Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR134220. The contractual start date was in June 2021. The draft manuscript began editorial review in December 2021 and was accepted for publication in December 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Souto-Ribeiro et al. This work was produced by Souto-Ribeiro et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Souto-Ribeiro et al.
Chapter 1 Background
Description of the health problem
Prostate cancer is the most commonly diagnosed cancer in men in the UK1 and for males born after 1960 in the UK the estimated lifetime risk of being diagnosed with prostate cancer is 1 in 6 (18%). 2 The risk of developing prostate cancer increases with age and it mainly affects people aged 50 years or more. 3 The risk of developing prostate cancer is also higher for people of African family origin and for people where there is a family history of prostate cancer. 4 Most people who are diagnosed when their prostate cancer is at its earliest stage will survive for 5 years or more. If any of the following symptoms cannot be attributed to other health conditions, prostate cancer might be suspected:
-
lower back or bone pain
-
lethargy
-
erectile dysfunction
-
haematuria
-
weight loss
-
lower urinary tract symptoms, such as frequency, urgency, hesitancy, terminal dribbling and/or overactive bladder.
Epidemiology
In 2018, there were 49,810 new diagnoses of prostate cancer in England, an increase of 7985 more registrations than the previous year. 5 The age-standardised incidence rate in England was 204.7 per 100,000 in 2018, which was an increase from 182.8 per 100,000 in 2009. 6 The incidence rate for prostate cancer in the UK is projected to rise to 233 cases per 100,000 males by 2035. 1
Prostate cancer accounts for 30% of all male cancer diagnoses and is the most commonly diagnosed cancer in males over 45 years old. In 2018, 55% of prostate cancers were diagnosed at stages 1–25 and despite an increased incidence rate the age-standardised mortality rate decreased between 2009 and 2018 from 51 per 100,000 to 46 per 100,000. 6
In England, the South East has the highest age–sex-standardised rate of prostate cancer (228 per 100,000 people), compared with the North West at 171 per 100,000 people. 5 Prostate cancer incidence rates in males in England are 17% lower in the most deprived quintile compared with the least deprived quintile (2013–7). 1 Cancer Research UK states that ‘Prostate cancer is most common in black males, then white males and least common in Asian males’. 1
Description of the diagnostic technologies under assessment
When a person presents to primary care with clinical signs and symptoms that may be indicative of prostate cancer (such as the above), the National Institute for Health and Care Excellence’s (NICE) guideline on suspected cancer: recognition and referral (NG127) advises the following:
-
consider a prostate-specific antigen (PSA) test and digital rectal examination (DRE) to assess for prostate cancer in men with:
-
any lower urinary tract symptoms, such as nocturia, urinary frequency, hesitancy, urgency or retention; or
-
erectile dysfunction; or
-
visible haematuria.
-
-
refer men using a suspected cancer pathway referral (for an appointment within 2 weeks) for prostate cancer if their:
-
PSA levels are above the age-specific reference range; or
-
prostate feels malignant on DRE.
-
The NICE guideline on prostate cancer: diagnosis and management (NG1318) recommends that a multiparametric magnetic resonance imaging (mpMRI) test should be offered to people referred with suspected clinically localised prostate cancer. The results of the mpMRI test should be reported using a five-point Likert scale. The Likert scale takes into account clinical factors and lesion size, where a score of 1 indicates prostate cancer is very unlikely and a score of 5 indicates prostate cancer is very likely. 9
-
People who have a Likert scale score of 3 or more should be offered a mpMRI-influenced prostate biopsy.
-
For people with a Likert scale score of 1 or 2, the risks and benefits of having a biopsy are discussed and other factors, such as family history, are taken into account so that a shared decision about whether to have a biopsy or not can be made. If that decision is to have a biopsy, a systematic prostate biopsy should be offered.
-
For people who are not able to have radical treatment (e.g. radical prostatectomy, radical radiotherapy, or docetaxel chemotherapy) NG131 states that mpMRI should not be routinely offered.
An alternative to Likert scale assessment of mpMRI results that is not mentioned in NG131 is the Prostate Imaging Reporting and Data System (PI-RADS). This system was developed in 201210 and updated in 201511 and 2019. 12 Each lesion is assigned a score from 1 to 5 indicating the likelihood of clinically significant (CS) cancer (where 1 is very low and 5 is very high). The 2018 National Health Service (NHS) England handbook on implementing a timed prostate cancer diagnostic pathway13 indicates that people with a Likert or PI-RADS score of 1 or 2 and people with a Likert or PI-RADS score of 3 who also have a PSA density ˂ 0.15 (or 0.12 in some centres) nanograms of PSA per ml of serum per ml of prostate volume can be discharged from the diagnostic pathway. This would only occur after a discussion of the risks and benefits of biopsy and consensus between the doctor and the person about the most appropriate course of action.
There are two main routes by which a prostate biopsy can be obtained, the transrectal route and the transperineal route. In addition to the route, there are also different approaches to sampling the prostate tissue. The site (or sites) for biopsy can be targeted based on the findings from mpMRI or the biopsies can be systematic (i.e. samples are taken in a systematic fashion from different regions of the prostate according to a predefined scheme). Sometimes, after targeting sites of interest for biopsy, additional biopsy cores are taken from the area around the target lesion, or a systematic biopsy may be done in addition to the targeted biopsy.
If a mpMRI is contraindicated, factors such as PSA density and family history of prostate cancer would influence a decision about whether a systematic biopsy would be appropriate.
Transrectal ultrasound prostate biopsy
During a transrectal ultrasound (TRUS) prostate biopsy a TRUS probe is inserted into the anus to image the prostate. Samples of prostate tissue are collected using a biopsy needle inserted via the anus, through the rectal wall, and into the prostate. This procedure is typically carried out under local anaesthetic in an outpatient setting but can also be carried out under general anaesthetic (e.g. if the patient is unlikely to be able to tolerate the procedure under local anaesthetic). However, because the biopsy needle is inserted through the rectal wall, biopsy-related infections can occur, including, in some cases, sepsis (estimated to be 0.8% in a 2016 systematic review). 14 Sepsis is a serious infection which requires a hospital admission and antibiotics.
Traditionally, most prostate biopsies in the NHS used the TRUS method. However, there has been an increase in the use of transperineal biopsy (TP), and this has been accelerated due to the COVID-19 pandemic. A strategy document issued by the British Association of Urological Surgeons (BAUS) Section of Oncology for the interim management of prostate cancer during the pandemic recommended that TRUS biopsies should be avoided if possible. 15
Transperineal prostate biopsy
In common with TRUS, a transperineal prostate biopsy also uses a TRUS probe inserted into the anus to image the prostate, but the samples of prostate tissue are collected using a biopsy needle inserted through the perineum (the skin area between the anus and the scrotum) rather than through the rectal wall. Transperineal prostate biopsy can be conducted using any of the following methods:
-
a grid and stepping unit
-
a coaxial needle (‘double freehand’)
-
a freehand device (using one of the six devices listed in the NICE scope for this assessment).
Transperineal prostate biopsy using a grid and stepping device
Traditionally, transperineal biopsies were performed (using a grid and stepping device). The biopsy needle is passed through the perineum multiple times, creating a new skin puncture for every biopsy taken, and a broad area of local anaesthetic coverage was needed, hence the procedure typically took place under general anaesthetic.
Stepping devices are used to cradle the ultrasound probe and the grid provides a guide for needle insertion. Grid and stepping units are also used to perform brachytherapy for prostate cancer, and therefore they are available in treatment centres for this purpose at least. Each biopsy of the prostate requires a separate skin puncture. Many steppers can be fitted to a variety of different ultrasound probes and the grids are typically disposable, consisting of rows and columns of holes spaced 5 mm apart. The stepping unit is usually fixed to a stabiliser that is either mounted onto a table or supported by a floor stand.
Transperineal prostate biopsy using a coaxial needle (double freehand)
More recent TP techniques use an access needle which acts as a cannula, through which the biopsy needle is passed, allowing multiple biopsy samples to be taken through one access point. The access needle can be separate from the ultrasound probe (e.g. a coaxial needle), in which case it is known as the ‘double freehand’ technique. However, it may be technically challenging to master because the needle and ultrasound probe have to be kept in line manually, and this procedure is not extensively used within the NHS.
Transperineal prostate biopsy using a freehand device
As an alternative to the double freehand approach, the access needle can also be inserted through a positioning guide which is attached to the ultrasound probe. When the access needle and the ultrasound probe are physically coupled together, the device may be referred to as a freehand TP device and the user can more easily track the location of the biopsy needle in relation to the ultrasound probe. The access needle is typically inserted only twice, once to the left of the anal verge and once to the right of the anal verge. This limited number of access points means the procedure can be routinely completed using local anaesthetic during an outpatient appointment. The NICE scope for this assessment identified six proprietary freehand devices which are available for use in clinical practice in the UK. We describe the key features of each device below.
PrecisionPoint™ Transperineal Access System (BXTAccelyon Ltd, Burnham, UK)
PrecisionPoint is a single-use transperineal access system distributed by the company BXTAccelyon in the UK (they are the sole distributor outside North America). The device consists of a rail/clamp assembly that is mounted onto a sliding carriage. The Perineologic 15-gauge, 7-cm access needle is inserted through one of the five apertures on the sliding carriage (the aperture used depends on the height of the prostate). Local anaesthetic is used to enable the access needle to puncture the skin. Typically, only two punctures are required – one on the right and one on the left side of the anal verge. A biopsy needle is then inserted via the access needle and used to deliver local anaesthetic to the tract of tissues between the skin and the prostate so that the access needle can be advanced more deeply into the subcutaneous tissue. Multiple biopsies from different locations can be taken from each puncture of the skin. The PrecisionPoint transperineal access system can be used to perform targeted or systematic biopsies, with no limitation on the size of the prostate or the number of biopsies.
UA1232 puncture attachment (BK Medical, MA, USA)
The UA1232 puncture attachment is a reusable needle guide and mounting ring with lock screw that is designed for transperineal puncture and biopsy. The mounting ring and lock screw are used to attach the device to a BK medical ultrasound probe with the needle guide parallel to the centreline of the ultrasound transducer. The needle guide has nine parallel guide channels, spaced 5 mm apart vertically, each with an internal diameter of 2.1 mm, which is suitable for a 14-gauge coaxial/access needle. The coaxial/access needle can be inserted at different heights using the vertical guide channels and then localisation to the left and right is achieved by rotating the ultrasound probe (and so the attachment). If necessary, the position of the coaxial/access needle in the vertical guide can be changed (requiring an additional skin puncture) to access anterior, middle and posterior regions of the prostate. The 14-gauge needle is used for access and a separate biopsy needle is inserted through this to obtain the biopsy samples. After completion of the procedure, all parts of the puncture attachment are sterilised by either autoclave or immersion in a suitable disinfectant solution.
Cambridge Prostate Biopsy Device (JEB Technologies Ltd, Suffolk, UK)
The Cambridge Prostate Biopsy Device (CamPROBE) is a single-use transperineal access system designed to enable integrated local anaesthetic delivery. The device comprises a stainless-steel cannula housing an integrated needle. The integrated needle is used to deliver local anaesthetic under ultrasound guidance enabling the access needle to be placed in position. When the access needle is correctly located, the integrated needle is removed, and a standard 18-gauge core biopsy needle (not supplied as part of the device) is inserted via the access needle to take the prostate biopsies. The device is inserted on the left and right sides of the perineum mid-line: two punctures. A new device is used for each puncture; therefore, two devices are used per person. There is no physical connection between the access needle and the ultrasound probe and there is no needle guide, so the CamPROBE is therefore used with double freehand technique to manually keep the device in phase with the ultrasound probe. The CamPROBE device was initially for research use only while an application for CE marking was prepared. JEB Technologies launched the CE marked device in November 2022.
Trinity® Perine (KOELIS®, NJ, USA)
The Trinity Perine system, manufactured by KOELIS and distributed in the UK by Kebomed UK, includes reusable-guide Perine grids. The reusable-guide Perine grids come in two sizes, to accommodate either a 17–20-gauge or 14–16-gauge needle and they are designed to adapt on to a KOELIS K3DEL00 ultrasound probe. Each Perine grid has 20 marked needle positions spaced 3 mm apart. Grids can be reused up to 100 times.
SureFire Guide (LeapMed, Jiangsu, China)
The SureFire disposable transperineal needle guide biopsy kit includes a sterile needle guide, a latex-free cover and a sterile gel packet. The vertical needle guide has nine guide channels at different height settings allowing vertical access to 8 cm, and an ultrasound probe clamp. The needle guide is designed to adapt to BK Medical Biplane probes 8648, 8848, 9048 and E14C4b or Hitachi Healthcare Biplane probes U533, C41L47RP and UST-672. The vertical needle guide can be rotated to reach different areas of the left and right sides of the prostate. The device is used freehand (i.e. without the need for a stepper or stabilising device) and is available in two sizes, to accommodate either 15-/16-gauge needles or 17-/18-gauge needles.
EZU-PA3U (Hitachi Ltd, Tokyo, Japan)
The reusable EZU-PA3U puncture guide fixture is available for attachment to either the Hitachi CC41R or C41L47RP biplane transducers. The needle holder can slide vertically within the guide and the fixing screw is secured to keep it firmly in the intended position. The scale on the puncture guide fixture is marked with 0.5 cm divisions ranging from 1 to 5 cm. The puncture guide fixture is compatible with 14- and 18-gauge needles.
Care pathway
Figure 1 illustrates the current NICE pathway for people referred to specialist care for suspected prostate cancer. 16 Following referral [e.g. from a general practitioner (GP)], individuals follow different pathways based on key decision points, which can be summarised as follows:
FIGURE 1.
NICE pathway for diagnosing and staging prostate cancer.

-
Pre-biopsy imaging to determine whether or not a biopsy is necessary at that time.
-
Initial biopsy to detect the absence or presence of prostate cancer. This is where a transperineal or a TRUS approach to biopsy would be considered.
-
If the biopsy is negative but there is ongoing suspicion of prostate cancer, a re-biopsy may be done after an appropriate interval.
-
If the initial biopsy (or re-biopsy) is positive it may be termed CS/insignificant based on a risk classification incorporating biopsy core length and cancer grade. The level of significance reflects the predicted spread of the cancer over time and is informative when deciding to undergo active surveillance, or radical treatment.
Clinically significant prostate cancer
When prostate cancer is diagnosed, it is often distinguished in terms of whether the cancer is CS or insignificant. The purpose is to assess how rapidly the cancer will progress and, hence, whether to recommend active surveillance or active treatment. Expert clinical opinion suggests there is no universally agreed definition of the term CS prostate cancer. There are varying definitions available in the literature. For example, clinicians at University College London (UCL) devised criteria for defining CS cancer, as localised cancer with a maximum total cancer core length of 10 mm, a maximum cancer core length of 6 mm and a Gleason score of at least 4 + 3 or 3 + 5 (UCL definition 1). A second set of criteria from this group defines CS cancer as a maximum total cancer core length of 6 mm, a maximum cancer core length of 4 mm and a Gleason score of at least 3 + 4 (UCL definition 2). These criteria have been used in clinical trials assessing different prostate biopsy modalities, including the PROMIS trial in the UK, which examined the diagnostic accuracy of mpMRI and TRUS biopsy in prostate cancer. 17
The NICE clinical guideline prostate cancer diagnosis and management (NG131) defines CS prostate cancer as any prostate cancer of Gleason score 7 and above. 18
Chapter 2 Definition of the decision problem
One of the potential benefits of more widespread use of local anaesthetic transperineal (LATP) biopsies in clinical practice would be fewer serious infections associated with puncture of the rectum by the biopsy needle during TRUS biopsy. Fewer infections will reduce the need for preventive antibiotics and the need for antibiotic treatment of infection-related hospital admissions. Another potential benefit of LATP compared to a TP approach conducted under general anaesthetic transperineal (GATP) biopsy is that the use of a limited number of access points in LATP biopsy could reduce pain during and after the biopsy and would release some operating-theatre time. The basis of this diagnostic assessment therefore is to evaluate the empirical evidence in support of these proposed benefits using an economic (cost-effectiveness) decision-making perspective, to inform guidance to the NHS.
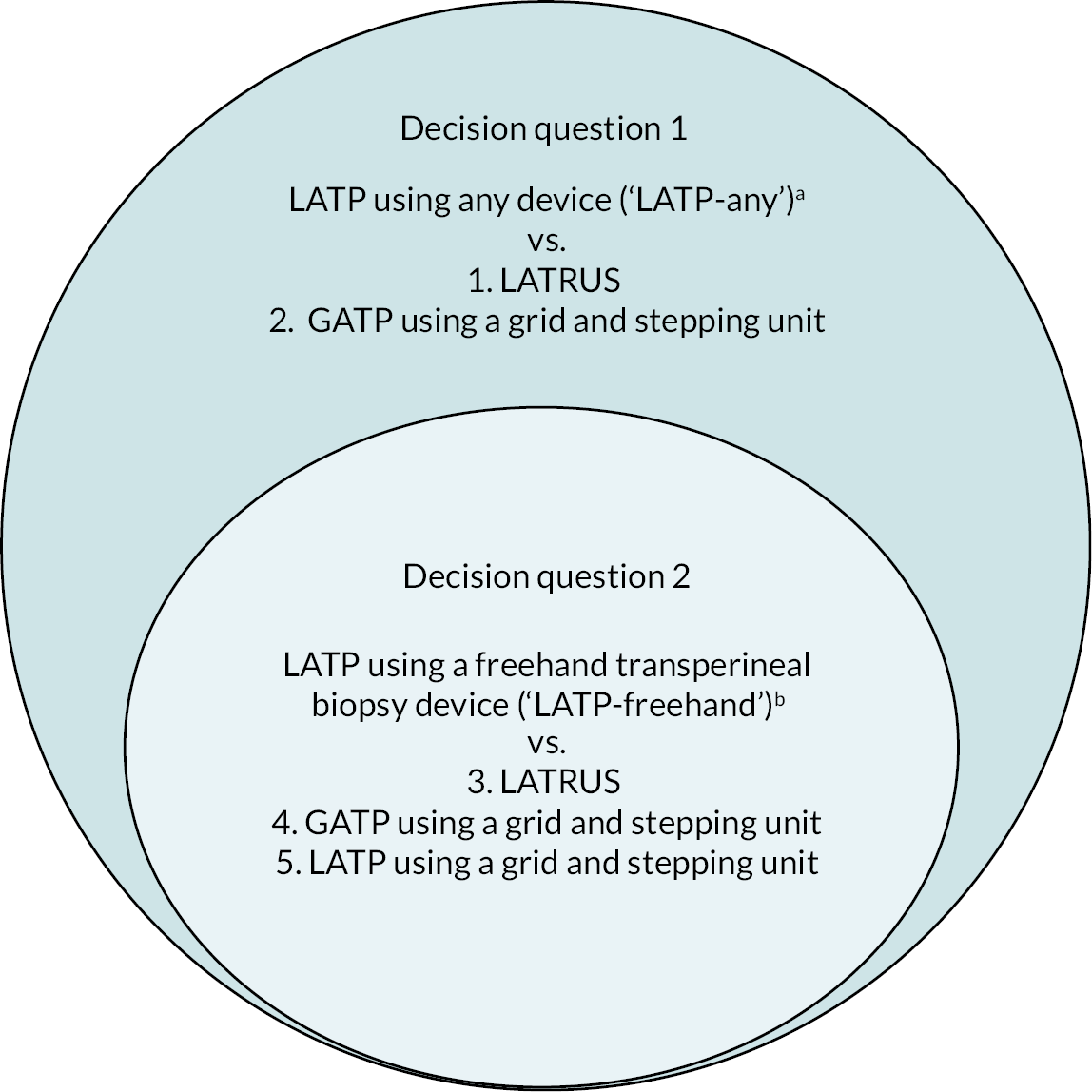
The NICE scope for this assessment includes two decision questions, which have been developed and prioritised by NICE in consultation with relevant stakeholders.
Decision question 1. Do LATP prostate biopsies in patients with suspected prostate cancer represent a clinically and cost-effective use of NHS resources?
Decision question 2. Do freehand TP devices for LATP prostate biopsies in patients with suspected prostate cancer represent a clinically effective and cost-effective use of NHS resources?
These two questions comprise the decision problem for this assessment. The following subsections define the parameters relevant to the decision problem.
Population and relevant subgroups
The relevant population for this assessment is people with suspected prostate cancer where prostate biopsy is indicated. People who have already been diagnosed with prostate cancer are not included (e.g. those receiving treatment for prostate cancer and those whose cancer is being monitored by either active surveillance or watchful waiting). People presenting with metastatic prostate cancer are also not included.
The intervention
The intervention relevant to this assessment is LATP prostate biopsy conducted using any of the following methods:
-
a grid and stepping device
-
a coaxial needle (‘double freehand’)
-
a freehand device within the NICE scope for this appraisal.
Details of these three types of biopsy are given above in Description of the diagnostic technologies under assessment. To recap, the six freehand devices within the NICE scope of this assessment are: PrecisionPoint, EZU-PA3U, CamPROBE, Trinity Perine, SureFire Guide and UA1232.
The comparator
There are three comparators relevant to this assessment:
-
local anaesthetic transrectal ultrasound biopsy (LATRUS)
-
LATP biopsy using a grid or template and stepping device
-
GATP using a grid or template and stepping device.
Details of these three types of biopsy are given above in Description of the diagnostic technologies under assessment.
For each of these three comparators the biopsy could be ‘targeted’ (i.e. mpMRI is used to identify lesions from which a small number of tissue samples or cores are taken) or ‘systematic’ (multiple samples are taken from different regions of the left and right side of the prostate).
Two of the three comparators apply to decision question 1, and all three comparators apply to decision question 2 as detailed in Table 1. Figure 2 depicts each of the five pairwise comparisons according to their relevant decision question.
| Decision question | Decision question |
|---|---|
| 1. Do LATP prostate LATP biopsies in people with suspected prostate cancer represent a clinically and cost-effective use of NHS resources? | 2. Do freehand TP devices for LATP prostate biopsies in people with suspected prostate cancer represent a clinically and cost-effective use of NHS resources? |
| Intervention LATP biopsy using a grid and stepping device, a coaxial needle (‘double freehand’) or a freehand device within the NICE scope |
Intervention LATP biopsy using a freehand TP device within the NICE scope |
| Comparator LATRUS |
Comparator LATRUS |
| Comparator GATP biopsy using a grid and stepping device |
Comparator GATP biopsy using a grid and stepping device |
| Comparator LATP biopsy using a grid and stepping device |
FIGURE 2.
Visual summary of the decision problem for this assessment. GATP is general anaesthetic transperineal biopsy; LATP is local anaesthetic transperineal biopsy; LATRUS is local anaesthetic transurethral biopsy. a, A grid and stepping device; a coaxial needle (‘double freehand’) or a freehand device within the NICE scope (see b). b, Freehand devices: PrecisionPointTM (BXTAccelyon) or UA1232 (BK Medical) or Trinity® Perine (KOELIS®) or CamPROBE (JEB) or SureFire Guide (LeapMed) or EZU-PA3U (Hitachi))
Outcomes
The outcomes of relevance to the decision problem are grouped into three overarching categories reflecting the effects of the biopsy procedure itself and the interpretation of the biopsy result and its impact on subsequent healthcare decisions.
Intermediate outcomes can include measures of diagnostic accuracy (e.g. sensitivity and specificity), cancer detection rates (CS/insignificant); low-, medium-, high-risk cancer detection rates; biopsy sample suitability/quality; number of biopsy samples taken; procedure completion rates and re-biopsy events within 6 months.
Clinical outcomes evaluate unintended adverse effects associated with prostate biopsy. These include short-term (acute) events including hospitalisation events after biopsy, rates of biopsy-related complications (infection, sepsis and haematuria), and rates of urinary retention. Medium- to longer-term measures include rates of erectile dysfunction, survival (including progression-free survival) and adverse events from prostate cancer treatment (in patients the biopsy diagnosed as having prostate cancer).
Patient-reported outcomes evaluate aspects that have an impact on patients on a personal and/or functional level. These reflect the experience of the biopsy itself, including tolerability (taking into account pain and discomfort) and also the longer-term impacts on health-related quality of life (HRQoL).
Overall aims and objectives of the assessment
The aim of this diagnostic assessment is to estimate the clinical effectiveness and cost-effectiveness of LATP prostate biopsies performed with or without available specialist devices and equipment (e.g. a grid and stepping unit), in people with suspected prostate cancer. The results will inform NICE guidance to the NHS on use of this diagnostic technology.
The objectives of this diagnostic assessment are as follows:
-
To conduct a systematic review of diagnostic test evaluation and clinical effectiveness of LATP prostate biopsies compared to alternative biopsy modalities in people with suspected prostate cancer.
-
To conduct systematic reviews of evidence to inform a health economic evaluation of LATP prostate biopsies. We will conduct a systematic review of cost-effectiveness studies of LATP prostate biopsies in people with suspected prostate cancer and of HRQoL (utility) studies. We will take a systematic approach to identifying relevant resource use and cost data relating to the diagnosis, monitoring and treatment of prostate cancer.
-
To conduct a health economic evaluation using decision-analytic modelling to assess the incremental cost-effectiveness of LATP prostate biopsies compared to alternative biopsy modalities in people with suspected prostate cancer.
Chapter 3 Methods of clinical and diagnostic assessments
The proposed methods to produce the systematic review of diagnostic test evaluation and clinical effectiveness were reported a priori in a published research protocol (PROSPERO registration number 266443). The final protocol was published on the NICE website shortly after the final scope of this assessment was published in June 2021. The following subsections report further detail on the methods used, noting instances where changes to the protocol were necessary, with a suitable justification.
Identification of studies
Comprehensive, systematic literature search strategies were designed and tested by an experienced information specialist from the project team to inform searches for the systematic review of diagnostic test evaluation and clinical effectiveness, and systematic reviews of cost-effectiveness evidence and economic model input parameters (see Chapter 5). The draft strategy for diagnostic test evaluation and clinical effectiveness was piloted on MEDLINE. We examined the relevance of the references identified, and whether any relevant evidence was not identified. The search terms and combined sets of terms were revised iteratively until an acceptable balance of sensitivity (comprehensiveness) and specificity (precision) of search results was achieved, upon which the strategy was finalised and implemented.
Health and medical research database searches were performed on 9 July 2021 on the following databases: MEDLINE (including Epub Ahead of Print, In-process & Other Non-indexed Citations); EMBASE; the Cochrane Database of Systematic Reviews (CDSR); the Cochrane CENTRAL register of controlled trials; Web of Science; the International Health Technology Assessment Database (INAHTA); the Database of Abstracts of Reviews of Effects (DARE); the NHS Economic Evaluations Database (NHS EED); Epistemonikos; Open Grey; and PROSPERO.
Databases of research in progress were searched on 10 June 2021: ClinicalTrials.gov, National Institute for Health and Care Research (NIHR) Be Part of Research and the NIHR Clinical Research Network Portfolio. We re-ran all of the above database searches on 19 October 2021 to identify relevant references added in the 3 months since our first search.
The proceedings of four international urology conferences were hand-searched in June 2021 covering the period from January 2018 to June 2021: American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium; American Urologic Association (AUA) Annual Meeting; BAUS Annual Scientific Meeting; European Association of Urology (EAU) Annual Meeting.
We screened the reference lists of relevant systematic reviews identified by the database searches, to identify any additionally relevant primary studies we had not already found from the above searches. Likewise, we examined the evidence submissions to NICE from manufacturers and/or distributors of the freehand TP devices, to identify any additionally relevant primary studies. We also screened references brought to our attention by our clinical experts and NICE specialist committee members.
Further details on literature searching, including the full search strategy applied to each database, are reported in Appendix 1.
Inclusion and exclusion criteria
The predefined inclusion and exclusion criteria are based on the decision problem as outlined earlier in Chapter 2, and are described below. An extended PICO (population, intervention, comparator, outcome) tabulation of these criteria is included in Table 50, Appendix 2. This table is the basis of the worksheet we used to systematically apply the criteria to each study screened.
Population
The relevant population is people with suspected prostate cancer where prostate biopsy is indicated. People included in the review may have a clinical suspicion of prostate cancer (e.g. raised PSA level or abnormal DRE findings), or people may have had a previous prostate biopsy that was negative for prostate cancer but have a continued clinical suspicion. People are not included if they have already been diagnosed with prostate cancer and are receiving treatment or monitoring by active surveillance or by watchful waiting, and likewise people are not included if they are known to have metastatic prostate cancer.
Interventions and comparators
Local anaesthetic transperineal prostate biopsy is the diagnostic procedure relevant to this review, and for the purposes of this report is considered as the intervention. The relevant LATP procedures vary according to two separate (though related) decision questions.
-
Decision question 1 compares any LATP prostate biopsy procedure versus LATRUS prostate biopsy or versus GATP prostate biopsy. For example:
-
LATP using a grid and stepping unit
-
LATP using a coaxial needle (‘double freehand’)
-
LATP using a freehand TP device (see decision question 2).
-
The comparison of LATP versus LATRUS assess differences/similarities in diagnostic and clinical outcomes between the transperineal and transrectal prostate biopsy respectively, both using local anaesthetic. The comparison of LATP versus GATP assesses differences or similarities in diagnostic and clinical outcomes between different anaesthetic modalities used during the transperineal prostate biopsy.
-
Decision question 2 compares LATP using any of the six freehand devices listed below versus LATRUS, GATP or LATP using a grid and stepping unit (NB: name of the company making/distributing the device in parentheses):
-
PrecisionPoint (BXTAccelyon)
-
UA1232 (BK Medical)
-
Trinity Perine (KOELIS/Kebomed)
-
CamPROBE (JEB)
-
SureFire Guide (LeapMed)
-
EZU-PA3U (Hitachi).
-
As evident from the above, the intervention relevant to decision question 2 (LATP using any of the six freehand devices) is nested within the broader range of biopsy interventions relevant to decision question 1 (any LATP prostate biopsy procedure). The comparators relevant to decision question 2 overlap with those relevant to decision question 1, but additionally, include LATP using a grid and stepping device (see Table 1 for a summary of the above).
No restriction was placed on the inclusion of specific biopsy protocols and procedures, such as number of biopsy cores taken, or whether prostate biopsy sampling was systematic and/or targeted, and whether mpMRI was used to determine whether a prostate biopsy is needed, and, if so, which prostate lesions should be targeted for core sampling. Cognitive fusion biopsies, also known as visual registration biopsies, were eligible, whereas software-based fusion biopsies were not. Biopsy techniques using sedation in place of local or general anaesthetic were not included.
Outcomes
We categorised relevant outcome measures according to which aspect of the prostate biopsy they evaluate, following the same approach used in the NICE scope for this diagnostic assessment. Our synthesis of the results of the studies is structured according to these categories for consistency and ease of report navigation (see Intermediate outcomes, Clinical outcomes and Patient-reported outcomes).
Intermediate and diagnostic outcomes of relevance were: measures of diagnostic accuracy (e.g. sensitivity/specificity); cancer detection rates; CS cancer detection rates; clinically insignificant cancer detection rates; low-, medium-, high-risk cancer detection rates; biopsy sample suitability/quality; number of biopsy samples taken; procedure completion rates; re-biopsy events within 6 months and length of time to perform the biopsy procedure (we added the latter outcome to inform biopsy cost estimates for potential inclusion in our economic model to assess cost-effectiveness; see Economic analysis).
Clinical effectiveness outcomes of relevance were hospitalisation events after biopsy; rates of biopsy-related complications, including infection, sepsis and haematuria; rates of urinary retention; rates of erectile dysfunction; survival; progression-free survival; adverse events from treatment.
Patient-reported outcomes of relevance were HRQoL and patient-reported tolerability. We added biopsy procedure time to the inclusion criteria for outcomes because it impacts on the cost of the procedure.
Study design
Any primary comparative research study evaluating the biopsy methods outlined in the ‘Interventions and comparators’ subheading above is included. We noted single-arm evaluations of LATP biopsy during screening so that we could potentially include them if there was insufficient available comparative evidence.
Inclusion screening process
At the first stage of screening, two reviewers independently applied the above criteria to the titles and abstracts using an inclusion/exclusion worksheet (see Table 50, Appendix 2). Any disagreements between reviewers in judgements about study eligibility were resolved through discussion or with the opinion of a third reviewer where necessary.
At the second stage of screening one reviewer screened the full texts of references judged potentially relevant on title and abstract screening. A second reviewer checked the first reviewer’s judgement on eligibility based on the full text. The reviewers discussed any discrepancies in judgement and before agreeing a final decision to include or exclude the reference. Where study eligibility remained unclear due to missing information to inform reviewers’ judgement, we contacted the authors of the study and requested the required information.
To ensure consistency between reviewers in the application of the inclusion/exclusion criteria, the Evidence Assessment Group (EAG) developed decision rules to be followed when screening studies with complex characteristics or ambiguously reported procedures.
-
Mixed populations: for example, a study population comprising people with clinical suspicion of prostate cancer and people on active surveillance following a previous diagnosis of prostate cancer. Such studies were eligible if:
-
the outcomes of relevance to this review were reported separately by participant subgroup, allowing us to extract only outcome data for the relevant subgroup, or
-
the proportion of the study population relevant to this review was at least 70%, based on a pragmatic threshold for inclusion agreed by the EAG.
-
-
Mixed types of anaesthesia: for example, a study in which some participants chose local anaesthesia for their biopsy and others chose general anaesthesia. We used the same decision rule as for mixed populations above. That is, we included if relevant outcomes were reported separately for participants having local and general anaesthesia, or if the proportion of participants in the study who received the anaesthesia relevant to the comparison of relevance to this review was at least 70%.
-
Definitions of local anaesthesia: described variously in the literature as local anaesthetic, spinal anaesthetic, periprostatic anaesthetic, periprostatic nerve block, caudal nerve block, etc. Consultation with our clinical experts confirmed that pain relief given in the region around the prostate could be described as a local anaesthetic procedure. We therefore used this as a decision rule for local anaesthesia when applying inclusion criteria. We did not include studies describing use of sedation rather than local anaesthesia.
-
Of note, NICE subsequently queried whether it is clinically appropriate to consider spinal anaesthesia and caudal block (used in two included trials) as local anaesthetic. We therefore excluded these two trials from our economic base case and retained them in scenario analyses, as will be discussed in Cancer detection rates.
-
Intraparticipant biopsy comparison: if a study performed transperineal and transrectal biopsies simultaneously (i.e. in the same session) on the same participant, the study was eligible for inclusion if relevant outcomes for each biopsy approach were reported separately.
Data-extraction strategy
Relevant data were extracted from each included study, including study design and methods, the socio-demographic characteristics and health and disease status of the study population, the intervention (i.e. the biopsy), and comparator(s) evaluated and the study outcomes. Each study underwent data extraction by a single reviewer, using a structured and piloted data-extraction form (see Appendix 3 for the data-extraction template). The extracted data were checked for accuracy and interpretation by a second reviewer, and any discrepancies between them were resolved through discussion. The finalised data-extraction form for each study comprised information identified from one or more publications describing that study, as applicable (NB: these can be made available on request).
Critical appraisal of study methodology
As stated in the research protocol, we planned to use the quality assessment of diagnostic accuracy studies (QUADAS) 2 tool to appraise the risk of bias of diagnostic test evaluation studies. 19 The tool assesses risk of bias and applicability across four key study domains relating to diagnostic evaluation: patient selection, index test, reference standard, and flow of patients through the study and timing of the index test(s) and reference standard. We began piloting QUADAS 2 on a sample of included studies but found that many of the questions were not applicable. For example, the reference standard domain features questions relating to the standard’s accuracy in correctly classifying disease, biases arising in the interpretation of reference standard results and the applicability of the reference standard to the condition under evaluation. As we report later (see Results of clinical and diagnostic assessments), studies meeting our inclusion criteria did not evaluate prostate biopsy in terms of diagnostic/prognostic accuracy and the use of a reference standard was rarely mentioned. Instead, the studies compared LATP prostate biopsy against comparators across a range of intermediate, clinical and patient-reported outcomes, reflecting a broader focus of investigation beyond diagnostic accuracy. It is for these reasons we decided not to use QUADAS 2 as a critical appraisal instrument in the review.
We assessed the internal validity of randomised controlled trials (RCTs) using the Cochrane risk of bias tool, version 1. 20 This is a validated and widely used tool designed for use in systematic reviews to assess the potential risk of bias in RCTs of health interventions. The tool covers six domains of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias (as relevant).
Non-randomised (observational) studies were appraised using The Joanna Briggs Institute (JBI) critical appraisal checklist for cohort studies/case series studies (as applicable). 21 These checklists are comprehensive in their consideration of potential risks of bias that affect observational studies. They cover factors such as similarity of study groups, measures to identify and address confounding variables, validity and reliability of data collection and analysis, loss to follow-up and addressing incomplete follow-up/missing data, and appropriateness of statistical analyses. We edited questions two and three in the checklist for cohort studies to replace ‘exposures’ with relevant biopsy details.
We consider the aforementioned tools for random and non-randomised evidence are relevant and comprehensive for an informed critical appraisal of the studies included in this diagnostic assessment. Omission of a diagnostic test-specific critical appraisal instrument from this review does not imply that relevant aspects of diagnostic evaluation validity have been overlooked. The results of our critical appraisal are summarised in Results of critical appraisal of study methodology and reported in full in Appendix 9.
Method of data synthesis
We summarised the characteristics of the included studies and study outcomes through a structured narrative synthesis. Numerical and statistical data were tabulated and summarised in the text. We assessed the appropriateness and feasibility of meta-analysis, taking into account factors including the availability of necessary study data and the degree of clinical and statistical heterogeneity across the included studies. We performed pairwise meta-analysis for the prostate biopsy comparisons relevant to the decision problem for the outcome of cancer detection rates, expressed as relative risk (RR). This outcome was selected because it directly informs estimates of biopsy clinical effectiveness in our economic model (see Economic analysis). Furthermore, cancer detection rates were the most consistently reported of the outcomes across the included studies, thus providing sufficient data for a meaningful meta-analysis.
We used Stata 17 (College Station, TX, USA) software to conduct pairwise meta-analysis of cancer detection rates, expressing effects as RRs with 95% confidence intervals (CIs). We conducted pairwise meta-analyses for each biopsy comparison relevant to the decision problem (e.g. LATP vs. LATRUS), where data were available. We analysed randomised and non-randomised studies separately, as recommended by methodological guidance,22 but we pooled both types of evidence for exploratory analysis purposes. This exploratory analysis assumed equal study weights regardless of design, which is clearly a limitation.
Where a connected study network was present, we performed indirect comparisons of the biopsy modalities via network meta-analysis (NMA). The purpose was to provide relative treatment effect estimates (cancer detection rates) to inform an incremental assessment of the biopsy modalities in our economic analysis (see Model parameters). The NMA was restricted to RCTs and was conducted using MetaInsight software using the frequentist netmeta package. 23 Effect estimates were presented as RRs, with LATRUS as the reference treatment. We used random effects (random-effects maximum likelihood REML) in preference to fixed-effect models due to apparent clinical heterogeneity between studies.
Chapter 4 Results of clinical and diagnostic assessments
Quantity and validity of research available
Initial literature searches (reported in Identification of studies and Appendix 1) identified a total of 1969 potentially relevant references after duplicate references were removed. Independent screening of titles and (where provided) abstracts by two reviewers determined that 1858 of these references did not meet the inclusion criteria, while the full texts of the remaining 111 references were obtained for further screening. Of the 111 full texts, it was unclear whether 36 met our inclusion criteria. Of the 36 unclear full texts, we were able to contact the authors of 32 for clarification. We received author clarification responses for 15 of the 32 full texts; two authors provided us with an additional full text each, and two confirmed they did not have access to the data to answer our clarification questions. The authors of the remaining 17 full texts did not respond.
Comparative studies were identified for one of the six freehand biopsy devices within the scope of this review (PrecisionPoint). We therefore modified our inclusion criteria to include single-arm (i.e. non-comparative) studies for the remaining five freehand devices, when reported. We considered that these studies may be informative to the NICE diagnostics advisory committee’s consideration when the only alternative would be no evidence at all for these devices.
Update searches (reported in Identification of studies and Appendix 1) identified a further 37 unique references that were independently screened by two reviewers, of which 31 did not meet our inclusion criteria and 6 (all conference abstracts, none reporting RCTs) reported insufficient information to determine eligibility. Authors of all six abstracts were contacted for clarification, of whom two responded.
In summary, the combined July 2021 and October 2021 searches of literature and other sources identified a total of 2008 references of which 1889 were excluded after screening titles and abstracts. Of 119 references subjected to full-text screening, 65 were excluded, the majority for reporting an intervention not relevant to the scope (reasons for exclusion are given in Appendix 3). A further 27 references did not report sufficient information to fully inform a screening decision to include or exclude. The remaining 27 publications reported a total 23 studies meeting the inclusion criteria for this systematic review. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow chart in Figure 3 shows the flow of records through the stages of inclusion/exclusion screening.
FIGURE 3.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 flow chart.

Table 2 lists the 23 included studies according to their relevant decision question(s), organised by pairwise comparisons, and stratified by study design. The comparison with the largest number of studies was ‘LATP-any’ [i.e. prostate biopsy using a grid and stepping device, a coaxial needle (‘double freehand’) or a freehand device within the NICE scope] versus LATRUS (n = 15 studies). Far fewer studies compared LATP-any versus GATP using a grid and stepping device (n = 4 studies). Nested within the LATP-any group is a subset of studies comparing LATP prostate biopsy using a freehand transperineal device (LATP-freehand) versus LATRUS (n = 7 studies). This comparison is the focus of decision question 2; hence these seven studies appear twice in Table 2 (bold type is used to highlight this). Of the six freehand TP devices in the NICE scope, relevant comparative evidence was identified for just one device, PrecisionPoint. Single-arm non-comparative studies were included for the remaining devices where available.
| Decision question 1 | Decision question 2 |
|---|---|
| Intervention: LATP biopsy using a grid and stepping device, a coaxial needle (‘double freehand’), or a freehand device within the NICE scope. (‘LATP-any’) | Intervention: LATP biopsy using a freehand TP device within the NICE scope. (‘LATP-freehand’) |
Comparator: LATRUS (n = 15 studies)
|
Comparator: LATRUS (n = 7 studies)
Comparator: GATP using a grid and stepping device (n = 1 study)
No studies met inclusion criteria Comparator: Noneb |
Characteristics of studies comparing local anaesthetic transperineal biopsy by any method versus local anaesthetic transrectal ultrasound prostate biopsy (decision question 1)
Overview of general study characteristics
Table 3 gives an overview of the LATP prostate biopsy versus LATRUS biopsy studies included in the review.
| Study | Country; no. centres | Design | Intervention | Comparator | Study population |
|---|---|---|---|---|---|
| RCTs | |||||
| Cerruto et al. 201424 | Italy; single centre | RCT; n = 108 randomised |
TRUS-guided LATP biopsy using coaxial needle; n = 54 | LATRUS biopsy; n = 54 | Prostate biopsy naïve participants with suspected prostate cancer |
| Guo et al. 201525 | China; single centre | RCT; n = 339 randomised |
TRUS-guided LATP biopsy (device not reported); n = 173 | LATRUS biopsy; n = 166 | Prostate biopsy naïve participants with suspected prostate cancer |
| Hara et al. 200826 | Japan; single centre | RCT; n = 246 randomised |
TRUS-guided LATP biopsy (device not reported); n = 126 | LATRUS biopsy; n = 120 | Prostate biopsy naïve participants with suspected prostate cancer |
| Lam et al. 2021 (AB)27 | Hong Kong; single centre | RCT; n = 266 randomised |
LATP biopsy using the PrecisionPoint freehand device (imaging guidance not reported); n = 134 | LATRUS biopsy; n = 132 | Prostate biopsy naïve participants with suspected prostate cancer |
| Takenaka et al. 200828 | Japan; single centre | RCT; n = 200 randomised |
TRUS-guided LATP biopsy using an attachment for needle guidance; n = 100 | LATRUS biopsy using an attachment for needle guidance; n = 100 | Prostate biopsy naïve participants with suspected prostate cancer |
| Other prospective studies | |||||
| Bojin 201929 | England; single centre | Case series with historical comparison group; n = 292 | TRUS-guided LATP biopsy using the PrecisionPoint device; n = 103 | LATRUS biopsy; n = 189 | Prostate biopsy naïve participants with suspected prostate cancer; participants who underwent repeat biopsy; participants on active surveillance |
| Chen et al. 202130,31 | Singapore; single centre | Prospective cohort with historical comparison group; n = 390 | TRUS-guided LATP biopsy using the PrecisionPoint freehand device; n = 212 | LATRUS biopsy; n = 178 | Prostate biopsy naïve participants (> 90%) |
| Emiliozzi et al. 200332 | Italy; single centre | Prospective single cohort study; transperineal and transrectal biopsies obtained in all patients in the same session; n = 107 | TRUS-guided LATP biopsy (device not reported); n = 107 | LATRUS biopsy; n = 107 | Prostate biopsy naïve participants with suspected prostate cancer |
| Hung et al. 2020 (AB)33 | Hong Kong; single centre | Prospective comparative study. How participants were assigned to each arm is not reported; n = 120 | LATP biopsy using the PrecisionPoint freehand device (imaging guidance not reported); n = 63 | LATRUS biopsy; n = 57 | Prostate biopsy naïve participants with suspected prostate cancer |
| Kum et al. 2018 (AB)34,35 | England; single centre | Cohort study with historical comparison group | TRUS-guided LATP biopsy using the PrecisionPoint freehand device; n = 176 | LATRUS biopsy; n = 77 | Prostate biopsy naïve participants with suspected prostate cancer; participants who underwent repeat biopsy; participants on active surveillance |
| Starmer et al. 202136,37 | England; single centre | Prospective cohort study; participants assigned to intervention or comparator for different reasons; n = 108 |
LATP biopsy using the PrecisionPoint freehand device (imaging guidance not reported); n = 56 | LATRUS biopsy; n = 52 | Prostate biopsy naïve participants with suspected prostate cancer; participants who underwent repeat biopsy; participants on active surveillance |
| Watanabe et al. 200538 | Japan; single centre | Prospective cohort study; transperineal and transrectal biopsies obtained in all patients in the same session; n = 402 | Ultrasound-guided LATP biopsy (device not reported); n = 402 | LATRUS biopsy; n = 402 | Prostate biopsy naïve participants with suspected prostate cancer |
| Retrospective studies | |||||
| Abdollah et al. 201139 | Italy; two centres | Retrospective cohort study; n = 280 propensity score matched |
TRUS-guided LATP biopsy using a coaxial needle; n = 140 | LATRUS biopsy; n = 140 | Participants with continued suspicion of prostate cancer who underwent a saturation repeat biopsy |
| Jiang et al. 201940 | China; two centres | Retrospective cohort study; n = 2962 (n = 752 propensity score matched) | TRUS-guided LATP biopsy (device not reported); n = 1746 (n = 376 propensity score matched) | LATRUS biopsy; n = 1216 (376 propensity score matched) | Prostate biopsy naïve participants with suspected prostate cancer |
| Szabo et al. 202141 | USA; single centre | Retrospective case series; n = 375 | (1) Ultrasound-guided LATP biopsy using the PrecisionPoint freehand device n = 242; (2) LATP using coaxial needle n = 62; |
LATRUS biopsy; n = 133 | Prostate biopsy naïve participants with suspected prostate cancer; participants who underwent repeat biopsy; participants on active surveillance |
Of the 15 included studies comparing LATP-any versus LATRUS biopsies, 5 are RCTs, 7 prospective cohort studies and 3 retrospective cohort studies.
The RCTs were conducted in Japan,26,28 China,25 Hong Kong27 and Italy,24 and all were single-centre studies. The participants in all RCTs were prostate biopsy naïve with suspected prostate cancer, and no study reported any pre-biopsy mpMRI. The LATP techniques varied: one study used a coaxial needle,24 another used an unnamed attachment for needle guidance,28 another used PrecisionPoint27 and two studies did not specify a device. 25,26
The seven prospective cohort studies are all single-centre studies, set in England,29,34–36 Hong Kong,33 Japan,38 Singapore30,31 and Italy. 32 They comprise two studies which carried out both transperineal and transrectal biopsies in the same participants in the same session,32,38 three studies where the LATRUS arm is a historical comparison group,29–31,34,35 one study that assigned participants to study arms according to pre-biopsy MRI findings and other criteria,36,37 and one study that does not report how it assigned participants to study arms. 33
The participants in the three English prospective cohort studies are a mixed population of those who were biopsy naïve, those who were undergoing repeat biopsy and a small proportion of participants on active surveillance. In all the other studies participants were exclusively prostate biopsy naïve. All English studies used the PrecisionPoint device to perform LATP,29,34–37 as did the Hong Kong study,33 and the earlier studies do not report any device. 32,38
One of the studies33 is reported only in a conference abstract and another is an unpublished slide-set presentation29 and so they have limited information. The other studies are reported in full publications.
The retrospective studies were set in Italy,39 China40 and the USA. 41 The Italian and Chinese studies were multicentre (two-centre) studies where LATP was performed at one centre and LATRUS was performed at the other. The USA study is a single-centre study. One study population consists entirely of repeat biopsy participants39 one study consists entirely of biopsy naïve participants,40 and one study included a mixed population of biopsy naïve, repeat biopsy and active surveillance participants. 41 Two studies performed propensity score matching of the participants: one study reports propensity score matched results only39 and the other reports both the unmatched and propensity score matched results. 40 The LATP techniques varied according to device used: one study used a coaxial needle,39 one study used the PrecisionPoint freehand device41 and one study did not report using a device. 40
Details of local anaesthetic transperineal prostate-any biopsy procedures
Table 53 in Appendix 4 gives details of the LATP-any biopsy procedures. Most studies used systematic biopsy sampling, with the number of cores taken (where reported) ranging from 6 to 24 across studies. Two studies based the number of cores taken on the size of the prostate, one by whether or not the prostate volume was above or below 50 ml,25 and another study reports that the samples were spaced 1 cm apart. 41
Where targeted biopsy sampling was performed this could be in addition to systematic sampling biopsies, or targeted sampling alone. 34,35 Reasons to prompt additional targeted sampling were: suspicious areas detected by TRUS or DRE,25 any hypoechoic areas noted,32 PI-RADS score > 2 on pre-biopsy mpMRI,36,37 hypoechoic lesions or palpable nodules on DRE,38 or participants with pre-biopsy mpMRI PI-RADS score of 4 or 5. 36
Additional variations to the biopsy procedures that are not reported above are: any other medications administered or ceased (e.g. anticoagulation medication), whether antibiotic prophylaxis was given (and how much), what position the participant was in (e.g. lithotomy or dorsal lateral) and where they were performed (e.g. in outpatient clinics or day theatres), thus further illustrating the heterogeneous nature of the biopsy procedures and the studies.
Participant characteristics
Most of the included studies reported age, PSA level, prostate volume and the proportion of participants with abnormal DRE or pre-biopsy imaging findings (see Table 54, Appendix 4).
Age is reported in various combinations of mean or median with interquartile range (IQR), range or standard deviation (SD), with a mean age of 63–72 years across the studies. PSA level is also reported in various combinations of mean or median with IQR, range or SD. It can be seen that mean PSA levels varied from around 7–8 to 12–19 ml, with one of the retrospective studies having participants with PSA levels 38–40 (Jiang et al.). 40 Only five studies reported PSA density. 28–31,34,35,41
The PI-RADS score, based on pre-biopsy imaging, is only reported in two studies neither of which correspond exactly with the NICE subgroups of interest (people with a Likert or PI-RADS score of 2 or less, or a score of 3, 4 or 5). One study reports the proportion of participants with PI-RADS 2/3, 3/4 and 5 separately, but only for the LATP arm. 34,35 The other reports the proportion of participants with PI-RADS 4 or 5. 41 None reported the location of lesions identified in pre-biopsy imaging.
Two studies reported body mass index,24,25 one study reported ethnicity. 41 None reported any family history of prostate cancer.
There is not enough evidence to review the efficacy of the biopsy procedures for several of the NICE subgroups (people with anterior lesions; people with posterior lesions; people with apical lesions; people with basal lesions; people with a Likert or PI-RADS score of 2 or less; people with a Likert or PI-RADS score of 3, 4 or 5).
Summary
The comparison of LATP-any versus LATRUS biopsy (decision question 1) is the largest in terms of number of included studies, comprising five RCTs, seven non-randomised prospective studies and three retrospective studies. This is not unsurprising given the broad scope of the LATP-any intervention grouping in this assessment, which encapsulates the spectrum of transperineal prostate biopsy techniques in use. Three studies (non-randomised) were set in England, but many were done in East Asian countries. The vast majority of study participants were prostate biopsy naïve with suspected prostate cancer, with just one study assessing the effects of repeat biopsies in people with suspected prostate cancer who had a previous negative biopsy. The TP biopsy protocols (e.g. device used/sampling method/number of cores taken) varied between studies, which may partly reflect local clinical practice guidelines in study host institutions, but also the evolution of transperineal prostate biopsy practices over time (e.g. increases in the number of cores sampled). Some of the more recently published studies used pre-biopsy mpMRI to inform biopsy sampling, but this constitutes a small proportion of the evidence base as a whole.
Characteristics of studies comparing local anaesthetic transperineal prostate biopsy by any method versus general anaesthetic transperineal prostate biopsy using a grid and stepping device (decision question 1)
Overview of general study characteristics
Table 4 gives an overview of the four studies comparing LATP-any biopsy versus GATP biopsy with grid and stepping device. Three of the studies are available only as conference abstracts currently; thus some of the necessary detail in the following subsections is limited. 43–45
| Study | Country; no. centres | Design | Intervention | Comparator | Study population |
|---|---|---|---|---|---|
| RCTs | |||||
| Lv et al. 202042 | China; single centre |
RCT; n = 216 randomised | TRUS-guided LATP biopsy using a stepper and grid; n = 108 | TRUS-guided GATP biopsy using a stepper and grid; n = 108 | All participants were suspected of prostate cancer. Prior biopsy experience is not reported |
| Other prospective studies | |||||
| Takuma et al. 2012 (AB)43 | Japan; single centre | Prospective comparative cohort study; n = 66 | LATP biopsy (imaging guidance not reported); n = 37 | GATP biopsy using a template (imaging guidance not reported); n = 29 | All participants had one or more previous negative biopsies |
| Walters et al. 2021 (AB)44 | England; single centre | Case series; n = 407 | LATP biopsy (imaging guidance not reported); n = 339 | GATP biopsy (imaging guidance not reported); n = 68 | All participants undergoing TP biopsy identified from a prospective prostate cancer diagnostic registry |
| Retrospective studies | |||||
| Rij and Chapman 2020 (AB)45 | New Zealand; single centre | Retrospective cohort study; n = 143 | LATP biopsy using the PrecisionPoint device (imaging guidance not reported); n = 72 | GATP biopsy using a brachytherapy grid (image guidance not reported); n = 71 | All participants undergoing TP biopsy. Prior biopsy experience and reasons for suspected prostate cancer are not reported |
Of the four studies, one was a RCT set in China,42 two were prospective non-randomised studies set in England44 and Japan43 respectively, while the fourth was a retrospective study set in New Zealand. 45
One study42 used a grid and stepping device to perform LATP biopsy; another performed LATP using the PrecisionPoint freehand device45 and two studies did not specify use of a device. 43,44
Details of prior biopsy history were not clearly reported, but in one study it is stated that all participants had previously had one or more negative biopsies. 43
Details of local anaesthetic transperineal-any biopsy procedures
Table 55 in Appendix 4 gives details of LATP-any biopsy procedures used. Reporting of details by the studies was limited, but the available information shows that systematic sampling was commonly performed, with additional targeting of cores based on pre-biopsy imaging. Details of image guidance and anaesthesia are limited.
Participant characteristics
Available information on the characteristics of study participants (e.g. age, PSA level, prostate volume) is extremely limited, and only one study gave adequate detail (see Table 56, Appendix 4). 42
Summary
This comparison (LATP vs. GATP, decision question 1) is based on a smaller evidence base: one RCT, two prospective observational studies and one retrospective observational study. The location of the studies is mixed, including two studies done in Asia, and one each from New Zealand and England, respectively. LATP was performed using a grid and stepping device in at least one study, and using a freehand device (PrecisionPoint) in another. Sampling was systematic with additional targeting of cores in some cases. With the exception of the RCT, the other three studies are reported in conference abstracts only, thus limited information is available.
Characteristics of studies comparing local anaesthetic transperineal prostate biopsy using a freehand device versus local anaesthetic transrectal ultrasound prostate biopsy (decision question 2)
Overview of general study characteristics
Seven studies were identified that compare LATP biopsy using a freehand device with LATRUS biopsy. All freehand devices are the PrecisionPoint device; see Table 10. In contrast, only one study compares LATP biopsy using a specific freehand device with GATP (n = 1, PrecisionPoint device); see Table 12. No studies were identified that compare LATP-freehand with LATP using a grid and stepping device.
As no comparative studies were identified for any devices other than PrecisionPoint, we included single-arm studies for devices where no comparative evidence was available. One study reports a single-cohort study (i.e. with no comparative biopsy group) reporting ‘the first in man’ evaluation of the CamPROBE device. 46 Three conference abstracts report three separate single-cohort studies that used the UA1232 device. 47–49 See Table 13.
| Study | Country; no. centres | Design | Intervention | Comparator | Study population |
|---|---|---|---|---|---|
| RCTs | |||||
| Lam et al. 2021 (AB)27 | Hong Kong; single centre | RCT; n = 266 randomised |
LATP biopsy using the PrecisionPoint device (imaging guidance not reported); n = 134 | LATRUS biopsy; n = 132 | Prostate biopsy naïve participants with suspected prostate cancer |
| Other prospective studies | |||||
| Bojin 201929 | England; single centre | Case series with historical comparison group; n = 292 | TRUS-guided LATP biopsy using the PrecisionPoint device; n = 103 | LATRUS biopsy; n = 189 | Prostate biopsy naïve participants with suspected prostate cancer; participants who underwent repeat biopsy; participants on active surveillance |
| Chen et al. 202130,31 | Singapore; single centre | Prospective cohort with historical comparison group; n = 390 | TRUS-guided LATP biopsy using the PrecisionPointdevice; n = 212 | LATRUS biopsy; n = 178 | Prostate biopsy naïve participants (> 90%) |
| Hung et al. 2020 (AB)33 | Hong Kong; single centre | Prospective comparative study. How participants were assigned to each arm is not reported; n = 120 | LATP biopsy using the PrecisionPoint device (imaging guidance not reported); n = 63 | LATRUS biopsy; n = 57 | Prostate biopsy naïve participants with suspected prostate cancer |
| Kum et al. 2018 (AB)34,35 | England; single centre | Cohort study with historical comparison group | TRUS-guided LATP biopsy using the PrecisionPoint device; n = 176 | LATRUS biopsy; n = 77 | Prostate biopsy naïve participants with suspected prostate cancer; participants who underwent repeat biopsy; participants on active surveillance |
| Starmer et al. 202136,37 | England; single centre | Prospective cohort study; participants assigned to intervention or comparator for different reasons; n = 108 |
LATP biopsy using the PrecisionPoint device (imaging guidance not reported); n = 56 | LATRUS biopsy; n = 52 | Prostate biopsy naïve participants with suspected prostate cancer; participants who underwent repeat biopsy; participants on active surveillance |
| Retrospective studies | |||||
| Szabo et al. 202141 | USA; single centre | Retrospective case series; n = 375 | Ultrasound-guided LATP biopsy using the PrecisionPoint device and LATP prior to using the PrecisionPoint device; n = 242 | LATRUS biopsy; n = 133 | Prostate biopsy naïve participants with suspected prostate cancer; participants who underwent repeat biopsy; participants on active surveillance |
Of the seven studies comparing LATP-PrecisionPoint to LATRUS, one is a RCT,27 five were prospective cohorts29–31,33–37 and one was a retrospective case series. 41 All studies were single-centre studies, with three conducted in the England, two in Hong Kong, one in Singapore and one in the USA. The English and American studies were of mixed populations, whereas the others were prostate biopsy naïve participants with suspected prostate cancer only, and only two studies reported the number of cores taken during biopsy: 12 cores30 and 24 cores. 29
Participant characteristics
Participant characteristics are reported for the LATP freehand device PrecisionPoint versus LATRUS studies and are summarised in Table 57 in Appendix 4.
Summary
The evidence for this comparison (LATP-freehand vs. LATRUS, decision question 2) is a subset of the evidence for the LATP-any versus LATRUS, decision question 1 comparison. All the evidence is for the PrecisionPoint freehand device as the intervention. Included within this set of seven studies is one RCT and the three non-randomised studies set in England.
Characteristics of studies comparing local anaesthetic transperineal prostate biopsy using a freehand device versus general anaesthetic transperineal prostate biopsy by grid and stepping device (decision question 2)
Overview of general study characteristics
Table 6 gives an overview of the single study comparing LATP-PrecisionPoint versus GATP biopsy. 45
| Study | Country; no. centres | Design | Intervention | Comparator | Study population |
|---|---|---|---|---|---|
| Retrospective | |||||
| Rij and Chapman 2020 (AB)45 | New Zealand; single centre | Retrospective cohort study; n = 143 | LATP biopsy using the PrecisionPoint device (imaging guidance not reported); n = 72 | GATP biopsy using a brachytherapy grid (image guidance not reported); n = 71 | All participants undergoing TP biopsy. Prior biopsy experience and reasons for suspected prostate cancer are not reported |
Rij and Chapman report a retrospective cohort study conducted in a single centre in New Zealand. 45 At the current time (November 2021) the study is available publicly only as a conference abstract. The precise details of the study methods and outcomes are therefore limited. This study did not report the indications for biopsy, nor the number of cores taken during the biopsies, nor any participant characteristics.
