stent (i.e. occlusion) in the stent-only group was obtained from Yang et al. 29 The study by Yang et al. 29 was the only study, to the best of our knowledge, that provided the mean and 95% CI for time to occlusion in the stent-only group. The time to occlusion was 3.4 (95% CI 2.4 to 6.5) months. It was not clear from the paper how the 95% CI was calculated, as the bounds are not equidistant from the mean on the natural timescale or when converted to the log-scale. It is possible that this was a sample interval, which would be consistent with a follow-up period of 18 months, a mean of 3.4 months, a sample group size of 33 and with 82% of patients still alive at 6 months. Despite this, to model the mean time to occlusion, a normal distribution was used (mean = 3.4, SE = 1.05), as this would overestimate the uncertainty in the mean if the 95% CI actually reflects sample variation.
The probability of an occlusion for the stent-only group was modelled using a gamma distribution. The mean of the gamma distribution was derived from the normal distribution. The variance of the gamma distribution was 1.1, based on the assumption that the 95% CI, stated above, reflects sample variation. Exponential, gamma and log-normal distributions were considered for modelling time to occlusion. Parameter values for the distributions were sought to match the mean and variance of the distribution to a mean of 3.4 and a sample variance of 1.1. Although this was not possible for the exponential distribution, it was possible for the log-normal and gamma distributions. The gamma distribution was selected as it had a broader lower tail. The best fit distributions for each of the three distributions are presented in Figure 15. The parameters of the gamma distribution were derived from the mean and SE of time to occlusion. The gamma distribution parameters were α = 10.5 (shape) and
FIGURE 15.
Density plots for exponential, gamma and log-normal distributions.

β = 3.1 (rate). The probability of occlusion in each cycle was the difference in the cumulative probability before and after each cycle obtained from the cumulative gamma distribution function. The probability of occlusion was conditional on survival in the model.
For RFA, mean time to occlusion was modelled as the sum of the time to occlusion in the stent-only group and the difference in time to occlusion between RFA and no RFA. The evidence for difference in time to occlusion is described in Effectiveness. Monte Carlo simulation ensured that the uncertainty in mean time to occlusion was propagated through the model. The gamma distribution has two parameters, which can be derived from the mean and variance of the distribution. The mean for the gamma distribution was obtained by adding the difference in mean time to occlusion to the stent-only group time to occlusion. The variance was assumed to be the standard deviation of the mean time to occlusion in the RFA group (1.17 months), obtained from Yang et al. ,29 again, assuming the variance to represent the sample variation.
Time to occlusion following a reintervention and subsequent reinterventions was assumed to be the same as following the initial intervention in the base-case analysis. However, the above approach to modelling time to occlusion, using a cumulative gamma distribution, cannot be adopted following subsequent reinterventions when a cohort modelling approach is used. The gamma distribution could be used only if microsimulation analysis was conducted and the time at which individuals experience a reintervention was tracked for each individual. As we conducted a cohort analysis, an exponential distribution was used to produce a constant risk of an occlusion each month following the first reintervention.
One of the problems with using an exponential distribution in this context is that the probability of an occlusion is far higher in the first and second months (roughly 0.25) following an intervention than when using the gamma distribution (0.0007 for month 1 and 0.07 for month 2), and this is a problem because this increases the cumulative probability of subsequent occlusions. The earlier some people have an occlusion, the greater the likelihood they will have yet another occlusion later on. To address this, it was assumed that the probability of an occlusion during the first month after a reintervention was zero. This is why a patient cannot remain in the ‘extra reintervention’ state in the model in Figure 11.
Bias adjustment
The clinical studies included in the systematic review were expected to be at risk of bias during the project planning stage. The reason for this was the small sample and often poor study design of published clinical studies, which often are non-randomised or have poor randomisation and may not adjust for confounding factors. Risk of bias produces uncertainty in the validity of the results, which is not reflected in the CI statistics reported for the effectiveness estimates. Risk of bias can mean that uncertainty in the cost-effectiveness of a technology is underestimated and the value of future research is underestimated. This section describes the methods used to adjust the HR of mortality and the difference in time to occlusion effect estimates for bias. The outcomes that were the object of the bias elicitation was the HR of mortality and the mean difference in time to occlusion. Every study included that informed the HR of mortality and mean difference in time to occlusion was included in the exercise.
As there were expected to be few comparative studies of RFA and the risk of bias was expected to be significant, the effect of bias on the cost-effectiveness and value of information results was investigated in two ways. The first way involved trying to quantify the bias elicited from clinical experts. The second way involved quantifying plausible degrees of bias based on reviewers’ internal risk-of-bias assessments and external risk of bias from the clinical experts and study populations.
Expert elicitation of bias
Four clinical experts were included in the expert elicitation exercise. Three of the clinical experts (Irfan Ahmed, Manu Nayar and Kofi Oppong) were in the Advisory Group, and one of the clinical experts (SP) was in the Advisory Group and was an author for the project. A review of bias adjustment methods by Verde et al. was identified. 95 The most practical method for adjustment of individual elements of internal and external bias of individual study results before a meta-analysis is conducted was considered to be that of Turner et al. 96 The method by Turner et al. 96 involved three steps. The first step was a consideration of the presence of bias-related factors in the studies. The second step was a group meeting of the clinical experts to discuss the bias elements. The third step was a qualitative assessment of the level of each bias on the outcome statistic of interest (i.e. HR of mortality, difference in time to occlusion) for the populations of interest (i.e. advanced bile duct cancer, advanced pancreatic cancer) and a marking on a scale to quantify that bias (Figures 16 and 17, and see Appendix 7). The internal bias elements included were selection bias, performance bias, attrition bias and detection bias. The external bias elements included were population bias, intervention bias, control bias and outcome bias.
FIGURE 16.
Cost-effectiveness plane for RFA vs. no RFA.
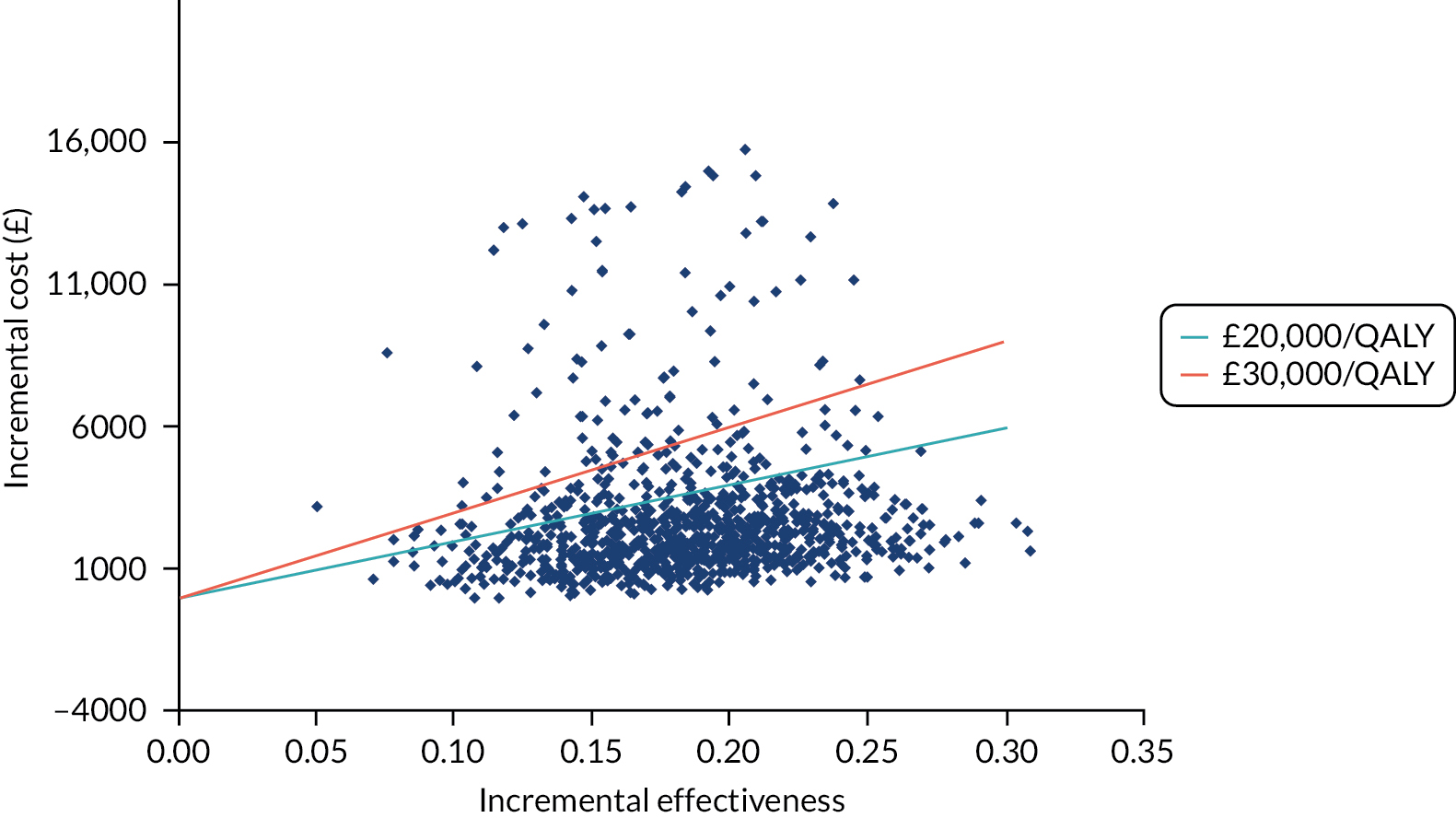
FIGURE 17.
Cost-effectiveness acceptability curves for RFA and no RFA.

Unfortunately, owing to late finalisation of the comparative studies to be included in the systematic review, the tight timelines from the end of the review to the end of the project and difficulty in arranging for the clinical experts to be available at the same time to discuss the bias results, the step 2 discussion did not happen. The clinical experts also needed more guidance on the assessment and marking of bias. In addition, the review update included a study that could not be reviewed by the clinical experts because of project time constraints.
The result was that there was only one response where the bias quantification could be assessed as consistent with the assessed bias factors in each study, and this did not include an assessment of the study by Gao et al. 28 Where only a qualitative assessment was provided, a quantitative value was inferred. A value of 1 indicates no bias. A value of 0.6 indicates that bias may reduce the hazard rate of mortality with a HR of 0.6 when, in fact, the intervention has no effect (HR 1). A range such as 0.85–0.6 indicates bias (0.6) more likely to favour RFA than the comparator. The qualitative and quantitative assessment of bias from this response for the HR outcome for the bile duct cancer population is presented in Table 3.
| Yang et al.29 | Dutta et al.34 | Sharaiha et al.16 | ||||
|---|---|---|---|---|---|---|
| Assessment | Bias | Value | Bias | Value | Bias | Value |
| Internal | ||||||
| Selection | None | 1 | High | 0.85–0.6 | Medium | 0.7–0.7 |
| Performance | None | 1 | Medium | 0.95–0.84 | Medium | 0.7–0.7 |
| Attrition | None | 1 | Medium | 0.8–0.75 | Low | 0.9–0.9 |
| Detection | None | 1 | Medium | 0.76–0.76 | Medium | 0.76–0.76 |
| Other | None | 1 | None | 1 | None | 1 |
| External | ||||||
| Population | None | 1 | Medium | 0.8–0.1 | Medium | 0.76–0.76 |
| Intervention | None | 1 | None | 1 | None | 1 |
| Control | None | 1 | None | 1 | None | 1 |
| Outcome | None | 1 | None | 1 | None | 1 |
The relationship between the qualitative assessment and quantitative assessment in Turner et al. 96 was based on the percentage increase in the SE of the log-odds ratio,96 and it assumed a rare occurrence of events. Bias assessment for the log-HR and the difference in time to occlusion requires an appropriate scale on which to mark the degree of bias (see Appendix 7). The sampling variances of studies with a small sample size from the included studies were used to derive scales comparable to the log-odds ratio scale used in Turner et al. 96 and comparable bias quantification guidance [e.g. low (0.92–1)] for the log-HR and the difference in time to occlusion (see Appendix 7). Bias was considered either additive or proportional to the outcome. The bias values are presented in Table 3.
The effectiveness statistics were adjusted using the formulae presented in Turner et al. 96
No risk of bias was identified for Yang et al. 29 for either the HR of mortality or difference in time to occlusion. For the HR of mortality, the bias-adjusted statistic was 0.28 (95% CI: 0.17 to 0.48), and this compares with 0.26 (95% CI 0.16 to 0.42) from a HR meta-analysis including Yang et al. ,29 Dutta et al. 34 and Sharaiha et al. 16 Yang et al. 29 had the greatest weight in the meta-analysis unadjusted for bias. As no risk of bias was identified for Yang et al. ,29 it increased its weight in the bias-adjusted meta-analysis.
Excluding Gao et al. ,28 only Yang et al. 29 reported a difference in stent patency. The between-study variance estimate from the meta-analysis including Gao et al. 28 was added to the variance of the effect estimate in Yang et al. 29 to ensure that the uncertainty in the average effect estimate was adequately captured.
Reviewer risk-of-bias assessment
A reviewer risk-of-bias assessment was conducted because of the limitations of the expert elicitation bias conducted. There were two RCTs28,29 and two comparative observational studies. 16,34 The risk of bias for the RCTs was assessed using the Risk of Bias 2.0 tool,24 and the risk of bias for the comparative observational studies was assessed using the ROBINS-I tool. 25
The risk-of-bias assessments using these tools were mapped to the bias categories used in Turner et al. 96 and an estimate of bias at the high end of the range was adopted. For example, a medium risk of bias range for the HR was assumed to be 0.76–0.92. If the risk of bias using the ROBINS-I tool was moderate, then the risk of bias for a bias category was assumed to be 0.76. 25 The objective was to obtain a bias estimate on the high side. Likewise, the assessment ‘some concern’ from the Risk of Bias 2.0 tool was assumed to be moderate bias with a value of 0.76. 24 There is no ‘no bias’ option. The lowest assessment is ‘low’ risk of bias. For ‘low’ risk of bias, a very small bias effect was assumed of 0.95.
The Risk of Bias 2.0 and ROBINS-I tools assess the risk of internal bias. 24,25 The risk of external bias for the population obtained from the clinical expert response was added to the reviewer risk of internal bias assessment. No assumption was made about the direction of bias. It was assumed that the risk of bias would increase the CIs for the effect estimate, but not change the estimated effect. The bias values are reported in Table 4.
| Assessment | Yang et al.29 | Dutta et al.34 | Sharaiha et al.16 | Gao et al.28 |
|---|---|---|---|---|
| Internal | ||||
| Selection | 0.95–0.95 | 0.6–0.6 | 0.95–0.95 | 0.6–0.6 |
| Performance | 0.7–0.7 | 0.95–0.95 | 0.95–0.95 | 0.95–0.95 |
| Attrition | 0.95–0.95 | 0.95–0.95 | 0.95–0.95 | 0.95–0.95 |
| Detection | 0.95–0.95 | 0.95–0.95 | 0.95–0.95 | 0.95–0.95 |
| Other | 0.7–0.7 | 1 | 0.6–0.6 | 0.7–0.7 |
| External | ||||
| Population | 1 | 0.76–0.76 | 0.76–0.76 | 1 |
| Intervention | 1 | 1 | 1 | 1 |
| Control | 1 | 1 | 1 | 1 |
| Outcome | 1 | 1 | 1 | 1 |
The Risk of Bias 2.0 and ROBINS-I tools do not assess whether the biases are additive in nature or proportional to the effect size, and so two bias-adjusted estimates were produced, one assuming that biases are additive and the other assuming that the biases are proportional. Assuming proportional bias, the difference in time to occlusion was 0.58 (95% CI –2.68 to 3.85), and this compares with the results of the meta-analysis presented in Chapter 4, Figure 10 of 1.16 (95% CI –2.50 to 4.83). Assuming additive bias, the difference in time to occlusion was 1.18 (95% CI –2.49 to 4.86).