Characteristics of single-arm studies evaluating local anaesthetic transperineal biopsy using a freehand device where no comparative evidence was identified
Overview of general study characteristics
No comparative evidence was identified for the LATP freehand devices CamPROBE, UA1232, SureFire, EZU-PA3U and Trinity Perine Grid. We therefore looked for any relevant single-arm (non-comparative) studies of these freehand devices. We did not identify any relevant single-arm studies with SureFire, the Trinity Perine Grid (for which all the studies we found used software-based fusion techniques outside the scope of this review) or EZU-PA3U. Table 7 gives an overview of the CamPROBE and UA1232 studies.
| Study | Country; no. centres | Design | Intervention | Study population |
|---|---|---|---|---|
| Prospective studies for CamPROBE | ||||
| Gnanapragasam et al. 202046 | England; multicentre (a lead centre provided training to the five other centres) | Prospective cohort study | LATP using the disposable single-use CamPROBE device | 56 men were screened over an 8-month period, and 40 were recruited. No further information reported; n = 40 (n = 80 biopsies, study counts right and left prostate biopsies separately, i.e. two CamPROBE devices per patient per biopsy) |
| Prospective studies for UA1232 | ||||
| Lau et al. 2020 (AB)47 | England; single centre | Prospective cohort study | LATP using a coaxial needle and a transducer-mounted needle guide (BK Medical). Use of UA1232 device as the mounted needle guide is implied by inclusion in the company submission |
Prostate biopsy naïve participants with suspected prostate cancer; n = 482 |
| Yamamoto et al. 2019 (AB)48 | England; single centre | Prospective cohort study | LATP using a transducer-mounted needle guide and a perineal coaxial needle. Use of UA1232 device is implied by inclusion in the company submission |
Prostate biopsy naïve participants with suspected prostate cancer; n = 200 |
| Yamamoto et al. 2020 (AB)49 | England. Single centre | Prospective cohort study | LATP using a co-axial needle and transperineal needle guide (BK Medical). Use of UA1232 device as the needle guide is implied by inclusion in the company submission |
Prostate biopsy naïve participants with suspected prostate cancer; n = 219 |
The one study evaluating CamPROBE was a prospective single-cohort study (i.e. with no comparative biopsy group) conducted in six centres in England. 46 It has a small (n = 40) study population. The indications for prostate biopsy were not reported and two devices were used per patient per biopsy, for the right and left sides of the prostate, respectively.
The three studies evaluating the UA1232 device are all single-centre prospective single-cohort studies conducted in England. 47–49 The study populations are larger (n = 482, n = 200, n = 219) and all the participants are biopsy naïve. All three studies were identified via the company submission as none of the abstracts explicitly report using the UA1232 device. All are conference abstracts and as such contain limited information.
Participant characteristics
The reporting of participant characteristics for the single-arm studies for CamPROBE and UA1232 is minimal: the CamPROBE study46 reports participants’ median and range for age, and one of the UA1232 studies47 reports median age and median PSA level.
Summary
The evidence available for LATP-freehand devices specified in the NICE scope, other than the PrecisionPoint device, is limited to single-arm studies: CamPROBE46 with a small population and UA1232 with limited information from three conference abstracts. 47–49 There is no evidence for the other devices in the NICE scope. Details of study characteristics and participant characteristics are limited.
Results of critical appraisal of study methodology
In this section, we the report results of our critical appraisal of the RCTs included in this systematic review, followed by our critical appraisal of the included observational studies.
Critical appraisal of randomised controlled trials
As mentioned earlier (see Critical appraisal of study methodology), we used the Cochrane risk of bias tool (version 1)20 to critically appraise the six RCTs in our review. 24–28,42 A key finding from this exercise is that we are unable to fully judge the studies’ overall risk of bias due to inadequate reporting of study methodological details in the available publications. Commonly, therefore, we recorded ‘unclear’ risk of bias for studies across the domains, notably those concerning reporting bias (due to selective outcome reporting), detection bias (due to lack of blinding of outcome assessors to type of prostate biopsy performed) and selection bias (due to inadequate randomisation of participants to trial arms, and/or inadequate concealment of the randomisation sequence). However, sufficient detail was available to inform judgements relating to other bias domains, including attrition bias. Overall, we advise caution in the interpretation of these study findings due to uncertainty regarding potential risks to their internal validity. Below is a brief summary of our findings; full details are reported in Table 59 in Appendix 5.
There was a lack of detail given on the methods used for random sequence generation in four of the trials,24,26–28 leading to uncertainty about whether or not ‘true’ randomisation had been achieved and selection bias avoided. Likewise, little or no information was given on whether adequate procedures were in place to conceal the random allocation sequence from study personnel, particularly those involved in enrolling participants to the study.
We judged all six trials to be at high risk of performance bias on the reasonable assumption that study participants and investigators knew which type of biopsy procedure participants had been randomly allocated to. This is an unavoidable consequence of this type of intervention, whereby the clinician performing the biopsy cannot be blinded to the type of biopsy the participant has been allocated to. Likewise, it is unlikely that the study participant would not be informed of their surgical procedure. It is also unclear whether any protocols were in place to reduce the risk of differential behaviours by participants and healthcare providers associated with knowledge of the type of biopsy performed. All six trials were judged at low risk of attrition bias, due to no or minimal reported participant loss to follow-up or study withdrawal.
Our judgements of the risk of bias across the five domains were identical for four of the six RCTs. 24,26–28 The trial by Guo et al. was at low risk of bias for the greatest number of domains:25 specifically, low risk of detection bias due to blinding of the outcome assessor (pathologist), low risk of selection bias due to adequate (computer-generated) randomisation (though we cannot rule out selection bias completely because details of allocation concealment were not reported) and low risk of reporting bias.
Critical appraisal of observational studies
As stated earlier (see Critical appraisal of study methodology) we used the checklists from the JBI suite of critical appraisal tools to critically appraise observational studies. 21 Eleven of the 13 observational studies were assessed using the JBI checklist for cohort studies50 and the remaining 2 studies were assessed using the JBI checklist for case series. 51
Most of the cohort studies recruited biopsy comparison groups from the same or a similar population. Likewise, the case series reported consecutive/complete inclusion of participants. However, limited reporting of study inclusion criteria and participants’ demographic and clinical information means it is unclear how comparable the biopsy groups within the studies are. Confounding factors were identified and handled in only about half of all the studies (both cohort studies and case series); the remainder are mostly unclear. Therefore, we judge the studies to have unclear risk of selection bias.
Follow-up times and methods to deal with loss to follow-up were mostly unclear, raising the potential for attrition bias. However, some key outcomes relevant to this diagnostic assessment are unlikely to be affected by loss to follow-up as they are measured/taken during the biopsy procedure itself (e.g. cancer detection rate based on biopsy samples) or immediately afterwards (e.g. pain questionnaires). Therefore, we judge the risk of attrition bias as low for cancer detection rate and pain/tolerability outcomes, but unclear for other outcomes.
The risk of detection bias was judged as generally low because in almost all the studies the biopsy methods are clearly reported and over half of the studies reported using a protocol or schema for the biopsy procedure. In addition, the cancer detection rate outcome was measured in a valid and reliable way in most of the studies, usually referring to a specific grade group or score. However, there may be a risk of detection bias when considering the validity and reliability of measurement of the other outcomes in several of the studies, for example complications, where for some studies only complications that occurred were reported and no time frame was stated for reporting any complications. Therefore, when considering different outcomes in the studies, detection bias is either low or unclear depending on the outcome in question (as for attrition bias).
There is a high risk of reporting bias (and several other bias domains) in studies available, at the time of writing, only as conference abstracts. Commonly, abstracts are restricted in word limits, prohibiting authors from reporting all intended outcome data. Clarity on reporting bias may improve if full text reports of studies are published (personal communication with study authors indicates that some are in the process of preparing manuscripts for publication). There is lack of clarity around several domains of bias due to the limited amount of information that can be conveyed in a conference abstract.
Our critical appraisal judgements for each cohort study and each case series are presented in Table 60 in Appendix 5.
Intermediate outcomes
Below we present a synthesis of studies measuring the diagnostic yield of LATP prostate biopsy in suspected prostate cancer. We regard the term diagnostic yield as synonymous with cancer detection rates, the most commonly reported outcome measure in the included studies. We take each pairwise biopsy comparison in the decision problem in turn (Figure 2) and present cancer detection rates for individual studies and for studies combined in meta-analyses (where meta-analysis was possible).
Prostate cancer detection (local anaesthetic transperineal-any biopsy vs. local anaesthetic transrectal ultrasound, decision question 1)
Prostate cancer detection was the most commonly reported of all the outcome measures relevant to this assessment (n = 14 of 15 studies). Only the study by Starmer et al. did not report this outcome. 36,37 In marked contrast, CS prostate cancer detection, informative for assessing the risk of rapid cancer progression, was reported in just five studies. 27,29,33–35,41 Table 8 reports study cancer detection rates, including CS cancer rates, where available.
| Study | Outcome measure | Intervention LATP-any |
Comparator LATRUS |
Statistical significance (p-value) |
|---|---|---|---|---|
| RCTs | ||||
| Cerruto et al. 201424 | Cancer detection rate, n/N (%) | 24/54 (44.4) | 25/54 (46.3) | 0.846 |
| Guo et al. 201525 | Cancer detection rate: positive rate, n/N (%) | 61/173 (35.3) | 53/166 (31.9) | 0.566 |
| Hara et al. 200826 | Cancer detection rate, n/N (%) | 53/126 (42.1) | 58/120 (48.3) | 0.323 |
| Lam et al. 2021 (AB)27 | Cancer detection rate, n/N (%) | 47/134 (35.1) | 33/132 (25.0) | < 0.05 |
| CS cancer detection ratea | 22/134 (16.4) | 19/132(14.4) | p = 0.74 | |
| Takenaka et al. 200828 | Cancer detection rates overall, n/N (%) | 47/100 (47.0) | 53/100 (53.0) | 0.333 |
| Other prospective studies | ||||
| Bojin 201929 | Cancer detection rates malignant, n/N (%) | 76/103 (73.7) | 117/189 (61.9) | Not reported |
| Cancer detection rates benign, n/N (%) | 27/103 (26.2) | 72/189 (38.1) | Not reported | |
| CS cancer pick up, n/N (%)b | 51/76 (67.1) | 48/117 (41.2) | Not reported | |
| Chen et al. 202130,31 | Cancer detection rate in biopsy naïve patients, n/N (%) | 127/200 (63.5) | 86/172 (50.0) | 0.0115 |
| Emiliozzi et al. 200332 | Cancer detection rate, n/N (%)c | 43/107 (40.0) | 34/107 (32.0) | 0.012 |
| Hung et al. 2020 (AB)33 | Cancer detection rate (%) | 20/63 (31.7) | 14/57 (24.6) | 0.851 |
| CS prostate cancer, (%) | 57.1 | 45.0 | 0.501 | |
| Kum et al. 2018 (AB)34,35 | Cancer detection rate, overall n/N (%) | 139/176 (79.0) | Not reported | Not reported |
| CS cancer detection n/N (%)d,e Systematic |
28/46 (60.9) | 25/43 (58.1) | p = 0.80 | |
| Targeted and systematic | 29/35 (82.9) | Not reported | Not reported | |
| Targeted | 33/38 (86.8) | Not reported | Not reported | |
| Watanabe et al. 200538 | Positive biopsy, n/N (%) | 166/402 (41.3) | 161/402 (40.0) | Not reported |
| Retrospective studies | ||||
| Abdollah et al. 201139 | Prostate cancer diagnosis rate, n/N (%) | 36/140 (25.7) | 44/140 (31.4) | 0.3 |
| Jiang et al. 201940 | Cancer detection rates Unmatched group |
785/1746 (45.0) | 524/1216 (43.1) | 0.314 |
| Propensity score matched group | 182/376 (48.4) | 184/376 (48.9) | 0.884 | |
| Szabo et al. 2021 I41 | Overall cancer detection rate, n/N (%) | 105/242 (43.4) | 52/133 (39.0) | 0.4451 |
| Szabo et al. 2021 II41 | Overall cancer detection rate, n/N (%) | 20/62 (32.0) | 52/133 (39.0) | Not reported |
| Szabo et al. 2021 I and II41 | CS cancer detection rate, n/N (%)f | 35/242 (14.0) | Not reported | Not reported |
There was variation between the studies in overall cancer detection rates, which highlights the heterogeneous nature of this evidence base. In terms of differences in detection rates between LATP and LATRUS, the results are mixed. Some studies reported similar rates for the two biopsy methods, while others reported differences in rates. There is not a clear pattern to these differences – in some cases LATP biopsy detects a greater proportion of cancers than LATRUS, but the opposite is also evident. We urge caution when interpreting these results given the predominance of observational study methods. The similarities and differences in cancer detection rates between the two biopsy methods may be driven, in part, by selection bias from lack of random allocation of participants to LATP biopsy or LATRUS biopsy study arms.
Figure 9 in Appendix 4 shows pooled study estimates from a random-effects meta-analysis of LATP-any versus LATRUS for detection of prostate cancer. There is no statistically significant difference between LATP-any biopsy and LATRUS biopsy in detection of prostate cancer based on RCTs (RR = 1, 95% CI 0.85 to 1.18) (n = 5 RCTs) and based on observational comparative studies (RR = 1.10, 95% CI 1.01 to 1.21) (n = 8 studies). Caution is advised in the interpretation of these results given that the overall risk of bias in the RCTs is unclear due to limited available study details (see Results of critical appraisal of study methodology). Furthermore, although heterogeneity was low and not statistically significant, we note the presence of clinical heterogeneity across the studies.
Figure 10 in Appendix 4 reports pooled study estimates from a random-effects meta-analysis of LATP-any versus LATRUS for detection of CS prostate cancer. There is no statistically significant difference between LATP-any biopsy and LATRUS biopsy in detection of CS prostate cancer, based on a single RCT (RR = 1.14, 95% CI 0.65 to 2.01) and based on observational comparative studies (RR 1.20, 95% CI 0.98 to 1.47) (n = 4 studies).
Prostate cancer detection (local anaesthetic transperineal-any vs. general anaesthetic transperineal grid and stepping device, decision question 1)
Table 9 reports study cancer detection rates from the four studies which compared LATP-any biopsy versus GATP biopsy using grid and stepping device, and Figure 11 in Appendix 4 shows a meta-analysis forest plot containing three of the four studies (NB: the study publication by Walters et al. did not provide numerical cancer detection rates and was therefore not included in the meta-analysis). 44 There was some inconsistency between the studies in the direction of effects, with two studies marginally favouring LATP-any42,45 and another (smaller) study showing a large effect in favour of GATP. 43 Overall, there is no statistically significant difference between the two biopsy modalities in detection of prostate cancer, as estimated by a single RCT (RR = 1.05, 95% CI 0.76 to 1.44) and observational comparative evidence (RR = 0.76, 95% CI 0.34 to 1.72) (n = 2 studies).
| Study | Outcome measure | Intervention LATP-any |
Comparator GATP |
Statistical significance (p-value) |
|---|---|---|---|---|
| RCTs | ||||
| Lv et al. 202042 | Cancer positive detectable rate, n (%) | 45 (41.7) | 43 (39.8) | 0.782 |
| Other prospective studies | ||||
| Takuma et al. 2012 (AB)43 | Cancer detection rate, n/N (%) | 9/37 (24.0) | 15/29 (51.0) | 0.041 |
| Walters et al. 2021 (AB)44 | Histology outcomes | ‘No significant differences in histology outcome’ between the different anaesthetic methods (LATP vs. LATRUS) | Not reported | |
| Retrospective studies | ||||
| Rij and Chapman 2020 (AB)45 | Cancers detected, n/N (%) | 65/72 (90.0) | 59/71 (83.0) | Not reported |
Prostate cancer detection (network meta-analysis of local anaesthetic transperineal-any vs. local anaesthetic transrectal ultrasound vs. general anaesthetic transperineal grid and stepping device, decision question 1)
We used MetaInsight software23 to conduct a frequentist random-effects NMA of cancer detection rates for the biopsy modalities relevant to decision question 1 (see Figure 12, Appendix 4). The NMA provides an indirect comparison between LATP-any, LATRUS and GATP grid and stepping device to provide clinical effect estimates used in our economic analysis (see Model parameters). We restricted the NMA to RCTs because, in principle, randomised study designs have greater internal validity than observational studies (notwithstanding the uncertain risk of bias in RCTs we discussed earlier– see Results of critical appraisal of study methodology).
Consistent with the results of the pairwise meta-analyses above, the results of the NMA show RRs just below or just above RR = 1 for the respective biopsy modalities. Confidence intervals cross 1, indicating no statistically significant differences in cancer detection rates between the three biopsy modalities (see Figure 13, Appendix 4).
Our original economic base case included these NMA cancer detection rates in the economic model; however, at the request of NICE the base case was subsequently revised to exclude the Hara et al. 26 and Takenaka et al. 28 trials from the NMA. This was due to uncertainties raised by NICE Specialist Committee Members about the most appropriate anaesthesia classification for these studies (i.e. LATP or GATP) arising from the clinically atypical approach to anaesthesia administration taken (spinal injection in the transperineal trial arm and caudal block in the transrectal trial arm). The revised economic base case was accompanied by two economic scenario analyses: scenario 1 in which Hara et al. 26 and Takenaka et al. 28 were retained in the NMA as originally classified (i.e. LATP, as Figure 13, Appendix 4); and scenario 2 in which Hara et al. 26 and Takenaka et al. 28 were reclassified as GATP. The results for all three NMAs are given in Table 31.
Prostate cancer detection (local anaesthetic transperineal-freehand vs. local anaesthetic transrectal ultrasound, decision question 2)
Cancer detection rates, including CS cancer rates (where available), for six of the seven studies comparing LATP-freehand versus LATRUS are reported in Table 10 (NB: the remaining study36,37 did not report cancer detection as an outcome). The PrecisionPoint freehand device was evaluated in all six studies, and collectively the studies comprise a subset of LATP-any studies for decision question 1 presented earlier.
| Study | Outcome measure | Intervention LATP-freehand |
Comparator LATRUS |
Statistical significance (p-value) |
|---|---|---|---|---|
| RCTs | ||||
| Lam et al. 2021 (AB)27 | Cancer detection rate, n/N (%) | 47/134 (35.1) | 33/132 (25.0) | < 0.05 |
| CS cancer detection ratea | 22/134 (16.4) | 19/132 (14.4) | 0.74 | |
| Prospective studies | ||||
| Bojin 201929 | Cancer detection rates malignant, n/N (%) | 76/103 (73.7) | 117/189 (61.9) | Not reported |
| Cancer detection rates benign, n/N (%) | 27/103 (26.2) | 72/189 (38.1) | Not reported | |
| CS cancer pick up, n/N (%)b | 51/76 (67.1) | 48/117 (41.2) | Not reported | |
| Chen et al. 202130,31 | Cancer detection rate in biopsy naïve patients, n/N (%) | 127/200 (63.5) | 86/172 (50.0) | 0.0115 |
| Hung et al. 2020 (AB)33 | Cancer detection rate (%) | 20/63 (31.7) | 14/57 (24.6) | 0.851 |
| CS prostate cancer, (%) | 57.1 | 45.0 | 0.501 | |
| Kum et al. 201834,35 | Cancer detection rate, overall, n/N (%) | 139/176 (79.0) | Not reported | Not reported |
| Malignant primary biopsy, n/N (%)c | ||||
| Systematic | 46/75 (61.3) | 43/77d (55.8) | 0.50 | |
| Targeted and systematic | 35/40 (88.6) | Not reported | Not reported | |
| Targeted | 38/41 (92.7) | Not reported | Not reported | |
| CS cancer detection n/N (%)e,f | ||||
| Systematic | 28/46 (60.9) | 25/43 (58.1) | 0.80 | |
| Targeted and systematic | 29/35 (82.9) | Not reported | Not reported | |
| Targeted | 33/38 (86.8) | Not reported | Not reported | |
| Retrospective studies | ||||
| Szabo et al. 2021 I41 | Overall cancer detection rate, n/N (%) | 105/242 (43.4)g | 52/133 (39.0) | 0.4451 |
| CS cancer detection rate, n/N (%)h | 35/242 (14.0) | Not reported | Not reported | |
Figure 14 in Appendix 4 reports results of the random-effects meta-analysis of cancer detection rates for LATP-freehand versus LATRUS. A borderline non-statistically significant difference favouring LATP-freehand was estimated by the single relevant RCT (RR = 1.40, 95% CI 0.96 to 2.04). A statistically significant difference favouring LATP-freehand was estimated by the pooled observational comparative studies (RR = 1.21, 95% CI 1.08 to 1.34) (n = 4 studies).
To permit an incremental assessment of biopsy modality effects in our economic model we considered splitting the ‘LAPT-any’ study category into its constituent biopsy subtypes, that is LATP-freehand, LATP grid and stepping device and LATP coaxial needle (double freehand). However, details of biopsy procedures were limited in some study publications and it was unclear whether studies of LATP grid and stepping device or LATP coaxial needle (double freehand) could be reliably classified as such. Hence, as a pragmatic adjustment to allow an assessment of incremental cost-effectiveness, we combined these two biopsy modalities into a general category we refer to as ‘LATP-other’. It should be acknowledged, however, that a potential limitation is the underlying assumption that LATP using a grid and stepping device and LATP with a coaxial needle are necessarily equivalent in effects.
When RCT and observational evidence are pooled in our exploratory meta-analysis there is a statistically significant effect in favour of LATP-freehand compared to LATRUS (RR 1.22, 95% CI 1.10 to 1.35) (see Figure 13, Appendix 4). In contrast, there is no statistically significant difference between LATP-other and LATRUS for RCT evidence or observational comparative evidence or the two combined (see Figure 15, Appendix 4).
In the random-effects meta-analysis of LATP-freehand compared to LATRUS for CS prostate cancer detection, RRs non-significantly favoured LATP-freehand, as based on a single RCT (RR = 1.14, 95% CI 0.65 to 2.01) (see Figure 16, Appendix 4). There was a borderline statistically significant difference favouring LATP-freehand based on the observational comparative studies (RR = 1.31, 95% CI 1.00 to 1.72) (n = 3 studies). When observational and RCT studies are pooled in our exploratory analysis, a statistically significant difference is estimated (see Figure 16, Appendix 4).
Prostate cancer detection (local anaesthetic transperineal-freehand vs. general anaesthetic transperineal grid and stepping device decision question 2)
A single study compared cancer detection rates between LATP-freehand (PrecisionPoint) versus GATP grid and stepping device. 45 The study is a retrospective review of people who underwent transperineal prostate biopsy under local anaesthetic or under general anaesthetic, performed by a single surgeon. There was a small difference of seven percentage points in cancer detection rates, favouring PrecisionPoint [cancers detected: 65/72 (90.0%) PrecisionPoint versus 59/71 (83.0%) GATP]. [NB; this is one of the studies included in the comparison of LATP-any vs. GATP grid and stepping device presented earlier: see Prostate cancer detection (LATP-any vs. GATP grid and stepping device, decision question 1)].
Prostate cancer detection (network meta-analysis of local anaesthetic transperineal-freehand vs. local anaesthetic transperineal-other vs. local anaesthetic transrectal ultrasound vs. general anaesthetic transperineal grid and stepping device, decision question 2)
We used MetaInsight software (Owen et al.)23 to conduct a frequentist random-effects NMA of cancer detection rates from RCTs for decision question 2 (see Figure 17, Appendix 4). This provided an indirect comparison between LATP-freehand versus LATP-other versus LATRUS versus GATP grid and stepping device, to inform an incremental assessment of cost-effectiveness in our economic analysis (see Model parameters).
Prostate cancer detection risk classification
Table 11 compares risk classification scores for people with detected prostate cancer biopsy for LATP-any versus LATRUS. The risk of the prostate cancer progressing aggressively was commonly assessed using Gleason scores (higher scores indicate greater progression risk), though other classification systems appear to have been used. 34 Not all studies provided risk classification for the comparator biopsy arm, but where comparative data were given Gleason scores were similar. Two of the studies34,41 are also relevant to the comparison of LATP-freehand versus LATRUS (decision question 2). 34,35,41
| Study | Risk classification of prostate cancer detected | Intervention LATP-any | Comparator LATRUS | Statistical significance (p-value) |
|---|---|---|---|---|
| RCTs | ||||
| Guo et al. 201525 | Gleason score, n/N (%) | |||
| ≤ 6 | 18/173 (10.4) | 18/166 (10.8) | 0.547 | |
| = 7 | 18/173 (10.4) | 15/166 (9.0) | 1.000 | |
| ≥ 8 | 25/173 (14.5) | 18/166 (10.8) | 0.564 | |
| Very LR prostate cancer, n/N (%) | 6/173 (3.5) | 5/166 (3.0) | 1.000 | |
| Other prospective studies | ||||
| Emiliozzi et al. 200332 | Gleason score, n/N (%) | |||
| Gleason 5 | 2/41 (5.0) | 0 (0) | Not reported | |
| Gleason 6 | 20/41 (49.0) | 19/34 (56.0) | ||
| Gleason 7 | 17/41 (41.0) | 14/34 (41.0) | ||
| Gleason 8–9 | 2/41 (5.0) | 1/34 (3.0) | ||
| Kum et al. 2018 (AB)34,35 | LRa, n/N (%) | |||
| Systematic | 36/91b (39.0) | Not reported | Not reported | |
| Targeted and systematic | 7/40b (17.0) | |||
| Targeted | 6/45b (13.0) | |||
| IRc, n/N (%) | ||||
| Systematic | 52/91b (57.0) | Not reported | Not reported | |
| Targeted and systematic | 28/40b (69.0) | |||
| Targeted | 26/45b (58.0) | |||
| HRa, n/N (%) | ||||
| Systematic | 4/91b (4.0) | Not reported | Not reported | |
| Targeted and systematic | 6/40b (14.0) | |||
| Targeted | 13/45b (29.0) | |||
| Watanabe et al. 200538 | Clinical staged, n/N (%) | |||
| T1c | 29/39 (74.4) | 25/39 (64.1) | Not reported | |
| T2 | 71/86 (82.6) | 70/86 (81.4) | ||
| T3-T4 | 66/70 (94.3) | 66/70 (94.3) | ||
| Gleason score, n/N (%) | ||||
| Gleason 2–4 | 25/37 (67.6) | 26/37 (70.3) | Not reported | |
| Gleason 5–6 | 59/70 (84.3) | 55/70 (78.6) | ||
| Gleason 7 | 47/52 (90.4) | 45/52 (86.5) | ||
| Gleason 8–9 | 35/36 (97.2) | 35/36 (97.2) | ||
| Retrospective studies | ||||
| Jiang et al. 201940 | Gleason score, n/N (%)b | |||
| ≤ 6 | 32/182 (17.6) | 58/184 (31.5) | Not reported | |
| 7 | 73/182 (40.1) | 90/184 (48.9) | < 0.001 | |
| ≥ 8 | 77/182 (42.3) | 36/184 (19.6) | Not reported | |
| Szabo et al. I41 | Gleason grade, n/N (%) | |||
| Grade group 1 | 70/105 (66.7) | Not reported | Not reported | |
| Grade group 2 | 20/105 (19.0) | |||
| Grade group 3 | 4/105 (3.8) | |||
| Grade group 4 | 2/105 (1.9) | |||
| Grade group 5 | 9/105 (8.6) | |||
A single (retrospective observational) study reported cancer risk classification for the comparison of LATP-any versus GATP grid and stepping device. 45 The study used the International Society of Urological Pathology (ISUP) grade group classification as ‘low risk’ to ‘Intermediate Favourable risk’. The LATP biopsy was done using the PrecisionPoint freehand device, thus this study is also relevant to ‘LATP-freehand versus GATP grid and stepping device (decision question 2)’. A higher percentage of participants were classified as ISUP > 2 by the LATP biopsy than GATP, but this was not statistically significant [n = 35/65 (53.8%) vs. n = 28/59 (47.5%), respectively, p = 0.48].
Diagnostic accuracy of prostate biopsy
None of the included studies fully reported the diagnostic or prognostic accuracy of LATP biopsy. Rather, as mentioned earlier, studies tended to report cancer detection rates without necessarily verifying the accuracy of cancer detected against a reference standard in terms of measures such as sensitivity and specificity.
One study reported the proportion of all cancers detected under LATP and under GATP (clinical sensitivity), but did not provide information on proportion of cancers not detected (clinical specificity). 45 A reference standard was not reported either. This study is currently available only as a conference abstract, hence limited information.
Another study reported the pathological accordance of Gleason scores based on biopsy with histological analysis of prostatectomy specimens (i.e. a reference standard). 43 This resulted in a small proportion of participants having their Gleason scores upgraded and upstaged.
Clinical outcomes
Hospitalisation events after biopsy
Hospitalisation following prostate biopsy was reported by a total of 10 studies, for 4 of the 5 biopsy comparisons relevant to the decision problem (see Tables 12–14). Studies tended to report the number of participants admitted to hospital at various time points after the biopsy (e.g. up to 30 days post biopsy), while others reported hospitalisation in response to serious complications such as fever and pneumonia. Less commonly reported was the duration of hospital stay. Overall, rates of hospitalisation were numerically higher for comparator biopsy approaches compared to LATP across the four biopsy comparisons. However, hospitalisation rates were very low in general, and it is therefore difficult to make definitive conclusions on the currently available evidence.
| Study | Hospitalisation outcome | LATP-any biopsy | LATRUS biopsy |
|---|---|---|---|
| RCTs | |||
| Takenaka et al. 200828 | Major complications, n/N (%)a | ||
| Total | 1/100 (1.0) | 4/100 (4.0) | |
| Macrohematuria | 0/100 (0) | 1/100 (1.0) | |
| Fever > 38.5 °C | 0/100 (0) | 2/100 (2.0) | |
| Urinary retention | 0/100 (0) | 1/100 (1.0) | |
| Other prospective studies | |||
| Chen et al. 202130,31 | Hospitalised for monitoring and discharged after 1 day, n/N (%) | 1/212 (0.5) | 0/178 (0) |
| Emiliozzi et al. 200332 | Post-biopsy hospitalisation, n/N (%) | 0/107 (0) | 0/107 (0) |
| Kum et al. 2018 (AB)34,35 | Hospitalisation overnight, n/N | 1/176 | Not reported |
| Starmer et al. 202136,37 | Readmission within 30 days, n/N (%) | 0/56 (0) | 1/52 (1.9)b |
| Pneumonia requiring readmission, n/N (%) | 0/56 (0) | 1/52 (1.9)b | |
| Watanabe et al. 200538 | Prolonged hospital stay, n/N (%) | 0/402 (0) | 0/402 (0) |
| Retrospective studies | |||
| Szabo et al. 2021 I41 | Hospital admission, n/N (%) | Not reported | 1/133 (0.75) |
| Szabo et al. 2021 II41 | Hospital admission, n/N (%) | Not reported | 1/133 (0.75) |
| Study | Hospitalisation outcome | LATP-any biopsy | GATP biopsy grid and stepping device |
|---|---|---|---|
| RCTs | |||
| Lv et al. 202042 | Duration of hospital stay, hours, mean (SD) | 23.50 (± 3.48) | 23.12 (± 2.85) |
| Retrospective studies | |||
| Rij and Chapman 2020 (AB)45 | Readmission to hospital post biopsy, n/N (%) | 0/72 (0)a | 0/71 (0)a |
| Study | Hospitalisation outcome | LATP-freehand biopsy | LATRUS biopsy |
|---|---|---|---|
| Other prospective studies | |||
| Chen et al. 202130,31 | Hospitalised for monitoring and discharged after 1 day, n/N (%) | 1/212 (0.5) | 0/178 (0) |
| Kum et al. 2018 (AB)34,35 | Hospitalisation overnight | 1/176 | Not reported |
| Starmer et al. 202136,37 | Readmission within 30 days, n/N (%) | 0/56 (0) | 1/52 (1.9)a |
| Pneumonia requiring readmission, n/N (%) | 0/56 (0) | 1/52 (1.9)a | |
| Retrospective studies | |||
| Szabo et al. 2021 I41 | Hospital admission, n/N (%) | Not reported | 1/133a (0.8) |
| Rij and Chapman 2020 (AB)45 | Readmission to hospital post biopsy, n/N (%) | 0/72 (0)b | 0/71 (0)b |
The cost of hospital stays can be influential in the assessment of cost-effectiveness of health care. We discuss the hospitalisation estimates which inform our economic analysis of prostate biopsy in Biopsy-related complications.
Overall biopsy-related complications
Six studies reported overall rates of complications following prostate biopsy. Some, but not all, of the studies reported overall rates in addition to rates of the constituent complications. We report here only studies which presented an overall complication rate; we did not sum rates of specific named complications to create an overall total complication rate for each study. All six studies were comparisons of LATP-any biopsy versus LATRUS biopsy and are relevant to decision question 1 (Table 15). Two of the six studies compared freehand transperineal devices versus LATRUS and therefore are also relevant to decision question 2. 30,31,34,35
| Study | Complication | LATP-any biopsy | LATRUS biopsy | Statistical significance |
|---|---|---|---|---|
| RCTs | ||||
| Cerruto et al. 201424 | Overall complication rate, n/N (%)a | 7/54 (13.0) | n = 7/54 (13.0) | Not significant |
| Guo et al. 201525 | All complications, n/N (%) | 76/167 (45.5) | 73 (45.3) | 0.912 |
| All minor complications, n/N (%) | 75/167 (44.9) | 66 (41.0) | 0.504 | |
| All major complications, n/N (%) | 1 (0.6) | 7 (4.3) | 0.034 | |
| Takenaka et al. 200828 | Total complications (inclusive of major complications), n/N (%) | 19/100 (19.0) | 20/100 (20.0) | |
| Other prospective studies | ||||
| Chen et al. 2021b,30,31 | Overall complication rate, n/N (%) | 13/212 (6.1) | 20/178 (11.2) | 0.0993 |
| Kum et al. 2018 (AB)b,34,35 | Complications (Clavien–Dindo I/II), n/N (%) | 5/176 (2.8) | Not reported | Not reported |
| Watanabe et al. 200538 | Adverse event, n/N (%) | 5/402 (1.2) | 5/402 (1.2) | Not reported |
Specific biopsy-related complications
Bleeding and haematuria
Various types of bleeding events were reported as biopsy-related complications, including rectal and urethral bleeding and haematuria (the presence of blood in urine). In some cases, the severity of these events was defined, ranging from mild symptoms to severe symptoms such as retention of blood clots in the bladder requiring urgent medical attention. In other cases there was little or no elaboration beyond stating the location of the bleed.
For the comparison of LATP-any versus LATRUS (decision question 1), 9 of the 15 included studies reported a relevant bleeding and/or haematuria outcome (Table 16). Generally, bleeding/haematuria rates were low (e.g. < 30% of participants), and in relative terms rates were higher with LATRUS than LATP-any. Conversely, urethral bleeding was more common with LATP-any in the study by Cerruto et al.,24 but the sample size for this analysis was very small (< 20 participants) and is unlikely to be sufficient to ensure a definitive effect.
| Study | Outcome | LATP-any | LATRUS | Statistical significance | |
|---|---|---|---|---|---|
| RCTs | |||||
| Cerruto et al. 201424 | Rectal bleeding, n/N (%)a | 0/7 (0) | 4/7 (57.2) | 0.04 | |
| Urethral bleeding, n/N (%)a | 5/7 (71.4) | 0/7 (0) | 0.022 | ||
| Guo et al. 201525 | Mild rectal bleeding, n/N (%) | 0/167 (0) | 14/161 (8.7) | < 0.001 | |
| Severe rectal bleeding, n/N (%) | 0/167 (0) | 2/161 (1.2) | Not reported | ||
| Mild haematuria, n/N (%) | 33/167 (19.8) | 37/161 (23.0) | 0.502 | ||
| Severe haematuria, n/N (%) | 0/167 (0) | 0/161 (0) | Not reported | ||
| Hara et al. 200826 | Major rectal bleeding, n/N (%) | 0 (0) | 0 (0) | N/A | |
| Haematuria > 1 day, n/N (%) | 2 (1.6) | 0 (0) | 0.166 | ||
| Takenaka et al. 200828 | Rectal bleeding, n/N (%) | 0/100 (0) | 1/100 (1.0) | Not reported | |
| Macrohaematuria, n/N (%) | 11/100 (11.0) | 12/100 (12.0) | Not reported | ||
| Other prospective studies | |||||
| Chen et al. 202130,31 | Haematuria, n/N (%) | 2/212 (0.9) | 3/178 (1.7) | 0.6640 | |
| Emiliozzi et al. 200332 | Temporary haematuria, n/N (%) | 33/107 (31.0)b | Not reported | ||
| Kum et al. 2018 (AB)34,35 | Clot retention (Clavien–Dindo Grade II), n/N (%) | 1/176 (0.6) | Not reported | Not reported | |
| Watanabe et al. 200538 | Significant haematuria requiring transurethral coagulation of prostatic bleeding, n/N (%) | 1/402 (0.2) | Not reported | ||
| Retrospective studies | |||||
| Szabo et al. 2021 I41 | Gross haematuria with clot retention, n/N (%) | 3/242 (1.2) | Not reported | Not reported | |
| Szabo et al. 2021 II41 | Gross haematuria with clot retention, n/N (%) | 1/62 (1.6) | Not reported | Not reported | |
For the comparison between LATP-any biopsy and GATP biopsy with grid and stepping device, two of the four included studies reported bleeding-related outcomes (Table 17). Observation of the data gives a faint suggestion that bleeding is potentially worse for GATP biopsy grid and stepping device than for LATP-any biopsy. However, this is based on a small number of events from a single RCT. 42 Rates of urethral bleeding were generally between the two biopsies, in stark contrast to the aforementioned comparison between LATP-any and LATRUS by Cerruto et al. 24
| Study | Outcome | LATP-any biopsy | GATP biopsy grid and stepping device | Statistical significance |
|---|---|---|---|---|
| RCTs | ||||
| Lv et al. 202042 | Blood loss, ml, mean (SD) | 3.35 (± 1.04) | 3.60 (± 1.13) | 0.092 |
| Perineal haematoma, n/N (%) | 0/108 (0) | 1/108 (0.93) | 0.996 | |
| Urethral bleeding, n/N (%) | 19/108 (17.59) | 25/108 (23.15) | 0.311 | |
| Retrospective studies | ||||
| Rij and Chapman 2020 (AB)45 | Prolonged haematuria, n/N (%) | 2/72 (3.0) | Not reported | Not reported |
| Perineal haematomas, n/N (%) | Not reported | 3/71 (4.0) | Not reported | |
Moving on to decision question 2, four of the seven LATP-freehand (PrecisionPoint) device studies (all observational studies) assessed bleeding as a biopsy complication (Table 18). Rates of bleeding were very low overall, and it is difficult to draw any definitive conclusions regarding whether they are more common with LATP-freehand versus LATRUS. Likewise, for the single-study comparison of LATP-freehand biopsy versus GATP biopsy grid and stepping device,45 data are very sparse and, thus, inconclusive at present.
| Study | Outcome | LATP-freehand | LATRUS | Statistical significance |
|---|---|---|---|---|
| Other prospective studies | ||||
| Chen et al. 202130,31 | Haematuria, n/N (%) | 2/212 (0.9) | 3/178 (1.7) | 0.6640 |
| Kum et al. 2018 (AB)34,35 | Clot retention (Clavien–Dindo Grade II), n/N (%) | 1/176 (0.6) | Not reported | Not reported |
| Retrospective studies | ||||
| Szabo et al. 2021 I41 | Gross haematuria with clot retention, n/N (%) | 3/242 (1.2) | 1/62 (1.6) | Not reported |
| Rij and Chapman 2020 (AB)45 | Prolonged haematuria, n/N (%) | 2/72 (3.0) | Not reported | Not reported |
| Perineal haematomas, n/N (%) | Not reported | 3/71 (4.0) | Not reported | |
Sepsis
Relatively few studies reported post-biopsy sepsis as an outcome. Where reported, rates of sepsis were generally low (< 10%) and exclusively to LATRUS biopsy participants; no LATP biopsy participants are recorded as having post-biopsy sepsis (see Tables 19 and 20).
| Study | Outcome | LATP-any | LATRUS | Statistical significance |
|---|---|---|---|---|
| RCTs | ||||
| Guo et al. 201525 | Major complications: sepsis, n (%) | 0 (0) | 1 (0.6) | Not reported |
| Hara et al. 200826 | Major complications: sepsis/mortality, n (%) | 0 (0) | 0 (0) | Not reported |
| Lam et al. 2021 (AB)27 | Post-biopsy sepsis | 0/0 (0) | 11/132 (8.3) | Not reported |
| Other prospective studies | ||||
| Chen et al. 202130,31 | Urosepsis, n/N (%)a | 0/212 (0) | 4/178 (2.2) | 0.0431 |
| Hung et al. 2020 (AB)33 | Sepsis, n/N (%) | 0/63 (0) | 3/57 (5.3) | 0.045 |
| Retrospective studies | ||||
| Szabo et al. 2021 I41 | Sepsis, n/N (%), Clavien grade |
0/242 (0) Not applicable |
1/133a (0.8) Clavien IVb |
Not reported |
| Szabo et al. 2021 II41 | Sepsis, n/N (%), Clavien grade |
0/62 (0) Not applicable |
1/133a (0.8) Clavien IVb |
Not reported |
| Study | Outcome | LATP-any | LATRUS | Statistical significance |
|---|---|---|---|---|
| RCTs | ||||
| Lam et al. 2021 (AB)27 | Post-biopsy sepsis | 0/0 (0) | 11/132 (8.3) | Not reported |
| Other prospective studies | ||||
| Chen et al. 202130,31 | Urosepsis, n/N (%)a | 0/212 (0) | 4/178 (2.2) | 0.0431 |
| Hung et al. 2020 (AB)33 | Sepsis, n/N (%) | 0/63 (0) | 3/57 (5.3) | 0.045 |
| Retrospective studies | ||||
| Szabo et al. I41 | Sepsis, n/N (%), Clavien grade |
0/242 (0) Not applicable |
1/133a (0.75) Clavien IVb |
Not reported |
| Szabo et al. II41 | Sepsis, n/N (%), Clavien grade |
0/62 (0) Not applicable |
1/133a (0.75) Clavien IVb |
Not reported |
None of the LATP-any versus GATP grid and stepping device studies (decision question 1) and none of the LATP-freehand biopsy versus GATP biopsy grid and stepping device studies (decision question 2) included sepsis as an outcome measure.
Fever
Post-biopsy fever was reported by four studies (all RCTs) all of which compared LATP-any versus LATRUS (decision question 1). None of the LATP biopsy procedures involved use of a freehand device (Table 21). Rates of high fever were numerically higher for LATRUS though the event rates are low overall, and it is difficult to make definitive conclusions on small numbers of participants.
| Study | Outcome | LATP-any | LATRUS | Statistical significance |
|---|---|---|---|---|
| RCTs | ||||
| Cerruto et al. 201424 | Fever > 38.5 °C, n/N (%) | 0/7 (0) | 1/7 (14.3) | 0.315 |
| Guo et al. 201525 | Low fever < 38.5 °C, n/N (%) | 2/167 (1.2) | 2/167 (1.2) | 0.099 |
| High fever > 38.5 °C, n (%) | 0 (0) | 2 (1.2) | Not reported | |
| Hara et al. 200826 | Fever > 38.5 oC, n (%) | 0 (0) | 2 (1.7) | 0.136 |
| Takenaka et al. 200828 | Fever > 38.5 oC, n/N (%) | 1/100 (1.0) | 2/100 (2.0) | Not reported |
Rates of urinary retention
Post-biopsy urinary retention is reported by nine studies in total across three biopsy comparisons (see Tables 22–24). Some studies reported retention data for the LATP biopsy but not the comparator. Where comparative evidence was available, retention rates were similar between biopsy modalities, though it is difficult to make definitive conclusions based on small event rates.
| Study | Outcome | LATP-any | LATRUS | Statistical significance |
|---|---|---|---|---|
| RCTs | ||||
| Lam et al. 2021 (AB)27 | Post-biopsy urinary retention | ‘No statistically significant difference between both arms’ p = 0.107 |
p = 0.107 | |
| Hara et al. 200826 | Urinary retention, n (%) | 2 (1.6) | 3 (2.5) | 0.612 |
| Takenaka et al. 200828 | Urinary retention, n (%) | 2/100 (2.0) | 3/100 (3.0) | Not reported |
| Other prospective studies | ||||
| Chen et al. 202130,31 | Acute urinary retention, n/N (%) | 8/212 (3.8) | 8/178 (4.5) | 0.8008 |
| Hung et al. 2020 (AB)33 | Urinary retention rate | ‘No statistically significant difference’ | Not reported | |
| Kum et al. 2018 (AB)34,35 | Urinary retention (Clavien-–Dindo Grade II), n/N (%) | 1/176 (0.6) | Not reported | Not reported |
| Watanabe et al. 200538 | Urinary retention requiring urethral catheterisation, n/N (%) | 2/402 (0.5) | Not reported | |
| Retrospective studies | ||||
| Szabo et al. 2021a41 | Acute urinary retention, n/N (%), Clavien grade | 1/242 (0.4) Clavien I |
Not reported | Not reported |
| Study | Outcome | LATP-any | GATP biopsy grid and stepping device | Statistical significance |
|---|---|---|---|---|
| RCT | ||||
| Lv et al. 202042 | Retention of urine, n (%) | 3 (2.8) | 2 (1.9) | 0.997 |
| Study | Outcome | LATP-any | LATRUS | Statistical significance | |
|---|---|---|---|---|---|
| Other prospective studies | |||||
| Chen et al. 202130,31 | Acute urinary retention, n/N (%) | 8/212 (3.8) | 8/178 (4.5) | 0.8008 | |
| Hung et al. 2020 (AB)33 | Urinary retention rate | ‘No statistically significant difference’ | Not reported | ||
| Kum et al. 2018 (AB)34,35 | Urinary retention (Clavien–Dindo Grade II), n/N (%) | 1/176 (0.6) | Not reported | Not reported | |
| Retrospective studies | |||||
| Szabo et al. 202141 | Acute urinary retention, n/N (%), Clavien grade | 1/242 (0.4) Clavien I |
Not reported | Not reported | |
No studies reported post-biopsy urinary retention for the comparison of LATP-freehand versus GATP/LATP using a grid and stepping device (decision question 2).
Rates of erectile dysfunction
Only two studies in this systematic review reported assessing post-biopsy erectile dysfunction. 27,33 Both used the International Index of Erectile Function (IIEF-5) instrument, in which lower scores indicate greater severity of erectile dysfunction. The observational study by Hung et al. reports that mean IIEF-5 change post biopsy was 2.74 in LATRUS and 6.03 in LATP, and was statistically significant (p = 0.023). 33
The RCT by Lam et al. reports a reduction in the IIEF-5 score that was ‘more significant in LATP arm’ p < 0.05. 27 No further detail is given to quantify this statement. Details of these two studies are publicly available only as a conference abstract at the time of writing. The EAG has been told, via personal communication with the lead investigator,27 that a manuscript is being prepared for submission to a journal.
Survival
None of the included studies reported survival outcomes for participants receiving biopsy.
Progression-free survival
None of the included studies reported progression-free survival for participants treated for prostate cancer detected on biopsy.
Adverse events from treatment
None of the included studies reported adverse events in participants treated for prostate cancer detected on biopsy.
Patient-reported outcomes
Patient-reported tolerability
A total of 12 studies reported data on the degree of pain and discomfort during prostate biopsy as rated by patients (see Tables 25 and 26). Tolerability was measured in a variety of ways across the studies, but often data are only presented for the LATP biopsy group, thus limiting comparisons to be drawn between types of biopsy.
| Study | Patient-reported tolerability | Intervention LATP-any |
Comparator LATRUS |
Statistical significance (p-value) |
|---|---|---|---|---|
| RCTs | ||||
| Cerruto et al. 201424 | VAS pain level, mean (SD) | 1.42 (1.37) | 1.56 (1.73) | 0.591 |
| Guo et al. 201525 | Pain, VAS score, median (IQR) | 4.0 (1.0–6.0) | 2.0 (0.0–4.0) | < 0.001 |
| Most painful procedure, n (%) | ||||
| None | 3 (1.7) | 37 (22.3) | < 0.001 | |
| Probe insertion | 30 (14.5) | 67 (42.2) | < 0.001 | |
| Anaesthesia | 110 (63.6) | 29 (17.5) | < 0.001 | |
| Sampling | 26 (15.0) | 25 (15.1) | 1.000 | |
| Others | 9 (5.2) | 5 (3.0) | 0.415 | |
| Additional anaesthesia, number of times, n (%) | 26 (15.0) | 2 (1.2) | < 0.001 | |
| Lam et al. 2021 (AB)27 | Patient tolerability comparison measured by VAS | ‘No statistically significant difference between both arms’ | p = 0.14 | |
| Other prospective studies | ||||
| Bojin 201929 | Tolerability, VAS pain score 0–6, median | 1.9 | Not reported | Not reported |
| Chen et al. 202130,31 | VAS pain score for the entire procedure, mean (SD, range) | 3.67 (2.57, 0–9) | Not reported | Not reported |
| Emiliozzi et al. 200332 | Mild post-biopsy perineal discomfort, n/N (%) | 7/107 (6.0) | Not reported | |
| Hung et al. 2020 (AB)33 | Overall pain scores | ‘No statistically significant difference’ | 0.527 | |
| Kum et al. 2018 (AB)34,35 | Procedure tolerability (100 mm VAS score) during three stages of procedure: ultrasound (US) probe insertion, local anaesthesia (LA) administration, biopsies, and an overall rating | Pain scores of the LATP group were not significantly different to TRUS at any procedural stage | Not reported | |
| Overall VAS rating of tolerability, median (IQR) | 27.5 (15, 49.25) | 45 (40–50) | p = 0.004 | |
| Starmer et al. 202136,37 | VAS scores, rated 0–9, for discomfort, median | |||
| At probe insertion | 3 | 4 | 0.66 | |
| Probe presence | 3 | 3 | 0.91 | |
| Local anaesthetic injection | 3 | 2 | 0.15 | |
| Taking biopsy | 3 | 3 | 0.18 | |
| VAS scores, rated 0–3, median | ||||
| Overall pain | 1 | 1 | 0.17 | |
| Embarrassment | 0 | 0 | 0.34 | |
| Describe to a friend | 1 | 1 | 0.2 | |
| Retrospective studies | ||||
| Szabo et al. 2021 I41 | VAS pain ratings, 0–10, average, median (range and SD) | 3.9, 4 (0–10, 1.9)a | Not reported | Not reported |
| Study | Patient-reported tolerability | Intervention LATP-any |
Comparator GATP |
Statistical significance (p-value) |
|---|---|---|---|---|
| RCTs | ||||
| Lv et al. 202042 | Degree of pain VAS scores during the perioperative period (0 = no pain, 10 = unbearable pain) mean (SD) | |||
| VAS1 (during anaesthesia) | 2.92 (± 0.96) | 0.00 (± 0.00) | Not calculated | |
| VAS2 (during biopsy) | 2.91 (± 1.09) | 0.00 (± 0.00) | Not calculated | |
| VAS3 (6 hours after biopsy) | 1.03 (± 0.76) | 1.06 (± 0.76) | 0.810 | |
| VAS4 (1 day after biopsy) | 1.04 (± 0.82) | 0.91 (± 0.78) | 0.238 | |
| Retrospective studies | ||||
| Rij and Chapman 2020 (AB)45 | Participants tolerating the procedure, n (%) | 72/72 (100.0) | Not reported | Not reported |
Ongoing studies
The EAG identified five ongoing studies relevant to this review, all of which are RCTs. Four studies are investigating LATP biopsy compared with LATRUS biopsy and one will investigate LATP biopsy compared with GATP biopsy. Below is a brief narrative summary of the five studies, with a tabular summary available in Table 58 in Appendix 4.
LATP versus LATRUS. The multicentre UK study (TRANSLATE) will provide evidence for freehand LATP using any ultrasound probe-mounted needle guidance device, including the PrecisionPoint and UA1232 devices. 52–54 As the study uses freehand devices to perform the biopsies it will assist with both decision question 1 (LATP-any vs. LATRUS) and decision question 2 (LATP-freehand vs. LATRUS). This will be the first comparative evidence to become available for the UA1232 device. As well as CS prostate cancer [Gleason grade (GG) > 2] detection rates and infection rates, this study will report on outcomes for which there is limited evidence in this review: erectile function and the number of subsequent biopsies within 4 months. It will also report cost outcomes. It is expected to have a larger study population (n = 1042) than any of the prospective studies included in this review.
The other three LATP versus LATRUS studies are based in the USA. ProBE-PC is a single-centre study and will report on sexual function, for which there is limited evidence in this review. 55 It will also report cost outcomes. Two multicentre studies (unnamed) run by the same institution differ in terms of the population: one study population is men with elevated PSA or abnormal DRE, and the other is men on active surveillance, or with prior negative prostate biopsy and a clinical concern for the presence of prostate cancer, which is partially relevant to this review. 56,57
All four LATP versus LATRUS studies incorporate using a pre-biopsy MRI to inform additional targeted biopsies that are performed during the procedure and will be relevant to the UK diagnostic pathway (not all included studies in this review reported the use of a pre-biopsy MRI).
LATP versus GATP. One Australian study (LATProBE), yet to start recruiting, will provide evidence for freehand LATP compared with GATP using a grid template. 58 It will report similar outcomes to studies already included in this review: cancer detection rates, costs, patient experience, pain, 30-day complications and HRQoL.
The earliest study completion date is December 2022 (ProBE-PC),55 the UK study is expected to complete the following year in October 2023 (TRANSLATE),52,53 and one study has not yet started recruiting (LATProBE). 58
Chapter 5 Economic analysis
The aim of this chapter is to assess the cost-effectiveness of LATP prostate biopsies in people with suspected prostate cancer. It comprises:
-
A systematic review of economic evidence comprising (1) a systematic review of cost-effectiveness studies of LATP prostate biopsies in people with suspected prostate cancer and (2) a systematic review of HRQoL (utility) for people with suspected or diagnosed prostate cancer.
-
An overview of evidence from company submissions to NICE.
-
An independent economic model developed by the EAG.
The EAG health economic base case originally submitted to NICE went through several changes to accommodate corrections and updated assumptions proposed by NICE and stakeholders. We report the results of a revised EAG base case in this monograph. The original EAG base case and the EAG addenda submitted to NICE can be accessed from the NICE website. 59
Systematic review of existing cost-effectiveness evidence
Methods for review of economic studies
The database searches for cost-effectiveness were carried out on 17 June 2021 and updated on 2 November 2021. The search strategies were based on an early version of the clinical-effectiveness searches with the addition of the Canadian Agency for Drugs and Technologies in Health (CADTH) filter for Economic Evaluations/Cost/Economic Models applied to the MEDLINE and EMBASE strategies and amended versions of the filter applied to the Cochrane Library and Web of Science strategies. 60 The INAHTA, DARE and NHS EED strategies were the same as for the clinical-effectiveness searches. In addition, the EconLit database was searched. An English-language limit was applied. The full search strategies are shown in Appendix 1 (Table 47).
The relevant population, interventions and comparators are the same as for the systematic review of test yield and clinical effectiveness (see Inclusion and exclusion criteria) but differed in terms of the relevant study design and outcomes. Studies were included if they were full economic evaluations, assessing both costs and consequences, for the specified diagnostic strategies. Outcomes included are those consistent with full economic evaluations, including measures of resource use and costs, and health outcomes: life-years (LYs) or quality-adjusted life-years (QALYs) gained. Each step of the review was completed by two health economists and any disagreements were resolved by discussion. Studies that reported resource use, costs or HRQoL in the area of prostate cancer were excluded if they did not meet the inclusion criteria above, but considered separately as possible sources of evidence to inform model structure and inputs.
The EAG planned to extract data related to the study design, methods, parameter sources, relevant model inputs and results of the included cost-effectiveness studies. The credibility of the included cost-effectiveness studies and their relevance to current UK practice were assessed using a pre-defined checklist, shown in Appendix 6, Table 64. This checklist was based on the International Society for Pharmacoeconomics and Outcomes Research (ISPOR)61 and Philips et al.'62 checklists.
Results of the review of economic studies
Starting with 724 potentially relevant references identified in the original (704) and updated (20) searches, 10 studies were retrieved for full-text screening (see flowchart in Appendix 6, Figure 19). After inspection, nine references were excluded (see Appendix 6, Table 62 for the reasons for exclusion).
Summary of included cost-effectiveness study: Wilson et al.
We identified one economic evaluation for inclusion within the scope of this assessment: Wilson et al. 63 Wilson et al. reported the cost-effectiveness of LATP (with the CamPROBE transperineal prostate biopsy device) versus LATRUS for use in the diagnosis of prostate cancer in men with suspected localised prostate cancer from the perspective of the UK NHS. The relevance and credibility checklist for this study and further details including a list of the model inputs are shown in Appendix 6, Tables 63 and 64.
Wilson et al. built a lifetime model comprising a decision tree with a Markov model at the terminal nodes. The model was informed by a prospective case series on the safety and acceptability of the CamPROBE device46 and published studies including an economic analysis of diagnostic strategies including mpMRI and TRUS biopsy based on data from the PROMIS study, reported by Faria et al. 64,65 The diagnostic pathway was based on NICE guidance8 and strategy ‘M7’ of the Faria study. The risks of biopsy complications were derived from a Cochrane review of antibiotic prophylaxis for transrectal prostate biopsy,66 with a base-case assumption of zero risk of infection with LATP. The analysis assumed equal diagnostic accuracy for LATP with the CamPROBE device and LATRUS.
Unit costs were taken from routine NHS sources for the price year 2018–9. The costs of biopsy were estimated from a sample of 17 CamPROBE and 17 LATRUS biopsies. Consumables were excluded from the incremental analysis if they were common to both procedures. Given the small sample, both procedures were assumed to take the same time and use the same volume of local anaesthetic. The price of the CamPROBE LATP biopsy device was unknown and set to zero for the base-case analysis, with sensitivity analysis used to estimate the maximum price for the device at which it would be cost-neutral, or cost saving compared with LATRUS. The incremental cost of LATRUS was therefore the difference in remaining consumable costs between the two biopsy techniques (£16.71). QALYs were based on disutility and duration of biopsy complications, and a disutility due to metastatic disease (MD).
Base-case results indicated that LATP (with the CamPROBE device at zero price) dominates LATRUS biopsy. At a threshold of £20,000 per QALY gained, the estimated probability that LATP is cost-effective compared with LATRUS was 59.0% and the maximum cost-effective price for CamPROBE was £81.17 per procedure (or £40.59 per CamPROBE device, as two are required per procedure). The maximum price at which CamPROBE was estimated to be cost-neutral was £40.82 per procedure. Two-way sensitivity analysis was used to explore uncertainty relating to the RR of infections and price of the CamPROBE device. At the £20,000 per QALY threshold, this indicated a maximum cost-effective procedure price of £14.50 for LATP with CamPROBE if the risk of infection was the same as with LATRUS. The results from the study by Wilson et al. are subject to a high degree of uncertainty. They also exclude other relevant comparators for the current evaluation, as specified in the two NICE decision questions.
Overview of other published economic studies of interest
Other studies retrieved by the systematic review were considered as possible sources of evidence to inform our model structure and inputs (see Appendix 6, Table 65). Most of these studies are evaluations of the use of mpMRI to inform TRUS biopsies versus TRUS alone in people with suspected prostate cancer, a prior negative or inconclusive biopsy or undergoing active surveillance. The remaining studies assessed screening or other diagnostic tests and assays (vs. TRUS or a PSA test) in men with suspected prostate cancer. Eight out of 13 studies used a decision tree plus a Markov model, while 2 used a decision tree only and another two used a Markov model only. One of the studies used a microsimulation model. Most studies applied a lifetime horizon and a 1-year Markov cycle length. All the studies reported costs and utilities and estimated the cost per QALY gained. Two economic studies were very influential in the development of our model and are discussed below.
Summary of other studies of interest: the PROMIS model
Firstly, the cost-effectiveness analysis conducted alongside the PROMIS study was reported in Brown et al.’ Health Technology Assessment (HTA) report and in Faria et al.’ paper. 64,65 This analysis assessed the cost-effectiveness of a range of diagnostic strategies using mpMRI, TRUS biopsy and/or a template prostate mapping biopsy (TPM) for men referred to secondary care in the UK NHS with suspected prostate cancer. It used a decision tree to model alternative diagnostic pathways consisting of sequences of up to three tests, followed by a Markov model that extrapolated from diagnostic outcomes to estimate long-term costs and QALYs. The PROMIS Markov model is illustrated in Faria et al. supplementary figure 1. It includes three basic health states: progression-free or localised disease, MD and death. But this simple three-state model is replicated for five groups of patients, based on ‘true disease’ status and treatment allocated at the end of the diagnostic pathway: patients with low-risk (LR) cancer on ‘watchful waiting’ and patients with intermediate-risk (IR) and high-risk (HR) cancer on either watchful waiting or with radical prostatectomy.
The analysis by Wilson et al., described above, relied heavily on the model structure and input parameters from Faria et al.’ model. We also used parameters from the PROMIS economic analysis to inform estimates of baseline prevalence of prostate cancer and diagnostic yield of TRUS biopsy in our model (see Baseline prevalence and Cancer detection rates). These estimates provided the baseline diagnostic outcomes for TRUS, against which other biopsy methods in the current scope were compared.
Summary of other studies of interest: the NG131 model
The second analysis that informed our model structure and parameters was that developed by the NICE Guideline Updates Team for the NICE guideline on prostate cancer published in May 2019 (NG131) to estimate the cost-effectiveness of follow-up protocols for people with a raised PSA, negative mpMRI and/or negative biopsy. 67
The NG131 Markov model includes 11 health states grouped in four categories: ‘true negatives’ (no prostate cancer or undiagnosed LR disease); ‘false negatives (FNs)’ (undiagnosed IR, HR or MD); ‘true positives’ (diagnosed disease from LR to metastatic); and death related to prostate cancer or from other causes (see NG131 health economic model report Table HE03). 67 We adapted this Markov model to predict long-term costs and outcomes based on diagnostic yield of the biopsy methods in the current decision problems (see Long-term consequences: the Markov model and Markov model structure).
Systematic review of health-related quality of life
Methods for review of health-related quality of life
We undertook searches to identify data on HRQoL for patients undergoing screening and diagnosis of prostate cancer, and for patients with diagnosed prostate cancer. The aim of these searches was to identify utility values that were suitable for use in the economic model.