Assuming proportional bias, the hazard rate of mortality was 0.44 (95% CI 0.24 to 0.8), and this compares with 0.34 (95% CI 0.21 to 0.55) from the HR meta-analysis presented in Chapter 4, Survival, and the estimate of 0.28 (95% CI 0.17 to 0.48) from the expert bias adjustment. Assuming additive bias, the hazard rate of mortality was 0.32 (95% CI 0.15 to 0.69).
The additive bias method hardly affected the mean HR of mortality effectiveness estimate. The CI of the HR was wider. The difference in stent patency CI from the additive bias-adjusted estimate was very similar to the unadjusted CI, and this is because there were only two studies28,29 in the random-effects meta-analysis and because there is significant statistical heterogeneity. Although the additive bias approach increased the individual SEs of the effectiveness estimates of the trials, this result also reduced the estimate of the between-study variance, and, overall, the CIs were similar.
The proportional bias method reduced the effectiveness estimate for both time to occlusion and HR of mortality and increased the CIs. The proportional bias method, therefore, represents a high estimate of bias and the additive bias method represents a low estimate of bias.
The limitations of the reviewer assessment of bias are summarised as follows: the reviewers are not experts in this clinical area; the reviewers use a risk-of-bias assessment tool that has less flexibility in the grading of bias than in the Turner et al. 96 risk-of-bias assessment; the reviewers’ assessment results in a categorical assessment of bias, which then needed to be mapped onto the bias scale; the reviewers assessed internal validity, not external validity; and the reviewers’ did not assess proportional or additive bias. Despite these limitations, a crude assessment of the potential impact of bias on the results could determined.
Adverse events
Relative risks
The relative risks of cholangitis, cholecystitis and pancreatitis were estimated from meta-analyses, including a subset of studies included in the meta-analyses of AEs reported in Chapter 4, Adverse events. The reason the estimates used in the economic model were different from the estimates reported in Chapter 4, Primary outcomes is that the AE meta-analyses were updated at a late stage of the project. The relative risks reported here are less precise, but both the clinical effectiveness and cost-effectiveness results report considerable uncertainty in their estimates. The estimates used in the base-case and scenario economic analyses were as follows: the relative risk of cholecystitis for RFA compared with no RFA was 7.07 (95% CI 1.31 to 38.26) and the relative risk of pancreatitis for RFA compared with no RFA was 2.08 (95% CI 0.55 to 7.89). The very wide 95% CIs reflects the very small numbers of events and sample sizes. No difference in cholangitis risk was assumed in the base case. The base-case analysis was re-run with the updated AE relative risks, as reported in Chapter 4, Primary outcomes, and the relative risk of cholecystitis for RFA compared with no RFA was 11.47 (95% CI 2.28 to 57.66), the relative risk of pancreatitis for RFA compared with no RFA was 1.34 (95% CI 0.55 to 3.25) and the relative risk of cholangitis for RFA compared with no RFA was 1.15 (95% CI 0.63 to 2.12).
In the base case, the relative risks were modelled on a log-scale as normal distributions with the following parameters: a mean of 1.96 and a SE of 0.86 for cholecystitis, and a mean of 0.73 and a SE of 0.68 for pancreatitis.
Adverse event risk
The risk of an AE was modelled each time a stent was inserted with or without RFA. The cost was a one-off cost. Based on clinical expert opinion, the occurrence of AEs was post procedure or shortly after discharge. There were few to no data on the time to AE or recurrence of AEs in the included studies. The AEs were modelled as one-off events when a stent was inserted.
The risks of cholangitis, cholecystitis and pancreatitis were estimated by conducting meta-analyses of AEs risks in the stent-only groups.
In the base-case analysis and scenario analyses, in the stent-only group, the risk of cholangitis was 0.077 (SE 0.089), the risk of cholecystitis was 0.00016 (SE 0.0015) and the risk of pancreatitis was 0.015 (SE 0.019). These risks were modelled as beta distributions where the parameters of the beta distribution were derived from the means and SEs of the risk estimates.
The studies included in the AE meta-analyses were updated late in the project. The revised mean estimates were 0.0495 (SE 0.03693) for cholangitis, 0.000041 (SE 0.000617) for cholecystitis and 0.03127 (SE 0.02458) pancreatitis, and these were included in a scenario analysis for the base case.
The risk of haemobilia was assumed to be 5%, which was obtained from clinical expert opinion.
Health utility
A focused literature review was conducted to identify utility values for patients with locally advanced or metastatic pancreatic cancer and cholangiocarcinoma, for stent or RFA procedures in these populations and for AEs, such as cholangitis, in these populations.
Web of Science (Clarivate) and EMBASE (Ovid) were searched. The search term ‘QALY OR health utility*’ was combined with free-text terms for the cancer, ‘pancreatic cancer OR pancreatic carcinoma OR cholangiocarcinoma OR bile duct cancer OR biliary tract’; free-text terms for cancer type, ‘locally advanced OR metastatic’; and a term for the UK or US context (e.g. ‘UK’). Searches were limited to English-language publications and excluded neuroendocrine tumours or resectable (operable) cancers.
The Cost-Effectiveness Analysis Registry was searched for health utilities using the terms ‘biliary’, ‘pancreas’ and ‘cancer’.
Limited utility data were identified. A set of utilities favourable to RFA was defined and a set of utilities conservative to RFA (i.e. favourable to stent only) was defined, and these were used in two scenario analyses. The scenarios are reported in Table 5.
| Condition | RFA conservative | RFA favourable | Source | Time period |
|---|---|---|---|---|
| Locally advanced cancer | 0.61 | 0.71 | 0.61 from Martinez et al.97 who reference Heiberg et al.98 (utility study population: delimited, locally advanced, metastatic pancreatic cancer; utility method: EQ-5D-3L index) | Per cycle |
| 0.71 from Roth and Carlson99 who reference Connock et al.100 (utility study population: advanced hepatocellular carcinoma; utility method: mapping FACT-G scores to TTO values) | ||||
| Stent/RFA procedure | 0.18 for 2 days | 0.18 for 3 days | 0.18 for 3 days from Martinez et al.97 who reference Jeurnink et al.101 EQ-5D-3L (utility study population: patients receiving ERCP; utility EQ-5D | 2 or 3 days |
| AEa | 0.57 | 0.5 | Assumption | 2 weeks |
| State | ||||
| Reintervention: per month | 0.046 | 0.054 | Reintervention (per year)/12 | |
| Reintervention: per month | 0.553 | 0.646 | Derived from Equation 2 | |
| Post-reintervention: per month | 0.051 | 0.059 | Post-reintervention (per year)/12 | |
| Post-reintervention: per month | 0.61 | 0.71 | The locally advanced cancer utilities | |
An average utility estimate of 0.61 for people living with unresectable bile duct cancer was used in the base-case analysis, which is conservative to RFA. A utility estimate of 0.71 was used in a scenario analysis favourable to RFA. Martinez et al. 97 used an EuroQol-5 Dimensions (EQ-5D) utility estimate for delimited, locally advanced and metastatic pancreatic cancer, obtained from Heiberg et al. ,98 of 0.61 for an economic evaluation of stents in patients with locally advanced or metastatic pancreatic cancer presenting with biliary obstruction. Roth and Carlson99 used a utility estimate for advanced hepatocellular carcinoma, obtained from Connock et al. ,100 of 0.71 for an economic evaluation of chemotherapy regimens in patients with advanced biliary tract cancer, and this estimate was derived by mapping Functional Assessment of Cancer Therapy – General scores to time trade-off values.
A utility estimate of 0.18 was assumed for a procedure for a duration of 2 days in the base-case analysis and a procedure for a duration of 3 days in a scenario analysis favourable to RFA. Martinez et al. 97 used an EQ-5D utility estimate of 0.18 for patients receiving ERCP, obtained from Jeurnink et al. ,101 for patients receiving ERCP and metal or plastic stents, and it was assumed to last for 2 days. No additional specific disutility for symptoms leading to the reintervention was modelled.
No reliable utility estimate was found for AEs in the study populations. A high estimate of 0.57 was used in the base-case analysis and a low estimate of 0.5 was used in a scenario analysis favourable to RFA. An AE was assumed to last for 2 weeks.
The QALYs for the model states are also reported in Table 5. A cycle length was 1 month. The QALY gain for a reintervention was a weighted average. For example, for the RFA favourable utility scenario, the QALY gain for a 1-month cycle involving a reintervention was:
where probAE is the probability of an AE. The QALYs for the ‘post-reintervention’ and ‘post extra reintervention’ states was the utility for locally advanced cancer over a 1-month period.
Resource use and unit costs
The resource use in the model was limited to the stent and RFA procedures, chemotherapy regimens, treatment for intraoperative complications and treatment for AEs. The unit costs were obtained from the 2018/19 NHS reference costs. 102 The unit costs and the NHS reference cost Healthcare Resource Group (HRG) codes associated with stent insertion are reported in Table 6, the total procedure-related costs associated with stent placement alone are reported in Table 7 and the total procedure-related costs associated with stent insertion with RFA are reported in Table 8.
| Parameter | Cost (£) | Source | Description |
|---|---|---|---|
| Day case procedure, no complications | 842 | NHS reference costs 2018/19102 | FE10A Endoscopic Insertion of Luminal Stent into Gastrointestinal Tract with CC Score 7 +; Day Case |
| Elective inpatient procedure, complications | 5320 | NHS reference costs 2018/19102 | FE10A Endoscopic Insertion of Luminal Stent into Gastrointestinal Tract with CC Score 7 +; Elective inpatient |
| Base-case total | 1289.80 | Assuming 10% inpatient stays due to complications | |
| Higher complications scenario | 1737.60 | Assuming 20% inpatient stays due to complications | |
| Lower complications scenario | 1065.90 | Assuming 5% inpatient stays due to complications |
| Resource unit | Unit cost (£) | Source | Description |
|---|---|---|---|
| Diagnostic ERCP | 1515 | NHS reference costs 2018/19102 | GB11Z Diagnostic Endoscopic Retrograde Cholangiopancreatography; Elective |
| Endoscopic stent insertion | 1290 | NHS reference costs 2018/19102 | FE10A Endoscopic Insertion of Luminal Stent into Gastrointestinal Tract with CC Score 7+; 90% Day Case and 10% Elective procedures |
| Total | 2805 |
| Parameter | Cost (£) | Source | Description |
|---|---|---|---|
| Diagnostic ERCP | 1515 | NHS reference costs 2018/19102 | GB11Z Diagnostic Endoscopic Retrograde Cholangiopancreatography; Elective |
| RFA catheter | 1203 | Navaneethan et al.103 | Cost of a Habib Endo HPB single-use catheter. Original cost: US(2017)$1495 (Navaneethan et al.103), converted to GBP (£2017) and inflated to 2019 prices using the NHSCII (PSSRU 2019)104 |
| Endoscopic stent insertion | 1290 | NHS reference costs 2018/19102 | FE10A Endoscopic Insertion of Luminal Stent into Gastrointestinal Tract with CC Score 7+; 90% Day Case and 10% Elective procedures |
| Total | 4007 |
The occurrence of stent and RFA procedures was determined by the parameters in the model. The unit cost of stent placement (£2804.80) was assumed to comprise diagnostic ERCP and endoscopic stent insertion, with 90% of patients having a stent insertion as a day case procedure and 10% of patients staying overnight because of complications. The assumption that 10% of patients stay overnight in hospital following an intervention is based on expert opinion, which stated that < 10% of patients would do so. It was also assumed that patients who experienced complications had an intervention with the highest complexity and comorbidity score in the tariff (i.e. a CC score of 7+), which had a mean length of stay of 8 days. The percentage of patients who had an intervention with complications that required hospital stay was varied in sensitivity analysis. This contrasts with the assumption made in the economic model for the use of stents for the management of biliary obstruction in people with unresectable pancreatic cancer in the National Institute for Health and Care Excellence (NICE) Guideline NG85, Chapter 12,105 where the mean length of stay for self-expanding metal stent is assumed to vary from 2.5 days to 3.5 days. The assumption used in NICE Guideline NG85105 was based on evidence from a RCT. 106
The unit cost of RFA and stent placement (£4007) was assumed to be the unit cost of stent placement plus the cost of a RFA catheter. It was assumed that the standard endoscopic RFA procedure is performed using a single-use Habib EndoHPB catheter. The cost of the catheter was obtained from the literature, and converted to 2019 GBP. 103 The unit costs and the NHS reference cost HRG codes are reported in Table 8.
An assumption was made in the model about the proportion of patients receiving chemotherapy. Patients in the cohort receiving chemotherapy incurred a chemotherapy cost every month for 24 months, except for months when a reintervention occurred.
For patients suitable for chemotherapy, the treatment differs according to cancer type. In cholangiocarcinoma, following the ABC-02 trial,107 cisplatin combined with gemcitabine is administered at in first 2 weeks of a 3-week cycle. 107
Drug costs were derived from the drugs and pharmaceutical electronic market information tool (2019/20) for 2019. 108 Chemotherapy delivery costs were derived from the 2018/19 NHS reference costs. 102 The unit costs and sources are reported in Table 9.
| Chemotherapy regimens | |||
|---|---|---|---|
| Parameter | Cost (£) | Source | Description |
| Cholangiocarcinoma first-time chemotherapy delivery | 458 | Valle et al.,107 eMIT 2019/20,108 NHS reference costs 2018/19102 | Deliver complex chemotherapy, including prolonged infusional treatment, at first attendance of a gemcitabine plus cisplatine regimen. Doses and cycles based on the ABC-02 trial.107 2019 prices obtained from eMIT 2019/20108 |
| Cholangiocarcinoma subsequent chemotherapy monthly cost | 580 | Valle et al.,107 eMIT 2019/20,108 NHS reference costs 2018/19102 | Gemcitabine plus cisplatine regimen. Doses and cycles based on the ABC-02 trial.107 2019 prices obtained from eMIT 2019/20108 |
The AEs that may be associated with stent placement or RFA that were most commonly reported in the systematic review were:
-
pancreatitis
-
cholangitis
-
cholecystitis
-
haemobilia
-
stent migration/occlusion.
Haemobilia is described here as a complication of the procedure. Pancreatitis, cholangitis and cholecystitis are described here as AEs that could occur after hospital discharge.
Pancreatitis, cholangitis and cholecystitis are generally assessed in an inpatient setting and can lead to patients staying for 24–48 hours in the hospital. The HRG code used reflects the cost of a hospital day case or an ordinary stay, assuming that the adverse effects are caused by an infection in the local area. The risk of an AE was obtained from the clinical evidence (see Chapter 4, Adverse events). The unit cost associated with haemobilia and the other AEs are reported in Table 10.
| Parameter | Cost (£) | Source | Description |
|---|---|---|---|
| Cholangitis/pancreatitis/ cholecystitis requiring inpatient stays | 498.00 | NHS reference costs 2018/19102 | WH07E Infections or Other Complications of Procedures, without Interventions, with CC Score 4+; Day Case |
The cost of a procedure, a complication and an AE are all assumed to be incurred during the month in which a reintervention takes place. The cost of the resinsertion state for a stent and RFA procedure is:
Summary of model parameters
A summary of the model parameters is provided in Table 11.
| Parameter | Value (95% CI) | Distribution | Source |
|---|---|---|---|
| Clinical parameter Survival HR of RFA |
|||
| Base case | 0.34 (0.2 to 0.55) | Log-normal | Meta-analysis |
| Additive bias | 0.32 (0.15 to 0.7) | Log-normal | Meta-analysis |
| Proportional bias | 0.44 (0.24 to 0.8) | Log-normal | Meta-analysis |
| Survival HR of chemotherapy | |||
| Base case | 0.61 (0.47 to 0.8) | Log-normal | Meta-analysis |
| Proportion receiving chemotherapy | |||
| Base case | 20% | Expert opinion | |
| Favourable case | 40% | Assumption | |
| Difference in stent patency months | for RFA | ||
| Base case | 1.16 (–2.5 to 4.8) | Normal | Meta-analysis |
| Additive bias | 1.18 (–2.49 to 4.86) | Normal | Meta-analysis |
| Proportional bias | 0.58 (–2.68 to 3.85) | Normal | Meta-analysis |
| AE risks stent only | |||
| Cholangitis | 0.077 (SE 0.089) | Beta | Meta-analysis |
| Pancreatitis | 0.015 (SE 0.019) | Beta | Meta-analysis |
| Cholecystitis | 0.00016 (SE 0.0015) | Beta | Meta-analysis |
| Intraoperative haemobilia | 0.05 | Expert opinion | |
|
Utility
Locally advanced cancer |
|||
| Base case | 0.61 | Martinez et al.;97 Heiberg et al.98 | |
| Favourable case | 0.71 | Roth and Carlson et al.;99 Connock et al.100 | |
| Moderately low | 0.5 | Assumption | |
| Very low | 0.4 | Assumption | |
| Reintervention | |||
| Base case | 0.18 for 2 days | Martinez et al.;97 Jeurnink et al.101 | |
| Favourable case | 0.18 for 3 days | Martinez et al.;97 Jeurnink et al.101 | |
| AEs | |||
| Base case | 0.57 for 2 weeks | Assumption | |
| Favourable case | 0.5 for 2 weeks | Assumption | |
| Moderately low | 0.46 for 2 weeks | Assumption | |
| Very low | 0.36 for 2 weeks | Assumption | |
|
Costs
Endoscopic stent insertion |
|||
| Day case procedure, no complications | £842 | NHS reference costs 2018/19102 | |
| Elective inpatient procedure, complications | £5320 | NHS reference costs 2018/19102 | |
| Diagnostic ERCP | £1515 | NHS reference costs 2018/19102 | |
| RFA catheter | £1203 | Navaneethan et al.103 | |
| Stent alone/reintervention | £2805 | ||
| RFA plus stent insertion | £4007 | ||
| Chemotherapy regimens | |||
| Cholangiocarcinoma first time chemotherapy delivery | £458 | Valle et al.,107 eMIT 2019/20,108 NHS reference costs 2018/19102 | |
| Cholangiocarcinoma subsequent chemotherapy monthly cost | £580 | Valle et al.,107 eMIT 2019/20,108 NHS reference costs 2018/19102 | |
| Postoperative AEs | |||
| Cholangitis/pancreatitis/ cholecystitis requiring inpatient stays | £498 | NHS reference costs 2018/19102 | |
Incremental cost-effectiveness analysis
The outcome of the cost-effectiveness analysis is incremental cost per QALY gained and this is calculated as the difference in the total discounted cost between the RFA plus stent group and the stent-only group, divided by the difference in the total discounted utility between the RFA plus stent group and the stent-only group:
The incremental cost per QALY gained is the incremental cost-effectiveness ratio (ICER). If the ICER of a health technology is less than the accepted cost-effectiveness threshold, then the health technology is considered to be cost-effective and the decision-maker is willing to adopt the technology. The £20,000 per QALY and £30,000 per QALY cost-effectiveness thresholds recommended by NICE are used as reference cost-effectiveness thresholds in this report. 109
Analysis of uncertainty
Probabilistic sensitivity analysis
The investigation of how much uncertainty in the evidence influences decision uncertainty, that is uncertainty in whether or not a health-care technology should be adopted, is a key part of economic evaluation. Where evidence is available, we specify probability distributions to represent the uncertainty in the effectiveness estimates. Parameter values for these distributions have been reported in each section. Monte Carlo simulation is then used in the analysis sampling from every distribution 1000 times to produce a joint distribution of the incremental costs and effects of RFA compared with no RFA. All analyses of uncertainty, including value of information, were performed using the TreeAge Pro software.
The production of statistics from the Monte Carlo simulation is probabilistic sensitivity analysis. The sampled incremental cost and incremental QALY estimates are presented on a cost-effectiveness plane. The net benefit of adopting a health technology is calculated for different cost-effectiveness thresholds using the following equation:
The proportion of the simulation estimates where the intervention has the highest net benefit represents the probability that the intervention is cost-effective. The probability that an intervention is cost-effective at different cost-effectiveness thresholds is presented in a cost-effectiveness acceptability curve (CEAC). 109
Uncertainty in effectiveness parameters
Some evidence on uncertainty in the effectiveness estimate, as well as a mean estimate, is essential to conduct probabilistic sensitivity analysis that has value in a cost-effectiveness analysis of a health technology. If there is only one study that provides evidence on effectiveness and that study is small, then there will be no evidence on the between-study variance that may arise from conducting several small studies. In that situation, some measure of between-study variance would need to be assumed and sensitivity analysis conducted on the between-study variance value.
For the HR of mortality, there were four studies16,92–94 included in the meta-analysis. For stent patency, there were only two studies28,29 and the estimate of between-study variance was high. A lot of uncertainty was reflected in the CI for difference in stent patency.
Uncertainty in the validity of study results is not reflected in the CI or SE of an effectiveness estimate. Consequently, basic estimates of bias were obtained, as described in Bias adjustment, to conduct bias-adjusted meta-analyses, and these provided a high estimate of uncertainty in the effectiveness parameters.
Scenario analyses
Where there is insufficient evidence to inform a probability distribution that can adequately represent the uncertainty in a parameter estimate, then the effect of assuming different values for the parameters on the economic results can be explored. An example is the paucity of evidence informing health utility in this population. This paucity of evidence led us to create favourable and conservative (with respect to RFA) sets of health utility values. The uncertainty in the effect of bias on the effectiveness estimates is another example. The full set of scenario analyses is presented in Table 12.
| Scenario | Parameter value | Base-case value |
|---|---|---|
| A: lower stent patency for secondary stents | Stent patency in stent group: mean 1.7, SE 0.505 | Stent patency in stent group: mean 3.4, SE 1.01 |
| Difference in stent patency (RFA vs. no RFA): mean 0.76, SE 0.73 | Difference in stent patency (RFA vs. no RFA): mean 1.52, SE 1.46 | |
| B: zero RFA effectiveness in increasing stent patency and zero uncertainty | Difference in stent patency (RFA vs. no RFA): mean 0, SE 0 | Difference in stent patency (RFA vs. no RFA): mean 1.16, SE 1.46 |
| C: 20% complications | Probability of a complication requiring inpatient stay: 20% | Probability of a complication requiring inpatient stay: 10% |
| D: 5% complications | Probability of a complication requiring inpatient stay: 5% | Probability of a complication requiring inpatient stay: 10% |
| E: low reviewer bias adjustment | HR (mortality): mean 0.32, 95% CI 0.15 to 0.69 | HR (mortality): mean 0.34, 95% CI 0.21 to 0.55 |
| Difference in stent patency: mean 1.18, 95% CI –2.49 to 4.86 | Difference in stent patency: mean 1.16, 95% CI –2.5 to 4.83 | |
| F: high reviewer bias adjustment | HR (mortality): mean 0.44, 95% CI 0.24 to 0.8 | HR (mortality): mean 0.34, 95% CI 0.21 to 0.55 |
| Difference in stent patency: mean 0.58, 95% CI –2.68 to 3.85 | Difference in stent patency: mean 1.16, 95% CI –2.5 to 4.83 | |
| G: expert bias adjustment without the Gao et al.28 study | HR (mortality): mean 0.31, 95% CI 0.14 to 0.7 | HR (mortality): mean 0.26, 95% CI 0.16 to 0.42 |
| Difference in stent patency: mean 3.4, 95% CI –1.4 to 8.2 | Difference in stent patency: mean 3.4, 95% CI –1.4 to 8.32 | |
| H: RFA favourable utilities | Locally advanced cancer utility: 0.71 | Locally advanced cancer utility: 0.61 |
| AE utility: 0.5 | AE utility: 0.57 | |
| I: moderately low advanced cancer utility | Locally advanced cancer utility: 0.5 | Locally advanced cancer utility: 0.61 |
| AE utility: 0.46 | AE utility: 0.57 | |
| J: very low advanced cancer utility | Locally advanced cancer utility: 0.4 | Locally advanced cancer utility: 0.61 |
| AE utility: 0.36 | AE utility: 0.57 | |
| K: greater survival for stent-only intervention | Number dying each period decreased by 10% | Yang et al.29 stent-only survival curve |
| L: lower survival for stent-only intervention | Number dying each period increased by 10% | Yang et al.29 stent-only survival curve |
| M: greater proportion of patients receive chemotherapy | 40% | 20% |
| N: lower proportion of patients receive chemotherapy | 10% | 20% |
The scenarios can be grouped into six broad categories:
-
stent patency
-
stent and RFA cost
-
bias
-
health utility
-
survival
-
chemotherapy.
Stent patency
Stent patency has a significant effect on cost because of the significant cost incurred for a reintervention. Despite the short life expectancy of these patients, around 67% of patients are modelled to have at least one reintervention, and many have two or more. There is no limit on the number of reinterventions in the model, and so the shorter the stent patency duration the more reinterventions there will be. If RFA increases survival, then there may also be an increase in stent reinsertions, and this will not be the case if RFA also increases stent patency by a certain degree. There is risk of bias associated with the stent patency effectiveness estimate, and this is covered in the bias category.
There was considerable uncertainty in the effect estimate for stent patency. The same stent patency for no RFA and stent patency effectiveness was assumed for subsequent reinterventions as for the first intervention. There is a lack of evidence for stent patency and the effectiveness of RFA in secondary RFA. A scenario analysis was designed to halve the duration of stent patency in a secondary RFA population in the stent-only group and halve the effectiveness of RFA in increasing stent patency in a secondary RFA population (see Table 12, scenario A).
Given the significance of stent patency on cost, a scenario was specified where the effectiveness of RFA on stent patency was zero and there was no uncertainty in this value (see Table 12, scenario B). The purpose of this scenario was to explore the effect of non-inferiority in stent patency on the cost-effectiveness of RFA.
Stent and radiofrequency ablation cost
The effect that increased survival has on the number of reinterventions is important because of the significant cost associated with a reintervention. The cost includes both the cost of a stent and RFA intervention and the cost of treating complications. The effect on the results of varying the cost of stent insertion and RFA was explored by increasing the probability of a complication to 20% (see Table 12, scenario C) and reducing the probability of a complication to 5% (see Table 12, scenario D).
Bias
The clinical studies identified were expected to be at risk of bias. Consequently, estimates of bias and the effect on the effectiveness estimates were sought. Two scenario analyses (see Table 12, scenarios E and F) with different methods of converting reviewer bias assessment of included studies into quantitative values and a scenario (see Table 12, scenario G) representing a clinical expert’s assessment of bias were specified to explore the effect of bias on the results. The results of the clinical expert bias assessment were compared with the base-case analysis excluding the Gao et al. 28 evidence, as that evidence was identified in the review update.
Health utility
The most important health utility value is the utility of living with advanced cancer, as this determines the value of additional survival that may be a result of RFA. There were limited data on the health utility for advanced bile duct cancer and advanced pancreatic cancer, and for AEs and the duration of events. Consequently, favourable and conservative sets of utilities were produced with respect to RFA. The conservative set was used in the base-case analysis. The favourable set was used in scenario H (see Table 12). In addition, lower utilities for cancer were assumed in alternative analyses (see Table 12, scenarios I and J).
Survival
Since some patients in the model received chemotherapy and others did not, and some patients received RFA and others did not, survival differed across these groups. The survival of patients in the stent-only group and not receiving chemotherapy was based on one study. 29 High and low survival estimates were assumed in scenario analyses (see Table 12, scenarios K and L).
Chemotherapy
Chemotherapy in this population is associated with longer life expectancy, and this may have particular importance in the model in terms of increasing the number of occlusions and reinterventions that may occur. A smaller effect is that any increase in stent patency leads to a longer uninterrupted period of receiving chemotherapy treatment. The proportion of patients fit to receive chemotherapy was based on clinical expert opinion. High and low values were specified in scenario analyses (see Table 12, scenarios M and N). These scenario analyses also help to determine the importance of the mortality HR for people who are fit to receive chemotherapy compared with people who are not fit to receive chemotherapy.
Expected value of information
Value of information analysis estimates the value of reducing decision uncertainty and there is an opportunity cost to the selection of the suboptimal intervention. Further information may reveal that an adopted intervention was suboptimal. The expected value of perfect information (EVPI) is the maximum expected gain in net benefit per patient that can be obtained from reducing uncertainty in model parameters. 110
The maximum expected gain in net benefit that could be achieved across the whole population is the population expected value of perfect information (PEVPI). PEVPI is calculated by multiplying the individual EVPI by the expected future population to benefit from the interventions. The total population to benefit was estimated using the equation:
where It is the incidence per year, t is the number of years and r is the annual discount rate.
The number of RFA procedures per year in the UK was assumed to be 2035 based on the number of stent placements reported in the NHS reference costs. 102 The population cost was discounted over a 10-year period and the annual discount rate was assumed to be 3.5%.
If PEVPI is not significantly greater than the cost of doing a specific piece of research, then there is no value in doing that research. A good-quality clinical trial to evaluate the effectiveness of a surgical intervention could cost up to £2M; however, the cost of a RCT varies considerably, and this is at the higher end. Examples of National Institute for Health and Care Research-funded trials of surgical interventions that cost less than, but close to, £2M are research awards NIHR128768111 and NIHR128815. 112
Expected value of perfect information and PEVPI can also be estimated for specific model parameters, either individually or in combination. For example, a clinical trial researching the effectiveness of RFA will likely provide information on both mortality and occlusion. A slightly more costly piece of research could add research into the quality of life of patients. EVPI when applied to a subset of parameters is known as EVPPI, and there is a corresponding value at the population level [i.e. the population expected value of partial perfect information (PEVPPI)]. The EVPPI methods used were those stated in Briggs et al. 110
Chapter 7 Cost-effectiveness results
Models were planned for patients with advanced bile duct cancer and patients with advanced pancreatic cancer, for both primary and secondary RFA. Owing to a lack of evidence surrounding the effectiveness of secondary RFA, no model was produced for secondary RFA. The studies included in the meta-analyses of the HR of mortality in Chapter 4, Survival, included both patients with bile duct cancer and patients with pancreatic cancer, although there was a higher proportion of patients with bile duct cancer. There was also a lack of evidence on difference in stent patency for pancreatic cancer separately to bile duct cancer. Consequently, one model was developed for primary RFA in patients with bile duct cancer.