A sequential approach was used to identify HRQoL studies:
-
Systematic searches of bibliographic databases were conducted for HRQoL data in people with suspected prostate cancer (searches ‘HRQoL 1’).
-
Additional systematic searches of bibliographic databases were conducted for HRQoL data in people with both suspected as well as diagnosed prostate cancer (searches ‘HRQoL 2’), to find additional utility values suitable for the economic model not identified in the ‘HRQoL 1’ searches.
The first set of searches (HRQoL 1) used the clinical-effectiveness search strategies with the addition of the CADTH search filter for Health Utilities/Quality of Life applied to the MEDLINE and EMBASE strategies and amended versions of the filter applied to the Cochrane Library and Web of Science strategies. The second set of database searches (HRQoL 2) were subsequently run with the biopsy terms removed to retrieve studies that would cover the whole disease pathway in addition to the diagnostic process. In order to save time, HRQoL 2 included terms specific to the European Quality of Life Working Group Health Status Measure 5 Dimensions (EQ-5D) utility measure (the CADTH search filter was not used), to reflect the NICE preferred method for utility assessment,68 with the option to expand the search to other utility measures if needed. The searches were carried out in MEDLINE, EMBASE, Web of Science and the Cochrane Library, and they were limited to the most recent 10 years. The strategies for HRQoL 1 and HRQoL 2 are shown in Appendix 1 (see Tables 48 and 49).
The inclusion and exclusion criteria for eligibility screening are given in Appendix 7, Table 66. The same eligibility criteria were used for screening both titles and abstracts and full-text records. Only primary research studies were included. The relevant population was people who had undergone screening or diagnostic tests for prostate cancer, or with diagnosed prostate cancer. We planned to extract data related to the study design, country and sample size, HRQoL instruments used, and health states assessed.
Results of the review of health-related quality-of-life studies
The systematic searches ‘HRQoL 1’ identified 244 potentially relevant studies (see Appendix 7, Figure 20). Of the 244 references, 34 were retrieved for full-text screening and 9 studies were included. 69–77 The excluded references and reasons for exclusion are shown in Appendix 7, Table 67. Study characteristics and results from the included studies are summarised in Appendix 7, Tables 68 and 69. However, these studies did not provide suitable utility values for our economic model.
The systematic searches ‘HRQoL 2’ identified 369 potentially relevant studies, of which 21 were retrieved for full-text screening, and 6 studies78–83 were included (see Appendix 7, Figure 21). The excluded references and reasons for exclusion are shown in Appendix 7 (Table 70).
The main characteristics and results of the six studies included from ‘HRQoL 2’ are presented in Tables 27 and 28 (further detail in Appendix 7, Table 71). Three studies were conducted in the UK and used the EQ-5D-5L, and three were conducted in Finland, from which two used the EQ-5D-3L version with a UK tariff and the other did not specify the version used. Overall, the studies reported EQ-5D scores associated with no cancer, early/localised prostate cancer and late/metastatic prostate cancer. All the studies, except one, have a sample size > 300. These papers are discussed in relation to their applicability to the EAG economic model in Utilities.
| First author, Year | N a | Country | Instrument | Health state(s) described |
|---|---|---|---|---|
| Booth et al. 201478 | 5516 | Finland | EQ-5D | No prostate cancer (screened and not screened); prostate cancer (screened and not screened); organ-confined prostate cancer (screened and not screened); advanced prostate cancer (screened and not screened) |
| Drummond et al. 201579 | 3348 | Republic of Ireland and Northern Ireland | EQ-5D-5L | Invasive prostate cancer (at least 20-month survivors) |
| Farkkila et al. 201480 | 30 | Finland | EQ-5D-3L | End-stage prostate cancer |
| Gavin et al. 201681 | 3348 | Republic of Ireland and Northern Ireland | EQ-5D-5L | Invasive prostate cancer, 2–18 years post treatment: early disease at diagnosis (stage I/II and Gleason grade 2–7), late disease at diagnosis (stage III/IV and any Gleason grade at diagnosis) |
| Torvinen et al. 201382 | 621 | Finland | EQ-5D-3L | Localised disease 6 months after diagnosis; localised disease in the following 12 months; remission; MD; palliative care |
| Watson et al. 201683 | 316 | UK | EQ-5D-5L | No/mild and moderate/severe problems due to prostate cancer treatment in patients diagnosed at least 9 months before |
| Health states | Utility | Source |
|---|---|---|
| No prostate cancer | ||
| No PC (screening programme) | 0.830 | Booth et al. 201478 |
| No PC (no screening programme) | 0.857 | Booth et al. 201478 |
| Prostate cancer | ||
| Difference of PC vs. no PC (screening programme) | + 0.005 | Booth et al. 201478 |
| Difference of PC vs. no PC (no screening programme) | − 0.031 | Booth et al. 201478 |
| Early disease | ||
| Difference of organ-confined PC vs. no PC (screening programme) | + 0.010 | Booth et al. 201478 |
| Difference of organ-confined PC vs. no PC (no screening programme) | − 0.031 | Booth et al. 201478 |
| Early disease PC (2–18 years post treatment) | 0.877 | Gavin et al. 201681 |
| Localised disease (6 months after diagnosis) n = 46 | 0.900 (0.840–0.960) | Torvinen et al. 201382 |
| Localised disease (18 months after diagnosis) n = 91 | 0.890 (0.860–0.920) | Torvinen et al. 201382 |
| Localised disease (remission) n = 309 | 0.870 (0.850–0.890) | Torvinen et al. 201382 |
| Advanced disease | ||
| Difference of advanced PC vs. no PC (screening programme) | − 0.039 | Booth et al. 201478 |
| Difference of advanced PC vs. no PC (no screening programme) | − 0.051 | Booth et al. 201478 |
| Invasive PC (at least 20 months after diagnosis) | 0.820 | Drummond et al. 201579 |
| Late disease PC (2–18 years post treatment) | 0.777 | Gavin et al. 201681 |
| MD n = 85 | 0.740 (0.690–0.800) | Torvinen et al. 201382 |
| Palliative disease n = 17 | 0.590 (0.480–0.700) | Torvinen et al. 201382 |
| End-stage PC | 0.551 (0.405–0.664) | Farkkila et al. 201480 |
| Adverse events after treatment for PC (diagnosed at least 9 months before) | ||
| Urine function (no/mild problems) | 0.868 (SD, 0.160) | Watson et al. 201683 |
| Urine function (moderate/severe problems) | 0.773 (0.222) | Watson et al. 201683 |
| Bowel function (no/mild problems) | 0.862 (0.166) | Watson et al. 201683 |
| Bowel function (moderate/severe problems) | 0.653 (0.195) | Watson et al. 201683 |
| Sexual function (no/mild problems) | 0.861 (0.176) | Watson et al. 201683 |
| Sexual function (moderate/severe problems) | 0.838 (0.170) | Watson et al. 201683 |
Overview of economic evidence in the company submissions
BXTAccelyon, the company that produces PrecisionPoint, submitted a cost-minimisation study. This was developed in 2020 by the York Health Economics Consortium (YHEC) using an economic model that compared the costs of LATP (with the PrecisionPoint device) against different combinations of LATRUS and GATP for UK NHS Trusts. YHEC assumed that LATP and GATP have the same rate of achieving a successful biopsy (with no need to repeat the procedure) and fewer complications than LATRUS biopsies. The majority of clinical experts providing feedback to the EAG reported that they would expect better diagnostic yield for transperineal biopsies compared with LATRUS. This suggests that the assumption of equal diagnostic yield may be conservative.
The YHEC model included costs associated with carrying out prostate biopsies and costs associated with biopsy complications from an HTA report by Ramsay et al. 84 YHEC concluded that it was not possible to calculate a cost per case that could be multiplied by the number of cases to show the total cost of each biopsy, as the costs of complications and the capital cost of a stepping device vary according to the number of cases. In addition, different NHS Trusts undertake different proportions of TRUS and GATP. Therefore, they conducted scenarios to estimate the economic impact of different combinations.
The results suggested that LATP using the PrecisionPoint device is cost saving, yielding higher savings as the proportion of biopsies that were previously performed as GATP increases. Assuming that an NHS Trust undertakes 500 biopsies per year (250 TRUS and 250 GATP), adopting PrecisionPoint yields a cost saving of £81,027.
We note that this study does not compare costs against LATP with grid and stepping device or with another freehand device.
Economic evaluation approach and rationale
The EAG developed a health economic model to compare the cost-effectiveness of alternative biopsy methods for people with suspected prostate cancer, as specified in the NICE scope (see Definition of the decision problem). The model comprises a decision tree to estimate short-term diagnostic outcomes and a cohort health state transition (Markov) model to predict the long-term consequences of the diagnostic pathway on disease progression and associated costs and patient outcomes. In this section, we introduce the EAG economic evaluation. Further detail and explanation are provided in subsequent sections of this chapter.
The modelled cohort
The base case population entering the model is a cohort referred for a first prostate biopsy for suspected localised prostate cancer after mpMRI with Likert score of 3 or more. We also conduct analysis for three other subgroups: mpMRI Likert score of 1 or 2 at first biopsy; mpMRI Likert score of 3 or more after a previous negative biopsy; and mpMRI Likert score of 1 or 2 after a previous negative biopsy. For our base case, we assume that there are no people with metastatic prostate cancer in the cohort because it is likely that people with overt MD and those for whom active treatment for diagnosed disease would not be appropriate would have been screened out of the cohort prior to biopsy. We tested the impact of including a proportion of people with pre-existing MD in scenario analysis (see Appendix 9, Table 88).
The diagnostic pathway: decision tree
The structure of the decision tree is described in detail in Decision tree structure. The design and parameter sources are largely based on the PROMIS economic analysis reported by Faria and colleagues, and the version of this analysis adapted by Wilson et al. to estimate cost-effectiveness for LATP (see Results of the review of economic studies and Overview of other published economic studies of interest above for discussion of these studies). 63–65
The cohort entering the decision tree is first stratified by baseline prevalence of LR, IR and HR localised disease, and MD (if included). The tree then models the diagnostic pathway with the alternative biopsy methods specified in the scope, and estimates resulting complication and cancer detection rates, costs and QALYs. The tree includes a second biopsy for a proportion of patients with a negative first biopsy, with the assumption that the second biopsy would be conducted with the LATRUS method. This is a simplification. In practice, methods for repeat biopsies are likely to vary, but evidence for the diagnostic yield of other biopsy methods after a previous negative first biopsy is sparse. The proportion undergoing repeat biopsy can be changed.
Inputs to the decision tree are:
-
Baseline prevalence stratified by level of risk, estimated from data reported by Faria et al. (see Baseline prevalence). 64,65,94
-
Probabilities of detecting CS and clinically non-significant (CNS) prostate cancer (see Cancer detection rates). For LATRUS, these probabilities are also estimated from data reported by Faria et al. 64,65,94 These baseline probabilities are adjusted for other biopsy methods using RR estimates from the EAG systematic reviews and meta-analyses (see Intermediate outcomes).
-
The probability of a repeat biopsy if the first biopsy is negative is estimated from a paper by Jimenez et al., identified from our clinical review. 85 Assumptions about how this probability differs according to the first biopsy method and result were tested in scenario analysis (see discussion in Probability of a repeat biopsy).
-
Probabilities of biopsy-related complications were estimated from various sources. 66,96–100 Relevant papers were identified from our clinical review and the review of economic evaluations (see Clinical outcomes, Results of the review of economic studies and Overview of other published economic studies of interest), with alternative sources tested in scenario analysis (see discussion in Biopsy-related complications).
-
Costs of the biopsy procedures and treatment for complications, see Costs of the biopsy procedure and Resource use and costs for management of suspected prostate cancer. We developed detailed cost estimates for different LATP approaches in decision question 2.
-
The impact of biopsy-related complications on patient HRQoL and survival (QALY loss) is based on assumptions as in the analysis by Wilson et al. (see Utilities). 63
Long-term consequences: the Markov model
We considered two designs for the Markov model based on existing studies:
-
a model with three health states (progression-free, MD and death), stratified by initial level of cancer risk and treatment, developed for the PROMIS economic evaluation by Faria et al.; and
-
a model developed by the NICE Guideline Updates Team for the 2019 update of the NICE prostate cancer guideline (NG131) evaluation of follow-up strategies for people with a negative mpMRI or biopsy result. 64,65,67
The NG131 model structure includes some features that make it more appropriate for the current decision problem. In particular, it explicitly models subsequent diagnosis for people with FN results after the biopsy pathway, based on estimated rates of symptomatic presentation and routine follow-up in primary care. This enables quantification of the monetary and QALY costs of a biopsy failing to diagnose CS disease and the resulting delay in treatment. The NG131 model also includes costs for diagnosis and follow-up and a wider range of treatments that reflect NICE guidance. We therefore decided to adapt the NG131 Markov model structure for our analysis.
The structure and input parameters of the NG131 model are described in the health economic model report available on the NICE website. 67 We also had access to a copy of the model. Input parameters and assumptions in our model were aligned with those in the NG131 model, except where more recent or relevant sources were identified. We included parameters to specify a schedule of primary care follow-up for people with one or more negative biopsy result. For our base-case analysis, we assumed a PSA test at 6 months, then annual tests for a maximum of 10 years for everyone with a FN biopsy result. Modelled treatment for diagnosed prostate cancer reflected NICE guidance at the time of the 2019 guideline update, with additional treatments for MD based on more recent technology appraisals. 86–88
See Markov model structure for further description of the NG131 model structure and explanation of how we adapted it for the current decision problem.
Parameters for the Markov model include:
-
Transition probabilities between the 11 health states. We used the same probabilities as in the NG131 model (Table HE07 in the online model report). 67 See Long-term transition probabilities for details and explanation of how these probabilities were derived.
-
Resource use and costs, including monitoring and follow-up, treatment of diagnosed prostate cancer, management of adverse events and end-of-life costs (see Resource use and costs for management of suspected prostate cancer for details).
-
Health outcomes are estimated in the form of QALYs, incorporating modelled survival and the impact of symptoms and adverse effects on utility. 91,92,109 See Utilities.
Framework for economic analysis
Analysis followed the NICE reference case at the time of analysis, as specified in section 15 of the Diagnostics Assessment Programme (DAP) manual. 68
-
The model uses a ‘lifetime’ time horizon (up to a maximum age of 100 years) to reflect the life-threatening consequences of misdiagnoses or serious biopsy-related complications. The Markov model uses a 3-month model cycle.
-
Health outcomes are estimated as QALYs, with utilities estimated from EQ-5D-3L data with NICE-recommended UK general population values.
-
Costs are estimated from an NHS and personal social services perspective. Biopsy costs are estimated with a micro-costing approach, informed by company submissions and expert judgement. Unit costs are taken from standard national and NHS sources. 89,90 The base case uses long-term average cost estimates for the interventions and comparators, with annuitised costs for capital equipment.
-
Standard rates of discounting for time preference over costs and QALYs are applied, as recommended by NICE (currently 3.5% per year for costs and QALYs).
Modelled decision problem
Population and subgroups
The model was designed to estimate costs and health outcomes for the population specified in the NICE scope: people with suspected prostate cancer referred for prostate biopsy. We aimed to reflect characteristics of this population in routine NHS practice, including age and probability of prostate cancer prior to biopsy.
The National Prostate Cancer Audit (NPCA) reported that 54% of people newly diagnosed with prostate cancer in England and Wales between April 2018 and March 2019 were aged 70 years or over (mean age at diagnosis was not reported) (NPCA 2020 Table 3). 92 However, one would expect the mean age at biopsy to be lower than the mean age at diagnosis. The mean age at referral for a first prostate biopsy in the PROMIS study was 63.4 years, but the mean age for those diagnosed with IR and HR cancers was 64.9 and 66.8 years, respectively. 64 For the base case, we assumed a mean age of 66 years at referral for biopsy, as this matches the assumption in the NG131 update analysis, as well as feedback from a specialist committee member. 67 We tested the effect of baseline age in scenario analysis (see Appendix 9, Table 88).
For the purposes of the economic evaluation, we assumed that the cohort had already had mpMRI as an investigation for suspected clinically localised prostate cancer prior to referral for biopsy, with results reported on a five-point Likert scale. This aligns with the NICE recommendation from the 2019 update of NG131 (recommendation 1.2.2). 8 Use of the Likert scale is also consistent with evidence of the diagnostic yield of mpMRI available from the PROMIS study, which we use to estimate the baseline prevalence of prostate cancer conditional on mpMRI results (see Baseline prevalence). We acknowledge that this does not necessarily align with clinical practice, as some centres use PI-RADS instead of Likert to report mpMRI results. There is also uncertainty over the generalisability of evidence on the comparative diagnostic yield of biopsy methods, as some studies did not report prior mpMRI use, and those that did report results in terms of PI-RADS rather than Likert scores (see Characteristics of studies comparing LATP prostate biopsy by any method versus LATRUS prostate biopsy (decision question 1), Characteristics of studies comparing LATP prostate biopsy by any method versus GATP prostate biopsy using a grid and stepping device (decision question 1), Characteristics of studies comparing LATP prostate biopsy using a freehand device versus LATRUS prostate biopsy (decision question 2), Characteristics of studies comparing LATP prostate biopsy using a freehand device versus GATP prostate biopsy by grid and stepping device (decision question 2) and Characteristics of single-arm studies evaluating LATP prostate biopsy using a freehand device where no comparative evidence was identified).
Faria et al. 65 evaluated ‘true disease’ status for the PROMIS population based on a combination of a TPM biopsy and TRUS biopsy (whichever was the most severe). They classified LR, IR and HR localised prostate cancer according to two sets of definitions. For the economic model, we used the following:
-
LR: Gleason ≤ 6, PSA ≤ 10 ng/ml and clinical stage T1 to T2a
-
IR: Gleason 7, PSA 10–20 ng/ml and clinical stage T2b
-
HR: Gleason 8–10, PSA > 20 ng/ml and clinical stage T2c or higher.
Intermediate- and high-risk localised disease are grouped together as CS disease. LR disease is classed as CNS.
We assume that the referred cohort does not include people with MD. NICE guidance is that people who are not going to be able to have radical treatment should not be routinely offered mpMRI (NG131 recommendation 1.2.1), and that those for whom clinical suspicion of prostate cancer is high because of high PSA value and evidence of bone metastases should not be routinely offered prostate biopsy for histological confirmation (NG131 recommendation 1.2.8). The PROMIS, which provides baseline estimates of prevalence for the model, excluded people with MD; 5 out of 740 men registered for the study were withdrawn due to having stage T4 or nodal disease (Brown et al., table 6). 64
Patient subgroups
In our base-case analysis, we focus on people referred for a first biopsy with a prior mpMRI Likert score of 3 or more (NG131 recommendation 1.2.3). NG131 recommends considering omission of a prostate biopsy for people with a mpMRI Likert score of 1 or 2, but only as a shared decision after discussion of the risks and benefits with the person concerned (NG131 recommendation 1.2.4). The NICE scope for the current assessment reports expert opinion that around 40% of people with Likert score of 1 or 2 are discharged based on the results of the mpMRI scan. This group are less likely to have CS prostate cancer than those with a mpMRI score of 3 or more. Similarly, the risk of prostate cancer, and hence cost-effectiveness, is likely to differ for people who have never had a prostate biopsy, and for those who have had a previous negative prostate biopsy and are referred back.
We assess cost-effectiveness separately for the following subgroups:
-
people referred for a first biopsy with a Likert score of 3 or more (base case)
-
people referred for a first biopsy with a Likert score of 1 or 2
-
people referred after a previous negative biopsy with a Likert score of 3 or more
-
people referred after a previous negative biopsy with a Likert score of 1 or 2.
We do not present subgroup analysis by location of lesions or enlarged prostate, due to a lack of evidence to differentiate prognosis or diagnostic yield for these groups.
The model uses prevalence of LR, IR and HR localised prostate cancer in subgroups A to D estimated from data on true disease status in the PROMIS cohort and diagnostic yield characteristics of mpMRI and TRUS biopsy reported by Faria et al. See Baseline prevalence for details of the prevalence calculations.
Biopsy methods and devices
The model was designed to evaluate the decision questions defined in the NICE scope. Following the naming conventions for interventions and comparators used in the pairwise and network meta-analyses in Intermediate outcomes, we conducted the following comparisons.
Decision question 1:
-
LATP prostate biopsy with a freehand device, grid and stepping device or coaxial needle (LATP-any)
-
LATRUS biopsy (LATRUS)
-
GATP using a grid and stepping device (GATP).
Decision question 2:
-
LATP prostate biopsy with a freehand device (LATP-freehand)
-
LATP prostate biopsy without a freehand device (LATP-other), including LATP conducted with a grid and stepping device or coaxial needle
-
LATRUS biopsy (LATRUS)
-
GATP with a grid and stepping device (GATP).
Model structure
The model comprises a decision tree which maps out the initial diagnostic pathway and a Markov model which estimates long-term treatment costs and health outcomes. See Model parameters for model input parameters and Model assumptions for a list of model assumptions.
Decision-tree structure
A simplified overview of the decision tree is shown in Figure 4. This tree is replicated for each biopsy method under comparison.
FIGURE 4.
Overview of decision tree. CNS, clinically non-significant cancer; HR, true high-risk; HR Dx, high-risk correctly diagnosed; HR FN, misdiagnosed intermediate-risk; IR, true intermediate-risk; IR Dx, intermediate-risk correctly diagnosed; IR FN, misdiagnosed intermediate-risk; LR, true low-risk; LR Dx, low-risk correctly diagnosed; LR FN, misdiagnosed low-risk; MS, true metastatic disease; MS Dx, metastatic correctly diagnosed; MS FN, misdiagnosed metastatic; NC Dx, no cancer correctly diagnosed.

The model starts with a cohort of interest, one of four subgroups A–D defined by mpMRI Likert score and history of previous biopsy. The cohort is first stratified by true prostate cancer status (no cancer, LR, IR or HR localised disease or MD). The decision tree then estimates diagnostic outcomes (the proportions of correct and FN biopsy results) and complications from the biopsy process. The end points of the decision tree, correct diagnoses (Dx) or FNs, represent the initial health states in the Markov model.
Biopsy-related complications are categorised as:
-
no adverse event: no or minor events for which the patient does not seek treatment
-
mild adverse events: mild/moderate events treated outside hospital
-
admission: admission within 28 days of the biopsy
-
mortality within 28 days of the biopsy.
Costs are estimated for the biopsy process, including costs of the initial and repeat biopsies, and costs for management of any biopsy-related complications. QALYs accumulated within the biopsy process are also estimated, based on initial utility values assigned to the cohort and allowing for any QALY loss attributable to complications, including disutility for transient adverse events and lifetime QALY loss for rare biopsy-related deaths.
The following sections describe the structure of the subtrees for people without prostate cancer (NC) and for those with CNS (LR) or CS (IR, HR) localised prostate cancer. The model also includes a subtree for MD, but this is not used in the current analysis.
No prostate cancer
See Figure 5 for an illustration of the biopsy process for people who do not have prostate cancer. We assume that all biopsy methods are perfectly specific: so there cannot be false positive results for people who truly do not have prostate cancer. However, it is possible that a patient may be referred for a repeat biopsy if there is a high level of clinical suspicion. Biopsy-related complications may occur, classified as above (mild, admission or mortality). End points for people without prostate cancer are correct diagnosis (NC Dx) or death from biopsy-related complications.
FIGURE 5.
Illustration of decision tree for people without prostate cancer. AE, adverse events; Bx, biopsy; NC Dx, no cancer correctly diagnosed.
Clinically non-significant prostate cancer
Figure 6 illustrates the biopsy process for people with CNS disease (LR prostate cancer). For this population, the biopsy may give a correct diagnosis, a false positive result of CS disease or a FN result of no cancer. In practice, there were no cases of people with LR cancer receiving a CS biopsy result in the PROMIS study (see Table 30). 64 Hence, the probability of this event in our model is zero.
FIGURE 6.
Illustration of decision tree for people with LR prostate cancer. AE, adverse event; Bx, biopsy; LR, true low-risk; LR Dx, low-risk correctly diagnosed (classified as CNS); LR FN, low-risk false negative (classified as no cancer); LR FP, low-risk false positive (classified as CS).

If the biopsy is negative (CNS or no cancer), a repeat biopsy may be performed. We assumed that the probability of a repeat biopsy is higher if the result of the first biopsy is CNS or if the prior mpMRI Likert score was 3 or more, than if the first biopsy result is ‘no cancer’ and the Likert score is ˂ 3. A second biopsy may give a CS, CNS or no cancer result, although the estimated probability of a CS result for a second TRUS biopsy with LR cancer is zero (as in the PROMIS model, based on the systematic review and meta-analysis by Schoots et al.). 65,93
Complications may occur after the first and/or second biopsy, classified as above (none, mild, admission or mortality). End points for people with LR disease are correct diagnosis (LR Dx), false positive (LR FP), false negative (LR FN) or death.
Clinically significant prostate cancer
The structure of the decision tree is the same for people with IR prostate cancer (illustrated in Figure 7) as for those with HR disease (figure not shown). We assume that the incidence of complications does not differ by cancer risk group. End points for intermediate and high risk are correct diagnosis (IR Dx; HR Dx), false negative (IR FN; HR FN) or death.
FIGURE 7.
Illustration of decision tree for people with IR prostate cancer. AE, adverse event; Bx, biopsy; IR, true intermediate-risk; IR Dx, intermediate-risk correctly diagnosed (classified as CS); IR FN, intermediate-risk FN (classified as CNS or no cancer).
Markov model structure
As discussed above (see Long-term consequences: the Markov model), we considered two designs for the Markov model to estimate long-term costs and QALYs from the diagnostic outcomes from our decision tree: the model developed for the economic evaluation of the PROMIS study by Faria et al., later adapted by Wilson et al.; and the model developed for the 2019 update of the NICE prostate cancer guideline (NG131). 63–65,67 We chose to use a replicated version of the NG131 Markov model as this gives a more flexible structure to model the costs and consequences of missed diagnoses, allowing for possible future diagnosis.
The structure of this model is illustrated in Figure 8. It includes health states based on prostate cancer and diagnostic status: no cancer (NC) and diagnosed and undiagnosed LR, IR and HR localised disease and MD. The initial distribution of the cohort across the Markov states is determined by the output from the biopsy pathway modelled in the decision tree: with some ‘overdiagnosis’ of LR disease and some missed diagnoses of CS disease. Rates of transition from undiagnosed to diagnosed health states can be set to reflect primary care monitoring and symptomatic presentation. The model includes simplified assumptions about sequential progression of prostate cancer: from incident LR disease through IR and HR localised disease to MD. Death from prostate cancer is assumed to only occur with MD, although death from other causes occurs from all states.
FIGURE 8.
Schematic of Markov model structure. PC, prostate cancer.

Model parameters
Baseline prevalence
Estimates of the true prevalence of prostate cancer for each of the subgroups, A–D, are shown in Table 29. These provide the starting proportions of the cohort allocated to LR, IR and HR disease in the decision-tree model. They were derived from the following PROMIS results reported by Faria et al.:65
| True disease status | First biopsy | Previous negative biopsy | ||
|---|---|---|---|---|
| Likert 3+ | Likert 1 or 2 | Likert 3+ | Likert 1 or 2 | |
| Subgroup A (%) | Subgroup B (%) | Subgroup C (%) | Subgroup D (%) | |
| No cancer | 19.4 | 47.7 | 40.0 | 59.4 |
| LR cancer | 12.4 | 25.7 | 25.7 | 32.0 |
| IR cancer | 63.8 | 26.6 | 34.3 | 8.6 |
| HR cancer | 4.4 | 0.0 | 0.0 | 0.0 |
-
True cancer status in the PROMIS cohort defined by the most severe of the template mapping biopsy and TRUS biopsy results (Faria et al. supplement table 5). 65
-
Diagnostic yield of mpMRI: probability of mpMRI result of no cancer, CNS or CS disease, given true cancer status (Faria et al. table 3, CNS definition 2, Likert cut-off ≥ 3). 65
-
Diagnostic yield of first TRUS biopsy: probability of finding of no cancer, CNS or CS disease, given true cancer status (Faria et al. table 2, Type 4, CNS definition 2). 65
We combined these results using Bayes formula. For example, the probability that a member of subgroup A does not have cancer is calculated from the probability that someone with no cancer had Likert ≥ 3, the proportion of the cohort with no cancer and the overall probability of Likert ≥ 3: p(NC | Likert ≥ 3) = (p(Likert ≥ 3 | NC) * p(NC))/p(Likert ≥ 3).
We note the zero probability of true HR localised prostate cancer for people with a Likert 1 or 2 result from mpMRI (Faria et al., supplementary table 7). We understand that such cases may occur in practice, although this may reflect inaccurate mpMRI scoring.
Cancer detection rates
Cancer detection rates for LATRUS prostate biopsy
Estimates of diagnostic yield for LATRUS biopsy were taken from the PROMIS economic evaluation (Table 30). These results correspond with definition 2 for a CS TRUS result, which reflects the definition in the optimal cost-effective strategy identified by Faria et al.. Methods of calculation for these results are reported in supplementary section 2.2 of Faria et al.. 65
| True cancer status | Probability of TRUS result | ||
|---|---|---|---|
| No cancer | CNS | CS | |
| First biopsy after a suspicious mpMRI resulta | |||
| LR cancer | 0.79 (0.66 to 0.89) | 0.21 (0.11 to 0.34) | |
| IR cancer | 0.15 (0.09 to 0.21) | 0.11 (0.06 to 0.16) | 0.74 (0.65 to 0.84) |
| HR cancer | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) |
| Second biopsy after a negative first biopsy and suspicious mpMRI resultb | |||
| LR cancer | 0.68 (0.02 to 1.00) | 0.32 (0.02 to 0.91) | |
| IR cancer | 0.05 (0.02 to 0.11) | 0.08 (0.03 to 0.18) | 0.87 (0.71 to 0.95) |
| HR cancer | 0.05 (0.02 to 0.11) | 0.08 (0.03 to 0.18) | 0.87 (0.71 to 0.95) |
Relative risks for cancer detection with other biopsy methods
Cancer detection rates for the other biopsy methods in decision questions 1 and 2 are estimated by adjusting the LATRUS rates using RRs from the EAG evidence synthesis described in Intermediate outcomes. Our original base case used results from the NMA of RCTs reported in Figures 13 and 18 in Appendix 4. These include results from the Hara et al. 26 and Takenaka et al. 28 trials classified as comparisons between LATP (without freehand device) and LATRUS. However, there are uncertainties over this classification due to the types of anaesthesia used in the Hara and Takenaka trials (spinal injection in the transperineal arm and caudal block in the transrectal arm). We therefore used a NMA excluding these two trials in a revised EAG economic base case. The results of the revised network meta-analyses results excluding the Hara and Takenaka trials are shown in Table 31, alongside two alternative NMA scenarios. We also conducted a scenario analysis based on the pairwise meta-analyses of observational studies. See Scenario analysis: cancer detection rates for discussion of these scenario analyses.
| Base case NMA excluding Hara and Takenaka26,28 |
Scenario 1 Hara and Takenaka26,28 trials included as LATP vs. LATRUS |
Scenario 2 Hara and Takenaka26,28 trials included as GATP vs. LATRUS |
|
|---|---|---|---|
| Decision question 1 | |||
| LATP-any | 1.15 (0.93 to 1.41) | 1.01 (0.85 to 1.18) | 1.09 (0.91 to 1.30) |
| GATP | 1.09 (0.75 to 1.60) | 0.96 (0.64 to 1.44) | 0.92 (0.77 to 1.09) |
| Decision question 2 | |||
| LATP-freehand | 1.40 (0.96 to 2.04) | 1.40 (0.96 to 2.04) | 1.40 (0.96 to 2.04) |
| LATP-other | 1.05 (0.83 to 1.34) | 0.94 (0.81 to 1.10) | 1.01 (0.82 to 1.24) |
| GATP | 1.01 (0.67 to 1.51) | 0.90 (0.63 to 1.29) | 0.90 (0.76 to 1.08) |
There is high uncertainty over comparative cancer detection rates between the included biopsy methods, as indicated by the confidence intervals in Table 31. In addition, there is uncertainty related to the nature of the evidence base, including potential risks to internal validity of the RCTs and clinical heterogeneity between studies, and various assumptions and simplifications that we had to make.
-
The NMA value for ‘LATP-freehand’ is based on a single RCT (Lam et al.), which used the PrecisionPoint device. 27 It is not clear whether this is representative of the list of freehand devices included in the scope. Given the lack of evidence for other devices, we model LATP-freehand for decision question 2 as a single intervention but test the impact of using different prices for the respective devices in scenario analyses (see Scenario analysis: biopsy costs).
-
The scope specifies LATP biopsy with a grid and stepping device as a comparator for decision question 2. However, reporting of LATP methods and devices in the clinical evidence base made it difficult to separate evidence relating to grid and stepping devices. We therefore use a pooled estimate for studies that did not report use of a freehand device (‘LATP-other’).
-
The value for GATP is based on a single RCT (Lv et al.), which compared with LATP. 42 This means that RR estimates from the NMA compared with LATRUS differ for decision questions 1 and 2.
Probability of a repeat biopsy
The probability of patients having a second biopsy after a negative first biopsy is based on a prospective cohort study reported by Jimenez et al.. 85 They assessed whether an initial GATP biopsy (systematic TP with 30 cores taken in theatre under general or spinal anaesthetic) translated into a lower risk of re-biopsy compared with LATRUS (systematic transrectal biopsy with 12–18 cores taken in office under local anaesthetic). Repeat biopsy was indicated based on a protocol defined by the authors (see table 1 in Jimenez et al.). 85 The number of patients having GATP in the cohort was much smaller than those having LATRUS, and patients with larger prostates were preferably selected for GATP. During the study period, 15.5% (95/615) and 5.4% (3/56) of patients had repeat biopsies after LATRUS and GATP respectively (p = 0.06). This difference was statistically significant in a multivariate analysis with adjustment for PSA density (p = 0.03). However, there are uncertainties over the generalisability of this result due to the lower sample size for GATP and differences in prostate volume and the numbers of core samples. We applied the LATRUS re-biopsy rate (15.5%) in our base case for all biopsy methods and varied this in scenario analyses (see Appendix 9, Table 87).
Biopsy-related complications
Comparative rates of complications from our systematic review are reported in Clinical outcomes. The included studies reported a variety of adverse outcomes, but the studies were too heterogeneous for meta-analysis and the individual studies lacked power to reliably estimate adverse-event rates. We therefore used other observational sources identified from our clinical and economic searches to estimate complication rates for the model. See Table 32 for a summary of sources used in our base case analysis.
| Biopsy | n | Mean (%) | 95% CI | |
|---|---|---|---|---|
| Tamhankar et al. 2020, analysis of Hospital Episode Statistics data (2017–9) 94 | ||||
| TRUS | Non-elective admission | 2845/76,106 | 3.74 | 3.60 to 3.87 |
| Mortality | 53/76,106 | 0.07 | 0.05 to 0.09 | |
| TP | Non-elective admission | 1314/37,077 | 3.54 | 3.36 to 3.73 |
| Mortality | 19/37,077 | 0.05 | 0.03 to 0.08 | |
| Rosario et al. 2012, prospective cohort associated with ProtecT study 95 | ||||
| TRUS | Consultation with GP or nurse | 119/1147 | 10.4 | 8.7 to 12.3 |
| Hospital admission | 15/1147 | 1.3 | 0.8 to 2.1 | |
| Pepe and Aregona 2013, cohort study 96 | ||||
| TP | Emergency department visit | 274/3000 | 9.1 | 8.1 to 10.2 |
| Hospital admission | 37/3000 | 1.2 | 0.9 to 1.7 | |
For admission and mortality, we used results from an analysis of NHS Hospital Episode Statistics by Tamhankar et al.. 94 They included all patients with a code of M702 (transperineal needle biopsy of prostate) or M703 (transrectal needle biopsy of prostate) between April 2008 and March 2019, and identified those who were readmitted or attended accident and emergency within 28 days after the biopsy. These data do not distinguish between transperineal biopsies conducted under local or general anaesthetic. We used results from the two most recent years (April 2017–March 2019), following advice that these are more reflective of current practice. In the 2017–9 cohort, non-elective admissions were lower after TP than after transrectal biopsy, but the difference was not statistically significant (3.54% and 3.74% respectively, p = 0.11). Infections were the main cause of non-elective admissions after transrectal biopsy, while urinary retention was the main cause after TP. Mortality within 28 days of the procedure was rare and similar after transperineal and transrectal biopsy (0.05% and 0.07%, respectively).
The decision-tree model also included biopsy-related complications treated outside hospital (‘mild’ adverse events) (see Decision tree structure). For this outcome, we used complication rates from different sources for transrectal and transperineal biopsies, as we could not identify a suitable source that included both. Rosario et al. reported healthcare contacts within 35 days of a TRUS biopsy for a prospective cohort of 1147 patients nested within the ProtecT trial. 95 Pepe and Aragona reported complications within 15–20 days of a TP in a single-centre study in Italy. 96 This cohort included 3000 patients who underwent biopsy under general or local anaesthetic, with 12, 18 or 24 or more biopsy cores.
In the base case, we assumed the same rates of complications for LATP and GATP. We have received conflicting expert advice over the relative incidence of complications for transperineal biopsies under local or general anaesthetic, although Pepe and Aragona reported that these rates were ‘superimposable’. 96
Scenario analyses for other estimates of biopsy-related hospital admission rates are reported in Scenario analysis: probability of biopsy complications. In addition to scenarios with admission rates from the Rosario and Pepe and Aragona studies (as reported in Table 32), we report scenarios based on a study by Berry et al.. 97
Berry et al. used data from the NPCA linked to Hospital Episode Statistics to compare readmission rates within 30 days of a transrectal or TP (type of anaesthesia not reported) conducted prior to a new diagnosis of prostate cancer. People who underwent a TP were less likely to be readmitted to hospital because of sepsis, but more likely to be readmitted because of urinary retention than patients who underwent a transrectal biopsy. The analysis also showed that an overnight stay was significantly more likely immediately after a TP than after a transrectal biopsy (12.25% and 2.36%, respectively, p < 0.001). However, NICE specialist committee members advised that this difference is not reflective of current practice, as the Berry et al. analysis used data from a period prior to March 2017 when TP was conducted under general anaesthetic.
Long-term transition probabilities
Transition probabilities for the Markov model were based on values used in the NICE 2019 guideline update NG131. 8 The natural history parameters used to calculate transition probabilities are reported in Table HE07 of the health economic model report available on the NICE website. 67
The base-case transition probabilities (per 3-month model cycle) are shown in Table 33. The matrix differs for model cycles in which primary care follow-up (PSA testing and LATRUS biopsy if indicated) is expected for people with a negative diagnosis, because the probability of diagnosis for FN cases is higher than in the other model cycles, when diagnosis is only related to symptomatic presentation.
| Non-screening cycle (diagnosis through symptomatic presentation only) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per cycle probability | NC | Undiagnosed | Diagnosed | PC death | Other death | ||||||||
| Progression | Diagnosis | LR FN | IR FN | HR FN | MS FN | LR Dx | IR Dx | HR Dx | MS Dx | ||||
| Screening cycle (diagnosis through primary care follow-up or symptomatic presentation) | |||||||||||||
| Per cycle probability | NC | Undiagnosed | Diagnosed | PC death | Other death | ||||||||
| Progression | Diagnosis | LR FN | IR FN | HR FN | MS FN | LR Dx | IR Dx | HR Dx | MS Dx | ||||
| NC | – | – | 1.000 | – | – | General population mortality | |||||||
| LR FN | 0.038 | 0.001 | 0.960 | 0.038 | 0.001 | 0.000 | |||||||
| IR FN | 0.085 | 0.010 | 0.906 | 0.084 | 0.009 | 0.001 | |||||||
| HR FN | 0.014 | 0.010 | 0.976 | 0.014 | 0.010 | 0.000 | |||||||
| MS FN | 0.073 | 0.927 | 0.073 | 13.380 | |||||||||
| LR Dx | 0.035 | 0.965 | 0.035 | ||||||||||
| IR Dx | 0.031 | 0.969 | 0.031 | ||||||||||
| HR Dx | 0.008 | 0.992 | 0.008 | ||||||||||
| MS Dx | 1.000 | 9.060 | |||||||||||
| NC | – | – | 1.000 | – | – | General population mortality | |||||||
| LR FN | 0.038 | 0.222 | 0.748 | 0.030 | 0.213 | 0.009 | |||||||
| IR FN | 0.085 | 0.604 | 0.362 | 0.034 | 0.553 | 0.051 | |||||||
| HR FN | 0.014 | 0.604 | 0.390 | 0.006 | 0.596 | 0.009 | |||||||
| MS FN | 0.630 | 0.370 | 0.630 | 13.380 | |||||||||
| LR Dx | 0.035 | 0.965 | 0.035 | ||||||||||
| IR Dx | 0.031 | 0.969 | 0.031 | ||||||||||
| HR Dx | 0.008 | 0.992 | 0.008 | ||||||||||
| MS Dx | 1.000 | 9.060 | |||||||||||
Costs of the biopsy procedure
We estimated the cost of each biopsy method using a micro-costing approach, including the following components:
-
cost of device (where applicable)
-
cost of general consumables (needles, antibiotics, anaesthesia, ultrasound, lithotomy bed, etc.)
-
staff time for training
-
staff time to perform biopsy (urologists, nurses, anaesthetists)
-
cost of the place of biopsy (outpatient room, theatre session)
-
cost of reprocessing for reusable devices
-
cost of histopathological analysis
-
urologist consultation to discuss biopsy results and disease management.
Estimates were based on information provided to NICE by the companies (including the YHEC study),84 from clinical experts, and from the study by Wilson et al.. 63 Where information was not available, we made assumptions. More details on the assumptions used in the estimation of biopsy costs are shown in Appendix 8. Costs of consumables are summarised in Appendix 8, Table 72.
We estimated a cost of £681 for LATRUS biopsy and £1251 for GATP (Table 34). The estimated cost varies between LATP methods and devices.
| Cost component | Cost per biopsy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LATP | GATP | LATRUS | ||||||||
| CamPROBE | PrecisionPoint | EZU-PA3U | UA1232 | Trinity Perine | SureFire Guide | Grid and stepping device | Double freehand | |||
| Device | £70 | £200 | £19 | £14 | £8 | £135 | £80 | – | £80 | – |
| Consumables | £87 | £87 | £108 | £87 | £89 | £87 | £87 | £109 | £170 | £86 |
| Training | £2 | £5 | £1 | £1 | £1 | £5 | £5 | £5 | £5 | £1 |
| Biopsy staff | ||||||||||
| Urologist | £49 | £40 | £44 | £44 | £44 | £44 | £44 | £44 | £119 | £37 |
| Nurse | £25 | £21 | £23 | £23 | £23 | £23 | £23 | £23 | £62 | £19 |
| Anaesthetist | – | – | – | – | – | – | – | – | £119 | – |
| Place of biopsy | £53 | £43 | £48 | £48 | £48 | £48 | £48 | £48 | £194 | £40 |
| Reprocessing | – | – | £5 | £5 | £5 | – | £5 | – | £5 | – |
| Histopathology | £439 | £439 | £439 | £439 | £439 | £439 | £439 | £439 | £439 | £439 |
| Urologist consult | £60 | £60 | £60 | £60 | £60 | £60 | £60 | £60 | £60 | £60 |
| Total | £785 | £894 | £746 | £721 | £715 | £826 | £791 | £727 | £1251 | £681 |
-
For decision question 1, we used a base case cost of £776 for LATP-any, which is the mean of the named LATP devices (CamPROBE, PrecisionPoint, EZY-PA3, UA1232, Trinity Perine and SureFire Guide), LATP with a grid and stepping device and LATP with a coaxial needle (‘double freehand’).
-
For decision question 2, we used a cost of £781 for ‘LATP freehand’: the mean cost for all named LATP devices (CamPROBE, PrecisionPoint, EZY-PA3, UA1232, Trinity Perine and SureFire Guide). For ‘LATP other’, we used the estimated cost for LATP with a grid and stepping device (£791).
See Scenario analysis: biopsy costs for scenario analysis with different estimates of biopsy costs.
Resource use and costs for management of suspected prostate cancer
In addition to the cost of biopsies, the model includes costs for subsequent follow-up and monitoring, diagnosis and the treatment of prostate cancer and adverse events. In this section, we summarise the key assumptions used for costing. Full details of resource use inputs to the base-case model are listed in Appendix 8, Table 73. Unit costs are listed in Appendix 8, Table 74.
Monitoring of suspected and diagnosed prostate cancer
We based our assumptions regarding the monitoring of suspected and diagnosed prostate cancer on the recommendations outlined in the 2019 update of NICE guideline NG1318 and the assumptions of the decision model that informed NG131. 67
Initial diagnostic pathway in the decision tree:
-
A proportion of patients with a first biopsy result of no cancer or CNS disease were assumed to repeat the biopsy.
-
MRI Likert score 3+: base-case assumption is that 5.0% of patients with biopsy result no cancer and 15.5% of patients with CNS repeat the biopsy.
-
MRI Likert score 1 or 2: base-case assumption is that 1.3% of patients with biopsy result no cancer and 5.0% of patients with CNS repeat the biopsy.
-
-
Patients without cancer are assumed to receive a correct diagnosis at first or second biopsy and are discharged with no additional costs at the end of the decision tree.
Primary care follow-up for suspected prostate cancer in the Markov model:
-
There is a probability that patients with undiagnosed prostate cancer (LR, IR, HR and metastatic) can be diagnosed in each 3-month model cycle due to symptomatic presentation or follow-up in primary care (Table 33).
-
We assume that primary care follow-up consists of a PSA velocity test after 6 months, and yearly thereafter. Patients with a positive PSA (threshold 0.75 mg/ml/year) are assumed to have a TRUS biopsy for disease confirmation. The proportion of patients having a positive PSA (69%) is the sensitivity of the PSA velocity test used in the NG131 economic model. 67
Monitoring of diagnosed disease in the Markov model:
-
Once diagnosed, patients are offered active surveillance, radical treatment, treatment for MD or watchful waiting, depending on their level of risk. We made the following assumptions regarding subsequent monitoring.
-
Active surveillance was assumed to include: PSA measurement every 3 months and DRE and mpMRI at 12 months in the first year; and subsequently, PSA measurement every 6 months and DRE every 12 months.
-
Following radical treatment, patients were assumed to have a PSA test every 6 months for 2 years and once a year thereafter.
-
Patients diagnosed with prostate cancer on watchful waiting were assumed to require a PSA measurement once a year.
-
Half of the patients diagnosed with IR, 70.0% diagnosed with HR and 100.0% diagnosed with metastatic prostate cancer were assumed to have a computerised tomography (CT) and a bone scan to monitor for metastases once.
The costs of repeat biopsy were based on the microcosting analysis (as in Table 34). The cost of PSA involves the costs of the test kit and the cost of a primary care nurse appointment to take the blood sample (assumed to last 10 minutes). Costs for PSA tests, mpMRI, CT and bone scans were obtained from NHS National Cost Collection Data Publication 2019–20. 90 The cost of DRE was assumed to be the cost of a 20-minute GP appointment. The costs of nurse and GP appointments were obtained from Personal Social Services Research Unit (PSSRU) 2020. 89 See Appendix 8, Table 74 for the unit costs.
Treatment for diagnosed prostate cancer
Patients with LR or IR localised prostate cancer will have one of the following treatments: active surveillance, radical prostatectomy or radical radiotherapy. Patients with HR localised prostate cancer will have radical prostatectomy or radical radiotherapy. Patients with no intent of curative treatment in the IR and HR groups may choose a watchful waiting approach.
The distributions of patients by risk group across treatments for localised disease were obtained from the NPCA Annual Report 2020. 92 This reported that around 5.0% of patients with LR and 71.0% of patients with HR localised disease have radical treatment. The distribution across radical treatments (radical prostatectomy and radical radiotherapy) were informed by a study by Gnanapragasam et al. (see Appendix 8, Table 73). 98
Radical prostatectomy was estimated as a robotic surgery. 92 Radical radiotherapy includes both brachytherapy and of hypofractionated radiotherapy using image-guided intensity-modulated radiation therapy. During radical radiotherapy, patients were assumed to receive androgen-deprivation therapy (ADT): bicalutamide 50 mg for 21 days followed by leuprorelin/triptorelin 11.25 mg or goserelin 3.6 mg for 3 months to patients with LR prostate cancer, 6 months to patients with IR prostate cancer and 2 years to patients with HR prostate cancer. 67,99
The management of MD, according to NG131, includes a course of docetaxel plus ADT for patients without significant comorbidities or ADT alone for patients not suitable to receive docetaxel. In addition, two drugs were more recently recommended for metastatic hormone-sensitive prostate cancer – apalutamide plus ADT (ID1534)86 and enzalutamide plus ADT (TA712). 87 The proportion of patients taking docetaxel for metastatic hormone-sensitive prostate cancer (36.0%) was obtained from the NPCA Annual Report 2020,92 while the proportion of patients taking ADT alone was assumed to be 50.0% and the remaining treatment options were assumed to be taken by the remaining patients (7.0% each).
The treatment with docetaxel consists of six cycles of 3 weeks at a dose of 75 mg/m2. ADT alone, apalutamide plus ADT and enzalutamide plus ADT were taken until disease progression, which we assumed to occur after 2 years.
Once patients progress to metastatic hormone-relapsed prostate cancer, we assumed that they could have one of the following:
-
abiraterone for 8 months
-
enzalutamide for 14 months
-
docetaxel for 9.5 cycles
-
best supportive care.
The distribution across metastatic treatments for hormone-relapse disease were informed by NICE Technology Appraisal (TA712)87 as reported in Appendix 8, Table 73. We assumed that patients could only have abiraterone or enzalutamide at this stage if they had not previously received these treatments.
The costs for radical treatment were obtained from NHS National Cost Collection Data Publication 2019–20,90 while the costs for ADT and drugs for MD were obtained from British National Formulary (BNF) 2020 and electronic market information tool (eMIT) 2020 (see Appendix 8, Table 74). 100,101
Managing adverse events associated with prostate biopsy and radical and metastatic treatment
Biopsy-related adverse events were categorised into mild (requiring consultation with a GP or other healthcare professional), hospital admission (including haematuria, urinary retention, sepsis) and death. See Biopsy-related complications for the estimated incidence of biopsy-related adverse events used in the base case model.
We modelled the most common adverse events associated with radical treatment: sexual, urinary and bowel dysfunction. Incidence data were sourced from the ProtecT study. 102 For metastatic treatment, we considered the adverse events from STAMPEDE103 for ADT and docetaxel plus ADT, from TITAN for apalutamide plus ADT104 and from ARCHES for enzalutamide plus ADT. 105 See Appendix 8, Table 73 for rates of treatment-related adverse events used in the base-case model.
The costs of biopsy-related adverse events were taken from the Tamhankar study (estimated cost per patient of non-elective admission), inflated to the cost year 2019–20 using inflation indices from PSSRU 2020. 89,94 Costs of the remaining adverse events were taken from the NHS National Cost Collection 2019–2090 and the decision model that informed NG13167 (see Appendix 8, Table 74).
We assume the same cost of adverse events for misdiagnosed patients (FN LR, IR, HR and metastatic) on primary care follow-up as for patients undergoing active surveillance.
Utilities
Health-related quality-of-life (utility) values for the decision model were derived from studies identified from the systematic review ‘HRQoL 2’ (see Tables 27 and 28) and from references in relevant economic evaluations (see Results of the review of economic studies and Overview of other published economic studies of interest).
Decision tree
The initial utility of the cohort on entry to the decision tree was based on age and gender-related utilities for the general population in England, estimated by Ara and Brazier. 107 This source was also used to adjust utility as the cohort aged within the Markov model.
We did not apply a direct loss of utility associated with the yield of a prostate biopsy, regardless of the method used (LATP, GATP or LATRUS). Evidence on the degree of pain and discomfort or tolerability of different prostate biopsy methods is sparse (see Tables 25 and 26). Faria et al. assumed that the impact on patient-reported EQ-5D from the PROMIS study was associated with the transperineal mapping biopsy, and assumed no utility loss from TRUS biopsy, based on results from a large European screening study. 65,70
The model does account for the utility impact of biopsy-related adverse events. The utility decrement for mild adverse events (treated without admission) and adverse events requiring admission was based on the estimates used by Wilson et al. 63 and Lee et al. 108 for urinary-tract infection (−0.289 for 3 days) and sepsis (−0.490 for 30 days) respectively. The decrement for urinary-tract infection is based on a study from 1997,109 which assessed suspected urinary-tract infection in healthy adult women. The decrement applied to sepsis is based on a study from 2001,110 which assessed the change in health status among sepsis survivors over a 6-month period.
We assumed a utility decrement of −0.490 for 30 days for patients who died due to biopsy adverse events, in addition to the QALY loss associated with lost years of life.
Markov model
For the localised disease health states (including LR, IR and HR), utilities were based on population norms with adjustment for age,107 since there is no evidence of worse HRQoL than in the general population. 81,82 We have, however, included utility decrements for adverse effects associated with treatments for localised disease. These were calculated as the difference between the EQ-5D utilities reported for no/mild complications and moderate/severe complications reported by Watson et al. 83 Utility decrements of 0.023, 0.095 and 0.209 were applied to sexual, urinary and bowel dysfunction, respectively (Table 28). Incidences of these complications with active monitoring, prostatectomy and radiotherapy were estimated from the ProtecT study:102 see Appendix 8, Table 73.
For the metastatic health state, we applied a utility decrement of 0.137 obtained from the study by Torvinen et al. 82 This decrement was calculated as the difference between the average EQ-5D score reported for localised cancer (0.877) and the EQ-5D score reported for metastatic cancer (0.740) (Table 28).
For patients with undiagnosed disease (FN LR, IR, HR localised or metastatic), we assumed the same disutility as for patients on active surveillance. This results in patients with undiagnosed MD having a much lower disutility (−0.019) than patients with diagnosed MD (−0.137). This can be explained as undiagnosed patients do not experience treatment-related adverse effects and patients with severe symptoms are unlikely to be undiagnosed. We have tested the impact of this assumption in scenario analysis, applying the disutility of diagnosed metastatic patients (−0.137) to undiagnosed metastatic patients.
Model assumptions
Table 35 lists the key assumptions in the de novo economic model.
| Population | Initial cohort have had mpMRI as a first-line investigation for suspected clinically localised prostate cancer. |
| Initial cohort does not include people with evidence of MD. | |
| Initial cohort does not include people for whom active treatment would not be appropriate – they would not be referred for biopsy. | |
| Initial mean age of the cohort is 66 years. | |
| Diagnostic accuracy | All biopsies are assumed to be perfectly specific – if the biopsy result is positive (CNS or CS), the person has true disease (LR, IR, HR or metastatic). Although we classify diagnosis of LR localised disease as a ‘true positive’, we note that treatment would not usually be indicated for this patient group. Hence, in NG131 a correct diagnosis of LR was labelled as a ‘true negative’. Despite this different terminology, assumptions about treatment for this group within our model are the same as in the NG131 analysis. |
| Biopsy pathway | A proportion of patients with a negative result from a first biopsy have a repeat biopsy. Second biopsies are assumed to be conducted with an LATRUS method. |
| Biopsy complications | The incidence of biopsy complications does not differ by true disease status (LR, IR or HR localised prostate cancer). |
| Natural history | The NG131 model makes the following assumptions about disease incidence and progression. True negative patients are at continuous risk of developing the disease; this is included in our model although we set the probability of incidence to zero for our base case. True negative patients who develop the disease must pass through FN states, starting on LR, before moving to true positive states. People with true disease (diagnosed or undiagnosed) are at continuous risk of progression. Progression occurs from LR to IR to HR and then to metastatic. Prostate-cancer-specific death occurs only among people with MD. |
| Utilities | Utility for localised disease is assumed equal to that of the general population plus disutilities from radical treatment adverse events. |
| Patients with a FN biopsy result (LR, IR, HR and metastatic) have the same disutility as patients on active surveillance. | |
| Follow-up pathway | A proportion of patients with a first biopsy result NC or CNS repeat the biopsy. The probability of a repeat biopsy will be higher with a prior mpMRI Likert score of 3 or more (5.0–15.5%) than with a score of 1 or 2 (1.3–5.0%). |
| Patients without cancer and a biopsy result NC are discharged and no additional costs are incurred. | |
| Patients with LR prostate cancer and a biopsy result NC as well as patients with IR, HR or metastatic and a biopsy result NC or CNS are followed up in primary care. | |
| Patients with LR prostate cancer and a biopsy result CNS as well as patients with IR and a biopsy result CS are offered a choice between radical treatment or active surveillance, while patients with HR prostate cancer and a biopsy result CS are not offered active surveillance. A proportion of patients with no intent of curative treatment have watchful waiting. Patients with MD are offered drugs for MD. | |
| Primary care follow-up consists of a PSA velocity test measurement at 6 months and yearly thereafter. Patients with a positive PSA (threshold 0.75 mg/ml/year) have a LATRUS biopsy for disease confirmation. | |
| Follow-up resource use | Active surveillance costs consist of a PSA measurement every 3 months, DRE and mpMRI at 12 months in the first year and PSA measurement every 6 months and DRE every 12 months in the subsequent years. |
| Patients having radical treatment have a PSA every 6 months for 2 years and once a year thereafter. | |
| Patients on watchful waiting require a PSA measurement once a year. | |
| Half of the patients diagnosed with IR disease, 70.0% of the patients with HR disease and 100.0% of the patients with MD have one CT and bone scan. | |
| Prostate cancer treatment | The proportion of patients taking ADT alone for metastatic hormone-sensitive prostate cancer is 50.0% and the proportions taking apalutamide plus ADT and enzalutamide plus ADT are 7.0% each. |
| ADT alone, apalutamide plus ADT and enzalutamide plus ADT are taken until disease progression, which we assumed to occur after 2 years of having metastatic hormone-sensitive disease. | |
| Once patients progress to metastatic hormone-relapsed prostate cancer, they can only have abiraterone or enzalutamide if they have not received apalutamide or enzalutamide before. | |
| All patients receiving radical radiotherapy receive ADT. | |
| Micro-costing analysis | A co-axial needle was assumed to be used for biopsies using double freehand devices and EZU-PA3U. |
| Antibiotic prophylaxis for TP biopsies is one prophylactic dose of ciprofloxacin (500 mg), while for LATRUS biopsies it is a course of ciprofloxacin 500 mg twice a day for 3 days. | |
| We assumed the average cost of the ultrasound machine costs of EZU-PA3U, UA1232 and Trinity Perine as the cost of the ultrasound machine and transducer of the remaining biopsy methods and devices. We also assumed the same lifetime, number of procedures and proportion of biopsies as for a stepper. | |
| We assumed that an average of five urologists per hospital have training each year regardless of the biopsy method. We assumed that a whole day (8 hours) of training would be required per person for SureFire Guide, LATP using grid and stepping device, LATP using double freehand devices and GATP. For LATRUS, we assumed that this would only require 1 hour of training – based on the assumption that urologists will already be familiar with this technique. | |
| We assumed that all biopsies are carried out by one urologist and that there are two nurses in the room for assistance. | |
| We assumed the mean procedure time for CamPROBE and PrecisionPoint (0.37 hours) for the remaining LATP devices and 1.00 hour for GATP. | |
| The cost of reprocessing was assumed to be £5, as advised by a Specialist Committee Member. | |
| For the base case, we assumed that 12 samples were taken from a prostate biopsy regardless of the biopsy method. | |
| We assumed that 1000 biopsies are carried out per year on average per hospital. This informed estimates of the cost per patient for capital equipment. |
Model validation
The model was developed by two health economists (JL and IR). The model was developed sequentially, starting with the cost and utility calculation sheets, then the parameter sheet, one copy of the decision tree, one copy of the Markov trace and the results sheets. Each element of the model was created independently by one member of the team and checked by the other before proceeding. One version of the decision-tree sheets was developed and double-checked before duplicating for other arms of the analysis. Similarly, one version of the Markov model was developed and checked first, and then duplicated. Calculations of the Markov probabilistic input parameters, the transition matrix and Markov trace were cross-checked against the calculations in the NG131 model, to which we had access.
Economic analysis results
Base-case results for decision question 1
Deterministic results
Deterministic cost-effectiveness results for decision question 1 are shown in Table 36. LATP-any is more costly but yields more QALYs than LATRUS for all subgroups. The incremental cost-effectiveness ratio (ICER) for LATP-any versus LATRUS increases from £5859 per QALY gained in subgroup A up to £16,792 per QALY gained for subgroup D. LATP-any dominates GATP in all subgroups, as it is less costly but no less effective.
| Biopsy method | Total | Incremental | INHB (QALYs) | ICERs | |||
|---|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | £20k | £30k | £/QALY | |
| Subgroup A: MRI Likert 3 + first biopsy | |||||||
| LATRUS | £19,878 | 9.299 | |||||
| LATP-any | £19,919 | 9.306 | £40 | 0.007 | 0.005 | 0.006 | £5859 |
| GATP | £20,405 | 9.304 | £486 | −0.002 | −0.021 | −0.013 | Dominated |
| Subgroup B: MRI Likert 1 or 2 first biopsy | |||||||
| LATRUS | £15,753 | 9.478 | |||||
| LATP-any | £15,805 | 9.483 | £51 | 0.004 | 0.002 | 0.003 | £11,610 |
| GATP | £16,286 | 9.482 | £482 | −0.001 | −0.023 | −0.014 | Dominated |
| Subgroup C: MRI Likert 3 + previous negative biopsy | |||||||
| LATRUS | £16,653 | 9.456 | |||||
| LATP-any | £16,703 | 9.461 | £50 | 0.004 | 0.002 | 0.003 | £11,111 |
| GATP | £17,185 | 9.460 | £482 | −0.001 | −0.023 | −0.014 | Dominated |
| Subgroup D: MRI Likert 1 or 2 previous negative biopsy | |||||||
| LATRUS | £14,066 | 9.547 | |||||
| LATP-any | £14,121 | 9.550 | £55 | 0.003 | 0.001 | 0.001 | £16,792 |
| GATP | £14,601 | 9.550 | £480 | 0.000 | −0.024 | −0.015 | Dominated |
Probabilistic results
Results of the probabilistic sensitivity analysis for decision question 1 are shown in Appendix 9, Table 75. The results are similar to the deterministic results, with slightly higher ICERs for LATP-any compared with LATRUS: £6710 per QALY gained in subgroup A up to £19,126 in subgroup D. GATP is dominated in all subgroups. The probabilistic results for subgroup A are illustrated in the scatterplot and cost-effectiveness acceptability curves (CEAC) in Appendix 9, Figures 22 and 23, respectively.