Base-case results
Cost-effectiveness results
The cost-effectiveness results for the base-case probabilistic analysis, which does not adjust for bias in the effectiveness estimates, are reported in Table 13. The average discounted cost for the RFA intervention is £2659 more than the average discounted cost without the RFA intervention. The average discounted QALYs for the RFA intervention is 0.18 more than the average discounted QALYs without the RFA intervention. The ICER is £14,392 per QALY. The ICER increased to £14,511 when the updated AE risk data, including cholangitis, were used.
| Intervention | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Stent | 7185 | 0.46 | |||
| RFA plus stent | 9845 | 2659 | 0.64 | 0.18 | 14,392 |
The cost-effectiveness plane presenting the joint distribution of the incremental cost and incremental QALY estimates from the probabilistic analysis is presented in Figure 16. The scatterplot shows that there is considerable variation in the incremental QALY estimates.
The probability that RFA plus stent is cost-effective at different cost-effectiveness thresholds is presented as a CEAC in Figure 15. The probability that RFA plus stent is cost-effective is 0.82 at a £20,000 per QALY cost-effectiveness threshold and 0.92 at a £30,000 per QALY cost-effectiveness threshold.
Population expected value of perfect information results
The PEVPI for the base-case analysis is £9.14M at a cost-effectiveness threshold of £20,000 per QALY and £5.66M at a cost-effectiveness threshold of £30,000 per QALY, indicating that there may be value in undertaking further research. When the updated AE risk data were used in the analysis, the PEVPI was £10.07M at a cost-effectiveness threshold of £20,000 per QALY and £6.64M at a cost-effectiveness threshold of £30,000 per QALY.
Scenario analyses
The cost-effectiveness results of the scenario analyses are reported in Table 14.
| Scenario | Incremental cost (£) | Incremental QALYs | ICER (£) | Probability cost-effective | PEVPI (£M; £/QALY threshold) | ||
|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | £20,000 | £30,000 | ||||
| Base case | 2659 | 0.184 | 14,436 | 0.82 | 0.92 | 9.14 | 5.66 |
| A: lower stent patency for secondary stents | 2753 | 0.183 | 15,038 | 0.73 | 0.88 | 9.27 | 3.66 |
| B: zero RFA effectiveness in increasing stent patency and zero uncertainty | 3017 | 0.183 | 16,459 | 0.8 | 0.99 | 1.74 | 0.09 |
| C: 20% complications | 2850 | 0.183 | 15,565 | 0.79 | 0.9 | 12.02 | 7.7 |
| D: 5% complications | 2615 | 0.194 | 14,264 | 0.83 | 0.92 | 8.68 | 5.5 |
| E: low reviewer bias adjustment | 2704 | 0.196 | 13,811 | 0.81 | 0.92 | 8.83 | 5.29 |
| F: high reviewer bias adjustment | 2764 | 0.140 | 19,758 | 0.67 | 0.83 | 14.94 | 10.20 |
| G: expert bias adjustment without the Gao et al.28 study | 1425 | 0.213 | 7165 | 0.94 | 0.97 | 2.93 | 1.85 |
| H: favourable utility to RFA | 2670 | 0.151 | 12,503 | 0.86 | 0.93 | 7.92 | 4.89 |
| I: cancer utility 0.5, AE utility 0.46 | 2687 | 0.121 | 17,820 | 0.74 | 0.88 | 11.79 | 7.47 |
| J: cancer utility 0.4, AE utility 0.36 | 2666 | 0.218 | 22,070 | 0.62 | 0.81 | 10.35 | 9.45 |
| K: 10% improved survival (stent) | 2952 | 0.141 | 13,564 | 0.84 | 0.92 | 10.24 | 6.48 |
| L: 10% worse survival (stent) | 2340 | 0.183 | 16,604 | 0.76 | 0.89 | 10 | 6.4 |
| M: 40% fit to receive chemotherapy | 2940 | 0.184 | 16,037 | 0.81 | 0.91 | 9.66 | 5.92 |
| N: 10% fit to receive chemotherapy | 2595 | 0.183 | 14,090 | 0.82 | 0.91 | 10.2 | 6.51 |
Stent patency
Halving the time to occlusion for secondary stents without RFA and halving the effectiveness of RFA in increasing stent patency significantly lowers the probability that RFA is cost-effective at the £20,000 per QALY threshold from 0.82 to 0.73 (see Table 14, scenario A). Lower stent patency increases the number of reinterventions and, therefore, cost. The number of reinterventions increases more with RFA than without RFA because the expected proportional increase in survival associated with RFA is greater than the expected proportional increase in stent patency. The cumulative first reinterventions and the cumulative additional reinterventions (i.e. extra reinterventions) for the base-case analysis and scenario A are reported in Table 15. A person can have more than one extra reintervention. In the base case, 65% of the cohort population have a reintervention during the course of the model in the RFA group not receiving chemotherapy, compared with 61% of the population in the stent-only group, and the difference in the cumulative number of extra reinterventions is greater. Reducing stent patency for secondary interventions further increases the number of additional reinterventions, with a proportionate increase in the RFA and stent-only groups. Halving the time to occlusion without RFA and halving the effectiveness of RFA for secondary stents does not increase the PEVPI because the value of information is related to the expected benefit of RFA and the effectiveness of RFA for secondary stents has been reduced.
| Base case | Scenario A | |||
|---|---|---|---|---|
| Intervention | Cumulative reintervention | Cumulative extra reintervention | Cumulative reintervention | Cumulative extra reintervention |
| RFA | 65% | 70% | 65% | 113% |
| Stent only | 61% | 54% | 61% | 84% |
The effectiveness of RFA in increasing stent patency is far less certain than the evidence of the effectiveness of RFA for HR of mortality. The CI for difference in stent patency leaves open the possibility that RFA is associated with shorter stent patency than stent only. To investigate whether the uncertainty in the cost-effectiveness of RFA is to do with RFA needing to be non-inferior or superior to stent only, a scenario analysis was run with zero difference in stent patency between RFA and stent only, and with zero uncertainty. The result was an ICER of £16,459 per QALY (see Table 14, scenario B). The probability that RFA is cost-effective would be 0.8 at a £20,000 per QALY threshold and 0.99 at a £30,000 per QALY threshold. Furthermore, the PEVPI reduces from £9.14M to £1.74M at a £20,000 threshold, and from £5.66M to £0.09M at a £30,000 threshold. This indicates that, given the costs associated with an intervention, RFA does not need to be superior to stent only in stent patency for RFA to be cost-effective.
Stent and radiofrequency ablation cost
Increasing the cost of a reintervention by increasing the probability of a person having an intervention with a complication has a greater effect on the PEVPI than on the probability of being cost-effective (see Table 14, scenarios C and D). The greater the cost of a reintervention, the greater the cost of making the wrong decision.
Bias
The high estimate of bias from the reviewer assessment (see Table 14, scenario F) significantly reduces the probability that RFA is cost-effective and increases the PEVPI. The PEVPI increases from £9.1M to £14.1M at a £20,000 threshold and from £5.7M to £10.2M at a £30,000 threshold. The low reviewer bias estimate does not have much effect on the results (see Table 14, scenario E), and this is because of the considerable uncertainty already present in the estimate unadjusted for bias. The scenario analysis with expert bias adjustment (see Table 14, scenario G) does not include evidence from Gao et al. ,28 as that evidence was identified in the review update late in the project. The probability that RFA was cost-effective was reduced to 0.94 from 0.97 at a £20,000 per QALY cost-effectiveness threshold when compared with the base-case analysis, without the Gao et al. 28 evidence.
Health utility
The favourable utility assumption does not have a significant effect on the results (see Table 14, scenario H). Reducing the estimated utility for someone with advanced cancer significantly reduces the probability that RFA is cost-effective and increases the PEVPI, but there is no strong evidence that average utility is that low in this population (see Table 14, scenarios I and J).
Survival and chemotherapy
Small changes in the median survival in the stent-only group (see Table 14, scenarios K and L) and changes in the proportion of patients being fit for chemotherapy (see Table 14, scenarios M and N) have little impact on the results. The fact that these changes in the proportion of patients fit to receive chemotherapy have little effect on the results means that the uncertainty in the mortality HR of people fit to receive chemotherapy compared with people not fit to receive chemotherapy will also have little impact on the results.
Population expected value of perfect information
The PEVPI for the base-case analysis is £9.14M at a cost-effectiveness threshold of £20,000 per QALY and £5.66M at a cost-effectiveness threshold of £30,000 per QALY, indicating that there may be value in undertaking further research. The PEVPI associated with each scenario analysis is presented in Table 14, and the impact of scenario analyses is described in Scenario analyses.
Across all of the scenarios, apart from assuming that there is no effect of RFA on stent patency, the PEVPI is greater than £2M, indicating that a future trial may have some value. The PEVPI increases with plausible levels of bias adjustment in the effectiveness estimates. If the cost associated with an intervention were significantly higher than assumed in the base case, then the PEVPI would be significantly higher. The majority of the PEVPI is removed when the stent patency effectiveness of RFA is assumed to be zero, and this is explored further in the estimation of PEVPPI.
Population expected value of partial perfect information
An EVPPI analysis was conducted for the HR of mortality, difference in stent patency and both parameters combined. These analyses were run for the base-case analysis, the scenario where stent patency and the effectiveness of RFA was halved following secondary RFA, and for the scenario analysis with the high bias estimate. The population EVPPI was calculated each time. The results are reported in Table 16. Assuming that the cost of a good clinical trial would be £2M, the PEVPPI for the HR of mortality is £42,084, which is much less than £2M (i.e. the cost of a good-quality clinical trial), indicating that uncertainty in the effectiveness estimate for the HR of mortality is not a reason to do further research on RFA, and this reflects the evidence strongly supporting greater mean survival with RFA.
| PEVPPI (£) | |||
|---|---|---|---|
| Scenario | Parameter | £20,000/QALY threshold | £30,000/QALY threshold |
| ln(HR) | 42,084 | ||
| Base case | Stent patency | 8,327,584 | 4,529,519 |
| ln(HR) and stent patency | 8,457,815 | 4,166,388 | |
| Half-patency duration | ln(HR) and stent patency | 3,116,139 | 767,124 |
| High bias adjustment | ln(HR) and stent patency | 14,044,549 | 10,151,764 |
The majority of the PEVPI is attributable to uncertainty in the effectiveness of RFA in increasing stent patency, and this is reflected in the PEVPPI values of £8.3M at a £20,000 per QALY threshold and £4.5M at a £30,000 per QALY threshold for stent patency. These PEVPPI values are greater than £2M, which is a high estimate of the cost of a good-quality trial. A clinical trial would not eliminate uncertainty in the effectiveness estimate; however, decision uncertainty could almost be eliminated by demonstrating RFA non-inferiority in stent patency in a quality clinical study.
Summary
A cost-effectiveness model was produced for primary RFA only. The effectiveness evidence came from studies with either a bile duct cancer population or a mixed population of patients with bile duct cancer and patients with pancreatic cancer, but with a higher proportion of patients with bile duct cancer.
The results are most applicable to an unresectable bile duct cancer population. Every study included in the meta-analysis of the HR of survival was at least at moderate risk of bias. Furthermore, only one of four studies16,28–29,34 was conducted in the UK.
The economic analysis showed that RFA was cost-effective at a cost-effectiveness threshold of £20,000 per QALY in almost all scenarios evaluated and there was moderate uncertainty in the results. If the effect of bias on the effectiveness results is in fact greater than the high estimate derived from the reviewers’ assessment of risk of bias due to, for example, unaccounted for external validity bias, then it is possible that the RFA would no longer be cost-effective at a threshold of £20,000 per QALY. With the high estimate of bias assumption, the probability that RFA is cost-effective is 0.69 at a £20,000 per QALY threshold and 0.85 at a £30,000 per QALY threshold. The effectiveness of RFA in increasing time to occlusion was the parameter that had the greatest impact on the results and for which there was very little evidence. Given the design of the economic model and the intervention cost assumptions, it was shown that RFA did not need to be superior to stent only in terms of increasing time to occlusion, but that it should not be significantly inferior. The value of information analysis showed that further research to evaluate the effect of RFA on stent patency may be warranted, and the higher PEVPI estimates after accounting for risk of bias lend greater support to this. There was also very little evidence on health-related quality of life to inform the health states and events in the model. It would be efficient for any future trial investigating the effectiveness of RFA to also evaluate health-related quality of life.
Chapter 8 Discussion
To the best of our knowledge, this is the largest and most comprehensive review of the role of RFA in malignant biliary obstruction to date. Previous reviews have suggested that RFA may be of benefit to overall survival but were based on analysis of largely retrospective, small, single-centre, non-randomised studies. In addition, previous reviews have not conducted an analysis of cost-effectiveness. To better understand the role of RFA in the management of malignant biliary obstruction, a full clinical effectiveness review was combined with a cost-effectiveness analysis using the most up-to-date data from published studies.
Summary
The clinical effectiveness review showed that primary RFA appears to be a beneficial adjunct to standard care in terms of increasing survival, agreeing with previous reviews. 113,114 Primary RFA reduces the hazard of mortality by at least 45% (i.e. the upper limit of CI in the main analysis). Five16,28,29,34,37 out of six of the studies in the subgroup analysis showed a statistically significant reduction in the hazard of mortality when primary RFA was used. None of the 18 comparative studies12,14,16,28–42 that reported mortality showed that primary RFA increased the risk of mortality compared with stent placement alone. There was no evidence that primary RFA increased two of the most common AEs (i.e. cholangitis and pancreatitis). Interestingly, there is some evidence that primary RFA may increase rates of cholecystitis, but this was significant in only one of the three studies reporting this outcome. 28,30,31 Cholecystitis is a well-recognised AE that can occur following insertion of covered metal stents at ERCP; however, the same stent types were used in both arms of these studies, suggesting that primary RFA adds to this risk. Cholecystitis was not reported in all studies and deserves further investigation. One possible mechanism is related to increased survival times in the primary RFA group, leading to a higher risk of developing cholecystitis over time. None of the studies reported the interval at which this cholecystitis occurred. The results appear to be generalisable, as the studies were conducted in many different countries and health-care systems.
There was insufficient evidence to be able to perform a meta-analysis for secondary RFA. Evaluation of the limited number of comparative studies in this area showed no difference in mortality rates between patients receiving RFA and patients receiving standard care. 35,42 There was insufficient information in the secondary RFA studies to determine whether or not there was any difference in AE rates.
Perhaps the most important finding was the lack of evidence reported about factors prioritised by our PPI colleagues (e.g. quality of life and well-being, personal costs and financial impact on carers) in either the primary or the secondary RFA studies.
The economic analysis showed that RFA was cost-effective at a cost-effectiveness threshold of £20,000 per QALY in almost all scenarios evaluated, and there was moderate uncertainty in the results. The effectiveness of RFA in increasing time to occlusion was the parameter that had the greatest impact on the results, but for which there was very little evidence. Given the design of the economic model and the intervention cost assumptions, it was shown that RFA did not need to be superior to stent only in terms of increasing time to occlusion. The value of information analysis showed that further research to evaluate the effect of RFA on stent patency may be warranted, and the higher PEVPI estimates after accounting for risk of bias lend greater support to this. There was also very little evidence on health-related quality of life to inform the health states and events in the model. It would be efficient for any future trial investigating the effectiveness of RFA to also evaluate health-related quality of life.
Strengths
This project benefited from a multidisciplinary team with clinical, methodological and expert-by-experience backgrounds. The systematic review of clinical effectiveness used robust methods, which involved a comprehensive search strategy (including for non-English-language studies), independent duplicate screening of results at title and abstract stage, and independent checking of the data extracted by a second reviewer.
The economic analysis used the best available evidence in the development of the economic model. Despite the limited evidence, there was enough evidence to enable the cost-effectiveness of RFA and the value of future research to be evaluated for primary RFA. Given the paucity of effectiveness and quality-of-life evidence for the use of RFA in advanced bile duct cancer and advanced pancreatic cancer populations, a thorough assessment of uncertainty through probabilistic and scenario analyses was conducted. Plausible estimates of the effect of risk of bias in the clinical studies on the effectiveness estimates were obtained to explore the value of doing further good-quality research.
Limitations
There are some limitations to this review. Only a small number of studies (n = 6) could be included in the meta-analysis looking at survival because of the differences in outcome measures, but none of the comparative studies (of a total of 18) reported a decrease in survival in the RFA group. Several studies needed to be excluded from either the base-case analysis and/or the sensitivity analysis because of a number of factors. The most common reasons were a lack of a comparator group9,13,17,20,43-88 or use of mixed populations within the study. 9,12,13,16,17,28,30,31,38,44,46-49,54,55,57,62,63,69,71,77 Many of the earliest publications were feasibility studies determining whether or not RFA was indeed deliverable in the biliary tract and, therefore, did not have a comparator group. Some studies used a mixture of tumour types (e.g. bile duct and pancreatic cancers) and some studies used both endoscopic and percutaneous methods to deliver RFA. 9,20,34,44,49,54,59,64,67,69 It was usually not possible to extract the data on patients having solely endoscopic RFA in these studies and, therefore, the studies were excluded on this basis. Similarly, it was not possible to extract the differences in outcomes between patients with bile duct cancer and patients with pancreatic cancer within an individual trial, and this may be important, as overall survival is generally longer in patients with bile duct cancer than in patients with pancreatic cancer. 115
One of the major findings was the lack of data on effectiveness of secondary RFA. There were insufficient data from the current studies to conduct the planned meta-analysis and data from the observational studies did not suggest a difference in survival. Given that patients in this group have had their cancer for longer and may be at a more advanced stage, survival may not be the most appropriate outcome to measure. There was limited evidence that stent patency is increased, but the evidence came from two very small studies. 35,42 There were few data on other potentially important outcomes in this group, such as reintervention rates, re-admission rates and quality of life.
A further limitation was the standard reporting of adverse outcomes. Some studies reported these outcomes as rates, whereas others gave average time to event. 12,14,16,28-39,42 In addition, some studies did not report AE rates between the intervention and control groups, confounding determination of potential harm. 12,34 The lack of detail in AE reports also meant that it was not possible to evaluate the consistency and similarity of case definitions across studies.
One other major limitation was the lack of standard reporting of other factors that may have had a positive effect on survival, particularly the use of chemotherapy. Some studies excluded patients who were suitable for chemotherapy12,29 and some studies included this as a variable in regression analysis, but this was not consistent. 14,16,34,40
Some studies used different stents to that of UK practice. 29,33 Metal stents are associated with better drainage and longer patency, and this might be a confounding factor, particularly in the secondary RFA group. 7 Variation in treatment practice that affects survival will also affect the cost-effectiveness results, although uncertainty related to study design and sample size is probably a greater factor.
There were also insufficient data to perform an analysis of outcomes between the different probe types, and this was because the majority of studies used the Habib EndoHPB probe rather than the StarMed (ELRA) probe. These two probes have slightly differing characteristics, with one being purely energy based (Habib EndoHPB)9 and the other using a temperature sensor to deliver pulsed RFA energy. 20 It is unknown whether or not the outcomes would be different with either probe, and future studies should document probe type and settings accurately.
Perhaps the biggest limitation was the lack of any data concerning quality of life. This is extremely important, as the groups of patients involved in these studies have non-curable cancers and, therefore, although affecting survival is desirable, improving or at least maintaining quality of life in this situation is paramount, and this was also a priority focus from the PPI group members.
The base-case meta-analysis included studies that were full-text papers and had adjusted for the proportion of patients receiving chemotherapy treatment, if that was necessary, given the study population inclusion criteria and the study design. Although the adjustment should ensure that the selection of studies in the base-case analysis is expected to be at less risk of bias than the studies excluded from the base-case analysis but included in the sensitivity analysis, all of the included studies in the base-case analysis were at moderate to high risk of bias, according to the assessments using the risk-of-bias tools. Consequently, there is a risk of bias associated with the meta-analysis estimates.
The lack of effectiveness evidence for RFA in a secondary RFA population also meant that no economic model could be developed for this population and no cost-effectiveness analysis was conducted. Given the different patient population, extrapolation from primary RFA may not be representative. There was also a lack of evidence to support separate economic analyses for patients with advanced bile duct cancer and patients with advanced pancreatic cancer, and this is an important consideration, as survival is generally longer in patients with bile duct cancers than in patients with pancreatic cancers and the majority of patients included in this review had bile duct cancers. 115
It was expected that the effectiveness evidence identified in the systematic review would be of poor quality. Consequently, a bias elicitation exercise was planned with the clinical experts on the advisory group identified as participants in the exercise. Owing to project delays due to the COVID-19 pandemic, late finalisation of the comparative studies to be included in the systematic review, the tight timelines from the end of the review to the end of the project, and difficulty in arranging for the clinical experts to be available at the same time to discuss the bias results, the step 2 discussion of the exercise did not happen. The clinical experts also needed more guidance on the assessment and marking of bias. In addition, the review update identified a study that could not be reviewed by the clinical experts because of project time constraints. 28 As a result, only one response could be identified where the bias quantification could be assessed as consistent with the assessed bias factors in each study. However, there was moderate uncertainty in the effectiveness results without accounting for bias, and this increased when accounting for the effect of bias estimated from reviewer bias assessments in the systematic review.
The economic model was limited by the available data. Limited data on time to occlusion meant that distribution assumptions needed to be made, and competing risk between death and occlusion could not be explicitly accounted for. There was no evidence on the effect on personal costs to patients and carers and, therefore, the study perspective was necessarily limited to the NHS and Personal Social Services perspective. Given the limitations in the data, the uncertainty associated with some parameters and assumptions could not be reflected in the base-case probabilistic sensitivity analysis results and, consequently, several scenario analyses were conducted to assess the impact on the cost-effectiveness results of different parameter assumptions. The lack of evidence meant that an economic model for only the primary RFA population, and largely bile duct population, could be developed.
Further research
Primary radiofrequency ablation
Assessment of quality of life in patients undergoing primary RFA at baseline and over the course of a trial is critically needed. High-quality prospective collection of data on AEs, particularly cholecystitis, is needed. An assessment is required of whether or not repeated application of RFA at specific intervals adds a further boost to survival. In all of these aspects, outcomes need to be adjusted for confounders, particularly chemotherapy. Given that stent patency is a crucial determinant of cost-effectiveness, the effect of RFA on stent patency should be accurately documented.
The majority of studies reported on patients with bile duct cancer and, therefore, a trial that allowed assessment of individual cancer types would be very useful.
All of the studies analysed were in unresectable cancers, as per the remit of the review; however, the effect of primary RFA as an adjunct in patients undergoing stent insertion prior to potentially curative surgery should be considered.
Assessment of the mechanism by which primary RFA leads to improved survival should be considered, and this may be achieved by looking at changes in markers for antitumour immunity, as has been previously suggested as a potential mechanism.
Secondary radiofrequency ablation
High-quality prospective RCTs with appropriate outcomes including quality of life, AE rates and survival, are needed. There would need to be adjustment for potential confounders, such as chemotherapy use prior to and after secondary RFA. Tumour type is also very important, with a reasonable proportion in this clinical group having metastatic tumours, such as colorectal cancer, which has generally better outcomes than pancreaticobiliary tract cancers.
Many studies are small and a further study would benefit from an appropriate sample size estimate. If the study has a sufficient sample size, then a study evaluating both time to occlusion and time to death could consider conducting competing risk survival analysis, as well as survival analysis and evaluating average stent duration, to allow comparison with existing studies.
Any future research should account for the research currently in progress.
Chapter 9 Conclusions
The current evaluation of the role of RFA in malignant biliary obstruction shows that there appears to be a significant positive effect on survival, particularly for primary RFA, and RFA is likely to be cost-effective. The cost-effectiveness of RFA seems to depend on RFA increasing stent patency and there is considerable uncertainty in the effect estimate. For primary RFA, there does not appear to be a negative effect on stent patency, but better-quality reporting of this outcome is needed. There appears to be a good AE profile, with no significant differences found in rates of abdominal pain, cholangitis and pancreatitis. There was an increased rate of cholecystitis in the RFA group compared with the stent-only group, which is not easy to explain. However, very few of the studies were large, prospective, multicentre, randomised trials and did not consistently report other important outcomes, including AEs, time to stent reocclusion, reintervention rates and, most importantly, effect on quality of life. As an adjunct to standard care, the addition of primary RFA appears to have a very high technical success rates and no effect on the ability to place a stent for drainage following application.
For secondary RFA, the evidence was far more limited, with no prospective randomised studies to inform decision-making. There was a lack of robust clinical effectiveness data and, therefore, more information is needed for this indication. In designing a trial to examine the effectiveness of RFA in this setting, outcomes would need to reflect the needs of the patients. Consistent reporting of AEs, time to stent occlusion and need for further admissions and interventions would be essential. Most importantly, information is needed on the effect of RFA on quality of life in this setting.
Endobiliary RFA has currently been used only in specialist centres (particularly in the UK) and largely in clinical trial settings, and has been carried out using careful patient selection. Therefore, wider routine clinical use would need to be performed using the same inclusion and exclusion criteria.
Implications for practice/decision-makers
The included studies were mostly assessed to be at moderate to high risk of bias. Despite the risk of bias, primary endobiliary RFA is likely to be cost-effective at the £20,000 per QALY and £30,000 per QALY cost-effectiveness thresholds, as there was a consistent and large survival benefit across the studies. The bias associated with these studies would need to be greater than that considered in this study for RFA to not be cost-effective at these thresholds. Alternatively, RFA would need to be associated with a higher risk of occlusion and, therefore, a shorter time to occlusion. There was some evidence of an increased risk of cholecystitis associated with RFA. The use of secondary RFA is currently lacking good effectiveness data and, as such, cannot be recommended for standard clinical practice.
Implications for research
The evidence for improved survival for primary RFA appears to be strong and RFA does not appear to be associated with an AE profile that would prevent the use of RFA. The evidence for secondary RFA is far less certain because there are far fewer studies examining this usage. There is a lack of data concerning very important outcomes for both primary and secondary RFA. The biggest driver of cost-effectiveness is stent patency, which is intimately related to quality of life, and the evidence for the effectiveness of RFA in increasing stent patency is weak. These areas should be the priority focus of future research.
Further research on endobiliary RFA should include the following:
-
Primary RFA effects on quality of life. Improvement in survival is important, but not at the cost of quality of life. Whether primary RFA improves, preserves or reduces quality of life is unknown, and this should be an essential measure in future studies.
-
Primary RFA AE profile. Cholecystitis can be a serious condition and is usually treated by surgical removal of the gallbladder. Undergoing such a procedure with an underlying advanced cancer is usually not advisable. Further evaluation of whether or not cholecystitis is related to patient factors, tumour factors or intervention factors should be conducted.
-
Mechanism of improved survival in primary RFA. An evaluation of effectiveness should be conducted to determine the mechanism by which primary RFA leads to improved survival.
-
Repeated applications of RFA. There is a lack of data on repeated applications of endobiliary RFA. The survival benefit seen could be postulated to be boosted by a further application at given time points, and this hypothesis should be tested.
-
Secondary RFA. More and higher-quality data are needed in this area specifically. Larger and higher-quality, preferably, prospective randomised studies with robust and patient-centred outcomes should be conducted to examine the clinical effectiveness and AE profiles. Data are also needed concerning the absolute requirement for further stent insertion in this group.
-
Secondary RFA. More and higher-quality data are needed on cost-effectiveness in this area, and this could be incorporated into a study that is examining clinical effectiveness.
-
Secondary RFA. If secondary RFA shows clinical effectiveness, as for primary RFA, then an evaluation of the mechanism by which secondary RFA works should be conducted.
Acknowledgements
The authors would like to warmly thank the following members of our Clinical Advisory Board who gave up their time to contribute to this project: Professor Irfan Ahmed (clinical expert), Jayne Fairburn (PPI colleague), Dr Manu Nayar (clinical expert) and Dr Kofi Oppong (clinical expert).
Patient and public involvement statement
Two PPI members were included in the Clinical Advisory Board for this project. Meetings were held via the Zoom video conferencing platform (Zoom Video Communications, San Jose, CA, USA) and chaired by John Leeds. Progress and data were presented with opportunities for Clinical Advisory Board members to give feedback. PPI members contributed to the design of the protocol, interpretation of the results and writing of the Plain English summary. Importantly, PPI members consulted on patient outcomes, identifying quality of life and well-being, personal costs and financial impact on carers as crucial outcomes of interest. As a result of PPI involvement, we identified a significant evidence gap surrounding quality-of-life outcomes. It also became apparent that patient-focused outcomes were lacking in all trials, and this has contributed to our recommendations for future research.
Contributions of authors
Fiona Beyer (https://orcid.org/0000-0002-6396-3467) (Senior Research Associate, Evidence Synthesis) co-led the writing of the protocol and the final report; led the conduct and write-up of the systematic reviews; supervised the design and running of the literature searches; and co-supervised the meta-analyses.
Stephen Rice (https://orcid.org/0000-0002-6767-0813) (Senior Research Associate, Health Economics) co-led the writing of the protocol and the final report; led the development of the cost-effectiveness model and the value of information analysis; and co-supervised the meta-analyses.
Giovany Orozco-Leal (https://orcid.org/0000-0002-7473-7318) (Research Assistant, Health Economics) contributed to the screening of studies for the systematic reviews, data extraction for the systematic review, development of the cost-effectiveness model and the value of information analysis.
Madeleine Still (https://orcid.org/0000-0003-0625-6325) (Research Assistant, Evidence Synthesis) contributed to the screening of studies, data extraction and risk-of-bias assessments for the systematic reviews; and contributed to the writing of the final report.
Hannah O’Keefe (https://orcid.org/0000-0002-0107-711X) (Training Fellow, Evidence Synthesis) designed and ran the searches for the systematic reviews; helped to co-ordinate the screening; produced the PRISMA flow charts; managed the references and referencing; and contributed to the writing of the final report.
Nicole O’Connor (https://orcid.org/0000-0002-6654-7178) (Training Fellow, Evidence Synthesis) contributed to screening and data extraction for the systematic reviews; and contributed to writing of the final report.
Akvile Stoniute (https://orcid.org/0000-0001-8279-5123) (Training Fellow, Evidence Synthesis) contributed to data extraction and meta-analyses for the systematic reviews; and contributed to writing of the final report.
Dawn Craig (https://orcid.org/0000-0002-5808-0096) (Professor of Practice in Evidence Synthesis) contributed to the design and writing of the protocol; provided advice on the systematic review, model and value of information analyses methods; and contributed to the final report.
Stephen Pereira (https://orcid.org/0000-0003-0821-1809) (Consultant Gastroenterologist and Hepatologist) was a clinical member of the Clinical Advisory Board; contributed to the design and writing of the protocol; provided clinical advice throughout; contributed to the bias elicitation assessment; and contributed to the writing of the final report.
Louise Carr (https://orcid.org/0000-0002-6730-4186) (PPI expert by experience) was a member of the Clinical Advisory Board; and contributed to the design of the protocol, the plain English outputs, the interpretation of the results and to the writing of the final report.