Intermediate outcomes
Outcomes related to the decision-tree biopsy pathway for decision question 1 are shown in Appendix 9, Table 77. The mean numbers of biopsies per person are lower for subgroup B than for subgroup A, reflecting base-case assumptions that the probability of repeat biopsy after a negative (no cancer or CNS) first biopsy result is lower for people with a Likert score of 1 or 2 than for people with a Likert score of 3 or more. Subgroups C and D, with a previous negative biopsy, are assumed not to have a repeat biopsy within the decision tree. The proportion of the cohort with undiagnosed CS prostate cancer at the end of the decision tree declines from subgroup A to D, in accordance with expected prevalence between the subgroups. The differences between the biopsy methods in the estimated proportions of undiagnosed CS prostate cancer (FNs) are due to small, non-statistically significant differences in cancer detection estimates (Table 31). We note that these parameters are highly uncertain; see Scenario analysis: cancer detection rates for scenario analysis using alternative estimates of comparative cancer detection rates.
Base-case estimates of biopsy-related adverse events result in a lower proportion of people with ‘mild’ adverse events (not requiring hospital admission) with TP than with transrectal biopsy. The estimated rate of admissions is also lower with the transperineal methods than with LATRUS. There is high uncertainty over differences in adverse-event rates with different biopsy methods, see scenario analyses in Scenario analysis: probability of biopsy complications.
Outcomes from the Markov model for decision question 1 are summarised in Appendix 9, Table 78. Deaths from prostate cancer decline and mean LYs and QALYs increase for the subgroups with lower baseline prevalence of CS prostate cancer. There are small differences in these outcomes between the biopsy methods, driven by the proportions of the cohort with FN biopsy results estimated from the decision tree.
Appendix 9, Table 79 summarises costs estimated from the decision tree and Markov models for decision question 1. Although the estimated costs of treating prostate cancer are high, cost differences between the biopsy methods from the Markov model are very small. Total costs are therefore driven by costs of the biopsy pathway, as estimated from the decision tree. We explore the impact of uncertainty over biopsy costs in Scenario analysis: cost of core samples and Scenario analysis: biopsy costs.
Base-case results for decision question 2
Deterministic results
For decision question 2, LATP-freehand dominates both LATP-other and GATP, yielding lower costs and more QALYs in all subgroups (Table 37). The ICER for LATP-freehand versus LATRUS is below £20,000 per QALY gained for all the subgroups (A–D). The QALY advantage for LATP-freehand in this analysis is driven by the favourable RR of cancer detection based on a single study;27 see Table 31.
| Biopsy method | Total | Incremental | INHB (QALYs) | ICERs | |||
|---|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | £20k | £30k | £/QALY | |
| Subgroup A: MRI Likert 3 + first biopsy | |||||||
| LATRUS | £19,878 | 9.299 | |||||
| LATP-freehand | £19,888 | 9.312 | £10 | 0.013 | 0.013 | 0.013 | £743 |
| LATP-other | £19,952 | 9.303 | £63 | −0.010 | 0.000 | 0.001 | Dominated |
| GATP | £20,420 | 9.301 | £468 | −0.001 | −0.025 | −0.016 | Dominated |
| Subgroup B: MRI Likert 1 or 2 first biopsy | |||||||
| LATRUS | £15,753 | 9.478 | |||||
| LATP-freehand | £15,788 | 9.486 | £35 | 0.008 | 0.006 | 0.006 | £4595 |
| LATP-other | £15,830 | 9.481 | £42 | −0.005 | −0.001 | 0.000 | Dominated |
| GATP | £16,295 | 9.480 | £465 | −0.001 | −0.025 | −0.016 | Dominated |
| Subgroup C: MRI Likert 3 + previous negative biopsy | |||||||
| LATRUS | £16,653 | 9.456 | |||||
| LATP-freehand | £16,699 | 9.461 | £46 | 0.005 | 0.003 | 0.003 | £9284 |
| LATP-other | £16,729 | 9.459 | £30 | −0.002 | −0.001 | 0.000 | Dominated |
| GATP | £17,195 | 9.458 | £466 | −0.001 | −0.025 | −0.016 | Dominated |
| Subgroup D: MRI Likert 1 or 2 previous negative biopsy | |||||||
| LATRUS | £14,066 | 9.547 | |||||
| LATP-freehand | £14,112 | 9.552 | £46 | 0.004 | 0.002 | 0.003 | £10,640 |
| LATP-other | £14,144 | 9.550 | £32 | −0.002 | −0.001 | 0.000 | Dominated |
| GATP | £14,608 | 9.549 | £464 | 0.000 | −0.025 | −0.016 | Dominated |
Probabilistic results
Appendix 9, Table 76 shows probabilistic results for decision question 2. As with question 1, the probabilistic results are similar to the deterministic results, with slightly higher ICERs for LATP-freehand compared with LATRUS in all subgroups, although these ICERs remain well under £20,000 per QALY gained: £2184 per QALY in subgroup A rising to £11,022 per QALY in subgroup D. LATP-other and GATP are dominated in all subgroups. The probabilistic results for this decision question are illustrated for subgroup A in Appendix 9, Figures 24 and 25.
Intermediate outcomes
Outcomes and costs for decision question 2 are shown in Appendix 9, Tables 80–82. Cancer detection rates for LATP-freehand are more favourable than for other comparators – driven by RR estimates from the NMA (Table 31). Other decision-tree results are very similar for LATP-freehand, LATP-other and GATP, as we use the same repeat biopsy and adverse-event rates for the transperineal methods in our base case. This might not be realistic, and we explore alternative scenarios in Scenario analysis: cancer detection rates and Scenario analysis: probability of biopsy complications.
The Markov outcomes for decision question 2 show the impact of the more favourable cancer detection rates for LATP-freehand biopsy, as deaths from prostate cancer are lower and life expectancy and QALYs are higher than for other comparators (see Appendix 9, Table 81). Costs of treatment from the Markov model are also slightly lower for LATP-freehand than for other comparators, although estimated biopsy costs are higher for LATP-freehand than for LATRUS (see Appendix 9, Table 82). We investigate alternative biopsy cost estimates in Scenario analysis: cost of core samples and Scenario analysis: biopsy costs.
Scenario analysis: cancer detection rates
Our original base case used RRs of cancer detection estimated from NMA of six RCTs, including the Hara 200826 and Takenaka 200828 trials classified as comparisons between LATP (without freehand device) and LATRUS. There are uncertainties over this approach due to the types of anaesthesia used in the Hara and Takenaka trials (spinal injection in the transperineal arm and caudal block in the transrectal arm). We therefore excluded the Hara and Takenaka trials from the revised base case, as reported above.
Alternative NMA scenarios were reported in Table 31:
-
Hara and Takenaka classified as LATP-any versus LATRUS (for decision question 1) and LATP-other versus LATRUS (for decision question 2).
-
Hara and Takenaka classified as GATP versus LATRUS.
Results of these scenarios for subgroup A decision question 1 are reported in Table 38. For NMA scenario 1, the ICER for LATP versus LATRUS is £28,322 per QALY in subgroup A, increasing to £31,261 per QALY in subgroup D. For NMA scenario 2, the ICER for LATP versus LATRUS is £10,096 per QALY in subgroup A, increasing to £21,322 per QALY in subgroup D. GATP remains dominated in NMA scenarios 1 and 2 for all subgroups.
| Biopsy method | RRa | Total | Incremental | ICERs | ||
|---|---|---|---|---|---|---|
| cost | QALYs | cost | QALYs | £/QALY | ||
| Network meta-analysis scenario 1: Hara and Takenaka classified as LATP-any vs. LATRUS26,28 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-any | 1.01 | £19,944 | 9.301 | £66 | 0.002 | £28,322 |
| GATP | 0.96 | £20,430 | 9.299 | £486 | −0.002 | Dominated |
| Network meta-analysis scenario 2: Hara and Takenaka classified as GATP vs. LATRUS26,28 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-any | 1.09 | £19,929 | 9.304 | £51 | 0.005 | £10,096 |
| GATP | 0.92 | £20,439 | 9.298 | £510 | −0.006 | Dominated |
For decision question 2, results are not sensitive to the NMA scenarios. This is because the RR for cancer detection with LATP-freehand versus LATRUS does not change between NMA scenarios and other comparators are dominated in all scenarios and subgroups (see Table 39 for subgroup A).
| Biopsy method | RRa | Total | Incremental | ICERs £/QALY |
||
|---|---|---|---|---|---|---|
| cost | QALYs | cost | QALYs | |||
| Network meta-analysis scenario 1: Hara and Takenaka classified as LATP-other vs. LATRUS26,28 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-freehand | 1.40 | £19,888 | 9.312 | £10 | 0.013 | £743 |
| LATP-other | 0.94 | £19,974 | 9.299 | £86 | −0.014 | Dominated |
| GATP | 0.90 | £20,444 | 9.297 | £470 | −0.002 | Dominated |
| Network meta-analysis scenario 2: Hara and Takenaka classified as GATP vs. LATRUS26,28 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-freehand | 1.40 | £19,888 | 9.312 | £10 | 0.013 | £743 |
| LATP-other | 1.01 | £19,960 | 9.301 | £71 | −0.011 | Dominated |
| GATP | 0.90 | £20,444 | 9.297 | £484 | −0.004 | Dominated |
We also investigated the effect of using observational data to estimate cancer detection rates: see Appendix 9, Tables 83 and 84. Results were not sensitive to the observational scenarios. For decision question 1, the ICER for LATP-any versus LATRUS was well below £30,000 per QALY for all observational scenarios and subgroups. For decision question 2, the ICER for LATP-freehand versus LATRUS was below £20,000 per QALY for all observational scenarios and subgroups. GATP and LATP-other in decision question 2 had high ICERs or were dominated in all observational analyses.
Scenario analysis: probability of biopsy complications
The rationale for choosing sources for the incidence of biopsy-related complications is explained in Biopsy-related complications above. For the base case, we used admission rates reported by Tamhankar et al.. 80 Additional scenario analyses to test the effect of alternative estimates of biopsy-related admission rates are reported below:
-
Inclusion of additional overnight stays immediately after biopsy as reported by Berry et al.. 94,97 This increases the overall admission rate for transperineal biopsies more than for transrectal biopsies.
-
Inclusion of additional overnight stays from the Berry study applied to GATP only. 97
-
Rosario et al. 95 used as the source of admissions. This reduces the admission rate for LATRUS compared with the base case.
-
Pepe and Aragona96 used as the source of admissions for transperineal biopsies. This reduces the admission rate for LATP and GATP.
-
Rosario study and Pepe and Aragona study as the sources of admissions for LATRUS and transperineal biopsies respectively.
Table 40 (decision question 1) and Table 41 (decision question 2) show the results of these scenarios for subgroup A (MRI Likert score 3+ at first biopsy).
| Biopsy method | Admission rate (%) | Total | Incremental | ICERs £/QALY |
||
|---|---|---|---|---|---|---|
| cost | QALYs | cost | QALYs | |||
| Scenario 1: include overnight stay from Berry et al . for LATRUS, LATP and GATP 97 | ||||||
| LATRUS | 6.10 | £19,940 | 9.298 | |||
| LATP all | 15.61 | £20,149 | 9.301 | £210 | 0.003 | £70,257 |
| GATP | 15.61 | £20,635 | 9.299 | £486 | −0.002 | Dominated |
| Scenario 2: include overnight stay from Berry et al. for GATP only 97 | ||||||
| LATRUS | 3.74 | £19,878 | 9.299 | |||
| LATP all | 3.54 | £19,919 | 9.306 | £40 | 0.007 | £5859 |
| GATP | 15.61 | £20,633 | 9.299 | £715 | −0.007 | Dominated |
| Scenario 3: Rosario et al. admission rate for LATRUS 95 | ||||||
| LATRUS | 1.31 | £19,815 | 9.300 | |||
| LATP all | 3.54 | £19,917 | 9.306 | £101 | 0.006 | £17,119 |
| GATP | 3.54 | £20,403 | 9.304 | £486 | −0.002 | Dominated |
| Scenario 4: Pepe and Aragona admission rate for LATP and GATP96 | ||||||
| LATP all | 1.23 | £19,875 | 9.307 | |||
| LATRUS | 3.74 | £19,878 | 9.299 | £3 | −0.008 | Dominated |
| GATP | 1.23 | £20,361 | 9.305 | £483 | 0.006 | Dominateda |
| Scenario 5: Rosario et al. for LATRUS; Pepe and Aragona for LATP and GATP95,96 | ||||||
| LATRUS | 1.31 | £19,815 | 9.300 | |||
| LATP all | 1.23 | £19,873 | 9.307 | £57 | 0.007 | £8395 |
| GATP | 1.23 | £20,359 | 9.305 | £486 | −0.002 | Dominated |
| Biopsy method | Admission rate (%) | Total | Incremental | ICERs | ||
|---|---|---|---|---|---|---|
| cost | QALYs | cost | QALYs | £/QALY | ||
| Scenario 1: include overnight stay from Berry et al. for LATRUS, LATP and GATP 97 | ||||||
| LATRUS | 6.10 | £19,940 | 9.298 | |||
| LATP-freehand | 15.61 | £20,119 | 9.307 | £179 | 0.009 | £19,140 |
| LATP-other | 15.61 | £20,182 | 9.298 | £63 | −0.010 | Dominated |
| GATP | 15.61 | £20,651 | 9.296 | £468 | −0.001 | Dominated |
| Scenario 2: include overnight stay from Berry et al. for GATP only 97 | ||||||
| LATRUS | 3.74 | £19,878 | 9.299 | |||
| LATP-freehand | 3.54 | £19,888 | 9.312 | £10 | 0.013 | £743 |
| LATP-other | 3.54 | £19,952 | 9.303 | £63 | −0.010 | Dominated |
| GATP | 15.61 | £20,649 | 9.296 | £697 | −0.006 | Dominated |
| Scenario 3: Rosario et al. admission rate for LATRUS 95 | ||||||
| LATRUS | 1.31 | £19,815 | 9.300 | |||
| LATP-freehand | 3.54 | £19,886 | 9.312 | £71 | 0.012 | £5750 |
| LATP-other | 3.54 | £19,950 | 9.303 | £64 | −0.010 | Dominated |
| GATP | 3.54 | £20,418 | 9.301 | £468 | −0.001 | Dominated |
| Scenario 4: Pepe and Aragona admission rate for LATP and GATP96 | ||||||
| LATP-freehand | 1.23 | £19,844 | 9.313 | |||
| LATRUS | 3.74 | £19,878 | 9.299 | £34 | −0.014 | Dominated |
| LATP-other | 1.23 | £19,908 | 9.304 | £30 | 0.005 | Dominated |
| GATP | 1.23 | £20,376 | 9.302 | £468 | −0.001 | Dominated |
| Scenario 5: Rosario et al. for LATRUS; Pepe and Aragona for LATP and GATP95,96 | ||||||
| LATRUS | 1.31 | £19,815 | 9.300 | |||
| LATP-freehand | 1.23 | £19,842 | 9.313 | £27 | 0.013 | £2035 |
| LATP-other | 1.23 | £19,906 | 9.304 | £64 | −0.010 | Dominated |
| GATP | 1.23 | £20,374 | 9.302 | £468 | −0.001 | Dominated |
For decision question 1, results are sensitive to the difference in admission rates between LATP and LATRUS. In scenario 1 with additional overnight stays included, the ICER for LATP is over £70,000 per QALY for subgroup A, and higher for other subgroups. In scenario 3, with a lower admission rate for LATRUS, the ICER for LATP versus LATRUS is £17,119 per QALY for subgroup A, and over £30,000 per QALY for other subgroups.
GATP is dominated in all scenarios.
For decision question 2, ICERs for LATP-freehand are higher in scenario 1 with the overnight stay included: £19,140 per QALY for subgroup A and over £30,000 for other subgroups. LATP-other and GATP are dominated in all scenarios.
We note that the excess overnight stays after TP from the Berry et al. study relate to Hospital Episode Statistics from 2014 to 2017, when the use of local anaesthetic for TP was rare. This suggests that scenario 2 may be a more appropriate interpretation of results from the Berry study than scenario 1.
Scenario analysis: cost of core samples
Number of core samples by biopsy method
The base-case assumption of equal numbers of core samples (12) per patient for all biopsy methods was based on a lack of data on the mean number of core samples taken in the clinical trials that contributed to the effectiveness (cancer detection) estimates in the model. Five of the six RCTs in the network meta-analyses reported protocols with the same number of cores for intervention and comparator arms. The exception was the Lam 2021 study, which used a ‘modified Ginsburg’ protocol for the LATP arm (with the PrecisionPoint freehand device), but a standard 12-core protocol for LATRUS. 27 The mean number of cores taken for patients in the LATP arm in the Lam study was not reported. The other RCT protocols included between 8 and 14 core samples,24–26,28,42 although two of these trials included additional targeted sampling as needed (mean per patient not reported). 25,42
Experts advising NICE reported that in practice the number of cores is likely to differ between biopsy methods. We understand that LATRUS biopsy is ‘almost always’ 10–12 cores, and that TP usually follows one of two protocols: the RAPID protocol (12–16 biopsies), mostly used for grid and stepping device LATP or GATP; and the Ginsburg protocol (24–34 biopsies), mostly used for freehand LATP or GATP. In a recent study, Lopez et al. reported a median of 24 cores (range 1–47) in a multicentre prospective cohort of 1218 patients who underwent LATP biopsy with the PrecisionPoint device according to the Ginsburg protocol (7 out of 10 centres from the UK). 111
We consider three alternative scenarios including costs for different numbers of core samples taken for the different biopsy procedures:
-
24 cores for LATP and GATP, 12 for LATRUS.
-
24 cores for LATP-freehand and 12 for the LATP-other, GATP and LATRUS.
-
24 cores for LATP-freehand, 16 for LATP-other and GATP and 12 for LATRUS.
Note that these scenarios only model changes to histopathology costs; we were not able to model the impact of the number of core samples on patient outcomes. In practice one would expect clinical parameters, including rates of repeat biopsy and adverse events as well as cancer detection rates, to be affected by the number of cores sampled. It may be argued that the scenario analyses with costs for 24 cores for LATP-freehand and 12 for LATRUS are more consistent with the clinical evidence from the Lam et al. trial. 27
Results are highly sensitive to the scenarios with 24 cores for transperineal biopsies and 12 for LATRUS. For decision question 1, the ICER for LATP-any versus LATRUS in subgroup A is over £60,000 per QALY (Table 42), and in decision question 2 the ICER for LATP-freehand compared with LATRUS is £33,813 per QALY (Table 43). These ICERs are higher for other subgroups.
| Biopsy method | Biopsy samples | Total | Incremental | ICERs | ||
|---|---|---|---|---|---|---|
| cost | QALYs | cost | QALYs | £/QALY | ||
| Core scenario 1: 24 core samples for all transperineal methods | ||||||
| LATRUS | 12 | £19,878 | 9.299 | |||
| LATP-any | 24 | £20,358 | 9.306 | £479 | 0.007 | £69,547 |
| GATP | 24 | £20,844 | 9.304 | £486 | −0.002 | Dominated |
| Biopsy method | Biopsy samples | Total | Incremental | ICERs | ||
|---|---|---|---|---|---|---|
| cost | QALYs | cost | QALYs | £/QALY | ||
| Core scenario 1: 24 cores for all transperineal methods | ||||||
| LATRUS | 12 | £19,878 | 9.299 | |||
| LATP-freehand | 24 | £20,327 | 9.312 | £449 | 0.013 | £33,813 |
| LATP-other | 24 | £20,391 | 9.303 | £63 | −0.010 | Dominated |
| GATP | 24 | £20,859 | 9.301 | £468 | −0.001 | Dominated |
| Core scenario 2: 24 cores for LATP-freehand only | ||||||
| LATRUS | 12 | £19,878 | 9.299 | |||
| LATP-other | 12 | £19,952 | 9.303 | £73 | 0.004 | £19,716 |
| LATP-freehand | 24 | £20,327 | 9.312 | £375 | 0.010 | £39,304 |
| GATP | 12 | £20,420 | 9.301 | £93 | −0.011 | Dominated |
| Core scenario 3: 24 cores for LATP-freehand and 16 for LATP-other and GATP | ||||||
| LATRUS | 12 | £19,878 | 9.299 | |||
| LATP-other | 16 | £20,098 | 9.303 | £220 | 0.004 | Dominateda |
| LATP-freehand | 24 | £20,327 | 9.312 | £229 | 0.010 | £33,813 |
| GATP | 16 | £20,566 | 9.301 | £239 | −0.011 | Dominated |
Histopathology costs
The above scenario results depend on histopathology costs in addition to the number of core samples. In the base case and core scenarios, we assumed £36.58 per core (code DAPS2, NHS cost collection 2019–20):90 £439 for 12 cores and £878 for 24 cores. However, it has been suggested that these are overestimates, as the unit cost may be applied per sample pot, which may contain more than one core.
An alternative source for estimating histopathology costs is available from an online report by the University of Surrey. 112 This reported a cost of £37.50 for ‘standard histology’ (1–2 sites/lesions) and £7 per additional site/lesion. Assuming that each core sample is one ‘site/lesion’, this gives a cost of £108 for 12 cores and £192 for 24 cores. There is uncertainty over the appropriateness of these estimates, as we had previously received feedback that they underestimate histopathology costs.
The ICERs for LATP in the core sample scenarios are more favourable with the Surrey histopathology costs (see Appendix 9, Tables 85 and 86) than with our base-case costs (see Tables 42 and 43). For example, the ICER for LATP-freehand (24 cores) versus LATRUS (12 cores) with Surrey costs is £8052 in subgroup A, increasing to £33,545 in subgroup D.
Scenario analysis: biopsy costs
Decision question 1
The cost of LATP in the base case for decision question 1 assumes an equal mix of methods, including grid and stepping device, double freehand and the six named devices included in the scope. The costs were obtained from our micro-costing analysis. We conducted two scenario analyses for the overall cost of the biopsy procedure for decision question 1 (Table 44).
| Biopsy method | Cost per biopsy | Total | Incremental | ICERs | ||
|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | £/QALY | ||
| Cost scenario 1: NHS cost data 2019–20 90 | ||||||
| LATP-any | £329 | £19,460 | 9.306 | |||
| LATRUS | £332 | £19,518 | 9.299 | £58 | −0.007 | Dominated |
| GATP | £1512 | £20,654 | 9.304 | £1136 | 0.005 | Dominated |
| Cost scenario 2: LATP mix (30% each for CamPROBE, PrecisionPoint and UA1232; and 10% grid and stepping device) | ||||||
| LATRUS | £681 | £19,878 | 9.299 | |||
| LATP-any | £799 | £19,942 | 9.306 | £64 | 0.007 | £9245 |
| GATP | £1251 | £20,405 | 9.304 | £463 | −0.002 | Dominated |
-
Biopsy costs from the National Schedule of NHS costs 2019–20:90 £332 for LATRUS (outpatient procedure LB76Z 101, urology), £329 for LATP (outpatient procedure B77Z, 101, urology) and £1512 for GATP (day-case procedure LB77Z). In this scenario, the cost for LATRUS is slightly higher than the cost for LATP, so LATP is dominant in all subgroups.
-
Microcosting, but with different assumptions about the proportion of LATP methods used: 10% conducted with a grid and stepping device and 30% with each of the transperineal devices that we understand are currently most common in the UK (CamPROBE, PrecisionPoint and UA1232). This increases the mean cost of LATP by £23 per biopsy, which increases the ICER for LATP in subgroup A from £5859 per QALY to £9245. The ICER for LATP in this scenario remains below £20,000 per QALY for subgroups B and C and below to £30,000 per QALY in subgroup D.
Decision question 2
For decision question 2, we report three scenarios for biopsy costs.
The first scenario relates to the cost of LATP-freehand. In our base case, we used a simple average of the cost of each named device as the cost for the LATP-freehand arm. In this scenario, we use the cost of the PrecisionPoint device, which was used in the clinical trial that provided the evidence on diagnostic yield for LATP-freehand. 27 This is the most costly of the included freehand devices: £200 for the device and total cost of the procedure estimated at £894. This increases the ICER for LATP-freehand versus LATRUS in subgroup A to £9230 per QALY (Table 45). The ICER for LATP-freehand remains below £20,000 per QALY for subgroup B, but exceeds £30,000 per QALY for subgroups C and D.
| Biopsy method | Cost per biopsy | Total | Incremental | ICERs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | £/QALY | ||||||
| Cost of LATP-freehand: cost of PrecisionPoint device | ||||||||||
| LATRUS | £681 | £19,878 | 9.299 | |||||||
| LATP-other | £791 | £19,952 | 9.303 | £73 | 0.004 | Dominateda | ||||
| LATP-freehand | £894 | £20,001 | 9.312 | £49 | 0.010 | £9230 | ||||
| GATP | £1251 | £20,420 | 9.301 | £419 | −0.011 | Dominated | ||||
| Cost of LATP-other scenario 1: cost of double freehand device | ||||||||||
| LATRUS | £681 | £19,878 | 9.299 | |||||||
| LATP-other | £727 | £19,888 | 9.303 | £10 | 0.004 | Dominateda | ||||
| LATP-freehand | £781 | £19,888 | 9.312 | £0 | 0.010 | £743 | ||||
| GATP | £1251 | £20,420 | 9.301 | £532 | −0.011 | Dominated | ||||
| Cost of LATP-other scenario 2: cost of CamPROBE | ||||||||||
| LATRUS | £681 | £19,878 | 9.299 | |||||||
| LATP-freehandb | £780 | £19,887 | 9.312 | £9 | 0.013 | £682 | ||||
| LATP-other | £785 | £19,946 | 9.303 | £59 | −0.010 | Dominated | ||||
| GATP | £1251 | £20,420 | 9.301 | £474 | −0.001 | Dominated | ||||
The second and third scenarios relate to the cost of LATP-other. In the base case, we grouped evidence relating to LATP biopsy without a named freehand device together as ‘LATP-other’, but we based the cost for this grouped comparator only on the cost for LATP biopsy using grid and stepping device (£791). We report additional scenarios below, using the cost of LATP biopsy with a double freehand technique (£727) or LATP biopsy with the CamPROBE double freehand device (£785) for the LATP-other comparator. These scenarios do not affect the cost-effectiveness results (see Table 45 for subgroup A). This is because LATP-other remains dominated for both scenarios, and for all subgroups.
Other scenario analysis
Other scenario analyses are presented in Appendix 9, including the probability of repeat biopsy, and RR of cancer detection from observational studies. Appendix 9, Table 88 presents scenario analyses conducted for decision questions 1 and 2 in subgroup A with a lower impact in the model results and that did not impact the final conclusions. The results for the other subgroups (B, C and D) follow the same tendency as the results presented in Appendix 9, Table 88.
Chapter 6 Discussion
Clinical effectiveness evidence
We conducted a comprehensive systematic review of studies assessing the diagnostic yield and clinical effectiveness outcomes of LATP prostate biopsy for people in whom prostate cancer is suspected.
We included 23 studies which we grouped into 5 pairwise comparisons of LATP prostate biopsy versus an alternative biopsy modality relevant to the decision problem. Each pairwise comparison was of primary relevance to one of two decision questions on the clinical and cost-effectiveness of LATP prostate biopsy.
The largest volume of available evidence is for comparison 1: LATP-any versus LATRUS. As its title suggests, this comparison incorporates the spectrum of LATP biopsy methods and hence has a diverse evidence base. The majority of the available LATP prostate-biopsy studies are relevant here. The strength of this evidence is mixed – some are RCTs, but the majority are observational studies of varying designs. The RCTs appear well designed and executed, but we are unclear on the potential for bias due to limitations in study reporting, as is the case for the observational studies. Decision question 2, nested within decision question 1, has a more specific focus – on the use of freehand biopsy devices. This is a smaller evidence base, in terms of number of studies, and less heterogeneous than that of the broader decision question.
We identified few differences between LATP prostate biopsy and alternatives, principally, LATRUS, in terms of key outcome measures, notably cancer detection rates. Our meta-analyses estimated RRs close to 1 for cancer detection rates, indicating similar effects. Our overall interpretation of the decision question 1 evidence is that LATP biopsy is similar to LATRUS biopsy in diagnostic yield, a conclusion shared by previous studies in this field. The strength of the evidence is adequate and there is reasonable certainty (based on relatively narrow confidence intervals in our meta-analyses).
Regarding post-biopsy complications, we discerned no definitive association between specific complications and biopsy modalities. Rates of complications were low, often occurring in a handful of participants; it would be unwise to interpret very small differences seen between biopsy methods as being definitive. This is a limitation of clinical trials and evaluations – they are often not statistically powered to detect differences in relatively rare events. Larger cohort studies and data sets often provide more certain estimates of rare events; hence we use these to inform estimates of complication rates in our cost-effectiveness analysis.
Generalisability
The TP protocols (e.g. device used/sampling method/number of cores taken) varied between studies, which may partly reflect local clinical practice guidelines in study host institutions, but also the evolution of transperineal prostate biopsy practices over time (e.g. increases in the number of cores sampled over time as protocols evolve). Some of the more recently published studies used pre-biopsy mpMRI to inform biopsy sampling, but this constitutes a small proportion of the whole evidence base. This presents a challenge to the applicability of the evidence to UK clinical practice, where mpMRI is recommended for routine use to inform the decision to refer for biopsy and may also be used to target biopsy sampling.
The studies were typically conducted in single centres by clinical investigators using local biopsy protocols to evaluate the optimum biopsy modality in their centre, on a range of outcomes such as use of general or local anaesthesia protocols, procedure time and related resources, biopsy complications and the patient’s ability to tolerate pain and discomfort during and after the biopsy. Many studies pre-date the introduction of mpMRI into prostate biopsy protocols, and given the preponderance of studies from East Asia use of mpMRI worldwide may differ from practice in the UK.
The multicentre UK study (TRANSLATE52–54) will provide evidence for freehand LATP using any ultrasound probe-mounted needle guidance device, including the PrecisionPoint and UA1232 devices. As the study uses freehand devices to perform the biopsies, it is expected to inform any future consideration of both decision question 1 (LATP-any vs. LATRUS) and decision question 2 (LATP-freehand vs. LATRUS). This will be the first available comparative evidence for the UA1232 device, and is expected to provide information on cancer detection, infection rates and other outcomes including cost.
Cost-effectiveness evidence
We developed an economic model to assess the cost-effectiveness of LATP prostate biopsies and freehand TP devices. The model includes a decision tree to evaluate short-term diagnostic outcomes and biopsy-related costs and adverse effects, and a Markov model that estimates the long-term costs and health consequences of failing to detect CS disease. The Markov model was replicated from a model previously developed by the NICE Guidelines Update Team to evaluate different follow-up strategies for people at increased risk of prostate cancer. 67 The model was also informed by the results and cost-effectiveness analysis of the PROMIS trial,65 and a recent economic evaluation that compared transperineal and transrectal prostate biopsies conducted under local anaesthetic. 63
We estimated cost-effectiveness for four subgroups of patients with suspected prostate cancer. The subgroups vary by prior likelihood of having CS prostate cancer: from the highest risk in the subgroup with mpMRI Likert 3 + and no previous biopsy to lowest in the subgroup with mpMRI Likert 1 or 2 and previous negative biopsy.
The model is designed to address both decision questions in the NICE scope, although limitations in the clinical evidence do impose some restrictions on the analysis for decision question 2: in particular, we do not have comparative evidence of the diagnostic yield or adverse-event rates of LATP with different freehand TP devices or with a grid and stepping device. Cancer detection rates for the different biopsy methods are estimated from our network meta-analyses in the base case, with scenarios using RRs from pairwise meta-analysis of observational evidence.
Relative rates of complications and repeat biopsy associated with the different biopsy methods are difficult to assess. There is good evidence from NHS practice, based on Hospital Episode Statistics and observational cohort studies. 94–96,113,114 However, this does not reliably distinguish between type of anaesthesia as well as biopsy route (transrectal vs. transperineal).
For decision question 1, the economic base-case analysis indicated that GATP is more expensive and less effective (yielding fewer QALYs) than LATP in all four subgroups. This result was based on sparse comparative evidence, with a single RCT reporting on the diagnostic yield of GATP compared with LATP. 42 The ICER for LATP based on pooled evidence for all LATP methods compared with LATRUS was below £20,000 per QALY gained in all subgroups, within the lower limit used for decision-making by NICE advisory committees. This conclusion was supported by probabilistic sensitivity analysis and a range of scenario analyses, although the results are sensitive to some uncertainties over relative cancer detection rates, rates of hospital admissions, the number of core samples and pathology costs.
For decision question 2, the economic analysis indicated that LATP with a freehand device was the most cost-effective strategy, with an ICER of £743 per QALY compared with LATRUS for the highest-risk subgroup with MRI Likert score of 3 or more at first biopsy, and £4595 per QALY for the subgroup with a MRI Likert score 1 or 2 at first biopsy. For the subgroups with a previous negative biopsy, the ICER remained below £20,000 per QALY. These favourable ICER estimates are driven by cancer detection rates from a single RCT for LATP with a freehand device (PrecisionPoint). 27 In the scenario based on observational evidence of cancer detection rates, the ICERs for LATP with a freehand device were higher but still below £20,000 per QALY in all subgroups. However, these results were sensitive to the number of core samples per biopsy and the cost of processing them, and to the cost of the freehand device.
Strengths and limitations of the assessment
Strengths
We conducted a systematic review of evidence related to the decision questions specified in the NICE scope, with pairwise and NMA of cancer detection outcomes from both randomised and observational studies.
A major strength of the economic analysis is that we could build on the work of previous researchers to develop an appropriate decision-tree structure and model parameters, including the economic evaluation of the PROMIS study by Faria et al., the adaptation of the PROMIS analysis by Wilson et al. and the economic model that informed the update of the NICE guideline (NG131). 63,65,67,115 The decision tree is based on prevalence and diagnostic yield data for TRUS from the PROMIS study, which used estimates of true disease status based on a template mapping biopsy as the reference standard.
Another strength is that the predicted impact of diagnostic yield on long-term costs and outcomes was based on the recent and high-quality economic model that was developed to inform an update of the NICE guideline for prostate cancer (NG131). The NICE economic model has gone through a rigorous process of development, review and discussion by members of the guideline committee (including topic specialists and methodological, patient and public experts) and consultation with stakeholders. We appreciate that the NICE Centre for Guidelines provided a copy of this model, as this helped us to replicate the transition probabilities accurately (in particular it provided access to the covariance matrices for the calibrated parameters).
The RR of cancer detection was directly informed by the clinical effectiveness systematic review and therefore we believe that the most relevant studies reporting data on cancer detection rates were considered.
Limitations
The clinical evidence base and economic model have several limitations. As discussed above, there are limitations to the generalisability of the clinical evidence to UK practice, including variations between centres and over time in TP protocols and the use of mpMRI to inform referral for biopsy, and biopsy sampling.
The definition of patient subgroups in the model was based on mpMRI Likert scores, in order to align with epidemiological data from the PROMIS study. However, we are aware that some UK centres use the PI-RADS method to summarise mpMRI results. We have not provided results for subgroups according to lesion site or prostate volume, due to lack of data to differentiate prognosis or diagnostic yield of the biopsy methods under assessment.
We extrapolated data on repeat biopsy from LATRUS and GATP (based on the Jimenez et al.’ study) to LATP, in the absence of specific evidence for LATP. 113 Moreover, the Jimenez et al. study assesses a Spanish cohort that may not be wholly generalisable to UK practice. However, a scenario analysis on the probability of repeat biopsy showed that the model results are not sensitive to this parameter.
We have assumed that patients with a negative biopsy result were discharged and no additional costs were incurred, since we are uncertain about the extent and nature of the follow-up of these patients in primary care. However, it is likely that a substantial proportion of people with a negative biopsy who develop prostate cancer later have a diagnosis based on symptoms, which is considered in the model. Lastly, although we included costs for recently recommended treatments for metastatic hormone-sensitive prostate cancer (apalutamide and enzalutamide), we did not incorporate survival benefit from these treatments in our model. Our scenario analysis showed that excluding apalutamide and enzalutamide from the treatment options for mHSPC has a low impact in the model results.
Uncertainties
Uncertainties in the clinical evidence base contribute to uncertainties in cost-effectiveness estimates. In particular, the RR for cancer detection for LATP-freehand is based on a single RCT which used the PrecisionPoint device. 27 The RR for ‘LATP-other’ is a pooled estimate of studies that did not report the use of a freehand device, so it is unclear whether this corresponds with the LATP using grid and stepping device comparator for decision question 2.
Sources of evidence for biopsy complications for the economic model were difficult to interpret, as results were not reported for LATP and GATP separately and therefore it is unclear how many complications (and which ones) relate to LATP or GATP.
The microcosting analysis is also associated with some uncertainty, although the majority of assumptions relate to values that cancel out across biopsy methods. A key uncertainty is the number of cores taken, which we assume to be 12 for every biopsy method. This is potentially an important factor, as the number of cores taken may have an impact on cancer detection rates, but oversampling can make the procedure more difficult for the patient to tolerate, as well as having a cost impact related to the duration of the procedure and pathology costs. The cost-effectiveness results were very sensitive to assumptions about differences in the number of core samples for LATP and LATRUS, and to estimates of the cost of histopathology.
There was no evidence on the disutility of biopsy procedures and limited evidence on the disutilities of biopsy complications. Although we have used the same disutilities for biopsy complication as Wilson et al.,63 these estimates were obtained from old studies not conducted in the population of interest. We assumed that misdiagnosed patients have the same rate of adverse events and disutility from adverse events as patients undergoing active surveillance, although it is uncertain if that reflects real practice.
Chapter 7 Conclusions
Pooled evidence from randomised trials indicates that transperineal prostate biopsy (using any available method) performed under local anaesthetic is equally effective at detecting prostate cancer as TRUS-guided prostate biopsy under local anaesthetic. One RCT estimated a non-significant improvement in the cancer detection rate for transperineal prostate biopsy using a freehand device under local anaesthetic compared with TRUS-guided prostate biopsy under local anaesthetic. This finding was supported by observational evidence. Comparative evidence on cancer detection rates with transperineal prostate biopsy conducted under local versus general anaesthetic is sparse. What evidence there is does not indicate a difference.
Evidence on complications associated with the different biopsy methods is sparse and difficult to interpret, for example because studies do not specify the anaesthetic approach or whether any specific device was used. The available evidence, supported by clinical opinion, suggests that LATP prostate biopsy is associated with more urinary retention whereas local anaesthetic TRUS-guided prostate biopsy has higher infection rates.
Based on pooled evidence for all types of LATP biopsy (with or without a specified freehand device), LATP is estimated to meet conventional criteria for cost-effectiveness in a UK context, with an incremental cost below £20,000 per QALY gained in comparison with LATRUS. LATP with a freehand device was also estimated to meet conventional criteria as a cost-effective alternative to LATRUS. These results are subject to some uncertainties over the cost of the freehand device, the number of core samples, and the sources for cancer detection rates and biopsy complication rates.
Implications for service provision
This analysis suggests that the use of LATP freehand TP devices is potentially cost-effective compared to LATRUS, with costs per QALY within the range generally considered acceptable by health services decision-makers. This conclusion is more certain for PrecisionPoint because, of all the freehand devices, it had most of the available evidence. Furthermore, until more evidence is available the comparative cost-effectiveness of the freehand TP devices is unknown. Our study also suggests that the additional expense of more costly biopsy procedures may not be warranted for patients at lower risk of having prostate cancer (according to Likert or PI-RADS scores, previous negative biopsy, prostate volume and site of lesions).
Suggested research priorities
-
Evidence for freehand devices. There was no evidence for several of the freehand devices in the NICE scope. The TRANSLATE study may address this question to some extent, as it is evaluating the PrecisionPoint, UA1232 and ‘any ultrasound probe-mounted needle guidance device’.
-
Outcomes not covered in included available evidence. We suggest that incidence of defined complications (standardised for grading of severity and length of follow-up), health-related quality of life, and longer-term clinical outcomes could be defined in a core outcome set.
-
LATP versus GATP. Evidence for this comparison is sparse (we identified one RCT reporting cancer detection rates).
-
Repeat biopsy population. There is a need for separate reporting of results for this subgroup, or a separate prospective RCT.
-
UK NHS setting. The three UK studies included in our review were single-centre observational studies with a limited set of outcomes. The TRANSLATE study is expected to remedy this; it is a multicentre randomised study across nine NHS Trusts in England.
Additional information
Contributions of authors
Inês Souto-Ribeiro (https://orcid.org/0000-0001-8464-4513) (Senior Research Assistant, health economics) carried out the review of economic evaluations, developed the independent economic model, and drafted the report.
Lois Woods (https://orcid.org/0000-0002-4587-9149) (Senior Research Assistant, evidence synthesis) conducted literature searches, carried out the systematic review of diagnostic test evaluation and clinical effectiveness, and drafted the report.
Emma Maund (https://orcid.org/0000-0002-3998-6669) (Research Fellow, evidence synthesis) carried out the systematic review of diagnostic test evaluation and clinical effectiveness, and drafted the report.
David Alexander Scott (https://orcid.org/0000-0001-6475-8046) (Principal Research Fellow, evidence synthesis) conducted the network meta-analysis and drafted the report.
Joanne Lord (https://orcid.org/0000-0003-1086-1624) (Professorial Research Fellow, health economics) carried out the review of economic evaluations, developed the independent economic model, and drafted the report.
Joanna Picot (https://orcid.org/0000-0001-5987-996X) (Senior Research Fellow, evidence synthesis) wrote the research protocol, carried out systematic review of diagnostic test evaluation and clinical effectiveness, drafted the report and provided a quality assurance review of the draft report.
Jonathan Shepherd (https://orcid.org/0000-0003-1682-4330) (Principal Research Fellow, evidence synthesis) carried out the systematic review of diagnostic test evaluation and clinical effectiveness, drafted the report, managed the project, and is the project guarantor.
Acknowledgements
We are grateful to the following for providing expert methodological/clinical advice and comments on the draft report:
-
Mr Alistair Grey, Consultant Urologist, University College London Hospital, London, UK.
-
Mr Jonathan Aning, Consultant Urological Surgeon, Bristol Urological Institute, Southmead Hospital, Bristol, UK.
-
Professor Mark Emberton, Professor of Interventional Oncology, Division of Surgery and Interventional Science, University College London, London, UK.
We also thank the following from NICE:
-
The NICE Specialist Committee Members (SCMs) on this assessment for their informative comments on a draft of this report and their expert clinical advice.
-
Joshua Pink and the NICE Guideline Update Team who developed the NG131 economic model which informed development of the model for this assessment, and also the NICE Centre for Guidelines for sharing the model.
-
The NICE Diagnostic Assessment Programme team for their assistance during the assessment.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to completed data extractions for all studies included in the systematic review of diagnostic test and clinical effectiveness may be provided following review.
Ethics statement
Ethical approval was not sought for this study as this is not a requirement for secondary research, including economic modeling. There was no direct access to human participants for data collection or analysis.
Information governance statement
The University of Southampton is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679.
Under the Data Protection legislation the University of Southampton is the Data Processor; the NIHR is the Data Controller and we process personal data in accordance with their instructions. You can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: www.southampton.ac.uk/about/governance/policies/privacy-policy.page.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/ZKTW8214.
Primary conflicts of interest: Joanne Lord was a member of the NIHR Evidence Synthesis Programme Advisory Group 2017–22. She is a co-investigator on the CONFIRM trial of nivolumab for the treatment of mesothelioma (CRUK/16/022), funded by the Stand Up to Cancer campaign for Cancer Research UK (award reference no. C16728/A21400). The investigational drug for this trial was provided by Bristol Myers Squibb.
The other authors have no competing interests to declare.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Cancer Research UK . Prostate Cancer Statistics: Prostate Cancer Incidence 2017. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer#heading-Zero (accessed 17 May 2021).
- Cancer Research UK . Lifetime Risk of Prostate Cancer 2018. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/risk-factors#heading-Zero (accessed 17 May 2021).
- Cancer Research UK . Prostate Cancer Incidence Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence#heading-One (accessed 17 May 2021).
- Cancer Research UK . Prostate Cancer Risk n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/risk-factors (accessed 17 May 2021).
- Public Health England . Cancer Registration Statistics, England: Final Release, 2018: Public Health England 2020. www.gov.uk/government/statistics/cancer-registration-statistics-england-2018-final-release/cancer-registration-statistics-england-final-release-2018 (accessed 17 May 2021).
- Public Health England . Cancer Registration Statistics: Cancer Mortality in England, 2018: Public Health England 2020. www.gov.uk/government/statistics/cancer-registration-statistics-cancer-mortality-in-england-2018/cancer-registration-statistics-cancer-mortality-in-england-2018 (accessed 17 May 2021).
- National Institute for Health and Care Excellence . Suspected Cancer: Recognition and Referral 2015. www.nice.org.uk/guidance/ng12 (accessed 22 August 2024).
- National Institute for Health and Care Excellence . Prostate Cancer: Diagnosis and Management. NG131 2019. www.nice.org.uk/guidance/ng131 (accessed 22 August 2024).
- National Institute for Health and Care Excellence . Prostate Cancer: Diagnosis and Management [D] Evidence Review for Diagnosing and Identifying Clinically Significant Prostate Cancer 2019. www.nice.org.uk/guidance/ng131/evidence/d-diagnosing-and-identifying-clinically-significant-prostate-cancer-pdf-6779081777 (accessed 22 August 2024).
- Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. European Society of Urogenital Radiology . ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. https://doi.org/10.1007/s00330-011-2377-y.
- Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging - reporting and data system: 2015, Version 2. Eur Urol 2016;69:16-40. https://doi.org/10.1016/j.eururo.2015.08.052.
- Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 2019;76:340-51. https://doi.org/10.1016/j.eururo.2019.02.033.
- NHS England . Implementing a timed prostate cancer diagnostic pathway. A Handbook for Local Health and Care Systems 2018.
- Bennett HY, Roberts MJ, Doi SA, Gardiner RA. The global burden of major infectious complications following prostate biopsy. Epidemiol Infect 2016;144:1784-91. https://doi.org/10.1017/s0950268815002885.
- British Association of Urological Surgeons (BAUS) Section of Oncology . COVID-19 Strategy for the Interim Management of Prostate Cancer 2020.
- National Institute for Health and Care Excellence . Prostate Cancer: Diagnosis and Managment 2019. www.nice.org.uk/guidance/ng131/resources (accessed 2 September 2022).
- Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. PROMIS study group . Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. https://doi.org/10.1016/s0140-6736(16)32401-1.
- Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014;370:932-42. https://doi.org/10.1056/NEJMoa1311593.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Moola S, Munn Z, Tufanaru C, . Joanna Briggs Institute Reviewer’s Manual. Adelaide: The Joanna Briggs Institute; 2017.
- Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley; 2019.
- Owen RK, Bradbury N, Xin Y, Cooper N, Sutton A. MetaInsight: an interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res Synth Methods 2019;10:569-81. https://doi.org/10.1002/jrsm.1373.
- Cerruto MA, Vianello F, D’Elia C, Artibani W, Novella G, . Transrectal versus transperineal 14-core prostate biopsy in detection of prostate cancer: a comparative evaluation at the same institution. Arch Ital Urol Androl 2014;86:284-7. https://doi.org/10.4081/aiua.2014.4.284.
- Guo L-H, Wu R, Xu H-X, Xu J-M, Wu J, Wang S, et al. Comparison between ultrasound guided transperineal and transrectal prostate biopsy: a prospective, randomized and controlled trial. Sci Rep 2015;5. https://doi.org/10.1038/srep16089.
- Hara R, Jo Y, Fujii T, Kondo N, Yokoyoma T, Miyaji Y, et al. Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology 2008;71:191-5. https://doi.org/10.1016/j.urology.2007.09.029.
- Lam W, Wong AHG, Chun S, Wong T, Hung WPL, Lie H, et al. P0999 Prostate cancer detection, tolerability and safety of transperineal prostate biopsy under local-anaesthesia versus standard transrectal biopsy in biopsy-naïve men: a pragmatic, parallel group, randomized-controlled study. Eur Urol 2021;79. https://doi.org/10.1016/S0302-2838(21)01372-5.
- Takenaka A, Hara R, Ishimura T, Fujii T, Jo Y, Nagai A, et al. A prospective randomized comparison of diagnostic efficacy between transperineal and transrectal 12-core prostate biopsy. Prostate Cancer Prostatic Dis 2008;11:134-8. https://doi.org/10.1038/sj.pcan.4500985.
- Bojin Z. TPLA Biopsies ULHT [PowerPoint Slide Set; AIC]: Provided by Company 2019.
- Chen KW, Pek G, Yufei Q, Toh PC, Kuek N, Lee JKC, et al. Comparing outcomes of transperineal to transrectal prostate biopsies performed under local anaesthesia. BJUI Compass 2021;1. https://doi.org/10.1002/bco2.112.
- Chen K, Chau TW, Qiao Y, Chun CM, Tan L, Chiong E, et al. Is transperineal prostate biopsy the emerging standard? a prospective comparison on outcomes of transperineal versus transrectal prostate biopsies. J Urol 2020;203. https://doi.org/10.1097/JU.0000000000000853.01.
- Emiliozzi P, Corsetti A, Tassi B, Federico G, Martini M, Pansadoro V. Best approach for prostate cancer detection: a prospective study on transperineal versus transrectal six-core prostate biopsy. Urology 2003;61:961-6. https://doi.org/10.1016/S0090-4295(02)02551-7.
- Hung WPL, Chun S, Wong T, Tsang C, Ho B, Ng A, et al. Transrectal vs. transperineal prostate biopsy under local anaesthesia: prospective comparative analysis of cancer detection, safety and tolerability using patient-reported outcome measures at a single centre. Eur Urol Open Sci 2020;19. https://doi.org/10.1016/S2666-1683(20)34175-6.
- Kum FE, Elhage O, Maliyil J, . Outcomes of local anaesthetic freehand transperineal biopsies in the outpatient setting: how does this compare to conventional biopsy methods?. J Endourol 2018;32.
- Kum F, Elhage O, Maliyil J, Wong K, Faure Walker N, Kulkarni M, et al. Initial outcomes of local anaesthetic freehand transperineal prostate biopsies in the outpatient setting. BJU Int 2020;125:244-52. https://doi.org/10.1111/bju.14620.
- Starmer B, Iordan N, McCabe J. Comparing tolerability of local anaesthetic transperineal and transrectal prostate biopsies. J Clin Urol 2021;16:147-51. https://doi.org/10.1177/20514158211024075.
- Starmer B, Iordan NJM, . Comparison of tolerability of LA transperineal and transrectal prostate biopsies 2020.
- Watanabe M, Hayashi T, Tsushima T, Irie S, Kaneshige T, Kumon H. Extensive biopsy using a combined transperineal and transrectal approach to improve prostate cancer detection. Int J Urol Off J Jpn Urol Assoc 2005;12:959-63. https://doi.org/10.1111/j.1442-2042.2005.01186.x.
- Abdollah F, Novara G, Briganti A, Scattoni V, Raber M, Roscigno M, et al. Trans-rectal versus trans-perineal saturation rebiopsy of the prostate: is there a difference in cancer detection rate?. Urology 2011;77:921-5. https://doi.org/10.1016/j.urology.2010.08.048.
- Jiang CY, Shen PF, Wang C, Gui H-J, Ruan Y, Zeng H, et al. Comparison of diagnostic efficacy between transrectal and transperineal prostate biopsy: a propensity score-matched study. Asian J Androl 2019;21:612-7. https://doi.org/10.4103/aja.aja_16_19.
- Szabo RJ. Free-hand transperineal prostate biopsy under local anesthesia in the office without antibiotic prophylaxis: experience with 304 cases. J Endourol 2021;35:518-24. https://doi.org/10.1089/end.2020.1086.
- Lv Z, Jiang H, Hu X, Yang C, Chand H, Tang C, et al. Efficacy and safety of periprostatic nerve block combined with perineal subcutaneous anaesthesia and intrarectal lidocaine gel in transrectal ultrasound guided transperineal prostate biopsy: a prospective randomised controlled trial. Prostate Cancer Prostatic Dis 2020;23:74-80. https://doi.org/10.1038/s41391-019-0155-0.
- Takuma K, Mikio S, Masashi I, . Transperineal ultrasound-guided multiple core biopsy using template for patients with one or more previous negative biopsies: comparison with systematic 10-core biopsy. Urology 2012;80:S306-S07. https://doi.org/10.1016/S0090-4295(12)00882-5.
- Walters U, Connor MJ, Bass EJ, Eldred-Evans D, Maynard W, Sarkar P, et al. P0888: switching from sedation to local anaesthetic transperineal prostate biopsies: a cost-benefit analysis. Eur Urol 2021;79. https://doi.org/10.1016/S0302-2838(21)01262-8.
- Rij SV, Chapman D. Transperineal prostate biopsy under local anaesthetic is ready for prime time – a comparison between general anaesthetic procedure for accuracy, tolerability and complications. BJU Int 2020;125:87-.
- Gnanapragasam VJ, Leonard K, Sut M, Ilie C, Ord J, Roux J, et al. Multicentre clinical evaluation of the safety and performance of a simple transperineal access system for prostate biopsies for suspected prostate cancer: the CAMbridge PROstate Biopsy DevicE (CamPROBE) study. J Clin Urol 2020;13:364-70. https://doi.org/10.1177/2051415820932773.
- Lau YZ, Henderson A, Russell G, Ghosh S, Naji M, Goel A, et al. Short term outcomes of zero-antibiotic co-axial needle-guided transperineal prostate biopsy under local anaesthesia (CTB): a prospective study. Eur Urol Open Sci 2020;19. https://doi.org/10.1016/S2666-1683(20)34168-9.
- Yamamoto H, Goshi S, Naji M, . Safety of zero-antibiotic co-axial needleguided transperineal prostate biopsy (TPB) under local anaesthesia: a prospective cohort study of 200 patients. J Clin Urol 2019;12.
- Yamamoto H, Lau YZ, Russell G, . Office-based transperineal prostate biopsies under local anaesthesia with cognitive registration: technique, immediate patient perception and cancer detection – a prospective study. Eur Urol Open Sci 2020;19.
- The Joanna Briggs Institute . Checklist for Cohort Studies (The Joanna Briggs Institute Critical Appraisal Tools for Use in JBI Systematic Reviews). 2017. https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Cohort_Studies2017_0.pdf (accessed 10 November 2021).
- The Joanna Briggs Institute . Checklist for Case Series (The Joanna Briggs Institute Critical Appraisal Tools for Use in JBI Systematic Reviews). 2017. https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Case_Series2017_0.pdf (accessed 10 November 2021).
- ISRCTN . A Study That Aims to Find Out in the NHS What Is the Best Way to Take a Prostate Biopsy to Determine If a Man Has Prostate Cancer (the TRANSLATE Trial) 2021. www.isrctn.com/ISRCTN98159689.
- Oxford Clinical Trials Research Unit . TRANSLATE Trial Website Oxford: Oxford Clinical Trials Research Unit 2021. https://translate.octru.ox.ac.uk/welcome-translate-trial (accessed 27 October 2021).
- Bryant RJ, Lamb AD, Campbell T, . Oxford: The University of Oxford and Oxford University Hospitals NHS Foundation Trust; 2021.
- Albany Medical College. Prostate Biopsy, Transrectal vs. Transperineal: Efficacy and Complications. 2021.
- NCT . Evaluation of Transperineal Biopsy under Local Anesthesia 2021. https://clinicaltrials.gov/show/NCT04843566 (accessed 22 August 2024).
- NCT . Randomized Trial Comparing Transperineal Vs. Transrectal MRI-Targeted Prostate Biopsy 2021. https://clinicaltrials.gov/study/NCT04815876 (accessed 22 August 2024).
- ANZCTR . Transperineal Prostate Biopsy Under Local Anaesthetic Compared to General Anaesthetic for Men With Suspected Prostate Cancer 2020. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379787&isReview=true (accessed 10 June 2021).
- National Institute for Health and Care Excellence . Transperineal Biopsy for Diagnosing Prostate Cancer; Project Documents 2022. www.nice.org.uk/guidance/indevelopment/gid-dg10043/documents (accessed 14 August 2022).
- Strings Attached: CADTH Database Search Filters. 2021. www.cadth.ca/strings-attached-cadths-database-search-filters (accessed 17 June 2021).
- Caro JJ, Eddy DM, Kan H, Kaltz C, Patel B, Eldessouki R, et al. Questionnaire to assess relevance and credibility of modeling studies for informing health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health 2014;17:174-82. https://doi/org/10.1016/j.jval.2014.01.003.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8:iiiix1-ivxi158. https://doi/org/10.3310/hta8360.
- Wilson ECF, Wreford A, Tamer P, Leonard K, Brechka H, Gnanapragasam VJ. Economic evaluation of transperineal versus transrectal devices for local anaesthetic prostate biopsies. PharmacoEcon Open 2021;5:737-53. https://doi/org/10.1007/s41669-021-00277-4.
- Brown LC, Ahmed HU, Faria R, El-Shater Bosaily A, Gabe R, Kaplan RS, et al. Multiparametric MRI to improve detection of prostate cancer compared with transrectal ultrasound-guided prostate biopsy alone: the PROMIS study. Health Technol Assess 2018;22:1-176. https://doi/org/10.3310/hta22390.
- Faria R, Soares MO, Spackman E, Ahmed HU, Brown LC, Kaplan R, et al. Optimising the diagnosis of prostate cancer in the era of multiparametric magnetic resonance imaging: a cost-effectiveness analysis based on the Prostate MR Imaging Study (PROMIS). Eur Urol 2018;73:23-30. https://doi/org/10.1016/j.eururo.2017.08.018.
- Zani EL, Clark OA, Rodrigues Netto N. Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database Syst Rev 2011 2011. https://doi/org/10.1002/14651858.CD006576.pub2.
- National Institute for Health and Care Excellence . Prostate Cancer: Diagnosis and Management 2019.
- National Institute for Health and Care Excellence (NICE) . Diagnostics Assessment Programme Manual 2011.
- Blazevski A, Scheltema MJ, Yuen B, Masand N, Nguyen TV, Delprado W, et al. Oncological and quality-of-life outcomes following focal irreversible electroporation as primary treatment for localised prostate cancer: a biopsy-monitored prospective cohort. Eur Urol Onco 2020;3:283-90. https://doi/org/10.1016/j.euo.2019.04.008.
- Essink-Bot ML, de Koning HJ, Nijs HG, Kirkels WJ, van der Maas PJ, Schröder FH. Short-term effects of population-based screening for prostate cancer on health-related quality of life. J Natl Cancer Inst 1998;90:925-31. https://doi/org/10.1093/jnci/90.12.925.
- Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. Active monitoring, radical prostatectomy and radical radiotherapy in PSA-detected clinically localised prostate cancer: the ProtecT three-arm RCT. Health Technol Assess 2020;24:1-176.
- Hamid S, Donaldson IA, Hu Y, Rodell R, Villarini B, Bonmati E, et al. The SmartTarget biopsy trial: a prospective, within-person randomised, blinded trial comparing the accuracy of visual-registration and magnetic resonance imaging/ultrasound image-fusion targeted biopsies for prostate cancer risk stratification. Eur Urol 2019;75:733-40. https://doi/org/10.1016/j.eururo.2018.08.007.
- Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378:1767-77. https://doi/org/10.1056/NEJMoa1801993.
- Peters M, Maenhout M, Van Der Voort Van Zyp JRN, . Focal salvage iodine-125 brachytherapy for prostate cancer recurrences after primary radiotherapy: a retrospective study regarding toxicity biochemical outcome and quality of life. Radiother Oncol 2014;112:77-82. https://doi/org/10.1016/j.radonc.2014.06.013.
- Sefik E, Eker A, Gunlusoy B, Celik S, Bozkurt IH, Basmaci I, et al. The effect of alpha blocker treatment prior to prostate biopsy on voiding functions, pain scores and health-related quality-of-life outcomes: a prospective randomized trial. Prog Urol 2020;30:198-204. https://doi/org/10.1016/j.purol.2019.12.006.
- Shankar PR, Maturen KE, George AK, Borza T, Ellimoottil C, Montgomery JS, et al. Temporary health impact of prostate MRI and transrectal prostate biopsy in active surveillance prostate cancer patients. J Am Coll Radiol 2019;16:1385-92. https://doi/org/10.1016/j.jacr.2018.11.031.
- Vasarainen H, Malmi H, Määttänen L, Ruutu M, Tammela T, Taari K, et al. Effects of prostate cancer screening on health-related quality of life: results of the Finnish arm of the European randomized screening trial (ERSPC). Acta Oncol 2013;52:1615-21. https://doi/org/10.3109/0284186X.2013.802837.
- Booth N, Rissanen P, Tammela TL, Määttänen L, Taari K, Auvinen A. Health-related quality of life in the Finnish trial of screening for prostate cancer. Eur Urol 2014;65:39-47. https://doi/org/10.1016/j.eururo.2012.11.041.
- Drummond FJ, Kinnear H, Donnelly C, O’Leary E, O’Brien K, Burns RM, et al. Establishing a population-based patient-reported outcomes study (PROMs) using national cancer registries across two jurisdictions: the Prostate Cancer Treatment, your experience (PiCTure) study. BMJ Open 2015;5. https://doi/org/10.1136/bmjopen-2014-006851.
- Farkkila N, Torvinen S, Roine RP, Sintonen H, Hänninen J, Taari K, et al. Health-related quality of life among breast, prostate, and colorectal cancer patients with end-stage disease. Qual Life Res 2014;23:1387-94. https://doi/org/10.1007/s11136-013-0562-y.
- Gavin AT, Donnelly D, Donnelly C, Drummond FJ, Morgan E, Gormley GJ, et al. Effect of investigation intensity and treatment differences on prostate cancer survivor’s physical symptoms, psychological well-being and health-related quality of life: a two country cross-sectional study. BMJ Open 2016;6. https://doi/org/10.1136/bmjopen-2016-012952.