John Leeds (https://orcid.org/0000-0002-5140-6225) (Consultant Gastroenterologist) was the principal investigator and oversaw the running of the project; provided clinical advice throughout; and contributed to the design and writing of the protocol, to the screening and data extraction for the systematic review, to the bias elicitation assessment and to the writing of the final report.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
Appendix 1 Search strategy: clinical effectiveness search
MEDLINE (via Ovid)
Search date: March 2020
Dates searched: January 2008 to March 2020
Search strategy
-
exp Radiofrequency Ablation/
-
(“radio?frequen* ablat*” or RFA).ti,ab,kw,kf.
-
exp Catheter Ablation/
-
“catheter ablat*”.ti,ab,kw,kf.
-
“coagulative necro*”.ti,ab,kw,kf.
-
“thermal* ablat*”.ti,ab,kw,kf.
-
(bipolar adj4 (catheter* or probe* or ablat*)).ti,ab,kw,kf.
-
or/1-7
-
exp Pancreatic Neoplasms/
-
pancreatic adenocarcinoma.ti,ab,kw,kf.
-
exp Bile Duct Neoplasms/
-
exp Cholangiocarcinoma/
-
gallbladder neoplasms/
-
adenoma, bile duct/
-
duodenal neoplasms/
-
common bile duct neoplasms/
-
cholangiocarcinom*.ti,ab,kw,kf.
-
((bile or biliar* or endobiliar* or bile duct or pacrea* or choliangio*) adj4 (obstruct* or occlu* or cancer* or carcinom* or adenocarcinom* or tumo?r* or malignan* or lump* or mass or masses or sarcom* or metastas* or stricture*)).ti,ab.
-
exp Biliary tract disease/
-
“stent*”.ti,ab.
-
Self Expandable Metallic Stents/
-
((intraductal or intraluminal or unresect*) adj4 (obstruct* or occlu* or cancer* or carcinom* or adenocarcinom* or tumo?r* or malignan* or lump* or mass or masses or sarcom* or metastas* or stricture*)).ti,ab.
-
or/9-22
-
8 and 23
-
(EndoHBP or ELRA).ti,ab,kw,kf.
-
24 or 25
-
exp Animals/ not exp Human/
-
26 not 27
-
limit 28 to yr=“2008-Current“
Appendix 2 Search strategy: cost-effectiveness search
MEDLINE (via Ovid)
Search date: May 2020
Dates searched: January 2008 to May 2020
Search strategy
-
exp Radiofrequency Ablation/
-
(“radio?frequen* ablat*” or RFA).ti,ab,kw,kf.
-
exp Catheter Ablation/
-
“catheter ablat*”.ti,ab,kw,kf.
-
“coagulative necro*”.ti,ab,kw,kf.
-
“thermal* ablat*”.ti,ab,kw,kf.
-
(bipolar adj4 (catheter* or probe* or ablat*)).ti,ab,kw,kf.
-
or/1-7
-
exp Pancreatic Neoplasms/
-
pancreatic adenocarcinoma.ti,ab,kw,kf.
-
exp Bile Duct Neoplasms/
-
exp Cholangiocarcinoma/
-
gallbladder neoplasms/
-
adenoma, bile duct/
-
duodenal neoplasms/
-
common bile duct neoplasms/
-
cholangiocarcinom*.ti,ab,kw,kf.
-
((bile or biliar* or endobiliar* or bile duct or pacrea* or choliangio*) adj4 (obstruct* or occlu* or cancer* or carcinom* or adenocarcinom* or tumo?r* or malignan* or lump* or mass or masses or sarcom* or metastas* or stricture*)).ti,ab.
-
exp Biliary tract disease/
-
“stent*”.ti,ab.
-
Self Expandable Metallic Stents/
-
((intraductal or intraluminal or unresect*) adj4 (obstruct* or occlu* or cancer* or carcinom* or adenocarcinom* or tumo?r* or malignan* or lump* or mass or masses or sarcom* or metastas* or stricture*)).ti,ab.
-
or/9-22
-
8 and 23
-
(EndoHBP or ELRA).ti,ab,kw,kf.
-
24 or 25
-
exp Animals/ not exp Human/
-
26 not 27
-
limit 28 to yr=“2008-Current”
-
Economics/
-
exp “costs and cost analysis”/
-
Economics, Dental/
-
exp economics, hospital/
-
Economics, Medical/
-
Economics, Nursing/
-
Economics, Pharmaceutical/
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic $).ti,ab.
-
(expenditure$ not energy).ti,ab.
-
value for money.ti,ab.
-
budget$.ti,ab.
-
or/30-40
-
((energy or oxygen) adj cost).ti,ab.
-
(metabolic adj cost).ti,ab.
-
((energy or oxygen) adj expenditure).ti,ab.
-
or/42-44
-
41 not 45
-
letter.pt.
-
editorial.pt.
-
historical article.pt.
-
or/47-49
-
46 not 50
-
bmj.jn.
-
“cochrane database of systematic reviews“.jn.
-
health technology assessment winchester england.jn.
-
or/52-54
-
51 not 55
-
29 and 56
Appendix 3 Excluded studies list: clinical effectiveness review
No articles were excluded based on language alone. All articles were screened at title/abstract level, regardless of language. Google Translate (Google Inc., Mountain View, CA, USA) was used to assess an article if the title/abstract was not available in English. Full-text articles of potentially eligible articles that were not in the English language were translated by individuals fluent in those languages. Studies were excluded where international interlibrary loans were required because of The British Library’s limitations during the COVID-19 pandemic.
The full references for all excluded articles are provided in Table 17. Articles were excluded for one of the following reasons (in order of hierarchical importance):
| Reason for exclusion | Reference |
|---|---|
| The paper focuses on an ineligible patient population (n = 72) |
|
| The paper did not focus on endoscopic RFA plus stenting as the intervention (n = 168) |
|
| The paper focuses on an ineligible comparator (n = 5) |
|
| The paper did not focus on the outcomes of interest (n = 4) |
|
| The paper describes an ineligible study design (n = 214) |
|
| The paper was not retrievable by international interlibrary loan (n = 8) |
|
| The paper is an exact duplicate (n = 109) |
|
| The paper was an animal study (n = 1) |
|
-
The paper focuses on an ineligible patient population (n = 72).
-
The paper did not focus on endoscopic RFA plus stenting as the intervention (n = 169).
-
The paper focuses on an ineligible comparator (n = 5).
-
The paper did not focus on the outcomes of interest (n = 4).
-
The paper describes an ineligible study design (n = 217).
-
The paper was not retrievable by international interlibrary loan (n = 7).
-
The paper is an exact duplicate (n = 111).
-
The paper was an animal study (n = 1).
Appendix 4 Included studies list: clinical effectiveness review
| Study | Article type | Reference |
|---|---|---|
| Gao 2020 | Paper | Gao DJ, Yang JF, Ma SR, Wu J,Wang TT, Jin HB, et al. Endoscopic radiofrequency ablation plus plastic stent placement versus stent placement alone for unresectable extrahepatic biliary cancer: a multicenter randomized controlled trial. Gastrointest Endosc 2021;94:91–100.e2 |
| Yang 2018 | Paper | Yang J,Wang J, Zhou H, Zhou Y,Wang Y, Jin H, et al. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy 2018;50:751–60. https://doi.org/10.1055/s-0043-124870 |
| Trial registry | ClinicalTrials.gov. Endobiliary Radiofrequency Ablation With S-1 for Unresectable Cholangiocarcinoma. URL: https://ClinicalTrials.gov/show/NCT02592538 (accessed 27 September 2022) | |
| Hu 2016 | Abstract | Hu B, Gao DJ, Zhang X, Zhang YC. Endobiliary radiofrequency ablation improve overall survival of cholangiocarcinoma: a multi-center randomized control study. Gastrointest Endosc 2016;83:AB126 |
| Abstract | Hu B, Gao D-j, Zhang X, Zhang Y-c. 121 Endobiliary radiofrequency ablation improve overall survival of cholangiocarcinoma: a multi-center randomized control study ... 2016 DDW (Digestive DiseaseWeek) ASGE (American Society for Gastrointestinal Endoscopy) program and abstracts 21 May 2016–24 May 2016, San Diego, California. Gastrointest Endosc 2016;83:AB126 | |
| Trial registry | ClinicalTrials.gov. Endobiliary RFA for Unresectable Malignant Biliary Strictures. URL: https://ClinicalTrials.gov/show/NCT01844245 (accessed 27 September 2022) | |
| Hucl 2018 | Abstract | Hucl T, Macinga P, Gogova D, Fronek J, Spicak J. Radiofrequency ablation plus stenting vs. stenting alone in the treatment of pancreatic cancer and cholangiocarcinoma. Pancreas 2018;47:1394 |
| Trial registry | ClinicalTrials.gov. RFA for Malignant Biliary Obstruction. URL: https://ClinicalTrials.gov/show/NCT03166436 (accessed 27 September 2022) | |
| Teoh 2018 | Abstract | Teoh AY, Cheung SY, Chong C, Lee KF, Ng EK, Lai PB, et al. Endoscopic biliary radiofrequency ablation for malignant distal common bile duct strictures does not improve survival. A randomized controlled trial. Gastrointest Endosc 2018;87:AB104–AB5 |
| Trial registry | ClinicalTrials.gov. Endoscopic Biliary Radiofrequency Ablation of Malignant Distal Common Bile Duct Strictures. URL: https://ClinicalTrials.gov/show/NCT01721174 (accessed 27 September 2022) | |
| Yang 2017 | Abstract | Yang J, Zhou Y, Jin H, Gu W, Zhang X. Endoscopic biliary radiofrequency ablation prolong the survival of patients with unrespectable extrahepatic cholangiocarcinoma. Gastrointest Endosc 2017;85:AB605 |
| Abstract | Yang J. Clinical effect and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma, a prospective study. United European Gastroenterol J 2017:A124–A5 | |
| Bokemeyer 2019 | Paper | Bokemeyer A, Matern P, Bettenworth D, Cordes F, Nowacki TM, Heinzow H, et al. Endoscopic radiofrequency ablation prolongs survival of patients with unresectable hilar cholangiocellular carcinoma – a case–control study. Sci Rep 2019;9:13685. https://doi.org/10.1038/s41598-019-50132-0 |
| Abstract | Bokemeyer A, Matern P, Bettenworth D, Cordes F, Nowacki T, Heinzow H, et al. Endoscopic radiofrequency ablation prolongs survival in patients with advanced hilar cholangiocellular carcinomas. Endoscopy 2019;51:S36 | |
| Kallis 2015 | Paper | Kallis Y, Phillips N, Steel A, Kaltsidis H, Vlavianos P, Habib N, Westaby D. Analysis of endoscopic radiofrequency ablation of biliary malignant strictures in pancreatic cancer suggests potential survival benefit. Dig Dis Sci 2015;60:3449–55. https://doi.org/10.1007/s10620-015-3731-8 |
| Sharaiha 2014 | Paper | Sharaiha RZ, Natov N, Glockenberg KS, Widmer J, Gaidhane M, Kahaleh M. Comparison of metal stenting with radiofrequency ablation versus stenting alone for treating malignant biliary strictures: is there an added benefit? Dig Dis Sci 2014;59:3099–102 |
| Abstract | Sharaiha RZ, Widmer J, Natov N, Gaidhane M, Kahaleh M. Comparison of self expanding metal stenting with radiofrequency ablation versus stenting alone in the treatment of malignant biliary strictures: is there an added benefit? United European Gastroenterol J 2013;1:A456 | |
| Trial registry | ClinicalTrials.gov. Radio Frequency Ablation in the Management of Pancreatico-biliary Disorders: A Multicenter Registry. URL: https://ClinicalTrials.gov/show/NCT01439698 (accessed 27 September 2022) | |
| Dutta 2017 | Paper | Dutta AK, Basavaraju U, Sales L, Leeds JS. Radiofrequency ablation for management of malignant biliary obstruction: a single-center experience and review of the literature. Expert Rev Gastroenterol Hepatol 2017;11:779–84. https://doi.org/10.1080/17474124.2017.1314784 |
| Abstract | Dutta AK, Basavaraju U, Sales L, Leeds JS. Radiofrequency ablation for management of malignant biliary obstruction. Gut 2015;64:A216–A7 | |
| Kadayifci 2016 | Paper | Kadayifci A, Atar M, Forcione DG, Casey BW, Kelsey PB, Brugge WR. Radiofrequency ablation for the management of occluded biliary metal stents. Endoscopy 2016;48:1096–101. https://doi.org/10.1055/s-0042-115938 |
| Abstract | Atar M, Kadayifci A, Forcione DG, Casey B, Kelsey PB, Brugge WR. 1061 Efficacy of radiofrequency ablation (RFA) for the management of occluded biliary metal stents. Gastrointest Endosc 2015;81:AB195 | |
| Andalib 2017 | Abstract | Andalib I, Tyberg A, Siddiqui A, Novikov AA, Gaidhane M, Kedia P, et al. Comparison of endoscopically applied radiofrequency ablation with stenting versus stenting alone in patients with unresectable malignant biliary obstruction: can we improve our biliary drainage? Gastrointest Endosc 2017;85:AB611–AB2 |
| Buerlein 2019 | Abstract | Buerlein R, Strand DS, Patrie JT, Sauer BG, Shami VM, Scheiman JM, et al. 544 ERCP-directed biliary ablation prolongs survival in patients with unresectable perihilar cholangiocarcinoma compared to stenting alone. Gastrointest Endosc 2019;89:AB91–AB2 |
| Kallis 2011 | Abstract | Kallis Y, Phillips N, Steel A, Baldwin C, Nicholls J, Jiao L, et al. First report of the long-term efficacy of a novel endoscopic radiofrequency ablation technique for malignant biliary obstruction. Gut 2011;60:A9 |
| Nair 2020 | Abstract | Nair P, Rao H, Koshy A, Venu RP. Safety and efficacy of intraluminal RFA for inoperable cholangiocarcinoma – a prospective cohort study. J Gastroenterol Hepatol 2019;34:622. https://doi.org/10.1111/jgh.14865 |
| Sampath 2016 | Abstract | Sampath K, Hyder SM, Gardner T, Gordon SR. The effect of endoscopic radiofrequency ablation on survival in patients with unresectable peri-hilar cholangiocarcinoma. Gastrointest Endosc 2016;83:AB595 |
| Schwarzer 2016 | Abstract | Schwarzer R, Hametner S, Ziachehabi A, Gerstl S, Fugger R, Schofl R, et al. Therapeutic options in patients with malignant biliary obstruction-a retrospective single center analyze. Z Gastroenterologie 2016;54 |
| Wu 2017 | Abstract | Wu J, Gao DJ, Hu B. Endoscopic radiofrequency ablation for management of occluded metal stents in malignant distal biliary obstruction. Gastrointest Endosc 2017;85:AB95 |