- Torvinen S, Färkkilä N, Sintonen H, Saarto T, Roine RP, Taari K. Health-related quality of life in prostate cancer. Acta Oncol 2013;52:1094-101. https://doi/org/10.3109/0284186x.2012.760848.
- Watson E, Shinkins B, Frith E, Neal D, Hamdy F, Walter F, et al. Symptoms, unmet needs, psychological well-being and health status in survivors of prostate cancer: implications for redesigning follow-up. BJU Int 2016;117:E10-19. https://doi/org/10.1111/bju.13122.
- Ramsay CR, Adewuyi TE, Gray J, Hislop J, Shirley MD, Jayakody S, et al. Ablative therapy for people with localised prostate cancer: a systematic review and economic evaluation. Health Technol Assess 2015;19:1-490. https://doi/org/10.3310/hta19490.
- Marenco Jimenez JL, Claps F, Ramón-Borja JC, Mascarós Martinez JM, Gutierrez AW, Lozano AGF, et al. Rebiopsy rate after transperineal or transrectal prostate biopsy. Prostate Int 2021;9:78-81. https://doi/org/10.1016/j.prnil.2020.10.001.
- National Institute for Health and Care Excellence . Apalutamide With Androgen Deprivation Therapy for Treating Hormone-Sensitive Metastatic Prostate Cancer [TA741] 2021. www.nice.org.uk/guidance/ta741 (accessed September 2021).
- National Institute for Health and Care Excellence . Enzalutamide for Treating Hormone-Sensitive Metastatic Prostate Cancer Technology Appraisal Guidance [TA712] 2021. www.nice.org.uk/guidance/ta712 (accessed September 2021).
- National Institute for Health and Care Excellence . Apalutamide With Androgen Deprivation Therapy for Treating High-Risk Hormone-Relapsed Non-Metastatic Prostate Cancer [TA740] 2021. www.nice.org.uk/guidance/ta740 (accessed September 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: Personal Social Services Research Unit, University of Kent; 2020.
- NHS England . 2019/20/National/Cost/Collection/Data/Publication 2019. https://www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication (accessed August 2024).
- Kirby RS, Roehrborn C, Boyle P, Bartsch G, Jardin A, Cary MM, et al. Prospective European Doxazosin and Combination Therapy Study Investigators . Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology 2003;61:119-26. https://doi/org/10.1016/s0090-4295(02)02114-3.
- The Royal College of Surgeons of England . Results of the NPCA Prospective Audit in England and Wales for Men Diagnosed from 1 April 2018 to 31 March 2019 2021.
- Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MGM. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68:438-50. https://doi/org/10.1016/j.eururo.2014.11.037.
- Tamhankar AS, El-Taji O, Vasdev N, Foley C, Popert R, Adshead J. The clinical and financial implications of a decade of prostate biopsies in the NHS: analysis of Hospital Episode Statistics data 2008–2019. BJU Int 2020;126:133-41. https://doi/org/10.1111/bju.15062.
- Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, Goodwin L, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 2012;344. https://doi/org/10.1136/bmj.d7894.
- Pepe P, Aragona F. Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology 2013;81:1142-6. https://doi/org/10.1016/j.urology.2013.02.019.
- Berry B, Parry MG, Sujenthiran A, Nossiter J, Cowling TE, Aggarwal A, et al. Comparison of complications after transrectal and transperineal prostate biopsy: a national population-based study. BJU Int 2020;126:97-103. https://doi/org/10.1111/bju.15039.
- Gnanapragasam VJ, Lophatananon A, Wright KA, Muir KR, Gavin A, Greenberg DC. Improving clinical risk stratification at diagnosis in primary prostate cancer: a prognostic modelling study. PLOS Med 2016;13. https://doi/org/10.1371/journal.pmed.1002063.
- Mowatt G, Scotland G, Boachie C, Cruickshank M, Ford JA, Fraser C, et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: a systematic review and economic evaluation. Health Technol Assess 2013;17:vii1-xix281. https://doi/org/10.3310/hta17200.
- Joint Formulary Committee . British National Formulary (October 2021) 2021.
- Department of Health and Social Care . Drugs and Pharmaceutical Electronic Market Information Tool (eMIT) 2020. www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit (accessed October 2021).
- Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425-37. https://doi/org/10.1056/NEJMoa1606221.
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. STAMPEDE Investigators . Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387:1163-77. https://doi/org/10.1016/s0140-6736(15)01037-5.
- Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019;381:13-24. https://doi/org/10.1056/NEJMoa1903307.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 2019;37:2974-86. https://doi/org/10.1200/jco.19.00799.
- Round J, Jones L, Morris S. Estimating the cost of caring for people with cancer at the end of life: a modelling study. Palliat Med 2015;29:899-907. https://doi/org/10.1177/0269216315595203.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi/org/10.1111/j.1524-4733.2010.00700.x.
- Lee K, Drekonja DM, Enns EA. Cost-effectiveness of antibiotic prophylaxis strategies for transrectal prostate biopsy in an era of increasing antimicrobial resistance. Value Health 2018;21:310-7. https://doi/org/10.1016/j.jval.2017.08.3016.
- Barry HC, Ebell MH, Hickner J. Evaluation of suspected urinary tract infection in ambulatory women: a cost–utility analysis of office-based strategies. J Fam Pract 1997;44:49-60.
- Drabinski A, Williams G, Formica C. Observational evaluation of health state utilities among a cohort of sepsis patients. Value Health 2001;4:128-9. https://doi/org/10.1046/j.1524-4733.2001.40202-165.x.
- Lopez JF, Campbell A, Omer A, Stroman L, Bondad J, Austin T, et al. Local anaesthetic transperineal (LATP) prostate biopsy using a probe-mounted transperineal access system: a multicentre prospective outcome analysis. BJU Int 2021;128:311-8. https://doi/org/10.1111/bju.15337.
- University of Surrey . Diagnostic Histopathology Pricing n.d. www.surrey.ac.uk/sites/default/files/Diagnostic-Histopathology-Prices.pdf (accessed 17 May 2021).
- Jimenez JLM, Claps F, Ramon-Borja JC, Mascarós Martinez JM, Gutierrez AW, Lozano AGF, et al. Rebiopsy rate after transperineal or transrectal prostate biopsy. Prostate Int 2021;9:78-81. https://doi/org/10.1016/j.prnil.2020.10.001.
- Berry B, Parry M, Sujenthiran A, Nossiter J, Cowling TE, Aggarwal A, et al. Comparison of complications after transrectal and transperineal prostate biopsy: a national population-based study. Eur Urol Open Sci 2020;19. https://doi/org/10.1016/S2666-1683(20)34171-9.
- Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010;362:1192-202. https://doi/org/10.1056/NEJMoa0908127.
- Adshead J, Simpson N, El-Taji O, . P12-1 Readmissions, sepsis and costs of 200,000 NHS prostate biopsies-interrogation of HES data for TRUS vs TP biopsy between 2012–2018. J Clin Urol 2019;12:77-8.
- Chae Y, Kim Y-J, Kim T, Yun SJ, Lee S-C, Kim W-J. The comparison between transperineal and transrectal ultrasound-guided prostate needle biopsy. Korean J Uro 2009;50:119-24. https://doi/org/10.4111/kju.2009.50.2.119.
- Eldred-Evans D, Brittain J, Servian P, Miah S, Tam H, Ahmed H, et al. The role of multi-parametric MRI as a triage test: a propensity-matched comparison of a MRI-triage and a TRUS-biopsy pathway. Eur Urol Suppl 2018;17:e695-96. https://doi/org/10.1016/S1569-9056(18)31319-8.
- Han KS, Lee KH. Korean Urologic Oncology Society Prostate Cancer Study Group . Factors influencing pain during transrectal ultrasonography-guided prostate biopsy. Prostate Cancer Prostatic Dis 2008;11:139-42. https://doi.org/10.1038/sj.pcan.4501004.
- Israël B, Immerzeel JJFM, Van Der Leest MMG, Hannink GJ, Zámecnik P, Bomers JGR, et al. P0943 – transrectal and transperineal MRI-guided prostate cancer pathways in biopsy-naïve men. Eur Urol 2021;79:S1327-8. https://doi/org/10.1016/S0302-2838(21)01316-6.
- Kasivisvanathan V, Arya M, Ahmed HU, Ahmed HU, Moore CM, Emberton M. A randomized controlled trial to investigate magnetic resonance imaging-targeted biopsy as an alternative diagnostic strategy to transrectal ultrasound-guided prostate biopsy in the diagnosis of prostate cancer. Urol Oncolo Semin Orig Investig 2015;33:156-7. https://doi/org/10.1016/j.urolonc.2014.12.003.
- Kawakami S, Yamamoto S, Numao N, Ishikawa Y, Kihara K, Fukui I. Direct comparison between transrectal and transperineal extended prostate biopsy for the detection of cancer. Int Jo Urol Off J Jpn Urol Assoc 2007;14:719-24. https://doi/org/10.1111/j.1442-2042.2007.01810.x.
- Lavoipierre AM. Transrectal ultrasound and prostate-specific antigen in prostate cancer. J Med Imaging Radiat Oncol 2008;52. https://doi/org/10.1111/j.1440-1673.2008.02009.x.
- Lim LY, Tan GH, Zainuddin ZM, . Prospective evaluation of using multi-parametric MRI in cognitive fusion prostate biopsy compared to standard systematic 12-core biopsy in the detection of prostate cancer. BJU Int 2018;122.
- Lim LY, Tan GH, Zainuddin ZM, Fam XI, Goh EH, Syaris OS, et al. Prospective evaluation of using multiparametric magnetic resonance imaging in cognitive fusion prostate biopsy compared to the standard systematic 12-core biopsy in the detection of prostate cancer. Urol Ann 2020;12:276-82. https://doi/org/10.4103/UA.UA_98_19.
- Lo KL, Chui KL, Leung CH, LIM K, Ng T, Wong J, et al. Outcomes of transperineal and transrectal ultrasound-guided prostate biopsy. Hong Kong Med J 2019;25:209-15. https://doi/org/10.12809/hkmj187599.
- NCT . Perineal Versus Rectal Approach for Prostate Biopsy to Prevent Iatrogenic Infections 2018. https://clinicaltrials.gov/study/NCT03496142 (accessed 22 August 2024).
- NCT . Whether Transperineal Prostate Biopsy Under Local-Anaesthesia Using a Transperineal-Access System Is Non-Inferior to Standard Transrectal Biopsy to Detect Prostate Cancer in Biopsy-naïve Men 2019. https://clinicaltrials.gov/study/NCT04108871 (accessed 22 August 2024).
- Neale A, Stroman L, Kum F, Jabarkhyl D, Di Benedetto A, Mehan N, et al. Targeted and systematic cognitive freehand-guided transperineal biopsy: is there still a role for systematic biopsy?. BJU Int 2020;126:280-5. https://doi/org/10.1111/bju.15092.
- Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR Imaging-guided strategies for detection of prostate cancer in biopsy-naïve men. Radiology 2017;285:157-66. https://doi/org/10.1148/radiol.2017162181.
- Pal RP, Ahmad R, Trecartan S, Voss J, Ahmed S, Bazo A, et al. A single center evaluation of the diagnostic accuracy of multiparametric magnetic resonance imaging against transperineal prostate mapping biopsy: an analysis of men with benign histology and insignificant cancer following transrectal ultrasound biopsy. J Urol 2018;200:302-8. https://doi/org/10.1016/j.juro.2018.02.072.
- Pepe P, Garufi A, Priolo GD, Pennisi M. Multiparametric MRI/TRUS fusion prostate biopsy: advantages of a transperineal approach. Anticancer Res 2017;37:3291-4.
- Postema AW, Scheltema MJV, Mannaerts CK, Van Sloun RJG, Idzenga T, Mischi M, et al. The prostate cancer detection rates of CEUS-targeted versus MRI-targeted versus systematic TRUS-guided biopsies in biopsy-naïve men: a prospective, comparative clinical trial using the same patients. BMC Urol 2017;17:1-8. https://doi/org/10.1186/s12894-017-0213-7.
- Presti JC, Chang JJ, Bhargava V, Shinohara K. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol 2000;163:163-7. https://doi/org/10.1016/S0022-5347(05)67995-5.
- Ristau BT, Allaway M, Cendo D, Hart J, Riley J, Parousis V, et al. Free-hand transperineal prostate biopsy provides acceptable cancer detection and minimizes risk of infection: evolving experience with a 10-sector template. Urol Oncol Semin Orig Investig 2018;36:528.e15-20. https://doi/org/10.1016/j.urolonc.2018.09.013.
- Roberts M, Macdonald A, Teloken P, . Morbidity and cost implications of shifting from routine transrectal ultrasound-guided prostate biopsy to mpMRI triage with selective transperineal prostate biopsy: a 12-year retrospective analysis from a tertiary referral centre. BJU Int 2020;125.
- Roberts MJ, Macdonald A, Ranasinghe S, Bennett H, Teloken PE, Harris P, et al. Transrectal versus transperineal prostate biopsy under intravenous anaesthesia: a clinical, microbiological and cost analysis of 2048 cases over 11 years at a tertiary institution. Prostate Cancer Prostatic Dis 2021;24:169-76. https://doi/org/10.1038/s41391-020-0263-x.
- Rochester MA, Griffin S, Chappell B, McLoughlin J. A prospective randomised trial of extended core prostate biopsy protocols utilizing 12 versus 15 cores. Urol Int 2009;83:155-9. https://doi/org/10.1159/000230016.
- Rodriguez Socarras ME, Gomez Rivas J, Cuadros Rivera V, . Prostate mapping for cancer diagnosis: the Madrid Protocol. Transperineal prostate biopsies using multiparametric magnetic resonance imaging fusion and micro-ultrasound guided biopsies. J Urol 2020;204:726-33. https://doi/org/10.1097/JU.0000000000001083.
- Roethke MC, Kuru TH, Schultze S, Tichy D, Kopp-Schneider A, Fenchel M, et al. Evaluation of the ESUR PI-RADS scoring system for multiparametric MRI of the prostate with targeted MR/TRUS fusion-guided biopsy at 3.0 tesla. Eur Radiol 2014;24:344-52. https://doi.org/10/1007/s00330-013-3017-5.
- Rojas Claros O, Muttin F, Barbosa RRT, Gallardo AC, Barret E, Cathala N, et al. Comparison of cancer detection rates in micro-ultrasound biopsies versus robotic ultrasound-magnetic resonance imaging fusion biopsies for prostate cancer. Eur Urol Suppl 2019;18. https://doi/org/10.1016/S1569-9056(19)34657-3.
- Salagierski M, Kania P, Wierzcholowski W, Poźniak-Balicka R. The role of a template-assisted cognitive transperineal prostate biopsy technique in patients with benign transrectal prostate biopsies: a preliminary experience. Cent Eur J Urol 2019;72:15-8. https://doi/org/10.5173/ceju.2018.1840.
- Satoh T, Matsumoto K, Fujita T, Tabata K-I, Okusa H, Tsuboi T, et al. Cancer core distribution in patients diagnosed by extended transperineal prostate biopsy. Urology 2005;66:114-8. https://doi/org/10.1016/j.urology.2005.01.051.
- Self D, Kam J, Louie-Johnsun M, . Clinical impact of magnetic resonance imaging (MRI)-guided biopsy on prostate cancer treatment: a single surgeon experience of cognitive-guided and MRI-ultrasound fusion biopsy. Can Urol Assoc J 2018;12.
- Shigemura K, Arakawa S, Yamanaka K, Kataoka N, Yuien K, Fujisawa M. Significance of lateral biopsy specimens during transrectal ultrasound-guided prostate biopsies in Japanese men. Int J Urol 2007;14:935-8. https://doi/org/10.1111/j.1442-2042.2007.01865.x.
- Sivaraman A, Sanchez-Salas R, Ahmed HU, Barret E, Cathala N, Mombet A, et al. Clinical utility of transperineal template-guided mapping biopsy of the prostate after negative magnetic resonance imaging-guided transrectal biopsy. Urol Oncol 2015;33:329.e7-11. https://doi/org/10.1016/j.urolonc.2015.04.005.
- Song W, Kang M, Jeong BC, Seo SI, Jeon SS, Lee HM, et al. The clinical utility of transperineal template-guided saturation prostate biopsy for risk stratification after transrectal ultrasound-guided biopsy. Investig Clin Urol 2019;60:454-62. https://doi/org/10.4111/icu.2019.60.6.454.
- Stabile A, Dell’Oglio P, Gandaglia G, Fossati N, Brembilla G, Cristel G, et al. Not all multiparametric magnetic resonance imaging-targeted bopsies are equal: the impact of the type of approach and operator expertise on the detection of clinically significant prostate cancer. Eur Urol Oncol 2018;1:120-8. https://doi/org/10.1016/j.euo.2018.02.002.
- Suga K, Akakura K, Ueda T, Furuya Y, Akimoto S, Ito H. Association between clinical stage of prostate cancer and pathological findings of random systematic transperineal core biopsy under transrectal ultrasonography. Int J Clin Oncol 1999;4:353-7. https://doi/org/10.1007/s101470050084.
- Sulaiman SK, Margaret S, Bob Y, . Is our local multi-parametric MRI reliable enough to pave the future for targeted image guided biopsies?. Br J Surg 2019;106.
- Taira AV, Merrick GS, Galbreath RW, Andreini H, Taubenslag W, Curtis R, et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis 2010;13:71-7. https://doi/org/10.1038/pcan.2009.42.
- Tamhankar A, El Taji O, Vasdev N, . P9-7 The clinical and financial implications of a decade of prostate biopsies in the NHS – interrogation of the Hospital Episode Statistics (HES) Data 2008–2019. J Clin Urol 2020;13.
- Taverna G, Bozzini G, Grizzi F, Seveso M, Mandressi A, Balzarini L, et al. Endorectal multiparametric 3-tesla magnetic resonance imaging associated with systematic cognitive biopsies does not increase prostate cancer detection rate: a randomized prospective trial. World J Urol 2016;34:797-803. https://doi/org/10.1007/s00345-015-1711-4.
- Taverna G, Bozzini G, Seveso M, de Francesco O, Bono P, Provenzano M, et al. Endorectal multiparametric 3-tesla magnetic resonance imaging associated to systematic cognitive biopsies do not increase prostate cancer detection rate: a randomized prospective trial. J Urol 2016;195. https://doi/org/10.1016/j.juro.2016.02.654.
- Teoh JYC, Yuen SKK, Tsu JHL, Wong CK, Ho BS, Ng AT, et al. Prostate cancer detection upon transrectal ultrasound-guided biopsy in relation to digital rectal examination and prostate-specific antigen level: what to expect in the Chinese population?. Asian J Androl 2015;17:821-5. https://doi/org/10.4103/1008-682X.144945.
- Tilak G, Tuncali K, Song SE, Tokuda J, Olubiyi O, Fennessy F, et al. 3T MR-guided in-bore transperineal prostate biopsy: a comparison of robotic and manual needle-guidance templates. J Magn Reson Imaging 2015;42:63-71. https://doi/org/10.1002/jmri.24770.
- Tschirdewahn S, Wiesenfarth M, Bonekamp D, Püllen L, Reis H, Panic A, et al. Detection of significant prostate cancer using target saturation in transperineal magnetic resonance imaging/transrectal ultrasonography-fusion biopsy. Eur Urol Focus 2021;7:1300-7. https://doi/org/10.1016/j.euf.2020.06.020.
- Valerio M, McCartan N, Freeman A, Punwani S, Emberton M, Ahmed HU. Visually directed vs. software-based targeted biopsy compared to transperineal template mapping biopsy in the detection of clinically significant prostate cancer. Urol Oncol Semin Orig Investig 2015;33:424.e9-16. https://doi/org/10.1016/j.urolonc.2015.06.012.
- Vanni AP, Schaal CH, Costa RP, Sala FC. Is the periprostatic anesthetic blockade advantageous in ultrasound-guided prostate biopsy?. Int Braz J Urol 2004;30:114-8. https://doi/org/10.1590/s1677-55382004000200005.
- Vezelis A, Platkevicius G, Kincius M, Gumbys L, Naruševičiūtė I, Briedienė R, et al. Systematic and MRI-cognitive targeted transperineal prostate biopsy accuracy in detecting clinically significant prostate cancer after previous negative biopsy and persisting suspicion of malignancy. Medicina 2021;57. https://doi/org/10.3390/medicina57010057.
- Wang L, Wang XF, Zhao WF, Zhao Z, Li Z, Fei S, et al. Surface-projection-based transperineal cognitive fusion targeted biopsy of the prostate: an original technique with a good cancer detection rate. BMC Urol 2019;19:1-7. https://doi/org/10.1186/s12894-019-0535-8.
- Westhoff N, Baessler B, von Hardenberg J, Hetjens S, Porubsky S, Siegel F, et al. Systematic prostate biopsy still matters: a comprehensive analysis of MRI/TRUS-fusion targeted prostate biopsies across different indications. Urol Oncol Semin Orig Investig 2019;37:678-87. https://doi/org/10.1016/j.urolonc.2019.07.004.
- Williams M, Ranasinghe SV, Donato P, . A retrospective analysis of transperineal cognitive fusion biopsy of high grade multi-parametric MRI lesions (PIRADS 4-5) in helping to detect clinically significant prostate cancer lesions. Asia-Pac J Clin Oncol 2018;14.
- Williams M, Ranasinghe SV, Donato P, . Multi-parametric magnetic resonance imaging (MPMRI) in detecting prostate cancer: an initial experience in a tertiary centre. Asia-Pac J Clin Oncol 2018;14.
- Yamada Y, Shiraishi T, Ueno A, Ueda T, Fujihara A, Naitoh Y, et al. Magnetic resonance imaging-guided targeted prostate biopsy: comparison between computer-software-based fusion versus cognitive fusion technique in biopsy-naïve patients. Int J Urol 2020;27:67-71. https://doi/org/10.1111/iju.14127.
- Yang B, Sulaiman S, Suhail N, . Introducing PrecisionPointTM transperineal biopsy into a district general hospital: a viable alternative to TRUS and templates?. Br J Surg 2019;106.
- Yaxley AJ, Yaxley JW, Thangasamy IA, Ballard E, Pokorny MR. Comparison between target magnetic resonance imaging (MRI) in-gantry and cognitively directed transperineal or transrectal-guided prostate biopsies for Prostate Imaging-Reporting and Data System (PI-RADS) 3-5 MRI lesions. BJU Int 2017;120:43-50. https://doi/org/10.1111/bju.13971.
- Yunkai Z, Yaqing C, Ren W, Yongchang Z. Are transition zone biopsies necessary in transrectal ultrasound-guided transperineal prostate biopsy protocol? Results of a Chinese population-based study. Clin Imaging 2010;34:43-6. https://doi/org/10.1016/j.clinimag.2009.02.003.
- Zhang K, Zhang Z, Liu M, Zhu G, Roobol MJ. Comparison of clinically significant prostate cancer detection by MRI cognitive biopsy and in-bore MRI-targeted biopsy for naïve biopsy patients. Transl Androl Urol 2020;9:243-9. https://doi/org/10.21037/tau.2020.02.20.
- Zhang K, Zhang Z, Zhu G, . Comparison of clinically significant prostate cancer detection by MRI cognitive biopsy and in-bore MRI-targeted biopsy for naïve biopsy patients. Int J Urol 2019;26.
- Zhang Q, Wang W, Zhang B, Shi J, Fu Y, Li D, et al. Comparison of free-hand transperineal mpMRI/TRUS fusion-guided biopsy with transperineal 12-core systematic biopsy for the diagnosis of prostate cancer: a single-center prospective study in China. Int Urol Nephrol 2017;49:439-48. https://doi/org/10.1007/s11255-016-1484-8.
- Zhang Q, Wang W, Yang R, Zhang G, Zhang B, Li W, et al. Free-hand transperineal targeted prostate biopsy with real-time fusion imaging of multiparametric magnetic resonance imaging and transrectal ultrasound: single-center experience in China. Int Urol Nephrol 2015;47:727-33. https://doi/org/10.1007/s11255-015-0957-5.
- Zhao HX, Zhu Q, Wang Z. Detection of prostate cancer with three-dimensional transrectal ultrasound: correlation with biopsy results. Br J Radiol 2012;85:714-9. https://doi/org/10.1259/bjr/68418881.
- Zhou SR, Chang E, Patankar A, Huang J, Marks LS, Natarajan S. Prostate cancer detection rate of freehand versus 3-dimensional template mapping biopsy using a magnetic resonance imaging-ultrasound fusion device in biopsy naïve men. J Urol 2020;203:699-705. https://doi/org/10.1097/JU.0000000000000587.
- Al-Dhahir W, Sharma A, Subin F, . Freehand transperineal ultrasound guided biopsy of the prostate under local anaesthetic: a cost effective and viable diagnostic procedure for prostate cancer?. Br J Surg 2019;106.
- Chan CK, Leung CH, Liu YZ, Ling L, Lo KL, Li KM, et al. PT432 - Road to Trexit: a territory-wide study on the predictors of 30-day complications in transrectal versus transperineal prostate biopsy. Eur Urol Open Sci 2020;19. https://doi/org/10.1016/S2666-1683(20)34166-5.
- Chan CK, Leung C-H, Liu Y-Z, Ling L, Lo K-L, Li K-M, et al. MP75-07 A territory-wide study on the predictors of 30-day complications in transrectal versus transperineal prostate biopsy. J Urol 2020;203:e1144-44. https://doi/org/10.1097/JU.0000000000000961.07.
- Cole A, Lee M, Raisky J, Qi J, Linsell S, Martin R, et al. MI trexit: pilot evaluation of a state-wide initiative for in-office transperineal versus transrectal prostate biopsy under local anesthesia. J Urol 2019;201. https://doi/org/10.1097/01.JU.0000555492.74947.ad.
- Cole A, QI J, Walker C, Wu R, Linsell S, Johnson A, et al. MP58-18 Transrectal MRI/US fusion biopsy doubles the risk of infectious hospitalization – analysis of a statewide collaboratives. J Urol 2020;203. https://doi/org/10.1097/JU.0000000000000927.018.
- Demozzi E, Cavicchioli F, Molinari A, Carbognin G, Cavalleri S. P211 – Detection of prostate cancer: Comparison between ‘In-bore’ MRI-guided biopsies and cognitive transperineal-ultrasound-guided biopsies. Eur Urol Suppl 2018;17:311-2. https://doi/org/10.1016/S1569-9056(18)33305-0.
- Di Franco CA, Jallous H, Porru D, Giliberto GL, Cebrelli T, Tinelli C, et al. A retrospective comparison between transrectal and transperineal prostate biopsy in the detection of prostate cancer. Arch Ital Urol Androl 2017;89:55-9. https://doi/org/10.4081/aiua.2017.1.55.
- Elkhoury J, Jiao C, Navani R, Lin Y, Johns-Putra L, Dodds L, et al. PT436: Transperineal template biopsy is both safer and more cost-effective than transrectal ultrasound-guided prostate biopsy in the Australian public hospital setting. Eur Urol Open Sci 2020;19. https://doi/org/10.1016/S2666-1683(20)34170-7.
- Ferrante S, Hawken S, Montie J, Miller D, Wu RC, Ellimoottil C, et al. MP20-03 Patient experience for transperineal biopsy under local anesthesia versus transrectal biopsy: results from the Michigan Urological Surgery Improvement Collaborative. J Urol 2020;203:e307-8. https://doi/org/10.1097/JU.0000000000000853.03.
- Ferriero MC, Flammia RS, Oderda M, Forte V, Peltier A, Kumar P, et al. MP36-02 Periprocedural and diagnostic outcomes of transrectal versus transperineal US/MRI guided fusion prostate biopsy: multi-institutional propensity score matched pair analysis. J Urol 2019;201:e514-15. https://doi/org/10.1097/01.JU.0000556012.63289.68.
- Islam M, Silva RDD, Gustafson D, Nogueira L, Clark N, Kim F. MP20-06 A comparison of infectious outcomes between in-office transperineal prostate biopsies without antibiotic prophylaxis and transrectal prostate biopsies. J Urol 2020;203. https://doi/org/10.1097/JU.0000000000000853.06.
- Islam M, Quach A, Kim F, Da Silva RD. V14-09 Infectious outcomes between in-office transperineal prostate biopsies without antibiotic prophylaxis and transrectal prostate biopsies. J Urol 2021;206. https://doi/org/10.1097/JU.0000000000002111.09.
- Lai J, Auffenberg G, Hudnall M, Pham M, Schaeffer E, Ghomrawi H, et al. MP17-14 Cost-effectiveness of transperineal versus transrectal prostate biopsy for the diagnosis of prostate cancer. J Urol 2021;206. https://doi/org/10.1097/JU.0000000000002002.14.
- Lovegrove C, Brown L, Miah S, . P5-4 Comparison of TRUS-biopsy to transperineal template mapping biopsies stratified by MRI score within the PROMIS trial. J Clin Urol 2019;12.
- Lovegrove C, Brown L, Miah S, El-Shater Bosaily A, Kaplan R, Freeman A, et al. MP30-08 Comparison of TRUS-biopsy to transperineal template mapping biopsies stratified by MRI score within the PROMIS trial. J Urol 2019;201:e425-6. https://doi/org/10.1097/01.JU.0000557563.97737.fd.
- Marra G, Eldred-Evans D, Van Hemelrijck M, Challacombe B, Cahill D, Polson A, et al. Concordance of transperineal, transrectal and transperineal sector-based template approaches with 305 radical prostatectomies: a multicentre study. BJU International Conference: 71st Annual Scientific Meeting of the British Association of Urological Surgeons, BAUS 2015 United Kingdom 2015;115. https://doi/org/10.1111/bju.13136.
- Maruf M, George A, Wei J, Montie J, Miller D, Wu RC, et al. PD38-08 Multi-institutional prospective validation of the novel Michigan urological surgery improvement collaborative transperineal biopsy template. J Urol 2020;203. https://doi/org/10.1097/JU.0000000000000917.08.
- Newman T, Stroman L, Hadjipavlou M, Mulhem W, Ioannides D, Chan K, et al. PT438 – a switch from transrectal biopsy to cognitive freehand transperineal prostate biopsy can reduce sepsis where targeted antibiotics for resistant rectal flora have failed. Eur Urol Open Sci 2020;19. https://doi/org/10.1016/S2666-1683(20)34172-0.
- Ng CK, Lo KL, Lim K, . Zero sepsis rate: a new era of transperineal ultrasound-guided prostate biopsy. BJU Int 2019;123.
- Sharma A, Al-Dhahir W, Subin F, Mulholland C. Post-MRI primary local anaesthetic freehand transperineal prostate biopsy significantly reduces biopsy rate in comparison to trans-rectal prostate biopsy: implications for service burden in a secondary-care referral centre in Northern Ireland. Eur Urol Suppl 2019;18. https://doi/org/10.1016/S1569-9056(19)32653-3.
- Stroman L, Neale A, Hage OE, . PD64-03 TREXIT – a year on. Making a clean break from trans-rectal prostate biopsy. J Urol 2019;201:e1181-2. https://doi/org/10.1097/01.JU.0000557442.00399.ce.
- Stroman L, Neale A, Kum F, Rusere J, Jabarkhyl D, Simpson N, et al. MP81-14 Local anesthetic, intravenous sedation or general anesthetic freehand guided transperineal prostate biopsies: does anesthetic modality impact cancer detection and adverse events?. J Urol 2020;203. https://doi/org/10.1097/JU.0000000000000973.014.
- Stroman L, Newman T, Hadjipavlou M, Ioannides D, Milliken C, Pinczes T, et al. MP81-18 A switch from transrectal biopsy to cognitive freehand transperineal prostate biopsy can reduce sepsis where targeted antibiotics for resistant rectal flora have failed. J Urol 2020;203. https://doi/org/10.1097/JU.0000000000000973.018.
- Ting F, Van Leeuwen PJ, Thompson J, Shnier R, Moses D, Delprado W, et al. Assessment of the performance of magnetic resonance imaging/ultrasound fusion guided prostate biopsy against a combined targeted plus systematic biopsy approach using 24-core transperineal template saturation mapping prostate biopsy. Prostate Cancer 2016;2016:1-8. https://doi/org/10.1155/2016/3794738.
- Urkmez A, Altok M, Demirel C, Davis J. Comparative outcomes of 2 different transperineal prostate biopsy techniques. J Urol 2020;203. https://doi/org/10.1097/JU.0000000000000973.05.
- Urkmez A, Demirel C, Altok M, Tharakeswara B, Shapiro D, Davis J. PD56-01 Freehand versus grid-based transperineal prostate biopsy: a comparison of anatomic region yield and complications. J Urol 2021;206. https://doi/org/10.1097/JU.0000000000002090.01.
- Zattoni F, Marra G, Kasivisvanathan V, . P0958: transperineal vs. transrectal MRI-targeted prostate biopsy: evaluation of cancer detection risk and biopsy accuracy for clinical significant prostate cancer. Eur Urol 2021;79:S1348-S49.
- NCT . Impact of Magnetic Resonance and Biomarkers for Screening for Prostate Cancer. Cost-Effectiveness Analysis 2020. https://clinicaltrials.gov/study/NCT04283032 (accessed 22 August 2024).
- Altok M, Kim B, Patel BB, Shih YT, Ward JF, McRae SE, et al. Cost and efficacy comparison of five prostate biopsy modalities: a platform for integrating cost into novel-platform comparative research. Prostate Cancer Prostatic Dis 2018;21:524-32. https://doi/org/10.1038/s41391-018-0056-7.
- Cheng LJ, Soon SS, Tan TW, Tan CH, Lim TSK, Tay KJ, et al. Cost-effectiveness of MRI targeted biopsy strategies for diagnosing prostate cancer in Singapore. BMC Health Serv Res 2021;21:1-16. https://doi/org/10.1186/s12913-021-06916-0.
- Cricco-Lizza E, Vanden Berg RNW, Laviana A, Pantuck M, Basourakos SP, Salami SS, et al. Comparative effectiveness and tolerability of transperineal MRI-targeted prostate biopsy under local versus sedation. Urology 2021;155:33-8. https://doi/org/10.1016/j.urology.2021.06.023.
- Popert R, Kum F, MacAskill F, Stroman L, Zisengwe G, Rusere J, et al. Our first month of delivering the prostate cancer diagnostic pathway within the limitations of COVID-19 using local anaesthetic transperineal biopsy. BJU Int 2020;126:329-32. https://doi/org/10.1111/BJU.15120.
- Nicholson A, Mahon J, Boland A, Beale S, Dwan K, Fleeman N, et al. The clinical effectiveness and cost-effectiveness of the PROGENSA® prostate cancer antigen 3 assay and the Prostate Health Index in the diagnosis of prostate cancer: a systematic review and economic evaluation. Health Technol Assess 2015;19:1-192. https://doi/org/10.3310/hta19870.
- Cerantola Y, Dragomir A, Tanguay S, Bladou F, Aprikian A, Kassouf W. Cost-effectiveness of multiparametric magnetic resonance imaging and targeted biopsy in diagnosing prostate cancer. Urol Oncol 2016;34:119.e1-9. https://doi/org/10.1016/j.urolonc.2015.09.010.
- de Rooij M, Crienen S, Witjes JA, Barentsz JO, Rovers MM, Grutters JP. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modelling study from a health care perspective. Eur Urol 2014;66:430-6. https://doi/org/10.1016/j.eururo.2013.12.012.
- Dijkstra S, Govers TM, Hendriks RJ, Schalken JA, Van Criekinge W, Van Neste L, et al. Cost-effectiveness of a new urinary biomarker-based risk score compared to standard of care in prostate cancer diagnostics – a decision analytical model. BJU Int 2017;120:659-65. https://doi/org/10.1111/bju.13861.
- Hao S, Karlsson A, Heintz E, Elfström KM, Nordström T, Clements M. Cost-effectiveness of magnetic resonance imaging in prostate cancer screening: a microsimulation study. Value Health 2021;24:1763-72. https://doi/org/10.1016/j.jval.2021.06.001.
- Patel S, Rongen JJ, Futterer JJ, Boltyenkov A, Rovers MM. The role of multiparametric magnetic resonance imaging in active surveillance for men with low-risk prostate cancer: a cost-effectiveness modeling study. Eur Urol Ogy 2018;1:476-83. https://doi/org/10.1016/j.euo.2018.05.007.
- Sathianathen NJ, Warlick CA, Weight CJ, Ordonez MA, Spilseth B, Metzger GJ, et al. A clinical prediction tool to determine the need for concurrent systematic sampling at the time of magnetic resonance imaging-guided biopsy. BJU Int 2019;123:612-7.
- Venderink W, Govers TM, de Rooij M, Fütterer JJ, Sedelaar JPM. Cost-effectiveness comparison of imaging-guided prostate biopsy techniques: systematic transrectal ultrasound, direct in-bore MRI, and image fusion. AJR Am J Roentgenol 2017;208:1058-63. https://doi/org/10.2214/ajr.16.17322.
- Ahmed HU, Freeman A, Kirkham A, Sahu M, Scott R, Allen C, et al. Focal therapy for localized prostate cancer: a phase I/II trial. J Urol 2011;185:1246-54. https://doi/org/10.1016/j.juro.2010.11.079.
- Aktas BK, Bulut S, Gokkaya CS, Ozden C, Salar R, Aslan Y, et al. Association of prostate volume with voiding impairment and deterioration in quality of life after prostate biopsy. Urology 2014;83:617-21. https://doi/org/10.1016/j.urology.2013.11.002.
- Awsare NS, Green JSA, Aldwinckle B, Hanbury DC, Boustead GB, McNicholas TA. The measurement of psychological distress in men being investigated for the presence of prostate cancer. Prostate Cancer Prostatic Dis 2008;11:384-9. https://doi/org/10.1038/pcan.2008.21.
- Azzouzi AR, Barret E, Moore CM, Villers A, Allen C, Scherz A, et al. TOOKAD Soluble vascular-targeted photodynamic (VTP) therapy: determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int 2013;112:766-74. https://doi/org/10.1111/bju.12265.
- Burns JF, Hurwitz LM, Levie KE, Caumont F, Brand TC, Rosner IL, et al. Impact of subsequent biopsies on comprehensive health related quality of life in patients with and without prostate cancer. J Urol 2019;201:916-22. https://doi/org/10.1097/ju.0000000000000024.
- Cantor SB, Spann SJ, Volk RJ, . Prostate cancer screening: a decision analysis. J Fam Pract 1995;41:33-41.
- Chaussy C, Thuroff S. Results and side effects of high-intensity focused ultrasound in localized prostate cancer. J Endourol 2001;15:437-40. https://doi/org/10.1089/089277901300189501.
- Dickinson L, Ahmed HU, Kirkham AP, Allen C, Freeman A, Barber J, et al. INDEX Study Group . A multi-centre prospective development study evaluating focal therapy using high intensity focused ultrasound for localised prostate cancer: the INDEX study. Contemp Clin Trials 2013;36:68-80. https://doi/org/10.1016/j.cct.2013.06.005.
- Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al. Prostate testing for cancer and treatment (ProtecT) feasibility study. Health Technol Assess 2003;7:1-88.
- Egan J, Marhamati S, Carvalho FLF, Davis M, O’Neill J, Lee H, et al. Retzius-sparing robot-assisted radical prostatectomy leads to durable improvement in urinary function and quality of life versus standard robot-assisted radical prostatectomy without compromise on oncologic efficacy: single-surgeon series and step-by-step guide. Eur Urol 2021;79:839-57. https://doi/org/10.1016/j.eururo.2020.05.010.
- Ganzer R, Hadaschik B, Pahernik S, Koch D, Baumunk D, Kuru T, et al. Prospective multicenter phase II study on focal therapy (hemiablation) of the prostate with high intensity focused ultrasound. J Urol 2018;199:983-9. https://doi/org/10.1016/j.juro.2017.10.033.
- Ghai S, Louis AS, Van Vliet M, Lindner U, Haider MA, Hlasny E, et al. Real-time MRI-guided focused ultrasound for focal therapy of locally confined low-risk prostate cancer: feasibility and preliminary outcomes. AJR Am J Roentgenol 2015;205:W177-84. https://doi/org/10.2214/AJR.14.13098.
- Gu M, Li W, Chen Q, Chen Y, Xu M, Wang Z. Prostate calculi can higher urinary retention probability and worsen uncomfortable feeling after prostate biopsy but not predict cancer. Int J Clin Exp Med 2015;8:6282-6.
- Koch MO, Gardner T, Cheng L, Fedewa RJ, Seip R, Sangvhi NT. Phase I/II trial of high intensity focused ultrasound for the treatment of previously untreated localized prostate cancer. J Urol 2007;178:2366-71. https://doi/org/10.1016/j.juro.2007.08.014.
- Kok ET, Groeneveld FPMJ, Gouweloos J, Jonkheijm R, Bosch JR, Thomas S, et al. Determinants of seeking of primary care for lower urinary tract symptoms: the Krimpen study in community-dwelling men. Eur Urol 2006;50:811-7. https://doi/org/10.1016/j.eururo.2006.03.024.
- Mettlin CJ, Murphy GP, Babaian RJ, Chesley A, Kane RA, Littrup PJ, et al. Observations on the early detection of prostate cancer from the American Cancer Society National Prostate Cancer Detection Project. Cancer 1997;80:1814-7. https://doi/org/10.1002/(sici)1097-0142(19971101)80:9<1814::aid-cncr20>3.0.co;2-7.
- Miki K, Kiba T, Sasaki H, Kido M, Aoki M, Takahashi H, et al. Transperineal prostate brachytherapy, using I-125 seed with or without adjuvant androgen deprivation, in patients with intermediate-risk prostate cancer: study protocol for a phase III, multicenter, randomized, controlled trial. BMC Cancer 2010;10. https://doi/org/10.1186/1471-2407-10-572.
- Natarajan S, Raman S, Priester AM, Garritano J, Margolis DJA, Lieu P, et al. Focal laser ablation of prostate cancer: phase I clinical trial. J Urol 2016;196:68-75. https://doi/org/10.1016/j.juro.2015.12.083.
- Naughton CK, Miller DC, Yan Y. Impact of transrectal ultrasound guided prostate biopsy on quality of life: a prospective randomized trial comparing 6 versus 12 cores. J Urol 2001;165:100-3. https://doi/org/10.1097/00005392-200101000-00025.
- Pane-Alemany R, Ramirez-Garcia I, Carralero-Martinez A, Blanco-Ratto L, Kauffmann S, Sánchez E. Efficacy of transcutaneous perineal electrostimulation versus intracavitary anal electrostimulation in the treatment of urinary incontinence after a radical prostatectomy: randomized controlled trial study protocol. BMC Urol 2021;21. https://doi/org/10.1186/s12894-020-00718-y.
- Pisters LL, Von Eschenbach AC, Scott SM, Swanson DA, Dinney CPN, Pettaway CA, et al. The efficacy and complications of salvage cryotherapy of the prostate. J Urol 1997;157:921-5. https://doi/org/10.1016/S0022-5347(01)65084-5.
- Soloway MS, Soloway CT, Eldefrawy A, Acosta K, Kava B, Manoharan M. Careful selection and close monitoring of low-risk prostate cancer patients on active surveillance minimizes the need for treatment. Eur Urol 2010;58:831-5. https://doi/org/10.1016/j.eururo.2010.08.027.
- Uchida T, Baba S, Irie A, . Transrectal high-intensity focused ultrasound in the treatment of localized prostate cancer: a multicenter study. Acta Urol Jpn 2005;51:651-58.
- Valerio M, Dickinson L, Ali A, Ramachandran N, Donaldson I, Freeman A, et al. A prospective development study investigating focal irreversible electroporation in men with localised prostate cancer: Nanoknife Electroporation Ablation Trial (NEAT). Contemp Clin Trials 2014;39:57-65. https://doi/org/10.1016/j.cct.2014.07.006.
- Van De Ven WJM, Barentsz JO. Prostate cancer: MRI/US-guided biopsy: a viable alternative to TRUS-guidance. Nat Rev Urol 2013;10:559-60. https://doi/org/10.1038/nrurol.2013.179.
- Sullivan PW, Ghushchyan V. Mapping the EQ-5D index from the SF-12: US general population preferences in a nationally representative sample. Med Decis Making 2006;26:401-9. https://doi/org/10.1177/0272989x06290496.
- Ara R, Brazier J. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health 2008;11:1131-43. https://doi/org/10.1111/j.1524-4733.2008.00352.x.
- Donnelly DW, Donnelly C, Kearney T, Weller D, Sharp L, Downing A, et al. Urinary, bowel and sexual health in older men from Northern Ireland. BJU Int 2018;122:845-57. https://doi/org/10.1111/bju.14182.
- Downing A, Wright P, Hounsome L, Selby P, Wilding S, Watson E, et al. Quality of life in men living with advanced and localised prostate cancer in the UK: a population-based study. Lancet Oncol 2019;20:436-47. https://doi/org/10.1016/S1470-2045(18)30780-0.
- Glaser AW, Fraser LK, Corner J, Feltbower R, Morris EJA, Hartwell G, et al. Patient-reported outcomes of cancer survivors in England 1–5 years after diagnosis: a cross-sectional survey. BMJ Open 2013;3. https://doi/org/10.1136/bmjopen-2012-002317.
- Kuppen MCP, Westgeest HM, van den Eertwegh AJM, Coenen JL, van Moorselaar RJA, van den Berg P, et al. Health-related quality of life and pain in a real-world castration-resistant prostate cancer population: results from the PRO-CAPRI study in the Netherlands. Clin Genitourin Cancer 2020;18:e233-53. https://doi/org/10.1016/j.clgc.2019.11.015.
- Lemanska A, Poole K, Manders R, Marshall J, Nazar Z, Noble K, et al. Patient activation and patient-reported outcomes of men from a community pharmacy lifestyle intervention after prostate cancer treatment. Support Care Cancer 2022;30:347-58. https://doi/org/10.1007/s00520-021-06404-5.
- Lloyd AJ, Kerr C, Penton J, Knerer G. Health-related quality of life and health utilities in metastatic castrate-resistant prostate cancer: a survey capturing experiences from a diverse sample of UK patients. Value Health 2015;18:1152-7. https://doi/org/10.1016/j.jval.2015.08.012.
- Loeb S, Curnyn C, Walter D, Fagerlin A, Siebert U, Mühlberger N, et al. Health state utilities among contemporary prostate cancer patients on active surveillance. Transl Androl Urol 2018;7:197-202. https://doi/org/10.21037/tau.2017.03.80.
- Lopez-Calderero I, Lopez-Fando L, Rios-Gonzalez E, Maisonobe P, Hernández-Yuste E, Sarmiento-Jordán M. Impact of locally advanced or metastatic prostate cancer on the quality of life. Actas Urol Esp 2017;41:368-75. https://doi/org/10.1016/j.acuroe.2017.05.005.
- Maguire R, Drummond FJ, Hanly P, Gavin A, Sharp L. Problems sleeping with prostate cancer: exploring possible risk factors for sleep disturbance in a population-based sample of survivors. Support Care Cancer 2019;27:3365-73. https://doi/org/10.1007/s00520-018-4633-z.
- Murasawa H, Sugiyama T, Matsuoka Y, Okabe T, Hino A, Tanaka N, et al. Health utility and health-related quality of life of Japanese prostate cancer patients according to progression status measured using EQ-5D-5L and FACT-P. Qual Life Res 2019;28:2383-91. https://doi/org/10.1007/s11136-019-02184-y.
- Smith L, Downing A, Norman P, Wright P, Hounsome L, Watson E, et al. Influence of deprivation and rurality on patient-reported outcomes of men living with and beyond prostate cancer diagnosis in the UK: a population-based study. Cancer Epidemiol 2020;69. https://doi/org/10.1016/j.canep.2020.101830.
- Uemura H, Ye D, Kanesvaran R, Chiong E, Lojanapiwat B, Pu Y-S, et al. United in Fight against prOstate cancer (UFO) registry: first results from a large, multi-centre, prospective, longitudinal cohort study of advanced prostate cancer in Asia. BJU Int 2020;125:541-52. https://doi/org/10.1111/bju.14980.
- Venderbos LDF, Deschamps A, Dowling J, Carl E-G, Remmers S, van Poppel H, et al. Europa Uomo Patient Reported Outcome Study (EUPROMS): descriptive statistics of a prostate cancer survey from patients for patients. Eur Urol Focus 2021;7:987-94. https://doi/org/10.1016/j.euf.2020.11.002.
- Wilding S, Downing A, Selby P, Cross W, Wright P, Watson EK, et al. Decision regret in men living with and beyond nonmetastatic prostate cancer in the United Kingdom: a population-based patient-reported outcome study. Psychooncology 2020;29:886-93. https://doi/org/10.1002/pon.5362.
- Yao HH, Crump RT, Charbonneau C, Khan A, Barton C, Brotherhood H, et al. Baseline patient reported outcomes data shows high prevalence of overactive bladder, sexual dysfunction, depression and anxiety in Canadian men with newly diagnosed localized prostate cancer. Transl Androl Urol 2020;9:2046-53. https://doi/org/10.21037/tau-20-689.
- York Health Economics Consortium . Economic Impact Evaluation Case Study: PrecisionPointTM Transperineal Prostate Biopsies 2020.
- Pilon D, Behl AS, Ellis LA, Emond B, Lefebvre P, Dawson NA. Duration of treatment in prostate cancer patients treated with abiraterone acetate or enzalutamide. J Manag Care Spec Pharm 2017;23:225-35. https://doi/org/10.18553/jmcp.2016.16233.
Appendix 1 Literature search strategies for the systematic reviews of clinical effectiveness, cost-effectiveness and health-related quality of life
All the database search strategies for the clinical effectiveness, cost-effectiveness and HRQoL searches are reported below. Each strategy was first developed in MEDLINE (Ovid) and then adapted for the other databases. Reference management and deduplication of search results were carried out in EndNote (Clarivate).
Searches for diagnostic test evaluation and clinical effectiveness studies
The searches for diagnostic test evaluation and clinical effectiveness had no date limits and the databases were searched from inception. An English-language limit was applied to the search strategy as a pragmatic decision due to the fixed time and resources available to this assessment for study-language translation. In order to be sensitive and retrieve all relevant studies, no study design search filters were used. Table 46 details the search strategies for the databases and the conference hand searches. See also the section Identification of studies in this report.
| Database, host, years searched, date searched | Literature search strategy | Results |
|---|---|---|
| Ovid MEDLINE(R) and Epub Ahead of Print, In-process, In-data-review & Other Non-indexed Citations, Daily and Versions(R) 1946–8 July 2021 Date of original search: 9 July 2021 Date of update search: 19 October 2021 |
|
Original search: 205 Update search: 6 |
|
||
| Ovid Embase Classic + Embase 1947–8 July 2021 Date of original search: 9 July 2021 Date of update search: 19 October 2021 |
|
Original search: 1348 Update search: 17 |
| Cochrane Library, Wiley (CDSR and CENTRAL) Date of original search: 9 July 2021 Date of update search: 19 October 2021 |
|
Original search: Reviews: 2 Trials: 122 Update search: Reviews: 0 Trials: 2 |
| Web of Science Indexes = SCI-EXPANDED, CPCI-S Timespan = 1970–2021 Date of original search: 9 July 2021 Date of update search: 19 October 2021 |
|
Original search: 491 Update search: 34 |
| Epistemonikos www.epistemonikos.org/ Date of original search: 9 July 2021 Date of update search: 19 October 2021 |
title:((prostate or prostatic) AND (cancer* OR carcinoma* OR malignan* OR neoplasm* OR tumour* OR tumor*)) AND ((title:(biops* AND (transperineal or perineal or transrectal)) OR (title:(precisionpoint OR ‘precision point’ OR BXTAccelyon OR UA1232 OR ‘BK Medical’ OR Trinity OR Perine OR Koelis OR camprobe OR ‘cambridge prostate biopsy device’ OR JEB OR SureFire OR LeapMed* OR EZU-PA3U OR Hitachi))) OR abstract:(precisionpoint OR ‘precision point’ OR BXTAccelyon OR UA1232 OR ‘BK Medical’ OR Trinity OR Perine OR Koelis OR camprobe OR ‘cambridge prostate biopsy device’ OR JEB OR SureFire OR LeapMed* OR EZU-PA3U OR Hitachi)) | Original search: 43 Update search: 2 |
| DARE and NHS EED www.crd.york.ac.uk/CRDWeb/ Date of original search: 9 July 2021 Date of update search: Not applicable (Database ceased to be updated after March 2015) |
|
Original search: 2 |
| International HTA Database (INAHTA) www.inahta.org/hta-database/ Date of original search: 9 July 2021 Date of update search: 19 October 2021 |
(((cognitive* and biops*)) OR (‘cognitive fusion biops*’) OR (‘cognitive MRI-targeted biops*’) OR ((‘transrectal ultraso*’ or TRUS) and biops* and (‘local an?esthesia’ or ‘local an?esthetic’)) OR (perineal and biops* and (‘general an?esthesia’ or ‘general an?esthetic’)) OR (perineal and biops* and (‘local an?esthesia’ or ‘local an?esthetic’)) OR (transperineal and biops* and (‘general an?esthesia’ or ‘general an?esthetic’)) OR (transperineal and biops* and (‘local an?esthesia’ or ‘local an?esthetic’)) OR (LATP and (biops* or prostat*)) OR (‘general an?esthesia transperineal’) OR (‘general an?esthetic transperineal’) OR (‘local an?esthesia transperineal’) OR (‘local an?esthetic transperineal’) OR ((freehand or free?hand) and (device* or needle* or biops*)) OR (device and (grid or guide or stepping or template)) OR (stepping and (device or grid or guide or template)) OR (needle and (device or grid or guide or template)) OR (Hitachi and prostat*) OR (EZU-PA3U) OR (LeapMed*) OR (SureFire) OR (JEB) OR (CamPROBE or ‘cambridge prostate biopsy device’) OR (Koelis) OR ((Trinity or Perine) and prostat*) OR (UA1232 or ‘BK Medical’) OR (Precisionpoint or BXTAccelyon)) AND ((((prostat* and (cancer* or carcinoma* or malignan* or neoplasm* or tumour* or tumor*))) OR (‘Prostatic Neoplasms’[mhe]))) English language filter |
Original search: 30 Update search: 0 |
| OpenGrey (DANS EASY Archive) Date of original search: 9 July 2021 |
Prostate and biops* – only useful search terms 82 results: 71 in French, 14 in English, 1 in German 0 relevant |
Original search: 0 |
| PROSPERO www.crd.york.ac.uk/prospero/ Date of original search: 9 July 2021 |
|
Original search: 73 |
| ClinicalTrials.gov www.clinicaltrials.gov/ Date of original search: 10 June 2021 |
Prostate cancer | transperineal = 93 studies Prostate cancer | perineal = 34 studies Prostate cancer | transrectal = 254 studies Prostate cancer | TRUS = 209 NB ‘Also searched for Prostatic Neoplasm, Prostatic, and Neoplasm’ Total 590, deduplicated = 346 |
Original search: 346 |
| Be Part of Research https://bepartofresearch.nihr.ac.uk/ Date of original search: 10 June 2021 |
Search terms: prostate cancer, biopsy, biopsies, prostate biopsy, transperineal, perineal, transrectal, TRUS | Original search: 0 |
| NIHR CRN Portfolio Search (NIHR website) Date of original search: 10 June 2021 |
272 results for prostate cancer. Title screen = 0 relevant/biopsy-related. | Original search: 0 |
| ASCO Genitourinary Cancers Symposium Date of original search: June 2021 Date of update search: Not applicable, no further conferences in 2021 |
Hand-search proceedings published in the Journal of Clinical Oncology supplements for 2018–21 Keywords: prostat*, biopsy, biopsies, transperineal, TRUS, etc. |
Original search: 16 |
| AUA Annual Meeting Date of original search: June 2021 Date of update search: 19 October 2021 |
Hand-search proceedings published in The Journal of Urology supplements for 2018–21 Keywords: prostat*, biopsy, biopsies, transperineal, TRUS, etc. |
Original search: 54 Update search: 3 |
| BAUS has an Annual Scientific meeting Date of original search: June 2021 Date of update search: 19 October 2021 |
Hand-search proceedings published in the Journal of Clinical Oncology supplements for 2018–21 Keywords: prostat*, biopsy, biopsies, transperineal, TRUS, etc. |
Original search: 9 Update search: 2 |
| EAU Annual Meeting Date of original search: June 2021 Date of update search: 19 October 2021 |
Hand-search proceedings published in European Urology Open Science (2020-), formerly European Urology Supplements (-2019). Keywords: prostat*, biopsy, biopsies, transperineal, TRUS, etc. |
Original search: 35 Update search: 4 |
Searches for cost-effectiveness studies
The database search strategies for the cost effectiveness searches were based on an early version of the clinical effectiveness searches with the addition of the CADTH filter for Economic Evaluations/Cost/Economic Models60 applied to the MEDLINE and EMBASE strategies, and amended versions of the filter applied to the Cochrane Library and Web of Science strategies. An English language limit was applied. In addition, the EconLit database was searched. The full strategies are in Table 47.
| Database, host, years searched, date searched | Literature search strategy | Results |
|---|---|---|
| Ovid MEDLINE(R) and Epub Ahead of Print, In-process, In-data-review & Other Non-indexed Citations, Daily and Versions(R) 1946–16 June 2021 Date of original search: 17 June 2021 Date of update search: 2 November 2021 |
|
Original search: 144 Update search: 10 |
| Ovid Embase Classic + Embase 1947–2021 Week 23 Date of original search: 17 June 2021 Date of update search: 2 November 2021 |
|
Original search: 378 Update search: 8 |
| Cochrane Library for CDSR and CENTRAL, Wiley Date of original search: 17 June 2021 Date of update search: 2 November 2021 |
|
Original search: Reviews: 1 Trials: 69 Update search: Reviews: 0 Trials: 3 |
| EconLit, EBSCO Date of original search: 17 June 2021 Date of update search: 2 November 2021 |
|
Original search: 4 Update search: 0 |
| Web of Science Indexes = SCI-EXPANDED, CPCI-S Timespan = 1970–2021 Date of original search: 17 June 2021 Date of update search: 2 November 2021 |
Custom year range 2021–2021 (+ deduplication in EndNote) Update search: |
Original search: 86 Update search: 21 |
(#45) AND LANGUAGE: (English)
|
||
| DARE and NHS EED www.crd.york.ac.uk/CRDWeb/ Date of original search: 7 June 2021 Date of update search: not applicable (Database ceased to be updated after March 2015) |
|
Original search: 6 |
| International HTA Database (INAHTA) www.inahta.org/hta-database/ Date of original search: 7 June 2021 Date of update search: 2 November 2021 |
(((cognitive* and biops*)) OR (‘cognitive fusion biops*’) OR (‘cognitive MRI-targeted biops*’) OR ((‘transrectal ultraso*’ or TRUS) and biops* and (‘local an?esthesia’ or ‘local an?esthetic’)) OR (perineal and biops* and (‘general an?esthesia’ or ‘general an?esthetic’)) OR (perineal and biops* and (‘local an?esthesia’ or ‘local an?esthetic’)) OR (transperineal and biops* and (‘general an?esthesia’ or ‘general an?esthetic’)) OR (transperineal and biops* and (‘local an?esthesia’ or ‘local an?esthetic’)) OR (LATP and (biops* or prostat*)) OR (‘general an?esthesia transperineal’) OR (‘general an?esthetic transperineal’) OR (‘local an?esthesia transperineal’) OR (‘local an?esthetic transperineal’) OR ((freehand or free?hand) and (device* or needle* or biops*)) OR (device and (grid or guide or stepping or template)) OR (stepping and (device or grid or guide or template)) OR (needle and (device or grid or guide or template)) OR (Hitachi and prostat*) OR (EZU-PA3U) OR (LeapMed*) OR (SureFire) OR (JEB) OR (CamPROBE or ‘cambridge prostate biopsy device’) OR (Koelis) OR ((Trinity or Perine) and prostat*) OR (UA1232 or ‘BK Medical’) OR (Precisionpoint or BXTAccelyon) OR ((transperineal or perineal or transrectal) AND (((needle or puncture or aspiration) and biops*) OR (‘Biopsy, Needle’[mhe]) OR (‘Biopsy’[mh]) OR (prostat* and biops*)))) AND ((suspected or suspicion or suspicious) AND ((((prostat* and (cancer* or carcinoma* or malignan* or neoplasm* or tumour* or tumor*)))) OR (‘Prostatic Neoplasms’[mhe]))) | Original search: 4 Update search: 0 |
| Epistemonikos www.epistemonikos.org/ Date of original search: 7 June 2021 Date of update search: 2 November 2021 |
title:(prostat* AND (cancer* OR carcinoma* OR malignan* OR neoplasm* OR tumour* OR tumor*)) AND (title:(suspected OR suspicion OR suspicious) OR abstract:(suspected OR suspicion OR suspicious)) AND (title:(biops* OR precisionpoint OR BXTAccelyon OR UA1232 OR ‘BK Medical’ OR Trinity OR Perine OR Koelis OR camprobe OR ‘cambridge prostate biopsy device’ OR JEB OR SureFire OR LeapMed* OR EZU-PA3U OR Hitachi) OR abstract:(biops* OR precisionpoint OR BXTAccelyon OR UA1232 OR ‘BK Medical’ OR Trinity OR Perine OR Koelis OR camprobe OR ‘cambridge prostate biopsy device’ OR JEB OR SureFire OR LeapMed* OR EZU-PA3U OR Hitachi)) | Original search: 129 Update search: 2 |
Searches for health-related quality-of-life studies
The first search for relevant HRQoL studies (‘HRQoL 1’) was carried out on 17 June 2021 and was similar to the clinical effectiveness searches but with the CADTH filter for Health Utilities/Quality of Life added. This was not sufficient as it only covered the biopsy aspects of the disease pathway. Therefore, a second search was performed on 15 September 2021 (‘HRQoL 2’) where the biopsy terms were removed in order to retrieve studies that would cover the whole disease pathway in addition to the diagnostic process. In order to save time, search terms were applied specifically for the EQ-5D utility measure, as the preferred method according to NICE guidance. The option to expand the search to other utility measures was considered, but after screening the results it was not deemed necessary. The searches were carried out in MEDLINE, EMBASE, Web of Science, and the Cochrane Library, and they were limited to the most recent 10 years. The strategies are in Tables 48 and 49.
| Database, host, years searched, date searched | Literature search strategy | Results |
|---|---|---|
| Ovid MEDLINE(R) and Epub Ahead of Print, In-process, In-data-review & Other Non-indexed Citations, Daily and Versions(R) 1946 to 17 June 2021 Date of original search: 17 June 2021 |
|
Original search: 75 |
| Ovid Embase Classic + Embase 1947–2021 Week 23 Date of original search: 17 June 2021 |
|
Original search: 138 |
| Web of Science – Science Citation Index Expanded (SCI-EXPANDED), Conference Proceedings Citation Index – Science (CPCI-S) Timespan = 1970–2021 Date of original search: 16 September 2021 |
|
Original search: |
| Cochrane Library, Wiley Date of original search: 18 June 2021 |
|
Original search: 35 |
| Database, host, years searched, date searched | Literature search strategy | Results |
|---|---|---|
| Ovid MEDLINE(R) and Epub Ahead of Print, In-process, In-data-review & Other Non-indexed Citations, Daily and Versions(R) 1946–14 September 2021 Date of original search: 15 September 2021 Date of update search: 29 January 2022 |
|
Original search: 89 Update search: |
| Ovid Embase Classic + Embase 1947–2021 Week 36 Date of original search: 15 September 2021 Date of update search: 29 January 2022 |
|
Original search: 261 Update search: |
| Web of Science – Science Citation Index Expanded (SCI-EXPANDED), Conference Proceedings Citation Index – Science (CPCI-S) Date of original search: 16 September 2021 Date of update search: 29 January 2022 |
(TS=(prostat* near/3 (cancer* or carcinoma* or malignan* or neoplasm* or tumour* or tumor*))) AND TS=(eq or euroqol or ‘euro qol’ or eq5d or ‘eq 5d’ or euroqual or ‘euro qual’) Publication date: 1 January 2011–16 September 2021 Refine by English language |
Original search: 133 Update search: |
| Cochrane Library, Wiley Date of original search: 16 September 2021 Date of update search: |
|
Original search: 146 Update search: |
Appendix 2 Further detail on inclusion/exclusion of studies
Extended inclusion/exclusion criteria for the systematic review of diagnostic test evaluation and clinical effectiveness
| Population (decision questions 1 and 2) | |
|---|---|
| Population: People with suspected prostate cancer where prostate biopsy is indicated |
|
| Inclusion criteria | Exclusion criteria |
|
|
| Interventions – relevant diagnostic procedures (decision question 1) | |
|
|
|
|
| Comparators – relevant alternative diagnostic procedures (decision question 1) | |
|
|
| Inclusion criteria | Exclusion criteria |
|
|
| Interventions – relevant diagnostic procedures (decision question 2) | |
|
|
| Comparators – relevant alternative diagnostic procedures (decision question 2) | |
|
|
| Inclusion criteria | Exclusion criteria |
|
|
| Outcomes (decision questions 1 and 2) | |
|
|
|
|
|
|
| Inclusion criteria | Exclusion criteria |
|
|
| Study design (decision questions 1 and 2) | |
| Inclusion criteria | Exclusion criteria |
|
|
| Publication type (decision questions 1 and 2) | |
| Inclusion criteria | Exclusion criteria |
|
|
| Language (decision questions 1 and 2) | |
| Inclusion criteria | Exclusion criteria |
| English | Non-English language |
List of studies excluded from the systematic review of diagnostic test evaluation and clinical effectiveness
Studies that have not been included in this review were either excluded or their eligibility remains unclear:
-
Excluded studies: studies excluded after full-text screening are listed in Table 51. Studies may have been excluded for not meeting more than one eligibility criteria, but only the first exclusion reason is recorded.