| Alis 2013 | Paper | Alis H, Sengoz C, Gonenc M, Kalayci MU, Kocatas A. Endobiliary radiofrequency ablation for malignant biliary obstruction. Hepatobiliary Pancreat Dis Int 2013;12:423–7 |
| Dolak 2014 | Paper | Dolak W, Schreiber F, Schwaighofer H, Gschwantler M, Plieschnegger W, Ziachehabi A, et al. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc 2014;28:854–60. https://doi.org/10.1007/s00464-013-3232-9 |
| Trial registry | ClinicalTrials.gov. Radiofrequency Ablation for Malignant Biliary Obstruction. URL: https://ClinicalTrials.gov/show/NCT01758341 (accessed 27 September 2022) | |
| Figueroa-Barojas 2013 | Paper | Figueroa-Barojas P, Bakhru MR, Habib NA, Ellen K, Millman J, Jamal-Kabani A, et al. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol 2013;2013:910897. https://doi.org/10.1155/2013/910897 |
| Abstract | Figueroa-Barojas P, Bakhru MR, Habib N, Ellen K, Gaidhane M, Kahaleh M. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. Gastrointest Endosc 2011;73:AB127 | |
| Trial registry | ClinicalTrials.gov. Endoscopic Bipolar Radiofrequency Probe (ENDOHPB) in the Management of Unresectable Bile Duct and Pancreatic Cancer. In: https://ClinicalTrials.gov/show/NCT01303159 (accessed 27 September 2022) | |
| Han 2020 | Paper | Han SY, Kim DU, Kang DH, Baek DH, Lee TH, Cho JH. Usefulness of intraductal RFA in patients with malignant biliary obstruction. Medicine 2020;99:e21724. https://doi.org/10.1097/MD.0000000000021724 |
| Lee 2019 | Paper | Lee YN, Jeong S, Choi HJ, Cho JH, Cheon YK, Park SW, et al. The safety of newly developed automatic temperature-controlled endobiliary radiofrequency ablation system for malignant biliary strictures: a prospective multicenter study. J Gastroenterol Hepatol 2019;34:1454–9. https://doi.org/10.1111/jgh.14657 |
| Ogura 2017 | Paper | Ogura T, Onda S, Sano T, Takagi W, Okuda A, Miyano A, et al. Evaluation of the safety of endoscopic radiofrequency ablation for malignant biliary stricture using a digital peroral cholangioscope (with videos). Dig Endosc 2017;29:712–17. https://doi.org/10.1111/den.12837 |
| Sharaiha 2015 | Paper | Sharaiha RZ, Sethi A, Weaver KR, Gonda TA, Shah RJ, Fukami N, et al. Impact of radiofrequency ablation on malignant biliary strictures: results of a collaborative registry. Dig Dis Sci 2015;60:2164–9. https://doi.org/10.1007/ s10620-015-3558-3 |
| Steel 2011 | Paper | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, et al. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc 2011;73:149–53. https://doi.org/10.1016/j.gie.2010.09.031 |
| Abstract | Steel A, Postgate A, Vlavianos P, Khorsandi S, Habib N, Westaby D. PTU-021 The use of a novel endoscopically placed radiofrequency probe for the management of malignant bile duct obstruction. Gut 2010;59:A56–A57 | |
| Tal 2014 | Paper | Tal AO, Vermehren J, Friedrich-Rust M, Bojunga J, Sarrazin C, Zeuzem S, et al. Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction. World J Gastrointest Endosc 2014;6:13–19. https://doi.org/10.4253/wjge.v6.i1.13 |
| Abstract | Tal AO, Rust FM, Trojan J, Sarrazin C, Zeuzem S, Albert JG. Endoscopic treatment of malignant biliary stricture by intraductal radio-frequency ablation (RFA)-safety concerns from a pilot study. Gastrointest Endosc 2013;77:AB305 | |
| Battish 2016 | Abstract | Battish R, Lewis ME, Dehel BA, Niesley ME, Vashi P. Efficacy and safety of endoscopic retrograde cholango-pancreatography (ERCP) guided biliary radiofrequency ablation (RFA). Gastrointest Endosc 2016;83:AB612 |
| De Nucci 2019 | Abstract | De Nucci G, Domenico M, elli E, Redaelli D, Reati R, Morganti D, et al. Endoscopic radiofrequency ablation for extrahepatic malignant biliary obstruction: safety and efficacy of a single center experience. Endoscopy 2019;51:S154–S5 |
| Ermerak 2018 | Abstract | Ermerak G, Wu N, Abi HD, Edwards P, Bassan M. Endoscopic biliary radiofrequency ablation for the palliative management of malignant biliary obstruction: a prospective case series. J Gastroenterol Hepatol 2018;33:4 |
| Han 2019 | Abstract | Han S, Kim DU, Lee MW, Lee SH, Baek DH, Lee BE, et al. The feasibility of temperature-controlled endobiliary radiofrequency ablation in patients with advanced hilar cholangiocarcinoma. Gastrointest Endosc 2019;89:AB219 |
| Hashimoto 2019 | Abstract | Hashimoto S, Tanoue S, Iwashita Y, Kamikihara Y, Tsuneyoshi K, Nakamura Y, et al. Short-Term Outcomes of Endoscopic Radiofrequency Ablation for Unresectable Malignant Hilar Biliary Obstruction. Vienna: United European Gastroenterology; 2019 |
| Kahaleh 2014 | Abstract | Kahaleh M, Sharaiha RZ, Sethi A, Gonda TA, Shah RJ, Fukami N, et al. Radiofrequency ablation for palliation of malignant biliary strictures: an American collaborative experience. Gastrointest Endosc 2014;79:AB232 |
| Kallis 2012 | Abstract | Kallis Y, Phillips N, Steel A, Dickinson R, Nicholls J, Jiao L, et al. Radiofrequency ablation for biliary metal stent occlusion: Evolution of a novel endoscopic technique and proof of concept. Gastrointest Endosc 2012;75:AB377–AB8 |
| Ribeiro 2017 | Abstract | Ribeiro AL. Endobiliary radiofrequency ablation for palliation of malignant biliary stricture. Gastrointest Endosc 2017;85:AB616 |
| Samuel 2020 | Abstract | Samuel GO, Asagbra EE, Samuel OB, Arhinful J, Mudireddy P. Safety and efficacy of radiofrequency ablation in the palliative management of malignant biliary strictures. Am J Gastroenterol 2020;115:S491 |
| Saraswat 2018 | Abstract | Saraswat VA, Nayak H, Mohindra S, Ey G, Butala SP, Bhadauria AS. Early experience with endobiliary radiofrequency ablation (Endo-RFA) in unresectable malignant hilar biliary obstruction. Indian J Gastroenterol 2018;37:A98 |
| Ueno 2019 | Abstract | Ueno S, Ogura T, Okuda A, Nishioka N, Imoto A, Masuda D, et al. Evaluation of the safety of endoscopic radiofrequency ablation for malignant biliary stricture using a digital peroral cholangioscope. Gastrointest Endosc 2019;89:AB228 |
| Marti Romero 2019 | Paper | Martí Romero L, Martínez Escapa V, Castelló Miralles I, Estellés Arnau J, Querol Ribelles JM. Intraductal ablation by radiofrequency for inoperable biliopancreatic neoplasms with jaundice: experience at a regional hospital. Rev Esp Enferm Dig 2019;111:485–7. https://doi.org/10.17235/reed.2019.5720/ 2018 |
| Mukund 2013 | Paper | Mukund A, Arora A, Rajesh S, Bothra P, Patidar Y. Endobiliary radiofrequency ablation for reopening of occluded biliary stents: a promising technique. J Vasc Interv Radiol 2013;24:142–4. https://doi.org/10.1016/j.jvir.2012.09.018 |
| Nayar 2018 | Paper | Nayar MK, Oppong KW, Bekkali NLH, Leeds JS. Novel temperature-controlled RFA probe for treatment of blocked metal biliary stents in patients with pancreaticobiliary cancers: initial experience. Endosc Int Open 2018;6:E513–E517. https://doi.org/10.1055/s-0044-102097 |
| Abstract | Nayar M, Oppong K, Bekkali N, Leeds J. Preliminary results of a novel temperature controlled endo luminal radio-frequency ablation (ELRA) electrode for treatment of blocked biliary stents in patients with inoperable pancreaticobiliary cancers. Gut 2017;66:A210 | |
| Lewis 2012 | Abstract | Lewis J, Mehendiratta V, Korenblit J, Siddiqui AA, Kowalski TE, Loren DE. Safety of an endoscopic bipolar radiofrequency probe in the management of malignant biliary strictures: a single center experience. Gastrointest Endosc 2012;75:AB388 |
| Morales 2017 | Abstract | Morales MJ, De La Mora-Levy JG, Ortega Espinosa CR, Alonso-Larraga JO, Ramirez-Solis ME, Del Monte JS, et al. Endoscopic radio-frequency ablation of biliary strictures unleashed: a case series in a variety of clinical scenarios. Gastrointest Endosc 2017;85:AB640 |
| Mukund 2014 | Abstract | Mukund A, Rajesh S, Arora A, Panda D. Endobiliary RFA and balloon sweep to restore the patency of occluded metallic biliary stents-a feasibility study. J Vasc Interv Radiol 2014;25:S75 |
| Watson 2012 | Abstract | Watson J, Habr F. Safety and efficacy of endoscopic radiofrequency ablation in non-resectable cholangiocarcinoma: a case series. Am J Gastroenterol 2012;107:S78 |
| Bastos 2018 | Paper | Bastos VR, Safatle-Ribeiro AV, Baba ER, da Costa Martins B, Maluf-Filho F. The impact of probe-based confocal endomicroscopy on the management of indeterminate bile duct strictures. VideoGIE 2018;3:26–7. https://doi.org/10.1016/j.vgie.2017.10.003 |
| Gunasingam 2019 | Paper | Gunasingam N, Craig PI. Cholangioscopy-directed radiofrequency ablation of complex biliary cholangiocarcinoma. VideoGIE 2019;4:211–13 |
| Han 2018 | Paper | Han SY, Song GA, Kim DU, Baek DH, Lee MW, Kim GH. Bile duct patency maintained after intraductal radiofrequency ablation in a case of hepatocellular cholangiocarcinoma with bile duct invasion. Clin Endosc 2018;51:201–5. https://doi.org/10.5946/ce.2017.097 |
| Inoue 2020 | Abstract | Inoue T, Kitano R, Ibusuki M, Kobayashi Y, Ito K, Yoneda M. Simultaneous triple stent-by-stent deployment following endobiliary radiofrequency ablation for malignant hilar biliary obstruction. Endoscopy 2021;53:E162–E163 |
| Kruger 2018 | Paper | Kruger AJ, Krishna SG. Unexpected outcome following radiofrequency ablation of a malignant biliary stricture. Turk J Gastroenterol 2018;29:230–2 |
| Abstract | Kruger AJ, Krishna SG. An unexpected outcome following radiofrequency ablation of a malignant biliary stricture. Am J Gastroenterol 2017;112:S1126 | |
| Laquiere 2016 | Paper | Laquière A, Boustière C, Leblanc S, Penaranda G, Désilets E, Prat F. Safety and feasibility of endoscopic biliary radiofrequency ablation treatment of extrahepatic cholangiocarcinoma. Surg Endosc 2016;30:1242–8. https://doi.org/10.1007/s00464-015-4322-7 |
| Lee 2020 | Paper | Lee YW, Kim HJ, Lee SY, Heo J, Jung MK. Palliative measures with ethanol gallbladder ablation and endobiliary radiofrequency ablation followed by endoscopic biliary stent placement in an advanced case of common bile duct cancer: a case report. Korean J Gastroenterol 2020;75:50–5. https://doi.org/10.4166/kjg.2020.75.1.50 |
| Lorenzo 2018 | Paper | Lorenzo D, Barret M, Bordacahar B, Leblanc S, Chaussade S, Cattan P, Prat F. Intraductal radiofrequency ablation of an intraductal papillary mucinous neoplasia of the main pancreatic duct. Endoscopy 2018;50:176–7. https://doi.org/10.1055/ s-0043-121459 |
| Lui 2013 | Paper | Lui KL, Li KK. Intraductal radiofrequency ablation of tumour ingrowth into an uncovered metal stent used for inoperable cholangiocarcinoma. Hong Kong Med J 2013;19:539–41. https://doi.org/10.12809/hkmj133867 |
| Mansilla-Vivar 2019 | Paper | Mansilla-Vivar R, Arguello-Viudez L, Sanchez-Montes C, Alonso-Lazaro N, Pons-Beltran V. Endoluminal radiofrequency ablation with SpyGlass™ in the management of cholangiocarcinoma. Rev Esp Enferm Dig 2019;111:803–5 |
| Abstract | Mansilla-Vivar R, Alonso-Lazaro N, Arguello-Viudez L, Ponce-Romero M, Bustamante-Balen M, Sanchez-Montes C, et al. Endoluminal radiofrequency ablation with spyglass in the management of cholangiocarcinoma. Endoscopy 2019;51:S235 | |
| Mok 2017 | Paper | Mok SRS, Khara HS, Johal AS, Confer BD, Diehl DL. Cholangioscopic appearance after radiofrequency ablation of cholangiocarcinoma. VideoGIE 2017;2:279–83 |
| Monga 2011 | Paper | Monga A, Gupta R, Ramchandani M, Rao GV, Santosh D, Reddy DN. Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality (with videos). Gastrointest Endosc 2011;74:935–7. https://doi.org/10.1016/j.gie.2010.10.018 |
| Abstract | Monga A, Wee EWL, Gupta R, Ramch, ani M, Reddy DN. Endoscopic radiofrequency ablation of unresectable malignant obstructive jaundice. Ann Acad MedSingap 2011;40:S132 | |
| Trial registry | ClinicalTrials.gov. Role of Endoscopic RFA in Prolonging the Patency of Metal Stents in Patients With Malignant Obstructive Jaundice. URL: https://ClinicalTrials.gov/show/NCT01275768 (accessed 27 September 2022) | |
| Linz 2016 | Abstract | Linz CM, Modi RM, Krishna SG. A dual-modality approach of endobiliary radiofrequency ablation and self-expandable metal stent placement to control malignant hemobilia. Endoscopy 2017;49:E21–E2 |
| Ludvik 2019 | Abstract | Ludvik N, Kumar M, Fahmawi Y, Mizrahi M. Fire in the hole! Role of radiofrequency ablation for biliary stent occlusion: prolonging the stent patency. Am J Gastroenterol 2019;114:S767 |
| Morais 2019 | Abstract | Morais R, Vilas-Boas F, Antunes J, Pereira P, Macedo G. Endoscopic radiofrequency ablation for palliative treatment of hilar cholangiocarcinoma. Endoscopy 2019;51:S87 |
| Musquer 2016 | Abstract | Musquer N, Ménager Tabourel E, Luet D, Caroli-Bosc FX, Métivier Cesbron E. Recanalization of obstructed metallic uncovered biliary stent using endobiliary radiofrequency ablation. Gastrointest Endosc 2016;83:256–7. https://doi.org/10.1016/j.gie.2015.07.010 |