-
Unclear studies: studies whose eligibility for inclusion remained unclear after full-text screening and after contacting the authors for further information are listed in Table 52.
| Study | Publication type | Exclusion reason |
|---|---|---|
| ACTRN12620001145998/LATProBE 202058 |
Trial register record | Ongoing study (no results) |
| ISRCTN98159689/TRANSLATE 202152 |
Trial register record | Ongoing study (no results) |
| Adshead 2019116 | Conference abstract | Intervention |
| Berry 202097 | Journal article | Population |
| Berry 2020114 | Conference abstract | Population |
| Chae 2009117 | Journal article | Language |
| Eldred-Evans 2018118 | Conference abstract | Intervention |
| Han 2008119 | Journal article | Intervention |
| Israel 2021120 | Conference abstract | Comparator |
| Kasivisvanathan 2015121 | Letter | Intervention |
| Kawakami 2007122 | Journal article | Intervention |
| Lavoipierre 2008123 | Letter | Outcomes |
| Lim 2018124 | Conference abstract | Intervention |
| Lim 2020125 | Journal article | Intervention |
| Lo 2019126 | Journal article | Comparator |
| NCT03496142 2018127 | Trial register record | Intervention |
| NCT04108871 2019128 | Trial register record | Ongoing study (no results) |
| NCT04815876 202157 | Trial register record | Ongoing study (no results) |
| NCT04843566 202156 | Trial register record | Ongoing study (no results) |
| Neale 2020129 | Journal article | Intervention |
| Pahwa 2017130 | Journal article | Outcomes |
| Pal 2018131 | Journal article | Intervention |
| Pepe 2017132 | Journal article | Design |
| Postema 2017133 | Journal article | Intervention |
| Presti 2000134 | Journal article | Design |
| Ristau 2018135 | Journal article | Comparator |
| Roberts 2020136 | Conference abstract | Intervention |
| Roberts 2021137 | Journal article | Intervention |
| Rochester 2009138 | Journal article | Comparator |
| Rodriguez Socarras 2020139 | Journal article | Intervention |
| Roethke 2014140 | Journal article | Intervention |
| Rojas Claros 2019141 | Conference abstract | Intervention |
| Salagierski 2019142 | Journal article | Intervention |
| Satoh 2005143 | Journal article | Comparator |
| Self 2018144 | Conference abstract | Design |
| Shigemura 2007145 | Journal article | Intervention |
| Sivaraman 2015146 | Journal article | Intervention |
| Song 2019147 | Journal article | Intervention |
| Stabile 2018148 | Journal article | Intervention |
| Suga 1999149 | Journal article | Population |
| Sulaiman 2019150 | Conference abstract | Design |
| Taira 2010151 | Journal article | Intervention |
| Tamhankar 2020152 | Conference abstract | Intervention |
| Taverna 2016153 | Journal article | Intervention |
| Taverna 2016154 | Conference abstract | Intervention |
| Teoh 2015155 | Journal article | Intervention |
| Tilak 2015156 | Journal article | Comparator |
| Tschirdewahn 2020157 | Journal article | Intervention |
| Valerio 2015158 | Journal article | Intervention |
| Vanni 2004159 | Journal article | Intervention |
| Vezelis 2021160 | Journal article | Intervention |
| Wang 2019161 | Journal article | Comparator |
| Westhoff 2019162 | Journal article | Intervention |
| Williams 2018163 | Conference abstract | Comparator |
| Williams 2018164 | Conference abstract | Comparator |
| Yamada 2020165 | Journal article | Intervention |
| Yang 2019166 | Conference abstract | Intervention |
| Yaxley 2017167 | Journal article | Intervention |
| Yunkai 2010168 | Journal article | Comparator |
| Zhang 2020169 | Journal article | Intervention |
| Zhang 2019170 | Conference abstract | Intervention |
| Zhang 2017171 | Journal article | Intervention |
| Zhang 2015172 | Journal article | Intervention |
| Zhao 2012173 | Journal article | Intervention |
| Zhou 2020174 | Journal article | Intervention |
| Study | Publication type | Reason unclear | Notes |
|---|---|---|---|
| Al-Dahir 2019175 | Conference abstract | Unclear comparator | No author contact details; no response via ResearchGate |
| Chan 2020176 | Conference abstract | Unclear population | No author response |
| Chan 2020177 | Conference abstract | Unclear population | No author response |
| Cole 2019178 | Conference abstract | Unclear population and comparator | Invalid author contact details |
| Cole 2020179 | Conference abstract | Unclear intervention | Invalid author contact details |
| Demozzi 2018180 | Conference abstract | Unclear comparator | No author response |
| Di Franco 2017181 | Journal article | Unclear population | No author response |
| Elkhoury 2020182 | Conference abstract | Unclear population | No author contact details |
| Ferrante 2020183 | Conference abstract | Unclear comparator | No author response |
| Ferriero 2019184 | Conference abstract | Unclear population | No author response |
| Islam 2020185 | Conference abstract | Unclear population | No author response |
| Islam 2021186 | Conference abstract | Unclear population | No author response |
| Lai 2021187 | Conference abstract | Unclear intervention | No author response |
| Lovegrove 2019188 | Conference abstract | Unstratified data | Not data ownera |
| Lovegrove 2019189 | Conference abstract | Unstratified data | Not data ownera |
| Marra 2015190 | Conference abstract | Unclear intervention and comparator | No author response |
| Maruf 2020191 | Conference abstract | Unclear population | No author response |
| Newman 2020192 | Conference abstract | Unclear population | No author response |
| Ng 2019193 | Conference abstract | Unclear population, intervention and comparator | No author response |
| Sharma 2019194 | Conference abstract | Unclear population | No author contact details |
| Stroman 2019195 | Conference abstract | Unclear intervention | No author response |
| Stroman 2020196 | Conference abstract | Unclear population | No author response |
| Stroman 2020197 | Conference abstract | Unclear population | No author response |
| Ting 2016198 | Journal article | Unclear intervention | No author response |
| Urkmez 2020199 | Conference abstract | Unclear population, intervention and comparator | No author response |
| Urkmez 2021200 | Conference abstract | Unclear population | No author response |
| Zattoni 2021201 | Conference abstract | Unclear intervention | No author response |
Appendix 3 Data-extraction template used in the systematic review of diagnostic test evaluation and clinical effectiveness
-
Study overview.
-
Relevant subgroup analyses (as per NICE scope).
-
Participant baseline characteristics.
-
Biopsy characteristics.
-
Results: intermediate outcomes (repeat for each subgroup reported).
-
Results: other intermediate outcomes.
-
Results: clinical outcomes.
-
Results: patient-reported outcomes.
-
Results: costs and resources.
-
General reviewer comments (e.g. importance, methodological issues).
1. Study overview
| Reviewer 1: Date: |
Reviewer 2: Date: |
Version: | |
|---|---|---|---|
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|
First author and ref ID:
Publication year: Linked papers: Study name/trial identifier: Study design: Country: Number of centres: Recruitment dates: Funding: Competing interests: |
Condition being diagnosed/detected:
Prostate cancer Emphasis here on describing the elements of the biopsy that define this DAR’s intervention and comparators: transperineal or transrectal approach, anaesthetic type. Further details are in the ‘Biopsy characteristics’ table below. Index test: Reference standard: Intervention: Comparator: |
Number of participants:
Sample attrition/dropout: Selection of participants: Inclusion criteria for study entry: Exclusion criteria for study entry: Sample size calculation: |
Primary outcome of study: Include definition where available. Other relevant outcomes: List other (secondary) outcomes briefly. If there are many, list a couple of key outcomes and then cross refer to the results tables below (see table 5 onwards) Definition of CS disease: State any definition or threshold(s) used. Relevant subgroup analyses: If relevant to NICE scope. None/See table 3 below. |
2. Relevant subgroup analyses (as per NICE scope)
| Subgroup in NICE scope | Subgroup in study |
|---|---|
| People with anterior lesions | |
| People with posterior lesions | |
| People with apical lesions | |
| People with basal lesions | |
| People with a Likert or PI-RADS score of 2 or less | |
| People with a Likert or PI-RADS score of 3, 4 or 5 | |
| People with enlarged prostate | |
| People who have never had a prostate biopsy | |
| People who have had a previous negative prostate biopsy and are referred back |
3. Participant baseline characteristics
| Characteristic, units and variance measure | Intervention: (write short description), n = |
Comparator: (write short description), n= |
p-value/ CI /other relevant statistic (e.g. ORs) |
|---|---|---|---|
| Age, years, mean (SD) | |||
| Ethnicity | |||
| BMI/Height/Weight | |||
| PSA level, ng/ml, mean (SD) | |||
| Prostate volume, ml, mean (SD) | |||
| DRE findings, (n, %) | |||
| Imaging findings (ultrasound, CT or MRI), (n, %) |
|||
| Family history of prostate cancer, (n, %) | |||
| Previous prostate biopsy experience, n (%) First biopsy Repeat biopsy |
|||
| MRI performed, n (%) | |||
| Likert or PI-RADs score | |||
| Lesion location (posterior, anterior, basal, apical) and number | |||
| Previous prostate biopsy was abnormal (e.g. HGPIN, ASAP) but not cancer, n (%) | |||
| Previous prostate biopsy was positive for cancer, n (%) |
4. Biopsy characteristics
| Characteristics | Intervention: | Comparator: |
|---|---|---|
| Device(s) | E.g. grid + stepper, or coaxial needle, or freehand device, e.g. PrecisionPoint | |
| Targeted/systematic/saturation, and sequence | ||
| Type of imaging used | E.g. TRUS or MRI/TRUS-guided fusion | |
| Number of cores | ||
| Location of cores | ||
| Anaesthetic used (type of anaesthesia – name of drug (strength), dose, method of admin., location of admin.) | Example: Periprostatic nerve block – lidocaine (1%) 10 ml injected at five injections sites from base to apex of prostate | |
| Antibiotic prophylaxis | ||
| Other medications administered as standard protocol procedure | ||
| Patient position | ||
| Clinician’s experience and training in prostate biopsy |
5. Results: intermediate outcomes (repeat for each subgroup reported)
| Prostate cancer on histopathology | No prostate cancer on histopathology | Total | |
|---|---|---|---|
| Index test positive | a | b | a + b |
| Index test negative | c | d | c + d |
| Total | a + c | b + d | a + b + c + d |
| Accuracy | |||
| Calculate clinical sensitivity, specificity, PPV, NPV if possible and note whether these agree with any values that may be reported in the paper. Use www.medcalc.org/calc/diagnostic_test.php to assist with calculations | |||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | |||
| Clinical specificity d/(b + d) | |||
| PPV a/(a + b) | |||
| NPV d/(c + d) | |||
| Positive likelihood ratio [sensitivity/(1−specificity)] | |||
| Negative likelihood ratio [(1−sensitivity)/specificity] | |||
| Diagnostic odds ratio (a × d)/(b × c) | |||
| Outcome in NICE scope | Specific outcome(s) measured in study Specify units and mean, median, range, SD, SE, % etc. as appropriate – for % report with (n/N). Add rows as necessary. When scope outcome was not measured, state ‘Not reported’. Delete examples below. |
Intervention: (write short description), n= |
Comparator: (write short description), n= |
p-value/ CI /other relevant statistic (e.g. ORs) |
|---|---|---|---|---|
| Cancer detection rates |
Examples:
Positive detectable rate, n (%) Cancer core rate, n (%) |
|||
| CS cancer detection rates | ||||
| Clinically insignificant cancer detection rates | ||||
| Low-, medium-, high-risk cancer detection rates |
Example:
Gleason score, n (%) Gleason 6 Gleason 7 Gleason 8 Gleason 9 Gleason 10 |
|||
| Interpretability of test | ||||
| Interobserver agreement | ||||
| Intraobserver agreement |
6. Results: other intermediate outcomes
| Outcome in NICE scope | Specific outcome(s) in the study | Intervention: (write short description), n = |
Comparator: (write short description), n = |
|---|---|---|---|
| Biopsy sample suitability/quality | |||
| Number of biopsy samples taken | |||
| Procedure completion rates | |||
| Re-biopsy events within 6 months | |||
| Outcome(s) added by EAG | |||
| Length of time to perform the biopsy | |||
7. Results: clinical outcomes
| Outcome in NICE scope | Specific outcome(s) in the study | Intervention: (write short description), n = |
Comparator: (write short description), n = |
|---|---|---|---|
| Hospitalisation events after biopsy | |||
| Rates of biopsy-related complications | |||
| Rates of urinary retention | |||
| Rates of erectile dysfunction | |||
| Survival | |||
| Progression-free survival | |||
| Adverse events from treatment |
8. Results: patient-reported outcomes
| Outcome in NICE scope | Specific outcome(s) in the study | Intervention: (write short description), n = |
Comparator: (write short description), n = |
|---|---|---|---|
| Health-related quality of life | |||
| Patient-reported tolerability |
9. Results: costs and resources
| Outcome in NICE scope | Specific outcome(s) in the study | Intervention: (write short description), n = |
Comparator: (write short description), n = |
|---|---|---|---|
| e.g. cost of biopsy devices (refer to the NICE scope for the full list of relevant costs) |
10. General reviewer comments (e.g. importance, methodological issues)
| Comments |
|---|
Appendix 4 Further information on studies included in the systematic review of diagnostic test evaluation and clinical effectiveness
| Study | LATP device/approach | Sampling | Number of cores taken | Pre-biopsy imaging (MRI) | Prostate biopsy image guidance | Anaesthesia |
|---|---|---|---|---|---|---|
| RCTs | ||||||
| Cerruto et al. 201424 | Coaxial needle | Systematic | 14 | Not reported | TRUS | Mepivacaine (1%) 2 ml at the level of the prostate apex |
| Guo et al. 201525 | Not reporteda | Systematic | 12 cores if PV > 50 ml; 8 cores if PV < 50 ml; 2 cores per suspicious area detected by TRUS/DRE |
Not reported | TRUS | Periprostatic nerve block: lidocaine (2%) 2 ml; additional lidocaine (2%) 2 ml administered where participant could not tolerate pain |
| Hara et al. 200826 | Not reporteda | Systematic | 12 | Not reported | TRUS | Spinal anaesthesia: bupivacaine (0.5%) |
| Lam et al. 2021 (AB)27 | Freehand PrecisionPoint | Systematic | Not reported (modified Ginsburg protocol) | Not reported | Not reported | Local anaesthetic (details not reported) |
| Takenaka et al. 200828 | Attachment for needle guidance | Systematic | 12 | Not reported | TRUS | ‘Saddle blockade’: bupivacaine (0.5%) |
| Other prospective studies | ||||||
| Bojin 201929 | Freehand PrecisionPoint | Systematic and targeted | Not reported (up to 24 for participants needing the full template) | Unclear | TRUS | Peri-prostatic block: lignocaine (1%) 13–20 ml |
| Chen et al. 202130,31 | Freehand PrecisionPoint | Systematic | 12 | 30% of participants had a pre-biopsy MRI | TRUS | Periprostatic nerve block: lignocaine (1%) at the perineal skin on both sides. Further, lignocaine (1%) 10 ml given on each side |
| Emiliozzi et al. 200332 | Not reporteda | Targeted and systematic (Fan technique but any hypoechoic area was also included) | 6 | Not reported | TRUS | Mepivacaine (2%) two 10 ml transperineal injections, one in each lobe |
| Hung et al. 2020 (AB)33 | Freehand PrecisionPoint | Not reported | Not reported | Not reported | Not reported | Local anaesthetic (details not reported) |
| Kum (AB) 201834,35 | Freehand PrecisionPoint | Systematic (52%), targeted (25%), and systematic and targeted (23%) | Not reported | Not reported | TRUS | Lidocaine (1%) approximately 10–12 ml (up to 30 ml in total) injected on each side, around perineal body and to the apex of the prostate, then laterally to the neurovascular bundles |
| Starmer et al. 202136,37 | Freehand PrecisionPoint | Systematic, plus targeted biopsies if a PI-RADSv2 > 2 lesion on MRI | Not reported | Pre-biopsy MRI assisted in assigning participants to groups | Not reported | Lidocaine (1%) 10 mla and chirocaineb (0.5%) 10 ml |
| Watanabe et al. 200538 | Not reporteda | Systematic with additional targeted biopsies for any hypoechoic lesions or palpable nodules on DRE | 6 | Not reported | Ultrasound | Spinal anaesthesia (details not reported) |
| Retrospective studies | ||||||
| Abdollah et al. 201139 | Coaxial needle | Saturation | 24 | Not reported | TRUS | Anaesthetic block of the periprostatic plexus: mepivacaine (1%) 2 ml at prostate apex |
| Jiang et al. 201940 | Not reporteda | Systematic | 12 | Pre-biopsy MRI performed in some participants (proportion not reported) | TRUS | Subcutaneous infiltration plus periprostatic nerve block: lidocaine (1%) |
| Szabo et al. 202141 | Freehand PrecisionPoint | Systematic Participants with PI-RADS 4 or 5 had additional cognitive (42/242) or software-based (6/242) targeted biopsy |
Varied with the size of the prostate (samples spaced 1 cm apart) | 31% had pre-biopsy MRI | Ultrasound | Lidocaine gel (2%) 10 ml into the rectum and lidocaine (0.5%) 5 ml mixed with 8.4% sodium bicarbonate injected into the perineal skin; additional 10 ml anaesthetic solution infiltrated into the ischiorectal fat, pelvic diaphragm, and periapical triangle. Maximum dose: 4.5 mg/kg |
| Szabo et al. 202141 | Freehand co-axial needle (without PrecisionPoint) | Not reported | Not reported | Not reported | Not reported | Not reported |
| Study | Age, years, mean (SD) | PSA ng/ml, mean (SD) | Prostate volume, cm3, mean (SD) | Abnormal DRE findings, n/N (%) | Abnormal pre-biopsy imaging findings |
|---|---|---|---|---|---|
| RCTs | |||||
| Cerruto et al. 201424 | |||||
| LATP | 66.50 (8.87) | 15.95 (41.04) | 56.29 (31.33) | 11/54 (20.4) | 10/54 (18.2) |
| LATRUS | 67.30 (8.05) | 12.36 (36.95) | 61.49 (33.39) | 10/54 (18.2) | 10/54 (18.2) |
| Guo et al. 201525 | |||||
| LATP | 67.18 (6.76) | 8.81 (3.6–56.0)a | 47.2 (12.9–97.7) | 20/173 (11.6) | 40/173 (23.1)b |
| LATRUS | 67.35 (7.28) | 10.48 (6.2–69.0)a | 45.9 (20.0–98.0) | 19/166 (11.5) | 30/166 (20.1)b |
| Hara et al. 200826 | |||||
| LATP | 71.0 (7.29) | 8.34 (3.44) | 33.2 (15.2) | 23/126 (18)b | |
| LATRUS | 71.7 (7.55) | 8.48 (3.90) | 36.0 (17.1) | 22/120 (18.0) | 12/120 (10)b |
| Lam et al. 2021 (AB)27 | |||||
| LATP | Not reported | Not reported | Not reported | Not reported | Not reported |
| LATRUS | Not reported | Not reported | Not reported | Not reported | Not reported |
| Takenaka et al. 200828 | |||||
| LATP | 71.1 (7.53) | 17.1 (30.1) | 34.5 (18.9)c | 16/100 (16.0) | 28/100 (28)b |
| LATRUS | 72.1 (7.42) | 19.6 (43.2) | 37.2 (19.7)c | 28/100 (28.0) | 22/100 (22)b |
| Other prospective studies | |||||
| Bojin 201929 | |||||
| LATP | 65 (45–82)e | 10.5 (3.6–89)i | 57 (15–210)e | Not reported | Unclear |
| LATRUS | 69 (43–88)e | 32.44 (1–1581)i | 51.6 (16–175)e | Not reported | Unclear |
| Chen et al. 202130,31 | |||||
| LATP | 69.40 (7.75) | 13.17 (6.82–47.13)a | 45.08 (26.78)c | 102/205 | Unclear |
| LATRUS | 68.24 (7.98) | 10.76 (6.45–50.97)a | 49.62 (27.76)c | 77/177 | Not reported |
| Emiliozzi et al. 200332 | |||||
| LATP and LATRUSd | 68 (52–88)e | 8.2 (4.1 to 240)e | Not reported | 26/107 (24.0) | 29/107 (27)b |
| Hung et al. 2020 (AB)33 | |||||
| LATP and LATRUSf | Median 68 | 7.66 (3.23) | Not reported | Not reported | Not reported |
| Kum et al. 2018 (AB)34,35 | |||||
| LATP | 65 (36–83)e | 7.9 (0.7–1374)e | 45 (15–157)e | Not reported | Not reported |
| LATRUS | Not reported | Not reported | Not reported | Not reported | Not reported |
| Starmer et al. 202136,37 | |||||
| LATP | 66.8 (53–80)g | 10.7 (2.2–55.6)g | 47.8 (20–100)g,h | Not reported | Not reported |
| LATRUS | 66.5 (52–78)g | 18.15 (1.2–160)g | 48.0 (14–147)g,h | Not reported | Not reported |
| Watanabe et al. 200538 | |||||
| LATP and LATRUSd | 72.5 (41 to 98)e | Median 10.3 | Not reported | 130 (32.3) | Not reported |
| Retrospective studies | |||||
| Abdollah et al. 201139 | |||||
| LATP | 66.4 (52.0–79.0)e | 10 (0.9 to 31.5)e | 62.3 (17.0–98.0)c | 15/140 (10.7) | Not reported |
| LATRUS | 66.2 (47.6–82.1)e | 9.7 (2.1 to 26.2)e | 65.4 (15.0–93.0)c | 16/140 (11.4) | Not reported |
| Jiang et al. 201940 | |||||
| LATP | 69.72 (8.93) | 38.02 (91.11) | 51.75 (23.94)c | Not reported | Not reported |
| LATRUS | 69.20 (8.03) | 40.31 (130.08) | 59.64 (33.44)c | Not reported | Not reported |
| Szabo et al. 202141 | |||||
| LATP using PrecisonPoint | 63 (9) | 7.2 (7.7) | 50 (35.7)c | Not reported | Not reported |
| LATP coaxial needle | Not reported | Not reported | Not reported | Not reported | Not reported |
| LATRUS | Not reported | Not reported | Not reported | Not reported | Not reported |
| Study | Device/approach | Sampling | Number of cores taken | Pre-biopsy imaging (MRI) | Prostate biopsy image guidance | Anaesthesia |
|---|---|---|---|---|---|---|
| RCTs | ||||||
| Lv et al. 202042 | Grid and stepper | Systematic and targeted | 12 + X targeted cores as per suspicious areas on MRI | Pre-biopsy MRI was performed | TRUS for systematic cores; MRI/TRUS cognitive fusion for targeted cores. | Subcutaneous perineal anaesthesia: lidocaine (2%) 5 ml and 1 : 200,000 adrenaline. Followed by deep periprostatic anaesthesia on right then left side of prostate |
| Other prospective studies | ||||||
| Takuma et al. 2012 (AB)43 | Not reported | Systematic and targeted | 10 + additional cores from suspicious lesions on DRE or ultrasound | Not reported | Not reported | Lumbar spinal anaesthesia (no details reported) |
| Walters et al. 2021 (AB)44 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Retrospective studies | ||||||
| Rij and Chapman 2020 (AB)45 | Freehand PrecisionPoint | Systematic (Ginsburg consensus method); plus targeted for 88% participants with a MRI abnormality; targeted only for 43% of participants | Median of 20.6 for the systematic biopsies | Pre-biopsy MRI was performed | Not reported | Local anaesthesia without sedation (no details reported) |
| Study | Age, years, mean (SD) | PSA ng/ml, mean (SD) | Prostate volume, ml, mean (SD) | Abnormal DRE findings, n/N (%) | Abnormal pre-biopsy imaging findings |
|---|---|---|---|---|---|
| RCTs | |||||
| Lv et al. 202042 | |||||
| LATP | 66.50 (9.48) | 22.00 (22.59) | 53.05 (15.43) | 90/108 (83.33) | 105/108 (97.22) |
| GATP | 67.06 (7.55) | 22.97 (24.78) | 54.00 (19.04) | 81/108 (75.00) | 102/108 (94.44) |
| Other studies (observational) | |||||
| No information reported by: Takuma et al. 2012 (AB)43 Walters et al. 2021 (AB)44 Rij and Chapman 2020 (AB)45 |
|||||
| Study | Age, years, mean (SD) | PSA ng/ml, mean (SD) | Prostate volume, cm3, mean (SD) | Abnormal DRE findings, n/N | Abnormal pre-biopsy imaging findings |
|---|---|---|---|---|---|
| RCTs | |||||
| Lam et al. 2021 (AB)27 | |||||
| LATP | Not reported | Not reported | Not reported | Not reported | Not reported |
| LATRUS | Not reported | Not reported | Not reported | Not reported | Not reported |
| Other prospective studies | |||||
| Bojin 201929 | |||||
| LATP | 65 (45–82)e | 10.5 (3.6–89)i | 57 (15–210)e | Not reported | Unclear |
| LATRUS | 69 (43–88)e | 32.44 (1–1581)i | 51.6 (16–175)e | Not reported | Unclear |
| Chen et al. 202130,31 | |||||
| LATP | 69.40 (7.75) | 13.17 (6.82–47.13)a | 45.08 (26.78)c | 102/205 | Unclear |
| LATRUS | 68.24 (7.98) | 10.76 (6.45–50.97)a | 49.62 (27.76)c | 77/177 | Not reported |
| Hung et al. 2020 (AB)33 | |||||
| LATP and LATRUSf | Median 68 | 7.66 (3.23) | Not reported | Not reported | Not reported |
| Kum et al. 2018 (AB)34,35 | |||||
| LATP using PrecisionPoint | 65 (36–83)a | 7.9 (0.7–1374)a | 45 (15–157)a | Not reported | Not reported |
| LATRUS | Not reported | Not reported | Not reported | Not reported | Not reported |
| Starmer et al. 202136,37 | |||||
| LATP using PrecisionPoint | 66.8 (53–80)b | 10.7 (2.2–55.6)b | 47.8 (20–100)b,c | Not reported | Not reported |
| LATRUS | 66.5 (52–78)b | 18.15 (1.2–160)b | 48.0 (14–147)b,c | Not reported | Not reported |
| Retrospective studies | |||||
| Szabo et al. 202141 | |||||
| LATP using PrecisonPoint | 63 (9) | 7.2 (7.7) | 50 (35.7)d | Not reported | Not reported |
| LATRUS | Not reported | Not reported | Not reported | Not reported | Not reported |
| Study | Population | Intervention | Comparator | Outcomes |
|---|---|---|---|---|
| LATP vs. LATRUS | ||||
| Study: TRANSLATE53,54 ISRCTN9815968952 Country: UK (multicentre RCT) Estimated completion date: October 2023 |
Men undergoing investigation for suspected prostate cancer Target recruitment: n = 1042 |
LATP biopsy using the PrecisionPoint and UA1232 devices; pre-biopsy MRI will influence any additional targeted biopsies | LATRUS biopsy; pre-biopsy MRI will influence any additional targeted biopsies | Detection rates; infection rates; hospital readmissions; HRQoL; tolerability; complications, e.g. bleeding, pain, erectile function; number of subsequent biopsies; cost |
| Study: ProBE-PC55 NCT04081636 Country: USA (single-centre RCT) Estimated completion date: December 2022 |
Men requiring prostate biopsy due to clinical suspicion of prostate cancer Estimated recruitment: n = 568 |
LATP biopsy (either with ultrasound-guided or with MRI-guided biopsy) | LATRUS biopsy (either with ultrasound-guided or with MRI-guided biopsy) | Rate of infectious complications; rate of bleeding complications; cancer detection rate; tolerability under local anaesthesia; urinary function; cost; sexual function |
| Study: aNCT0484356656 Country: USA (multicentre RCT) Estimated completion date: June 2025 |
Men with elevated PSA or abnormal digital rectal exam Estimated recruitment: n = 400 |
MRI-targeted LATP biopsy | MRI-targeted LATRUS biopsy | Infection adverse events; pain and discomfort; anxiety; detection of CS disease; change in adverse events |
| Study: aNCT0481587657 Country: USA (multicentre RCT) Estimated completion date: April 2025 |
Men on active surveillance, or with prior negative prostate biopsy and a clinical concern for the presence of prostate cancer Estimated recruitment: n = 1302 |
MRI-targeted LATP biopsy | MRI-targeted LATRUS biopsy | Infection adverse events; pain and discomfort; anxiety; detection of CS disease; change in adverse events |
| LATP vs. GATP | ||||
| Study: LATProBE58 ACTRN12620001145998p Country: Australia (multicentre RCT) Estimated completion date: Not yet recruiting. |
Men with suspected prostate cancer Target recruitment: n = 620 |
Freehand LATP biopsy (no device reported) | GATP biopsy using a template grid | Cancer detection rates; costs; patient experience; pain; 30-day complications; HRQoL |
FIGURE 9.
Meta-analysis forest plot of cancer detection rates for LATP-any vs. LATRUS (decision question 1). REML, random-effects maximum likelihood.
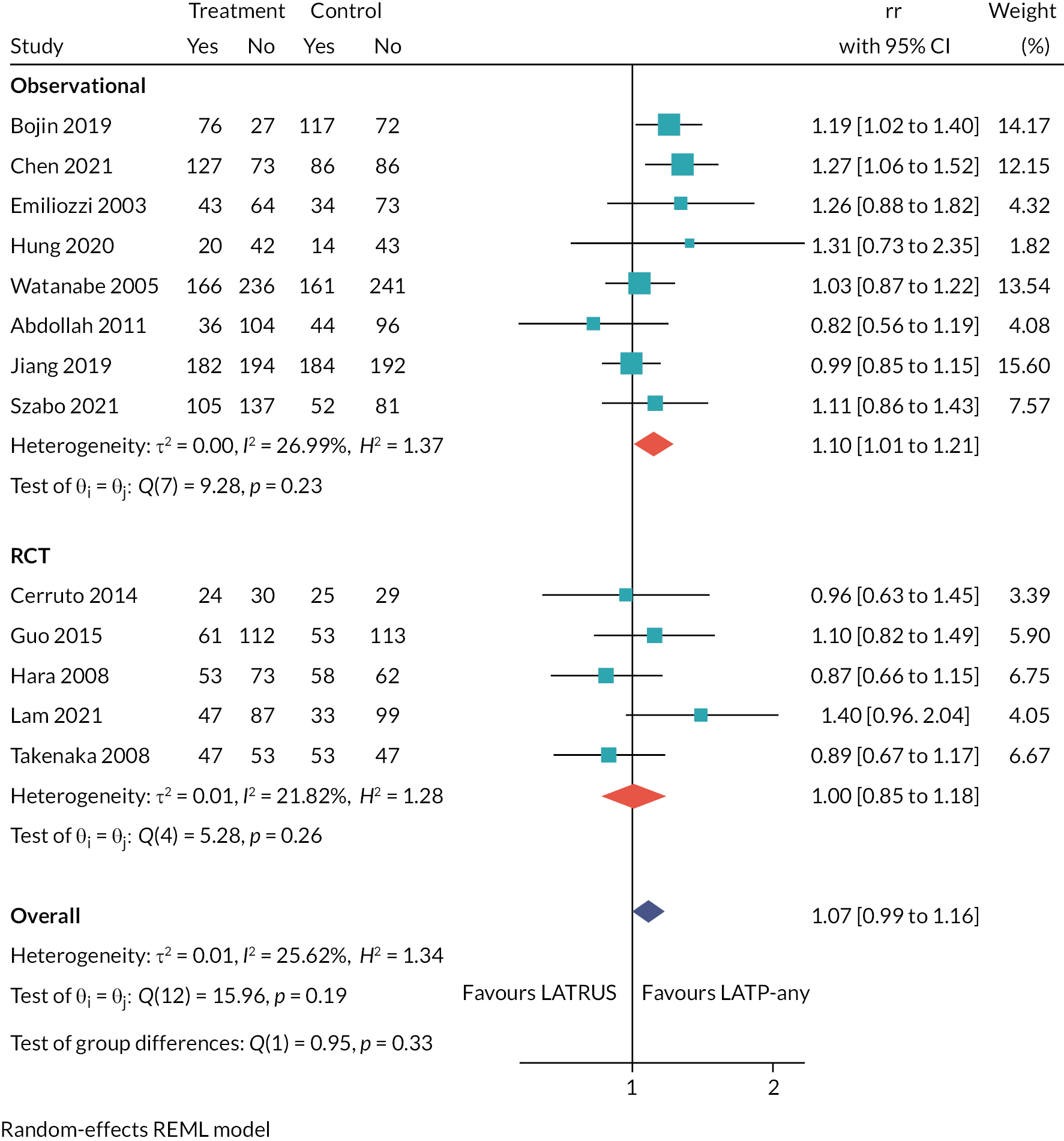
FIGURE 10.
Meta-analysis forest plot of CS cancer detection rates for LATP-any vs. LATRUS (decision question 1). REML, random-effects maximum likelihood.
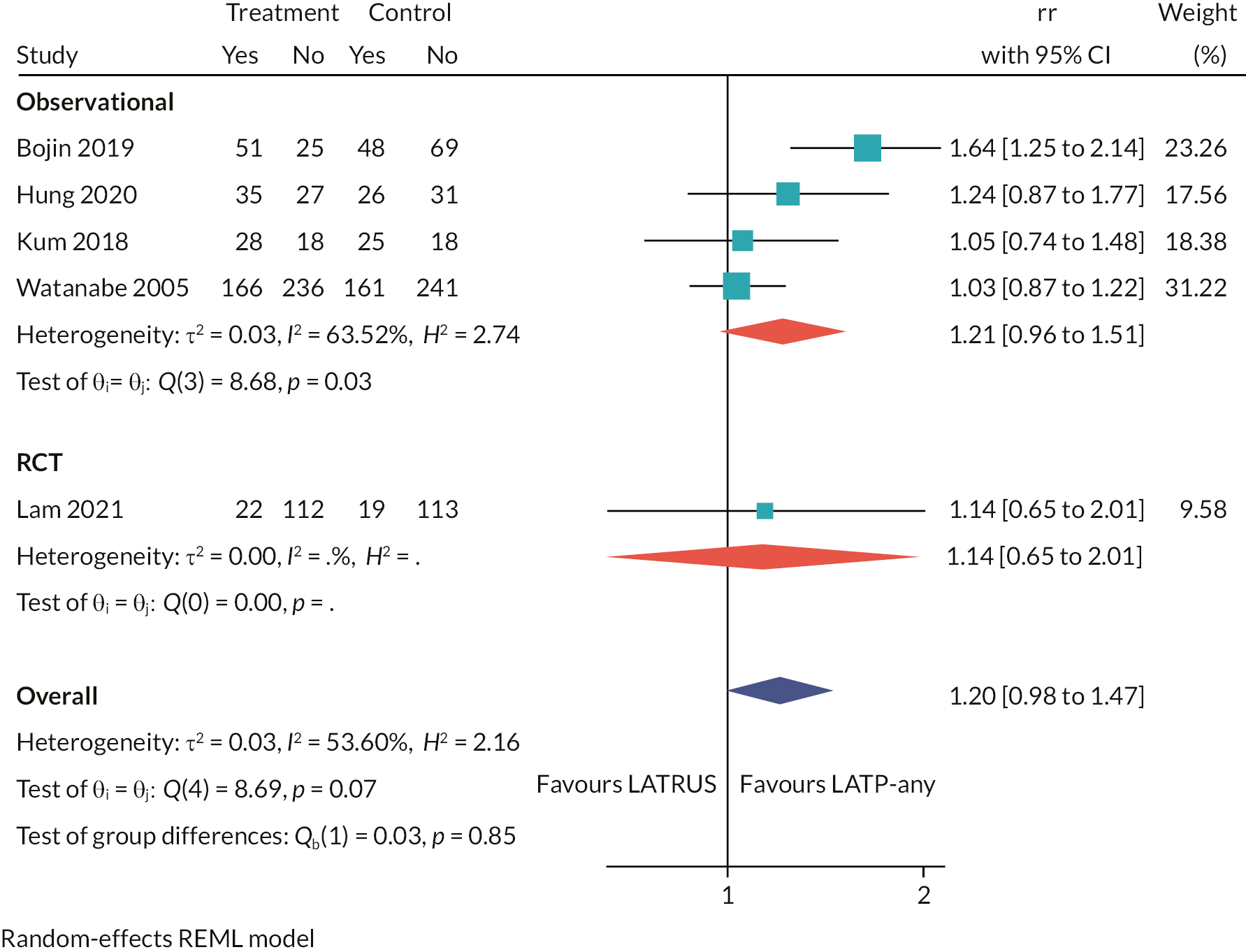
FIGURE 11.
Meta-analysis forest plot of cancer detection rates for LATP-any vs. GATP grid and stepping device (decision question 1).

FIGURE 12.
Evidence network for indirect comparison of LATP-any, LATRUS, and GATP grid and stepping device (decision question 1). NB: LATRUS is the reference treatment to which all other treatments are compared against.
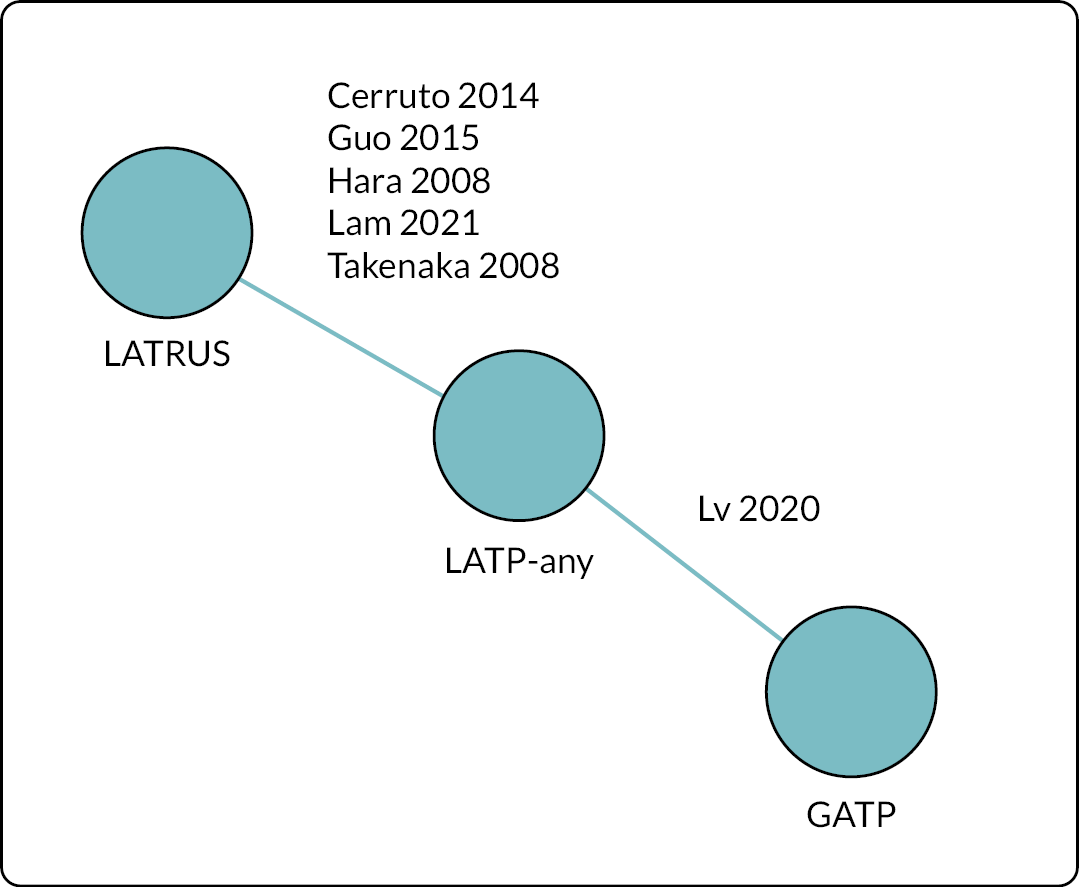
FIGURE 13.
Network meta-analysis forest plot of cancer detection rates for LATP-any vs. LATRUS vs. GATP grid and stepping device (decision question 1).

FIGURE 14.
Meta-analysis forest plot of cancer detection rates for LATP-freehand vs. LATRUS (decision question 2).
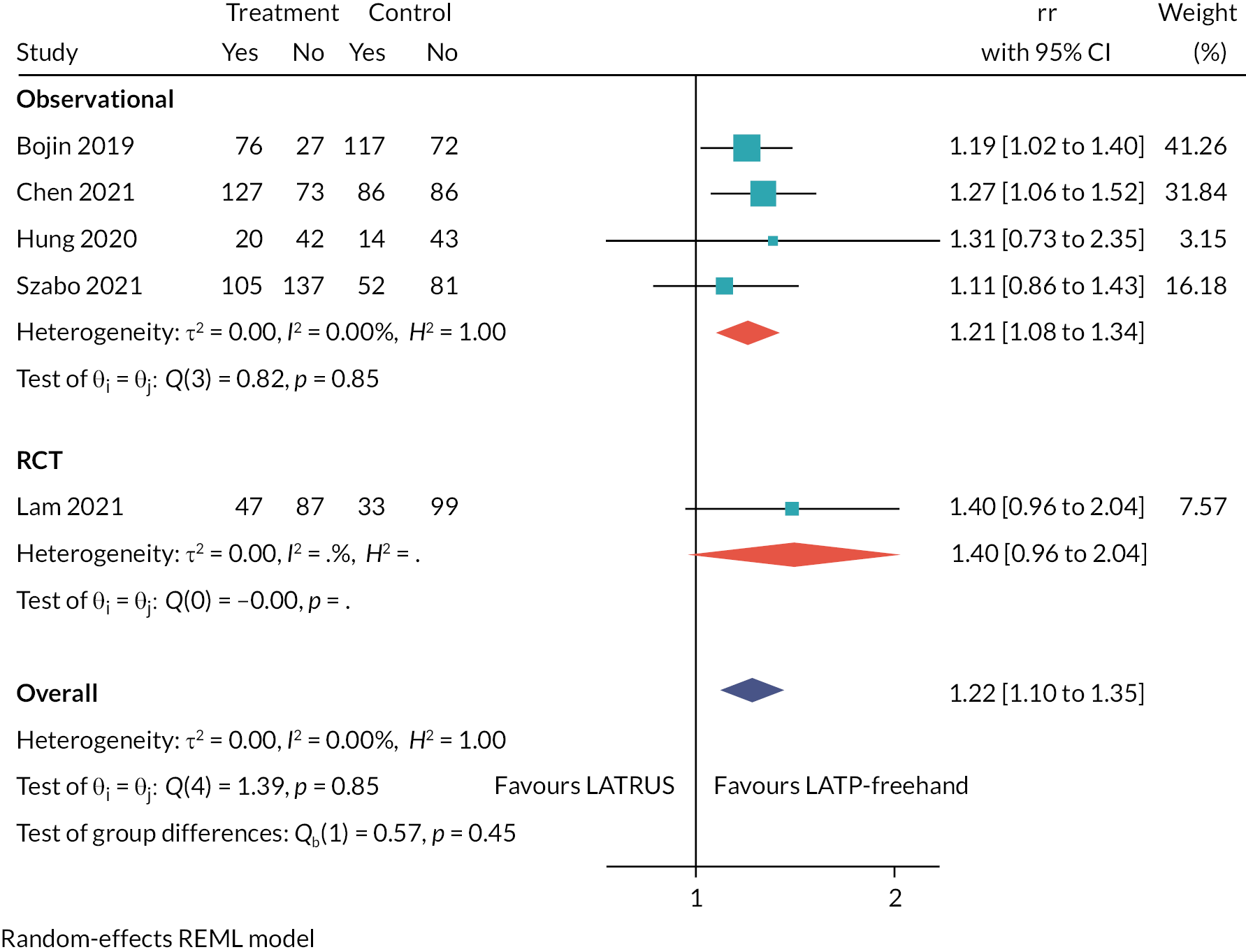
FIGURE 15.
Meta-analysis forest plot of cancer detection rates for LATP-other vs. LATRUS (decision question 2).
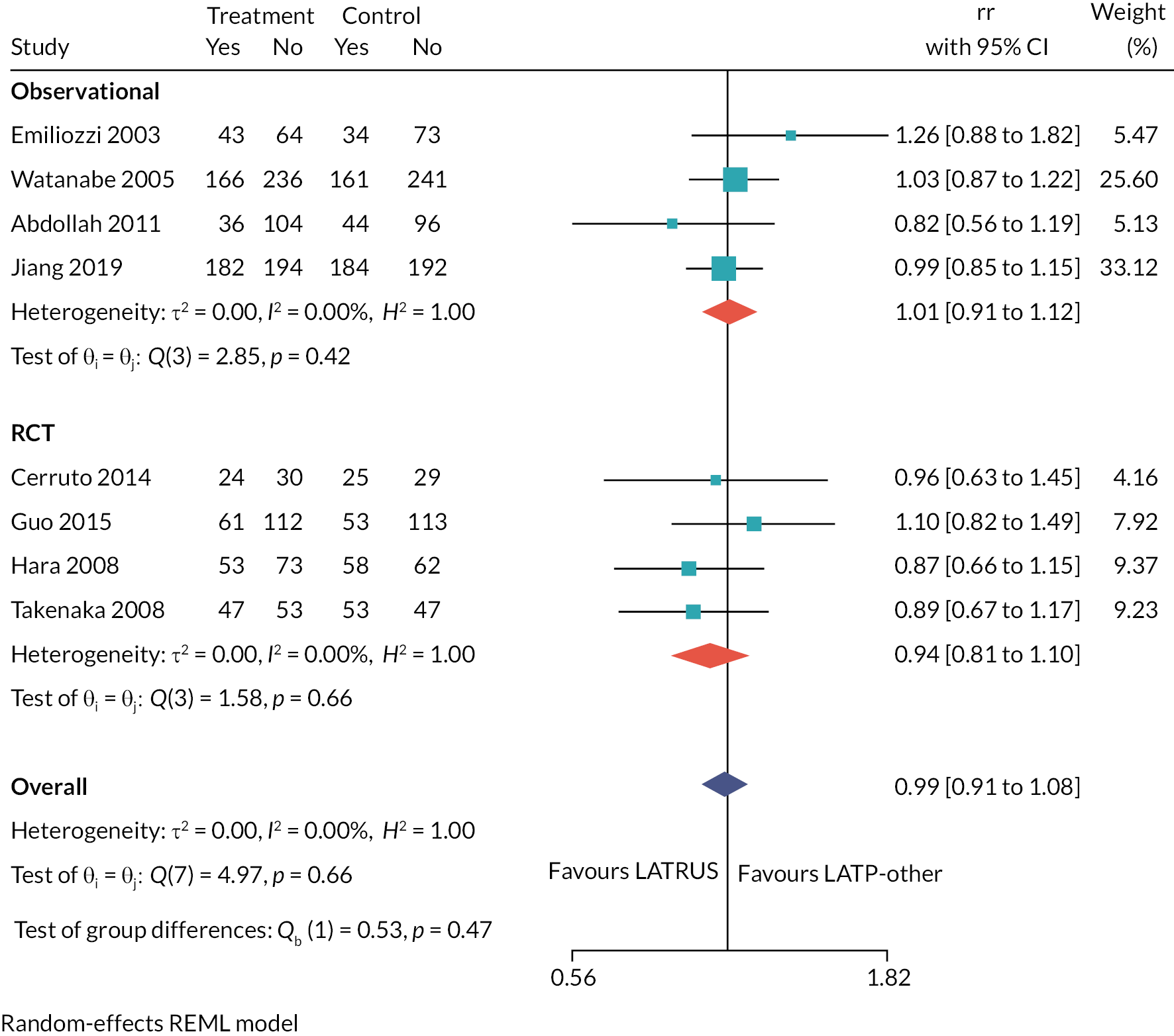
FIGURE 16.
Meta-analysis forest plot of CS cancer detection rates for LATP-freehand vs. LATRUS (decision question 2).
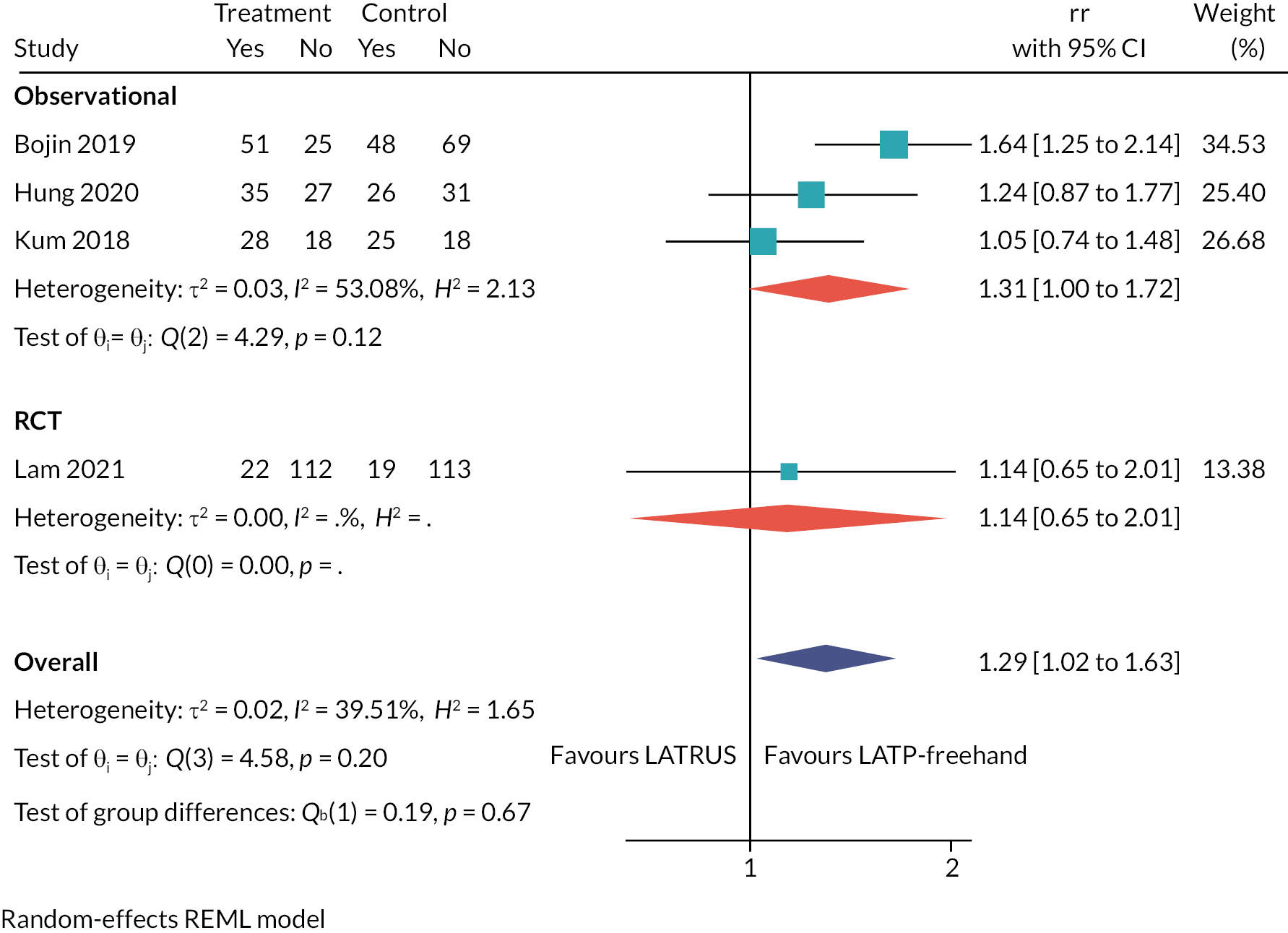
FIGURE 17.
Evidence network for indirect comparison of LATP-freehand, LATP-other, LATRUS, and GATP grid and stepping device (decision question 2). NB: LATRUS is the reference treatment to which all other treatments are compared.

Consistent with the pairwise meta-analyses described above, the NMA shows no statistically significant differences in cancer detection rates from RCTs between biopsy modalities (see Figure 18, Appendix 4).
FIGURE 18.
Forest plot of NMA results comparing cancer detection rates for LATP freehand, LATP other, GATP grid and stepping device and LATRUS.
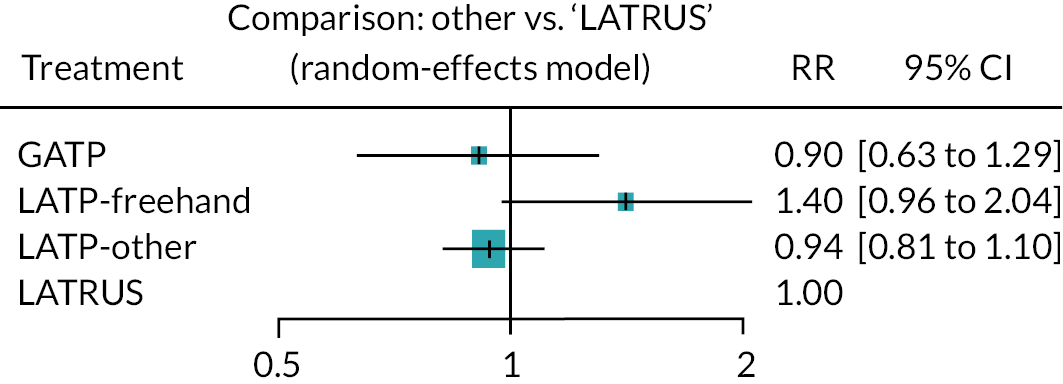
Appendix 5 Critical appraisal assessments of studies included in the systematic review of diagnostic test evaluation and clinical effectiveness
| Study | Random sequence generation | Allocation concealment | Blinding (participants; personnel) | Blinding (outcome assessors) | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| Cerruto et al. 201424 | Unclear | Unclear | High | Unclear | Low | Unclear |
| Guo et al. 201525 | Low | Unclear | High | Low | Low | Low |
| Hara et al. 200826 | Unclear | Unclear | High | Unclear | Low | Unclear |
| Lam et al. 2021 (AB)27 | Unclear | Unclear | High | Unclear | Low | Unclear |
| Lv et al. 202042 | Low | Unclear | High | Unclear | Low | Unclear |
| Takenaka et al. 200828 | Unclear | Unclear | High | Unclear | Low | Unclear |
Individual risk-of-bias assessments of included RCTs using the Cochrane Risk of Bias tool (version 1)
Cerruto et al . 2014 24
| Domain | Type of bias | Assessment (low, high, unclear) |
|---|---|---|
| Random sequence generation | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | UNCLEAR
States ‘with a randomisation ratio of 1:1.’ (p285). No further information provided. |
| Allocation concealment | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment | UNCLEAR
States ‘with a randomisation ratio of 1:1.’ (p285), but no further information provided. |
| Blinding of participants and personnel | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | HIGH
No details reported but blinding highly unlikely due to nature of study. Unclear whether there was any protocol in place to reduce the risk of differential behaviours by patients and healthcare provider. |
| Blinding of outcome assessors | Detection bias due to knowledge of the allocated interventions by outcome assessment | UNCLEAR
No information provided. |
| Incomplete outcome data | Attrition bias due to amount, nature, or handling of incomplete outcome data | LOW
Not explicitly reported but number of people in analysis is equal to number of people randomised |
| Selective outcome reporting | Reporting bias due to selective outcome reporting. | UNCLEAR
Insufficient information to permit judgement |
| Other sources of bias | Not applicable | Not applicable |
Guo et al . 2015 25
| Domain | Type of bias | Assessment (low, high, unclear) |
|---|---|---|
| Random sequence generation | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | LOW
‘The randomization procedure was carried out before biopsy using a computer-generated random-number sequence to assign patients to two groups’ (p. 2) |
| Allocation concealment | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment | UNCLEAR
‘two independent investigators were in charge of the randomization procedure, data recording, and follow-up’ (p. 2) |
| Blinding of participants and personnel | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | HIGH
‘All patients and investigators were aware of study group assignments except for the pathologist’ (p. 2). Unclear whether there was any protocol in place to reduce the risk of differential behaviours by patients and healthcare provider. |
| Blinding of outcome assessors | Detection bias due to knowledge of the allocated interventions by outcome assessment | LOW
‘One pathologist with 20 years’ experience made all the pathological diagnoses. Besides, two independent investigators were in charge of the randomization procedure, data recording, and follow-up. All patients and investigators were aware of study group assignments except for the pathologist’ |
| Incomplete outcome data | Attrition bias due to amount, nature, or handling of incomplete outcome data | LOW
All participants analysed on intention-to-treat basis, except for post-biopsy complications where 6 from TP were lost to follow-up and 5 from transrectal biopsy were lost to follow-up |
| Selective outcome reporting | Reporting bias due to selective outcome reporting. | UNCLEAR
Insufficient information to permit judgement |
| Other sources of bias | Not applicable | Not applicable |
Hara et al . 2008 26
| Domain | Type of bias | Assessment (low, high, unclear) |
|---|---|---|
| Random sequence generation | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | UNCLEAR
‘a prospective randomized study of transperineal vs. transrectal 12-core biopsy’, ‘we performed a prospective randomized Study’. No further information provided. |
| Allocation concealment | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment | UNCLEAR
‘a prospective randomized study of transperineal vs. transrectal 12-core biopsy’, ‘we performed a prospective randomized study’. No further information provided |
| Blinding of participants and personnel | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | HIGH
No details reported but blinding highly unlikely due to nature of study. Unclear whether there was any protocol in place to reduce the risk of differential behaviours by patients and healthcare provider |
| Blinding of outcome assessors | Detection bias due to knowledge of the allocated interventions by outcome assessment | UNCLEAR
No information provided |
| Incomplete outcome data | Attrition bias due to amount, nature, or handling of incomplete outcome data | LOW
Not explicitly reported but denominator for overall cancer detection rate is the same as that randomised |
| Selective outcome reporting | Reporting bias due to selective outcome reporting. | UNCLEAR
Insufficient information to permit judgement |
| Other sources of bias | Not applicable | Not applicable |
Lam et al . 2021 27
| Domain | Type of bias | Assessment (low, high, unclear) |
|---|---|---|
| Random sequence generation | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | UNCLEAR
‘A parallel group randomized study of men suspected with Pca were allocated in a 1 : 1 ratio.’ No further information provided |
| Allocation concealment | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment | UNCLEAR
‘A parallel group randomized study of men suspected with Pca were allocated in a 1 : 1 ratio.’ No further information provided |
| Blinding of participants and personnel | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | HIGH
No details reported but blinding highly unlikely due to nature of study. Unclear whether there was any protocol in place to reduce the risk of differential behaviours by patients and healthcare provider |
| Blinding of outcome assessors | Detection bias due to knowledge of the allocated interventions by outcome assessment | UNCLEAR
No information provided |
| Incomplete outcome data | Attrition bias due to amount, nature, or handling of incomplete outcome data | LOW
Not explicitly reported but denominator for overall cancer detection rate is the same as that randomised reported |
| Selective outcome reporting | Reporting bias due to selective outcome reporting. | UNCLEAR
Insufficient information to permit judgement |
| Other sources of bias | Not applicable | Not applicable |
Lv et al . 2020 42
| Domain | Type of bias | Assessment (low, high, unclear) |
|---|---|---|
| Random sequence generation | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | LOW
‘All patients were randomly assigned to the control group or the experimental group at a ratio of 1 : 1. The randomisation was implemented with SPSS 19.0 for Windows, which randomly generated a series of numbers. The randomisation was conducted by an independent doctor to ensure that membership in each group could not be predicted’ |
| Allocation concealment | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment | UNCLEAR
‘All patients were randomly assigned to the control group or the experimental group at a ratio of 1 : 1. The randomisation was implemented with SPSS 19.0 for Windows, which randomly generated a series of numbers. The randomisation was conducted by an independent doctor to ensure that membership in each group could not be predicted’ |
| Blinding of participants and personnel | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | HIGH
‘it was not possible to blind the groups and the operator. The lack of blinding may have affected the operator’s perceptions and led to measurement bias in the questionnaire results’ |
| Blinding of outcome assessors | Detection bias due to knowledge of the allocated interventions by outcome assessment | UNCLEAR
‘The secondary outcomes included changes in vital signs during the procedure, the operative time, the volume of blood loss, the duration of hospitalisation and the incidence of postoperative complications. The operative time was the combined anaesthetic time and puncture time. The postoperative complications were infection, perineal haematoma, urethral bleeding, haematospermia, retention of urine and dysuresia. All the observed indexes mentioned above were recorded by an independent urologist’ |
| Incomplete outcome data | Attrition bias due to amount, nature, or handling of incomplete outcome data | LOW
Figure 1. Consort diagram of patient enrolment shows that no patients were lost to follow-up or excluded from the analyses |
| Selective outcome reporting | Reporting bias due to selective outcome reporting. | UNCLEAR
Insufficient information to permit judgement |
| Other sources of bias | Not applicable | Not applicable |
Takenaka et al . 2008 28
| Domain | Type of bias | Assessment (low, high, unclear) |
|---|---|---|
| Random sequence generation | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | UNCLEAR
‘We prospectively randomized’; ‘The randomly assigned groups of 100 patients underwent TP 12-core biopsy or TR 12-core biopsy’ |
| Allocation concealment | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment | UNCLEAR
‘We prospectively randomized’; ‘The randomly assigned groups of 100 patients underwent TP 12-core biopsy or TR 12-core biopsy’ |
| Blinding of participants and personnel | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | HIGH
No details reported but blinding highly unlikely due to nature of study. Unclear whether there was any protocol in place to reduce the risk of differential behaviours by patients and healthcare provider |
| Blinding of outcome assessors | Detection bias due to knowledge of the allocated interventions by outcome assessment | UNCLEAR
No information provided |
| Incomplete outcome data | Attrition bias due to amount, nature, or handling of incomplete outcome data | LOW
Not explicitly reported but number of people in analysis is equal to number of people randomised |
| Selective outcome reporting | Reporting bias due to selective outcome reporting. | UNCLEAR
Insufficient information to permit judgement |
| Other sources of bias | Not applicable | Not applicable |
Summary of risk-of-bias assessments of included non-randomised observational studies using The Joanna Briggs Institute Critical Appraisal Checklists
The tables below show reviewer responses to the JBI checklist questions for critical appraisal of included cohort studies (see Table 60) and included case series (see Table 61) The reasons for the responses are documented in a spreadsheet available from the review authors on request.
| JBI Checklist for cohort studies50 | Study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdollah et al. 201139 | Bojin 201929 | Chen et al. 202130 | Emiliozzi et al. 200332 | Hung et al. 202033 | Jiang et al. 201940 | Kum et al. 201834 | Rij et al. 202045 |
Starmer et al. 202136 | Takuma et al. 201243 | Watanabe et al. 200538 | |
| 1. Were the two groups similar and recruited from the same population? | Yes | Unclear | Unclear | Yes | Yes | Yes | Unclear | Unclear | Yes, and No | Yes | Yes |
| 2. Was each biopsy method clearly defined and described to enable reviewers to assess whether or not the participants received the biopsies of interest? a | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | No | Yes |
| 3. Were the biopsies carried out in a valid and reliable way? E.g. use of a protocol or schema for sampling of cores, other protocols, staff carrying out the procedure a | Yes | Yes | Unclear | Yes | Unclear | Yes | Unclear | Yes | Yes | Unclear | Unclear |
| 4. Were confounding factors identified? | Yes | No | Yes | NA | Unclear | Yes | No | No | Yes | No | NA |
| 5. Were strategies to deal with confounding factors stated? | Yes | No | Yes | NA | No | Yes | No | No | Yes | No | NA |
| 6. Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | Unclear | Unclear | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| 7. Were the outcomes measured in a valid and reliable way? | Unclear | Yes for CDR; Unclear for other outcomes | Yes | Yes | Unclear for CDR; Yes for other outcomes | Yes | Yes for CDR; Unclear for pain and complications | Yes for CDR; Unclear for complications | Yes for tolerability and CDR; Unclear for complication | Unclear | Yes |
| 8. Was the follow-up time reported and sufficient to be long enough for outcomes to occur? | NA | Unclear/NA | Unclear | Yes | Unclear | NA | Unclear | Unclear | Yes | Unclear | Unclear |
| 9. Was follow-up complete, and if not, were the reasons to loss to follow-up described and explored? | NA | Unclear | Unclear | Yes | Unclear | NA | Unclear | Unclear | Yes/Unclear | Unclear | Unclear |
| 10. Were strategies to address incomplete follow-up utilised? | NA | Unclear | Unclear | Unclear | Unclear | NA | Unclear | Unclear | Unclear | Unclear | Unclear |
| 11. Was appropriate statistical analysis used? | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| JBI Checklist for case series51 | Study | |
|---|---|---|
| Szabo et al. 202141 | Walters et al. 202144 | |
| 1. Were there clear criteria for inclusion in the case series? | No | No |
| 2. Was the condition measured in a standard, reliable way for all participants included in the case series? | Yes | Unclear |
| 3. Were valid methods used for identification of the condition for all participants included in the case series? | Unclear | Unclear |
| 4. Did the case series have consecutive inclusion of participants? | Yes | Yes |
| 5. Did the case series have complete inclusion of participants? | Yes | Yes |
| 6. Was there clear reporting of the demographics of the participants in the study? | Unclear | No |
| 7. Was there clear reporting of clinical information of the participants? | Unclear | No |
| 8. Were the outcomes or follow-up results of cases clearly reported? | Yes | Unclear |
| 9. Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | Unclear | Unclear |
| 10. Was statistical analysis appropriate? | Yes | Unclear |
Appendix 6 Systematic review of cost-effectiveness studies
FIGURE 19.
Flow chart for the identification of economic studies.

| Study | Reasons for exclusion |
|---|---|
| Actrn 202058 | Only protocol/No results posted |
| NCT 2020202 | Only protocol/No results posted |
| Altok 2018203 | Does not include the interventions of interest |
| Brown 201864 | Does not include the interventions of interest |
| Faria 201865 | Does not include the interventions of interest |
| Cheng 2021204 | Does not include the interventions of interest |
| Cricco-Lizza 2021205 | Does not include the interventions of interest |
| Jimenez 2021113 | Not an economic evaluation |
| Popert 2020206 | Not an economic evaluation |
| Study | Wilson et al.63 | |
|---|---|---|
| Year | 2021 | |
| Country | UK | |
| Research question | What is the cost effectiveness of transperineal vs. TRUS-guided local anaesthesia procedures for prostate biopsy in the diagnosis of prostate cancer in a secondary care setting? | |
| Perspective of analysis | UK NHS | |
| Population | Men with suspected localised prostate cancer | |
| Interventions | TP biopsy (CamPROBE) vs. TRUS biopsy | |
| Type of model | Decision tree (diagnostic and short-term treatment pathway) Markov model (long-term consequences; composed by three health states: PF, metastatic and death) |
|
| Time horizon | Lifetime | |
| Cycle length | 1 year | |
| Discount rate | 3.5% | |
| Diagnostic pathway | Based on NICE guideline and on strategy ‘M7’ of the Faria et al. decision model, men referred to secondary care are offered a mpMRI:
|
|
| Model inputs | ||
| Prevalence of PC | No cancer: 27.9% Non-clinically significant cancer: 16.0% Intermediate-risk cancer: 52.9% High-risk cancer: 3.2% |
Source: PROMIS |
| Diagnostic accuracy | mpMRI mpMRI (NC)|NC: 0.33 (0.26–0.4) mpMRI (CNS)|NC: 0.17 (0.11–0.23) mpMRI (CS)|NC: 0.50 (0.43–0.58) mpMRI (NC)|CNS: 0.28 (0.19–0.38) mpMRI (CNS)|CNS: 0.16 (0.08–0.24) mpMRI (CS)|CNS: 0.56 (0.46–0.67) mpMRI (NC)|IR: 0.08 (0.05–0.11) mpMRI (CNS)|IR: 0.05 (0.02–0.07) mpMRI (CS)|IR: 0.87 (0.83–0.91) mpMRI (NC)|HR: 0.00 mpMRI (CNS)|HR: 0.00 mpMRI (CS)|HR: 1.00 |
Source: PROMIS, as reported in Faria et al.,65 definition 2, cutoff 3. Assumption, as per Faria et al. PROMIS, Schoots et al., as reported in Faria et al.,65 test 4, definition 2. Assumption, as per Faria et al.65 PROMIS, Schoots et al., as reported in Faria et al.,65 test 5, definition 2. Assumption, as per Faria et al.65 Assumption, as per NC findings above (see Faria et al.65) |
| First mpMRI-targeted TRUS/TPUS biopsy (if mpMRI = CS) Biopsy1 (NC)|NC: 1.00 Biopsy1 (CNS)|NC: 0.00 Biopsy1 (CS)|NC: 0.00 Biopsy1 (NC)|CNS: 0.79 (0.66–0.89) Biopsy1 (CNS)|CNS: 0.21 (0.11–0.34) Biopsy1 (CS)|CNS: 0.00 Biopsy1 (NC)|IR: 0.15 (0.09–0.21) Biopsy1 (CNS)|IR: 0.11 (0.06–0.16) Biopsy1 (CS)|IR: 0.74 (0.65–0.84) Biopsy1 (NC)|HR: 0.00 Biopsy1 (CNS)|HR: 0.00 Biopsy1 (CS)|HR: 1.00 |
||
| Second mpMRI-targeted TRUS/TPUS biopsy If first biopsy = NC and mpMRI = CS Biopsy2 (NC)|NC: 1.00 Biopsy2 (CNS)|NC: 0.00 Biopsy2 (CS)|NC: 0.00 Biopsy2 (NC)|CNS: 0.68 (0.02–1.00) Biopsy2 (CNS)|CNS: 0.32 (0.02–0.91) Biopsy2 (CS)|CNS: 0.00 Biopsy2 (NC)|IR: 0.05 (0.02–0.11) Biopsy2 (CNS)|IR: 0.08 (0.03–0.18) Biopsy2 (CS)|IR: 0.87 (0.71–0.95) Biopsy2 (NC)|HR: 0.05 (0.02–0.11) Biopsy2 (CNS)|HR: 0.08 (0.03–0.18) Biopsy2 (CS)|HR: 0.87 (0.71–0.95) |
||
| If first biopsy = CNS and mpMRI = CS Biopsy2 (NC)|NC: 1.00 Biopsy2 (CNS)|NC: 0.00 Biopsy2 (CS)|NC: 0.00 Biopsy2 (NC)|CNS: 0.68 (0.02–1) Biopsy2 (CNS)|CNS: 0.32 (0.02–0.91) Biopsy2 (CS)|CNS: 0.00 Biopsy2 (NC)|IR: 0.05 (0.02–0.11) Biopsy2 (CNS)|IR: 0.08 (0.03–0.18) Biopsy2 (CS)|IR: 0.87 (0.71–0.95) Biopsy2 (NC)|HR: 0.05 (0.02–0.11) Biopsy2 (CNS)|HR: 0.08 (0.03–0.18) Biopsy2 (CS)|HR: 0.87 (0.71–0.95) |
||
| Biopsy complications | TRUS biopsy No infection: 0.92 Mild infection: 0.04 (0.03–0.07) UTI: 0.033 (0.02–0.06) Sepsis: 0.00 (0.00–0.02) TP biopsy No infection: 1.00 Mild infection: 0.00 UTI: 0.00 Sepsis: 0.00 Mortality from sepsis: 0.04 (0.03–0.05) |
Source: Zani et al.66 Assumption Lee et al.108 |
| Long-term transition probabilities | CNS cancer PF to metastatic: 0.01 (0.00–0.01) PF to dead: 0.05 (0.04–0.06) Metastatic to dead: 0.14 (0.06–0.23) Intermediate-risk cancer Active surveillance PF to metastatic: 0.02 (0.01–0.03) PF to dead: 0.06 (0.05–0.08) Metastatic to dead: 0.15 (0.07–0.22) Radical prostatectomy PF to metastatic: 0.01 (0.00–0.01) PF to dead: 0.05 (0.05–0.06) Metastatic to dead: 0.14 (0.06–0.23) High-risk cancer Active surveillance PF to metastatic: 0.02 (0.01–0.03) PF to dead: 0.08 (0.06–0.10) Metastatic to dead: 0.16 (0.09–0.23) Radical prostatectomy PF to metastatic: 0.01 (0.00–0.01) PF to dead: 0.07 (0.05–0.09) Metastatic to dead: 0.15 (0.07–0.23) |
Source: Fit from figures reported in Faria et al.65 |
| Treatment complications | Following radical prostatectomy Sexual dysfunction: 34.6% Urinary incontinence: 8.2% Bowel dysfunction: 5.9% Following active surveillance Sexual dysfunction: 20.1% Urinary incontinence: 3.1% Bowel dysfunction: 5.5% |
Source: Will et al., converted to 1-year probabilities as per Faria et al.65 |
| Unit costs | Diagnosis mpMRI: £217 TRUS biopsy: £17 TP biopsy: £0 Complications Fever: £40 UTI: £46 Sepsis: £2206 Treatments Watchful waiting (per year): £123 Radical prostatectomy: £6667 Radical prostatectomy AEs (per year): £207 MD (per year): £1990 |
Source: NHS Ref Costs 2018/19, Imaging: Outpatient, RD03Z Difference in cost between TP and TR. GP + 3-day trimethoprim GP + urinalysis + 7-day trimethoprim NHS Ref Costs 2018/19, Total HRGs, weighted average WJ06A to WJ06J 1 × follow-up visit + 3 × PSA test Surgery + 1 × first visit + 2 × follow-up visits |
| Components for compound costs Radical prostatectomy surgery: £6330 Surgical consultation pre surgery: £127 Surgical consultation follow-up: £105 Primary care PSA test: £6 Sexual dysfunction management: £217 Urinary incontinence management: £296 Bowel dysfunction management: £1810 GP visit: £39 Trimethoprim, 3 days: £0.40 Trimethoprim, 7 days: £0.93 Urinalysis: £6 |
Weighted average of 1-year probabilities As calculated by Faria et al.65 NHS Ref Costs 2018/19, EL, weighted average LB21A, LB21B, LB22Z NHS Ref Costs 2018/19, CL, WF01B, 101, urology. NHS Ref Costs 2018/19, CL, WF01A, 101, urology. NHS Ref Costs 2018/19, DAPS, DAPS09. NHS Ref Costs 2018/19, Total HRGs, LB43Z. Inflated to 2018/19 from Faria et al.65 Inflated to 2018/19 from Faria et al.65 PSSRU 2019, p. 120 Drug Tariff, March 2019, trimethoprim 200 mg × 6 Drug Tariff, March 2019, trimethoprim 200 mg × 14 Assumption (same as PSA test) |
|
| Utilities | QALY loss Fever: 0.001 UTI: 0.006 Sepsis: 0.040 Utility of progression free: age-dependent Disutility of MD: 0.137 |
Source: Assumption Barry et al.109 Faria et al.65 Faria et al.65 |
| Key assumptions |
|
|
| Results | ||
| Base-case results | TRUS biopsy Cost: £5052, QALYs: 10.291 TP biopsy Cost: £5022, QALYs: 10.292 Increment Cost: −£30, QALYs: 0.002, ICER: TPUS biopsy dominates TRUS biopsy |
|
| Sensitivity-analysis results | 1. One-way sensitivity analysis on the price of TP biopsy device, identifying the price associated with an ICER of £20,000. Increment results: Cost: £29, QALYs: 0.002, ICER: £19,999 2. One-way sensitivity analysis on risk of infection with TPUS biopsy, varying the risk between 0.0% and 100.0% of that of TRUS biopsy (base-case assumes zero risk of infection). Results: not reported 3. Two-way sensitivity analysis showing the maximum cost-effective per-procedure price of the TPUS biopsy device as a function of the infection risk. Results: maximum per-procedure cost-effective price of £15. |
|
| Conflicts of interest | Vincent J. Gnanapragasam is the inventor and patent holder of the CamPROBE device. All other authors confirm they have no conflicts of interest to declare. | |
| Funding | NIHR i4i Product Development Award (II-LB-0716-20001). | |
| Item | Wilson et al. 202163 | Comments | |
|---|---|---|---|
| RELEVANCE | |||
| 1 | Is the population relevant? E.g. demographics, risk factors, medical condition … |
Yes | |
| 2 | Are any critical interventions missing? | No | |
| 3 | Are any relevant outcomes missing? | No | |
| 4 | Is the context (settings and circumstances) applicable? E.g. geographic location, health care system, time horizon, perspective of analysis, discount rate … |
Yes | |
| CREDIBILITY | |||
| Design | |||
| 1 | Is the modelling methodology appropriate? Is the model structure described and does it reflect the disease process? Are its assumptions listed and justified? | Yes | |
| Data inputs | |||
| 2 | Are the data inputs for the model described and justified? | Yes | |
| Uncertainty | |||
| 3 | Has uncertainty been assessed? | Yes | |
| Validation | |||
| 4 | Has the model been validated? | Yes | |
| Study | Decision problem | Model | Parameters of interest | ||||
|---|---|---|---|---|---|---|---|
| type | Time horizon | Cycle length | Epidemiology, clinical, diagnostic | Utilities | Resource use/costs | ||
| Brown, 2018 (UK)64 |
Cost-effectiveness of diagnostic strategies using mpMRI, TRUS-guided biopsy and TPM biopsy (under general/spinal anaesthesia) in men with suspected localised prostate cancer | Decision tree + Markov model | – | – | Tables 26, 35 | Tables 28, 35 | Tables 29, 35 |
| Faria, 2018 (UK)65 |
Cost-effectiveness of diagnostic strategies using mpMRI, TRUS-guided biopsy and TPM biopsy (under general/spinal anaesthesia) in men with suspected localised prostate cancer | Decision tree + Markov model | Lifetime | – | Tables 2, S9 and S11 | Table S10 | Tables S11, S12 |
| Mowatt, 2013 (UK)99 |
Cost-effectiveness of using alternative MRS/MRI sequences to direct TRUS-guided biopsies compared to systematic TRUS-guided biopsy alone in patients with suspected prostate cancer and a prior negative/inconclusive biopsy | Decision tree + Markov model | 30 years | 3 months | Tables 16, 17, 18, 19 | Table 25 | Tables 18, 22, 23, 24 |
| Nicholson, 2015 (UK)207 |
Cost-effectiveness of PCA3 assay or phi, in combination with existing tests, scans and clinical judgement, in the diagnosis of prostate cancer in men suspected of having malignant disease in whom the results of an initial prostate biopsy were negative or equivocal | Decision tree | 3 years | – | Table 32 | Page 81–82 | Tables 32, 34, 35 |
| Cerantola, 2016 (Canada)208 |
Cost-effectiveness of MRI-cognitive targeted biopsy compared to TRUS-guided biopsy in diagnosing patients with suspected prostate cancer | Markov model | 5, 10, 15 and 20 years | 1 year | Table 1 | Section 2.4 | Tables 1, 2 |
| de Rooij, 2014 (The Netherlands)209 |
Cost-effectiveness of mpMRI followed by MRI-guided biopsy compared to TRUS-guided biopsy in diagnosing prostate cancer in patients with an elevated PSA | Decision tree + Markov model | 10 years | 1 year | Table 1 | Table 3 | Tables 1, 2 |
| Dijkstra, 2017 (The Netherlands)210 |
Cost-effectiveness of SelectMDx to identify patients for TRUS-guided biopsy compared to the use of PSA only to select for TRUS-guided biopsy in patients with an elevated PSA | Decision tree + Markov model | 18 years | 1 year | Table 1 | Table 2 | Tables 1, 3 |
| Hao, 2021 (Sweden)211 | Cost-effectiveness of MRI with combinations of targeted biopsy and systematic biopsy (at outpatient care) for early detection of prostate cancer within the context of organised quadrennial PSA screening among men aged 55–69 years | Microsimulation model | Lifetime | – | Table 1 | Table 1, S4 | Table S2 |
| Pahwa, 2017 (USA)130 |
Cost-effectiveness of mpMRI followed by MRI-guided biopsy compared to TRUS-guided biopsy to detect prostate cancer in biopsy-naïve men presenting with clinical suspicion of cancer | Decision tree | Lifetime | – | Table 1 | Table E2 | Table 2, E1 |
| Patel, 2018 (The Netherlands)212 |
Cost-effectiveness of three active surveillance strategies (TRUS-guided biopsy, mpMRI followed by MRI-guided biopsy, mpMRI alone) for patients with LR prostate cancer | Markov model | Lifetime | 1 year | Table 1 | Table 2 | Table 2 |
| Sathianathen, 2018 (USA)213 |
Cost-effectiveness of four biomarker tests (PHI, 4Kscore, SelectMDx and the EPI) to determine which individuals require biopsy compared to TRUS-guided biopsy alone in men with elevated PSA | Decision tree + Markov model | Lifetime | – | Supplementary table, appendix 2 | Supplementary table | Supplementary table |
|
Venderink, 2017
(The Netherlands) 214 |
Cost-effectiveness of three prostate biopsy approaches (TRUS-guided biopsy, direct in-bore MRI-guided biopsy and image fusion guided biopsy) for biopsy-naïve patients in whom CS prostate cancer was suspected | Decision tree + Markov model | 18 years | 1 year | Tables 1, 3 | Table 3 | Tables 1, 2 |
| NG131 model, 2019 (UK)67 | Cost-effectiveness of different follow-up strategies (including screening test, based on PSA and its derivatives at given intervals, and diagnostic procedures) for people who have a raised PSA, negative MRI and/or negative biopsy | Decision tree + Markov model | Lifetime | 3 months | Tables HE02, HE05, HE07, HE09, HE11 | Table HE14 | Tables HE08, HE12, HE13 |
Appendix 7 Systematic review of health-related quality of life
Inclusion/exclusion criteria for health-related quality of life review
| Inclusion criteria | Searches ‘HRQoL 1’ | Searches ‘HRQoL 2’ |
|---|---|---|
| Research type | Primary research studies | Primary research studies |
| Population | People undergoing screening/diagnostic tests for prostate cancer People diagnosed with prostate cancer |
People undergoing screening/diagnostic tests for prostate cancer People diagnosed with prostate cancer |
| Outcomes | SF-36, SF-12, SF-6D, EQ-5D, HUI-1, -2 and -3 and 15D | EQ-5D |
| Value set | – | UK |
| Exclusion criteria | Searches ‘HRQoL 1’ | Searches ‘HRQoL 2’ |
| Reference type | Conference abstracts, letters, protocols, case reports | Conference abstracts, letters, protocols, case reports |
| Language | Studies not in English language | Studies not in English |
| Others | – | Studies assessing the quality of life of specific treatments |
Results of the systematic searches ‘HRQoL 1’
FIGURE 20.
Flow chart for the identification of HRQoL studies (searches ‘HRQoL 1’).
| Study | Reasons for exclusion |
|---|---|
| Ahmed et al. 2011215 | Different HRQoL outcome |
| Aktas et al. 2014216 | Different HRQoL outcome |
| Awsare et al. 2008217 | Different HRQoL outcome |
| Azzouzi et al. 2013218 | Different HRQoL outcome |
| Burns et al. 2019219 | Can’t find full text |
| Cantor et al. 1995220 | Can’t find full text |
| Chaussy and Thüroff 2001221 | HRQoL outcome not specified |
| Dickinson et al. 2013222 | Protocol |
| Donovan et al. 2003223 | Can’t find full text |
| Egan et al. 2021224 | Different HRQoL outcome |
| Ganzer et al. 2018225 | Different HRQoL outcome |
| Ghai et al. 2015226 | Can’t find SF-12 results |
| Gu et al. 2015227 | HRQoL outcome not specified |
| Koch et al. 2007228 | Can’t find results |
| Kok et al. 2006229 | Different HRQoL outcome |
| Mettlin et al. 1997230 | No HRQoL outcomes |
| Miki et al. 2010231 | Different HRQoL outcome |
| Natarajan et al. 2016232 | Different HRQoL outcome |
| Naughton et al. 2001233 | Different HRQoL outcome |
| Pane-Alemany et al. 2021234 | Protocol |
| Pisters et al. 1997235 | Different HRQoL outcome |
| Soloway et al. 2010236 | Different HRQoL outcome |
| Uchida et al. 2005237 | Different HRQoL outcome |
| Valerio et al. 2014238 | Protocol |
| Van de Ven et al. 2013239 | Different study design |
| First author, year | N a | Country | Instrument | Health state(s) described |
|---|---|---|---|---|
| Blazevski et al. 202069 | 84 | Australia | SF-12 | At baseline, 6 weeks, 3, 6, 12 and 24 months after treating patients with localised prostate cancer with irreversible electroporation |
| Essink-Bot, 199870 | 1126 | Netherlands | SF-36, EQ-5D |
3 weeks before the screening for prostate cancer, waiting room preceding the screening, 1 week after receiving the unsuspicious results of the initial screening tests, during the 2-week waiting period for the biopsy result, and 1 week after receiving the negative results of the biopsy |
| Hamdy, 202071 | 1413 | UK | SF-12, EQ-5D |
At the recruitment phase to test for prostate cancer, at the moment of confirmatory biopsy, 6 and 12 months following randomisation to treatment strategy and yearly thereafter for at least 10 years |
| Hamid, 201972 | 110 | UK | EQ-5D-5L | Before repeat biopsy, at 1 and 6 weeks after repeat biopsy |
| Kasivisvanathan, 201873 | 483 | Severalb | EQ-5D-5L | At baseline, 24 hours and 30 days after the interventions (MRI-targeted biopsy or TRUS biopsy) |
| Peters, 201474 | 14 | Netherlands | SF-36 | At baseline, 1 and 6 months and then annually after focal salvage treatment for prostate cancer |
| Sefik, 202075 | 114 | Turkey | SF-36 | Before and 1 month after TRUS biopsy |
| Shankar, 201976 | 110 | USA | SF-12 | 1 to 3 days after the diagnostic test (mpMRI or TRUS biopsy) as part of active surveillance |
| Vasarainen, 201377 | 386 | Finland | SF-36 | At invitation to participate in the trial, after PSA blood sample collection, after DRE (unaware of its result but aware of PSA result), after TRUS biopsy (unaware of its results but aware of PSA result) |
| Health states | Utilitya | Source |
|---|---|---|
| Pre-screening | ||
| 3 weeks before | 0.86785 | Essink-Bot et al. 199870 |
| Before screening | 0.9387 | Vasarainen et al. 201377 |
| Screening | ||
| Right after collecting blood for PSA analysis | 0.936 | Vasarainen et al. 201377 |
| PSA result known (positive or negative) | 0.920 | Vasarainen et al. 201377 |
| Right after DRE (result unknown) | 0.906 | Vasarainen et al. 201377 |
| Screening negative result | 0.88215 | Essink-Bot et al. 199870 |
| Screening positive result | 0.908 | Kasivisvanathan et al. 201873 |
| 0.692 | Sefik et al. 202075 | |
| Diagnostic | ||
| 24 hours after MRI-targeted biopsy | 0.907 | Kasivisvanathan et al. 201873 |
| 30 days after MRI-targeted biopsy | 0.917 | Kasivisvanathan et al. 201873 |
| After TRUS biopsy (result unknown) | 0.936 | Vasarainen et al. 201377 |
| 24 hours after TRUS biopsy | 0.894 | Kasivisvanathan et al. 201873 |
| 30 days after TRUS biopsy | 0.921 | Kasivisvanathan et al. 201873 |
| 0.790 | Sefik et al. 202075 | |
| 30 days after TRUS biopsy (with tamsulosine) | 0.791 | Sefik et al. 202075 |
| Repeat biopsy | 0.879 | Hamid et al. 201972 |
| Biopsy negative result | 1.14889 | Essink-Bot et al. 199870 |
| Biopsy positive result | 0.883 | Hamdy et al. 202071 |
| Treatment | ||
| Active surveillance | ||
| Before procedure (mpMRI or TRUS biopsy) | 0.961 | Shankar et al. 201976 |
| Before mpMRI | 0.965 | Shankar et al. 201976 |
| Before TRUS biopsy | 0.956 | Shankar et al. 201976 |
| Irreversible electroporation | ||
| Before treatment | 0.979 | Blazevski et al. 202069 |
| Between 6 weeks and 24 months after | 0.979 | Blazevski et al. 202069 |
| Focal salvage treatment | ||
| Before treatment | 1.015 | Peters et al. 201474 |
| 1 month | 0.967 | Peters et al. 201474 |
| 6 months | 0.937 | Peters et al. 201474 |
| 3 years | 0.977 | Peters et al. 201474 |
Results of the systematic searches ‘HRQoL 2’
FIGURE 21.
Flow chart for the identification of HRQoL studies (searches ‘HRQoL 2’). QoL, quality of life.
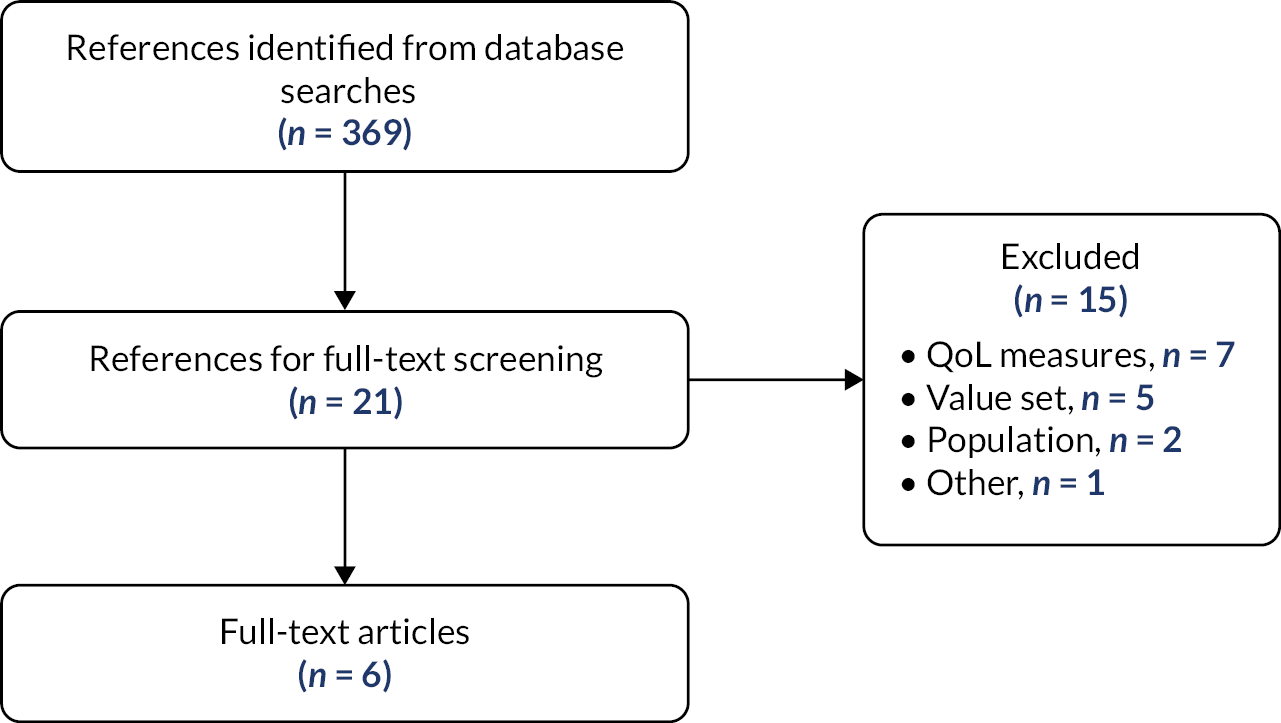
| Study | Reasons for exclusion |
|---|---|
| Donnelly et al. 2018242 | No prostate cancer |
| Downing et al. 2019243 | No relevant results |
| Glaser et al. 2013244 | No relevant results |
| Kuppen et al. 2020245 | Non-UK value set |
| Lemanska et al. 2021246 | Different population |
| Lloyd et al. 2015247 | Assess specific interventions |
| Loeb et al. 2018248 | Non-UK value set |
| Lopez-Calderero et al. 2017249 | Unclear value set |
| Maguire et al. 2019250 | No relevant results |
| Murasawa et al. 2019251 | Non-UK value set |
| Smith et al. 2020252 | No relevant results |
| Uemura et al. 2020253 | Unclear value set |
| Venderbos et al. 2020254 | No relevant results |
| Wilding et al. 2020255 | No relevant results |
| Yao et al. 2020256 | No relevant results |
| Study | Drummond et al.79 | |||||
|---|---|---|---|---|---|---|
| Year | 2015 | |||||
| Country | Republic of Ireland and Northern Ireland | |||||
| Type of study | Cross-sectional study | |||||
| Study objective | To perform an international population-based PROMs study form among short-term (<5 years), long-term (5–9.9 years) and very long-term (≥10 years postdiagnosis) prostate cancer survivors | |||||
| Population | Men registered with invasive prostate cancer diagnosed between 1 January 1995 and 31 March 2010, and alive in November 2011 | |||||
| Sample size | 3348 responders (1010 from Northern Ireland) | |||||
| HRQoL instrument | EORTC QLQ-C30 and QLQ-PR25, EQ-5D-5L (UK value set) | |||||
| Mapping | Mean utility scores were calculated using a crosswalk algorithm to convert EQ-5D-5L to the three-level version (Herdman et al. Qual Life Res 2011;20:1727–36) | |||||
| Health states | Invasive prostate cancer (alive at least 20 months after diagnosis) | |||||
| Results | Utility: 0.82 | |||||
| Conclusions/Limitations | Overall HRQoL of prostate cancer survivors in Ireland, measured by EQ-5D-5L, was similar to that of short-term prostate cancer survivors in the UK Limitations: no baseline (prediagnosis) HRQoL data |
|||||
| Study | Booth et al. 78 | |||||
| Year | 2014 | |||||
| Country | Finland | |||||
| Type of study | Surveys conducted among men in the Finnish trial of screening for prostate cancer | |||||
| Study objective | To quantify the long-term HRQoL impact associated with screening for prostate cancer | |||||
| Population | Men born in from 1929 to 1944 who resided in the Helsinki or Tampere region during recruitment period (1996–9) without a diagnosis of prostate cancer before date of randomisation. | |||||
Two groups of men from the trial received the questionnaires concerning HRQoL:
|
||||||
| Sample size | 5516 | |||||
| HRQoL instrument | 15D, EQ-5D (UK value set) and SF-6D. | |||||
| Health states | Surveys completed by men diagnosed with prostate cancer, organ-confined prostate cancer and advanced prostate cancer and men from the trial subsample (without prostate cancer) in four different time points (1998, 1999, 2003 and 2011) | |||||
| Results | Utilities | EQ-5D results from 2011 | ||||
| Screening arm | Control arm | |||||
| Men free of PC from trial subsample | 0.830 | 0.857 | ||||
| Men with PC (vs. no PC) | +0.005 | −0.031 | ||||
| Men with organ-confined PC (vs. no PC) | +0.01 | −0.031 | ||||
| Men with advanced PC (vs. no PC) | −0.039 | −0.051 | ||||
| Conclusions/limitations | Small advantage in mean HRQoL scores for the screening arm over the control arm for men diagnosed with prostate cancer in the 13-year follow-up. Lower HRQoL associated with more advanced age and lower socioeconomic status | |||||
| Study | Farkkila et al. 80 | |||||
| Year | 2014 | |||||
| Country | Finland | |||||
| Type of study | Cross-sectional study | |||||
| Study objective | To explore end-stage breast, prostate and colorectal cancer patients’ HRQoL. To compare results obtained by different HRQoL instruments and to explore factors related to impaired HRQoL | |||||
| Population | Patients with metastatic breast, prostate and colorectal cancer and receiving palliative treatments only (no chemotherapy or radiotherapy) and patients who died due to cancer within 6 months of responding to the questionnaire (irrespective of treatment given) | |||||
| Sample size | 114 (30 with prostate cancer) | |||||
| HRQoL instrument | 15D, EQ-5D-3L (UK value set), EORTC QLQ-C30 | |||||
| Health states | End-stage prostate cancer | |||||
| Results | EQ-5D utility for prostate cancer patients: 0.551 (0.405–0.664) | |||||
| Conclusions/limitations | With patients closer to death, HRQoL scores were lower and symptom burden increased. Symptoms, especially fatigue, leading to the impairment of both activities of daily living and psychological functioning seemed to be the most significant deteriorating factors | |||||
| Study | Gavin et al.81 | |||||
| Year | 2016 | |||||
| Country | Republic of Ireland and Northern Ireland | |||||
| Type of study | Cross-sectional study | |||||
| Study objective | To investigate effects on men’s health and well-being of higher prostate cancer investigation and treatment levels in similar populations | |||||
| Population | Prostate cancer survivors in Ireland, where Republic of Ireland has a 50% higher prostate cancer incidence than Northern Ireland | |||||
| Sample size | 3348 responders (781 from Northern Ireland) | |||||
| HRQoL instrument | EORTC QLQ-C30, EQ-5D-5L (UK value set) | |||||
| Mapping | EQ-5D-5L were converted to EQ-5D-3L | |||||
| Health states | Early (stage I/II and Gleason grade 2–7) and late disease prostate cancer (stage III/IV and any Gleason grade at diagnosis) – 2–18 years post treatment | |||||
| Results | Utilities | Early disease | Late disease | |||
| Northern Ireland | N = 269 0.8 |
N = 282 0.7 |
||||
| Republic of Ireland | N = 1431 0.9 |
N = 407 0.8 |
||||
| Conclusions/limitations | Patient-reported outcomes are very similar between Republic of Ireland and Northern Ireland despite different levels of PSA testing and diagnosed prostate cancer | |||||
| Study | Torvinen et al.82 | |||||
| Year | 2013 | |||||
| Country | Finland | |||||
| Type of study | Cross-sectional study | |||||
| Study objective | To assess HRQoL scores in different health states of prostate cancer, compare the results obtained by different HRQoL instruments, compare the HRQoL of prostate cancer patients with that of the Finnish general population, and explore the factors associated with the resultant HRQoL scores | |||||
| Population | Patients over 18 years of age diagnosed with prostate cancer | |||||
| Sample size | 621 | |||||
| HRQoL instrument | 15D, EQ-5D-3L (UK value set), EORTC QLQ-C30 | |||||
| Health states |
|
|||||
| Results | EQ-5D | N | Mean | SD | 95% CI | ∆ vs. general population |
| Loc1 | 46 | 0.90 | 0.19 | 0.84 to 0.96 | +0.103 | |
| Loc2 | 91 | 0.89 | 0.14 | 0.86 to 0.92 | +0.089 | |
| Loc3 | 309 | 0.87 | 0.19 | 0.85 to 0.89 | +0.043 | |
| Metastatic | 85 | 0.74 | 0.27 | 0.69 to 0.80 | −0.054 | |
| Palliative | 17 | 0.59 | 0.22 | 0.48 to 0.70 | −0.157 | |
| All patients | 621 | 0.85 | 0.03 | 0.83 to 0.86 | – | |
| Study | Watson et al.83 | |||||
| Year | 2016 | |||||
| Country | UK | |||||
| Type of study | Cross-sectional study | |||||
| Study objective | To explore ongoing symptoms, unmet needs, psychological well-being, self-efficacy and overall health status in prostate cancer survivors | |||||
| Population | Men diagnosed 9–24 months previously, regardless of treatment modality, whose condition was considered stable as judged by the most recent PSA test result | |||||
| Sample size | 316 | |||||
| HRQoL instrument | EPIC-26, EQ-5D-5L | |||||
| Mapping | Conversion to EQ-5D-3L using a crosswalk algorithm (van Hout et al. Value Health 2012;15:708–715) | |||||
| Health states | Adverse events after treatment for prostate cancer:
|
|||||
| Results | Utilities | No/mild problems | Moderate/big problems | p -value | ||
| Urine function | 0.868 (0.160) | 0.773 (0.222) | 0.001 | |||
| Bowel function | 0.862 (0.166) | 0.653 (0.195) | 0.000 | |||
| Sexual function | 0.861 (0.176) | 0.838 (0.170) | 0.261 | |||
| Conclusions/limitations | Treatment ongoing symptoms have an impact on the quality of life of patients Limitations: volunteer bias cannot be excluded, those with the greatest need may be less or more likely to participate in such a study (although no significant differences were found between respondents and non-respondents); two areas included may not be representative of the wider UK population; cross-sectional design |
|||||
| HRQoL of prostate cancer patients appears to be surprisingly good prior to metastatic progression of the disease. Both generic instruments produced higher scores in the Loc1 and Loc2 groups – and the EQ-5D also in the Loc3 group – than those found among the general population standardised for gender and age. A significant proportion of patients entering prostate cancer treatment because of elevated PSA levels found in opportunistic testing can explain this finding. As PSA testing has not been recommended at the national level, such opportunistic testing in Finland is currently limited mainly to occupational health services Limitation: cross-sectional design (different patients in groups representing different states); response rate of 61.5% (it is possible that non-respondents may have had more severe disease, although there’s no reason to expect significant differences regarding disease severity between respondents and non-respondents based on previous experiences with similar surveys) |
||||||
Appendix 8 Resource use and cost estimates
Details of micro-costing of biopsy procedures
The component costs included in the base case are explained in further detail below.
Cost of devices
-
LATP biopsies
-
CamPROBE: cost of £35 (provided by JEB), with each biopsy requiring two devices – resulting in a cost per biopsy of £70.
-
PrecisionPoint: cost of £200 (provided by BXTAccelyon), with each biopsy requiring one device – resulting in a cost per biopsy of £200.
-
EZU-PA3U: cost of £1826 for orders with quantity > 5 and £2000 for orders with quantity < 5 (provided by Hitachi). We assumed that half of EZU-PA3U orders is for a quantity > 5. Each device is reusable, and we assumed that it can be reprocessed 100 times (as for Trinity Perine, see below) – resulting in a cost per biopsy of £19.
-
UA1232: cost of £1400 (provided by BK Medical). Each device is reusable, and we assumed that it can be reprocessed 100 times (as for Trinity Perine, see below) – resulting in a cost per biopsy of £14.
-
Trinity Perine: cost of £754 for a Perine Mini Grid (provided by KOELIS). Each device is reusable and can be reprocessed 100 times, as advised by the company – resulting in a cost per biopsy of £8.
-
SureFire Guide: cost of £120 (provided by Delta Surgical), with each biopsy requiring one device – resulting in a cost per biopsy of £120.
-
Grid and stepping device:
-
- Grid: cost of £78 per biopsy (obtained from YHEC study).
-
- Stepper: cost of £22,000 (obtained from YHEC study) apportioned by the number of procedures carried out per stepper per year (18 procedures per week, of which 15 are biopsies) for a lifetime of 10 years (informed by our clinical expert) – resulting in a cost per biopsy of £2.
-
-
Double freehand device: not applicable.
-
-
GATP biopsy: we assumed the same cost of the grid and stepping device as for the LATP biopsy – resulting in a cost per biopsy of £78 for grid and £2 for stepper.
-
LATRUS biopsy: not applicable.
Cost of consumables
General consumables
-
See below for the cost and quantity of each consumable per type of biopsy.
-
LATP biopsies
-
LATP biopsies using freehand devices (except EZU-PA3U) and using grid and stepping device: we summed up the costs of the consumables that are common to all biopsies (£62) with the costs for the consumables that are used for TP biopsies (£3), for biopsies carried out under local anaesthesia (£18) – resulting in a cost per biopsy of £83.
-
LATP biopsies using double freehand devices and EZU-PA3U: we assumed the same costs as above (£83) plus the cost of the co-axial needle (£21) – resulting in a cost per biopsy of £104.
-
-
GATP biopsy: we summed up the costs of the consumables that are common to all biopsies (£62) with the costs for the consumables that are used for TP biopsies (£3) and the cost of general anaesthesia (£100) – resulting in a cost per biopsy of £165.
-
LATRUS biopsy: we summed up the costs of the consumables that are common to all biopsies (£62) with the costs for the consumables that are used for TRUS biopsies (£2) and for biopsies carried out under local anaesthesia (£18) – resulting in a cost per biopsy of £81.
Lithotomy bed
-
The cost of a lithotomy bed was applied in the EAG revised base case after an expert has commented that it would be required for all types of TP but not for LATRUS. This expert informed us that the estimated cost of a lithotomy bed was £10,000 and we have apportioned this cost over an estimated lifetime of 10 years and an assumed 1000 biopsies per year – resulting in a cost per biopsy of £3.
Ultrasound
-
Hitachi, BK Medical and KOELIS provided the cost of the ultrasound machine required to perform a biopsy using EZU-PA3U, UA1232 and Trinity Perine, respectively. For the remaining devices and methods, we assumed that the cost of the ultrasound machine and transducer is the average cost of the ultrasound machine costs of EZU-PA3U, UA1232 and Trinity Perine. We assumed the same lifetime (10 years) as for stepper and an estimated number of biopsies per year of 1000.
-
EZU-PA3U: cost of £38,000 for a FUJIFILM transperineal transducer and FUJIFILM ultrasound system – resulting in a cost per biopsy of £3.
-
UA1232: cost of £40,050 for a BK ultrasound system, urology software with 9048 transducer – resulting in a cost per biopsy of £3.
-
Trinity Perine: cost of £68,509 for a Trinity 3D Prostate Suite (£45,000) plus Koelis Sidefire ultrasound probe (£23,509) – resulting in a cost per biopsy of £5.
-
Remaining devices and methods: cost of £48,853 as the average of the abovementioned machines – resulting in a cost per biopsy of £3.
Cost of staff time spent on training
-
We considered that five urologists have a given amount of training each year regardless of the biopsy method. The cost per working hour of a urologist (£119) was based on the cost per working hour of a consultant (medical) hospital-based doctor reported by Curtis and Burns. 89 We assumed that 1000 biopsies are carried out per year on average (as advised by our experts). The amount of time spent on training was provided by some companies, as follows:
-
LATP biopsies:
-
CamPROBE: half day (4 hours) spent on training per person – resulting in a cost per biopsy of £2.
-
PrecisionPoint: 1 day (8 hours) spent on training per person – resulting in a cost per biopsy of £5.
-
EZU-PA3U: 1 hour spent on training per person – resulting in a cost per biopsy of £0.60.
-
UA1232: 2 hours spent on training per person – resulting in a cost per biopsy of £1.
-
Trinity Perine: 1 hour spent on training per person – resulting in a cost per biopsy of £0.60.
-
For the remaining LATP biopsies (SureFire Guide, LATP using grid and stepping device and LATP using double freehand devices), as no data are available, we assumed that a whole day (8 hours) of training would be required per person – resulting in a cost per biopsy of £5.
-
-
GATP biopsy: again, as no data are available, we assumed that a whole day (8 hours) of training would be required per person – resulting in a cost per biopsy of £5.
-
LATRUS biopsy: we assumed that this would only require 1 hour of training per person since we believe this is a well-known and also easy to use method – resulting in a cost per biopsy of £0.60.
Cost of staff time spent on performing the biopsy
-
We assumed that all biopsies are carried out by one urologist and that there are two nurses in the room for assistance. For GATP biopsies, we considered the cost of one anaesthetist as well. The cost per working hour of the urologist and anaesthetist (£119) was informed by Curtis and Burns89 as explained above. The cost per working hour of each nurse was based on the cost per working hour of a Band 4 hospital-based nurse (£31) reported by Curtis and Burns. 89
-
LATP biopsies:
-
CamPROBE: a procedure time of 0.41 hour was based on the study by Wilson et al. 63 – resulting in a cost per biopsy of £49 for the urologist and £25 for the two nurses.
-
PrecisionPoint: a procedure time of 0.33 hour was based on the study by Szabo41 – resulting in a cost per biopsy of £40 for the urologist and £21 for the two nurses.
-
For the remaining LATP biopsies, due to lack of data on procedure time, we assumed the average between CamPROBE and PrecisionPoint (0.37 hour) – resulting in a cost per biopsy of £44 for the urologist and £23 for the two nurses.
-
-
GATP biopsy: a procedure time of 1.00 hour was assumed – resulting in a cost per biopsy of £119 for the urologist and anaesthetist and £62 for the two nurses.
-
LATRUS biopsy: a procedure time of 0.31 hour was assumed. This was obtained by multiplying the average procedure time of LATP biopsies (0.37 hour) by the LATRUS/LATP procedure time ratio (0.84) derived from Guo et al. 25 This study reported a procedure time of 14.73 minutes for LATRUS and 17.51 minutes for LATP – resulting in a cost per biopsy of £37 for the urologist and £19 for the two nurses.
Cost of place of biopsy
-
The YHEC study reported a cost per biopsy for an outpatient room of £43 and for a theatre session of £194. We assumed that the cost of the outpatient room corresponds to a procedure time of 0.33 hour (based on Szabo 2021), being the cost per hour of £129. The cost of the theatre session was assumed for a procedure time of 1.00 hour.
-
LATP biopsies.
-
CamPROBE: assuming the use of an outpatient room and a procedure time of 0.41 hour results in a cost per biopsy of £53.
-
PrecisionPoint: assuming the use of an outpatient room and a procedure time of 0.33 hour results in a cost per biopsy of £43.
-
For the remaining LATP biopsies, assuming the use of an outpatient room and a procedure time of 0.37 hour results in a cost per biopsy of £48.
-
-
GATP biopsy: assuming the use of a theatre session and a procedure time of 1.00 hour results in a cost per biopsy of £194.
-
LATRUS biopsy: assuming the use of an outpatient room and a procedure time of 0.31 hours results in a cost per biopsy of £40.
Cost of reprocessing
-
The cost of reprocessing was applied to reusable devices only – the LATP devices EZU-PA3U, UA1232 and Trinity Perine and the LATP and GATP using a grid and stepping device.
-
The cost of reprocessing was assumed to be £5 per biopsy as advised by a Specialist Committee Member. This might include the cost of use of an autoclave, the blood cleaning, the item packaging in sterile cloth or paper and the technician time.
Cost of histopathology
-
The cost of histopathological analysis was applied to all biopsy methods. We used the cost of £37 per sample from NHS Cost Data 2019–20 (diagnostic code DAPSO2). 90 For the base case, we assumed that 12 samples were taken from a prostate biopsy – resulting in a cost per biopsy of £439.
Costing for diagnosis, monitoring and treatment
Base-case estimates of the quantities of healthcare resources used for diagnosis, monitoring and treatment of prostate cancer and adverse events are listed in Table 73. Unit costs are listed in Table 74.