| Saumoy 2017 | Abstract | Saumoy M, Dawod E, Xu MM, Kahaleh M. Two-step endoscopic radiofrequency ablation for metastatic cholangiocarcinoma. Endoscopy 2017;49:E210–E211. https://doi.org/10.1055/s-0043-111714 |
| Schlosser 2019 | Abstract | Schlosser SH, Casty A, Netzer P. Endobiliary radiofrequency ablation (ELRA) for malignant billiary obstruction over 24 months follow-up. Swiss Med Wkly 2019;149:11S |
| Sonpal 2012 | Abstract | Sonpal N, Saitta P, Haber G. Maintaining stent patency with radiofrequency ablation and interim plastic stent placement for Klatskin tumors. Am J Gastroenterol 2012;107:S337 |
| Tian 2017 | Abstract | Tian Q,Wang G, Zhang Y, Jin Y, Cui Z, Sun X, Shen Z. Endoscopic radiofrequency ablation combined with fully covered self-expandable metal stent for inoperable periampullary carcinoma in a liver transplant patient: a case report. Medicine 2017;96:e5790. https://doi.org/10.1097/MD. 0000000000005790 |
| Tyberg 2015 | Abstract | Tyberg A, Zerbo S, Sharaiha RZ, Kahaleh M. Digital cholangioscopy: assessing the impact of radiofrequency ablation. Endoscopy 2015;47:E544. https://doi.org/10.1055/s-0034-1393144 |
| Yoon 2012 | Abstract | Yoon WJ, Brugge WR. Radiofrequency ablation of malignant biliary obstruction. Gastrointest Endosc 2012;75:AB116 |
| Trial registry | ClinicalTrials.gov. Endoscopic Therapy of Malignant Bile Duct Strictures. URL: https://ClinicalTrials.gov/show/NCT01543607 (accessed 27 September 2022) | |
| Ogura 2019 | Paper | Ogura T, Ueno S, Nishioka N, Yamada M, Higuchi K. Success of stent-in-stent deployment after intraductal radiofrequency ablation for hepatic hilar obstruction. Endoscopy 2020;52:E206–E207 |
| ClinicalTrials.gov | Trial registry | ClinicalTrials.gov. Cholangioscopic Assessment of Occluded Biliary Stent and Role of Biliary Radiofrequency Ablation. URL: https://ClinicalTrials.gov/show/NCT03133026 (accessed 27 September 2022) |
| ClinicalTrials.gov | Trial registry | ClinicalTrials.gov. Intra-luminal Radiofrequency Ablation for Inoperable Malignant Biliary Stenosis. URL: https://ClinicalTrials.gov/show/NCT02841800 (accessed 27 September 2022) |
| ClinicalTrials.gov | Trial registry | ClinicalTrials.gov. Endoscopic Biliary RFA of Malignant Bile Duct Obstruction. URL: https://ClinicalTrials.gov/show/NCT02582541 (accessed 27 September 2022) |
| ClinicalTrials.gov | Trial registry | ClinicalTrials.gov. Radiofrequency Ablation for Biliopancreatic Malignancy. URL: https://ClinicalTrials.gov/show/NCT02468076 (accessed 27 September 2022) |
| ClinicalTrials.gov | Trial registry | ClinicalTrials.gov. Endoscopic Biliary Co-axial Stent Placement Plus/Minus Use of Radiofrequency Ablation (RFA) for Clearance of Occluded Self Expandable Metal Stents (SEMS) in Patients With Distal Biliary Obstruction From Unresectable Biliary-pancreatic Malignancies. In: https://ClinicalTrials.gov/show/NCT02340728 (accessed 27 September 2022) |
| ClinicalTrials.gov | Trial registry | ClinicalTrials.gov. RFA RCT for Pancreatic or Bile Duct Cancer. URL: https://ClinicalTrials.gov/show/NCT02166190 (accessed 27 September 2022) |
| ClinicalTrials.gov | Trial registry | ClinicalTrials.gov. Radiofrequency Probe for Management of Unresectable Bile Duct and Pancreatic Cancer. URL: https://ClinicalTrials.gov/show/NCT02042859 (accessed 27 September 2022) |
| Kct0003373 | Trial registry | World Health Organization International Clinical Trials Registry Platform Search Portal. Treatment of Endobiliary Radiofreqeuncy Ablation for the Treatment of Malignant Extrahepatic Biliary Stricture. URL: https://trialsearch.who.int/Trial2.aspx?TrialID=KCT0003373 (accessed 16 November 2022) |
| Kct0004623 | Trial registry | World Health Organization International Clinical Trials Registry Platform Search Portal. Comparison of Endoscopic Radiofrequency Ablation Versus Stenting Alone for the Treatment of Unresectable Malignant Biliary Obstruction. URL: https://trialsearch.who.int/Trial2.aspx?TrialID=KCT0004623 (accessed 16 November 2022) |
| Kct0003275 | Trial registry | World Health Organization International Clinical Trials Registry Platform Search Portal. Efficacy of Additional Radiofrequency Ablation in Malignant Hilar Biliary Obstruction. 2018. URL: https://trialsearch.who.int/Trial2.aspx?TrialID= KCT0003275 (accessed 16 November 2022) |
| TCTR2019070 4002 | Trial registry | Thai Clinical Trials Registry. Endobiliary Radiofrequency Ablation in Recurrence and Unresectable Cholangiocarcinoma. URL: www.thaiclinicaltrials.org/show/TCTR20190704002 (accessed November 2021) |
| Jprn, U | Trial registry | N Kato. Exploratory Survey of the Safety and Efficacy of Endoscopic Radiofrequency Ablation for Malignant Biliary Stricture. URL: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000036026 (accessed November 2021) |
| Jprn, U | Trial registry | S Hashimoto. Endoscopic Biliary Radiofrequency Ablation with Multiple Metal Stents in Patients with Unresectable Malignant Hilar Biliary Stenosis: A Multicenter Prospective Observation Study. URL: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000030078 (accessed November 2021) |
| Jprn, U | Trial registry | K Ogura. Multicenter Prospective Study of Feasibility and Safety of Radio Frequency Ablation for Malignant Biliary Stricture Under ERCP Guidance. URL: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000030476 (accessed November 2021) |
| Jprn, U | Trial registry | HY Choi. Endobiliary Radiofrequency Ablation Using a New Catheter for Malignant Biliary Strictures: a Prospective Multicenter Study. URL: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000029721 (accessed November 2021) |
| Gastroenterology TOSCMCDo | Trial registry | UMIN-CTR Clinical Trial. Efficacy and Safety of the Endoscopic Radiofrequency Ablation for Unresectable Cholangiocarcinoma: The Pilot Study. 2017. URL: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000040330 (accessed 16 November 2022) |
| Gastroenterology TOSCMCDo | Trial registry | M Inoue. Endoscopic Radiofrequency Ablation Combined with Bilateral Metal Stent Placement for Malignant Hilar Biliary Obstruction. URL: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000037989 (accessed November 2021) |
| Gastroenterology TOSCMCDo | Trial registry | T Inoue. Prospective Evaluation of Radiofrequency Ablation for Stent Occlusion After Bilateral Metal Stent Placement in Patients with Malignant Hilar Biliary Obstructions. URL: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000045784 (accessed November 2021) |
| Gastroenterology TOSCMCDo | Trial registry | T Inoue. Prospective Evaluation of Radiofrequency Ablation Combined with a Novel Uncovered Metal Stent Placement for distal Malignant Biliary Obstruction. URL: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000045117 (accessed November 2021) |
Appendix 5 Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for cost-effectiveness review
FIGURE 18.
A PRISMA flow diagram for cost-effectiveness review. 116 Reproduced with permission from Moher et al. 116 This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The figure above includes minor additions and formatting changes to the original figure.
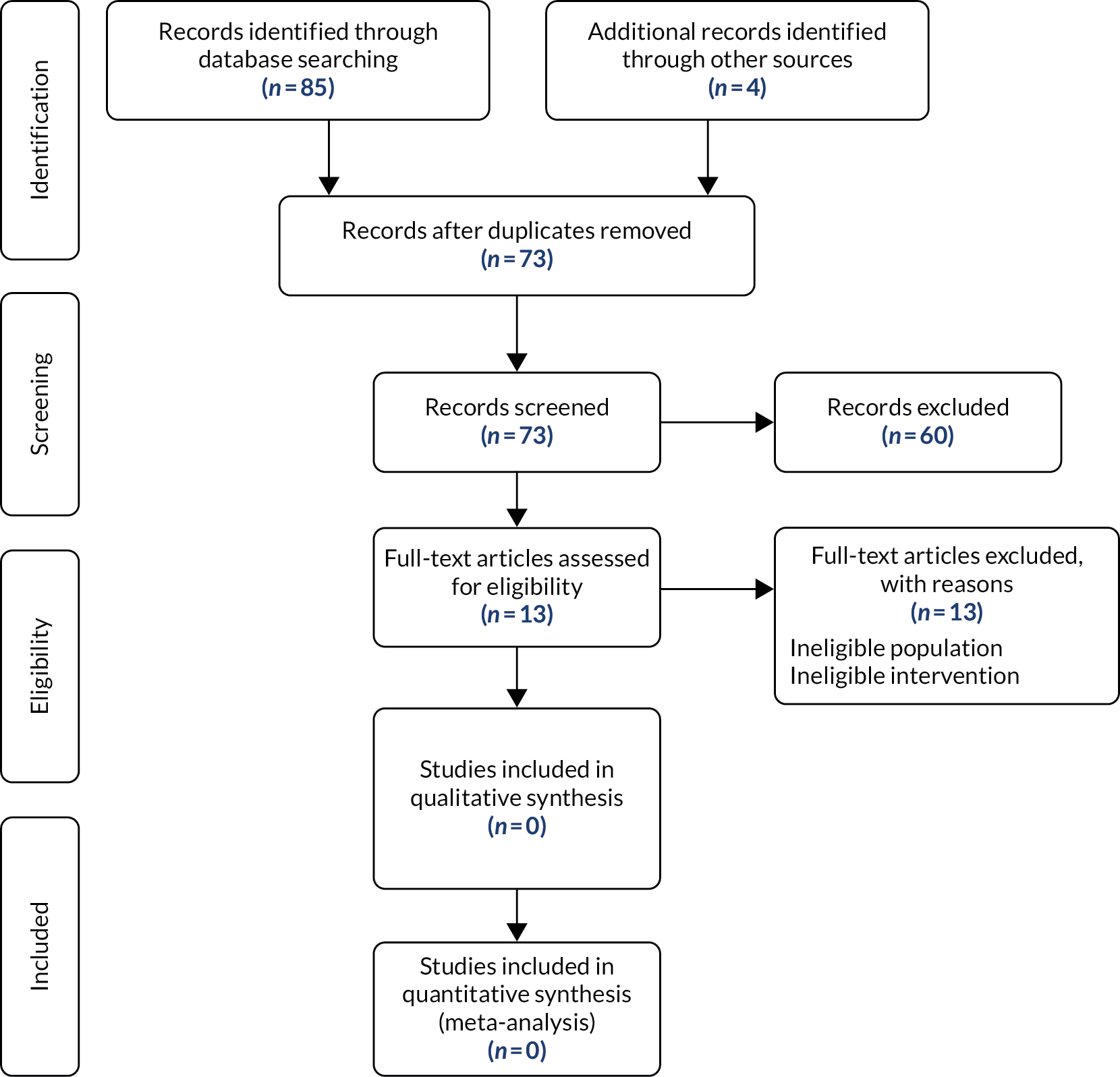
Appendix 6 Excluded studies list: cost-effectiveness review
No articles were excluded based on language alone. All articles were screened at title/abstract level, regardless of language. Google Translate was used to assess an article if the title/abstract was not available in English. Full-text articles of potentially eligible articles that were not in the English language were translated by individuals fluent in those languages. Studies were excluded where international interlibrary loans were required because of The British Library’s limitations during the COVID-19 pandemic.
The full references for all excluded articles are provided in Table X. Articles were excluded for one of the following reasons (in order of hierarchical importance):
-
The paper focuses on an ineligible patient population (n = 1).
-
The paper did not focus on endoscopic RFA plus stenting (n = 3).
-
The paper describes an ineligible study design (n = 9).
| Reason for exclusion | Reference |
|---|---|
| The paper focuses on an ineligible patient population (n = 1) |
|
| The paper did not focus on endoscopic RFA plus stenting (n = 3) |
|
| The paper describes an ineligible study design (n = 9) |
|
Appendix 7 Bias estimates
Turner et al. 96 report low, medium and high bias estimates on the log-odds ratio scale, and these estimates relate to 2.7%, 28% and 84% increases in the SE, respectively. Estimates for the log-HR scale and the mean difference scale were derived by find the same per cent increase in the SE assuming additive bias. Although Turner et al. 96 estimated the bias estimates assuming rare events, SEs from included studies for stent patency and HR of mortality were used to determine the bias estimates. The bias estimates used are reported in Table 20.
| % increase of SE | log-odds ratio | log-HR | Mean difference |
|---|---|---|---|
| 2.7 | 0.9, 0.9 | 0.92, 0.92 | 0.075, 0.075 |
| 28 | 0.7, 0.7 | 0.76, 0.76 | 0.255, 0.255 |
| 84 | 0.5, 0.5 | 0.59, 0.59 | 0.5, 0.5 |
Bias checklists
The bias checklists can be found in Turner et al. 96
Bias scales
Examples of bias scales are presented in Figures 19 and 20. 96
FIGURE 19.
Adjustment scale independent of the effect scale. None (1); low (0.92–1); medium (0.76–0.92); high (< 0.76). Adapted from Turner et al. 96 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 2.5) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited.
FIGURE 20.
Adjustment scale dependent on the effect scale. None (1); low (0.9–1.0); medium (0.7–0.9); high (< 0.7). Adapted from Turner et al. 96 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 2.5) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited.

Selection bias
Independent of effect scale
For bias that the assessor has indicated that the bias does NOT depend on the magnitude of the intervention effect, mark the degree of bias on the ‘independent of effect’ scale (Figure 19) and do this by dragging the crosses to a point on the line. Answer the following question: ‘even if there were no intervention effect in this study, what apparent effect (ignoring sampling variation) might be induced by this bias?’.
Dependent on effect scale
For bias that the assessor has indicated that the bias DOES depend on the magnitude of the intervention effect, mark the degree of bias on the dependent on effect scale (Figure 20). The assessor answers the question ‘What proportional change to the intervention effect (represented by the log-HR, ignoring sampling variation) might this bias induce?’.
Bias adjustment
The bias-adjusted mean and SE estimates for the individual studies were calculated using the formulae reported in section 6 of Turner et al. 96
Glossary
- Bacteraemia
- Presence of bacteria in the bloodstream.
- Bile duct
- A thin tube that goes from the liver to the small intestine.
- Cholangiocarcinoma
- Cancer of the bile duct.
- Cholangitis
- Inflammation of the bile duct system.
- Cholecystitis
- Inflammation of the gallbladder.
- Cohort study
- A prospective or retrospective non-randomised comparative study.
- Gallbladder
- A small, pear-shaped organ on the right side of the abdomen, beneath the liver.
- Haemobilia
- Bleeding in the biliary tree.
- Hepatic abscess
- A mass filled with pus inside the liver.
- Hyperamylasaemia
- An elevated level of serum amylase beyond the upper limit of the normal range.
- Liver infarction
- Areas of coagulative necrosis from hepatocyte cell death.
- Lumen
- The cavity or channel within a tube or tubular organ.
- Necrosis
- A form of cell injury that results in the premature death of cells in living tissue.
- Oesophageal tumours
- Tumours in the oesophageal area. The oesophagus is the long tube that carries food from the throat to the stomach.
- Pancreatitis
- Inflammation in the pancreas.
- Perforation
- A hole that develops through the wall of a body organ.
- Photodynamic therapy
- A treatment that involves light-sensitive medicine and a light source to destroy abnormal cells.
- Stenosis
- Narrowing or restriction of a blood vessel or valve that reduces blood flow.
List of abbreviations
- AE
- adverse event
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CRD
- Centre for Reviews and Dissemination
- EQ-5D
- EuroQol-5 Dimensions
- ERCP
- endoscopic retrograde cholangiopancreatography
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- PEVPI
- population expected value of perfect information
- PEVPPI
- population expected value of partial perfect information
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RFA
- radiofrequency ablation
- ROBINS-I
- Risk Of Bias In Non-randomized Studies – of Interventions
- SE
- standard error