| Consumables | Cost per biopsy | Unit cost | Source | Pack | Source | Quantity | Source | Notes |
|---|---|---|---|---|---|---|---|---|
| All biopsies | ||||||||
| Biopsy gun | £26.00 | £26 | YHEC 2020257 | 1 | Assumption | 1 | Assumption | YHEC study reported the cost per biopsy directly |
| Biopsy needle | £27.00 | £135 | Wilson 202163 | 5 | Wilson 202163 | 1 | Wilson 202163 | |
| Condoms | £0.06 | £28 | Wilson 202163 | 500 | Wilson 202163 | 1 | Wilson 202163 | |
| Ultrasound lubricant gel | £0.01 | £4 | Wilson 202163 | 5000 | Wilson 202163 | 10 | Wilson 202163 | ml |
| Sterile gloves | £3.00 | £79 | Wilson 202163 | 50 | Wilson 202163 | 2 | Wilson 202163 | |
| Dressing towel | £0.20 | £0.20 | Wilson 202163 | 1 | Wilson 202163 | 1 | Wilson 202163 | |
| Syringe | £0.07 | £4 | Wilson 202163 | 100 | Wilson 202163 | 2 | Wilson 202163 | |
| Antiseptic wash | £0.04 | £3 | Wilson 202163 | 600 | Wilson 202163 | 10 | Wilson 202163 | ml |
| Sterile saline | £0.04 | £4 | Wilson 202163 | 1000 | Wilson 202163 | 10 | Wilson 202163 | ml |
| Sponges/cassettes | £0.68 | £0.10 | Wilson 202163 | 1 | Wilson 202163 | 12 | Wilson 202163 | Average between cost reported by Wilson 2021 and YHEC; YHEC reported the cost per biopsy directly |
| £0.16 | YHEC 2020257 | 1 | Assumption | 1 | Assumption | |||
| Balloon/probe cover | £5.00 | £5 | YHEC 2020257 | 1 | Assumption | 1 | Assumption | YHEC study reported the cost per biopsy directly |
| TP biopsies | ||||||||
| Orange needles | £0.06 | £3 | JEB/ Wilson 202163 |
100 | JEB/ Wilson 202163 |
2 | JEB/ Wilson 202163 |
|
| Green needles | £0.04 | £2 | JEB/ Wilson 202163 |
100 | JEB/ Wilson 202163 |
2 | JEB/ Wilson 202163 |
|
| Marker skin pen with ruler | £0.33 | £2 | JEB | 5 | JEB | 1 | JEB | |
| Cotton gauze | £0.09 | £0.90 | JEB/ Wilson 202163 |
100 | JEB/ Wilson 202163 |
10 | JEB/ Wilson 202163 |
|
| Steri-Strips | £0.31 | £8 | JEB/ Wilson 202163 |
50 | JEB/ Wilson 202163 |
2 | JEB/ Wilson 202163 |
|
| Sterile drapes/gowns | £2.00 | £111 | JEB/ Wilson 202163 |
50 | JEB/ Wilson 202163 |
1 | JEB/ Wilson 202163 |
Average between cost reported by Wilson 2021 and YHEC; YHEC reported the cost per biopsy directly |
| £2 | YHEC 2020257 | 1 | Assumption | 1 | Assumption | |||
| Shallow sterile plastic tray | £0.36 | £18 | JEB/ Wilson 202163 |
50 | JEB/Wilson 202163 | 1 | JEB/ Wilson 202163 |
|
| Antibiotics prophylaxis | £0.31 | £3 | emiT 2020101 | 10 | emiT 2020101 | 1 | Expert opinion | Assumed as one prophylactic dose of ciprofloxacin (500 mg), as advised by EAG expert |
| TP biopsies using double freehand devices and EZU-PA3U device | ||||||||
| Co-axial needles | £21.00 | £107 | Hitachi | 5 | Hitachi | 1 | Hitachi | |
| TRUS biopsies | ||||||||
| Antibiotics course | £2.00 | £3 | emiT 2020101 | 10 | emiT 2020101 | 6 | SmPC/Assumption | Assumed as a course of ciprofloxacin 500 mg twice a day for 3 days, according to SmPC and as advised by EAG expert |
| LA biopsies | ||||||||
| Spinal needles | £6 | £6 | YHEC 2020257 | 1 | Assumption | 1 | Assumption | YHEC study reported the cost per biopsy directly |
| Local anaesthetic | £12 | £11 | Wilson 202163 | 20 | Wilson 202163 | 20 | Wilson 202163 | ml; Average between cost reported by Wilson 2021 and YHEC study; YHEC study reported the cost per biopsy directly |
| £13 | YHEC 2020257 | 20 | Assumption | 20 | Assumption | |||
| GA biopsies | ||||||||
| General anaesthetic | £100 | £100 | YHEC 2020257 | 1 | Assumption | 1 | Assumption | YHEC study reported the cost per biopsy directly |
| Parameter | Input | Source | Notes | |
|---|---|---|---|---|
| Distribution of LATP biopsy methods | ||||
| CamPROBE | 12.5% | Assumption | ||
| PrecisionPoint | 12.5% | Assumption | ||
| EZU-PA3U | 12.5% | Assumption | ||
| UA1232 | 12.5% | Assumption | ||
| Trinity Perine | 12.5% | Assumption | ||
| SureFire Guide | 12.5% | Assumption | ||
| Grid and stepping device | 12.5% | Assumption | ||
| Double freehand | 12.5% | Assumption | ||
| BSA | 1.91 | Sacco et al. 2010 (from NG131 model)67 | ||
| Proportion of patients that repeat biopsy after a first biopsy result NC or CNS | ||||
| MRI Likert score 3+ | ||||
| Result first biopsy: CNS | 15.5% | Jimenez et al. 2021113 | ||
| Result first biopsy: NC | 5.0% | Assumption | Fewer patients with NC than CNS result repeat biopsy | |
| MRI Likert score 1 or 2 | ||||
| Result first biopsy: CNS | 5.0% | Assumption | Fewer patients with MRI score 1 or 2 than 3 + repeat biopsy | |
| Result first biopsy: NC | 1.3% | Assumption | Fewer patients with NC than CNS result repeat biopsy | |
| Frequency of follow-up (per year) | ||||
| FN LR, IR, HR or metastatic that did not repeat biopsy or after repeat biopsy | ||||
| PSA | 1 | NG131 economic model67 | ||
| Nurse appointment | 1 | NG131 economic model67 | ||
| TRUS | 1 | NG131 economic model67 | ||
| % having TRUS | 69.0% | NG131 economic model67 | Sensitivity of PSA test | |
| True positive LR (receiving active surveillance) | ||||
| PSA (1st year) | 4 | NG13167 | ||
| PSA (subs years) | 2 | NG13167 | ||
| Nurse appointment (1st year) | 4 | NG13167 | ||
| Nurse appointment (subs years) | 2 | NG13167 | ||
| DRE | 1 | NG13167 | ||
| mpMRI (1st year) | 1 | NG13167 | ||
| True positive LR (receiving radical treatment) | ||||
| PSA (1st and 2nd year) | 4 | NG13167 | ||
| PSA (subs years) | 1 | NG13167 | ||
| Nurse appointment (1st year) | 4 | NG13167 | ||
| Nurse appointment (subs years) | 1 | NG13167 | ||
| True positive IR (receiving active surveillance) | ||||
| PSA (1st year) | 4 | NG13167 | ||
| PSA (subs years) | 2 | NG13167 | ||
| Nurse appointment (1st year) | 4 | NG13167 | ||
| Nurse appointment (subs years) | 2 | NG13167 | ||
| DRE | 1 | NG13167 | ||
| mpMRI (1st year) | 1 | NG13167 | ||
| CT scan (1st year) | 1 | Clinical expert advice | ||
| Bone scan (1st year) | 1 | Clinical expert advice | ||
| % having CT and bone scan | 50.0% | Clinical expert advice | ||
| True positive IR (receiving radical treatment) | ||||
| PSA (1st and 2nd year) | 4 | NG13167 | ||
| PSA (subs years) | 1 | NG13167 | ||
| Nurse appointment (1st year) | 4 | NG13167 | ||
| Nurse appointment (subs years) | 1 | NG13167 | ||
| CT scan (1st year) | 1 | Clinical expert advice | ||
| Bone scan (1st year) | 1 | Clinical expert advice | ||
| % having CT and bone scan | 50.0% | Clinical expert advice | ||
| True positive IR (receiving watchful waiting) | ||||
| PSA | 1 | NG13167 | ||
| Nurse appointment | 1 | NG13167 | ||
| CT scan (1st year) | 1 | Clinical expert advice | ||
| Bone scan (1st year) | 1 | Clinical expert advice | ||
| % having CT and bone scan | 50.0% | Clinical expert advice | ||
| True positive HR (receiving radical treatment) | ||||
| PSA (1st and 2nd year) | 4 | NG13167 | ||
| PSA (subs years) | 1 | NG13167 | ||
| Nurse appointment (1st year) | 4 | NG13167 | ||
| Nurse appointment (subs years) | 1 | NG13167 | ||
| CT scan (1st year) | 1 | Clinical expert advice | ||
| Bone scan (1st year) | 1 | Clinical expert advice | ||
| % having CT and bone scan | 70.0% | Assumption | ||
| True positive HR (receiving watchful waiting) | ||||
| PSA | 1 | NG13167 | ||
| Nurse appointment | 1 | NG13167 | ||
| CT scan (1st year) | 1 | Clinical expert advice | ||
| Bone scan (1st year) | 1 | Clinical expert advice | ||
| % having CT and bone scan | 70.0% | Assumption | ||
| True positive metastatic | ||||
| CT scan (1st year) | 1 | Clinical expert advice | ||
| Bone scan (1st year) | 1 | Clinical expert advice | ||
| % having CT and bone scan | 100.0% | Assumption | ||
| Treatment distribution | ||||
| Localised disease (LR) | ||||
| Active surveillance | 95.0% | NPCA Annual Report 202092 | ||
| Radical treatment | 5.0% | NPCA Annual Report 202092 | ||
| Radical prostatectomy | 2.0% | Gnanapragasam et al. 201698 | Weighted proportions based on Gnanapragasam et al. 2016 | |
| External radiotherapy | 2.3% | Gnanapragasam et al. 201698 | ||
| Brachytherapy | 0.7% | Gnanapragasam et al. 201698 | ||
| Watchful waiting | 0.0% | Assumption | Assume that no patients with LR have watchful waiting | |
| ADT therapies | 3.0% | Assumption | All patients on radical radiotherapy receive ADT | |
| Localised disease (IR) | ||||
| Active surveillance | 12.7% | Gnanapragasam et al. 201698 | Assumed that half of patients not receiving radical treatment are on active surveillance and the other half on watchful waiting | |
| Radical prostatectomy | 21.9% | Gnanapragasam et al. 201698 | ||
| External radiotherapy | 48.7% | Gnanapragasam et al. 201698 | ||
| Brachytherapy | 4.1% | Gnanapragasam et al. 201698 | ||
| Watchful waiting | 12.7% | Gnanapragasam et al. 201698 | ||
| ADT therapies | 52.8% | Assumption | All patients on radical radiotherapy receive ADT | |
| Localised disease (HR) | ||||
| Active surveillance | 0.0% | Assumption | Assume that no patients with HR have active surveillance | |
| Radical treatment | 71.0% | NPCA Annual Report 202092 | ||
| Radical prostatectomy | 17.6% | Gnanapragasam et al. 201698 | Weighted proportions based on Gnanapragasam et al. 2016 | |
| External radiotherapy | 52.5% | Gnanapragasam et al. 201698 | ||
| Brachytherapy | 0.9% | Gnanapragasam et al. 201698 | ||
| Watchful waiting | 29.0% | NPCA Annual Report 202092 | ||
| ADT therapies | 53.4% | Assumption | All patients on radical radiotherapy receive ADT | |
| ADT market share (localised disease) | ||||
| Leuprorelin | 33.3% | Assumption | Assumed that LHRH therapies are used at the same rate | |
| Triptorelin | 33.3% | Assumption | ||
| Goserelin | 33.3% | Assumption | ||
| Bicalutamide | 100.0% | Assumption | ||
| Metastatic hormone-sensitive disease | ||||
| ADT alone | 50.0% | Assumption | ||
| Docetaxel + ADT | 36.0% | NPCA Annual Report 202092 | ||
| Apalutamide + ADT | 7.0% | Assumption | ||
| Enzalutamide + ADT | 7.0% | Assumption | ||
| ADT market share (mHSPC) | ||||
| Leuprorelin | 33.3% | Assumption | Assumed that LHRH therapies are used at the same rate | |
| Triptorelin | 33.3% | Assumption | ||
| Goserelin | 33.3% | Assumption | ||
| Bicalutamide | 50.0% | Assumption | ||
| Metastatic hormone-relapsed disease | ||||
| Abiraterone | 28.3% | NICE TA71287 | Weighted proportions according to treatment for metastatic hormone-sensitive prostate cancer | |
| Docetaxel | 22.4% | NICE TA71287 | ||
| Enzalutamide | 30.1% | NICE TA71287 | ||
| Best supportive care | 19.2% | |||
| Duration of drug therapies | ||||
| Localised disease | ||||
| LHRH drugs | ||||
| Low risk | 3 months | NG131 model, Mowatt et al. 201367,99 | ||
| Intermediate risk | 6 months | NG131 model, Mowatt et al. 201367,99 | ||
| High risk | 2 years | NG131 model, Mowatt et al. 201367,99 | ||
| Bicalutamide | 21 days | NG131 model, Mowatt et al. 201367,99 | ||
| Metastatic hormone-sensitive disease | ||||
| ADT alone | 2 years | Assumption | ||
| Docetaxel + ADT | 6 cycles | STAMPEDE (from NG131 model)67,103 | Cycles of 3 weeks | |
| Apalutamide + ADT | 2 years | Assumption | Same as ADT | |
| Enzalutamide + ADT | 2 years | Assumption | Same as ADT | |
| Metastatic hormone-relapsed disease | ||||
| Abiraterone | 8 months | COU-AA-301 (from NG131 model)67 | ||
| Docetaxel | 9.5 cycles | TAX327 (from NG131 model)67 | Cycles of 3 weeks | |
| Enzalutamide | 14 months | Pilon et al. 2017258 | ||
| Adverse events | ||||
| Incidence of biopsy adverse events (TRUS) | ||||
| Mild AEs | 10.4% | Rosario et al. 201295 | ||
| AEs requiring admission | 3.7% | Tamhankar et al. 202094 | ||
| Mortality | 0.1% | Tamhankar et al. 202094 | ||
| Incidence of biopsy adverse events (TP) | ||||
| Mild AEs | 9.1% | Pepe and Aragona 201396 | ||
| AEs requiring admission | 3.5% | Tamhankar et al. 202094 | ||
| Mortality | 0.1% | Tamhankar et al. 202094 | ||
| Incidence of radical treatment adverse events | ||||
| Active surveillance/watchful waiting | ||||
| Erectile dysfunction | 50.9% | ProtecT study71 | 1-year FUP (table 2; table S2B, erect not firm f/intercourse) | |
| Urinary incontinence | 4.2% | ProtecT study71 | 1-year FUP (table 2; table S2A, one/more pads per day) | |
| Bowel dysfunction | 1.7% | ProtecT study71 | 1-year FUP (table S2C, mod/sev impact on QoL) | |
| Radical prostatectomy | ||||
| Erectile dysfunction | 85.4% | ProtecT study71 | 1-year FUP (table 2; table S2B, erect not firm f/intercourse) | |
| Urinary incontinence | 26.2% | ProtecT study71 | 1-year FUP (table 2; table S2A, one/more pads per day) | |
| Bowel dysfunction | 2.5% | ProtecT study71 | 1-year FUP (table S2C, mod/sev impact on QoL) | |
| Radical radiotherapy | ||||
| Erectile dysfunction | 62.4% | ProtecT study71 | 1-year FUP (table 2; table S2B, erect not firm f/intercourse) | |
| Urinary incontinence | 3.6% | ProtecT study71 | 1-year FUP (table 2; table S2A, one/more pads per day) | |
| Bowel dysfunction | 5.8% | ProtecT study71 | 1-year FUP (table S2C, mod/sev impact on QoL) | |
| Incidence of metastatic treatment adverse events | ||||
| ADT | ||||
| Cardiac disorder | 3.0% | STAMPEDE (from NG131 model)67,103 | ||
| Endocrine disorder | 12.2% | STAMPEDE (from NG131 model)67,103 | ||
| Gastrointestinal disorder | 3.0% | STAMPEDE (from NG131 model)67,103 | ||
| General disorder | 3.9% | STAMPEDE (from NG131 model)67,103 | ||
| Musculoskeletal disorder | 5.8% | STAMPEDE (from NG131 model)67,103 | ||
| Nervous system disorder | 1.7% | STAMPEDE (from NG131 model)67,103 | ||
| Neutropenia | 1.8% | STAMPEDE (from NG131 model)67,103 | ||
| Renal disorder | 6.0% | STAMPEDE (from NG131 model)67,103 | ||
| Respiratory disorders | 2.3% | STAMPEDE (from NG131 model)67,103 | ||
| Docetaxel + ADT | ||||
| Cardiac disorder | 2.9% | STAMPEDE (from NG131 model)67,103 | ||
| Endocrine disorder | 10.4% | STAMPEDE (from NG131 model)67,103 | ||
| Gastrointestinal disorder | 8.2% | STAMPEDE (from NG131 model)67,103 | ||
| General disorder | 6.2% | STAMPEDE (from NG131 model)67,103 | ||
| Musculoskeletal disorder | 5.8% | STAMPEDE (from NG131 model)67,103 | ||
| Nervous system disorder | 3.5% | STAMPEDE (from NG131 model)67,103 | ||
| Neutropenia | 27.3% | STAMPEDE (from NG131 model)67,103 | ||
| Renal disorder | 4.2% | STAMPEDE (from NG131 model)67,103 | ||
| Respiratory disorder | 5.3% | STAMPEDE (from NG131 model)67,103 | ||
| Apalutamide + ADT | ||||
| Blood disorder | 2.1% | TITAN study (Kim et al. 2019)104 | table 4 | |
| Cardiac disorder | 8.4% | TITAN study (Kim et al. 2019)104 | table 4 | |
| Gastrointestinal disorder | 1.1% | TITAN study (Kim et al. 2019)104 | table 4 | |
| General disorder | 3.4% | TITAN study (Kim et al. 2019)104 | table 4 | |
| Musculoskeletal disorder | 6.5% | TITAN study (Kim et al. 2019)104 | table 4 | |
| Nervous system disorder | 0.2% | TITAN study (Kim et al. 2019)104 | table 4 | |
| Renal disorder | 0.8% | TITAN study (Kim et al. 2019)104 | table 4 | |
| Skin disorder | 6.5% | TITAN study (Kim et al. 2019)104 | table 4 | |
| Enzalutamide + ADT | ||||
| Cardiac disorder | 4.9% | ARCHES study (Armstrong 2019)105 | table 3 | |
| Endocrine disorder | 0.3% | ARCHES study (Armstrong 2019)105 | table 3 | |
| Gastrointestinal disorder | 0.5% | ARCHES study (Armstrong 2019)105 | table 3 | |
| General disorder | 2.8% | ARCHES study (Armstrong 2019)105 | table 3 | |
| Musculoskeletal disorder | 4.4% | ARCHES study (Armstrong 2019)105 | table 3 | |
| Nervous system disorder | 2.1% | ARCHES study (Armstrong 2019)105 | table 3 | |
| Neutropenia | 0.3% | ARCHES study (Armstrong 2019)105 | table 3 | |
| Skin disorder | 0.3% | ARCHES study (Armstrong 2019)105 | table 3 | |
| Parameter | Cost | Source | Notes |
|---|---|---|---|
| Follow-up costs | |||
| PSA | £1 | NHS Cost Data 2019/2090 | DAPS: DAPS04 |
| Primary care nurse | £10 | PSSRU 202089 | 10-minute appointment with a Band 7 community-based nurse (p.129) |
| DRE | £78 | PSSRU 202089 | Assumed as a 20-minute GP appointment |
| mpMRI | £211 | NHS Cost Data 2019/2090 | IMAG: RD03Z (outpatient) |
| CT scan | £126 | NHS Cost Data 2019/2090 | IMAG: RD21A |
| Bone scan | £331 | NHS Cost Data 2019/2090 | NM: RN15A |
| Treatment costs | |||
| Localised disease | |||
| Radical prostatectomy | |||
| Surgery | £8331 | NHS Cost Data 2019/2090 | EL: LB69Z |
| First appointment | £247 | NHS Cost Data 2019/2090 | OPROC: WF01B |
| Follow-up appointment | £214 | NHS Cost Data 2019/2090 | OPROC: WF01A |
| Number of follow-up appointments | 2 | Wilson et al. 202163 | |
| External radiotherapy | £3114 | NHS Cost Data 2019/2090 | RAD: weighted average of SC40Z and SC41Z (outpatient) plus SC21Z (outpatient) multiplied by 20 fractions |
| Brachytherapy | £3106 | NHS Cost Data 2019/2090 | RAD: SC55Z + SC30Z (weighted average of inpatient, day case and outpatient) |
| ADT therapies | |||
| Low risk | £246 | BNF 2021, eMIT 2020100,101 | 21-day course of bicalutamide + 1 injection of LHRH + admin costs |
| Intermediate risk | £489 | BNF 2021, eMIT 2020100,101 | 21-day course of bicalutamide + 2 injections of LHRH + admin costs |
| High risk | £1947 | BNF 2021, eMIT 2020100,101 | 21-day course of bicalutamide + 8 injections of LHRH + admin costs |
| Metastatic hormone-sensitive disease | |||
| ADT alone | £1946 | BNF 2021, eMIT 2020 | 28-day course of bicalutamide + 2-year LHRH drugs |
| Docetaxel + ADT | £4076 | eMIT 2020101 | Cost of ADT alone + 6 cycles of 75 mg/m2 docetaxel + admin costs |
| Apalutamide + ADT | £73,300 | BNF 2021100 | Cost of ADT alone + 2-year apalutamide |
| Enzalutamide + ADT | £73,291 | BNF 2021100 | Cost of ADT alone + 2-year enzalutamide |
| Metastatic hormone-relapsed disease | |||
| Abiraterone | £23,785 | BNF 2021100 | 8 months (from NG131 model) |
| Docetaxel | £3411 | eMIT 2020101 | 9.5 cycles of 75 mg/m2 docetaxel + admin costs |
| Enzalutamide | £41,618 | BNF 2021100 | 14 months |
| Best supportive care | £0 | Assumption | Assumed no costs as they are negligible |
| Administration costs | |||
| LHRH drugs | £13 | PSSRU 202089 | 15.5 minutes with a Band 6 hospital-based nurse (p.155) |
| Docetaxel (IV, 1st attendance) | £300 | NHS Cost Data 2019/2090 | CHEM: SB12Z |
| Docetaxel (IV, subs attendances) | £366 | NHS Cost Data 2019/2090 | CHEM: SB15Z |
| Adverse event costs | |||
| Biopsy adverse events | |||
| Mild AEs (urinary infection) | £48 | Wilson et al. 202163 | GP visit + urinalysis + 7-day trimethoprim |
| GP visit | £39 | PSSRU 202089 | 10.3b GP (unit costs per patient contact lasting 9.22 minutes) |
| Urinalysis | £8 | NHS Cost Data 2019/2090 | DAPS: DAPS07 |
| 7-day trimethoprim | £0.23 | eMIT 2020101 | 200 mg × 14 tablets |
| Non-elective admission (TRUS) | £2503 | Tamhankar et al. 202094 | Inflated to 2019/20 |
| Non-elective admission (TP) | £1895 | Tamhankar et al. 202094 | Inflated to 2019/20 |
| Overnight stay | £602 | PSSRU 202089 | 7.1 NHS reference costs for hospital services – average cost per episode of non-elective short stay (˂2 days) |
| Mortality | £9740 | NHS Cost Data 2019/2090 | NE: WJ06A (weighted average of short and long stay) |
| Radical treatment adverse events | |||
| Erectile dysfunction | £174 | NHS Cost Data 2019/2090 | OPROC: LB43Z (weighted average) |
| Urinary incontinence | £308 | NG131 model67 | Managed by containment pads. Inflated to 2019/20 |
| Bowel dysfunction | £1883 | NG131 model67 | Mean weighted cost including costs associated with sigmoidoscopy, laser therapy, enemas and blood transfusion. Inflated to 2019/20 |
| Metastatic treatment adverse events | |||
| Blood disorder | £1831 | NHS Cost Data 2019/2090 | NE: SA03G-SA03H, SA08G-SA08J, SA12G-SA12K (weighted average of short and long stay) |
| Cardiac disorder | £1592 | NHS Cost Data 2019/2090 | NE: EB10 (weighted average of short and long stay) |
| Endocrine disorder | £174 | Assumption | Same as erectile dysfunction |
| Gastrointestinal disorder | £1492 | NHS Cost Data 2019/2090 | NE: FD10 (weighted average of short and long stay) |
| General disorder | £40 | Assumption | Same as fever |
| Musculoskeletal disorder | £1061 | NHS Cost Data 2019/2090 | NE: HD26 (weighted average of short and long stay) |
| Nervous system disorder | £1513 | NHS Cost Data 2019/2090 | NE: AA26 (weighted average of short and long stay) |
| Neutropenia | £6605 | NHS Cost Data 2019/2090 | NE: PM45 (weighted average of short and long stay) |
| Renal disorder | £48 | Assumption | Same as urinary infection |
| Respiratory disorders | £657 | NHS Cost Data 2019/2090 | NE: DZ19 (weighted average of short and long stay) |
| Skin disorder | £1615 | NHS Cost Data 2019/2090 | NE: JD07 (weighted average of short and long stay) |
| Other costs | |||
| End of life | £16,052 | Round et al. 2015106 | From initiation of strong opioids until death (expected survival 243 days); inflated to 2019/20 |
Appendix 9 Additional cost-effectiveness results
Probabilistic sensitivity analyses: decision question 1
Results for the probabilistic sensitivity analysis for decision question 1 are shown in Table 75. These results are illustrated for subgroup A in the scatterplot and CEAC in Figures 22 and 23, respectively.
| Biopsy method | Total | Incremental | INHB (QALYs) | ICERs | |||
|---|---|---|---|---|---|---|---|
| cost | QALYs | cost | QALYs | £20k | £30k | £/QALY | |
| Subgroup A: MRI Likert 3 + first biopsy | |||||||
| LATRUS | £19,938 | 9.296 | |||||
| LATP-any | £19,982 | 9.303 | £44 | 0.007 | 0.004 | 0.005 | £6710 |
| GATP | £20,479 | 9.300 | £497 | −0.003 | −0.023 | −0.014 | Dominated |
| Subgroup B: MRI Likert 1 or 2 first biopsy | |||||||
| LATRUS | £15,825 | 9.474 | |||||
| LATP-any | £15,880 | 9.479 | £55 | 0.004 | 0.002 | 0.003 | £12,544 |
| GATP | £16,362 | 9.477 | £482 | −0.002 | −0.024 | −0.015 | Dominated |
| Subgroup C: MRI Likert 3 + previous negative biopsy | |||||||
| LATRUS | £16,679 | 9.452 | |||||
| LATP-any | £16,730 | 9.456 | £51 | 0.004 | 0.001 | 0.002 | £14,141 |
| GATP | £17,207 | 9.454 | £477 | −0.002 | −0.024 | −0.015 | Dominated |
| Subgroup D: MRI Likert 1 or 2 previous negative biopsy | |||||||
| LATRUS | £14,109 | 9.543 | |||||
| LATP-any | £14,168 | 9.546 | £58 | 0.003 | 0.000 | 0.001 | £19,126 |
| GATP | £14,639 | 9.546 | £530 | −0.001 | −0.024 | −0.015 | Dominated |
FIGURE 22.
Cost-effectiveness scatterplot: subgroup A (decision question 1).
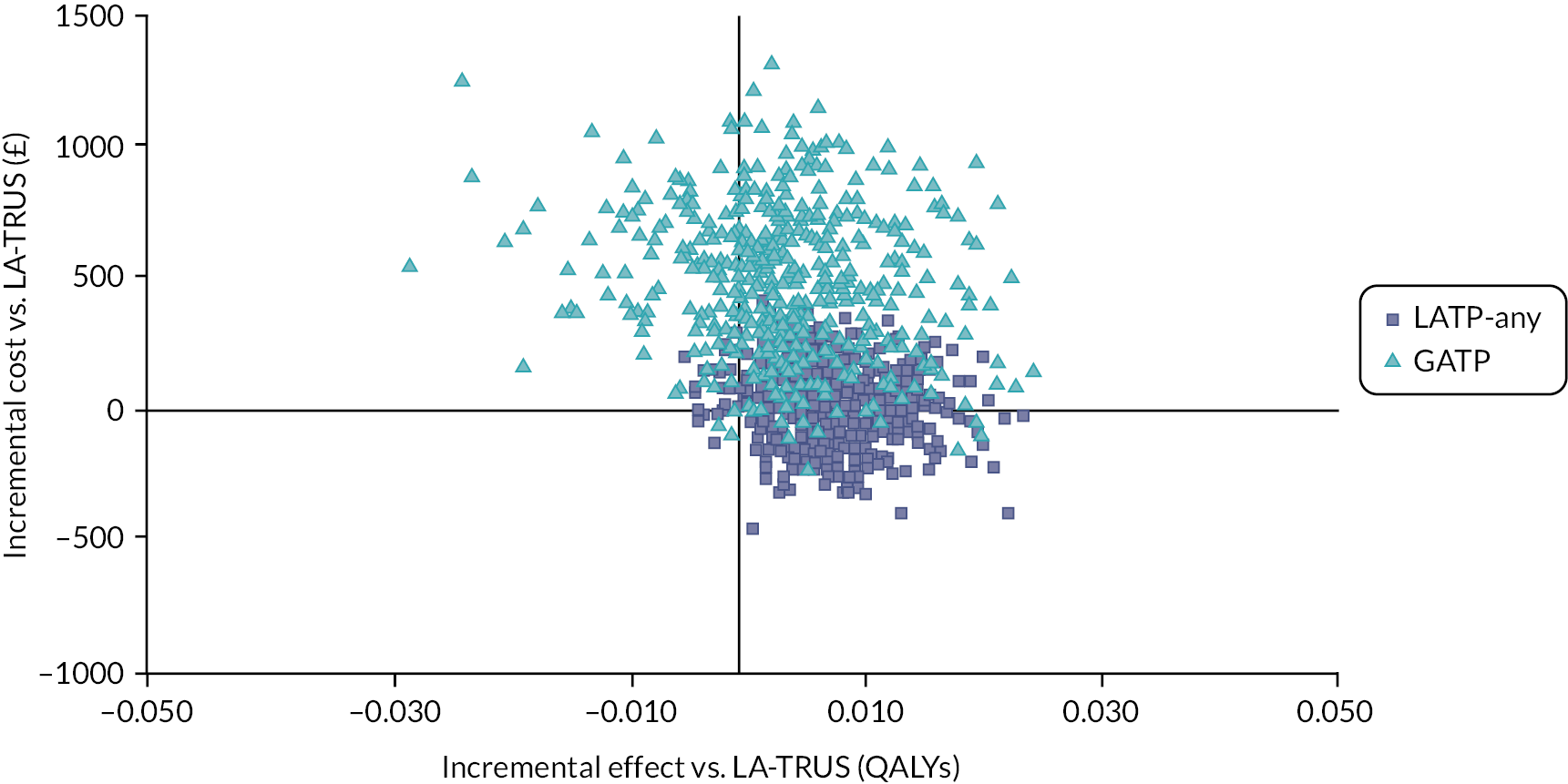
FIGURE 23.
Cost-effectiveness acceptability curve: subgroup A (decision question 1).

Probabilistic sensitivity analyses: decision question 2
Table 76 shows probabilistic results for decision question 2. The probabilistic results for subgroup A are illustrated in the scatterplot and CEACs in Figures 24 and 25, respectively.
| Biopsy method | Total | Incremental | INHB (QALYs) | ICERs | |||
|---|---|---|---|---|---|---|---|
| cost | QALYs | cost | QALYs | £20k | £30k | £/QALY | |
| Subgroup A: MRI Likert 3 + first biopsy | |||||||
| LATRUS | £19,859 | 9.299 | |||||
| LATP-freehand | £19,882 | 9.309 | £23 | 0.010 | 0.009 | 0.010 | £2184 |
| LATP-other | £19,932 | 9.302 | £50 | −0.007 | −0.000 | 0.001 | Dominated |
| GATP | £20,414 | 9.300 | £482 | −0.002 | −0.027 | −0.017 | Dominated |
| Subgroup B: MRI Likert 1 or 2 first biopsy | |||||||
| LATRUS | £15,740 | 9.473 | |||||
| LATP-freehand | £15,784 | 9.480 | £45 | 0.007 | 0.004 | 0.005 | £6846 |
| LATP-other | £15,818 | 9.476 | £34 | −0.004 | −0.001 | 0.000 | Dominated |
| GATP | £16,291 | 9.475 | £473 | −0.001 | −0.026 | −0.017 | Dominated |
| Subgroup C: MRI Likert 3 + previous negative biopsy | |||||||
| LATRUS | £16,756 | 9.452 | |||||
| LATP-freehand | £16,806 | 9.456 | £50 | 0.004 | 0.002 | 0.003 | £11,330 |
| LATP-other | £16,836 | 9.454 | £30 | −0.002 | −0.002 | −0.000 | Dominated |
| GATP | £17,298 | 9.453 | £462 | −0.001 | −0.026 | −0.017 | Dominated |
| Subgroup D: MRI Likert 1 or 2 previous negative biopsy | |||||||
| LATRUS | £14,076 | 9.543 | |||||
| LATP-freehand | £14,121 | 9.547 | £45 | 0.004 | 0.002 | 0.003 | £11,022 |
| LATP-other | £14,153 | 9.545 | £32 | −0.002 | −0.002 | −0.000 | Dominated |
| GATP | £14,612 | 9.544 | £459 | −0.001 | −0.025 | −0.016 | Dominated |
FIGURE 24.
Cost-effectiveness scatterplot: subgroup A (decision question 2).

FIGURE 25.
Cost-effectiveness acceptability curve: subgroup A (decision question 2).

Intermediate outcomes: decision question 1
Intermediate outcomes related to the decision-tree biopsy pathway are shown in Table 77. Outcomes from the Markov model are summarised in Table 78. Table 79 summarises costs estimated from the decision-tree and Markov models.
| Biopsy method | Mean biopsies | Undiagnosed | Biopsy-related AE | AE QALY loss | |||
|---|---|---|---|---|---|---|---|
| CNS (%) | CS (%) | Mild (%) | Admissions (%) | Deaths (%) | |||
| Subgroup A: MRI Likert 3 + first biopsy | |||||||
| LATRUS | 1.034 | 9.92 | 15.22 | 10.7 | 3.9 | 0.07 | −0.0018 |
| LATP-any | 1.034 | 9.62 | 12.23 | 9.5 | 3.7 | 0.05 | −0.0017 |
| GATP | 1.034 | 9.74 | 13.36 | 9.5 | 3.7 | 0.05 | −0.0017 |
| Subgroup B: MRI Likert 1 or 2 first biopsy | |||||||
| LATRUS | 1.013 | 20.40 | 6.73 | 10.5 | 3.8 | 0.07 | −0.0018 |
| LATP-any | 1.013 | 19.72 | 5.47 | 9.3 | 3.6 | 0.05 | −0.0017 |
| GATP | 1.013 | 19.99 | 5.95 | 9.3 | 3.6 | 0.05 | −0.0017 |
| Subgroup C: MRI Likert 3 + previous negative biopsy | |||||||
| LATRUS | 1.000 | 17.44 | 4.45 | 10.4 | 3.7 | 0.07 | −0.0018 |
| LATP-any | 1.000 | 16.46 | 3.28 | 9.1 | 3.5 | 0.05 | −0.0017 |
| GATP | 1.000 | 16.85 | 3.71 | 9.1 | 3.5 | 0.05 | −0.0017 |
| Subgroup D: MRI Likert 1 or 2 previous negative biopsy | |||||||
| LATRUS | 1.000 | 21.74 | 1.12 | 10.4 | 3.7 | 0.07 | −0.0018 |
| LATP-any | 1.000 | 20.53 | 0.82 | 9.1 | 3.5 | 0.05 | −0.0017 |
| GATP | 1.000 | 21.01 | 0.93 | 9.1 | 3.5 | 0.05 | −0.0017 |
| Biopsy method | Deaths (% of whole cohort) | Undiscounted | Discounted | ||||
|---|---|---|---|---|---|---|---|
| Prostate cancer | Other cause | All | LYs | QALYs | LY | QALY | |
| Subgroup A: MRI Likert 3 + first biopsy | |||||||
| LATRUS | 19.60 | 80.31 | 99.90 | 16.010 | 12.578 | 11.717 | 9.301 |
| LATP-any | 19.52 | 80.41 | 99.92 | 16.024 | 12.589 | 11.726 | 9.307 |
| GATP | 19.55 | 80.38 | 99.92 | 16.020 | 12.586 | 11.723 | 9.306 |
| Subgroup B: MRI Likert 1 or 2 first biopsy | |||||||
| LATRUS | 10.86 | 89.03 | 99.89 | 16.780 | 12.960 | 12.138 | 9.480 |
| LATP-any | 10.82 | 89.09 | 99.91 | 16.789 | 12.967 | 12.143 | 9.484 |
| GATP | 10.84 | 89.08 | 99.91 | 16.787 | 12.965 | 12.142 | 9.483 |
| Subgroup C: MRI Likert 3 + previous negative biopsy | |||||||
| LATRUS | 12.64 | 87.26 | 99.90 | 16.638 | 12.903 | 12.063 | 9.458 |
| LATP-any | 12.60 | 87.32 | 99.92 | 16.647 | 12.910 | 12.069 | 9.462 |
| GATP | 12.61 | 87.31 | 99.92 | 16.645 | 12.908 | 12.067 | 9.462 |
| Subgroup D: MRI Likert 1 or 2 previous negative biopsy | |||||||
| LATRUS | 7.32 | 92.57 | 99.89 | 17.087 | 13.111 | 12.304 | 9.549 |
| LATP-any | 7.30 | 92.61 | 99.91 | 17.093 | 13.115 | 12.308 | 9.552 |
| GATP | 7.31 | 92.60 | 99.91 | 17.092 | 13.114 | 12.308 | 9.552 |
| Biopsy method | Decision-tree costs | Markov model, undiscounted costs | Discounted total costs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Biopsies | AE | Total cost | Treatment | AE | Follow-up | End of life | Total | ||
| Subgroup A: MRI Likert 3 + first biopsy | |||||||||
| LATRUS | £704 | £109 | £813 | £8965 | £2709 | £662 | £16,042 | £28,378 | £19,065 |
| LATP-any | £799 | £80 | £879 | £8942 | £2715 | £651 | £16,043 | £28,351 | £19,040 |
| GATP | £1274 | £80 | £1354 | £8951 | £2713 | £655 | £16,043 | £28,363 | £19,050 |
| Subgroup B: MRI Likert 1 or 2 first biopsy | |||||||||
| LATRUS | £690 | £107 | £796 | £5118 | £1715 | £639 | £16,040 | £23,513 | £14,957 |
| LATP-any | £785 | £78 | £863 | £5107 | £1718 | £630 | £16,042 | £23,498 | £14,942 |
| GATP | £1260 | £78 | £1338 | £5112 | £1717 | £634 | £16,042 | £23,505 | £14,948 |
| Subgroup C: MRI Likert 3 + previous negative biopsy | |||||||||
| LATRUS | £681 | £105 | £786 | £5953 | £1987 | £654 | £16,041 | £24,634 | £15,867 |
| LATP-any | £776 | £76 | £852 | £5942 | £1990 | £643 | £16,042 | £24,617 | £15,851 |
| GATP | £1251 | £76 | £1328 | £5947 | £1989 | £647 | £16,042 | £24,625 | £15,857 |
| Subgroup D: MRI Likert 1 or 2 previous negative biopsy | |||||||||
| LATRUS | £681 | £105 | £786 | £3568 | £1303 | £607 | £16,039 | £21,516 | £13,280 |
| LATP-any | £776 | £76 | £852 | £3563 | £1304 | £597 | £16,041 | £21,505 | £13,269 |
| GATP | £1251 | £76 | £1328 | £3565 | £1304 | £601 | £16,041 | £21,510 | £13,273 |
Intermediate outcomes: decision question 2
Intermediate outcomes and costs for decision question 2 are shown in Tables 80, 81 and 82.
| Biopsy method | Mean biopsies | Undiagnosed (%) | Biopsy-related AE (%) | ||||
|---|---|---|---|---|---|---|---|
| CNS | Mild | Admission | Death | AE QALY loss | |||
| Subgroup A: MRI Likert 3 + first biopsy | |||||||
| LATRUS | 1.0342 | 9.92 | 15.22 | 10.7 | 3.9 | 0.07 | −0.0018 |
| LATP-freehand | 1.0344 | 9.15 | 8.38 | 9.5 | 3.7 | 0.05 | −0.0017 |
| LATP-other | 1.0342 | 9.82 | 14.16 | 9.5 | 3.7 | 0.05 | −0.0017 |
| GATP | 1.0342 | 9.90 | 15.01 | 9.5 | 3.7 | 0.05 | −0.0017 |
| Subgroup B: MRI Likert 1 or 2 first biopsy | |||||||
| LATRUS | 1.0132 | 20.40 | 6.73 | 10.5 | 3.8 | 0.07 | −0.0018 |
| LATP-freehand | 1.0139 | 18.64 | 3.85 | 9.3 | 3.6 | 0.05 | −0.0017 |
| LATP-other | 1.0132 | 20.17 | 6.29 | 9.3 | 3.6 | 0.05 | −0.0017 |
| GATP | 1.0132 | 20.35 | 6.64 | 9.3 | 3.6 | 0.05 | −0.0017 |
| Subgroup C: MRI Likert 3 + previous negative biopsy | |||||||
| LATRUS | 1.0000 | 17.44 | 4.45 | 10.4 | 3.7 | 0.07 | −0.0018 |
| LATP-freehand | 1.0000 | 14.95 | 3.59 | 9.1 | 3.5 | 0.05 | −0.0017 |
| LATP-other | 1.0000 | 17.11 | 4.02 | 9.1 | 3.5 | 0.05 | −0.0017 |
| GATP | 1.0000 | 17.38 | 4.37 | 9.1 | 3.5 | 0.05 | −0.0017 |
| Subgroup D: MRI Likert 1 or 2 previous negative biopsy | |||||||
| LATRUS | 1.0000 | 21.74 | 1.12 | 10.4 | 3.7 | 0.07 | −0.0018 |
| LATP-freehand | 1.0000 | 18.64 | 0.90 | 9.1 | 3.5 | 0.05 | −0.0017 |
| LATP-other | 1.0000 | 21.33 | 1.01 | 9.1 | 3.5 | 0.05 | −0.0017 |
| GATP | 1.0000 | 21.66 | 1.09 | 9.1 | 3.5 | 0.05 | −0.0017 |
| Biopsy method | Deaths (% of whole cohort) | Undiscounted | Discounted | |||||
|---|---|---|---|---|---|---|---|---|
| Prostate cancer | Other cause | All | LYs | QALYs | LY | QALY | ||
| Subgroup A: MRI Likert 3 + first biopsy | ||||||||
| LATRUS | 19.60 | 80.31 | 99.90 | 16.010 | 12.578 | 11.717 | 9.301 | |
| LATP-freehand | 19.41 | 80.51 | 99.92 | 16.037 | 12.599 | 11.734 | 9.314 | |
| LATP-other | 19.57 | 80.35 | 99.92 | 16.017 | 12.584 | 11.722 | 9.304 | |
| GATP | 19.59 | 80.33 | 99.92 | 16.014 | 12.581 | 11.720 | 9.303 | |
| Subgroup B: MRI Likert 1 or 2 first biopsy | ||||||||
| LATRUS | 10.86 | 89.03 | 99.89 | 16.780 | 12.960 | 12.138 | 9.480 | |
| LATP-freehand | 10.77 | 89.15 | 99.91 | 16.795 | 12.972 | 12.147 | 9.487 | |
| LATP-other | 10.85 | 89.06 | 99.91 | 16.785 | 12.964 | 12.141 | 9.483 | |
| GATP | 10.86 | 89.05 | 99.91 | 16.784 | 12.963 | 12.140 | 9.482 | |
| Subgroup C: MRI Likert 3 + previous negative biopsy | ||||||||
| LATRUS | 12.64 | 87.26 | 99.90 | 16.638 | 12.903 | 12.063 | 9.458 | |
| LATP-freehand | 12.58 | 87.33 | 99.92 | 16.648 | 12.911 | 12.069 | 9.463 | |
| LATP-other | 12.62 | 87.29 | 99.92 | 16.643 | 12.907 | 12.067 | 9.461 | |
| GATP | 12.64 | 87.28 | 99.92 | 16.642 | 12.906 | 12.066 | 9.460 | |
| Subgroup D: MRI Likert 1 or 2 previous negative biopsy | ||||||||
| LATRUS | 7.32 | 92.57 | 99.89 | 17.087 | 13.111 | 12.304 | 9.549 | |
| LATP-freehand | 7.28 | 92.63 | 99.91 | 17.096 | 13.117 | 12.310 | 9.553 | |
| LATP-other | 7.32 | 92.60 | 99.91 | 17.092 | 13.114 | 12.307 | 9.551 | |
| GATP | 7.32 | 92.59 | 99.91 | 17.091 | 13.113 | 12.307 | 9.551 | |
| Biopsy method | Decision-tree costs | Markov model, undiscounted costs | Discounted total costs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Biopsies | AE | Total | Treatment | AE | Follow-up | End of life | Total | ||
| Subgroup A: MRI Likert 3 + first biopsy | |||||||||
| LATRUS | £704 | £109 | £813 | £8965 | £2709 | £662 | £16,042 | £28,378 | £19,065 |
| LATP-freehand | £805 | £80 | £885 | £8909 | £2721 | £637 | £16,043 | £28,309 | £19,004 |
| LATP-other | £814 | £80 | £894 | £8958 | £2711 | £658 | £16,043 | £28,371 | £19,058 |
| GATP | £1275 | £80 | £1355 | £8965 | £2710 | £661 | £16,043 | £28,380 | £19,066 |
| Subgroup B: MRI Likert 1 or 2 first biopsy | |||||||||
| LATRUS | £690 | £107 | £796 | £5118 | £1715 | £639 | £16,040 | £23,513 | £14,957 |
| LATP-freehand | £791 | £78 | £868 | £5092 | £1721 | £618 | £16,042 | £23,472 | £14,920 |
| LATP-other | £800 | £78 | £877 | £5115 | £1717 | £636 | £16,042 | £23,510 | £14,952 |
| GATP | £1260 | £78 | £1338 | £5119 | £1716 | £638 | £16,042 | £23,515 | £14,957 |
| Subgroup C: MRI Likert 3 + previous negative biopsy | |||||||||
| LATRUS | £681 | £105 | £786 | £5953 | £1987 | £654 | £16,041 | £24,634 | £15,867 |
| LATP-freehand | £781 | £76 | £858 | £5942 | £1990 | £633 | £16,042 | £24,607 | £15,841 |
| LATP-other | £791 | £76 | £867 | £5950 | £1988 | £650 | £16,042 | £24,630 | £15,862 |
| GATP | £1251 | £76 | £1328 | £5953 | £1987 | £653 | £16,042 | £24,636 | £15,867 |
| Subgroup D: MRI Likert 1 or 2 previous negative biopsy | |||||||||
| LATRUS | £681 | £105 | £786 | £3568 | £1303 | £607 | £16,039 | £21,516 | £13,280 |
| LATP-freehand | £781 | £76 | £858 | £3560 | £1305 | £583 | £16,041 | £21,489 | £13,254 |
| LATP-other | £791 | £76 | £867 | £3566 | £1303 | £603 | £16,041 | £21,514 | £13,277 |
| GATP | £1251 | £76 | £1328 | £3568 | £1303 | £606 | £16,041 | £21,518 | £13,280 |
Scenario analysis: relative risk of cancer detection from observational data
The base-case analysis uses cancer detection rates from network meta-analyses of RCT data only. We tested the effect of using estimates from observational studies, based on pairwise meta-analysis comparisons reported in Intermediate outcomes above (see Appendix 4, Figures 9, 14 and 15). Observational data for GATP are only available in comparison with LATP (method not specified). Therefore, the RR for GATP versus LATRUS has to be adjusted by the RR for LATP versus LATRUS for use in the model. This yields different estimates for the effectiveness of GATP in decision questions 1 and 2: 1.45 (1.31 × 1.10) or 1.33 (1.31 × 1.01) respectively in the base case.
Following questions raised by NICE specialist committee members for this assessment, we conducted additional scenario analysis to investigate the impact of uncertainty over which observational studies should be included. These include scenarios excluding the Bojin study or excluding the Watanabe study, a scenario including a study by Walters et al. and a scenario including Walters but excluding the Takuma study. 29,38,43,44
Table 83 shows the results for decision question 1 subgroup A. The ICERs for LATP-any compared with LATRUS are higher in the observational scenarios (from £7609 to £11,175 per QALY) than in the base case analysis with RCT data (£5859 per QALY). The ICERs for LATP-any remain below £20,000 per QALY for all observational scenarios and subgroups, with the exception of scenario 2 in subgroup D, for which the ICER is £22,260 per QALY. GATP has a high ICER (above £30,000 per QALY) or is dominated in all observational scenarios and subgroups.
| Biopsy method | RRa | Total | Incremental | ICERs | ||
|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | £/QALY | ||
| Observational scenario 1: original pairwise meta-analysis | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-any | 1.10 | £19,927 | 9.304 | £49 | 0.005 | £9159 |
| GATP | 1.45 | £20,359 | 9.312 | £431 | 0.008 | £54,953 |
| Observational scenario 2: excluding Bojin 29 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-any | 1.08 | £19,931 | 9.304 | £53 | 0.005 | £11,175 |
| GATP | 1.42 | £20,358 | 9.312 | £427 | 0.009 | £49,771 |
| Observational scenario 3: excluding Watanabe 38 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-any | 1.12 | £19,924 | 9.305 | £46 | 0.006 | £7609 |
| GATP | 1.47 | £20,359 | 9.312 | £435 | 0.007 | £61,058 |
| Observational scenario 4: including Walters 44 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-any | 1.10 | £19,927 | 9.304 | £49 | 0.005 | £9159 |
| GATP | 1.16 | £20,393 | 9.306 | £466 | 0.002 | £263,212 |
| Observational scenario 5: including Walters and excluding Takuma 43 , 44 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-any | 1.10 | £19,927 | 9.304 | £49 | 0.005 | £9159 |
| GATP | 0.97 | £20,428 | 9.300 | £500 | −0.004 | Dominated |
Table 84 shows the observational scenario results for decision question 2 subgroup A. The ICERs for LATP-freehand versus LATRUS are higher when based on observational data than in the base case (£743 per QALY), but they remain below £20,000 per QALY in all observational scenarios and subgroups. LATP-other and GATP are dominated or have high ICERs in all observational scenarios and subgroups. This remains the case if we use the same RR for GATP versus TRUS as in decision question 1.
| Biopsy method | RRa | Total | Incremental | ICERs | ||
|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | £/QALY | ||
| Observational scenario 1: original pairwise meta-analysis | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-freehand | 1.21 | £19,915 | 9.308 | £36 | 0.009 | £4209 |
| LATP-other | 1.01 | £19,960 | 9.301 | £45 | −0.006 | Dominated |
| GATP | 1.33 | £20,367 | 9.311 | £408 | 0.009 | £148,623 |
| Observational scenario 2: excluding Bojin 29 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-freehand | 1.22 | £19,913 | 9.308 | £35 | 0.009 | £3904 |
| LATP-other | 1.01 | £19,960 | 9.301 | £46 | −0.007 | Dominated |
| GATP | 1.33 | £20,367 | 9.311 | £408 | 0.009 | £163,869 |
| Observational scenario 3: excluding Watanabe 38 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-freehand | 1.21 | £19,915 | 9.308 | £36 | 0.009 | £4209 |
| LATP-other | 1.00 | £19,962 | 9.301 | £47 | −0.007 | Dominated |
| GATP | 1.32 | £20,369 | 9.310 | £408 | 0.009 | £166,422 |
| Observational scenario 4: including Walters 44 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-freehand | 1.21 | £19,915 | 9.308 | £36 | 0.009 | £4209 |
| LATP-other | 1.01 | £19,960 | 9.301 | £45 | −0.006 | Dominated |
| GATP | 1.06 | £20,410 | 9.303 | £450 | 0.002 | Dominated |
| Observational scenario 5: including Walters and excluding Takuma 43 , 44 | ||||||
| LATRUS | 1.00 | £19,878 | 9.299 | |||
| LATP-freehand | 1.21 | £19,915 | 9.308 | £36 | 0.009 | £4209 |
| LATP-other | 1.01 | £19,960 | 9.301 | £45 | −0.006 | Dominated |
| GATP | 0.89 | £20,445 | 9.297 | £486 | −0.005 | Dominated |
Scenario analysis: cost of histopathology
We reported results for three scenarios with alternative assumptions on the numbers of core samples for the different biopsy methods in Tables 42 and 43. These analyses used the base-case histopathology cost of £36.58 per core sample. Tables 85 and 86 report results for the core scenarios with alternative unit costs for histopathology from an online report by the University of Surrey: £37.50 for ‘standard histology’ (1–2 sites/lesions) and £7 per additional site/lesion. 112
| Biopsy method | Biopsy samples | Total | Incremental | ICERs | ||
|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | £/QALY | ||
| Core scenario 1: 24 core samples for all transperineal methods | ||||||
| LATRUS | 12 | £19,467 | 9.299 | |||
| LATP-any | 24 | £19,597 | 9.306 | £130 | 0.007 | £18,852 |
| GATP | 24 | £20,081 | 9.304 | £484 | −0.002 | Dominated |
| Biopsy method | Biopsy samples | Total | Incremental | ICERs | ||
|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | £/QALY | ||
| Core scenario 1: 24 cores for all transperineal methods | ||||||
| LATRUS | 12 | £19,467 | 9.299 | |||
| LATP-freehand | 24 | £19,574 | 9.312 | £107 | 0.013 | £8052 |
| LATP-other | 24 | £19,626 | 9.303 | £52 | −0.010 | Dominated |
| GATP | 24 | £20,093 | 9.301 | £467 | −0.001 | Dominated |
| Core scenario 2: 24 cores for LATP-freehand only | ||||||
| LATRUS | 12 | £19,467 | 9.299 | |||
| LATP-other | 12 | £19,542 | 9.303 | £75 | 0.004 | Dominateda |
| LATP-freehand | 24 | £19,574 | 9.312 | £32 | 0.010 | £8052 |
| GATP | 12 | £20,009 | 9.301 | £435 | −0.011 | Dominated |
| Core scenario 3: 24 cores for LATP-freehand and 16 for LATP-other and GATP | ||||||
| LATRUS | 12 | £19,467 | 9.299 | |||
| LATP-other | 16 | £19,570 | 9.303 | £103 | 0.004 | Dominateda |
| LATP-freehand | 24 | £19,574 | 9.312 | £4 | 0.010 | £8052 |
| GATP | 16 | £20,037 | 9.301 | £463 | −0.011 | Dominated |
Scenario analysis: probability of repeat biopsy
The base-case value for the probability of repeat biopsy for MRI Likert score 3 + after first biopsy result CNS was 15.45% for LATRUS, LATP and GATP, informed by the rate after a first LATRUS biopsy reported by Jimenez et al. (see Probability of a repeat biopsy). 85 Jimenez et al. also reported the rate of re-biopsy after a first GATP biopsy (5.36%), which we have not used in the base case because it is associated with some uncertainty – a much lower sample size and prostates with higher volume than for LATRUS.
Jimenez et al. do not report the probability of repeat biopsy after a first LATP biopsy. It is unclear whether this is closer to the rate after LATRUS or after GATP: whether the likelihood of repeat biopsy is more related to the route of biopsy or the type of anaesthesia. The route of biopsy may affect accessibility of different areas of the prostate, which could influence the proportion of unexpected negative biopsy results when there is a high suspicion of prostate cancer. On the otherhand, we understand that it can be possible to take more and better samples of the prostate under general anaesthetic, when patients cannot tolerate a prolonged procedure under local anaesthetics.
Experts advising NICE stated that they would expect rates of repeat biopsy to be lower for GATP than for LATP and LATRUS. They stated a preference for an analysis with the rate for LATP assumed equal to that for LATRUS (15.45%), but with a lower rate for GATP (5.36%). The view that the likelihood of repeat biopsy is similar for LATRUS and LATP was supported by a stakeholder comment. This attributed the lower rate of repeat biopsy for GATP compared with LATRUS in the Jimenez study to the greater number of biopsy core samples taken for GATP (reported as 12–18 for LATRUS and 30 for GATP).
We therefore report results for two repeat biopsy scenarios:
-
In the first scenario we use the lower repeat biopsy rate from Jimenez (5.36%) for LATP and GATP.
-
In the second scenario, we retain the high repeat biopsy rate for LATP (assumed equal to LATRUS) but use the lower rate for GATP. Note that these scenarios are not relevant for the other subgroups because we assume lower rates of repeat biopsy for patients with a MRI Likert score of 1 or 2, and no repeat biopsy after a second biopsy.
Table 87 shows that the ICER for LATP-any versus LATRUS is lower when the lower rate of repeat biopsy (5.36%) observed after TP in the Jimenez et al. study85 is used for LATP (rather than 15.45% as observed after LATRUS). LATP-freehand dominates all other comparators when the lower rate of repeat biopsy is assumed. These scenarios do not change overall conclusions in subgroup A: the ICER for LATP-any or LATP-freehand versus LATRUS remains below the £20,000 per QALY threshold.
| Biopsy method | Total | Incremental | ICERs | ||
|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | £/QALY | |
| Decision question 1: lower rate for LATP and GATP | |||||
| LATRUS | £19,878 | 9.299 | |||
| LATP all | £19,908 | 9.305 | £29 | 0.006 | £5094 |
| GATP | £20,394 | 9.303 | £486 | −0.002 | Dominated |
| Decision question 1: lower rate for GATP only | |||||
| LATRUS | £19,878 | 9.299 | |||
| LATP all | £19,919 | 9.306 | £40 | 0.007 | £5859 |
| GATP | £20,394 | 9.303 | £475 | −0.003 | Dominated |
| Decision question 2: lower rate for LATP and GATP | |||||
| LATP-freehand | £19,877 | 9.311 | |||
| LATRUS | £19,878 | 9.299 | £2 | −0.012 | Dominated |
| LATP-other | £19,941 | 9.301 | £63 | 0.003 | Dominated |
| GATP | £20,410 | 9.300 | £469 | −0.001 | Dominated |
| Decision question 2: lower rate for GATP only | |||||
| LATRUS | £19,878 | 9.299 | |||
| LATP-freehand | £19,888 | 9.312 | £10 | 0.013 | £743 |
| LATP-other | £19,952 | 9.303 | £63 | −0.010 | Dominated |
| GATP | £20,410 | 9.300 | £458 | −0.003 | Dominated |
We also tested the impact of changing the probability of repeat biopsy after a ‘no cancer’ biopsy result (assumed to be 5% for all biopsy methods in the base case). This did not change the cost-effectiveness conclusions, even when we increased this probability to 15.45% for LATP (the same as if the biopsy had detected CNS disease) but left the probability at 5% for other comparators.
| Element | Base case | Scenario analysis | Justification | ICER (£ per QALY gained) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Decision question 1 | Decision question 2 | ||||||||
| LATP vs. LATRUS | LATP vs. GATP | LATP-fh vs. LATRUS | LATP-fh vs. LATP-other | LATP-fh vs. GATP | |||||
| Base-case results | £5859 | Dominant | £743 | Dominant | Dominant | ||||
| 1 | Time horizon | 40 years | 20 years | Test the impact of an alternative time horizon | £5913 | Dominant | £68 | Dominant | Dominant |
| 2 | Discount rate | 3.5% | 0.0% | Test the impact of alternative discount rates as recommended by NICE | £3591 | Dominant | £66 | Dominant | Dominant |
| 3 | 1.5% QALYs 1.5% costs |
£4404 | Dominant | £228 | Dominant | Dominant | |||
| 4 | 1.5% QALYs 3.5% costs |
£4641 | Dominant | £580 | Dominant | Dominant | |||
| 5 | Initial age of the cohort | 66 years | 55 years | Test the impact of a younger cohort | £4586 | Dominant | £231 | Dominant | Dominant |
| 6 | 63 years | Mean age at referral for a first prostate biopsy in PROMIS trial | £5290 | Dominant | £493 | Dominant | Dominant | ||
| 7 | 75 years | Test the impact of an older cohort | £9859 | Dominant | £2987 | Dominant | Dominant | ||
| 8 | Proportion of initial cohort with MD | 0.0% | 5.0% | It is likely that a small proportion of patients with MD undergo biopsy | £5859 | Dominant | £743 | Dominant | Dominant |
| 9 | Probability of CS result for LR disease (at first/second LATRUS) | 0.0% | 5.0% | As advised by SCM it’s unlikely that there are no false positive results of CS for patients with LR disease | £5701 | Dominant | £585 | Dominant | Dominant |
| 10 | Probability of CNS/NC result for HR disease (first/second LATRUS) | 0.0% | CNS: 8.0% NC: 5.0% |
Test the impact of FN results by using the probabilities of CNS and NC from second biopsy | £5689 | Dominant | £734 | Dominant | Dominant |
| 11 | Incidence of prostate cancer | 0.0% | 0.8% per 3 month Markov model cycle | Assume some incident cases as in practice | £5871 | Dominant | £749 | Dominant | Dominant |
| 12 | Proportion of patients in primary care follow-up having PSA | 100.0% per year | 50.0% per year | It is unlikely that all patients comply and measure their PSA every year | £3401 | Dominant | £587 | Dominant | Dominant |
| 14 | Radical treatment: probability of erectile dysfunction | AS/WW: 50.9% RP: 85.4% RT: 62.4% |
AS/WW: 70.0% RP: 90.0% RT: 80.0% |
As suggested by expert the probability of erectile dysfunction is likely to be higher | £5862 | Dominant | £751 | Dominant | Dominant |
| 15 | Distribution of treatments for mHSPC | ADT: 50.0% DOX + ADT: 36.0% APA + ADT: 7.0% ENZA + ADT: 7.0% |
ADT: 25.0% ENZA + ADT: 32.0% |
Expert opinion that use of enzalutamide is growing and ADT alone is reducing | £4658 | Dominant | Dominant | Dominant | Dominant |
| 16 | Exclusion of APA + ADT and ENZA + ADT for mHSPC | Included | Excluded | The model is not coded to account for the long-term benefits of these treatments | £6514 | Dominant | £1540 | Dominant | Dominant |
| 17 | Duration of ADT alone APA + ADT and ENZA + ADT for mHSPC | 2 years | 3 years | According to a SCM | £5754 | Dominant | £615 | Dominant | Dominant |
| 18 | Disutility for mild biopsy-related AE | −0.289 for 3 days | −0.289 for 30 days | To test sensitivity to QALY loss for less serious complications | £6642 | Dominant | £729 | Dominant | Dominant |
| 19 | Disutility for biopsy-related admission | −0.490 for 30 days | −0.490 for 10 days | To test sensitivity to QALY loss for serious complications | £5904 | Dominant | £746 | Dominant | Dominant |
| 20 | −0.490 for 90 days | £5729 | Dominant | £735 | Dominant | Dominant | |||
| 21 | Disutility for patients with FN result and true MD | −0.019 | −0.137 | Apply the same disutility as for patients diagnosed with MD | £5884 | Dominant | £747 | Dominant | Dominant |
Glossary
- Active surveillance
- Monitoring of a person following a diagnosis of prostate cancer with a view to the person having radical treatment if the cancer progresses. One of the aims of active surveillance is to avoid the risk of overtreatment by avoiding immediate radical intervention.
- Adverse event
- Any undesirable experience associated with the use of a medical product or procedure in a patient.
- Benign
- Not cancerous. Benign tumours do not spread to tissues around them or to other parts of the body.
- Biopsy
- Sampling of tissue from a specific area of the body (e.g. the prostate) to check for abnormalities such as cancer.
- Cancer
- Growth of abnormal cells in the body in an uncontrolled manner.
- Digital rectal exam
- The doctor inserts a gloved, lubricated finger into the rectum and feels the rectum, anus and prostate to check for anything abnormal.
- Erectile dysfunction
- The inability to get or maintain an erection.
- Fusion biopsy
- A fusion biopsy combines the pre-biopsy magnetic resonance imaging image with the ultrasound image during the biopsy procedure in order to more accurately target any suspicious areas of the prostate. Cognitive fusion, or visual registration, is when the urologist views both sets of images and mentally translates the multiparametric magnetic resonance imaging target lesions onto the real-time ultrasound images during the biopsy procedure. Software-based fusion uses technology to fuse the images from the pre-biopsy multiparametric magnetic resonance imaging and the real-time ultrasound, creating a detailed three-dimentional image for the urologist to use.
- General anaesthetic transperineal biopsy grid and stepping device
- For the purpose of this assessment report, ‘general anaesthetic transperineal biopsy grid and stepping device’ refers to general anaesthetic transperineal prostate biopsy done using a grid and stepping device
- Gleason system
- A commonly used system used to grade prostate cancer cells to estimate how quickly they are likely to grow (the Gleason grade). The overall Gleason score is calculated by adding together the two most common Gleason grades. Grade Group 1 is the least aggressive, indicating that the cancer is likely to grow very slowly, if at all. Grade Group 5 is the most aggressive, indicating the cells look very abnormal and the cancer is likely to grow quickly.
- Grade
- Describes the degree of severity of a cancer.
- Haematuria
- The presence of blood in a person’s urine.
- Heterogeneous/heterogeneity
- Composed of a diverse mixture of different kinds or subgroups.
- Local anaesthetic transperineal prostate-any (LATP-any)
- For the purpose of this assessment report, ‘LATP-any’ refers to local anaesthetic transperineal prostate biopsy done by any method with the National Institute for Health and Care Excellence scope [i.e. prostate biopsy using a grid and stepping device, a coaxial needle (‘double freehand’), or a freehand device].
- Local anaesthetic transperineal prostate-freehand (LATP-freehand)
- For the purpose of this assessment report, ‘LATP-freehand’ refers to local anaesthetic transperineal prostate biopsy done using one of the six freehand devices within the National Institute for Health and Care Excellence scope. This is a subcategory of the LATP-any grouping of biopsy methods.
- Local anaesthetic transperineal prostate-other (LATP-other)
- For the purpose of this assessment report, ‘LATP-other’ refers to local anaesthetic transperineal prostate biopsy done without a freehand device. This includes LATP done with a coaxial needle or with a grid and stepping device.
- Likert score
- A Likert score is reported using a five-point Likert scale. The Likert scale, when used in the diagnosis of prostate cancer, takes into account clinical factors and lesion size on multiparametric magnetic resonance imaging. A score of 1 indicates prostate cancer is very unlikely and a score of 5 indicates prostate cancer is very likely. Likert scores are used to help decide whether or not to have a prostate biopsy at the current time. The Likert score differs from the Prostate Imaging Reporting and Data System (PI-RADS) score in that it takes into account clinical factors and does not require specific sequential review of magnetic resonance imaging sequences.
- Magnetic resonance imaging (MRI)
- MRIs use magnetic fields to create clear images of tissues, muscles, nerves and bones. MRIs makes better images of organs and soft tissue than other scanning techniques, such as computed tomography or X-ray.
- Malignant
- Cancerous. Malignant tumours can invade and destroy nearby tissue and can spread to other parts of the body.
- Multiparametric MRI-influenced prostate biopsy (mpMRI)
- The information from the mpMRI scan taken before prostate biopsy is used to determine the best needle placement. In rare cases, the biopsy may be MRI-guided (the needle is inserted within the MRI machine). In most cases, the biopsy that follows the mpMRI will be ultrasound-guided, but the specific area(s) targeted will be predetermined by the mpMRI data.
- Prostate
- A walnut-sized gland surrounding the urethra, located immediately below the bladder in males. The prostate gland produces a thick, white fluid that gets mixed with sperm to create semen.
- Prostate Imaging Reporting and Data System (PI-RADS) score
- The PI-RADS score is a system whereby each lesion identified by mpMRI is assigned a score from 1 to 5 to indicate the likelihood of clinically significant cancer (where 1 is very low and 5 is very high). PI-RADS v2 is the current validated version. It differs from the Likert score in that it does not take into account clinical factors and it requires specific sequential review of MRI sequences.
- Prostate-specific antigen (PSA)
- PSA is a substance made by the prostate gland. A small amount of PSA in the blood is normal. If the prostate becomes enlarged, inflamed, infected or cancerous, larger amounts of PSA get into the blood.
- Rectum
- The rectum, also known as the back passage, is the last 6 inches of the large bowel and connects the colon to the anus.
- Scrotum
- A bag of skin near the penis that contains the testicles.
- Sepsis
- Sepsis, also known as septicaemia or blood poisoning, is a life-threatening reaction to an infection. It happens when the body’s immune system overreacts to an infection and starts to damage the body’s own tissues and organs.
- Transrectal ultrasound
- A small wand (probe) is put into the patient’s rectum. It gives off sound waves and picks up the echoes as they bounce off the prostate gland. The echoes are made into a picture on a computer screen.
- Urinary retention
- Difficulty in urinating fully or inability to completely empty the bladder.
- Watchful waiting
- Monitoring of a person diagnosed with prostate cancer where any potential treatment offered is aimed at controlling rather than trying to cure the prostate cancer (palliative rather than curative).
List of abbreviations
- ADT
- androgen deprivation therapy
- AE
- adverse event
- ASCO
- American Society of Clinical Ontology
- AUA
- American Urologic Association
- BAUS
- British Association of Urological Surgeons
- BNF
- British National Formulary
- BSA
- body surface area
- CADTH
- Canadian Agency for Drugs and Technologies in Health
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CNS
- clinically non-significant
- CS
- clinically significant
- CSDR
- Cochrane Database of Systematic Reviews
- CT
- computerised tomography
- DAP
- Diagnostics Assessment Programme
- DARE
- Database of Abstracts of Reviews of Effects
- DRE
- digital rectal examination
- EAG
- Evidence Assessment Group
- EAU
- European Association of Urology
- EED
- Economic Evaluations Database
- eMIT
- electronic market information tool
- EQ-5D-3L
- European Quality of Life Working Group Health Status Measure 5 Dimensions, 3 Levels
- EQ-5D-5L
- European Quality of Life Working Group Health Status Measure 5 Dimensions, 5 Levels
- FN
- false negative
- GATP
- general anaesthetic transperineal biopsy
- GP
- general practitioner
- HR
- high risk
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IIEF
- International Index of Erectile Function
- INAHTA
- International Health Technology Assessment Database
- IQR
- interquartile range
- IR
- intermediate risk
- ISPOR
- The Professional Society for Health Economics and Outcomes Research
- ISUP
- International Society of Urological Pathology
- JBI
- Joanna Briggs Institute
- LATP biopsy
- local anaesthetic transperineal biopsy
- LATRUS biopsy
- local anaesthetic transrectal ultrasound biopsy
- LHRH
- luteinising hormone-releasing hormone
- LR
- low risk
- LY
- life-years
- MD
- metastatic disease
- mpMRI
- multiparametric magnetic resonance imaging
- MRI
- magnetic resonance imaging
- NPCA
- National Prostate Cancer Audit
- NG131
- NICE Guideline 131
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMA
- network meta-analysis
- PC
- prostate cancer
- PI-RADS
- prostate imaging – reporting and data system
- PSA
- prostate-specific antigen
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk/risk ratio
- SCM
- Specialist Committee Member
- SD
- standard deviation
- SF-6D
- short-form questionnaire – 6 items
- SF-12
- short-form questionnaire – 12 items
- SF-36
- short-form questionnaire – 36 items
- TP
- transperineal biopsy
- TPM
- template prostate mapping
- TRUS
- transrectal ultrasound
- UTI
- urinary-tract infection
- VAS
- visual analogue scale
- YHEC
- York Health Economics Consortium
